Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 14/186/11. The contractual start date was in January 2017. The final report began editorial review in February 2020 and was accepted for publication in October 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Littlewood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Depression and long-term conditions
Depression is the largest cause of disease burden of all mental health problems and is estimated to become the highest among all health problems by 2030. 1 It accounts for 4.3% of the global disease burden and causes 63 million disability-adjusted life-years each year. 1 Approximately 30% of the UK population have a long-term health condition (LTC) (e.g. diabetes mellitus or asthma), and in those who do there is a two- to threefold increase in the prevalence of depression. 2 This combination of depression and LTCs can result in poorer health outcomes, lower quality of life and a reduced ability to self-manage, and contributes substantially to health inequalities. 2–4 Furthermore, people with depression are more likely to have two or more LTCs and this group experience the greatest decrements in quality of life. 5
The importance of comorbid depression and LTCs (mental–physical multimorbidity) is well established from the epidemiological and economic perspectives. The cost of care for people with mental–physical multimorbidity is 45% higher than for those without. 2 The NHS Five Year Forward View6 recognises that 30% of the population live with a LTC, the treatment of which accounts for 70% of total NHS costs; the most common and disabling multimorbidity is physical health alongside common mental disorders. 7 There is a need for an integrated patient-centred approach to support the mental–physical care of this underserved population. 7 The NHS Long Term Plan8 emphasises the need to provide better care for people with LTCs with expanded neighbourhood teams comprising a range of staff, such as general practitioners (GPs), pharmacists, district nurses, community geriatricians, dementia workers and allied health professionals (e.g. physiotherapists and podiatrists/chiropodists), joined by social care and the voluntary sector. 8
Subthreshold depression
Subthreshold depression is highly prevalent and is a major risk factor for progression to major depression. 9 It is defined as between two and four symptoms of depression, at least one of which must be either low mood or loss of interest or pleasure. 10 With comparable rates of excess mortality to those of major depression,11 estimates suggest that the prevalence of subthreshold depression in community samples ranges from 1.4% to 17.9%. 12 Prevalence estimates increase to up to 20% in those with LTCs; for this population, clinical guidelines recommend brief psychological support. 13 Although psychological interventions can reduce symptoms of depression in those with subthreshold depression (thus reducing the incidence of major depression), over 80% of people with ‘below-threshold’ mental health conditions remain untreated in psychological health-care services because they are often unable to meet the demand for treatment of major depression. 14 However, when people with frailty or physical health problems are referred to mental health services, they often do not make use of such services because of access issues and they often find the psychological approaches on offer unacceptable. 15 There is perhaps a need to move beyond traditional reliance on health services delivery; a public health approach to subthreshold depression in at-risk groups, such as those with LTCs, may offer alternative opportunities to provide health-care interventions in innovative and accessible settings.
Community pharmacies
In England, community pharmacies (CPs) are the most accessible and available health-care provider in the community, with 89% of the population able to access a CP from home within a 20-minute walk. 16 Importantly, in areas of the highest deprivation, this figure increases to almost 100%, an observation termed the positive pharmacy care law. 17,18 In terms of delivering health-care services to the people who need the services the most, CPs are uniquely placed in our local communities to achieve this. At present, there are approximately 11,600 CPs in England. Many of these are open in evenings and at weekends, which allows a range of different people, including those who work during the day, access to health care and public health services. 16 They offer people the opportunity to consult with a community pharmacist without a prior appointment, at a convenient time and in an accessible location. Published qualitative research also suggests that people often form trusting relationships with their local community pharmacist, which means that they are more likely to consult with them and ask for health advice than they are to consult with some other health-care professionals. 19 Further work has also shown that people with mental health conditions value CP services, particularly those reflecting patient-centred care, and are happy to engage with such services. 20 Examining experiences of CP users more broadly showed that people do not expect to routinely receive public health services in this setting, but that when they do they are highly satisfied with such services; however, there were mixed views on the ability of the pharmacist to do this effectively. 21
Community pharmacies, therefore, have the potential to deliver services that are aimed at promoting health and preventing disease. Recent examples of these services have focused on health promotion, including smoking cessation and weight management services. 22 These opportunities are also supported by recent policy drivers that allow CPs to deliver more patient-focused services, as outlined in the recently published Summary of the Five-Year Deal on the Community Pharmacy Contractual Framework. 23 The potential of the CP network has also been acknowledged by policy-makers as a way of helping the NHS to deliver on its long-term strategic plan. 24
An example at the national level of the changing role of the CP is the Healthy Living Pharmacy (HLP) programme. This is a tiered and structured commissioning framework that aims to deliver high-quality, patient-focused services from CPs to improve the health and well-being of the local population. 25 Crucially, the framework has provision to involve the wider pharmacy team who are able to train as Healthy Living Champions (HLCs): a formal qualification in understanding health improvement, accredited by the Royal Society of Public Health. Since April 2020, all CPs in England have been required to obtain HLP status as part of the agreement between the Pharmaceutical Services Negotiating Committee, NHS England and NHS Improvement, and the Department of Health and Social Care.
These developments support and complement the existing patient-focused services available through CPs, such as the flu vaccination and smoking cessation. A recent review of reviews26 examined the effectiveness of CP-delivered public health services and concluded that there are a number of CP services that have positive intervention effects on health outcomes. These services are predominantly focused on primary disease prevention and include smoking cessation, weight management programmes, syringe exchange programmes and inoculation services. The review also concluded that, although there is evidence of CP-delivered services being able to target people with physical health conditions, there is a dearth of literature exploring if (and how) CPs could deliver public mental health services and, given the reach of CPs in deprived areas, how these services could impact on health inequalities.
Developments such as the HLP programme lend support to the NHS and Public Health England’s Five Year Forward View6 plan, which calls for a radical upgrade in public health, including new partnerships that ‘break down barriers’ to support people with multiple health problems. In support of this, and owing to the socioeconomic disparities and the impact of physical–mental comorbidities, the Marmot27 report on health inequalities recommended a focus on the mental health of people with LTCs. There are many similarities between the behaviour change/management approaches to subthreshold depression (i.e. goal-setting, facilitated self-help and diary-keeping) and the public health interventions already being delivered by HLCs in CPs. CPs may, therefore, be well placed to offer and deliver opportunistic enhanced support to people with a range of health problems, such as comorbid subthreshold depression and LTCs, alongside the health promotion services and behaviour change programmes that they already provide. 28
Evidence for enhanced support interventions
A number of non-pharmacological treatments are recommended by the National Institute for Health and Care Excellence (NICE) for depression in people with LTCs. 12,13 A recent Cochrane review29 highlighted collaborative care as having particular relevance to people with multimorbidity. Collaborative care ensures delivery of effective forms of treatment30 and uses session-by-session symptom profiling, medication management, patient education and brief evidence-based psychological interventions to optimise outcomes. 31,32 The evidence supporting collaborative care for people with depression33,34 is strong, especially when it includes a structured psychological intervention. 35 Collaborative care for depression is also effective when delivered by non-mental health specialists and for comorbid LTCs. 36
This collaborative care framework has been adapted to include a low-intensity psychosocial intervention for subthreshold depression. 37,38 The Collaborative Care for Screen Positive Elders (CASPER) trial38 developed an effective intervention for people with subthreshold depression (90% of whom also had comorbid LTCs) within primary care. This collaborative care intervention involved facilitated self-help and was based on behavioural activation (BA) supported by non-mental health specialists. This study reported a significant reduction in depression symptoms at 4 and 12 months (with an effect size of 0.30) for those people in the intervention group compared with those in the usual-care group. Importantly, significantly fewer people in the intervention group than in the usual-care group progressed to major depression at 12 months. 38 This finding supports existing depression prevention literature30 and lends support to the use of similar non-mental health specialist-supported interventions to target at-risk populations. 39,40 Further research is now needed to establish the suitability and effectiveness of such a prevention intervention as a public health initiative.
Rationale for CHEMIST
The Community Pharmacies Mood Intervention Study (CHEMIST) aimed to evaluate the potential for delivery of a behavioural change depression prevention intervention (similar to that implemented in the CASPER trial) within the important public health setting of CPs. Reviews of the literature at the time of the design of CHEMIST indicated that the existing evidence was not sufficiently robust, or experience in this area of research sufficiently developed, to support a definitive randomised controlled trial (RCT) at this stage in the evolution of this public health intervention. CHEMIST, therefore, sought to adapt ‘what works’ for people with subthreshold depression in primary care38,41 and evaluate this for use within a CP public mental health setting for people experiencing comorbid subthreshold depression and LTCs.
To the best of our knowledge, CHEMIST is the first to test the feasibility of an intervention (and associated study procedures) developed to utilise the unique opportunity of CPs to offer depression prevention alongside their other health promotion activities. CHEMIST aimed to provide high-quality evidence to inform the design and delivery of such a service and deliver the important preparatory work ahead of a definitive RCT that is needed to evaluate the clinical effectiveness and cost-effectiveness of such an intervention. CHEMIST adds to the existing limited evidence base on the use of CPs for the management of people’s mental health-care needs. 42,43
Research objectives
Feasibility study
-
To refine the bespoke enhanced support intervention (ESI) (including patient self-help materials, ESI manual and training) for implementation by CP staff to people with subthreshold depression and LTCs based on evidence-supported interventions in primary care.
-
To develop and refine study procedures (CP set-up and recruitment strategies; participant screening, recruitment and assessment; suitability of outcome measures; and data collection procedures) for testing in the external pilot RCT. 42
External pilot randomised controlled trial
-
To quantify the flow of participants (eligibility, recruitment and follow-up rates).
-
To evaluate proposed recruitment, assessment and outcome measure collection.
-
To examine the delivery of the ESI in a CP setting (ESI uptake, retention and dose) to inform the process evaluation.
-
To conduct a process evaluation, using semistructured interviews with participants, pharmacy staff and GPs across a range of socioeconomic settings, to explore the acceptability of the ESI within the CP setting, elements of the ESI that were considered useful (or not), and the appropriateness of study procedures. 42
Progression criteria
Progression criteria would inform the decision of the Trial Steering Committee (TSC) and the funder in the continuation of the study from the feasibility study to the external pilot RCT, and in the feasibility of conducting a definitive RCT (to test the clinical effectiveness and cost-effectiveness of the ESI for people with subthreshold depression and LTCs within a CP setting).
Feasibility study to external pilot randomised controlled trial
-
Recruit five participants at each of four to six CPs by month 6.
-
For 80% of participants to receive two or more ESI sessions within the 4-month post-recruitment period.
-
Collect valid (intended) primary outcome measure on 80% of recruited participants at the 4-month post-recruitment follow-up.
-
A fidelity score of ‘acceptable’ (3) or above to be achieved in at least 90% of assessed audio-recorded ESI sessions.
External pilot randomised controlled trial to a definitive randomised controlled trial
-
Recruit and randomise 100 participants across five or six CPs by month 20.
-
For 80% of participants randomised to the ESI to receive two or more ESI sessions within the 4-month post-randomisation period.
-
Collect valid (intended) primary outcome measure on 80% of participants at the 4-month post-randomisation follow-up.
Chapter 2 Feasibility study: methods
Parts of this chapter have been reported in Littlewood et al. 44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Some of the information in this chapter is reported in Chew-Graham et al. (paper under review).
Study design
This was a feasibility intervention study with a nested qualitative evaluation. Participants with subthreshold depression and at least one LTC were recruited and offered the intervention. Quantitative data were collected at baseline and at 4 months. Qualitative feedback and data were collected throughout the study.
A number of modifications were made throughout the delivery of the feasibility study; the majority of these were considered minor and did not involve changes to key aspects of the study design (e.g. eligibility criteria). These changes are detailed in Chapter 5. Analysis of the qualitative data was initially intended to involve normalisation process theory (NPT),45 but instead the data were analysed following a framework analysis46 using the theoretical framework of acceptability (TFA). 47 The rationale for this revised analysis plan is detailed in Qualitative Study, Analysis.
Study approvals
Ethics approval was sought and granted on 18 November 2016 by North-East – Newcastle and North Tyneside 2 Research Ethics Committee (16/NE/0327). Approval was also obtained from the North East and North Cumbria Clinical Research Network on behalf of study sites. The study is registered with the International Standard Randomised Controlled Trials Number (ISRCTN) registry (ISRCTN11290592).
Study sites
The study was conducted in eight CPs in the north of England. Five CPs were involved from the commencement of the study and three additional CPs were recruited during the recruitment period.
Participant eligibility
Inclusion criteria
To be eligible for inclusion in the feasibility study, participants needed to meet the following inclusion criteria:
-
had subthreshold depression
-
had at least one LTC
-
were aged ≥ 18 years.
Subthreshold depression was defined as two to four symptoms of depression, at least one of which must be either low mood or loss of interest or pleasure on the major depressive episode module of the Mini International Neuropsychiatric Interview (MINI). 48
Long-term conditions were defined according to the Department of Health and Social Care definition:
. . . a condition that cannot, at present, be cured; but can be controlled by medication and other therapies.
Department of Health and Social Care. 49 Contains public sector information licensed under the Open Government Licence v3.0
The LTCs were those that were included in the NHS Quality and Outcomes Framework50 at the time of recruitment and included exemplar conditions, such as arthritis, cancer, cardiovascular conditions, diabetes, respiratory conditions, stroke and progressive conditions.
Exclusion criteria
People were ineligible for the study if they met any of the following exclusion criteria:
-
had alcohol or drug dependence
-
had cognitive impairment
-
had bipolar disorder, psychosis or psychotic symptoms
-
had active suicidal ideation
-
were in receipt of psychological therapy.
Participant recruitment
Potential participants were identified via CPs or general practices using a number of recruitment methods. These recruitment methods were refined and/or implemented as the feasibility study progressed (see Chapter 5).
Potential participants were deemed eligible to receive study information via their CP or general practice if they met the following inclusion criteria (from those described above):
-
were aged ≥ 18 years
-
had a least one LTC.
An assessment of depression symptoms was conducted by the study team as part of the screening assessment with those potentially eligible participants who returned a consent form to the CHEMIST study team (see Screening for eligibility).
Study information pack
Potentially eligible participants were given the opportunity to receive a study information pack (see Community pharmacy-based recruitment and General practice-based recruitment). This contained an invitation letter (printed on CP- or general practice-headed paper, as appropriate), a patient information sheet (PIS), a consent form, a background information sheet (see Report Supplementary Material 1) and a prepaid return envelope. The PIS advised interested CP customers/patients to complete and return the consent form and background information sheet to the study team in the prepaid envelope provided. They would then be contacted by the study team to discuss the study and to assess their suitability to take part.
Community pharmacy-based recruitment
Four methods of CP-based recruitment were employed during the feasibility study. All of the approaches were recorded (including the method of approach) on the customer’s patient medical record (PMR) to avoid duplication of approach via these recruitment methods.
Opportunistic approach by community pharmacy staff
Potentially eligible participants (CP customers) who attended the CP were approached by CP staff (see Study training) and were advised that the CP was taking part in a research study involving people with LTCs and were invited to receive information about the study. Interested CP customers were provided with a brief information sheet (BIS) (one page) to read while they were waiting to collect their prescription or to take away and read at home (see Report Supplementary Material 2). The BIS included the two short Whooley depression case-finding questions51 recommended by NICE. 13,52 A positive response to one or both of these two questions indicated suitability for further assessment of depression symptoms. The BIS advised CP customers that if they responded positively to either/both questions they may be suitable for inclusion in CHEMIST, and to request a study information pack from their CP to take away and read. The BIS included contact details for the CHEMIST study team if the individuals had any questions about the study. CP customers may have been attending the CP for a variety of reasons related to their LTC, for example to access services, such as the medicines use review (MUR) and new medicines service, or to collect their prescription, or for reasons unrelated to their LTC; therefore, the study approach could come from any CP staff member.
Community pharmacy customers who requested and were provided with a study information pack were asked for their ‘verbal consent to contact’. This was a verbal agreement for a member of the CHEMIST study team to contact them to discuss the study. CP customers providing their ‘verbal consent for contact’ gave the CP staff their name and contact details. These were then securely faxed to the CHEMIST study team who would then contact the CP customer a minimum of 2 days following the date of verbal consent to discuss the study and answer any questions.
Home-delivered prescriptions
Potentially eligible CP customers who received their prescriptions via the CP home delivery service were provided with a study information pack with their delivered prescription.
Community pharmacy system search
Community pharmacy staff conducted searches on their systems to identify potentially eligible CP customers. Lists of potentially eligible CP customers were reviewed by appropriately trained CP staff for exclusion of any customers whom it would be inappropriate to invite or any customers who had been previously invited by an alternative method. Following this check, the CP posted a study information pack to potentially eligible CP customers.
Study posters
Study posters were displayed in recruiting CPs (see Report Supplementary Material 3). These encouraged CP customers to contact the CHEMIST study team directly or speak to the CP staff for more information about the study and to request a study information pack.
General practice-based recruitment
General practices that were located nearby participating CPs conducted database searches on their systems to identify potentially eligible patients. Searches were restricted to patient postal codes within 2 miles of the participating CP(s) to ensure patient suitability of access, and included the exclusion criteria (see Participant eligibility). Lists of potentially eligible patients were reviewed by practice staff to exclude patients whom it would be inappropriate to invite. Practice staff then posted a study information pack to eligible patients.
Screening for eligibility
On receipt of a completed consent form, the potential participant was contacted by telephone by a trained CHEMIST researcher. The researcher provided a detailed explanation of the study, emphasising that the person may withdraw their consent to participate at any time without providing a reason, and that this would not affect the care that they received from their CP or GP. Potential participants were provided with an opportunity to ask any questions that they may have about the study. The researcher then confirmed the person’s LTC(s); if the person did not report having a LTC they were ineligible to take part and the screening interview was terminated at that point. The major depressive episode module of the MINI48 was then administered to assess for depression symptoms and disorders (subthreshold depression/major depression). The MINI is a standardised diagnostic interview that determines the presence or absence of depression (and other common mental disorders) in accordance with internationally recognised criteria. 53,54
Potentially eligible participants who met the criteria for subthreshold depression (indicated by two to four depression symptoms on the MINI) were then asked additional screening questions to check exclusion criteria (i.e. alcohol/drug use, bipolar disorder/psychosis/psychotic symptoms and current use of psychological therapy). Those who met any of the study exclusion criteria were not eligible to take part and were advised to speak with their GP, where appropriate. Eligible participants were then informed of their eligibility and arrangements were made to complete the baseline assessment (see Baseline assessment). Participants were reminded that their GP would be informed that they were taking part in the study (participants were required to provide their written consent for this to be included in the study).
Those who did not reach criteria for subthreshold depression (indicated by fewer than two depression symptoms on the MINI) or who met criteria for major depression (indicated by five or more depression symptoms on the MINI) were ineligible to participate, and the additional screening questions were not asked. If major depression was present, the person was informed of the probable presence of depressive symptoms and were advised to speak with their GP. In addition, a letter was sent to the person’s GP (with the person’s consent) informing them of the probable presence of depression and that further assessment/treatment may be required.
A telephone appointment screening letter was sent to those potential participants who could not be reached by telephone following several attempts. If this appointment was not attended, potential participants were sent a second letter that advised them to contact the study team if they would like to take part, otherwise there would be no further contact from the study team.
Baseline assessment
Eligible participants were posted a baseline questionnaire to complete and return to the study team in a prepaid envelope (see Outcome measures; see Appendix 1 for non-validated feasibility baseline questionnaires). The questionnaire included a £5 note where participants had provided their consent for this. If participants indicated that postal completion would be difficult, a telephone appointment was arranged with a researcher. A blank copy of the questionnaire (and £5 note where applicable) was posted to the participant to facilitate the subsequent telephone interview. A copy of the participant’s completed consent form was sent with their baseline questionnaire.
On receipt of a completed baseline questionnaire, a researcher contacted the participant by telephone to advise them that they would shortly be contacted by a ‘Healthy Living Advisor’ (a member of the CP team trained to deliver the CHEMIST intervention) to arrange their first pharmacy support session (see Intervention). They were then posted a copy of the self-help workbook and associated materials. Where applicable during this contact, the researcher also obtained any missing data or clarified any unclear responses on the returned baseline questionnaire (the date and method of any additional data collected/clarified was recorded on the questionnaire).
Participants who did not return a baseline questionnaire were sent a reminder letter (with a further copy of the baseline questionnaire) and/or were contacted by telephone to confirm receipt and check if they required help with completing the questionnaire. Participants who did not return a baseline questionnaire following these reminders were judged to be no longer interested and were not considered as recruited to the study.
Sample size
Given that the feasibility study was designed to refine the intervention and develop the study procedures for testing in the pilot RCT, the sample size required for the feasibility study was small: between 20 and 30 participants.
Intervention
No treatment was withheld from study participants.
The intervention is described in line with the Template for Intervention Description and Replication (TIDieR) checklist. 55
Intervention name
The intervention was an ESI: a brief psychological intervention (BA) delivered within a collaborative care framework.
Rationale
The ESI was based on a collaborative care approach and involved BA as the core psychological component.
The CHEMIST ESI was adapted from existing training and intervention materials. These materials have undergone extensive development exploring the theoretical framework, acceptability and validity in previous multicentre RCTs. 38,56 The existing intervention and training materials were refined for use in CHEMIST to provide a focus on functioning in people with mental–physical multimorbidity. This was achieved through discussions with CHEMIST co-applicants, members of the research team and with a range of stakeholders (see Patient and public involvement).
Central to the ESI were CP staff (referred to as ‘ESI facilitators’ hereafter; referred to as ‘Healthy Living Advisors’ in participant-facing information) who were trained to deliver the ESI with study participants. ESI facilitators were responsible for employing BA techniques to support study participants to work through a self-help workbook and for proactively following up with participants, monitoring depression symptoms and facilitating communication with other members of the participant’s health-care team to ensure synergy with any current care being provided. These key elements of the ESI are described in the following sections. This approach is responsive to individual patient needs and fits the core elements of BA and collaborative care (see the following sections).
Collaborative care
Collaborative care is based on chronic disease management models and may be useful in the management of common mental health problems. 35 It is a way of managing the treatment of an individual that seeks to maximise the integration of their care across all of the health-care professionals who make up their health-care team. This is achieved through the input of a person who forms part of the patient’s care team, often called a case manager (referred to as ‘ESI facilitator’ within CHEMIST), who liaises with all members of the team when necessary. A brief psychological intervention (such as BA) may be delivered by the case manager in addition to their liaison role. They may also help with medication management and treatment adherence.
Behavioural activation
At the core of the ESI is a brief psychological intervention based on utilising BA. 57 BA focuses on addressing the behavioural deficits common among those with depression and long-term health problems by reintroducing positive reinforcement of functional behaviours and reducing avoidance. Supporting people to identify and reintroduce valued activities that they have stopped doing by reason of physical frailty, or activities they would like to take up, is an important component of BA; previous work has demonstrated that this is helpful for people with functional deficits secondary to long-term health problems. 58
Behavioural activation is a simple intervention that has four key elements. The initial step introduces the BA cycle and how life changes, such as a long-term health condition, have resulted in a reduction in positive reinforcement from valued activities. Resultant low mood then leads to increased avoidance to mitigate the moment of distress. The cycle highlights how this avoidance subsequently further distances the person from the valued activity, which results in a downward spiral of mood and functioning. Using the combination of the collaborative care and BA structure, this treatment explanation is individualised to the specific patient and draws on their priorities and values to understand what changes are important for them.
Building from this, the core elements of BA (self-monitoring, activity scheduling and functional analysis) are then used to break the cycle. Self-monitoring identifies the link between mood and activity/functioning. Activity scheduling targets behaviours that are important and useful to the individual patient in the context of their health priorities and plans these in a diary to increase positive reinforcement. This approach introduces the concept of ‘rather than waiting to feel better to do something, do something to feel better’. Functional analysis introduces an ‘Antecedent, Behaviour, Consequence’ breakdown of problem areas identified in the course of the intervention to support the change process. In CHEMIST, the adapted ESI utilised these core BA strategies to address problems of functioning and subthreshold depression associated with long-term health problems. BA strategies were individualised based on individual patient’s needs using a BA patient self-help workbook and contact with and support from the ESI facilitator (or case manager, as described earlier).
The CHEMIST enhanced support intervention
The CHEMIST ESI consisted of four main elements:
-
Self-help support – this focused on BA (as described above) with the provision of a self-help patient workbook, supported by the ESI facilitator.
-
Proactive follow-up – the participant’s use of the self-help patient workbook was supported by the ESI facilitator via ESI sessions (face-to-face and/or telephone delivery) conducted at regular (weekly) intervals. ESI facilitators were encouraged to be proactive in scheduling and following up patient contact/sessions where necessary.
-
Depression symptom monitoring – depression symptoms were monitored at each ESI session by the ESI facilitator, using the depression scale from the Depression Anxiety Stress Scale (DASS). 59 The DASS is widely used and validated in a UK community context and is sensitive to change over time. 60 It is brief and simple to score with clear clinical cut-off scores (non/mild/moderate/severe depression symptoms).
-
Decision-supported signposting – scores on the DASS were used to guide decision-making by the ESI facilitator and guided by supervision provided by clinical members of the research team. Where significant clinical/symptom deterioration was observed, the participant was supported to access more formal health-care interventions and was encouraged to contact their GP to discuss this, or the ESI facilitator would talk with their GP directly if the participant so wished. Through discussion with the participant, other areas where functioning deficits may exist could be identified. These might include necessary adaptations within the home, access to transport or financial advice. If it was felt that the involvement of other services, such as social services or voluntary sector organisations, was warranted, the ESI facilitator could help to enable access to these (with support provided where necessary via clinical supervision from the CHEMIST study team).
Materials and procedures
Study participants received a 28-page self-help workbook that was divided into eight stages (‘stages to keeping well’). The stages covered recognising symptoms of low mood/depression, diary-keeping, defining activity types, breaking activities down, identifying benefits of activities, identifying ways to be active, spotting symptoms of depression and setting an action plan. The workbook also included a number of tasks for participants to complete (e.g. making a list of activities that keeps the person well). A notes section was included at the end of the workbook, and participants were provided with additional copies of the diary and task pages. The workbook was used during and between contacts with the ESI facilitator. Participants were also provided with a blank copy of the DASS for use during each session.
The ESI was supported by a detailed ESI facilitator manual that included an outline of the key elements of the ESI, detailed session-by-session content, the DASS and the risk protocol (see Risk management). The ESI facilitator manual was supplemented by participant treatment logs, in which session information (e.g. scores on DASS, outcome of risk assessment and general session notes) was recorded for each participant and used to guide clinical supervision.
The CHEMIST ESI and training materials are not currently available outside the context of the study.
Enhanced support intervention facilitators were provided with information about study participants via the study team (following randomisation in the pilot RCT; see Chapter 6). The assigned ESI facilitator would contact the participant to arrange their first intervention session (to take place face to face in the pharmacy consultation room or over the telephone). Subsequent sessions were arranged during the previous session. Participants were encouraged to contact their ESI facilitator if they were unable to attend sessions so that these could be rearranged. ESI facilitators were advised to proactively contact participants who did not attend prearranged sessions. Contacts were monitored during clinical supervision.
Enhanced support intervention sessions followed a schedule outlined in the ESI facilitator manual. Each session involved a discussion between the participant and the ESI facilitator based on the stages within the self-help workbook. The first session included discussing how the participants were, including how they felt physically and mentally. Information was gathered on how the participant’s activities were being affected by their mood or physical health, as well as their understanding of the self-help workbook. Subsequent sessions began by gathering information related to the participant’s understanding of the previous session, discussion of the work undertaken since the previous session and reflection in relation to the BA approach. Opportunities to collaborate with health-care or social care professionals and voluntary sector organisations were identified and discussed. Support to access such services was provided if the participant wished. The DASS was administered during all sessions to monitor depression symptoms and the outcome was discussed. Potential risk was checked at the beginning of all sessions and the risk protocol was activated if necessary (see Risk management). Finally, the next stage(s) of the self-help workbook was discussed and the ESI facilitator and participant agreed the work to be undertaken before the next session.
Intervention providers
The CP pharmacist and/or pharmacy manager identified and approached suitable CP support staff about the study and the ESI facilitator role. ESI facilitators were CP support staff experienced in the delivery of pharmacy extended roles (such as smoking cessation behavioural–change approaches) and/or training to Royal Society of Public Health standards (Understanding Health Improvement Level 2).
Enhanced support intervention facilitators attended a 2-day training workshop prior to delivering the ESI. The workshop included presentations (copies of presentation slides were provided) that outlined the study and explained the concepts of BA and collaborative care. Clinical scenarios were used to outline the ESI rationale and process. The materials to be used as part of the ESI (patient self-help workbook, ESI facilitator manual and participant treatment logs) were provided and described. Clinical role-play exercises were used to practise skills and ESI session content based around the self-help workbook. Opportunities were provided to discuss the ESI and the role-play exercises. The workshop also included training on risk assessment (with opportunities to practise this via role-play) and other important study procedures [including serious adverse event (SAE) reporting and participant withdrawal]. ESI facilitators were required to undertake and pass a telephone-based competency assessment before they could be assigned an ESI participant.
Enhanced support intervention facilitators received telephone supervision from a clinical member of the research team on a session-by-session basis.
Mode of delivery
The ESI was designed to be delivered on a one-to-one basis by a combination of face-to-face and telephone contact (based on participant preference). Participants were offered the first session as a face-to-face contact within the CP, with telephone delivery encouraged for subsequent sessions.
Location
The ESI was delivered via CPs located in the north of England.
Personalisation
Although all participants received the same self-help workbook, the ESI was designed to be personalised by the ESI facilitator to the participant’s situation and long-term health problems using the self-help workbook as a guide.
Intervention dose
The ESI was designed to be between four and six sessions, delivered where feasible on a weekly basis over a period of up to 4 months. The first session was designed to take up to 60 minutes, with subsequent sessions taking between 20 and 30 minutes.
Modifications
The ESI was adapted from existing intervention and training materials. The ESI was not modified during the course of the feasibility study. Modifications were made following the feasibility study in advance of the pilot RCT; these modifications are reported and described in Chapters 5 and 6.
Competency and intervention fidelity
The competency of ESI facilitators to deliver the ESI in line with training and the ESI facilitator manual was assessed using procedures adapted from previous related studies. 61 Following the ESI training workshop, mock telephone ESI sessions were conducted with ESI facilitators, and behaviours and performance were evaluated against a competency checklist. ESI facilitators were offered additional training, if necessary, to enable them to achieve the required standard to pass the competency assessment.
Fidelity to the ESI was supported by the ESI facilitator manual, which included ESI session guides. Telephone supervision was offered by clinical members of the research team after every ESI session. ESI facilitators also had access to ad hoc telephone supervision if they required further support. Fidelity assessment also involved obtaining audio-recordings of ESI sessions (where participant consent was provided for this). A random selection (10–20%) of audio-recordings across different phases of the ESI (early/late) was to be independently reviewed and assessed against a fidelity checklist. The feasibility of obtaining these audio-recordings was to be explored in the feasibility study, given that the delivery of the ESI to research participants was a new approach for CPs.
Follow-up
Participants were followed up 4 months after completion of their baseline questionnaire (see Outcome measures; see Appendix 2 for non-validated follow-up questionnaires).
Those participants who completed their baseline questionnaire via post were sent a 4-month follow-up questionnaire to complete and return to the study team in a prepaid envelope. Participants who completed a telephone baseline questionnaire were contacted by telephone by a researcher to determine how they wanted to complete the follow-up questionnaire. Telephone follow-ups were arranged where this was requested and a blank copy of the questionnaire was posted in advance of the telephone appointment. A £5 note was included with posted follow-up questionnaires where participant consent for this was provided. Participants who did not return a follow-up questionnaire were sent a reminder letter (with a further copy of the follow-up questionnaire) and/or were contacted by telephone to confirm receipt and check if they required help with completing the questionnaire. Where applicable, participants were contacted by telephone to obtain missing data or to clarify any unclear responses on returned follow-up questionnaires (the date and method of any collection of additional data or clarification were recorded on the questionnaire).
Participants were sent a study completion letter on receipt of a completed follow-up questionnaire.
Outcome measures
Participants completed a number of measures to assess the quality and acceptability of data collection at the various time points during the feasibility study and to inform the pilot RCT phase and any future definitive RCT (see Chapter 1).
Participants completed a background information sheet at the point of consent to the study (see Report Supplementary Material 1). This included the two Whooley depression case-finding questions51 and demographic information. The Whooley questions and a subset of the demographic information were completed again as part of the 4-month follow-up questionnaire. It was also planned for participants to complete the 9-item Behavioural Activation for Depression Scale (BADS)62 during ESI sessions in order to inform the process evaluation in the pilot RCT. The BADS is a measure of activation and is a tool commonly used in studies involving behavioural activation for depression. Additional information (to include depression onset age and number of episodes) obtained via the MINI at baseline would also inform the pilot RCT process evaluation.
Intended primary and secondary outcome measures were collected at baseline and at the 4-month follow-up.
See Appendix 3 for the data collection schedule.
Intended primary outcome measure
The intended primary outcome measure was self-reported depression severity at the 4-month follow-up, as measured by the Patient Health Questionnaire-9 (PHQ-9). 63 The PHQ-9 is widely used in clinical and research settings and has good sensitivity and specificity in a UK population. 64
Intended secondary outcome measures
-
Self-reported binary depression severity (PHQ-9),63 using a score ≥ 10 to indicate moderate depression caseness at the 4-month follow-up (thus providing a measure of the potential preventative aspects of the intervention in their ability to prevent progression of depression).
-
Anxiety [Generalised Anxiety Disorder-7 (GAD-7) scale]. 65
-
Physical/somatic health problems (PHQ-15). 66
-
Health-related quality of life [Short Form-12, version 2 (SF-12v2)]. 67
-
Health state utility [EuroQol-5 Dimensions, three-level version (EQ-5D-3L)]. 68
-
Health and social services use collected via a bespoke resource use questionnaire.
Study completion and participant withdrawal
Participants were considered to have exited the study when (1) the participant had completed their 4-month follow-up (and qualitative interview if this was to be conducted following the 4-month follow-up; see Qualitative Study), (2) the participant wished to fully withdraw from the study, (3) the participant’s GP advised full withdrawal from the study or (4) the participant had died.
Where participants expressed a wish to withdraw from the study, they were given the choice of (1) withdrawal from the ESI sessions only (participants were still followed up at 4 months and could participate in a qualitative interview), (2) withdrawal from follow-up (participants could continue to receive the ESI sessions up to the 4-month follow-up time point) or (3) full withdrawal from the study, including the ESI and follow-up. Where withdrawal from the study was because of a SAE, the SAE standard operating procedure (SOP) was followed (see Serious adverse events).
Where possible, data were collected on reasons for withdrawal. Participants were reminded that withdrawal from the study would not affect the care that they received from the CP or their GP. Data collected from participants up to the point of full withdrawal were still included in any data analyses, unless the participant requested that their data were not used in the analysis.
Quantitative data analysis
Owing to the small sample size for the feasibility study, no formal analysis was planned. A single analysis was performed at the end of the feasibility study using Stata® v15 (StataCorp LP, College Station, TX, USA). Feasibility baseline data and all standardised measures are summarised descriptively, and the number of missing responses detailed. Continuous data are reported using means, standard deviations, medians, and the minimum and maximum. Categorical data are reported as counts and percentages. The flow of participants through the study will be reported.
Outcomes
In the feasibility study, the outcomes of interest were recruitment and retention rates, the quality of data collected and engagement with the ESI. The number of pharmacy customers/patients approached and the number of participants recruited and who completed the 4-month follow-up are detailed, along with the completeness of the standardised measures used. The number of ESI sessions attended and the number of participants who did and did not start the ESI sessions will be detailed, with reasons provided where possible.
Serious adverse event data were summarised descriptively by treatment arm.
Qualitative study
A qualitative evaluation was nested within the feasibility study with the primary aim of exploring the feasibility and acceptability of the study, ESI training and delivery, and study procedures. Qualitative interviews were conducted with study participants and ESI facilitators, and a focus group was held with CP staff. The qualitative findings would inform adaptations for the pilot RCT (see Chapter 5).
Participants and recruitment
Semistructured interviews
The aim was to conduct in-depth semistructured interviews with (1) up to 10 study participants (purposively sampled, where feasible, across recruiting CPs and with a mix of LTCs and from different areas of deprivation) to explore the acceptability of the ESI and delivery within a CP setting; and (2) approximately 10 ESI facilitators following ESI training and/or delivery of the ESI, to explore the acceptability and feasibility of the ESI training and delivery, and the study procedures.
Study participants provided their consent to participate at the same time as consenting to the feasibility study (see Report Supplementary Material 1 for a copy of the participant consent form). Participants who declined to consent to an interview were still eligible to participate in the feasibility study.
Study participants who had provided their consent for an interview were contacted by telephone by a member of the research team once they had finished their ESI sessions. Study participants who did not commence ESI sessions or who started ESI sessions but then dropped out were also approached to take part in an interview (where they had provided consent for this) to explore their reasons for disengagement. Study participants were advised that interviews would last around 45 minutes and could be conducted at a time and location convenient to them (e.g. at home, in the pharmacy or by telephone).
All ESI facilitators were invited to participate in an interview. Following completion of the ESI training, ESI facilitators were sent a study invitation pack (containing an invitation letter, PIS, consent form and prepaid return envelope; see Report Supplementary Material 4) in the post (individually addressed to them at the pharmacy). Interested ESI facilitators were required to send their completed consent form directly to the study team. This process was carried out independently from the pharmacy (including pharmacy managers and co-workers) to ensure that ESI facilitators felt comfortable to talk about their experiences.
Enhanced support intervention facilitators who returned a consent form following the ESI training were contacted by telephone by a member of the research team to arrange an interview to explore their experiences and views of the ESI training. Once delivery of the ESI had commenced, those ESI facilitators who had participated in an initial (training) interview and who had supported at least one participant through the ESI were invited to participate in a brief second interview to explore their experiences of delivering the ESI. A second reminder invitation pack was posted to those ESI facilitators who did not respond to the initial invite. Those ESI facilitators who returned a consent form following this second invite participated in a single interview to explore their experiences of the ESI training and ESI delivery. ESI facilitators could indicate whether they wanted to be interviewed in person, in a private room at the pharmacy or over the telephone, and a time convenient to them.
To ensure confidentiality and to encourage interviewees to speak freely, interviews with ESI facilitators were not conducted with those study researchers who had been involved in their training (either ESI training or general study/recruitment training). Only anonymised interview transcripts could be accessed by those researchers involved in their training.
Focus group
The aim was to hold a focus group consisting of 8–10 CP staff, to include a range of CP roles from across the recruiting CPs. All CP staff (including ESI facilitators) within each recruiting CP were invited to take part in a focus group to explore their experiences of the study, including recruitment, delivery of the ESI, study procedures and impact on pharmacy routine practice. CP staff were invited to participate following the procedure described above (see Report Supplementary Material 5). Interested CP staff returned a completed consent form directly to the study team indicating their availability for a focus group. The focus group was held at a community facility away from their place of work.
Topic guides
Individual topic guides were used for each participant type (study participants, ESI facilitators, focus group with CP staff) (see Report Supplementary Material 6). Interview topic guides were developed based on existing literature and were amended iteratively throughout the data collection process.
Analysis
The interviews and focus group were recorded using an encrypted digital audio-recorder and were professionally transcribed verbatim. All data were analysed thematically initially using constant comparison69 followed by a framework analysis46 using the TFA to sensitise the analysis. 47 Although it was initially intended that a secondary analysis using NPT45 would be undertaken, as the analysis progressed the TFA was felt to be more appropriate as this framework offers the opportunity to focus on acceptability of the ESI and ESI training, and what modifications are needed for the pilot RCT. The analyses were undertaken by a team of researchers with varying professional backgrounds to increase the reliability of the analysis. 70 Regular coding and analysis meetings were held to ensure that the emerging themes remained grounded in the data, and that the TFA appropriately represented the data.
Health economic analysis
Economic analysis was conducted with the aim to evaluate the feasibility of collecting data on costs and health-related quality-of-life outcomes to inform the pilot RCT. Data were collected at baseline and at 4 months post recruitment. The economic analysis evaluated the overall response rates, item completion rates and the range of values provided in response to the bespoke resource use questionnaire and health-related quality-of-life questionnaire, EuroQol-5 Dimensions (EQ-5D) (described below). 71
Health service resource use data were collected using a bespoke self-reported resource utilisation questionnaire (adapted from the Adult Service Use Schedule72) (see Chapter 2 and Appendix 1). The questionnaire collected data on the following service use categories in the previous 4 months:
-
General health and community service use (i.e. appointments with GP, nurse, NHS direct and walk-in centre, occupational health services, social worker or community support worker or drug and alcohol support worker). Participants were also asked how many of each of these visits were because of low mood.
-
Mental health services (i.e. appointments with psychotherapist or counsellor, clinical psychologist, community mental health team or community psychiatric nurse or consultant psychiatrist).
-
Hospital-based services [i.e. outpatient appointments, accident and emergency department visits, urgent care centre or minor injuries unit, or inpatient admission(s) with or without overnight stays].
Health-related quality of life was measured using the EQ-5D-3L questionnaire. 73 Individual-level responses on the EQ-5D were used to estimate health-related quality of life based on a UK population valuation set. The EQ-5D, originally developed by the EuroQol group, is a widely used measure of health-related quality of life. Using this measure, respondents are able to classify their own health on a three-point scale: 1 = no problems, 2 = some problems and 3 = severe problems. Health-related quality of life is measured over five dimensions: mobility, self-care, usual activities, pain and/or discomfort, and anxiety and/or depression. All questions refer to the participant’s health state ‘today’.
Quality of life was also measured using the SF-12v2 health questionnaire. 74 The SF-12v2 measures health by asking participants about eight domains of physical and mental health. These include the following:
-
general health – one item (SF-1) provides a rating of general health (levels 1–5: 1 = excellent, 5 = poor)
-
physical health – two items measure health limitations on moderate (SF-2) physical activities (e.g. moving a table) and more demanding (SF-3) physical activities (e.g. climbing several flights of stairs) (levels 1–3: 1 = limited a lot, 3 = not limited at all)
-
role physical (SF-4 and SF-5) – two items measure lower accomplishment (SF-4) and limited activity (SF-5) at work owing to physical problems (levels 1–5: 1 = all of the time, 5 = none of the time)
-
role emotional (SF-6 and SF-7) – two items assess limitations in work or daily activities owing to emotional problems (levels 1–5: 1 = all of the time, 5 = none of the time)
-
bodily pain (SF-8) – one item related to the presence of and limitations as a result of pain (levels 1–5: 1 = not at all, 5 = extremely)
-
mental health (SF-9 and SF-11) – two items assess feeling calm (SF-9) and feeling downhearted/low (SF-11) (levels 1–5: 1 = all of the time, 5 = none of the time)
-
vitality (SF-10) – one item assesses abundance of energy level and fatigue (levels 1–5: 1 = all of the time, 5 = none of the time)
-
social functioning (SF-12) – one item assesses limitations owing to physical or emotional problems (levels 1–5: 1 = all of the time, 5 = none of the time).
The SF-12v2 items refer to the participant’s health in the past 4 weeks (except general health and physical health that do not specify a time period).
Finally, the results of the cost and health-related quality-of-life data were reported in terms of the overall response rate for each questionnaire, rate of missing items within each questionnaire and level of health service resource use and health-related quality of life (by item/domain) at baseline and follow-up.
Serious adverse events
A study-specific SOP was implemented for the identification and reporting of suspected SAEs. Standard criteria for the definition of a SAE were used. Study researchers and ESI facilitators were instructed to report any suspected SAEs to the trial manager and chief investigator via a standard SAE reporting form.
All suspected SAEs were reviewed by a clinician independent to the study team. Any SAEs judged to be related to study treatment or procedures, or that were unexpected, were referred to the Research Ethics Committee. SAEs were reviewed by the TSC and Data Monitoring and Ethics Committee.
Risk management
Study-specific risk protocols were implemented for the identification, assessment and reporting of any risk relating to suicide or self-harm during all designated participant contacts (screening assessment, baseline and follow-up questionnaires, ESI sessions and qualitative interviews). Participants were asked specific questions about risk as part of the MINI (screening assessment) and PHQ-9 (baseline and follow-up questionnaires), and during each intervention session. A risk assessment was conducted immediately following a positive response to any of the specific risk questions asked, or in instances where participants voluntarily expressed thoughts of suicide or self-harm during any participant contact. The researcher asked the participant a set of questions to determine the level of risk and where necessary this was then discussed (within the same day) with a clinical member of the study team. Where any risk was identified, regardless of level of risk, the participant was advised to speak with their GP. Risk was reported to the participant’s GP (with the participant’s consent) where deemed necessary by a clinical member of the study team. Where risk was identified on a returned postal questionnaire, the participant was contacted within 24 hours of knowledge of the potential risk to conduct the risk assessment. Where contact could not be made within this time frame, the potential risk was discussed with a clinical member of the study team and relevant actions taken (this may include continuing to make contact with the participant after the 24-hour time frame or contacting the participant’s GP). At least one clinical member of the study team was on call to respond to risk during working hours (unless otherwise arranged) while participants were involved in the study. All instances in which a risk protocol was implemented were recorded and signed by the researcher conducting the risk assessment and the clinician who provided the risk advice.
Study training
All study researchers and ESI facilitators completed study-specific training on all those activities relevant to their role (to include assessment of risk) before commencing contact with participants. ESI facilitators completed a 2-day training workshop on the ESI (see Intervention). Study researchers and ESI facilitators were provided with support following risk assessments or any participant contact, if required, from relevant members of the study team. Study researchers completed good clinical practice training.
Study researchers delivered face-to-face study training with CP staff (including pharmacists/pharmacy managers and ESI facilitators where possible) from all recruiting CPs. The training provided an overview of the study, a detailed explanation of recruitment methods and processes, and associated study paperwork requiring regular completion. Training emphasised that all CP staff could be involved in the recruitment process. Study training was supported by a training document that CPs stored in their investigator site files. Recruiting CPs received regular telephone calls and/or visits from study researchers to discuss recruitment progress and to gather feedback on the study processes (including recruitment methods).
Patient and public involvement
CHEMIST benefited throughout its duration from the involvement from people who disclosed lived experience of mental health problems (including depression) and/or long-term health conditions. The study was developed in partnership with people (including service users and those accessing CP services) from the University of Durham Pharmacy patient and public involvement (PPI) Group and the Tees, Esk and Wear Valleys NHS Foundation Trust Service User Research Consultation Group. Members of these two groups provided feedback on the study research topic/question and the study and intervention design in advance of submission of the funding application via face-to-face group discussions and through follow-up e-mail communication.
A CHEMIST PPI advisory group (AG) was established from those members involved in the initial consultation and feedback process. Members were involved in the development of various recruitment materials (such as the PIS) and the patient self-help workbook, and were consulted on various study procedures. For example, the patient self-help workbook was amended to include less age-specific activity examples in the light of feedback from this group, and members suggested useful ways in which participant recruitment might be improved. Members were also consulted on various planned changes ahead of the pilot RCT phase. This lead to the restructuring of the PIS and a clearer explanation of instructions for completion of study questionnaires.
Expressions of interest were sought from PPI AG members for membership of the Trial Management Group (TMG) and the independent TSC. Two PPI AG members sat on the TMG and two (different) PPI AG members sat on the TSC. These four members contributed to TMG/TSC meetings where possible, either in person or via teleconference, and provided input into the running of CHEMIST (although two of these PPI AG members, one from each of the TMG and TSC, resigned from their roles on these committees and the PPI AG before the study finished).
In addition to the above PPI input, a CHEMIST special interest group consisting of public health specialists and CP staff (including pharmacists and counter staff) convened on three occasions throughout the development of the study and funding application to advise on aspects including local pharmacy recruitment procedures and materials. Throughout the duration of the study, meetings were held with Local Pharmacy Committees, Local Pharmacy Networks and those CP staff and public health specialists involved with the study (including those from recruiting CPs). This provided an opportunity to discuss issues relating to study delivery and progress (with a particular focus on recruitment processes and strategies) and study promotion within local CP community/networks.
The final study findings were discussed at a results and interpretation meeting before submission of this report; representation from the PPI AG was sought for this meeting, although, unfortunately, attendance was not possible. Two PPI AG members were invited to help with drafting the Plain English summary for this report and one member provided useful comments. Members of the PPI AG will be provided with a summary of the study findings and will be invited to provide input on the development and implementation of an appropriate dissemination strategy (to include the development of an accessible summary of the study findings to distribute to study participants). A summary of the findings will also be made available to all recruiting CPs and general practices, those involved in the special interest group, and local CP community/networks.
Chapter 3 Feasibility study: quantitative and health economic findings
Some of the information in this chapter is reported in Chew-Graham et al. (paper under review).
Quantitative results
Recruitment
The target sample size for the feasibility study was 20–30 participants, who were to be recruited over 5 months (February to June 2017) from four to six CPs (see Chapter 1, Progression criteria).
A total of 24 participants were recruited to the study over a period of 9 months (April to December 2017) and from across a total of eight CPs, indicating that recruitment was slower than anticipated. Five CPs commenced recruitment in April 2017; however, owing to the observed slow recruitment, the recruitment period was extended (by 4 months) and three additional CPs were recruited to the study (from August 2017). One general practice conducted a database search and mailed out study information packs to eligible patients (eligible and recruited participants were associated with a single recruiting CP). Figure 1 shows a breakdown of recruitment over the 9-month recruitment period.
FIGURE 1.
Feasibility study: target and actual recruitment rates. Reproduced with permission from Chew-Graham et al. 75 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
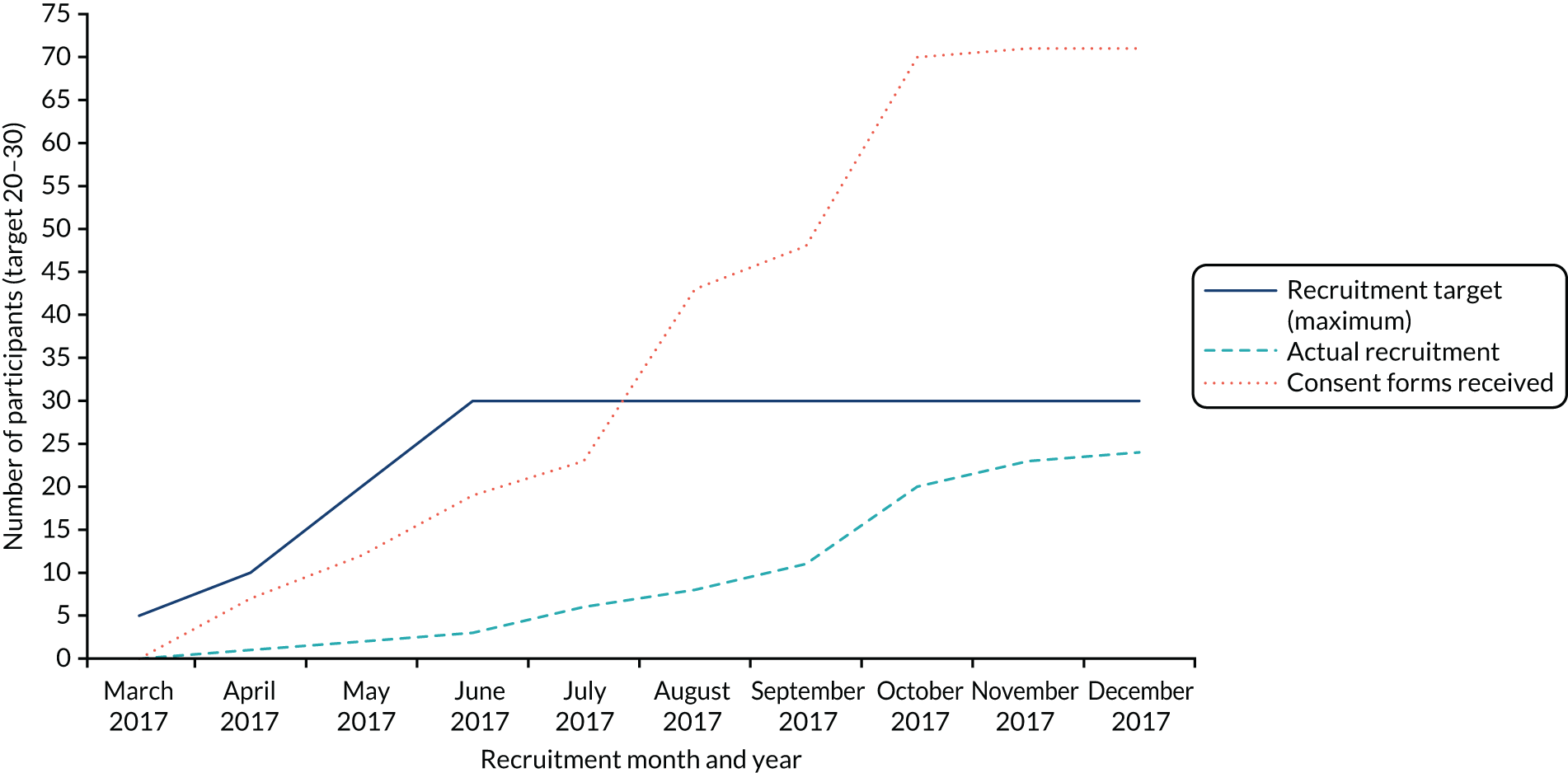
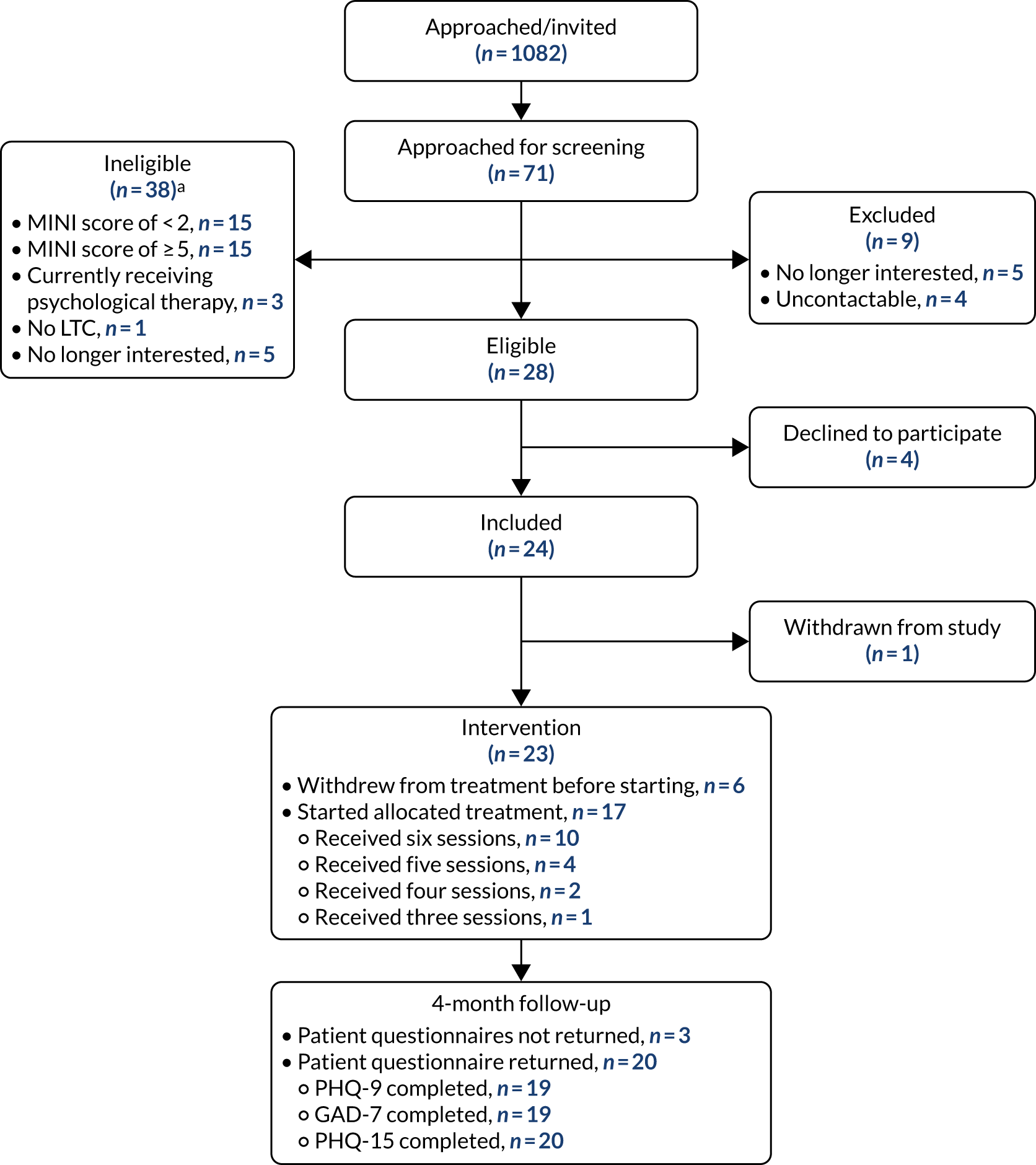
Overall, 1082 study information packs were distributed: 882 packs via pharmacy-based recruitment methods (opportunistic approach within the CP, via home-delivered prescriptions and via pharmacy system searches) and 200 packs via GP-based recruitment methods. From this, 71 pharmacy customers/patients returned a consent form to the study team to indicate their interest in the study (6.6%), of whom 28 were eligible to participate in the study following eligibility screening (39.4% of the 71 screened) and 24 (85.7%) decided to take part in the study. Reasons for ineligibility can be found in Table 1, with the most common reason being either non-depressed (34.9%) or currently experiencing an episode of major depression (34.9%). Table 2 details the distribution of study information packs, returned consent forms and resultant screened and recruited participants. Recruitment activity by pharmacy is shown in Table 3.
| Reason for ineligibility | Number of people ineligible, n (%) (N = 43) |
|---|---|
| Non-depressed (MINI score < 2) | 15 (34.9) |
| Major depressive episode (MINI score ≥ 5) | 15 (34.9) |
| Currently receiving any form of psychological therapy | 3 (7.0) |
| Does not have a CHEMIST LTC | 1 (2.3) |
| No longer interested when contacted for eligibility screening | 5 (11.6) |
| Uncontactable | 4 (9.3) |
| Recruitment method | Study information packs given out (n) | Returned consent forms, n (%)a | Recruited, n (%)a |
|---|---|---|---|
| In the CP | 168 | 32 (19.0) | 14 (8.3) |
| Via home-delivered prescriptions | 414 | 23 (5.6) | 6 (1.4) |
| CP system searches (one conducted) | 300 | 9 (3.0) | 2 (0.7) |
| General practice searches (one conducted) | 200 | 7 (3.5) | 2 (1.0) |
| Total | 1082 | 71 (6.6) | 24 (2.3) |
| Pharmacy | Study information packs given out (n) | Returned consent forms, n (%) | Participants recruited, n (%) | |||
|---|---|---|---|---|---|---|
| Pharmacy | GP | Pharmacya | GPa | Pharmacya | GPa | |
| 1 | 347 | 0 | 11 (3.2) | – | 2 (0.6) | – |
| 2 | 17 | 200 | 4 (23.5) | 7 (3.5) | 0 (0.0) | 2 (1.0) |
| 3 | 28 | 0 | 12 (42.9) | – | 5 (17.9) | – |
| 4 | 147 | 0 | 15 (10.2) | – | 7 (4.8) | – |
| 5 | 39 | 0 | 4 (10.3) | – | 2 (5.1) | – |
| 6 | 34 | 0 | 1 (2.9) | – | 0 (0.0) | – |
| 7 | 129 | 0 | 9 (7.0) | – | 5 (3.9) | – |
| 8 | 141 | 0 | 8 (5.7) | – | 1 (0.7) | – |
| Total | 882 | 200 | 64 (7.3) | 7 (3.5) | 22 (2.5) [34.4]b | 2 (0.2) [28.6]b |
The active recruitment period across CPs varied from 2.7 months to 7.2 months, with an average period of 5.5 months; however, it should be noted that the three pharmacies that were recruited part-way through the 9-month recruitment period were open to recruitment for a maximum period of 5 months. This gives an average recruitment rate of 0.55 participants per pharmacy per month. Rates of recruitment varied across the pharmacies, with the proportion of those participants screened who were subsequently recruited to the study ranging from 0 to 0.55 and with, on average, 30.3% of those screened participating in the study; Figure 2 details the screening and recruitment for each pharmacy. The flow of participants through the study is shown in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram in Figure 3.
FIGURE 2.
Feasibility study: overall screening and recruitment per pharmacy. Reproduced with permission from Chew-Graham et al. 75 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

FIGURE 3.
Feasibility study: CONSORT flow diagram. a, Multiple reasons for exclusion may be given. Reproduced with permission from Chew-Graham et al. 75 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Participant characteristics
Baseline data for the 24 recruited participants are detailed fully in Table 4. There were no missing data with respect to participant characteristics. The average age of the participants was 66.8 years, ranging from 51.3 years to 83.6 years. One-quarter of the participants were male and most (87.5%) responded positively to Whooley question 1 (‘During the last month, have you often been bothered by feeling down, depressed or hopeless?’) and to Whooley question 2 (‘During the past month, have you often been bothered by having little interest or pleasure in doing things?’) (95.8%). There was a wide variety of health problems, with the most common being high blood pressure (n = 16). All of the participants classified themselves as being of white ethnicity; it is noted that all of the recruiting CPs were located in the north-east of England, which has a largely white population. The majority of participants (n = 14) did not continue with education after the minimum school leaving age; however, six participants indicated that they had a degree or equivalent-level qualifications. See Table 6 for the baseline standardised measures alongside those at the 4-month follow-up.
| Baseline characteristic | Participants (N = 24) |
|---|---|
| Age (years) | |
| Mean (SD) | 66.8 (9.8) |
| Median (minimum, maximum) | 65.9 (51.3, 83.6) |
| Gender, n (%) | |
| Male | 6 (25.0) |
| Female | 18 (75.0) |
| During the last month, have you often been bothered by feeling down, depressed or hopeless, n (%) | |
| Yes | 21 (87.5) |
| No | 3 (12.5) |
| During the past month, have you often been bothered by having little interest or pleasure in doing things? n (%) | |
| Yes | 23 (95.8) |
| No | 1 (4.2) |
| On average, do you drink 3 or more units of alcohol each day? n (%) | |
| Yes | 1 (4.2) |
| No | 23 (95.8) |
| Do not know | 0 (0.0) |
| Smoking status, n (%) | |
| Non-smoker | 12 (50.0) |
| Current smoker | 4 (16.7) |
| Ex-smoker | 8 (33.3) |
| Health problems,a n (%) | |
| Diabetes mellitus | 7 (29.2) |
| Osteoporosis | 2 (8.3) |
| High blood pressure | 16 (66.7) |
| Rheumatoid arthritis | 3 (12.5) |
| Osteoarthritis | 9 (37.5) |
| Stroke | 5 (20.8) |
| Cancer | 2 (8.3) |
| Respiratory conditions | 7 (29.2) |
| Eye conditions | 3 (12.5) |
| Heart disease | 8 (33.3) |
| Other | 14 (58.3) |
| Did your education continue after the minimum school leaving age? n (%) | |
| Yes | 10 (41.7) |
| No | 14 (58.3) |
| Do you have a degree or equivalent professional qualification? n (%) | |
| Yes | 6 (25.0) |
| No | 18 (75.0) |
| Ethnicity, n (%) | |
| White | 24 (100.0) |
| Asian or Asian British | 0 (0.0) |
| Black or black British | 0 (0.0) |
| Other ethnic group | 0 (0.0) |
| Number of children, n (%) | |
| 0 | 4 (16.7) |
| 1 | 7 (29.2) |
| 2 | 8 (33.3) |
| 3 | 5 (20.8) |
| ≥ 4 | 0 (0.0) |
| Marital status, n (%) | |
| Single | 1 (4.2) |
| Divorced/separated | 1 (4.2) |
| Widowed | 5 (20.8) |
| Cohabiting | 2 (8.3) |
| Civil partnership | 3 (12.5) |
| Married | 12 (50.0) |
Intervention delivery
All of the 24 participants were offered the ESI, of whom 17 (70.8%) commenced the ESI. Engagement with the ESI sessions is detailed in Table 5. In total, 10 out of the 17 participants completed all six sessions of the ESI (58.8%), with all those participants who commenced the ESI receiving at least three of the ESI sessions. A total of 91 ESI sessions were conducted, of a possible 102, giving an average completion of 86.1% for those who commenced the ESI.
| Total number of ESI sessions completed | Number of participants |
|---|---|
| 6 | 10 |
| 5 | 4 |
| 4 | 2 |
| 3 | 1 |
| 2 | 0 |
| 1 | 0 |
| Did not commence ESI | 7 |
A total of 17 ESI facilitators completed the ESI training as part of the feasibility study, and 13 were available to deliver the ESI during the intervention period, of whom nine supported at least one participant through the ESI. The average number of participants supported by an ESI facilitator was 1.9 participants (ranging from one to four participants).
All seven participants who did not start the ESI sessions withdrew from the ESI before the sessions commenced. Reasons (where known) for not commencing the ESI sessions included no longer feeling that the intervention was relevant, ill health, feeling better, the family feeling that the study was not suitable for the participant, and being unable to contact the participant to arrange ESI sessions.
Follow-up and withdrawal
Seven participants (29.2%) withdrew from the ESI before commencing the sessions (see Intervention delivery); of these, one participant withdrew fully from the study.
Twenty of the remaining 23 participants completed and returned a 4-month follow-up questionnaire, giving a return rate of 87.0%; this included five participants who did not commence the ESI. Overall, the retention rate for the feasibility study at 4 months was 83.3% (20 out of the 24 participants).
Standardised measures
A range of standardised measures were collected during the feasibility study (see Chapter 2) to assess for completeness and suitability for use in the pilot RCT. These measures included the PHQ-9, GAD-7 and Patient Health Questionnaire-15 (PHQ-15). 63,65,66 Scores for these measures at baseline and at the4-month follow-up can be found in Table 6.
| Measure | Overall (N = 24) |
|---|---|
| PHQ-9 depression (range 0–27)a | |
| Baseline (N = 24) | |
| Mean (SD) | 13.5 (5.8) |
| Median (minimum, maximum) | 13 (2, 24) |
| No depression (0–4), n (%) | 1 (4.2) |
| Mild depression (5–9), n (%) | 4 (16.7) |
| Moderate depression (10–14), n (%) | 12 (50.0) |
| Moderately severe depression (15–19), n (%) | 2 (8.3) |
| Severe depression (20–27), n (%) | 5 (20.8) |
| 4-month follow-up (N = 19) | |
| Mean (SD) | 9.9 (6.1) |
| Median (minimum, maximum) | 9 (3, 20) |
| No depression (0–4), n (%) | 5 (26.3) |
| Mild depression (5–9), n (%) | 7 (36.8) |
| Moderate depression (10–14), n (%) | 1 (5.3) |
| Moderately severe depression (15–19), n (%) | 5 (26.3) |
| Severe depression (20–27), n (%) | 1 (5.3) |
| GAD-7 anxiety (range 0–21)a | |
| Baseline (N = 24) | |
| Mean (SD) | 10.3 (5.0) |
| Median (minimum, maximum) | 10 (1, 21) |
| No anxiety (0–5), n (%) | 2 (8.3) |
| Mild anxiety (5–10), n (%) | 9 (37.5) |
| Moderate anxiety (10–15), n (%) | 8 (33.3) |
| Severe anxiety (15–21), n (%) | 5 (20.8) |
| 4-month follow-up (N = 19) | |
| Mean (SD) | 6.5 (4.6) |
| Median (minimum, maximum) | 6 (0, 16) |
| No anxiety (0–5), n (%) | 7 (36.8) |
| Mild anxiety (5–10), n (%) | 8 (42.1) |
| Moderate anxiety (10–15), n (%) | 2 (10.5) |
| Severe anxiety (15–21), n (%) | 2 (10.5) |
| PHQ-15 somatic (range 0–30)a | |
| Baseline (N = 24) | |
| Mean (SD) | 14.8 (4.7) |
| Median (minimum, maximum) | 15 (4, 25) |
| Minimal (0–5), n (%) | 1 (4.2) |
| Low (5–10), n (%) | 2 (8.3) |
| Medium (10–15), n (%) | 8 (33.3) |
| High (15–30), n (%) | 13 (54.2) |
| 4-month follow-up (N = 20) | |
| Mean (SD) | 13.2 (4.5) |
| Median (minimum, maximum) | 14 (5, 23) |
| Minimal (0–5), n (%) | 0 (0.0) |
| Low (5–10), n (%) | 4 (20.0) |
| Medium (10–15), n (%) | 7 (35.0) |
| High (15–30), n (%) | 9 (45.0) |
Overall, the level of completion for these standardised measures in the feasibility study was excellent: 100% at baseline for all measures and between 95% and 100% at the 4-month follow-up (of those who returned the questionnaires). Although there is only a small sample of data, and for this reason no formal comparisons can be made, it does appear that the results change slightly between baseline and 4 months.
Although the intention had been for participants to complete the BADS during ESI sessions (see Chapter 2, Outcome measures), this measure was not administered during the feasibility study. In addition, additional information (relating to depression history) was also not obtained during the feasibility study. These data will be collected in the pilot RCT described in Chapters 6–10.
Competency and intervention fidelity
Of the 17 ESI facilitators who completed the ESI training, nine passed the competency assessment on the first attempt and, following additional telephone-based support, six passed on the second attempt and one on the third attempt (one ESI facilitator left the pharmacy before attempting the competency assessment). All ESI facilitators who went on to support participants through the ESI received session-by-session clinical supervision.
Although the intention was to audio-record ESI sessions (with participant consent) to assess fidelity to the ESI, the decision was made not to record these during the feasibility study. CHEMIST represented a new challenge for the recruiting CPs (the majority of which had no prior experience of research). Many of the ESI facilitators had limited experience of mental health, were new to working with the participant group (people with subthreshold depression and LTCs) and were engaging with new experiential training methods. The study team felt, on reflection, that asking ESI facilitators to record ESI sessions in addition to delivering the ESI within the setting of a CP would be an unfair burden on this group, and may risk ESI facilitator dropout. This decision was made in consultation with the wider study team, the CHEMIST stakeholders and the funder.
Although recordings of ESI sessions were not obtained, close monitoring of ESI delivery and adherence to the ESI manual took place (as originally planned). This was achieved through session-by-session clinical supervision at which the content and delivery of each ESI session was discussed, allowing the clinical supervisor to offer support and advice (to include adherence to the ESI manual) following each delivered ESI session. ESI facilitators were also required to complete a participant treatment log for each delivered ESI session, which was reviewed during each clinical supervision session.
Fidelity to the ESI would be examined in the pilot RCT, by which point it was anticipated that study processes would be more embedded in CPs and ESI facilitators would have increased confidence in these new training and assessment procedures. ESI facilitators would be advised of the need to audio-record sessions during the pilot RCT ESI training workshop.
Serious adverse events
There were no SAEs reported in the feasibility study.
Clinical results: summary
The feasibility study suggests that it is possible to recruit to the study; however, recruitment was much slower than anticipated, which highlights the novelty of conducting a mental health research study within a busy CP setting. The completeness of the outcome measures and the return rate of the questionnaires indicate that this patient population were willing to complete the questionnaires and were keen to stay active participants in the study. Equally, the high completeness (86.1%) of the ESI (for those participants who commenced the ESI) suggests that participants find the intervention acceptable and are willing to participate in the ESI sessions (see Chapter 4). This also suggests that CP staff can be successfully trained to deliver the ESI in a CP setting.
With respect to the predetermined criteria for progressing from the feasibility study to the pilot RCT (see Chapter 1) as follows:
-
Recruit five participants at each of four to six CPs by month 6 – 24 participants were recruited across eight CPs over a 9-month recruitment period (the last participant was recruited in month 12).
-
For 80% of participants to receive two or more ESI sessions within the 4-month post-recruitment period – 17 out of the 24 recruited participants (70.8%) received two or more ESI sessions (100% of those commencing the intervention) within the 4-month post-recruitment period. The remaining seven participants did not commence the ESI.
-
Collect valid (intended) primary outcome measure on 80% of recruited participants at the 4-month post-recruitment follow-up – the retention rate was 83.3% at the 4-month post-recruitment follow-up, with 95% data completeness for the PHQ-9.
-
A fidelity score of ‘acceptable’ (3) or above to be achieved in a least 90% of assessed audio-recorded ESI sessions – to be examined in the pilot RCT (see Competency and intervention delivery).
Interviews with participants and ESI facilitators conducted as part of the feasibility qualitative study will further explore the feasibility and acceptability of the study, ESI training and delivery, and study procedures (see Chapter 4).
Following discussions with the TSC and the funder, progression to the external pilot RCT was recommended.
Health economic results
Health services resource use
Data completion
At baseline and at the 4-month follow-up, all participants responded to the bespoke resource use questionnaire. Moreover, there were no missing data for any of the resource use items at either time point. Four participants did not complete the 4-month follow-up and, therefore, had no resource use information available (note that this is also reflected in the overall questionnaire completion rate in the study). The analysis also explored if the participants who did not provide follow-up data had a higher level of resource use at baseline (i.e. any sign of non-random dropout), but found their resource use to be similar to the participants who provided follow-up data.
Frequency of health service use
Tables 7 and 8 show the frequency of use of each type of health service in the last 4 months, measured at baseline and at the 4-month follow-up. The aim was to evaluate if responses were within the expected range of health service use and not to draw statistical inference regarding the differences between baseline and follow-up.
| Primary care | Time point, mean (range) | |||
|---|---|---|---|---|
| Baseline | 4-month follow-up | |||
| Number of appointments for any reason | Number of appointments for low mood | Number of appointments for any reason | Number of appointments for low mood | |
| GP at the clinic | 3.08 (0–16) | 0.3 (0–3) | 3.3 (0–10) | 0.2 (0–4) |
| GP at home | 0.63 (0–6) | 0 (0–0) | 0.2 (0–2) | 0 (0–0) |
| GP on the telephone | 0.79 (0–5) | 0 (0–0) | 0.55 (0–3) | 0 (0–0) |
| Nurse at the clinic | 0.96 (0–4) | 0 (0–0) | 0.75 (0–4) | 0.05 (0–1) |
| Nurse at home | 0.67 (0–10) | 0 (0–0) | 0.3 (0–3) | 0 (0–0) |
| Nurse on the telephone | 0.25 (0–5) | 0 (0–0) | 0.1 (0–2) | 0 (0–0) |
| NHS Direct | 0.13 (0–2) | 0 (0–0) | 0.1 (0–2) | 0 (0–0) |
| NHS walk-in centre | 0.17 (0–2) | 0 (0–0) | 0.1 (0–2) | 0 (0–0) |
| Occupational health services | 1 (0–14) | 0 (0–0) | 0.1 (0–1) | 0 (0–0) |
| Social worker or community support worker | 0.17 (0–4) | 0 (0–0) | 0.25 (0–5) | 0 (0–0) |
| Drug and alcohol support worker | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Type of service | Time point, mean (range) | |
|---|---|---|
| Baseline | 4-month follow-up | |
| Mental health services | ||
| Psychotherapist or counsellor | 0.5 (0–10) | 0.15 (0–3) |
| Clinical psychologist | 0 (0–0) | 0 (0–0) |
| Community mental health team or community psychiatric nurse | 0 (0–0) | 0 (0–0) |
| Consultant psychiatrist | 0 (0–0) | 0 (0–0) |
| Hospital-based services | ||
| Outpatient appointments | 1.7 (0–8) | 0.76 (0–3) |
| Accident and emergency | 0.15 (0–1) | 0.1 (0–2) |
| Urgent care centre or minor injuries unit | 0 (0–0) | 0.05 (0–1) |
| Admission with overnight stay | 0.25 (0–1) | 0.14 (0–1) |
| Admission without overnight stay | 0.1 (0–1) | 0 (0–0) |
At baseline and the 4-month follow-up, GP consultations were the most common category of resource use. The mean number of GP consultations at the clinic was 3.08 and 3.3 for baseline and the 4-month follow-up, respectively. Only a small proportion of visits were because of low mood. Resource use in other categories was low at both time points. The numerical values of the number of visits per participant was within plausible range for all resource use categories at both time points. In addition, the mean number of visits did not show any unexpected changes over time.
During the feasibility study it was noticed that participants had a number of appointments with the pharmacist that were not recorded by the resource use questionnaire.
Health-related quality of life
Health-related quality-of-life data were collected using the EQ-5D73 and SF-12v267 questionnaires at baseline and at the 4-month follow-up.
Data completion, frequency and pattern of EuroQol-5 Dimensions and Short Form-12 item responses
Data completion was 100% for all domains of EQ-5D and SF-12v2 at baseline and the 4-month follow-up (except for one non-response to item 2 in SF-12v2 at follow-up). This indicates that participants found it feasible to complete these questions. Moreover, responses were within the plausible range (i.e. 1–3 for EQ-5D and 1–5 for SF-12v2). The frequency and pattern of EQ-5D and SF-12v2 responses at both time points were also assessed. Based on the EQ-5D questionnaire, at baseline and the 4-month follow-up most respondents had some level of problem in at least one of the five domains; participants reported the lowest level of problems in the self-care domain. For the anxiety/depression domain, 3 out of 24 participants, 17 out of 24 and 4 out of 24 reported no problems, some problems and severe problems, respectively, at baseline (i.e. 87.5% of respondents reported experiencing symptoms of anxiety/depression) (Table 9). This is not surprising given that participants with subthreshold depression were recruited to the study. At the 4-month follow-up, there was little change in most domains except for anxiety/depression, with a slight decrease in the number of participants experiencing moderate anxiety/depression. However, the numbers are too small to draw any conclusions about the trend.
| EQ-5D domain | Time point | No problems | Some problems | Severe problems |
|---|---|---|---|---|
| Mobility | Baseline | 5 | 19 | 0 |
| 4-month follow-up | 5 | 15 | 0 | |
| Self-care | Baseline | 12 | 12 | 0 |
| 4-month follow-up | 13 | 7 | 0 | |
| Usual activities | Baseline | 3 | 17 | 4 |
| 4-month follow-up | 4 | 13 | 3 | |
| Pain/discomfort | Baseline | 0 | 11 | 13 |
| 4-month follow-up | 0 | 12 | 8 | |
| Anxiety/depression | Baseline | 3 | 17 | 4 |
| 4-month follow-up | 7 | 11 | 2 |
The SF-12v2 questionnaire produced similar responses to the EQ-5D questionnaire, with most respondents having some level of problem in at least one of the domains (Table 10). A large proportion of study participants reported having fair or poor general health (SF-1). Participants reported the highest level of problems in the physical health and vitality domains. For the mental health domain (SF-9 and SF-11), all except one participant reported at least some level of problem at baseline. This is consistent with the EQ-5D responses and in line with the study eligibility criteria. At the 4-month follow-up, there was little change in most domains, except small improvements in the mental health (SF-11) and vitality (SF-10) domains. However, the numbers are too small to draw any conclusions about the trend.
| Item | Time point | Level | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| SF-1 | Baseline | 0 | 1 | 2 | 6 | 15 |
| Follow-up | 0 | 1 | 3 | 12 | 4 | |
| SF-2 | Baseline | 12 | 9 | 3 | 0 | 0 |
| Follow-up | 10 | 7 | 2 | 0 | 0 | |
| SF-3 | Baseline | 12 | 9 | 3 | 0 | 0 |
| Follow-up | 10 | 9 | 1 | 0 | 0 | |
| SF-4 | Baseline | 4 | 9 | 6 | 5 | 0 |
| Follow-up | 3 | 5 | 10 | 1 | 1 | |
| SF-5 | Baseline | 1 | 13 | 7 | 3 | 0 |
| Follow-up | 2 | 6 | 10 | 1 | 1 | |
| SF-6 | Baseline | 1 | 7 | 10 | 5 | 1 |
| Follow-up | 0 | 3 | 9 | 4 | 4 | |
| SF-7 | Baseline | 1 | 6 | 9 | 3 | 5 |
| Follow-up | 2 | 2 | 7 | 3 | 6 | |
| SF-8 | Baseline | 1 | 4 | 1 | 14 | 4 |
| Follow-up | 2 | 3 | 1 | 11 | 3 | |
| SF-9 | Baseline | 0 | 3 | 4 | 13 | 4 |
| Follow-up | 0 | 7 | 6 | 5 | 2 | |
| SF-10 | Baseline | 0 | 4 | 3 | 5 | 12 |
| Follow-up | 0 | 2 | 5 | 4 | 9 | |
| SF-11 | Baseline | 0 | 10 | 10 | 3 | 1 |
| Follow-up | 1 | 6 | 6 | 4 | 3 | |
| SF-12 | Baseline | 4 | 8 | 9 | 3 | 0 |
| Follow-up | 3 | 5 | 7 | 4 | 1 | |
Health economic results: summary
The economic analysis evaluated the overall response rates, the item completion rates and the range of values provided in response to the bespoke resource use questionnaire and quality-of-life (EQ-5D and SF-12v2) questionnaires. Both health service resource use and EQ-5D questionnaires had 100% completion rate among participants at baseline and at the 4-month follow-up. There were no missing items in either questionnaire. GP visits were the most common types of resource use items. Overall, the economic analysis suggested a high level of questionnaire completion rate, a low level of item missingness and no out of range responses.
Chapter 4 Feasibility study: qualitative findings
Some of the information in this chapter is reported in Chew-Graham et al. (paper under review).
Participants
Study participants
Eleven study participants were interviewed from five of the eight participating CPs. Nine study participants had completed between four and six ESI sessions (seven study participants had completed all six ESI sessions) and two study participants did not start the ESI sessions (ESI non-completers). All of the study participants were of white ethnicity. Table 11 provides demographic details for the interviewed study participants. Interviews with participants explored their experiences of receiving the ESI and their acceptability of the ESI.
| Demographic | Total participants (N = 11) |
|---|---|
| Age (years) | |
| Mean (SD) | 64.3 (7.7) |
| Median (minimum, maximum) | 62.9 (53.1, 79.8) |
| Gender, n (%) | |
| Male | 7 (63.6) |
| Female | 4 (36.3) |
| Health problems,a n (%) | |
| Diabetes | 3 (27.2) |
| High blood pressure | 8 (72.7) |
| Rheumatoid arthritis | 2 (18.1) |
| Osteoarthritis | 3 (27.2) |
| Stroke | 2 (18.1) |
| Cancer | 1 (0.9) |
| Respiratory conditions | 4 (29.2) |
| Eye conditions | 1 (0.9) |
| Heart disease | 3 (27.2) |
| Other | 5 (45.5) |
Enhanced support intervention facilitators
A total of 17 ESI facilitators completed the ESI training as part of the feasibility study and 13 were available to deliver the ESI during the intervention period, of whom nine supported at least one participant through the ESI. Thirteen interviews were conducted with nine ESI facilitators from seven of the recruiting CPs (see Table 12). Four ESI facilitators completed two interviews (the first focused on their experiences of the ESI training and the second focused on their experience of delivering the ESI). Five ESI facilitators completed a single interview on their experiences of ESI training and delivery. Table 12 details the demographic details of the interviewed ESI facilitators.
| Demographic | Total facilitators, n (%) |
|---|---|
| Gender | |
| Male | 1 (11.1) |
| Female | 8 (88.9) |
| Job role | |
| Accuracy checking technician | 1 (11.1) |
| Counter assistant | 2 (22.2) |
| Dispenser | 5 (55.5) |
| Trainee dispenser | 1 (11.1) |
Five pharmacy staff from three participating CPs participated in the focus group.
Thematic analysis
The initial thematic analysis is presented in Table 13, with illustrative data included. Illustrative data are provided to support the analysis and are labelled by identifier and number.
| Main theme | Subtheme | Illustrative data |
|---|---|---|
| Linking mood and physical health conditions | I do focus on the physical disabilities now as causing me great unhappiness and depression because I can’t see any improvement, I, I, and I couldn’t look to any improvement, I just felt as if my life was overP049Well it’s gotta be [linked] cos if you’re getting up every day feeling poorly it’s, you’re not gonna be feeling happy, are you, really? So obviously if you get up, you’re not well, you stop doing things, makes yer pretty miserable, doesn’t it?ESI2I can’t hold things in me right hand and so it does get us depressed not being able to do what I want to doP6[I’ve] always been quite active for me age, and when something stops you from being that same person active-wise it sort of like kicks you in yer teeth . . .PNC 038 | |
| The pharmacy as a place to deliver psychosocial support: enablers | Non-stigmatising | We have a lotta people that come in and tend to, for whatever reason, see it, us in a pharmacy, as someone as a, who they can talk to openly and honestlyESI1I know [the facilitator], I can speak to [her] and I know it’ll be confidential and it wouldn’t go any, any further, it wouldn’t go any further with any of the staff or anything like that, she would give me a good result and I would give her a good result . . .P22 |
| Pharmacy is local | I wouldn’t go into town or something like that, it would give you the excuse not to actually go. But I, I, it, it it’s the locality, was the best thing about itP18 | |
| Pharmacy is trusted | . . . but knowing the pharmacy, this particular pharmacy as I do, I wasn’t surprised that they were interested in it . . .P18 | |
| ESI facilitator already known | . . . I think a big part of it is like how the staff are with the patients, like you said you know your patients very well . . .FG P3 | |
| Acceptability of the intervention | The intervention makes sense | Yes, it did. It was particularly in the workbook, it mentioned things like can’t be bothered, not going out, making excuses; and I thought yes, that, that, that’s me, but I thought it was just getting older and, you know, not wanting to do things like that, so. That was, that was, that was very useful.P18I thought, and I, I, perfectly honest, it has done me the world of good. It’s been therapeutic, because I was getting a regular call on a Wednesday, and I would work to that call on the Wednesday, because I was given a plan, I was given advice, keeping me diary, for example, on how I felt from day to day, planning to do tasks, even though they seemed insurmountable at times . . .P49 |
| Personal contact | The personal contact, the personal support, the personal understanding, I think that was, that was really really valuable to me and as a motivatorP49 | |
| Acceptability of the intervention | The patient self-help workbook | Yeah, I thought it was a bit [sighs] a bit strange at first, and then when you read and understand what everybody’s trying to say and get to and get your answers from you, I think it, it’s done in a very sensitive way and I think it, it’s positiveP14 |
| The pharmacy as a place to deliver psychosocial support: challenges | Introducing the study | Like my big problem was how to initially say to people ‘do you want to participate?’, ‘cos I didn’t want to say it’s about low mood or subthreshold depression, I just didn’t wanna mention those terms ‘cos I just knew people would be put off. So I was like I need some key words to throw in here [laughs] and she said ‘Say it’s a psychological well-being study’. And as soon as she said that I was like, right, that’s much easier [laughs]. I approached a lot more people then, yeahESI4 |
| Opportunity costs | So I think it’s got a big impact on the pharmacy, if you’re working in a busy pharmacy, ‘cos you’re taking somebody away for a good 30 minutes and then, which is a good hour, because then you have to call [supervisor] and then you go through the session with [supervisor]; so you’re talking about, sometimes that can be another 15/20 minutes, depends. So you’re taking like a, a body out of the pharmacy; so while that, while that’s getting done my work at the back’s not getting doneFG ESI1 |
Theoretical framework of acceptability analysis
The main results will be reported following the analysis mapping onto the TFA:47 intervention coherence, perceived effectiveness, self-efficacy, burden, opportunity costs and affective attitudes. There were no data related to the construct ethicality.
Intervention coherence
This construct assessed the extent to which the ESI participants understood the ESI and how it worked.
The identification of mood problems and the provision of subsequent support made sense to participants who received (‘intervention participants’) and delivered (‘ESI facilitators’) the ESI. Intervention participants and ESI facilitators acknowledged the link between physical and mental health and ill health:
I think they are linked. I think if somebody gets diagnosed with, whether it be asthma or diabetes, we, we initially think oh my goodness, how am I gonna cope with this? Some people are really strong and can cope with it and take it all on board, other people really struggle, and in that struggle it affects the day-to-day life of everything else, it’s not just the fact they’ve got diabetes, unfortunately it affects every aspect of their life.
ESI1
. . . it, I can’t do, there’s things that you can’t do; I can’t hold things in me right hand and; so it does get us depressed not being able to do what I want to do. Me colitis sometimes gets us depressed because if I’ve got a flare up I can’t go out the house, because I need to use the toilet all the time. Me COPD [chronic obstructive pulmonary disease], as long as I’m taking me inhalers I’m not too bad, you know. But yes, it definitely reflects on me mood, on me moods and things and, yeah.
P6
The ESI made sense to those who undertook it, with the intervention participants reflecting on the usefulness of the workbook, the structured nature of the ESI, the homework required of them and the benefits of the ESI to them as an individual:
Yes, it did [make sense]. It was particularly in the workbook, it mentioned things like can’t be bothered, not going out, making excuses; and I thought yes, that, that, that’s me, but I thought it was just getting older and, you know, not wanting to do things like that, so. That wa, that was, that was very useful.
P18
I thought, and I, I, perfectly honest, it has done me the world of good. It’s been therapeutic, because I was getting a regular call on a Wednesday, and I would work to that call on the Wednesday, because I was given a plan, I was given advice, keeping me diary, for example, on how I felt from day to day, planning to do tasks, even though they seemed insurmountable at times.
P49
The CP was seen as an appropriate and logical setting in which to deliver the ESI, with the location seen as familiar and non-stigmatising by both intervention participants and ESI facilitators:
We have a lotta people that come in and tend to, for whatever reason, see it, us in a pharmacy, as someone as a, who they can talk to openly and honestly.
ESI1
Perceived effectiveness
The TFA construct of perceived effectiveness describes the extent to which participants perceive that the intervention will achieve its purpose. 76
A number of aspects of the ESI were perceived as likely to achieve their purpose. Although some intervention participants disclosed initial uncertainty about the preliminary assessment, these feelings were quashed once the assessment had been undertaken:
Yeah, I thought it was a bit [sighs] a bit strange at first, and then when you read and understand what everybody’s trying to say and get to and get your answers from you, I think it, it’s done in a very sensitive way and I think it, it’s positive.
P14
The materials were thought to achieve their purpose; in particular, the patient workbook was well received by intervention participants and also by ESI facilitators who described working through them with the participants:
We went through it together. So she was like asking us the questions out the book that I already had, you know, so went through together. So yes, it was all right, I understood it, yeah.
P6
I thought that was fine, I just, I thought it made more sense once I got the self-help work book, everything, once I got that, I read this from beginning to end. So I didn’t even, you know, I, once I got this I thought oh so we’re gonna be doing this and gonna be doing that; but that’s the person I am. Some people might just do one stage as, at a time, I read the whole thing to see what it was gonna entail before; so I was well aware what I was going into, and if there was something I didn’t like I would have said; but I thought oh this is all right, seems all right to me. So, there you go.
P2
Intervention participants said that the activity planner in the workbook was particularly valuable:
I gave up filling the end part of the book up cos I thought what activities do yer like to keep, help yer keep well? There’s a lotta things that I could, I could be doing more, but that’s gonna come in time. I’m more worried about, I’m, was more interested in filling this chart to say, right, this is what I did.
P2
Very, yeah. Ess, essential in fact, cos that’s a whole part of the, the, the study, isn’t it, the kind of, help the, the patient, if you will, to use the book to help themselves. So yeah, very good, kind of well set out and easy to understand.
ESI6
Intervention participants reflected on the importance of confidentiality and trust in the ESI facilitator, which appeared to give them confidence to engage in the ESI and faith that it could produce a positive outcome or ‘good result’:
I know [the facilitator], I can speak to [her] and I know it’ll be confidential and it wouldn’t go any, any further, it wouldn’t go any further with any of the staff or anything like that, she would give me a good result and I would give her a good result . . .
P22
Self-efficacy
Enhanced support intervention facilitators expressed mixed views about whether or not they had developed the confidence to deliver the ESI following the ESI training. Notably, anxiety and uncertainty particularly focused around participant risk assessment:
. . . OK. I think one of the questions I would, I would find it quite awkward asking people where you had to just say have you thought about killing yerself in the last week; I think that might come across as, I don’t know what the word is, a bit blunt [laughs]. But yeah, apart from that . . .
ESI6
However, some ESI facilitators described how they gained confidence with increasing experience of ESI delivery:
I didn’t really find it difficult. I found, I did, at first, at first, when I first started, I thought asking the questions, the risk questions I’d asked be quite daunting and impersonal and whether they would be quite negative, but when we’ve done it on a regular basis I can see that there’s a need for it and you can see, especially with one, with one of them, even with the one that decided at 4 weeks, decided she didn’t want to go any further, it was a noticeable difference in the scores that it had improved, so at least there was some benefit. So with regards to that, I think it was quite OK, to be honest, I didn’t really have any problems with that.
ESI1 (second interview)
Intervention participants all described confidence in goal-setting, keeping diaries and being monitored by their ESI facilitator:
. . . in particular when we were talking about breaking activities down into smaller parts, because again me mobility has really restricted me with a lot of things, and even a simple thing like making the bed [laughs] you, you know, we used that as an ex, one, one example, and it was a case of not making it and changing the blankets all in one go and, you know, so we did it sort of like, you know, with [names ESI facilitator] help we, we broke that down into smaller steps.
P46
Not all intervention participants were confident that they could continue to practice what they had learned during their work with the ESI facilitators:
I think I try to now, yeah, I think on things a bit more. Whereas before I would just sit, now I try to get up and do something and I try to have a different outlook on, on the way it was to the way it is now, even though I’ve got the pressure of my wife it’s still, it’s still a bit different than what it was.
P14
However, other intervention participants described continued use of the BA techniques learned, including monitoring mood, and indeed their own adaptions to the study materials:
Having a mood chart but, I mean I’ve, I’m using me own calendar and I’m putting me score; so I just do, I just do morning and night, because morning I’m usually, nine times out of ten, I’m very high in a morning, because I like getting up, I’ll get up early, I do everything on a morning, so my mood’s quite high, it’s when I get, after work I’m tired. Obviously, it lowers and things like that, and at the minute it’s, it’s on about a 5 when I come back from work, cos when I come back from work I’m thinking about me sister, so. But it’ll, it’ll be like that, and I’m not worried about that being low when it’s been an 8 on the morning, because you haven’t had time to think, you know, things like that . . .
P2
Burden
Both ESI facilitators and intervention participants alluded to the burden that participating in the study entailed. For example, ESI facilitators described the challenge of discussing the study with pharmacy customers and specifically the difficulty of finding the words to describe the purpose of the study and the description of ‘mood’:
Like my big problem was how to initially say to people ‘do you want to participate?’, cos I didn’t want to say it’s about low mood or subthreshold depression, I just didn’t wanna mention those terms cos I just knew people would be put off. So I was like I need some key words to throw in here [laughs] and she said ‘Say it’s a psychological well-being study’. And as soon as she said that I was like, right, that’s much easier [laughs]. I approached a lot more people then, yeah.
ESI4
All ESI facilitators and pharmacy staff described some difficulty in fitting aspects of the study into their daily routine:
. . . it’s sort of 15/20 minutes taken off my time and I’m very needed on the shop floor. So, it means that somebody has to drop down, and then I feel like I’ve left two very capable dispensers having to cover a shop floor with a queue full of people, which sometimes I do feel guilty about.
ESI1 (second interview)
However, this ESI facilitator added:
But then when you think of the impact it’s gonna have on the customers that we see, it’s worthwhile doing; regardless of how many staff we’ve got on the counter, if we can help just one person it’s been worthwhile.
ESI1
In addition, the impact of the study, in particular the recruitment process, was echoed by the wider pharmacy staff in the participating CPs:
A bit, like you said, like it’s the, the, [facilitator] would have to go, she’d want to sit and prepare before somebody came in, to read the notes, to see where she was at. I think the first person that came in took about three-quarters of an hour and then, then there was the phone call with [names clinical supervisor] to then catch up; it just seemed to take a lotta time and I know, obviously I, there was another three pharmacies in my group, one of them didn’t do it because they do like 17,000, like 18,000 items, just don’t have time to do it, they said they just don’t have time to get the staff to go and give the packs out, never mind seeing people; and it’s, it’s unfortunately about money sometimes that they . . .
FG PS5
Despite the difficulties experienced, pharmacy staff were keen to stress the generalisability of the training and skills learned in being part of the study:
Yeah, and it’s expanded my knowledge, it’s made me aware to, to look for what’s in our community, not further afield, because people don’t want to travel, but to see what’s openly available. So now I’ve got a list of what’s openly available for people who are overweight or struggling with anything and; men’s football clubs for the over fifties, running clubs. I’ve been looking at everything so that if anybody says to me I need to do that, I’ve got a folder; and I would never have done that if it hadn’t have done the CHEMIST study.
ESI1 (second interview)
Descriptions of burden from intervention participants, however, were seen infrequently; rather, the sessions with the ESI facilitators were viewed positively:
In fact when she said when it was the last one I was quite disappointed really because it was becoming a regular on the Wednesday morning, you know, about an hour we would have talking together.
P49
A minority of intervention participants described how a lack of motivation affected their enthusiasm to attend ESI sessions:
. . . but it was just the, just the, just the mood I was in, I would say to [the facilitator] ‘I’ll come in tomorrow’. And then when the, the tomorrow come, I thought oh I, I really didn’t want to come, you know. but I did, I mean I did do it all, I finished it; took a bit of a while. But just, like I said, all depending; I mean today, the way my hand is, I, I found difficulty driving, to tell you the truth, ‘cos I had to drive with them two fingers. So there is times where I’ve got to push meself, otherwise I’m just in the house, you know. But, but yeah, I, I, I, I think it’s, like I say, I still come, but it was a bit difficult sometimes not wanting to come, so.
P6
Opportunity costs
The TFA domain of opportunity costs describes if benefits, profits or values need to be relinquished to engage in the intervention. 76
Discussion of opportunity costs was reported by ESI facilitators and pharmacy staff in terms of the time cost on regular pharmacy work as a result of the ESI facilitator delivering the ESI intervention:
So I think it’s got a big impact on the pharmacy, if you’re working in a busy pharmacy, ‘cos you’re taking somebody away for a good 30 minutes and then, which is a good hour, because then you have to call [supervisor] and then you go through the session with [supervisor]; so you’re talking about, sometimes that can be another 15/20 minutes, depends. So you’re taking like a, a body out of the pharmacy; so while that, while that’s getting done my work at the back’s not getting done.
FG PS1
Intervention participants did not feel that being involved in the study had any impact on their day-to-day activities (‘opportunity costs’), but one intervention participant acknowledged the difficulty of fully participating in the study owing to a recent bereavement:
But I can only do; and obviously with just losing me sister, everything’s gone a bit pear-shaped at the minute, but I can only do so much and it, it, it is what it is, but I will get there.
P2
Affective attitudes
The construct affective attitudes describes participants’ feelings about the intervention. 76
The ESI facilitators were generally known to the intervention participants and this appeared to be a key enabler of their positive feelings towards participating in the study and working with the ESI facilitator to complete the ESI:
. . . but knowing the pharmacy, this particular pharmacy as I do, I wasn’t surprised that they were interested in it.
P18
. . . I think a big part of it is like how the staff are with the patients, like you said you know your patients very well . . .
FG PS3
In addition, the personal qualities of the ESI facilitators were deemed to be important in relationship building and persisting with the ESI:
Oh great, absolutely great, great, because she’s [the facilitator] friendly, she’s approachable, you know what I mean, so; and I mean that, that’s what you need because it, it, some people can find it intimidating.
P2
No, no, I just, I really found [names ESI facilitator] to be very helpful, very understanding; there was one time, again it was me mobility, I couldn’t get to the chemist, she was quite happy to change the appointment, you know, and, and she was just really, really helpful.
P46
The training was valued not just to help in the delivery of the study, but to assist in their usual, routine work within the pharmacy:
Yeah, and it’s expanded my knowledge, it’s made me aware to, to look for what’s in our community, not further afield, because people don’t want to travel, but to see what’s openly available. So now I’ve got a list of what’s openly available for people who are overweight or struggling with anything and; men’s football clubs for the over fifties, running clubs. I’ve been looking at everything so that if anybody says to me I need to do that, I’ve got a folder; and I would never have done that if it hadn’t have done the CHEMIST study.
ESI1, second transcript
Reflections on the use of the theoretical framework of acceptability
We used the TFA47 to explore the acceptability of an intervention in this feasibility study. This provided a useful analytical framework and allowed a more in-depth, multidimensional, theoretically informed analysis of acceptability across the data sets than the initial thematic analysis of each data set allowed.
The TFA can be used to investigate acceptability in three ways: prospective acceptability (prior to participation in the intervention), concurrent acceptability (while participating in the intervention) and retrospective acceptability (after participating in the intervention). We collected data at one time point (after participants had participated in the intervention) and, therefore, undertook a retrospective assessment of acceptability.
Sekhon et al. 47 present the constructs of the TFA in alphabetical order and outline the extent to which they may cluster or influence each of the temporal assessments of acceptability as an empirical question. The process of mapping subthemes from the thematic analysis to TFA constructs was not always straightforward, as some subthemes were aligned with more than one TFA construct. When this happened the research team made decisions about which construct best represented the theme. Our analysis suggests that the constructs are interconnected. For example, intervention participants’ perceptions about how they felt towards the ESI (affective attitudes) were often linked with feelings about how recommendations from the ESI facilitator added extra work (burden).
Although all of the TFA constructs represented in these findings were important for assessing acceptability of the ESI, some were more populated than others. For instance, most of the data that contributed the most insight and support for intervention participant acceptability of the ESI were mapped across three TFA constructs (affective attitudes, intervention coherence and perceived effectiveness). Less data were mapped to the TFA constructs of self-efficacy and opportunity costs, and no data were mapped to the TFA construct of ethicality. This lack of findings linked to ethics issues may be explained by the fact that the topic guide was not developed using the TFA constructs.
Chapter summary
The ESI was found to be acceptable to the intervention participants as well as to the ESI facilitators who found that the ESI training was valuable and that it enabled them to deliver the ESI. There were examples of burden, opportunity costs and low self-efficacy for a small number of intervention participants, but more opportunity costs for the CPs in which the ESI was delivered, with challenges described by pharmacy staff and ESI facilitators in allocating time to incorporate CHEMIST work into routine work.
Learning from the feasibility study that informed the pilot RCT is described in Chapter 5. The barriers to and facilitators of implementation of the ESI were the focus of exploration in the pilot RCT.
Chapter 5 Feasibility study: protocol changes, summary of learning and progression to a pilot randomised controlled trial
This chapter details the changes that were made to the study and study protocol during the feasibility study and in preparation for the pilot RCT.
Many of the adaptations detailed were made in response to (1) analysis of semistructured interviews conducted with study participants and ESI facilitators, and a focus group with pharmacy staff (see Chapter 4); (2) discussions with pharmacy staff from recruiting CPs as part of their regular telephone calls and/or visits with the CHEMIST study team; and (3) meetings with the CHEMIST special interest group (see Chapter 2). This chapter, therefore, serves as a record of protocol changes made and a summary of the learning from the feasibility study.
Examples of findings/quotations that informed/supported these adaptations can be found in Tables 14 and 15.
| Item | Data | Lessons for pilot trial |
|---|---|---|
| Links between physical and mental health | I mean obviously after doing the training I’ve, I’ve realised it is. But yeah, I think obviously you can, you can tell when you speak to some people that they are quite, quite down and it probably is affecting, affecting their health, whether they realise it or not. But looking back, I think I can definitely, yeah . . .ESI6 |
|
| Importance of the broader social context of patients | Hmm [sighs] I, I, I, I’ve got a feeling; obviously it, it does link in somewhere along the line. First experience of me first participant that I’ve done the study with, she hasn’t had much of an improvement going through the booklet and I think more it’s, it’s been sort of other things that have been a knock-on effect of her long-term health condition. So she’s lost her job, she’s had a knee replacement, all sorts going on; so obviously everything is a knock-on effect of that, but the way, I don’t think she sees the long-term health condition as the factor, even though that’s the cause of all the other knock-on effects that’s causing, well-being the end cause of things anywayESI8 |
|
| Physical health problems can limit people’s ability to engage in BA | Basically he had a, a lot of health conditions and it wasn’t the fact that it was his mood that was stopping him from doing things, it was more his kind of physical health conditions; had severe arthritis so he couldn’t grip anything, severe stomach reflux problems so he couldn’t really eat very much or go out and eat, and spondylosis, which is kind of like a compressed nerve type of problem, so he couldn’t walk, he couldn’t sit down, he can’t, he couldn’t, can’t stand for very long. So a lot of the problems were he just couldn’t do anything; so it was trying to suggest alternative things to things he used to do, but he just physically can’t do very much, not because of his mood, because of his physical health condition. So it was very difficult in that sense . . .ESI6 (second interview) |
|
| Awareness impacts on understanding for usual work | In the CHEMIST study, it’s made me realise that there’s more people with underlying low mood than you could ever, ever imagineESI1 (second interview) |
|
| Implications for training | Well really [would have liked more information before attending the course], yeah, cos it was all rushed because we were, [researchers] came in on the Monday and we went on the training the following Thursday/Friday, cos I was on holiday, I had to cancel me holidays. So we went on the training the following Thursday and Friday. We work in a busy pharmacy and there’s no way that we had the time to even really look at the information. So we didn’t really look at the information until we were actually sat in the courseESI2Uh huh that was far too intense, it was horrible, it was absolutely awful [laughter], not [trainer’s] fault [laughter] . . . and there was far too much role-play, the pressure was awful. Like after we’d done the training I was absolutely gutted that I’d decided to take part in it, but obviously once I’d gotten out into doing the sessions it was a lot more comfortable and easierESI8 (second interview)No. I think that [role-play] should probably be mentioned, cos I think a lot of people are quite uncomfortable with it. So I think possibly in the introduction to the whole thing, people should be made aware that actually there’s going to be quite a lot of role-playingESI8Yeah, good. I think a lot of people find role-play daunting, and I do as well, to be honest with you, it was kind of, not embarrassing but daunting at the time. But I think it is needed, because I’ve been on training in the past, for example, the health, health, healthy living champion, where they just kinda say, there’s something, read it, right, you’re a healthy living champion, but you haven’t actually put it into practice. So I think that part of it was good, you need to be able to, when you do come to do an interview you need to be able to do it, and if, if you hadn’t actually practised it, rather than just reading a bit of paper, I think it would show and it . . .ESI6 |
|
| Did training prepare for intervention delivery? | I would say I wasn’t very prepared [after the training], I wasn’t prepared, you can never be prepared enough, and I just think I don’t want to feel like I’ve let my participants down; I don’t think I have with the last two, but the one than fell by the wayside, I think she needed more than I could possibly ever have given her; even with 3 weeks of training I don’t think I would have been at the standard she wanted me to be, whatever standard that is, so . . .ESI1 (second interview)Very good, very good. I think the, I know nobody likes it, I think the role-play was good, because I’ve done training in the past for things in here where they’re saying read this, read this, read this, right, go; and then you go, what was I supposed to do again? Whereas when you’ve actually, you’ve had some, [names supervisors] sitting there listening, doing the role-play and they kinda suggest things, you’ve already kind of had like a, almost a test patient so you’ve already ran through it, instead of reading a, reading a book and doing like a multiple choice questionnaire at the end, you know, when, when it comes to speaking to somebody face to face you, you’re trying to think back to that bit of paper you read, whereas when you’ve done it face to face you’ve already picked up a few kind of . . .ESI6 (second interview) |
|
| Pharmacy as a delivery site | We have a lotta people that come in and tend to, for whatever reason, see it, us in a pharmacy, as someone as a, who they can talk to openly and honestlyESI1I think it would be a good thing to do in pharmacies, rather than people going to the doctors all the time, when they don’t really need to at first, they could come into the pharmacyESI3It’s good for people who’ve lost sort of, we have, it’s awful to say, a lot of elderly patients and they lose loved ones, so obviously when they’re coming in and they’re getting quite upset, they fetch the prescriptions back, they fetch medication back, and they get quite weepy, which is understandableESI1 |
|
| Knowing customers | No, ‘cos they know, everybody knows everybody; that could put people offFG PS2 |
|
| Dealing with complex study participants | . . . the one thing that was a bit daunting was like my first patient was quite a complex case and I didn’t think we were going to get anybody who was depressed. I know it’s meant to be subthreshold depression, but she’s actually, I would say, quite severely depressed; I’m not an expert. But every single time she’s answered the DAS it’s been quite a high, well it’s been a very high score, she’s in the top end. That’s one thing that I didn’t think I was going to have to deal with; and sometimes when she’s told us stuff I’ve been like, oh I’m not, don’t feel qualified enough to give you any information. But then as I’d spoke to [names supervisor], who said, you know, like ‘You’re not there, just direct her to her GP, all you’re there for is to sort of work her through the booklet’ . . .ESI8 |
|
| The value of the intervention | I think they felt quite comfortable speaking to us, well I would like to think that they did, and they, they did give feedback saying that they’d sort of felt like it had benefited them and it was nice to offload onto somebody who wasn’t sort of too involved in their personal life, somebody who sort of they could offload to and then go away and then forgotten aboutESI8 (second interview) |
|
| Yeah, the structure, yeah, yeah, because obviously there was, you know, like there was plenty for [the participant] to do and concentrate on, the diary was very good, and she did fill that in, she was good at the diary, so yesESI19 |
|
|
| Asking about risk | Asking the risk question, except it was a little bit daunting, you know, it seems like a very, it’s sort of almost looking a bit too far into people’s minds, but then at the same time I, I explained it was just basically for their safety; and people were pretty OK with that. And then obviously as the sessions went on it just became like a standard question, yeah, so wasn’t too bad, to be honestESI8 (second interview)OK, to be honest, I didn’t really find it difficult. I found, I did, at first, at first, when I first started, I thought asking the questions, the risk questions I’d ask’d be quite daunting and impersonal and whether they would be quite negative, but when we’ve done it on a regular basis I can see that there’s a need for it and you can see, especially with one, with one of them, even with the one that decided at 4 weeks decided she didn’t want to go any further, it was a noticeable difference in the scores that it had improved, so at least there was some benefit. So with regards to that, I think it was quite OK, to be honest, I didn’t really have any problems with thatESI1 (second interview) |
|
| Materials | Oh it’s fine, it’s self-explanatory; and then if you just go through the booklet, I mean it, it more or less tells yer, you just have to follow the booklet and follow yer client, you know. But yeah, I think, I think the booklet is, is good, for me, whether it is for the client; I mean people have different opinions. They didn’t like to, they didn’t like to fill in any of this, they didn’t want to put on this, their activities or . . .ESI2 (second interview) |
|
| Recruitment procedures | So me and [PS4] are from the same pharmacy and it’s quite a low income, low socioeconomic area. So we have a lot of patients that can’t necessarily read, write; and we’ve found, with relation to this, that was a big part of the problem with recruitmentFG PS3Like my big problem was how to initially say to people ‘do you want to participate?’, cos I didn’t want to say it’s about low mood or subthreshold depression, I just didn’t wanna mention those terms cos I just knew people would be put off. So I was like I need some key words to throw in here [laughs] and she said ‘Say it’s a psychological well-being study’. And as soon as she said that I was like, right, that’s much easier [laughs]. I approached a lot more people then, yeahESI4So initially it’s difficult to, to talk to every person that you want to talk to; it tends to be, you’re giving a prescription out, there’s a queue, so you’re just literally giving another prescription out or serving a customer. After a few weeks, we made an effort to, obviously, start talking to more customers, so asking people [staff] to drop down on the counter so I could discuss aspects with peopleESI1I would say you can’t, me personally, cos I’m on the counter, the, the thing that I feel, if you’ve got the time to actually hand out a leaflet or be drawn into a conversation about low mood, to me that’s a lot better than just sticking a leaflet in a bag and walking away, taking the medication out and the leaflet goes in the bin. If you can have that contact with a client it makes it a lot better, and you can explain a lot more than a leaflet can. So I feel, personally, that that is more beneficial, and people, if they were interested, were openly talking about it, but due to ti, time restraints I couldn’t always do thatESI1 (second interview)So initially it was difficult; it got easier when we were allowed to put the leaflet information into bags, because when I was handing the prescription over it would have CHEMIST written on it, and it was an ideal talking point. So it was a lot easier when it became available that we could pop them in the bagsESI1I mean a, quite a few of the customers seen the posters, so that was good, they like, you know, they approached us, so that was nice. And, like I say, I do know quite a few people in the, the chemist, so like looking at prescriptions and things like that sometimes could give you a little bit of an idea that I, you know, and then I could approach them and, like I say, they’re all lovely, so, you know, they either say, oh yes or no, you know, so they’re, they’re good reallyESI9 |
|
| Impact on the pharmacy | I mean initially it [pause] staff-wise, I think if we’d had more staff we could have gotten, we could have recruited more people, it was finding the time to get out, ‘cos [. . .] you haven’t got time to look up when you’re on the counter; and I think there’s probably a lot of people that we haven’t asked, but it’s just getting that time. And we ha, now when they come in and now the questions are different and you’ve just got to ask them that brief sentence, and if I spot people I’ll go out and ask them, but it’s, it’s, it’s finding the time to, to get to themESI2 (second interview)I would say yes, as long as the funding’s there to cover it, because obviously pharmacies are like really restricted on what we can do at the moment, there’s cuts left, right and centre, so fitting extra things in with no extra funding would be very difficult. It was hard fitting in the time, to be honest; I think that’s one of the things that was tough, because we’re so busy, we’re, there’s only so many members of staff, we don’t have extra staff in when we’re sort of doing the CHEMIST study, we’re trying to fit it in around everything else that we do, because we are a busy little pharmacy and we never stop. So that’s probably one of the things that could be put maybes as a, as a negative, sort of trying to fit it in around putting it in with yer usual sort of jobESI8 (second interview)No [laughs] apart from losing us for half an hour/40 minutes a week. We’ve struggled in the pharmacy because it, it’s a really busy pharmacy and you do a session and it’s probably half an hour/40 minutes and then we would talk it over with [names supervisor] the next day, so then we’d taken away then. So I think for the pharmacy it was maybes a bit frustrating. I think like [the pharmacist] said, it works, it does work, but whether it works in a busy pharmacyESI2 (second interview) |
|
| Item | Data | Changes made |
|---|---|---|
| Self-help booklet | . . . it could have been set out in the stages, that would have made life a lot easier, however sort of the, sort of the idea of setting the diary out and; things are laid out pretty well, I just think it needs re-grouping into sort of sessions rather than stages, because, like I said, in, in the beginning where you do the introduction and then part of stage one or however it’s, part of, part one of the booklet makes it a bit too confusing, especially for sort of older people and people who sort of struggle with absorbing things, maybes that’s the way to put it. It would be, be a lot easier if it was laid out session 1, session 2, session 3ESI8 (second interview). . . so you’ve got the introduction and then the, the cycle. So that’s stage, well session 1, but they, they stage different, differently throughout the booklet, so you’ve only got six sessions but eight stages. [Q laughs] So that’s, that’s the, that’s the harder thing to try and sort of, you know, manoeuvre into each session, which one you’re going to do correctly, instead of it just being, you know, like, you know, today this is session 1 in the booklet, this is what we’re going to do; that would be a lot easier than going, you know, we’re, we’re going to do session 1 and 2 today, session 1 was stage . . .ESI8Like I would have thought like a session 1 would have been put into a session 1 and then a session 2 into a session 2 where it was just on its own; I just thought it was a little bit like all overESI9. . . once people kinda make their mind up and think I’m not depressed and I don’t need to do it, you’ve lost them, I thinkESI5. . . a lot of people are in denial about it, aren’t they? Yeah, it’s kind of a big, scary word to some people, isn’t it? Especially in an area like this, you know, men, they live in the dark ages [laughs] so it’s like something that would never be spoken about. So if they read that word, even the subclinical depression, that’s just gonna tip them over the edge. So yeah, I think people would presume that it was about people who were depressed and if they think they’re not then they’re not gonna bother, they’re not gonna bother filling out the, the rest of itESI5 |
|
| Recruitment methods | . . . we do about 276 deliveries, and you’re missing all those people. So I think that [home deliveries] should have been looked into before we got this far down the lineESI2Yeah, I, I mean obviously I, it was mostly me had a lot of time to do with, with the recruitment process; I did a lot of the postal packs which we found use, useful, the kind of walk-in side, the face-to-face approach was very challengingESI6 (second interview). . . about, and I didn’t think there was enough information on that one sheet for them to then make a decision, and I did find that I’d say that this is, this is about can I give you a pack; so they would take a pack. So we found a lot more people recruited that way, rather than just off this one sheet; and I think we found giving the packs out on delivery tend to have a bigger response because they had, I think, more informationFG PS5Do you feel, yeah, low; I think a lot of people thought oh my God, I don’t get depressed, I haven’t got mental health issues, and I don’t think they liked it. I didn’t, and they didn’t like to be asked at the counter, they didn’t like to be asked at the counter, whereas if you just were saying now, oh the university are doing a trial on people with long-term conditions and how it affects their mood, would you be interested; they’re fine with that. But actually when we were doing it the other way, where you were asking the questions at the counter, they, they, it wasn’t, no, wasn’t goodESI2 (second interview). . . but we know everybody; and what worked better, when it came to the end of the trial when [name] was saying ‘Instead of asking them these questions, why don’t you just say, when you’re handing the prescriptions out, you’ve got a long-term condition, would you be interested in helping out on a trial about how it affects yer mood?’ And that was much better, that was much better than asking the questions . . . [talking together]FG PS1 |
|
| Paperwork | Paperwork; the amount of paperwork that’s involved is colossal, we’ve got paperwork on top of paperworkESI1There is a lot of paperwork; when we were doing, obviously the bag, popping them in the bag, there was a lot of paperwork involved there because obviously they had to take the names down. So the way we, obviously, did it, we had an A4 sheet of paper, just wrote the initials down, and the illness that they qualified for, and then we were waiting until we got sorta 10 and 20 on the sheet and then I would fax it acrossESI1 (second interview)I think maybe the, the self-help book, possibly if we’d had that before, just to have a read through and just get a general idea what it was going to be about. That might have, might have been something, yeahESI6 |
|
| Case report forms | . . . exactly what [PS 3] says, even if people can read and write, the forms are difficult. I’ve had two people that have had to come in and I’ve had to sit down with them and fill it in with them; they found the forms really difficult. And you don’t get to see these forms when you’re training; so it was new to me when they brought this form in, with loads of pages with questions that they obviously thought were, were getting duplicated all the time; well you’ve just asked us that question, that’s just the same as that, isn’t it? And I said well not exactly. But they found it quite difficult; and I think a lot of people might get the telephone call, call, agree to do the trial, get the forms, and I think will take a look of it and think I’m not filling that in, I really doFG PS1Yeah, well I’m, I’m going through like with two customers and it’s on about sexual intercourse; why do we, why do we need to . . .FG PS1 |
|
Adaptations and protocol changes made during the feasibility study
Recruitment methods/strategies
The original protocol involved sending the baseline questionnaire to all those potential participants who returned a consent form and background information sheet to the study team. On receipt of a completed baseline questionnaire, potential participants would then be contacted by the study team to determine study eligibility (including the MINI diagnostic interview48), with eligible participants thereafter being included in the study. This procedure was amended prior to the start of participant recruitment to reduce participant burden and mitigate potential disappointment (for those potential participants who completed and returned a baseline questionnaire but who were then found to be ineligible to participate following eligibility screening). Instead, on receipt of a completed consent form, potential participants were first contacted by the study team to conduct the telephone-based eligibility screening. The baseline questionnaire was then sent to eligible participants to complete and return to the study team before their involvement in the study commenced. To capture information about all of the potential participants, the demographic questions originally contained within the baseline questionnaire were removed and included on the consent form and background information sheet.
A number of adaptations were made to the recruitment methods during the feasibility study, often with the dual aim of providing the wider pharmacy community with the opportunity to learn about the study and to improve the slower than anticipated rate of recruitment. CP staff provided consistent feedback to the CHEMIST study team that they felt unable to approach all eligible pharmacy customers about the study owing to limited time and resources, especially during busy periods (see Chapter 4), and that this lack of time affected recruitment. A number of additional recruitment strategies were, therefore, implemented. These are detailed in the following sections.
Providing study information with pre-ordered prescriptions
Community pharmacy staff reported that they were often unable to approach those pharmacy customers who visited the CP to collect pre-ordered prescriptions because these customers are not required to wait in the pharmacy for their medication to be dispensed. CP staff suggested including the BIS in pre-ordered prescription bags. Customers could then read the study information in their own time and request the full study information pack the next time that they visited the pharmacy. This additional recruitment method was added after the study had been recruiting for 2 months.
Providing study information with home-delivered prescriptions
During regular discussions with recruiting CPs, CP staff highlighted that those pharmacy customers who received their prescriptions/medication via the pharmacy home delivery service, rather than visiting the pharmacy in person, were not likely to receive information about the study. An additional recruitment method was, therefore, introduced that allowed CP staff to provide those eligible pharmacy customers who received home-delivered prescriptions with information about the study. This protocol change initially allowed CP staff to include the BIS in home-delivered prescriptions; however, this was amended so that the full study information pack was provided, thereby reducing the burden of participants initiating contact with their CP and/or the CHEMIST study team to request a full study information pack. This additional recruitment method was introduced in month 5 of recruitment.
Community pharmacy system search
An additional recruitment method was implemented after the study had been recruiting for 3 months that allowed CPs to conduct searches on their pharmacy database/systems to identify customers eligible to receive study information in the post. This recruitment method was added to aid CPs with the identification of eligible pharmacy customers and to increase recruitment to the study by reducing (although not removing) the need for CP staff to approach all eligible customers who visit the pharmacy in person.
Other changes
The time frame in which members of the CHEMIST study team could contact potential participants following receipt of ‘verbal consent to contact’ was reduced from a minimum of 1 week to a minimum of 2 days in month 5 of recruitment. Feedback from the CHEMIST study team who were contacting potential participants in this way suggested that the original 1-week time frame was perhaps too long because some pharmacy customers struggled to remember the study information that they had received 1 week earlier. This revised time frame is often used in primary care mental health studies and was considered to represent an adequate length of time in which the participant had time to read, digest and discuss the study with their family/friends/GP.
Qualitative study
Semistructured interviews with ESI facilitators aimed to explore their experiences of undertaking the ESI training and delivering the ESI in a single interview following ESI delivery. However, given the slow recruitment of participants, there was a delay in ESI facilitators gaining experience of delivering the ESI and, in order not to lose valuable information as to the utility of the study and ESI training, initial interviews focused on ESI facilitators’ experiences of the study and ESI training. These ESI facilitators indicated that they would be happy to participate in a second interview to discuss their experiences of delivering the ESI. The option of conducting a second interview was, therefore, included, along with the option of sending a reminder study invitation pack to those ESI facilitators who did not respond to the first invitation pack. In addition, to achieve data saturation, the target number of qualitative interviews to be conducted with ESI facilitators was increased from 4 to 10. These protocol changes were implemented after the study had been recruiting for 8 months.
Adaptations made in preparation for the pilot randomised controlled trial
An important aim of the feasibility study was to develop and refine the study procedures and materials for use in the pilot RCT. The learning acquired from the feasibility study facilitated the refinement of the study procedures and documentation in preparation for the pilot RCT. Many of the protocol changes that were made during the feasibility study were maintained in the design of the pilot RCT. Additional protocol changes made prior to the commencement of the pilot RCT and other changes are detailed in the following sections. These changes were discussed in collaboration with CP staff and study stakeholders (including the CHEMIST PPI AG and the CHEMIST special interest group; see Chapter 2) and were informed by the results of the feasibility qualitative study.
Recruitment methods
In the feasibility study, eligible pharmacy customers who visited the pharmacy in person were approached by CP staff about the study and were provided with the BIS in the first instance; this advised them to request the full study information pack for further information. Some CP staff advocated for the potential benefit of providing the full study information pack directly to those eligible pharmacy customers visiting the pharmacy, reporting that pharmacy customers may often not get time to read the BIS while they are visiting the pharmacy and so they may not request further information about the study during that visit. They felt that this may improve recruitment to the study. Given the small number of full study information packs that were requested by pharmacy customers (potential participants), following receipt of the BIS during the feasibility study the protocol was amended to enable the full study information pack to be provided at initial contact (in line with other recruitment methods: home-delivered prescriptions, CP system searches and general practice database searches). The BIS was, therefore, not used in the pilot RCT.
Recruitment materials
Participant recruitment materials were revised for the pilot RCT. Feedback from the feasibility study (both via semistructured interviews with study participants and ESI facilitators and via general discussions with CP staff) suggested that some participants may experience difficulties reading the PIS because this was felt to be long and contained a lot of information. CP staff highlighted that because the study was recruiting in low socioeconomic areas, people may struggle to read and understand the PIS and, therefore, poor literacy may be a barrier to recruitment. The PIS was re-formatted to improve its appearance, with the information separated into clear sections. The complexity and length of some of the sentences were reduced and the language/words used were simplified to improve readability (this was reflected in a lower reading age score). The majority of the information provided in the feasibility PIS was maintained in the pilot RCT PIS, but an ‘Important things you need to know’ and a ‘contents’ section were added to the front page to facilitate understanding and readability. Revisions to the pilot PIS were made in consultation with the CHEMIST PPI AG.
An additional version of the PIS was developed to be used when recruiting participants through general practices. Participants recruited via this method may be asked to access a different CP to their usual one for their involvement in CHEMIST. CHEMIST researchers reported that some participants were not aware of this when they were contacted to discuss the feasibility study and that this may have contributed to some potential participants changing their mind about their interest in the study. The ‘GP PIS’ included information about which CP participants would be required to liaise with if they were to be included in the pilot RCT but otherwise was identical to the ‘pharmacy PIS’. The ‘GP PIS’ was discussed and developed in agreement with CP stakeholders and the CHEMIST special interest group.
In the feasibility study, a number of potential participants placed a tick/cross in the consent statement boxes on the consent form (parts 1 and 2), rather than placing their initials as required. This often resulted in a delay to them commencing their involvement in the study, as participants were required to update the consent form or complete a second form. To reduce participant burden and the potential delay in starting the study, regulatory approval was secured to accept a consent form as valid if participants (including those participating in semistructured interviews; see Chapter 6) placed a tick/cross in the consent statement boxes, provided that they had printed their name and signed and dated the consent form.
Questionnaires
Feedback from both CP staff and study participants in the feasibility study suggested that some participants found the baseline and follow-up questionnaires too long, that they sometimes struggled to understand how to complete these properly and that some of the questions asked were repeated. Some participants took their questionnaire into the pharmacy for help completing it, and some CP staff felt that the questionnaires may lead to some participants questioning their further participation in the study.
In response to this feedback, the PHQ-1566 was removed as an outcome measure to reduce the number of questionnaires that participants were required to complete. Some participants reported feeling uncomfortable answering some of the PHQ-15 questions [e.g. those questions about menstruation (women only) and sexual intercourse]; this was not a questionnaire that was originally proposed as an outcome measure in the original funding application.
The questionnaire instructions for study participants were revised to facilitate better understanding and explanation as to how to complete the questionnaire. These highlighted why the questions were important for the study and that, although some of the questions may appear repetitive, all of the questions provide valuable information. Some participants reported finding the bespoke resource use questionnaire difficult to complete: the structure of this questionnaire was revised to aid ease of completion in the pilot RCT. A further question was added to ask about the number of pharmacy visits to further inform the cost-effectiveness evaluation. The CHEMIST PPI AG provided feedback on the revised questionnaires and found that these were to easier to read, understand and complete.
To improve participant experience of questionnaire completion in the pilot RCT, participants were routinely given the option of completing the questionnaires over the telephone with a CHEMIST researcher. Participants could still request to complete the questionnaires through the post if that was their preferred method.
Qualitative study
Although a number of CP staff participated in a semistructured interview and/or focus group in the feasibility study, their subsequent (informal) feedback suggested that some staff were initially reticent about their involvement in this aspect of the study. Reference to an ‘interview’ in the recruitment materials was reported to concern some staff, as they equated this term with going for a job interview. Some CP staff discussed their invitation with the study team and, following an explanation of what the interview would involve, agreed to participate. The qualitative recruitment materials to be used in the pilot RCT were, therefore, revised to invite participants to provide their ‘feedback’ rather than to participate in an ‘interview’.
Given that the pilot RCT ESI training workshop would introduce the qualitative aspect of CHEMIST (including an overview of the findings from the feasibility qualitative study), ESI facilitators were provided with the study invitation pack following completion of this training workshop. It was anticipated that this may lead to increased consent rates to the qualitative study.
The target interview number for CP staff was increased from 10 to 20 to allow interviews to be conducted with CP staff from across a range of pharmacy roles (to include ESI facilitators).
Self-help patient workbook
The self-help workbook used in the feasibility study was positively received by study participants and ESI facilitators (see Chapter 4); therefore, relatively minor revisions were made to this for the pilot RCT. Feedback (via semistructured interviews, informally during ESI facilitator supervision and reflections from the CHEMIST study team and various stakeholders) led to minor revisions that fell broadly within five areas, as briefly described in the following sections.
Revised stages
The self-help workbook contained eight stages delivered across up to six ESI sessions. Although the workbook was designed to be used flexibly and in response to individual participant circumstances and experiences, some CP staff reported that they might find it easier to work with the workbook if the stages aligned with the sessions. As a result, some of the stages were combined to create a six-stage workbook for use in the pilot RCT (see Chapter 6).
Removal of reference to depression
Feedback suggested that the workbook should not reference ‘depression’ because some participants may not understand or equate their current symptoms with this term (given the study concerned people with subthreshold depression/low mood). Reference to the term depression was, therefore, removed from the workbook for the pilot RCT. Examples included revising ‘Understanding what depression is, so it may be recognised in future’ to ‘Understanding what low mood is, so that it doesn’t get worse in the future’, and ‘Some people lose their appetite and start to lose weight when they are depressed’ to ‘Some people lose their appetite and start to lose weight when they feel low in mood’.
Links to physical health problems
The workbook was revised to include additional references to physical health problems to further emphasise how mental and physical health are linked. For example, when describing the cycle of low mood (‘In order to break the cycle it is important to plan activities that reconnect or maintain your connections with things in your world that are healthy for you. We call this planning to maintain a healthy mood’) the following sentence was added: ‘This is particularly important for people who have physical health problems to help them stay well’. When discussing breaking down tasks into smaller more manageable steps, the wording was amended from ‘They do not need to be done all in one day’ to ‘Remember, it does not need to be done all in one day, often when you have health problems doing too much in one go is very exhausting. This can lead to feeling overwhelmed and then not doing other things that you like doing’.
Positive wording
Some of the wording was amended to emphasise positive action. Examples included amending stage titles such as ‘An 8-stage plan to help you keep well and prevent depression’ to ‘A 6-stage plan to help you keep a healthy mood when you have long-term health problems’, and amending ‘Spotting the symptoms of depression’ to ‘Spotting the symptoms of low mood and making an action plan to stay well’.
Further clarifications/explanations/descriptions
Other minor revisions were made to aid clarification or to provide further explanation or descriptions of concepts or situations described.
Enhanced support intervention facilitator manual
Minor changes were made to the ESI facilitator manual in the light of feedback from ESI facilitators and to align the ESI facilitator manual with the revised self-help patient workbook.
The word ‘supervisor’ was considered anxiety-provoking for some ESI facilitators, so this word was replaced with ‘study team advisor’. Further clarification was added around the timeline and process for contacting newly recruited participants and an explanation of the process of randomisation was included to align this with the pilot RCT.
Other changes
Numerous additional minor changes were made to the pilot RCT study documentation, including revisions to participant and GP letters and the development of new participant letters to better inform and maintain contact with participants throughout the duration of the pilot RCT.
Training
An important aim of the feasibility study was to evaluate the training delivered during the study. This included the ESI training for ESI facilitators and general study and recruitment training for CP staff within recruiting CPs. Feedback was obtained via semistructured interviews and focus groups, and informally via discussions between CP staff and CHEMIST study team members.
Enhanced support intervention training
The ESI training is described in detail in Chapter 2.
Adaptations were made to the ESI training in response to formal and informal feedback from those ESI facilitators who completed the ESI training as part of the feasibility study (see Tables 14 and 15 for examples of interview quotations that informed/supported these adaptations).
Information about the ESI training was sent in advance of the pilot RCT training dates to all those CP staff identified to undertake the training. This included a copy of the self-help patient workbook, a brief description of the purpose of the study and what would be covered in the training, including role-play (described as skills practice sessions). This would allow CP staff to attend the ESI training with prior knowledge and relevant expectations.
Key aspects of the ESI training delivered in the feasibility study were identified and the training was modified to address/acknowledge the following areas: (1) preparing the ESI facilitator for complex participants (e.g. those with multiple LTCs); (2) discussing the importance of risk assessment; (3) the need for ESI facilitators to feel confident and comfortable to use the study materials (e.g. self-help patient workbook and the ESI facilitator manual) flexibly in response to participants’ circumstances and LTCs; (4) discussion of familiarity and confidentiality with pharmacy customers and the associated strengths and limitations; and (5) importance of good listening skills.
The training was further adapted to better emphasise/highlight the links between physical and mental health with case/patient stories used to illustrate the complexity of these links. The self-help patient workbook was also modified to emphasise this important link (see Self-help patient workbook). The updated training sought to increase understanding and awareness that a person’s LTCs can limit their ability to engage with the BA component of the ESI (identified as a key component of the ESI), and work out how to effectively and appropriately modify the BA in response to a person’s LTCs.
An important training update was to describe the experiences gained from the feasibility study, including findings from the interviews/focus group with ESI facilitators and CP staff. This not only served to highlight how valuable and vital this feedback is to the study, but also served to provide real-life context to the study by providing concrete examples.
A key theme from the feasibility study was the importance of ensuring that ESI facilitators (and those CP staff involved in recruitment) felt supported to engage with the study. The clinical supervision provided to ESI facilitators was seen as a vital mechanism by which to provide ongoing support throughout the delivery of the ESI. ESI facilitators were made aware during training that they would receive regular clinical supervision and that they could contact their supervisor (or any member of the CHEMIST study team) at any time if they had any questions or concerns.
General study and recruitment training
A brief description of the general study and recruitment training delivered to CP staff is described in Chapter 2.
A number of adaptations were made to the study training and related study documentation and procedures in the light of feedback from CP staff and the experiences of those CHEMIST researchers who designed and delivered the feasibility study training.
Community pharmacy staff suggested that the study training session was too long (4 hours) and involved too much information/detail about the study (although it was noted from semistructured interviews that some CP staff felt that more information was needed to explain what was required of the CP). Study training in the pilot RCT was, therefore, split over two separate 2-hour sessions and the presentation/training slides were simplified to include the main/important information. Splitting the training into two sessions allowed CP staff to discuss the various study recruitment methods and procedures and how these might best fit into their existing routines; any issues or challenges could then be discussed within the CP and then discussed/confirmed during the second training session. The training also included the learning gained from the feasibility study in terms of what worked well and ways to mitigate or respond to problems, and highlighted how recruitment methods had been adapted in the light of feedback from CP staff.
Community pharmacy staff reported that they felt uncomfortable with approaching pharmacy customers directly about the study, especially given the mental health/low mood aspect. As was the case in the feasibility study, time was spent during training going through ways in which eligible pharmacy customers could be approached in person, with example ‘scripts’ provided (based in part on feedback/suggestions from CP staff involved in the feasibility study) that could be adapted to allow CP staff to feel more comfortable with how they approached customers. CP staff were advised that there was no requirement to mention ‘low mood’ or ‘mental health’ and that any questions that they might receive about the study from customers could be directed to the CHEMIST study team. Concerns could also be discussed during the regular telephone calls between the CP and the CHEMIST study team.
The paperwork that documented daily recruitment activity at each CP was streamlined with only minimal information required in an attempt to reduce the time taken for CP staff to complete this. The need for all CPs involved in CHEMIST to distribute a large number of study information packs to recruit (a relatively small number of) people into the study was discussed; this highlighted the importance of approaching and providing study information to as many eligible pharmacy customers as possible (not least to provide the opportunity for pharmacy customers to take part in the study).
Chapter summary
The feasibility study provided an important opportunity to develop and refine the ESI (materials and training) and study procedures (identification, screening, recruitment and assessment of participants, suitability of outcome measures and data collection processes) for use within a CP setting. Semistructured interviews with participants and ESI facilitators, and a focus group with CP staff, provided rich and detailed data on the acceptability and experiences of the study. These data were further informed by ongoing discussions with recruiting CPs, CP staff and CHEMIST stakeholders (including CP and public health specialists and the CHEMIST PPI AG) throughout the duration of the feasibility study and via the study team’s experience of conducting the study. These data identified a number of challenges to the delivery of the feasibility study (most notably with respect to participant recruitment) but also provided an opportunity to work closely and collaboratively with recruiting CPs and CP staff to identify and explore alternative/additional strategies/or solutions. This combination of qualitative data collection and collaborative discussions provided a critical learning opportunity that fully informed the design and delivery of the pilot RCT.
The CP is a new area for mental health research and represents a relatively novel setting in which to conduct depression prevention studies, such as CHEMIST. Many of the CPs involved in the CHEMIST feasibility study had limited experience of research and, for this reason, had few established processes for research delivery. The feasibility study provided important reflections on how to embed research and study processes in this busy public health setting. The learning acquired from conducting the feasibility study was critical in refining study procedures, training and materials for use in the pilot RCT and will also be useful in the design and delivery of future studies within pharmacy. Clinical research networks are keen to expand the delivery of research within this important public health setting and the establishment of primary care networks will further facilitate important collaborative and interdisciplinary working across key stakeholders.
Chapter 6 Pilot randomised controlled trial: methods
Study design
This was an external pilot two-arm parallel-group individually randomised controlled trial with a nested qualitative process evaluation and economic evaluation.
A number of modifications were made throughout the delivery of the pilot RCT (as detailed in Chapter 7). The majority of these were considered minor and did not involve changes to key aspects of the study design. The analysis of the qualitative data was initially intended to involve both a thematic analysis and a NPT framework analysis; however, a thematic analysis was not conducted as the NPT framework45 appeared to capture the interview data sufficiently well (see Chapter 9, Data analysis, for a detailed justification).
Study approvals
Study approvals are detailed in Chapter 2.
Study sites
Fifteen CPs in the north of England took part. Thirteen CPs were involved from the commencement of the study and two additional CPs were recruited during the recruitment period. Six CPs were involved in both the feasibility study and the pilot RCT.
Participant eligibility
The inclusion and exclusion criteria were as described for the feasibility study (see Chapter 2).
Participant recruitment
Four CP-based recruitment methods and one GP-based recruitment method were employed for the pilot RCT. These are described in Chapter 2 (see Report Supplementary Material 7 for the participant study information pack for the pilot RCT). Refinements were made following the feasibility study, including removing the use of the BIS (see Chapter 5).
Screening for eligibility
The process of screening for participant eligibility was as detailed in Chapter 2.
Baseline assessment
The process for participant baseline assessment was as described in Chapter 2, with the exception that participants could opt to complete the baseline questionnaire over the telephone with a study researcher or complete and return the questionnaire via post (see Chapter 5; see Appendix 4 for non-validated baseline pilot RCT questionnaires).
Randomisation
Individual randomisation was employed and carried out by the York Trials Unit online randomisation service, independent of the study team. Following completion of the baseline questionnaire, participants were randomised on a 1 : 1 basis to either the ESI arm or the usual-care arm (see Intervention and Comparator).
Following randomisation, participants were contacted by the ESI clinical supervisor (or a researcher not involved in follow-up assessments) to advise them of their treatment arm allocation. Participants randomised to the ESI arm were advised that a ‘Healthy Living Advisor’ from the local participating CP would contact them within the next few days to arrange their first pharmacy support session (ESI session). All participants (and their GPs) received a letter confirming their treatment arm allocation; those randomised to the ESI arm also received copies of the ESI materials.
Owing to the nature of the intervention it was not possible to blind participants, ESI facilitators or GPs to participant treatment arm allocation. Study researchers were blinded to participant treatment arm allocation, where possible.
Sample size
To estimate recruitment and attrition, and the standard deviation of the PHQ-9 (the intended primary outcome to be used in a main trial), a sample size of 100 participants was used. Allowing for 20% attrition, with 100 participants, the 95% confidence interval (CI) for this level of attrition would be 8 percentage points and would provide robust estimates of recruitment and follow-up. Equally, this sample size would allow for robust estimates of the standard deviation of the intended primary outcome in this patient population, to inform a future sample size for a main trial.
Intervention
The ESI is described in detail in Chapter 2.
A number of minor adaptations were made to the ESI (as used in the feasibility study) for implementation in the pilot RCT. These are described in detail in Chapter 5.
Comparator
The control arm (‘usual-care’ arm) was the usual primary care management of subthreshold depression offered by the participant’s GP and any other local community provision.
Follow-up
The process for participant follow-up assessment was as described in Chapter 2, with the exception that participants could opt to complete the follow-up questionnaire over the telephone with a study researcher or complete and return the questionnaire via post (see Chapter 5; see Appendix 5 for non-validated pilot RCT follow-up questionnaires). Telephone follow-ups and participant contacts to obtain missing data following receipt of postal questionnaires were conducted by researchers blinded to participant treatment arm allocation, where this was feasible.
Outcome measures
The intended primary and secondary outcome measures were the same as those described in Chapter 2 with the exception of the PHQ-1566 (physical/somatic health problems), which was removed from the pilot RCT (see Chapter 5).
In addition, as part of the process evaluation (see Qualitative study), participants were invited to complete and return (using a pre-paid envelope) the 9-item BADS62 at 5 weeks post randomisation in order to assess response rates (to inform a future definitive RCT).
Study completion and participant withdrawal
The criteria for participant exit from the pilot RCT and the criteria and process for participant withdrawal were the same as reported in Chapter 2.
Clinical data analysis
All planned analysis was detailed in a statistical analysis plan and was agreed with the TSC prior to completion of data collection. A single analysis was performed at the end of the pilot RCT using Stata® v15. Because this was a pilot RCT with a small sample size, the planned analysis was mainly descriptive. The flow of participants through the pilot RCT will be reported. All data are presented descriptively by treatment arm and overall. Continuous data are reported using means, standard deviations, medians, the upper and lower quartiles, and the minimum and maximum. Categorical data are reported as counts and percentages. Baseline data are summarised descriptively, with no formal statistical comparisons undertaken.
Serious adverse events will be detailed by treatment arm.
Primary analysis
The intended primary outcome for the pilot RCT was the PHQ-963 at 4 months post randomisation. This will be summarised descriptively overall and by treatment arm. To explore any potential effect from the ESI, a linear regression model, adjusted for age, gender and baseline PHQ-9 score, will be undertaken. Given the small sample size, the results will be exploratory and should be interpreted with caution.
Secondary outcomes
All secondary outcomes will be reported descriptively, as detailed above. No formal statistical comparisons of these measures will be undertaken.
In addition, the pharmacy effect will be explored using the intracluster correlation coefficient and the associated 95% CI to determine whether or not the effect of the intervention differed across the recruiting CPs.
The average caseload per ESI facilitator will be detailed.
Quantitative outcomes associated with the process evaluation (see Qualitative study) will be reported descriptively.
Interim analysis and stopping rules
No interim analysis was planned for this study and there were no stopping rules in place.
Qualitative study
The pilot RCT included a qualitative process evaluation utilising semistructured interviews with study participants, pharmacy staff (including ESI facilitators) and GPs. The aim of the process evaluation was to explore the acceptability of the ESI within CPs, the elements of the intervention that were considered useful (or not) and the appropriateness of study procedures.
Participants
The aim was to purposively sample up to 15 study participants who completed the ESI to explore acceptability and important elements of the ESI, and up to 15 study participants who started the ESI but then dropped out (had less than two sessions) to explore their reasons for disengagement. All study participant interviews were conducted after the proposed primary outcome measure had been collected (4 months post randomisation) and at a time and location convenient to the participant (e.g. at home, in the pharmacy or by telephone).
The aim was to interview up to 20 pharmacy staff (ESI facilitators, general pharmacy staff and pharmacists) to explore important aspects of the ESI, ESI training and clinical supervision, and barriers to/facilitators of study participation and implementation. Interviews were conducted in person in a private room at the pharmacy or over the telephone, according to participant preference, at a time convenient to them.
To ensure confidentiality and to encourage interviewees to speak freely, interviews with ESI facilitators were not conducted with those study researchers who had been involved in their training (either ESI training or general recruitment training). Interviews with general pharmacy staff were conducted by a study researcher who had not previously been in contact with them as part of the study (i.e. had not been involved in their training and was not their CP’s main contact for the study). Study researchers who had had previous contact with interviewees were able to access anonymised interview transcripts only.
Interviews were also planned with up to eight GPs to explore their views and knowledge of the study, the ESI, how this may impact on routine primary care and the barriers to/facilitators of implementation. Interviews took place at a time and location according to GP preference (e.g. in the general practice, over the telephone).
Interview topic guides varied between participant treatment arms, but incorporated core questions used in the feasibility study (see Report Supplementary Material 8). These were developed by the research team and modified iteratively as data generation and analysis proceeded.
Recruitment
Study participants were recruited as described in Chapter 2 (see Report Supplementary Material 7).
Enhanced support intervention facilitators were provided with a study information pack (containing an invite letter, PIS, consent form and prepaid envelope) following completion of the ESI training (see Report Supplementary Material 9 for examples). The remaining pharmacy staff from the recruiting CPs (including general pharmacy staff and pharmacists) were posted a study information pack by the study team (see Report Supplementary Material 9). Interested pharmacy staff were asked to complete and return the consent form to the study team. A reminder letter was sent to all pharmacy staff once ESI delivery had ceased at each pharmacy. This process was carried out independently of the pharmacy to ensure that it was free from pressure from managers or co-workers.
General practitioners of recruited participants and GPs from those practices involved in the identification of potential participants were posted the study information pack (see Report Supplementary Material 10). Interested GPs were asked to complete and return the consent form to the study team.
Analysis
Interviews were recorded, with participant consent, using an encrypted digital audio-recorder and transcribed verbatim independently of the study team. Transcripts were then anonymised by members of the research team to ensure the confidentiality of participants. A framework analysis was conducted using NPT. 45 NPT supports the identification of key factors that enable or constrain the successful normalisation of an intervention into routine working practices. 77 By applying the NPT domains, the pilot RCT process evaluation explored how participant groups made sense of (coherence) and engaged with (cognitive participation) the ESI. In addition, the work completed and needed to facilitate the intervention (collective action), as well as participant’s appraisal (reflexive monitoring) of the intervention, was explored.
Data were coded directly to the NPT domains (coherence, cognitive participation, collective action and reflexive monitoring) by three researchers (CS, LN and AP). These researchers were from a variety of professional backgrounds to increase the reliability of the analysis,70 and all researchers received supervision from a researcher experienced in the use of NPT (CCG).
Before formal data coding began, general reliability for coding data to the NPT domains was established. This involved researchers independently coding transcripts and then subsequently meeting to discuss and agree coding at a series of meetings supported by CCG. Although it is noted that different interpretations of the NPT domains can occur, this exercise was completed to begin to immerse researchers in the NPT framework and data engagement, as well as to establish a general agreement on coding. The coding process was supported by regular memo taking, with the purpose of recording emergent understanding of the data and to inform discussion and analysis between researchers.
Following the coding process, the analysis drew on principles of the constant comparison method. 69 This process involved comparing and contrasting coding both within and across participant groups under each NPT domain, with the purpose to explicate phenomena within NPT categories. This was an iterative process that also involved moving back and forth between the meaning of the NPT categories described by Murray et al. 77 and the emergent phenomena observed in the data. Deviant case analysis was sought throughout the process. Subsequently derived categories and phenomena with NPT domains were discussed and agreed by researchers (CS and CCG) and are presented in Chapter 9.
A future definitive RCT would involve a mixed methods process evaluation incorporating mediational and moderational analyses to investigate mechanisms of change. 78 Mediational analysis would examine outcomes such as number of ESI sessions, content of ESI sessions and changes in activation (using the BADS). Moderational analyses would examine baseline variables that may moderate outcomes such as age, depression onset age, number of episodes and socioeconomic status. Although the current pilot RCT would be underpowered to undertake these analyses, data will be reported descriptively, where feasible.
Health economic analysis
The health economic analysis aimed to collect resource use and quality-of-life data to estimate levels and changes in costs and health outcomes over the study period to understand the potential economic impact of the ESI in a RCT. Resource use data focused on primary and secondary care services, including mental health services; service use data were multiplied by unit costs to arrive at the total cost in each treatment arm. Unit costs of health service use were obtained from the UK national database of reference costs, the Department of Health and Social Care,79 and the Unit Costs of Health and Social Care report produced by the Personal and Social Services Resource Unit. 80 All costs are reported for year 2018–19 using Great British pounds. Quality-of-life data were collected using the standard EQ-5D-3L and SF-12v267,73 questionnaires; these were then used to estimate health-related quality of life (i.e. utility) using the UK tariff. 73 The pilot RCT analysis was not intended to provide a definite estimate of relative cost-effectiveness but to inform a larger RCT.
The pilot RCT used the same bespoke resource use, EQ-5D and SF-12v2 questionnaires, as the feasibility study (except for the addition of a question on the number of ‘pharmacy visits’ in the resource use questionnaire; this was based on feedback from the feasibility study). Individual-level data were collected on health service resource use (including primary, community-based and secondary care services, including mental health consultations), as well as quality of life. In addition, intervention-related data were collected in terms of the time spent by the ESI facilitator with each participant to deliver the ESI (i.e. number of ESI sessions multiplied by the duration of each session + total administration time and clinical supervision time to deliver the ESI). Resource use was multiplied by unit costs to arrive at the total cost in each treatment arm. Participants were offered up to six sessions with an ESI facilitator. ESI sessions were delivered at the individual level and the length of each session was recorded. The time spent by the ESI facilitator delivering the session was multiplied by their hourly rate.
The analysis was conducted from the UK health services perspective. Data were collected at baseline and at 4 months post randomisation. The analysis of resource use and health-related quality-of-life data was conducted in terms of the overall response rate for each questionnaire, the rate of missing items within each questionnaire and the level of health service resource use and health-related quality of life (by item/domain) at baseline and at the 4-month follow-up.
Serious adverse events
The process for the identification, reporting and reviewing of suspected SAEs was the same as that detailed in Chapter 2.
Risk management
The same study-specific risk protocols for the identification, assessment and reporting of suicide or self-harm were followed as those described in Chapter 2.
Study training
The training of study researchers and ESI facilitators was as described in Chapter 2.
Study researchers were informed of any changes to study procedures from the feasibility study, and any study researchers who had not worked on the feasibility study received training on all study procedures.
Enhanced support intervention facilitators who had not taken part in the feasibility study completed the 2-day ESI training workshop (see Chapter 5, see Intervention and see Tables 14 and 15 for a description of the adaptions made to the pilot RCT ESI training workshop). Those ESI facilitators who had taken part in the feasibility study and were continuing in that role in the pilot RCT were offered telephone-based refresher training.
Study researchers delivered face-to-face general study and recruitment training to all those recruiting CPs who had not taken part in the feasibility study. Study training was adapted following the feasibility study and was streamlined and delivered over two shorter sessions to aid understanding and to encourage discussion regarding potential challenges to study delivery (see Chapter 5 for a detailed description of these study training adaptations).
Those CPs that were involved in the feasibility study and that were continuing to the pilot RCT were given face-to-face refresher training lasting around 1 hour to update CP staff on the changes made to the study and recruitment procedures from the feasibility study.
Patient and public involvement
There was PPI throughout the duration of CHEMIST (feasibility study and pilot RCT). This is described in detail in Chapter 2.
Chapter 7 Pilot randomised controlled trial: protocol changes
The current protocol is version 4.2 (dated 14 June 2018).
The general practice database search criteria included refining the search to patient postal codes within 2 miles of the participating CHEMIST CP. Feedback from several general practices suggested that this particular search criteria was complex and time-consuming for practice staff to implement, resulting in some general practices withdrawing their initial involvement in the study. So as not to exclude patients from receiving information about the study owing to the practicalities of conducting searches on general practice systems, this postal code restriction was removed from the search criteria in month 5 of recruitment.
A request to extend the pilot RCT recruitment period by 9 months was submitted to the funder after the study had been recruiting for 9 months (excluding a 3-month delay to the start of recruitment). Both costed and non-costed extension requests were rejected by the funder. Given that recruitment had continued for a longer period of time (14 months) than had been initially planned (8 months), a time-only extension was subsequently approved to allow collection of planned data for all those participants recruited to the pilot RCT (n = 44). The full study was, therefore, extended by 7 months.
Chapter 8 Pilot randomised controlled trial: quantitative results
Recruitment
The target sample size for the pilot RCT was 100 participants, who were to be recruited over 8 months (January to August 2018) from five CPs (see Chapter 1, Progression criteria).
Recruitment activity took place over a period of 14 months (March 2018 to April 2019) and from across a total of 15 CPs, indicating that recruitment was slower than anticipated, as was the case in the feasibility study. Thirteen CPs were recruited from the start of the pilot RCT (a larger number of CPs were recruited from the outset of the pilot RCT than originally planned given the difficulties experienced with recruitment in the feasibility study); two additional CPs were recruited after the commencement of the pilot RCT (from May 2018) and the original recruitment period of 8 months was extended (by 6 months). Five general practices conducted database searches and mailed out study information packs to potentially eligible patients (eligible participants recruited via general practices were associated with five recruiting CPs). Figure 4 shows a breakdown of recruitment over the 14-month recruitment period.
FIGURE 4.
Pilot RCT: target and actual recruitment rates.
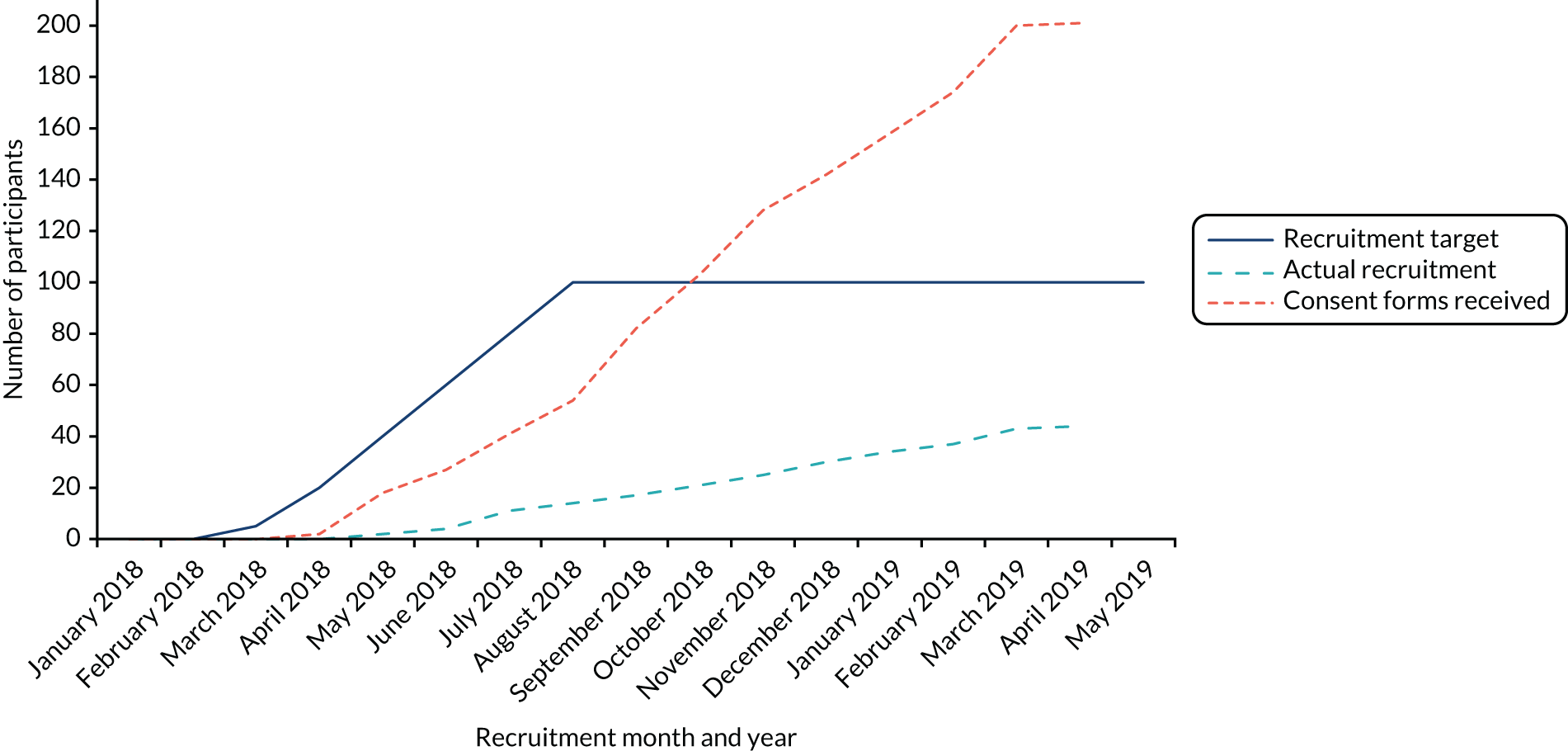
Overall, 7342 study information packs were distributed: 4892 via pharmacy-based recruitment methods and 2450 via GP-based recruitment methods. This resulted in 201 (2.7%) pharmacy customers/patients returning a consent form indicating their interest in the study, and 166 (82.6% of those contacted) agreeing to complete a screening interview. Table 16 details the distribution of study information packs, returned consent forms and resultant screened and recruited participants. Recruitment activity by pharmacy is shown in Table 17.
| Recruitment method | Packs given out (n) | Customers/patients who consented to contact, n (%) | Screened, n (%) | Randomised, n (%) |
|---|---|---|---|---|
| In the CP | 1480 | 44 (3.0) | 37 (2.5) | 8 (0.5) |
| Via home-delivered prescriptions | 404 | 20 (5.0) | 18 (4.5) | 5 (1.2) |
| CP system searches (nine conducted) | 3008 | 66 (2.2) | 50 (1.7) | 14 (0.5) |
| General practice searches (five conducted) | 2450 | 71 (2.9) | 61 (2.5) | 17 (0.7) |
| Total | 7342 | 201 (2.7) | 166 (2.3) | 44 (0.6) |
| Pharmacy | Packs given out (n) | Returned consent forms, n (%)a | Screened, n (%)a | Randomised, n (%)a | ||||
|---|---|---|---|---|---|---|---|---|
| Pharmacy | GP | Pharmacy | GP | Pharmacy | GP | Pharmacy | GP | |
| 1 | 435 | 600 | 23 (5.3) | 18 (3.0) | 21 (5.1) | 14 (2.3) | 1 (0.2) | 2 (0.3) |
| 2 | 570 | 0 | 18 (3.2) | – | 15 (2.6) | – | 5 (0.9) | – |
| 3 | 454 | 0 | 10 (2.2) | – | 8 (1.8) | – | 0 (0.0) | – |
| 4 | 141 | 0 | 4 (2.8) | – | 2 (1.4) | – | 1 (0.7) | – |
| 5 | 285 | 0 | 5 (1.8) | – | 5 (1.8) | – | 3 (1.1) | – |
| 6 | 449 | 0 | 6 (1.3) | – | 5 (1.1) | – | 1 (0.2) | – |
| 7 | 45 | 400 | 4 (8.9) | 7 (1.8) | 4 (8.9) | 7 (1.8) | 2 (4.4) | 2 (0.5) |
| 8 | 408 | 450 | 13 (3.2) | 7 (1.6) | 7 (1.7) | 5 (1.1) | 1 (0.2) | 1 (0.2) |
| 9 | 828 | 600 | 11 (1.3) | 24 (4.0) | 11 (1.3) | 21 (3.3) | 2 (0.2) | 8 (1.3) |
| 10 | 467 | 0 | 13 (2.8) | – | 10 (2.1) | – | 2 (0.4) | – |
| 11 | 216 | 0 | 8 (3.7) | – | 7 (3.2) | – | 3 (1.4) | – |
| 12 | 487 | 0 | 9 (1.8) | – | 5 (1.0) | – | 2 (0.4) | – |
| 13 | 0 | 400 | – | 15 (3.8) | – | 14 (3.5) | – | 4 (1.0) |
| 14 | 106 | 0 | 5 (4.7) | – | 4 (3.8) | – | 4 (3.8) | – |
| 15 | 1 | 0 | 1 (100.0) | – | 1 (100.0) | – | 0 (0.0) | – |
| Total | 4892 | 2450 | 130 (2.7) | 71 (2.9) | 105 (2.1) | 61 (2.5) | 27 (0.6) [20.8]b | 17 (0.7) [23.9]b |
Reasons for not fully completing the screening interview are given for all patients; these included uncontactable (n = 17), no longer interested (n = 14), found to be ineligible (n = 1) and other (n = 3). A total of 159 participants (95.8% of those who agreed to a screening interview) were found to be eligible based on the presence of a LTC.
Overall, 45 participants (27.1% of the 166 screened; 22.3% of the 201 contacted) were found to be eligible for inclusion in the study. The reasons for ineligibility can be found in Table 18. The most common reasons for exclusion were being non-depressed (35.9%) or currently experiencing a major depressive episode (31.4%), similar to the results of the feasibility study.
| Reason | Number of patients excluded (N = 156), n (%) |
|---|---|
| Non-depressed | 56 (35.9) |
| Major depression | 49 (31.4) |
| Current psychological treatment | 3 (1.9) |
| Serious mental illness present | 1 (0.6) |
| Alcohol or drug dependence | 4 (2.6) |
| Actively suicidal | 2 (1.3) |
| No relevant LTC | 6 (3.9) |
| No longer interested | 14 (9.0) |
| Not contactable | 16 (10.3) |
| Other | 5 (3.2) |
A total of 44 (97.8% of those eligible) participants were recruited to the study, from 13 out of 15 recruiting CPs (86.7%). The average number of participants recruited per pharmacy was 2.9, ranging from 0 to 10 participants.
Randomisation
Of the 44 participants who were recruited to the study, 24 (54.6%) were randomised to the intervention arm and 20 (45.5%) were randomised to the usual-care arm. The number of participants randomised by pharmacy, and to which treatment arm of the study, can be seen in Figure 5, which also details the number of potential participants screened. The flow of participants through the study is shown in Figure 6.
FIGURE 5.
Pilot RCT: overall number of participants screened and randomised by pharmacies (by treatment arm).

FIGURE 6.
Pilot RCT: CONSORT flow diagram. a, Multiple reasons for exclusion may be given.
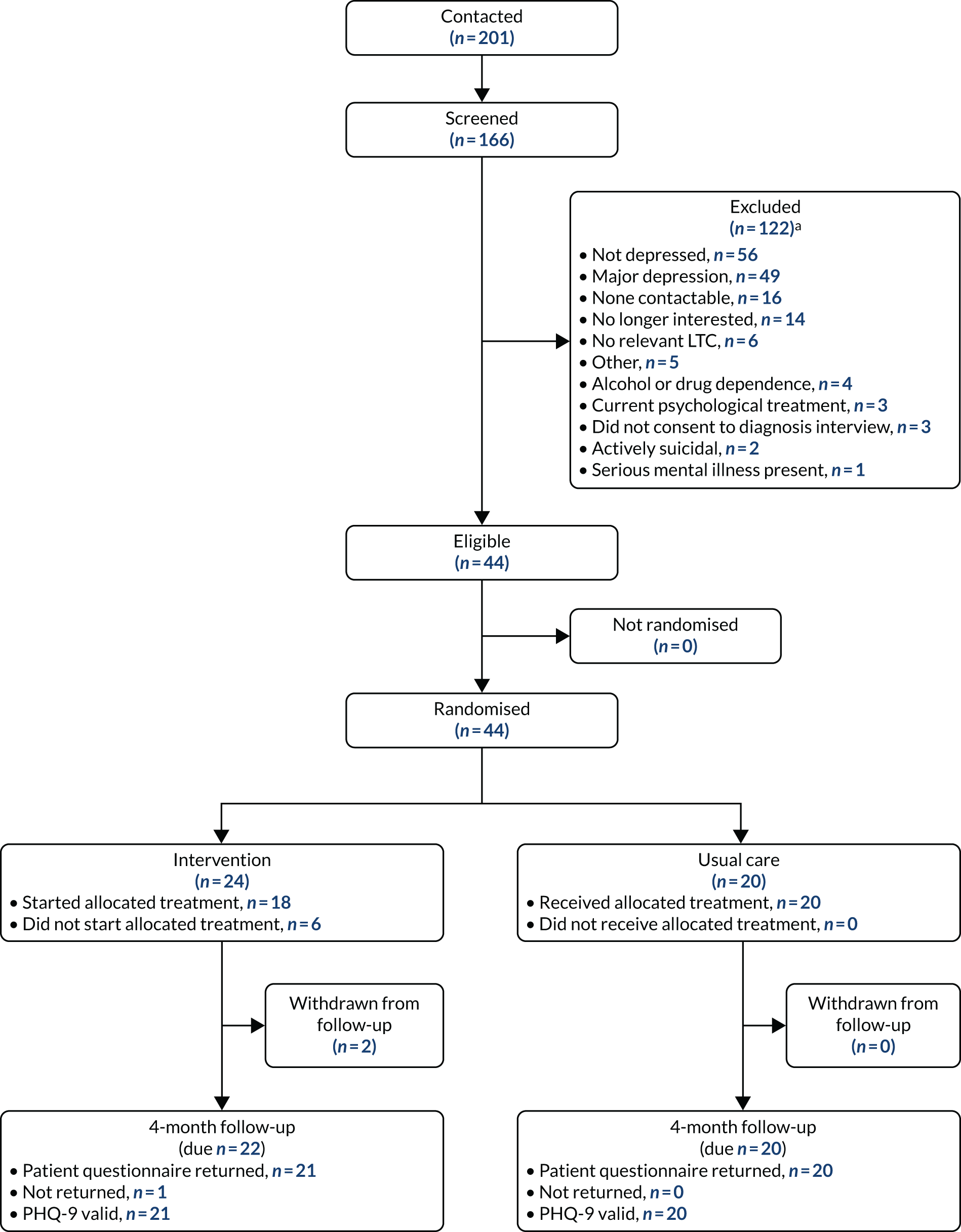
As can be seen in Figure 5, the number of packs and resulting number of randomised participants varied largely between the CPs, especially as not all CPs benefited from associated recruitment via a general practice search. Table 17 provides additional information on recruitment rates and conversion rates (from the number of packs given out to the number of participants randomised into the trial) via the different recruitment methods (pharmacy based or general practice based) and indicates variability across CPs. Overall, pharmacy-based and general practice-based recruitment methods resulted in a similar number of participants being randomised: 0.6% for pharmacy-based methods and 0.7% for general practice-based methods.
Participant characteristics
The demographic characteristics of the randomised participants can be found in Table 19. The average age of the participants was 67.6 years, ranging from 20.8 to 89.1 years. There was a roughly equal split of male and female participants, and all except one participant classed themselves as of white ethnicity. The most common health problem reported was arthritis, which 30 participants stated that they experienced, followed by cardiovascular (n = 24) and respiratory (n = 19) conditions.
| Characteristic | Treatment arm | Overall (N = 44) | |
|---|---|---|---|
| Intervention (N = 24) | Usual care (N = 20) | ||
| Age (years) | |||
| Mean (SD) | 69.0 (14.7) | 65.8 (14.6) | 67.6 (14.6) |
| Median (minimum, maximum) | 71.6 (20.8, 89.2) | 70.5 (27.0, 89.1) | 71.1 (20.8, 89.1) |
| Gender, n (%) | |||
| Male | 11 (45.8) | 10 (50.0) | 21 (47.7) |
| Female | 13 (54.2) | 10 (50.0) | 23 (52.3) |
| During the last month, have you often been bothered by feeling down, depressed or hopeless? n (%) | |||
| Yes | 22 (91.7) | 19 (95.0) | 41 (93.2) |
| No | 2 (8.3) | 1 (5.0) | 3 (6.8) |
| During the past month, have you often been bothered by having little interest or pleasure in doing things? n (%) | |||
| Yes | 19 (79.2) | 19 (95.0) | 38 (86.4) |
| No | 5 (20.8) | 1 (5.0) | 6 (13.6) |
| On average, do you drink ≥ 3 units of alcohol each day? n (%) | |||
| Yes | 4 (16.7) | 2 (10.0) | 6 (13.6) |
| No | 20 (83.3) | 18 (90.0) | 38 (86.4) |
| Do not know | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Smoking status, n (%) | |||
| Non-smoker | 10 (41.7) | 7 (35.0) | 17 (38.6) |
| Current smoker | 2 (8.3) | 0 (0.0) | 2 (4.6) |
| Ex-smoker | 12 (50.0) | 13 (65.0) | 25 (56.8) |
| Health problems,a n (%) | |||
| Arthritis | 18 (75.0) | 12 (60.0) | 30 (68.2) |
| Diabetes mellitus | 6 (25.0) | 8 (40.0) | 14 (31.8) |
| Stroke | 1 (4.2) | 0 (0.0) | 1 (2.3) |
| Cancer | 1 (4.2) | 5 (25.0) | 6 (13.6) |
| Respiratory conditions | 12 (50.0) | 7 (35.0) | 19 (43.2) |
| Progressive conditions | 2 (8.3) | 2 (10.0) | 4 (9.1) |
| Cardiovascular conditions | 15 (62.5) | 9 (45.0) | 24 (54.6) |
| Other | 6 (25.0) | 10 (50.0) | 16 (36.4) |
| Did your education continue after the minimum school leaving age? n (%) | |||
| Yes | 12 (50.0) | 13 (65.0) | 25 (56.8) |
| No | 12 (50.0) | 7 (35.0) | 19 (43.2) |
| Do you have a degree or equivalent professional qualification? n (%) | |||
| Yes | 10 (41.7) | 8 (40.0) | 18 (40.9) |
| No | 14 (58.3) | 12 (60.0) | 26 (59.1) |
| Ethnicity, n (%) | |||
| White | 23 (95.8) | 20 (100.0) | 43 (97.7) |
| Asian or Asian British | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Black or black British | 1 (4.2) | 0 (0.0) | 1 (2.3) |
| Other ethnic group | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Number of children, n (%) | |||
| 0 | 4 (16.7) | 5 (25.0) | 9 (20.5) |
| 1 | 3 (12.5) | 1 (5.0) | 3 (6.8) |
| 2 | 10 (41.7) | 4 (20.0) | 14 (31.8) |
| 3 | 6 (25.0) | 4 (20.0) | 10 (22.7) |
| ≥ 4 | 1 (4.2) | 6 (30.0) | 7 (15.9) |
| Marital status, n (%) | |||
| Single | 3 (12.5) | 2 (10.0) | 5 (11.4) |
| Divorced/separated | 0 (0.0) | 4 (20.0) | 4 (9.1) |
| Widowed | 6 (25.0) | 5 (25.0) | 11 (25.0) |
| Cohabiting | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Civil partnership | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Married | 15 (62.5) | 9 (45.0) | 24 (54.6) |
Intervention delivery
Of the 24 participants randomised to the ESI, 18 commenced the ESI sessions (75.0%). The average number of ESI sessions completed was four, with nine participants completing all six sessions. A total of 79 ESI sessions were completed by these 18 participants, out of a possible 108 (73.1%). Of the 18 participants who commenced the ESI, 16 completed at least two ESI sessions (88.9%) (Table 20).
| Total number of ESI sessions completed | Number of participants |
|---|---|
| 6 | 9 |
| 5 | 0 |
| 4 | 3 |
| 3 | 3 |
| 2 | 1 |
| 1 | 2 |
| Did not commence ESI | 6 |
A total of 18 ESI facilitators completed the ESI training as part of the pilot RCT (these were different ESI facilitators to those completing the feasibility study ESI training). For the pilot RCT, a total of 21 ESI facilitators (from those trained during the feasibility study or pilot RCT) were available to deliver the ESI, of whom 11 supported at least one participant through the ESI (five ESI facilitators delivered the ESI in both the feasibility study and the pilot RCT). On average, the number of participants per ESI facilitator was 1.6 (ranging from one to three participants).
All six of the participants who did not start the ESI withdrew before commencing the ESI sessions.
Follow-up and withdrawal
A total of 41 of the 44 randomised participants completed a 4-month follow-up questionnaire, indicating a retention rate of 93.2%.
A total of eight participants withdrew from the ESI. Six participants withdrew from the ESI before commencing any sessions (25.0%). A further two participants withdrew from the ESI after having commenced ESI sessions (one participant withdrew after session 2 and one participant withdrew after session 1). The reasons given for stopping the ESI sessions were that the intervention was unsuitable and that they did not think the intervention was helping.
Two participants fully withdrew from the pilot RCT prior to the 4-month follow-up.
Standardised measures
Primary outcome measure
The intended primary outcome measure for the pilot RCT was the PHQ-9,63 measuring depression severity at 4 months. The PHQ-9 is scored between 0 and 27, where higher scores represent a higher level of depression.
In addition to the descriptive overview of the PHQ-9 scores presented in Table 21, a linear regression model adjusting for allocation, age, gender and baseline PHQ-9 score was undertaken to explore any potential effect of the ESI. This found that, at the 4-month follow-up, the average PHQ-9 score was 0.77 points higher (indicating a higher level of depression) among participants who received the ESI than in the usual-care arm (95% CI –2.05 to 0.97 points). The CI suggests a potential benefit of the intervention of up to 2.05 points or a potential detriment of up to 0.97 points. These results are exploratory only and should be interpreted with caution, as the sample size for this estimate is small (n = 41).
| Treatment arm | Overall | ||
|---|---|---|---|
| Intervention | Usual care | ||
| Baseline | |||
| Raw score | N = 24 | N = 20 | N = 44 |
| Mean (SD) | 11.1 (5.2) | 10.8 (5.0) | 11.0 (5.0) |
| Median (Q1, Q3) | 11 (7, 13.5) | 11.5 (6.5, 14) | 11.0 (7.0, 14) |
| Minimum, maximum | 4, 24 | 0, 20 | 0, 24 |
| Categorised, n (%) | |||
| No depression (0–4) | 1 (4.2) | 1 (5.0) | 2 (4.6) |
| Mild depression (5–9) | 10 (41.7) | 7 (35.0) | 17 (38.6) |
| Moderate depression (10–14) | 7 (29.2) | 8 (40.0) | 15 (34.1) |
| Moderately severe depression (15–19) | 4 (16.7) | 3 (15.0) | 7 (15.9) |
| Severe depression (20–27) | 2 (8.3) | 1 (5.0) | 3 (6.8) |
| 4-month follow-up | |||
| Raw score | N = 21 | N = 20 | N = 41 |
| Mean (SD) | 10.0 (6.3) | 8.7 (4.4) | 9.3 (5.4) |
| Median (Q1, Q3) | 9.0 (6.0, 13.0) | 8.0 (6.0, 11.0) | 9.0 (6.0, 12.0) |
| Minimum, maximum | 0, 22 | 0, 17 | 0, 22 |
| Categorised, n (%) | |||
| No depression (0–4) | 4 (19.1) | 3 (15.0) | 7 (17.1) |
| Mild depression (5–9) | 8 (38.1) | 10 (50.0) | 18 (43.9) |
| Moderate depression (10–14) | 4 (19.1) | 4 (20.0) | 8 (19.5) |
| Moderately severe depression (15–19) | 3 (14.3) | 3 (15.0) | 6 (14.6) |
| Severe depression (20–27) | 2 (9.5) | 0 (0.0) | 2 (4.9) |
To further explore this result, Figure 7a and b shows the breakdown of the change in PHQ-9 categories between baseline and the 4-month follow-up by treatment arm. This shows that the baseline PHQ-9 categories that saw the most change between the two time points were the moderate and moderately severe depression, and that those at the top and bottom of the PHQ-9 scale (those not depressed and those most depressed) had no change in PHQ-9 score regardless of which treatment arm of the trial they were in. This figure also indicates that all participants who were in the usual-care arm of the trial and had moderately severe depression showed a reduction in depression severity, despite not being offered the ESI as part of CHEMIST.
FIGURE 7.
Pilot RCT: (a) the change in the PHQ-9 category from baseline (left) to the 4-month follow-up (right) for the intervention arm; and (b) the change in the PHQ-9 category from baseline (left) to the 4-month follow-up (right) for the usual-care arm.
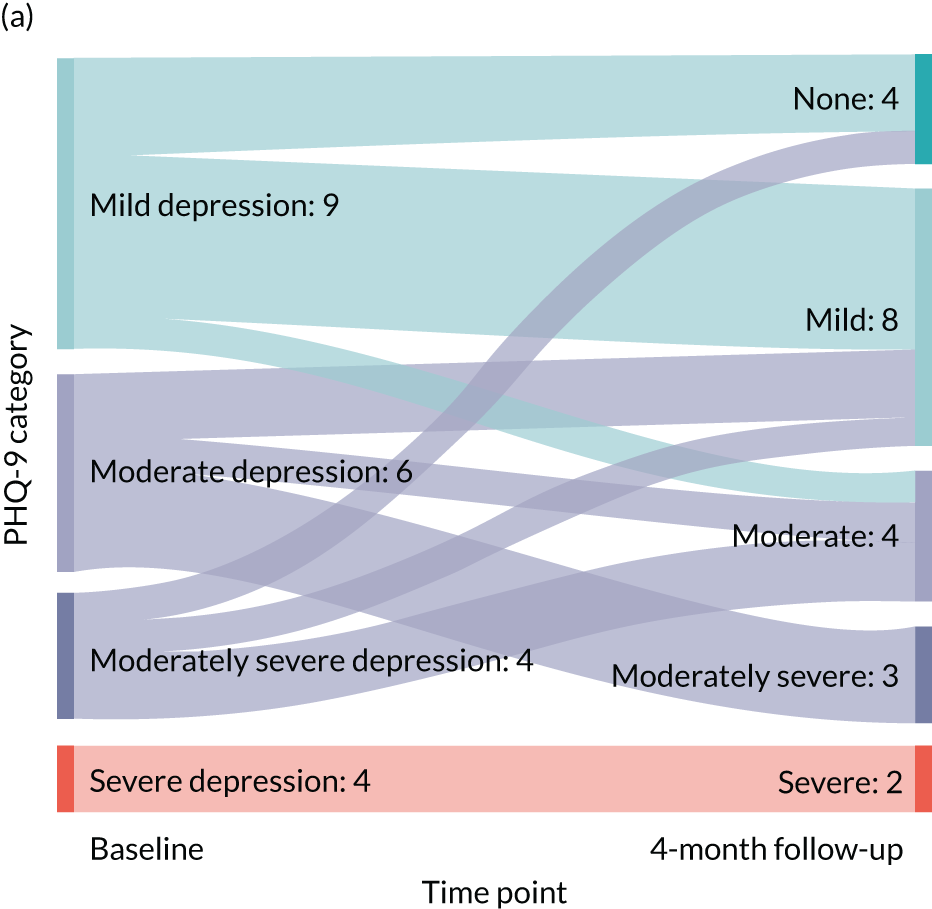
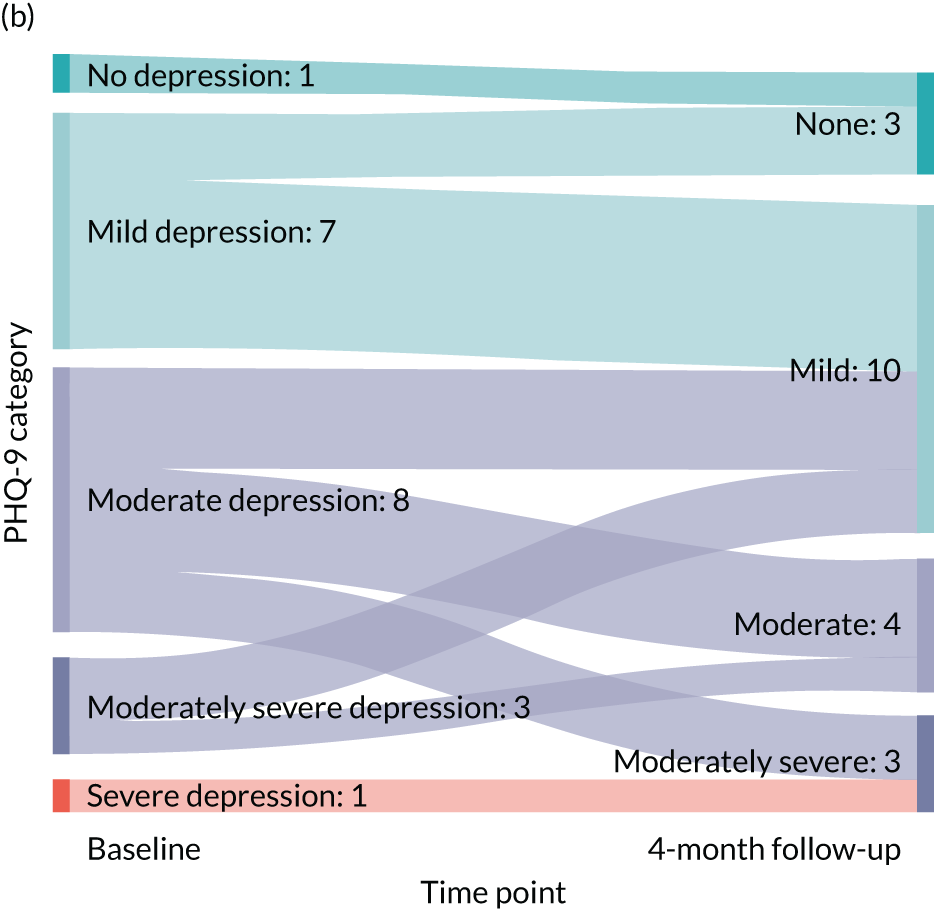
Results are shown only for those participants who provided PHQ-9 scores at both baseline and the 4-month follow-up.
Given the limited number of participants recruited per pharmacy (average 2.9 participants, range 0–10 participants), the pharmacy effect was not explored.
Secondary outcome measures
As in the feasibility study, the GAD-765 was also completed both at baseline and at the 4-month follow-up; the results are presented in Table 22. This measure was well completed, with the scores calculated for every returned questionnaire.
The BADS was sent to a total of 43 participants (the BADS was not sent to one participant in the intervention group as this was deemed by the study clinicians as inappropriate at that time). Thirty participants completed and returned the questionnaire (69.8%), with similar response rates across the intervention group (69.6%) and usual care group (70.0%).
Mediational and moderational analyses were not conducted (see Chapter 6, Qualitative study). However, data on age, LTC, depression severity and number of ESI sessions are reported in Table 19 (age and LTC), Table 21 (depression severity, PHQ-9) and Table 20 (number of ESI sessions). The number of episodes of depression (obtained via the MINI at baseline) was provided by 40 of the 44 randomised participants. On average, the participants had experienced 6.8 episodes (standard deviation 17.1), ranging from 0 to 100 episodes, although the data was skewed as the median number of episodes was 1.5. The mean was similar between the two groups: 6.9 episodes (standard deviation 20.7) for the intervention group and 6.6 episodes (standard deviation 11.2) for the usual care group. Twenty-nine participants who reported experiencing at least one episode of depression provided an age at which they experienced their first episode. This was slightly older in the intervention group (mean 34.4 years, standard deviation 23.6) than in the usual care group (28.9 years, standard deviation 16.0), and ranged from 9 to 85 years.
| Treatment arm | Overall | ||
|---|---|---|---|
| Intervention | Usual care | ||
| Baseline | |||
| Raw score | N = 24 | N = 20 | N = 44 |
| Mean (SD) | 8.1 (5.0) | 6.6 (4.7) | 7.4 (4.9) |
| Median (Q1, Q3) | 8.0 (4.5, 11.0) | 5.0 (3.0, 9.5) | 7.0 (4.0, 11.0) |
| Minimum, maximum | 0, 20 | 0, 18 | 0, 20 |
| Categorised, n (%) | |||
| No anxiety (0–5) | 6 (25.0) | 8 (40.0) | 14 (31.8) |
| Mild anxiety (5–10) | 8 (33.3) | 7 (35.0) | 15 (34.1) |
| Moderate anxiety (10–15) | 8 (33.3) | 3 (15.0) | 11 (25.0) |
| Severe anxiety (15–21) | 2 (8.3) | 2 (10.0) | 4 (9.1) |
| 4-month follow-up | |||
| Raw score | N = 21 | N = 20 | N = 41 |
| Mean (SD) | 6.0 (5.1) | 5.2 (4.8) | 5.6 (4.9) |
| Median (Q1, Q3) | 5.0 (3.0, 7.0) | 3.0 (2.0, 7.5) | 5.0 (2.0, 7.0) |
| Minimum, Maximum | 0, 18 | 0, 18 | 0, 18 |
| Categorised, n (%) | |||
| No anxiety (0–5) | 9 (42.9) | 11 (55.0) | 20 (48.8) |
| Mild anxiety (5–10) | 8 (38.1) | 6 (50.0) | 14 (34.2) |
| Moderate anxiety (10–15) | 2 (9.5) | 2 (10.0) | 4 (9.8) |
| Severe anxiety (15–21) | 2 (9.2) | 1 (10.0) | 3 (7.3) |
Competency and intervention fidelity
Of the 18 ESI facilitators who completed the ESI training as part of the pilot RCT, 10 passed the competency assessment on the first attempt and seven passed on the second attempt following additional telephone-based support. One ESI facilitator did not undertake the competency assessment for personal reasons and did not go on to deliver the ESI. All ESI facilitators who went on to support participants through the ESI received session-by-session clinical supervision.
Enhanced support intervention facilitators were advised during the ESI training workshop (or via the clinical supervisor for those ESI facilitators who completed their ESI training during the feasibility study) about the need to audio-record some of their ESI sessions to undertake the ESI fidelity assessment. ESI facilitators were approached to record ESI sessions once they had delivered the ESI to a minimum of one participant to enable them to gain experience and confidence in delivery of the ESI. A total of four out of a possible six ESI facilitators (the figure of six does not include one ESI facilitator who had previously refused to audio-record ESI sessions during the feasibility study, a decision supported by their manager) were approached to audio-record ESI sessions. Two ESI facilitators were not asked to record sessions as it was deemed not to be appropriate by the clinical supervisor (in one case owing to concerns raised by the ESI facilitator and the complexity of the presenting participant, and in the other case owing to concerns expressed by the ESI facilitator). Of the four ESI facilitators who were approached, two agreed to record their ESI sessions and were supplied with an encrypted digital recorder (the study team had access to only two digital recorders at any one time because of the significant increase in the cost of these). One of these ESI facilitators successfully recorded one ESI session (ESI session number 2); reasons for not recording additional sessions included participant cancellation of planned session and anxiety about recording sessions on the part of the ESI facilitator. The second ESI facilitator advised that they attempted to record ESI sessions on several occasions but reported technical problems with the digital recorder (e.g. the digital recorder had no charge, or despite putting the recorder on charge the recorder still did not work) that prevented them from recording any ESI sessions.
In total, one ESI session was recorded by one ESI facilitator during the course of the pilot RCT.
Serious adverse events
There was one SAE reported in the pilot RCT. A participant in the intervention arm experienced a fall and was subsequently hospitalised. This event was reviewed by an independent clinician (as per study protocol) and was judged to be unrelated to the study. This participant later withdrew fully from the study.
Chapter summary
The pilot RCT demonstrated that recruitment remained a significant barrier to the delivery of the study, with the average recruitment being 3.1 participants per month. The target sample size of 100 participants was not achieved, which limits the feasibility of progressing to a main RCT. However, the intended future primary outcome (PHQ-9) was scoreable for all returned follow-up questionnaires, as was the GAD-7. This indicates a high level of data quality and completeness. Similar to the feasibility study, the ESI was well attended, with 75% of participants allocated to the intervention taking up the ESI and 89% completing at least two sessions. This suggests that participants are willing to undertake the ESI. Although the primary analysis was not powered to detect any effect of the ESI, this does suggest that there may be a detrimental effect from the ESI (0.77, 95% CI –2.05 to 0.97). Assessment of fidelity to the ESI was difficult to conduct via audio-recordings of ESI sessions. Completion rates for the BADS was acceptable, although it is noted that this was collected at only one time point. The pilot RCT process evaluation will further explore some of these issues (see Chapter 9).
The pilot RCT findings informed the predetermined progression criteria that would assess the feasibility of conducting a definitive RCT as follows:
-
Recruit and randomise 100 participants across five or six CPs by month 20 – a total of 44 participants were recruited and randomised across 13 out of 15 CPs over a 14-month recruitment period (last participant recruited in month 23).
-
For 80% of participants randomised to the ESI to receive two or more ESI sessions within the 4-month post-randomisation period – 16 out of the 24 participants randomised to the ESI (66.7%) received two or more ESI sessions (88.9% of those who commenced the ESI received two or more ESI sessions) within the 4-month post-randomisation period. The remaining six participants did not commence the ESI.
-
Collect valid (intended) primary outcome measure on 80% of participants at the 4-month post-randomisation follow-up – the retention rate was 93.2% at the 4-month post-randomisation follow-up, with 100% data completeness for the PHQ-9.
-
A fidelity score of ‘acceptable’ (3) or above to be achieved in at least 90% of assessed audio-recorded ESI session (criterion rolled over from feasibility study) – one ESI session was recorded.
Chapter 9 Pilot randomised controlled trial: qualitative findings
The procedures that were used to recruit participants to the pilot RCT qualitative study are described in Chapter 6.
Study participants
Given that recruitment to the pilot RCT was slower than expected, all those participants randomised to the ESI arm who provided their consent to participate in the qualitative study (n = 18) were approached to take part in a semistructured interview.
Thirteen of the participants who had completed the ESI (termed ESI completers), and who were recruited from nine participating CPs, took part in a semistructured interview; two ESI completers declined to be interviewed. The age of the interviewed ESI completers was between 48 and 87 years (mean 71.7 years). Five participants were female, and the number of ESI sessions completed by those interviewed ranged from two to six (mean 5.4 sessions). The LTCs reported for this treatment arm included heart problems, diabetes mellitus, arthritis, chronic obstructive pulmonary disease (COPD), emphysema, psoriasis, coeliac disease, cancer, hypertension and lung disease.
In addition, a total of three study participants from three participating CPs who either decided not to start the ESI sessions or started the ESI sessions but did not complete these consented and took part in a semistructured interview (these participants will be referred to as ESI non-completers). ESI non-completers were aged between 65 and 67 years (mean 66.3 years) and two ESI non-completers were female. One ESI non-completer completed two ESI sessions but then decided not to continue, reporting no benefit from the sessions. The other two ESI non-completers who were interviewed reported an operative procedure (in one case) and a miscommunication in the pharmacy (in the other case) as reasoning for not starting the ESI sessions. LTCs reported by the ESI non-completers were COPD, arthritis, heart problems, joint problems and asthma.
All interviewed study participants described themselves as being of white ethnicity.
General pharmacy staff, enhanced support intervention facilitators and general practitioner participants
A total of 20 pharmacy staff from nine recruiting CPs participated in a semistructured interview. Nine of these participants were ESI facilitators, of whom five had experience of delivering the ESI. The remaining four ESIs received training in the ESI but did not deliver the ESI. All ESI facilitators were female and described their role in the pharmacy as dispensing; however, several ESI facilitators also mentioned additional pharmacy roles, such as delivering smoking cessation services, serving customers and labelling prescriptions. ESI facilitators’ years of pharmacy experience ranged from 5 to 24 (mean 12.3) years. Of the 11 general pharmacy staff (non-ESI facilitators) who were interviewed, nine were female, and their roles included counter staff, dispensers, pharmacists, senior pharmacy management roles and academic pharmacists. Years of experience for general pharmacy staff interviewed ranged from 3 to 22 (mean 12.22) years. In addition, three GPs, linked with two participating CPs (two females and one male), consented and participated in a semistructured interview.
Data analysis
As described in Chapter 6, data analysis focused on a theory-driven approach that was guided by the four main constructs of NPT:81 coherence, cognitive participation, collective action and reflexive monitoring (Table 23 shows a description of the NPT domains). Although the initial intention was to carry out both a thematic analysis and a NPT analysis of interview data, coding researchers discussed that the NPT framework appeared to capture most phenomena observed in the interview transcripts. In addition, the process of constant comparison69 in the framework domains allowed for the production of inductively derived categories. The process of data exploration appeared to negate the need to carry out an additional thematic analysis, and it was decided that data analysis would take the form of a primary NPT analysis. 81
| NPT domain | Example |
|---|---|
| Coherence | This is the sense-making work that people do individually and collectively when they are faced with the problem of operationalising some set of practices |
| Cognitive participation | This is the relational work that people do to build and sustain a community of practice around a new technology or complex intervention |
| Collective action | This is the operational work that people do to enact a set of practices, whether these represent a new technology or complex health-care intervention |
| Reflexive monitoring | This is the appraisal work that people do to assess and understand the ways that a new set of practices affect them and others around them |
Taking this rigorous approach, it was not thought that important data were overlooked, and the NPT framework was applied as a sensitising theory for data analysis. 82 In addition, as discussed previously, given that initial coding was carried out independently and researchers met regularly to discuss emerging themes, the possibility of overlooking any key themes not captured by the NPT-sensitised analysis was reduced. Disconfirmatory evidence was sought in the data throughout the analysis.
The qualitative analysis of interview data by the four NPT domains are presented in the following sections. Illustrative data are provided to support analysis and are labelled by identifier and number (e.g. P = ESI completer, PNC = ESI non-completer, ESI = ESI facilitator, ESI NDI = ESI facilitator who did not deliver the ESI and PS = pharmacy staff).
Coherence
CHEMIST involved a novel intervention that was predicated on the relationship between physical and mental health. Several pharmacy staff described not considering these links prior to their involvement in the study:
I didn’t really like link them [physical and mental health] together, does that make sense?
ESI3
To be honest I wasn’t really aware of it until I went to the CHEMIST study training, until we went through it, but I can see why now.
PS10
For those pharmacy staff who undertook the ESI training, it appeared that the coherence of the relationship between physical and mental health became clearer following training:
I hadn’t really actually associated it with it, so it was quite, you know, it made us . . . it made us stop and think about it and think about the effects that long-term health conditions do have on people, you know. But I hadn’t . . . I hadn’t thought about it previously.
ESI3
For ESI facilitators, the experience of delivering the ESI seemed to consolidate their understanding of the links between physical and mental health:
I understand it more than I thought, you know, obviously the more you speak to people who do have long-term health problems more things come up like, you know, not being able to get out to see family, not being able to do something simple like going to do some gardening. So I understand, you know, the things that we maybe take for granted, you know, if that was taken away from us how that would affect, massively how would say not being able to even go to the shop, you know, simple things in life aren’t always so simple are they?
ESI2
The three GPs who were interviewed described CHEMIST clearly, indicating that the premise of the ESI made sense:
. . . so my understanding is that, um, that pharmacists, er, will be able to provide low-level interventions, er, to people, er, who were screened as having, um, symptoms of, er, lower-level anxiety and depression . . . um, which would hopefully have a positive impact on their consultation rate, and their psychological well-being, um, er, to, to, to really ultimately see whether there may be scope for preventing people, um, with more significant depressive symptoms hitting the GP longer term.
GP3
The ESI was generally seen by pharmacy staff as clearly distinct from other interventions that they had received training on or that had been carried out previously in pharmacies. Some pharmacy staff cited the process of keeping in contact with study participants as part of its distinction:
We’ve never really had to like make appointments and things and see people like that. It’s normally on just a walk-in basis that we generally see people and then they just go. It’s more like if we were going to test their blood pressure or whatever it would be something we would offer when they came into the pharmacy and then if they chose not to, then that’s up to them. Obviously with the CHEMIST study when you have got a participant, it’s obviously keeping in contact with them which is something that we would do in the pharmacy.
ESI7 NDI
The distinction between the CHEMIST ESI and current practice at the pharmacy was also cited by some pharmacy staff as making CHEMIST more challenging to engage with than previous training undertaken within the pharmacy:
It is quite a lot different. I mean everything we do in the pharmacy we need training for, like we needed in the study. It was just . . . I don’t know how to word it, it was a different type of . . . I think it was a lot harder to get to grips with than other training that we’ve had for the pharmacy. I don’t know whether it’s just because everything you do in the pharmacy is just part of your role whereas this CHEMIST study was something new altogether.
ESI8 NDI
Some pharmacy staff, however, drew similarities between their current operational roles and the CHEMIST ESI. For example, one pharmacy staff member discussed that trust is established between pharmacy staff and customers during routine interactions in pharmacies, such as the medication review. Thus, it was thought that trusting the pharmacy staff might facilitate disclosure of how pharmacy customers might be feeling, and this made for coherent links with the CHEMIST ESI in relation to delivery and engagement:
. . . and in the pharmacies in the past, I used to find that people coming to the pharmacy and that trust is there automatically and people came to disclose a lot of their feelings to the pharmacist and when we started doing Medicines Use Review, which I know this study was sort of based around, I encountered a lot of people talking to me about how they feel rather than their long-term condition and that’s where I think the link between the two is very evident within pharmacy as well.
PS03
The perspective that pharmacists were well placed to pick up on patients’ health concerns was shared by GPs:
So, they certainly have a, a very valuable role in, in identifying patients, um, at an early stage . . . and their knowledge of a patient and their circumstances and their social circumstances is often as good as ours really.
GP3
Enhanced support intervention completers agreed with the view that the pharmacy was a potentially suitable place for a wider role in health-care delivery:
I’m interested in anything like that. I can see that there is potentially more of a role for pharmacies particularly as doctors get so busy. I always use the pharmacist if I’ve got any questions about medications rather than the doctors at least for first call because they do know their stuff, you know. They know what goes with what. If you’ve got a problem with a reaction or anything like that, they tend to be the ones that will know.
P03
However, some study participants suggested that they might prefer more traditional routes to help-seeking for mental health problems. One ESI non-completer said that they were not expecting the pharmacy to be interested in mood and that if they felt ‘really low’ they would prefer to consult their GP:
. . . mm I don’t know, I haven’t thought about that. I mean I don’t think I’ll go and say to [pharmacy staff member]. ‘Well, I’m feeling really low’, because if I felt really low I think I would go and see the doctor. But I do ask [pharmacy staff member] things when I go in about various things and say ‘do you think I should see the doctor about this?’ But it’s usually a physical thing that I’m asking about.
PNC01
Varying levels of sense-making work were required to integrate the ESI into a coherent activity for the pharmacy. It was recognised that mental health is increasing in profile and it seemed to make sense that this could become part of the remit of the pharmacy:
I think it’s becoming, I wouldn’t say more common knowledge now, but I think because mental health is rising the profile continuously, I think it will become more accepted to be done in the pharmacy as well.
PS03
However, for some pharmacy staff who did not undertake the ESI training, the rationale for the ESI seemed to have less coherence:
. . . well it might be a bit excessive but regards to the role that we played as recruiters, even if it was half a day’s training where we came with the intervention, the people doing the intervention training, just for the introduction and to listen to the slides being spoken about the rationale of why it’s being done so that everyone had a clear understanding of why we were doing it, how we were doing it and then we could leave.
PS01
One pharmacy staff participant discussed how they encountered initial resistance to implementing the ESI in the pharmacy, with staff raising questions about the rationale for the pharmacy being involved in and carrying out the intervention. This resistance was countered by other pharmacy staff who felt that the study offered an extension to the pharmacy role, which was positive for the pharmacy profession:
. . . think if I’m being a little bit negative there was a little bit of discussion around why are we doing this and obviously my argument around that it’s good, it’s good for the profession, we’re extending the role but there was a little bit of resistance to change.
PS09
Other pharmacy staff also drew on this narrative of CHEMIST being an extension of their current skill set. This rationale seemed to be, in part, how pharmacy staff made sense of their pharmacy hosting the CHEMIST ESI:
. . . again, it’s just showing that we can be involved in more than just the basic day-to-day dispensing. We’re there for all health-care needs not just the obvious ones.
PS11
Cognitive participation
Cognitive participation was required to assist both pharmacy staff and study participants to engage with the ESI. 45
Cognitive participation for pharmacy staff
Pharmacies came to participate in CHEMIST via a number of routes. In some cases, senior pharmacy staff volunteered their pharmacy to participate in CHEMIST by responding to an ‘expression of interest’ e-mail that was sent to pharmacies:
It was me colleague that signed the branch up for it but I think there was an e-mail that was put round and we just signed up that way because we’re keen to involve, we do lots of services, and we’re keen to involve other new services at this branch.
PS10
Other pharmacy staff were approached directly by their manager and asked to deliver the ESI:
Yeah it was just my manager put me forward for it. I was never really got given any information, it was just, ah this is what you’re doing and I read into it and I thought actually it might not be too bad.
ESI8 NDI
My boss just approached me but I think [they] knew I was a bit of a mental health geek and it would be something I would enjoy; I think more than anything and something that I would probably put 100% to because it’s of interest to me.
ESI2
Other pharmacy staff reported that they had volunteered for the role of ESI facilitator:
The pharmacist like spoke about it to all of us and asked if anybody would like to do it, so me and one of the other girls said that we would.
ESI3
More senior pharmacy staff often suggested that a reason for participating in CHEMIST was wanting to be (more) research active:
. . . it was in regards to being research ready, I had never taken part in research as a [job role] or let alone be a [role in the study]. So from a continuing professional development point of view that was very useful for me.
PS03
In addition, some pharmacy staff and ESI facilitators described already having an interest in mental health, and others described altruistic motives that the intervention might help others:
I just think it’s interesting, mental health and I just wanted to know more and see if I could help people. I was interested to see if, when they were saying that something that pharmacy, community pharmacy could help with, I was interested to see how or why.
ESI9
Other respondents suggested that CHEMIST might be something that the pharmacy customers were interested in:
Yeah it was a bit strange at first but then I thought, no, you know, something that some people might be interested, it might do them good to talk and things like that, so you know, that didn’t bother me in the end because like you say I know my customers well enough to, you know, speak to them more freely.
ESI5 NDI
Expanding the role of the pharmacy to deliver a talking therapy was also something that was highlighted by GPs as a rationale for their participation in CHEMIST:
We’ve got a pharmacy right next to us, so we said that it would actually be quite a good thing for us to do, but also we realised that we can’t deliver all of this mental health support and things. The resources aren’t there in the NHS and we need to think about alternative ways of dealing with things.
GP1
However, for some pharmacy staff, the process of recruiting pharmacy customers to CHEMIST face to face seemed initially difficult to operationalise. One pharmacy staff member reported that pharmacy staff were ‘scared of what they were going to uncover’ (PS07) in the process of initiating discussions about a mental health intervention with their customers during the recruitment process. Conversely, discussions around physical health were thought of as ‘easier’ because they were seen as within the usual role of pharmacy staff:
I think it’s slightly easier to talk to someone about long-term conditions if it’s not necessarily associated with mental health, plus some of the feedback initially I got from the team members involved is that they were scared of what they were going to uncover.
PS07
Part of this apprehension was concern about managing risk, including suicide risk, with study participants:
I think initially they were a little bit unsure of themselves. I think mainly because they heard back from the intervention facilitators that the type of consultations that we were having would happen during the intervention and the fact that they had to talk a little bit more about mental health and about whether the patient had any suicidal thoughts and I think in the beginning they thought, am I going to have to have these kind of conversations at the point of recruitment? But then once they realised that that wasn’t the case, then they were fine after that.
PS03
The concept of risk management, which was covered in ESI facilitator training and an integral part of the ESI, appeared a difficult practice to embed, as the ESI facilitators saw this as far removed from their usual work; moving beyond these fears required reassurance and support from others, including the ESI clinical supervisors:
Well I got in contact with [clinical supervisor] and we spoke about it. Like that was fine. It’s obviously it’s just I felt out of me depth. Like I don’t think I could have helped kind of thing because I don’t have obviously no experience in anything like mental health or anything, so that was like the issue really. I didn’t think I could help them.
ESI3
One pharmacy staff member thought that some of the initial anxiety about CHEMIST was in part because of some pharmacy staff not knowing what the ESI involved before they were put forward to deliver it:
I think the best way to manage it is to let them know exactly what it was before they were put forward.
PS11
In addition, some pharmacy staff raised questions about the compatibility of the CHEMIST ESI with their role:
. . . think amongst certain staff in the sense that, you know, the role has massively changed in recent years and, you know, we’ve got to do more and more things and why do I now need to do this?
PS09
However, those ESI facilitators who went on to deliver the ESI described how the process of delivering it allowed them to work through any initial discomfort and ultimately feel pride in delivering the ESI:
What I got out of it? Knowing that I’d helped these two people who, well one who I knew anyway but the [person] I didn’t know. How can I explain? As I say, I didn’t want to do it, I’ll be honest. I just did not want to do it in the beginning. I was sort of like, no this is what you’re doing, you’re doing it and I wasn’t happy. But now that I’ve done it, I’m so glad I did it because ‘em for what I’ve done for these two customers. Do you know I just find I’m quite proud of myself!
ESI1
This was something that was also recognised by the general pharmacy staff who were not trained to deliver the ESI, but observed their colleagues doing so:
Yeah they all felt incredibly uncomfortable doing the intervention, but they were proud of it, you know, they were really proud of how much the patients had benefited.
PS11
Cognitive participation for study participants
Perhaps because of the relative novelty of the CHEMIST intervention in the pharmacy setting, study participants did not seem to have a strong sense of expectation on what taking part could mean:
I was very apprehensive and thought how daft is all this, but never mind we’ll go on.
P06
Yeah just I thought maybe something could maybe help us, I don’t know.
P03
One study participant described how they thought the CHEMIST intervention might benefit them by providing a ‘sympathetic ear’ and how it might ‘cheer them up’:
Yeah I thought it might cheer me up a little bit. Just have a break and have a talk to them. I’ve done some counselling myself over the years. I’m reasonably well qualified, so I wasn’t expecting an awful lot because as we went through it I’m doing most of the things that I can, so there wasn’t an awful lot that they can add really other than just a sympathetic ear which actually was quite helpful in itself.
P05
Other study participants described feelings of altruism and reported wanting to help the health service:
My thoughts were I’ve received such a lot from the NHS, I was happy to do anything which might help.
P01
Flexibility of the ways in which the ESI could be delivered was also discussed as a factor for continuing to participate in CHEMIST:
Well at the beginning I thought I don’t know if it would do us any good because I have a job getting out and things but [they] said [they] would speak to us on the phone if I couldn’t manage to get down. So yeah I was quite happy to do it because I did know I had odd days where I felt a bit depressed.
P12
The degree to which confidentiality could be ensured in the pharmacy also appeared to influence how study participants felt about participation in the study:
It’s a slightly odd fit [pharmacy] but I think one of the things that I wondered about, they say you sort of get a lot of safe-guarding stuff within a [place] and you do wonder slightly when there are a lot of staff that are local whether you want to be talking an awful lot. It would be depend who the staff were I think and where you are. I’m not sure it would translate to every pharmacy.
P05
For example, one study participant expressed how they were pleased that their ESI facilitator did not live locally, as they did not want to talk to local pharmacy staff who would then have information about them:
. . . but it’s a local pharmacy with . . . manned by local people serving local people . . . However, that’s the plus, the minus there’d be some people who think I don’t want to talk to somebody here because they’ll know my business . . . [Name] was a great choice because [they are] not local [they] . . . [they] doesn’t live in the [area].
P08
General practitioner interviewees also raised the issue that the pharmacy may not be perceived by patients as operating the same rules of confidentiality as a general practice:
. . . suppose really there is an element of trust there and it can be something quite tricky. I mean at least the patient’s know that when they come into the GP surgery whatever you say within the four walls is confidential and that side of things I don’t know whether or not they class the pharmacy staff as holding that same situation, if you see what I mean, and I suppose really as well the concern if they said no or if they didn’t think it was successful or helpful then would they feel comfortable about going back to the pharmacy again in the future.
GP1
However, most study participants appeared reassured that the work with the ESI facilitator was confidential:
It’s personal. You can say things that you want to say, nobody else knows about it, just [name]. [They] writes things down, so to me that is good. It’s a good thing and you’ve got that bit of extra time.
P04
In addition, one GP thought that familiarity with pharmacy staff might potentially facilitate participation in the study:
I think that for some patients there’s a pre-existing relationship with their pharmacist, um, and they, and they, they view that positively.
GP2
Furthermore, some study participants noted that it was the possibility of developing positive relationships with pharmacy staff that facilitated engagement with CHEMIST:
Well I thought it was good for anybody else that was, you know, really because I thought like if I had anything else to talk to him about I could obviously talk and get to know me pharmacist which it has done, you know, talking about them and I was really pleased that I did it.
P12
Collective action
The operational work required for the study fell into two areas: recruitment to the study and delivering the ESI.
This first section describes the collective action involved in the recruitment of study participants (i.e. the identification of pharmacy customers/patients who were eligible to be approached about the study), as detailed in Chapters 2, 5 and 6.
Collective action of recruitment to the pilot randomised controlled trial
Pharmacy staff at participating CPs received training via the study team on recruitment methods. This training appeared to be received positively:
Yeah the two [people] that came out, obviously some of it was on screen on the laptop. Obviously, we talked through everything on the screen as well and [they] went through everything, so it was really clear. Very clear, you know, dead easy to understand. Asked us if we had any questions. We actually discussed certain scenarios in certain circumstances where we’d come across things, you know, and they agreed with things we’d said and done and obviously interacted with each other. It was quite good actually, so yeah it was fine, it was great.
PS02
In addition, it appeared that following recruitment, training pharmacy staff had a clear understanding of the study recruitment criteria:
The recruitment part was quite . . . quite interesting because you had the criteria that had to be met, so, therefore, you knew what patients you were looking at so we could actually, actually implement our PMR [patient medication records] records . . .
PS04
Given the apparent simplicity of identifying potential participants through patient medication records (PMRs), it was initially anticipated by some pharmacy staff that the collective action required to recruit to the study would complement existing working practices:
. . . it goes back to kind of engaging the staff in the recruitment phase. I explained to them that it wasn’t anything that they weren’t already used to doing and what we were looking at was if you think about anyone that’s on long-term medication, they’re eligible for an MURs and we’d already identified patients MURs, so I said it’s only an extra identification that if you stick an MUR sticker on the bag then this person is eligible to be recruited for the CHEMIST study. So that engaged the staff straightaway because I wasn’t giving them any extra to do on top of their normal daily job.
PS03
Medicines use reviews were discussed as a potential opportunity for study recruitment:
. . . now those people are normally associated or the medicine is normally associated with the long-term condition that again fits in the criteria within the CHEMIST study and there are lots of opportunities of that, I can’t remember the numbers now but with new medicine service every time someone comes in with that it potentially opens a door to have a discussion with CHEMIST.
PS07
Other opportunities for recruitment included when customers attended for flu vaccinations:
. . . we linked it to the flu vaccination campaign and so when they were coming in they were sitting one to one with the pharmacist anyway, so we were able to . . . a lot of the people who were having the flu vaccinations were on the whole long-term medication as well, so it was a good way to link in with that as well.
PS05
However, for other CPs, having to consider CHEMIST was perceived to impede the work of recruiting to the flu vaccination programme. Thus, CHEMIST recruitment was not prioritised at the time of the flu clinic:
You can’t recruit for two services at once, so when we had to recruit for the flu vaccine unfortunately the CHEMIST study we weren’t able to recruit as what we had been because we had to concentrate . . . I find the staff can only concentrate on recruiting well for one big service at a time. So, if you’re having a massive push for flu for which we do in September then you can’t also be having a massive push for the CHEMIST study. It’s too much to do at the counter in a very limited time, if that makes sense.
PS10
Most participating CPs adopted the work of identifying potential pharmacy customers face to face as they collected prescriptions from the pharmacy:
We highlighted through the medication records who was suitable and then we would highlight it on the bag to have a word with them and explain that we were running a study, I can’t remember the exact saying we had but we had a little speech to say, you’ve been selected, would you like us to put some information in the bag? If you’ve got any more queries you can ask us; that kind of thing.
PS11
Some pharmacy staff described the process of approaching customers as compatible with their current practices:
No negative impact. It [recruitment] didn’t interfere with our day to day at all. We just merged it in with what we were doing normally every day anyway, so there was nothing . . . I didn’t have any feedback from anyone to say that it was anything extra on top of what they were already doing.
PS03
Others discussed how some pharmacy customers were dismissive of the study when approached face to face. This may have limited the success of face-to-face recruitment:
. . . it sounds like you’re saying if you approach people face to face then it seemed like they would prefer not to?
Yeah they were sort of quick to dismiss it face to face.
ESI9
In addition, several pharmacy staff talked about knowing their customers; it appeared that some pharmacy staff preferred to approach customers whom they knew and already had a relationship with:
I think it’s just a general, er, relationship that the counter girls have with their patients, you know. I think, you know, they kind of knew the ones that were easier to approach, the ones that they wanted to give information to, who . . . who were a bit standoffish, and kind of used their own judgement of how they were, you know, giving the information packs.
ESI4
One pharmacy staff participant talked about the context of the pharmacy as a place where some customers do not anticipate spending any length of time and how this could affect conversations about the study, which could impact recruitment and engagement with the ESI:
I think you’ve got to recognise when somebody is busy and they don’t want to have that discussion in the consultation room and if they do that’s great, I’d be happy to have it. But sometimes people just want to come in, you know, they’re parked on double yellow lines or they’ve got to go and get the bus and they just want their medicine very quick.
PS09
In addition, ESI facilitators thought that, when pharmacy staff were busy with the usual work of the pharmacy, the added work of face-to-face recruitment was not prioritised and did not take place:
I don’t know to be honest because obviously we’re really busy so it was like the whole, when you were labelling them all and putting CHEMIST study on it so that we knew to ask people and it was when you were busy, like remembering to do it and remembering to when you give it out to ask people and if you were busy you didn’t. So it was like time-consuming that way.
ESI3
To ensure that customers were not approached about the study more than once, pharmacy staff were required to record the approach on the customer’s PMRs. This, again, affected the routine work of the pharmacy:
No it was just getting a system in place to flag patients’ records to say that they’ve had it before rather than keeping trying to, you know, ask the same patients every time, that we weren’t asking them twice, you know, the way we did that is we flagged it on their medication records, produced an extra label that went onto the prescription to say, you CHEMIST, so that when the lasses got to the counter they knew that they didn’t have to ask them again.
PS05
This additional work for pharmacy staff was reported as having operational difficulties, as some staff may forget to record an approach:
You see, I don’t know whether it was just the system we use or what but it was quite difficult to keep track of who we’d actually spoken to because quite a lot of people, like we wouldn’t have anything written on their records to say that we’d already spoken to them about it, but I don’t know whether that was just because someone who had spoken to them had forgotten to put it on their records; does that make sense?
ESI8 NDI
In an attempt to increase recruitment to the study, pharmacy system searches (involving pharmacy staff posting out the study information to eligible customers) were implemented as an additional recruitment method (see Chapter 2). Pharmacy staff felt that they could give time to the postal mail-out only at quiet times in the pharmacy, and this was seen as an additional task that did not complement existing working practices. In addition, one pharmacy staff participant suggested that when postal recruitment was employed, face-to-face recruitment was sidelined:
. . . so we had multiple components to that, so one of the things we did was we identified people according to their medication histories to see if they would like to become part of it and we did that really through mailing the participant information sheet and that was very . . . it was good in a sense that we could do it when we were quiet in the pharmacy but I found that once we did that we took the focus of actively approaching people.
PS09
Although initially it appeared that individual recruitment strategies could complement existing working practices, the cumulative effects of numerous recruitment strategies could impede the working practices of the pharmacy. Thus, recruitment within the pharmacy could be difficult to embed and routinise:
I think it had a bigger impact on them is what they initially thought and initially it was let’s do it through the MUR process, right let’s add [unclear 11.46] process now. Let’s do it MUR, [unclear 11.44] and flu process and by the way [name] is now going to send you a load of data from your PMR search and [name] is going to send you loads of packs; let’s label those and send them all out. So I think the combined effect soon added up to quite an additional amount of workload that wasn’t there initially.
PS07
One recruitment strategy involved general practices conducting searches and posting out the study information to eligible patients. One GP who was interviewed reported that, owing to the study eligibility criteria including factors that are not routinely coded for in consultations, the process of having to look through lists of patients to assess patient suitability for the study was a burden to the practice:
. . . um, but this one, um, it, um, there wasn’t a prebuilt search, um, and, and, er, searching for each of the individual conditions was, was really quite huge.
GP2
Recruitment through home-delivered prescriptions was an additional method implemented during the feasibility study (as described in Chapter 5). However, one pharmacy staff member discussed how they did not have the opportunity to discuss the study with potential participants during the prescription home delivery process:
We weren’t going into as much depth in our home deliveries, but face-to-face customers who we were getting we were getting to speak to them a bit more. Whereas out on deliveries it was just our delivery driver taking them out, so you couldn’t speak to them as much.
PS10
In addition, some pharmacy staff did not perceive this method of recruitment as the most efficacious because they thought that they may have had less potential participant responses from home delivery recruitment:
I think we had a couple look at that, but quite a lot in the end we just never heard anything back from them really. Some of them just thought, I don’t know. I mean because we thought we were [unclear 14.21] quite a lot of them will be willing to like do it and things but I don’t think we really got a lot of response from the delivery ones.
ESI8 NDI
Collective action of enhanced support intervention training for pharmacy staff
Given that delivery of a psychosocial intervention is fairly novel for the pharmacy setting, intervention delivery required ESI facilitators to acquire a new skill set by undertaking a 2-day ESI training workshop that was delivered by clinical members of the study team (see Chapter 2). Owing to the novelty of the ESI, it is perhaps unsurprising that this training was reported by ESI facilitators as requiring greater understanding than previous training that they had undertaken to deliver new interventions to pharmacy customers:
. . . because it was a 2-day training it was probably longer than we get for like other things in the pharmacy but it was . . . I think the training we get, like sometimes in-house training so we’ll have a course to do online, so it’s just like concise facts: this is what you do, this is what you do where. The CHEMIST was a lot more to take in, like understanding-wise.
ESI8 NDI
A central feature of training for skill development in talking therapies, and for the CHEMIST ESI training, is the use of role-play. This was something that was reported by some ESI facilitators as a process that they found challenging:
Yeah sometimes I did find it [role-play] a bit intimidating to be quite honest, to think that I’ve been doing my job for that many years and, you know, I was trying to do this role-play which was totally like, you know, I just couldn’t grasp it, no.
ESI5 NDI
Conversely, other ESI facilitators reported that role-play was helpful for skills development:
I think when we’re doing like the, er, like role-play and stuff like that, you know, that . . . that helps a lot, you know, how you would . . . and [name], like [they] role-played with us as well, and you know, made us think, and you know, gave us like words and you know, phrases to use and everything. And that . . . that . . . that helped an awful lot.
ESI4
Following the ESI training, ESI facilitators were required to complete a telephone-based competency assessment to evaluate if the training had equipped them with the skills and knowledge required to deliver the ESI. This process was reported as inducing some anxiety in the ESI facilitators and required additional work outside the training workshop:
. . . think there was impact on the staff in terms of when they were going through the training. Staff were certainly anxious around the training and the assessment, the assessment in particular. I know staff were taking their, you know, their training materials home to read through in preparation for their exam the next day . . .
PS09
Overall, it appeared that the ESI training was reported positively in providing the ESI facilitator with the skills to deliver the ESI, and some ESI facilitators reported that training helped them to communicate with pharmacy customers more generally:
No, I think it all went well in general, you know, it was, er . . . it was like, you know, easy, you know, to . . . to listen to and, you know, easily explained and everything, so you know, I think it all went well.
ESI4
However, it was noted that the ESI training took place some time before some ESI facilitators began to work with study participants:
The training was obviously the last time I had training was [month] last year and afterwards I felt quite motivated to do it but because we didn’t start for a while after and I didn’t get anybody recruited for a while after.
ESI9
The ESI training affected the usual work of the pharmacy to the extent that other pharmacy staff needed to cover the work of the ESI facilitators when they attended the ESI training, with staffing in the pharmacy requiring a resource reallocation:
Umm, no, just really, like, a time point of view when [name] was taken out of the equation obviously for sessions or retraining and that . . . that was the only thing.
PS01
In addition, ESI facilitators reported needing to plan in advance when they could deliver the ESI sessions. This was carried out to mitigate the impact of them being taken out of their usual role in the pharmacy. The most appropriate times to deliver the ESI were reported as quiet times in the pharmacy or when additional staff could be present:
I think if you’d seen our branch, we only have at any one time there’s only three of us in, so say like today, there’s me, the pharmacist and another dispenser in. If we, say if I had two or three CHEMIST [participants] in today, it might put a bit of a strain on it. But then, well actually on a [day], we normally have four people in so I tended to try and keep the appointments for a [day] where I know we’ve got more staff and it’s not going to become, ‘oh God [they] in there for ages again’, because that can happen.
ESI2
To ensure privacy the face-to-face ESI sessions required the use of the pharmacy consultation room, and some pharmacy facilities were limited to one consultation room. Thus, if ESI facilitators were using this room for CHEMIST participants, this prevented its use by other pharmacy customers who might wish to speak privately with the pharmacist, for example. Thus, delivery of the CHEMIST ESI could affect the usual work of the pharmacy:
. . . just a very minor thing, these things tend to happen in the consultation room of the pharmacy and sometimes they might have tied up the consultation room a little bit. So if somebody was having a 30-minute consultation with somebody and then somebody else wanted to come in and they wanted to talk to the pharmacist, they were kind of unable to but that was only very minor thing.
PS09
In addition, some pharmacy staff indicated that not all staff were involved in doing the work required to support CHEMIST:
When, because I think somebody came into branch and spoke to the other girls about the CHEMIST study, so maybe at that point say, you know, you can all help with the admin and giving the packs out, sending the faxes out, sort of thing. I mean I wasn’t here when that talk happened, so that could have happened.
ESI2
Collective action of the intervention for participants
This section describes the actions required by study participants to enact the CHEMIST intervention.
Study participants who received the ESI described participating through a number of routes. Many talked about being recruited to CHEMIST via a letter from their GP:
. . . but I mean yet again I’ve always been one that don’t argue against doctors unless you know what you’re talking about and if the doctor says you should do that, I do that. So there’s no argument. I wouldn’t query it. I would follow it through because we pay them to look after you and if you do exactly what you’re asked to do then you can go and knock on the door.
P06
Other study participants discussed being recruited by pharmacy staff:
I went to the chemist and I can’t think, is it [name]? I think they call the [person name] and they were asking people if they would participate in this study by the pharmacy and I can’t exactly remember who I spoke to but it could have been [name] because nobody was agreeing to do it, so I said, yeah I’ll do it. I’ll give it a go and that’s how it started.
P13
In addition, participants reported that they had read the advertising materials displayed in the pharmacy:
How did you come to be involved in the CHEMIST study at the beginning?
I saw a leaflet on the desk in the chemist.
PNC02
With regard to mode of delivery of the ESI, it was planned that initial meetings would be face to face in the pharmacy, after which time the participants would receive the ESI over the telephone (as described in Chapter 2). Although some participants received the ESI this way, others preferred to receive the entire ESI face to face, which was accommodated by ESI facilitators:
I guess it was more convenient [face-to-face delivery] and also I think perhaps she would get a better understanding of some of the things I would say because [they] could see how I looked.
P11
In addition, sensory problems sometimes prevented study participants from speaking to the ESI facilitator over the telephone:
Because if they’re on the phone I can’t hear. Left ear I’ve got a hearing aid and a clef pallet and that’s damaged the left ear altogether and right ear has gone because of using chain saws and everything from the noise.
P02
Furthermore, some study participants discussed how attending the pharmacy was a method of treatment in itself:
Getting out to the pharmacy, making yourself get ready and go out to the pharmacy and come back. I used to go to the shops and feel like a new [person] when I came out.
P07
However, one study participant who received the ESI face to face, but then needed to switch to telephone delivery, reported that as they then needed to call the ESI facilitator, they could not always reach them at the allocated time:
Face to face if I’m honest because the telephone ones, [name] the [person] who was doing the interviewing I would have to ring [them] because [they] was busy and [they] didn’t get chance to ring, so I just felt, what’s the word I’m looking for? Slightly put out, shall we say, where when I went to the pharmacy to do the face to face it was easy, [they] finished whatever [they] was doing and then we did the interview.
P11
In addition, although one study participant had arranged to receive the first ESI session in the pharmacy, they described arriving for their first session and finding that the expected meeting was not known to the general pharmacy staff:
Well I went in and nobody knew anything about it and after sort of about 10, 15 minutes they realised it was this particular [person] who wasn’t there, [they’d] either gone home sick or taken somebody sick and I sat for about 40 minutes waiting for [them] to turn up and [they] didn’t turn up. So in the end I just said, look I’m going home. I’m not really well enough to sit here waiting but nobody in the pharmacy really knew what it was about and I never saw anyone.
PNC02
This study participant later decided not to participate in the ESI sessions, citing the young age of their potential ESI facilitator as an additional reason for their decision not to participate. Subsequent discussions with the pharmacy suggest that this situation was down to communication issues within the pharmacy team.
Engagement with the ESI involved study participants speaking with the ESI facilitator up to six times and working through the ESI self-help workbook, supported by the ESI facilitator.
The self-help workbook was reported as accessible and understandable:
It was quite nice to have a booklet that sort of said, right, OK, these are strategies, try these strategies and think about, and it’s something that I’ve kept it and, you know, I think what I’ll probably do every now and again when I’m feeling really miserable, I’ll look through it and go right is there anything that I could be doing here?
P05
One study participant reported that the workbook contained ‘too much information’:
But my observation was there was too much information, do, you know, it could have been simpler to my mind.
PNC02
However, most study participants completing the ESI described the workbook as being of use and being used:
Right, well the first thing we did was to read all the literature and discuss that with this lady at the chemist, who was excellent, absolutely excellent.
P06
We kind of went through, there was a structured booklet wasn’t there and we kind of went through that and what I was doing at the moment. It does make you take a step back and think actually what am I doing? Am I doing all that I could be doing?
P05
Study participants also discussed how the interaction with the ESI facilitator involved talking and being listened to:
We just talked and she explained why I was there and I said, yes I understand that I’ve come for a talk and, you know, I opened up and told her why I’ve got this anxiety and why I don’t think it will ever go and, you know, she was very, very helpful and listened.
P07
Participants described the workbook and speaking with the ESI facilitators as helping them to make links between activity and mood, and understanding the underlying theory of BA:
I’ve got this to do, I’ve got that to do and you just do them without really thinking about what makes you feel better and when she was sitting there and sort of saying, right OK, how are you feeling this morning? How are you feeling? Why are you feeling better this morning? Why are you feeling better this evening? It’s because I walked the dog and I think that was the most useful thing that came out of it . . . My mood is better.
P05
The diary in the workbook supported study participants to monitor the links between mood and behaviour:
. . . I think it was doing the diary, it really made me realise that I wasn’t doing certain things as often as I used to and it made me more conscious of them.
P11
The diary also supported action-planning:
She had half an hour and we discussed everything over the half an hour. There was no panic, you know. But we did fulfil the half-hour chat. We didn’t just say, oh I’m alright today, no problem, I feel a lot better. We didn’t. We made it into, what’s the word I’m after? An action plan.
P06
Most study participants reported the number of ESI sessions that would be suitable:
I think we did enough with six. I think what it was, it was the same questions and I thought to me self, can’t just keep going over the same thing really [unclear 28.16] . . . if I wanted anything, I could always come in and see [them].
P02
Conversely, one study participant discussed that they would have liked more sessions to embed their learning:
I thought for long-term change, 6 weeks only just identifies sort of areas I think for longer-term change. You need longer.
P10
Reflexive monitoring
The following section considers the reflexive monitoring of the participants by participant group. Both sections are supplemented with information from across participant groups where their voice is a key part of the discussion.
Reflexive monitoring of pharmacy staff, enhanced support intervention facilitators and general practitioners
It appeared that a central way that the pharmacy staff appraised the ESI was through its observed effects on the study participants who had received it. These observed effects appeared to be collectively appraised positively in some pharmacies:
I’ve heard really good things, so the pharmacy in [place] definitely said to me that they’ve seen the patient transform. I think I was explaining to you earlier, they’ve gone from someone with no confidence whatsoever, quite low mood, and they then started doing the intervention because the patient was successful and they’ve now seen the patient, you know, a completely different person. Very confident, changed their looks as well, even appearance looked better as well, involved in the community and they couldn’t talk enough good about how they’ve seen the transformation.
PS07
As well as observing the positive effects of the study on participants, one ESI facilitator described how a participant who they had worked with over the telephone had attended the pharmacy following the conclusion of the ESI to express their thanks:
Yeah. [They] said to me, I can’t come in, [health reason] and that sort of thing, and about 2 weeks after the study had finished [they] came in to see me and I’d never met [them] the whole I’d done it and [they] said, do you know who I am? I said I do actually, and [they’d] come in. I’ve come in to thank ya. I was like wow!
ESI2
These interactions would have provided positive feedback to pharmacy staff/ESI facilitators and reinforced the NPT domains of cognitive participation and the coherence of CHEMIST. In addition, positive appraisal of the benefits of the ESI was described as validating the NPT domain of collective action required to operationalise the ESI:
. . . also we started to see on the back of the interventions that the girls did, we saw a difference in those people after they’d finished the 6 weeks, and I think if I was to talk to all the girls, I know we mentioned it briefly between ourselves around the time we were still doing the study, that we felt good about what we had done even though I think it was only two people, but we feel like we made a difference for those two people and that kind of validated what we were doing as well.
PS03
As well as the benefits observed for study participants, some pharmacy staff talked about the increased confidence that they had gained through exposure to the study, such as recognising signs of low mood in other customers and subsequent consideration of how they interacted with them:
Obviously seeing the effects of the CHEMIST study it was quite beneficial really to see how we could help people and obviously, you know, help other people as well in the experience you have and the confidence you get to speak to people and obviously helping other people over the counter to even recognise that sign and recognising when someone is a bit low and even just an odd word or an odd smile sometimes can help, so it was quite good.
PS02
Furthermore, some pharmacy staff and ESI facilitators described how a benefit of their involvement in CHEMIST was that they had gained skills in mental health. Some staff spoke about how perceiving themselves as helping others offered personal reward:
The chance to help someone for me. Just to help somebody feel better. It was just a fab opportunity to be able to get some knowledge around this subject and just like, as I say, put things into place to help people because to me if I’ve made someone happy I’m the happiest person going [laugh].
ESI2
Another pharmacy staff member discussed how the CHEMIST intervention had added value to their practice by allowing them to view their customers more holistically:
So I felt that it added a lot of value to my practice and it helped me kind of remember that these people that we see day in days out, that they have a lot of other things going on in their lives aside from their prescription, which is mainly the reason that they would come to see us, and it kind of reminds you that it’s a person you’re dealing with and not necessarily a patient.
PS03
However, some ESI facilitators who had not delivered the intervention appeared to attach less value to the study:
I don’t think there is any, because I mean the value for me would have been better if I had seen somebody but because I didn’t get anybody, I feel like nothing at the end of it type of thing, if that makes sense. You didn’t get to put it into practice.
And what about the pharmacy. Do you think there was a value for the pharmacy?
I don’t think so, no.
ESI5 NDI
The emotional burden of delivering a mental health intervention was acknowledged by one ESI facilitator. However, they appraised their overall experience of delivering the ESI as positive:
Yeah I mean there were a couple of times after they’d gone, after they went, I came down in the [place] and I had a few tears because I was like, oh my God these people. But, no, I’m quite proud of myself. I’m so glad I did it. So glad I did it.
ESI01
Several pharmacy staff reflected that delivering a mental health intervention from the pharmacy was a novel enterprise and that this required a change in perception about the role of the pharmacy, for both pharmacy staff and customers. Customers who participated in the ESI generally appeared to be accepting of receiving a talking therapy via the pharmacy or, in some cases, over the telephone. However, it was acknowledged that those customers who did not participate in the study may have thought differently about the suitability of the pharmacy as a place to deliver a talking therapy:
I think really the main barriers would actually just be down to the customer themselves. There is still a certain type of customer that it doesn’t matter what you want to discuss with them even if it’s something that’s going to benefit them, they’re not interested and there’s always going to be that for anything that we try and do in the pharmacy.
PS03
As discussed under Cognitive participation, issues around confidentiality and customer familiarity with pharmacy staff, particularly in smaller communities, were cited as a potential barrier to participating in CHEMIST:
The other thing is that somebody coming in as the client or patient or whatever you want to call them, there . . . there has to be an element of trust and part of that element of trust is that the person sitting on the other side of the table knows what they’re doing, and in a way, it needs to be somebody who is distant from them.
P08
Concerns around customer familiarity with pharmacy staff delivering the ESI were also recognised by some pharmacy staff. A potential solution was offered that participants could choose which pharmacy they received the ESI at:
If you’re a patient that goes to the pharmacy day in and day out, it may be that you don’t want to go to the pharmacy for the intervention. You may want to go to another setting, so you may want to go to, I don’t know, another pharmacy or GP practice or no pharmacy at all.
PS07
In addition, one study participant expressed concern around maintaining privacy regarding the reason for attendance at pharmacy when receiving a mental health intervention:
I think maybe possibly privacy, but that’s something that you would work out if you were doing something regularly. You wouldn’t want to be saying to somebody, umm you can come into the room for your, I don’t know, your counselling session or whatever. You’d just have to be a bit careful how you worded things . . .
P05
Study recruitment materials were also discussed as an area for potential modification. For example, some pharmacy staff suggested that the length of these materials could be reduced:
I think because when you see the paperwork it is quite lengthy, there’s quite a few pages for the patients to read and I think when you go home, I think some people don’t bother. They don’t bother looking at it but obviously when the patients have read through and thought ah yes, and filled it in and sent it off but not everyone is going to read it because it’s quite in depth isn’t it. It’s quite a lot to look through and if patients are taking it away, obviously we don’t have time to sit in here and go through each one with the patient; that wouldn’t be feasible.
PS10
The language of the recruitment materials was also highlighted as an additional area for future consideration by one GP interviewee:
. . . and I think that, um, it’s recognised that health-care professionals often . . . even when they’re trying desperately hard not to use the jargon, use a vocabulary that is not always clear.
GP 2
Some pharmacy staff raised the problem of increased workload that was related to recruitment activity; when this work did not result in participant recruitment, this was found to be demotivating:
So if we had any further referrals through to the service, that would have been better. It’s just a bit demotivating when you’re not seeing any positive outcomes in the end apart from at the very, very final end.
PS05
Allowing pharmacists to be trained in the ESI was highlighted as a potential modification because it was felt that this would allow pharmacists to potentially better support the ESI facilitators and complement existing working structures:
If I had the opportunity to have the same training as the intervention facilitators I feel I could have supported them through what they were doing a lot more because I was reading all the information that they had been trained on and they had the verbal training as well, so I felt I would have taken in a lot more that side a lot better if I was at the training.
PS03
Finally, pharmacy staff participants discussed how the study would need to consider relevant pharmacy funding and staffing were it to be rolled out further:
I don’t know if this study was funded to an extent for the interventions or be it in a sort of roundabout way but I think that if it ever had to come back to pharmacy I think it needs to be appropriately funded and recognise the really important role that team members play in the management of the person’s holistic health, so their long-term as well as mental conditions as well.
PS07
Reflective monitoring for study participants
Study participants who had completed the ESI repeatedly offered positive appraisal. For example, positive changes in mood and behaviour were discussed:
. . . it’s funny how something you can read to make you realise, you know, you’ve got to change a thing and I have actually, I have. People have remarked I’m looking better to what I used to, so that makes you feel good as well.
P04
For many study participants, the relationship and support of the ESI facilitator was an important ingredient of the ESI’s success:
I’ve established a lovely relationship with the one who helped me, we got away from the abyss because it either sounds dramatic, I knew I was going down and down and down and I’m now going up and up. I can’t go too far, me legs won’t take it, but never mind!
P06
Yeah at the time it was, as I say, when I was relaying my mood there was encouragement from this particular [person], you know, ‘that’s good, do some more of that’, you know, that was good in regards to the whole exercise.
P09
Numerous study participants discussed that they specifically valued how the ESI had offered up a space in which they could evaluate or consider the relationship between their health condition and their mood:
Particularly with [health condition] you tend not to talk to people because it becomes boring after a while because it doesn’t change. It doesn’t get any better and other people kind of want you to get better. It’s like, oh are you feeling better today? Actually no I’m not and I won’t, you know; if anything I’m not going to get any better. So it’s getting your head around that and going, how much am I going to let it get me down?
P05
These benefits were also reported for study participants who had received previous psychological treatments:
. . . we had some very interesting conversations and [they] did say at one stage, with your experience do you find these of any use then? I said, well, yes, because what it’s doing is it’s giving me a chance to, err, to evaluate where I’ve come.
P08
Most of the changes that were reported as a result of the ESI were in mood:
Not so many physical changes I would have said but more mental changes at saying, right, actually, you know, up with this I will not put.
P05
However, one study participant reported no changes despite completing the ESI:
Do you think that being involved in the study has helped you manage any difficulties or problems that you might have been experiencing?
Err, well I haven’t really bothered so not really.
P03
One ESI non-completer who completed two ESI sessions, but subsequently decided not to continue with the ESI, described the ESI as not making a difference for them:
. . . so you didn’t continue working with the pharmacy worker, can you tell me a little bit about why that was?
Err, don’t know why really, I just didn’t think it made any difference.
PNC01
As discussed in Collective action, study participants generally appraised the self-help workbook positively. In addition, study participants discussed how the study had influenced how they would use the pharmacy in the future, suggesting that they would seek help and advice from the pharmacy, a practice that could reduce burden on primary care:
Yes because [facilitator] has opened up and said, you know, if I need to go back and help with my mood or anything, she’s more than willing to do that and I think if I started feeling down again that would be my first port of call. Now whether if [facilitator] moves on, I don’t know what the position of the other staff would be, but it would be me first port of call.
P11
Chapter summary
This qualitative evaluation has presented the perspectives of study participants, pharmacy staff, ESI facilitators and GPs, analysed through the interpretive framework of NPT. 81 The use of NPT allowed for a theory-driven evaluation of the pilot RCT study processes. For example, it appeared that reflexive monitoring of the positive effects of the ESI for pharmacy customers promoted both coherence and cognitive participation for pharmacy staff and ESI facilitators. Gaining confidence in using the skills acquired through the ESI training appeared to promote coherence and cognitive participation for ESI facilitators. Increased cognitive participation and coherence would have theoretically positively promoted the collective action of the CHEMIST intervention. 77 However, some ESI facilitators who did not deliver the ESI reported less positive reflexive monitoring, which could theoretically have reduced their coherence and cognitive participation of the ESI. Once the ESI was operational, the collective action, or work of the ESI itself, seemed to have the potential to be embedded and normalised in the pharmacy setting, provided adequate facilities were available and privacy and confidentiality concerns were addressed. However, a primary factor that appeared to inhibit the normalisation of CHEMIST appeared to be the collective action involved in recruitment and the prohibiting effect of this practice on routine pharmacy working.
Most study participants who completed the ESI reflected on subsequent positive changes in mood and behaviour that appeared important and significant to them, although fewer perceived benefits were reported by ESI non-completers. In addition, study materials were generally well received and used by those who completed the ESI. In addition, the role of the ESI facilitator was seen by some study participants as central to the process of supporting the ESI. This was a relationship that they appeared to value highly and most likely promoted coherence, cognitive participation and overall study retention. However, for some study participants, familiarity with the staff who they encounter in the pharmacy may have prohibited participation in the study, owing to concerns about the privacy and confidentiality of the pharmacy. In addition, although participants who entered the study had few expectations about the study, it is not known how coherent other pharmacy customers might find the prospect of receiving a mental health intervention in their pharmacy setting. However, given that CHEMIST was the first study to evaluate the delivery of a talking therapy in the pharmacy setting, it is perhaps to be expected that issues around coherence, related to the novelty of the study, are to be expected.
This qualitative work has benefited from a range of perspectives obtained from across participant groups; however, the views of the ESI non-completers and GPs are less well illuminated because of their smaller numbers in the interviewee sample. In addition, the application of NPT has provided an established theoretical framework in which to interpret and elucidate pilot study phenomena. However, criticisms of the use of the theoretical framework in qualitative data analysis include the possibility that alternative themes could be occluded if they are applied too rigidly. In the CHEMIST pilot data, however, the NPT framework provided a conceptual theory to sensitise the data analysis, rather than constrain the inductive development of categories during the process of constant comparison. 69 Thus, a thorough exploration of the pilot qualitative data was possible within the NPT framework.
To promote the normalisation of the ESI, future work could explore potential issues around the coherence of receiving talking therapies in the pharmacy setting for pharmacy customers. In addition, consideration of if smaller pharmacies would have the available facilities from which to deliver the ESI to larger numbers of study participants would be needed. In addition, training non-ESI-delivering pharmacists in the ESI could complement existing pharmacy working structures, promoting ESI normalisation.
Chapter 10 Pilot randomised controlled trial: health economic analysis
The aim of the economic analysis was to evaluate the level and changes in health service resource use and quality of life to understand the potential economic impact of the CHEMIST ESI in a pilot RCT. For this reason, the economic analysis compared the ESI with the usual-care arm in terms of overall response rates, item completion rates and any trends in the level and changes in health service resource use and health-related quality of life. The pilot RCT analysis was not intended to provide a definite estimate of relative cost-effectiveness, but to inform the design of a larger RCT.
Health services resource use
Data completion
At baseline and 4-month follow-up, all of the participants responded to at least one question in the resource use questionnaire (i.e. there were no participants who completely failed to answer any resource use items). Three participants in the ESI arm did not complete 4-month follow-up and, therefore, had no data available (note that this is also reflected in the overall questionnaire completion rate in the study).
However, a small number of data items were missing. Table 24 shows each resource use item with the corresponding number of missing data at each time point. The data completion rate was high in both treatment arms and at both time points: only one participant had missing data on certain items in each treatment arm. As a result, the response rate was comparable in the two treatment arms. When missing response items were compared, this indicated that the question about consultations with a GP at home and on the telephone may be more likely to have missing data (although this is observed in the case of only two participants in the study). Overall, the results indicate that participants were able to respond to the resource use items.
| Time point | ||||
|---|---|---|---|---|
| Baseline | 4-month follow-up | |||
| Intervention (n = 24) | Usual care (n = 20) | Intervention (n = 21) | Usual care (n = 20) | |
| Primary care | ||||
| GP at the clinic | 0 | 0 | 1 | 0 |
| GP at home | 0 | 1 | 1 | 0 |
| GP on the telephone | 0 | 1 | 1 | 0 |
| Nurse at the clinic | 0 | 0 | 1 | 0 |
| Nurse at home | 1 | 1 | 1 | 0 |
| Nurse on the telephone | 1 | 1 | 1 | 0 |
| NHS direct | 0 | 1 | 1 | 0 |
| NHS walk-in centre | 0 | 1 | 1 | 0 |
| Occupational health services | 0 | 1 | 1 | 0 |
| Social worker or community support worker | 0 | 1 | 1 | 0 |
| Drug and alcohol support worker | 0 | 1 | 1 | 0 |
| Pharmacy | 0 | 0 | 1 | 0 |
| Hospital-based services | ||||
| Outpatient appointments | 0 | 0 | 0 | 0 |
| Accident and emergency | 0 | 0 | 1 | 0 |
| Urgent care centre or minor injuries unit | 0 | 0 | 1 | 0 |
| Admission with overnight stay | 0 | 1 | 1 | 0 |
| Admission without overnight stay | 0 | 0 | 1 | 0 |
| Mental health services | ||||
| Psychotherapist or counsellor | 0 | 0 | 1 | 0 |
| Clinical psychologist | 0 | 0 | 1 | 0 |
| Community mental health team or community psychiatric nurse | 0 | 0 | 1 | 0 |
| Consultant psychiatrist | 0 | 0 | 1 | 0 |
Frequency of health service use
Table 25 shows the frequency of use of each type of health service in the last 4 months, measured at baseline and the 4-month follow-up. Given the small number of participants, the aim of this section was to summarise the results for each treatment arm, but not to draw statistical inference regarding differences between arms.
| Intervention, mean (range) | Usual care, mean (range) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4-month follow-up | Baseline | 4-month follow-up | |||||
| Number of appointments for any reason | Number of appointments for low mood | Number of appointments for any reason | Number of appointments for low mood | Number of appointments for any reason | Number of appointments for low mood | Number of appointments for any reason | Number of appointments for low mood | |
| GP at the clinic | 4.21 (0–15) | 0.21 (0–1) | 2.25 (0–6) | 0.55 (0–10) | 1.8 (0–6) | 0.22 (0–2) | 1.65 (0–4) | 0.37 (0–3) |
| GP at home | 0.17 (0–2) | 0 (0–0) | 0.1 (0–2) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.05 (0–1) | 0.05 (0–1) |
| GP on the telephone | 1.46 (0–6) | 0.04 (0–1) | 1.05 (0–7) | 0 (0–0) | 0.42 (0–4) | 0.06 (0–1) | 0.75 (0–6) | 0 (0–0) |
| Nurse at the clinic | 3 (0–20) | 0 (0–0) | 1.75 (0–10) | 0 (0–0) | 1.65 (0–5) | 0 (0–0) | 1.85 (0–6) | 0 (0–0) |
| Nurse at home | 0.35 (0–6) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.2 (0–4) | 0 (0–0) |
| Nurse on the telephone | 0.09 (0–1) | 0 (0–0) | 0.1 (0–2) | 0 (0–0) | 0.11 (0–1) | 0 (0–0) | 0.15 (0–2) | 0 (0–0) |
| NHS direct | 0.17 (0–3) | 0 (0–0) | 0.05 (0–1) | 0 (0–0) | 0.63 (0–12) | 0 (0–0) | 0.35 (0–4) | 0 (0–0) |
| NHS walk-in centre | 0.21 (0–2) | 0 (0–0) | 0.1 (0–1) | 0 (0–0) | 0.21 (0–2) | 0 (0–0) | 0.05 (0–1) | 0 (0–0) |
| Occupational health services | 0.04 (0–1) | 0 (0–0) | 0.05 (0–1) | 0 (0–0) | 0.11 (0–2) | 0 (0–0) | 0.3 (0–3) | 0 (0–0) |
| Social or community support worker | 0.25 (0–5) | 0.22 (0–5) | 0 (0–0) | 0 (0–0) | 0.05 (0–1) | 0 (0–0) | 0.05 (0–1) | 0 (0–0) |
| Drug and alcohol support worker | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Pharmacy | 5.5 (0–16) | 0.71 (0–6) | 5.75 (0–18) | 0.45 (0–3) | 4.75 (0–16) | 0.47 (0–3) | 5.35 (0–40) | 0.53 (0–3) |
At baseline, GP consultations and pharmacy visits were the most common categories of resource use. The mean number of GP consultations at the clinic was 4.21 and 1.65 in the intervention and usual-care arms, respectively, whereas the mean number of pharmacy visits was 5.5 and 4.75, respectively. At baseline, only a small proportion of visits in both treatment arms were because of low mood. The numerical range of the number of visits per participant was plausible for all resource use categories for both treatment arms. For almost all categories of primary/community care, the number of visits reported at baseline (except NHS direct visits) was higher in the intervention arm than in the usual-care arm. However, given the small sample size, the difference between treatment arms can be driven by a small number of observations; for instance, in the present case, the difference in GP visits was primarily driven by four participants in the intervention arm who had 10–15 consultations in the last 4 months. The observed pattern of resource use indicates that the cost-effectiveness analysis should control for any differences in baseline level of resource use.
At the 4-month follow-up, participants in the ESI arm reported a reduction in the number of all primary/community care consultations except for pharmacy and occupational therapist visits, the number of which had increased slightly. The usual-care arm showed a mixed pattern, with a slight reduction in GP consultations, NHS direct visits, and walk-in visits and a small increase in all other categories of resource use.
Table 26 shows the frequency of use of mental health and hospital-based services in both treatment arms at the two time points. The use of mental health services was very low in both treatment arms at baseline, with further reduction at the 4-month follow-up. None of the study participants had consulted a clinical psychologist or psychiatrist, which is not surprising given that the study participants were recruited into CHEMIST with subthreshold depression.
| Time point | ||||
|---|---|---|---|---|
| Baseline | 4-month follow-up | |||
| Intervention (n = 24) | Usual care (n = 20) | Intervention (n = 21) | Usual care (n = 20) | |
| Mental health services | ||||
| Psychotherapist or counsellor | 0.25 (0–6) | 0.2 (0–4) | 0 (0–0) | 0.05 (0–1) |
| Clinical psychologist | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Community mental health team or community psychiatric nurse | 0.21 (0–5) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Consultant psychiatrist | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Hospital-based services | ||||
| Outpatient appointments | 2.63 (0–11) | 1.95 (0–16) | 1.1 (0–4) | 2.1 (0–24) |
| Accident and emergency | 0.17 (0–4) | 0.24 (0–2) | 0.14 (0–3) | 0.14 (0–2) |
| Urgent care centre or minor injuries unit | 0.21 (0–3) | 0.19 (0–2) | 0.05 (0–1) | 0.1 (0–1) |
| Admission with overnight stay | 0.29 (0–4) | 0.05 (0–1) | 0.24 (0–3) | 0 (0–0) |
| Admission without overnight stay | 0.25 (0–3) | 0.67 (0–8) | 0.29 (0–3) | 0.29 (0–2) |
Table 26 also shows the frequency of use of hospital-based services in the two treatment arms at both time points. Only a small proportion of participants used hospital services. In the intervention arm, the number of outpatient appointments at baseline was 2.63 appointments over a 4-month period, which reduced to 1.1 appointments at follow-up. In the usual-care arm, the number of outpatient appointments was 1.95 appointments at baseline, which increased slightly to 2.1 appointments at the 4-month follow-up. Resource use in other categories was low in both treatment arms and time points, and the range of values were within plausible range. It should be noted that given that hospital inpatient visits (particularly those with overnight stays) are costly, a small number of outliers in this category can be highly influential in determining the difference in overall costs between the treatment arms. In the current study, one participant in the intervention arm had inpatient stays lasting 23 nights over three admissions. This was clearly an outlier in the data set and, therefore, was not included in the final cost analysis.
Unit costs of health service use
Unit costs were obtained from national databases, when available, and otherwise from other published sources. Sources and assumptions for unit costs are presented in Table 27.
| Service use | Unit cost (£) | Source |
|---|---|---|
| GP (per consultation) | 39.00 | PSSRU 2019 (p. 120, assumed to be 9.2 minutes) |
| Nurse (per consultation) | 10.50 | PSSRU 2019 (p. 118, assumed to be 15 minutes) |
| Pharmacy visit | 8.80 | NHS prescription cost |
| Pharmacy support staff consultation (per hour) | 9.88 | Scale 4, local government pay rates83 |
| NHS direct (telephone advice line) | 20.53 | Tam and O’Brien 2016 |
| NHS walk-in centre | 99.00 | Thompson et al. 2018 |
| Occupational health services | 34.00 | PSSRU 2019 (p. 113) |
| Social or community support worker (per visit) | 23.00 | McCrone et al. 2017 |
| Drug and alcohol support worker (per visit) | 57.00 | PSSRU 2019 (p. 51, assumed to be 45 minutes) |
| Psychotherapist or counsellor (per visit) | 45.00 | PSSRU 2019 (p. 113, assumed to be 60 minutes) |
| Clinical psychologist (per visit) | 54.00 | PSSRU 2019 (p. 113, assumed to be 60 minutes) |
| Community mental health team or community psychiatric nurse | 28.00 | PSSRU 2019 (p. 114, assumed to be 20 minutes) |
| Consultant psychiatrist (per visit) | 104.00 | NHS Reference Costs 2017/18 79 |
| Outpatient appointments (per visit) | 125.00 | NHS Reference Costs 2017/18 79 |
| Accident and emergency (per visit) | 160.00 | NHS Reference Costs 2017/18 79 |
| Urgent care centre or minor injuries unit (per visit) | 149.00 | NHS Reference Costs 2017/18 79 |
| Admission with overnight stay (per night): this was multiplied by the number of nights | 431.00 | PSSRU 2019 (p. 82) |
| Admission without overnight stay | 742.00 | NHS Reference Costs 2017/18 79 |
| Mental health worker (per hour) | 39.00 | PSSRU 2017 (p. 186)80 |
Intervention cost
The intervention consisted of up to six sessions that were delivered by a trained member of pharmacy support staff (ESI facilitator). Table 28 summarises the number and duration of ESI sessions as well as the cost of the ESI. The mean number of sessions was four, with most participants having at least three sessions, and the range was one to six sessions (one session, n = 2 participants; two sessions, n = 1 participant; three sessions, n = 3 participants; four sessions, n = 3 participants; six sessions, n = 9 participants). Each session lasted an average of 31 minutes, with the range being 5–90 minutes. The total session delivery time per participant (across all sessions) was 136 minutes (mean), with the range being 20–305 minutes. In addition, data were also collected on the time spent on administrative tasks related to the sessions. The average administrative time (across all sessions) was 49 minutes per participant, with the range being 5–170 minutes.
| Mean | Minimum | Maximum | ||||
|---|---|---|---|---|---|---|
| Sessions/minutes | Cost (£)a | Sessions/minutes | Cost (£) | Sessions/minutes | Cost (£) | |
| Number of ESI sessions | 4 | – | 1 | – | 6 | – |
| Total time of all ESI sessions | 136 | 66.00 | 20 | 10.00 | 305 | 147.00 |
| Total administration time | 49 | 24.00 | 5 | 2.00 | 170 | 82.00 |
| Clinical supervision time | 60 | 20.90 | 15 | 5.20 | 90 | 31.40 |
| Total time: total ESI session time plus administration time plus clinical supervision | 245 | 51.40 | 40 | 9.30 | 450 | 91.00 |
The total ESI time (i.e. session plus administration time) was 185 minutes (mean), with the range being 25–360 minutes. In addition, each session required clinical supervision, lasting an average of 15 minutes, equal to 60 minutes over four sessions per study participant. The total time of the ESI facilitator was multiplied by their hourly cost (assumed to be a member of pharmacy support staff, employed at £9.88 per hour, i.e. scale 4 for local government employees). 83 Clinical supervision was provided by a grade 6 researcher (employed at £20.90 per hour). 84 The mean total cost of the ESI was £51.40 per study participant, with the range being £9–91 per study participant.
In addition to the above, the cost of training ESI facilitators on the CHEMIST intervention was estimated using the following information: each ESI facilitator attended a 2-day training workshop conducted by a mental health researcher (academic grade 6; hourly rate of £20.90) and a nurse consultant (NHS band 8b; hourly rate of £80.30), and the average cost of venue hire, catering and travel claims per workshop was £500. The average number of ESI facilitators trained in each workshop was 11.7. Based on this information, the cost of training an ESI facilitator was £181.70. This cost is in addition to the intervention delivery cost presented in Table 26.
Total cost of health service resource use
Figure 8 presents the total health service resource use cost in the last 4 months, by treatment arm and time points. Four cost components are presented separately: primary/community care resource use cost, mental health service use, hospital-based service use and intervention cost. These are in line with the data presented above.
FIGURE 8.
Pilot RCT: health service resource use cost, by time point and treatment arm.
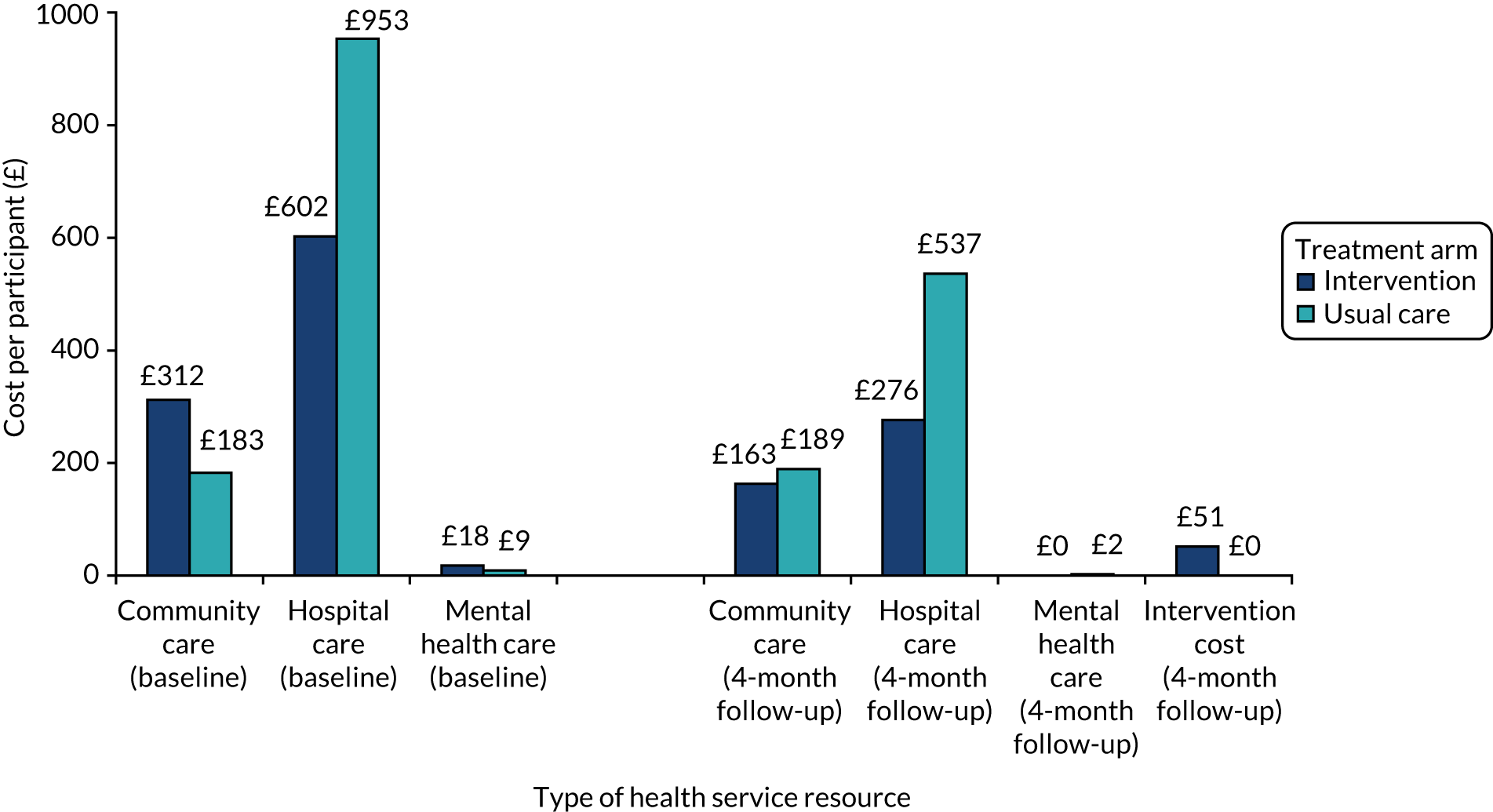
At baseline, primary/community care resource use cost was higher in the intervention arm than in the usual-care arm. This is mainly driven by the larger number of GP consultations in the intervention arm. At the 4-month follow-up, these costs reduced significantly for the intervention arm, but remained stable for the usual-care arm.
The cost of mental health services was very small at baseline and follow-up for both treatment arms. The cost of hospital care was significantly higher in the usual-care arm at baseline and remained higher at the 4-month follow-up, although the difference became smaller. These differences were primarily driven by outpatient appointments and inpatient admissions without an overnight stay.
The total cost per study participant at baseline was £1145 in the usual-care arm compared with £933 in the intervention arm (difference: £212). The total costs were lower for both treatment arms at the 4-month follow-up (i.e. £728 for the usual-care arm and £490.70 for the intervention arm, including the cost of the intervention). The difference in cost was slightly higher at the 4-month follow-up (i.e. £237.30). However, these figures should be interpreted with caution because of the small number of participants.
Health-related quality of life
Health-related quality-of-life data were collected using the EQ-5D and the SF-12v267,73 questionnaires at baseline and at the 4-month follow-up. EQ-5D data were subsequently used to derive utility values using the UK population tariff.
Data completion
Data completion was 100% for all domains of EQ-5D and SF-12v2 at baseline and the 4-month follow-up in both the intervention and the usual-care arms. This indicates that participants found it feasible to complete these questions. This finding was consistent with results of the feasibility study.
Frequency and pattern of EuroQol-5 Dimensions item responses
Figure 9 presents the distribution of completed responses for each domain or item of the EQ-5D questionnaire at baseline and the 4-month follow-up in the intervention arm. At baseline, most participants had some level of problem in at least one of the five domains; study participants reported lowest level of problems in the self-care domain (i.e. 71% participants reported no problems). For the anxiety/depression domain, 6 out of 24, 15 out of 24 and 3 out of 24 participants reported no problems, some problems and severe problems, respectively (i.e. 75% of participants reported experiencing symptoms of anxiety/depression). This is not surprising given that participants with subthreshold depression were recruited in the study. At the 4-month follow-up, there was little change in the mobility, self-care and usual activities domains, a slight increase in the number of study participants reporting severe pain/discomfort and a slight decrease in the number of participants experiencing moderate anxiety/depression. However, the numbers are too small to draw any conclusions about the trend.
FIGURE 9.
Pilot RCT: EQ-5D responses at baseline and at the 4-month follow-up for the intervention arm.
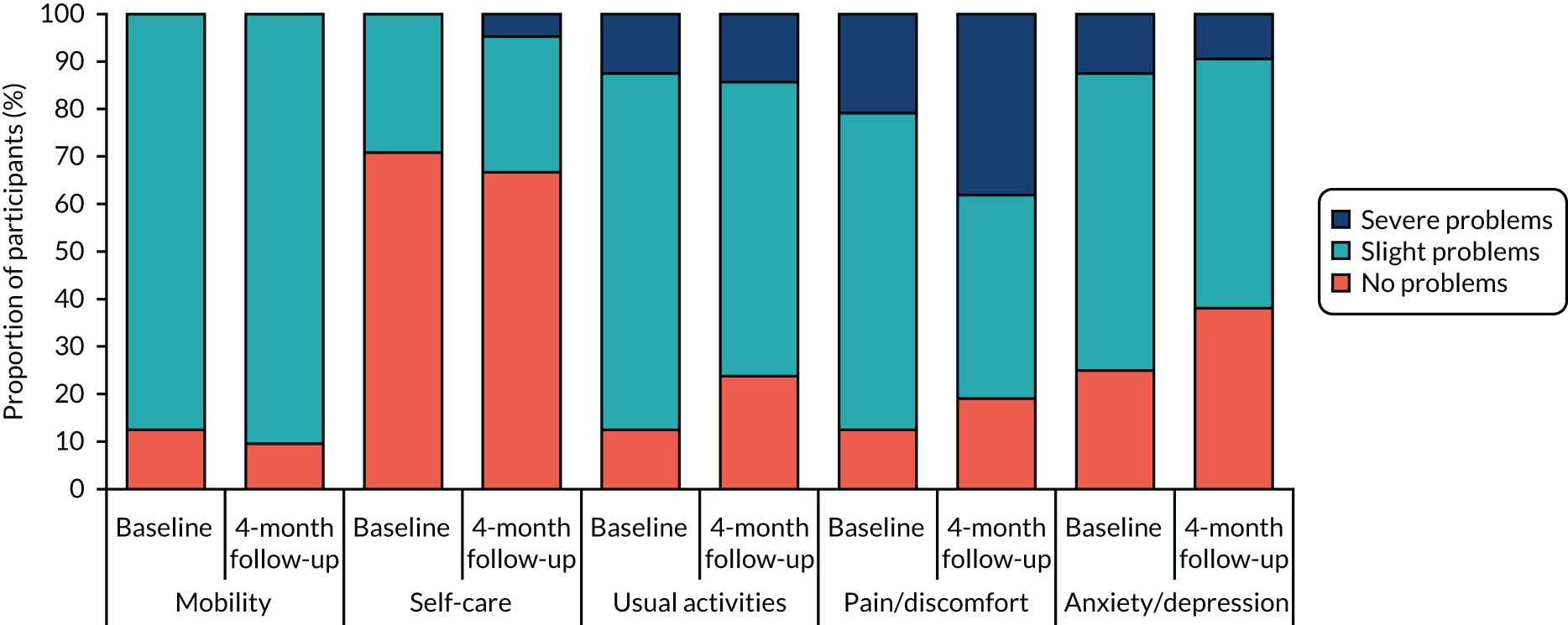
Figure 10 presents the distribution of completed responses for each domain or item of the EQ-5D questionnaire at baseline and at the 4-month follow-up in the usual-care arm. At baseline, most participants had some level of problem in at least one of the five domains; as with the intervention arm, study participants reported the lowest level of problems in the self-care domain (i.e. 80% participants reported no problems). For the anxiety/depression domain, 75% of participants reported experiencing symptoms of anxiety/depression (i.e. the same as the intervention arm at baseline). However, in general, compared with the intervention arm, fewer study participants in the usual-care arm reported problems at baseline: this was true for all EQ-5D domains. This is important to note for a full-scale RCT, which would need to take account of differences in baseline quality of life between treatment arms: adjusting for baseline utility is recommended in the literature to avoid biasing results of the cost-effectiveness analysis. 85 At the 4-month follow-up, there was little change in the mobility, self-care and usual activities domains (same as the intervention arm), a slight increase in the number of study participants reporting severe pain/discomfort and a decrease in the number of participants experiencing severe anxiety/depression. However, the numbers are too small to draw any conclusions about the trend.
FIGURE 10.
Pilot RCT: EQ-5D responses at baseline and at the 4-month follow-up for the usual-care arm.
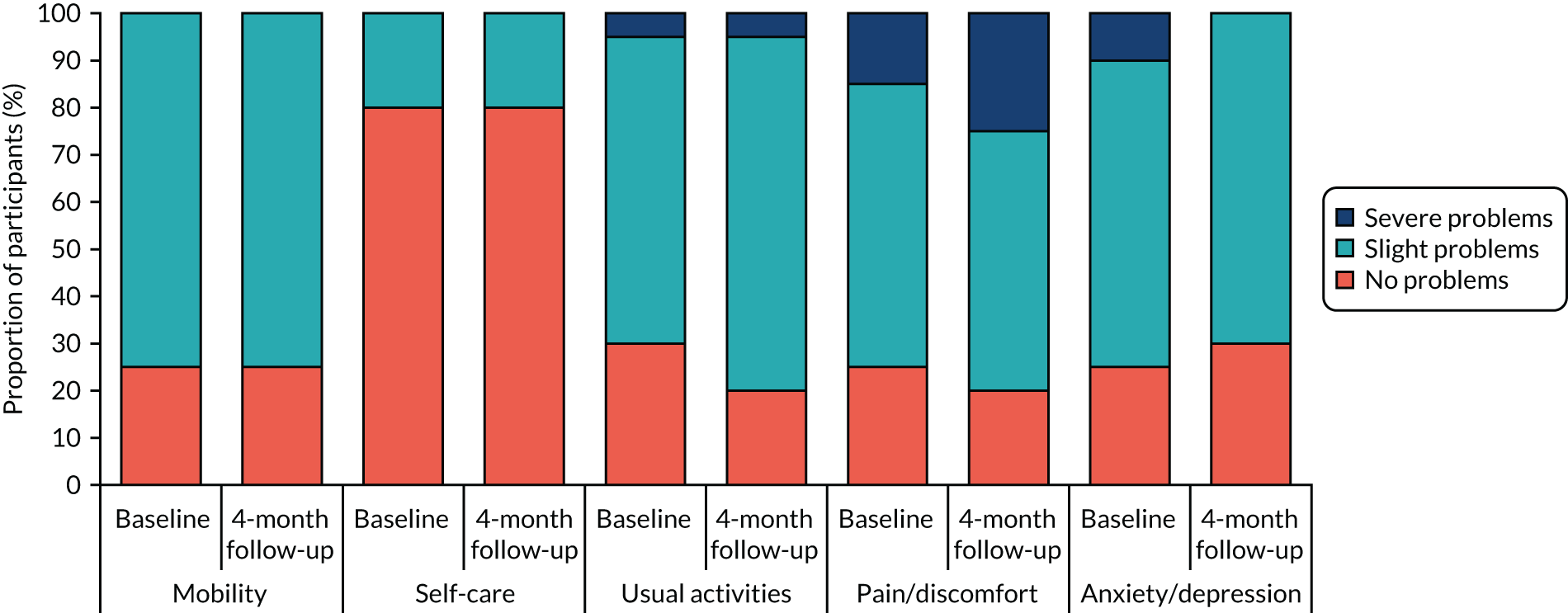
Frequency and pattern of Short Form-12 item responses
Figure 11 presents the distribution of responses for each item and domain of the SF-12v2 questionnaire at baseline and at the 4-month follow-up in the intervention arm. All items have five levels, except the two physical health questions (SF-2 and SF-3) that have three levels (see Chapter 2 for scoring of levels). At baseline, most participants had some level of problem in at least one of the domains; participants reported the lowest level of problems in the social functioning domain (which asks about physical health or emotional problems interfering with social activities): in this domain, at least 20% of participants reported no problems at baseline and at the 4-month follow-up. The domains with the highest level of problems were ‘general health’, ‘physical health’, ‘role physical’ and ‘vitality’. On the mental health domain, most participants (around 90%) reported some level of problems at baseline, with most of them reporting problems ‘some of the time’. This is consistent with subthreshold depression in this population. At the 4-month follow-up, there was a trend towards slight improvement in general health (SF-1). In addition, physical health problems (SF-2 and SF-3) appeared to get marginally worse over time. At the same time, the proportion of participants reporting extreme problems decreased in one domain (i.e. SF-11: feeling downhearted/low). Overall, although there was a slight trend towards health improvement in some domains, the numbers are too small to draw any conclusions about the trend.
FIGURE 11.
Pilot RCT: SF-12v2 responses at baseline and at the 4-month follow-up for the intervention arm. 4M, 4-month follow-up; BL, baseline. a, For SF-1, SF-8, SF-9 and SF-10, lower level (i.e. 1) indicates better health. For all other questions, lower level indicates worse health.

Figure 12 presents the distribution of SF-12v2 responses for the usual-care arm. At baseline, the pattern was similar to that in the intervention arm, that is most participants had some level of problem in at least one of the domains. Study participants reported the lowest level of problems in the social functioning and bodily pain domains: for these domains, at least 15% of participants reported no problems at baseline and at the 4-month follow-up. The domains with the highest level of problems were ‘general health’, ‘physical health’ and ‘vitality’. On the mental health domain, almost all study participants reported some level of problems at baseline. At the 4-month follow-up, there was no clear trend in quality of life, with some domains showing some improvement and others showing slight worsening. The proportion of participants reporting extreme problems remained stable at the 4-month follow-up. Overall, the trend was stable with marginal changes in some domains; however, the numbers are too small to draw any conclusions about the trend.
FIGURE 12.
Pilot RCT: SF-12v2 responses at baseline and at the 4-month follow-up for the usual-care arm. a, For SF-1, SF-8, SF-9 and SF-10, lower level (i.e. 1) indicates better health. For all other questions, lower level indicates worse health.
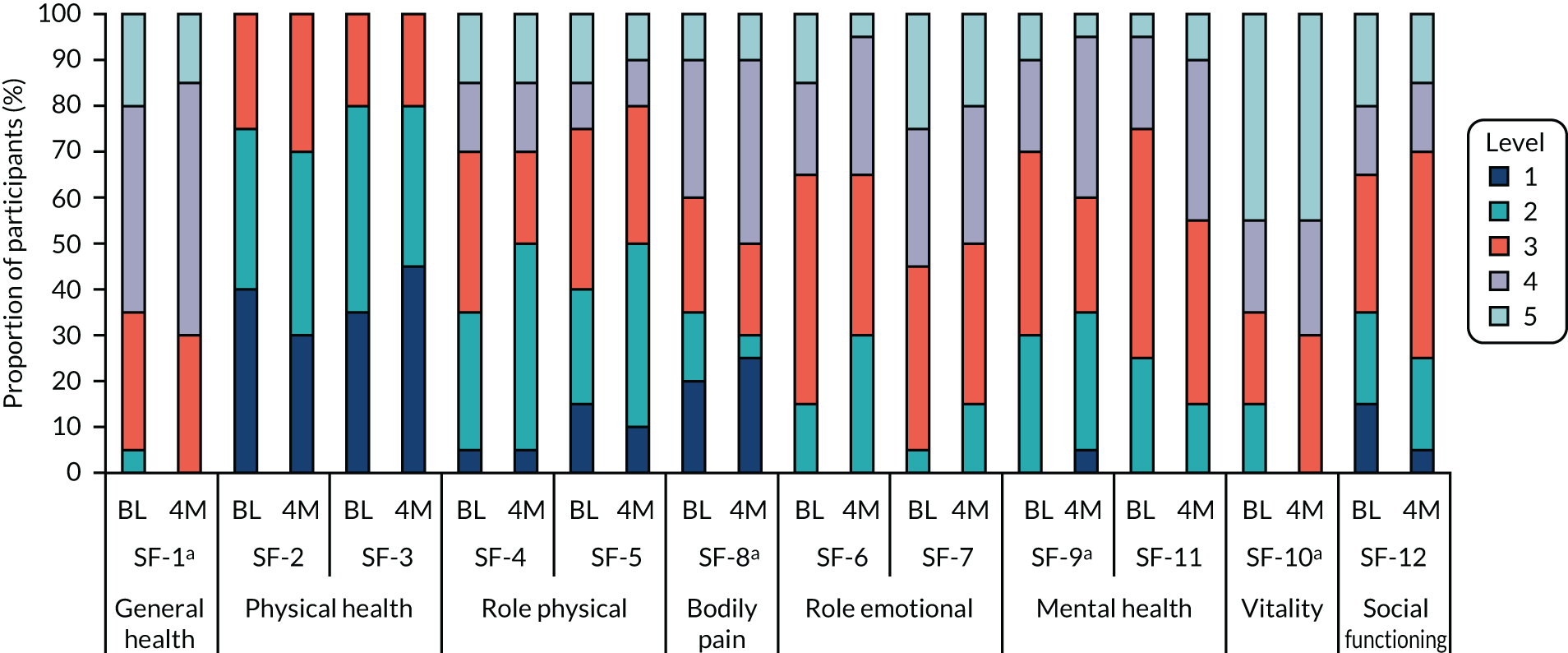
Health-related quality of life (utility values)
Figure 13 presents health-related quality of life (or utility) at baseline and at the 4-month follow-up (based on EQ-5D 3L responses, using UK tariff). The figure shows that participants in both treatment arms had low quality of life at baseline, which reduced further during the follow-up period. This is not surprising given the eligibility criteria of the study (i.e. patients with LTCs and subthreshold depression). On average, baseline utility level was lower among participants in the intervention arm than among those in the usual-care arm (difference: 0.096). During the 4-month post-randomisation period, utility values fell slightly in both treatment arms, but the difference remained almost the same (i.e. 0.101). However, as with the cost data, these results should be interpreted with caution as they are based on a small sample size.
FIGURE 13.
Pilot RCT: utility values based on EQ-5D 3L responses at baseline and at the 4-month follow-up by treatment arm. Numbers are rounded to second decimal place.
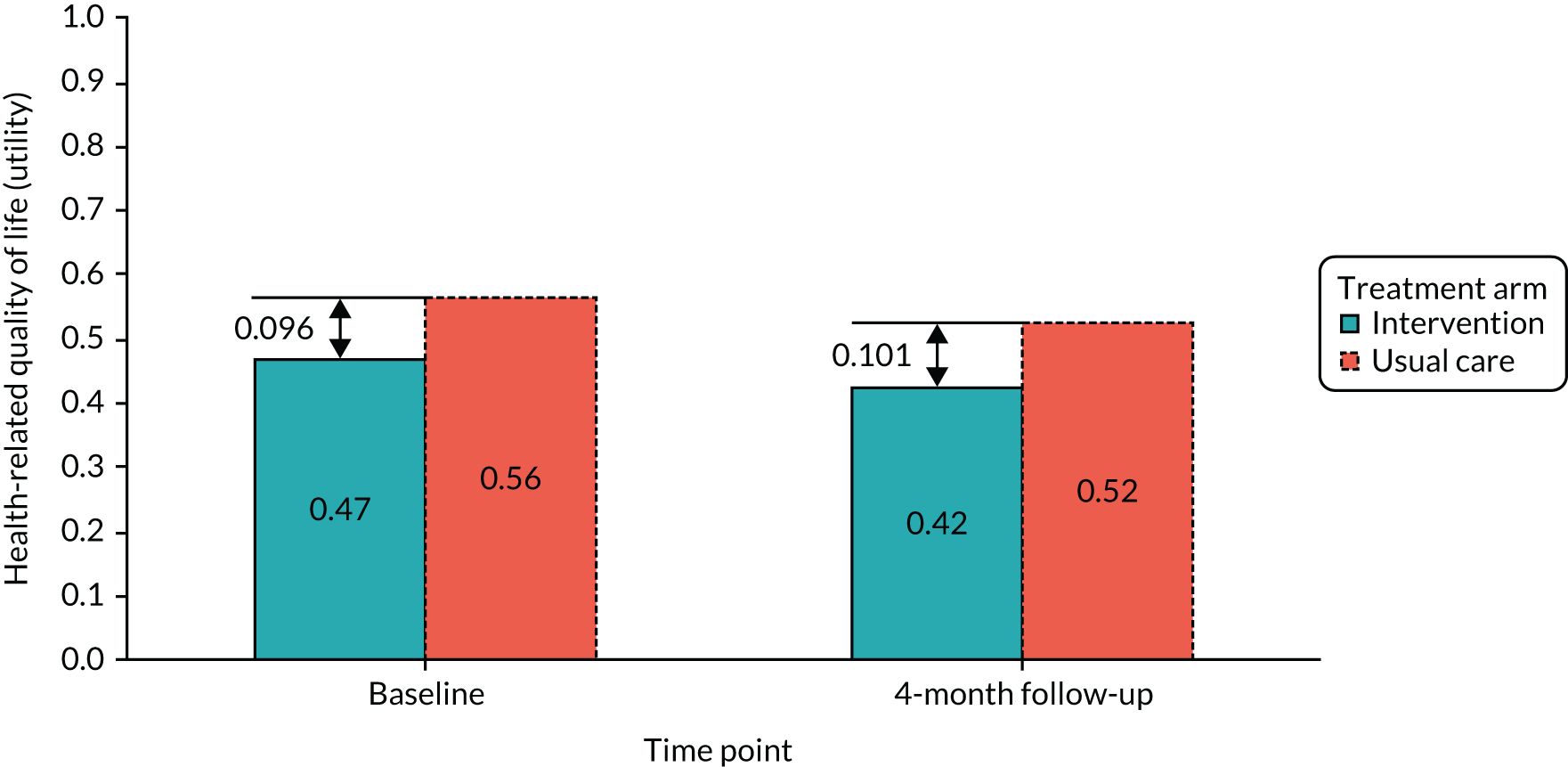
Chapter summary
The economic analysis evaluated the overall response rates, item completion rates and trends in the level and changes in health service resource use and health-related quality of life. The ESI cost per study participant was £51.40 (range £9.30–91), including the cost of ESI sessions, administrative work and clinical supervision. Questionnaire response rate, item completion rate and changes in resource use and quality of life over the trial follow-up period were also evaluated. Both health service resource use and quality-of-life questionnaires had very high completion rates among study participants. The overall rate of missing items was also very low. Visits to the GP, nurse and pharmacist were the most common types of resource use items. It was noted that a very small number of study participants had inpatient admissions with high costs. These may not be related to the intervention; therefore, analysis of the full trial should be conducted with and without the cost of inpatient admissions. Regarding the EQ-5D and SF-12v2 questionnaires, there were no missing data among participants; however, an imbalance in baseline utility was observed. Given the observed difference in cost and utility at baseline, the analysis of any future full-scale RCT should adjust for these in a regression analysis.
During the study follow-up, the level of primary and community care use reduced in the intervention arm compared with the usual-care arm, which could be partly attributable to the intervention; however, the sample size was too small to draw an inference. In addition, during the follow-up period there were small changes across quality-of-life domains in both treatment arms, but the overall utility values reduced by the same quantity in both treatment arms. Overall, the economic analysis suggested a high level of questionnaire completion, a low level of item missingness and no out-of-range responses. Moreover, there were differences between treatment arms at baseline that should be adjusted for. Finally, there was an indication that the ESI might reduce primary care costs, although the study sample size was insufficient to draw any statistical conclusions.
Chapter 11 Discussion and conclusions
Overview of study objectives
CHEMIST is the first study, to our knowledge, to evaluate the feasibility of conducting research into psychological approaches for depression prevention in a CP setting. The aim of the feasibility study was to adapt an existing depression prevention intervention38 for implementation by CP staff for people with comorbid subthreshold depression and LTCs, and to develop and refine study procedures for use in this setting. The pilot RCT sought to quantify and evaluate important study parameters, including recruitment and retention rates, assessment and data collection methods, and engagement with the ESI. Process evaluations during both the feasibility and the pilot RCT phases aimed to determine the acceptability and appropriateness of the ESI and study procedures, and to explore the barriers to and facilitators of implementation.
Summary of main findings
Feasibility study
Eight CPs participated in the feasibility study and 17 pharmacy staff completed the ESI facilitator training. The recruitment target (20–30 participants) was achieved, with 24 participants recruited across seven of the eight CPs and one general practice over a 9-month period. Initial face-to-face pharmacy recruitment was slower than expected, with pharmacy staff reporting difficulties in finding the time to raise the study with pharmacy customers owing to the busy nature of the pharmacy setting. To achieve the recruitment target, a number of additional recruitment methods were implemented, the recruitment period was extended and additional pharmacies were recruited (see Chapter 5).
Despite these various recruitment methods, the number of study information packs distributed was relatively small (n = 1082), with recruitment activity varying across CPs. Response rates to the study information were lower than anticipated (7.3% via pharmacy methods; 3.5% via general practice database searches) and are lower than those typically seen in primary care studies (18%). 38 The highest response rates were for face-to-face recruitment in the pharmacy, although these rates are based on small numbers. Conversion rates (from return of consent form to recruited participant) were similar across pharmacy (34.4%) and general practice recruitment (28.6%), and are comparable to those reported in primary care studies (28%). 38
Data completion across all outcome measures was excellent (95–100% of returned questionnaires), indicating that participants found it feasible to complete these. The retention rate at the 4-month follow-up was good (83%) and exceeded the target of 80%; one participant withdrew from the study.
Nine of the 17 trained ESI facilitators delivered the ESI. Engagement with the ESI was good, with 17 out of the 24 recruited participants (71%) commencing the ESI sessions. This level of engagement is comparable to engagement with psychological intervention services in routine care, in which 68% of people commence treatment, although is slightly less than that reported in primary care depression research studies (84%). 38,86 For those participants who commenced the ESI, 86% of all possible sessions were completed, with all participants completing at least two sessions, and 10 participants completing all six sessions. This compares favourably with routine psychological intervention services, in which 56% of people who enter the service attend two or more clinical appointments. 86
Depressive symptoms (as measured by the PHQ-963) appeared to reduce slightly from baseline to follow-up (mean reduction of 3.5 points), which reflected findings of the nested qualitative study, which found that participants reported positive benefits from the ESI. However, as the sample size is small and randomisation was not employed, this finding must be viewed with caution. Health economic analysis indicated that GP visits were the most common type of health service resource accessed, with only a small proportion of these visits being for low mood.
Data generated from semistructured interviews suggested that study participants felt that the CP was an appropriate place to offer a mental health intervention; that the ESI made sense and was acceptable; and that the ESI supported self-management of mood and LTCs. Study participants reported that they had engaged with the self-help materials and valued the contact with the ESI facilitator. Analysis of data generated by semistructured interviews with ESI facilitators suggested that ESI facilitators found the ESI to be acceptable. Despite initial concerns about delivering the ESI, ESI facilitators reported increased confidence with ESI delivery. The ESI training was reported as acceptable and useful, and suggestions were made to modify this training for the pilot RCT. ESI facilitators and pharmacy staff reported concerns about increased work burden and the practical challenges of study delivery in the CP, which were especially problematic at busy times. Fidelity to the ESI was not assessed as originally planned owing to concerns over the additional burden and anxiety this may cause ESI facilitators.
Learning from the feasibility study and progression to the external pilot randomised controlled trial
The feasibility study generated important learning that led to a number of refinements to the recruitment and study processes during the delivery of the study, and in preparation for the pilot RCT. Analysis of qualitative data informed further refinements to the ESI and the associated training materials (including the ESI training workshop). The participant study materials (including recruitment materials and study questionnaires) were also modified in an attempt to improve recruitment to the pilot RCT. The feasibility study highlighted the complexities of embedding mental health research in the new setting of the CP and the need to adapt study processes.
The findings from the feasibility study demonstrated that the ESI could be delivered in the CP setting and that pharmacy staff could be trained by the research team to deliver the ESI. Although not all of the feasibility study progression criteria were met, the TSC and the funder approved progression to the pilot RCT.
Pilot randomised controlled trial
Fifteen CPs participated in the pilot RCT, including six CPs that also took part in the feasibility study. An additional 18 pharmacy staff completed the ESI facilitator training. Recruitment remained a challenge and, despite greater engagement with recruitment activities in CPs and via general practice database searches, the recruitment period had to be extended by 6 months.
Forty-four participants (out of the target of 100 participants) were recruited and randomised (24 to the ESI and 20 to usual care) from across 12 of the 15 CPs and from five general practices. As in the feasibility study, recruitment activity varied across pharmacies. The response rate to pharmacy-based recruitment (2.7%) was lower than in the feasibility study (7.3%), although in the case of general practice recruitment response rates were similar (2.9% in the pilot RCT, 3.5% in the feasibility study). In addition, the conversion rate (from return of consent form to randomised participant) was lower in the pilot RCT (20.8% for pharmacy recruitment, 23.9% for general practice recruitment) than in the feasibility study; however, these conversion rates are still comparable to those reported in other primary care studies with similar patient populations (28%). 38 By contrast to the feasibility study, the different pharmacy-based recruitment methods yielded similar response rates (although numbers were again small).
As with the feasibility study, data completion of all outcome measures was excellent at baseline and follow-up (100% completion of returned questionnaires), confirming the suitability of these measures for use in this study. Retention at the 4-month follow-up was again excellent (93%), providing further evidence that it is feasible to retain and follow up this patient population. Two participants withdrew from the study.
Eleven ESI facilitators delivered the ESI (including five ESI facilitators who delivered the ESI in the feasibility study). Engagement with the ESI remained positive, with similar levels of engagement to that observed in the feasibility study. Of the 24 participants randomised to receive the ESI, 18 commenced the sessions (75%); this rate is above that seen in standard Improving Access to Psychological Therapy services in the NHS (68%)86 and slightly lower than that observed in similar research studies (84%). 38 For those 18 participants who commenced the ESI, 73% of all possible sessions were completed, with 16 participants (90%) completing two or more sessions and nine completing all six sessions. This level of treatment engagement is above that found in routine secondary care and is comparable to that reported in similar research studies. 38
There was a slight decrease in depressive symptoms at the 4-month follow-up in both the ESI and the usual-care arms, with a slightly larger decrease observed in the usual-care arm (mean reduction of 2.1 points and 1.1 points on the PHQ-9, respectively); however, the sample size (n = 41 at follow-up) is too small to draw any conclusions and the findings should be interpreted with caution. It is of note that the size of the decrease in depressive symptoms was less than that observed in the feasibility study, although baseline PHQ-9 scores were lower across both treatment arms in the pilot RCT than in the feasibility study.
Data generated from semistructured interviews with study participants, pharmacy staff (including ESI facilitators) and GPs were analysed using NPT. 45 The findings demonstrated that study participants found that the intervention made sense and was acceptable, that the support provided was perceived to be useful and that the self-help workbook was accessible and understandable, supporting and extending the qualitative findings from the feasibility study. Most participants who engaged with the intervention reported positive changes in mood and behaviour and viewed the relationship with the ESI facilitator as central to the intervention. Familiarity with pharmacy staff was viewed by some participants as a positive reason to participate in the study, whereas others felt that this may prohibit involvement because of concerns around privacy and confidentiality.
Pharmacy staff and GPs generally provided positive feedback about the intervention. ESI facilitators felt that the ESI training had extended their skill set and those who delivered the ESI reported gaining positive feedback from observing beneficial changes in the participants. Although some pharmacy staff found the study processes feasible to embed within their current pharmacy practice, others reported that the cumulative effect of numerous recruitment methods, and issues with limited availability of facilities for private consultations, impeded the working practices of the pharmacy. Delivering a mental health intervention in the CP was viewed as novel by some pharmacy staff, requiring a change in perception (for pharmacy staff and customers) about the role of pharmacy.
Considerable attempts were made in the pilot RCT to record ESI sessions to assess fidelity to the ESI. This was a new process for the ESI facilitators and the levels of anxiety reported were underestimated. Although some ESI facilitators agreed to record ESI sessions, this was achieved for only one session.
Economic analysis demonstrated high completion rates of resource (health service) use and quality-of-life questionnaires, with low levels of missing items. Visits to the GP, nurse and pharmacy were the most common types of resource use accessed. A small number of participants reported inpatient admissions (not related to mental health) during the study; however, owing to high unit cost, these inpatient attendances may have a significant impact on incremental cost and should be carefully considered in any future economic analysis. An imbalance in baseline quality-of-life scores between the two treatment arms was observed. There was some indication that the ESI might reduce the use of primary care and community services and hence costs, although the sample size was insufficient to draw any statistical conclusions. The ESI cost per study participant was £51.40, including the cost of delivering ESI sessions, administrative work and clinical supervision (based on four ESI sessions).
The pilot RCT incorporated progression criteria for a definitive RCT (although this was not part of the funded CHEMIST study) (see Chapter 1). The pilot RCT did not meet the recruitment target of 100 participants; 67% of participants randomised to the ESI completed at least two sessions (lower than the target of 80%, although it is worth noting that 90% of those participants who did commence the ESI completed a minimum of two sessions) and fidelity to the ESI was difficult to assess. However, retention at the 4-month follow-up was 93% (exceeding the target of 80%) and data completeness was excellent (100%).
Discussion of main findings across feasibility study and pilot randomised controlled trial
CHEMIST represented a new experience for the majority of the CPs taking part, both in terms of undertaking research and in terms of undertaking training in and delivering a mental health depression prevention intervention. Recruitment was a challenge; pharmacy staff found it difficult to reliably embed the study recruitment processes into their existing pharmacy routines for a variety of reasons. It is difficult to know whether or not recruitment to similar research studies will improve as CPs become more research active and accustomed to study and recruitment processes. Increased experience of research would facilitate the development of more streamlined processes to better embed research into existing pharmacy workloads and practices.
Response rates were lower than those reported for similar research studies conducted with similar patient populations within primary care. This may be related to the relative novelty of offering and delivering a mental health intervention via the CP, as identified in our process evaluations. The feasibility study demonstrated that the response rate was higher for face-to-face recruitment than for other (non-face-to-face) pharmacy-based methods; this is despite pharmacy staff reporting that this recruitment method was challenging to implement. This perhaps highlights the beneficial effects of existing relationships between pharmacy staff and customers for engagement with the research. The low response rates observed may also indicate that recruitment materials require modification to ensure that these are suitable for this patient population and/or this research setting. It is of note that many of the CPs involved in CHEMIST were located in socially deprived regions. A large proportion of those people who received study information chose not to indicate their interest in the study and the reasons for this are unknown.
Delivery of a mental health intervention in the CP is at this time relatively uncommon. Findings from the process evaluation suggest that preparatory work may be needed to provide context (or to ‘set the scene’) for such mental health support in this setting. This may to some degree explain the lower response rates to study information observed in this study than in primary care, in which such mental health interventions are more routine.
Our qualitative work highlighted that there may be a perception that the role of the CP does not include delivery of a talking treatment for a mental health problem. Pharmacies sit in the communities that they serve and concerns raised as to the confidentiality of information disclosed within this setting suggest that this may not be comparable to the perception of confidentiality in other settings, such as primary care. Some participants who were interviewed indicated a preference to speak to their GP about mental health concerns. Such concerns may have impacted on people’s willingness to take part in the study at the outset or on their willingness to engage with the ESI following recruitment to the study.
There were some indications from our process evaluation that intervention delivery posed some difficulties in the routine activities within the pharmacy. The consulting room is a key and limited resource in most CPs, and was occupied more often owing to the CHEMIST intervention. Further consideration is needed of intervention structures that are suited to the CP setting. Although we found that the CHEMIST intervention (which was based on BA) was acceptable and that we could train pharmacy staff to deliver this, the structure of the intervention placed additional burden on other pharmacy staff and the ability to meet the daily demands posed by a busy CP. If a new intervention impacts on the usual work of the CP, it is likely to encounter barriers to roll out. Our process evaluation indicates that further consideration of this factor is required to support interventions, such as the intervention that we implemented.
The issue of risk raised anxiety for CP staff in relation to both recruitment and intervention delivery activities. These anxieties appeared to ease across time but highlight the need for attention in study set up.
Based on these observations, further preparatory work may be required to help better embed research and related intervention activities, such as those used in CHEMIST, into the CP setting.
An additional factor that may have contributed to the low recruitment activity by the CPs relates to funding cuts that were experienced around the time of CHEMIST. In December 2015, the Department of Health and Social Care revealed that it was planning to reduce CP funding for delivering the contractual framework (essential and advanced services). This had a substantial impact on CPs, with reports that up to one in four pharmacies may face closure causing ‘shockwaves’ through the sector. 87,88 It was within this context that CHEMIST was conducted. It is perhaps not surprising that CPs participating in CHEMIST found it challenging to deliver on the additional activity that the study necessitated in an already overstretched workforce. If these funding restrictions were reversed and the funding climate were to improve, it may be that the additional activities required by participation in research may become more feasible for CPs.
The depression symptom severity of study participants is worthy of note. A structured diagnostic interview (MINI)48 was administered by the study team at screening to confirm diagnosis of subthreshold depression. Despite this, participant PHQ-9 scores at baseline in both the feasibility study and the pilot RCT indicated the presence of moderate and severe depression. Although the overall mean baseline PHQ-9 score was lower in the pilot RCT than in the feasibility study (mean PHQ-9 scores of 11.0 and 13.5, respectively), this score is still higher than what would be expected, with 57% (25/44) of participants having a PHQ-9 score indicative of moderate or more severe depression. This degree of depressive symptoms is somewhat higher than reported in research studies with similar patient populations. 38
The small sample size in the pilot RCT indicates uncertainty as to whether or not all potential confounders were distributed equally across the ESI and usual-care arms; however, the two arms appeared equal at baseline in terms of key characteristics. Differences observed between the two treatment arms in the primary outcome (PHQ-9) at the 4-month follow-up were minimal and less than seen in similar research studies. 38,76 Even if the sample size of 100 participants had been achieved in the pilot RCT, the study was not powered to test for intervention effects and, for this reason, the findings should be viewed with caution.
Promising levels of engagement with the ESI were demonstrated across both the feasibility study and the pilot RCT. Process evaluations indicated that the ESI was acceptable to both study participants and those ESI facilitators delivering the ESI; both study participants and ESI facilitators reported positive benefits. Across both phases of the study, data (questionnaire) completeness and participant follow-up rates were good. These findings suggest that it would be possible to collect the relevant information to conduct analysis in a definitive RCT of the ESI delivered within the CP setting, provided that the issues discussed above regarding recruitment and intervention implementation could be resolved.
Strengths and limitations
CHEMIST explores the feasibility of a psychological intervention (BA) for people with comorbid subthreshold depression and LTCs, and is delivered in the CP setting.
It demonstrated that CPs are interested in mental health research and are keen to participate, despite the challenges they face with funding cuts and an often understaffed workforce. A key strength of the study is the finding that CP staff can be successfully trained to deliver a psychological intervention (BA). This builds on previous work exploring the capacity for this simple treatment to be delivered by non-mental health specialists and has potential implications for the roll out of psychological interventions, such as BA, by non-specialist groups. 38–40
Qualitative work permitted an in-depth exploration of a range of aspects important to CHEMIST. These data demonstrated that the ESI was often found to be acceptable to both those delivering and those receiving the intervention, although with some important implications for the routine activity in the CP setting.
People with comorbid subthreshold depression and LTCs were willing to engage in CHEMIST. They were able to complete study questionnaires and were willing to be followed up. Those participants who were offered the ESI generally engaged well with this, despite the finding that some concerns relating to confidentiality existed.
A further strength is the learning that has been gained from conducting this study. This was critical throughout the duration of the study and allowed procedures and materials to be developed and refined for use in a CP setting. This learning will also be crucial in the design and delivery of future research studies within this setting.
The study benefited from the input of key stakeholders (including pharmacy and public health stakeholders) and PPI from the inception of the study and throughout its management. This allowed issues to be discussed as these arose. Involvement of the PPI AG was instrumental in the refinement of the ESI and study materials, and ensured that the study remained focused on supporting people with mental and physical health-care needs, and supporting CP staff to engage effectively with the study.
We adopted usual care as our comparator. This was seen to be suitable in this study and reflects what is usually provided to people with LTCs and subthreshold depression. There is ongoing debate about the use of attention placebos in psychological therapy research and no gold standard is available. 89 The approach taken in CHEMIST is consistent with other large-scale research in similar areas38,76 and was considered to be appropriate.
The main limitation is the small sample size in the pilot RCT. Although no formal statistical comparisons were planned, the smaller than expected sample size precludes the drawing of any conclusion from the comparison of primary outcome data. It also limits any reliable sample size estimates for a definitive RCT.
Although a range of interviews were conducted, the small sample size of some participant groups may have limited the range of views gathered. The views of those people who decided not to participate in the study are unknown. A further limitation is the predominantly white patient population in both phases of the study.
Owing to the problems with the delivery of the study as described above, not all those ESI facilitators trained to deliver the ESI had the opportunity to support participants through the intervention; ESI facilitators may have found this frustrating and this may have affected their engagement with the study. However, it is worth noting that, of the 35 pharmacy staff who attended the ESI facilitator training, only 21 were available to deliver the ESI during the intervention periods (reasons for unavailability included ESI facilitators leaving their pharmacy role, concerns over existing workloads and personal circumstances). The problems experienced with recording ESI sessions prohibited an independent assessment of fidelity to the ESI. This posed a further limitation in assessing the feasibility of the intervention for future research.
Recruitment via general practice database searches was limited across both the feasibility study and the pilot RCT owing to research-active general practices not being close or linked to those CPs participating in the study. Recruitment may have been improved if a larger number of general practices within the vicinity of the recruiting CPs were research active.
Conclusions
The CP is a relatively new setting for mental health research and often constitutes a novel setting in which to conduct depression prevention studies, such as CHEMIST.
The findings from the feasibility study and the pilot RCT suggest that there would be challenges to conducting a large-scale definitive RCT of a structured psychological intervention for people with comorbid subthreshold depression and LTCs in the CP. Although key aspects of the study were promising (e.g. suitability and completion of outcome measures, retention rates and engagement with the ESI), a significant issue was recruitment to the study.
Importantly, CHEMIST has provided important reflections on how to effectively embed research and study processes in this busy public health setting, and the barriers that may be encountered. The learning acquired from conducting the feasibility study was critical in refining study procedures, training and materials for use in the pilot RCT. Such findings will also be useful in the design and delivery of future studies within a CP setting.
The CP would appear to be ideally placed to provide support to people with mental–physical multimorbidity. Prevention of depression in people with long-term health problems is important because they are at increased risk. The Community Pharmacy Contractual Framework for 2019/20 to 2023/24: Supporting Delivery for the NHS Long Term Plan24 encourages CPs to play a greater role in clinical service delivery and in helping people to stay well. The CHEMIST intervention would seem well placed to reflect this new focus for the CP, which has prevention acknowledged as a core role. The limitations highlighted in this study suggest that more research is required to consider effective methods to recruit people to studies and successfully embed interventions within the CP setting. The evidence base for such activities, especially in relation to depression, is lacking43 and further definitive research is required. Targeted approaches, such as the CHEMIST intervention, require more research.
Clinical Research Networks are keen to expand the delivery of research in this important public health setting and the establishment of primary care networks, with the aim of fostering wider relationships with their partners to deliver integrated care, which will further facilitate important collaborative and interdisciplinary working. The CP has been identified as one such key partner, especially in relation to public health and prevention. It may be that embedding CP research within a primary care network may offer an effective platform to better implement research within this setting and help explore ways to improve and facilitate recruitment.
Implications for health care
Mental–physical multimorbidity poses a significant challenge for the future. The identification of a suitable depression prevention intervention that can be offered and delivered in the CP setting could offer an important option to communities, particularly in areas of socioeconomic deprivation. The ability to train CP staff to deliver the CHEMIST intervention does indicate that further work may be warranted to make a robust evaluation of clinical effectiveness and cost-effectiveness possible.
Recommendations for future research
Further research is required to inform the design and delivery of a definitive RCT to test the clinical effectiveness and cost-effectiveness of depression prevention interventions within the CP setting.
Such research should seek to explore and address barriers to recruitment in this setting. This could include further exploration to understand the issues of confidentiality and disclosure of mental health concerns to CP staff, and to gain further knowledge about people’s perceptions of the CP as a setting to deliver a psychological intervention for people with comorbid mental and physical health conditions. Future research may also benefit from consideration of joined up working across CP and primary care with respect to mental health support, which may promote engagement in mental health studies conducted within the CP setting.
The factors promoting effective implementation of a depression prevention intervention in the CP setting need more detailed investigation. Consideration of intervention design and the impact of intervention delivery on the usual activities within the CP is recommended to help overcome some of the barriers to recruitment and study/intervention implementation described in this report. Such research will facilitate a better understanding of the organisational, operational and cultural factors to consider when designing research for implementation within this setting.
Future work should also seek to involve a more ethnically diverse sample. Further work to review the numbers of people with subthreshold depression who can be identified and who would be willing to engage in a mental health intervention delivered within the CP setting may be needed.
Consideration may need to be given to developing and testing a more pragmatic approach to study inclusion criteria and assessment that uses a depression scale rather than a diagnostic interview. Such processes may improve recruitment rates and reflect what may be adopted in the future in this type of setting, although the methodological limitations of such approaches would need to be considered.
Future research may also benefit from preparatory cross-disciplinary learning and training between research and CP environments to better facilitate more effective embedding of research and intervention processes and structures within the CP setting.
Acknowledgements
Our first thanks are to all those participants who agreed to take part in this study. We would also like to thank CP staff for their hard work in identifying participants, delivering the ESI, completing study documentation and for their general support of the study; all those researchers who assisted with recruitment, data collection and data coding/checking including Andrew Henry, Deborah Kemp and Jacqueline Vicars; Cath Robson for being the (unofficial) ‘Pharmacy Research Champion’; members of the TSC and the Data Monitoring and Ethics Committee for overseeing the study; members of the CHEMIST special interest group for their guidance and support; colleagues in Research and Development at Tees, Esk and Wear Valleys NHS Foundation Trust and the North East and North Cumbria Clinical Research Network for their continued support of the study; and Megan Russell for providing additional administrative support. Special thanks to Rose NcNulty for providing exceptional administrative support (always with a smile), to all of the individuals who formed the CHEMIST PPI AG and to Sarah Speight for her contribution to the PPI AG and membership of the TSC.
CHEMIST benefited from the support of the North East and North Cumbria Clinical Research Network.
Trial Steering Committee independent members
Professor David Kessler (chairperson), Professor of Primary Care, Bristol Medical School, Population Health Sciences, The University of Bristol, Bristol.
Professor Rachel Elliott, Professor in Health Economics, Division of Population Health, Health Services Research and Primary Care, The University of Manchester, Manchester.
Dr Richard Jacques, Senior Lecturer in Medical Statistics, School of Health and Related Research, The University of Sheffield, Sheffield.
Sarah Speight, PPI member, North Yorkshire.
Data Monitoring and Ethics Committee independent members
Professor Chris Dickens (chairperson), Professor of Psychological Medicine, College of Medicine and Health (Medicine, Nursing and Allied Health Professions), University of Exeter, Exeter.
Professor Richard Emsley, Professor of Medical Statistics and Trials Methodology, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London.
Professor David Alldred, Professor of Medicines Use and Safety, School of Healthcare, University of Leeds, Leeds.
Contributions of authors
Elizabeth Littlewood (https://orcid.org/0000-0002-4606-4590) (Research Fellow, Applied Health Services Research) was the trial manager and provided study oversight; was a member of the TMG; supervised the research team; refined the protocol; recruited participants to the study, collected study data, provided feedback on and helped to refine the study procedures, and attended researcher meetings; refined the procedures for the process evaluation work; and led and drafted the final report as part of the report writing team.
Carolyn A Chew-Graham (https://orcid.org/0000-0002-9722-9981) (Professor of General Practice Research and Academic GP, Primary Care) was a co-applicant on the Public Health Research funding application and a member of the TMG; refined the protocol; designed the process evaluations and provided oversight for this work; refined the procedures for the process evaluation work; analysed the feasibility and pilot RCT interview data; and drafted the final report as part of the report writing team.
Elizabeth Coleman (https://orcid.org/0000-0003-4210-1865) (Trainee Statistician, Statistics and Trials Methodology) refined the protocol; drafted the statistical analysis plan and conducted the data analysis; and drafted the final report as part of the report writing team.
Samantha Gascoyne (https://orcid.org/0000-0001-7763-2776) (Research Fellow, Applied Health Services Research) was the trial co-ordinator; refined the protocol; recruited participants to the study, collected study data, provided feedback on and helped to refine the study procedures, and attended researcher meetings; refined the procedures for the process evaluation work; conducted semistructured interviews with participant groups; coded and analysed the feasibility interview data with supervision provided by Carolyn Chew-Graham; coded the pilot RCT interview data; and drafted the final report as part of the report writing team.
Claire Sloan (https://orcid.org/0000-0003-1589-5145) (Research Fellow, Qualitative Applied Health Research) refined the protocol; refined the procedures for the process evaluation work; led the pilot RCT process evaluation; conducted semistructured interviews with participant groups; coded and analysed the pilot RCT interview data; and drafted the final report as part of the report writing team.
Shehzad Ali (https://orcid.org/0000-0002-8042-3630) (Associate Professor, Public Health Economics) was a co-applicant on the Public Health Research funding application and a member of the TMG; designed and conducted the economic analysis; refined the protocol; and drafted the final report as part of the report writing team.
Jay Badenhorst (https://orcid.org/0000-0001-5539-6776) (Superintendent Pharmacist, Pharmacy Practice) was a co-applicant on the Public Health Research funding application and a member of the TMG; refined the protocol; and read and reviewed the final report.
Della Bailey (https://orcid.org/0000-0002-6059-2111) (Research Fellow, Applied Health Services Research) refined the protocol; supervised delivery of the intervention; recruited participants to the study, collected study data, provided feedback on and helped to refine the study procedures, and attended researcher meetings; and drafted the final report as part of the report writing team.
Suzanne Crosland (https://orcid.org/0000-0001-6658-6815) (Research Fellow, Applied Health Services Research) refined the protocol; recruited participants to the study, collected study data, provided feedback on and helped to refine the study procedures, and attended researcher meetings; conducted semistructured interviews with participant groups; and read and reviewed the final report.
Charlotte EW Kitchen (https://orcid.org/0000-0002-9323-0061) (Research Fellow, Applied Health Services Research) refined the protocol; recruited participants to the study, collected study data, provided feedback on and helped to refine the study procedures, and attended researcher meetings; refined the procedures for the process evaluation work; led the feasibility study process evaluation with supervision from Carolyn Chew-Graham; facilitated the focus group with pharmacy staff; conducted semistructured interviews with participant groups; coded and analysed the feasibility interview data with supervision provided by Carolyn Chew-Graham; and read and reviewed the final report.
Dean McMillan (https://orcid.org/0000-0002-2901-8410) (Professor; Applied Health Services Research) was a co-applicant on the Public Health Research funding application and a member of the TMG; refined the protocol; and read and reviewed the final report.
Caroline Pearson (https://orcid.org/0000-0003-0018-8464) (Trial Support Officer; Applied Health Services Research) refined the protocol; recruited participants to the study, collected study data, provided feedback on and helped to refine the study procedures, and attended researcher meetings; refined the procedures for the process evaluation work; conducted semistructured interviews with participant groups; and read and reviewed the final report.
Adam Todd (https://orcid.org/0000-0003-1496-9341) (Reader and Pharmacist; Pharmacy Practice and Research) was a co-applicant on the Public Health Research funding application and a member of the TMG; refined the protocol; and drafted the final report as part of the report writing team.
Cate Whittlesea (https://orcid.org/0000-0002-4951-2272) (Professor; Pharmacy Practice and Research) was a co-applicant on the Public Health Research funding application and a member of the TMG; refined the protocol; and read and reviewed the final report.
Clare Bambra (https://orcid.org/0000-0002-1294-6851) (Professor; Public Health) was a co-applicant on the Public Health Research funding application and a member of the TMG; and read and reviewed the final report.
Catherine Hewitt (https://orcid.org/0000-0002-0415-3536) (Professor; Statistics and Trials Methodology) was a co-applicant on the Public Health Research funding application and a member of the TMG; refined the protocol; designed the data analysis and provided oversight for the conduct of the data analysis; and read and reviewed the final report.
Claire Jones (https://orcid.org/0000-0003-0799-6798) (Public Health Pharmacist; Public Health) was a co-applicant on the Public Health Research funding application and a member of the TMG; refined the protocol; and read and reviewed the final report.
Ada Keding (https://orcid.org/0000-0002-1182-887X) (Statistician; Statistics and Trials Methodology) was a co-applicant on the Public Health Research funding application and a member of the TMG; refined the protocol; provided supervision to Elizabeth Coleman; and read and reviewed the final report.
Elizabeth Newbronner (https://orcid.org/0000-0003-2366-9981) (Research Fellow; Qualitative Health Research) coded and analysed the pilot RCT interview data; and read and reviewed the final report.
Alastair Paterson (https://orcid.org/0000-0002-8565-3304) (Clinical Pharmacist; Pharmacy) coded and analysed the pilot RCT interview data; and read and reviewed the final report.
Shelley Rhodes (https://orcid.org/0000-0001-7195-8759) (Senior Trial Manager; Trials) drafted the ethics submission; refined the protocol; and read and reviewed the final report.
Eloise Ryde (https://orcid.org/0000-0003-4597-7774) (Research Assistant; Applied Health Services Research) recruited participants to the study, collected study data, provided feedback on and helped to refine the study procedures, and attended researcher meetings; and read and reviewed the final report.
Paul Toner (https://orcid.org/0000-0001-7801-7636) (Lecturer; Qualitative and Addiction Research) refined the procedures for the process evaluation work; facilitated the focus group with pharmacy staff; and read and reviewed the final report.
Michelle Watson (https://orcid.org/0000-0002-1790-9953) (Research Fellow; Applied Health Services and Pharmacy Research) was the trial co-ordinator; refined the protocol; and read and reviewed the final report.
Simon Gilbody (https://orcid.org/0000-0002-8236-6983) (Professor; Psychological Medicine and Applied Health Services Research) wrote the original protocol; was joint lead applicant on the Public Health Research funding application and a member of the TMG; was joint chief investigator and provided oversight to the study; refined the protocol; and approved the final report.
David Ekers (https://orcid.org/0000-0003-3898-3340) (Professor; Mental Health Nursing and Applied Health Services Research) wrote the original protocol; was joint lead applicant on the Public Health Research funding application and a member of the TMG; was joint chief investigator and provided oversight to the study; refined the protocol; drafted the final report as part of the report writing team; and approved the final report.
Publications
Littlewood E, Ali S, Badenhorst J, Bailey D, Bambra C, Chew-Graham C, et al. Community Pharmacies Mood Intervention Study (CHEMIST): feasibility and external pilot randomised controlled trial protocol. Pilot Feasibility Stud 2019;5:71.
Chew-Graham CA, Kitchen CEW, Gascoyne S, Littlewood E, Coleman E, Bailey D, et al. The feasibility and acceptability of a brief psychological intervention for adults with long-term health conditions and subthreshold depression delivered via community pharmacies: a mixed methods evaluation – the Community Pharmacies Mood Intervention Study (CHEMIST). Pilot Feasibility Stud 2022;8:27.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following this.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care.
References
- World Health Organization (WHO) . The Global Burden of Disease: 2004 Update 2008.
- Naylor C, Parsonage M, McDaid D, Knapp M, Fossey M, Galea A. Long Term Conditions and Mental Health: The Cost of Co-morbidities. London: The King’s Fund and Centre for Mental Health; 2012.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370:851-8. https://doi.org/10.1016/S0140-6736(07)61415-9.
- Mercer SW, Watt GC. The inverse care law: clinical primary care encounters in deprived and affluent areas of Scotland. Ann Fam Med 2007;5:503-10. https://doi.org/10.1370/afm.778.
- Mujica-Mota RE, Roberts M, Abel G, Elliott M, Lyratzopoulos G, Roland M, et al. Common patterns of morbidity and multi-morbidity and their impact on health-related quality of life: evidence from a national survey. Qual Life Res 2015;24:909-18. https://doi.org/10.1007/s11136-014-0820-7.
- NHS England and Public Health England . Five Year Forward View n.d. www.england.nhs.uk/ourwork/futurenhs/5yfv-fore/ (accessed 30 October 2019).
- Naylor C, Das P, Ross S, Honeyman M, Thompson J, Gilburt H. Bringing Together Physical and Mental Health. London: The King’s Fund and Centre for Mental Health; 2016.
- NHS . The NHS Long Term Plan n.d. www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf (accessed 30 October 2019).
- Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord 2004;79:71-9. https://doi.org/10.1016/S0165-0327(02)00348-8.
- National Collaborating Centre for Mental Health, National Institute for Health Clinical Excellence, British Psychological Society, Royal College of Psychiatrists . Common Mental Health Disorders: Identification and Pathways to Care. 2011.
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry 2013;202:22-7. https://doi.org/10.1192/bjp.bp.112.112169.
- Rodríguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry 2012;12. https://doi.org/10.1186/1471-244X-12-181.
- National Institute for Health and Care Excellence (NICE) . Depression in Adults With a Chronic Physical Health Problem. Clinical Guidance [CG91] 2009.
- McManus S, Meltzer H, Brugha T, Bebbington P, Jenkins R. Adult Psychiatric Morbidity in England 2007: Results of a Household Survey. Leeds: NHS Digital; 2009.
- Dowrick C, Chew-Graham C, Lovell K, Lamb J, Aseem S, Beatty S, et al. Increasing equity of access to high-quality mental health services in primary care: a mixed-methods study. Programme Grants Appl Res 2013;1. https://doi.org/10.3310/pgfar01020.
- Pharmaceutical Services Negotiating Committee . About Community Pharmacy n.d. https://psnc.org.uk/psncs-work/about-community-pharmacy/ (accessed 30 October 2019).
- Todd A, Copeland A, Husband A, Kasim A, Bambra C. The positive pharmacy care law: an area-level analysis of the relationship between community pharmacy distribution, urbanity and social deprivation in England. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005764.
- Todd A, Copeland A, Husband A, Kasim A, Bambra C. Access all areas? An area-level analysis of accessibility to general practice and community pharmacy services in England by urbanity and social deprivation. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007328.
- Lindsey L, Husband A, Steed L, Walton R, Todd A. Helpful advice and hidden expertize: pharmacy users’ experiences of community pharmacy accessibility. J Public Health 2017;39:609-15. https://doi.org/10.1093/pubmed/fdw089.
- Knox K, Kelly F, Mey A, Hattingh L, Fowler JL, Wheeler AJ. Australian mental health consumers’ and carers’ experiences of community pharmacy service. Health Expect 2015;18:2107-20. https://doi.org/10.1111/hex.12179.
- Eades CE, Ferguson JS, O’Carroll RE. Public health in community pharmacy: a systematic review of pharmacist and consumer views. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-582.
- Steed L, Kassavou K, Madurasinghe VW, Edwards EA, Todd A, Summerbell CD, et al. Community pharmacy interventions for health promotion: effects on professional practice and health outcomes (Protocol). Cochrane Database Syst Rev 2014;7. https://doi.org/10.1002/14651858.CD011207.
- Pharmaceutical Services Negotiating Committee . PSNC Briefing 026 19: A Summary of the Five-Year Deal on the Community Pharmacy Contractual Framework n.d. https://psnc.org.uk/wp-content/uploads/2019/07/PSNC-Briefing-026.19-A-Summary-of-the-Five-Year-Deal-on-the-Community-Pharmacy-Contractual-Framework.pdf (accessed 30 October 2019).
- Department of Health and Social Care, NHS England and NHS Improvement, Pharmaceutical Services Negotiating Committee . The Community Pharmacy Contractual Framework for 2019 20 to 2023 24: Supporting Delivery for the NHS Long Term Plan n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/819601/cpcf-2019-to-2024.pdf (accessed 3 February 2020).
- Pharmaceutical Services Negotiating Committee . PSNC Healthy Living Pharmacies n.d. https://psnc.org.uk/services-commissioning/locally-commissioned-services/healthy-living-pharmacies/ (accessed 30 October 2019).
- Thomson K, Hillier-Brown F, Walton N, Bilaj M, Bambra C, Todd A. The effects of community pharmacy-delivered public health interventions on population health and health inequalities: a review of reviews. Prev Med 2019;124:98-109. https://doi.org/10.1016/j.ypmed.2019.04.003.
- Patel C, North F, Head J, . The Marmot Review. Fair Society, Healthy Lives: Strategic Review of Health Inequalities in England Post-2010. London: University College London; 2010.
- NHS Confederation . Health on the High Street: Rethinking the Role of Community Pharmacy 2013.
- Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD006560.pub3.
- van Zoonen K, Buntrock C, Ebert DD, Smit F, Reynolds CF, Beekman AT, et al. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. Int J Epidemiol 2014;43:318-29. https://doi.org/10.1093/ije/dyt175.
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness?. Eff Clin Pract 1998;1.
- Gunn J, Diggens J, Hegarty K, Blashki G. A systematic review of complex system interventions designed to increase recovery from depression in primary car. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-88.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton A. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314-21. https://doi.org/10.1001/archinte.166.21.2314.
- Gilbody S, Whitty P, Grimshaw J, Thomas R. Educational and organizational interventions to improve the management of depression in primary care: a systematic review. JAMA 2003;289:3145-51. https://doi.org/10.1001/jama.289.23.3145.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD006525.pub2.
- Ekers D, Murphy R, Archer J, Ebenezer C, Kemp D, Gilbody S. Nurse-delivered collaborative care for depression and long-term physical conditions: a systematic review and meta-analysis. J Affect Disord 2013;149:14-22. https://doi.org/10.1016/j.jad.2013.02.032.
- Mitchell N, Hewitt C, Adamson J, Parrott S, Torgerson D, Ekers D, et al. A randomised evaluation of CollAborative care and active surveillance for Screen-Positive EldeRs with sub-threshold depression (CASPER): study protocol for a randomized controlled trial. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-225.
- Gilbody S, Lewis H, Adamson J, Atherton K, Bailey D, Birtwistle J, et al. Effect of collaborative care vs usual care on depressive symptoms in older adults with subthreshold depression: the CASPER randomized clinical trial. JAMA 2017;317:728-37. https://doi.org/10.1001/jama.2017.0130.
- Kingstone T, Bartlam B, Burroughs H, Bullock P, Lovell K, Ray M, et al. Can support workers from AgeUK deliver an intervention to support older people with anxiety and depression? A qualitative evaluation. BMC Fam Pract 2019;20. https://doi.org/10.1186/s12875-019-0903-1.
- Kingstone T, Burroughs H, Bartlam B, Ray M, Proctor J, Shepherd T, et al. Developing a community-based psycho-social intervention with older people and third sector workers for anxiety and depression: a qualitative study. BMC Fam Pract 2017;18. https://doi.org/10.1186/s12875-017-0648-7.
- Gilbody S, Bower P, Whitty P. Costs and consequences of enhanced primary care for depression: systematic review of randomised economic evaluations. Br J Psychiatry 2006;189:297-308. https://doi.org/10.1192/bjp.bp.105.016006.
- Sampson SJ, Todd A, Walton N, Steele R, Webster L, Churchill R, et al. Pharmacy based management for depression in adults. Cochrane Database Syst Rev 2019;4. https://doi.org/10.1002/14651858.CD013299.
- Brown JVE, Walton N, Meader N, Todd A, Webster LA, Steele R, et al. Pharmacy-based management for depression in adults. Cochrane Database Syst Rev 2019;12. https://doi.org/10.1002/14651858.CD013299.pub2.
- Littlewood E, Ali S, Badenhorst J, Bailey D, Bambra C, Chew-Graham C, et al. Community Pharmacies Mood Intervention Study (CHEMIST): feasibility and external pilot randomised controlled trial protocol. Pilot Feasibility Stud 2019;5. https://doi.org/10.1186/s40814-019-0457-y.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Ritchie J, Spencer L. Qualitative Data Analysis for Applied Policy Research. London: Routledge; 1994.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- Department of Health and Social Care . Improving the Health and Well-Being of People With Long-Term Conditions. World Class Services for People With Long-Term Conditions – Information Tool for Commissioners 2010.
- NHS England . 2019/20/General/Medical/Services/(GMS)/Contract/Quality/and/Outcomes/Framework/(QOF) 2019.
- Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med 1997;12:439-45. https://doi.org/10.1046/j.1525-1497.1997.00076.x.
- National Institute for Care Excellence (NICE) . Management of Depression in Primary and Secondary Care 2009.
- American Psychiatric Association (APA) . Diagnostic and Statistical Manual of Mental Disorders 2000.
- World Health Organization (WHO) . ICD-10: International Statistical Classification of Diseases and Related Health Problems. 2004.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Overend K, Lewis H, Bailey D, Bosanquet K, Chew-Graham C, Ekers D, et al. CASPER plus (CollAborative care in Screen-Positive EldeRs with major depressive disorder): study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-451.
- Martell CR, Dimidjian S, Herman-Dunn R. Behavioral Activation for Depression: A Clinician’s Guide. New York, NY: Guilford Press; 2013.
- Pasterfield M, Bailey D, Hems D, McMillan D, Richards D, Gilbody S. Adapting manualized behavioural activation treatment for older adults with depression. Cogn Behav Ther 2014;7. https://doi.org/10.1017/S1754470X14000038.
- Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol 2005;44:227-39. https://doi.org/10.1348/014466505X29657.
- Page AC, Hooke GR, Morrison DL. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in depressed clinical samples. Br J Clin Psychol 2007;46:283-97. https://doi.org/10.1348/014466506X158996.
- Lovell K, Bower P, Gellatly J, Byford S, Bee P, McMillan D, et al. Clinical effectiveness, cost-effectiveness and acceptability of low-intensity interventions in the management of obsessive–compulsive disorder: the Obsessive–Compulsive Treatment Efficacy randomised controlled Trial (OCTET). Health Technol Assess 2017;21. https://doi.org/10.3310/hta21370.
- Manos RC, Kanter JW, Luo W. The behavioral activation for depression scale–short form: development and validation. Behav Ther 2011;42:726-39. https://doi.org/10.1016/j.beth.2011.04.004.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Gilbody S, Richards D, Barkham M. Diagnosing depression in primary care using self-completed instruments: UK validation of PHQ-9 and CORE-OM. Br J Gen Pract 2007;57:650-2.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258-66. https://doi.org/10.1097/00006842-200203000-00008.
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Glaser BG. The constant comparative method of qualitative analysis. Soc Probl 1965;12:436-45. https://doi.org/10.2307/798843.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Mulhern B, Mukuria C, Barkham M, Knapp M, Byford S, Soeteman D, et al. Using generic preference-based measures in mental health: psychometric validity of the EQ-5D and SF-6D. Br J Psychiatry 2014;205:236-43. https://doi.org/10.1192/bjp.bp.112.122283.
- Byford S, Harrington R, Torgerson D, Kerfoot M, Dyer E, Harrington V, et al. Cost-effectiveness analysis of a home-based social work intervention for children and adolescents who have deliberately poisoned themselves. Results of a randomised controlled trial. Br J Psychiatry 1999;174:56-62. https://doi.org/10.1192/bjp.174.1.56.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Chew-Graham CA, Kitchen CEW, Gascoyne S, Littlewood E, Coleman E, Bailey D, et al. The feasibility and acceptability of a brief psychological intervention for adults with long-term health conditions and subthreshold depression delivered via community pharmacies: a mixed methods evaluation – the Community Pharmacies Mood Intervention Study (CHEMIST). Pilot Feasibility Stud 2022;8. https://doi.org/10.1186/s40814-022-00992-7.
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f4913.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- Kraemer H, Wilson G, Fairburn CG, Agras W. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877-83. https://doi.org/10.1001/archpsyc.59.10.877.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017 18 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 30 October 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- May C, Rapley T, Mair FS, Treweek S, Murray E, Ballini L, et al. Normalization Process Theory On-Line Users’ Manual, Toolkit and Nomad Instrument n.d. www normalizationprocess.org (accessed 27 August 2020).
- May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0758-1.
- NHS . Pay Rates in Local Government n.d. www.healthcareers.nhs.uk/working-health/working-public-health/employers-public-health-staff/local-government/pay-rates-local-government (accessed 2 February 2020).
- University of York . Grades 1–8R n.d. www.york.ac.uk/admin/hr/pay-and-grading/pay-scales/grades-1-8/ (accessed 2 February 2020).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Community and Mental Health team, NHS Digital . Psychological Therapies: Annual Report on the Use of IAPT Services, England 2018–19 2019. https://files.digital.nhs.uk/1C/538E29/psych-ther-2018-19-ann-rep.pdf (accessed 3 February 2020).
- The Pharmaceutical Journal . Pharmacy Funding Cuts: The Story so Far n.d. www.pharmaceutical-journal.com/news-and-analysis/features/pharmacy-funding-cuts-the-story-so-far/20202223.article?firstPass=false (accessed 3 February 2020).
- Kennedy B. Everything You Need to Know About the Pharmacy Cuts n.d. www.chemistanddruggist.co.uk/feature/everything-you-wanted-know-about-funding-cuts (accessed 3 February 2020).
- Popp L, Schneider S. Attention placebo control in randomized controlled trials of psychosocial interventions: theory and practice. Trials 2015;16. https://doi.org/10.1186/s13063-015-0679-0.
Appendix 1 Non-validated baseline questionnaires (feasibility study)
Appendix 2 Non-validated 4-month follow-up questionnaire (feasibility study)
Appendix 3 Data collection schedule
| Data checked/obtained | Approach | Invitation and recruitment pack | Diagnostic interview | Baseline questionnaires | Randomisation | 4-month follow-up |
|---|---|---|---|---|---|---|
| Permission to contact | ✗ | |||||
| Two Whooley questions | ✗ | ✗ | ||||
| Consent/decline | ✗ | ✗ (re-checked) | ✗ (re-checked) | ✗ (re-checked) | ||
| Demographic questionnaire | ✗ | ✗ | ||||
| Physical health condition(s) | ✗ | ✗ | ||||
| PHQ-9 | ✗ | ✗ | ||||
| SF-12v2 | ✗ | ✗ | ||||
| EQ-5D | ✗ | ✗ | ||||
| GAD-7 | ✗ | ✗ | ||||
| PHQ-15 | ✗ | ✗ | ||||
| Bespoke resource use questionnaire | ✗ | ✗ | ||||
| MINI | ✗ |
Appendix 4 Non-validated baseline questionnaires (pilot randomised controlled trial)
Appendix 5 Non-validated 4-month follow-up questionnaire (pilot randomised controlled trial)
List of abbreviations
- AG
- advisory group
- BA
- behavioural activation
- BADS
- Behavioural Activation for Depression Scale
- BIS
- brief information sheet
- CASPER
- Collaborative Care for Screen Positive Elders
- CHEMIST
- Community Pharmacy Mood Intervention Study
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CP
- community pharmacy
- DASS
- Depression and Anxiety Stress Scale
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ESI
- enhanced support intervention
- GAD-7
- General Anxiety Disorder-7
- GP
- general practitioner
- HLP
- Health Living Pharmacy
- LTC
- long-term condition
- MINI
- Mini International Neuropsychiatric Interview
- MUR
- medicines use review
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- PHQ-9
- Patient Health Questionnaire-9
- PHQ-15
- Patient Health Questionnaire-15
- PIS
- patient information sheet
- PMR
- patient medical record
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SF-12v2
- Short Form-12 item, version 2
- SOP
- standard operating procedure
- TFA
- theoretical framework of acceptability
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
Notes
-
Enhanced support intervention (ESI) facilitators study information pack (qualitative interview – feasibility study)
-
Study information pack for general pharmacy staff – focus group (feasibility study)
-
Example topic guides-study participants, ESI facilitators and focus group
-
Example topic guide for GP qualitative interview (pilot RCT)
-
ESI facilitator/general pharmacy staff study information pack (qualitative interview – pilot RCT)
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/EKZE0617).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
