Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 13/164/51. The contractual start date was in September 2015. The final report began editorial review in January 2020 and was accepted for publication in November 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Stathi et al. This work was produced by Stathi et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Stathi et al.
Chapter 1 Background
Parts of this chapter have been adapted from Stathi et al. 1 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text includes minor additions and formatting changes to the original text.
Scientific background and explanation of rationale
Healthy ageing is defined as ‘the process of developing and maintaining the functional ability that enables wellbeing in older age’. 2 Functional ability comprises the intrinsic capacities, both mental and physical, that people can draw on, relevant environmental characteristics and demands, and how people respond to these demands.
With increasing age, there is a population-wide decline in physical function. 3–5 Frailty and associated comorbidities compromise quality of life for older adults and contribute major societal costs directly to people who live with frailty, to friends and family providing care and losing productivity, and to health-care and social care services. 6,7 The impact of this transition is further heightened by an ever-increasing ageing population in the UK (18.2% in 2017 over the age of 65 years, rising to 20.7% by 20278) that is also reflected worldwide. 9,10
There is strong evidence that physical activity has a positive impact, slowing or preventing disability in later life. 11–14 A fit and active older person has 36% lower risk of developing functional limitations and 38% lower risk of hip fracture than inactive older people. 15 In the UK-based OPAL PLUS cohort study,16 older people who undertook at least 25 minutes of moderate to vigorous physical activity (MVPA) per day at baseline received fewer prescriptions and were less likely to be admitted to hospital in an emergency 4–5 years later. 16 Despite these significant benefits, as people age they engage in less physical activity and spend more time being sedentary,13,17 with 31% of 65- to 74-year-olds reporting < 30 minutes of MVPA per week, rising to 53% of people aged ≥ 75 years. 5
This toxic mix of reduced physical activity leading to compromised physical function and increasing pressure on health-care and social care support services has shifted the focus towards supporting the maintenance of functional capacity in later life, with healthy ageing identified as a key public health priority. 18,19 Clinical trials have provided robust evidence that physical features of frailty, such as reduced muscular strength or endurance, can be reversed or their progression slowed by undertaking an appropriate exercise programme. 14,20,21 According to data from the Health Survey for England, 31% of women and 22% of men aged ≥ 65 years report needing help in the last month with one or more activities of daily living (ADLs), such as getting up and down stairs, dressing, getting around indoors or shopping. 5 These people are in transition from independence to frailty and have a great deal to gain if loss of function can be reversed and independence maintained.
Physical inactivity and mobility limitations in older people are more prevalent in socioeconomically deprived sectors of the population. 22,23 Ethnically diverse groups in the USA have a significantly greater risk of developing a range of physical and mental health problems than their white counterparts, and subsequently suffer higher rates of morbidity and premature mortality. 24,25 Self-reported data from the Health Survey for England and the Active Lives survey indicate that older (≥ 55 years) Bangladeshi, Pakistani and Indian adults in England are less likely than their white counterparts to meet physical activity guidelines. 26–28 Thus, exercise interventions that can successfully engage and retain inactive and ethnically diverse groups of older adults could contribute to the reduction of health and social inequalities.
Lifestyle Interventions and Independence for Elders (LIFE) was a landmark US study in the field of physical activity promotion in older adults. 21 LIFE was a multicentre randomised controlled trial (RCT) comparing the effects of a physical activity programme with a successful ageing educational programme in more than 1600 functionally impaired older persons, over an average follow-up period of 2.6 years. The intervention reduced the risk of developing major mobility disability (defined as the inability to complete a 400-metre walk test within 15 minutes) by 18% relative to the control group [hazard ratio (HR) 0.82, 95% confidence interval (CI) 0.69 to 0.98]. It also reduced the risk of persistent mobility disability by 28% (major mobility disability at consecutive time points) (HR 0.72, 95% CI 0.57 to 0.91). The intervention group maintained an increase of 40 minutes per week (95% CI 29 to 52 minutes; p < 0.001) in objectively assessed lifestyle intensity activity (≥ 760 counts/minute compared with the control group at 24 months of follow-up). These estimates are likely to be conservative because the study utilised an active control group that received a substantial health education/lifestyle intervention, including weekly workshops for 6 months and monthly sessions for a further 18 months. Being an efficacy trial, however, LIFE was highly resource intensive, and there was no post-intervention long-term follow-up. The challenge now is to build on this evidence base and develop affordable, scalable interventions that are suitable for delivery in specific contexts and demonstrate maintenance of effect in the long term.
Building on the findings of the LIFE RCT, we designed REtirement in ACTion (REACT), a pragmatic effectiveness trial to test whether or not the LIFE intervention could be adapted into an effective real-life, community programme for older adults at high risk of mobility limitations in the UK. To the best of our knowledge, REACT is the first large-scale UK-based study of its kind. If effective and cost-effective, this programme would offer important health-care and social care benefits, while sustaining health and independence among older adults.
Objectives and hypotheses
The REACT study aimed to test the effectiveness and cost-effectiveness of a community, group-based physical activity and behaviour maintenance intervention based on the US LIFE programme for reducing or reversing the progression of functional limitations in older people who are at high risk of mobility-related disability.
A nested substudy, led by the Wellcome Centre for Integrative Neuroimaging, University of Oxford, tested the hypothesis that the REACT intervention slows the rate of brain atrophy and cognitive function decline. Measures included a brief battery of tests (paper and pencil and computerised) to assess memory, attention and executive function; structural and functional brain magnetic resonance imaging (MRI) measures; and gait analysis for a subsample of participants. This substudy was funded by the National Institute for Health and Care Research (NIHR) Oxford Biomedical Centre, University of Oxford, and is reported elsewhere.
Primary hypothesis
The null hypothesis is that participants allocated to receive the REACT programme will have the same mobility-related limitations, as indicated by Short Physical Performance Battery (SPPB) score, at 24 months of follow-up as the control group.
Secondary hypotheses
The null hypothesis is that participants allocated to the REACT intervention will have the same levels of moderate-intensity physical activity, health-related quality of life, cognitive function, ability to perform the ADL, and mental and social well-being at 24 months as the control group.
Primary objectives
-
To adapt the LIFE intervention from the USA for delivery in UK community settings.
-
To conduct an internal pilot study to evaluate and optimise the feasibility and acceptability of the REACT intervention for older people and intervention providers and of the proposed trial methods across a diverse sample, spanning multiple ethnic groups and geographic areas varying in deprivation index.
-
To conduct a full-scale, pragmatic, multicentre RCT of the REACT intervention, with data collection at baseline and at 6, 12 and 24 months’ follow-up.
-
To explore how intervention effectiveness varies with deprivation index and ethnicity (i.e. to explore potential effects on health inequalities).
Secondary objectives
To compare intervention and control groups in terms of:
-
minutes of moderate-intensity physical activity, as measured by accelerometer data
-
sedentary time and breaks in sedentary time
-
self-reported physical activity
-
hand-grip strength of the dominant hand
-
performance on a brief test of cognitive function
-
the rate of brain atrophy and performance on more detailed tests of cognitive function and gait analysis tests (functional MRI substudy)
-
mental and social well-being, energy, sleep quality and pain
-
health-related quality of life
-
ADL scores.
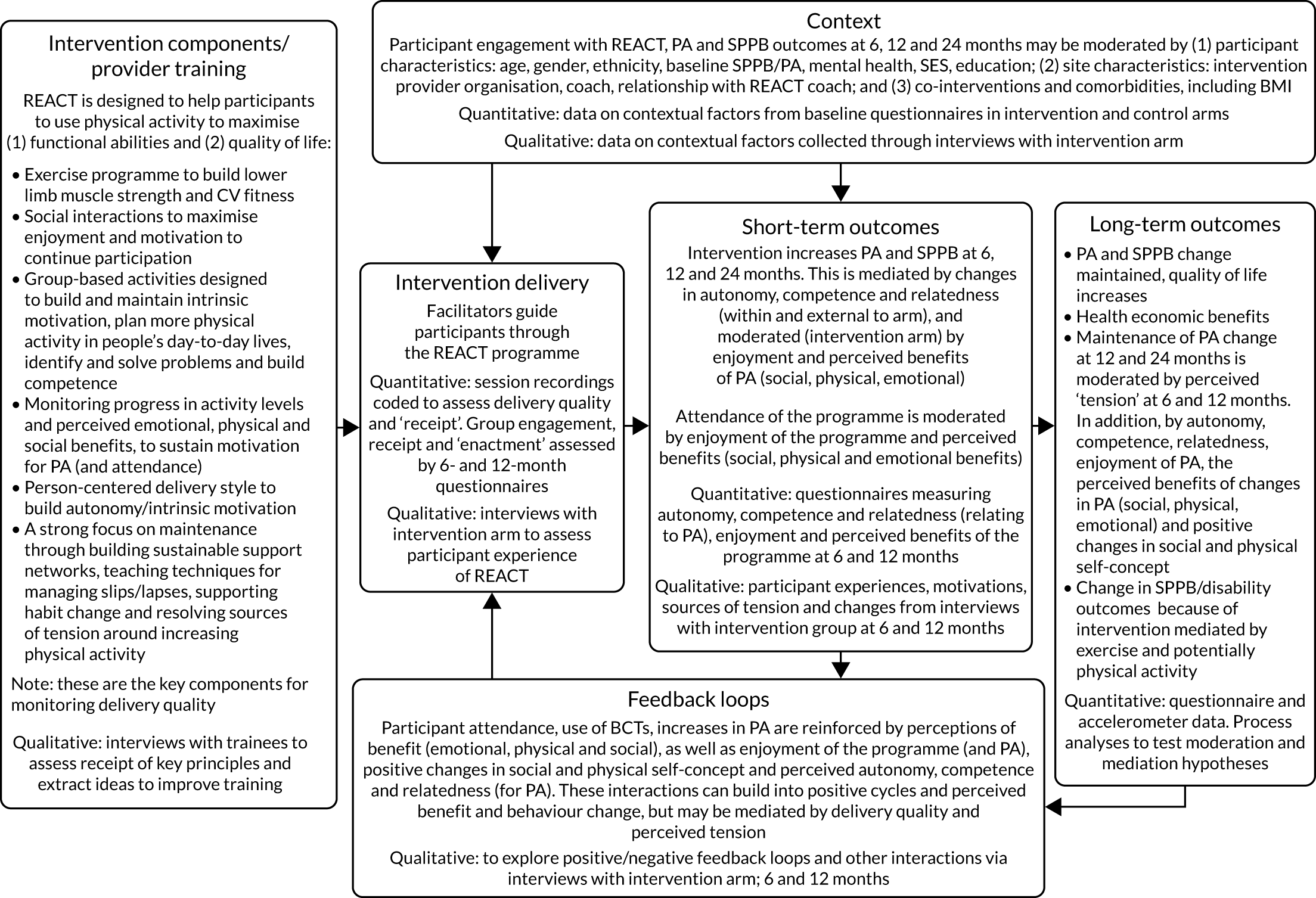
To conduct a full economic evaluation to estimate the incremental cost-effectiveness of the REACT intervention compared with the control group. To conduct a multimethod process evaluation to evaluate the feasibility of intervention implementation and inform future implementation and possible refinements of the intervention; to evaluate the intervention delivery and inform conclusions about intervention effectiveness; to investigate the proposed mechanisms of change outlined in the REACT logic model (Figure 1) and seek alternative explanations if this model is not supported; and to understand the role of context to inform whether or not and how the findings can be generalised.
FIGURE 1.
The REACT logic model and associated data collection. CV, cardiovascular; PA, physical activity.
Chapter 2 Trial design and methods
Parts of this chapter have been adapted from Stathi et al. 1 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text includes minor additions and formatting changes to the original text.
Parts of this chapter have been adapted from Withall et al. 29 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text includes minor additions and formatting changes to the original text.
Ethics and governance
The REACT study was reviewed and approved by the NHS South East Coast – Surrey Research Ethics Committee (15/LO/2082).
A substantial amendment (approved 7 March 2016) was submitted to change the SPPB inclusion criteria from 4–8 to 4–9; to change the randomisation process in the internal pilot from 1 : 1 to 2 : 1; and to streamline the recruitment process.
A second substantial amendment (approved 28 October 2016) was submitted to revert to 1 : 1 randomisation in the main trial without balancing the sample imbalance owing to 2 : 1 randomisation in the pilot; to make a change to the randomisation process to balance sites on key variables; to make a change to the process of informing MRI study participants of incidental findings; to include an additional case report form to be used with a REACT group funded by Bristol Ageing Better (Bristol, UK); to add questions to the Telephone Screening Questionnaire; to randomise couples together; and to make an addition to the consent form regarding audio-recording of REACT sessions. Finally, we refined the recruitment materials and processes after recommendations by participants during the internal pilot study.
A third substantial amendment (approved 2 October 2017) was submitted to add socioeconomic status (SES), marital status and caring-related questions to the Telephone Screening Form; and to enable the requesting of participants’ e-Frailty data from general practitioners (GPs) and also summary data from GP mailing databases to allow comparison of responders with non-responders.
A fourth substantial amendment (approved 9 January 2018) was submitted to gain approval for documentation relating to session leader interviews and to exclude planned hospitalisation from serious adverse event (SAE) reporting.
A fifth substantial amendment (approved 16 May 2018) was submitted because three co-applicants changed institution, and a small change was made allowing disclosure of a MRI scan to a GP at the participant’s request. No further protocol changes were made. All research and development approvals were sought and obtained for each site and amendment. The study is registered as a current RCT (ISRCTN45627165).
Public involvement and engagement
The REACT study was built on several years of multidisciplinary work by the study team aimed at understanding influences on the adoption and maintenance of physical activity in community-dwelling older adults. Our Avon Network for the Promotion of Active Ageing in the Community (AVONet) [Medical Research Council (MRC) Lifelong Health and Wellbeing – Collaborative Development Network; ref 90543] used focus groups and workshops with service providers, older people, international experts and service commissioners to assess the needs of older people and their communities for physical activity promotion. 30 The REACT study was considered by our AVONet service user, service provider and commissioner stakeholders to be suitable for delivery across a range of socioeconomic and cultural populations. The REACT protocol was developed based on this input. The Trial Management Group (TMG) was closely involved in the development of the study protocol, and all co-applicants, the trial manager and three people from our service user advisory group (research partners) formed part of that committee. One of the research partners also served on the Trial Steering Committee (TSC). The draft protocol was open to consultation by our service user representatives, our public health expert and members of community organisations. In addition, our Advisory Group (six members) reviewed REACT study materials prior to use.
Trial design
The REACT study was a multicentre, pragmatic, two-arm, single-blind, parallel-group RCT with an internal pilot phase that incorporated comprehensive process and economic evaluations. Following identification and recruitment, 777 participants who met the study inclusion criteria were randomised to either the intervention arm or the control arm. The internal pilot phase assessed the feasibility of recruitment methods (allowing for some refinement) and confirmed that the prespecified criteria for retention to the intervention sessions and the study were met, prior to progressing with the main trial. A nested substudy, led by the Wellcome Centre for Integrative Neuroimaging at the University of Oxford, employed MRI to test the hypothesis that the REACT intervention slows the rate of brain atrophy and decline in cognitive function. This substudy was funded by the NIHR Oxford Biomedical Centre, University of Oxford, and is reported elsewhere. 31 Outcome data were collected at baseline and 6, 12 and 24 months. The protocol of this study was published as an open access publication in 2018. 1
Changes to the trial design
A substantial amendment was accepted (7 March 2016) to change the SPPB inclusion criterion (described below) from scores 4–8 (inclusive) to 4–9 (inclusive) out of 12. This change was deemed to be appropriate to support the recruitment efforts and to adopt the same inclusion criterion as the US LIFE trial. 21
A further substantial amendment was accepted (28 October 2016) to (1) revert to 1 : 1 randomisation in the main trial without balancing the sample owing to 2 : 1 randomisation in the internal pilot study and (2) clarify the randomisation process regarding balancing sites on key variables [study site, age group, sex and initial functional ability (SPPB)].
These changes were discussed and agreed by the TMG and the TSC.
Participants
The eligibility criteria were intended to identify sedentary, community-dwelling, older people aged ≥ 65 years, who were not in full-time employment and had functional limitations (i.e. who were at risk of major mobility limitations), but who were still ambulatory (i.e. they could still walk). This was measured using a battery of objective physical function tests, the SPPB, a group of measures that combines the results of the gait speed, chair rise and balance tests:
-
gait speed test – a timed walk over 4 metres at normal walking pace (minimum score 0, maximum score 4)
-
chair rise test– time taken to stand up and sit down five times from a dining/kitchen-style chair, arms crossed across the chest (minimum score 0, maximum score 4)
-
balance test – unsupported balances for 10 seconds in three positions – feet side by side, feet in semi-tandem and feet in full tandem position (minimum score 0, maximum score 4).
Summed, these tests generate a physical function score from 0 to 12. Older adults with scores of 4–9 (inclusive) out of 12 were eligible to take part in REACT. This criterion was based on data showing that older adults with SPPB scores of ≤ 9 have substantially higher risk of major mobility disability 3 years later [odds ratio (OR) 8.3, 95% CI 3.3 to 20.67] than those with a score of 12. 21,32 Furthermore, the European Medicines Agency has published specific cut-off points for SPPB to identify three stages of frailty (< 7, frail; 8–9, pre-frail; and 10–12, fit/normal function), which support the REACT study inclusion criterion. 33
During recruitment, participants were informed that they would receive £15 shopping vouchers for attending each of the 6-, 12- and 24-month assessment sessions.
Eligibility screening
The eligibility of respondents was assessed in a three-step sequential screening process:
-
Initial self-selection. The participant approach letters and participant information sheet (PIS) clearly stated that we were recruiting people who were still able to carry out, but had some difficulty with, daily activities, such as walking, climbing stairs and getting out of a chair.
-
Phone-based screening. After gaining verbal consent, a preliminary phone screen checked inclusion and exclusion criteria that could be assessed by telephone, including a check on medical exclusion criteria. We excluded people who were unable to walk across a room, who were living in residential care, who were awaiting hip or knee surgery, who were receiving radiation therapy or chemotherapy, who had received recent heart or spinal surgery, or who had severe illness that would prevent participation, including severe arthritis, diagnosed dementia, severe kidney disease, unstable heart disease and severe psychiatric illness. Availability to attend intervention sessions was also checked. Participants who did not meet the eligibility criteria were thanked for their time and provided with the Age UK (London, UK)/NHS guide to healthy ageing and other sources of further advice and information. 34
-
Face-to-face screening sessions. Potentially eligible participants were invited to a group-based assessment session at a local community centre, at which they could ask questions about the study and were asked to give written informed consent. The SPPB gait speed test was conducted first, and those who failed to complete the 4-metre walk or did not meet the SPPB inclusion criterion (a score of 4–9 inclusive) were thanked for their time and provided with an information pack (as above). Participants who met the eligibility criteria were invited to take part in the remainder of the baseline assessments.
Full inclusion and exclusion criteria are summarised in Table 1.
| Criteria | Details |
|---|---|
| Inclusion criteria |
|
| Exclusion criteria |
|
| Temporary exclusion criteria |
|
Consent
Older adults who were willing to take part in REACT were asked to provide verbal informed consent at the beginning of the telephone screening call and written informed consent prior to commencement of the face-to-face screening sessions.
Verbal consent
At the beginning of the telephone screening interview, the researcher checked that the participant had read the PIS that had been mailed to them, summarised the study verbally and then offered the participant the chance to ask questions. Verbal consent to the telephone screening was then requested. If the participant failed to give consent, the telephone screen was not conducted. If a participant provided verbal consent, the assignment of a study identification (ID) number was taken as positive evidence that consent was obtained.
Written consent
The environment for consent
The setting in which written consent was obtained at the face-to-face screening session was as private as possible, so that participants could ask questions freely and without embarrassment. To avoid pressuring the participant, only one person associated with the study was present when the participant reviewed the consent form.
The consent process
The consent process involved a full explanation of the study given by the person taking consent [research assistant (RA) or other authorised researcher], with reference to the PIS that had been mailed to each participant prior to any of the face-to-face screening processes commencing. Potential participants were informed that they may, at any time, withdraw their consent to participate without giving a reason and without it affecting their relationship with their GP, the referring organisation and/or their future treatment and care. The PIS provided details of a contact point at which participants could obtain further information about the study. Following these discussions, people who were willing to participate were asked to complete, sign and date the study consent form, which was also be signed and dated by the person obtaining consent.
Capacity to consent
To be eligible for participation, participants had to have the capacity to give their own informed consent. If a member of the research team considered a participant to be incapable of understanding what was expected of them as a subject in the study, it was not permissible for informed consent to be obtained from a guardian. The study required daily responsibilities that could not be easily assumed by other people. In line with Health Research Authority guidance (www.hra-decisiontools.org.uk/consent/; accessed 13 October 2022), consent to participate in the REACT study was presumed to remain legally valid after loss of capacity (provided that the REACT protocol did not change significantly). In all cases, we took note of any signs of objection or distress from participants and consulted closely with them.
Study settings
The study was conducted in Bath, Bristol, Birmingham and Devon, UK, allowing recruitment of a socioeconomically and ethnically diverse sample, comprising participants from urban, rural and semirural locations.
A range of recruitment strategies to identify suitable participants was employed.
Primary care
General practices were invited to participate through their local Clinical Research Network and through the research teams’ existing networks in primary care. Where possible, we selected practices to maximise diversity in terms of ethnicity, SES and, in Devon, rurality.
Practice staff searched their electronic patient databases for potentially eligible patients using the trial entry criteria that were routinely coded in the database. Lists generated from the searches were further screened for suitability by a GP. GPs focused on screening for items that were not included/partially covered by the electronic searches (e.g. recent bereavement, awaiting knee or hip surgery). A recruitment pack consisting of a participant approach letter printed on the practice headed notepaper, a reply form and the PIS was sent to suitable patients, with a reply-paid envelope addressed to the local research team. GPs and practice nurses also offered the recruitment pack in surgery to patients they considered may be eligible.
Third-sector organisations
The principal investigators and RAs at each trial site engaged with third-sector and community-based organisations who worked with adults over the age of 65 years. Professionals in these services were asked to approach potentially eligible service users, provide a brief summary of the study and offer recruitment packs. Researchers also attended relevant community groups to present the study and distribute recruitment packs. Publicity materials were also made available through libraries, supermarkets, post offices and general practices.
Word of mouth and snowball sampling
To enhance recruitment, we used word of mouth and snowball sampling techniques and employed the assistance of bilingual community champions. This approach specifically focused on increasing engagement with ethnically diverse groups.
Local media
Recruitment was supported by a public relations campaign targeting local newspapers, magazines, radio and community events. This was supported by the public relations team at the University of Bath at no cost to the study.
Each REACT trial site tracked recruitment methods to determine the most successful recruitment strategy, in particular for recruiting from diverse ethnic groups.
Study intervention
Intervention arm
The intervention arm received a manualised 12-month exercise and behavioural maintenance programme designed for delivery in leisure/community centres by qualified exercise professionals. Sessions were organised as group activities, with up to 15 participants per group. A comprehensive guidance manual outlining the content and structure of the types of exercise to be delivered, methods for progression, safety considerations, methods for tailoring exercises and progression to individual capabilities and the behavioural maintenance sessions was distributed to the session leaders prior to their training event dedicated to the delivery of the REACT intervention. REACT session leaders were qualified to at least Register of Exercise Professionals (REPS) Level 3 (Exercise Referral Diploma or equivalent) and were experienced in delivering safe and effective exercise sessions to older adults. The 1-hour exercise sessions were delivered twice weekly for 12 weeks, reducing to once weekly for a further 40 weeks (64 sessions in total over 12 months) to groups of around 15 participants. Despite being delivered in a group setting, exercise programmes were personalised to each participant, based on their functional status and goals. Towards the end of each session, games-based activities of 15–20 minutes’ duration were delivered at ‘light to moderate’ intensities (points 8 to 13 according to the Rate of Perceived Exertion scale). 35 This individualised approach to exercise prescription enabled each participant to progress at their own pace.
The exercise sessions were each followed by 20 minutes of refreshments and socialising to promote session attendance and contribute to participants’ social well-being. After 9 weeks, the behavioural maintenance programme commenced as a 45-minute session delivered once per week (usually immediately following the exercise class). The maintenance sessions were designed to encourage a ‘social club’ atmosphere. They provided physical activity and health information and emphasised long-term maintenance of an active lifestyle, including the promotion of ongoing engagement in exercise classes, home-based exercise, neighbourhood walking and active travel. They incorporated behaviour change techniques (BCTs) derived from social cognitive theory (SCT), self-determination theory (SDT)22,23 and the skills for maintenance model. 24 These techniques included building intrinsic motivation; making realistic plans for sustainable activity; pre-empting and overcoming barriers; maximising enjoyment, social interaction and group identity; engaging external social support; and using self-monitoring and self-regulatory techniques to support the maintenance of behaviour change. From week 25 of the intervention, the behaviour maintenance programme reduced to one meeting per month for the remainder of the programme (six further meetings in total). If participants missed two consecutive sessions, REACT session leaders were asked to contact them by telephone to problem solve ways for them to re-engage with the programme.
REACT was delivered in three progressive phases (adoption, transition and maintenance), and established BCTs were used to enhance motivation, to make realistic plans for sustainable activity, to pre-empt and overcome barriers, to engage social support and to use self-monitoring and self-regulatory techniques to support the maintenance of behaviour change. 36
Start-up (adoption: weeks 1–8)
The purpose of this phase was to stimulate initial increases in physical activity and fitness, to reduce any anxieties or concerns about exercise, and to build confidence and a sense of attachment to the programme. Each participant received a 30-minute individualised, face-to-face introductory session with the session leader. This was used to personalise the programme for starting levels and progression. Two 60-minute group-based physical activity sessions per week, plus 15–20 minutes of social time, were then delivered by the session leader.
Build-up (transition: weeks 9–24)
A 45-minute interactive behavioural maintenance session delivered by session leaders was added to one of the two weekly sessions. These sessions used evidence-based, person-centred behaviour change strategies to build intrinsic motivation and self-efficacy. Sessions were designed to maximise enjoyment, social interaction and group identity. 37 They incorporated BCTs to address theoretical determinants of behaviour change relating to SCT, SDT38,39 and the skills for maintenance model:40,41 for example, to build intrinsic motivation, to make realistic plans, to pre-empt and overcome barriers, to maximise enjoyment, social interaction and group identity, to engage external social support and to use self-monitoring and self-regulatory techniques to support maintenance of behaviour change. A key focus was on exploring and planning transition to more daily lifestyle activities. After week 12, the exercise session frequency reduced to once per week, with an expectation that participants find an hour per week to exercise at home or in the neighbourhood or to attend a local physical activity session.
Taking charge (maintenance: weeks 25–52)
The maintenance stage focused further on home-based and neighbourhood-based activities, while continuing with a weekly centre-based physical activity session followed by a 20-minute social session. The 45-minute behavioural maintenance sessions reduced to once per month and focused on enacting participants’ action plans for physical activity outside the REACT programme. We encouraged groups to self-organise and to consider doing activities together beyond the scope of the study. Participants were informed about other local opportunities for physical activity in their community. They were also introduced to the REACT ambassador programme during the maintenance stage. This was a novel element whereby participants could contribute to the group as a co-ordinator. The REACT ambassadors’ main role was to sustain activities after the end of the intervention at 12 months by supporting ongoing group meetings and activities without adding to intervention costs, thereby supporting sustainability.
Control arm
After completion of baseline assessments, participants allocated to the control arm were given information regarding healthy ageing. They were invited to three 60- to 90-minute group sessions over the 2 years of the study. These consisted of presentations and discussion groups on various aspects of healthy ageing, such as healthy eating, living with dementia and volunteering. There was no physical activity content.
Intervention delivery
REACT session leaders were qualified to at least REPS Level 3 (Exercise Referral Diploma or equivalent) and were experienced in delivering safe and effective exercise sessions to older adults. Behavioural maintenance sessions were usually delivered by the same session leader but were occasionally delivered by other staff from the same organisation. The REACT co-applicants provided a 2-day intervention delivery training to session leaders, including detailed session plans to ensure consistency in and fidelity to programme delivery based on a written programme of materials and manuals.
Intervention fidelity
We included a range of the strategies outlined by the National Institutes of Health Behaviour Change Consortium to reinforce intervention fidelity. 42 We (1) ensured ‘design fidelity’ by building our intervention around a clear logic model (see Figure 1); (2) recruited REACT session leaders with appropriate skills and experience; (3) developed an accessible, standardised intervention manual; (4) implemented the standardised REACT session leader training programme; (5) trained more REACT session leaders than needed to accommodate illness or withdrawal; (6) monitored delivery fidelity by recording consultation meetings for a sample of three or four sessions per intervention provider and by applying a fidelity checklist; and (7) checked for intervention ‘receipt’ and ‘enactment’ of appropriate levels of physical activity outside the REACT sessions by checking participant understanding of the correct performance of exercises and regularly reviewing progress in the behavioural maintenance sessions. In addition, fidelity was enhanced by incorporating a gradual transition to daily activity within the structure of the REACT intervention (i.e. withdrawal of one session per week after 12 weeks, reduction of behavioural maintenance sessions to once per month after the first 6 months, with targeted planning of ongoing daily lifestyle physical activities around each transition).
Health economics
Full details of the REACT economic evaluation are given in Chapter 5, but, in summary, we used data collected during the trial to estimate the resource use and costs associated with the delivery of the intervention and the wider NHS, social care and participant-level resource use and costs over a 24-month follow-up. The primary economic outcome measure is the quality-adjusted life-year (QALY) gain, derived from participant-reported EuroQol-5 Dimensions, five-level version (EQ-5D-5L), data.
Given the long-term nature of the potential benefits from the REACT intervention, we conducted decision-analytic modelling to assess the longer-term (lifelong) consequences of the intervention compared with the control, including consequences in terms of health-care and social care costs.
Process evaluation
The REACT study follows the principles of the UK MRC guidance on process evaluation. 43 For full details of the process evaluation see Chapter 4; however, the aims and methods are briefly summarised below. The purpose of the process evaluation was to:
-
evaluate the feasibility of implementation, including barriers to and facilitators of implementation, to inform future implementation and possible refinements of the intervention
-
evaluate the quality and quantity of intervention delivery to inform conclusions about intervention effectiveness
-
investigate the proposed mechanisms of change outlined in the REACT logic model (see Figure 1) and seek alternative explanations if this model is not supported
-
understand the role of context to inform whether or not and how the findings can be generalised.
Methods used in the process evaluation included:
-
a mixed-methods assessment of intervention fidelity (quality of intervention delivery) using a checklist applied to audio-recordings of the REACT social education sessions
-
a qualitative analysis of semistructured interviews conducted with participants and staff providing the intervention
-
quantitative testing of hypotheses derived from the logic model, using study data on demographics and outcomes, as well as a set of questionnaires to measure processes of behaviour change proposed by the REACT logic model.
Sample size calculation
Power calculations for the primary outcome (SPPB score) at 24 months were based on the published definition of a clinically minimum meaningful change in SPPB score of 0.5 points,21,44 an expected SD for change in SPPB scores from baseline to 2 years of 2.2,21 a two-sided significance level of 0.05 and an expected cumulative loss to follow-up of 12.5% per year. To provide 90% power, this required a sample size of 384 participants per arm (768 in total).
To detect a change of 0.5 points with a SD of 2.0, assuming that loss to follow-up accumulates at 12.5% per year throughout REACT’s 2-year follow-up period, the required sample size was 384 participants per arm for 85% power using two-sided 5% significance. The REACT study, therefore, looked to recruit a total sample of 768 participants. This sample size also provides 90% power to detect a difference in moderate-intensity physical activity of 50 minutes per week [standard deviation (SD) 185 minutes per week] with 5% significance. A 2 : 1 randomisation process was applied in the internal pilot phase. A 1 : 1 randomisation process without rebalancing was applied in the main trial, resulting in an allocation ratio of 1.11 : 1. On the assumption that the effect size, the dropout rate and the significance level that we were interested in remained unchanged, our power reduced to 84.9% (from 85% power using two-sided 5% significance if the sample was rebalanced).
Interim analyses and stopping guidelines
The TSC, with advice from the Data Monitoring and Ethics Committee (DMEC), assessed the feasibility of the trial during the internal pilot phase, taking into account findings on the acceptability of trial procedures, intervention adherence and recruitment rates. After 6 months, recruitment data were reviewed by the TSC and, as outlined in the study protocol,1 when recruitment rates were found to be less than predicted,29 the research team took actions to increase them (increasing the number of people approached and adapting recruitment procedures). The TSC was happy with the impact on recruitment rates and recommended that we proceed to the main trial. Retention rates (proportion of people providing follow-up data) were also checked at 6 months. Receipt of strong negative feedback from the majority of either participants or intervention providers about the intervention or trial methods would have been considered a stopping criterion. No such negative feedback was received.
Randomisation
Randomisation type
When 30 eligible participants had been recruited (enough to form one study group), they were randomised to one of the two arms (intervention arm or control arm) using a secure, centralised web-based randomisation website built by Peninsula Clinical Trials Unit. Randomisation was carried out using a minimisation algorithm45,46 to balance arms in terms of study site (Bath/Bristol, Birmingham, Devon), age group (65–74 years or ≥ 75 years), sex and baseline functional ability (SPPB score of 4–7 or 8–9). This algorithm was built by the Peninsula Clinical Trials Unit and uses the method proposed by Taves45 and extended by Pocock and Simon. 46 It maintains a stochastic element by computing probabilities proportional to the existing imbalance at the point of randomisation.
Participants were allocated to a specific exercise group within 1 or 2 days of randomisation. This was carried out via a telephone call from a researcher and followed up with a confirmation letter. Session leaders were then provided with contact details for their group members and contacted them directly to arrange a one-to-one meeting prior to the sessions starting.
During the pilot phase, the randomisation ratio was 2 : 1 (intervention to control) to enable feasibility testing of intervention engagement and retention as early as possible. The main trial randomisation ratio was 1 : 1. The arms were not re-balanced following the pilot. Couples presenting together at the screening, with both people eligible and willing to be involved in the study, were randomised together to reduce contamination between study arms.
Randomisation: allocation concealment mechanism
To carry out randomisation, an authorised member of the research team accessed the randomisation website using unique username and password log-in details. The website required entry of patient’s initials, date of birth and stratification variables [study site, age group (65–74 years or ≥ 75 years), sex and baseline functional ability (SPPB score of 4–7 or 8–9)]. The randomisation website generated a unique study ID number for the participant when they were randomised.
Each REACT group consisted of 30 participants. In the event that more than 30 individuals (15 intervention, 15 controls) were recruited within a study site, a small waiting list was maintained. If an individual dropped out of the study (intervention or control arm) during the first 2 weeks, they were replaced by a randomised member of the waiting list, although the original participant was still followed up and included in the ITT analysis. This ensured that the group nature of the intervention was maintained. If a new group was subsequently started at the site, then the members of the waiting list were randomised into the new group.
Randomisation: implementation
Confirmation that randomisation had been carried out was communicated in a blinded fashion to local site staff and key members of the research team via e-mails automatically generated by the randomisation website.
The clinical trials unit sent the study ID numbers of intervention and control participants to the RA at the local site. The RA, who was not involved in collecting primary outcome data, telephoned participants to inform them of their allocation and sent them a confirmation letter using the contact details collected at the baseline assessment.
Letters to participants in the intervention arm advised participants of the date and venue of their REACT sessions.
Blinding
It is not possible to blind study participants to treatment allocation in behavioural intervention studies, and this is not a problem in pragmatic trial designs, which aim to estimate the benefits of the intervention over and above usual or standardised care. However, we took steps to ensure that data collectors, statisticians and the research team remained blinded to allocation, excluding one RA at each site. The chief investigator was unblinded when needed to allow assessment of SAEs. At follow-up data collection visits, participants were asked not to reveal which arm they were in.
Data were coded so that those undertaking the statistical and economic analyses were blinded. Given the study design, we did not anticipate a substantial risk of contamination (i.e. exposure of the control arm participants to the REACT intervention). However, as part of their briefing on entry to the study, participants in the intervention arm were asked not to share or discuss the content of the intervention sessions with any control arm participants whom they may be in touch with for the duration of the study. The possibility of contamination of control arm participants by REACT session leaders was minimised by giving them clear instructions not to provide intervention materials or information to any participants not assigned to the intervention arm. Attrition bias was minimised by having robust trial procedures to prevent data loss and also by analysing the data using an intention-to-treat (ITT) approach.
Case report forms did not contain any data that would enable the identification of the participant, so staff entering data remained blinded.
Unblinding
The DMEC undertook regular safety data reviews after recruitment began, and all SAEs were reported to it. The DMEC was responsible for identifying any need for unblinding and periodically reviewed unblinded safety data to determine patterns and trends of events, or to identify safety issues, which would not be apparent on an individual case basis.
Statistical analyses
The REACT analysis populations were as follows:
-
Population 0. The primary analysis was performed on an ITT basis including all participants consented and randomised.
-
Population 1. Analysis of all consented participants who completed ≥ 50% of the intervention (minimum required dose analysis).
-
Population 2. Analysis of all consented participants who completed ≥ 75% of the intervention (high adherers analysis).
There is no published consensus on what constitutes a minimum dose of attendance for behaviour change interventions. Our choice of 50% as a minimum/sufficient dose and 75% as a high dose was based on (1) attendance rates for other successful behaviour change interventions promoting physical activity range from 36%47 to 61%,21 and (2) expert opinion within the TMG.
Primary and secondary outcome data analysis
Analyses were prespecified in the published protocol. Primary outcome analysis was undertaken blinded to group allocation. Primary comparative analysis was undertaken using the principles of ITT with due emphasis placed on CIs. Using appropriate descriptive statistics, we assessed any imbalance between the trial arms at baseline and described the characteristics of participants. The comparison of primary interest was the difference between the intervention and the control arms in SPPB score at the 2-year follow-up (24 months after randomisation). Factors in the model comprised baseline age, sex and study site (Bath/Bristol, Devon or Birmingham). Baseline SPPB scores were added as a covariate in the model and, in addition, we adjusted the estimates for clustering by exercise group within the intervention arm, with control arm participants entered as individual groups following the method cited by Flight et al. 48 Analyses were conducted in Stata® SE version 15.0 (StataCorp LP, College Station, TX, USA). The randomisation stratification factors (site, age and sex) were entered into the analysis model as categorical variables.
Secondary outcome analyses were undertaken using the same approach as for the primary analysis (excluding the sensitivity analyses), using the baseline, 6-month, 12-month and 24-month follow-up data and linear mixed-regression models. Health-related quality of life, as assessed by EuroQol-5 Dimensions (EQ-5D), will be reported elsewhere as part of the health economic evaluation.
As an exploratory analysis, the effect of several predefined factors was further investigated and is presented. These included the stratification variables [age categories (65–74 years and ≥ 75 years), sex and study site (Bath/Bristol, Devon, Birmingham)], as well as comorbidity levels at baseline (none or one chronic medical conditions vs. two or more chronic medical conditions), socioeconomic subgroups (using education, home ownership and quintiles of area deprivation), history of falls (recorded fall or not during 6 months prior to baseline) and the uptake of any co-interventions during the 24-month study period. Health-related quality of life, as assessed by EQ-5D, is reported in Chapter 10.
Intervention costs were estimated by identifying key resources (programme co-ordination, session leader time and expenses, venue hire, equipment, consumables, and programme-specific training) and assigning values to the resources used (see Chapter 5). The data were collected by the REACT session leaders and trial manager.
Adjustment in the models
The intention was to adjust the models for the four stratification factors and the intervention group (clustered owing to the group nature of the intervention) and the intervention arm as follows: age group and sex were included as fixed effects, site was included as a random effect, baseline functional ability was included as a covariate, the intervention group was included as a random effect and the intervention arm (intervention or control) was included as a fixed factor. The intervention group effect is the effect due to the intervention being delivered to groups of individuals. In the control arm, the clusters are specified by the individual. 48
Subgroup analyses
To examine the association between dose and response, we conducted subgroup analyses comparing participants attending ≥ 50% and ≥ 75% of sessions with (all) controls (the three defined analysis populations above). Mirroring the primary analysis, variables representing age group, sex, baseline SPPB and exercise group (within the intervention arm) were entered into the model.
Missing data
We expected a relatively low level of dropout and missing data. Therefore, the primary analysis was undertaken without imputation of missing values. However, a comparison of baseline covariates between completers and non-completers was undertaken to assess the impact of dropouts on the results.
Multiple testing
The primary analysis (SPPB at 24 months in the ITT population) is a single test, and, therefore, adjustment for multiple testing was not considered to be appropriate.
The analyses of the secondary outcome measures and the primary outcome at other time points were considered as exploratory analyses, and, therefore, there was no adjustment to account for multiple testing. The results of these analyses were interpreted in the light of the potential for an increased risk of making a type 1 error.
Safety data
The safety population was all trial participants (see Chapter 3, Main outcome results, for the analysis population for the primary outcome).
All SAEs were reported regardless of relatedness to the trial. Non-SAEs (regardless of relatedness) were not reported. All reportable events were followed up until resolution, where possible, or until the end of the data collection period.
No formal comparisons between groups were made because the numbers of events were relatively small. Full details of SAEs are presented in Chapter 8.
Amendments to the statistical analysis plan
Following the provision of baseline scores, but prior to the primary analysis, concerns were raised by members of the TMG about the inclusion of the adjustment for clustering in the primary analysis. Given the group nature of the intervention, there were concerns that adjusting for the clustering (given the lack of clustering in the control arm) would reduce the apparent significance of the intervention effect. Two teleconference meetings were held between the TSC and the TMG: one on 8 January 2020 and one on 27 January 2020. This addendum reflects additions to the analysis following those discussions.
This addendum is an addition to the main REACT statistical analysis plan v5. The statistical analysis plan outlines the primary analysis, including (but not limited to) adjusting the primary analysis for the group nature of the delivery of the intervention in the intervention arm.
In the submitted proposal30 and the protocol paper,1 no consideration was given to the clustering in the intervention arm. Therefore, by adjusting for the clustering in the intervention arm in the analysis, there would be a potential reduction in the power of the study. It was also agreed that the clustering in the intervention arm is a potentially important aspect to the structure of the data and, therefore, not including this may result in an increased risk of making a type 1 error. The impact of this, potentially, would be to marginally increase the size of the standard errors associated with the treatment effect estimates. Flight et al. 48 provided three case studies suggesting that for moderate levels of intracluster correlation these are unlikely to affect the conclusions. However, this may be the case where a result is borderline significant or where there is a strong clustering effect. 48
Sensitivity analysis for the primary outcome data analysis
The structure of the study was such that participants in the intervention arm met in groups whereas participants in the control arm had minimal interaction. Therefore, in the primary analysis, the primary outcome (SPPB at 24 months) was modelled using the approach recommended by Flight et al. 48 for a partially clustered design; an analysis of covariance (ANCOVA) was undertaken, adjusting for the site as a fixed effect (Birmingham, Bath/Bristol, Devon) and group as a random effect (the groups within the intervention arm that met for exercise). Following this primary analysis, an additional sensitivity analysis was undertaken without the inclusion of the treatment groupings in the model. This analysis is in line with the analysis proposed in the protocol paper. 1
The sensitivity analysis was further enhanced by an investigation of the intracluster correlation at each data collection period once other factors (primarily site) had been accounted for. Investigating how the amount of clustering (represented by the intracluster correlation) changes from baseline to 6 months (peak intervention intensity) to 12 and then 24 months may help to indicate the extent to which the intervention itself was associated with any clustering effects (e.g. owing to treatment groups forming close activity-supporting bonds or having strong leadership that may cause the outcomes to cluster by intervention delivery group). This could aid in our interpretation of any discrepancies between effectiveness estimates produced by the clustered and the un-clustered analysis.
Statistical significance
For the hypothesis test, two-tailed p-values of ≤ 0.05 are considered statistically significant. Given that the additional sensitivity analyses outcomes here are exploratory in nature, there was no adjustment for multiple testing.
Model assumptions
For all methods outlined, underlying assumptions were checked using standard methods (e.g. residual plots). If assumptions were violated, alternative methods of analysis were sought. In particular, the underlying assumption of the linear impact of baseline covariates was assessed to ensure that baseline covariates were categorised and fitted as factors in the model. The assumption of the consistent clustering effect between the intervention and the control arm was evaluated.
Outcomes
Assessments
A full list of measures and time points at which outcome data were collected is presented in Appendix 1, Table 20. The person conducting the assessments checked for completion of questionnaires before participants left the assessment premises and made every effort to ensure that missed or spoiled questions were addressed.
Primary hypothesis
The primary hypothesis was that, compared with the participants in the control arm, participants allocated to receive the REACT programme would have significantly reduced mobility-related limitations, as indicated by SPPB score, at 24 months.
Secondary hypotheses
The secondary hypotheses were that, compared with the control arm, participants allocated to receive the REACT programme would have significantly increased their levels of moderate-intensity physical activity, health-related quality of life, cognitive function, ability to perform the ADL, and mental and social well-being at 24 months.
Primary outcome
The primary outcome was the SPPB score at 24 months.
Secondary outcomes
-
Change in minutes of moderate-intensity physical activity, as measured by wrist-worn accelerometers.
-
Sedentary time and breaks in sedentary time per day, as measured by wrist-worn accelerometers.
-
Self-reported physical activity [Physical Activity Scale for the Elderly (PASE questionnaire)]. 49
-
Self-reported adherence to government guidelines on muscle-strengthening activity, assessed by the Muscle-Strengthening Exercise Questionnaire.
-
Hand-grip strength of the dominant hand, as measured by a digital dynamometer.
-
Ageing Well Profile Social scale (six items) score. 50
-
ADL [EQ-5D, Short Form questionnaire-36 items (SF-36) and Mobility Assessment Tool-Short Form (MAT-SF)] score. 51–53
-
Simple processing speed, episodic memory, fluid intelligence, working memory, visual attention and complex processing speed as determined using the UK Biobank Healthy Minds Questionnaire. 54,55
-
Cognitive impairment, assessed by the Montreal Cognitive Assessment (MoCA). 56
-
The incremental cost-effectiveness of the REACT intervention (EQ-5D, SF-36, Health and Social Service Usage). 51,52
-
Pain [assessed using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)]. 57
-
Sleep quality, assessed by the Sleep Condition Indicator. 58
-
Medical history.
-
Falls Inventory. 59
Health economics outcomes
-
Intervention costs. Each component of resource use was estimated at aggregated and at site level. A mean cost per participant of delivering the intervention in REACT was estimated.
-
Health-care, social care and other resource use. Resource use is presented for baseline and the 6-, 12- and 24-month follow-up periods and for the adoption, transition and maintenance phases.
-
Effectiveness/health-related quality-of-life outcomes. SPPB score at 24 months and QALY data were derived from trial data on EQ-5D-5L, using the UK algorithms/tariffs. Derived health state utility values were used to estimate QALYs through application of standard area-under-the-curve methods using baseline and the 6-, 12- and 24-month assessments.
-
Discounting. Costs and health outcomes were discounted using a rate of 3.5%, as recommended by the National Institute for Health and Care Excellence (NICE).
-
For full details of the REACT health economic evaluation, see Chapter 5.
Process evaluation outcomes
-
Evaluation of the feasibility of implementation, including barriers to and facilitators of it, to inform future implementation and possible refinements of the intervention.
-
Evaluation of the quality and quantity of intervention delivery to inform conclusions about intervention effectiveness.
-
Investigation of the proposed mechanisms of change outlined in the REACT logic model and identification of alternative explanations if this model is not supported.
-
Evaluation of the role of context to inform whether or not and how the findings can be generalised.
For full details of the REACT process evaluation, see Chapter 4.
Changes to the study outcomes during the course of the study
There were no changes to the study outcomes during the course of the study.
Chapter 3 Results: randomised controlled trial
Parts of this chapter have been adapted from Stathi et al. 60 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Parts of this chapter have been adapted from Withall et al. 29 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text includes minor additions and formatting changes to the original text.
In this chapter, we report our findings for the REACT RCT, including recruitment and adherence data, participant baseline characteristics and quantitative study outcomes.
In line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting parallel-group randomised trials,61 we outline the flow of participants through the study and report baseline and outcome data for each trial group (Figure 2; see Table 3).
FIGURE 2.
The CONSORT flow diagram of participants through the REACT study. a, Loss to follow-up data are cumulative.

Participant flow
Between June 2016 and September 2017, 3116 people were telephone screened (of whom 1077 were not eligible and 825 declined to participate further) and 1214 attended for baseline screening. Of these, 804 were found to be eligible and 777 were randomised (intervention, n = 410; control, n = 367). Throughout the study, 39 couples or pairs of close friends who were both eligible were randomised together to minimise contamination between study arms. The number of participants included in the primary analysis at 24 months was 628 (80.8%): 334 (81.5%) in the intervention arm and 294 (80.1%) in the control arm. Figure 2 shows the flow of participants through the study.
Recruitment
The trial was successful in recruiting. In fact, slightly more participants were recruited than had been planned (target, n = 768; actual, n = 777). Between February 2016 and September 2017, we contacted 25,559 people (via 35 GP practices, community partners and a PR campaign). A total of 3116 people responded and were telephone screened by the local site RA, 1214 people were screened face to face at local community centres and 777 were randomised (intervention, n = 410; control, n = 367), slightly exceeding the recruitment goal of 768 participants. 29
Baseline data
Baseline characteristics were similar between the two study arms (Table 2). The mean age of the participants was 77.6 years (SD 6.8 years) and 66.2% were female. The majority of participants (95.1%) were Caucasian, 1.2% were Asian, 3.0% were African/Caribbean and 0.8% were of other/mixed ethnicity. The mean SPPB score was 7.37 (SD 1.56) and the mean body mass index (BMI) was 29.25 kg/m2 (SD 5.71 kg/m2). Just over half of participants were educated beyond secondary school level (n = 417; 53.7%) and the majority were overweight/obese (n = 588; 76.5%). The study aimed to broadly represent the diversity of deprivation and ethnicity for individuals over 65 years of age within the UK population. Comparisons of the REACT cohort and the population aged over 65 years in England and Wales are shown in Appendix 1, Table 21. 62–64 In total, 11.1% of REACT participants fell within quintile 1 (most deprived) of the Index of Multiple Deprivation (IMD), compared with 14.3% of the general UK population aged over 65 years. In quintile 2, these figures were 20.2% and 17.6%, respectively. In terms of ethnicity, REACT under-recruited Asian participants (2.6% in the UK population aged over 65 years, 1.2% in the study) but over-recruited African/Caribbean participants (1.3% in the UK population aged over 65 years, 3.0% in the study).
| Characteristic | Study arm | |
|---|---|---|
| Control (N = 367) | Intervention (N = 410) | |
| Demographic characteristics | ||
| Age (years), mean (SD); n | 77.3 (6.64); 367 | 77.8 (6.93); 410 |
| Sex, n (%) | ||
| Female (n = 514) | 240 (65.6) | 274 (66.7) |
| Male (n = 263) | 126 (34.4) | 137 (33.3) |
| Ethnicity, n (%) | ||
| Caucasian/white (n = 739) | 352 (96.17) | 387 (94.16) |
| African/Caribbean (n = 23) | 9 (2.46) | 14 (3.41) |
| Asian (n = 9) | 3 (0.32) | 6 (1.46) |
| Other/mixed (n = 6) | 2 (0.55) | 4 (0.97) |
| BMI (kg/m2), mean (SD); n | 29.34 (5.51); 363 | 29.20 (5.67); 404 |
| Cognitive impairment (MoCA), mean (SD); n | 24.29 (3.62); 354 | 24.45 (3.70); 399 |
| Highest education level, n (%) | ||
| Less than secondary school (n = 64) | 32 (8.74) | 32 (7.79) |
| Completed secondary school (n = 295) | 154 (42.08) | 141 (34.31) |
| Some college/vocational training (n = 206) | 89 (24.32) | 117 (28.47) |
| College or university degree (n = 162) | 72 (19.67) | 90 (21.90) |
| Graduate degree, or higher (n = 49) | 18 (4.92) | 31 (7.54) |
| IMD, n (%) | ||
| Quintile 1 (n = 86) | 43 (11.75) | 43 (10.46) |
| Quintile 2 (n = 157) | 73 (19.95) | 84 (20.44) |
| Quintile 3 (n = 159) | 70 (19.13) | 89 (21.65) |
| Quintile 4 (n = 156) | 73 (19.95) | 83 (20.19) |
| Quintile 5 (n = 219) | 107 (29.23) | 112 (27.25) |
| Caring responsibilities, n (%) | ||
| Yes (n = 86) | 37 (11.9) | 49 (14.4) |
| No (n = 564) | 273 (88.1) | 291 (85.6) |
| Marital status, n (%) | ||
| Married or living with partner (n = 334) | 158 (50.5) | 176 (51.8) |
| Widowed (n = 200) | 90 (28.8) | 110 (32.4) |
| Divorced/separated (n = 79) | 48 (15.3) | 31 (9.1) |
| Single and never married (n = 39) | 17 (5.4) | 22 (6.5) |
| Other (n = 1) | 1 (0.3) | |
| Home ownership, n (%) | ||
| Own home (n = 553) | 259 (83.0) | 294 (86.5) |
| Renting/other (n = 99) | 53 (17.0) | 46 (13.5) |
| Number of chronic illnesses, n (%) | ||
| None (n = 173) | 90 (25.0) | 83 (20.5) |
| One (n = 260) | 129 (35.8) | 131 (32.4) |
| Two or more (n = 331) | 141 (39.2) | 190 (47.0) |
| Outcomes | ||
| SPPB total score, mean (SD); n | 7.36 (1.54); 367 | 7.38 (1.58); 410 |
| Accelerometry | ||
| MVPA (minutes/day): time spent at > 100 milligravitational units in at least 10-minute bouts, mean (SD); n | 5.80 (8.62); 330 | 5.94 (8.91); 374 |
| Unbouted MVPA (minutes/day): all time spent at > 100 milligravitational units, mean (SD); n | 58.82 (32.18); 330 | 55.10 (29.86); 374 |
| Very low PA/sedentary time, excluding sleep (minutes/day), mean (SD); n | 804 (91.66); 318 | 804 (91.52); 362 |
| Breaks in sedentary time (n/day), mean (SD); n | 43.23 (13.40); 328 | 43.53 (13.36); 375 |
| Subjective PA (PASE), mean (SD); n | 119.90 (57.61); 359 | 112.33 (58.13); 400 |
| Muscle-strengthening exercise (MSEQ), mean (SD); n | 3.18 (2.12); 338 | 2.90 (2.01); 388 |
| Hand-grip strength (kg), mean (SD); n | 24.92 (8.66); 361 | 24.68 (8.49); 404 |
| Ageing Well Profile Social Wellbeing subscale, mean (SD); n | 23.92 (7.30); 347 | 23.91 (6.74); 387 |
| Sleep Condition Indicator, mean score (SD); n | 21.95 (7.90); 333 | 22.53 (7.55); 342 |
| Pain (WOMAC), mean score (SD); n | 10.12 (3.77); 351 | 9.73 (3.94); 399 |
| Loneliness, n/N (%) | 135/361 (37.2) | 135/403 (33.7) |
| SF-36, mean score (SD); n | ||
| Physical component | 30.01 (10.61); 392 | 29.70 (10.96); 353 |
| Mental component | 53.77 (8.66); 392 | 54.55 (8.33); 353 |
| MAT-SF, mean score (SD); n | 49.89 (8.88); 357 | 49.06 (9.75); 403 |
| UK Biobank Healthy Minds Questionnaire, mean score (SD); n | ||
| Simple processing speed | 866.92 (277.42); 337 | 865.70 (282.35); 383 |
| Fluid intelligence | 3.60 (1.70); 332 | 3.75 (1.59); 377 |
| Executive function | 59,849.78 (31,733.12); 254 | 61,269.89 (38,594.83); 283 |
| Working memory 1 | 4.29 (1.44); 335 | 4.37 (1.46); 382 |
| Working memory 2 | 14.09 (6.43); 336 | 13.73 (6.14); 383 |
| Episodic memory | 5.94 (4.80); 333 | 6.08 (4.29); 377 |
| Falls inventory | ||
| Number of falls in last 6 months, mean (SD); n | 0.72 (1.15); 359 | 0.69 (1.08); 401 |
| Fall-related injury in last 6 months, n/N (%) | 45/355 (12.5) | 56/399 (14.2) |
The proportion of Caucasian/white participants was slightly lower than in the general population (95.1% vs. 95.5%, respectively) while the proportions of other/mixed ethnicities were very similar (0.8% vs. 0.7%, respectively). In terms of sex, 45.6% of the over-65 years population of England and Wales are male, compared with the 33.85% of REACT participants. However, compared with the over-65 years population of England and Wales, the REACT cohort was skewed towards the older age ranges, where the proportion of females increases. 29
Main outcome results
At the 24-month follow-up, the mean SPPB score (adjusted for baseline SPPB, age, sex, study site and exercise group) was significantly higher in the intervention arm (mean 8.08, SD 2.87) than in the control arm (mean 7.59, SD 2.61), with an adjusted mean difference of 0.49 (95% CI 0.06 to 0.92; p = 0.014). Only one instance of unblinding was reported during the collection of data at 24 months.
The primary and secondary outcomes at 24 months are presented in Table 3, and all outcomes at 6 and 12 months are reported in Appendix 1, Tables 25 and 26. The SPPB score was significantly higher in the intervention arm than in the control arm at 6 months (adjusted mean difference 0.68 points, 95% CI 0.39 to 0.96 points; p < 0.001) and 12 months (adjusted mean difference 0.77 points, 95% CI 0.40 to 1.14 points; p < 0.001). Self-reported physical activity was significantly higher in the intervention arm than in the control arm at 6 months (adjusted mean difference in PASE score of 16.3 points, 95% CI 6.78 to 25.9 points; p = 0.001), 12 months (adjusted mean difference 10.8 points, 95% CI 3.18 to 18.5 points; p = 0.006) and 24 months (adjusted mean difference 10.7 points, 95% CI 2.62 to 18.8 points; p = 0.010). Self-reported engagement in muscle-strengthening exercise showed a similar pattern, with highly significant differences (p < 0.001) at all three follow-up time points.
| Study arm | Estimated mean difference (95% CI) | p-valuea | ||
|---|---|---|---|---|
| Control | Intervention | |||
| Primary outcome | ||||
| SPPB total score, mean (SD); n | 7.59 (2.61); 294 | 8.08 (2.87); 334 | 0.49 (0.06 to 0.92) | 0.014 |
| Secondary outcomes | ||||
| MVPA (minutes/day): time spent at > 100 milligravitational units in at least 10-minute bouts, mean (SD); n | 4.50 (6.61); 250 | 5.15 (5.99); 290 | 0.65 (–0.48 to 1.78) | 0.255 |
| MVPA (minutes/day): all time spent at > 100 milligravitational units, mean (SD); n | 48.76 (19.48); 250 | 51.22 (17.20); 290 | 2.46 (–0.52 to 5.44) | 0.105 |
| Sedentary time, excluding sleep (minutes/day), mean (SD); n | 798 (65.80); 249 | 804 (64.04); 287 | 6.43 (–4.81 to 17.67) | 0.259 |
| Breaks in sedentary time (n/day), mean (SD); n | 42.33 (13.54); 248 | 40.76 (13.21); 287 | –1.57 (–3.89 to 0.75) | 0.184 |
| Subjective PA (PASE), mean score (SD); n | 113.17 (52.10); 301 | 123.90 (49.79); 328 | 10.73 (2.62 to 18.84) | 0.010 |
| Muscle-strengthening exercise (MSEQ), mean (SD); n | 3.18 (1.88); 276 | 3.86 (2.30); 307 | 0.68 (0.33 to 1.02) | < 0.001 |
| Hand-grip strength (kg), mean (SD); n | 23.43 (4.08); 291 | 23.74 (3.86); 328 | 0.31 (–0.33 to 0.94) | 0.343 |
| Ageing Well Profile Social Well-being subscale, mean score (SD); n | 24.68 (5.85); 295 | 24.88 (7.07); 306 | 0.20 (–0.84 to 1.24) | 0.700 |
| Sleep Condition Indicator, mean score (SD); n | 21.97 (6.10); 285 | 22.50 (6.65); 311 | 0.53 (–0.49 to 1.54) | 0.306 |
| Pain (WOMAC), mean score (SD); n | 10.20 (3.28); 290 | 9.63 (3.95); 324 | –0.57 (–1.15 to 0.00) | 0.052 |
| Loneliness, n/N (%) | 107/300 (35.7) | 110/330 (33.3) | 0.037 (–0.064 to 0.074)b | 0.914 |
| SF-36, mean score (SD); n | ||||
| Physical component | 29.38 (9.39); 295 | 30.84 (10.04); 306 | 1.46 (–0.09 to 3.01) | 0.065 |
| Mental component | 54.73 (7.64); 295 | 54.33 (9.18); 306 | –0.40 (–1.78 to 0.98) | 0.563 |
| EQ–5D, mean (SD); n | 0.67 (0.16); 302 | 0.69 (0.16); 330 | 0.02 (–0.01 to 0.04) | 0.220 |
| MAT-SF, mean (SD); n | 47.96 (8.13); 289 | 49.99 (8.96); 319 | 2.03 (0.66 to 3.40) | 0.004 |
| UK Biobank Healthy Minds Questionnaire, mean score (SD); n | ||||
| Simple processing speed | 811.28 (240.15); 264 | 801.67 (246.72); 286 | –9.61 (–52.47 to 33.24) | 0.657 |
| Fluid intelligence | 4.03 (1.41); 262 | 4.19 (1.61); 282 | 0.16 (–0.11 to 0.43) | 0.234 |
| Executive function | 64,770.62 (38,677.48); 210 | 58,515.77 (35,648.79); 236 | –6254.85 (–13,498.22 to 988.52) | 0.090 |
| Working memory 1 | 4.59 (1.29); 263 | 4.46 (1.22); 282 | –0.13 (–0.35 to 0.06) | 0.260 |
| Working memory 2 | 14.27 (5.24); 264 | 14.62 (5.15); 285 | 0.36 (–0.56 to 1.28) | 0.439 |
| Episodic memory | 5.84 (4.19); 263 | 5.36 (6.85); 286 | –0.48 (–1.49 to 0.53) | 0.347 |
| Falls inventory | ||||
| Number of falls in last 6 months, mean (SD); n | 0.73 (1.05); 300 | 0.70 (1.05); 330 | –0.02 (–0.19 to 0.14) | 0.772 |
| Fall-related injury in last 6 months, n/N (%) | 51/297 (17.2) | 57/326 (17.5) | 0.3 (–5.92 to 6.46)b | 0.809 |
Accelerometer data indicated a significant difference favouring the intervention group at 12 months for total MVPA (adjusted mean difference 3.11 minutes per day, 95% CI 0.00 to 6.23 minutes; p = 0.05) and MVPA accumulated in bouts of at least 10 minutes (1.24 minutes per day, 95% CI 0.22 to 2.26; p = 0.018). This equates to a difference of 22 minutes per week of MVPA lasting ≤ 10 minutes. Significant differences favouring the intervention arm were also observed in the SF-36 physical component score (at 6 and 12 months), hand-grip strength (at 12 months) and the MAT-SF self-reported lower limb physical functioning scale (at 6, 12 and 24 months).
Numbers analysed
All data were analysed based on the participants’ originally assigned groups (ITT). The number of participants included in the analysis of each outcome measure is shown in the outcome tables (see Table 3; see Appendix 1, Tables 25–26).
Losses and exclusions
Over the 2-year measurement period, we had a relatively low level of withdrawals from the study. Only 14.3% (n = 111) of participants withdrew from the study: 59 from the intervention arm and 52 from the control arm. The reasons were personal choice (30.6%), health issues (25.6%), unknown reasons (18.9%), death (14.4%), family health issues (7.2%) and being excluded by the intervention delivery organisation (3.6%) (see Appendix 1, Table 22). Final 24-month data collection was completed in October 2019. Given that the number of missing data did not differ substantially between the intervention and the control arm, the primary analysis was undertaken without imputation of missing values.
Data for physical function (SPPB score) at 24 months (primary outcome) were available for 628 participants (80.8%): 334 (81.5%) participants in the intervention arm and 294 (80.1%) participants in the control arm. Compared with the predicted loss to follow-up of 12.5% per year (25% cumulative for two years), the actual loss to follow-up over the 2 years of the study was 19.2% (see Appendix 1, Table 23).
Programme adherence
Of the 410 participants allocated to the intervention arm, 16.1% did not engage with any of the intervention sessions (non-starters), 19.0% attended < 50% of the sessions offered, 20.2% attended 50–74% of the sessions offered and 44.6% attended ≥ 75% of the sessions offered. Among all participants allocated to the intervention arm (including the non-starters), the mean percentage of sessions attended was 56.8% (95% CI 53.6% to 60.1%). Among participants allocated to the intervention arm who engaged with the programme (starters only), this figure was 67.7% (95% CI 65.1% to 70.4%). An association between dose and response was observed (see Appendix 1, Table 28), with an adjusted mean SPPB difference of 0.64 (95% CI 0.23 to 1.05; p = 0.002) for those attending ≥ 50% of intervention sessions and 0.81 (95% CI 0.38 to 1.23; p < 0.001) for those attending ≥ 75% of intervention sessions.
Sensitivity analysis
Sensitivity analyses, including imputation of missing values and not adjusting for clustering by exercise group, did not significantly change the above results (see Appendix 1, Table 29). The intracluster correlation coefficient for SPPB scores relating to clustering by exercise group within the intervention arm was 0.02 (95% CI 0.0085 to 0.129). Subgroup analyses for age, education levels and SES (key inequality populations) or other characteristics found no significant interactions with study arm, indicating that the intervention worked equally well for all of the pre-identified subgroups (see Appendix 1, Table 29).
Health economic results
The full 12-month REACT programme, as delivered in the trial, was estimated to cost £9466 per group, an average of £622 per participant. For more details on health economic analysis, see Chapter 5.
Non-adherence to the protocol
As determined in the REACT statistical analysis plan, the following protocol violations were considered:
-
Enrolment protocol violations were considered to occur if a member of the research team failed to appropriately apply the study’s eligibility criteria, resulting in the enrolment of an inappropriate patient into the trial.
-
A randomisation protocol violation was defined as a technical or human error leading to the violation of the intended randomisation sequence or any attempts to subvert allocation concealment.
-
A study intervention protocol violation was defined as a delivery error (incorrect number of sessions delivered) in the study intervention attributable to members of the research team. The research team included members of the study co-ordinating centre, site investigators and research co-ordinators.
-
Data collection protocol violations encompassed errors in which the research team failed to comply with specific trial guidelines for data collection and/or outcome evaluation for avoidable reasons.
No type 3 or 4 violations occurred. One type 1 error occurred when a participant scored 3 on the SPPB (inclusion criterion: SPPB score of 4–9) but was mistakenly included in the trial and randomised to the control arm. Data for this participant were included in the analysis. Three type 2 errors occurred. Prior to a protocol change to allow close friends to be randomised together (to avoid contamination), two pairs of friends were randomised separately and allocated to different arms. After discussions with the TMG and the TSC, it was agreed to submit an ethics amendment for the protocol change and both pairs of friends were allowed to attend the intervention programme. There was also one erroneous allocation letter sent to a participant informing them that they were allocated to the control arm when, in fact, their allocation was to the intervention arm. In all cases, analysis was conducted on the basis of original allocation (ITT), and the flow diagram (see Figure 2) reflects this.
Harms
In the 56 months of the study, 93 events were classified as SAEs: 59 from the intervention arm and 34 from the control arm (see Appendix 1, Table 24). SAE data were collected when inviting participants to attend assessment sessions, at assessment sessions (6, 12 and 24 months), when inviting control arm participants to attend one of their three social and education sessions and via session leaders reporting SAEs among intervention participants. The somewhat larger number of SAEs reported for intervention arm participants is likely to be a result of the higher frequency of contact with this group by session leaders, providing increased opportunity for reporting illness, injury and hospitalisation.
In the early phases of the study, all hospitalisations were reported as SAEs, including hospitalisation for planned procedures. On 9 January 2018, a substantial ethics amendment was approved to exclude planned hospitalisation from SAE reporting. From that date, any adverse event or adverse reaction was regarded as serious if it:
-
resulted in death
-
was life-threatening
-
required non-elective hospitalisation, prolongation of existing hospitalisation or elective hospitalisation that may be related to taking part in the study
-
resulted in persistent or significant disability or incapacity.
In line with the REACT protocol, SAEs were reported within 24 hours to the chief investigator, the Data Management and Ethics Committee chairperson for consultation on the relatedness to the study, and the sponsor of the trial. Ninety of these SAEs were classified as events unrelated to the study and, therefore, were not reported for further consultation with the TSC. One case was related to the study, and two cases considered to possibly have been related to the study were investigated and deemed not to be related to the study. These were reported to the appropriate regulatory body, the sponsor of the REACT trial and the DMEC, as per trial protocol.
Discussion
In support of the primary hypothesis, older adults with mobility limitations who received the 12-month REACT programme experienced statistically and clinically significant improvements in lower limb physical function compared with control arm participants at the 24-month follow-up (12 months after the end of the intervention) and also at 6 and 12 months, indicating a sustained benefit over time. Higher intervention effects were associated with increased programme attendance.
The baseline SPPB scores were almost identical to the LIFE study population,21 enabling comparison. The observed difference in SPPB score of 0.49 at 24 months was three times larger than the between-group difference reported in the LIFE trial. In the LIFE trial, this smaller difference in SPPB was sufficient to reduce the subsequent risk of major mobility disability (defined as the objectively assessed inability to walk 400 metres) by 18% and the risk of persistent mobility disability (defined as two consecutive major mobility disability assessments or assessment of major mobility disability followed by death) by 28%. The ability to walk a distance of 400 metres strongly relates to maintenance of independent living and reduced risk of mortality. 65 These meaningful impacts from lower levels of SPPB change in the LIFE study suggest that the minimum clinically meaningful difference in SPPB may be considerably lower than the difference of 0.50 used in calculating our sample size. Indeed, other evidence suggests that changes in SPPB score of ≥ 0.28 are meaningful in frail or pre-frail older adults (SPPB score of 4–9). 66
At the completion of the intervention (12 months post baseline), significant differences were observed in SF-36 physical component score, MAT-SF, MVPA, self-reported physical activity and adherence to muscle-strengthening exercise, and hand-grip strength. These are consistent with the idea that the intervention increased engagement in muscle-strengthening, balance and endurance exercise that mediated the observed effects on physical functioning.
The strong association between session attendance and increased lower limb physical function (see Appendix 1, Table 28) also suggests that the intervention worked through engagement in the REACT programme. However, causality cannot be implied from this association and there may be other explanations; for example, older adults who became frailer owing to injuries or life events, or who did not feel they were benefiting from the intervention, may have been less likely to attend the intervention.
It is worth noting the methodological issue of how to choose cut-off points representing a ‘sufficient’ or minimum dose for behavioural interventions, and this study provides data that may be informative for future studies of group-based exercise interventions. Our data show that attending ≥ 50% of sessions was associated with a clinically meaningful effect on SPPB (0.64 points) and that a minimum of 75% attendance was associated with a considerably stronger effect (0.81 points).
The increase in MVPA (9 minutes per week bouted or 22 minutes per week unbouted) was small, although it is worth noting that any increase in MVPA has effects on health67 and these increases must be set against very low levels of initial MVPA in this sample of frail or pre-frail older people (the baseline level of bouted MVPA was just 41 minutes per week). The 2.6-point change in SF-36 physical component score was small (a clinically meaningful difference being cited as around 4 points),68,69 as was the change in hand-grip strength (0.8 kg compared with a clinically important difference of 5.0 kg). 70 This demonstrates that functional benefits of the REACT programme were specific to the focus of the exercise intervention programme (lower limb mobility) and did not generalise to upper body physical functioning.
At 24 months, of the secondary outcomes, only changes in self-reported physical activity, muscle-strengthening exercise and MAT-SF were sustained. Other differences found at 12 months were reduced (17 minutes per week of unbouted MVPA, 1.5 points in SF-36 physical component score) but fell below the level of significance. This may reflect deterioration of the effects on exercise behaviours over time, as well as a lack of statistical power to detect smaller differences.
To the best of our knowledge, the REACT trial is the first trial targeting physical function with a long-term (12-month) intervention and a 24-month follow-up in this population. It was a well-powered definitive study, being the largest of its kind conducted in the UK. It was a robustly designed and conducted study, with low attrition rates (19% at 24 months) and high adherence.
Although only 3% of those invited to take part were recruited, it should be noted that the REACT study invited everyone in our study area aged ≥ 65 years and then applied a two-stage screening process. Based on the screening data from the study, we estimate that over 80% of those invited were likely to be ineligible because their SPPB score was outside the target range or because they met other exclusion criteria. On this basis, the response rate among the eligible population was 17%. Although this still leaves some uncertainty around the generalisability of the findings, it is reassuring that the recruited sample was representative of the UK population aged over 65 years in terms of deprivation and ethnicity, except for an under-representation of South Asian older adults. 29,65
The main limitation was that, as with other studies of behavioural interventions, blinding of participants to the study arm was not possible, introducing the possibility of social desirability bias in patient-reported measures. However, the primary outcome here consisted of a battery of physical tests assessed by independent observers, with the data collectors blinded to study arm allocation. Given that the secondary outcome analyses were exploratory, there was no adjustment for multiple testing, and the significance of the analyses needs to be interpreted accordingly. A further limitation is that it was not possible to explore variation in outcomes by ethnicity owing to insufficient numbers of ethnic minority participants.
Measurement of physical activity using accelerometers is held to be a gold standard for large-scale field trials and superior to self-report questionnaires. However, in REACT we observed that, despite improved physical function, Muscle-Strengthening Exercise Questionnaire (MSEQ) and self-reported PASE scores, there were no meaningful improvements in accelerometer-measured physical activity over the course of the study. The lack of agreement between objectively measured and self-reported measures could be explained by (1) wrist-mounted accelerometers lacking the sensitivity to detect muscle-strengthening and balance exercise and (2) the fact that the MSEQ is designed to capture muscle-strengthening activity and the PASE includes an item that captures muscle-strengthening exercises and weights it heavily in its scoring algorithm. Furthermore, a focus on MVPA using absolute cut-off points derived from healthier populations may not be appropriate for a pre-frail older adult population whose capacity for aerobic physical activity is likely to be lower than that of healthier, younger adults. Further work is, therefore, needed to understand how valid measures of physical activity and of engagement in muscle-strengthening exercise/activities can be derived from accelerometer data, particularly in older adults.
The subgroup analyses suggested consistency of intervention effects across different subgroups of the population, including both sexes and people of different ages, education levels and SES (as assessed by area deprivation). This indicates that, if rolled out, the intervention has potential to reduce health inequalities, if targeted towards underserved populations.
Implications for practice and/or future research
Programmes such as REACT could help to sustain the health and independence of vulnerable older adults at risk of mobility limitations. The observed dose–response relationship supports the importance of group, multimodal physical activity at least once per week in the initial stage and then at least once per fortnight in the maintenance stage for achieving sustained clinically meaningful changes in physical function. This is a strong clinical and public health message to give to older adults in terms of defining the relatively low level of commitment required to maintain their lower limb physical function.
The REACT exercise intervention provides important evidence supporting the World Health Organization, US and UK physical activity recommendations for multimodal exercise in adults aged over 65 years.
Given that recruitment to this trial was challenging, further research is needed to identify a simple, sensitive and specific assessment process to identify older adults who are likely to benefit from this type of intervention (i.e. likely to have a SPPB score of 4–9). This will be useful both for future research and for implementation of the intervention in this population. Indeed, we hope to generate such a measure from further analyses of the REACT data set.
Future studies will need to focus specifically on people of black, Asian and other non-white ethnic backgrounds to examine the effectiveness of the REACT intervention in these populations, and to identify and address any barriers that might deter them from engaging with this programme.
Finally, research is needed to optimise the implementation of REACT at scale and further evaluate/extend its reach, effectiveness and cost-effectiveness. It may be possible and synergistic, for instance, to integrate the REACT intervention with existing mobility-related prevention and rehabilitation services.
In conclusion, among older adults at risk of mobility limitations, the REACT intervention prevented decline in physical function over a 24-month period. Contrary to the belief that older age comes with an inevitable decline in physical functioning, the REACT study shows that this decline can be slowed or even prevented with modest lifestyle changes.
Chapter 4 Process evaluation
Parts of this chapter have been reproduced from Stathi et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Parts of this chapter have been reproduced from Cross et al. 71 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
In this chapter, we report our mixed-methods process evaluation of the REACT RCT, based on MRC guidance on process evaluation43 and the REACT logic model (see Figure 1). This chapter reports the experiences of receiving and delivering the REACT intervention, aiming to identify modifications for future implementation; it explores:
-
whether or not the components of the intervention were delivered as intended
-
the mechanisms which influenced (1) engagement with the programme and (2) changes in lower limb physical functioning (SPPB).
Aims
The purposes of the process evaluation in the REACT trial were to:
-
explore participants’ and facilitators’ experiences of the intervention to identify possible refinements of the intervention for future implementation
-
evaluate the quality and quantity of intervention delivery to inform conclusions about intervention effectiveness
-
investigate proposed mechanisms of change outlined in the REACT logic model and seek alternative explanations if this model is not supported
-
understand the role of context (e.g. setting, area deprivation, ethnicity and sex) to inform whether or not and how the findings can be generalised.
The dose of the intervention delivered and the associations of outcomes with dose are reported in Chapter 3. Subgroup analyses exploring differential impacts of the intervention in different population subgroups are also reported in Chapter 3, but are combined with the data presented in this chapter to help to inform refinements of the REACT logic model.
The REACT logic model
The logic model for REACT is shown in Figure 1. It identifies:
-
the REACT intervention components and how they were intended to be delivered to participants
-
hypothesised mechanisms of action of the REACT intervention (the model incorporates a number of causal assumptions about the process by which the intervention was intended to effect change in health behaviours and outcomes)
-
hypothesised contextual variables that might moderate or mediate mechanisms of change in motivations and behaviour
-
hypothesised interactions between participation in the intervention, delivery quality, motivation, behaviour and outcomes.
Study 1: intervention fidelity
Parts of this section have been reproduced from Cross et al. 71 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Methods
Design
We conducted a mixed-methods assessment of intervention fidelity (quantity and quality of intervention delivery). This included collecting observational data on intervention exposure (dose) and participant characteristics, as described in Chapter 3. We also applied an intervention fidelity checklist to in vivo audio-recordings of a purposive sample of the REACT health behavioural maintenance programme (social education) sessions to generate a descriptive summary of delivery quality scores. Examples of good practice in the delivery of the health behavioural maintenance programme were also extracted from the audio data to inform recommendations for future programme delivery and training. Qualitative data (see Study 2: qualitative evaluation of intervention processes) added further context to these data and possible explanations for any deviations from the intended delivery processes.
We did not formally evaluate intervention fidelity with respect to delivery of the group exercise component of REACT. However, we did send observers to one exercise group per site to check that exercise delivery (1) included the intended range of exercises, (2) was individually tailored and (3) promoted progression of exercises. The observers reported that the exercise delivery was close or very close to what the exercise protocol (developed by Dr Ladlow, co-applicant and specialist in strength and conditioning) had specified in all cases.
Participants’ sampling
Audio-recordings of the REACT health behaviour maintenance group sessions were purposively sampled to include a diverse sample of sessions based on (1) intended inclusion of key BCTs in the session plan, (2) intervention provider (organisations responsible for delivering the intervention groups) and (3) key transition points in the intervention (where the intervention changed in frequency or focus). Key transition points and BCTs in the selected sessions are shown in Table 4. Following the internal pilot stage of the study, we asked all providers to record the nine selected sessions for each exercise group that they delivered the REACT intervention to (a total of 54 sessions). Participants in the recorded sessions were REACT intervention arm participants and REACT session leaders.
| Intervention BCT | Intervention weeks sampled | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 9a | 12 | 13b | 16 | 20 | 24c | 28 | 48d | 52e | |
| Person-centred delivery | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Facilitating enjoyment | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Monitoring progress | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Self-monitoring | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| Managing setbacks and problem-solving | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Action-planning and goal-setting | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Modelling | ✗ | ✗ | |||||||
| Promoting autonomy | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Supporting competence and self-efficacy | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Supporting relatedness | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
Measures
Fidelity scoring
We applied a ‘fidelity checklist’ to assess the delivery fidelity of the session audio-recordings. The 11-item checklist was developed by REACT intervention developers (CG and AS) and a postgraduate researcher (RC) to assess key components of the REACT logic model (see Figure 1) and to measure the extent to which REACT intervention facilitators delivered the intervention BCTs and delivery processes as intended (see Appendix 2, Table 31).
We used a 6-point Dreyfus scale72 to measure the session leaders’ adherence to the use of intended intervention BCTs and delivery processes, as well as the skill with which they were implemented. The response scale ranges from 0 (very poor/no delivery) to 5 (near-perfect/expert delivery) (see Appendix 2, Table 32).
Detailed scoring instructions can be found in Report Supplementary Material 1. As suggested by other studies,73,74 the coding was anchored to the key heuristic that a score of 3 was considered to represent ‘competent delivery’, that is delivery that was considered to be sufficient to deliver the intended behaviour change processes.
In an effort to reduce subjectivity in scoring, two coders – an expert coder (CG) and a postgraduate researcher (RC) – independently coded a sample of 10 sessions. If discrepancies in scoring between coders exceeded more than 1 point on the Likert scale, the sessions were discussed to produce a consensus about how to apply the scoring system. The remaining sessions were coded by the postgraduate researcher (RC).
Examples of good practice
When coding for intervention fidelity, the researchers (RC and CG) noted the time stamps in the recordings of examples of theorised and non-theorised intervention processes in practice, as well as examples of good and poor intervention delivery. This enabled extraction of examples of good or poor practice and of the intended delivery of specific intervention processes for future intervention training courses.
Analysis
Intervention dose and delivery quality
For analysis of the association of dose with the primary outcome, see Chapter 3.
Intervention fidelity
Fidelity checklist scores were summarised by calculating either a mean or a maximum score for each item across all coded sessions. Items representing delivery processes or change techniques that were intended to be delivered in every session (e.g. person-centred delivery, managing setbacks and problem-solving) were summarised with a mean score, whereas items representing processes or techniques that were intended to be delivered in only some of the sessions (e.g. self-monitoring and modelling) were summarised with a maximum score (see Appendix 2, Table 33).
A mean item score was then calculated for (1) each BCT, (2) each exercise group and (3) overall delivery fidelity (the mean of all checklist item scores for all exercise groups).
Examples of good and suboptimal practice
Examples of good and poor practice for each checklist item were transcribed and tabulated.
Results
Characteristics of the recorded sessions
From a sample of 54 requested audio-recordings, 25 (46%) were suitable for analysis. Missing data were attributable to equipment failure (n = 10), session leaders failing to record the session (n = 12), communication issues between researchers and session leaders (resulting in session leaders not recording sessions) (n = 5) or sound problems that led to poor quality audio-files (n = 2). Table 5 reports the characteristics of the sessions analysed.
| Intervention group | Intervention site | Intervention provider | Facilitator | Participants (n) | Sessions sampled (n) | Sessions suitable for analysis, n (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | F1 | 13 | 9 | 6 (67) |
| 2 | 1 | 2 | F2 | 15 | 9 | 4 (44) |
| 3 | 1 | 3 | F1 | 16 | 9 | 7 (78) |
| 4 | 2 | 4 | F3 | 15 | 9 | 2 (22) |
| 5 | 1 | 2 | F4 | 14 | 9 | 5 (56) |
| 6 | 3 | 5 | F5 | 3 | 9 | 1 (11) |
The audio-recordings revealed that the mean session length was 24.6 minutes (SD 16.7 minutes) rather than the intended 45 minutes.
Intervention delivery fidelity
The overall delivery fidelity for the intervention (the mean of the scores for each checklist item taken across all exercise groups) was 2.5 points (SD 0.45 points), indicating that, overall, intervention delivery fidelity was suboptimal (see Table 6). The mean fidelity scores broken down by group ranged from 2.4 to 2.9. However, one group (group 4) had consistently lower delivery fidelity scores, with a mean overall fidelity score of 1.7. The fidelity scores for each checklist item (aggregated across all delivery groups) are outlined in Table 6.
| Group | Facilitator ID | Item number | Overall score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Person-centred delivery | Facilitating enjoyment | Monitoring progress: acknowledge and review | Monitoring progress: eliciting benefits of PA | Self-monitoring | Managing setbacks and problem-solving | Action-planning and goal-setting | Modelling | Promoting autonomy | Supporting competency and self-efficacy | Supporting relatedness | |||
| 1 | F1 | 3.3 | 3.5 | 2.2 | 1.8 | 3.0 | 1.9 | 1.8 | 3.0 | 3.0 | 2.2 | 2.2 | 2.5 |
| 2 | F2 | 2.6 | 2.8 | 2.6 | 1.5 | 3.0 | 1.0 | 2.6 | 3.0 | 2.4 | 1.9 | 2.9 | 2.4 |
| 3 | F1 | 3.4 | 3.5 | 3.3 | 2.0 | 4.0 | 2.6 | 2.5 | 3.0 | 3.0 | 2.7 | 2.4 | 2.9 |
| 4 | F3 | 2.5 | 2.5 | 2.0 | 2.0 | 1.0 | 1.0 | 0 | 2.0 | 2.5 | 2.0 | 1.0 | 1.7 |
| 5 | F4 | 3.2 | 3.2 | 2.6 | 2.2 | 3.0 | 1.9 | 3.0 | 3.0 | 3.2 | 2.9 | 2.5 | 2.8 |
| 6 | F5 | 4.0 | 3.0 | 3.5 | 2.5 | 3.0 | 3.0 | 3.0 | 0 | 4.0 | 2.5 | 2.0 | 2.8 |
| Mean item score | 3.2 | 3.1 | 2.7 | 2.0 | 2.8 | 1.9 | 2.2 | 2.3 | 3.0 | 2.4 | 2.2 | 2.5 | |
| Standard deviation | 0.55 | 0.39 | 0.59 | 0.34 | 0.98 | 0.81 | 1.14 | 1.21 | 0.57 | 0.39 | 0.65 | 0.46 | |
Examples of good and suboptimal practice
Some examples of both good practice and practices requiring improvement in delivery of each BCT are provided in Appendix 2, Table 34.
Discussion
Summary of findings
The delivery of the exercise component of REACT was considered to be good or very good across all sites. However, the overall score for intervention delivery fidelity (2.5, SD 0.45) indicated that, on average, across the sample the delivery of the behaviour maintenance sessions was suboptimal. There were numerous examples of good practice, but also numerous inconsistencies and examples of practice that contradicted the intended delivery model. There was considerable variation in delivery fidelity between intervention components (BCTs and delivery processes), between session leaders and between intervention groups. Key areas identified for future improvement were monitoring progress/eliciting benefits of physical activity (mean score of 2.0), action-planning and goal-setting (mean score of 2.2), modelling (mean score of 2.3), supporting competence and self-efficacy (mean score of 2.2), supporting relatedness (mean score of 2.3) and managing setbacks and problem-solving (mean score of 1.9).
Relation to other literature and possible explanations
The current study adds to an emerging body of work on intervention fidelity. 73–76 It is consistent with this evidence in finding that the quality of delivery of complex interventions varies considerably between session leaders and from group to group. This illustrates the importance of ensuring consistency of delivery in group-based interventions, as poor facilitation in one group or centre could undermine effectiveness for multiple participants (15 per group in the case of REACT). High-quality training and quality assurance processes may, therefore, be crucial to ensuring that the effectiveness of the intervention is maintained in transitioning from the context of a research study to wider-scale community-based implementation. This might, for example, involve rating of delivery fidelity for each trainee post training (by independent observation or self-rating), performance monitoring or other methods for identifying further training needs.
The fact that the REACT intervention generated clinically meaningful changes in SPPB at both 12 and 24 months (see Chapter 3) suggests that, despite scope for improvements in delivery, the intervention still worked. This apparent discrepancy may reflect one or more of several underlying phenomena:
-
The approach to rating delivery fidelity may have been overly conservative (i.e. the delivery was actually ‘adequate’ and so merited a mean score of at least 3.0). However, the authors who reviewed the session delivery are confident that there is clear scope for improvement in delivery of the behaviour maintenance sessions.
-
The intervention worked by processes beyond those that were specified in the REACT logic model. However, although some additional processes were identified in the qualitative process evaluation (see Study 2: qualitative evaluation of intervention processes), the logic model was broadly supported.
-
It may be the case that participants who were more ready to engage and had more intrinsic capacity to overcome the shortcomings of the programme (i.e. to succeed despite suboptimal delivery) did particularly well, lifting the group mean effect to the level of significance. The qualitative data suggest that this may be a possibility and that more individual tailoring could help to engage and benefit a larger proportion of the participants.
-
The groups of participants may have had sufficient mutual resources within the group to self-generate some of the intended intervention processes, such as forming a strong sense of relatedness or social identity around the idea of doing exercise together, mutually supporting motivation and helping each other to problem-solve.
-
It may be that the shortcomings in the delivery of the health behaviour maintenance sessions have more effect on longer-term outcomes (i.e. they affect maintenance more than short-term changes in physical functioning). This might help to explain the decline in intervention effectiveness from 12 to 24 months (see Appendix 1, Tables 26–28). Given that some of the lower-scoring checklist items related to self-regulatory processes, such as managing setbacks, problem-solving, reinforcing benefits and planning future actions, this idea seems plausible. This implies a need to focus more on implementing these and other maintenance-focused behaviour change processes in future implementations of REACT.
-
The health behaviour maintenance sessions may not be an important part of the REACT intervention, compared with the structured exercise classes. However, this seems unlikely as the evidence base for the long-term effects on physical functioning of structured exercise programmes delivered without behavioural support is poor.
In relation to item (e) above, a systematic review and meta-regression of physical interventions in older adults suggested that some self-regulation techniques may not be acceptable to older adults. 76 This may be because older people are less likely to be concerned with attaining a particular level of physical activity and more concerned with the enjoyment and social connectedness that can be derived. 77–80 Hence, the poor delivery of self-regulation techniques may to some extent reflect resistance to such techniques from the participants, which the session leaders responded to by downplaying these elements of the intervention. Participant ‘pushback’ has been cited as a factor in lower delivery fidelity for physical activity promotion in at least one other behavioural intervention. 74
Scores were low not only for self-regulation, but also for the social processes of supporting relatedness and modelling. Hence, important elements of the intervention’s underlying theories (SDT and SCT) were not proactively delivered by session leaders. Despite this, it may be the case that participants gained significant motivation from social interactions which developed spontaneously as a result of the group setting [as suggested in item (d) above]. This process is confirmed as being present in several groups by the qualitative process evaluation (see Study 2: qualitative evaluation of intervention processes). Encouraging the development of positive intra-group dynamics is also suggested as a key intervention process in other literature81–83 and a recent framework of processes for the delivery of group-based intervention. 84
The discrepancy between planned session length (45 minutes) and mean session length determined from the audio-recordings (24.6 minutes, SD 16.74 minutes) is also an issue of concern, suggesting that workload pressure on the provider staff may have prevented delivery of the sessions as intended A further possible explanation for the variations between session leaders in fidelity scores may be differences in interest or ‘buy-in’ between session leaders.
Strengths and limitations
Assessing intervention fidelity using coding of audio-recorded intervention delivery sessions is considered a gold-standard method. 42 Although time-consuming and labour intensive, this method allowed direct observation of intervention delivery and an assessment that was specifically tailored to the REACT intervention and its associated logic model. The fidelity scores along with the examples of good practice extracted from the session recordings provide direct feedback to the intervention designers about the ways in which the intervention and its training course can be improved in the future. Furthermore, scoring was based on a validated response scale designed for coding the acquisition of skills and reliability. 72 This was enhanced by using independent coders for the first 10 sessions to calibrate the coding and minimise subjective bias, as well as sampling of recordings across a diverse range of intervention components, intervention sites, session leaders and intervention providers.
However, several limitations need to be acknowledged. The data came from a relatively small sample of participants (around 90 of the 410 intervention arm participants). There is a strong potential for sampling bias, given that we were able to score fidelity for only 25 of our intended 54 sessions. This may have led to overestimation of intervention fidelity, assuming that recordings were more likely to be missing at sites at which performance was low. Furthermore, the rating approach used was unavoidably subjective, so there is no definitive way to ensure that a score of 3 represents ‘adequate’ delivery. Despite this, the raters were confident that there was clear scope for improvement in the delivery of health behaviour maintenance programme.
Implications for practice/future research directions
The variations observed have implications for intervention design, training and implementation, as well as for the specific refinement of the REACT intervention:
-
Both participants and session leaders should be involved in the refinement/adaptation of the REACT health behaviour maintenance programme, the REACT training course, and strategies for translation of theoretical constructs and BCTs into deliverable and acceptable intervention components.
-
Refinement of the intervention should focus on improving the delivery of intervention techniques and processes identified as having lower fidelity in this study (i.e. monitoring progress/eliciting benefits of physical activity, action-planning and goal-setting, modelling, supporting competence and self-efficacy, supporting relatedness and managing setbacks/problem-solving).
-
Refinement of the intervention should include ideas on promoting intragroup support for the delivery of intended intervention processes.
-
The examples of good and suboptimal practice may be helpful in constructing future REACT training materials.
-
For implementation, future training of REACT session leaders should invite the session leaders who have been identified (using the checklist developed here or an adapted version) as delivering with competent or high delivery fidelity.
-
For implementation, it may be helpful to consider what organisation-level governance or performance monitoring systems might support high-quality delivery of the REACT intervention. This might include incentives for, or monitoring of, intervention sessions to ensure that sufficient time is given to the social education sessions.
However, there is debate concerning whether perfect fidelity is feasible or even desirable. 85 Strict adherence to protocol does not always account for the individual needs of the participants and the context in which the delivery takes place. The intended intervention components and delivery processes need to be considered with reflection on the need for individual tailoring, the receptiveness of participants and session leaders to the intended techniques, and the feasibility of delivering those components in the implementation setting, which may be resource poor in terms of time and facilitator training.
Future research might seek to explore why some BCTs and intervention delivery processes were not delivered as well as others, particularly self-regulation techniques and techniques to build social identity and/or relatedness.
Conclusions
Delivery of the health behaviour maintenance part of the REACT intervention was suboptimal, with considerable scope for improvement in the delivery of both self-regulation processes and social/relatedness-building processes. However, this may have been mitigated by mutual support and self-delivery of some of the intended processes within the groups during the exercise part of the REACT intervention. Two encouraging conclusions that can be drawn are that (1) the REACT intervention can work even with lower than intended delivery quality and (2) there is scope for improvement of delivery quality, which may lead to increased effectiveness and better maintenance of effects.
This study also highlighted the importance of assessing fidelity in the evaluation of complex behavioural interventions and the value that this adds in (1) helping to explain research outcomes, (2) understanding the theoretical underpinnings of health behaviour change interventions and (3) identifying ways to refine interventions and their training courses for future implementation (and further evaluation).
Study 2: qualitative evaluation of intervention processes
Design
We conducted a qualitative study to evaluate the effectiveness of intervention processes and how the REACT programme was received by participants and service providers. This integrated data from:
-
51 semistructured, longitudinal individual interviews at 6, 12 and 24 months with a purposive sample of participants in the intervention arm (designed to sample diversity in terms of SPPB scores, session attendance, ethnicity, age, sex, service provider and site location)
-
interviews with a sample of session leaders after intervention completion.
For sampling purposes, SPPB scores of 8 and 9 were classified as pre-frail (high SPPB score) and scores of 4–7 were classified as frail (low SPPB score). Attendance was classified as high if participants attended ≥ 50% of the intervention sessions and low if they attended < 50% of the intervention sessions.
Research questions
-
Research question 1: why did older adults engage with the REACT intervention and what were the perceived benefits of this?
-
Research question 2: what were the factors (barriers and enablers) associated with adherence to REACT intervention sessions and to daily physical activity outside the REACT sessions?
-
Research question 3: what was the apparent mechanism of physical activity behaviour change for older adults participating in REACT, as observed through participants’, session leaders’ and organisation providers’ experiences of receiving/delivering the intervention, and how did this compare to the mechanisms proposed in the REACT logic model?
-
Research question 4: was the intervention delivered as planned?
-
Research question 5: what changes to the programme were recommended for future delivery of REACT?
Methods
Sampling: participants
Using stratified purposive sampling strategy, we selected a diverse sample of participants with a wide range of experience. This provided rich data to explore factors associated with adherence to REACT, the mechanisms of change proposed by the logic model and factors that may be associated with variation in intervention outcomes. 86 Participants were stratified and assigned to one of four groups creating a 2 × 2 matrix of SPPB status and 3-month programme attendance per intervention group.
Sampling: session leaders
All session leaders and organisation providers involved in the delivery of the REACT programme were invited to participate in focus groups after the completion of the 12-month intervention for the groups that they were leading.
Data collection: participant interviews
A semistructured topic guide was developed and piloted with the REACT service user advisory group prior to use. The topic guide was divided into four sections, for 6-, 12- and 24-month interviews:
-
factors associated with REACT intervention effectiveness, attendance and adherence to a daily physical activity
-
participant experiences of barriers to and enablers of participation in the REACT intervention and daily physical activity
-
impact of baseline factors, such as physical activity levels, physical function (SPPB), physical activity motivations and past physical activity behaviours, on attendance of the REACT intervention and/or adherence to daily physical activity
-
observed mechanisms of behaviour change for older adults participating in REACT compared with the mechanisms proposed in the REACT logic model.
Data collection: session leader interviews
A semistructured topic guide was developed and divided into four sections:
-
reasons for and personal benefits from participating in REACT
-
evaluation of the training programme, the structure and content of exercise and behavioural maintenance sessions and suggestions for future delivery
-
impact of REACT on physical function and well-being of participants
-
strengths and weaknesses of the programme.
Data collection: procedures and informed consent
Individual interviews were conducted with participants, and a combination of individual interviews and focus groups were conducted with session leaders and providers. REACT participants were recruited following purposive sampling to achieve diversity in ethnicity, sex, variation in SPPB score and adherence at 3 and 6 months (for 6 and 12 month interviews, respectively). Interviews with participants were also conducted at 24 months to examine reasons for maintenance or non-maintenance of physical activity and physical function, with purposive sampling based on the 24-month SPPB scores.
At 6 months, 12 semistructured face-to-face interviews were conducted in participants’ homes and five were conducted in community centres. At 12 months, 10 interviews were conducted at home and five were conducted at community centres. At 24 months, 19 interviews were conducted at participants’ homes. Twelve session leaders agreed to participate in interviews and group discussions (Bath/Bristol, n = 4; Birmingham, n = 4; Devon, n = 4), while three had left their organisations and were uncontactable. One research team member who had delivered social and education sessions at three groups and two members of staff from one provider organisation were also interviewed. Interviews were recorded using an Olympus VN-741PC digital recorder (Olympus Vietnam Ltd, Long Thành, Vietnam). The average interview lasted approximately 48 minutes (range 22–89 minutes). Informed consent for interview was obtained from participants when they were recruited to the study. Verbal consent was also obtained and recorded at the beginning of each interview. Participants were reminded that their interview would be confidential and that they could withdraw from the interview at any time without any repercussions. No participants declined to participate at that stage.
Data analysis: participant interviews
The MRC process evaluation framework was used to explore and understand how the REACT programme was implemented, the causal mechanisms involved and the contextual factors at play that link intervention theory to intervention outcomes. 43 Qualitative data from 6-, 12- and 24-month interviews were used to explore (1) participant interactions with the REACT intervention (analysed using framework analysis87) and (2) the accuracy and validity of the REACT logic model. The analysis aimed to capture individual narratives/within-person processes of change, as well as to draw out common themes. Emergent themes were compared and contrasted with the theorised processes of change specified in the logic model. Processes of engagement with the intervention were also explored. Using data collected at 12 and 24 months, factors influencing the maintenance of physical activity/exercise were assessed and linked to participant responses at 6 months. This allowed a qualitative description of participants’ experiences, potential pathways and barriers to adoption, and maintenance of active lifestyle to be evaluated at three time points. Techniques to enhance the trustworthiness of the analysis included cross-tabulation, negative case analysis and hypothesis testing. 88
Data analysis: session leader interviews and focus groups
Data from both the individual interviews and the focus group audio-recordings were transcribed verbatim. Data analyses were conducted by members of the research team. Themes were predetermined based on the process evaluation research protocol guide (https://www.fundingawards.nihr.ac.uk/award/13/164/51; accessed 17 October 2022).
Data trustworthiness
To assure rigour and reduce bias, we employed the following strategies:
-
Credibility – via prolonged engagement with the participants. Repeated encounters gave the researchers who were collecting data the context needed to understand the participant experiences and how these changed over time.
-
Credibility – via researcher triangulation. Two researchers (Dr Rosina Cross and Professor Afroditi Stathi, chief investigator) conducted coding on the first three transcripts, discussing codes and reaching a consensus before developing the coding framework. The coding framework was discussed with the process evaluation research team (including Professor Colin J Greaves and Dr Janet Withall) to ensure that there was a consensus in the way in which it had been applied. This framework was then applied to the remaining transcripts by Dr Cross, with regular discussion with Professor Stathi.
-
Reflexivity – via regular discussions within the research team, including members with diverse scientific backgrounds and experiences of conducting qualitative research. These diverse voices allowed a better interpretation of data and refinement of coding framework.
-
Transferability – via mapping the findings of the qualitative study onto the logic model of the REACT intervention and highlighting similarities and differences among groups with high and low levels of (1) SPPB and (2) adherence to the intervention. This process enables other researchers to transfer the findings of this study to similar contexts and/or settings.
-
Auditability – via keeping records of the raw data, field notes, transcripts, analytical decisions made and the way data were interpreted, which created a clear audit trail.
Results
Six-, 12- and 24-month semistructured interview findings
Participant characteristics
At the 6-month interviews, the study sample comprised 12 women (71%) and five men (29%). The baseline age of the participants ranged from 68 to 88 years. The SPPB score was relatively balanced across the sample (47% frail, 53% pre-frail), as was attendance (47% low attendance, 53% high attendance).
At the 12-month interviews, as it was not possible to contact four women and one man from the 6-month cohort, three additional participants were recruited and the sample comprised 11 women (73%) and four men (27%). The age of the participants ranged from 68 to 89 years. At the 12-month interviews, most participants were classified as pre-frail (73%). Programme attendance was relatively balanced across the sample (40% low attendance, 60% high attendance).
At the 24-month interviews, the study sample comprised 13 women (68%) and six men (32%). The age of the sample ranged from 68 to 89 years. At the 24-month interviews, the majority of the participants were classified as being pre-frail (79%) and most (63%) were classified as having high attendance. Table 7 summarises participant characteristics and reports baseline and 24-month SPPB scores and programme attendance.
| Pseudonym | Group | Sex | Age (years) (baseline) | SPPB score | Attendance | Interview | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline: frail | Baseline: pre-frail | 24 months | 6 months | 12 months | 24 months | |||||
| Dorothy | 1 | Female | 68 | 7 | 12 | 72%a | ✗ | ✗ | ✗ | |
| Cordelia | 1 | Female | 88 | 4 | 5 | 27% | ✗ | |||
| Etta | 1 | Female | 70 | 8 | 10 | 98%a | ✗ | ✗ | ✗ | |
| Anandi | 1 | Female | 76 | 9 | 9 | 49% | ✗ | ✗ | ✗ | |
| Darsha | 1 | Female | 77 | 9 | 8 | 81%a | ✗ | ✗ | ||
| Iris | 2 | Female | 89 | 9 | 8 | 79%a | ✗ | ✗ | ✗ | |
| Mary | 2 | Female | 76 | 4 | 4 | 35% | ✗ | |||
| Cecil | 2 | Male | 69 | 4 | 12 | 65% | ✗ | |||
| Frederick | 2 | Male | 87 | 8 | 9 | 90%a | ✗ | ✗ | ✗ | |
| Geraldine | 3 | Female | 78 | 8 | 10 | 61% | ✗ | ✗ | ✗ | |
| Arman | 3 | Male | 71 | 8 | 9 | 95%a | ✗ | ✗ | ✗ | |
| Valerie | 3 | Female | 86 | 8 | 9 | 61% | ✗ | ✗ | ||
| Rita | 3 | Female | 76 | 8 | 10 | 75%a | ✗ | ✗ | ||
| Beatrice | 4 | Female | 80 | 9 | 9 | 96%a | ✗ | ✗ | ||
| Alvita | 4 | Female | 76 | 5 | 8 | 91%a | ✗ | ✗ | ||
| Arthur | 4 | Male | 76 | 8 | 9 | 65% | ✗ | ✗ | ||
| Roger | 4 | Male | 84 | 5 | 2 | 87%a | ✗ | ✗ | ||
| Ann | 5 | Female | 74 | 7 | 6 | 78%a | ✗ | ✗ | ||
| Evelyn | 5 | Female | 69 | 7 | 5 | 35% | ✗ | |||
| Flora | 5 | Female | 79 | 8 | 5 | 30% | ✗ | |||
| John | 4 | Male | 83 | 6 | 1 | 56% | ✗ | |||
| Sam | 2 | Male | 88 | 5 | 2 | 51% | ✗ | |||
| Betty | 4 | Female | 88 | 7 | 7 | 98%a | ✗ | |||
| Timothy | 6 | Male | 70 | 9 | 7 | 5% | ✗ | |||
| Angelina | 6 | Female | 73 | 9 | 9 | 65% | ✗ | |||
| Mark | 7 | Male | 73 | 9 | 9 | 63% | ✗ | |||
| Jane | 2 | Female | 84 | 8 | 10 | 35% | ✗ | |||
| Rachel | 4 | Female | 83 | 6 | 11 | 82%a | ✗ | |||
| Eleanor | 6 | Female | 68 | 8 | 12 | 30% | ✗ | |||
Participant characteristics: session leaders
Following completion of the study, all (n = 15) session leaders and organisation providers were approached and asked to participate in either an interview or a focus group regarding their experiences of REACT. Twelve agreed to participate (Bath/Bristol, n = 4; Birmingham, n = 4; Devon, n = 4) but three had left their organisations and were uncontactable. In addition, one research team member who had delivered some of the health behaviour maintenance sessions with three groups (Devon) and two members of staff from one provider organisation (Bath/Bristol) were also interviewed. The total number of interviewees was 15 (male, n = 7; female, n = 8).
Thematic tree at 6, 12 and 24 months
The four predetermined central themes were supported by participant descriptions of their experiences of the REACT study and participation in daily physical activity. This section presents the four central themes, higher-order themes (HOTs) and subthemes (Figures 3 and 4) and the comparison of responses among participants classified as either pre-frail or frail and participants with low and high attendance, and presents these findings in the context of the REACT logic model.
FIGURE 3.
Thematic trees identified from participant experiences: reasons to engage in the REACT study and benefits of participation.

FIGURE 4.
Thematic trees identified from participant experiences: barriers to and enablers of participation.
Reasons to engage in REACT
Where relevant, and in an effort to further understand themes generated by participant response, we triangulate these data with relevant data provided by the session leaders.
The central theme, ‘reasons to engage in REACT’, comprised six HOTs: seeking better health, seeking social connectedness, appeal of research, appeal of physical activity, being influenced by others and having no reason not to take part in the 6-month interviews. Four HOTs persisted at the 12-month interviews: seeking better health, seeking social connectedness, appeal of research and appeal of REACT physical activity. This central-order theme was made up of 15 subthemes at the 6-month interviews and seven subthemes at the 12-month interviews. All 12-month subthemes remained from the 6-month interviews, with no new subthemes identified. A total of 19 subthemes remained at the 24-month interviews, with one new subtheme identified.
Reasons to engage in REACT for people classified as frail and pre-frail
Reasons for engagement in REACT were very similar between people classified as frail and people classified as pre-frail. At the 6-month interviews, health was the predominant reason to engage in REACT for those classified as frail:
And then I thought about it and I thought ‘well give it a try’, anything that will help me to walk better.
Cordelia, 6 months, SPPB score of 4, attendance 72%
By contrast, people classified as pre-frail based on their SPPB scores were more concerned with the social aspect of engaging in REACT and the appeal of taking part in research:
I thought it was interesting that they were studying older people to find out if they do have enough exercise ’cos I don’t think they do . . . I’ve done these sort of things before [participate in research] . . .
Iris, 6 months, SPPB score of 9, attendance 79%
At the 12-month interviews, participants classified as frail or pre-frail were concerned with losing the physical benefits that they experienced during the REACT programme:
I just wanted to carry on with what we’d been doing, because like you know I could see the benefits.
Beatrice, 12 months, SPPB score of 9, attendance 96%
Reasons to engage in REACT for people with low and high attendance
At the 6-month interviews, the key difference between people with low and people with high attendance was that those with high attendance reported that increasing their activity levels was a key reason to participate in REACT:
. . . just hoping to get a bit of exercise out it . . . yes, I think I’ve always wanted to be more active really . . .
Beatrice, 12 months, SPPB score of 9, attendance 96%
This was less commonly reported by those with low attendance, who more often reported improving health and avoiding age-related decline as key reasons for participation. At the 12-month interviews, those with high attendance had multiple reasons to attend, whereas those with low attendance tended to report fewer reasons to continue attending, citing social connectedness as one of the most important reasons.
Findings in the context of the REACT logic model
Participants reported concerns about their poor physical health affecting their independence and social connectedness. Consequently, they sought to participate in exercise that they perceived to be potentially beneficial. The group-based delivery was particularly appealing. Social interactions are proposed by the REACT logic model as a means of maximising enjoyment. At the 6-month interviews, participants confirmed the importance of social interaction and reported that it could be beneficial to their well-being and a key reason to engage in REACT. This was further confirmed at the 12-month interviews, at which participants highlighted the supportive role of this enhanced social connectedness in forming social support networks. This was reported as a motivator of continued participation and confirmed the logic model, which anticipated that changes in relatedness would mediate short-term REACT attendance. The appeal of physical activity reported at 6 months was strengthened with the perceived benefits of participation in REACT reported at 12 months, which motivated participants to maintain these benefits in the long term. This supports the logic model, which highlights that perceived benefits from participation in the REACT sessions would influence attendance of REACT sessions and levels of daily physical activity (see Figure 2). One finding, which was not identified in the logic model, was the appeal of participating in research. Initial curiosity in participation translated to appreciation of the value of the research beyond individual gain and a sense of obligation to continue to participate in REACT. These findings in the context of the logic model are shown in a modified REACT logic model (see Appendix 2, Figure 28).
Benefits of participation in REACT
Under the central theme ‘benefits to participation in REACT’, participants reported five main benefits: improved physical health, improved social connectedness, changes in knowledge and attitudes, better mental well-being and positive behaviour change. These benefits (HOTs) were reported at both the 6-month and the 12-month interviews. They were made up of 23 subthemes at the 6-month interviews, with 19 subthemes identified at the 12-month interviews: four were new subthemes and 15 remained from the 6-month interviews. A total of 16 subthemes remained at the 24-month interviews, with the emergence of one new subtheme (see Figure 3).
At the 6-month interviews, those classified as pre-frail reported experiencing more physical health benefits, whereas those classified as frail seemed to experience more benefits related to mental well-being and social connectedness. Similarly, at the 12-month interviews, participants classified as pre-frail reported more physical health improvements than participants classified as frail:
Yes it was . . . very interesting, sometimes challenging uh, it was interesting to see what your body could do and how you improved, you know we all improved balance wise.
Beatrice, 12 months, SPPB score of 9, attendance 96%
At the 24-month interviews, participants identified four main categories of physical activity participation:
-
walking (for leisure or active travel)
-
everyday activity (housework, gardening)
-
structured exercise (group or gym based)
-
independent exercises (prescribed by a health-care professional, learned through the REACT programme).
Although not described as physical activity, social activities reported by participants included meeting friends, caring for family and friends, being church members and being members of community groups.
At 24 months, physical activity maintenance was related to specific benefits, including increased mobility and ability to carry out ADLs. Experiencing physical gains from physical activity promoted feelings of competence, which further facilitated physical activity maintenance:
My health has got better, I’m more motivated and I can get around a lot better. I don’t use my stick now whereas I always had my stick. I just got better and better [during REACT], now I walk all round the cycle track.
Geraldine, 24 months, SPPB score of 10, attendance 64%
Four participants reported increased physical activity levels post REACT. Three attributed improved motivation to be active directly to REACT. They described multiple benefits gained physically, developing new friendships and engaging in new activities (e.g. flower arranging and bowling). Two individuals wanted to capitalise on the benefits gained:
I think because I enjoyed REACT so much, and because before I used to do a tiny bit of exercise, but I enjoyed it so much and I just thought it’s a waste if I just leave it.
Dorothy, 24 months, SPPB score of 12, attendance 72%
People with both high and low SPPB scores reported experiencing improved self-efficacy for physical activity, improved social connectedness and increased physical activity levels:
I think what we did at REACT programme did give me bit of more confidence in walking.
Darsha, 12 months, SPPB score of 9, attendance 81%
Being supported to reach achievable goals enhanced participants’ confidence. One participant was spurred on to do more vigorous physical activity (running):
I was really getting better. So, I better do something with my good health . . . They [REACT] have opened my mind on what I can do. I cannot think of not going to exercise or not go for a walk or anything. I just have to go and do it and I am happy to do it.
Angelina, 24 months, SPPB score of 9, attendance 65%
REACT prompted participants to self-monitor and seek opportunities for exercise outside the REACT programme, in which support from session leaders was important to maintain self-efficacy:
It’s giving me an incentive to be more aware of what I need to do . . . I don’t exercise but I do stretching exercises . . . I never did it before . . . this basically has to do with REACT encouraging me. In a way you know, otherwise I wouldn’t be doing it because I never did.
Eleanor, 24 months, SPPB score of 12, attendance 30%
I said [to session leader] that I hadn’t really enjoyed it so I wanted to do something much more active, so he said, come to the gym, and then I found out I was in the gym on my own and I didn’t like that. So he then said well do aqua aerobics on a Friday.
Jane, 24 months, SPPB score of 10, attendance 35%
Benefits of participation in REACT findings and the REACT logic model
Participants experienced physical, social, emotional and behavioural benefits that supported the mediating role of perceived benefits on REACT attendance and physical activity outcomes, as stated in the REACT logic model. Physical activity outcomes or positive behaviour change in the form of increased physical activity levels and trying new exercise classes were mediated by perceptions of improved self-efficacy for physical activity and improved motivation for physical activity, with participants feeling more capable and motivated to exert autonomy over their choice to participate in daily physical activity and new exercise classes. The logic model proposed that relatedness would act as a mediator of physical activity outcomes. Participants indicated that improved social connectedness not only was a benefit of participation in REACT, but also enhanced enjoyment of REACT and acted as a driver for continued participation. The findings suggested that connectedness, shared identity and sense of relatedness were moderated by the specific intervention groups that participants were members of. The logic model currently identifies site characteristics that could moderate REACT attendance and physical activity outcomes; however, the moderating role of the specific intervention group that participants join on social connectedness and relatedness was not specifically identified. Group dynamics and coherence may differ among different groups and their members, and these processes need more attention and targeting. These findings are shown in the context of the logic model in a modified REACT logic model (see Appendix 2, Figure 29).
Barriers to participation in REACT and daily physical activity
Under the central theme ‘barriers to participation in REACT and daily physical activity’, participants identified nine main barriers (HOTs): being time poor, declining mental well-being, physical health barriers, REACT programme features, difficulty accessing transport, cost of daily physical activity, negative life experiences, bad weather and proposed reasons for dropouts at the 6-month interviews. These HOTs comprise 24 subthemes at 6 months. At 12 months, all nine HOTs identified at 6 months remained, in addition to three new HOTs: not perceiving benefits, barriers in the local area and lack of past physical activity. The 12 HOTs at 12 months comprised 32 subthemes, nine newly identified at 12 months, while 23 remained from the 6-month interviews. At the 24-month interviews, 12 subthemes remained, with the emergence of one new subtheme. Although all HOTs and subthemes within this central theme are presented in Figure 4, only the key themes in terms of addressing the research questions of this evaluation are discussed below. Furthermore, we distinguish between barriers that influenced attendance at REACT sessions and those that affected daily physical activity participation.
Barriers to participation in REACT and daily physical activity and the REACT logic model
Although people classified as either frail or pre-frail experienced physical health barriers to REACT and daily physical activity, those classified as frail experienced several health problems simultaneously:
My muscles, because I have this muscle weakness . . . because like sitting down I’m fine but getting up I struggle . . . I used to fall over quite often . . . I’d lose my balance like . . . And then I’ve seems to got no muscles to hold me back . . . I’m just blop! [laughs] . . . that’s my big problem.
Alvita, 12 months, SPPB score of 5, attendance 91%
In addition to physical health barriers, those classified as frail also reported experiencing other barriers to participation, such as having other commitments and difficulty accessing transport. A difference in physical health barriers experienced between people classified as frail and people classified as pre-frail was less evident in the 12-month interviews, with both groups reporting similar physical health barriers. Although the barriers to physical activity for participants classified as frail were dominated by physical health barriers in the 12-month interviews, participants classified as pre-frail reported barriers that related to the REACT programme features, including issues with some venues, some REACT session leaders, the delivery of the health behaviour maintenance programme, some faulty pedometers and moving from two sessions per week to one session per week from week 12 onwards:
I’m a bit disappointed in we had it [health behaviour maintenance programme] a couple of times when it first started but most of the people don’t seem to want to stay behind you know and have a coffee and a chat . . . They seem to have other things they want to do.
Cecil, 6 months, SPPB score of 4, attendance 65%
One participant recounted there being no formal discussion of goal-setting:
. . . we have talked about the step counter [pedometer] uh but we’ve sort I don’t think we’ve never talked about the actual, about any actual goals you know.
Beatrice, 6 months, SPPB score of 9, attendance 96%
The barriers that were related to REACT programme features were confirmed in session leader interviews, in which it was reported that, although the use of pedometers was popular in some groups, a number of groups faced difficulties with poor-quality pedometers that did not accurately record steps.
Participants classified as frail reported experiencing numerous and chronic health problems that remained throughout the REACT programme. By contrast, those classified as pre-frail often reported experiencing either acute illness or injuries that were relatively short-lived or health concerns that were stable or they were accustomed to dealing with, such as diabetes. At 12 months, both participants classified as frail and those considered pre-frail shared their experiences of the REACT programme and identified where they faced challenges. These experiences also varied depending on the site of the intervention group and how some session leaders delivered different components of the programme. Reports from interviews with session leaders also noted the challenges involved when working with older adults experiencing numerous physical health barriers, which often included adapting exercise:
When you’re working with 15 people with multiple health conditions you need to be flexible with your delivery.
At the 24-month interviews, physical health barriers were the most cited reasons for lapses in physical activity participation, together with leading busy lives and having competing commitments. Participants who articulated strategies to overcome ‘tensions’ to physical activity maintenance had both (1) lived active lives and (2) been engaged in a wide array of activities prior to REACT. Self-regulatory strategies to overcome challenges, such as declining health, competing commitments, motivational barriers and adverse weather, included adjusting expectations or actual physical activity, self-monitoring and making plans. These are discussed in more detail in Enablers of participation in REACT and daily physical activity.
Barriers to participation in REACT and daily physical activity for people with low and high attendance
Although the responses between the two groups were similar in the 6-month interviews, in the 12-month interviews there was a distinction between participants with high attendance scores, who tended to experience a single barrier to participation (predominantly physical health), and participants with low attendance scores, who reported multiple barriers to participation (predominantly physical health barriers and having other commitments). When considering participants who were in both the low SPPB score and the low attendance strata, it is important to note that two of the three participants interviewed at 6 months dropped out shortly before the 6-month interview, having experienced multiple barriers to participation relating to physical health, time commitments and travel difficulties. The third participant dropped out a few months after the 6-month interview, having experienced significant physical health barriers and reporting having other commitments. None of the three was available for follow-up at the 12-month interviews.
Being time poor and its associated subthemes – having competing commitments and not having the time – were expected to be a barrier to physical activity, especially in the short term; however, BCTs were incorporated into the REACT programme to help participants to identify and resolve sources of tension around increasing physical activity. Reports that time was a barrier to participation remained throughout the 12 months, indicating that participants were not able to successfully utilise BCTs, the BCTs were not suitable for resolving these tensions or the BCTs were not delivered as planned. The intervention fidelity study, which assessed delivery fidelity of health behaviour maintenance sessions (not exercise sessions), triangulates this finding, as it demonstrated that BCTs, such as managing setbacks and problem-solving, were delivered with low fidelity. Furthermore, reports from session leader interviews at follow-up indicated that reasons for poor delivery fidelity could include (1) not feeling comfortable delivering the content or (2) participants not being engaged with the health behaviour maintenance sessions. This, together with real life issues (e.g. lack of access to transport or competing interests) that participants may have faced, may explain why issues with time availability and tensions with having competing commitments remained at 12 months:
I mean I have been playing indoor bowls quite recently up and till about a month ago but I’ve had to give up my car so I can’t get around quite as much so it looks like I am not able to drive. And also I’ve got to find someone to take me.
BT2013, 24-month interview
I didn’t pursue any others. I should have done, I think, but it may be difficult to explain this to you but despite my age I’m very busy, I don’t have much spare time. I just deferred starting my last book due to lack of time, I’m going to write it some time. I’ve written 16 altogether but . . . I’ve got one more I want to write but certainly things get in the way, but I’ll get there. I’ve got to make sure I live long enough to do it!
AT2624, 24-month interviews
Although REACT programme features such as issues with the REACT session leader and issues with REACT structure and content were not reported to have affected REACT attendance, REACT venue issues were prohibitive of full engagement in REACT sessions. This shows how context in the form of intervention provider and site can, by moderating the way in which interventions are implemented, affect the way that participants experience an intervention and engage with it, as illustrated in the logic model. For example, in the case of issues with REACT structure and content, reports suggest that in some cases BCTs (managing setbacks and problem-solving/goal-setting and action-planning) were not discussed widely in some groups, suggesting a lack of competent delivery of health behaviour maintenance sessions on behalf of the REACT session leader, which confirms findings from the intervention fidelity study and reports from session leaders that some could not remember content from health behaviour maintenance training sessions. The intervention fidelity study showed that the delivery of action-planning and goal-setting was rated as needing improvement (mean score 2.2, SD 1.14), while managing setbacks and problem-solving were delivered with low fidelity (mean score 1.9, SD 0.81).
Participants did not report the delivery fidelity of these BCTs during health behaviour maintenance sessions as being prohibitive of either daily physical activity or REACT attendance; however, intervention outcomes may indicate whether or not this influenced long-term physical activity outcomes as the logic model anticipates it could. Participants with low motivation for physical activity or low self-efficacy for physical activity did not report this as a barrier to REACT attendance, which suggests that participants had the motivation for specifically engaging with REACT and felt a sense of self-efficacy and competence in the REACT context. It is expected that this would not necessarily translate to physical activity in other contexts. This supports the logic model, which anticipated that changes in intrinsic motivation and competence/self-efficacy would mediate changes in physical activity outcomes, in either a negative or a positive feedback loop. Participants reporting physical health barriers seemed to overcome them during REACT classes. This is perhaps because feelings of low motivation for physical activity or low self-efficacy for physical activity were reported more in relation to daily physical activity rather than in REACT itself (i.e. physical health barriers were not further compounded by low self-efficacy for physical activity and low motivation for physical activity). Many participants reported that having the autonomy to manage their own exercise throughout the class enabled them to overcome physical health barriers. Furthermore, the fact that session leaders were able to adapt exercises for those struggling with physical health barriers supported the mediating effect that participants’ autonomy over their route of progression and ability to carry out an adapted exercise had on REACT attendance.
Participants and session leaders reported difficulty in accessing transport as an important contextual variable affecting REACT participants, who used a range of strategies to overcome this barrier, such as carpooling and accessing community transport.
The organisation of carpooling is an example of participants building support networks to enable REACT attendance. The logic model anticipated that this could be a determinant of physical activity outcomes and could be mediated by feelings of relatedness among REACT peers. Findings from ‘barriers to participation in REACT and daily physical activity’ in the context of the logic model are shown in a modified REACT logic model (see Appendix 2, Figure 30).
Enablers of participation in REACT and daily physical activity
Within the subtheme ‘enablers of participation in REACT and daily physical activity’ there was a clear distinction between pre-existing motivation and components of the REACT project targeting participant motivation. This was reflected in the two HOTs, characteristics of REACT and personal and psychosocial characteristics of participants, including 10 subthemes. At the 12-month interviews, all 10 subthemes remained, with the addition of a new subtheme. At the 24-month interviews, 11 subthemes remained, with the emergence of two new subthemes (see Appendix 2, Figure 31).
Enablers of participation in REACT and daily physical activity for people classified as frail and pre-frail
At the 6-month interviews, participants classified as either frail or pre-frail reported similar enablers of participation to REACT. However, both groups demonstrated a shift in importance of enablers from the 6-month to the 12-month interviews. At 6 months, the predominant enabling factor was the support received from REACT session leaders; however, although this is also discussed at the 12-month interviews, the emphasis was on enjoyment of REACT and supportive group dynamics:
Just being with everybody and seeing how we could all do, and everybody said ‘oh well done’ . . . we all encouraged each other I think you know.
Beatrice, 12 months, SPPB score of 9, attendance 96%
This suggests a transition from REACT session leader-supported participation to a group network of support for physical activity. When focusing on daily physical activity, participants reported that their REACT session leaders’ encouragement and support of other activities within the community were important factors in their continuation of activity independent of REACT. Furthermore, having motivations to return to previous physical activity behaviours seemed to be enabling of daily physical activity for both frail and pre-frail participants. Previous physical activity behaviours were also important at the 24-month interviews, with participants who overcame barriers to physical activity participation being those who had been historically active and engaged in a variety of activities before enrolling in REACT.
In contrast to reports at the 6-month and 12-month interviews, in which self-regulatory techniques were not commonly cited, at the 24-month interviews self-regulatory techniques were reportedly used in an effort to overcome challenges such as declining health, competing commitments, motivational barriers and adverse weather. These self-regulatory strategies included:
-
Adjusting expectations of or actual physical activity in accordance with their health limitations and other commitments –Valerie, 24 months, SPPB score of 9, attendance 61%
If I can’t [attend exercise class] then I shall do what I do now which is to do light exercises for my back before I get out of bed in the morning and walk and [partner’s] corridor is about the same length as my path so we walk up and down that several times . . .
-
Walking indoors was a coping strategy for staying active when the weather was poor –Darsha, 24 months, SPPB score of 8, attendance 81%
I make a habit of a daily walk you know, of course if the weather is very very bad. Like it was so windy I try to go but then I just walk up and down the corridor.
-
Doing ‘something rather than nothing’ was another coping strategy –Frederick, 24 months, SPPB score of 9, attendance 90%Rachel, 24 months, SPPB score of 11, attendance 82%Frederick, 24 months, SPPB score of 9, attendance 90%
. . . now instead of just going and getting my paper and coming straight back, I could do a circuit if it’s a nice day, you know? I’m not counting them [steps] now but it’s still reasonable.
You have to make yourself sometimes, you know I think to myself, ‘come on get up get up’ and I do I reckon every day I reckon to go out somewhere, even if I only walk up the village and back down.
I’m pretty active in silly ways. It sounds nothing, but I don’t use the lift . . . it is a small thing . . . so not to use the lift, I have to walk to the far end of the building . . . Go down the stairs and walk back half-way through the building. It’s far enough to be an activity.
-
Repeating the same walking route enabled participants to self-monitor their progress –Mark, 24 months, SPPB score of 9, attendance 63%
That’s a favourite walk because we can sort of tell where we were last year and last week.
-
‘Habit’, ‘routine’ and ‘making plans’ were key enablers for physical activity, whereas participants did stress that not being as able as before was evident in generally being slow with all activities, which led to frustration –Dorothy, 24 months, SPPB score of 12, attendance 72%
I make a plan to walk from home to (city centre) and back . . . and then at home I walk, because I have a lot of stairs, I walk up and down, up and down until I get tired.
Post REACT at the 24-month interviews, individuals who reported frequent physical activity engagement described gaining pleasure and enjoyment from physical activity:
I love seeing things, I love being out in the fresh air I love noticing things. I don’t get up and say right I’ve gotta do a couple of miles this morning, no I go when I want to enjoy myself.
Jane, 24 months, SPPB score of 10, attendance 35%
Another participant described how physical activity was an enabler to participation in meaningful activities:
I’ve got no real interest in . . . just exercise as exercise. My interest was always in exercise really incidental to some proper activity, and that would be a social activity among people I liked.
Frederick, 24 months, SPPB score of 9, attendance 90%
Despite the importance of a supportive group network as an enabling factor during the REACT programme, post REACT, social support was a less prominent theme in explaining physical activity maintenance. None of the interviewed participants had utilised support from community agencies (e.g. health visitors, health friend scheme):
I personally did not need it, because you know I am quite fulfilled with the activities that I am doing, and then I go on holiday and visit my daughters and granddaughters.
Arman, 24 months, SPPB score of 8, attendance 95%
For some participants, friendships developed during REACT and motivational support from friends and spouses facilitated continued physical activity engagement:
Well [friend] bullied me . . . she went and it [attending REACT] was a good way of meeting up and perhaps getting a coffee after . . . and then it developed from there . . .
Valerie, 24 months, SPPB score of 9, attendance 61%
I’m not very good at self-motivation . . . if you said, oh, you know, go for a run every morning I wouldn’t, but if the whole group of us were meeting and I said, ‘oh we’re going to go for a short walk meet at 10 o’clock at [location]’ then I’d go.
Rita, 24 months, SPPB score of 10, attendance 75%
Illness also brought about changes in some friendships and had an impact on people’s social activities:
It was quite hard for me because she [friend] used to come and ring me up every day and we’d meet for an evening meal nearly every day, and all of a sudden it was gone!
Rita, 24 months, SPPB score of 10, attendance 75%
Social isolation and lack of support can have detrimental effects, as vividly reported by one participant:
I’m less active than I ever was in the past and it’s getting worse because I have no friends, no relatives. I don’t have children . . . They’ve all died or they’ve got hip or knee problems . . . or they’re involved with family, you know, so I have to create things to do to keep myself occupied but in [city] it’s a big fat zero. I have nothing, no one, and I don’t want to go walking by myself because it can be dangerous. If I fall there’s no one you see?
Eleanor, 24 months, SPPB score of 12, attendance 30%
Enablers of participation in REACT and daily physical activity of people with low and high attendance
It was apparent from both the 6-month and the 12-month interviews that people with high attendance were more motivated by REACT than people with low attendance. This indicates that motivation could act to mediate REACT attendance but barriers could moderate the positive loop between motivation and REACT attendance, as we observed that people in the low-attendance group experienced more physical health barriers. The role of supportive group dynamics was reported more often in the 12-month interviews by people in the high-attendance group. Three participants in the group with low attendance and low SPPB score who reported supportive group dynamics at the 6-month interviews still dropped out, showing that, although supportive group dynamics may be a strong enabling factor, it might not be sufficient to overcome the multiple barriers (physical health, having competing commitments and difficulties accessing transport) that these participants may had faced before they dropped out. People with high attendance reported more participation in previous physical activity behaviours, suggesting a moderating role of previous physical activity behaviours in the attendance of REACT sessions in the short term; however, at the 12-month interviews, this relationship was no longer evident, suggesting that previous experiences may not be a moderator of long-term REACT attendance.
Enablers of participation in REACT and daily physical activity findings in the context of the REACT logic model
The logic model anticipated that enjoyment of REACT would mediate REACT attendance and physical activity outcomes. Participant reports suggest that enjoyment was a key reason to participate in REACT and start new physical activity classes beyond REACT. Participants reported being motivated by REACT, deriving motivation from their REACT session leaders who supported participants’ competence and sense of autonomy, and by watching their REACT peers improve their fitness levels. Participants’ perceived competence mediated both short-term and long-term physical activity outcomes. High levels of competence and self-efficacy at baseline, mainly among participants who had previously been active, may influence outcome expectations and need to be accounted for by the REACT logic model (see Appendix 2, Figure 31).
Key REACT programme components
The key programme components responsible for influencing participants’ lifestyles at 24 months are summarised below.
Behaviour change techniques
Techniques for managing slips/lapses, supporting habit change and resolving sources of tension around increasing physical activity were utilised by participants to support physical activity at 24 months. Participants were able to overcome ‘tensions’ to physical activity by employing self-regulatory strategies (e.g. incorporating more physical activity into daily life, finding alternative physical activity and monitoring techniques). Routine was an enabler for maintaining PA.
REACT facilitator delivery style
REACT session leaders used a person-centred delivery style to build autonomy/intrinsic motivation. Individuals with formerly low physical activity levels expressed increased motivation to be active through mastery and improved self-efficacy. This was sustained post REACT.
Group-based physical activity
The group-based format of the REACT programme promoted social connectedness among participants. The social benefits of physical activity were recognised by most participants. Individuals with established social networks reported more physical activity. The success of REACT in supporting sustainable social networks varied between groups. Where this was most successful, social contact continued post intervention.
Themes identified from session leader and organisation provider interviews
The REACT programme session leaders and organisation providers were interviewed at follow-up (12 months) to explore whether or not the intervention was delivered as planned and what, if any, changes could be made to the REACT programme for future delivery (research questions 4 and 5). The analysis of session leader interview data identified 10 themes, which address whether or not the programme was delivered as planned and propose further improvements to the programme components. The programme components presented are manual and training, one-to-one sessions and group exercise classes, health behaviour maintenance sessions, group size/common start time, equipment/recording attendance, ambassadors programme, non-REACT activities, continuation session, transport and cost/funding.
The REACT manual was a substantial document and was given to session leaders at the training session. It was designed to be used as a reference document throughout the 12-month programme. The manual could appear daunting at first sight, but the training session explained/eased its use and session leaders were very positive about using it:
. . . it scares a lot of people . . . when you’ve done a course you realise it’s actually quite simple.
Exercise specialist, AT9001, Male
Another session leader recounted:
I do refer to the training manual, I have it with me at all times.
Exercise specialist, AT9002, Male
Feedback on the REACT 2-day training course delivered by three or four members of the research team was largely positive, with session leaders stressing that it informed them of what they needed to deliver. Some session leaders thought that the sessions focusing on the exercise component of the programme might have been too detailed given that the leaders were well qualified. However, others thought that these sessions were a good refresher:
I found it very, in depth and possibly . . . I didn’t need it to be that in depth. But I didn’t mind sometimes you need reminders.
Exercise specialist, AT9002, Male
Suggestions for improvements included a practical demonstration of how an entire class would run, and provision of online materials for use during the programme delivery:
. . . maybe a demonstration of classes . . . this is what session one would look like.
Exercise specialist, AT9004, Female
Another suggestion for improvement was the provision of ‘an online resource; an online clip of an hour session can you just watch it when you got time to kind of format and layout’ (exercise specialist, AT9001, male).
Feedback was less detailed about the health behaviour change training. Some leaders did not recall the training session, and some recalled only some aspects:
I’m not quite sure what you mean. I’ve got to be honest I don’t.
Exercise specialist, BT001, Female
One-to-one sessions and group exercise classes
Feedback about these sessions, at which participants met with their session leader on a one-to-one basis, was overwhelmingly positive. One session leader reported that they had taken their participants on a familiarisation tour of the facility as part of the one-to-one session.
There was positive feedback about the structure of the classes, especially the warm-up and games, although ‘when you’re working with 15 people with multiple health conditions you need to be flexible with your delivery’ (exercise specialist, AT9003, male). Some sessions leaders reported that they had to make some adjustments, including a lengthier warm-up.
Reducing the exercise sessions from two sessions per week to one was perceived as challenging by some, who reported that participants did not want this reduction, as they were engaged with the programme and were making good progress. Some session leaders agreed that moving to one session reduced the rate of progression of the participants.
A change in session leader was challenging for the incoming leader, who reported that the groups were attached to their session leaders and were not happy with changes:
I took on the first group probably about 4/5 months into it, and I think it was quite hard to engage with them as an outside instructor coming into an already established group.
Exercise specialist, AT9003, Male
Health behaviour maintenance sessions
Feedback about the health behaviour maintenance sessions was mixed, with some session leaders reporting that they did not deliver these sessions as planned because they were uncomfortable with either the content or the format of the sessions:
I ended up just incorporating what needed to be covered within the classes ’cause I found it really hard to structure specific sessions for that.
Exercise specialist, AT9002, Male
Alternatively, session leaders reported that they did not deliver sessions as planned because their participants did not want to engage with them:
I’ve got to say a lot of the time they just wanted to have a coffee and a chat as a group, it was quite difficult to, you know, get all their attention.
Exercise specialist, ET9001, Male
However, some session leaders reported that, although it was challenging to deliver these sessions, on the whole participants enjoyed them:
. . . they had a few problems with them but on the whole they enjoyed that.
Exercise specialist, BT9004, Female
Group size/common start time
The fact that all REACT participants joined the group at the same time for the 12-month duration was viewed as both a positive (‘being in a group starting off starting off them as a group to get them to know one another which is fantastic’; exercise specialist, BT9003, male) and a negative (‘if a few people drop out . . . if you don’t have a flow of people to keep fresh blood, it’s just not sustainable’; exercise specialist, AT9003, male).
Equipment/recording attendance
Everyone was happy using Therabands (Akron, Ohio, USA), but getting ankle weights on and off participants was an issue: ‘they took quite a long time to get them on an off so we were kind of losing a bit of time’. Using pedometers as part of the health behaviour maintenance programme was popular, but some groups experienced issues with poor-quality pedometers that did not accurately record steps:
They really enjoyed having that kind of visual tool [map] to do it and they got really on board with counting their steps and I think that was good motivator.
Exercise specialist, AT9003, Male
There were issues around recording attendance at sessions. Some session leaders did not have access to online systems in the exercise room, so needed to keep paper-based registers. Several session leaders suggested the provision of an online register in future REACT delivery.
Ambassadors’ programme
Most session leaders found it difficult to engage participants in a formal ambassador programme because participants ‘didn’t want that full-on responsibility’. However, in many cases, some elements of the ambassador roles were taken up by participants in a more informal manner:
There wasn’t a specific ambassador . . . they just naturally started to pick their own roles.
Exercise specialist, BT9002, Male
I think it’s a really good idea because it just gives them that more sort of ownership over the programme. But it just didn’t work.
Exercise specialist, ET9001, Male
Non-REACT activities
A main focus of the REACT programme was to encourage participants to engage with other community activities. This was particularly the case when the frequency of sessions changed from twice per week to once per week. Session leaders reported some level of success with some groups, but some participants ‘were not easily persuaded and were very wedded to their group and session’ (exercise specialist, AT9002, male). However, ‘one or two of them jumped on quite quickly were very eager to do it’ (exercise specialist, AT9001, male). Despite some having concerns about new activities, ‘a lot of them were a bit worried about going out and trying other things’ (exercise specialist, AT9001, male).
Continuation sessions
Many groups did not want the sessions to end after the 12-month programme:
When I had to leave the group, it’s been a bit traumatic. They were really really upset. So the majority have continued (in some form) with participants paying to attend.
Exercise specialist, AT9002, Male
As a result, additional sessions were created by trusts for the REACT participants:
There was so much demand for the trust to continue with some form of exercise class that we created the [name] weekly exercise class [REACT continuation].
Exercise specialist, AT9008, Male
Transport
Despite the fact that during the REACT screening process potential participants were given specific information about the location of the REACT classes and asked if they would be able to attend sessions, some still faced transport issues. These were usually solved by community transport or, more commonly, through lift-sharing.
Cost/funding
Some session leaders reported that the programme was a large investment for a small number of people, but they acknowledged the importance of free sessions to improve engagement with exercise sessions:
But I think you need to hook them with the free yeah just to get them settled.
Exercise specialist, ET9001, Male
Discussion
Summary of findings
The longitudinal qualitative evaluation identified the perceived benefits, barriers to and enablers of participation in REACT and daily physical activity at 6, 12, and 24 months, while exploring the perspectives of the session leaders on what worked well and necessary changes for a community roll-out. Exploring the theorised mechanisms proposed by the REACT logic model helped us to understand the way that psychological processes mediate and moderate the programme’s impact on physical activity behaviour change. In addition, the interviews with participants and providers helped us to better understand the variation in delivery fidelity that was illustrated by the quantitative fidelity study and whether or not this variation affected participant experiences and outcomes. Exploration of the logic model in this way highlighted where the logic model can be refined to inform changes and adaptations to the REACT programme for a community roll-out.
The findings demonstrate that older adults engaged with the REACT programme, seeking better health, both physical and mental, as well as better social connectedness. During the 12-month programme, they experienced improvements in physical and mental health, improved social connectedness and positive behaviour change. Most benefits described at the 6-month interviews were also reported at 12 months; however, the way in which participants related to these benefits changed. For instance, at 6 months, participants described the physical health benefits that they experienced (e.g. improved balance); however, at 12 months, they described how these benefits translated to better day-to-day quality of life (walking without a stick). Although competence and self-efficacy at the 6-month interviews was derived mainly from the REACT session leader and REACT peers, at the 12-month interviews, participants described deriving self-efficacy and competence from reflecting on their own achievements. This enabled them to explore other exercise opportunities in their community. At 24 months, there was a strong focus on maintenance of functional ability improvements, with participants reporting strategies for long-term maintenance.
There was a distinct transition in how participants experienced enabling factors during the REACT programme. The chief enabling factor at 6 months was the support received from the REACT session leaders; however, the emphasis shifted at 12 months to the support derived from the REACT groups, indicating the creation of a network of group support and a shared identity around the REACT programme.
Personal and psychosocial enablers of REACT attendance and daily physical activity included having good levels of competence, self-efficacy and motivation to be physically active, alongside a history of being physically active. Despite these enablers, daily physical activity was impacted to a greater extent by barriers than REACT attendance. Barriers experienced by participants usually persisted throughout the programme; these were seemingly overcome during REACT exercise sessions but seemed to continue to affect daily physical activity. For example, physical and mental well-being barriers persisted throughout the REACT programme but were overcome with the help of REACT session leaders or REACT peers. Not having enough time affected daily physical activity but not REACT, as did low self-efficacy and competence or low motivation. Furthermore, physical health barriers, such as mobility issues, pain and discomfort and tiredness, were more prohibitive of daily physical activity than REACT attendance. These barriers were seemingly overcome either with the support of the REACT session leader or peers or, in the case of lack of time as a barrier, by prioritising attendance of REACT sessions. However, when participants were outside the REACT programme, the environment was not conducive to overcoming these barriers to the same extent. With the removal of the REACT session leader and a supportive group of peers, these barriers were less likely to be overcome. Planning for the community delivery of REACT needs to focus on a tailored, personalised approach, while at the same time a whole-systems approach needs to be implemented to target barriers operating outside the personal level.
At 24 months, participants’ accounts mostly aligned with the hypothesised mechanisms of change depicted in the REACT logic model, although some aspects were more effective than others in promoting long-term behaviour change. There was mixed evidence for the programme’s effectiveness in improving relatedness. Different ability levels among group members interfered with the creation of group relatedness and connectedness. Participants often described having supportive social networks independent of the REACT programme. This may suggest that participants’ social support needs were fulfilled elsewhere. However, this was not universally true, and people with limited social networks reported that diminished social interactions influenced their health and well-being and their opportunities for daily activity.
Themes identified at 24 months largely mirror those reported at 6 and 12 months. However, whereas the 6- and 12-month interviews found that social support was a key reason for engaging in REACT, at 24 months individual-level factors were more prominent themes in explaining physical activity maintenance. Self-regulatory strategies were also discussed, which were not identified in the earlier interviews. Key components of the REACT programme that positively influenced maintenance of physical activity at 24 months were (1) techniques for managing slips/lapses, supporting habit change and resolving sources of tension around increasing physical activity; (2) person-centred delivery style to build autonomy/intrinsic motivation; and (3) group-based delivery promoting social connectedness.
The findings from interviews with session leaders and participants triangulated the findings of the fidelity of intervention delivery study. The health behaviour change programme received mixed evaluation. Participants and session leaders highlighted the importance of the programme and its focus on self-regulation, strategies to resolve tensions and competing interests, and increasing knowledge and understanding of the importance of physical activity and ways to incorporate physical activity into daily lives. However, for a REACT community delivery, this programme requires fine-tuning and a good communication and support system for REACT session leaders to enable them to deliver the programme as planned.
Considerations for refining the REACT logic model
One of the main aims of the qualitative process evaluation was to refine the logic model further with new knowledge based on participants’ and providers’ experiences and evaluation of the REACT programme. The interview findings confirm many of the processes and mechanisms of impact anticipated by the logic model. There are some aspects of the logic model that this study was not designed to address, such as the objectively measured physical activity and SPPB outcomes. The analysis of the qualitative data has highlighted six key modifications for further refining the REACT logic model:
-
Social interactions, formation of shared identity, support networks and feelings of relatedness are moderated by intervention group as a contextual variable. This should be identified in the logic model to enable the assessment of this contextual variable on intervention processes (relatedness) and outcomes (REACT attendance and physical activity outcomes).
-
The responses of participants in the qualitative study stressed that perceived benefits (physical, social and emotional) may be influenced by baseline SPPB score. Consequently, baseline SPPB score needs to be recognised in the logic model as having the potential to impact the mediating effect of perceived benefits on REACT attendance and physical activity outcomes.
-
The delivery of BCTs (monitoring progress, goal-setting and action-planning, and managing setbacks and problem-solving) was moderated by intervention group and session leaders. Session leaders are recognised in the REACT logic model as a key contextual variable and, similarly, intervention group should be identified as a contextual variable potentially moderating intervention delivery. This will highlight the need to monitor and assess delivery fidelity, how this changes from one context to another and any impact on perceived benefits, ability to overcome barriers, REACT attendance and physical activity outcomes.
-
Baseline self-efficacy and competence were identified in the interviews as key enablers of REACT attendance and daily physical activity. Therefore, participant baseline self-efficacy and competence should be identified in the logic model alongside baseline SPPB and physical activity as a key contextual variable in the moderation of REACT attendance and physical activity outcomes.
-
Access to transport was identified as an important environmental barrier to daily physical activity and, in some cases, REACT attendance. Subsequently, it should be recognised within the logic model as an important contextual variable with the potential to moderate both REACT attendance and physical activity outcomes.
-
Competence is recognised in the logic model for its role in mediating REACT attendance and physical activity outcomes; however, interview findings show that the source of the competence needs to be recognised. The source of competence transitioned from external sources (REACT peers and session leaders) to internal sources (drawn from own capabilities).
Strengths and limitations
A key strength of this qualitative process evaluation was the longitudinal study design, with the collection of data from study participants at multiple time points, including interviews at 6 months (during), 12 months (post intervention) and 24 months (12 months post intervention). 89 This approach provided a robust, in-depth account of participant experiences and the psychological processes responsible for behaviour change, while identifying the interactions that these processes had with context and time over the 24-month period. As previously described, there are few longitudinal qualitative studies of older adults’ experiences of physical activity interventions, and this study adds to this limited body of research by highlighting the dynamic processes involved in older adults’ engagement with a physical activity intervention, and the uptake and maintenance of daily physical activity over a 24-month period. 90–93
Having a logic model that clearly defines an intervention helps to identify where evidence agrees with underlying theory and anticipated pathways or if there is limited agreement and the intervention needs to be refined. 43,94,95 The analysis of findings in the context of the REACT logic model enabled the evaluation of the programme and highlighted successful areas of delivery, the mechanisms by which the core elements of the programme work and how they are affected by contextual variables to produce short-term and long-term changes. The integration of the REACT logic model with the qualitative and the quantitative findings will inform the refinement and scale-up of the REACT programme and help to explain variation in outcomes, for example why some participants respond to the programme more successfully than others.
The purposive sampling strategy adopted allowed for sampling of REACT participants across a wide range of characteristics (SPPB, attendance, age, sex, ethnicity, intervention group, intervention session leader and provider). This strategy maximised the diversity in participant experience and provided rich data grounded in the real experiences of community-dwelling older adults. In addition, this sampling strategy facilitated a subgroup analysis of themes based on SPPB and REACT attendance,96 which highlighted key differences in the way that low (frail) and high (pre-frail) SPPB participants experienced both benefits of REACT participation and barriers to REACT participation and daily physical activity. These differences have important implications for the way that physical activity interventions are designed and delivered.
The purposive sampling ensured diversity of participant characteristics and that thematic saturation was reached during the analysis. However, we cannot be sure that the addition of data from the participants who declined a follow-up interview would not have affected the findings, and we accept this as a limitation of longitudinal qualitative studies. Consequently, we need to be careful when we generalise findings to other populations, especially those less represented in the study sample, for example frail older adults.
A common limitation associated with interviews is the generation of responses from participants that are socially desirable, undermining the credibility of the results. Furthermore, researcher bias when analysing interview data is also possible. Several strategies (prolonged engagement, reflexivity in approach to analysis, involving discussions with other researchers, paraphrasing participant responses to ensure correct interpretations) were employed to increase credibility and minimise bias in the study. This could have been further limited by using multiple interviewers as a form of triangulation; however, it was deemed that rapport and an awareness of previous interviews was an important factor in facilitating in-depth narratives. 89
Implications for practice
Moving forwards to a potential community roll-out, the findings from the interviews with the session leaders highlighted some key actions to fine-tune the REACT programme while providing some key implications for the design of active ageing programmes (Table 8).
| Key actions | Details |
|---|---|
| Promote social interaction and enjoyment | REACT attendance and physical activity outcomes were moderated by enjoyment and mediated by relatedness. This was a successful REACT component and a future roll-out should continue targeting enjoyment and social interaction. Active ageing programmes should promote exercise that is enjoyable and facilitate a sense of belonging and social interaction |
| Health behaviour maintenance sessions | Revise the content and delivery of these sessions and provide ongoing support to session leaders to deliver the content of these sessions with confidence. Ensure that content of these sessions is engaging and interactive and avoid the ‘classroom’ effect. Capitalise on opportunities to reinforce key messages during the group exercise sessions and during tea and coffee breaks |
| Transition to non-REACT activities | Evidence of successful transition arrangements during REACT reinforce the importance of focusing on incorporating such arrangements in future REACT roll-out. Provision of information and support to identify meaningful and enjoyable activities and facilitation of taster sessions during the REACT delivery would enable physical activity maintenance post REACT. Furthermore, participants should be given the opportunity to continue with the REACT programme if they wish, so no official programme finish is recommended |
| Session leaders | The role of the session leader is key in the delivery of a group-based programme. REACT session leaders use social interactions to maximise enjoyment, encourage social bonding and foster a sense of belonging. Their delivery is positive and person centred, and they constantly seek to create opportunities for social interaction, such as exercises in pairs/small groups, water breaks and tea and coffee time afterwards. Focusing on individual progress, adapting exercise to suit individual needs and ensuring that each participant exercises at the right level for them is challenging in a group delivery but achievable. That seems to be particularly important for people with frailty and low SPPB scores, who may face a range of complex barriers, and they may require a more holistic approach to ensure their continuous involvement with the REACT programme |
| Recruitment | Being able to contribute to research that may benefit the community was a key source of motivation for older adults engaging in REACT. In future roll-out of REACT, the contribution of participants in the group programme, their commitment to their group and ways to facilitate the contribution older adults in the delivery of the programme, especially the health behaviour maintenance programme, could increase the value attached to their engagement. Responsibility to the group, sense of belonging and sense of sharing similar challenges were important motivators for REACT participants |
Considerations for research
Key considerations for research, based on the findings of this study, are presented in Table 9.
| Key actions | Details |
|---|---|
| Understanding the experiences of frail older people | More targeted qualitative research is needed to understand how frail older adults self-evaluate or relate to this, how this affects their experience of physical activity interventions and how they can be supported in the face of numerous barriers to physical activity and fewer perceived benefits. This knowledge can be used in the design of physical activity interventions targeting older adults and the training of intervention session leaders to successfully support them |
| Utilising longitudinal methodology | Qualitative researchers exploring the processes involved in the long-term behaviour change of older adults need to consider longitudinal study design. This study design can provide in-depth data and a robust narrative over time, from which to understand older adults’ experiences of physical activity interventions and the processes involved in long-term physical activity behaviour change |
Study 3: quantitative evaluation of intervention mechanisms
Aims
-
To investigate the mediating and moderating effects of changes in physical activity on the primary outcome (change in physical function) and other outcomes of interest (cognitive function, sleep quality, well-being, depression).
-
To assess changes in proposed psychosocial determinants of physical activity behaviour suggested by SCT and SDT (the theoretical underpinnings of the intervention model).
-
To explore the role of contextual variables (e.g. age, sex and ethnicity) in moderating the effects of the REACT intervention.
Methods
Design
A detailed set of hypotheses (outlined in the following section) were derived from the logic model and quantitatively tested using a range of process measures, including questionnaires and accelerometer data collected at baseline and 6 (short term), 12 (medium term) and 24 months (long term). The hypotheses were stated and published ‘a priori’, prior to unlocking or any analysis of the REACT data set. 1 Some minor edits were made prior to analysis (e.g. owing to non-availability of some data), and these are indicated in footnotes to the hypotheses below.
Hypotheses for quantitative testing of the REACT logic model
The hypotheses were derived from the stated and implied assumptions in the REACT logic model. The time from baseline to 12 months represents the intervention period (0–6 months was the ‘initial change phase’ and 6–12 months was the ‘supported maintenance phase’) and the 12- to 24-month time frame represents the post-intervention (unsupported maintenance) phase.
Hypothesis 1 was that being in the intervention arm would lead to changes in physical activity (MVPA, steps and sedentary time) and engagement in muscle-strengthening exercise from 0 to 6 months.
Hypothesis 2 was that increased exposure to the intervention would correlate with increased change in physical activity (MVPA, steps and sedentary time) and engagement in muscle-strengthening exercise from 0 to 6 months.
Hypothesis 3 was that increased exposure to the intervention would correlate with increased change in physical activity (MVPA, steps and sedentary time), engagement in muscle-strengthening exercise and SPPB score from 0 to 12 months.
Hypothesis 4 was that increased exposure to the intervention would lead to increased maintenance of physical activity and engagement in exercise. Therefore, within the intervention arm, intervention dose would correlate negatively with decreases in physical activity, engagement in muscle-strengthening exercise and SPPB score from 6 to 12 months (during the supported maintenance period) and from 12 to 24 months (the unsupported maintenance period).
Hypothesis 5 was that exposure to the intervention would lead to changes in key psychosocial determinants of physical activity and exercise from baseline to 6 and 12 months:
-
Compared with the control arm, the intervention arm would experience increases in autonomy, competence (self-efficacy), relatedness for MVPA and exercise, perceived intrinsic benefits of exercise (social, physical and emotional) and enjoyment of physical activity and exercise from 0 to 6 months. An originally intended measure of physical activity-related self-concept was removed from the questionnaire pack to reduce patient burden following review of the process evaluation measures by the TMG and patient and public involvement members. Hence the words ‘self-concept’ have been removed from the original wording of this hypothesis.
-
Compared with the control arm, the intervention arm would experience increases in physical activity-related autonomy, competence (self-efficacy), relatedness for physical activity and exercise, perceived intrinsic benefits of physical activity and exercise (social, physical and emotional) and enjoyment of physical activity and exercise from 0 to 12 months. As with the hypothesis above, the words ‘self-concept’ have been removed from the original wording of this hypothesis.
-
Increased exposure to the intervention (total contact time from baseline to the relevant time point) would correlate with increased change in the above determinants (and in the expected direction).
Hypothesis 6 was that the intervention effect on SPPB (intervention vs. control) would be mediated by changes in muscle-strengthening exercise and by changes in MVPA, changes in lower intensity physical activity or walking activity (Figure 5). The amount of variance in SPPB explained by the different types of activity/exercise will be of interest. The words ‘changes in balance and co-ordination exercise’ have been removed from the original wording of this hypothesis, as this could not be distinguished from muscle-strengthening exercise by the measure used.
FIGURE 5.
Causal diagram for mediators and moderators of intervention effects on physical function.
Hypothesis 7 was that the intervention effect on SPPB score and on physical activity and exercise from 0 to 6 and 0 to 24 months may be moderated by a number of potential moderating variables, including age, sex, ethnicity, baseline physical activity and SPPB, co-interventions, comorbidities, BMI, mental health, SES, education level (see Figure 5, and Appendix 2, Figure 30). The words ‘and on PA and exercise’ have been added to this hypothesis, as the moderating influence of contextual variables on changes in physical activity behaviours (as well as physical functioning) is of key scientific interest. The time point 0–12 months was removed to reduce the number of analyses. The moderation of short-term (0–6 months) and long-term (0–24 months) effects was considered to be of the most interest from the three time points available.
Participants
We applied analyses to the whole sample where data were available; however, some hypotheses applied only to the intervention arm.
Measures
Brief questionnaires assessing the following mechanisms of change suggested by SCT and SDT (the main theoretical underpinnings of the intervention model) were administered at baseline and 6, 12 and 24 months (see Table 2 for full details):
-
autonomy in relation to physical activity
-
competence for physical activity
-
relatedness for physical activity
-
enjoyment of physical activity
-
perceived intrinsic benefits of physical activity (social, physical and emotional)
-
autonomy for strength-building exercise
-
competence for strength-building exercise
-
relatedness for strength-building exercise
-
enjoyment of strength-building exercise
-
perceived intrinsic benefits of strength-building exercise (social, physical and emotional).
To test the hypotheses, we also used measures of intervention engagement (session attendance) and intervention effectiveness [SPPB scores, physical activity/time doing MVPA (by accelerometer and PASE questionnaire), walking (PASE walking subscale), sedentary time (accelerometer), self-reported engagement in muscle strength exercise (MSEQ adherence score)] and secondary outcomes of interest (cognitive function, falls, pain, sleep quality, well-being and grip strength).
Variables used to define individual context were age, sex, ethnicity, baseline physical activity, baseline SPPB, co-interventions, physical comorbidities, BMI, mental health status (any diagnosed mental health conditions in the 6 months prior to baseline), IMD (from postcode) and education level. All of the measures are described in detail in Chapter 2.
Analysis
The above hypotheses were tested using a variety of statistical analyses, as described in Table 10. To maximise sensitivity to change and avoid difficulties in constructing the mediation analyses (which require specialist software),97 the analyses testing hypotheses 1 to 19 have been constructed without accounting for clustering by exercise group within the intervention arm.
| Hypothesis | Analysis |
|---|---|
| Hypothesis 1: the intervention will increase physical activity and muscle-strengthening exercise from 0 to 6 months | Comparison of change scores between intervention and control arms, using univariate ANCOVA models with the baseline value of the outcome, age (continuous variable), sex (male, female) and study site (Devon, Bath/Bristol, Birmingham) entered as covariates |
| Hypothesis 2: intervention exposure will correlate with change in physical activity and muscle-strengthening exercise from 0 to 6 months | Bivariate correlation between the outcome variable and intervention exposure (% of sessions attended) within the intervention arm only. Given the skewed distribution for intervention exposure (46/409 intervention participants did not attend any sessions), we categorised attendance as high (≥ 75%), medium (51–74%) or low (0–50%) and used ANCOVA analysis to look for a linear trend in the mean outcome scores for these three groups. These models also controlled for age, sex and study site (the covariates used in the primary trial analysis) |
| Hypothesis 3: intervention exposure will correlate with change in physical activity, muscle-strengthening exercise and SPPB from 0 to 12 months | The analyses were the same as for testing hypothesis 2, except for changes to the dependent variable (changes from 0 to 12 months rather than from 0 to 6 months) |
| Hypothesis 4: intervention exposure will correlate with maintenance of physical activity and engagement in exercise from 6 to 12 and from 12 to 24 months | The analyses were the same as for testing hypothesis 2, except for changes to the dependent variable |
| Hypothesis 5: intervention will change key psychosocial determinants of physical activity and exercise from baseline to 6 and 12 months. Such increases will correlate with intervention exposure within the intervention arm | Comparison of change scores between intervention and control arms, using univariate ANCOVA models with the baseline value of the outcome, age (continuous variable), sex (male, female) and study site (Devon, Bath/Bristol, Birmingham) entered as covariates |
| Hypothesis 6: the intervention effect on SPPB (and on key secondary outcomes) will be mediated by changes in muscle-strengthening exercise and in physical activity | Mediation analyses were conducted using linear regression models set up in SPSS v26, using the PROCESS v3.4 macro.97 Model 4 was selected, which uses a single regression model to estimate coefficients for (1) the direct effect of the independent variable (intervention arm) on the dependent variable (e.g. SPPB) and (2) the indirect effect of the independent on the dependent variable when mediated by a third variable (e.g. physical activity). Bootstrapping with 5000 samples was used to estimate CIs. Age, sex and study site were entered into the models as covariates |
| Hypothesis 7: the intervention effect on physical activity and exercise from 0 to 6 and from 0 to 24 months may be moderated by a number of contextual variables (11 variables specified) | Linear regression models were used to estimate the interaction effects of individual moderators with trial arm. We followed procedures for moderation analysis recommended by Hayes.97 Any significant interactions were probed by calculating point estimates at the 16th, 50th and 84th centiles for continuous variables (equivalent to the mean plus or minus one SD for normally distributed data) or at distinct category values where categorical moderators were included. Any significant interactions were visualised to aid interpretation and checked for floor or ceiling effects. The analyses were conducted in SPSS V26 using the PROCESS macro.97 The p-value representing significance was not adjusted for multiple testing as these analyses are considered to be exploratory (as indicated by the words ‘may be’ in the hypothesis). It is worth noting that adjusting for the 11 tests conducted at each time point/for each outcome would yield a p-value of 0.0045. Any findings not meeting this criterion are, therefore, potentially vulnerable to analytic bias owing to multiple testing. Note that, for these analyses, ethnic groups were collapsed into two groups owing to numbers being too small to allow finer-grained analysis: (1) black, Asian, Chinese and other ethnic groups and (2) white |
Results
Hypothesis 1 to hypothesis 5: effects of the intervention on mediators of lower limb physical function
Full data tables are available in Report Supplementary Material 2. Key statistics are provided in the following text for significant findings only.
Hypothesis 1
Hypothesis 1 was that being in the intervention arm would lead to changes in physical activity (MVPA, steps, sedentary time) and engagement in muscle-strengthening exercise from 0 to 6 months.
There was mixed evidence in support of hypothesis 1, with measures of self-reported physical activity, walking and muscle-strengthening exercise appearing to be more sensitive to the intervention than accelerometer measures. Unbouted MVPA seemed to be more sensitive than bouted MVPA to the intervention for the accelerometer measures. Hence, unbouted MVPA was used to assess changes in physical activity in testing the following hypotheses (hypotheses 2–7). Based on accelerometer data, there were no significant differences between the intervention arm and the control arm in bouted MVPA (in bouts of at least 10 minutes) or sedentary time at 6 months, controlled for baseline MVPA, site, sex and age. However, there was a close to significant difference of 20.2 minutes of unbouted MVPA per week (2.88 minutes per day, 95% CI 5.82 to –0.06 minutes per day; p = 0.054) favouring the intervention arm.
Using questionnaire data, significant differences favouring the intervention arm were observed in self-reported physical activity (PASE total score) (mean difference 16.54 points, 95% CI 7.89 to 25.20 points; p < 0.001), self-reported walking (PASE walking outside items: days per week × hours per day) (mean difference 0.727 hours per week, 95% CI 0.293 to 1.162 hours per week; p = 0.001) and self-reported muscle-strengthening exercise (MSEQ adherence score) (mean difference 0.73 scale points, 95% CI 0.42 to 1.04 scale points; p < 0.001).
Hypothesis 2
Hypothesis 2 was that increased exposure to the intervention would correlate with increased change in physical activity (MVPA, steps, sedentary time) and engagement in muscle-strengthening exercise from 0 to 6 months.
Hypothesis 2 was broadly supported, with a consistent pattern of data across all measures (albeit with two out of the five measures achieving only marginal significance in the linear trend analysis). There were significant, weak correlations between intervention attendance and changes from 0 to 6 months in unbouted MVPA (R = 0.147, p = 0.012, n = 292), PASE total score (R = 0.136, p = 0.012, n = 339) (see Appendix 2, Figure 34), hours per week spent walking outside (from PASE walking items) (R = 0.165, p = 0.002, n = 332) and change in MSEQ adherence score (R = 0.122, p = 0.029, n = 320), but not in sedentary time (R = –0.063, p = 0.290, n = 281).
Significant linear trends, whereby positive changes in outcomes were associated with increased session attendance, were identified for unbouted MVPA (Figure 6; difference between low and high attenders 9.8 minutes per day, p = 0.002 for the linear trend, n = 265), PASE walking outside (see Appendix 2, Figure 32) (difference between low and high attenders: 1.24 hours per week, p = 0.011, n = 302) and MSEQ adherence score (see Appendix 2, Figure 33) (difference between low and high attenders 0.82 points, p = 0.049, n = 293). Linear trends that were in the predicted direction were observed for 0- to 6-month changes in sedentary time (difference between low and high attenders 28.7 minutes per day, p = 0.056, n = 255) and PASE total score (difference between low and high attenders 17.8 points, p = 0.051, n = 308).
FIGURE 6.
Changes in daily unbouted MVPA from 0 to 6 months for different levels of attendance within the intervention arm (error bars: 95% CI).
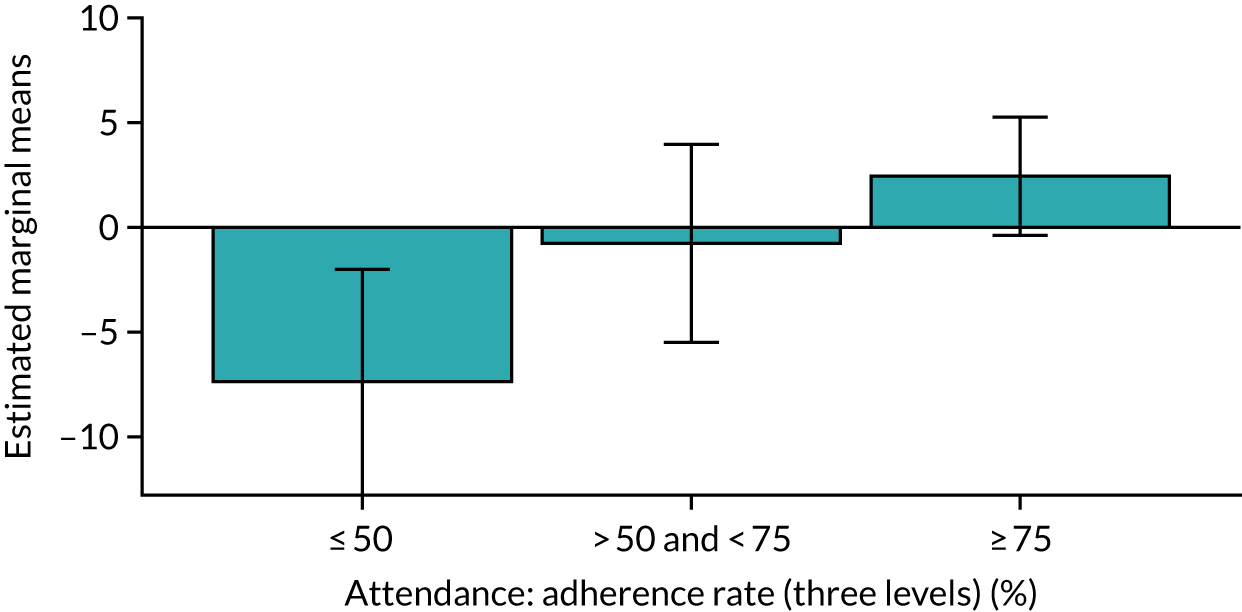
Hypothesis 3
Hypothesis 3 was that increased exposure to the intervention would correlate with increased change in physical activity (MVPA, steps, sedentary time), engagement in muscle-strengthening exercise and SPPB score from 0 to 12 months.
Hypothesis 3 was broadly supported, although not with respect to accelerometer-assessed sedentary time and with two out of the five measures achieving only marginal significance in the linear trend analysis. There were significant, weak correlations between intervention attendance and changes from 0 to 12 months in unbouted MVPA (R = 0.174, p = 0.004, n = 278), PASE total score (R = 0.134, p = 0.015, n = 329), PASE walking score (R = 0.158, p = 0.004, n = 321), MSEQ adherence score (R = 0.252, p < 0.001, n = 315) and SPPB score (R = 0.175, p = 0.001, n = 346), but not in sedentary time (R = –0.076, p = 0.215, n = 268).
Significant linear trends, whereby positive changes in outcomes were associated with increased session attendance, were identified for PASE total score (see Appendix 2, Figure 35) (difference between low and high attenders 17.9 points, p = 0.043 for the linear trend, n = 301), MSEQ adherence score (Figure 7) (difference between low and high attenders 1.28 points, p = 0.001, n = 292) and SPPB (Figure 8) (difference between low and high attenders 0.89 points, p = 0.004, n = 316). A linear trend that was in the predicted direction was observed for 0- to 12-month changes in unbouted MVPA (difference between low and high attenders 5.1 minutes per day, p = 0.051, n = 256) and for the PASE walking outside score (difference between low and high attenders 0.805 hours per week, p = 0.083, n = 295). No significant trend was observed for sedentary time.
FIGURE 7.
Changes in muscle-strengthening exercise (MSEQ adherence score) from 0 to 12 months for different levels of attendance in the intervention arm (error bars: 95% CI).
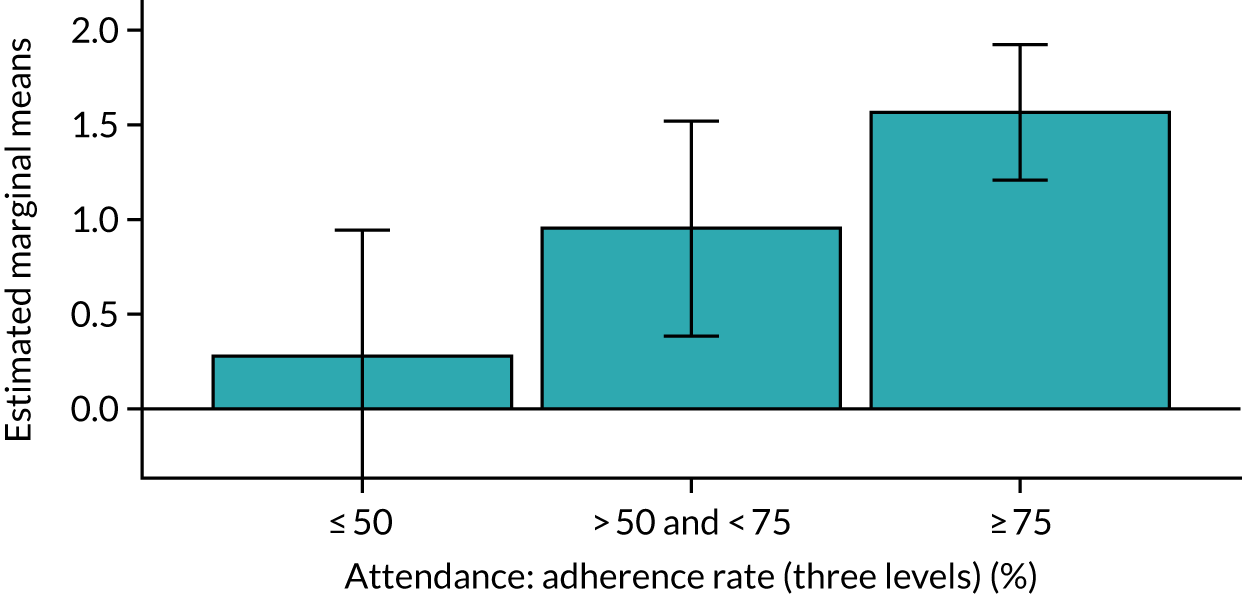
FIGURE 8.
Changes in SPPB total score from 0 to 12 months for different levels of attendance in the intervention arm (error bars: 95% CI).
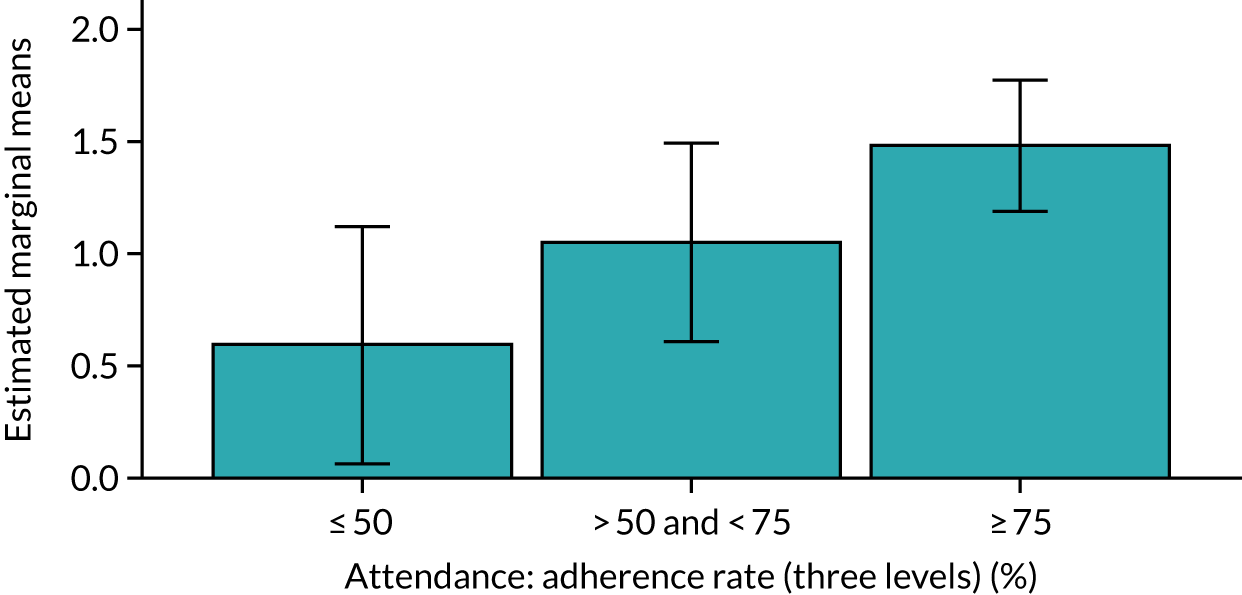
Hypothesis 4
Hypothesis 4 was that increased exposure to the intervention would lead to increased maintenance of physical activity and engagement in exercise. Therefore, within the intervention arm, intervention dose would correlate negatively with decreases in physical activity, engagement in muscle-strengthening exercise and SPPB score from 6 to 12 months (during the supported maintenance period) and from 12 to 24 months (the unsupported maintenance period).
Hypothesis 4 was not supported by the data (in 13 out of 14 analyses). These analyses were applied in the intervention arm only. No significant correlations were found between percentage session attendance and changes from 6 to 12 months in bouted MVPA, unbouted MVPA, sedentary time, PASE total score, PASE walking score, MSEQ adherence score or SPPB score. There was a weak negative correlation between percentage session attendance and changes in bouted MVPA from 12 to 24 months (R = –0.125, p = 0.047, n = 252). No other changes in outcomes from 12 to 24 months were significantly correlated with session attendance. No significant linear relationship were found for any of the outcomes across the three attendance groups, controlling for age, sex, study site and baseline SPPB score.
Hypothesis 5
Hypothesis 5 was that exposure to the intervention would lead to changes in key psychosocial determinants of physical activity and exercise from baseline to 6 and 12 months.
Hypothesis 5a was that, compared with controls, the intervention arm will experience increases in autonomy and competence (self-efficacy); relatedness for MVPA, exercise and perceived intrinsic benefits of exercise (social, physical and emotional); and enjoyment of physical activity and exercise from 0 to 6 months.
Hypothesis 5a was broadly supported: we saw an intervention effect on most psychosocial determinants specified by the REACT logic model at 6 months. However, no signal was detected in relation to autonomy (for either muscle-strengthening or MVPA) or for enjoyment of muscle-strengthening exercise.
In relation to MVPA, at the 6-month follow-up, there was a significantly greater increase in the intervention arm compared with the control arm for perceived competence (mean difference 0.343 points, 95% CI 0.148 to 0.539 points, p = 0.001, n = 627), relatedness (mean difference 0.278 points, 95% CI 0.138 to 0.419 points, p < 0.001, n = 610) and enjoyment of MVPA (mean difference 0.149 points, 95% CI 0.015 to 0.282, p = 0.030, n = 627). However, there was no significant difference in autonomy related to MVPA (mean difference 0.065 points, 95% CI –0.040 to 0.170 points, p = 0.223, n = 625).
In relation to muscle-strengthening exercise, at the 6-month follow-up, there was a significant increase in the intervention arm compared with the control arm for perceived competence (mean difference 0.705 points, 95% CI 0.490 to 0.921 points, p < 0.001, n = 625) and relatedness (mean difference 0.283 points, 95% CI 0.040 to 0.525 points, p = 0.023, n = 254). The perceived intrinsic benefits of exercise were higher in the intervention arm than in the control arm at 6 months (mean difference 0.862, 95% CI 0.672 to 1.053, p < 0.001, n = 609). However, there were no significant differences in enjoyment (mean difference 0.185 points, 95% CI –0.037 to 0.407 points, p = 0.103, n = 261) or in autonomy related to muscle strengthening (mean difference –0.042 points, 95% CI –0.199 to 0.115 points, p = 0.597, n = 257).
Hypothesis 5b was that, compared with control subjects, the intervention arm will experience increases in physical activity-related autonomy and competence (self-efficacy); relatedness for physical activity, exercise and perceived intrinsic benefits of exercise (social, physical and emotional); and enjoyment of physical activity and exercise from 0 to 12 months.
Hypothesis 5b was broadly supported: we saw an intervention effect on most psychosocial determinants specified by the REACT logic model at 12 months. However, no signal was detected in relation to autonomy (for either muscle-strengthening or MVPA) or enjoyment of MVPA.
In relation to MVPA, at the 12-month follow-up, there was a significant increase in the intervention arm compared with the control arm for perceived competence (mean difference 0.279 points, 95% CI 0.073 to 0.486 points, p = 0.008, n = 615) and relatedness (mean difference 0.211 points, 95% CI 0.064 to 0.357 points, p = 0.005, n = 594). However, there was no significant difference in autonomy related to MVPA (mean difference –0.052 points, 95% CI –0.064 to 0.168 points, p = 0.378, n = 613) or in enjoyment of MVPA (mean difference 0.083 points, 95% CI –0.051 to 0.218 points, p = 0.225, n = 612).
In relation to muscle-strengthening exercise, at the 12-month follow-up, there was a significant increase in the intervention arm compared with the control arm for perceived competence (mean difference 0.625 points, 95% CI 0.403 to 0.847 points, p < 0.001, n = 609), relatedness (mean difference 0.438 points, 95% CI 0.196 to 0.679 points, p < 0.001, n = 265) and enjoyment (mean difference 0.250 points, 95% CI 0.023 to 0.476 points, p = 0.031, n = 268). The perceived intrinsic benefits of exercise were also higher in the intervention arm than in the control arm at 12 months (mean difference 0.757, 95% CI 0.565 to 0.950, p < 0.001, n = 598). However, there were no significant differences for autonomy related to muscle-strengthening (mean difference –0.023 points, 95% CI –0.174 to 0.128 points, p = 0.769, n = 268).
Hypothesis 5c was that increased exposure to the intervention (total contact time from baseline to the relevant time point) will correlate with increased change in the above determinants (and in the expected direction).
Hypothesis 5c was largely supported in relation to muscle-strengthening exercise but not in relation to MVPA.
In relation to MVPA, at the 12-month follow-up, we identified one significant linear trend, whereby an increase in MVPA relatedness was associated with increased session attendance (Figure 9) (difference between low and high attenders 0.564 points in the predicted direction, p = 0.002 for the linear trend, n = 295). No significant trend was observed for MVPA autonomy, MVPA competence or MVPA enjoyment.
FIGURE 9.
Changes in relatedness in relation to MVPA from 0 to 12 months for different levels of attendance in the intervention arm (error bars: 95% CI).

In relation to muscle-strengthening exercise, at the 12-month follow-up, we identified significant linear trends whereby positive changes in psychosocial determinants were associated with increased session attendance for muscle-strengthening competence (Figure 10) (difference between low and high attenders 0.633 points in the predicted direction, p = 0.021, n = 298), relatedness (Figure 11) (difference between low and high attenders 0.619 points in the predicted direction, p = 0.041 for the linear trend, n = 149), enjoyment (Figure 12) (difference between low and high attenders 0.745 points in the predicted direction, p = 0.014, n = 153) and perceived benefits of muscle-strengthening exercise (Figure 13) (difference between low and high attenders 1.142 points in the predicted direction, p < 0.001, n = 291). No significant trend was observed for autonomy for muscle-strengthening exercise.
FIGURE 10.
Changes in competence in relation to muscle-strengthening exercise from 0 to 12 months by level of attendance in the intervention arm (error bars: 95% CI).
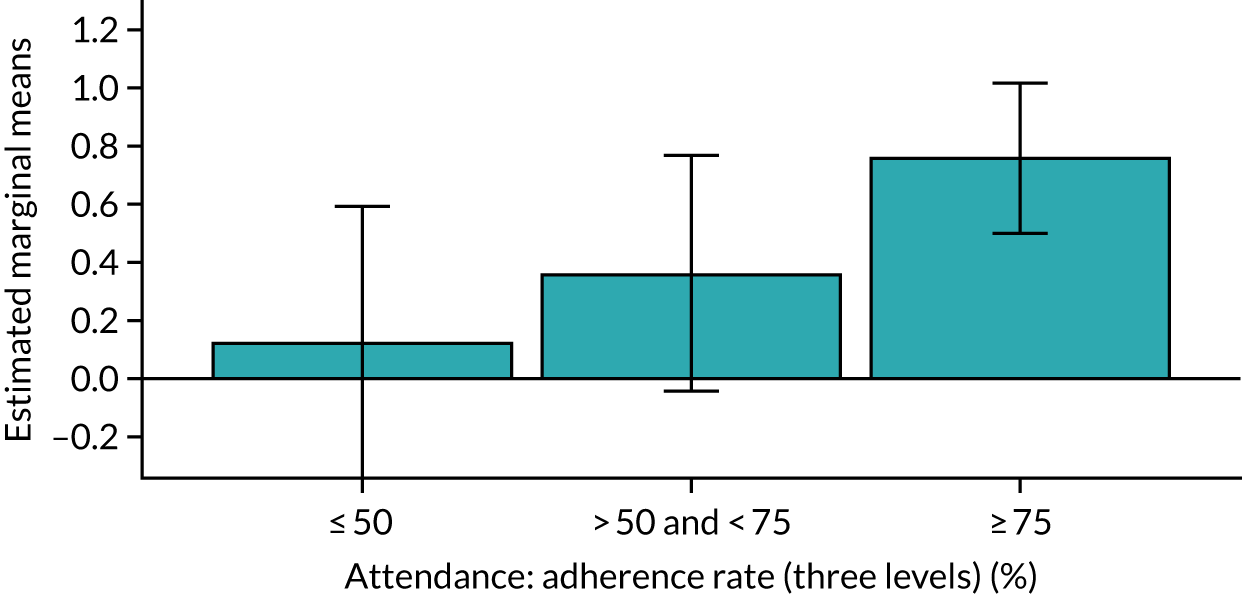
FIGURE 11.
Changes in relatedness for muscle-strengthening exercise from 0 to 12 months by level of attendance in the intervention arm (error bars: 95% CI).
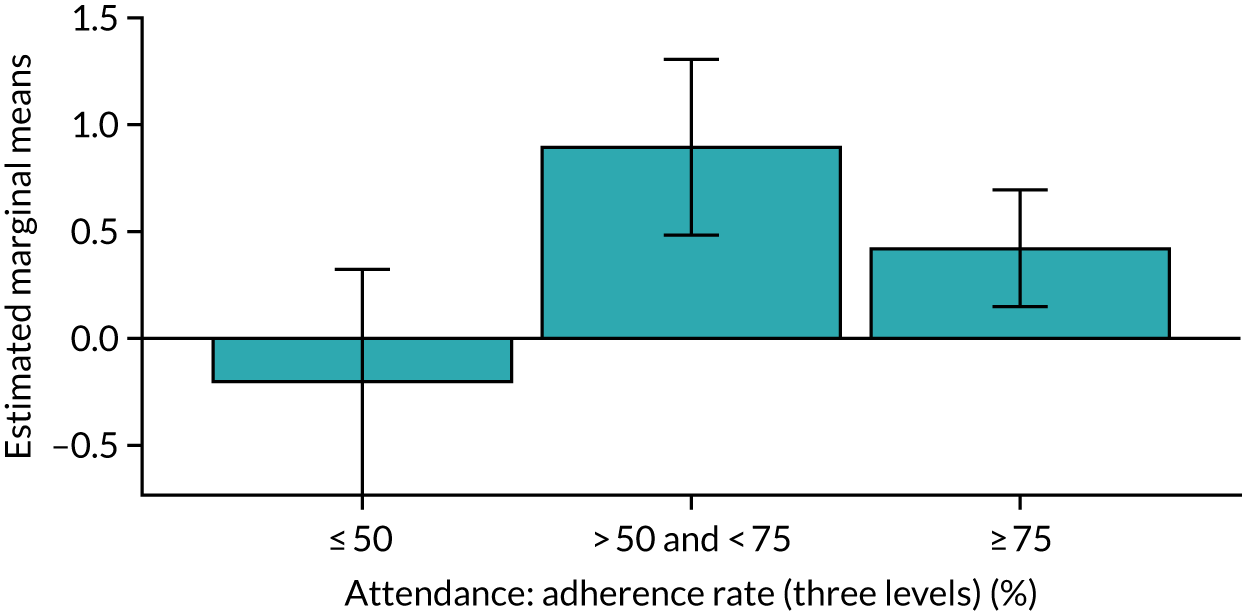
FIGURE 12.
Changes in enjoyment in relation to muscle-strengthening exercise from 0 to 12 months by level of attendance in the intervention arm (error bars: 95% CI).
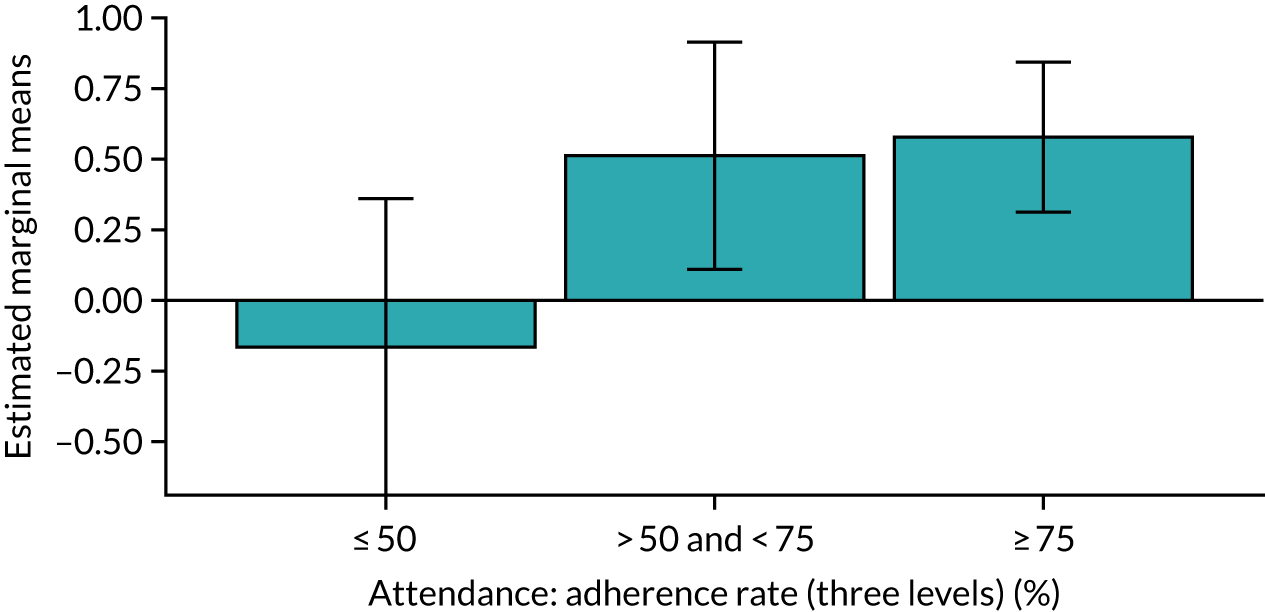
FIGURE 13.
Changes in perceived benefits of muscle-strengthening exercise from 0 to 12 months by level of attendance in the intervention arm (error bars: 95% CI).
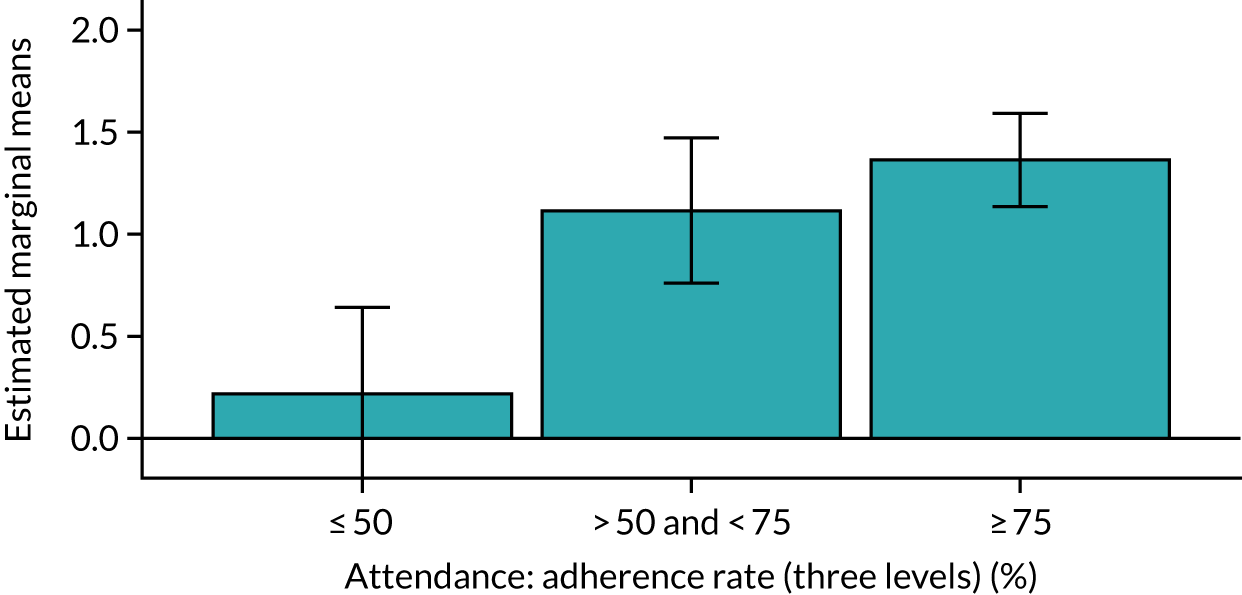
Hypotheses 6 and 7 mediation and moderation of intervention effects on lower limb physical function
Hypothesis 6
Hypothesis 6 was that the intervention effect on SPPB (intervention vs. control) will be mediated by changes in muscle-strengthening exercise and by changes in MVPA, lower-intensity physical activity or walking activity.
Lower-intensity physical activity was assessed using our accelerometer measures of sedentary time (also called ‘very low physical activity’).
Hypothesis 6 was largely supported by the data, with the proviso that accelerometer-based measures did not follow the same pattern as the self-reported measures. The intervention effect on SPPB score was partly or completely mediated by changes in self-reported muscle-strengthening exercise and self-reported changes in physical activity (both total activity and walking) between 0 and 12 months and between 0 and 24 months. No accelerometer measures of physical activity significantly mediated the intervention effect on SPPB score at any time point. The standardised mediation effects for muscle-strengthening exercise were generally higher than those for physical activity, and walking seemed to account for the majority of the mediating effect identified by the PASE questionnaire (Table 11).
| Mediating variable | Standardised coefficient (95% CI); n | ||
|---|---|---|---|
| 6 months | 12 months | 24 months | |
| Muscle-strengthening (MSEQ) | – | 0.051 (0.018 to 0.092); 579 | 0.053 (0.020 to 0.094); 569 |
| Total activity (PASE) | – | 0.025 (0.004 to 0.054); 616 | 0.039 (0.008 to 0.075); 598 |
| Walking (PASE) | – | 0.017 (0.000 to 0.041) 598 | 0.029 (0.004 to 0.059); 590 |
| MVPA (accelerometer) | – | – | – |
| VLPA (accelerometer) | – | – | – |
Hypothesis 7
Hypothesis 7 was that the intervention effect on SPPB score, physical activity and exercise from 0 to 6 months and from 0 to 24 months may be moderated by a number of variables, including age, sex, ethnicity, baseline physical activity and SPPB score, co-interventions, comorbidities, BMI, mental health, SES and education level.
In the main trial analysis (see Chapter 3 and Appendix 2, Figure 34), intervention effects were found for PASE total score and muscle-strengthening exercise (MSEQ adherence score), as well as the primary outcome (SPPB score) at both 6 and 24 months. Report Supplementary Material 2 shows the moderation effects of all of the variables specified in the hypothesis of these three variables. The text below gives an overview of these data, highlighting any significant associations.
Intervention effects on SPPB, physical activity and engagement in muscle-strengthening exercise (MSEQ) were not significantly moderated by age, sex, baseline SPPB score, co-morbidity (number of chronic physical conditions at baseline) or BMI. However, the following significant interactions were observed (Table 12).
| Moderating Variable | SPPB | Muscle-strengthening (MSEQ) | Total activity (PASE) | |||
|---|---|---|---|---|---|---|
| 6 months | 24 months | 6 months | 24 months | 6 months | 24 months | |
| Age | ||||||
| Sex | ||||||
| Ethnicity | –1.426, SE 0.714; p = 0.048 | –69.519, SE 24.500; p = 0.005 | ||||
| SES (deprivation quintile) | R2 change 0.022, F = 3.818 (df = 4, 649); p = 0.0045 | R2 change 0.017, F = 2.729 (df = 4, 587); p = 0.029 | R2 change 0.017, F = 2.715 (df = 4, 604); p = 0.029 | |||
| Baseline physical activity | 0.188, SE 0.079, p = 0.018 | |||||
| Baseline SPPB | ||||||
| Co-interventions | 26.741, SE 11.664, p = 0.022 | |||||
| Comorbidities | ||||||
| BMI | ||||||
| Mental health | –2.335, SE 0.972; p = 0.017 | –2.450, SE 1.057; p = 0.021 | ||||
| Education level | R2 change 0.016, F = 2.447 (df = 4, 572); p = 0.045 | |||||
At 6 months, the estimated effects of the intervention on SPPB score and the PASE total score were higher for ethnic minority participants than for white participants [1.43 points higher for SPPB score (beta 1.43; p = 0.048); 69.52 points higher for PASE total score (beta 69.52; p = 0.005)]. The interactions are visualised in Report Supplementary Material 2. These results need to be treated with considerable caution owing to (1) multiple testing (the Bonferroni adjusted p-value for multiple testing would be 0.0045) and (2) unbalanced group sizes (31 ethnic minority participants vs. 628 white participants for SPPB score, and 25 ethnic minority participants vs. 625 white participants for PASE total score).
Socioeconomic status (deprivation quintile) significantly moderated the effects of the intervention on SPPB score (p = 0.0045) and MSEQ score at 6 months (p = 0.029), and on PASE total score at 24 months (p = 0.029) (see Table 12). The interactions are visualised in Report Supplementary Material 2. For SPPB score at 6 months, the intervention worked best in the highest deprivation group (estimated mean difference 1.78 points, 95% CI 0.88 to 2.67 points, n = 70) and worst in the lowest deprivation group (estimated mean difference –0.13 points, 95% CI –0.67 to 0.41, n = 191), with a general pattern of increasing effect size as area deprivation decreased. However, there was no clear pattern of effect for MSEQ score or physical activity. These results need to be treated with considerable caution owing to (1) multiple testing (the Bonferroni adjusted p-value for multiple testing would be 0.0045) and (2) unbalanced group sizes (ranging from 63 to 171).
Physical activity level at baseline significantly moderated the effects of the intervention on physical activity at 6 months (beta 0.188; p = 0.018), with intervention effects being greater in those who were more physically active at baseline. The interaction is visualised in Report Supplementary Material 2. This result needs to be treated with caution owing to multiple testing (the adjusted p-value would be 0.0045).
The intervention effect from 0 to 24 months on self-reported physical activity (PASE total score) was significantly moderated by engagement in co-interventions during the 24 months of the study (beta 26.741; p = 0.022). The intervention effect on physical activity for people who engaged in additional physical activity interventions during the course of the study was 26.741 PASE points higher than for the subgroup who did not engage in such interventions. This result needs to be treated with caution owing to multiple testing (the adjusted p-value would be 0.0045).
Mental health status at baseline significantly moderated the effects of the intervention on MSEQ score at both 6 months (beta –2.335; p = 0.017) and 24 months (beta –2.450; p = 0.021), with intervention effects being greater in those who had experienced no diagnosed mental health condition in the last 6 months. The interactions are visualised in Report Supplementary Material 2, which shows that the effects in both cases are largely driven by large increases over time in MSEQ adherence for people with mental health conditions in the control arm. This may reflect a regression to the mean effect in participants who had a transient mental health condition at baseline (they may have been less engaged in exercise at baseline), or it may reflect some kind of responding bias (i.e. a tendency for people with a mental health condition to under-report activity at baseline). This result needs to be treated with considerable caution owing to (1) multiple testing (the adjusted p-value would be 0.0045) and (2) unbalanced group sizes (29 participants with a recent mental health condition at baseline vs. 567 participants with none).
The intervention effect from 0 to 24 months on MSEQ adherence score was also significantly moderated by education level. The explanatory value of adding the arm × education interaction term to the regression model (R2 change) was 0.016 (p = 0.045). The interaction was largely driven by an unexpected increase in MSEQ score from 0 to 24 months in the least educated control arm participants. This result needs to be treated with caution owing to multiple testing (adjusting for this would require a p-value of 0.0045 to denote significance).
Discussion
Summary of findings
Testing of the hypotheses supported many elements of the logic model for the REACT intervention (see Figure 1). Changes in physical activity in terms of muscle-strengthening exercise, walking and overall physical activity were observed during the initial intervention period (0–6 months). In the intervention arm, these changes, as well as changes in physical activity and SPPB score from 0 to 1 month, were linearly related to the number of intervention sessions attended. No significant changes in sedentary time were observed, however, and maintenance of physical activity from 6 to 12 months and from 12 to 24 months was not associated with intervention exposure.
The intervention generated significant changes in most of the proposed mechanisms of behaviour change at both 6 and 12 months, including (1) changes in perceived competence, relatedness and enjoyment (at 6 months only) relating to MVPA; and (2) changes in perceived competence, relatedness, enjoyment (at 12 months only) and intrinsic benefits relating to muscle-strengthening exercise. However, no signal was detected in relation to perceived autonomy for either muscle-strengthening or MVPA. Changes in the psychosocial determinants of muscle-strengthening exercise were linearly related to session attendance (for competence, relatedness, enjoyment and perceived benefits, but not autonomy). Changes in the psychosocial determinants of MVPA were not related to intervention exposure, except for relatedness.
Mediation analyses showed that the intervention effects on SPPB at 12 and 24 months were mediated by self-reported engagement in muscle-strengthening exercise (MSEQ adherence score). These effects were also mediated by self-reported measures of physical activity (PASE total and PASE walking), but not by accelerometer-assessed physical activity.
Moderation analyses found that the short-term (0–6 months) intervention effects on SPPB were moderated by ethnicity and area deprivation (with ethnic minorities and more-deprived population groups benefiting more from the intervention than white and less-deprived populations). Longer-term (0–24 months) intervention effects on SPPB were not moderated by any of the variables tested, indicating that the intervention worked equally well for participants irrespective of their age, sex, ethnicity, baseline fitness (as indicated by baseline physical activity) and physical functioning, co-interventions, physical and psychological comorbidities, BMI, SES and education level. All of the above are consistent with the idea that the intervention worked through increases in resistance/muscle-strengthening exercise, which in turn was associated with session attendance and changes in the theorised psychosocial variables, as specified in the REACT logic model (with the exception of perceived autonomy).
Relation to other literature and interpretation
These findings add to the body of literature on the processes of behaviour change for physical activity interventions, particularly in relation to older people. A recent systematic review of the literature on process evaluation in trials of physical activity interventions, conducted as part of the PhD associated with the REACT project,71 found that, since publication of the MRC guidance on process evaluation in 2014,66 only 21% of trials (24 papers) quantitatively evaluated mechanisms of behaviour change and only 15% of trials (17 papers) evaluated the moderating effects of context on outcomes. Studies of such processes in older people and in relation to longer-term intervention effects (beyond 12 months) are even scarcer.
The findings on the psychosocial process of behaviour change are consistent with, and help to validate, the REACT logic model and its underpinning theories, in particular SDT41 and SCT. 98 However, it is worth noting that the SDT process of increasing autonomy did not seem to be associated with increases in either muscle-strengthening exercise or overall physical activity. This may reflect the structured, centre-based nature of the intervention, including formal exercise sessions (the only element of choice being whether or not to attend), although we did try to promote autonomy and choice in the social education sessions around making plans for physical activity and for continuation of exercise outside the REACT context (and our intervention fidelity analysis indicated that this element was adequately delivered). Nevertheless, the data suggest that promoting autonomy/choice may not be necessary for promoting physical activity, and particularly muscle-strengthening exercise, in older people. This may reflect a preference among some older people for more structured/guided interventions that are enjoyable and socially engaging (and that deliver noticeable physical, social and mental benefits), rather than for individual choice-based activities. In theory, promoting autonomy should be important for longer-term maintenance of exercise behaviours, particularly when the REACT intervention comes to an end. However, this did not seem to be substantiated by the data in this case.
Alternatively, the data on autonomy processes may reflect a measurement error: the items that we used to assess autonomy in relation to physical activity and exercise (i.e. ‘I feel free to make my own decisions about muscle-strengthening exercises’; ‘I feel like I am in charge of how often I do muscle-strengthening exercises’) were taken from a validated scale and had good internal reliability (Cronbach’s α = 0.71 for the two-item physical activity autonomy scale and α = 0.76 for the two-item muscle-strengthening exercise autonomy scale). However, the scores were skewed towards the high end of the scale (mean scores 8.1 and 7.7, respectively, on a 0 to 10 scale), suggesting a possible ceiling effect for this measure. The items selected were also part of a longer scale and, therefore, may not function well in isolation.
Importantly, the moderation analyses found that the intervention appeared to perform well in relation to health inequalities. People from ethnic minority backgrounds and areas of high deprivation responded as well to the intervention (and seemingly better at 6 months) as white participants and people from areas of low deprivation. Furthermore, no differences in intervention effect were noted by sex, baseline physical functioning or other demographic characteristics. It is worth noting that the lack of any quantitative difference between people with high or people with low SPPB score at baseline contrasts with the qualitative data reported in Chapter 4, Study 2: qualitative evaluation of intervention processes. This may reflect non-generalisation of the reported experiences from the relatively small qualitative sample, or it may reflect a difference between subjectively perceived and objectively measured changes in physical functioning. In particular, the perceptions of benefit for those with lower SPPB score at baseline may have been diminished by comparison with their peers who ended the intervention having achieved (and also started with) better physical functioning. Negative effects of social comparisons involving ‘upwards contrasts’ on health perceptions have been noted in other literature. 99
In contrast to the increasingly dominant paradigm favouring objective measurement of physical activity, the findings here suggest that self-reported measures of physical activity may be more valid indicators of engagement in the physical activities targeted by exercise interventions than accelerometer measures. 100,101 In this study, we found that overall physical activity and walking activity, as measured by the PASE questionnaire, and muscle-strengthening activity, as measured by the two-item MSEQ adherence score that we developed specifically for this study, were sensitive to change (producing highly significant differences between groups) and linearly associated with increasing intervention attendance. They, therefore, seem to be valid indicators of participant engagement in the activities targeted by the REACT intervention. In this study, we are assured that physical activity, especially muscle-strengthening exercise, changed as a result of the intervention because this is the only way that physical functioning (as assessed using the SPPB) could have been modified at 12 and 24 months. However, the self-reported measures were better able than the accelerometers to detect these changes. Other literature acknowledges the potentially complementary roles of questionnaires and accelerometers for assessing physical activity in older people. 102
Strengths and limitations
The REACT process evaluation benefited from having access to a large data set with good levels of retention and completion for most measures. This offered good statistical power for most analyses. However, some of the moderation analyses (e.g. the analyses including ethnicity and mental health conditions) need to be treated with caution owing to multiple testing (11 analyses per time point and per variable), small/unbalanced group sizes (e.g. only around 30 participants in the ethnic minority group) and the inability to distinguish between different ethnic minority subgroups (owing to the small numbers available for analysis).
Implications for practice/future research directions
The process evaluation identified ways in which both the theoretical basis and the delivery of the intervention could be further improved.
The data from the quantitative process evaluation broadly support the theoretical basis for behaviour change in the REACT logic model. However, they also imply that some intended processes (notably promoting autonomy) were not necessary for change to occur. Further research is needed to explore the role of autonomy in the REACT intervention and whether or not this construct should be retained in the logic model.
The suggestions of individual-level contextual influences on intervention effectiveness in the logic model (relating to age, sex, etc.) were not supported. However, further research is needed to examine the associations of systemic influences and facilitator-level factors with intervention effectiveness.
Hypotheses about theoretical processes associated with the maintenance of behaviour change (e.g. autonomy, relatedness and enjoyment of physical activity) were not supported and need further investigation. This might involve analyses focused on maintenance of changes in those participants who made initial changes (context-specific analyses), rather than looking for such associations in the whole sample. Further research is also needed to explore the role of perceived tension following changes in physical activity in relation to maintenance of behaviour changes. This element of the logic model has not yet been tested owing to time and resource limitations.
Chapter 5 Health economic evaluation
Parts of this chapter have been adapted from Stathi et al. 1 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text includes minor additions and formatting changes to the original text.
Parts of this chapter have been adapted from Snowsill et al. 103 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
The economic evaluation assesses whether or not the community-based physical activity and behavioural maintenance intervention (REACT) is cost-effective for reducing the progression of functional limitation in retired or semi-retired men and women aged ≥ 65 years who are at risk of mobility-related disability compared with a minimal intervention control group. The perspective used was the NHS and Personal Social Services (PSS), which was complemented with out-of-pocket costs expenditure. All health-care resources used by participants from baseline to 24 months after randomisation were included. The additional (incremental) costs associated with delivering the REACT intervention were estimated using the resource data collected within the trial. Incremental cost-effectiveness ratios (ICERs) were estimated against the primary outcome measure (SPPB) and also for cost per QALYs gained. In addition, the cost-effectiveness of the REACT programme beyond the trial-end time horizon (24 months) up to death was estimated.
Within-trial economic evaluation
Aims and objectives
To assess if the REACT programme (plus usual care) was cost-effective compared with usual care alone in retired or semi-retired adults at risk of mobility-related disability from the perspective of the NHS and PSS.
Methods
Target population and subgroups
Participants were those eligible for the REACT study, that is adults aged ≥ 65 years, retired or semi-retired, with a SPPB score between 4 and 9 (inclusive).
Subgroup analyses were conducted as follows:
-
baseline SPPB score – 4–7 compared with 8–9
-
SES by beyond secondary school compared with not beyond secondary school and by IMD decile – divided to achieve a split of the trial participants as close as possible to 50 : 50
-
age – 65–74 compared with ≥ 75 years
-
known medical conditions – 0–1 compared with ≥ 2
-
sex – women compared with men
-
site – Devon compared with Bath/Bristol compared with Birmingham
-
number of falls in the 6 months before randomisation – 0 compared with ≥ 1.
Setting and location
The REACT intervention was delivered in Bath, Bristol, Devon and Birmingham.
Perspective
The base-case analysis adopts an NHS and a PSS perspective, in line with the NICE reference case. 104 A scenario analysis considers the costs from a societal perspective, in which the voluntary and out-of-pocket expenditure of long-term care is included, along with an estimate of the value of informal care provided by friends and relatives, and an estimate of private expenditure on health care.
Intervention and comparators
The REACT programme is a group-based physical activity and behavioural maintenance intervention that aims to prevent decline in those at risk of mobility-related disability. The programme runs for 12 months, with sessions initially twice weekly and then once weekly, for a total of 64 sessions. Sessions are led by a fitness instructor. REACT participants also receive support as usual from NHS services.
Time horizon
The time horizon for the within-trial economic evaluation is 2 years.
Measurement of effectiveness
The SPPB score was directly assessed by study investigators at 24 months.
Measurement and valuation of preference-based outcomes
Health-related quality of life was measured at baseline and at 6, 12 and 24 months using the EQ-5D-5L and the SF-36 (self-completed in face-to-face sessions).
The EQ-5D-5L covers five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) measured at five different levels. 51 EQ-5D-5L health states were valued through mapping from EQ-5D-5L to EuroQol-5 Dimensions, three-level version (EQ-5D-3L), utilities using the crosswalk method. 105–107 SF-36 responses were converted to Short Form questionnaire-6 Dimensions (SF-6D) health states. The SF-6D has six dimensions (physical functioning, role limitation, social functioning, bodily pain, mental health and vitality), with four to six levels per dimension. 108 Within-trial QALYs were estimated from EQ-5D utilities109 for the base-case and SF-6D utilities in a scenario analysis.
Estimation of costs
There are two cost components:
-
costs of delivering the REACT programme
-
costs associated with the use of health-care and social care services.
Data on the use of NHS and PSS resources were collected at the face-to-face sessions using a resource use questionnaire, which asked participants to recall their usage over the previous 6 months at baseline and at 6, 12 and 24 months.
Valuation of resources
Valuation of resources used the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care110 and NHS National Cost Collection (NCC)111 (Table 13). Further details are presented in Appendix 3.
| Item | Unit costa | Source |
|---|---|---|
| Primary and/or community-based services (per contact or consultation) | ||
| GP at surgery/health centre | 39.19 | PSSRU 2019110 (surgery consultation lasting 9.22 minutes, including direct care staff costs, including training) |
| GP via telephone | 15.52 | PSSRU 2019110 |
| GP home visit | 78.92 | PSSRU 2014112 and PSSRU 2019110 [£156 per hour of GMS activity, 11.4-minute visit, 1 : 0.61 direct-to-indirect time ratio (7.0 minutes indirect), 12 minutes of travel] |
| Practice nurse at surgery/health centre | 12.43 | PSSRU 2014112 and PSSRU 2019110 (£37 per hour, 15.5 minutes per contact, 1 : 0.30 direct-to-indirect time ratio) |
| Practice nurse via telephone | 7.80 | PSSRU 2019110 |
| Practice nurse at home | 39.68 | NCC 2018/19111 (N02AF: district nurse, face to face) |
| Physiotherapist | 62.90 | NCC 2018/19111 (A08A1) |
| Occupational therapist | 83.17 | NCC 2018/19111 (A06A1) |
| Dietitian | 89.90 | NCC 2018/19111 (A03) |
| Podiatrist | 42.51 | NCC 2018/19111 (A09 A) |
| Counsellor | 45.00 | PSSRU 2019110 (Agenda for Change band 6 for 1 hour) |
| Walk-in centre | 21.00 | PSSRU 2019110 (assume 15 minutes of Band 6 nurse time) |
| Day care services | ||
| Per day care centre | 40.20 (60.00) | PSSRU 2019110 |
| Overnight hospital stays (per attendance) | ||
| General medical ward or long-stay ward | 385.16 plus 503.96 per day | NHS reference costs 2017/18113 |
| Intensive care unit | 3532.09 | NCC 2018/19111 |
| Other hospital usage | ||
| Outpatient appointment | 126.85 | NCC 2018/19111 (weighted average of all consultant-led and non-consultant-led outpatient attendances) |
| Day case treatment | 751.90 | NCC 2018/19111 (weighted average of all day-case episodes) |
| A&E attendance | 166.05 | NCC 2018/19111 (weighted average of all A&E episodes) |
| Convalescent or nursing home | ||
| Convalescent or nursing home | 79.73 (119.00) | PSSRU 2019110 |
| Support from relatives and/or friends | ||
| Help with tasks at home (per hour) | 0.00 (11.06) | van den Berg and Ferrer-I-Carbonell (2007);114 OECD;115 and Office for National Statistics116 [€9.65 per hour (Netherlands, 2002) at 0.690 : 0.901 conversion (OECD PPP 2002) and inflated according to nominal earnings growth 2002 (390.9) to 2019 (584.9)] |
| Stayed off work to help (per day) | 0.00 (99.53) | Office for National Statistics116 |
| Other (provided as free text) | ||
| Chiropractor | 0.00 (40.06) | Newell et al. (2016)117 |
| Osteopath | 0.00 (44.95) | General Osteopathic Council118 |
| Paid care at home (per hour) | 18.95 (28.29) | PSSRU 2019110 |
Total within-trial costs were estimated by interpolation, assuming that utilisation between months 12 and 18 was midway between utilisation from months 6 to 12 and utilisation from months 18 to 24.
Currency, price date and conversion
Great British pounds (2018/19) were used, and purchasing power parity exchange rates were used when converting currencies, as well as appropriate inflation rates for adjusting to the price year 2018/19.
Discounting
The discount rate for costs and QALYs is 3.5% per year. 104
Analytical methods
All analyses were conducted using the ITT principle.
Aggregating costs of NHS/Personal Social Services resource use
Costs were aggregated for each time point using the following aggregate categories:
-
primary care – GP (at surgery/health centre, via telephone, at home), practice nurse (at surgery/health centre, via telephone, at home), physiotherapist (at surgery/health centre, at home), occupational therapist (at surgery/health centre, at home), nutritionist, chiropodist, counsellor and walk-in centre
-
day care – day care attendances
-
hospital overnight stays – general medical ward, long-stay ward and intensive care unit
-
other hospital attendances – outpatient attendances, day case procedures, and accident and emergency attendances
-
convalescent/nursing home – days spent in convalescent/nursing home
-
informal care – hours of help from friends/relatives around the home and days friends/relatives took off work (not included in base-case perspective)
-
other (from free text) – chiropractor and osteopath (not included in base-case perspective), paid care at home.
Statistical analysis
A generalised linear model with gamma family and log link was used to model cost data. 119 Within-trial QALYs could not exceed 2 (perfect health for 24 months), so a generalised linear model with gamma family and log link was used to model the difference of 2 minus QALYs and estimates then transformed to QALYs.
Costs and QALYs were adjusted for baseline differences by including the following as covariates:
-
age at randomisation (grouped in 5-year age bands)
-
sex
-
site (Bath/Bristol, Birmingham, Devon)
-
baseline SPPB score
-
baseline health-care resource use
-
baseline utility values120
-
unadjusted analyses are used in a sensitivity analysis.
Primary analysis was carried out with imputation. Patterns of missingness among resource use and utility value data were explored graphically and through examining the correlation of indicator variables for missingness across follow-up time points. Logistic regression was used to explore the influence of key predictors individually (randomised allocation, sex, age, site and baseline SPPB), with p-values calculated from the likelihood ratio test. For details of the analysis of missing data, see Appendix 3, Figure 36.
Complete-case analysis (CCA) was used in a sensitivity analysis. Vital status was not recorded in the analysis data set, so any participants who died during the trial would appear as having missing data after they died. They would, therefore, have costs and QALYs imputed just as any participant missing data for other reasons.
Given that REACT is a group-based intervention, analyses producing cluster-robust standard errors were used throughout. In the control arm, each participant was treated as an individual cluster.
Uncertainty in results due to sampling variation was estimated either through cluster-robust Huber–White standard errors [multiple imputation (MI) analyses] or via bootstrapping (1000 iterations for CCAs, 100 iterations for MI analyses where used).
The sensitivity of the results to changing the values assigned to resources by ± 20% was estimated through one-way sensitivity analysis deviating from the CCA, presented in the form of a tornado diagram. Subgroup analyses were conducted by refitting models with interaction terms between the intervention allocation and the subgroup covariate, and producing predictive means for costs and QALYs. These were based on MI, but as these are exploratory we do not report standard errors or CIs. ICER 95% CIs are produced using the bootstrap percentile method. 121
Results
Intervention costs
The cost of the REACT intervention was estimated to be £9465.69 per arm, or £621.83 per participant (see Appendix 3, Table 35). The cost was somewhat sensitive to underlying assumptions, with the cost per participant ranging from £554.54 (–11%) to £775.13 (+25%) across the sensitivity analyses (see Appendix 3, Table 36). The methods for estimating these costs are described in detail in Appendix 3.
Resource use and utility values
A total of 367 and 410 participants were randomised to the control arm and the intervention arm, respectively. Resource use at baseline was generally well balanced, but there was some evidence of higher baseline resource use in the intervention arm (see Appendix 3, Table 37).
The average total value of NHS and PSS resource utilisation in the 6 months prior to randomisation was £653 and £797 in the control arm and the intervention arm, respectively. After multiple imputation and adjusting for covariates, the difference in pre-randomisation resource utilisation was £107 (95% CI –£50 to £263). Over the course of the REACT study, we estimate that NHS and PSS resource use was £724.74 lower in the intervention arm (95% CI £25.79 to £1423.69) (see Appendix 3, Table 38). There was generally a trend towards greater resource use over time, and the difference in costs was greatest in the first 6 months. Expanding to a societal perspective moved the expected cost difference towards zero (£645.65), meaning that the cost difference was no longer statistically significant (95% CI –£63.73 to £1355.02).
Table 14 explores which cost categories lead to savings between the intervention arm and the control arm. In available case analyses and MI analyses, the category ‘other hospital’ (which includes day-case procedures, outpatients and A&E attendances) was the primary category in which intervention arm participants had lower costs. The difference between MI and CCAs appears to be driven by the difference in hospital overnight stay costs: the unadjusted difference in mean costs is £72.80 among available cases compared with –£78.62 with MI, and this difference may be exacerbated by adjustment.
| Cost category | Mean value (SD); n | Difference | ||
|---|---|---|---|---|
| Intervention arm | Control arm | Unadjusted | Adjusted (95% CI) | |
| Available case analysis | ||||
| Primary care | 820.49 (680.47); 299 | 838.12 (704.30); 260 | –17.63 | –14.40 (–129.17 to 100.37) |
| Hospital overnight stay | 1104.98 (3670.8); 289 | 1032.18 (3282.4); 246 | 72.80 | 254.25 (–358.29 to 866.80) |
| Other hospital | 1372.61 (1799.1); 299 | 1856.01 (3281.2); 263 | –483.40 | –484.43 (–931.70 to –37.16) |
| Day care | 1.78 (13.53); 276 | 7.71 (95.31); 232 | –5.93 | –8.74 (–31.88 to 14.40) |
| Convalescent | 27.18 (199.77); 299 | 24.66 (312.97); 263 | 2.52 | 26.74 (–7.47 to 60.95) |
| Other | 26.16 (332.18); 410 | 10.66 (187.38); 366 | 15.49 | b |
| Day carea | 2.65 (20.20); 276 | 11.51 (142.26); 232 | –8.85 | –13.04 (–47.58 to 21.49) |
| Convalescenta | 40.56 (298.17); 299 | 36.80 (467.12); 263 | 3.76 | 39.91 (–11.14 to 90.96) |
| Informal carea | 169.93 (590.27); 253 | 128.99 (364.44); 217 | 40.94 | 46.69 (–40.82 to 134.19) |
| Othera | 46.56 (501.27); 410 | 22.86 (285.68); 366 | 23.70 | 9.96 (–39.66 to 59.58) |
| MI analysis | ||||
| Primary care | 848.02 (685.56); 410 | 853.80 (717.95); 367 | –5.79 | –25.36 (–127.81 to 77.08) |
| Hospital overnight stay | 1064.98 (3440.2); 410 | 1143.60 (3492.6); 367 | –78.62 | –107.76 (–628.11 to 412.60) |
| Other hospital | 1441.98 (2039.3); 410 | 1836.89 (3255.0); 367 | –394.91 | –462.03 (–861.98 to –62.07) |
| Day care | 3.72 (37.73); 410 | 6.64 (82.25); 367 | –2.92 | –4.79 (–21.69 to 12.12) |
| Convalescent | 69.34 (674.84); 410 | 35.57 (354.83); 367 | 33.77 | 16.02 (–87.99 to 120.04) |
| Other | 26.16 (332.18); 410 | 10.64 (187.13); 367 | 15.52 | b |
| Day carea | 5.56 (56.32); 410 | 9.91 (122.76); 367 | –4.35 | –7.15 (–32.38 to 18.09) |
| Convalescenta | 103.50 (1007.2); 410 | 53.09 (529.60); 367 | 50.40 | 23.92 (–131.33 to 179.17) |
| Informal carea | 190.51 (589.62); 410 | 163.07 (451.56); 367 | 27.44 | 23.79 (–54.63 to 102.21) |
| Othera | 46.56 (501.27); 410 | 22.80 (285.29); 367 | 23.76 | 9.30 (–37.78 to 56.37) |
Participants randomised to receive the intervention had marginally better EQ-5D utility values than those randomised to the control arm at baseline (0.689 vs. 0.677, respectively) and this difference diminished after MI and adjustment (0.007, 95% CI –0.016 to 0.029). However, for SF-6D utility values, there was no difference between the arms at baseline (0.000, 95% CI –0.014 to 0.014) (Table 15).
| HRQoL | Mean value (SD); n | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention arm | Control arm | |||||||
| Baseline | 6 months | 12 months | 24 months | Baseline | 6 months | 12 months | 24 months | |
| EQ-5D value | ||||||||
| Crosswalk to EQ-5D-3L | 0.689 (0.158); 397 | 0.708 (0.167); 346 | 0.705 (0.170); 337 | 0.686 (0.200); 330 | 0.677 (0.165); 352 | 0.670 (0.181); 299 | 0.680 (0.183); 293 | 0.661 (0.195); 302 |
| EQ-5D-VAS | 70.6 (17.3); 399 | 72.6 (16.7); 349 | 71.4 (17.8); 338 | 70.4 (18.4); 329 | 72.1 (16.9); 362 | 72.0 (17.3); 298 | 70.6 (17.0); 294 | 69.4 (19.2); 301 |
| EQ-5D-5L value set | 0.789 (0.149); 397 | 0.805 (0.160); 346 | 0.801 (0.158); 337 | 0.782 (0.180); 330 | 0.781 (0.152); 352 | 0.770 (0.177); 299 | 0.785 (0.162); 293 | 0.767 (0.174); 302 |
| SF-36 | ||||||||
| PCS | 29.7 (11.0); 393 | 32.8 (11.5); 342 | 31.9 (11.5); 334 | 30.9 (12.0); 326 | 30.0 (10.6); 352 | 30.6 (10.9); 293 | 29.8 (10.9); 293 | 29.2 (10.8); 295 |
| MCS | 54.6 (8.3); 393 | 54.4 (8.6); 342 | 54.0 (8.8); 334 | 54.3 (8.6); 326 | 53.8 (8.7); 352 | 54.0 (9.1); 293 | 54.3 (9.4); 293 | 54.5 (9.1); 295 |
| SF-6D | 0.622 (0.095); 365 | 0.637 (0.103); 323 | 0.637 (0.098); 313 | 0.630 (0.105); 312 | 0.623 (0.089); 326 | 0.621 (0.103); 280 | 0.622 (0.100); 280 | 0.619 (0.096); 283 |
The resulting predicted mean QALYs (calculated from the EQ-5D and SF-6D) and incremental QALYs are shown in Table 16. The intervention is associated with increased utility values at all follow-up time points, and this difference is statistically significant at 6 months using EQ-5D and at 12 months using SF-6D. The within-trial QALYs estimated using EQ-5D were 1.354 for the intervention arm and 1.314 for the control arm, a statistically significant difference of 0.040 (95% CI 0.006 to 0.074).
| Timepoint | EQ-5D utility (crosswalk) | SF-6D utility | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention arm | Control arm | Difference | 95% CI | Intervention arm | Control arm | Difference | 95% CI | |
| Baseline | 0.685 | 0.678 | 0.007 | –0.016 to 0.029 | 0.622 | 0.622 | 0.000 | –0.014 to 0.014 |
| 6 months | 0.703 | 0.668 | 0.035 | 0.012 to 0.058 | 0.634 | 0.621 | 0.012 | –0.003 to 0.027 |
| 12 months | 0.692 | 0.676 | 0.016 | –0.007 to 0.039 | 0.635 | 0.617 | 0.018 | 0.004 to 0.031 |
| 24 months | 0.676 | 0.657 | 0.019 | –0.007 to 0.046 | 0.627 | 0.616 | 0.011 | –0.002 to 0.024 |
| Within-trial QALYs | 1.354 | 1.314 | 0.040 | 0.006 to 0.074 | 1.241 | 1.216 | 0.025 | 0.006 to 0.044 |
Sensitivity analyses
Varying the unit costs for NHS resources each by ± 20% did not result in a large impact on total costs (Figure 14). Four NHS resources had the potential to affect incremental total costs by more than £20:
-
Increasing the cost of a day-case procedure (base-case value £752) by 20% increased the costs for the intervention relative to the control arm by £39.
-
Increasing the cost of a hospital inpatient bed-day (base-case value £504) by 20% decreased the costs for the intervention relative to the control arm by £32.
-
Increasing the cost of an intensive care unit stay (base-case value £3532) by 20% increased the costs for the intervention relative to the control arm by £25.
-
Increasing the cost of an outpatient appointment (base-case value £127) by 20% increased the costs for the intervention relative to the control arm by £24.
FIGURE 14.
Tornado diagram for one-way sensitivity analyses of resource values (NHS/PSS perspective).
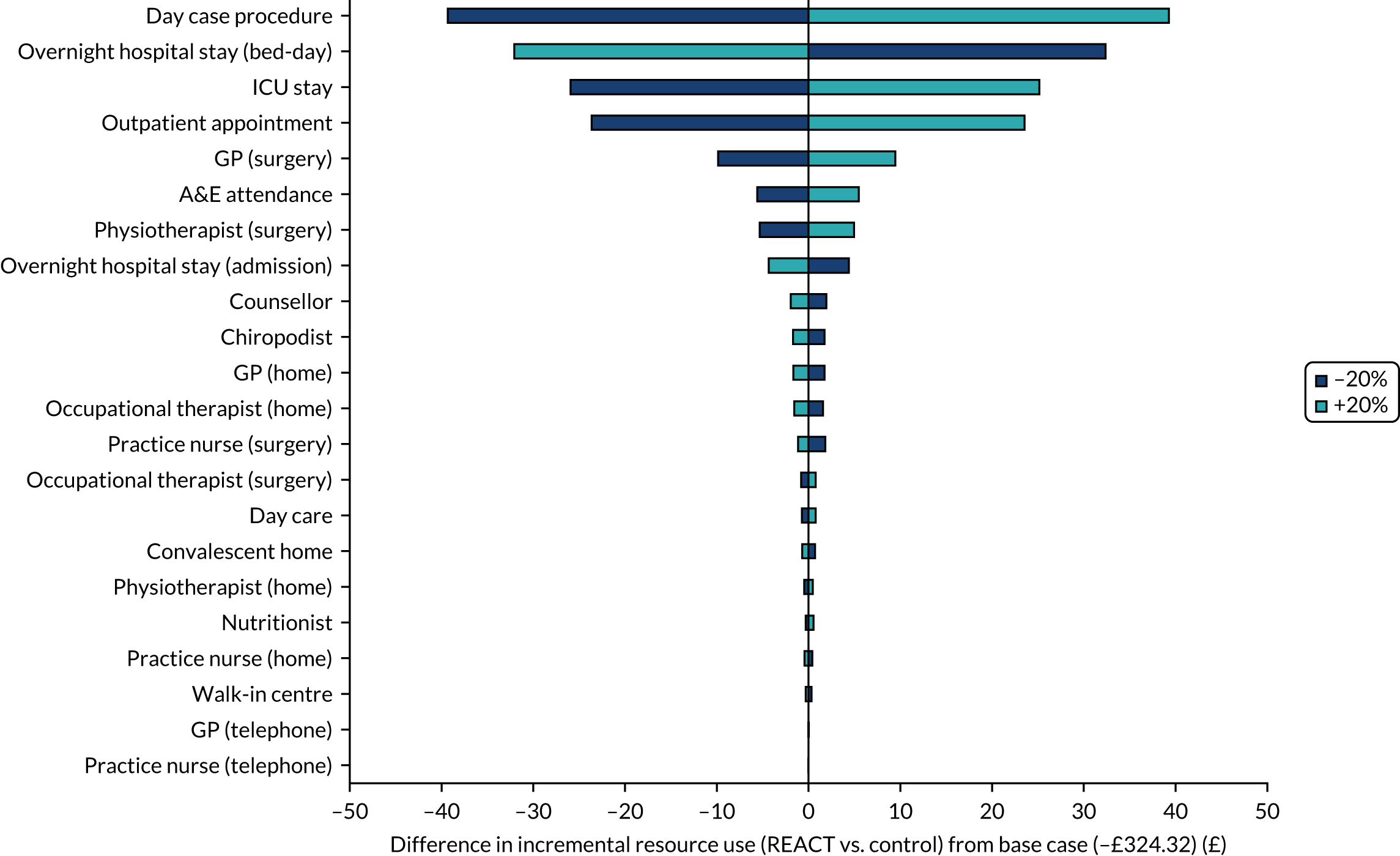
For costs affecting the societal perspective only, one-way sensitivity analyses showed very limited sensitivity to unit costs. Only the cost of a friend/relative taking a day off work and the cost of a chiropractor affected relative costs by more than £5 when varied by ± 20%.
The sensitivity analysis for the plausible extreme values of the societal value of an hour of help around the house revealed some sensitivity in costs from a societal perspective. When the value was reduced to £0.35 per hour, the total incremental costs for the intervention compared with usual care became more negative (to –£541, from a base case of –£458), while when the value was increased to £335 per hour the difference in costs narrowed (to –£347).
Cost-effectiveness
Cost-effectiveness estimates include intervention costs (£621.83 per participant), as well as the impact of REACT on within-trial resource use, SPPB score and QALYs, as described above. Considering cost per 1-point increase in SPPB score at 24 months, the intervention is dominant in the base case (saves £103 and results in an increase in mean SPPB of 0.49 points).
When using QALYs as the measure of health benefit, as shown in Table 17, the intervention is cost-effective, either being dominant or having ICER below the £20,000 per QALY cost-effectiveness threshold. In the base case, the 95% upper confidence limit is below £20,000 per QALY, suggesting reasonable confidence that the intervention is cost-effective within the trial time horizon. However, uncertain results are found when there is no adjustment for covariates in the MI analysis or when only participants with complete data are included in the analysis adjusting for baseline covariates, because the upper 95% confidence limit for the ICER extends beyond the £20,000 per QALY cost-effectiveness threshold in these analyses.
| Trial arm | Costs (£) | QALYs | Including costs (£) | Including QALYs | ICER | ||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||||
| Base case: MI adjusted | |||||||||
| Control | 4046 | 1.314 | |||||||
| Intervention | 3943 | 1.354 | –103 | –695 to 489 | 0.040 | 0.009 to 0.071 | Dominant | Dominant | 16,950 |
| Scenario analysis | |||||||||
| MI unadjusted | |||||||||
| Control | 3887 | 1.319 | |||||||
| Intervention | 4076 | 1.364 | 189 | –401 to 778 | 0.045 | –0.001 to 0.091 | 4200 | Dominant | Dominated |
| CCA adjusted | |||||||||
| Control | 3573 | 1.323 | |||||||
| Intervention | 3871 | 1.372 | 297 | –421 to 1016 | 0.049 | 0.010 to 0.089 | 6000 | Dominant | 71,250 |
| CCA unadjusted | |||||||||
| Control | 3609 | 1.325 | |||||||
| Intervention | 3856 | 1.389 | 247 | –498 to 992 | 0.063 | 0.005 to 0.122 | 3900 | Dominant | 18,000 |
The uncertainty in cost-effectiveness is demonstrated in cost-effectiveness scatter plots (Figure 15) and cost-effectiveness acceptability curves (Figure 16). The proportion of estimates cost-effective at £20,000 per QALY is 98% in the base case, 84% in the MI unadjusted analysis, 89% in the CCA adjusted analysis, and 98% in the CCA unadjusted analysis.
FIGURE 15.
Cost-effectiveness scatter plot for within-trial analysis. a, CCA adjusted; b, CCA unadjusted; c, MI adjusted; d, MI unadjusted.


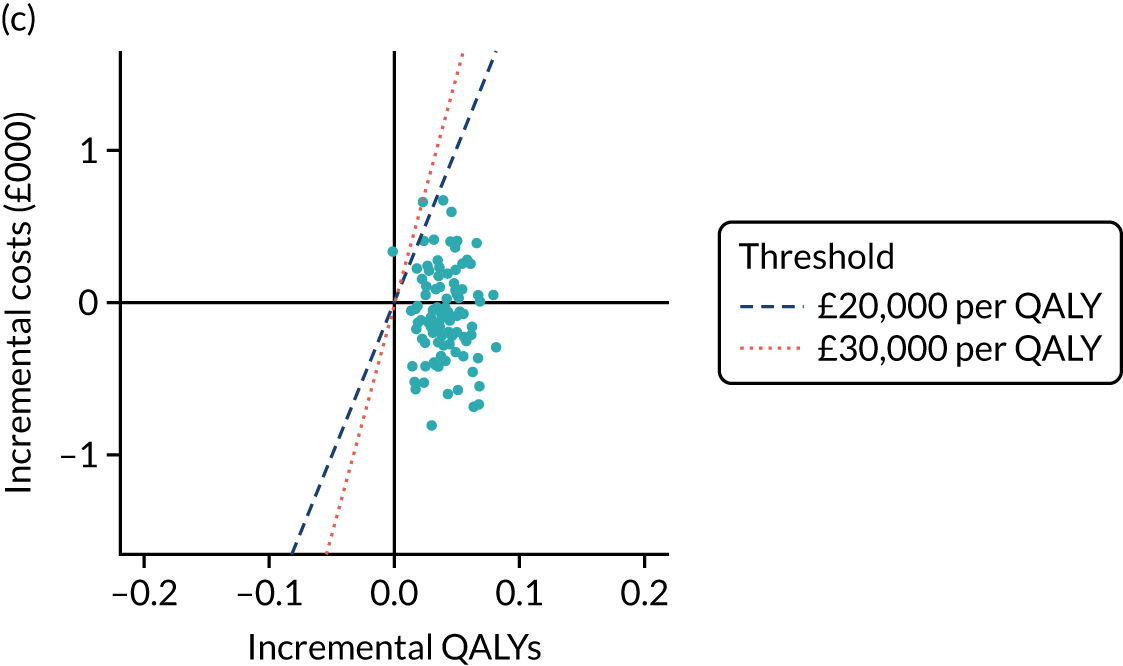
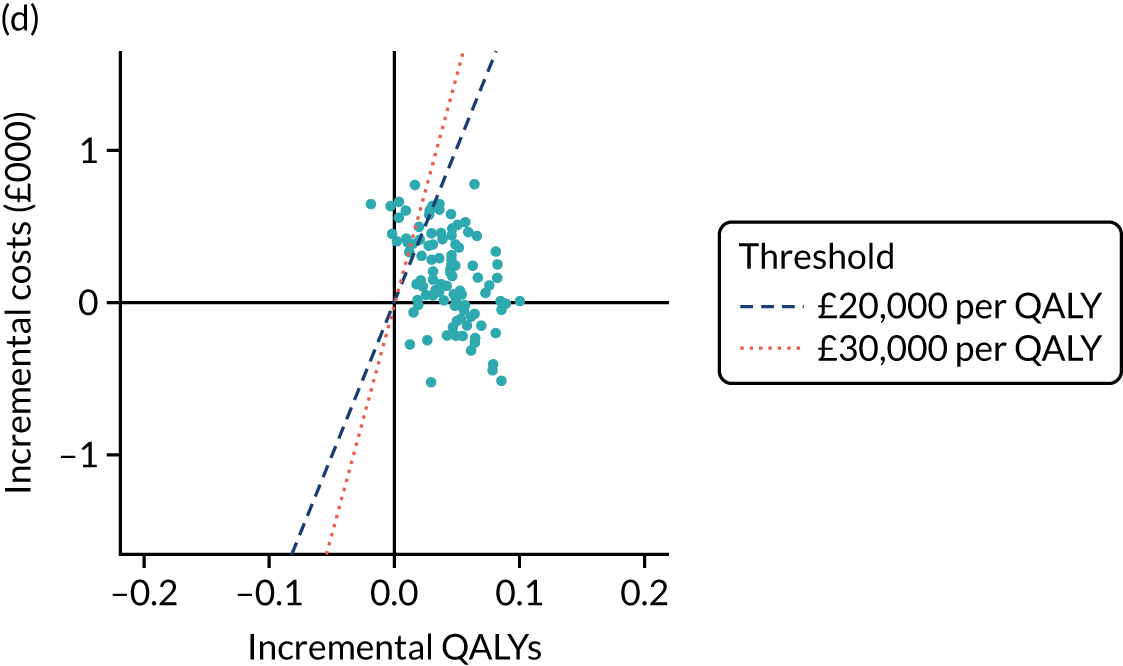
FIGURE 16.
Cost-effectiveness acceptability curves for within-trial analysis.
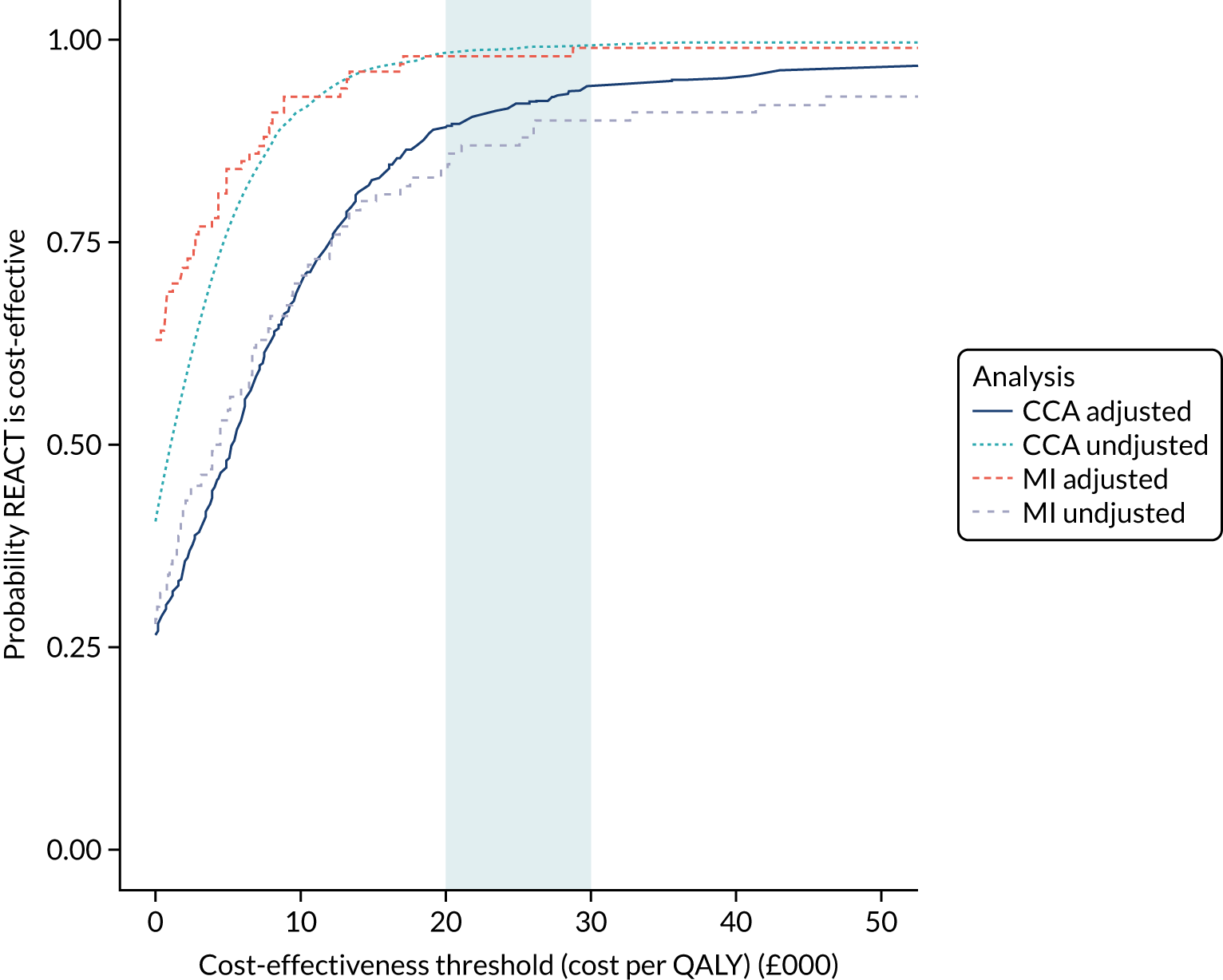
Scenario analyses
In Table 18, a number of scenarios were explored. Removing the resource use impact observed in the REACT study makes the intervention less cost-effective but is not enough by itself to make the intervention not cost-effective at a £20,000 per QALY threshold. If the resource use impact is removed and SF-6D utilities are used to estimate QALYs (instead of EQ-5D), then the ICER for the intervention increases to £25,050 per QALY, which would not be considered cost-effective at a £20,000 per QALY threshold but would be considered cost-effective at a £30,000 per QALY threshold.
| Scenario | Cost (£) | QALYs | ICER | ||||
|---|---|---|---|---|---|---|---|
| Control arm | Intervention arm | Difference | Control arm | Intervention arm | Difference | ||
| Base case | 4046 | 3943 | –103 | 1.314 | 1.354 | 0.040 | Dominant |
| No resource use impact | 0 | 622 | 622 | Unchanged from base case | 15,650 | ||
| Societal perspective | 4240 | 4216 | –24 | Unchanged from base case | Dominant | ||
| SF-6D QALYs | Unchanged from base case | 1.216 | 1.241 | 0.025 | 4150 | ||
| No resource use impact and SF-6D QALYs | 0 | 622 | 622 | 1.216 | 1.241 | 0.025 | 25,050 |
| Group size 12 | 4046 | 4097 | 50 | Unchanged from base case | 1250 | ||
Subgroup analyses
Subgroup analyses were conducted by refitting models for within-trial costs and QALYs, including interaction terms for the subgroup of interest with the group allocation variable. The REACT study appears to be cost-effective across all subgroups, except when the trial population is divided by baseline SPPB score. Participants with a baseline SPPB score of 4–7 do appear to obtain some health benefit in terms of QALYs, but there is no beneficial effect of the intervention on NHS/PSS resource use (see Appendix 3, Table 39).
Discussion
The results indicate that the intervention plus usual care is cost-effective compared with usual care alone over the 2-year time horizon of the REACT study within-trial analysis. We estimated that the REACT intervention would cost £622 per participant to deliver, although this was sensitive to the costs of major inputs (REACT session leader, venue hire) and the group size. We also estimated that the intervention would lead to a reduction in NHS/PSS resource use, corresponding to savings of £725, although the magnitude of cost savings was subject to uncertainty owing to sampling uncertainty and methodological choices (handling of missing data and baseline imbalances). We also estimated that the intervention would lead to an increase in discounted QALYs and alongside estimated cost saving the intervention is cost-effective (dominant), as it reduces costs to the NHS and society while increasing patient health benefits terms of mobility-related disability compared with usual care alone.
The REACT study had a similar gain in within-trial QALYs as the LIFE study (0.044 to 0.063 over 2 years in REACT, depending on analysis methods, vs. 0.047 over 2.6 years in LIFE),21 although it should be noted that different preference-based instruments were used [EQ-5D-5L vs. Quality of Well-Being Self-Administered (QWB-SA)]. The cost of the intervention is cheaper than the cost of the LIFE intervention [£622 (2018/19 Great British pounds) vs. £1770 (US$2301, 2013 US dollars122)], and has demonstrated a reduction in health-care utilisation of £324–724 compared with an increase of £1220 [US$1583 (2013 US dollars122)].
This economic evaluation shows that the effect of the intervention on maintaining mobility, as demonstrated through SPPB score, the primary outcome of our study, also led to the expected improvement in self-reported health-related quality of life and health-care utilisation. It included a number of sensitivity and scenario analyses to explore the uncertainty in the results associated with missing data and imbalance in baseline characteristics between trial arms.
The principal limitation of the within-trial analysis is that it covers only 2 years and it is likely (given the persistence of differences in SPPB between the study arms) that further differences in costs and QALYs would occur beyond this time horizon. This particular limitation will be addressed in the next subsection. We have conducted regressions using only the gamma family and log link; we have not explored alternative parameterisations. There were missing data in the study, and it is not possible to rule out the possibility that data were missing not at random. It is estimated that 33% of long-term care spending comes from voluntary and out-of-pocket expenditure,123 and this is excluded from the base-case perspective.
Model-based economic evaluation
A pragmatic review identified two economic evaluations of physical activity interventions for older adults at risk of frailty (see Appendix 3, Figure 39). In both cases, the authors concluded that the intervention was cost-effective; however, both studies have certain limitations. The study by Alhambra-Borrás et al. 124 is at high risk of treatment selection bias, and the authors may have introduced further bias into their modelling by estimating costs and utility values separately for the intervention and control arms. Their modelling approach was based on dividing participants into two states based on the risk of falls/frailty, but this was poorly reported and was, therefore, of little value for informing the development of an economic model for REACT.
In the cost-effectiveness analysis study by Groessl et al. ,125 uncertainty is introduced on the impact of health-care resource utilisation, which in turn makes the cost-effectiveness of the LIFE study uncertain; the authors also did not attempt to estimate costs and QALYs beyond mean follow-up from the trial.
Although there is some evidence that group-based physical activity interventions can be cost-effective within their own settings, these findings are unlikely to be applicable to the REACT intervention owing to differences in the intervention designs (e.g. lasting 12 months compared with 36 months in the LIFE study).
Aims and objectives
To estimate the cost-effectiveness of the REACT programme beyond the trial end time horizon (24 months) up to death.
Decision problem
What is the cost-effectiveness of the REACT programme evaluated in line with the NICE reference case, specifically adopting:
-
a lifetime time horizon
-
NHS and PSS perspective (a societal perspective is adopted in a scenario analysis)
-
benefit measured in QALYs
-
3.5% discount rate for costs and QALYs?
Methods
A new decision-analytic model was developed in R (version 4.0.0) using the heemod package126 (version 0.12.0.9000).
Model structure and methodology
The decision analytic model focuses on projecting mobility over time and the consequences of mobility on utility values and costs for the NHS and PSS. The cohort of REACT participants enter the model at the end of the trial follow-up, distributed across a set of mutually exclusive mobility states defined by SPPB score. The following year, the patients face the probability of remaining in their end of trial mobility state, of moving to a worse or better health state, or of dying. This annual cycle process is repeated separately for intervention and control arms of the trial, until all cohort members are dead. At the end of the lifetime simulation, the costs and QALYs are aggregated and compared between trial arms to assess the differences in expected costs and QALYs and cost per QALY gained by receiving the intervention over usual care.
In this Markov cohort simulation model, the transition probabilities are allowed to vary with time but the model retains the Markov memoryless property,127 with 13 health states reflecting different levels of mobility (SPPB score of 0 to SPPB score of 12) and a death state. SPPB score has been shown to be a prognostic for loss of ability to walk 400 metres32 and development of mobility disability and disability in ADL. 128 We have further found it to be correlated with utility values and NHS/PSS resource use within the REACT study.
The utility values and NHS/PSS costs are related to the SPPB score based on statistical models fitted to the control arm of the REACT study. Transition probabilities between the mobility states are related to the current SPPB score, age and sex, estimated using statistical models fitted to the control arm of the REACT study. It conservatively assumed that mobility does not affect the mortality rate, which means that the model predicts only differences in QALYs based on utility values. Studies have shown an association between SPPB and mortality;129 however, it was considered plausible that long-term health conditions could affect both mobility and mortality, and that mobility has not been established sufficiently as a surrogate for mortality risk. 130
The model has a cycle length of 1 year and runs for 35 cycles, at which point the cohort is aged 100–130 years, depending on the starting age, which ranges from 65 to 95 years in 5-year intervals. The model is run separately for men and women. In the first two annual cycles, the costs and QALYs are taken directly from the REACT study, after which the distribution of SPPB values in the cohort is established based on predicted values from the REACT study according to age and sex.
Model inputs
Population
In the base-case analysis, we assume a population of women aged 75 years given that women were the majority of participants (66%) in REACT and the age 75 years is close to the modal age in the study of 77 years for men and women. We also analyse cost-effectiveness for men and women aged 65–95 years in 5-year intervals.
The distribution of SPPB values (after 24 months) for each combination of age, sex and group (intervention or control) is estimated from the REACT study using ordered logistic regression, as shown in Figure 17. Specifically, an ordered logistic regression was fitted to trial-end SPPB score, with intervention allocation, age, sex, SPPB score at screening into the study and study site as predictors, and predicted probabilities of each trial-end SPPB score category were computed based on age, sex and intervention allocation using the margins command in Stata.
FIGURE 17.
Expected trial-end (24-month) SPPB score by age, sex and group (error boxes show ± 1 standard error). a, male; b, female.

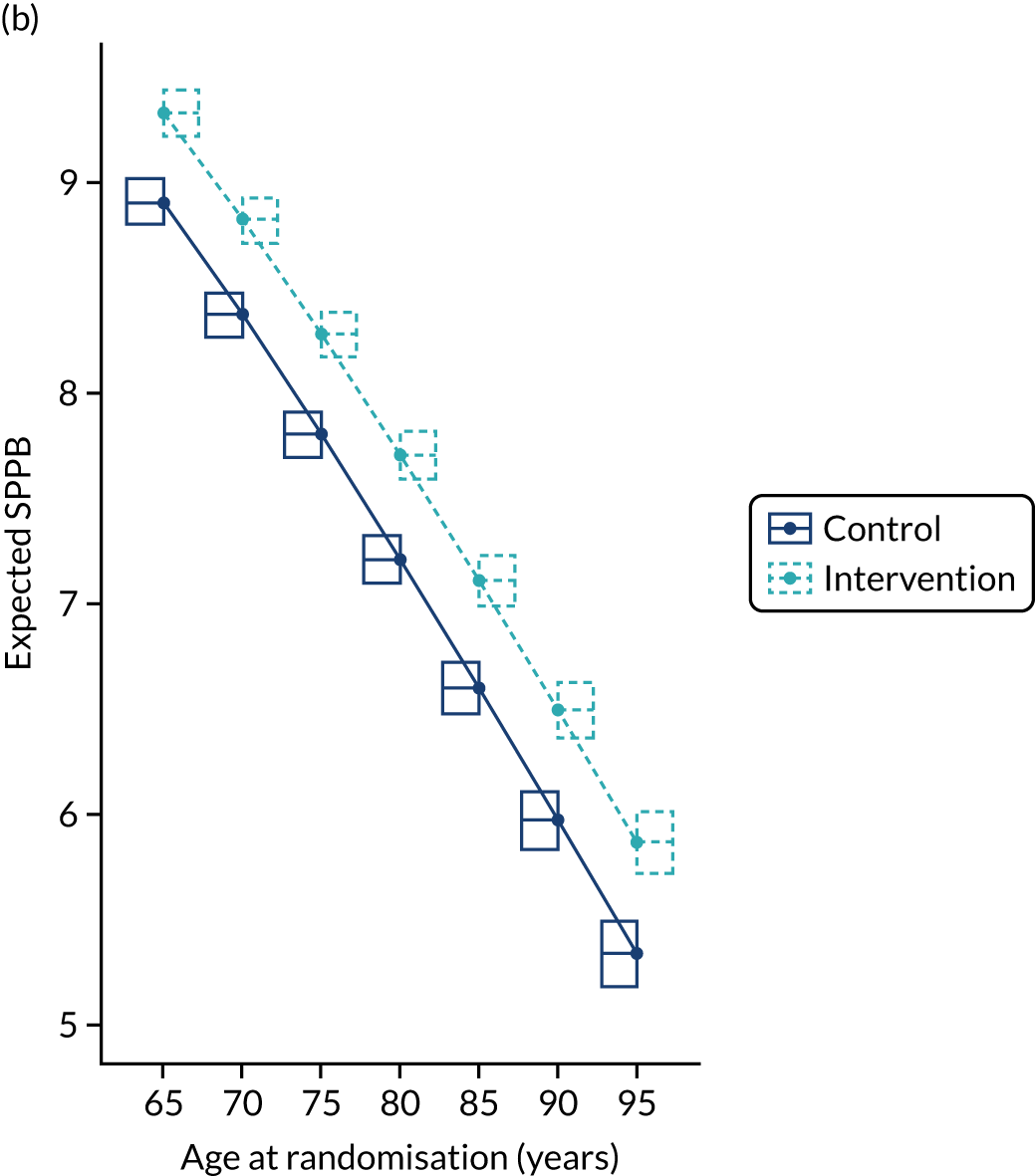
To estimate the average population costs and benefits of REACT, we use two reference populations:
In each case, we estimate weights based on the number of individuals of a given sex whose nearest age is 65, 70, 75, . . . and 95 years.
Intervention effectiveness
The effectiveness of the intervention is incorporated in two components:
-
the directly observed effect of the intervention on costs (excluding the intervention cost) and QALYs within the REACT study (i.e. up to 24 months after randomisation)
-
the SPPB score distribution realised at the end of the trial period.
The first of these components is taken from the analysis in which MI is used to account for missing data and bootstrapping is used to characterise uncertainty. This analysis (see Results) produces cost estimates of £4043 [standard error (SE) £254] in the control arm and £3319 (SE £173) in the intervention arm (excluding the cost of delivering the intervention), and produces QALY estimates of 1.348 (SE 0.012) and 1.388 (SE 0.014) in the control arm and the intervention arm, respectively.
The second of these has been described above (see Figure 17).
Extrapolation of the intervention effectiveness on health outcomes and costs is modelled as a function of the primary outcome in the trial, SPPB, under variable scenarios about the rate at which the effect of REACT on this outcome wears off.
Natural history
There are two natural history components in the model:
-
the evolution of mobility with age
-
the changing mortality rate with age.
These components do not interact, that is there is no differential mortality rate according to mobility level. Both components apply equally to the intervention and control arms. This means, for example, that there is no assumption that participants in the intervention arm are better able to maintain their mobility after the 24-month trial period. The natural history model is fully described in Appendix 3.
Costs
NHS and PSS (annual) costs were estimated as a function of mobility (SPPB score, categorised as 0–3, 4–7, 8–9 or 10–12) from the control arm of the REACT study. For each time point, we estimated the current cost accrual rate based on the cost of recalled health-care resource usage in the 6 months leading up to the time point, which we denote xm for the m-month follow-up time point. At baseline, we estimated costs to be accrued at a rate of:
We further estimate:
A linear regression with dummy variables for the SPPB categories was fitted, adjusting for clustering within participants, as shown in Figure 18.
FIGURE 18.
Cost by SPPB score range.
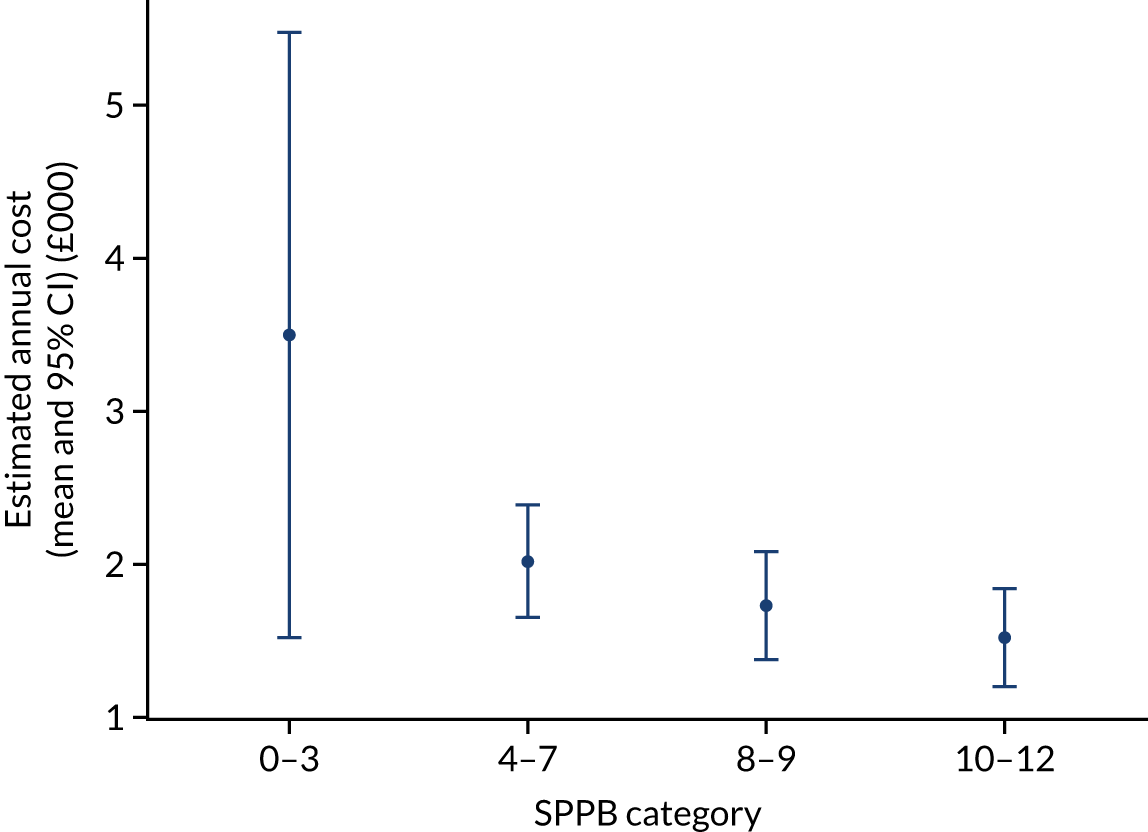
The model shows that costs generally increase with decreasing mobility, but there is considerable uncertainty about costs for those with very poor mobility (SPPB score of < 4); this is partly because the REACT study selected participants with a SPPB score of ≥ 4, so there are fewer data available (n = 48 observations with SPPB score of < 4 out of 1269 SPPB observations in the control arm).
Utility values
Utility values were estimated from SPPB using a linear regression with a restricted cubic regression spline for SPPB. 133 Observations from control arm participants were included, and the analysis was adjusted for clustering within participants (Figure 19).
FIGURE 19.
Predicted EQ-5D utility according to SPPB score.
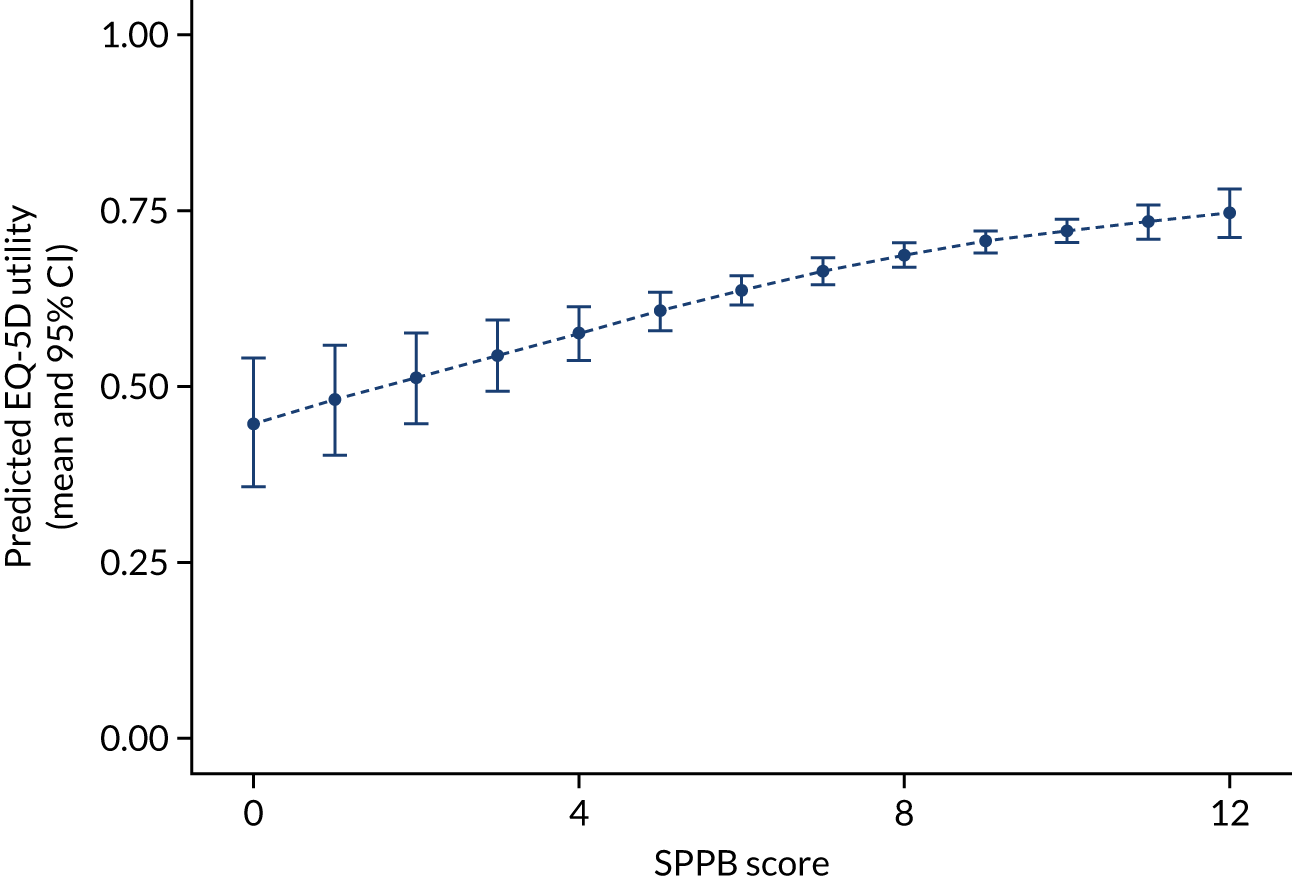
Given that SPPB score declines with age (see Appendix 3), this means that utility values also decline with age. In the REACT study, a Pearson’s chi-squared test showed significant associations of baseline SPPB score with baseline EQ-5D self-care (p < 0.001), usual activities (p < 0.001) and pain/discomfort (p < 0.001) scores. In addition, age was not negatively associated with EQ-5D utility before or after adjusting for baseline SPPB score.
Analysis of uncertainty
Deterministic one-way sensitivity analyses of model parameters and probabilistic sensitivity analysis (PSA) were considered. Correlations between model parameters in the PSA are incorporated through sampling from suitable multivariate normal distributions or sampling from bootstrap estimates. We assume that the cost of the intervention follows a gamma distribution with coefficient of variation of 20%.
We also consider a number of scenario analyses:
-
all model inputs re-estimated using CCA rather than MI
-
societal costs instead of NHS/PSS costs
-
SF-6D utilities instead of EQ-5D
-
four alternative models for the evolution of SPPB (as described in Appendix 3).
A summary of all model parameters is provided in Appendix 3, Tables 45–48.
Results
Base case
The model predicts a decline in SPPB over time for a woman aged 75 years with no difference in mortality between the two arms, as shown in Figure 20.
FIGURE 20.
Mean SPPB score and proportion alive in the Markov model.

In the lifetime simulation, the cohort receiving the intervention spends more time in higher SPPB states (8–12) but less time in lower SPPB states (< 8), and there is no difference in total life years lived. Spending more time in higher SPPB states results in accruing more QALYs and incurring lower costs (see Appendix 3, Table 48).
In our base-case analysis, a woman aged 75 years receiving the intervention is expected to gain 0.072 discounted QALYs over her lifetime and to reduce lifetime discounted costs by £290 (see Appendix 3, Table 49). This compares with the trial-end incremental costs of –£103 and 0.040 QALYs. The intervention is both more effective and less costly than usual care and, therefore, cost-effective regardless of the threshold at which the NHS is willing to pay for a QALY.
Figure 21 shows the cumulative incremental discounted QALYs over time. More than half of the lifetime incremental discounted QALYs come in the first 2 years. After 10 years, there are very limited further incremental QALYs, which reflects the fact that SPPB score distributions have largely converged by this point and an increasing proportion of the population is dying and, hence, unable to accrue further QALYs.
FIGURE 21.
Cumulative incremental discounted QALYs over time.
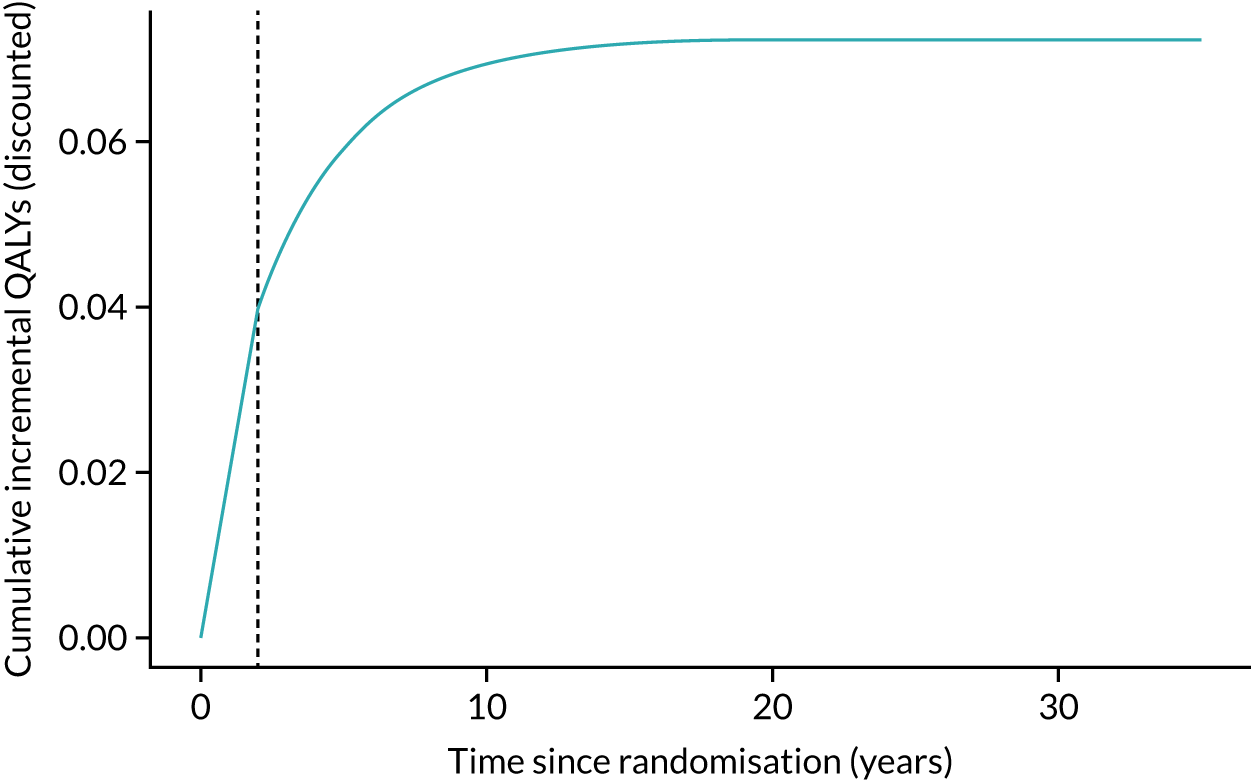
Figure 22 shows the cumulative incremental discounted costs over time. In contrast to QALYs, the majority of the lifetime cost savings do not come in the first 2 years, although, similar to QALYs, there is very limited change after 10 years. The intervention is predicted to lead to QALY gains and cost savings and, therefore, be cost-effective at any time horizon beyond 2 years.
FIGURE 22.
Cumulative incremental discounted costs over time.
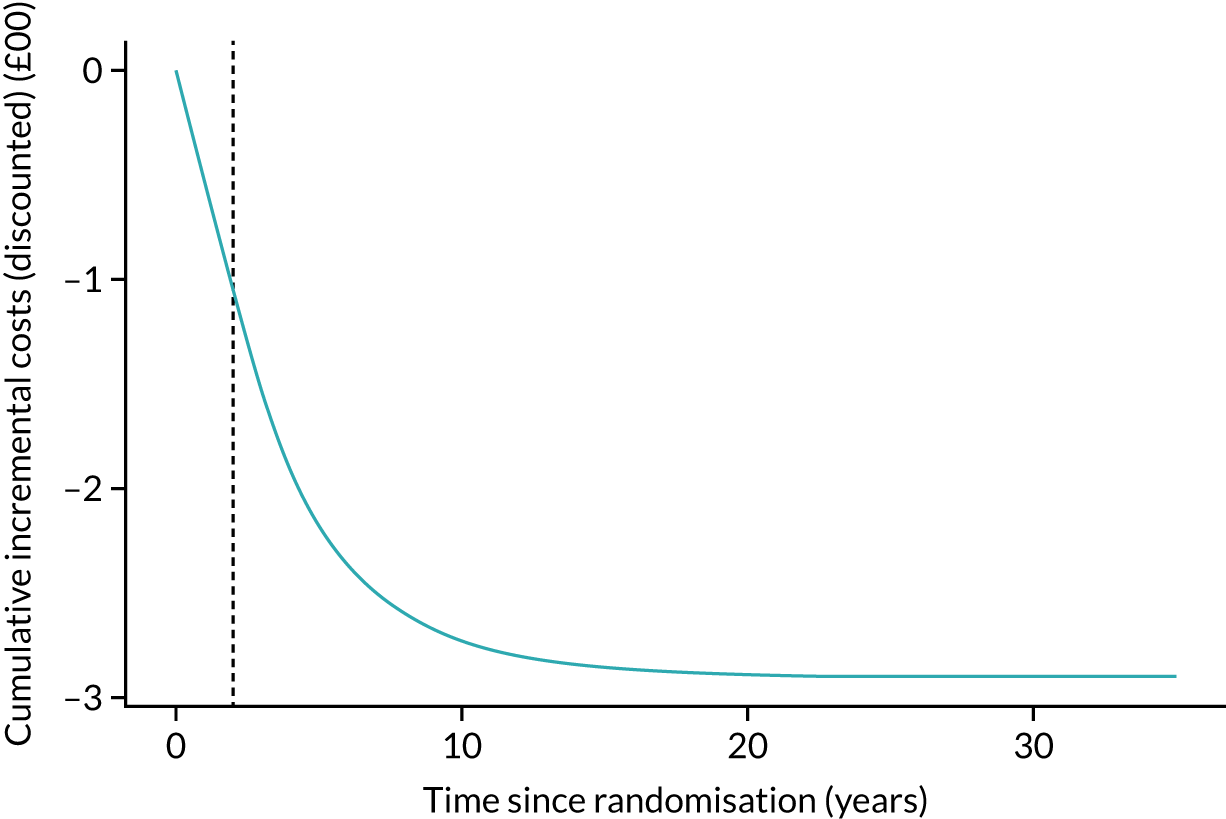
Heterogeneity analysis
As shown in Figure 23, it is expected that discounted lifetime costs and QALYs are higher for younger participants than for older participants, and are higher for women than for men. This is consistent with the fact that life expectancy is greater for women than for men.
FIGURE 23.
Heterogeneity analysis, absolute lifetime discounted costs and QALYs. a, Lifetime costs; b, lifetime QALYs.
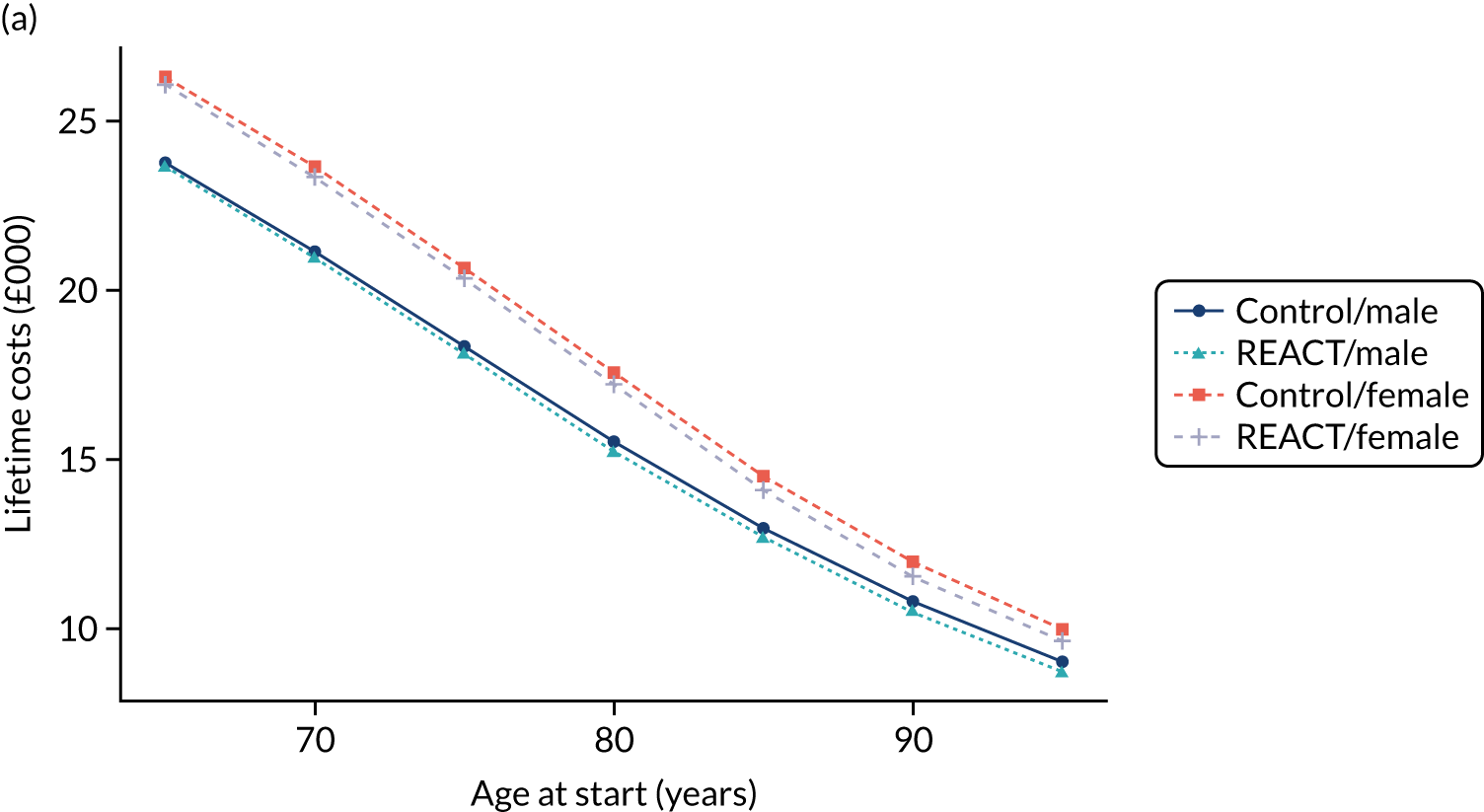
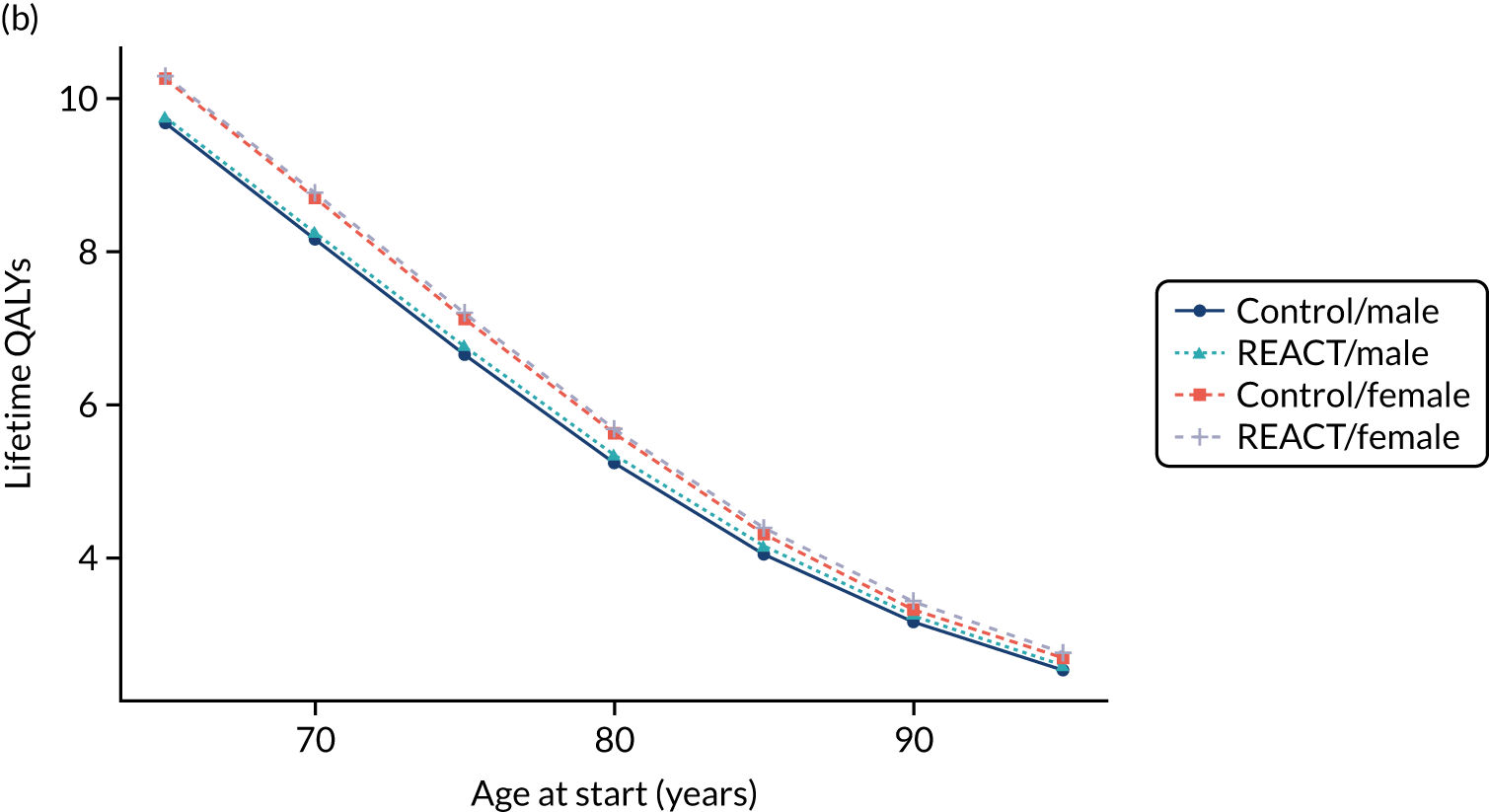
Analysis of the incremental costs and QALYs reveals that the REACT programme is most beneficial to those aged 80–85 years and is more beneficial for women than for men (Figure 24). The REACT programme is expected to reduce lifetime costs for participants of any age and sex (Figure 25), which means that it is dominant across the population. The incremental net monetary benefit (INMB) at a willingness-to-pay threshold of £20,000 per QALY is shown in Figure 26, which demonstrates that the cost of the intervention could increase by £1350 (to around £1970) and still be marginally cost-effective for 65-year-old men and cost-effective for men of other ages and women across the age range.
FIGURE 24.
Heterogeneity analysis: incremental QALYs.
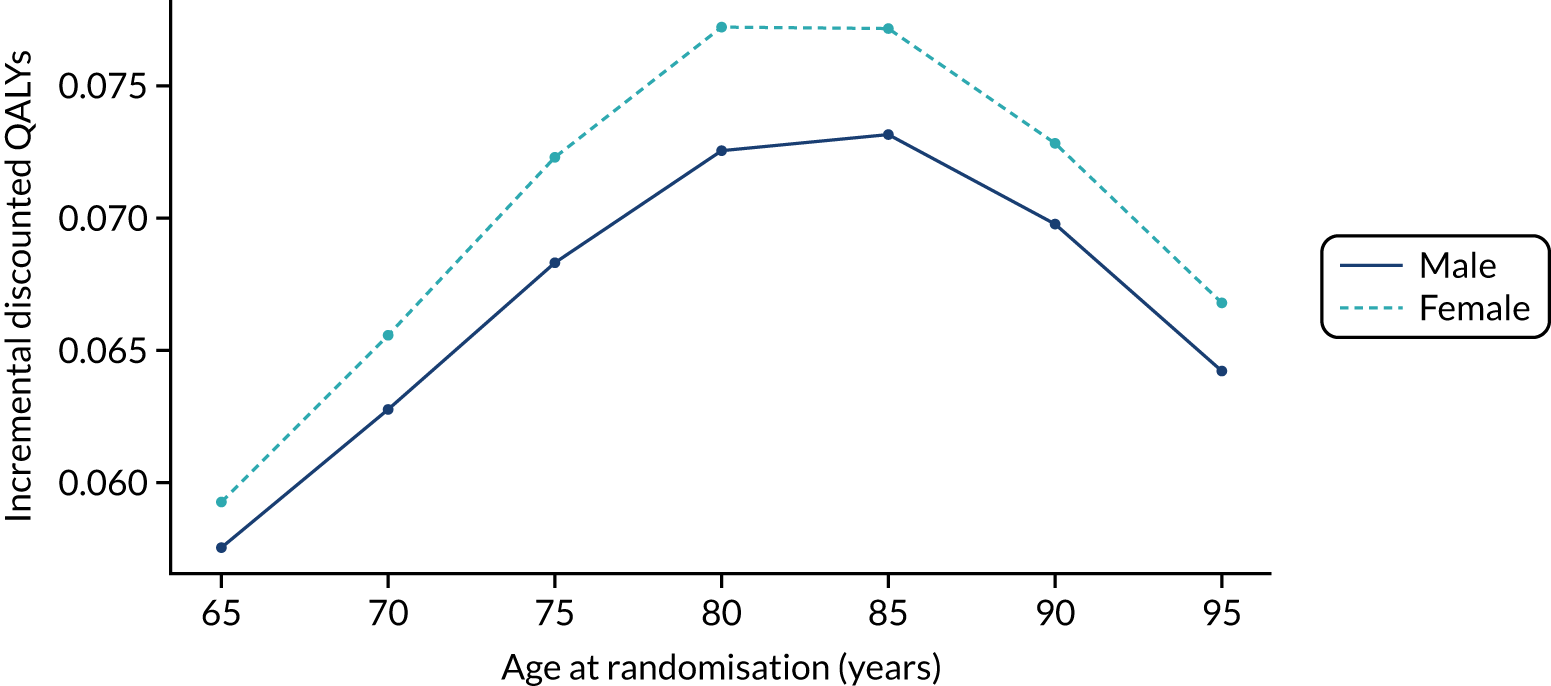
FIGURE 25.
Heterogeneity analysis: incremental costs.
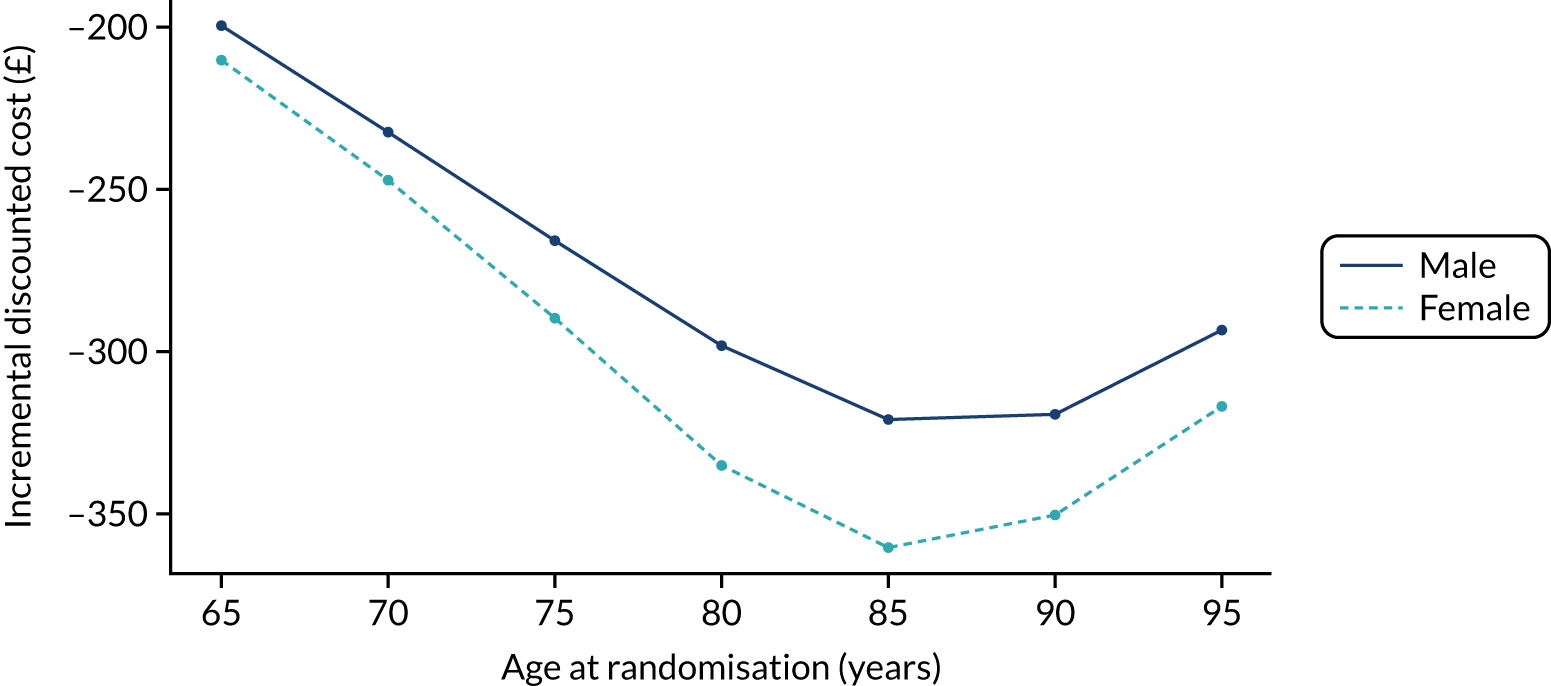
FIGURE 26.
Heterogeneity analysis: INMB.
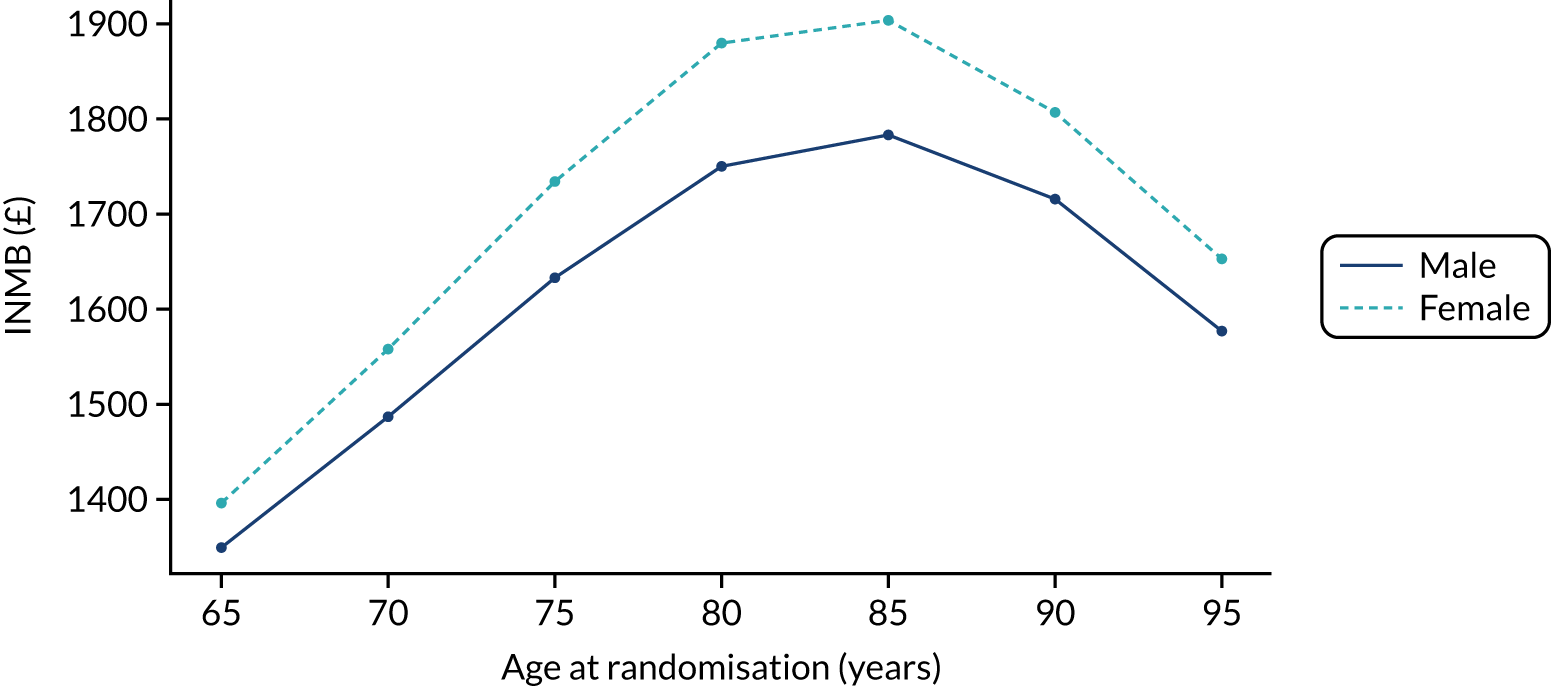
The base case produces similar incremental costs and QALYs to the REACT study participants, on average. When the reference population was the UK general population aged 65–100 years, REACT was still dominant but produced slightly smaller cost savings and incremental QALYs (see Appendix 3, Figure 41).
Sensitivity analyses
A PSA was conducted with 500 iterations. The 95% credible interval for INMB for the base case was £659 to £3550 at a willingness-to-pay threshold of £20,000 per QALY. The 95% credible interval for the ICER was –£14,626 (dominant) to £5537 per QALY. Further heterogeneity analysis is presented in Appendix 3, Figure 42.
One-way sensitivity analyses
One-way sensitivity analyses were conducted for all model parameters (Figure 27). The results show that the within-trial QALY and cost estimates strongly influence cost-effectiveness overall, as do the effects of age on utility values and SPPB evolution. There are no parameters that independently cause the intervention to have an ICER above £20,000.
FIGURE 27.
Tornado diagram for one-way sensitivity analyses.
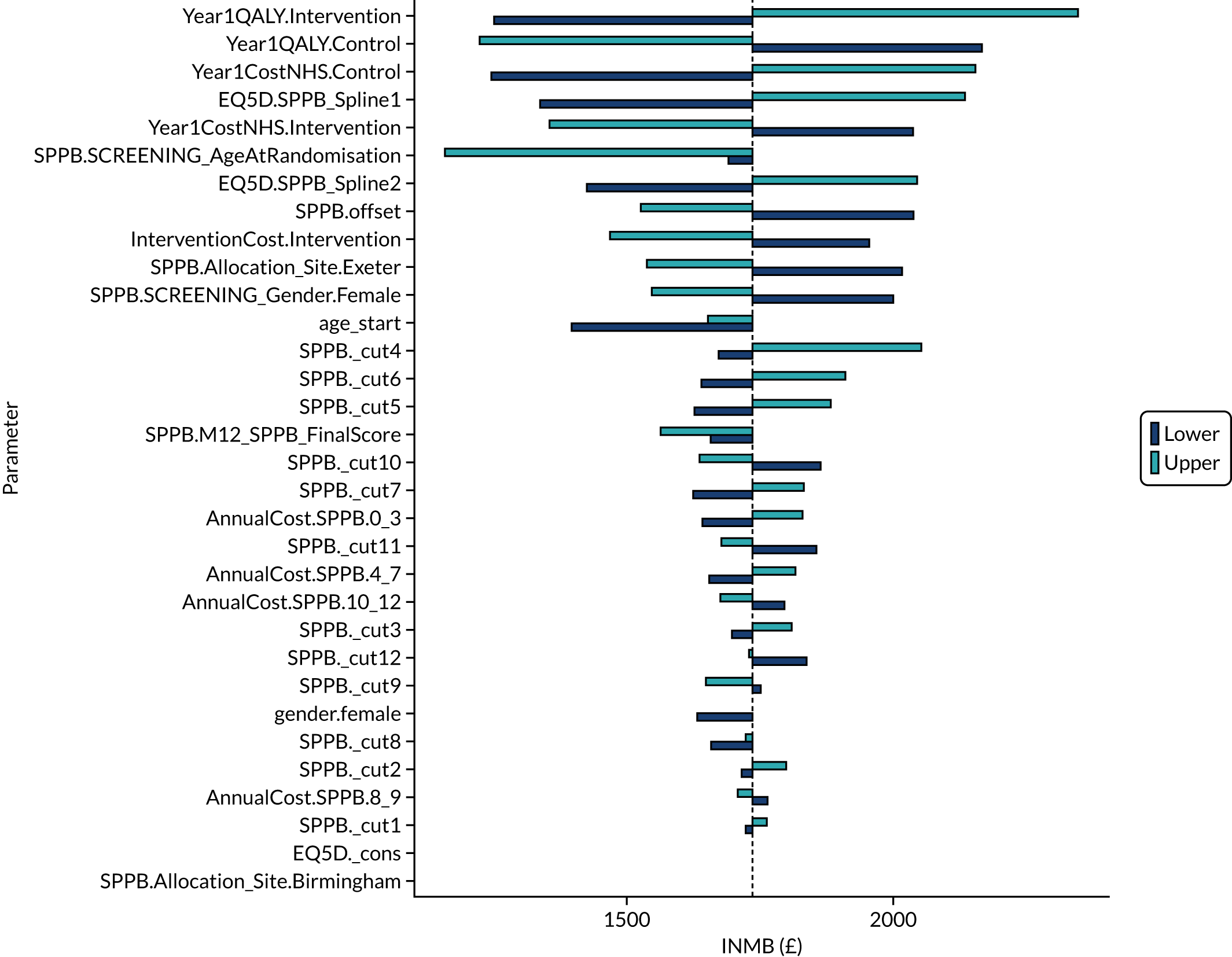
Scenario analyses
The results of the scenario analyses with a population of women aged 75 years (base-case analysis) are shown in Table 19.
| Scenario | Cost (£) | QALY | INMB (£) | ICER (£/QALY) | ||
|---|---|---|---|---|---|---|
| Control arm | Intervention arm | Control arm | Intervention arm | |||
| Base case | 20,627 | 20,338 | 7.111 | 7.183 | 1735 | Dominant |
| Inputs from CCAs | 19,580 | 19,690 | 7.177 | 7.259 | 1528 | 1345 |
| Societal perspective | 21,168 | 20,891 | 7.111 | 7.183 | 1723 | Dominant |
| SF-6D utilities | 20,627 | 20,338 | 6.579 | 6.623 | 1165 | Dominant |
| Mobility model 2 (simplified ordered logistic) | 21,057 | 20,690 | 7.044 | 7.126 | 2008 | Dominant |
| Mobility model 3 (static mobility distributions) | 20,502 | 19,838 | 7.171 | 7.302 | 3281 | Dominant |
| Mobility model 4 (alternative method for inclusion of REACT SPPB changes) | 23,843 | 23,268 | 6.749 | 6.855 | 2697 | Dominant |
| Mobility model 5 (artificial transition matrix with 38% annual convergence) | 23,192 | 22,932 | 6.834 | 6.896 | 1501 | Dominant |
All model inputs were re-estimated using CCAs rather than MI analyses. This was the only scenario in which lifetime costs in the intervention arm exceeded those in the control arm (although costs for both arms were lower than in the base-case analysis). The intervention is predicted to be cost-effective in this scenario because the ICER is well below £20,000 per QALY.
Societal perspective on costs had a limited effect on cost-effectiveness. QALYs were unchanged and costs in both arms increased, although incremental costs were marginally smaller.
The SF-6D utilities had a moderate effect on QALYs, reducing lifetime QALYs in both arms and reducing the difference in QALYs between the arms. Costs were unchanged and REACT was still the dominant strategy.
The finding that the intervention produced cost savings and more QALYs than usual care remained in all scenarios analysis of structural uncertainty associated with the model extrapolation.
Discussion
The results of the model-based economic evaluation reinforce the findings that the intervention is cost-effective compared with usual care alone. Further cost savings and QALY gains are expected to be accrued up to around 15 years from randomisation, which are a result of the intervention reducing and/or preventing frailty, which is then associated with lower costs to the NHS and PSS, as well as better health-related quality of life. Adopting a broader societal perspective had little effect on the cost-effectiveness of the intervention.
The main strengths of the model-based economic evaluation are that it extends the trial-based economic evaluation to a lifetime horizon and assumes no lasting effect of the intervention beyond the end of the trial for maintaining mobility, and no effect on mortality or chronic conditions from REACT. Making these assumptions means that it is less likely that the effects of the intervention will be exaggerated, but also risks underestimating the total benefits of the REACT programme.
Although we model transitions across 13 mobility health states, our granularity came at the cost of statistical model simplicity, given that our ordinal logit regression model relies on the strong untested assumption that the effects of the variables conform to the proportional odds pattern, which is unlikely to apply across all predictors in the model, SPPB at 12 months and covariates in the base-case analysis.
A limitation of our model analysis is that the estimation of costs and utilities fully relied on the REACT trial data, which enrolled relatively mobile participants with SPPB levels of ≥ 4. The predicted model of costs and utility values may, therefore, be biased because it relied on very limited numbers of participants at the lowest mobility levels and may not have accounted for the full spectrum of severity and its impact on health status and resource use. Further research will be required to validate the costs and utility values in representative samples, particularly those at increasing risk of permanent disability.
Chapter 6 Discussion
This chapter discusses the key findings, process and economic evaluations of the REACT RCT and their implications for future research. Recommendations for scaling up the REACT programme are also provided.
Key findings and interpretation
The primary REACT hypothesis was supported. Older adults with mobility limitations who received the 12-month, community-based group physical activity and behavioural maintenance programme had significantly better lower limb function at the 24-month follow-up than participants who attended a socioeducation-only control programme (adjusted mean difference 0.49 SPPB points, 95% CI 0.06 to 0.92 SPPB points). The difference in lower limb function between participants in the intervention and control arms was clinically meaningful at 6, 12 and 24 months. The intervention was also found to have significantly increased self-reported physical activity and performance of muscle-strengthening activity, and decreased perceived joint pain, after 24 months’ follow-up. However, there was no significant effect on accelerometer-measured physical activity or other secondary outcomes, and some differences found at 12 months were reduced at 24 months. This may reflect deterioration of the effects of exercise behaviours over time.
In qualitative interviews, participants reported that they enjoyed the programme and being part of a research study, and they reported better mental and social well-being, especially higher physical confidence, improved motivation and feeling more outgoing. Improved social connectedness and bonding with REACT groups were key outcomes for participants in the intervention arm, who also cited numerous physical health benefits, including improvements in mobility, strength, balance, walking, fitness, sleep and physical independence. Themes identified at 24 months largely mirrored those reported at 6 and 12 months. However, whereas the 6- and 12-month interviews found that social support was a key reason for engaging in REACT, at 24 months individual-level factors, such as perceived benefits, were more prominent themes in explaining physical activity maintenance. Key components of the REACT programme that seemed to positively influence maintenance of physical activity at 24 months were (1) techniques for managing slips/lapses, supporting habit change and resolving sources of tension around increasing physical activity; (2) the person-centred delivery style to build autonomy/intrinsic motivation; and (3) the group-based delivery promoting social connectedness.
The quantitative process evaluation confirmed that, compared with the control arm, the intervention arm reported experiencing greater benefits from exercising in terms of their physical, mental and social well-being (see Chapter 4, Results). In particular, the hypothesis that increased exposure to the intervention will be associated with positive changes in competence, relatedness, enjoyment and perceived benefits (hypothesis 5c) was largely supported in relation to muscle-strengthening exercise only. Increased exposure to the intervention was associated with positive changes in psychosocial determinants for muscle-strengthening exercise 12 months after baseline, but not with changes in determinants of MVPA. Moderation analyses (see Chapter 4, Results) and subgroup interactions (see Appendix 2, Table 33) showed that REACT appeared to work equally well with men and women, and the positive effects were consistently seen irrespective of age, SES (assessed by education, home ownership and quintiles of area deprivation), baseline physical function, comorbidities and study site. However, the findings in relation to ethnicity and people with mental health problems need to be treated with caution owing to small group sizes.
The intervention fidelity analysis confirmed good delivery of the exercise programme. However, it identified considerable scope for improvement in the delivery of the behaviour maintenance sessions, particularly in terms of support for monitoring progress/eliciting benefits of physical activity, action-planning/goal-setting, modelling of behaviour, supporting competence/self-efficacy, supporting relatedness and managing setbacks/problem-solving.
Taken together, the qualitative and quantitative process evaluations broadly supported the logic model for the REACT intervention. They identified several ways that the intervention and its implementation could be improved. This included possible changes to the logic model (from the qualitative and quantitative studies) and changes to delivery processes (from the intervention fidelity and qualitative studies). Recommendations for refinement/implementation of the REACT intervention are listed in the discussion sections of the relevant chapters.
Our health economic analyses indicated that REACT offers good value for money. The intervention plus usual care was cost-effective compared with usual care alone over the 2-year time period of the REACT intervention. In the base-case scenario, the intervention saved £103 in NHS/PSS costs per participant, with a QALY gain of 0.04 within the 2-year trial window. Lifetime horizon modelling estimated that further cost savings and QALY gains were accrued up to 15 years post randomisation. This is primarily because the intervention reduced frailty and its associated costs to the NHS and PSS, as well as improving health-related quality of life.
Stronger intervention effects were associated with a higher frequency of session attendance. An association between dose and response was observed, with an adjusted mean SPPB score difference of 0.64 points (95% CI 0.23 to 1.05 points; p = 0.002) for those attending ≥ 50% of intervention sessions and of 0.81 points (95% CI 0.38 to 1.23 points; p < 0.001) for those attending ≥ 75% of sessions. This strong dose–response relationship could simply reflect the effectiveness of the intervention or it could be explained in terms of participants being less likely to attend if their physical function deteriorated over the course of the study (e.g. owing to injury or other life events).
To situate our findings in the context of existing evidence, we searched Google Scholar (Google Inc., Mountain View, CA, USA), the Cochrane Database of Systematic Reviews, the NIHR Journals Library and PubMed, focusing on recent systematic reviews and meta-analyses from inception to 2014, using terms representing ‘older people’, ‘physical activity’, ‘physical function’ and ‘randomised controlled trials’.
Based on these searches, the REACT study findings are consistent with a robust existing body of evidence demonstrating the positive impact of physical activity on physical functioning,11–14 independent living, mobility-related disability, falls, hospital admissions and mortality. 16,134,135
For example, the largest efficacy trial worldwide in this population, the US LIFE efficacy trial, has already shown that an exercise and behavioural support intervention can reduce the risk of developing major mobility disability by 18%. However, prior to the publication of the current findings, there has been a lack of evidence regarding the effectiveness and cost-effectiveness of long-term (≥ 1 year) exercise programmes administered at a community level in a real-world setting and tailored for a UK population of older adults at risk of mobility disability. Furthermore, our database searches found no long-term, community-based interventions reporting effects for longer than 12 months post intervention.
The REACT study, therefore, provides robust evidence that a relatively low-resource, 1-year exercise intervention can improve physical functioning in real-world community settings in the UK, with clinically meaningful benefits that are sustained over at least 24 months.
The baseline SPPB scores were almost identical with the baseline score of the LIFE study, which enables comparison. The observed difference in SPPB score of 0.49 points at 24 months was three times larger than the between-group difference reported in the LIFE trial. 21 The REACT intervention cost an estimated £622 per participant, which also compares favourably with the cost of the LIFE intervention (£1770 per participant).
Strengths, limitations and generalisability
To the best of our knowledge, the REACT trial is the first trial in the UK to target physical function with a long-term exercise-based intervention in a population of older adults who have restricted mobility. It was a robustly designed and conducted study with low attrition rates (19% at 24 months) and a comprehensive health economic evaluation. The comprehensive process evaluation increases our understanding of the mechanisms of engagement and behaviour change underpinning the REACT intervention. This will inform further refinement and implementation of the intervention and its training, as well as further development of the logic model and behaviour change theory. As well as informing any future implementation of the REACT intervention, this may help to design other interventions promoting active ageing.
Attendance compared favourably with that reported in other exercise programmes. Of the 83.9% who started the programme, 67.7% (95% CI 65.1% to 70.4%) of participants attended ≥ 50% of the sessions. However, it is notable that 16.1% of participants allocated to the intervention arm did not attend any intervention sessions, so there is scope to improve initial engagement in the programme. The intervention fidelity analysis also identified scope for improvement in several aspects of delivery of the health behaviour maintenance sessions. Taken together, these intervention delivery deficits may have led to underestimation of intervention effects.
There are some limitations that need to be acknowledged. As with other studies of behavioural interventions, blinding of participants to the study arm was not possible, which introduces the possibility of social desirability bias in patient-reported measures. 136 However, the primary outcome was assessed by independent observers who were blinded to study arm allocation, so this is unlikely to affect the primary outcome.
The generalisability of the findings to a wider UK population is also a key issue. The REACT study aimed to test a programme that was acceptable to as wide a range of the older adult population as possible and to recruit a diverse population in terms of sex, area deprivation and ethnic background. Our attempt to recruit a representative cohort for REACT in terms of deprivation and ethnicity was reasonably successful. Across the IMD quintiles, we recruited 3.2% less than the general population of those most deprived (quintile 1), but 2.6% more than those in quintile 2 (see Table 2). Overall, we avoided a substantial skewing towards affluence, which has proved challenging with this age group in previous research. 23 The sample recruited was also representative of the older adult UK population in terms of deprivation and ethnicity, except for an under-representation of South Asian older adults. 29
Although only 3% of those invited to take part were recruited, the response rate among the eligible population was 17% (see Chapter 3). Given this, and the small numbers of ethnic minority participants recruited, the generalisability of the findings (particularly) to ethnic minority populations should be explored further in future studies or as part of a REACT implementation evaluation study.
The mixed findings on the secondary outcomes indicate that the effects of the intervention were limited to lower limb physical function and did not extend to substantial increases in physical activity or other domains of physical function (e.g. grip strength). The secondary outcome analyses were exploratory, with no adjustment for multiple testing, and should be interpreted accordingly.
Furthermore, the trial analyses did not show an impact on quality of life. However, the more sophisticated, time-integrated approach used in the health economic analysis revealed a significant difference in EQ-5D (as well as a saving in health-care costs). Indeed, the health economic analyses indicated that the increases in physical function observed were associated with substantial quality-of-life and health economic benefits, both within the 24-month trial window and across a lifetime horizon.
Although the overall results for REACT were positive, the process evaluation indicated, as with most service-based interventions, that there was considerable scope for improvement by session leaders in the facilitation of important self-regulation processes and social/relatedness-building processes during the delivery of the behavioural maintenance programme. To some extent, this may have been mitigated by mutual support among participants and self-delivery of some of the intended processes within the groups during the exercise sessions. However, future implementations of the REACT intervention should aim to improve the training and delivery of the programme accordingly.
Considerations for further research
A key consideration for scaling up REACT and for future research in this population is how to identify people who would benefit from interventions (those with SPPB scores of 4–9). Although we met our recruitment target, from invitation to randomisation we had a success rate of only 3%. This recruitment yield is comparable to that of other behavioural interventions targeting older populations. 29 However, finding ways to make recruitment more efficient and more inclusive would be of benefit to future research in this field.
One means by which this might be achieved is via the UK’s electronic Frailty Index (which is recorded in GP databases) or similar schemes being tested in Europe. 33 However, evidence of the electronic Frailty Index’s ability to identify people with mobility limitations is required to reveal whether or not its use would be beneficial in recruitment for studies targeting improved mobility. Another option might be to develop an easy-to-use, parsimonious self-report measure that has strong predictive value for lower limb physical function. One candidate measure is the MAT-SF measure (a video-scenario-based self-assessment tool designed to assess lower limb physical function) that we used as a secondary outcome of REACT. 53 Further analyses of the REACT (and other) data will reveal whether or not this might prove to be a useful screening tool for use in practice and in future studies. A third option would involve relationship-building with multiple charities, local GPs and social prescribers, and community groups to help identify suitable candidates for intervention.
More research is needed in ethnic minority communities, particularly South Asian communities, to identify strategies for greater inclusivity and any necessary programme modifications to optimise the effectiveness of the REACT programme for ethnic minority populations. Further evaluation of the effectiveness of the programme for people from diverse ethnic backgrounds is also warranted.
The exclusion criteria for REACT meant that some groups of older adults or those with particular conditions were not able to participate, despite the fact that their needs suggest that they may have benefited. Examples include people with diagnosed dementia or people living in a residential care home. Future consideration should be given to strategies and conditions for their inclusion and for assessing the effectiveness of the REACT intervention in these populations.
Further research is also needed to optimise the implementation of REACT at scale and further evaluate and extend its reach, effectiveness and cost-effectiveness.
Using comprehensive participant and public involvement techniques to engage with session leaders, older adults eligible for the REACT programme, together with the research team, will help to fine-tune the behavioural maintenance programme (in terms of both content and training) and its delivery to ensure better fidelity of delivery when REACT is implemented at scale. The examples of good practice identified in the intervention fidelity analysis and the feedback from the qualitative process evaluation will be helpful in refining the REACT training materials.
It may be possible and synergistic to integrate the REACT intervention with existing mobility-related prevention and rehabilitation services, and provide support for participants who do not meet the criteria for specific programmes targeting falls. Given their cost-effectiveness, research into the cost-effectiveness of ongoing/continuous exercise programmes may also be fruitful; this may further improve the maintenance profile of the intervention, thereby increasing the available cost savings. There is also scope for longer-term health outcomes of the REACT programme to be evaluated. For example, maintained or increased mobility in older adults may influence longer-term mental well-being and delay onset of or deterioration through other diseases and conditions, such as dementia, diabetes and heart disease. Finally, the estimated effects of the programme on long-term health service costs, such as medications, primary care visits and emergency secondary care usage, should be directly measured to verify the conclusions of the health economic modelling, particularly to identify more definitive estimates of the cost consequences of delivering the REACT intervention at scale.
The REACT study provides robust evidence that the trajectory of declining physical functioning with age is modifiable and may even be reversible for many adults. It is particularly timely, allowing for some optimism post the COVID-19 pandemic, during which deconditioning was one of the key health challenges that older people experienced.
Future research will need to examine the feasibility of new ways to increase access to the programme, including virtual attendance, which might be useful in future lockdown/pandemic situations for people who are housebound, who may live more rurally or who prefer to engage in home-based interventions in later life. Similar to other programmes,137 REACT has the potential to be delivered online in its entirety, but we need to evaluate the reach/accessibility of this delivery style and its impact on physical and psychological well-being.
Implications for decision-makers and for practice
The REACT study has provided robust evidence that a group-based exercise programme with long duration (12 months) that encourages participants to build strong social networks and the habit of exercising in a safe and fun environment can improve their prognosis for maintaining mobility and save costs in both the short and the long term. This adds to the existing literature indicating that physical activity is crucial for older people’s physical functioning and health. It should strengthen the case for consideration by funders and policy-makers to support exercise programmes for the critical at-risk population of older people with lower limb frailty or pre-frailty (as defined by a SPPB score of 4–7).
The intervention delivered positive results on mobility across different settings in three different geographical areas of the UK, using a range of session leaders who were already active in the field of exercise programme delivery. For this reason, the REACT intervention is suitable for delivery by existing service providers, including city council providers, voluntary sector organisations, commercial organisations, the leisure industry and the private sector; therefore, the results should generalise well to other real-world settings. However, attention needs to be paid to maintaining (or improving) delivery fidelity when rolling out complex interventions. 43 Therefore, it may be helpful to consider what organisation-level governance or performance-monitoring systems might support high-quality delivery of the REACT intervention.
Given that the intervention appeared to work equally well irrespective of age, gender, SES (assessed by education, home ownership and quintiles of area deprivation) and baseline physical function, REACT may have the potential to reduce health inequalities if delivery is targeted towards underserved populations.
The REACT exercise intervention also provides important evidence supporting both the US and the UK physical activity recommendations for multimodal exercise programmes. 138,139 In particular, it demonstrates the beneficial effect of engaging in at least one exercise session per week. This is a strong public health message for older adults in terms of the level of commitment that they need to demonstrate to achieve maintenance of their lower limb physical function. Contrary to the widespread belief that ageing causes an inevitable decline in physical functioning, the REACT study has proved that this decline can be delayed or reversed with relatively modest lifestyle changes.
The health economic analysis indicates that REACT provides a feasible and cost-effective means of helping older people stay mobile, and this will enhance their chances of remaining independent for longer. Based on the moderation and subgroup analyses presented above, it also has potential to help reduce health inequalities among the elderly. Given this and the cost-saving nature of the intervention, REACT should be given serious consideration by health and public health commissioners.
Although recruitment of older participants to a research study that includes a RCT is much more of a commitment than recruiting to an exercise programme offered by leisure or community services, many lessons have been learned that may help to inform real-world implementation. These lessons are available through our recruitment publication. 29 Furthermore, given that the NIHR Public Health Research programme does not fund intervention costs, the researchers needed to work closely with multiple partners to deliver the REACT intervention programme, which was an excellent test of programme pragmatism and sustainability. The delivery of the REACT study provides an important template for this partnership model.
Two further implications that may be useful for future practice and/or decision-making are that (1) the REACT exercise intervention seems to work even with attendance as low as 50% and a lower-than-intended quality of delivery of the behavioural maintenance programme, and (2) there is scope for improvement of delivery quality through refinements to the behavioural maintenance programme and its training course. This may lead to increased effectiveness and better maintenance of effects in future implementations of the programme.
Finally, it is important to position the REACT programme within the COVID-19 pandemic context and the potential for REACT delivery in the post-COVID-19 era. The COVID-19 pandemic has resulted in the target population of the REACT intervention following stay-at-home guidance during lockdowns and, in some cases, shielding outside lockdowns. During these periods, it would not have been possible to deliver the REACT intervention as originally designed and evaluated. It is unclear whether or not a digital alternative to REACT, delivered in people’s homes or online, would be feasible, safe and effective.
As the UK exits from COVID-19 restrictions and a large proportion of the target population have been vaccinated, it is likely that the REACT intervention will once again be deliverable. There may be some concerns around travel to the REACT venues (if the target population are less comfortable taking public transport than previously), which could affect take-up. On the other hand, the demand for appropriate venues may have dropped owing to an increasing number of people working from home, which could lead to a reduction in the cost of delivering REACT (in the short to medium term).
Considering the possibility of future pandemics (e.g. further novel coronaviruses or influenza pandemics), the REACT intervention may be expected to allow more people to maintain their mobility and continue to live in private homes, rather than entering residential or nursing homes because of frailty. A significant burden of COVID-19 in the UK resulted from SARS-CoV-2 spreading through care homes and leading to the hospitalisation and/or death of residents; this may give an additional incentive for local governments and the NHS to fund interventions that stave off frailty.
Conclusion
The REACT study provides robust evidence that a relatively low-resource, 1-year exercise intervention delivered alongside a behaviour maintenance programme helped UK older adults to retain their lower limb physical function over a 24-month period. The REACT intervention is cost saving from an NHS/PSS perspective within a 2-year window, with further cost savings and QALY benefits estimated in the longer term. The results indicate that the well-established trajectory of declining physical functioning in older age is modifiable and in some cases reversible. Following further refinements guided by the findings of the process evaluation, the REACT intervention is suitable for large-scale implementation. The findings also suggest that there is potential for improvement in the design and delivery of the REACT intervention, and implementation should be evaluated to confirm the generalisability of these findings to the wider population, especially in ethnic minority populations.
Some text in this chapter has been reproduced from Stathi et al. ,1 Withall et al. 29 and Cross et al. 71 These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Acknowledgements
Non-author contributors
We wish to express our thanks to the entire REACT research team, the Clinical Research Networks at each REACT site and all of the general practices and community organisations that supported REACT recruitment. Delivery of the REACT programme was possible only because of the support of our partners Bath and North East Somerset Council; Exeter and Solihull City Councils; Westbank Charity, Exminster; St Monica Trust, Bristol; Bristol Ageing Better; St John’s Hospital, Bath; Age UK, Birmingham; Agewell, West Midlands; Sandwell and West Birmingham Hospitals NHS Trust; the Portway Lifestyle Centre, Oldbury; and Solihull Borough Council, Birmingham.
Our Trial Steering Committee, chaired by Professor Yoav Ben-Shlomo, Data Management and Ethics Committee, chaired by Professor Dawn Skelton, and independent statistician, Professor Peter Thomas, provided hugely valuable expert guidance and support.
Dr Andrew F Hayes of The Ohio State University Department of Psychology provided valuable advice on the conduct and interpretation of the moderation analyses.
Finally, we would like to thank the 777 REACT participants without whom this research would not have been possible.
Contribution of authors
Afroditi Stathi (https://orcid.org.uk/0000-0003-2162-777X) (Professor of Physical Activity and Community Health) obtained funding for the research, acted as chief investigator and principal investigator at the Bath site, led the design of the study and drafted the report manuscript.
Janet Withall (https://orcid.org.uk/0000-0003-0196-5309) (Trial Manager, Active Ageing) led study co-ordination, contributed to the design of the study, was involved in the collection and processing of data and drafted the report manuscript.
Colin J Greaves (https://orcid.org.uk/0000-0003-4425-2691) (Professor of Psychology Applied to Health, Health Psychology) obtained funding for the research, acted as principal investigator at the Devon site, contributed to the design of the study, led the process evaluation and drafted the report manuscript.
Janice L Thompson (https://orcid.org.uk/0000-0002-7312-3226) (Professor of Public Health Nutrition and Exercise) obtained funding for the research, acted as principal investigator at the Birmingham site, contributed to the design of the study and reviewed the report manuscript.
Gordon Taylor (https://orcid.org.uk/0000-0003-1715-0913) (Professor of Medical Statistics) obtained funding for the research, contributed to the design of the study, developed the statistical analysis plan and undertook the analyses, and reviewed the report manuscript.
Antonieta Medina-Lara (https://orcid.org.uk/0000-0001-7325-8246) (Associate Professor in Health Economics) obtained funding for the research, contributed to the design of the health economic study, estimated the cost of the intervention and reviewed the report manuscript.
Colin Green (https://orcid.org.uk/0000-0001-6140-1287) (Honorary Professor of Health Economics) obtained funding for the research, contributed to the design of the study and reviewed the report manuscript.
Tristan Snowsill (https://orcid.org.uk/0000-0001-7406-2819) (Senior Research Fellow, Health Economics) was involved in the collection and processing of data, estimated the cost of the intervention and reviewed the report manuscript.
Heidi Johansen-Berg (https://orcid.org.uk/0000-0002-4134-9730) (Professor of Cognitive Neuroscience) obtained funding for the research, contributed to the design of the study (MRI substudy) and reviewed the report manuscript.
James Bilzon (https://orcid.org.uk/0000-0002-6701-7603) (Professor of Human and Applied Physiology, Rehabilitation Medicine) obtained funding for the research, acted as principal investigator at the Bath site, contributed to the design of the study and reviewed the report manuscript.
Selena Gray (https://orcid.org.uk/0000-0001-6257-4024) (Professor of Public Health) obtained funding for the research, contributed to the design of the study and reviewed the report manuscript.
Rosina Cross (https://orcid.org.uk/0000-0002-2548-2398) (Postdoctoral Research Fellow, Primary Care) was involved in the collection and processing of data and contributed to the process evaluation report manuscript.
Max J Western (https://orcid.org.uk/0000-0003-1107-8498) (Lecturer, Behavioural Science) was involved in the collection and processing of data and reviewed the report manuscript.
Jolanthe L de Koning (https://orcid.org.uk/0000-0001-6811-5726) (Research Assistant, Active Ageing) was involved in the collection and processing of data and reviewed the report manuscript.
Peter Ladlow (https://orcid.org.uk/0000-0002-9891-9714) (Higher Scientific Officer, Strength and Reconditioning/Rehabilitation) contributed to the design of the study, led the design of the exercise intervention and reviewed the report manuscript.
Jessica C Bollen (https://orcid.org.uk/0000-0002-6437-5923) (Research Assistant, Active Ageing) was involved in the collection and processing of data and reviewed the report manuscript.
Sarah J Moorlock (https://orcid.org.uk/0000-0003-4473-7226) (Research Assistant, Active Ageing) was involved in the collection and processing of data and reviewed the report manuscript.
Jack M Guralnik (https://orcid.org.uk/0000-0001-5108-7263) (Professor in Epidemiology and Public Health, Epidemiology and Ageing) obtained funding for the research, contributed to the design of the study and reviewed the report manuscript.
W Jack Rejeski (https://orcid.org.uk/0000-0003-2281-4649) (Professor of Health and Exercise Science, Behaviour Change and Disability in Ageing) obtained funding for the research, contributed to the design of the study and reviewed the report manuscript.
Melvyn Hillsdon (https://orcid.org.uk/0000-0003-2818-3278) (Associate Professor in Physical Activity Epidemiology and Public Health, Physical Activity and Health) contributed to the processing and analysis of accelerometer data and reviewed the report manuscript.
Kenneth R Fox (https://orcid.org.uk/0000-0003-2216-4287) (Emeritus Professor of Exercise and Health Sciences, Physical Activity and Health) obtained funding for the research, contributed to the design of the study and reviewed the report manuscript.
Publications
Stathi A, Withall J, Greaves CJ, Thompson JL, Taylor G, Medina-Lara A, et al. A community-based physical activity intervention to prevent mobility-related disability for retired older people (REtirement in ACTion (REACT)): study protocol for a randomised controlled trial. Trials 2018;19:228.
Withall J, Greaves CJ, Thompson JL, de Koning JL, Bollen JC, Moorlock SJ, et al. The tribulations of trials: lessons learnt recruiting 777 older adults into REtirement in ACTion (REACT), a trial of a community, group-based active aging intervention targeting mobility disability. J Gerontol 2020;75:2387–95.
Cross R, Greaves CJ, Withall J, Rejeski WJ, Stathi A. Delivery fidelity of the REACT (REtirement in ACTion) physical activity and behaviour maintenance intervention for community dwelling older people with mobility limitations. BMC Public Health 2022;22:1112.
Demnitz N, Stathi A, Withall J, Stainer C, Seager P, de Koning J, et al. Hippocampal maintenance after a 12-month physical activity intervention in older adults: the REACT MRI study. Neuroimage Clin 2022;35:102762.
Snowsill T, Stathi A, Green C, Withall J, Greaves C, Thompson J, et al. Cost-effectiveness of a community-based physical activity and behaviour maintenance intervention for preventing decline in physical functioning in older people: an economic evaluation of the REACT (Retirement in Action) intervention. Lancet Public Health 2022;7:E327–34.
Stathi A, Greaves C, Thompson J, Withall J, Ladlow P, Taylor G, et al. Effect of a physical activity and behaviour maintenance programme on functional mobility decline in older adults: the REACT (REtirement in ACTion) randomised controlled trial. Lancet Public Health 2022;7:E316–26.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PHR programme or the Department of Health and Social Care.
References
- Stathi A, Withall J, Greaves CJ, Thompson JL, Taylor G, Medina-Lara A, et al. A community-based physical activity intervention to prevent mobility-related disability for retired older people (REtirement in ACTion (REACT)): study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2603-x.
- World Health Organisation . Healthy Ageing and Functional Ability 2018. www.who.int/news-room/questions-and-answers/item/healthy-ageing-and-functional-ability (accessed February 2021).
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752-62. https://doi.org/10.1016/S0140-6736(12)62167-9.
- Nguyen QD, Wu C, Odden MC, Kim DH. Multimorbidity patterns, frailty, and survival in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2019;74:1265-70. https://doi.org/10.1093/gerona/gly205.
- NHS Digital . Adults Health-Related Behaviours Health Survey for England 2018 2019. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2018 (accessed February 2021).
- Health and Social Care Information Centre . Health Survey for England 2014: Health, Social Care and Lifestyles 2014. http://healthsurvey.hscic.gov.uk/media/33473/HSE-2014-summary-report.pdf (accessed February 2021).
- Kojima G. Increased healthcare costs associated with frailty among community-dwelling older people: a systematic review and meta-analysis. Arch Gerontol Geriatr 2019;84. https://doi.org/10.1016/j.archger.2019.06.003.
- Office for National Statistics . Overview of the UK Population: November 2018 2018. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/november2018 (accessed February 2021).
- World Health Organisation . World Population Ageing 2017. www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf (accessed February 2021).
- United States Census Bureau . Older Population and Aging 2019. www.census.gov/topics/population/older-aging.html (accessed January 2021).
- Dipietro L, Campbell WW, Buchner DM, Erickson KI, Powell KE, Bloodgood B, et al. Physical activity, injurious falls, and physical function in aging: an umbrella review. Med Sci Sports Exerc 2019;51:1303-13. https://doi.org/10.1249/MSS.0000000000001942.
- Englund DA, Price LL, Grosicki GJ, Iwai M, Kashiwa M, Liu C, et al. Progressive resistance training improves torque capacity and strength in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 2019;74:1316-21. https://doi.org/10.1093/gerona/gly199.
- Stathi A, Western M, De Koning J, Perkin O, Withall J, Nyman S. The Palgrave Handbook of Ageing and Physical Activity Promotion. London: Palgrave Macmillan; 2018.
- García-Hermoso A, Ramirez-Vélez R, Sáez de Asteasu ML, Martínez-Velilla N, Zambom-Ferraresi F, Valenzuela PL, et al. Safety and effectiveness of long-term exercise interventions in older adults: a systematic review and meta-analysis of randomized controlled trials. Sports Med 2020;50:1095-106. https://doi.org/10.1007/s40279-020-01259-y.
- Cooper R, Kuh D, Cooper C, Gale CR, Lawlor DA, Matthews F, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing 2011;40:14-23. https://doi.org/10.1093/ageing/afq117.
- Simmonds B, Fox K, Davis M, Ku PW, Gray S, Hillsdon M, et al. Objectively assessed physical activity and subsequent health service use of UK adults aged 70 and over: a four to five year follow up study. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0097676.
- Jacob ME, Yee LM, Diehr PH, Arnold AM, Thielke SM, Chaves PHM, et al. Can a healthy lifestyle compress the disabled period in older adults?. J Am Geriatr Soc 2016;64:1952-61. https://doi.org/10.1111/jgs.14314.
- Department of Health and Social Care . Ageing Well and Supporting People Living With Frailty 2018. www.england.nhs.uk/ourwork/clinical-policy/older-people/frailty/ (accessed March 2021).
- World Health Organization . More Active People for a Healthier World. The Global Action Plan on Physical Activity 2018–2030 2018. www.who.int/news-room/initiatives/gappa (accessed March 2021).
- Giné-Garriga M, Roqué-Fíguls M, Coll-Planas L, Sitjà-Rabert M, Salvà A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med Rehabil 2014;95:753-69.e3. https://doi.org/10.1016/j.apmr.2013.11.007.
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014;311:2387-96. https://doi.org/10.1001/jama.2014.5616.
- The Marmot Review . Fair Society, Healthy Lives: Strategic Review of Health Inequalities in England Post-2010 2010.
- Shaw RJ, Čukić I, Deary IJ, Gale CR, Chastin SF, Dall PM, et al. Relationships between socioeconomic position and objectively measured sedentary behaviour in older adults in three prospective cohorts. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016436.
- Centers for Disease Control and Prevention . CDC Health Disparities and Inequalities Report. Morbidity and Mortality Weekly Report 2011. www.cdc.gov/mmwr/pdf/other/su6001.pdf (accessed February 2021).
- Bor J, Cohen GH, Galea S. Population health in an era of rising income inequality: USA, 1980–2015. Lancet 2017;389:1475-90. https://doi.org/10.1016/S0140-6736(17)30571-8.
- NHS Digital . Health Survey for England – 2010, Trend Tables 2011. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2010-trend-tables (accessed January 2021).
- Sport England . Ethnicity Facts and Figures 2019. www.ethnicity-facts-figures.service.gov.uk/health/diet-and-exercise/physical-activity/latest#data-sources (accessed January 2021).
- GOV.UK . Ethnicity Facts and Figures 2020. www.ethnicity-facts-figures.service.gov.uk/health/diet-and-exercise/physical-activity/latest (accessed February 2021).
- Withall J, Greaves CJ, Thompson JL, de Koning JL, Bollen JC, Moorlock SJ, et al. The tribulations of trials: lessons learnt recruiting 777 older adults into REtirement in ACTion (REACT), a trial of a community, group-based active aging intervention targeting mobility disability. J Gerontol A Biol Sci Med Sci 2020;75:2387-95. https://doi.org/10.1093/gerona/glaa051.
- Stathi A, Fox KR, Withall J, Bentley G, Thompson JL. Promoting Physical Activity in Older Adults: A Guide for Local Decision Makers 2014. https://purehost.bath.ac.uk/ws/portalfiles/portal/32338941/AVON_strategies_report_2014_Feb.pdf (accessed 17 October 2022).
- Demnitz N, Stathi A, Withall J, Stainer C, Seager P, de Koning J, et al. Hippocampal maintenance after a 12-month physical activity intervention in older adults: the REACT MRI study. Neuroimage Clin 2021;35. https://doi.org/10.1016/j.nicl.2021.102762.
- Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, et al. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2009;64:223-9. https://doi.org/10.1093/gerona/gln022.
- European Medicines Agency . Reflection Paper on Physical Frailty: Instruments for Baseline Characterisation of Older Populations in Clinical Trials 2018. www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-physical-frailty-instruments-baseline-characterisation-older-populations-clinical_en.pdf (accessed January 2021).
- NHS England . A Practical Guide to Healthy Ageing 2015. www.england.nhs.uk/publication/practical-guide-to-healthy-ageing/ (accessed January 2021).
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377-81. https://doi.org/10.1249/00005768-198205000-00012.
- Ntoumanis N, Ng JYY, Prestwich A, Quested E, Hancox JE, Thøgersen-Ntoumani C, et al. A meta-analysis of self-determination theory-informed intervention studies in the health domain: effects on motivation, health behavior, physical, and psychological health. Health Psychol Rev 2021;15:214-44. https://doi.org/10.1080/17437199.2020.1718529.
- Ryan RM, Deci EL. A self-determination theory approach to psychotherapy: the motivational basis for effective change. Canadian Psychology 2008;49:186-93. https://doi.org/10.1037/a0012753.
- Deci EL, Ryan RM. Handbook of Self-determination Research. Rochester, NY: The University of Rochester Press; 2002.
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychology &Amp; Health 1998;13:623-49. https://doi.org/10.1080/08870449808407422.
- Greaves C, Poltawski L, Garside R, Briscoe S. Understanding the challenge of weight loss maintenance: a systematic review and synthesis of qualitative research on weight loss maintenance. Health Psychol Rev 2017;11:145-63. https://doi.org/10.1080/17437199.2017.1299583.
- Poltawski L, van Beurden SB, Morgan-Trimmer S, Greaves C. The dynamics of decision-making in weight loss and maintenance: a qualitative enquiry. BMC Public Health 2020;20. https://doi.org/10.1186/s12889-020-08664-y.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci 2005;60:894-900. https://doi.org/10.1093/gerona/60.7.894.
- Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 1974;15:443-53. https://doi.org/10.1002/cpt1974155443.
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103-15. https://doi.org/10.2307/2529712.
- Sampson M, Clark A, Bachmann M, Garner N, Irvine L, Howe A, et al. Lifestyle intervention with or without lay volunteers to prevent type 2 diabetes in people with impaired fasting glucose and/or nondiabetic hyperglycemia: a randomized clinical trial. JAMA Internal Med 2020;181:168-78. https://doi.org/10.1001/jamainternmed.2020.5938.
- Flight L, Allison A, Dimairo M, Lee E, Mandefield L, Walters SJ. Recommendations for the analysis of individually randomised controlled trials with clustering in one arm – a case of continuous outcomes. BMC Med Res Methodol 2016;16. https://doi.org/10.1186/s12874-016-0249-5.
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153-62. https://doi.org/10.1016/0895-4356(93)90053-4.
- Stathi A, Fox K. The dimensions of a well-being scale designed for older adults: the Ageing-Well Profile. J Aging Phys Act 2004;12.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Rejeski WJ, Rushing J, Guralnik JM, Ip EH, King AC, Manini TM, et al. The MAT-sf: identifying risk for major mobility disability. J Gerontol A Biol Sci Med Sci J 2015;70:641-6. https://doi.org/10.1093/gerona/glv003.
- Lyall DM, Cullen B, Allerhand M, Smith DJ, Mackay D, Evans J, et al. Cognitive Test Scores in UK biobank: data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0154222.
- Cornelis MC, Wang Y, Holland T, Agarwal P, Weintraub S, Morris MC. Age and cognitive decline in the UK Biobank. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0213948.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833-40.
- Espie CA, Kyle SD, Hames P, Gardani M, Fleming L, Cape J. The Sleep Condition Indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004183.
- Kempen GI, Todd CJ, Van Haastregt JC, Zijlstra GA, Beyer N, Freiberger E, et al. Cross-cultural validation of the Falls Efficacy Scale International (FES-I) in older people: results from Germany, the Netherlands and the UK were satisfactory. Disabil Rehabil 2007;29:155-62. https://doi.org/10.1080/09638280600747637.
- Stathi A, Greaves C, Thompson J, Withall J, Ladlow P, Taylor G, et al. Effect of a physical activity and behaviour maintenance programme on functional mobility decline in older adults: the REACT (REtirement in ACTion) randomised controlled trial. Lancet Public Health 2022;7:E316-26. https://doi.org/10.1016/S2468-2667(22)00004-4.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-18.
- Office for National Statistics . Ethnicity Facts and Figures 2018. www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/age-groups/latest#age-profile-by-ethnicity (accessed January 2021).
- Office for National Statistics . Population Estimates for the UK, England and Wales, Scotland and Northern Ireland: Mid-2018 2019. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2018 (accessed January 2021).
- Office for National Statistics . Populations by Sex, Age Group and Index of Multiple Deprivation (IMD) Quintile, England, 2001 to 2017 2018. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/adhocs/009283populationsbysexagegroupandimdquintileengland2001to2017 (accessed January 2021).
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006;295:2018-26. https://doi.org/10.1001/jama.295.17.2018.
- Perera S, Studenski S, Newman A, Simonsick E, Harris T, Schwartz A, et al. Study. Are estimates of meaningful decline in mobility performance consistent among clinically important subgroups? (Health ABC study). J Gerontol A Biol Sci Med Sci 2014;69:1260-8. https://doi.org/10.1093/gerona/glu033.
- Jefferis BJ, Parsons TJ, Sartini C, Ash S, Lennon LT, Papacosta O, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation?. Br J Sports Med 2019;53:1013-20. https://doi.org/10.1136/bjsports-2017-098733.
- Badhiwala JH, Witiw CD, Nassiri F, Akbar MA, Jaja B, Wilson JR, et al. Minimum clinically important difference in SF-36 scores for use in degenerative cervical myelopathy. Spine 2018;43:E1260-E1266. https://doi.org/10.1097/BRS.0000000000002684.
- Carreon LY, Glassman SD, Campbell MJ, Anderson PA. Neck Disability Index, short form-36 physical component summary, and pain scales for neck and arm pain: the minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J 2010;10:469-74. https://doi.org/10.1016/j.spinee.2010.02.007.
- Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci 2019;31:75-8. https://doi.org/10.1589/jpts.31.75.
- Cross R, Greaves CJ, Withall J, Rejeski JW, Stathi A. Delivery fidelity of the REACT (REtirement in ACTion) physical activity and behaviour maintenance intervention for community dwelling older people with mobility limitations. BMC Public Health 2022;22. https://doi.org/10.1186/s12889-022-13496-z.
- Dreyfus SE. The five-stage model of adult skill acquisition. Bull Sci Technol Soc 2004;24:177-82. https://doi.org/10.1177/0270467604264992.
- Frost J, Wingham J, Britten N, Greaves C, Abraham C, Warren FC, et al. Home-based rehabilitation for heart failure with reduced ejection fraction: mixed methods process evaluation of the REACH-HF multicentre randomised controlled trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026039.
- Thompson TP, Lambert JD, Greaves CJ, Taylor AH. Intervention delivery fidelity assessment of a counselling based intervention for promoting smoking reduction and increasing physical activity. Health Psychol 2018;37:627-37. https://doi.org/10.1037/hea0000613.
- Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol 2008;41:327-50. https://doi.org/10.1007/s10464-008-9165-0.
- Lambert JD, Greaves CJ, Farrand P, Cross R, Haase AM, Taylor AH. Assessment of fidelity in individual level behaviour change interventions promoting physical activity among adults: a systematic review. BMC Public Health 2017;17. https://doi.org/10.1186/s12889-017-4778-6.
- Devereux-Fitzgerald A, Powell R, Dewhurst A, French DP. The acceptability of physical activity interventions to older adults: A systematic review and meta-synthesis. Soc Sci Med 2016;158:14-23. https://doi.org/10.1016/j.socscimed.2016.04.006.
- Zubala A, MacGillivray S, Frost H, Kroll T, Skelton DA, Gavine A, et al. Promotion of physical activity interventions for community dwelling older adults: a systematic review of reviews. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0180902.
- French DP, Olander EK, Chisholm A, Mc Sharry J. Which behaviour change techniques are most effective at increasing older adults’ self-efficacy and physical activity behaviour? A systematic review. Ann Behav Med 2014;48:225-34. https://doi.org/10.1007/s12160-014-9593-z.
- Ashford S, Edmunds J, French DP. What is the best way to change self-efficacy to promote lifestyle and recreational physical activity? A systematic review with meta-analysis. Br J Health Psychol 2010;15:265-88. https://doi.org/10.1348/135910709X461752.
- Borek AJ, Abraham C. How do small groups promote behaviour change? An integrative conceptual review of explanatory mechanisms. Appl Psychol Health Well Being 2018;10:30-61. https://doi.org/10.1111/aphw.12120.
- Farrow C, Tarrant M, Khan S, Buckinham S, Best D. Addiction, Behavioral Change and Social Identity: The Path to Resilience and Recovery. New York, NY: Routledge; 2016.
- Khan SS, Tarrant M, Kos K, Daly M, Gimbuta C, Farrow CV. Making connections: social identification with new treatment groups for lifestyle management of severe obesity. Clin Psychol Psychother 2020;27:686-96. https://doi.org/10.1002/cpp.2454.
- Borek AJ, Smith JR, Greaves CJ, Gillison F, Tarrant M, Morgan-Trimmer S, et al. Developing and applying a framework to understand mechanisms of action in group-based, behaviour change interventions: the MAGI mixed-methods study. Efficacy and Mech Eval 2019;6. https://doi.org/10.3310/eme06030.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:52-63. https://doi.org/10.1111/j.1752-7325.2011.00233.x.
- Patton MQ. Qualitative Evaluation and Research Methods. London: SAGE Publications Ltd; 1990.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Lincoln YS, Guba EG. Naturalistic Inquiry. London: SAGE Publications Ltd; 1985.
- Nevedal AL, Ayalon L, Briller SH. A qualitative evidence synthesis review of longitudinal qualitative research in gerontology. Gerontologist 2019;59:e791-e801. https://doi.org/10.1093/geront/gny134.
- Maula A, LaFond N, Orton E, Iliffe S, Audsley S, Vedhara K, et al. Use it or lose it: a qualitative study of the maintenance of physical activity in older adults. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1366-x.
- Moore AJ, Holden MA, Foster NE, Jinks C. Therapeutic alliance facilitates adherence to physiotherapy-led exercise and physical activity for older adults with knee pain: a longitudinal qualitative study. J Physiother 2020;66:45-53. https://doi.org/10.1016/j.jphys.2019.11.004.
- Sims-Gould J, Miran-Khan K, Haggis C, Liu-Ambrose T. Timing, experience, benefits, and barriers: older women’s uptake and adherence to an exercise program. Act Adapt Aging 2012;36:280-96. https://doi.org/10.1080/01924788.2012.729188.
- Walker KC, Valentiner LS, Langberg H. Motivational factors for initiating, implementing, and maintaining physical activity behavior following a rehabilitation program for patients with type 2 diabetes: a longitudinal, qualitative, interview study. Patient Prefer Adherence 2018;12:145-52. https://doi.org/10.2147/PPA.S150008.
- Curnan S, LaCava L, Langenburg D, Lelle M, Reece M. WK Kellogg Foundation Evaluation Handbook 1998. www.wkkf.org/pubs/Tools/Evaluation/Pub770.pdf (accessed January 2021).
- W.K Kellogg Foundation. Logic Model Development Guide. Battle Creek, MI: W.K Kellogg Foundation; 2004.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Hayes AF. Introduction to Mediation, Moderation and Conditional Process Analysis: A Regression Based Approach. New York, NY: Guildford Press; 2018.
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986.
- Graham Beaumont J, Kenealy P. Quality of life perceptions and social comparisons in healthy old age. Ageing Soc 2004;24:755-69. https://doi.org/10.1017/S0144686X04002399.
- Silfee VJ, Haughton CF, Jake-Schoffman DE, Lopez-Cepero A, May CN, Sreedhara M, et al. Objective measurement of physical activity outcomes in lifestyle interventions among adults: a systematic review. Prev Med Rep 2018;11:74-80. https://doi.org/10.1016/j.pmedr.2018.05.003.
- Guo W, Key TJ, Reeves GK. Accelerometer compared with questionnaire measures of physical activity in relation to body size and composition: a large cross-sectional analysis of UK Biobank. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-024206.
- Harris TJ, Owen CG, Victor CR, Adams R, Ekelund U, Cook DG. A comparison of questionnaire, accelerometer, and pedometer: measures in older people. Med Sci Sports Exerc 2009;41:1392-402. https://doi.org/10.1249/MSS.0b013e31819b3533.
- Snowsill T, Stathi A, Green C, Withall J, Greaves C, Thompson J, et al. Cost-effectiveness of a community-based physical activity and behaviour maintenance intervention for preventing decline in physical functioning in older people: an economic evaluation of the REACT (Retirement in Action) intervention. Lancet Public Health 2022;7:E327-34.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/the-reference-case (accessed February 2021).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 17 October 2022).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Kharroubi SA, Brazier JE, Roberts J, O’Hagan A. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ 2007;26:597-612. https://doi.org/10.1016/j.jhealeco.2006.09.002.
- Brazier J, Ratcliffe J, Salomon J, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2007.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2018.
- NHS Improvement . National Cost Collection for the NHS 2020. https://improvement.nhs.uk/resources/national-cost-collection/ (accessed January 2021).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- NHS Improvement . Reference Costs, 2018 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed January 2021).
- van den Berg B, Ferrer-i-Carbonell A. Monetary valuation of informal care: the well-being valuation method. Health Econ 2007;16:1227-44. https://doi.org/10.1002/hec.1224.
- Organisation for Economic Cooperation and Development (OECD) . Purchasing Power Parities (PPP) 2017. www.oecd-ilibrary.org/content/data/1290ee5a-en (accessed January 2021).
- Office for National Statistics . Employee Earnings in the UK: 2019 2019. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2019 (accessed January 2021).
- Newell D, Diment E, Bolton JE. An electronic patient-reported outcome measures system in UK chiropractic practices: a feasibility study of routine collection of outcomes and costs. J Manipulative Physiol Ther 2016;39:31-4. https://doi.org/10.1016/j.jmpt.2015.12.001.
- General Osteopathic Council . Visiting an Osteopath: What to Expect 2020. www.osteopathy.org.uk/visiting-an-osteopath/what-to-expect/ (accessed January 2021).
- Nixon RM, Thompson SG. Parametric modelling of cost data in medical studies. Stat Med 2004;23:1311-31. https://doi.org/10.1002/sim.1744.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Campbell Collaboration, ePPI-Centre . CCEMG – EPPI-Centre Cost Converter 2020. https://eppi.ioe.ac.uk/costconversion/default.aspx (accessed January 2021).
- Incisive Health . An International Comparison of Long-Term Care Funding and Outcomes: Insights for the Social Care Green Paper 2018. www.ageuk.org.uk/globalassets/age-uk/documents/reports-and-publications/reports-and-briefings/care--support/rb_aug18_international_comparison_of_social_care_funding_and_outcomes.pdf (accessed January 2021).
- Alhambra-Borrás T, Durá-Ferrandis E, Ferrando-García M. Effectiveness and estimation of cost-effectiveness of a group-based multicomponent physical exercise programme on risk of falling and frailty in community-dwelling older adults. Int J Environ Res Public Health 2019;16. https://doi.org/10.3390/ijerph16122086.
- Groessl EJ, Kaplan RM, Castro Sweet CM, Church T, Espeland MA, Gill TM, et al. Cost-effectiveness of the LIFE physical activity intervention for older adults at increased risk for mobility disability. J Gerontol A Biol Sci Med Sci 2016;71:656-62. https://doi.org/10.1093/gerona/glw001.
- Filipovic-Pierucci A, Zarca K, Durand-Zaleski I. Markov Models for Health Economic Evaluation: The R Package Heemod 2017. https://doi.org/10.1016/j.jval.2016.09.133 (accessed January 2021).
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13:322-38. https://doi.org/10.1177/0272989X9301300409.
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221-31. https://doi.org/10.1093/gerona/55.4.m221.
- Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0763-7.
- Ciani O, Buyse M, Drummond M, Rasi G, Saad ED, Taylor RS. Time to review the role of surrogate end points in health policy: state of the art and the way forward. Value Health 2017;20:487-95. https://doi.org/10.1016/j.jval.2016.10.011.
- Office for National Statistics . Estimates of the Population for the UK, England and Wales, Scotland and Northern Ireland 2019. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed February 2021).
- Office for National Statistics . Mid-Year Population Estimates of the Very Old, Including Centenarians: UK 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/datasets/midyearpopulationestimatesoftheveryoldincludingcentenariansunitedkingdom (accessed January 2021).
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551-61. https://doi.org/10.1002/sim.4780080504.
- Callahan KE, Lovato L, Miller ME, Marsh AP, Fielding RA, Gill TM, et al. Self-reported physical function as a predictor of hospitalization in the lifestyle interventions and independence for elders study. J Am Geriatr Soc 2018;66:1927-33. https://doi.org/10.1111/jgs.15468.
- Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci 2000;55:M691-7. https://doi.org/10.1093/gerona/55.11.m691.
- Hebert JR, Hurley TG, Peterson KE, Resnicow K, Thompson FE, Yaroch AL, et al. Social desirability trait influences on self-reported dietary measures among diverse participants in a multicenter multiple risk factor trial. J Nutr 2008;138:226S-234S. https://doi.org/10.1093/jn/138.1.226S.
- Maddison R, Rawstorn JC, Stewart RAH, Benatar J, Whittaker R, Rolleston A, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart 2019;105:122-9. https://doi.org/10.1136/heartjnl-2018-313189.
- US Department of Health & Human Services . Physical Activity Guidelines for America 2018. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf (accessed January 2021).
- Department for Health and Social Care . UK Chief Medical Officers’ Physical Activity Guidelines 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf (accessed January 2021).
- Department of Health and Social Care . NHS Reference Costs 2013 to 2014 2015. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed January 2021).
- Mentzakis E, Ryan M, McNamee P. Using discrete choice experiments to value informal care tasks: exploring preference heterogeneity. Health Econ 2011;20:930-44. https://doi.org/10.1002/hec.1656.
- Mentzakis E, McNamee P, Ryan M, Sutton M. Valuing informal care experience: does choice of measure matter?. Soc Indic Res 2012;108:169-84. https://doi.org/10.1007/s11205-011-9873-y.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Office for National Statistics . EMP04: Employment by Occupation (April to June 2018) 2018. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/datasets/employmentbyoccupationemp04 (accessed February 2021).
- Callaghan L, Thompson TP, Creanor S, Quinn C, Senior J, Green C, et al. Individual health trainers to support health and well-being for people under community supervision in the criminal justice system: the STRENGTHEN pilot RCT. Public Health Res 2019;7. https://doi.org/10.3310/phr07200.
- Department for Communities and Local Government . English Indices of Deprivation 2019 – LSOA Level 2019. http://opendatacommunities.org/data/societal-wellbeing/imd2019/indices (accessed January 2021).
- Glanville J, Kaunelis D, Mensinkai S. How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. Int J Technol Assess Health Care 2009;25:522-9. https://doi.org/10.1017/S0266462309990523.
- Andresen EM, Rothenberg BM, Kaplan RM. Performance of a self-administered mailed version of the Quality of Well-Being (QWB-SA) questionnaire among older adults. Med Care 1998;36:1349-60. https://doi.org/10.1097/00005650-199809000-00007.
- Cabrero-García J, Muñoz-Mendoza CL, Cabañero-Martínez MJ, González-Llopís L, Ramos-Pichardo JD, Reig-Ferrer A. Short physical performance battery reference values for patients 70 years-old and over in primary health care. Aten Primaria 2012;44:540-8. https://doi.org/10.1016/j.aprim.2012.02.007.
- Bergland A, Strand BH. Norwegian reference values for the Short Physical Performance Battery (SPPB): the Tromsø Study. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1234-8.
- Ramírez-Vélez R, Pérez-Sousa MA, Venegas-Sanabria LC, Cano-Gutierrez CA, Hernández-Quiñonez PA, Rincón-Pabón D, et al. Normative values for the Short Physical Performance Battery (SPPB) and their association with anthropometric variables in older Colombian adults. The SABE study, 2015. Front Med 2020;7. https://doi.org/10.3389/fmed.2020.00052.
- Office for National Statistics . Mortality Rates (qx), Principal Projection, UK: 2018-Based Edition of This Dataset 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/mortalityratesqxprincipalprojectionunitedkingdom (accessed January 2021).
Appendix 1 Randomised controlled trial
Parts of this appendix have been adapted from Stathi et al. 1 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text includes minor additions and formatting changes to the original text.
Parts of this appendix have been adapted from Withall et al. 29 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text includes minor additions and formatting changes to the original text.
Parts of this appendix have been adapted from Stathi et al. 60 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
| Outcome measure | Visit type | ||||
|---|---|---|---|---|---|
| Screening | Follow-up | ||||
| Visit code | SV1 | F06 | F12 | F24 | |
| Visit number | 1 | 2 | 3 | 4 | |
| Telephone call | 1 | ||||
| Activity/assessment month | –0.5 | 0 | 6 | 12 | 24 |
| Form name | |||||
| Verbal consent | ✗ | ||||
| Telephone screening | ✗ | ||||
| Written informed consent | ✗ | ||||
| Contact information update | ✗ | ✗ | ✗ | ✗ | ✗ |
| Demographic data | |||||
| Age | ✗ | ||||
| Sex | ✗ | ||||
| Ethnicity | ✗ | ||||
| BMI (weight only at 12 months) | ✗ | ✗ | ✗ | ||
| MoCA | ✗ | ✗ | ✗ | ✗ | |
| Highest education level | ✗ | ||||
| IMD | ✗ | ||||
| Caring responsibilities | ✗ | ||||
| Marital status | ✗ | ✗ | ✗ | ✗ | |
| Home ownership | ✗ | ||||
| Number of chronic illnesses | ✗ | ✗ | ✗ | ✗ | |
| SPPB | ✗ | ✗ | ✗ | ✗ | |
| Accelerometry | ✗ | ✗ | ✗ | ✗ | |
| Subjective Physical Activity (PASE questionnaire-10 item) | ✗ | ✗ | ✗ | ✗ | |
| Muscle-Strengthening Exercise Adherence Questionnaire | ✗ | ✗ | ✗ | ✗ | |
| Dynamometer (hand-grip strength) | ✗ | ✗ | ✗ | ✗ | |
| Ageing Well profile (6 items social well-being scale, only at Bath/Bristol site) | ✗ | ✗ | ✗ | ||
| Sleep Condition Indicator | ✗ | ✗ | ✗ | ✗ | |
| Pain (WOMAC) | ✗ | ✗ | |||
| Loneliness | ✗ | ✗ | ✗ | ✗ | |
| Health-related quality of life (EQ-5D, SF-36) | ✗ | ✗ | ✗ | ✗ | |
| MAT-SF | ✗ | ✗ | ✗ | ✗ | |
| Cognitive function (UK Biobank Healthy Minds Questionnaire) | ✗ | ✗ | ✗ | ✗ | |
| Falls Inventory | ✗ | ✗ | ✗ | ✗ | |
| Health and Social Service Usage | ✗ | ✗ | ✗ | ✗ | |
| Process evaluation | |||||
| Session attendance (intervention group only) | ✗ | ✗ | |||
| Total contact time for each participant | ✗ | ✗ | |||
| Physical activity-related self-concept | ✗ | ✗ | ✗ | ||
| Perceived tension of maintaining current physical activity | ✗ | ✗ | ✗ | ||
| Perceived tension of maintaining current exercise | ✗ | ✗ | ✗ | ||
| Autonomy in relation to physical activity | ✗ | ✗ | ✗ | ✗ | |
| Competence for physical activity | ✗ | ✗ | ✗ | ✗ | |
| Relatedness for physical activity | ✗ | ✗ | ✗ | ✗ | |
| Enjoyment of physical activity | ✗ | ✗ | ✗ | ✗ | |
| Perceived intrinsic benefits of physical activity (social, physical and emotional) | ✗ | ✗ | ✗ | ✗ | |
| Autonomy for strength-building exercise | ✗ | ✗ | ✗ | ✗ | |
| Competence for strength-building exercise | ✗ | ✗ | ✗ | ✗ | |
| Relatedness for strength-building exercise | ✗ | ✗ | ✗ | ✗ | |
| Enjoyment of strength-building exercise | ✗ | ✗ | ✗ | ✗ | |
| Perceived intrinsic benefits of strength-building exercise (social, physical and emotional) | ✗ | ✗ | ✗ | ✗ | |
| Enjoyment of the REACT programme (intervention group) | ✗ | ✗ | |||
| Functional MRI imaging sub-study | |||||
| MRI scan, detailed cognitive assessment and gait analysis | ✗ | ✗ | ✗ | ||
| Characteristic | REACT (N = 777), n (%) | UK general population aged over 65 years (N = 9,223,073), n (%) |
|---|---|---|
| Age (years) | ||
| 65–69 | 95 (12.2) | 2,674,161 (29.0) |
| 70–74 | 191 (24.6) | 2,178,672 (23.6) |
| 75–79 | 190 (24.5) | 1,777,547 (19.3) |
| 80–84 | 160 (20.6) | 1,338,005 (14.5) |
| ≥ 85 | 141 (18.1) | 1,254,688 (13.6) |
| Sex | ||
| Female | 514 (66.2) | 6,617,318 (54.4) |
| Male | 263 (33.9) | 5,548,239 (45.6) |
| Race/ethnicity | ||
| Caucasian/whitea | 739 (95.1) | 8,806,190 (95.5) |
| African/Caribbean | 23 (3.0) | 115,288 (1.3) |
| Asian | 9 (1.2) | 238,878 (2.6) |
| Other/mixed | 6 (0.8) | 60,872 (0.7) |
| IMDb | ||
| Quintile 1 | 86 (11.1) | 1,321,666 (14.3) |
| Quintile 2 | 157 (20.2) | 1,618,649 (17.6) |
| Quintile 3 | 159 (20.5) | 1,975,582 (21.4) |
| Quintile 4 | 156 (20.1) | 2,127,763 (23.7) |
| Quintile 5 | 219 (28.2) | 2,180,334 (23.6) |
| Group | No. of participants (%) | ||
|---|---|---|---|
| Total | Control arm | Intervention arm | |
| Control | 52 (46.85) | ||
| Intervention | 59 (53.15) | ||
| Male | 45 (40.54) | 25 (48.08) | 25 (42.37) |
| Female | 66 (59.46) | 27 (51.92) | 39 (66.10) |
| Bath/Bristol | 47 (42.34) | 24 (46.15) | 23 (38.98) |
| Birmingham | 18 (16.22) | 11 (21.15) | 7 (11.86) |
| Devon | 46 (41.44) | 17 (32.69) | 29 (49.15) |
| 65–74 years | 24 (21.62) | 14 (29.92) | 10 (16.95) |
| ≥ 75 years | 87 (78.38) | 38 (73.08) | 49 (83.05) |
| Reasons | |||
| 1: Excluded | 4 (3.60) | 0 (0.00) | 4 (6.78) |
| 2: Choice | 34 (30.63) | 17 (32.69) | 17 (28.81) |
| 3: Health issues (participant) | 28 (25.23) | 13 (25.00) | 15 (25.42) |
| 4: Health issues (family) | 8 (7.21) | 4 (7.69) | 4 (6.78) |
| 5: Death | 16 (14.41) | 8 (15.38) | 8 (13.56) |
| 6: Other | 21 (18.92) | 10 (19.23) | 11 (18.64) |
| Total | 111 (100) | 52 (100) | 59 (100) |
| Group | No. of participants enrolled | 6 months | 12 months | 24 months | |||
|---|---|---|---|---|---|---|---|
| No. with valid SPPB score | No. (%) with missing SPPB score | No. with valid SPPB score | No. (%) with missing SPPB score | No. with valid SPPB score | No. (%) with missing SPPB score | ||
| Bath/Bristol | 335 | 283 | 52 (15.5) | 280 | 55 (16.4) | 275 | 60 (17.9) |
| Devon | 268 | 222 | 46 (17.2) | 215 | 53 (19.8) | 209 | 59 (22.0) |
| Birmingham | 174 | 154 | 20 (11.5) | 154 | 20 (11.5) | 144 | 30 (17.2) |
| Overall | 777 | 659 | 118 (15.2) | 649 | 128 (16.5) | 628 | 149 (19.2) |
| Study number | Allocation | Type | Related to intervention |
|---|---|---|---|
| AT1464 | Intervention | Deceased: unknown cause | Not related |
| AT1564 | Intervention | Stroke | Not related |
| AT1767 | Intervention | Scheduled hip operation | Not related |
| AT1832 | Intervention | Heart attack | Not related |
| AT1905 | Control | Deceased: unknown cause | Not related |
| AT1948 | Intervention | Deceased: unknown cause | Not related |
| AT2171 | Control | Deceased: unknown cause | Not related |
| AT2187 | Intervention | Stomach ulcer | Not related |
| AT2275 | Intervention | Mini stroke | Possible |
| AT2305 | Intervention | Fall | Not related |
| AT2341 | Intervention | Deceased: unknown cause | Not related |
| AT2414 | Intervention | Deceased: stroke | Not related |
| AT2486 | Intervention | Deceased: unknown cause | Not related |
| AT2536 | Intervention | Fall: fractured hip | Related |
| AT2630 | Control | Deceased: unknown cause | Not related |
| AT2648 | Intervention | Deceased: unknown cause | Not related |
| AT2675 | Intervention | Deceased: frailty in old age | Not related |
| BT1231 | Intervention | Deceased: Alzheimer’s disease | Not related |
| BT1348 | Intervention | Cardiac event (non-fatal) | Not related |
| BT1585 | Control | Cancer diagnosis | Not related |
| BT1636 | Intervention | Severe nose bleeds | Not related |
| BT1704 | Control | Deceased: cardiac event | Not related |
| BT1753 | Control | Deceased: suspected cardiac arrest | Not related |
| BT1770 | Intervention | Cancer diagnosis | Not related |
| BT1785 | Intervention | Heart attack | Not related |
| BT1979 | Control | Fall: broken shoulder | Not related |
| BT2002 | Control | Bowel cancer diagnosis | Not related |
| ET1036 | Control | Fall: injured arm and head (hospitalised). Diagnosed with pneumonia and kidney issues | Not related |
| ET1044 | Control | Stroke | Not related |
| ET1073 | Intervention | Diarrhoea, dehydration and rheumatoid arthritis | Not related |
| ET1295 | Control | Stomach pains, a hernia and memory deterioration | Not related |
| ET1392 | Intervention | Sciatica which aggravated MS | Not related |
| ET1392 | Intervention | Spinal surgery, fractured sacrum owing to surgery | Not related |
| ET1393 | Control | Fall: fractured head of femur, led to half-hip replacement | Not related |
| ET1425 | Intervention | Non-Hodgkin’s lymphoma | Not related |
| ET1439 | Intervention | Spine compression | Not related |
| ET1506 | Intervention | Elective surgery resulting in sepsis and pneumonia | Not related |
| ET1565 | Intervention | Fall: socket of femur break. Hip replacement needed | Not related |
| ET1603 | Control | Bad reaction to flu injection necessitating cardioversion | Not related |
| ET1689 | Control | Sepsis | Not related |
| ET1717 | Intervention | Pneumonia | Not related |
| ET1752 | Control | Deceased: pneumonia, emphysema and lung cancer | Not related |
| ET1754 | Intervention | Terminal cancer diagnosis | Not related |
| ET1804 | Control | Asthma attack (hospitalised), followed by fall resulting in brain bleed | Not related |
| ET1844 | Control | Collapsed at home: suspected bleeding ulcer | Not related |
| ET1866 | Intervention | Deceased: colon cancer | Not related |
| ET1953 | Intervention | Knee injury | Not related |
| ET1959 | Control | Fall: double fracture and dislocation of left ankle | Not related |
| ET1969 | Intervention | Fall: cracked ribs | Not related |
| ET1977 | Intervention | Fall: broken hip | Not related |
| ET1999 | Intervention | Elective surgery: swollen testicles | Not related |
| ET2017 | Control | TIA (hospitalised) | Not related |
| ET2024 | Control | Fall: broken shoulder | Not related |
| ET2024 | Control | Dementia diagnosis | Not related |
| ET2035 | Control | Two stents fitted and aortic valve replaced | Not related |
| ET2036 | Intervention | Hernia (surgery performed) | Not related |
| ET2045 | Intervention | Chest pains (high troponin levels), contracted flu in hospital | Not related |
| ET2067 | Intervention | Pneumonia (hospitalised). Minor heart attack while hospitalised | Not related |
| ET2067 | Intervention | Pneumonia, second bout (hospitalised) | Not related |
| ET2069 | Control | Fall: fractured humerus | Not related |
| ET2069 | Control | Planned knee replacement | Not related |
| ET2081 | Intervention | TIA | Not related |
| ET2092 | Control | Sepsis | Not related |
| ET2117 | Intervention | Bleeding (details unknown) | Not related |
| ET2128 | Control | Deceased: unknown cause | Not related |
| ET1281 | Intervention | Cardiac issues | Not related |
| ET2191 | Intervention | Triple fusion of ankle joint | Not related |
| ET2205 | Intervention | Cellulitis | Not related |
| ET2255 | Intervention | Stroke | Not related |
| ET2255 | Intervention | Fall (hospitalised) | Not related |
| ET2290 | Intervention | Stroke | Not related |
| ET2309 | Intervention | Lung cancer | Not related |
| ET2374 | Control | Accident: fractured hip resulting in replacement | Not related |
| ET2377 | Control | Stroke | Not related |
| ET2402 | Control | Fall: haematoma on leg | Not related |
| ET2401 | Intervention | Pneumonia (hospitalised). Treatment caused AF and bleed to abdomen | Not related |
| ET2416 | Intervention | Planned knee replacement | Not related |
| ET2416 | Intervention | Problems with knee replacement | Not related |
| ET2425 | Intervention | Terminal bone cancer | Not related |
| ET2449 | Intervention | Deceased: heart attack and possible appendicitis | Not related |
| ET2455 | Intervention | Heart attack: three stents fitted | Not related |
| ET2472 | Control | Chest pains: stents fitted | Not related |
| ET2481 | Control | TIA | Not related |
| ET2511 | Intervention | Big toe removed | Not related |
| ET2528 | Control | Cardiac ‘episode’ (hospitalised) | Not related |
| ET2357 | Intervention | Hospitalisation (pneumonia) | Not related |
| ET2588 | Intervention | COPD (hospitalised). On palliative care | Not related |
| ET2709 | Intervention | Fall: broke left femur | Not related |
| ET2710 | Intervention | Sepsis, pneumonia and pleurisy | Not related |
| ET2710 | Intervention | Deceased: ischaemic heart disease, COPD and diabetes | Not related |
| ET2716 | Control | Gall stone in bowel | Not related |
| ET2733 | Control | Fall (hospitalised): pacemaker fitted | Not related |
| ET2733 | Intervention | Hematoma (hospitalised) | Not related |
| Outcome | n (control, intervention) | Study arm | Estimated mean difference (95% CI) | p-valueb | |
|---|---|---|---|---|---|
| Controla | Interventiona | ||||
| Primary outcome | |||||
| SPPB (total score) | 305, 354 | 8.07 (1.91) | 8.74 (1.87) | 0.68 (0.39 to 0.96) | < 0.001 |
| Secondary outcomes | |||||
| Accelerometry | |||||
| MVPA (minutes per day) | |||||
| Time spent at > 100 milligravitational units in at least 10-minute bouts | 270, 314 | 5.68 (7.36) | 6.56 (7.09) | 0.86 (–0.37 to 2.12) | 0.165 |
| All time spent at > 100 milligravitational units | 270, 314 | 53.74 (18.17) | 56.59 (22.42) | 2.85 (–0.67 to 6.37) | 0.11 |
| Sedentary time, excluding sleep (minutes per day) | 261, 304 | 810 (78.84) | 809 (95.02) | –0.91 (–15.52 to 13.71) | 0.902 |
| Breaks in sedentary time (n per day) | 269, 312 | 41.48 (9.51) | 42.91 (9.71) | 1.42 (–0.17 to 3.02) | 0.080 |
| Subjective physical activity score (PASE) | 296, 346 | 115.49 (58.21) | 131.82 (61.76) | 16.33 (6.78 to 25.89) | 0.001 |
| Muscle-strengthening exercise score (MSEQ) | 277, 320 | 3.22 (1.90) | 3.93 (1.90) | 0.70 (0.39 to 1.01) | < 0.001 |
| Hand-grip strength (kg) | 302, 350 | 24.78 (3.93) | 25.34 (4.09) | 0.55 (–0.08 to 1.18) | 0.085 |
| Sleep Condition Indicator score | 255, 288 | 24.34 (5.74) | 24.23 (5.45) | –0.16 (–1.05 to 0.81) | 0.806 |
| Loneliness | 301, 345 | 111 (36.8%) | 115 (33.3%) | –0.009 (–0.070 to 0.052) | 0.778 |
| SF-36 score | |||||
| Physical component | 293, 342 | 30.64 (8.68) | 32.75 (8.42) | 2.11 (0.73 to 3.48) | 0.003 |
| Mental component | 293, 342 | 54.19 (7.46) | 54.41 (6.88) | 0.23 (–0.93 to 1.38) | 0.699 |
| EQ-5D | 299, 346 | 0.70 (0.15 | 0.68 (0.15) | 0.026 (0.003 to 0.05) | 0.028 |
| MAT-SF | 297, 345 | 50.00 (7.05) | 51.40 (6.53) | 1.40 (0.32 to 2.48) | 0.012 |
| UK Biobank Healthy Minds Questionnaire score | |||||
| Simple processing speed | 287, 333 | 811.79 (243.82) | 821.25 (259.59) | 9.45 (–32.48 to 51.39) | 0.655 |
| Fluid intelligence | 286, 327 | 3.87 (1.42) | 3.89 (1.32) | 0.02 (–0.21 to 0.25) | 0.854 |
| Executive function | 240, 270 | 62,291.45 (39,262.35) | 60,343.81 (39,565.45) | –1947.64 (–8835.47 to 4940.18) | 0.576 |
| Working memory 1 | 286, 331 | 4.34 (1.21) | 4.42 (1.13) | 0.08 (–0.12 to 0.29) | 0.423 |
| Working memory 2 | 286, 330 | 13.59 (5.53) | 13.83 (5.77) | 0.24 (–0.70 to 1.19) | 0.609 |
| Episodic memory | 287, 331 | 5.97 (4.98) | 5.96 (4.59) | –0.00 (–0.80 to 0.79) | 0.994 |
| Falls Inventory | |||||
| No. of falls in last 6 months | 295, 335 | 0.61 (0.93) | 0.58 (1.02) | –0.03 (–0.18 to 0.12) | 0.712 |
| Fall-related injury in last 6 months, n (%) | 291, 328 | 40 (13.7) | 43 (13.1) | 0.64 (–4.96 to 6.35)c | 0.728 |
| Outcome | n (control, intervention) | Controla | Interventiona | Estimated mean difference (95% CI) | p-valueb |
|---|---|---|---|---|---|
| Primary outcome | |||||
| SPPB (total score) | 303, 346 | 7.85 (2.05) | 8.62 (2.58) | 0.77 (0.40 to 1.14) | < 0.001 |
| Secondary outcomes | |||||
| Accelerometry | |||||
| MVPA (minutes per day) | |||||
| Time spent at > 100 milligravitational units in at least 10-minute bouts | 277, 299 | 4.98 (5.51) | 6.22 (6.20) | 1.24 (0.22 to 2.26) | 0.018 |
| All time spent at > 100 milligravitational units | 277, 299 | 52.04 (15.92) | 55.15 (19.67) | 3.11 (–0.00 to 6.23) | 0.05 |
| Sedentary time, excluding sleep (minutes per day) | 274, 300 | 790 (67.54) | 789 (66.51) | –0.92 (–12.11 to 10.27) | 0.871 |
| Breaks in sedentary time (number per day) | 275, 301 | 42.92 (9.62) | 43.49 (10.93) | 0.57 (–1.15 to 2.28) | 0.512 |
| Subjective physical activity (PASE) | 296, 306 | 120.22 (49.34) | 131.05 (46.23) | 10.84 (3.18 to 18.50) | 0.006 |
| Muscle-strengthening exercise (MSEQ) | 266, 315 | 3.32 (1.84) | 3.98 (2.22) | 0.67 (0.33 to 1.00) | < 0.001 |
| Hand-grip strength (kg) | 297, 340 | 24.42 (3.64) | 25.23 (4.05) | 0.81 (0.20 to 1.43) | 0.010 |
| Ageing Well Profile Social Wellbeing subscale | 284, 322 | 24.34 (5.74) | 24.23 (5.44) | –0.12 (–1.05 to 0.81) | 0.805 |
| Sleep Condition Indicator | 266, 303 | 22.44 (5.28) | 23.30 (4.89) | 0.86 (–0.05 to 1.77) | 0.064 |
| Loneliness | 296, 340 | 104 (35.1%) | 111 (32.6) | –0.003 (–0.069 to 0.064) | 0.935 |
| SF-36 | |||||
| Physical component | 293, 334 | 29.66 (8.53) | 32.25 (8.23) | 2.59 (1.22 to 3.95) | < 0.001 |
| Mental component | 293, 334 | 54.52 (7.52) | 53.95 (7.81) | –0.57 (–1.82 to 0.67) | 0.362 |
| EQ-5D | 293, 337 | 0.70 (0.14) | 0.69 (0.14) | 0.018 (0.004 to 0.041) | 0.112 |
| MAT-SF | 292, 328 | 49.25 (7.51) | 51.47 (7.79) | 2.21 (1.00 to 3.43) | 0.001 |
| UK Biobank Healthy Minds Questionnaire | |||||
| Simple processing speed | 257, 297 | 829.66 (241.06) | 842.97 (253.15) | 13.31 (–30.01 to 56.63) | 0.543 |
| Fluid intelligence | 254, 295 | 4.18 (1.47) | 4.22 (1.54) | 0.04 (–0.23 to 0.31) | 0.768 |
| Executive function | 204, 244 | 66,552.53 (44,421.06) | 62,642.94 (44,187.88) | –3909.59 (–12,564.60 to 4745.42) | 0.371 |
| Working memory 1 | 257, 294 | 4.48 (1.35) | 4.36 (1.52) | –0.13 (–0.38 to 0.13) | 0.322 |
| Working memory 2 | 259, 296 | 14.22 (5.68) | 13.74 (5.83) | –0.49 (–1.50 to 0.53) | 0.343 |
| Episodic memory | 258, 297 | 5.91 (4.87) | 5.86 (4.45) | –0.05 (–0.88 to 0.77) | 0.896 |
| Falls inventory | |||||
| Number of falls in last 6 months | 300, 330 | 0.73 (1.05) | 0.70 (1.05) | –0.02 (–0.19 to 0.14) | 0.772 |
| Fall-related injury in last 6 months, n (%) | 297, 326 | 51 (17.2) | 57 (17.5) | 0.3 (–5.92 to 6.46)c | 0.809 |
| Outcome | n (control, intervention) | Control arma | Intervention arma | Estimated mean difference (95% CI) | p-valueb |
|---|---|---|---|---|---|
| Primary outcome | |||||
| SPPB total score | 294, 334 | 7.59 (2.61) | 8.08 (2.87) | 0.49 (0.06 to 0.92) | 0.014 |
| Secondary outcomes | |||||
| MVPA (minutes per day) | |||||
| Time spent at > 100 milligravitational units in at least 10-minute bouts | 250, 290 | 4.50 (6.61) | 5.15 (5.99) | 0.65 (–0.48 to 1.78) | 0.255 |
| All time spent at > 100 milligravitational units | 250, 290 | 48.76 (19.48) | 51.22 (17.20) | 2.46 (–0.52 to 5.44) | 0.105 |
| Sedentary time, excluding sleep (minutes per day) | 249, 287 | 798 (65.80) | 804 (64.04) | 6.43 (–4.81 to 17.67) | 0.259 |
| Breaks in sedentary time (n/day) | 248, 287 | 42.33 (13.54) | 40.76 (13.21) | –1.57 (–3.89 to 0.75) | 0.184 |
| Subjective physical activity (PASE) | 301, 328 | 113.17 (52.10) | 123.90 (49.79) | 10.73 (2.62 to 18.84) | 0.010 |
| Muscle-strengthening exercise (MSEQ) | 276, 307 | 3.18 (1.88) | 3.86 (2.30) | 0.68 (0.33 to 1.02) | < 0.001 |
| Hand-grip strength (kg) | 291, 328 | 23.43 (4.08) | 23.74 (3.86) | 0.31 (–0.33 to 0.94) | 0.343 |
| Ageing Well Profile Social Wellbeing subscale | 295, 306 | 24.68 (5.85) | 24.88 (7.07) | 0.20 (–0.84 to 1.24) | 0.700 |
| Sleep Condition Indicator | 285, 311 | 21.97 (6.10) | 22.50 (6.65) | 0.53 (–0.49 to1.54) | 0.306 |
| Pain (WOMAC) | 290, 324 | 10.20 (3.28) | 9.63 (3.95) | –0.57 (–1.15 to 0.00) | 0.052 |
| Loneliness, n (%) | 300, 330 | 107 (35.7) | 110 (33.3) | 0.037 (–0.064 to 0.074)c | 0.914 |
| SF-36 | |||||
| Physical component | 295, 306 | 29.38 (9.39) | 30.84 (10.04) | 1.46 (–0.09 to 3.01) | 0.065 |
| Mental component | 295, 306 | 54.73 (7.64) | 54.33 (9.18) | –0.40 (–1.78 to 0.98) | 0.563 |
| EQ-5D | 302, 330 | 0.67 (0.16) | 0.69 (0.16) | 0.02 (–0.01 to 0.04) | 0.220 |
| MAT-SF | 289, 319 | 47.96 (8.13) | 49.99 (8.96) | 2.03 (0.66 to 3.40) | 0.004 |
| UK Biobank Healthy Minds Questionnaire | |||||
| Simple processing speed | 264, 286 | 811.28 (240.15) | 801.67 (246.72) | –9.61 (–52.47 to 33.24) | 0.657 |
| Fluid intelligence | 262, 282 | 4.03 (1.41) | 4.19 (1.61) | 0.16 (–0.11 to 0.43) | 0.234 |
| Executive function | 210, 236 | 64,770.62 (38,677.48) | 58,515.77 (35,648.79) | –6254.85 (–13,498.22 to 988.52) | 0.090 |
| Working memory 1 | 263, 282 | 4.59 (1.29) | 4.46 (1.22) | –0.13 (–0.35 to 0.06) | 0.260 |
| Working memory 2 | 264, 285 | 14.27 (5.24) | 14.62 (5.15) | 0.36 (–0.56 to 1.28) | 0.439 |
| Episodic memory | 263, 286 | 5.84 (4.19) | 5.36 (6.85) | –0.48 (–1.49 to 0.53) | 0.347 |
| Falls inventory | |||||
| Number of falls in last 6 months | 300, 330 | 0.73 (1.05) | 0.70 (1.05) | –0.02 (–0.19 to 0.14) | 0.772 |
| Fall-related injury in last 6 months, n (%) | 297, 326 | 51 (17.2) | 57 (17.5) | 0.3 (–5.92 to 6.46)c | 0.809 |
| Sensitivity analysis | n; mean SPPB total score (SD) | Estimated mean difference (95% CI) | p-valuea | |
|---|---|---|---|---|
| Control | Intervention | |||
| MI (n = 777) | 367; 7.50 (2.44) | 410; 7.87 (2.93) | 0.38 (0.02 to 0.73) | 0.040 |
| With no clustering by exercise groupb (n = 628) | 294; 7.59 (2.61) | 334; 8.7 (2.21) | 0.49 (0.14 to 0.84) | 0.006 |
| Population 0 (whole sample) (n = 628) | 294; 7.59 (2.61) | 334; 8.08 (2.87) | 0.49 (0.06 to 0.92) | 0.014 |
| Population 1 (≥ 50% adherence) (n = 546) | 294; 7.59 (2.20) | 252; 8.23 (2.49) | 0.64 (0.23 to 1.05) | 0.002 |
| Population 2 (≥ 75% adherence) (n = 471) | 294; 7.64 (2.12) | 177; 8.45 (2.28) | 0.81 (0.38 to 1.23) | < 0.001 |
| Factor/covariate | Coefficient (SE); p | Coefficient for interaction with study arm (SE); pa | Control arm, mean (SE) | Intervention arm, mean (SE) |
|---|---|---|---|---|
| Baseline SPPB | ||||
| 4–7 | 0 | 6.20 (0.20) | 6.71 (0.22) | |
| 8–9 | 2.35 (0.19); < 0.001 | 0.094 (0.361); 0.794 | 8.59 (0.17) | 9.03 (0.18) |
| Education level | ||||
| 1 | 0 | 0 | 7.41 (0.44) | 7.79 (0.45) |
| 2 | 0.28 (0.35); 0.423 | 0.229 (0.690); 0.740 | 7.57 (0.20) | 8.18 (0.23) |
| 3 | 0.39 (0.36); 0.278 | –0.317 (0.718); 0.659 | 8.00 (0.27) | 8.06 (0.24) |
| 4 | 0.15 (0.37); 0.696 | 0.303 (0.738); 0.681 | 7.39 (0.29) | 8.08 (0.27) |
| 5 | –0.06 (0.46); 0.901 | 0.760 (0.926); 0.412 | 6.89 (0.54) | 8.03 (0.42) |
| Deprivation level | ||||
| 1 | 0 | 0 | 7.14 (0.36) | 7.68 (0.42) |
| 2 | 0.03 (0.34); 0.935 | 0.344 (0.670); 0.607 | 6.98 (0.30) | 7.86 (0.29) |
| 3 | 0.28 (0.34); 0.410 | –0.343 (0.670); 0.609 | 7.60 (0.29) | 7.80 (0.27) |
| 4 | 0.38 (0.35); 0.280 | 0.040 (0.668); 0.952 | 7.48 (0.29) | 8.06 (0.27) |
| 5 | 1.04 (0.34); 0.002 | –0.211 (0.645); 0.743 | 8.28 (0.24) | 8.61 (0.25) |
| Age (years) | ||||
| 65–74 | 0 | 8.35 (0.21) | 8.78 (0.21) | |
| ≥ 75 | –1.20 (0.19); < 0.001 | –0.016 (0.368); 0.965 | 7.16 (0.17) | 7.57 (0.18) |
| Comorbidity | ||||
| No | 0 | 7.86 (0.17) | 8.17 (0.18) | |
| Yes | –0.41 (0.18); 0.022 | –0.283 (0.476); 0.553 | 7.19 (0.20) | 7.97 (0.20) |
| Sex | ||||
| Male | 0 | 7.54 (0.23) | 7.85 (0.22) | |
| Female | 0.22 (0.19); 0.238 | 0.273 (0.375); 0.466 | 7.62 (0.16) | 8.20 (0.17) |
| Site | ||||
| Bath/Bristol | 0 | 7.18 (0.19) | 7.95 (0.22) | |
| Birmingham | 0.70 (0.25); 0.007 | –0.193 (0.497); 0.698 | 7.96 (0.27) | 8.54 (0.29) |
| Devon | 0.39 (0.23); 0.090 | –0.711 (0.574); 0.116 | 7.88 (0.23) | 7.94 (0.25) |
| Fallers | ||||
| Yes | 0 | 7.48 (0.22) | 7.76 (0.21) | |
| No | 0.39 (0.18); 0.033 | 0.331 (0.365); 0.366 | 7.69 (0.16) | 8.30 (0.17) |
| Housing type | ||||
| Home owner | 0 | 7.73 (0.14) | 8.19 (0.14) | |
| Other | –0.935 (0.26); 0.007 | 0.012 (0.509); 0.981 | 6.79 (0.32) | 7.27 (0.36) |
| Other interventions: 24 monthsb | ||||
| No | 0 | 7.73 (0.25) | 8.43 (0.31) | |
| Yes | 0.08 (0.30); 0.777 | –0.291 (0.634); 0.513 | 7.82 (0.16) | 8.22 (0.15) |
| Time point | Control arm, mean (SE) | Intervention arm, mean (SE) | Estimated mean differencea (95% CI) | p-value |
|---|---|---|---|---|
| Baseline | 7.37 (0.12) | 7.45 (0.28) | – | |
| 6 months | 8.02 (0.13 | 8.75 (0.28) | 0.65 (0.25 to 1.03) | 0.001 |
| 12 months | 7.83 (0.13) | 8.65 (0.28) | 0.74 (0.34 to 1.13) | < 0.001 |
| 24 months | 7.54 (0.13) | 8.11 (0.28) | 0.48 (0.08 to 0.88) | 0.018 |
Appendix 2 Process evaluation
Parts of this appendix have been reproduced from Cross et al. 71 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Intervention fidelity
| Checklist item | Intended delivery strategies |
|---|---|
| 1. Person-centred delivery style communication should be participant focused, maximising participant autonomy (delivery style) | Use of open-ended questions |
| Affirmations for positive behaviours, recognising efforts to change, as well as participants’ autonomy | |
| Reflective listening (actively engage with participant, empathise, reflect emotional state) | |
| Summaries of the discussion, drawing together the ideas discussed (e.g. around motivation, overcoming barriers) | |
| Using the ask-tell-discuss technique to exchange/deliver key information | |
| 2. Facilitating enjoyment (delivery style) | Using the techniques associated with person-centred delivery (as above), session leaders should encourage and reinforce enjoyment of social interactions within the group by making the social interactions positive, supportive and enjoyable |
| 3. Monitoring progress (acknowledging and reviewing) (self-regulatory technique) | Using the techniques associated with person-centred delivery (as above), session leaders should regularly acknowledge and review the progress of group members in terms of their physical activity levels |
| 4. Monitoring progress (eliciting and reinforcing the benefits of physical activity) (self-regulatory technique) | Using the techniques associated with person-centred delivery (as above), session leaders should encourage discussion on the emotional, social and physical benefits of physical activity |
| 5. Self-monitoring (self-regulatory technique) | Using techniques associated with person-centred delivery (as above), session leaders should encourage participant self-monitoring or acknowledge participant attempts to self-monitor |
| 6. Managing setbacks and problem-solving (self-regulatory technique) | Using techniques associated with person-centred delivery (as above), session leaders should encourage discussion on setbacks participants have experienced and encourage problem-solving. This should include reframing and normalising setbacks. Problems should be broken down, and the sustainability of coping plans and the support others can provide should also be considered |
| 7. Action-planning and goal-setting (self-regulatory technique) | Using techniques associated with person-centred delivery (as above), session leaders should work with the participants to agree on action plans, including goal-setting and identifying any barriers that may arise |
| 8. Modelling (SCT) | Using techniques associated with person-centred delivery (as above), session leaders should give participants the opportunity to observe others engaging appropriately with the programme |
| 9. Promoting autonomy (SDT) | Using techniques associated with person-centred delivery (as above), session leaders should encourage proactive involvement in the classes and discussion and create opportunities for participant input, while acknowledging participant perspectives, encouraging participants to be the driver of change and develop a sense of control |
| 10. Supporting competence and self-efficacy (SDT and SCT) | Using techniques associated with person-centred delivery (as above), session leaders should encourage participants to identify and break down barriers to change, set achievable goals/encourage gradual progress and encourage problem-solving |
| 11. Supporting relatedness (SDT) | Using techniques associated with person-centred delivery (as above), session leaders should fulfil participants’ needs for relatedness (social engagement/acceptance, approval of one’s behaviour and giving support to others). This can be promoted by encouraging engagement in physical activity, where there are opportunities for positive social interactions, as well as highlighting physical activity as a social opportunity |
| Competence level72 | Scoring | Examples | REACT delivery fidelity categories |
|---|---|---|---|
| Absence | 0 | Absence of feature and/or highly inappropriate performance | Low fidelity |
| Novice | 1 | Minimal use of feature and/or inappropriate performance | Low fidelity |
| Advanced beginner | 2 | Evidence of competence, but numerous problems or inconsistencies | Scope for improvement |
| Competent | 3 | Competent, good features but some minor problems or inconsistencies | Competent |
| Proficient | 4 | Very good features, but minimal problems or inconsistencies | Proficient |
| Expert | 5 | Excellent features, no problems or inconsistencies | Expert |
| Checklist item | Criterion for summarising scores across multiple sessions |
|---|---|
| Person-centred delivery style | Mean |
| Facilitating enjoyment | Mean |
| Monitoring progress | Mean |
| Self-monitoring | Maximum |
| Managing setbacks and problem-solving | Mean |
| Action-planning and goal-setting | Mean |
| Modelling | Maximum |
| Promoting autonomy | Mean |
| Supporting competence and self-efficacy | Mean |
| Supporting relatedness | Mean |
| Checklist item | Examples of good practice | Examples of practice requiring improvement |
|---|---|---|
| Person-centred delivery | Group 5, week 12: the facilitator starts a discussion with open-ended questions, reflects on the responses of participants, responding where appropriate. The facilitator gives options throughout the discussion, but lets the group direct the conversation. The facilitator highlights the group social aspect. The conversation is natural, and the facilitator confirms and summarises details at the end before praising them | Group 4, week 9: the facilitator’s communication is not participant focused. For example, the facilitator asks a question but does not reflect on answers, quickly moving on to ask another question. The facilitator frequently talks over participants and directs conversation back to themselves |
| Facilitating enjoyment | Group 3, week 9: the facilitator introduces a name game as a means of getting to know each other. The whole group is involved. The facilitator supports the idea by going first, dispelling awkwardness and encourages ‘banter’ as she goes along. Throughout the game, the facilitator praises the group and reinforces positive comments that are made | Group 3, week 12: the session leader is slow to react when groups are not interacting or the mood of the group has dropped |
| Monitoring progress: acknowledge and review | Group 6, week 16: the facilitator asks how the group got on with their ‘activity snacks’. Group discussion ensues with a bit of joking, a positive light-hearted environment in which to discuss progress. One participant reflects on a failure to progress, but the facilitator reframes it positively as an improvement (since starting), asking how the lady felt about it. The facilitator and participant move on to discuss a specific goal and action plan for keeping it up | Group 4, week 12: the facilitator jokes that a participant is finding it too easy and needs more weight but fails to use it as an opportunity to formally praise them and highlight the progress made |
| Monitoring progress: eliciting benefits of physical activity | Group 6, week 16: the facilitator praises the group for the progress made in increasing activity levels and opens a discussion about the benefits. The facilitator asks participants about ways they feel they have benefited. The session leaders’ approach to the discussion is participant focused | Group 6, week 16: the facilitator affirms that the group is more active and learning different movements, but fails to use the opportunity to explore the benefits of this with the group |
| Self-monitoring | Group 3, week 9: the facilitator describes how to use the pedometers to monitor steps, constantly checking for understanding and clarifying where needed. The facilitator praises those who ask questions about the device and highlights barriers they may face and how they might go about overcoming them. The facilitator summarises and encourages participants to do their best to improve their steps and not to compare their efforts with those of others | Group 4, week 28: a participant reminds the facilitator to discuss and record their pedometer steps. Session leader records them and asks for them to reset their pedometers to zero for the following week. The facilitator offers no praise or feedback on participants’ efforts to self-monitor steps |
| Managing setbacks and problem-solving |
Group 3, week 13: the facilitator asks an open question about a participant and the participant reports she has not done well. The facilitator is positive and reframes the comment, highlighting the fact that the participant has shown up to REACT and goes on to offer her praise Group 3, week 48: the facilitator asks for progress on a participant’s leg injury. Despite the participant’s negative outlook on it, the facilitator is positive, reassuring her that her plan of rehabilitation is a good one. The facilitator then focuses on the fact that organising a physiotherapy appointment is a positive step |
Group 6, week 16: the group brings up the fact that they are struggling to exercise in the hot weather; the facilitator agrees but does not encourage them or offer any ways to overcome this as a barrier Group 6, week 16: a participant starts a discussion about managing their knee injury and the effect that this has had on their activity; however, the facilitator misses the opportunity to offer any reassurance, support or problem-solving strategies |
| Action-planning and goal-setting | Group 6, week 16: the facilitator discusses an action plan for keeping up recent progress and encourages participant input, making positive affirmations about the action plan that participants have devised | Group 5, week 48: the facilitator asks about participants’ plans for exercise post REACT. The facilitator does not take the opportunity to discuss the specificity of goals or plans, how participants will execute them or discuss barriers or problems that may arise |
| Modelling | Group 1, week 13: the facilitator asks a participant to share their experiences of starting a new activity with the rest of the group. The facilitator highlights their success and praises the participant for attending | Group 4, week 28: the facilitator highlights a participant’s correct execution of a particular exercise but does not explain why it is correct or congratulate the participant for successful engagement |
| Promoting autonomy | Group 5, week 12: the facilitator supports participant choice during a discussion about new activities that they are thinking of joining. The facilitator offers options for a planned group activity but insists it is ‘their choice’ and asks open questions about how they feel about this. The facilitator lets them discuss and offers help in summarising the discussion, asking again how they feel about the particular plan that they have formulated | Group 5, week 13: the facilitator consistently points out that participants are able to choose the way in which they complete exercises, but does not facilitate a discussion about this or encourage participant input |
| Supporting competence and self-efficacy |
Group 6, week 16: after discussing an action plan with a participant, the facilitator asks the participant if she feels that she could manage it. The facilitator goes on to affirm that she believes that the participant can Group 3, week 28: while action-planning, the facilitator asks participants for barriers that they face to joining a new class. The facilitator breaks each down, offering up solutions that the group goes on to discuss. The interaction is very participant focused |
Group 5, week 13: the facilitator works well at identifying barriers to change but then does not continue the discussion adequately to include breaking down the barriers, and seeking the input of participants was limited in this instance |
| Supporting relatedness | Group 5, week 12: the facilitator supports discussion about organising a group walk, encouraging participants to choose their own pace but support each other through it. The facilitator continues to encourage a social aspect by organising coffee afterwards. The facilitator supports a participant-focused discussion, during which most of the interaction occurs between the participants | Group 6 week 16: a participant discusses problems that they experience with their knees and that they do not want to drop out of REACT. Another participant offers to work with the first, as both have limited mobility, but the facilitator fails to encourage this pairing and does not use it as an opportunity to discuss the social benefits of physical activity or how participants may support each other in the future |
Qualitative evaluation of intervention processes
FIGURE 28.
The REACT logic model in the context of findings: reasons to engage in REACT. CV, cardiovascular; PA, physical activity.
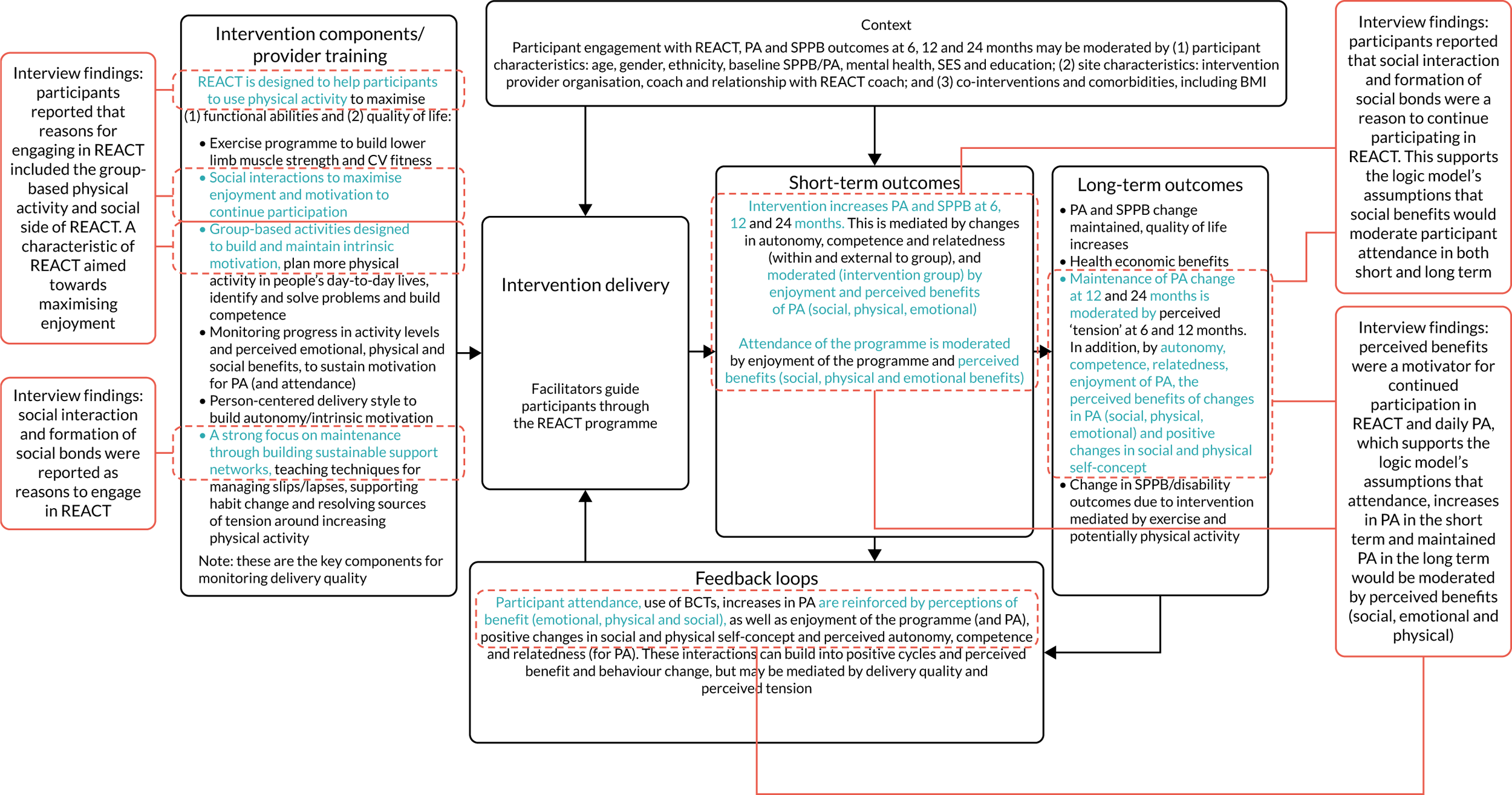
FIGURE 29.
The REACT logic model in the context of findings: benefits of participation in REACT. CV, cardiovascular; PA, physical activity.
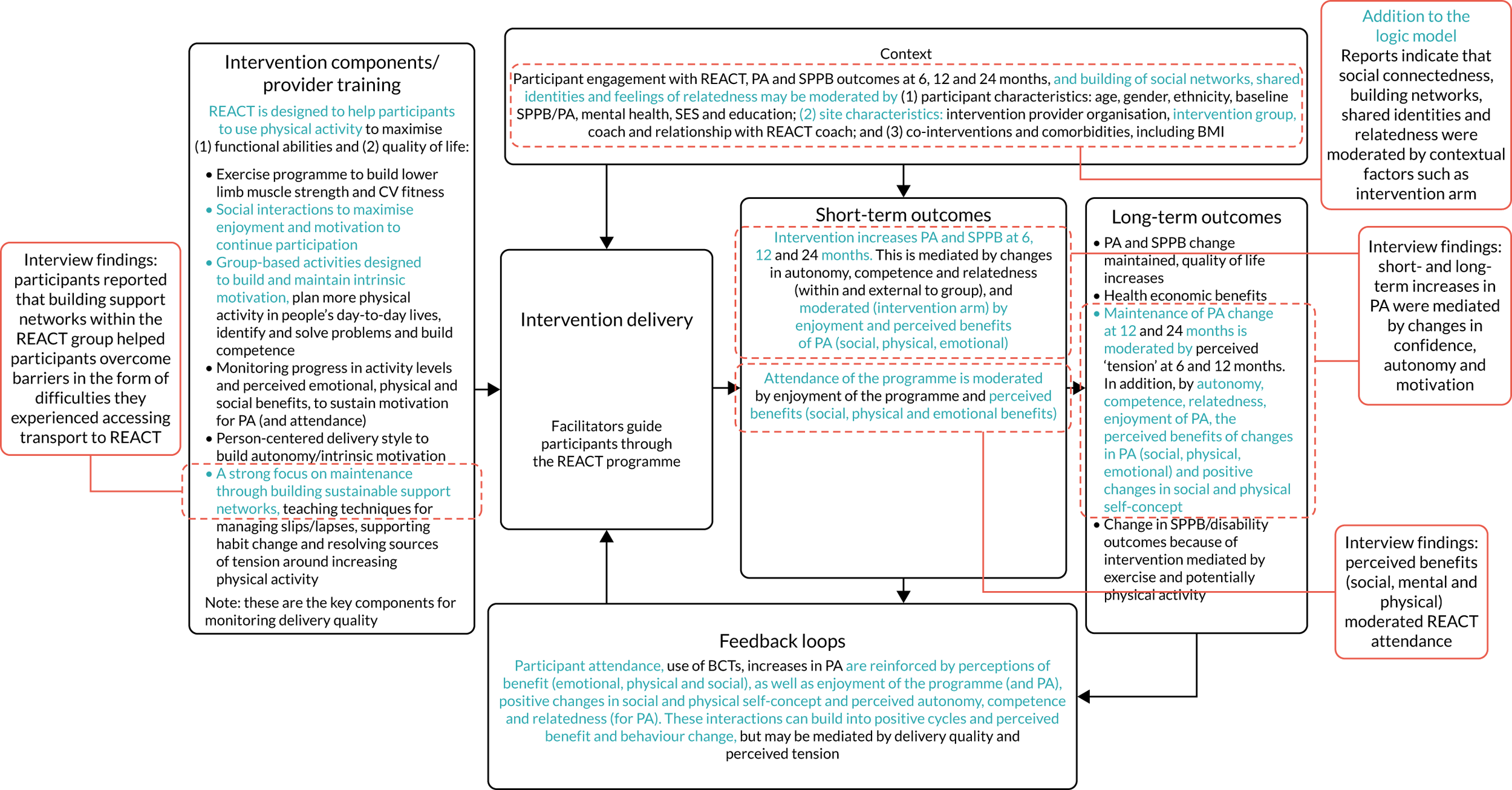
FIGURE 30.
The REACT logic model in the context of findings: barriers to participation in REACT and daily physical activity. CV, cardiovascular; PA, physical activity.

FIGURE 31.
The REACT logic model in the context of findings: enablers of participation in REACT and daily physical activity. CV, cardiovascular; PA, physical activity.
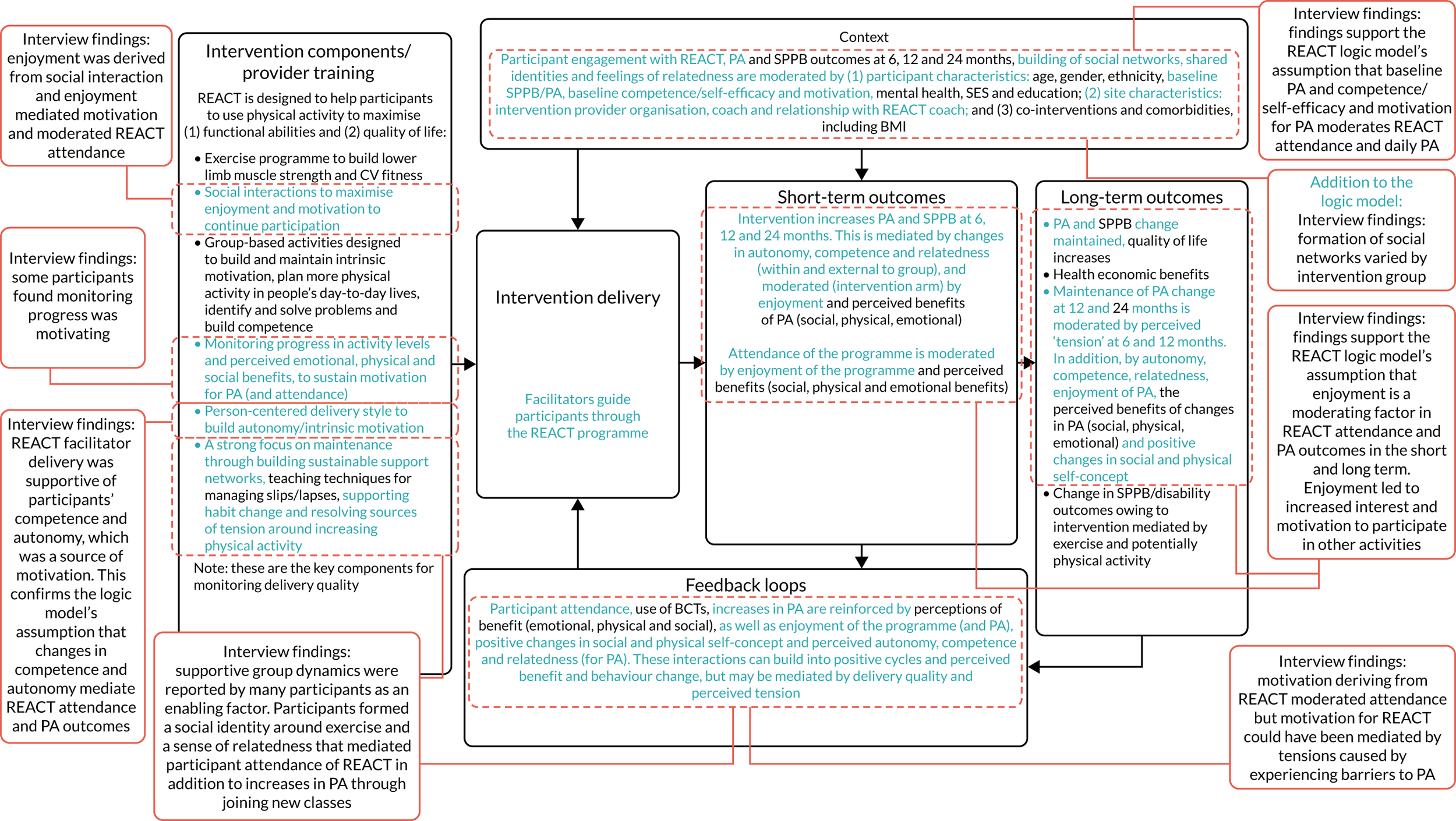
Quantitative process evaluation
FIGURE 32.
Changes in hours spent walking outside from 0 to 6 months for different levels of attendance within the intervention arm (error bars: 95% CI).
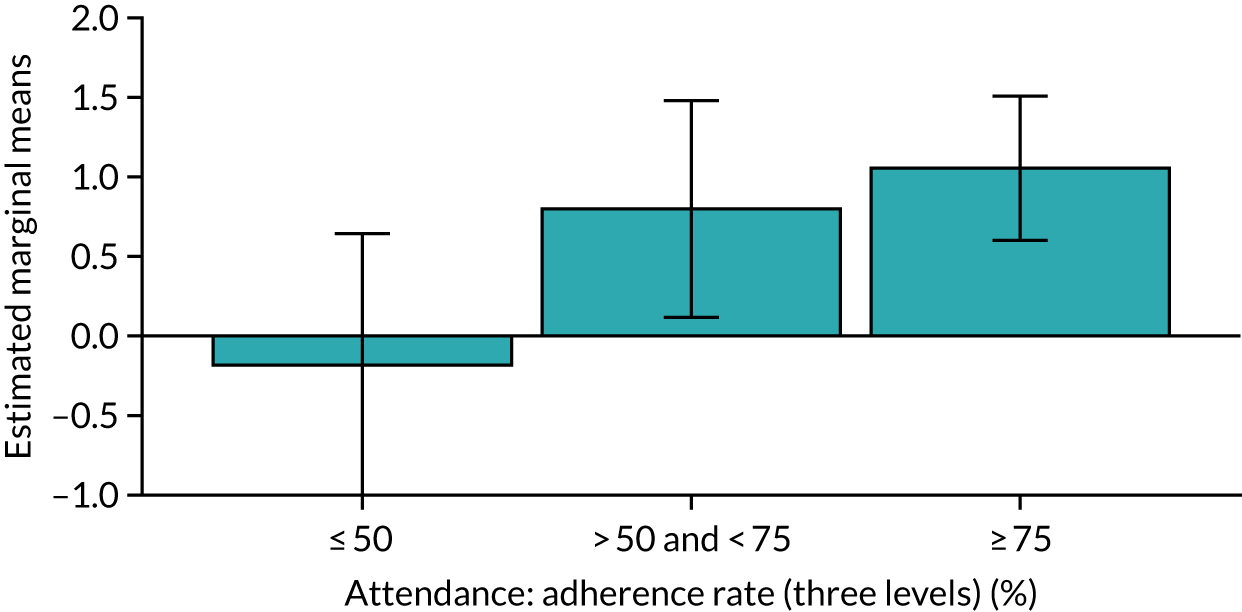
FIGURE 33.
Changes in adherence to muscle-strengthening exercise (MSEQ adherence score) from 0 to 6 months for different levels of attendance within the intervention arm (error bars: 95% CI).
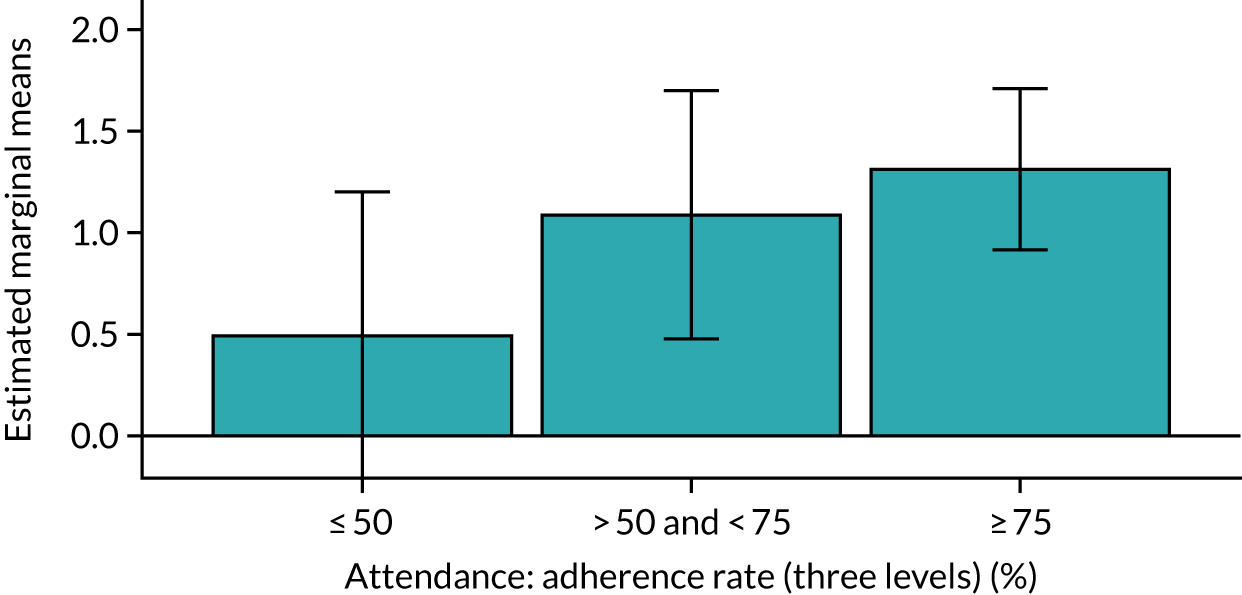
FIGURE 34.
Changes in PASE total score from 0 to 6 months for different levels of attendance within the intervention arm (error bars: 95% CI).
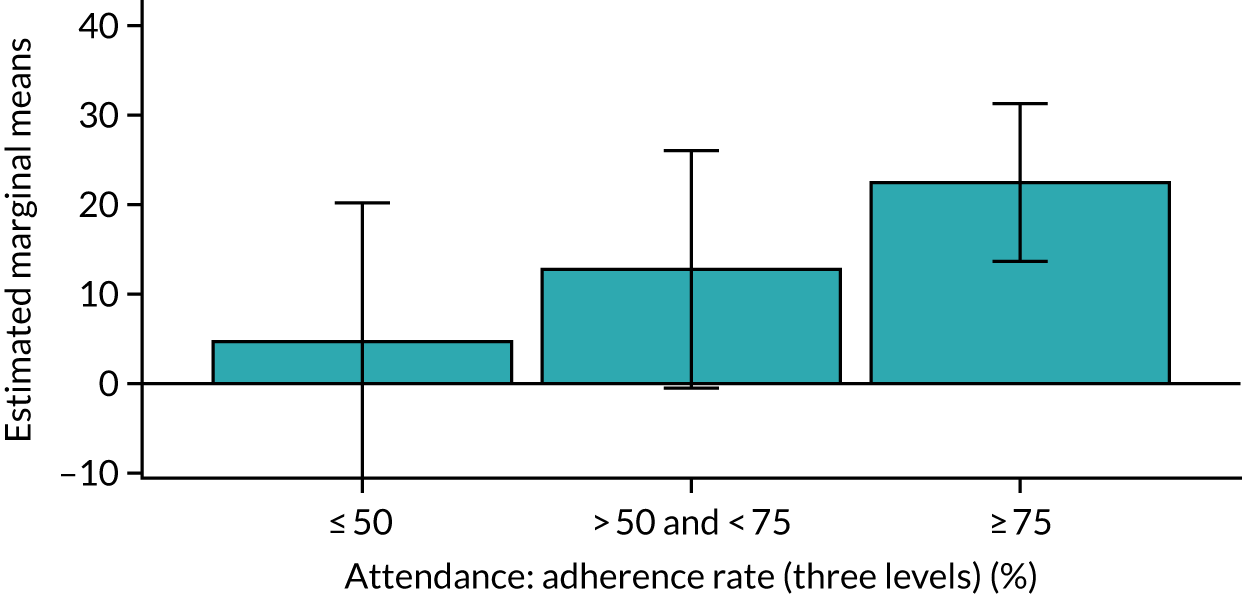
FIGURE 35.
Changes in PASE total score from 0 to 12 months for different levels of attendance within the intervention arm (error bars: 95% CI).
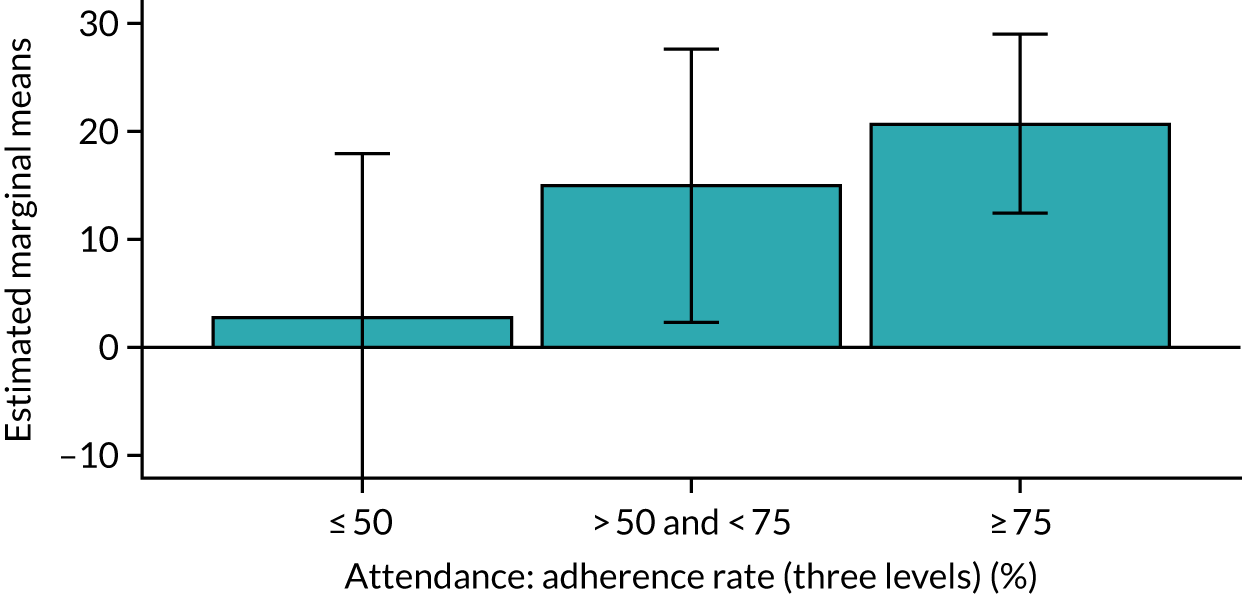
Appendix 3 Health economic evaluation
Parts of this appendix have been adapted from Snowsill et al. 103 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Valuation of resources
The costs of hospital overnight stays were estimated from the 2017/18 NHS reference costs113 and the 2018/19 NCC for the NHS schedule of NHS costs. 111 Unlike previous editions, the current edition does not present costs for excess bed-days (or the number of bed-days contributing to estimated costs), so it is challenging to estimate costs per bed-day.
We examined the number of admissions and number of bed-days blinded to treatment allocation. A total of 186 general medical ward admissions were reported, accumulating 810 bed-days (mean 4.4 days per admission). In the case of long-stay wards, there were eight admissions totalling 101 bed-days (mean 12.6 days per admission), and there were 20 admissions to intensive care units, totalling 108 bed-days (mean 5.4 days per admission).
The cost for general medical ward and long-stay ward admissions were estimated using the 2017/18 NHS reference costs113 as follows:
-
All data for elective, non-elective long stay and non-elective short stay finished consultant episodes were collated.
-
Any Healthcare Resource Groups (HRGs) clearly not applicable to the population (e.g. paediatric or relating to maternity, or specifically giving an age range for children and young people) were filtered out.
-
The total number of bed-days for each HRG was calculated from the ‘inlier’ and excess bed-days.
-
A linear regression (weighted for activity) was used to relate the mean cost for a HRG to the mean bed-days for that HRG.
As a result, it was assumed that the cost for an admission would be £376.47 plus £492.58 per bed-day, and these figures were then inflated by 2.31% to 2018/19 prices. 110 Estimating a cost for spending time in an intensive care unit is challenging because critical care reference costs are ‘unbundled’, meaning that they refer only to the costs of critical care specifically and not to the costs of treating the underlying disease that caused admission, which are reported and reimbursed separately. In addition, current reference costs refer to a period spent in critical care rather than bed-days. The most recent reference costs using bed-days as activity are the 2013/14 reference costs,140 which suggest an average cost of £1190 per bed-day after excluding paediatric and neonatal critical care (£1274 in 2018/19 prices). For comparison, the most recent NCC estimates are an average cost of £1428 per critical care period (again, after excluding paediatric and neonatal critical care).
We assumed that a period of critical care would cost £1428.09 (specifically for critical care) plus £2104.00 (the average cost for admissions after exclusions as above), for a total cost of £3532.09.
The societal cost of individuals paying for a chiropractor or osteopath was estimated from market prices. For a chiropractor, we used estimates from a study by Newell et al.,117 who estimated, based on data from 33 clinics relating to 1895 patients, a mean of 5.4 chiropractic consultations per patient over a 90-day period, with associated mean per-patient costs of £202.15. The resulting mean cost per appointment (£37.44) was then inflated to £40.06 in 2018/19 prices. The cost of an appointment with an osteopath was estimated from the public reporting of a private survey by the General Osteopathic Council that the average follow-up session cost £42,118 which was inflated to £44.95 in 2018/19 prices. The costs of care at home were estimated from the PSSRU 2019 costs for a home care worker (£28 per hour on weekdays, £29 per hour at the weekend).
The societal value of an hour of help around the home from friends or relatives was estimated based on a compensating variation study among informal caregivers in the Netherlands. 114 This provided an estimated value of €9.65 per hour, which was converted to £7.39 in 2002 prices using purchasing power parity rates,115 and then inflated to £11.06 in 2019 prices according to nominal weekly earnings growth. 116 Sensitivity analyses for plausible extreme values for an hour of help around the home came from two separate British studies. 141,142 First, a discrete choice experiment with 270 respondents from Scotland was analysed with a latent class model,141 which suggested three classes with average class probabilities of 0.528, 0.192 and 0.204. The value at which respondents were willing to accept an hour of household tasks in each class was £0.25, £0.58 and £0.53, respectively, giving a weighted average willingness-to-accept value of £0.35 per hour of help with household tasks. Second, a compensating income variation analysis of the British Household Panel Survey142 suggested that a compensation of £5020 per week would be required to offset the loss to well-being for an individual providing 10–19 hours per week of informal care, which is approximately £335 per hour (assuming 15 hours per week).The societal value of a day taken off work by a friend or relative was estimated from median daily earnings (£99.53). 116
Missing data
Multiple imputation was used to account for missing data on the assumption that cost and QALY data were missing at random (i.e. their missingness can be predicted from observed data). Fifty imputation sets were generated using MI chained equations with predictive mean matching (five nearest neighbours). The number of imputation sets was chosen based on the rule of thumb that the number of imputations should be similar to (or exceed) the percentage of cases that are incomplete. 143
The variables used in the predictive mean matching were:
-
random allocation variable (intervention vs. control)
-
adjustment variables (sex, age, baseline SPPB score and site)
-
SPPB scores at each follow-up time point
-
EQ-5D and SF-6D utilities at each follow-up time point
-
disaggregated costs at each follow-up time point.
Of the 777 trial participants, 451 (58%) provided complete data for cost-effectiveness analysis (i.e. resource use and EQ-5D-5L at all time points, including baseline). A further 25 participants provided complete data for an unadjusted cost-effectiveness analysis, that is they provided all data specified above apart from baseline resource use.
Figure 36 shows that, although most participants provided baseline data, a fair proportion of the participants failed to provide one or more measurement of EQ-5D-5L or NHS/PSS resource use, meaning that QALYs and/or total costs could not be estimated.
FIGURE 36.
Missing data for EQ-5D and NHS/PSS costs.
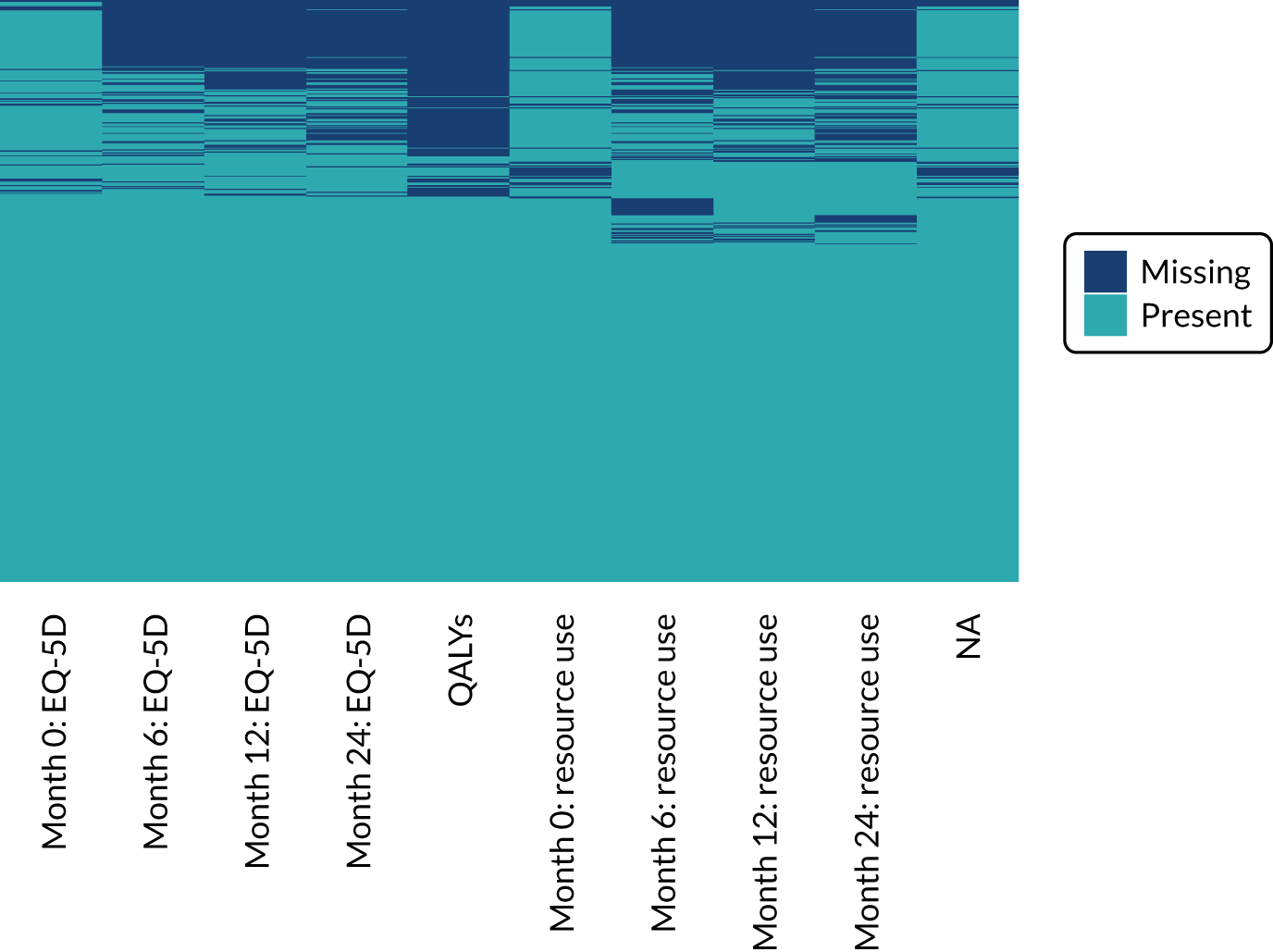
Patterns of missing data for EQ-5D and SF-6D were examined. At each time point, EQ-5D was more likely to be present than SF-6D, but the missingness of EQ-5D and SF-6D was highly correlated. In total, 359 (46%) participants had at least one utility value missing for at least one time point: 230 (30%) were missing EQ-5D for at least one time point and 346 (46%) were missing SF-6D for at least one time point (Figure 37). Participants who had an instrument missing for one of the follow-up time points were more likely to have that instrument missing for the other follow-up time points. Logistic regression on an indicator variable for missingness revealed that treatment allocation did not appear to influence missingness (EQ-5D, p = 0.95; SF-6D, p = 0.61). Age at randomisation appeared to have an effect on missingness, with a fairly consistent pattern that older participants were more likely than younger participants to have missing EQ-5D and SF-6D (EQ-5D, p < 0.001; SF-6D, p < 0.001) data. Study site appeared to influence missingness; however, when age was also included in the logistic model, study site was no longer statistically significant (EQ-5D, p = 0.12; SF-6D, p = 0.063). Sex did not appear to influence missingness (EQ-5D, p = 0.49; SF-6D, p = 0.22).
FIGURE 37.
Correlogram of missingness of health-related quality-of-life measures at different time points.
For resource use, there was naturally a relationship between the missingness of societal costs and the missingness of NHS/PSS costs, given that all resources included in societal costs are also included in NHS/PSS costs (although sometimes with different values). Having missing resource use at one follow-up time point increased the probability of having missing resource use at subsequent time points (Figure 38). Treatment allocation and sex did not appear to influence missingness (treatment allocation, p = 0.2; sex, p = 0.74). Increased age was positively associated with the likelihood of costs being missing (p < 0.001). Site was also associated with missingness of costs, with participants in Devon most likely and participants in Birmingham least likely to be missing costs (p < 0.001). This relationship was preserved when age was also included in the logistic model.
FIGURE 38.
Correlogram of missingness of costs at different timepoints from NHS/PSS and societal perspectives.
Intervention costs
Referral and co-ordination
Our base-case assumption is that there would be no incremental GP time cost associated with referral, as the time taken to make a referral is not expected to be significant or notably different from inputs in the usual care arm, such as GP advice to increase physical activity, for which no costs have been included in the analysis. We included 30 minutes of time for a social prescribing link worker per patient, whose job is to ensure that the patient is appropriate for referral, briefly describe the REACT intervention and refer the patient to a REACT co-ordinator (see below). REACT study investigators estimated that it took 30 minutes to screen an individual for the REACT study. 29 As a sensitivity analysis, we replace these costs with the estimated recruitment costs for the REACT study, which included screening GP databases to identify suitable individuals, mailing invitations, a PR campaign and other recruitment methods, at an estimated total cost of £81,597 to recruit 777 participants (£105.02 per participant recruited). 29
The REACT co-ordinator would perform activities such as:
-
identifying appropriate venues and making bookings
-
recruiting session leaders
-
ensuring that session leaders are trained appropriately to deliver REACT and are intermittently supervised for intervention fidelity
-
maintaining a waiting list
-
administering related payments (to venues, session leaders and other expenses)
-
sending schedules and reminders to participants.
We assume that these activities will require 36 hours of co-ordinator time (approximately 1 working week) per REACT group.
REACT session leader
In general, the NHS does not employ sport and fitness instructors. The mean hourly salary for an employed fitness instructor was estimated to be £10.63. 134 However, over half of fitness instructors are self-employed144 and the hourly charge for these is not well estimated by national statistics. In the REACT study, all session leaders were qualified to at least level 3 (whereas many fitness instructors may be qualified only to level 2). One REACT study provider reported a base salary cost of £15.50 per hour and one freelance trainer charged £26 per hour. Using the Agenda for Change band 4 cost information from the PSSRU, a recent economic evaluation of individual health trainers145 estimated the cost of a health trainer to be £28 per hour (revised to £29.43 in the most recent version of PSSRU unit costs). 110 The base annual salary for band 4 community-based scientific and professional staff is £22,256, which, spread over 1618 working hours per year (as assumed for band 4 workers by PSSRU), corresponds to an hourly gross rate of £13.76. We assume that the cost of REACT session leaders will be £29.43 per hour, and this should not be highly sensitive to whether REACT session leaders are employed or freelance. We, therefore, assume that this includes the cost of any supervision required, a suitable location to meet REACT participants and perform any administrative duties, and any travel expenses. In sensitivity analyses we vary the cost by ± 20%.
Venue hire
The NHS does not (in general) have facilities to accommodate an intervention such as REACT, which requires a hall or large room suitable for group exercise with up to 15 participants. If the REACT intervention were to be rolled out, it is likely that a venue would need to be hired from a private sector or charity/non-profit sector organisation or from a public body (e.g. a local authority community centre or school). The costs of hiring such a venue will probably vary significantly depending on geographical location, type of space and facilities. The REACT intervention would operate during off-peak hours for health centres. The cost of venue hire was reported by the investigators as £15–25 per hour for some sites and £32.50 per session for a multisite provider. In the case of some sites, an overall cost was reported, including costs for staff and refreshments, so no specific venue cost could be identified.
To attempt to identify a representative sample of venue hire costs from outside the REACT trial, we randomly sampled 10 lower-layer super output areas in England, one from each decile of the IMD. 146 The retrieved prices ranged from £12 to £25 per hour, with a mean of £17.32 per hour, which is used as the base-case cost per hour of venue hire. In sensitivity analyses, we vary the cost from £15 per hour to £25 per hour.
One-to-one introductory sessions
We assume that the 45-minute introductory sessions are attended by all individuals allocated to the intervention. As this may be an overestimate (some may not attend the introductory session and it is unlikely that the opportunity cost of a non-attendance is equal to the opportunity cost of a session), we do not include other preparation or travel costs for these sessions. In a sensitivity analysis, we include an additional 30 minutes of REACT trainer time for travel and/or preparation.
Cost per session
The cost per session of REACT is estimated from the REACT session leader time (preparation, travel and delivery), REACT session leader travel expenses, venue hire and consumables. The nominal session lengths are 80 minutes (41 sessions) and 105 minutes (23 sessions), giving a mean nominal session length of 89 minutes. Session leaders were requested to note the start and end times of each session, which provides empirical data on the length of sessions (719 sessions provided these data out of a maximum of 1728). The data suggest that a significant proportion of 80-minute sessions were shortened to 75 minutes or extended to 90 minutes. The mean session length was 86.3 minutes. We estimate the cost per session by assuming that each session requires 90 minutes of the session leader time and venue hire. In a sensitivity analysis, we vary this from 80 minutes to 105 minutes.
Session leaders were heterogeneous in their recording of preparation time. We assumed 90 minutes per session and 45 minutes for 15 participants of one-to-one introductory sessions: this leaves ≈ 51 hours (≈ 48 minutes per session) for preparation. In the base case, we assume 30 minutes of preparation per session. In sensitivity analyses, we vary this from 10 minutes to 90 minutes.
Session leader travel time was recorded in only 139 out of 737 (18.9%) session records, with a mean travel time of 22.2 minutes in those sessions. In a sensitivity analysis, we remove travel time to represent the cost if staff are already on site.
Consumables per session were assumed to be limited to refreshments and to cost £1 per attendee per session. We assumed that refreshments would be ordered for the full class for the first session (mean 14.63) and thereafter would be ordered for the number attending the first session (mean 9.63).
Printed materials
We assume a cost per participant of £2, suggested by the investigators.
Group size
In total, there were 410 individuals randomised, who were allocated to 27 groups; therefore, the average group size was 15.2 individuals. In sensitivity analyses we vary the group size from 12 to 17 individuals.
Overall costs
The breakdown of the costs is presented in Table 35.
| Item | Resource use per group | Unit cost (£) | Cost per group (£) | Cost per participant (£) |
|---|---|---|---|---|
| Link worker | 30 minutes per participant | 33.83 | 257.48 | 16.92 |
| Co-ordinator | 36 hours per group | 33.83 | 1217.88 | 80.01 |
| Introductory sessions | 45 minutes per participant | 29.43 | 335.99 | 22.07 |
| Equipment | ||||
| Pedometers | One per participant | 9.95 | 151.46 | 9.95 |
| Other | Ankle weights and Therabands, assumes reuse and sharing between groups | 29.16 | 29.16 | 1.92 |
| REACT session leader | ||||
| Preparation | 30 minutes per session | 29.43 | 941.76 | 61.87 |
| Travel time | 30 minutes per session | 29.43 | 941.76 | 61.87 |
| Delivery | 90 minutes per session | 29.43 | 2825.28 | 185.60 |
| Consumables | ||||
| Refreshments | Approximately one per participant per session | 1.00 | 621.32 | 40.82 |
| Printed materials | One set per participant | 2.00 | 30.44 | 2.00 |
| Venue hire | 90 minutes per session | 17.32 | 1662.72 | 109.23 |
| Training session leaders | ||||
| Training leaders | One leader to four trainees | 33.83 | 22.20 | 1.46 |
| REACT session leaders time | 1.5 days for every four programmes delivered | 29.43 | 77.25 | 5.08 |
| Training venue | As above | 29.43 | 77.25 | 5.07 |
| Training manual | One per REACT trainer | 12.00 | 3.00 | 0.20 |
| Total | 9465.69 | 621.83 | ||
The cost was somewhat sensitive to underlying assumptions, as shown in Table 36. The cost per participant ranged from £554.54 (–11%) to £775.13 (25%) across the sensitivity analyses.
| Sensitivity analysis | Cost per group (£) | Cost per participant (£) |
|---|---|---|
| REACT study recruitment costs (£105.02 per recruited participant) | 10,806.84 | 709.94 |
| REACT session leader | ||
| 20% less per hour | 8441.28 | 554.54 |
| 20% more per hour | 10,490.10 | 689.13 |
| Additional 30 minutes for introductory one-to-one sessions | 9689.68 | 636.55 |
| Venue cost | ||
| £15 per hour | 9242.97 | 607.20 |
| £25 per hour | 10,202.97 | 670.27 |
| Session duration | ||
| 80 minutes | 8967.02 | 589.07 |
| 105 minutes | 10,213.69 | 670.97 |
| REACT session leader | ||
| 10 minutes preparation per session | 8837.85 | 580.59 |
| 90 minutes preparation per session | 11,349.21 | 745.57 |
| Zero travel time | 8523.93 | 559.97 |
| Group size | ||
| n = 12 | 9301.56 | 775.13 |
| n = 17 | 9556.24 | 562.13 |
Baseline resource use and utility values and within-trial costs analysis
| Characteristic | Unit | Control arm (N = 367) | Intervention arm (N = 410) |
|---|---|---|---|
| Primary care resource use | |||
| Primary care usage reported, n (%) | Participants | 359 (98) | 404 (99) |
| GP, mean (SD) | Visits | 2.91 (4.03) | 2.75 (2.99) |
| Practice nurse, mean (SD) | Visits | 1.40 (2.41) | 1.54 (2.68) |
| Physiotherapist, mean (SD) | Visits | 0.28 (1.05) | 0.67 (2.54) |
| Occupational therapist, mean (SD) | Visits | 0.03 (0.26) | 0.04 (0.42) |
| Nutritionist, mean (SD) | Visits | 0.04 (0.56) | 0.04 (0.33) |
| Chiropodist, mean (SD) | Visits | 0.84 (1.63) | 0.87 (1.66) |
| Counsellor, mean (SD) | Visits | 0.09 (1.29) | 0.03 (0.37) |
| Walk-in centre, mean (SD) | Visits | 0.03 (0.22) | 0.03 (0.21) |
| Hospital overnight stay resource use | |||
| Overnight stay usage reported, n (%) | Participants | 350 (95) | 392 (96) |
| General medical ward, mean (SD) | Visits | 0.04 (0.22) | 0.06 (0.25) |
| Long-stay ward, mean (SD) | Visits | 0.00 (0.00) | 0.00 (0.05) |
| Intensive care unit, mean (SD) | Visits | 0.00 (0.00) | 0.01 (0.07) |
| Other hospital resource use | |||
| Other hospital usage reported, n (%) | Participants | 362 (99) | 406 (99) |
| Outpatient appointment, mean (SD) | Visits | 1.19 (2.44) | 1.11 (1.86) |
| Day case, mean (SD) | Visits | 0.30 (0.92) | 0.31 (0.88) |
| A&E attendance, mean (SD) | Visits | 0.09 (0.34) | 0.12 (0.37) |
| Social care resource use, mean (SD) | |||
| Day care | Visits | 0.01 (0.16) | 0.07 (1.26) |
| Convalescent home | Visits | 0.00 (0.00) | 0.03 (0.70) |
| Relatives/friends help | Hours | 1.60 (6.80) | 2.20 (7.90) |
| Relatives/friends off work | Days | 0.07 (0.83) | 0.10 (1.34) |
| Outside NHS/PSS perspective resource use, mean (SD) | |||
| Chiropractor | Visits | 0.03 (0.34) | 0.02 (0.30) |
| Osteopath | Visits | 0.06 (0.67) | 0.02 (0.23) |
| Care at home | Hours | 0.00 (0.00) | 0.00 (0.00) |
| Summary costs (NHS/PSS perspective) | |||
| Complete cases, n (%) | Participants | 347 (95) | 387 (94) |
| Primary care, mean (SD) | £ | 185 (218) | 212 (247) |
| Overnight stay, mean (SD) | £ | 68 (491) | 188.16 (1461.92) |
| Other hospital, mean (SD) | £ | 394 (825) | 390 (762) |
| Other care (excluding informal), mean (SD) | £ | 0 (6) | 6 (75) |
| Total costs from NHS/PSS perspective, mean (SD) | £ | 653 (1006) | 797 (1765) |
| Summary costs (societal perspective) | |||
| Complete cases, n (%) | Participants | 306 (83) | 358 (87) |
| Other care (excluding informal), mean (SD) | £ | 4 (35) | 10 (114) |
| Informal care, mean (SD) | £ | 25 (116) | 36 (204) |
| Total societal costs, mean (SD) | £ | 659 (1025) | 841 (1890) |
| Baseline health-related quality of life | |||
| Complete cases, n (%) | Participants | 320 (87) | 364 (89) |
| EQ-5D, mean (SD) | Utility | 0.68 (0.17) | 0.69 (0.16) |
| SF-6D, mean (SD) | Utility | 0.62 (0.09) | 0.62 (0.10) |
| Analysis | n | Intervention cost (£), mean (SE) | Control cost (£), mean (SE) | Difference in cost (£), mean (95% CI) | ||
|---|---|---|---|---|---|---|
| MI, NHS/PSS perspective | ||||||
| 0–6 months | 777 | 634.56 (51.35) | 877.47 (104.39) | –242.91 (–464.94 to –20.89) | ||
| 6–12 months | 777 | 751.24 (68.15) | 908.26 (97.83) | –157.01 (–366.61 to 52.59) | ||
| 18–24 months | 777 | 1107.04 (106.83) | 1224.89 (131.04) | –117.84 (–426.60 to 190.91) | ||
| Total within-trial costs | 777 | 3321.39 (203.36) | 4046.13 (310.05) | –724.74 (–1423.69 to –25.79) | ||
| MI, societal perspective | ||||||
| 0–6 months | 777 | 681.59 (53.43) | 915.78 (106.40) | –234.19 (–459.73 to 8.64) | ||
| 6–12 months | 777 | 804.36 (73.77) | 964.86 (102.52) | –160.50 (–378.93 to 57.93) | ||
| 18–24 months | 777 | 1201.00 (113.23) | 1280.22 (134.01) | –79.23 (–398.96 to 240.51) | ||
| Total within-trial costs | 777 | 3594.56 (212.52) | 4240.21 (317.77) | –645.65 (–1355.02 to 63.73) | ||
| Complete case analysis, NHS/PSS perspective | ||||||
| 0–6 months | 578 | 623.34 (53.96) | 876.30 (118.93) | –252.96 (–508.74 to 2.82) | ||
| 6–12 months | 584 | 724.98 (73.49) | 793.90 (83.25) | –68.91 (–266.53 to 128.71) | ||
| 18–24 months | 579 | 1129.23 (123.52) | 1217.96 (147.71) | –88.72 (–450.07 to 272.63) | ||
| Total within-trial costs | 477 | 3248.86 (257.30) | 3573.37 (306.69) | –324.51 (–1086.45 to 437.43) | ||
| Complete case analysis, societal perspective | ||||||
| 0–6 months | 496 | 653.83 | 64.22 | 782.55 | 85.71 | –128.73 (–352.09 to 94.64) |
| 6–12 months | 501 | 669.51 | 67.25 | 766.94 | 81.81 | –97.43 (–295.12 to 100.26) |
| 18–24 months | 492 | 1164.12 | 148.57 | 1297.73 | 173.41 | –133.61 (–561.72 to 294.50) |
| Total within-trial costs | 377 | 3114.50 | 241.16 | 3572.72 | 330.51 | –458.23 (–1254.94 to 338.49) |
Subgroup analyses
| Subgroup | Costs (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Intervention arm | Control arm | Intervention arm | Control arm | ||
| Baseline SPPB score | |||||
| 4–7 | 4842.42 | 4156.20 | 1.315 | 1.288 | 25,008 |
| 8–9 | 3291.05 | 3771.44 | 1.394 | 1.343 | Dominant |
| Age (years) | |||||
| 65–74 | 3394.97 | 3589.01 | 1.390 | 1.333 | Dominant |
| ≥ 75 | 4230.99 | 4257.29 | 1.333 | 1.305 | Dominant |
| Sex | |||||
| Female | 3822.75 | 3796.69 | 1.347 | 1.313 | 784 |
| Male | 4154.65 | 4540.45 | 1.373 | 1.321 | Dominant |
| Known medical conditions | |||||
| 0–1 | 3791.61 | 3367.29 | 1.376 | 1.340 | 11,881 |
| ≥ 2 | 4028.48 | 4867.41 | 1.339 | 1.286 | Dominant |
| SES (by education) | |||||
| Not beyond secondary school | 4361.20 | 4239.89 | 1.350 | 1.324 | 4725 |
| Beyond secondary school | 3614.64 | 3799.94 | 1.359 | 1.307 | Dominant |
| SES (by IMD decile) | |||||
| 1–6 | 3871.42 | 3989.77 | 1.343 | 1.292 | Dominant |
| 7–10 | 4004.43 | 4089.17 | 1.371 | 1.341 | Dominant |
| Site | |||||
| Bath/Bristol | 3833.29 | 3603.76 | 1.364 | 1.308 | 4075 |
| Birmingham | 4301.44 | 4235.21 | 1.360 | 1.306 | 1235 |
| Devon | 3841.29 | 4509.19 | 1.342 | 1.333 | Dominant |
| Falls in previous 6 months | |||||
| None | 4016.40 | 3815.74 | 1.377 | 1.330 | 4266 |
| One or more | 3857.19 | 4273.30 | 1.326 | 1.293 | Dominant |
Review of economic evaluations of interventions to prevent or reduce mobility-related disability
We have conducted a pragmatic review to identify existing economic evaluations of interventions similar to REACT, performed a within-trial economic evaluation and developed a new decision analytic model to extrapolate any long-term costs and benefits that may arise from the REACT intervention.
Introduction
The aim of this review was to identify any economic evaluations of interventions with similar aims to REACT, that is group-based physical activity interventions for older adults with the intention to maintain mobility or delay frailty. The purpose of identifying any such evaluations was to explore modelling approaches that may be appropriate and to be able to put the results of the economic evaluation of REACT in the wider evidence base for any existing competing interventions in the same patient population.
The broader set of economic evaluations of physical activity interventions (individual and group based) was not considered in this review because these studies often focus on the impact of physical activity on long-term health conditions, such as cardiovascular disease, which is not considered to be a primary aim of REACT.
Methods
A pragmatic review of economic evaluations of interventions to prevent or reduce mobility-related disability in older people was undertaken. To be eligible for inclusion, a study needed to be an economic evaluation comparing at least two options, with at least one including a physical activity component. Studies must incorporate costs and a health outcome measure. The study population must be older adults (mostly or all aged ≥ 65 years) and must not be selected according to the presence and/or history of specific health conditions. The primary purpose of the intervention must be to maintain mobility or delay the incidence of frailty, and its effect may be evaluated in terms of maintaining or improving general mobility or balance or reducing falls.
MEDLINE was searched via Ovid from inception to 25 March 2020 by using medical subject heading (MeSH) terms and the study design filter used by the NHS Economic Evaluation Database (NHS EED) to restrict records of full economic evaluations:
-
exp Frailty/or exp Aging/or exp Mobility Limitation/ [n = 247,743]
-
exp Exercise/or exp Exercise Therapy/ [n = 221,376]
-
1 and 2 [n = 8623]
-
(NHS EED search filter begins)
. . .
29. (NHS EED search filter ends) [n = 827,917]
30. 3 and 29 [n = 296].
The MeSH terms were identified by the reviewer (TS) as relevant to the study question by considering the MeSH terms associated with REACT protocol1 and the LIFE study,21 and also searching for relevant MeSH terms. Free-text terms were not included because this was a pragmatic review. The NHS EED study design filter is one that has been used for many systematic reviews of economic evaluations. 147
Results
One reviewer (TS) screened the titles and abstracts from the 296 retrieved records and selected 21 to retrieve as full texts for assessment of eligibility. One citation could not be retrieved as full text, so 20 full texts were assessed for eligibility (see Figure 39). Forward citation chasing from protocol papers was also conducted using Google Scholar to find published results.
A significant proportion of the full texts was protocols for studies with no associated economic evaluation published, and, in addition to this, several studies were excluded because they did not include physical activity as a core intervention component (e.g. it was an optional component in a multicomponent intervention or the intervention was designed to facilitate physical activity) or because they did not complete a full economic evaluation. For a list of excluded studies at the full-text screening stage, see Report Supplementary Material 3.
FIGURE 39.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram for pragmatic review of economic evaluations.
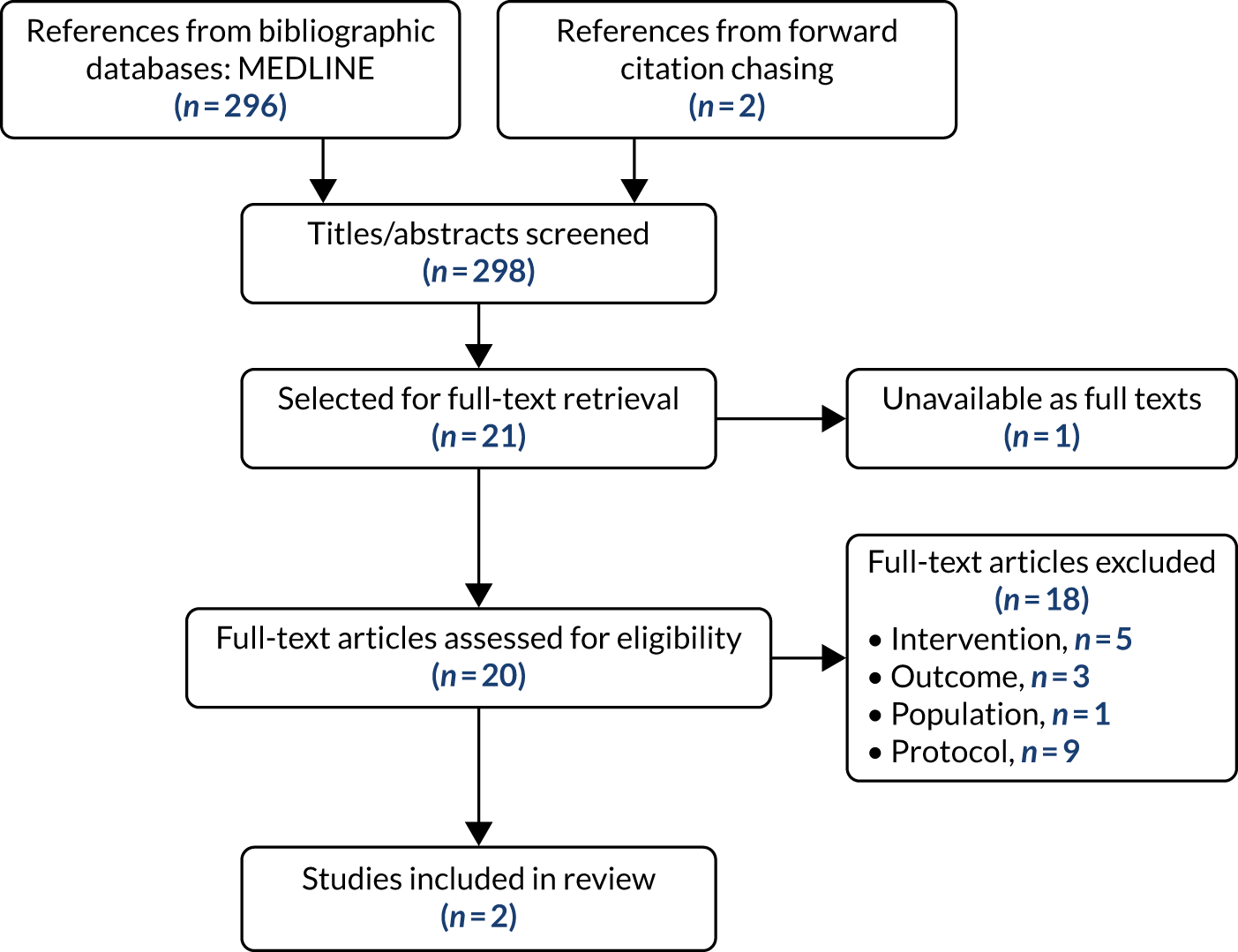
Two studies124,125 were ultimately judged to be eligible, and these are described briefly below.
Alhambra-Borrás et al. 2019
Alhambra-Borrás et al. 124 investigated the effectiveness and cost-effectiveness of a group-based exercise programme for community-dwelling adults aged ≥ 65 years in Valencia, Spain. Their study was quasi-experimental, with participants from two different health centres being recruited concurrently and all participants from one centre being offered the intervention, and all participants from the other centre receiving usual care. However, an estimated 100 out of 258 participants in the ‘intervention centre’ did not agree to participate in the physical exercise programme and were, therefore, excluded. This introduces a substantial risk of bias into all of the study analyses in addition to the normal caveats associated with the lack of randomisation in allocating patients to treatments.
Health-care resource utilisation was estimated by asking participants to recall doctors’ visits and hospitalisations for the previous 12 months (at baseline) or 9 months (at the 9-month follow-up). The SF-12 was used to assess health-related quality of life (at baseline and at the 9-month follow-up). The cost of the intervention was estimated solely from the time of the physiotherapist delivering the intervention, suggesting that no cost was used for the venue or any equipment. It is not clear whether the cost per hour for the physiotherapist is salary cost only or if it includes overhead costs.
A within-trial cost-effectiveness analysis was not conducted. Instead, trial data were used to estimate the associated health-care costs and quality of life (SF-12 mapped to EQ-5D-3L utility) of participants in the ‘baseline state’ (improvement in risk of falls/frailty after 9 months) and ‘deteriorated state’ (no improvement or worsening of risk of falls/frailty after 9 months). These costs and utility values were estimated separately for the two centres (intervention and control). This may be problematic because differences in costs and health-related quality of life could arise from pre-existing differences between the centres rather than owing to the effect of the intervention. The authors do not report what proportion of participants was expected to be in each health state or how future transitions between these health states were accounted for in the analysis.
The authors conclude that the intervention, if offered continuously, would lead to reductions in overall costs (from a health-care perspective) and increases in QALYs, meaning that the intervention would be dominant. For all age groups (up to 92 years), the intervention is predicted to be dominant, with younger participants experiencing greater QALY gains and realising greater cost savings. The analysis predicted average cost savings of €44,833 (approximately £48,500, converted using 2019 PPP122) and QALY gains of 0.513 (both discounted at 3.5% per year).
The authors did not report the uncertainty in incremental costs and QALYs, but they did conduct a sensitivity analysis, which suggested that participants who were older at the start of the intervention would achieve smaller cost savings and gain fewer QALYs.
Groessl et al. 2016
Groessl et al. 125 investigated the cost-effectiveness of the LIFE physical activity programme. Adults aged 70–89 years at risk of developing major mobility disability were recruited and randomised to either the LIFE physical activity programme (including group sessions and home-based activity plans) or a health education programme as a control. Every 6 months, participants reported their health-care resource utilisation using a published resource use questionnaire and reported their health-related quality of life using the QWB-SA tool. 148
Intervention costs were estimated from personnel costs and adjusted for overheads, such as facilities. The longest follow-up time point was at 36 months after randomisation and mean follow-up was 2.6 years, which served as the time horizon for the analysis.
The physical activity intervention was estimated to cost $2300 more than the health education programme per participant and to lead to 0.047 additional QALYs, for an ICER of $49,000 per QALY in 2013 prices. Health-care resource utilisation costs were not included in the base-case analysis, with the justification that the difference did not reach statistical significance; however, when these were included in a sensitivity analysis, the ICER was $83,000 per QALY. Limited sensitivity analyses were conducted, and showed that, if overheads, wages or fringe benefits were higher, the physical activity intervention would be less cost-effective.
No attempt was made to extrapolate with decision analytic modelling to estimate lifetime costs and QALYs. It is not clear whether or not extrapolation would have made a material difference to the conclusions given that differences between the arms in resource use and QWB-SA score were not statistically significant at 36 months, but there was some evidence that fewer participants in the physical activity arm had persistent mobility disability at 36 months.
Natural history model
There are two natural history components in the model:
-
the evolution of mobility with age
-
the changing mortality rate with age.
The evolution of mobility with age
It has been demonstrated in a number of population studies from countries other than the UK that SPPB score is negatively correlated with age, with the mean SPPB score typically decreasing by around 1 point per decade of life beyond 60 years of age, and that women typically have lower SPPB scores. 149–151 However, it should be noted that these are cross-sectional studies rather than longitudinal studies; therefore, these may not correspond to a typical pattern of SPPB scores over an individual’s life course. For example, it may be that those with high SPPB scores are able to maintain their mobility, whereas those with some impairment are at high risk of becoming more impaired. It is also possible that surgical management (e.g. hip replacement) could result in an increase in SPPB score. Mortality may also be associated with mobility impairment (although not necessarily directly caused by poor mobility),129 which could lead to a less pronounced decline of SPPB with increasing age when assessed in a cross-section of the population compared to if SPPB were measured over a typical life course. Population studies have generally shown that very few older people fall in the lowest SPPB category (0–3),149,150 although it is possible that such individuals were selected out (or selected themselves out) of the studies by not completing the SPPB, as well as being less likely to survive into old age.
There is a trend to convergence in the predicted mean SPPB score observed over the course of the REACT study, as described in Chapter 3. In particular, there is an increase in the benefit of the intervention from 6 to 12 months, which is followed by a decline from the 12- to 24-month follow-up points, which amounts to yearly reduction of 36%.
The extrapolation of mobility from the end of the trial follow-up to lifetime was derived from an ordered logistic regression of SPPB score at 24 months as a function of SPPB score at 12 months post randomisation, age at randomisation, sex and allocation site (Bath/Bristol, Birmingham, Devon) in the base-case analysis (model 1) and a modified model that included only SPPB score at 12 months post randomisation (model 2) in sensitivity (scenario) analysis. The model may be described by the following equation:
where SPPB^24i is the value of a latent (unobserved) continuous mobility status variable for individual i, a trial participant allocated to the control arm at 24 months, which is determined by that person’s own observed SPBB12i value at 12 months with coefficient α plus a linear combination of the product of variables and their coefficients, β′Xi, which in the case of model 1 is equal to:
where Devon takes the value of 1 if the individual is from the Devon trial site and is zero otherwise, while DBirmingham is similarly defined (with Bath and Bristol as the reference); β′Xi is omitted from model 2. The residual term, SPPB^24i, is a random unobserved variable following a logistic distribution. The continuous dependent variable, [inline], is not observed; instead the discrete variable, SPBB24i, is observed, taking the value of 0, 1, 2, . . ., 11, 12, with probabilities determined by the cumulative logistic distribution centred on the mean value of αSPBB12i + β′Xi relative to a set of fixed thresholds defining the boundaries of SPPB response categories.
To assess convergence, we used the appropriate 24-month SPPB distributions in the control and intervention arms, as described above, and then used the model to evolve the SPPB distribution over time in the absence of mortality. The mean SPPB at 24 months, 36 months (3 years) and 120 months (10 years) was calculated. Convergence is given by the reduction in the difference in mean SPPB score between intervention and control from 24 to 36 months.
The two models resulted in a convergence (i.e. a decline in the benefit of the intervention over usual care) rate for the first year of extrapolation ranging from 30% at age 65 years to 15% at age 95 years in women and from 31% to 15% in men in the base-case analysis (see Table 40); the corresponding predicted convergence rates in scenario analysis were 22% to 16% in women and 22% to 14% in men (see Table 42). Details of the models are presented below. We also consider three other models for the evolution of mobility. Model 3 is a static distribution, that is all individuals maintain their 24-month end-of-trial SPPB status until death, so there is no convergence of SPPB distributions between the intervention group and the control group. Model 4 uses the observed frequency of changes in SPPB between month 12 and month 24 in the REACT control group (annual convergence 5.2–7.3%, depending on age and sex). Model 5 assumes a normal distribution for changes in SPPB that does not achieve long-term decline of SPPB but is matched to the 36% annual convergence between the intervention group and the control group in the last year of the trial.
Predictions from model 1 are shown in Table 40 (predictions have been made assuming that the site is Devon). Notable features are that the convergence is not as fast as observed between month 12 and month 24 in the REACT study (36%), that the mean SPPB is predicted to increase for younger individuals (e.g. women aged 65 years, men aged 75 years), and is predicted to become very low on average for older individuals (especially women).
| Sex | Age (years) | Mean SPPB score after 2 years | Mean SPPB score after 10 years | Convergence |
|---|---|---|---|---|
| Female | 65 | 8.91 | 9.26 | 30% |
| Female | 75 | 7.81 | 7.41 | 23% |
| Female | 85 | 6.60 | 4.77 | 19% |
| Female | 95 | 5.34 | 1.81 | 15% |
| Male | 65 | 8.79 | 9.80 | 31% |
| Male | 75 | 7.67 | 8.17 | 24% |
| Male | 85 | 6.45 | 5.78 | 19% |
| Male | 95 | 5.19 | 2.74 | 15% |
| Sex | Age (years) | Mean SPPB score after 2 years | Mean SPPB score after 10 years | Convergence |
|---|---|---|---|---|
| Female | 65 | 8.91 | 7.06 | 22% |
| Female | 75 | 7.81 | 6.81 | 19% |
| Female | 85 | 6.60 | 6.49 | 16% |
| Female | 95 | 5.34 | 6.09 | 14% |
| Male | 65 | 8.79 | 7.04 | 22% |
| Male | 75 | 7.67 | 6.77 | 19% |
| Male | 85 | 6.45 | 6.44 | 16% |
| Male | 95 | 5.19 | 6.22 | 13% |
We use model 1 in the base-case and model 2 (see Table 41) in a scenario analysis. We also consider three other models for the evolution of mobility. Model 3 is a static distribution, that is all individuals maintain their 24-month SPPB status until death. This implies no convergence of the SPPB distributions between the REACT group and the control group. Model 4 is based on the frequency of observed changes in SPPB between month 12 and month 24 in the REACT control group (annual convergence 5.2–7.3% depending on age and sex). Model 5 is an artificial model (based on a normal distribution for changes in SPPB) that does not aim to achieve long-term decline of SPPB but does achieve 38% annual convergence between the REACT and the control groups.
Details of mobility models
The evolution of mobility was initially estimated from the REACT study using ordered logistic regression (model 1) with mobility at 24 months post randomisation in the control arm as the dependent variable, with estimated coefficients for mobility at 12 months post randomisation, age at randomisation, sex and allocation site (Bath/Bristol vs. Birmingham vs. Devon) as independent variables (Table 42).
| Term | Estimate | 95% CI |
|---|---|---|
| SPPB score at 12 months | 0.787 | 0.660 to 0.915 |
| Age at randomisation | –0.052 | –0.088 to –0.016 |
| Sex: female | –0.208 | –0.646 to 0.230 |
| Site | ||
| Birmingham | 0.050 | –0.542 to 0.642 |
| Devon | 0.572 | 0.107 to 1.037 |
| Threshold SPPB score | ||
| 0/1 | –3.988 | –7.070 to –0.906 |
| 1/2 | –3.291 | –6.379 to –0.203 |
| 2/3 | –2.476 | –5.592 to 0.640 |
| 3/4 | –1.830 | –4.931 to 1.270 |
| 4/5 | –0.617 | –3.755 to 2.521 |
| 5/6 | 0.119 | –3.028 to 3.265 |
| 6/7 | 1.060 | –2.079 to 4.200 |
| 7/8 | 1.972 | –1.177 to 5.120 |
| 8/9 | 3.115 | –0.024 to 6.255 |
| 9/10 | 4.181 | 1.029 to 7.332 |
| 10/11 | 5.188 | 2.038 to 8.337 |
| 11/12 | 6.877 | 3.616 to 10.14 |
As shown in Table 43, the SPPB score at 12 months was positively associated with SPPB score at 24 months. Furthermore, age had a negative independent effect; for example, the predicted SPPB score distribution at 24 months for a 75-year-old with a SPPB score of 8 at 12 months would be worse than the predicted SPPB score distribution at 24 months for a 65-year-old also with a SPPB score of 8. There was no evidence to suggest that the decline in SPPB score was faster for women (because the coefficient CI included the null). Participants in Devon had a lower predicted probability than those from other sites of finding themselves in a low-mobility health state at 24 months. The mean distance between thresholds was 0.988, meaning that the SPPB distribution predicted at any one time will be concentrated in three to four SPPB categories (i.e. unlikely to have large jumps in SPPB). The thresholds appear uncertain owing to wide CIs, but the thresholds are highly positively correlated, so the distance between them remains relatively stable.
A more parsimonious model was fitted, removing the terms for sex, site and age, leaving only SPPB score at 12 months to predict SPPB score at 24 months (model 2). Table 43 shows the terms for this model.
| Term | Estimate | 95% CI |
|---|---|---|
| SPPB score at 12 months | 0.799 | 0.676 to 0.922 |
| Threshold SPPB score | ||
| 0/1 | 0.196 | –0.888 to 1.280 |
| 1/2 | 0.868 | –0.091 to 1.827 |
| 2/3 | 1.681 | 0.813 to 2.549 |
| 3/4 | 2.320 | 1.456 to 3.183 |
| 4/5 | 3.512 | 2.625 to 4.399 |
| 5/6 | 4.227 | 3.332 to 5.122 |
| 6/7 | 5.135 | 4.161 to 6.109 |
| 7/8 | 6.014 | 4.978 to 7.050 |
| 8/9 | 7.121 | 6.019 to 8.222 |
| 9/10 | 8.146 | 6.973 to 9.318 |
| 10/11 | 9.129 | 7.897 to 10.36 |
| 11/12 | 10.796 | 9.404 to 12.19 |
The predicted transitions across states using this model will (in the absence of death) converge to a steady state distribution with mean SPPB score of 6.53 (Figure 40).
FIGURE 40.
Steady state distribution for Model 2.
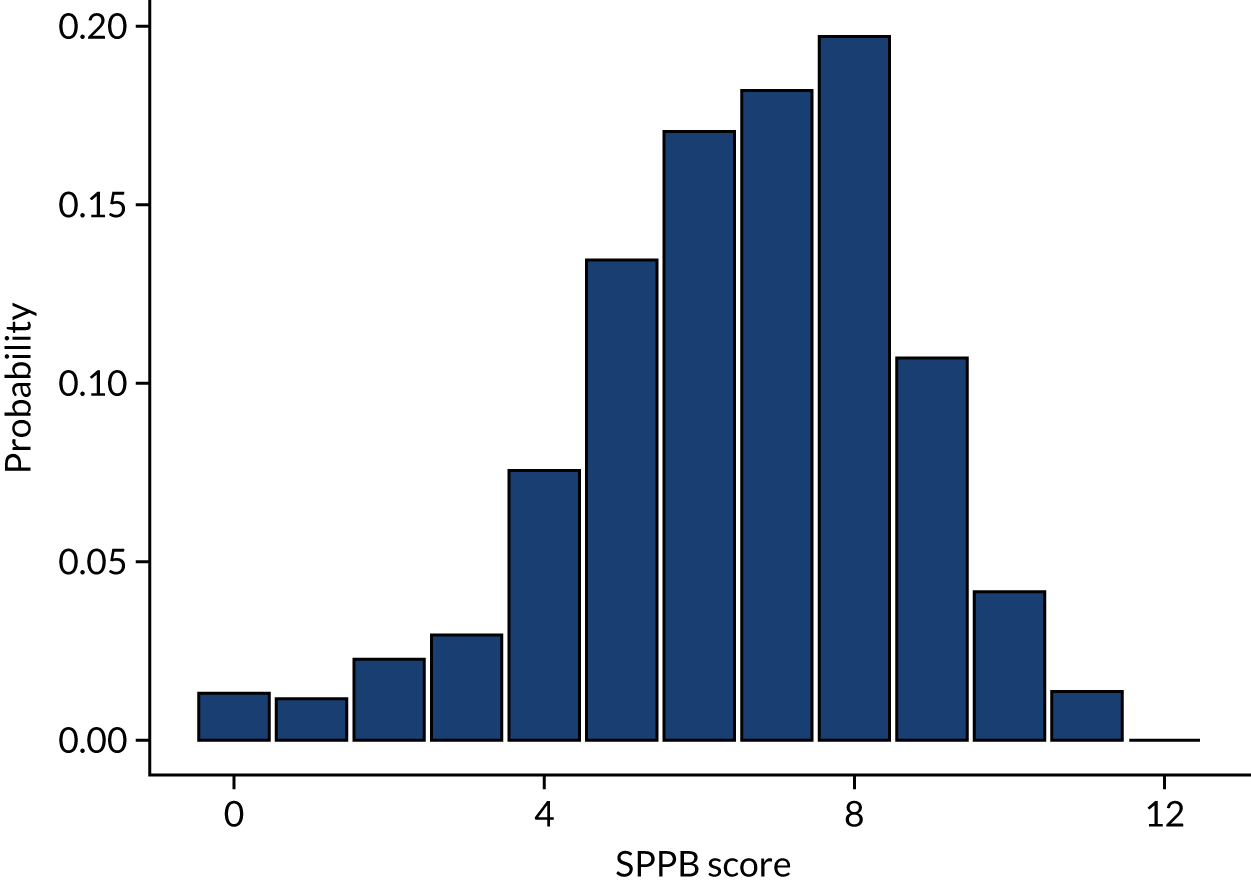
The changing mortality rate with age
The mortality rate for individuals was estimated from UK life tables for men and women aged 65–100 years. 152 For men and women aged > 100 years, a Gompertz model was fitted to the data for men and women aged 65–100 years and was used to extrapolate to age 135 years. It is assumed that the population that would participate in a REACT programme is well approximated by the general population. There are two reasons why this may not be an ideal approximation, but these operate in opposite directions: (1) to participate in a REACT programme an individual will need to have a minimum level of mobility and be able to attend (which is likely to exclude those in residential or nursing homes or with severe health conditions) and (2) to participate in a REACT programme an individual must have some level of mobility impairment (which would exclude those in very good health).
The scenario analyses considered four alternative models for the evolution of SPPB (Table 44):
-
model 2 – ordered logistic model fitted to REACT study control arm with no terms for age, sex or site
-
model 3 – no transitions between SPPB states (i.e. remain in the same SPPB state until death)
-
model 4 – transitions based on distribution of changes in SPPB in the REACT study control arm between month 12 and month 24
-
model 5 – artificial transition matrix designed to achieve fast convergence between REACT and control arms (difference in mean SPPB cut by 36% each year).
| Parameter name | Description | Base-case value | One-way sensitivity analysis | PSA | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Distribution | Parameter 1 | Parameter 2 | |||
| Dr | Discount rate for costs and QALYs | 3.5% | |||||
| age_start | Age at the start of the model (years) | 75 | 65 | 95 | |||
| sex.female | Population is female? | 1 | 0 | 1 | |||
| TrialEnd_SPPB.Control.0 | Probability of having given SPPB (0 to 12) in control arm at 24 months | 0.008 | Multinomial | 5 | |||
| TrialEnd_SPPB.Control.1 | 0.015 | 8.9 | |||||
| TrialEnd_SPPB.Control.2 | 0.015 | 8.9 | |||||
| TrialEnd_SPPB.Control.3 | 0.028 | 16.4 | |||||
| TrialEnd_SPPB.Control.4 | 0.051 | 30.1 | |||||
| TrialEnd_SPPB.Control.5 | 0.069 | 41 | |||||
| TrialEnd_SPPB.Control.6 | 0.089 | 52.8 | |||||
| TrialEnd_SPPB.Control.7 | 0.134 | 79.5 | |||||
| TrialEnd_SPPB.Control.8 | 0.149 | 88.1 | |||||
| TrialEnd_SPPB.Control.9 | 0.164 | 97.3 | |||||
| TrialEnd_SPPB.Control.10 | 0.121 | 71.6 | |||||
| TrialEnd_SPPB.Control.11 | 0.104 | 61.6 | |||||
| TrialEnd_SPPB.Control.12 | 0.053 | 31.3 | |||||
| TrialEnd_SPPB.Intervention.0 | Probability of having given SPPB (0 to 12) in REACT arm at 24 months | 0.006 | Multinomial | 3.1 | |||
| TrialEnd_SPPB.Intervention.1 | 0.010 | 5.7 | |||||
| TrialEnd_SPPB.Intervention.2 | 0.011 | 5.9 | |||||
| TrialEnd_SPPB.Intervention.3 | 0.020 | 11.2 | |||||
| TrialEnd_SPPB.Intervention.4 | 0.039 | 21.4 | |||||
| TrialEnd_SPPB.Intervention.5 | 0.056 | 30.6 | |||||
| TrialEnd_SPPB.Intervention.6 | 0.075 | 41.3 | |||||
| TrialEnd_SPPB.Intervention.7 | 0.119 | 65.5 | |||||
| TrialEnd_SPPB.Intervention.8 | 0.142 | 77.8 | |||||
| TrialEnd_SPPB.Intervention.9 | 0.171 | 94.2 | |||||
| TrialEnd_SPPB.Intervention.10 | 0.141 | 77.3 | |||||
| TrialEnd_SPPB.Intervention.11 | 0.135 | 74.2 | |||||
| TrialEnd_SPPB.Intervention.12 | 0.075 | 41.4 | |||||
| site.BathBristol | Input to mobility model 1 | 0 | Multinomial | 335 | |||
| site.Birmingham | Input to mobility model 1 | 0 | 174 | ||||
| site.Devon | Input to mobility model 1 | 1 | 268 | ||||
| SPPB.M12_SPPB_FinalScore | Coefficient for SPPB at 12 months in mobility model 1 | 0.787 | 0.660 | 0.915 | Multivariate normal (parameter 1 = mean vector, parameter 2 = variance-covariance matrix) | 0.787 | See Appendix 3, Table 45 |
| SPPB.Allocation_Site.Birmingham | Coefficient for site = Birmingham in mobility model 1 | 0.050 | –0.542 | 0.642 | 0.050 | ||
| SPPB.Allocation_Site.Devon | Coefficient for site = Devon in mobility model 1 | 0.572 | 0.107 | 1.037 | 0.572 | ||
| SPPB.SCREENING_AgeAtRandomisation | Coefficient for age at randomisation in mobility model 1 | –0.052 | –0.088 | –0.016 | –0.052 | ||
| SPPB.SCREENING_Sex.Female | Coefficient for sex = female in mobility model 1 | –0.208 | –0.646 | 0.230 | –0.208 | ||
| SPPB._cut1 | Mobility model 1 ordinal logistic regression cut points | –3.988 | –7.070 | –3.291 | –3.988 | ||
| SPPB._cut2 | –3.291 | –3.988 | –2.476 | –3.291 | |||
| SPPB._cut3 | –2.476 | –3.291 | –1.830 | –2.476 | |||
| SPPB._cut4 | –1.830 | –2.476 | –0.617 | –1.830 | |||
| SPPB._cut5 | –0.617 | –1.830 | 0.119 | –0.617 | |||
| SPPB._cut6 | 0.119 | –0.617 | 1.060 | 0.119 | |||
| SPPB._cut7 | 1.060 | 0.119 | 1.972 | 1.060 | |||
| SPPB._cut8 | 1.972 | 1.060 | 3.115 | 1.972 | |||
| SPPB._cut9 | 3.115 | 1.972 | 4.181 | 3.115 | |||
| SPPB._cut10 | 4.181 | 3.115 | 5.188 | 4.181 | |||
| SPPB._cut11 | 5.188 | 4.181 | 6.877 | 5.188 | |||
| SPPB._cut12 | 6.877 | 5.188 | 10.139 | 6.877 | |||
| SPPB.offset | Additional offset parameter (drives mobility up or down) | 0 | –0.5 | 0.5 | Normal | 0 | 0.25 |
| EQ5D.SPPB_Spline1 | Coefficient for SPPB in EQ-5D utility function | 0.032 | 0.017 | 0.046 | Multivariate normal | 0.032 | See Appendix 3, Table 46 |
| EQ5D.SPPB_Spline2 | Coefficient for SPPB (spline) in EQ-5D utility function | –0.011 | –0.025 | 0.003 | –0.011 | ||
| EQ5D._cons | Intercept in EQ-5D utility function | 0.449 | 0.357 | 0.540 | 0.449 | ||
| SPPB_Spline2.SPPB.0 | SPPB spline | 0 | |||||
| SPPB_Spline2.SPPB.1 | 0 | ||||||
| SPPB_Spline2.SPPB.2 | 0 | ||||||
| SPPB_Spline2.SPPB.3 | 0 | ||||||
| SPPB_Spline2.SPPB.4 | 0 | ||||||
| SPPB_Spline2.SPPB.5 | 0.020 | ||||||
| SPPB_Spline2.SPPB.6 | 0.163 | ||||||
| SPPB_Spline2.SPPB.7 | 0.551 | ||||||
| SPPB_Spline2.SPPB.8 | 1.306 | ||||||
| SPPB_Spline2.SPPB.9 | 2.503 | ||||||
| SPPB_Spline2.SPPB.10 | 4.027 | ||||||
| SPPB_Spline2.SPPB.11 | 5.714 | ||||||
| SPPB_Spline2.SPPB.12 | 7.429 | ||||||
| AnnualCost.SPPB.0_3 | Annual cost with SPPB 0–3 | 3494.62 | 1516.90 | 5472.34 | Multivariate normal | 3494.62 | See Appendix 3, Table 47 |
| AnnualCost.SPPB.4_7 | Annual cost with SPPB 4–7 | 2019.66 | 1652.31 | 2387.00 | 2019.66 | ||
| AnnualCost.SPPB.8_9 | Annual cost with SPPB 8–9 | 1730.03 | 1376.41 | 2083.64 | 1730.03 | ||
| AnnualCost.SPPB.10_12 | Annual cost with SPPB 10–12 | 1520.00 | 1200.29 | 1839.72 | 1520.00 | ||
| Year1CostNHS.Control | Within-trial costs for control arm | 4043.00 | 3553.79 | 4462.12 | Bootstrap samples (n = 100) | ||
| Year1CostNHS.Intervention | Within-trial costs for REACT arm (excluding intervention) | 3319.00 | 3018.50 | 3699.12 | |||
| Year1QALY.Control | Within-trial QALYs for control arm | 1.348 | 1.327 | 1.374 | |||
| Year1QALY.Intervention | Within-trial QALYs for REACT arm | 1.388 | 1.364 | 1.419 | |||
| InterventionCost.Control | Intervention costs (control arm) | 0 | |||||
| InterventionCost.Intervention | Intervention costs (REACT arm) | 621.83 | 402.42 | 888.22 | Gamma | Shape 25 | Scale 24.87 |
| p_survive | Probability of surviving 1 year (given at age 75; varies with age) | 0.978 | |||||
| Parameter | Variance-covariance matrix | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPPB.M12_SPPB_FinalScore | 0.004 | 0.000 | 0.000 | –0.002 | 0.000 | 0.030 | 0.031 | 0.033 | 0.035 | 0.038 | 0.039 | 0.043 | 0.045 | 0.047 | 0.049 | 0.051 | 0.053 |
| SPPB.Allocation_Site.Birmingham | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.027 | 0.027 | 0.028 | 0.028 | 0.028 | 0.028 | 0.028 | 0.028 | 0.027 | 0.027 | 0.027 | 0.027 |
| SPPB.Allocation_Site.Devon | 0.000 | 0.000 | 0.050 | 0.004 | –0.004 | 0.059 | 0.061 | 0.058 | 0.054 | 0.058 | 0.061 | 0.060 | 0.060 | 0.060 | 0.057 | 0.056 | 0.054 |
| SPPB.SCREENING_AgeAtRandomisation | –0.002 | 0.000 | 0.004 | 0.091 | 0.022 | 0.012 | 0.015 | 0.016 | 0.014 | 0.016 | 0.018 | 0.015 | 0.012 | 0.013 | 0.013 | 0.013 | 0.011 |
| SPPB.SCREENING_Sex.Female | 0.000 | 0.000 | –0.004 | 0.022 | 0.056 | 0.018 | 0.013 | 0.014 | 0.015 | 0.019 | 0.021 | 0.023 | 0.023 | 0.022 | 0.021 | 0.023 | 0.027 |
| SPPB._cut1 | 0.030 | 0.027 | 0.059 | 0.012 | 0.018 | 2.472 | 2.402 | 2.402 | 2.379 | 2.402 | 2.409 | 2.394 | 2.391 | 2.376 | 2.374 | 2.358 | 2.410 |
| SPPB._cut2 | 0.031 | 0.027 | 0.061 | 0.015 | 0.013 | 2.402 | 2.482 | 2.456 | 2.424 | 2.437 | 2.443 | 2.426 | 2.425 | 2.407 | 2.406 | 2.389 | 2.436 |
| SPPB._cut3 | 0.033 | 0.028 | 0.058 | 0.016 | 0.014 | 2.402 | 2.456 | 2.528 | 2.489 | 2.499 | 2.502 | 2.487 | 2.486 | 2.470 | 2.469 | 2.456 | 2.503 |
| SPPB._cut4 | 0.035 | 0.028 | 0.054 | 0.014 | 0.015 | 2.379 | 2.424 | 2.489 | 2.502 | 2.507 | 2.506 | 2.491 | 2.492 | 2.476 | 2.476 | 2.462 | 2.511 |
| SPPB._cut5 | 0.038 | 0.028 | 0.058 | 0.016 | 0.019 | 2.402 | 2.437 | 2.499 | 2.507 | 2.563 | 2.557 | 2.541 | 2.542 | 2.527 | 2.526 | 2.514 | 2.563 |
| SPPB._cut6 | 0.039 | 0.028 | 0.061 | 0.018 | 0.021 | 2.409 | 2.443 | 2.502 | 2.506 | 2.557 | 2.576 | 2.559 | 2.558 | 2.543 | 2.543 | 2.530 | 2.581 |
| SPPB._cut7 | 0.043 | 0.028 | 0.060 | 0.015 | 0.023 | 2.394 | 2.426 | 2.487 | 2.491 | 2.541 | 2.559 | 2.566 | 2.564 | 2.548 | 2.548 | 2.537 | 2.588 |
| SPPB._cut8 | 0.045 | 0.028 | 0.060 | 0.012 | 0.023 | 2.391 | 2.425 | 2.486 | 2.492 | 2.542 | 2.558 | 2.564 | 2.581 | 2.563 | 2.563 | 2.552 | 2.603 |
| SPPB._cut9 | 0.047 | 0.027 | 0.060 | 0.013 | 0.022 | 2.376 | 2.407 | 2.470 | 2.476 | 2.527 | 2.543 | 2.548 | 2.563 | 2.566 | 2.565 | 2.553 | 2.606 |
| SPPB._cut10 | 0.049 | 0.027 | 0.057 | 0.013 | 0.021 | 2.374 | 2.406 | 2.469 | 2.476 | 2.526 | 2.543 | 2.548 | 2.563 | 2.565 | 2.586 | 2.571 | 2.621 |
| SPPB._cut11 | 0.051 | 0.027 | 0.056 | 0.013 | 0.023 | 2.358 | 2.389 | 2.456 | 2.462 | 2.514 | 2.530 | 2.537 | 2.552 | 2.553 | 2.571 | 2.582 | 2.627 |
| SPPB._cut12 | 0.053 | 0.027 | 0.054 | 0.011 | 0.027 | 2.410 | 2.436 | 2.503 | 2.511 | 2.563 | 2.581 | 2.588 | 2.603 | 2.606 | 2.621 | 2.627 | 2.769 |
| Parameter | Variance–covariance matrix | ||
|---|---|---|---|
| EQ5D.SPPB_Spline1 | 5.24E-05 | –4.6E-05 | –0.00033 |
| EQ5D.SPPB_Spline2 | –4.6E-05 | 5.05E-05 | 0.000267 |
| EQ5D._cons | –0.00033 | 0.000267 | 0.002181 |
| Parameter | Variance–covariance matrix | |||
|---|---|---|---|---|
| AnnualCost.SPPB.0_3 | 1,018,202 | 12,910 | 3949 | –7816 |
| AnnualCost.SPPB.4_7 | 12,910 | 35,128 | 7045 | 5855 |
| AnnualCost.SPPB.8_9 | 3949 | 7045 | 32,551 | 5919 |
| AnnualCost.SPPB.10_12 | –7816 | 5855 | 5919 | 26,609 |
Model-based economic evaluation results
Results are presented in Tables 48–50.
| State | Control arm | Intervention arm | Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Life-years | QALYs | Costs (£) | Life-years | QALYs | Costs (£) | Life-years | QALYs | Costs (£) | |
| Within-trial | |||||||||
| 2.00 | 1.31 | 4046 | 2.00 | 1.35 | 3943 | = 0.000 | + 0.040 | –103 | |
| Markov model extrapolation | |||||||||
| 0 | 0.09 | 0.02 | 188 | 0.09 | 0.02 | 167 | –0.007 | –0.003 | –21 |
| 1 | 0.09 | 0.03 | 197 | 0.08 | 0.02 | 169 | –0.010 | –0.004 | –28 |
| 2 | 0.18 | 0.06 | 394 | 0.16 | 0.05 | 345 | –0.017 | –0.007 | –49 |
| 3 | 0.25 | 0.09 | 564 | 0.22 | 0.08 | 497 | –0.023 | –0.010 | –66 |
| 4 | 0.85 | 0.33 | 1148 | 0.78 | 0.29 | 1035 | –0.068 | –0.032 | –113 |
| 5 | 0.86 | 0.36 | 1211 | 0.80 | 0.33 | 1110 | –0.060 | –0.030 | –101 |
| 6 | 1.55 | 0.70 | 2233 | 1.47 | 0.66 | 2100 | –0.078 | –0.042 | –133 |
| 7 | 1.96 | 0.96 | 2925 | 1.91 | 0.93 | 2829 | –0.054 | –0.032 | –97 |
| 8 | 2.57 | 1.35 | 3390 | 2.58 | 1.35 | 3398 | 0.011 | 0.003 | 8 |
| 9 | 1.81 | 1.01 | 2480 | 1.90 | 1.06 | 2607 | 0.089 | 0.052 | 127 |
| 10 | 0.94 | 0.55 | 1169 | 1.04 | 0.62 | 1305 | 0.105 | 0.065 | 136 |
| 11 | 0.47 | 0.29 | 607 | 0.56 | 0.35 | 731 | 0.093 | 0.060 | 124 |
| 12 | 0.05 | 0.04 | 75 | 0.07 | 0.05 | 101 | 0.019 | 0.013 | 27 |
| Total | 13.68 | 7.11 | 20,627 | 13.68 | 7.18 | 20,338 | 0.000 | 0.072 | –290 |
| Group | Discounted cost (£) | Discounted QALYs | Net monetary benefit (£) | ICER (£/QALY) |
|---|---|---|---|---|
| Control | 20,627 | 7.111 | 121,594 | |
| Intervention | 20,338 | 7.183 | 123,330 | |
| Difference | –290 | 0.072 | 1735 | Dominant |
| Population | Incremental costs | Incremental QALYs | INMB | ICER |
|---|---|---|---|---|
| Base case (75-year-old woman) | –290 | 0.072 | 1735 | Dominant |
| REACT study participants | –296 | 0.071 | 1718 | Dominant |
| UK general population aged 65–100 years | –273 | 0.068 | 1631 | Dominant |
Heterogeneity analysis
As demonstrated in heterogeneity analysis, the intervention is least cost-effective in 65-year-old men, so we repeated the PSA in 65-year-old men. All other parameters were left unchanged from the original PSA.
The 95% credible intervals for INMB and the ICER were £387 to £2811 and –£14,133 (dominant) to £10,147 per QALY, respectively.
Figures 41 and 42 show the cost-effectiveness scatter plot and cost-effectiveness acceptability curve.
FIGURE 41.
Probabilistic sensitivity analysis in 65-year-old men: cost-effectiveness plane.
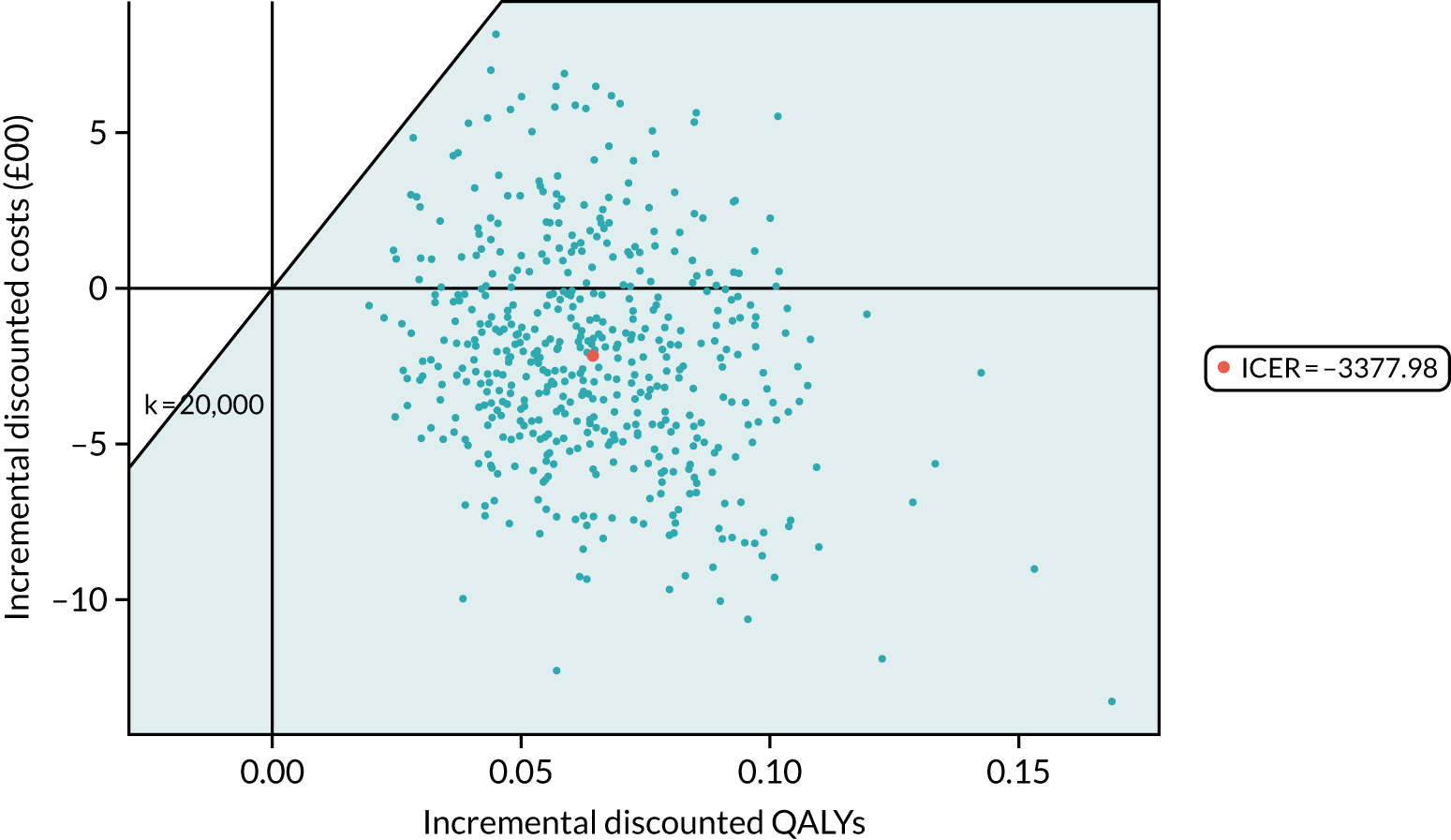
FIGURE 42.
Probabilistic sensitivity analysis in 65-year-old men: cost-effectiveness acceptability curve.
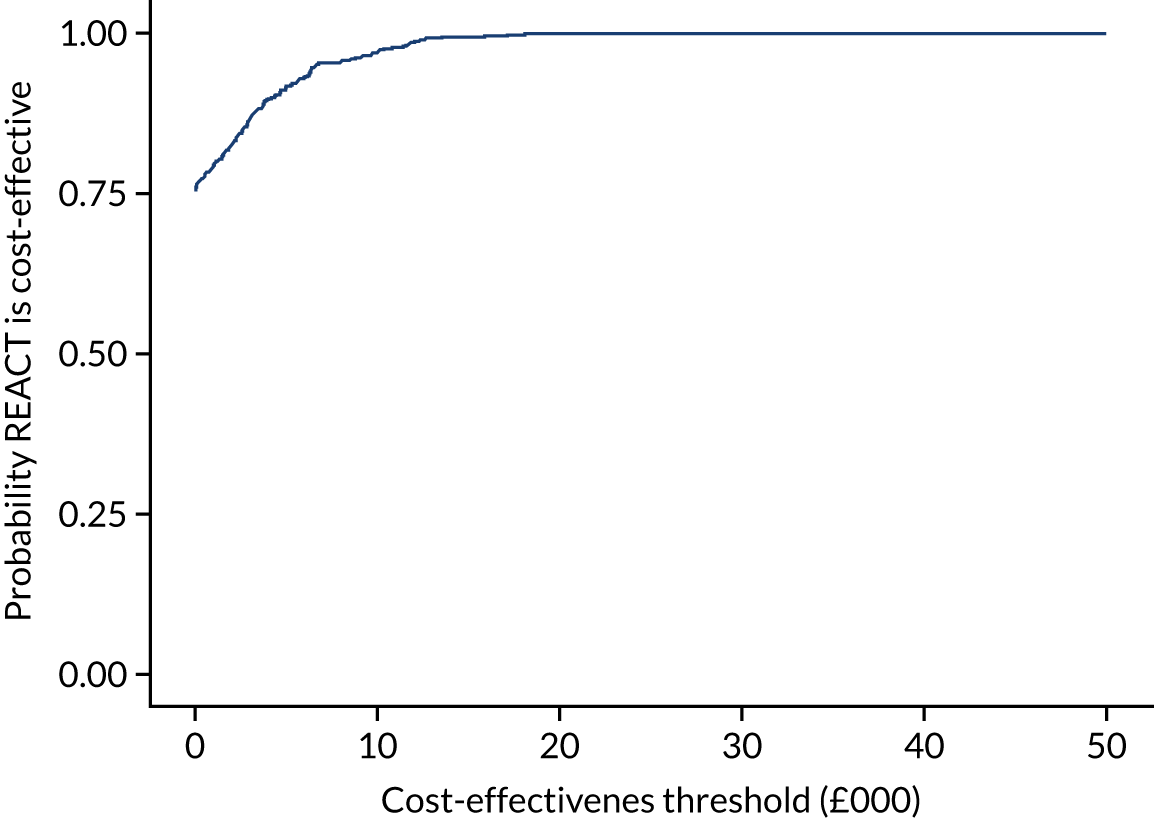
List of abbreviations
- A&E
- accident and emergency
- ADL
- activities of daily living
- ANCOVA
- analysis of covariance
- BCT
- behaviour change technique
- BMI
- body mass index
- CCA
- complete-case analysis
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HOT
- higher-order theme
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- ID
- identification
- IMD
- Index of Multiple Deprivation
- INMB
- incremental net monetary benefit
- ITT
- intention to treat
- LIFE
- Lifestyle Interventions and Independence for Elders
- MAT-SF
- Mobility Assessment Tool-Short Form
- MeSH
- medical subject heading
- MI
- multiple imputation
- MoCA
- Montreal Cognitive Assessment
- MRI
- magnetic resonance imaging
- MSEQ
- Muscle-Strengthening Exercise Questionnaire
- MVPA
- moderate to vigorous physical activity
- NCC
- National Cost Collection
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- OR
- odds ratio
- PASE
- Physical Activity Scale for the Elderly
- PIS
- participant information sheet
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QWB-SA
- Quality of Well-Being Self-Administered
- RA
- research assistant
- RCT
- randomised controlled trial
- REACT
- REtirement in ACTion
- REPS
- Register of Exercise Professionals
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SCT
- social cognitive theory
- SDT
- self-determination theory
- SES
- socioeconomic status
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SPPB
- Short Physical Performance Battery
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- WOMAC
- Western Ontario and McMaster Universities Osteoarthritis Index
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/MQBW6832).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
