Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 15/190/42. The contractual start date was in June 2017. The final report began editorial review in September 2021 and was accepted for publication in May 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research, or similar, and contains language which may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Clemes et al. This work was produced by Clemes et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Clemes et al.
Chapter 1 Introduction
Background and rationale
Truck driving is essential to the economy. Approximately 75% of all goods delivered in the UK are transported via road freight, with the road freight transport sector contributing over £13B to the UK economy. 1 The UK logistics sector currently employs just under 300,000 heavy goods vehicle (HGV) drivers, with a HGV being defined as having a gross vehicle weight between 3.5 and 44 tonnes. 1 Owing to the nature of their occupation, long-distance HGV drivers are exposed to a multitude of health-related risk factors and have been identified as working within one of the most hazardous professions. 2,3 The working environment of long-distance HGV drivers and their job demands (i.e. long irregular hours, enforced sedentarism, poor dietary options, high stress) constrain the enactment of healthy behaviours, leaving drivers vulnerable to a myriad of physical and mental health conditions. 4
Our own systematic review-level evidence has shown that HGV drivers globally exhibit high levels of physical inactivity and accumulate large amounts of sedentary (sitting) behaviour. HGV drivers also tend to make poor dietary choices, have high alcohol intakes and have a high prevalence of smoking. 4 Furthermore, long and variable working hours, including shift work, contributes to sleep deprivation,5,6 and this can lead to metabolic disturbances and further promote the uptake of unhealthy behavioural choices. 3,5–8 The isolated nature of driving a HGV can result in a lack of peer social support and poor mental health. 9,10 Within this occupational group, adverse mental health conditions can be exacerbated by intense job demands and low levels of perceived job control, as a result of chronic time pressures, compounded by tight delivery schedules and traffic conditions. 11 Indeed, our systematic review identified high levels of mental ill-health within HGV drivers. 4
As a result of HGV drivers’ working environment and poor health behaviours, review-level evidence has demonstrated that they nationally and internationally exhibit high rates of obesity and cardiometabolic risk factors. 4,12–14 In addition to elevating their risk of cardiovascular disease (CVD) and type 2 diabetes, the incidence of obesity-related comorbidities in HGV drivers is increasing, suggesting that the trajectory of HGV driver health is declining. 2,3,15–18 These factors likely culminate in HGV drivers having an increased risk of accidents, higher rates of chronic diseases and reduced life expectancies in comparison with other occupational groups. 2,19–24 Despite this, HGV drivers are currently underserved in terms of health promotion efforts. 25
To compound the high-risk health profile observed in HGV drivers nationally and internationally,4,12–14 within the UK’s logistics sector, HGV drivers are an ageing workforce, with an average age of 48 years. 26 A report prepared by an All Party Parliamentary Group for Freight Transport has highlighted the challenges that the industry is facing with an ageing workforce, and the health impact of this ageing, at-risk workforce driving such large and potentially dangerous vehicles. 27
The UK’s logistics sector is also experiencing a serious shortfall in HGV drivers, which has recently been described as reaching a ‘crisis point’, with this shortage rising from 60,000 drivers in 201528 to an estimated 100,000 drivers in 2021. 29 Factors responsible for the sharp decrease in driver numbers include the uncertainties around Brexit, with a number of European drivers returning home; the COVID-19 pandemic, with the resulting national lockdowns further encouraging international drivers to return to their home countries and seeing HGV licence testing suspended; and a large number of drivers retiring. 29 Barriers to driver recruitment have been reported to include a lack of roadside facilities, medical concerns and long working hours. 27 Recommendations on how to address this shortfall and attract younger employees to the sector made by the All Party Parliamentary Group for Freight Transport include increasing awareness within the industry of the need to address driver health risks and health behaviours. 27 Indeed, now more than ever, the government and sector urgently need to address working conditions and the poor health profile of this ageing workforce to attract employees to the role. Driver recruitment and a prioritisation of driver health is essential to combat the current challenges seen in maintaining critical supply chains.
A systematic review25 of health promotion interventions in HGV drivers, including only eight studies, observed that the interventions generally led to improvements in health and health-related behaviours. However, the review25 concluded that the strength of the evidence was limited because of poor study designs, no control groups, small samples and no or limited follow-up periods. 25 Since the publication of the systematic review,25 studies have examined the impact of a weight loss intervention in US HGV drivers30 and a smartphone application (app) on physical activity and diet in Australian HGV drivers. 31 Although positive findings were observed, the studies were limited by having relatively small samples and no comparison groups. It has been suggested that health and well-being programmes that focus on health education and improvements in health literacy should be implemented and prioritised across the logistics industry. 4 For example, international research has shown that HGV drivers with higher educational levels are more likely to have higher levels of physical activity32 and lower body mass index (BMI)33 than HGV drivers with lower levels of education. Where they exist, health and well-being programmes within the logistics industry have been considered to have the potential to have a positive impact on employee health4,25 and, in turn, potentially benefit employers through increased employee retention and reductions in health-care costs. 4 Furthermore, health promotion initiatives targeting HGV drivers will likely have a broader public health impact through improving road safety for all users. 25 Research in the USA, for example, has shown that HGV drivers with obesity were 55% more likely to have an accident than normal-weight drivers. 34 In the UK, although only accounting for 12% of all vehicle traffic on motorways, 41% of accident-related fatalities involved HGVs in 2017,35 highlighting the wider public safety impact of health improvement programmes in this at-risk occupational group.
Development of the SHIFT programme
We developed the Structured Health Intervention For Truckers (SHIFT) programme, which is a multicomponent theory-driven health behaviour intervention designed to promote positive lifestyle changes in relation to physical activity, diet and sitting in HGV drivers. This SHIFT intervention has been informed by extensive public and patient involvement (PPI), which has included drivers and relevant stakeholders, a qualitative study exploring the perceived barriers to healthy lifestyle behaviours in drivers,7 an observational study (n = 157) exploring lifestyle health-related behaviours in HGV drivers and markers of health,36 and a pre–post pilot intervention (n = 57)37 with a full process evaluation. 38 Initial pilot testing of our intervention delivery, over a 3-month period, revealed potentially favourable increases in physical activity, with 81% of the sample increasing their daily step counts by an average of 1646 [standard deviation (SD) 2156] steps per day. Significant increases in fruit and vegetable intake were also observed (4.5 vs. 5.4 portions/day), along with favourable changes in markers of cardiometabolic health. 37
The current study extends this work by evaluating the multicomponent SHIFT programme within a cluster randomised controlled trial (RCT), with the inclusion of full process and cost-effectiveness evaluations. As the intervention was administered within the worksite setting, a cluster RCT design was employed with delivery sites/depots (i.e. individual worksites) as the unit of allocation to minimise any potential contamination occurring between intervention and control participants.
Aim and objectives
The aim of this study was to evaluate the effectiveness and cost-effectiveness of the multicomponent SHIFT programme, compared with usual care, in a sample of long-distance HGV drivers at both 6 months and 16–18 months.
Primary objective
-
To investigate the impact of the 6-month SHIFT programme, compared with usual care, on device-measured physical activity (expressed as steps/day) at 6 months’ follow-up.
Secondary objectives
-
To investigate the impact of the SHIFT programme, compared with usual care, at 6 months’ follow-up on:
-
time spent in light physical activity and moderate or vigorous physical activity (MVPA)
-
sitting time
-
measures of adiposity (i.e. BMI, per cent body fat, waist–hip ratio, neck circumference)
-
cardiometabolic risk markers [i.e. glycated haemoglobin (HbA1c), total cholesterol, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C)]
-
fruit and vegetable intake and dietary quality
-
blood pressure
-
psychophysiological reactivity
-
sleep duration and quality
-
functional fitness (i.e. grip strength)
-
cognitive function
-
mental well-being (i.e. anxiety and depression symptoms, and social isolation)
-
work-related psychosocial variables (i.e. work engagement, job performance and satisfaction, occupational fatigue, presenteeism, sickness absence and driving-related safety behaviour)
-
health-related quality of life (HRQoL)
-
health-related resource use [i.e. general practitioner (GP) visits].
-
-
To investigate the longer-term impact of the SHIFT programme, compared with usual care, at 16–18 months’ follow-up on:
-
steps per day
-
time spent in light physical activity and in MVPA
-
sitting time
-
fruit and vegetable intake and dietary quality
-
sleep
-
mental well-being (i.e. anxiety and depression symptoms, and social isolation)
-
work-related psychosocial variables (i.e. work engagement, job performance and satisfaction, occupational fatigue, presenteeism, sickness absence and driving-related safety behaviour)
-
HRQoL.
-
-
To conduct a mixed-methods process evaluation throughout the implementation of the intervention (using qualitative and quantitative measures) with participating drivers and site managers.
-
To undertake a full economic analysis of the SHIFT programme.
Chapter 2 Study design and methods
This chapter summarises the study protocol for this RCT as originally funded. Some of the material, including tables and figures, has already appeared in Clemes et al. 39 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Study design and setting
The SHIFT trial was a two-armed cluster RCT, which incorporated an internal pilot phase and included a mixed-methods process and economic evaluations. The trial was registered with the International Standard Randomised Controlled Trial Number registry before participant recruitment commenced (URL: www.isrctn.com/ISRCTN10483894; accessed 13 July 2021). The trial protocol paper was published in November 2019,39 and protocol revisions can be accessed via the National Institute for Health and Care Research (NIHR) Journals Library (URL: www.journalslibrary.nihr.ac.uk/programmes/phr/1519042/; accessed 13 July 2021). A summary of the amendments to the original protocol are listed in Table 1.
| Amendment number | Date | Description |
|---|---|---|
| 1 | 8 November 2018 | Owing to one pilot site [a BP (London, UK) site] not allowing participants to wear the accelerometers during working hours for health and safety reasons and, therefore, limiting the collection of the primary outcome measure [i.e. activPAL™ (PAL Technologies Ltd, Glasgow, UK)-determined steps/day] to non-working hours only, the TSC approved the recruitment of an additional site in the main trial phase. The total site recruitment target changed from 24 to 25 |
| 2 | 5 April 2019 | Owing to the time needed to undertake baseline measurements in the main trial phase, sites (i.e. clusters) were randomised into the study arms in blocks of three following completion of baseline measures, as opposed to randomising all sites after all baseline measures were completed |
| 3 | 13 July 2020 |
Owing to COVID-19, face-to-face 12-month follow-up measures were no longer viable in the majority of sites. The primary outcome was assessed following completion of the 6-month intervention, with the sustainability of the intervention assessed by the self-report questionnaire-based measures at approximately 10–12 months following intervention completion The process evaluation conducted with sites within the main trial phase involved telephone interviews as opposed to face-to-face interviews and/or focus groups An additional ‘COVID-19’ online questionnaire was distributed to participants in May–June 2020 The trial was extended by 15 months because of delays due to COVID-19 |
The trial took place within the worksite setting of a major international logistics and transport company, DHL Supply Chain (Milton Keynes, UK). DHL Supply Chain agreed to provide the setting and gave access to their drivers and sites for our research. Transport sites/depots formed individual clusters. Following the completion of baseline measurements, clusters were randomised 1 : 1 to receive either the SHIFT programme or to continue with usual practice (i.e. the control condition). Outcome measurements were undertaken at baseline and at 6 months’ follow-up. A third set of outcome measures were originally planned to take place 6 months following completion of the intervention (i.e. 12 months’ follow-up); however, owing to the COVID-19 pandemic, these measurements were unable to be completed within this time frame for the majority of sites. As a result, the primary outcome was assessed following completion of the 6-month intervention (at 6 months’ follow-up) to mitigate potential confounding factors associated with the pandemic, along with a threat of increased rates of loss to follow-up caused by drivers on furlough/isolating or drivers being re-deployed. The easing of government COVID-19 restrictions enabled a range of secondary outcome measures to be collected approximately 10–12 months following completion of the intervention (i.e. 16–18 months’ follow-up), informing an assessment of the potential longer-term impact of the intervention. The study methods are reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) extension statement for cluster RCTs. 40
Ethics approval
The trial was approved by the Loughborough University Ethics Approvals (Human Participants) Sub-Committee (reference R17-P063). Loughborough University (Loughborough, UK) sponsored the study.
Cluster recruitment and eligibility
The health and safety director of DHL Supply Chain, UK and Ireland, nominated individual DHL Supply Chain transport sites/depots for participation in this study. Sites were eligible for participation if they contained at least 20 long-distance HGV drivers and were located within a 2-hour drive of Loughborough University. Depots containing HGV drivers who made many delivery stops, for example drivers who delivered consumer goods to domestic customers throughout the day, were excluded. During enrolment into the study, transport managers were informed that their site would have a 50% chance of being randomised to the current practice control condition.
Participant recruitment
Within the nominated sites, transport managers were provided with recruitment material to promote the study. Posters advertising the study were displayed in participating sites for up to 4 weeks prior to the scheduling of baseline measurements. In addition, all drivers within participating sites received a letter and a participant information sheet informing them of the study. Following the distribution of the marketing material (e.g. posters and participant information sheets), members of the research team visited each site for at least 1 day. During these visits, the research team had stands in the lobby area with posters showcasing the study, along with example materials used in the SHIFT education session (see The SHIFT programme) and example devices used as part of the outcome measures (e.g. a grip strength dynamometer). Interested drivers could ask the research team any questions about the study before providing a member of the research team their name, if they were interested in taking part. On completion of these visits, the researchers provided a list of the drivers’ names who had signed up to the trial to their transport manager, who then scheduled a time for participating drivers within their sites to attend the baseline (and follow-up) measurements. The baseline measurements were scheduled for at least 1 week after the site recruitment visits to enable drivers to have sufficient time to fully decide on their willingness to participate. All outcome measurements were undertaken in a private room at each participating DHL Supply Chain site. In the UK logistics industry, 1% of HGV drivers are women. 41 At the time of participant recruitment, the proportion of female HGV drivers employed by DHL Supply Chain reflected this national average. All drivers (male and female) at participating sites were invited to participate in this study.
Participant eligibility
All HGV drivers within participating sites were eligible to participate, unless they met any of our exclusion criteria. Drivers were excluded from the trial if they were suffering from clinically diagnosed CVD, had mobility limitations that prevented them from increasing their daily activity levels, were suffering from haemophilia or any blood-borne virus, or were unable to provide written informed consent.
Informed consent
During the baseline measurement session, the study details were verbally reiterated to potential participants, including full details of the study procedures. The expectations of participating in the trial were explained, along with participants’ right to withdraw. This information was provided by a member of the research team who was suitably qualified and who was authorised to do so by the principal investigator. Written informed consent was obtained prior to any measurements being taken at baseline, and at each follow-up assessment.
Trial allocation arms
The SHIFT programme
The SHIFT programme is a multicomponent lifestyle–behaviour intervention that is designed to target behaviour changes in physical activity, diet and sitting in HGV drivers. The 6-month intervention, grounded within social cognitive theory (SCT) for behaviour change,42 consists of a group-based (4–6 participants) 6-hour structured education session, tailored for HGV drivers and delivered by two trained educators. The education session includes information about physical activity, diet and sitting, and details risk factors for type 2 diabetes and CVD. The educational component is founded on the approach used in the award-winning suite of DESMOND (Diabetes Education and Self-Management for Ongoing and Newly Diagnosed) programmes, including the PREPARE (Prediabetes Risk Education and Physical Activity Recommendation and Encouragement)43 and Let’s Prevent Diabetes programmes,44 created by researchers at the Leicester Diabetes Centre (Leicester, UK) and used throughout the NHS,45 while being tailored to meet the needs of HGV drivers. 7 During the education session, participants are not ‘taught’ in a formal way but are supported to work out knowledge through group discussions. Participants are also encouraged to develop individual goals and plans based on detailed individual feedback received during their health assessments (see Outcome measurements) to achieve over the 6-month intervention period. The education session is supported by specially developed resources and participant support materials for HGV drivers. The education session includes the discussion of feasible strategies for participants to increase their physical activity, improve their diet and reduce their sitting time (when not driving) during working and non-working hours. The content of the educational session is summarised in Table 2.
| Section name | Theoretical underpinning | Main aims and educator activities | Duration (minutes) |
|---|---|---|---|
| Welcome and introduction | Participants are introduced to the SHIFT programme and are made aware of both the content and style of the session | 10 | |
| Driver story | Dual process theory46 and common sense model47 | Participants are asked about their beliefs about how being a HGV driver can affect health, the causes of these health problems and controllability of these problems | 30 |
| Risks and health problems | Dual process theory,46 common sense model47 and social learning theory48 | The facilitator uses participant stories to help participants work out why they may be at risk of future health problems, and what to do to reduce/manage risk | 55 |
| Physical activity | Dual process theory46 and social learning theory48 |
The facilitator supports participants to develop knowledge and skills to support confidence, to increase personal activity levels and to set personal goals, which can be self-monitored through the use of a Fitbit® (Fitbit Inc., San Francisco, CA, USA) Introduction and practical demonstration of the ‘cab workout’ |
80 |
| Depression, sleeping, smoking | Dual process theory46 and social learning theory48 | The facilitator supports participants to develop strategies to manage depression, poor sleep and smoking | 30 |
| Food choices | Dual process theory46 and social learning theory48 | The facilitator supports participants to develop knowledge and skills for food choices to reduce cardiovascular risk factors and to improve overall health | 90 |
| Self-management plan | Dual process theory46 and social learning theory48 | Participants are supported in developing personal self-management plans | 15 |
| Questions | Common sense model47 and social learning theory48 | The facilitator checks that all questions raised by participants throughout the programme have been answered and understood | 5 |
| What happens next | Social learning theory48 | Follow-up care is outlined | 5 |
During the education session, participants were provided with a Fitbit Charge 2 (Fitbit, Inc., San Francisco, CA, USA) activity tracker. Participants were encouraged to use the Fitbit activity tracker to set goals (agreed at the session) and gradually increase their physical activity, predominantly through walking-based activity. The Fitbit activity tracker and associated smartphone app provided participants with information on their daily step counts and was used as a tool for self-monitoring and self-regulation. Physical activity tracking using step counters (traditionally pedometers) has been associated with significant reductions in BMI and blood pressure, with interventions incorporating goal-setting being the most effective. 49 Participants were provided with instructions on how to link their Fitbit account to an online monitoring system (Fitabase, Small Steps Labs LLC, San Diego, CA, USA). Participants were encouraged to link their account to Fitabase and to regularly upload their Fitbit data from their device to their mobile phone via Bluetooth. When participants sync their Fitbit through the Fitbit app, their step count data are automatically updated on the Fitabase website. Participants’ data on the Fitabase website were accessible to only two members of the research team, who used the step count data to provide participants with individually tailored step count challenges throughout the 6-month intervention period.
The education session adopted the promotion of the ‘small changes’ philosophy, using the specific, measurable, achievable, relevant, time bound (SMART) principle50 to encourage participants to gradually build-up their daily activity levels, within the confines of their occupation, to meet the UK physical activity guidelines. 51 For example, participants were encouraged to establish their own personalised action plan, which may have included making dietary improvements in addition to increases in physical activity, with SMART goals throughout the 6-month intervention. ‘Step count challenges’ were run every 6 weeks throughout the 6-month intervention and were facilitated by members of the research team via a text messaging service (TextMagic™, TextMagic Ltd, Cambridge, UK).
A ‘cab workout’ was introduced and practised at the education session, and participants were provided with resistance bands and balls, and grip strength dynamometers to take away. Participants were encouraged to undertake the cab workout during breaks when they were not permitted to leave their vehicle. Participants were able to keep the intervention tools beyond the 6-month intervention period; however, the step count challenges, as well as the supportive text messages sent by members of the research team, ended after the 6-month intervention period. A logic model detailing the underlying theory behind the intervention components is shown in Figure 1.
FIGURE 1.
A logic model for the SHIFT programme. CPC, Certificate of Professional Competence; PA, physical activity.
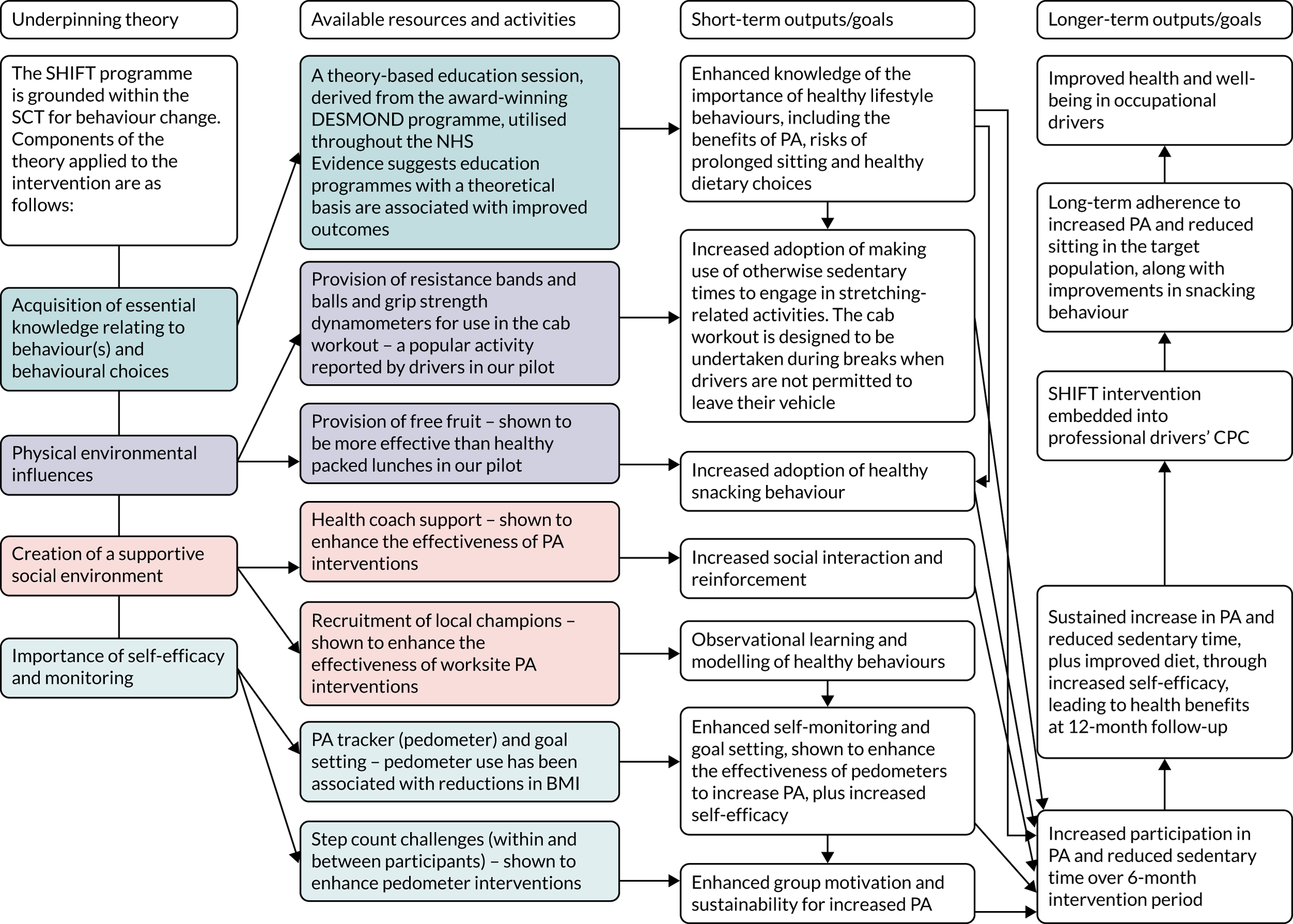
The structured education session was delivered by trained members of the research team in collaboration with trained personnel from DHL Supply Chain. Individuals from DHL Supply Chain co-delivering the education session were predominantly HGV drivers who also acted as driver trainers in each site as part of their role. The ‘driver trainers’ were trained by specialist educators from the Leicester Diabetes Centre and mentored by trained members of the research team. The education sessions took place within appropriate training rooms within the intervention depots. Personnel co-delivering the education sessions in each intervention depot were also trained to act as local champions, which has been shown to enhance the effectiveness of worksite physical activity interventions. 52 They provided ongoing health coach support, along with members of the research team (who provided support via the text messaging service), to intervention participants (during the 6-month intervention period).
The control arm
Sites assigned to the control arm (i.e. usual practice) were asked to continue with their usual-practice conditions. Participants in the control sites received an educational leaflet at the outset, detailing the importance of healthy lifestyle behaviours (i.e. undertaking regular physical activity, breaking up periods of prolonged sitting and consuming a healthy diet) for the promotion of health and well-being. Control participants completed the same study measurements as participants in the intervention worksites, at the same time points, and received the same health feedback immediately following their health assessments (i.e. outcome measurements).
Outcome measurements
This section describes the outcome measurements, as explained in the original trial protocol. 39 The outcome measurements were undertaken as intended at baseline and following the completion of the 6-month intervention for all sites bar one intervention site, where these measurements had been due to take place the same week as the first national lockdown commenced. A change in protocol was required for the final set of measurements, originally intended to take place 6 months following completion of the intervention (i.e. 12 months’ follow-up). The protocol for these measurements is described below.
Protocol for the outcome measurements assessed at baseline and at 6 months’ follow-up
Baseline measurements took place prior to randomisation of the sites into the two study arms. A second set of identical measurements occurred at 6 months’ follow-up. The two sets of measurements were undertaken in suitable rooms within participating DHL Supply Chain sites by trained researchers and lasted approximately 2 hours per participant. Participants were scheduled to attend these measurements, during their working time by their transport manager either before or following their driving shift.
Participants completed a range of self-report questionnaires and had a series of physiological health assessments taken (described below) at baseline and immediately following the completion of the 6-month intervention. Participants were also issued with two devices [an activPAL and a GENEActiv (Activinsights, Kimbolton, UK) accelerometer] to wear over a period of 8 days following the measurement sessions. Participants received detailed feedback on their physiological health assessment measures during these two measurement sessions. If a potential health issue was evident during the measurements, such as undiagnosed hypertension or high cholesterol levels, then participants were advised to visit their GP for further checks. A standard referral letter was provided for participants to give to their GP, which summarised the findings from our point-of-care (i.e. blood markers) and automated (i.e. blood pressure) measures.
Protocol for the 16- to 18-month follow-up assessments (undertaken during the COVID-19 pandemic)
A third set of outcome measures were originally planned to take place 6 months following completion of the intervention (i.e. a 12-month follow-up); however, owing to the COVID-19 pandemic, these measurements were unable to be completed within this time frame. The easing of government COVID-19 restrictions enabled a range of secondary outcome measures to be collected at 16–18 months (approximately 10–12 months following completion of the intervention). Owing to restrictions on external visitors to DHL Supply Chain sites throughout the pandemic, face-to-face physiological measurements were not able to be conducted at the final follow-up phase. Instead, the case report form (CRF), which contained a series of self-report questionnaires and recording sheets for the physiological measures used during data collection at baseline and immediately following the intervention (see Report Supplementary Material 1), was modified into a self-administered questionnaire booklet (see Report Supplementary Material 2).
Individual participant packs were prepared, which contained an instruction leaflet, a questionnaire booklet, a consent form, an activPAL and logbook, and a return envelope. On prior arrangement with transport managers, a member of the research team delivered the participant packs to each site. The transport managers distributed the packs to participating drivers, who completed the relevant paperwork in their own time and, on request, wore the activPAL for a period of 8 continuous days. After this 8-day period, participants returned their activPAL and their completed logbook, questionnaire booklet and consent form in a sealed envelope to a collection point within their site. Once all packs were returned, the packs were collected from the site by a member of the research team. This protocol was also followed for the one remaining intervention site for its 6-month follow-up (which had initially been due to take place at the beginning of the first national lockdown and, therefore, was unable to be completed as intended). Table 3 summarises all measurements collected at the three time points during the trial. All measurements are described in detail in the following sections.
| Information collected | Time point | ||
|---|---|---|---|
| Baseline | 6-month follow-up | 16- to 18-month follow-up | |
| Informed consent | ✗ | ✗ | ✗ |
| Physiological measures (i.e. blood pressure, height, weight, body composition, grip strength, finger-prick blood samples, waist, hip and neck circumferences) | ✗ | ✗ | Self-reported weight only |
| Cognitive function and psychophysiological reactivity | ✗ | ✗ | |
| Health Screen Questionnaire and medication use | ✗ | ✗ | Medication use only |
| Demographic information | ✗ | ✗ | ✗ |
| QRISK3 | ✗ | ✗ | |
| Short-form FFQ | ✗ | ✗ | ✗ |
| Smoking and alcohol use | ✗ | ✗ | ✗ |
| Nordic Musculoskeletal Questionnaire | ✗ | ✗ | ✗ |
| HADS | ✗ | ✗ | ✗ |
| Social Isolation Short Form | ✗ | ✗ | ✗ |
| Utrecht Work Engagement Scale | ✗ | ✗ | ✗ |
| OFER scale | ✗ | ✗ | ✗ |
| Job satisfaction | ✗ | ✗ | ✗ |
| Job performance | ✗ | ✗ | ✗ |
| Self-reported sickness absence | ✗ | ✗ | ✗ |
| Self-reported presenteeism | ✗ | ✗ | ✗ |
| Work ability scale | ✗ | ✗ | ✗ |
| Work Demands Questionnaire | ✗ | ✗ | ✗ |
| Karolinska Sleepiness Scale | ✗ | ✗ | ✗ |
| MEQ | ✗ | ✗ | ✗ |
| Driver Safety Behaviour Questionnaire (self-reported) | ✗ | ✗ | ✗ |
| EQ-5D-5L | ✗ | ✗ | ✗ |
| Health-related resource use questionnaire | ✗ | ✗ | ✗ |
| activPAL | ✗ | ✗ | ✗ |
| GENEActiv | ✗ | ✗ | |
Primary outcome
The primary outcome was device-measured physical activity, expressed as average steps per day, at the 6-month follow-up (originally intended to be measured at 6 months following completion of the intervention, i.e. at 12 months). Physical activity was measured using the activPAL micro accelerometer, which provides a valid measure of walking and posture (i.e. sitting and standing) in adults. 53–55 As the physical activity component of the intervention predominantly included the promotion of walking-based activity, and as participants were provided with a Fitbit, which provided information on daily step counts and promoted goal-setting to increase daily steps, steps per day was chosen as the primary physical activity-related outcome.
We have previously observed56 that the activPAL provides a more accurate measure of physical activity and sitting in occupational drivers than waist-worn accelerometers. As a further validity check within the current trial, we attached two activPAL devices to the underneath and lateral side of a driver’s seat within a HGV cab for a 24-hour period. Vehicle movement times were extracted from the vehicle’s tachograph data, and the activPAL outputs were assessed during these time periods. No accelerations were detected by the activPAL, confirming that the device is not affected by vehicle accelerations, the suspension system or movement of the driver’s seat during driving time.
During each measurement session, participants were provided with an activPAL and requested to wear the device continuously (i.e. 24 hours/day) for the following 8 days. The activPALs were initialised using the default manufacturer settings and recorded data at a sampling frequency of 20 Hz. The device was waterproofed using a nitrile sleeve and attached (by the participant) to the midline anterior aspect of their non-dominant thigh using Hypafix® transparent dressing (BSN medical, Hull, UK). Participants were provided with a daily logbook in which they were requested to record the times that they got into bed, went to sleep, woke up and got out of bed. Participants were also requested to indicate on the logbook whether each day was a workday or a non-workday, and whether or not the activPAL had been removed for any periods (and, if so, the duration), throughout the 8-day period. Following the completion of the wear period, the activPALs and logbooks were returned to the site, where they were collated by a transport manager and, subsequently, collected by a member of the research team. activPALs were downloaded and visually checked for adequate wear, if a sufficient number of valid days of data were not obtained, then participants were contacted and asked if they would be willing to re-wear the device.
Secondary outcomes
A number of secondary outcomes were assessed during each measurement time point (see below).
Secondary activPAL variables
Sitting, standing, time in light intensity physical activity and time in MVPA were assessed using the activPAL micro accelerometer. The activPAL is regarded as the most accurate method of assessing sitting behaviour in free-living settings,55,57,58 and is recommended for use in interventions when sitting is an outcome measure. 54 From the data provided by the device, the following variables were derived by calculating the average across the number of valid days provided during each measurement period:
-
average total daily sitting time (minutes/day)
-
average total daily sitting time (minutes/day) accumulated in prolonged bouts lasting ≥ 30 minutes
-
average total daily standing time (minutes/day)
-
average total daily stepping time (minutes/day)
-
average number of transitions from sitting to an upright posture
-
average total daily time in MVPA (minutes/day), calculated as total stepping time at a step cadence threshold of 100 steps per minute (in bouts lasting ≥ 1 minute)
-
average total daily time in light physical activity (minutes/day)
-
number of valid days
-
average waking wear time (minutes/day)
-
average percentage of the day spent sitting
-
average percentage of the day spent standing
-
average percentage of the day spent stepping
-
average percentage of total sitting time spent in prolonged sitting bouts (lasting ≥ 30 minutes).
The variables below were calculated and summarised for three different time periods within each measurement period: (1) daily (i.e. across all waking hours on all valid days), (2) during workdays only and (3) during non-workdays only.
Anthropometry and markers of adiposity
Height was measured at baseline only, without shoes and to the nearest millimetre, using a portable stadiometer (seca 206, seca Ltd, Birmingham, UK). Weight (kg) and body fat percentage were assessed via bio-impedance analysis using Tanita DC-360S body composition scales (Tanita Corporation, Tokyo, Japan). A clothing allowance of 1.5 kg was entered into the scales, along with participants’ age, sex and height. BMI (kg/m2) was calculated as weight (kg)/height (m2). Waist, hip and neck circumferences (cm) were measured using standard anthropometric measuring tape (seca Ltd, Birmingham, UK), and waist-to-hip ratio was calculated.
Biochemical assessments
Capillary blood samples were collected via finger-prick blood sampling. Participants were requested to place their hand in a bowl of warm water (provided) for 5 minutes prior to the sample being collected. Participants were also requested to fast for at least 4 hours prior to attending their health assessment. HbA1c (mmol/mol) was measured using an A1CNow®+ point-of-care analyser (PTS Diagnostics, Indianapolis, IN, USA). Triglycerides (mmol/l), HDL-C (mmol/l) and total cholesterol (mmol/l) levels were assessed using a Cardiocheck® point-of-care analyser (PTS Diagnostics, Indianapolis, IN, USA). LDL-C (mmol/l) was calculated using Friedewald’s formula. 59
Dietary quality and fruit and vegetable intake
Dietary quality and fruit and vegetable intake (g/day) were assessed using a short-form Food Frequency Questionnaire (FFQ). 60 Using this measure, a dietary quality score was derived from reported fruit, vegetable, oily fish, non-milk extrinsic sugar and fat intakes. The dietary quality score calculated using this short-form FFQ has been shown to demonstrate a significant agreement (κ = 0.38) with dietary quality determined using a 217-item FFQ. 60
Sleep duration and quality, subjective sleepiness and chronotype
Sleep duration and quality were assessed using a GENEActiv tri-axial accelerometer (ActivInsights Ltd., Huntingdon, UK), which was worn (concurrently with the activPAL) on the non-dominant wrist continuously for 8 days. The GENEActiv has been shown to provide an accurate measure of sleep and activity behaviour patterns over a 24-hour period. 61 The device collected data at 100 Hz with a ± 8 g dynamic range. Participants were asked to note any time they removed this device on the same logbook used for the activPAL.
Situational sleepiness was assessed using the Karolinska Sleepiness Scale, which has been shown to be a valid measure of sleepiness when validated against electroencephalography and performance outcomes. 62,63 Participants’ chronotype was determined using the short version of the Morningness–Eveningness Questionnaire (MEQ). 64
Blood pressure
Blood pressure and heart rate were measured from the left arm of the driver after a 20-minute period of quiet sitting using an automated monitor (Omron HEM-907, Omron Corporation, Kyoto, Japan), in accordance with recommendations from the European Society of Hypertension. 65 Three separate measurements of blood pressure and heart rate were taken at 5-minute intervals. The mean systolic and diastolic blood pressures, and heart rate, recorded from the second and third assessments, were calculated and used in the analyses.
Cognitive function and psychophysiological reactivity
The Stroop test was administered over a 5-minute period using a validated software package (SuperLab 5, Cedrus Corporation, San Pedro, CA, USA) to provide a measure of reaction time, sensitivity to interference and the ability to suppress an automated response (i.e. reading colour names in favour of naming the font colour). 66 The Stroop test was utilised to provide a measure of cognitive function and as part of a battery of measures to induce acute stress to support the assessment of psychophysiological reactivity.
The mirror tracing task (Campden Instruments Auto Scoring Mirror Tracer 58024E, Campden Instruments LTD, Loughborough, UK) was used as the second stress task, which has been routinely used to induce stress in field- and laboratory-based studies. 67 The mirror tracing task immediately followed the Stroop test. The mirror tracing task involved tracing an adonised star pattern using a metal-tipped stylus with the right hand continuously for 5 minutes. Participants were, however, permitted to use only the reflection of the star in an adjacent mirror for reference. The machine beeped if the metal-tipped stylus left the star pattern, and each mistake was recorded on the machine. Participants were told to aim for at least five complete stars in the time frame. 68 Measurements of blood pressure and heart rate were repeated during the mirror tracing task at 2 minutes 15 seconds, and again at 4 minutes 35 seconds, into the task to measure psychophysiological reactivity to acute stress. The mean stress-induced blood pressure and heart rate readings were calculated from these two measurements. Blood pressure and heart rate psychophysiological reactivity were calculated by subtracting the average resting systolic and diastolic blood pressures, and resting heart rate, from the average systolic and diastolic blood pressures, and heart rate, taken during the stress task.
Functional fitness
Grip strength (kg) was assessed from both hands using the Takei Hand-Grip dynamometer (Takei Scientific Instruments Co., Ltd, Niigata, Japan).
Mental well-being
Depression and anxiety symptoms were self-reported using the validated Hospital Anxiety and Depression Scale (HADS). 69 The HADS consists of two subscales containing seven questions for anxiety symptoms and seven questions for depressive symptoms. The Cronbach’s alpha for HADS anxiety and HADS depression has been reported as 0.83 and 0.82, respectively. 70 Each answer is scored on a scale from 0 to 3. Therefore, total scores for each construct range from 0 to 21. For each construct, a score of ≤ 7 would be classified as ‘no symptoms’, whereas scores of 8–10, 11–14 and 15–21 are classified as the presence of mild, moderate and severe symptoms, respectively. 70 Social isolation was assessed using the 8-item Social Isolation Short Form from the Patient-Reported Outcomes Measurement Information System. 71,72
Musculoskeletal symptoms
Musculoskeletal symptoms were assessed using the standardised Nordic Musculoskeletal Questionnaire, which is a self-reported measure of musculoskeletal pain covering nine body regions. 73
Work-related psychosocial variables
A series of self-reported questionnaires assessed a range of work-related psychosocial variables. Work engagement (characterised by vigour, dedication and absorption) was measured using the Utrecht Work Engagement Scale. 74 Occupational fatigue was measured using the Occupational Fatigue Exhaustion Recovery (OFER) scale. 75 Perceived job performance76 and job satisfaction77 were measured using single-item 7-point Likert scales. Perceived work ability was assessed using the single-item Work Ability Index. 78 Sickness presenteeism and absenteeism were assessed using a single-item questionnaires. Participant’s perceptions of their work demands and support was assessed using four subscales from the Health and Safety Executive Management Standards Indicator Tool. 79 Reported driving-related safety behaviour was assessed using a six-item measure. 80
Health-related quality of life and resource use
Health-related quality of life was measured using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L). 81,82 The EQ-5D-5L measure comprises a short descriptive questionnaire and a visual analogue scale. On the descriptive questionnaire, participants rate their current health state across five dimensions (i.e. mobility, self-care, usual activities, pain/discomfort, anxiety/depression) and across five levels of severity (ranging from ‘no problem’ to ‘unable to/extreme problems’). The visual analogue scale (which ranges from 0 to 100) records the participant’s overall current health, where the end points are labelled ‘the best health you can imagine’ (100) and ‘the worst health you can imagine’ (0).
Information on health-related resource use was collected using a questionnaire designed for this study. Using this tool, participants were asked to report information on the quantity and duration of GP and nurse practitioner visits, inpatient and outpatient appointments, and visits with other relevant health professionals. The information obtained from the EQ-5D-5L and health-related resource use questionnaire was used to inform the within-trial cost-effectiveness analysis (see Chapter 4).
Demographics and additional lifestyle health-related behaviour and risk measures
At baseline, participants completed a brief questionnaire collecting basic demographic information, including date of birth, sex, ethnicity, highest level of education, marital status, postcode (to determine Index of Multiple Deprivation as an indicator of neighbourhood socioeconomic status), working hours, years worked as a HGV driver, shift pattern and years worked at DHL Supply Chain. At each follow-up assessment, participants were asked if there have been any changes in these variables. During each assessment, information on smoking status and typical alcohol intake [using questions 1 and 2 from the Alcohol Use Disorders Identification Test (AUDIT)83] was gathered. Using information collected from the self-report questionnaires, and data collected within the health assessments (i.e. systolic blood pressure, cholesterol/HDL-C ratio, height and weight), participants’ 10-year risk of having a cardiovascular event was calculated using the Cardiovascular Risk Score (QRISK3) calculator [URL: https://qrisk.org/2017/ (accessed 16 July 2021)].
Accelerometer data processing
activPAL
activPALs were initialised and downloaded using manufacturer proprietary software (activPAL Professional v.7.2.38, PAL Technologies Ltd, Glasgow, UK). Event files were generated and processed using the freely available Processing PAL software [URL: https://github.com/UOL-COLS/ProcessingPAL (accessed 24 August 2022), version 1.3, University of Leicester, Leicester, UK]. The software provides information on valid waking wear time, sleep time, extended non-wear time and invalid data, according to a validated algorithm. 84 Once data were processed, heat maps were created, showing valid waking wear data and invalid data. The heat maps were visually checked independently by two researchers for any occasions where the algorithm had misclassified waking wear data, and vice versa. On any occasion where suspected misclassifications had occurred, the participant’s self-reported logbook wake and sleep times were compared with the processed data. If a misclassification was confirmed, then the data were corrected. The logbooks were also checked for scenarios where data should be removed, for example if participants reported removing the device for any reason. Once this process was completed, summary variables were calculated (see Secondary activPAL variables). A valid activPAL wear-day was defined as having ≥ 10 hours wear time per day, ≥ 1000 steps per day and < 95% of the day spent in any one behaviour (e.g. sitting, standing or stepping). Participants were included in the primary outcome analysis if they provided at least 1 valid wear-day at both baseline and 6 months’ follow-up (i.e. immediately following completion of the 6-month intervention). One valid day was chosen to maximise our sample and is in line with previous studies. 85,86
GENEActiv
GENEActiv devices were initialised and downloaded using manufacturer proprietary software (GENEActiv v.3.1, Activinsights Ltd, Huntingdon, UK). Accelerometer files were processed in the R package GGIR version 1.11-0 (The R Foundation for Statistical Computing, Vienna, Austria)87 to generate sleep outcome variables, with sleep duration (i.e. minutes/24-hour period) and sleep efficiency [i.e. sleep duration/sleep window duration × 100 (%)] the variables of interest for this report. ‘Sleep windows’ (i.e. the time between ‘lights out’ and out of bed time) were detected from the accelerometer data using a validated algorithm. 88 Sleep duration within the sleep window period was calculated using a validated sleep detection algorithm, which has been shown to demonstrate high sensitivity and specificity in detecting sleep periods. 89 A device wear time of ≥ 16 hours per 24-hour period was required to determine a valid night of sleep data. 89 Individual nights of data with a sleep window > 13 hours or < 2 hours or sleep duration > 12 hours or < 1 hour were identified as erroneous and removed. As with the activPAL data, participants were required to have provided at least 1 valid wear-day at both baseline and follow-up (i.e. immediately following completion of the 6-month intervention) to be included in the analyses within this report.
From the data provided by the GENEActiv, the following variables were derived by calculating the average across the number of valid days provided during each measurement period:
-
sleep window duration [i.e. average duration between ‘lights out’ and ‘out of bed’ time (minutes)]
-
sleep duration [i.e. average time spent asleep during the sleep window (minutes)]
-
sleep efficiency [i.e. sleep duration/sleep window duration × 100 (%)]
-
average number of valid days (days).
The variables below were calculated and summarised for three different time periods within each measurement period: (1) daily (i.e. across all 24-hour periods on all valid days), (2) on workdays only and (3) on non-workdays only.
Cost-effectiveness evaluation
Full details of the methods for the cost-effectiveness analysis are in Chapter 4. In brief, the economic evaluation assessed whether or not the SHIFT programme, compared with a control arm, was likely to be cost-effective at commonly used threshold values. The economic analysis consisted of a cost–consequences analysis based on the observed results within the trial period and a cost-effectiveness analysis in which differences between groups in the trial were extrapolated to the longer term.
Within-trial analysis
Within the trial, resource use estimates were collected during each assessment point using the health-related resource use questionnaire. This questionnaire was based on a variant of the Client Service Receipt Inventory and included services that this population are likely to utilise, such as GPs and practise nurse appointments, occupational health visitors and counsellors. Costs of resources were calculated by applying published national unit cost estimates (e.g. NHS reference costs or Personal Social Services Research Unit unit costs of health and social care90,91), where available, to estimates of relevant resource use. A range of trial outcomes were assessed as part of this economic evaluation, including HRQoL, measured using the EQ-5D-5L. 81,82 The within-trial analysis evaluated incremental results for the primary and secondary outcomes [including EuroQol-5 Dimensions (EQ-5D)] in both intervention and control arms and compared the incremental costs mentioned above.
Longer-term analysis
Existing models linking physical activity to quality-adjusted life-years (QALYs)92 were utilised to extrapolate costs and effects of the intervention beyond the trial period to a more appropriate time horizon. An incremental cost-effectiveness ratio (ICER) for the extrapolated period was reported using the QALY. Costs and effects were discounted at the prevailing recommended rate (currently 1.5% per annum on both costs and effects), and a sensitivity analysis was also conducted to reflect the ongoing uncertainty around appropriate discount rates for public health interventions. Sensitivity analyses were performed to determine the robustness of the results to altering certain assumptions, such as the discount rate or inclusion/exclusion of productivity losses.
Process evaluation
Full details of the methods for the process evaluation are included in Chapter 5. In brief, the process evaluation aimed to examine any discrepancies between expected and observed outcomes, increase our understanding of the influence of each intervention component and context on the observed outcomes, and provide insight for any further intervention development and implementation. 93 Throughout the trial, we monitored the implementation fidelity, dose, attrition, adaptation, contamination, barriers and facilitators, and sustainability, using the Medical Research Council (MRC) framework. 94 The process evaluation adopted a mixed-methods approach. Self-report questionnaires that were provided to study participants were used to evaluate the various intervention components (e.g. structured education session, Fitbit, cab workout). Interviews with participants and transport managers examined further engagement in the various components of the intervention, along with any perceived barriers to and facilitators of participating in these components.
Sample size
Our earlier exploratory pre–post study revealed that, on average, HGV drivers accumulated 8786 steps per day across both workdays and non-workdays, with a SD of 2919 steps. 37 This trial was powered to look for a difference in step counts (i.e. the primary outcome) of 1500 steps per day (equivalent to approximately 15 minutes of moderately paced walking) between the intervention and control groups. Evidence demonstrates a linear association between step counts and a range of morbidity and mortality outcomes, as well as markers of health status, including inflammation and adiposity, insulin sensitivity and HDL-C in adults. 95–97 The linear association between step counts and health outcomes indicates that, regardless of an individual’s baseline value, even modest increases in daily step counts can yield clinically meaningful health benefits. For example, a difference in daily steps of 1500 steps per day has been associated with around a 5–10% lower risk of all-cause mortality and cardiovascular morbidity and mortality in the general population and in those with a high risk of type 2 diabetes, respectively. 98,99 This proposed level of change was chosen based on findings from our exploratory pre–post intervention,37 while also being clinically meaningful.
Based on a cluster size of 10, a conservative intraclass correlation coefficient (ICC) of 0.05 (as there were no previous data to inform this, we were guided by recommendations of Campbell et al. 100), an alpha of 0.05, power of 80% and a coefficient of variation to allow for variation in cluster size of 0.51 (based on information provided by DHL Supply Chain), we required 110 participants from 11 clusters per arm. From experience in conducting such studies, it was originally estimated that retention and compliance rates would be approximately 70% at 12 months’ follow-up, and, therefore, the sample size was inflated by 30% to ensure that we had adequate power in the final analysis. The number of clusters was also inflated by two to allow for whole-cluster drop out. Therefore, we aimed to recruit 24 clusters (i.e. DHL Supply Chain sites), with an average of 14 participants per cluster, providing a total target sample size of 336 drivers.
Owing to one pilot site [i.e. a BP (London, UK) site] not allowing participants to wear the accelerometers during working hours for health and safety reasons and, therefore, limiting the collection of the primary outcome measure (i.e. activPAL-determined steps/day) to non-working hours only, the Trial Steering Committee (TSC) approved the recruitment of an additional site in the main trial phase (in November 2018) (see Internal pilot). The total number of sites recruited increased, therefore, from 24 to 25.
Internal pilot
The trial incorporated an internal pilot, which was conducted using the first six clusters (i.e. sites) recruited. The internal pilot examined issues surrounding worksite and participant recruitment, randomisation, compliance to the primary outcome and retention rates at 6 months’ follow-up. The following progression criteria were reviewed by the TSC on the completion of the measurements collected from these six sites at 6 months, and the trial was considered eligible to progress to the main trial phase if it confirmed the following:
-
All 24 sites required for the full sample size agreed to take part.
-
A minimum of 84 drivers (based on an average of 14 participants per cluster, across the six pilot sites) had provided informed consent to participate in the internal pilot.
-
An average of 75% of drivers opting into the study, randomised into the intervention arm, attended the education session across the three intervention sites in the internal pilot phase. This figure was based on the intervention uptake rate seen in our exploratory pre–post intervention study (i.e. 87%),37 but the figure also recognises that take-up rates tend to be lower when moving from an efficacy study to a larger multicentre effectiveness trial.
-
No more than 20% of participants failed to provide valid data for the primary outcome measure (i.e. activPAL-determined step counts) at baseline and at 6 months’ follow-up (i.e. immediately following completion of the intervention), or had withdrew or were lost to follow-up during the 6-month intervention phase.
If the final two progression criteria were not fully met, then it was agreed that strategies to improve these metrics for the full trial would be discussed with the TSC and the TSC would have the final say on whether or not the trial progressed to the main trial phase.
Allocation to treatment groups
Clusters (i.e. individual DHL Supply Chain sites) were randomised at the worksite level into the two trial arms (i.e. intervention and control), using an allocation ratio of 1 : 1. Randomisation was conducted by a statistician from the Leicester Clinical Trials Unit using a pregenerated list. The statistician was blinded to any identifiable cluster features and all clusters were represented by a unique cluster identifier. Randomisation took place in two phases, initially as part of the internal pilot phase and then as part of the main trial phase. Within both trial phases, the research team were responsible for co-ordinating the deployment of the intervention across sites and were, therefore, unable to be blinded to allocation arm. Similarly, owing to the nature of the intervention, participants were unable to be blinded to their assigned trial arm.
Internal pilot
Within the internal pilot, the six sites were randomised into the two trial arms following the completion of baseline measurements across the sites, using simple randomisation.
Main trial
Within the main trial phase, sites/clusters were randomised in blocks of three on completion of the baseline measures in these sites. Sites were also stratified by cluster size [i.e. small (< 40 drivers) vs. large (≥ 40 drivers)].
COVID-19: impact of a temporary change in driving hour regulations on SHIFT participants
As a response to the COVID-19 pandemic, the government temporarily relaxed the driving regulations during the first national lockdown in England, extending the permitted fortnightly driving limit from 90 hours to 99 hours for HGV drivers. 101 To investigate the impact of the changes in driving regulations, along with the impact of the pandemic on SHIFT participants’ mental health and health-related behaviours, participants were invited to complete an additional optional short online survey in May 2020. The online survey also asked if participants had been furloughed and if participating in the study had an impact on their lifestyle behaviours during the initial government lockdown.
Ethics approval for this additional survey was obtained from the Loughborough University Ethics Approvals (Human Participants) Sub-Committee (reference 2020-1444-1221). The online survey was created and distributed via the Jisc Online Surveys platform (Jisc, Bristol, UK), which is a General Data Protection Regulation-compliant online survey tool designed for academic research. Participants were contacted via the study’s text messaging service and were invited to participate in the survey. A link to the online survey was included in the text message. In addition, a participant information sheet and a consent statement were included on the opening page of the survey.
The following measures were included in the online survey:
-
Working situation (whether participants continued to work or had been furloughed).
-
Working hours, driving hours, in-cab waiting hours and between-shift resting hours before and during the pandemic.
-
Sitting, standing and moving time before and during the pandemic.
-
Whether or not participants had commenced any new forms of physical activity during the pandemic.
-
Symptoms of anxiety and depression during the pandemic, assessed using the HADS.
-
Work-related chronic and acute fatigue during the pandemic, assessed using the OFER scale.
-
Whether or not participants habitually spent time in nature before the pandemic, and whether or not they were spending time in nature during the pandemic. Nature was defined as spaces such as gardens, parks, sports fields, allotments, woodland, lakes, rivers, coastline, beaches or mountains. Participants also indicated the frequency with which they spent time in nature, before and during the pandemic, using the following options: no time in nature, once per week, 2–3 times per week, almost every day and every day. 102
-
Whether or not participants had made any changes to their activity levels, diet, smoking status or alcohol intake during the pandemic.
-
Sleep duration over the past 14 days.
-
Whether or not participating in the SHIFT study had provided participants with the right knowledge to maintain a healthy lifestyle during the COVID-19 restrictions.
Statistical analysis
A detailed statistical analysis plan (SAP) (see Report Supplementary Material 3) was created and signed off before the independent statistician had access to the data. Cluster- and participant-level baseline characteristics were summarised by trial arm and for the sample as a whole. In addition, we carried out a descriptive comparison of baseline data (specifically cluster size, age, BMI, number of years as a HGV driver, number of steps/day) between completers (i.e. participants who provided valid activPAL data at baseline and at 6 months) and non-completers, within randomisation groups and overall.
Primary outcome analysis
The primary analysis was performed using a mixed-effect linear regression model, with each participant’s daily average number of steps (measured using the activPAL) at 6 months’ follow-up as the outcome, adjusting for the participant’s daily average number of steps at baseline and for the average waking wear time at baseline and at 6 months. The model also included a categorical variable for randomisation group (control as reference) and a term for the stratification factor [i.e. cluster size: small (< 40 drivers) vs. large (≥ 40 drivers)]. Depot was included as a random effect to model driver heterogeneity within participating sites. The structure of the variance–covariance matrix for the random effect was assumed to be identity and the models were estimated using restricted maximum likelihood. The primary analysis examined the effect of the intervention using a complete-case population. All clusters randomised, and the recruited participants in these clusters, excluding participants with missing outcome data (i.e. without at least 1 valid day of activPAL data at baseline and follow-up), were included in the primary analysis, which followed the intention-to-treat (ITT) principle (i.e. participants were analysed in the arm to which they were randomised). The estimate of the difference between the SHIFT arm and the control arm for daily average number of steps at 6 months and the corresponding 95% confidence intervals (CIs) and p-values are presented. Statistical tests were two sided. Furthermore, the ICC was estimated to assess the strength of the clustering effect.
Sensitivity analyses
Sensitivity analyses were conducted (see Full intention-to-treat analysis and Effects on the number of valid activPAL days), using similar methodology as the primary outcome analysis. There was no formal adjustment for multiple significance testing. The sensitivity analyses were conducted for the primary outcome (i.e. average daily step counts at 6 months’ follow-up). All tests and reported p-values were two sided. Estimates are presented with 95% CIs.
Per-protocol analysis
The effect size was also estimated using a per-protocol analysis. The per-protocol population were participants who did not exhibit any protocol deviations, and excluded participants who:
-
did not provide valid activPAL data at baseline or at the 6 months’ follow-up (as applied in the primary outcome analysis)
-
had time window deviations for their follow-up (> ± 2 months) assessment.
Full intention-to-treat analysis
Sensitivity analyses were performed to assess the impact of missing data on the primary results and to account for uncertainty associated with imputing data (full ITT analysis). To allow for analysis of the full data set, missing data from variables included in the primary analysis model (i.e. average daily steps at baseline and immediately following the intervention) were imputed using a multiple imputation procedure, which substituted predicted values from a regression equation. The following variables were used as predictors of the primary outcome in the regression equation: baseline BMI, sex, ethnicity, age, cluster size category, years worked as HGV driver and average waking wear time across baseline and 6 months. Missing values for these predictor variables were also imputed if needed. The imputation was carried out by the MI command in Stata® (StataCorp LP, College Station, TX, USA). MI replaced missing values with multiple sets of simulated values to complete the data, performed standard analysis on each completed data set and adjusted the obtained parameter estimates for missing data uncertainty using Rubin’s rules to combine estimates. 103 Twenty imputations were estimated and a seed was set to allow reproducibility.
Additional worst- and best-case scenario ITT analyses using basic imputation methods were also carried out. A simple worst-case scenario ITT analysis was carried out, where missing covariate data in the final analysis model were replaced using cluster means. Where it was not possible to impute using the cluster mean, the mean for the respective arm was used instead. Missing outcome data in the final analysis model (i.e. at baseline and 6 months) were replaced using the mean for the standard care arm. Furthermore, a simple best-case scenario ITT analysis was also carried out using the same approach as above, but outcome data were replaced using the mean for the respective arm.
Effects on the number of valid activPAL days
We carried out further sensitivity analyses by assessing the effect of the number of valid activPAL days on the primary outcome analysis. This analysis was performed by including participants who provided valid activPAL data (including weekdays and weekend days) on:
-
≥ 2 valid days at both baseline and 6 months
-
≥ 3 valid days at both baseline and 6 months
-
≥ 4 valid days at both baseline and 6 months.
Secondary outcome analysis
Secondary outcomes, including those measured at 6 months and at 16–18 months, were analysed using similar methodology to the primary outcome. Owing to the volume of secondary outcomes assessed, statistical analysis of secondary outcome variables was restricted to the following key secondary outcomes: steps per day (16–18 months’ follow-up), activPAL-determined time spent sitting, standing and stepping, and time in light intensity physical activity and MVPA daily, during workdays and during non-workdays (at both 6 months’ follow-up and 16–18 months’ follow-up). The models for each of these secondary outcomes were adjusted for their respective variable at baseline and for the respective average wear time period (i.e. daily, workdays or non-workdays) at baseline and follow-up.
Fruit and vegetable intake (g/day) and dietary quality score were also analysed at 6 months and at 16–18 months. The models for each of these outcomes were adjusted for their respective baseline levels. Furthermore, the following markers of cardiometabolic health were also compared statistically at 6 months’ follow-up: weight, BMI, per cent body fat, waist circumference, HbA1c (mmol/mol), triglycerides (mmol/l), HDL-C (mmol/l), LDL-C (mmol/l) and total cholesterol (mmol/l). The models for each of these outcomes were adjusted for their respective baseline levels.
The models above included a categorical variable for intervention group (control as reference) and the stratification factor (cluster size). No corrections for multiple testing were made. In all models, estimates of the difference between the SHIFT arm and the control arm for the variables examined are presented, along with corresponding 95% CIs and p-values. Statistical tests were two sided.
For the other secondary outcomes (see Secondary outcomes), continuous data that were approximately normally distributed were summarised in terms of the mean and SD. Skewed data are presented in terms of the medians and interquartile range (IQR). Ordinal and categorical data are summarised in terms of frequency counts and percentages. All variables are summarised by trial arm.
Statistical analysis plan deviations
Mixed-effect linear regression models were fitted, instead of analysis of covariance models, because the analysis of covariance set-up in Stata did not allow all of the options specified in the SAP. The MEQ data were added together to create a total MEQ score, which was analysed as a continuous variable. Where BMI at 6 months was missing but weight data were available, baseline height was used to calculate BMI at 6 months, and likewise for BMI at 16–18 months. Medians (IQR) were calculated for AUDIT scores and job satisfaction and performance in addition to the planned descriptive statistics in the SAP.
Analysis of the COVID-19 questionnaire
The data were downloaded from the Jisc platform and imported into Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA), where all data cleaning and reduction took place. Data were then imported into SPSS v25 (SPSS Inc., Chicago, IL, USA) for analysis. Continuous data that were approximately normally distributed were summarised in terms of the mean and SD, whereas skewed data are presented in terms of the medians and IQR. Comparisons between questionnaire responses from control and SHIFT arm participants, and between participants who had been furloughed and participants who were working at the time of questionnaire completion, were conducted using between-samples tests. Baseline characteristics in terms of age, duration working as a HGV driver, duration working for DHL Supply Chain, hours worked per week, BMI, per cent body fat, waist circumference, self-reported symptoms of anxiety and depression, musculoskeletal complaints, physical activity levels, and sleep duration and efficiency were compared between participants completing the additional COVID-19 questionnaire and participants not using between-samples tests. For participants completing the online questionnaire, comparisons were made, using repeated measures tests, between participants’ working, driving, in-cab waiting or rest hours reported before and during the pandemic. Similarly, comparisons were made between participants’ reported time spent sitting, standing and walking/moving around on a workday before and during the pandemic, along with reported symptoms of anxiety and depression and fatigue. The impact of spending time in nature before and during the pandemic on symptoms of anxiety, depression and fatigue were explored. The impact of participating in the study on maintaining a healthy lifestyle during the pandemic, along with any lifestyle- or work-related changes experienced by participants, were explored descriptively.
Public and patient involvement
The initial development and refinement of the SHIFT intervention, and the implementation and running of this trial, have been informed by extensive PPI. The preparatory work,7,36–38 which informed the original grant application, was the result of a 3-year partnership between the research team and a large transport and logistics company (not DHL Supply Chain) located in the East Midlands, UK. This preparatory work was instigated by the company. The company requested help in improving the lifestyle behaviours and health of their long-distance drivers, who were proving difficult to engage. As part of the preparatory work, the SHIFT programme was developed in collaboration with long-distance HGV drivers and health and safety personnel working within the logistics sector. Following pilot testing,37 and input from drivers and associated stakeholders,38 the intervention and outcome measures were refined. Specifically, the duration of the intervention increased from 3 months to 6 months, as it was felt that a longer intervention duration would lead to more sustainable changes in health behaviours. The provision of free fruit at the participating DHL Supply Chain sites was removed as an intervention component, as senior health and safety personnel at DHL Supply Chain felt that this would not be feasible to implement across the wide range of sites across their business. Assessments of lung function were removed from the collection of outcome measures, as the relevance of this particular measure was questioned.
As part of the implementation planning for the trial, an initial meeting was held with transport managers from a range of DHL Supply Chain sites. The feedback obtained during this meeting informed our driver recruitment plans and highlighted effective strategies for informing and engaging office staff and drivers about the study across the individual sites. Extensive input and feedback were obtained from DHL Supply Chain health and safety personnel and human resources staff on our study marketing materials and on our health assessment feedback booklet produced for drivers.
There was extensive PPI regarding the creation and refinement of the project CRFs. We sought opinions and feedback from HGV drivers (independent from DHL Supply Chain), DHL Supply Chain transport managers and DHL Supply Chain health and safety personnel regarding the development of the drivers’ health-related resource use questionnaire to inform part of our economic evaluation. We also asked independent HGV drivers, via research team contacts, to complete draft versions of our CRFs. Initial concerns were raised over the length of time taken to complete the CRFs, and this led to subtle changes being made to reduce the overall length of the included self-report questionnaires (e.g. a shorter version of the Nordic Musculoskeletal Questionnaire was used).
To inform the best practice procedures for undertaking the health assessments (i.e. outcome measurements), independent HGV drivers (i.e. contacts of the research team) were invited to undertake health assessments at Loughborough University. The HGV drivers provided further feedback on the length of the CRF and on the general procedures adopted for the physiological health assessments. Based on feedback, the order in which a number of the physiological measures were conducted as part of the health assessments was revised. In addition, we piloted the updated SHIFT education session on two independent HGV drivers, prior to running these sessions in the trial. A half-day workshop was arranged for senior DHL Supply Chain health and safety personnel at Loughborough University, where the personnel experienced our health assessments and a shortened version of the education session. This workshop was organised to enable colleagues to experience aspects of the health assessments and intervention that would be undertaken by their participating drivers, and to obtain feedback on these components from senior staff.
Throughout the study, members of the research team have presented the project at the DHL Supply Chain Transport Safety Conference (2017 and 2020), which is attended by transport managers, health and safety personnel, and drivers. The conferences have enabled the team to update a wider audience of DHL Supply Chain staff about the project and to initiate discussions about the sustainability of the intervention throughout the company. We have also attended a range of events throughout this project with industry stakeholders [e.g. attending events organised by the Chartered Institute of Logistics and Transport (CILT) (Corby, UK), the East Midlands Chamber of Commerce (Chesterfield, UK) and Women in Logistics (Corby, UK)], which have enabled us to provide updates to stakeholders and gain feedback on the project as it has progressed. We have had regular engagement with colleagues from CILT, and we have kept CILT informed with the project progress.
In addition, throughout the project, we organised workshops and events at Loughborough University, with a wide range of stakeholders invited. An initial workshop was organised in 2018, the purpose of which was to increase awareness of the project within the logistics sector and to gain feedback from personnel working in the sector. Attendees included representatives from 3t Logistics Ltd (Leicester, UK), Foster Logistics Consulting Ltd (Ashby-de-la-Zouch, UK), Tarmac Ltd (Solihull, UK), the Road Haulage Association Ltd (Weybridge, UK), Keltruck Scania (West Bromwich, UK), CILT and UK-Aggregates (Nottingham, UK), a local haulage company, and a member of the public interested in the project. We gained valuable feedback from participants attending this workshop with regard to both the project and how the SHIFT intervention could potentially be rolled out to all HGV drivers in the future.
In December 2019, we hosted a 1-day conference entitled ‘A healthier workforce for a healthier UK’, which focused on health within the logistics and transport sector. The conference included presentations from a variety of speakers [including the SHIFT team, a HGV driver, a local council representative, Unite the Union (London, UK) and Public Health England (London, UK)]. The varied audience included representatives from companies with logistics and transport/delivery departments [e.g. DHL Supply Chain, John Lewis & Partners (London, UK), Forterra plc (Northampton, UK), Wincanton plc (Chippenham, UK), Wren Kitchens (Barton-upon-Humber, UK), Bibby Distribution (Edinburgh, UK), PepsiCo, Inc. (London, UK) and Tower Transit (London, UK)], along with other stakeholders, policy-makers (including the Health and Safety Executive, the Department for Work and Pensions, Institution of Occupational Safety and Health, County Councils, CILT, the Road Haulage Association) and academics. The day concluded with all delegates agreeing that driver health should be considered a priority, and there was resounding support for policy change within the sector to promote drivers’ health and well-being.
An independent HGV driver and a manager working within the logistics sector were members of our TSC (note that the manager was a member of the TSC for the first 18 months of the project only), and both members provided invaluable insight into the design, set-up, conduct and dissemination of this research as it progressed. The inclusion of the COVID-19 questionnaire within the trial was the result of discussions with our health and safety colleagues at DHL Supply Chain, who expressed concerns about drivers’ physical and mental health following the government’s relaxation in driving hours for HGV drivers during the height of the first wave of the pandemic. The questionnaire content was designed in partnership with DHL Supply Chain colleagues. Draft versions of the questionnaire were piloted with four HGV drivers who were independent to the study, and this was facilitated through our public member of the TSC (a HGV driver). The questionnaire, and its appearance and formatting on the online platform, was modified following feedback obtained from these drivers, prior to it being finalised and issued to SHIFT participants.
Data management and research governance
Data were entered in an anonymised format into the Clinical Data Management System (InferMed Macro v4, Elsevier Ltd, Oxford, UK) provided by the Leicester Clinical Trials Unit. The validated system included a number of quality control mechanisms to ensure that the data entered were complete and accurate. This trial was sponsored by Loughborough University. Two groups were created to oversee the trial, including an independent TSC and a Project Committee. As applied elsewhere,104 and because the study was regarded as low risk, the TSC took on the role of a Data Monitoring Committee to monitor progress with data collection and to review any serious adverse events should they have arisen. The TSC met every 6 months and included the principal investigator (SAC), an independent chairperson (a medical statistician), two independent academics, including a health economist, an independent delivery driver and a logistics industry manager. The Project Committee comprised the principal investigator, all co-investigator and those concerned with the day-to-day running of the study. The Project Committee provided update reports for the TSC. 104
Chapter 3 Results
Parts of this chapter have been reproduced from Clemes et al. 105 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Internal pilot
Site recruitment for the internal pilot phase, which involved six sites, commenced in August 2017, with participant recruitment across these sites commencing in October 2017. Participants consented into the study and baseline measurements were undertaken between January and August 2018. A delay in recruitment was experienced in one site. Baseline measurements were undertaken in five sites between January and April 2018 and between July and August 2018 in one site.
Data regarding worksite and participant recruitment, randomisation, compliance to the primary outcome and retention rates at 6 months’ follow-up were examined to determine whether or not the trial should progress to the main trial phase. The study’s TSC agreed to base the progression criteria review on the baseline data collected from all six sites, which included site and participant recruitment numbers and activPAL compliance data, and to base the follow-up progression criteria (in terms of retention rates and activPAL compliance at follow-up) on the data collected from the five sites that had completed the 6-month follow-up measures by November 2018. This enabled the progression criteria to be reviewed by the end of 2018, as opposed to waiting until March 2019, when the follow-up measures were due to be completed in the final site, thereby minimising further delays to the trial.
Recruitment, compliance and retention outcomes
Table 4 summarises the recruitment, compliance and retention outcomes observed from the internal pilot sites. Outcomes were reviewed by the TSC on the 11 December 2018; on the basis of the data reviewed, the TSC recommended continuation of the trial.
| Progression criterion | Observed outcome |
|---|---|
| All 24 sites required for the full sample size agree to take part in the study | Twenty-four sites were identified and agreed to participate in the trial by November 2018. Following agreement by the TSC, an additional site was recruited into the main trial phase because of participants in one pilot site (a BP site) not being able to wear the activPAL during working hours for health and safety reasons |
| A minimum of 84 drivers agree to participate in the internal pilot | Ninety-eight drivers across the six internal pilot sites provided informed consent and participated in the baseline measures, of which 84% provided valid activPAL data at baseline |
| An average of 75% of drivers, randomised into the intervention arm, attended the education session across three intervention depots | Seventy-four per cent of drivers in the intervention sites attended the education workshop |
| No more than 20% of participants fail to provide valid data for the primary outcome measure (i.e. activPAL-determined step counts) at baseline and at 6 months’ follow-up, or withdraw or are lost to follow-up during the 6-month intervention phase |
Across the five sites completing the 6-month follow-up assessments by November 2018, 57% of participants provided valid activPAL data at baseline and follow-up Strategies were discussed with, and approved by, the TSC for how activPAL compliance could be improved for the main trial phase |
Main trial
Participant recruitment
Participant recruitment across the remaining 19 sites commenced in January 2019, and baseline measurements for participants consented into the study were undertaken across these sites between February and July 2019.
As experienced in the internal pilot, a number of delays were encountered during the main trial phase. The delays were predominantly associated with challenges across sites in scheduling drivers for their measurement sessions, which required drivers to be released from their duties for 2 hours. The challenges were further exacerbated in the sites randomised to the SHIFT arm, which required drivers to attend the 6-hour structured education session, which was scheduled during work time. As a consequence, delays in the 6-month follow-up measures were experienced across the majority of sites.
Overall cluster and participant numbers
Figure 2 shows the flow of all participants through the study, combining data from the internal pilot and main trial phases. Overall, 386 participants across 25 clusters (i.e. sites) were recruited and consented into the study. The 25 sites were located across the Midlands region of the UK (1502 drivers were employed across these sites), and the sites operated within the transport, retail, hospitality, health-care, pharmaceutical, construction, oil and gas, and automotive industries. Of the 386 participants recruited, 382 participants were randomised into the two trial arms and four participants withdrew prior to randomisation. Thirteen sites (n = 199 participants) were randomised to the control arm and 12 sites (n = 183 participants) were randomised to the SHIFT arm. Between baseline and 6-month follow-up measures, two sites (i.e. one intervention site and one control site) dropped out of the trial. For both sites, this was because of site closures due to the collapse of the contracting companies.
FIGURE 2.
A CONSORT diagram of participant flow through the study.
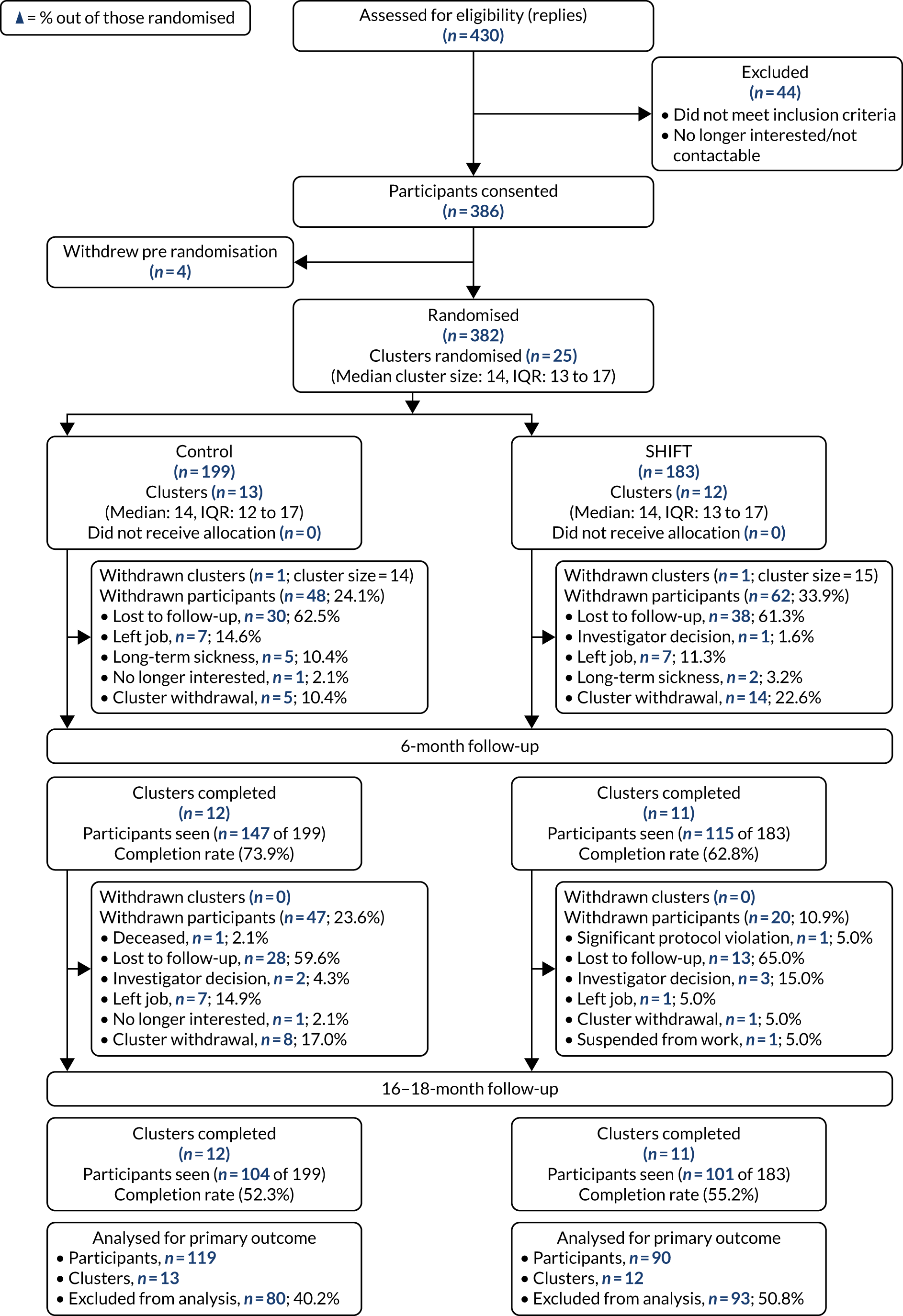
Baseline characteristics
Characteristics of the clusters and baseline demographic characteristics of participants within each trial arm, and overall, are shown in Table 5. Table 6 displays the biometric measurements collected from the sample overall, and according to trial arm, at baseline. Table 7 displays the accelerometer-derived measures (i.e. physical activity, sitting time and sleep) collected at baseline. Descriptive comparisons between baseline characteristics of participants completing the trial and non-completers are shown in Appendix 1, Table 42. There were no noticeable differences between completers (i.e. participants who provided valid activPAL data at baseline and at 6 months) and non-completers in terms of cluster size, age, BMI, number of years as a HGV driver and number of steps per day at baseline.
| Characteristic | Trial arm | Overall (clusters, n = 25; participants, n = 382) | |
|---|---|---|---|
| Control (clusters, n = 13; participants, n = 199) | SHIFT intervention (clusters, n = 12; participants, n = 183) | ||
| Cluster level | |||
| Cluster size category, n (%) | |||
| Small (< 40 drivers) | 81 (40.7) | 93 (50.8) | 174 (45.6) |
| Large (≥ 40 drivers) | 118 (59.3) | 90 (49.2) | 208 (54.4) |
| Participant level | |||
| Cluster size | |||
| Median (IQR) | 14 (12–17) | 14 (13–17) | 14 (13–17) |
| Minimum, maximum | 9, 24 | 11, 25 | 9, 25 |
| Age (years), mean (SD) | 48.3 (9.7) | 48.6 (9.1) | 48.4 (9.4) |
| Sex, n (%) | |||
| Male | 196 (98.5) | 182 (99.5) | 378 (99.0) |
| Female | 3 (1.5) | 1 (0.5) | 4 (1.0) |
| Ethnicity, n (%) | |||
| White British | 154 (77.4) | 152 (83.1) | 306 (80.1) |
| Other ethnicity | 45 (22.6) | 30 (16.4) | 75 (19.6) |
| Shift pattern, n (%) | |||
| Morning | 146 (73.4) | 124 (67.8) | 270 (70.7) |
| Afternoon | 29 (14.6) | 31 (16.9) | 60 (15.7) |
| Night | 35 (17.6) | 45 (24.6) | 80 (20.9) |
| Duration working at DHL Supply Chain (years), median (IQR) | 6.17 (3.67–11.50) | 9.30 (4.06–14.27) | 7.75 (3.88–13.42) |
| Duration working as a HGV driver (years), median (IQR) | 15.00 (6.00–26.00) | 17.00 (10.00–25.02) | 16.00 (9.00–25.17) |
| Average hours worked per week, median (IQR) | 48 (45–50) | 48 (45–50) | 48 (45–50) |
| IMD rank, median (IQR) | 16,779.0 (8499.5–22,903.5) | 16,040.0 (7934.0–22,171.0) | 16,591.0 (8165.0–22,544.0) |
| Marital status, n (%) | |||
| Married | 133 (66.8) | 113 (61.8) | 246 (64.4) |
| Living with partner | 34 (17.1) | 31 (16.9) | 65 (17.0) |
| Separated/divorced | 9 (4.5) | 13 (7.1) | 22 (5.8) |
| Single | 22 (11.1) | 25 (13.7) | 47 (12.3) |
| Widowed | 1 (0.5) | 1 (0.6) | 2 (0.5) |
| Level of education: degree or above, n (%) | 16 (8.0) | 10 (5.5) | 26 (6.8) |
| Diabetes history: yes, n (%)a | 15 (7.5) | 9 (4.9) | 24 (6.3) |
| Smoking status, n (%) | |||
| Never smoked | 73 (36.7) | 77 (42.1) | 150 (39.3) |
| Ex-smoker | 84 (42.2) | 73 (39.9) | 157 (41.1) |
| Current smoker | 42 (21.1) | 32 (17.5) | 74 (19.4) |
| Biometric measurement | Missing values (n) | Trial arm | Overall (clusters, n = 25; participants, n = 382) | |
|---|---|---|---|---|
| Control (clusters, n = 13; participants, n = 199) | SHIFT intervention (clusters, n = 12; participants, n = 183) | |||
| Anthropometric measures and markers of adiposity | ||||
| Weight (kg), median (IQR) | 2 | 94.0 (84.2–106.9) | 95.7 (84.0–106.4) | 94.8 (84.1–106.5) |
| Body fat (%), mean (SD) | 11 | 26.8 (5.8) | 27.3 (6.0) | 27.0 (5.9) |
| Fat mass (kg), median (IQR) | 11 | 25.3 (19.6–32.3) | 25.6 (19.9–32.7) | 25.5 (19.6–32.4) |
| Fat-free mass (kg), mean (SD) | 12 | 69.3 (8.6) | 69.6 (7.8) | 69.5 (8.2) |
| BMI (kg/m2), median (IQR) | 2 | 29.6 (27.0–32.8) | 29.9 (26.9–33.7) | 29.8 (26.9–33.2) |
| Waist circumference (cm), median (IQR) | 2 | 104.4 (94.6–113.1) | 103.0 (95.0–113.5) | 103.7 (95.0–113.4) |
| Hip circumference (cm), median (IQR) | 2 | 106.5 (101.0–111.8) | 107.5 (103.0–114.0) | 107.0 (102.0–112.5) |
| Waist–hip ratio (cm), mean (SD) | 2 | 0.97 (0.07) | 0.97 (0.07) | 0.97 (0.07) |
| Neck circumference (cm), median (IQR) | 2 | 40.2 (38.9–42.5) | 41.0 (38.3–42.5) | 40.5 (38.4–42.5) |
| Resting blood pressure and heart rate | ||||
| Systolic blood pressure (mmHg), median (IQR) | 2 | 130 (122–140) | 130 (122–138) | 130 (122–139) |
| Diastolic blood pressure (mmHg), median (IQR) | 2 | 82 (76–90) | 81 (76–88) | 82 (76–88) |
| Heart rate (b.p.m.), mean (SD) | 4 | 68 (10) | 68 (10) | 68 (10) |
| Biochemical assessments, median (IQR) | ||||
| HbA1c (mmol/mol) | 14 | 35 (32–38) | 34 (31–38) | 35 (31–38) |
| HbA1c (%) | 14 | 5.4 (5.1–5.6) | 5.3 (5.0–5.6) | 5.4 (5.0–5.6) |
| Triglycerides (mmol/l) | 5 | 1.3 (1.0–2.1) | 1.3 (0.9–2.1) | 1.3 (0.9–2.1) |
| HDL-C (mmol/l) | 5 | 1.1 (1.0–1.4) | 1.1 (1.0–1.4) | 1.1 (1.0–1.4) |
| LDL-C (mmol/l) | 6 | 2.8 (2.3–3.5) | 2.8 (2.4–3.5) | 2.8 (2.3–3.5) |
| Total cholesterol (mmol/l) | 5 | 4.4 (3.8–5.1) | 4.4 (3.8–5.1) | 4.4 (3.8–5.1) |
| Accelerometer measurement | Missing values (n) | Trial arm | Overall (clusters, n = 25; participants, n = 382) | |
|---|---|---|---|---|
| Control (clusters, n = 13; participants, n = 199) | SHIFT intervention (clusters, n = 12; participants, n = 183) | |||
| Physical activity and sitting time | ||||
| Steps/day, median (IQR) | 41 | 8471 (6774–10,160) | 8725 (7033–11,298) | 8583 (6922–10,696) |
| Sitting (minutes/day), mean (SD) | 41 | 678 (91) | 651 (97) | 665 (95) |
| Prolonged (i.e. ≥ 30 minutes) sitting (minutes/day), mean (SD) | 41 | 428 (118) | 389 (128) | 409 (124) |
| Standing (minutes/day), median (IQR) | 41 | 195 (165–238) | 213 (180–244) | 203 (169–243) |
| Stepping (minutes/day), median (IQR) | 41 | 112 (90–134) | 116 (93–149) | 114 (92–139) |
| Number of sit-to-stand transitions (transitions/day), median (IQR) | 41 | 49 (38–59) | 47 (39–58) | 48 (39–58) |
| MVPA (minutes/day), median (IQR) | 41 | 10 (6–18) | 11 (6–21) | 10 (6–19) |
| Light physical activity (minutes/day), median (IQR) | 41 | 97 (81–114) | 102 (83–129) | 99 (82–123) |
| Number of valid days, median (IQR) | 41 | 8 (6–8) | 7 (5–8) | 7 (6–8) |
| Waking wear time (minutes/day), median (IQR) | 41 | 993 (955–1033) | 989 (950–1022) | 990 (953–1032) |
| Sitting (%/day), median (IQR) | 41 | 69 (64–73) | 67 (61–72) | 68 (62–72) |
| Prolonged (≥ 30 minutes) sitting (%/day), median (IQR) | 41 | 63 (56–70) | 62 (51–69) | 63 (54–70) |
| Standing (%/day), median (IQR) | 41 | 20 (16–24) | 22 (18–25) | 21 (17–24) |
| Stepping (%/day), median (IQR) | 41 | 11 (9–13) | 11 (10–15) | 11 (9–14) |
| Sleep, median (IQR) | ||||
| Sleep window duration (minutes/day) | 36 | 426 (393–465) | 424 (387–459) | 425 (390–460) |
| Sleep duration (minutes/day) | 36 | 371 (336–405) | 371 (340–407) | 371 (337–406) |
| Sleep efficiency (%) | 36 | 88.5 (84.2–91.3) | 88.9 (84.6–92.0) | 88.6 (84.3–91.5) |
| Number of valid nights | 36 | 6 (6–6) | 6 (5–6) | 6 (6–6) |
Primary outcome analysis
A mixed-effect linear regression model revealed a statistically significant difference in mean daily step counts at 6 months’ follow-up, in favour of the SHIFT group [SHIFT group mean change: 32 (SD 2939) steps/day; control group mean change: –716 (SD 2109) steps/day], in the complete-case analysis (1008 steps/day, 95% CI 145 to 1871 steps/day; p = 0.022) (Table 8). The ICC for the model was 0.112. Mixed results were seen in the ITT and per-protocol analyses (see Table 8).
| Analysis | Number of clusters | Number of participants | Baseline, mean (SD) | 6-month follow-up, mean (SD) | Mean (SD) change from baseline to 6 monthsa | SHIFT intervention vs. control at 6 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Adjusted mean difference (95% CI)b | p-value | |
| Primary analysis (complete case)c | 13 | 12 | 119 | 90 | 8932 (2922) | 9355 (3305) | 8216 (2767) | 9387 (3455) | –716 (2109) | 32 (2939) | 1008 (145 to 1871) | 0.022 |
| Per protocolc | 12 | 2 | 98 | 2d | 8887 (2856) | 9531 (263) | 8175 (2758) | 9613 (1231) | –711 (2002) | 82 (1494) | 929 (–1705 to 3563) | 0.489 |
| ITTc | ||||||||||||
| Multiple imputatione | 13 | 12 | 199 | 183 | 335 (–471 to 1141) | 0.414 | ||||||
| Worst-case scenario | 13 | 12 | 199 | 183 | 8788 (2843) | 9394 (3134) | 8244 (2270) | 8846 (2527) | –544 (2499) | –548 (3056) | 399 (–129 to 927) | 0.139 |
| Best-case scenario | 13 | 12 | 199 | 183 | 8788 (2843) | 9464 (3127) | 8244 (2270) | 9344 (2466) | –543 (2499) | –120 (2985) | 868 (398 to 1338) | < 0.001 |
| Sensitivity analyses: effect of number of valid activPAL days (complete case) | ||||||||||||
| ≥ 2 days | 13 | 12 | 118 | 88 | 8960 (2919) | 9427 (3291) | 8238 (2768) | 9411 (3488) | –722 (2117) | –16 (2949) | 981 (102 to 1860) | 0.029 |
| ≥ 3 days | 13 | 12 | 116 | 87 | 8925 (2921) | 9459 (3296) | 8278 (2770) | 9456 (3484) | –647 (2015) | –4 (2963) | 906 (27 to 1784) | 0.043 |
| ≥ 4 days | 13 | 12 | 113 | 79 | 8832 (2877) | 9385 (3290) | 8179 (2710) | 9480.69 (3509) | –653 (2020) | 96 (3028) | 973 (76 to 1870) | 0.034 |
Sensitivity analyses
Sensitivity analyses showed similar results to the primary analysis, with significant differences observed between groups in terms of daily step counts measured at 6 months’ follow-up, when including participants with ≥ 2, ≥ 3 and ≥ 4 valid days of activPAL data (see Table 8).
Secondary outcomes: statistical analyses
activPAL-assessed secondary outcomes
Steps per day, time spent sitting, standing and stepping, and time in light physical activity and MVPA across all monitored days
In complete-case analyses, at 6 months’ follow-up, a series of mixed-effect linear regression models revealed statistically significant differences in favour of the SHIFT group in time spent sitting, standing and stepping, and time in MVPA. At 6 months, daily sitting time was significantly shorter in the SHIFT arm (–24 minutes/day, 95% CI –43 to –6 minutes/day), whereas times spent standing (14 minutes/day, 95% CI 2 to 26 minutes/day) and stepping (11 minutes/day, 95% CI 2 to 21 minutes/day) and time in MVPA (6 minutes/day, 95% CI 0.3 to 11 minutes/day) were greater, than in the control arm. There were no statistically significant differences between groups at 6 months’ follow-up in time spent in light physical activity (Table 9). There were no statistically significant differences observed between groups in activPAL variables at 16–18 months’ follow-up (see Table 9).
| Daily variable | Number of clusters | Number of participants | Baseline, mean (SD) | Follow-up, mean (SD) | Mean (SD) change from baseline to follow-upa | SHIFT intervention vs. control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Adjusted mean difference (95% CI)b | p-value | |
| Steps/dayc | ||||||||||||
| 16–18 months | 12 | 10 | 90 | 74 | 8978 (3226) | 9663 (3122) | 8789 (3148) | 9259 (3105) | –189 (2169) | –404 (2688) | 94 (–878 to 1066) | 0.849 |
| Time (minutes/day) spent sittingc | ||||||||||||
| 6 months | 13 | 12 | 119 | 90 | 675 (92) | 664 (92) | 696 (87) | 655 (93) | 21 (79) | –9 (77) | –24 (–43 to –6) | 0.011 |
| 16–18 months | 12 | 10 | 90 | 74 | 676 (97) | 651 (87) | 679 (98) | 647 (77) | 4 (82) | –4 (90) | –12 (–34 to 9) | 0.268 |
| Time (minutes/day) spent standingc | ||||||||||||
| 6 months | 13 | 12 | 119 | 90 | 204 (55) | 209 (53) | 194 (56) | 210 (61) | –10 (37) | 1 (48) | 14 (2 to 26) | 0.024 |
| 16–18 months | 12 | 10 | 90 | 74 | 200 (54) | 216 (61) | 197 (58) | 211 (64) | –3 (45) | –5 (77) | 11 (–5 to 27) | 0.183 |
| Time (minutes/day) spent steppingc | ||||||||||||
| 6 months | 13 | 12 | 119 | 90 | 116 (34) | 122 (40) | 107 (32) | 122 (40) | –8 (23) | –0.4 (32) | 11 (1 to 21) | 0.024 |
| 16–18 months | 12 | 10 | 90 | 74 | 117 (36) | 125 (38) | 114 (37) | 120 (36) | –2 (22) | –5 (31) | 1 (–9 to 11) | 0.818 |
| Time (minutes/day) in LPAc | ||||||||||||
| 6 months | 13 | 12 | 119 | 90 | 101 (29) | 107 (34) | 94 (28) | 104 (33) | –6 (19) | –3 (25) | 5 (–2 to 12) | 0.152 |
| 16–18 months | 12 | 10 | 90 | 74 | 102 (29) | 109 (34) | 100 (32) | 104 (32) | –2 (18) | –5 (26) | –1 (–8 to 7) | 0.863 |
| Time (minutes/day) in MVPAc | ||||||||||||
| 6 months | 13 | 12 | 119 | 90 | 15 (15) | 15 (14) | 13 (10) | 18 (18) | –2 (14) | 3 (19) | 6 (0.3 to 11) | 0.038 |
| 16–18 months | 12 | 10 | 90 | 74 | 14 (15) | 16 (14) | 14 (13) | 16 (16) | –1 (15) | –0.1 (16) | 2 (–3 to 7) | 0.539 |
Steps per day, time spent sitting, standing and stepping, and time in light physical activity and MVPA on workdays
There were no statistically significant differences observed between groups in any activPAL variables measured on workdays at 6 months’ follow-up or at 16–18 months’ follow-up (Table 10).
| Daily variable | Number of clusters | Number of participants | Baseline, mean (SD) | Follow-up, mean (SD) | Mean (SD) change from baseline to follow-upa | SHIFT intervention vs. control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Adjusted mean difference (95% CI)b | p-value | |
| Steps/dayc | ||||||||||||
| 6 months | 13 | 12 | 111 | 84 | 9308 (3154) | 9547 (3458) | 8890 (3041) | 9357 (3241) | –418 (2046) | –190 (2649) | 541 (–269 to 1351) | 0.190 |
| 16–18 months | 12 | 10 | 88 | 66 | 9394 (3177) | 9881 (3472) | 9491 (3388) | 9456 (3390) | 97 (2817) | –425 (3067) | –325 (–1578 to 928) | 0.611 |
| Time (minutes/day) spent sittingc | ||||||||||||
| 6 months | 13 | 12 | 111 | 84 | 720 (95) | 713 (118) | 740 (94) | 700 (96) | 20 (83.41) | –13 (101) | –14 (–36 to 8) | 0.215 |
| 16–18 months | 12 | 10 | 88 | 66 | 718 (98) | 701 (122) | 726 (117) | 707 (91) | 8 (97) | 6 (115) | 0.1 (–22 to 22) | 0.995 |
| Time (minutes/day) spent standingc | ||||||||||||
| 6 months | 13 | 12 | 111 | 84 | 191 (58) | 195 (61) | 186 (52) | 194 (59) | –5 (52) | –2 (60) | 10 (–3 to 23) | 0.129 |
| 16–18 months | 12 | 10 | 88 | 66 | 191 (57) | 201 (68) | 190 (60) | 195 (48) | –2 (55) | –6 (64) | 3 (–12 to 18) | 0.708 |
| Time (minutes/day) spent steppingc | ||||||||||||
| 6 months | 13 | 12 | 111 | 84 | 120 (37) | 124 (43) | 115 (37) | 123 (42) | –5 (24) | –2 (30) | 7 (–3 to 16) | 0.162 |
| 16–18 months | 12 | 10 | 88 | 66 | 122 (37) | 128 (43) | 122 (40) | 122 (42) | 0.05 (32) | –5 (31) | –3 (–17 to 10) | 0.621 |
| Time (minutes/day) in LPAc | ||||||||||||
| 6 months | 13 | 12 | 111 | 84 | 105 (35) | 110 (38) | 102 (34) | 109 (40) | –3 (22) | –1 (26) | 4 (–5 to 13) | 0.343 |
| 16–18 months | 12 | 10 | 88 | 66 | 109 (33) | 112 (39) | 108 (36) | 108 (39) | –1 (27) | –4 (24) | –2 (–13 to 8) | 0.692 |
| Time (minutes/day) in MVPAc | ||||||||||||
| 6 months | 13 | 12 | 111 | 84 | 14 (13) | 14 (13) | 13 (10) | 14 (11) | –2 (10) | –0.1 (14) | 2 (–2 to 6) | 0.357 |
| 16–18 months | 12 | 10 | 88 | 66 | 13 (11) | 16 (13) | 14 (11) | 15 (16) | 1 (10) | –1 (19) | –0.4 (–5 to 5) | 0.875 |
Steps per day, time spent sitting, standing and stepping, and time in light physical activity and MVPA on non-workdays
In complete-case analyses, at 6 months’ follow-up, mixed-effect linear regression models revealed statistically significant differences in favour of the SHIFT group in daily step counts, time spent sitting and stepping, and time in light physical activity and MVPA on non-workdays. At 6 months, on non-workdays, daily step counts were larger in the SHIFT group than in the control group (2012 steps/day, 95% CI 480 to 3545 steps/day). In the SHIFT group, non-workday sitting time was shorter (–40 minutes/day, 95% CI –65 to –14 minutes/day), whereas time spent stepping was greater (21, 95% CI 6 to 37 minutes/day), as was time in light physical activity (10 minutes/day, 95% CI 2 to 17 minutes/day) and time in MVPA (11 minutes/day, 95% CI 1 to 20 minutes/day), than in the control group. There were no statistically significant differences observed between groups in activPAL variables measured at 16–18 months’ follow-up (Table 11).
| Daily variable | Number of clusters | Number of participants | Baseline, mean (SD) | Follow-up, mean (SD) | Mean (SD) change from baseline to follow-upa | SHIFT intervention vs. control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Adjusted mean difference (95% CI)b | p-value | |
| Steps/dayc | ||||||||||||
| 6 months | 13 | 12 | 102 | 77 | 8467 (5248) | 8733 (3894) | 6897 (3331) | 9077 (4895) | –1570 (4754) | 344 (4150) | 2012 (480 to 3545) | 0.010 |
| 16–18 months | 12 | 9 | 81 | 65 | 8348 (5935) | 9252 (3994) | 7397 (4116) | 9096 (4167) | –951 (5561) | –156 (4031) | 1392 (–277 to 3060) | 0.102 |
| Time (minutes/day) spent sittingc | ||||||||||||
| 6 months | 13 | 12 | 102 | 77 | 577 (118) | 587 (121) | 610 (131) | 584 (132) | 33 (110) | –4 (123) | –40 (–65 to –14) | 0.003 |
| 16–18 months | 12 | 9 | 81 | 65 | 585 (122) | 568 (105) | 595 (140) | 563 (114) | 11 (116) | –5 (112) | –20 (–62 to 23) | 0.360 |
| Time (minutes/day) spent standingc | ||||||||||||
| 6 months | 13 | 12 | 102 | 77 | 233 (75) | 234 (73) | 214 (88) | 240 (88) | –18 (67) | 6 (82) | 20 (–1 to 41) | 0.059 |
| 16–18 months | 12 | 9 | 81 | 65 | 222 (75) | 243 (69) | 213 (84) | 230 (74) | –9 (68) | –12 (81) | 7 (–22 to 36) | 0.630 |
| Time (minutes/day) spent steppingc | ||||||||||||
| 6 months | 13 | 12 | 102 | 77 | 110 (54) | 114 (42) | 93 (40) | 117 (51) | –17 (48) | 3 (44) | 21 (6 to 37) | 0.008 |
| 16–18 months | 12 | 9 | 81 | 65 | 109 (62) | 120 (42) | 100 (47) | 117 (47) | –9 (54) | –3 (47) | 14 (–5 to 32) | 0.155 |
| Time (minutes/day) in LPAc | ||||||||||||
| 6 months | 13 | 12 | 102 | 77 | 93 (36) | 96 (31) | 81 (33) | 94 (35) | –12 (31) | –2 (31) | 9 (2 to 17) | 0.017 |
| 16–18 months | 12 | 9 | 81 | 65 | 92 (42) | 101 (32) | 87 (39) | 97 (40) | –5 (27) | –3 (40) | 5.28 (–7 to 17) | 0.381 |
| Time (minutes/day) in MVPAc | ||||||||||||
| 6 months | 13 | 12 | 102 | 77 | 17 (37) | 18 (21) | 12 (14) | 23 (29) | –6 (37) | 4 (29) | 11 (1 to 20) | 0.027 |
| 16–18 months | 12 | 9 | 81 | 65 | 17 (42) | 19 (22) | 13 (21) | 20 (21) | –4 (45) | 0.5 (18) | 6 (–2 to 14) | 0.123 |
Anthropometry and markers of adiposity
There were no statistically significant differences observed between groups in anthropometric measures or markers of adiposity at 6 months’ follow-up, although differences in weight and BMI were marginal, with these differences being in favour of the SHIFT group (weight: –1.2 kg, 95% CI –2.6 kg to 0.1 kg; BMI: –0.35 kg/m2, 95% CI –0.75 kg/m2 to 0.05 kg/m2) (Table 12).
| Anthropometric measure | Number of clusters | Number of participants | Baseline, mean (SD) | Follow-up, mean (SD) | Mean (SD) change from baseline to follow-upa | SHIFT intervention vs. control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Adjusted mean difference (95% CI)b | p-value | |
| Weight (kg) | ||||||||||||
| 6 months | 13 | 12 | 143 | 112 | 94.9 (17.5) | 96.9 (16.0) | 94.8 (17.4) | 95.5 (16.2) | –0.1 (4.48) | –1.4 (5.2) | –1.2 (–2.6 to 0.1) | 0.078 |
| BMI (kg/m2) | ||||||||||||
| 6 months | 13 | 12 | 143 | 112 | 29.9 (5.2) | 30.7 (5.0) | 29.9 (5.1) | 30.3 (5.1) | –0.0 (1.4) | –0.4 (1.6) | –0.4 (–0.8 to 0.1) | 0.086 |
| Per cent body fat | ||||||||||||
| 6 months | 13 | 10 | 141 | 96 | 26.3 (5.9) | 27.3 (5.9) | 26.4 (6.0) | 27.1 (5.8) | 0.1 (1.7) | –0.2 (2.0) | –0.2 (–0.7 to 0.3) | 0.435 |
| Waist circumference (cm) | ||||||||||||
| 6 months | 13 | 11 | 143 | 103 | 103.7 (13.7) | 104.9 (12.7) | 103.8 (13.9) | 103.6 (12.7) | 0.1 (5.1) | –1.3 (6.7) | –1.1 (–2.7 to 0.5) | 0.195 |
Biochemical assessments
There were no statistically significant differences observed between groups in any biochemical measures at 6 months’ follow-up (Table 13).
| Biochemical measure | Number of clusters | Number of participants | Baseline, mean (SD) | Follow-up, mean (SD) | Mean (SD) change from baseline to follow-upa | SHIFT intervention vs. control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Adjusted mean difference (95% CI)b | p-value | |
| HbA1c (mmol/mol) | ||||||||||||
| 6 months | 13 | 10 | 139 | 89 | 36.8 (9.4) | 35.6 (10.3) | 37.1 (10.4) | 35.0 (9.0) | 0.2 (6.0) | –0.6 (6.9) | –1.9 (–4.9 to 1.2) | 0.229 |
| Triglycerides (mmol/l) | ||||||||||||
| 6 months | 13 | 10 | 143 | 98 | 1.7 (1.1) | 1.6 (0.9) | 1.7 (1.1) | 1.7 (1.1) | 0.1 (1.0) | 0.04 (0.9) | –0.08 (–0.3 to 0.2) | 0.530 |
| HDL-C (mmol/l) | ||||||||||||
| 6 months | 13 | 10 | 143 | 98 | 1.2 (0.4) | 1.2 (0.3) | 1.3 (0.3) | 1.3 (0.3) | 0.02 (0.2) | 0.1 (0.2) | 0.04 (–0.02 to 0.1) | 0.241 |
| LDL-C (mmol/l) | ||||||||||||
| 6 months | 13 | 10 | 143 | 98 | 2.9 (0.8) | 2.8 (0.8) | 2.9 (0.9) | 2.8 (0.9) | –0.01 (0.9) | –0.03 (0.8) | 0.0 (–0.2 to 0.2) | 0.973 |
| Total cholesterol (mmol/l) | ||||||||||||
| 6 months | 13 | 10 | 143 | 98 | 4.4 (0.9) | 4.4 (0.9) | 4.5 (1.0) | 4.4 (1.0) | 0.02 (0.9) | 0.1 (0.9) | 0.02 (–0.2 to 0.2) | 0.868 |
Dietary quality and fruit and vegetable intake
There were no statistically significant differences observed between groups in reported fruit and vegetable intake or overall dietary quality at 6 months’ follow-up or at 16–18 months’ follow-up (Table 14).
| Fruit and vegetable intake and dietary quality | Number of clusters | Number of participants | Baseline, mean (SD) | Follow-up, mean (SD) | Mean (SD) change from baseline to Follow-upa | SHIFT intervention vs. control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Adjusted mean difference (95% CI)b | p-value | |
| Fruit (g/day) | ||||||||||||
| 6 months | 13 | 12 | 147 | 124 | 100.8 (122.2) | 135.4 (158.1) | 123.9 (142.3) | 120.6 (153.6) | 23.1 (128.1) | –14.8 (159.5) | –20.6 (–64.5 to 23.3) | 0.359 |
| 16–18 months | 12 | 10 | 112 | 102 | 92.2 (109.4) | 127.4 (152.7) | 89.6 (111.8) | 112.1 (148.0) | –2.7 (105.3) | –15.2 (162.8) | 7.4 (–30.3 to 45.2) | 0.700 |
| Vegetables (g/day) | ||||||||||||
| 6 months | 13 | 12 | 147 | 124 | 110.5 (135.3) | 127.7 (165.8) | 106.1 (142.1) | 131.9 (184.6) | –4.4 (167.3) | 4.1 (207.0) | 27.3 (–24.8 to 79.4) | 0.305 |
| 16–18 months | 12 | 10 | 112 | 102 | 95.1 (98.1) | 127.3 (167.8) | 100.2 (145) | 90.0 (101.0) | 5.1 (148.5) | –37.2 (157.4) | –25.3 (–68.5 to 17.9) | 0.251 |
| Dietary quality scorec | ||||||||||||
| 6 months | 13 | 12 | 147 | 124 | 11.1 (2.0) | 11.1 (2.1) | 11.4 (1.7) | 11.1 (2.0) | 0.3 (2.2) | –0.01 (2.4) | –0.2 (–0.7 to 0.2) | 0.241 |
| 16–18 months | 12 | 11 | 112 | 102 | 11.1 (1.8) | 11.0 (2.0) | 11.3 (1.6) | 11.3 (1.8) | 0.1 (2.0) | 0.3 (2.3) | 0.07 (–0.4 to 0.5) | 0.778 |
Secondary outcomes: descriptive analyses
Further activPAL variables
Across all monitored days and workdays, for the SHIFT group, there were no noticeable differences in time spent sitting in prolonged bouts (> 30 minutes), the number of transitions from sitting to standing and the proportions of time spent sitting, standing and stepping, and the proportion of sitting spent in prolonged bouts, between baseline and 6 months’ follow-up. Similar findings were observed for the control group, except for the time spent sitting in prolonged bouts, which tended to increase at 6 months’ follow-up (Table 15). On non-workdays, the control group exhibited increases in the time spent sitting (and the proportion of sitting) in prolonged bouts at 6 months’ follow-up, relative to baseline. The control group also exhibited an increase in the overall proportion of time spent sitting and a decrease in the proportion of time spent standing on non-workdays at 6 months’ follow-up. For the SHIFT group, no noticeable differences were observed for any variables on non-workdays between baseline and 6 months (see Table 15).
| Variable | Number of participants | Baseline, mean (SD) | 6-month follow-up, mean (SD) | Mean (SD) change from baseline to 6-month follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| All daysa | ||||||||
| Valid days (n) | 119 | 92 | 7 (2) | 6 (3) | 7 (1) | 7 (1) | 1 (3) | 1 (3) |
| Waking wear time (minutes/day) | 119 | 92 | 995 (66) | 997 (56) | 997 (64) | 987 (67) | 2 (67) | –10 (60) |
| Sitting time (minutes/day) in prolonged bouts (i.e. > 30 minutes) | 119 | 92 | 430 (116) | 396 (127) | 456 (115) | 395 (113) | 26 (92) | –1 (90) |
| Number of transitions | 119 | 92 | 50 (16) | 53 (18) | 49 (18) | 52 (19) | –1 (12) | –1 (14) |
| Per cent of day sitting | 119 | 92 | 68 (8) | 67 (8) | 70 (7) | 67 (8) | 2 (5) | 0 (6) |
| Per cent of day standing | 119 | 92 | 21 (6) | 21 (5) | 19 (5) | 21 (6) | –2 (4) | 0 (5) |
| Per cent of day stepping | 119 | 92 | 12 (3) | 12 (4) | 11 (3) | 12 (4) | –1 (2) | 0 (3) |
| Per cent of day sitting in prolonged bouts (i.e. > 30 minutes) | 119 | 92 | 63 (11) | 58 (13) | 65 (12) | 59 (11) | 2 (8) | 1 (9) |
| Workdaysa | ||||||||
| Valid days (n) | 114 | 87 | 4 (2) | 4 (2) | 5 (1) | 5 (2) | 1 (2) | 1 (3) |
| Waking wear time (minutes/day) | 114 | 87 | 1033 (75) | 1037 (96) | 1032 (126) | 1017 (86) | –1 (133) | –20 (94) |
| Sitting time (minutes/day) in prolonged bouts (i.e. > 30 minutes) | 114 | 87 | 481 (128) | 452 (171) | 494 (140) | 433 (139) | 13 (118) | –19 (142) |
| Number of transitions | 114 | 87 | 51 (21) | 54 (23) | 49 (21) | 54 (26) | –2 (14) | 0 (21) |
| Per cent of day sitting | 114 | 87 | 70 (7) | 69 (9) | 70 (10) | 69 (8) | 0 (9) | 0 (8) |
| Per cent of day standing | 114 | 87 | 19 (6) | 19 (7) | 18 (5) | 19 (5) | –1 (5) | 0 (7) |
| Per cent of day stepping | 114 | 87 | 12 (4) | 12 (4) | 11 (4) | 12 (4) | –1 (3) | 0 (3) |
| Per cent of day sitting in prolonged bouts (i.e. > 30 minutes) | 114 | 87 | 66 (13) | 61 (16) | 66 (15) | 61 (14) | 0 (12) | 0 (13) |
| Non-workdaysa | ||||||||
| Valid days (n) | 102 | 80 | 2 (1) | 2 (1) | 3 (1) | 3 (1) | 1 (2) | 1 (2) |
| Waking wear time (minutes/day) | 102 | 80 | 920 (78) | 937 (86) | 917 (107) | 937 (103) | –3 (107) | 0 (107) |
| Sitting time (minutes/day) in prolonged bouts (i.e. > 30 minutes) | 102 | 80 | 325 (139) | 329 (137) | 375 (156) | 325 (140) | 50 (137) | –4 (124) |
| Number of transitions | 102 | 80 | 48 (21) | 47 (14) | 46 (18) | 47 (15) | –2 (16) | 0 (14) |
| Per cent of day sitting | 102 | 80 | 63 (11) | 63 (11) | 66 (12) | 62 (12) | 3 (10) | –1 (11) |
| Per cent of day standing | 102 | 80 | 25 (8) | 25 (8) | 23 (9) | 26 (9) | –2 (7) | 1 (9) |
| Per cent of day stepping | 102 | 80 | 12 (6) | 12 (5) | 10 (4) | 12 (5) | –2 (5) | 0 (5) |
| Per cent of day sitting in prolonged bouts (i.e. > 30 minutes) | 102 | 80 | 55 (16) | 54 (13) | 60 (16) | 54 (14) | 5 (15) | 0 (12) |
Across all monitored days, workdays and non-workdays, for both groups, there were no noticeable differences in time spent sitting in prolonged bouts (> 30 minutes), the number of transitions from sitting to standing, the proportions of time spent sitting, standing and stepping, and the proportion of sitting spent in prolonged bouts, between baseline and 16–18 months’ follow-up (Table 16).
| Variable | Number of participants | Baseline, mean (SD) | 16- to 18-month follow-up, mean (SD) | Mean (SD) change from baseline to 16- to 18-month follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| All daysa | ||||||||
| Valid days (n) | 90 | 74 | 7 (2) | 6 (2) | 7 (1) | 7 (1) | 0 (2) | 1 (3) |
| Waking wear time (minutes/day) | 90 | 74 | 993 (67) | 992 (54) | 991 (59) | 978 (65) | –2 (62) | –14 (78) |
| Sitting time (minutes/day) in prolonged bouts (i.e. > 30 minutes) | 90 | 74 | 434 (124) | 380 (121) | 434 (134) | 383 (114) | 0 (107) | 3 (115) |
| Number of transitions | 90 | 74 | 49 (18) | 54 (17) | 51 (21) | 53 (19) | 2 (15) | –1 (19) |
| Per cent of day sitting | 90 | 74 | 68 (8) | 66 (8) | 69 (8) | 66 (7) | 1 (6) | 0 (9) |
| Per cent of day standing | 90 | 74 | 20 (6) | 22 (6) | 20 (6) | 21 (5) | 0 (5) | –1 (7) |
| Per cent of day stepping | 90 | 74 | 12 (4) | 13 (4) | 12 (4) | 12 (4) | 0 (2) | –1 (3) |
| Per cent of day sitting in prolonged bouts (i.e. > 30 minutes) | 90 | 74 | 63 (12) | 58 (14) | 63 (14) | 58 (14) | 0 (11) | 0 (12) |
| Workdaysa | ||||||||
| Valid days (n) | 89 | 68 | 5 (2) | 4 (2) | 5 (2) | 5 (2) | 0 (2) | 1 (3) |
| Waking wear time (minutes/day) | 89 | 68 | 1031 (78) | 1028 (84) | 1026 (141) | 994 (192) | –5 (137) | –34 (189) |
| Sitting time (minutes/day) in prolonged bouts (i.e. > 30 minutes) | 89 | 68 | 480 (132) | 429 (163) | 478 (161) | 419 (163) | –2 (131) | –10 (163) |
| Number of transitions | 89 | 68 | 51 (23) | 55 (22) | 51 (25) | 55 (24) | 0 (20) | 0 (21) |
| Per cent of day sitting | 89 | 68 | 70 (7) | 68 (10) | 69 (11) | 67 (14) | –1 (10) | –1 (15) |
| Per cent of day standing | 89 | 68 | 19 (5) | 20 (8) | 18 (6) | 18 (6) | –1 (6) | –2 (8) |
| Per cent of day stepping | 89 | 68 | 12 (4) | 12 (4) | 12 (4) | 12 (5) | 0 (4) | 0 (4) |
| Per cent of day sitting in prolonged bouts (i.e. > 30 minutes) | 89 | 68 | 66 (13) | 60 (17) | 65 (16) | 60 (17) | –1 (12) | 0 (15) |
| Non-workdaysa | ||||||||
| Valid days (n) | 83 | 69 | 2 (1) | 2 (1) | 2 (1) | 3 (2) | 0 (2) | 1 (2) |
| Waking wear time (minutes/day) | 83 | 69 | 915 (76) | 928 (75) | 886 (163) | 858 (230) | –29 (164) | –70 (221) |
| Sitting time (minutes/day) in prolonged bouts (i.e. > 30 minutes) | 83 | 69 | 342 (149) | 305 (115) | 344 (174) | 286 (145) | 2 (146) | –19 (143) |
| Number of transitions | 83 | 69 | 45 (21) | 48 (15) | 47 (25) | 46 (20) | 2 (21) | –2 (21) |
| Per cent of day sitting | 83 | 69 | 64 (12) | 61 (10) | 64 (17) | 58 (18) | 0 (15) | –3 (19) |
| Per cent of day standing | 83 | 69 | 24 (8) | 26 (8) | 23 (10) | 24 (10) | –1 (8) | –2 (10) |
| Per cent of day stepping | 83 | 69 | 12 (6) | 13 (4) | 11 (6) | 12 (6) | –1 (6) | –1 (5) |
| Per cent of day sitting in prolonged bouts (i.e. > 30 minutes) | 83 | 69 | 57 (16) | 53 (14) | 57 (17) | 52 (16) | 0 (16) | –1 (16) |
Sleep duration and quality, subjective situational sleepiness and chronotype
Across all monitored days, between baseline and 6 months’ follow-up, both groups exhibited a decrease in their sleep window duration (defined as the time between ‘lights out’ and out of bed time) and a decrease in their overall sleep duration. These changes appeared to be driven by large reductions in sleep window duration and sleep duration on workdays at 6 months’ follow-up. In contrast, on non-workdays, increases in sleep window duration and sleep duration were observed for both groups at 6 months. There were no noticeable changes in sleep efficiency across any types of day between baseline and 6 months’ follow-up for either group (Table 17). There were no changes in ratings of situational sleepiness or chronotype score between baseline and either follow-up period for both groups (Table 18).
| Variable | Number of participants | Baseline, mean (SD) | 6-month follow-up, mean (SD) | Mean (SD) change from baseline to 6-month follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| All days | ||||||||
| Number of valid nights | 118 | 89 | 6 (1) | 5 (2) | 5 (1) | 5 (1) | –1 (1) | 0 (2) |
| Sleep windowa duration (minutes) | 118 | 89 | 425 (54) | 419 (54) | 410 (62) | 405 (65) | –15 (70) | –14 (58) |
| Sleep duration (minutes) | 118 | 89 | 370 (54) | 368 (55) | 355 (57) | 357 (59) | –15 (57) | –11 (51) |
| Sleep efficiencyb (%) | 118 | 89 | 87 (7) | 88 (6) | 87 (6) | 89 (6) | 0 (4) | 1 (5) |
| Workdays | ||||||||
| Number of valid nights | 100 | 67 | 3 (1) | 3 (1) | 3 (1) | 3 (1) | 0 (1) | 0 (2) |
| Sleep windowa duration (minutes) | 100 | 67 | 420 (61) | 402 (67) | 363 (74) | 369 (79) | –57 (80) | –33 (70) |
| Sleep duration (minutes) | 100 | 67 | 366 (56) | 354 (65) | 317 (68) | 329 (72) | –49 (69) | –25 (64) |
| Sleep efficiencyb (%) | 100 | 67 | 87 (8) | 88 (7) | 88 (7) | 89 (6) | 1 (6) | 1 (5) |
| Non-workdays | ||||||||
| Number of valid nights | 96 | 60 | 2 (1) | 2 (1) | 2 (1) | 2 (1) | 0 (1) | 0 (1) |
| Sleep windowa duration (minutes) | 96 | 60 | 422 (79) | 429 (78) | 482 (92) | 462 (103) | 60 (107) | 33 (123) |
| Sleep duration (minutes) | 96 | 60 | 367 (74) | 377 (78) | 416 (78) | 406 (95) | 49 (90) | 29 (111) |
| Sleep efficiencyb (%) | 96 | 60 | 87 (7) | 88 (7) | 87 (7) | 88 (8) | 0 (6) | 0 (5) |
| Variable | Number of participants | Baseline, median (IQR) | Follow-up, median (IQR) | Median (IQR) change from baseline to follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| Karolinska Sleepiness Scale ratinga | ||||||||
| 6 months | 144 | 113 | 3 (2–5) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 0 (–1 to 1) | 0 (–1 to 1) |
| 16–18 months | 99 | 90 | 3 (2–5) | 3 (2–5) | 3 (3–6) | 3 (2–5) | 0 (–1 to 1) | 0 (0–2) |
| MEQ scoreb | ||||||||
| 6 months | 144 | 113 | 19 (15–21) | 17 (14–20) | 18 (16–21) | 17 (14–20) | 0 (–1 to 1) | 0 (–1 to 1) |
| 16–18 months | 99 | 90 | 18 (15–21) | 16 (13–20) | 19 (16–21) | 17 (14–20) | 0 (–1 to 2) | 0 (–1 to 1) |
Blood pressure and psychophysiological reactivity
Small reductions in resting systolic and diastolic blood pressure were observed for both groups between the baseline and 6-month follow-up measures, but no noticeable changes were observed in resting heart rate for either group (Table 19). Mean blood pressure and heart rate measures increased during the mirror tracing task; however, the differences between resting values and values recorded during the task tended to be smaller for both groups during the 6-month follow-up measures (see Table 19). There were no differences in perceived stress ratings during this task between baseline and follow-up for either group. The control group tended to have fewer errors while undertaking this task at 6-months follow-up, whereas no evidence of a change in performance was observed for the SHIFT group (see Table 19).
| Variable | Number of participants | Baseline, median (IQR) | 6-month follow-up, median (IQR) | Median (IQR) change from baseline to 6-month follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| Resting blood pressure and heart ratea | ||||||||
| Systolic blood pressure (mmHg) | 145 | 104 | 130 (120–139) | 130 (122–138) | 127 (118–135) | 127 (118–136) | –2 (–9 to 4) | –3 (–10 to 5) |
| Diastolic blood pressure (mmHg) | 145 | 104 | 82 (76–88) | 82 (78–88) | 80 (74–86) | 80 (75–87) | –2 (–6 to 4) | –1 (–6 to 4) |
| Heart rate (b.p.m.) | 143 | 104 | 67 (60–74) | 68 (60–74) | 66 (60–73) | 66 (58–72) | 0 (–5 to 5) | –1 (–6 to 3) |
| Blood pressure and heart rate during the mirror tracing taskb | ||||||||
| Systolic blood pressure (mmHg) | 138 | 100 | 146 (134–159) | 145 (137–158) | 142 (132–156) | 143 (131–153) | –3 (–12 to 5) | –4 (–10 to 4) |
| Diastolic blood pressure (mmHg) | 138 | 100 | 91 (85–98) | 92 (85–103) | 89 (81–95) | 89 (84–96) | –2 (–9 to 3) | –3 (–9 to 1) |
| Heart rate (b.p.m.) | 136 | 100 | 74 (67–80) | 72 (66–82) | 72 (66–78) | 72 (65–78) | –1 (–7 to 4) | –2 (–7 to 3) |
| Psychophysiological reactivityc | ||||||||
| ΔSystolic blood pressure (mmHg) | 138 | 100 | 17 (10–22) | 16 (9–26) | 16 (9–24) | 15 (8–22) | –1 (–8 to 8) | –3 (–9 to 6) |
| ΔDiastolic B blood pressure (mmHg) | 138 | 100 | 10 (6–14) | 10 (7–14) | 8 (4–12) | 8 (4–14) | –2 (–6 to 4) | –1 (–6 to 3) |
| ΔHeart rate (b.p.m.) | 136 | 100 | 7 (2–10) | 6 (3–10) | 5 (2–8) | 6 (4–10) | –1 (–6 to 2) | 0 (–4 to 5) |
| Number of errors and feelings of stress | ||||||||
| Number of errors | 132 | 100 | 28 (12–48) | 32 (17–54) | 23 (8–52) | 34 (14–56) | –5 (–15 to 11) | –1 (–17 to 11) |
| Perceived stressd | 136 | 100 | 3 (2–4) | 3 (2–4) | 2 (1–3) | 3 (2–4) | 0 (–1 to 0) | 0 (–1 to 0) |
Cognitive function
There were no noticeable differences in reaction times, measured using the Stroop test, between baseline and 6 months’ follow-up for both groups. No noticeable differences were observed between groups at baseline and 6 months (Table 20).
| Reaction time (ms) | Number of participants | Baseline, median (IQR) | 6-month follow-up, median (IQR) | Median (IQR) change from baseline to 6-month follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| Congruent conditiona | 111 | 68 | 988 (880 to 1112) | 998 (888 to 1110) | 959 (895 to 1058) | 976 (878 to 1058) | –17 (–104 to 68) | 4 (–102 to 59) |
| Incongruent conditionb | 111 | 68 | 1121 (992 to 1325) | 1125 (994 to 1420) | 1078 (977 to 1247) | 1095 (968 to 1268) | –41 (–145 to 36) | –44 (–135 to 43) |
Functional fitness
No noticeable changes in grip strength were observed between baseline and 6 months’ follow-up in the control group, whereas modest improvements in grip strength for both hands were observed following completion of the intervention in the SHIFT group (Table 21).
| Grip strength | Number of participants | Baseline, mean (SD) | 6-month follow-up, mean (SD) | Mean (SD) change from baseline to 6-month follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| Right hand (kg) | 144 | 104 | 52.0 (9.6) | 51.9 (8.5) | 52.2 (10.2) | 52.7 (8.9) | 0.2 (6.9) | 0.8 (6.2) |
| Left hand (kg) | 144 | 104 | 50.0 (9.2) | 49.1 (7.5) | 50.0 (9.8) | 50.6 (8.0) | 0.02 (6.6) | 1.5 (5.1) |
| Average (kg) | 143 | 104 | 51.0 (9.0) | 50.5 (7.6) | 51.0 (9.5) | 51.6 (7.9) | 0.09 (5.7) | 1.1 (4.9) |
Mental well-being
There were no noticeable differences in self-reported scores for symptoms of anxiety, depression or social isolation between baseline and 6 months’ follow-up for both groups, and similar findings were also observed for symptoms of anxiety and depression at 16–18 months’ follow-up. There was a tendency in both groups for perceived social isolation scores to increase marginally at 16–18 months’ follow-up (Table 22). No noticeable differences were observed between groups at any assessment point.
| Variable | Number of participants | Baseline, median (IQR) | Follow-up, median (IQR) | Median (IQR) change from baseline to follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| HADS anxietya | ||||||||
| 6 months | 145 | 113 | 5 (3–7) | 4 (2–7) | 4 (2–6) | 4 (2–7) | –1 (–3 to 1) | 0 (–2 to 1) |
| 16–18 months | 100 | 88 | 5 (3–7) | 4 (2–7) | 4 (2–7) | 4 (2–7) | –1 (–2 to 1) | 0 (–1 to 1) |
| HADS depressiona | ||||||||
| 6 months | 145 | 113 | 3 (2–7) | 3 (1–5) | 3 (1–6) | 3 (1–5) | –1 (–2 to 1) | 0 (–1 to 1) |
| 16–18 months | 100 | 88 | 3 (2–6) | 3 (1–4) | 3 (1–6) | 3 (1–5) | 0 (–2 to 1) | 0 (–1 to 1) |
| Social isolationb | ||||||||
| 6 months | 145 | 113 | 44 (39–51) | 41 (34–49) | 44 (34–49) | 43 (34–50) | 0 (–5 to 2) | 0 (0 to 5) |
| 16–18 months | 101 | 88 | 44 (39–51) | 41 (34–49) | 47 (39–52) | 44 (34–51) | 0 (–2 to 4) | 0 (0 to 5) |
Musculoskeletal symptoms
Table 23 provides a summary of the prevalence of musculoskeletal discomfort reported in the past month for each body site, along with discomfort scores by body region, reported over the three time points. There was a tendency for the prevalence of musculoskeletal discomfort across the majority of body sites to decrease at the two follow-up assessments in both groups, with similar changes in prevalence occurring between groups. Similarly, there were no noticeable differences in discomfort scores (i.e. upper extremity, lower extremity and overall) between baseline and 6 months’ follow-up, and between baseline and 16–18 months’ follow-up, for both groups. No noticeable differences in discomfort scores were observed between groups at any assessment point.
| Prevalence of musculoskeletal discomfort in the past month per body areaa | Number of participants | Baseline, proportion (%) | Follow-up, proportion (%) | Change in proportion (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| Neck | ||||||||
| 6 months | 145 | 112 | 39 | 34 | 36 | 34 | –3 | 0 |
| 16–18 months | 101 | 91 | 42 | 31 | 38 | 37 | –4 | 7 |
| Shoulder | ||||||||
| 6 months | 145 | 112 | 40 | 44 | 41 | 43 | 1 | –1 |
| 16–18 months | 101 | 91 | 44 | 42 | 49 | 43 | 5 | 1 |
| Upper back | ||||||||
| 6 months | 145 | 112 | 21 | 27 | 17 | 20 | –4 | –7 |
| 16–18 months | 101 | 91 | 22 | 29 | 25 | 26 | 3 | –2 |
| Elbow | ||||||||
| 6 months | 145 | 112 | 21 | 19 | 19 | 24 | –2 | 5 |
| 16–18 months | 101 | 91 | 23 | 15 | 29 | 18 | 6 | 2 |
| Wrist/hand | ||||||||
| 6 months | 145 | 112 | 26 | 33 | 29 | 31 | 3 | –2 |
| 16–18 months | 101 | 91 | 30 | 34 | 38 | 33 | 8 | –1 |
| Lower back | ||||||||
| 6 months | 145 | 112 | 57 | 56 | 50 | 49 | –7 | –7 |
| 16–18 months | 101 | 91 | 59 | 57 | 53 | 47 | –6 | –10 |
| Hip/thigh | ||||||||
| 6 months | 145 | 112 | 26 | 24 | 14 | 13 | –12 | –11 |
| 16–18 months | 101 | 91 | 24 | 25 | 22 | 22 | –2 | –3 |
| Knee | ||||||||
| 6 months | 145 | 112 | 45 | 44 | 41 | 40 | –3 | –4 |
| 16–18 months | 101 | 91 | 47 | 48 | 42 | 42 | –5 | –7 |
| Ankle/feet | ||||||||
| 6 months | 145 | 112 | 28 | 27 | 21 | 21 | –8 | –5 |
| 16–18 months | 101 | 91 | 32 | 26 | 29 | 29 | –3 | 2 |
| Discomfort scores | Median (IQR) | Median (IQR) | Median (IQR) change from baseline to follow-up | |||||
| Upper extremity discomfortb | ||||||||
| 6 months | 145 | 112 | 0.5 (0.0–1.5) | 1.0 (0.0–2.0) | 0.5 (0.0–1.8) | 0.8 (0.0–2.0) | 0.0 (–0.5 to 0.5) | 0.0 (–0.5 to 0.5) |
| 16–18 months | 101 | 91 | 0.5 (0.0–1.5) | 0.8 (0.0–1.9) | 1.0 (0.0–2.5) | 0.8 (0.0–2.0) | 0.0 (–0.3 to 1.0) | 0.0 (–0.5 to 0.6) |
| Lower extremity discomfortc | ||||||||
| 6 months | 145 | 112 | 0.7 (0.0–2.0) | 0.3 (0.0–1.7) | 0.3 (0.0–1.7) | 0.3 (0.0–1.7) | 0.0 (–1.0 to 0.3) | 0.0 (–0.3 to 0.3) |
| 16–18 months | 101 | 91 | 1.0 (0.0–2.0) | 0.7 (0.0–2.0) | 0.3 (0.0–1.7) | 0.3 (0.0–2.0) | 0.0 (–0.7 to 0.3) | 0.0 (–0.3 to 0.7) |
| Overall discomfortd | ||||||||
| 6 months | 145 | 112 | 1.0 (0.3–1.8) | 1.0 (0.3–1.9) | 0.9 (0.2–1.8) | 0.9 (0.3–1.7) | 0.0 (–0.7 to 0.4) | 0.0 (–0.6 to 0.3) |
| 16–18 months | 101 | 91 | 1.1 (0.4–1.7) | 1.0 (0.4–1.9) | 1.3 (0.3–2.2) | 1.0 (0.2–2.1) | 0.0 (–0.3 to 0.8) | 0.0 (–0.4 to 0.6) |
Work-related psychosocial variables
Table 24 provides a descriptive summary of a range of work-related psychosocial variables assessed across the three time points. There were no noticeable differences in any of the outcome measures (i.e. work engagement, occupational fatigue, perceived job satisfaction and performance, sickness absence, presenteeism, perceived work ability and perceived job demands) between baseline and 6 months’ follow-up, and between baseline and 16–18 months’ follow-up, for both groups. No noticeable differences in any measure were observed between groups at any assessment point.
| Variable | Number of participants | Baseline, median (IQR) | Follow-up, median (IQR) | Median (IQR) change from baseline to follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| Utrecht Work Engagement Scalea | ||||||||
| Vigour | ||||||||
| 6 months | 144 | 113 | 4.0 (3.3–5.0) | 4.0 (3.0–4.7) | 4.0 (3.0–5.0) | 4.0 (2.7–5.0) | 0.0 (–0.7 to 0.3) | 0.0 (–0.7 to 0.3) |
| 16–18 months | 100 | 89 | 4.0 (3.3–5.0) | 3.7 (3.0–4.7) | 4.3 (3.3–4.7) | 3.7 (2.3–4.7) | 0.0 (–0.7 to 0.7) | –0.3 (–0.7 to 0.0) |
| Dedication | ||||||||
| 6 months | 144 | 113 | 4.3 (3.3–5.3) | 4.0 (3.0–5.0) | 4.3 (3.3–5.0) | 4.3 (3.0–5.0) | 0.0 (–0.7 to 0.3) | 0.0 (–0.3 to 0.7) |
| 16–18 months | 100 | 89 | 3.7 (4.7–6.0) | 4.0 (3.0–5.0) | 4.3 (3.6–5.0) | 3.7 (2.7–5.0) | –0.2 (–0.7 to 0.3) | 0.0 (–0.7 to 0.3) |
| Absorption | ||||||||
| 6 months | 144 | 113 | 4.0 (3.0–5.0) | 3.7 (2.7–4.7) | 3.7 (2.7–4.7) | 4.0 (2.7–4.7) | 0.0 (–0.7 to 0.3) | 0.0 (–0.7 to 0.7) |
| 16–18 months | 100 | 89 | 3.3 (3.7–5.0) | 3.7 (2.3–4.7) | 4.0 (3.3–5.0) | 3.7 (2.3–4.7) | 0.0 (–1.0 to 0.7) | 0.0 (–0.7 to 0.3) |
| Overall summary score | ||||||||
| 6 months | 144 | 113 | 4.3 (3.2–5.0) | 3.9 (3.0–4.9) | 4.1 (3.0–4.8) | 3.9 (2.8–4.8) | –0.1 (–0.7 to 0.3) | 0.0 (–0.4 to 0.4) |
| 16–18 months | 100 | 89 | 3.7 (4.4–5.2) | 3.8 (2.9–4.9) | 4.2 (3.4–4.8) | 3.7 (2.7–4.6) | –0.1 (–0.7 to 0.6) | –0.1 (–0.7 to 0.3) |
| OFER scaleb | ||||||||
| Chronic fatigue | ||||||||
| 6 months | 144 | 113 | 33.3 (16.7–60.8) | 33.3 (16.7–53.3) | 36.7 (16.7–56.7) | 36.7 (16.7–56.7) | 0.0 (–6.7 to 10.8) | 0.0 (–10.0 to 13.3) |
| 16–18 months | 100 | 90 | 31.7 (16.7–53.3) | 31.7 (16.7–53.3) | 36.7 (20.0–57.5) | 33.3 (20.0–53.3) | 3.3 (–3.3 to 13.3) | 1.7 (–13.3 to 16.7) |
| Acute fatigue | ||||||||
| 6 months | 144 | 113 | 46.7 (32.5–60.0) | 50.0 (30.0–63.3) | 43.3 (26.7–63.3) | 46.7 (30.0–66.7) | 0.0 (–10.8 to 10.0) | 0.0 (–13.3 to 6.7) |
| 16–18 months | 100 | 90 | 50.0 (33.3–60.8) | 48.3 (26.7–63.3) | 50.0 (30.0–63.3) | 50.0 (30.8–66.7) | –3.3 (–14.2 to 13.3) | 3.3 (–6.7 to 13.3) |
| Intershift recovery | ||||||||
| 6 months | 144 | 113 | 55.0 (40.0–80.0) | 60.0 (43.3–76.7) | 60.0 (43.3–76.7) | 56.7 (43.3–76.7) | 0.0 (–13.3 to 10.0) | 0.0 (–10.0 to 6.7) |
| 16–18 months | 100 | 90 | 53.3 (42.5–80.0) | 60.0 (43.3–80.0) | 56.7 (40.0–77.5) | 53.3 (40.8–80.0) | 0.0 (–10.0 to 10.0) | 0.0 (–10.0 to 12.5) |
| Job satisfaction ratingc | ||||||||
| 6 months | 144 | 113 | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 0.0 (–1.0 to 0.0) | 0.0 (–1.0 to 0.0) |
| 16–18 months | 100 | 90 | 6.0 (4.0–6.0) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 0.0 (–1.0 to 0.0) | 0.0 (–1.0 to 1.0) |
| Job performance ratingc | ||||||||
| 6 months | 144 | 113 | 6.0 (5.0–7.0) | 6.0 (6.0–7.0) | 6.0 (6.0–7.0) | 6.0 (6.0–7.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| 16–18 months | 100 | 90 | 6.0 (5.0–6.3) | 6.0 (6.0–7.0) | 6.0 (5.0–7.0) | 6.0 (6.0–7.0) | 0.0 (–1.0 to 1.0) | 0.0 (0.0–0.0) |
| Sickness absence (days)d | ||||||||
| 6 months | 142 | 113 | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) | 0.0 (0.0–3.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| 16–18 months | 100 | 90 | 0.0 (0.0–2.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (–1.3 to 0.0) | 0.0 (0.0–0.0) |
| Presenteeism (days)d | ||||||||
| 6 months | 141 | 113 | 2.0 (0.0–5.0) | 2.0 (0.0–5.0) | 1.0 (0.0–5.0) | 1.0 (0.0–5.0) | 0.0 (–2.0 to 1.0) | 0.0 (–1.3 to 3.0) |
| 16–18 months | 98 | 89 | 2.0 (0.0–5.0) | 2.0 (0.0–5.0) | 0.0 (0.0–2.0) | 0.0 (0.0–5.0) | 0.0 (–4.0 to 0.0) | 0.0 (–2.0 to 2.0) |
| Work ability ratinge | ||||||||
| 6 months | 144 | 113 | 9.0 (8.0–10.0) | 8.5 (8.0–10.0) | 8.0 (7.0–9.0) | 9.0 (8.0–9.0) | 0.0 (–1.0 to 1.0) | 0.0 (–1.0 to 1.0) |
| 16–18 months | 96 | 87 | 9.0 (8.0–9.0) | 9.0 (8.0–10.0) | 8.0 (8.0–9.0) | 9.0 (8.0–10.0) | 0.0 (–1.0 to 0.0) | 0.0 (–1.0 to 1.0) |
| Work demands (Health and Safety Executive Management Standards Indicator Tool)f | ||||||||
| Demand summary score | ||||||||
| 6 months | 144 | 113 | 2.1 (1.8–2.5) | 2.1 (1.6–2.9) | 2.1 (1.8–2.6) | 2.1 (1.6–2.6) | 0.1 (–0.3 to 0.4) | 0.0 (–0.4 to 0.3) |
| 16–18 months | 100 | 90 | 2.1 (1.8–2.7) | 2.2 (1.6–2.9) | 2.2 (1.6–2.8) | 2.3 (1.6–2.8) | 0.1 (–0.4 to 0.5) | 0.0 (–0.5 to 0.4) |
| Control summary score | ||||||||
| 6 months | 144 | 113 | 3.3 (2.7–3.8) | 3.3 (2.8–3.8) | 3.3 (2.7–3.8) | 3.2 (2.8–3.8) | 0.0 (–0.3 to 0.5) | 0.0 (–0.5 to 0.3) |
| 16–18 months | 100 | 90 | 3.2 (2.6–3.8) | 3.2 (2.8–3.8) | 3.3 (2.7–3.9) | 3.2 (2.8–4.0) | 0.0 (–0.5 to 0.7) | 0.0 (–0.3 to 0.5) |
| Support summary score | ||||||||
| 6 months | 144 | 113 | 3.3 (2.8–3.8) | 3.3 (2.7–3.9) | 3.2 (2.6–3.8) | 3.2 (2.7–3.9) | –0.1 (–0.4 to 0.3) | –0.1 (–0.4 to 0.4) |
| 16–18 months | 100 | 90 | 3.4 (2.9–4.0) | 3.3 (2.7–4.0) | 3.2 (2.8–3.9) | 3.3 (2.8–3.9) | 0.0 (–0.4 to 0.3) | 0.1 (–0.3 to 0.4) |
Driving-related safety behaviour
There were no noticeable differences in self-reported driving-related safety behaviour between baseline and 6 months’ follow-up, and between baseline and 16–18 months’ follow-up, for both groups (Table 25). No noticeable differences were observed between groups at any assessment point.
| Variable | Number of participants | Baseline, median (IQR) | Follow-up, median (IQR) | Median (IQR) change from baseline to follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| Occasionally jump to get out of lorry quickly | ||||||||
| 6 months | 144 | 113 | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–2) | 0 (–1 to 0) | 0 (–1 to 0) |
| 16–18 months | 100 | 89 | 2 (1–3) | 2 (1–3) | 1 (1–3) | 1 (1–2) | 0 (–1 to 0) | 0 (–1 to 0) |
| Compliance with posted speed limits | ||||||||
| 6 months | 144 | 113 | 4 (4–5) | 4 (4–5) | 4 (3–5) | 4 (4–5) | 0 (–1 to 0) | 0 (0–0) |
| 16–18 months | 100 | 89 | 4 (4–5) | 4 (4–5) | 4 (4–5) | 5 (4–5) | 0 (0–1) | 0 (0–1) |
| Occasionally drive without getting enough sleep | ||||||||
| 6 months | 144 | 113 | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0 (0–0) | 0 (0–1) |
| 16–18 months | 100 | 89 | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0 (–1 to 0) | 0 (0–1) |
| Always use logbook legally | ||||||||
| 6 months | 144 | 113 | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0 (0–0) | 0 (0–0) |
| 16–18 months | 100 | 89 | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0 (0–0) | 0 (0–0) |
| Skip the daily vehicle inspection when tired or rushed | ||||||||
| 6 months | 144 | 113 | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0 (0–0) | 0 (0–0) |
| 16–18 months | 100 | 89 | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) |
| Sometimes get in a difficult situation without having a way out | ||||||||
| 6 months | 144 | 113 | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–2) | 0 (–1 to 0) | 0 (0–0) |
| 16–18 months | 100 | 89 | 2 (1–3) | 2 (1–3) | 2 (1–3) | 1 (1–2) | 0 (–1 to 1) | 0 (–1 to 0) |
Health-related quality of life
There were no noticeable differences in perceived markers of HRQoL between baseline and 6 months’ follow-up, and between baseline and 16–18 months’ follow-up, for both groups (Table 26). No noticeable differences were observed between groups at any assessment point.
| Variable | Number of participants | Baseline, median (IQR) | Follow-up, median (IQR) | Median (IQR) change from baseline to follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| Mobility | ||||||||
| 6 months | 144 | 113 | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) |
| 16–18 months | 100 | 90 | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) |
| Self-care | ||||||||
| 6 months | 144 | 113 | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) |
| 16–18 months | 100 | 90 | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) |
| Usual activities | ||||||||
| 6 months | 144 | 113 | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) |
| 16–18 months | 100 | 90 | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) |
| Pain/discomfort | ||||||||
| 6 months | 144 | 113 | 2 (1–2) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0 (–1–0) | 0 (0–0) |
| 16–18 months | 100 | 90 | 2 (1–2) | 2 (1–2) | 2 (1–2) | 2 (1–3) | 0 (0–1) | 0 (0–1) |
| Anxiety/depression | ||||||||
| 6 months | 144 | 113 | 1 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) |
| 16–18 months | 100 | 90 | 1 (1–2) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 0 (0–0) | 0 (0–0) |
| Overall health today | ||||||||
| 6 months | 144 | 113 | 80 (70–90) | 80 (75–90) | 80 (74–90) | 80 (75–90) | 0 (–5 to 5) | 0 (–9 to 9) |
| 16–18 months | 100 | 90 | 80 (70–89) | 82 (75–90) | 80 (70–85) | 85 (71–90) | 0 (–5 to 10) | 0 (–5 to 8) |
Lifestyle health-related behaviours and risk measures
There were no noticeable differences in reported alcohol intake between baseline and 6 months’ follow-up, and between baseline and the final follow-up, for both groups (Table 27). No noticeable differences were observed between groups at any assessment point.
| Variable | Number of participants | Baseline, median (IQR) | Follow-up, median (IQR) | Median (IQR) change from baseline to follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | Control | SHIFT intervention | |
| AUDIT scorea | ||||||||
| 6 months | 144 | 113 | 4 (2–5) | 4 (3–6) | 3 (2–5) | 4 (2–5) | 0 (0–0) | 0 (0–0) |
| 16–18 months | 101 | 91 | 3 (2–5) | 4 (3–6) | 3 (2–5) | 4 (2–6) | 0 (–5 to 10) | 0 (–5 to 8) |
| QRISK3 scoreb | ||||||||
| 6 months | 144 | 111 | 5.6 (2.4–9.6) | 5.3 (2.5–9.7) | 5.3 (2.3–10.6) | 5.7 (2.2–9.3) | 0.2 (–0.6 to1.2) | 0.0 (–0.9 to 0.7) |
| Proportion (%) | Proportion (%) | Change in proportion (%) | ||||||
| Smoking prevalencec | ||||||||
| 6 months | 145 | 113 | 19 | 14 | 20 | 12 | 1 | –2 |
| 16–18 months | 101 | 91 | 21 | 15 | 20 | 14 | 0 | –1 |
There were no differences in QRISK3 scores between baseline and 6 months’ follow-up for both groups (see Table 27). Likewise, there were no noticeable differences in QRISK3 scores between the control group and the SHIFT group at any assessment point. When examining the proportion of participants with an estimated CVD risk of ≥ 10% over the next 10 years, 23.6% of control participants fell into this category at baseline, with this proportion increasing to 26.4% at 6 months. In contrast, 24.3% of participants in the SHIFT group exhibited a ≥ 10% risk of CVD over the next 10 years at baseline, but this fell slightly to 23.4% at 6 months.
Table 27 also provides a summary of the smoking prevalence reported by participants at each time point. There was a tendency for a higher smoking prevalence to be seen in the control group, than in the SHIFT group, across all assessment points. Between baseline and 6 months, two participants in the control group and one participant in the SHIFT group reported changing from a past smoker to a current smoker (i.e. re-starting smoking), and four participants in the SHIFT group reported stopping smoking. No control participants reported stopping smoking between baseline and 6 months. The average number of cigarettes smoked per day by current smokers in the SHIFT group (baseline, n = 16; 6 months, n = 13) was 14 (SD 5) cigarettes per day at both baseline and 6 months’ follow-up. The average number of cigarettes smoked per day by current smokers in the control group (baseline, n = 27; 6 months, n = 29) was 14 (SD 6) cigarettes per day at baseline and 13 (SD 7) cigarettes per day at 6 months.
Between baseline and 16–18 months’ follow-up, one participant in the SHIFT group reported re-starting smoking at and three participants (control group, n = 1; SHIFT group, n = 3) reported stopping smoking. The average number of cigarettes smoked per day by current smokers in the SHIFT group (baseline, n = 14; 16–18 months, n = 13) was 15 (SD 5) cigarettes per day at both baseline and 16–18 months. The average number of cigarettes smoked per day by current smokers in the control group (baseline, n = 21; 16–18 months, n = 20) was 14 (SD 6) cigarettes per day at both baseline and 16–18 months.
COVID-19: impact of a temporary change in driving hour regulations on SHIFT participants
Participants
Of the 220 participants who were still enrolled in the study in May 2020, 91 (41.4%; control group, n = 48; SHIFT group, n = 43; 99% male) participants completed an additional online questionnaire that captured data on the effect of the pandemic on their working hours, mental well-being and health-related behaviours. The questionnaire was completed during the UK’s first national lockdown. At the time of completing the questionnaire, 20 (22%) participants [control group, n = 15 (31%); SHIFT group, n = 5 (12%)] were on furlough, and 44 (48%) participants [control group, n = 32 (67%); SHIFT group, n = 12 (28%)] reported being on furlough at some point during the pandemic.
There were no statistically significant differences in questionnaire responses between intervention or control participants, or between participants who were/had been furloughed and participants not furloughed. As a result, the responses received for the COVID-19 questionnaire are presented for the group as a whole. The only measure where a difference was reported was ‘sleep duration in the past 14 days’. Participants who had been or who were still on furlough reported a longer sleep duration [median 7.0 (IQR 5.5–8.5) hours/night] than participants who were not furloughed [median 6.5 (IQR 5.5–7.5) hours/night].
Baseline characteristics and measures of the 91 participants completing the COVID-19 questionnaire did not differ significantly from the remainder of the participants within the SHIFT study, and this suggests that the subsample of 91 participants are largely representative of the wider sample. Specifically, there were no differences at baseline between participants completing the COVID-19 questionnaire and the wider sample in terms of age, duration working as a HGV driver, duration working for DHL Supply Chain, hours worked per week, BMI, per cent body fat, waist circumference, self-reported symptoms of anxiety and depression, musculoskeletal complaints, physical activity levels, and sleep duration and efficiency.
Working hours and activity levels before and during the pandemic
Participants reported no changes to their working, driving, in-cab waiting or rest hours during the pandemic. Similarly, participants reported no changes in the time spent sitting, standing and walking/moving around on a workday during the pandemic (Table 28).
| Variable | Numbera | Before COVID-19, median (IQR) | During COVID-19, median (IQR) | Difference (p-value)b |
|---|---|---|---|---|
| Working hours (hours/week) | 46 | 48.0 (44.0–50.0) | 47.5 (43.0–51.0) | 0.46 |
| Driving hours (hours/day) | 46 | 7.0 (6.0–7.8) | 7.0 (6.0–8.0) | 0.75 |
| In-cab waiting hours (hours/shift) | 46 | 1.5 (1.0–2.0) | 1.5 (1.0–2.9) | 0.15 |
| Rest hours between shifts (hours) | 46 | 12.0 (11.0–14.0) | 12.0 (11.0–14.0) | 0.70 |
| Sitting time (hours/day) | 86 | 7.0 (6.0–8.6) | 7.0 (5.0–9.0) | 0.81 |
| Standing time (hours/day) | 86 | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.78 |
| Walking/moving time (hours/day) | 86 | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.56 |
Anxiety, depression and fatigue before and during the pandemic
In comparison with baseline, there was a tendency for participants who completed the additional COVID-19 questionnaire to report lower levels of symptoms of anxiety during the pandemic (Table 29). There were no differences observed in symptoms of depression, levels of acute and chronic fatigue or intershift recovery during the pandemic, compared with baseline, for this subsample (see Table 29).
| Variable | Numbera | Before COVID-19, median (IQR)b | During COVID-19, median (IQR) | Difference (p-value)c |
|---|---|---|---|---|
| HADS anxietyd | 90 | 3.0 (1.0–5.0) | 3.0 (1.0–6.0) | 0.01 |
| HADS depressiond | 90 | 4.0 (3.0–7.0) | 1.0 (0.3–5.0) | 0.27 |
| Chronic fatiguee | 69 | 26.7 (16.7–43.3) | 30.0 (8.3–51.7) | 0.98 |
| Acute fatiguee | 69 | 46.7 (30.0–63.3) | 46.7 (21.7–63.3) | 0.77 |
| Intershift recoverye | 69 | 63.3 (46.7–83.3) | 56.7 (45.0–83.3) | 0.12 |
The impact of exposure to green space on anxiety, depression and fatigue before and during the pandemic
Within the online questionnaire, 72% (n = 65) of participants reported that they regularly spent time in nature (e.g. spending time in their garden/allotment, in parks and woodland, at the coast and in open green spaces) prior to the onset of the pandemic. Data collected at baseline revealed that participants who reported spending time in nature also reported significantly lower amounts of chronic fatigue associated with their work than participants who reported that they did not spend time in nature (n = 25) [median chronic fatigue score: 23.3 (IQR 10.0–40.0) vs. 43.3 (IQR 26.7–66.7); p = 0.008]. There were no other differences between groups in terms of other markers of fatigue and symptoms of anxiety and depression at baseline.
During the pandemic, 78% (n = 70) of participants reported spending time in nature. Examining data from participants working at the time of completing the online questionnaire revealed that participants who reported spending time in nature (n = 51) also reported significantly lower amounts of chronic and acute fatigue associated with their work than participants who reported that they did not spend time in nature (n = 17) [median chronic fatigue score: 20.0 (IQR 3.3–41.7) vs. 51.7 (IQR 35.8–65.0), p = 0.002; median acute fatigue score: 40.0 (IQR 16.7–56.7) vs. 55.0 (IQR 47.5–79.2), p = 0.009]. The differences between groups for intershift recovery were marginal, but in favour of the group spending time in nature [median intershift recovery score: 63.3 (IQR 50.0–83.3) vs. 50.0 (IQR 36.7–66.7); p = 0.06]. There were no differences between groups in symptoms of anxiety and depression reported during the pandemic.
The impact of the pandemic on drivers’ lifestyle health-related behaviours
Twenty-three (25%) participants reported engaging in a new form of physical activity since the COVID-19 outbreak. New activities reported by participants included cycling (n = 10), walking (n = 5), gardening (n = 3), running (n = 2), weights at home (n = 2), boxing (punchbag training) (n = 1), exercises at home (n = 1), home workouts with a personal trainer (n = 1) and DIY (‘do it yourself’) (n = 1) (note that some participants reported more than one new activity). Seven of 47 (15%) participants who had not been furloughed reported engaging in a new activity, compared 16 of 44 (36%) participants who had been furloughed during the pandemic.
Twenty per cent of participants reported that their consumption of snacks (e.g. cakes, biscuits, crisps, chocolate and sweets) had decreased during the pandemic, whereas 19% reported an increase. Twenty-three per cent of participants reported that their consumption of fresh fruit and vegetables had increased during the pandemic, whereas 7% reported a decrease. Thirty-four per cent of participants reported not being able to access healthy food while at work during the pandemic, whereas 45% reported accessing healthy food, all stating that they brought their own from home.
Five per cent of participants reported a change to their smoking status during the pandemic. One participant reported starting smoking, two participants reported smoking less and two participants reported stopping smoking. Five per cent of participants reported a decrease in their alcohol intake during the pandemic, whereas 26% reported an increase.
Twenty-seven per cent of participants reported that their sleep duration had increased during the pandemic, whereas 13% reported a decrease. Of the participants reporting an increase in sleep duration, 23 of 25 participants had been furloughed. Participants currently furloughed at the time of completing the questionnaire (n = 20) reported a median sleep duration over the past 14 days of 8 (IQR 6–10) hours per day. Participants currently working (n = 70) reported a median sleep duration over the past 14 days of 7 (IQR 6–8) hours per day.
The impact of involvement in the SHIFT study on health behaviours during the pandemic, and lifestyle changes experienced
Participants were asked within the online questionnaire whether or not they felt that participating in the SHIFT study had given them the right knowledge to maintain a healthy lifestyle during the COVID-19 restrictions. A total of 63% of both intervention and control participants answered ‘yes’ to this question. Overall, 63% of both intervention and control participants answered ‘yes’. A range of qualitative quotes were provided by respondents on how the study had helped them maintain a healthy lifestyle during the pandemic. The responses received were similar between intervention and control participants, and largely centred around an increased understanding of the importance of activity and a better diet. The quotes are shown in Appendix 2, Box 1.
When asked within the questionnaire whether or not they had experienced any changes in lifestyle and/or work that had either a positive or negative impact on health, 40 (44%) participants answered ‘yes’. Of them, 20 (22%) participants reported that these changes had a positive impact and 20 (22%) reported that these changes had a negative impact. A range of qualitative quotes were provided from respondents regarding changes in lifestyle experienced (see Appendix 2, Box 2). Participants reporting positive changes tended to refer to having time off work as a result of being furloughed and, therefore, having more time to be physically active. Participants reporting negative changes tended to refer to a lack of access to facilities (e.g. gyms, swimming pools) to enable them to be active, increased snacking behaviours due to being at home and reductions in the overall quality of their diets due to limited food choices.
Adverse events
No serious adverse events were reported during the trial.
Chapter 4 Economic evaluation
Methodology overview
The economic evaluation considers the resource use, costs and cost-effectiveness of the SHIFT intervention compared with usual practice, using evidence from the SHIFT RCT and other sources. Costs were measured in GBP (2019–20) from a public sector perspective (i.e. NHS and Personal Social Services). A private sector perspective (haulage firm) was also considered for secondary analyses. 106,107 Health outcomes were primarily measured in QALYs and based on the EQ-5D-5L questionnaire. 81,108 Other measures, including productivity, employee well-being and absenteeism, were also considered. Missing data were populated using multiple imputation by chained equations. 109 Within-trial costs and QALYs were estimated using multilevel econometric modelling to control for participant co-variables and cross-cluster variation. 110 Decision-analytic models were used to extrapolate the results over a longer time horizon, based on any observed differences in physical activity between trial arms. 111 In line with UK guidelines,106 costs and QALYs were discounted at 3.5% per annum. Cost-effectiveness was measured using ICERs and incremental net health benefits (INHBs) at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY. 106,112
Probabilistic sensitivity analysis was used to characterise the uncertainty, and decision uncertainty was assessed across alternate cost-effectiveness thresholds. Scenario, sensitivity and threshold analyses were also conducted to consider the implications of alternate methods and modelling assumptions on study findings. 106 Further details are available in the health economic analysis plan (see Report Supplementary Material 4).
Resource use and costs
Health-related resource use was collected from participants using a service use questionnaire at baseline and at 6 months and 16–18 months. Responses at each time point related to participants’ resource use in the 6 months prior. Health-care costs were calculated for each trial participant by resource use category and at each follow-up period by applying unit costs to any resources used. It was assumed that the 16- to 18-month follow-up occurred at 18 months, and resource use between 6 and 12 months was equal to resource use between 12 and 18 months. Health-related resources and costs were categorised into primary care, secondary care, mental health care and occupational services. Unit costs were measured in GBP (2019–20) and were sourced from published UK sources (see Appendix 3, Table 43). 91,113 Unit costs were inflated to 2019–20 prices using inflation indices where necessary. 91 Costs of absenteeism were calculated based on firm-reported full-day driver-replacement costs.
The resource use and associated intervention costs for the SHIFT intervention comprised (1) exercise-related devices, equipment and materials [e.g. wearable device (i.e. a Fitbit Charge 2), THERABAND® bands (Akron, OH, USA), intervention booklet] and (2) a 6-hour education session that required driver time, course materials and staff training. It was assumed that the education session would require a full worker day for each attendee and that the session was delivered by existing facilitators and, therefore, incurred no additional costs. The average SHIFT intervention cost per driver was treated as an up-front cost (i.e. with no follow-up costs) and calculated on an ITT basis. Usual practice was assumed to incur no intervention-related costs. Intervention unit costs were based directly on those incurred during the trial.
Outcomes
The primary outcome used in the cost-effectiveness analysis was QALYs, which is a generic measure of health that combines both length of life and HRQoL (1 QALY is equal to 1 year in perfect health). 108 Participants’ HRQoL was assessed using the EQ-5D-5L questionnaire, collected at baseline and at 6 months and 16–18 months. The EQ-5D-5L is a descriptive HRQoL instrument that comprises five levels of severity across the following five health dimensions: (1) mobility, (2) self-care, (3) usual activity, (4) pain/discomfort and (5) anxiety/depression. 81 In accordance with National Institute for Health and Care Excellence (NICE) recommendations, HRQoL weights were calculated from a published mapping of EQ-5D-5L responses onto HRQoL values calculated for the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) instrument from a survey of the UK general public. 106,114 Trial QALYs were estimated through an area under the curve approach, with linear interpolation between observations. 108 It was assumed at the 16- to 18-month follow-up occurred at 18 months to allow for a common time horizon for estimation of QALYs. Longer-term cost-effectiveness analyses calculated QALYs using decision-analytic models (see Decision-analytic model and longer-term cost-effectiveness), which combined estimated within-trial QALY differences (i.e. within-trial analysis) with QALYs estimated over a longer time horizon, based on any observed impacts on levels of physical activity. It was assumed that the HRQoL of drivers beyond the trial were equal between arms (i.e. any within-trial differences did not persist beyond the trial period) and equivalent to age-specific values observed in the trial. The removal of estimated within-trial differences in outcomes and the use of HRQoL weights from the EQ-5D-5L value set were explored in scenario analyses.
Secondary outcomes included productivity, employee well-being and absenteeism. Productivity considered employee-assessed job performance and work ability on Likert scales that ranged from 0 to 7 and 0 to 10, respectively (with higher scores denoting more favourable outcomes). Participants’ work-related well-being considered employee-assessed job satisfaction on a Likert scale that ranged from 0 to 7 (with higher scores denoting greater satisfaction) and presenteeism according to the number of days drivers have worked despite feeling unwell.
Methods for analysis
Analysis
A health economics analysis plan (see Report Supplementary Material 4) was created before the health economics analysts had access to the data. From a public sector perspective, the cost-effectiveness of the SHIFT intervention (compared with usual practice) was assessed according to (1) the estimated differences in the QALYs gained by drivers and (2) the incremental costs to public services incurring over a 16- to 18-month time horizon (i.e. within trial) and over the longer term. Secondary analyses considered cost-effectiveness from a private sector perspective by assessing the differences in changes to measures of productivity and employee well-being at 6 months and 16–18 months from baseline, as well as the incremental costs. From a public perspective, intervention costs and all costs relating to health resource usage were considered. Only intervention and absenteeism costs were considered from the private sector perspective. Both public and private sector perspectives included SHIFT intervention costs, as these could feasibly be financed by either source.
For the public sector analysis, estimated costs and outcomes in each arm, and their differentials, are presented alongside ICERs and INHBs. ICERs represented the cost per additional unit of outcome for the SHIFT intervention compared with usual practice. The INHBs for the SHIFT intervention measured the intervention’s health gain less the health that would have otherwise been generated elsewhere had the additional resources (compared with usual practice) been allocated for alternative purposes (i.e. the opportunity cost estimated using a given cost-effectiveness threshold). Three cost-effectiveness thresholds (i.e. measures of health opportunity cost) were considered in the analysis: (1) £15,000 per QALY [i.e. the Department of Health and Social Care’s usual threshold (based on recent empirical estimates)],112,115 (2) £20,000 per QALY and (3) £30,000 per QALY. The thresholds (i.e. £15,000, £20,000 and £30,000) are used by NICE to assess cost-effectiveness of health-care interventions. 106 At a given threshold, the SHIFT intervention is considered cost-effective, compared with usual practice, when its ICER is below the chosen cost-effectiveness threshold and it has positive INHB. For the private sector perspective, ICERs are presented, showing the cost per unit change in the measure of productivity or well-being.
Within-trial analysis
Estimated within-trial costs and QALYs in each treatment arm were obtained using multilevel linear regression models on multiply imputed data that controlled for a set of relevant participant co-variables and accounted for cross-cluster variation (i.e. variation between sites). Regression analyses controlled for age (i.e. 40–49 years, 50–59 years and ≥ 60 years vs. < 40 years), sex (i.e. female vs. male), ethnicity (i.e. white vs. other), BMI category (i.e. overweight, morbidly obese and obese vs. healthy), presence of diabetes, smoking status (i.e. ex-smoker and current smoker vs. never smoked) and cluster size. QALY regression analyses also controlled for baseline EQ-5D scores to account for differences in baseline HRQoL. 116 Scenarios considered the following alternative approaches: (1) a generalised linear model using a log-link transformation and gamma family form to account for the positive and right-skewed nature of the cost data; (2) excluding the costs from inpatient-related services, given the potential for random imbalances in hospital procedures to affect cost differentials between arms; and (3) an analysis that considers only participants with complete data (i.e. complete-case analysis).
Missing data
Missing cost and outcome data were populated via multiple imputation using chained equations, with predictive mean matching used to match predicted missing values with the closest observed value. 116 The imputation model controlled for all the covariates considered in the within-trial regression models, including clusters. Using Rubin’s rules, overall imputed mean estimates and standard errors were calculated from 20 imputed data sets to reflect the variability within and across imputations. 116,117
Decision-analytic model and longer-term cost-effectiveness
The longer-term cost-effectiveness of the SHIFT intervention was assessed using decision-analytic models that sought to capture the longer-term benefits of physical activity for HGV drivers. Given the uncertainties associated with modelling the impact of physical activity on public health,118 alternative measures of physical activity and modelling approaches were considered to estimate outcomes.
The first decision-analytic model was a two-state Markov model, where the cohort starts in an alive state and will either remain in that state or transition into an absorbing dead state. Beyond the first year, the model captured QALYs using the age-specific HRQoL observed in the trial and did not consider additional costs. For usual practice, transitions to the death state were based on age- and sex-adjusted English general population mortality rates. 119 Mortality rates for the SHIFT arm were then adjusted according to estimated changes in one of two alternative measures of physical activity: (1) time spent in MVPA (i.e. the MVPA-based model) and (2) time spent sedentary (i.e. the sedentary-based model) (see Chapter 2). The dose–response relationship between changes in accelerometer-measured physical activity (specifically MVPA and sedentary time) and all-cause mortality was based on hazard estimates reported in Ekelund et al. ’s meta-analysis. 120 Polynomial functions were used to interpolate between all-cause mortality hazard ratios, relative risks and 95% CI point estimates (see Appendix 3, Figures 4 and 5). Physical activity in the usual-practice arm was assumed to follow baseline values (across arms) and to remain constant over time (see Table 7). In the SHIFT arm, the average change in physical activity relative to usual practice in the first year was assumed to be equal to estimated differences at the yearly mid-point (i.e. 6 months) (see Table 9). After the first year, SHIFT-associated differentials in physical activity were reduced exponentially at a 50% decay rate per annum from estimated differences at final follow-up, although the decay rate was varied in sensitivity analyses. The model was run using average participant characteristics over a lifetime horizon.
An alternative decision-analytic model considered was the Model for estimating the Outcomes and Values in the Economics of Sport (MOVES) tool, version 2.0, which was developed for Sport England. 92,121 The MOVES tool estimates risk reductions across seven completing diseases (i.e. dementia, depression, colon cancer, type 2 diabetes, breast cancer, ischaemic heart disease and stroke) from changes in physical activity measured by metabolic-equivalent hours per week. The model was modified to have the same treatment effect schedule as the first decision-analytic model, with first year exercise differences equal to those estimated at 6 months, second year differences equal to those estimated at 16–18 months, and differences thereafter reduced exponentially from 16–18 months at a 50% decay rate per annum. The underlying HRQoL of the cohort over time was aligned with the age-specific HRQoL observed in the trial. Minutes in MVPA were translated into metabolic-equivalent hours per week by assuming the intensity of activity within MVPA follows a uniform distribution between 3 (i.e. light moderate exercise) and 6 (i.e. vigorous exercise). Metabolic-equivalent hours per week in each arm were defined according to baseline MVPA minutes (pooled across arms), estimated treatment differentials in MVPA minutes and presumed metabolic intensity (drawn from identical uniform distributions) (see Table 9). Exercise differentials were bounded such that no individual could undertake a negative number of minutes exercise. The model was run using average participant characteristics over a 25-year time horizon.
In all models, treatment-associated changes in physical activity were applied as common effects (i.e. irrespective of participant characteristics). Estimated within-trial cost and QALY treatment differences were incorporated in the first year, but were not extrapolated thereafter. In accordance with NICE guidance,106 costs and QALYs were discounted at a 3.5% discount rate. Scenario analyses considered removing all estimated trial-specific treatment differentials in QALYs and non-intervention costs, as well as an annual discount rate of 1.5%. 122
Uncertainty
Monte Carlo simulation was used to propagate the uncertainty in the analyses to estimate overall decision uncertainty surrounding the adoption of the SHIFT intervention. It was assumed that (1) event probabilities followed beta distributions (MOVES92,121), (2) baseline physical activity (i.e. usual practice), treatment effects and all-cause mortality hazard ratios (Ekelund et al. 120) were normally distributed and (3) regression coefficients (i.e. within-trial non-intervention costs and outcomes) followed multivariate normality. 111 Regression parameter correlations were accounted for using Cholesky transformations of the variance–covariance matrix. 111
Uncertainty was reported at 95% credible intervals around mean cost, QALY and INHB values, alongside the probabilities of the SHIFT intervention/usual practice being the most costly, clinically effective and cost-effective alternative. INHB and the probability of being cost-effective are presented for cost-effectiveness thresholds of £15,000, £20,000, and £30,000 (i.e. the health that would have been generated elsewhere using the same resources). Cost-effectiveness acceptability curves illustrated the probability of the SHIFT intervention or usual practice being cost-effective up to a threshold of £50,000 per QALY.
Key uncertainties were explored in a range of scenario analyses involving alternative methodological approaches (see above) and the removal of all estimated within-trial differences in non-intervention costs and QALYs between trial arms. Sensitivity and threshold analyses explored the impacts of two alternative degrees of treatment maintenance on study findings: (1) the annual rate of decay in the treatment effect on physical activity beyond the first year (i.e. a 50% decay rate used in the base case) and (2) the continuation of the treatment effect on physical activity observed at 6 months.
Results
The SHIFT trial involved 382 participants (SHIFT intervention, n = 183; usual practice, n = 199). Health-care resource use forms were fully completed by approximately 93.5%, 63.9% and 46.9% of participants at baseline, 6 months and 16–18 months, respectively. Complete-case resource use was achieved by 40.8% of participants. EQ-5D-5L questionnaires were complete for 98.2% of participants at baseline, and for 66.8% and 49.2% of participants at 6 months and 16–18 months, respectively. Complete-case EQ-5D-5L responses amounted to 44.5% of participants. Secondary outcome data (i.e. productivity, employee work-related well-being and absenteeism) had a comparable degree of missingness, ranging between 98.7% and 99.5% at baseline, between 67.0% and 67.3% at 6 months and between 48.4% and 50.5% at 16–18 months. Participant characteristics are reported in Table 5.
Treatment effect
Baseline physical activity (i.e. usual practice) was modelled at 15.36 minutes per day in MVPA and 670.31 minutes of sedentary time per day. In the first year, the SHIFT intervention was associated with an additional 5.84 minutes per day in MVPA and a reduction in sedentary time of 24.37 minutes per day (see Table 9). In the second year, the SHIFT intervention was associated with an additional 1.6 minutes per day in MVPA and a reduction in sedentary time of 12.16 minutes per day. In the subsequent years, the treatment effect was extrapolated from second year differentials (i.e. a 1.6-minute/day increase in MVPA and a 12.16-minute/day reduction in sedentary time).
Resource use and costs
Intervention-level costs
Table 30 provides the average per driver costs of delivering each element of the SHIFT intervention. The average total cost of delivering the SHIFT intervention was £369.57 per driver. The equipment (£182.49) and education (£187.08) elements of the SHIFT intervention had comparable costs. Education costs mostly comprised driver replacement costs.
| SHIFT intervention component | Cost (£) per driver |
|---|---|
| Equipment and materials | 182.49 |
| THERABAND bands | 12.17 |
| Exercise balls | 3.80 |
| Fitbit Charge 2 | 90.99 |
| Fitabase software | 65.53 |
| Duffel bag | 2.20 |
| Text messaging service | 2.80 |
| Intervention booklet | 5.00 |
| 6-hour education session | 187.07 |
| Individual driver’s time | 180.00 |
| Printing of curriculum and laminates | 1.21 |
| Creation of resources | 0.74 |
| Training staff facilitators | 5.12 |
| Total cost per participant | 369.56 |
Health-care resource use and non-intervention costs
Resource use and associated costs were broadly balanced between the SHIFT intervention and usual practice over the course of the trial (Table 31). Differences within and across resource categories were small in magnitude and inconsistent in direction of effect (see Appendix 3, Tables 44–53). The total imputed costs were lower for usual practice (£637.66) than for the SHIFT intervention (£1,162.50). When controlling for participant covariates, the SHIFT intervention was associated with an additional £181.50 in non-intervention costs compared with usual care (see Appendix 3, Table 57). Appendix 3, Tables 44–47, present available- and complete-case breakdowns of resource use for each comparator by resource category and follow-up period. Appendix 3, Tables 48–51, describe available-case, complete-case and imputed costs by resource category and follow-up period for each trial arm.
| Imputed cost | SHIFT intervention | Usual practice | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | 95% CI | n | Mean (SD) | 95% CI | |
| SHIFT intervention | 185 | 369.57 (0) | 0 to 0 | 201 | – | – |
| Primary care | ||||||
| GP: surgery visit | 185 | 52.37 (104.03) | 37 to 68 | 201 | 57.72 (115.81) | 41 to 74 |
| GP: home visit | 185 | 5.29 (62.01) | –4 to 14 | 201 | 3.46 (183.48) | –22 to 29 |
| GP: telephone call | 185 | 11.85 (44.81) | 5 to 18 | 201 | 13.66 (40.7) | 8 to 19 |
| General practice nurse: surgery visit | 185 | 3.08 (9.63) | 2 to 4 | 201 | 3.13 (9.06) | 2 to 4 |
| General practice nurse: home visit | 185 | 2.74 (26.9) | –1 to 7 | 201 | 1.76 (23.3) | –2 to 5 |
| General practice nurse: telephone call | 185 | 1.14 (7.65) | 0 to 2 | 201 | 1.17 (7.82) | 0 to 2 |
| Secondary care | ||||||
| Inpatient days | 185 | 440.24 (3405.4) | –63 to 943 | 201 | 306.95 (2740.31) | –80 to 694 |
| Outpatient visits | 185 | 102.67 (331.89) | 54 to 151 | 201 | 112.97 (348.93) | 64 to 162 |
| Accident and emergency visits | 185 | 37.88 (137.35) | 18 to 58 | 201 | 33.32 (133.51) | 14 to 52 |
| NHS walk-in centre visit | 185 | 3.86 (24.99) | 0 to 8 | 201 | 4.17 (23.75) | 1 to 7 |
| NHS urgent care centre visit | 185 | 2.01 (20.01) | –1 to 5 | 201 | 3.42 (23.05) | 0 to 7 |
| Other hospital-based services | 185 | 7.68 (64.73) | –2 to 17 | 201 | 3.75 (48.38) | –3 to 11 |
| Mental health care | ||||||
| Mental health nurse | 185 | 3.80 (44.29) | –3 to 10 | 201 | 8.02 (53.42) | 1 to 15 |
| Occupational services | ||||||
| Occupational health nurse | 185 | 3.93 (25.71) | 0 to 8 | 201 | 1.93 (20.61) | –1 to 5 |
| Physiotherapist | 185 | 114.4 (408.15) | 55 to 174 | 201 | 82.24 (462.83) | 17 to 147 |
| Total costs | ||||||
| Overall total observed costs | 185 | 1162.50 (3976.2) | 576 to 1749 | 201 | 637.66 (3251.62) | 179 to 1096 |
| Total costs, excluding inpatient-related services | 185 | 722.26 (873.19) | 595 to 850 | 201 | 330.71 (809.67) | 217 to 444 |
Outcomes
Quality-adjusted life-years and HRQoL scores were similar between the arms, albeit with modest differences in baseline values and changes at 6 months’ follow-up (Table 32). In the SHIFT arm, EQ-5D-5L and mapped EQ-5D-3L scores fell over the trial period. In the usual-practice arm, scores rose between baseline and 6 months, before declining to levels below baseline (see Appendix 3, Table 54). Adjusted QALY estimates (i.e. QALY estimates estimated using imputed data while controlling for participant covariates) found –0.028 and –0.015 QALY decrements associated with the SHIFT intervention compared with usual practice over the trial horizon when using mapped EQ-5D-3L and EQ-5D-5L preference weights, respectively (see Appendix 3, Tables 61 and 62). Secondary outcomes are reported in Table 33. There were small and largely inconsistent changes within and between arms for each outcome considered.
| Outcome | SHIFT intervention, mean (SD) | Control, mean (SD) | Differential, mean (95% CI) |
|---|---|---|---|
| Preference scores | |||
| EQ-5D-3L (base case) | |||
| Baseline | 0.852 (0.146) | 0.839 (0.141) | 0.013 (–0.016 to 0.042) |
| 6 months | 0.838 (0.155) | 0.864 (0.147) | –0.026 (–0.056 to 0.003) |
| 16–18 months | 0.797 (0.188) | 0.795 (0.197) | 0.002 (–0.039 to 0.042) |
| EQ-5D-5L (scenario) | |||
| Baseline | 0.909 (0.113) | 0.902 (0.108) | –0.016 (–0.039 to 0.007) |
| 6 months | 0.905 (0.121) | 0.922 (0.103) | –0.016 (–0.016 to 0.029) |
| 16–18 months | 0.875 (0.153) | 0.869 (0.166) | 0.006 (–0.027 to 0.040) |
| QALYs | |||
| EQ-5D-3L (base case) | |||
| 0–6 months | 0.422 (0.063) | 0.426 (0.061) | –0.003 (–0.016 to 0.009) |
| 16–18 months | 0.817 (0.146) | 0.830 (0.145) | –0.012 (–0.042 to 0.017) |
| Total | 1.240 (0.198) | 1.256 (0.194) | –0.016 (–0.054 to 0.023) |
| EQ-5D-5L (scenario) | |||
| 0–6 months | 0.454 (0.051) | 0.456 (0.045) | –0.002 (–0.012 to 0.007) |
| 16–18 months | 0.890 (0.115) | 0.895 (0.114) | –0.005 (–0.028 to 0.018) |
| Total | 1.344 (0.157) | 1.351 (0.148) | –0.007 (–0.038 to 0.023) |
| Secondary outcome: imputed | Baseline | Month 6 | Months 16–18a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SHIFT intervention, mean (SD) | Usual practice | Differential (95% CI) | SHIFT intervention, mean (SD) | Usual practice | Differential (95% CI) | SHIFT intervention, mean (SD) | Usual practice | Differential (95% CI) | |
| Absenteeism | |||||||||
| Number of sick days | 3.492 (15.405) | 3.814 (12.866) | –0.322 (–3.154 to 2.51) | 1.776 (6.796) | 3.039 (8.241) | –1.263 (–2.745 to 0.218) | 6.388 (24.251) | 5.015 (22.726) | 1.372 (–3.028 to 5.773) |
| Productivity | |||||||||
| Employee-assessed job performanceb | 6.019 (0.907) | 5.974 (0.859) | 0.045 (–0.132 to 0.222) | 6.026 (1.076) | 5.981 (1.004) | 0.045 (–0.148 to 0.238) | 6.059 (1.253) | 5.933 (1.236) | 0.126 (–0.112 to 0.364) |
| Employee-assessed work abilityc | 8.349 (1.385) | 8.275 (1.512) | 0.074 (–0.218 to 0.365) | 8.342 (1.989) | 8.12 (1.714) | 0.222 (–0.15 to 0.595) | 8.363 (1.731) | 8.134 (2.045) | 0.228 (–0.145 to 0.602) |
| Employee work-related well-being | |||||||||
| Presenteeism | 4.846 (11.993) | 3.944 (7.786) | 0.902 (–1.104 to 2.909) | 7.521 (25.216) | 4.262 (12.841) | 3.259 (–0.707 to 7.224) | 5.851 (21.833) | 6.138 (26.285) | –0.287 (–4.482 to 3.908) |
| Job satisfaction | 4.798 (1.422) | 4.997 (1.337) | –0.199 (–0.475 to 0.077) | 4.692 (1.827) | 4.855 (1.539) | –0.163 (–0.523 to 0.197) | 4.845 (1.927) | 4.893 (1.598) | –0.048 (–0.39 to 0.294) |
Cost-effectiveness analysis
The within-trial and longer-term base-case mean cost, QALY and cost-effectiveness estimates for each arm and modelling approach are reported in Table 34.
| Analysis | Costs (£) (95% CI) [p (most costly)] | QALYs (95% CI) [p (most effective)] | ΔCost (95% CI) | ΔQALYs (95% CI) | ICER | INHB (95% CI) [p (cost-effective)] | ||
|---|---|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||||
| Within-trial cost-effectiveness | ||||||||
| Usual practice | 403.76 (–215.63 to 1045.02) [0.049] | 1.24624 (1.21873 to 1.27376) [0.96] | [0.989] | [0.99] | [1] | |||
| SHIFT intervention | 958.51 (299.02 to 1639.83) [0.951] | 1.21818 (1.1888 to 1.2466) [0.04] | 554.75 (–119.64 to 1228.65) | –0.02806 (–0.059 to 0.002) | Dominated | –0.065 (–0.118 to –0.013) [0.011] | –0.056 (–0.099 to –0.013) [0.01] | –0.047 (–0.081 to –0.011) [0.009] |
| Cost-effectiveness analysis: Ekelund et al.120 MVPA minutes/day (1) | ||||||||
| Usual practice | 403.76 (–215.63 to 1045.02) [0.049] | 16.15429 (16.15429 to 16.15429) [0.931] | [0.978] | [0.982] | [0.983] | |||
| SHIFT intervention | 958.51 (299.02 to 1639.83) [0.951] | 16.13240 (16.10182 to 16.16267) [0.069] | 554.75 (–119.64 to 1228.65) | –0.02190 (–0.052 to 0.008) | Dominated | –0.059 (–0.113 to –0.004) [0.022] | –0.050 (–0.094 to –0.003) [0.018] | –0.040 (–0.078 to –0.002) [0.017] |
| Cost-effectiveness analysis: Ekelund et al.120 sedentary minutes/day (2) | ||||||||
| Usual practice | 403.76 (–215.63 to 1045.02) [0.049] | 16.15429 (16.15429 to 16.15429) [0.908] | [0.981] | [0.982] | [0.983] | |||
| SHIFT intervention | 958.51 (299.02 to 1639.83) [0.951] | 16.13350 (16.10327 to 16.16419) [0.092] | 554.75 (–119.64 to 1228.65) | –0.02079 (–0.051 to 0.01) | Dominated | –0.058 (–0.114 to –0.004) [0.019] | –0.049 (–0.095 to –0.004) [0.018] | –0.039 (–0.076 to –0.002) [0.017] |
| Cost-effectiveness analysis: MOVES MVPA minutes translated into metabolic equivalents (3) | ||||||||
| Usual practice | 13,336.41 (7657.31 to 19,927.97) [0.066] | 14.16817 (13.78574 to 14.56869) [0.833] | [0.956] | [0.952] | [0.946] | |||
| SHIFT intervention | 13,843.20 (8049.98 to 20,393.84) [0.934] | 14.15197 (13.7727 to 14.54723) [0.167] | 506.79 (–145.31 to 1180.41) | –0.0162 (–0.049 to 0.019) | Dominated | –0.050 (–0.103 to 0.009)[0.044] | –0.042 (–0.088 to 0.008) [0.048] | –0.033 (–0.071 to 0.008) [0.054] |
The within-trial analysis found the SHIFT intervention to be more costly and less effective than usual practice, resulting in it being dominated. The probability of the SHIFT intervention being cost-effective in the within-trial period was low, with a probability of between 0.009 and 0.011 for the range of cost-effectiveness thresholds considered.
For the MVPA-based model, when using Ekelund et al. 120 all-cause mortality estimates with changes to MVPA minutes per day, the SHIFT intervention was found to be more costly and less effective than usual practice and, thereby, dominated. Incremental costs (£555) were the same as the within-trial analysis (the model did not extrapolate costs); however, QALY decrements were reduced to –0.022 per driver because of the increased physical activity in the SHIFT group reducing mortality. Similar results were found when using the sedentary-based model, with costs aligned to within-trial results and QALY decrements of –0.021.
The SHIFT intervention was also found to be more costly and less effective when using the MOVES tool. The inclusion of lifetime costs in the MOVES model increased overall cost estimates, but resulted in a small reduction in the incremental costs for the SHIFT group (£507). QALY decrements were reduced to –0.016 in the SHIFT group, relative to usual practice.
In all base-case analyses, the SHIFT intervention was found to be dominated by usual practice. The 95% credible intervals around incremental QALYs overlapped zero in the MOVES model, suggesting a significant level of uncertainty in the QALY differentials of the SHIFT intervention compared with usual practice. Appendix 3, Tables 57–63, present each regression analysis used to inform the cost-effectiveness analysis. Appendix 3, Figure 8, shows the cost-effectiveness acceptability curves for each modelling approach.
Scenario analyses
Table 35 presents the incremental cost, incremental QALY and associated ICER estimates for the scenario analyses considered for the long-term analyses, based on the three decision-analytic models. Base-case estimates are also provided for reference. A more detailed breakdown of each scenario can be found in Appendix 3, Tables 64–69.
| Scenario analysis | Ekelund et al.:120 MVPA minutes/daya | Ekelund et al.:120 sedentary minutes/dayb | MOVES modelc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALY | ICER (£) | Incremental cost (£) | Incremental QALY | ICER (£) | Incremental cost (£) | Incremental QALY | ICER (£) | |
| Base case | 555 | –0.02190 | Dominated | 555 | –0.02079 | Dominated | 507 | –0.01620 | Dominated |
| No within-trial differences in costs and QALYs | 364 | 0.00559 | 65,072 | 364 | 0.00711 | 51,173 | 340 | 0.01161 | 29,287 |
| EQ-5D-5L preference values | 555 | –0.00918 | Dominated | 555 | –0.00737 | Dominated | 507 | –0.00215 | Dominated |
| 1.5% discount rate (costs and QALYs) | 555 | –0.02037 | Dominated | 555 | –0.01827 | Dominated | 424 | –0.01343 | Dominated |
| Costs estimated using generalised linear models | 548 | –0.02190 | Dominated | 548 | –0.02079 | Dominated | 518 | –0.01620 | Dominated |
| Inpatient-related costs removed | 383 | –0.02190 | Dominated | 383 | –0.02079 | Dominated | 353 | –0.01620 | Dominated |
| Complete-case analysis | 751 | –0.01975 | Dominated | 751 | –0.02018 | Dominated | 721 | –0.01581 | Dominated |
In each modelling approach, removing estimated trial-specific differentials in non-intervention costs and QALYs between arms resulted in smaller incremental costs for the SHIFT intervention and positive incremental QALYs, compared with usual practice. For the MVPA- and sedentary-based models, the resulting ICERs were £65,072 and £51,174 per QALY, respectively, which are above the cost-effectiveness thresholds considered. For the MOVES model, SHIFT was associated with an ICER of £29,287 per QALY, thereby falling below the highest cost-effectiveness threshold considered. Under this scenario, for the MOVES model, the SHIFT intervention had a 12.4%, 26.1% and 46.6% probability of being cost-effective at the cost-effectiveness thresholds £15,000, £20,000 and £30,000 per QALY, respectively (see Appendix 3, Table 64).
Scenarios concerning the application of EQ-5D-5L preference weights, a 1.5% discount rate, generalised linear models to estimate costs, participant costs omitting inpatient-related resource utilisation and a complete-case analysis framework did not have marked impacts on results and, therefore, did not change base-case findings (i.e. the SHIFT intervention dominated by usual services).
Sensitivity and threshold analyses
Study findings were largely insensitive to changes in the decay rate of the treatment effect on physical activity. At base-case settings, the INHB of the SHIFT intervention remained negative for each threshold considered across the range of decay rates examined (i.e. 10–100%) in all models considered (Figure 3). When removing estimated trial-specific differentials in non-intervention costs and QALYs between arms, the INHB for the SHIFT intervention was positive at a decay rate of approximately 20% at a £30,000 per QALY threshold for the sedentary-based model. Likewise, for MVPA-based model, the SHIFT intervention was positive at a decay rate of approximately 15% at a £30,000 per QALY threshold (see Appendix 3, Figure 6). For the MOVES model, when within-trial differences were removed, the SHIFT intervention was cost-effective at all cost-effectiveness threshold values considered at a decay rate at, or below, approximately 20%.
FIGURE 3.
Incremental net health benefit of the SHIFT intervention relative to usual practice for alternative rates of treatment decay: MOVES model.

The base-case cost-effectiveness results were also largely insensitive to extensions in the duration of treatment effect on physical activity observed at 6 months. A comparison of SHIFT intervention ICERs (relative to usual practice) for alternative additional intervention costs and extension periods in treatment benefit are displayed in Table 36. With all other things remaining equal, the SHIFT intervention remained dominated for extensions up to and including 7 years for the MVPA- and sedentary-based models. For the MOVES model, ICERs only fell below £30,000 at a 6-year extension (see Table 36). Cost-effectiveness results were more sensitive to extensions in treatment effect when removing estimated trial-specific differentials in non-intervention costs and QALYs between arms. For the MVPA-based model, ICERs fell below £30,000 per QALY at 3 years, below £20,000 per QALY at 5 years and below £15,000 per QALY at 7 years. For the sedentary-based model, ICERs fell below £30,000 per QALY at 3 years, below £20,000 per QALY at 5 years and below £15,000 per QALY at 6 years. For the MOVES model, ICERs fell below £20,000 per QALY at a 1-year extension and below £15,000 per QALY for a 2-year extension (see Appendix 3, Table 72).
| ΔCost (£) | Continuation of SHIFT treatment benefit and additional cost profiles: ICER (£) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1): Ekelund et al.120 MVPA | (2): Ekelund et al.120 sedentary | (3): MOVES model | ||||||||||||||||
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | |
| –370 | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 69,399 | Dominated | Dominated | 28,422 | 8480 | 4742 | 2309 |
| –200 | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 133,256 | Dominated | Dominated | 85,269 | 29,189 | 19,027 | 12,303 |
| –100 | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 170,819 | Dominated | Dominated | 118,709 | 41,370 | 27,430 | 18,181 |
| Base case | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 208,382 | Dominated | Dominated | 152,148 | 53,551 | 35,833 | 24,060 |
| 100 | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 245,945 | Dominated | Dominated | 185,588 | 65,733 | 44,236 | 29,938 |
| 200 | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 283,508 | Dominated | Dominated | 219,028 | 77,914 | 52,640 | 35,817 |
| 370 | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 347,204 | Dominated | Dominated | 275,732 | 98,570 | 66,889 | 45,785 |
| 500 | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 396,197 | Dominated | Dominated | 319,347 | 114,459 | 77,849 | 53,452 |
| 1000 | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | 584,012 | Dominated | Dominated | 486,545 | 175,366 | 119,865 | 82,845 |
Secondary cost-effectiveness analysis from a private sector perspective
Absenteeism-related cost differentials between the SHIFT intervention and usual practice at baseline equated to a cost saving of £57.98 between the two arms (SHIFT intervention, £628.50; usual practice, £686.46). At the 6-month follow-up, larger reductions in absenteeism in the SHIFT arm (see Table 33) resulted in a cost saving equivalent to £169.43, compared with baseline differences. At the 16- to 18-month follow-up, an increase in absenteeism amounted to an additional £552.05, compared with baseline differences. Over the course of the trial, changes in absenteeism costs were £324.64 higher in the SHIFT group than in the usual-practice group, relative to baseline values. Incremental private costs per driver equated to £200.16 at 6 months and amounted to £694.23 at final follow-up (see Appendix 3, Table 72).
Employee-assessed job performance and presenteeism at 6 months were less favourable in the SHIFT arm, relative to the differences in changes from baseline. At 6 months, differences in employee-assessed work ability and satisfaction rose relative to baseline differences by approximately 0.148 and 0.036, respectively, in favour of the SHIFT arm, equating to a £1353 and £5560 cost per unit increase on each respective Likert scale. At the final follow-up, relative to baseline differences, differences in employee-assessed job performance, work ability, presenteeism and satisfaction changed in favour of the SHIFT intervention by approximately 0.081, 0.154, 1.19 and 0.151 less days worked while sick, respectively, equating to a £6816, £3585, £465 and £3656 cost per unit increase on each respective Likert scale. Average difference in results over the trial equated to the SHIFT intervention being dominated in presenteeism (compared with usual service), while having ICERs of £17,142, £4598 and £7425 with respect to one unit increases in employee-assessed job performance, work ability and job satisfaction, respectively (see Appendix 3, Table 72). Given the modest changes over time and largely inconsistent differences between arms, caution must be taken when interpreting the differences in costs, outcomes and associated ICERs.
Given the additional productivity costs (i.e. lost driver days), QALY decrements and higher public costs associated with the SHIFT intervention, relative to usual practice, a broader perspective that considers public costs and productivity would fail to alter base-case study findings.
Chapter 5 Process evaluation
Text from this chapter has been reproduced from Guest et al. 123 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Overview
Process evaluations provide a contextual understanding of how a particular intervention or programme was delivered, how participants reacted to it and why it was successful/unsuccessful in influencing behaviour change. In the context of complex and multicomponent interventions, such as the SHIFT intervention, which was delivered across multiple heterogeneous working environments throughout the UK, process evaluations provide useful information relating to all aspects of programme delivery and potential success, helping to inform which intervention components contributed the most and least to overall effectiveness. Therefore, process evaluations allow potential modification of the intervention, if required, and can inform the future implementation of the intervention (e.g. the SHIFT intervention as a training resource to HGV drivers across the logistics sector). 94
The purpose of this process evaluation, therefore, was to investigate the application of the SHIFT intervention to understand the context within which the intervention was applied and the key elements of its implementation, including fidelity (i.e. components of the intervention), adaptations, contamination, sustainability, barriers and facilitators. To determine the implementation fidelity (which is the extent to which the programme adhered to the protocol model initially developed124), we aimed to understand whether or not components were used as intended (e.g. dosage, attrition rates) and if any adaptations were made to the intervention. A further aim was to describe and understand the contextual factors that may have influenced the intervention’s implementation and/or effectiveness. In addition, the process evaluation will recommend refinement of the intervention for future sustainability to ensure that the intervention can be optimally embedded into the stakeholders’ routine policies. Finally, this process evaluation will also help to support the development of further effective RCTs that are evaluating lifestyle health-related behaviours in HGV drivers (and employees with similar enforced sedentary occupations).
Methods
The MRC guidance for process evaluation of complex interventions was the most suitable framework for this process evaluation. 94 The MRC guidance offers key comprehensive direction to describe the intervention, implementation and mechanisms of change, while understanding the contextual factors throughout. This integrated process evaluation proceeded in a series of steps and took place alongside the RCT. Mixed methods were used to deepen analytic understanding of a specific issue and, in turn, triangulate results. Fidelity and dose were measured both quantitatively and qualitatively. Qualitative research techniques were used to identify how context affects implementation, barriers, mechanisms of impact and future sustainability. Using a feedback questionnaire, we aimed to collect data on key aspects from both control and intervention participants, and combined these data with information gained from in-depth interviews and focus groups with drivers and managers who were purposively sampled from each depot. Feedback questionnaires were given to every participant 1 month after baseline measures and 1 month after 6-month follow-up measures. Interviews were conducted with one participant and one manager from each site after the 16- to 18-month follow-up measures. Table 37 summarises each of the evaluation outcomes examined, how the evaluation outcomes were defined and how the evaluation outcomes were measured. Table 38 provides a detailed overview of the process evaluation data collected through each method. The process evaluation results are presented according to the key outcomes examined (see Table 37).
| Implementation outcome | Definition | Data source | Time point |
|---|---|---|---|
| Context of the intervention | Contextual factors that affected the implementation, intervention mechanisms and outcomes | Initial discussions with site managers prior to intervention implementation | Pre study |
| Interviews with participants and managers | 16–18 months | ||
| First-hand experience of data collection | Continuous | ||
| Fidelity | The extent to which the intervention was delivered as planned | Project records | Post-study |
| Interviews with participants and managers | 16–18 months | ||
| Dose | How frequently participants engaged with intervention components | 6-month follow-up questionnaires | 6 months |
| TextMagic statistical reporting | Continuous | ||
| Adaptations | Changes made to improve the delivery of the intervention | Interviews with the drivers and managers | 16–18 months |
| Fortnightly research team meetings | Continuous | ||
| Sustainability |
Were changes in health behaviours following the 6-month intervention period maintained? The extent to which participants and managers can envisage the SHIFT intervention becoming sustainable in the future |
Interviews with the drivers and managers | 16–18 months |
| Mechanisms of impact | What strategies were put in place by intervention participants to facilitate behaviour change | Interviews with the participants and managers | 16–18 months |
| 6-month follow-up questionnaires | 6 months | ||
| Contamination | Did intervention and control participants/managers interact with one another? | Interviews with the participants and managers | 16–18 months |
| Component | SHIFT intervention group | Control group | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline questionnaire | 6-month questionnaire | Driver interviews/focus groups | Interview with managers | Baseline questionnaire | 6-month questionnaire | Driver interviews/focus groups | Interview with managers | |
| Heath assessment | ||||||||
| Did the health assessment encourage participation? | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Did the health assessment meet expectations? | ✓ | ✓ | ✓ | ✓ | ||||
| Did the health assessment increase awareness of health? | ✓ | ✓ | ✓ | ✓ | ||||
| Was the health assessment understandable? | ✓ | ✓ | ✓ | ✓ | ||||
| What was the most useful measurement? | ✓ | ✓ | ✓ | ✓ | ||||
| Did the health assessment motivate lifestyle change? | ✓ | ✓ | ✓ | ✓ | ||||
| Intervention component | ||||||||
| Education session | ||||||||
| Was the education session understandable? | ✓ | ✓ | ||||||
| Was the education session engaging? | ✓ | ✓ | ||||||
| Was the booklet informative? | ✓ | |||||||
| Did the education session increase awareness of health? | ✓ | ✓ | ||||||
| Did the education session motivate lifestyle change? | ✓ | ✓ | ||||||
| Did participants create action plans? | ✓ | |||||||
| What were the key messages? | ✓ | |||||||
| Did the booklets increase awareness? | ✓ | |||||||
| Cab workout | ||||||||
| Regularity of use? | ✓ | ✓ | ||||||
| What was the most common equipment? | ✓ | ✓ | ||||||
| Where was the most common location? | ✓ | ✓ | ||||||
| Barriers to the cab workout? | ✓ | ✓ | ||||||
| Fitbit | ||||||||
| Was the Fitbit understandable? | ✓ | ✓ | ||||||
| Did the Fitbit increase awareness of health? | ✓ | ✓ | ||||||
| Did the Fitbit motivate changes to lifestyle? | ✓ | ✓ | ||||||
| Step challenges | ||||||||
| Did step challenges increase awareness of daily steps? | ✓ | ✓ | ||||||
| Did step challenges motivate an increase in step count? | ✓ | ✓ | ||||||
| Text messages | ||||||||
| Was the frequency enough? | ✓ | ✓ | ||||||
| Was the content relevant? | ✓ | ✓ | ||||||
| Did the text messages motivate participants? | ✓ | ✓ | ||||||
| Did participants feel supported? | ✓ | ✓ | ||||||
| Were text messages efficient? | ✓ | ✓ | ||||||
| Overall impact of the study, factors influencing support and sustainability of the SHIFT intervention | ||||||||
| Workplace managerial support | ✓ | ✓ | ✓ | ✓ | ||||
| Knowledge changes due to study participation | ✓ | ✓ | ||||||
| Lifestyle changes due to study participation | ✓ | ✓ | ||||||
| Fluctuations of changes | ✓ | |||||||
| Barriers to a healthy lifestyle | ✓ | |||||||
| Most important component | ✓ | |||||||
| Future direction of the SHIFT intervention into CPC module | ✓ | |||||||
| External impact on lifestyle | ✓ | ✓ | ✓ | ✓ | ||||
| External fitness/diet tracking | ✓ | ✓ | ✓ | |||||
| Pandemic affecting study participation | ✓ | ✓ | ✓ | ✓ | ||||
| Thoughts of being in the control group | ✓ | ✓ | ||||||
| Contamination between control and intervention | ✓ | ✓ | ✓ | ✓ | ||||
| Managerial role | ✓ | ✓ | ||||||
| Management outcomes hoped to achieve | ✓ | ✓ | ||||||
| Operational difficulties | ✓ | ✓ | ||||||
| Sustainability of the SHIFT intervention | ✓ | ✓ | ||||||
Quantitative data collection
To inform attrition rates, records were gathered from all participants, including participation uptake per site (i.e. the number of participants who completed the baseline health assessment per site), dropout rate per site (i.e. the number of participants who failed to complete each follow-up), engagement with text messaging service (i.e. the number of responses) and compliance with activity monitor wear.
Feedback questionnaires
All participants were asked to fill out feedback questionnaires 1 month after the baseline health assessment and 1 month after the 6-month follow-up health assessment. The questionnaires included a mix of multiple-choice, open-ended and Likert scale questions. At baseline, the questionnaires sought feedback about the usefulness of the baseline health assessment for awareness of health and what the most useful measurements were. The intervention follow-up questionnaire (given to intervention participants 1 month after the 6-month follow-up health assessment) sought feedback about the quality and usefulness of different elements of the health intervention, including the education sessions, booklets, cab workout equipment, Fitbit, step challenges, text message feedback and any other lifestyle or work changes that may have affected the results. In the questionnaire that was completed after their 6-month follow-up assessment, control participants were asked about both health assessments and any other lifestyle or work changes that may have affected the results. Open-ended questions were analysed by listing key responses into themes and calculating frequencies in each theme.
Qualitative data collection
Managers in intervention sites were asked to schedule three to six participants for an on-site focus group, during the drivers’ working hours. A semistructured focus group schedule was developed and piloted with participants from six pilot sites. Questions related to each SHIFT intervention component, barriers to achieving health goals and recommendations for improvements to the SHIFT intervention. A semistructured interview schedule was developed and piloted for the managers in the pilot sites. Managers were also asked to schedule time for an interview on the same day as participants. These interview schedules were then analysed and revised, as needed, before commencing data collection for the main trial sites (n = 19).
Main trial interviews
Owing to the outbreak of COVID-19 and a national lockdown (March to July 2020), when face-to-face data collection (e.g. in-person interviews) was no longer feasible, mobile telephone interviews were completed for the main trial sites. As these interviews occurred during non-work hours, all participants were sent a text message to ask if they would be interested in participating, with the offer of a £5 high street voucher and a chance to win a Fitbit. The main liaison manager from each site was asked to participate via e-mail.
Data analysis
All focus groups and interviews were carried out by one or two researchers (AG and YLC). The focus groups were recorded on a digital audio device and transcribed verbatim (by AG) into Microsoft Word® (Microsoft Corporation, Redmond, WA, USA), before inputting into NVivo 12 software (QSR International, Warrington, UK) for analysis. Transcripts were then re-read, coded and developed into themes using the deductive method of thematic analysis, where themes were already preconceived based on the interview schedule and existing knowledge. This was followed by an inductive method where themes were identified entirely based on the data (by AG). 125 Each stage of the analysis was critically analysed from an informed external perspective. (Dr Anna Chalkley, an expert in qualitative research methods and programme evaluation, was independent to the research team and acted as a ‘critical friend’ throughout the analysis and reporting stage of this process evaluation.) Quantitative data, including dose, attrition and questionnaire data, such as multiple-choice and Likert scale answers, were analysed descriptively in IBM SPSS v25 (IBM Corporation, Armonk, NY, USA).
Results
Questionnaires
A total of 71.2% (n = 272) and 57.8% (n = 167) of the participants invited to complete a feedback questionnaire following the baseline and 6-month follow-up health assessments, respectively, responded.
Focus groups and interviews
During the internal pilot, on completion of the final follow-up measures, four focus groups and three interviews with 13 participants (i.e. an 83.3% site response rate) and eight individual interviews with managers (i.e. an 83.3% site response rate) were carried out to capture their views on and experiences of implementation. In the main trial phase, 15 telephone interviews were conducted with participants from the remaining 19 sites (i.e. an 82.4% site response rate), and 13 telephone interviews were conducted with managers (i.e. a 76.5% site response rate) after the 16- to 18-month follow-up assessments.
Through synthesising the data, we found there to be no substantial differences between pilot testing and main trial sites in both the questionnaire responses and the content of the interviews and focus groups. Therefore, the combined findings from both the pilot and main trial sites are presented below.
Dose
It was intended that participants in the SHIFT arm would receive a total of 12 hours of face-to-face contact with the research team over 12 months, broken down into three 2-hour health assessments and one 6-hour education session. Control participants would receive 6 hours of face-to-face contact, consisting of three 2-hour health assessments. Owing to the COVID-19 pandemic, a national lockdown prevented all main trial participants from receiving a face-to-face final follow-up health assessment, hence a reduction of 2 hours of face-to-face contact with participants from the main trial.
Text messages
Throughout the duration of the study, the average number of text messages sent to each driver was 20.2 and the average number of text messages received from each driver was 3.8, which resulted in a 18.8% response rate. Although drivers did not engage much with the text messaging, it appeared that the overall use of text messages was relatively positive, and a number of drivers used this service at the end of the trial to relay their appreciation to the research team:
I haven’t really text backwards and forwards, but when you sent me the, the little challenges like I thought good, yeah, I enjoyed that.
Intervention participant, P06 – text messages
. . . it’s been interesting and enlightening and hopefully you and your team have added a few years to my life and others with the result of your research.
Intervention participant, P03 – text messages
Thank you to yourselves [students] your university, staff, teachers, etc., for all your endeavours helping me to take my health more seriously. Wishing you all the very best in the future.
Intervention participant, M18 – text messages
Cab workout equipment
Fifty-nine per cent of intervention participants said that they had used their cab workout equipment in the last 6 months, with few planning to use it in the future. For participants who did use it, the most common piece of equipment was the hand gripper, followed by the resistance bands. There was minimal reported use of the fitness ball. Of the participants who used the cab workout equipment, only 16.3% agreed/strongly agreed that it increased their physical activity levels. Reasons for low adherence levels were explained in more depth during the interviews. Some participants suggested that it was impractical to use the workout equipment in their cabs:
I mean to start doing all the . . . [demonstrates exercising], you know, just completely impractical. Really is . . . but . . . It’s all right if you’re office based.
Intervention participant, P03, FG2 – cab workout
Participants also reported that they prioritised sleeping and eating on their breaks:
. . . the only thing I do on my break is sleep.
Intervention participant, P01, FG1 – cab workout
I don’t, I don’t think it’s practical. You have 50 minutes’ break and by the time you’ve been to the toilet, you’ve had your dinner, you need a nap, if you’ve done 3.5, 4 hours of driving, you need shut eye for 10, 15 minutes. There’s just not enough time . . . To do those things.
Intervention participant, M17 – cab workout
Participants also suggested that, although not embarrassing for them, cab workouts would be embarrassing for some of the older drivers:
So the thing is . . . I think with a lot of like the old boys, like the drivers. Maybe they wouldn’t want to do something like that because they’d feel like, stupid doing it to be honest. Do you know what you mean? Whereas I don’t care. But like older drivers, they’d have just been like ‘oh I’m not doing that’.
Intervention participant, P03, FG1 – cab workout
However, a small number of participants did carry out cab workouts regularly and seemed to benefit from this:
Yeah, the hand gripper, I use that, the stretchy bands, I used to use them more, because I could tie it to the bottom of the handrail in the cab and I could exercise my arms, both arms. And you could do it whilst you were driving. Right hand only. I mean obviously you’ve got to keep holding the steering wheel all the time, but when I were parked up, I used the gripper, and the ball. And you know, I kept up with that. I’ve still got them, and I still use them. So you know, they are, they are quite, you know. They’re all right, they do what they’re meant to do.
Intervention participant, M14 – cab workout
Fitbit
The majority (92.4%) of intervention participants were still using their Fitbit 6 months later, and 6.1% of participants had previously used their Fitbit but no longer use it. Only 1.5% of participants had never used their Fitbit and did not intend to in the future.
Participants who did not use the Fitbit mainly said that it was due to the Fitbit causing skin irritation, already having an activity monitor or not knowing how to use the Fitbit:
I haven’t, I have been wearing it, but I had a rash on my wrist so I’ve had to take it off.
Intervention participant, M17 – Fitbit
Umm. Fitbit no, because to be honest, I’ve got one of these . . . I’ve got my own.
Intervention participant, M18 – Fitbit
If I remember, we were given the Fitbit at the end of the lesson weren’t we? I have, still no idea. I have no idea how to use the watch. I have no idea how to . . . Yes. I have no idea how to use it. The only thing I do is, press the button, and it tells me the time and that’s it. I mean, seriously. I mean, I mean, I’m hoping that this Fitbit thing is going to show me just how bone idle I am.
Intervention participant, P03, FG2 – Fitbit
Fidelity
Health assessment
Eighty-three per cent of main trial respondents agreed/strongly agreed that the baseline health assessment met their expectations, whereas 86.0% of respondents agreed/strongly agreed that the baseline health assessment made them more aware of their current health status. Most (90.3%) respondents agreed/strongly agreed that the health assessment was thorough and understandable. Participants were asked to rank the measurements in terms of most interesting and useful, and cholesterol was considerably the most valued measurement, with waist circumference the least valued measurement (Table 39).
| Health assessment component | Frequency (n) |
|---|---|
| Cholesterol | 147 |
| BMI | 108 |
| Blood pressure | 93 |
| Blood sugar | 90 |
| Fat percentage | 85 |
| Grip strength | 57 |
| Waist circumference | 31 |
| None | 5 |
Most (87.8%) intervention participants agreed/strongly agreed that the first health assessment motivated them to want to change aspects of their lifestyle, including increasing physical activity (73.2%) or improving diet (70.7%). Fewer control participants agreed/strongly agreed (63.6%) that the first health assessment motivated them to want to change aspects of their lifestyle, including increasing physical activity (49.5%) or improving diet (52.5%). Participants mentioned that it was ‘eye-opening’ to see their results within the health assessment:
But when you see it wrote down in front of you, it sort of clicked something in the back of your mind, thinking ‘oh God’. You know what I mean. Numbers, numbers look a lot worse than looking at yourself because you’re used to looking at yourself . . . you look in the mirror and you just tap your belly, and you say ‘blimey I’ve got to lose some weight’. You know what I mean. But, when you’re sitting there, and someone writes down, like . . . I can’t think what it was, it was bleeding high, I know that! Yeah they said your BMI is this and that, then you start to think . . . You know what I mean. Because as I say, you look at the numbers and then it triggers something in you and you think ‘oh blimey, I’ve gotta sort this out’. You know what I mean.
Intervention driver, M15 – health assessment, eye-opening
Participants (including control participants) also mentioned that it was motivational to improve their health:
I didn’t realise I was overweight or, I’ve got a bit of belly but it’s quite literally, yeah it’s not too big but when they turned around and said you’re obese and your BMI is like a little bit not where it should be and then you’ve got the graph and they point to you in a certain section . . . Mine wasn’t too bad it was just a little bit above where I should have been so it gave me confidence that I could get there.
Control driver, M23 – health assessment, motivating
Six-hour structured education session
Most (90.9%) participants agreed/strongly agreed that the structured education session was the correct length of time, and participants agreed/strongly agreed that it motivated them to increase their physical activity (77.3%), reduce their sitting time while not at work (72.7%) and improve their diet (71.2%). When asked in the questionnaire about the key messages participants took away from the education session, 41% of 97 responses mentioned dietary knowledge changes and 31% mentioned exercise knowledge changes.
Based on the interviews and focus groups, participants were happy overall with the information they received. Most participants recalled learning about diet, more so than other elements (e.g. physical activity, smoking, alcohol, sleep and mental health):
Yeah, I enjoyed the workshop with the, with all the information we received about the food, I thought it was really enjoyable. And the understanding, gave me a better understanding of food. And gave me a better idea of how I could balance my diet. So I thought that was one of the best things of the study, was actually that, that part of it and the information that was available to us.
Intervention participant, P03, FG1 – education session feedback
The way it was put across was great, the people we had in there was, was brilliant, and everyone partook in it brilliantly. But it was the information, the information that was there and available to us. And it was given to us in a way, it was given to us because it gives us a lasting knowledge of what was good for you and how easy it is to have the things you like, alongside the things you maybe no like to make a balanced diet, so you don’t have to be daft and cut everything out and just have fruit and veg, you can have a bit of everything and still be healthy. So I mean it was really good.
Intervention participant, P03, FG1 – education session feedback
Fitbit
Participants agreed/strongly agreed that the Fitbit increased their awareness of their physical activity (90.5%), sitting time (73.8%) and sleep pattern (88.1%). In addition, the Fitbit motivated participants to make changes to their physical activity (81.0%) and their sitting time (69.0%), but less so their sleep pattern (40.5%). Three-quarters (75.4%) of participants engaged in the step challenges, 59.4% agreed/strongly agreed that step challenges were motivating and 54.7% agreed/strongly agreed that participating in the step challenges helped to increase their step count. Some participants reported that the free Fitbit was the main motivation for taking part:
. . . so why did you guys decide to take part in the SHIFT study?
Truthful answer . . . the free Fitbit.
The Fitbit was also mentioned as a useful tool for goal-setting and for providing a consistent sense of achievement:
. . . with having the tracker thing [points at Fitbit]. It’s like a reminder like, in better weather, I’d look at it, and if I hadn’t got enough steps in I’d go and take the dogs around the block again, you know what I mean, just to get my counts up.
Intervention participant, P06, FG1 – Fitbit, goal-setting/achieving
Because of course, if you haven’t done your 250 steps, it buzzes, so I’d actually get up and start walking up down the living room to get those 250 steps in! If it’s nearly to midnight, I haven’t done me 10,000 steps, I’d be up and walking wherever I could.
Intervention participant, M18, Fitbit
I think the Fitbit, because I think that motivated me more than anything else because it challenged us all the time, you know it buzzes and said come on let’s go, 250 steps. That’s . . . where I live on my boat we’ve got washrooms we can use, that’s walking to the washrooms and coming back, I get my 250 steps so I maybe do that four times a day, whether I want to or not, I still like the challenge. You know, and I’m still getting that challenge now, which I try my best to carry out and get done. But I would say the Fitbit is the . . . it gets into your head, you get addicted to it. I am addicted, I always have it on, when the armband broke I actually used an elastic band and I wrapped it around so it stayed on my arm, ha ha ha! But that really got me addicted, and I’m still addicted to it now like you know.
Intervention participant, M24 – Fitbit
Text messages
Participants agreed/strongly agreed that the frequency (84.5%) and content (91.1%) of the text messages was appropriate and informative. Based on the focus groups and interviews, the consensus of participants was that the text messages were good at providing logistical information for the upcoming tasks and that it was beneficial to know that the SHIFT team were readily contactable:
Well, they were sent as they were needed, you know it wasn’t an overload for the brain or anything but they were you know, when something had to be done, and yeah they were fine, unless you wanted . . . because I had the number I knew if I had any problems, any time I actually messaged you I did get a reply back . . . I did. Yeah every time I always got my reply back, and that’s what I was happy about. At least I, I knew I was doing something at my end, and you’s were taking it serious at your end . . . You were always there, because you said if you need us, we’re there. You kept your word, like I said, I messaged you, you got back, and you had kept your word.
Intervention participant, M24 – SHIFT components, text messages
However, participants suggested that if the messages were to be used in a motivational capacity, then more frequent messages would be beneficial:
The only thing the only thing I could think of personally, would be to just ramp them up a little bit more. You know what I mean . . . Because they were quite well spaced out. You know what I mean. If I’m right, there were only about two of them, weren’t there?
Intervention participant, M15 – SHIFT components, text messages
Adaptations
All baseline health assessments were conducted at the beginning of the drivers’ shift. Carrying out baseline health assessments for all drivers was logistically challenging for both the researchers and transport managers because of the 24 hours a day, 7 days a week (24/7) nature of the job (i.e. drivers started their shifts at different times of the day and night). As a result, post-intervention measures were carried out at a time of day that was more feasible and occasionally this was at the end of the drivers’ shifts. Drivers were still asked and reminded to fast for 4 hours prior to the assessment; however, this change in protocol may have affected body weight and blood pressure measurements.
The initial target was to include six participants per education session; however, it was logistically challenging for the transport managers to take six drivers off the road at any one time, particularly for the smaller sites, and, as a result, most education sessions consisted of fewer drivers per workshop (mean: 3.4 drivers; range: 1–7 drivers).
Owing to the COVID-19 pandemic, a national lockdown prevented all main trial participants from receiving a face-to-face final follow-up health assessment. Instead, in the 16- to 18-month follow-up health assessment, participants were asked to fill in the questionnaire, self-report their weight and wear the activPAL device (to monitor physical activity and sitting) while filling in the wear log continuously for 8 days. The pandemic also caused a 4- to 6-month delay in the final follow-up assessments for sites in the main trial phase, resulting in these taking place 16–18 months after randomisation.
Attrition
A total of 382 participants (SHIFT intervention, n = 183; control group, n = 199) received the baseline health assessment. Two hundred and sixty-two participants (68.6%) took part in the 6-month follow-up health assessments. The retention rate at 6 months was higher in the control group (73.9%) than in the intervention group (62.8%), and this may be because of intervention participants being ineligible to continue with the study if they failed to take part in the 6-hour structured education session. Of the 183 intervention participants, 145 (79.2%) took part in the education session. At 6 months, the retention rate for intervention participants who were eligible to continue after the education session (n = 145) was 79.3%. Participants who did not attend the session were removed from the trial. The dropout rate in the intervention group may have been a result of their expectations for greater health improvements among them:
I think that for them that dropped out they just . . . I think they’d realised that they’d not really made that much of a change, or they made them, but they were short-lived if you know what I mean?
Intervention manager, M11, FG1 – feedback from drivers (manager perspective) – reasons for dropping out
Table 40 shows reasons for discontinuation of the study.
| Trial arm, n/N (%) | Overall, n/N (%) | ||
|---|---|---|---|
| Control | SHIFT intervention | ||
| At baseline | |||
| Consented at baseline | 199 | 183 | 382 |
| Entered trial and gave data | 199 | 183 | 382 |
| At 6 months’ follow-up | |||
| Attended and gave data | 147/199 (73.9) | 115/183 (62.8) | 262/382 (68.6) |
| At 16–18 months’ follow-up | |||
| Completed trial | 104/199 (52.3) | 101/183 (55.2) | 205/382 (53.7) |
| Reasons for discontinuation | |||
| Participant deceased | 1/199 (0.5) | 0/183 (0.0) | 1/382 (0.3) |
| Lost to follow-up | 61/199 (30.7) | 51/183 (27.9) | 112/382 (29.3) |
| Investigator decision | 2/199 (1.0) | 4/183 (2.2) | 6/382 (1.6) |
| Left job | 14/199 (7.0) | 8/183 (4.4) | 22/382 (5.8) |
| Long-term sickness | 5/199 (2.5) | 2/183 (1.1) | 7/382 (1.8) |
| No longer interested | 3/199 (1.5) | 0/183 (0.0) | 3/382 (0.8) |
| Cluster withdrawal | 13/199 (6.5) | 15/183 (8.2) | 28/382 (7.3) |
| Significant protocol violation | 0/199 (0.0) | 1/183 (0.6) | 1/382 (0.3) |
| Suspended from work | 0/199 (0.0) | 1/183 (0.6) | 1/382 (0.3) |
Retention rate between the 6- and 16- to 18-month follow-ups was higher (78.2%). In this instance, retention was better in the intervention group (87.8%) than in the control group (70.7%). It may be that overall retention rate was higher after 6 months because participants who were disinterested in the programme had already dropped out. Participants in the intervention group were possibly more invested in the intervention, and yet little time spent engaged in the project was required of them during this time. From start to finish, 46.3% (n = 177) of participants dropped out of the RCT. Mangers were asked in interviews if they knew the reasons for participants dropping out. One of the primary reasons mentioned was that truck drivers are notoriously transient workers, with a high staff turnover rate. It was evident that a lot of drivers had left their company before the cessation of the programme:
Like I say, the drivers that had to drop out, they left, obviously that’s one of those things, they left and went on to different contracts.
Control manager, M12, interview – feedback from drivers (manager perspective) – reasons for dropping out
Other reasons for participants dropping out included long-term illness, and a few participants were isolating or on furlough:
Yeah, a couple on long term sick, and I think a couple that were shielding as well.
Intervention manager, M11, interview – feedback from drivers (manager perspective) – reasons for dropping out
Another commonly mentioned reason for participants dropping out was that some saw the timings of the health assessments as too inconvenient:
They just want to come in and go home. Yeah, they don’t want to do anything else that adds on to their day. Which I totally get.
Control manager, P05, interview – feedback from drivers (manager perspective) – reasons for dropping out
Barriers to behaviour change
In the 6-month questionnaires, all participants were open-endedly asked to identify their main barriers to a healthy lifestyle. Among the 145 responses, 46% suggested that the biggest barriers were work related, predominantly the length, irregularity and start times of their shifts. The second biggest barrier was family (e.g. child care) commitments (12%) and this was followed by self-motivation (10%), with drivers referring to themselves as being ‘lazy’ or ‘need[ing] more discipline’. Table 41 shows a summary of the mentioned health barriers.
| Barrier | Frequency (%) |
|---|---|
| Work related | 67 |
| Long hours | 23 |
| Shift pattern | 16 |
| Diet at work | 10 |
| Lack of routine | 5 |
| Family | 18 |
| Diet | 16 |
| Self-motivation | 14 |
| Time (not explicitly work related) | 10 |
| Weather | 4 |
| Injury/illness | 3 |
| Sleep | 3 |
| Embarrassed to exercise in public | 1 |
Using an inductive approach in the thematic analysis, we discovered that participants discussed barriers to living a healthy lifestyle both at work and at home. We deemed it important to mention these barriers, as this may influence guidance for future health interventions in this demographic. Participants mentioned that there was little time in the day to fulfil a healthy lifestyle:
The trouble is, is when do you get the time, isn’t it? . . . You know, you finished at 10 o’clock in the morning, from 8 o’clock at night. All you want to do is go back and have a shower go to bed.
Intervention participant, P03, FG2 – barriers, time
You’ve got all the things to do, you know I don’t have any chance to do any exercise, I don’t.
Intervention participant, P03, FG2 – barriers, time
Linked to time pressure are the long, irregular shift patterns and early start times that almost all drivers from both control and intervention sites mentioned at least once in their interview:
. . . well, yeah, you know and the start times as well, I start between midnight and 4 a.m. in the morning and that’s no, no good for your body rhythm if you like. It’s not good for your body. By 2 o’clock in the afternoon you want to go to sleep.
Control participant, M09 – barriers, shift pattern
But I’ve done this for 35, 36 years now, umm, and it’s just, it’s just part of being a lorry driver, it is how it is, it’s the territory that you’re in. You know, the uncertainty of where you’re going to work, whether you’re going to go home that night, or it might be 5 days later.
Intervention participant, M14 – barriers, shift pattern
I think there should be, once you have worked a 12-hour shift, you should have a minimum of 12 hours off. Whereas you can actually work 15 hours and have 9 off. That’s the legal requirement, 9, 9 minimum . . . because it’s 15 and 9, that’s your 24 hours in a day isn’t it. Alright, so you could start at 6 in the morning, work till 9 at night and realistically you could start at 6 o’clock in the morning the next day.
Intervention participant, M14 – barriers, shift pattern
And, I mean, to say that the trouble with the HGV world is uh, it’s poor wages long hours, you know. You can make a good life out of it, but you’ve got to put a lot of hours in, and when you put the hours in, it is detrimental to your health at the end of the day. You know, unfortunately that’s the aim of the game, you know.
Control participant, M25 – barriers, shift pattern
Therefore, there’s often unrealistic, unrealistic expectations required of drivers, and long hours. And short breaks. And that doesn’t help at all with this side of things, which is looking at trying to keep healthy. Try to have a diet, a decent diet. When you’ve got guys going out for 13, 14 hours a day, when you get home . . . when I’m on 12 hours a day, I get home, I have a snack and go to bed practically. A snack, a shower and bed and up in the morning. And it’s a snack, and into work, when I’m on my 12-hour shift. So the guys that are getting a couple of 15 hour shifts on the bounce, they get home, go straight to bed. And they’re actually back the next day driving, munching as they go down the road because that’s their time for having something to eat. So, the fact that the pressures that are put on us in the workplace, and often or not the uhhh . . . the mismanagement of that is, is a factor towards the healthiness and well-being of the driver himself.
Intervention participant, P03 – barriers, shift pattern
A lack of managerial support and excessive expectations also affected drivers:
It’s operational requirements, like. It’s, it’s operational requirements, because you can’t always eat when you’re supposed to eat, or sleep when you’re supposed to sleep or anything like that. It all revolves around your working day. And that’s how it is.
Intervention participant, P03, FG2 – barriers, shift pattern
It’s not doing one 12-hour shift or one 15-hour shift, it’s one after another, after another, after another. You know, they think you’re machines like these trucks, you can drive them for 24 hours a day, 7 days a week, they’re just trucks, put some more diesel in, put some more oil in, they’ll go forever, we can’t do that. They think we can.
Intervention participant, M14 – barriers, shift pattern
I’ll be honest, well, me and my partner have now gone part time, because it had just got ridiculous. And they just expect so much of you. And like, especially . . . for some reason, I don’t know why. In our place, the night drivers are just the dirt on their feet . . . They’ve just, no respect for them . . . but you try working nights. They don’t seem to understand that, you know, your natural body clock, even no matter how many years you work nights, your natural body clock is, you go sleep at night.
Intervention participant, M18 – barriers, shift pattern
Another key barrier included unavailability of healthy foods while at work:
Well, umm, basically being lorry driver is . . . the diet is shocking, you can’t really get healthy food it’s all fast food. Basically working the shift that I do I finish my day at the end of the night, around 11 o’clock at night. And if I’m parked up somewhere, the only thing that’s available is McDonald’s [McDonald’s Corporation, San Bernardino, CA, USA], or Burger King [Jacksonville, FL, USA] or sandwiches from WHSmith [WHSmith, Swindon, UK].
Intervention participant, M14 – barriers, diet at work
. . . you’re feeling knackered, you’re feeling drowsy, you’re feeling, you know, you need to be doing something, you eat. And unfortunately that’s the nature of the beast. And it’s not necessarily good for you, but you can also have the mindset, well, it’s better that I eat than crash. Because I could kill somebody, or myself, you know. And it’s one of those, umm, it’s not ideal, and I do try and eat more healthily when I’m snacking, but sometimes you are thinking an apple ain’t doing it, a banana ain’t doing it, grape ain’t doing it, dried fruit’s not doing it. **** it, I’m having a choccy bar.
Control participant, M22 – barriers, diet at work
But yeah, the biggest problem is, when you go to a service station, what’s the first thing you see there? It’s fast food. They are trying to change it but it is fast food, burger bars, so . . . They are popping up aren’t they. You don’t see enough healthy food options, certainly in the UK, and whether that’s because of the climate we have I don’t know.
Intervention manager, P01 – barriers, diet at work
Less mentioned, and more debated, was physical activity, with some drivers suggesting that they do a lot of physical activity at work and others saying that they do not:
Well, the thing is my exercise regime did go out the window a little bit. Because I was starting an hour earlier . . . I was starting at . . . getting up at 2 and starting at 3 in the morning. And of course you know, that knocks you right up, you know, you come in and all you want to do is have a sit down an hour, half an hour, you have your food and then you’re back in the bed, you know.
Control participant, M25 – barriers, physical activity
It’s a shame, we haven’t sort of got a place, a room or something with gym equipment in it. Because I mean, you know, for example, like drivers, they sometimes sat down there for like 2 or 3 hours waiting for a job. I mean, if you have like gym facilities on site, rather than just sitting there, you could just come in and do a bit of something, you know what I mean?
Intervention participant, P03 – barriers, physical activity
Yeah, so, and the pumping weights, well I do enough of that at work, loading and unloading trailers.
Intervention participant, M11 – exercise
My job is quite physical anyway. Umm and you know you unload like 300, 400 tyres, umm, so yeah you know that’s quite a good workout.
Intervention participant, M19 – exercise
Self-motivation was also a key barrier that was mentioned, and this appeared to be at both home and work:
Just being lazy . . . That’s it. Me just telling myself ‘oi get your big fat ass out of there and go for a walk!’ or do this, or do that!
Intervention participant, M24 – barriers, self-motivation
I just think it’s that lazy mindset really, like I said I’ve been preparing, like when I get home now like, my fruit or anything that I’m going to bring with me today, umm, it was a lot easier before to maybe just get to shop to buy sandwich. It’s pretty packed. It’s just lazy that mindset. Isn’t it?
Intervention participant, P03, FG1 – barriers, self-motivation
Suggested improvements to the SHIFT intervention
By a significant margin, the most frequently suggested improvement from both drivers and managers was that the SHIFT team could have had more regular contact and engagement with the participants:
I suppose, how could I say it. I think if I was to say anything, and this is not being negative or nothing, but you just asked to think of something so . . . Umm, maybe just the odd phone call just to cheer you up and keep you going. You know what I mean . . . Barring that, I couldn’t imagine anything else. You know what I mean. But yeah, a bit of a phone call every now and again, how are you keeping, how are you doing? We’re keeping an eye on you like. If you know like, someone is watching you, it makes you, it pushes you along a bit more. You know what I mean.
Intervention participant, M15 – improvements to SHIFT
I haven’t really text backwards and forwards, but when you sent me the, the little challenges like I thought good, yeah, I enjoyed that. And that’s one thing I would say. There could have been a lot more of that. Yeah, a lot more challenges, like, you know, you know, let’s say, look, everybody’s gotta hit 15 . . . But if there was more goals in there . . . And sort of push us along because I would rise to the occasion with the goals. I like that sort of thing. If I’ve got a target, that’s my goal that, you know me. But when there’s no targets, you tend to take a backward step.
Intervention participant, P06 – improvements to SHIFT
I think for them they were given all the information and then there was quite a long gap, so that was the only negative thing I would say, that gap was too long, they were saying what’s happening and they were starting to lose interest.
Intervention manager, P01 – improvements to SHIFT
Lots of drivers and managers would have liked to have seen more feedback about the results:
Yeah, just better . . . more publication of results for, for me, personally, I would have got something out for that. And I would have probably been able to get more of the guys talking about it if they have that data as well.
Control participant, M22 – improvements to SHIFT
I’d have liked to have been able to have access to the results of the monitors that we were wearing to see over the period of time that we were wearing them how things fluctuated as well.
Control participant, M22 – improvements to SHIFT
From the perspective of the managers, more clarity at the beginning would have been helpful in organising and managing expectations:
Umm, I think the only thing for me would be, at the very, very beginning, having a more understanding of what was involved . . . [exhales] it was a little bit, um, it was a little bit, ‘it won’t affect your business, it won’t affect your everyday’ bla bla bla, bla bla bla. But it does affect it when it’s 2 or 3 hours and it, and you’ve got to umm, plan that around the drivers’ times, rest days, and all that kind of stuff. Umm you know that alone, the admin side from myself or one of my managers, to prep it, umm, probably, it’s a few hours in a day, each time, to try and prep that. And it’s not about not doing that part of the role, I don’t mind doing it, but it’s about understanding that’s what is involved. Because at first it was sort of addressed, that ‘yeah, there’s no problems, there’s no impact to yourselves’, etc., etc., etc., but that is 2 or 3 hours of the drivers days that then have to rearrange his job for the day so that he can complete his job within legal time.
Intervention manager, M17 – improvements to SHIFT
Future sustainability
All managers were asked if they could see the SHIFT intervention becoming sustainable in the future, and if they would support it. All managers said that they would support the SHIFT intervention:
Absolutely. 100%. I can say that without even speaking to my, my higher tiers . . . But I know they’ll be on board, they will, yes . . . Again, it’s all about the well-being of drivers isn’t it?
Control manager, M12 – support SHIFT in the future
I think to have it as part of a CPC [Certificate of Professional Competence] I think certainly, would be, be a much easier route and you’d obtain a lot more numbers to be involved.
Intervention manager, M17 – support SHIFT in the future
However, some managers caveated this support with comments about issues with practicalities:
Umm, I suppose within DHL to get everybody done for the assessments and stuff would take some major planning. You know what I mean, just on my contract alone you’ve got 650 drivers. Umm, within DHL you’ve got 7500 drivers. Umm, so you imagine trying to have consultations and assessments with their health and things on site with 7500 people . . . Umm, and that’s just like 7500 driving colleagues, staff at the moment. Within DHL . . . It’s quite big . . . If you want to start planning your days around, you can plan 7500 in if you want to haha . . . Keep you busy until 2029 I think! Haha.
Intervention manager, M18 – support SHIFT in the future
Yes, it could. Ummm, you would have to work around sort of different areas or different drivers, because not all drivers would want to participate. Not everybody would want to be part of it. Like I said, in the planning of the drivers and getting the drivers back, it’s sometimes not that easy to be able to manage small contracts and get drivers back on site, the time that you need them.
Control manager, M22 – support SHIFT in the future
Intervention participants were asked about their thoughts on turning the education session into a Certificate of Professional Competence (CPC) module, and all drivers said that it would be a good idea:
Now that would be interesting that would. That would be interesting . . . yeah, I think that would be a good thing actually. It would be well educational actually for a lot of the drivers.
Intervention participant, M24 – education session, CPC module
However, almost all participants said that the education session would need a medical professional rather than a driver trainer to lead the workshop:
Well I think I’d prefer it to be a health professional, uh, because it just seems more appropriate that it comes from a health professional. You’d think that you’d take it more seriously.
Intervention participant, M14 – education session, CPC module, driver trainer
But no with that thing I think a health professional could present it better than a driver trainer because driver trainers understand driving but they don’t understand . . . other things . . . it’s always better to have an expert talking about something they know, then somebody talking about something they’ve been told to talk about.
Intervention participant, M17 – education session, CPC module, driver trainer
No . . . because they haven’t got the depth of knowledge that you guys have, I don’t think it would work. I really don’t think it would work. All they’d do is just read it. And then ask you five questions at the end of it and say right, you sign that, then sign the form to say that you understood. And people will just be saying, god is it time to go home?
Intervention participant, P03, FG2 – education session, CPC module, driver trainer
One driver mentioned that cost would prevent DHL Supply Chain from doing the CPC module:
They have a programme that the whole of DHL use and that’s all you’re doing, not doing nothing else. Because we may have to register for something else, or we may have to pay out for something else. So we’re not going to do that. So . . . companies would then have to pay for it, they’d have less interest . . . Is it going to help them get a truck down the road?
Intervention participant, P03 FG1 – education session, CPC module
All drivers said that moving the education session to an app-based workshop would be detrimental:
I mean if you’re in the classroom and that, with other people and that, then you get involved more, I think if you’re on your own, doing an app, I think a lot of people probably won’t bother.
Intervention participant, M19 – education session, app based
I’d rather have face to face because you can ask questions and that can’t you really. And if you’re there with a group of people, which you can’t do at the moment, everybody would be coming up with different questions . . . I might be asking one question and the guy next to me asks another question that I never even thought about asking.
Intervention participant, M24 – education session, app based
Being honest. I wouldn’t have paid much attention to it at all. I’d have flicked it on and flicked it back off just to say I’d been on it! I’m being totally honest. I’ve always worked with face to face, and I work well that way. I’m more of your audio kinetic sort of learner and everything. Umm, on a web page or on an app, you’re going to have loads of writing and all that sort of thing. I’m not going to read it and that’s being honest.
Intervention participant, P03, FG1 – education session, app based
Control site participants
There was a lack of awareness from participants and managers in the control group with regard to whether they were in the control or intervention arm, suggesting that the SHIFT team could have provided more communication and explanation to both the managers and participants of their allocated arms and what this means for participants:
So were you aware that there was two different groups in the SHIFT study, one was called control and one was called intervention?
Yeah, yeah, yeah. That’s what we’ve been told before, yeah, before we started . . .
Do you know which one your site was put into?
The . . . I think that second one as far as I can remember . . .
Oh, the intervention?
Yes.
Were you aware that there were two different groups in the SHIFT study, one called control and one called intervention?
Umm I can’t remember, it possibly was mentioned to me but it’s that long ago that I can’t remember to be honest with you.
OK, and did you know that there were two different groups in the SHIFT study, one was called control and one was called intervention?
Yes, yes I did.
OK, and did you know which one your site was allocated to?
No.
Contamination
Randomisation occurred at the site level to ensure that there was a minimal risk of contamination between intervention and control participants. All participants and managers were asked in the interviews if they had spoken about the SHIFT intervention to anybody from any other depot, and all participants and managers confirmed that they had not.
The COVID-19 pandemic
A large, unforeseen confounding variable to the SHIFT intervention was the COVID-19 pandemic, which caused three major lockdowns in the UK from March 2020 to June 2021. Fortunately, almost all (254/262, 96.9%) participants who were still enrolled in the trial at the time had completed the 6-month follow-up health assessment before the national lockdown. However, the final follow-up was greatly affected by the pandemic and, as such, all main trial sites required remote data collection, where participants were asked to fill out the questionnaires, self-report their weight, wear the activPAL and complete the sleep/work diary by themselves. Although this method has clear shortcomings, this was deemed the most appropriate, pragmatic and safe form of data collection at this time to assess the key main outcomes. When the COVID-19 pandemic was discussed in the interviews, it appeared that all sites were affected by the pandemic in different ways. For example, participants who delivered essential items were busier and worked longer hours:
In fact, we went through a stage where we were actually really short of drivers.
Oh wow, was that due to sickness?
No it was due to operational demand because people were panic buying so our stores we deliver to, were ordering bigger and things like that . . . Oh lots of hours and double shifts and things like that, just to keep the business going and to keep stocks up and things like that.
Some drivers mentioned that it was business as usual:
It was the same run on the same start times, so not many changes if you like.
Control participant, M12 - Contamination, COVID-19, business as usual
The same, whatever happens. Our job is the same. It never ever changes. We go out, two runs a day. Fourteen pallets, it might be a bit less. But it all depends how much the shop’s selling. But yeah, that’s it, it never changes. It’s just the same every day.
Intervention participant, M15 - contamination, COVID-19, business as usual
Some drivers mentioned being on furlough for most of the year. However, there was ambiguity between the furlough status of all sites. Several sites furloughed staff on a rotational basis, some sites furloughed all staff, other sites made it optional for drivers to choose furlough and the remainder of sites, which provided essential goods, did not make furlough an option:
I was put onto furlough, I was put on that until August . . . then we were locked down again in November, so we were put on furlough then . . . Since April I have probably done less than . . . 10 days’ work?
Control participant, M21 – contamination, COVID-19, furlough
Some managers also mentioned that the pandemic halted the enthusiasm for the study, with priorities moving elsewhere:
It’s just after that the, the communication and the engagement because of the COVID-19, where that stopped, then obviously, this . . . the SHIFT stopped as well, well in the drivers’ minds. So they just said ‘it’s not continuing anymore obviously’. Nobody’s ringing us, and everybody else had obviously their personal interests in their minds during this pandemic as well. So they just lost focus on it.
Intervention manager, M10 – contamination, COVID-19, impacted study enthusiasm
Behavioural changes
The primary outcome measure was the difference in steps per day at 6 months between the SHIFT and control arms, measured using the activPAL3 accelerometer. Although a significant difference in daily steps was observed at 6 months, in favour of the SHIFT arm, drivers (from both trial arms) commented that the biggest modifications they made were to their diet, with 57 comments/references to dietary changes recorded, whereas only 27 comments/references were made with regard to changes in physical activity. There were no significant differences in fruit and vegetable intake or dietary quality observed between trial arms at either follow-up period:
Well, as I say I cut down my sugars and the, is it . . . saturated fats? Is it? . . . I try and do a little bit more exercise although that’s a little bit more difficult haha!
Mmm, yeah yeah, understandable. Right OK, and you mentioned there that exercise was a bit harder to control than the diet, what makes you think . . . what makes you say that?
Well, I mean it’s easy to change from butter to margarine you just buy a different one on the shop haha, whereas to get your mind to want to go out and do a bit of exercise is a bit harder, ha ha.
Control participant, M09 – behavioural changes, dietary
Well, the barriers are still there, but you’ve just got to, it just puts thoughts in your mind because of the training you’ve had, I consider this to be like a training exercise to me, you know, I’m thinking about things before you just go in. So I used to like, when I got meals at McDonalds, I used to get the large meal. Now, I never do that. Always just your standard meal. I know there might not be many calories difference between a large and a medium meal, but there is some difference. So, that’s, that’s what I do now, and I manage with that. You know, I used I used to think I’ve got to have a large meal to be sort of, satisfied but now I don’t. I just have a standard meal, I have that, and that’s it.
Intervention participant, M14 – behavioural changes, dietary
I don’t eat as much chocolate as I used to do now. I take an apple or something like that for work, whilst I’m travelling down the motorway.
Intervention participant, M17 – behavioural changes, dietary
The small snack thing, before I just, I don’t know. I’d eat . . . in the truck for example, I’d always have a . . . I don’t know, say humbugs or something. And I’m eating a packet of biscuits and you’d just happily munch on them. Whereas now, I deliberately won’t have them in the vehicle, because if they’re not in the vehicle, I can’t . . . I can’t . . . do you know what I mean? I can’t eat them . . . I’d rather take like a bag of like roasted monkey nuts or something like that instead.
Control participant, M20 – behavioural changes, dietary
I’ve started eating more fruit . . . Rather than when I get hungry going for a chocolate bar, I go for some grapes, a banana or an orange or something like that.
Control participant, M23 – behavioural changes, dietary
Well put it this way, I now eat vegetables around four times a week now, never touched them before.
Oh OK, you never ate them before?
Never. I ate them once a week on Sunday if I had a Sunday dinner . . . But like I say, I live on a marina and there’s a pub, when it was open we’d go across for a meal, but I’d get burger and chips or, steak and chips, and I never really touched the vegetables. But since the lockdown as well like, I just sort of looked at them and said ‘I’ll try them’ and I tried them and like I say I’m now on four times a week on vegetables. In summer every night I was having salad. So my way of thinking about food now, I’m trying as they said in the study, how to portion your food as well.
Intervention participant, M24 – behavioural changes, dietary
My diet changed very much straight away . . . from not eating any fruit because I wasn’t interested, because I was a biscuit sweet person, I take fruit with me at work, I got down from nearly 14 stone to 13 [stone], 3 [pounds].
Intervention participant, P01, FG1 – behavioural changes, dietary
At 6 months, significant differences in steps, sitting and standing time were evident on non-workdays between trial arms, with no differences in these variables seen between groups on workdays, and this is reflected by the drivers’ and managers’ opinions about the inherent characteristics of the job:
Were the shifts a barrier to actually live the healthy lifestyle, did you just do it around it?
Just had to do it around it.
It’s more just, not doing it at work, just everything at home.
Chapter 6 Discussion
Drivers of HGVs drivers have been identified as a high-risk occupational group who have traditionally been underserved in terms of health promotion initiatives. 4,25 This trial aimed to evaluate the effectiveness and cost-effectiveness of the multicomponent SHIFT intervention in a sample of long-distance HGV drivers. Participants were recruited across 25 transport sites across the Midlands region of the UK, with sites operating within the transport, retail, hospitality, health-care, pharmaceutical, construction, oil and gas, and automotive industries. The average age of our sample at baseline [48 (SD 9) years] and our sex split (99% male) matches the average age of HGV drivers and the sex proportions seen nationally. 26
A high prevalence of overweight and obesity were observed in our sample at baseline, which exceeds the prevalence of overweight and obesity seen in males aged 45–54 years across the general population (89% vs. 79%). 126 Five per cent of our sample had severe obesity (i.e. a BMI ≥ 40 kg/m2) at baseline, which is more than double the prevalence of severe obesity seen in a national sample of aged-matched males (2%). 126 Furthermore, over half the sample had pre-hypertension (51%) or hypertension (28%), 84% had clinically elevated circulating LDL-C concentrations (i.e. > 2 mmol/l), and 67% had high total cholesterol levels (i.e. > 4 mmol/l). Participants accumulated high volumes of sitting, particularly on workdays, and high levels of physical inactivity. The characteristics of our recruited sample support previous observations of the high-risk health profile of UK-based HGV drivers,36 and highlight the need for health promotion initiatives to be prioritised in this workforce.
Main findings from the randomised controlled trial
Primary outcome
The primary outcome was device-measured physical activity, expressed as mean steps per day across all monitored days, assessed at 6 months. At baseline, the sample accumulated 8583 steps per day, which is comparable to daily step counts recorded previously in a sample of UK-based HGV drivers,36 and to daily step counts seen in office-based workers. 127 The complete-case analysis revealed a statistically significant difference in mean daily step counts at 6 months’ follow-up, in favour of the SHIFT group, with this group accumulating 1008 more steps per day than the control group. The findings showed a similar pattern in the sensitivity analyses (examining the effect of the number of valid activPAL days), although the results were mixed in the ITT and per-protocol analyses. Although the difference in the primary outcome measure between the SHIFT and control arms at 6 months (i.e. 1008 steps/day) was lower than 1500 steps per day, which formed the basis of our sample size calculation, it has recently been reported that 500 steps per day is the minimum clinically important difference for inactive individuals, applying equally to men and women. 128 Therefore, the difference observed in the intervention group relative to the control group is potentially clinically meaningful and potentially of a sufficient magnitude to impact longer-term health and mortality risk. 128
Closer inspection of the changes in mean daily step counts recorded between baseline and 6 months revealed that a decrease in daily steps occurred in the control group, whereas activity levels (i.e. steps/day) measured at baseline were maintained in the SHIFT group. Although large increases in overall daily steps were not observed in the intervention group, the SHIFT intervention appears to be effective in mitigating a reduction in overall activity over at least a 6-month period, observed in the control group. As baseline and 6-month follow-up measures were distributed evenly over a 6-month data collection period (i.e. baseline measures were undertaken between the months of January and July) for all groups, with the corresponding follow-up measures taking place 6–8 months later, it is unlikely that the reduction in steps seen in the control group could be explained by seasonal effects. As physical inactivity is widely associated with an increased risk of many adverse health conditions,129 the prevention of a decline in habitual activity in any population/individual is important when considering longer-term health outcomes. Therefore, the observed differences in steps between groups at 6 months remain potentially clinically important. 128
Despite the high-risk health profile of HGV drivers globally,4 limited health promotion interventions have been conducted in this occupational group. A systematic review25 of health promotion interventions in HGV drivers (which included only eight studies) observed that the interventions generally led to improvements in health and health behaviours; however, the review cautioned that the strength of the evidence was limited because of poor study designs, with no control groups, small samples and no or limited follow-up periods. 25 Of the available literature, only one other study31 of HGV drivers has examined the potential impact of a wrist-worn device to help monitor and self-regulate physical activity levels and healthy dietary choices. In a sample of 26 Australian HGV drivers, similar to the present findings, Gilson et al. 31 observed that participants’ daily step counts [measured using the Jawbone UP accelerometer (Jawbone, San Francisco, CA, USA)] remained constant across the 20-week intervention, with daily steps averaging 8743 steps per day across the first 4 weeks, and averaging 8944 steps per day across the last 4 weeks. Across the 20-week intervention, the logging of dietary choices using the associated Jawbone UP app declined steadily, and the authors concluded that step counts were more successfully monitored than dietary choices. 31
The process evaluation revealed that the Fitbit was a favoured component of the SHIFT intervention. Fitbits, along with similar commercially available wearable activity trackers, and their associated apps, contain a number of behaviour change techniques, including self-monitoring, feedback and goal-setting. 130 Recent systematic reviews and meta-analyses131–133 have revealed that commercially available wearables are associated with favourable increases in physical activity in controlled trials in adults over the short term (note that the duration of the interventions included in these reviews typically ranged between 3 and 6 months). In their meta-analysis, which included 12 controlled trials that incorporated the use of a commercial wearable as an intervention tool, Brickwood et al. 131 reported greater intervention effects when the wearable was part of a multicomponent intervention (as applied in the present study), as opposed to when the wearable was utilised as the primary intervention tool. Within both trial types, however, meta-analyses revealed significant increases in daily step counts in intervention groups relative to control groups (multicomponent interventions, +685 steps/day; wearable-only interventions, +475 steps/day).
Findings from a systematic review and meta-analysis,133 which specifically examined the use of Fitbits as an intervention tool, reported significant increases in daily steps across 16 studies, with a mean difference of +951 steps per day seen in intervention participants, relative to control participants. The majority of RCTs included in this meta-analysis examined the effectiveness of multicomponent interventions, and had a duration of < 5 months. Only five studies incorporated a 6-month follow-up (as applied in the present trial), with only two further studies including a 12-month follow-up. 133 Given the unique population targeted in the present study, and the limited scope to compare the present findings with other studies using HGV drivers,31 the difference in our primary outcome (i.e. +1008 steps/day) observed between intervention and control participants at 6 months appears promising, especially when compared with the findings reported in the recent meta-analyses of wearable interventions, highlighted above. 131,133
Secondary outcomes
activPAL variables on workdays and non-workdays
Complete-case analyses for these secondary outcomes revealed statistically significant differences, in favour of the SHIFT group, in time spent sitting, standing and stepping, and time in MVPA, at 6 months’ follow-up, across all monitored days. Further analyses revealed that the positive changes in overall activity and sitting seen at 6 months were driven by differences in these behaviours occurring between groups on non-workdays. No statistically significant differences were observed in any variables assessed using the activPAL between groups at 6 months (or at 16–18 months) on workdays.
A common theme, which emerged as part of the process evaluation, was the irregularities of shifts and the long duration of shift patterns, which many drivers reported as a barrier to being able to engage in beneficial health behaviours. Owing to the constraints of their job, it appears, therefore, that participants in the SHIFT arm were more likely to adopt positive behaviours in terms of physical activity and reduced sitting on non-workdays than on workdays. Relative to the control group, at 6 months, participants in the SHIFT group accumulated 2012 more steps per day on non-workdays. This was accompanied by an extra 21 minutes per day spent stepping, which was broken down into an extra 10 minutes per day spent in light physical activity and 11 minutes per day spent in MVPA. Similarly, participants in the SHIFT arm accumulated 40 minutes per day less sitting, relative to control participants, at 6 months. The mean differences in MVPA and sitting observed between groups on non-workdays are greater in the present study than those observed in Ringeval et al. ’s133 meta-analysis of Fitbit interventions, where mean differences in MVPA of 6 minutes more per day and 11 minutes less per day of sedentary time were seen in intervention groups, relative to control groups.
As with the primary outcome, further interrogation of the data revealed that the favourable changes in behaviours observed on non-workdays at 6 months between intervention and control participants were largely driven by the reductions in physical activity and increases in sitting seen in control group participants, alongside small positive behaviour changes seen in the SHIFT group. It appears, therefore, that the SHIFT intervention was successful in mitigating the unhealthy behaviour changes seen at 6 months in the control group. Furthermore, as highlighted in the recent World Health Organization physical activity guidelines update,134 doing some physical activity is better than none, and even modest increases in activity seen in the SHIFT arm on non-workdays could be beneficial to health.
At baseline, all participants accumulated high volumes of sitting on workdays (≈ 12 hours/day) and non-workdays (≈ 9 hours, 40 minutes), which, unsurprisingly, owing to the nature of their work, demonstrates that HGV drivers accumulate greater sitting times than most occupational groups. 135 At baseline, there was no evidence that participants compensated for their highly sedentary occupation by being more active on non-workdays, with participants actually accumulating less physical activity on non-workdays. Sedentary behaviour, defined as ‘any waking behaviour characterised by an energy expenditure ≤ 1.5 metabolic equivalents while in a sitting, reclining or lying posture’,136 has been identified as a risk factor for a number of chronic conditions, including CVD, type 2 diabetes and all-cause mortality. 120,137–140 Although recent studies suggest that the detrimental effects of sedentary behaviour can be mitigated by engagement in regular MVPA, with at least 150 minutes of moderate intensity activity accumulated per week required,141 the relatively low volumes of MVPA seen in the present sample is unlikely to reduce the risk of the detrimental health effects associated with sedentary behaviour. Furthermore, recent studies have reported potential thresholds, ranging from 6–8 hours per day140 to 9.5 hours per day,120 spent sedentary where all-cause mortality risk is substantially increased, independent of physical activity. Our sample exceed both of these thresholds when looking at their overall daily sitting times.
Periods of prolonged sitting have been associated with negative health outcomes, and regularly breaking up sitting (every 20–30 minutes) has been associated with favourable changes in blood glucose control, particularly in individuals who are overweight or have obesity and/or individuals who are at high risk of type 2 diabetes. 142 Accumulating prolonged periods of sitting is unavoidable in long-distance HGV drivers on workdays; however, non-workdays provide an opportunity where prolonged bouts of sitting can be minimised. A noticeable observation from the descriptive analyses of the activPAL data revealed that, at 6 months, control participants exhibited an increase in the time spent sitting (and the proportion of sitting) in prolonged bouts. No such changes were observed in the intervention group, again suggesting that the SHIFT intervention likely mitigated increases in time spent in prolonged sitting bouts at 6 months.
Despite the favourable differences seen in the SHIFT arm, relative to the control arm, at 6 months, particularly on non-workdays, limited differences between groups were seen in the majority of activPAL variables assessed at 16–18 months’ follow-up. Although not statistically significant (p = 0.10), at 16–18 months, daily step counts on non-workdays were 1391 steps per day more in the SHIFT group, relative to the control group, suggesting some evidence of sustainability. The COVID-19 pandemic, however, is a major confounding factor that occurred for the majority of participants between the 6- and 16- to 18-month follow-up assessments. Furthermore, a disproportionately larger number of control participants (58%) were furloughed at some point between the 6- and 16- to 18-month follow-up assessments, relative to participants in the SHIFT arm (24%). Questionnaire-based data collected from a subsample of participants during the first national lockdown, along with qualitative responses provided on the 16- to 18-month follow-up CRFs, indicated that participants who were furloughed were more likely to engage in new forms of physical activity while away from work. In contrast, it is likely that drivers who continued to work throughout the national lockdowns had extended driving hours and, therefore, even less time to engage in healthy lifestyle behaviours because of the relaxation in drivers’ hours that came into force. 101
Markers of cardiometabolic health and functional fitness
The changes in weight and BMI observed at 6 months demonstrated favourable trends in the direction of the SHIFT group. At 6 months, participants in the SHIFT arm recorded an average weight loss of 1.4 kg (i.e. a change of –1.2 kg relative to control participants; p = 0.08) and a reduction in BMI of 0.4 kg/m2 (i.e. a change of –0.4 kg/m2 relative to control participants; p = 0.09). Fifty-eight per cent of participants in the SHIFT arm experienced a reduction in weight at 6 months, compared with 48% of control participants. Although these findings look promising, it should be cautioned that this level of change in weight (≈ 1.4%) would not be considered clinically meaningful and could be an artefact of natural variations in hydration status occurring between measurement sessions. Interventions predominantly focusing on physical activity have been shown to have small to no effects on weight loss. 143 To have a bigger impact on weight, the SHIFT intervention could be revised to include a greater emphasis on diet. In a weight-loss intervention conducted in US truck drivers, Thiese et al. 30 reported a median weight loss of 3.2 kg in participants following the completion of their 12-week intervention. However, this was a single-arm trial involving only 12 participants. 30
There were no other beneficial changes in markers of cardiometabolic health (e.g. blood pressure, waist circumference, waist–hip ratio, biochemical measures) seen in the SHIFT arm relative to the control group at 6 months. Given the strong links between adiposity and a number of these cardiometabolic markers, and the small change in weight, it is perhaps not surprising that no changes in markers of cardiometabolic health were observed. Albeit in a smaller sample, similar findings were observed in the weight-loss intervention in US truck drivers reported by Thiese et al. 30 Similarly, no noticeable differences were observed between groups in the present study in their psychophysiological reactivity to stress at 6 months.
Descriptive analyses suggested that the SHIFT group demonstrated favourable increases in average grip strength at 6 months, whereas no changes were detected in the control group. Lower hand grip strength, indicative of lower muscle function, has been shown to be strongly associated with a wide range of adverse health outcomes, including all-cause mortality and incidence of, and mortality from, CVD, respiratory diseases and cancer. 144,145 The potential improvements in grip strength observed in the SHIFT group are promising, and are likely to be linked to the inclusion of a hand gripper as part of the cab workout equipment. Although the process evaluation revealed that the cab workout was the least-favoured part of the intervention, participants did highlight that they enjoyed using the hand gripper. Therefore, this simple piece of equipment, which could help maintain and/or improve upper-limb muscle function, holds promise as an effective tool for drivers to use during breaks.
Dietary quality and fruit and vegetable intake
There were no statistically significant differences observed between groups in reported fruit and vegetable intake or overall dietary quality at both 6 months and 16–18 months. These findings contrast with the numerous comments made as part of the process evaluation from drivers, where favourable changes to their diets were reported. This contrast in findings may be attributable to the sensitivity of the FFQ used to assess diet, as previous studies146 have demonstrated questionable validity of FFQs when compared with 4-day weighed food records. However, the feasibility of assessing dietary intake using weighed records in the present study population was uncertain at the planning stages of this trial. The overall dietary quality score derived from the FFQ for our sample (11/15 at baseline) is comparable to that observed from a large randomly selected general population sample from Northern England (11.4/15). 60 In comparison to this population sample, overall intake of fruit and vegetables appears to be lower in our driver sample (≈ 240 g/day), with intake decreasing further at 16–18 months (≈ 200 g/day), indicating that participants are falling short of the government’s recommendations of at least 400 g/day of a variety of fruit and vegetables. 147 This finding suggests that more needs to be done to support drivers in making healthier dietary choices, with improved access to fresh fruit and vegetables.
Sleep
A notable observation within this trial was the short sleep duration observed across the sample at baseline and at 6 months’ follow-up. Although the SHIFT intervention did not specifically target sleep in detail, sleep duration and efficiency were assessed in the present study as secondary outcomes using a wrist-worn device, and processed using a validated algorithm. 89 Of the participants providing valid GENEActiv data at baseline (n = 349), the average sleep duration across all monitored days for the whole sample was 6 hours and 10 minutes (SD 54 minutes), and this reduced slightly to 6 hours (SD 60 minutes) on workdays. At baseline, 41% of participants exhibited an average sleep duration across all monitored days of < 6 hours per 24-hour period, and 82% of participants exhibited an average sleep duration of < 7 hours per 24-hour period. These proportions increased further on workdays to 45% and 85%, respectively.
Of concern, a consistent finding observed across both the SHIFT and control groups at 6 months’ follow-up was a further reduction in sleep duration. The average sleep duration for the sample at 6 months across all monitored days was 5 hours and 56 minutes (SD 57 minutes), and this fell to an average of just 5 hours and 21 minutes (SD 69 minutes) on workdays. At 6 months, just over half of the sample (51%) providing valid GENEActiv data (n = 221) exhibited an average sleep duration across all monitored days of < 6 hours per 24-hour period, and 87% exhibited an average sleep duration of < 7 hours per 24-hour period. These proportions increased further on workdays to 71% and 91%, respectively. The reductions in sleep duration appear to be solely driven by reductions in the duration of the sleep window (i.e. at follow-up, drivers were allowing themselves less time in bed to sleep, as opposed to reductions in overall sleep quality). At the 6-month follow-up, although there was a consistent trend across groups for sleep duration (and sleep window duration) to increase on non-workdays [mean increase across the sample: 41 (SD 99) minutes/24-hour period], participants were still only accumulating 6 hours and 52 minutes (SD 85 minutes) of sleep on these days, which falls short of the recommended minimum of 7 hours per 24-hour period required for optimum health. 148 Similar to that discussed above in relation to the activPAL data (see Primary outcome), as both baseline and 6-month follow-up measures were distributed evenly over a 6-month data collection period for both groups, it is unlikely that seasonal changes can fully explain the net reduction in sleep observed.
Systematic review-level evidence has demonstrated that people habitually sleeping less than 6–7 hours per night have a significantly increased prevalence of type 2 diabetes, obesity and CVD, higher cortisol and cholesterol levels, reduced cognitive functioning, depression and other psychiatric conditions, and premature all-cause mortality. 149,150 Some of these associations may be mediated by sleep-related changes in glucose metabolism and appetite regulation. Sleep restriction impairs glucose tolerance,151 reduces circulating leptin, and increases hunger and the consumption of carbohydrate-rich foods. 152 Short sleep can also lead to daytime fatigue and suppresses the volume and intensity of physical activity undertaken. 153 Indeed, a common theme that emerged from the process evaluation when discussing the cab workout component of the intervention was that a high proportion of participants reported prioritising trying to catch-up with their sleep when at a rest stop, as opposed to using the cab workout equipment. As a result, the cab workout was a less favourable intervention component.
In addition to the individual-level cardiometabolic risks associated with short sleep duration,150 and of particular relevance and concern within the present sample, is the association between short sleep duration and reduced driving performance and increased accident risk,154,155 as this has wider public health and safety implications for all road users. For example, a US Department for Transportation study observed that both severe sleep apnoea (a condition common in commercial drivers, which drivers are required to inform the Driver and Vehicle Licensing Agency about156) and sleeping < 6 hours per night were equally, and independently, associated with impaired driver performance. 157
A limitation of the measurement of sleep used in the present study is the fact that naps were not assessed, and it appears from the process evaluation that a number of participants did attempt to nap during their breaks. Therefore, it is possible that total sleep durations are underestimated in this study. Nevertheless, sleep duration was a recurrent theme highlighted within the process evaluation, and this, in combination with the sleep data collected from the GENEActiv, suggests that the drivers in this sample are chronically sleep deprived. These findings have important implications, suggesting that participants are at an increased risk of excessive daytime sleepiness, road traffic accidents and chronic disease. 158 Indeed, a UK Department for Transport review concluded that insufficient sleep, leading to daytime sleepiness, impaired vigilance and poor concentration, is responsible for the ‘disproportionately high number of fatigue related accidents’ involving drivers of large goods vehicles (contains public sector information licensed under the Open Government Licence v3.0). 159
The findings from this secondary outcome measure, along with the concerning observation of a further reduction in sleep duration and sleep window duration at 6 months in this sample of drivers, suggests that the SHIFT intervention should be expanded to include a much greater focus on sleep. Increasing sleep quantity through interventions targeting improved sleep management in drivers will potentially offer dual public health benefits of reducing accident risk (through reduced fatigue and improved vigilance performance) and reducing cardiometabolic risk within the individual (through improved glucose tolerance and appetite regulation, and increased engagement in physical activity). This recommendation is particularly pertinent at the present time, given the increased number of HGV driver shortages within the UK29 and the relaxation of drivers’ hours rules as a result of COVID-19 and Brexit. 160 There is a risk that the current sleep profile of HGV drivers may be even worse than that observed in this study, given our 6-month follow-up assessments were completed just prior to the COVID-19 outbreak and Brexit, and the associated relaxation in drivers’ hours rules and substantial increase in driver shortages. The long hours worked by our participants suggests that drivers may also not completely recover from work-related fatigue between shifts. High levels of ‘need for recovery’ have been associated with sleep complaints in coach drivers,161 and with longer-term sickness absence in HGV drivers. 162 Further work examining interventions to improve drivers’ sleep should also take into account, therefore, working hours and the potential impact of the need for recovery between shifts.
In the present study, within both groups, no changes in device-measured sleep quality (i.e. sleep efficiency) or chronotype score were observed between baseline and follow-up. Despite the reduction in device-measured sleep duration observed in both groups at 6 months, there were no changes in ratings of situational sleepiness observed across groups, although this measure should be treated with caution because of the variability in the exact timing within the day/night that this questionnaire was completed across follow-up periods. Furthermore, other studies have demonstrated no associations between self-reported sleepiness and reduced cognitive performance across a range of tasks, including driving, resulting from sleep deprivation. 157,163
Mental well-being, cognitive function, musculoskeletal symptoms and work-related psychosocial variables
In contrast to previous observations of relatively high levels of poor mental health within drivers,4 reported symptoms of anxiety and depression were low in the present sample at baseline, with limited changes in symptoms occurring across the follow-up assessments in either group. At baseline, 13% of participants reported borderline symptoms of depression and 17% of participants reported borderline symptoms of anxiety, whereas 2% and 5% of participants reported abnormal scores for depression and anxiety, respectively. Similarly, low levels of social isolation were reported across all assessment points throughout this study. No noticeable differences in changes in cognitive function were observed between groups at 6 months’ follow-up. Likewise, there were no observable differences between groups in changes in musculoskeletal symptoms or any work-related psychosocial variables (i.e. work engagement, occupational fatigue, job satisfaction and performance, sickness absence and presenteeism, work ability and perceived job demands) occurring at either follow-up. In addition, no differences were observed between groups in terms of reported driving-related safety behaviours.
Lifestyle-related behaviours and cardiovascular disease risk
At baseline, 25% of participants reported drinking more than 14 units per week of alcohol, and this is a lower proportion than that reported126 in a nationally representative sample of aged-matched males, where 35% of the sample reported drinking more than 14 units per week of alcohol. No noticeable differences were observed between groups at any assessment point in terms of alcohol intake, and alcohol intakes observed in the present sample appear lower overall than what has been reported elsewhere in HGV drivers from other countries. 4 However, this observation should be treated with caution, as the tools used to assess alcohol intake in HGV drivers have varied extensively across studies, making it difficult to draw comparisons. 4
The prevalence of smoking within the sample at baseline (19.4%) was similar to that seen in males aged 45–54 years living in England (20%). 126 When split by study group, there was a tendency for a higher smoking prevalence to be seen in the control group than in the SHIFT group across all assessment points. For participants completing the baseline and 6-month follow-up assessments, smoking prevalence changed from 17% to 19% in the control group, and from 13% to 11% in the SHIFT group. In the smaller sample of participants who completed the baseline and 16- to 18-month follow-up assessments, smoking prevalence decreased by 1% in both groups at 16–18 months. The impact of the SHIFT intervention on smoking is, therefore, uncertain, and limited effects on smoking (and alcohol intake) are perhaps to be anticipated, as these topics were covered only briefly in the structured education session, with the focus of this session being predominantly on physical activity, diet and sitting.
When examining the proportion of participants with an estimated CVD risk of ≥ 10% over the next 10 years, 23.6% of control participants and 24.3% of SHIFT participants fell into this category at baseline, and this increased to 26.4% in the control group and reduced to 23.4% in the SHIFT group at 6 months. These findings suggest that participants in the SHIFT group experienced a modest reduction in risk of a cardiovascular event over the next 10 years, relative to control participants. Reducing the risk of a CVD-related event in HGV drivers has important implications, not only for the individual, but also for the wider public, given the serious consequences should a driver have a CVD event while driving. Although not specifically related to CVD events, Ronna et al. 23 reported that, based on 10-year CVD risk calculated using the Framingham Risk Scale, the odds of having an accident doubled in US truck drivers with a Framingham Risk Scale score > 13. Ronna et al. 23 also observed a statistically significant association between prevalence of accidents and increased risk scores, further highlighting the public health importance of improving the overall health of this occupational group.
COVID-19
The COVID-19 pandemic had a large impact on the overall running of this trial, with the first government national lockdown occurring at the time that the final follow-up measurements within the main trial phase were about to commence. In addition, 6-month follow-up measurements were scheduled to take place in one intervention site during the week commencing 23 March 2020 (i.e. the start of the first national lockdown), and this was the last site to undergo the 6-month follow-up measurements. As a result of the national lockdowns that followed, the 6-month follow-up assessments in this intervention site, as well as all final follow-up assessments, were severely delayed. A change to the original protocol was approved in June 2020, where it was confirmed that the primary outcome would be daily steps recorded at the 6-month follow-up assessment, as opposed to daily steps recorded at 12-month follow-up, which was not feasible given the suspension of data collection. This required change in protocol is a limitation of the trial, as the switch in timing of the primary outcome analysis (from 12 months to 6 months) means that we cannot completely rule out any seasonal changes in behaviour affecting our findings. However, it should also be acknowledged that this change in timing affects both the intervention and control arms.
Within the main trial phase, the easing of government COVID-19 restrictions enabled a range of secondary outcome measures to be collected approximately 16–18 months after randomisation in sites. Owing to restrictions on external visitors to DHL Supply Chain sites throughout the pandemic, face-to-face physiological measurements were not able to be conducted at the final follow-up phase. These follow-up assessments, therefore, did not contain the complete set of measures included at baseline and at 6 months. Furthermore, for the one intervention site due their 6-month measures at the start of the first national lockdown, the delayed 6-month assessments did not contain the physiological health measures included for all other sites, and this led to a reduction in the sample size within the intervention arm for some of these secondary outcomes. Although a strength of this study is the fact that we were able to follow-up participants at 16–18 months, the pandemic presents a major confounding factor that limits our ability to draw firm conclusions regarding the sustainability of the SHIFT intervention. In particular, a greater proportion (58%) of control participants than intervention participants (24%) reported being furloughed, which may have had a large impact on their lifestyle health behaviours and markers of well-being at the final follow-up assessments.
Despite the associated challenges, the pandemic also provided an opportunity to collect further information on its impact on our sample of HGV drivers, who were classed as a key worker group. A subsample of participants completed an additional questionnaire during the first national lockdown. The questionnaire was developed in partnership with colleagues at DHL Supply Chain in response to the relaxation of permitted maximum driving hours. 101 Despite the change in permitted driving hours, respondents to our COVID-19 questionnaire did not report any changes to their working, driving, in-cab waiting or rest hours. Similarly, participants reported no changes in the time spent sitting, standing and walking/moving around on a workday during the pandemic, and there were no negative impacts on symptoms of anxiety or depression, or markers of occupational fatigue. The responses to the COVID-19 questionnaire should be treated with caution, however, as the responses represent only 41% of the sample invited to complete the questionnaire, and non-responders may have been experiencing the pandemic very differently.
The questionnaire did enquire whether or not participating in the study had provided participants with the right knowledge to maintain a healthy lifestyle during the COVID-19 restrictions, and, interestingly, 63% of both intervention and control participants answered ‘yes’. Responses to this question were similar between intervention and control participants, and largely centred around an increased understanding of the importance of activity and diet. The responses received from control participants to this question support observations from the process evaluation that a number of control participants were not aware of the two trial arms, with some participants believing that they were experiencing an intervention as a result of the regular health assessments they were invited to (note that control participants received the same feedback on their physiological measures as the intervention participants).
The questionnaire also enquired whether or not participants had spent time in nature (which could include time in their garden/allotment, in parks, in woodland, at the coast and in open green spaces) during the pandemic, along with whether or not participants habitually spent time in nature prior to the pandemic. These questions were included following recent reports of a wide range of both physiological and psychological health and well-being benefits associated with exposure to nature. 164,165 In this subsample, we observed novel associations between reported time in nature and reductions in measures of occupational fatigue. Further analyses, reported elsewhere,102 revealed that after controlling for covariates, drivers who visited nature at least once a week exhibited 16% less chronic fatigue prior to the COVID-19 pandemic, and 23% less chronic fatigue and 20% less acute fatigue during the COVID-19 pandemic. These novel findings suggest that nature exposure may have the potential to provide a promising remedy for many of the negative health outcomes associated with HGV driving,102 and further research into the use of nature exposure as a potential low-cost intervention to promote physical and mental health in drivers is recommended.
Main findings from the cost-effectiveness analysis
The within-trial analysis showed that the SHIFT intervention reduced QALYs and increased costs. The small improvements in physical activity seen as a result of the intervention generated potential for slight improvements in QALYs in the longer term. Despite this, under a range of alternative scenarios and assumptions, the SHIFT intervention in its current delivery format is unlikely to be considered cost-effective when compared with usual practice at commonly used threshold values of a QALY.
Main findings from the process evaluation
The process evaluation indicated that the SHIFT intervention had a positive impact on the intervention participants, as reported in both the questionnaire and interview responses. Participants reported an increase in knowledge, awareness and motivation regarding the importance of increased physical activity and a healthy diet. The Fitbit was the most favoured component of the intervention, whereas the cab workout appeared the least favoured and too cumbersome for the majority of participants. The most common suggested improvement to the intervention was to increase the frequency of communication with participants. The barriers to health were still very apparent throughout, with the irregularity and long duration of their shift patterns highlighted by many drivers. These barriers required a high level of extrinsic motivation to overcome within this at-risk occupational group to enable them to change health-related behaviours, and, therefore, regular contact from those administering any future interventions would likely be needed to help motivate participants to maintain improved behaviours.
Using the MRC process evaluation framework,94 the discussion of findings from the process evaluation will focus on the implementation process and the mechanisms of impact that influenced the findings, followed by the contextual factors that may have affected the RCT outcomes.
Implementation process
This RCT was complex in terms of multiple components, environments and outcome measures. The intervention comprised the amalgamation of five different components (the 6-hour structured education session, the Fitbit, step count challenges, cab workout equipment and text messages) among 25 heterogeneous worksites (pilot sites, n = 6; main trial sites, n = 19) and aimed to influence the health behaviours of participants in numerous ways (e.g. daily steps, sitting and standing time, time spent in MVPA and nutritional intake).
The structured education session was regarded as valuable by all interviewed intervention participants, who reported that it increased their knowledge, particularly about healthy diets. However, only 145 of 183 (79.2%) intervention participants took part in the education session, mainly because of logistical challenges and operational requirements, which made scheduling the education sessions across sites and ensuring driver availability particularly challenging. This shows that although the education session was beneficial to those participants who attended, it was not wholly feasible in this occupational group, with key issues being the varying start times, operational demand and time-critical deliveries. However, in a ‘real-world’ context, it is estimated that currently only 15–30% of people in the UK newly diagnosed with diabetes attend structured education sessions organised through the NHS, despite high referral rates by GPs. 166 Based on this information, it could, therefore, be argued that, although challenging to organise, if such sessions can be embedded within the workplace of at-risk occupational groups, then their reach could be substantially improved. In the context of HGV drivers, if such health promotion programmes can be embedded within compulsory professional competency training that drivers are required to undertake to maintain their licenses, which take place within working hours, the potential reach and impact of such programmes could be considerable.
The Fitbit worked as an important tool for increasing understanding of current activity levels, providing participants with feedback on their activity and acting as a motivational tool to increase daily steps. There was high adherence to the Fitbit throughout the intervention, suggesting that it was an effective tool to encourage behaviour change, specifically physical activity, but less so regarding sleep (although the Fitbit provides feedback on sleep, this was not a primary focus of the SHIFT intervention). There was less agreement on the step count challenges. Some participants liked the competition of the step count challenges, but other participants did not like competing with ‘strangers’. The text messages were regarded as useful for logistical purposes (e.g. for reminding participants about their up-and-coming health assessments); however, overall, there were minimal replies to the messages, with an average response rate of 18.8%. Participants mentioned that more frequent, personalised messages would be required to stimulate motivation.
The cab workout was a less favourable intervention component, with participants stating that they had more important priorities than using this equipment in their breaks, particularly catching up on lost sleep and eating. However, some participants did use the cab workout equipment, with the most popular device provided being the hand gripper, and 20% of participants agreed the cab workout equipment increased their overall levels of activity. As the adherence to the cab workout equipment appeared low overall, however, the cab workout is regarded as a poor tool to encourage behaviour change within this occupational group.
Mechanisms of impact
The SHIFT intervention used Bandura’s SCT as the theory of behaviour change for intervention development. 42 Bandura’s SCT suggests that learning can occur through observing and imitating someone else’s behaviour, and is most effective when the observer witnesses a model with similarities (e.g. another HGV driver) carrying out the behaviour. Bandura’s SCT focuses on the triadic model, in which personal factors, environmental influences and behaviour continually interact. 167 Bandura argues that goal-setting and self-monitoring are relevant components in effective interventions. In addition, Bandura suggests that the key concepts that affect health behaviour change interventions include self-control, self-efficacy, observational learning and reinforcement. Based on the SHIFT logic model (see Figure 1), self-efficacy and self-monitoring were to be utilised with the Fitbit. The supportive social environment was to be facilitated via the education session and through health coach support from the text messaging service. The acquisition of the essential knowledge relating to behaviours came from the education session. However, the SCT has a shortcoming regarding this RCT, as truck drivers are inherently isolated from each other and, therefore, they rarely learn behaviours from each other’s doing. A further model applicable to the SHIFT intervention is the behaviour change wheel, which uses the capability, opportunity, motivation – behaviour framework, where participants require capability, opportunity and motivation to change their health behaviours. 168 The opportunities to foster motivation can be created through the health assessments, notifying the individual of their current health status and that they may be at risk of certain lifestyle-related diseases and conditions. Capabilities are highlighted through the education sessions, where individuals acquire essential knowledge relating to health behaviours and lifestyle choices. Opportunity is derived from receiving the Fitbit and cab workout equipment, and then turning these changes into habits through regular reminders and feedback from the Fitbit, step count challenges and health coach support from the text messages.
It is also important to recognise that there is no ‘one size fits all’ solution with regard to behaviour change, and this is explained by Resnicow and Vaughan’s169 chaos theory and complex dynamic systems. Theories such as the SCT view change as an interaction of self-efficacy, belief, knowledge, attitude and intention, which creates a linear mechanism for an individual to assess the positives and negatives in a consistent manner. However, Resnicow and Vaughan’s169 chaos theory and complex dynamic systems argue that it is impossible to make predictions on human behaviour, likening this to the impossibility of mathematically predicting the course of two identical balls rolling down a rocky mountain, with the balls ending up in two very different places because of an almost infinite number of variables. Behaviour change encompasses these infinite interacting variables that impact the outcome. 169 According to Resnicow and Vaughan,169 regarding human behaviour, there may be common patterns of behaviour change that occur across and within individuals that may follow complex non-linear patterns. Resnicow and Vaughan169 highlight that identifying these recurrent patterns of change will be useful to aid identification of target groups who could benefit from common intervention components.
Context
All participants were asked in the follow-up questionnaires during each measurement session about any major changes to their life over the past 6 months. The biggest changes reported were moving house, followed by family illness and relationship break-ups. There were no apparent biases between trial groups regarding external factors influencing study participation.
The COVID-19 pandemic caused three major lockdowns in the UK from March 2020 to July 2021, which had wide-ranging impacts on each site that was involved in the study. Although there appeared no systematic differences between intervention and control sites in terms of the impact of the pandemic, it was a rapidly changing, dynamic situation that was unable to be adequately reported. We cannot, therefore, say with certainty that there were no differences in the impact of the pandemic between intervention and control groups. Indeed, as highlighted above, a greater proportion of control participants reported being furloughed than intervention participants, which may have affected participants’ lifestyle health behaviours and markers of well-being, either positively or negatively, prior to the final follow-up assessments.
The outcomes of the study were measured using health assessments, which all intervention and control participants attended. The health assessments were followed by short feedback sessions where the results were explained to each participant. Although not part of the intervention, the health assessments did have an impact on awareness and knowledge about a healthy lifestyle in both intervention and control participants, and this was an unintended outcome of the study, which, although it did not in turn lead to observed behavioural changes in control participants, provided participants with a more holistic understanding of their own current health status.
Process evaluation strengths and limitations
The triangulation of data led to a more comprehensive understanding and rigorous analysis, as we were able to capture data using different dimensions of the same phenomenon. 170 Data were also collected at multiple levels, including driver-, manager- and site-level data, to provide a more complete understanding of the specific context of the RCT. Data for the process evaluation were collected from baseline to the completion of the study (i.e. 16–18 months later), and this enabled us to follow the participants’ reflections throughout their experience of the study. The length of follow-up at the end gives the participant and managers time to reflect and provide more holistic responses about their experiences. The representativeness of each depot was considered when stratified sampling of the drivers and managers for the interviews took place. This method gives the reader a more thorough comprehension of the study, as every site was heterogeneous. The process evaluation was undertaken primarily by a single integrated evaluator, which was beneficial for effective communication, avoids duplication of efforts and reduces participant burden. 94 Very much part of the intervention team, the evaluator used this first-hand experience to understand thoroughly every part of the intervention, and this, in turn, helped to minimise the Hawthorne effect while collecting observational data about the operational challenges for both for the implementation team and the sites.
Assessing the reach of the SHIFT intervention across the included 25 depots was not appropriate or feasible within the context of the programme, as the present trial aimed to recruit approximately 14 participants per site because of financial and time restrictions. It was apparent that in most sites there was a large interest in the study, highlighting the necessity of such health interventions in this at-risk population. Indeed, at baseline, the trial over-recruited, with 382 participants providing informed consent, which exceeded our recruitment target of 336 participants from our sample size calculation. However, despite the initial high interest in the study, the total loss to follow-up was high (46.3%) and this potentially may have resulted in attrition bias, whereby there may have been systematic differences between participants who left and participants who stayed.
All participants were asked to participate in the interviews and incentivised to do so, and this may have led to a sampling bias, although this was mitigated as best as possible by involving one participant from each depot. The limitation of having an integrated process evaluator may increase risk of potential biases in the process evaluation outcome. However, this was mitigated through having an external, and independent from the trial, ‘critical friend’ (Dr Anna Chalkley), and all findings were discussed with the principal investigator (SC). 171 As the process evaluation data were analysed without the knowledge of the main trial outcomes, bias was also minimised so as to reduce influenced interpretations.
Process evaluation conclusions and recommendations
The SHIFT intervention demonstrated effectiveness in the primary outcome (i.e. daily steps); however, future replication and extension of this study should consider more valid measures of nutritional intake to best capture dietary behavioural changes, as regularly reported in the interviews. More frequent contact with both control and intervention participants was suggested as a key improvement, which, in turn, would lessen attrition rates. Attrition rates were high throughout the study, which supports the existing understanding that HGV drivers are a hard-to-reach population,7 not least due to the transient nature of the workforce. The COVID-19 pandemic had a mixed impact on participating sites, which would make any conclusions about the final follow-up uncertain. Overall, participants were enthusiastic about the SHIFT intervention, with particular emphasis on the dietary lessons from the education session and the activity monitoring and motivation from the Fitbit.
Trial strengths and limitations
A major strength of this study was the implementation of a lifestyle health behaviour intervention within the workplace environment of a very underserved and at-risk occupational group. The characteristics of our sample at baseline highlight the poor health profile of HGV drivers within the UK, and emphasise the urgent need to improve the health of this shrinking, yet essential, workforce. 29 The study involved 25 different transport sites spread throughout the Midlands region, operating within subcontracts across eight different industries. The range of industries represented by these sites, together with the demographic characteristics of our sample (mean age at baseline 48 years and 99% male, which matches exactly the characteristics of UK HGV drivers26), suggests that the included sample likely represents the 278,700 HGV drivers currently in employment. 1
Our multicomponent lifestyle health behaviour intervention (i.e. the SHIFT intervention) was evaluated through a fully powered cluster RCT, where randomisation occurred at the site level (reducing the risk of contamination) after baseline assessments had been undertaken (reducing bias). The trial incorporated immediate (6-month) and longer-term (16- to 18-month) follow-up periods to enable the examination of the effectiveness and potential sustainability of the SHIFT intervention. The trial also included a mixed-methods process evaluation and a full economic analysis. To the best of our knowledge, this is the first cluster RCT to examine the effectiveness and cost-effectiveness of a lifestyle health behaviour intervention within HGV drivers, with the few earlier intervention studies25,30,31 reported in this workforce limited by small sample sizes, no control groups and limited follow-up durations. The SHIFT intervention has a strong theoretical underpinning. 42 The SHIFT intervention was created and refined based on our earlier work,7,36–38 and the planning of this study, and subsequent conducting of it, has been informed by extensive PPI.
The use of the activPAL accelerometer as the primary outcome measure is a further strength, with this device being shown to provide a highly accurate measure of steps and posture. 53–55 Furthermore, we were able to confirm the validity of this device in our particular sample by demonstrating that, within the HGV cab, the activPAL is not affected by vehicle vibrations. Compliance to the activPAL wear protocol was relatively high in the present study, and this was facilitated by checking the activPAL data on return of the devices and requesting re-wears where possible. At baseline, 90% of participants provided at least 1 day of activPAL data. Of the sample of participants returning the device at 6 months, 89% provided at least 1 day of activPAL data, of whom 84% provided valid activPAL data at both baseline and 6 months. On average, participants wore the activPAL for 6.8 days at baseline and 7.2 days at 6 months. These compliance rates are similar to those seen recently in a large sample of office-based workers. 172 Although a minimum number of days of device wear are usually specified to allow for day-to-day variation in behaviours,173 to maximise our sample, owing to the high loss to follow-up experienced (discussed below), in our main analysis we included all participants who provided at least 1 day of activPAL data, as applied elsewhere. 86 However, to test the robustness of our findings, we performed a sensitivity analysis including only participants who provided more valid days of activPAL data, and our findings remained unchanged. Although the activPAL provides a device-based measure of physical activity (and participants were blinded to the data recorded), reducing bias associated with self-report measures, participants were still aware of the purpose of the activPAL. Therefore, reactivity to this measure may have occurred, although any potential reactivity is likely to have affected the SHIFT and control groups equally. The trial included a range of validated secondary outcomes, enabling a comprehensive evaluation of the SHIFT intervention on markers of adiposity and cardiometabolic risk, mental well-being, a range of lifestyle health-related behaviours and measures of work-related psychosocial factors.
A major limitation of the present study was the high loss to follow-up experienced, which was beyond that initially predicted. We experienced a 31.4% loss to follow-up at the 6-month assessments, with the sample included in the primary outcome analysis reduced further (55% of the initial randomised sample) after taking into account activPAL compliance across the two assessment points. Further losses to follow-up were experienced at the final follow-up, with 54% of the original sample attending this assessment. We also lost two sites/clusters during the trial due to the collapse of their contracting companies. It was emphasised by managers as part of our process evaluation that HGV drivers are notoriously transient workers, with a high staff turnover rate. A large proportion of drivers not completing this study had left their role before the cessation of the programme. Sick leave and missed assessment sessions were also common reasons for non-completion. Future trials with this, or similar, occupational groups will need to take into account potentially high loss to follow-up rates within sample size calculations, along with consideration of compliance rates to device-based measures, if appropriate. Within the present study, we overrecruited at baseline, which is perhaps further evidence of the need for such health improvement interventions in HGV drivers. Nevertheless, the initial larger sample recruited meant that the larger than expected loss to follow-up rates were mitigated to a certain extent within our primary analysis, where sufficient statistical power remained to detect a significant difference between trial arms in our primary outcome.
The overall day-to-day running of the trial was extremely complex, and it was very challenging to schedule the measurement sessions in some sites because of the demand on the workforce, which led to overall delays with data collection. Owing to the 24/7 working nature of the logistics sector, a number of site visits took place during the night/very early hours of the morning, which led to further challenges for the research team in terms of scheduling and undertaking these visits. It was also extremely challenging to schedule the 6-hour education sessions within intervention sites, as, owing to the pressures faced by the industry, a number of managers found it difficult to facilitate the time for their drivers to be away from their driving duties. The overall challenges associated with the scheduling of measurement visits and education sessions, along with the challenges faced by the drivers to incorporate healthy lifestyle behaviours on workdays, emphasise and confirm the hard-to-reach nature of this male-dominated occupational group.
Owing to the multicomponent nature of our intervention, it should be highlighted that the SHIFT structured education session did not focus on one specific element of lifestyle health behaviours. It was not focused on physical activity, diet or sitting alone; all three elements were included. With the education session linked to the feedback participants had received from their baseline health measurements, intervention participants could choose to work on and improve any single behaviour or a combination of behaviours. Therefore, for some participants, step count targets may have increased if this is what they chose to focus on; for other participants, it could have been dietary choices and/or weight. Therefore, in some respects, owing to the multiple health behaviours covered, our overall results for each individual behaviour (i.e. steps, diet, weight, sitting) could have been ‘watered down’.
Conclusions and recommendations
The SHIFT intervention may have had a degree of success in positively impacting physical activity levels and reducing sitting time in HGV drivers at 6 months’ follow-up. Owing to the nature and demands of the occupation, the statistically significant differences observed between groups in these behaviours were largely driven by changes occurring on non-workdays, and are also largely attributable to the maintenance of physical activity levels in the SHIFT arm and a decline in physical activity levels in the control arm. The process evaluation revealed favourable attitudes towards the SHIFT intervention from both drivers and managers, with drivers highlighting that the education session, Fitbit and step count challenges were particularly effective for facilitating behavioural changes. Managers and participants reported enthusiasm and a sense of necessity for the SHIFT intervention to be included in future CPC training for professional drivers in the UK.
Although most intervention participants reported positive improvements to both knowledge and behaviour around their dietary intake within the process evaluation, the dietary outcome measures did not substantiate these findings within the RCT. Owing to the modest differences in physical activity seen between groups, and there being no differences between dietary variables, no statistically significant differences were observed between groups in terms of markers of adiposity or cardiometabolic outcomes. No differences in any outcome measure were seen between groups during the final follow-up assessments, suggesting that the positive impacts of the SHIFT intervention were not sustained beyond the duration of the 6-month intervention. However, the pandemic presents a major confounding factor that limits our ability to draw firm conclusions regarding the sustainability of the SHIFT intervention, particularly in light of the imbalance in participants on furlough between the two trial arms. The economic evaluation revealed that the SHIFT intervention is not likely to be cost-effective in its current delivery format.
The high prevalence of drivers with obesity, along with the poor cardiometabolic health profile and sleep deprivation seen in our sample, accompanied by the challenges experienced in scheduling data collection and the education sessions, highlight substantial health inequalities in this at-risk and hard-to-reach occupational group. Given the current, and increasing, shortfall of HGV drivers in the UK, which has risen from 60,00028 to an estimated 100,000 in 2021,29 the government and sector urgently need to address working conditions and the poor health profile of this ageing workforce to attract employees to the role. The already challenging working conditions are likely to be only exacerbated currently, as the small number of drivers have to compensate for driver shortages by expanding their own working hours, as relaxations in drivers’ hours rules have been re-introduced as a result of driver shortages, COVID-19 and Brexit. 160 Driver recruitment and a prioritisation of driver health is essential to combat the current challenges seen in maintaining critical supply chains, and to support the UK’s economic recovery from the COVID-19 pandemic. In addition, improving drivers’ health has significant implications, not only for the individual or their employer (through reductions in sickness absence and staff turnover), but also for the wider public through improving road safety for all users. Although the longer-term impact of the SHIFT intervention is unclear, the intervention (with ongoing development and refinement) offers potential to be incorporated into driver training courses to promote activity in this at-risk, underserved and hard-to-reach essential occupational group.
Based on the findings of the present study, we recommend the following:
-
To support the development and implementation of the SHIFT intervention as a CPC training module for HGV drivers, further work involving stakeholder engagement is needed to refine the content of the intervention, based on findings of the present study, and to examine an appropriate delivery mode that is cost-effective with maximal reach. On the translation of the SHIFT intervention into a CPC module, further work should be conducted to evaluate the scaling-up of this intervention over the longer term, in a real-world setting.
-
Effective strategies targeting improvements in dietary behaviours that, in turn, promote weight loss in HGV drivers need to be researched and incorporated into the SHIFT intervention to further impact the high prevalence of drivers with obesity.
-
Effective interventions targeting improvements in drivers’ sleep duration need to be created and evaluated and, subsequently, incorporated into the SHIFT intervention to combat the high levels of sleep deprivation observed in this study. Increasing sleep quantity through interventions targeting improved sleep management in drivers will potentially offer dual public health benefits of reducing accident risk (through reduced fatigue and improved vigilance performance) and reducing cardiometabolic risk within the individual.
Further research
Based on the findings of the present study relating to the high levels of sleep deprivation seen in our sample, members of the research team, along with colleagues with expertise in sleep science, have been awarded a MRC Public Health Intervention Development grant (reference MR/W004070/1; principal investigator Dr Iuliana Hartescu; start date 1 November 2021) to co-develop (with target users and stakeholders) an app-based intervention to improve sleep quality and quantity in commercial drivers within the road freight sector.
Acknowledgements
We gratefully acknowledge the support provided by senior health and safety personnel and transport managers at our partner logistics company in facilitating this research. We gratefully acknowledge all participating drivers in this study for their involvement in the trial. We thank all the casual researchers who are not named on this report but contributed to data collection. Particular thanks must go to Mr Ash Newton and Mr Cameron Wilson for their support of the study during their undergraduate studies. We are very grateful to the independent members of the TSC for their continued support and advice throughout the trial: Dr Derrick Bennett (chairperson, Nuffield Department of Population Health, University of Oxford), Professor Emma McIntosh (Institute of Health and Wellbeing, University of Glasgow), Professor Petra Wark (Institute of Health and Well-being, Coventry University) and Mr Paul Gardiner (independent HGV driver). We are very grateful to Dr Anna Chalkley (School of Sport, Exercise and Health Sciences, Loughborough University) for acting as an independent ‘critical friend’ during the analysis and write-up of our process evaluation, and to Dr Iuliana Hartescu (School of Sport, Exercise and Health Sciences, Loughborough University) and Dr Alex Rowlands (Diabetes Research Centre, University of Leicester) for their support with the processing of and interpretation of the sleep data collected. We are grateful to Professor Mark Hamer (Division of Surgery and Interventional Science, University College London) for his advice on the protocol used to assess psychophysiological reactivity. We also gratefully acknowledge the support provided by Professor David Stensel (School of Sport, Exercise and Health Sciences, Loughborough University), on behalf of the NIHR Leicester Biomedical Research Centre, who kindly provided funds to cover research associate time (for Dr Aron Sherry) on this project. We acknowledge the contribution of Mr Nishal Bhupendra Jaicim (former Medical Statistician at the Leicester Clinical Trials Unit), who wrote the SAP. We are also extremely grateful to Mrs Alison Stanley (School of Sport, Exercise and Health Sciences, Loughborough University) for all her help and support throughout the trial, particularly with regard to our public engagement activities. We are very grateful to Miss Helen Buxton (Research Manager, NIHR) for her continued support and advice throughout the running of this study. We also acknowledge the helpful feedback received from the NIHR’s anonymous reviewers on the first draft of this report.
This project was funded by the National Institute for Health and Care Research (NIHR) Public Health Research programme (reference 15/190/42). The study was also supported by the NIHR Leicester Biomedical Research Centre which is a partnership between University Hospitals of Leicester NHS Trust, Loughborough University, and the University of Leicester. Funding to cover intervention costs (e.g. Fitbits, cab workout equipment) was provided by the Higher Education Innovation Fund, via the Loughborough University Enterprise Projects Group. The Colt Foundation provided funding for a PhD studentship, awarded to Amber Guest (reference JD/618), which covered Amber Guest’s time and contributions to this project.
Contributions of authors
Stacy A Clemes (https://orcid.org/0000-0001-5612-5898) (Reader in Active Living and Public Health) the principal investigator, had overall responsibility for the study (including funding acquisition, study design and methods development) and report writing, drafted Chapters 1–3 and 6, and provided a detailed review and edit of Chapters 4 and 5.
Veronica Varela-Mato (https://orcid.org/0000-0003-4070-6609) (Research Associate) was responsible for the day-to-day management of the project (years 1–3), conducted and oversaw all fieldwork and data collection, co-delivery of the SHIFT education sessions and quantitative process evaluation data, contributed to the study design and methods development, and obtained funds to complete the project.
Danielle H Bodicoat (https://orcid.org/0000-0002-2184-4865) (Medical Statistician) was responsible for the statistical analysis and the preparation and presentation of the quantitative results in Chapter 3.
Cassandra L Brookes (https://orcid.org/0000-0002-0084-0400) (Principal Statistician) contributed to the study design, methods development, oversight of trial statistics and analysis plan.
Yu-Ling Chen (https://orcid.org/0000-0002-6976-4055) (Research Associate) supported the day-to-day management of the project (years 1–2.5), conducted fieldwork, co-delivered the SHIFT education sessions and supported the processing of the activPAL data (blinded).
Edward Cox (https://orcid.org/0000-0001-8981-0699) (Research Fellow) contributed to the health economic analysis plan, conducted the economic analysis and drafted the economic analysis reported in Chapter 4.
Charlotte L Edwardson (https://orcid.org/0000-0001-6485-9330) (Associate Professor in Physical Activity, Sedentary Behaviour and Health) contributed to the study design and methods development, and obtained funds to complete the project.
Laura J Gray (https://orcid.org/0000-0002-9284-9321) (Professor of Medical Statistics) contributed to the study design, methods development, trial statistics and analysis plan, and obtained funds to complete the project.
Amber Guest (https://orcid.org/0000-0002-3610-347X) (Doctoral Researcher) supported the day-to-day running of the project, conducted fieldwork, co-delivered the SHIFT education sessions, and undertook the process evaluation and drafted Chapter 5.
Vicki Johnson (https://orcid.org/0000-0001-6709-7634) (Education and Research Associate) contributed to the design and delivery of the SHIFT education programme, and obtained funds to complete the project.
Fehmidah Munir (https://orcid.org/0000-0002-5585-0243) (Professor of Health Psychology) contributed to the study design and methods development, and obtained funds to complete the project.
Nicola J Paine (https://orcid.org/0000-0001-9988-9310) (Lecturer in Health Psychology) contributed to the study methods and supported the process evaluation.
Gerry Richardson (https://orcid.org/0000-0002-2360-4566) (Professor of Health Economics) designed and oversaw the economic analysis, and obtained funds to complete the project.
Katharina Ruettger (https://orcid.org/0000-0002-8820-4272) (Doctoral Researcher) supported the day-to-day running of the project, conducted fieldwork, co-delivered the SHIFT education sessions and processed the activPAL data (blinded).
Mohsen Sayyah (https://orcid.org/0000-0002-6453-9086) (Research Associate) was responsible for the day-to-day management of the project (year 4), conducted and oversaw data collection, supported data entry and quality control checking, and undertook the processing of the activPAL and Stroop test data (blinded).
Aron Sherry (https://orcid.org/0000-0001-7489-253X) (Research Associate) was responsible for the day-to-day management of the project (year 4), conducted and oversaw data collection, supported data entry and quality control checking, and undertook the processing of the GENEActiv data (blinded).
Ana Suazo Di Paola (https://orcid.org/0000-0002-8523-8557) (Medical Statistician) assisted and facilitated the statistical analysis and reporting of the trial (including data queries and analysis validation), and facilitated the data transfer between collaborators for statistical and health economics analysis.
Jacqui Troughton (https://orcid.org/0000-0003-3690-9534) (Senior Clinical Research Associate) contributed to the design and delivery of the SHIFT education programme, and obtained funds to complete the project.
Simon Walker (https://orcid.org/0000-0002-5750-3691) (Research Fellow) contributed to the health economic analysis plan, conducted the economic analysis and drafted the economic analysis reported in Chapter 4.
Thomas Yates (https://orcid.org/0000-0002-5724-5178) (Professor) contributed to the study design and methods development, and obtained funds to complete the project.
James King (https://orcid.org/0000-0002-8174-9173) (Senior Lecturer in Exercise Physiology) contributed to the study design and methods development, supported the principal investigator with project oversight and management, and obtained funds to complete the project.
All authors were members of the internal Project Committee for the trial. All authors read drafts and provided revisions on the content of the report and have given final approval for submission.
Publications
Clemes SA, Varela Mato V, Munir F, Edwardson CL, Chen YL, Hamer M, et al. Cluster randomised controlled trial to investigate the effectiveness and cost-effectiveness of a Structured Health Intervention For Truckers (the SHIFT study): a study protocol. BMJ Open 2019;9:e030175.
Guest AJ, Chen YL, Pearson N, King JA, Paine NJ, Clemes SA. Cardiometabolic risk factors and mental health status among truck drivers: a systematic review. BMJ Open 2020;10:e038993.
Guest AJ, Clemes SA, King JA, Chen YL, Ruettger K, Sayyah M, et al. Attenuated cardiovascular reactivity is related to higher anxiety and fatigue symptoms in truck drivers. Psychophysiology 2021;58:e13872.
Longman DP, Shaw CN, Varela-Mato V, Sherry AP, Ruettger K, Sayyah M, et al. Time in nature associated with decreased fatigue in UK truck drivers. Int J Environ Res Public Health 2021;18:3158.
Clemes SA, Varela-Mato V, Bodicoat DH, Brookes CL, Chen YL, Edwardson CL, et al. The effectiveness of the Structured Health Intervention For Truckers (SHIFT): a cluster randomised controlled trial (RCT). BMC Med 2022;20:195.
Guest AJ, Paine NJ, Chen YL, Chalkley A, Munir F, Edwardson CL, et al. The Structured Health Intervention For Truckers (SHIFT) cluster randomised controlled trial: a mixed methods process evaluation. Int J Behav Nutr Phys Act 2022;19:79.
Ruettger K, Clemes SA, Chen YL, Edwardson C, Guest A, Gilson N, et al. Drivers with and without obesity respond differently to a multi-component health intervention in heavy goods vehicle drivers. Int J Environ Res Public Health 2022;19:15546.
Ruettger K, Varela-Mato V, Chen YL, Edwardson CL, Guest A, Gilson ND, et al. Physical activity, sedentary time and cardiometabolic health in heavy goods vehicle drivers: a cross-sectional analysis. J Occup Environ Med 2022;64:e217–23.
Sherry AP, Clemes SA, Chen YL, Edwardson C, Gray LJ, Guest A, et al. Sleep duration and sleep efficiency in UK long-distance heavy goods vehicle drivers. Occup Environ Med 2022;79:109–15.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PHR programme or the Department of Health and Social Care.
References
- Department for Transport . Domestic Road Freight Statistics, United Kingdom 2020. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1006792/domestic-road-freight-statistics-2020.pdf (accessed 6 September 2021).
- Apostolopoulos Y, Sonmez S, Shattell MM, Belzer M. Worksite-induced morbidities among truck drivers in the United States. AAOHN J 2010;58:285-96. https://doi.org/10.3928/08910162-20100625-01.
- Sieber WK, Robinson CF, Birdsey J, Chen GX, Hitchcock EM, Lincoln JE, et al. Obesity and other risk factors: the national survey of U.S. long-haul truck driver health and injury. Am J Ind Med 2014;57:615-26. https://doi.org/10.1002/ajim.22293.
- Guest AJ, Chen YL, Pearson N, King JA, Paine NJ, Clemes SA. Cardiometabolic risk factors and mental health status among truck drivers: a systematic review. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-038993.
- Garbarino S, Guglielmi O, Sannita WG, Magnavita N, Lanteri P. Sleep and mental health in truck drivers: descriptive review of the current evidence and proposal of strategies for primary prevention. Int J Environ Res Public Health 2018;15. https://doi.org/10.3390/ijerph15091852.
- Hege A, Lemke MK, Apostolopoulos Y, Sönmez S. Occupational health disparities among U.S. long-haul truck drivers: the influence of work organization and sleep on cardiovascular and metabolic disease risk. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0207322.
- Caddick N, Varela-Mato V, Nimmo MA, Clemes S, Yates T, King JA. Understanding the health of lorry drivers in context: a critical discourse analysis. Health 2017;21:38-56. https://doi.org/10.1177/1363459316644492.
- Garbarino S, Durando P, Guglielmi O, Dini G, Bersi F, Fornarino S, et al. Sleep apnea, sleep debt and daytime sleepiness are independently associated with road accidents. A cross-sectional study on truck drivers. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0166262.
- Hatami A, Vosoughi S, Hosseini AF, Ebrahimi H. Effect of co-driver on job content and depression of truck drivers. Saf Health Work 2019;10:75-9. https://doi.org/10.1016/j.shaw.2018.06.001.
- Shattell M, Apostolopoulos Y, Collins C, Sönmez S, Fehrenbacher C. Trucking organization and mental health disorders of truck drivers. Issues Ment Health Nurs 2012;33:436-44. https://doi.org/10.3109/01612840.2012.665156.
- da Silva-Junior FP, de Pinho RS, de Mello MT, de Bruin VM, de Bruin PF. Risk factors for depression in truck drivers. Soc Psychiatry Psychiatr Epidemiol 2009;44:125-9. https://doi.org/10.1007/s00127-008-0412-3.
- Abu Dabrh AM, Firwana B, Cowl CT, Steinkraus LW, Prokop LJ, Murad MH. Health assessment of commercial drivers: a meta-narrative systematic review. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003434.
- Prince SA, Elliott CG, Scott K, Visintini S, Reed JL. Device-measured physical activity, sedentary behaviour and cardiometabolic health and fitness across occupational groups: a systematic review and meta-analysis. Int J Behav Nutr Phys Act 2019;16. https://doi.org/10.1186/s12966-019-0790-9.
- Mabry JE, Hosig K, Hanowski R, Zedalis D, Gregg J, Herbert WG. Prevalence of metabolic syndrome in commercial truck drivers: a review. J Transp Health 2016;3:413-21. https://doi.org/10.1016/j.jth.2016.06.012.
- Martin BC, Church TS, Bonnell R, Ben-Joseph R, Borgstadt T. The impact of overweight and obesity on the direct medical costs of truck drivers. J Occup Environ Med 2009;51:180-4. https://doi.org/10.1097/JOM.0b013e3181965d6e.
- Thiese MS, Moffitt G, Hanowski RJ, Kales SN, Porter RJ, Hegmann KT. Commercial driver medical examinations: prevalence of obesity, comorbidities, and certification outcomes. J Occup Environ Med 2015;57:659-65. https://doi.org/10.1097/JOM.0000000000000422.
- Garbarino S, Guglielmi O, Campus C, Mascialino B, Pizzorni D, Nobili L, et al. Screening, diagnosis, and management of obstructive sleep apnea in dangerous-goods truck drivers: to be aware or not?. Sleep Med 2016;25:98-104. https://doi.org/10.1016/j.sleep.2016.05.015.
- Thiese MS, Moffitt G, Hanowski RJ, Kales SN, Porter RJ, Hegmann KT. Repeated cross-sectional assessment of commercial truck driver health. J Occup Environ Med 2015;57:1022-7. https://doi.org/10.1097/JOM.0000000000000522.
- Crizzle A, Bigelow P, Adams D, Gooderham S, Myers A, Thiffault P. Health and wellness of long-haul truck and bus drivers: a systematic literature review and directions for future research. J Transp Health 2017;7:90-109. https://doi.org/10.1016/j.jth.2017.05.359.
- Thiese MS, Hanowski RJ, Moffitt G, Kales SN, Porter RJ, Ronna B, et al. A retrospective analysis of cardiometabolic health in a large cohort of truck drivers compared to the American working population. Am J Ind Med 2018;61:103-10. https://doi.org/10.1002/ajim.22795.
- Office for National Statistics . Trend in Life Expectancy at Birth and at Age 65 by Socio-Economic Position Based on the National Statistics Socio-Economic Classification, England and Wales: 1982–1986 to 2007–2011 2015.
- Laberge-Nadeau C, Dionne G, Ekoé JM, Hamet P, Desjardins D, Messier S, et al. Impact of diabetes on crash risks of truck-permit holders and commercial drivers. Diabetes Care 2000;23:612-17. https://doi.org/10.2337/diacare.23.5.612.
- Ronna BB, Thiese MS, Ott U, Effiong A, Murtaugh M, Kapellusch J, et al. The association between cardiovascular disease risk factors and motor vehicle crashes among professional truck drivers. J Occup Environ Med 2016;58:828-32. https://doi.org/10.1097/JOM.0000000000000806.
- Thiese MS, Hanowski RJ, Kales SN, Porter RJ, Moffitt G, Hu N, et al. Multiple conditions increase preventable crash risks among truck drivers in a cohort study. J Occup Environ Med 2017;59:205-11. https://doi.org/10.1097/JOM.0000000000000937.
- Ng M, Yousuf B, Bigelow P, Van Eerd D. Effectiveness of health promotion programmes for truck drivers: a systematic review. Health Educ J 2015;74:270-86. https://doi.org/10.1177/0017896914533953.
- The Freight Transport Association . Logistics Report 2019 2019. www.santandercb.co.uk/sites/default/files/documents/fta_logistics_report_2019.pdf (accessed 6 September 2021).
- The All Party Parliamentary Group for Freight Transport . Barriers to Youth Employment in the Freight Transport Sector 2015. www.fta.co.uk/export/sites/fta/_galleries/downloads/events/driver_crisis_delegate/mp_report_barriers_to_youth_employment.pdf (accessed 1 August 2017).
- The Freight Transport Association . Logistics Report 2015: Freight Transport Association Limited 2015. www.fta.co.uk/export/sites/fta/_galleries/downloads/logistics_report/Web_files/LR15_WEB_270415.pdf (accessed 1 August 2017).
- RHA . A Report on the Driver Shortage 2021. www.rha.uk.net/LinkClick.aspx?fileticket=ICI0C-FWmVo%3d%26portalid=0%26timestamp=1627564639720 (accessed 8 September 2021).
- Thiese MS, Effiong AC, Ott U, Passey DG, Arnold ZC, Ronna BB, et al. A clinical trial on weight loss among truck drivers. Int J Occup Environ Med 2015;6:104-12. https://doi.org/10.15171/ijoem.2015.551.
- Gilson ND, Pavey TG, Vandelanotte C, Duncan MJ, Gomersall SR, Trost SG, et al. Chronic disease risks and use of a smartphone application during a physical activity and dietary intervention in Australian truck drivers. Aust N Z J Public Health 2016;40:91-3. https://doi.org/10.1111/1753-6405.12501.
- Codarin MAF, Moulatlet EM, Nehme P, Ulhôa M, de Castro Moreno CR. Association between physical activity, educational level and food intake profile among truck drivers. Saude E Sociedade 2010;19:418-28. https://doi.org/10.1590/S0104-12902010000200017.
- Rosso GL, Montomoli C, Candura SM. Poor weight control, alcoholic beverage consumption and sudden sleep onset at the wheel among Italian truck drivers: a preliminary pilot study. Int J Occup Med Environ Health 2016;29:405-16. https://doi.org/10.13075/ijomeh.1896.00638.
- Anderson JE, Govada M, Steffen TK, Thorne CP, Varvarigou V, Kales SN, et al. Obesity is associated with the future risk of heavy truck crashes among newly recruited commercial drivers. Accid Anal Prev 2012;49:378-84. https://doi.org/10.1016/j.aap.2012.02.018.
- Department for Transport . Reported Road Casulaties Great Britain: 2016 Annual Report 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/648081/rrcgb2016-01.pdf (accessed 6 July 2021).
- Varela-Mato V, O’Shea O, King JA, Yates T, Stensel DJ, Biddle SJ, et al. Cross-sectional surveillance study to phenotype lorry drivers’ sedentary behaviours, physical activity and cardio-metabolic health. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013162.
- Varela Mato V, Caddick N, King JA, Johnson V, Edwardson C, Yates T, et al. The impact of a novel Structured Health Intervention for Truckers (SHIFT) on physical activity and cardiometabolic risk factors. J Occup Environ Med 2018;60:368-76. https://doi.org/10.1097/JOM.0000000000001128.
- Varela-Mato V, Caddick N, King JA, Yates T, Stensel DJ, Nimmo MA, et al. A Structured Health Intervention for Truckers (SHIFT): a process evaluation of a pilot health intervention in a transport company. J Occup Environ Med 2018;60:377-85. https://doi.org/10.1097/JOM.0000000000001258.
- Clemes SA, Varela Mato V, Munir F, Edwardson CL, Chen YL, Hamer M, et al. Cluster randomised controlled trial to investigate the effectiveness and cost-effectiveness of a Structured Health Intervention For Truckers (the SHIFT study): a study protocol. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-030175.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group . CONSORT 2010 statement: extension to cluster randomised trials. BMJ 2012;345. https://doi.org/10.1136/bmj.e5661.
- Department for Transport . Domestic Road Freight Statistics, United Kingdom 2019 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/898747/domestic-road-freight-statistics-2019.pdf (accessed 6 July 2021).
- Bandura A. Health promotion by social cognitive means. Health Educ Behav 2004;31:143-64. https://doi.org/10.1177/1090198104263660.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care 2009;32:1404-10. https://doi.org/10.2337/dc09-0130.
- Gray LJ, Yates T, Troughton J, Khunti K, Davies MJ. Engagement, Retention, and progression to type 2 diabetes: a retrospective analysis of the cluster-randomised ‘Let’s Prevent Diabetes’ trial. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002078.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. https://doi.org/10.1136/bmj.39474.922025.BE.
- Chaiken S, Zanna M, Olson J, Herman C. Social Influence: the Ontaio Symposium. Bergen County, NJ: Erlbaum; 1987.
- Leventhal H, Meyer D, Nerenz D, Rachman S. Contributions to Medical Psychology. New York, NY: Pergamon; 1980.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. https://doi.org/10.1037//0033-295x.84.2.191.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296-304. https://doi.org/10.1001/jama.298.19.2296.
- Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev 1981;70:35-6.
- Chief Medical Officers of England, Scotland, Wales, and Northern Ireland . UK Chief Medical Officers’ Physical Activity Guidelines. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf (accessed 6 July 2021).
- Conn VS, Hafdahl AR, Cooper PS, Brown LM, Lusk SL. Meta-analysis of workplace physical activity interventions. Am J Prev Med 2009;37:330-9. https://doi.org/10.1016/j.amepre.2009.06.008.
- Ryan CG, Grant PM, Tigbe WW, Granat MH. The validity and reliability of a novel activity monitor as a measure of walking. Br J Sports Med 2006;40:779-84. https://doi.org/10.1136/bjsm.2006.027276.
- Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc 2011;43:1561-7. https://doi.org/10.1249/MSS.0b013e31820ce174.
- Atkin AJ, Gorely T, Clemes SA, Yates T, Edwardson C, Brage S, et al. Methods of measurement in epidemiology: sedentary behaviour. Int J Epidemiol 2012;41:1460-71. https://doi.org/10.1093/ije/dys118.
- Varela Mato V, Yates T, Stensel D, Biddle S, Clemes SA. Concurrent validity of actigraph-determined sedentary time against the Activpal under free-living conditions in a sample of bus drivers. Meas Phys Educ Exerc Sci 2017;21:212-22. https://doi.org/10.1080/1091367x.2017.1335204.
- Edwardson CL, Rowlands AV, Bunnewell S, Sanders J, Esliger DW, Gorely T, et al. Accuracy of posture allocation algorithms for thigh- and waist-worn accelerometers. Med Sci Sports Exerc 2016;48:1085-90. https://doi.org/10.1249/MSS.0000000000000865.
- Edwardson CL, Winkler EAH, Bodicoat DH, Yates T, Davies MJ, Dunstan DW, et al. Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Health Sci 2017;6:162-78. https://doi.org/10.1016/j.jshs.2016.02.002.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502. https://doi.org/10.1093/clinchem/18.6.499.
- Cleghorn CL, Harrison RA, Ransley JK, Wilkinson S, Thomas J, Cade JE. Can a dietary quality score derived from a short-form FFQ assess dietary quality in UK adult population surveys?. Public Health Nutr 2016;19:2915-23. https://doi.org/10.1017/S1368980016001099.
- Buman MP, Hu F, Newman E, Smeaton AF, Epstein DR. Behavioral periodicity detection from 24-h wrist accelerometry and associations with cardiometabolic risk and health-related quality of life. Biomed Res Int 2016;2016. https://doi.org/10.1155/2016/4856506.
- Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci 1990;52:29-37. https://doi.org/10.3109/00207459008994241.
- Kaida K, Takahashi M, Akerstedt T, Nakata A, Otsuka Y, Haratani T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol 2006;117:1574-81. https://doi.org/10.1016/j.clinph.2006.03.011.
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol 1976;4:97-110. https://doi.org/10.1037/t02254-000.
- O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003;21:821-48. https://doi.org/10.1097/00004872-200305000-00001.
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull 1991;109:163-20. https://doi.org/10.1037/0033-2909.109.2.163.
- Feldman PJ, Cohen S, Lepore SJ, Matthews KA, Kamarck TW, Marsland AL. Negative emotions and acute physiological responses to stress. Ann Behav Med 1999;21:216-22. https://doi.org/10.1007/BF02884836.
- Guest AJ, Clemes SA, King JA, Chen YL, Ruettger K, Sayyah M, et al. Attenuated cardiovascular reactivity is related to higher anxiety and fatigue symptoms in truck drivers. Psychophysiology 2021;58. https://doi.org/10.1111/psyp.13872.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. https://doi.org/10.1016/S0022-3999(01)00296-3.
- Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res 2007;16:133-41. https://doi.org/10.1007/s11136-007-9204-6.
- Johnston KL, Lawrence SM, Dodds NE, Yu L, Daley DC, Pilkonis PA. Evaluating PROMIS® instruments and methods for patient-centered outcomes research: patient and provider voices in a substance use treatment setting. Qual Life Res 2016;25:615-24. https://doi.org/10.1007/s11136-015-1131-3.
- Kuorinka I, Jonsson B, Kilbom A, Vinterberg H, Biering-Sørensen F, Andersson G, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon 1987;18:233-7. https://doi.org/10.1016/0003-6870(87)90010-X.
- Schaufeli W, Salanova M, González-Romá V, Bakker A. The measurement of engagement and burnout: a confirmative analytic approach. J Happiness Stud 2002;3:71-92. https://doi.org/10.1023/A:1015630930326.
- Winwood PC, Lushington K, Winefield AH. Further development and validation of the Occupational Fatigue Exhaustion Recovery (OFER) scale. J Occup Environ Med 2006;48:381-9. https://doi.org/10.1097/01.jom.0000194164.14081.06.
- Bond FW, Bunce D. Job control mediates change in a work reorganization intervention for stress reduction. J Occup Health Psychol 2001;6:290-302. https://doi.org/10.1037/1076-8998.6.4.290.
- Nagy M. Using a single-item approach to measure facet job satisfaction. J Org Occup Psychol 2002;75:77-86. https://doi.org/10.1348/096317902167658.
- Ilmarinen J. The Work Ability Index (WAI). Occup Med 2007;57. https://doi.org/10.1093/occmed/kqm008.
- Health and Safety Executive . Health and Safety Executive Management Standards Indicator Tool 2013. www.hse.gov.uk/stress/standards/index.htm (accessed 1 February 2015).
- Huang Y, Roetting M, McDevitt J, Melton D, Smith G. Feedback by technology: attitudes and opinions of truck drivers. Transp Res Part F Traffic Psychol Behav 2005;8:277-97. https://doi.org/10.1016/j.trf.2005.04.005.
- EuroQol G. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption – II. Addiction 1993;88:791-804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Winkler EA, Bodicoat DH, Healy GN, Bakrania K, Yates T, Owen N, et al. Identifying adults’ valid waking wear time by automated estimation in activPAL data collected with a 24 h wear protocol. Physiol Meas 2016;37:1653-68. https://doi.org/10.1088/0967-3334/37/10/1653.
- Healy GN, Eakin EG, Owen N, Lamontagne AD, Moodie M, Winkler EA, et al. A cluster randomized controlled trial to reduce office workers’ sitting time: effect on activity outcomes. Med Sci Sports Exerc 2016;48:1787-97. https://doi.org/10.1249/MSS.0000000000000972.
- Edwardson CL, Yates T, Biddle SJH, Davies MJ, Dunstan DW, Esliger DW, et al. Effectiveness of the Stand More AT (SMArT) work intervention: cluster randomised controlled trial. BMJ 2018;363. https://doi.org/10.1136/bmj.k3870.
- Migueles JH, Rowlands AV, Huber F, Sabia S, Van Hees VT. GGIR: a research community-driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Meas Phys Behav 2019;2:188-96. https://doi.org/10.1123/jmpb.2018-0063.
- van Hees VT, Sabia S, Jones SE, Wood AR, Anderson KN, Kivimäki M, et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep 2018;8. https://doi.org/10.1038/s41598-018-31266-z.
- van Hees VT, Sabia S, Anderson KN, Denton SJ, Oliver J, Catt M, et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0142533.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2012–2013 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: PSSRU, University of Kent; 2020.
- Sport England, University of East Anglia’s Medical School Health Economics Consulting Group . Measuring Impact 2016. www.sportengland.org/our-work/health-and-inactivity/what-is-moves/ (accessed 2 September 2021).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud 2013;50:587-92. https://doi.org/10.1016/j.ijnurstu.2012.09.010.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Ewald B, Attia J, McElduff P. How many steps are enough? Dose–response curves for pedometer steps and multiple health markers in a community-based sample of older Australians. J Phys Act Health 2014;11:509-18. https://doi.org/10.1123/jpah.2012-0091.
- Thomson JL, Landry AS, Zoellner JM, Tudor-Locke C, Webster M, Connell C, et al. Several steps/day indicators predict changes in anthropometric outcomes: HUB City Steps. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-983.
- Dwyer T, Ponsonby AL, Ukoumunne OC, Pezic A, Venn A, Dunstan D, et al. Association of change in daily step count over five years with insulin sensitivity and adiposity: population based cohort study. BMJ 2011;342. https://doi.org/10.1136/bmj.c7249.
- Dwyer T, Pezic A, Sun C, Cochrane J, Venn A, Srikanth V, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0141274.
- Yates T, Haffner SM, Schulte PJ, Thomas L, Huffman KM, Bales CW, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet 2014;383:1059-66. https://doi.org/10.1016/S0140-6736(13)62061-9.
- Campbell MK, Fayers PM, Grimshaw JM. Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research. Clin Trials 2005;2:99-107. https://doi.org/10.1191/1740774505cn071oa.
- Department for Transport and Driver and Vehicle Standards Agency . Temporary Relaxation of the Enforcement of the Drivers’ Hours Rules: All Sectors Carriage of Goods by Road 2020. www.gov.uk/government/publications/temporary-relaxation-of-the-enforcement-of-the-drivers-hours-rules-all-sectors-carriage-of-goods-by-road (accessed 5 May 2020).
- Longman DP, Shaw CN, Varela-Mato V, Sherry AP, Ruettger K, Sayyah M, et al. Time in nature associated with decreased fatigue in UK truck drivers. Int J Environ Res Public Health 2021;18. https://doi.org/10.3390/ijerph18063158.
- Rubin DB. Inference and missing data. Biometrika 1976;63:581-92. https://doi.org/10.1093/biomet/63.3.581.
- Clemes SA, Bingham DD, Pearson N, Chen YL, Edwardson C, McEachan R, et al. Sit–stand desks to reduce sedentary behaviour in 9- to 10-year-olds: the Stand Out in Class pilot cluster RCT. Public Health Esearch 2020;8. https://doi.org/10.3310/phr08080.
- Clemes SA, Varela-Mato V, Bodicoat DH, Brookes CL, Chen YL, Edwardson CL, et al. The effectiveness of the Structured Health Intervention For Truckers (SHIFT): a cluster randomised controlled trial (RCT). BMC Med 2022;20. https://doi.org/10.1186/s12916-022-02372-7.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 2 July 2021).
- Walker S, Griffin S, Asaria M, Tsuchiya A, Sculpher M. Striving for a societal perspective: a framework for economic evaluations when costs and effects fall on multiple sectors and decision makers. Appl Health Econ Health Policy 2019;17:577-90. https://doi.org/10.1007/s40258-019-00481-8.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge: Cambridge University Press; 2007.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Hernandez-Villafuerte K, Zamora B, Towse A. R&D, Issues Surrounding the Estimation of the Opportunity Cost of Adopting a New Health Care Technology: Areas for Further Research. London: Office of Health Economics; 2018.
- NHS . Reference Costs 2018–19 2019. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 7 September 2021).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons, Inc.; 2002.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Candio P, Meads D, Hill AJ, Bojke L. Modelling the impact of physical activity on public health: a review and critique. Health Policy 2020;124:1155-64. https://doi.org/10.1016/j.healthpol.2020.07.015.
- Office for National Statistics . National Life Tables: UK 2021. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 3 March 2021).
- Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, et al. Dose–response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019;366. https://doi.org/10.1136/bmj.l4570.
- Public Health England . Guide to Online Tools for Valuing Physical Activity, Sport and Obesity Programmes 2014. www.be-activeltd.co.uk/assets/Online-tools-briefing.pdf (accessed 2 September 2021).
- National Institute of Health and Care Excellence . Methods for the Development of NICE Public Health Guidance (Third Edition) 2012. www.nice.org.uk/process/pmg4/resources/methods-for-the-development-of-nice-public-health-guidance-third-edition-pdf-2007967445701 (accessed 5 October 2020).
- Guest AJ, Paine NJ, Chen YL, Chalkley A, Munir F, Edwardson CL, et al. The Structured Health Intervention For Truckers (SHIFT) cluster randomised controlled trial: a mixed methods process evaluation. Int J Behav Nutr Phys Act 2022;19. https://doi.org/10.1186/s12966-022-01316-x.
- Mowbray CT, Holter MC, Teague GB, Bybee D. Fidelity criteria: development, measurement, and validation. Am J Eval 2003;24:315-40. https://doi.org/10.1177/109821400302400303.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- NHS Digital . Health Survey for England 2019: Data Tables 2020. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019#data-sets (accessed 6 September 2021).
- Clemes SA, Patel R, Mahon C, Griffiths PL. Sitting time and step counts in office workers. Occup Med 2014;64:188-92. https://doi.org/10.1093/occmed/kqt164.
- Rowlands A, Davies M, Dempsey P, Edwardson C, Razieh C, Yates T. Wrist-worn accelerometers: recommending ∼1.0 mg as the minimum clinically important difference (MCID) in daily average acceleration for inactive adults. Br J Sports Med 2021;55:814-15. https://doi.org/10.1136/bjsports-2020-102293.
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Lancet Physical Activity Series Working Group . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219-29. https://doi.org/10.1016/S0140-6736(12)61031-9.
- Düking P, Tafler M, Wallmann-Sperlich B, Sperlich B, Kleih S. Behavior change techniques in wrist-worn wearables to promote physical activity: content analysis. JMIR Mhealth Uhealth 2020;8. https://doi.org/10.2196/20820.
- Brickwood KJ, Watson G, O’Brien J, Williams AD. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR Mhealth Uhealth 2019;7. https://doi.org/10.2196/11819.
- Tang MSS, Moore K, McGavigan A, Clark RA, Ganesan AN. Effectiveness of wearable trackers on physical activity in healthy adults: systematic review and meta-analysis of randomized controlled trials. JMIR Mhealth Uhealth 2020;8. https://doi.org/10.2196/15576.
- Ringeval M, Wagner G, Denford J, Paré G, Kitsiou S. Fitbit-based interventions for healthy lifestyle outcomes: systematic review and meta-analysis. J Med Internet Res 2020;22. https://doi.org/10.2196/23954.
- Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451-62. https://doi.org/10.1136/bjsports-2020-102955.
- Kazi A, Haslam C, Duncan M, Clemes S, Twumasi R. Sedentary behaviour and health at work: an investigation of industrial sector, job role, gender and geographical differences. Ergonomics 2019;62:21-30. https://doi.org/10.1080/00140139.2018.1489981.
- Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. Sedentary Behavior Research Network (SBRN) – Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act 2017;14. https://doi.org/10.1186/s12966-017-0525-8.
- Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 2012;55:2895-905. https://doi.org/10.1007/s00125-012-2677-z.
- Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123-32. https://doi.org/10.7326/M14-1651.
- Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016;388:1302-10. https://doi.org/10.1016/S0140-6736(16)30370-1.
- Patterson R, McNamara E, Tainio M, de Sá TH, Smith AD, Sharp SJ, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 2018;33:811-29. https://doi.org/10.1007/s10654-018-0380-1.
- Stamatakis E, Gale J, Bauman A, Ekelund U, Hamer M, Ding D. Sitting time, physical activity, and risk of mortality in adults. J Am Coll Cardiol 2019;73:2062-72. https://doi.org/10.1016/j.jacc.2019.02.031.
- Benatti FB, Ried-Larsen M. The effects of breaking up prolonged sitting time: a review of experimental studies. Med Sci Sports Exerc 2015;47:2053-61. https://doi.org/10.1249/MSS.0000000000000654.
- Twells LK, Harris Walsh K, Blackmore A, Adey T, Donnan J, Peddle J, et al. Nonsurgical weight loss interventions: a systematic review of systematic reviews and meta-analyses [published online ahead of print]. Obes Rev 2021. https://doi.org/10.1111/obr.13320.
- Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266-73. https://doi.org/10.1016/S0140-6736(14)62000-6.
- Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018;361. https://doi.org/10.1136/bmj.k1651.
- Steinemann N, Grize L, Ziesemer K, Kauf P, Probst-Hensch N, Brombach C. Relative validation of a food frequency questionnaire to estimate food intake in an adult population. Food Nutr Res 2017;61. https://doi.org/10.1080/16546628.2017.1305193.
- NHS . 5 a Day Portion Sizes 2018. www.nhs.uk/live-well/eat-well/5-a-day-portion-sizes/ (accessed 7 September 2021).
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health 2015;1:233-43. https://doi.org/10.1016/j.sleh.2015.10.004.
- Kurina LM, McClintock MK, Chen JH, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol 2013;23:361-70. https://doi.org/10.1016/j.annepidem.2013.03.015.
- Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med 2017;32:246-56. https://doi.org/10.1016/j.sleep.2016.08.006.
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435-9. https://doi.org/10.1016/S0140-6736(99)01376-8.
- Spiegel K, Leproult R, L’hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004;89:5762-71. https://doi.org/10.1210/jc.2004-1003.
- Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr 2009;90:1476-82. https://doi.org/10.3945/ajcn.2009.27984.
- Stern HS, Blower D, Cohen ML, Czeisler CA, Dinges DF, Greenhouse JB, et al. Data and methods for studying commercial motor vehicle driver fatigue, highway safety and long-term driver health. Accid Anal Prev 2019;126:37-42. https://doi.org/10.1016/j.aap.2018.02.021.
- Lemke MK, Apostolopoulos Y, Hege A, Sönmez S, Wideman L. Understanding the role of sleep quality and sleep duration in commercial driving safety. Accid Anal Prev 2016;97:79-86. https://doi.org/10.1016/j.aap.2016.08.024.
- Driver and Vehicle Licensing Agency . Excessive Sleepiness and Driving 2020. www.gov.uk/excessive-sleepiness-and-driving (accessed 6 September 2021).
- Pack AI, Dinges DF, Maislin G. A Study of Prevalence of Sleep Apnea Among Commercial Truck Drivers. Washington, DC: US Department of Transportation; 2002.
- Sherry AP, Clemes SA, Chen YL, Edwardson C, Gray LJ, Guest A, et al. Sleep duration and sleep efficiency in UK long-distance heavy goods vehicle drivers. Occup Environ Med 2022;79:109-15. https://doi.org/10.1136/oemed-2021-107643.
- Jackson P, Hilditch C, Holmes A, Reed N, Merat N, Smith L. Fatigue and Road Safety: A Critical Analysis of Recent Evidence. London: Department for Transport; 2011.
- Department for Transport and Driver and Vehicle Standards Agency . Coronavirus (COVID-19) and Brexit: Guidance on Drivers’ Hours Relaxations 2020. www.gov.uk/government/publications/covid-19-guidance-on-drivers-hours-relaxations/coronavirus-covid-19-guidance-on-drivers-hours-relaxations (accessed 6 September 2021).
- Sluiter JK, van der Beek AJ, Frings-Dresen MH. The influence of work characteristics on the need for recovery and experienced health: a study on coach drivers. Ergonomics 1999;42:573-83. https://doi.org/10.1080/001401399185487.
- de Croon EM, Sluiter JK, Frings-Dresen MH. Need for recovery after work predicts sickness absence: a 2-year prospective cohort study in truck drivers. J Psychosom Res 2003;55:331-9. https://doi.org/10.1016/S0022-3999(02)00630-X.
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose–response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003;26:117-26. https://doi.org/10.1093/sleep/26.2.117.
- Frumkin H, Bratman GN, Breslow SJ, Cochran B, Kahn PH, Lawler JJ, et al. Nature contact and human health: a research agenda. Environ Health Perspect 2017;125. https://doi.org/10.1289/EHP1663.
- Hansen MM, Jones R, Tocchini K. Shinrin-Yoku (forest bathing) and nature therapy: a state-of-the-art review. Int J Environ Res Public Health 2017;14. https://doi.org/10.3390/ijerph14080851.
- NHS . Improved Access to and Uptake of Diabetes Structured Education for People With Diabetes 2021. www.england.nhs.uk/ltphimenu/diabetes-prevention/improved-access-to-and-uptake-of-diabetes-structured-education-for-people-with-diabetes/ (accessed 13 September 2021).
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1986.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Resnicow K, Vaughan R. A chaotic view of behavior change: a quantum leap for health promotion. Int J Behav Nutr Phys Act 2006;3. https://doi.org/10.1186/1479-5868-3-25.
- Salkind N, Salkind NJ. Encyclopedia of Research Design. New York, NY: SAGE Publications Ltd; 2010.
- O’Cathain A, Murphy E, Nicholl J. Multidisciplinary, interdisciplinary, or dysfunctional? Team working in mixed-methods research. Qual Health Res 2008;18:1574-85. https://doi.org/10.1177/1049732308325535.
- Edwardson CL, Biddle SJH, Clarke-Cornwell A, Clemes S, Davies MJ, Dunstan DW, et al. A three arm cluster randomised controlled trial to test the effectiveness and cost-effectiveness of the SMART Work. Life intervention for reducing daily sitting time in office workers: study protocol. BMC Public Health 2018;18. https://doi.org/10.1186/s12889-018-6017-1.
- Barreira TV, Hamilton MT, Craft LL, Gapstur SM, Siddique J, Zderic TW. Intra-individual and inter-individual variability in daily sitting time and MVPA. J Sci Med Sport 2016;19:476-81. https://doi.org/10.1016/j.jsams.2015.05.004.
- Curtis L. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Department of Health and Social Care . NHS Reference Costs 2017–2018 2018.
- National Institute for Health and Care Excellence . Emergency and Acute Medical Care in Over 16s: Service Delivery and Organisation 2018. www.nice.org.uk/guidance/ng94 (accessed 1 September 2022).
Appendix 1 Baseline characteristics: completers versus non-completers
| Characteristic | Control | SHIFT intervention | Total | |||
|---|---|---|---|---|---|---|
| Non-completers (n = 69) | Completersa (n = 130) | Non-completers (n = 83) | Completers (n = 100) | Non-completers (n = 152) | Completers (n = 230) | |
| Cluster size, n (%) | ||||||
| Small | 31 (44.9) | 50 (38.5) | 52 (62.7) | 41 (41.0) | 83 (54.6) | 91 (39.6) |
| Large | 38 (55.1) | 80 (61.5) | 31 (37.4) | 59 (59.0) | 69 (45.4) | 139 (60.4) |
| Demographics | ||||||
| Age (years), median (IQR) | 49.95 (42.50–56.41) | 49.25 (40.64–55.16) | 49.82 (43.77–54.96) | 50.04 (41.66–55.40) | 49.82 (43.07–55.47) | 49.45 (41.12–55.24) |
| Number of years as a HGV driver, median (IQR) | 19.50 (11.33–28.25) | 12.34 (5.17–24.02) | 17.50 (9.00–25.83) | 17.00 (10.50–25.00) | 17.88 (10.00–27.00) | 14.50 (7.38–25.00) |
| Biometric measures | ||||||
| BMI (kg/m2), median (IQR) | 30.57 (27.86–32.84) | 28.89 (26.64–32.90) | 29.58 (26.56–33.54) | 30.03 (26.93–34.30) | 30.06 (27.08–32.96) | 29.71 (26.87–33.47) |
| activPAL variables | ||||||
| Number steps at baseline (steps/day) (IQR) | 7969.4 (6718.7–9894.9) | 8579.5 (6920.0–10,327.0) | 8813.6 (7208.6–11,973.3) | 8605.5 (6978.6–11,067.7) | 8531.7 (6879.8–10,678.6) | 8597.5 (6964.9–10,695.7) |
Appendix 2 Quotes provided by participants completing the additional online COVID-19 questionnaire
Eating, exercise, sleep.
Makes you more aware of your health.
Eating healthy and taking daily exercise.
Confirmation of prior knowledge.
Understanding how food effect your body.
Aware of a better way of living.
It has given me an insight to what I should be doing.
Better understanding of health and well-being. The importance of exercise.
I am quite sporty in my home life, but shift has given me tools to make some small but good changes in my work life.
Maintenance of exercise and trying to eat healthier and in moderation.
I’m more aware of the effects of not enough sleep.
Motivation to eat healthy and exercise.
Started eating better and going for a walk most days.
Learnt the importance of a balanced diet – conscious of my sugar intake.
Knowing what diet I should follow.
I’m eating more fruit and cycling every other day.
Being more conscious of my diet and health.
I’ve taken action appropriately given the results from the continued assessments in an effort to improve health and fitness.
Healthy outlook on life diet.
Finding out my blood pressures, good and bad cholesterol. Which has made me think more about what I put in my body and fitness.
Been given exercise and healthy food advice.
By making me aware of a healthy lifestyle using websites.
I understand what I should eat better and in what amounts. Keeping to the limit is still hard though.
More fruit better diet.
Health check feedback during visit at work.
Responses from participants in the intervention group
Maintaining a healthy diet and getting enough exercise.
Learning that doing a little bit every day is better than doing nothing.
It made me realise that it’s not that difficult to eat healthier by thinking about what I really need to eat.
Take more care at checking calories and fat in foods before buying.
Exercise healthy eating body and mind balance.
Cut out the crap and keep moving.
Keep off the junk food.
It has shown me that even small changes can make a big difference.
Understanding my calorie intake has had the most effect on my health.
It helped me chose the correct diet.
More aware of the minor alterations to make in diet to maintain good healthy weight.
I was more educated on sugar content in some foods I was regularly eating and also cut down on alcohol consumption.
Its given me the knowledge not necessarily stuck to it.
Full health check showed how unhealthy I was and how close to becoming diabetic I was, this has changed my eating habits and taking care of my body more seriously.
Small changes can make a big difference, plus help focus and motivate to do more exercise.
Healthy eating and exercise is the key to life.
Made me aware how unhealthy I am with not enough exercise I get the food that is good and bad And the problem this causes.
Choosing healthy options and understandings calories.
Healthy lifestyle booklet.
Education and highlighting the advantages of better eating.
To keep doing my steps.
Keeping moving and standing as much as possible, eating more healthier diet.
Health workshop.
Eating better.
Insight into healthy diet and exercise needs.
Cycle/walk more when furloughed.
Felt mentally better while off work, feeling a bit stressed and anxious now back working.
More sleep. Better diet.
Increased exercise.
Being on furlough gave me time to de-stress. It was a very positive experience.
More time to do things, like walking, golfing, gardening and DIY.
Change of shift at work, better sleep, feeling more alert and energetic.
I’ve started landscaping again and I feel healthier for moving more in the day.
More exercise.
Cycling.
Rediscovered the joy of cycling.
Positive impact on sleeping and eating.
Not so tired eating at regular times bit more exercise.
Exercising more.
The roads were not as busy as usual and so less stressful.
Everybody seems anxious . . . although I’m not . . . I think it’s been blown up out of all proportion.
Running more and healthy eating.
Going for more walks than ever before.
Getting more quality sleep but due to social distancing I’m not jogging or going for really long walks or bike rides.
Cycling to work.
Participants reporting a negative impact
Unable to go to the gym cannot sustain the same level of fitness as before.
Less movement. No work.
Shielding.
Eating more treats at home, picking.
Poor eating choices out on the road.
More drinking alcohol and eating slightly worse.
Not getting as much exercise as sitting longer.
Am doing a lot less physical activity.
Working days, less chance of preparing dinner and end up buying food out instead.
Access to the right sort of food.
I haven’t done as much exercise while being off work.
Can’t go swimming.
Gyms closed.
More difficult to create motivation, getting lazier, eating less veg and fruit.
Had a very sore knee for the last month.
I have become considerably lazier.
Nothing available at the services. I had to rediscover pot noodles to survive on nights out.
Less walking.
Increase in weight.
DIY, do it yourself.
Appendix 3 Tables and figures associated with the health economics analysis
| Resource | Unit cost (£) | Source |
|---|---|---|
| Primary care | ||
| GP: surgery visit | 34.09 | PSSRU 2019174 |
| GP: home visit | 110.60 | PSSRU 2010175 |
| GP: telephone call | 15.83 | PSSRU 2019174 |
| General practice nurse: surgery visit | 5.88 | PSSRU 2019174 |
| General practice nurse: home visit | 32.48 | PSSRU 2010175 |
| General practice nurse: telephone call | 6.20 | PSSRU 2019174 |
| Secondary care | ||
| Outpatient appointment | 142.12 | NHS reference costs 2017/18176 [General Surgery] |
| Accident and emergency visits | 116.11 | PSSRU 2010175 |
| NHS walk-in centre visit | 47.52 | NICE 2018177 |
| NHS urgent care centre visit | 69.21 | NICE 2018177 |
| Mental health care | ||
| Mental health nurse | 92.00 | PSSRU 2019174 |
| Occupational services | ||
| Occupational health nurse | 39.42 | NHS reference costs 2017/18176 |
| Physiotherapist | 88.35 | NHS reference costs 2017/18176 [Adult, One to One] |
| Available-case resource use | Baseline | Month 6 | Months 16–18a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| Primary care | |||||||||
| GP: surgery visit | 177 | 0.9 (1.87) | 0, 20 | 110 | 0.59 (0.99) | 0, 4 | 90 | 0.43 (0.91) | 0, 4 |
| GP: home visit | 184 | 0 (0) | 0, 0 | 114 | 0 (0) | 0, 0 | 92 | 0.02 (0.21) | 0, 2 |
| GP: telephone call | 181 | 0.14 (0.72) | 0, 8 | 112 | 0.11 (0.45) | 0, 3 | 89 | 0.31 (1.27) | 0, 10 |
| General practice nurse: surgery visit | 176 | 0.24 (0.7) | 0, 6 | 112 | 0.24 (0.66) | 0, 4 | 89 | 0.1 (0.37) | 0, 2 |
| General practice nurse: home visit | 184 | 0.01 (0.07) | 0, 1 | 114 | 0 (0) | 0, 0 | 92 | 0.03 (0.31) | 0, 3 |
| General practice nurse: telephone call | 183 | 0.01 (0.15) | 0, 2 | 114 | 0.04 (0.26) | 0, 2 | 91 | 0.05 (0.35) | 0, 3 |
| Secondary care | |||||||||
| Inpatient days | 183 | 0.09 (0.57) | 0, 5 | 114 | 0.11 (0.7) | 0, 6 | 92 | 0.57 (3.87) | 0, 34 |
| Outpatient visits | 178 | 0.23 (0.61) | 0, 4 | 112 | 0.23 (0.78) | 0, 5 | 90 | 0.22 (0.7) | 0, 4 |
| Accident and emergency visits | 182 | 0.08 (0.62) | 0, 8 | 114 | 0.09 (0.31) | 0, 2 | 92 | 0.11 (0.38) | 0, 2 |
| NHS walk-in centre visit | 183 | 0.04 (0.22) | 0, 2 | 113 | 0.01 (0.09) | 0, 1 | 91 | 0.02 (0.15) | 0, 1 |
| NHS urgent care centre visit | 184 | 0.01 (0.1) | 0, 1 | 114 | 0 (0) | 0, 0 | 92 | 0.01 (0.1) | 0, 1 |
| Other hospital-based services | 180 | 0.1 (0.95) | 0, 12 | 111 | 0.01 (0.09) | 0, 1 | 90 | 0.12 (0.58) | 0, 4 |
| Mental health care | |||||||||
| Mental health nurse | 184 | 0.01 (0.07) | 0, 1 | 114 | 0 (0) | 0, 0 | 91 | 0.01 (0.1) | 0, 1 |
| Occupational services | |||||||||
| Occupational health nurse | 184 | 0.04 (0.22) | 0, 2 | 114 | 0.03 (0.21) | 0, 2 | 92 | 0.03 (0.23) | 0, 2 |
| Physiotherapist | 183 | 0.34 (1.39) | 0, 12 | 113 | 0.67 (2.41) | 0, 20 | 90 | 0.34 (1.36) | 0, 10 |
| Available-case resource use | Baseline | Month 6 | Months 16–18a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| Primary care | |||||||||
| GP: surgery visit | 190 | 1.18 (2.37) | 0, 20 | 140 | 0.86 (1.43) | 0, 10 | 101 | 0.34 (0.85) | 0, 5 |
| GP: home visit | 201 | 0 (0.07) | 0, 1 | 146 | 0 (0) | 0, 0 | 102 | 0 (0) | 0, 0 |
| GP: telephone call | 200 | 0.25 (1.5) | 0, 20 | 145 | 0.12 (0.42) | 0, 2 | 97 | 0.27 (0.6) | 0, 3 |
| General practice nurse: surgery visit | 196 | 0.34 (1.32) | 0, 16 | 143 | 0.29 (0.68) | 0, 4 | 99 | 0.07 (0.29) | 0, 2 |
| General practice nurse: home visit | 201 | 0 (0.07) | 0, 1 | 146 | 0 (0) | 0, 0 | 102 | 0 (0) | 0, 0 |
| General practice nurse: telephone call | 201 | 0.01 (0.1) | 0, 1 | 146 | 0.08 (0.83) | 0, 10 | 101 | 0.02 (0.14) | 0, 1 |
| Secondary care | |||||||||
| Inpatient days | 201 | 0.06 (0.4) | 0, 3 | 146 | 0.01 (0.17) | 0, 2 | 102 | 0.02 (0.14) | 0, 1 |
| Outpatient visits | 197 | 0.31 (1.05) | 0, 10 | 145 | 0.21 (0.53) | 0, 3 | 102 | 0.2 (0.78) | 0, 7 |
| Accident and emergency visits | 201 | 0.1 (0.39) | 0, 3 | 145 | 0.06 (0.29) | 0, 2 | 102 | 0.07 (0.29) | 0, 2 |
| NHS walk-in centre visit | 200 | 0.04 (0.25) | 0, 3 | 144 | 0.04 (0.29) | 0, 3 | 100 | 0.02 (0.14) | 0, 1 |
| NHS urgent care centre visit | 201 | 0.01 (0.1) | 0, 1 | 146 | 0.01 (0.12) | 0, 1 | 102 | 0.02 (0.14) | 0, 1 |
| Other hospital-based services | 200 | 0.21 (1.67) | 0, 20 | 146 | 0.1 (0.53) | 0, 5 | 102 | 0.09 (0.55) | 0, 5 |
| Mental health care | |||||||||
| Mental health nurse | 201 | 0.01 (0.14) | 0, 2 | 146 | 0.03 (0.34) | 0, 4 | 102 | 0.03 (0.22) | 0, 2 |
| Occupational services | |||||||||
| Occupational health nurse | 200 | 0.02 (0.17) | 0, 2 | 146 | 0.01 (0.08) | 0, 1 | 102 | 0 (0) | 0, 0 |
| Physiotherapist | 199 | 0.34 (2.94) | 0, 40 | 146 | 0.42 (3.36) | 0, 40 | 100 | 0.14 (0.67) | 0, 5 |
| Complete-case resource use | Baseline | Month 6 | Months 16–18a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| Primary care | |||||||||
| GP: surgery visit | 67 | 0.88 (2.53) | 0, 20 | 72 | 0.56 (0.96) | 0, 4 | 72 | 0.4 (0.93) | 0, 4 |
| GP: home visit | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 | 72 | 0.03 (0.24) | 0, 2 |
| GP: telephone call | 70 | 0.17 (0.61) | 0, 3 | 72 | 0.06 (0.29) | 0, 2 | 72 | 0.35 (1.4) | 0, 10 |
| General practice nurse: surgery visit | 67 | 0.15 (0.4) | 0, 2 | 72 | 0.19 (0.52) | 0, 2 | 72 | 0.08 (0.37) | 0, 2 |
| General practice nurse: home visit | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 |
| General practice nurse: telephone call | 71 | 0.03 (0.24) | 0, 2 | 72 | 0.03 (0.24) | 0, 2 | 72 | 0.07 (0.39) | 0, 3 |
| Secondary care | |||||||||
| Inpatient days | 72 | 0.1 (0.63) | 0, 5 | 72 | 0.08 (0.71) | 0, 6 | 72 | 0.51 (4.02) | 0, 34 |
| Outpatient visits | 69 | 0.25 (0.63) | 0, 3 | 72 | 0.28 (0.91) | 0, 5 | 72 | 0.25 (0.75) | 0, 4 |
| Accident and emergency visits | 70 | 0.14 (0.97) | 0, 8 | 72 | 0.1 (0.34) | 0, 2 | 72 | 0.13 (0.41) | 0, 2 |
| NHS walk-in centre visit | 72 | 0.06 (0.23) | 0, 1 | 72 | 0.01 (0.12) | 0, 1 | 72 | 0 (0) | 0, 0 |
| NHS urgent care centre visit | 72 | 0.03 (0.17) | 0, 1 | 72 | 0 (0) | 0, 0 | 72 | 0.01 (0.12) | 0, 1 |
| Other hospital-based services | 70 | 0.06 (0.48) | 0, 4 | 72 | 0 (0) | 0, 0 | 72 | 0.06 (0.37) | 0, 3 |
| Mental health care | |||||||||
| Mental health nurse | 72 | 0.01 (0.12) | 0, 1 | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 |
| Occupational services | |||||||||
| Occupational health nurse | 72 | 0.04 (0.2) | 0, 1 | 72 | 0.03 (0.24) | 0, 2 | 72 | 0.04 (0.26) | 0, 2 |
| Physiotherapist | 72 | 0.18 (0.78) | 0, 4 | 72 | 0.69 (2.76) | 0, 20 | 72 | 0.31 (1.34) | 0, 10 |
| Complete-case resource use | Baseline | Month 6 | Months 16–18a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| Primary care | |||||||||
| GP: surgery visit | 80 | 1 (1.68) | 0, 10 | 84 | 0.9 (1.36) | 0, 7 | 84 | 0.37 (0.9) | 0, 5 |
| GP: home visit | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 |
| GP: telephone call | 84 | 0.1 (0.33) | 0, 2 | 84 | 0.1 (0.37) | 0, 2 | 84 | 0.24 (0.55) | 0, 2 |
| General practice nurse: surgery visit | 84 | 0.25 (0.64) | 0, 3 | 84 | 0.24 (0.55) | 0, 2 | 84 | 0.07 (0.3) | 0, 2 |
| General practice nurse: home visit | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 |
| General practice nurse: telephone call | 84 | 0 (0) | 0, 0 | 84 | 0.13 (1.1) | 0, 10 | 84 | 0.02 (0.15) | 0, 1 |
| Secondary care | |||||||||
| Inpatient days | 84 | 0.07 (0.46) | 0, 3 | 84 | 0 (0) | 0, 0 | 84 | 0.02 (0.15) | 0, 1 |
| Outpatient visits | 83 | 0.33 (1.22) | 0, 10 | 84 | 0.24 (0.57) | 0, 3 | 84 | 0.23 (0.86) | 0, 7 |
| Accident and emergency visits | 84 | 0.1 (0.4) | 0, 3 | 84 | 0.04 (0.24) | 0, 2 | 84 | 0.08 (0.32) | 0, 2 |
| NHS walk-in centre visit | 83 | 0.02 (0.15) | 0, 1 | 84 | 0.04 (0.19) | 0, 1 | 84 | 0.02 (0.15) | 0, 1 |
| NHS urgent care centre visit | 84 | 0.01 (0.11) | 0, 1 | 84 | 0.02 (0.15) | 0, 1 | 84 | 0.02 (0.15) | 0, 1 |
| Other hospital-based services | 84 | 0.46 (2.55) | 0, 20 | 84 | 0.12 (0.63) | 0, 5 | 84 | 0.11 (0.6) | 0, 5 |
| Mental health care | |||||||||
| Mental health nurse | 84 | 0.02 (0.22) | 0, 2 | 84 | 0.05 (0.44) | 0, 4 | 84 | 0.04 (0.24) | 0, 2 |
| Occupational services | |||||||||
| Occupational health nurse | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 |
| Physiotherapist | 84 | 0.65 (4.49) | 0, 40 | 84 | 0.65 (4.41) | 0, 40 | 84 | 0.17 (0.73) | 0, 5 |
| Available-case cost | Totala | Baseline | Month 6 | Months 16–18b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SHIFT intervention | 185 | 369.57 (0) | 370, 370 | 185 | 0 (0) | 0, 0 | 185 | 369.57 (0) | 370,370 | 185 | 0 (0) | 0, 0 |
| Primary care | ||||||||||||
| GP: surgery visit | 81 | 46.68 (79.63) | 0, 404 | 177 | 30.82 (63.85) | 31, 64 | 110 | 20.14 (33.71) | 0, 136 | 90 | 29.05 (61.16) | 0, 268 |
| GP: home visit | 85 | 5.12 (47.17) | 0, 435 | 184 | 0 (0) | 0, 0 | 114 | 0 (0) | 0, 0 | 92 | 4.73 (45.34) | 0, 435 |
| GP: telephone call | 81 | 11.36 (42.94) | 0, 311 | 181 | 2.27 (11.46) | 2, 11 | 112 | 1.7 (7.16) | 0, 47 | 89 | 9.79 (39.42) | 0, 311 |
| General practice nurse: surgery visit | 81 | 2.16 (6.4) | 0, 35 | 176 | 1.4 (4.12) | 1, 4 | 112 | 1.42 (3.89) | 0, 24 | 89 | 1.17 (4.29) | 0, 23 |
| General practice nurse: home visit | 85 | 0 (0) | 0, 0 | 184 | 0.18 (2.39) | 0, 2 | 114 | 0 (0) | 0, 0 | 92 | 2.08 (19.97) | 0, 192 |
| General practice nurse: telephone call | 84 | 0.87 (5.63) | 0, 49 | 183 | 0.07 (0.92) | 0, 1 | 114 | 0.22 (1.64) | 0, 12 | 91 | 0.67 (4.21) | 0, 37 |
| Secondary care | ||||||||||||
| Inpatient days | 83 | 380.24 (2305.49) | 0, 18,984 | 178 | 132.78 (839.69) | 133, 840 | 112 | 59.09 (566.01) | 0, 5978 | 91 | 339.78 (2203.86) | 0, 18,984 |
| Outpatient visits | 82 | 99.47 (235.03) | 0, 1118 | 178 | 32.74 (86.49) | 33, 86 | 112 | 32.99 (111.21) | 0, 711 | 90 | 62.1 (195.48) | 0, 1118 |
| Accident and emergency visits | 85 | 36.42 (106.36) | 0, 573 | 182 | 8.93 (71.65) | 9, 72 | 114 | 10.19 (36.43) | 0, 232 | 92 | 24.81 (86) | 0, 457 |
| NHS walk-in centre visit | 84 | 1.68 (11.38) | 0, 93 | 183 | 1.82 (10.41) | 2, 10 | 113 | 0.42 (4.47) | 0, 48 | 91 | 2.05 (13.77) | 0, 93 |
| NHS urgent care centre visit | 85 | 1.6 (14.76) | 0, 136 | 184 | 0.75 (7.2) | 1, 7 | 114 | 0 (0) | 0, 0 | 92 | 1.48 (14.19) | 0, 136 |
| Other hospital-based services | 85 | 8.47 (54.86) | 0, 360 | 184 | 4.93 (66.94) | 5, 67 | 114 | 0.4 (4.24) | 0, 45 | 92 | 7.82 (52.76) | 0, 360 |
| Mental health care | ||||||||||||
| Mental health nurse | 84 | 0 (0) | 0, 0 | 184 | 0.52 (7.01) | 1, 7 | 114 | 0 (0) | 0, 0 | 91 | 2.05 (19.59) | 0, 187 |
| Occupational services | ||||||||||||
| Occupational health nurse | 85 | 3.78 (21.12) | 0, 160 | 184 | 1.55 (8.9) | 2, 9 | 114 | 1.07 (8.5) | 0, 81 | 92 | 2.61 (18.58) | 0, 160 |
| Physiotherapist | 82 | 106.91 (435.38) | 0, 3504 | 183 | 29.93 (122.69) | 30, 123 | 113 | 59.42 (212.66) | 0, 1767 | 90 | 59.83 (236.04) | 0, 1737 |
| Total costs | ||||||||||||
| Overall total observed costs | 74 | 1008.56 (2646.22) | 370, 22348 | 170 | 251.85 (906.77) | 252, 907 | 106 | 563.24 (724.97) | 370, 7267 | 84 | 480.84 (2421.34) | 0, 21,787 |
| Total costs excluding inpatient-related services | 74 | 704.3 (813.55) | 370, 5761 | 170 | 113.7 (237.36) | 114, 237 | 106 | 503.12 (310.13) | 370, 2137 | 84 | 217.49 (551.33) | 0, 3624 |
| Available-case cost | Totala | Baseline | Month 6 | Months 16–18b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SHIFT intervention | 201 | 0 (0) | 0, 0 | 201 | 0 (0) | 0, 0 | 201 | 0 (0) | 0, 0 | 201 | 0 (0) | 0, 0 |
| Primary care | ||||||||||||
| GP: surgery visit | 90 | 40.91 (65.08) | 0, 307 | 190 | 40.19 (80.81) | 0, 682 | 140 | 29.22 (48.65) | 0, 341 | 101 | 11.48 (29.04) | 0, 170 |
| GP: home visit | 96 | 0 (0) | 0, 0 | 201 | 0.55 (7.8) | 0, 111 | 146 | 0 (0) | 0, 0 | 102 | 0 (0) | 0, 0 |
| GP: telephone call | 91 | 5.57 (10.65) | 0, 32 | 200 | 3.88 (23.72) | 0, 317 | 145 | 1.97 (6.7) | 0, 32 | 97 | 4.24 (9.56) | 0, 47 |
| General practice nurse: surgery visit | 90 | 1.76 (3.89) | 0, 18 | 196 | 1.98 (7.76) | 0, 94 | 143 | 1.69 (3.99) | 0, 24 | 99 | 0.42 (1.73) | 0, 12 |
| General practice nurse: home visit | 96 | 0 (0) | 0, 0 | 201 | 0.16 (2.29) | 0, 32 | 146 | 0 (0) | 0, 0 | 102 | 0 (0) | 0, 0 |
| General practice nurse: telephone call | 95 | 0.85 (6.43) | 0, 62 | 201 | 0.06 (0.62) | 0, 6 | 146 | 0.47 (5.15) | 0, 62 | 101 | 0.12 (0.87) | 0, 6 |
| Secondary care | ||||||||||||
| Inpatient days | 95 | 51.25 (460.47) | 0, 4475 | 197 | 73.28 (557.02) | 0, 5978 | 145 | 26.28 (316.4) | 0, 3810 | 102 | 47.74 (444.42) | 0, 4475 |
| Outpatient visits | 95 | 61.34 (153.25) | 0, 1137 | 197 | 44.73 (149.36) | 0, 1421 | 145 | 29.4 (74.71) | 0, 426 | 102 | 27.87 (111.42) | 0, 995 |
| Accident and emergency visits | 96 | 14.51 (53.94) | 0, 348 | 201 | 12.13 (45.57) | 0, 348 | 145 | 7.21 (34.13) | 0, 232 | 102 | 7.97 (33.72) | 0, 232 |
| NHS walk-in centre visit | 92 | 2.58 (10.83) | 0, 48 | 200 | 1.66 (12.03) | 0, 143 | 144 | 1.98 (13.62) | 0, 143 | 100 | 0.95 (6.69) | 0, 48 |
| NHS urgent care centre visit | 96 | 2.88 (13.9) | 0, 69 | 201 | 0.69 (6.89) | 0, 69 | 146 | 0.95 (8.07) | 0, 69 | 102 | 1.36 (9.64) | 0, 69 |
| Other hospital-based services | 96 | 1.01 (9.9) | 0, 97 | 201 | 14.06 (198.06) | 0, 2808 | 146 | 0.66 (8.02) | 0, 97 | 102 | 0 (0) | 0, 0 |
| Mental health care | ||||||||||||
| Mental health nurse | 96 | 6.93 (44.14) | 0, 380 | 201 | 0.95 (13.41) | 0, 190 | 146 | 3.25 (32.38) | 0, 380 | 102 | 2.8 (20.96) | 0, 190 |
| Occupational services | ||||||||||||
| Occupational health nurse | 96 | 0 (0) | 0, 0 | 200 | 0.81 (7.02) | 0, 81 | 146 | 0.28 (3.37) | 0, 41 | 102 | 0 (0) | 0, 0 |
| Physiotherapist | 94 | 67.67 (377.83) | 0, 3534 | 199 | 29.75 (259.98) | 0, 3534 | 146 | 37.52 (297.1) | 0, 3534 | 100 | 12.37 (58.93) | 0, 442 |
| Total costs | ||||||||||||
| Overall total observed costs | 84 | 277.02 (695.27) | 0, 4800 | 187 | 232.92 (815.96) | 0, 6542 | 138 | 147.23 (481.52) | 0, 4168 | 95 | 125.3 (529.02) | 0, 4800 |
| Total costs excluding inpatient-related services | 84 | 217.9 (466.85) | 0, 3602 | 187 | 140.61 (457.84) | 0, 5474 | 138 | 118.92 (336.89) | 0, 3602 | 95 | 74.05 (181.03) | 0, 1409 |
| Complete-case cost | Totala | Baseline | Month 6 | Months 16–18b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SHIFT intervention | 72 | 369.57 (0) | 370, 370 | 72 | 0 (0) | 0, 0 | 72 | 369.57 (0) | 370, 370 | 72 | 0 (0) | 0, 0 |
| Primary care | ||||||||||||
| GP: surgery visit | 72 | 45.94 (80.19) | 0, 404 | 67 | 30.02 (86.31) | 30, 86 | 72 | 18.94 (32.81) | 0, 136 | 72 | 27 (62.29) | 0, 268 |
| GP: home visit | 72 | 6.04 (51.26) | 0, 435 | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 | 72 | 6.04 (51.26) | 0, 435 |
| GP: telephone call | 72 | 11.69 (45.33) | 0, 311 | 70 | 2.71 (9.71) | 3, 10 | 72 | 0.88 (4.52) | 0, 32 | 72 | 10.81 (43.44) | 0, 311 |
| General practice nurse: surgery visit | 72 | 2.11 (6.5) | 0, 35 | 67 | 0.88 (2.35) | 1, 2 | 72 | 1.14 (3.06) | 0, 12 | 72 | 0.96 (4.23) | 0, 23 |
| General practice nurse: home visit | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 |
| General practice nurse: telephone call | 72 | 1.02 (6.08) | 0, 49 | 71 | 0.17 (1.47) | 0, 1 | 72 | 0.17 (1.46) | 0, 12 | 72 | 0.85 (4.72) | 0, 37 |
| Secondary care | ||||||||||||
| Inpatient days | 72 | 312.72 (2262.17) | 0, 18,984 | 69 | 121.29 (989.59) | 121, 990 | 72 | 5.47 (46.43) | 0, 394 | 72 | 307.24 (2262.44) | 0, 18,984 |
| Outpatient visits | 72 | 109.34 (247.36) | 0, 1118 | 69 | 35.02 (89.24) | 35, 89 | 72 | 39.48 (128.94) | 0, 711 | 72 | 69.86 (208.42) | 0, 1118 |
| Accident and emergency visits | 72 | 39.83 (112.38) | 0, 573 | 70 | 16.59 (112.32) | 17, 112 | 72 | 11.29 (39.75) | 0, 232 | 72 | 28.54 (93.36) | 0, 457 |
| NHS walk-in centre visit | 72 | 0.66 (5.6) | 0, 48 | 72 | 2.64 (10.96) | 3, 11 | 72 | 0.66 (5.6) | 0, 48 | 72 | 0 (0) | 0, 0 |
| NHS urgent care centre visit | 72 | 1.89 (16.04) | 0, 136 | 72 | 1.92 (11.45) | 2, 11 | 72 | 0 (0) | 0, 0 | 72 | 1.89 (16.04) | 0, 136 |
| Other hospital-based services | 72 | 0 (0) | 0, 0 | 72 | 12.61 (107.01) | 13, 107 | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 |
| Mental health care | ||||||||||||
| Mental health nurse | 72 | 0 (0) | 0, 0 | 72 | 1.32 (11.2) | 1, 11 | 72 | 0 (0) | 0, 0 | 72 | 0 (0) | 0, 0 |
| Occupational services | ||||||||||||
| Occupational health nurse | 72 | 4.47 (22.9) | 0, 160 | 72 | 1.7 (8.19) | 2, 8 | 72 | 1.13 (9.6) | 0, 81 | 72 | 3.34 (20.98) | 0, 160 |
| Physiotherapist | 72 | 114.43 (462.6) | 0, 3504 | 72 | 15.95 (68.48) | 16, 68 | 72 | 61.35 (243.49) | 0, 1767 | 72 | 53.08 (232.58) | 0, 1737 |
| Total costs | ||||||||||||
| Overall total observed costs | 72 | 1019.69 (2682.07) | 370, 22,348 | 66 | 248.31 (1044.38) | 248, 1044 | 72 | 510.09 (348.44) | 370, 2137 | 72 | 509.6 (2612.97) | 0, 21,787 |
| Total costs excluding inpatient-related services | 72 | 706.97 (823.8) | 370, 5761 | 66 | 123.75 (283.76) | 124, 284 | 72 | 504.62 (341.69) | 370, 2137 | 72 | 202.35 (577.39) | 0, 3624 |
| Complete-case cost | Totala | Baseline | Month 6 | Months 16–18b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SHIFT intervention | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 |
| Primary care | ||||||||||||
| GP: surgery visit | 84 | 43.42 (66.59) | 0, 307 | 80 | 34.09 (57.4) | 0, 341 | 84 | 30.84 (46.32) | 0, 239 | 84 | 12.58 (30.76) | 0, 170 |
| GP: home visit | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 |
| GP: telephone call | 84 | 5.28 (10.52) | 0, 32 | 84 | 1.51 (5.28) | 0, 32 | 84 | 1.51 (5.82) | 0, 32 | 84 | 3.77 (8.73) | 0, 32 |
| General practice nurse: surgery visit | 84 | 1.82 (3.98) | 0, 18 | 84 | 1.47 (3.75) | 0, 18 | 84 | 1.4 (3.24) | 0, 12 | 84 | 0.42 (1.78) | 0, 12 |
| General practice nurse: home visit | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 |
| General practice nurse: telephone call | 84 | 0.96 (6.84) | 0, 62 | 84 | 0 (0) | 0, 0 | 84 | 0.81 (6.79) | 0, 62 | 84 | 0.15 (0.95) | 0, 6 |
| Secondary care | ||||||||||||
| Inpatient days | 84 | 57.96 (489.64) | 0, 4475 | 83 | 110.89 (741.81) | 0, 5978 | 84 | 0 (0) | 0, 0 | 84 | 57.96 (489.64) | 0, 4475 |
| Outpatient visits | 84 | 65.98 (161.28) | 0, 1137 | 83 | 46.23 (173.51) | 0, 1421 | 84 | 33.84 (81.42) | 0, 426 | 84 | 32.15 (121.54) | 0, 995 |
| Accident and emergency visits | 84 | 13.82 (52.25) | 0, 348 | 84 | 11.06 (46.37) | 0, 348 | 84 | 4.15 (28.19) | 0, 232 | 84 | 9.68 (36.97) | 0, 232 |
| NHS walk-in centre visit | 84 | 2.83 (11.31) | 0, 48 | 83 | 1.15 (7.33) | 0, 48 | 84 | 1.7 (8.87) | 0, 48 | 84 | 1.13 (7.29) | 0, 48 |
| NHS urgent care centre visit | 84 | 3.3 (14.83) | 0, 69 | 84 | 0.82 (7.55) | 0, 69 | 84 | 1.65 (10.61) | 0, 69 | 84 | 1.65 (10.61) | 0, 69 |
| Other hospital-based services | 84 | 1.15 (10.58) | 0, 97 | 84 | 0.21 (1.96) | 0, 18 | 84 | 1.15 (10.58) | 0, 97 | 84 | 0 (0) | 0, 0 |
| Mental health care | ||||||||||||
| Mental health nurse | 84 | 7.92 (47.14) | 0, 380 | 84 | 2.26 (20.74) | 0, 190 | 84 | 4.53 (41.48) | 0, 380 | 84 | 3.39 (23.08) | 0, 190 |
| Occupational services | ||||||||||||
| Occupational health nurse | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 | 84 | 0 (0) | 0, 0 |
| Physiotherapist | 84 | 72.57 (398.71) | 0, 3534 | 84 | 57.85 (397.13) | 0, 3534 | 84 | 57.85 (389.96) | 0, 3534 | 84 | 14.72 (64.08) | 0, 442 |
| Total costs | ||||||||||||
| Overall total observed costs | 84 | 277.02 (695.27) | 0, 4800 | 80 | 277.24 (1023.41) | 0, 6542 | 84 | 139.42 (412.11) | 0, 3602 | 84 | 137.6 (561.3) | 0, 4800 |
| Total costs excluding inpatient-related services | 84 | 217.9 (466.85) | 0, 3602 | 80 | 161.96 (624.95) | 0, 5474 | 84 | 138.27 (412.37) | 0, 3602 | 84 | 79.64 (190.43) | 0, 1409 |
| Imputed cost | Totala | Month 6 | Months 16–18b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SHIFT intervention | 185 | 369.57 (0) | 0, 0 | 185 | 369.57 (0) | 370, 0 | 185 | 0 (0) | 0, 0 |
| Primary care | |||||||||
| GP: surgery visit | 185 | 52.37 (104.03) | 37, 68 | 185 | 22.55 (53.73) | 23, 54 | 185 | 29.82 (81.91) | 18, 42 |
| GP: home visit | 185 | 5.29 (62.01) | –4, 14 | 185 | 0 (0) | 0, 0 | 185 | 5.29 (62.01) | –4, 14 |
| GP: telephone call | 185 | 11.85 (44.81) | 5, 18 | 185 | 1.79 (8.74) | 2, 9 | 185 | 10.05 (41.75) | 4, 16 |
| General practice nurse: surgery visit | 185 | 3.08 (9.63) | 2, 4 | 185 | 1.55 (4.75) | 2, 5 | 185 | 1.53 (6.38) | 1, 2 |
| General practice nurse: home visit | 185 | 2.74 (26.9) | –1, 7 | 185 | 0 (0) | 0, 0 | 185 | 2.74 (26.9) | –1, 7 |
| General practice nurse: telephone call | 185 | 1.14 (7.65) | 0, 2 | 185 | 0.21 (2.87) | 0, 3 | 185 | 0.93 (6.34) | 0, 2 |
| Secondary care | |||||||||
| Inpatient days | 185 | 440.24 (3405.4) | –63, 943 | 185 | 75.05 (690.61) | 75, 691 | 185 | 365.19 (3048.45) | –85, 816 |
| Outpatient visits | 185 | 102.67 (331.89) | 54, 151 | 185 | 35.3 (134.49) | 35, 134 | 185 | 67.37 (284.68) | 25, 109 |
| Accident and emergency visits | 185 | 37.88 (137.35) | 18, 58 | 185 | 12.58 (52.22) | 13, 52 | 185 | 25.3 (107.95) | 9, 41 |
| NHS walk-in centre visit | 185 | 3.86 (24.99) | 0, 8 | 185 | 0.78 (10.58) | 1, 11 | 185 | 3.08 (22.07) | 0, 6 |
| NHS urgent care centre visit | 185 | 2.01 (20.01) | –1, 5 | 185 | 0.13 (1.55) | 0, 2 | 185 | 1.88 (19.48) | –1, 5 |
| Other hospital-based services | 185 | 7.68 (64.73) | –2, 17 | 185 | 0.58 (8.27) | 1, 8 | 185 | 7.1 (63.86) | –2, 16 |
| Mental health care | |||||||||
| Mental health nurse | 185 | 3.8 (44.29) | –3, 10 | 185 | 0.77 (35.23) | 1, 35 | 185 | 3.03 (35.16) | –2, 8 |
| Occupational services | |||||||||
| Occupational health nurse | 185 | 3.93 (25.71) | 0, 8 | 185 | 1.44 (11.68) | 1, 12 | 185 | 2.49 (23.34) | –1, 6 |
| Physiotherapist | 185 | 114.4 (408.15) | 55, 174 | 185 | 58.72 (259.05) | 59, 259 | 185 | 55.68 (235.03) | 21, 90 |
| Total costs | |||||||||
| Overall total observed costs | 185 | 1162.5 (3976.2) | 576, 1749 | 185 | 581.03 (881.33) | 581, 881 | 185 | 581.47 (3456.29) | 71, 1092 |
| Total costs excluding inpatient-related services | 185 | 722.26 (873.19) | 595, 850 | 185 | 505.98 (379.27) | 506, 379 | 185 | 216.28 (629.36) | 124, 308 |
| Imputed cost | Totala | Month 6 | Months 16–18b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SHIFT intervention | 201 | 0 (0) | 0, 0 | 201 | 0 (0) | 0, 0 | 201 | 0 (0) | 0, 0 |
| Primary care | |||||||||
| GP: surgery visit | 201 | 57.72 (115.81) | 41, 74 | 201 | 29.11 (54.24) | 22, 37 | 201 | 15 (44.78) | 9, 21 |
| GP: home visit | 201 | 3.46 (183.48) | –22, 29 | 201 | 0 (0) | 0, 0 | 201 | 2.42 (71.08) | –7, 12 |
| GP: telephone call | 201 | 13.66 (40.7) | 8, 19 | 201 | 2.16 (7.73) | 1, 3 | 201 | 5.69 (18.51) | 3, 8 |
| General practice nurse: surgery visit | 201 | 3.13 (9.06) | 2, 4 | 201 | 1.73 (4.49) | 1, 2 | 201 | 0.71 (2.97) | 0, 1 |
| General practice nurse: home visit | 201 | 1.76 (23.3) | –2, 5 | 201 | 0 (0) | 0, 0 | 201 | 0.87 (10.67) | –1, 2 |
| General practice nurse: telephone call | 201 | 1.17 (7.82) | 0, 2 | 201 | 0.44 (5.46) | 0, 1 | 201 | 0.41 (3.04) | 0, 1 |
| Secondary care | |||||||||
| Inpatient days | 201 | 306.95 (2740.31) | –80, 694 | 201 | 37.91 (473.08) | –28, 104 | 201 | 161.45 (1458.6) | –45, 368 |
| Outpatient visits | 201 | 112.97 (348.93) | 64, 162 | 201 | 32.1 (109.8) | 17, 48 | 201 | 38.92 (164.33) | 16, 62 |
| Accident and emergency visits | 201 | 33.32 (133.51) | 14, 52 | 201 | 8.52 (45.8) | 2, 15 | 201 | 12.54 (60.59) | 4, 21 |
| NHS walk-in centre visit | 201 | 4.17 (23.75) | 1, 7 | 201 | 1.73 (13.19) | 0, 4 | 201 | 1.5 (12.4) | 0, 3 |
| NHS urgent care centre visit | 201 | 3.42 (23.05) | 0, 7 | 201 | 0.83 (8.11) | 0, 2 | 201 | 1.14 (10.59) | 0, 3 |
| Other hospital-based services | 201 | 3.75 (48.38) | –3, 11 | 201 | 0.78 (9.19) | –1, 2 | 201 | 2 (68.34) | –7, 11 |
| Mental health care | |||||||||
| Mental health nurse | 201 | 8.02 (53.42) | 1, 15 | 201 | 2.58 (29.5) | –2, 7 | 201 | 2.84 (27.44) | –1, 7 |
| Occupational services | |||||||||
| Occupational health nurse | 201 | 1.93 (20.61) | –1, 5 | 201 | 0.55 (6.81) | 0, 2 | 201 | 0.98 (9.95) | 0, 2 |
| Physiotherapist | 201 | 82.24 (462.83) | 17, 147 | 201 | 45.98 (335.9) | –1, 93 | 201 | 17.43 (114.71) | 1, 34 |
| Total costs | |||||||||
| Overall total observed costs | 201 | 637.66 (3251.62) | 179, 1096 | 201 | 164.41 (724.49) | 63, 266 | 201 | 263.9 (1673.24) | 27, 501 |
| Total costs excluding inpatient-related services | 201 | 330.71 (809.67) | 217, 444 | 201 | 125.72 (411.2) | 68, 183 | 201 | 100.45 (336.25) | 53, 148 |
| Primary outcome | Available case, mean (SD) | Complete case, mean (SD) | Imputed analysis, mean (SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | |
| Preference scores | |||||||||
| EQ-5D-3L (base case) | |||||||||
| Baseline | 0.853 (0.146) | 0.838 (0.14) | 0.014 (–0.014 to 0.043) | 0.846 (0.143) | 0.835 (0.135) | 0.011 (–0.031 to 0.052) | 0.852 (0.146) | 0.839 (0.141) | 0.013 (–0.016 to 0.042) |
| 6 months | 0.832 (0.135) | 0.867 (0.129) | –0.035 (–0.067 to –0.002) | 0.831 (0.138) | 0.862 (0.13) | –0.031 (–0.071 to 0.008) | 0.838 (0.155) | 0.864 (0.147) | –0.026 (–0.056 to 0.003) |
| 16–18 months | 0.794 (0.173) | 0.801 (0.139) | –0.007 (–0.052 to 0.037) | 0.796 (0.174) | 0.801 (0.14) | –0.005 (–0.052 to 0.041) | 0.797 (0.188) | 0.795 (0.197) | 0.002 (–0.039 to 0.042) |
| EQ-5D-5L (scenario) | |||||||||
| Baseline | 0.909 (0.113) | 0.902 (0.108) | 0.007 (–0.015 to 0.029) | 0.906 (0.114) | 0.902 (0.101) | 0.005 (–0.027 to 0.037) | 0.909 (0.113) | 0.902 (0.108) | –0.016 (–0.039 to 0.007) |
| 6 months | 0.9 (0.112) | 0.922 (0.094) | –0.023 (–0.048 to 0.002) | 0.899 (0.115) | 0.92 (0.094) | –0.021 (–0.052 to 0.01) | 0.905 (0.121) | 0.922 (0.103) | –0.016 (–0.016 to 0.029) |
| 16–18 months | 0.869 (0.142) | 0.877 (0.112) | –0.008 (–0.044 to 0.028) | 0.871 (0.141) | 0.878 (0.114) | –0.007 (–0.045 to 0.031) | 0.875 (0.153) | 0.869 (0.166) | 0.006 (–0.027 to 0.04) |
| QALYs | |||||||||
| EQ-5D-3L (base case) | |||||||||
| 0–6 months | 0.419 (0.065) | 0.427 (0.06) | –0.008 (–0.023 to 0.008) | 0.42 (0.062) | 0.424 (0.058) | –0.004 (–0.022 to 0.014) | 0.422 (0.063) | 0.426 (0.061) | –0.003 (–0.016 to 0.009) |
| 16–18 months | 0.813 (0.143) | 0.832 (0.117) | –0.019 (–0.058 to 0.019) | 0.813 (0.143) | 0.831 (0.116) | –0.017 (–0.056 to 0.021) | 0.817 (0.146) | 0.83 (0.145) | –0.012 (–0.042 to 0.017) |
| Total | 1.235 (0.197) | 1.253 (0.168) | –0.018 (–0.073 to 0.036) | 1.235 (0.197) | 1.253 (0.168) | –0.018 (–0.073 to 0.036) | 1.24 (0.198) | 1.256 (0.194) | –0.016 (–0.054 to 0.023) |
| EQ-5D-5L (scenario) | |||||||||
| 0–6 months | 0.451 (0.053) | 0.456 (0.045) | –0.005 (–0.017 to 0.007) | 0.452 (0.051) | 0.455 (0.044) | –0.003 (–0.017 to 0.011) | 0.454 (0.051) | 0.456 (0.045) | –0.002 (–0.012 to 0.007) |
| 16–18 months | 0.885 (0.117) | 0.899 (0.089) | –0.014 (–0.045 to 0.016) | 0.885 (0.117) | 0.898 (0.089) | –0.013 (–0.044 to 0.018) | 0.89 (0.115) | 0.895 (0.114) | –0.005 (–0.028 to 0.018) |
| Total | 1.339 (0.161) | 1.352 (0.128) | –0.014 (–0.057 to 0.03) | 1.339 (0.161) | 1.352 (0.128) | –0.014 (–0.057 to 0.03) | 1.344 (0.157) | 1.351 (0.148) | –0.007 (–0.038 to 0.023) |
| Available case | Baseline | Month 6 | Months 16–18 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | |
| Absenteeism | |||||||||
| Number of sick days | 3.478 (15.303) | 3.825 (12.895) | –0.347 (–3.17 to 2.476) | 1.456 (4.639) | 3.048 (7.214) | –1.592 (–3.117 to –0.067) | 7.076 (21.701) | 2.386 (11.269) | 4.69 (–0.128 to 9.507) |
| Productivity | |||||||||
| Employee-assessed job performancea | 6.022 (0.902) | 5.975 (0.859) | 0.047 (–0.13 to 0.223) | 5.991 (0.955) | 5.986 (0.897) | 0.005 (–0.221 to 0.231) | 6.011 (0.994) | 5.96 (0.871) | 0.051 (–0.213 to 0.314) |
| Employee-assessed work abilityb | 8.37 (1.363) | 8.275 (1.51) | 0.095 (–0.195 to 0.385) | 8.377 (1.525) | 8.138 (1.517) | 0.239 (–0.134 to 0.612) | 8.371 (1.562) | 8.25 (1.376) | 0.121 (–0.303 to 0.544) |
| Employee work-related well-being | |||||||||
| Presenteeism (days worked while sick) | 4.852 (11.99) | 3.854 (7.173) | 0.997 (–0.968 to 2.962) | 7.886 (25.554) | 3.34 (5.504) | 4.546 (0.253 to 8.838) | 4.652 (10.989) | 4.273 (16.335) | 0.379 (–3.599 to 4.358) |
| Job satisfaction | 4.803 (1.42) | 4.995 (1.336) | –0.192 (–0.468 to 0.084) | 4.737 (1.476) | 4.924 (1.354) | –0.187 (–0.533 to 0.158) | 4.846 (1.541) | 5.079 (1.339) | –0.233 (–0.641 to 0.174) |
| Complete case | Baseline | Month 6 | Months 16–18 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | SHIFT intervention, mean (SD) | Usual practice, mean (SD) | Differential, mean (95% CI) | |
| Absenteeism | |||||||||
| Number of sick days | 3.059 (16.84) | 2.245 (7.261) | 0.814 (–2.923 to 4.551) | 1.494 (5.068) | 2.596 (7.119) | –1.102 (–2.929 to 0.726) | 5.541 (18.469) | 2.298 (11.474) | 3.243 (–1.216 to 7.703) |
| Productivity | |||||||||
| Employee-assessed job performancea | 6.048 (0.923) | 5.957 (0.793) | 0.091 (–0.162 to 0.345) | 6 (0.969) | 6 (0.842) | 0 (–0.266 to 0.266) | 6.036 (0.924) | 5.957 (0.891) | 0.078 (–0.189 to 0.345) |
| Employee-assessed work abilityb | 8.575 (1.3) | 8.539 (1.349) | 0.036 (–0.365 to 0.436) | 8.463 (1.307) | 8.258 (1.45) | 0.205 (–0.21 to 0.62) | 8.317 (1.578) | 8.326 (1.304) | –0.009 (–0.441 to 0.424) |
| Employee work-related well-being | |||||||||
| Presenteeism | 5.298 (14.304) | 3.934 (7.254) | 1.364 (–1.959 to 4.686) | 8.271 (27.89) | 3.462 (5.907) | 4.809 (–1.056 to 10.674) | 4.6 (11.251) | 3.923 (15.958) | 0.677 (–3.429 to 4.782) |
| Job satisfaction | 4.88 (1.292) | 5.183 (1.375) | –0.303 (–0.699 to 0.092) | 4.774 (1.434) | 5.106 (1.121) | –0.333 (–0.709 to 0.044) | 4.857 (1.522) | 5.106 (1.372) | –0.249 (–0.674 to 0.176) |
| Trial cost | Coefficient | SE | t-value | p-value | 95% CI | Significant |
|---|---|---|---|---|---|---|
| Treatment | 181.495 | 348.230 | 0.520 | 0.603 | –506.132 to 869.121 | |
| Female | 250.552 | 1536.169 | 0.160 | 0.871 | –2768.186 to 3269.289 | |
| Age: 40–49 years | 356.792 | 472.001 | 0.760 | 0.450 | –571.187 to 1284.772 | |
| Age: 50–59 years | 944.670 | 533.017 | 1.770 | 0.079 | –110.112 to 1999.451 | * |
| Age: ≥ 60 years | 981.036 | 864.525 | 1.130 | 0.260 | –743.358 to 2705.429 | |
| Overweight | –1031.631 | 624.262 | –1.650 | 0.101 | –2268.692 to 205.429 | |
| Obese | –530.971 | 607.802 | –0.870 | 0.384 | –1733.962 to 672.019 | |
| Morbidly obese | –361.152 | 954.029 | –0.380 | 0.705 | –2244.036 to 1521.731 | |
| A Levels | –249.189 | 524.562 | –0.480 | 0.635 | –1280.347 to 781.968 | |
| University graduate | –63.010 | 674.593 | –0.090 | 0.926 | –1385.772 to 1259.753 | |
| Master’s degree | –427.618 | 1233.818 | –0.350 | 0.729 | –2845.937 to 1990.702 | |
| Other education | –333.910 | 509.610 | –0.660 | 0.513 | –1336.781 to 668.961 | |
| Non-white | –398.073 | 711.787 | –0.560 | 0.577 | –1804.189 to 1008.043 | |
| Diabetes | –539.520 | 726.935 | –0.740 | 0.459 | –1975.310 to 896.269 | |
| Ex-smoker | 114.403 | 372.197 | 0.310 | 0.759 | –619.019 to 847.825 | |
| Current smoker | 825.426 | 562.951 | 1.470 | 0.147 | –296.766 to 1947.617 | |
| Cluster size | 6.735 | 36.909 | 0.180 | 0.855 | –65.873 to 79.342 | |
| Work years | –30.626 | 26.244 | –1.170 | 0.245 | –82.388 to 21.137 | |
| _cons | 802.571 | 900.506 | 0.890 | 0.374 | –970.736 to 2575.878 |
| Trial cost | Coefficient | SE | t-value | p-value | 95% CI | Significant |
|---|---|---|---|---|---|---|
| Treatment | 368.025 | 323.623 | 1.14 | 0.255 | –266.26 to 1002.31 | |
| Female | 839.412 | 1958.667 | 0.43 | 0.668 | –2999.51 to 4678.33 | |
| Age: 40–49 years | 132.369 | 461.301 | 0.29 | 0.774 | –771.76 to 1036.50 | |
| Age: 50–59 years | 499.180 | 465.811 | 1.07 | 0.284 | –413.79 to 1412.15 | |
| Age: ≥ 60 years | 480.275 | 789.373 | 0.61 | 0.543 | –1066.87 to 2027.42 | |
| Overweight | –1312.879 | 503.439 | –2.61 | 0.009 | –2299.60 to –326.15 | *** |
| Obese | –1182.842 | 514.908 | –2.3 | 0.022 | –2192.05 to –173.64 | ** |
| Morbidly obese | –60.663 | 879.860 | –0.07 | 0.945 | –1785.16 to 1663.84 | |
| A Levels | –354.948 | 498.249 | –0.71 | 0.476 | –1331.50 to 621.60 | |
| University graduate | 159.329 | 681.412 | 0.23 | 0.815 | –1176.21 to 1494.87 | |
| Master’s degree | –360.348 | 999.944 | –0.36 | 0.719 | –2320.20 to 1599.51 | |
| Other education | –200.862 | 482.003 | –0.42 | 0.677 | –1145.57 to 743.85 | |
| Non-white | –416.044 | 728.603 | –0.57 | 0.568 | –1844.08 to 1011.99 | |
| Diabetes | –460.849 | 828.743 | –0.56 | 0.578 | –2085.16 to 1163.46 | |
| Ex-smoker | 69.339 | 356.872 | 0.19 | 0.846 | –630.12 to 768.80 | |
| Current smoker | 985.603 | 464.262 | 2.12 | 0.034 | 75.67 to 1895.54 | ** |
| Cluster size | 11.619 | 33.660 | 0.35 | 0.73 | –54.35 to 77.59 | |
| Work years | –19.340 | 22.171 | –0.87 | 0.383 | –62.79 to 24.11 | |
| _cons | 1006.965 | 838.3473 | 1.2 | 0.23 | –636.1671 to 2650.098 |
| Trial cost | Coefficient | SE | t-value | p-value | 95% CI | Significant |
|---|---|---|---|---|---|---|
| Treatment | 14.939 | 61.372 | 0.24 | 0.808 | –105.895 to 135.774 | |
| Female | 200.851 | 278.603 | 0.72 | 0.471 | –346.038 to 747.740 | |
| Age: 40–49 years | 16.952 | 82.734 | 0.2 | 0.838 | –145.341 to 179.245 | |
| Age: 50–59 years | 73.872 | 85.762 | 0.86 | 0.389 | –94.544 to 242.289 | |
| Age: ≥ 60 years | 133.119 | 127.140 | 1.05 | 0.296 | –116.911 to 383.150 | |
| Overweight | –90.753 | 100.704 | –0.9 | 0.368 | –288.705 to 107.200 | |
| Obese | –42.250 | 98.658 | –0.43 | 0.669 | –236.075 to 151.576 | |
| Morbidly obese | 203.445 | 163.397 | 1.25 | 0.214 | –117.670 to 524.560 | |
| Female | –46.492 | 91.606 | –0.51 | 0.612 | –226.158 to 133.174 | |
| A Levels | 50.390 | 133.168 | 0.38 | 0.705 | –211.140 to 311.920 | |
| University graduate | –118.803 | 230.057 | –0.52 | 0.606 | –569.717 to 332.112 | |
| Master’s degree | –44.462 | 95.787 | –0.46 | 0.643 | –233.023 to 144.100 | |
| Other education | –40.019 | 122.656 | –0.33 | 0.744 | –281.296 to 201.257 | |
| Non-white | 26.475 | 127.226 | 0.21 | 0.835 | –223.925 to 276.875 | |
| Diabetes | –25.188 | 72.128 | –0.35 | 0.727 | –167.611 to 117.234 | |
| Ex-smoker | 41.225 | 92.479 | 0.45 | 0.656 | –141.639 to 224.090 | |
| Current smoker | –2.328 | 6.611 | –0.35 | 0.725 | –15.312 to 10.657 | |
| Cluster size | –3.905 | 4.704 | –0.83 | 0.407 | –13.164 to 5.354 | |
| Work years | 314.568 | 153.726 | 2.05 | 0.041 | 12.936 to 616.199 | ** |
| _cons | 14.939 | 61.372 | 0.24 | 0.808 | –105.895 to 135.774 |
| Trial cost | Coefficient | SE | t-value | p-value | 95% CI | Significant |
|---|---|---|---|---|---|---|
| Treatment | 0.062 | 0.383 | 0.16 | 0.872 | –0.695 to 0.819 | |
| Female | 0.604 | 1.460 | 0.41 | 0.679 | –2.262 to 3.469 | |
| Age: 40–49 years | 0.513 | 0.624 | 0.82 | 0.412 | –0.721 to 1.748 | |
| Age: 50–59 years | 1.028 | 0.608 | 1.69 | 0.093 | –0.176 to 2.233 | * |
| Age: ≥ 60 years | 0.788 | 0.928 | 0.85 | 0.399 | –1.061 to 2.638 | |
| Overweight | –0.893 | 0.651 | –1.37 | 0.173 | –2.183 to 0.397 | |
| Obese | –0.134 | 0.555 | –0.24 | 0.809 | –1.226 to 0.958 | |
| Morbidly obese | 0.366 | 0.876 | 0.42 | 0.677 | –1.355 to 2.086 | |
| Female | –0.136 | 0.646 | –0.21 | 0.833 | –1.414 to 1.141 | |
| A Levels | 0.451 | 0.842 | 0.54 | 0.593 | –1.207 to 2.109 | |
| University graduate | –0.455 | 1.323 | –0.34 | 0.731 | –3.050 to 2.140 | |
| Master’s degree | –0.332 | 0.543 | –0.61 | 0.541 | –1.402 to 0.737 | |
| Other education | –0.285 | 0.889 | –0.32 | 0.75 | –2.058 to 1.489 | |
| Non-white | –0.196 | 0.716 | –0.27 | 0.785 | –1.608 to 1.217 | |
| Diabetes | 0.184 | 0.418 | 0.44 | 0.661 | –0.643 to 1.011 | |
| Ex-smoker | 0.625 | 0.556 | 1.12 | 0.263 | –0.476 to 1.726 | |
| Current smoker | –0.005 | 0.040 | –0.14 | 0.892 | –0.084 to 0.074 | |
| Cluster size | –0.040 | 0.030 | –1.3 | 0.194 | –0.100 to 0.020 | |
| Work years | 6.272 | 1.056 | 5.94 | 0 | 4.187 to 8.358 | *** |
| _cons | 0.062 | 0.383 | 0.16 | 0.872 | –0.695 to 0.819 |
| Trial QALYs | Coefficient | SE | t-value | p-value | 95% CI | Significant |
|---|---|---|---|---|---|---|
| Baseline EQ-5D-3L cw | 0.796 | 0.056 | 14.160 | 0.000 | 0.685 to 0.907 | *** |
| Treatment | –0.028 | 0.015 | –1.820 | 0.070 | –0.059 to 0.002 | * |
| Age: 40–49 years | –0.042 | 0.020 | –2.060 | 0.040 | –0.081 to –0.002 | ** |
| Age: 50–59 years | –0.056 | 0.021 | –2.690 | 0.007 | –0.096 to –0.015 | *** |
| Age: ≥ 60 years | –0.076 | 0.032 | –2.340 | 0.021 | –0.140 to –0.012 | ** |
| Overweight | 0.002 | 0.023 | 0.090 | 0.926 | –0.043 to 0.047 | |
| Obese | –0.024 | 0.025 | –0.940 | 0.347 | –0.074 to 0.026 | |
| Morbidly obese | –0.030 | 0.041 | –0.740 | 0.459 | –0.110 to 0.050 | |
| Female | –0.043 | 0.078 | –0.560 | 0.577 | –0.197 to 0.110 | |
| A Levels | 0.003 | 0.022 | 0.130 | 0.899 | –0.041 to 0.047 | |
| University graduate | 0.032 | 0.033 | 0.990 | 0.325 | –0.032 to 0.096 | |
| Master’s degree | –0.023 | 0.055 | –0.420 | 0.675 | –0.131 to 0.085 | |
| Other education | 0.000 | 0.025 | –0.020 | 0.987 | –0.050 to 0.049 | |
| Non-white | 0.028 | 0.029 | 0.960 | 0.338 | –0.029 to 0.085 | |
| Diabetes | –0.029 | 0.034 | –0.850 | 0.395 | –0.097 to 0.039 | |
| Ex-smoker | –0.003 | 0.016 | –0.170 | 0.868 | –0.035 to 0.030 | |
| Current smoker | –0.028 | 0.019 | –1.450 | 0.147 | –0.067 to 0.010 | |
| Cluster size | 0.001 | 0.002 | 0.680 | 0.495 | –0.002 to 0.004 | |
| Work years | 0.001 | 0.001 | 0.620 | 0.533 | –0.001 to 0.003 | |
| _cons | 0.608 | 0.062 | 9.880 | 0.000 | 0.487 to 0.730 | *** |
| Trial QALYs | Coefficient | SE | t-value | p-value | 95% CI | Significant |
|---|---|---|---|---|---|---|
| Baseline EQ-5D-5L cw | 0.839 | 0.059 | 14.210 | 0.000 | 0.721 to 0.956 | *** |
| Treatment | –0.015 | 0.012 | –1.270 | 0.207 | –0.038 to 0.008 | |
| Age: 40–49 years | –0.034 | 0.016 | –2.170 | 0.030 | –0.065 to –0.003 | ** |
| Age: 50–59 years | –0.040 | 0.016 | –2.520 | 0.012 | –0.072 to –0.009 | ** |
| Age: ≥ 60 years | –0.065 | 0.026 | –2.540 | 0.012 | –0.116 to –0.014 | ** |
| Overweight | 0.002 | 0.018 | 0.120 | 0.906 | –0.034 to 0.038 | |
| Obese | –0.019 | 0.020 | –0.940 | 0.349 | –0.059 to 0.021 | |
| Morbidly obese | –0.034 | 0.032 | –1.050 | 0.294 | –0.098 to 0.030 | |
| Female | –0.058 | 0.065 | –0.890 | 0.375 | –0.186 to 0.071 | |
| A Levels | –0.005 | 0.017 | –0.310 | 0.755 | –0.039 to 0.028 | |
| University graduate | 0.018 | 0.025 | 0.750 | 0.455 | –0.030 to 0.067 | |
| Master’s degree | –0.017 | 0.042 | –0.410 | 0.680 | –0.100 to 0.065 | |
| Other education | –0.010 | 0.019 | –0.510 | 0.613 | –0.048 to 0.028 | |
| Non-white | 0.028 | 0.021 | 1.300 | 0.195 | –0.014 to 0.070 | |
| Diabetes | –0.029 | 0.027 | –1.050 | 0.298 | –0.083 to 0.026 | |
| Ex-smoker | 0.006 | 0.013 | 0.490 | 0.622 | –0.018 to 0.031 | |
| Current smoker | –0.011 | 0.016 | –0.680 | 0.499 | –0.043 to 0.021 | |
| Cluster size | 0.001 | 0.001 | 0.620 | 0.539 | –0.002 to 0.003 | |
| Work years | 0.001 | 0.001 | 0.740 | 0.462 | –0.001 to 0.002 | |
| _cons | 0.605 | 0.058 | 10.390 | 0.000 | 0.490 to 0.720 | *** |
| Trial QALYs | Coefficient | SE | t-value | p-value | 95% CI | Significant |
|---|---|---|---|---|---|---|
| Baseline EQ-5D-3L cw | 0.863 | 0.067 | 12.96 | 0 | 0.732 to 0.993 | *** |
| Treatment | –0.027 | 0.019 | –1.44 | 0.149 | –0.063 to 0.010 | |
| Age: 40–49 years | –0.036 | 0.027 | –1.34 | 0.182 | –0.089 to 0.017 | |
| Age: 50–59 years | –0.069 | 0.027 | –2.62 | 0.009 | –0.121 to –0.017 | *** |
| Age: ≥ 60 years | –0.097 | 0.045 | –2.16 | 0.031 | –0.185 to –0.009 | ** |
| Overweight | –0.009 | 0.031 | –0.29 | 0.769 | –0.070 to 0.052 | |
| Obese | –0.056 | 0.032 | –1.72 | 0.085 | –0.120 to 0.008 | * |
| Morbidly obese | 0.000 | 0.054 | 0 | 0.998 | –0.105 to 0.105 | |
| Female | 0.016 | 0.085 | 0.19 | 0.849 | –0.150 to 0.183 | |
| A Levels | 0.023 | 0.029 | 0.79 | 0.429 | –0.034 to 0.079 | |
| University graduate | 0.050 | 0.040 | 1.24 | 0.215 | –0.029 to 0.129 | |
| Master’s degree | –0.006 | 0.061 | –0.09 | 0.927 | –0.125 to 0.114 | |
| Other education | –0.021 | 0.029 | –0.71 | 0.477 | –0.078 to 0.036 | |
| Non-white | 0.007 | 0.043 | 0.16 | 0.876 | –0.077 to 0.090 | |
| Diabetes | –0.053 | 0.045 | –1.19 | 0.236 | –0.141 to 0.035 | |
| Ex-smoker | 0.010 | 0.021 | 0.45 | 0.65 | –0.032 to 0.051 | |
| Current smoker | –0.023 | 0.026 | –0.88 | 0.379 | –0.074 to 0.028 | |
| Cluster size | 0.003 | 0.002 | 1.64 | 0.1 | –0.001 to 0.007 | |
| Work years | 0.000 | 0.001 | 0.05 | 0.957 | –0.002 to 0.003 | |
| _cons | 0.532 | 0.076 | 6.97 | 0 | 0.383 to 0.682 | *** |
| Analysis | Cost (£) (95% CI) [p (most costly)] | QALYs (95% CI) [p (most effective)] | ΔCost (95% CI) | ΔQALYs (95% CI) | ICER (£) | INHB (95% CI) [probability of being cost-effective] | ||
|---|---|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||||
| Cost-effectiveness analysis: Ekelund et al.120 MVPA minutes/day (1) | ||||||||
| Usual practice | 419.18 (–243.05 to 1073.04) [0.151] | 16.15429 (16.15429 to 16.15429) [0.007] | [0.784] | [0.752] | [0.704] | |||
| SHIFT intervention | 783.15 (139.94 to 1480.03) [0.849] | 16.15989 (16.1552 to 16.16918) [0.993] | 363.97 (–352.74 to 1067.48) | 0.00559 (0.001 to 0.015) | 65,071.33 | –0.019 (–0.068 to 0.03) [0.216] | –0.013 (–0.05 to 0.025) [0.248] | –0.007 (–0.031 to 0.019) [0.296] |
| Cost-effectiveness analysis: Ekelund et al.120 sedentary minutes/day (2) | ||||||||
| Usual practice | 419.18 (–243.05 to 1073.04) [0.151] | 16.15429 (16.15429 to 16.15429) [0.002] | [0.774] | [0.736] | [0.664] | |||
| SHIFT intervention | 783.15 (139.94 to 1480.03) [0.849] | 16.16141 (16.1562 to 16.16798) [0.998] | 363.97 (–352.74 to 1067.48) | 0.00711 (0.002 to 0.014) | 51,173.91 | –0.017 (–0.065 to 0.032) [0.226] | –0.011 (–0.048 to 0.026) [0.264] | –0.005 (–0.03 to 0.02) [0.336] |
| Cost-effectiveness analysis: MOVES MVPA minutes translated into metabolic equivalents (3) | ||||||||
| Usual practice | 13,336.41 (7657.31 to 19927.97) [0] | 14.16817 (13.78574 to 14.56869) [0.003] | [0.876] | [0.739] | [0.534] | |||
| SHIFT intervention | 13,676.33 (8011.48 to 20230.24) [1] | 14.17978 (13.8056 to 14.57744) [1] | 339.92 (286.86 to 369.46) | 0.01161 (0 to 0.029) | 29,286.70 | –0.011 (–0.024 to 0.009) [0.124] | –0.005 (–0.018 to 0.014) [0.261] | 0.000 (–0.012 to 0.019) [0.466] |
| Analysis | Cost (£) (95% CI) [p (most costly)] | QALYs (95% CI) [p (most effective)] | ΔCost (95% CI) | ΔQALYs (95% CI) | ICER (£) | INHB (95% CI) [probability of being cost-effective] | ||
|---|---|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||||
| Cost-effectiveness analysis: Ekelund et al.120 MVPA minutes/day (1) | ||||||||
| Usual practice | 403.76 (–215.63 to 1045.02) [0.049] | 17.79441 (17.79441 to 17.79441) [0.777] | [0.967] | [0.968] | [0.958] | |||
| SHIFT intervention | 958.51 (299.02 to 1639.83) [0.951] | 17.78524 (17.76123 to 17.8101) [0.223] | 554.75 (–119.64 to 1228.65) | –0.00918 (–0.033 to 0.016) | Dominated | –0.046 (–0.098 to 0.002) [0.033] | –0.037 (–0.079 to 0.003) [0.032] | –0.028 (–0.061 to 0.006) [0.042] |
| Cost-effectiveness analysis: Ekelund et al.120 sedentary minutes/day (2) | ||||||||
| Usual practice | 403.76 (–215.63 to 1045.02) [0.049] | 17.79441 (17.79441 to 17.79441) [0.732] | [0.96] | [0.957] | [0.942] | |||
| SHIFT intervention | 958.51 (299.02 to 1639.83) [0.951] | 17.78705 (17.76319 to 17.81083) [0.268] | 554.75 (–119.64 to 1228.65) | –0.00737 (–0.031 to 0.016) | Dominated | –0.044 (–0.094 to 0.005) [0.04] | –0.035 (–0.074 to 0.005) [0.043] | –0.026 (–0.056 to 0.006) [0.058] |
| Cost-effectiveness analysis: MOVES MVPA minutes translated into metabolic equivalents (3) | ||||||||
| Usual practice | 13,336.41 (7657.31 to 19927.97) [0.066] | 13.96258 (13.5914 to 14.34303) [0.568] | [0.894] | [0.883] | [0.85] | |||
| SHIFT intervention | 13,843.20 (8049.98 to 20393.84) [0.934] | 13.96043 (13.58123 to 14.34193) [0.432] | 506.79 (–145.31 to 1180.41) | –0.00215 (–0.03 to 0.026) | Dominated | –0.036 | –0.027 | –0.019 |
| (–0.089 to 0.019) | (–0.071 to 0.017) | (–0.056 to 0.018) | ||||||
| [0.106] | [0.117] | [0.15] | ||||||
| Analysis | Cost (£) (95% CI) [p (most costly)] | QALYs (95% CI) [p (most effective)] | ΔCost (95% CI) | ΔQALYs (95% CI) | ICER (£) | INHB (95% CI) [probability of being cost-effective] | ||
|---|---|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||||
| Cost-effectiveness analysis: Ekelund et al.120 MVPA minutes/day (1) | ||||||||
| Usual practice | 430.66 (–249.8 to 1063.12) [0.063] | 21.81795 (21.81795 to 21.81795) [0.902] | [0.978] | [0.975] | [0.97] | |||
| SHIFT intervention | 984.76 (298 to 1692.76) [0.937] | 21.79758 (21.76676 to 21.82906) [0.098] | 554.1 (–143.82 to 1219.61) | –0.02037 (–0.051 to 0.011) | Dominated | –0.057 (–0.115 to –0.001) [0.022] | –0.048 (–0.097 to 0) [0.025] | –0.039 (–0.08 to 0.001) [0.03] |
| Cost-effectiveness analysis: Ekelund et al.120 sedentary minutes/day (2) | ||||||||
| Usual practice | 430.66 (–249.8 to 1063.12) [0.063] | 21.81795 (21.81795 to 21.81795) [0.874] | [0.975] | [0.975] | [0.967] | |||
| SHIFT intervention | 984.76 (298 to 1692.76) [0.937] | 21.79968 (21.7684 to 21.83111) [0.126] | 554.1 (–143.82 to 1219.61) | –0.01827 (–0.05 to 0.013) | Dominated | –0.055 (–0.112 to 0.001) [0.025] | –0.046 (–0.093 to 0) [0.025] | –0.037 (–0.077 to 0.003) [0.033] |
| Cost-effectiveness analysis: MOVES MVPA minutes translated into metabolic equivalents (3) | ||||||||
| Usual practice | 18,504.27 (11013.09 to 28649.04) [0.071] | 17.00305 (16.47525 to 17.53415) [0.777] | [0.941] | [0.929] | [0.914] | |||
| SHIFT intervention | 19,030.49 (11421.75 to 29261.56) [0.929] | 16.98962 (16.4497 to 17.51692) [0.223] | 526.22 (–160.34 to 1184.87) | –0.01343 (–0.048 to 0.027) | Dominated | –0.049 (–0.105 to 0.013) [0.059] | –0.040 (–0.088 to 0.013) [0.071] | –0.031 (–0.071 to 0.014) [0.086] |
| Analysis | Cost (£) (95% CI) [p (most costly)] | QALYs (95% CI) [p (most effective)] | ΔCost (95% CI) | ΔQALYs (95% CI) | ICER (£) | INHB (95% CI) [probability of being cost-effective] | ||
|---|---|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||||
| Cost-effectiveness analysis: Ekelund et al.120 MVPA minutes/day (1) | ||||||||
| Usual practice | 433.24 (–232.16 to 1126.61) [0.056] | 16.15429 (16.15429 to 16.15429) [0.931] | [0.987] | [0.987] | [0.983] | |||
| SHIFT intervention | 980.85 (302.95 to 1659.46) [0.944] | 16.13240 (16.10182 to 16.16267) [0.069] | 547.61 (–153.37 to 1251.63) | –0.0219 (–0.052 to 0.008) | Dominated | –0.058 (–0.115 to –0.006) [0.013] | –0.049 (–0.096 to –0.005) [0.013] | –0.040 (–0.078 to –0.002) [0.017] |
| Cost-effectiveness analysis: Ekelund et al.120 sedentary minutes/day (2) | ||||||||
| Usual practice | 433.24 (–232.16 to 1126.61) [0.056] | 16.15429 (16.15429 to 16.15429) [0.931] | [0.987] | [0.987] | [0.983] | |||
| SHIFT intervention | 980.85 (302.95 to 1659.46) [0.944] | 16.13240 (16.10182 to 16.16267) [0.069] | 547.61 (–153.37 to 1251.63) | –0.0219 (–0.052 to 0.008) | Dominated | –0.058 (–0.115 to -0.006) [0.013] | –0.049 (–0.096 to –0.005) [0.013] | –0.040 (–0.078 to –0.002) [0.017] |
| Cost-effectiveness analysis: MOVES MVPA minutes translated into metabolic equivalents (3) | ||||||||
| Usual practice | 13,336.41 (7657.31 to 19,927.97) [0.063] | 14.16817 (13.78574 to 14.56869) [0.833] | [0.963] | [0.955] | [0.947] | |||
| SHIFT intervention | 13,854.37 (8093.36 to 20,362.03) [0.937] | 14.15197 (13.7727 to 14.54723) [0.167] | 517.96 (–176.82 to 1230.79) | –0.0162 (–0.049 to 0.019) | Dominated | –0.051 (–0.108 to 0.004) [0.037] | –0.042 (–0.091 to 0.004) [0.045] | –0.033 (–0.074 to 0.009) [0.053] |
| Analysis | Cost (£) (95% CI) [p (most costly)] | QALYs (95% CI) [p (most effective)] | ΔCost (95% CI) | ΔQALYs (95% CI) | ICER (£) | INHB (95% CI) [probability of being cost-effective] | ||
|---|---|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||||
| Cost-effectiveness analysis: Ekelund et al.120 MVPA minutes/day (1) | ||||||||
| Usual practice | 203.21 (94.95 to 321.97) [0] | 16.15429 (16.15429 to 16.15429) [0.931] | [0.999] | [0.995] | [0.988] | |||
| SHIFT intervention | 585.47 (472.91 to 700.95) [1] | 16.13240 (16.10182 to 16.16267) [0.069] | 382.26 (260.23 to 504.72) | –0.0219 (–0.052 to 0.008) | Dominated | –0.047 (–0.079 to –0.016) [0.001] | –0.041 (–0.073 to –0.01) [0.005] | –0.035 (–0.066 to –0.004) [0.012] |
| Cost-effectiveness analysis: Ekelund et al.120 sedentary minutes/day (2) | ||||||||
| Usual practice | 203.21 (94.95 to 321.97) [0] | 16.15429 (16.15429 to 16.15429) [0.908] | [0.998] | [0.992] | [0.984] | |||
| SHIFT intervention | 585.47 (472.91 to 700.95) [1] | 16.13350 (16.10327 to 16.16419) [0.092] | 382.26 (260.23 to 504.72) | –0.02079 (–0.051 to 0.01) | Dominated | –0.046 (–0.077 to –0.014) [0.002] | –0.040 (–0.07 to –0.008) [0.008] | –0.034 (–0.064 to –0.002) [0.016] |
| Cost-effectiveness analysis: MOVES MVPA minutes translated into metabolic equivalents (3) | ||||||||
| Usual practice | 13,336.41 (7657.31 to 19,927.97) [0] | 14.16817 (13.78574 to 14.56869) [0.833] | [0.982] | [0.965] | [0.937] | |||
| SHIFT intervention | 13,689.02 (8025.72 to 20,233.77) [1] | 14.15197 (13.7727 to 14.54723) [0.167] | 352.61 (220.95 to 479.72) | –0.0162 (–0.049 to 0.019) | Dominated | –0.040 (–0.075 to –0.003) [0.018] | –0.034 (–0.068 to 0.003) [0.035] | –0.028 (–0.062 to 0.009) [0.063] |
| Analysis | Cost (£) (95% CI) [p (most costly)] | QALYs (95% CI) [p (most effective)] | ΔCost (95% CI) | ΔQALYs (95% CI) | ICER (£) | INHB (95% CI) [probability of being cost-effective] | ||
|---|---|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||||
| Cost-effectiveness analysis: Ekelund et al.120 MVPA minutes/day (1) | ||||||||
| Usual practice | 240.34 (–374.56 to 846.11) [0.009] | 16.15429 (16.15429 to 16.15429) [0.848] | [0.996] | [0.995] | [0.983] | |||
| SHIFT intervention | 990.74 (290.55 to 1695.8) [0.991] | 16.13454 (16.09685 to 16.17373) [0.152] | 750.39 (154.47 to 1338.79) | –0.01975 (–0.057 to 0.019) | Dominated | –0.070 (–0.123 to –0.016) [0.004] | –0.057 (–0.104 to –0.011) [0.005] | –0.045 (–0.088 to –0.003) [0.017] |
| Cost-effectiveness analysis: Ekelund et al.120 sedentary minutes/day (2) | ||||||||
| Usual practice | 240.34 (–374.56 to 846.11) [0.009] | 16.15429 (16.15429 to 16.15429) [0.866] | [0.995] | [0.992] | [0.987] | |||
| SHIFT intervention | 990.74 (290.55 to 1695.8) [0.991] | 16.13411 (16.09789 to 16.16793) [0.134] | 750.39 (154.47 to 1338.79) | –0.02018 (–0.056 to 0.014) | Dominated | –0.070 (–0.123 to –0.018) [0.005] | –0.058 (–0.104 to –0.012) [0.008] | –0.045 (–0.085 to –0.005) [0.013] |
| Cost-effectiveness analysis: MOVES MVPA minutes translated into metabolic equivalents (3) | ||||||||
| Usual practice | 13,336.41 (7657.31 to 19,927.97) [0.01] | 14.16817 (13.78574 to 14.56869) [0.781] | [0.989] | [0.98] | [0.961] | |||
| SHIFT intervention | 14,057.15 (8253.15 to 20,585) [0.99] | 14.15236 (13.76664 to 14.55025) [0.219] | 720.74 (125.86 to 1303.86) | –0.01581 (–0.056 to 0.026) | Dominated | –0.064 (–0.119 to –0.006) [0.011] | –0.052 (–0.101 to –0.002) [0.02] | –0.040 (–0.085 to 0.005) [0.039] |
| Time period | Ekelund et al.:120 MVPA | Ekelund et al.:120 sedentary | MOVES model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALY | ICER (£) | Incremental cost (£) | Incremental QALY | ICER (£) | Incremental cost (£) | Incremental QALY | ICER (£) | |
| Base case | 364 | 0.00559 | 65,071 | 364 | 0.00711 | 51,174 | 340 | 0.01161 | 29,287 |
| 1 year | 364 | 0.00844 | 43,130 | 364 | 0.01072 | 33,938 | 320 | 0.01882 | 17,024 |
| 2 years | 364 | 0.01111 | 32,751 | 364 | 0.01429 | 25,464 | 302 | 0.02522 | 11,988 |
| 3 years | 364 | 0.01419 | 25,644 | 364 | 0.01842 | 19,763 | 288 | 0.03080 | 9354 |
| 4 years | 364 | 0.01676 | 21,714 | 364 | 0.02201 | 16,538 | 273 | 0.03602 | 7572 |
| 5 years | 364 | 0.02035 | 17,884 | 364 | 0.02558 | 14,227 | 260 | 0.03971 | 6536 |
| 6 years | 364 | 0.02243 | 16,228 | 364 | 0.03000 | 12,131 | 242 | 0.04482 | 5408 |
| 7 years | 364 | 0.02641 | 13,780 | 364 | 0.03399 | 10,708 | 229 | 0.04947 | 4633 |
| 8 years | 364 | 0.02920 | 12,464 | 364 | 0.03786 | 9613 | 214 | 0.05299 | 4043 |
| 9 years | 364 | 0.03190 | 11,410 | 364 | 0.04279 | 8506 | 204 | 0.05615 | 3637 |
| 10 years | 364 | 0.03545 | 10,267 | 364 | 0.04677 | 7782 | 178 | 0.06171 | 2884 |
| ΔCost (£) | Continuation of SHIFT treatment benefit and additional cost profiles: ICER (£) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ekelund et al.:120 MVPA | Ekelund et al.:120 sedentary | MOVES model | ||||||||||||||||
| 1 years | 2 years | 3 years | 4 years | 5 years | 6 years | 1 years | 2 years | 3 years | 4 years | 5 years | 6 years | 1 years | 2 years | 3 years | 4 years | 5 years | 6 years | |
| –370 | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant |
| –200 | 19,430 | 14,754 | 11,553 | 9782 | 8057 | 7311 | 15,289 | 11,471 | 8903 | 7450 | 6409 | 5465 | 6397 | 4058 | 2861 | 2020 | 1500 | 946 |
| –100 | 31,280 | 23,753 | 18,599 | 15,748 | 12,970 | 11,770 | 24,613 | 18,468 | 14,333 | 11,994 | 10,318 | 8798 | 11,710 | 8023 | 6108 | 4796 | 4018 | 3177 |
| Base case | 43,130 | 32,751 | 25,644 | 21,714 | 17,884 | 16,228 | 33,938 | 25,464 | 19,763 | 16,538 | 14,227 | 12,131 | 17,024 | 11,988 | 9354 | 7572 | 6536 | 5408 |
| 100 | 54,980 | 41,750 | 32,690 | 27,680 | 22,798 | 20,687 | 43,262 | 32,460 | 25,193 | 21,081 | 18,135 | 15,464 | 22,337 | 15,953 | 12,601 | 10,348 | 9054 | 7639 |
| 200 | 66,830 | 50,748 | 39,736 | 33,646 | 27,711 | 25,146 | 52,587 | 39,456 | 30,622 | 25,625 | 22,044 | 18,797 | 27,651 | 19,918 | 15,848 | 13,125 | 11,573 | 9871 |
| 370 | 86,924 | 66,007 | 51,684 | 43,763 | 36,043 | 32,706 | 68,398 | 51,320 | 39,830 | 33,330 | 28,672 | 24,448 | 36,661 | 26,642 | 21,353 | 17,832 | 15,843 | 13,654 |
| 500 | 102,380 | 77,743 | 60,874 | 51,544 | 42,452 | 38,522 | 80,560 | 60,445 | 46,912 | 39,257 | 33,771 | 28,796 | 43,592 | 31,814 | 25,588 | 21,453 | 19,127 | 16,564 |
| 1,000 | 161,630 | 122,736 | 96,103 | 81,374 | 67,021 | 60,816 | 127,182 | 95,426 | 74,061 | 61,975 | 53,315 | 45,460 | 70,160 | 51,639 | 41,821 | 35,335 | 31,718 | 27,719 |
| Secondary cost-effectiveness analysis | Baseline, mean differential | Month 6 | Months 16–18 | Average total trial results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean differential | Difference in differencea | ICER (£) | Mean differential | Difference in differencea | ICER (£) | Mean differential | Difference in differencea | ICER (£) | ||
| Costs (£) | ||||||||||
| Intervention | 0 | 369.59 | 369.59 | 0 | 0 | 369.59 | 369.59 | |||
| Absenteeism | –57.98 | –227.41 | –169.43 | 494.07 | 552.05 | 266.66 | 324.64 | |||
| Total | –57.98 | 142.18 | 200.16 | 494.07 | 552.05 | 636.25 | 694.23 | |||
| Outcomes and cost-effectiveness | ||||||||||
| Employee-assessed job performancea | 0.045 | 0.045 | 0.000 | Dominated | 0.126 | 0.081 | 6816 | 0.086 | 0.041 | 17,142 |
| Employee-assessed work abilitya | 0.074 | 0.222 | 0.148 | 1353 | 0.228 | 0.154 | 3585 | 0.225 | 0.151 | 4598 |
| Presenteeism (days worked while sick) | 0.902 | 3.259 | 2.357 | Dominated | –0.287 | –1.189 | 465 | 1.486 | 0.584 | Dominated |
| Job satisfaction | –0.199 | –0.163 | 0.036 | 5560 | –0.048 | 0.151 | 3656 | –0.101 | 0.094 | 7425 |
FIGURE 4.
Interpolated dose–response relationship between all-cause mortality hazard ratios and sedentary behaviour: Ekelund et al. 120 Poly., interpolated dose–response relationship.

FIGURE 5.
Interpolated dose–response relationship between all-cause mortality hazard ratios and time spent in MVPA: Ekelund et al. 120 Poly., interpolated dose–response relationship.

FIGURE 6.
Incremental net health benefit of the SHIFT trial relative to usual practice for alternative rates of treatment decay using Ekelund et al. 120 all-cause mortality estimates with changes to MVPA minutes/day.
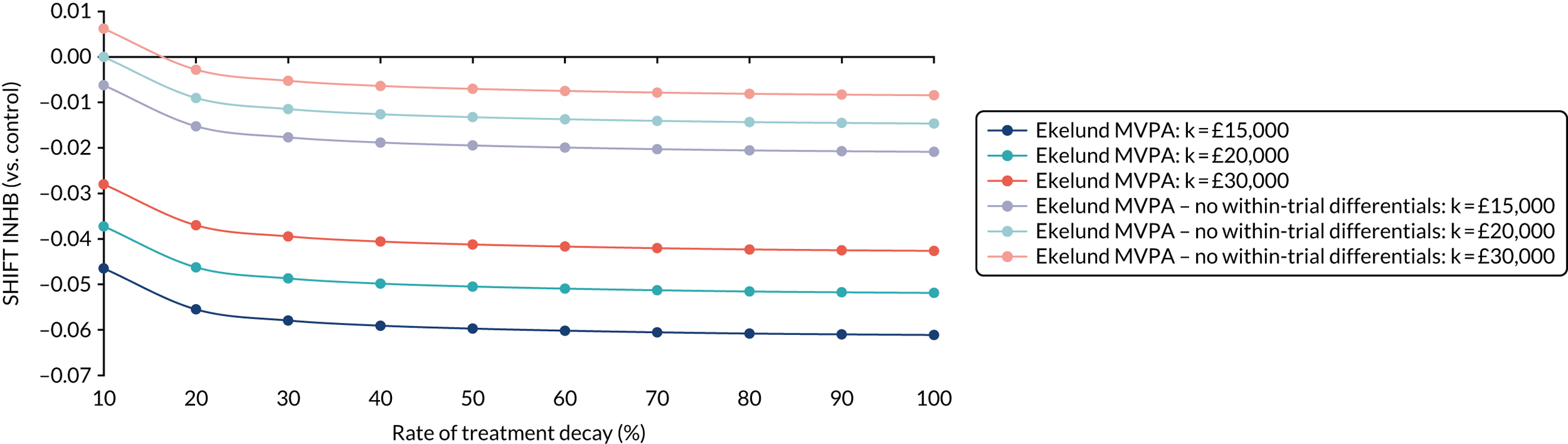
FIGURE 7.
Incremental net health benefit of the SHIFT trial relative to usual practice for alternative rates of treatment decay using Ekelund et al. 120 all-cause mortality estimates with changes to sedentary minutes/day.
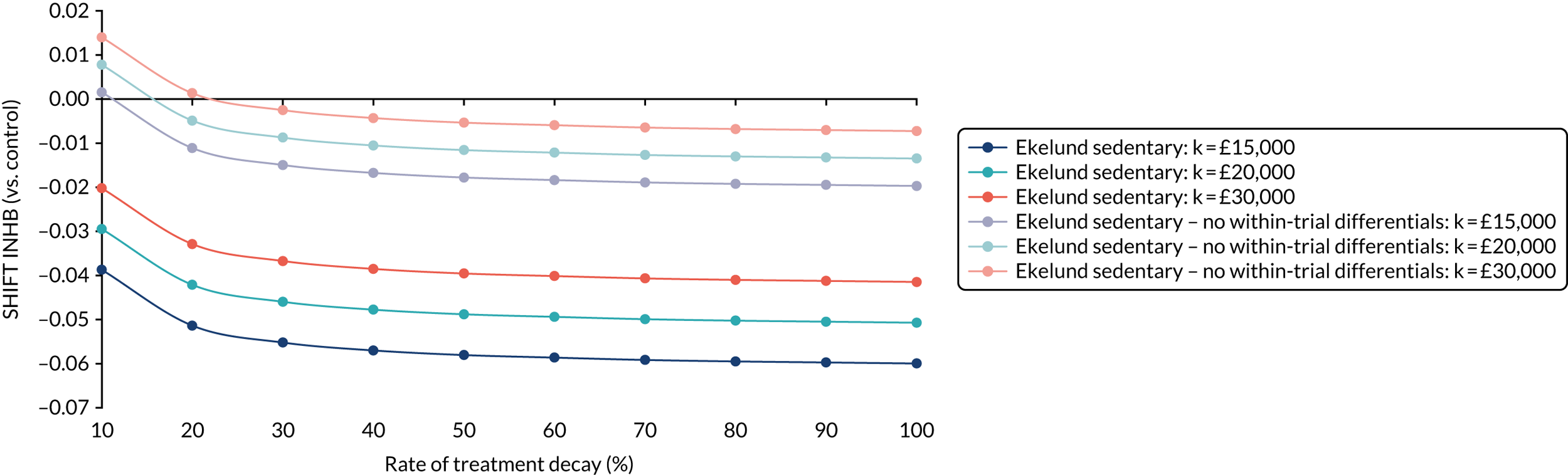
FIGURE 8.
Cost-effectiveness acceptability curves using Ekelund et al. 120 all-cause mortality estimates with changes to MVPA minutes/day, including and excluding within-trial differentials. (a) No within-trial differences; and (b) within-trial differences.
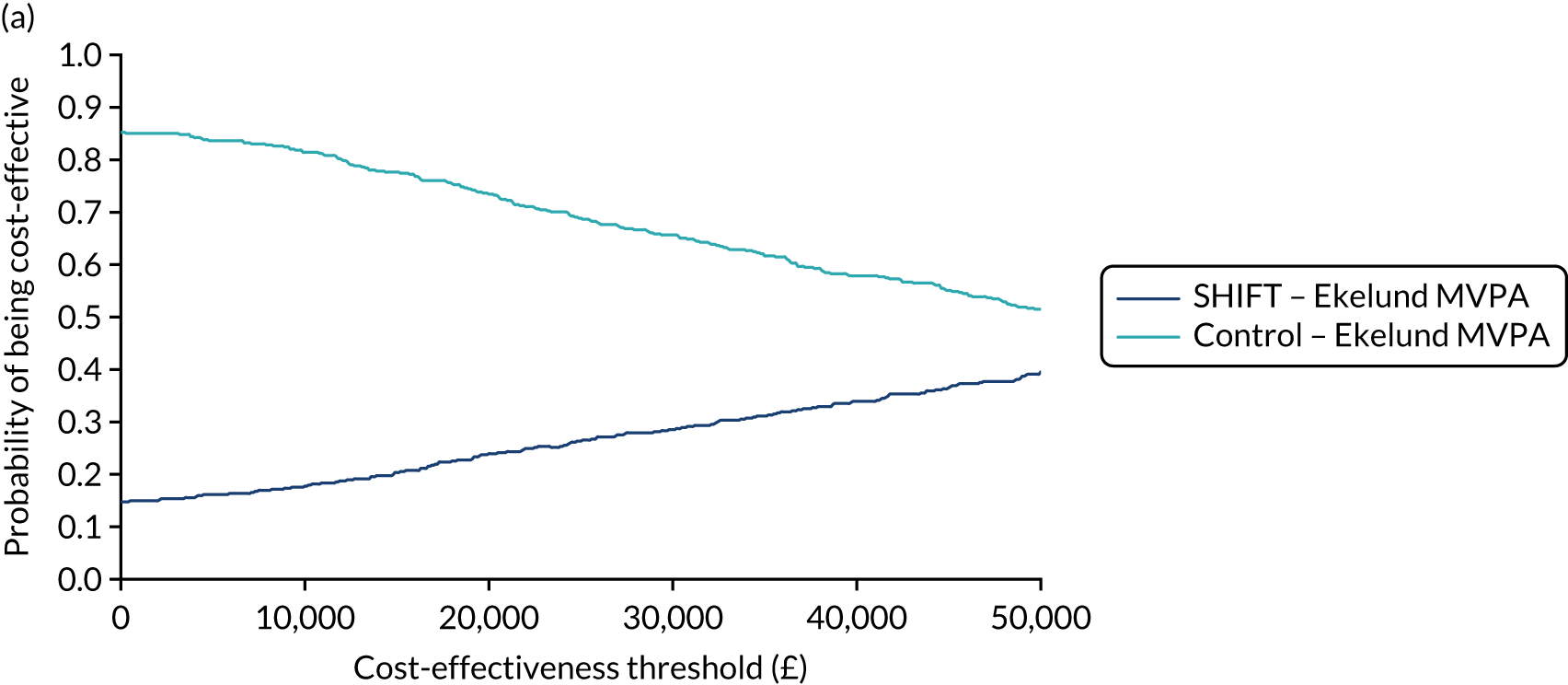

FIGURE 9.
Cost-effectiveness acceptability curves using Ekelund et al. 120 all-cause mortality estimates with changes to sedentary minutes/day, including and excluding within-trial differentials. (a) No within-trial differences; and (b) within-trial differences.
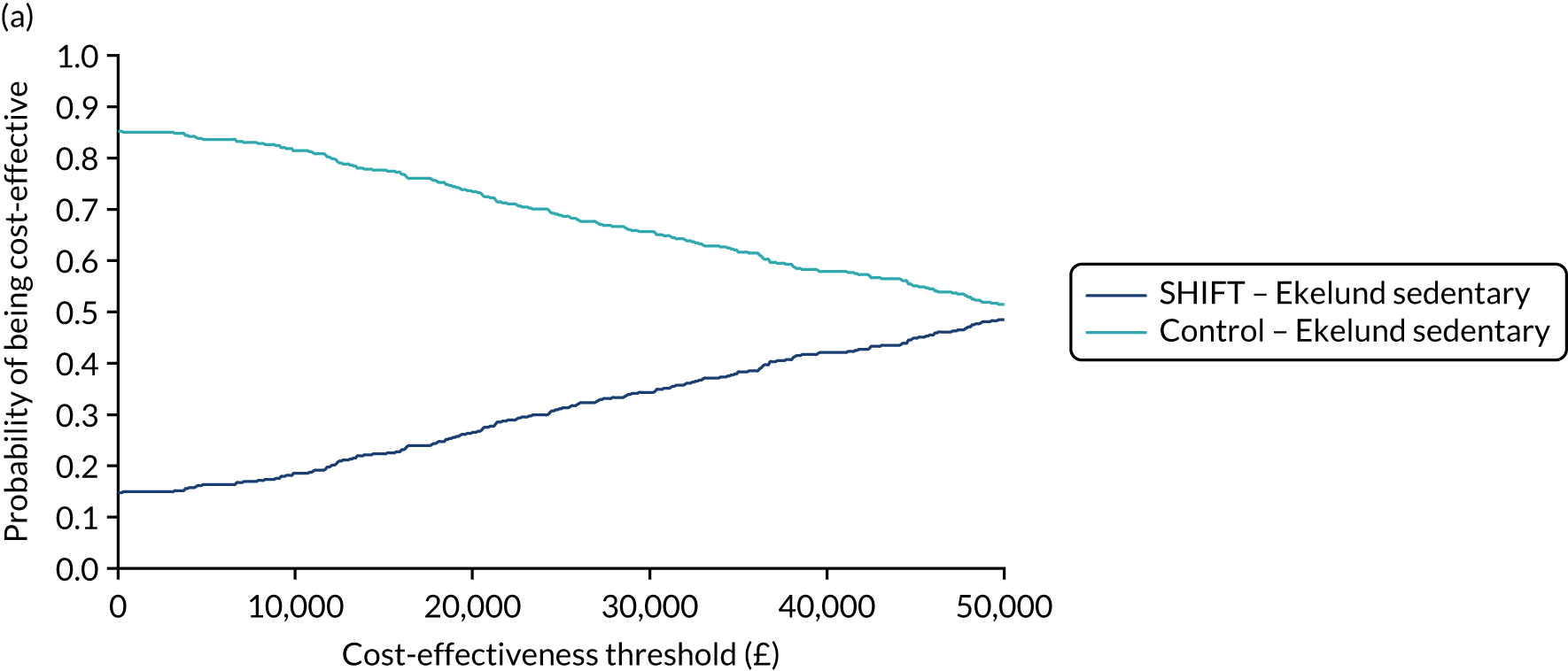
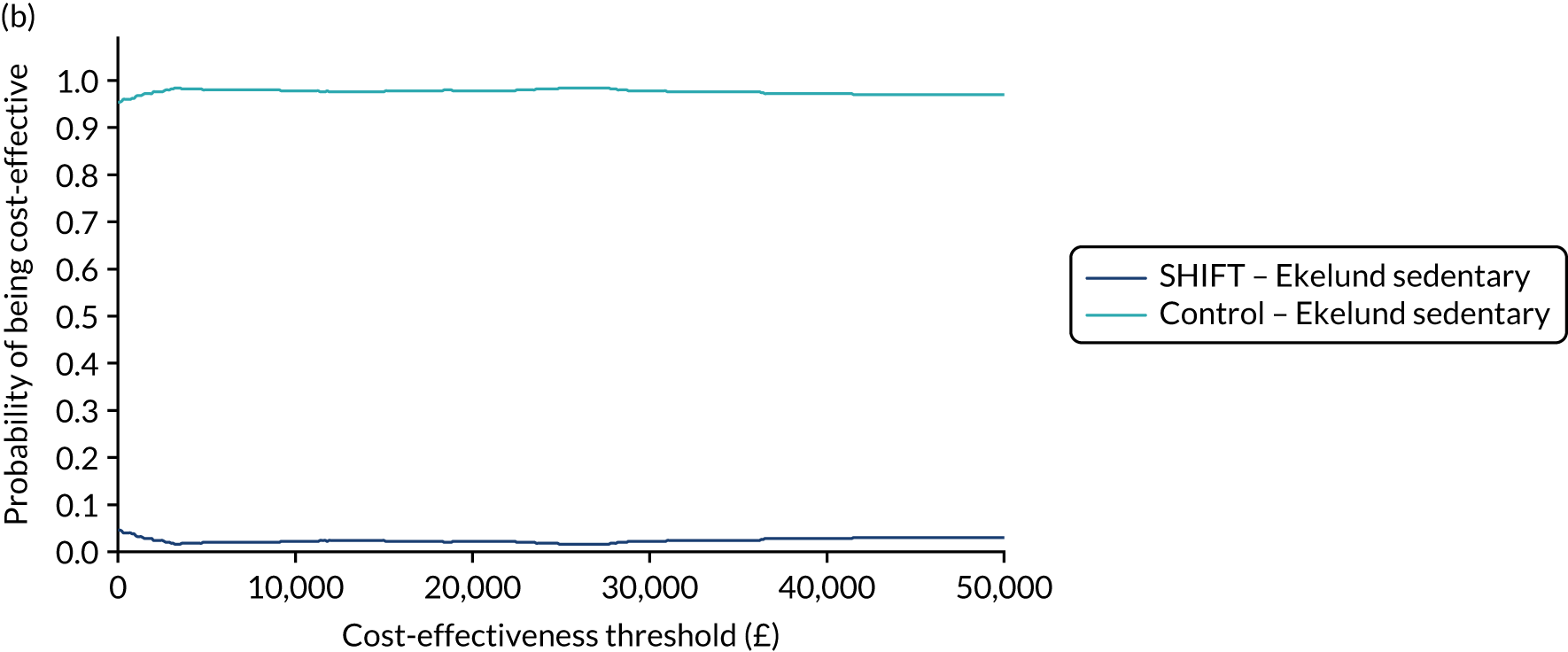
FIGURE 10.
Cost-effectiveness acceptability curves using MOVES model with changes to sedentary minutes/day, including and excluding within-trial differentials. (a) No within-trial differences; and (b) within-trial differences.
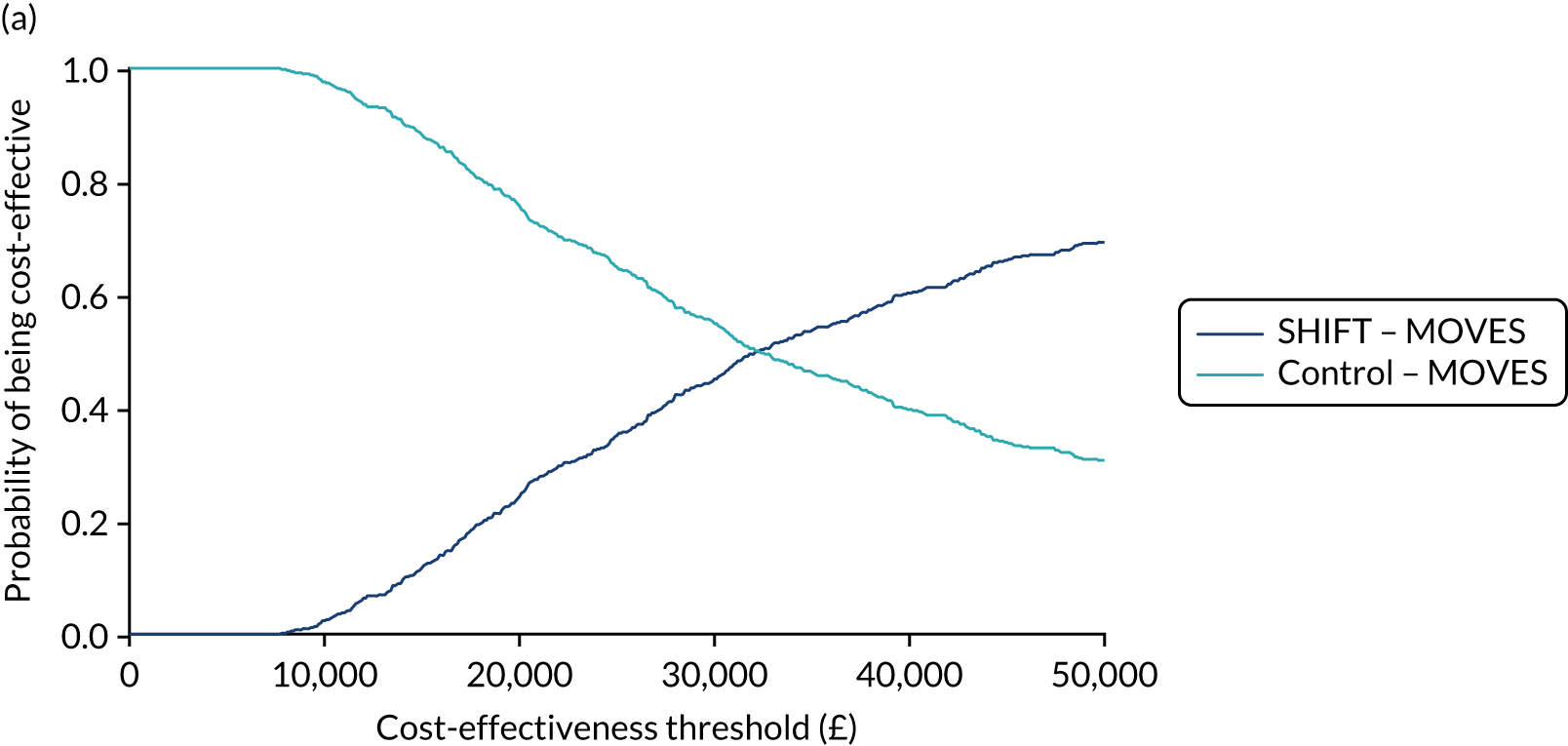
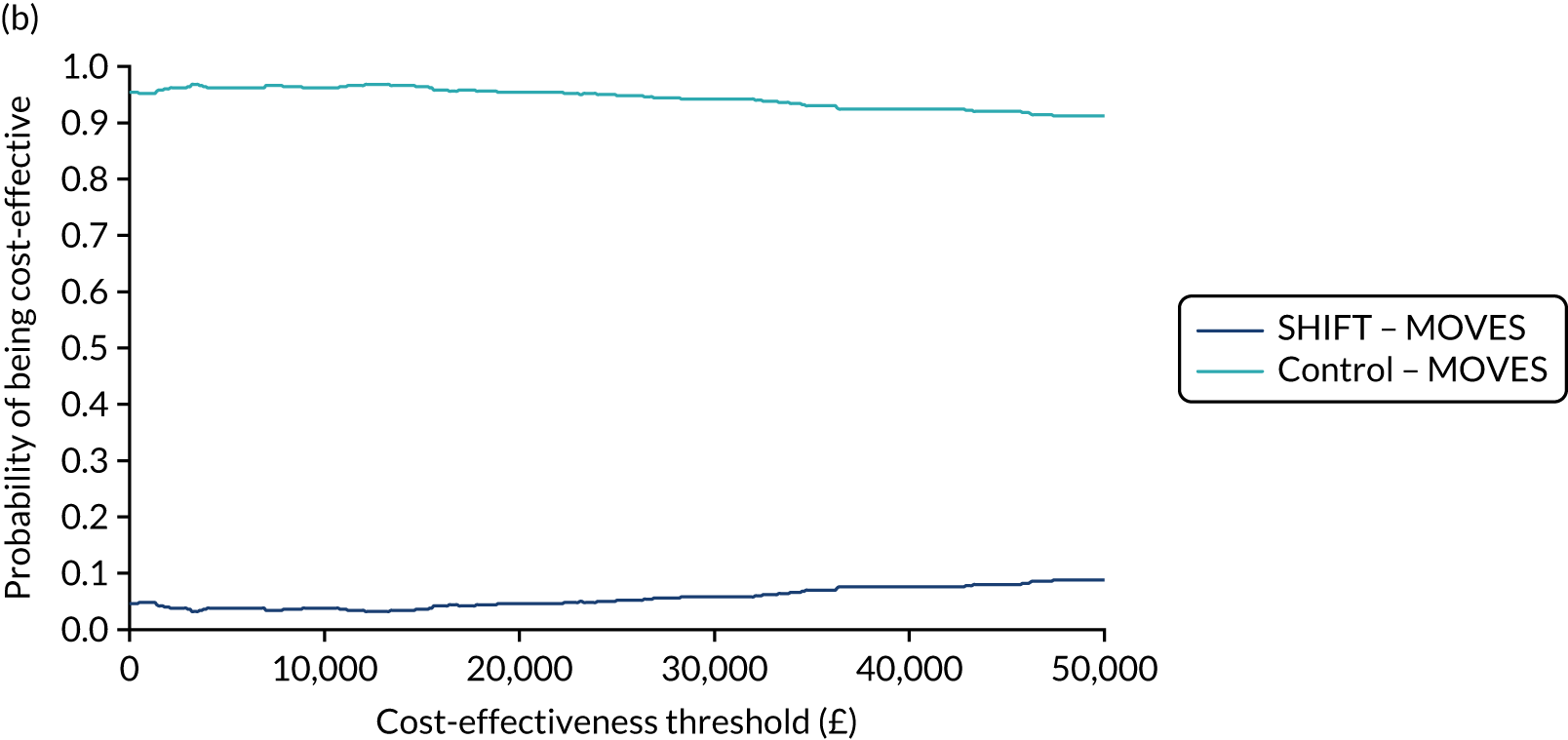
List of abbreviations
- 24/7
- 24 hours a day, 7 days a week
- app
- application
- AUDIT
- Alcohol Use Disorders Identification Test
- BMI
- body mass index
- CI
- confidence interval
- CILT
- Chartered Institute of Logistics and Transport
- CONSORT
- Consolidated Standards of Reporting Trials
- CPC
- Certificate of Professional Competence
- CRF
- case report form
- CVD
- cardiovascular disease
- DESMOND
- Diabetes Education and Self-Management for Ongoing and Newly Diagnosed
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FFQ
- Food Frequency Questionnaire
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HbA1c
- glycated haemoglobin
- HDL-C
- high-density lipoprotein cholesterol
- HGV
- heavy goods vehicle
- HRQoL
- health-related quality of life
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- INHB
- incremental net health benefit
- IQR
- interquartile range
- ITT
- intention to treat
- LDL-C
- low-density lipoprotein cholesterol
- MEQ
- Morningness–Eveningness Questionnaire
- MOVES
- Model for estimating the Outcomes and Values in the Economics of Sport
- MRC
- Medical Research Council
- MVPA
- moderate or vigorous physical activity
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- OFER
- Occupational Fatigue Exhaustion Recovery
- PPI
- public and patient involvement
- QALY
- quality-adjusted life-year
- QRISK3
- Cardiovascular Risk Score
- RCT
- randomised controlled trial
- SAP
- statistical analysis plan
- SCT
- social cognitive theory
- SD
- standard deviation
- SHIFT
- Structured Health Intervention For Truckers
- SMART
- specific, measurable, achievable, relevant, time bound
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/PNOY9785).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
