Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 12/178/17. The contractual start date was in November 2014. The final report began editorial review in December 2019 and was accepted for publication in August 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Allen et al. This work was produced by Allen et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Allen et al.
Chapter 1 Introduction
Paediatric mortality rates in the UK are among the highest in Europe. 1 Although perinatal events account for a major part, there continues to be evidence to suggest that missed deterioration and difference in hospital performance contribute. 2–4 More than a decade ago, the Confidential Enquiry into Maternal and Child Health highlighted identifiable failures in a child’s direct care in just over 25% of deaths; for an additional 43% of deaths, potentially avoidable factors were highlighted. 2 More than 700,000 children are admitted to hospital overnight in the UK annually, with 8000 of these admitted to paediatric intensive care units (PICUs) as an emergency. 5 Half of these are from wards in the same hospital, suggesting that patients deteriorated acutely or had a cardiopulmonary arrest. These missed opportunities to detect and intervene in hospital are instances of failures in care, with physiological, psychological and social costs to the child and family. 6,7 There is significant short-term added cost to the NHS from the rising cost of litigation (£1.1B). 8,9 For a society that values its NHS, this is widely recognised to be a situation that needs to be reversed. It is estimated that 1951 child deaths would need to be prevented to compare with the best performers in Europe. 10
Track-and-trigger tools (TTTs), otherwise known as early warning scores, have been a popular response to address missed deterioration in both adults and children. 11 A TTT consists of sequential recording and monitoring of physiological, clinical and observational data. 12 When a certain score or trigger is reached, this directs a clinical action, including, but not limited to, altered frequency of observation, a senior clinical review or more appropriate treatment or management. Tools may be paper based or electronic, and monitoring can be automated or undertaken manually by staff. 13 Research in the adult care context has shown that acute in-hospital deterioration is often preceded by a period of physiological instability, which, when recognised, provides an opportunity for earlier intervention, and improved outcome. 14,15 As a result, the Royal College of Physicians endorsed the implementation of a national early warning TTT for adults to standardise the assessment of acute illness severity, predicting that 6000 lives would be saved. 16
Standardising the use of TTTs to detect deterioration in children has been more challenging. Variation in accepted physiological normal parameters for respiratory rate, heart rate and blood pressure across the age range makes it challenging to develop a standardised tool suitable for generic application for all hospitalised children. Some single-site studies have reviewed the performance of individual TTTs, with preliminary data on the sensitivity of different cut-off points for physiological measurements. 17–19 However, it was difficult to prove an ‘effect’ based on the outcome measures described, because the event rate of in-hospital cardiac arrest or death is low. Subsequent systematic reviews demonstrated potential benefits but no clear improvement in patient outcomes. 20,21 Furthermore, a 2014 review of paediatric track-and-trigger tools (PTTTs) throughout Great Britain found that 85% of units were using a tool; however, there was huge variability in the tools used, and most were unpublished and unvalidated. 22 The ad hoc use of unvalidated TTTs and variance in organisational capacity to respond to a deteriorating child has been felt to represent a serious clinical risk.
The Paediatric early warning system Utilisation and Morbidity Avoidance (PUMA) study was commissioned by the National Institute for Health Research (NIHR) under a call for studies that generated interventions to reduce in-hospital mortality. The original aims of the study were to:
-
identify, through a systematic review of the literature, the evidence for the core components of a PTTT
-
identify, through a systematic review of the literature, the evidence for the core components of an effective paediatric early warning system.
-
identify, through a systematic review of the literature, the contextual factors that are consequential for PTTT and paediatric early warning system effectiveness
-
develop a PTTT implementation package
-
evaluate the ability of the PTTT to identify serious illness and reduce clinical events by examining core outcomes
-
identify the contextual factors that influence PTTT effectiveness
-
identify the key ingredients of successful implementation and normalisation.
The findings of the systematic reviews, which are presented in detail in Chapter 3, did not support an exclusive focus on PTTTs. The quality of studies evaluating PTTTs was generally low and there was limited evidence of the effectiveness of PTTTs in reducing adverse events in hospitalised children. Most of the studies reviewing the effectiveness of PTTTs also simultaneously implemented changes to the system, making it difficult to disentangle effects of the tool from system changes. However, the systematic reviews provided evidence of multiple failure points in systems for detecting and responding to deterioration, particularly around detection, preparation and action. There was also emerging evidence on common issues in traditional approaches to implementation and improvement, which often are solution driven, fail to engage the expertise of those responsible for implementing in practice, and focus on the use of a tool or intervention, rather than considering how an issue might be addressed in context.
As a result of the systematic reviews, the focus of the PUMA study shifted from PTTTs to a system-wide approach. The revised aims were to:
-
identify, through a systematic review of the literature, the evidence for the core components of an effective PTTT and paediatric early warning systems
-
identify, through a systematic review of the literature, the contextual factors that are consequential for PTTT and paediatric early warning system effectiveness
-
develop and implement an evidence-based paediatric early warning system improvement programme (i.e. the PUMA programme)
-
evaluate the effectiveness of the PUMA programme by examining changes in clinical practice and core outcomes trends
-
identify the key ingredients of successful implementation and normalisation of the PUMA programme.
Drawing on evidence from improvement and implementation literature, the PUMA programme was underpinned by a novel approach, developed as part of the study, that aimed to create a better understanding of system strengths and weaknesses in each setting, to capitalise on the expertise of those with knowledge of how the system worked, and to focus on the goals (improving detection and response to paediatric deterioration), rather than prescribing specific interventions.
Chapter 2 describes the study methods. Chapter 3 presents the key findings from the systematic reviews. Chapter 4 describes the development and implementation of the PUMA programme. Chapters 5–8 describe the four case studies, drawing on both quantitative and qualitative data. Chapter 9 provides a cross-comparative analysis of the effects of the PUMA programme across the four sites. Chapter 10 presents the findings of the parallel process evaluation of the delivery of and response to the PUMA programme and key areas of learning. Chapter 11 summarises the findings of the PUMA study, considers their implications for policy, practice and research, and signposts next steps.
Chapter 2 Methods
This chapter outlines the study methods. Parts of this chapter are reproduced from Thomas-Jones et al. 23 © The Authors. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Parts of this chapter are reproduced from Jacob et al. 13 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are reproduced from Trubey et al. 24 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Ethics approval was granted on 13 April 2015 by the National Research Ethics Service Committee South West, registration number 15/SW/0084 (see Report Supplementary Material 1).
Study design
The research was a prospective, mixed-methods, before-and-after study, divided into two workstreams:
-
Workstream 1 involved the development of an evidence-based paediatric early warning system improvement programme (the PUMA programme), drawing on three systematic reviews of the literature. 13,24
-
Workstream 2 involved the implementation and prospective evaluation of the PUMA programme in four UK hospitals, with an embedded process evaluation. Evaluation was conducted both quantitatively (comparing trends in rates of adverse outcomes on inpatient wards before, during and after implementation) and qualitatively (through ethnographic observations and interviews, and evaluating the implementation process and clinical practice before and after implementation).
Theoretical framework
Translational mobilisation theory
Translational mobilisation theory (TMT) was deployed in order to think systematically about paediatric early warning systems and the sociotechnical contexts into which an improvement programme would be introduced. 25,26 TMT is a practice theory that describes projects of goal-oriented collective action in conditions of emergence and complexity. The ‘project’ is the basic unit of analysis in TMT and refers to an institutionally sanctioned sociomaterial network of time-bounded co-operative action and actors that follows a trajectory in time and space: in this case the detection of physiological deterioration and timely intervention in the care of sick children. TMT directs attention to the institutional contexts in which projects are progressed and that provide the sociomaterial resources that condition collective action and the mechanisms through which projects are mobilised. TMT was deployed to identify the core components and mechanisms of action central to achieving the goal of detecting and acting on deterioration in hospitalised children, the elements of context that are most salient to enacting the goal and the processes by which that may be achieved.
Normalisation process theory
Normalisation process theory (NPT), which has a high degree of conceptual affinity with TMT, provided an additional theoretical lens to inform the evaluation of implementation processes. 27 NPT is concerned with ‘how and why things become, or don’t become, routine and normal components of everyday work’28 and it defines four mechanisms that shape the social processes of implementation, embedding and integrating ensembles of social practices. These are inter-related and dynamic domains, and include:
-
coherence (the extent to which an intervention is understood as meaningful, achievable and desirable)
-
cognitive participation (the enrolment of those actors necessary to deliver the intervention, which, for our purposes, can be human and non-human)
-
collective action (the work that brings the intervention into use)
-
reflexive monitoring (the ongoing process of adjusting the intervention to keep it in place).
Settings
A convenience sample of four UK hospitals was selected to represent inpatient units of varying size: two tertiary centres with integrated PICUs (Alder Hey Children’s Hospital and Noah’s Ark Children’s Hospital for Wales) and two large district general hospitals (DGHs) without a PICU (Arrowe Park Hospital and Morriston Hospital) (Table 1). Two hospitals had a PTTT in place for the duration of the PUMA study and two did not.
| Site | Number of beds (excluding PICU) | Annual inpatients (excluding day cases) (n) | PTTT in use |
|---|---|---|---|
| Alder Hey Hospital, Liverpool | 337 inpatient, 15 HDU | > 200,000 | Yes |
| Arrowe Park Hospital, Wirral | 32 inpatient, 2 HDU | 2500 | Yes |
| Noah’s Ark Children Hospital for Wales, Cardiff | 61 inpatient, 4 HDU | 23,000 | No |
| Morriston Hospital, Swansea | 38 inpatient, 7 HDU | 7500 | No |
Patient and public involvement
The project’s patient and public involvement (PPI) liaison officer, Jenny Preston, convened a PPI group, consisting of four parents with direct experience of a child deteriorating in hospital.
The group met at several stages of the project. Table 2 summarises those meetings, their objectives and outputs, and the way in which the group’s feedback was incorporated into the project. More detailed outputs of those meetings and reflections on the challenges of integrating PPI into the project are considered in Appendix 1, Table 19.
| Meeting date | Objectives | Summary of feedback | Summary of changes made |
|---|---|---|---|
| November 2014 |
|
|
|
| December 2015 |
|
As a result of the systematic review, the group was informed that the PUMA study team had revised the original aims of the study away from developing a single PTTT to the development of a paediatric early warning system improvement programme |
|
| March 2016 |
|
Suggested changes to SHINE |
|
| June 2018 |
|
Suggested changes to language, structure and content of FFT |
|
| March 2019 |
|
Suggested changes to funding proposal for project focusing on family involvement in detection of deterioration |
|
Workstream 1: evidence reviews
Quantitative systematic reviews
Two systematic reviews aimed to assess in depth the evidence base for the validity of PTTTs for predicting inpatient deterioration and the effectiveness of broader early warning systems at reducing instances of mortality and morbidity in paediatric settings. The review questions were as follows:
-
Review 1: how well validated are existing PTTTs and their component parts for predicting inpatient deterioration?
-
Review 2: how effective are paediatric early warning systems (with or without a PTTT) at reducing mortality and critical events?
Search strategy
A comprehensive search was conducted across a range of databases to identify relevant studies in the English language. Published and unpublished literature was considered if publicly available, as were studies in press. The following databases were searched from inception to May 2018: British Nursing Index, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, EMBASE, Health Management Information Consortium (HMIC), MEDLINE, MEDLINE In-Process, Scopus and Web of Knowledge (Science Citation Index). To identify additional papers, either published or unpublished, or research reported in the grey literature, a range of relevant websites and trial registers were searched, including ClinicalTrials.gov. To identify published papers that had not yet been catalogued in the electronic databases, recent editions of key journals were hand-searched. The search terms included ‘early warning scores’, ‘alert criteria’, ‘rapid response’, ‘track and trigger’ and ‘early medical intervention’. See Appendix 2, Tables 20 and 21, for the search strategies and results.
Eligibility screening and study selection
Population, intervention, control/comparison, outcomes (PICO) parameters guided inclusion criteria for the validation and effectiveness studies (see Appendix 3, Tables 22 and 23). Papers reporting development of validation of a PTTT were included for review 1, whereas papers reporting the implementation of any broader ‘paediatric early warning system’ (with or without a PTTT) were eligible for review 2. Both reviews were limited to studies that involved inpatients aged 0–18 years. Outcome measures considered were mortality and critical events, including: unplanned admission to a higher level of care, cardiac arrest, respiratory arrest, medical emergencies requiring immediate assistance, children reviewed by PICU staff on the ward (in specialist centres) or reviewed by external PICU staff (for non-specialist centres), acuity at PICU admission and PICU outcomes. A range of study designs was considered for both reviews.
Two of the review authors independently screened the titles and abstracts yielded in the search. Full texts were reviewed independently by six reviewers against the above eligibility criteria and were assigned to the relevant review question if included. Reasons for exclusion were recorded. Separate data extraction forms were developed for validation and effectiveness studies. The forms had common elements (study design, country, setting, study population, description of the PTTT or early warning system, statistical techniques used, outcomes assessed). Additional data items for validation studies included the items in the PTTT, modifications to the PTTT from previous versions, predictive ability of individual items and the overall tool, sensitivity and specificity and inter-rater and intrarater reliability. Effectiveness studies included an assessment of outcomes in terms of mortality and various morbidity variables. Two reviewers carried out data extraction; discrepancies were resolved by discussion. For effectiveness studies, effect sizes and 95% confidence intervals (CIs) were calculated or reported as risk ratios (RRs) or odds ratios (ORs) as appropriate, with p-values reported to assess statistical significance. Data analysis was conducted using an online medical statistics tool.
Quality appraisal
The methodological quality and risk of bias were assessed for each included study using a modified version of the Downs and Black rating scale29 (see Appendix 4, Tables 24 and 25).
Qualitative systematic review
A third, qualitative, systematic review addressed the following question:
-
What sociomaterial and contextual factors are associated with successful or unsuccessful paediatric early warning systems (with or without PTTTs)?13
Study design
We performed a hermeneutic systematic review of the relevant literature. A hermeneutic systematic review is an iterative process, integrating analysis and interpretation of evidence with literature searching and is designed to develop a better understanding of the field. 30 The popularity of the method is growing in health services research, for which it has value in generating insights from heterogeneous literatures that cannot be synthesised through standard review methodology, and which would otherwise produce inconclusive findings. 21,31 The purpose of the review was not exhaustive aggregation of evidence, but to develop an understanding of the social, material and contextual factors associated with successful or unsuccessful paediatric early warning systems.
Theoretical framework
Translational mobilisation theory and NPT informed the data extraction strategy, interpretation of the evidence and the development of a propositional model of the minimal conceptual requirements of a paediatric early warning system. 25,27,28,32
Focus of the review
The literature in this field identifies four integrated components that work together to provide a safety system for at-risk patients: (1) the afferent component, which detects deterioration and triggers a response; (2) the efferent component, which consists of the people and resources providing a response; (3) a process improvement component, which includes system auditing and monitoring; and (4) an administrative component focusing on organisational leadership and education required to implement and sustain the system. 33 Our focus was limited to the afferent components of the system.
Stages of the review
Stage 1: scoping the literature
Literature was identified through a recent scoping review,34 team members’ knowledge of the field, hand-searches and snowball sampling techniques. The purpose was to (1) inform our review question and eligibility criteria and (2) identify emerging themes and issues. Although we drew on several reviews of the literature, we always consulted original papers. 20,34,35 Data were extracted using data extraction template 1 (see Appendix 5) and analysed to produce a provisional conceptual model of the core components of paediatric early warning systems. Additional themes of relevance were identified: family involvement, situational awareness, structured handover, observations and monitoring, and the impact of electronic systems and new technologies.
Stage 2: searching for the evidence
We undertook systematic searches of the paediatric and adult early warning system literature (the goals and mechanisms of collective action in detecting and acting on deterioration are the same) (searches 1 and 2). For the adult literature, we used the same search strategies but added a qualitative filter to limit the scope to studies most likely to yield the level of sociomaterial and contextual detail of value to the review. Literature informing additional areas of interest was located through a combination of systematic searches and hand-searches. Systematic searches (searches 3 and 4) were undertaken in areas for which we anticipated locating evidence of the effectiveness of specific interventions to strengthen early warning systems. Theory-driven searches reflected the conceptual requirements of the developing analysis.
Four systematic searches were conducted across a range of databases from 1995 to September 2016 to identify relevant studies in English-language papers reporting on:
-
paediatric early warning systems
-
adult early warning systems
-
interventions to improve situational awareness
-
structured communication tools for handover and handoff.
Detailed information on the search methodology can be found in Appendix 6.
Theory-driven searches were conducted in the following areas:
-
family involvement
-
observations and monitoring
-
the impact of electronic systems.
These were a combination of exploratory, computerised, snowball and hand-searches. As the analysis progressed, we continued to review new literature on early warning systems as it was published.
After removing duplicates, 5256 references were identified for screening. Papers were screened by title to assess eligibility and then by full text to assess relevance for data extraction. Searches 1 and 2 were screened by two researchers; searches 3 and 4 were screened by the lead reviewer. Grey literature was excluded to keep the scale of the review manageable.
Stage 3: data extraction and appraisal
Data extraction template 2 (see Appendix 7) was applied to all papers included in the review. Evidential fragments and partial lines of inquiry formed the unit of analysis, rather than whole papers. Fragments were drawn from papers that were assessed for quality according to study type and the contribution made to the developing analysis. Data extraction and quality appraisal were undertaken concurrently and checked by a second reviewer. Disagreements and areas of uncertainty were resolved by discussion.
Intervention development
Building on the findings of the qualitative systematic review, the intervention (the PUMA programme) was developed iteratively and modified in the light of experience in use. Chapter 4 describes the intervention and its development in detail.
Workstream 2: implementation and prospective evaluation of the PUMA programme
Overview
The study deployed an interrupted time series (ITS) design, in conjunction with ethnographic case studies, to evaluate changes in practice and outcomes over time. Ethnographic methods were also deployed to evaluate implementation processes.
The ITS analysis involved tracking aggregate monthly rates of mortality and morbidity outcomes for up to 18 months before implementation of the PUMA programme, for 12 months during implementation, and for a further 12 months during the post-implementation period.
Embedded ethnographic case studies enabled evaluation of each site’s paediatric early warning system prior to implementation of the PUMA programme and the impact of the PUMA programme on each hospital’s paediatric early warning system post implementation. Ethnographic approaches were also deployed in a parallel process evaluation.
Quantitative evaluation
The quantitative evaluation of the PUMA programme involved tracking monthly aggregate outcomes at each of the four hospitals over a period of up to 42 months (May 2015–October 2018). The purpose was to evaluate the PUMA programme’s effect on measures of inpatient deterioration over time. This section gives details on the way in which data were collected from sites over the study, the outcome measures used to evaluate deterioration and the way in which data were analysed.
Data collection
A customised online PUMA database was created for site staff to upload monthly data forms. Staff were able to log in to the database via a password-protected home screen and were only ever able to access and submit forms for their own site. Data were uploaded by either principal investigators (PIs) or research nurses, and all staff responsible for entering data at each site were trained in using the database prior to entering data. Each monthly submission was quality assessed in real time by a member of the PUMA study team to allow timely resolution of any data queries or missing values.
Outcome measures
A provisional set of outcome measures was drawn up based on preliminary findings from systematic reviews 1 and 2. As part of the systematic reviews, an evaluation was conducted of the most commonly used outcome metrics reported in the literature for assessment of the validity and effectiveness of PTTTs and paediatric early warning systems. The feasibility of collecting these outcomes at each site was explored through preliminary piloting work, prior to commencement of data collection in August 2015.
Appendix 8, Table 26, shows the final outcomes that were selected as the most suitable proxies for inpatient deterioration, and the definition of each outcome that was agreed with the four sites. It is important to note that these cannot be used, in any way, to infer the processes leading to that outcome; for example, it is impossible to determine if the deaths were the result of missed deterioration or an unavoidable consequence of a disease process.
Primary outcome measure
For the primary outcome, we chose a composite outcome metric (‘adverse events’), representing the aggregate number of children in a given month that experienced at least one of the following events:
-
mortality
-
cardiac arrest
-
respiratory arrest
-
unplanned admission to a PICU
-
unplanned admission to a high-dependency unit (HDU).
Children who experienced more than one of these adverse events were counted only once, to avoid double-counting. The primary outcome was expressed per 1000 patient bed-days, using the aggregate number of events and the denominator (total number of patient-days) for each month.
Secondary outcome measures
Secondary outcome measures were the aggregate number of children experiencing the following adverse events each month, with each event recorded individually as a separate outcome:
-
mortality
-
cardiac arrest
-
respiratory arrest
-
unplanned admission to a PICU
-
unplanned admission to a HDU
-
other medical emergencies requiring immediate assistance
-
reviews by PICU staff.
Secondary outcome measures were also expressed as a rate per 1000 patient bed-days.
Timing of outcome assessments
Primary and secondary outcomes, and the patient bed-day denominator, were entered by site staff into the PUMA database on a monthly basis at each of the four sites. Table 3 summarises the various data points for each of the hospitals, for the pre-implementation, implementation and post-implementation stages.
| Outcome | Pre implementation (18 months) | Implementation (12 months) | Post implementation (12 months) | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | |||||||||||||||||||||||||||||||||||||||
| May | June | July | August | September | October | November | December | January | February | March | April | May | June | July | August | September | October | November | December | January | February | March | April | May | June | July | August | September | October | November | December | January | February | March | April | May | June | July | August | September | October | |
| Primary (composite) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Mortality | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Cardiac arrest | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Respiratory arrest | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Unplanned PICU admission | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Unplanned HDU admission | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Other medical emergency | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| PICU review | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Non-ICU patient bed-days | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
Sample size calculation
We used a simulation-based approach to calculate power, as it was challenging to calculate an accurate sample size. 36,37 Our initial calculation was based on the original aim of the study to implement a PTTT in each site.
We obtained historical data for one tertiary centre (Alder Hey) and one DGH (Morriston Hospital). These data showed a 1% prevalence (i.e. 10 events per 1000 patients) for unplanned transfers to a PICU. Tibballs et al. 38 have previously shown that the implementation of paediatric calling criteria with a rapid response team resulted in a RR of 0.65 in terms of total avoidable hospital mortality. We assumed that the PUMA intervention might result in a similar RR of 0.65. 38 The monthly recorded data for unplanned admission in both hospitals were used to estimate monthly death rate pre and post intervention. From obtained data, the estimated effect size, mean difference and common standard deviation were 2.8, 2.0 and 0.7, respectively. We estimated that we would have 90% power with a total of 24 months of observations (12 pre and 12 post intervention) for an effect size of at least 2. 37
The initial aims of the study were changed from the implementation of PTTT to a complex intervention: the PUMA programme. We retained the focus on collecting data 12 months pre and 12 months post intervention, but allowed 12 months for phase-in of the intervention, to give a total of 36 months. During the lifetime of the project, we were able to collect up to 6 additional months of data retrospectively for the pre-intervention period. This gave us 42 months of data and increased the sample size.
Data analysis
An ITS analysis was used to assess the effectiveness of the PUMA programme at reducing rates of inpatient deterioration over time. An ITS approach allows exploration of the longitudinal effect of the intervention through regression modelling. This approach controls for pre-intervention trends and assesses the extent to which an intervention ‘interrupted’ the trajectory of this trend. 39 See Appendix 9, Figure 27, for further details about an ITS approach.
The most common approach to an ITS analysis is to compare trends across two separate time periods: a pre- and a post-intervention phase. Typically, the intervention being studied is relatively clear-cut, for example a change in national policy, which would be expected to have an immediate effect (i.e. level change) on the outcome being studied. In this study, however, we expected that the complete implementation of the PUMA programme was likely to take longer, but that we might be able to observe gradual changes in measures of inpatient deterioration. Therefore, we decided to investigate both the short-term effect of the PUMA programme (by comparing pre-intervention levels in outcomes with implementation and post-intervention period levels in outcomes) and the longer-term effect (by exploring trends in outcomes during the implementation and after the intervention, in the post-intervention period, when teams would have had some time to embed local initiatives). Data from each hospital were analysed separately as independent case studies.
Primary analysis
There are different approaches to conducting an ITS. 39 We elected to fit a segmented linear regression on data from each hospital using an autoregressive integrated moving average (ARIMA) method to analyse the primary and secondary outcomes. 39,40 The residual plot corresponding to each model was investigated to check the assumptions of linear regression. The Durbin–Watson statistic, together with autocorrelation and partial autocorrelation function, was used to identify the order of autocorrelation and moving average.
To model the trajectory for all pre-implementation, implementation and post-implementation periods, two intervention start points were considered:
-
November 2016, the start of the implementation period (when sites began their improvement initiatives)
-
November 2017, the start of the post-implementation period.
Prior to observing the data, we decided to use one of the most commonly used impact models that allows immediate (level) and trend (slope) change after introducing or completing the implementation. 41 Any statistically significant change in either level or trend would imply that the intervention (i.e. the PUMA programme) had had an effect on outcomes.
For some secondary outcomes, we observed several zero monthly counts, particularly for the DGH sites. 42 For these cases, it was easy neither to transform the time series into a stationary series nor to detect a trend. Depending on the number of zero-count months, we adopted different mitigating strategies. If there were not many zero count months (e.g. a maximum of two per time period), an indicator variable was added to the model to account for its effect. Alternatively, if there were more zero counts per time period, we combined data into blocks of 2 months and, when possible, the trajectory was modelled. Otherwise, if there were still too many zero-count months and we were unable to fit a model, trajectory of the outcome only was plotted.
Exploratory and sensitivity analyses
In addition to the main analysis of the primary outcome (conducted using three time periods), we also conducted two sets of additional exploratory analyses to explore the data using different conceptual approaches to designate pre- and post-intervention time periods for analysing changes in trends.
In the first analysis, we simply excluded data collected during the 12-month implementation period (November 2016–October 2017), to create a binary pre- and post-intervention comparison of slopes. In the second analysis, we explored the pattern of changes in level and slope from the start of implementation phase until the end, given the potential for the different local initiatives to exert their effects over different time periods in different sites.
Finally, we conducted a sensitivity analysis of the primary outcome for each site, fitting a segmented Poisson regression model to the data. See Appendix 10, Tables 27–34 and Figure 28, for details.
Qualitative evaluation: ethnographic case studies
Overview
Embedded ethnographic case studies were undertaken on one ward in each of the four hospitals. Qualitative methods (observation, interviews and documentary analysis) were deployed to undertake a pre- and post-implementation review of the local paediatric early warning system in everyday clinical practice.
The aim of the pre-implementation stage was to understand current practice at baseline: evaluating the paediatric early warning system in practice and observing how the system was shaped by local context. The aim of the post-implementation stage was to evaluate any changes to the paediatric early warning system after implementation of the PUMA programme, in order to understand the impact of the intervention.
Case study wards
In contrast to the quantitative analysis, which summarised aggregate outcome measures at a hospital-wide level, the ethnographic case studies were conducted on a single ward. Table 4 summarises the wards selected at each site.
| Site | Number of paediatric inpatient wards | Case study ward selected |
|---|---|---|
| Alder Hey Children’s Hospital | 10 | Cardiac surgical ward |
| Arrowe Park Hospital | 1 | General paediatric ward |
| Noah’s Ark Children’s Hospital | 8 | General medical ward |
| Morriston Hospital | 2 | General medical ward |
Data collection
Table 5 shows the qualitative data collected at each case study site. In both the pre- and post-implementation phase, data were generated through:
-
ethnographic observation of everyday practice (by shadowing individuals – nurses, doctors, support staff – and discussing their practice, and attending key meetings and events)
-
interviews with clinical team members, service managers, PIs and family members or carers
-
analysis of relevant documents and artefacts.
| Site | Pre-implementation data collection (from March 2015 to October 2016) | Post-implementation data collection (from November 2017 to October 2018) |
|---|---|---|
| Alder Hey |
|
|
| Arrowe Park |
|
|
| Noah’s Ark |
|
|
| Morriston |
|
|
Observations were conducted at different times of day/night and on different days of the week, including weekends, to ensure that a range of time periods were covered. Our concern was with understanding the network of actors (people, processes, technologies and artefacts) and their inter-relationships in each paediatric early warning system. Drawing on the theoretical framework and the systematic review findings, we developed a template to guide the observations and interviews as shown in Figure 1 (see Report Supplementary Material 2). However, data generation was not absolutely constrained by this; rather, in each case, the strategy was to ‘follow the actors’ (human and non-human). This ensured that there was a consistent approach across case studies to facilitate comparative analyses, but flexibility to modify data generation in response to the singular features of each site. We focused on what participants did, the tools they used, the concepts they deployed and the factors that facilitated and constrained action. Adopting a TMT lens directed attention to the sociomaterial relationships within each paediatric early warning system and how the local institutional context conditioned the possibilities for action. 25,26
FIGURE 1.
Observations template.
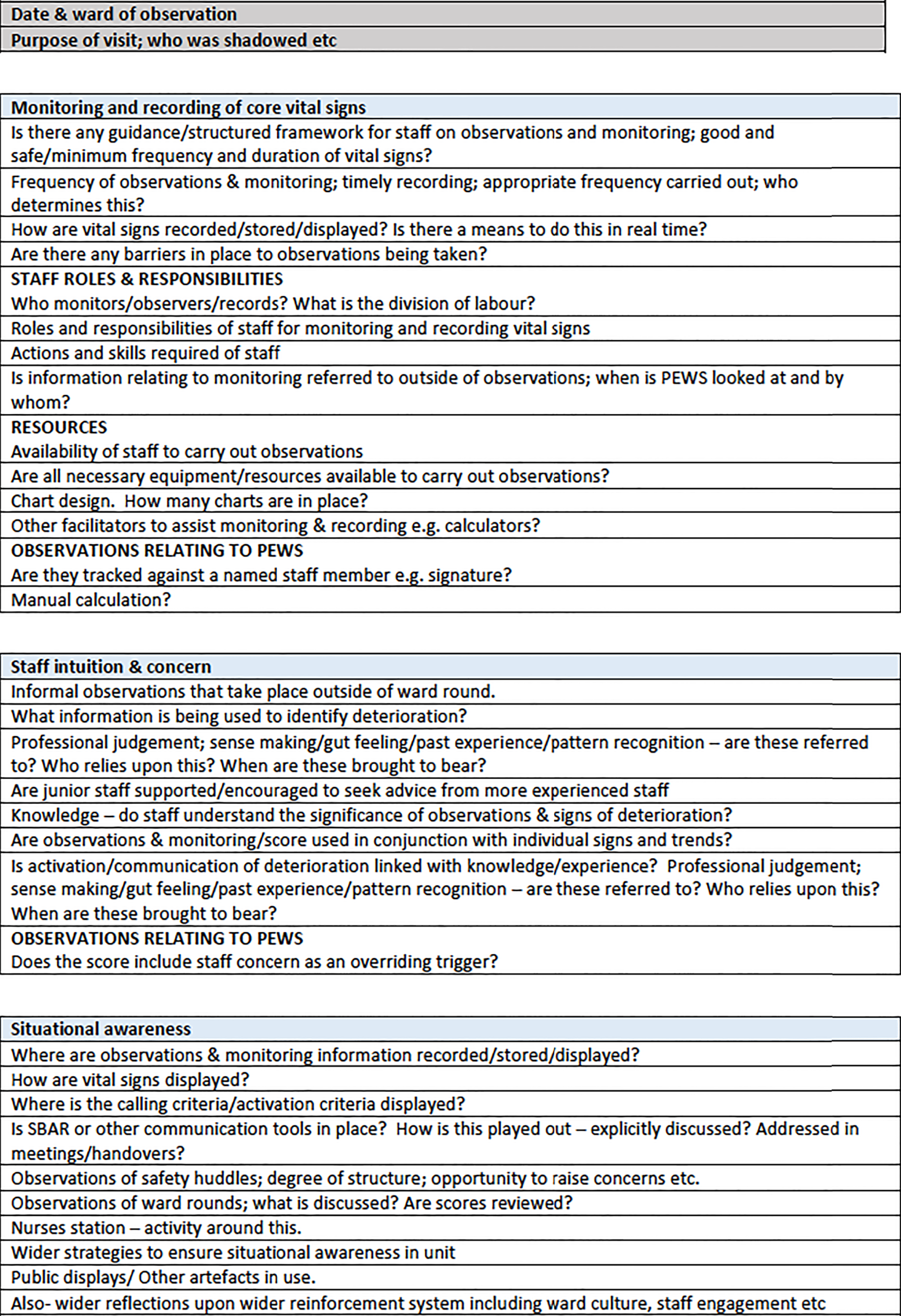

In addition, we also undertook a series of semistructured interviews with parents/carers to explore their views and experiences, and semistructured interviews with a sample of clinical staff and relevant service managers.
Ethnographic observations and embedded interviews were recorded contemporaneously as low inference-style field notes and expanded on as soon as possible after the data were collected. Staff interviews were digitally recorded with consent and organised to take place either in private offices or by telephone. Interviews with an opportunistic sample of parents who had a physiologically unstable child were undertaken when the child was still an inpatient, but at a time when their condition was considered by clinical staff to be stable. For the purposes of this study, we did not include parents whose child had died, but we interviewed parents whose child had (1) been monitored only, (2) received intervention to prevent deterioration or (3) experienced a critical event. Documents/records were treated as both a resource and a topic. Their content was analysed to inform our understanding of organisational processes and practices. Their form was analysed to develop a better understanding of their design, affordances and inter-relationships.
We replicated this ethnographic process (both non-participant observations and interviews) in the post-implementation period, modifying the interview style and content, as well as the primary focus of the observations, to explore, in detail, staff experiences of the PUMA programme, changes to the system, factors consequential for impact and any unintended consequences. We also reassessed the paediatric early warning system using a structured template based on the PUMA Standard as a guide to observation, in order to analyse changes in these relationships brought about by the improvement programme, and the implications this had for normalisation.
Analysis
At all stages, data collection and analysis were undertaken concurrently, facilitating a progressive narrowing of focus designed to develop an in-depth understanding of the paediatric early warning systems and the implications of the improvement programme for practice. The various materials collected (field notes, interview transcripts and documents) were coded using a common framework and used to develop concrete descriptions of relevant aspects of paediatric early warning systems, which were mapped onto the PUMA Standard. Local PIs also contributed to this sense-making process.
Analysis was undertaken in two main stages:
-
Stage 1 involved developing a description and analysis of the pre- and post-implementation paediatric early warning system in each hospital and the implementation process. This entailed the development of richly descriptive accounts, extending to up to 25,000 words, which were then subject to further analysis, refined and condensed into summaries for the purposes of the report.
-
Stage 2 involved cross-case analysis to understand the relationship between the intervention, context, mechanisms and outcomes to inform the extension and development of the PUMA programme.
Qualitative PUMA programme process evaluation
Overview
The process evaluation focused on the implementation of the PUMA programme in order to understand participants’ experiences, but also to identify where and how the programme might be strengthened.
Data
Observations
Observations, recorded as fieldnotes, were made of all facilitated sessions (set-up, action planning) and monthly PUMA study meetings during which PIs provided progress updates on local implementation efforts at their site (some of which were also audio-recorded).
Interviews with principal investigators and staff
Face-to-face digitally recorded interviews were conducted with PIs and clinical staff from each of the four sites at the end of the implementation phase, to gain an understanding of participants’ experiences of, and response to, different elements of the PUMA programme.
Other data sources
Written notes were made on telephone-based facilitation discussions held between one of the PUMA study team members and site PIs.
We also drew on documents shared by sites, including minutes of local improvement team meetings and policies or procedures created as a result of PUMA programme initiatives.
Analysis
Analysis was thematic, focusing on delivery and response to the core components of the PUMA programme, communication of PUMA, understanding of PUMA, barriers to change and implementation, facilitators of change and implementation, and sustainability.
Chapter 3 Evidence reviews
Parts of this chapter are reproduced from Jacob et al. 13 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are reproduced from Trubey et al. 24 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Two linked quantitative reviews were conducted to explore the evidence base for the validity and effectiveness of existing PTTTs and paediatric early warning systems (reviews 1 and 2). A third, qualitative, review was conducted to explore the wider contextual factors associated with successful (or unsuccessful) paediatric early warning systems (review 3).
The results of the reviews are described separately, with the overall findings synthesised in the conclusions. This chapter draws substantially on two published papers. 13,24
The quantitative reviews
Two linked quantitative reviews were undertaken to address the following questions:
-
Review 1: how well validated are existing PTTTs and their component parts for predicting inpatient deterioration?
-
Review 2: how effective are paediatric early warning systems (with or without a tool) at reducing mortality and critical events?
Study characteristics
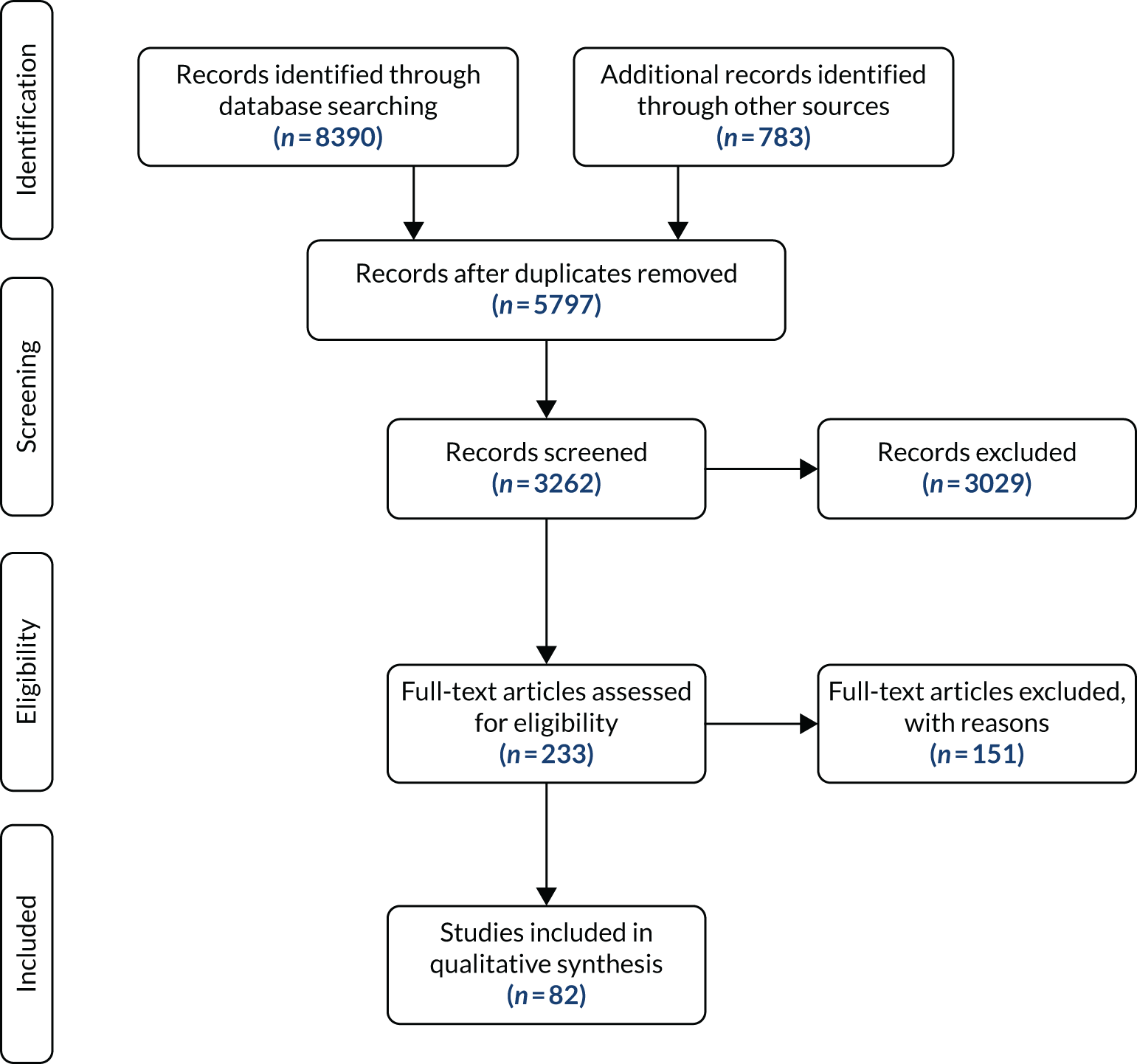
Figure 2 presents a summary of the study characteristics of the 36 validation (question 1) and 30 effectiveness (question 2) papers included in the reviews. Full details are provided in Appendix 11, Table 35.
FIGURE 2.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for study screening and selection: reviews 1 and 2. PEWS, Paediatric Early Warning Score; RQ, review question.

Types of paediatric track-and-trigger tools and components
Across 66 studies, we identified 27 unique PTTTs. Twenty PTTTs were based on one of four different tools: Monaghan’s44 Brighton Paediatric Early Warning Score (PEWS), the Bedside PEWS,19 the Bristol Paediatric Early Warning Tool45 and the Melbourne Activation Criteria (MAC). 38 Other PTTTs described in the literature included the NHS Institute for Innovation and Improvement (NHS III) PEWS, which is the second most frequently used PTTT in UK paediatric settings;22 rapid response team (RRT) and medical emergency team (MET) activation criteria;46–49 and one prediction algorithm developed from a large data set of electronic health data. 50 [Please note that, although the abbreviation PEWS is defined as Paediatric Early Warning Score in the main body of the text, this definition is not necessarily applicable to the wider literature where, for the purposes of the review, the original use is retained.]
The range of physiological and behavioural parameters underpinning PTTTs is illustrated in Appendix 12, Table 36. Common parameters included heart rate (present in 26 out of 27 PTTTs), respiratory rate (in 24 PTTTs), respiratory effort (in 24 PTTTs) and level of consciousness or behavioural state (in 24 PTTTs). All PTTTs required at least six different parameters to be collected.
Review 1: how well validated are paediatric track-and-trigger tools and component parts for predicting inpatient deterioration?
Nine validation papers meeting inclusion criteria were excluded from analysis: eight51–58 did not report any performance characteristics of the PTTT for predicting deterioration, and one study45 calculated incorrect sensitivity/specificity outcomes (see Appendix 13, Table 37).
The remaining 27 validation studies, evaluating the performance of 18 unique PTTTs, are described in Appendix 14, Table 38. Four studies evaluated multiple PTTTs,50,59–61 and one paper described three separate studies of the same PTTT. 62
Five cohort studies were included, three based on the same data set. 17,63–66 All other studies were either case–control studies or chart reviews. Thirteen papers implemented the PTTT in practice,54,62,64,66–75 whereas the remaining studies ‘bench-tested’ the PTTT, that is researchers retrospectively calculated the score based on data abstracted from medical charts and records. All studies were conducted in specialist centres, with only one multicentre study reported. 76
Outcome measures
Paediatric track-and-trigger tools were evaluated for their ability to predict a wide range of clinical outcomes. Composite measures were used in eight studies,17,54,61,63,65,77–79 cardiac/respiratory arrest or a ‘code call’ was used (singularly or as part of a composite outcome) in six studies,18,54,60,61,77,78 and 22 studies used transfer to a PICU or a paediatric HDU as the main outcome. 17,19,50,54,59–62,64–66,68,71,73–77,79–81
Predictive ability of individual paediatric track-and-trigger tool components
Three validation papers reported on the performance characteristics of individual components of the tool for predicting adverse outcomes. 19,65,73 Parshuram et al. ,19 for instance, reported area under receiver operating characteristic (AUROC) curve values for individual PTTT items of a pilot version of the Bedside PEWS: ranging from 0.54 (bolus fluid) to 0.81 (heart rate), compared with 0.91 for the overall PTTT. 19 All other studies reported outcomes for the PTTT as a whole.
Paediatric early warning system score
The predictive ability of the 16-item PEWS was assessed by one internal (AUROC = 0.90)18 and two external case–control studies (AUROC range 0.82–0.88)60,61 with a range of outcome measures and scoring thresholds. One case–control study18 used an observed prevalence rate to calculate a positive predictive value (PPV) of 4.2% for the tool in predicting code calls (i.e. for every 1000 patients triggering the PTTT, 42 would be expected to deteriorate).
Bedside Paediatric Early Warning Score and derivatives
The Bedside PEWS was evaluated in one internal (AUROC = 0.91)19 and five external case–control studies (AUROC range 0.73–0.90)50,60,61,76,79 for a range of different outcome measures and at different scoring thresholds. One case–control study50 calculated a PPV of 2.1% for identifying children requiring urgent PICU transfer within 24 hours of admission, based on locally observed prevalence rates. A modified version of the Bedside PEWS (with temperature added) demonstrated an AUROC of 0.86 in an external case–control study with a composite outcome of death, arrest or unplanned PICU transfer. 61
Brighton Paediatric Early Warning Score and derivatives
Six different PTTTs based on the original Brighton PEWS were evaluated across 11 studies,50,61,64,69,71–74,77,78,81 The Modified Brighton PEWS (a) was evaluated for its ability to predict PICU transfers in one large prospective cohort study (AUROC = 0.92, PPV = 5.8%),64 and an external case–control study tested the same score for predicting urgent PICU transfers within 24 hours of admission (AUROC = 0.74, PPV = 2.1%). 50
An external case–control study used a composite measure of death, arrest or PICU transfer to evaluate the Modified Brighton PEWS (b) (AUROC = 0.79) and the Modified Brighton PEWS (d) (AUROC = 0.74). 61 The latter tool was evaluated in a further internal case–control study for predicting PICU transfer (AUROC = 0.82). 80
The Children’s Hospital Early Warning Score had a reported AUROC of 0.90 for predicting PICU transfers or arrests in a large internal case–control study. 69 A modification for cardiac patients, the Cardiac Children’s Hospital Early Warning Score (C-CHEWS), was evaluated by one internal study on a cardiac unit (AUROC = 0.90)77 looking at arrests or unplanned PICU transfers, and by two external studies of oncology/haematology units for the same outcome (AUROC = 0.95). 73,74 Finally, the Children’s Hospital Los Angeles PEWS was evaluated in a small internal case–control study for prediction of re-admission to PICU after initial PICU discharge (AUROC = 0.71). 72
Melbourne Activation Criteria and derivatives
The MAC was assessed by one external case–control study with an outcome of death, arrest or unplanned PICU transfer (AUROC = 0.71)61 and by a large external cohort study with an outcome of death or unplanned PICU or HDU transfer (AUROC = 0.79, PPV = 3.6%). 65 A derivative of the MAC, using an aggregate score, the Cardiff & Vale Paediatric Early Warning Score (C&VPEWS), was tested using the same cohort and outcome measures as used in a previously mentioned external study (AUROC = 0.86, PPV = 5.9%)17 and was the best-performing PTTT in an external case–control study evaluating multiple PTTTs (AUROC = 0.89). 61
Bristol Paediatric Early Warning Tool
The Bristol Paediatric Early Warning Tool was evaluated by five external validation studies: two chart review studies (no AUROC);59,67 one small cohort study of PICU transfers (AUROC = 0.91, PPV = 11%);66 and two case–control studies looking at code calls (AUROC = 0.75)60 and a composite of death, arrests and PICU transfers (AUROC = 0.62). 61
Other paediatric track-and-trigger tools
The NHS III PEWS was tested by one external cohort study looking at a composite of death or unplanned transfers to PICU or HDU (AUROC = 0.88, PPV = 4.3%)63 and one external case–control study looking at a composite of death, arrests and PICU transfers (AUROC = 0.82). 61 Zhai et al. 50 developed and retrospectively evaluated a logistic regression algorithm in an internal case–control study looking at urgent PICU transfers in the first 24 hours after admission (AUROC = 0.91, PPV = 4.8%).
Across PTTTs, studies reporting performance characteristics of a tool at a range of different scoring thresholds demonstrate the expected interaction and trade-off between sensitivity and specificity: at lower triggering thresholds, sensitivity is high but specificity is low; at higher thresholds, the opposite is true.
Inter-rater reliability and completeness of data
Accurate assessment of the ability of a PTTT to predict clinical deterioration is contingent on accuracy and reliability of tool scoring (whether by bedside nurses in practice or by researchers abstracting data) and the availability of underpinning observations. Only five papers made reference to accuracy or reliability of scoring,60,64,73,77,78 with mixed results; for example, two nurses separately scoring a subset of patients on the Modified Brighton PEWS (a) achieved an intra-class coefficient of 0.92,64 but a study nurse and bedside nurse achieved only 67% agreement in scoring the C-CHEWS. 77 Completeness of data was reported in 11 studies. 17–19,50,61–63,65,73,76,78 An evaluation of the Modified Bedside PEWS (a) reported that ‘the PEWS was correctly performed and could be used for inclusion in the study’ in 59% of cases,62 a prospective study bench-testing the C&VPEWS found an average completeness rate of 44% for the seven different parameters in daily practice,17 and a multicentre study of the Bedside PEWS reported that ‘only 5.1% [of observation sets] had measurements on all 7 items’. 76
Box 1 presents a summary of the review 1 findings.
Given a growing understanding and emphasis on the importance of local context in health-care interventions, it is perhaps not surprising that such a wide range of PTTTs have been developed and evaluated internationally, and modifications to existing PTTTs are common. The result, however, is that, although numerous versions of PTTTs have been narrowly validated, none has been broadly validated across a variety of different settings and populations. With only one exception,76 all studies evaluating the validity of PTTTs have been single-centre reports from specialist units, greatly limiting the generalisability of the findings.
Paediatric track-and-trigger tools such as the Bedside PEWS, the C&VPEWS, the NHS III PEWS and the C-CHEWS have demonstrated very good (AUROC of ≥ 0.80) or excellent (AUROC of ≥ 0.90) diagnostic accuracy, typically for predicting PICU transfers, in internal and external validation studies. 17,19,50,61,63,73,76,77 However, common methodological issues mean that these results need to be interpreted with caution.
First, each of the studies was conducted in a clinical setting where paediatric inpatients are subject to various forms of routine clinical intervention throughout their stay. There are numerous statistical modelling techniques that can account for co-occurrence of clinical interventions and the longitudinal nature of the predictors,82,83 but none of these was used in the validation studies and so estimates of predictive ability are likely to be distorted. Indeed, most outcomes used in the validation studies are clinical interventions themselves (e.g. PICU transfer). Second, although it is understandable that most studies ‘bench-tested’ the PTTT rather than implemented it into practice before evaluation, the process of abstracting PTTT scores retrospectively from patient charts and medical records introduces potential bias or inaccuracy. For instance, several studies reported either high numbers of missing data (i.e. some of the observations required to populate the PTTT score being evaluated were not routinely collected or recorded, and so were scored as ‘normal’)17,19,50,76,78 or difficulty in abstracting certain descriptive or subjective PTTT components. 50,60,74,80 Assuming missing values are normal or excluding some PTTT items for analysis, are both likely to result in underscoring of the PTTT and skew the results. Finally, studies that evaluated a PTTT that had been implemented in practice are at risk of overestimating the ability of the PTTT to predict proxy outcomes such as PICU transfer, inasmuch as high PTTT scores or triggers automatically direct staff towards escalation of care, or clinical actions that make escalation of care more likely.
The findings reported in several PTTT studies point towards two potential challenges for some centres in implementing and sustaining a PTTT in clinical practice. As noted previously, several studies that retrospectively ‘bench-tested’ a PTTT reported that the observations that were required to score the tool were not always routinely collected or recorded in their centre. It may be that the introduction of a PTTT into practice would help create a framework to ensure that core vital signs and observations were collected more routinely (as demonstrated by Parshuram et al. 84), but this would obviously have resource implications that could be a potential barrier for some centres. Such considerations are important, as evidence from the adult literature points to the potential for tools to inadvertently mask deterioration when core observations are missing. 85
Furthermore, PPVs reported in cohort studies, and case–control studies that adjusted for outcome prevalence were uniformly low (between 2.3% and 5.9%). 17,18,50,63–65 They demonstrate that even PTTTs that demonstrate good predictive performance are likely to generate a large number of ‘false alarms’ because adverse outcomes are so rare. For some centres, these issues may be mitigated, to some extent, by dedicated response teams or other available resources, but other hospitals may not be able to sustain the increased workload of responding to PTTT triggers.
Key messages-
A wide range of PTTTs has been studied in the literature, although the majority are closely derived from a smaller handful of tools.
-
Many of these have demonstrated good predictive value for proxy measures of deterioration; transfers to higher level of care is the most used metric.
-
However, cohort studies suggest very high ‘false alarm’ rates are likely when tools are used in clinical practice.
-
No one PTTT has been broadly validated across different settings. The majority of research studies have been conducted in North America in specialist settings; therefore, generalisability of findings is limited.
Review 2: how effective are early warning systems at reducing the rates of mortality and critical events in hospitalised children?
Eleven papers meeting inclusion criteria were excluded from analysis for providing insufficient statistical information (e.g. denominator data, absolute numbers of events) to calculate effect sizes. 71,86–95 Further details on papers excluded from analysis are provided in Appendix 15, Table 39. Findings from the 19 studies included in the analysis are summarised in Appendix 16, Table 40.
Type of early warning system interventions
Seventeen interventions involved the introduction of a new PTTT,38,46–49,84,96–107 one intervention introduced a mandatory triggering element to an existing PTTT106 and one study reported a large, multicentre analysis of MET introduction with no details on PTTT use. 108 Twelve interventions included the introduction of a new MET or RRT,38,46–49,84,96–100,104 and four further interventions87,89,100,106 introduced a new PTTT in a hospital with an existing MET or RRT. Therefore, only three studies evaluated a PTTT in the absence of a dedicated response team. 102,103,105 A staff education programme was explicitly described in 10 interventions. 38,46,48,84,97,98,102,103,105,107
Of the 18 studies that used a PTTT, only seven used a tool that had been formally evaluated for validity: three used the Bedside PEWS,84,100,105 two used the MAC,38,98 one used the Modified Brighton PEWS (b)107 and one used the C-CHEWS. 102 One study did not report the PTTT used,97 and 10 studies used a variety of calling criteria and local modifications to validated tools that had not been evaluated for validity. 46–49,96,99,101,103,104,106
Mortality (ward or hospital wide)
Two uncontrolled before-and-after studies (both with a MET/RRT) reported significant mortality rate reductions post intervention: one in hospital-wide deaths per 100 discharges (RR 0.82, 95% CI 0.70 to 0.95)48 and one in total hospital deaths per 1000 admissions (RR 0.65, 95% CI 0.57 to 0.75) and deaths on the ward (‘unexpected deaths’) per 1000 admissions (RR 0.35, 95% CI 0.13 to 0.92). 98 Seven studies found no reductions in mortality, including two high-quality multicentre studies. 38,46,84,96,99,100,108 Parshuram et al. 84 conducted a cluster randomised trial and found no difference in all-cause hospital mortality rates between 10 hospitals randomly selected to receive an intervention centred around use of the Bedside PEWS and 11 usual care hospitals 1 year post intervention (OR 1.01, 95% CI 0.61 to 1.69). Kutty et al. 108 assessed the impact of MET implementation in 38 US paediatric hospitals with an ITS study and reported no difference in the slope of hospital mortality rates 5 years post intervention and the expected slope based on pre-implementation trends (OR 0.94, 95% CI 0.93 to 0.95).
Paediatric intensive care unit mortality
Two uncontrolled before-and-after studies (both with a MET/RRT) reported a significant post-intervention reduction in rates of PICU mortality among ward transfers (RR 0.31, 95% CI 0.13 to 0.72),49 and PICU mortality rates among patients re-admitted within 48 hours (RR 0.43, 95% CI 0.17 to 0.99). 99 Six studies (including a high-quality cluster randomised trial and an ITS study) reported no post-intervention change in PICU mortality rates using a variety of metrics. 84,100–104
Cardiac and respiratory arrests
Two uncontrolled before-and-after studies (both with a RRT/MET) reported significant post-intervention rate reductions in subcategories of cardiac arrests: one in ‘near cardiopulmonary arrests’ (RR 0.54, 95% CI 0.52 to 0.57),99 but not in ‘actual cardiopulmonary arrests’, and one in ‘preventable cardiac arrests’ (RR 0.45, 95% CI 0.20 to 0.97),98 but not ‘unexpected cardiac arrests’. One uncontrolled before-and-after study (with a RRT/MET) reported a significant post-intervention reduction in rates of ward respiratory arrests per 1000 patient-days (RR 0.27, 95% CI 0.07 to 0.95). 47 Seven studies (including one high-quality cluster randomised trial and one high-quality ITS study) found no change in cardiac arrest rates using a variety of metrics,38,46,47,84,97,100 or cardiac and respiratory arrests combined. 96
Calls for urgent review/assistance
Two uncontrolled before-and-after studies (all with RRTs/METs) reported significant post-intervention reductions in the rates of code calls (RR 0.29, 95% CI 0.10 to 0.65;48 RR 0.71, 95% CI 0.61 to 0.8399), whereas three studies found no change in the rate of code calls. 46,49,107 One uncontrolled before-and-after study in a community hospital (without a RRT/MET) found significant post-intervention reductions in rates of urgent calls to the in-house paediatrician (RR 0.23, 95% CI 0.11 to 0.46) and respiratory therapist (RR 0.36, 95% CI 0.13 to 0.95). 105 Two uncontrolled before-and-after studies (with RRTs/METs) found increases in the rates of RRT calls (RR 1.59, 95% CI 1.33 to 1.90)107 and outreach team calls (RR 1.92, 95% CI 1.79 to 2.07). 101 One study found no change in the rate of RRT calls. 106
Paediatric intensive care unit transfers
One uncontrolled before-and-after study (without a RRT/MET) found a significant post-intervention decrease in the rate of unplanned PICU transfers per 1000 patient-days (RR 0.70, 95% CI 0.56 to 0.88). 102 Four studies (including one high-quality cluster randomised trial and one high-quality ITS study) found no change in the rates of PICU admissions post intervention. 84,100,101,105
Paediatric intensive care unit outcomes
Two studies, one ITS study and one multicentre cluster randomised trial (both with RRTs/METs), found significant reductions in rates of ‘critical deterioration events’ (life-sustaining interventions administered within 12 hours of PICU admission) relative to pre-implementation trends (incidence rate ratio 0.38, 95% CI 0.20 to 0.75)100 and relative to control hospitals (OR 0.77, 95% CI 0.61 to 0.97). 84 One controlled before-and-after study (without a RRT/MET) reported a significant reduction in rates of invasive ventilation given to emergency PICU admissions post intervention (RR 0.83, 95% CI 0.72 to 0.97), with no significant change observed in a control group of patients admitted to PICU from outside the hospital. 103 One uncontrolled before-and-after study reported a significant post-intervention decrease in the rate of PICU admissions receiving mechanical ventilation (RR 0.85, 95% CI 0.73 to 0.99), but an increase in the rate of early intubation (RR 1.87, 95% CI 1.33 to 2.62). 104
Implementation outcomes
Only three studies reported outcomes relating to the quality of implementation of the intervention. One study reported that 99% of audited observation sets of the Bedside PEWS had at least five vital signs present post intervention, up from 76% pre intervention (no change in control hospitals). 84 A previous study of the same PTTT reported that 3% of audited cases had used the incorrect age chart, but reported an intraclass coefficient of 0.90 for agreement between bedside nurses scoring the PTTT in practice and research nurses retrospectively assigning scores. 105 Finally, error rates in C-CHEWS scoring were reported to have reduced from an initial 47% to < 10% by the end of the study. 102
Box 2 presents a summary of the review 2 findings.
We found limited evidence of early warning system interventions reducing mortality or arrest rates among hospitalised children. Although some effectiveness papers did report significant reductions in the rates of mortality (on the ward or in the PICU) or cardiac arrests after implementation of different early warning system interventions,47–49,98,99 they were all uncontrolled before-and-after studies, which have inherent limitations in terms of establishing causality. They do not preclude the possibility that outcome rates would have improved over time regardless of the intervention,109 or that changes were caused by other factors, and their inclusion is accordingly discouraged by some Cochrane review groups. 110 Three high-quality multicentre studies – two ITS studies and a 2018 cluster randomised trial – found no changes in rates or trends of mortality or arrests post intervention. 84,100,108
There was also limited evidence for early warning systems reducing PICU transfers or calls for urgent review. Again, a small number of uncontrolled before-and-after studies reported significant reductions post intervention,46,48,99 but several other studies reported significant increases in transfers or calls for review,98,107 or no post-intervention changes. We did find moderate evidence across four studies – including a controlled before-and-after study, a multicentre ITS study and a multicentre cluster randomised trial – for early warning system interventions reducing rates of early critical interventions in children transferred to a PICU. 84,100,103,104 Such results are promising, but corresponding reductions in hospital or PICU mortality rates have not yet been reported.
Implementing complex interventions in a health-care setting is challenging and evidence from the adult literature points to challenges and barriers to successfully implementing TTTs in practice. 111–113 However, given that so few effectiveness studies reported on implementation outcomes, it is difficult to know whether negative findings reflect poor effectiveness or poor implementation of early warning systems. Again, effectiveness studies were predominantly carried out in specialist centres, and, in all but three cases,102,103,105 involved the use of a dedicated response team, which greatly limits the generalisability of findings outside these contexts.
Key messages-
Only a handful of studies have reported significant changes in mortality or arrests among hospitalised children as a result of implementing a paediatric early warning system intervention; however, they have typically been uncontrolled before-and-after studies, which limits confidence in their findings.
-
Three high-quality multicentre studies have failed to find any significant reduction in mortality or arrests after paediatric early warning system interventions.
-
There is moderate evidence that paediatric early warning systems may reduce the rates of unplanned transfers to a higher level of care, but corresponding reductions in rates of hospital-wide or PICU mortality have not been reported.
-
Paediatric early warning system interventions are typically multifaceted (often including the use of dedicated response teams) and most studies have been conducted in specialist centres, thereby limiting generalisability of the results.
-
There is very little evidence on how well implemented interventions are in clinical practice, and their corresponding effects on wider system functioning.
The qualitative review
Review 3: what sociomaterial and contextual factors are associated with successful or unsuccessful paediatric early warning systems (with or without track-and-trigger tools)?
A parallel hermeneutic qualitative review was undertaken to address this question. The focus was limited to the afferent components of the system (see Chapter 2).
Search results
Eighty-two papers were included in the review (Figure 3). Forty-six papers focused on TTT implementation and use in paediatric and adult contexts (24 from the paediatric search and the remaining 22 from the adult-focused search); the remaining 36 papers contributed supplementary data on factors related to the wider early warning system. No studies were located that adopted a whole-systems approach to detecting and responding to deterioration. See Appendix 17, Table 41, for a detailed breakdown of the search process.
FIGURE 3.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for study screening and selection: review 3.
Translational mobilisation theory was used to analyse the evidence to identify the sociomaterial and contextual factors associated with successful and unsuccessful paediatric early warning systems. In TMT, the primary unit of analysis is the ‘project’, which defines the social and material actors (people, materials, technologies) and their relationships involved in achieving a goal. The goals of the afferent paediatric warning system are as follows: first, the child is identified as at risk and a vital signs monitoring regime is instigated; second, evidence of deterioration is identified through monitoring and categorised as such; and, third, timely and appropriate action is initiated in response to deterioration.
The analysis of the literature suggested that three subsystems within the afferent component of early warning systems support the following:
-
the detection of signs of deterioration
-
the planning needed to ensure that teams are ready to act when deterioration is detected
-
the initiation of timely action.
Detection
The goal of the ‘detection’ subsystem is to recognise early signs of deterioration, so the child becomes the focus of further clinical attention (see Appendix 18, Table 42). This requires, first, that the child is identified as at risk and a vital signs monitoring regime is instigated and, second, that the child is identified as showing signs of deterioration.
Although the evidence on TTT effectiveness in predicting adverse outcomes in hospitalised children is weak,24 the literature does suggest that TTTs have value in supporting process mechanisms in the detection subsystem. Vital signs monitoring is undertaken on all hospital inpatients and, like other high-volume routine activity, is often delegated to junior staff,44,114–132 who may not have the necessary skills to interpret results. 116,117,131 TTTs have value in mitigating these risks: by specifying physiological thresholds that indicate deterioration, they take knowledge to the bedside and act as prompts to action,114,133 which can lead to a more systematic approach to monitoring and improved detection of deterioration. 113,134
The effectiveness of TTTs in fulfilling these functions depends on certain preconditions. The review highlighted that TTT use was affected by the availability of appropriate and functioning equipment,112,117,122,123,128,133,135–138 (in)adequate staffing and night-time pressures112,113,117,121,123,124,131,135,136,139–144 and an appropriately skilled workforce. 44,89,121,130,136,141,142,145–148 On this third point, although several papers report on education packages to improve the detection of deterioration, the evidence is not robust enough to recommend specific programmes. 44,87,89,118–120,124,129,149,150 There is also evidence that nursing staff prioritise sleep over waking a patient to take vital signs. 138,151
Track-and-trigger tools are also used differently depending on the experience of the user. For juniors, they provide a methodology and structure for monitoring clinical instability and identifying deterioration, whereas more experienced staff reportedly use TTTs as confirmatory technologies. 44,114–126 The importance of professional intuition in detecting deterioration is extensively reported across the literature,113–117,121,123,125,126,130–133,135–138,140,142–145,150,152–158 and several authors recommend the inclusion of ‘staff concern’ in tool criteria. 121,140,143,148 This is important: TTTs may be of less value among patients with long-term conditions because of altered normal physiology, or where subtle changes are difficult to detect. 154 It is also the case that TTTs are implemented in contexts governed by competing organisational logics, which affect their value and use. 87,136,146 For example, Mohammmed Iddrisu et al. 148 show that TTTs have limited value immediately after surgery because acceptable vital signs parameters are different in the immediate post-operative period.
There is growing interest in the literature on strategies that facilitate patient and relative involvement in the early detection of deterioration. 159,160 Health-care professionals depend on families to explain their child’s normal physiological baseline and to identify subtle changes in their child’s condition, but this information is not always systematically obtained. 161,162 Some authors propose family involvement in interdisciplinary rounds,163 but this requires parents to have detailed information about the signs and symptoms they should be attending to,162 and, as yet, there is little evidence on effective strategies for how they might be involved in the detection of deterioration. 163
Although much of the literature reports on intermittent manual vital signs monitoring and paper-based recording systems, across the developed world, there is a growing use of electronic technologies, which has important implications for the wider detection subsystem. 164 We considered several evaluations of new technologies, which indicated that electronic vital signs recording is associated with some positive outcomes, particularly timeliness and accuracy, when compared with paper-based systems. 165,166 They can provide prompts or alerts for monitoring,167–169 which facilitates better recognition of deterioration and is associated with a reduction in mortality. 168,170 These studies tend to evaluate new technologies in isolation, however, and do not engage with the literature highlighting alarm fatigue, a factor known to mitigate effectiveness over time, or documented concerns about overburdening staff with alerts. 171–173 Moreover, the successful implementation of new technologies is conditioned by the local context. For instance, when manual input into an electronic device is required, access to computers is an essential precondition. When computers are not available staff ‘batch’ the collection of vital signs before data entry, thereby delaying the timely detection of deterioration. 122,137,174 In one study in which the electronic system was found to be cumbersome and separated the collection and entry of data from the review of vital signs, verbal reports were favoured to ensure timely communication of information. 175
Planning
Detecting and responding to deterioration involves the co-ordination of action in conditions of uncertainty and competing priorities. The goal of the ‘planning’ subsystem is to ensure that the clinical team are ready to act in the event of evidence of deterioration, and is reflected in the growing interest in the literature on structures to facilitate team situational awareness, group decisions and preparation (see Appendix 19, Table 43). 152
Track-and-trigger tools have been found to support situational awareness. Their use enabled clinicians to have a ‘bird’s-eye’ view of children at risk over all admitted patients on a ward, as well as encouraging staff to consider the projected acuity levels of the ward. 176 A number of studies also report on ‘huddles’ in facilitating situational awareness. 126,155,177,178 A huddle is a multidisciplinary event, scheduled at predetermined times, during which members discuss specific risk factors around deterioration and develop mitigation plans. One study combined the introduction of huddles with a ‘watchstander’, a role fulfilled by a charge nurse or senior resident, whose primary function is to know which patients are at high risk of deterioration. 178 These initiatives were associated with a near 50% reduction in transfers from acute to intensive care caused by failures of situation awareness. A further strategy identified by Goldenhar et al. 177 describes the use of the ‘watcher’ category to designate a patient as at risk when staff have a ‘gut feeling’ that deterioration is likely. A 2017 study179 used the category of ‘watcher’ to create a bundle of expectations to standardise communication and contingency planning. Once a patient was labelled ‘a watcher’, five specific tasks, such as documentation of physician awareness of watcher status and notification of the family of the patient’s changed status, had to be completed within 2 hours. 179
Handovers are integral to clinical communication and contribute to situational awareness. The extensive literature on handover indicates that information-sharing can be of variable quality,139,146,180 and there is growing evidence that structured approaches improve this. 124,139,146,153,177,180–184 Ranging from a checklist system183,185 to a cognitive aid developed through consensus,118,184 most of the published interventions are variations of the situation, background, assessment, recommendation (SBAR) tool. 146,180–182 Although effective handover depends on communicative forms that extend beyond the information transfer that is typically the focus of structured handover tools,180 in the context of early warning systems, a lack of standardisation allows greater margin for individualistic practices and difficulty in accessing complementary knowledge and establishing shared understandings. 139
There is also a literature on the use of common information spaces, such as whiteboards, in facilitating situational awareness among the health-care team. 87,89,118,127,139,145,157 These should be in a visible location and colour-coded to correspond with the TTT score, when relevant. 87,89,139 Electronic systems automate this information and allow information to be reviewed remotely. However, they disconnect vital signs data from the patient, and hence other indicators of clinical status, and access to data is contingent on the availability of computers. 122,137,139,174,186
The literature indicates that situational awareness can be facilitated in different ways in different contexts, and that it is the relationship between system elements that is important. 139 In their study on situational awareness in delivery suites, Mackintosh et al. 139 discuss the three main supports for situational awareness (i.e. whiteboard, handover and co-ordinator role) and illustrate how these worked together in organisations with strong situational awareness, compared with those with reduced levels of situational awareness. Crucially, this ‘interplay’ between the different activities was highly context dependent: ‘the same supports used differently generate different outcomes’. 139
Action
The goal of the ‘action’ subsystem is to initiate appropriate action in response to evidence of deterioration (see Appendix 20, Table 44).
The literature suggests that mobilising action across professional boundaries is challenging, with differences in language between doctors and nurses and power dynamics contributory factors. 113,122,134,142,144,148,150,187 TTTs are, in part, a response to the challenges of communication in marshalling action in response to deterioration. By transforming a series of discrete observations into a summative indicator of deterioration, such as a score or a trigger, TTTs ‘translate’ and package the patient’s status into a form that can be readily communicated, enabling individual-level clinical data to be synthesised, made sense of and shared. 44,114–123,127,133–135,138,140,142,143,147,152,156,164,176 One study, however, found that TTTs were regarded as a nursing tool and were therefore not valued by clinicians; consequently, nurses encountered difficulties summoning a response. 138
Several studies also report on the use of the SBAR tool in this context. Similar to TTTs, the SBAR tool translates information into a form that provides structure, consistency and predictability when presenting patient information. The SBAR tool has been shown to help establish common language and expectations, minimising differences in training, experience and hierarchy, and facilitating nurse–clinician communication. Although several papers advocate combining the SBAR tool with TTTs,118,120,122,124,129,137,142 none specifically evaluated SBAR tool use. Mackintosh et al. 122 highlight that audit data suggest resistance to the SBAR tool, cautioning that overextending SBAR tool use carries the risk of SBAR tool fatigue and attenuation of its effects.
Structured communication tools such as TTTs and the SBAR tool do not solve all the challenges of acting in response to evidence of deterioration. Barriers to action were widely reported in the literature where these tools were in place. These include a general disinclination to seek help,114–116,120,122,123,125,130–133,135,140,142,143,147,154,157 concerns about appearing inadequate in front of colleagues115,117,130,132,142,157 and failure of staff to invest in the escalation or calling criteria. 116,117,141 Several papers also reported negative attitudes to RRT or MET use. METs and RRTs operate outside the immediate medical team and create different issues in paediatric warning systems than when the escalation response is managed by the treating team. These include a reluctance to activate because of the perceived busyness of PICU or medical staff,115,123,133,140,142,143 because previous expectations about an appropriate response were not met or because of a sense that the situation was under control (particularly when the physiological instability is in the area of expertise of the treating team). 117,123,125,132,135,142,144,154
No literature reported on successful interventions to facilitate RRT use, but several propose strategies to support escalation where there was no designated response team. These include informal peer support, whereby inexperienced staff team up with more experienced staff;116,123,142,154,157 clear structures to support action; and a supportive culture that does not penalise individual decision-making, including the use of a ‘no false alarms’ policy so staff are not deterred from escalating care. 116,123,130,163 Senior leadership is consistently identified as important;87,115–118,120,122,124,126,127,129,139,144,156,157,188 lack of support from superiors means that staff are less likely to escalate and more likely to adhere to hierarchies within the current system. 99,155,189 There is some evidence to suggest that any escalation policy should be linked to an administrative arm that reinforces the system, measures outcomes and works to ensure an effective system. 122,124
There is a small amount of literature on family involvement in the ‘action’ subsystem. Several studies190–192 report on Condition Help, a programme developed in the USA to support families to directly activate the RRT if they have concerns about their child’s condition. Families are also becoming increasingly recognised as playing a key role in the activation of RRTs in Australia. 193 Research has evaluated the appropriateness of calls that were made by patients or relatives,127,190–194 but has not considered why calls were not made. 160 Involving family members in escalation demands vigilance, requiring them to take a proactive and interactive role with staff, with potentially some degree of confrontation, particularly if challenging the appropriateness of decisions taken. 163,193 Families need both cognitive and emotional resources to raise concerns that involve negotiating hierarchies and boundaries. 129,160 The literature points to a degree of professional resistance to family involvement in activation, with reports of physician concern that their role would be undermined, that resources would be stretched with an increase in calls and that it might divert attention away from those in need,50,161,190,193,195 although these fears are not supported by the evidence. 50,161,196
Box 3 presents a summary of the review 3 findings.
The literature in this field is heterogeneous and stronger on the sociomaterial barriers to successful afferent component paediatric early warning systems than it is on solutions. Although several different single interventions have been proposed and some have been evaluated, there is limited evidence to recommend their use beyond the specific clinical contexts described in the papers. This reflects both the weight and quality of the evidence; the extent to which paediatric systems are conditioned by the local clinical context; and the need to attend to the relationship between system components and interventions, which work in concert, not in isolation.
Although there is a growing consensus of the need to think beyond PTTTs to consider the whole system, no frameworks exist to support such an approach. Clinical teams wishing to improve rescue trajectories should take a whole-systems perspective focused on the constellation of factors necessary to support detection, planning and action, and consider how these relationships can be managed in their local setting. TTTs have value in paediatric early warning systems, but they are not the sole solution and they depend on certain preconditions for their use. An emerging literature highlights the importance of planning and indicates that combinations of interventions may facilitate situation awareness. Professional judgement is also important in detecting and acting on deterioration, and the evidence points to the importance of a wider organisational culture that facilitates this. Innovative approaches are needed to support family involvement in all aspects of paediatric early warning systems, which are sensitive to the cognitive and emotional resources this requires. System effectiveness requires attention to the sociomaterial relationships in the local context, senior support and leadership and continuous monitoring and evaluation.
New technologies, such as moving from paper-based to electronic TTTs, have important implications for all three subsystems, and critical consideration should be given to their wider impacts and the preconditions for their integration into practice.
Key messages-
Attempts to improve hospitals’ paediatric early warning systems should not be limited to consideration of PTTTs only.
-
Clinical teams seeking to improve rescue trajectories for hospitalised children should take a whole-systems approach.
-
The afferent limb of an early warning system comprises three subsystems that must function in concert: detection (a child must be identified as being at risk), planning (ensuring teams are able to act when deterioration is identified) and action (ensuring an appropriate response to a deteriorating child).
Limitations of the reviews
There are several limitations of the quantitative reviews (reviews 1 and 2). First, despite purposely widening the scope of the effectiveness review question to include paediatric ‘early warning systems’ with or without a PTTT, we identified very few studies that did not employ a PTTT as part of the intervention. In part, this probably reflects the fact that PTTTs have become almost synonymous with early warning systems, but it is also possible that our search strategy may have missed some broader early warning system initiatives that were not explicitly labelled as such. Second, the inclusion criteria for study selection were deliberately broad, and so resulted in the inclusion of several validation and effectiveness studies that were subsequently excluded from analysis because of insufficient statistical detail or methodological issues. Third, the scope of reviews 1 and 2 was limited to consideration of quantitative validation and effectiveness studies. As our qualitative review (review 3) has identified, implementing PTTTs in practice may confer secondary benefits including, but not limited to, improvements in communication, teamwork and empowerment of junior staff to call for assistance. 116,118,119 Finally, we opted not to conduct a meta-analysis of effectiveness findings because of the heterogeneity of outcome metrics, interventions and study designs, populations and settings. Given the large sample sizes required to detect changes in rare adverse events, further work is needed to standardise outcome measures used to evaluate early warning system interventions internationally, to facilitate aggregating findings across studies.
The literature included in the qualitative review (review 3) was heterogeneous and better at identifying system weakness than effective improvement interventions. It was only by deploying social theories and a hermeneutic review methodology that we were able to develop ideas about the core elements of an afferent component paediatric early warning system. The findings are drawn from logical inferences, drawing on the overall evidence synthesis, social theories (TMT) and clinical expertise, rather than strong empirical evidence of single intervention effectiveness.
Conclusions
The three reviews were conducted to examine the current evidence base for paediatric early warning systems, and to understand the sociomaterial and contextual factors associated with their success or otherwise. Collectively, the evidence generated suggests that most of the validation and effectiveness research into paediatric early warning systems has, to date, focused narrowly on PTTTs and has been carried out in predominantly specialist, single-site settings. Moreover, there is currently limited evidence of PTTTs’ effectiveness when introduced into practice, in terms of reductions in rates of mortality or clinical deterioration over time. The work carried out in the qualitative review suggests that it is important to look beyond PTTTs when considering effective strategies for detecting and acting on deterioration. Although it is not possible to make empirical recommendations for practice, a hermeneutic review methodology enabled the generation of theoretical inferences about the core components of an early warning system. These informed the development of the PUMA Standard, expressed as a propositional model that provided the foundations for the development of the PUMA programme.
Chapter 4 Intervention development and implementation
Development of the PUMA programme
This chapter describes how we drew on the evidence from the three systematic reviews to develop the PUMA programme, and the strategies used to implement it across four UK hospitals.
Background
Sustained and replicable improvement in health care is a global challenge. The inspiring success of some improvement efforts is undermined by a history of uneven outcomes, and system-wide progress has been elusive. 197–199 The PUMA programme is founded on OUTCOME, a novel approach to improvement, which was developed as part of the study and designed to overcome some of the weaknesses of orthodox approaches to health-care improvement (Box 4). OUTCOME inverts the realist evaluation question of ‘what works, for whom, in what way and in what circumstances?’ to ask ‘what is our desired outcome and how might this be achieved in a given context?’.
-
Solutions are often identified before problems are properly understood. 197–199
-
Interventions are implemented without an understanding of the local systems of work in which they must have their effects. 27,200
-
The desire for standardisation limits freedom to adapt to local context. 185
-
When an intervention is imposed from outside the organisation, there is little ownership and limited opportunity to capitalise on local expertise. 201
-
Service-led projects that do utilise local expertise often lack adequate evaluation and reportage, which precludes shared learning. 202
-
The form of an intervention is often given more consideration than its function, with a tendency to give precedence to a tool that can be implemented over an adjustment to the system. 32
-
Improvement efforts are often time-limited and not sustained over the longer term. 201
OUTCOME builds on insights from quality improvement (QI) and implementation science (IS) to offer principles, structures and theories to support scalable and sustainable locally embedded improvements to achieve an agreed outcome.
Both IS and QI have enhanced understanding of how to effect change in health-care improvement. IS has focused on evidence-based interventions, with context a central concern, typically conceptualised as a source of confounding factors that interfere with implementation. 185 Extensive training and facilitation to ensure enrolment in the initiative and intervention fidelity are often core components of implementation efforts. IS has generated theories and empirical research instruments to understand the complex interactions between context and interventions that influence implementation processes. 203
Quality improvement projects start in practice, and aim to support and empower health-care professionals to create change; therefore, they can be designed and delivered to fit the context in which they must work. However, although overcoming some of the challenges of externally imposed initiatives that characterise IS projects, QI has been criticised for being atheoretical,204 and for applying insufficient attention to rigorous evaluation and improvement or sharing the lessons of successes and failures in order to facilitate sustainability and spread. 205
Despite increasing calls for closer integration of QI and IS for faster and more effective improvement,206–208 there are few examples of improvement initiatives that explicitly use the terminology and concepts of both IS and QI. OUTCOME is designed to capitalise on the extensive learning from both QI and IS, to simultaneously attend to local contexts, deliver improvements at scale, and allow for robust evaluation.
The OUTCOME framework
The OUTCOME framework is informed by TMT,26,32 NPT26,200 and the Model for Improvement (Table 6). 209 It comprises six principles and associated structures, and is designed to support the improvements necessary to achieve an agreed outcome in context-specific ways. In the section that follows, we describe the OUTCOME framework and illustrate its application in the PUMA study.
| Theory | Overview | Core constructs | Application |
|---|---|---|---|
| TMT | TMT is a theory of collective action that focuses on the goal of a particular system of work, the elements of context that are most salient to enacting the goal and the mechanisms by which that may be achieved |
|
TMT is a relatively new theory; to our knowledge, this is the first time it has been deployed for QI purposes, for which it provides the logical scaffolding to link theories and insights from IS and QI |
| NPT | NPT shares the domain assumptions of TMT and may be used to inform the support required to enable context-appropriate solutions to be selected and embedded |
|
NPT is traditionally used by IS researchers and focuses on the work that is done around an intervention or new set of activities to embed them into routine practice |
| Model for Improvement | The model outlines five steps for improvement: forming the team, setting aims, establishing measures, selecting changes and testing changes using plan–do–study–act cycles | Three fundamental questions form the foundation of this approach:
|
The Model for Improvement is traditionally used in QI programmes as a framework for developing, testing and implementing changes |
Principle 1: outcomes directed
The first principle of OUTCOME is that improvement is driven by an agreed outcome, rather than by predefined interventions. This reflects a growing concern that health-care improvement is often solution driven, rather than focused on improving practice. The emphasis on outcomes in the framework is informed by the concept of ‘projects’, which, in TMT, refers to a ‘goal-oriented enterprise, constructed by the interests that gather around it, and which has an associated division of labour, tools, technologies, practices, norms, rules and conventions’. 32 Thinking about improvement in terms of the associated project helps to define the boundaries of the initiative. In the PUMA study, this focused improvement efforts on the afferent component of a paediatric early warning system, which detects deterioration and triggers timely and appropriate action, and excluded the efferent component, which consists of the people and resources providing a response. 24
Principle 2: functions oriented
The second principle of OUTCOME is that improvement is oriented towards the functions necessary to achieve the goal. This requires specification of the primary mechanisms of action that are necessary in an overall process for the goal to be achieved. In the PUMA study, the core functions of an afferent early warning system were identified through the application of TMT to the systematic review and refined through discussions with clinicians to produce seven functions in total: monitor, record, interpret, review, prepare, escalate and evaluate. 13
Principle 3: systems focused
The third principle of OUTCOME is that improvement is focused on the sociomaterial resources, processes and mechanisms needed to enact the essential functions for achieving the goal. This requires specification of the minimum system requirements and draws on the concept of the strategic action field in TMT. Strategic action fields provide the structures, organising logics, technologies and materials, and interpretative repertoires that condition projects of collective action. 32
In the PUMA study, the system standard was specified in a propositional model of minimal conceptual requirements organised around the seven functions of an afferent paediatric early warning system (PUMA Standard). The model drew together two kinds of evidence from the systematic review: evidence of the challenges that must be overcome in detecting and acting on deterioration and evidence on proposed and/or evaluated solutions to challenges. The propositional model was reviewed and refined by parents with experience of a child’s deterioration and by clinical experts on the PUMA study team. The model was refined during the implementation phase of the PUMA programme to provide a more easily accessible version of the original (Figure 4) and summarised in the PUMA Wheel (Figure 5). Please note that the original, branded colour scheme differs from the colours present in the tables and figures of this report; the original colours can be seen in Report Supplementary Material 3.
FIGURE 4.
The core components of a paediatric early warning system: the PUMA Standard. Reproduced from Allen et al. 43 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.


FIGURE 5.
The core components of a paediatric early warning system: the PUMA Wheel. Reproduced from Allen et al. 43 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
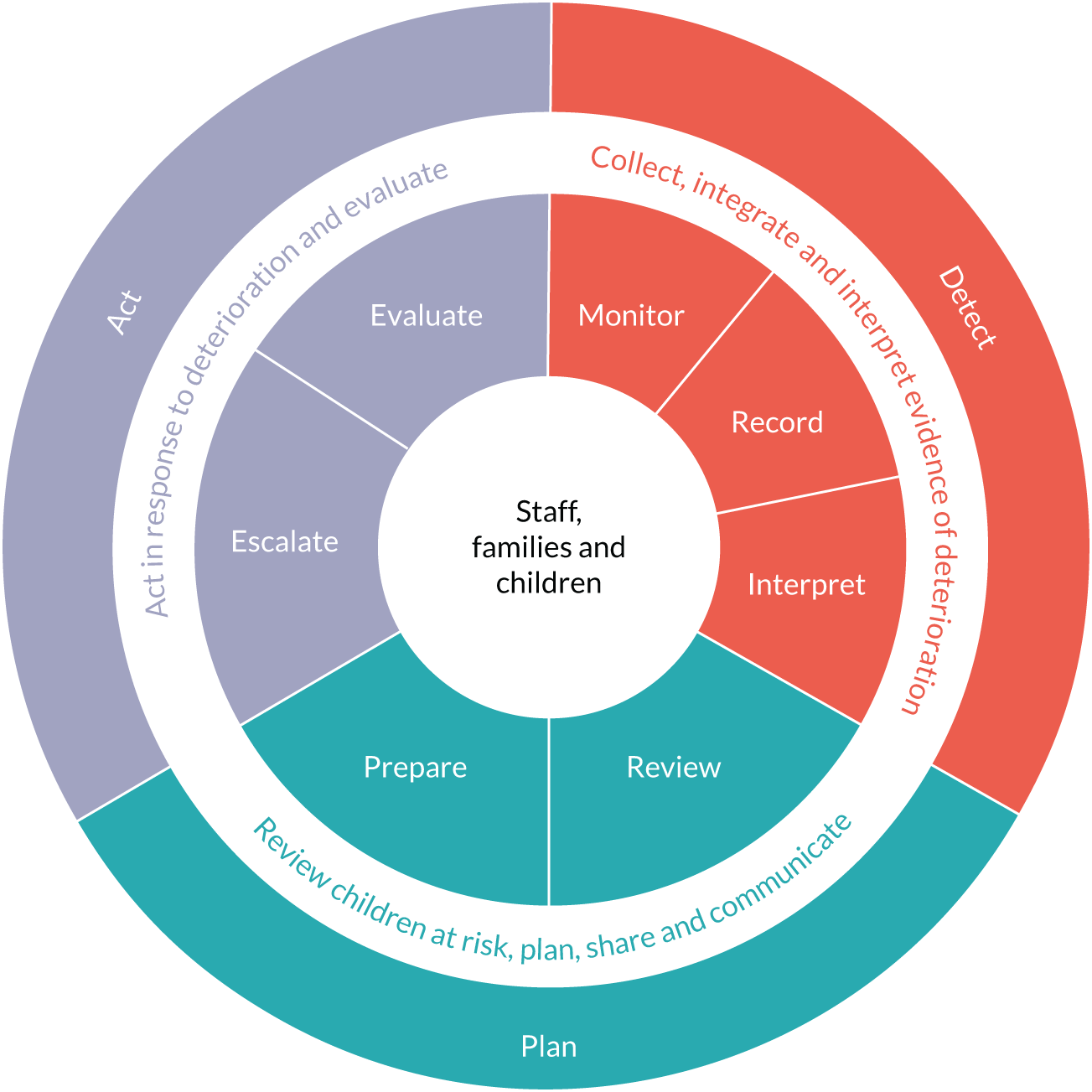
Principle 4: context specific
The fourth principle of OUTCOME is that improvement is focused on the development of context-specific initiatives to achieve the goal. Proponents of change often favour top-down approaches to bring about improvements; the list of interventions and improvement efforts that flounder when spread or scaled up continues to grow,197–199 however, in part because of failures to normalise and embed interventions into local contexts. As Braithwaite201 has argued, all meaningful improvement is local, and yet QI has been criticised for applying insufficient attention to rigorous evaluation and sharing the lessons of successes and failures to facilitate sustainability and spread. Avoiding these pitfalls requires structures to support systematic and rigorous local improvement efforts in relation to a service standard. In addition to specification of the minimum system requirements to support an improvement project, OUTCOME also involves the development of associated assessment tools that can be deployed to improve understanding of the local system and identify areas for improvement.
In the PUMA study, two complementary assessment tools were developed from the PUMA Standard: the Staff System Assessment Tool (SSAT) and the Family Feedback Tool (FFT). These were refined during the course of the study, along with the PUMA Standard. The tools were designed to prompt wider discussion among the improvement team, in order to reach a shared understanding of the local afferent paediatric warning system and areas that might be targeted for improvement.
Principle 5: locally led
The fifth principle of OUTCOME is that improvement capitalises on the expertise and knowledge of those delivering services. This is intended to encourage local ownership of the improvement initiative. An amended version of the Model for Improvement supports teams in driving their own improvement processes and is designed to operationalise the core constructs of NPT. It is based on a five-step process:
-
form an improvement team
-
assess the system
-
select and plan improvement initiatives
-
implement and review initiatives
-
sustain progress.
In the PUMA study, local leadership of the improvement process was supported through workshops, facilitation and written guidance.
Principle 6: learning systems
The final principle of OUTCOME is to create a learning system around the improvement project, with participants attuned to system features with strong feedback loops. 201 Health-care systems are dynamic, and wider changes to the system may be consequential for an area of practice, resulting in ‘drift’,210 or the need for further adjustments to the system. OUTCOME deploys the use of assessment tools to keep systems under review, and structures for supporting local leadership.
In the PUMA study, PIs were provided with written guidance on how to ‘sustain progress’ and were encouraged to repeat the system assessment every 12–24 months to reflexively monitor performance, select and plan initiatives, and implement and review initiatives. Table 7 summarises the principles, structures and theory informing the OUTCOME framework and its application to the PUMA study.
| Principles | Structures | Theory | PUMA |
|---|---|---|---|
| Outcome directed | |||
| Improvement is directed towards achieving an agreed outcome or goal | Specification of the collective action to be targeted for improvement and its overarching goal |
|
The goal of the PUMA study was to improve collective action in relation to the afferent component of a paediatric early warning system, which detects deterioration and triggers timely and appropriate action, and excluded the efferent component, which consists of the people and resources providing a response |
| Functions oriented | |||
| Improvement is oriented towards the functions necessary to achieve the goal | Specification of the core components, mechanisms of action and their relationships necessary to achieve the overarching goal |
|
Collective action in detecting and acting in response to deterioration includes detection (monitoring, recording, interpreting), preparation (reviewing, planning) and action (escalation, evaluation) |
| System focused | |||
| Improvement is focused on the sociomaterial system required to enact the functions necessary to achieve the goal | Minimum standards required to achieve the goal across contexts are specified (e.g. sociomaterial resources: people, materials, knowledge, processes and mechanisms) |
|
In the PUMA study, the minimal standards for a system for detecting and acting on deterioration was specified in propositional model structured around the seven core functions |
| Context specific | |||
| Improvement is focused on the development of locally appropriate initiatives to achieve the goals | Tools developed to assess systems against the standard |
|
|
| Locally led | |||
| Improvement capitalises on the expertise and knowledge of those delivering services | Five-step process to support improvement:
|
|
|
| Learning systems | |||
| Improvement is sustained by the creation of a learning system to optimise outcomes through the application of system assessment tools, to keep systems under review, and through structures for supporting local leadership |
|
|
Improvement guide provided guidance on repeating the system assessment every 12–24 months to reflexively monitor performance, select and plan initiatives, and implement and review initiatives |
Implementation of the PUMA programme
Implementation of the PUMA programme took place between June 2016 and November 2017. The improvement process was founded on the PUMA Standard, and implementation followed a structure to guide study sites through the PUMA programme’s five steps for improvement (Figure 6).
FIGURE 6.
Summary of PUMA programme.
First, PIs were encouraged to create a local improvement team to oversee the improvement process. Second, sites used the assessment tools to identify their own system’s strengths and weaknesses and considered potential solutions. Third, improvement teams planned their initiatives. Fourth, improvement teams implemented initiatives. The support and resources provided to teams during each of the steps is outlined in Table 8.
The specific implementation strategies adopted were designed to (1) disseminate information on, and clarify understanding of, the PUMA programme, and (2) facilitate and support each site’s engagement and ongoing participation.
| Improvement step | Facilitated workshop | Materials and resources sent to PIs | Additional facilitation strategies |
|---|---|---|---|
| 1. Form an improvement team | ‘Set-up’ session |
|
|
| 2. Assess the system | ‘Set-up’ session |
|
|
| 3. Select and plan improvement initiatives | ‘Action planning’ session |
|
|
| 4. Implement and review initiatives | ‘Action planning’ session |
|
|
| 5. Sustain progress |
|
Materials and resources were refined iteratively during the course of the PUMA study and later collated in an implementation guide (see Report Supplementary Material 3).
Summary
The OUTCOME framework for health-care improvement was developed as part of the study and designed to overcome the weaknesses of orthodox approaches to health-care improvement. OUTCOME draws on IS and QI to provide a framework to support teams to implement context-specific initiatives to achieve an overall improvement goal. In the PUMA study, OUTCOME offered a systematic approach to context-specific improvement around the shared goal of improving paediatric early warning systems. It provided a standardised approach across different settings, but still enabled those responsible for implementing interventions to select solutions that were more likely to work within the structures, organising logics, material and interpretative repertoires in the local context. 32 The PUMA Standard and associated assessment tools were central to the improvement programme. Limited facilitation was provided. In Chapters 5–8, we describe the impact of the PUMA programme in each of the four sites.
Chapter 5 Case study 1: Alder Hey Children’s Hospital
Please note that, although the abbreviation PEWS is defined as Paediatric Early Warning Score in the main body of the text, this definition is not necessarily applicable to data extracts, in which natural language use is maintained and no assumptions are made about the referent of the abbreviation (e.g. Paediatric Early Warning Score or Paediatric Early Warning System).
Pre-implementation phase
Paediatric early warning system in context
The hospital
Case study 1 was undertaken at Alder Hey Children’s Hospital, a large (260-bed) tertiary paediatric hospital in the north-east of England. The hospital has a national profile as a centre of excellence, and had recently moved into a new bespoke building, with dedicated family accommodation.
The ward
The ward case study focused on a specialist cardiac medical/surgical ward, which cared for cardiac and cardiac–surgical patients. The patient population was diverse and included children with established diagnoses and/or relatively stable conditions, others with more recent, unexpected or uncertain diagnoses and/or acute critical care needs, and day-surgery cases.
The ward had 24 beds divided into three ‘pods’, each with a nurses’ station. The orange pod comprised two four-bedded bays and was used for day-surgery patients and low-acuity children. The green pod was used for non-critical cardiac inpatients, with the sickest children allocated to beds visible from the nurses’ station. The blue pod was used for the most unwell and highest-dependency patients, and was classified as having ‘HDU’ beds (see Report Supplementary Material 4 for details).
Staffing
Ward staff comprised a ward manager, 38 band 5 nurses, six band 6 nurses, a nurse educator, four health-care assistants (HCAs) and a play specialist. Student nurses had placements on the ward. Many of the nurses were very experienced and had specialist skills, although none was Advanced Paediatric Life Support (APLS) trained. All qualified staff worked across regular and HDU patient beds. Staff turnover was low and agency staff usage was rare, with gaps in the rota filled by ward staff working ‘bank’ shifts.
Nurses and HCAs worked 12-hour shifts (07.00–19.00). The ward manager and nurse educator worked 08.00–16.00 weekdays, but frequently exceeded these hours. Nurses worked in one of the pods. A band 6 nurse was designated shift co-ordinator, but also carried a case load.
The cardiology medical team managed all patients; cardiology and surgical teams jointly managed surgical patients. The medical team comprised cardiology consultants, registrars and other junior doctors. Junior doctors rotated every 6 months. The cardiology team provided weekday and weekend cover, with day shifts starting around 08.00 and ending around 20.00. An on-site registrar and on-call cardiology consultant provided night cover. Medical staff from the PICU, which was adjacent to the ward, provided occasional ad hoc support to the cardiac registrar during night shifts. There was no RRT.
Routines
Nursing handover was at 07.00 and 19.00.
A shift co-ordinator’s handover was at 07.00 and 19.00.
There were two daily bed management meetings, attended by shift co-ordinators.
The medical team’s daily routine included (1) handover between the night and day shift registrars, (2) a daily ward round attended by the medical team and shift co-ordinator and (3) an evening ‘board round’ between the consultant, registrar and shift co-ordinator.
Family involvement
The ward environment was designed to accommodate parents, and staff encouraged them to be present on the ward. Sixteen single-occupancy rooms on the ward could accommodate parents overnight. Relationships between the ward team and families were highly valued and very positive, but, although the new infrastructure provided high levels of privacy, it prevented easy nurse–family communication.
Paediatric early warning system assessment
Detect
A detailed trust policy specified that observations were to be conducted on admission and during each clinical assessment. Nurses were required to conduct observations at least every 4 hours, but most children needed more frequent monitoring. Awareness of the detail of the formal policy was uneven, but appeared to be embedded in practice. There was a high level of awareness of the need for children with complex diagnoses and/or medical histories to have extra/additional vital signs monitored, or for certain key vital signs to be monitored more frequently. An electronic recording and scoring system was in use (MEDITECH-6; Medical Information Technology, Inc., Westwood, MA, USA), which included a ‘red clock’ that indicated when observations were due and acted as a prompt to ensure that all relevant vital signs were recorded, although nurses sometimes struggled to complete observations within schedule. Trust policy specified that the minimum frequency of observations for each patient was set by nursing staff on admission and reviewed at each nursing handover. Observation frequency for each patient was entered into MEDITECH-6.
Children admitted to the green and blue pods were monitored continuously. Real-time vital signs were displayed on bedside screens and terminals at the nurses’ stations. When vital signs fell outside predefined parameters, an alarm sounded at both the patient bedside and the nurses’ station. Elective day-surgery patients in the orange pod were monitored intermittently using portable equipment, which triggered alarms and could be connected to the nurses’ station as required.
Although continuous monitoring was widely used, scheduled observations were conducted manually and nurses were encouraged to look beyond the vital signs readings in assessing a child’s status, with nursing work allocated to ensure continuity of care and facilitate pattern recognition:
[I]t’s no good just looking at a monitor, because you need to be looking at their chest expansion and if they’re using extra muscles and whether its equal and whether they’ve got any other signs like a nasal flow, or other signs that they’re struggling to breathe.
Senior nurse
Relocation to the new hospital had created major challenges; the old accommodation was open plan and afforded high levels of surveillance over children, and colleagues and families were visible. With the new ward layout, this was lost, and affected how the unit worked. During the pre-implementation phase, nurses were still adjusting to these disruptive effects.
Although monitoring equipment was functional and available at all bedsides, nurses put effort into generating ‘good’ observational data; some of the ‘probes’ used to gather information on oxygen saturations or respiratory rate, for instance, did not fit small babies, and nurses had to actively work with the monitors, adjusting and calibrating different pieces of equipment, to ensure that vital signs readings were accurate. MEDITECH-6 used vital signs data and other variables to automatically calculate and communicate a ‘score’. This included ‘nurse concern of deterioration’, which scored 1, and ‘parental concern of deterioration’, which scored 2.
MEDITECH-6 was a relatively new intervention, and nurses were adjusting to its use in practice. Although MEDITECH-6 replicated the paper-based PTTT that it replaced, it had different affordances and its integration into practice had implications for nurses’ workflow. First, unlike the paper system, it did not immediately provide an overview of vital signs trends. Accessing this information required navigating through several screens (see Report Supplementary Material 5). Second, compared with recording observations on a paper chart, data entry in the electronic system was more time-consuming. Third, the requirement to access mobile computers resulted in nurses recording observations on scrap paper before later entering data into MEDITECH-6. Such practices carried the risk that vital information could be lost or misplaced, as illustrated by the following extract from the field notes:
[entering observation data into computer] I’m sure I did [9]’s obs[ervation].
I’m sure you did, you wrote it on a paper towel.
I can’t find it; I’m sure I did all three.
There are some pieces of paper towel here, are any of these yours? [3 × paper towel with numbers written on, no patient names or room numbers].
No, those are all [other nurses’].
The ward staff had good relationships with families, and often had cared for children previously. Many families had experience with, and understanding of, their child’s condition, but others were orienting themselves to an unexpected and/or new diagnosis. Although parents were encouraged to monitor their child and develop an understanding of bedside monitoring equipment, staff understood and responded to differences in capacity, and tended to give very knowledgeable parents more involvement in patient care. During the ward round, the consultant asked parents (when present) about their own understanding of their child’s health. Parents of children with long-term conditions were regarded as an important source of knowledge on baseline vital signs parameters.
Plan
There were a number of processes for reviewing individual patients and unit capacity, and these produced different levels of situation awareness across the nursing and medical teams.
Nursing handover was the key mechanism by which nurses formally collated and communicated information on patients’ statuses. Separate handovers were conducted in each pod; thus, nurses had an awareness of the patients in their area only. There were no formal face-to-face meetings between day and night staff; handovers were audio-recordings created by the nurse caring for the patient. All staff from the incoming shift reviewed this information by listening to the recording and annotating electronic pre-populated printed handover sheets. The quality of recordings was variable, however, and there was no formal opportunity for discussion between nurses from each shift.
In the nursing team, it was only the shift co-ordinator who had overall situation awareness of the ward and related units. A co-ordinator’s handover took place at 07.00 and 19.00. This was a face-to-face meeting in which co-ordinators shared detailed patient information, expected admissions, discharges and ‘step-downs’ from PICU, highlighted the ward’s most unwell patients and discussed concerns, and reviewed nurse staffing. The shift co-ordinators liaised with the ward nurses for updates on their patients before the meeting.
After the co-ordinator’s meeting, there was a bed management huddle attended by senior nurses from all critical care units. There were updates on the current status of HDU and PICU patients and information on planned and potential ‘step-down’ admissions to the ward. The shift co-ordinator communicated the ward’s current staffing levels and capacity.
Medical handover was an important mechanism for doctors to review children’s status. This took place at 08.00 and 20.00, and involved the night and day shift registrars only. All children were discussed consecutively and in detail, focusing on the patients of most concern. Outstanding work tasks were also identified. The registrar also attended PICU handover for an update on cardiac patients and expected discharges to the ward. Junior doctors received updated information on patients through an electronic handover sheet, which would have been updated by the outgoing medical team.
There was a 09.00 daily ward round led by the cardiac consultant and attended by the registrar and junior doctors. This was the primary mechanism by which the additional needs of ‘at-risk’ patients were identified and shared between nursing and medical staff. Ward rounds lacked a consistent format, however. In one model the medical team was accompanied by the shift co-ordinator and saw all their patients in turn. The nurse looking after each patient either remained in the patient’s room and communicated with the medical team directly, or, if possible, they liaised with the co-ordinator, who consulted the medical team on their behalf. There were other instances when the ward round was shared and conducted in two pods simultaneously, so the co-ordinator could attend only part of the round. In a third model, the ward round was conducted from an office; nurses were unable to attend because they could not leave the patients, and this created communication difficulties. Bedside ward rounds were markedly more formal events when compared with interprofessional communication at all other times. Communication between the senior doctors and parents was prioritised, with the nurse often left outside the room. Shift co-ordinators carried a case load and would frequently be called away to address other issues.
There was an evening ‘board round’ at 18.00 attended by the registrar, consultant, junior doctors and shift co-ordinator. The consultant led a team review of each patient to identify priority tasks and concerns. The co-ordinator found it more challenging to attend this handover, because of other competing priorities. It was also difficult to predict who would be present, and whether or not the results of the board round would be fed into nurse handover information for the next shift.
The ward had an electronic board linked to MEDITECH-6, which was intended to display up-to-date information on patients’ statuses across the ward. This was unused because it was inaccurate owing to delays in entering vital signs data, and was infrequently updated when patients were moved/discharged.
Act
There was a hospital-wide PTTT score built in to MEDITECH-6. Pop-up boxes prompted staff to identify and record a course of action when a score of > 3 was generated. Nurses were also required to indicate whether there had been a senior nurse or medical review during each observation, or if it was needed. The formal policy provided general guidance on escalation, but made reference to following instructions on the observation chart, which specified how to make use of the score, in what circumstances escalation should be triggered and the actions indicated. Nurses were well informed of the escalation policy and the respective responsibilities of nursing and medical staff.
When vital signs fell outside predefined parameters, an alarm sounded at the patient bedside and at the nurses’ station. However, many patients had vital signs readings (e.g. oxygen saturation levels) outside acceptable parameters for the general paediatric population, but normal for them because of their condition. MEDITECH-6 allowed vital signs thresholds to be adjusted, but this was the responsibility of the consultant and was rarely done. Nurses checked if parameters had been adjusted in order to assess whether or not to worry about alarms. Alarms activated frequently, and in the vast majority of circumstances were quickly silenced by either nursing staff or a parent/family member. Staff frequently discussed limitations of the MEDITECH-6/PTTT system, and repeatedly emphasised the importance of using their own professional judgement, alongside scores, when making patient assessments.
The ward had a supportive culture; junior nurses raised concerns first with the shift co-ordinator and were confident that these would be attended to. Interprofessional relationships were more variable. During the day, doctors had a high presence on the ward and, although nurses were mainly positive about the quality of relationships with medical staff, they also made reference to, and we observed, instances when they struggled to get doctors to act on their concerns:
I think the doctors and the nurse relationship is really, really good here [. . .] it depends which doctor [. . .] sometimes doctors don’t realise how serious you mean [. . .] like this little boy, [. . .] I knew him very well, so I knew that he really wasn’t right, whereas the doctor that, who happened to be on that day didn’t really know and he said ‘oh for that condition it can be that, that can be normal for that condition’ and I was thinking ‘yeah, but for him it’s not normal’ [. . .] so it’s often a problem there when the doctors don’t really understand what you’re trying to tell them, because they’ve got other ideas or something like that, you know.
Staff nurse
There was evidence that nurses could not always persuade doctors to respect their subjective assessments, and often they would make use of the more heavily weighted ‘parental concern’ variable in the PTTT to generate a score that better reflected their own clinical judgement. There were particular challenges at night when there were insufficient band 6 nurses to ensure consistent cover on all shifts, and junior nurses reportedly had more challenges in communicating with doctors.
There was a high level of contact between nursing staff (particularly) and parents, and this required the filtering of expressions of parental concern, to ensure that only relevant concerns were factored into clinical decision-making and the management of patient care. Report Supplementary Material 6, Table 1, shows a summary of the pre-implementation system strengths and weaknesses identified by the PUMA team.
Implementation phase
Process
The Alder Hey improvement team was led by two PIs (a consultant and a senior nurse), and included ward managers, cardiology consultants, cardiology liaison nurses and non-clinical staff involved in trust-level QI work. Membership fluctuated; some individuals remained throughout the implementation period, others were involved for a short time or to a limited extent. The team leaders identified three wards for the implementation of the PUMA programme: cardiac, medical and a third ward. The SSAT and FFT were completed on all three of these wards and the results (Figure 7) were used to identify areas for improvement.
FIGURE 7.
Alder Hey system assessment radar diagram. A score of 0 was used to indicate poor alignment with the system standard; a score of 10 indicated optimal alignment with the standard.

The team focused on elements of the wheel found to be the weakest (the involvement of families in the detection of deterioration, the reviewing of information, and identifying and preparing for risk) and used the process to develop a deeper understanding of these system weaknesses. These insights informed the development of the action plan, which evolved over time in response to local staff feedback, ongoing interactions with the PUMA team and significant organisational events. Interventions were not strongly branded as PUMA. Report Supplementary Material 7, Table 1, shows a summary of the pre-implementation system strengths and weaknesses identified by the improvement team, and Figure 8 shows the timeline for implementation of each initiative.
FIGURE 8.
Alder Hey implementation process timeline.

Context
Key organisational-level changes in response to critical incidents that related explicitly to the paediatric early warning system had significant implications for the implementation of the PUMA programme at Alder Hey. In November 2016, a patient died of sepsis as a result of a failure to recognise and act on signs of deterioration. This was immediately classified as a serious incident and triggered two inspections by the Care Quality Commission (CQC), which drew attention to inadequacies in recording observations that had a negative impact on the accuracy of PEWS and the timeliness of care escalation. 211 The critical event and subsequent CQC inspections led to numerous trust-mandated changes to the paediatric early warning system (see Initiatives).
Other organisational changes included a reduction in the number of PICU and HDU beds because of nursing shortages.
The trust also introduced a new ‘Global Digital Excellence’ project, with the aim of standardising all electronic documentation used at the site, which increased the workload of the PIs and took time away from the PUMA programme.
Initiatives
PUMA initiatives
Five PUMA initiatives were proposed at Alder Hey (see Report Supplementary Material 8, Table 1):
-
monthly critical deterioration review
-
out-of-hours standard operating procedure (SOP) for on-call doctors
-
family engagement tool
-
training clinical staff on the Alder Hey PEWS, recognition and response to deterioration, and National Institute for Health and Care Excellence (NICE) sepsis screening
-
SOP for improving ward rounds.
Monthly critical deterioration review
Led by a consultant and senior nurse, these meetings were intended to address weaknesses in the organisation’s approach to reviewing and learning from critical deterioration events. It related to the evaluate function in the propositional model.
The initiative was to comprise ‘critical deterioration review meetings’, held between a senior nurse and consultant paediatrician, and an online, anonymous survey of staff members involved in cases of critical deterioration. In a series of (three or four) meetings, multiple cases of critical deterioration were reviewed in detail. Eleven survey questions were designed and piloted with nursing and medical teams to generate ‘direct feedback’ from the teams involved through an anonymous staff survey, but the project then stalled and the initiative did not become embedded in practice. The work of collating information in preparation for each meeting was significant, as was the time required to review each case in the necessary detail, and the initiative was not sustained.
Out-of-hours standard operating procedure for on-call doctors
The cardiac ward had its own out-of-hours cover, whereas a medical and surgical team (one consultant and one registrar) covered the rest of the hospital. The out-of-hours SOP was developed in response to weaknesses in identifying and planning for risk. Normal practice was for doctors to review every patient currently admitted to the hospital, regardless of clinical acuity. The initiative was led by a consultant, building on previous activity around the organisation of medical work, who began by observing current junior doctors’ working practices and established that the handover was particularly poor at sharing key information on the ‘sickest kids’ across the hospital between doctors, and between medical and nursing teams. The new SOP introduced a system of working in which on-call medical teams were able to prioritise workload according to clinical need. It also introduced a third member of on-call medical staff (an additional registrar) and required nursing teams to follow a SBAR technique model of communication when requesting an out-of-hours medical review. The initiative was successfully introduced and embedded across the organisation.
Family engagement tool
This initiative aimed to ‘improve family satisfaction’ and ‘increase family involvement in care planning’ through the design and implementation of a communication tool to be implemented on the cardiac ward. A senior nurse led the initiative in partnership with a cardiology consultant, with additional input from a ward manager, a senior nurse, a junior doctor and a play specialist. Possible approaches were discussed in a series of meetings, but progress stalled because the technological infrastructure was not available. The team decided to implement ideas and approaches that had been successfully embedded in practice elsewhere and selected MyPad, a communication board used in the general HDU. However, although some progress was made in tailoring the tool for use on the cardiac ward, the initiative ceased as a result of significant changes in the structure of the senior nursing team, and the discovery of plans to implement communication technologies, namely iPads (Apple Inc., Cupertino, CA, USA), across the organisation.
Training clinical staff
This initiative resulted from organisational-level mandated changes in response to the serious critical incident. As outlined in one of the Improvement Team planning documents, the CQC identified a number of areas of system weakness, which aligned with the priority areas identified through the system assessment, namely ‘Staff were not compliant with Trust policy in obtaining and recording observations as per PEW tool guidance’ and ‘Lack of a standardised tool to recognise sepsis across the Trust’. The selected initiative consisted of retraining clinical staff on the Alder Hey PEWS, recognition and response to patient deterioration, and the NICE-endorsed sepsis screening pathway (Sepsis 6). 212 A ‘staff competency’ document was used alongside training sessions to assess and record each staff member’s level of understanding, and data on staff receiving and completing training were collected across the trust. Initiative four was successfully introduced across the whole organisation. Because this initiative was a mandated change in response to the CQC report, organisational-level support was guaranteed from the outset; therefore, a high level of practical, financial and regulatory assistance was provided throughout the development and implementation process. However, site leads indicated that the initiative ‘absorbed a high percentage of time’ and required ‘very tight project management’, with negative consequences for other PUMA initiatives.
Standard operating procedure for improving ward rounds
This initiative was designed to improve processes for reviewing children and planning for action on the cardiac ward and was led by a senior nurse working with a cardiology consultant. In the pre-implementation period, sometimes the ward round was divided into two medical teams undertaken simultaneously or conducted away from the ward. This meant that the shift co-ordinator could not attend both rounds, and therefore lacked situational awareness; the medical team also had a fragmented understanding of all patients. There was also significant variation in the way evening ‘board rounds’ were conducted: it was difficult to predict, on a regular basis, who would be present, and whether or not the results of the board round would be fed into nurse handover information for the next shift.
Some progress was made in getting key staff members to discuss the issue, but the momentum was not sustained and no ward round SOP was implemented. For the initiative to be successful, both nursing and medical teams needed to be involved; securing the direct involvement of senior medical staff was a particular challenge:
[T]he consultant team couldn’t agree what they wanted, how they wanted it to look. So we’ve spent months trying to get some sort of consensus.
Staff nurse
Non-PUMA initiatives
In addition to the PUMA programme initiatives, a number of other initiatives were implemented that affected the paediatric early warning system. These included additional organisational changes as a result of the CQC visit and other system changes implemented by ward-level staff.
Lower trigger threshold
The threshold on which a score triggered senior (medical) review was lowered after the critical incident took place (November 2016).
Sepsis 6 pathway
The Sepsis 6 pathway212 was implemented as a direct result of the CQC visit and the recognition that there was no standardised tool to recognise sepsis across the trust. Training for the pathway was implemented as part of the PUMA training initiative. In addition, it was introduced to the MEDITECH-6 system. This required nurses to answer an additional range of questions while inputting patient data. This was extra work, but the disruptive effects were mitigated by the fact that, at the time of this change, the electronic system had become normalised.
Appointment of additional staff: specialist sepsis nurses
A number of specialist sepsis nurses were employed from June 2017. This specialist team covered the whole hospital. They worked in advisory capacity if there were incidents involving sepsis and were also involved in training.
Development of a standard operating procedure to define and clarify the shift co-ordinator role
In a further development, the trust implemented a SOP for the shift co-ordinator role, to address a perceived lack of consistency. The aims were to generate role clarity, to ensure that the co-ordinator was able to support and direct the team to ensure that child safety was maintained, and to make ward staff aware of the roles and responsibilities of the co-ordinator.
Introduction of safety huddle
The cardiac ward implemented a formal safety huddle, which was led by the ward manager, who had been involved in the system assessment on the cardiac ward. The huddle was a 5-minute meeting immediately prior to both the morning and evening nursing handover meetings. The outgoing shift co-ordinator presented information relating to both ward and patient concerns to all incoming nursing staff. A handover sheet was introduced to help support this process that contained sections on ‘key messages’, ‘watchers/PEW triggers’, ‘safeguarding concerns’ and ‘recent incidents’.
Ward-specific escalation plan
A ward-specific ‘Escalation Plan for a Deteriorating Cardiac Patient’ was introduced, led by the ward manager and nurse educator, working in partnership with senior medical (cardiology) colleagues. The policy was developed in response to the fact that the particular clinical characteristics of many cardiac patients did not ‘fit’ the trust-level PTTT system and required vital signs parameters to be adjusted. This long-recognised issue was identified in the PUMA system assessment, although not prioritised in the action plan.
The final policy specified cardiac-specific guidance on the electronic PTTT system and provided updated detail on the role of the shift co-ordinator within the escalation pathway. Specific time frames were provided (‘medical review within 30 minutes’) and instances when consultant review must be sought were highlighted (‘consultant must be alerted to a new PEWS of 4 or nursing or parental concern or increased lactate above 3’). Printed copies of the new plan were placed at each nurses’ station, and, echoing the changes brought about by the out-of-hours SOP, were bundled with the SBAR technique sheets (with the aim of aiding clear and direct communication).
Communication checklist for high-dependency unit and paediatric intensive care unit transfers
A communication checklist for improving communication between staff and the family members of patients transferred from the HDU or PICU was developed and introduced to the cardiac ward in June 2018. This detailed information to be discussed with parents on admission, including information on the electronic monitoring system and a reminder to inform families that they can ask for help at any time. Nurses were required to sign off each completed task before returning the completed sheet to the ward manager. However, although the checklist was in regular use for a short period of time, it did not become embedded as a result of significant changes in the senior nursing team. Progress on alternative approaches was halted because it was anticipated that the trust would be introducing a hospital-wide system for communicating with parents. Report Supplementary Material 9, Table 1, summarises the implementation of action plan initiatives for Alder Hey and Appendix 21, Table 45, summarises all the embedded changes over the course of the study.
Post-implementation phase
Paediatric early warning system in context
The ward also experienced contextual changes that were consequential for system functioning in the post implementation period.
The number of HDU beds on the cardiac ward was increased from 8 to 10 (two beds in the ‘green’ zone were converted from standard care to HDU), and there was an associated increase in the number of qualified nursing staff employed on the ward. There were several new band 5 appointments in response to internal promotions and the loss of several staff. Overall, there was an increase in band 6 nurses, which ensured consistent senior nursing cover for both day and night shifts. All new and existing band-6 nurses received APLS training; the consistent presence of APLS-trained staff had been highlighted as a requirement by the CQC, in line with Royal College of Nursing (RCN) guidelines. 213 Two of the ward’s band 6 nurses were undertaking additional training to qualify as advanced nurse practitioners (ANPs).
The ward received an additional number of mobile computers; these were reported to be quicker, and to have a better battery life, than the ward’s previous computers.
The hospital escalation plan was updated in March 2018.
Paediatric early warning system assessment
Detect
In the post-implementation phase, it was evident that the MEDITECH-6 had become normalised, and the addition of more mobile computers helped to obviate some of the challenges nurses had experienced with timely data entry. In addition, the ‘Sepsis 6 pathway’212 was in routine use. Although there was a loss of some senior experienced members of the team, all nursing staff had received training on PEWS/Sepsis 6, and all senior nursing staff had received APLS training.
More recently qualified nurses who had joined the ward appeared to value the PTTT score.
The new cardiac-specific escalation policy was in use. This document provided staff with clear details on when to escalate (according to PTTT score and key vital signs). The policy formalised the co-ordinator’s level of seniority and responsibility within the escalation pathway, and the specific care requirements and typical vital signs observations of cardiac patients. Laminated, printed copies were positioned at each of the nurses’ stations, and electronic copies were available on the trust’s intranet.
Plan
The main mechanisms for reviewing and planning for action remained the same, but situational awareness across the nursing team had been improved through the introduction of the safety huddle.
Some of the challenges with the ward round remained; some ward rounds continued to be conducted away from the patient bedside, and thus excluded nursing staff and families. Co-ordinators were still allocated their own patient case load, and frequently ‘pulled away’ from the morning ward round because of multiple competing demands.
The out-of-hours working SOP was designed to address issues with the organisation of doctors’ work, but had minimal impact on the ward system because the cardiac ward had historically received a high level of out-of-hours medical cover. It was reported that cardiology team members could be more difficult to reach during the night if rostered to provide hospital-wide cover.
Although the timing and frequency of the evening board round remained variable, senior nurses reported that they are able to attend in the majority of instances, and the electronic board used during the meeting was more frequently updated, but rarely used at any other time.
Act
Consistent band 6 cover, coupled with the new ward-specific escalation policy – which clearly states that the nurse in charge may escalate directly to registrar or consultant without having to ‘go through’ the junior medical team – provides a clear, formalised mechanism for effectively communicating concerns across professional boundaries: roles and responsibilities are better understood.
All senior nursing staff had received APLS training, which included responding to deterioration.
Summary
Three of the five PUMA programme initiatives were successfully developed and implemented during the PUMA implementation phase. The out-of-hours SOP for on-call medical teams and the training of clinical staff became embedded throughout the organisation. The Monthly Critical Deterioration Review meeting was implemented, but did not become embedded, and the Family Engagement Tool and SOP for improving ward rounds did not progress beyond the planning stage. Beyond the formal PUMA initiatives, there were extensive changes to the paediatric early warning system during the lifetime of the study. Organisational-level initiatives, many of which were in response to the CQC report, included the introduction of a lower-trigger PTTT threshold and a specific Sepsis 6 pathway,212 the appointment of specialist sepsis nurses, APLS training for band 6 nurses and the development of a SOP to define and clarify shift co-ordinator roles. In addition, a number of ward-level initiatives were implemented and embedded, notably the introduction of a safety huddle, which improved the situation awareness of the ward nurses and support staff; a ward-specific escalation plan; and a communication checklist for HDU and PICU transfers. Report Supplementary Material 10, Table 1, shows a summary of post-implementation system strengths and weaknesses identified by the PUMA study team mapped against the PUMA Standard.
Wider impact of the PUMA programme
There was evidence of a continued interest in adopting the PUMA approach to provide a structure for continuous review of the paediatric early warning system on the cardiac ward and one other ward, as well as evidence of a systems approach to improving detecting and acting on deterioration on the part of the cardiac ward and improvement team.
Quantitative analysis
Monthly aggregate-level data were collected at a whole-hospital level in Alder Hey, between May 2015 and October 2018. Across all paediatric inpatient wards, patient bed-days averaged 6316 per month.
Further details on the approach to analysis of the data are described in Chapter 2. Appendix 10 shows the full statistical report for Alder Hey, including a series of exploratory (see Appendix 10, Figure 28 and Table 27) and sensitivity analyses (see Appendix 10, Table 28) performed on the primary outcome.
Primary outcome
Figure 9 shows fitted lines for pre-intervention, implementation and post-intervention rates of adverse events, per 1000 patient bed-days. For all figures, solid red lines represent observed fitted trend lines, dotted red lines represent projected trends based on a continuation of pre-intervention trajectory and dotted green lines represent 95% CIs around the observed fitted trend lines.
FIGURE 9.
Alder Hey scatterplot with fitted line from segmented linear regression for the primary outcome.
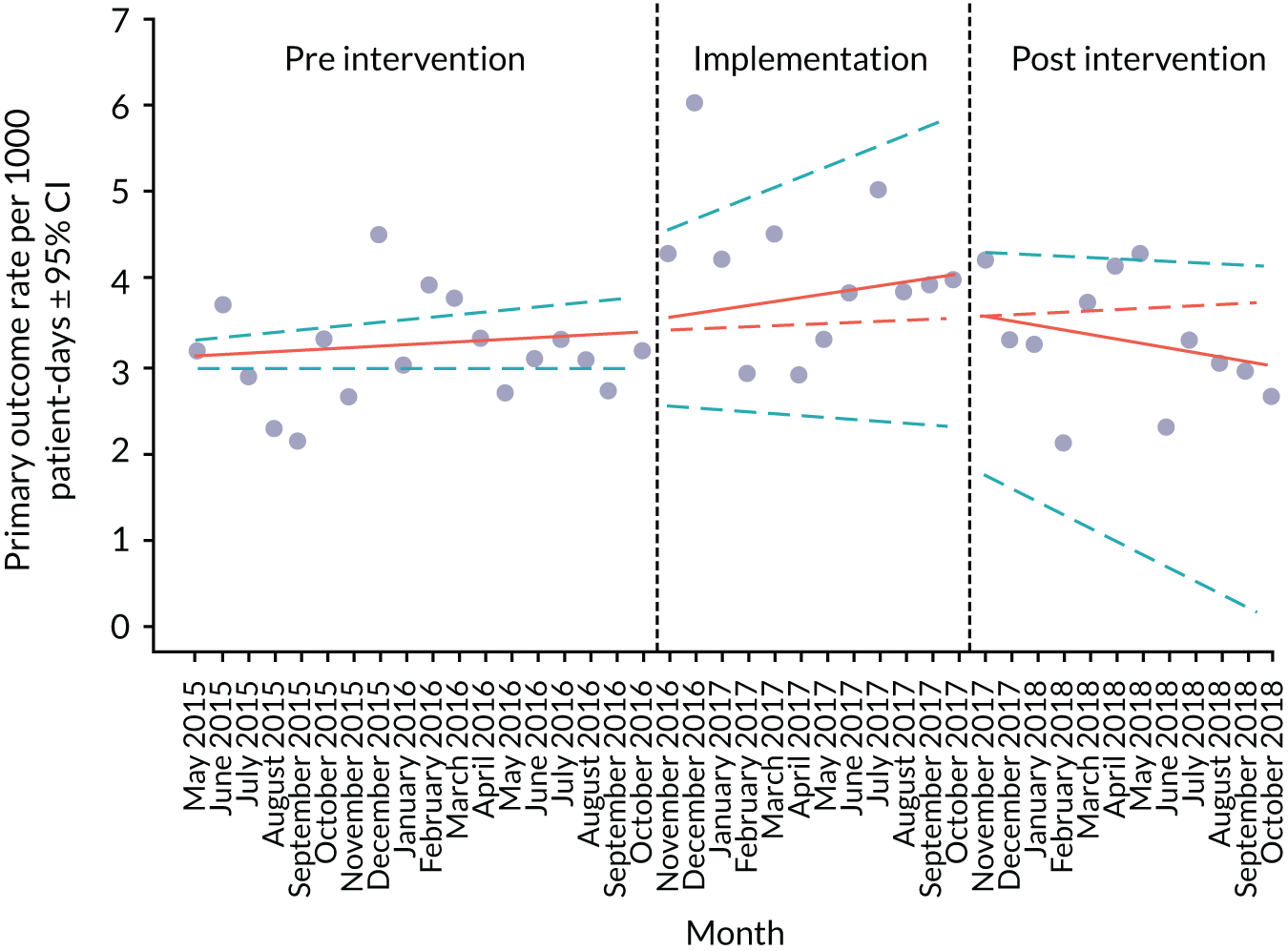
The overall rate of adverse events per 1000 patient-bed-days was 3.15 in the pre-intervention period, 4.08 in the implementation phase and 3.29 in the post-intervention phase.
Table 9 presents estimates from segmented linear regression for adverse events, including an interpretation of key findings. In the pre-intervention period, Alder Hey showed a slight upwards trajectory in rates of adverse events over time (β = 0.02, 95% CI 0.00 to 0.03). During the implementation phase, the observed rate of adverse events trended further upwards (β = 0.03, 95% CI –0.03 to 0.09), but with no significant difference from the projected pre-intervention trend (p = 0.29). However, during the post-intervention period, there was a downwards trajectory to the trend in adverse outcomes (β = –0.09, 95% CI –0.15 to –0.09), which was significantly different from the projected implementation trend (p < 0.001).
| Outcome | Estimate, β (95% CI) | p-value | Interpretation |
|---|---|---|---|
| Adverse events | |||
| Intercept | 3.08 (2.93 to 3.24) | < 0.00001 | |
| Pre-intervention trend | 0.02 (0.00 to 0.03) | 0.04 | Adverse events were very gradually, but significantly, increasing during this period. Given the low overall rates, the clinical impact of this increase is difficult to determine |
| Change in slope (implementation period vs. pre-intervention period) | 0.03 (–0.03 to 0.09) | 0.29 | There was a trend towards an increasing rate of adverse events (against the expected trend), but this was not significant. The wide CIs mean that the trend could have been in either direction should a greater sample size have been available |
| Immediate change in level (implementation period vs. pre-intervention period) | 0.15 (–0.34 to 0.64) | 0.55 | |
| Change in slope (post-intervention period vs. implementation period) | –0.09 (–0.15 to –0.05) | < 0.001 | Adverse event rates decreased by nearly 10% in this period, compared with the implementation period, which was statistically significant |
| Immediate change in level (post-intervention period vs. implementation period) | –0.43 (–1.03 to 0.17) | 0.16 | |
Secondary outcomes
Figure 10 shows fitted trends (or raw data, where fitted trends were not possible) for individual secondary outcome rates across the three time periods, per 1000 patient bed-days. Table 10 presents estimates from segmented linear regression for secondary outcomes, including an interpretation of key findings.
FIGURE 10.
Alder Hey scatterplot with fitted line from segmented linear regression for secondary outcomes. (a) All-cause mortality; (b) cardiac arrests; (c) respiratory arrests; (d) unplanned transfers to PICU; (e) unplanned transfers to HDU; (f) PICU staff reviews; and (g) other medical emergencies.


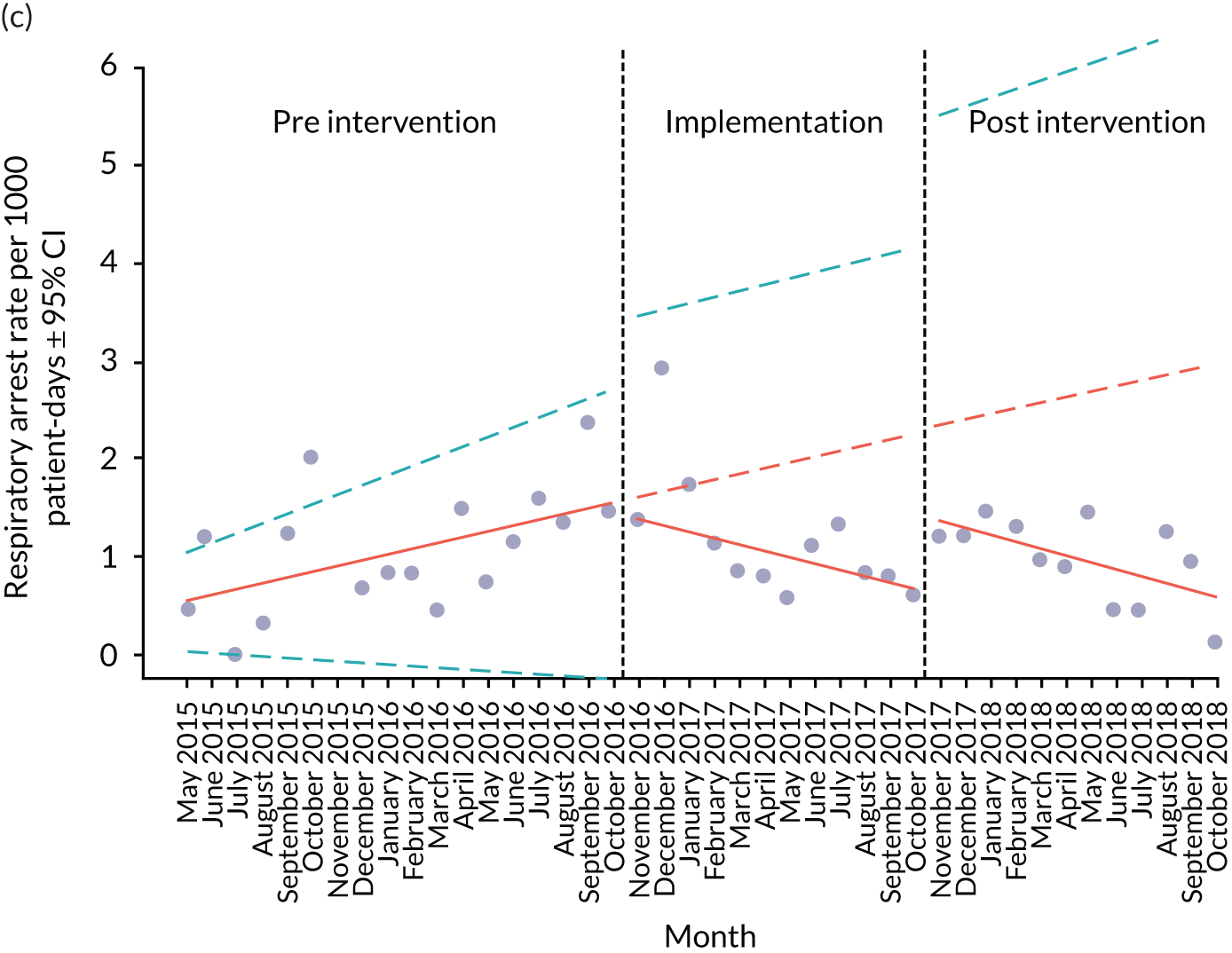
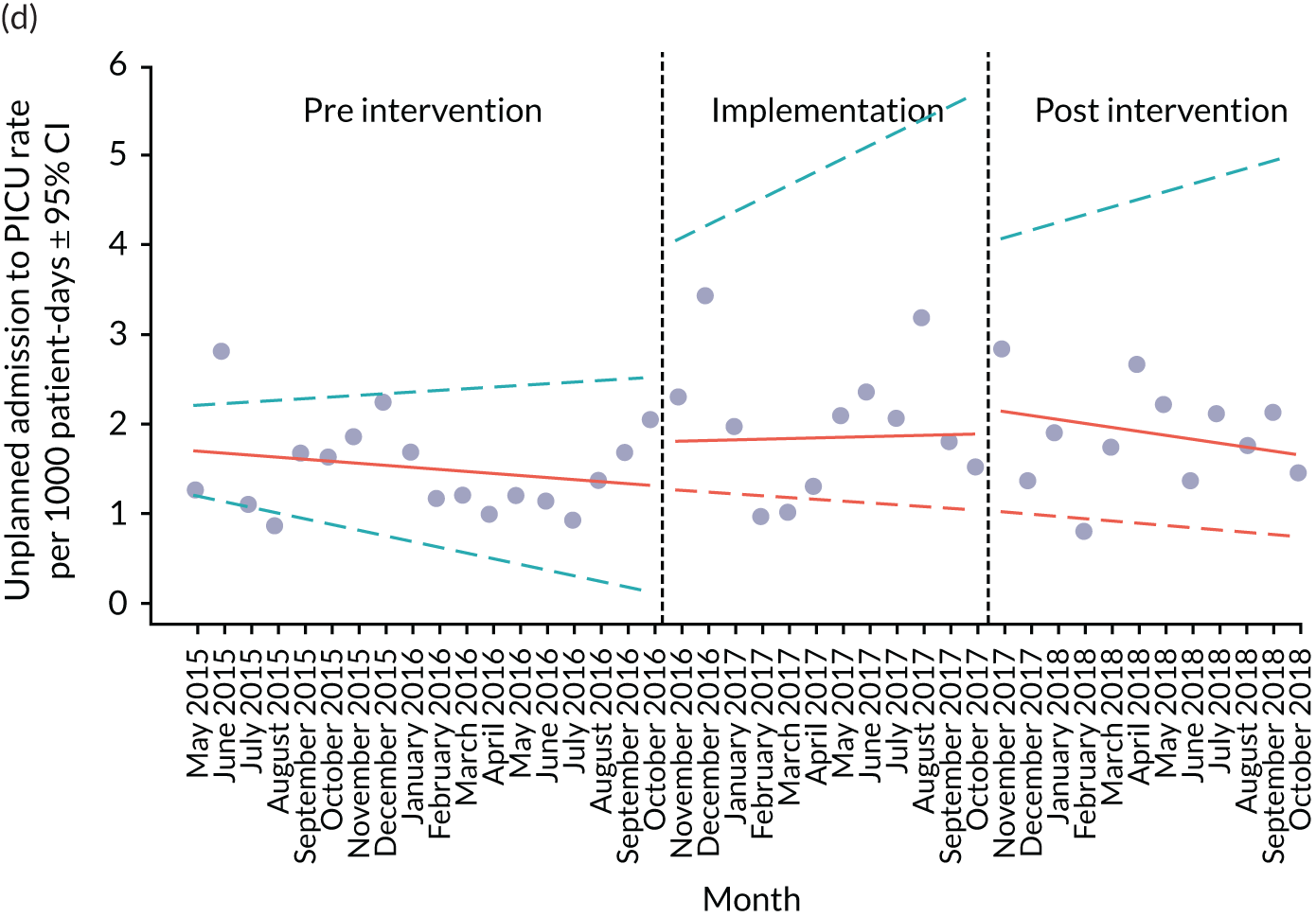
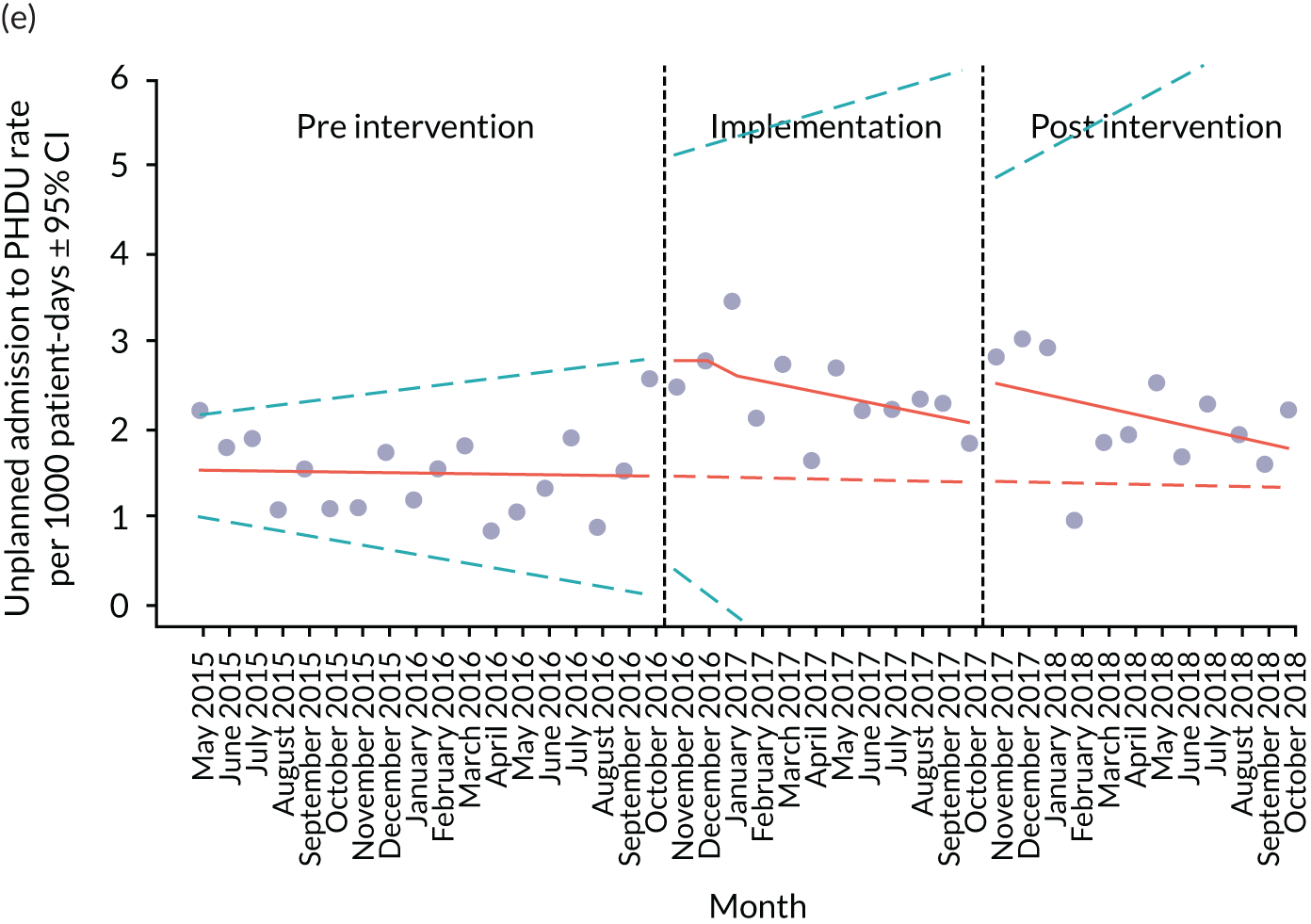

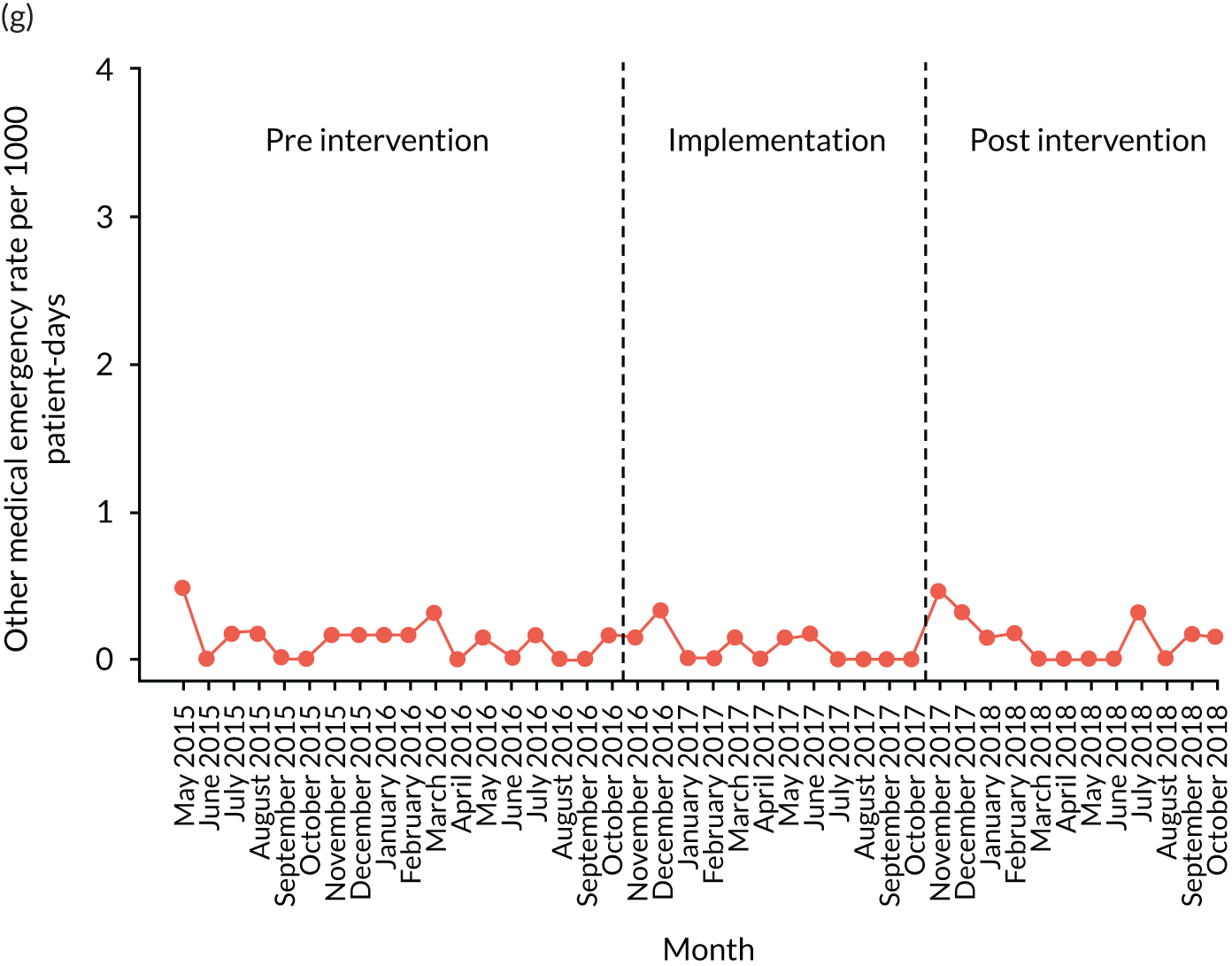
| Outcome | Estimate (95% CI) | p-value | Interpretation |
|---|---|---|---|
| All-cause mortality | |||
| Intercept | 0.85 (0.64 to 1.07) | ||
| Pre-intervention trend | 0.02 (0.00 to 0.04) | 0.15 | There was a very gradual, non-significant trend towards increasing all-cause mortality, but this could be natural variation, as opposed to a specific cause |
| Change in slope (implementation phase vs. pre-intervention phase) | –0.01 (–0.05 to 0.03) | 0.68 | The pre-implementation phase was not significantly different from the implementation phase |
| Immediate change in level (implementation period vs. pre-intervention period) | –0.22 (–0.58 to 0.13) | 0.22 | |
| Change in slope (post-intervention phase vs. implementation phase) | –0.03 (–0.09 to 0.02) | 0.20 | There was a gradual non-significant trend towards a reduction in all-cause mortality |
| Immediate change in level (post-intervention period vs. implementation period) | –0.18 (–0.57 to 0.21) | 0.21 | |
| Respiratory arrests | |||
| Intercept | 0.51 (0.08 to 0.94) | ||
| Pre-intervention trend | 0.06 (0.02 to 0.10) | < 0.01 | Respiratory arrest rates significantly increased during the pre-intervention period |
| Change in slope (implementation phase vs. pre-intervention phase) | –0.12 (–0.21 to –0.03) | 0.01 | There was a significant reduction in the respiratory arrest rate during the implementation phase |
| Immediate change in level (implementation period vs. pre-intervention period) | –0.09 (–0.82 to 0.64) | 0.81 | |
| Change in slope (post-intervention phase vs. implementation phase) | –0.01 (–0.11 to 0.10) | 0.92 | The direction of the trend was similar to the implementation phase, so no significant difference in slope was observed |
| Immediate change in level (post-intervention period vs. implementation period) | 0.75 (0.03 to 1.48) | 0.04 | |
| Unplanned PICU transfers | |||
| Intercept | 1.72 (1.26 to 2.17) | The slope matches the trend during the pre-intervention period, but the CIs do not support this being a statistically significant change | |
| Pre-intervention trend | –0.02 (–0.07 to 0.02) | 0.31 | |
| Change in slope (implementation phase vs. pre-intervention phase) | 0.03 (–0.07 to 0.13) | 0.55 | This trend did not persist during the implementation period, but the change in slope was not significant |
| Immediate change in level (implementation period vs. pre-intervention period) | 0.50 (–0.34 to 1.34) | 0.25 | |
| Change in slope (post-intervention phase vs. implementation phase) | –0.05 (–0.17 to 0.06) | 0.38 | The trend was towards an ongoing reduction in unplanned transfers during pre-intervention period, but the data indicate that the trend is not significant |
| Immediate change in level (post-intervention period vs. implementation period) | 0.29 (–0.61 to 1.20) | 0.53 | |
| Unplanned HDU transfers | |||
| Intercept | 1.55 (1.04 to 2.07) | Although the projected trend of HDU transfers was essentially level, deviations from this are difficult to evaluate because of the very wide CIs and because the overall rate of HDU transfers increased in the post-intervention phase, compared with the pre-intervention phase | |
| Pre-intervention trend | –0.01 (–0.05 to 0.04) | 0.80 | |
| Change in slope (implementation phase vs. pre-intervention phase) | –0.06 (–0.16 to 0.05) | 0.27 | |
| Immediate change in level (implementation period vs. pre-intervention period) | 1.36 (0.50 to 2.23) | 0.01 | |
| Change in slope (post-intervention phase vs. implementation phase) | –0.01 (–0.14 to 0.12) | 0.91 | |
| Immediate change in level (post-intervention period vs. implementation period) | 0.57 (–0.29 to 1.43) | 0.19 | |
| PICU reviews | |||
| Intercept | 0.47 (0.32 to 0.61) | ||
| Pre-intervention trend | 0.06 (0.04 to 0.07) | < 0.0001 | PICU reviews significantly increased during the pre-intervention period |
| Change in slope (implementation phase vs. pre-intervention phase) | –0.05 (–0.08 to –0.03) | < 0.001 | This rate of increase was reversed during the implementation phase and was highly significant |
| Immediate change in level (implementation period vs. pre-intervention period) | 0.08 (–0.20 to 0.36) | 0.58 | |
| Change in slope (post-intervention phase vs. implementation phase) | –0.03 (–0.07 to 0.00) | 0.08 | This downwards trend continued in the post-implementation phase, but did not reach significance |
| Immediate change in level (post-intervention period vs. implementation period) | –0.23 (–0.59 to 0.14) | 0.23 | |
Mortality
The overall all-cause mortality rate was 0.98 per 1000 patient bed-days in the pre-intervention period, 0.96 in the implementation phase and 0.61 in the post-intervention phase. Mortality rates trended slightly upwards in the pre-intervention period, but not significantly (β = 0.02, 95% CI 0.00 to 0.04; p = 0.15). The trend in mortality rates flattened during the implementation phase, representing a slight decrease relative to the pre-intervention trend (β = –0.01, 95% CI –0.05 to 0.03; p = 0.68), and then trended downwards in the post-intervention phase (β = –0.03, 95% CI –0.09 to 0.02; p = 0.20) (see Figure 10a).
Arrests
The overall cardiac arrest rate was 0.32 per 1000 patient bed-days in the pre-intervention period, 0.23 in the implementation period and 0.10 in the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in cardiac arrests over the different time periods (see Figure 10b).
The overall respiratory arrest rate was 1.06 per 1000 patient bed-days in the pre-intervention period, 1.19 in the implementation period and 0.99 in the post-intervention period. Rates of respiratory arrests trended significantly upwards in the pre-intervention period (β = 0.06, 95% CI 0.02 to 0.10; p < 0.01). This trend reversed in the implementation period, representing a significant downwards shift in the rate of respiratory arrests, compared with the post-intervention trajectory (β = –0.12, 95% CI –0.21 to –0.03; p = 0.01). The rate of arrests continued to trend downwards in the post-intervention phase, but with no significant change in the slope, compared with the implementation phase (β = –0.01, 95% CI –0.11 to 0.10; p = 0.92) (see Figure 10c).
Unplanned transfers
The overall rate of unplanned transfers from inpatient wards to PICU during the pre-intervention period was 1.50 per 1000 patient bed-days, compared with 1.99 for the implementation period and 1.87 for the post-intervention period. The equivalent overall rates for HDU transfers were 1.51 for the pre-intervention period, 2.41 for the implementation period and 2.17 for the post-intervention period.
In the pre-intervention period, there was a slight downwards trend in both PICU transfer rates (β = –0.02, 95% CI –0.07 to 0.02; p = 0.31) and HDU transfer rates (β = –0.01, 95% CI –0.05 to 0.04; p = 0.80). PICU transfer rates trended upwards in the implementation period (β = 0.03, 95% CI –0.07 to 0.13), then downwards in the post-intervention period (β = –0.05, 95% CI –0.17 to 0.06; p = 0.38). For HDU transfers, there was a non-significant downwards trend in the implementation period (β = –0.06, 95% CI –0.16 to 0.05; p = 0.27), which continued on a similar trajectory in the post-intervention period (β = –0.01, 95% CI –0.14 to 0.12; p = 0.91) (see Figures 10d and e).
Paediatric intensive care unit reviews
The overall rate of PICU reviews during the pre-intervention period was 0.96 per 1000 patient bed-days, compared with 1.66 for the implementation period and 1.30 for the post-intervention period. The rate of PICU reviews trended significantly upwards in the pre-intervention period (β = 0.06, 95% CI 0.04 to 0.07; p < 0.00001), before a significant downwards trend in the implementation phase (β = –0.05, 95% CI –0.08 to –0.03; p < 0.00001). There was a further downwards trend in PICU reviews in the post-intervention period, but the change in slope between post intervention and implementation was not significant (β = –0.03, 95% CI –0.07 to 0.00; p = 0.08) (see Figure 10f).
Other medical emergencies
The overall rate of other medical emergencies during the pre-intervention period was 0.13 per 1000 patient bed-days, compared with 0.08 for the implementation period and 0.14 for the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in other medical emergencies over the different time periods (see Figure 10g).
Synthesis
In this final section, we consider how some of the clearer quantitative findings relate to qualitative observations. Assessing the impact of the intervention on quantitative outcomes was challenging; therefore, interpreting the quantitative outcomes in relation to the ethnographic observations should be treated with caution.
Alder Hey implemented multiple organisational-level changes over the lifetime of the study, mandated in response to a critical CQC report. During the implementation period, the ITS analysis showed a short-term increase in the composite measure of adverse events, driven largely by increasing rates of HDU and PICU transfers. These coincided with local changes to PTTT escalation thresholds, which lowered the scoring threshold at which children had to be reviewed by a senior medical staff member. Over time, as further mandated changes were implemented, the adverse event rate markedly decreased in the post-intervention period, including decreasing rates of all-cause mortality and respiratory arrests. Although many of the changes implemented in Alder Hey were not formally identified as PUMA initiatives, they were in alignment with the areas of improvement identified in the site’s initial system assessment, and show how mandated organisational-level system change can have a positive impact.
Chapter 6 Case study 2: Arrowe Park Hospital
Pre-implementation phase
Paediatric early warning system in context
The hospital
Case study 2 was undertaken at Arrowe Park, a medium to large district general teaching hospital in the north-west of England. Children’s services comprised one ward and a paediatric assessment unit (PAU). There is no PICU, but there are links with a tertiary children’s hospital to refer children with high acuity and/or complex needs.
The ward
The ward case study focused on the 32-bed children’s ward, comprising 16 individual rooms and three four-bed bays, two HDU beds and an emergency room. See Report Supplementary Material 11 for details.
The ward cared for children with a wide range of conditions: children with breathing difficulties, those living with long-term complex conditions, children with feeding problems associated with being a premature neonate, elective/day surgery cases, and children and young people experiencing a mental health crisis.
Staffing
The ward team included a ward manager and deputy, qualified nurses (27 band 5 and five band 6), three HCAs and a play specialist. Student nurses had placements on the ward. Nurses and HCAs worked 12-hour shifts (07.30–19.30). A small number of staff worked 07.30–14.30. The ward manager and deputy worked office hours. External agency staff were used on occasion.
Nurses worked in one of three ward sections, with patients allocated to ensure continuity of care. There was a designated nurse in charge for the shift, who carried a case load. HCAs were allocated patients, but worked under the supervision of a registered nurse. Children admitted to the HDU had one-to-one nursing care. Not all nurses were HDU trained, so HDU admissions affected nursing work on the ward.
The medical team comprised consultants, registrars, senior house officers (SHOs) and ANPs. Junior doctors rotated between specialties every 6 months. The medical team provided weekday and weekend cover, spanning a variety of shifts. Day cover, from 09.00 to 17.00, included two to four SHOs, two registrars and one consultant. From 05.00 to 21.00, one SHO and one registrar would provide cover, with one consultant working until 19.00 in the summer and until 20.30 in the winter. A SHO, a registrar and an on-call consultant provided night-shift cover, with a second SHO working until 23.00. On the weekend, during the day, there were two SHOs and one registrar, with the consultant staying until 15.00. Weekend night-shift cover mirrored that during the week, except that there was no SHO overlap and the consultant was on call from 15.00. Some children were cared for by adult specialist and surgical teams located in the main hospital.
Routines
Nursing handover was at 07.30 and 19.30.
The medical team worked on both the ward and the PAU. Morning handover took place on the ward at 09.00. The evening handover took place in the PAU at 21.00.
The ward round followed the morning handover at 09.30.
Family involvement
Involving families was central to patient care. Parents were encouraged to stay with their child and the single-occupancy rooms were designed to accommodate a parent or carer overnight.
Paediatric early warning system assessment
Detect
A PTTT was used on the ward and PAU (see Report Supplementary Material 12). This was an adaption of the Brighton PEWS. In the pre-implementation phase, this was a paper-based system laid out in a four-page A4 booklet, which included instructions on escalation, parameters to be monitored (see Report Supplementary Material 13) and space for medical staff to determine the frequency of observations. Vital signs were recorded on the chart, which showed trends and included a traffic-light system indicating the significance of observations.
There were five versions of the chart, reflecting aged-based parameters. An observations policy was in place, which required nurses to generate a score at every observation, adhering to the following monitoring guidelines.
Doctors were responsible for setting observation frequency on admission. This rarely happened, however, and was usually determined by nurses based on their assessment of the child and family concern. HCAs and junior nurses sought direction from more experienced staff in determining observation frequency. Within the ward team, roles and responsibilities in relation to monitoring were well understood and work was allocated to ensure that the nurse had the appropriate monitoring skills.
Monitoring was conducted manually and equipment was available when required. A couple of rooms had continuous monitoring facilities, and were reserved for the sickest children. Readings could not be reviewed remotely, but baby monitors linked to the nurses’ station were routinely used. The large number of individual cubicles made routine surveillance of children difficult, and there was a degree of dependence on parents to summon help.
Monitoring and recording largely took place concurrently, with vital signs documented on the chart at the bedside. The ward was preparing to implement a new electronic monitoring system and staff were required to record observations on the paper chart and the electronic system to familiarise themselves with the system. A lack of available computers made this difficult, however, and, although some HCAs were observed adding information, nursing staff considered this to be unnecessary duplication.
Nursing and medical handovers were the main mechanisms for sharing information about prior risk factors that might increase a child’s risk of deterioration. Knowing an individual patient was highly valued by nurses, and pattern recognition was often used by more experienced staff to question an elevated score. Nurses were extremely supportive of one another, with frequent sense-checking about normal parameters, providing learning opportunities for more junior staff. In addition, staff engaged with parents to establish their child’s normal baseline on admission, but also subsequently during patient examinations and ward rounds. Indeed, nurses relied on parents staying overnight to monitor the patient. Although parents were not provided with formal instructions on how to raise concerns, nurses involved them in monitoring activity and encouraged them to alert staff if worried. Parents reported that they felt able to alert staff by asking them directly when they had minor concerns about their child, which was facilitated by the high visibility of staff on the ward and understanding that buzzers could be used.
Plan
The principal mechanisms for reviewing children’s statuses and identifying children of concern were the nursing and medical handovers and the ward round.
Nursing handover followed a narrative format to provide a comprehensive picture of the patient; salient details were recorded on a blank handover sheet. The nursing handover was divided into the two sections of the ward and the nurses had an overview of all patients in their section, but not the ward as a whole. As both handovers took place at the same time, the nurse in charge attended handover for one section and then received a second ‘mini-handover’ from the senior nurse at the other end of the ward. The purpose was to share information to develop a ward overview.
The doctors’ handover was attended by all doctors on that shift, ANPs and, when possible, the nurse in charge. The registrar from the previous shift handed over patients to the oncoming team, focusing on patients of most concern, and highlighting to-do lists for the upcoming shift. A printed handover sheet was distributed that contained information on the patient’s diagnosis, treatment plans and outstanding jobs (see Report Supplementary Material 14). The doctors’ handover covered all general medical patients on the ward, but excluded children under the care of a specialty or surgical team, who were managed by the relevant adult team.
The ward round followed handover; the registrar and the consultant divided patients between them, and were accompanied by the junior doctors and ANPs. Sickest children were seen first, although sometimes discharges could take priority. The consultant and registrar led discussions with parents/family members (and children with capacity). The nurse looking after the patient was usually present during the ward round and communicated with the medical team directly, often acting as an advocate for the parent or reminding them of conversations that they might have had earlier. This was not always possible, however, particularly if the nurse was busy with another patient. In these instances, it was usual for doctors to find the relevant nurse to update them on any decisions made.
Beyond formal handovers and the daily ward round, information on patients was routinely relayed throughout the day. Doctors were involved in these conversations when on ward during the morning; the majority of doctors moved to PAU during the afternoon and evening, and these conversations were less frequent.
The nurse in charge and the ward manager maintained an overview of ward activity (new admissions, changing patient acuity) and considered the implications for staff organisation. These were ongoing conversations throughout the shift, but were focused on their capacity to cope with the workload after a patient had deteriorated, rather than proactively planning for the management of at-risk patients.
There was also a mechanism called ‘the block’: a folder containing information on staffing levels and patient acuity. The block was supposed to be completed by the senior nursing staff to proactively manage capacity. However, it was not used systematically and was primarily completed at the end of the shift to document, rather than manage, activity.
Act
There was a graded escalation policy, linked to the severity of the score, and outlined on the PTTT chart. Staff had a good understanding of who to contact without having to refer to the chart itself. Although the score was the formal trigger for action, a decision about whether or not to escalate depended on who was calculating the score and how they defined the level of risk. Elevated scores were routinely not acted on by more experienced nurses, who justified their decision-making in the light of professional judgement. HCAs, by contrast, were far more likely to adhere to the escalation policy. In addition, although staff concern was not included in the score, the chart encouraged staff to raise concerns and all expressed their confidence in doing this:
This tool is intended to augment clinical judgement and NOT replace it. If you are worried about any child please escalate your concerns.
All ward staff appeared comfortable communicating with doctors whenever they had a concern about a patient, with or without an elevated score. Nursing staff clearly asserted that professional hierarchies did not prevent them from expressing concerns to the relevant staff, but they did encounter challenges in getting medical staff to act on their concerns. There was also difficulty reported around escalating care for non-general paediatric patients, again particularly for junior staff unfamiliar with procedures.
During the admission process, parents were encouraged to raise concerns, and were instructed to use the bedside buzzers or come to the nurses’ station if they required anything. Parents reported that they felt able to alert staff by asking them directly when they had concerns about their child, which was facilitated by the high visibility of staff on the ward. No written instructions on raising concerns or escalating care were provided, however, and some parents reported having been too worried on admission to process the guidance given by nursing staff.
Usual practice following a critical event or death would be to debrief and then discuss it during the weekly care improvement meeting, which focused on potential learning from key events of the week, for instance incorrect medication given. See Report Supplementary Material 6, Table 2, for a summary of the pre-implementation system strengths and weaknesses identified by the PUMA study team.
Implementation phase
Process
The improvement team at Arrowe Park comprised the medical PI, the ward manager and two ANPs. The improvement process was launched in November 2016 at the grand round. The SSAT and FFT were completed on the children’s ward and the results (Figure 11) were used to identify areas for improvement.
FIGURE 11.
Arrowe Park system assessment radar diagram. A score of 0 was used to indicate poor alignment with the system standard; a score of 10 indicated optimal alignment with the standard.
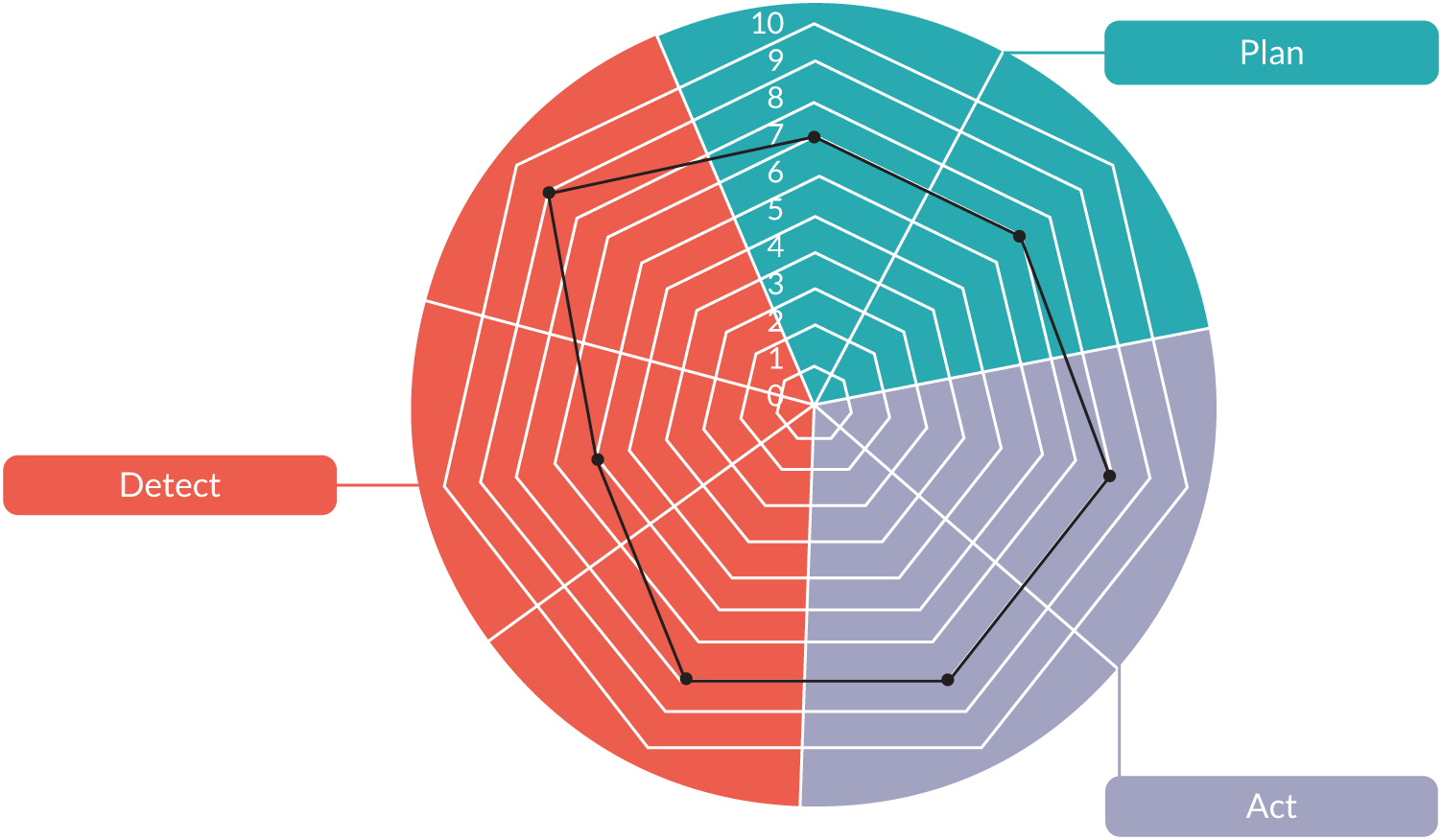
The team focused on the three elements of the wheel they found to be the weakest – the involvement of families in detecting deterioration, reviewing. information and planning, and escalation and response processes – using the process to develop a deeper understanding of these system weaknesses. Report Supplementary Material 7, Table 2, shows a summary of pre-implementation system strengths and weaknesses identified by the improvement team. The implementation strategy did not entail an explicit branding of the initiatives as linked to the PUMA study. Figure 12 shows the timeline for the implementation of each initiative.
FIGURE 12.
Arrowe Park implementation process timeline.

Context
Thresholds for HDU admissions changed at the start of the study in September 2015 to focus on the needs of the individual patient, rather than the treatment plan. In practice, this meant that patients who would have previously been admitted to the HDU were cared for on the ward.
Initiatives
Four PUMA initiatives were identified (see Report Supplementary Material 8, Table 2):
-
nurse education
-
introduction of second daily huddle
-
introduction of SHINE leaflet and poster
-
joint handover sheet, using the SBAR technique.
In addition, a number of other system improvements were introduced into the study site during the implementation phase. This section examines the implementation of all system changes.
The PUMA initiatives
Nurse education
This initiative was intended to address the lack of a structured approach to professional development. Led by the ward manager with assistance from two ANPs, a programme was developed on a range of subjects from raising concerns and escalation to asthma, tracheostomy and emergency management. A regular weekly time slot was established for a period of 10 weeks over the summer of 2017, and the ward manager made provision for staff who attended on days off to claim back their time. Although all staff valued the opportunity for training, attendance was challenging, because of either the busyness of the ward or the constraints of individuals’ outside commitments. Enthusiastic staff attended the programme, whereas others with greater training needs were less inclined to attend. In the light of their experience, the team proposed organising an annual study day and online resources, with nurses expected and rostered to attend the day.
Afternoon huddle
The afternoon huddle was designed to improve planning for risk when there was reduced communication between nursing staff and doctors because the latter were based in the PAU. The afternoon huddle was organised to take place in the PAU and was limited to doctors and the nurse in charge. The initiative failed to get off the ground, however, largely because the nurse in charge (who carried a case load) was unable to leave the ward:
If you were in charge, you couldn’t get off the ward, so.
ANP
Although a formal huddle was possible, an awareness of the need to increase communication between the ward and the PAU did become embedded in practice. Increased telephone contact between the two areas was evident, with ‘check-ins’ happening frequently:
It is getting better and the nurse in charge will go if they are not busy. But even if they don’t, they will phone down to the assessment unit to let them know what is going on. [. . .] they are getting better at letting each other know what their situation is like.
Senior nurse
In addition, the two areas had been brought together through the rotation of band-6 nurses working in the PAU.
Modified SHINE leaflet and poster
The SHINE leaflet and poster (see Report Supplementary Material 15) was a structured parental communication bundle, developed by Birmingham Children’s Hospital, that aimed to address the lack of formal processes for encouraging families to express concerns. The intervention, which was adapted for local use, included:
-
a leaflet for parents and carers that included a diagram to help recognise and describe changes in their child and what to do if concerned
-
tips on how to have an effective conversation with staff
-
a ‘Planning Care Together’ form for parents and staff to share, discuss and document parental concerns, to be signed by the nurse looking after the patient.
An ANP working on the ward was charged with implementing the communication bundle, but questioned its legitimacy. They had had no prior involvement in the PUMA study and had not been informed of the purpose of the intervention. Unsurprisingly, there was little evidence of the tool becoming embedded in practice. We found no evidence of posters on the ward, and subsequent interviews with staff confirmed a lack of enthusiasm for the initiative. First, nurses could not differentiate the SHINE communication tool from their normal practice:
I think you verbally do it all the time anyway. So you constantly say ‘if there’s a problem, will you let me know?’ and you just know that they’ll come to you anyway.
Nurse
Second, staff considered that families already effectively raised their concerns. Third, there was a lot of resistance to the idea of drawing up a plan with parents. Staff regarded the task as time-consuming and ‘setting them up to fail’, as it committed staff to actions within timescales that they knew they would not always be able to meet because of the unpredictability of the workflow. Fourth, the necessity for the tool posed a threat to nurses’ professional identities. Nurses considered that communication with families was a key element of their role. The introduction of the SHINE tool implied that they were not carrying out this crucial element of their work.
The SHINE tool was never implemented, but the ward manager argued that the challenge still needed to be addressed:
I am sure that we all think we are great nurses and great communicators, but we need a minimum standard in place, we don’t all communicate exactly the same way with all people.
Joint handover sheet
The joint handover sheet was intended to address the different and separate handovers between doctors and nurses. It was considered useful to combine information on patients to be passed on from day to night staff. However, this proved too difficult to implement because doctors and nurses had different information requirements.
Some changes were made to each of the handover sheets. The doctors’ handover sheet was changed to include all patients, not just the medical paediatric patients they are responsible for. The nurses’ handover sheet was changed from an unstructured sheet to the SBAR format, which was already used to hand over patients from the PAU to the ward (see Report Supplementary Material 16). Although nurses did report difficulty in initially getting used to the change in format, they largely accepted the reasons why it was being implemented and could see the benefits, compared with the previous ways of working:
You tend to go off your, like, a story, as in like, oh, and it go, like an SBAR is probably a better way to do it if you can stay focused [. . .] it has more of [. . .] a structure.
Nurse
There were different iterations of the sheet, which was modified in the light of experience of its use in practice. However, the narrative communication format is deeply embedded in nursing practice,214 and, 3 weeks after its introduction, there was some evidence of nurses reverting to an unstructured format. Furthermore, the SBAR technique was designed to support focused communication in acute situations; unsurprisingly, some nurses questioned how appropriate the format was for patients with long-term conditions.
Non-PUMA study initiatives
Two changes were introduced during the implementation phase, the planning for which pre-dated PUMA. This included the introduction of a safety huddle and the roll-out of an electronic recording and scoring system.
Safety huddle
The ward manager, as a result of the ward’s involvement in the Royal College of Paediatrics and Child Health’s (RCPCH’s) Situation Awareness for Everyone programme, implemented a safety huddle. The framework aimed to improve situation awareness in recognising and responding to children at risk of deterioration.
The safety huddle took place at 09.00, after the nursing handover but before the doctors’ handover. It was attended by all grades of staff, from domestic staff to consultants, with the objective of communicating which patients had the potential to deteriorate, categorised as ‘watchers’. The huddle began by running through each patient and highlighting any concerns. Safeguarding and safety issues were also relayed.
Nurses and allied health workers saw the huddle as an improvement over previous ways of working, whereby information would be cascaded in an ad hoc manner. Doctors were less positive. The medical doctors could not see the value in receiving information on the surgical patients and, because the huddle was before the doctors’ handover, they did not always know the patients. In addition, the huddle could delay the medical handover, which resulted in night staff going home later. The team responded to these difficulties by giving out the medical handover sheet at the start of the safety huddle so that everyone had a list of patients. In addition, the value of night staff taking part in the safety huddle came to be recognised. One year after the safety huddle was established, it still took place routinely every morning:
It did seem very strange at first; it was quite a big change, it was something that was out of our previous experience. But I think it has really embedded, I think we would feel strange if we didn’t do it now.
Consultant
Electronic early warning scoring system
The implementation of the electronic early warning recording system was designed to replicate the paper chart familiar to staff. Monitoring continued to be manual, but there was now a computerised system for inputting vital signs, as well as updating all notes, test results, medication and other information. The electronic system automatically calculated the score for the patient.
The implementation of the new system caused a number of problems for nurses. First, staff expressed concern about the accuracy of the scores calculated by the system; which tended to score lower than the paper version. These assumed-incorrect scores created an additional step in nurses’ monitoring tasks, whereby they had to manually make a note of what the correct score should be:
I haven’t got a lot of confidence [. . .] that they’re adding the PEWS up correctly as that, the electronic versus the paper, I think the parameters might be different.
Senior nurse
The new technology also disrupted nursing workflow, as there were often insufficient computers available to allow nurses to enter vital signs data, leading to a delay between monitoring and recording activity.
Doctors did not report problems with the technology. Indeed, the implementation of the electronic system enabled doctors to access patient data remotely, which was particularly useful when they were working off the ward. For a summary of all initiatives, see Report Supplementary Material 9, Table 2.
Post-implementation phase
Paediatric early warning system in context
In addition to the (non-PUMA study) modifications to the paediatric early warning system, site 2 also experienced contextual changes, which were consequential for the functioning of the paediatric early warning system in the post-implementation period. The ward had an increase in the number of band 6 nurses, from five at the beginning of the pre-implementation phase to 12 at the end of the post-implementation phase. Although regarded positively by senior staff, the benefits were less evident to staff on the ground, possibly because the band-6 nurses were fairly junior (having lost more senior band-6 nurses). In addition, there was an increased reliance on agency staff, because the winter of 2017 saw increased acuity for several months. From April 2019, a nursing bank of trust staff was introduced, so the ward reduced its reliance on external agencies. See Appendix 21, Table 46, for a summary of the embedded PUMA initiatives.
Paediatric early warning system assessment
Detect
There were major changes to the detection components of the paediatric early warning system in the post-implementation phase as a result of the introduction of the electronic recording and scoring system. When staff worked with the paper-based chart, it was straightforward to identify the vital signs that needed to be monitored for each patient. With the introduction of the electronic system, this was more difficult, and some more junior staff struggled to remember precisely what needed to be observed. The new electronic system required staff to manually select a patient’s chart, which often meant that the nurse would be aware of only the most recent vital sign, rather than the overall pattern, with the potential to negatively affect nurses’ use of professional judgement.
The electronic system had no impact on ensuring that doctors specified the frequency of observations, as per the hospital policy. In practice, this was still undertaken by nurses.
The electronic system also required manual input of vital signs data. The requirement for nurses to manually input data, and a general lack of availability of computers, particularly during busy times such as ward round, resulted in batched data entry. This would mean that the input of patient data into the system would be delayed, although everyone emphasised that, if a patient was scoring, they would escalate if needed. Delays to data entry also created a risk of errors being introduced into the process:
So often your first set of, um, of obs[ervations] that you do when you come out of handover, um, sits in your pocket, um, and sometimes a child could be seen by the doctor, not knowing what the current PEWS are, because they’re sat in your pocket.
Senior nurse
The loss of experienced nursing staff increased the need to support others:
Our problem is our skills set. We have been losing seniors who have been replaced by juniors.
Senior nurse, field notes
Plan
Nursing and medical handover and the ward round continued to be important mechanisms for identifying patients at risk and for forward planning. Changes to this process included the inclusion of all patients on the medical handover sheet. Although these patients were not formally handed over, it gave doctors an awareness of all children on the ward. In addition, when concern had been expressed about a patient during the safety huddle, whether they were medical or under the care of an adult specialist, the paediatric doctors would see the patient during their ward round.
The nursing handover sheet changed to follow a SBAR format. Although largely accepted, this was dependent on the individual nurse handing over, and there was some drift towards a narrative format. However, nursing handovers were reported as being quicker and more succinct than in the pre-implementation phase.
An important change in the post-implementation paediatric early warning system was the use of a morning safety huddle. Attended by all staff groups, it allowed quick identification of children at risk of deterioration, alongside bed management, safeguarding concerns and key messages of the day. For support staff, the huddle provided them the opportunity to obtain information that was not previously passed on to them. It also gave nurses a snapshot of acuity levels at the ‘other end of the ward’, whereas previously they would have had an awareness of the status of children in the area that they were working in only. One effect of the introduction of the safety huddle was to change the language used to identify potentially at-risk patients, with use of the term ‘watcher’ and ‘one to watch’ used more uniformly across all staff groups.
The safety huddle, in conjunction with the changes to the handover sheet, also enabled doctors to be aware of all patients on the ward, whereas previously they would have handed over only the general medical patients.
The introduction of the electronic scoring and recording system increased doctors’ ability to review children’s vital signs at a distance from the ward. There was also evidence of closer working between the ward and PAU:
It is getting better and the nurse in charge will go if they are not busy. But even if they, they will phone down to the Assessment Unit to let them know what is going on. So I think that the safety huddle has had an impact on how the two teams communicate – they are getting better at letting each other know what their situation is like.
Ward manager, field notes
The block was still used to review staffing, but this continued to be more to do with reporting what has happened, rather than a mechanism for actively managing the ward.
The ward manager or deputy now attended the post-ward round doctors’ meeting. This was largely in relation to bed management and patient flows.
Although the safety huddle had been embedded in practice, the nurse in charge was attending the medical handover less regularly than in the pre-implementation phase. Thus, at the end of the study, although the wider team situational awareness of the ward as a whole was more comprehensive, the exchange of information between the nurse in charge and doctors was less systematic than it was during the pre-implementation phase. Doctors considered that nursing attendance at medical handover was important, indeed more important than the safety huddle, whereas the nurse in charge had to balance this with their other commitments on the ward.
Act
Since the introduction of the electronic monitoring system, the escalation pathway was less accessible than it had been with the paper-based system. The significance of this change was compounded by the increased use of agency and new staff, who were unclear of the escalation procedure. In such cases, the first point of call was invariably the nurse in charge, regardless of the concern. Although the majority of nurses and HCAs made an effort to state that they would have no reservations in activating an alert to medical staff should a patient need it, there was some indication that the newer staff would speak to a senior nurse in the first instance.
Difficulties in escalating care for non-general paediatric patients, particularly for junior staff unfamiliar with procedures, remain unchanged in the post-implementation phase. There was also the suggestion that, with doctors able to access children’s monitoring information remotely from the ward, doctors were less inclined to act on nurses’ concerns.
Summary
There were a number of intentional changes to the paediatric early warning system in Arrowe Park, some of which were formally included in the PUMA programme, and some not.
Two planned initiatives pre dating the PUMA study were successfully implemented. The morning safety huddle had positive impacts on the overall situation awareness across the team, and, although less enthusiastically embraced by doctors, became embedded in practice. The new electronic recoding and scoring system improved access of medical staff to patient information, but has possibly made them less inclined to act on the nurses’ concerns and professional judgements, and it negatively affected nursing workflows and pattern recognition.
Few of the PUMA initiatives were embedded as planned: efforts to formalise engagement with parents through the implementation of the SHINE tool were not successful, but there was an expressed intent to develop more structured mechanisms in the future. The nurse education initiative was abandoned because of challenges with attendance, but plans were in place to address this perceived need in a different way. The implementation of a second safety huddle was not possible because of the difficulties of the nurse in charge leaving the ward areas, but alternative mechanisms for strengthening communication and awareness between ward-based nurses and PAU-based doctors were implemented. Finally, the implementation of a joint medical–nursing handover sheet was not realised, although both doctors and nurses made changes to their sheets, with nurses adopting the SBAR structured format, which brought nurse communication more in line with the medical model, and doctors including all children on the ward, rather than just the medical patients.
During the implementation period, the ward also experienced a loss of experienced staff and new appointments, which increased uncertainty over escalation processes. See Appendix 21, Table 46, for a summary of the embedded PUMA initiatives.
As a result of these changes, by the end of the study, there had been changes to the plan functions aligned to the PUMA Standard, compared with the pre-implementation phase, a deterioration in detect functions and little change to functions related to act. Report Supplementary Material 10, Table 2, shows post-implementation system strengths and weaknesses identified by the PUMA team.
Wider impact of the PUMA programme
We were unable to detect any wider impacts of the PUMA programme in the study site; however, the team continued to work on improvements in a number of the identified areas of system weakness.
Quantitative analysis
Monthly aggregate-level data were collected at a whole-hospital level in Arrowe Park between May 2015 and October 2018. Across all paediatric inpatient wards, patient bed-days averaged 330 per month.
Further details on the approach to analysis of the data are given in Chapter 2. Appendix 10 shows a full statistical report for this site, including a series of exploratory (Appendix 10, Table 29) and sensitivity analyses (Appendix 10, Table 30) performed on the primary outcome.
Primary outcome
For this site, adverse events were extremely rare. For 10 of the 41 months that we recorded outcomes, there were zero adverse events recorded. To reduce the number of months with no events, we created 2-month groupings (Figures 13 and 14) for the purpose of analysing trends. This, in turn, reduced the number of time periods, and so we changed the number of time periods from three (pre intervention, implementation and post intervention) to two (pre intervention and post intervention). For the primary analysis, we chose to designate October 2016 onwards as the beginning of post intervention (other cut-off points were considered in the exploratory analyses; see Appendix 10).
FIGURE 13.
Arrowe Park adverse event rates (unadjusted).
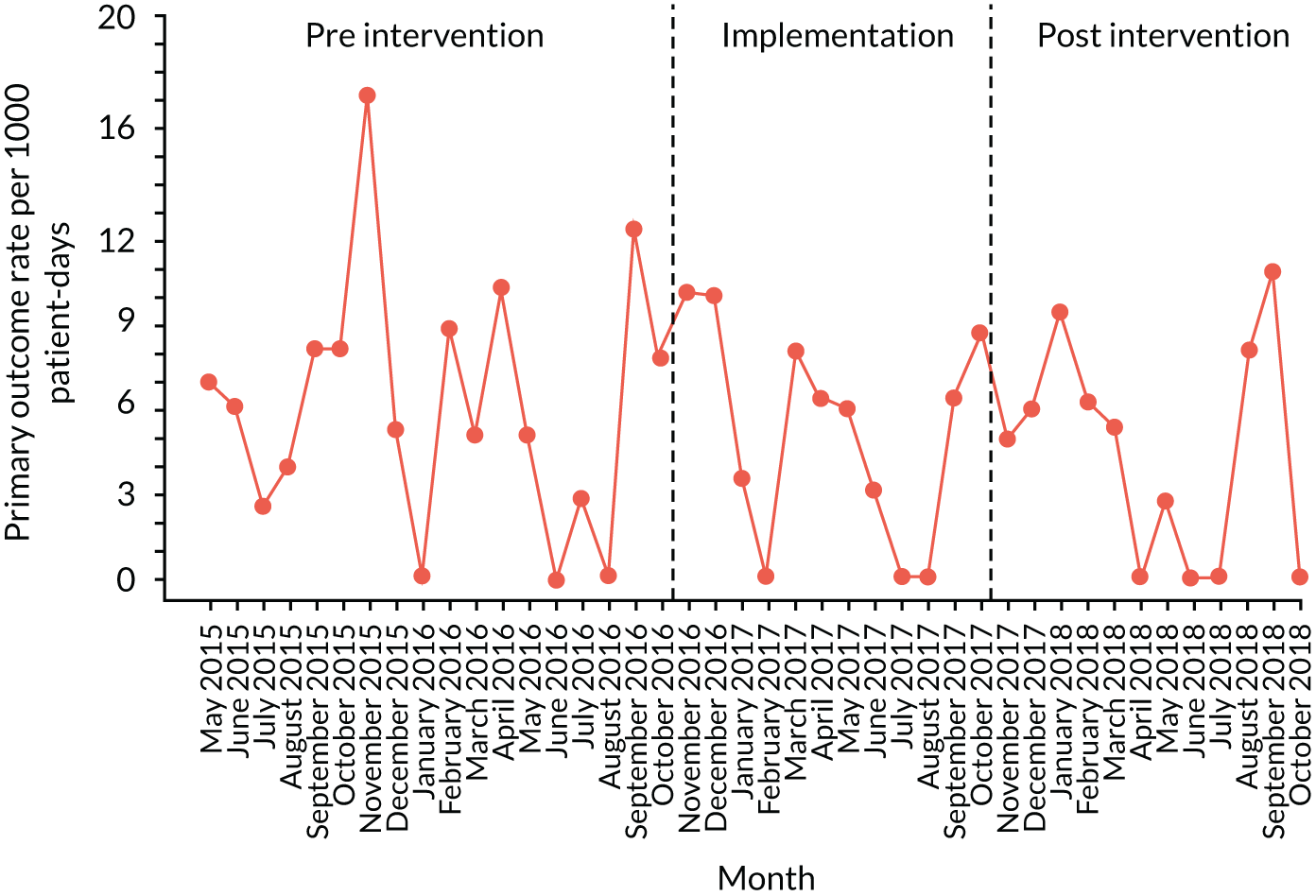
FIGURE 14.
Arrowe Park adverse event rates (2-month groups).
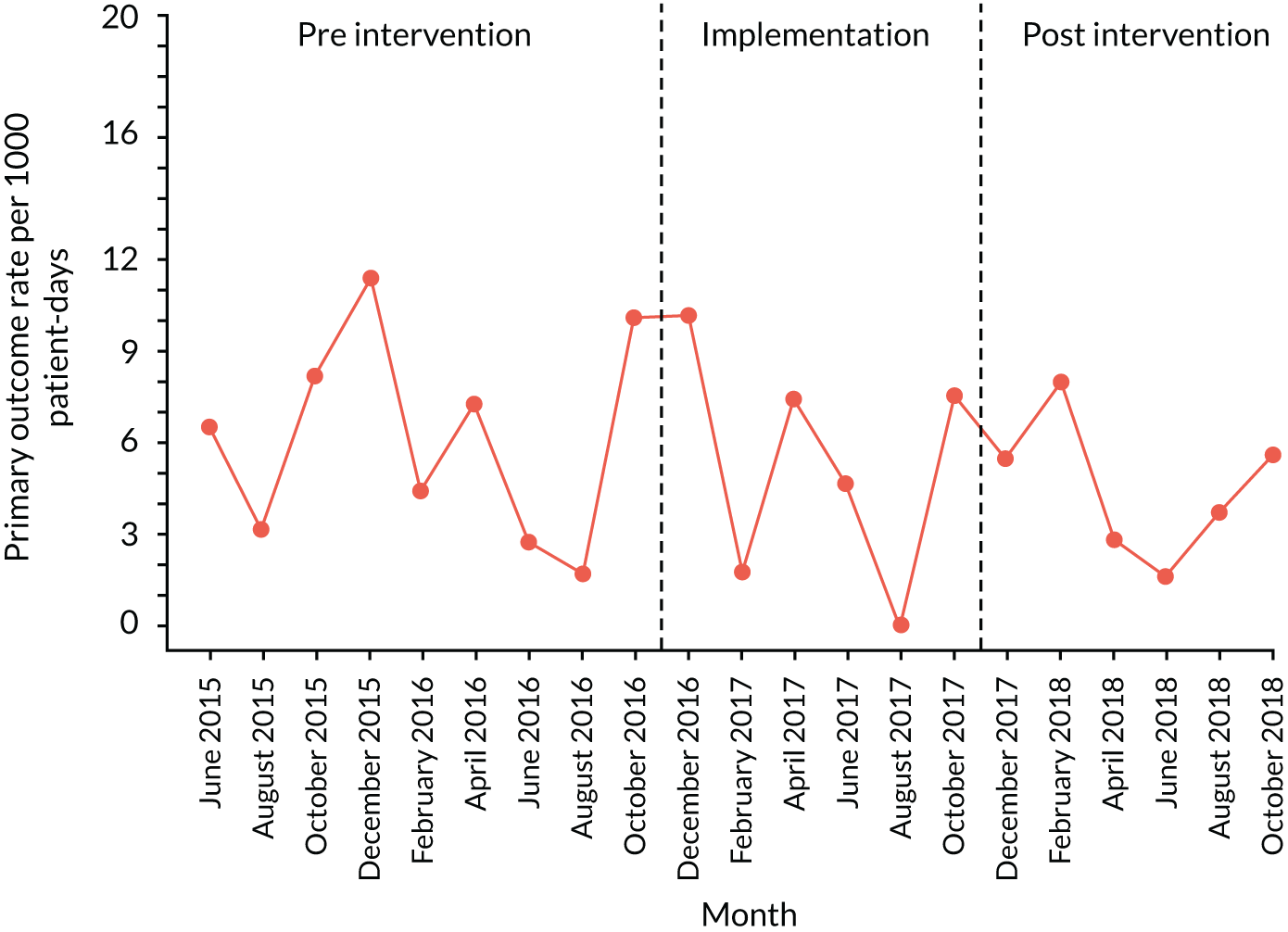
Figure 15 shows fitted trends for pre-intervention and post-intervention rates of adverse events, per 1000 patient bed-days. Solid red lines represent observed fitted trend lines, dotted red lines represent projected trends based on a continuation of the pre-intervention trajectory, and dotted green lines represent 95% CIs around the observed fitted trend lines.
FIGURE 15.
Arrowe Park scatterplot with fitted line from segmented linear regression for adverse events.

Table 11 summarises the ITS outcomes for adverse events, including an interpretation of key findings. The overall rate of adverse events per 1000 patient bed-days was 6.21 in the pre-intervention period and 4.49 in the post-intervention phase. In the pre-intervention stage, there was a non-significant downwards trend in adverse events (β = –0.17, 95% CI –0.49 to 0.17; p = 0.29). The observed rate of adverse events continued to slope downwards in the post-intervention period, with no significant difference in the slope of the trend, compared with the pre-intervention trajectory (β = 0.02, 95% CI –0.30 to 0.33; p = 0.98).
| Outcome | Estimate, β (95% CI) | p-value | Interpretation |
|---|---|---|---|
| Adverse events | |||
| Intercept | 3.08 (2.93 to 3.24) | ||
| Pre-intervention trend | –0.17 (–0.49 to 0.17) | 0.29 | There is a trend (non-significant) for reducing events, but the paucity of them occurring (in relation to raw numbers) makes it difficult to draw concrete conclusions |
| Change in slope (implementation period vs. pre-intervention period) | 0.02 (–0.30 to 0.33) | 0.98 | The trend does not appear to change, but the confidence limits around this are large |
| Immediate change in level (implementation period vs. pre-intervention period) | 0.29 (–1.74 to 2.32) | 0.78 | |
Secondary outcomes
Figure 16 shows fitted trends (or raw data, if fitted trends were not possible) for individual secondary outcome rates across the three time periods, per 1000 patient bed-days. Table 12 presents estimates from segmented linear regression for secondary outcomes, including an interpretation of key findings.
FIGURE 16.
Arrowe Park scatterplot with fitted line from segmented linear regression for secondary outcomes. (a) All-cause mortality; (b) cardiac arrests; (c) respiratory arrests; (d) unplanned transfers to PICU; (e) unplanned transfers to HDU; (f) PICU staff reviews; and (g) other medical emergencies.
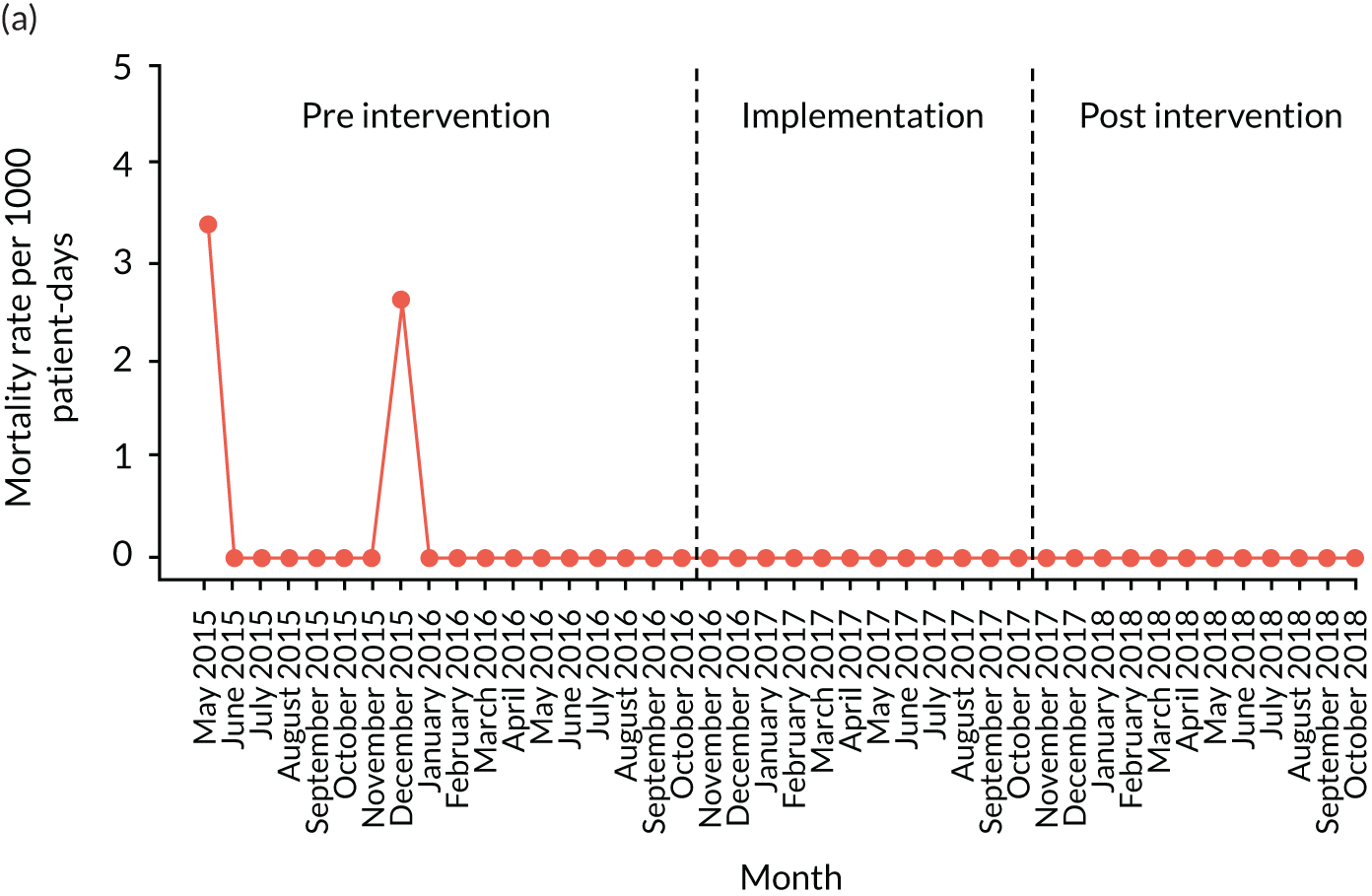

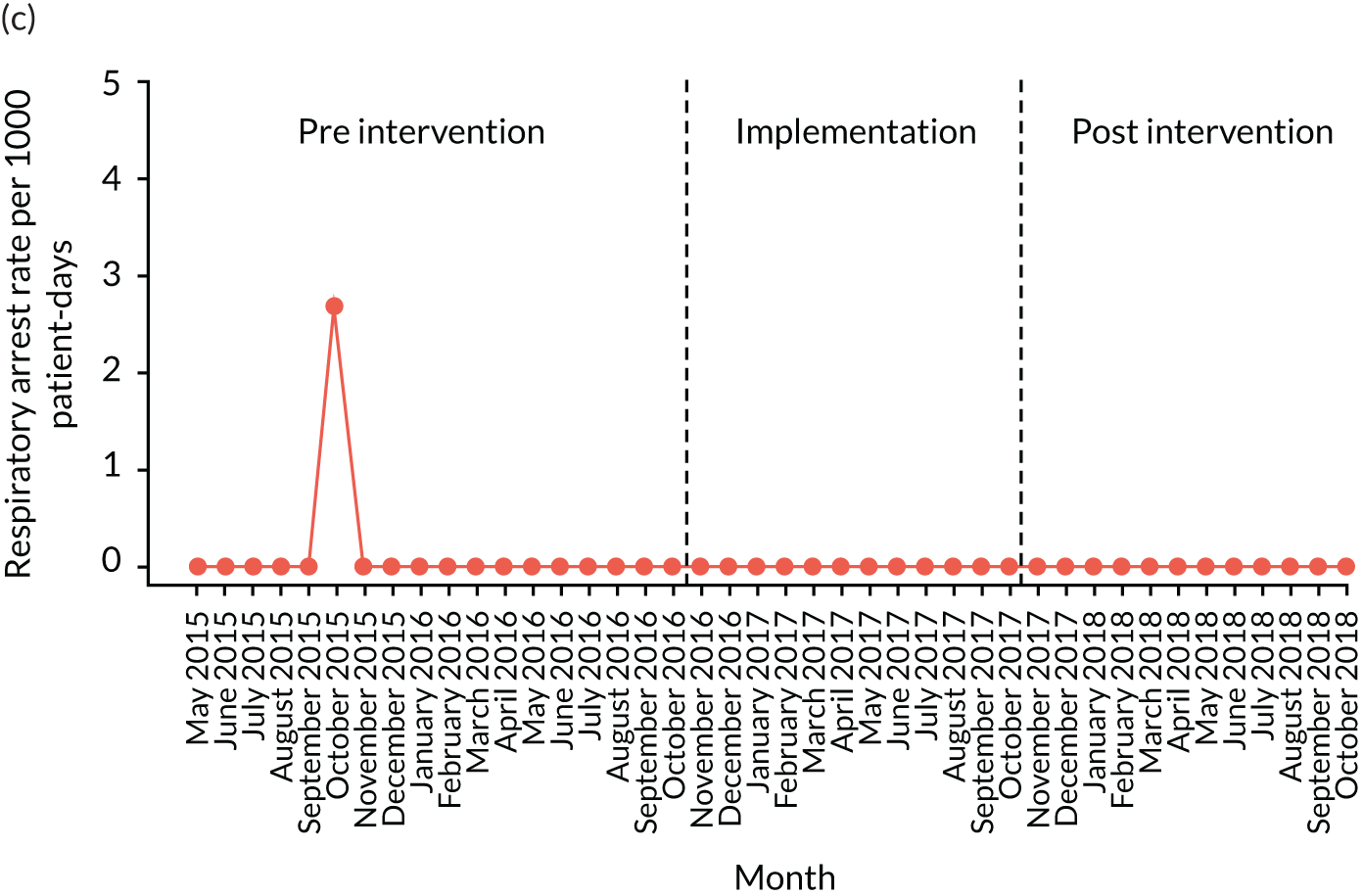
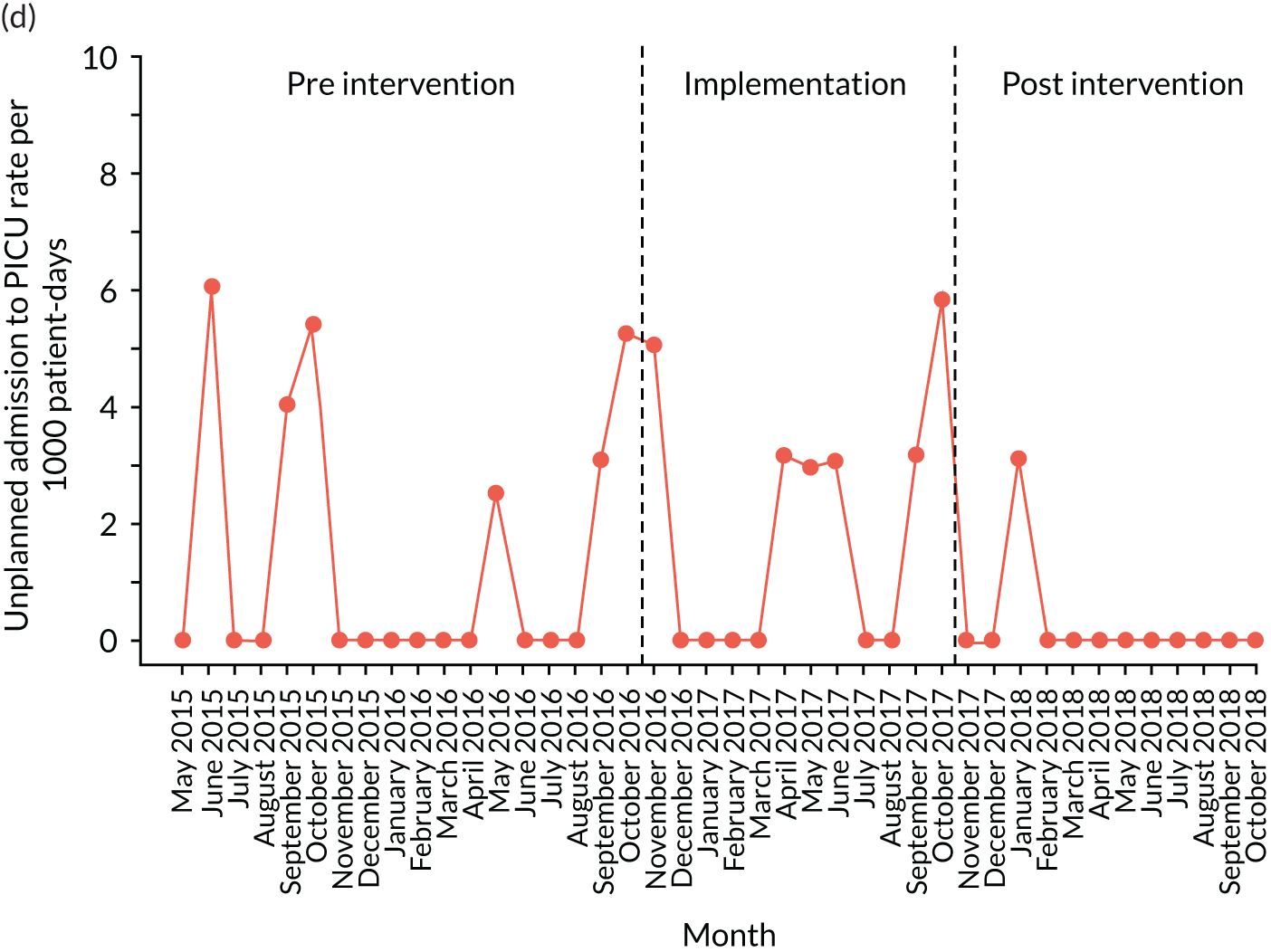
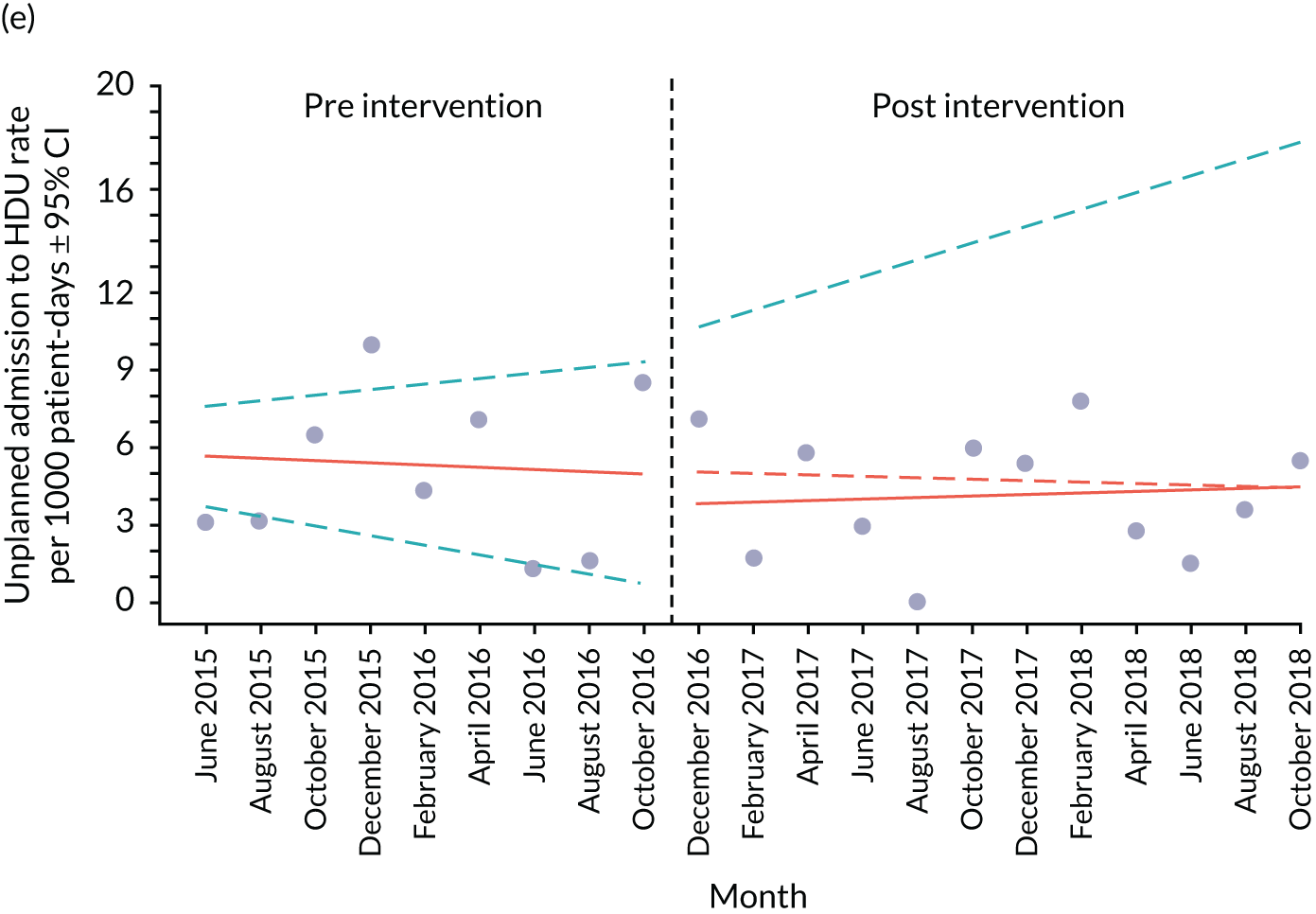
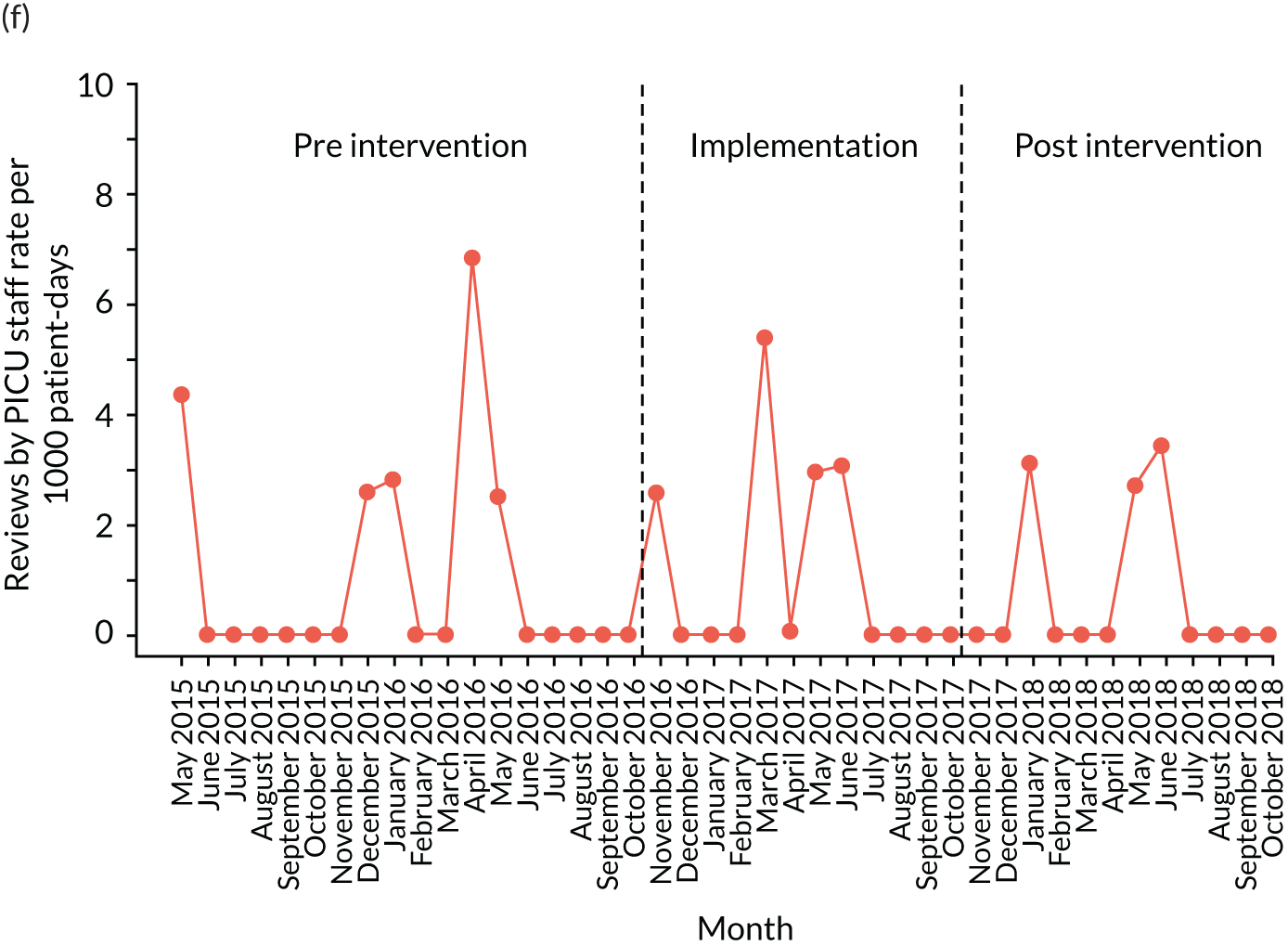
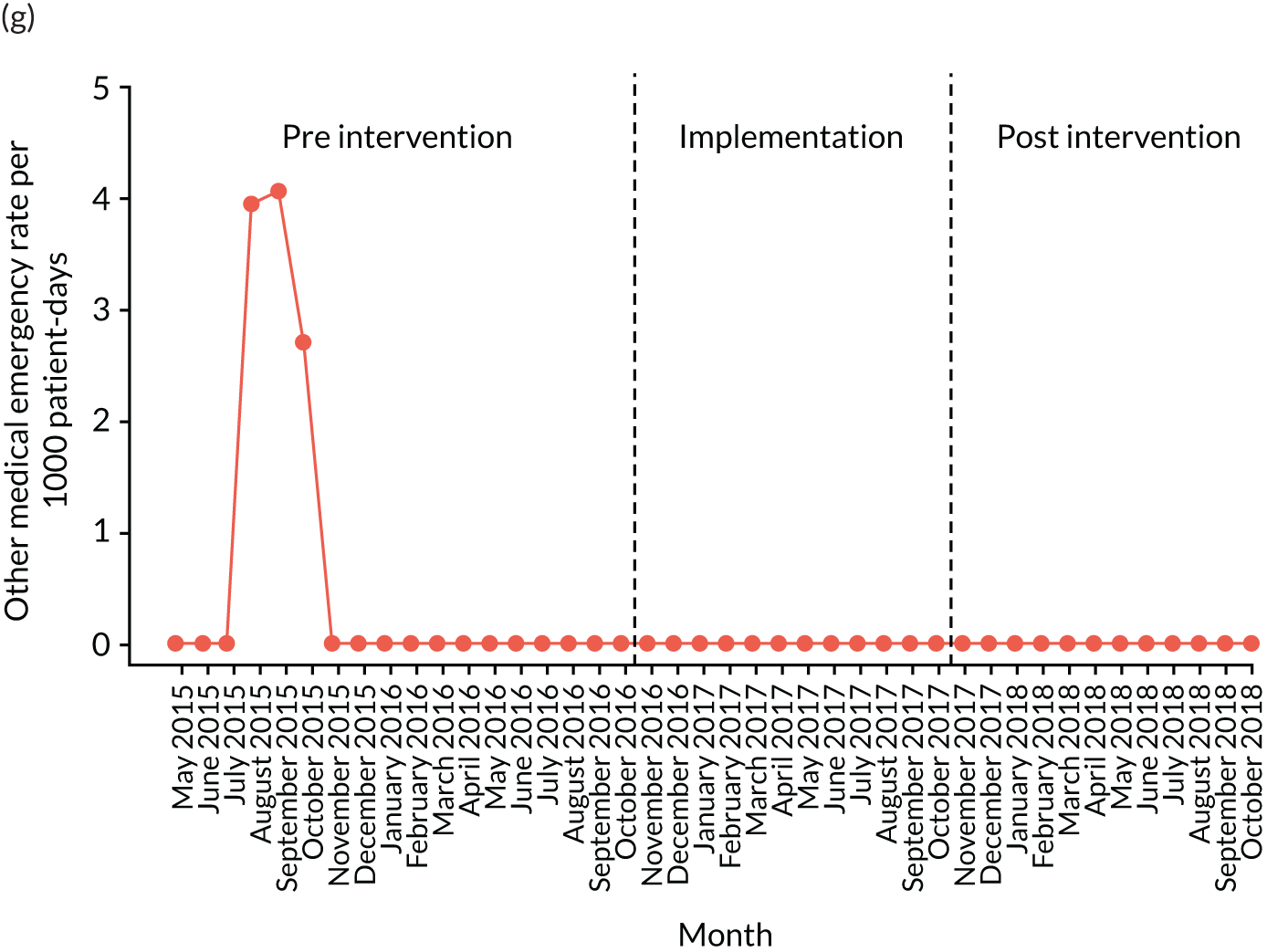
| Outcome | Estimate, β (95% CI) | p-value | Interpretation |
|---|---|---|---|
| Unplanned HDU transfers | |||
| Intercept | 5.72 (4.09 to 7.35) | ||
| Pre-intervention trend | –0.06 (–0.36 to 0.23) | 0.68 | HDU transfers probably did not consistently alter during the post-intervention phase, when compared with the pre-intervention trend |
| Change in slope (implementation period vs. pre-intervention period) | 0.12 (–0.17 to 0.41) | 0.42 | |
| Immediate change in level (implementation period vs. pre-intervention period) | 0.12 (–0.17 to 0.41) | 0.42 | |
Mortality
The overall all-cause mortality rate was 0.33 per 1000 patient bed-days in the pre-intervention period and 0.00 in the post-intervention period. Owing to a low rate of occurrence, it was not possible to model mortality trends over the different time periods (see Figure 16a).
Arrests
The overall cardiac arrest rate was 0.00 per 1000 patient bed-days in the pre-intervention period and 0.25 in the post-intervention period. The overall respiratory arrest rate was 0.16 per 1000 patient bed-days in the pre-intervention period and 0.00 in the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in cardiac or respiratory arrests over the different time periods (see Figures 16b and c).
Unplanned transfers
The overall PICU transfer rate was 1.47 per 1000 patient bed-days in the pre-intervention period and 0.25 in the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in PICU transfers over the different time periods (see Figure 16d).
The overall HDU transfer rate was 5.23 per 1000 patient bed-days in the pre-intervention period and 4.49 in the post-intervention period. In the pre-intervention period, there was a non-significant downwards trend in transfers (β = –0.06, 95% CI –0.36 to 0.23; p = 0.68), with a slight upwards trend in HDU transfers in the post-intervention period (β = 0.12, 95% CI –0.17 to 0.41). There was no significant difference between the pre- and post-intervention slopes (p = 0.42) (see Figure 16e).
Paediatric intensive care unit reviews
The overall PICU review rate was 1.03 per 1000 patient bed-days in the pre-intervention period and 0.75 in the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in PICU review rates over the different time periods (see Figure 16f).
Other medical emergencies
The overall rate of other medical emergencies was 0.49 per 1000 patient bed-days in the pre-intervention period and 0.00 in the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in the rate of other medical emergencies over the different time periods (see Figure 16g).
Synthesis
In this final section, we consider how some of the clearer quantitative findings relate to qualitative observations. Assessing the impact of the intervention on quantitative outcomes was challenging, and so interpreting the quantitative outcomes in relation to the ethnographic observations should be treated with caution.
Arrowe Park introduced a safety huddle, and electronic recording and PTTT, which strengthened some aspects of the local system and weakened others. From an ITS perspective, this was the smallest site with the lowest event rates. Quantitatively, there was no obvious ‘interruption’ to the adverse event rate over time: it appeared to be gently declining during the pre-intervention phase and continued to do so over time (albeit, again, with wide CIs due to event rates and having to combine months to avoid zero values). Very early on in the pre-intervention period, there was a change in the nurse lead for the children’s ward, with interviews and observational work suggesting that this member of staff was keen to reduce HDU transfers (the primary driver of ‘adverse events’ in this site) and instead manage some sicker children on the ward. This may have been one contributory factor in the declining event rates over the study period.
Chapter 7 Case study 3: Noah’s Ark Children’s Hospital
Pre-implementation phase
Paediatric early warning system in context
The hospital
Case study 3 was undertaken at the Noah’s Ark Children’s Hospital for Wales, a large tertiary (120 beds) service located in Cardiff. The purpose-built facility, which opened in 2015, has five wards [general medical (× 2), specialist cardiac/renal medical, surgical and oncology], a HDU and a PICU.
The ward
The ward case study focused on a medical ward, where children were cared for by general medical and specialist paediatric and adult consultants. The ward comprised 18 beds divided into two zones, ‘Turtle’ and ‘Lemur’, with a central nurses’ station. The ward was a mix of one single-occupancy room, four double bays, one four-bedded bay and a family room. See Report Supplementary Material 17 for the ward layout.
Staffing
Ward staff comprised a ward manager, two deputies shared across the two medical wards, band 6 nurses (n = 4), band 5 nurses (n = 29), HCAs (n = 5) and play specialists. The ward also had student nurses on placement. Staff turnover was high; many experienced staff had left for specialist roles, diluting the ward’s skill mix. There was widespread use of external agency staff.
Nurses and HCAs worked from 07.00 to 19.30. Typically, five registered nurses and a HCA were on shift, but staff could be moved in response to changing demand in the hospital. There was a designated nurse in charge of the shift (typically a senior band 6, although, at night, this could also be a band 5), who also carried a case load. Nurses were allocated patients from both sections of the ward. More experienced HCAs were allocated patients, but all observations had to be supervised by a qualified nurse.
The medical team covered the two general medical wards and worked 08.00–20.00. The eight-person rota included two consultants, two registrars and junior doctors (who rotated every 6 months), supported by ANPs who worked variable shift patterns. Generally, doctors divided themselves between the two general medical wards, the PAU and accident and emergency (A&E). General medical doctors were present on the ward immediately following ward round, with one SHO often based on the ward throughout the day. The hospital worked with the Hospital at Night model:215 two registrars and a SHO covered the general, specialty and surgical wards, and PAU and A&E, supported by an on-call consultant.
The specialist teams included paediatric specialists (oncology/renal/cardiac) and adult specialist with paediatric training (e.g. orthopaedics; ear, nose and throat).
Routines
Nursing handovers were at 07.00 and 19.00. Handover commenced with a safety briefing led by the nurse in charge of the previous shift, followed by a walking handover of individual patients.
The medical handovers were at 08.30 and 20.30 and followed the same format. They were led by the outgoing registrar and attended by all doctors on the incoming shift. Information was shared on all medical patients in the hospital. ANPs were present as part of the medical team. No senior nurses attended the doctors’ handover. Patients of concern were not systematically identified; handover would proceed in order of beds, with any concerns being highlighted as the patient was discussed.
The medical ward round was at approximately 09.00.
Many ward patients were under the care of specialist consultants; these were seen on the specialist ward rounds.
Paediatric early warning system assessment
Detect
Monitoring core vital signs was integral to nurses’ work, although not all nurses were equally skilled in monitoring techniques. The hospital’s observation policy specified which observations should be conducted routinely and set a minimum frequency of every 4 hours, unless otherwise indicated (see Report Supplementary Material 18). The policy was well understood by nurses, who undertook a range of ‘additional assessments’: ‘eyeballing’ patients, touching patients to assess temperature and assessing tracheal tug (a proxy for increased effort of breathing). Nurses often increased the frequency of observations based on their assessments. They also made professional judgements in deciding which vital signs to monitor, which may have been non-compliant with observation policy, but took into account wider considerations about a child’s care:
He’s not too hot, so I won’t do his temperature now. The fact his heart rate is low and I’ve touched him and he’s not hot, we’ll just let him go into a deeper sleep and then do his temperature, because they don’t want to wake him up just for that.
Nurse, field notes
Nurses had a high level of awareness of conditions that required additional observations. Patient categorisations such as ‘bronch baby’, ‘premie’ and ‘ex-25 weeker’ oriented staff to risk factors and additional observations required. Beyond formal observations, nurses valued having oversight of patients, although the physical layout of the ward made this challenging:
It is wonderful for the parents, the parents love it, but it is really tricky for the staff. We can’t see everything that is going on.
Nurse, field notes
The four-bedded bay and two single cubicles had fixed continuous monitoring. Higher-acuity patients were allocated to these beds or rooms immediately adjacent to the nurses’ station, which allowed greater visibility. Mobile continuous monitoring units could be provided if required.
The sound of alarms was commonplace, triggered by a drop in oxygen saturation levels or if a monitor had been disconnected. Nurses always responded, but often to check that the equipment was working correctly. Staff frequently readjusted probes to encourage oxygen saturation level readings to improve. There were no monitors at the nurses’ station, however, and nurses were required to determine the alarm’s location. Depending on the patient, nurses also permitted parents to turn off monitors:
[T]hey’ve said ‘you can switch it off if you want to because, as long as we check it every now and again’, I just turn it on and have a look [laughter] and check it and turn it off again.
Parent
A monitoring equipment trolley was located in the middle of the ward, which was inconvenient for staff working in more distant rooms. In addition, field observations revealed that nurses were often unable to locate a specific item, and/or the equipment was broken. Thermometers were a particular issue and, once located, would be held by staff rather than being returned to the equipment trolley.
You don’t know what I’ve done with my thermometer do you?
I don’t but I can tell you where I’ve stashed mine.
Field notes
There were no formal routines for involving families, although staff considered themselves to regularly involve families in defining baselines, as well as encouraging them to talk about any concerns. Nurses also actively engaged parents in monitoring their child’s condition. During interviews, two parents reported that nursing staff had explained to them acceptable ranges in observation numbers and that they then played an active role in monitoring their child. One parent noted that staff had spent time explaining what the numbers on continuous monitoring equipment related to, and the parent reported that she was not concerned as long as the patient’s heart rate stayed at < 200 beats per minute.
Observations were recorded on paper charts. There were no colour-coded vital signs thresholds and no PTTT score. Although charts allowed nurses to clearly see patterns and trends, staff with previous experience of working with PTTTs favoured the colour-coded vital signs thresholds, as these were useful triggers for action:
I really don’t like the documentation here so much. [. . .] Because it’s just black and white, [. . .] the obs[ervation] chart is not so nice. [. . .] When you see a heart rate up to a hundred per cent, in the dark red, so you see, everybody would see, OK, err and a student would see that is not right.
Nurse
There were no formal resources on the ward that allowed staff to check a child’s observations against normal thresholds, with an assumption that these should be known by all staff.
Observation charts were located in a folder stored outside a patient’s room. Nurses were often unable to find a folder immediately, typically because medical staff were using it. During these times, nursing staff still performed a full round of observations on their patients, noting the results on informal pieces of paper and later transferring these to observation charts.
Continuity of care was ensured whenever possible, and was prioritised during patient allocation. Nurses had high levels of engagement with parents and involved them in interpretations of a child’s vital signs:
He is very clammy. I asked mum if he gets clammy at night and she said he does but not like this, this is more than normal.
Nurse, field notes
Family concern was also regularly highlighted during nursing handover. Sometimes this was described as something relevant, and other times it was described as parental anxiety or a lack of understanding of the child’s condition. Not all parents were treated as equally legitimate in raising concerns.
Plan
There were a number of mechanisms for reviewing individual patients and unit capacity, which produced different levels of situational awareness across the nursing and medical teams.
Ward staff received a safety briefing at 07.00. This was used to highlight patients at risk of deterioration, or those with particularly challenging pre-existing conditions, as well as general information. The shift’s handover sheet was also distributed during the safety briefing. This was electronically generated, containing information relating to diagnosis, treatment, medication and tests needed or awaiting results (see Report Supplementary Material 19).
More detailed information on all patients was shared during nursing handover, which was conducted outside the patients’ rooms, to enable a visual review of the children. Nurses asked questions to make sense of the case, for example when a point was not clear, or if they felt that the account being provided contradicted their experience of looking after that child during a previous shift. Nurses received handover for all patients and so had an overview of the whole ward. Both the morning and evening handovers followed the same format. During the week, the ward manager, who was a highly experienced clinician, reviewed all patients to identify individuals at risk.
A whiteboard indicated the location of patients on the ward, nurse–patient allocation and the on-shift consultant. A yellow star denoted the sickest patients, although was not always used. The board was regularly updated at nursing handover.
A bed management meeting took place at 14.00 and 20.00 on the PAU. This was held with the patient flow co-ordinators and ward managers, to review organisational capacity. The medical handover took place twice a day at 08.30 and 20.30, and was an opportunity to review all patients in the medical service, with on-coming doctors and ANPs making notes on an electronic pre-populated handover sheet. However, there was no systematic mechanism for identifying children of concern and no ward nurse in attendance.
The general medical team ward round was an opportunity to review all patients and talk to parents. The format varied. Sometimes, the team saw all patients; on other occasions, the team divided into two, with the consultant focused on the sickest patients and the registrar leading the other team. The whole team came together after the ward round to review decisions and plan. In the week, the ward manager always attended the ward round. Children under the care of specialist teams would be seen during the morning ward round for that team, but the timing of this was less predictable and nurse involvement was inconsistent. Nurses would often have to check the medical notes to establish the plans for a child’s care.
Although there were mechanisms in place to ensure that nursing and medical teams had an overview of the patients in their care, there was no opportunity for a shared nursing and medical understanding, and information flows were fragmented. Doctors were not always aware of the most at-risk children on the ward, and did not always prioritise activity appropriately. In addition, the general medical team’s purview extended to all the medical patients in the hospital across two wards, the PAU and A&E, whereas the purview of nurses included children under the care of specialists and the medical patients on a single ward.
Act
There was no hospital-wide PTTT score and no formal escalation policy. Junior nurses typically escalated concerns to the nurse in charge, who then made a decision about escalating to medical colleagues, working up through the medical hierarchy if they were not satisfied with the response. One doctor, usually a SHO, was ward based during the day and was visible at the nurses’ station, which meant that concerns were often addressed informally. Although this seemed to work satisfactorily in practice, there was sometimes a lack of clarity about the process when escalating; this could be particularly challenging at night, when there was more limited medical cover and/or when the nurse raising concerns was more junior. There were particular challenges contacting the on-call consultant at night and some of the specialist consultants, especially if they were from the adult service:
The [name of specialist consultant] was phoned, and said he would come in in 2 hours to review [the patient], but I said, ‘sorry that’s not good enough’. He then told me off for forcing him to make decisions under pressure. But I wasn’t happy at all, I didn’t want to wait, so I sent her to HDU.
Consultant, field notes
At interview, many parents described feeling able to raise concerns about their child with nurses, although this was observed to be more difficult for families for whom English was not their first language. However, involvement was uneven, with not all parents instructed on the use of buzzers to alert the nurse. The physical layout of the ward could make it difficult for families to locate the nurses. See Report Supplementary Material 6, Table 3, for a summary of the pre-implementation system strengths and weaknesses identified by the PUMA team.
Implementation phase
Process
The Noah’s Ark improvement team was led by the two PIs: a consultant and a senior nurse. Together, they invested significant time and energy sharing the PUMA programme across the organisation through formal events, meetings, and activities and engagement with front-line staff. The aim was to raise awareness of the research and enrol key individuals in the team. However, although they successfully assembled an improvement team, it was not sustained:
There were people there with good intentions who wanted to help, but would be moved on or were too busy and had lots of various things to do. Or there were people who were sent along to help who had no intention of helping.
PI
The SSAT and FFT were completed on the two medical wards and on the surgical, oncology and specialty wards, with the results used to identify areas for improvement (Figure 17). Report Supplementary Material 7, Table 3, shows a summary of the pre-implementation system strengths and weaknesses identified by the improvement team.
FIGURE 17.
Noah’s Ark system assessment radar diagram. A score of 0 was used to indicate poor alignment with the system standard; a score of 10 indicated optimal alignment with the standard.

Although multiple system weaknesses were identified, the team focused on the three elements of the wheel that they found to be the weakest – the involvement of families in detecting deterioration, reviewing information and planning; and escalation and response processes – using the process to develop a deeper understanding of these system weaknesses. The implementation strategy entailed an explicit branding of the initiatives as linked to the PUMA programme. Figure 18 presents the implementation timeline.
FIGURE 18.
Noah’s Ark implementation timeline.

Context
A number of organisational factors at Noah’s Ark had implications for the implementation of the PUMA programme and the paediatric early warning systems in the site.
One of the study’s chief investigators, who held a senior clinical position at the hospital, moved to a new post. Although not involved in any of the implementation activities, he was the face of the PUMA programme, and a reminder to front-line staff of the initiatives associated with the study.
High levels of staff turnover proved to be a major challenge, with the PIs struggling to find the time to maintain momentum with a changing population.
The organisation also experienced changes in the thresholds for admission to the PICU and HDU, which increased the likelihood of admission and increased referrals from other hospitals.
The handover and organisation of the nursing team on the ward case study were changed. Nurses were allocated to work in a particular section of the ward and received handover on those patients only. The aim was to increase the efficiency of handover in the context of the new ward layout.
Nurses had also been given responsibility for registering parents’ parking, which had created additional burden on an already overstretched workforce.
Advanced nurse practitioners were no longer included in the medical team, which changed from an eight-person rota to a 10-person rota, which increased capacity to cope with sickness or training.
There were also improvements in monitoring equipment on the case study ward, including more Optiflow™ Nasal High Flow therapy devices (Fisher & Paykel Healthcare Corporation Limited, Auckland, New Zealand) and saturation monitors, as well as a central monitoring station.
Initiatives
The PUMA programme initiatives
The team proposed four initiatives (see Report Supplementary Material 8, Table 3):
-
modified SHINE posters in clinical areas
-
electronic site board at nurses’ stations
-
reviewing and adjusting existing communication mediums
-
escalation plan.
Modified SHINE posters
The SHINE poster was intended to address weakness in family involvement in monitoring and detecting deterioration (see Report Supplementary Material 20). The initiative, as expressed in the action planning documentation, aimed to ensure ‘continuity of clear information for parents’ and to ‘empower parents to report the deterioration of their child’s condition when it happens’.
The senior nurse PI led the initiative, having failed to enlist the help of others. The poster was developed in February 2017, and implemented throughout June 2017. Trust policy stipulated that posters should be placed on pin boards only; these were unavailable in the surgical unit, and the intervention was never extended to the specialist or oncology wards. Implementation on the medical wards was facilitated through an additional poster designed to engage nurses in the use of the tool, and was also promoted through nursing and medical induction programmes.
A small number of SHINE posters were observed in patient rooms during the post-implementation fieldwork, but their use had not been normalised on the medical ward. Many staff were unfamiliar with the tool, and those who were familiar with it maintained that they had not received any information on its purpose. When the intent of the poster was explained, staff indicated that, in formalising a process that was deeply embedded in their professional practice, it undermined their role as nurses. There was criticism of the poster’s content and format (volume of information and font size), which, it was suggested, would discourage people from reading it. There was also resistance to the idea of formally requesting parents to monitor their child:
[It is] a lot of responsibility for a parent to recognise change. You know, it’s different saying ‘I think his breathing has changed, can you have a look, I’m a bit worried’, than actually sort of rely on the parents to look at the rolling signs that their child’s . . . I don’t think I agree with that.
Senior nurse
Reviewing and adjusting existing communication mediums
This initiative was intended to address system weakness relating to nursing–medical communication of at-risk children. Doctors, in particular, were not always aware of the sickest children on the ward; therefore, prioritising high-risk children was not as efficient as it could be.
In February 2017, a consultant (the PI) observed medical handovers to assess the extent to which at-risk children were identified and prioritised. This confirmed that there was no clear mechanism for achieving this. A ‘common safety briefing’ attended by both nurses and medical staff was proposed, but little progress was made because of difficulties in accommodating different shift patterns.
The initiative was abandoned, with the team electing to focus on the implementation of the electronic whiteboard to support situational awareness.
Electronic site board
The electronic site board was intended as a common information space for doctors and nurses to identify the sickest patients across all wards. However, it quickly became apparent that this ‘became too big a mountain to climb’ because it needed to be managed by the health board’s information technology department, which had other priorities.
An alternative intervention was developed: a whiteboard displaying the ‘4Ss’ (sickest patients, bed status, safeguarding issues and staffing) in the general, specialty and surgical doctors’ handover rooms. The intent was that, prior to medical handovers, the registrar handing over would run through the ‘4Ss’ and then add a yellow star next to ‘at-risk children’.
Whiteboards were installed in the general medical doctors’ handover room. The board displayed the sickest patients across the general wards, as well as highlighting staff, bed management and safeguarding issues, and was completed at the start of every handover (see Report Supplementary Material 21). The purpose of this was to quickly orient medical staff to issues surrounding the planning of risk and to ensure that there was common understanding. For this to be achieved across teams, nursing staff were required to telephone the doctors’ room before each medical handover to share their own interpretation of the ‘4Ss’. Staff were enrolled in the initiative through a variety of means: presentations to all junior doctors during orientation, and meetings were held with new registrars and with senior nurses. A second whiteboard was also extended to specialty medicine in September 2017.
The success of the initiative was uneven. During the early period of implementation, the presence of the clinical chief investigator during handover may have helped to embed the new format, but, over time, practice became variable and was largely dependent on the registrar handing over.
Some medical staff found the new approach to be beneficial; it had the desired impact of improving multidisciplinary communication and at-risk children were identified on one system:
Before this, we probably wouldn’t have had a clue about bed status or erm, the nursing side of things. It was kind of, like, very separate.
Junior Doctor
Telephone calls between senior nurses and the doctors at the start of handover appeared to happen routinely during the post-implementation observations. Concern regarding any child raised during the nursing handover would also be communicated during medical handover. The ward manager and senior nurses all mentioned that they were now able to get information on to the doctors’ handover if needed. The initiative did not appear to have been implemented on the specialty medical ward. Although the whiteboard was installed, it was not used. This is arguably down to the very different culture on this ward. Rather than one team on the general medical ward, the specialty medicine ward is made up of a number of different teams, each responsible for their own patients. A consensus on what patient is at greatest risk of deteriorating is far more challenging in this environment.
Escalation plan
The purpose of this initiative, as expressed in the action planning documentation, was to articulate a clear procedure for escalating care, with the aim of embedding ‘a more consistent approach, to empower staff to call for help and to make clear the roles and expectation of senior staff in responding to escalation requests’. The policy was initially intended to be implemented across all wards and was developed by the senior nurse PI working with ward managers. The policy specified to whom concerns should be escalated and subsequent steps if there was ‘ongoing deterioration or an inadequate response’ (see Report Supplementary Material 22). Once the Children’s Board approved the policy, a number of implementation strategies were employed, such as inclusion in staff induction, and nursing and medical study days. However, difficulties were encountered in practice with implementing this across multiple wards. There were certain wards that were more difficult to change than others; the surgery ward, in particular, was very resistant to the idea of having a formal escalation policy, despite this being the ward where difficulties in escalation were highlighted during the system assessment process. Consequently, implementation was limited to the two general medical wards.
In the post-implementation fieldwork, there was a low level of awareness on the new policy, but this may be because the policy simply formalised usual practice. This lack of awareness of the formal policy, made it challenging to assess whether or not it empowered staff to escalate.
See Report Supplementary Material 9, Table 3, for a summary of initiatives implemented at Noah’s Ark, and Appendix 21, Table 47, for a summary of all embedded system improvements.
Post-implementation paediatric early warning system assessment
Detect
There were no discernible changes to monitoring, recording or interpreting activities.
We observed a few SHINE posters on the ward, but did not see that nurses oriented parents to them on admission and we observed no examples of their use in practice. Parent interviews revealed no awareness of the tool, although parents did show confidence in alerting nurses to any concerns regarding deterioration.
There had been some improvements to equipment with the introduction of central monitoring, and an increased number of saturation machines and breathing machines (Optiflow Nasal High Flow therapy devices) provided since 2015, although it was not possible to discern their impact.
Plan
The nurses’ morning safety briefing had been getting ‘longer and longer’, with a growing list of additional items of information, with the same announcements appearing over the week. This carried the risk of core information on at-risk children being crowded out. The nurses had started putting some of the information on an information board to keep the briefing shorter (see Report Supplementary Material 23).
To work more efficiently, nurses were now allocated to work in one area of the ward, and received handover on these patients only, rather than on the whole ward. At one level, this reduced the scope of nurses’ situational awareness. At another level, the shortened handover improved nurses’ concentration.
There has been an improvement in the shared situational awareness of children at risk as a result of the implementation of the ‘4Ss’ whiteboard. The senior nurse telephones through to the doctors’ handover to alert them about bed status and the sickest patients, which allowed the medical team to plan ahead before the ward round.
Act
As part of the PUMA programme, the hospital had implemented a formal escalation policy. Although there were low levels of awareness that a formal policy existed, staff had high levels of awareness of the escalation process.
The appointment of new PICU consultants had generated variation in thresholds for admission to PICU, which increased the likelihood of acceptance.
Summary
Noah’s Ark was under considerable pressure during the study, and high staff turnover made the implementation of initiatives challenging. Although a number of organisation-wide initiatives were planned, organisational-level change proved challenging. Those initiatives that were implemented were more limited in scope, with most changes restricted to the two general medical wards. Nevertheless, they did successfully develop and implement a formal escalation policy on the two medical wards. It has not been possible to assess whether or not this has been normalised in practice, but there is evidence that the ward manager on the case study ward was resistant to change and did not promote the policy. Ironically, implementation was not possible on the surgical ward, where the system assessment had identified particular problems around escalation. They also had some success in implementing changes to strengthen reviewing and planning for deterioration to ensure a shared situational awareness between nursing and medical teams; this was achieved after abandoning their original plans because they were not workable, and is a good example of the strengths of a functions-based approach to improvement. Once again, however, during the study, these changes were more limited in scope than originally planned, but were subsequently extended to include the surgical ward. The modified SHINE poster was developed and distributed on the general medical wards only, but it failed to be embedded in practice, again with some evidence of a lack of support from the ward manager in the ward case study. Report Supplementary Material 10, Table 3, shows a summary of the post-implementation system strengths and weaknesses identified by the PUMA team.
Wider impact of the PUMA programme
At the end of the study, the team indicated that they had plans to use the PUMA Standard as a structure for systematically reviewing critical incidents in the hospital. Used in this way, there is significant potential for the PUMA programme to support the development of a learning system. In addition, although the initiatives were more limited in scope than originally planned, there was evidence that they were continuing to be extended across the organisation.
Quantitative analysis
Monthly aggregate-level data were collected at a whole-hospital level in Noah’s Ark Hospital between May 2015 and October 2018. Across all paediatric inpatient wards, patient bed-days averaged 1956 per month. Further details on the approach to the analysis of the data are described in Chapter 2. Appendix 10 shows a full statistical report for this site, including a series of exploratory (Appendix 10, Figure 29 and Table 31) and sensitivity (Appendix 10, Table 32) analyses performed on the primary outcome.
Primary outcome
Figure 19 shows fitted trends for the pre-intervention, implementation period and post-intervention rates of adverse events, per 1000 patient bed-days. For all graphs, solid red lines represent observed fitted trend lines, dotted red lines represent projected trends based on a continuation of pre-intervention trajectory, and dotted green lines represent 95% CIs around the observed fitted trend lines. The mean rate of adverse events per 1000 patient bed-days was 3.99 in the pre-intervention period, 5.41 in the implementation phase and 6.00 in the post-intervention phase.
FIGURE 19.
Noah’s Ark scatterplot with fitted line from segmented linear regression for adverse events.
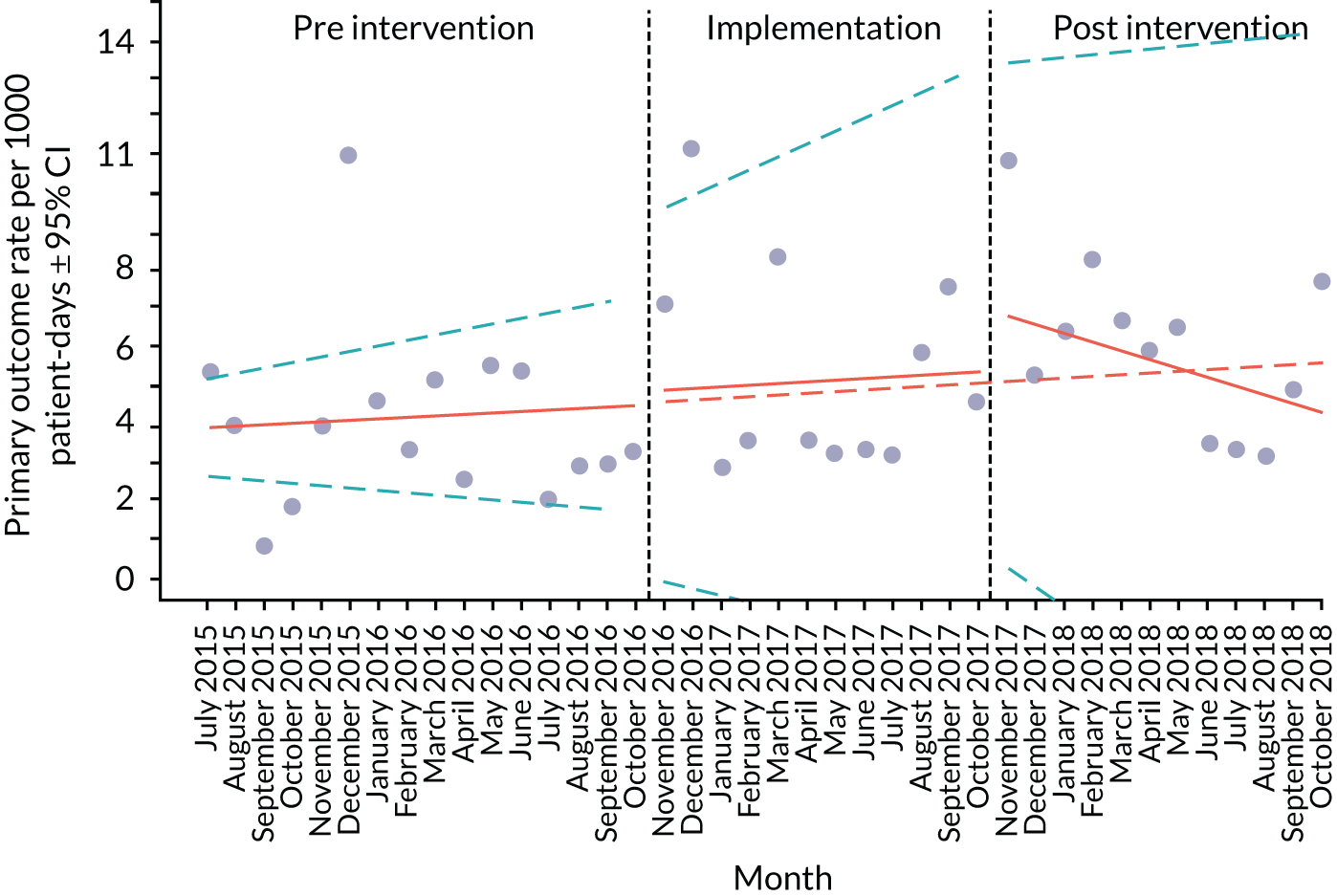
Table 13 presents estimates from segmented linear regression for adverse events, including an interpretation of key findings. The adverse event rate was trending upwards in the pre-intervention period, but not significantly so (β = 0.04, 95% CI –0.06 to 0.15; p = 0.42). During the implementation phase, there was little change in the slope (β = 0.01, 95% CI –0.16 to 0.18; p = 0.92). However, during the post-intervention period, there was a downwards trajectory to the trend in adverse outcomes (β = –0.27, 95% CI –0.47 to –0.07), which was significantly different from the projected trend (p = 0.01).
| Outcome | Estimate, β (95% CI) | p-value | Interpretation |
|---|---|---|---|
| Adverse events | |||
| Intercept | 3.27 (2.12 to 4.42) | ||
| Pre-intervention trend | 0.04 (–0.06 to 0.15) | 0.42 | There is a trend towards increasing event rates, although this is not significant |
| Change in slope (implementation period vs. pre-intervention period) | 0.01 (–0.16 to 0.18) | 0.92 | The event rate does not change, but, given the wide CIs, it is difficult to be precise about whether or not this is a true effect |
| Immediate change in level (implementation period vs. pre-intervention period) | 0.21 (–1.55 to 1.97) | 0.81 | |
| Change in slope (post-intervention period vs. implementation period) | –0.27 (–0.47 to –0.07) | 0.01 | The trend significantly reduced over this period (although the overall number of events per patient bed-day increases) |
| Immediate change in level (post-intervention period vs. implementation period) | 1.98 (–0.22 to 4.18) | 0.09 | |
Secondary outcomes
Figure 20 shows fitted trends (or raw data, when fitted trends were not possible) for individual secondary outcome rates across the three time periods, per 1000 patient bed-days. Table 14 presents estimates from segmented linear regression for secondary outcomes, including an interpretation of key findings.
FIGURE 20.
Noah’s Ark scatterplots with fitted line from segmented linear regression for secondary events. (a) All-cause mortality (raw data); (b) cardiac arrests (raw data); (c) respiratory arrests; (d) unplanned transfers to PICU; (e) unplanned transfers to HDU; (f) PICU staff reviews; and (g) other medical emergencies (raw data).
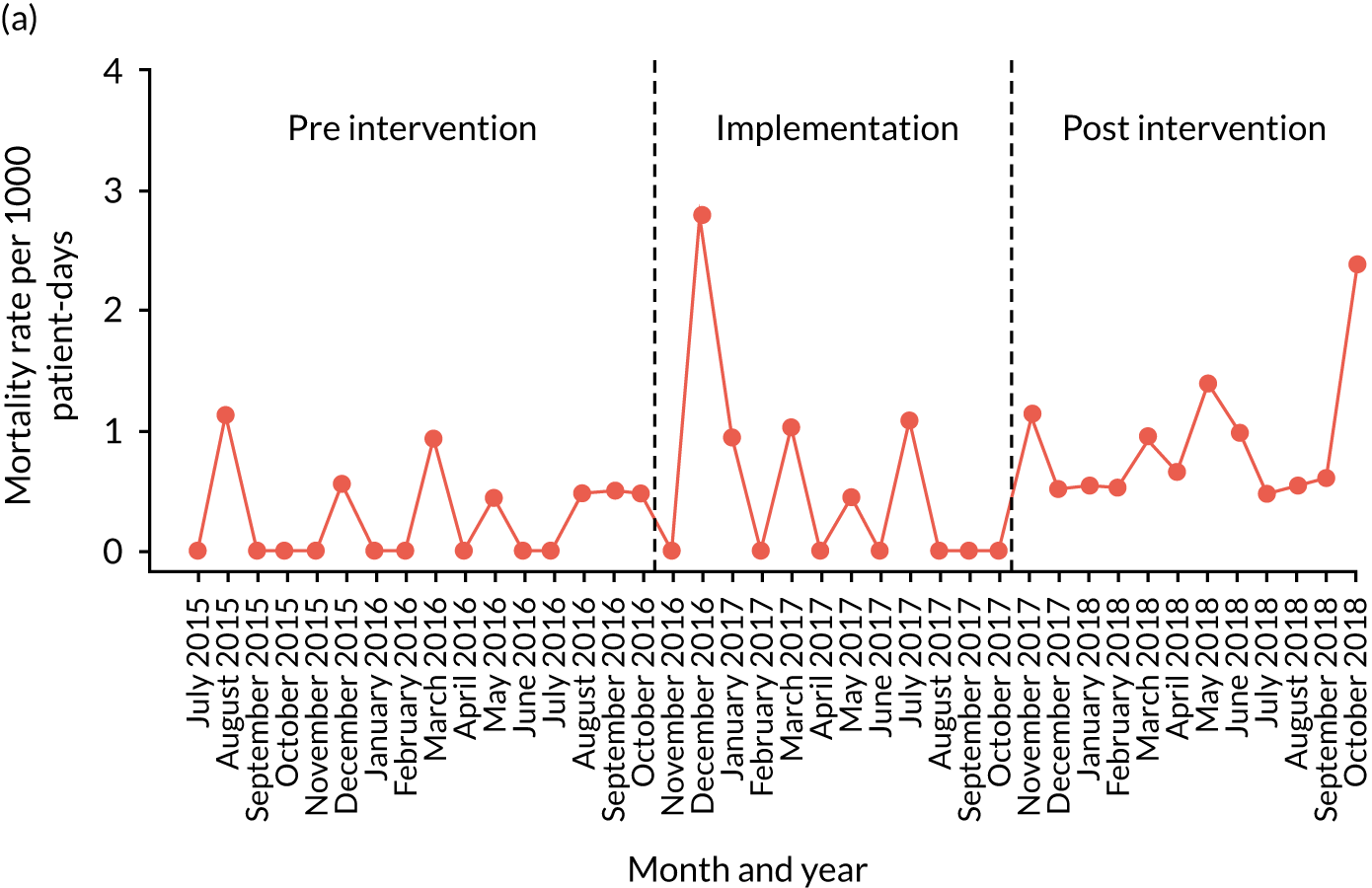
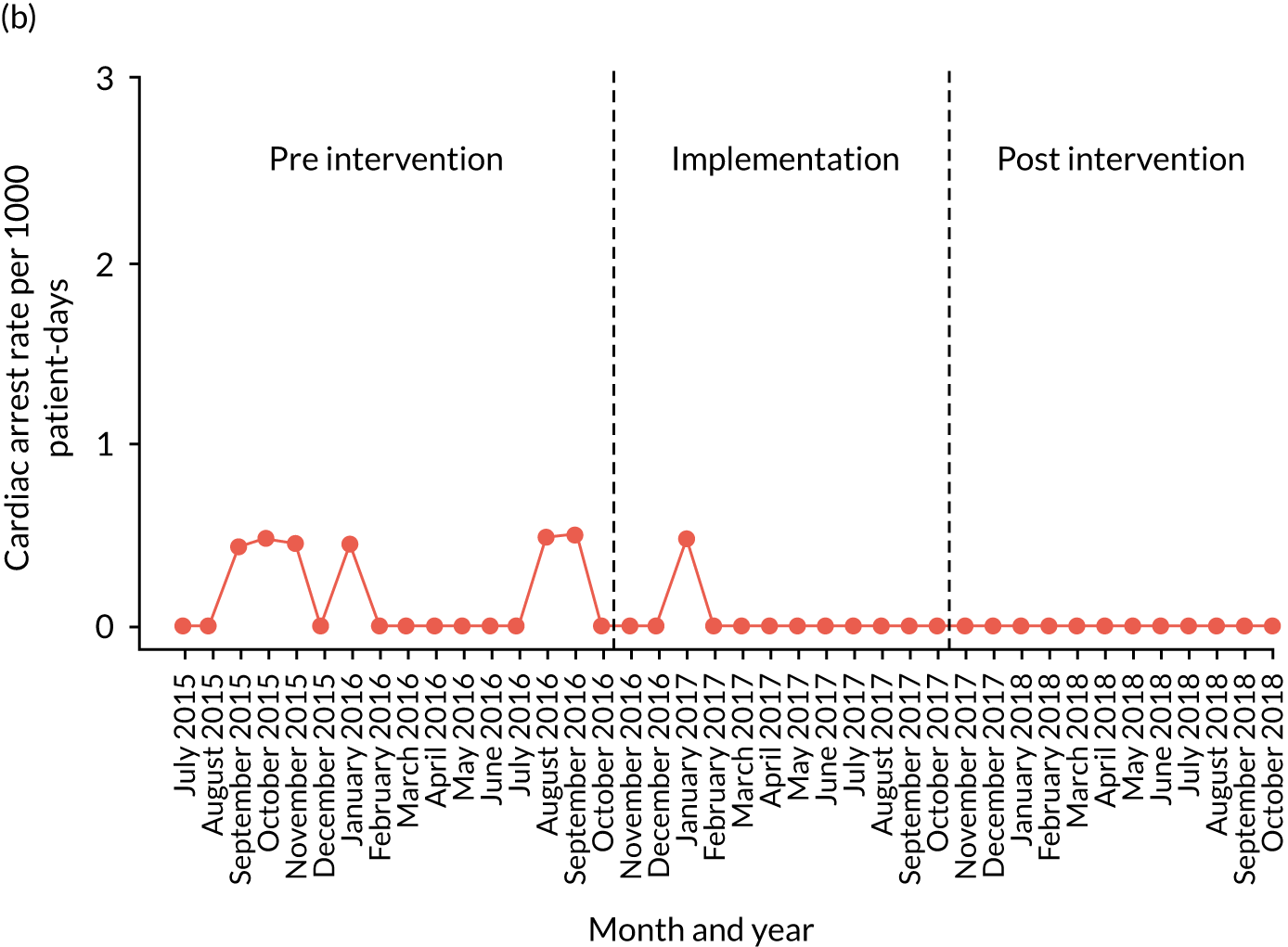
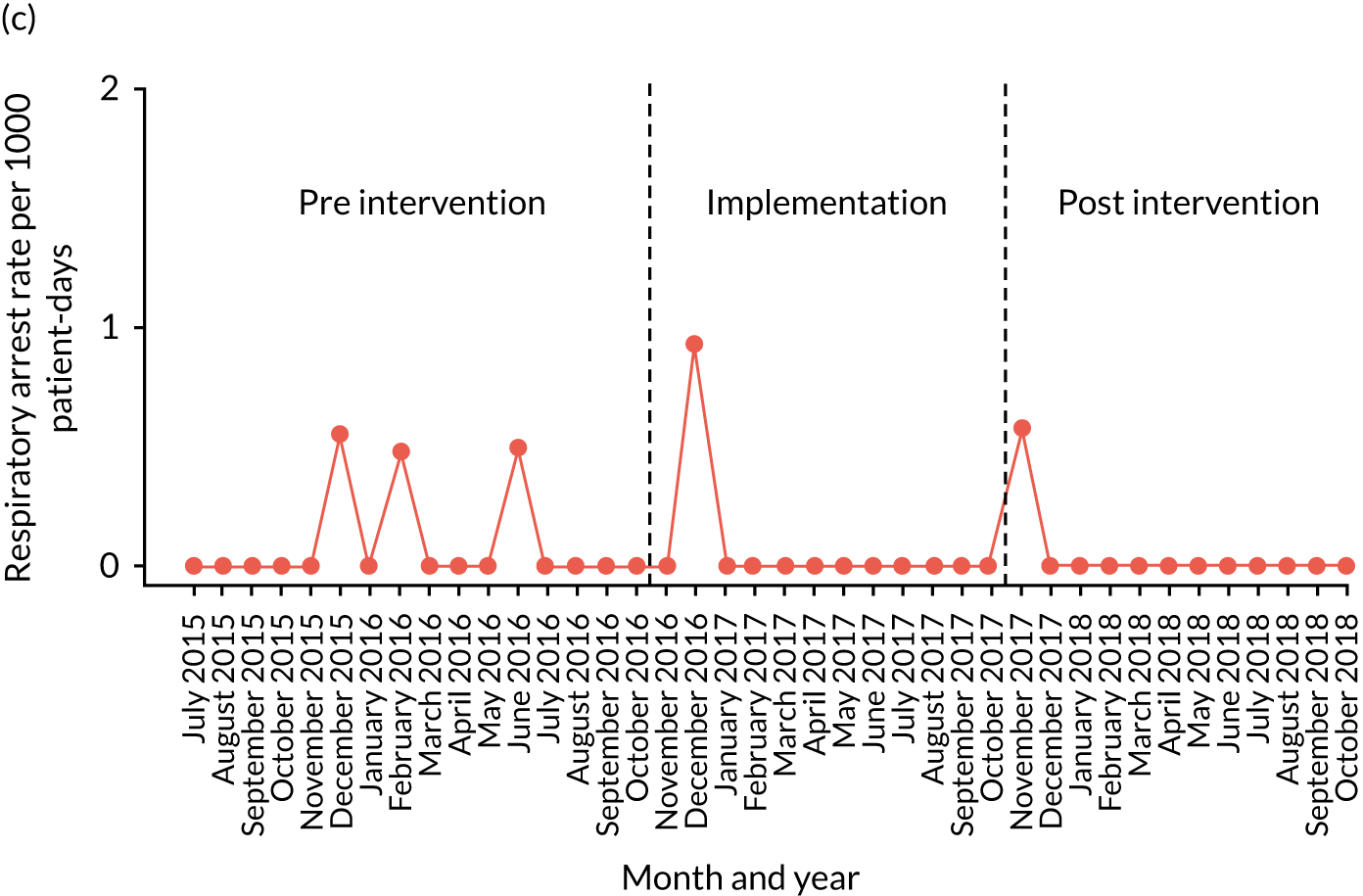
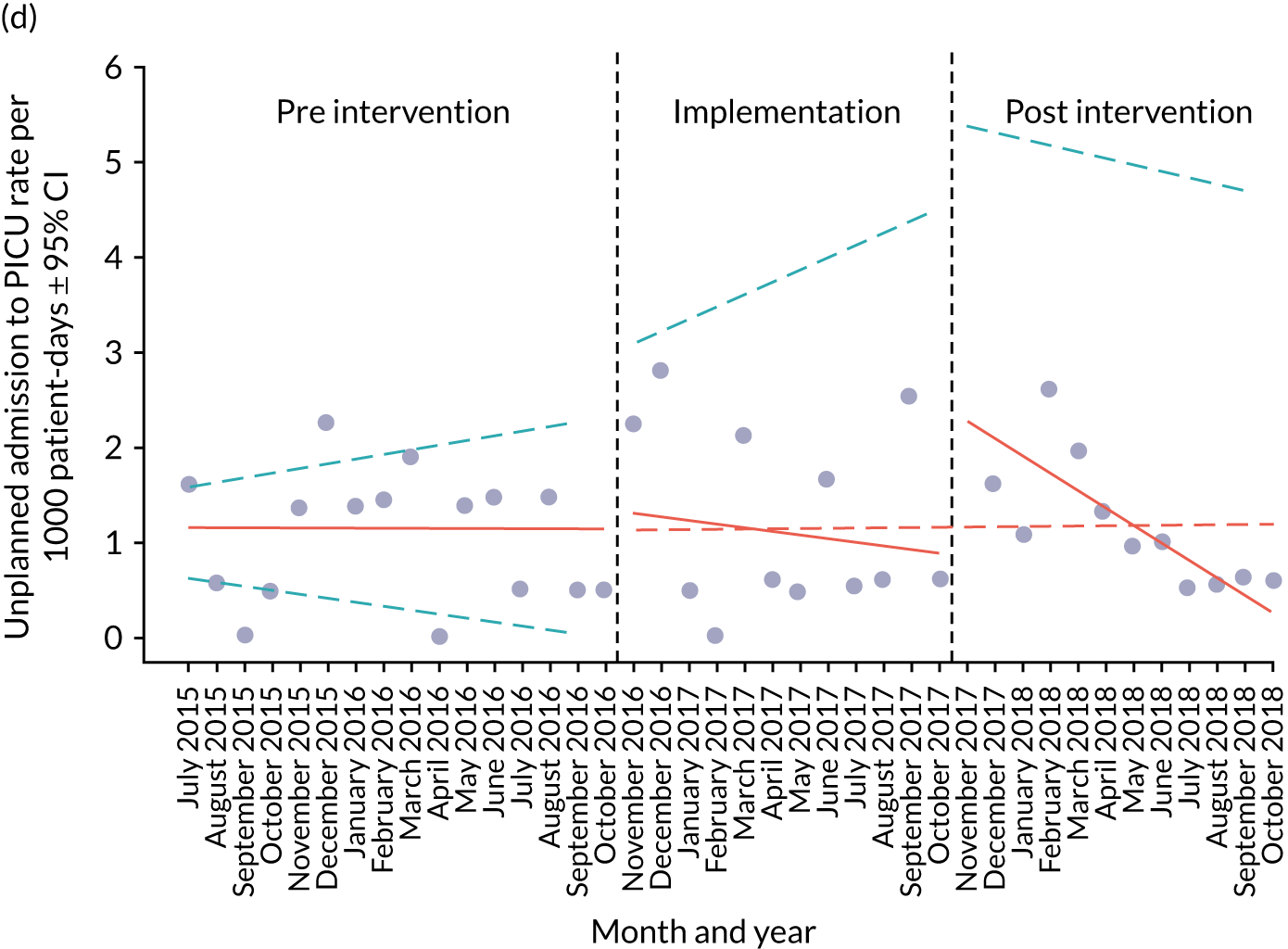
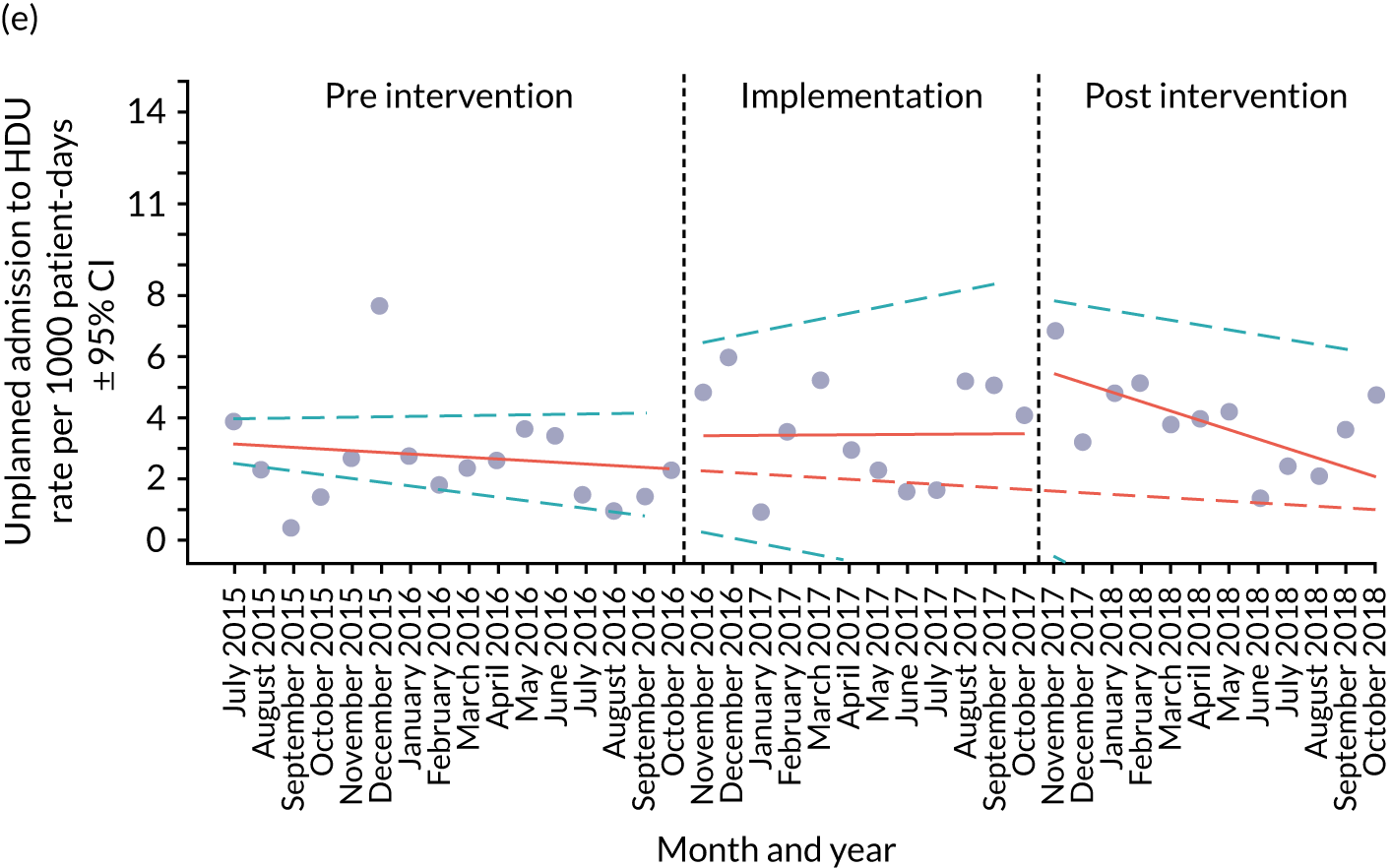
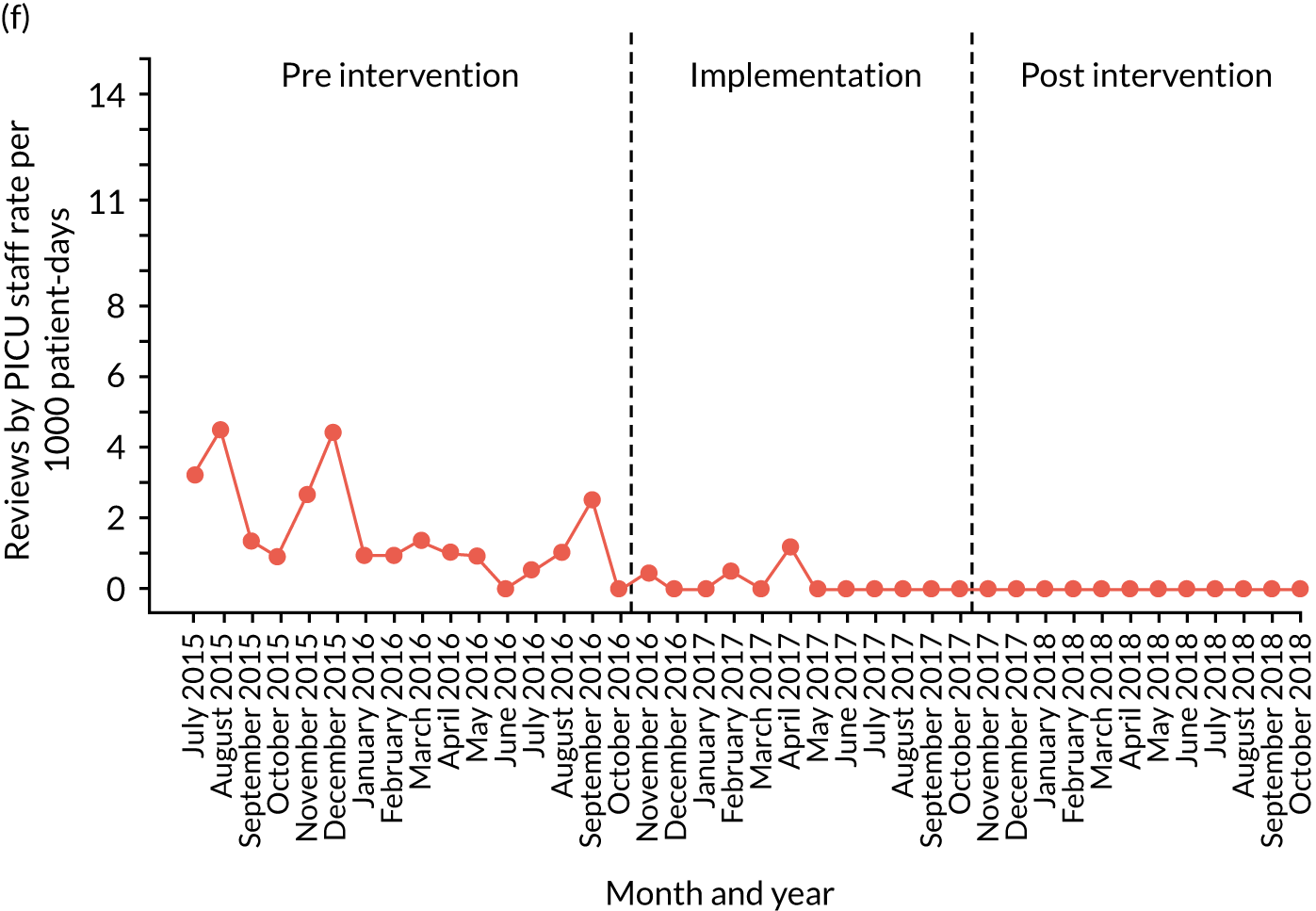
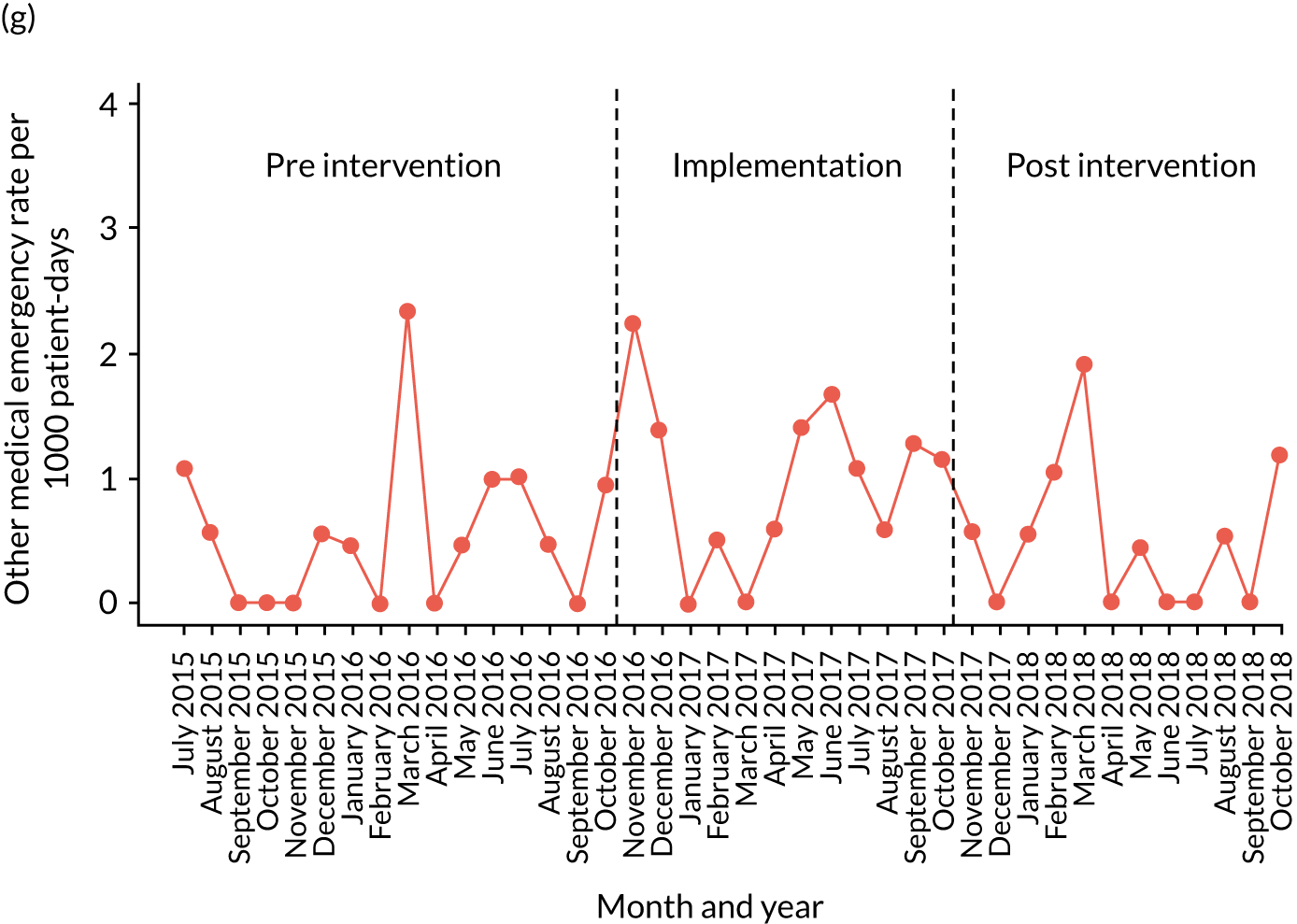
| Outcome | Estimate (95% CI) | p-value | Interpretation |
|---|---|---|---|
| Unplanned PICU transfers | |||
| Intercept | 1.13 (0.77 to 1.49) | ||
| Pre-intervention trend | 0.00 (–0.04 to 0.04) | 0.99 | There is a relatively static trend in unplanned PICU transfers between the implementation and pre-implementation periods |
| Change in slope (implementation period vs. pre-intervention period) | –0.04 (–0.11 to 0.03) | 0.23 | |
| Immediate change in level (implementation period vs. pre-intervention period) | 0.22 (–0.38 to 0.81) | 0.48 | |
| Change in slope (post-intervention period vs implementation period) | –0.14 (–0.22 to -0.07) | < 0.00001 | The rate of transfers significantly decreased in the post-intervention period, but the overall rate of events was actually higher during this period |
| Immediate change in level (post-intervention period vs. implementation period) | 1.60 (0.94 to 2.26) | < 0.00001 | |
| Unplanned HDU transfers | |||
| Intercept | 3.28 (2.63 to 3.93) | ||
| Pre-intervention trend | –0.06 (–0.13 to 0.01) | 0.12 | Similar to the PICU transfer rate, there is relatively little change in rates over time between implementation and pre implementation |
| Change in slope (implementation period vs. pre-intervention period) | 0.06 (–0.06 to 0.18) | 0.31 | |
| Immediate change in level (implementation period vs. pre-intervention period) | 1.03 (–0.12 to 2.18) | 0.09 | |
| Change in slope (post-intervention period vs implementation period) | –0.31 (–0.46 to -0.17) | < 0.00001 | Like PICU transfers, the trend in HDU transfers changes downwards in the post-intervention phase with statistical significance, but the overall rate is higher |
| Immediate change in level (post-intervention period vs. implementation period) | 2.34 (0.94 to 3.73) | < 0.00001 | |
Mortality
The overall mortality rate during the pre-intervention period was 0.27 per 1000 patient bed-days, compared with 0.57 for the implementation period and 0.89 for the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in mortality over the different time periods (see Figure 20a).
Arrests
The overall cardiac arrests rate during the pre-intervention period was 0.18 per 1000 patient bed-days, compared with 0.04 in the implementation period and 0.00 in the post-intervention stage. Respiratory arrests averaged 0.09 per 1000 patient bed-days in the pre-intervention period, 0.09 in the implementation period and 0.04 in the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in cardiac or respiratory arrests over the different time periods (see Figures 20b and c).
Unplanned transfers
The overall rate of unplanned transfers from inpatient wards to PICU during the pre-intervention period was 1.04 per 1000 patient bed-days, compared with 1.22 for the implementation period and 1.24 for the post-intervention period. The equivalent rates for HDU transfers were 2.53 for the pre-intervention period, 3.62 for the implementation period and 3.82 for the post-intervention period.
In the pre-intervention period, there was a non-significant downwards trend in HDU transfer rates (β = –0.06, 95% CI –0.13 to 0.01; p = 0.12), whereas PICU transfer rates were flat (β = 0.00, 95% CI –0.04 to 0.04; p = 0.99). For HDU transfers, there followed a flattening of the trend in the implementation period (β = 0.06, 95% CI –0.06 to 0.18; p = 0.31), before a significant downwards trend in the post-intervention period (β = –0.31, 95% CI –0.46 to –0.17, p < 0.00001). PICU transfer rates trended slightly downwards in the implementation period (β = 0.03, 95% CI –0.07 to 0.13), then significantly downwards in the post-intervention period (β = –0.05, 95% CI –0.17 to 0.06; p < 0.00001) (see Figures 20d and e).
Paediatric intensive care unit reviews
The overall rate of PICU reviews during the pre-intervention period was 1.58 per 1000 patient bed-days, compared with 0.17 for the implementation period and 0.00 for the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in PICU reviews over the different time periods (see Figure 20f).
Other medical emergencies
The overall rate of other medical emergencies during the pre-intervention period was 0.55 per 1000 patient bed-days, compared with 1.00 for the implementation period and 0.53 for the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in other medical emergencies over the different time periods (see Figure 20g).
Synthesis
In this final section, we consider how some of the clearer quantitative findings relate to qualitative observations. Assessing the impact of the intervention on quantitative outcomes was challenging, and so interpreting the quantitative outcomes in relation to the ethnographic observations should be treated with caution.
Noah’s Ark made a number of improvements in certain wards, but no organisational-level changes were introduced during the lifetime of the study. It is possible that changes in consultants resulted in lowered thresholds for HDU and PICU admission. The ITS analysis gave a mixed picture: although the overall adverse event rate actually increased during the implementation and post-intervention periods (driven in part by the increase in HDU and PICU admission rates), the rate of events was trending downwards over the course of both time periods. The downwards trends suggest a possible regression to the mean after some outlying months; in this case, the picture would probably have been clearer if we had continued to collect data over a longer period.
Chapter 8 Case study 4: Morriston Hospital
Pre-implementation phase
Paediatric early warning system in context
The hospital
Case study 4 was undertaken at Morriston Hospital, a 700-bedded DGH, and part of the newly formed Swansea Bay University Health Board (previously Abertawe Bro Morgannwg University Health Board). The paediatric service included a medical ward and a surgical ward, a PAU, a four-bedded medical HDU and a three-bedded surgical HDU. There was no PICU; very sick children were transferred to the tertiary centre approximately 40 miles away.
The ward
The ward case study focused on a 16-bedded medical ward that provided care for children with long-term conditions; acute episodes of illness, including breathing difficulties; or physical, developmental or feeding problems. Some children regularly attended the ward for treatments. HDU beds were in a separate area in the ward and staffed on a 1 : 2 nurse-to-patient ratio. See Report Supplementary Material 24 for details of the layout.
Staffing
The ward team comprised a ward manager, band 6 (× 2) and band 5 (× 16) nurses, three HCAs, four nursery nurses and two play specialists. Student nurses undertook educational placements on the ward. A complex shift system operated, including long days (07.00–19.30), early shift (07.00–15.00), normal days (07.00–16.30), middle shift (11.30–19.30) and night shifts (19.00–07.30). HCAs worked 07.00–14.30. Staffing was highest between 11.30 and 14.30. The nurse in charge carried a case load. All nurses who worked in the HDU area were HDU trained, but not all ward nurses were HDU trained. The nursing team included both experienced (60%) and recently qualified staff (40%). Nurses worked with the named-nursing model; patients were allocated according to their clinical needs. HCAs had their own patients, but these were typically non-complex cases, and they worked under the supervision of a registered nurse. New admissions to the ward were allocated to a suitably qualified nurse, and the allocation of work revised as necessary to optimise skill mix.
The medical team provided 24-hour cover. There were a variety of shift patterns that were different for the SHOs and the middle grades. Six doctors provided weekday cover: two consultants and four junior doctors (registrar and SHOs). The junior doctors rotated every 4–6 months. Throughout the day, at least two junior doctors were based on the case study ward, dealing with medications, blood tests and reviewing patients as requested by nursing staff. At night and at weekends, a registrar, a SHO and an on-call consultant covered the inpatient paediatric service.
Routines
The main nursing handover at 07.00 was an oral handover from one of the night staff to the day staff. Patients were allocated after handover; this was recorded on a whiteboard in the treatment room. Additional handovers took place at shift changes, typically a one-to-one handover from the outgoing to the remaining or incoming nurses. Handover to the night staff at 19.00 was by the nurse in charge, who handed over all patients for the ward.
The morning doctors’ handover took place at 08.30, and was a full department handover from two doctors who had been on night duty across the paediatric whole department. There was an afternoon handover at 16.30 between the consultant of the week, the on-call consultant and junior doctors, and a 20.00 handover from the middle-grade doctors, who handed over to the doctors working the night shift. A consultant would usually telephone or attend the 20.00 handover in person.
The medical ward round took place at approximately 09.30. This was followed by the nursing–medical board round, attended by the doctors and the nurse in charge.
Family involvement
Involving families was central to patient care, but there were limited facilities for parents to stay with their child over night. Parents slept in a chair next to the child and the ward policy restricted this to one parent only.
Paediatric early warning system assessment
Detect
Observation policy was based on RCN guidelines,213 although staff did not explicitly orient to this in practice. All children were required to have vital signs monitored at least every 4 hours, unless otherwise directed by the doctor. The normal set of vital signs observations consisted of heart rate, oxygen levels, temperature and respiratory rate (see Report Supplementary Material 25). In the exceptional cases when a child required additional clinical observations, this was communicated at handover and recorded on a whiteboard, which functioned as a common information space and was located in the treatment room. Roles and responsibilities in relation to detection appeared to be reasonably well understood.
Equipment was problematic: ward staff often had to search for an equipment trolley to perform observations. In addition, there was a shortage of paediatric probes for measuring oxygen saturation and pulse, and a lack of the correct size of cuffs for monitoring blood pressure; equipment was often found not to be working. There was no facility to perform continuous monitoring, which nurses perceived would be of value in cases when obtaining accurate observations could be difficult, such as in young children for whom the process could be distressing.
Formal observation policy required that heart/pulse rate should be checked manually for 1 minute. Throughout the period of observation, the use of a manual pulse was not observed at all, and heart rate was routinely taken from automated readings. Nurses’ reliance on machine readings reflected the additional time it took to manually obtain observations, and a lack of skills in manual approaches in the case of more junior staff. It was also difficult to conduct observations as required at night and during weekends, when there were only two nurses working.
There was no PTTT in use on the ward. There were local guidelines for observation frequency for some conditions, but nurses were encouraged to observe the child as a whole, to understand the normal parameters for each individual patient and to share any concerns. Increasing the frequency of observations was largely left to nurses’ discretion. There were also posters specifying normal physiological thresholds.
On admission, the named nurse encouraged parents to ask for help if the child’s status changed, and established baseline details of the child’s condition, which initiated parental involvement in detection. Nurses took pride in ‘knowing their patients’, and much of this knowledge of ‘what is normal’ for each child was derived from the parents in these first interactions. Nurses also had established relationships with families whose child regularly attended the ward. Nurses at the start of each shift also reinforced parental involvement. During medical review and ward rounds, doctors sought parents’ views on their child’s status and checked if they had specific concerns.
Vital signs were recorded on a paper-based observation chart, which displayed trends. A different observation chart was used in the PAU, which undermined monitoring continuity, as it was necessary to start a new chart on admission to the ward. There was no visual reminder or guidance for staff on the observation charts to indicate whether the plotted observation readings were within normal ranges or a cause for concern. There was no system in place for storing charts, and so these were sometimes difficult to locate, which could result in observations being recorded on pieces of paper before formal charting. Separating the time of collecting observations from the time in which they were recorded on the chart delayed the opportunity to interpret the vital signs alongside previous observations.
Both doctors and nurses asked parents regularly for their perspectives on their child, and this was combined with observations, knowledge of baseline parameters and test results, to provide up-to-date reviews. Parental engagement was uneven. Although there were many examples of constructive and supportive relationships, it could be challenging for nurses to manage parental concerns not considered to have a clinical foundation. Conversely, nurses reported that parents did not always raise concerns with doctors, even though they had raised their concerns with nurses beforehand. This offered an interesting insight into interprofessional relationships on the ward: nurses expressed frustration with this, because they considered that parents had more authority to question the doctors than they themselves, and this was a missed opportunity.
Nurses and support workers continuously reviewed the statuses of children in their care, undertaking additional observations as required and discussing the case with colleagues. From this, a plan for increasing the observation of children or referral to a doctor was made, collaboratively. Nurses emphasised that assessing a child went far beyond physiological indicators:
Paediatrics is very much observation of the whole patient, not just the figures. [. . .] sometimes you just look at the child, as a nurse, and you know this child is seriously sick. And it goes by nasal flaring, tracheal tugs, you don’t even need to do the respiratory rate, you can actually see the child is seriously ill. [. . .] You take in the whole picture of the patient [. . .] not just a parameter.
Senior Nurse
Plan
There were a number of mechanisms for reviewing individual patients: the separate nursing and medical handovers, the ward round and the post-ward round nursing–medical board round. The 07.00 nursing handover was the primary means for nurses to review all patients and ward status. Nurses received handover on all patients and made notes on a printed bed plan of the ward. There was no pre-populated electronic handover sheet. Each child was considered in bed order. Patients at risk of deterioration were not explicitly highlighted in one single section of nursing handover, but nurses generally noted if they were concerned about a patient. At the evening nursing handover, individual nurses handed over their patients to the night staff. Smaller handovers occurred at shift changes, but related only to the patients being handed over, not the whole ward.
The medical staff handover took place at 08.30. This was a full department handover attended by the doctors working the day shift on the PAU and medical and surgical wards. Doctors worked with pre-populated electronic handover sheets, which were updated, at a minimum, at the end of each shift. It included information on concerns and treatment plans on all paediatric patients. Additional handovers took place at 16.30 between the consultant and consultant on call and at 20.00 between the middle-grade doctors and the night-shift team. Consultants often called in for the 20.00 handover.
An explicit purpose of the medical handover was to identify the children of most concern who should be reviewed first, including children in the HDU:
[O]ne of the first questions they’ll say is ‘which ones are the children that you’re a bit concerned about or that we need to be more aware of, or that needs to be seen sooner rather than later?’, so we’ve already got an idea, often, from leaving handover which ones to keep an eye out for.
Registrar
Children were reviewed and treatment plans agreed on the ward rounds. On the medical ward round, the team divided into two groups, one led by the consultant and the other led by the registrar. The consultant saw the most ill patients, starting in the HDU. Although senior medical staff expressed a preference for a nurse to attend the ward round, this was considered impractical by the nursing team because the ward round took place at the same time as nurses were taking breaks and the nurse in charge carried a case load.
The main opportunity for a joint review by doctors and nurses was the board round that immediately followed the ward round. The nurse in charge, or their nominee, attended the board round and communicated information to colleagues. The board round took place in the treatment room and information was reviewed and updated on the whiteboard. This was consulted frequently by both medical and nursing staff, and was an important central resource for sharing information. Nurses took responsibility for maintaining the board, but did not always have time to update it.
Beyond these formal mechanisms, reviews of patient status and ward activity were woven through daily activities. Doctors and nurses discussed patients and planned for action; a new admission could prompt a review of overall acuity across the ward and the identification of patients considered to be at risk:
Because, with our assessment of when people come in, kids come into the ward, we will often rearrange, if we think somebody is a potential to get unwell, we will try to get them closest to this end, to the desk and the HDU area of the ward, rather than being, like, 30 feet down the corridor. So we’ll have them up here anyway.
Nurse
Act
This site did not use a PTTT; thus, there was no standardised score or trigger that prompted action. The escalation policy was based on the RCN guidelines,213 which recommend additional checks of vital signs, but no other forms of action. There were some general indicators in place for actions related to vital signs, but there was low awareness of a formal graded escalation policy. Decisions about when and how to escalate care was at the discretion of clinical staff.
Nurses reported that they would have no hesitation in seeking a senior medical review if this was indicated. Nurses typically first discussed the situation with each other; there was a strong informal network of peer support on the ward, and official support from more senior experienced nurses. Furthermore, during the day, doctors were often present on the ward or in the attached HDU, and so, in practice, nurses were pragmatic and, rather than working their way through the medical hierarchy, would speak to doctors who were on the ward or nearby. The doctors also preferred that junior nurses consulted with more senior nurse colleagues initially, with one doctor noting the tension between nurses reporting concerns and doctors’ ability to manage requests:
And so I do think it’s a good idea for junior nurses to speak to somebody senior on their ward, but if they can’t, if they’re off the ward, then they shouldn’t feel that they have to wait . . . And that’s, and that’s a challenge, I think, in trying to balance those two things, so encouraging people to not always ring, but not wanting them to feel like they can’t ring.
Consultant
Escalation could be a delicate balance, and we observed that tensions could sometimes surface, both within the nursing team and between nurses and doctors. HDU staff routinely reviewed cases of mortality or critical incidents. See Report Supplementary Material 6, Table 4, for a summary of the Morriston pre-implementation system strengths and weaknesses identified by the PUMA study team.
Implementation phase
Process
The Morriston improvement team was led by the PI (a consultant) and comprised a practice development nurse, an associate practice development nurse, the lead nurse for paediatrics, the head of nursing, a consultant paediatrician, a consultant paediatrician at a linked site and a lecturer in nursing from the partner university. A number of additional people from the paediatric service led the implementation of specific initiatives.
The SSAT and FFT were administered on the medical and surgical wards and the PAU, and highlighted many areas of weakness (Figure 21), which key members of the improvement team were unprepared for and found troubling. The implementation team met several times to discuss where to focus their efforts.
FIGURE 21.
The Morriston system assessment radar diagram. A score of 0 was used to indicate poor alignment with the system standard; a score of 10 indicated optimal alignment with the standard.
The implementation strategy entailed explicitly framing initiatives as everyday QI, rather than as PUMA-badged interventions. From the perspective of the improvement team, this was necessary to secure enrolment in the initiatives, which they believed would be difficult if the changes were perceived as ‘research’, and thus time-limited. However, from the perspective of the research, this did make it more challenging to trace impacts in the post-implementation phase. See Report Supplementary Material 7, Table 4, for a summary of the pre-implementation system strengths and weaknesses identified by the improvement team.
Context
At the time of the study, the hospital was involved in a wider restructuring of the organisation; this had some impacts on the implementation of certain initiatives, which required governance and institutional approvals.
The implementation process was also affected by a period of unscheduled absence on the part of the PI. Figure 22 presents the Morriston implementation process timeline.
FIGURE 22.
Morriston implementation process timeline.

Initiatives
The PUMA programme initiatives
Fifteen initiatives were proposed at this site (see Report Supplementary Material 8, Table 4):
-
Update and disseminate the observation policy.
-
Create posters and cards for staff to signpost abnormal thresholds for vital signs.
-
Update observation charts to include normal range thresholds for vital signs.
-
Conduct inventory of equipment.
-
Formally establish Deteriorating Child Study Day across the health board.
-
Roll out an in-house electronic learning (e-learning) package for nursing and medical staff.
-
Ward nursing staff to spend more time observing HDU staff.
-
Move to adopt three daily huddles/board rounds.
-
Introduce process for identifying ‘watchers’ at each huddle, for example with markers on a whiteboard.
-
Review handover content. Possibility of including nursing staff in medical handover.
-
Re-establish a nursing supernumerary role.
-
Establish a staff training course on situational awareness.
-
Review and disseminate existing escalation policy.
-
Review communication tools to aid escalation of patient care.
-
Explore tools for family/parental involvement.
Although work in some areas had already started before the PUMA programme, these were all included in the site action plan. Together, these were designed to improve the following: detection, by making changes to monitoring, recording and interpreting activity; planning for escalation, by prioritising/formalising reviewing of patients and using advance planning for escalation; and action, by timely escalation and confident planned response to deterioration.
Some initiatives were designed to come into effect as soon as possible. This was driven strongly by the leads of each initiative and enabled, in part, by earlier development work.
Update and disseminate observation policy
The paediatric early warning assessment revealed a lack of awareness of the observations policy, and that further clarity was required to guide staff regarding frequency of observations and the core vital signs to be observed for all children. The policy was updated to reflect the most recent RCN guidelines213 and was led by the PI and senior consultant. An e-mail and poster campaign was used to raise awareness of the policy, with staff required to sign to confirm that they had seen the policy. There was also an intention to monitor implementation of the policy weekly through spot checks, but we found no evidence that this process measure was implemented.
Posters and cards with information on normal physiological parameters
The development of posters and cards aimed to make access to information on normal physiological parameters easier for staff, as these were not included on the observation chart. The clinical educator, working with the support of five additional nursing team members, led the initiative. The cards and posters were easily produced without the need for additional resources, and staff kept the laminated card in their identification (ID) holder, which made it accessible and easily incorporated it into routine practice.
New observation chart
Development work on a new observation chart had already commenced prior to the PUMA programme. The practice development nurse led this, with support from others (a lead nurse, an associate practice development nurse and three consultant paediatricians). Although not a PTTT, the chart had been updated to include colour-coded age-related normal physiological vital signs thresholds (see Report Supplementary Material 26). Although a staff survey showed high levels of support for the new chart, implementation was delayed for 12 months because of the need to secure organisational governance with a sister hospital, and concerns about its use in some clinical areas. It was implemented on the medical ward, the PAU and the surgical ward, but not A&E, because it was felt that a bespoke chart was required.
Inventory of ward equipment
The paediatric lead nurse led an inventory of medical ward equipment in the surgical and medical wards and PAU. It was carried out early in the implementation period and the problems of inadequate and unsuitable equipment were rectified within a matter of weeks. Observation trolleys were cleaned daily by the ward cleaning staff, and all breakages or stock issues were reported at the time. An annual plan of maintenance was created with the medical physics department.
Deteriorating Child Study Day
This initiative was to ‘formally establish the Deteriorating Child Study Day’. This was already in the calendar of educational sessions offered by paediatric service, but the intent was to modify the content to include situation awareness and human factors, get it accredited by the RCN and RCPCH and make it a biannual event to increase the number of nursing and medical staff attending. The study day was arranged and then postponed at least twice during the PUMA project, but a study afternoon was implemented. At the end of the study, the initiative had not been implemented, but there was still a commitment to moving things forward.
Online learning materials
Another educational initiative was to encourage e-learning, to overcome some of the challenges of releasing staff to attend study days. This started with the intention of getting staff to complete the RCPCH RCN paediatric emergency e-learning package, ‘Spotting the Sick Child’. However, having experienced numerous ‘technical issues’, they moved forward with their own custom e-learning package (a simpler version, focusing on determining abnormal vital signs in children) for medical/nursing staff. Led by a medical student, work on this initiative predated the PUMA programme. At the end of the study the resource had been developed, but it was awaiting organisational approval before it could be implemented.
Nurse shadowing of high-dependency unit staff
A further educational initiative was to enable ward nurses to have an opportunity to shadow HDU-trained staff. Although well intentioned, this was not possible to implement because of nurse staffing levels.
Safety huddles
The post-ward round board round was considered a valuable mechanism for ensuring situation awareness between the medical and nursing teams, and this initiative sought to increase the frequency to three daily huddles (at 10.30, 16.30 and 21.00) and to develop a protocol specifying purpose, information and attendance, with the intention to audit practice. The initiative did not progress, however, in large part because not all staff considered it to be necessary.
Categorising children of concern as ‘watchers’
This initiative was intended to improve situational awareness through the routine designation of children considered to be at risk of deterioration as ‘watchers’ and adding this information to the whiteboard and handover sheets using a standard signifier. Implementation required considerable effort on the part of the PI in leading by example; by the end of the study, watchers were consistently mentioned in the board round, the team having implemented the ‘5Ss’: safeguarding, same name, bed status, sick children and staffing at every handover. A new acuity tool was also introduced for use by nurses, which highlighted watchers.
Review of nursing and medical handovers
This was a two-pronged initiative aimed at improving information-sharing, and improved situational awareness. First, the intention was to explore the possibility of adjusting nursing and medical shift patterns to enable a joint nursing–medical handover. However, this was not considered practical. Second, there was a plan to review the content and structure of nursing and medical handovers to assess whether or not there was potential for greater standardisation, to improve situation awareness. Work on the review was ongoing at the end of the study.
Establish a supernumerary nurse role
The RCN guidelines213 recommend that the nurse in charge of the shift should be supernumerary to ensure effective management, training and support of staff. In addition, and in the context of paediatric early warning systems, this individual has an important role in ensuring situational awareness. In the pre-implementation phase, we observed the difficulties of the nurse in charge attending ward rounds and board rounds because they also carried a case load. The team aimed to implement the RCN guidelines,213 but staffing levels in the study site prevented the implementation of this initiative, and increasing the nursing establishment was beyond the immediate control of the ward.
Situational awareness training
This initiative was proposed to fill a gap in staff education about risk management. The first situational awareness study afternoon took place in July 2018.
Review and dissemination of the existing escalation policy
The escalation policy was reviewed alongside the observation policy. An e-mail and poster campaign was used to raise awareness of the policy, with staff required to sign to confirm that they had read it. There was also an intention to monitor implementation of the policy weekly through spot checks, but there is no evidence that this process measure was implemented.
Review communication tools to aid escalation of care
This initiative was intended to strengthen the clarity of communication during escalation of care, through the identification and implementation of a structured communication tool. However, the site lead for this initiative had a long period of sick leave; thus, it never got off the ground.
Explore available tools for family engagement
This initiative aimed to identify more structured mechanisms for engaging parents in the care of their child, with staff expressing concern that parents did not always understand the information they were given. The initiative was led by the PI and resulted in amendments to a parent information booklet given to parents when the child was in the PAU, which emphasised the parents’ role in their child’s hospital stay, and that parents could be confident that staff would be open to their concerns.
See Report Supplementary Material 9, Table 4, for a summary of the implementation of action plan initiatives and Appendix 21, Table 48, for a summary of all embedded improvements to the system.
Post-implementation phase
Paediatric early warning system in context
During the implementation phase, we identified two contextual changes with potential implications for the functioning of the paediatric early warning system. The ward shift system was revised to reduce its complexity and the impact that this complexity had on continuity of care and communications.
There had also been ward-level changes to the storage and management of patient information.
Paediatric early warning system assessment
Detect
As a result of the equipment inventory, access to monitoring equipment on the ward had improved. Each cubicle/room had its own monitoring equipment, so that searching for the right tools was no longer necessary. New mobile observation trolleys with appropriate equipment (paediatric probes) and multiple operational capacity to record temperature, oxygen saturation, respiratory rate and blood pressure were held in the central corridor of the ward and used by staff in the patient bay areas. Observation trolleys were cleaned daily, and breakages or stock issues were reported at the same time. Ward staff unanimously considered the new equipment to have made their work much easier, with equipment much more accessible.
A laminated copy of the RCN observation and escalation guidelines213 was included in every patient file on admission, as a reminder to staff. However, despite the initiative to raise awareness of the new policy, this was not evident in the post-implementation fieldwork. This was one of the earliest initiatives implemented as part of the PUMA programme; therefore, it is possible that the policy had been normalised by the post-implementation period.
The detection of vital signs outside normal physiological parameters had been addressed in several ways. First, by early implementation of easily accessible information for staff through updated posters and cards showing normal/abnormal thresholds. Although the posters were not in evidence in the post-implementation period, the majority of staff were aware of the cards, and card use continued, albeit infrequently. Second, the accessible information campaign was subsequently followed by the introduction of the observation chart, which was colour-coded to indicate normal vital signs thresholds. The same chart was implemented across the medical ward, the PAU and the surgical ward, which improved monitoring continuity and recognition of vital signs trends.
The challenges nurses had in locating observation charts in the pre-implementation period, which created delays in formally recording observations, had been mitigated by ward-level changes to the storage and management of patient information. Observation notes were kept in the patient notes in individual red ring binders and stored in a trolley in the treatment room. There was a notice to the doctors to remind them to replace the red binders in the trolley as soon as possible, and not to separate the contents. Nursing staff observed that one or two regular offenders needed to be reminded, but, for the most part, staff appeared to take binders and return them appropriately, and there was no confusion over the location of the observation charts.
Plan
Although the improvement team decided that there was no need to increase the frequency of board rounds/safety huddles, the post-implementation fieldwork indicated that there was increased attention to situation awareness. The team had implemented the 5Ss at every handover. At-risk children were consistently designated a watcher status at board round and the contents of the whiteboard appeared to be more regularly updated and more complete. Nurses appeared to update the board during their work, while they performed the required tasks shown and those decided during handover. Doctors looked at the whiteboard frequently in between seeing patients in the ward round. We were not able to assess the impact of changes to the nurses’ shifts on communication.
Act
There was little evidence of increased awareness about the escalation policy, as in the pre-implementation phase, and no discernible differences in staff accounts of summoning medical teams. However, staff interviews in the post-implementation period suggested that, when there was a risk of deterioration, patients were moved into the HDU more quickly than previously:
The children who are sick in our cubes are escalated quickly to HDU [. . .] You know all the doctors seem to be very quick [. . .] quick and prompt, like, on moving them before, you know, things deteriorate.
Nurse
Summary
The system assessment at Morrison highlighted multiple areas for improvement to align with the PUMA Standard, which led to the inclusion of a large number of initiatives in the site action plan. The team was able to implement a small number of initiatives quickly, for example an equipment inventory, thresholds information cards, and efforts to raise staff awareness of the observations and escalation policies. Other initiatives were more ambitious to develop and implement, such as training programmes, new observation charts and e-learning, because they required higher-level organisational approvals and/or involvement of stakeholders beyond the improvement team. Staff shortages were also important constraints and prevented the implementation of HDU shadowing and the supernumerary status for the nurse in charge. Some small modifications were made to parent information sheets, but, otherwise, mechanisms for parental involvement were unchanged. Report Supplementary Material 10, Table 4, shows a summary of the post-implementation system strengths and weaknesses identified by the PUMA study team.
Wider impact of the PUMA programme
A number of initiatives were not implemented and/or embedded during the study lifetime, but remain ongoing: a review of the nursing and medical handover, an annual training programme and an online training programme. In addition, the nursing staff reflected positively on the PUMA programme in encouraging them to think differently about the improvement process:
It’s given us a purpose for changing things. I think it has improved it. Because we have put better things in place, haven’t we? I think we are more aware, our focus was on the [nurses], on their education, rather than proving we didn’t need a score, that was our drive.
Senior nurse, meeting, field notes
As a result of the work undertaken by the improvement team in reviewing educational provision, the PI became involved in an initiative with the university to develop a 30-credit nursing module on recognising the sick child.
Quantitative analysis
Monthly aggregate-level data were collected across all paediatric inpatient wards at Morriston Hospital between July 2015 and October 2018. Across all paediatric inpatient wards, patient bed-days averaged 655 per month.
Further details on the approach to the analysis of the data are described in Chapter 2. Appendix 10 shows a full statistical report for this site, including a series of exploratory (Appendix 10, Figure 30 and Table 33) and sensitivity (Appendix 10, Table 34) analyses performed on the primary outcome.
Primary outcome
Figure 23 shows fitted trends for pre-intervention, implementation period and post-intervention rates of adverse events, per 1000 patient bed-days. Solid red lines represent observed fitted trend lines, dotted red lines represent projected trends based on a continuation of pre-intervention trajectory, and dotted green lines represent 95% CIs around the observed fitted trend lines.
FIGURE 23.
Morriston scatterplot with fitted line from segmented linear regression for adverse events.
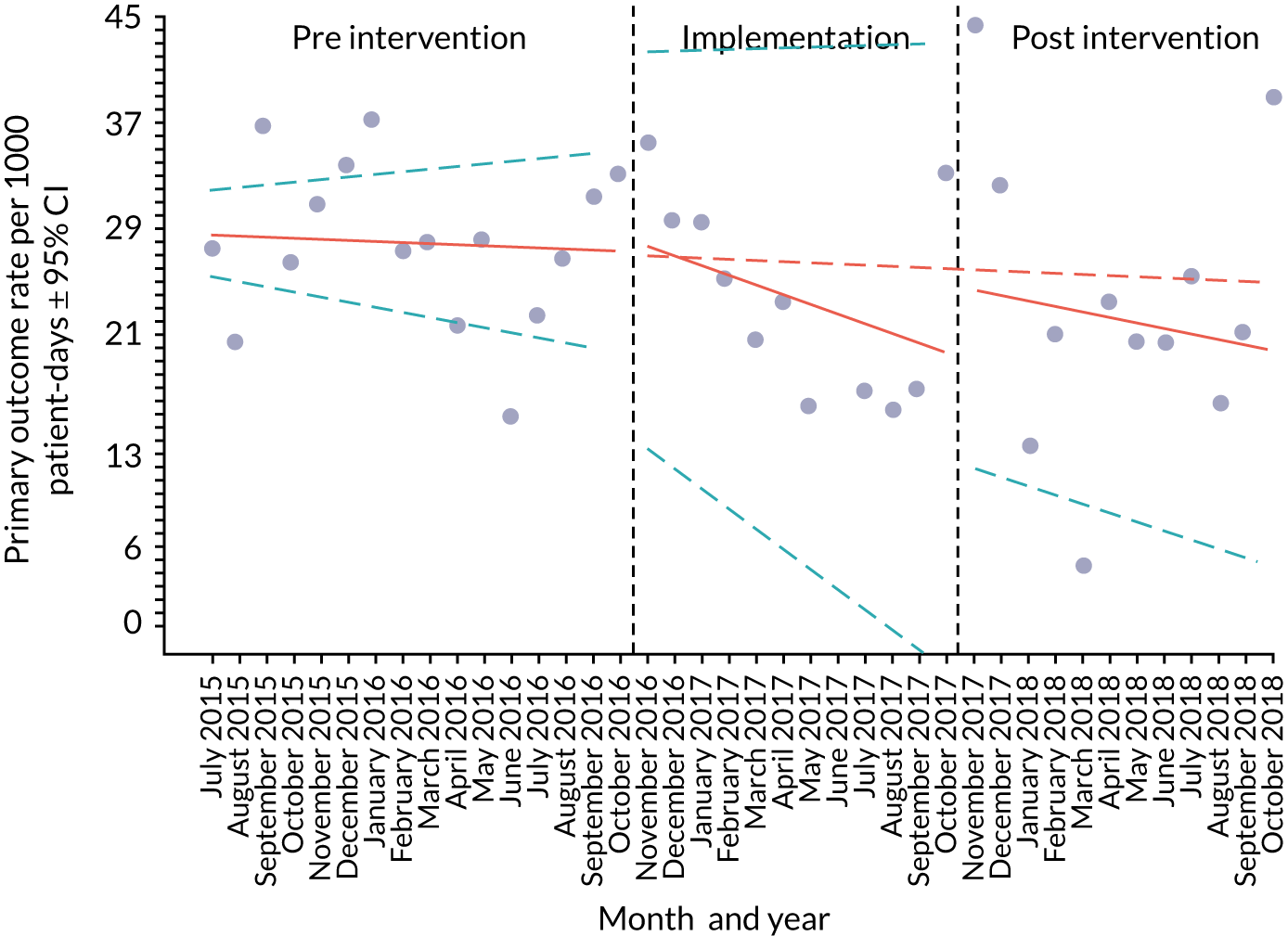
Table 15 presents estimates from segmented linear regression for adverse events, including an interpretation of key findings. The overall rate of adverse events per 1000 patient bed-days was 29.07 in the pre-intervention period, 24.83 in the implementation period and 24.24 in the post-intervention period. In the pre-intervention period, there was a downwards slope in rates of adverse events over time, but with wide CIs (β = –0.10, 95% CI = 0.40 to 0.21; p = 0.55). During the implementation phase, the adverse event rate trended significantly downwards, relative to the pre-intervention trajectory (β = –0;64, 95% CI –1.15 to –0.13). In the post-intervention period, the rate trended downwards, but flattened relative to the implementation period (β = 0.32, 95% CI –0.29 to 0.93; p = 0.31).
| Outcome | Estimate, β (95% CI) | p-value | Interpretation |
|---|---|---|---|
| Adverse events | |||
| Intercept | 29.69 (26.89 to 32.49) | ||
| Pre-intervention trend | –0.10 (–0.40 to 0.21) | 0.55 | There was no apparent significant trend in the overall adverse event rate |
| Change in slope (implementation phase vs. pre-intervention phase) | –0.64 (–1.15 to –0.13) | 0.02 | There was a significant deviation in the event rate during the implementation phase, which probably represents a real clinical impact |
| Immediate change in level (implementation period vs. pre-intervention period) | 1.57 (–4.05 to 7.18) | 0.59 | |
| Change in slope (post-intervention phase vs. implementation phase) | 0.32 (–0.29 to 0.93) | 0.31 | This trend was maintained, but was not significantly different from that of the implementation phase |
| Immediate change in level (post-intervention period vs. implementation period) | 0.32 (–0.29 to 0.93) | 0.31 | |
Secondary outcomes
Figure 24 shows fitted trends (or raw data, when fitted trends were not possible) for individual secondary outcome rates across the three time periods, per 1000 patient bed-days. Table 16 presents estimates from segmented linear regression for secondary outcomes, including an interpretation of key findings.
FIGURE 24.
Morriston scatterplots with fitted line from segmented linear regression for secondary outcomes. (a) All-cause mortality; (b) cardiac arrests; (c) respiratory arrests; (d) unplanned transfers to PICU; (e) unplanned transfers to HDU; and (f) PICU staff reviews.
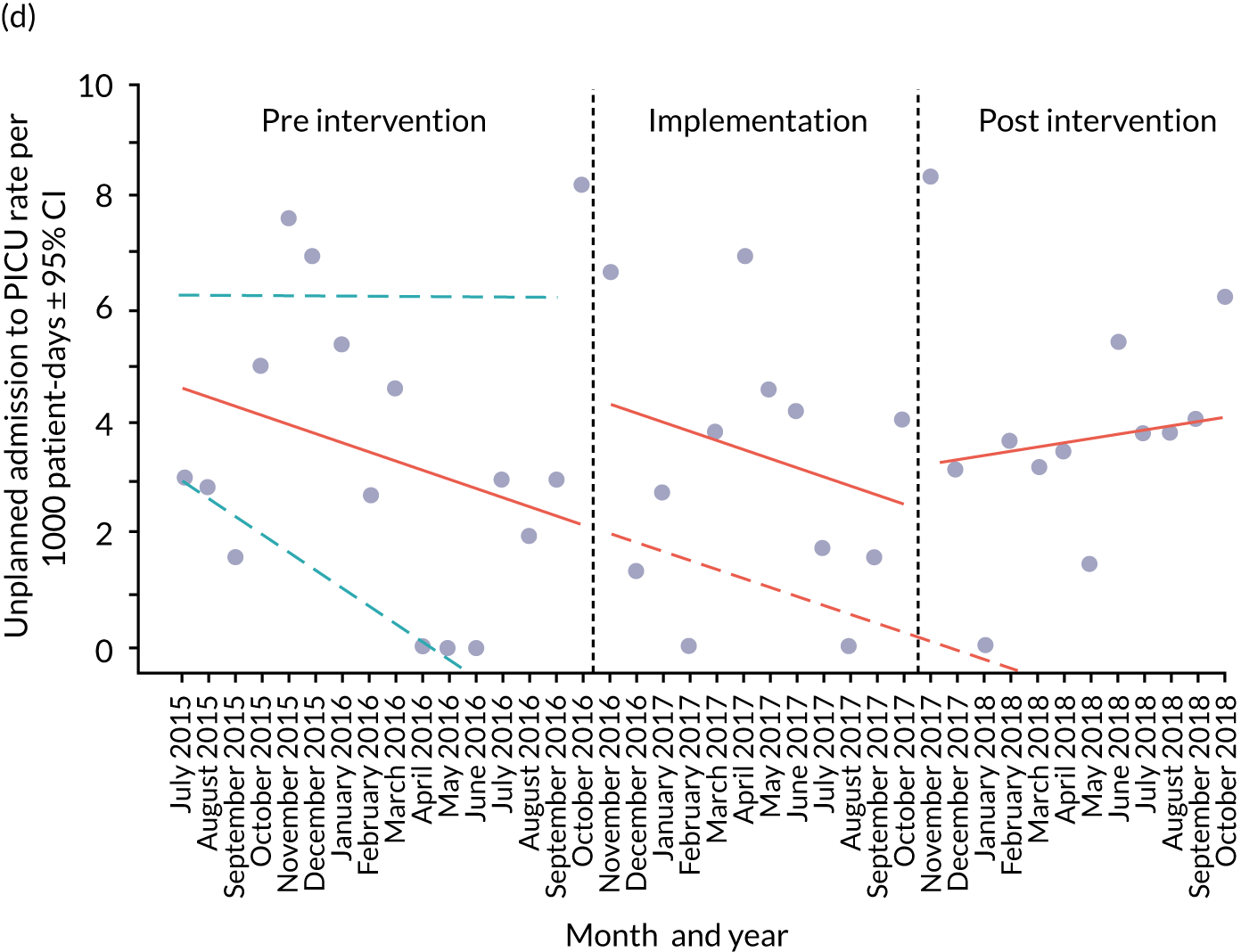
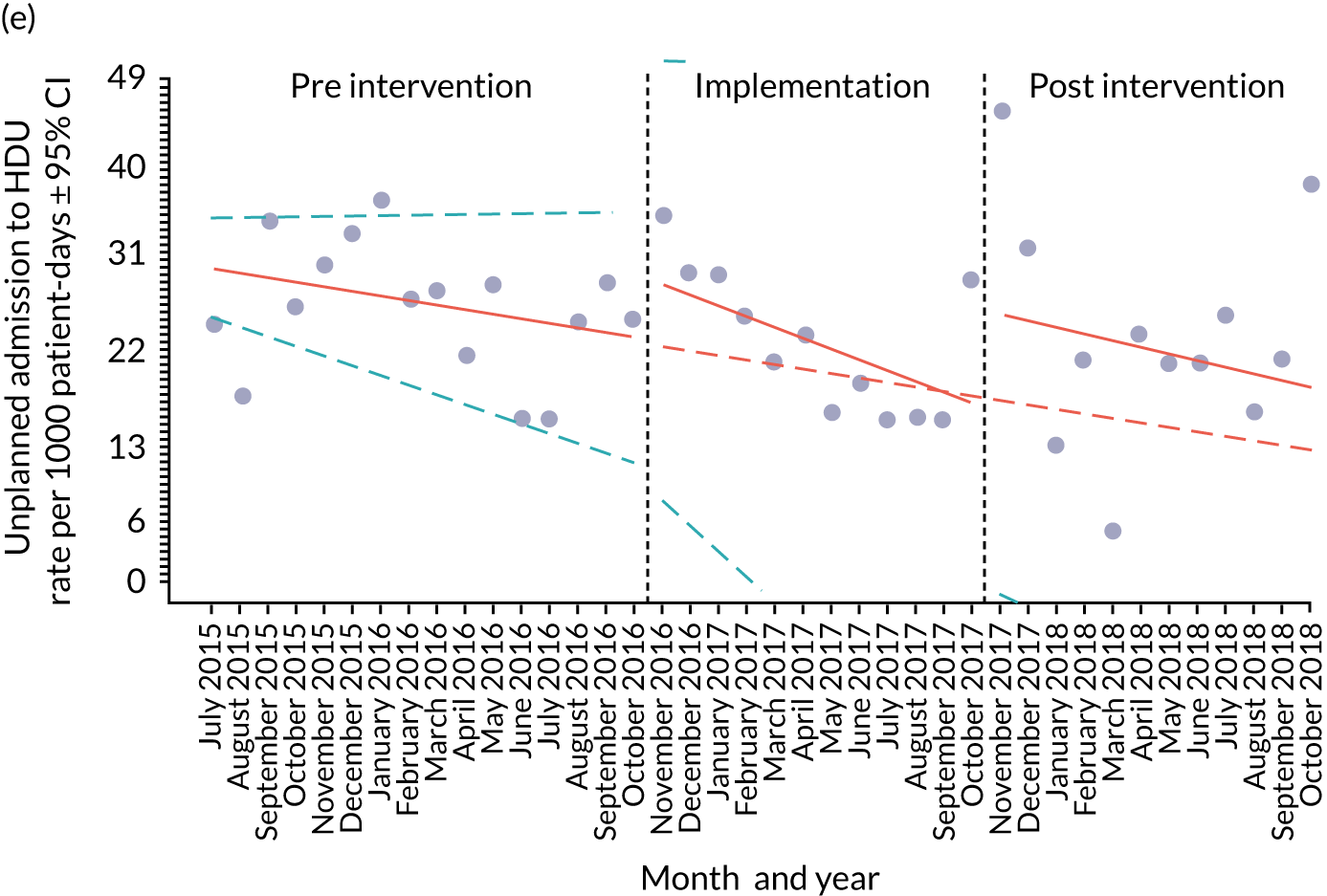
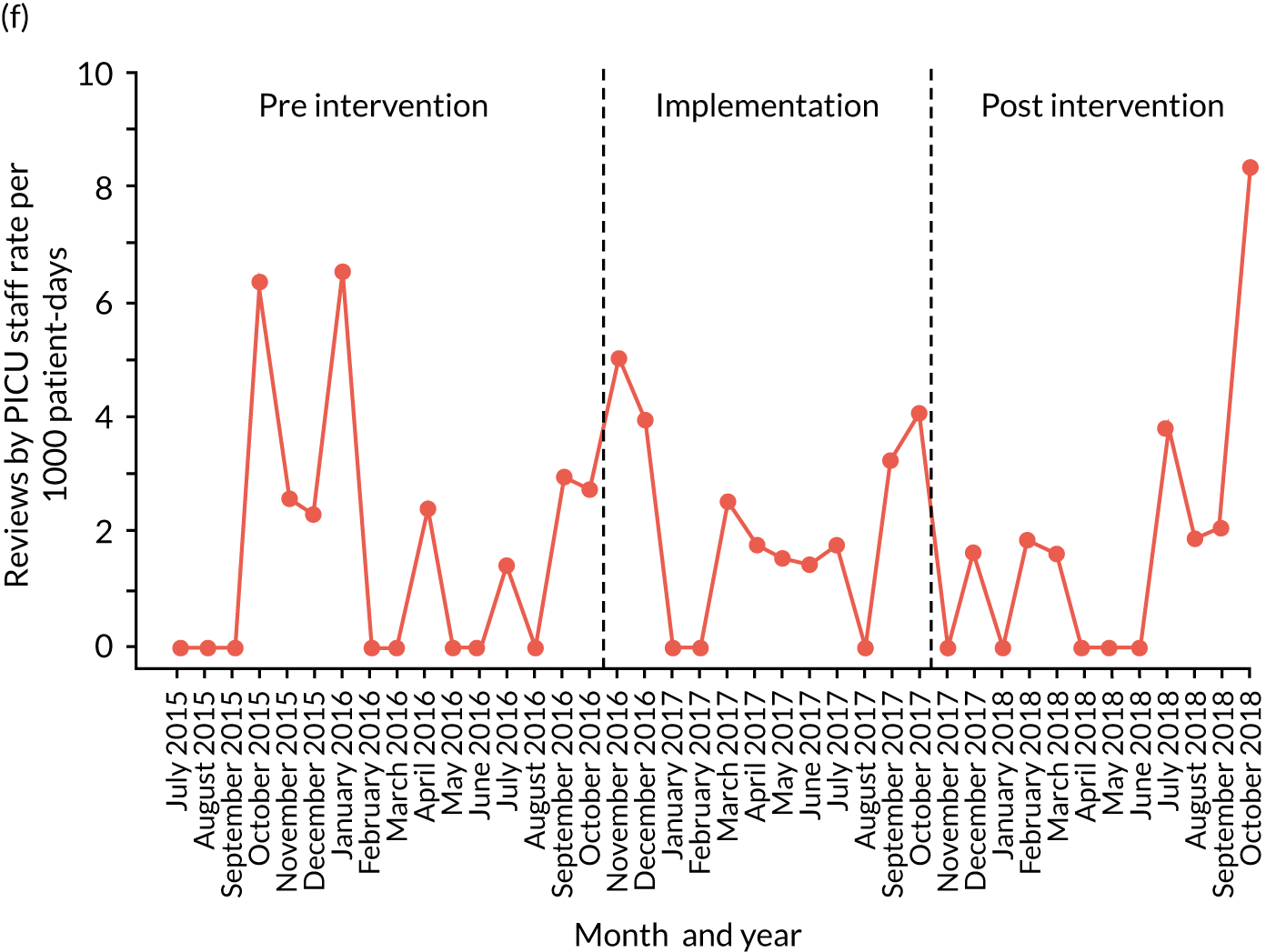
| Outcome | Estimate (95% CI) | p-value | Interpretation |
|---|---|---|---|
| Unplanned HDU transfers | |||
| Intercept | 31.66 (27.14 to 36.18) | ||
| Pre-intervention trend | –0.47 (–0.96 to 0.02) | 0.07 | There was a non-significant trend towards reduction in unplanned HDU transfers, even before the implementation phase |
| Change in slope (implementation phase vs. pre-intervention phase) | –0.65 (–1.49 to 0.18) | 0.14 | There was a trend towards a further reduction in the transfer rate, but the very large CIs make this difficult to interpret |
| Immediate change in level (implementation period vs. pre-intervention period) | 6.77 (–1.63 to 15.17) | 0.12 | |
| Change in slope (post-intervention phase vs. implementation phase) | 0.43 (–0.59 to 1.44) | 0.41 | The direction of the trend changes to be more in line with pre-intervention trends, but the range of data makes it difficult to interpret if this was a real change |
| Immediate change in level (post-intervention period vs. implementation period) | 10.05 (–0.25 to 20.34) | 0.06 | |
| Unplanned PICU transfers | |||
| Intercept | 4.78 (3.33 to 6.23) | ||
| Pre-intervention trend | –0.16 (–0.32 to 0.00) | 0.06 | There is a suggestion that unplanned PICU transfers were slowly reducing, but this did not reach statistical significance |
| Change in slope (implementation phase vs. pre-intervention phase) | 0.00 (–0.29 to 0.28) | 0.97 | No conclusions can be drawn. although it is unlikely that there was a major positive or negative shift during this period |
| Immediate change in level (implementation period vs. pre-intervention period) | 2.33 (–0.49 to 5.14) | 0.11 | |
| Change in slope (post-intervention phase vs. implementation phase) | 0.24 (–0.11 to 0.59) | 0.19 | The unplanned transfer rate appears to increase, but with wide CIs |
| Immediate change in level (post-intervention period vs. implementation period) | 0.66 (–2.55 to 3.88) | 0.19 | |
Mortality
The overall all-cause mortality rate was 0.09 per 1000 patient bed-days in the pre-intervention period, 0.00 in the implementation phase and 0.00 in the post-intervention phase. Owing to a low rate of occurrence, it was not possible to model mortality trends over the different time periods (see Figure 24a).
Arrests
The overall cardiac arrest rate was 0.09 per 1000 patient bed-days in the pre-intervention period, 0.00 in the implementation period and 0.00 in the post-intervention period. The overall respiratory arrest rate was 0.09 per 1000 patient bed-days in the pre-intervention period, 0.00 in the implementation period and 0.00 in the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in cardiac or respiratory arrests over the different time periods (see Figures 24b and 24c).
Unplanned transfers
The overall rate of unplanned transfers from inpatient wards to PICU during the pre-intervention period was 3.66 per 1000 patient bed-days, compared with 3.12 for the implementation period and 3.84 for the post-intervention period. The equivalent rates for HDU transfers were 27.46 for the pre-intervention period, 23.92 for the implementation period and 24.38 for the post-intervention period.
In the pre-intervention period, there was a downwards trend in both HDU transfer rates (β = –0.47, 95% CI –0.96 to 0.02; p = 0.07) and PICU transfer rates (β = –0.16, 95% CI –0.32 to 0.00; p = 0.06) over time (see Figures 24d and 24e). The downwards slope of the HDU transfer rate was even steeper during the implementation period (β = –0.65, 95% CI –1.49 to 0.18; p = 0.14), before levelling off in relative terms (but continuing to slope downwards) during the post-intervention stage (β = –0.43, 95% CI –0.59 to 1.44; p = 0.41). The PICU transfer rate trend was relatively unchanged in the implementation period (β = –0.00, 95% CI –0.29 to 0.28; p = 0.97), and then trended upwards during the post-intervention stage (β = –0.24, 95% CI –0.11 to 0.59; p = 0.19).
Paediatric intensive care unit reviews
The overall rate of PICU reviews during the pre-intervention period was 1.87 per 1000 patient bed-days, compared with 2.08 for the implementation period and 1.63 for the post-intervention period. Owing to a low rate of occurrence, it was not possible to model trends in PICU review rates over the different time periods (see Figure 24f).
Synthesis
In this final section, we consider how some of the clearer quantitative findings relate to qualitative observations. Assessing the impact of the intervention on quantitative outcomes was challenging, and so interpreting the quantitative outcomes in relation to the ethnographic observations should be treated with caution.
Although a smaller hospital, Morriston implemented a number of organisational-level system changes at an early stage in the ‘implementation period’; this coincided with a decreased slope in adverse event rates during this initial 12-month period. As with the other DGH, this reduction was largely driven by a decreasing trend in HDU transfers.
Chapter 9 Comparative analysis
In Chapters 5–8, we presented the cases’ study results; in this chapter, we present the cross-case comparative analysis.
Paediatric early warning systems in context
A central premise of the PUMA programme is that local context conditions the paediatric early warning system in a health-care organisation. Case study differences [e.g. patient populations, information and communication technology (ICT) and physical infrastructures, organisation of medical and nursing work] affected the operation of local paediatric early warning systems. Shifting contextual factors during the study were also consequential for the functioning of paediatric early warning systems.
Paediatric early warning system assessments
The pre-implementation system assessments revealed that each site had its own fingerprint (Figure 25); although this is not an objective measure as such, there are several points of interest when comparing the four sites.
FIGURE 25.
System assessment radar diagrams for all sites. (a) Site 1; (b) site 2; (c) site 3; and (d) site 4. A score of 0 was used to indicate poor alignment with the system standard; a score of 10 indicated optimal alignment with the standard.

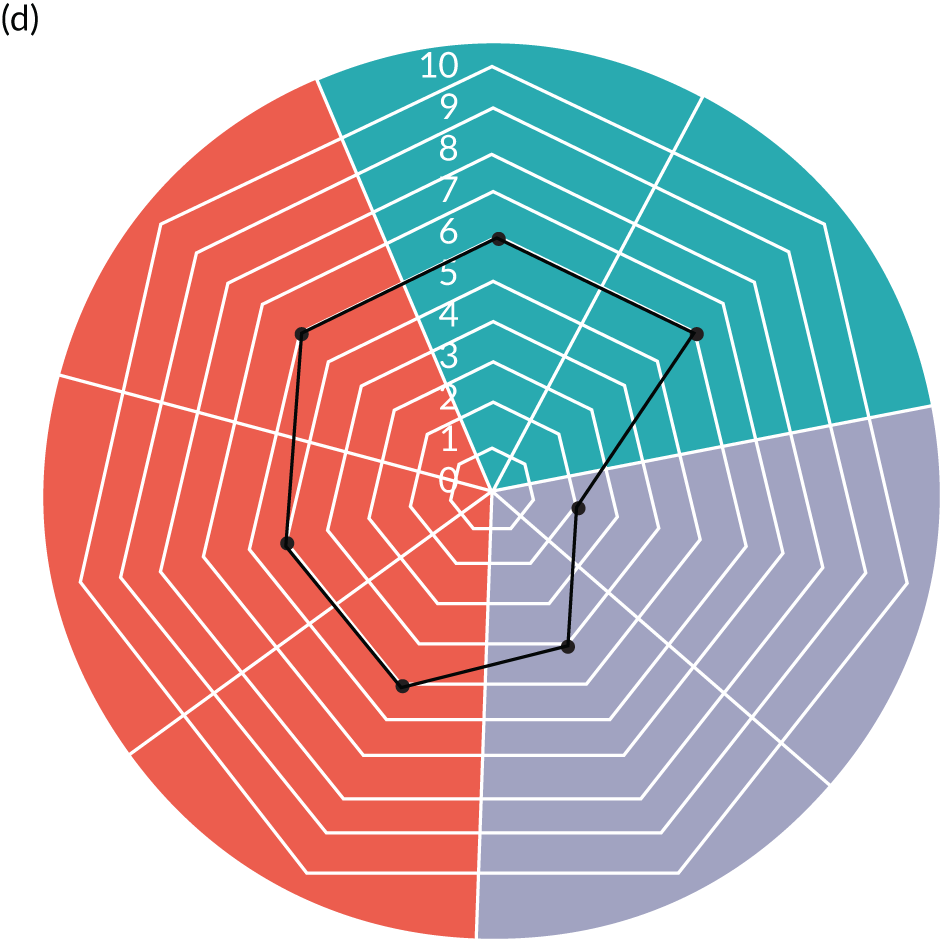
First, Morriston had weaker self-assessed performance across all PUMA Standard paediatric early warning system functions than the other three sites. Second, in all cases, both the plan function and parental involvement in the detect function scored lower than other areas of the system. All sites regarded parental involvement as central to their work, but none had formal processes in place for facilitating this. There were challenges in all sites in ensuring shared situation awareness between nursing and medical teams. Third, Noah’s Ark Hospital did not have PTTT, but the system assessment related to the relevant part of the wheel compares favourably with Arrowe Park and Alder Hey, where PTTTs were in use. Morriston did not have a PTTT, and the relevant self-assessment was lower than for the other three sites, but this is in the context of lower overall scores. Fourth, when we scrutinise the underlying reasons for the self-assessment score in relation to the act function, three of the sites (Alder Hey, Noah’s Ark Hospital and Morriston) identified specific challenges related to collective action across the nursing–medical boundary at nights and weekends.
Improvement initiatives
Three sites identified four or five improvement initiatives, with Morriston a clear outlier, selecting 15, perhaps reflecting the overall lower score.
Across the sites, many initiatives related to issues for which existing interventions were unavailable or inappropriate, and often involved multiple small interventions that adjusted existing processes at different places in the system (see Appendix 22, Table 49). For example, Morriston achieved some early changes to the ‘detect’ component of the paediatric early warning system through an equipment inventory and the production of cards with information on normal vital signs parameters, which could be carried in nurses’ ID holders.
In some cases, the team used the PUMA programme as a vehicle for implementing changes under consideration for some time, for example the new SOP selected at Alder Hey for on-call medical team handover at night and the weekend.
Some teams included initiatives in their action plan for which work had already started, for example the new observations chart and bespoke e-learning package adopted at Morriston. In some sites, changes were made to the paediatric early warning system, but not formally included in the action plans because planning preceded the PUMA programme, for example, the implementation of an electronic system in Arrowe Park.
Finally, in some cases, the system assessment highlighted areas for improvement not included in the action plan, but which were addressed during the implementation phase. For example, at Alder Hey, an enthusiastic ward manager in the cardiac ward introduced safety huddles for the nursing staff and a step-down checklist from HDU and PICU, addressing some weaknesses around planning.
Implementation
There were different implementation trajectories in each site, reflecting several factors.
First, it depended on the specific initiatives selected and whether these were relatively quick fixes or minor adjustments to existing processes, or whether they required more investment in development work, such as agreeing a new escalation policy.
Second, it reflected the scale of work undertaken to embed the interventions, which related to organisational size and complexity. With only one ward, implementation at Arrowe Park was relatively straightforward. For the larger sites, the process was more difficult and required more extensive engagement work and decisions about which initiatives should be implemented across the whole organisation, and which could be left to the local determination of wards.
Third, it reflected the capacity of the improvement teams. The single site PI in Morriston provided strong leadership for implementation, and delegated responsibility for leading on specific initiatives to identified individuals. But an unplanned absence from work led to a loss of momentum during the implementation phase, and highlights the potential risks of investing leadership exclusively in one person. In Noah’s Ark, staff turnover made sustaining an improvement team challenging, and most of the initiatives were progressed exclusively by the site PIs. Membership of the improvement team at Alder Hey also fluctuated, and, at this site, the energy of PIs was taken up by the requirement to oversee large-scale changes relating to the CQC inspection. In Arrowe Park, there was a clearly defined implementation/improvement team that took on responsibility for different initiatives, which meant that some of the initiatives were implemented fairly quickly.
Initiatives
All sites embedded changes to their paediatric early warning systems. Selected initiatives were often adjustments to current processes, rather than the introduction of new, or externally developed, interventions. Sites also selected different initiatives to address similar issues (e.g. improving staff awareness of children most at risk of deterioration was achieved through changes to handovers in one site, and through use of a whiteboard in another).
Although all sites successfully embedded system changes, some initiatives were more difficult to implement. Some initiatives were implemented but never embedded in practice, and some initiatives were never implemented. See Appendix 22, Table 49, for a summary. In several cases, initiatives required the negotiation of organisational constraints. For example, the development and implementation of a new observation chart (Morriston) and a new escalation policy (Noah’s Ark) necessitated the navigation of complex governance processes, which delayed progress. Aspirations to implement an electronic whiteboard at Noah’s Ark had to be abandoned, because of insufficiencies in the organisation’s ICT infrastructure. Efforts to restructure nursing and medical handovers at Morriston and Noah’s Ark were unable to proceed because of the challenges of adjusting medical and nursing shift patterns. The aspiration to implement supernumerary status for the nurse in charge at Morriston was not possible within the nursing establishment figures.
There were also examples of teams finding alternative ways of improving their systems when their first initiatives could not be implemented. When efforts to restructure nursing and medical handovers proved too difficult, the teams implemented other mechanisms for improving shared situational awareness, for example through the use of a whiteboard at Noah’s Ark, and by the nurse in charge routinely communicating children of concern to inform medical handover. Similarly, although implementation of the second safety huddle at Arrowe Park proved impractical, efforts to implement the initiative increased interactions with nurses and doctors based in the PAU.
Many of the cases included training initiatives in their action plans. Arrowe Park and Morriston both encountered challenges in releasing staff to attend, and yet a comprehensive training programme was implemented across the whole organisation at Alder Hey as a CQC-mandated requirement.
In all sites, action plans included initiatives intended to implement more systematic approaches to involving parents in detecting and acting on deterioration, but with limited success. An important barrier in this context was the impact that formalised approaches had on the professional identities of nurses.
Several initiatives intended to integrate nursing and medical practices had to be abandoned. It may be that the work practices and priorities of nursing and medical staff are too divergent to be integrated, and that alternative initiatives that create common ground are required. A number of our cases implemented relatively simple interventions that achieved this aim. For example, in Morriston, minor modifications to the board round ensured that nurses and medical staff had a shared understanding of children of concern.
Implementing all selected initiatives was simply not possible within the available time scales, either because of the need to implement across multiple wards (Noah’s Ark) or the desire to make multiple adjustments to the paediatric early warning system (Morriston) or because of other competing demands on the time and resources of the improvement team (Alder Hey, Noah’s Ark).
Post-intervention paediatric early warning systems
All sites successfully brought about changes aligned with the PUMA Standard, most notably in relation to the planning function. Indeed, an important change across all sites was an increased awareness of the importance of shared understanding, and an embedding of a new language of ‘watchers’ (Morriston, Alder Hey, Arrowe Park) or ‘sickest’ (Noah’s Ark) in all four organisations, sometimes as an explicit initiative and sometimes as a by-product of another intervention.
Addressing equipment shortages was also important in a number of sites, bringing about improvements in the detect function. Improvements in the availability of monitoring equipment was an important early improvement in Morriston, an increase in the number of portable computers reduced the delays between observing and recording activities in Alder Hey, and there were also improvements to monitoring equipment in Noah’s Ark.
All sites recognised the importance of involving parents in detecting and acting on deterioration, but had limited success in implementing changes to the system. Although staff prided themselves on engaging with parents in the care of their children, not all parental concern was accorded the same legitimacy, and more needs to be done to support staff in filtering concerns that are consequential for detection and escalation, and those for which interventions are required to address parent anxiety.
Both sites with a PTTT in place at the start of the study continued using these. Neither of the sites without a PTTT or score at the start of the study elected to introduce one as part of their improvement initiatives, although Morriston did implement a new observation chart with colour-coded vital signs thresholds. The new observation chart was not implemented in A&E, however, because key senior staff advocated PTTT and score use.
Several sites also experienced significant paediatric early warning system changes, not formally included in their action plans, but which affected overall system functioning.
Wider impact of PUMA
Hawe et al. 216 have suggested that interventions should be conceptualised as events within systems. There were a number of examples of the impact of the PUMA programme beyond the specific initiatives that were implemented within the lifetime of the study.
First, there was evidence that the intervention had encouraged both a systems orientation and a structure for thinking systematically about paediatric early warning system(s). At Noah’s Ark, the team planned to use the PUMA Standard as a framework for reviewing critical incidents, and the critical deterioration review, which was implemented but not embedded, at Alder Hey showed clear evidence of systems thinking. Furthermore, at the close of the study, in Morriston, work was under way to develop training programmes with the university deploying the PUMA Standard and Wheel.
Second, although the PUMA programme did not eliminate power relationships, several of the nurses claimed that the process had been empowering, and valued working in a systems-oriented multidisciplinary way. A number of senior nurses who had been associated with PUMA implemented initiatives outside the formal organisational action plan.
Chapter 10 The PUMA programme evaluation
Introduction
The PUMA programme was designed to support teams to develop contextually appropriate initiatives to optimise their paediatric early warning systems, drawing on local systems expertise. It was underpinned by the OUTCOME approach, which was developed as part of the study, and intended to address many of the shortcomings of orthodox approaches to quality improvement (see Box 4). In this chapter, we evaluate the PUMA programme, and reflect on the lessons arising from the process and their implications for the development and extension of the OUTCOME approach.
Process overview
The PUMA programme was developed in collaboration with site PIs, who, as members of the Study Management Group, were involved in the decision to change the research aims. The changes to the study focus were multifaceted. First, a shift away from PTTTs towards a whole-systems approach was required. Second, the implementation and evaluation of an improvement programme with initiatives tailored to local context, rather than a pre-determined PTTT, was required. Third, PIs were required to take on an improvement leadership role. The study’s change in focus was significant, and, despite the PIs close involvement in decision-making, they had ongoing concerns. First, there was evidence of disquiet that the study would not address the debate about PTTTs within the paediatric community. Second, some reported feeling ‘uncomfortable’ with the change in focus to an improvement-oriented model of research, a situation that, in part, reflected the fact that the OUTCOME framework (outlined in Chapter 4) was less well articulated at this stage in the study. Third, there was concern about the additional time commitment that the new study focus required. Working with and overcoming these concerns was ongoing over the lifetime of the study, producing insights that informed subsequent refinements to the PUMA programme.
Set-up
The aim of the set-up session was to formally introduce the PUMA programme. Attended by all site PIs and a clinician from Arrowe Park, it covered the background to the PUMA programme, the OUTCOME principles, the PUMA Standard and visual summary (PUMA Wheel), and instructions for administering the SSAT and FFT.
A key message was the changed focus from PTTTs to the whole system. The PUMA Wheel, which visualised the PUMA Standard, was received positively as a useful way of communicating the core components of a systems approach. PIs embraced this new focus to varying degrees. The PUMA team’s explanations of the evidence base, underlying theory and how this had informed the key elements of the PUMA programme were persuasive for some:
When we started the whole project, I thought it was all about the PEWS score [. . .] then what transpires . . . was that, actually, the PEWS is only one small part of it, and in fact . . . in itself may not be critical . . . that was quite a shock in a way, when that was suggested, that the PEWS wasn’t the most important thing, it was quite a shock to me, but, having thought about it and having sort of, um, you know, listened to the evidence and listened to the, you know, to the thoughts behind it, it did make sense.
PI
For others, the shift away from a focus on PTTTs to the whole system was more difficult, and the continued expectation that the study would involve implementation of a PTTT was evident several months after the set-up session.
There were also different views on the evidence base for the whole-systems approach, with medical PIs accustomed to orthodox hierarchies of evidence, rather than the evidence that informed the PUMA Standard:
It makes sense that you have a whole-systems approach . . . the evidence is there, but it’s a different type than we are used to dealing with.
PI
One PI expressed concern that the changed focus of the study no longer constituted ‘research’:
Felt that this wasn’t now a definitive randomized controlled trial, but a quality improvement programme; an exercise would be best run internally rather than as part of a wider study. [PI] said they needed to speak to [PUMA chief investigator] [. . .] Wanted to review the protocol.
PUMA team facilitation notes
The challenges of instigating change in complex and resource-pressured environments, and securing organisational support, were repeatedly raised as potential barriers to success:
You know, honestly there’s so many people employed in different hospitals whose job it is to be safety, quality, whatever, but the reality is that . . . that job is ticking boxes, providing reports. And what you need are people who are out helping make sure these things happen.
PI, fieldnotes
Following the set-up session, sites were charged with forming their improvement teams and undertaking an assessment of their local systems. They were provided with multiple copies of the SSAT and FFT and a set of instructions for their use, guidance on improvement team creation, a template to record improvement team membership and system assessment completion, and a slide set for use by PIs to introduce the PUMA programme in their organisations.
Formation of improvement teams
Principal investigators formed their improvement teams by first raising awareness of the need for improvement (e.g. by presenting at local meetings, or through informal discussions with colleagues), and subsequently enrolling those who were most engaged with the goal and who had sufficient capacity to take on an improvement team role. The PIs adopted the role of implementation lead, but also recruited staff with these skills/this background. For example, at Morriston, a medical team member had specific QI expertise, and, at Alder Hey, the team engaged with QI staff from the organisation during the early stages of implementation. PIs selected individuals who were trusted and well known to them, were considered to be improvement oriented, were capable of taking action and had sufficient workload flexibility to contribute:
I knew that they would be hands-on with regards to get people to, to change where perhaps I couldn’t get the change [. . .] . . . they are completer finishers. [. . .] you’ve got to know your team [. . .] And I think you’ve got to keep it as small enough team with the same sort of goals. [. . .] So you’ve got to identify that people think, do you know something?
PI
I think the key people have bee, um, the ward manager and the two ANPs, and I think we’ve been very lucky, very blessed to have two ANPs who are both very keen; they, they also have got quite a lot of general wisdom, and have some flexibility within their work.
PI
At Noah’s Ark, the PIs made progress without an improvement team and only sought to recruit members during the implementation phase. One consequence of this is that the wider team had not been involved in the formative stages of the improvement process when decisions were taken about the changes that were required:
I kept getting nominated different names of people that would help me [. . .] they didn’t really ever appear out of the woodwork. That was partially me not having the time to engage them [. . .] the few experiences I had of people coming to help me just made it more troublesome [. . .] Because you think ‘no that’s not what I wanted implemented’ and ‘no, that’s not how we’re doing it’.
PI
Only Alder Hey had a high level of organisational support for its initiatives, reflecting the CQC-mandated changes, rather than alignment with the PUMA programme.
System assessment
The SSAT and FFT were administered on a different number of wards in each site, ranging from one in Arrowe Park to five in Noah’s Ark Hospital. Questionnaires were completed by representatives of all staff groups and by any family member on the ward on a given day and returned anonymously. All teams found the task of administering the tools time-consuming and, at times, found it challenging to ensure that all staff groups were represented. The large workload in the clinical environments was frequently cited as a barrier:
There’s 101 other things going on . . . And then you dish it out . . . ‘Have you done that form? Have you done that form?’.
PI, field note
The extent to which the SSAT and the FFT were successfully administered in practice differed across both sites and individual wards. The number of questionnaires completed in each site ranged from 22 to 72 for staff, and from 7 to 78 for families. SSAT returns from mandatory staff group members were missing in some cases, and the quality of SSAT completion varied greatly.
Principal investigators were tasked with collating the results of the SSATs and FFTs and completing a ‘summary assessment’ in collaboration with their improvement teams, with the aim of ranking their system against the PUMA Standard. The original SSATs and FFTs were paper based and the work of collating the information was time-consuming. This task was particularly demanding for the two larger sites, which had to review returns from a greater number of staff, working across multiple wards. To facilitate the successful completion of the summary assessment, the PUMA study team provided support for the two larger sites. If the approach is to be extended, there is scope for information technology to both accelerate and automate this process.
Notwithstanding the demands of the process, all four teams considered the system assessment to have value. Discussing results and agreeing how to rank their system against the PUMA Standard was considered to be important. They also proposed that the system assessment made the process of improvement easier, as it allowed them to engage staff groups from an early stage, providing on-the-ground evidence of good practice and evidence of areas for improvement:
It wasn’t just [site leads] plucking out what did we want to take forward, this is what everybody on the team has said needs improving.
PI
The original version of the FFT generated little information of value, with high scores being achieved on all measures. Nevertheless, the SSAT allowed staff to identify areas of weakness in family engagement. This area of weakness was identified across all four sites. The FFT was subsequently revised and expanded (the new version was co-developed by the PUMA study team and the PPI group); an additional number of free-text questions were included, and the language used was clarified.
To evaluate the utility of the system assessment tools, the qualitative team, which was blinded to the site self-assessment results, carried out independent assessments, drawing on the pre-implementation qualitative data. There was considerable overlap in the results of the self-assessment and independent assessments, conferring confidence that the SSAT provides an accurate assessment of paediatric early warning systems in practice (Figure 26).
FIGURE 26.
System assessment comparison across sites, based on (a) observation data from qualitative case studies; and (b) site self-assessment using the SSAT.
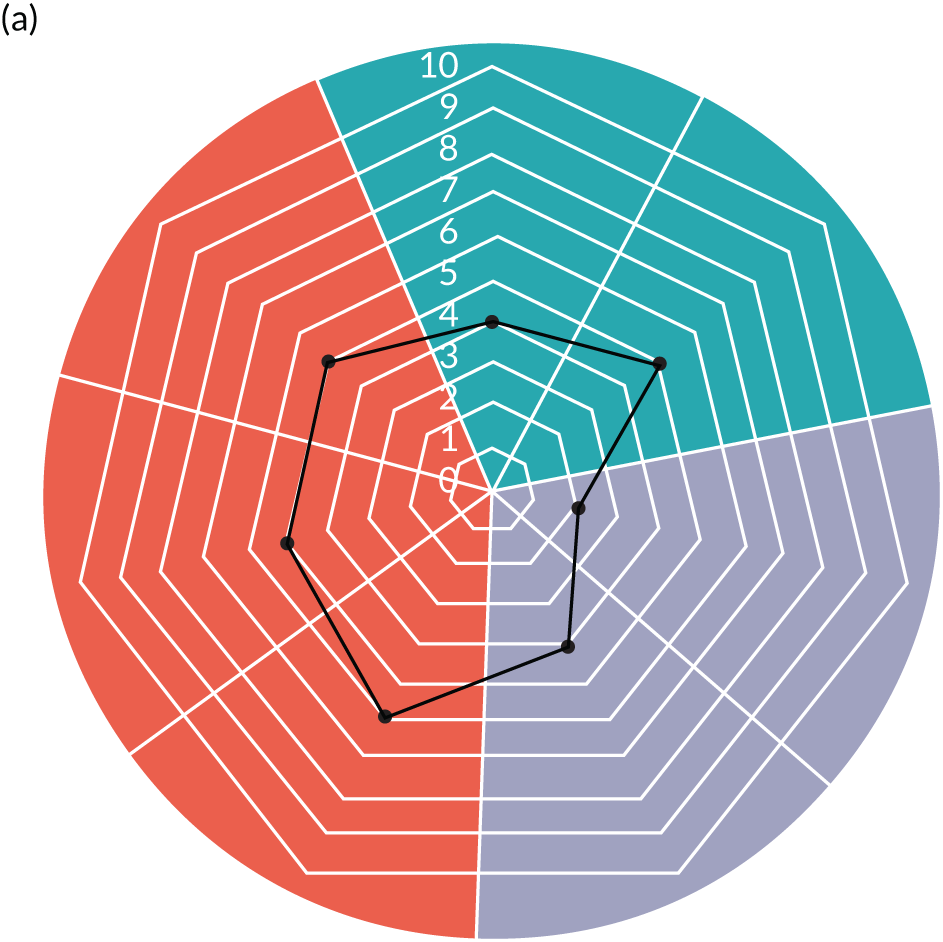
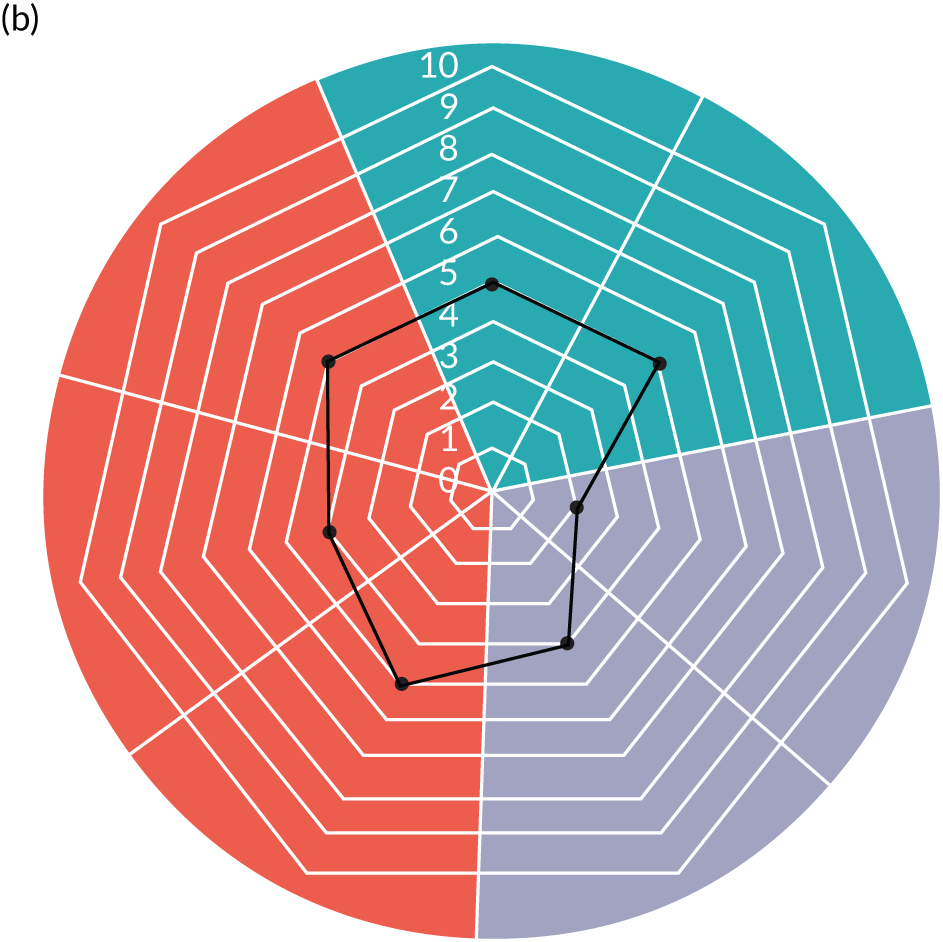
System assessment results
The final stages of the system assessment involved teams using their SSAT and FFT results to facilitate discussions around their system strengths and weaknesses, produce their radar diagram and prioritise areas for improvement. One of the purposes of the assessment process was to highlight different perspectives on the functioning of the paediatric early warning system, providing a basis for a shared understanding of the potential areas for improvement. For team members with a strong connection to front-line practice, the PUMA system assessment results rarely presented new or unexpected information and did not disrupt established perceptions of their site’s system. For these team members, the results of the system assessment were considered to be informative and valuable, producing a 360-degree understanding of their local system, and providing clear evidence of key strengths and weaknesses. The system assessment was also considered to be an effective method for producing such information, which compared favourably with other methods, such as audit:
I mean, we do do audits occasionally, but again, they’re time-consuming, aren’t they? [. . .] I think it just helped confirm some things that we already probably had an idea of, but just getting the evidence, and saying ‘actually yeah, we do need to look at this. Look, this is the area’ . . . And it also gave us a bit of ‘actually, some of what we’re doing is really good’. Which is nice feedback, isn’t it, as well?
Improvement team member
This did not mean that there was always agreement on the issues, but discussions around areas that lacked consensus were considered to be a valuable part of the process:
[W]e were looking particularly at the, you know, at the PEWS, um, you know, everybody had different views . . . I think we got a little bit confused when we’re talking about the PEWS, more of the problems are more with our computer system, rather than the actual PEW score, but [. . .] it’s good to hear from the mixed audience [. . .] that was beneficial as well, to get some other viewpoints on it.
Improvement team member
Although, mostly, the results provided evidence of known issues in the system, at times collating views of different staffing groups challenged the assumptions about how effectively the system was working.
The PUMA programme was designed to encourage local ownership and the system assessment processes appeared to be effective in achieving this aim. The importance of selecting initiatives that addressed issues relevant to front-line staff was repeatedly emphasised:
And, for me, the two big things have been it needs to be something developed on the ground [. . .] It needs to be on the ward. And it needs to be completely clear, transparent that this isn’t about payment or coding or finances or activity or any kind of bullshit that none of us care about. It needs to be about children.
PI
Principal investigators capitalised on the persuasive power of their system assessment to enrol front-line staff in improvement initiatives, and to explain the rationale that lay behind the approach:
I found it exceptionally beneficial . . . to use [system assessment results] as a whipping tool to say ‘this is what you have answered as to what is wrong in your institution. It’s not what we think is what’s wrong with your institution, you are telling us that this is what you think is wrong’.
PI
Action planning meetings
Improvement teams attended a whole-day workshop to begin planning their interventions. These were facilitated by the PUMA team using system assessment radar diagrams to guide discussions around areas for improvement and the development of specific initiatives.
Two sites attended the action planning session (North); attendance was good with a large number of clinical staff attending from both sites, alongside PIs and improvement team members. Attendance at the action planning session (South) was more limited: just two clinical staff were able to join the PIs from one site and another PI attended alone. PUMA study members guided the content of the discussion and helped to ensure that intervention rationale and measurement strategies were discussed. Although the action planning sessions were run separately, attendees at the action planning session (North) actively shared thoughts and perspectives, whereas there was less sharing of ideas and experiences at the action planning session (South).
After discussion of strengths and weaknesses, improvement teams considered possible initiatives to address the issues identified. Many of the identified solutions were for issues for which existing interventions were either unavailable or inappropriate, and often involved multiple small interventions that adjusted and harmonised existing processes at different places in the system.
Developing action plans
Following the action planning meetings, improvement teams developed their action plans, supported by a number of resources: ‘action planning guidance’, worksheets and the slides used during the action planning sessions.
The ‘action planning guidance’ provided comprehensive details on the five steps to improvement and contained worksheets to record priority concerns, improvement aims and intended timelines. The structure of the document reflected the OUTCOME approach. The focus was on the intended goals or functions to be achieved by the initiatives, rather than their form, and teams were asked to specify the rationale for their selected initiatives. Three sites identified broad areas for improvement and/or specific initiatives relating to areas of system weakness immediately following the workshop. A fourth site took longer, as the PI, who attended alone, wanted discussion with others in the service. Although all sites successfully worked through the process of identifying areas for improvement and the proposed initiatives, they found it tedious to document their thinking on the templates provided. Feedback from the PIs suggested that they found the document complex and difficult to use, especially within the time available. As an interim measure, the PUMA team completed some of the templates on behalf of the PIs. These materials were subsequently revised and simplified and incorporated into the implementation guide.
The PUMA programme is founded on a functions approach to improvement, intended to facilitate context-specific interventions. However, one of the main points of discussion in developing the action-planning guidance was whether or not to include examples of off-the-shelf interventions to support improvement teams. This reflected a concern that too little concrete information would prove daunting for teams more accustomed to top-down improvement initiatives. The systematic review identified multiple interventions that had been developed to strengthen paediatric early warning systems, but there was little evidence that one was more effective than another in all contexts. For example, there was evidence of multiple approaches to improving situational awareness of at-risk children, including whiteboards, huddles, structured and standardised handover, and supernumerary staff. As a compromise, the guidance provided described interventions that had been used in different settings (e.g. PTTTs, educational interventions, parental empowerment tools and communication tools). Only three tools were specified, but as examples of what others had done, rather than as recommended interventions: Recognising Signs of Paediatric hOspital iNpatients Deterioration (RESPOND) (an educational intervention),217 SHINE (a parent communication bundle)218 and the SBAR technique (a communication tool). 219 Detailed information about these interventions and how to implement them was not included. Although all teams successfully developed contextually appropriate improvements, early in the process, some expressed surprise at the lack of resources or references that were made available.
Although the exemplar tools (SHINE, the SBAR technique, RESPOND) were intended to function only as illustrations of what clinical teams had done to improve detection of deterioration elsewhere, two of the four PUMA sites included the SHINE tool in their final action plans. Their inclusion was not a result of an outcomes-oriented action planning process, and was not directed towards improving specific weaknesses in their local system, and attempts at implementation were not successful; improvement team members and clinical staff reported that there had been little ‘buy-in’ from on-the-ground clinical staff at both sites. In the final version of the implementation guide, we included different examples of contextually appropriate initiatives developed in the case study sites.
Measuring progress
The action planning guidance directed PIs to consider how they would meaningfully measure progress relating to each of their improvement initiatives. Teams were asked to identify specific process, outcome and balancing measures. This was achieved to a limited extent; some PIs engaged in auditing activities, asking staff to confirm via signature their review and understanding of a written policy, and observing clinical teams to gain an understanding of how often they highlighted at-risk patients during handover. For most of the initiatives implemented, however, sites struggled to identify achievable strategies for measuring progress, and once again asked for further guidance:
[W]hen [PUMA study team member] came out to talk to us and we’re saying ‘how is that measurable? How is that . . .?’ It does make you think more about the entire process and making sure that it is measurable, because it could be open to criticism if you don’t.
Discussion about how to assess certain initiatives [. . .] talked about huddles and the difference between a tick-box exercise in confirming that they were happening and evaluating how good they actually were – was keen for guidance/resources on how to achieve that.
PI, field notes
The fact that sites struggled to develop methods for measuring progress in implementation has parallels in the wider literature. 220
Facilitation
In addition to the set-up and action planning events, the PUMA team provided support to PIs throughout the implementation process. This was in recognition of the fact that the OUTCOME approach and PUMA programme resources were being refined and developed in parallel with the implementation process, through a process of reciprocal learning. These took the form of individual telephone- and/or e-mail-based support and site-specific face-to-face meetings. All PIs either attended or contributed to the face-to-face meetings, and two sites chose to use facilitated telephone calls, during which a PUMA study team member provided tailored support, reviewing and explaining the intended aims and improvement steps of the PUMA programme, and assisting with problem-solving in relation to specific initiatives. Those who most frequently took up opportunities to engage in PUMA-led facilitation valued regular contact with the study team, not simply because it helped advance their understanding of the programme, but because it helped to sustain momentum:
To a certain extent, kept me going at the times when I was just thinking ‘this is too much’. I didn’t realise the work I was going to have to put into it . . . [PUMA study team member] would say ‘yes that’s great’ or ‘that isn’t’ or ‘you should have done that’. They’d just listen, and say ‘well that’s been difficult because of this, this and this’, and that is invaluable.
PI
[T]he catch-ups were very beneficial . . . Because they kept you on path, they made both [PI] and I make the time in our diaries to sit there and go through what we had achieved [. . .] if you didn’t have that [. . .] whether you would put it to the back burner [. . .] without the steer.
PI
Sustainability
The aim of the PUMA programme was to create structures to support a learning system. Leading a system-wide improvement programme demanded a considerable commitment of time and resources, different skills and a changed perspective. Building and sustaining commitment in these circumstances required substantial effort. At the end of the study, there was evidence of continuing improvements in some sites, a recognition that the PUMA programme was a cyclical process, and proposals in some sites to use the PUMA Standard as a structure for systematically assessing critical events. Nevertheless, it was difficult to predict how far the PUMA programme had created a learning system so that improvements are sustained over time. Only two sites, Noah’s Ark and Alder Hey, completed a second system assessment and expressed interest in annually repeating the process.
Refinements to the PUMA programme
The PUMA programme was developed iteratively over the lifetime of the study, and the materials and resources were refined in response to feedback from the PIs and our experiences of the materials in use. This included a simplification of the PUMA Standard, PUMA Wheel, the SSAT and the FFT, and revisions to the resources and templates to support improvements. These were collated in an implementation guide (see Report Supplementary Material 3). Although ongoing facilitation was necessary in the context of the study because of the iterative nature of the programme’s development, PUMA is intended to be a parsimonious intervention, so that it might be adapted and replicated widely if proven successful. In the light of our experiences, we added a third formal facilitated workshop to the programme support, based on the assumption that ongoing facilitation is unlikely to be practical. These changes were evaluated in an additional three sites with no prior involvement with the study.
The three sites received the implementation guide and three facilitated sessions, including the additional session on implementation (Table 17). There was strong engagement with the process and the sites were quick to understand and adopt the systems approach. There was a similar gap between the set-up and action planning session (3 months for pilot sites; 4 months for original sites). When pilot sites returned for the new implementation session 4 months later, they had already made considerable progress with implementing initiatives identified during the action planning session.
| Improvement step | Facilitated workshop | Materials and resources sent to PIs |
|---|---|---|
| 1. Form an improvement team | Set-up session | Implementation Guide, including:
|
| 2. Assess the system | Set-up session | |
| 3. Select and plan improvement initiatives | Action planning session | |
| 4. Implement and review initiatives | Action planning session | |
| Implementation session | ||
| 5. Sustain progress | Implementation session |
Two sites had introduced safety huddles, two had updated their observation and escalation policy, and one site had developed written parent information about raising concerns and added ‘parent concern’ to their PTTT. Further work was planned to improve communication between different staff groups, and to develop a staff training programme on paediatric early warning systems. The pace of implementation appeared to be much quicker than in the original four sites, although this may, in part, be because of their size (all DGHs).
The impact of these changes was not formally evaluated in each site because of study resource limitations. However, a questionnaire evaluating the PUMA programme was completed by PIs in all three sites, and all stated that they would repeat the process of assessing the system, identifying areas for improvement, and selecting and implementing solutions, to attend to contextual changes occurring in practice and assess the impact of improvement processes:
We will have new junior doctors – so will be very useful to repeat the exercise and compare the two results.
Questionnaire – site 5
There have been operational and strategic changes to the trust and ward which necessitates a repeated system assessment.
Questionnaire – site 6
Going to use the PUMA system assessment to see whether changes have improved scoring in area’s identified using system.
Questionnaire – site 7
Summary
The shift from PTTT to a whole-system approach, and the process of implementing and iteratively developing the PUMA programme, made the implementation process more challenging for the original four sites. Feedback from the teams led to revisions of resources and support provided, which were piloted in an additional three sites. These sites made significant progress in a shorter time scale, suggesting that the revised PUMA programme, including implementation guide and three facilitated sessions, could be rolled out without additional facilitation.
Chapter 11 Discussion and conclusions
The PUMA journey
The PUMA study was commissioned to implement and evaluate a PTTT based on best available research evidence. Three linked systematic reviews did not support the continued focus on PTTTs and the study aims were revised to focus on the development, implementation and evaluation of a novel paediatric early warning system improvement programme: the PUMA programme. This chapter summarises the key study findings; considers their implications for practice, policy and research; and sets out next steps.
Summary of findings
Aim 1: identify, through systematic literature review, evidence for the core components of effective paediatric track-and-trigger tools and paediatric early warning systems
Two quantitative reviews of the literature found little high-quality evidence on the validity and effectiveness of PTTTs at reducing mortality and critical events among hospitalised children. Nevertheless, qualitative evidence suggests that, as part of a wider paediatric early warning system, PTTTs have value: they take knowledge to the bedside, offering support for less experienced staff; they act as prompts to action and lead to more systematic approaches to monitoring and detection; they facilitate situational awareness; and they can support nursing–medical communication by providing a common language.
The literature in this field is heterogeneous and stronger on the sociomaterial barriers to successful paediatric early warning systems than on the effectiveness of individual core components. These barriers include lack of access to appropriate monitoring equipment, inadequate staffing levels, insufficient staff skills and knowledge, lack of situational awareness, poor communication across professional boundaries, uncertain escalation policies and unsupportive organisational cultures that deter escalation.
An emerging literature highlights the importance of planning and indicates that combinations of interventions (nurse co-ordinator, whiteboards, safety huddles) may facilitate situational awareness of at-risk children and escalation plans across the wider clinical team. Professional judgement is also important in detecting and acting on deterioration, and the evidence points to the importance of a wider organisational culture that facilitates its use, with or without a PTTT. Family involvement in detecting and acting on deterioration is a growing area of interest; innovative approaches are required that are sensitive to the cognitive and emotional resources this requires.
A number of interventions to improve paediatric early warning systems have been proposed and some have been evaluated, but there is limited evidence to recommend their wider use. This reflects both the weight and quality of the evidence, the extent to which paediatric systems are conditioned by the local clinical context, and the need to attend to the relationship between system components and interventions, which work in concert, not in isolation.
No studies were located that adopted a whole-systems approach to improving processes for detecting and acting on deterioration in hospitalised adults or children.
Aim 2: identify, through a systematic literature review, contextual factors consequential for paediatric track-and-trigger tool and early warning system effectiveness
The hermeneutic qualitative literature review highlighted a number of preconditions for PTTT use: the availability of appropriate and functioning equipment, adequate staffing and an appropriately skilled workforce. TTTs are also deployed differently depending on the experience of the user. For juniors and health-care support workers, they provide a methodology and structure for monitoring clinical instability and identifying deterioration, whereas more experienced staff reportedly use TTTs as technologies for augmenting professional judgement. This is important; TTTs may be of less value in detecting deterioration in patients with long-term conditions because of altered normal physiology or where subtle changes are difficult to detect.
System effectiveness also requires attention to the sociomaterial relationships in the local context, senior support and leadership, and continuous monitoring and improvement.
Although an empirical synthesis was not possible because of the heterogeneity of the literature, by deploying social theories we were able to draw logical inferences from the review, paying particular attention to the evidence on barriers to successful paediatric warning systems. We analysed the evidence using TMT to develop a propositional model specifying the core functions (monitor, record, interpret, review, prepare, escalate, and evaluate) and minimum sociomaterial requirements of a paediatric early warning system (the PUMA Standard). The PUMA Standard was expressed as conceptual requirements, rather than specific interventions, to allow the development of locally tailored approaches. Informed by clinical experts and parents, the PUMA Standard laid the foundations for the PUMA programme (see Figure 4).
Aim 3: develop and implement an evidence-based paediatric early warning system improvement programme (the PUMA programme)
The PUMA programme is based on OUTCOME, a novel approach to service improvement informed by TMT and NPT, which was developed as part of the study. OUTCOME draws on insights from IS and QI, and is designed to overcome the limitations of orthodox approaches to improvement by harnessing local expertise in seeking contextually appropriate initiatives to improve systems. Rather than specifying an intervention and asking the realist evaluation question of ‘what works, for whom, in what way, and in what circumstances?’, OUTCOME inverts this logic to ask ‘what is our desired outcome and how might this be achieved in a particular context?’. The PUMA programme comprised the PUMA Standard, the PUMA Wheel, the SSAT, the FFT, the implementation guide, workshops and structured facilitation.
The PUMA programme was implemented in four study sites and refined iteratively in response to user feedback and our experiences of the materials in use. The improvement teams positively evaluated the system assessment process as a mechanism for generating a shared understanding of system strengths and weakness. Site system assessment results concurred with independent system assessments undertaken by the qualitative researchers, affording confidence in the process. There was considerable variation in assessments of strengths and weaknesses between the sites, indicating that each system had its own fingerprint, which reflected the sociomaterial conditions of the local context. All sites identified the need to strengthen the planning process to ensure team situational awareness and to develop more formalised processes for involving parents in the care of their child.
Each site developed an action plan intended to bring about system changes in alignment with the PUMA Standard. Many of the initiatives identified by improvement teams were intended to address issues for which existing interventions were either unavailable or inappropriate, and often involved multiple small interventions that adjusted and harmonised existing processes. Sites also selected different initiatives to address similar issues (e.g. improving staff awareness of children most at risk of deterioration was achieved through handovers in one site and through an electronic site board in another). Table 18 presents a summary of initiatives.
| PUMA Standard system component | Proposition | Site 1 initiatives | Site 2 initiatives | Site 3 initiatives | Site 4 initiatives |
|---|---|---|---|---|---|
| Detect | Detection of deterioration depends on timely and appropriate monitoring, recording and interpretation of vital signs and relevant risk factors |
1. Developed a tool to encourage family engagement 2. Retraining on PEWS recognition and response to deterioration including NICE sepsis screening for front-line clinical staff |
1. Observation policy updated and disseminated 2. Posters and cards for staff used to signpost abnormal thresholds for vital signs 3. Observation charts updated to include normal age-related thresholds 4. Inventory of equipment conducted |
||
| Plan | Planning depends on reviewing indicators of deterioration for each patient, staff being aware at ward level of the status of individual patients and the availability of skills and resources, and preparing an appropriate response | 3. Implement SOP for out-of-hours working for on-call medical teams – prioritising sickest children (hospital-wide) |
|
1. Introduction of electronic site board 2. Senior nurses now telephone through to doctors’ handover if they have any concerns about a particular patient |
5. Plans to establish a staff training course on situational awareness were amended; situational awareness now included in statutory training days |
| Act | Action depends on clear escalation and response and evaluation processes | 3. Introduction of new escalation policy | 6. Escalation policy reviewed and disseminated |
Aim 4: evaluate the effectiveness of the PUMA programme by examining changes in clinical practice and core outcomes trends
All sites successfully embedded system changes aligned with the PUMA Standard. All sites brought about system changes so that there was a shared understanding of children at risk. Equipment shortages were also addressed in several sites. At Alder Hey, implementation of the PUMA programme coincided with a CQC report, which precipitated large-scale mandated organisational-level system changes, all of which addressed areas of weakness in the site system assessment, but were not formally implemented as part of the PUMA study.
Across all sites, some initiatives were implemented but never embedded in practice, and some initiatives were never implemented. Several initiatives intended to integrate nursing and medical practices to improve situational awareness were abandoned, and all sites endeavoured to implement more systematic approaches to involving parents in detecting and acting on deterioration, but with limited success.
All sites experienced wider system changes that affected their paediatric early warning systems; in some sites, new interventions had mixed effects across the system, conferring improvements in some areas and generating challenges in others.
Developing and implementing the PUMA programme was a significant change in focus in the PUMA study, and building and sustaining the commitment of site PIs required considerable effort. At the end of the study, it was difficult to predict how far the PUMA programme had created a learning system (principle 6 of OUTCOME) to sustain improvements over time.
Assessing the impact of the PUMA programme on quantitative outcomes was challenging because of the low event rates for hard clinical outcomes, particularly in the DGHs with smaller inpatient populations. Nevertheless, several of the clearer quantitative findings appeared to relate to qualitative observations. The multiple organisational-level system changes implemented at Alder Hey were associated with significant improvements in clinical outcomes. Morriston implemented several organisational-level system changes at an early stage in the study, which coincided with a decreased slope in adverse event rates. Arrowe Park introduced a safety huddle and electronic recording, which strengthened some aspects of the local system, and weakened others. Quantitatively, there was no obvious ‘interruption’ to the adverse event rate over time. Noah’s Ark introduced several initiatives in certain wards, but implemented no organisational-level changes. The ITS analysis gave a mixed picture.
Aim 5: identify the key ingredients of successful implementation and normalisation of the PUMA programme
Overall, the findings indicate a number of key ingredients for successful implementation and normalisation of the PUMA programme.
First, improvement teams need to understand the OUTCOME approach and how this differs from orthodox improvement processes in the UK NHS (see Table 7 and Box 4). The process evaluation and our experiences from the additional pilot sites indicate that improvement teams engaged with the OUTCOME approach and have a strong sense of ownership over the improvement process, but require support and encouragement to develop local approaches to system problems, rather than reaching for off-the-shelf solutions.
Second, planning for improvement needs to include a mechanism for identifying where in the organisation initiatives are to be implemented (ward, department, hospital), the implications of improvement plans for governance processes, and who is formally responsible for leading improvement project initiatives.
Third, it is important that improvement leaders enrol others in identifying areas for improvement and agreeing shared goals. This is necessary to ensure that views and experiences from a broad range of staff are captured and that staff are engaged in the process of improvement.
Fourth, improvement teams need organisational support and resources for improvement. Although front-line staff and service managers are best placed to identify issues and implement solutions, in the PUMA study, improvement leads did not have dedicated time to undertake improvement work. Improvement activity should be factored in to the overall workload to ensure that those best placed to do the work are able to commit the time. Furthermore, as others have observed, there is a need to invest in improvement skills in health care.
Fifth, to generate learning systems, teams need to build the PUMA Standard into routine improvement processes.
Additional insights
In addition to findings relating to the research aims, the study generated important insights into the qualities of paediatric early warning systems. First, our in-depth ethnographic analysis of the study sites highlighted how wider changes in the organisation – workforce, key individuals (consultants, ward managers, senior nurses), fluctuating demand, infrastructure and technology – influenced the operation of paediatric early warning systems. Second, we have shown how interventions can have different impacts across the system and distributed costs and benefits. The insights indicate the importance of continuous improvement processes and regular assessments of system function, as well as processes to map the implications of planned change across the system and the staff involved.
Implications for policy and practice
From paediatric track-and-trigger tools to the formalisation of a whole-systems approach
In the paediatric community, there has been considerable interest and debate about the potential of PTTTs to improve processes for detecting and acting on deterioration among hospitalised children. Although there is little evidence for the effectiveness of any specific tool in reducing mortality or critical events, PTTTs do have value as mechanisms for co-ordinating action across clinical teams, but they depend on certain preconditions for their use. In addition, paper-based and electronic PTTTs function differently in the overall system. At the time of writing, there is a policy impetus for the implementation of national PTTTs; on the basis of these findings, there is no good reason to question such initiatives. It is increasingly clear, however, that PTTTs are not the sole solution to improving processes for detecting and acting on deterioration; they should be implemented as part of a wider systems approach. Indeed, over the life of the study, there has been a growing professional awareness of the need for a whole-systems approach to improving processes for detecting and acting on deterioration, but hitherto no formal framework has existed for improvement purposes. The PUMA Standard addresses this need.
Localisation and standardisation
A central tenet of the PUMA programme is that paediatric early warning systems are shaped by the local organisational context; the overall philosophy of OUTCOME is to enable context-appropriate approaches to improvement. These findings highlight how factors such as technology, architecture, shift patterns and the social organisation of nursing and medical work all affect the functioning of the system and condition the options for intervening to bring about improvement. We have shown how the PUMA programme facilitated locally tailored approaches oriented to a common standard across the varied service contexts in four study and three pilot sites; therefore, it has value as a framework for continuous improvement across diverse national and international contexts. Implemented at scale, there is also the potential for shared learning across health-care systems, whereby organisations with similar contextual conditions share successful approaches to the same problem.
Although the overall philosophy of the PUMA programme highlights the importance of local context-appropriate approaches to improving paediatric early warning systems, the study findings point to a number of areas where common standards may have value.
First, clinical expertise is an essential component of any paediatric early warning system, and staff turnover has potentially disruptive effects. Professional development is thus a critical component of all systems. Two sites struggled to embed educational programmes, yet, where such programmes were a mandated requirement, they were successfully implemented. There are compelling examples elsewhere, whereby mandated multidisciplinary training has brought about improvements in practice,221,222 an approach that merits further consideration in the context of paediatric early warning systems.
Second, lack of access to appropriate monitoring equipment affects the system negatively. A process to ensure that the correct equipment is available and functioning is a prerequisite of any paediatric early warning system, irrespective of the singular features of local context.
Third, all sites recognised the importance of involving parents in detecting and acting on deterioration, but had limited success in implementing changes to the system. Parental involvement in the detection of deterioration is difficult to address outside wider strategies to facilitate parental involvement in their child’s care.
Learning systems
The PUMA programme provides structures to support a learning system and our insights on the dynamic qualities of paediatric early warning systems indicate that regular assessment of system functioning has value, in order to intervene to ensure alignment with the PUMA Standard. At the end of the study, some sites proposed to use the PUMA Standard as a structure for systematically assessing critical events. Beyond critical incident analysis, however, the PUMA Standard and assessment tools offer resources for systematically appraising paediatric early warning system functioning as part of a continuous improvement culture. In other areas of health-care practice, checklists have value in ensuring that all the elements necessary to an activity are lined up in the right place and at the right time. However, even when their use is mandated, checklists all too often lose their effectiveness as staff bypass processes that they feel are redundant. PUMA offers a self-evaluation approach that engages staff in understanding local challenges that they need to address and offers structures to support systematic and rigorous local improvement efforts in relation to a service standard. Embedded in PUMA is the understanding that the context of care is continuously changing; therefore, it encourages a framework for deterioration review and the ability to set local stands for audit.
Detecting and acting on deterioration beyond paediatric hospital wards
The PUMA Standard is functions oriented, so it has applicability beyond paediatric inpatient wards. Work is already under way to implement the PUMA programme in Qatar, where it has been deployed to bring about improvements in a paediatric emergency department, which has now been open for 2 years, with 100,000 patients presenting per year. There is also scope for extending the approach to address global health challenges in developing health-care systems, where rescue trajectories must be implemented in conditions of significant sociomaterial constraint. Beyond paediatrics, TTTs have been deployed widely in adult care contexts to identify signs of deterioration. Although there is stronger quantitative evidence for TTT use in terms of clinical outcomes, here too more work is needed to understand the core components of early warning systems and the mechanisms of action of a TTT within an overall system. 223 Because it is underpinned by a functions-based approach, with minor adjustments, the PUMA programme has applicability for use across other patient populations beyond paediatrics.
Wider application of OUTCOME to health-care improvement
In the face of disappointing results in more than a decade of activity to bring about improvements in health-care quality and safety, there is a growing recognition of the need to move away from top-down solutions informed by a cause-and-effect logic, and to embrace more locally tailored approaches founded on an understanding of health care as a complex adaptive system. 201 In the context of this paradigm shift, the OUTCOME approach could be extended to other areas of health-care organisation and delivery, where there is a desire to adopt new approaches to service improvement.
In PUMA, the PUMA Standard and associated assessment tools were central to the improvement programme and a distinctive feature of the OUTCOME approach. The development of the PUMA Standard, through three systematic reviews and consultation with clinicians and parents, required significant expertise and resources. Alternative less resource-intensive approaches might include the use of existing clinical guidelines or service-level specifications. Standards could also be agreed through conducting a Delphi study or through professional consensus. In the longer term, a logical corollary of an OUTCOME approach is the generation of outcomes-focused systems standards through the use of new methods for systematic reviews and guideline development.
OUTCOME also provides a mechanism for PPI in QI processes. In the PUMA study, there was PPI in the initial stages of the project in agreeing the goals to be achieved (principle 1) and local system assessment (principle 4), but, going forward, there is scope for this to be extended to include defining local initiatives (principle 5) and learning systems (principle 6).
Implications for research
Quantitative outcomes for evaluating deterioration in paediatric contexts
Determining the impact and effectiveness of the PUMA programme using quantitative measures of inpatient deterioration was challenging. The original commissioning brief related to interventions to reduce mortality, and so our primary outcome (‘adverse events’) was a composite measure that included this measure and other related clinical metrics such as respiratory arrests and unplanned intensive care presentations.
The decision to use a composite metric for the primary outcome mirrors what has been done in many other single-site effectiveness studies of paediatric early warning system interventions. 199 As with other studies, it was largely a pragmatic decision, reflecting the low event rates of individual clinical outcomes such as mortality and arrests in hospitalised children. Even using this composite outcome, incorporating unplanned HDU and PICU transfers, we observed several zero months in the smallest DGH. Low event rates for key outcome metrics in DGHs point to the difficulty in assessing changes over time in smaller hospitals, and is a key reason that the literature on paediatric early warning systems is currently dominated by studies conducted in large specialist centres.
Mortality is significantly lower in children than in adult inpatient settings;1,224 there is an ongoing decline in child mortality over time,2 and even inpatient deterioration is a relatively infrequent occurrence in the context of large numbers of children moving through a hospital in a short space of time. 3,4 Our review of the literature also indicated huge variations in the definition of outcome measures, which makes synthesis and comparison difficult (see Chapter 3). Analytic approaches to rare event modelling, such as Bayesian belief networks, could be adapted from other fields to support the focus on preventing these events; however, a clear assessment of potential is required. Much of the safety literature on rare events requires clear causal pathways to be identifiable and measurable; the complexity of child deterioration and death may not be amenable to such approaches.
Including HDU and PICU transfers as markers of inpatient deterioration is common in the literature, but not without its problems. As we were able to demonstrate in the qualitative work, use varies in response to other system pressures or changes in clinical practices of senior staff.
The findings lend weight to debates about the appropriateness of downstream individual-level outcome measures in this field, and point to the need to reach agreement on upstream indicators of paediatric early warning system performance that are aligned with the PUMA Standard, for example monitoring compliance, situational awareness, parental involvement, staff knowledge and skills, or organisational culture in relation to escalation. The PUMA Standard offers a valuable framework for progressing this thinking, through consensus methods, such as a Delphi study.
Relational co-ordination in paediatric early warning systems
All of the study sites identified the need to address communication between nursing and medical teams to bring about improvements in situational awareness. Sites identified a mixture of structural and relational initiatives to improve interprofessional co-ordination. All sites experienced challenges in implementing structural approaches to co-ordination by closer alignment of nursing and medical organisational arrangements; in two sites, efforts to augment relational co-ordination through the introduction of supernumerary status of the nurse in charge were not successful. The findings suggest that senior nurses have a central role in ensuring situational awareness in paediatric early warning systems, but this is constrained by the requirement to carry a clinical case load. Research in other areas of health care has shown the importance of nurses in co-ordinating care across the interdisciplinary team. 214 Given the challenges of structural approaches to co-ordination, new research is necessary to explore the costs and consequences of models of nursing that facilitate implementation of a supernumerary nurse co-ordinator role.
Extension of the approach
There is potential for further research to examine the extension of the approach to other paediatric contexts and other areas of health-care practice. Of particular interest is whether or not the PUMA programme has value in improving paediatric early warning systems in developing health-care systems, where the sociomaterial contexts for practice differ widely from those of the UK NHS, and how far the PUMA Standard may have applicability in the adult care context. In addition, there would be value in further research that deploys the OUTCOME framework to address other areas of health-care practice, beyond paediatric early warning systems, where system complexity continues to confound service quality and patient safety, for example the organisation of hospital discharges or the management of complex continuing care arrangements in the community.
Evaluation of impacts of mandated and voluntary system improvement programmes
The PUMA programme was explicitly designed to harness local systems expertise to bring about contextually appropriate improvements. The approach was informed by insights from a critical body of work in improvement science, which has highlighted the limitations of the top-down, solutions-driven models typical of modern health-care systems. These findings highlight the challenges of locally led improvement in the absence of organisational sponsorship (in three of the study sites), as well as the potential impacts on clinical outcomes on goal-oriented mandated system-level change, as illustrated by the example of Alder Hey. Further research is needed to explore how approaches to improvement that are goal-oriented and locally owned could be strengthened through a mandatory framework. In particular, research to examine the probable impacts of mandating a system-wide improvement programme in the context of paediatric early warning systems merits serious consideration.
The value of translational mobilisation theory as a theoretical framework for complex systems research
Translational mobilisation theory is a new theory, which was used for the first time, to our knowledge, in this study to inform the hermeneutic systematic review, the development of the PUMA Standard and qualitative data generation processes. As Davidoff et al. 225 argue, there is an urgent need for the use of more formal theory in improvement research, not least because it facilitates learning, accumulative understanding and knowledge transfer. The PUMA study indicates that TMT offers a useful framework for understanding complex organisational systems that is grounded in the material and cognitive processes through which health-care activities are accomplished and the relational mechanisms that support or inhibit concerted action, and thus has value for future research.
Study strengths and limitations
We carried out three linked reviews of the literature, which provided strong evidence that PTTTs were not the sole solution to improving detecting and acting on deterioration among hospitalised children, and that a whole-systems approach was indicated. We analysed the review evidence using TMT to specify the core functions of paediatric early warning systems and the minimum sociomaterial requirements to enact these functions. TMT is a new theory, which has been applied for the first time, to our knowledge, in the PUMA study, where it provided the framework to apply a whole-systems approach to service improvement around a particular service goal (rather than a discrete intervention) and informed the development of the PUMA (system) Standard.
Building on the PUMA Standard, we developed a paediatric early warning system improvement programme, underpinned by an innovative approach (OUTCOME). Informed by NPT, TMT and the model for improvement, the OUTCOME approach was designed to overcome some of the weaknesses of orthodox approaches to improvement, building on insights from QI and IS to offer principles, structures and theories to support sustainable locally embedded improvements to achieve an agreed outcome. Despite increasing calls for closer integration of QI and IS for faster and more effective improvement,206–208 there are few examples of improvement initiatives that explicitly use the terminology and concepts of both IS and QI. Not only does the OUCTOME approach shift the focus away from top-down approaches to improvement efforts, it moves away from the implementation of single interventions, such as PTTTs, to consider how clinical systems, in all their social and material complexity, affect service outcomes. Therefore, the approach has promise in the context of an emerging paradigm shift in QI away from linear cause-and-effect approaches to models that acknowledge that health care is a complex adaptive system. The inclusion of longitudinal theoretically informed ethnographic case studies on the paediatric early warning systems in four sites allowed us to not only assess the impact of the PUMA programme, but to also highlight the dynamic qualities of paediatric early warning systems and the impact of external factors on system functioning.
District general hospitals represent the majority of hospitals where children are cared for, so understanding the systems in these institutions is critical to making impactful change. Studies in this field do not typically include DGHs; their inclusion in the PUMA study has yielded insights into the operation of paediatric early warning systems and improvement processes, as well as challenges in relation to event rates of quantitative clinical outcomes.
Implementation of the PUMA programme was not a one-shot event, which created challenges for the ITS, particularly in relation to the conceptualisation of the ‘implementation’ period and ‘post-intervention’ period. Had the hospitals implemented a ‘tool’, there would probably have been a shorter, well-defined ‘implementation’ period. In this study, we saw teams each take varying degrees of time to develop action plans, form teams, win organisational and staff buy-in and attempt to implement complex initiatives. Although we conceptualised the ‘implementation period’ as being 12 months for each site for the purpose of our quantitative analysis, it is important to reflect that, in reality, this probably varied between each site (and within sites, on an initiative-by-initiative basis) and is less well defined than in some intervention studies.
See Appendix 23 for a summary of next steps for the PUMA programme.
Conclusions
The PUMA study has shown that PTTTs are not the sole solution in improving processes for detecting and acting on deterioration in hospitalised children, and that a whole-systems focus is required. Drawing on the literature, we developed a system standard and implemented a novel whole-systems approach to improving paediatric early warning systems in four contrasting case study sites. All sites were successful in bringing about changes to their systems in line with the PUMA Standard. Paediatric early warning systems in all sites also changed over time in response to other external factors. Locally led service improvement is challenging without adequate resources, skills and organisational support, and alternative outcome measures are required to support research and QI efforts in this context. The findings from Alder Hey, the largest of the study sites, where system-level change was mandated in response to the CQC report, show that organisational-level whole-systems change can bring about positive impacts on clinical outcomes.
Over the lifetime of the study, there has been a growing consensus within the paediatric community about the need to think beyond PTTTs and to consider the whole system. Those who wish to improve health-care organisations need to clearly understand how they work, to think carefully about the nature of the interventions they are planning to implement and to find pathways to improvement that take the sociotechnical relationships into account. The PUMA programme offers a number of tools for clinicians and service managers wanting to improve their systems, and the underpinning OUTCOME approach has the potential to be used more widely.
Acknowledgements
We would like to thank and acknowledge the input from Debra Smith (Research Associate, October 2015–February 2017), who contributed to the systematic reviews, pre-implementation ethnographic data collection and preliminary data summaries.
We would also like to thank Marie-Jet Bekker, Fiona Lugg-Widger and Mala Mann for their contribution to the systematic reviews.
We would also like to thank James Bunn, Enitan Carrol and Brendon Mason for their clinical contribution to the study design, and Ian Sinha for his contribution as PI at Alder Hey Children’s Hospital.
Finally, we would like to thank Lena Meister, Rhys Thomas, Laura Tilly and Vicky Winters for their contribution as study administrators; Vincent Poile for the development of the study database; and Catherine Lisles for proofreading the report.
Independent members of the Study Steering Committee
We would like to thank the Trial Steering Committee members for their continued support: Professor Gordon Taylor (chairperson, University of Exeter), Professor Ann Greenhough (King’s College London), Dr Jennifer McGaughtey (Queen’s University Belfast), Dr Roger Paslow (University of Leeds), Dr Gale Pearson (Birmingham University Hospital) and Ms Jayne Wheway (NHS England).
Research networks
We also wish to thank the providers of nursing/clinical studies officer and administrative support in all sites. These include staff at the Health and Care Research Wales Workforce and the local NIHR Clinical Research Networks.
Contributions of authors
We have deployed alphabetical authorship from Dawn Edwards to Lyvonne Tume.
Davina Allen (https://orcid.org/0000-0002-6729-7502) (Professor of Health Care Organisation and Delivery, Sociologist and Nurse) was co-chief investigator, contributed to the study design, contributed to the quantitative review, led the qualitative systematic review and ethnographic case studies, led the theoretical framework, made a substantial contribution to intervention development, and led the writing of final report and final approval for submission.
Amy Lloyd (https://orcid.org/0000-0001-9181-4488) (Research Fellow, IS) was the study manager (qualitative, April 2015–October 2019), made a substantial contribution to intervention development, led implementation of the intervention, and made a substantial contribution to drafting and approval of the final report.
Dawn Edwards (https://orcid.org/0000-0002-3778-5468) (Consultant Paediatrician) was a co-investigator; contributed to study design, the quantitative systematic review and intervention development; was a PI (at Morriston); and commented critically on the final report.
Aimee Grant (https://orcid.org/0000-0001-7205-5869) (Research Associate, Qualitative methods, March 2016–September 2017) conducted pre-implementation and process evaluation ethnographic data collection, contributed to pre-implementation case study analysis and commented critically on the final report.
Kerenza Hood (https://orcid.org/0000-0002-5268-8631), (Professor of Trials and Director of Centre for Trials Research) was a co-investigator, contributed to the study design, led the quantitative systematic review and statistical analysis, contributed to intervention development and commented critically on the final report.
Chao Huang (https://orcid.org/0000-0002-3627-7218) (Senior Lecturer, Statistics) was a co-investigator; contributed to study design, quantitative systematic review and statistical analysis; and commented critically on the final report.
Jacqueline Hughes (https://orcid.org/0000-0002-9498-8376) (Research Associate, Qualitative methods, November 2017–October 2019) contributed to post-implementation ethnographic data collection, overall case study analysis (site 4) and drafting of final report.
Nina Jacob (https://orcid.org/0000-0002-3240-4179) (Research Associate, Qualitative methods, April 2015–October 2019) made a substantial contribution to the qualitative systematic review, contributed to intervention development, conducted pre- and post-implementation and process evaluation ethnographic data collection and overall case study analysis (sites 2 and 3), and contributed to the drafting of the final report.
David Lacy (https://orcid.org/0000-0002-3388-5728) (Consultant Paediatrician) was a co-investigator; contributed to study design, quantitative systematic review and intervention development; was a PI (at Arrowe Park); and commented critically on the final report.
Yvonne Moriarty (https://orcid.org/0000-0002-7608-4699) (Research Assistant, Qualitative methods, April 2015–April 2017) contributed to the qualitative systematic review, conducted pre-implementation and process evaluation ethnographic data collection and pre-implementation case study analysis and commented critically on the final report.
Alison Oliver (https://orcid.org/0000-0002-0893-2457) (Senior Paediatric Nurse) was a co-investigator; contributed to study design, quantitative systematic review and intervention development; was a PI (at Noah’s Ark); and commented critically on the final report.
Jennifer Preston (https://orcid.org/0000-0003-4800-234X) (PPI lead) was a co-investigator, contributed to the study design and led PPI activity.
Gerri Sefton (https://orcid.org/0000-0003-4159-6341) (ANP) was a co-investigator; contributed to study design, the qualitative and quantitative systematic reviews and intervention development; was a PI (at Alder Hey); and commented critically on the final report.
Richard Skone (https://orcid.org/0000-0001-7351-5273) (Consultant Paediatrician) was a co-investigator; contributed to the study design, the quantitative systematic review and intervention development; was a PI (at Noah’s Ark); and commented critically on the final report.
Heather Strange (https://orcid.org/0000-0002-5758-8445) (Research Associate, qualitative methods, June 2016–October 2019) conducted post-implementation and process evaluation ethnographic data collection and overall case study analysis (site 1), led the programme process evaluation and contributed to the drafting of the final report.
Khadijeh Taiyari (https://orcid.org/0000-0003-1220-0598) (Research Associate, statistics, July 2018–October 2019) conducted the statistical analysis and contributed to the drafting of the final report.
Emma Thomas-Jones (https://orcid.org/0000-0001-7716-2786) (Senior Research Fellow) was a co-investigator; was study manager (quantitative); and contributed to the study design, the quantitative systematic review, intervention development, and drafting and approval of the final report.
Robert Trubey (https://orcid.org/0000-0002-9550-1785) (Research Associate, quantitative methods, June 2015–October 2019) made a substantial contribution to the quantitative systematic reviews, contributed to intervention development, managed the quantitative data collection, contributed to implementation of the intervention and process evaluation data generation and contributed to the drafting of the final report.
Lyvonne Tume (https://orcid.org/0000-0002-2547-8209) (Principal Lecturer, Nursing) was a co-investigator; contributed to study design, intervention development and the quantitative and qualitative systematic reviews; and commented critically on the final report.
Colin Powell (https://orcid.org/0000-0001-8181-875X) (Consultant Paediatrician) was co-chief investigator (November 2014–September 2017) and contributed to the study design, both systematic reviews, intervention development and drafting and approval of the final report.
Damian Roland (https://orcid.org/0000-0001-9334-5144) (Consultant Paediatrician) was a co-investigator and co-chief investigator (October 2017–October 2019), contributed to the study design and both systematic reviews, made a substantial contribution to intervention development and implementation of the intervention and contributed to drafting and approval of the final report.
Publications
Thomas-Jones E, Lloyd A, Roland D, Sefton G, Tume L, Hood K, et al. A prospective, mixed-methods, before and after study to identify the evidence base for the core components of an effective Paediatric Early Warning System and the development of an implementation package containing those core recommendations for use in the UK: paediatric early warning system – utilisation and mortality avoidance– the PUMA study protocol. BMC Pediatr 2018;18:244.
Jacob N, Moriarty Y, Lloyd A, Mann M, Tume LN, Sefton G, et al. Optimising paediatric afferent component early warning systems: a hermeneutic systematic literature review and model development. BMJ Open 2019;9:e028796.
Trubey R, Huang C, Lugg-Widger FV, Hood K, Allen D, Edwards D, et al. Validity and effectiveness of paediatric early warning systems and track and trigger tools for identifying and reducing clinical deterioration in hospitalised children: a systematic review. BMJ Open 2019;9:e022105.
Allen D, Lloyd A, Edwards D, Hood K, Huang C, Hughes J, et al. Development, implementation and evaluation of an evidence-based paediatric early warning system improvement programme: the PUMA mixed methods study. BMC Health Serv Res 2022;22:9.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. However, some of the data are qualitative; therefore, the data generated are not suitable for sharing beyond those contained in the report.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HSDR programme or the Department of Health and Social Care.
References
- Wolfe I, Cass H, Thompson MJ, Craft A, Peile E, Wiegersma PA, et al. Improving child health services in the UK: insights from Europe and their implications for the NHS reforms. BMJ 2011;342. https://doi.org/10.1136/bmj.d1277.
- Pearson GA. Why Children Die: A Pilot Study 2006. London: Confidential Enquiry into Maternal and Child Health; 2008.
- Gephart SM, McGrath JM, Effken JA. Failure to rescue in neonatal care. J Perinat Neonatal Nurs 2011;25:275-82. https://doi.org/10.1097/JPN.0b013e318227cc03.
- NHS . NHS Atlas of Variation in Healthcare for Children and Young People: Reducing Unwarranted Variation to Increase Value and Improve Quality 2012. www.rightcare.nhs.uk (accessed 23 February 2014).
- PICANet . Paediatric Intensive Care Audit Network 2014. www.picanet.org.uk (accessed June 2018).
- Marmot M. Fair Society, Healthy Lives: The Marmot Review. Strategic Review of Health Inequalities in England Post-2010. London: Department for International Development; 2010.
- Balluffi A, Kassam-Adams N, Kazak A, Tucker M, Dominguez T, Helfaer M. Traumatic stress in parents of children admitted to the pediatric intensive care unit. Pediatr Crit Care Med 2004;5:547-53. https://doi.org/10.1097/01.PCC.0000137354.19807.44.
- Duncan HP, Frew E. Short-term health system costs of paediatric in-hospital acute life-threatening events including cardiac arrest. Resuscitation 2009;80:529-34. https://doi.org/10.1016/j.resuscitation.2009.02.018.
- House of Commons Health Committee . Complaints and Litigation. 6th Report of Session 2010–2012 2011.
- Wolfe I, Thompson M, Gill P, Tamburlini G, Blair M, van den Bruel A, et al. Health services for children in western Europe. Lancet 2013;381:1224-34. https://doi.org/10.1016/S0140-6736(12)62085-6.
- Smith ME, Chiovaro JC, O’Neil M, Kansagara D, Quiñones AR, Freeman M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc 2014;11:1454-65. https://doi.org/10.1513/AnnalsATS.201403-102OC.
- Roland D. Paediatric early warning scores: holy grail and Achilles’ heel. Arch Dis Child Educ Pract Ed 2012;97:208-15. https://doi.org/10.1136/archdischild-2011-300976.
- Jacob N, Moriarty Y, Lloyd A, Mann M, Tume LN, Sefton G, et al. Optimising paediatric afferent component early warning systems: a hermeneutic systematic literature review and model development. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028796.
- Kause J, Smith G, Prytherch D, Parr M, Flabouris A, Hillman K. Intensive Care Society (UK) . A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom – the ACADEMIA study. Resuscitation 2004;62:275-82. https://doi.org/10.1016/j.resuscitation.2004.05.016.
- Goldhill DR, McNarry AF. Physiological abnormalities in early warning scores are related to mortality in adult inpatients. Br J Anaesth 2004;92:882-4. https://doi.org/10.1093/bja/aeh113.
- Royal College of Physicians . National Early Warning System (NEWS) Standardising the Assessment of Acute Illness Severity in the NHS 2012.
- Edwards ED, Powell CV, Mason BW, Oliver A. Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system. Arch Dis Child 2009;94:602-6. https://doi.org/10.1136/adc.2008.142026.
- Duncan H, Hutchison J, Parshuram CS. The pediatric early warning system score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care 2006;21:271-8. https://doi.org/10.1016/j.jcrc.2006.06.007.
- Parshuram CS, Hutchison J, Middaugh K. Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care 2009;13. https://doi.org/10.1186/cc7998.
- Chapman SM, Wray J, Oulton K, Peters MJ. Systematic review of paediatric track and trigger systems for hospitalised children. Resuscitation 2016;109:87-109. https://doi.org/10.1016/j.resuscitation.2016.07.230.
- Lambert V, Matthews A, MacDonell R, Fitzsimons J. Paediatric early warning systems for detecting and responding to clinical deterioration in children: a systematic review. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014497.
- Roland D, Oliver A, Edwards ED, Mason BW, Powell CVE. Use of paediatric early warning systems in Great Britain: has there been a change of practice in the last 7 years?. Arch Dis Child 2014;99:26-9. https://doi.org/10.1136/archdischild-2012-302783.
- Thomas-Jones E, Lloyd A, Roland D, Sefton G, Tume L, Hood K, et al. A prospective, mixed-methods, before and after study to identify the evidence base for the core components of an effective Paediatric Early Warning System and the development of an implementation package containing those core recommendations for use in the UK: paediatric early warning system – utilisation and mortality avoidance – the PUMA study protocol. BMC Pediatr 2018;18. https://doi.org/10.1186/s12887-018-1210-z.
- Trubey R, Huang C, Lugg-Widger FV, Hood K, Allen D, Edwards D, et al. Validity and effectiveness of paediatric early warning systems and track and trigger tools for identifying and reducing clinical deterioration in hospitalised children: a systematic review. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-022105.
- Allen D. Translational mobilisation theory: a new paradigm for understanding the organisational elements of nursing work. Int J Nurs Stud 2018;79:36-42. https://doi.org/10.1016/j.ijnurstu.2017.10.010.
- Allen D. Analysing healthcare coordination using translational mobilization. J Health Organ Manag 2018;32:358-73. https://doi.org/10.1108/JHOM-05-2017-0116.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. https://doi.org/10.1136/jech.52.6.377.
- Boell SK, Cecez-Kecmanovic D. A hermeneutic approach for conducting literature reviews and literature searches. Commun Assoc Inf Syst 2014;34:257-86. https://doi.org/10.17705/1CAIS.03412.
- Greenhalgh T, A’Court C, Shaw S. Understanding heart failure; explaining telehealth – a hermeneutic systematic review. BMC Cardiovasc Disord 2017;17. https://doi.org/10.1186/s12872-017-0594-2.
- Allen D, May C. Organizing practice and practicing organization: an outline of translational mobilization theory. SAGE Open 2017;7:35-43. https://doi.org/10.1177/2158244017707993.
- van der Jagt EW. Improving pediatric survival from resuscitation events: the role and organization of hospital-based rapid response systems and code teams. Curr Pediatr Rev 2013;9:158-74. https://doi.org/10.2174/1573396311309020009.
- Lambert V, O’Shea M, Walshe C, Matthews A, Corbally M, O’Mathuna D, et al. A Systematic Literature Review to Support the Development of a National Clinical Guideline – Paediatric Early Warning System (PEWS) 2014. http://health.gov.ie/wp-content/uploads/2014/03/PEWS-Sytematic-Literature-Review-Oct-2014.pdf (accessed June 2018).
- Department of Health . The Irish Paediatric Early Warning System (PEWS) (NCEC National Clinical Guideline No. 12) 2015.
- Zhang F, Wagner AK, Ross-Degnan D. Simulation-based power calculation for designing interrupted time series analyses of health policy interventions. J Clin Epidemiol 2011;64:1252-61. https://doi.org/10.1016/j.jclinepi.2011.02.007.
- Gottman J. Time-Series Analysis: A Comprehensive Introduction for Social Scientists. Cambridge: Cambridge University Press; 1981.
- Tibballs J, Kinney S, Duke T, Oakley E, Hennessy M. Reduction of paediatric in-patient cardiac arrest and death with a medical emergency team: preliminary results. Arch Dis Child 2005;90:1148-52. https://doi.org/10.1136/adc.2004.069401.
- Nelson BK. Statistical methodology: V. Time series analysis using autoregressive integrated moving average (ARIMA) models. Acad Emerg Med 1998;5:739-44. https://doi.org/10.1111/j.1553-2712.1998.tb02493.x.
- Prais SJ, Winsten CB. Trend Estimators and Serial Correlation. Chicago, IL; 1954.
- Lopez Bernal J, Soumerai S, Gasparrini A. A methodological framework for model selection in interrupted time series studies. J Clin Epidemiol 2018;103:82-91. https://doi.org/10.1016/j.jclinepi.2018.05.026.
- Zhang F, Wagner AK, Ross-Degnan D. Simulation-based power calculation for designing interrupted time series analyses of health policy interventions. J Clin Epidemiol 2011;64:1252-61. https://doi.org/10.1016/j.jclinepi.2011.02.007.
- Allen D, Lloyd A, Edwards D, Hood K, Huang C, Hughes J, et al. Development, implementation and evaluation of an evidence-based paediatric early warning system improvement programme: the PUMA mixed methods study. BMC Health Serv Res 2022;22. https://doi.org/10.1186/s12913-021-07314-2.
- Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs 2005;17:32-5. https://doi.org/10.7748/paed2005.02.17.1.32.c964.
- Haines C, Perrott M, Weir P. Promoting care for acutely ill children-development and evaluation of a paediatric early warning tool. Intensive Crit Care Nurs 2006;22:73-81. https://doi.org/10.1016/j.iccn.2005.09.003.
- Brilli RJ, Gibson R, Luria JW, Wheeler TA, Shaw J, Linam M, et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med 2007;8:236-46. https://doi.org/10.1097/01.PCC.0000262947.72442.EA.
- Hunt EA, Zimmer KP, Rinke ML, Shilkofski NA, Matlin C, Garger C, et al. Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children’s center. Arch Pediatr Adolesc Med 2008;162:117-22. https://doi.org/10.1001/archpediatrics.2007.33.
- Sharek PJ, Parast LM, Leong K, Coombs J, Earnest K, Sullivan J, et al. Effect of a rapid response team on hospital-wide mortality and code rates outside the ICU in a children’s hospital. JAMA 2007;298:2267-74. https://doi.org/10.1001/jama.298.19.2267.
- Anwar-ul-Haque, Saleem AF, Zaidi S, Haider SR. Experience of pediatric rapid response team in a tertiary care hospital in Pakistan. Indian J Pediatr 2010;77:273-6. https://doi.org/10.1007/s12098-010-0032-2.
- Zhai H, Brady P, Li Q, Lingren T, Ni Y, Wheeler DS, et al. Developing and evaluating a machine learning based algorithm to predict the need of pediatric intensive care unit transfer for newly hospitalized children. Resuscitation 2014;85:1065-71. https://doi.org/10.1016/j.resuscitation.2014.04.009.
- Garlick J, Furlong-Dillard J, Alexander M, Burnley J, Rettiganti MR, Bhutta A, et al. Modified pediatric early warning scores, a predictor of appropriate transfer to higher level of care. Crit Care Med 2013;41:A166-7. https://doi.org/10.1097/01.ccm.0000439917.60065.34.
- Medar S, Weingarten-Arams J, Katyal C. Pediatric early warning score (PEWS) is a reliable predictor of rapid response team (RRT) call. Crit Care Med 2015;43. https://doi.org/10.1097/01.ccm.0000474735.10884.b3.
- Bell D, Mac A, Ochoa Y, Gordon M, Gregurich MA, Taylor T. The Texas Children’s Hospital pediatric advanced warning score as a predictor of clinical deterioration in hospitalized infants and children: a modification of the PEWS tool. J Pediatr Nurs 2013;28:e2-9. https://doi.org/10.1016/j.pedn.2013.04.005.
- McLellan MC, Connor JA. The Cardiac Children’s Hospital Early Warning Score (C-CHEWS). J Pediatr Nurs 2013;28:171-8. https://doi.org/10.1016/j.pedn.2012.07.009.
- Rahman ZH, Leahy NE, Sessler K, Greenway A, Sorensen LR, Rabbitts A, et al. Application of the burn center pediatric early warning score (PEWS) tool to clinical practice: a pilot validation study. J Burn Care Res 2014;35.
- Hopkins M, Rowan C, Rigby M, Tori A. PEWS Score Predicts the Need for PICU Transfer and Critical Care Interventions n.d.
- Gawronski O, Bertaina A, Broccati F, Cecchetti C, Ciaralli I, Ciscato C, et al. Bedside pews in an Italian pediatric bone marrow transplant unit: preliminary results of a longitudinal retrospective chart review. Intensive Care Med 2013;39.
- Sefton G, Lane S, Carrol ED. Performance of individual predictors of deterioration used in a paediatric early warning score. Pediatr Crit Care Med 2014;15:29-30. https://doi.org/10.1097/01.pcc.0000448835.89804.43.
- Tume L. The deterioration of children in ward areas in a specialist children’s hospital. Nurs Crit Care 2007;12:12-9. https://doi.org/10.1111/j.1478-5153.2006.00195.x.
- Robson MA, Cooper CL, Medicus LA, Quintero MJ, Zuniga SA. Comparison of three acute care pediatric early warning scoring tools. J Pediatr Nurs 2013;28:e33-41. https://doi.org/10.1016/j.pedn.2012.12.002.
- Chapman SM, Wray J, Oulton K, Pagel C, Ray S, Peters MJ. ‘The Score Matters’: wide variations in predictive performance of 18 paediatric track and trigger systems. Arch Dis Child 2017;102:487-95. https://doi.org/10.1136/archdischild-2016-311088.
- Fuijkschot J, Vernhout B, Lemson J, Draaisma JM, Loeffen JL. Validation of a Paediatric Early Warning Score: first results and implications of usage. Eur J Pediatr 2015;174:15-21. https://doi.org/10.1007/s00431-014-2357-8.
- Mason BW, Edwards ED, Oliver A, Powell CVE. Cohort study to test the predictability of the NHS Institute for Innovation and Improvement Paediatric Early Warning System. Arch Dis Child 2016;101:552-5. https://doi.org/10.1136/archdischild-2015-308465.
- Tucker KM, Brewer TL, Baker RB, Demeritt B, Vossmeyer MT. Prospective evaluation of a pediatric inpatient early warning scoring system. J Spec Pediatr Nurs 2009;14:79-85. https://doi.org/10.1111/j.1744-6155.2008.00178.x.
- Edwards ED, Mason BW, Oliver A, Powell CV. Cohort study to test the predictability of the Melbourne criteria for activation of the medical emergency team. Arch Dis Child 2011;96:174-9. https://doi.org/10.1136/adc.2010.187617.
- O’Loughlin K, Ruparelia K, Vince T, Bedford S, Drysdale SB, Broughton S. The effectiveness of the Paediatric Early Warning Tool (PEWT) in identifying children requiring admission to a critical care unit. Arch Dis Child 2012;97. https://doi.org/10.1136/archdischild-2012-301885.362.
- Wright D, Sefton G, Horan M. Charted observations and the use of a Paediatric Early Warning Tool did not predict the majority of cardio-respiratory arrest calls in a paediatric hospital. Arch Dis Child 2011;96. https://doi.org/10.1136/adc.2011.212563.134.
- Ahmed M, Sobithadevi D, Lall R, Ghose A, Boswell S, Reynolds T. Burton Paediatric Early Warning System score. Arch Dis Child 2012;97. https://doi.org/10.1136/archdischild-2012-302724.1483.
- McLellan M, Gauvreau K, Connor JA. Validation of the children’s hospital early warning scoring system for identifying hospitalized children at risk for arrest or ICU transfer. Pediatr Crit Care Med 2014;15. https://doi.org/10.1097/01.pcc.0000448836.27923.e3.
- Clayson R, Selby A, Tume L. Is a Paediatric Early Warning Tool (PEWT) useful in cardiac patients?. Pediatr Crit Care Med 2014;15:35-6. https://doi.org/10.1097/01.pcc.0000448865.31975.d1.
- Fenix JB, Gillespie CW, Levin A, Dean N. Comparison of pediatric early warning score to physician opinion for deteriorating patients. Hosp Pediatr 2015;5:474-9. https://doi.org/10.1542/hpeds.2014-0199.
- Mandell IM, Bynum F, Marshall L, Bart R, Gold JI, Rubin S. Pediatric early warning score and unplanned readmission to the pediatric intensive care unit. J Crit Care 2015;30:1090-5. https://doi.org/10.1016/j.jcrc.2015.06.019.
- Agulnik A, Méndez Aceituno A, Mora Robles LN, Forbes PW, Soberanis Vasquez DJ, Mack R, et al. Validation of a pediatric early warning system for hospitalized pediatric oncology patients in a resource-limited setting. Cancer 2017;123:4903-13. https://doi.org/10.1002/cncr.30951.
- Agulnik A, Forbes PW, Stenquist N, Rodriguez-Galindo C, Kleinman M. Validation of a pediatric early warning score in hospitalized pediatric oncology and hematopoietic stem cell transplant patients. Pediatr Crit Care Med 2016;17:e146-53. https://doi.org/10.1097/PCC.0000000000000662.
- Blackstone B, Sharmila A, Pienaar A, Bevan R, Cheung R. G453(P) ‘Between the flags’ Paediatric Early Warning System is a sensitive tool to detect deterioration in children from district general hospitals admitted to paediatric intensive care. Arch Dis Child 2017;102:A178 LP-A179. https://doi.org/10.1136/archdischild-2017-313087.446.
- Parshuram CS, Duncan HP, Joffe AR, Farrell CA, Lacroix JR, Middaugh KL, et al. Multicentre validation of the bedside Paediatric Early Warning System score: a severity of illness score to detect evolving critical illness in hospitalised children. Crit Care 2011;15. https://doi.org/10.1186/cc10337.
- McLellan MC, Gauvreau K, Connor JA. Validation of the Cardiac Children’s Hospital Early Warning Score: an early warning scoring tool to prevent cardiopulmonary arrests in children with heart disease. Congenit Heart Dis 2014;9:194-202. https://doi.org/10.1111/chd.12132.
- Akre M, Finkelstein M, Erickson M, Liu M, Vanderbilt L, Billman G. Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics 2010;125:e763-9. https://doi.org/10.1542/peds.2009-0338.
- Gawronski O, Ciofi Degli Atti ML, Di Ciommo V, Cecchetti C, Bertaina A, Tiozzo E, et al. Accuracy of Bedside Paediatric Early Warning System (BedsidePEWS) in a pediatric stem cell transplant unit. J Pediatr Oncol Nurs 2016;33:249-56. https://doi.org/10.1177/1043454215600154.
- Skaletzky SM, Raszynski A, Totapally BR. Validation of a modified pediatric early warning system score: a retrospective case-control study. Clin Pediatr 2012;51:431-5. https://doi.org/10.1177/0009922811430342.
- Ross C, Harrysson I, Goel V, Knight L, Poole S, Franzon D, et al. Sensitivity and specificity of a pediatric early warning system using data-driven vital signs. Crit Care Med 2015;43. https://doi.org/10.1097/01.ccm.0000473979.19857.a8.
- Guerra MW, Shults J, Amsterdam J, Ten-Have T. The analysis of binary longitudinal data with time-dependent covariates. Stat Med 2012;31:931-48. https://doi.org/10.1002/sim.4465.
- Selig JP, Preacher KJ, Little TD. Modeling time-dependent association in longitudinal data: a lag as moderator approach. Multivariate Behav Res 2012;47:697-716. https://doi.org/10.1080/00273171.2012.715557.
- Parshuram CS, Dryden-Palmer K, Farrell C, Gottesman R, Gray M, Hutchison JS, et al. Effect of a pediatric early warning system on all-cause mortality in hospitalized pediatric patients: the EPOCH randomized clinical trial. JAMA 2018;319:1002-12. https://doi.org/10.1001/jama.2018.0948.
- Clifton DA, Clifton L, Sandu D, . ‘Errors’ and omissions in paper-based early warning scores: the association with changes in vital signs – a database analysis. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007376.
- Mistry KP, Turi J, Hueckel R, Mericle JM, Meliones JN. Pediatric rapid response teams in the academic medical center. Clin Pediatr Emerg Med 2006;7:241-7. https://doi.org/10.1016/j.cpem.2006.08.010.
- Demmel KM, Williams L, Flesch L. Implementation of the pediatric early warning scoring system on a pediatric hematology/oncology unit. J Pediatr Oncol Nurs 2010;27:229-40. https://doi.org/10.1177/1043454209358410.
- Sandhu S, Ralph T, Bradbury K. The Paediatric Early Warning Tool in combination with a nurse-led outreach team: impact on outcomes for critically ill children. Pediatr Res 2010;68. https://doi.org/10.1203/00006450-201011001-00726.
- Randhawa S, Roberts-Turner R, Woronick K, DuVal J. Implementing and sustaining evidence-based nursing practice to reduce pediatric cardiopulmonary arrest. West J Nurs Res 2011;33:443-56. https://doi.org/10.1177/0193945910379585.
- Camacho EA, Holman D, Badgett V, . Modification of a pediatric early warning tool for use with patients in a specialized cardiac/renal unit. J Pediatr Nurs 2011;26:e2-3. https://doi.org/10.1016/j.pedn.2010.12.021.
- Heyden MV, Mudge B, Casella S. Initiation of a dedicated pediatric rapid response team reduces cardiopulmonary arrests outside of the intensive care unit in a small children’s hospital. Crit Care Med 2012;40. https://doi.org/10.1097/01.ccm.0000424810.76242.a7.
- Norville R, Wills-Bagnato P, Staton S, Kennedy-Nasser A. Improvement in early recognition of deteriorating pediatric bone marrow transplant patients. Biol Blood Marrow Transplant 2013;19. https://doi.org/10.1016/j.bbmt.2012.11.152.
- Ambati SR, Sweberg T, Silver P, Gangadharan S. Effect of simulation based curriculum on the utilization of rapid response team activations (RRT). Crit Care Med 2014;42. https://doi.org/10.1097/01.ccm.0000457794.10321.00.
- Ocholi T, Downing E, Rzeskiewicz A, Pang R, Broughton S. Impact of the Bedside PEWS on early detection of deteriorating patients. Arch Dis Child 2014;99:A89-90. https://doi.org/10.1136/archdischild-2014-306237.207.
- Somberg CM. Implementing a pediatric early warning system in a community hospital setting. Clinical Nurse Specialist 2013;27:E18-19.
- Zenker P, Schlesinger A, Hauck M, Spencer S, Hellmich T, Finkelstein M, et al. Implementation and impact of a rapid response team in a children’s hospital. Jt Comm J Qual Patient Saf 2007;33:418-25. https://doi.org/10.1016/S1553-7250(07)33048-1.
- Hanson CC, Randolph GD, Erickson JA, Mayer CM, Bruckel JT, Harris BD, et al. A reduction in cardiac arrests and duration of clinical instability after implementation of a paediatric rapid response system. Postgrad Med J 2010;86:314-18. https://doi.org/10.1136/qshc.2007.026054.
- Tibballs J, Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med 2009;10:306-12. https://doi.org/10.1097/PCC.0b013e318198b02c.
- Kotsakis A, Lobos AT, Parshuram C, Gilleland J, Gaiteiro R, Mohseni-Bod H, et al. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics 2011;128:72-8. https://doi.org/10.1542/peds.2010-0756.
- Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr 2014;168:25-33. https://doi.org/10.1001/jamapediatrics.2013.3266.
- Duns C, Ceely B, Festa M, . Introduction of a track and trigger system is associated with increased paediatric intensive care outreach utilisation and a trend towards improved patient outcomes in a tertiary children’s hospital. Aust Crit Care 2014;27. https://doi.org/10.1016/j.aucc.2013.10.058.
- Agulnik A, Mora Robles LN, Forbes PW, Soberanis Vasquez DJ, Mack R, Antillon-Klussmann F, et al. Improved outcomes after successful implementation of a pediatric early warning system (PEWS) in a resource-limited pediatric oncology hospital. Cancer 2017;123:2965-74. https://doi.org/10.1002/cncr.30664.
- Sefton G, McGrath C, Tume L, Lane S, Lisboa PJ, Carrol ED. What impact did a Paediatric Early Warning system have on emergency admissions to the paediatric intensive care unit? An observational cohort study. Intensive Crit Care Nurs 2015;31:91-9. https://doi.org/10.1016/j.iccn.2014.01.001.
- Kolovos NS, Gill J, Michelson PH, Doctor A, Hartman ME. Reduction in mortality following pediatric rapid response team implementation. Pediatr Crit Care Med 2018;19:477-82. https://doi.org/10.1097/PCC.0000000000001519.
- Parshuram CS, Bayliss A, Reimer J, Middaugh K, Blanchard N. Implementing the Bedside Paediatric Early Warning System in a community hospital: a prospective observational study. Paediatr Child Health 2011;16:e18-22. https://doi.org/10.1093/pch/16.3.e18.
- Panesar R, Polikoff LA, Harris D, Mills B, Messina C, Parker MM. Characteristics and outcomes of pediatric rapid response teams before and after mandatory triggering by an elevated pediatric early warning system (PEWS) score. Hosp Pediatr 2014;4:135-40. https://doi.org/10.1542/hpeds.2013-0062.
- Douglas K, Collado JC, Keller S. implementation of a pediatric early warning scoring system at an academic medical center. Crit Care Nurs Q 2016;39:363-70. https://doi.org/10.1097/CNQ.0000000000000130.
- Kutty S, Jones PG, Karels Q, Joseph N, Spertus JA, Chan PS. Association of pediatric medical emergency teams with hospital mortality. Circulation 2018;137:38-46. https://doi.org/10.1161/CIRCULATIONAHA.117.029535.
- Joffe AR, Anton NR, Burkholder SC. Reduction in hospital mortality over time in a hospital without a pediatric medical emergency team: limitations of before-and-after study designs. Arch Pediatr Adolesc Med 2011;165:419-23. https://doi.org/10.1001/archpediatrics.2011.47.
- Goodacre S. Uncontrolled before-after studies: discouraged by Cochrane and the EMJ. Emerg Med J 2015;32:507-8. https://doi.org/10.1136/emermed-2015-204761.
- Niegsch M, Fabritius ML, Anhøj J. Imperfect implementation of an early warning scoring system in a Danish teaching hospital: a cross-sectional study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0070068.
- Hands C, Reid E, Meredith P, Smith GB, Prytherch DR, Schmidt PE, et al. Patterns in the recording of vital signs and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf 2013;22:719-26. https://doi.org/10.1136/bmjqs-2013-001954.
- Lydon S, Byrne D, Offiah G, Gleeson L, O’Connor P. A mixed-methods investigation of health professionals’ perceptions of a physiological track and trigger system. BMJ Qual Saf 2016;25:688-95. https://doi.org/10.1136/bmjqs-2015-004261.
- Andrews T, Waterman H. Packaging: a grounded theory of how to report physiological deterioration effectively. J Adv Nurs 2005;52:473-81.
- Azzopardi P, Kinney S, Moulden A, Tibballs J. Attitudes and barriers to a medical emergency team system at a tertiary paediatric hospital. Resuscitation 2011;82:167-74. https://doi.org/10.1016/j.resuscitation.2010.10.013.
- Bonafide CP, Roberts KE, Weirich CM, Paciotti B, Tibbetts KM, Keren R, et al. Beyond statistical prediction: qualitative evaluation of the mechanisms by which pediatric early warning scores impact patient safety. J Hosp Med 2013;8:248-53. https://doi.org/10.1002/jhm.2026.
- Braaten JS. Hospital system barriers to rapid response team activation: a cognitive work analysis. Am J Nurs 2015;115:22-3. https://doi.org/10.1097/01.NAJ.0000460672.74447.4a.
- Ennis L. Paediatric Early Warning Scores on a children’s ward: a quality improvement initiative. Nurs Child Young People 2014;26:25-31. https://doi.org/10.7748/ncyp.26.7.25.e478.
- Kaul M, Snethen J, Kelber ST, Zimmanck K, Maletta K, Meyer M. Implementation of the Bedside Paediatric Early Warning System (BedsidePEWS) for nurse identification of deteriorating patients. J Spec Pediatr Nurs 2014;19:339-49. https://doi.org/10.1111/jspn.12092.
- Lobos AT, Fernandes R, Ramsay T, McNally JD. Patient characteristics and disposition after pediatric medical emergency team (MET) activation: disposition depends on who activates the team. Hosp Pediatr 2014;4:99-105. https://doi.org/10.1542/hpeds.2013-0032.
- McDonnell A, Tod A, Bray K, Bainbridge D, Adsetts D, Walters S. A before and after study assessing the impact of a new model for recognizing and responding to early signs of deterioration in an acute hospital. J Adv Nurs 2013;69:41-52. https://doi.org/10.1111/j.1365-2648.2012.05986.x.
- Mackintosh N, Rainey H, Sandall J. Understanding how rapid response systems may improve safety for the acutely ill patient: learning from the frontline. BMJ Qual Saf 2012;21:135-44. https://doi.org/10.1136/bmjqs-2011-000147.
- Pattison N, Eastham E. Critical care outreach referrals: a mixed-method investigative study of outcomes and experiences. Nurs Crit Care 2012;17:71-82. https://doi.org/10.1111/j.1478-5153.2011.00464.x.
- Pearson G, Duncan H. Early warning systems for identifying sick children. Paediatr Child 2011;21:230-3. https://doi.org/10.1016/j.paed.2011.02.007.
- Shearer B, Marshall S, Buist MD, Finnigan M, Kitto S, Hore T, et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi-campus Australian metropolitan healthcare service. BMJ Qual Saf 2012;21:569-75. https://doi.org/10.1136/bmjqs-2011-000692.
- Stewart J, Carman M, Spegman A, Sabol VK. Evaluation of the effect of the modified early warning system on the nurse-led activation of the rapid response system. J Nurs Care Qual 2014;29:223-9. https://doi.org/10.1097/NCQ.0000000000000048.
- Van Voorhis KT, Willis TS. Implementing a pediatric rapid response system to improve quality and patient safety. Pediatr Clin North Am 2009;56:919-33. https://doi.org/10.1016/j.pcl.2009.05.017.
- Wheatley I. The nursing practice of taking level 1 patient observations. Intensive Crit Care Nurs 2006;22:115-21. https://doi.org/10.1016/j.iccn.2005.08.003.
- McCabe A, Duncan H, Heward Y. Paediatric Early Warning Systems: where do we go from here?. Paediatr Nurs 2009;21:14-7. https://doi.org/10.7748/paed.21.1.14.s20.
- Chua WL, Mackey S, Ng EK, Liaw SY. Front line nurses’ experiences with deteriorating ward patients: a qualitative study. Int Nurs Rev 2013;60:501-9. https://doi.org/10.1111/inr.12061.
- James J, Butler-Williams C, Hunt J, Cox H. Vital signs for vital people: an exploratory study into the role of the Healthcare Assistant in recognising, recording and responding to the acutely ill patient in the general ward setting. J Nurs Manag 2010;18:548-55. https://doi.org/10.1111/j.1365-2834.2010.01086.x.
- Mackintosh N, Humphrey C, Sandall J. The habitus of ‘rescue’ and its significance for implementation of rapid response systems in acute health care. Soc Sci Med 2014;120:233-42. https://doi.org/10.1016/j.socscimed.2014.09.033.
- Endacott R, Westley M. Managing patients at risk of deterioration in rural hospitals: a qualitative study. Aust J Rural Health 2006;14:275-9. https://doi.org/10.1111/j.1440-1584.2006.00829.
- Sønning K, Nyrud CRI. A survey of healthcare professionals’ experiences with the Paediatric Early Warning Score (PEWS). Nor J Clin Nurs Sykepl Fors 2018;12. https://doi.org/10.4220/Sykepleienf.2017.64605en.
- Cioffi J, Salter C, Wilkes L, Vonu-Boriceanu O, Scott J. Clinicians’ responses to abnormal vital signs in an emergency department. Aust Crit Care 2006;19:66-72. https://doi.org/10.1016/s1036-7314(06)80011-1.
- Endacott R, Kidd T, Chaboyer W, Edington J. Recognition and communication of patient deterioration in a regional hospital: a multi-methods study. Aust Crit Care 2007;20:100-5. https://doi.org/10.1016/j.aucc.2007.05.002.
- Watson A, Skipper C, Steury R, Walsh H, Levin A. Inpatient nursing care and early warning scores: a workflow mismatch. J Nurs Care Qual 2014;29:215-22. https://doi.org/10.1097/NCQ.0000000000000058.
- Jensen CS, Nielsen PB, Olesen HV, Kirkegaard H, Aagaard H. Pediatric early warning score systems, nurses perspective – a focus group study. J Pediatr Nurs 2018;41:e16-22. https://doi.org/10.1016/j.pedn.2018.02.004.
- Mackintosh N, Berridge EJ, Freeth D. Supporting structures for team situation awareness and decision making: insights from four delivery suites. J Eval Clin Pract 2009;15:46-54. https://doi.org/10.1111/j.1365-2753.2008.00953.x.
- Cioffi J. Nurses’ experiences of making decisions to call emergency assistance to their patients. J Adv Nurs 2000;32:108-14. https://doi.org/10.1046/j.1365-2648.2000.01414.x.
- Adelstein BA, Piza MA, Nayyar V, Mudaliar Y, Klineberg PL, Rubin G. Rapid response systems: a prospective study of response times. J Crit Care 2011;26:e11-18. https://doi.org/10.1016/j.jcrc.2011.03.013.
- Astroth KS, Woith WM, Stapleton SJ, Degitz RJ, Jenkins SH. Qualitative exploration of nurses’ decisions to activate rapid response teams. J Clin Nurs 2013;22:2876-82. https://doi.org/10.1111/jocn.12067.
- Cioffi J. Recognition of patients who require emergency assistance: a descriptive study. Heart Lung 2000;29:262-8. https://doi.org/10.1067/mhl.2000.108327.
- Mackintosh N, Watson K, Rance S, Sandall J. Value of a Modified Early Obstetric Warning System (MEOWS) in managing maternal complications in the peripartum period: an ethnographic study. BMJ Qual Saf 2014;23:26-34. https://doi.org/10.1136/bmjqs-2012-001781.
- Davies O, DeVita MA, Ayinla R, Perez X. Barriers to activation of the rapid response system. Resuscitation 2014;85:1557-61. https://doi.org/10.1016/j.resuscitation.2014.07.013.
- McCrory MC, Aboumatar H, Custer JW, Yang CP, Hunt EA. ‘ABC-SBAR’ training improves simulated critical patient hand-off by pediatric interns. Pediatr Emerg Care 2012;28:538-43. https://doi.org/10.1097/PEC.0b013e3182587f6e.
- Salamonson Y, van Heere B, Everett B, Davidson P. Voices from the floor: nurses’ perceptions of the medical emergency team. Intensive Crit Care Nurs 2006;22:138-43. https://doi.org/10.1016/j.iccn.2005.10.002.
- Mohammmed Iddrisu S, Hutchinson AF, Sungkar Y, Considine J. Nurses’ role in recognising and responding to clinical deterioration. J Clin Nurs 2018;27:1920-30. https://doi.org/10.1111/jocn.14331.
- McKay H, Mitchell IA, Sinn K, Mugridge H, Lafferty T, Van Leuvan C, et al. Effect of a multifaceted intervention on documentation of vital signs and staff communication regarding deteriorating paediatric patients. J Paediatr Child Health 2013;49:48-56. https://doi.org/10.1111/jpc.12019.
- Radeschi G, Urso F, Campagna S, Berchialla P, Borga S, Mina A, et al. Factors affecting attitudes and barriers to a medical emergency team among nurses and medical doctors: a multi-centre survey. Resuscitation 2015;88:92-8. https://doi.org/10.1016/j.resuscitation.2014.12.027.
- Hope J, Recio-Saucedo A, Fogg C, Griffiths P, Smith GB, Westwood G, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs 2018;27:1860-71. https://doi.org/10.1111/jocn.14234.
- de Groot JF, Damen N, de Loos E, van de Steeg L, Koopmans L, Rosias P, et al. Implementing paediatric early warning scores systems in the Netherlands: future implications. BMC Pediatr 2018;18. https://doi.org/10.1186/s12887-018-1099-6.
- Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf 2014;23:153-61. https://doi.org/10.1136/bmjqs-2012-001747.
- Donohue LA, Endacott R. Track, trigger and teamwork: communication of deterioration in acute medical and surgical wards. Intensive Crit Care Nurs 2010;26:10-7. https://doi.org/10.1016/j.iccn.2009.10.006.
- Claussen D, Garner D, Crow B. Early warning signs and the EHR: at the intersection of technology and care. Nurs Manage 2013;44:14-6. https://doi.org/10.1097/01.NUMA.0000433384.49766.b4.
- Jones D, Baldwin I, McIntyre T, Story D, Mercer I, Miglic A, et al. Nurses’ attitudes to a medical emergency team service in a teaching hospital. Qual Saf Health Care 2006;15:427-32. https://doi.org/10.1136/qshc.2005.016956.
- Massey D, Chaboyer W, Aitken L. Nurses’ perceptions of accessing a Medical Emergency Team: a qualitative study. Aust Crit Care 2014;27:133-8. https://doi.org/10.1016/j.aucc.2013.11.001.
- Burns KA, Reber T, Theodore K, Welch B, Roy D, Siedlecki SL. Enhanced early warning system impact on nursing practice: a phenomenological study. J Adv Nurs 2018;74:1150-6. https://doi.org/10.1111/jan.13517.
- Rainey H, Ehrich K, Mackintosh N, Sandall J. The role of patients and their relatives in ‘speaking up’ about their own safety – a qualitative study of acute illness. Health Expect 2015;18:392-405. https://doi.org/10.1111/hex.12044.
- Mackintosh NJ, Davis RE, Easter A, Rayment-Jones H, Sevdalis N, Wilson S, et al. Interventions to increase patient and family involvement in escalation of care for acute life-threatening illness in community health and hospital settings. Cochrane Database Syst Rev 2017;10. https://doi.org/10.1002/14651858.CD012829.
- Paciotti B, Roberts KE, Tibbetts KM, Paine CW, Keren R, Barg FK, et al. Physician attitudes toward family-activated medical emergency teams for hospitalized children. Jt Comm J Qual Patient Saf 2014;40:187-92. https://doi.org/10.1016/S1553-7250(14)40024-2.
- Graedon J, Graedon T. Enlisting families as patient safety allies. Clin Pediatr Emerg Med 2006;7:265-7. https://doi.org/10.1016/j.cpem.2006.08.006.
- Entwistle V. Nursing shortages and patient safety problems in hospital care: is clinical monitoring by families part of the solution?. Health Expect 2004;7:1-5. https://doi.org/10.1046/j.1369-7625.2003.00259.x.
- Downey CL, Tahir W, Randell R, Brown JM, Jayne DG. Strengths and limitations of early warning scores: a systematic review and narrative synthesis. Int J Nurs Stud 2017;76:106-19. https://doi.org/10.1016/j.ijnurstu.2017.09.003.
- Mohammed M, Hayton R, Clements G, Smith G, Prytherch D. Improving accuracy and efficiency of early warning scores in acute care. Br J Nurs 2009;18:18-24. https://doi.org/10.12968/bjon.2009.18.1.32072.
- Sefton G, Lane S, Killen R, Black S, Lyon M, Ampah P, et al. Accuracy and efficiency of recording pediatric early warning scores using an electronic physiological surveillance system compared with traditional paper-based documentation. Comput Inform Nurs 2017;35:228-36. https://doi.org/10.1097/CIN.0000000000000305.
- Jones S, Mullally M, Ingleby S, Buist M, Bailey M, Eddleston JM. Bedside electronic capture of clinical observations and automated clinical alerts to improve compliance with an early warning score protocol. Crit Care Resusc 2011;13:83-8.
- Schmidt PE, Meredith P, Prytherch DR, Watson D, Watson V, Killen RM, et al. Impact of introducing an electronic physiological surveillance system on hospital mortality. BMJ Qual Saf 2015;24:10-2. https://doi.org/10.1136/bmjqs-2014-003073.
- Bellomo R, Ackerman M, Bailey M, Beale R, Clancy G, Danesh V, et al. A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit Care Med 2012;40:2349-61. https://doi.org/10.1097/CCM.0b013e318255d9a0.
- Subbe CP, Duller B, Bellomo R. Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit Care 2017;21. https://doi.org/10.1186/s13054-017-1635-z.
- Bonafide CP, Zander M, Graham CS, Weirich Paine CM, Rock W, Rich A, et al. Video methods for evaluating physiologic monitor alarms and alarm responses. Biomed Instrum Technol 2014;48:220-30. https://doi.org/10.2345/0899-8205-48.3.220.
- Fagan K, Sabel A, Mehler PS, MacKenzie TD. Vital sign abnormalities, rapid response, and adverse outcomes in hospitalized patients. Am J Med Qual 2012;27:480-6. https://doi.org/10.1177/1062860611436127.
- Bonafide CP, Localio AR, Holmes JH, Nadkarni VM, Stemler S, MacMurchy M, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr 2017;171:524-31. https://doi.org/10.1001/jamapediatrics.2016.5123.
- Wager KA, Schaffner MJ, Foulois B, Swanson Kazley A, Parker C, Walo H. Comparison of the quality and timeliness of vital signs data using three different data-entry devices. Comput Inform Nurs 2010;28:205-12. https://doi.org/10.1097/NCN.0b013e3181e1df19.
- Stevenson JE, Nilsson G. Nurses’ perceptions of an electronic patient record from a patient safety perspective: a qualitative study. J Adv Nurs 2012;68:667-76. https://doi.org/10.1111/j.1365-2648.2011.05786.x.
- de Vries A, Draaisma JMT, Fuijkschot J. Clinician perceptions of an early warning system on patient safety. Hosp Pediatr 2017;7:579-86. https://doi.org/10.1542/hpeds.2016-0138.
- Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf 2013;22:899-906. https://doi.org/10.1136/bmjqs-2012-001467.
- Brady PW, Muething S, Kotagal U, Ashby M, Gallagher R, Hall D, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics 2013;131:e298-308. https://doi.org/10.1542/peds.2012-1364.
- Parker MW, Carroll M, Bolser B, Ballinger J, Brewington J, Campanella S, et al. Implementation of a communication bundle for high-risk patients. Hosp Pediatr 2017;7:523-9. https://doi.org/10.1542/hpeds.2016-0170.
- Pezzolesi C, Manser T, Schifano F, Kostrzewski A, Pickles J, Harriet N, et al. Human factors in clinical handover: development and testing of a ‘handover performance tool’ for doctors’ shift handovers. Int J Qual Health Care 2013;25:58-65. https://doi.org/10.1093/intqhc/mzs076.
- Abraham J, Kannampallil T, Patel B, Almoosa K, Patel VL. Ensuring patient safety in care transitions: an empirical evaluation of a handoff intervention tool. AMIA Annu Symp Proc 2012;2012:17-26.
- Donahue M, Smith L, Dykes P, Fitzpatrick JJ. Phase 2 of the EMPOWER project: enhancing communication for paraprofessionals. J Contin Educ Nurs 2010;41:197-8. https://doi.org/10.3928/00220124-20100423-08.
- Mullan PC, Macias CG, Hsu D, Alam S, Patel B. A novel briefing checklist at shift handoff in an emergency department improves situational awareness and safety event identification. Pediatr Emerg Care 2015;31:231-8. https://doi.org/10.1097/PEC.0000000000000194.
- Weiss MJ, Bhanji F, Fontela PS, Razack SI. A preliminary study of the impact of a handover cognitive aid on clinical reasoning and information transfer. Med Educ 2013;47:832-41. https://doi.org/10.1111/medu.12212.
- May CR, Johnson M, Finch T. Implementation, context and complexity. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0506-3.
- Wong D, Bonnici T, Knight J, Morgan L, Coombes P, Watkinson P. SEND: a system for electronic notification and documentation of vital sign observations. BMC Med Inform Decis Mak 2015;15. https://doi.org/10.1186/s12911-015-0186-y.
- Preece MH, Hill A, Horswill MS, Karamatic R, Watson MO. Designing observation charts to optimize the detection of patient deterioriation: reliance on the subjective preferences of healthcare professionals is not enough. Aust Crit Care 2012;25:238-52. https://doi.org/10.1016/j.aucc.2012.01.003.
- Almblad AC, Siltberg P, Engvall G, Målqvist M. Implementation of pediatric early warning score; adherence to guidelines and influence of context. J Pediatr Nurs 2018;38:33-9. https://doi.org/10.1016/j.pedn.2017.09.002.
- Lydon S, Byrne D, Offiah G, Gleeson L, O’Connor P. A mixed-methods investigation of health professionals’ perceptions of a physiological track and trigger system. BMJ Qual Saf 2016;25:688-95. https://doi.org/10.1136/bmjqs-2015-004261.
- Dean BS, Decker MJ, Hupp D, Urbach AH, Lewis E, Benes-Stickle J. Condition HELP: a pediatric rapid response team triggered by patients and parents. J Healthc Qual 2008;30:28-31. https://doi.org/10.1111/j.1945-1474.2008.tb01139.x.
- Greenhouse PK, Kuzminsky B, Martin SC, Merryman T. Calling a condition H(elp). Am J Nurs 2006;106:63-6. https://doi.org/10.1097/00000446-200611000-00021.
- Hueckel RM, Mericle JM, Frush K, Martin PL, Champagne MT. Implementation of Condition Help: family teaching and evaluation of family understanding. J Nurs Care Qual 2012;27:176-81. https://doi.org/10.1097/NCQ.0b013e318235bdec.
- Gill FJ, Leslie GD, Marshall AP. Family initiated escalation of care for the deteriorating patient in hospital: family centred care or just ‘box ticking’. Aust Crit Care 2016;29:195-200. https://doi.org/10.1016/j.aucc.2016.07.004.
- Bogert S, Ferrell C, Rutledge DN. Experience with family activation of rapid response teams. Medsurg Nurs 2010;19:215-22.
- Gerdik C, Vallish RO, Miles K, Godwin SA, Wludyka PS, Panni MK. Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation 2010;81:1676-81. https://doi.org/10.1016/j.resuscitation.2010.06.020.
- Bavare AC, Thomas JK, Elliott EP, Morgan AC, Graf JM. Family-initiated pediatric rapid response: characteristics, impetus, and outcomes. J Healthc Qual 2018;40:103-9. https://doi.org/10.1097/JHQ.0000000000000096.
- Dixon-Woods M, Leslie M, Tarrant C, Bion J. Explaining matching Michigan: an ethnographic study of a patient safety program. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-70.
- Leape LL. The checklist conundrum. N Engl J Med 2014;370:1063-4. https://doi.org/10.1056/NEJMe1315851.
- Stephens TJ, Peden CJ, Pearse RM, Shaw SE, Abbott TEF, Jones EL, et al. Improving care at scale: process evaluation of a multi-component quality improvement intervention to reduce mortality after emergency abdominal surgery (EPOCH trial). Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0823-9.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- Braithwaite J. Changing how we think about healthcare improvement. BMJ 2018;361. https://doi.org/10.1136/bmj.k2014.
- Reed JE, Card AJ. The problem with plan-do-study-act cycles. BMJ Qual Saf 2016;25:147-52. https://doi.org/10.1136/bmjqs-2015-005076.
- Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0242-0.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Qual Saf 2015;24:228-38. https://doi.org/10.1136/bmjqs-2014-003627.
- Dixon-Woods M, Martin GP. Does quality improvement improve quality?. Futur Hopsital J 2016;3:191-4. https://doi.org/10.7861/futurehosp.3-3-191.
- Koczwara B, Stover AM, Davies L, Davis MM, Fleisher L, Ramanadhan S, et al. Harnessing the synergy between improvement science and implementation science in cancer: a call to action. J Oncol Pract 2018;14:335-40. https://doi.org/10.1200/JOP.17.00083.
- Ovretveit J, Mittman B, Rubenstein L, Ganz DA. Using implementation tools to design and conduct quality improvement projects for faster and more effective improvement. Int J Health Care Qual Assur 2017;30:755-68. https://doi.org/10.1108/IJHCQA-01-2017-0019.
- Ovretveit J, Dolan-Branton L, Marx M, Reid A, Reid J, Agins B. Adapting improvements to context: when, why and how?. Int J Qual Heal Care 2018;30:20-3. https://doi.org/10.1093/intqhc/mzy013.
- Langley G, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide. San Fransisco, CA: Jossey-Bass; 2009.
- Berg M, Hughes JF, Prinz W, Rodden T, Schmidt K. Proceedings of the Fifth European Conference on Computer-Supported Cooperative Work. Dordrecht: Kluwer Academic Publishers; 1997.
- Care Quality Commission . Alder Hey Children’s NHS Foundation Trust. Alder Hey Children’s Hospital: Quality Report 2017. https://api.cqc.org.uk/public/v1/reports/0e8881dd-4adc-411c-b4e1-d703b109bc65?20171004235506 (accessed 21 December 2020).
- The UK Sepsis Trust . Clinical n.d. https://sepsistrust.org/professional-resources/clinical/ (accessed 21 December 2020).
- Royal College of Nursing . Defining Staffing Levels for Children’s and Young People’s Services: RCN Guidance for Clinical Professionals and Service Managers 2003. www.umsts.org/umst/uploaded/Defining%20staffing%20levels.pdf (accessed 21 December 2020).
- Allen D. The Invisible Work of Nurses: Hospitals, Organisation and Healthcare. London: Routledge; 2015.
- Hamilton-Fairley D, Coakley J, Moss F. Hospital at night: an organizational design that provides safer care at night. BMC Med Educ 2014;14. https://doi.org/10.1186/1472-6920-14-S1-S17.
- Hawe P, Shiell A, Riley T. Theorising interventions as events in systems. Am J Community Psychol 2009;43:267-76. https://doi.org/10.1007/s10464-009-9229-9.
- Tume LN, Sefton G, Arrowsmith P. Teaching paediatric ward teams to recognise and manage the deteriorating child. Nurs Crit Care 2014;19:196-203. https://doi.org/10.1111/nicc.12050.
- Heath G, Montgomery H, Eyre C, Cummins C, Pattison H, Shaw R. Developing a tool to support communication of parental concerns when a child is in hospital. Healthcare 2016;4. https://doi.org/10.3390/healthcare4010009.
- Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf 2006;32:167-75. https://doi.org/10.1016/S1553-7250(06)32022-3.
- Woodcock T, Liberati EG, Dixon-Woods M. A mixed-methods study of challenges experienced by clinical teams in measuring improvement. BMJ Qual Saf 2021;30:106-15. https://doi.org/10.1136/bmjqs-2018-009048.
- Draycott T, Sibanda T, Owen L, . Does training in obstetric emergencies improve neonatal outcome?. BJOG 2006;113:177-82. https://doi.org/10.1111/j.1471-0528.2006.00800.x.
- Draycott TJ, Crofts JF, Ash JP, Wilson LV, Yard E, Sibanda T, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol 2008;112:14-20. https://doi.org/10.1097/AOG.0b013e31817bbc61.
- Hogan H, Hutchings A, Wulff J, Carver C, Holdsworth E, Welch J, et al. Interventions to reduce mortality from in-hospital cardiac arrest: a mixed-methods study. Health Serv Deliv Res 2019;7. https://doi.org/10.3310/hsdr07020.
- Parshuram CS, Dryden-Palmer K, Farrell C, Gottesman R, Gray M, Hutchison JS, et al. Effect of a pediatric early warning system on all-cause mortality in hospitalized pediatric patients: the EPOCH randomized clinical trial. JAMA 2018;319:1002-12. https://doi.org/10.1001/jama.2018.0948.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Qual Saf 2015;24:228-38. https://doi.org/10.1136/bmjqs-2014-003627.
- Brady PW, Zix J, Brilli R, Wheeler DS, Griffith K, Giaccone MJ, et al. Developing and evaluating the success of a family activated medical emergency team: a quality improvement report. BMJ Qual Saf 2015;24:203-11. https://doi.org/10.1136/bmjqs-2014-003001.
- Langley A, Denis JL. Beyond evidence: the micropolitics of improvement. BMJ Qual Saf 2011;20:i43-6. https://doi.org/10.1136/bmjqs.2010.046482.
- Scholes K, Johnson G, Scholes K. Exploring Public Sector Strategy. London: Prentice-Hall; 2001.
- Carter P, Ozieranski P, McNicol S, Power M, Dixon-Woods M. How collaborative are quality improvement collaboratives: a qualitative study in stroke care. Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-32.
Appendix 1 Summary of patient and public involvement
Patient and public involvement
The aim of PPI was to gain meaningful parent perspectives and input throughout the PUMA study.
To engage with parents with experience of being in hospital with a sick child was vital to the successful conduct of the PUMA study. Prior to the study commencing, we consulted with parents of children previously admitted to paediatric intensive care, and used this to canvas opinion regarding the study proposal. Feedback was positive, with recounted instances of when signs of deterioration were not acted on and parents feeling their concerns were not acknowledged.
Methods
The PUMA study had an experienced PPI lead (Jenny Preston) who co-ordinated parent involvement throughout the study to address topics such as advising on the tool and implementation package development; information leaflets for research ethics purposes; the design of interview schedules and the data generation templates; and qualitative data analysis, particularly parent interviews and dissemination strategies.
In the original PUMA study application, we set out to form a parent advisory group made up of approximately six to eight parents who had experience of their children being in hospital, who would meet face to face on a regular basis and throughout the duration of the study. However, it proved difficult to recruit the desired six to eight parents to meet on a regular basis for a number of reasons, such as caring responsibilities, work and geographical distance. However, we did manage to recruit four parents to the group, with an additional two members joining in the final year, as two had dropped out owing to family and work commitments.
Parents were contacted through a variety of channels including social media, existing contacts and parent organisations. Parents had varied experiences of looking after a sick child with conditions such as acute lymphoblastic leukaemia, severe asthma and complex needs associated with autism, and a child who was non-verbal and deaf. All had experience of being in hospital with their child.
Meetings
Five face-to-face meetings took place in Liverpool during the lifespan of the study. The preferred form of communication in between meetings was via e-mail.
Summary of parent input into the PUMA study
Despite using a variety of recruitment methods to involve between six and eight parents with experience of attending hospital with a sick child, we managed to recruit only four parents. It also proved quite challenging to stick to our intended two meetings per year because of family and work demands on those involved. We did, however, manage to meet once per year, with e-mail discussions in between.
The PUMA study took place over a 4-year period, which generated additional challenges to keep parents motivated and interested in between meetings. This resulted in two parents leaving the advisory group in the final year, but another two joined the group as they had an interest in this area and experience of long hospital stays with their children.
Changes in the focus of the study, from PTTTs to system-wide improvements, made it more challenging for PPI members to input. Rather than being directed by the study team, PIs were responsible for identifying and addressing potential weaknesses in their paediatric early warning systems; this made it harder for the PPI group to be involved and to follow what was going on in each of the sites. In retrospect, it may have been more fruitful to create site-specific PPI groups. However, that would have presupposed that family involvement was an issue that needed addressing.
Despite these limitations, the information we gathered provided us with valuable insights into the study and potential ideas for future studies focusing on the needs of parents in hospital settings. One idea, for example, is to explore the development of a parent/carer tool to crowdsource information to define what the key things are that parents need to know when they arrive on the ward, and define what would help them feel comfortable enough to identify and raise concerns about their child.
The study team acted on most of the parent feedback received, and gave reasons why feedback could not be acted on. Table 19 presents responses to the feedback on the FFT.
| Parent advisory group feedback/suggested changes | What we have done | Notes |
|---|---|---|
| Change language used throughout document. It needs to be clearer and more family friendly (at the moment it sounds overly clinical and somewhat alienating) | Phrasing of introductory paragraph, sign-off and questions altered throughout | |
| Restructure questionnaire so that it opens with the question on what is normal for your child | Restructured as suggested – this is now the first question | |
| Include question addressing how able (or not) parents feel to communicate with staff on the ward. Ask if parents know who to speak to, and when they will be around | We have added a new Likert scale question to the tool, asking about how able parents feel to communicate with staff (question 3c) | Unfortunately, it is not within scope of the tool to ask about communication in depth as we are focused on looking for change – but we would be very interested to think about how this may be addressed elsewhere |
| Add introductory question asking upfront whether or not family have been told about how to communicate their concerns. Ask if parents have understood this, rather than ‘been informed’ | New question added as suggested (question 2a) | The purpose of the PUMA FFT is very focused: we need to find out about what ward staff are routinely doing to tell parents about the importance of their involvement – whether or not they are talking to parents about deterioration at all, and if so, how (we agree however that what needs to be addressed in subsequent studies is how we work out what forms of communication best enables parent involvement and understanding) |
| Restructure so that the questionnaire opens with question on what is normal for your child | Restructured as suggested – this is now the first substantial question (question 1) | |
| Include a question that asks parents about their experiences of orientation/introduction onto the ward (and ask same of staff via the SSAT) | Free-text question added asking parents about their experience of orientation/induction onto the ward (question 6) | |
| Include cover sheet/information sheet tailored to parents – explaining purpose of the work, why it is helpful for them to complete it, where to deposit it once complete and including an explanation of anonymity/confidentiality | We have included details on this in the introduction/sign-off (confidentiality, where to deposit form) | |
| Think about exactly how the tool will be delivered in practice (e.g. will staff be encouraged to assist those who find written English challenging, will information on this be included in the implementation guide?) and how anonymity/confidentiality will be ensured (e.g. by providing sealable envelopes and a study-branded box for forms to be deposited into as part of the implementation package) | Information on ensuring confidentiality and making deposit box available is included in the staff implementation guide |
|
Appendix 2 Systematic reviews 1 and 2: search strategy
Database search
A range of databases were searched from their inception to January 2015. An update was carried out in September 2016, and a second update in May 2018.
A preliminary search strategy was developed using a set of key papers known to the group for Ovid MEDLINE using both text words and medical subject headings. The search strategy was modified according to the indexing systems of the other databases.
| Database and database platform | Hits (n) | ||
|---|---|---|---|
| Original search results, January 2015 | Update, September 2016 | Update, May 2018 | |
| British Nursing Index (ProQuest) | 19 | 12 | 25 |
| CINAHL (EBSCOhost) | 206 | 17 | 29 |
| Cochrane Central Register of Controlled Trials (The Cochrane Library) | 43 | 4 | 30 |
| EMBASE (Ovid) | 1065 | 206 | 431 |
| HMIC (Ovid) | 70 | 1 | 75 |
| MEDLINE (Ovid) | 943 | 135 | 328 |
| MEDLINE In-Process & Other Non-Indexed Citations (Ovid) | 43 | 69 | 45 |
| Scopus (Elsevier) | 747 | 85 | 234 |
| Web of Knowledge (Science Citation Indexes) (Thomson Reuters) | 400 | 82 | 166 |
| Total (prior to removing duplicates and irrelevant studies) | 3536 | 611 | 1363 |
Supplementary search
PUMA search information
Note that each of the following searches were restricted by date: 1 January 2016 to 16 May 2018.
| Source | Hits (n) | ||
|---|---|---|---|
| January 2015 | Update, September 2016 | Update, June 2018 | |
| Trials registers | |||
| ClinicalTrials.gov (https://clinicaltrials.gov/; accessed June 2018) | 6 | 4 | 0 |
| UK Clinical Trials Gateway (www.ukctg.nihr.ac.uk/default.aspx; accessed June 2018) | 3 (duplicates) | 5 (1 duplicate) | 0 |
| The WHO trial search portal for studies worldwide (http://apps.who.int/trialsearch; accessed June 2018) | 1 (duplicate) | 0 | 0 |
| Journal site | |||
| Archives of Disease in Childhood (http://adc.bmj.com/; accessed June 2018) | 14 | 4 | 7 |
| BMJ (www.bmj.com/theBMJ; accessed June 2018) | 1 | 0 | 1 |
| BMJ Quality and safety (http://qualitysafety.bmj.com/; accessed June 2018) | 7 | 4 | 2 |
| JAMA Pediatrics (http://archpedi.jamanetwork.com/journal.aspx; accessed June 2018) | 1 | 0 | 0 |
| Journal of Critical Care (www.jccjournal.org/; accessed June 2018) | 3 | 1 | 0 |
| Journal of Pediatrics (American) (www.jpeds.com/; accessed June 2018) | 1 | 0 | 2 |
| Journal of Paediatrics and Child Health (Australian) (http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1440-1754; accessed June 2018) | 2 | 2 | 0 |
| The Lancet (www.thelancet.com/; accessed June 2018) | 0 | 0 | 0 |
| New England Journal of Medicine (www.nejm.org/; accessed June 2018) | 0 | 0 | 0 |
| Pediatrics (http://pediatrics.aappublications.org/; accessed June 2018) | 6 | 2 | 0 |
| Pediatric Critical Care Medicine (http://journals.lww.com/pccmjournal/pages/default.aspx; accessed June 2018) | 14 | 6 | 3 |
| Websites and organisations | |||
| American Society of Anesthetists (www.asahq.org/; accessed June 2018) | 1 | 0 | 0 |
| American Academy of Pediatrics (www.aap.org/en-us/Pages/Default.aspx; accessed June 2018) | 1 | 0 | |
| Association of Anaesthetists of Great Britain and Ireland (www.aagbi.org/; accessed June 2018) | 0 | 0 | 0 |
| Australian Medical Council (www.amc.org.au/; accessed June 2018) | 1 | 0 | 0 |
| Royal College of Paediatrics and Child Health (https://www.rcpch.ac.uk/; accessed June 2018) | 1 | 0 | 4 |
| Paediatric Nursing Association Europe (www.rcn.org.uk/; accessed June 2018) | 9 | 0 | |
| European Federation of Critical Care Nursing Associations (www.efccna.org/; accessed June 2018) | No search option | No search option | No search option |
| Royal Australasian College of Physicians (Division of Child Health) (www.racp.edu.au/page/paed-policy; accessed June 2018) | 0 | 0 | 0 |
| Royal College of Physicians (inclusive of National Clinical Guideline Centre) (www.rcplondon.ac.uk/; accessed June 2018) | 2 | 0 | 0 |
| NHS III (www.institute.nhs.uk/; accessed June 2018) | 4 | Site had ceased to exist | Site had ceased to exist |
| NICE: Eyes on Evidence (www.evidence.nhs.uk/about-evidence-services/bulletins-and-alerts/eyes-on-evidence; accessed June 2018) | 4 | 1 | 1 |
| Total | 82 | 30 | 20 |
Search strategies
British Nursing Index
“Paediatric Early Warning” OR (“pediatric early warning” OR “pediatric rapid response”) OR (“paediatric rapid response” OR “Bedside paediatric early warning”) OR (“Pediatric Advanced Warning Score” OR “Paediatric Advanced Warning Score”)
Cochrane Central Register of Controlled Trials
Search name: PUMA update.
Last saved: 16 May 2018, 11:39:08.703.
Description
#1 “early warning score*”
#2 “early warning system*”
#3 “early warning tool*”
#4 “VitalPAC Early Warning Score”
#5 “activation criteria”
#6 “Rapid Response Team”
#7 “Rapid Response system*”
#8 “Track and trigger”
#9 “trigger tools”
#10 “calling criteria”
#11 “Alert criteria”
#12 “Rapid Response”
#13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 pediatric* or paediatric* or infant* or child* or baby or toddler or babies or teen* or adolescent*
#15 #13 and #14
#16 “Pediatric Early Warning”
#17 “Paediatric Early Warning”
#18 “p?ediatric alert”
#19 “Pediatric Rapid Response”
#20 “Pediatric Advanced Warning Score*”
#21 “Paediatric Advanced Warning Score*”
#22 “infant early warning”
#23 “Bedside PEWS”
#24 “Bedside paediatric early warning”
#25 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24
#26 #15 or #25 Publication Year from 2016 to 2018
Cumulative Index to Nursing and Allied Health Literature via EBSCO
| Search ID# | Search terms |
|---|---|
| S11 | S7 OR S10 |
| S10 | S1 AND S8 |
| S9 | S2 AND S8 |
| S8 | S3 AND S4 |
| S7 | S5 OR S6 |
| S6 | TX “infant early warning” OR TX “bedside PEWS” OR TX “Bedside paediatric early warning” |
| S5 | TX “p?ediatric early warning system” OR TX “P?ediatric Early Warning” OR TX “p?ediatric early warning score” OR TX “p?ediatric risk of mortality” OR TX “P?ediatric Rapid Response Team” OR TX “P?ediatric alert” |
| S4 | AB pediatric* or paediatric* or infant*1 or child* or baby or toddler or babies or teen* or adolescent* |
| S3 | TX “track-and-trigger” OR TX “VitalPAC Early Warning Score” OR TX “activation criteria”. OR TX “trigger tool*” OR TX “Rapid Response” OR TX “activation criteria”. OR TX “early warning” OR TX “Alert criteria” OR TX outreach N3 emergency |
| S2 | Detecting W3 deterioration |
| S1 | “early warning” |
Database of Abstracts of Reviews of Effects
(Paediatric early warning) OR (pediatric early warning) OR (Paediatric Rapid Response) IN Database of Abstracts of Reviews of Effects (DARE).
(early warning) OR (track-and-trigger system) OR (Rapid Response) IN DARE.
(emergency team) AND (early warning) IN DARE.
EMBASE
Date range searched: 1947 to May 2018.
Search strategy
-
(“early warning” adj5 scor*).ab,ti. (568)
-
(“early warning” adj5 system* adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti. (51)
-
“acute illness severity”.mp. (38)
-
early intervention/and ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti. (1185)
-
(“early medical intervention” adj5 (tool* or scor* or index* or indicator* or indice* or assessment* or guide* or instrument* or criteria or parameter* or deteriorat* or mortality or death or monitor* or outcome* or harm* or safety)).ab,ti. (10)
-
*”severity of illness index”/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj5 ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti. (3)
-
exp Health Status Indicators/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj3 ((prevent* or reduc* or improv*) adj3 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti. (7)
-
rapid response team/(849)
-
“alarm monitor”/and (prevent* or reduc* or improv*).mp. (245)
-
(“clinical alarm” adj5 (prevent* or reduc* or improv*)).mp. (2)
-
(outreach adj3 emergency).tw. (46)
-
VitalPAC Early Warning Score.tw. (15)
-
medical emergency team.tw. (395)
-
Rapid Response Systems.mp. (140)
-
(“rapid response” adj5 (prevent* or reduc* or improv*)).tw. (191)
-
(“medical device” adj3 (prevent* or reduc* or improv*)).mp. (187)
-
(((Detecting or managing) adj3 deterioration) and warning).tw. (11)
-
track-and-trigger system.tw. (24)
-
(Track adj trigger).tw. (4)
-
(Track and trigger).tw. (241)
-
trigger tools.tw. (47)
-
(“alert criteria” or “activation criteria” or “calling criteria”).tw. (209)
-
SBAR technique*.mp. (5)
-
(score adj3 severity of illness).tw. (393)
-
or/1-24 (4295)
-
limit 25 to (infant <to one year> or child <unspecified age> or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>) (533)
-
P?ediatric Early Warning.mp. (120)
-
p?ediatric alert.tw. (7)
-
p?ediatric early warning systems.mp. (4)
-
p?ediatric risk of mortality.tw. (527)
-
P?ediatric Rapid Response Team.tw. (14)
-
Point-of-Care Systems/and ((paediatric or pediatric) adj3 (improve or identify or detect* or outcome or early or critical or emergency)).tw. (23)
-
P?ediatric Advanced Warning Score.tw. (3)
-
neonatal early warning.tw. (1)
-
infant early warning.tw. (0)
-
p?ediatric rapid response.tw. (31)
-
Bedside paediatric early warning.tw. (5)
-
Bedside PEWS.tw. (7)
-
or/27-38 (707)
-
26 or 39 (1155)
-
limit 40 to human (1065)
Health Management Information Consortium
Search strategy
-
(“early warning” adj5 scor*).ab,ti. (23)
-
(“early warning” adj5 system* adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti. (6)
-
“acute illness severity”.mp. (3)
-
“early medical intervention”/and ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti. (0)
-
(“early medical intervention” adj5 (tool* or scor* or index* or indicator* or indice* or assessment* or guide* or instrument* or criteria or parameter* or deteriorat* or mortality or death or monitor* or outcome* or harm* or safety)).ab,ti. (0)
-
Health Status Indicators.mp. and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj3 ((prevent* or reduc* or improv*) adj3 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti. (0)
-
exp “Severity of illness index”/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj5 ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti. (0)
-
“activation criteria”.ab,ti. (2)
-
exp Rapid response teams/(39)
-
Clinical Alarms.mp. (0)
-
(outreach adj3 emergency).tw. (2)
-
VitalPAC Early Warning Score.tw. (0)
-
medical emergency team.tw. (15)
-
Rapid Response Systems.mp. (8)
-
Rapid Response Team.tw. (27)
-
((Detecting or managing) adj3 deterioration).tw. (1)
-
track-and-trigger system.tw. (2)
-
(Track adj trigger).tw. (1)
-
(Track and trigger).tw. (8)
-
trigger tools.tw. (4)
-
Calling criteria.tw. (1)
-
Alert criteria.mp. (1)
-
Rapid response.tw. (111)
-
(score adj3 severity of illness).tw. (3)
-
or/1-24 (171)
-
(pediatric* or paediatric* or infant*1 or child* or baby or toddler or babies or teen* or adolescent*).mp. (40,161)
-
25 and 26 (14)
-
p?ediatric alert.tw. (0)
-
p?ediatric early warning systems.mp. (1)
-
p?ediatric risk of mortality.tw. (4)
-
Pediatric Rapid Response Team.tw. (0)
-
Point-of-Care.mp. and ((paediatric or pediatric) adj3 (improve or identify or detect* or outcome or early or critical or emergency)).tw. (0)
-
Pediatric Advanced Warning Score.tw. (0)
-
neonatal early warning.tw. (0)
-
infant early warning.tw. (0)
-
paediatric rapid response.tw. (1)
-
pediatric rapid response.tw. (0)
-
Bedside paediatric early warning.tw. (0)
-
Bedside PEWS.tw. (0)
-
p?ediatric early warning.mp. (2)
-
care.mp. and ((paediatric or pediatric) adj3 (improve or identify or detect* or outcome or early or critical or emergency)).tw. [mp = title, other title, abstract, heading words] (57)
-
or/28-41 (59)
-
27 or 42 (70)
MEDLINE via Ovid
Date range searched: 1946 to January Week 2 2015.
Search strategy
-
(“early warning” adj5 scor*).ab,ti. (260)
-
(“early warning” adj5 system* adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti. (24)
-
“acute illness severity”.mp. (21)
-
“early medical intervention”/and ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti. (99)
-
(“early medical intervention” adj5 (tool* or scor* or index* or indicator* or indice* or assessment* or guide* or instrument* or criteria or parameter* or deteriorat* or mortality or death or monitor* or outcome* or harm* or safety)).ab,ti. (7)
-
exp Health Status Indicators/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj3 ((prevent* or reduc* or improv*) adj3 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti. (166)
-
“Severity of Illness Index”/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj5 ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti. (274)
-
exp Hospitals/and ((Detecting or managing) adj3 deterioration).tw. (2)
-
(“medical device” adj3 (prevent* or reduc* or improv*)).mp. (58)
-
(“alert criteria” or “activation criteria” or “calling criteria”).tw. (121)
-
Hospital Rapid Response Team/(334)
-
Clinical Alarms/(332)
-
(outreach adj3 emergency).tw. (32)
-
VitalPAC Early Warning Score.tw. (10)
-
medical emergency team.tw. (247)
-
Rapid Response Systems.mp. (87)
-
Rapid Response Team.tw. (185)
-
(((Detecting or managing) adj3 deterioration) and warning).tw. (8)
-
track-and-trigger system.tw. (14)
-
(Track adj trigger).tw. (2)
-
(Track and trigger).tw. (137)
-
trigger tools.tw. (22)
-
SBAR technique*.mp. (3)
-
(“rapid response” adj5 (prevent* or reduc* or improv*)).tw. (117)
-
(score adj3 severity of illness).tw. (243)
-
or/1-25 (2286)
-
limit 26 to (humans and “all child (0 to 18 years)”) (453)
-
P?ediatric Early Warning.mp. (38)
-
p?ediatric alert.tw. (5)
-
p?ediatric early warning systems.mp. (3)
-
p?ediatric risk of mortality.tw. (400)
-
P?ediatric Rapid Response Team.tw. (6)
-
Point-of-Care Systems/and ((paediatric or pediatric) adj3 (improve or identify or detect* or outcome or early or critical or emergency)).tw. (79)
-
P?ediatric Advanced Warning Score.tw. (2)
-
neonatal early warning.tw. (0)
-
infant early warning.tw. (0)
-
p?ediatric rapid response.tw. (20)
-
Bedside paediatric early warning.tw. (2)
-
Bedside PEWS.tw. (2)
-
or/28-39 (542)
-
27 or 40 (943)
Scopus
(TITLE-ABS-KEY (“Paediatric Early Warning” OR “Pediatric Early Warning” OR “Pediatric Advanced Warning Score” OR “Paediatric Advanced Warning Score” OR “neonatal early warning” OR “infant early warning” OR “pediatric rapid response” OR “Paedatric rapid response”)) OR (((TITLE-ABS-KEY (“early warning” W/5 scor*)) OR (TITLE-ABS-KEY (“Rapid Response”)) OR (TITLE-ABS-KEY (“track-and-trigger system”)) OR (TITLE-ABS-KEY (“track and trigger”)) OR (TITLE-ABS-KEY (“trigger tool*”)) OR (TITLE-ABS-KEY (“alert criteria”)) OR (TITLE-ABS-KEY (“activation criteria”)) OR (TITLE-ABS-KEY (“VitalPAC Early Warning Score”))) AND (TITLE-ABS-KEY (pediatric* OR paediatric* OR infant* OR child* OR baby OR toddler OR babies OR teen* OR adolescent*))) AND (LIMIT-TO (SUBJAREA, “MEDI”) OR LIMIT-TO (SUBJAREA, “NURS”) OR LIMIT-TO (SUBJAREA, “NEUR”)).
Web of Science
| Search ID | Hits (n) | Search terms |
|---|---|---|
| # 19 | 400 |
#17 OR #1 Refined by: [excluding] WEB OF SCIENCE CATEGORIES: (PARASITOLOGY OR PUBLIC ENVIRONMENTAL OCCUPATIONAL HEALTH OR BIOCHEMISTRY MOLECULAR BIOLOGY OR OPTICS OR HEALTH CARE SCIENCES SERVICES OR MYCOLOGY OR MANAGEMENT OR LINGUISTICS OR INSTRUMENTS INSTRUMENTATION OR MICROBIOLOGY OR INFORMATION SCIENCE LIBRARY SCIENCE OR MATHEMATICAL COMPUTATIONAL BIOLOGY OR GERIATRICS GERONTOLOGY OR ENGINEERING BIOMEDICAL OR FOOD SCIENCE TECHNOLOGY OR ENVIRONMENTAL STUDIES OR ENGINEERING ENVIRONMENTAL OR ENGINEERING ELECTRICAL ELECTRONIC OR HEALTH POLICY SERVICES OR TOXICOLOGY OR EDUCATION EDUCATIONAL RESEARCH OR NUTRITION DIETETICS OR SUBSTANCE ABUSE OR ECONOMICS OR MEDICINE RESEARCH EXPERIMENTAL OR STATISTICS PROBABILITY OR DEVELOPMENTAL BIOLOGY OR MEDICAL INFORMATICS OR SOCIOLOGY OR DENTISTRY ORAL SURGERY MEDICINE OR PSYCHOLOGY EXPERIMENTAL OR COMPUTER SCIENCE ARTIFICIAL INTELLIGENCE OR METEOROLOGY ATMOSPHERIC SCIENCES OR CHEMISTRY ANALYTICAL OR MEDICAL LABORATORY TECHNOLOGY OR CELL BIOLOGY OR DEMOGRAPHY OR BUSINESS FINANCE OR COMPUTER SCIENCE INTERDISCIPLINARY APPLICATIONS OR AUDIOLOGY SPEECH LANGUAGE PATHOLOGY OR PSYCHOLOGY DEVELOPMENTAL OR COMPUTER SCIENCE INFORMATION SYSTEMS OR PLANNING DEVELOPMENT) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 18 | 499 |
#17 OR #1 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 17 | 487 |
#16 AND #15 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 16 | 8044 |
#14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 15 | 1,689,232 |
TOPIC: ((pediatric* OR paediatric* OR infant* OR child* OR baby OR toddler OR babies OR teen* OR adolescent*)) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 14 | 130 |
TOPIC: (“Severity of Illness Index” and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) SAME ((prevent* or reduc* or improv*) SAME (deteriorat* or mortality or death or outcome* or harm* or safety)))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 13 | 63 |
TOPIC: ((“early medical intervention” SAME (tool* or scor* or index* or indicator* or indice* or assessment* or guide* or instrument* or criteria or parameter* or deteriorat* or mortality or death or monitor* or outcome* or harm* or safety))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 12 | 28 |
TOPIC: (“early medical intervention” and ((prevent* or reduc* or improv*) SAME (deteriorat* or mortality or death or outcome* or harm* or safety))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 11 | 1206 |
TOPIC: (“early warning” SAME system* SAME (deteriorat* or mortality or death or outcome* or harm* or safety)) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 10 | 2 |
TOPIC: (“SBAR technique”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 9 | 7 |
TOPIC: (“VitalPAC Early Warning Score”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 8 | 123 |
TOPIC: (“activation criteria”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 7 | 16 |
TS = (“alert criteria”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 6 | 159 |
TS = (“trigger tool*”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 5 | 45 |
TS = (“track and trigger”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 4 | 15 |
TS = (“track-and-trigger system”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 3 | 6100 |
TS = (“Rapid Response”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 2 | 604 |
TS = (“early warning” SAME scor*) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 |
| # 1 | 88 |
TS = (“Paediatric Early Warning” OR “Pediatric Early Warning” OR “Pediatric Advanced Warning Score” OR “Paediatric Advanced Warning Score” OR “neonatal early warning” OR “infant early warning” OR “pediatric rapid response” OR “Paedatric rapid response”) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = 1900-2015 PUMA Supplementary searches Search terms to use: “Pediatric Early warning” “Paediatric Early warning” “Pediatric Rapid Response Team” “Paediatric Rapid Response Team” PEWS “Paediatric trigger tools” “Pediatric trigger tools” |
Appendix 3 Systematic reviews 1 and 2: population, intervention, control/comparison and outcomes criteria
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Children aged 0–18 years who are inpatients in a hospital | Adult patients; children in emergency departments or neonatal unit |
| Intervention | Development or validation of a PTTT | Acuity or triage tools, tools developed for use in emergency departments |
| Comparator | Not applicable | |
| Outcomes | Mortality and critical events, including arrests, code calls, transfer to higher level of care (e.g. ICU/HDU), senior review, RRT/MET activation, acuity at PICU admission and critical interventions on the ward or PICU | |
| Study design | Chart or case reviews; cohort studies; case–control studies, observational studies | Reviews, editorials or opinion pieces |
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Patients | Children aged 0–18 years who are inpatients in a hospital | Adult patients; children in emergency departments or neonatal units |
| Intervention | Implementation of any ‘paediatric early warning system’ intervention (with or without a PTTT) – including implementing a new PTTT, RRT/MET implementation, educational initiatives or communications tools aimed at improving identification of deteriorating inpatients | Acuity or triage tools, tools developed for use in emergency departments, interventions whose purpose was not identification of deteriorating inpatients |
| Comparator | Not applicable | |
| Outcomes | Mortality and critical events, including arrests, code calls, transfer to higher level of care (e.g. ICU/HDU), senior review, RRT/MET activation, acuity at PICU admission and critical interventions on the ward or PICU | |
| Study design | Randomised controlled trials, non-randomised controlled trials, before-and-after studies (controlled or uncontrolled); ITS studies | Reviews, editorials or opinion pieces |
Appendix 4 Systematic reviews 1 and 2: Downs and Black rating scale
| Number | Criteria | Yes (2) | Partial (1) | No (0) | N/A | Score |
|---|---|---|---|---|---|---|
| 1 | Is the hypothesis/aim/objective of the study clearly described? | Easily identified in introduction/method | Vague/incomplete or found in other parts of paper (than introduction/method) | Aim/objective not reported | ||
| 2 | Was the score developed comprehensively? | Evidence base/expert opinion/Delphi method | Decided within research team | No information/unclear | ||
| 3 | Are the characteristics of the patients in the study clearly described? | Reproducible criteria used to categorise participants | Poorly defined criteria/incomplete information | No baseline/demographic information | ||
| 4 | Is the study design well described and appropriate? | Well described, easy to find in paper | Design not clearly described/design only partially answers the question | Design poorly described or does not answer study question | ||
| 5 | Are the study sample representative of the intended population? | A full description of the target population is given with the sample selected in a non-biased manner | Sample selected from a known population; however, selection strategy probably introduces bias, but not enough to seriously distort results | Sample recruited from an unknown population in an opportunistic fashion | ||
| 6 | Are population characteristics controlled for and adequately described? | Appropriate control at design/analysis stage | Incomplete control/description, or not considered, but unlikely to seriously influence results | Not controlled for and likely to seriously influence results | ||
| 7 | Was compliance/use of the PEWS reliable? | Compliance/use was well described and reliably implemented | Compliance/use was not well described or not reliably implemented | Compliance/use was not reported | ||
| 8 | Was consideration given for data collected at different times/sites | Well-described reason why data were collected at different time points | Data were collected at different times owing to specific opportunity | No explanation for data collection at different time points | Data were collected at the same time point | |
| 9 | Are the main findings clearly described? | Simple outcome data reported for all major findings | Incomplete or inappropriate descriptive statistics | No/inadequate descriptive statistics | ||
| 10 | Are methods of analysis adequately described and appropriate? | Described and appropriate | Not reported, but probably appropriate or some tests appropriate, some not | Methods not described and cannot be determined | ||
| 11 | Are the conclusions supported by the results? | All conclusions supported by data | Some of the major conclusions are supported by the data; some are not or speculative interpretations are not indicated as such | None/few of the major conclusions are supported by the data | ||
| 12 | How were missing data handled? | Missing data were reported and handled appropriately | Missing data were reported, but unable to determine how they were handled or they were not handled appropriately | Missing data were not reported | No missing data | |
| Total | ||||||
| Number | Criteria | Yes (2) | Partial (1) | No (0) | N/A | Score |
|---|---|---|---|---|---|---|
| 1 | Is the hypothesis/aim/objective of the study clearly described? | Easily identified in introduction/method | Vague/incomplete or found in other parts of paper (than introduction/method) | Aim/objective not reported | ||
| 2 | Was the score developed comprehensively? | Evidence base/expert opinion/Delphi method | Decided within research team | No information/unclear | ||
| 3 | Are the characteristics of the patients in the study clearly described? | Reproducible criteria used to categorise participants | Poorly define criteria/incomplete information | No baseline/demographic information | ||
| 4 | Is the study design well described and appropriate? | Well described, easy to find in paper | Design not clearly described/design only partially answers the question | Design poorly described or does not answer study question | ||
| 5 | Are the study sample representative of the intended population? | A full description of the target population is given, with the sample selected in a non-biased manner | Sample selected from a known population; however, selection strategy probably introduces bias, but not enough to seriously distort results | Sample recruited from an unknown population in an opportunistic fashion | ||
| 6 | Was the PEWS well implemented? | Implementation was well reported and appropriately applied | Implementation was not well reported or not appropriate | No information/unclear | ||
| 7 | Are population characteristics controlled for and adequately described? | Appropriate control at design/analysis stage | Incomplete control/description, or not considered, but unlikely to seriously influence results | Not controlled for and likely to seriously influence results | ||
| 8 | Was compliance/use of the PEWS reliable? | Compliance/use was well described and reliably implemented | Compliance/use was not well described or not reliably implemented | Compliance/use was not reported | ||
| 9 | Was consideration given for data collected at different times/sites | Well described reason why data were collected at different time points | Data were collected at different times owing to specific opportunity | No explanation for data collection at different time points | Data were collected at the same time point | |
| 10 | Are the main findings clearly described? | Simple outcome data reported for all major findings | Incomplete or inappropriate descriptive statistics | No/inadequate descriptive statistics | ||
| 11 | Are methods of analysis adequately described and appropriate? | Described and appropriate | Not reported but probably appropriate or some tests appropriate, some not | Methods not described and cannot be determined | ||
| 12 | Are the conclusions supported by the results | All conclusions supported by data | Some of the major conclusions are supported by the data; some are not or speculative interpretations are not indicated as such | None/few of major conclusions supported by the data | ||
| 13 | How were missing data handled | Missing data were reported and handled appropriately | Missing data were reported, but unable to determine how it was handled or it was not handled appropriately | Missing data were not reported | No missing data | |
| Total | ||||||
Appendix 5 Systematic review 3: data extraction template 1
Appendix 6 Systematic review 3: search methodology
Search methods
Database search
A comprehensive search was conducted across a wide-ranging set of databases from 1995 to September 2016, which was then extended to May 2018, to identify relevant evidence/studies in English language on paediatric early warning systems (all study types).
A preliminary search strategy was developed using a set of key papers known to the group for Ovid MEDLINE using both text words and medical subject headings. A further three systematic searches were conducted across a range of databases from 1995 to September 2016 to identify relevant studies in the English language papers reporting on:
-
adult early warning systems (qualitative studies only)
-
interventions to improve situational awareness (all study types)
-
structured communication tools for handover and handoff (all study types).
The MEDLINE search strategy was translated to use across the rest of the databases.
The focus of the search strategy was to achieve high sensitivity and specificity for retrieving studies relevant to the review question. The MEDLINE search strategy was modified according to the indexing systems of the other databases.
Databases searched
-
British Nursing Index.
-
CINAHL.
-
Cochrane Central Register of Controlled Trials.
-
DARE.
-
EMBASE.
-
HMIC.
-
MEDLINE.
-
MEDLINE In-Process.
-
Scopus.
-
Web of Knowledge (Science Citation Indexes).
Additional searches
In addition to these databases, we searched both published and unpublished literature. To identify supplementary papers, information on studies in progress, unpublished research or research reported in the grey literature was identified through searching a range of relevant websites and trial registers, including ClinicalTrials.gov. To identify published resources that had not yet been catalogued in the electronic databases, recent editions of key journals were hand-searched.
Trial registers
-
ClinicalTrials.gov: https://clinicaltrials.gov/.
-
UK Clinical Trials Gateway: www.ukctg.nihr.ac.uk/default.aspx.
-
The World Health Organization trial search portal for studies worldwide: https://apps.who.int/trialsearch.
Journal websites
-
British Medical Journal: www.bmj.com/theBMJ.
-
BMJ Quality and Safety: http://qualitysafety.bmj.com/.
Websites and organisations
-
The Health Foundation.
Identify relevant studies
The search results would be imported into reference management database EndNote [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA]. Duplicate references and clearly irrelevant citations will be removed. All remaining studies will then be downloaded to Dropbox (Dropbox, Inc., San Francisco, CA, USA) for reviewers to screen for relevance.
MEDLINE search strategy: adult early warning systems
-
(“early warning” adj5 scor*).ab,ti.
-
(“early warning” adj5 system* adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti.
-
“acute illness severity”.mp.
-
“early medical intervention”/and ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti.
-
(“early medical intervention” adj5 (tool* or scor* or index* or indicator* or indice* or assessment* or guide* or instrument* or criteria or parameter* or deteriorat* or mortality or death or monitor* or outcome* or harm* or safety)).ab,ti.
-
exp Health Status Indicators/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj3 ((prevent* or reduc* or improv*) adj3 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti.
-
“Severity of Illness Index”/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj5 ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti.
-
exp Hospitals/and ((Detecting or managing) adj3 deterioration).tw.
-
(“medical device” adj3 (prevent* or reduc* or improv*)).mp.
-
(“alert criteria” or “activation criteria” or “calling criteria”).tw.
-
Hospital Rapid Response Team/
-
Clinical Alarms/
-
(outreach adj3 emergency).tw.
-
VitalPAC Early Warning Score.tw.
-
medical emergency team.tw.
-
Rapid Response Systems.mp.
-
Rapid Response Team.tw.
-
(((Detecting or managing) adj3 deterioration) and warning).tw.
-
track-and-trigger system.tw.
-
(Track adj trigger).tw.
-
(Track and trigger).tw.
-
trigger tools.tw.
-
SBAR technique*.mp.
-
(“rapid response” adj5 (prevent* or reduc* or improv*)).tw.
-
(score adj3 severity of illness).tw.
-
or/1-25
-
(qualitative or ethnograph* or thematic analysis or grounded theory or audio-recorded or transcribed or verbatim or ethnograph* or content analysis technique).ti,ab.
-
((“semi-structured” or semistructured or unstructured or informal or “in-depth” or indepth or “face-to-face” or structured or guide) adj3 (interview* or discussion* or questionnaire*)).ti,ab.
-
((field or case) adj (stud* or research)).ti,ab.
-
Focus groups/or Qualitative research/or Interviews as topic/or Interview, Psychological/or ((focus or discussion) adj group*1).ti,ab.
-
(Questionnaires/or interviews as topic/or interview, psychological/) and (experience* or predictor* or determinant* or barrier* or facilitator* or enabler* or factor* associat* or perception* or perceive* or attitude* or view*1 or viewpoint* or standpoint* or encounter* or experience* or story or stories or narrative*1 or theme*1 or opinion* or concerns or motivat* or need*1).ti,ab.
-
(cross-sectional studies/or cross-sectional survey.ti,ab. or correlation study.ti,ab.) and (predictor* or determinant* or barrier* or facilitator* or enabler* or factor* associat* or perception* or perceive* or attitude* or view*1 or viewpoint* or standpoint* or encounter* or experience* or story or stories or narrative*1 or theme*1 or opinion* or concerns or motivat* or need*).ti,ab.
-
process evaluation/or process evaluation.ti,ab.
-
mixed method*1.ti,ab.
-
((assoc* factor*1 or predictor* or determinant* or barrier* or facilitator* or enabler*) adj3 (interview* or survey* or questionnaire* or study)).ti,ab.
-
*motivation/
-
((perception* or perceive* or attitude* or view*1 or viewpoint* or standpoint* or encounter* or experience* or story or stories or narrative*1 or description* or theme* or opinion* or need*1 or concerns or motivat*) adj3 (interview* or survey* or questionnaire* or study or explor* or evaluate or investigate* or analys* or collect*)).ti,ab.
-
(themes adj3 (identif* or analy* or review or explor* or investigat*)).ti,ab.
-
“attitude of health personnel”/or *attitude to health/
-
exp emotions/
-
consumer satisfaction/
-
personal satisfaction/
-
exp professional-patient relations/
-
exp interprofessional relations/
-
“Health Services Needs and Demand”/
-
or/27-45
-
26 and 46
-
limit 47 to (english language and humans and yr = “1995 -Current”).
MEDLINE search strategy: structured communication tools for handover and handoff
-
Situation Background Assessment Recommendation.tw.
-
SBAR.mp.
-
(ABC and “collaborative care”).tw.
-
Patient Handoff/
-
Patient Discharge/and ABC.mp.
-
exp Patient Transfer/and checklist.mp.
-
(handoff adj3 communication).tw.
-
(handoff adj5 (tool or approach or technique or method)).tw.
-
(“information transfer” and “emergency care”).tw.
-
Patient Discharge/and information transfer.tw.
-
(“information transfer” and “critical care”).tw.
-
(“information transfer” and handoff).tw.
-
written checklist.tw.
-
(“Rapid Syndrome Validation Project” or RSVP).tw.
-
“Communication tools”.tw.
-
“Escalation of care”.tw.
-
or/1-16
-
limit 17 to (english language and humans and yr = “1995 -Current”).
MEDLINE search strategy: paediatric early warning systems to include observation and training
-
(“early warning” adj5 scor*).ab,ti.
-
(“early warning” adj5 system* adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti.
-
“acute illness severity”.mp.
-
“early medical intervention”/and ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety)).ab,ti.
-
(“early medical intervention” adj5 (tool* or scor* or index* or indicator* or indice* or assessment* or guide* or instrument* or criteria or parameter* or deteriorat* or mortality or death or monitor* or outcome* or harm* or safety)).ab,ti.
-
exp Health Status Indicators/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj3 ((prevent* or reduc* or improv*) adj3 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti.
-
“Severity of Illness Index”/and ((tool* or scor* or index* or indicator* or indice* or assessment* or instrument* or criteria or parameter*) adj5 ((prevent* or reduc* or improv*) adj5 (deteriorat* or mortality or death or outcome* or harm* or safety))).ab,ti.
-
exp Hospitals/and ((Detecting or managing) adj3 deterioration).tw.
-
(“medical device” adj3 (prevent* or reduc* or improv*)).mp.
-
(“alert criteria” or “activation criteria” or “calling criteria”).tw.
-
Hospital Rapid Response Team/
-
Clinical Alarms/
-
(outreach adj3 emergency).tw.
-
VitalPAC Early Warning Score.tw.
-
medical emergency team.tw.
-
Rapid Response Systems.mp.
-
Rapid Response Team.tw.
-
(((Detecting or managing) adj3 deterioration) and warning).tw.
-
track-and-trigger system.tw.
-
(Track adj trigger).tw.
-
(Track and trigger).tw.
-
trigger tools.tw.
-
SBAR technique*.mp.
-
(“Situation Background Assessment Recommendation” or SBAR).tw.
-
(patient* adj3 deteriorat*).tw.
-
(deterioration adj3 hospital).tw.
-
(patient deterioration adj4 hospital).tw.
-
(Patients adj4 adverse event).tw.
-
clinical deterioration.tw.
-
(“rapid response” adj5 (prevent* or reduc* or improv*)).tw.
-
(score adj3 severity of illness).tw.
-
Vital signs.tw.
-
or/1-26
-
P?ediatric Early Warning.mp.
-
p?ediatric alert.tw.
-
p?ediatric early warning systems.mp.
-
p?ediatric risk of mortality.tw.
-
P?ediatric Rapid Response Team.tw.
-
Point-of-Care Systems/and ((paediatric or pediatric) adj3 (improve or identify or detect* or outcome or early or critical or emergency)).tw.
-
P?ediatric Advanced Warning Score.tw.
-
neonatal early warning.tw.
-
infant early warning.tw.
-
p?ediatric rapid response.tw.
-
Bedside paediatric early warning.tw.
-
Bedside PEWS.tw.
-
or/34-45
-
33 or 46
-
Health Plan Implementation/
-
(implement* or applicat* or execute).tw.
-
(observ* or monitoring or monitor or education).tw.
-
Risk Assessment/
-
Education/
-
Education, Continuing/
-
“Hospitals, Teaching”/
-
Decision Making/
-
Safety Management/
-
Patient Simulation/
-
Awareness/
-
Knowledge/
-
*”Attitude of Health Personnel”/
-
*”Education, Medical, Continuing”/
-
*”Interdisciplinary Communication”/
-
Communication/
-
Monitoring, Physiologic/
-
Decision Making/
-
Judgment/
-
Needs Assessment/
-
Interprofessional Relations/
-
Interdisciplinary Communication/
-
((organi#ation* adj2 (structur* or form* or function* or determinant* or factors or environme nt* or process* or culture*)) and (outcome? or perform* or satisf* or efficien* or effective* or equ* or growth or develop* or justice or quality or culture* or manage* or leader*)).tw.
-
exp *psychology, industrial/or *absenteeism/or *efficiency/or *job satisfaction/or *”task performance and analysis”/or *”time and motion studies”/or *work simplification/or *time management/or *vocational guidance/
-
Decision Support Systems, Clinical/
-
Workflow/
-
or/48-73
-
Nurses/
-
Physicians/
-
(nurse* or physician* or doctor*).tw.
-
Medical Staff/
-
Medical Staff, Hospital/or Nursing Staff, Hospital/or Intensive Care Units/
-
or/75-79
-
47 and 74 and 80
-
limit 81 to (english language and humans and yr = “1995 -Current”).
MEDLINE search strategy: interventions to improve situational awareness
-
Patient Safety/(6742)
-
patient safety.tw. (13,735)
-
1 or 2 (18,307)
-
“Situation* Awareness”.tw. (488)
-
3 and 4 (69)
-
limit 5 to (english language and humans and yr = “1995 -Current”).
Appendix 7 Systematic review 3: data extraction template 2
Appendix 8 Summary of outcomes used as proxies for inpatient deterioration
| Outcome | Agreed definition |
|---|---|
| Mortality | All-cause mortality among any children admitted to the hospital children’s ward, HDU or PICU |
| Excludes children who died before arrival at A&E | |
| Cardiac arrest | A child admitted to the hospital’s children’s ward or HDU who subsequently had a cardiac arrest |
| Respiratory arrest | A child admitted to the hospital’s children’s ward or HDU who subsequently had a respiratory arrest |
| Unplanned admission to PICU | A child who has an unplanned admission to a PICU bed from the hospital’s children’s ward(s) or HDU |
Excludes:
|
|
| Unplanned admission to HDU | A child who has an unplanned admission to a designated/funded HDU bed from the hospital’s children’s ward(s) |
Excludes:
|
|
| PICU reviews | A child admitted to the hospital’s children’s ward or HDU who is reviewed by an internal member of PICU staff (tertiary) or who is the subject of a telephone call to external PICU (DGH) for advice, regardless of whether review leads to a PICU admission |
| Other medical emergency necessitating immediate assistance | A child admitted to the hospital’s children’s ward or HDU who subsequently required an arrest call/code for any emergency other than a cardiac or respiratory arrest |
| Non-ICU patient days (≤ 16 years) |
|
Appendix 9 Interrupted time series
Interrupted time series is a time series (repeated observations of a particular event collected over time) that is interrupted by intervention. In a simple version, an ITS analysis involves comparison of pre intervention with post intervention, controlling for the counterfactual baseline trend, within the same population, where the counterfactual scenario refers to predicting how the outcome would have continued over time if no intervention had been implemented (see Figure 27 for an ITS example).
FIGURE 27.
An ITS example.
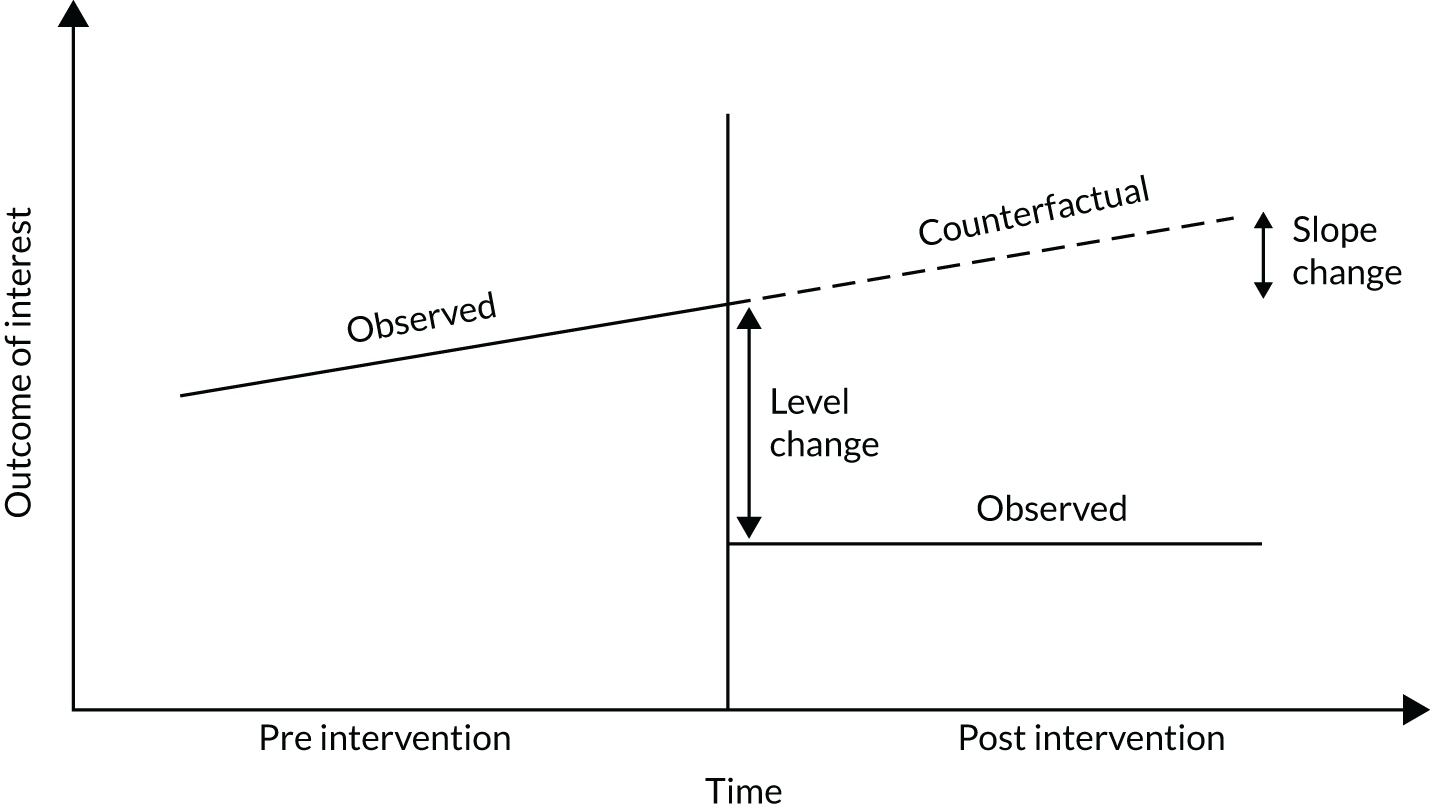
The most common method for an ITS analysis involves fitting segmented regression on an ITS data set using ARIMA method to account for correlation of data across time points. Segmented regression enables a researcher to measure statistically the changes in immediate (level) changes and trend (slope). We used segmented linear regression to analyse data from our study.
A minimum of three variables are required for an ITS analysis: (1) T – the time elapsed since the start of the study in with the unit representing the frequency with which observations are taken (e.g. month or year); (2) Xt – a dummy variable indicating the pre-intervention period (coded 0) or the post-intervention period (coded 1); and (3) Yt – the outcome at time t.
In standard ITS analyses, the following segmented regression model is used:
where β0 represents the baseline level at T = 0, β1 is interpreted as the change in outcome associated with a time unit increase (representing the underlying pre-intervention trend), β2 is the level change following the intervention and β3 indicates the slope change following the intervention (using the interaction between time and intervention (TXt).
Appendix 10 Summary of exploratory and sensitivity analyses
Exploratory analysis
Primary outcomes from each hospital were analysed using common approach, that is data from the implementation period were excluded from the analysis and changes in level and slope were examined by comparing data from the pre- and post-implementation period. In addition, we examined changes in the level and slope of trajectory by fitting the ITS model at each individual month of the implementation period, resulting in 12 separate models per site. The reason for the latter analysis was to assess the pattern of changes in level and slope from the start of implementation phase until the end, given the potential for the different local initiatives to exert their effects over different time periods in different sites.
Sensitivity analysis
As the outcomes are counts, segmented Poisson regression might be considered appropriate. Therefore, segmented Poisson regression was also used to analyse primary outcomes as a sensitivity analysis. Zero inflated segmented Poisson regression was used if there are many zero counts in the primary outcome. Of note, Poisson regression has memoryless characteristic, which means there is no need to model residuals to account for autocorrelation. However, the interpretation of the results is not as straightforward as linear regression. With regards to formulation, there are two big differences from the one introduced in equation 1: the required link function is log function rather than identity function, and log (person bed-days) needs to be added to the left side of the equation as an offset term.
Results
Alder Hey
Exploratory analysis 1
This analysis showed a pre-intervention upwards trajectory in adverse events (β = 0.02, 95% CI 0.00 to 0.03), compared with a downwards trend in adverse events in the post-intervention period, which was significantly different from the pre-intervention trajectory (β = –0.09, 95% CI –0.16 to –0.01; p = 0.03).
FIGURE 28.
Alder Hey exploratory analysis 1.
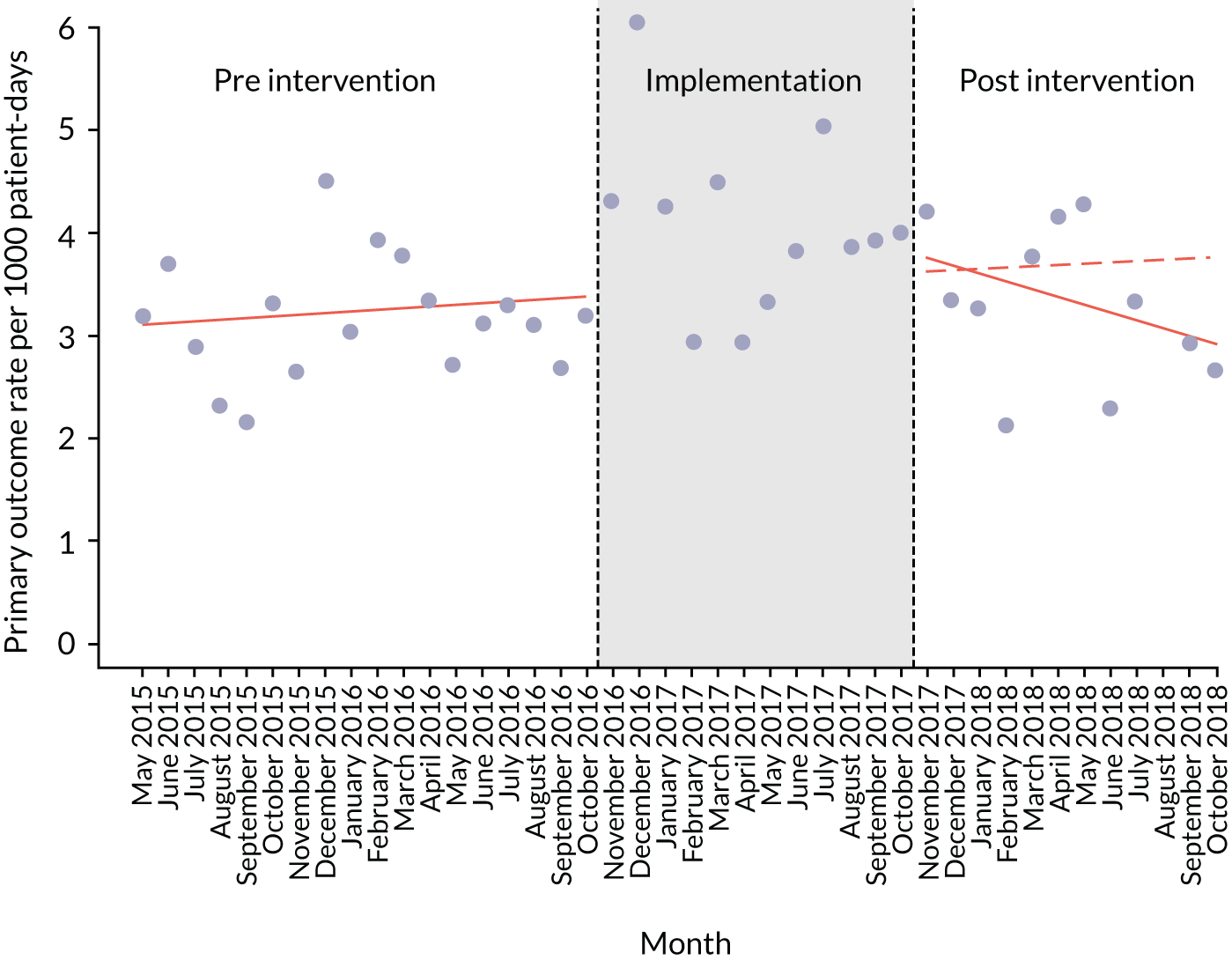
Exploratory analysis 2
In this analysis, we explored the effect of choosing different cut-off points for the pre- and post-intervention periods, across each of the 12 months of the implementation period. This created 13 separate models, with different pre- and post-intervention inflection points. Each of these cut-off points showed a significant downwards trajectory for post-intervention trends of adverse events, relative to a slight upwards trend in the pre-intervention period.
| Cut-off point | Test | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| October 2016 | Intercept | 3.06 | 2.72 to 3.41 | < 0.00001 |
| Pre-intervention trend | 0.01 | –0.02 to 0.05 | 0.50 | |
| Immediate effect of intervention (change in level) | 0.85 | 0.39 to 1.31 | < 0.001 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.05 | –0.09 to –0.02 | < 0.01 | |
| November 2016 | Intercept | 2.95 | 2.68 to 3.22 | < 0.00001 |
| Pre-intervention trend | 0.03 | 0.00 to 0.05 | < 0.05 | |
| Immediate effect of intervention (change in level) | 0.76 | 0.38 to 1.15 | < 0.001 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.07 | –0.10 to –0.05 | < 0.01 | |
| December 2016 | Intercept | 2.96 | 2.50 to 3.43 | < 0.00001 |
| Pre-intervention trend | 0.02 | –0.02 to 0.06 | 0.27 | |
| Immediate effect of intervention (change in level) | 0.65 | 0.01 to 1.29 | 0.05 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.07 | –0.12 to –0.02 | 0.01 | |
| January 2017 | Intercept | 3.07 | 2.94 to 3.20 | < 0.00001 |
| Pre-intervention trend | 0.02 | 0.01 to 0.03 | 0.01 | |
| Immediate effect of intervention (change in level) | 0.76 | 0.62 to 0.91 | < 0.00001 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.07 | –0.09 to –0.06 | < 0.00001 | |
| February 2017 | Intercept | 3.07 | 2.94 to 3.20 | < 0.00001 |
| Pre-intervention trend | 0.02 | 0.01 to 0.03 | < 0.00001 | |
| Immediate effect of intervention (change in level) | 0.75 | 0.61 to 0.91 | 0.01 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.08 | –0.09 to –0.06 | < 0.00001 | |
| March 2017 | Intercept | 2.91 | 2.68 to 3.13 | < 0.00001 |
| Pre-intervention trend | 0.03 | 0.01 to 0.05 | 0.01 | |
| Immediate effect of intervention (change in level) | 0.57 | 0.15 to 0.98 | < 0.00001 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.09 | –0.11 to –0.07 | < 0.00001 | |
| April 2017 | Intercept | 3.00 | 2.88 to 3.12 | < 0.00001 |
| Pre-intervention trend | 0.02 | 0.01 to 0.04 | < 0.00001 | |
| Immediate effect of intervention (change in level) | 0.68 | 0.47 to 0.91 | < 0.00001 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.10 | –0.11 to –0.09 | < 0.00001 | |
| May 2017 | Intercept | 2.99 | 2.87 to 3.10 | < 0.00001 |
| Pre-intervention trend | 0.03 | 0.01 to 0.04 | < 0.00001 | |
| Immediate effect of intervention (change in level) | 0.68 | 0.41 to 0.94 | < 0.00001 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.11 | –0.13 to –0.06 | < 0.00001 | |
| June 2017 | Intercept | 2.94 | 2.83 to 3.06 | < 0.00001 |
| Pre-intervention trend | 0.03 | 0.02 to 0.04 | < 0.00001 | |
| Immediate effect of intervention (change in level) | 0.58 | 0.26 to 0.90 | < 0.00001 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.12 | –0.14 to –0.10 | < 0.00001 | |
| July 2017 | Intercept | 2.87 | 2.70 to 3.03 | < 0.00001 |
| Pre-intervention trend | 0.04 | 0.02 to 0.04 | < 0.00001 | |
| Immediate effect of intervention (change in level) | 0.25 | –0.19 to 0.70 | 0.27 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.12 | –0.15 to –0.08 | < 0.00001 | |
| August 2017 | Intercept | 2.86 | 2.70 to 3.01 | < 0.00001 |
| Pre-intervention trend | 0.04 | 0.03 to 0.05 | 0.01 | |
| Immediate effect of intervention (change in level) | 0.11 | –0.36 to 0.58 | 0.64 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.11 | –0.16 to –0.07 | < 0.00001 | |
| September 2017 | Intercept | 2.87 | 2.78 to 3.21 | < 0.00001 |
| Pre-intervention trend | 0.04 | 0.03 to 0.04 | 0.01 | |
| Immediate effect of intervention (change in level) | 0.12 | –0.33 to 0.57 | 0.60 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.13 | –0.18 to –0.09 | < 0.00001 | |
| October 2017 | Intercept | 2.82 | 2.68 to 2.97 | < 0.00001 |
| Pre-intervention trend | 0.04 | 0.03 to 0.05 | < 0.00001 | |
| Immediate effect of intervention (change in level) | –0.31 | –0.80 to 0.19 | 0.23 | |
| Change in slope (post-intervention period vs. pre-intervention period) | –0.10 | –0.16 to –0.04 | < 0.00001 |
Sensitivity analysis
In this section, the results of fitting Poisson regression on primary outcome are presented. The results from Poisson regression are fairly similar to those from linear regression. Even though change in slope (post-intervention period vs. implementation period) is in the same direction, it is no longer significant.
| Test | Estimate | 95% CI | p-value |
|---|---|---|---|
| Intercept | –5.78 | –6.00 to –5.56 | < 0.00001 |
| Trend pre intervention | 0.00 | –0.02 to 0.02 | 0.83 |
| Immediate effect after start of intervention (change in level) | 0.18 | –0.16 to 0.52 | 0.30 |
| Change in slope (implementation period vs. pre-intervention period) | 0.00 | –0.04 to 0.04 | 0.98 |
| Immediate effect of intervention (change in level) | –0.05 | –0.38 to 0.28 | 0.78 |
| Change in slope (post-intervention period vs. implementation period) | –0.02 | –0.07 to 0.03 | 0.33 |
Arrowe Park Hospital
Exploratory analysis 1
No exploratory analysis was possible because of the small sample size.
Exploratory analysis 2
For this site, because of the small sample size, we could fit only seven monthly models. No significant trend was observed.
| Cut-off point | Test | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| October 2016 | Intercept | 6.73 | 5.18 to 8.28 | 0.00 |
| Trend pre intervention | –0.09 | –0.35 to 0.16 | 0.49 | |
| Immediate effect of intervention (change in level) | –0.47 | –2.52 to 1.58 | 0.66 | |
| Change in trend post intervention | –0.01 | –0.29 to 0.27 | 0.93 | |
| December 2016 | Intercept | 5.63 | 1.02 to 10.23 | 0.02 |
| Trend pre intervention | 0.16 | –0.59 to 0.90 | 0.66 | |
| Immediate effect of intervention (change in level) | –2.87 | –8.76 to 3.03 | 0.32 | |
| Change in trend post intervention | –0.15 | –1.13 to 0.84 | 0.76 | |
| February 2017 | Intercept | 6.92 | 6.07 to 7.77 | 0.00 |
| Trend pre intervention | –0.13 | –0.26 to –0.01 | 0.05 | |
| Immediate effect of intervention (change in level) | –0.04 | –1.30 to 1.23 | 0.95 | |
| Change in trend post intervention | 0.00 | –0.17 to 0.16 | 0.98 | |
| April 2017 | Intercept | 6.91 | 5.66 to 8.16 | 0.00 |
| Trend pre intervention | –0.13 | –0.30 to 0.05 | 0.16 | |
| Immediate effect of intervention (change in level) | –0.21 | –2.39 to 1.97 | 0.85 | |
| Change in trend post intervention | 0.02 | –0.30 to 0.33 | 0.92 | |
| June 2017 | Intercept | 6.69 | 5.54 to 7.83 | 0.00 |
| Trend pre intervention | –0.09 | –0.24 to 0.05 | 0.23 | |
| Immediate effect of intervention (change in level) | –1.19 | –3.57 to 1.19 | 0.34 | |
| Change in trend post intervention | 0.14 | –0.26 to 0.54 | 0.49 | |
| August 2017 | Intercept | 7.02 | 5.94 to 8.10 | 0.00 |
| Trend pre intervention | –0.15 | –0.28 to –0.02 | 0.04 | |
| Immediate effect of intervention (change in level) | 0.06 | –2.71 to 2.82 | 0.97 | |
| Change in trend post intervention | 0.02 | –0.54 to 0.58 | 0.96 | |
| October 2017 | Intercept | 6.95 | 5.93 to 7.98 | 0.00 |
| Trend pre intervention | –0.14 | –0.25 to –0.02 | 0.03 | |
| Immediate effect of intervention (change in level) | –0.40 | –3.89 to 3.08 | 0.82 | |
| Change in trend post intervention | 0.12 | –0.75 to 0.99 | 0.79 |
Sensitivity analysis
The results of fitting zero-inflated Poisson regression on primary outcome using original data (i.e. not 2-monthly data) are reported. Similar to the primary analysis of the primary outcome, zero-inflated segmented Poisson regression does not show any significant trend change in the pre-intervention, implementation or post-intervention phases. In addition, by every unit increase in month, the log odds of inflated zero increases by 0.37, which is not significant.
| Test | Estimate | 95% CI | p-value |
|---|---|---|---|
| Intercept | –5.01 | –5.61 to –4.41 | < 0.00001 |
| Trend pre intervention | –0.01 | –0.06 to 0.05 | 0.80 |
| Immediate effect after start of intervention (change in level) | 0.28 | –0.61 to 1.17 | 0.54 |
| Change in trend implementation | –0.04 | –0.17 to 0.08 | 0.49 |
| Immediate effect of intervention (change in level) | 0.18 | –0.78 to 1.15 | 0.71 |
| Change in trend post intervention | 0.08 | –0.06 to 0.22 | 0.26 |
| Log odds of inflated zero | 0.37 | –0.01 to 0.75 | 0.05 |
Noah’s Ark Hospital
Exploratory analysis 1
In this analysis, the downwards trend in adverse events in the post-intervention period did not reach significance when compared with the pre-intervention trajectory (β = –0.20, 95% CI –0.65 to 0.25; p = 0.37).
FIGURE 29.
Noah’s Ark exploratory analysis 1.
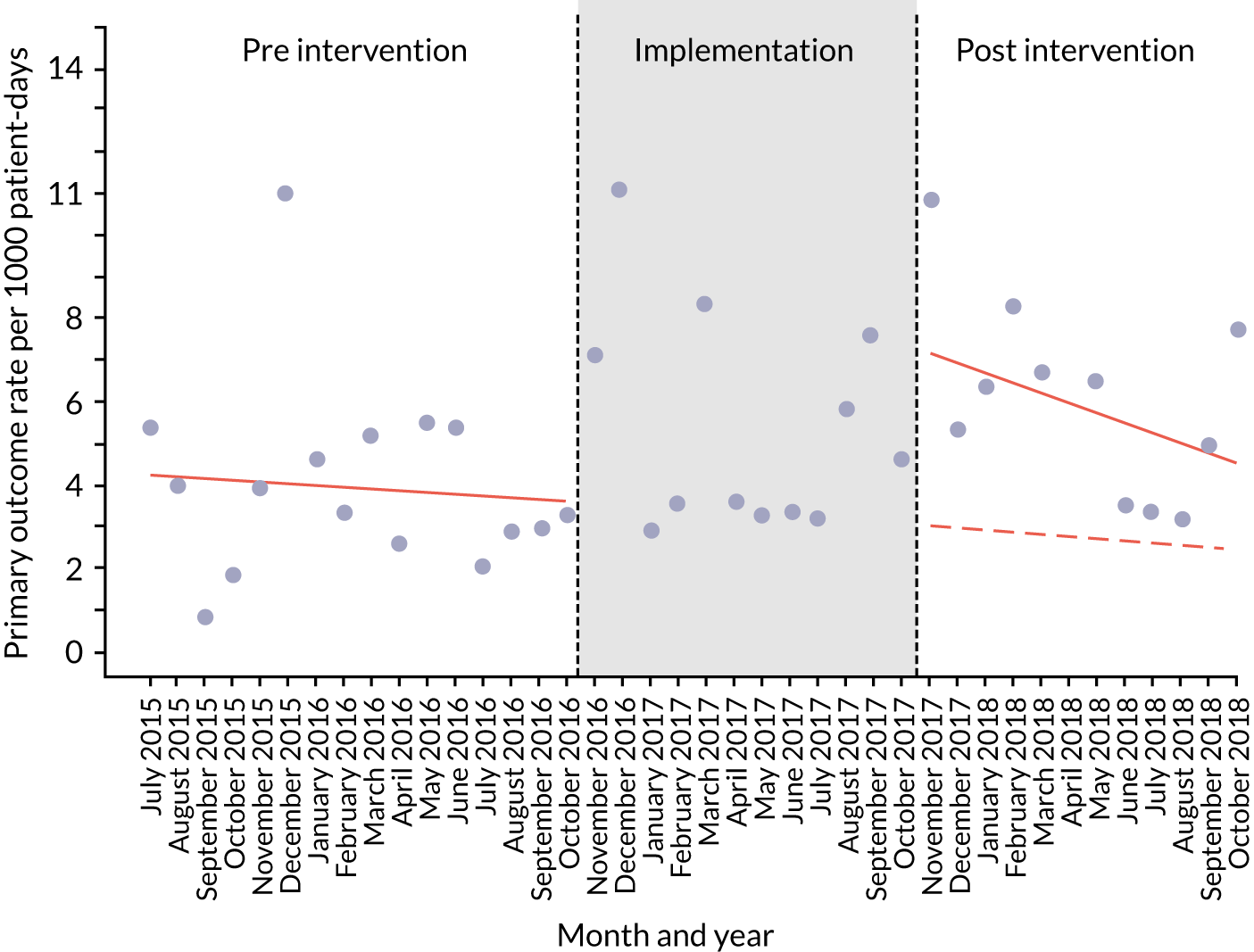
Exploratory analysis 2
In the second analysis, we explored the effect of choosing different cut-off points for the pre- and post-intervention periods, across each of the 12 months of the implementation period. This created 13 separate models, with different pre- and post-intervention inflection points. From June 2017, there was a significant downwards trajectory for post-intervention trends of adverse events, relative to a slight upwards trend in the pre-intervention period.
| Cut-off point | Test | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| October 2016 | Intercept | 3.00 | 1.71 to 4.28 | < 0.00001 |
| Trend pre intervention | 0.05 | –0.07 to 0.18 | 0.41 | |
| Immediate effect of intervention (change in level) | 0.25 | –1.21 to 1.72 | 0.74 | |
| Change in trend post intervention | 0.01 | –0.12 to 0.14 | 0.88 | |
| November 2016 | Intercept | 3.06 | 1.90 to 4.23 | < 0.00001 |
| Trend pre intervention | 0.04 | –0.06 to 0.15 | 0.43 | |
| Immediate effect of intervention (change in level) | 0.48 | –0.90 to 1.85 | 0.50 | |
| Change in trend post intervention | 0.01 | –0.11 to 0.13 | 0.86 | |
| December 2016 | Intercept | 2.90 | 1.81 to 3.99 | < 0.00001 |
| Trend pre intervention | 0.06 | –0.03 to 0.16 | 0.21 | |
| Immediate effect of intervention (change in level) | 0.17 | –1.17 to 1.51 | 0.80 | |
| Change in trend post intervention | 0.00 | –0.11 to 0.11 | 0.98 | |
| January 2017 | Intercept | 2.90 | 1.81 to 3.99 | < 0.00001 |
| Trend pre intervention | 0.06 | –0.03 to 0.16 | 0.21 | |
| Immediate effect of intervention (change in level) | 0.17 | –1.17 to 1.51 | 0.80 | |
| Change in trend post intervention | 0.00 | –0.11 to 0.11 | 0.98 | |
| February 2017 | Intercept | 3.04 | 2.06 to 4.01 | < 0.00001 |
| Trend pre intervention | 0.04 | –0.03 to 0.12 | 0.28 | |
| Immediate effect of intervention (change in level) | 0.79 | –0.52 to 2.09 | 0.24 | |
| Change in trend post intervention | –0.01 | –0.11 to 0.09 | 0.84 | |
| March 2017 | Intercept | 2.95 | 2.02 to 3.88 | < 0.00001 |
| Trend pre intervention | 0.05 | –0.02 to 0.12 | 0.14 | |
| Immediate effect of intervention (change in level) | 0.64 | –0.65 to 1.93 | 0.34 | |
| Change in trend post intervention | –0.02 | –0.12 to 0.08 | 0.68 | |
| April 2017 | Intercept | 2.96 | 2.07 to 3.84 | < 0.00001 |
| Trend pre intervention | 0.05 | –0.01 to 0.12 | 0.10 | |
| Immediate effect of intervention (change in level) | 0.78 | –0.48 to 2.05 | 0.23 | |
| Change in trend post intervention | –0.04 | –0.14 to 0.07 | 0.50 | |
| May 2017 | Intercept | 3.01 | 2.17 to 3.85 | < 0.00001 |
| Trend pre intervention | 0.05 | –0.01 to 0.11 | 0.09 | |
| Immediate effect of intervention (change in level) | 1.07 | –0.15 to 2.30 | 0.09 | |
| Change in trend post intervention | –0.06 | –0.16 to 0.04 | 0.28 | |
| June 2017 | Intercept | 3.09 | 2.29 to 3.89 | < 0.00001 |
| Trend pre intervention | 0.05 | 0.00 to 0.10 | 0.07 | |
| Immediate effect of intervention (change in level) | 1.38 | 0.16 to 2.61 | 0.03 | |
| Change in trend post intervention | –0.09 | –0.20 to 0.02 | 0.11 | |
| July 2017 | Intercept | 3.10 | 2.31 to 3.88 | < 0.00001 |
| Trend pre intervention | 0.05 | 0.00 to 0.10 | 0.04 | |
| Immediate effect of intervention (change in level) | 1.49 | 0.22 to 2.76 | 0.03 | |
| Change in trend post intervention | –0.12 | –0.24 to –0.01 | 0.04 | |
| August 2017 | Intercept | 3.17 | 2.43 to 3.92 | < 0.00001 |
| Trend pre intervention | 0.05 | 0.01 to 0.09 | 0.02 | |
| Immediate effect of intervention (change in level) | 1.80 | 0.50 to 3.10 | 0.01 | |
| Change in trend post intervention | –0.18 | –0.30 to –0.05 | 0.01 | |
| September 2017 | Intercept | 3.10 | 2.38 to 3.83 | < 0.00001 |
| Trend pre intervention | 0.06 | 0.02 to 0.10 | 0.01 | |
| Immediate effect of intervention (change in level) | 1.73 | 0.36 to 3.10 | 0.02 | |
| Change in trend post intervention | –0.21 | –0.36 to –0.06 | 0.01 | |
| October 2017 | Intercept | 3.12 | 2.41 to 3.82 | < 0.00001 |
| Trend pre intervention | 0.06 | 0.02 to 0.10 | < 0.00001 | |
| Immediate effect of intervention (change in level) | 1.92 | 0.48 to 3.36 | 0.01 | |
| Change in trend post intervention | –0.27 | –0.45 to –0.10 | < 0.00001 |
Sensitivity analysis
Apart from the pre-intervention level and level change in post intervention, the results from Poisson regression are fairly similar to those from linear regression. Even though change in slope (post-intervention period vs. implementation period) is in the same direction, it is no longer significant.
| Test | Estimate | 95% CI | p-value |
|---|---|---|---|
| Intercept | –5.59 | –5.99 to –5.20 | < 0.00001 |
| Trend pre intervention | 0.00 | –0.04 to 0.04 | 0.93 |
| Immediate effect after start of intervention (change in level) | 0.29 | –0.27 to 0.84 | 0.31 |
| Change in trend implementation | 0.00 | –0.07 to 0.07 | 0.99 |
| Immediate effect of intervention (change in level) | 0.22 | –0.40 to 0.84 | 0.49 |
| Change in trend post intervention | –0.01 | –0.08 to 0.06 | 0.69 |
Morriston Hospital
Exploratory analysis 1
This analysis showed a slight downwards trajectory in adverse events during the pre-intervention period that was not significant (β = –0.14, 95% CI –1.12 to 0.85; p = 0.78). In the post-intervention period, there was a slight downwards change in the post-intervention slope, compared with the trajectory, but this was not significant (β = –0.10, 95% CI –1.90 to 1.71; p = 0.91).
FIGURE 30.
Morriston Hospital exploratory analysis 1.
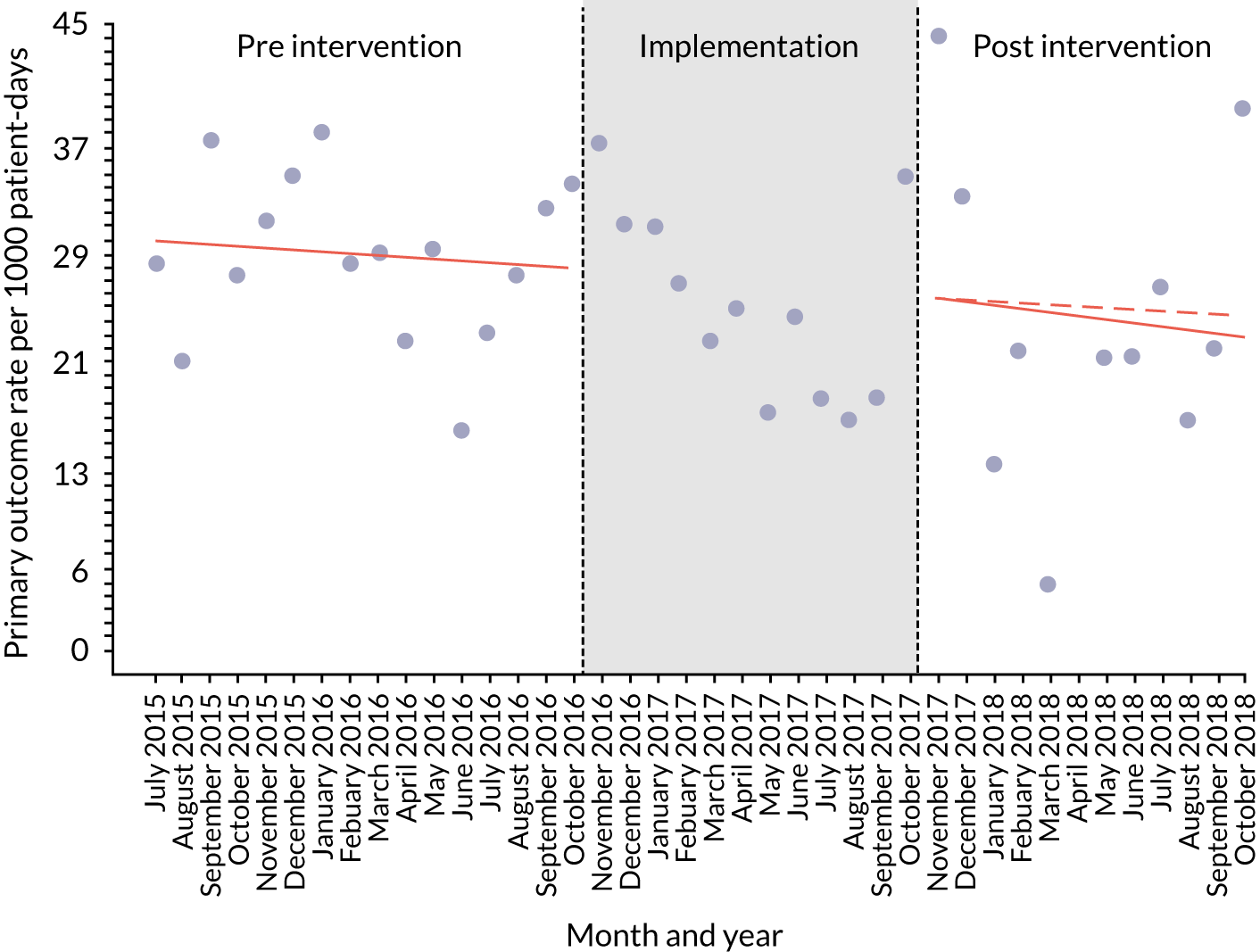
Exploratory analysis 2
There was a small, but non-significant, change in post-intervention trend in all the models.
| Cut-off point | Test | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| October 2016 | Intercept | 28.42 | 25.62 to 31.22 | < 0.00001 |
| Trend pre intervention | 0.07 | –0.22 to 0.36 | 0.64 | |
| Immediate effect of intervention (change in level) | –3.27 | –6.36 to –0.19 | 0.04 | |
| Change in trend post intervention | –0.26 | –0.54 to 0.01 | 0.07 | |
| November 2016 | Intercept | 29.21 | 26.92 to 31.50 | < 0.00001 |
| Trend pre intervention | –0.03 | –0.25 to 0.18 | 0.77 | |
| Immediate effect of intervention (change in level) | –3.20 | –6.07 to –0.33 | 0.04 | |
| Change in trend post intervention | –0.13 | –0.34 to 0.08 | 0.24 | |
| December 2016 | Intercept | 29.47 | 27.37 to 31.57 | < 0.00001 |
| Trend pre intervention | –0.06 | –0.25 to 0.12 | 0.50 | |
| Immediate effect of intervention (change in level) | –3.30 | –6.11 to –0.50 | 0.03 | |
| Change in trend post intervention | –0.07 | –0.27 to 0.13 | 0.49 | |
| January 2017 | Intercept | 29.47 | 27.37 to 31.57 | < 0.00001 |
| Trend pre intervention | –0.06 | –0.25 to 0.12 | 0.50 | |
| Immediate effect of intervention (change in level) | –3.37 | –6.21 to –0.53 | 0.03 | |
| Change in trend post intervention | –0.07 | –0.27 to 0.13 | 0.49 | |
| February 2017 | Intercept | 29.47 | 27.37 to 31.57 | < 0.00001 |
| Trend pre intervention | –0.06 | –0.25 to 0.12 | 0.50 | |
| Immediate effect of intervention (change in level) | –3.37 | –6.21 to –0.53 | 0.03 | |
| Change in trend post intervention | –0.07 | –0.27 to 0.13 | 0.49 | |
| March 2017 | Intercept | 29.51 | 27.40 to 31.62 | < 0.00001 |
| Trend pre intervention | –0.07 | –0.26 to 0.12 | 0.48 | |
| Immediate effect of intervention (change in level) | –3.42 | –6.31 to –0.53 | 0.03 | |
| Change in trend post intervention | –0.07 | –0.27 to 0.13 | 0.52 | |
| April 2017 | Intercept | 29.53 | 27.40 to 31.66 | < 0.00001 |
| Trend pre intervention | –0.07 | –0.26 to 0.12 | 0.48 | |
| Immediate effect of intervention (change in level) | –3.47 | –6.42 to –0.51 | 0.03 | |
| Change in trend post intervention | –0.06 | –0.27 to 0.14 | 0.54 | |
| May 2017 | Intercept | 29.57 | 27.41 to 31.72 | < 0.00001 |
| Trend pre intervention | –0.07 | –0.26 to 0.12 | 0.47 | |
| Immediate effect of intervention (change in level) | –3.49 | –6.52 to –0.46 | 0.03 | |
| Change in trend post intervention | –0.06 | –0.28 to 0.16 | 0.58 | |
| June 2017 | Intercept | 29.61 | 27.43 to 31.79 | < 0.00001 |
| Trend pre intervention | –0.08 | –0.27 to 0.12 | 0.45 | |
| Immediate effect of intervention (change in level) | –3.50 | –6.62 to –0.38 | 0.03 | |
| Change in trend post intervention | –0.06 | –0.29 to 0.18 | 0.63 | |
| July 2017 | Intercept | 29.65 | 27.44 to 31.85 | < 0.00001 |
| Trend pre intervention | –0.08 | –0.28 to 0.12 | 0.44 | |
| Immediate effect of intervention (change in level) | –3.50 | –6.70 to –0.30 | 0.04 | |
| Change in trend post intervention | –0.06 | –0.31 to 0.20 | 0.67 | |
| August 2017 | Intercept | 29.71 | 27.48 to 31.93 | < 0.00001 |
| Trend pre intervention | –0.08 | –0.29 to 0.12 | 0.42 | |
| Immediate effect of intervention (change in level) | –3.47 | –6.76 to –0.18 | 0.05 | |
| Change in trend post intervention | –0.05 | –0.33 to 0.23 | 0.73 | |
| September 2017 | Intercept | 29.77 | 27.53 to 32.01 | < 0.00001 |
| Trend pre intervention | –0.09 | –0.29 to 0.11 | 0.39 | |
| Immediate effect of intervention (change in level) | –3.42 | –6.79 to –0.05 | 0.05 | |
| Change in trend post intervention | –0.04 | –0.35 to 0.27 | 0.79 | |
| October 2017 | Intercept | 31.29 | 29.71 to 32.87 | < 0.00001 |
| Trend pre intervention | –0.25 | –0.35 to –0.15 | < 0.00001 | |
| Immediate effect of intervention (change in level) | –2.61 | –7.90 to 2.68 | 0.34 | |
| Change in trend post intervention | 0.48 | –0.22 to 1.19 | 0.19 |
Sensitivity analysis
Similar to the segmented linear regression, segmented Poisson regression did not show any significant trend in the Morriston data set.
| Test | Estimate | 95% CI | p-value |
|---|---|---|---|
| Intercept | –3.50 | –3.72 to –3.28 | < 0.00001 |
| Trend pre intervention | 0.00 | –0.03 to 0.02 | 0.70 |
| Immediate effect after start of intervention (change in level) | 0.14 | –0.21 to 0.50 | 0.43 |
| Change in trend implementation | –0.04 | –0.09 to 0.01 | 0.11 |
| Immediate effect of intervention (change in level) | 0.35 | –0.09 to 0.79 | 0.12 |
| Change in trend post intervention | 0.03 | –0.03 to 0.09 | 0.39 |
Appendix 11 Summary study of characteristics of validation and effectiveness papers in reviews 1 and 2
| Characteristic | n | % |
|---|---|---|
| Validation studies (N = 36) | ||
| Type | ||
| Full text | 22 | 61.1 |
| Abstract | 14 | 38.9 |
| Country | ||
| USA | 15 | 41.7 |
| UK | 12 | 33.3 |
| Canada | 2 | 5.5 |
| Australia | 0 | 0.0 |
| Other | 5 | 13.9 |
| Multiple | 1 | 2.8 |
| Unclear | 1 | 2.8 |
| Year of study | ||
| Pre 2012 | 10 | 27.8 |
| 2012 | 3 | 8.3 |
| 2013 | 6 | 16.7 |
| 2014 | 5 | 13.9 |
| 2015 | 7 | 19.4 |
| 2016 | 2 | 5.6 |
| 2017 | 3 | 8.3 |
| 2018 | 0 | 0.0 |
| Setting | ||
| Specialist/tertiary | 33 | 91.7 |
| Non-specialist/community | 0 | 0.0 |
| Unclear | 3 | 8.3 |
| Single/multicentre | ||
| Single centre | 35 | 97.2 |
| Multicentre | 1 | 2.8 |
| Study population | ||
| General inpatients | 23 | 63.9 |
| Specialist population | 11 | 30.6 |
| Unclear | 2 | 5.6 |
| Study design | ||
| Case-control | 18 | 50.0 |
| Case/chart review | 10 | 27.8 |
| Cohort | 7 | 19.4 |
| Pilot study | 1 | 2.8 |
| Effectiveness studies (N = 30) | ||
| Type | ||
| Full text | 21 | 70.0 |
| Abstract | 9 | 30.0 |
| Country | ||
| USA | 18 | 60.0 |
| UK | 3 | 10.0 |
| Canada | 2 | 6.7 |
| Australia | 3 | 10.0 |
| Other | 3 | 10.0 |
| Multiple | 1 | 3.3 |
| Unclear | 0 | 0.0 |
| Year of study | ||
| Pre 2012 | 15 | 50.0 |
| 2012 | 1 | 3.3 |
| 2013 | 2 | 6.7 |
| 2014 | 6 | 20.0 |
| 2015 | 0 | 0.0 |
| 2016 | 2 | 6.7 |
| 2017 | 1 | 3.3 |
| 2018 | 3 | 10.0 |
| Setting | ||
| Specialist/tertiary | 29 | 96.7 |
| Non-specialist/community | 1 | 3.3 |
| Unclear | 0 | 0.0 |
| Single/multicentre | ||
| Single centre | 28 | 93.3 |
| Multicentre | 2 | 6.7 |
| Study population | ||
| General inpatients | 20 | 66.6 |
| Specialist population | 5 | 16.7 |
| Unclear | 5 | 16.7 |
| Study design | ||
| Uncontrolled before-and-after | 26 | 86.7 |
| Controlled before-and-after | 1 | 3.3 |
| ITS | 2 | 6.7 |
| Cluster randomised trial | 1 | 3.3 |
Appendix 12 Range of physiological and behavioural parameters underpinning paediatric track-and-trigger tools
| PTTT name | Development/modification details | Score/trigger | Choice of thresholds/parameters | Age-dependent thresholds? | Number of items in the toola | PTTT parameters | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Respiratory rate | Heart rate | Respiratory effort/distress | LOC/behaviour | Oxygen saturation | Capillary refill time | Oxygen therapy | Systolic blood pressure | Pain | Staff concern | Skin colour | Airway problems | Temperature | Pulses | Family concern | Other items | ||||||
| Paediatric early warning system score and derivatives | |||||||||||||||||||||
| Paediatric Early Warning System (PEWS) score18,54 | Developed for use in Canadian tertiary centre.18 Nurse-generated candidate items reduced by focus groups/Delphi and evaluation with clinical data set (code blue calls, n = 87; controls, n = 128). Development and validation data sets not independent | Score | Expert opinion | Yes | 16 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Bolus fluid, medications, home oxygen, any previous admission to an ICU, central venous line in situ, transplant recipient, severe cerebral palsy, gastrostomy tube, > 3 medical specialties involved in care | |||||
| Bedside Paediatric Early Warning Score (PEWS)45,50,51,54,70,73,78,88,93,99 | Developed for use in US tertiary centre.19 Routinely collected items assessed for discriminatory ability using clinical data set (PICU admission, n = 60; controls, n = 120). Development and validation set not independent | Score | Expert opinion | Yes | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Modified Bedside PEWS (a)56 | Modification to Bedside PEWS for use in Dutch tertiary centre. Added temperature; modified wording of respiratory effort and oxygen therapy items | Score | Expert opinion | Yes | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Modified Bedside PEWS (b)75 | Modification to Bedside PEWS for use in US tertiary centre. Changed normal thresholds for heart rate and respiratory rate based on analysis of local clinical data | Score | Heart rate/respiratory data driven | Yes | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Brighton PEWS and derivatives | |||||||||||||||||||||
| Brighton PEWS38,83 | Initial development for use in UK tertiary centre. Adapted from existing adult scores but amended based on local clinical consensus. Small audit of patients (n = 30) described, but no formal validation | Score | Expert opinion | No | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Nebulisers every 15 minutes, persistent vomiting post surgery | ||||||||
| Modified Brighton PEWS (a)45,57,65 | Modification of Brighton PEWS for use in general medical ward of a US tertiary centre. Altered thresholds for oxygen therapy, changed wording for respiratory effort, modified escalation algorithm | Score | Expert opinion | No | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Nebulisers every 15 minutes, persistent vomiting post surgery | ||||||||
| Modified Brighton PEWS (b)72,97 | Modification of Brighton PEWS for use in US tertiary centre. Added age-dependent thresholds for heart rate and respiratory rate | Score | Expert opinion | Yes | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Nebulisers every 15 minutes, persistent vomiting post surgery | ||||||||
| Modified Brighton PEWS (c)81 | Modification of Brighton PEWS for use in a US haematology/oncology unit. Altered thresholds; changed respiratory effort wording; modified escalation algorithm; added and removed items. No formal validation study reported | Score | Expert opinion | No | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Modified Brighton PEWS (d)74 | Modification of Brighton PEWS for use in a US tertiary centre. Modified wording of behaviour component, added age-dependent thresholds for heart rate and respiratory rate; removed nebulisers and persistent vomiting | Score | Expert opinion | Yes | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Modified Brighton PEWS (e)96 | Modification of Brighton PEWS for use in a US tertiary centre. Modified wording of behaviour and respiratory effort items; altered thresholds for O2 therapy; removed nebulisers and persistent vomiting items. No formal validation study reported | Score | Expert opinion | No | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Texas Children’s Hospital PAWS47 | Modification of Brighton PEWS for use in a US tertiary centre. Modified wording of behaviour category; added scoring items to respiratory and cardiovascular categories; changed O2 therapy thresholds; modified escalation algorithm | Score | Expert opinion | No | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Hourly respiratory treatments; persistent vomiting post surgery | |||||||
| Children’s Hospital Early Warning Score (CHEWS)62 | Modification of Brighton PEWS for use in a US tertiary centre. Altered thresholds for O2 therapy; changed wording for behaviour and respiratory categories; added staff and family concern; removed nebulisers and vomiting; modified escalation algorithm | Score | Expert opinion | No | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| C-CHEWS67,68,96 | Modification of Brighton PEWS for cardiac ward of a US tertiary centre. Altered O2 therapy thresholds; added items to behaviour, respiratory and cardiovascular categories; added family and staff concern; added age-related thresholds; removed nebulisers and vomiting items; modified escalation algorithm | Score | Expert opinion | Yes | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Burn-specific PEWS49 | Modification of Brighton PEWS, for use in the specialist burn centre of a US tertiary centre. Added temperature; added intake and output scoring items; added skin component | Score | Expert opinion | No | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Intake; outputs; skin | ||||||
| Children’s Hospital Los Angeles PEWS66 | Modification of Brighton PEWS for use in a US tertiary centre. Added medical history scoring item; added single ventricle physiology scoring item; changed O2 therapy thresholds; added items to respiratory category | Score | Expert opinion | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | RRT, code blue, or transfer from/to PICU in previous 2 weeks; single ventricle physiology; any assisted ventilation | |||||||||
| MAC and derivatives | |||||||||||||||||||||
| MAC40,53,58,91 | Initial development for use in an Australian tertiary centre to activate MET. Adapted from adult MET calling criteria, using age-appropriate thresholds. No formal validation study reported | Trigger | Expert opinion | Yes | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Cardiac or respiratory arrest | |||||||
| Modified MAC92 | Modification of the MAC for use in a Canadian tertiary centre, to activate a rapid response system. Removed cardiac/respiratory arrest outcome. No formal validation study reported | Trigger | Expert opinion | Yes | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| C&VPEWS17,58 | Modification of the MAC for evaluation in a UK tertiary centre. Removed cardiac/respiratory arrest outcome; altered thresholds of some items; evaluated as an aggregate score rather than single-item trigger | Score | Expert opinion | Yes | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Bristol Paediatric Early Warning Tool and derivatives | |||||||||||||||||||||
| Bristol PEWT39,53,54,59,60 | Initial development for use in a UK tertiary centre. Initial candidate items drawn from unvalidated Plymouth tool – retrospectively evaluated for ability to predict adverse events among cases (n = 360, HDU or PICU transfers). Development and validation data set not independent | Trigger | APLS values | Yes | 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Required nebulised adrenaline; hyperkalaemia; suspected meningococcus; diabetic ketoacidosis; persistent convulsion | |||||
| Modified Bristol PEWT (a)97 | Modification of Bristol PEWT for a UK tertiary centre. Adjusted wording of Airway parameters; added respiratory items; added AVPU evaluation; removed suspected meningococcus and diabetic ketoacidosis; added pH of < 7.2 and unresolved pain. No formal validation study reported | Trigger | APLS values | Yes | 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Required nebulised adrenaline or no improvement after nebulisers; pH of < 7.2; unresolved pain or current analgesic therapy; fitting | ||||
| Modified Bristol PEWT (b)64 | Modification of Bristol PEWT for cardiac ward of a UK tertiary centre. Amended heart rate and respiratory rate thresholds. Adjusted wording of airway parameters; added respiratory items; added AVPU evaluation; removed suspected meningococcus and diabetic ketoacidosis; added pH of < 7.2 and unresolved pain | Trigger | Heart rate/respiratory rate data driven | Yes | 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Required nebulised adrenaline or no improvement after nebulisers; pH of < 7.2; unresolved pain or current analgesic therapy; fitting | ||||
| Other PTTTs | |||||||||||||||||||||
| NHS III PEWS41 | Designed as part of a NHS Institute fellowship project. Adapted from adult scores and Brighton PEWS. No formal development or internal validation study published | Score | APLS values | Yes | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Paediatric MET-triggering criteria (a)42 | Initial development for use in a US tertiary centre to activate a MET. Retrospective chart review of case patients (n = 44, code calls) used to generate candidate items. Clinical judgement used to select final items. No formal validation of final tool reported | Trigger | Expert opinion | No | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Worsening retractions; cyanosis | |||||||||
| Paediatric MET-triggering criteria (b)24 | Initial development for use in a US tertiary centre to activate a MET. Minimal description of tool development – authors deliberately chose broad criteria and categories of illness, rather than specific vital signs. No formal validation study reported | Trigger | Expert opinion | Unclear | 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Cardiac or respiratory arrest; seizures with apnoea; progressive lethargy; circulatory compromise/acute shock syndrome | ||||||||
| Paediatric RRT-triggering criteria (a)13 | Initial development for use in a US tertiary centre, to activate a RRT. Triggering items elected through expert consensus locally – reference to similarity to MAC and paediatric MET-triggering criteria (a). No formal validation study reported | Trigger | Expert opinion | No | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Paediatric RRT-triggering criteria (b)44 | Initial development for use in calling RRT in a tertiary centre in Pakistan. Minimal explanation for selection of calling criteria. No formal validation study reported in the literature | Trigger | Unclear | Yes | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Convulsion | ||||||||
| Logistic regression algorithm45 | Initial development based on data-mining of electronic health records in US tertiary-centre. Extracted 24 hours of clinical data from inpatients (n = 6722 controls, 526 PICU transfers) and used logistic regression model to select 29-item tool. Validation performed on subset of development data set | Score | Expert opinion | Yes | 29 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Acuity level (local measure); tissue perfusion and oxygenation | ||||||
Appendix 13 Validation papers excluded from analysis
| PTTT | First author, year | Country | Study population | Study design | Number of centres | PTTT used in practice? | Internal/external validation study? | Outcome measures | Sample size (n) | Score or trigger? | Study overview and reason for exclusion from validation results | Quality score (maximum = 24) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modified Brighton PEWS (a) | Garlick 201351 | USA | All inpatients (MET calls only) | Case–control study (retrospective) | 1 | No | External | Transfer to PICU | 267 (116 cases) | Score | Describes review of MET calls (n = 267) to evaluate predictive ability of Modified Brighton PEWS tool for identifying children requiring transfer to PICU (n = 116). Results presented in terms of association between PEWS and odds of transfer to higher level of care – no evaluation of performance characteristics such as AUROC, sensitivity or specificity | 8 |
| Medar 201552 | Unclear | RRT calls only | Chart review (retrospective) | 1 | NR | External | RRT call | 61 | Score | Describes retrospective review of RRT calls (n = 61) to evaluate Modified Brighton PEWS at time of admission and time of RRT call. Report higher median PEWS score for patients at time of RRT call than at time of admission. No evaluation of performance characteristics such as AUROC, sensitivity or specificity | 6 | |
| Texas Children’s Hospital PAWS | Bell 201353 | USA | General medical ward and two specialist units | Chart review (retrospective) | 1 | Yes | Internal | Other validated scales (e.g. Glasgow Coma Scale) | 150 | Score | Describes development and implementation of the Texas Children’s Hospital PAWS tool in three wards of a specialist paediatric unit in the USA. Texas Children’s Hospital PAWS amended locally from the Brighton PEWS. Reports on internal reliability (correlation coefficients between 3 categories of the score) and inter-rater reliability of scoring among nurses. Also compares scores on subcategories to other measures (e.g. the behavioural subscore is compared with the Glasgow Coma Scale). No evaluation of performance characteristics such as AUROC, sensitivity or specificity | 12 |
| C-CHEWS | McLellan 201354 | USA | Cardiac unit | Tool development | 1 | Yes | Internal | Cardiac ICU transfer | 27 | Score | Describes the development and implementation of a modified version of the Children’s Hospital Early Warning Score for cardiac patients. Results focus on tool modification and implementation challenges – no evaluation of performance characteristics such as AUROC, sensitivity or specificity. Validation of the tool described in a separate paper | 9 |
| Burn-specific PEWS | Rahman 201455 | USA | Specialist burn unit | Chart review (retrospective) | 1 | Yes | Internal | Burn injuries | 50 | Score | Conference abstract only. Describes development and implementation of a modified version of the Brighton PEWS, for use with inpatients with burn injuries. Analysis of 50 randomly selected charts – results focus on compliance with scoring and relationship between PTTT score and extent of burn injuries. No evaluation of performance characteristics such as AUROC, sensitivity or specificity | 13 |
| Bedside Paediatric Early Warning Score (PEWS) | Hopkins 201356 | USA | All inpatients (code blue and RRT calls only) | Chart review (retrospective) | 1 | No | External | PICU transfer and critical intervention in PICU among RRT and code calls | 113 (64 cases) | Score | Conference abstract only. Describes retrospective chart review of code blue and RRT calls over 1 year – Bedside PEWS scores calculated and comparisons drawn between patients eventually transferred to PICU and those who stayed on ward. Preliminary analysis given in terms of mean PEWS scores for different groups – no evaluation of performance characteristics such as AUROC, sensitivity or specificity | 6 |
| Gawronski 201357 | Italy | Bone marrow transplant unit | Case–control study (retrospective) | 1 | No | External | Urgent PICU transfer, PICU consult or death | 21 (11 cases) | Score | Conference abstract only. Describes case–control study evaluating Bedside PEWS in an Italian bone marrow transplant unit, in relation to urgent PICU transfers or consultations. Preliminary analysis only – comparison of mean PTTT scores for cases and controls. No evaluation of performance characteristics such as AUROC, sensitivity or specificity | 6 | |
| Bristol PEWT | Haines 200645 | UK | All inpatients | Chart review (retrospective) | 1 | Yes | Internal | Transfer to PICU or HDU | 360 (180 cases) | Trigger | Describes development and piloting of the Bristol PEWT in a UK tertiary centre. Only included children who would have triggered the pilot version of the tool (n = 360) and then identified PICU or HDU transfers from this population. Paper presents specificity and sensitivity outcomes, but they are incorrectly calculated, so results not included in analysis | 9 |
| Modified Bristol PEWT (a) | Sefton 201458 | UK | All inpatients | Chart review (retrospective) | 1 | Yes | Internal | Transfer to PICU, cardiac/respiratory arrest or unexpected death | Unclear | Trigger | Conference abstract only. Describes a retrospective review of 5 years of data from locally implemented PTTT in a UK tertiary centre, presenting a multiple regression model identifying seven components (including age) most strongly associated with subsequent adverse event if triggered. Of the six clinical elements, all were associated with increased odds of an adverse event, except nurse concern, which was significantly associated with decreased odds of an adverse event. No evaluation of overall PTTT performance characteristics such as AUROC, sensitivity or specificity | 10 |
Appendix 14 Summary of paediatric track-and-trigger tool validation study outcomes
| PTTT | First author, year | Country | Study population | Study design | Number of centres | PTTT used in practice? | Internal/external validation study? | Outcome measures | Sample size | Score or trigger? | Score tested/maximum score | Which score used (frequency of scoring)?a | AUROC | Sensitivity | Specificity | PPV | NPV | Notes on accuracy/reliability of scoring and missing data | Quality score (maximum = 24) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paediatric Early Warning System (PEWS) score | Duncan 200618 | Canada | All inpatients | Case–control study (retrospective) | 1 | No | Internal | Code blue call for actual or impending cardiopulmonary arrest | 215 (87 cases) | Score | 5/26 | Maximum 24 hours before event (hourly) | 0.90 | 78.0 | 95.0 | 4.2b | No details on data abstraction. 13% of eligible cases and 84% of eligible controls excluded because of incomplete clinical data | 14 | |
| Robson 201360 | USA | All inpatients | Case–control study (retrospective) | 1 | No | External | Code blue call | 192 (96 cases) | Score | 5/32 | Maximum 24 hours before event (every 6 hours) | 0.85 | 86.6 | 72.2 | Four researchers scored PTTT from 20 charts; inter-rater reliability of 0.95. No details on number of missing data | 8 | |||
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or unplanned PICU transfer | 608 (297 cases) | Score | 7/32 | Maximum 48 hours before event (per usual practice) | 0.82 | 70.0 | 75.0 | 72.6 | 72.0 | Data abstraction by single researcher. 36% of observation sets contained heart rate, respiratory rate, O2 saturation, systolic blood pressure, temperature and assessment of consciousness | 17 | |
| Bedside PEWS | Parshuram 200919 | Canada | All inpatients | Case–control study (retrospective) | 1 | No | Internal | Urgent PICU transfer (without code blue call) | 180 (60 cases) | Score | 8/26 | Maximum 24 hours before event (hourly) | 0.91 | 82.0 | 93.0 | Availability of scoring items in medical records varied from 27% (capillary refill time) to 93% (oxygen therapy) | 21 | ||
| Parshuram 201884 | Canada and UK | All inpatients | Case–control study (prospective) | 4 | No | External | Urgent PICU transfer or immediate call to resuscitation team | 2074 (686 cases) | Score | 7/26 | Maximum 24 hours before event (hourly) | 0.87 | 64.0 | 91.0 | PTTT scores calculated electronically after abstraction by research nurse; 5.1% of records had all seven items recorded, 31% had at least five items | 22 | |||
| Robson 201360 | USA | All inpatients | Case–control study (retrospective) | 1 | No | External | Code blue call | 192 (96 cases) | Score | 7/26 | Maximum 24 hours before event (every 6 hours) | 0.73 | 56.3 | 78.1 | See above | 8 | |||
| Zhai 201450 | USA | All inpatients | Case–control study (retrospective) | 1 | No | External | Urgent PCU transfer within 24 hours of admission | 6352 (53 cases) | Score | 7/26 | Maximum 24 hours before event (hourly) | 0.82 | 73.6 | 71.7 | 2.1b | Data extracted from electronic health records. Excluded two items of Bedside PEWS (oxygen therapy and respiratory effort) due to difficulty abstracting | 17 | ||
| Gawronski 201679 | Italy | Stem cell transplant unit | Case–control study (retrospective) | 1 | No | External | Unexpected death, urgent consult with RRT or urgent PICU transfer | 99 (19 cases) | Score | 6/26 | Score 4 hours before event | 0.90 | 79.0 | 97.5 | Data abstracted by research nurses. No details on extent of missing data. Conflicting/missing observations resolved by interviews with clinical staff | 15 | |||
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or PICU transfer | 608 (297 cases) | Score | 6/26 | Maximum 48 hours before event (per usual practice) | 0.88 | 72.0 | 89.0 | 86.0 | 77.0 | See above | 17 | |
| Modified Bedside PEWS (a) | Fuijkschot 201562 (study 1) | Netherlands | Oncology ward | Case–cohort study (retrospective) | 1 | Yes | Internal | Emergency medical intervention or reviewed by PICU staff or staff concern | 118 (15 cases) | Score | 8/28 | Unclear (at least every 8 hours) | 73.0 | 41% of admissions excluded from study because of incomplete PTTT scores | 10 | ||||
| Fuijkschot 201562 (study 2) | Netherlands | All inpatients | Case–cohort study (retrospective) | 1 | Yes | Internal | PICU transfer | Unclear (24 cases) | Score | 8/28 | Score 2–6 hours before event (at least every 8 hours) | 66.6 | High rate of exclusions reported as a result of missing data | 10 | |||||
| Fuijkschot 201562 (study 3) | Netherlands | All inpatients | Case–cohort study (prospective) | 1 | Yes | Internal | Emergency medical intervention | Unclear (14 cases) | Score | 8/28 | Unclear (at least every 8 hours) | 100 | No details on missing data | 10 | |||||
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or PICU transfer | 608 (297 cases) | Score | 7/28 | Maximum 48 hours before event (per usual practice) | 0.87 | 69.0 | 91.0 | 87.9 | 79.0 | See above | 17 | |
| Modified Bedside PEWS (b) | Ross 201581 | USA | All inpatients | Case–control study (retrospective) | 1 | No | Internal | Urgent PICU transfer | 4628 (848 cases) | Score | 8/26 | Maximum during admission | 70.0 | 84.0 | No details on data abstraction. Respiratory effort category excluded because of difficulty abstracting. No details on missing data | 9 | |||
| Modified Brighton PEWS (a) | Tucker 200964 | USA | General medical unit | Cohort study (prospective) | 1 | Yes | Internal | PICU transfer | 2979 (51 cases) | Score | 3/11 | Maximum during admission (every 4 hours) | 0.89 | 90.2 | 74.4 | 5.8 | 99.8 | Intraclass coefficient of 0.92 reported for two bedside nurses scoring 55 patients. No details on missing data | 14 |
| Zhai 201450 | USA | All inpatients | Case–control study (retrospective) | 1 | No | External | Urgent PICU transfer within 24 hours of admission | 6352 (53 cases) | Score | 2/11 | Maximum 24 hours before event (hourly) | 0.74 | 68.4 | 81.6 | 2.3 | Data extracted from electronic health records. Only included records with complete PEWS score: 64% of eligible cases and 51% of eligible controls excluded | 17 | ||
| Fenix 201571 | USA | PICU transfers among all inpatients (excluding haematology oncology, surgical and cardiac wards) | Case–control study (retrospective) | 1 | Yes | External | Non-elective PICU transfer followed by deterioration event | 97 PICU transfers (51 cases of PICU transfer followed by ‘deterioration event’) | Score | 3/11 | Maximum during admission | 80.0 | 43.0 | 61.0 | 67.0 | No details on missing data | 15 | ||
| Modified Brighton PEWS (b) | Akre 201078 | USA | All inpatients | Chart review study (retrospective) | 1 | No | Internal | RRT call or code blue call | 186 cases (170 RRT calls, 16 code calls) | Score | 4/13 | Maximum 24 hours before event (at least every 4 hours) | 85.5 | Scores abstracted from charts by single nurse, having calibrated with ANP. Categories scored missing if any items missing. 25% of charts missing behavioural state, 26% missing cardiovascular colour | 14 | ||||
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or PICU transfer | 608 (297 cases) | Score | 4/13 | Maximum 48 hours before event (per usual practice) | 0.79 | 61.0 | 84.0 | 78.4 | 69.0 | See above | 17 | |
| Modified Brighton PEWS (d) | Skaletzky 201280 | USA | Medical surgical wards | Case–control study (retrospective) | 1 | No | Internal | PICU transfer | 350 (100 cases) | Score | 2.5/9 | Maximum 48 hours before event (every 4 hours) | 0.81 | 62.0 | 89.0 | Data abstracted from medial charts and notes. Behaviour category abstracted from level of consciousness. No details on missing data | 15 | ||
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or PICU transfer | 608 (297 cases) | Score | 4/9 | Maximum 48 hours before event (per usual practice) | 0.74 | 46.0 | 90.0 | 81.3 | 63.0 | See above | 17 | |
| Children’s Hospital Early Warning Score (CHEWS) | McLellan 201477 | USA | All inpatients | Case–control study (retrospective) | 1 | Yes | Internal | Arrest or unplanned PICU transfer | 1136 (360 cases) | Score | 4/12 | Maximum in admission (every 4 hours) | 0.90 | 84.2 | 80.9 | No details on missing data | 10 | ||
| C-CHEWS | McLellan 201354 | USA | Cardiovascular unit | Case–control study (retrospective) | 1 | Yes | Internal | Arrest or unplanned PICU transfer | 312 (64 cases) | Score | 3/12 | Maximum 18 hours before event (every 4 hours) | 0.86 | 95.3 | 76.2 | 50.8 | 98.4 | Study nurse and bedside nurses assessed scores for 37 patients: 67% agreement. No details on missing data | 9 |
| Agulnik 201674 | USA | Oncology unit | Case–control study (retrospective) | 1 | Yes | External | Unplanned PICU transfer | 330 (110 cases) | Score | 4/12 | Maximum 24 hours before event (every 4 hours) | 0.96 | 86.0 | 95.0 | PTTT scores abstracted by researcher. Did not abstract if vital signs were present, but no PTTT score calculated by nurse. No details on missing data | 14 | |||
| Agulnik 201773 | Guatemala | Oncology unit | Case–control study (retrospective) | 1 | Yes | External | Unplanned PICU transfer | 258 (129 cases) | Score | 4/12 | Maximum 24 hours before event (every 4 hours) | 91.0 | 88.0 | Researcher evaluated charts and calculated scores, reporting 14% error rate (PTTT score calculated incorrectly) and 3% omission rate (vital signs recorded but no PTTT score calculated). One out of 130 cases excluded because of missing PTTT documentation | 16 | ||||
| Children’s Hospital Los Angeles PEWS | Mandell 201572 | US | Inpatients discharged from PICU to ward | Case–control study (retrospective) | 1 | Yes | Internal | Early unplanned re-admission to PICU (within 48 hours of discharge from PICU to ward) | 189 (38 cases) | Score | 2/10 | First score assigned on ward, post PICU discharge | 0.71 | 76.0 | 56.0 | No details on missing data | 12 | ||
| MAC | Tume 200759 | UK | Inpatients with an unplanned PICU transfer | Chart review study (retrospective) | 1 | No | External | Unplanned PICU transfer | 33 cases | Trigger | N/A | Unclear | 87.8 | Data abstracted by two reviewers. Reference to ‘large number of missing records and observation charts’59 | 11 | ||||
| Tume 200759 | UK | Inpatients with an unplanned PHDU transfer | Chart review study (retrospective) | 1 | No | External | Unplanned PHDU transfer | 32 cases | Trigger | N/A | Unclear | 87.5 | See above | 11 | |||||
| Edwards 201165 | UK | All inpatients | Cohort study (retrospective) | 1 | No | External | Death or unplanned PICU or HDU transfer | 1,000 (16 cases) | Trigger | N/A | Any trigger over admission (per usual practice) | 0.79 | 68.3 | 83.2 | 3.6 | 99.7 | Observation charts altered to include all PTTT parameters. 56% of records missing at least one component. Missing data assumed to be normal | 17 | |
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or PICU transfer | 608 (297 cases) | Trigger | N/A | Maximum 48 hours before event (per usual practice) | 0.71 | 93.0 | 49.0 | 64.0 | 88.0 | See above | 17 | |
| C&VPEWS | Edwards 200917 | UK | All inpatients | Cohort study (prospective) | 1 | No | Internal | Death or unplanned PICU or HDU transfer | 1000 (16 cases) | Score | 2/8 | Maximum score during admission (per usual practice) | 0.86 | 69.5 | 89.9 | 5.9 | 99.7 | Observation charts altered to include all PTTT parameters. 56% of records missing at least one component. Missing data assumed to be normal | 18 |
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or PICU transfer | 608 (297 cases) | Score | 3/8 | Maximum 48 hours before event (per usual practice) | 0.89 | 80.0 | 86.0 | 84.0 | 82.0 | See above | 17 | |
| Bristol Paediatric Early Warning Tool (PEWT) | Tume 200759 | UK | Inpatients with an unplanned PICU transfer | Chart review (retrospective) | 1 | No | External | Unplanned PICU transfer | 33 cases | Trigger | N/A | Unclear | 87.8 | See above | 11 | ||||
| Tume 200759 | UK | Inpatients with an unplanned PHDU transfer | Chart review (retrospective) | 1 | No | External | Unplanned PHDU transfer | 32 cases | Trigger | N/A | Unclear | 84.4 | See above | 11 | |||||
| Wright 201167 | UK | All inpatients | Chart review (retrospective) | 1 | Yes | External | Cardiac arrest | 55 cases | Trigger | N/A | If triggered 24 hours before event | 49.1 | One case excluded because of missing notes. No details on missing data | 11 | |||||
| O’Loughlin 201266 | UK | All inpatients | Cohort study (prospective) | 1 | Yes | External | PICU transfer | 331 (7 cases) | Trigger | N/A | Triggered during admission (every 12 hours) | 0.91 | 100 | 81.0 | 11.0 | No details on missing data | 6 | ||
| Robson 201360 | USA | All inpatients | Case–control study (retrospective) | 1 | No | External | Code blue call | 192 (96 cases) | Trigger | N/A | Triggered 24 hours before event (every 6 hours) | 0.75 | 76.3 | 61.5 | See above | 8 | |||
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or PICU transfer | 608 (297 cases) | Trigger | N/A | If triggered 48 hours before event (per usual practice) | 0.62 | 96.0 | 28.0 | 56.0 | 88.0 | See above | 17 | |
| Modified Bristol Paediatric Early Warning Tool (PEWT) (b) | Clayson 201470 | UK | Cardiac ward | Cohort study (prospective) | 1 | Yes | Internal | ‘A deteriorating patient’70 | 126 (unclear number of cases) | Trigger | N/A | Unclear | 12.5 | 97.0 | No details on missing data | 5 | |||
| NHS III PEWS | Mason 201663 | UK | All inpatients | Cohort study (retrospective) | 1 | No | External | Death or unplanned PICU or HDU transfer | 1000 (16 cases) | Score | 2/7 | Maximum score over admission (per usual practice) | 0.88 | 80.0 | 81.0 | 4.3 | 99.7 | Observation charts altered to include all PTTT parameters. 56% of records missing at least one component. Missing data assumed to be normal | 15 |
| Chapman 201761 | UK | All inpatients | Case–control study (retrospective) | 1 | No | External | Death, arrest or PICU transfer | 608 (297 cases) | Score | 2/7 | Maximum 48 hours before event (per usual practice) | 0.82 | 83.0 | 65.0 | 69.6 | 80.0 | See above | 17 | |
| Logistic regression algorithm | Zhai 201450 | USA | All inpatients | Case–control study (retrospective) | 1 | No | External | Urgent PICU transfer within 24 hours of admission | 6352 (53 cases) | Score | > 0.5 | Maximum 24 hours before event (hourly) | 0.91 | 84.9 | 85.9 | 4.8 | Data extracted from electronic health records. No details on number of missing data but authors report that ‘missing data was a major cause of incorrect prediction’50 | 17 | |
| Burton Paediatric Early Warning Score (BPEWS) | Ahmed 201268 | UK | PICU admissions only | Chart review (retrospective) | 1 | Yes | Internal | PICU admission | 23 | Score | 4/19 | Maximum 24 hours before event (unclear) | 93.0 | Data extracted from case notes by two reviewers. No details on missing data | 4 | ||||
| ‘Between the Flags’ Paediatric Early Warning System (PEWS) | Blackstone 201775 | UK | Urgent PICU admissions only | Chart review (retrospective) | 1 | Yes | External | Urgent PICU admission | 100 | Trigger | N/A | Unclear | 91.0 | Data extracted from health records. No details on missing data | 8 |
Appendix 15 Effectiveness papers excluded from analysis
| Study | Intervention | PTTT | Country | Number of centres | Specialist unit? | Existing RRT/MET? | Population | Study design | Study duration in months (before and after intervention) | Description and reason for excluding from analysis | Quality score (maximum = 26) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Implemented a new PTTT | Implemented new RRT/MET | Modified escalation process | Staff training/education | |||||||||||
| Mistry 200686 | ✓ | ✓ | ✓ | Paediatric RRT activation criteriaa | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 11 (6 before, 5 after) | Describes implementation of a paediatric RRT with calling criteria (not defined). Looked at impact on mortality, cardiac arrests and PICU outcomes among PICU transfers. Reports absolute decreases in numbers of deaths and arrests post intervention, but no denominator data provided or further statistical details given | 3 | |
| Demmel 201087 | ✓ | Modified Brighton PEWS (e) | USA | 1 | Yes | Yes | Haematology/oncology patients | Uncontrolled before-and-after study (prospective) | Unclear (unclear, 8 after) | Implemented a locally modified version of the Brighton PEWS in a specialist haematology/oncology unit. Discusses challenges in the development and implementation of the tool. Refers to time between cardiopulmonary arrests being 299 days immediately before implementation, and 1053 days 8 months after implementation; however, no denominator data or further statistical details given | 8 | |||
| Sandhu 201088 | ✓ | Unclear | UK | 1 | Yes | No | Unclear | Uncontrolled before-and-after study (retrospective) | Unclear (unclear, 3 months) | Conference abstract only. Reported implementing an ‘outreach response team’ alongside an existing ‘paediatric early warning tool’ (unclear which tool) in a UK tertiary centre. Reference to comparable triggering rate of PTTT before (28% of patients) and after (28% of patients) piloting the outreach team, and two arrests before piloting, and 0 after – but no denominator data or further statistical details given | 8 | |||
| Randhawa 201189 | ✓ | ✓ | ✓ | Brighton PEWS | USA | 1 | Yes | Yes | All inpatients | Uncontrolled before-and-after study (prospective) | Unclear | Describes implementation of the Brighton PEWS in a specialist paediatric centre. Details various cycles of change during implementation of the tool across different wards, and efforts at staff education. Reports reduction in rate of cardiopulmonary arrests post intervention, but no absolute numbers, denominator data or further statistical details given | 12 | |
| Camacho 201190 | ✓ | Modified Brighton PEWS (a)b | USA | 1 | Yes | NR | Cardiac and renal patients | Uncontrolled before-and-after study (prospective) | 8 (3 before, 5 after) | Conference abstract only. Reported piloting and modifying Tucker’s modified Brighton PEWS for specialist cardiac and renal population. Unclear if RRT/MET in place. Referred to there being five code calls in the quarter (3 months) before implementation, and 0 in the following 5 months. However, no denominator data or further statistical details given | 8 | |||
| Heyden 201291 | ✓ | ✓ | Paediatric RRT activation criteriaa | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (retrospective) | 72 (24 before, 48 after) | Conference abstract only. Describes implementation of a RRT in a US tertiary centre, with an associated ‘broad calling criteria’ (limited details given). Reports number of cardiac arrests on ward and PICU before and after intervention, and refers to increase in RRT calls over time. No denominator data or further statistical details given | 7 | ||
| Somberg 201395 | ✓ | ✓ | Unclear | USA | 1 | No | No | All inpatients | Uncontrolled before-and-after study (unclear) | Unclear | Conference abstract only. Reported developing and implementing a PTTT (tool not named) and RRT for a paediatric unit in a community hospital. Reference to no intubation or code calls since intervention, but no pre-intervention comparison, time frames, denominator data or further statistical details given | 2 | ||
| Norville 201392 | ✓ | Texas Children’s Hospital PAWSb | USA | 1 | Yes | Yes | Bone marrow transplant patients | Uncontrolled before-and-after study (unclear) | 23 (12 before, 11 after) | Conference abstract only. Describes implementation of the Texas Children’s Hospital PAWS, with amended algorithm for specialist bone marrow transplant unit. Looked at impact on code calls and RRT calls – refers to 3 code calls and 18 RRT calls pre intervention, compared with 0 codes and 25 RRT calls post intervention. No denominator data or further statistical details given | 5 | |||
| Ambati 201493 | ✓ | Not applicable | USA | 1 | Yes | Yes | Unclear | Uncontrolled before-and-after study (unclear) | 48 (12 before, 36 after) | Conference abstract only. Reported effect of implementing a ‘simulation based curriculum’ for clinical staff on subsequent RRT utilisation. Reference to increase in RRT calls year on year post implementation, but no denominator data or further statistical details given | 3 | |||
| Ocholi 201494 | ✓ | Bedside Paediatric Early Warning Score (PEWS) | UK | 1 | Yes | No | Unclear | Uncontrolled before-and-after study (unclear) | 12 months (6 before, 6 after) | Conference abstract only. Describes implementation of Bedside PEWS in a UK tertiary centre. Looked at impact of intervention on ward outcomes and outcomes of children transferred to PICU. Reference to impact of tool on number of ‘adverse incidents’ (not defined) on the ward and median length of stay in PICU among PICU transfers, but no denominator data or further statistical details given | 6 | |||
| Fenix 201571 | ✓ | ✓ | Unclear | USA | 1 | Yes | NR | Two general paediatric wards | Uncontrolled before-and-after study (retrospective) | 46 months (16 before, 30 after) | Conference abstract only. Describes implementation of a ‘Situational Awareness’ tool, with integrated PTTT (unclear which tool) in a tertiary centre. Retrospective review of rates of critical deterioration events on two of seven general paediatric wards. Reports a significant decrease in trend and trajectory of critical deterioration events post implementation, but no event numbers, denominator data or further statistical details given | 6 | ||
Appendix 16 Summary of early warning system effectiveness study outcomes
| Outcome | Study | Intervention | PTTT | Country | Number of centres | Specialist unit? | Existing RRT/MET? | Population | Study design | Study duration in months | Events before, n (rate) | Events after, n (rate) | Effect size (95% CI) | p-value | Quality score (maximum = 26) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Implemented a new PTTT | Implemented new RRT/MET | Modified escalation process | Staff training/education | |||||||||||||||
| Mortality | ||||||||||||||||||
| Deaths on ward (per 1000 admissions) | Tibballs 200538 | ✓ | ✓ | ✓ | MAC | Australia | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 53 (41 before, 12 after) | 13 (0.12) | 2 (0.06) | RR 0.45 (0.10 to 1.99)a | 0.29 | 10 | |
| Hospital-wide deaths (per 100 discharges) | Sharek 200748 | ✓ | ✓ | ✓ | Paediatric RRT-triggering criteria | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 84 (67 before, 17 after) | 547 (1.01) | 158 (0.83) | RR 0.82 (0.70 to 0.95) | 0.007 | 15 | |
| Hospital-wide deaths, excluding neonate ICU and ED (per 1000 discharges) | Zenker 200796 | ✓ | ✓ | RRT activation criteriab | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 34 (23 before, 11 after) | 97 (4.30) | 52 (4.45) | RR 1.04 (0.74 to 1.45)a | 0.57 | 12 | ||
| Deaths outside ICU (per 1000 non-ICU patient-days) | Brilli 200746 | ✓ | ✓ | ✓ | Paediatric MET-triggering criteria (a) | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 27 (15 before, 12 after) | 9 (0.10) | 2 (0.04) | RR 0.39 (0.08 to 1.80)a | 0.13 | 14 | |
| Ward death rate (per 1000 ward admissions) | Hanson 201097 | ✓ | ✓ | ✓ | Not described | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (retrospective) | 36 (24 before, 12 after) | 13 (1.50) | 2 (0.45) | RR 0.30 (0.07 to 1.31)a | 0.07 | 18 | |
| Total hospital deaths (per 1000 admissions) | Tibballs 200998 | ✓ | ✓ | ✓ | MAC | Australia | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 89 (41 before, 48 after) | 459 (4.38) | 398 (2.87) | RR 0.65 (0.57 to 0.75) | < 0.0001 | 15 | |
| Deaths on ward (per 1000 admissions) | Tibballs 200998 | ✓ | ✓ | ✓ | MAC | Australia | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 89 (41 before, 48 after) | 13 (0.12) | 6 (0.04) | RR 0.35 (0.13 to 0.92) | 0.03 | 15 | |
| All-cause hospital mortality (per 1000 admissions) | Kotsakis 201199 | ✓ | ✓ | Modified MAC | Canada | 4 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 553 (9.97) | 540 (9.65) | RR 0.97 (0.83 to 1.12) | 0.65 | 18 | ||
| All-cause hospital mortality (per 1000 discharges) | Parshuram 201884 | ✓ | ✓ | ✓ | Bedside PEWS | Belgium, Ireland, Netherlands, England, Italy, Canada, New Zealand | 21 | Yes | No | All inpatients | Cluster randomised trial (prospective) | 18 (6 pre, 12 post) | Control: 61 (1.31) | Control: 147 (1.56) | OR 1.01 (0.61 to 1.69) | 0.96 | 23 | |
| Intervention: 52 (1.95) | Intervention: 97 (1.93) | |||||||||||||||||
| Hospital mortality (per 1000 admissions) | Kutty 2018108 | ✓ | NR | USA | 38 | Yes | No | All inpatients | ITS (retrospective) | 180 (60 before, 120 after) | NA | NA | OR 0.94 (0.93 to 0.95) | 0.98 | 20 | |||
| PICU mortality | ||||||||||||||||||
| PICU mortality after PICU admission from ward (per PICU admission) | Anwar-ul-Haque 201049 | ✓ | ✓ | Paediatric RRT-triggering criteria (b) | Pakistan | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (retrospective) | 18 (9 before, 9 after) | 23 (51.11) | 5 (15.63) | RR 0.31 (0.13 to 0.72)a | 0.007 a | 6 | ||
| PICU mortality after PICU re-admission within 48 hours of discharge (per 1000 admissions) | Kotsakis 201199 | ✓ | ✓ | Modified MAC | Canada | 4 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 16 (0.29) | 7 (0.13) | RR 0.43 (0.17 to 0.99) | < 0.05 | 18 | ||
| PICU mortality after urgent PICU admission from ward (per 1000 admissions) | Kotsakis 201199 | ✓ | ✓ | Modified MAC | Canada | 4 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 70 (1.3) | 61 (1.1) | RR 0.90 (0.70 to 1.00) | 0.25 | 18 | ||
| Death prior to discharge (per unplanned PICU transfer) | Bonafide 2014100 | ✓ | ✓ | Bedside PEWS | USA | 1 | Yes | No | All inpatients | ITS study (prospective) | 59 (32 before, 27 after) | 51 (6.3) | 56 (6.5) | RR 1.03 (0.72 to 1.49)a | 0.99 | 23 | ||
| PICU mortality (per PICU admission) | Duns 2014101 | ✓ | Between the Flags (BTS) toolb | Australia | 1 | Yes | Yes | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 30 (8.57) | 20 (5.49) | RR 0.64 (0.37 to 1.11)a | 0.14 | 7 | |||
| Death in PICU (per 1000 patient-days) | Agulnik 2017102 | ✓ | ✓ | C-CHEWS | Guatemala | 1 | Yes | No | Oncology unit | Uncontrolled before-and-after study (retrospective) | 24 (12 before, 12 after) | 21 (1.25) | 22 (1.10) | RR 0.89 (0.49 to 1.61)a | 0.76 | 19 | ||
| Death in PICU (per emergency PICU admission) | Sefton 2015209 | ✓ | ✓ | ✓ | Modified Bristol PEWT (a) | UK | 1 | Yes | No | All PICU admissions | Controlled before-and-after study (retrospective) | 24 (12 before, 12 after) | 17 (10.8) | 14 (8.4) | RR 0.78 (0.40 to 1.53)a | 0.47 | 16 | |
| Deaths in PICU (per unplanned PICU admission) | Kolovos 2018104 | ✓ | ✓ | RRT activation criteriab | USA | 1 | Yes | No | All unplanned PICU admissions | Uncontrolled before-and-after study (retrospective) | 78 (42 before, 36 after) | 54a (4.9) | 40a (3.8) | RR 0.77 (0.52 to 1.15)a | 0.20a | 12 | ||
| PICU mortality (per 1000 discharges) | Parshuram 201884 | ✓ | ✓ | ✓ | Bedside PEWS | Belgium, Ireland, Netherlands, England, Italy, Canada, New Zealand | 21 | Yes | No | All inpatients | Cluster randomised trial (prospective) | 18 (6 pre, 12 post) | Control: 34 (0.73) | Control: 91 (0.96) | OR 0.95 (0.48 to 1.86) | 0.88 | 23 | |
| Intervention: 33 (1.24) | Intervention: 56 (1.12) | |||||||||||||||||
| Cardiac arrest | ||||||||||||||||||
| Cardiac arrests on ward (per 1000 admissions) | Tibballs 200538 | ✓ | ✓ | ✓ | MAC | Australia | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 53 (41 before, 12 after) | 20 (0.19) | 4 (0.11) | RR 0.58 (0.20 to 1.70) | 0.33 | 10 | |
| Cardiopulmonary arrests (per 1000 non-ICU patient-days) | Brilli 200746 | ✓ | ✓ | ✓ | Paediatric MET triggering criteria (a) | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 27 (15 before, 12 after) | 7 (0.08) | 2 (0.04) | RR 0.50 (0.10 to 2.42)a | 0.11 | 14 | |
| Ward cardiac arrest rate (per 1000 ward admissions) | Hanson 201097 | ✓ | ✓ | ✓ | Not described | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (retrospective) | 36 (24 before, 12 after) | 11 (1.27) | 2 (0.45) | RR 0.35 (0.08 to 1.58)a | 0.13 | 18 | |
| Ward cardiopulmonary arrests (per 1000 patient-days) | Hunt 200847 | ✓ | ✓ | Paediatric MET triggering criteria | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 24 (12 before, 12 after) | 5 (0.10) | 5 (0.10) | RR 0.98 (0.22 to 4.24) | 0.97 | 17 | ||
| Preventable cardiac arrests (per 1000 admissions) | Tibballs 200998 | ✓ | ✓ | ✓ | MAC | Australia | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 89 (41 before, 48 after) | 17 (0.16) | 10 (0.07) | RR 0.45 (0.20 to 0.97) | 0.04 | 15 | |
| Unexpected cardiac arrests (per 1000 admissions) | Tibballs 200998 | ✓ | ✓ | ✓ | MAC | Australia | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 89 (41 before, 48 after) | 20 (0.19) | 24 (0.17) | RR 0.91 (0.50 to 1.64) | 0.75 | 15 | |
| Actual cardiopulmonary arrests (per 1000 ward admissions) | Kotsakis 201199 | ✓ | ✓ | Modified MAC | Canada | 4 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 69 (1.9) | 66 (1.8) | RR 0.95 (0.76 to 1.96) | 0.68 | 18 | ||
| Near cardiopulmonary arrests (per 1000 admissions) | Kotsakis 201199 | ✓ | ✓ | Modified MAC | Canada | 4 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 123 (3.4) | 67 (1.9) | RR 0.54 (0.52 to 0.57) | < 0.001 | 18 | ||
| Cardiac arrests on ward (per 1000 non-ICU patient-days) | Bonafide 2014100 | ✓ | ✓ | Bedside PEWS | USA | 1 | Yes | No | All inpatients | ITS study (prospective) | 59 (32 before, 27 after) | 6a (0.03) | 2a (0.01) | RR 0.36 (0.07 to 1.78)a | 0.21 | 23 | ||
| Cardiac arrests (per 1000 patient-days) | Parshuram 201884 | ✓ | ✓ | ✓ | Bedside PEWS | Belgium, Ireland, Netherlands, England, Italy, Canada, New Zealand | 21 | Yes | No | All inpatients | Cluster randomised trial (prospective) | 18 (6 pre, 12 post) | Control: 18 (0.11) | Control: 32 (0.10) | RR 1.02 (0.65 to 1.62) | 0.92 | 23 | |
| Intervention: 15 (0.12) | Intervention: 27 (0.11) | |||||||||||||||||
| Respiratory arrest | ||||||||||||||||||
| Ward respiratory arrests (per 1000 patient-days) | Hunt 200847 | ✓ | ✓ | Paediatric MET triggering criteria | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 24 (12 before, 12 after) | 11 (0.23) | 3 (0.06) | RR 0.27 (0.07 to 0.95) | 0.04 | 17 | ||
| Cardiac or respiratory arrest | ||||||||||||||||||
| Cardiac or respiratory arrest (per 1000 discharges) | Zenker 200796 | ✓ | ✓ | RRT activation criteriab | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 34 (23 before, 11 after) | 180 (7.98) | 60 (5.13) | RR 0.64 (0.48 to 0.86)a | 0.19 | 12 | ||
| Code calls (per 1000 non-ICU patient-days) | Brilli 200746 | ✓ | ✓ | ✓ | Paediatric MET triggering criteria (a) | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 27 (15 before, 12 after) | 25 (0.27) | 6 (0.11) | RR 0.42 (0.17 to 1.03)a | 0.06a | 14 | |
| Code calls (per 1000 non-ICU patient-days) | Sharek 200748 | ✓ | ✓ | ✓ | Paediatric RRT triggering criteria | USA | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 84 (67 before, 17 after) | 53 (0.52) | 5 (0.15) | RR 0.29 (0.10 to 0.65) | 0.008 | 15 | |
| Code calls (per 1000 admissions) | Anwar-ul-Haque 201049 | ✓ | ✓ | Paediatric RRT triggering criteria (b) | Pakistan | 1 | Yes | No | All inpatients | Uncontrolled before-and-after study (retrospective) | 18 (9 before, 9 after) | 26 (5.25) | 12 (2.73) | RR 0.52 (0.26 to 1.03) | 0.06 | 6 | ||
| Calls for urgent review/assistance | ||||||||||||||||||
| Urgent calls to respiratory therapist (per 1000 patient-days) | Parshuram 2011105 | ✓ | ✓ | ✓ | Bedside PEWS | Canada | 1 | No | No | All inpatients | Uncontrolled before-and-after study (prospective) | 8 (3 before, 5 after) | 8 (9.5) | 8 (3.4) | RR 0.36 (0.13 to 0.95)a | 0.04 a | 23 | |
| Urgent calls to paediatrician (per 1000 patient-days) | Parshuram 2011105 | ✓ | ✓ | ✓ | Bedside PEWS | Canada | 1 | No | No | All inpatients | Uncontrolled before-and-after study (prospective) | 8 (3 before, 5 after) | 19 (22.6) | 12 (5.1) | RR 0.23 (0.11 to 0.46)a | < 0.0001 | 23 | |
| Code blue calls on the ward (per 1000 admissions) | Kotsakis 201199 | ✓ | ✓ | Modified MAC | Canada | 4 | Yes | No | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 210 (3.75) | 150 (2.70) | RR 0.71 (0.61 to 0.83) | < 0.0001 | 18 | ||
| Urgent calls to outreach team (per 1000 admissions) | Duns 2014101 | ✓ | Between the Flags toolb | Australia | 1 | Yes | Yes | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 1058 (39.5) | 2120 (76.0) | RR 1.92 (1.79 to 2.07)b | 0.02 | 7 | |||
| RRT calls (per 1000 patient-days) | Panesar 2014106 | ✓ | Modified Brighton PEWS (e) | USA | 1 | Yes | Yes | All inpatients | Uncontrolled before-after study (retrospective) | 42 (18 before, 24 after) | 44 (3.14) | 69 (4.23) | RR 1.35 (0.92 to 1.96)a | 0.11 | 15 | |||
| RRT calls (per 1000 patient days) | Douglas 2016107 | ✓ | ✓ | ✓ | Modified Brighton PEWS (b) | USA | 1 | Yes | Yes | All inpatients | Uncontrolled before-and-after study (retrospective) | 24 (12 before, 12 after) | 194 (6.17) | 292 (9.80) | RR 1.59 (1.33 to 1.90)a | < 0.001 | 12 | |
| Code calls (per 1000 patient days) | Douglas 2016107 | ✓ | ✓ | ✓ | Modified Brighton PEWS (b) | USA | 1 | Yes | Yes | All inpatients | Uncontrolled before-and-after study (retrospective) | 24 (12 before, 12 after) | 31 (0.98) | 20 (0.67) | RR 0.68 (0.39 to 1.19)a | 0.21 | 12 | |
| PICU transfers | ||||||||||||||||||
| Transfers from ward to other specialist units (per 1000 patient-days) | Parshuram 2011105 | ✓ | ✓ | ✓ | Bedside PEWS | Canada | 1 | No | No | All inpatients | Uncontrolled before-and-after study (prospective) | 8 (3 before, 5 after) | 5 (5.9) | 19 (8.1) | RR 1.37 (0.51 to 3.63)a | 0.54a | 23 | |
| Clinical deterioration events on ward prior to transfer to specialist unit (per 1000 patient-days) | Parshuram 2011105 | ✓ | ✓ | ✓ | Bedside PEWS | Canada | 1 | No | No | All inpatients | Uncontrolled before-and-after study (prospective) | 8 (3 before, 5 after) | 2 (2.4) | 1 (0.43) | RR 0.18 (0.02 to 1.97)a | 0.16a | 23 | |
| PICU transfers (per 1000 admissions) | Duns 2014101 | ✓ | Between the Flags toolb | Australia | 1 | Yes | Yes | All inpatients | Uncontrolled before-and-after study (prospective) | 48 (24 before, 24 after) | 350 (13.1) | 364 (13.1) | RR 1.00 (0.86 to 1.16)a | 0.98 | 7 | |||
| Unplanned PICU transfers from ward (per 1000 non-ICU patient-days) | Bonafide 2014100 | ✓ | ✓ | Bedside PEWS | USA | 1 | Yes | No | All inpatients | ITS study (prospective) | 59 (32 before, 27 after) | 874 (4.54) | 936 (5.25) | IRR 0.73 (0.46 to 1.14) | 0.16 | 23 | ||
| Unplanned transfers to PICU from ward (per 1000 patient-days) | Agulnik 2017102 | ✓ | ✓ | C-CHEWS | Guatemala | 1 | Yes | No | Oncology unit | Uncontrolled before-and-after study (retrospective) | 24 (12 before, 12 after) | 157 (9.3) | 130 (6.5) | RR 0.70 (0.56 to 0.88)a | 0.003 | 19 | ||
| Urgent PICU admissions (per 1000 patient-days) | Parshuram 201884 | ✓ | ✓ | ✓ | Bedside PEWS | Belgium, Ireland, Netherlands, England, Italy, Canada, New Zealand | 21 | Yes | No | All inpatients | Cluster randomised trial (prospective) | 18 (6 pre, 12 post) | Control: 652 (4.01) | Control: 1178 (3.83) | RR 0.95 (0.82 to 1.09) | 0.45 | 23 | |
| Intervention: 469 (3.62) | Intervention: 828 (3.29) | |||||||||||||||||
| PICU outcomes | ||||||||||||||||||
| Critical deterioration events after PICU transfer (per 1000 non-ICU patient-days) | Bonafide 2014100 | ✓ | ✓ | Bedside PEWS | USA | 1 | Yes | No | All inpatients | ITS study (prospective) | 59 (32 before, 27 after) | 260a (1.35) | 282a (1.58) | IRR 0.38 (0.20 to 0.75) | 0.01 | 23 | ||
| Mechanical ventilation within 1 hour of unplanned PICU transfer (per unplanned transfer to PICU) | Bonafide 2014100 | ✓ | ✓ | Bedside PEWS | USA | 1 | Yes | No | All inpatients | ITS study (prospective) | 59 (32 before, 27 after) | 45 (5.1) | 42 (4.5) | RR 0.87 (0.58 to 1.31)a | 0.51 | 23 | ||
| Mechanical ventilation within 12 hours of unplanned PICU transfer (per unplanned transfer to PICU) | Bonafide 2014100 | ✓ | ✓ | Bedside PEWS | USA | 1 | Yes | No | All inpatients | ITS study (prospective) | 59 (32 before, 27 after) | 112 (12.8) | 103 (11.0) | IRR 0.17 (0.07 to 0.44) | < 0.001 | 23 | ||
| Vasopressor within 1 hour of unplanned PICU transfer (per unplanned transfer to PICU) | Bonafide 2014100 | ✓ | ✓ | Bedside PEWS | USA | 1 | Yes | No | All inpatients | ITS study (prospective) | 59 (32 before, 27 after) | 41 (4.7) | 16 (1.7) | RR 0.36 (0.21 to 0.64)a | < 0.001 | 23 | ||
| Vasopressors within 12 hours of unplanned PICU transfer (per unplanned transfer to PICU) | Bonafide 2014100 | ✓ | ✓ | Bedside PEWS | USA | 1 | Yes | No | All inpatients | ITS study (prospective) | 59 (32 before, 27 after) | 71 (8.1) | 57 (6.1) | IRR 0.20 (0.06 to 0.62) | 0.006 | 23 | ||
| Invasive ventilation in PICU (per emergency PICU admission) | Sefton 2015103 | ✓ | ✓ | ✓ | Modified Bristol PEWT (a) | UK | 1 | Yes | No | All PICU admissions | Controlled before-and-after study (retrospective) | 24 (12 before, 12 after) | 118 (75.2) | 104 (62.7) | RR 0.83 (0.72 to 0.97)a | 0.002 | 16 | |
| Inotropes in PICU (per emergency PICU admission) | Sefton 2015103 | ✓ | ✓ | ✓ | Modified Bristol PEWT (a) | UK | 1 | Yes | No | All PICU admissions | Controlled before-and-after study (retrospective) | 24 (12 before, 12 after) | 50 (31.8) | 40 (24.1) | RR 0.76 (0.53 to 1.08)a | 0.12 | 16 | |
| Intubation within 24 hours of PICU admission (per 1000 patient-days) | Agulnik 2017102 | ✓ | ✓ | C-CHEWS | Guatemala | 1 | Yes | No | Oncology unit | Uncontrolled before-and-after study (retrospective) | 24 (12 before, 12 after) | 11 (0.65) | 18 (0.90) | RR 1.38 (0.65 to 2.92)a | 0.46 | 19 | ||
| Vasopressors within 24 hours of PICU admission (per 1000 patient-days) | Agulnik 2017102 | ✓ | ✓ | C-CHEWS | Guatemala | 1 | Yes | No | Oncology unit | Uncontrolled before-and-after study (retrospective) | 24 (12 before, 12 after) | 29 (1.72) | 37 (1.86) | RR 1.08 (0.66 to 1.75)a | 0.60 | 19 | ||
| Mechanical ventilation during PICU admission (per PICU admission) | Kolovos 2018104 | ✓ | ✓ | RRT activation criteriab | USA | 1 | Yes | No | All unplanned PICU admissions | Uncontrolled before-and-after study (retrospective) | 78 (42 before, 36 after) | 285 (25.98) | 233 (22.09) | RR 0.85 (0.73 to 0.99)a | 0.03 a | 12 | ||
| Intubation within 1 hour of PICU admission (per PICU admission) | Kolovos 2018104 | ✓ | ✓ | RRT activation criteriab | USA | 1 | Yes | No | All unplanned PICU admissions | Uncontrolled before-and-after study (retrospective) | 78 (42 before, 36 after) | 49 (4.47) | 88 (8.34) | RR 1.87 (1.33 to 2.62) | 0.0003 | 12 | ||
| Significant clinical deterioration events (per 1000 patient-days) | Parshuram 201884 | ✓ | ✓ | ✓ | Bedside PEWS | Belgium, Ireland, Netherlands, England, Italy, Canada, New Zealand | 21 | Yes | No | All inpatients | Cluster randomised trial (prospective) | 18 (6 pre, 12 post) | Control: 144 (0.89) | Control: 259 (0.84) | RR 0.77 (0.61 to 0.97) | 0.03 | 23 | |
| Intervention: 80 (0.62) | Intervention: 127 (0.50) | |||||||||||||||||
Appendix 17 Screening breakdown of included papers for review 3
| Evidence screening process | Included papers (n) | |||||||
|---|---|---|---|---|---|---|---|---|
| PEWS | EWS | Structured handover | Situational awareness | Electronic systems | Observations and monitoring | Family involvement | Snowball sample | |
| Database searching | 3564 | 1155 | 3369 | 302 | / | / | / | / |
| Additional sources | 83 | 7 | 150 | 46 | / | / | / | / |
| Records after duplicates removed | 2194 | 751 | 2156 | 199 | / | / | / | / |
| Hand-searches | 431 | / | / | / | 26 | 20 | 15 | 5 |
| Title screening | 90 | 751 | 2156 | 199 | 26 | 20 | 15 | 5 |
| Abstract screening | 62 | 106 | N/A | N/A | 26 | 20 | 15 | 5 |
| Full-paper screening | 39 | 65 | 37 | 26 | 26 | 20 | 15 | 5 |
| Included in syntheses | 24 | 22 | 4 | 6 | 10 | 2 | 9 | 5 |
Appendix 18 Summary of detection evidence
| Study | Country | Methodology | Analysis | Search area | Evidence contribution |
|---|---|---|---|---|---|
| Andrews and Waterman114 2005 | UK | Interviews and observations | Grounded theory | EWS |
|
| Astroth et al.142 2013 | USA | Semistructured interviews with nurses | Coding categories were generated from the data, and consensus on final themes was achieved through an iterative process | EWS |
|
| Azzopardi et al.115 2011 | Australia | Survey | Statistical analysis | PEWS |
|
| Bellomo et al.169 2012 | International: USA, Sweden, UK, the Netherlands, Australia | Before-and-after multicentred international controlled trial | Automated vital signs monitoring and early warning score calculated, international study, blinded trial, hospitals retained own score and escalation policy | Electronic systems |
|
| Bonafide et al.116 2013 | USA | Semistructured interviews | Grounded theory | PEWS |
|
| Bonafide et al.171 2014 | USA | Prospective feasibility study | Video-recording and electronic patient data collected prospectively. Pragmatic observational study of VitalPAC deployment in two large hospitals | Electronic systems |
|
| Bonafide et al.173 2017 | USA | Video review and response time outcome | Statistical analysis | PEWS |
|
| Braaten117 2015 | USA | Document review and interviews using the principles of cognitive work analysis | Inductive and deductive forms of analysis – cognitive work analysis, framework and directed content analysis | EWS |
|
| Brady and Goldenhar153 2014 | USA | Focus groups × 7 – held in groups of participants with similar roles | Constant comparison | Situational awareness |
|
| Burns et al.158 2018 | USA | Semistructured interviews were used, drawing on a descriptive phenomenological methodology | Iterative thematic analysis | Snowball sample |
|
| Chua et al.130 2013 | Singapore | A qualitative survey using critical incident technique | Inductive content analysis | EWS |
|
| Cioffi140 2000 | Australia | Unstructured interviews with nurses who had activated the MET | Simple code and retrieve | EWS |
|
| Cioffi143 2000 | Australia | Unstructured interviews | Simple code and retrieve | EWS |
|
| Cioffi et al.135 2006 | Australia | Focus groups with clinicians and nurses exploring their responses to abnormal vital signs | Constant comparison | EWS |
|
| Claussen et al.155 2013 | USA | Retrospective review of calls to the RRT and cardiac arrest calls to evaluate impact of evidence-based guidelines (pre intervention). Modified Early Warning Score and huddles implemented. Electronic health record available throughout to compare pre and post | Descriptive statistics | Electronic systems |
|
| Davies et al.145 2014 | USA | Survey looking at barriers to RRS activation | Statistical analysis | EWS |
|
| de Groot et al.152 2018 | Netherlands | Retrospective patient review and semistructured interviews with professionals | Descriptive statistics and grounded theory | PEWS |
|
| Donohue and Endacott154 2010 | UK | Qualitative design with critical incident technique. Semistructured interviews with nurses and the outreach team | Thematic analysis | EWS |
|
| Downey et al.164 2017 | UK | Narrative review | Patterns were identified and translated to themes, which were further refined using an iterative process164 | PEWS |
|
| Endacott et al.136 2007 | UK | Mixed-methods case study – semistructured interviews and audit of charts | Qualitative content analysis and descriptive statistics | Observations and monitoring |
|
| Endacott and Westley133 2006 | Australia | Questionnaire, in-depth interviews and observations | Content analysis and constant comparison | EWS |
|
| Entwistle163 2004 | USA | Editorial | N/A | Family involvement |
|
| Fagan et al.172 2012 | USA | Observational cohort comparison study | Descriptive statistics | Electronic systems |
|
| Graedon and Graedon162 2006 | USA focus | Opinion piece | N/A | Family involvement |
|
| Hands et al.112 2013 | UK | The vital signs and early warning data for all inpatients for 1 year to investigate patterns of vital signs observations collected | Statistical | Observations and monitoring |
|
| Hope et al.151 2018 | UK | Semistructured interviews with 17 registered nurses | Constant comparative method informed by grounded theory | Snowball sample |
|
| James et al.131 2010 | UK | Postal survey with HCAs using closed and open questions | Descriptive statistics and content analysis of qualitative data | Observations and monitoring |
|
| Jensen et al.138 2018 | Denmark | Focus group exploring nurses’ experiences with a paediatric early warning score | Qualitative meaning condensation analysis | PEWS |
|
| Jones et al.156 2006 | Australia | Questionnaire about understandings and barriers to activating a MET | Simple descriptive statistics | EWS |
|
| Jones et al.167 2011 | UK | Electronic capture of physiological data to see if automated clinical alerts increase compliance with an early warning score and improve patient outcomes | Statistical | Electronic systems |
|
| Kaul et al.119 2014 | USA | Descriptive cross-sectional study; nurse and medical staff survey | Descriptive statistics | PEWS |
|
| Lobos et al.120 2014 | Canada | Implementation report | Simple descriptive statistics | PEWS |
|
| Lydon et al.113 2016 |
|
Deductive content analysis | PEWS |
|
|
| Mackintosh et al.132 2014 | UK | Ethnographic perspective; observations, semistructured interviews | Data were inductively and deductively coded using NVivo version 8 (QSR International, Warrington, UK) and organised thematically | EWS |
|
| Mackintosh et al.122 2012 | UK | Comparative case study of a RRS using ethnographic methods including observations, interviews and documentary review | Inductive and deductive coding facilitated by NVivo. Also used theme-building and structuring methods from framework analysis; also informed by other theoretical frameworks such as ‘technology-in-practice’ | EWS |
|
| Mackintosh et al.144 2014 | UK | Ethnographic study using observations (> 120 hours), semistructured interviews (n = 45) and documentary review | Thematic analysis with data analysed iteratively in addition to a more strategic and policy-focused coding framework | EWS |
|
| Massey et al.157 2014 | Australia | In-depth semistructured interviews | Inductive approach – thematic analysis | EWS |
|
| McDonnell et al.121 2013 | UK | Single-centre, mixed-method before-and-after study including a survey to measure changes in nurses’ knowledge after implementation of a track-and-trigger system; also, qualitative interviews | Statistical analysis and thematic framework analysis | EWS |
|
| McKay et al.149 2013 | Australia | Prospective, controlled before-and-after intervention trial | Statistical analysis | PEWS |
|
| Mohammed et al.165 2009 | UK | Three phases; the first two were based in a classroom and asked nurses to calculate an EWS from vignettes using pen and paper, followed by a hand-held computer. The third phase followed the previous approach but was based on the ward after nurses had been using the device for 4 weeks | Statistical | Electronic systems |
|
| Mohammmed Iddrisu et al.148 2018 | Australia | To explore nurses’ role in recognising and responding to deteriorating postoperative patients through focus groups | Thematic analysis | Snowball sample |
|
| Paciotti et al.161 2014 | USA | Semistructured interviews with clinicians | Grounded theory and constant comparison | Family involvement |
|
| Pattison and Eastham123 2012 | UK | Mixed-method study looking at the impact of a critical care outreach team | Statistical analysis and grounded theory | EWS |
|
| Radeschi et al.150 2015 | Italy | Multicentre survey to identify the attitudes and barriers to MET use | Statistical analysis | EWS |
|
| Schmidt et al.168 2015 | UK | Retrospective analysis of data collected routinely. Pragmatic observational study of VitalPAC deployment in two large hospitals | Statistical analyses | Electronic systems |
|
| Sefton et al.166 2017 | UK | Controlled study of vital signs documentation and PEWS calculation and a survey of acceptability | Descriptive statistics | PEWS |
|
| Shearer et al.125 2012 | Australia | A mixed-method study | Iterative coding | EWS |
|
| Sønning and Nyrud134 2018 | Norway | Questionnaire of a sample of staff who use a PEWS | Descriptive statistics | PEWS |
|
| Stevenson and Nilsson175 2012 | Sweden | Qualitative; focus groups with 21 registered nurses | Content analysis of interviews | Electronic systems |
|
| Stewart et al.126 2014 | Sweden | Mixed method. Retrospective review of records and nurse-led focus groups | Statistical analysis and content analysis | EWS |
|
| Subbe et al.170 2017 | UK | A before-and-after study of an electronic automated advisory vital signs monitoring and notification system. Elevated scores were relayed to a RRT | Statistical analysis | Snowball sample |
|
| Wager et al.174 2010 | USA | Observational study | Descriptive statistics | Electronic systems |
|
| Watson et al.137 2014 | USA | Mixed method, retrospective medical record observations and observations of nurse interactions in 1-minute blocks | Observation analysis, although this is not described, and statistical analysis | PEWS |
|
| Wheatley128 2006 | UK | Ethnographic approach; participant observation and semistructured interviews | Thematic and content analysis | Observations and monitoring |
|
Appendix 19 Summary of planning evidence
| Study | Country | Methodology | Analysis | Search area | Evidence contribution |
|---|---|---|---|---|---|
| Abraham et al.181 2012 | USA | Pre-and-post prospective study | The quality and completeness of the hand-off note – both tools – was assessed by a multiprofessional round | Structured handover |
|
| Brady and Goldenhar153 2014 | USA | Focus groups × 7 – held in groups of participants with similar roles | Constant comparison | Situational awareness |
|
| Brady et al.178 2013 | USA | Statistical process control charts | Situational awareness |
|
|
| Claussen et al.155 2013 | USA | Retrospective review of calls to the RRT and cardiac arrest calls to evaluate impact of evidence-based guidelines | Descriptive statistics | Electronic systems |
|
| Davies et al.145 2014 | USA | Survey looking at barriers to RRS activation | Statistical analysis | EWS |
|
| Demmel et al.87 2010 | USA | Discussion of the set-up and implementation of a paediatric early warning scoring tool and an associated algorithm | Rapid PDSA cycles were implemented using small tests of change. The data from the PDSA cycles were continuously collected, analysed and reviewed with the multidisciplinary staff and planning team and used to give ongoing direction to the implementation plan | PEWS |
|
| Donahue et al.182 2010 | USA | Focus group evaluation of a training programme that was developed to teach paraprofessionals the SBAR communication tool | Not clear | Structured handover |
|
| Ennis118 2014 | Ireland | Description of implementation of PEWS and subsequent audit (prospective cohort observational study) | Simple descriptive statistics of the numbers of children triggering the PEWS and compliance with escalation protocol | PEWS |
|
| Goldenhar et al.177 2013 | USA | Semistructured interviews and focus groups to develop a deeper understanding of a newly implemented huddle systems | Constant comparison | Situational awareness |
|
| Mackintosh et al.139 2009 | UK |
|
Initial thematic analysis and search for negative cases | Situational awareness |
|
| Mackintosh et al.122 2012 | UK | Comparative case study of a RRS using ethnographic methods, including observations, interviews and documentary review | Inductive and deductive coding facilitated by NVivo. Theme-building and structuring methods from framework analysis were also used; informed by other theoretical frameworks such as ‘technology-in-practice’ | EWS |
|
| Massey et al.157 2014 | Australia | In-depth semistructured interviews | Inductive approach – thematic analysis | EWS |
|
| McCrory et al.146 2012 | USA | Prospective, pre-interventional and post-interventional study to evaluate the educational intervention of teaching ABC-SBAR | Two blinded reviewers assessed 52 video-recorded hand-offs for inclusion, order and elapsed time to essential hand-off information using a scoring tool | Structured handover |
|
| Mullan et al.183 2015 | USA | Descriptive observational study | Checklists were evaluated for rates of use, completion and identification of potential safety events | Situational awareness |
|
| Parker et al.179 2017 | USA | Manual review of all eligible patient records | Descriptive statistics | PEWS |
|
| Pearson and Duncan124 2011 | UK | Brief review of the evidence base surrounding the paediatric early warning score | N/A | PEWS |
|
| Pezzolesi et al.180 2013 | UK | Delphi study for tool development |
|
Situational awareness |
|
| Randhawa et al.89 2011 | USA | Description of the implementation process with cardiopulmonary arrest statistics pre and post implementation | Once a cycle from the implementation has been completed, this is evaluated and then another cycle begins | PEWS |
|
| Stewart et al.126 2014 | Sweden | Mixed method. Retrospective review of records and nurse-led focus groups | Statistical analysis and content analysis | EWS |
|
| Van Voorhis and Willis127 2009 | USA | Discussion paper highlighting the process of developing a paediatric RRS | N/A | PEWS |
|
| de Vries et al.176 2017 | Netherlands | Semistructured interview | Qualitative content analysis | PEWS |
|
| Wager et al.174 2010 | USA |
|
Descriptive statistics | Electronic systems |
|
| Watson et al.137 2014 | USA | Mixed method, retrospective medical record observations and observations of nurse interactions | Observation analysis, although this is not described, and statistical analysis | PEWS |
|
| Weiss et al.184 2013 | Canada | A randomised controlled trial in an academic PICU of 20 handover events | Differences between intervention and control groups were assessed using the Mann–Whitney U-test and multivariate linear regression | Structured handover |
|
| Wong et al.186 2015 | UK | Description of user-focused design process for use of electronic monitoring and numbers of observations taken using the system. Acceptability questionnaire | Descriptive statistics on the number of observations recorded using the SEND system and the number of active users | Electronic systems |
|
Appendix 20 Summary of action evidence
| Study | Country | Methodology | Analysis | Search area | Evidence contribution |
|---|---|---|---|---|---|
| Adelstein et al.141 2011 | Australia | Prospective comparison of RRT criteria breaches | Statistical | EWS |
|
| Almblad et al.188 2018 | Sweden | Retrospective review of electronic patient record and a context assessment of the work environment using the Alberta Context Tool | Statistical | Snowball sample |
|
| Andrews and Waterman114 2005 | UK | Interviews and observations | Grounded theory | EWS |
|
| Astroth et al.142 2013 | USA | Semistructured interviews with nurses | Coding categories were generated from the data, and consensus on final themes was achieved through an iterative process | EWS |
|
| Azzopardi et al.115 2011 | Australia | Survey | Statistical analysis | PEWS |
|
| Bavare et al.196 2018 | USA | Retrospective observational study of rapid response events | Descriptive statistics | PEWS |
|
| Bogert et al.194 2010 | USA | Implementation of Condition Help (ConditionH) | Descriptive statistics | Family involvement |
|
| Bonafide et al.116 2013 | USA | Semistructured interviews | Grounded theory | PEWS |
|
| Braaten117 2015 | USA | Document review and interviews using the principles of cognitive work analysis | Inductive and deductive forms of analysis – cognitive work analysis, framework and directed content analysis | EWS |
|
| Brady et al.178 2013 | USA | Statistical process control charts | Situational awareness |
|
|
| Brady et al.226 2015 | USA | A retrospective cohort study looking at the association between family and clinician activations and transfer to the intensive care unit following a MET call | QI methods and statistical process control charts were used to assess the rate of family activation of METs | Family involvement |
|
| Chua et al.130 2013 | Singapore | A qualitative survey using critical incident technique | Inductive content analysis | EWS |
|
| Cioffi140 2000 | Australia | Unstructured interviews with nurses who had activated the MET | Simple code and retrieve | EWS |
|
| Cioffi143 2000 | Australia | Unstructured interviews | Simple code and retrieve | EWS |
|
| Cioffi et al.135 2006 | Australia | Focus groups with clinicians and nurses exploring their responses to abnormal vital signs | Constant comparison | EWS |
|
| de Groot et al.152 2018 | Netherlands | Retrospective patient review and semistructured interviews with professionals | Descriptive statistics and grounded theory | PEWS |
|
| Dean et al.190 2008 | USA | Two-year reflection following implementation of Condition Help (ConditionH) | Descriptive statistics | Family involvement |
|
| Demmel et al.87 2010 | USA | Discussion of the set-up and implementation of a paediatric early warning scoring tool and an associated algorithm | Rapid PDSA cycles were implemented using small tests of change | PEWS |
|
| Donohue and Endacott154 2010 | UK |
|
Thematic analysis | EWS |
|
| Downey et al.164 2017 | UK | Narrative review | Patterns were identified and translated to themes, which were further refined using an iterative process164 | PEWS |
|
| Endacott and Westley133 2006 | Australia | Questionnaire, in-depth interviews and observations | Content analysis and constant comparison | EWS |
|
| Ennis118 2014 | Ireland | Description of implementation of PEWS and subsequent audit (prospective cohort observational study) | Simple descriptive statistics of numbers of children triggering PEWS and compliance with escalation protocol | PEWS |
|
| Entwistle163 2004 | USA | Editorial | N/A | Family involvement |
|
| Gerdik et al.195 2010 | USA | Routine data collection for number of RRT calls and the result of these activations and patient/family survey relating to RRT activation | Statistical analysis | Family involvement |
|
| Gill et al.193 2016 | Australia | Commentary drawing together family-centred care concepts, the NSQHS Standards and the development of family-initiated care in Australia | N/A | PEWS |
|
| Greenhouse et al.191 2006 | USA focus | Discussion about the implementation of Condition Help (ConditionH) | Descriptive statistics | Family involvement |
|
| Hueckel et al.192 2012 | USA | Scripted family teaching about RRT activation at the time of patient admission from Condition Help (ConditionH) | Descriptive statistics about delivery of educational programme and RRT call-out; survey testing family understanding | Family involvement |
|
| James et al.131 2010 | UK | Postal survey with HCAs using closed and open questions | Descriptive statistics and content analysis of qualitative data | Observations and monitoring |
|
| Jensen et al.138 2018 | Denmark | Focus group exploring nurses’ experiences with PEWS | Qualitative meaning condensation analysis | PEWS |
|
| Kaul et al.119 2014 | USA | Descriptive cross-sectional study; nurse and medical staff survey | Descriptive statistics | PEWS |
|
| Lobos et al.120 2014 | Canada | Implementation discussion | Simple descriptive statistics | PEWS |
|
| Mackintosh et al.122 2012 | UK | Comparative case study of a RRS using ethnographic methods including observations, interviews and documentary review | Inductive and deductive coding facilitated by NVivo; also used theme-building and structuring methods from framework analysis, while also informed by other theoretical frameworks such as ‘technology-in-practice’ | EWS |
|
| Mackintosh et al.132 2014 | UK | Ethnographic perspective; observations, semistructured interviews | Data were inductively and deductively coded and organised thematically | EWS |
|
| Massey et al.157 2014 | Australia | In-depth semistructured interviews | Inductive approach – thematic analysis | EWS |
|
| McCabe et al.129 2009 | UK | Opinion piece about lessons to be learnt from the adult experience of implementing early warning systems | N/A | PEWS |
|
| McDonnell et al.121 2013 | UK | Single-centre, mixed-method before-and-after study including a survey to measure changes in nurses’ knowledge after implementation of a TTS; also, qualitative interviews | Statistical analysis and thematic framework analysis | EWS |
|
| Monaghan44 2005 | UK focus | Commentary on the development of the Brighton PEWS and setting up a paediatric critical care outreach team | Simple descriptive statistics of all activations, actions and outcomes during the first 3 months of implementation | PEWS |
|
| Paciotti et al.161 2014 | USA | Semistructured interviews with clinicians to explore physicians’ viewpoints on families facilitating the identification of children with a deteriorating condition | Grounded theory and constant comparison | Family involvement |
|
| Pattison and Eastham123 2012 | UK | Mixed-method study looking at the impact of a CCOT | Statistical analysis and grounded theory | EWS |
|
| Pearson and Duncan124 2011 | UK | Brief review of the evidence base surrounding PEWS, together with reflections from their own experiences from the Birmingham Children’s Hospital | N/A | PEWS |
|
| Salamonson et al.147 2006 | Australia | Survey with closed and open questions to examine perceptions of and satisfaction with the MET | Descriptive statistics and content analysis | EWS |
|
| Shearer et al.125 2012 | Australia |
|
Iterative coding | EWS |
|
| Sønning and Nyrud134 2018 | Norway | Questionnaire of a sample of staff who use PEWS | Descriptive statistics | PEWS |
|
| Stewart et al.126 2014 | Sweden | Mixed method. Retrospective review of records and nurse-led focus groups | Statistical analysis and content analysis | EWS |
|
| Van Voorhis and Willis127 2009 | USA |
|
N/A | PEWS |
|
| de Vries et al.176 2017 | Netherlands | Semistructured interview | Qualitative content analysis | PEWS |
|
| Watson et al.137 2014 | USA | Mixed method; retrospective medical record observations and observations of nurse interactions in 1-minute blocks | Observation analysis, although this is not described, and statistical analysis | PEWS |
|
Appendix 21 Summary of embedded paediatric early warning system improvement initiatives across all case studies
| Embedded initiatives | PUMA or non-PUMA |
|---|---|
| Out-of-hours SOP for on-call medical teams | PUMA |
| Training clinical staff on (1) PEWS, (2) recognition and response to deterioration and (3) NICE sepsis screening | PUMA |
| Lower trigger threshold | Non-PUMA |
| Sepsis 6 pathway | Non-PUMA |
| Appointment of additional staff: specialist sepsis nurses | Non-PUMA |
| Introduction of a safety huddle | Non-PUMA |
| Ward-specific escalation plan | Non-PUMA |
| Embedded initiatives | PUMA or non-PUMA |
|---|---|
| Doctors’ handover sheet changed to include all patients | PUMA |
| Nurses’ handover sheet changed from unstructured sheet to SBAR format | PUMA |
| Electronic PEWS | Non-PUMA |
| Safety huddle | Non-PUMA |
| Embedded initiatives | PUMA or non-PUMA |
|---|---|
| Whiteboard | PUMA |
| New escalation policy | PUMA |
| Embedded initiatives | PUMA or non-PUMA |
|---|---|
| Create posters and cards for staff to signpost abnormal thresholds for vital signs | PUMA |
| Update observation charts to include normal age-related thresholds | PUMA |
| Update and disseminate observation policy | PUMA |
| Review and disseminate existing escalation policy | PUMA |
| Conduct inventory of equipment | PUMA |
| Establish a staff training course on situational awareness | PUMA |
| Introduce process for identifying ‘watchers’ at each ‘huddle’ and handover, for example with markers on a whiteboard | PUMA |
Appendix 22 Summary of paediatric early warning system improvement initiatives across all case studies
| Number | Site | Proposed initiative | Element of system being addressed | Understanding/source of the problem | Implemented?/initiative changed | Embedded? | Promoting/hindering factors |
|---|---|---|---|---|---|---|---|
| 1 | Alder Hey | Develop a tool to encourage family engagement | Detect |
|
Yes | Yes |
|
| 12 | Alder Hey | Retraining on PEWS recognition and response to deterioration, including NICE sepsis screening for front-line clinical staff | Detect, plan, act | Need to improve recognition and response to deteriorating patients . . . Evidence that signs of deterioration including sepsis have not always been managed as quickly as desired | Yes | Yes |
|
| 13 | Alder Hey | Implement SOP for out-of-hours working for on-call medical teams – prioritising sickest children (hospital-wide) | Detect, plan, act | Need to improve how on-call junior medical team prioritise workload to identify and respond to the sickest patients across the hospital . . . the weekend ward round is often still ongoing at night-time, with all patients being seen and no structured focus on the sickest patients | Yes | Yes |
|
| 14 | Alder Hey | Establish a monthly Critical Deterioration Review Panel to learn lessons about which aspects of the system need improvement | Detect | Need to tighten process of identifying and responding to sick children in the hospital . . . there is occasionally some complacency regarding increased PEWS and response is less than adequate . . . We want to have a review process for all cases of critical deterioration and look for opportunities for prevention that can be fed back to teams in real time. The goal is to learn and continually improve | Yes | No |
|
| 20 | Alder Hey | Implement SOP for ward 1C ward round structure | Plan |
|
No | No |
|
| 6 | Arrowe Park | Introduction of a second daily huddle | Plan | Communication between senior nurses and doctors is more challenging in the afternoon/evening when medical staff are located away from the ward on the PAU | Changed | Yes |
|
| 9 | Arrowe Park | Joint handover sheets, using the SBAR technique | Plan | Currently, nursing and medical handovers are conducted separately (although nurses occasionally attend medical handover). However, there is a feeling that the doctors handover sheets contain information that would be useful for the nurses – and vice versa | Changed | Yes |
|
| 15 | Arrowe Park | Nurse education | Detect | At present, there is no structured approach to ongoing nurse education – particularly with regard to PEWS, and identifying potential deterioration on the ward | Yes | No |
|
| 16 | Arrowe Park | Introduction of the SHINE leaflets and poster | Detect | Feeling that there is currently no formal process for encouraging family members to input their concerns about possible deterioration | Yes | No |
|
| 7 | Noah’s Ark Hospital | Introduction of electronic site board | Plan | No clear mechanisms for highlighting and communicating the most at-risk children between teams. As a result, clinical staff are not always aware of the most at-risk/sick children in their area – so not as efficient as could be at allocating of resources/prioritising high-risk children | Yes | Yes |
|
| 10 | Noah’s Ark Hospital | Introduction of new escalation policy | Act |
|
Yes | Yes |
|
| 17 | Noah’s Ark Hospital | Introduction of parent posters (based on SHINE tool, designed to inform parents about how to communicate concerns) | Detect | Inconsistency in information given to parents/family members when children are admitted. Perception that some family members do not feel empowered to report deterioration of child’s condition when it happens | Yes | No |
|
| 2 | Morriston | Create posters and cards for staff to signpost abnormal thresholds for vital signs | Detect | No normal ranges on current observation charts; need to be clearer, and signpost staff to escalation of care | Yes | Yes |
|
| 3 | Morriston | Update observation charts to include normal age-related thresholds | Detect | Existing observation charts outdated. Need more clarity, for ease of use as a signpost to escalation | Yes | Yes |
|
| 4 | Morriston | Update and disseminate observation policy | Detect |
|
Yes | N/A |
|
| 5 | Morriston | Conduct inventory of equipment | Detect | Not enough suitable equipment to enable staff to conduct observations effectively | Yes | N/A |
|
| 8 | Morriston | Establish a staff training course on situational awareness | Plan | There is no regular training on risk management; staff not routinely trained in situational awareness | Changed | Yes |
|
| 11 | Morriston | Review and disseminate existing escalation policy | Act | Lack of awareness of policy. Some staff unsure of roles and responsibilities around escalation | Yes | Yes |
|
| 18 | Morriston | Explore tools for family/parental involvement | Detect |
|
Yes | No |
|
| 19 | Morriston | Introduce process for identifying ‘watchers’ at each ‘huddle’ and handover, for example with markers on a whiteboard | Plan | Board rounds and ward rounds could be improved. Increase and maintain staff awareness of children at risk | Yes | No |
|
| 21 | Morriston | Formally establish Deteriorating Child Study Day across health board | Plan | Staff not always able to go to training for identifying risk because of staffing issues. Desire to formalise course with health board approval, make a biannual event | No | No |
|
| 22 | Morriston | Roll out in-house e-learning package for nursing and medical staff | Plan |
|
No | No |
|
| 23 | Morriston | Ward nursing staff to spend more time observing HDU staff | Plan | Inexperienced staff to gain more knowledge, enhance their learning about critically ill children | No | No |
|
| 24 | Morriston | Move to adopt 3 × daily ‘huddles’/board rounds | Plan |
|
No | No |
|
| 25 | Morriston | Review handover content. Possibility of including nursing staff in medical handover | Plan | Handover content could be standardised to aid identification of potential deterioration. Opportunity for information-sharing, improved situational awareness, less chance of missing information in separate handovers | No | No |
|
| 26 | Morriston | Re-establish a nursing supernumerary role | Plan |
|
No | No |
|
| 27 | Morriston | Review communication tools to aid escalation of patient care | Act |
|
No | No |
|
Appendix 23 Next steps in the development of the PUMA programme
The PUMA programme was developed iteratively over the lifetime of the study, and the materials and resources were refined in response to feedback from the PIs and our experiences of the materials in use. If the PUMA programme is to be made more widely available, then several additional refinements are indicated.
First, in PUMA, the assessment tools were completed on hard copy and, in large sites, the study team assisted in collating and summarising results. If system assessment is to be facilitated to sustain improvement and generate a learning system, then there is a need for ICT to facilitate these processes.
Second, complex interventions tend to have distributed costs and benefits, which need to be taken into account in implementing change. 227 There are numerous examples in our case studies of when interventions had positive impacts for some actors, but increased the burdens of others and/or challenged power relationships between groups. Others have advocated the use of stakeholder mapping to trace these relationships before implementing a change and build the necessary support for improvement; this would be a useful addition to the PUMA programme resources. 228
Third, we also see scope for developing practical guidance to involving parents in improvement processes. This would include parental involvement in the improvement team (OUTCOME principle 5) and the use of routinely collected patient experience and family feedback data in the local learning system (OUTCOME principle 6).
Fourth, although ongoing facilitation was necessary in the context of the study, PUMA is intended to be a parsimonious intervention, so that it might be adapted and replicated widely if proven successful. Findings from the pilot sites suggest that the PUMA programme could be implemented with minimal resource. Moving forward, further work is required to explore cost-effective models of facilitation, for example using a manualised train-the-trainer approach. Peer-to-peer support could also be an option for scaling and spreading. 229
Fifth, all materials will need to be made available as online resources.
List of abbreviations
- 4Ss
- sickest patients, bed status, safeguarding issues and staffing
- 5Ss
- safeguarding, same name, bed status, sick children and staffing
- A&E
- accident and emergency
- ANP
- advanced nurse practitioner
- APLS
- Advanced Paediatric Life Support
- ARIMA
- autoregressive integrated moving average
- AUROC
- area under receiver operating characteristic
- C&VPEWS
- Cardiff and Vale Paediatric Early Warning Score
- C-CHEWS
- Cardiac Children’s Hospital Early Warning Score
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CQC
- Care Quality Commission
- DARE
- Database of Abstracts of Reviews of Effects
- DGH
- district general hospital
- FFT
- Family Feedback Tool
- HCA
- health-care assistant
- HDU
- high-dependency unit
- HMIC
- Health Management Information Consortium
- ICT
- information and communication technology
- ID
- identification
- IS
- implementation science
- ITS
- interrupted time series
- MAC
- Melbourne Activation Criteria
- MET
- medical emergency team
- NHS III
- NHS Institute for Improvement and Innovation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- OR
- odds ratio
- PAU
- paediatric assessment unit
- PEWS
- Paediatric Early Warning Score
- PI
- principal investigator
- PICU
- paediatric intensive care unit
- PPI
- patient and public involvement
- PPV
- positive predictive value
- PTTT
- paediatric track-and-trigger tool
- PUMA
- Paediatric early warning system Utilisation and Morbidity Avoidance
- QI
- quality improvement
- RCN
- Royal College of Nursing
- RCPCH
- Royal College of Paediatrics and Child Health
- RESPOND
- Recognising Signs of Paediatric hOspital iNpatients Deterioration
- RR
- risk ratio
- RRT
- rapid response team
- SBAR
- situation, background, assessment, recommendation
- SHO
- senior house officer
- SOP
- standard operating procedure
- SSAT
- Staff System Assessment Tool
- TMT
- translational mobilisation theory
- TTT
- track-and-trigger tool
Notes
-
Screenshots from Alder Hey’s MEDITECH 6 electronic recording and scoring system
-
Pre-implementation system strengths and weaknesses identified by the PUMA team across all case studies
-
Pre-implementation system strengths and weaknesses identified by the improvement team across all case studies
-
Summary of the implementation of PUMA action plan initiatives and non-PUMA initiatives across all case studies
-
Post-implementation system strengths and weaknesses identified by the PUMA team across all case studies
-
Paediatric track-and-trigger tool observation chart, Arrowe Park
-
Monitoring guidance included in the Arrowe Park paediatric track-and-trigger tool
-
The situation, background, assessment and recommendation technique nursing handover sheet, Arrowe Park
-
Whiteboard in the general medical doctors’ handover room, Noah’s Ark Children’s Hospital
-
Additional safety briefing information, Noah’s Ark Children’s Hospital
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/CHCK4556).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
