Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/69/02. The contractual start date was in June 2018. The draft report began editorial review in December 2020 and was accepted for publication in August 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Reeve et al. This work was produced by Reeve et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Reeve et al.
Chapter 1 Background
Overview
Polypharmacy is common practice in modern health care, offering benefits to many patients. However, a report by The King’s Fund1 on polypharmacy recognised growing awareness of the potential for harm and waste associated with the long-term use of multiple medicines, especially in patients with complex health problems (e.g. multimorbidity). It recommended that all medication reviews include a consideration of whether or not medicines could be stopped. Deprescribing was thus recognised as an important component in optimising the use of medicines in a polypharmacy context, with a call for practice to be tailored to individual circumstances. The need for new evidence to support patient-centred understanding of deprescribing practice was therefore identified. This is the focus of the TAILOR synthesis.
Describing the problem: managing problematic polypharmacy
Polypharmacy, the concurrent use of multiple medicines in a single person, is on the rise, driven by an expanding population living with multiple long-term conditions (multimorbidity). It is estimated that around 1 in 5 patients takes five or more medicines per day. 1 Polypharmacy can be appropriate, extending life expectancy and improving quality of life. 1 However, 40% of people taking five or more medicines per day report feeling significantly burdened by their medication. 2 These individuals are experiencing what has been described as problematic polypharmacy: the use of multiple medicines on a long-term basis when the intended benefit of the medicines is not achieved, or the potential risks outweigh the intended benefits. 1 Problematic polypharmacy is associated with treatment burden, potential harm and waste (through non-concordance). 1 It is, therefore, a challenge for patients, professionals and health services alike.
In its 2013 review1 of the challenge of polypharmacy, The King’s Fund highlighted the potential importance of deprescribing as part of the response to problematic polypharmacy. The report recommended that consideration of stopping medicines should be an integral part of all medication reviews. The report also underlined the importance of adopting a person-centred approach when making decisions about medicines use, recognising that the perspectives and priorities of the medicines taker (patient) and indeed their family and carers may or may not match the priorities of the prescriber. 1,3 The King’s Fund report1 recognised that compromises may be needed between a prescriber’s goal to optimise medical management and a patient’s choices based on individual circumstances. This compromise was described in Denford et al. ’s3 review as a process of balancing the benefits and harms from medication use through the mutually agreed tailoring of medicines.
A strong and growing body of evidence-based guidelines recognises benefit and harms, and so describes best practice, in starting medication for various conditions. Equivalent guidance on stopping medicine (deprescribing) has been slower to appear. Notable exceptions include the Scottish polypharmacy guidelines (first published in 2012, and updated in 2015, 2018 and 2019). 4,5 Similar to the report by The King’s Fund,1 the guidelines4,5 advocate individualised reviews of the merits of each medicine prescribed to an individual, including consideration of whether or not it should be continued. However, although deprescribing (in the context of person-centred care) was increasingly seen as good practice in principle, there remained a shortage of evidence-informed guidelines on how to deprescribe in practice. This gap was recognised by a call in 2017 from the National Institute for Health and Care Research (NIHR) (17/69), ‘Safely and effectively stopping medications in older people with multimorbidity and polypharmacy’. The call asked for research to ‘describe the benefits, harms and optimal strategies for the safe withdrawal of medication in older people with multimorbidity to reduce polypharmacy and treatment burden’. TAILOR was a response to that call.
Addressing the problem: what we already know about deprescribing
Dealing with problematic polypharmacy means knowing how to safely and effectively taper, withdraw or stop medications that may be offering more harm than benefit. 6 However, discontinuing long-term medicines is a process that causes anxiety and concern for clinicians and patients alike. 6,7 Deprescribing is the process of supervised withdrawal of potentially inappropriate medication,8 a planned/supervised process of dose reduction or the stopping of medicines that may be causing harm or conferring no additional benefit. However, clinicians remain concerned about the safety and impact of stopping medicines, including the potential consequences for them as decision-makers. 7,8
Part of the challenge lies in recognising what is ‘inappropriate medication’ that can or should be withdrawn. The withdrawal of medicines that are causing acute harm to patients (e.g. following an acute adverse reaction) is a common experience for patients and prescribers alike. In such situations, the risk–benefit ratio of acute discontinuation, and hence the clinical decision to be made, is usually clear. Long-term medication can be more challenging. Guidance on stopping longer-term, potentially inappropriate, medicines (defined on biomedical grounds) has been around for a number of years [e.g. Beers criteria9 and the Screening Tool of Older Person’s Prescriptions/Screening Tool to Alert doctors to the Right Treatment (STOPP/START) tool10]. Such tools help identify potentially inappropriate drugs, considering dose and duration, but do not provide explicit support on stopping the medicines. A particular challenge comes in knowing how and when to stop medication that may be seen as ‘appropriate’ from a clinical perspective (including condition-specific guidelines) but potentially ‘not right for this individual’ as judged by the patient or their clinician (e.g. discontinuation of primary prevention medication). 6
The second challenge comes in managing the process of withdrawal, including understanding potential issues related to safety and impact. The Scottish polypharmacy guidelines4 address this issue by offering clinicians clear guidance on the potential absolute benefit of medication use (e.g. the numbers needed to treat with warfarin to prevent one stroke in people living with atrial fibrillation). This offers clinicians useful data to discuss likely benefit with patients, and so, if appropriate, support a conversation about discontinuation. However, to our knowledge, there is no comprehensive review, or data set, describing absolute effects of stopping medication.
Since NIHR published the funding call that supports this TAILOR project, we have seen publication of a range of resources to support deprescribing. These include a National Institute for Health and Care Excellence (NICE) guideline11 focused specifically on the deprescribing of hypnotics and a number of institutional resources describing best practice aimed at supporting staff managing the problem in the field,12–14 as well as expert commentaries from academics working in the field. 15,16 All seek to support professionals in the complex process of tailoring medication use to individual circumstances and in making ‘defendable decisions’ with regard to the individual tailoring of medicines. 6,17
Much of the guidance to date draws on the principles of good prescribing practice, supported by data on prescribing for specific conditions. Both the principles of good prescribing practice and data on prescribing for specific conditions offer support for deprescribing practice in highlighting the absolute (limitations to) benefit of medication in given conditions, and in offering permission in principle for person-centred care. 18
However, our previous research has revealed four barriers to tailored care, and specifically tailored prescribing, which would suggest that the guidance to date could have a limited impact. 6,17 Professionals involved in the complex decision-making (knowledge work)19 of providing beyond-guideline (tailored) care report a lack of confidence in undertaking this work because of a lack of perceived support in four areas (Table 1).
| Barrier | Description |
|---|---|
| Permission to work beyond guidelines and outside specialist frameworks | Guideline care is perceived as ‘best’ practice; beyond guideline care is ‘exceptional’ practice and needs to be justified. Lines of responsibility are unclear for generalists (e.g. GPs) reviewing medication started in specialist practice20 |
| Prioritisation of the greater workload involved | Practice workflow is designed to support delivery of usual care, with insufficient time17 and headspace6 built into the day to support the extended conversations and considerations (including justifications) for beyond-protocol care |
| Professional skills in complex decision-making and the confidence to use them | The extended skills of expert generalist (tailored) decision-making are not consistently taught (often learnt through experience and apprenticeship), with professionals describing lack of confidence in using the skills they have |
| Performance management supportive of the task | Complex decision-making is often not adequately recognised and rewarded by performance management processes (e.g. the Quality and Outcomes Framework), and may even be criticised (e.g. excessive exception reporting) |
Research therefore describes whole-system barriers to tailored (de)prescribing practice at consultation, organisation of practices and policy levels. 21 Structural changes, such as the design of health-care systems (including workflow) and performance management tools, may require evidence that different models of care provision offer efficient, effective and equitable care. But this research also points to work6,17 that may support individual clinicians and patients (consultation-level changes) in tackling problematic polypharmacy through tailored care. It does this specifically by providing them with evidence-based support that addresses ‘permission’ (why you could tailor care) and professional skills and confidence (how you could tailor care).
Addressing the gaps in our knowledge: describing the TAILOR evidence synthesis
Based on our overview of the current literature on problematic polypharmacy, stakeholder discussions and our own research in this field, we identified two specific additional areas of knowledge needed to support clinicians in the decision-making (knowledge work) of tailored deprescribing. These form the basis for the work of the TAILOR evidence synthesis.
Data on safety and impact of discontinuing medication
Advice for clinicians published by NICE,22 which it recognises as ‘guidelines not tramlines’ provides non-mandatory advice to inform, but not dictate, best practice. The limitations of guidelines for clinical practice are well recognised, for example in being ‘condition specific’ and ‘context blind’. 23 Guideline development has been criticised for using evidence that often excludes patients with multimorbidity,20 or for overlooking, or placing less weight on, evidence related to patients’ lived experiences of illness or treatment (e.g. theoretical or qualitative work). 24 Clinical practice for person-centred care inevitably involves working beyond guidelines, especially for people with multimorbidity, to reduce the risk of burden and iatrogenic harm. 25
In practice, guidelines are one source of data used by clinicians in the complex task of interpreting individual patient need,26–28 with their limitations well recognised. 29 Clinicians delivering tailored, whole-person care engage in a complex task of data collection, described by Donner-Banzhoff and Hertwig30 as inductive foraging, which draws on data from patient consultation, external data sources (including guidelines and evidence) and professional experience and expertise. 28
Access to data that helps clinicians in this process (to discuss the safety and impact of discontinuing medication with their patients and/or carers) is therefore key to tailored prescribing. 6 TAILOR addresses that gap through a scoping review to summarise the current available evidence on deprescribing in a form that makes a useful reference source for clinicians.
Framework for judging ‘best’ practice for tailored prescribing decisions
Tailored decisions require professional interpretation of multiple data sets, often in varied and varying circumstances, to generate individualised assessments of potential for risk and benefit. 14,28 Tailored prescribing may require the use of ‘clinically appropriate overrides’ of evidence-based guidance. 31 Outcomes of patient-centred care focus on clinical decisions that optimise health-related capacity for daily living, not simply condition- or medication-specific outcomes. 32–37
Defining and delivering best practice in tailored (de)prescribing can therefore be understood as a ‘wicked problem’: a complex (and often messy) problem that cannot be fixed because of incomplete, competing and changing requirements, but can be managed through iterative, adaptive and ongoing responses,38,39 resulting in solutions and outcomes that are better described as ‘better or worse’ rather than ‘right or wrong’. 40–42 Clinicians, patients and wider stakeholders, therefore, need a framework using which they can judge ‘better or worse’.
Medicines optimisation is the framework currently used to guide best practice, emphasising outcomes focused on clinical effectiveness, cost-effectiveness and minimising both harmful effects and waste. 43 However, it is known that patients define benefit from medicines differently from clinicians. What a medical perspective may describe as effective or optimal care may be experienced as burdensome by patients. 1,32,44 Evidence highlights that patients prioritise the impact of care (including medicines use) on their continued daily living33,44–47 over the management of disease. 48,49 Assessing best, or better, practice by adherence to guidelines will be insufficient.
Tailored care actively incorporating patients’ priorities and perspectives into clinical decision-making may produce varied outcomes depending on individual patient circumstances and priorities. Outcomes of tailored prescribing decisions may also not be immediately apparent. For example, a tailored decision to stop primary prevention medication may not produce any recordable effect for some time, if at all. Assessing best, or better, practice using simple outcome measures may not be sufficient.
The research demonstrates that if we are to address the identified barriers to tailored (de)prescribing of permission and supporting professional confidence in the knowledge work of complex decision-making, clinicians need additional tools to support judgement of better practice. 6 Clinicians describe needing ‘permission’ to work ‘beyond guidelines’6,28 and so seek a validated framework against which they can judge and defend these interpretations. 28 TAILOR addresses that gap through developing a realist programme theory that describes best practice for tailored deprescribing, and so provides critical framework for practitioners, patients and managers to judge the quality of tailored care. The TAILOR framework will also provide the additional evidence needed to address the identified organisational barriers to delivery and so describe practice redesign.
Our review of the literature, previous research and stakeholder engagement described a number of elements that may be important in developing this framework. These were incorporated into a draft programme theory used to inform the TAILOR realist review (Figure 1).
FIGURE 1.
Draft programme theory describing elements needed for individual tailoring of medicines.
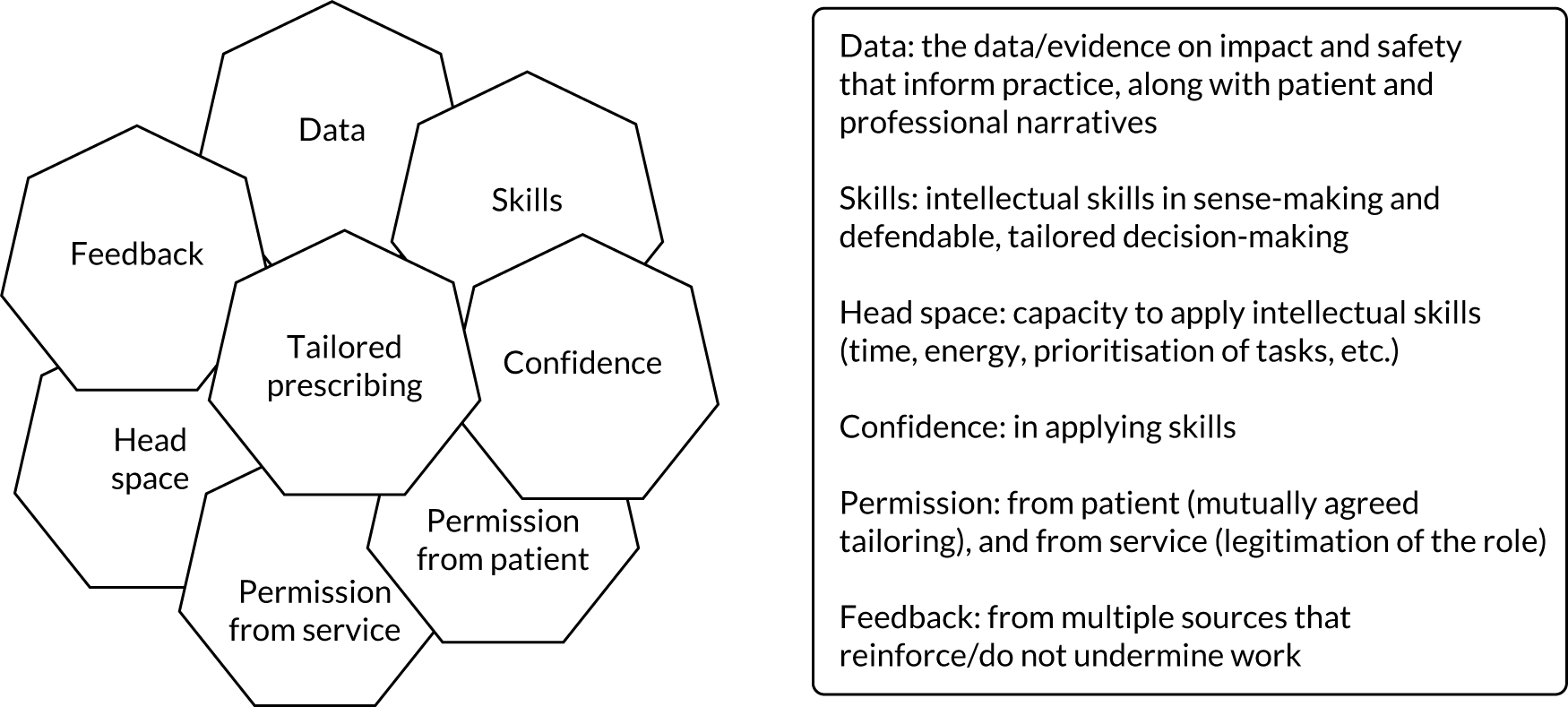
In Chapter 2, we describe how these findings and observations shaped the design of the TAILOR evidence synthesis.
Chapter 2 Research questions and design
Overview
In outlining the problem in supporting tailored deprescribing in the person-centred management of problematic polypharmacy, we have identified two key gaps in the existing body of knowledge available to clinicians to support robust and safe tailored decision-making around deprescribing. First, we recognise the need for a structured overview of the data on safety and effectiveness of deprescribing to provide clinicians with a key resource for interpretive practice. 28 Second, we need a robust, evidence-informed framework describing the key components of good clinical practice for tailored prescribing. In this chapter, we outline the decision to address these needs using two distinct review methodologies, interlinked through a shared initial search strategy and ongoing combined critical reflection.
Research questions
Our literature review in Chapter 1 led us to formulate two research questions for TAILOR to address:
-
What quantitative and qualitative evidence exists to support the safe, effective and acceptable stopping of medication in older people with multimorbidity and polypharmacy?
-
How, for whom and in what contexts can safe and effective individual tailoring of clinical decisions related to medication use work to produce desired outcomes?
Aims and objectives
Our aim was to deliver to clinicians, patients and policy-makers the resources that they need to support safe and effective compromise when tailoring medicines to individual needs and circumstances. Our two research questions prompted the use of different methodological approaches to answer them and so generated our first two objectives. The third objective recognised our commitment to delivering outputs that can have an impact on clinical care.
We therefore describe three objectives:
-
to complete a robust scoping review of the literature on stopping medicines in this group to describe what is being done, where and for what effect
-
to undertake a realist synthesis review to construct a programme theory that describes ‘best practice’ and helps explain the heterogeneity of deprescribing approaches
-
to translate findings into resources to support tailored prescribing in clinical practice.
Our intended outputs were to deliver (1) a reference data set for clinicians describing the approaches to deprescribing being used and what is known on effectiveness, safety and acceptability; and (2) a framework describing best (‘better’)41 practice in the individual tailoring of medicines, generating a set of recommendations for practice.
Justification for design
The research questions identified by our review of the literature (see Chapter 1) led us to recognise the need for different methodological approaches to answer each question.
Justification for a scoping review
Preliminary searches undertaken in preparing our bid demonstrated that the current body of evidence on deprescribing is disparate with significant heterogeneity. Studies cover many topic areas (e.g. clinical problems and research methods used), although the volume of scholarship in each area appears to be relatively small. We concluded that standard systematic review methods (including meta-analysis) would not allow us to adequately describe and integrate the diverse literature in a way that met our goals to offer clinicians, patients and policy-makers resources to support safe and effective tailored deprescribing.
We therefore opted for a scoping review to identify, map and draw together data in a useable form. Scoping reviews are recognised to be the most appropriate methodology when reviewing the evidence on complex interventions, allowing for the variability and complexity of the intervention and evaluation methods. 50 We chose a scoping review to enable us to systematically distil, from a diverse literature, the data that would support clinical decision-making.
Justification for a realist review
Our second goal was to provide a robust, evidence-informed framework describing the key components needed to deliver person-centred (tailored) deprescribing. Our intention was that this framework provide clinicians with a model of ‘best practice’ to support them in their daily work, and a model that could explain the heterogeneity in the literature on deprescribing.
We recognised tailored deprescribing as a complex intervention. 1 An intervention is defined as complex (rather than complicated) because it consists of numerous components interacting in non-linear ways and is sensitive to context. 51 As discussed in Chapter 1, Addressing the problem: what we already know about deprescribing, addressing problematic polypharmacy needs a tailored approach to prescribing that recognises compromise between biomedical (condition-specific factors increasingly in the context of multimorbidity) and biographical (individual context, preferences and priorities) perspectives. 1 Decisions involve weighing up multiple factors that vary in themselves and through interaction with each other. 21 Tailored deprescribing is therefore an example of a complex intervention, in which controlled and uncontrollable variation is inevitable and the active ingredient(s) may behave differently in varying contexts and for different people. 52
The realist review methodology is particularly useful for understanding and illuminating the relationships and impact of the interaction between the components of a complex intervention. 53,54 Realist reviews ask ‘what works, for whom, in what circumstances, to what extent, how and why?’ and consider the interaction between context, mechanism and outcome [how particular contexts (e.g. people, practices) trigger or interfere with mechanisms to generate the observed outcomes]. 55 Realist reviews generate explanations about the mechanisms by which stopping medication may (or may not) achieve impact in different settings and within different subgroups.
We therefore opted to use a realist review methodology to address our second research question, providing clinicians with a framework that describes and explains what is needed to support ‘better’ practice. 41 Our intention was to provide a framework against which to ‘defend’ good practice, addressing the recognised barriers to tailored care of permission, professional confidence and performance management (see Table 1). We also anticipated that that framework could help explain the heterogeneity revealed in reviews of the literature.
Justification for a combined literature search
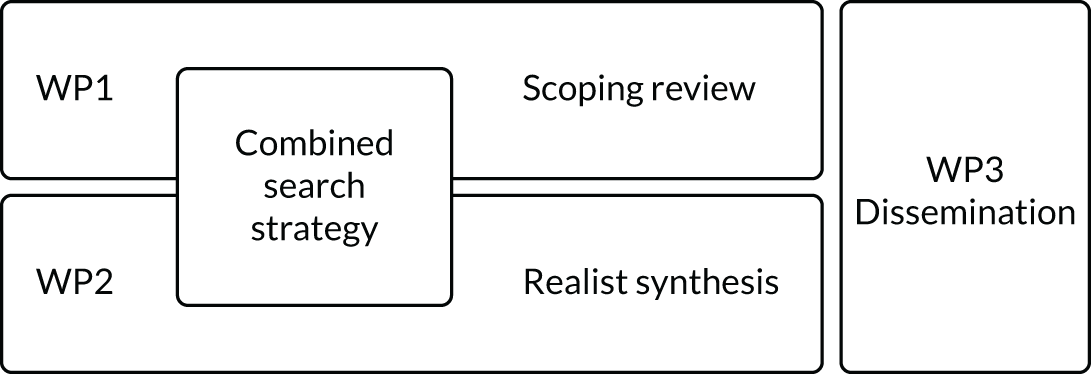
Although we identified a need for two distinct analytical approaches, our common goal was to deliver outputs that supported the clinical task of tailored deprescribing to address problematic polypharmacy. Our funders had stipulated a particular focus on an older population living with multimorbidity. Our initial proposal, therefore, was to use a combined search strategy to collect the initial data for analysis using both methodological approaches (Figure 2).
FIGURE 2.
Outlining the initial workflow for the TAILOR medication synthesis. WP, work package.
Refining the work plan
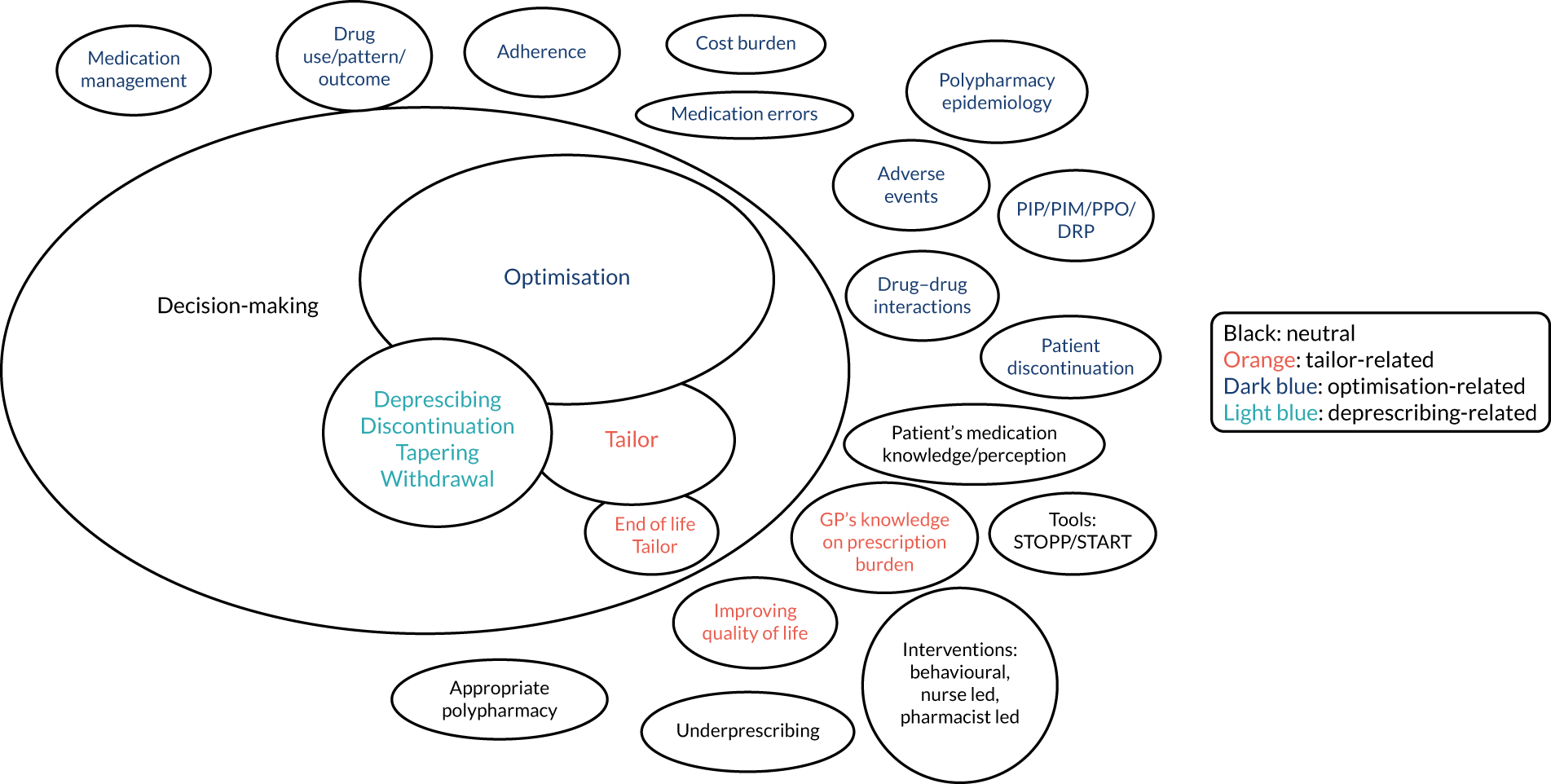
During the set-up stages of TAILOR, Nia Roberts (co-applicant) ran an initial literature search using our combined search strategy (described in Chapter 6). This generated an initial list of > 2000 studies. Kat Kavalidou (a research fellow working with us temporarily during the set-up stages) undertook initial work to categorise these studies to inform detailed discussions on developing the scoping review. Kat Kavalidou presented an initial thematic overview of the data set.
This work revealed that studies addressed a wide range of goals for practice. Three inter-related but distinct health-care goals could be identified: medicines optimisation (with a predominant focus on safety and biomedical effectiveness), deprescribing (the specific act of stopping medication) and tailored care (person-centred care around medication use). A wide range of research goals was also described, including research that aimed to define appropriate polypharmacy, improve appropriate medication use, recognise patterns of medication behaviour, improve adherence, develop and evaluate tools to recognise/address potentially inappropriate medication, and support end-of-life care.
Kavalidou’s summary of the complexity of the field is shown in Figure 3.
FIGURE 3.
Mapping the themes identified from a descriptive overview of the literature on deprescribing. ‘De-prescribing’-related refers to studies looking at stopping specific medicines, ‘optimisation’-related studies focused on safety and biomedical effectiveness, ‘tailor-related’ studies were person focused and ‘neutral’ refers to studies that did not clearly state the underlying goal. DRP, drug-related problems; PIM, potentially inappropriate medication; PIP, potentially inappropriate polypharmacy; PRO, potential prescribing omissions.
Finalising work plans
Kavalidou’s findings were discussed at an extended team meeting. We recognised that this data set provided the richness needed for a realist review. However, we were concerned that it would not allow us to meet our goal to provide a useful resource to clinicians from a scoping review. We therefore opted for a revised and refocused scoping review based on a revised search strategy.
The revised final work packages for TAILOR are shown in Table 2.
| Work package | Led by | Chapters |
|---|---|---|
| 1: scoping review | Michelle Maden, Ruaraidh Hill; Liverpool University | 3–5 |
| 2: realist synthesis | Amadea Turk, Kamal Mahtani, Geoff Wong; Oxford University | 6–8 |
| 3: dissemination | Joanne Reeve; Hull University | 10 |
Joanne Reeve provided overview of, and support for, all work packages.
Detailing the research team
As described in our protocol (version 1.1, July 2019), we assembled a team of people to undertake this work including:
-
core research team (project working group) – responsible for delivery of the work as detailed in Table 2
-
academic advisory group – the additional co-authors of this report (see Chapter 10, Reviewing how we went about the work) responsible for overseeing the academic rigour of the research
-
stakeholder group – consisting of end users of our work, responsible for ensuring that the research remains relevant and for supporting the dissemination activities.
Chapter 3 Scoping review design and methods
Overview
In outlining the problem related to supporting tailored deprescribing in the person-centred management of problematic polypharmacy, we recognised the need for a structured overview of the evidence on the safety and effectiveness of deprescribing to provide clinicians with a key resource for interpretive practice. 27
Through our scoping review, we aimed to produce this reference set by outlining the approaches to the use of deprescribing and what is known about its effectiveness, safety and acceptability. Having described the heterogeneity of the literature based on an initial search (see Chapter 2, Refining the work plan), we identified the need for a refocused scoping review. This chapter details the approach used.
Aim
The aim of the scoping review was to map and characterise the available evidence on the approaches, effectiveness, safety and acceptability of interventions to taper/tailor and stop medication in older people living with multimorbidity and polypharmacy.
Methods
Scoping reviews allow for the mapping of research findings and identification of gaps in the evidence base. 56 The methodology allowed us to identify, map and draw together the current evidence base on strategies to support safe medication withdrawal in this population, including recognising the impact of health systems and context on prescribing practice. The TAILOR scoping review was specifically designed to signpost health-care professionals and policy-makers to the quantitative and qualitative data they need to support decisions about when, if and how to stop medications. We also sought to provide valuable information to researchers and funders on the gaps in the current evidence base where new research can be prioritised. We aimed, for example, to determine the feasibility of conducting further evidence syntheses, and identify the types of synthesis needed (e.g. meta-analysis of effectiveness or meta-synthesis).
This scoping review followed the methodology for conducting a scoping review as set out by the Joanna Briggs Institute. 57 This draws on the methodological framework from Arksey and O’Malley56 and is enhanced by Levac et al. ,58 which has been used to map the evidence of complex interventions. Five stages are described: (1) setting the research question, (2) identifying studies, (3) selecting studies, (4) charting the data, and (5) collating and reporting. Consistent with the scoping review methodology, risk of bias was not assessed.
Stage 1: setting the research question
The scoping review questions were agreed by the research team in collaboration with our stakeholder and advisory groups. The overarching research question was to identify what recent quantitative and qualitative evidence exists to support the safe, effective and acceptable stopping of medication in older people with multimorbidity and polypharmacy. We wanted to offer clinicians a resource (data) set to inform their clinical judgement when making tailored prescribing decisions. Our intention was therefore to produce a map of the current evidence base for deprescribing practice outlining what is being done, where and for what effect. Our map was also to describe the ongoing gaps in our knowledge: areas where clinical judgement is particularly necessary. We therefore described a focused set of subquestions for the scoping review:
-
What research methods (study designs) have been used in the studies that focus on this topic? This offers clinicians an overview of what types of research have been done and where there are gaps (e.g. clinical trials with a biomedical outcome and/or intervention studies with a patient-centred outcome). It allows clinicians to judge the value and limitations of the reported TAILOR data set in relation to the specific clinical challenges they face.
-
What clinical strategies, contexts and outcomes have been studied on this topic? This offers clinicians an overview of what types of clinical interventions have been studied, and where there are gaps. It allows clinicians to judge the value and limitations of the reported TAILOR data set in addressing the specific clinical challenges they face.
-
What tools are available to support addressing problematic pharmacy in older people with multimorbidity and polypharmacy? This offers clinicians an overview of what tools for clinical practice exist and what the data tell us about the use and effectiveness of these tools.
Eligibility criteria
From these questions, we identified a refocused set of eligibility criteria for the scoping review, outlined in Table 3.
| Inclusion criteria | Explanation/justification |
|---|---|
| Populations |
Eligible studies included patients and/or health-care professionals Patients: patients with polypharmacy (i.e. five or more long-term medications) and multimorbidity (two or more long-term conditions) and aged ≥ 50 yearsa Health-care professionals: health-care professionals (e.g. clinicians, pharmacists) involved in deprescribing for people (aged ≥ 50 years) with multimorbidity (two or more long-term conditions) and polypharmacy (five or more long-term medications)b |
| Interventions | Eligible studies included those assessing a strategy or strategies used to safely deprescribe (withdraw) medications in older people with multimorbidity and polypharmacy and the outcomes used to measure the success of these strategies in relation to effectiveness, safety and acceptability (may include, but were not restricted to, patient benefits and harms, acceptability to patients and prescribers, health-related quality of life/functional status, treatment burden, safety including adverse events, and service use) |
| Context |
Studies in any context were eligible for inclusion We defined context as relating to personal context (e.g. gender, ethnicity), wider environmental context (country), setting or service (e.g. general practice, pharmacy, home, acute/interface care, secondary/tertiary care, outreach from secondary care or community pharmacy), care context (e.g. end-of-life care, dementia care) and/or deprescribing intervention context (e.g. medicines optimisation, deprescribing, tailoring) |
| Study design |
All study designs using quantitative [e.g. experimental (randomised controlled trials, quasi-randomised controlled trials, non-randomised clinical trials), quasi-experimental (interrupted time series, controlled before–after studies), observational (cohort, case–control, cross-sectional, case series)] or qualitative (e.g interviews, open-ended questionnaires, focus groups) methodologies were eligible for inclusion We excluded case reports. Practice guidelines or deprescribing manuals were excluded, unless reporting outcome data or process outcomes Relevant systematic reviews were retained and their reference lists scanned for other potentially relevant studies |
| Limits |
From 2009 English language No conference abstracts Our focus was on the most recent evidence to support deprescribing in the elderly. Analysis of records in PubMed indicated that studies focusing on deprescribing were published after 2009 |
Stage 2: search strategy
We used a comprehensive, broad and iterative approach to identify relevant literature. We conducted an initial exploratory search using search terms identified by the review team and PubMed PubReminer [URL: https://hgserver2.amc.nl/cgi-bin/miner/miner2.cgi (accessed 16 June 2021)] in MEDLINE (via Ovid). We then identified a set of key relevant studies identified in a recent scoping exercise undertaken by Kat Kavalidou (see Chapter 2, Refining the work plan). Free-text and thesaurus terms of MEDLINE records of the relevant key studies were analysed and the search strategy was amended to ensure that it captured all key relevant records. We conducted a sensitivity analysis on the search by comparing the retrieval of different search techniques (e.g. proximity operators, phrase searching and field searching) to develop a scoping search strategy that ensured the retrieval of all key relevant studies.
The exploratory search was then peer-reviewed by a second reviewer (RH). The following keywords formed the main structure of the search: A – multimorbidity terms combined with OR; B – polypharmacy terms combined with OR; C – deprescribing terms combined with OR; D – aged terms combined with OR. The initial findings suggested that not all relevant studies would be captured by combining A AND B AND C AND D; therefore, a multisearch combination approach was developed: search 1 – (A OR B) AND C AND D, Search 2 – A AND B AND C, Search 3 – (A OR B) AND C AND Qualitative terms. The results of search 1, search 2 and search 3 were combined with OR to obtain a single set of search results. The final version of the exploratory search was then translated into other databases (see Appendix 1 for full details of the search strategies).
We conducted comprehensive searches in MEDLINE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, EMBASE, The Cochrane Library [Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL)], Joanna Briggs Institute Database of Systematic Reviews and Implementation Reports, Google (Google Inc., Mountain View, CA, USA) and Google Scholar (targeted searches for both Google sources). The search was limited to studies published in English between 2009 and 30 August 2019. The search was then updated on 23 June 2020 with an addition to the search of ‘five or more’ as a free-text term in polypharmacy concept (searches with this new term were also backdated to 2009 to capture any earlier studies that may have been missed in the initial search). An additional supplementary PubMed search was also conducted to ensure that online preprints were captured. An experienced information specialist (MM) conducted the searches. All searches were peer-reviewed by at least one other member of the review team. We also scanned through the reference lists of eligible articles to identify additional relevant studies. Finally, we conducted an abbreviated version of the CLUSTER search approach,60 using key relevant studies to identify sibling studies and additional relevant studies (via citation searching, lead author searching and project/tool searching).
Stage 3: selecting studies
Search results were downloaded into EndNote [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA], deduplicated and then uploaded into Covidence software (Melbourne, VIC, Australia) for screening. A two-stage screening process was conducted. First, all titles and abstracts were screened. Records that clearly met the inclusion criteria, or records for which it was not possible to tell from the title and abstract whether or not the study was relevant, were sent through to full-text screening. Full-texts were then screened against the eligibility criteria. One reviewer screened all records (MM) and a second reviewer (Gerlinde Pilkington, Yenal Dundar and Katherine Edwards) independently screened all records. Disagreements were resolved by a third reviewer (RH).
Stage 4: charting the data
Data were extracted on study design, population characteristics, intervention characteristics [using the Template for Intervention Description and Replication (TIDieR) framework],61 health inequalities [using the PROGnosis RESearch Strategy partneship+ (PROGRESS+) framework],62 and outcomes of interest. The template was piloted and all data were extracted by two reviewers (MM, Katherine Edwards) independently and cross-checked using Microsoft Access® (Microsoft Corporation, Redmond, WA, USA).
Stage 5: collating and reporting
The results were synthesised to address the aims of the review (i.e. provide a map of the evidence in relation to the effects, safety and acceptability of interventions to support deprescribing in the elderly with multimorbidity and polypharmacy). A narrative descriptive approach to the synthesis was adopted to map the evidence on research methods, contexts, tools and outcomes used in deprescribing interventions for elderly people with multimorbidity and polypharmacy. Outcomes were categorised as effects, safety or acceptability. In addition, intervention outcome results were summarised as having a positive, negative or equivocal effect. A framework synthesis approach was adopted using the TIDieR framework61 to synthesise data on deprescribing intervention characteristics. TIDieR is a checklist designed to unpick complex intervention components; however, it is a generic checklist designed to be applied to all different types of complex interventions. Item 4 of the TIDieR framework (‘What procedures’; see Table 7) asks the reviewer to ‘Describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities’. 61 Given the complexity of the ‘procedures, activities and/or processes’ that we observed in the reported deprescribing studies, we expanded this TIDieR item using Reeve et al. ’s63 published framework detailing the expected elements of the deprescribing process. This allowed us to extract in greater detail and in a consistent manner the specific deprescribing processes from across multiple studies. Full details are described in Appendix 2, Table 23. In some studies, insufficient information was reported to rate an item as a full ‘yes’ and therefore a ‘partial yes’ was assigned. Based on the findings of the scoping review, a stage 1 logic model64 [i.e. a static (visual) model of components of the logic rather than the interactions/interdependencies] was developed to summarise the evidence in terms of population, intervention, context, outcome (PICO).
Chapter 4 Results of scoping review
Overview
The results of our review described the diversity of research approaches and the range of clinical strategies, contexts and outcomes being used, and identified a set of tools available to support tailored deprescribing in this patient group.
Search and screening result
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)65 flow chart (Figure 4) outlines the search and screening results.
FIGURE 4.
The PRISMA flow chart for the scoping review.
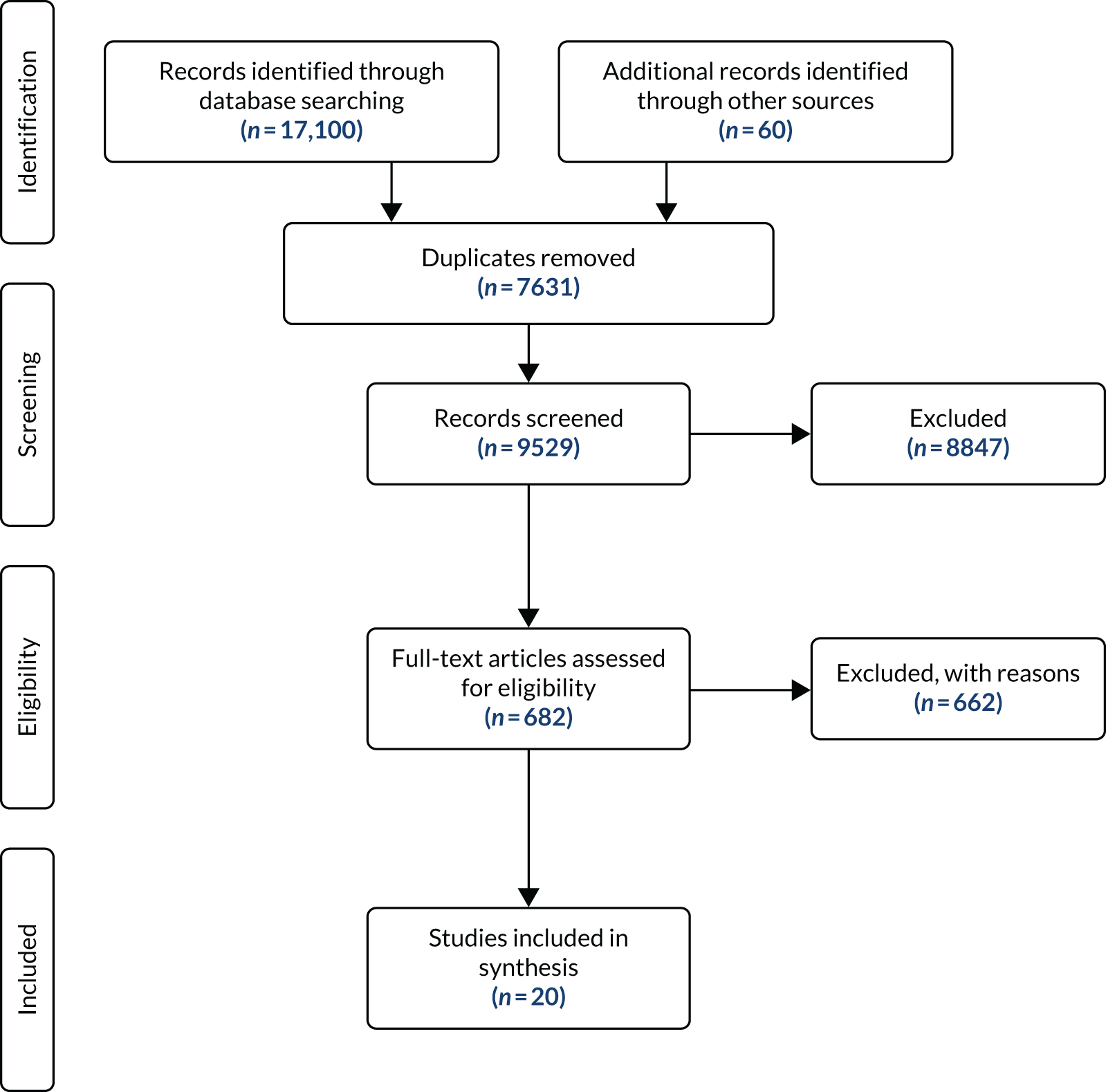
This scoping review found that between 2009 and 2020, 20 studies (reported in 27 references) examined the effectiveness, safety and acceptability of deprescribing in older adults (aged ≥ 50 years) with polypharmacy (five or more prescribed medications) and multimorbidity (two or more long-term conditions) (see Appendix 3, Table 24, with additional detail on study characteristics in Report Supplementary Material 1, Table 27; assessed effectiveness, impacts and outcomes for included studies are detailed in Report Supplementary Material 2, Table 28). 66–85
Of the 662 studies excluded at full-text stage, 148 were not explicit in stating or did not meet the number of multimorbidities [i.e. did not define their population as multimorbid (two or more long-term conditions), or reported mean/medians] and did not define or meet polypharmacy as being five or more drugs, 99 studies met the polypharmacy criteria (five or more prescribed medications) but did not meet/state the number of multimorbidities as two or more long-term conditions and 26 met the multimorbidity criteria but did not meet or define the polypharmacy criteria (see Report Supplementary Material 3, Table 29).
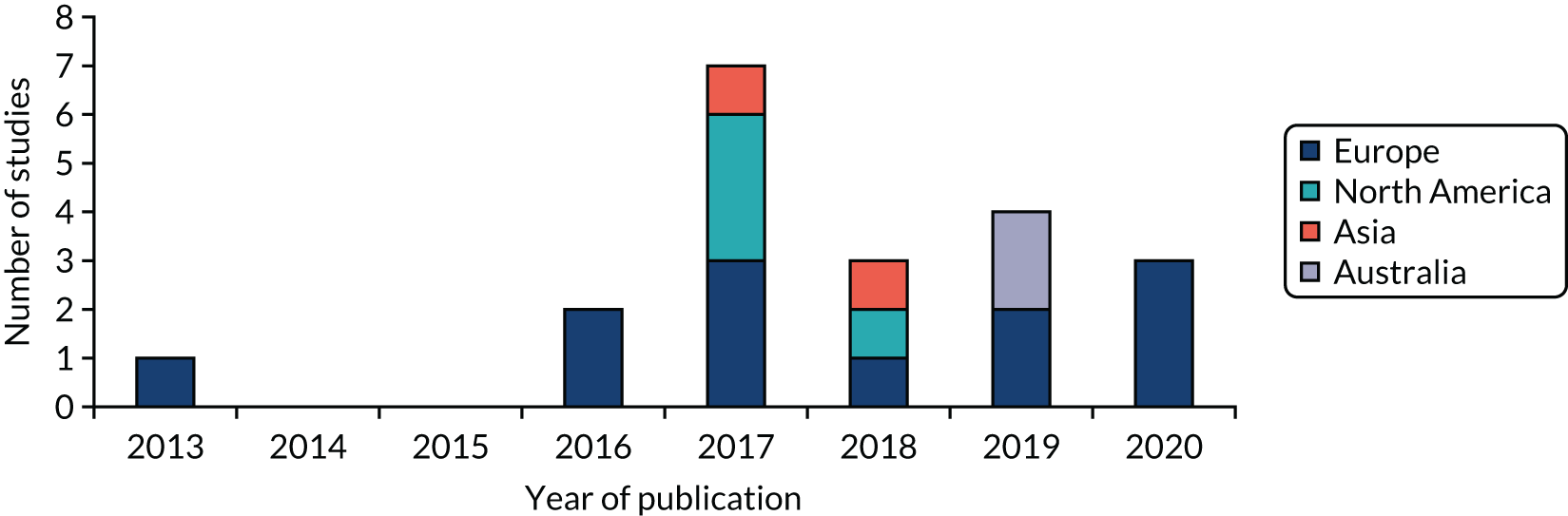
Figure 5 displays the region and year of publication of the included studies. Studies were published from 2013 onwards (our earliest publication date searched for was 2009) and were carried out across Europe, North America, Asia and Australia. Table 27 in Report Supplementary Material 1 provides more detailed study characteristics.
FIGURE 5.
Region and year of publication of included studies: scoping review.
Findings
What research methods are being used in the studies on this topic?
Our first review question asked, ‘what type of research methods are used to explore deprescribing in this patient group?’. Our findings revealed variability in the study designs used, study populations and durations, and the definitions of multimorbidity applied.
Study designs
Table 4 outlines the study designs of the included studies. Just under half were interventional studies and just over half were observational studies. Specifically, 13 (65%) used an intervention design, six (30%) used observational designs and one (5%) used an exploratory design. Nineteen (95%) were quantitative studies and one (5%) was a qualitative study. Seven randomised controlled trials (RCTs) (35%) were included, featuring three cluster RCTs (one was also a stepped-wedge design), two pragmatic RCTs and one open-label, multicentre RCT. Five (25%) were pilot studies.
| Study design | Frequency, n (%) |
|---|---|
| RCT | 7 (35) |
| Non-RCT | 2 (10) |
| Pre/post study | 4 (20) |
| Prospective cohort | 2 (10) |
| Retrospective cohort | 2 (10) |
| Cross-sectional | 2 (10) |
| Exploratory | 1 (5) |
Seven papers provided additional information on the studies above, four related to protocols and one was a validation study that provided further details on the intervention. One publication that covered multiple studies reported on additional outcomes and one study was a retrospective analysis of a RCT.
Inclusion criteria, sample size and length of follow-up
Table 5 outlines the inclusion criteria, sample size and length of follow-up in the included studies. Most studies focused on populations of people who were aged ≥ 65 years and taking five or more medicines per day. Around half the studies were classed as small (sample size < 100 participants); 35% were moderate (sample size 100–500 participants). Follow-up times were short, with only one study being > 12 months, and 30% of studies did not report the duration. All studies included multimorbid populations, but only 6 out of the 20 studies were explicit in recruiting multimorbid patients. In the remaining 14 studies, all patients included were multimorbid (according to the Charlson Comorbidity Index),59 but the researchers did not specify multimorbidity in their inclusion criteria. None of the studies focused specifically on the deprescribing of a single drug or category of drug.
| Inclusion criteria | Frequency, n (%) |
|---|---|
| Population age (years) | |
| ≥ 60 | 2 (10) |
| ≥ 65 | 13 (65) |
| ≥ 70 | 2 (10) |
| ≥ 75 | 3 (15) |
| Polypharmacy (number of drugs) | |
| ≥ 4a | 1 (5) |
| ≥ 5 | 16 (80) |
| ≥ 7 | 1 (5) |
| ≥ 8 | 1 (5) |
| ≥ 15 | 1 (5) |
| Multimorbidity (number of diseases) | |
| ≥ 2 | 2 (10) |
| ≥ 3 | 4 (20) |
| Not explicit (CCI) | 14 (70) |
| Sample size (number of participants) | |
| 1–100 | 9 (45) |
| 101–500 | 7 (35) |
| 501–1000 | 2 (10) |
| 1001–5000 | 1 (5) |
| > 5000 | 1 (5) |
| Length of follow-up (months) | |
| Up to 3 | 6 (30) |
| Up to 6 | 2 (10) |
| Up to 8 | 1 (5) |
| Up to 12 | 4 (20) |
| > 12 | 1 (5) |
| Not applicable | 6 (30) |
Multimorbidities
Fourteen studies report on the type of multimorbidities included in the study samples (Table 6). In Van Summeren et al. ’s study,84 cardiovascular disease was the focus of the study population. In Muth et al. ’s study,78 patients had to have diseases affecting at least two different organ systems (not including diseases of the eyes and ears and diseases of the thyroid gland without hyperthyroidism). The remaining 12 studies reported various multimorbidities in their populations. Six (30%) studies did not specify any type of multimorbidity.
| Multimorbidity | Frequency |
|---|---|
| Addictions | 2 |
| Asthma | 4 |
| Cancer | 5 |
| Cardiovascular disease | 12 |
| Cerebrovascular disease | 8 |
| Chronic kidney disease | 5 |
| COPD | 6 |
| Dementia | 7 |
| Diabetes | 10 |
| Endocrine | 2 |
| Gastrointestinal disorders | 4 |
| Gout | 1 |
| Haematological disorders | 2 |
| Hypertension | 5 |
| Liver disorders | 4 |
| Mental health disorders | 4 |
| Musculoskeletal disorders | 5 |
| Neurological diseases | 2 |
| Peripheral vascular disorders | 2 |
| Vision disorders | 1 |
| Others (not stated) | 9 |
In summary, the review identified studies that were mainly interventional or observational in design, small to moderate in size, undertaken on older populations (aged > 65 years) and with clinically short follow-up times.
What clinical strategies, contexts and outcomes have been studied on this topic?
Clinical strategies
We used the TIDieR framework61 supplemented by the Reeve et al. 63 framework for deprescribing to describe the clinical strategies used as interventions in these studies.
Owing to the complex nature of the deprescribing interventions employed, the TIDieR framework was found to be insufficient on its own to allow for a rich description of the deprescribing strategies. Specifically, this related to the lack of a detailed description of the deprescribing intervention components. Therefore, we used a novel approach in supplementing the TIDieR framework with Reeve et al. ’s63 deprescribing process framework (see Appendix 2, Table 23). Reeve et al. 63 described seven steps needed to support robust deprescribing practice: (1) a comprehensive medical history, (2) an assessment of risk/harm, (3) an identification of potentially inappropriate medicines, (4) a shared decision on whether or not to stop, (5) communication of a plan, (6) implementation and monitoring and (7) documenting the process.
The purpose of using both these frameworks to describe and assess included studies was twofold: first, to assess the quality of the reporting in deprescribing studies and, second, to identify specific intervention components and delivery modes of the deprescribing strategies to allow for an assessment of the replicability of the deprescribing strategies in practice. In using the two frameworks together, therefore, we can provide clinicians with a more detailed map of what deprescribing strategies are used and how they are used.
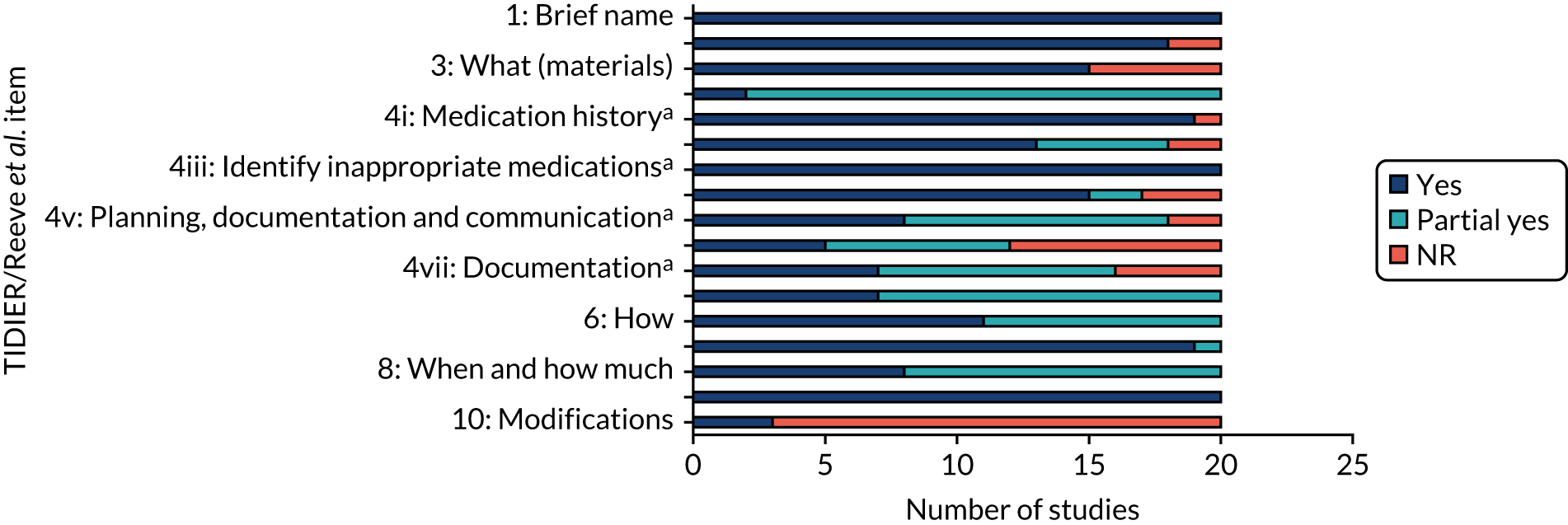
The extent to which individual studies (Table 7) and the studies collectively (Figure 6) reported on each of the TIDieR items is shown on the following pages (see also Appendix 2, Table 23, for further details of the frameworks).
| Study (first author and year) | 1: brief name | 2: why | 3: what (materials) | 4: what (procedures code) | 4a: medication historya | 4b: assess risk and patient factorsa | 4c: identify inappropriate medicationsa | 4d: shared decision-makinga | 4e: planning, documentation and communicationa | 4f: monitoring and supporta | 4g: documentationa | 5: who provided | 6: how | 7: where | 8: when and how much | 9: tailoring | 10: modifications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boersma 201966 | Y | Y | Y | P | Y | P | Y | P | P | NR | NR | P | Y | Y | P | Y | Y |
| Caffiero 201767 | Y | Y | NR | P | Y | Y | Y | Y | Y | NR | Y | Y | Y | Y | Y | Y | NR |
| Campins 201768 | Y | Y | Y | P | NR | NR | Y | Y | P | P | P | P | P | Y | P | Y | NR |
| Chiarelli 202069 | Y | Y | Y | P | Y | P | Y | NR | P | NR | Y | P | P | Y | Y | Y | NR |
| Curtin 202070 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR |
| Fried 201771 | Y | Y | Y | P | Y | Y | Y | Y | Y | NR | NR | P | Y | Y | P | Y | NR |
| Köberlein-Neu 201672 | Y | Y | NR | P | Y | Y | Y | Y | Y | Y | P | Y | Y | Y | Y | Y | NR |
| Komagamine 201773 | Y | NR | Y | P | Y | Y | Y | Y | NR | P | P | P | P | Y | P | Y | NR |
| Komagamine 201874 | Y | Y | NR | P | Y | P | Y | NR | NR | P | NR | P | P | Y | P | Y | NR |
| Martín Lesende 201380 | Y | Y | Y | P | Y | P | Y | P | P | NR | NR | P | P | Y | P | Y | NR |
| McCarthy 201775 | Y | Y | Y | P | Y | Y | Y | Y | P | P | P | P | P | Y | P | Y | Y |
| McDonald 201976 | Y | Y | Y | P | Y | Y | Y | Y | Y | Y | Y | P | Y | Y | Y | Y | NR |
| Muth 201677 | Y | Y | Y | P | Y | Y | Y | Y | P | NR | P | Y | Y | Y | Y | Y | Y |
| Muth 201878 | Y | Y | Y | P | Y | Y | Y | Y | P | NR | P | Y | Y | Y | Y | Y | NR |
| Petersen 201879 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | P | P | Y | P | Y | NR |
| Potter 201981 | Y | Y | NR | P | Y | Y | Y | Y | Y | Y | P | P | Y | Y | P | Y | NR |
| Russell 201982 | Y | Y | NR | P | Y | NR | Y | Y | P | NR | P | P | P | Y | P | Y | NR |
| San-José 202083 | Y | NR | Y | P | Y | Y | Y | NR | P | P | Y | Y | Y | Y | P | Y | NR |
| van Summeren 201784 | Y | Y | Y | P | Y | P | Y | Y | Y | P | Y | Y | Y | Y | Y | Y | NR |
| Zechmann 201985 | Y | Y | Y | P | Y | Y | Y | Y | P | NR | P | P | P | P | P | Y | NR |
A more detailed description of each criterion from the TIDieR framework is offered below, including items 11 and 12.
Item 1: brief name
All included studies (100%) reported the name of or a phrase that described the intervention. Eleven studies provided precise names for the intervention. The remaining studies provided a brief phrase or description.
Item 2: why (rationale, theoretical framework, goal)
Eighteen (90%) of the included studies provided the rationale for the intervention. None of the studies was explicit in reporting a named theory (e.g. theory of planned behaviour) to underpin their intervention. The rationale or underlying theories provided were largely based on the findings of previous research with reference to intervention components [e.g. academic detailing, medication review and specific tools (e.g. STOPP/START criteria) known to be effective], barriers to and facilitators of deprescribing (e.g. availability of health-care specialists with familiarity in managing polypharmacy in multimorbid populations and patient priorities) or setting (e.g. patients in hospital are seen as a captive audience and therefore more likely to be motivated to stop medications, which provides time for patients to discuss their options and the opportunity to observe patient outcomes closely).
Item 3: what (materials)
Fourteen (70%) studies reported using 20 different tools to guide the deprescribing process. Seven types of tools were identified: six studies (30%) used a clinical decision support system (CDSS), five (25%) studies used a criteria-led tool, one (5%) study used an algorithm, one (5%) used a CDSS plus criteria-led tool and one (5%) used an algorithm plus criteria-led tool (see What tools are available to support addressing problematic pharmacy in older people with multimorbidity and polypharmacy? and Table 27 in Report Supplementary Material 1 for details on the specific tools used). Twelve (60%) studies report using a single tool. Two (10%) studies used more than one tool to guide deprescribing. Six (30%) studies did not use a specific tool to guide the deprescribing process. Instead, pharmacists and clinicians were free to use any tool they wished or rely on their own expertise to propose and implement medication changes.
Item 4: what (procedures)
The seven elements of the deprescribing process as reported in Reeve et al. 63 were used to further define the procedures involved in the intervention. Only two (10%) studies reported on all seven items.
Nearly all studies (19/20, 95%) were explicit in detailing the taking of a patient medication history. One study, by Boersma et al. ,66 reported using a tool [Structured History taking of Medication use (SHiM)]66 to inform the medication review process.
Eighteen (90%) studies recorded an assessment of risk of harm and benefit and/or patient factors. Two studies did not report an assessment of these factors in the deprescribing process. Two studies used a checklist-based pre-consultation interview tool, Medication-Monitoring-List (MediMoL), to assess risk and patient factors. 77,78
All 20 (100%) studies recorded details of how potentially inappropriate medications were identified (see Chapter 3, Methods, for details on the tools used).
Seventeen (85%) studies reported incorporating patient preferences into the deprescribing process. Seven studies (35%) incorporated patient preferences into the process before providers decided on the medications to deprescribe. 72,75,77–79,84,85 Of these, six were conducted in the primary care setting and one was conducted in a tertiary setting. Seven studies (35%) involved patient discussion at the end of the process, only after medications for deprescribing had been identified. 67,68,70,71,73,76,79 Three studies utilised tools to elicit patient preferences prior to the identification of medicines to be deprescribed; Muth et al. 77,78 used MediMoL, whereas van Summeren et al. 84 used the outcome prioritisation tool. 84 Patient preference was the focus of the deprescribing process in van Summeren et al. 84 In three studies it was unclear at what point in the deprescribing process the patient was involved.
Eight (40%) studies described planning the tapering or withdrawal process with documentation and communication among health-care professionals. Ten (50%) studies lacked information on the tapering and withdrawal process. Two (10%) studies did not state a plan for the tapering or withdrawal of medications.
Twelve (60%) studies detailed monitoring and/or support for patients following deprescribing. This involved the symptom and safety monitoring of patients (e.g. for adverse drug withdrawal events or disease relapse). Support offered included additional consultations and telephone follow-ups.
Sixteen (80%) studies described the process for documenting the outcome of deprescribing (e.g. dose reduced or medication ceased). Of these, seven (35%) describe sharing the documentation with all relevant health-care professionals.
Item 5: who provided
In more than half (n = 11, 55%) of the studies a physician led the deprescribing process (i.e. identified the medications to deprescribe). Of these, seven studies were general practitioner (GP)/primary care physician led and one was led by a specialist registrar in geriatric medicine. Five studies (25%) were pharmacist led and in four studies (20%) the deprescribing recommendations were made by the team involved in patient care. Three studies involved a single intervention provider (two GP led, one clinician led). The majority (n = 17, 85%) involved more than one provider in the deprescribing process. Pharmacists and specialist geriatric physicians were more likely to be involved in the deprescribing process in secondary care settings than in primary care settings. Table 27 (see Report Supplementary Material 1) provides more information on the personnel involved in the provision of the intervention.
Item 6: how
All studies (n = 20, 100%) detailed to some extent the mode of delivery of the deprescribing intervention. Multiple methods of delivery were reported involving face-to-face, online, telephone, electronic health record, fax and written modes of delivery. All studies (n = 20, 100%) employed an individual delivery format.
Item 7: where
Ten studies (50%) carried out the intervention in primary care settings, seven (35%) studies were set in secondary care settings, two (10%) studies were set in tertiary care settings and one (5%) study was set in a pharmacy call centre (Table 8).
| Setting | Frequency (%) |
|---|---|
| Primary care | 10 (50) |
| Secondary care | 7 (35) |
| Tertiary care | 2 (10) |
| Other (pharmacy call centre) | 1 (5) |
Item 8: when and how much
All studies (100%) described when the deprescribing intervention took place. Seven (35%) studies invited patients for a medication review. In seven (35%) studies patients were invited to participate upon or during hospital admission. Four (20%) studies invited patients who were attending another GP or outpatient appointment or were awaiting a primary care appointment. Two (10%) studies referred patients from primary care or hospital. Four (20%) studies reported the intervention as being delivered on a single occasion and one (5%) study offered an optional second consultation. In two studies a medication review was offered twice (once at hospital admission and again at discharge, and once after invitation for medication review and again at 6 months) and one study offered an annual medication review with quarterly targeted reviews. In 12 studies (60%) it was unclear how many times the intervention was delivered.
Item 9: tailoring
All 20 (100%) studies reported tailoring their interventions with decisions to deprescribe based on individual patient requirements as described under item 4d. Additional tailoring approaches described included protected time for individual consultations to discuss prescribing decisions and incorporation of individual patient medication-related problems. These were in addition to medications that physicians judged to be inappropriate or unnecessary but were sometimes continued owing to patients’ preference, and recommendations made regarding the need to simplify the regimen of patients with problems with adherence, compliance and poor social support.
Item 10: modifications
Three (15%) studies reported modifying the intervention. Boersma et al. 66 modified the intervention during the study by introducing consensus-based instructions to standardise the prescribing recommendations. Two pilot studies reported on making changes to the intervention after completion of the study to inform larger studies. Muth et al. 77 intensified the provider training and written CDSS manual. McCarthy et al. 75 made minor modifications to the training videos and medication review template to improve clarity of instruction and reduce repetition.
Item 11: adherence to the study recommendations
One (5%) study reported training pharmacists and home care specialists with a planned assessment of the training on 10 patients. Eleven studies (55%) reported that training of intervention providers was undertaken but planned assessment was not reported. Six studies planned assessment of patient adherence.
Item 12: outcome of training
None of the 11 studies that reported training intervention providers reported on the outcome of the training. Five of the six studies that planned to assess patient adherence reported on adherence outcomes.
Overall, in summary, our analysis revealed that studies offered clear accounts of the goals of the deprescribing interventions used and to whom they were offered (i.e. which patients were included). However, there was often less detail reported on who delivered the intervention and how (what specifically was done).
Using Reeve et al. ’s63 framework to further analyse details of the interventions revealed that most studies offered clear accounts of the assessment of patients leading to a decision to potentially deprescribe. Details on subsequent actions, including communication, documentation and planning, follow-up monitoring and support, and documentation of the clinical plan were all less clearly described.
Contexts
We sought to understand the context in which deprescribing interventions were being delivered by considering the clinical focus of the study (whether on stopping medication, or improving prescribing–medicines optimisation). We also examined the extent to which researchers considered the population context in which studies took place, through an examination of assessment of markers of inequalities.
Focus of the intervention
Deprescribing was the focus of the intervention in eight studies. The remaining 12 studies involved deprescribing as part of a wider medicines optimisation context (Table 9).
| Focus of the intervention | Frequency (%) |
|---|---|
| Deprescribing | 8 (40) |
| Medicines optimisation | 12 (60) |
Inequalities
By assessing population characteristics using the PROGRESS+ framework,62 we sought to offer end users of our review an analysis of which populations the findings could be generalised to. Given the focus of our research question, all studies considered age and comorbidity inequalities. We assessed the extent to which other population contextual factors were also considered through an assessment of inequalities using the PROGRESS+ framework. This describes nine characteristics that stratify health opportunities and outcomes within populations, namely place, race/ethnicity, occupation, gender, religion, education, socioeconomic status, social capital and other.
Only two studies (10%) explicitly report a PROGRESS+ inequality, namely Medicare status, as being the focus of their study population.
PROGRESS+ inequalities collected
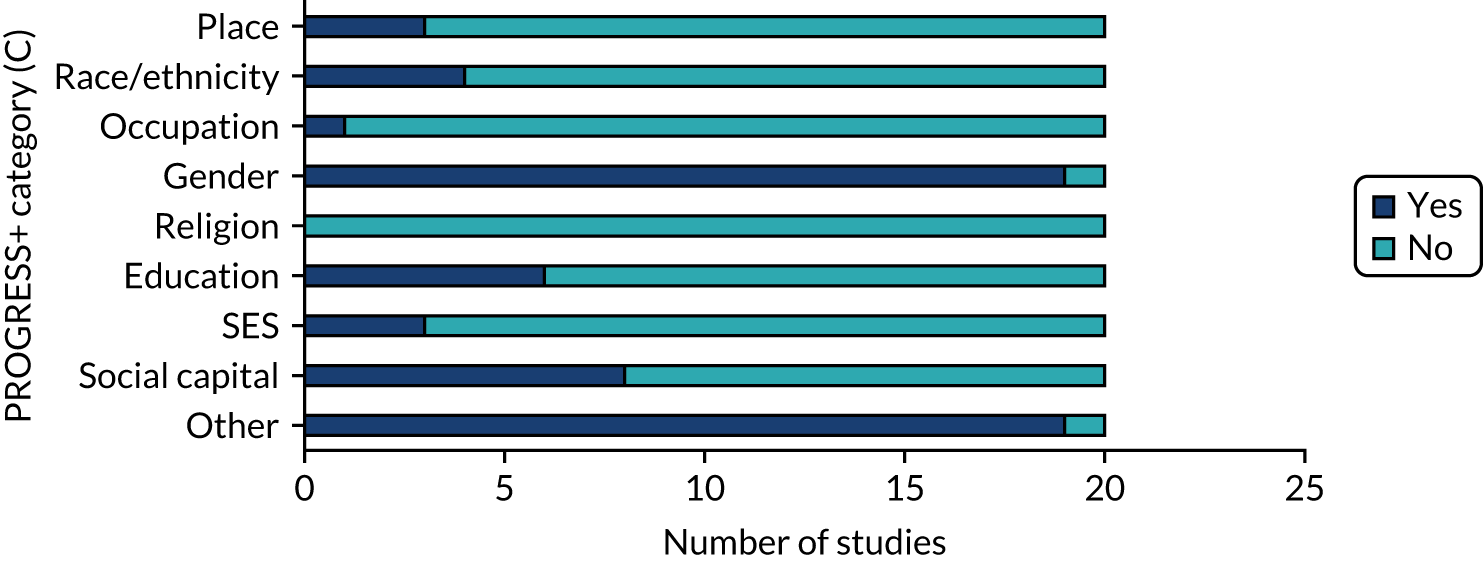
Nineteen studies (95%) collected baseline data from participants on characteristics described by the PROGRESS+ inequalities (Figure 7). All 19 studies collected data on gender. The one study that did not report baseline inequality characteristics reported pilot data within a study protocol. Other inequalities collected were age and comorbidity status (which reflected the target population) and Medicare status (i.e. health insurance).
FIGURE 7.
PROGRESS+ inequalities collected. SES, socioeconomic status.
PROGRESS+ inequalities analysed
Nine studies (45%) analysed data on PROGRESS+ inequalities (Figure 8). The most common inequality analysed was gender (nine studies). Other inequalities analysed were age and comorbidity status (which reflected the target population) and Medicare status (i.e health insurance). Inequality variables were mostly adjusted for in statistical analyses (e.g. through logistic regression models). However, only one study discussed the effect of population characteristics and inequalities on outcomes (Figure 9).
FIGURE 8.
PROGRESS+ inequalities data analysed. SES, socioeconomic status.
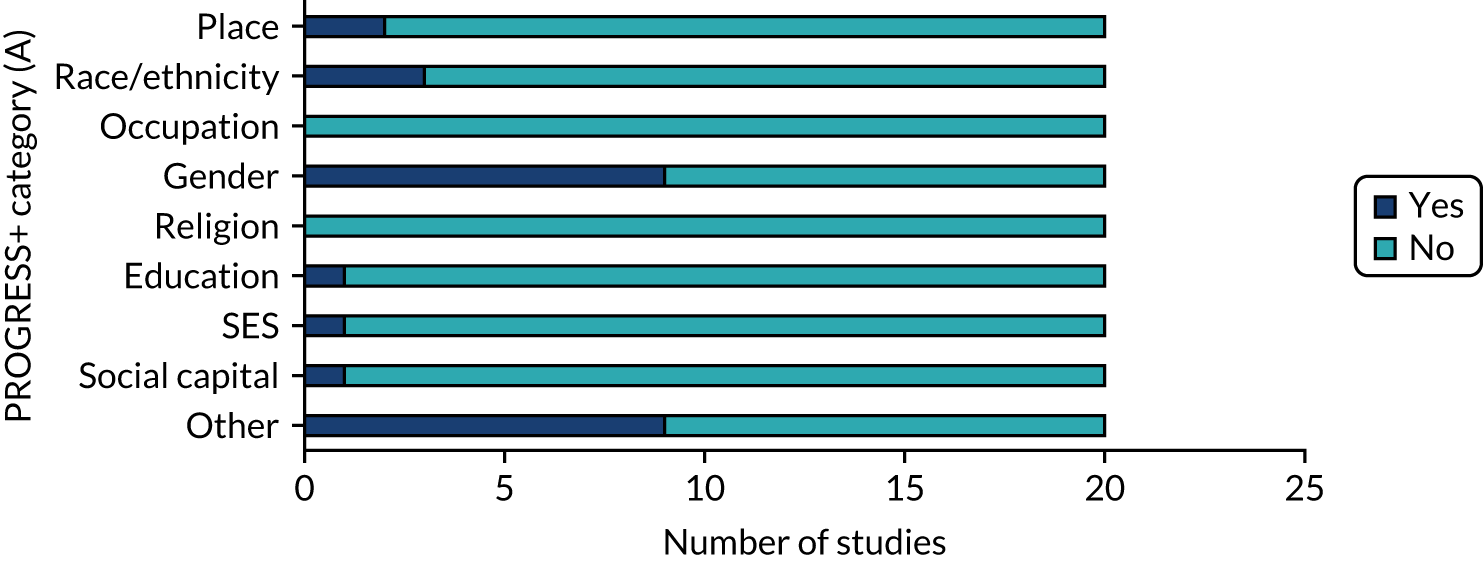
FIGURE 9.
PROGRESS+ inequalities data discussed. SES, socioeconomic status.
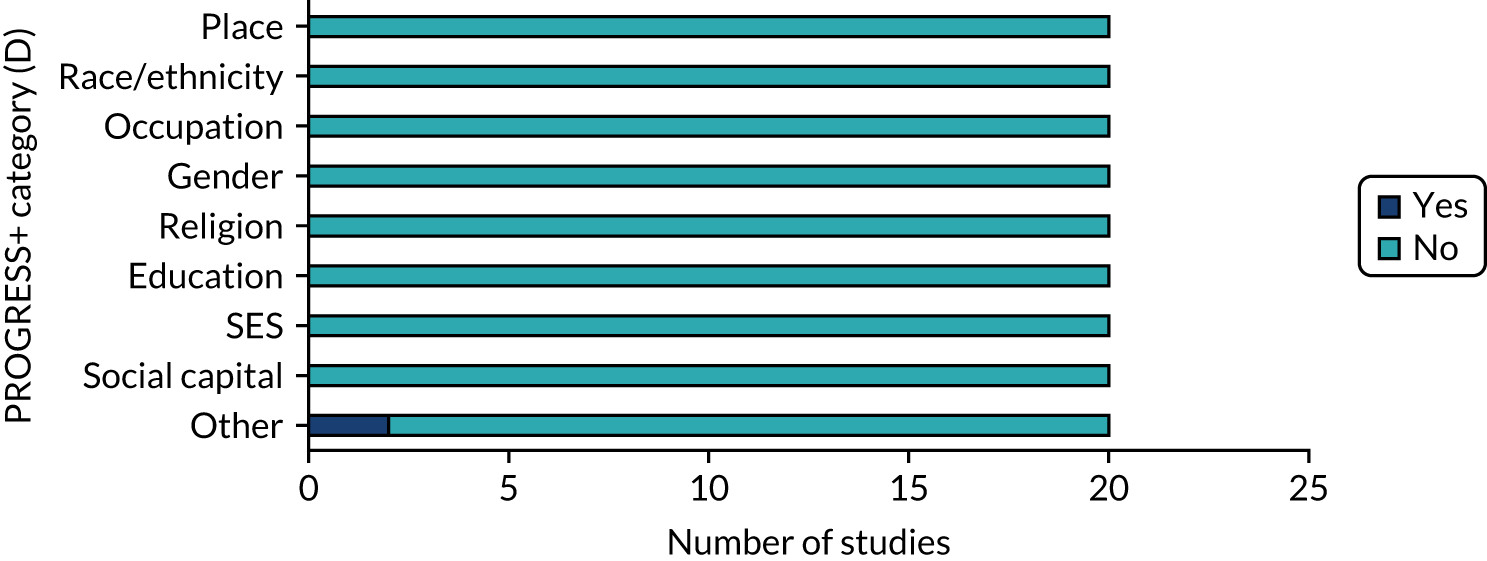
In summary, population-level contextual information in the form of key characteristics known to have an impact on inequalities was poorly reported and discussed across the studies. Reporting of clinical contextual information (deprescribing vs. medicines optimisation) was present in most studies, with approximately half looking at deprescribing.
Outcomes
Our review demonstrated that studies used multiple outcomes relating to the effectiveness, safety and acceptability of interventions. These are summarised in Table 10. Altogether, 461 outcomes were reported relating to effectiveness (n = 382), acceptability (n = 49), safety (n = 23) and other (n = 7) (see also Report Supplementary Material 2, Table 28).
| Effects/safety/acceptability | Type of outcome | Frequency |
|---|---|---|
| Effects (prescribing) | Drugs deprescribed (stopped/withdrawn/tapered/dose reduced, etc.) | 9 |
| Drug dosage (increased, decreased, application interval shortened/prolonged, pill splitting started/stopped) | 36 | |
| Drug discontinuation | 24 | |
| Drug addition | 6 | |
| Drug substitution | 10 | |
| Drug restart | 6 | |
| Drug strength | 8 | |
| Drug administration method | 3 | |
| Number of drugs | 32 | |
| Active pharmaceutical ingredient changes | 3 | |
| Inappropriate prescribing | 25 | |
| Medication change | 9 | |
| Proposed change in medication | 22 | |
| Implemented change in medication | 25 | |
| Medication interaction | 3 | |
| Medication complexity | 4 | |
| Medication appropriateness | 13 | |
| Patient/drug monitoring | 2 | |
| Drug burden | 2 | |
| Medication discrepancy | 4 | |
| Medication errors | 1 | |
| Number of START criteria | 10 | |
| Number of STOPP criteria | 10 | |
| Prescribing omission | 4 | |
| Drug-related problems | 2 | |
| Cost | 2 | |
| Effects (clinical) | Hospital admission/readmission/visits | 23 |
| Health appointments/visits/tests | 11 | |
| Mortality | 10 | |
| Fracture | 2 | |
| Falls | 3 | |
| Functional status | 8 | |
| Pain | 4 | |
| Depression | 2 | |
| Frailty | 1 | |
| Adherence | 20 | |
| Beliefs about medication | 16 | |
| Prioritised health outcome | 8 | |
| Quality of life | 15 | |
| Social support | 1 | |
| Mental ability | 1 | |
| Safety | Adverse event (unspecified) | 2 |
| Adverse drug event (unspecified) | 4 | |
| Delirium | 1 | |
| Cardiovascular event | 2 | |
| In-hospital death | 3 | |
| Infection | 1 | |
| Acceptability (patient) | Pursue offer to change drugs | 23 |
| Acceptability (provider) | Satisfaction (usability/experience) | 16 |
| Acceptability( patient/provider) | Time | 3 |
| Communication | 5 | |
| Participation | 1 |
We summarise the outcomes reported across the 20 studies included in this review in Table 11.
| Studies | Outcomes reported | Effects | Safety | Acceptability | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparative data on prescribing behaviour | Comparative data on clinical outcomes | Single data point on prescribing behaviour | AE reported | ADE reported | AE clinical outcome reported | Adverse effect single data point | Patient | Provider | Patient and Provider | ||
| Setting | |||||||||||
| Primary care, n = 9 | 310 | ↑41 | ↑25 | • 84 | ↑1 | ↑2 | • 1 | ↑4 | ↑2 | • 2 | |
| ↓35 | ↓36 | ||||||||||
| • 18 | • 5 | ↓1 | • 3 | • 31 | |||||||
| ↔8 | ↔11 | ||||||||||
| Secondary care, n = 7 | 114 | ↑21 | ↑6 | • 49 | ↑6 | ↑2 | ↑2 | • 3 | • 1 | • 2 | |
| • 3 | ↓9 | ||||||||||
| ↔2 | ↓4 | ||||||||||
| • 4 | |||||||||||
| Tertiary care, n = 2 | 28 | ↑17 | • 4 | • 1 | • 4 | ||||||
| ↓2 | |||||||||||
| Other, n = 2 | 2 | ↑1 | |||||||||
| ↓1 | |||||||||||
| Intervention tool | |||||||||||
| Algorithm, n = 1 | 2 | • 1 | • 1 | ||||||||
| Algorithm and criteria led, n = 1 | 55 | ↑11 | ↑6 | • 13 | • 5 | ||||||
| ↓4 | ↓12 | ||||||||||
| • 1 | • 3 | ||||||||||
| CDSS, n = 6 | 210 | ↑30 | ↑17 | • 40 | ↑1 | ↑2 | ↑2 | • 4 | ↑4 | ↑2 | • 1 |
| ↓29 | ↓19 | ||||||||||
| ↔8 | ↔8 | ↓1 | • 2 | • 15 | |||||||
| • 20 | • 5 | ||||||||||
| CDSS and criteria led, n = 1 | 22 | • 21 | • 1 | ||||||||
| Criteria led, n = 5 | 99 | ↑36 | ↑8 | • 24 | ↑5 | ↑1 | ↑2 | • 8 | • 4 | ||
| ↓2 | ↓8 | ||||||||||
| ↔1 | |||||||||||
| No tool, n = 6 | 66 | ↑3 | ↓6 | • 39 | ↓4 | • 1 | • 1 | • 4 | |||
| ↓3 | ↔4 | ||||||||||
| • 1 | |||||||||||
| Lead prescriber | |||||||||||
| GP/primary care physician led, n = 7 | 211 | ↑16 | ↑17 | • 59 | ↑1 | ↑2 | • 1 | ↑4 | ↑2 | • 2 | |
| ↓29 | ↓18 | ||||||||||
| ↔8 | ↔8 | ↓1 | • 3 | • 22 | |||||||
| • 17 | • 1 | ||||||||||
| Pharmacist led, n = 5 | 99 | ↑14 | ↑6 | • 39 | • 1 | • 10 | |||||
| ↓7 | ↓14 | ||||||||||
| • 1 | ↔3 | ||||||||||
| • 4 | |||||||||||
| Secondary care physician led, n = 4 | 55 | ↑20 | ↑6 | • 6 | ↑5 | ↑1 | ↑2 | • 1 | • 1 | ||
| ↓5 | |||||||||||
| • 3 | ↔1 | ||||||||||
| • 4 | |||||||||||
| Team, n = 4 | 89 | ↑30 | ↑2 | • 33 | ↑1 | ↑1 | ↓4 | • 3 | • 4 | ||
| ↓2 | ↓8 | ||||||||||
| ↔1 | |||||||||||
| Context | |||||||||||
| Deprescribing focus, n = 8 | 88 | ↑22 | ↑5 | • 31 | ↑2 | ↑1 | ↓4 | • 4 | • 2 | • 1 | • 5 |
| ↓3 | ↓7 | ||||||||||
| ↔1 | |||||||||||
| Medicines optimisation, n = 12 | 366 | ↑58 | ↑26 | • 106 | ↑4 | ↑2 | ↑4 | • 1 | ↑4 | ↑2 | • 1 |
| ↓35 | ↓38 | ||||||||||
| ↔8 | ↔12 | ↓1 | • 2 | • 32 | |||||||
| • 21 | • 9 | ||||||||||
| Total: 454 per category | |||||||||||
Outcomes from the included studies have been grouped by setting, intervention modality, profession of lead prescriber(s) implementing the intervention and context. Outcomes are reported under the three headings of effects, safety and acceptability.
Table 11 reports the total number of outcomes reported for each category of study setting, intervention, prescriber and context. It also details the number of outcomes reported under each heading with the direction of effect (positive, negative or neutral) when comparative data were reported and able to be interpreted.
For example, nine primary care studies (row 1) reported a total of 310 outcome measures (column 2). Of these, 102 related to prescribing behaviour [such as number of medications (de)prescribed], with 41 showing a positive effect, 35 showing a negative effect and 18 being unclear (column 3). When outcome data either showed mixed effects or were uncertain, the number of outcomes is indicated with the ‘dot’ symbol. Outcomes were classified as uncertain if the effect was not reported or was unclear, or if the data were observational in nature.
The outcomes are reported under three headings of effects, safety and acceptability. These included ‘clinical outcomes’ experienced by the patient or service impacts, such as mortality or hospital admission; safety-related adverse effects classed as ‘AE’ (adverse effect) or ‘ADE’ (adverse drug effect); or ‘AE clinical outcome reported’ [framed in the source paper as relating to the patient experiencing an adverse outcome related to (de)prescribing]. Acceptability outcomes were taken from stated acceptability measures but also derived form study ‘process outcomes’, such as number of patients accepting or number of professionals applying the deprescribing intervention. Acceptability outcomes are mapped as patient, provider and a combination of patient and provider.
In summary, Table 11 reveals considerable variation in the reported effects of deprescribing work with both improvement and decline in reported outcomes. Safety outcomes were reported only for clinician-led (rather than pharmacist-led) interventions. The majority of safety outcomes reported were positive, but safety concerns were noted for general clinical outcomes in secondary care-based studies where no clinical tool was used. Acceptability was variably reported and was usually based on observation. When reported, studies indicated acceptability of interventions to professionals, with patient acceptability less clearly reported.
What tools are available to support addressing problematic pharmacy in older people with multimorbidity and polypharmacy?
Studies reported a range of tools used to support deprescribing. A CDSS is a computer application designed to aid clinicians in making deprescribing decisions. It may incorporate algorithm- or criteria-led tools in its design. Algorithms are a set of rules or steps that guide deprescribing and are followed in a pre-determined way to lead to an outcome. Criteria-led decision-making involves the use of a list of criteria to consider when making decisions to deprescribe medications. Five studies69,72,77–79 reported the use of tools within a wider structured framework involving a process-driven approach to deprescribing, which details a set of rules or steps to be followed from patient identification through to discharge and follow-up.
Table 12 details the tools used in the deprescribing interventions. The seven studies employing a CDSS reported using seven different CDSSs. Many were created by the clinical team or study team for the purposes of the study, commonly drawing on previously published criteria or algorithms (see Table 12). In addition, five studies report using other tools to inform medication reviews. One used a tool to identify patient priorities and one study used a protocol for the withdrawal and reinstatement of drugs associated with potential for adverse drug withdrawal events. The most reported tool was the criteria-led STOPP/START tool.
| Tool type (number of studies) | Tool name | Tool references provided in included studies |
|---|---|---|
| CDSS (7) | AiDKinik®77 | Not reported |
| AiD®78 | Not reported | |
| INTERcheck69 | Ghibelli et al.86 | |
| MedSafer76 | Available online at URL: www.medsafer.org (accessed 16 June 2021) | |
| SPPiRE online medication review 75 | SPPiRE medication review process template reported in McCarthy et al.75 | |
| STRIP Assistant66 | References studies evaluating STRIP Assistant: Meulendijk et al.87 and Willeboordse et al.88 | |
| TRIM71 | Components of the TRIM clinical decision support system described in related study (Niehoff et al.89) | |
| Criteria led (6) | STOPP/START (version 2)68,69,80,83 |
O’Mahony et al. 10 Delgado et al. 90 |
|
STOPP79 STOPPFrail70 |
Gallagher and O’Mahony91 Lavan et al. 92 |
|
| Beers69,79 |
American Geriatrics Society 2012 Beers Criteria Update Expert Panel93 (in Chiarelli et al. 69) American Geriatrics Society 2015 Beers Criteria Update Expert Panel94 (in Petersen et al. 79) |
|
| SPC69 | SPC provided by the Italian Medicine Agency [URL: www.aifa.gov.it/note-aifa (accessed 16 June 2021)] | |
| Author reported criteria in Komagamine et al.73 | As reported in Komagamine et al.73 | |
| Algorithm (2) | Adapted GPGP algorithm85 | As reported in Zechmann et al.85 |
| GPGP algorithm68 | Garfinkel and Mangin95 | |
| Other (5) | MediMoL to identify medication-related problems and patient preferences77,78 | Not reported |
| SHiM to inform medication review66 | Drenth van Maanen et al.96 | |
| Outcome Prioritisation Tool to elicit patients’ prioritisation84 | Available online at URL: www.optool.nl (accessed 16 June 2021) | |
| Author-reported protocol for withdrawal and reinstatement of drugs associated with potential for adverse drug withdrawal events70 | As reported in Curtin et al.70 |
Seven of the CDSS tools report incorporating criteria-led or algorithm tools or other frameworks (Table 13).
| CDSS | Incorporated criteria-led or algorithm tools |
|---|---|
| Adapted GPGP algorithm85 |
|
| INTERcheck69 |
|
| MedSafer76 |
|
| ShedMEDS79 | |
| SPPiRE (online medication review)75 |
|
| STRIP Assistant66 |
|
| TRIM71 |
|
As described in Table 11, acceptability for all tools was poorly reported. Clinicians reported acceptability across all tools, but patient perceptions of acceptability were not generally recorded. Clinical safety concerns were described for secondary care studies not using any tool but inconsistently reported (positive or negative) in other contexts. Effectiveness reports were varied for all tools.
Integrating our findings
To further consolidate observations across population, settings, interventions, outcomes and inequalities, we developed a simple ‘static’ logic model as a visual map (Figure 10). We used a systems-level logic model development guidance by Rohwer et al. 64 The model groups our key categories (identified as population, contexts, interventions and outcomes) and recognises the variability and complexity of components within each, as well as the potential interplay.
FIGURE 10.
Systems-based logic model for deprescribing (based on the 20 included studies). SES, socioeconomic status.
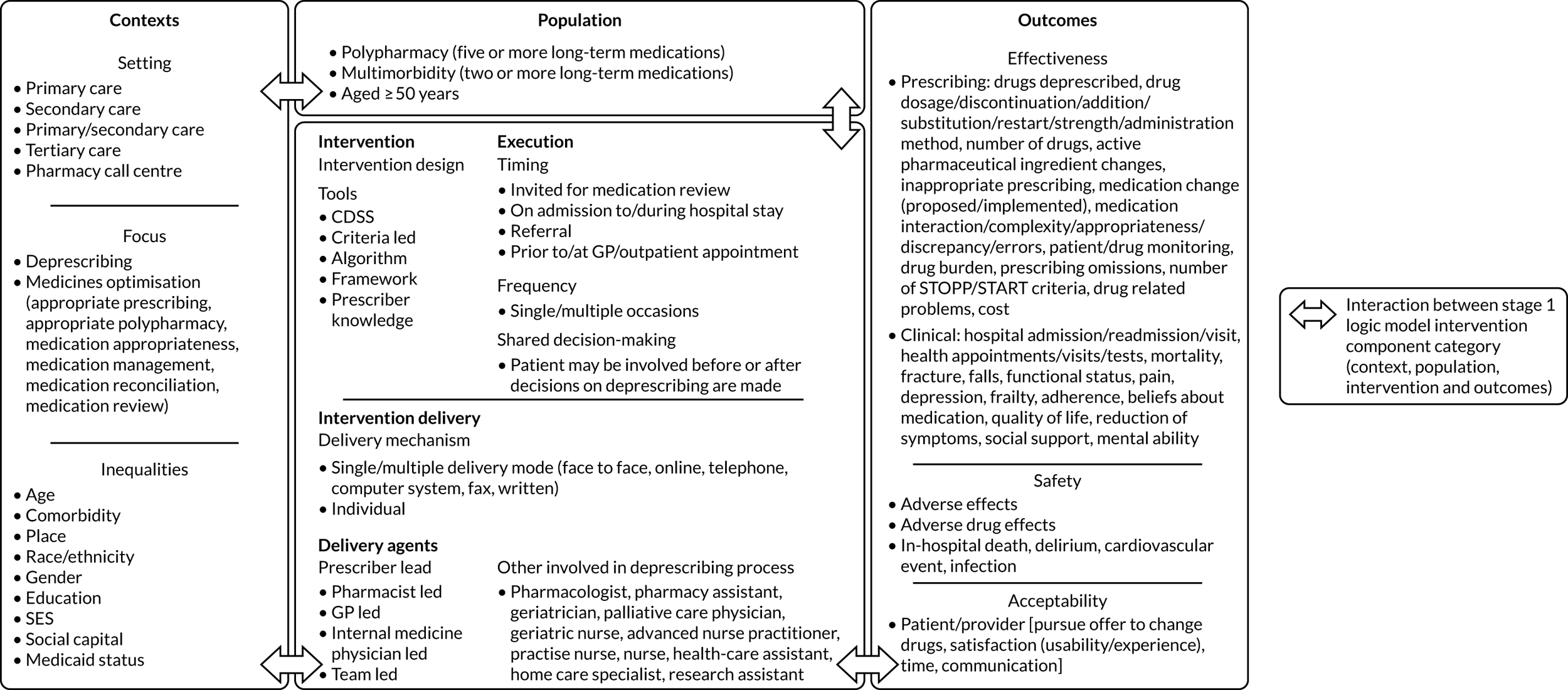
Chapter 5 Scoping review discussion
Overview
The goal of the scoping review was to describe a map of deprescribing practice to provide a resource for clinicians engaged in deprescribing. We sought to map what is being done, where and for what effect with regard to deprescribing in older patients living with multimorbidity and polypharmacy. In this way, we sought to develop a resource (data set) for clinicians to inform their clinical judgement when making tailored prescribing decisions. Our map would include recognition of ongoing gaps in our knowledge and so areas of practice in which clinical judgement is particularly necessary.
We deliberately chose to conduct a very focused search of the literature to examine only studies that clearly described assessment of deprescribing practice for this patient group. Despite our strict entry criteria, our analysis still revealed significant heterogeneity in both the research and the clinical methods used, as well as variability in the quality of reporting.
Our carefully conducted scoping review therefore reveals that a map of current evidence (even within tightly defined parameters) does not provide clinicians with a ‘what to do’ toolkit. However, it does provide data that can support the interpretive practice of clinical decision-making. We now discuss the challenges and opportunities for research and clinical practice this reveals.
Key findings
We summarise our key findings with reference to the three sub-questions we set for the scoping review (see Chapter 3, Methods, Stage 1: setting the research question):
-
What research methods (study designs) have been used in the studies that focus on this topic?
-
What strategies, contexts and outcomes have been studied on this topic?
-
What tools are available to support addressing problematic pharmacy in older people with multimorbidity and polypharmacy?
Reviewing the research methods used
To understand the utility of knowledge/evidence for clinical practice, we have to understand how it has been generated. The first part of our review examined the research methods used by studies in this area. As outlined in Chapter 1, and underlined by our patient partners, TAILOR sought to look in particular at a person-centred understanding of deprescribing. Here, therefore, we also consider the extent to which the published research methods support a whole-person understanding of deprescribing approaches.
Quantitative study designs were the most common approach used to exploring issues around effectiveness, safety and applicability, with the RCT as the most common method within that group. Around half of the studies were intervention studies, and half used observational designs (e.g. cohort). Interventional designs were associated with the consistent reporting of outcomes related to impact and safety. The studies therefore have the potential to provide clinicians with robust data on focused outcomes.
The patient’s voice was less clearly reported in the study set. For example, patient reports on acceptability were generally missing. Quantitative study designs can capture patient perspectives on treatments, but qualitative designs are often used to ensure that the patient’s voice is heard. Although we identified an abundance of qualitative evidence around deprescribing during our screening of studies, this work related to an understanding of deprescribing approaches in general, rather than an examination of the specific effects of an intervention. These studies were therefore excluded from our review at screening stage. Only one qualitative design met our inclusion criteria. 85 This reported that patients generally found a discussion about deprescribing to be acceptable, with conservative practice (hesitancy to change) and fragmented care both described as barriers to stopping medicines in practice.
Previous reviews32 have raised concerns about evidence relating to patients’ experiences of medications being overlooked in evidence synthesis work such as guideline preparation. Therefore, although our scoping review can provide importance clinical data supporting clinician interpretation, it misses an important wider area of study. This will be addressed in our realist review (see Chapters 6–8).
Our review noted that most studies were small to moderate in size with a short follow-up period (all < 1 year, and 30% ≤ 3 months). Study size and duration potentially affect the generalisability of findings to a clinical setting with divergent populations and continuity of care in effect.
We noted that studies on deprescribing in the elderly were not necessarily explicit in defining multimorbidity in their inclusion criteria (as evidenced by the large number of studies excluded for not meeting the multimorbidity criteria). This could be because there is difficulty in defining ‘multimorbidity’ or that there is an underlying assumption that anyone who is taking five or more drugs is considered to have multimorbidity (even though this may not strictly be true). This meant that a large number of studies were excluded purely on the basis that they did not report on the number of multimorbidities in the inclusion criteria. Again, this may have implications for clinicians using our findings. Multimorbidity is rarely a diagnosis/criterion used explicitly in clinical practice. Translating research findings based on strict clinical criteria to the practice context in which patient needs may be more uncertain is challenging. Our decision in applying a strict definition for the review was to ensure that we could offer clinicians a clear account of which patients our findings relate to, and which they do not.
In summary, our review findings offer clinicians a defined data set on the clinical outcomes related to deprescribing practice in a defined population of patients aged > 50 years living with two or more long-term conditions and taking five or more medicines per day. The TAILOR data set is, however, incomplete and does not provide insights into patient experience. This will be addressed by our realist review.
Reviewing the clinical interventions used
Our review next examined what type of interventions are being conducted, where and to what effect. By using a modified TIDieR framework, we offer a detailed account of what has been done in the published studies (see Table 7). Our outcomes data set (see Table 11) offers a visual overview of outcomes related to effectiveness, safety and acceptability.
The TIDieR analysis (see Table 7) and the logic model (see Figure 10) highlight the complexity and diversity of the deprescribing process. The included studies are heterogenous not only in terms of their populations, but also in terms of the intervention components, the types of outcomes assessed and the contexts within which deprescribing takes place. We discuss this further through consideration of our three foci of interest: clinical strategies used, context of the studies and outcomes assessment.
Clinical strategies
Our analysis revealed that studies offered clear accounts of the goals of the deprescribing interventions used and to whom they were offered (which patients). However, there was often incomplete detail reported on who delivered the intervention and how (what specifically was done). Using Reeve et al. ’s63 framework to analyse details of the interventions revealed that most studies offered clear accounts of the assessment of patients leading to a decision to potentially deprescribe. However, details on subsequent actions, including communication, documentation and planning, follow-up monitoring and support, and documentation of the clinical plan were all less consistently described.
Deprescribing interventions were highly variable. The interventions in studies included in this review differ not only in the extent to which they incorporated different components of the deprescribing process (see Table 7), but also in the way in which the individual intervention components were implemented. Despite this variability, and regardless of the context in which the study was conducted and the way in which the intervention was designed and delivered, positive impacts on the same outcomes can be seen (see Table 11). This suggests that there may be multiple pathways to achieving a positive impact on the same deprescribing outcome, with the presumed mechanism of impact depending not only on the deprescribing context and the specific intervention components but also on the way in which the intervention components are implemented.
For example, although the majority of interventions included shared decision-making within the deprescribing process, the point at which a patient was involved in the decision-making process differed. In some studies, patients were involved before deprescribing recommendations were made, whereas in others, patients were involved only after deprescribing recommendations were made. Both approaches were associated with both positive and neutral/negative impacts on outcomes (adherence). Patient-centred strategies that incorporate patient preferences before deprescribing recommendations are made may reflect or affect the patient–prescriber relationship through an impact on the relationship (including trust) between the patient and prescriber. We recognise that it may be the effect of the doctor–patient relationship, rather than the timing of the shared decision-making process, that influences outcomes. However, we also noted that not all patient-centred strategies included in this scoping review had a positive impact on patient adherence, which suggests that the presence and implementation of this single intervention element is not sufficient on its own to trigger a positive deprescribing impact.
Our findings support our opening discussion (see Chapter 1) that deprescribing is not a linear process but rather a complex intervention with multiple interacting component elements. Although the Medical Research Council Complex Interventions framework99 highlights the necessity of strong theoretical underpinnings in research evaluating such interventions, the theoretical underpinnings of most of the interventions included in this scoping review were often low-level programme theories based on the findings of previous research. This, along with the heterogeneity displayed in the deprescribing strategies, presents us with challenges in identifying and explaining what interventions may work, for whom and under what circumstances. Similar concerns have been expressed in reviews of condition-specific deprescribing work. 100
Context
Reporting of clinical contextual information (deprescribing vs. medicines optimisation) was present in most studies, with 40% of studies focusing specifically on deprescribing. The majority of the studies included deprescribing as part of a wider intervention in the context of medicines optimisation. Although the original funding call for this research invited research examining the ‘intervention’ of deprescribing, the reality of clinical practice (and practice-based research) sees deprescribing as a variable component in complex interventions that address medicines optimisation, reducing treatment burden and delivering person-centred care. These findings highlight implications for future research funding calls.
With regard to population characteristics, the included study samples involved patients on different numbers and types of drugs, with different comorbidities and number of comorbidities. There is a lack of evidence around the impact of deprescribing on people with specific comorbidities (e.g. people with dementia).
Population-level contextual information in the form of key characteristics known to have an impact on inequalities was poorly reported across the studies. Our contextual analysis considered the assessment of inequalities within study populations. Although nearly all studies collected baseline data that could be used to consider the impact of inequalities (specifically gender), fewer than half analysed the data on inequalities. Those that did analysed to adjust data for inequality characteristics, rather than analyse the data to assess the impact on inequalities. Two studies67,79 focused on a population from deprived communities and so discussed the impact of their findings on inequalities.
There is evidence of increased risk of treatment burden among deprived communities,101,102 with a potential, therefore, for them to be considered for deprescribing interventions. Clinicians are urged to consider deprivation status as well as disease status when making clinical decisions. 102 Our review highlights that, as yet, inequality issues are not routinely incorporated into research studies.
Outcomes
We described considerable variation in the reported effects of deprescribing with both improvement and decline in reported outcomes. Interventions were generally acceptable to clinicians, although patient perspectives were commonly not reported. Reporting of safety outcomes was generally positive, although concerns were flagged for general clinical outcomes in secondary care-based studies in which no clinical tools were used. Safety outcomes were reported for clinician-led interventions but not for pharmacist-led interventions. Clinicians may be wary of using our findings to support pharmacy-led deprescribing interventions. The findings do, however, offer support for deprescribing approaches incorporated into clinician-led prescribing approaches.
The use of a range of numbers and types of outcomes by the included studies, particularly for effectiveness, makes it difficult to meaningfully compare the findings across studies. The recent publication of studies describing core outcome sets for multimorbid older populations with polypharmacy, if implemented, may improve the ability to compare the findings from across different studies in future. 103,104
The variability in direction of impact of deprescribing on the effectiveness outcomes (see Table 11) also suggests that there is still a lot of uncertainty associated with deprescribing practice. These findings resonate with a 2018 Cochrane review105 of the effectiveness of interventions to improve appropriate polypharmacy. This review reported substantial variation in outcomes, concluding that overall it was unclear whether or not reported interventions had clinically significant effects.
The recognised variability and uncertainty may arise because we do not yet fully understand the mechanisms involved with deprescribing practice. However, our TAILOR review highlights a body of positive evidence that offers reason to think that deprescribing practice can be both safe and acceptable to patients and health-care professionals. Included studies all scored well against Reeve et al. ’s63 seven steps for good deprescribing practice (see Table 7), a framework that aligns well with the Scottish polypharmacy guidelines. 4 All reported that they were offering tailored care (see Table 7). Outcomes showed mixed evidence of effectiveness, but reasonable safety. More than half the studies included in our TAILOR review incorporate deprescribing within the wider context of medicines optimisation (see Table 9), and it is not possible to isolate the effect of deprescribing. Our findings thus offer support to deprescribing practice within the context of a broader complex intervention of tailored prescribing.
Most studies (n = 12) collected outcome data only up to 3 months. However, several studies that collected outcome data at multiple time points beyond 3 months showed different impacts of the effect of deprescribing over time; therefore, studies with longer-term follow-up are needed.
What tools are available to address problematic pharmacy in older people with multimorbidity and polypharmacy?
The review highlighted the diversity in the tools available for deprescribing, with studies evaluating a range of CDSSs, criteria-led guidance, algorithms and frameworks. In addition, the reported amount of training and level of experience required to deploy these tools also varied.
More importantly, the review findings highlight that although a variety of tools and guidance is available to provide decision support for deprescribing by identifying eligible patients and identifying the medications to deprescribe, the use of these tools alone is not enough to ensure a successful outcome. The lack of a clear direction of effectiveness impact by tool type (see Table 9) suggests that deprescribing is about more than just a set of tools: it is a patient-centred decision-making process that needs to recognise and understand diversity across populations, deprescribing processes, implementation and outcomes.
Generating a reference set for clinicians
The TAILOR scoping review demonstrates that deprescribing is widely used and studied. Deprescribing can be safe and effective, particularly in managing single problems within the context of supporting patients living with multimorbidity. However, our review demonstrates an ongoing lack of evidence on deprescribing, specifically in the management of multimorbidity, for example evidence to be used in prescribing decisions that seek to address burden by choosing between medication used for different conditions or contexts. This may reflect, at least in part, that the concept of multimorbidity does not define a (single) clinical entity. Tailored decisions here will remain the remit of the clinician.
However, our review does highlight that deprescribing within the context of clinician-led reviews of medication, informed by existing frameworks of good practice as described, for example, by Reeve et al. ,63 is usually safe and acceptable to professionals and possibly patients, although with mixed evidence of meaningful impact on outcomes. Our review supports professional calls to recognise deprescribing as part of good prescribing practice. 7,18
Our goal was to generate a reference data set to support clinicians in the complex task of making tailored interpretations of benefit and risk in specific deprescribing decisions. The Scottish polypharmacy guidelines,4,5 for example, include reference sources providing details of numbers needed to treat or harm for a range of medications that a clinician may commonly be assessing within a medication review. Such data can be useful to clinicians discussing the pros and cons of medication use with patients. We have considered whether or not it might be useful to generate something similar focused specifically on deprescribing outcomes. However, the variability in outcomes data described in Table 11 indicates that generating a similar resource based on these deprescribing data could have limited benefit for clinicians’ discussions with patients. We therefore propose to invite clinicians and patients to help us consider how best to present the findings of this review to support clinical practice in our dissemination work (see Chapter 10).
Review of the review
Methodological and topic-specific issues arising from our work
We experienced considerable challenges in identifying a focused data set for this review, related in part to problems in defining and reporting multimorbidity criteria in the title and abstracts of papers. We therefore had to amend our search strategy to merge two concepts relating to polypharmacy and multimorbidity into a single concept (multimorbidity or polypharmacy) to ensure that we were picking up key studies (a change needed/recognised by our sensitivity analysis). As a result, large numbers of studies were picked up and underwent full-text screening. Even then, the majority of studies failed to define what they meant by multimorbidity, or failed to include it as part of their inclusion criteria.
Similar challenges relate to the multiple definitions of both polypharmacy and deprescribing (e.g. ‘five or more’ and stopping medication, respectively). Therefore, we had to use an iterative approach to search strategy development that added in new terms when papers were found through supplemental searching that were not captured by the database searches.
If we are to meaningfully synthesise future work in this area, we need to pay further attention to how research and clinical teams are both describing their interventions, and the quality of reporting within published work.
Strengths and limitations of our work
One of the key strengths of this scoping review was the comprehensive, iterative approach used in the identification of studies. In a highly varied field of clinical practice and research, we describe a focused review of a carefully defined element of that practice. We can therefore be sure that the reported variability is a feature of the published research and not a limitation of our search strategy.
Use of the TIDieR framework61 to extract data on intervention components and the amendment of this to incorporate the Reeve et al. 63 framework allowed for greater detail of the reporting of deprescribing intervention processes than would have otherwise been possible. Our review therefore offers a clear account of what the deprescribing intervention was in each of our included studies. Again, we can therefore be confident that the reported variability reflects the reality of the approaches being used.
Limitations include the lack of quality assessment of included studies. This is not a requirement of a scoping review in which the goal is to map the field of study57 (and given that no statistical meta-analysis was planned). Applying a quality assessment standard may simply have limited our data set and our ability to comment meaningfully on the evidence base that we do have. Furthermore, we used strict inclusion criteria in defining multimorbidity, requiring that study participants had two or more long-term conditions. This potentially excluded many studies that would otherwise have met the remaining inclusion criteria. To address this issue we refined the population criteria to include studies that were not explicit in defining the number of multimorbidities, but that either reported their population as multimorbid, or gave a detailed report of their population that revealed it to be multimorbid. Strict application of this criterion enabled us to confidently offer a detailed analysis of a clearly defined data set. However, it flags two issues relevant to the application of findings to clinical practice. The first issue is about the quality of reporting of studies, that is how well study authors detail the population included and so allow clinicians to judge applicability to their own patients. The second issue is an ongoing concern about the clinical applicability of the concept of ‘multimorbidity’. In clinical practice, clinicians and patients will be discussing a given patient-centred concern (e.g. treatment burden, quality of life or specific symptoms) within a context of a patient living with multiple long-term conditions (multimorbidity), but it is rare that a clinician will be specifically ‘treating multimorbidity’. The careful definition of multimorbidity as an inclusion criterion in this review represents both an academic strength of our work and an applied limitation.
Implications for future work
Our review demonstrates through rigorous analysis of the literature something that is commonly recognised within clinical practice: deprescribing is a complex process. Our findings demonstrate that we need to understand deprescribing not as a technical process akin to a single diagnostic test for which the mechanisms of action are easily understood. Instead, we need to recognise it as a complex process (potentially part of a wider complex intervention of tailored prescribing practice) in which the mechanisms of action are not yet fully understood. Clinical judgement remains paramount in the rigorous application of this process. We consider the implications of this in the light of our realist findings in Chapter 10.
Chapter 6 Realist synthesis: methods
Overview
In outlining the problem in supporting tailored deprescribing in the person-centred management of problematic polypharmacy, we recognised the need for a robust evidence-informed framework that describes the key components of good clinical practice for tailored prescribing. We identified a realist synthesis as the appropriate method to support this. In this chapter, we outline the methodology and methods used in this work.
Outlining the realist approach
Realist reviews ask ‘what works, for whom, in what circumstances, to what extent, how and why?’ and consider the interaction between context, mechanism and outcome [i.e. how particular contexts (e.g. people and practices) trigger or interfere with mechanisms to generate the observed outcomes]. 55 The realist review methodology is particularly useful for understanding and illuminating the relationships between component parts and the impact of the interaction between component parts in a complex intervention. 53,54 It generates explanations about the mechanisms by which stopping medication may (or may not) achieve an impact in different settings and within different subgroups. (Some people with multimorbidity and problematic polypharmacy may respond well to stopping medication, whereas others might respond better to a different approach, or not at all.)
Our realist review followed the methodological and publication standards for realist reviews described by the Realist And Meta-narrative Evidence Syntheses: Evolving Standards (RAMESES) group. 106 The realist approach is widely recognised as a robust methodology that is particularly appropriate when seeking to explain and understand the outcomes observed under different contexts in a complex intervention. Our review followed the five key steps of conducting a realist review outlined by Pawson et al:55
-
clarifying the scope
-
searching for the evidence
-
selecting articles
-
extracting and organising data
-
synthesising the evidence and drawing conclusions.
Step 1: clarifying the scope and constructing a more refined initial programme theory
A realist review begins with an initial ‘draft’ theory of how any intervention is understood to work, also known as a programme theory. Our prestudy draft programme theory is shown in Figure 11 and was further developed in stakeholder discussion in the first 2 months of the project. To develop our initial programme theory further we held a half-day face-to-face meeting at which four members of the project team (JR, JK, GW and DM) discussed and debated the processes and assumptions behind deprescribing. We drew on our content expertise for this process as well as our initial programme theory. At the end of this meeting we developed a more refined programme theory that set out the important concepts we needed to consider in our realist review, as well as putative inter-relationships between these processed. This more refined programme theory was circulated to the wider project team by e-mail and further refined based on their feedback (Figure 12).
FIGURE 11.
Prestudy draft programme theory: elements needed for tailored prescribing.
FIGURE 12.
Refined version of the initial programme theory.
This more refined initial programme theory informed the realist review in the following ways:
-
Searching – we developed our searches so that they would capture the concepts found within our programme theory.
-
Data analysis – the concepts contained within the programme theory informed our sense making of the data. The programme theory also provided a means for us to organise our emerging context–mechanism–outcome configurations (CMOCs).
As the review progressed, we made further gradual refinements to the programme theory when relevant data emerged. During the review, we focused our CMOC development on parts of the programme theory that we judged to be the most important in providing explanations of deprescribing.
Step 2: searching for the evidence
Our search strategy describes a comprehensive, structured approach to identifying relevant literature from as many relevant sources as possible on the complex intervention that is stopping medication (in the context of individual tailoring of medication use). Petticrew50 explains that a search strategy for a review of a complex intervention needs to adopt broader eligibility criteria than those used in traditional systematic reviews, going beyond PICO to include context, processes and theory (mechanisms of action). Similarly, Peters et al. 57 propose that scoping reviews need to also consider populations (i.e. types of participants), context and ‘concepts’ (the interventions being examined and the outcomes used to assess their success). We combined these approaches to describe the search eligibility criteria we used for the realist review in the TAILOR study (Table 14).
| Inclusion criteria | Explanation/justification |
|---|---|
|
Populations: all participants aged ≥ 50 years with multimorbidity (two or more long-term conditions) and polypharmacy (five or more long-term medications) Excluding: response to acute adverse reactions/toxicity |
Aged ≥ 50 years as this is the age when multimorbidity starts to rise: 20% have more than two long-term conditions and 10% have more than three. 107 A growing group facing the challenges of problematic polypharmacy so inclusion in this study future-proofs our work Burden from medicines use (problematic polypharmacy) does not correlate directly to disease burden or number of medications. 2 We therefore kept a broad definition of multimorbidity Polypharmacy as five or more medicines as this is a common approach used by researchers and so will ensure that we capture the key studies |
|
Interventions (concepts/process and theory): any systematic intervention process used to safely withdraw medications in older people with multimorbidity and polypharmacy and the outcomes used to measure the effectiveness of these strategies Excluding: no comparator group |
Including deprescribing, individual/mutually agreed tailoring, medicines optimisation assessments, stopping medication and personalised prescribing, including individual/mutually agreed tailoring. Involving discrete/multifaceted/blended strategies. 108 Noting details of comparators, theories of mechanisms of actions and outcomes used to measure success (may include patient benefits and harms; acceptability to patients and prescribers; health-related quality of life/functional status; treatment burden; safety including adverse events; and service use) For the scoping review, we will exclude studies without a comparator group. However, these studies will potentially be used within the realist review |
| Context: studies conducted in any appropriate setting | Including primary care (general practice, pharmacy, home settings), acute/interface care and secondary or tertiary care. Noting details of settings to inform explanation of variability in mechanisms of action and outcomes |
|
Study design: any comparative studies including RCTs, cohort or case-control studies, qualitative studies Excluding: single case reports, case series, studies in which results for intervention and control groups are not presented separately |
We used a modified version of the 6S Pyramid109 to frame the types of included evidence that will include both quantitative study designs110 (experimental, before-and-after studies, and observational studies), as well as qualitative studies with recognised methodological frameworks. We will include studies using any recognised structured review methodology and scan reference list of reviews for previously unidentified studies. We will include any national or international clinical guidelines that provide information on the safe withdrawal of medications in multimorbid patients with polypharmacy Again, excluded studies will be reconsidered for inclusion in the realist review |
The criteria described in Table 14 were used to produce a detailed search strategy in conjunction with our information specialist (Dr Nia Roberts). The initial search was tested and refined to the Ovid MEDLINE, Cochrane Library and EMBASE databases by analysing words contained in the title, abstract, and index terms of identified studies.
The refined search terms were then applied to the following databases: Ovid MEDLINE, EMBASE, CINAHL, The Cochrane Library [including CENTRAL and the Database of Abstracts of Reviews of Effects (DARE)], Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register, Campbell Collaboration Library of Systematic Reviews, Joanna Briggs Institute Database of Systematic Reviews and Implementation Reports, PsycInfo, Allied and Complementary Medicine Database (AMED) and CAB Abstracts (see Appendix 4 for details of our search strategy).
We also searched:
-
Trial registries – https://clinicaltrials.gov/ and https://apps.who.int/trialsearch/.
-
Grey literature – Google (Google Inc., Mountain View, CA, USA) and Google Scholar websites and websites of relevant stakeholders {including Royal College of General Practitioners Bright Ideas, National Clinical Guideline Centre, Royal Pharmaceutical Society and conference abstracts [e.g. Prescribing and Research in Medicines Management (PRIMM)]}. We also used personal communications to contact experts in the field who may have been able to signpost us to further relevant information.
We also used ‘pearling’, in which we examined the reference list of finally included relevant studies to identify additional documents.
In the scoping review, we described a search strategy to systematically identify the current literature on the approaches used, impact, safety and acceptability of interventions for stopping medication. The realist review method also specifically examines the mechanism of action of an intervention in different contexts. We therefore identified that two additional search elements would be needed for this work package:
-
‘Sister papers’ (qualitative studies, process evaluations, etc.) for any studies identified in the above search,57 along with purposive searching to find relevant data that would enable us to develop and then confirm, refute or refine (‘test’) aspects of the draft programme theory.
-
For each theory area in our draft programme theory, we generated a sequence of search questions. For example, Figure 1 highlights ‘sense making’ as a concept in our draft programme theory. Emerging questions might include ‘what impact does the interaction between individual (patient and professional) beliefs and values and setting have on individual tailoring of medicines?’ From this, we drew up a series of specific search terms: a systematic search strategy that sought to identify research (as ‘data’) related to the targeted programme theories. This searching captured the additional relevant data necessary for our developing programme theory that were not captured within existing specific studies of stopping medications.
In addition to the formal searches for the realist review, we also drew on the search conducted for the scoping review described in Chapter 4. The 20 studies identified for inclusion in the scoping review were also screened for eligibility to be included in the realist review. Furthermore, we also screened qualitative studies identified by the scoping review search for their eligibility to be included in the realist review, as these were likely to contain rich information relevant for the development of the programme theory.
Step 3: selecting articles
Our inclusion and exclusion criteria for the review were identified based on our research questions, draft programme theory and discussion in the team. Our inclusion and exclusion criteria are outlined in Table 15.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Populations: all participants aged ≥ 50 years with multimorbidity | Response to acute adverse reactions/toxicity |
| Interventions (concepts/process and theory): any systematic intervention process used to safely withdraw medications in older people with multimorbidity and polypharmacy and the outcomes used to measure the effectiveness of these strategies | |
| Context: studies conducted in any appropriate setting (general practice/pharmacy) | Studies from low- and middle-income countries, studies not published in English |
|
Study design:a any comparative studies including RCTs, cohort or case-control studies, qualitative studies Grey literature |
Criteria were applied to the data set in a first phase of screening conducted at title and abstract level by Amadea Turk and a random sample of 10% of these was reviewed independently by Kamal Mahtani and Geoff Wong to help ensure that the criteria were applied consistently, and disagreements were resolved through discussion.
From these, the selection of full-text documents primarily focused on the extent to which the articles included data that could contribute to the development and refinement of the programme theory. Documents were assessed on their relevance (whether or not they contributed to the development of the programme theory) and rigour (whether or not the data contained in the documents were trustworthy). 106 Documents that did not include mention of involvement from patients in the deprescribing/medication management process were deemed to be of low relevance to our research question, which explored individually tailored approaches to medication management, and were therefore excluded from the review.
Steps 4 and 5: extracting, organising and synthesising evidence
Included full text documents were uploaded into NVivo (QSR international, Warrington, UK) a qualitative data software tool supporting analysis, and study characteristics were recorded in a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) file. The coding of relevant extracts from documents was largely inductive, although consideration of concepts within the initial programme theory enabled a degree of deductive coding, as did discussions with members of the project team and stakeholder groups.
The initial stages of coding focused on the conceptual level and classified content into broad descriptive categories. This initial process helped us manage the data as well as make sense of the landscape of the literature, and helped us make decisions about whether or not we had captured enough data to further develop and refine the programme theory. During this first stage of coding, data were not immediately categorised into contexts, mechanisms and outcomes, but instead the focus was on looking at the data with an open mind to understand key issues. The data within these broad categories were then reread and, when needed, recoded and reclassified. Once this conceptual-level coding was completed, we started to consider whether or not these categories and the subcategories within them included sections relating to contexts, mechanisms and outcomes.
The development of CMOCs began by considering an outcome and using interpretations of the data to develop explanations of how specific contexts might have triggered different mechanisms to produce the outcome. A list of potential CMOCs was created by Amadea Turk and then shared and discussed with Geoff Wong, Joanne Reeve and Kamal Mahtani as well as with our patient and public involvement (PPI) partners. Developing CMOCs were then incorporated into the refined programme theory. Diagrams of partial theories or subsections of the programme theory were created to help guide and illustrate our findings. This process continued iteratively until we were able to develop CMOCs that explained what we judged were the most important parts of the programme theory.
The CMOCs were considered to have sufficient explanatory value when they met the key criteria for programme theory coherence. These criteria included:
-
consilience – when the CMOC was able to account for as much of the possible data related to that CMOC
-
simplicity: when the theory/CMOC was simple and did not have to have special (or ‘ad hoc’) assumptions made to explain data
-
analogy: when the theory/CMOC fitted in with what we currently know/and or substantive theory.
Engagement with substantive theory
In this review, substantive theories were drawn on to help substantiate and develop the inferences about CMOCs, as well as to act as lenses through which to bring together the findings of the review. Some of the theoretical ideas that informed the development of some of the CMOCs were derived from the documents included in the review and other theoretical frameworks were sought to help situate findings within a wider context. These other theories were identified through team discussions and drew on the expertise and knowledge of members of the research team and aided the iterative process of programme theory refinement.
Chapter 7 Realist synthesis: results
Overview
Our findings deliver on our goal to move beyond describing barriers to and facilitators of patient-centred deprescribing to reach an explanation of how and why health-care professionals and patients engage with deprescribing in different contexts. We offer an understanding of what drives the medication management behaviours and decisions of health-care professionals and patients in the presence of uncertainty and complexity.
Our starting literature (see Chapter 1) recognised a number of challenges to deprescribing, including a culture of diagnosing and prescribing; evidence-based guidance focused on single diseases; a lack of evidence-based guidance for the care of older people with multimorbidity; a lack of shared communication, decision-making systems, tools and resources; professional etiquette; and fragmented care. 111 These challenges were all discussed extensively in the articles included in this review. Our analysis offers an explanation of why and how some of these challenges affect deprescribing, and identifies some potential intervention strategies that may be helpful in navigating some of the uncertainties and complexities involved in the deprescribing process.
Our analysis developed a total of 34 CMOCs. The first 19 of these explain the deprescribing landscape and the different organisational-/system-, health-care professional- and patient-level factors that affect the deprescribing process. The remaining 15 CMOCs explain how potential intervention strategies, including shared decision-making, continuity of care, monitoring and multidisciplinary teams, may help navigate some of the complexities described in CMOCs 1–19. These are presented as partial programme theories or subsections that illustrate and evidence our final programme theory, which describes five high-level concepts to help inform policy and practice. These comprise providing an enabling infrastructure, access to data to inform decision-making, creating a shared understanding of meaning and purpose of medications, trial and learn, and building trust.
Data set
In total, 119 documents6,18,32,71,74,75,79,84,85,103,112–217 were included in the review (Figure 13). Articles were published between 1997 and 2020 and included a mixture of study designs and article types (see Appendix 5, Table 26, for a detailed table of included studies). The initial search also included documents relating to the topic of medication adherence. We included some of these when we believed that they could help us refine some of our CMOCs about how patients value their medication.
FIGURE 13.
The PRISMA flow diagram for the TAILOR realist review.
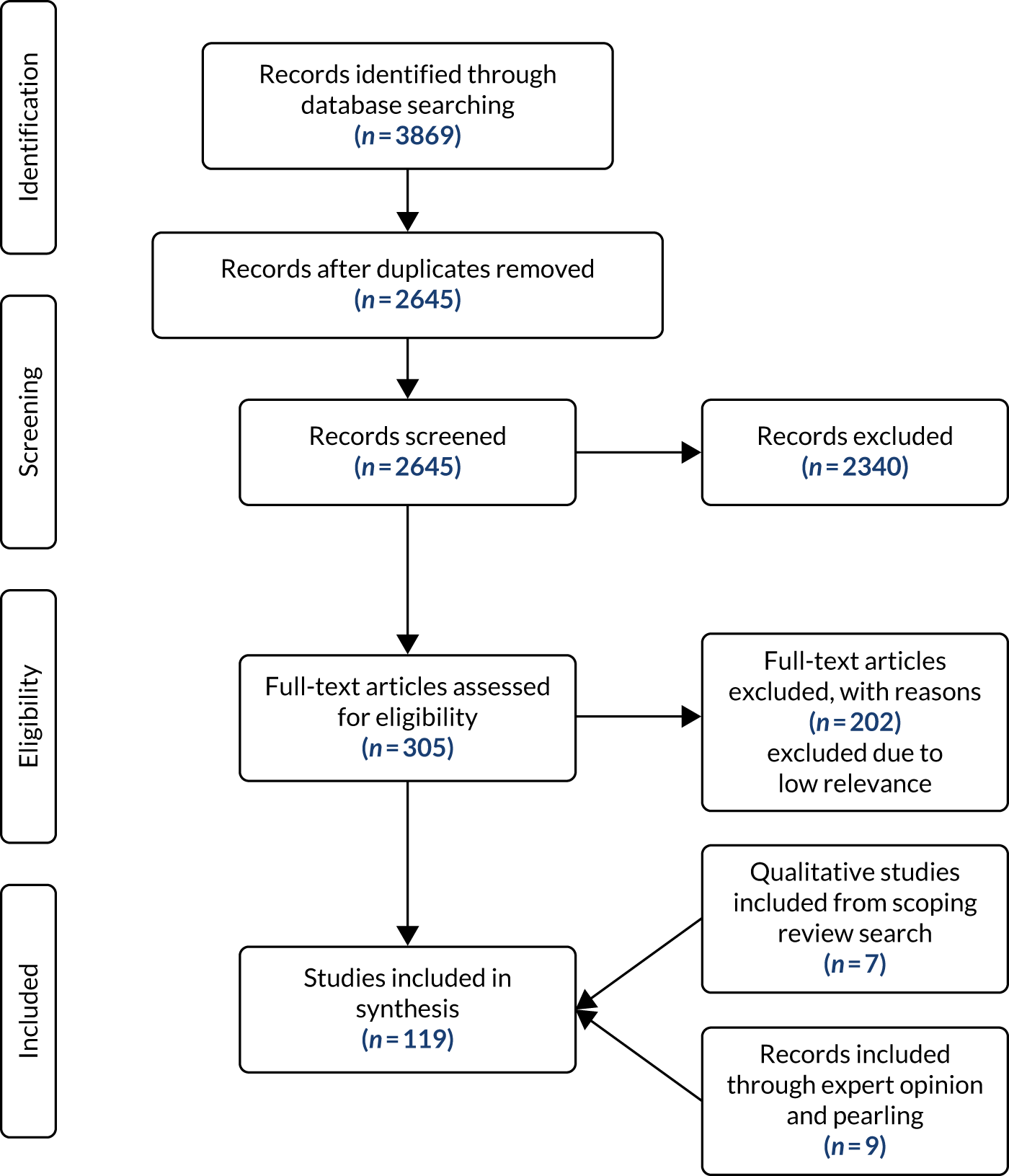
Six of the studies included in the realist review were also identified by the scoping review search and included in the scoping review described in Chapter 4. 71,74,75,79,84,85 The additional 14 studies identified for inclusion in the scoping review were also screened; however, none contributed to the further development of the programme theory.
The findings of this review explain the various factors that shape the medication management and deprescribing process. They also highlight potential intervention strategies/contexts that need to be present to mitigate some of the challenges and complexities presented by the factors shaping the landscape of medication management.
The results are presented under two main sections. First, we discuss the deprescribing landscape, and then we consider potential solutions to enhance deprescribing.
Each of the sections will first provide a narrative of the findings based on the analysis of the included literature followed by a realist analysis that contains one or more CMOCs. Additional data describing and supporting development of each of the CMOCs can be found in Report Supplementary Material 4, Tables 30–36.
The deprescribing landscape
The factors that shape the activity of deprescribing can broadly be grouped into organisational/system-level factors, health-care provider-level factors and patient-level factors. Each of these factors interacts with the others both within and across different levels.
Organisational/system-level factors
Guidelines and policies
Health-care professionals managing polypharmacy are faced with a number of challenges posed by treatment and policy guidelines, which may limit their willingness or ability to consider deprescribing.
Although numerous guidelines for medication management exist, these are often based on the management of single conditions and on evidence from trials in younger populations. 111 Data from the included studies suggest that health-care professionals struggle to apply these guidelines to older patients with multimorbidity, therefore making it difficult for them to tailor medicines for individual patients and difficult to feel that the decisions they do make are safe and/or defendable (CMOCs 1 and 2).
Furthermore, incentive structures and administrative rules can make it difficult for healthcare practitioners to dedicate the time necessary to undertake the complex and time-consuming deprescribing process [CMOC 3 (also see related CMOCs 10 and 11)].
Transitions in care and difficulty accessing patient information
Multiple prescribers, transitions between primary and secondary care, and the poor documentation of changes made to treatments mean that health-care professionals sometimes do not have an accurate understanding of patients’ current medication regimens. 48 Data from the literature highlight health-care professionals’ frustration at the lack of central and universalised access to patient medical records and at the delays in communication from other sectors of care (CMOC 4).
Optimising medicines relies on having an accurate understanding of the medications a patient is taking, and in the absence of this information health-care professionals may struggle to make decisions about deprescribing.
Unclear roles and responsibilities
The lack of clarity surrounding the roles and responsibilities for deprescribing among health-care professionals is thought to both contribute to inappropriate prescribing and limit the extent to which health-care professionals engage in deprescribing. 112 The literature reveals disparities in opinion among health-care professionals regarding to whom the assignment of medication management responsibilities should fall. 113 Although GPs are often recognised as being well placed to take on the role of co-ordinating and managing medications, the ‘lack of a clear line of responsibility’114 for this role, alongside the additional challenges posed by the wider system (CMOCs 1–4), may mean that GPs struggle to engage with this role. This lack of clear responsibility for deprescribing may leave health-care professionals feeling like they do not have ownership of the process and, therefore, reluctant to engage with it (CMOC 5).
Realist analysis of organisational/system-level factors
Our realist analysis of organisational and system-level factors is summarised in Figure 14 below, with further details in Table 16.
FIGURE 14.
Partial programme theory CMOCs 1–5: influence of organisational/system-level factors.
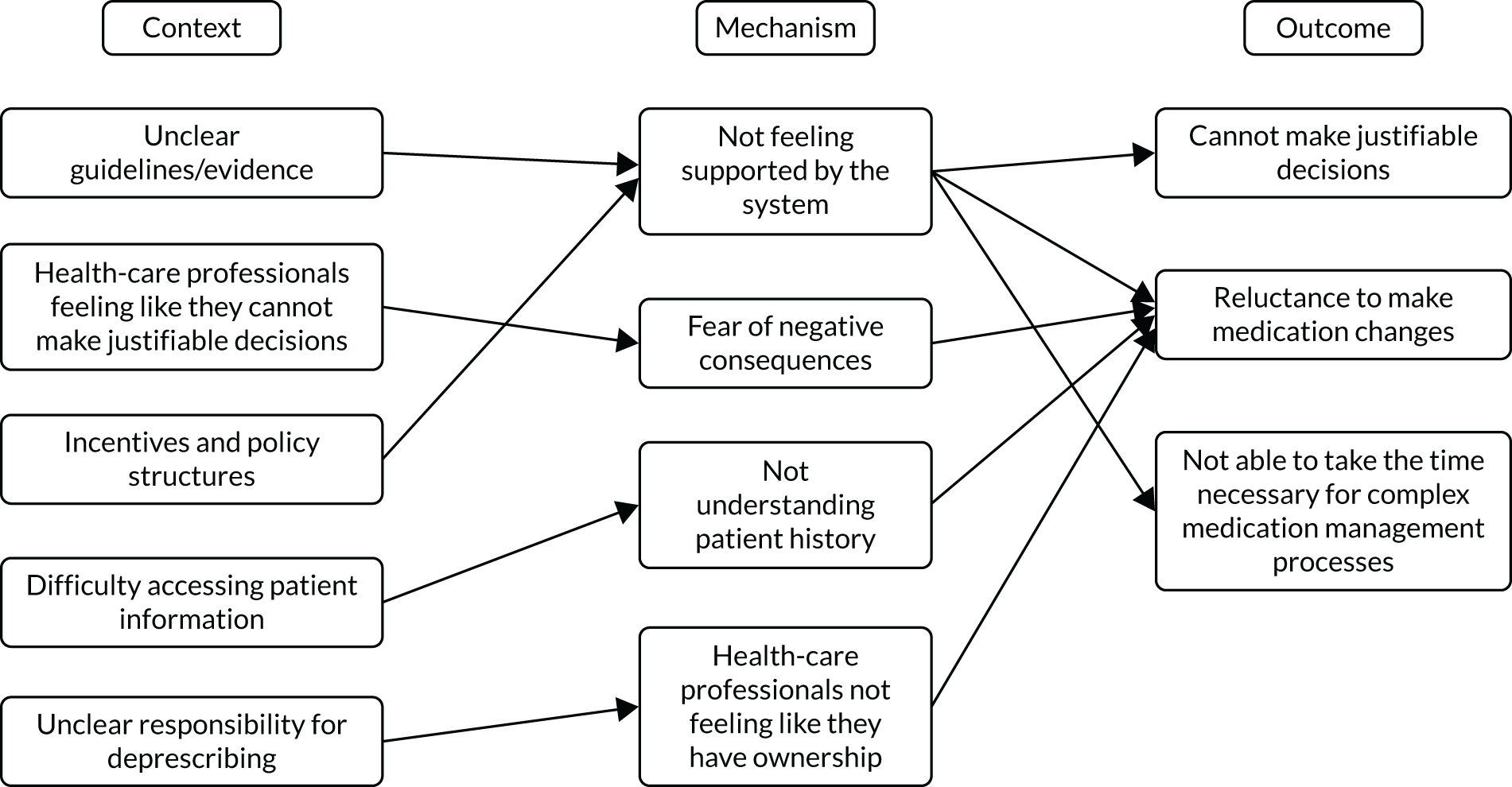
| CMOC | Description | Supporting data |
|---|---|---|
| 1 | In the absence of applicable deprescribing guidelines and evidence (C), health-care professionals may feel that they cannot make justifiable decisions regarding medication changes (O) because they do not feel that these decisions are supported by the system (M) | The GP quoted as follows: we can’t justify that you can just stop [prescribing] it. We can’t really do that because of how our treatment criteria look, where there aren’t any defined criteria for when we can stopNixon and Vendelø173GPs generally felt insufficiently supported by the guidelines in their efforts to treat hypertension in older people . . . I would really like to have a guideline that states: in elderly you have pay attention to this, this and thisvan Middelaar et al.212 |
| 2 | When health-care professionals feel that they cannot make justifiable decisions that are supported by guidelines (C) they may be reluctant to make changes to medications (O) because they are afraid of negative consequences (M) | Physicians reported comfort in deprescribing preventive medication, but fewer were comfortable with deprescribing guideline-recommended therapeutic medications in patients with poor life expectancy. One explanation may be the fear of adverse withdrawal effects, which were also mentioned by physicians as a potential factor that prevented them from deprescribingDjatche et al.132Although most ‘guidelines’ were not proven in older people, particularly in the very old, those with co-morbidity, dementia, frailty, and limited life-expectancy, doctors are afraid of lawsuits and of the patient/family reaction if they do not follow all experts’ recommendationsGarfinkel138The Australian GPs were overwhelmingly negative about aged care and expressed dissatisfaction at the financial reimbursement provided for ACF services. Their attitudes towards deprescribing for ACF residents were influenced by concerns of blame in the case of negative health outcomesBolmsö et al.121 |
| 3 | When health-care professionals are not supported by incentive and policy structures (C) they may not be able to take the time necessary for complex medication management processes (O) and they may be reluctant to make changes (O) because they do not feel supported to do so (M) | Inability to maintain follow-up to support a gradual process of deprescribing was a major frustration. One participant cited administrative rules as an impediment to follow-up: I think that the 2-year window [for reimbursement] makes it difficult to follow-up, especially for the complex patients that need that stepwise approachAnderson et al.117Patients who are most in need of a home medication review and most complex were considered less likely to receive equally detailed reviews because pharmacists seemed unwilling to substantially extend the review duration without additional remunerationMc Namara et al.166 |
| 4 | When health-care professionals cannot access information about a patient’s medication regimen (C) they do not have an accurate understanding of the medication regimen (O) because they do not understand the patient’s history (M) | GPs found that there were discrepancies between the systems [across sectors] and that they were not properly informed about the changes made at the hospital . . . ‘Often we are not informed about the changes. It is us, the GPs, that must try and figure it all out, that isn’t easy’Laursen et al.160There were mixed views on the quality and extent of documentation of medicines in patient records . . . ‘The problem, many times there is no documentation about the medication. Most people write documentation. Some don’t write it. Some write incoherent handwriting’Al Shemeili et al.115All groups discussed health system structure concerns including limited funding and incomplete medical and medication histories. Medication histories rarely detail why and when medications were initiated and which prescriber is responsible for ongoing monitoringTurner et al.208 |
| 5 | When health-care professionals are unsure about whose responsibility medication management is (C) they may struggle to engage in making medication changes (O) because they do not feel that they have ownership over the process (M) | Some GPs felt responsible towards overseeing patients’ medicines. Others were insecure about their task and desired ‘a clear line of responsibility’Bokhof et al.120Some physicians were of the opinion that they dealt with the management and control of their specialist condition only, and while this may involve an element of polypharmacy in the use of several medicines, they considered polypharmacy to be the responsibility of othersAl Shemeili et al.115Whilst an oncologist may be in a good position to judge prognosis and whether a medication is appropriate, he or she may defer to a patient’s GP regarding such medications which are usually initiated in primary care. Thus neither party may feel that they have ownership of the problemCashman et al.122 |
Health-care professional factors
Health-care professionals’ ability to engage in deprescribing is shaped by a number of individual and interpersonal factors including their skills and experience, their professional etiquette, and the amount of time and headspace they have available to engage in a complex decision-making process.
Skills and experience
The level of experience and expertise of a health-care professional may play a significant role in how comfortable they feel in engaging in deprescribing, particularly in the absence of applicable guidelines (CMOC 6; also see CMOC 1). More experienced health-care professionals may feel more comfortable making recommendations without clear guidelines because they have had to balance quality of life against risks and benefits of medicines before, and know what to do and what to expect (CMOC 6). 121
Medication management in older populations is seen to require specific skills and knowledge owing to physiological changes associated with ageing and changes in pharmacokinetics and pharmacodynamics (CMOCs 22 and 25). 115,130 When health-care professionals feel that they lack the skills in what they see as an area that requires specific training or experience, they may not feel confident making changes to their patients’ medicines (CMOC 7), particularly if these medications have been prescribed by a specialist (CMOC 8).
Professional etiquette
Patients with polypharmacy and multimorbidity are often managed and cared for by health-care professionals across different specialties and health-care settings. Health-care professionals may be reluctant to deprescribe medications that have been prescribed by other professionals either because they do not feel that they have the expertise (CMOC 8) or because they are worried about damaging the relationship with the original prescriber (CMOC 9). This is compounded by working in a system in which it can sometimes be difficult to understand why a medication was prescribed in the first place (CMOC 4) and in which the health-care professionals do not have the time to contact the original prescriber to ask for clarification. Furthermore, health-care professionals may be worried about damaging the relationship between the patient and the original prescriber, as presenting patients with conflicting recommendations may damage patient trust (CMOC 23).
Time
Deprescribing and medication management is a process that requires the careful consideration of the benefits and harms of medicines, as well as balancing these against patients’ goals of care, and may require a period of follow-up. Health-care professionals are often limited by set consultation times (related to CMOC 3), which may force them to prioritise how they spend their time (see CMOC 11). This can have an impact on health-care professionals’ headspace (cognitive and emotional capacity) to allow them to consider and balance the potential benefits and harms of medication changes in a context in which there may not be adequate guidelines (CMOC 4). It can also affect the extent to which health-care professionals are able to engage with patients to understand their treatment goals and explain the reasons behind recommended changes, and therefore deliver tailored person-centred care (CMOC 11). Health-care professionals may therefore be reluctant to make changes to a patient’s medications (CMOC 10), particularly when the patient is judged to be stable.
Realist analysis of health-care professional factors
Our realist analysis of health-care professional factors is summarised in Figure 15 below, with details in Table 17.
FIGURE 15.
Partial programme theory CMOCs 6–11: influence of health-care professional factors.
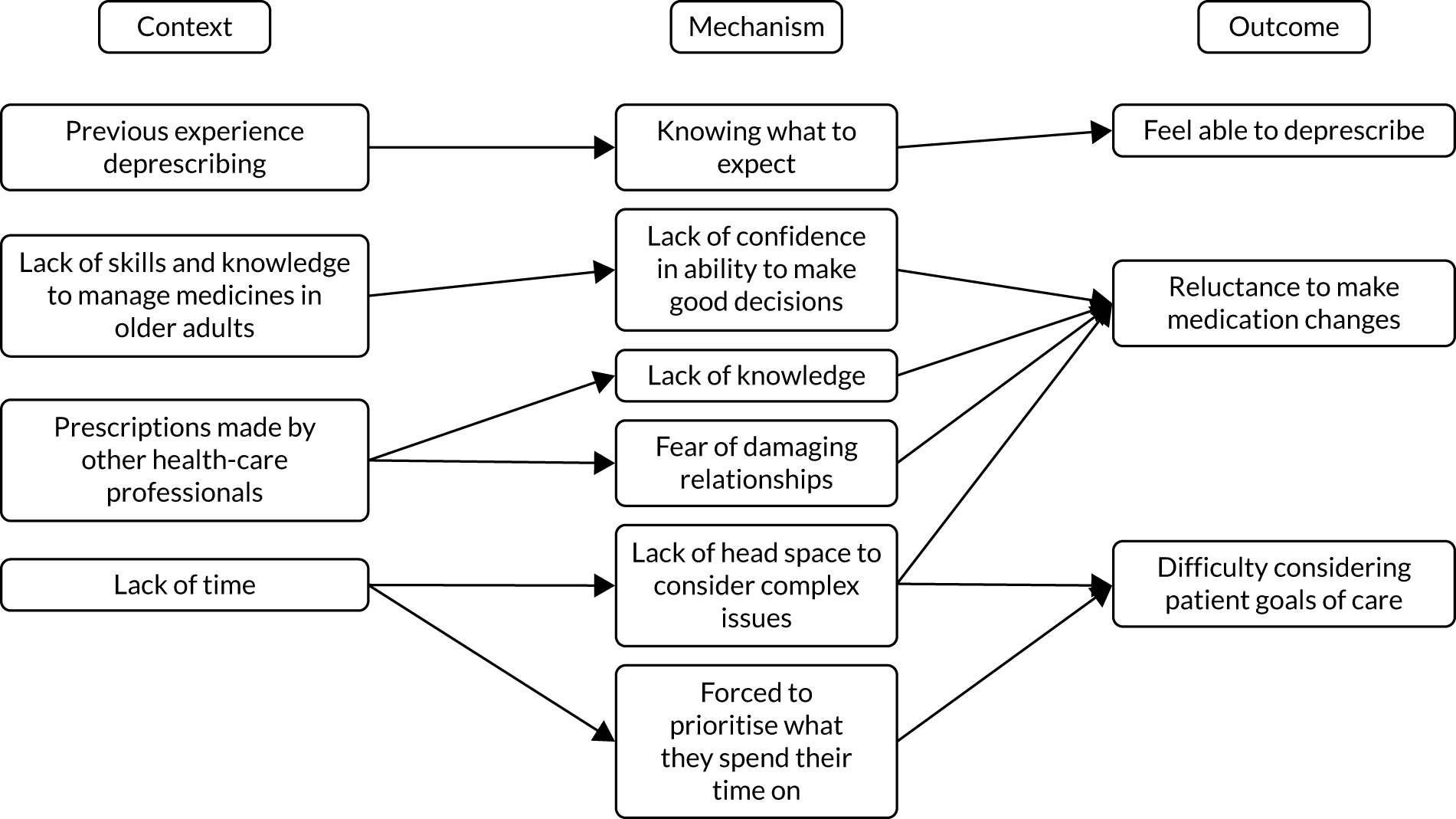
| CMOC | Description | Supporting data |
|---|---|---|
| 6 | When a health-care professional has previous experience deprescribing medication (C) they are more likely to feel able to deprescribe (O) because they know what to do and expect (M) | Best guesses were also required because ‘you don’t have guidelines for every situation — there are times when you just have to make a decision as best you can’ (GP6). GPs relied heavily on their prior knowledge and experience of the patient in this processSinnott et al.199Later on now in my career, I’ve taken on a different approach. I understand that managing polypharmacy is an art as much as it is a science. You have to balance quality of life, risks and benefits, when prescribing medications. I don’t feel the need to fix everythingHernandez148Negative experiences reinforced a tendency to opt for the status quo, whereas positive or neutral experiences fostered open-mindedness toward deprescribing . . . ‘As you get older, you realize that is not really true because you have done it so many times and they have not had a stroke the next week’ (GP4)Anderson et al.117 |
| 7 | When health-care professionals feel that they do not have the necessary skills and knowledge to manage medicines in older adults (C) they are less likely to make changes to patients’ medicine regimes (O) because they are not confident in their ability to make good decisions (M) | ‘I’m not clever enough to have all the statistics in my head to be able to say, well, that Statin is stopping all that absolute relative . . . It would be a great help [with deprescribing] to have further training and to meet with GPs in the same situation’Bolmsjö et al.121Often, the GPs needed to discuss the patient’s treatment because they did not feel they had the knowledge or skills to make correct therapeutic decisions. As one GP stated, ‘A specialized treatment belongs at the hospital, where the specialist can use their expertise’Laursen et al.160 |
| 8 | When medicines have been prescribed by a specialist (C), other health-care professionals from other specialties may be reluctant to make changes to patients’ medicine regimens (O) because they do not feel that they have the knowledge to make a safe decision (M) | Often they will stop these days and just go back to one so I would question the dipyridamole but not necessarily stop it. Looks like the cardiologist has prescribed dipyridamole so I guess we would accept that (GP6)Ailabouni et al.113Physicians may be reluctant to review or alter decisions that were made by experts from other specialties, or to deviate from recommended therapeutic guidelines that were derived from younger populationsDjatche et al.132Prescription by a specialist or other practitioner is a factor identified in this survey that inhibits many FPs from deprescribing medications . . . this may be due to lack of confidence in their own knowledge and experience or being unclear about the indication for the medication chosen by the specialistHarriman et al.146 |
| 9 | When medicines have been prescribed by another health-care professional (C), health-care professionals may be reluctant to make changes to patients’ medicines (O) because they are worried about damaging relationships with the original prescriber as well as between the original prescriber and the patient (M) | GPs discussed being intimidated by specialist physicians for deprescribing medications they initiated, with one recounting being ‘scorned by a colleague’. Furthermore, GPs expressed disappointment when deprescribed medications were restarted by a specialist physician or in hospital. These factors have also been identified in previous researchTurner et al.208External factors GPs were reluctant to discontinue medication prescribed by other medical specialists without contacting them. Contacting the specialist to change medication, however, took additional effort and GPs feared that it would be difficult to reach a consensus as the specialists often have a different viewpointRieckert et al.186There was also a reluctance to ‘interfere’ with other healthcare providers’ prescribing driven by fear of disturbing therapeutic relationships, hesitation to contradict prescribing by other healthcare providersMcNamara et al.166 |
| 10 | When health-care professionals do not have dedicated time (C) they may be less likely to make changes to patients’ medications (O) because they do not have the emotional and cognitive capacity to consider complex issues (M) | It is important to consider a patient’s goals of care, prognosis, and functional level when considering which medications are potentially inappropriate. The process of deprescribing also requires special attention and timePruskowski and Handler183Participants emphasized, consistent with the importance of sense-making, that communication should go beyond checks on understanding of information to communicating the benefits of any changes and being responsive to patient concerns. Both patients and professionals agreed this would require dedicated, protected time to enable issues to be exploredKnowles et al.155 |
| 11 | When health-care professionals do not have time (C) they may find it difficult to fully consider a patient’s care goals (O) because they are forced to prioritise what they spend their time on (M) | There is time taken away during a visit because of a variety of screenings that must take place during a patient assessment, such as asking questions about falls, depression, and abuse. This is impossible to achieve in our health care system, which demands high efficiency and throughputChen and Buonano125Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue themMcGrath et al.165Key barriers to engagement related to the practical constraints of workload placing limits on the necessary time and ‘head space’ needed to engage with this complex form of clinical practice. ‘limited by time, caseload and so lack of mental capacity’ (GP) ‘I barely get through the day reacting’ (GP)Reeve et al.6 |
Patient-level factors
Many studies75,79,114,143,162 included in the review suggested that, in principle, patients are open-minded about deprescribing and may be willing to discontinue one or more medications that are considered to be ‘inappropriate’ or unnecessary. However, patients’ willingness to engage with, and consider, deprescribing is shaped by the relationship patients have with their medicines and the value they place on them, as well as the involvement from their families and carers.
Perceived value of medicines
Medicines may be perceived to be symbols of good care and healing and, therefore, an outcome that is to be expected following a consultation with a health-care professional. 138,183 The suggestion to withdraw a medicine may therefore also be perceived as being a withdrawal of care and may make health-care professionals reluctant to pursue deprescribing, because justifying this action to the patient could be emotionally and cognitively taxing (CMOC 12).
Patients may also believe that their medicines provide them with a range of benefits, including the maintenance of their independence and identities, by controlling symptoms that may interfere with their daily lives, and may even see them as keeping them alive. Patients may therefore feel reluctant to consider discontinuing these medicines because they do not want to lose these benefits, and they worry about the possible negative consequences (CMOCs 13–17). Medicines may also be a sign of hope and patients may be eager to continue using them because they feel that the medicines are doing something for their condition and they may see an improvement in the future (CMOC 14). In the context of these beliefs held by patients, health-care professionals may find it difficult to discuss deprescribing because doing so might be viewed as withdrawing care or abandoning the patient, and explaining and justifying deprescribing may be emotionally taxing (CMOCs 12, 13 and 15).
Influence of families and carers
Patients’ families and carers can play an important role in influencing the medication management process. Families may have strong expectations of medicines keeping their family members alive and may make it difficult for health-care professionals to have conversations about what realistic goals of care might be. 179 These expectations may put pressure on health-care professionals to maintain patients’ medication regimens (CMOC 18).
Families and carers who are involved in patients’ care can also be a source of support for patients by helping them access information about their medication and support them to be independent and actively involved in their care. This may result in patients being more able to engage in decisions about their medicines (CMOC 19).
Realist analysis of patient-level factors
Our realist analysis of patient-level factors is summarised in Figure 16, with details in Table 18.
FIGURE 16.
Partial programme theory CMOCs 12–19: influence of patient-level factors.
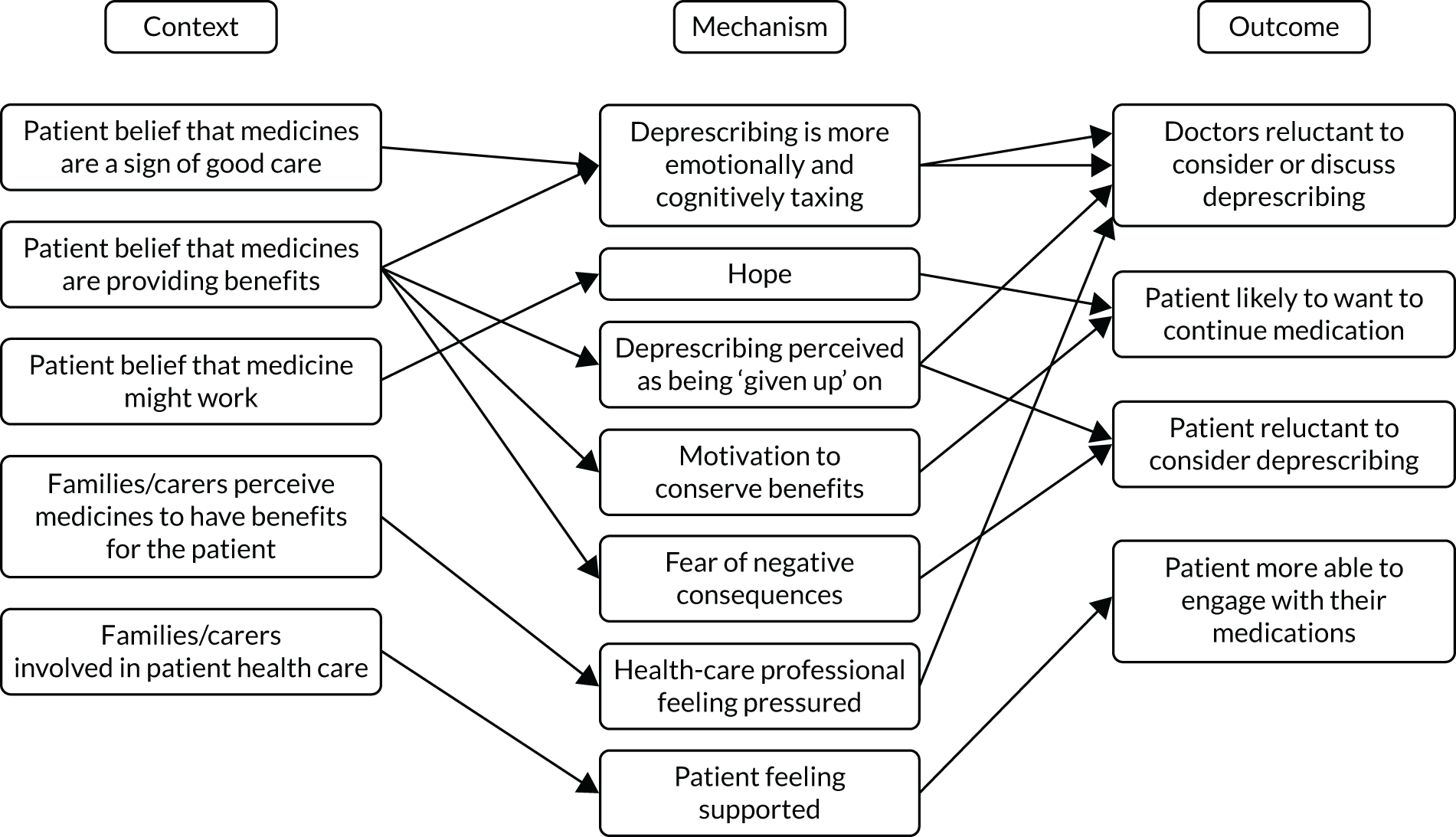
| CMOC | Description | Supporting data |
|---|---|---|
| 12 | When patients believe that medicines are a sign of good care (C) doctors may be reluctant to consider deprescribing (O) because explaining and justifying any deprescribing is more emotionally and cognitively taxing (M) than not doing this | Patients and providers alike possess psychological connections to medications. This may be because medications are the most visible form of health carePruskowski and Handler183Moreover, in the absence of ‘low-hanging fruit’ or a clear trigger to cease therapy, deprescribing, compared with initiating therapy, appears a riskier, less certain, and more cognitively and socially demanding process, with minimal decision supportAnderson et al.117GPs had specific difficulties talking to multimorbid patients about stopping medications; they feared this could be interpreted by the patient as a withdrawal of care and potentially damage the doctor–patient relationshipSinnott et al.200 |
| 13 | When patients believe that their medicines are providing them with benefits (C) doctors may find it difficult to discuss deprescribing (O) because explaining and justifying any deprescribing is more emotionally taxing (M) than not doing this | Factors influencing GPs’ deprescribing were beliefs concerning patients; patients have no problem with polypharmacy; patients may interpret a proposal to stop preventive medication as a sign of having been given up on; and confronting the patient with a discussion of life expectancy vs. quality of life is ‘not done’Schuling et al.195Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or ‘downgraded’McGrath et al.165GPs acknowledged that in the terminal phase it would be rational to discontinue anti-hypertensive medication. However, they often hesitated to take this step to avoid the impression they were giving up on the patient or unnecessarily deprive them of a sense of being in control with adequate BP measurementsvan Middelaar et al.212 |
| 14 | When patients believe that a medicine might be working or will work in the future (C) they are likely to want to continue taking it (O) because they hope that they are doing something to help their condition (M) | Patients feel an improvement when they start taking the drug or hope for a future improvement and for that reason they do not want to discontinue it. Some patients think that they are doing something that can help their condition and in doing so they feel reassuredGonçalves144Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementiaMcGrath et al.165 |
| 15 | When patients believe that their medicines are keeping them alive (C) health-care professionals may find it difficult to discuss deprescribing (O) because they do not want their patients to feel that they have abandoned them (M) | They also considered the reaction of the patients, who might have come to value their medicines or feel that deprescribing is a sign of abandonmentBokhof and Junius-Walker120It is important to anticipate barriers to deprescribing and to discuss these with patients and carers. Barriers may include psychological discomfort when ceasing a medication they have been taking for many years, or feeling their situation is hopeless since medications for chronic diseases are being ceasedHardy and Hilmer145Moreover, GPs are reluctant to initiate a discussion about stopping medication because they are concerned that patients may interpret this as a sign of being given up on. People may then get the feeling, ‘Don’t I count anymore, am I not important?’Schuling et al.195 |
| 16 | When patients view medicines as prolonging their lives (C) they may be reluctant to stop taking them (O) because they view deprescribing as a sign that they are not worth keeping alive any more (M) | Patients may raise difficult questions that the doctor may wish to avoid, for example: ‘am I not worth treating anymore?’, ‘I was told I should take this for the rest of my life, does this mean I am going to die?’, ‘won’t I get ill without the tablets?’Cashman et al.122Still other physicians voice concerns that patients will feel the physician is ‘giving up on them’ or ‘leading them to quicker deaths’Harriman et al.146 |
| 17 | When patients believe that medicines are providing them with benefits (C) they may be reluctant to discontinue them (O) because they are afraid of negative consequences (M) | For some participants, a complex drug regimen was the only means through which they could gain equilibrium, relief from distressing symptoms, or a sense of having a ‘normal’ life (though this varied in degree of success and setbacks)Townsend et al.207The overarching pattern of ‘preserving self’ was a surprising and clear finding. Taking medication was closely tied to self-identity and manifested in various ways, described in the ensuing sections. Taking multiple medications was significant and personalVandermause et al.213Mrs. D derives an important sense of empowerment from her supplement use that should be respected by her physiciansPitkälä et al.181 |
| 18 | When families or carers perceive medicines to have a benefit for the patient (C) health-care professionals may be reluctant to consider deprescribing (O) because they feel pressured not to do so (M) | Several GPs talked about the challenge of keeping patients on potentially unnecessary medication at the urging of family membersJansen et al.152Ms L: The family is all guilt-ridden and they tell themselves that they have to keep dear old dad alive . . . and his caregivers face choices about using medications that may increase his longevity but negatively affect his quality of lifeSteinman and Hanlon203GPs discussed exercising caution with initiating medication changes, particularly where they assumed a resident’s family had strong expectations of medicines keeping their relative alive. ‘We really need to be in a situation where we’re educating relatives about what is realistic, it’s very hard to initiate the discussion with relatives’Palagyi et al.179 |
| 19 | When families/carers are involved in a patient’s health-care (C) patients may be more able to engage in decision-making about their medicines (O) because they feel supported by them (M) | Families can facilitate exchange of information and encourage patient engagement in their healthcare. Studies have found that office visits in which the older adult patient was accompanied by a companion who prompted their involvement were 4.5 times more likely to be involved in decision making than their counterpartsHernandez148However, it was also evident that daily routine medication-work can and does go beyond the self, with network members being involved or called upon selectively to provide ad-hoc and/or regular support in the performance of a particular type(s) of workCheraghi-Sohi et al.126This patient also mentioned relatives as an important means of support. As they are more used to the internet, her daughters look up information for her. In addition, she discusses her disease and treatment with her daughters, who motivate her to go to the doctor and address difficult topicsSchöpf et al.194 |
Potential intervention strategies to improve appropriate deprescribing
The results discussed above highlight the complex system in which deprescribing and medication management in general take place. Our review has identified potential intervention strategies and/or contexts that may need to be present to mitigate some of the challenges and complexities posed by operating in a complex system. These intervention strategies comprise shared-decision-making, continuity of care and the development of trust, monitoring and a multidisciplinary approach. These work by modifying some of the contexts laid out in CMOCs 1–19 to trigger different mechanisms that produce desired outcomes.
Shared decision-making
Shared decision-making was widely discussed in the included documents as an important strategy in the management of problematic polypharmacy and deprescribing. Shared decision-making allows health-care professionals and patients to make collaborative decisions about treatment priorities. This model of care recognises the patient experience and embodied learning that equips them with the knowledge to make decisions regarding their treatments.
By drawing on shared expertise, patients and health-care professionals may be able to navigate some of the complexities and uncertainties associated with the deprescribing process by establishing treatment priorities and situating changes within the context of patients’ lives and understanding of their medicines (CMOCs 12–19).
As described in CMOCs 12–18, withdrawal of medications may be perceived by patients as also being a withdrawal of care, which in turn makes them reluctant to consider deprescribing and also makes it difficult for health-care professionals to discuss that option with them. By engaging in shared decision-making, health-care professionals are likely to become more aware of patients’ beliefs about their medicines and their goals of care, making them more likely to achieve patient-centred outcomes (CMOC21). However, it is worth noting that these beliefs may not always be in line with the recommendations made by healthcare professionals. 85
Involving patients in the decision-making process by making them aware of the risks and benefits, as well as drawing on their expertise (CMOC 20), also allows health-care professionals to share the responsibility for deprescribing and can help them make decisions that are defendable (CMOC 22), therefore helping to address some of the issues laid out in CMOCs 1 and 2.
Our realist analysis of shared decision-making factors is summarised in Figure 17, with details in Table 19.
FIGURE 17.
Partial programme theory CMOCs 20–22: shared decision-making.
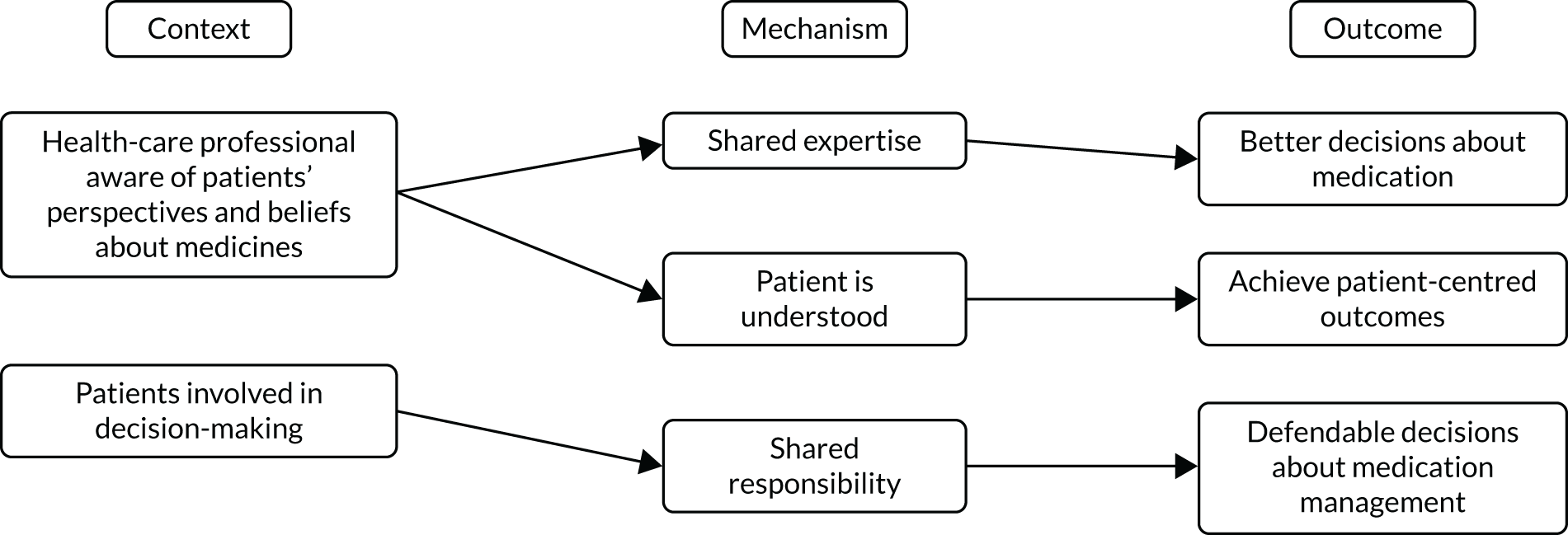
| CMOC | Description | Supporting data |
|---|---|---|
| 20 | When health-care professionals involve patients in the medication management process (C) they are more likely to make better decisions about medication (O) because of their shared expertise (M) | For older adults, decades of observing their physical reactions to various medicines and other dietary behaviours locates them as experts of their own bodies. Health care teams must recognize this knowledge if deprescribing programs are to become standard medical care for older adultsRoss and Gillett189The stories of patients and caregivers, their representations, perceptions, experiences and preferences can reduce the risks of inappropriate exams and treatments . . . Using the patients’ and caregivers’ stories, the interdisciplinary teams can interact better, focussing not on the single pathology, but on the individual as a complex systemCenci123The aim of the medication assessment is to collect information about medication use and to gain an understanding of the patient and their wishes, experiences, and beliefs. This information will enable the doctor to make rational decisions, with the patient, and to determine whether patient needs are being metDrenth-van Maanen et al.133 |
| 21 | When health-care professionals are aware of a patient’s perspectives and beliefs about medicines and their goals of care (C) they are more likely to achieve patient-centred outcomes (O) because the patient is understood (M) | Establishing the importance of symptoms and outcomes with patients and carers will help guide deprescribing decisions. Such discussions may require decisions about relaxing targets for therapy . . . Also important when discussing goals is to anticipate barriers to deprescribing and to discuss any barriers with patients and carersHardy and Hilmer145Perhaps the minimum requirement for shared decision making in this context is establishing awareness of the option to be involved; discussing preferences over time, as they may change; and, if a person is interested in being involved, creating the circumstances for thisWeir et al.218High levels of patient involvement and shared decision-making do not necessarily mean that patients will pursue deprescribing . . . The joint decision between GP and informed patient to continue a medication rather than deprescribing it could be the best decision for the patient if in line with their values and preferencesZechman et al.84 |
| 22 | When health-care professionals involve patients in the decision-making process (C) they are more likely to make defendable decisions about medications (O) because of their shared responsibility (M) | Patients’ attitudes to change could relieve the clinician of any responsibility for deprescribing: ‘If you don’t know what right and wrong is, you don’t necessarily have to provide the answer. The patient will provide the answer as to how willing they are to stop . . .’Anderson et al.117While stopping a medicine could be regarded as dangerous from a medicolegal perspective, particularly when a clinical guideline suggests its use (Barnett and Kelly 2017), the patient has the right to make that decision provided they have the capacity and the information necessary to make an informed choiceKaufman et al.154The GP shared the uncertainty and responsibility for a decision with the patient . . . ‘You have to go “this is your life, your decision” and then give them my advice but they have to make the decision for themselves.’Sinnott et al.199 |
Continuity of care and development of trust
Continuity of care, understood as ‘The extent to which a person experiences an ongoing relationship with a clinical team or member of a clinical team and the co-ordinated clinical care that progresses smoothly as the patient moves between different parts of the health service,’219 featured prominently across the studies included in our review. Three main types of continuity of care were identified in the literature. 220 The first is informational continuity, which refers to the use of information on past events and personal circumstances to make patient-centred decisions about care. The second is management continuity, which involves a consistent and coherent approach to the management of a patient’s changing needs. Finally, relational continuity, which refers to the ongoing relationship between a patient and one or more health providers. Continuity can help contribute to the building of trust by providing the opportunity to amass cumulative experiences of trustworthy behaviour and establish norms of co-operation and reciprocity. 221 This trust may also contribute to the effectiveness of medical care. 222
Within the context of medication management and deprescribing, siloed care and difficulty accessing up-to-date patient information can influence whether or not health-care professionals make changes to patients’ medications (CMOC 4). Lack of continuity, be it informational, management or relational, can damage the trust patients have in health-care professionals (CMOC 23). Relational continuity can help ensure that patients feel like they are being managed by a professional that knows them and their situation personally, and is therefore tailoring recommendations to them and knows what is in their best interest (CMOC 26). In turn, patients may then be more willing to consider recommendations for medication change made by that professional (CMOCs 24 and 25). Furthermore, management continuity can help reassure health-care professionals and patients that any potential harms of medication changes will be managed and may make them more likely to consider making these changes (CMOC 27).
As described in CMOCs 23–27, continuity of care may help build patient trust, which may make deprescribing less emotionally taxing for the health-care professional (CMOCs 4, 12 and 13). If patients trust health-care professionals, they may be more likely to listen when their beliefs about their medications are challenged and be more likely to take recommendations on board (CMOCs 14–18).
By engendering trust, continuity of care (as described in CMOCs 23–27) may also help facilitate shared-decision-making (CMOCs 20–22).
Our realist analysis of continuity of care is summarised in Figure 18, with details in Table 20.
FIGURE 18.
Partial programme theory CMOCs 23–27: continuity of care.
| CMOC | Description | Supporting data |
|---|---|---|
| 23 | When patients are presented with conflicting recommendations about their medication by health-care professionals (C), their trust may decrease (O) because they do not know whom to believe (M) | Further patients distrusted the health-care system after having poor experiences such as no follow-up, not being heard, or conflicting advice given by different doctorsBokhof et al.120Participants emphasised that there is a need for continuity of care to support safe deprescribing. Otherwise, different health-care providers might have different pieces of advice for their clients, then it would be difficult to build a therapeutic relationship between the care providers and care recipientsSun et al.205The second reason was a more general distrust of the complex health-care system and its various stakeholders. It was repeatedly seen as a problem that consulting several doctors (GPs and specialists) frequently led to uncoordinated prescription of multiple different medicinesUhl et al.211 |
| 24 | When patients and their carer/family are asked to change their usual medication by a health-care professional they are unfamiliar with (C), they may be reluctant (O), because they are concerned the person does not know what is best for them personally (M) | Residents commented that unfamiliar nurses were unlikely to be aware of their medical, social and medication history and preferences. This was perceived to potentially result in residents’ voices not being heard, which was a barrier to deprescribingTurner et al.208Pharmacists’ perceived lack of ‘continuity of nursing staff’ limited their ability to determine residents’ goals of careTurner et al.208Unfamiliarity with the medical team during hospitalisation may lead to resistance among patients and family members when deprescribingNadarajan et al.170 |
| 25 | When a health-care professional demonstrates to a patient that they understands their needs and goals (C), the patient is more likely to trust them (O) because they believe that the heath care professional is acting in their best interest (M) | Physicians can counsel patients as to why a medication isn’t indicated. This counseling often requires the development of trust, and the patient having a sense that the physician understands what they feel. Physicians must listen and explain, patients must believe that the physician has their best interest in mind.Chen and Buonanno125Reasons participants gave for this were trust, a long relationship, and a belief that their doctor is aware of their preferences. ‘Well, um, he knows best. He knows my condition. I’ve been with him for 20 odd years. So he knows me inside and out sort of thing’Weir et al.218This study highlights the importance of establishing trust in the physician as a pre-requisite in order to influence change in attitudes and practices of patients . . . Physician trust can be enhanced via continuity of care by the same physician, physician personality and behaviour and patients’ perceived freedom to select choiceNg et al.172 |
| 26 | When a patient trusts their health-care provider (C), they may be more likely to consider changes to their medication (O) because they believe that their health-care professional is acting in their best interest (M) | The results showed that the majority of the participants were willing to stop a regular medication if their physician thought it was no longer required. A high physician trust score and a younger age group were significant factors influencing this attitudeNg et al.172When studied against the backdrop of polypharmacy and deprescribing, trust remains an essential ingredient in the health-care needs of the older adults of this studyWeir et al.218Trust becomes necessary if the patient placed on a deprescribing plan is vulnerable to future consequences. It could be the case that remaining on certain medications may lead to problematic outcomes; or perhaps discontinuing certain medications that the older adult believes are vital may cause significant uncertainty and stressRoss and Gillett189 |
| 27 | When health-care professionals know that they will be able to follow up with a patient (C), they are more likely to try deprescribing (O), because they are reassured they will be able to manage potential harms (M) | A continuous therapeutic relationship with a patient was critical to better assessing harms and benefits and committing to the potentially protracted process of deprescribing . . . ‘Until you know what the relationship is – whether it is an ongoing or an episodic one; that would lead to where you take the consultation’Anderson et al.117 |
Monitoring
Deprescribing and medication management in general are complex interventions comprising a number of interactions between components, different groups, variable outcomes and a number of uncertainties (CMOCs 1–19).
Putting a monitoring process in place following a decision to make a change to a patient’s medication regimen may help to alleviate some of the concerns relating to the fear of negative consequences of withdrawing medications (CMOCs 15–17) and worries around deprescribing symbolising the withdrawal of care. Monitoring does this by reassuring both health-care professionals and patients that potential harms will be managed (CMOCs 28 and 29, and also related to CMOC 27), providing an opportunity for patient perspectives, which may change over time, to be taken into account (CMOC 31) and allowing patient feedback to further inform the deprescribing process (CMOC 30). Monitoring may also help to contribute to the continuity of care by providing opportunities for management and relational continuity (CMOC 27).
Our realist analysis of monitoring is summarised in Figure 19 below with details in Table 21 following.
FIGURE 19.
Partial programme theory CMOCs 28–31: monitoring.
| CMOC | Description | Supporting data |
|---|---|---|
| 28 | When a clinician judges that a patient may benefit from a change in medication (C), they are likely make small, incremental changes (O) because they are concerned about causing harm to the patient (M) | If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a conditionMcGrath et al.165Reluctance to deprescribe due to ‘fear of deterioration’ was highly ranked by GPs, and discussed by the pharmacist, resident and multidisciplinary groups . . . This concern may be mitigated by gradual individual medication withdrawal, allowing restart if the condition/symptoms returnTurner et al.208Once a discontinuation regimen has been decided, selected drugs can then be ceased or weaned, one at a time, while monitoring the patient closely for disease recrudescence or onset of withdrawal or rebound syndromesScott et al.196 |
| 29 | When a harms minimisation process is provided by clinicians during medication changes (C), patients are more willing to make these changes (O) because they feel reassured (M) | Patients may be afraid of the adverse events after stopping a medication and are likely to be more receptive to deprescribing when they are assured that a discontinued medication can be restarted if necessaryNadarajan et al.170 |
| 30 | When a patient provides feedback to a clinician about the effects of a medication change (C), the clinician can make an informed decision about its value (O) because of their new knowledge (M) | At times, discontinued medication had to be restarted. However, in these cases the GPs were glad to have tried the discontinuation because then the decision to prescribe the medication was made more consciously and the necessity of the medication was confirmedRieckert et al.186GPs proposed medication changes that seemed partly aligned with patient’s priorities. In patients with ‘remaining alive’ as the highest prioritised outcome, GPs proposed to stop or decrease symptom-relieving medication. Few of these proposed changes were observed at follow-up, but the proposed dose decreases for macrogol resulted in medication stopsvan Summeren et al.84 |
| 31 | When health-care professionals are aware of a patient’s current perspective and beliefs about their medication (C), patients are more likely to consider medication change (O) because they feel understood (M) | The patient’s problems and goals will change over time and are likely to differ considerably in an acute situation compared with a stable one. Taking the patient’s perspective into account means that the problems and goals they see as most important and needing attention are those dealt with firstKrska157Perhaps the minimum requirement for shared decision making in this context is establishing awareness of the option to be involved; discussing preferences over time, as they may change; and, if a person is interested in being involved, creating the circumstances for thisWeir et al.218Preferences are not stable and can change over time and should therefore never simply be assumed; Type 1 or Type 3 participants, if provided with appropriate support, may come to value information about their medicines and desire a more active role in decision-makingWeir et al.218 |
Multidisciplinary teams
Working in multidisciplinary teams to make treatment decisions for patients was another commonly cited intervention strategy for the management of polypharmacy across the literature. 118,198,201
Health-care professionals may sometimes feel that they do not have the specialist skills or experience necessary to make complex decisions about patients’ medications (CMOCs 5–7), and although additional training may help to increase their knowledge and confidence, working in multidisciplinary teams may allow them to draw on the expertise and experience of colleagues (CMOCs 31 and 32). Being able to discuss complex cases with colleagues may help to reassure health-care professionals that the changes they plan to make to a patient’s medications are safe and may give them the support necessary to help them feel confident about these decisions (CMOCs 31 and 32). It may also help them feel that they are making defendable decisions in the absence of adequate guidelines (see CMOC 1).
Working in multidisciplinary teams may also contribute to continuity of care (CMOC 33) by encouraging informational continuity (countering effects of CMOC 23), thereby helping to increase patient trust (CMOC 25). Working collaboratively also allows health-care professionals to share the responsibility and workload associated with deprescribing (CMOC 34), thereby helping to mediate some of the challenges imposed by limited time (CMOCs 10 and 11).
Our realist analysis of multidisciplinary collaboration is summarised in Figure 20 below with further details in Table 22 following.
FIGURE 20.
Partial programme theory CMOCs 32–34: multidisciplinary collaboration.
| CMOC | Description | Supporting data |
|---|---|---|
| 32 | When health-care professionals can draw on the skills and expertise of colleagues (C) they feel more confident in making prescription changes (O) because they feel reassured that they are making safe and optimal prescribing decisions (M) | ‘It is not such a bad idea to do [talking about complex patients], and to discuss them together, like we are doing right now. When doing so, you come up with new ideas sooner, like, I should pay more attention to those factors.’ (GP 10)Sinnige et al.198A general standpoint was that the GPs, as generalists, did not feel they had the necessary knowledge or backup from hospital-based specialists to conduct critical medication reviews, and this highlighted the need for better cross-sectoral collaboration as well as greater educationLaursen et al.160Articulating and justifying patients’ medications to another GP appeared to be the most important component of the intervention. GPs who experimented with conducting reviews on their own (checklist only) reported that the collaborative approach was better as it revealed their prescribing ‘blind spots’ and quicker than doing it aloneSinnott et al.201 |
| 33 | When health-care professionals can discuss complex cases with colleagues (C) they feel more confident about making medication changes (O) because they feel supported (M) | Peer support appeared to be key in generating recommendations for medication optimisation. While other professional sources were reported to be useful, conducting the review with patients only was not; therefore, professional social support will be a compulsory component of any future iterations of the interventionSinnott et al.201A further issue raised by the interviewees, was the team support within the hospital environment. Particularly, the hospital pharmacist was considered a useful team member and a reliable resourceCullinan et al.130 |
| 34 | When health-care professionals work collaboratively (C) they can improve continuity of care (O) and their understanding of their patients’ needs (O) because they can share workload (M) | Nurses’ prescribing permission differs among countries and in some of them they have no permission at all. Even so, they may influence prescribing because they observe and can communicate to physicians the treatment burden associated with polypharmacy, particularly those with elevated levels of frailtyGonçalves144Results of the medication review were discussed within a multidisciplinary team. Residents were monitored for adverse events by care-home staff and followed up by the pharmacist to ensure suspected negative effects were managed. The process was iterative, with rapid feedback from each clinic used to improve the processBaqir et al.118Medication review and optimization should involve other health professionals. Nurses can assist patients with adherence and in clarifying the accuracy of a medication list. Collaboration with clinical pharmacists has been shown to be an important strategy to reduce inappropriate medications and to help deprescribe as appropriateFrank137 |
Chapter 8 Discussion of realist review findings
Summary
Through our realist review we set out to describe a robust evidence-informed framework outlining the key components of good clinical practice for tailored deprescribing. Our review and analysis of the literature generated 34 CMOC statements that describe and explain the various factors that shape deprescribing at the system, health-care professional and patient levels. The review also identified four key potential strategies that may help produce more desirable outcomes: shared decision-making, continuity of care, monitoring and multidisciplinary collaboration.
Based on these findings, discussion with stakeholders and PPI contributors identified five high-level concepts to help inform policy and practice, namely providing an enabling infrastructure, having consistent access to high-quality, relevant data, creating a shared understanding of the meaning and purpose of medications, trial and learn, and building trust. These concepts may be used to develop interventions to support effective tailored deprescribing.
Enabling infrastructure
Managing patients living with complex multimorbidity, including managing (de)prescribing, is an inherently uncertain process. Our review consistently identified fear of harm as a barrier to change, including deprescribing. For clinicians and patients already juggling the challenges of managing complex (and uncertain) multimorbidity, overcoming the ‘inertia’ associated with fear of change may be one task too many. If we are to ask clinicians to take on the work of managing the uncertainty of tailored (de)prescribing, we need to provide them with supportive guidance to do so. Current guidance describes the principle of person-centred care as ‘good practice’, with policy documents recognising tailored care as good practice. 1,7,8,43 However, our analysis suggests that these documents, to date, do not provide adequate permission to counteract the fear described by clinicians.
Existing guidance describes the steps within the consultation that support good practice around deprescribing. 63 But CMOCs 1–5 highlight that organisational context also affects professionals’ confidence and ability to deprescribe. Supportive guidance, therefore, needs to offer more than a consultation model/set of consultation steps, and describe at an organisational/system level a framework offering ‘safe boundaries’ for uncertain practice. This may include clarity on whose responsibility is tailored (de)prescribing (CMOC 5), recommendations on time and resource allocation to this complex task (supporting the work by prioritising time for it – CMOCs 10 and 11), and guidance on how feedback is offered to support and reinforce best practice.
Supportive guidance may also be important for legitimising the deprescribing role. Health-care professionals taking on the responsibility of deprescribing may be described as having ‘boundary spanning’ roles. 223 Boundary spanners are agents that relate practices in one field to practices in another by negotiating the meaning and terms of the relationship between them. 224 They enable the translation, co-ordination and alignment of different perspectives and practices. 223 Health-care professionals taking on the responsibility of tailoring medications need to negotiate and reconcile different sources of information (see Conservation of resource theory and loss aversion bias) and perspectives (CMOCs 12–17), as well as develop and co-ordinate a plan to manage these changes (CMOCs 28–31).
For boundary spanning roles to be realised and to take hold, they need to be seen as legitimate within the broader system around them. 223 By clarifying whose responsibility medication tailoring and (de)prescribing is and by allocating sufficient time and resource to this complex task, supportive guidance may act to formalise the deprescribing role, making it legitimate and accepted within the wider system, and therefore granting health-care professionals ‘permission’ to undertake deprescribing.
A key source of support for health-care may also come from taking a multidisciplinary approach to managing patients with complex medications. Our analysis shows that being able to work with colleagues to manage deprescribing may allow health-care professionals to draw on the expertise of their colleagues (CMOCs 31 and 32) and help share the workload (CMOC34) and responsibility.
Access to high-quality, relevant data
Our review highlighted that access to data matters (CMOC 4). This was perhaps a surprising finding given that health systems routinely collect large numbers of data. However, our review highlighted that it is contextual data that professionals need – an understanding of history and context (CMOCs 15–17) to support the complex, and sometimes uncertain, tailored decision-making (CMOC 4). In the general practice setting, these data have perhaps traditionally been held in the head of the practitioner (often a GP) who knows the patient. In a world of extended clinical teams, the data highlight the importance of ensuring access for all to these data. These data may also help support shared decision-making (CMOCs 20–2), as well as provide informational continuity (CMOC 25), which may be key in supporting patient trust (CMOCs 23 and 25).
Access to data also applies when dealing with (and potentially reversing) decisions that have been made by other professionals (CMOC 8). Our findings highlight that when communicating between teams/sectors, we need to convey more than what medicines have been started, but also the contextual information on why – what impact is anticipated, how is this to be judged and what conversation has been had with the patient. The TAILOR findings explain why we need to look differently at what and how data are generated for, and used in, practice to support tailored (de)prescribing decisions.
Shared understanding of meaning and purpose of medicines
Prescribing decisions need to stem from tailored understanding and explanation of a patient’s illness in context – through an understanding of the patient’s values and beliefs about the role of their medicines within their wider health care and daily living (CMOCs 12–18). Shared decision-making is a process that can achieve such tailoring (CMOCs 20–2). Tailored explanations convey to the patient that this professional understands them (CMOC 24) and is acting in their best interests (CMOC 26).
Tailored explanations require the professional to have a good understanding of the individual patient context (see Access to high-quality, relevant data), and the value and importance of medicines to the patient and carer (CMOCs 12–16 and 17–19).
Tailored explanations require the elimination of conflicting information/advice from different members of an extended clinical team to avoid the effect of conflict undermining the patient’s trust in their health-care professionals and the advice being offered (CMOC 23). This requires attention to sharing data (see Access to high-quality, relevant data) and to recognising the team-wide nature of care for patients with complex comorbidities (see Trial and learn).
Trial and learn
The data describe the professional inertia associated with the uncertainty and complexity of deprescribing (CMOC 7) and fear of negative consequences (CMOCs 15–17). A trial and learn process incorporating small incremental changes to medicines (CMOC 28) and a harms minimisation process (CMOC 29) with follow-up and continuity (CMOCs 27, 30 and 31) that enables patient perspectives to be heard (CMOCs 30 and 31) may enhance patient trust (CMOC 31) and increase chances of patients considering medication changes and patient-centred outcomes being achieved.
Trust
The need for trust is implicit in all that health services and health professionals do, and particularly so in situations of vulnerability such as managing complexity, uncertainty and new relationships. 225–227 But what that involves, beyond good communication skills and a good relationship between patient and professional, is rarely articulated. Our analysis highlights key elements including:
-
professional trust (confidence) in the professional’s own decisions, for example stemming from guidance supportive of this form of practice, and shared responsibility for decisions (with patients and other members of the team) (CMOCs 1–5, 7 and 8)
-
patient trust in the professional through a sense that this decision is made in their best interests, generated through production of tailored explanations (CMOCs 12–18 and 23–7)
-
the importance of building consistency of care across a team, for example to minimise conflicting advice and also because team decision-making supports professionals in taking complex decisions in situations of uncertainty (CMOCs 32–4)
-
the importance of planned follow-up to review (CMOC 27), and if necessary amend, decisions made based on feedback (CMOC 30) – an approach that supports harms minimisation (CMOC 29) as well as trust.
In summary, we can thus describe a more finalised and revised programme theory describing the contextual and consultation factors needed to support tailored (de)prescribing. Our final analysis recognises that tackling problematic polypharmacy needs a tailored approach to (de)prescribing. Tailored prescribing commonly involves beyond-protocol decision-making, a complex process that involves emotional and cognitive (knowledge) work for clinician and patient alike. The impact of this cognitive and emotional load can contribute to inertia: a failure to implement tailored decisions even if recognised. Our final programme theory therefore describes and explains the components needed to enable tailored prescribing through addressing this cognitive and emotional load (Figures 21 and 22).
FIGURE 21.
TAILOR programme theory: core elements needed to support effective tailored prescribing.
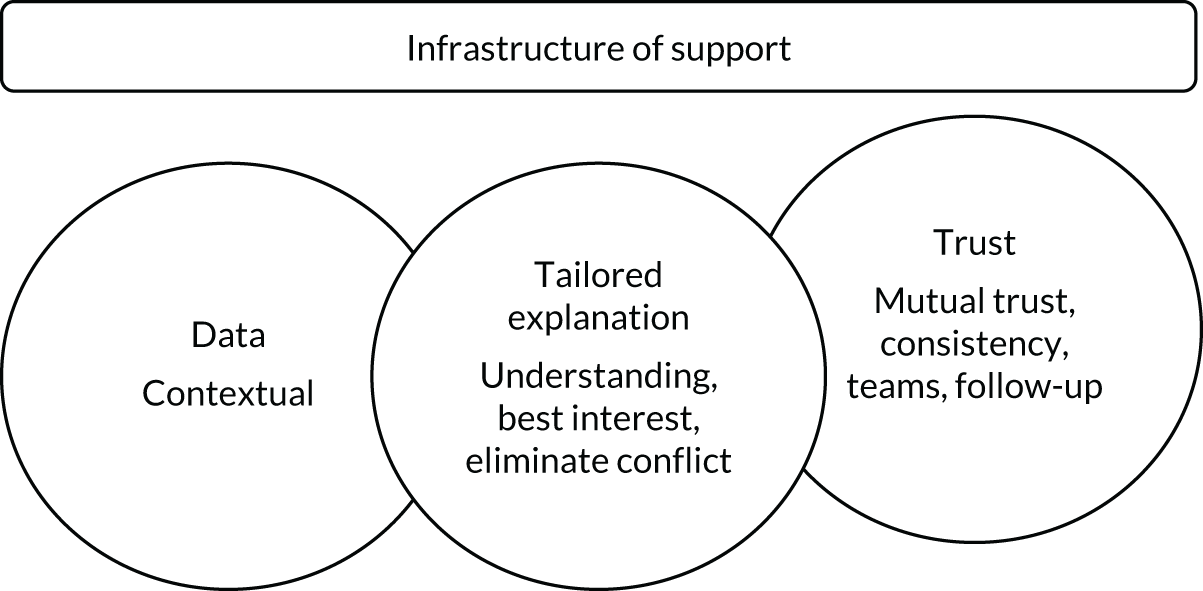
FIGURE 22.
Detailed final programme theory: delivering TAILORED deprescribing. Numbers in brackets refer to CMOCs.
Alignment with substantive theory and other literature
As discussed in Chapter 6, we drew on substantive theories to help us understand how our findings are analogous to (or ‘fit in with’) substantive theory. We did this to help provide additional support to any inferences we have made.
Conservation of resource theory and loss aversion bias
Conservation of resource theory is a theory of motivation that posits that individuals are motivated to protect their current resources and acquire new ones. 228 A resource can be loosely defined as anything that an individual believes can help them attain their goals and can include objects, states, conditions, relationships, social support and other things that people value. 229 The value of resources varies between individuals and is tied to the experiences and the realities in which they live. 229 This basic principle of conservation and acquisition lead to other principles within the theory, such as the primacy of resource loss and the idea that it is more psychologically damaging for individuals to lose resources than it is beneficial for them to gain the resources that they have lost. 229
Deprescribing involves a change from the status quo and the removal or loss of medications (resources) that patients see as having value in their lives (CMOCs 12–18). Conservation of resource theory, and especially the primacy of resource loss, may help to explain the prominent phenomena reported in the literature of inertia experienced by health-care professionals and patients when needing to consider deprescribing.
Discussions with our PPI contributors (see Chapter 9) led to the suggestion that it may be more helpful to change the framing of deprescribing (stopping something) to ‘re-prescribing’ (reviewing medication that may lead to multiple outcomes, including starting, stopping or maintaining the status quo). Given the primacy of resource loss highlighted above, reframing deprescribing, which by definition entails the removal/loss of something, to something more neutral, ‘re-prescribing’, which does not necessarily entail a loss to resources, may help overcome this inertia. This may be a potentially fruitful area for future research.
Social support theory
Social support refers to the broad process through which social relationships promote health and well-being. 230 Information that leads individuals to believe that they are cared for, held in esteem and belong to a network of communication is a form of social support that can help individuals cope and adapt in times of stress. 231
Deprescribing often involves dealing with high levels of complexity and uncertainty, which can be psychologically and emotionally taxing for health-care professionals (CMOCs 12 and 13) as well as patients (CMOCs 16 and 17). Across our review, a number of mechanisms identified relate to health-care professionals and patients needing to feel supported and understood for desired outcomes to be achieved (CMOCs 1, 2, 19, 21, 31, 33 and 34).
Creating a supportive infrastructure as described in Enabling infrastructure may provide health-care professionals with the support and resources they need to be able to cope and manage when dealing with the uncertainties and complexities of deprescribing.
Providing data (see Access to high-quality, relevant data), tailored explanations (see Shared understanding of meaning and purpose of medicines) and building trust (see Trust) may enable patients to feel supported by reassuring them that health-care professionals understand and value their goals and needs.
Therapeutic relationships
Positive relationships between health-care professionals and patients have been shown to improve patient satisfaction and professional fulfilment, increase compliance with prescribed medication, help save time and reduce the number of complaints from patients. 232
Our analysis has shown that relationships between patients and health-care professionals may also be central to supporting tailored deprescribing (CMOCs 25 and 26). The social support (see Social support theory) provided by these relationships may be key to working through the uncertainties deprescribing entails,117 perhaps mediated through a process of generating and maintaining trust. The importance of this relationship to both patients and health-care professionals may, however, sometimes also act as a barrier to deprescribing. When considering making changes to prescriptions, health-care professionals may be hesitant to make these changes because of the fear of damaging the therapeutic relationship (and trust) between the patient and the original prescriber (CMOC 9). Furthermore, deprescribing a medication that a health-care professional knows a patient values, even though it may be inappropriate, may be especially difficult in the light of the possibility of damaging their relationship with their patient. 160
Given the centrality of therapeutic relationships and the potential dual role they play as both facilitators of and barriers to deprescribing, shared decision-making (in which both health-care professionals and patients actively participate, share information and reach consensus) may be key to negotiating some of these possible tensions to reach desired outcomes (CMOCs 20–2). Shared decision-making has long been recognised as a principle of best practice in the NHS. 233 Our findings explain why it matters (how it contributes to meaningful outcomes), and also highlights the need for supportive infrastructure (the potential need for organisational change) for this to be effective.
Trust has been described as one of the most important components of well-functioning relationships,234 and may also contribute to the effectiveness of medical care. 60 Continuity of care has been shown to be associated with higher levels of trust between patients and healthcare professionals. Our analysis has emphasised the importance of all forms of continuity (relational, informational and management) for the building of trust necessary for successful engagement in deprescribing (CMOCs 23–6).
Strengths and limitations
Our realist review provides a synthesis of data from a variety of documents and study designs. It moves beyond describing the barriers to and facilitators of tailored (de)prescribing to provide a framework that describes and explains the key components of good clinical practice for tailored medication management.
Our realist synthesis followed a systematic and transparent process for the screening, analysis and synthesis of the data. The CMOCs and programme theory were developed in iterative stages through in-depth, reflective discussions within the project team as well as with PPI partners and stakeholder groups. Our wider project team and stakeholder group included individuals with varied academic and clinical backgrounds, and conversations with them played an important role in confirming and refining aspects of the CMOCs and programme theory.
Evidence syntheses rely on the evidence that is available. The evidence included in our review discussed the many barriers to and facilitators of, and attitudes towards, medication management and provided a good overview of the factors influencing the engagement with deprescribing in a broad sense. We found, however, that individual deprescribing interventions were often not described in enough detail to be able to draw conclusions on how their different components resulted in desired outcomes. This therefore limits the scope for analysing the role of specific intervention components in producing desired outcomes. However, by including data from across 119 documents, we have been able to explain what factors shape the engagement with deprescribing and have highlighted the contexts that may need to be changed for appropriate deprescribing to be more likely to be considered by both healthcare professionals and patients.
Implications
In generating and describing our TAILOR programme theory, we have demonstrated how and why deprescribing is a systems-wide issue.
As was also highlighted by our scoping review, we need to recognise deprescribing as a component in the complex intervention that is tailored prescribing and use of medicines.
Solutions to improving deprescribing practice, and so addressing problematic polypharmacy, will not be found in technical solutions alone, for example tools and algorithms (see Chapter 5).
We will discuss this further in Chapter 10.
Chapter 9 Working with patient and public involvement partners
Overview
The research topic for this work (deprescribing) was set by the funders. Our PPI co-applicant (ER) helped shape our response to the call in setting the focus for our work as a ‘patient-centred understanding of tackling deprescribing’.
This focus shaped the research questions we asked, the methods we used (a double review to capture the breadth needed) and our interpretation of the data.
We have found it difficult to engage new patient and public partners with the work. Our research findings offer a possible an explanation for this.
In 2015, we published a paper235 with the two PPI partners who have helped us with TAILOR which argued that the patient’s voice needs to be heard in the evidence used to shape prescribing practice and policy. Our patient partners have helped to ensure that the completed TAILOR work offers an important contribution to meeting that goal.
Patient and public involvement aims
Our goal was to ensure that a patient-centred perspective was maintained at all stages when seeking to answer our two research questions:
-
What quantitative and qualitative evidence exists to support the safe, effective and acceptable stopping of medication in older people with multimorbidity and polypharmacy?
-
How, for whom and in what contexts can safe and effective individual tailoring of clinical decisions related to medication use work to produce desired outcomes?
For each question, our PPI partners ensured that the team considered whether or not, and how, a patient view of the data and analysis offered additional insights.
Methods used
A patient-centred focus was built in to the theoretical framework that informed all stages of this work. We also used a number of specific steps to actively engage the patient voice in our work.
Inviting a patient co-applicant on the study team
Ed Ranson was a participant in a previous study47 we ran to understand personal experiences of living with long-term conditions and the implication for health care. Ed has personal experience of long-term health issues and polypharmacy, and of seeking to improve care through research as a lay member of a team.
Ed Ranson played three key roles:
-
supporting the core team in project planning, including PPI engagement activities – Ed has commented on and amended project plans at all stages, from the original application through to reporting and dissemination plans
-
as a member of the extended project team, taking part in 6-monthly review meetings with the full team to consider data collection and analysis
-
as lead for PPI activities, working in partnership with Joanne Reeve in leading the design of planned PPI meetings and the review and revision of PPI activities and engagement with comments from our second PPI support (Michelle Dickenson; see Acknowledgements).
Planned patient and public involvement stakeholder events
Ed Ranson and Joanne Reeve planned for, and invited lay members to, four attempted PPI meetings in March and August 2019 and February and October 2020. Flyers were prepared and distributed through stakeholder networks (including practice patient participation groups, local GP networks, ‘generic’ PPI networks within host universities and personal contacts) as well as via social media. For example, Joanna Reeve attended a lay health-care network meeting at Castle Hill Hospital in Hull in August 2019 to introduce the study and invite people to join the next planned PPI meeting.
Ed Ranson supported the writing, and revision, of all invitations. For example, he advised that including the word ‘deprescribing’ in the event title was not likely to encourage engagement and that we needed to focus on patient experience of using medicines. Later versions instead described the project as being about ‘making medicines work better for you’. Ed was directly engaged in contacting his own patient participation group network, and provided advice to Joanne Reeve when invitations were unsuccessful.
In the light of the difficulties we experienced, we got in touch with PPI contacts from previous studies. Michelle Dickenson offered to support the project through reviewing and providing feedback on written documents.
Presentation of collated findings and draft report to patient and public involvement members
We were unsuccessful in recruiting members of the public to join our final (virtual) PPI meeting to discuss draft findings (October 2020). We therefore modified our plans to two actions. We held a virtual meeting with Ed Ranson, Joanne Reeve and Amadea Turk to present the realist review findings to Ed Ranson. A copy of the presentation and notes from the meeting were subsequently sent to Michelle Dickenson for further review and comment. Ed Ranson and Joanne Reeve met to discuss Michelle Dickenson’s reflections.
Co-authorship of this section of the report
Reflections on the opportunities and challenges of PPI engagement within TAILOR have been discussed at core team and extended team meetings throughout the project. These were collated in the preparation of this report. Joanne Reeve drafted this section, with Ed Ranson editing and offering comment.
Outcomes of patient and public involvement
Our PPI engagement with our work flagged two key findings that have shaped our final report: the importance of meaning, and of trust.
Challenges of recruiting to face-to-face patient and public involvement meetings – recognising the importance of meaning
The challenge in recruiting new PPI members to the project has led us to reflect on the importance of meaning – reflections that have also informed the realist review work.
We arranged four PPI meetings over the duration of the project, refining the invitation and design of the meeting each time to seek to improve engagement. In August 2019, Joanne Reeve met with a Hull-based PPI group to discuss the work. People at that meeting told us that tailoring of medicines/care was an area of interest. We were approached by one potential participant after that meeting, but he indicated that his particular interest was in the use of genomics to tailor care. When advised that this was not the focus of our meeting, he withdrew.
Ed Ranson supported us to reflect on our recruitment challenges as viewed from the patient-centred focus of our study. For example, he proposed that we change the wording of our invitation flyers. In 2019, we invited people to come to talk with us about potential ‘burden’ of using medicines, to help us understand how to ‘safely’ tailor medicines. Burden and safety were both concepts identified from previous research and conversations. When people did not take up our invitations, Ed suggested that our invitations were not yet tapping in to the issues that were important to potential participants. This led us to change the invitations in 2020 to invite people to discuss ‘making medicines work for you’.
Our realist review identified the need to recognise the meaning and value of medicines to individuals when thinking about deprescribing (see Chapter 7). While reflecting on our recruitment challenges, we have recognised how our invitations to participants may have misrepresented their understanding of the meaning of medicines. Our invitations placed a (evidence-informed) focus on burden. Our PPI partners encouraged us to reflect on ‘a prescription . . . [as] the currency of the doctor–patient transaction’, in which any proposed changes are ‘likely to be seen as being motivated by the desire to save money’ (Michelle Dickenson, 2020, personal communication). With hindsight, we needed a different approach to establish shared meaning in inviting PPI engagement with our research.
In the light of our experiences, Ed Ranson has proposed the need to change from talking about deprescribing (stopping something) to ‘re-prescribing’ (reviewing medication, which may lead to multiple outcomes including starting, stopping or maintaining the status quo) (see details in Conservation of resource theory and loss aversion bias).
Shifting outcomes – the importance of trust
The importance of establishing and maintaining trust when considering changes in prescribing was a key finding from our data analysis (see Figure 22). The issue of trust also underpins our reflections on the importance of establishing shared meaning when engaging PPI partners. Trust was therefore recognised as key to successful engagement in discussions about prescribing, whether in the clinical context or in research conversations about how to improve practice.
Trust is recognised in our analysis as a key element in the successful delivery of tailored prescribing decisions. Reflections from our PPI discussions also considered whether establishing or strengthening trust can be seen as an outcome of good practice around prescribing, as well as research into prescribing practice.
As highlighted in our examination of an extensive literature in our scoping review, previous research on (de)prescribing interventions has focused on the impact on medication and biomedical outcomes (e.g. changes in the number or types of medication used, or effect on measures of illness or risk). Both our analysis and our experience of seeking to engage with PPI partners in the process of doing the research, have highlighted that other factors matter. We need to recognise what medicines mean to individuals in the context of their daily life. A patient must be able to trust that a clinician, or research team, understands that.
Impact of patient and public involvement on TAILOR findings
Having PPI partners working with us throughout all stages of this research has kept a number of key points in the foreground of our work. First, that the use of medicines, including deprescribing, is not simply a technical process but also a deeply personal one grounded in individual meaning. Both clinical management and research activities must recognise that prescribing cannot be reduced to a mechanical process. Second, that establishing and maintaining trust is a key finding from the TAILOR project. A patient must trust the doctor who is discussing/considering deprescribing with them, and patient/public partners must trust the research team who are inviting them to discuss the topic.
These observations are reflected in our analysis, and are also shaping our dissemination plans.
For our dissemination work, we propose to use Ed Ranson’s suggestion to talk about ‘represcribing’ rather than ‘deprescribing’ to help shape perceptions of an interest in personalising the use of medicines over stopping them (e.g. for cost-saving purposes).
During our dissemination activities, we will seek to actively engage PPI stakeholders in the next stages of our research. We will use sharing these findings as the mechanism to recruit PPI partners to the next stages of our research, with the goal being to develop and establish the necessary trust between all partners at the earliest stages.
Ed Ranson also led our reflections on the implications of our work for future research, and specifically for engaging PPI partners in future research. He led our discussions on the challenges both of recruiting any public partners to stakeholder events and of engaging those with a general interest in medicines to consider the specific issues that we were being funded to address. He highlighted the importance of PPI partners having contextual understanding of the research in order that they are able to make meaningful contributions.
These conversations recognised a body of research literature on how patients engage with medicines in the context of their clinical care. For example, Pound et al. ’s review48 described four groups of medicine users – noting examples of both active and passive behaviours among people who use (take) or resist their prescribed medicines. We considered whether or not related behaviours may influence people’s decision-making and mode of engagement when considering getting involved in research.
There are implications for thinking about future research. Ed Ranson has been working with our team for a number of years (including supporting previous unsuccessful research bids) before joining us for this work. His prior contributions were often over and above the limited funding we could offer for ‘scoping and set up’ activities. TAILOR has been extremely fortunate in having this ongoing support. Ed brought experience of using data to inform project development (albeit not health-care related) to his working with the group: a skill that was beneficial to him and the team.
We had planned (and budgeted) for PPI training in this work but were unable to recruit new PPI partners to train up. Ed Ranson and Michelle Dickenson ensured that we still added the patient voice at the heart of our team’s work and discussions. We deliberately chose to keep our PPI recruitment approaches as ‘open’ as possible, inviting anyone with interest (with a plan to define the ‘job role’ ‘once they were engaged with the team’). In the light of Ed’s reflections on the importance of contextual understanding and active or passive engagement in supporting PPI work, for future projects, we will consider seeking to actively recruit to a specified job role with personal characteristics described. We will budget for, and timetable in, the additional work involved.
Reflections
Patient and public involvement has been a critical part of the TAILOR work from the outset.
The findings from TAILOR offer distinct new insights into managing the challenge of problematic polypharmacy. The need to recognise the human and personal aspects of the use of medicines, and so attend to tailored explanation and trust as key outcomes of good practice, is not fully recognised in descriptions of good practice on deprescribing. Maintaining a strong patient voice in our research team has offered a key source of curiosity, creativity and inspiration, encouraging us to move beyond a focus on describing outcomes of deprescribing interventions to understanding the meaning and impact of tailored prescribing activities in context.
The work involved in achieving this, and continuing through into our dissemination activities, has been, and will be, considerable, predominantly in the time and headspace required from all involved. We continue to reflect as a team on how to make this sustainable beyond and between individual funded projects.
For now, recognising, celebrating and championing the importance and value of PPI at the heart of good research continue to be at the core of what we do.
Chapter 10 Integrated discussion: supporting the knowledge work of tailored prescribing
Recapping what we set out to do
The TAILOR project was designed to tackle the challenges of problematic polypharmacy. Our work is grounded in The King’s Fund’s call1 to better support practice in achieving the ‘compromise’ needed to effectively and safely achieve what Denford et al. 3 described as the mutually agreed tailoring of medicines. Our goal was therefore to provide an evidence base to support the complex clinical decision-making (knowledge work) needed. Our study was designed to address two identified gaps in our knowledge base: providing clinicians with data on the safety and acceptability of stopping medicines, and a framework by which they could judge ‘best’ practice when making decisions that are inevitably ‘beyond guideline care’. 6,26 Recognising the importance of organisational context in shaping the decision-making process,6,14,17 we also sought to offer guidance to policy-makers on the practice-level changes that may be needed to support clinicians and patients in their daily work.
Clinicians make tailored decisions about the use of medicines every day, especially in the context of patients living with and managing polypharmacy and multimorbidity. To interpret individual need and potential benefit from medicines, a clinician needs access to biomedical data on potential benefit. They integrate this with data ‘foraged’ from the consultation and clinical record describing individual circumstances, goals and values to generate management plans. 30 Existing polypharmacy guidelines (e.g. the Scottish guidelines4,5) provide clinicians with accessible data on the absolute benefit of some medicines for some conditions in given populations. 4,5 These data can be used to inform discussions with patients on the potential benefit of starting medication, and also considerations on what would be ‘lost’ if the medicine were to be discontinued. However, to our knowledge, there is no equivalent database describing the absolute benefits and risks of stopping particular medicines. Our scoping review sought to map the available evidence base to describe what we know about deprescribing and to determine whether or not a similar dataset to the Scottish guidelines4,5 could be generated for deprescribing, and so describe what gaps exist that may require further research.
Tailored prescribing is inherently variable because individuals and their contexts are different and changing. The clinical decision-making (knowledge work) of tailored prescribing practice is, therefore, characterised by managing uncertainty. It is still possible to differentiate ‘good’ practice – albeit recognised as ‘better or worse’ rather than ‘right or wrong’. 41,236 Yet clinicians report lacking a clear framework to support them in judging the appropriateness of their ‘beyond guideline’ practice. 6 Scientific practice can be used to generate evidence-informed models that describe/define the parameters of practice that, if employed, predict a likelihood of better outcomes. Our realist review was designed to generate just such a framework.
Reviewing how we went about the work
In Chapter 2, Detailing the research team, we outlined the workplan for the TAILOR project: how we proposed to deliver the work. The Project Working Group were to be supported by a PPI group, an Academic Advisory Group (AAG) and a Stakeholder Group. PPI has been discussed extensively in Chapter 9. Here, we describe the working of the additional groups and consider their role/impact on our reported findings.
Academic Advisory Group
The realities of pressures on, and availability of, all parties led to some modifications of these groups. Competing priorities for members of the Project Working Group not involved in detailed day-to-day generation and analysis of data meant that their input to the project was refocused as academic advisory roles. Some original members of our AAG changed roles (T Fahey), one relocated to another country (T Walley) and time pressures limited engagement of others. Our academic teams therefore consisted of the core team (JR, MM, RH, AT, GW and KM) that was responsible for day-to-day delivery of the work, and a revised AAG (DL, DM, JK and RB – all listed as co-authors – along with Dr Nia Roberts, an information specialist providing input into the search strategies). The core team met monthly to ensure cross review between the two reviews. The AAG originally planned to meet quarterly. As work progressed, we recognised a need for flexibility to optimise the use of AAG members’ time, as well as to ensure timely input into the emerging work. The AAG therefore met on five occasions at key stages in the project:
-
set-up – reviewing the draft programme theory and focus for the scoping review (May and September 2018)
-
data collection – reviewing and revising the data collection process (June 2019)
-
interpretation – reviewing and revising the data analysis (January 2020)
-
integration – reviewing the integration of findings (March 2020).
The AAG were also actively involved in the preparation of the initial draft final report (submitted December 2020) as co-authors of the work presented. This work was undertaken remotely because of the COVID-19 pandemic.
Stakeholder Group
Competing pressures and changes in roles also led to changes in the makeup and working of our Stakeholder Group. We originally intended that this group would meet twice per year. The focus for discussion would be on the relevance of the research for end users, and on supporting dissemination activities. Mirroring our experiences with PPI (see Chapter 9), we experienced some difficulties in recruiting people to attend stakeholder meetings. Feedback from our first event clarified that busy professionals needed to prioritise time for work with more ‘tangible outputs’ than research at the set-up stages.
However, we were able to hold two stakeholder meetings during the project. A mixed audience of clinicians, NHS managers and clinical academics joined us to review and feed back on our progress and emerging observations. Nine external partners, along with co-applicants and researchers from the TAILOR team, met face to face in Birmingham in March 2019. Stakeholders reviewed and commented on the proposed direction of the research, and guided our development of search strategies. In September 2020, eight external partners (including four new members) met with the team using a virtual platform. At this meeting we reviewed the draft results of both analyses and invited stakeholder discussion on the interpretation (i.e. meaning and value) of the findings. Our stakeholder meetings contributed to ensuring that our work remained grounded in the needs of the end-users of our research, namely clinicians and managers. We also held a third stakeholder meeting in December 2021, following the submission of our draft final report. We shared the key findings from TAILOR, discussing their contribution to the challenges posed in the National Overprescribing Review. 237 Stakeholders fed into our described dissemination work, as discussed further in Dissemination activities: continuing our work to optimise the impact of TAILOR.
Reviewing what we found and what it means
Scoping review
Our scoping review identified a broad and complicated field of research. We therefore opted to conduct an in-depth look at a clearly delineated part of that picture, focusing on research that explicitly examined deprescribing effects in populations living with defined multimorbidity. Our focused inclusion/exclusion criteria led us to identify just 20 papers from a large and diverse field. However, even within this dataset, there was considerable variation in what was done, to whom and with what effect.
Our analysis revealed that deprescribing under ‘research conditions’ mapped well to expert guidance on the steps needed for good clinical practice (see Table 7). 63 When reported, interventions were generally safe (see Table 11) with an observed ‘pocket’ of negative outcomes on safety in four studies of deprescribing conducted in secondary care without the use of a defined tool or framework. Interventions were commonly reported as acceptable to clinicians, although with fewer data available on acceptability to patients. Effect outcomes were variable across our data set, a perhaps unsurprising finding given the variability in interventions used and context of practice, along with methodological issues of studies with mainly small to moderate sample sizes and short follow-up periods. However, there was evidence of a positive impact on prescribing behaviour, although there was less clear evidence of clinical effect. Similar observations of hard-to-interpret variability in the outcomes dataset was described for a systematic (Cochrane) review of the range of interventions being used to enhance appropriate polypharmacy. 105 However in our review, although a wide range of indicators was used, of those reported in Table 11, positive impacts were seen for 444 reported outcomes (upward arrows), 328 reported negative impact (downward arrows), 84 reported no change (side-to-side arrow), and for 208 the effect could not be interpreted (dots).
We can therefore conclude that our map of the evidence offers clinicians evidence-informed support for the safety, clinician acceptability and potential effectiveness of deprescribing approaches that demonstrate structured approaches to deprescribing decisions (conclusion 1).
Our scoping review was not designed to offer clinicians specific details on the absolute benefits/risks associated with specific deprescribing decisions (e.g. stopping drug X in condition Z produces outcomes A, B or C) but rather to consider whether such work might be possible or desirable to do. In conducting this scoping review, we have taken a detailed look at an extensive body of literature. Our observations lead us to believe that attempts to undertake the detailed subanalyses needed to generate a deprescribing dataset would experience similar challenges of hard-to-interpret variability recognised in the Cochrane review discussed above. 105 We are not confident that the data available for such a piece of work (namely the conducted/published research studies to date) would support generation of meaningful synthesis or meta-analysis because of the considerable heterogeneity of clinical and research methods used.
It is also unclear whether or not clinicians would find such a resource useful in their daily practice. Datasets describing absolute benefits associated with the use of named medicines in specified patient groups already exist [e.g. the Scottish polypharmacy guidelines previously discussed4,5 and the Database of Treatment Effects linked to the NICE guideline on multimorbidity assessment and treatment (NG56)]. 238 However, we have been unable to identify any evaluation studies describing if and how such datasets are being used by clinicians and patients in practice. Our realist review offers insight into why this might be the case. Our realist review findings describe the complexity of the tailored decision-making process, with outcome data playing a limited role in the broader knowledge work of practice.
The findings of our scoping review, therefore, resonate with our realist review in highlighting the need to recognise deprescribing as a complex intervention (see Figure 10). Our analysis leads us to conclude that the currently available data (published studies) do not readily support the production of a ‘deprescribing outcomes data set’ owing to significant heterogeneity in both the conduct and reporting of the studies to date. We suggest that academics and research funders would need to consider the development and use of both a core outcome set and clearly defined reporting guidelines to achieve this outcome. Our critical observations in undertaking both reviews lead us to question the utility to clinicians, and, therefore, patients, of such work. Discussions at our final stakeholder meeting in December 2021 supported this stance.
In the final dissemination stage of TAILOR, we will therefore use the presentation of our findings to ask clinicians and patients whether or not a dataset on the absolute benefit and harm associated with specific deprescribing decisions would change their decision-making practice (conclusion 2).
Our analysis highlighted two key challenges for the research community to consider in generating evidence to support patient-centred clinical practice. First, we recognised the need for research that recognises, and examines, deprescribing in context (see Chapter 5, Implications for future work). Our review highlights why deprescribing cannot be researched as a linear, single intervention but requires the use of methodologies to evaluate complex interventions. Second, our review highlighted the challenges in synthesising data (whether as a clinician or researcher) from such a fragmented research base. In the absence of a coherent and co-ordinated map describing what research questions are needed in the context of current practice, using clear agreed definitions and measures, we will continue to generate a dataset that is hard to interpret meaningfully. Again, the Cochrane review of measures to support appropriate polypharmacy highlights this point. 105 We propose the need to consider if and how we might address this issue through new (potentially international) thinking on decision-making on how research is generated and prioritised. Our PPI partners also recognised the importance of this issue in highlighting the need for patient partners involved in research to also have the ‘contextual understanding’ of the research necessary to support development of a coherent body of understanding (see Chapter 9, Challenges of recruiting to face-to-face patient and public involvement meetings – recognising the importance of meaning).
A common theme across this discussion is the importance of recognising the need for research in context. To optimise the impact of research on complex health care, knowledge generation cannot be understood solely as a ring-fenced task to be done and then translated into practice. Evans and Scarbrough239 challenged the research community to consider the benefits as well as risks of generating knowledge in context, blurring the roles between clinicians and researchers. As Green240 described, if we want evidence-based practice, we need to generate practice-based evidence.
Our review recognises the importance of generating practice-based evidence for complex health care, and raises questions for the research community about how we best achieve that (conclusion 3).
Realist review
Our realist review critically examined a complex body of research to understand the mechanisms behind the outcomes reported in the scoping review, and so generate a new theory describing tailored deprescribing practice. We sought to describe how tailored prescribing happens, and to explain the variability in practice by understanding for whom and in what context practice occurs.
Our realist review recognised the significant cognitive (intellectual) and emotional load involved in the knowledge work of producing tailored explanations and decisions about medication use, working ‘beyond guidelines’, managing uncertainty, and maintaining continuity of approach and trust across a team and across an extended timeline. Our analysis identified that the complexity of this work can contribute to inertia, with both patient and practitioner maintaining a prescribing status quo even when it was recognised as not ideal. Our programme theory described a number of components needed to manage/reduce this load and so support tailored deprescribing.
Specifically, our analysis described four key concepts necessary to support strong tailored deprescribing practice:
-
an enabling infrastructure that provides clear guidance on, and support for, professional responsibilities; enables multidisciplinary working; and supports continuity of consistent care
-
consistent access to high-quality, relevant (notably contextual) data
-
support for development and maintenance of tailored explanations – a shared understanding of the meaning, purpose and impact of medicine
-
attention to generating and maintaining trust through monitoring and continuity that supports a mutual ‘trial and learn’ approach.
Our concepts recognise that deprescribing is a complex intervention: an interpretive practice28 that occurs in the interaction between patient and practitioner to generate a tailored understanding of priorities (including the meaning and value of medicines) and possibilities (exploring what is known – data – on the potential impact of use or discontinuation of medicines in the context of an individual’s conditions and circumstances – contextual data). It is the generation of a tailored explanation of medicines use in context that is necessary for effective care, and needed also to support and maintain the trust that is necessary to sustain management of complex health-care needs and so optimise outcomes. Trust is also enhanced and maintained by planned follow-up to review, evaluate and, if necessary, amend decisions.
Tailored deprescribing therefore relies on both the interaction between clinician and patient and the context in which the interaction occurs. Context includes the recognition that health care is delivered by multiple professionals in multiple settings. Continuity of approach across teams is vital to avoid conflicting explanations, and so the undermining of trust. In addition, we recognise that both clinician and patient need external resources that offer support for the complex task they undertake, including how to recognise if and when things are going well, or could be ‘better or worse’ (an infrastructure of support).
The TAILOR realist synthesis therefore provides a framework for ‘better’ tailored deprescribing as an interpretive practice based on the four key elements of the need for an enabling infrastructure for person-centred health care; consistent access to high-quality, person-centred (including contextual) data; supporting the generation of tailored explanation and so shared understanding of medicines use; and continuing review to enable mutual learning and so the development and maintenance of trust (conclusion 4).
Our framework resonates with existing descriptions of best practice (e.g. Reeve et al. 63) in recognising the need to explicitly consider patient priorities for care, and interpret potential harm and benefit of decisions in individual contexts. However, our work extends these existing frameworks in two ways, first, by highlighting the need to also recognise the importance of the perceived value and meaning of medicines to patients (see Chapter 7, Perceived value of medicines) and explicitly include these in decisions about their health-care needs and medication use. The impact of stopping medicines, from a patient perspective, may not relate to the effect on a given clinical condition.
Second, we recognise development and maintenance of trust as key and necessary components of ‘better’ practice. Recognising the importance of trust as both an element and outcome in prescribing practice potentially requires a redesign of health systems and health-care practices in areas such as data management and workflow planning. Data systems need to ensure consistent access to the contextual data (both biomedical and biographical) needed to support understanding, and so trust, between clinician and patient. Care models need to be designed to provide adequate time for discussions and robust follow-up arrangements.
Our analysis therefore highlights the complexity of the knowledge work involved in tailored prescribing, and suggests that tailored deprescribing requires models of health care in which all parties have consistent access to the resources needed to generate, implement and review tailored, shared decisions about medication use. Our work provides data highlighting the importance of professional roles (including permission to deliver tailored, ‘beyond guideline’ care, and clarity of roles across diverse and changing teams/communities of practice); enhanced resources for practice (including extended professional skills, consistent access to high-quality data, and prioritisation of protected time for review and shared learning); and the review of expected outcomes for the delivery of quality person-centred care.
Our TAILOR deprescribing framework extends existing models of good practice by recognising the need to consider the potential impact of prescribing decisions beyond biomedical or pharmacological effects by demonstrating the need to include organisational/contextual factors in models of better practice (conclusion 5).
Our findings have implications for the development of clinical guidance and research activities.
Our findings describe why an infrastructure of support is necessary for person-centred prescribing that offers ‘permission’ as well as the resources necessary for practice. We now seek to work with clinicians and patients to translate the principle described in our analysis into a clear description of what is needed on the ground. We have incorporated the principles outlined in Figure 22 into the logic model for a new research Programme Grant looking at deprescribing of sleep medication for people living with dementia. 241 During the dissemination stages of the TAILOR project, we will share our robustly generated, evidence-informed framework (guidance) on better practice with stakeholders and ask (how) could this model support the changes in practice needed if we are to consistently deliver person-centred prescribing practice? We will invite stakeholders, and especially patients (see Chapter 9), to help us to consider how best to share our findings to stimulate recognition of, and action on, the changes needed.
In the dissemination stages of TAILOR, we will continue our work with stakeholders to help us optimise the impact of our work through translating our findings into resources for front-line practice (conclusion 6).
Current guidelines and guidance largely focus on the management of specific (single) conditions (e.g. epilepsy or hypertension) or interventions (e.g. interventional radiology), and less commonly on complex conditions or interventions (e.g. multimorbidity or medicines optimisation). TAILOR has focused instead on an outcome of care (tailored, person-centred outcomes) albeit in the context of a given intervention (deprescribing). Although delivering person-centred care is an NHS England policy priority,242 survey data highlight that success in delivery of person-centred care has been declining in some areas of care. 243 Our findings raise a question about an opportunity for future outcomes-focused guidance on ‘better’ delivery of person-centred prescribing as part of developing an ‘infrastructure of support’.
Our findings therefore raise the question as to whether or not there is an opportunity for new NICE guidance on person-centred care using tailored prescribing as one example (conclusion 7).
Our scoping review described a diverse set of outcomes being used to assess the impact of deprescribing initiatives. Our realist review findings help to explain the variability in practice in recognising the key importance of offering tailored explanations. ‘Better’ outcomes of practice may, therefore, vary considerably between different individuals when priorities, meaning and context are actively included in considerations on what care is needed and why. We discussed the potential value of work to generate outcome sets for deprescribing research in Chapter 5, Generating a reference set for clinicians. Findings from our realist review offer additional insights in to what these outcome sets could consider and include. Specifically, we query the need for outcome sets to include measures of delivery of tailored (meaningful) explanation and trust. Current measures for both exist,244,245 although our data highlight that they may need to be adapted for the tailored prescribing context.
Our realist review also adds further insights to our discussion on the need to support the generation of practice-based evidence (see Scoping review). The data/analysis demonstrates that deprescribing is a complex intervention. Research methodologies designed for the study of complex interventions have been published;99 these include the robust development of the theoretical frameworks needed to support complex interventions, as well as the co-delivered implementation methods needed to evaluate impact.
Our findings highlight the importance of recognising person-centred health care, including deprescribing, as a complex intervention needing robust practice-based evidence to support delivery of quality care. The methodological implications of these observations should inform future research funding and prioritisation setting (conclusion 8).
Dissemination activities: continuing our work to optimise the impact of TAILOR
Sharing our work has been an integral part of our research strategy throughout this project.
Our PPI activities are described in Chapter 9. We also held three stakeholder meetings during the project. A mixed audience of clinicians, NHS managers and clinical academics joined us to review and feed back on our progress and emerging observations. The COVID-19 pandemic had a significant impact on our original plans for stakeholder events. However, nine external partners, along with co-applicants and researchers from the TAILOR team, met face to face in Birmingham in March 2019. Stakeholders reviewed and commented on the proposed direction of the research, and guided our development of search strategies. In September 2020, eight external partners (including four new members) met with the team using a virtual platform. At this meeting we reviewed the draft results of both analyses and invited stakeholder discussion on the interpretation (i.e. meaning and value) of the findings.
We held our third and final stakeholder meeting in December 2021, which discussed the key questions identified in our discussion. The stakeholder meeting recognised that a deprescribing data set detailing absolute benefit and harm was not practical to produce and would not address the clinical challenges faced. Participants agreed that our core findings around broadening data and sharing conversations across teams were key to tailored deprescribing. The group recognised the potential value of educational resources to translate our findings into practice, along with the need for ongoing learning and evaluation of practice innovation. Our stakeholder meetings have contributed to ensuring that our work remains grounded in the needs of the end-users of our research: clinicians and managers.
Publication of this report, along with academic papers focused on different elements of our work, will ensure that our work receives a wide peer review and response, and that the data and findings integrate into the collective body of research knowledge in this area. Our dissemination plans extend beyond that with work targeting different audiences.
Working with clinicians and policy-makers
We have been sharing the preliminary findings of our work at clinical educational meetings including the Avoiding Harm event at the Royal College of Physicians (November 2019),21 and within the Humber Coast and Vale CATALYST programme for new-to-practice GPs (URL: www.hyms.ac.uk/catalyst; accessed 11 March 2022).
In our original bid, we described a plan to hold an 1-day professional learning event, in collaboration with the Royal College of General Practitioners. In the light of the changes resulting from the ongoing pandemic, we have negotiated a change in that plan. We are now in the process of developing a massive online open course (MOOC) to make our findings widely accessible as a learning resource to clinicians. We will use the feedback from the MOOC and our other professional development activities to inform ongoing discussions with policy-makers about future practice in this area.
The MOOC will be launched in summer 2022, following discussion with our stakeholder group.
Working with patients
We are producing a short video for patients highlighting the key findings from our work. We had originally planned for this to be shown publicly (e.g. in GP practice waiting rooms) but are revising our ideas in the light of the current pandemic. We now aim to produce a short animation for social media sharing the findings of our work and highlighting the opportunity of engaging with research. Ed Ranson as our PPI lead will continue to offer support for these discussions.
Working with researchers and research funders
The TAILOR protocol describes an anticipated output of a submitted application for funding to support implementation research using the developed TAILOR model. This was discussed at our stakeholder event in December 2021, which highlighted a need and an opportunity to integrate recent policy and practice changes into the emerging plans.
However, the work from this review has already supported an additional successful application for funding. Tailored prescribing is a rapidly developing field of interest for the clinical and academic community. During this project, including through our stakeholder work, we have developed new collaborations with partners focusing on deprescribing in specific contexts. Joanne Reeve and Geoff Wong are co-applicants on a successful NIHR Programme Grants for Applied Research application looking at tailored sleep management for people living with dementia, which will include work to both avoid initiating medicines and deprescribe hypnotics (starting February 2022). 241 The TAILOR programme theory (see Figure 22) has informed the logic model for this work, with the practice model to be developed and refined using co-design methods. Other bids are also in preparation.
We are also preparing a briefing paper highlighting the implications/recommendations for future research calls and activities identified from our work. We will share our briefing paper with other researchers through our networks including the Society for Academic Primary Care network, the British Pharmacological Society and the Royal Pharmaceutical Society, and so invite commentary and contribution from other projects that have raised similar issues. In this way we seek to generate a discipline-wide report on implications for future research that we will take to key research funding bodies and policy-makers.
Reviewing our objectives
In Chapter 1, we described a problem facing modern health care: how to support tailored deprescribing in the person-centred management of problematic polypharmacy. We identified two key gaps in the existing body of knowledge available to clinicians to support robust and safe tailored decision-making around deprescribing. These were (1) the need for a structured summary of the data on the safety and effectiveness of deprescribing, and (2) a credible framework describing good clinical practice for tailored prescribing. We therefore described three distinct objectives:
-
complete a robust scoping review of the literature on stopping medicines adults aged ≥ 50 years with polypharmacy and multimorbidity group to describe what is being done, where and for what effect
-
undertake a realist synthesis review to construct a programme theory that describes ‘best practice’ and helps explain the heterogeneity of deprescribing approaches
-
translate findings into resources to support tailored prescribing in clinical practice.
Our report demonstrates successful completion of the first two objectives, discussed in Reviewing what we found and what it means, Scoping review and Reviewing what we found and what it means, Realist review; with synergy between the two objectives providing further support for our research design decision to combine two review methods (see Chapter 2, Justification for design). For example, the extensive variation and variability seen within the scoping review is explained, in part, by the findings of the realist review. The findings from our realist review suggest that a degree of variability in clinical outcomes is inevitable in tailored care and that a different set of outcomes (e.g. trust and safety) may be more important than biomedical or pharmacological outcomes in guiding future care and research. Our scoping review used a current framework for best deprescribing practice63 to describe what interventions have been evaluated in research studies. Our realist review highlighted that there are key elements missing from this framework (e.g. meaning of medicines and trust), which limit its capacity to support person-centred, tailored deprescribing. A key synergistic outcome of both reviews is in describing and explaining why the person-centred deprescribing needed to address the challenge of problematic polypharmacy (see Chapter 1) must be understood as a complex intervention, not a linear technical process. The findings outline why both clinical practice and research must change to better support person-centred (tailored) care.
Finally, our work demonstrates the importance and value of theory-informed research to improve complex clinical practice. By combining the theory-based outcomes of the realist review with an assessment of the empirical/quantitative outcomes of the scoping review, we are better able to make recommendations for future practice.
Our third objective was to translate findings into useful resources to support practice on the ground. Dissemination activities: continuing our work to optimise the impact of TAILOR outlines our ongoing dissemination activities to provide outputs targeted to our various stakeholder groups, work that has been and will continue to be shaped by conversations with our stakeholders and PPI partners. Our protocol also described a goal to use our findings to refine our working model of a complex intervention (PRIME Prescribing)246 to support tailored prescribing in primary care practice. Our working model previously recognised the importance of shared understanding (see Figure 22). However, the new findings from this review of the importance of data and trust require us to do some rethinking and modification. We will be exploring the application of these elements within the TIMES (TaIlored ManagEment of Sleep) project241 (see Reviewing what we found and what it means), although this work will focus on a defined population. We discussed the implications for primary care prescribing practice at our final stakeholder meeting in December 2021. These discussions inform the development of dissemination work, including publications and the production of a MOOC, together with preparation of a new funding application for a study to co-design a feasibility pilot and full trial of a new complex intervention supporting person-centred, primary care prescribing practice.
Chapter 11 Implications and recommendations
Summary of conclusions
Chapter 10 discussed the key conclusions from our findings:
C1. Our findings provide evidence-informed support for the safety, clinician acceptability and potential effectiveness of deprescribing approaches that demonstrate structured approaches to deprescribing decisions.
C2. We need to ask clinicians and patients whether or not a dataset detailing the absolute benefit and harm associated with specific deprescribing decisions would change their decision-making practice.
C3. (De)prescribing is a complex intervention that must be understood, supported and assessed in context, including through practice-based research.
C4. The TAILOR realist synthesis describes a framework defining ‘better’ tailored deprescribing based on the four key elements of an enabling infrastructure for person-centred care, consistent access to quality data (including contextual), support for the generation of tailored explanations, and continuity of review supporting development and maintenance of trust.
C5. The TAILOR deprescribing framework extends existing models of good practice by recognising the need to consider the potential impact of prescribing decisions beyond biomedical or pharmacological effects, and by demonstrating the need to include organisational/contextual factors in models of better practice.
C6. In the final dissemination stages of TAILOR, we will continue our work with stakeholders to help translate our findings into resources for front-line practice.
C7. Our findings question whether or not there is an opportunity for new NICE guidance on person-centred care, using tailored prescribing as one example.
C8. Our findings highlight the importance of recognising person-centred health care, including prescribing, as a complex intervention needing robust practice-based evidence to support the delivery of quality care. The methodological implications of these observations should inform future research funding and prioritisation setting.
These inform our recommendations for research.
Implications for health care
| 1 | Clinicians can be advised that the evidence review suggests that deprescribing approaches using explicit approaches to clinical decision-making are often safe and acceptable to clinicians (with some limited data on acceptability for patients). However, clinical tools alone are insufficient for decision-making; clinical judgement will always be necessary |
| 2 | Deprescribing is a complex task involving many interacting elements and requires a supportive infrastructure to be done well. Practices may wish to review their prescribing review processes in the light of our findings |
| 3 | The TAILOR review provides evidence highlighting the need to recognise the impact of trust on prescribing decisions for best practice, describing the mechanism by which trust potentially affects patient outcomes. Trust is generated and maintained through clinicians’ awareness and understanding of patient context (as well as priorities), and its integration into generating and reviewing tailored explanations of health and health care. Trust is also developed and maintained by clear commitment to follow-up and review. Inconsistent advice across an extended clinical team undermines trust |
Recommendations for research
| 1 | Future research into deprescribing practice should make use of complex interventions approaches, with explicit attention to the theoretical underpinnings of the intervention (including proposed mechanism of impact) and consideration of whether/or not how proposed methodological approaches support generation of practice-based evidence180 (e.g. through embedding researchers within the practice context187) |
| 2 | The research community (including funders, researchers and wider stakeholders) should consider opportunities to optimise the impact of a combined research field through considering how research activities can be co-ordinated, including consistency in definitions and measures used |
| 3 | PPI partners played a crucial role in the TAILOR project through their contextual understanding of the work: the topic of prescribing and the process of using research to understand and improve care. We recommend that recruitment of, and support for, PPI partners in future research should focus on developing and maintaining this contextual understanding |
Acknowledgements
The research team offers our sincere thanks to the following people:
Dr Kat Kaviladou, research fellow who worked with us in the initial set-up stages and produced the analysis described in Chapter 2.
Scoping review contributors – Katherine Edwards (screening, data extraction), Gerlinde Pilkington (screening) and Yenal Dundar (screening).
Multiple stakeholders who have supported the work through input at several stakeholder and PPI meetings throughout the project.
Michelle Dickenson – our additional PPI partner who has offered invaluable advice and commentary throughout the project.
Ali Waring, who provided administrative support to the project throughout.
Contributions of authors
Professor Joanne Reeve (https://orcid.org/0000-0002-3184-7955) (Professor, Primary Care Research) was chief investigator for the work and led the co-design of the TAILOR study; supported both review teams in the work described in this report, including preparation of the final report; led the PPI and stakeholder engagement; wrote Chapters 1, 2, 9, 10 and 11 and oversaw preparation and editing of the full manuscript; and leads dissemination activities. Joanne Reeve also acts as corresponding author.
Dr Michelle Maden (https://orcid.org/0000-0003-4419-6343) (Research Associate, Evidence Synthesis) undertook the scoping review and prepared Chapters 3–5.
Dr Ruaraidh Hill (https://orcid.org/0000-0002-2801-0505) (Lecturer, Evidence Synthesis) led the scoping review design and delivery and co-wrote Chapters 3–5 with Michelle Maden.
Ms Amadea Turk (https://orcid.org/0000-0002-5139-0016) (Researcher, Primary Care) undertook the realist review under the supervision of Geoff Wong and Kamal Mahtani and led the writing of Chapters 6–8.
Dr Kamal Mahtani (https://orcid.org/0000-0002-7791-8552) (Associate Professor, Evidence-Based Medicine) co-supervised Amadea Turk, supporting the writing of Chapters 6–8.
Dr Geoff Wong (https://orcid.org/0000-0002-5384-4157) (Associate Professor of Primary Care, Realist Methodology) provided expert oversight and supervised Amadea Turk in the delivery of the realist review, contributing to Chapters 6–8.
Professor Dan Lasserson (https://orcid.org/0000-0001-8274-5580) (Professor, Ambulatory Medicine) was a member of the Academic Advisory Group and offered academic expertise at all project stages: set up, analysis, interpretation and meaning, and dissemination.
Professor Janet Krska (https://orcid.org/0000-0002-4148-5652) (Professor, Clinical and Professional Pharmacy) was a member of the Academic Advisory Group and offered academic expertise at all project stages: set up, analysis, interpretation and meaning, and dissemination.
Professor Dee Mangin (https://orcid.org/0000-0003-2149-9376) (Professor, Primary Care Research Including Rational Prescribing) was a member of the Academic Advisory Group and offered academic expertise at all project stages: set up, analysis, interpretation and meaning, and dissemination.
Professor Richard Byng (https://orcid.org/0000-0001-7411-9467) (Professor, Primary Care Research Including Person-Centred Care) was a member of the Academic Advisory Group and offered academic expertise at all project stages: set up, analysis, interpretation and meaning, and dissemination.
Dr Emma Wallace (https://orcid.org/0000-0002-9315-2956) (Senior Lecturer, Primary Care Research Including Prescribing Practice) was a member of the Academic Advisory Group and offered academic expertise at all project stages: set up, analysis, interpretation and meaning, and dissemination.
Mr Ed Ranson (PPI Co-applicant) worked with Joanne Reeve to lead the PPI work described in Chapter 9; both co-authored the chapter.
Publications
Maden M, Wong G, Mahtani K, Turk A, Reeve J, Hill R. Deprescribing in older people with multimorbidity: a scoping review. Target journal: BMC HSR
Turk A, Wong G, Mahtani K, Madden M, Hill R, Ranson E, et al. Optimising a person-centred approach to stopping medicines in older people with multimorbidity and polypharmacy using the DExTruS Framework: a realist review. BMC Med 2022; in press.
Data-sharing statement
No new data have been generated by this work, which involves secondary analysis of published data sources. The appendices and supplementary files give full details of the data search and extraction processes.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Duerden M, Avery T, Payne R. Polypharmacy and Medicines Optimisation: Making It Safe and Sound 2013. www.kingsfund.org.uk/sites/files/kf/field/field_publication_file/polypharmacy-and-medicines-optimisation-kingsfund-nov13.pdf (accessed 16 June 2021).
- Krska J, Katusiime B, Corlett SA. Validation of an instrument to measure patients’ experiences of medicine use: the Living with Medicines Questionnaire. Patient Prefer Adherence 2017;11:671-9. https://doi.org/10.2147/PPA.S126647.
- Denford S, Frost J, Dieppe P, Cooper C, Britten N. Individualisation of drug treatments for patients with long-term conditions: a review of concepts. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004172.
- Scottish Government Polypharmacy Model of Care Group . Polypharmacy Guidance, Realistic Prescribing 3rd Edition, 2018 2018. www.therapeutics.scot.nhs.uk/wp-content/uploads/2018/04/Polypharmacy-Guidance-2018.pdf (accessed 16 June 2021).
- Scottish Government Polypharmacy Model of Care Group . Polypharmacy: Managing Medicines n.d. https://managingmeds.scot.nhs.uk/ (accessed 16 June 2021).
- Reeve J, Fleming J, Britten N, Byng R, Krska J, Heaton J. Identifying enablers and barriers to individually tailored (expert generalist) prescribing: a survey of healthcare professionals in the UK. BMC Fam Pract 2018;19. https://doi.org/10.1186/s12875-017-0705-2.
- Barnett N, Kelly O. Deprescribing: is the law on your side?. Eur J Hosp Pharmacy 2017;24:21-5. https://doi.org/10.1136/ejhpharm-2016-000949.
- Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol 2015;80:1254-68. https://doi.org/10.1111/bcp.12732.
- Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med 1997;157:1531-6. https://doi.org/10.1001/archinte.157.14.1531.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213-18. https://doi.org/10.1093/ageing/afu145.
- National Institute for Health and Care Excellence . Benzodiazepine/Hypnotics/De-Prescribing 2019. www.nice.org.uk/sharedlearning/benzodiazepine-hypnotics-deprescribing (accessed 16 June 2021).
- Bruyère Research Institute . What Is Deprescribing n.d. https://deprescribing.org/what-is-deprescribing/ (accessed 16 June 2021).
- Derby and Derbyshire Clinical Commissioning Group . Deprescribing: A Practical Guide 2019. www.derbyshiremedicinesmanagement.nhs.uk/assets/Clinical_Guidelines/clinical_guidelines_front_page/Deprescribing.pdf (accessed 16 June 2021).
- Mangin D, Bahat G, Golomb B, Mallery L, Moorhouse P, Onder G, et al. International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): position statement and ten recommendations for action. Drugs Aging 2018;35:575-87. https://doi.org/10.1007/s40266-018-0554-2.
- Farrell B, Mangin D. Deprescribing is an essential part of good prescribing. Am Fam Physician 2019;99:7-9.
- Le Bosquet K, Barnett N, Minshull J. Deprescribing: practical ways to support person-centred, evidence-based deprescribing. Pharmacy 2019;7. https://doi.org/10.3390/pharmacy7030129.
- Reeve J, Dowrick CF, Freeman GK, Gunn J, Mair F, May C, et al. Examining the practice of generalist expertise: a qualitative study identifying constraints and solutions. JRSM Short Rep 2013;4. https://doi.org/10.1177/2042533313510155.
- Barnett NL, Oboh L, Smith K. Patient-centred management of polypharmacy: a process for practice. Eur J Hosp Pharm 2016;23:113-17. https://doi.org/10.1136/ejhpharm-2015-000762.
- BJGP Life . Future Doctors As Knowledge Workers n.d. https://bjgplife.com/future-doctors-as-knowledge-workers/ (accessed 11 March 2022).
- Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ 2015;350. https://doi.org/10.1136/bmj.h176.
- Reeve J. Avoiding harm: tackling problematic polypharmacy through strengthening expert generalist practice. Br J Clin Pharmacol 2021;87:76-83. https://doi.org/10.1111/bcp.14531.
- National Institute for Health and Care Excellence . David Haslam: Getting the Guidance Right 2016. www.nice.org.uk/news/feature/david-haslam-getting-the-guidance-right (accessed 16 June 2021).
- Montori V. Clinical Evidence for the Brave New World of Multimorbidity 2015. https://blogs.bmj.com/bmj/2015/03/19/victor-montori-clinical-evidence-for-the-brave-new-world-of-multimorbidity/ (accessed 16 June 2021).
- Fearon D, Hughes S, Brearley SG. A philosophical critique of the UK’s National Institute for Health and Social Care Excellence guideline ‘Palliative care for adults: strong opioids for pain relief’. Br J Pain 2018;12:183-8. https://doi.org/10.1177/2049463717753021.
- Hughes LD, McMurdo M, Guthrie B. Guidelines for people not diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Aging 2013;42:62-9. https://doi.org/10.1093/ageing/afs100.
- Gabbay J, le May A. Mindlines: making sense of evidence in practice. Br J Gen Pract 2016;66:402-3. https://doi.org/10.3399/bjgp16X686221.
- Straus SE, McAlister FA. Evidence-based medicine: a commentary on common criticisms. CMAJ 2000;163:837-41.
- Reeve J. Interpretive medicine: supporting generalism in a changing primary care world. Occas Pap R Coll Gen Pract 2010;88:1-20.
- Greenhalgh T, Snow R, Ryan S, Rees S, Salisbury H. Six ‘biases’ against patients and carers in evidence-based medicine. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0437-x.
- Donner-Banzhoff N, Hertwig R. Inductive foraging: improving the diagnostic yield of primary care consultations. Eur J Gen Pract 2014;20:69-73. https://doi.org/10.3109/13814788.2013.805197.
- Halm EA, Atlas SJ, Borowsky LH, Benzer TI, Metlay JP, Chang YC, et al. Understanding physician adherence with a pneumonia practice guideline: effects of patient, system, and physician factors. Arch Intern Med 2000;160:98-104. https://doi.org/10.1001/archinte.160.1.98.
- Heaton J, Britten N, Krska J, Reeve J. Person-centred medicines optimisation policy in England: an agenda for research on polypharmacy. Prim Health Care Res Dev 2017;18:24-3. https://doi.org/10.1017/S1463423616000207.
- The Health Policy Partnership . The State of Play in Person-Centred Care n.d. www.healthpolicypartnership.com/example-work/person-centred-care/ (accessed 16 June 2021).
- University of Plymouth . Person-Centred Coordinated Care n.d. www.plymouth.ac.uk/research/primarycare/person-centred-care (accessed 16 June 2021).
- World Health Organization . Primary Care, Now More Than Ever 2008. www.who.int/whr/2008/chapter3/en/ (accessed 16 June 2021).
- Williamson DL. Health as a resource for living: advancing the conceptualisation. Crit Pub Heal 2009;19:107-22. https://doi.org/10.1080/09581590802376234.
- Tinetti ME, Fried T. The end of the disease era. Am J Med 2004;116:179-85. https://doi.org/10.1016/j.amjmed.2003.09.031.
- Hunter DJ. Leading for health and wellbeing: the need for a new paradigm. J Public Health 2009;31:202-4. https://doi.org/10.1093/pubmed/fdp036.
- Rittel HWJ, Webber MM. Dilemmas in general theory of planning. Pol Sc 1973;4:155-69. https://doi.org/10.1007/BF01405730.
- Burns D, Hyde P, Killett A. Wicked problems or wicked people? Reconceptualising institutional abuse. Sociol Health Illn 2013;35:514-28. https://doi.org/10.1111/j.1467-9566.2012.01511.x.
- Riviett G. Our Health Service Presents Wicked Problems 2012. www.nuffieldtrust.org.uk/news-item/our-health-service-presents-wicked-problems (accessed 16 June 2021).
- Pawson R, Greenhalgh J, Brennan C. Demand management for planned care: a realist synthesis. Health Serv Deliv Res 2016;4. https://doi.org/10.3310/hsdr04020.
- NHS England . Medicines Optimisation n.d. www.england.nhs.uk/medicines-2/medicines-optimisation/ (accessed 16 June 2021).
- Tran VT, Barnes C, Montori VM, Falissard B, Ravaud P. Taxonomy of the burden of treatment: a multi-country web-based qualitative study of patients with chronic conditions. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0356-x.
- Gallacher K, Morrison D, Bautesh J, MacDonald S, May CR, Montori V, et al. Uncovering treatment burden as a key concept for stroke care. PLOS Med 2013. https://doi.org/10.1371/journal.pmed.1001473.
- Demain S, Gonçalves AC, Areia C, Oliveira R, Marcos AJ, Marques A, et al. Living with, managing and minimising treatment burden in long term conditions: a systematic review of qualitative research. PLOS One 2015;10. https://doi.org/10.1371/journal.pone.0125457.
- Reeve J, Cooper L. Rethinking how we understand individual healthcare needs for people living with long-term conditions: a qualitative study. Health Soc Care Community 2016;24:27-38. https://doi.org/10.1111/hsc.12175.
- Pound P, Britten N, Morgan M, Yardley L, Pope C, Daker-White G, et al. Resisting medicines: a synthesis of qualitative studies of medicine taking. Soc Sci Med 2005;61:133-55. https://doi.org/10.1016/j.socscimed.2004.11.063.
- Wallace E, McDowell R, Bennett K, Fahey T, Smith SM. Impact of potentially inappropriate prescribing on adverse drug events, health related quality of life and emergency hospital attendance in older people attending general practice: a prospective cohort study. J Gerontol A Biol Sci Med Sci 2017;72:271-7. https://doi.org/10.1093/gerona/glw140.
- Petticrew M. Complex Interventions: Some Definitions, Examples and Challenges n.d. www.evidencebasedpublichealth.de/download/Complex_interventions_Petticrew.pdf (accessed 19 April 2022).
- Petticrew M. When are complex interventions complex? When are simple interventions simple?. Eur J Public Health 2011;21:297-8. https://doi.org/10.1093/eurpub/ckr084.
- Reeve J, Cooper L, Harrington S, Rosbottom P, Watkins J. Developing, delivering and evaluating primary mental health care: the co-production of a new complex intervention. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1726-6.
- Meads C, Nyssen OP, Wong G, Steed L, Bourke L, Ross CA, et al. Protocol for an HTA report: does therapeutic writing help people with long-term conditions? Systematic review, realist synthesis and economic modelling. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004377.
- Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-33.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review – a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34. https://doi.org/10.1258/1355819054308530.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19-32. https://doi.org/10.1080/1364557032000119616.
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141-6. https://doi.org/10.1097/XEB.0000000000000050.
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-69.
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676-82. https://doi.org/10.1093/aje/kwq433.
- Booth A, Harris J, Croot E, Springett J, Campbell F, Wilkins E. Towards a methodology for cluster searching to provide conceptual and contextual ‘richness’ for systematic reviews of complex interventions: case study (CLUSTER). BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-118.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- O’Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol 2014;67:56-64. https://doi.org/10.1016/j.jclinepi.2013.08.005.
- Reeve E, Thompson W, Farrell B. Deprescribing: a narrative review of the evidence and practical recommendations for recognizing opportunities and taking action. Eur J Intern Med 2017;38:3-11. https://doi.org/10.1016/j.ejim.2016.12.021.
- Rohwer A, Booth A, Pfadenhauer L, Brereton L, Gerhardus A, Mozygemba K, et al. Guidance on the Use of Logic Models in Health Technology Assessments of Complex Interventions n.d. www.integrate-hta.eu/wp-content/uploads/2016/02/Guidance-on-the-use-of-logic-models-in-health-technology-assessments-of-complex-interventions.pdf (accessed 16 June 2021).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Boersma MN, Huibers CJA, Drenth-van Maanen AC, Emmelot-Vonk MH, Wilting I, Knol W. The effect of providing prescribing recommendations on appropriate prescribing: a cluster-randomized controlled trial in older adults in a preoperative setting. Br J Clin Pharmacol 2019;85:1974-83. https://doi.org/10.1111/bcp.13987.
- Caffiero N, Delate T, Ehizuelen MD, Vogel K. Effectiveness of a clinical pharmacist medication therapy management program in discontinuation of drugs to avoid in the elderly. J Manag Care Spec Pharm 2017;23:525-31. https://doi.org/10.18553/jmcp.2017.23.5.525.
- Campins L, Serra-Prat M, Gózalo I, López D, Palomera E, Agustí C, et al. Randomized controlled trial of an intervention to improve drug appropriateness in community-dwelling polymedicated elderly people. Fam Pract 2017;34:36-42. https://doi.org/10.1093/fampra/cmw073.
- Chiarelli MT, Antoniazzi S, Cortesi L, Pasina L, Novella A, Venturini F, et al. Pharmacist-driven medication recognition/reconciliation in older medical patients. Eur J Intern Med 2020;83:39-44. https://doi.org/10.1016/j.ejim.2020.07.011.
- Curtin D, Jennings E, Daunt R, Curtin S, Randles M, Gallagher P, et al. Deprescribing in older people approaching end of life: a randomized controlled trial using STOPP Frail Criteria. J Am Geriatr Soc 2020;68:762-9. https://doi.org/10.1111/jgs.16278.
- Fried TR, Niehoff KM, Street RL, Charpentier PA, Rajeevan N, Miller PL, et al. Effect of the tool to reduce inappropriate medications on medication communication and deprescribing. J Am Geriatr Soc 2017;65:2265-71. https://doi.org/10.1111/jgs.15042.
- Köberlein-Neu J, Mennemann H, Hamacher S, Waltering I, Jaehde U, Schaffert C, et al. Interprofessional medication management in patients with multiple morbidities. Dtsch Arztebl Int 2016;113:741-8. https://doi.org/10.3238/arztebl.2016.741.
- Komagamine J, Hagane K. Intervention to improve the appropriate use of polypharmacy for older patients with hip fractures: an observational study. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0681-3.
- Komagamine J, Sugawara K, Hagane K. Characteristics of elderly patients with polypharmacy who refuse to participate in an in-hospital deprescribing intervention: a retrospective cross-sectional study. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0788-1.
- McCarthy C, Clyne B, Corrigan D, Boland F, Wallace E, Moriarty F, et al. Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE): a cluster randomised controlled trial protocol and pilot. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0629-1.
- McDonald EG, Wu PE, Rashidi B, Forster AJ, Huang A, Pilote L, et al. The MedSafer Study: a controlled trial of an electronic decision support tool for deprescribing in acute care. J Am Geriatr Soc 2019;67:1843-50. https://doi.org/10.1111/jgs.16040.
- Muth C, Harder S, Uhlmann L, Rochon J, Fullerton B, Güthlin C, et al. Pilot study to test the feasibility of a trial design and complex intervention on PRIoritising MUltimedication in Multimorbidity in general practices (PRIMUMpilot). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011613.
- Muth C, Uhlmann L, Haefeli WE, Rochon J, van den Akker M, Perera R, et al. Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-017740.
- Petersen AW, Shah AS, Simmons SF, Shotwell MS, Jacobsen JML, Myers AP, et al. Shed-MEDS: pilot of a patient-centred deprescribing framework reduces medications in hospitalized older adults being transferred to inpatient postacute care. Ther Adv Drug Saf 2018;9:523-33. https://doi.org/10.1177/2042098618781524.
- Martín Lesende I, Mendibil Crespo I, Maiz López G, Gabilondo Zelaia I, Aretxabaleta Parra JC, Mota Goicoechea A. Potentiality of STOPP/START criteria used in primary care to effectively change inappropriate prescribing in elderly patients. Eur Geriatr Med 2013;4:293-8. https://doi.org/10.1016/j.eurger.2013.06.006.
- Potter EL, Lew TE, Sooriyakumaran M, Edwards AM, Tong E, Aung AK. Evaluation of pharmacist-led physician-supported inpatient deprescribing model in older patients admitted to an acute general medical unit. Australas J Ageing 2019;38:206-10. https://doi.org/10.1111/ajag.12643.
- Russell P, Laubscher S, Roberts GW, Mangoni AA, McDonald C, Hendrix I, et al. A pilot cohort study of deprescribing for nursing home patients acutely admitted to hospital. Ther Adv Drug Saf 2019;10. https://doi.org/10.1177/2042098619854876.
- San-Jose A, Perez-Bocanegra C, Agusti A, Laorden H, Gost J, Vidal X, et al. Integrated health intervention on polypharmacy and inappropriate prescribing in elderly people with multimorbidity: results at the end of the intervention and at 6 months after the intervention. Med Clin (Barc) 2021;156:263-9. https://doi.org/10.1016/j.medcli.2020.04.030.
- van Summeren JJ, Schuling J, Haaijer-Ruskamp FM, Denig P. Outcome prioritisation tool for medication review in older patients with multimorbidity: a pilot study in general practice. Br J Gen Pract 2017;67:e501-6. https://doi.org/10.3399/bjgp17X690485.
- Zechmann S, Trueb C, Valeri F, Streit S, Senn O, Neuner-Jehle S. Barriers and enablers for deprescribing among older, multimorbid patients with polypharmacy: an explorative study from Switzerland. BMC Fam Pract 2019;20. https://doi.org/10.1186/s12875-019-0953-4.
- Ghibelli S, Marengoni A, Djade CD, Nobili A, Tettamanti M, Franchi C, et al. Prevention of inappropriate prescribing in hospitalized older patients using a computerized prescription support system (INTERcheck®). Drugs Aging 2013;30:821-8. https://doi.org/10.1007/s40266-013-0109-5.
- Meulendijk MC, Spruit MR, Drenth-van Maanen AC, Numans ME, Brinkkemper S, Jansen PAF, et al. Computerized decision support improves medication review effectiveness: an experiment evaluating the STRIP Assistant’s usability. Drugs Aging 2015;32:495-503. https://doi.org/10.1007/s40266-015-0270-0.
- Willeboordse F, Schellevis FG, Hung Chau S, Hugtenburg JG, Elders PJM. The effectiveness of optimised clinical medication reviews for geriatric patients: Opti-Med a cluster randomised controlled trial. Fam Pract 2017;34:437-45. https://doi.org/10.1093/fampra/cmx007.
- Niehoff KM, Rajeevan N, Charpentier PA, Miller PL, Goldstein MK, Fried TR. Development of the Tool to Reduce Inappropriate Medications (TRIM): a clinical decision support system to improve medication prescribing for older adults. Pharmacotherapy 2016;36:694-701. https://doi.org/10.1002/phar.1751.
- Delgado E, Munõz M, Montero B, Sánchez C, Gallagher PF, Cruz-Jentoft AJ. [Inappropriate prescription in older patients: the STOPP/START criteria.]. Rev Esp Geriatr Gerontol 2009;44:273-9. https://doi.org/10.1016/j.regg.2009.03.017.
- Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing 2008;37:673-9. https://doi.org/10.1093/ageing/afn197.
- Lavan AH, Gallagher P, Parsons C, O’Mahony D. STOPPFrail (screening tool of older persons prescriptions in frail adults with limited life expectancy): consensus validation. Age Ageing 2017;46:600-7. https://doi.org/10.1093/ageing/afx005.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel . American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012;60:616-31. https://doi.org/10.1111/j.1532-5415.2012.03923.x.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel . American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63:2227-46. https://doi.org/10.1111/jgs.13702.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010;170:1648-54. https://doi.org/10.1001/archinternmed.2010.355.
- Drenth-van Maanen AC, Spee J, van Hensbergen L, Jansen PAF, Egberts TCG, van Marum RJ. Structured history taking of medication use reveals iatrogenic harm due to discrepancies in medication histories in hospital and pharmacy records. J Am Geriatr Soc 2011;59:1976-7. https://doi.org/10.1111/j.1532-5415.2011.03610_11.x.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006;166:605-9. https://doi.org/10.1001/archinte.166.6.605.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med 2012;125:529-37.e4. https://doi.org/10.1016/j.amjmed.2011.09.021.
- O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029954.
- Pollmann AS, Murphy AL, Bergman JC, Gardner DM. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol 2015;16. https://doi.org/10.1186/s40360-015-0019-8.
- Rosbach M, Anderson JS. Patient-experienced burden of treatment in patients living with multimorbidity. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0179916.
- Taylor-Robinson DC, Smyth RL, Diggle PJ, Whitehead M. The effect of social deprivation on clinical outcomes and the use of treatments in the UK cystic fibrosis population: a longitudinal study. Lancet Respir Med 2013;1:121-8. https://doi.org/10.1016/S2213-2600(13)70002-X.
- Beuscart JB, Knol W, Cullinan S, Schneider C, Dalleur O, Boland B, et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med 2018;16. https://doi.org/10.1186/s12916-018-1007-9.
- Rankin A, Cadogan CA, Ryan C, Clyne B, Smith SM, Hughes CM. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc 2018;66:1206-12. https://doi.org/10.1111/jgs.15245.
- Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2018;9. https://doi.org/10.1002/14651858.CD008165.pub4.
- Wong G, Greenhalgh T, Westhorp G, Pawson R. Development of methodological guidance, publication standards and training materials for realist and meta-narrative reviews: the RAMESES (Realist And Meta-narrative Evidence Syntheses: Evolving Standards) project. Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02300.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. https://doi.org/10.1016/S0140-6736(12)60240-2.
- Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendation for specifying and reporting. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-139.
- Dicenso A, Bayley L, Haynes RB. Accessing pre-appraised evidence: fine-tuning the 5S model into a 6S model. Evid Based Nurs 2009;12:99-101. https://doi.org/10.1136/ebn.12.4.99-b.
- Grimes DA, Schulz KF. An overview of clinical research: the lay of the land. Lancet 2002;359:57-61. https://doi.org/10.1016/S0140-6736(02)07283-5.
- Doherty AJ, Boland P, Reed J, Clegg AJ, Stephani AM, Williams NH, et al. Barriers and facilitators to deprescribing in primary care: a systematic review. BJGP Open 2020;4. https://doi.org/10.3399/bjgpopen20X101096.
- Ahmad A, Hugtenburg J, Welschen LM, Dekker JM, Nijpels G. Effect of medication review and cognitive behaviour treatment by community pharmacists of patients discharged from the hospital on drug related problems and compliance: design of a randomized controlled trial. BMC Public Health 2010;10. https://doi.org/10.1186/1471-2458-10-133.
- Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. General practitioners’ insight into deprescribing for the multimorbid older individual: a qualitative study. Int J Clin Pract 2016;70:261-76. https://doi.org/10.1111/ijcp.12780.
- Akinbolade O, Husband A, Forrest S, Todd A. Deprescribing in advanced illness. Prog Palliat Care 2016;24:268-71. https://doi.org/10.1080/09699260.2016.1192321.
- Al Shemeili S, Klein S, Strath A, Fares S, Stewart D. An exploration of health professionals’ experiences of medicines management in elderly, hospitalised patients in Abu Dhabi. Int J Clin Pharm 2016;38:107-18. https://doi.org/10.1007/s11096-015-0212-2.
- Altiner A, Schäfer I, Mellert C, Löffler C, Mortsiefer A, Ernst A, et al. Activating GENeral practitioners dialogue with patients on their Agenda (MultiCare AGENDA) study protocol for a cluster randomized controlled trial. BMC Fam Pract 2012;13. https://doi.org/10.1186/1471-2296-13-118.
- Anderson K, Foster M, Freeman C, Luetsch K, Scott I. Negotiating ‘unmeasurable harm and benefit’: perspectives of general practitioners and consultant pharmacists on deprescribing in the primary care setting. Qual Health Res 2017;27:1936-47. https://doi.org/10.1177/1049732316687732.
- Baqir W, Barrett S, Desai N, Copeland R, Hughes J. A clinico-ethical framework for multidisciplinary review of medication in nursing homes. BMJ Qual Improv Rep 2014;3. https://doi.org/10.1136/bmjquality.u203261.w2538.
- Bartlett Ellis RJ, Welch JL. Medication-taking behaviours in chronic kidney disease with multiple chronic conditions: a meta-ethnographic synthesis of qualitative studies. J Clin Nurs 2017;26:586-98. https://doi.org/10.1111/jocn.13588.
- Bokhof B, Junius-Walker U. Reducing polypharmacy from the perspectives of general practitioners and older patients: a synthesis of qualitative studies. Drugs Aging 2016;33:249-66. https://doi.org/10.1007/s40266-016-0354-5.
- Bolmsjö BB, Palagyi A, Keay L, Potter J, Lindley RI. Factors influencing deprescribing for residents in Advanced Care Facilities: insights from General Practitioners in Australia and Sweden. BMC Fam Pract 2016;17. https://doi.org/10.1186/s12875-016-0551-7.
- Cashman J, Wright J, Ring A. The treatment of co-morbidities in older patients with metastatic cancer. Support Care Cancer 2010;18:651-5. https://doi.org/10.1007/s00520-010-0813-1.
- Cenci C. Narrative medicine and the personalisation of treatment for elderly patients. Eur J Intern Med 2016;32:22-5. https://doi.org/10.1016/j.ejim.2016.05.003.
- Centeno M, Fullerton C. Got pills? A pharmacist’s impact on chronic disease and older adults in transitions of care. Int J Qual Heal Care 2016;28. https://doi.org/10.1093/intqhc/mzw104.35.
- Chen Z, Buonanno A. Geriatric polypharmacy: two physicians’ personal perspectives. Clin Geriatr Med 2017;33:283-8. https://doi.org/10.1016/j.cger.2017.01.008.
- Cheraghi-Sohi S, Jeffries M, Stevenson F, Ashcroft DM, Carr M, Oliver K, et al. The influence of personal communities on the self-management of medication taking: a wider exploration of medicine work. Chronic Illn 2015;11:77-92. https://doi.org/10.1177/1742395314537841.
- Christensen LD, Petersen J, Andersen O, Kaae S. Physicians’ non-uniform approach to prescribing drugs to older patients – a qualitative study. Basic Clin Pharmacol Toxicol 2017;121:505-11. https://doi.org/10.1111/bcpt.12837.
- Cimmino K, Pisano M. A patient’s last wish at end-of-life. J Am Geriatr Soc 2016;1:375-80. https://doi.org/10.4140/TCP.n.2016.375.
- Clyne B, Cooper JA, Hughes CM, Fahey T, Smith SM. OPTI-SCRIPT study team . ‘Potentially inappropriate or specifically appropriate?’ Qualitative evaluation of general practitioners views on prescribing, polypharmacy and potentially inappropriate prescribing in older people. BMC Fam Pract 2016;17. https://doi.org/10.1186/s12875-016-0507-y.
- Cullinan S, Raae Hansen C, Byrne S, O’Mahony D, Kearney P, Sahm L. Challenges of deprescribing in the multimorbid patient. Eur J Hosp Pharm 2017;24:43-6. https://doi.org/10.1136/ejhpharm-2016-000921.
- Cullinan S, Fleming A, O’Mahony D, Ryan C, O’Sullivan D, Gallagher P, et al. Doctors’ perspectives on the barriers to appropriate prescribing in older hospitalized patients: a qualitative study. Br J Clin Pharmacol 2015;79:860-9. https://doi.org/10.1111/bcp.12555.
- Djatche L, Lee S, Singer D, Hegarty SE, Lombardi M, Maio V. How confident are physicians in deprescribing for the elderly and what barriers prevent deprescribing?. J Clin Pharm Ther 2018;43:550-5. https://doi.org/10.1111/jcpt.12688.
- Drenth-van Maanen AC, Leendertse AJ, Jansen PAF, Knol W, Keijsers CJPW, Meulendijk MC, et al. The Systematic Tool to Reduce Inappropriate Prescribing (STRIP): combining implicit and explicit prescribing tools to improve appropriate prescribing. J Eval Clin Pract 2018;24:317-22. https://doi.org/10.1111/jep.12787.
- Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm 2017;24:37-42. https://doi.org/10.1136/ejhpharm-2016-000967.
- Edelman M, Jellema P, Hak E, Denig P, Blanker MH. Patients’ attitudes towards deprescribing alpha-blockers and their willingness to participate in a discontinuation trial. Drugs Aging 2019;36:1133-9. https://doi.org/10.1007/s40266-019-00712-6.
- Elliott RA, Ross-Degnan D, Adams AS, Safran DG, Soumerai SB. Strategies for coping in a complex world: adherence behavior among older adults with chronic illness. J Gen Intern Med 2007;22:805-10. https://doi.org/10.1007/s11606-007-0193-5.
- Frank C. Deprescribing: a new word to guide medication review. CMAJ 2014;186:407-8. https://doi.org/10.1503/cmaj.131568.
- Garfinkel D. IGRIMUP . Overview of current and future research and clinical directions for drug discontinuation: psychological, traditional and professional obstacles to deprescribing. Eur J Hosp Pharm 2017;24:16-20. https://doi.org/10.1136/ejhpharm-2016-000959.
- Gaup MA, Halvorsen KH. Physicians’ experiences with NORGEP criteria and the use of inappropriate medication in elderly patients in nursing home and home care service. Int J Clin Pharm 2015;37:14-5.
- Geijteman ECT, Huisman BAA, Dees MK, Perez RSGM, van der Rijt CCD, van Zuylen L, et al. Medication discontinuation at the end of life: a questionnaire study on physicians’ experiences and opinions. J Palliat Med 2018;21:1166-70. https://doi.org/10.1089/jpm.2017.0501.
- Gillespie R, Mullan J, Harrison L. Deprescribing for older adults in Australia: factors influencing GPs. Aust J Prim Health 2018;24:463-9. https://doi.org/10.1071/PY18056.
- Gnjidic D, Le Couteur DG, Kouladjian L, Hilmer SN. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med 2012;28:237-53. https://doi.org/10.1016/j.cger.2012.01.006.
- Gnjidic D, Le Couteur DG, Hilmer SN. Discontinuing drug treatments: we need better evidence to guide deprescribing. BMJ 2014;349. https://doi.org/10.1136/bmj.g7013.
- Gonçalves F. Deprescription in advanced cancer patients. Pharmacy 2018;6. https://doi.org/10.3390/pharmacy6030088.
- Hardy JE, Hilmer SN. Deprescribing in the last year of life. J Pharm Pract Res 2011;41:146-51. https://doi.org/10.1002/j.2055-2335.2011.tb00684.x.
- Harriman K, Howard L, McCracken R. Deprescribing medication for frail elderly patients: a survey of Vancouver family physicians. BCMJ 2014;56:436-41.
- Hasler S, Senn O, Rosemann T, Neuner-Jehle S. Effect of a patient-centered drug review on polypharmacy in primary care patients: study protocol for a cluster-randomized controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0915-7.
- Hernandez J. Medication management in the older adult: a narrative exploration. J Am Assoc Nurse Pract 2017;29:186-94. https://doi.org/10.1002/2327-6924.12427.
- Hilmer SN, Gnjidic D, Le Couteur DG. Thinking through the medication list – appropriate prescribing and deprescribing in robust and frail older patients. Aust Fam Physician 2012;41:924-8.
- Howland RH. Questions to ask when selecting medication. J Psychosoc Nurs Ment Health Serv 2012;50:13-5. https://doi.org/10.3928/02793695-20120607-01.
- Jäger C, Steinhaeuser J, Freund T, Szecsenyi J, Goetz K. Medication lists and brown bag reviews: potential positive and negative impacts on patients beliefs about their medicine. Biomed Res Int 2015;2015. https://doi.org/10.1155/2015/874067.
- Jansen J, McKinn S, Bonner C, Irwig L, Doust J, Glasziou P, et al. General practitioners’ decision making about primary prevention of cardiovascular disease in older adults: a qualitative study. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0170228.
- Jones BA. Decreasing polypharmacy in clients most at risk. AACN Clin Issues 1997;8:627-34. https://doi.org/10.1097/00044067-199711000-00014.
- Kaufman G, Bellerby A, Kitching M. Considering patient experience and evidence-based choice of medicines in medicines optimisation. Nurs Stand 2017;31:54-63. https://doi.org/10.7748/ns.2017.e10883.
- Knowles S, Hays R, Senra H, Bower P, Locock L, Protheroe J, et al. Empowering people to help speak up about safety in primary care: Using codesign to involve patients and professionals in developing new interventions for patients with multimorbidity. Health Expect 2018;21:539-48. https://doi.org/10.1111/hex.12648.
- Köberlein J, Gottschall M, Czarnecki K, Thomas A, Bergmann A, Voigt K. General practitioners’ views on polypharmacy and its consequences for patient health care. BMC Fam Pract 2013;14. https://doi.org/10.1186/1471-2296-14-119.
- Krska J. Factoring in frailty when optimising medication. Prescriber 2018;29:32-4. https://doi.org/10.1002/psb.1698.
- Krska J, Morecroft CW, Rowe PH, Poole H. Measuring the impact of long-term medicines use from the patient perspective. Int J Clin Pharm 2014;36:675-8. https://doi.org/10.1007/s11096-014-9970-5.
- Kuruvilla L, Weeks G, Eastman P, George J. Medication management for community palliative care patients and the role of a specialist palliative care pharmacist: a qualitative exploration of consumer and health care professional perspectives. Palliat Med 2018;32:1369-77. https://doi.org/10.1177/0269216318777390.
- Laursen J, Kornholt J, Betzer C, Petersen TS, Christensen MB. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: how can a focus on the pharmacotherapy in an outpatient clinic support GPs?. Health Serv Res Manag Epidemiol 2018;5. https://doi.org/10.1177/2333392818792169.
- Maidment I, Booth A, Mullan J, McKeown J, Bailey S, Wong G. Developing a framework for a novel multi-disciplinary, multi-agency intervention(s), to improve medication management in community-dwelling older people on complex medication regimens (MEMORABLE) – a realist synthesis. Syst Rev 2017;6. https://doi.org/10.1186/s13643-017-0528-1.
- Manias E, Claydon-Platt K, McColl GJ, Bucknall TK, Brand CA. Managing complex medication regimens: perspectives of consumers with osteoarthritis and healthcare professionals. Ann Pharmacother 2007;41:764-71. https://doi.org/10.1345/aph.1H623.
- Mantelli S, Jungo KT, Rozsnyai Z, Reeve E, Luymes CH, Poortvliet RKE, et al. How general practitioners would deprescribe in frail oldest-old with polypharmacy – the LESS study. BMC Fam Pract 2018;19. https://doi.org/10.1186/s12875-018-0856-9.
- Marengoni A, Nobili A, Onder G. Best practices for drug prescribing in older adults: a call for action. Drugs Aging 2015;32:887-90. https://doi.org/10.1007/s40266-015-0324-3.
- McGrath K, Hajjar ER, Kumar C, Hwang C, Salzman B. Deprescribing: a simple method for reducing polypharmacy. J Fam Pract 2017;66:436-45.
- Mc Namara KP, Breken BD, Alzubaidi HT, Bell JS, Dunbar JA, Walker C, et al. Health professional perspectives on the management of multimorbidity and polypharmacy for older patients in Australia. Age Ageing 2017;46:291-9. https://doi.org/10.1093/ageing/afw200.
- Modig S, Kristensson J, Ekwall AK, Hallberg IR, Midlöv P. Frail elderly patients in primary care – their medication knowledge and beliefs about prescribed medicines. Eur J Clin Pharmacol 2009;65:151-5. https://doi.org/10.1007/s00228-008-0581-8.
- Mudge A, Radnedge K, Kasper K, Mullins R, Adsett J, Rofail S, et al. Effects of a pilot multidisciplinary clinic for frequent attending elderly patients on deprescribing. Aust Health Rev 2016;40:86-91. https://doi.org/10.1071/AH14219.
- Molokhia M, Majeed A. Current and future perspectives on the management of polypharmacy. BMC Fam Pract 2017;18. https://doi.org/10.1186/s12875-017-0642-0.
- Nadarajan K, Balakrishnan T, Yee ML, Soong JL. The attitudes and beliefs of doctors towards deprescribing medications. Proc Singapore Healthc 2018;27:41-8. https://doi.org/10.1177/2010105817719711.
- Naughton C, Hayes N. Deprescribing in older adults: a new concept for nurses in administering medicines and as prescribers of medicine. Eur J Hosp Pharm 2017;24:47-50. https://doi.org/10.1136/ejhpharm-2016-000908.
- Ng WL, Tan MZ, Koh EY, Tan NC. Deprescribing: what are the views and factors influencing this concept among patients with chronic diseases in a developed Asian community?. Proc Singapore Healthc 2017;26:172-9. https://doi.org/10.1177/2010105817699633.
- Nixon MS, Vendelø MT. General practitioners’ decisions about discontinuation of medication: an explorative study. J Health Organ Manag 2016;30:565-80. https://doi.org/10.1108/JHOM-01-2015-0011.
- Frailty, polypharmacy and deprescribing. Drug Ther Bull 2016;54:69-72. https://doi.org/10.1136/dtb.2016.6.0408.
- Oboh L, Qadir MS. Deprescribing and managing polypharmacy in frail older people: a patient-centred approach in the real world. Eur J Hosp Pharm 2017;24:58-62. https://doi.org/10.1136/ejhpharm-2016-001008.
- O’Brien CP. Withdrawing medication: managing medical comorbidities near the end of life. Can Fam Physician 2011;57:304-7.
- Ouellet GM, Ouellet JA, Tinetti ME. Principle of rational prescribing and deprescribing in older adults with multiple chronic conditions. Ther Adv Drug Saf 2018;9:639-52. https://doi.org/10.1177/2042098618791371.
- Page AT, Potter K, Clifford R, Etherton-Beer C. Deprescribing in older people. Maturitas 2016;91:115-34. https://doi.org/10.1016/j.maturitas.2016.06.006.
- Palagyi A, Keay L, Harper J, Potter J, Lindley RI. Barricades and brickwalls – a qualitative study exploring perceptions of medication use and deprescribing in long-term care. BMC Geriatr 2016;16. https://doi.org/10.1186/s12877-016-0181-x.
- Rodríguez Pérez A, Alfaro Lara ER, Nieto Martín MD, Ruiz Cantero A, Santos Ramos B. Deprescribing in patients with multimorbidity: a necessary process. Eur J Intern Med 2015;26:e18-9. https://doi.org/10.1016/j.ejim.2015.06.011.
- Pitkälä KH, Suominen MH, Bell JS, Strandberg TE. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Med 2016;48:586-602. https://doi.org/10.1080/07853890.2016.1197414.
- Le Couteur DG, McLachlan AJ, Hilmer SN. Polypharmacy in older people: when should you deprescribe?. Med Today 2016;17:16-24.
- Pruskowski J, Handler SM. The DE-PHARM Project: a pharmacist-driven deprescribing initiative in a nursing facility. Consult Pharm 2017;32:468-78. https://doi.org/10.4140/TCP.n.2017.468.
- Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmacol 2014;78:738-47. https://doi.org/10.1111/bcp.12386.
- Reeve E, Bell JS, Hilmer SN. Barriers to optimising prescribing and deprescribing in older adults with dementia: a narrative review. Curr Clin Pharmacol 2015;10:168-77. https://doi.org/10.2174/157488471003150820150330.
- Rieckert A, Sommerauer C, Krumeich A, Sönnichsen A. Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA-eDS study): a qualitative study of practical implementation in primary care. BMC Fam Pract 2018;19. https://doi.org/10.1186/s12875-018-0789-3.
- Rigby D. Medication review: interview crucial to HMR success. Aust J Pharm 2013;94:28-9.
- Rose O, Richling I, Voigt K, Gottschall M, Köberlein-Neu J. Patient selection and general practitioners’ perception of collaboration in medication review. Res Soc Adm Pharm 2019;15:521-7. https://doi.org/10.1016/j.sapharm.2018.06.019.
- Ross A, Gillett J. Confronting medicine’s dichotomies: older adults’ use of interpretative repertoires in negotiating the paradoxes of polypharmacy and deprescribing. Qual Health Res 2020;30:448-57. https://doi.org/10.1177/1049732319868981.
- Ross A, Gillett J. ‘At 80 I know myself’: embodied learning and older adults’ experiences of polypharmacy and perceptions of deprescribing. Gerontol Geriatr Med 2019;5. https://doi.org/10.1177/2333721419895617.
- Ross A, Gillett J. Forms of trust and polypharmacy among older adults. Ageing Soc 2020;41:1-16. https://doi.org/10.1017/S0144686X20000483.
- Ryan R, Hill S. Making rational choices about how best to support consumers’ use of medicines: a perspective review. Ther Adv Drug Saf 2016;7:159-64. https://doi.org/10.1177/2042098616651198.
- Schäfer I, Kaduszkiewicz H, Mellert C, Löffler C, Mortsiefer A, Ernst A, et al. Narrative medicine-based intervention in primary care to reduce polypharmacy: results from the cluster-randomised controlled trial MultiCare AGENDA. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-017653.
- Schöpf AC, von Hirschhausen M, Farin E, Maun A. Elderly patients’ and GPs’ perspectives of patient–GP communication concerning polypharmacy: a qualitative interview study. Prim Health Care Res Dev 2018;19:355-64. https://doi.org/10.1017/S1463423617000883.
- Schuling J, Gebben H, Veehof LJ, Haaijer-Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract 2012;13. https://doi.org/10.1186/1471-2296-13-56.
- Scott IA, Gray LC, Martin JH, Pillans PI, Mitchell CA. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med 2013;18:121-4. https://doi.org/10.1136/eb-2012-100930.
- Sheppard JP, Burt J, Lown M, Temple E, Benson J, Ford GA, et al. OPtimising Treatment for MIld Systolic hypertension in the Elderly (OPTiMISE): protocol for a randomised controlled non-inferiority trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-022930.
- Sinnige J, Korevaar JC, van Lieshout J, Westert GP, Schellevis FG, Braspenning JC. Medication management strategy for older people with polypharmacy in general practice: a qualitative study on prescribing behaviour in primary care. Br J Gen Pract 2016;66:e540-51. https://doi.org/10.3399/bjgp16X685681.
- Sinnott C, Hugh SM, Boyce MB, Bradley CP. What to give the patient who has everything? A qualitative study of prescribing for multimorbidity in primary care. Br J Gen Pract 2015;65:e184-91. https://doi.org/10.3399/bjgp15X684001.
- Sinnott C, Mercer SW, Payne RA, Duerden M, Bradley CP, Byrne M. Improving medication management in multimorbidity: development of the MultimorbiditY COllaborative Medication Review And DEcision Making (MY COMRADE) intervention using the Behaviour Change Wheel. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0322-1.
- Sinnott C, Byrne M, Bradley CP. Improving medication management for patients with multimorbidity in primary care: a qualitative feasibility study of the MY COMRADE implementation intervention. Pilot Feasibility Stud 2017;3. https://doi.org/10.1186/s40814-017-0129-8.
- St Peter WL. Management of polypharmacy in dialysis patients. Semin Dial 2015;28:427-32. https://doi.org/10.1111/sdi.12377.
- Steinman MA, Hanlon JT. Managing medications in clinically complex elders: ‘there’s got to be a happy medium’. JAMA 2010;304:1592-601. https://doi.org/10.1001/jama.2010.1482.
- Straßner C, Steinhäuser J, Freund T, Szecsenyi J, Wensing M. German healthcare professionals’ perspective on implementing recommendations about polypharmacy in general practice: a qualitative study. Fam Pract 2018;35:503-10. https://doi.org/10.1093/fampra/cmx127.
- Sun W. A scaling-up approach to educating home care nurses about deprescribing to promote active living of frail older adults at home. J Am Geriatr Soc 2018;66. https://doi.org/10.1093/geroni/igy023.478.
- Thomas R, Kilbey C. Complex medicines management. Nurs Times 2011;107:20-2.
- Townsend A, Hunt K, Wyke S. Managing multiple morbidity in mid-life: a qualitative study of attitudes to drug use. BMJ 2003;327. https://doi.org/10.1136/bmj.327.7419.837.
- Turner JP, Edwards S, Stanners M, Shakib S, Bell JS. What factors are important for deprescribing in Australian long-term care facilities? Perspectives of residents and health professionals. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009781.
- Turner JP, Shakib S, Bell JS. Is my older cancer patient on too many medications?. J Geriatr Oncol 2017;8:77-81. https://doi.org/10.1016/j.jgo.2016.10.003.
- Twigg MJ, Wright D, Kirkdale CL, Desborough JA, Thornley T. The UK Pharmacy Care Plan service: description, recruitment and initial views on a new community pharmacy intervention. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0174500.
- Uhl MC, Muth C, Gerlach FM, Schoch GG, Müller BS. Patient-perceived barriers and facilitators to the implementation of a medication review in primary care: a qualitative thematic analysis. BMC Fam Pract 2018;19. https://doi.org/10.1186/s12875-017-0707-0.
- van Middelaar T, Ivens SD, van Peet PG, Poortvliet RKE, Richard E, Pols AJ, et al. Prescribing and deprescribing antihypertensive medication in older people by Dutch general practitioners: a qualitative study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020871.
- Vandermause R, Neumiller JJ, Gates BJ, David P, Altman M, Healey DJ, et al. Preserving self: medication-taking practices and preferences of older adults with multiple chronic medical conditions. J Nurs Scholarsh 2016;48:533-42. https://doi.org/10.1111/jnu.12250.
- Voigt K, Gottschall M, Köberlein-Neu J, Schübel J, Quint N, Bergmann A. Why do family doctors prescribe potentially inappropriate medication to elderly patients?. BMC Fam Pract 2016;17. https://doi.org/10.1186/s12875-016-0482-3.
- Waller DG. Rational prescribing: the principles of drug selection and assessment of efficacy. Clin Med 2005;5:26-8. https://doi.org/10.7861/clinmedicine.5-1-26.
- Wilchesky M, Mueller G, Morin M, Marcotte M, Voyer P, Aubin M, et al. The OptimaMed intervention to reduce inappropriate medications in nursing home residents with severe dementia: results from a quasi-experimental feasibility pilot study. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0895-z.
- Williams ME, Pulliam CC, Hunter R, Johnson TM, Owens JE, Kincaid J, et al. The short-term effect of interdisciplinary medication review on function and cost in ambulatory elderly people. J Am Geriatr Soc 2004;52:93-8. https://doi.org/10.1111/j.1532-5415.2004.52016.x.
- Weir K, Nickel B, Naganathan V, Bonner C, McCaffery K, Carter SM, et al. Decision-making preferences and deprescribing: perspectives of older adults and companions about their medicines. J Gerontol B Psychol Sci Soc Sci 2018;73:e98-e107. https://doi.org/10.1093/geronb/gbx138.
- Royal College of General Practitioners . Continuity of Care n.d. www.rcgp.org.uk/clinical-and-research/our-programmes/innovation/continuity-of-care.aspx (accessed 16 June 2021).
- Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, Mckendry R. Education and Debate Continuity of Care: A Multidisciplinary Review n.d. www.euro.who.int/AboutWHO/Policy/20010927_5 (accessed 16 June 2021).
- Tarrant C, Dixon-Woods M, Colman AM, Stokes T. Continuity and trust in primary care: a qualitative study informed by game theory. Ann Fam Med 2010;8:440-6. https://doi.org/10.1370/afm.1160.
- Mainous AG, Baker R, Love MM, Gray DP, Gill JM. Continuity of care and trust in one’s physician: evidence from primary care in the United States and the United Kingdom. Fam Med 2001;33:22-7.
- Kislov R, Hyde P, Mcdonald R, Manchester A. New game, old rules? Mechanisms and consequences of legitimation in boundary spanning activities. Organ Stud 2017;38:1421-44. https://doi.org/10.1177/0170840616679455.
- Levina N, Vaast E. The emergence of boundary spanning competence in practice: implications for implementation and use of information systems. MIS Q Manag Inf Syst 2005;29:335-63. https://doi.org/10.2307/25148682.
- Dang BN, Westbrook RA, Njue SM, Giordano TP. Building trust and rapport early in the new doctor-patient relationship: a longitudinal qualitative study. BMC Med Educ 2017;17. https://doi.org/10.1186/s12909-017-0868-5.
- Skirbekk H, Middelthon AL, Hjortdahl P, Finset A. Mandates of trust in the doctor-patient relationship. Qual Health Res 2011;21:1182-90. https://doi.org/10.1177/1049732311405685.
- Petrocchi S, Iannello P, Lecciso F, Levante A, Antonietti A, Schulz PJ. Interpersonal trust in doctor-patient relation: evidence from dyadic analysis and association with quality of dyadic communication. Soc Sci Med 2019;235. https://doi.org/10.1016/j.socscimed.2019.112391.
- Hobfoll SE. Conservation of resources. A new attempt at conceptualizing stress. Am Psychol 1989;44:513-24. https://doi.org/10.1037//0003-066x.44.3.513.
- Halbesleben JRB, Neveu J-P, Westman M. Getting to the ‘COR’: understanding the role of resources in conservation of resources theory. J Manage 2014;40:1334-64. https://doi.org/10.1177/0149206314527130.
- Hupcey JE. Clarifying the social support theory-research linkage. J Adv Nurs 1998;27:1231-41. https://doi.org/10.1046/j.1365-2648.1998.01231.x.
- Leahy-Warren P, Fitzpatrick JJ, McCarthy G. Theories Guiding Nursing Research and Practice: Making Nursing Knowledge Development Explicit. New York, NY: Springer Publishing Company; 2014.
- Stewart M. Reflections on the doctor-patient relationship: from evidence and experience. Br J Gen Pract 2005;55:793-801.
- NHS England . Shared Decision Making n.d. www.england.nhs.uk/shared-decision-making/ (accessed 16 June 2021).
- Crits-Christoph P, Rieger A, Gaines A, Gibbons MBC. Trust and respect in the patient-clinician relationship: preliminary development of a new scale. BMC Psychol 2019;7. https://doi.org/10.1186/s40359-019-0347-3.
- Reeve J, Dickenson M, Harris J, Ranson E, Dohnhammer U, Cooper L, et al. Solutions to problematic polypharmacy: learning from the expertise of patients. Br J Gen Pract 2015;65:319-20. https://doi.org/10.3399/bjgp15X685465.
- Grint K. Wicked problems and clumsy solutions: the role of leadership. Clinical Leader 2008;1:54-68.
- Department of Health and Social Care . National Overprescribing Review Report 2021. www.gov.uk/government/publications/national-overprescribing-review-report (accessed 19 April 2022).
- National Institute for Health and Care Excellence (NICE) . Multimorbidity: Clinical Assessment and Management 2016. www.nice.org.uk/guidance/ng56/resources (accessed 16 June 2021).
- Evans S, Scarbrough H. Supporting knowledge translation through collaborative translational research initiatives: ‘bridging’ versus ‘blurring’ boundary-spanning approaches in the UK CLAHRC initiative. Soc Sci Med 2014;106:119-27. https://doi.org/10.1016/j.socscimed.2014.01.025.
- Green LW. Making research relevant: if it is an evidence-based practice, where’s the practice-based evidence?. Fam Pract 2008;25:i20-4. https://doi.org/10.1093/fampra/cmn055.
- Fox GC, Lazar A, Hilton A, Wong G, Maidment I, Khondoker M, et al. The Clinical, Social and Cost Effectiveness of a Decision Support Tool to Optimise Community-Based Tailored Management of Sleep (TIMES) for People Living With Dementia or Mild Cognitive Impairment and Sleep Disturbance: TIMES TaIlored ManagEment of Sleep n.d. https://fundingawards.nihr.ac.uk/award/NIHR202345 (accessed 14 March 2022).
- NHS England . Involving People in Their Own Care n.d. www.england.nhs.uk/ourwork/patient-participation/ (accessed 16 June 2021).
- National Voices . Person-Centred Care in 2017. Evidence from Service Users n.d. www.nationalvoices.org.uk/publications/our-publications/person-centred-care-2017 (accessed 16 June 2021).
- Gopichandran V, Wouters E, Chetlapalli SK. Development and validation of a socioculturally competent trust in physician scale for a developing country setting. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007305.
- Holzinger A, Carrington A, Muller H. Measuring the Quality of Explanations: The System Causability Scale (SCS). Comparing Human and Machine Explanations n.d. https://arxiv.org/abs/1912.09024 (accessed 16 June 2021).
- Royal College of Physicians . Avoiding Harm from Over-Prescribing: How to Reduce Waste and Dependence on Prescription Drugs n.d. www.rcplondon.ac.uk/events/avoiding-harm-over-prescribing-how-reduce-waste-and-dependence-prescription-drugs (accessed 19 April 2022).
Appendix 1 Scoping review search strategies
Searches were run on 30 June 2019 and updated on 23 June 2020 (additional term ‘five or more’ was added to the updated search and backdated to 2009). The databases were searched from 2009 to 30 August 2019.
MEDLINE (via OVID)
-
exp Multimorbidity/
-
Multimorbid*.ti,ab,kw.
-
Multi-morbid*.ti,ab,kw.
-
“Multiple morbidit*”.ti,ab,kw.
-
exp Comorbidity/
-
comorbid*.ti,ab,kw.
-
co-morbid*.ti,ab,kw.
-
polymorbid*.ti,ab,kw.
-
poly-morbid*.ti,ab,kw.
-
(multiple adj3 (disease* or condition* or disorder* or illness*)).ti,ab,kw.
-
exp chronic disease/
-
((chronic or longterm or long-term) adj2 (disease* or condition* or disorder* or
-
illness*)).ti,ab,kw.
-
or/1-12
-
exp Polypharmacy/
-
polypharma*.ti,ab,kw.
-
polymedic*.ti,ab,kw.
-
poly-pharma*.ti,ab,kw.
-
Poly-medic*.ti,ab,kw.
-
polydrug*.ti,ab,kw.
-
Poly-drug*.ti,ab,kw.
-
multipharm*.ti,ab,kw.
-
multi-pharm*.ti,ab,kw.
-
multimedic*.ti,ab,kw.
-
multi-medic*.ti,ab,kw.
-
multidrug*.ti,ab,kw.
-
multi-drug.ti,ab,kw.
-
multi-prescri*.ti,ab,kw.
-
((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad*
-
or combination* or combined or “five or more”) adj (medicine* or medicat* or prescrib* or prescription*
-
or drug* or pharma*)).ti,ab,kw.
-
((multi-drug* or multidrug* or multi-medic* or multimedic*) adj2 (prescrib* or
-
prescription* or regimen* or therap* or treatment*)).ti,ab,kw.
-
(copharm* or comedic* or codrug* or co-pharm* or co-medic* or codrug*).
-
ti,ab,kw.
-
or/14-30
-
exp Deprescriptions/
-
exp Withholding treatment/and exp Drug prescriptions/
-
De-prescrib*.ti,ab,kw.
-
deprescrib*.ti,ab,kw.
-
deprescript*.ti,ab,kw.
-
((medicine* or medicat* or prescrib* or prescription* or drug* or overprescrib*)
-
adj3 (cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or
-
withhold* or remov* or minim* or tailor* or personaliz* or personalis* or individualiz*
-
or individualis* or revers*)).ti,ab,kw.
-
“stopp criter*”.ti,ab,kw.
-
“stopp list*”.ti,ab,kw.
-
((forta or rasp or priscus) adj3 (criter* or list* or instrument*)).ti,ab,kw.
-
((beer* or shan* or mcleod*) adj3 criter*).ti,ab,kw.
-
(“fit for the aged” adj3 (criter* or list* or instrument or classif*)).ti,ab,kw.
-
“medication appropriateness index”.ti,ab,kw.
-
“Screening Tool of Older Person’s Prescriptions”.ti,ab,kw.
-
exp Inappropriate Prescribing/
-
exp Potentially Inappropriate Medication List/
-
(prescri* adj cascad*).ti,ab,kw.
-
((overprescrib* or inappropriate prescri*) adj3 (review* or reconcil* or
-
manag*)).ti,ab,kw.
-
or/32-48
-
exp aged/or exp middle aged/
-
“older adult*”.ti,ab,kw.
-
“older person*”.ti,ab,kw.
-
“older people”.ti,ab,kw.
-
“older patient*”.ti,ab,kw.
-
elder*.ti,ab,kw.
-
“over 50*”.ti,ab,kw.
-
“over 60*”.ti,ab,kw.
-
“over 65*”.ti,ab,kw.
-
ageing.ti,ab,kw.
-
aging.ti,ab,kw.
-
senior*.ti,ab,kw.
-
geriatric*.ti,ab,kw.
-
pensioner*.ti,ab,kw.
-
octogenerian*.ti,ab,kw.
-
nonagenarian*.ti,ab,kw.
-
or/50-65
-
13 or 31
-
49 and 66 and 67
-
limit 68 to (english language and yr = “2009 -Current”)
-
13 and 31 and 49
-
limit 70 to (english language and yr = “2009 -Current”)
-
69 or 71
-
13 or 31
-
49 and 73
-
limit 74 to “qualitative (maximizes sensitivity)”
-
limit 75 to (english language and yr = “2009 - 2019”)
-
exp Qualitative Research/
-
qualitative.af.
-
77 or 78
-
74 and 79
-
limit 80 to (english language and yr = “2009 - 2019”)
-
76 or 81
-
72 or 82
-
limit 83 to (case reports or editorial or letter)
-
83 not 84
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
-
S1 TI multi-morbid* OR AB multi-morbid* OR KW multi-morbid*
-
S2 TI “Multiple morbidit*” OR AB “Multiple morbidit*” OR KW “Multiple morbidit*”
-
S3 TI multimorbid* OR AB multimorbid* OR KW multimorbid*
-
S4 (MH “Comorbidity”)
-
S5 TI comorbid* OR AB comorbid* OR KW comorbid*
-
S6 TI co-morbid* OR AB co-morbid* OR KW co-morbid*
-
S7 TI polymorbid* OR AB polymorbid* OR KW polymorbid*
-
S8 TI poly-morbid* OR AB poly-morbid* OR KW poly-morbid*
-
S9 TI (multiple N3 (disease* or condition* or disorder* or illness*)) OR AB (multiple N3 (disease* or condition* or disorder* or illness*)) OR KW (multiple N3 (disease* or condition* or disorder* or illness*))
-
S10 (MH “Chronic Disease+”)
-
S11 TI (((chronic or longterm or long-term) N2 (disease* or condition* or disorder* or illness*))) OR AB (((chronic or longterm or long-term) N2 (disease* or condition* or disorder* or illness*))) OR KW (((chronic or longterm or long-term) N2 (disease* or condition* or disorder* or illness*)))
-
S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11
-
S13 (MH “Polypharmacy”)
-
S14 TI polypharma* OR AB polypharma* OR KW polypharma*
-
S15 TI polymedic* OR AB polymedic* OR KW polymedic*
-
S16 TI poly-pharma* OR AB poly-pharma* OR KW poly-pharma*
-
S17 TI Poly-medic* OR AB Poly-medic* OR KW Poly-medic*
-
S18 TI polydrug* OR AB polydrug* OR KW polydrug*
-
S19 TI Poly-drug* OR AB Poly-drug* OR KW Poly-drug*
-
S20 TI multipharm* OR AB multipharm* OR KW multipharm*
-
S21 TI multi-pharm* OR AB multi-pharm* OR KW multi-pharm*
-
S22 TI multimedic* OR AB multimedic* OR KW multimedic*
-
S23 TI multi-medic* OR AB multi-medic* OR KW multi-medic*
-
S24 TI multidrug* OR AB multidrug* OR KW multidrug*
-
25 TI multi-drug* OR AB multi-drug* OR KW multi-drug*
-
S26 TI multi-prescri* OR AB multi-prescri* OR KW multi-prescri*
-
S27 TI (((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad* or combination* or combined or “five or more”) N1 (medicine* or medicat* or prescrib* or prescription* or drug* or pharma*))) OR AB (((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad* or combination* or combined or “five or more”) N1 (medicine* or medicat* or prescrib* or prescription* or drug* or pharma*))) OR KW (((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad* or combination* or combined or “five or more”) N1 (medicine* or medicat* or prescrib* or prescription* or drug* or pharma*)))
-
S28 TI (((multi-drug* or multidrug* or multi-medic* or multimedic*) N2 (prescrib* or prescription* or regimen* or therap* or treatment*))) OR AB (((multi-drug* or multidrug* or multi-medic* or multimedic*) N2 (prescrib* or prescription* or regimen* or therap* or treatment*))) OR KW (((multi-drug* or multidrug* or multi-medic* or multimedic*) N2 (prescrib* or prescription* or regimen* or therap* or treatment*)))
-
S29 TI ((copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*)) OR AB ((copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*)) OR KW ((copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*))
-
S30 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29
-
S31 TI De-prescrib* OR AB De-prescrib* OR KW De-prescrib*
-
S32 TI deprescrib* OR AB deprescrib* OR KW deprescrib*
-
S33 TI deprescript* OR AB deprescript* OR KW deprescript*
-
S34 TI (((medicine* or medicat* or prescrib* or prescription* or drug* or overprescrib*) N3 (cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or withhold* or remov* or minim* or tailor* or personaliz* or personalis* or individualiz* or individualis* or revers*))) OR AB (((medicine* or medicat* or prescrib* or prescription* or drug* or overprescrib*) N3 (cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or withhold* or remov* or minim* or tail...
-
S35 TI “stopp criter*” OR AB “stopp criter*” OR KW “stopp criter*”
-
S36 TI “stopp list*” OR AB “stopp list*” OR KW “stopp list*”
-
S37 TI (((forta or rasp or priscus) N3 (criter* or list* or instrument*))) OR AB (((forta or rasp or priscus) N3 (criter* or list* or instrument*))) OR KW (((forta or rasp or priscus) N3 (criter* or list* or instrument*)))
-
S38 TI (((beer* or shan* or mcleod*) N3 criter*)) OR AB (((beer* or shan* or mcleod*) N3 criter*)) OR KW (((beer* or shan* or mcleod*) N3 criter*))
-
S39 TI ((“fit for the aged” N3 (criter* or list* or instrument or classif*))) OR AB ((“fit for the aged” N3 (criter* or list* or instrument or classif*))) OR KW ((“fit for the aged” N3 (criter* or list* or instrument or classif*)))
-
S40 TI “medication appropriateness index” OR AB “medication appropriateness index” OR KW “medication appropriateness index”
-
S41 TI “Screening Tool of Older Person’s Prescriptions” OR AB “Screening Tool of Older Person’s Prescriptions” OR KW “Screening Tool of Older Person’s Prescriptions”
-
S42 TI (prescri* N1 cascad*) OR AB (prescri* N1 cascad*) OR KW (prescri* N1 cascad*)
-
S43 TI (((overprescrib* or inappropriate prescri*) N3 (review* or reconcil* or manag*))) OR AB (((overprescrib* or inappropriate prescri*) N3 (review* or reconcil* or manag*))) OR KW (((overprescrib* or inappropriate prescri*) N3 (review* or reconcil* or manag*)))
-
S44 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43
-
S45 (MH “Middle Age”) OR (MH “Aged+”)
-
S46 TI “older adult*” OR AB “older adult*” OR KW “older adult*”
-
S47 TI “older person*” OR AB “older person*” OR KW “older person*”
-
S48 TI “older people” OR AB “older people” OR KW “older people”
-
S49 TI “older patient*” OR AB “older patient*” OR KW “older patient*”
-
S50 TI elder* OR AB elder* OR KW elder*
-
S51 TI “over 50*” OR AB “over 50*” OR KW “over 50*”
-
S52 TI “over 60*” OR AB “over 60*” OR KW “over 60*”
-
S53 TI “over 65*” OR AB “over 65*” OR KW “over 65*”
-
S54 TI ageing OR AB ageing OR KW ageing
-
S55 TI aging OR AB aging OR KW aging
-
S56 TI senior* OR AB senior* OR KW senior*
-
S57 TI geriatric* OR AB geriatric* OR KW geriatric*
-
S58 TI pensioner* OR AB pensioner* OR KW pensioner*
-
S59 TI octogenerian* OR AB octogenerian* OR KW octogenerian*
-
S60 TI nonagenarian* OR AB nonagenarian* OR KW nonagenarian*
-
S61 S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60
-
S62 S12 OR S30
-
S63 S44 AND S61 AND S62
-
S64 S12 AND S30 AND S44
-
S65 S44 AND S62
-
S66 (MH “Qualitative Studies+”)
-
S67 TI (qualitative* or interview* or “focus group*”) OR AB (qualitative* or interview* or “focus group*”) OR KW (qualitative* or interview* or “focus group*”)
-
S68 S66 OR S67
-
S69 S65 AND S68
-
S70 S63 OR S64 OR S69
-
S71 S63 OR S64 OR S69 Limiters - Publication Year: 2009-2019
-
S72 S44 AND S62 Limiters - Publication Year: 2009-2019; Clinical Queries: Qualitative - High Sensitivity
-
S73 S71 OR S72
Web of Science
-
# 1 TS = ((Multimorbid* or Multi-morbid* or “Multiple morbidit*” or comorbid* or co-morbid* or polymorbid* or poly-morbid* or (multiple NEAR/3 (disease* or condition* or disorder* or illness*)) or ((chronic or longterm or long-term) NEAR/2 (disease* or condition* or disorder* or illness*))))
-
# 2 TS = ((polypharma* or polymedic* or poly-pharma* or poly-medic* or polydrug* or poly-drug* or multipharm* or multi-pharm* or multimedic* or multi-medic* or multidrug* or multi-drug* or multi-prescri*) OR (((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad* or combination* or combined or “five or more”) NEAR/1 (medicine* or medicat* or prescrib* or prescription* or drug* or pharma*))) OR (((multi-drug* or multidrug* or multi-medic* or multimedic*) NEAR/2 (prescrib* or prescription* or regimen* or therap* or treatment*))) OR (copharm* or comedic* or codrug* or co-pharm* or co-medic* or codrug*))
-
# 3 TS = (“older adult*” or “older person*” or “older people” or “older patient*” or elder* or “over 50*” or “over 60*” or “over 65*” or ageing or aging or senior* or geriatric* or pensioner* or octogenerian* or nonagenerian*)
-
# 4 TS = ((De-prescrib* or Deprescrib* or deprescript* or ((medicine* or medicat* or prescrib* or prescription* or drug* or overprescrib*) NEAR/3 (cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or withhold* or remov* or minim* or tailor* or personaliz* or personalis* or individualiz* or individualis* or revers*))) OR (“stopp criter*” or “stopp list*” or ((forta or rasp or priscus) NEAR/3 (criter* or list* or instrument*)) or ((beer* or shan* or mcleod*) NEAR/3 criter*) or (“fit for the aged” NEAR/3 (criter* or list* or instrument or classif*))) OR (“medication appropriateness index” or “Screening Tool of Older Person’s Prescriptions” or prescri* NEAR cascad* or (inappropriate prescri*) NEAR/3 (review* or reconcil* or manag*) or (overprescrib*) NEAR/3 (review* or reconcil* or manag*)))
-
# 5 #2 OR #1
-
# 6 #5 AND #4 AND #3
-
# 7 #4 AND #2 AND #1
-
# 8 #5 AND #4
-
# 9 TS = (qualitative* or interview* or “focus group*”)
-
# 10 #9 AND #8
-
# 11 #10 OR #7 OR #6
-
# 12 #10 OR #7 OR #6
-
Refined by: PUBLICATION YEARS: (2019 OR 2011 OR 2018 OR 2010 OR 2017 OR 2009 OR 2016 OR 2015 OR 2014 OR 2013 OR 2012) AND [excluding] DOCUMENT TYPES: (MEETING ABSTRACT OR LETTER OR EDITORIAL MATERIAL) AND LANGUAGES: (ENGLISH)
EMBASE (via OVID)
-
exp multiple chronic conditions/
-
Multimorbid*.ti,ab,kw.
-
Multi-morbid*.ti,ab,kw.
-
“Multiple morbidit*”.ti,ab,kw.
-
exp comorbidity/
-
comorbid*.ti,ab,kw.
-
co-morbid*.ti,ab,kw.
-
polymorbid*.ti,ab,kw.
-
poly-morbid*.ti,ab,kw.
-
(multiple adj3 (disease* or condition* or disorder* or illness*)).ti,ab,kw.
-
exp chronic disease/
-
((chronic or longterm or long-term) adj2 (disease* or condition* or disorder* or
-
illness*)).ti,ab,kw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
-
exp polypharmacy/
-
polypharma*.ti,ab,kw.
-
polymedic*.ti,ab,kw.
-
poly-pharma*.ti,ab,kw.
-
Poly-medic*.ti,ab,kw.
-
polydrug*.ti,ab,kw.
-
Poly-drug*.ti,ab,kw.
-
multipharm*.ti,ab,kw.
-
multi-pharm*.ti,ab,kw.
-
multimedic*.ti,ab,kw.
-
multi-medic*.ti,ab,kw.
-
multidrug*.ti,ab,kw.
-
multi-drug.ti,ab,kw.
-
multi-prescri*.ti,ab,kw.
-
((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad*
-
or combination* or combined or “five or more”) adj (medicine* or medicat* or prescrib* or prescription*
-
or drug* or pharma*)).ti,ab,kw.
-
((multi-drug* or multidrug* or multi-medic* or multimedic*) adj2 (prescrib* or
-
prescription* or regimen* or therap* or treatment*)).ti,ab,kw.
-
(copharm* or comedic* or codrug* or co-pharm* or co-medic* or
-
codrug*).ti,ab,kw.
-
or/14-30
-
exp deprescription/
-
De-prescrib*.ti,ab,kw.
-
deprescrib*.ti,ab,kw.
-
deprescript*.ti,ab,kw.
-
((medicine* or medicat* or prescrib* or prescription* or drug* or overprescrib*)
-
adj3 (cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or
-
withhold* or remov* or minim* or tailor* or personaliz* or personalis* or individualiz*
-
or individualis* or revers*)).ti,ab,kw.
-
“stopp criter*”.ti,ab,kw.
-
“stopp list*”.ti,ab,kw.
-
((forta or rasp or priscus) adj3 (criter* or list* or instrument*)).ti,ab,kw.
-
((beer* or shan* or mcleod*) adj3 criter*).ti,ab,kw.
-
(“fit for the aged” adj3 (criter* or list* or instrument or classif*)).ti,ab,kw.
-
“medication appropriateness index”.ti,ab,kw.
-
“Screening Tool of Older Person’s Prescriptions”.ti,ab,kw.
-
exp inappropriate prescribing/
-
exp potentially inappropriate medication/
-
(prescri* adj cascad*).ti,ab,kw.
-
((overprescrib* or inappropriate prescri*) adj3 (review* or reconcil* or
-
manag*)).ti,ab,kw.
-
or/32-47
-
exp aged/
-
exp middle aged/
-
exp adult/
-
“older adult*”.ti,ab,kw.
-
“older person*”.ti,ab,kw.
-
“older people”.ti,ab,kw.
-
“older patient*”.ti,ab,kw.
-
elder*.ti,ab,kw.
-
“over 50*”.ti,ab,kw.
-
“over 60*”.ti,ab,kw.
-
“over 65*”.ti,ab,kw.
-
ageing.ti,ab,kw.
-
aging.ti,ab,kw.
-
senior*.ti,ab,kw.
-
geriatric*.ti,ab,kw.
-
pensioner*.ti,ab,kw.
-
octogenerian*.ti,ab,kw.
-
nonagenarian*.ti,ab,kw.
-
or/49-66
-
13 or 31
-
48 and 67 and 68
-
13 and 31 and 48
-
69 or 70
-
48 and 68
-
limit 72 to “qualitative (maximizes sensitivity)”
-
exp qualitative research/
-
qualitative.af.
-
74 or 75
-
72 and 76
-
73 or 77
-
69 or 70 or 78
-
limit 79 to (english language and yr = “2009 -Current”)
-
limit 80 to (conference abstract or editorial or letter or note)
-
80 not 81
Cochrane
-
#1 MeSH descriptor: [Multimorbidity] explode all trees
-
#2 Multimorbid*:ti,ab,kw
-
#3 Multi-morbid*:ti,ab,kw
-
#4 “Multiple morbidit*”:ti,ab,kw
-
#5 MeSH descriptor: [Comorbidity] explode all trees
-
#6 comorbid*:ti,ab,kw
-
#7 co-morbid*:ti,ab,kw
-
#8 polymorbid*:ti,ab,kw
-
#9 poly-morbid*:ti,ab,kw
-
#10 (multiple NEAR/3 (disease* or condition* or disorder* or illness*)):ti,ab,kw
-
#11 MeSH descriptor: [Chronic Disease] explode all trees
-
#12 ((chronic or longterm or long-term) NEAR/2 (disease* or condition* or disorder* or illness*)):ti,ab,kw
-
#13 {OR #1-#12}
-
#14 MeSH descriptor: [Polypharmacy] explode all trees
-
#15 polypharma*:ti,ab,kw
-
#16 polymedic*:ti,ab,kw
-
#17 poly-pharma*:ti,ab,kw
-
#18 Poly-medic*:ti,ab,kw
-
#19 polydrug*:ti,ab,kw
-
#20 Poly-drug*:ti,ab,kw
-
#21 multipharm*:ti,ab,kw
-
#22 multi-pharm*:ti,ab,kw
-
#23 multimedic*:ti,ab,kw
-
#24 multi-medic*:ti,ab,kw
-
#25 multidrug*:ti,ab,kw
-
#26 multi-drug:ti,ab,kw
-
#27 multi-prescri*:ti,ab,kw
-
#28 ((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad* or combination* or combined or “five or more”) NEAR/1 (medicine* or medicat* or prescrib* or prescription* or drug* or pharma*)):ti,ab,kw
-
#29 ((multi-drug* or multidrug* or multi-medic* or multimedic*) NEAR/2 (prescrib* or prescription* or regimen* or therap* or treatment*))
-
#30 (copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*):ti,ab,kw
-
#31 {OR #14-#30}
-
#32 MeSH descriptor: [Deprescriptions] explode all trees
-
#33 MeSH descriptor: [Withholding Treatment] explode all trees
-
#34 MeSH descriptor: [Drug Prescriptions] explode all trees
-
#35 #33 AND #34
-
#36 #32 OR #35
-
#37 De-prescrib*:ti,ab,kw
-
#38 deprescrib*:ti,ab,kw
-
#39 deprescript*:ti,ab,kw
-
#40 ((medicine* or medicat* or prescrib* or prescription* or drug* or overprescrib*) NEAR/3 (cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or withhold* or remov* or minim* or tailor* or personaliz* or personalis* or individualiz* or individualis* or revers*)):ti,ab,kw
-
#41 “stopp criter*”:ti,ab,kw
-
#42 “stopp list*”:ti,ab,kw
-
#43 ((forta or rasp or priscus) NEAR/3 (criter* or list* or instrument*)):ti,ab,kw
-
#44 ((beer* or shan* or mcleod*) NEAR/3 criter*):ti,ab,kw
-
#45 (“fit for the aged” NEAR/3 (criter* or list* or instrument or classif*)):ti,ab,kw
-
#46 “medication appropriateness index”:ti,ab,kw
-
#47 “Screening Tool of Older Person’s Prescriptions”:ti,ab,kw
-
#48 MeSH descriptor: [Inappropriate Prescribing] explode all trees
-
#49 MeSH descriptor: [Potentially Inappropriate Medication List] explode all trees
-
#50 (prescri* NEAR/1 cascad*):ti,ab,kw
-
#51 ((overprescrib* or inappropriate prescri*) NEAR/3 (review* or reconcil* or manag*)):ti,ab,kw
-
#52 {OR #36-#51}
-
#53 MeSH descriptor: [Aged] explode all trees
-
#54 MeSH descriptor: [Middle Aged] explode all trees
-
#55 older NEXT adult*:ti,ab,kw
-
#56 older NEXT person*:ti,ab,kw
-
#57 older NEXT people:ti,ab,kw
-
#58 older NEXT patient*:ti,ab,kw
-
#59 elder*:ti,ab,kw
-
#60 over NEXT 50*:ti,ab,kw
-
#61 over NEXT 60*:ti,ab,kw
-
#62 over NEXT 65*:ti,ab,kw
-
#63 ageing:ti,ab,kw
-
#64 aging:ti,ab,kw
-
#65 senior*:ti,ab,kw
-
#66 geriatric*:ti,ab,kw
-
#67 pensioner*:ti,ab,kw
-
#68 octogenerian*:ti,ab,kw
-
#69 nonagenarian*:ti,ab,kw
-
#70 {OR #53-#69}
-
#71 #13 OR #31
-
#72 #71 AND #70 AND #52
-
#73 #13 AND #31 AND #52
-
#74 #72 OR #73
PsycInfo (via EBSCOhost)
-
S1 TI Multimorbid* OR AB Multimorbid* OR KW Multimorbid*
-
S2 TI Multi-morbid* OR AB Multi-morbid* OR KW Multi-morbid*
-
S3 TI “Multiple morbidit*” OR AB “Multiple morbidit*” OR KW “Multiple morbidit*”
-
S4 DE “Comorbidity”
-
S5 TI comorbid* OR AB comorbid* OR KW comorbid*
-
S6 TI co-morbid* OR AB co-morbid* OR KW co-morbid*
-
S7 TI polymorbid* OR AB polymorbid* OR KW polymorbid*
-
S8 TI poly-morbid* OR AB poly-morbid* OR KW poly-morbid*
-
S9 TI ((multiple N3 (disease* or condition* or disorder* or illness*))) OR AB ((multiple N3 (disease* or condition* or disorder* or illness*))) OR KW ((multiple N3 (disease* or condition* or disorder* or illness*)))
-
S10 DE “Chronic Illness”
-
S11 TI (((chronic or longterm or long-term) N2 (disease* or condition* or disorder* or illness*))) OR AB (((chronic or longterm or long-term) N2 (disease* or condition* or disorder* or illness*))) OR KW (((chronic or longterm or long-term) N2 (disease* or condition* or disorder* or illness*)))
-
S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11
-
S13 DE “Polypharmacy”
-
S14 TI polypharma* OR AB polypharma* OR KW polypharma*
-
S15 TI polymedic* OR AB polymedic* OR KW polymedic*
-
S16 TI poly-pharma* OR AB poly-pharma* OR KW poly-pharma*
-
S17 TI Poly-medic* OR AB Poly-medic* OR KW Poly-medic*
-
S18 TI polydrug* OR AB polydrug* OR KW polydrug*
-
S19 TI Poly-drug* OR AB Poly-drug* OR KW Poly-drug*
-
S20 TI multipharm* OR AB multipharm* OR KW multipharm*
-
S21 TI multi-pharm* OR AB multi-pharm* OR KW multi-pharm*
-
S22 TI multimedic* OR AB multimedic* OR KW multimedic*
-
S23 TI multi-medic* OR AB multi-medic* OR KW multi-medic*
-
S24 TI multidrug* OR AB multidrug* OR KW multidrug*
-
S25 TI multi-drug* OR AB multi-drug* OR KW multi-drug*
-
S26 TI multi-prescri* OR AB multi-prescri* OR KW multi-prescri*
-
S27 TI (((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad* or combination* or combined or “five or more”) N1 (medicine* or medicat* or prescrib* or prescription* or drug* or pharma*))) OR AB (((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad* or combination* or combined) N1 (medicine* or medicat* or prescrib* or prescription* or drug* or pharma*))) OR KW (((concomitant* or concurren* or simultaneous* or multi* or excess* or cascad* or combination* or combined or “five or more”) N1 (medicine* or medicat* or prescrib* or prescription* or drug* or pharma*)))
-
S28 TI (((multi-drug* or multidrug* or multi-medic* or multimedic*) N2 (prescrib* or prescription* or regimen* or therap* or treatment*))) OR AB (((multi-drug* or multidrug* or multi-medic* or multimedic*) N2 (prescrib* or prescription* or regimen* or therap* or treatment*))) OR KW (((multi-drug* or multidrug* or multi-medic* or multimedic*) N2 (prescrib* or prescription* or regimen* or therap* or treatment*)))
-
S29 TI ((copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*)) OR AB ((copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*)) OR KW ((copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*))
-
S30 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29
-
S31 TI De-prescrib* OR AB De-prescrib* OR KW De-prescrib*
-
S32 TI deprescrib* OR AB deprescrib* OR KW deprescrib*
-
S33 TI deprescript* OR AB deprescript* OR KW deprescript*
-
S34 TI (((medicine* or medicat* or prescrib* or prescription* or drug* or overprescrib*) N3 (cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or withhold* or remov* or minim* or tailor* or personaliz* or personalis* or individualiz* or individualis* or revers*))) OR AB (((medicine* or medicat* or prescrib* or prescription* or drug* or overprescrib*) N3 (cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or withhold* or remov* or minim* or tail...
-
S35 TI “stopp criter*” OR AB “stopp criter*” OR KW “stopp criter*”
-
S36 TI “stopp list*” OR AB “stopp list*” OR KW “stopp list*”
-
S37 TI (((forta or rasp or priscus) N3 (criter* or list* or instrument*))) OR AB (((forta or rasp or priscus) N3 (criter* or list* or instrument*))) OR KW (((forta or rasp or priscus) N3 (criter* or list* or instrument*)))
-
S38 TI (((beer* or shan* or mcleod*) N3 criter*)) OR AB (((beer* or shan* or mcleod*) N3 criter*)) OR KW (((beer* or shan* or mcleod*) N3 criter*))
-
S39 TI ((“fit for the aged” N3 (criter* or list* or instrument or classif*))) OR AB ((“fit for the aged” N3 (criter* or list* or instrument or classif*))) OR KW ((“fit for the aged” N3 (criter* or list* or instrument or classif*)))
-
S40 TI “medication appropriateness index” OR AB “medication appropriateness index” OR KW “medication appropriateness index”
-
S41 TI “Screening Tool of Older Person’s Prescriptions” OR AB “Screening Tool of Older Person’s Prescriptions” OR KW “Screening Tool of Older Person’s Prescriptions”
-
S42 TI (prescri* N1 cascad*) OR AB (prescri* N1 cascad*) OR KW (prescri* N1 cascad*)
-
S43 TI (((overprescrib* or inappropriate prescri*) N3 (review* or reconcil* or manag*))) OR AB (((overprescrib* or inappropriate prescri*) N3 (review* or reconcil* or manag*))) OR KW (((overprescrib* or inappropriate prescri*) N3 (review* or reconcil* or manag*)))
-
S44 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43
-
S45 (ZG “aged (65 yrs & older)”) or (ZG “middle age (40-64 yrs)”) or (ZG “very old (85 yrs & older)”)
-
S46 TI “older adult*” OR AB “older adult*” OR KW “older adult*”
-
S47 TI “older person*” OR AB “older person*” OR KW “older person*”
-
S48 TI “older people” OR AB “older people” OR KW “older people”
-
S49 TI “older patient*” OR AB “older patient*” OR KW “older patient*”
-
S50 TI elder* OR AB elder* OR KW elder*
-
S51 TI “over 50*” OR AB “over 50*” OR KW “over 50*”
-
S52 TI “over 60*” OR AB “over 60*” OR KW “over 60*”
-
S53 TI “over 65*” OR AB “over 65*” OR KW “over 65*”
-
S54 TI ageing OR AB ageing OR KW ageing
-
S55 TI aging OR AB aging OR KW aging
-
S56 TI senior* OR AB senior* OR KW senior*
-
S57 TI geriatric* OR AB geriatric* OR KW geriatric*
-
S58 TI pensioner* OR AB pensioner* OR KW pensioner*
-
S59 TI octogenerian* OR AB octogenerian* OR KW octogenerian*
-
S60 TI nonagenarian* OR AB nonagenarian* OR KW nonagenarian*
-
S61 S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60
-
S62 S12 OR S30
-
S63 S44 AND S61 AND S62
-
S64 S12 AND S30 AND S44
-
S65 S44 AND S62
-
S66 DE “Qualitative Methods” OR DE “Focus Group” OR DE “Grounded Theory” OR DE “Interpretative Phenomenological Analysis” OR DE “Narrative Analysis” OR DE “Semi-Structured Interview” OR DE “Thematic Analysis”
-
S67 TI (qualitative* or interview* or “focus group*”) OR AB (qualitative* or interview* or “focus group*”) OR KW (qualitative* or interview* or “focus group*”)
-
S68 S66 OR S67
-
S69 S65 AND S68
-
S70 S63 OR S64 OR S69
-
S71 S63 OR S64 OR S69
-
Limiters - Published: 20090101-20191231
Joanna Briggs Database of Systematic Reviews and Implementation Reports
https://journals.lww.com/jbisrir/Pages/default.aspx
Full text:
(multimorbidit* or “multi-mobidit*” or comorbidit* or “co-morbidit*” or “Multiple morbidit*” or “multiple disease*” or “multiple condition*” or “multiple disorder*” or “multiple illness” or polymorbidit* or “poly-morbidit*” or chronic* or “long-term” or longterm) AND (polypharma* or “poly-pharma*” or polymedic* or “poly-medic*” or polydrug* or “poly-drug*” or multipharma* or “multi-pharma*” or “multi-drug*” or multidrug* or multimedic* or “multi-medic*” or “multi-prescri*” or “co-pharma*” or “co-medic*” or “co-drug” or copharma* or comedic* or “co-drug*”) AND (deprescrib* or “deprescrip*” or “de-prescrib” or cessation or cease* or withdraw* or discontinu* or stop* or taper* or reduc* or withhold* or remov* or minim* or tailor* or personaliz* or personalis* or individualiz* or individualis* or revers* or “stopp criter*” or “stopp list*” or forta or rasp or priscus or beer* or shan* or mcleod* or “fit for the aged” or “medication appropriateness index” or “Screening Tool of Older Person’s Prescriptions” or “prescri* cascad*”) AND (aged or elder* or ageing or aging or “older adult*” or “older person*” or “older patient*” or “older people” or “over 50*” or “over 60*” or “over 65*” or senior* or geriatric* or pensioner* or octogenerian* or nonagenarian*)
National Institute for Health and Care Excellence Evidence
-
1. Deprescri*, Limit to 2009-2019 - 308
-
www.evidence.nhs.uk/search?from%20=%2001%2F01%2F2009&to%20=%2016%2F08%2F2019&q%20=%20deprescri*
-
2. polypharma* AND multimorbid*, Limit to 2009-2019 – 434
-
3. polypharma* AND comorbid*, Limit to 2009-2019 – 421
Google Scholar
(multimorbidity OR multimorbidities OR multi-morbidity OR multi-mobidities OR comorbidity OR comorbidities OR polypharmacy OR “multiple medications”) (deprescribing) (“older adults” OR elderly OR “older people”) Limit 2009-2019
(multimorbidity OR multimorbidities OR multi-morbidity OR multi-mobidities OR comorbidity OR comorbidities) (polypharmacy OR “multiple medications”) (deprescribing) Limit 2009-2019
(multimorbidity OR multimorbidities OR multi-morbidity OR multi-mobidities OR comorbidity OR comorbidities OR polypharmacy OR “multiple medications”) (deprescribing) (qualitative OR interview OR “focus group”) Limit 2009-2019
Websites
-
British Geriatrics Society
-
deprescribing.org
-
NHS Evidence
-
NICE Guidance
-
Kings Fund
-
Pharmaceutical Care Network Europe
-
PrescQIPP
-
Royal College of General Practitioners
-
Royal Pharmaceutical Society
-
Senator Project
-
UK Clinical Pharmacy
Supplementary PubMed search strategy
(((medication[Title] OR medicines[Title] OR polypharmacy[Title] OR drugs[Title] OR prescriptions[Title]) AND (management[Title] OR review[Title] OR reviews[Title] OR optimisation[Title] OR optimization[Title] OR reconciliation[Title] OR inappropriate[Title])) AND (decrease*[Title/Abstract] OR fall[Title/Abstract] OR deprescri*[Title/Abstract] OR reduce[Title/Abstract] OR reduces[Title/Abstract] OR stop*[Title/Abstract] OR withdrawal[Title/Abstract] OR taper*[Title/Abstract] OR reducing[Title/Abstract] OR reduction[Title/Abstract] OR drop[Title/Abstract] OR fell[Title/Abstract])) AND (elderly[Title/Abstract] OR aged[Title/Abstract] OR “older people”[Title/Abstract])
Appendix 2 Scoping review: amended TIDieR template
Reeve et al. 63 items were considered described if they mentioned the fact that the process was undertaken, and additional levels of detail were not sought (e.g. for the medication review we sought to highlight whether or not a medication review was undertaken, not whether or not it included all of the elements described by Reeve et al. 63 as being part of a medication review).
| Item | Description | Decision rule for Yes, Partial Yes, NR, NA |
|---|---|---|
| 1 | Brief name: provide the name or a phrase that describes the intervention | Gives precise name or details a description of the intervention (usually always a yes): YES, otherwise NR |
| 2 | Why: describe any rationale, theory or goal of the elements essential to the intervention | Details whether or not they think that the intervention is likely to be successful in their population, perhaps based on previous research in other settings/populations: YES, otherwise NR |
| 3 | What (materials): describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g. online appendix, URL) |
Describe what tools were used in the deprescribing process: If any of the above are documented then YES If no tools are mentioned or used then NR |
| 4 | What (procedures): |
If they outline ALL of the Reeve et al. 63 processes (4i-4vii): YES If they outline at least 1 of the Reeve et al. 63 processes (4i-4vii): PARTIAL YES, otherwise NR |
| 4ia | Collect a complete and comprehensive medication history | Details taking a medication history: YES, otherwise NR |
| 4iia | Assess overall risk of harm and benefit and individual patient factors that may affect deprescribing |
If detail assessment of BOTH risk AND patient factors (e.g. through clinical examination): YES If details only one of risk OR patient factors: PARTIAL YES, otherwise NR |
| 4iiia | Identify potentially inappropriate medications | Details identification of potentially inappropriate medications: YES, otherwise NR |
| 4iva | Decide on medication withdrawal (shared-decision-making) | Details how/who was involved in withdrawal decision-making: YES, otherwise NR |
| 4va | Plan, monitor, communicate: plan tapering or withdrawal process and monitoring with documentation and communication to all persons relevant to care |
Details discussions on appropriate timing of withdrawal AND whether/method of documentation AND communicates plan to all involved in health care, including patient: YES If only 1 or 2 of above are detailed: PARTIAL YES, otherwise NR |
| 4via | Conduct monitoring and support |
Details any monitoring of BOTH patient AND support for patient during deprescribing process: YES Details either monitoring OR support for patient during deprescribing process: PARTIAL YES, otherwise NR |
| 4viia | Documentation |
Document reasons for, process and outcome (e.g. medication ceased, dose reduced or withdrawal attempted with reasons for failure) of deprescribing AND shares documentation with all relevant health-care professionals: YES Document reasons for, process and outcome (e.g. medication ceased, dose reduced or withdrawal attempted with reasons for failure) of deprescribing OR shares documentation with all relevant health-care professionals: PARTIAL YES, otherwise NR |
| 5 | Who provided: for each category of intervention provider (e.g. psychologist, nursing assistant) describe their expertise, background and any specific training given |
Lists who provided AND provides details on expertise/background AND training given: YES Details who provided OR provides details on expertise/background OR training given: PARTIAL YES, otherwise NR |
| 6 | How: describe the modes of delivery (e.g. face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group |
Details how ALL parts of the intervention were delivered: YES Details how SOME parts of the intervention were delivered: PARTIAL YES, otherwise NR |
| 7 | Where: describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features | Setting and location detailed: YES, otherwise NR |
| 8 | When and how much: describe the number of times the intervention was delivered and over what period of time, including the number of sessions, their schedule, and their duration, intensity or dose |
Details when the intervention took place AND how many times it took place: YES Details when the intervention took place OR how many times it took place: PARTIAL YES, otherwise NR |
| 9 | Tailoring: if the intervention was planned to be personalised, titrated or adapted, then describe what, why, when, and how | If deprescribing interventions are personalised to the individual with outcome dependent on individual patient review: YES, otherwise NR |
| 10 | Modifications: if the intervention was modified during the course of the study, describe the changes (what, why, when and how) | Details modifications to intervention: YES, otherwise NR |
| 11 | How well (planned): if intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them | Measures process outcomes: YES, otherwise NA |
| 12 | How well (actual): if intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned | Measures process outcomes: YES, otherwise NA |
Appendix 3 Scoping review: included studies
| Study ID | Main study | Supplementary studies |
|---|---|---|
| 1 | Boersma et al.,66 2019 | |
| 2 | Caffiero et al.,67 2017 | |
| 3 | Campins et al.,68 2017 | |
| 4 | Chiarelli et al.,69 2020 | |
| 5 | Curtin et al.,70 2020 | Medication Rationalization for Older People awaiting long-term nursing home care: a randomized controlled trial using the STOPPfrail criteria. Trial Protocol. 2020 |
| 6 | Fried et al.,71 2017 | Niehoff KM, Rajeevan N, Charpentier PA, Miller PL, Goldstein MK, Fried TR. Development of the Tool to Reduce Inappropriate Medications (TRIM): a clinical decision support system to improve medication prescribing for older adults. Pharmacother 2016;36:694–701 |
| 7 | Köberlein-Neu et al.,72 2016 |
Rose O, Schaffert C, Czarnecki K, Mennemann HS, Waltering I, Hamacher S, et al. Effect evaluation of an interprofessional medication therapy management approach for multimorbid patients in primary care: a cluster-randomized controlled trial in community care (WestGem study protocol). BMC Fam Pract 2015;16:84 Rose O, Mennemann H, John C, Lautenschlager M, Mertens-Keller D, Richling K, et al. Priority setting and influential factors on acceptance of pharmaceutical recommendations in collaborative medication reviews in an ambulatory care setting – analysis of a cluster randomized controlled trial (WestGem-Study). PLOS ONE 2016;11:e0156304 |
| 8 | Komagamine and Hagane,73 2017 | |
| 9 | Komagamine et al.,74 2018 | |
| 10 | Martin Lesende et al.,80 2013 | |
| 11 | McCarthy et al.,75 2017 | |
| 12 | McDonald et al.,76 2019 | |
| 13 | Muth et al.,77 2016 | |
| 14 | Muth et al.,78 2018 |
Muth C, Uhlmann L, Haefeli WE, Rochon J, van den Akker M, Perera R, et al. Prioritising and optimising multiple medications in elderly multi-morbid patients in general practice. - A pragmatic cluster-randomised controlled trial. [PRIMUM] 2018 (Study protocol) von Buedingen F, Hammer MS, Meid AD, Muller WE, Gerlach FM, Muth C. Changes in prescribed medicines in older patients with multimorbidity and polypharmacy in general practice. BMC Fam Pract 2018;19:131 |
| 15 | Petersen et al.,79 2018 | |
| 16 | Potter et al.,81 2019 | |
| 17 | Russell et al.,82 2019 | |
| 18 | San-Jose et al.,83 2020 | |
| 19 | van Summeren et al.,84 2017 | |
| 20 | Zechmann et al.,85 2019 | Hasler S, Senn O, Rosemann T, Neuner-Jehle S. Effect of a patient-centered drug review on polypharmacy in primary care patients: study protocol for a cluster-randomized controlled trial. Trials 2015;16:380 |
Appendix 4 Realist review: summary of search strategy and results
Search strategy for MEDLINE
| # | Searches | Results |
|---|---|---|
| 1 | exp Chronic Disease/ | 251,136 |
| 2 | exp Comorbidity/ | 95,886 |
| 3 | (multimorbid* or multi-morbid* or comorbid* or co-morbid* or polymorbid* or poly-morbid*).ti,ab. | 149,284 |
| 4 | (multiple adj3 (disease? or condition? or disorder? or illness*)).ti,ab. | 32,914 |
| 5 | ((chronic or longterm or long-term) adj2 (disease? or condition? or disorder? or illness*)).ti,ab. | 227,085 |
| 6 | 1 or 2 or 3 or 4 or 5 | 652,517 |
| 7 | exp polypharmacy/ | 4133 |
| 8 | (polypharm* or polymedic* or polydrug* or poly-pharm* or poly-medic* or poly-drug*).ti,ab. | 8646 |
| 9 | (multipharm* or multimedic* or multidrug* or multi-pharm* or multi-medic* or multi-drug*).ti,ab. | 57,390 |
| 10 | (copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*).ti,ab. | 2379 |
| 11 | ((multiple* or simultaneous* or concurren* or concomitant* or combination* or combined*) adj3 (medication? or drug? or treatment? or pharmacotherap* or therap*)).ti,ab. | 239,595 |
| 12 | 7 or 8 or 9 or 10 or 11 | 303,700 |
| 13 | Medication Therapy Management/ | 1633 |
| 14 | “Drug Utilization Review”/ | 3559 |
| 15 | deprescriptions/ | 162 |
| 16 | Inappropriate Prescribing/ | 2346 |
| 17 | ((medication? or medicines or drugs or prescri* or overprescri*) adj3 (review? or reconcil* or manage*)).ti,ab. | 17,234 |
| 18 | ((medication? or medicines or drugs or prescri* or overprescri*) adj5 (reduc* or withdraw* or discontinu* or continu* or stop* or minim* or personaliz* or peronalis* or tailor*)).ti,ab. | 52,097 |
| 19 | ((overprescri* or inappropriate prescri*) and (review? or reconcil* or manage*)).ti,ab. | 662 |
| 20 | ((overprescri* or inappropriate prescri*) and (reduc* or withdraw* or discontinu* or stop* or minim*)).ti,ab. | 797 |
| 21 | (deprescri* or de-prescri*).ti,ab. | 414 |
| 22 | 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 | 73,764 |
| 23 | 6 and 12 and 22 | 1252 |
Search strategy for EMBASE
| Searches | Results |
|---|---|
| exp Chronic Disease/ | 162,610 |
| exp Comorbidity/ | 216,387 |
| (multimorbid* or multi-morbid* or comorbid* or co-morbid* or polymorbid* or poly-morbid*).ti,ab. | 261,808 |
| (multiple adj3 (disease? or condition? or disorder? or illness*)).ti,ab. | 47,219 |
| ((chronic or longterm or long-term) adj2 (disease? or condition? or disorder? or illness*)).ti,ab. | 324390 |
| 1 or 2 or 3 or 4 or 5 | 788,047 |
| exp polypharmacy/ | 13,257 |
| (polypharm* or polymedic* or polydrug* or poly-pharm* or poly-medic* or poly-drug*).ti,ab. | 13,942 |
| (multipharm* or multimedic* or multidrug* or multi-pharm* or multi-medic* or multi-drug*).ti,ab. | 70,961 |
| (copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*).ti,ab. | 4080 |
| ((multiple* or simultaneous* or concurren* or concomitant* or combination* or combined*) adj3 (medication? or drug? or treatment? or pharmacotherap* or therap*)).ti,ab. | 346,788 |
| 7 or 8 or 9 or 10 or 11 | 432,085 |
| Medication Therapy Management/ | 8804 |
| “Drug Utilization Review”/ | 265 |
| deprescription/ | 192 |
| exp Inappropriate Prescribing/ | 4026 |
| ((medication? or medicines or drugs or prescri* or overprescri*) adj3 (review? or reconcil* or manage*)).ti,ab. | 28,845 |
| ((medication? or medicines or drugs or prescri* or overprescri*) adj5 (reduc* or withdraw* or discontinu* or continu* or stop* or minim* or personaliz* or peronalis* or tailor*)).ti,ab. | 80,522 |
| ((overprescri* or inappropriate prescri*) and (review? or reconcil* or manage*)).ti,ab. | 1112 |
| ((overprescri* or inappropriate prescri*) and (reduc* or withdraw* or discontinu* or stop* or minim*)).ti,ab. | 1381 |
| (deprescri* or de-prescri*).ti,ab. | 584 |
| 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 | 113,130 |
| 6 and 12 and 22 | 2233 |
Search strategy for Cochrane Libraries
MeSH descriptor: [Chronic Disease] explode all trees
MeSH descriptor: [Comorbidity] explode all trees
(multimorbid* or multi-morbid* or comorbid* or co-morbid* or polymorbid* or poly-morbid*):ti,ab,kw
(multiple NEAR/3 (disease? or condition? or disorder? or illness*)):ti,ab,kw
(((chronic or longterm or long-term) NEAR/2 (disease* or condition* or disorder* or illness*))):ti,ab,kw
#1 or #2 or #3 or #4 or #5
MeSH descriptor: [Polypharmacy] explode all trees
(polypharm* or polymedic* or polydrug* or poly-pharm* or poly-medic* or poly-drug*):ti,ab,kw
(multipharm* or multimedic* or multidrug* or multi-pharm* or multi-medic* or multi-drug*):ti,ab,kw
(copharm* or comedic* or codrug* or co-pharm* or co-medic* or co-drug*):ti,ab,kw
(((multiple* or simultaneous* or concurren* or concomitant* or combination* or combined*) NEAR (medication* or drug* or treatment* or pharmacotherap* or therap*))):ti,ab,kw
#7 or #8 or #9 or #10 or #11
MeSH descriptor: [Medication Therapy Management] explode all trees
MeSH descriptor: [Drug Utilization Review] explode all trees
MeSH descriptor: [Deprescriptions] explode all trees
MeSH descriptor: [Inappropriate Prescribing] explode all trees
(((medication* or medicines or drugs or prescri* or overprescri*) NEAR/3 (review* or reconcil* or manage*))):ti,ab,kw
(((medication* or medicines or drugs or prescri* or overprescri*) NEAR (reduc* or withdraw* or discontinu* or continu* or stop* or minim* or personaliz* or peronalis* or tailor*))):ti,ab,kw
(((overprescri* or “inappropriate prescri*”) and (review* or reconcil* or manage*))):ti,ab,kw
((overprescri* or “inappropriate prescri*”) and (reduc* or withdraw* or discontinu* or stop* or minim*)):ti,ab,kw
(deprescri* or de-prescri*):ti,ab,kw
#13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21
#6 and #12 and #22
| Database | Interface | Coverage | Date | Hits |
|---|---|---|---|---|
| Cochrane Database of Systematic Reviews | Cochrane Library, Wiley | Issue 10 of 12, October 2018 | 16 October 2018 | 23 |
| Cochrane Central Register of Controlled Trials | Cochrane Library, Wiley | Issue 9 of 12, September 2018 | 16 October 2018 | 361 |
| EMBASE | OvidSP | 1974 to 2018 May 23 | 16 October 2018 | 2233 |
| MEDLINE | OvidSP | 1946-present | 16 October 2018 | 1252 |
| Total | 3869 | |||
| Excluded studies | 90 | |||
| Animal | 8 | |||
| 0–18 years | 82 | |||
| Duplicates | 1134 | |||
| Final total | 2645 | |||
| Systematic reviews | 58 | |||
| Articles | 1786 | |||
| Trial protocols | 63 | |||
| Conference abstracts | 738 |
Appendix 5 Studies included in the realist synthesis
| Author | Year | Country | Title | Study design/methods | Sample/setting | Objectives |
|---|---|---|---|---|---|---|
| Ahmad et al.112 | 2010 | The Netherlands | Effect of medication review and cognitive behaviour treatment by community pharmacists of patients discharged from the hospital on drug related problems and compliance: design of a randomized controlled trial | Protocol for RCT | Patients aged > 60 years discharged from general academic hospitals | To examine the effect of medication review and cognitive behaviour therapy of discharged patients by community pharmacists to minimise the occurrence of drug-related problems |
| Ailabouni et al.113 | 2016 | New Zealand | General practitioners’ insight into deprescribing for the multimorbid older individual: a qualitative study | Qualitative interview study | GPs prescribing for patients living in residential care | To explore GPs’ opinions and awareness of deprescribing for a hypothetical older multimorbid patient in residential care |
| Akinbolade et al.114 | 2016 | UK | Deprescribing in advanced illness | Literature review | Patients with advanced illness | To review reviews’ research on prescribing medicines to patients with advanced illness, focusing on the identification of the prevalence of inappropriate or unnecessary medicines to the initiation of the deprescribing process |
| Al Shemeili et al.115 | 2016 | United Arab Emirates | An exploration of health professionals’ experiences of medicines management in elderly, hospitalised patients in Abu Dhabi | Qualitative interview study | Health-care professionals working in hospitals involved in medication management. The sample included nurses, pharmacists and doctors | To describe and understand health professionals’ views and experiences of medicines management health-care structures, processes and outcomes for elderly, hospitalised patients |
| Altiner et al.116 | 2012 | Germany | Activating GENeral practitioners dialogue with patients on their Agenda (MultiCare AGENDA) study protocol for a cluster randomized controlled trial | Protocol for cluster RCT | General practice patients aged 65–84 years with at least three chronic conditions | To investigate the efficacy of a complex, multifaceted intervention aimed at increasing the quality of care of GPs for patients with multimorbidity through enhancing the doctor–patient dialogue and identifying the patient’s agenda and needs |
| Anderson et al.117 | 2017 | Australia | Negotiating ‘unmeasurable harm and benefit’: perspectives of general practitioners and consultant pharmacists on deprescribing in the primary care setting | Qualitative focus group study | GPs and consultant pharmacists working in south-east Queensland | To explore GPs’ and consultant pharmacists’ views about inappropriate polypharmacy, the reasoning they apply to deprescribing in primary care and to identify factors that support or inhibit this process |
| Baqir et al.118 | 2014 | UK | A clinico-ethical framework for multidisciplinary review of medication in nursing homes | Quality improvement project | Pharmacists undertaking medication reviews with nursing home residents | To optimise medicines in care homes while involving all residents in decision-making |
| Barnett et al.18 | 2016 | UK | Patient-centred management of polypharmacy: a process for practice | Review | Current UK literature around polypharmacy | To provide an overview of key guidance from the UK about polypharmacy and to introduce a tool to support patient-centred practice |
| Bartlett Ellis and Welch119 | 2016 | USA | Medication-taking behaviours in chronic kidney disease with multiple chronic conditions: a meta-ethnographic synthesis of qualitative studies | Systematic review: meta-ethnography | Literature on medication-taking behaviour in chronic kidney disease | To identify behaviours associated with taking medications and medication adherence reported in qualitative studies of adults with chronic kidney disease and coexisting multiple chronic conditions |
| Beuscart et al.103 | 2018 | Belgium | International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy | Mixed methods: systematic review, semistructured interviews, Delphi survey | Older patients with multimorbidity and polypharmacy | To describe a method that could be used to develop a core outcome set for use in trials of older patients with multimorbidity |
| Bokhof and Junius-Walker120 | 2016 | Germany | Reducing polypharmacy from the perspectives of general practitioners and older patients: a synthesis of qualitative studies | Systematic review, meta-ethnography | GPs and older patients | To synthesise qualitative studies exploring the perspectives and experiences of GPs and older patients in reducing polypharmacy and to discover approaches already being practised |
| Bolmsjö et al.121 | 2016 | Sweden and Australia | Factors influencing deprescribing for residents in advanced care facilities: insights from general practitioners in Australia and Sweden | Qualitative synthesis of two interview studies | General practitioners serving patients in long-term care facilities | To compare and contrast behavioural factors influencing the prescribing practices of GPs providing care in advanced care facilities in two different countries; to review health policy and aged care facility systems in each setting for their potential impact on the prescribing of medications; based on these findings provide recommendations |
| Cashman et al.122 | 2010 | UK | The treatment of co-morbidities in older patients with metastatic cancer | Review of medical records and patient interviews | Patients with metastatic cancer | To determine whether or not older patients with metastatic cancer continue to take medications for the treatment of pre-existing comorbidities after the diagnosis of metastatic disease |
| Cenci123 | 2016 | Italy | Narrative medicine and the personalisation of treatment for elderly patients | Literature review | Patients with multimorbidity and polypharmacy | To provide an overview of how narrative medicine can promote the development of a systematic, integrated and multidisciplinary approach to older patients |
| Centeno and Fullerton124 | 2016 | USA | Got pills? A pharmacist’s impact on chronic disease and older adults in transitions of care | Conference abstract | Quality improvement project | To assess the impact of medication reconciliation by clinical pharmacist on patient outcomes during transitions of care |
| Chen and Buonanno125 | 2017 | USA | Geriatric polypharmacy: two physicians’ personal perspectives | Opinion piece | Two clinicians discussing experiences of managing polypharmacy | To discuss geriatric polypharmacy from two practitioners’ viewpoints |
| Cheraghi-Sohi et al.126 | 2015 | United Kingdom | The influence of personal communities on the self-management of medication taking: a wider exploration of medicine work | Qualitative interview study | Patients with long-term conditions | To explicate the nature of the work that people with multiple long-term conditions, and their network members, do in attempting to take their medications on a daily basis, the division of labour among these members and when and why network members become involved in that work |
| Christensen et al.127 | 2017 | Denmark | Physicians’ non-uniform approach to prescribing drugs to older patients – a qualitative study | Qualitative interview study | Medical specialists working with older patients | To explore physicians’ approach to prescribing drugs to older patients, including identifying the drugs that physicians perceive to be risk drugs for older patients and comparing them with established lists of potentially inappropriate medications |
| Cimmino and Pisano128 | 2016 | USA | A patient’s last wish at end-of-life | Case study | Patients at the end of life | A case study discussing managing polypharmacy at the end of life |
| Clyne et al.129 | 2016 | Ireland | ‘Potentially inappropriate or specifically appropriate?’ Qualitative evaluation of general practitioners views on prescribing, polypharmacy and potentially inappropriate prescribing in older people | Qualitative interview study | GPs participating in a RCT of an intervention to decrease potentially inappropriate prescribing in older patients (aged ≥ 70 years) in Ireland | To explore GP perspectives regarding prescribing and potentially inappropriate prescribing in older primary care patients |
| Cullinan et al.130 | 2017 | Ireland | Challenges of deprescribing in the multimorbid patient | Literature review | Literature on challenges to deprescribing in patients with multimorbidity | To highlight some of the potential reasons for this lack of deprescribing and the challenges to discontinuing drugs for these patients |
| Cullinan et al.131 | 2015 | Ireland | Doctors’ perspectives on the barriers to appropriate prescribing in older hospitalized patients: a qualitative study | Qualitative interview study | Hospital doctors prescribing for older people | To identify hospital doctors’ perceptions as to why potentially inappropriate prescribing occurs, to identify the barriers to addressing the issues identified and to determine which intervention types would be best suited to improving prescribing |
| Djatche et al.132 | 2017 | Italy | How confident are physicians in deprescribing for the elderly and what barriers prevent deprescribing? | Survey | Primary care physicians | To assess the perceptions of primary care physicians on deprescribing for elderly patients and potential barriers to deprescribing that physicians experience in the local health authority of Parma, Emilia Romagna, Italy |
| Drenth-van Maanen et al.133 | 2017 | The Netherlands | The Systematic Tool to Reduce Inappropriate Prescribing (STRIP): combining implicit and explicit prescribing tools to improve appropriate prescribing | Presentation of a prescribing tool | n/a | To describe STRIP and its ability to identify potentially inappropriate prescribing |
| Duncan et al.134 | 2017 | UK | Deprescribing: a primary care perspective | Literature review | Literature on deprescribing and polypharmacy in primary care | To describe trends in polypharmacy and explanations for why it is increasing; outline the harms associated with overtreatment; outline the rationale for deprescribing and different approaches to deprescribing within general practice, including the role of the pharmacist; outline the barriers to and enablers of deprescribing; and make recommendations for future practice |
| Edelman et al.135 | 2019 | The Netherlands | Patients’ attitudes towards deprescribing alpha-blockers and their willingness to participate in a discontinuation trial | Questionnaire | Men aged ≥ 30 years with lower urinary tract symptoms and who were first prescribed an alpha-blocker in 2015 or 2016 | To gain insights into the attitudes of men with lower urinary tract symptoms towards deprescribing alpha-blockers and to assess their willingness to participate in a planned discontinuation trial |
| Elliott et al.136 | 2007 | USA | Strategies for coping in a complex world: adherence behavior among older adults with chronic illness | Qualitative interview study | Older adults taking multiple medications | To explore how older adults with multiple illnesses make choices about medicines |
| Frank137 | 2014 | Canada | Deprescribing: a new word to guide medication review | Commentary | n/a | To describe deprescribing |
| Fried et al.71 | 2017 | USA | Effect of the Tool to Reduce Inappropriate Medications (TRIM) on medication communication and deprescribing | RCT | 128 veterans aged ≥ 65 years prescribed seven medications, randomised to receipt of TRIM or usual care | To examine the effect of TRIM, a web tool linking the EHR to a clinical decision support system, on medication communication and prescribing |
| Garfinkel138 | 2017 | Israel | Overview of current and future research and clinical directions for drug discontinuation: psychological, traditional and professional obstacles to deprescribing | Literature review/commentary | n/a | To provide an overview of, and future research and clinical directions for, drug discontinuation |
| Gaup and Halvorsen139 | 2015 | Norway | Physicians’ experiences with NORGEP criteria and the use of inappropriate medication in elderly patients in nursing home and home care service | Conference abstract for qualitative interview study | Nursing home physicians and GPs | To investigate how nursing home physicians and GPs cope with inappropriate prescribing, their own experiences of using NORGEP criteria in clinical work, and how inappropriate prescribing could be reduced |
| Geijteman et al.140 | 2018 | The Netherlands | Medication discontinuation at the end of life: a questionnaire study on physicians’ experiences and opinions | Questionnaire | General practitioners and clinical specialists working in three regions in the Netherlands | To explore physicians’ opinions and experiences regarding medication discontinuation during the last phase of life, and to identify factors influencing the continuation of potentially inappropriate medications |
| Gillespie et al.141 | 2018 | Australia | Deprescribing for older adults in Australia: factors influencing GPs | Survey | GPs | To explore factors that influence deprescribing among Australian GPs using a new 21-item survey to measure GP attitudes and practices |
| Gnjidic et al.142 | 2012 | Australia | Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes | Literature review | Literature on interventions designed to reduce polypharmacy on prescribing and clinical outcomes | To highlight the evidence for the impact of various types of interventions designed to reduce polypharmacy on prescribing and clinical outcomes in older adults from community, nursing home, and hospital settings |
| Gnjidic et al.143 | 2014 | Australia | Discontinuing drug treatments: we need better evidence to guide deprescribing | Commentary | n/a | To describe the evidence base for deprescribing |
| Gonçalves144 | 2018 | Portugal | Deprescription in advanced cancer patients | Literature review | n/a | To describe deprescribing in cancer patients and propose a six-step method for deprescription |
| Hardy and Hilmer145 | 2011 | Australia | Deprescribing in the last year of life | Literature review | n/a | To provide an algorithm to guide safe, rational deprescribing for patients who are believed to be in their last year of life |
| Harriman et al.146 | 2015 | Canada | Deprescribing medication for frail elderly patients in nursing homes: a survey of Vancouver family physicians | Survey | Family physicians | To understand the beliefs and approaches of experienced FPs to help identify ways to improve current practices and reduce polypharmacy among frail elderly patients |
| Hasler et al.147 | 2015 | Switzerland | Effect of a patient-centered drug review on polypharmacy in primary care patients: study protocol for a cluster-randomized controlled trial | Protocol for a cluster RCT | Primary care physicians | To determine whether or not a patient-centred systematic review leads to more appropriate medication use in patients without negatively affecting quality of life and the course of the disease |
| Heaton et al.32 | 2017 | UK | Person-centred medicines optimisation policy in England: an agenda for research on polypharmacy | Review of policy, documentary analysis of reports on medicines optimisation | Policy reports on medicines optimisation published by the RPS, The King’s Fund and NICE since 2013 | To examine how patient perspectives and person-centred care values have been represented in documents on medicines optimisation policy in England |
| Hernandez148 | 2017 | USA | Medication management in the older adult: a narrative exploration | Qualitative interview study | Nurse practitioners caring for older adults | To characterise the meaning NPs ascribe to personal experiences of providing care to older adults who take multiple medications to manage complex conditions |
| Hilmer et al.149 | 2012 | Australia | Thinking through the medication list: appropriate prescribing and deprescribing in robust and frail older patients | Literature review | n/a | To provide an ethically sound, evidence-based discussion of the benefits and harms of medications commonly used in primary care among older patients |
| Howland150 | 2012 | USA | Questions to ask when selecting medication | Commentary/opinion piece | n/a | To explore eight questions that should be considered when selecting medication for a patient |
| Jäger et al.151 | 2015 | Germany | Medication lists and brown bag reviews: potential positive and negative impacts on patients beliefs about their medicine | Cross-sectional study with survey | Patients aged > 50 years taking more than four drugs and enrolled into the ‘Polypharmacy in Multimorbid Patients’ study | To explore whether or not patients’ use of a medication list is associated with their beliefs about their medicine and their memory of structured medication counselling |
| Jansen et al.152 | 2017 | Australia | General Practitioners’ decision-making about primary prevention of cardiovascular disease in older adults: a qualitative study | Qualitative interview study | GPs | To explore GPs’ decision-making about primary CVD prevention in patients aged ≥ 75 years |
| Jones153 | 1997 | USA | Decreasing polypharmacy in clients most at risk | Commentary/opinion piece | n/a | To give an overview of decreasing polypharmacy |
| Kaufman et al.154 | 2017 | UK | Considering patient experience and evidence-based choice of medicines in medicines optimisation | CPD module | n/a | To discuss the challenges of medicines optimisation, a patient-focused approach to supporting patients to gain maximum benefit from their medicines |
| Knowles et al.155 | 2017 | UK | Empowering people to help speak up about safety in primary care: using co-design to involve patients and professionals in developing new interventions for patients with multimorbidity | Accelerated experience-based co-design and the future workshop approach | Health-care professionals and patients | To explore whether or not co-production methodologies could enhance intervention development and provide a mechanism to translate available evidence into patient-centred intervention proposals for multimorbidity and safety |
| Köberlein et al.156 | 2013 | Germany | General practitioners’ views on polypharmacy and its consequences for patient health care | Study protocol for a retrospective cross-sectional study using mixed methods | GPs and patients | To detect the status quo of the health-care situation in Saxony’s general practices for multimorbid patients receiving multiple medications |
| Komagamine et al.74 | 2018 | Japan | Characteristics of elderly patients with polypharmacy who refuse to participate in an in-hospital deprescribing intervention: a retrospective cross-sectional study | Retrospective cross-sectional study | Patients aged ≥ 65 years who reported the use of five or more medications on admission to the orthopaedic ward from January 2015 to December 2016 and who were approached by a pharmacist for polypharmacy screening | To evaluate the prevalence of PIM use in elderly patients accepting and refusing a deprescribing intervention and to investigate factors associated with deprescribing refusal |
| Krska157 | 2018 | UK | Factoring in frailty when optimising medication | Opinion piece/commentary | n/a | To give advice to help identify frailty and adopt a patient-centred approach to medicines optimisation |
| Krska et al.158 | 2014 | UK | Measuring the impact of long-term medicines use from the patient perspective | Commentary | n/a | To discuss measuring the impact of long-term medicines use from the patient perspective |
| Kuruvilla et al.159 | 2018 | Australia | Medication management for community palliative care patients and the role of a specialist palliative care pharmacist: a qualitative exploration of consumer and health care professional perspectives | Qualitative focus group study | Palliative care consumers and clinicians specifically patients, caregivers, physicians, nurses and pharmacists | To explore the perspectives of stakeholders about the gaps in the current model of community palliative care services in relation to medication management and to assess their opinions pertaining to the role of a specialist palliative care pharmacist in addressing some of those gaps |
| Laursen et al.160 | 2018 | Denmark | General practitioners’ barriers towards medication reviews in polymedicated multimorbid patients: how can a focus on the pharmacotherapy in an outpatient clinic support GPs? | Qualitative interview study | GPs | To explore whether or not GPs experienced barriers towards medication reviews in polymedicated, multimorbid patients, and how a clinical pharmacologist with a focus on pharmacotherapy can support the GPs in an outpatient clinic |
| Maidment et al.161 | 2017 | UK | Developing a framework for a novel multidisciplinary, multiagency intervention(s), to improve medication management in community-dwelling older people on complex medication regimens (MEMORABLE)–a realist synthesis | Protocol for a realist synthesis | Literature on medication management in older people on complex medication regimes residing in the community | To understand how, why, for whom and in what context interventions to improve medication management in older people on complex medication regimes residing in the community work |
| Mangin et al.14 | 2018 | Canada | International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): position statement and 10 recommendations for action | Opinion piece | n/a | To present the first position statement of IGRIMUP on the international co-operative effort and recommendations for actions needed to prevent and counter IMUP and its drivers globally |
| Manias et al.162 | 2007 | Australia | Managing complex medication regimens: perspectives of consumers with osteoarthritis and healthcare professionals | Qualitative focus group and interview study | Patients and health-care professionals | To examine medication management for osteoarthritis and other chronic conditions from the perspectives of community-dwelling consumers and health-care professionals, using a qualitative approach |
| Mantelli et al.163 | 2018 | Switzerland | How general practitioners would deprescribe in frail oldest-old with polypharmacy – the LESS study | Survey | GPs | To determine whether or not, how and why Swiss GPs deprescribe for the oldest-old (aged > 80 years) with multimorbidity and polypharmacy |
| Marengoni et al.164 | 2015 | Italy | Best practices for drug prescribing in older adults: a call for action | Opinion piece | n/a | To propose a multicomponent intervention with the goal of achieving the best-tailored pharmacotherapy |
| McCarthy et al.75 | 2017 | Ireland | Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE): a cluster randomised controlled trial protocol and pilot | Protocol for a cluster RCT | General practice patients (aged ≥ 65 years with ≥ 15 prescribed medications) and GPs | To assess the effectiveness of a complex intervention designed to support GPs to reduce potentially inappropriate prescribing and consider deprescribing in older people with multimorbidity and significant polypharmacy in Irish primary care |
| McGrath et al.165 | 2017 | USA | Deprescribing: a simple method for reducing polypharmacy | Commentary/opinion piece using a case study | n/a | To present a four-step plan to aid the safe deprescribing in older adults |
| Mc Namara et al.166 | 2017 | Australia | Health professional perspectives on the management of multimorbidity and polypharmacy for older patients in Australia | Qualitative interview study | Health-care professionals including nurses, doctors, dentists, pharmacists and physiotherapists working in a range of settings | To explore current approaches to multimorbidity management, and perceived barriers to and enablers of delivering appropriate medications management for community-dwelling patients with multimorbidity and polypharmacy from a broad range of health-care professional perspectives in Australia |
| Modig et al.167 | 2009 | Sweden | Frail elderly patients in primary care – their medication knowledge and beliefs about prescribed medicines | Questionnaire | Patients aged ≥ 65 years with multiple illnesses | To describe elderly patients’ knowledge about and attitudes towards their medicines in Swedish primary care |
| Molokhia and Majeed169 | 2017 | UK | Current and future perspectives on the management of polypharmacy | Opinion piece | n/a | To review trends in polypharmacy and how clinicians can try to ensure that they maximise the benefits of prescribing and minimise the associated complications, particularly in the increasing number of frail, elderly patients whom physicians are now seeing in health systems across the world |
| Mudge et al.168 | 2016 | Australia | Impact of a pilot multidisciplinary clinic for frequent attending elderly patients on deprescribing | Retrospective study | Patients with frequent medical admissions | To examine the impact of the THRIVE model on medication count, tablet load and PIMs |
| Nadarajan et al.170 | 2018 | Singapore | The attitudes and beliefs of doctors towards deprescribing medications | Survey | Hospital doctors | To explore the attitudes and beliefs of deprescribing medications among doctors in the DIM in SGH, and to see if differences exist among junior and senior doctors in their attitudes towards deprescribing |
| Naughton and Hayes171 | 2016 | UK | Deprescribing in older adults: a new concept for nurses in administering medicines and as prescribers of medicine | Literature review | n/a | To examine the context of deprescribing from the perspective of nurses in medicines administration and prescribing practices and to outline the nature of the nursing contribution to this emerging topic |
| Ng et al.172 | 2017 | Singapore | Deprescribing: what are the views and factors influencing this concept among patients with chronic diseases in a developed Asian community? | A cross-sectional study using the validated PATD questionnaire | Patients on regular follow-up at the clinics for chronic disease management and with at least five regular prescription medications | To elucidate patients’ attitudes towards the number of medications they were taking and identify factors that might influence acceptance of deprescription |
| Nixon and Vendelø173 | 2016 | Denmark | General practitioners’ decisions about discontinuation of medication: an explorative study | Qualitative interviews and observations | GPs | To investigate how GPs’ decisions about discontinuation of medication are influenced by their institutional context |
| Drug and Therapeutics Bulletin174 | 2016 | UK | Frailty, polypharmacy and deprescribing | Commentary | n/a | To provide an overview of frailty, polypharmacy and deprescribing |
| Oboh and Qadir175 | 2017 | UK | Deprescribing and managing polypharmacy in frail older people: a patient-centred approach in the real world | Case report | A 73-year-old diabetic man taking multiple medications, with GI and pain symptoms as well as poor adherence to medicines | To describe a pharmacist-led, patient-centred approach to deprescribing in a 73-year-old diabetic man taking multiple medication, with GI and pain symptoms as well as poor adherence to medicines |
| O’Brien176 | 2011 | Canada | Withdrawing medication managing medical comorbidities near the end of life | Case report | A 67-year-old woman with a long smoking history, presenting with dyspnoea, cough with haemoptysis, fatigue, and weight loss, as well as low back and left hip pain | To discuss withdrawing medication in a patient with multimorbidity near the end of life |
| Ouellet et al.177 | 2018 | USA | Principle of rational prescribing and deprescribing in older adults with multiple chronic conditions | Literature review | n/a | To provide a reasoned approach to medication prescribing and deprescribing decisions for older adults with multiple chronic conditions, which aims to achieve clinical outcomes that matter most to each individual patient |
| Page et al.178 | 2016 | Australia | Deprescribing in older people | Narrative literature review | Literature on deprescribing | To describe the genesis of deprescribing as an increasingly accepted medical and pharmaceutical intervention. It also provides an overview of deprescribing |
| Palagyi et al.179 | 2016 | Australia | Barricades and brickwalls – a qualitative study exploring perceptions of medication use and deprescribing in long-term care | Qualitative focus group and interview study | GPs, staff members, residents and their relatives within LTCFs | To report the perceptions of medication use and the concept of deprescribing for LTCF residents, as identified by the RELEASE study participants. The application of these findings in informing the development of deprescribing initiatives within the aged care sector is discussed. RELEASE aims to improve understanding of the attitudes towards medication reduction held by the frail elderly in residential care |
| Petersen et al.79 | 2018 | USA | Shed-MEDS: pilot of a patient-centred deprescribing framework reduces medications in hospitalised older adults being transferred to inpatient post-acute care | Cross-sectional study | 40 Medicare-eligible, hospitalised patients with at least five prescribed medications | To describe a hospital-based, patient-centred deprescribing protocol (Shed-MEDS) and report pilot results |
| Pitkälä et al.181 | 2016 | Finland | Herbal medications and other dietary supplements. A clinical review for physicians caring for older people | Literature review | Literature regarding older people’s use of dietary supplements with special reference to polypharmacy | To conduct a literature review on clinical considerations associated with dietary supplement use, focusing on benefits and harms, motivations for use and contribution to polypharmacy among older people |
| Le Couteur et al.182 | 2016 | Australia | Polypharmacy in older people: when should you deprescribe? | Opinion piece/commentary | n/a | To describe the challenges of managing multimorbidity and polypharmacy and present an individualised, person-centred approach that takes into account multimorbidity |
| Pruskowski and Handler183 | 2017 | USA | The DE-PHARM Project: a pharmacist-driven deprescribing initiative in a nursing facility | Quality improvement project | Residents in a nursing facility | To reduce the number of PIMs via accepted recommendations from the clinical pharmacist to the primary team |
| Reeve et al.184 | 2014 | Australia | Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process | Literature review | n/a | To describe the development of a patient-centred deprescribing process |
| Reeve et al.185 | 2015 | Australia | Barriers to optimising prescribing and deprescribing in older adults with dementia: a narrative review | Narrative review of the literature | Literature on optimising medications in older adults with dementia | To explore barriers to optimising prescribing and deprescribing of medication as the goal of care shifts from prolonging life to optimising quality of life |
| Reeve et al.6 | 2018 | UK | Identifying enablers and barriers to individually tailored prescribing: a survey of healthcare professionals in the UK | Survey | 419 health professionals across the UK | To examine health professionals’ perceptions of enablers and barriers to delivering individually tailored prescribing |
| Rieckert et al.186 | 2018 | Germany | Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA-eDS study): a qualitative study of practical implementation in primary care | Qualitative interview study | General practitioners belonging to the intervention group of the PRIMA-eDS study | To examine how GPs experienced the use of the PRIMA-eDS tool, how GPs adopted the recommendations provided by the CMR and explores GPs’ ideas on the future implementation of the tool |
| Rigby187 | 2013 | Australia | Interview crucial to HMR success | Opinion piece/commentary | n/a | To discuss the importance of interviews in home medications review |
| Rodriguez Perez180 | 2015 | Spain | Deprescribing in patients with multimorbidity: a necessary process | Opinion piece/commentary | n/a | To discuss the importance of deprescribing in patients with multimorbidity |
| Rose et al.188 | 2019 | Germany | Patient selection and general practitioners’ perception of collaboration in medication review | Qualitative interview study | GPs | To gain information on patient selection for a medication review by GPs. GP selection was compared with objective selection criteria on identifying patients who would benefit from a medication review the most. A secondary objective of this study was to get insight into GPs’ perceptions on interprofessional collaboration with pharmacists |
| Ross and Gillett189 | 2020 | Canada | Confronting medicine’s dichotomies: older adults’ use of interpretative repertoires in negotiating the paradoxes of polypharmacy and deprescribing | Qualitative interview study | Older adults aged > 70 years taking part in the TAPER trial | To identify the medication paradoxes experienced by older adults taking multiple medications and describe the work that older adults do to bring them to resolution |
| Ross and Gillet190 | 2020 | Canada | ‘At 80 I know myself’: embodied learning and older adults’ experiences of polypharmacy and perceptions of deprescribing | Qualitative interview study | Older adults aged > 70 years taking part in the TAPER trial | To examine the forms of expertise that inform older adults’ decisions about how to use medications given concerns over polypharmacy and a clinical focus on deprescribing |
| Ross and Gillett191 | 2020 | Canada | Forms of trust and polypharmacy among older adults | Qualitative interview study | Older adults aged > 70 years taking part in the TAPER trial | To examine how older adults make decisions about their medications through interconnected axes of trust that operate across social networks |
| Ryan and Hill192 | 2016 | Australia | Making rational choices about how best to support consumers’ use of medicines: a perspective review | Literature review | n/a | To present perspectives on how to support consumers’ use of medicines |
| Schäfer et al.193 | 2017 | Germany | Narrative medicine-based intervention in primary care to reduce polypharmacy: results from the cluster-randomised controlled trial MultiCare AGENDA | Two-arm cluster RCT | 604 patients aged 65–84 years with at least three chronic conditions in general practice | To determine if patient-centred communication leads to a reduction in the number of medications taken without reducing health-related quality of life |
| Schöpf et al.194 | 2018 | Germany | Elderly patients’ and GPs’ perspectives of patient–GP communication concerning polypharmacy: a qualitative interview study | Qualitative interview study | Patients aged ≥ 65 years with polypharmacy (five or more medications) and their GPs in a German primary health-care centre | To explore elderly patients’ and GPs’ perceptions of communication about polypharmacy, medication safety and approaches for empowerment |
| Schuling et al.195 | 2012 | The Netherlands | Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study | Qualitative focus group study | GPs with a minimum of five years’ experience and active as GP trainers | To explore how experienced GPs feel about deprescribing medication in older patients with multimorbidity and to what extent they involve patients in these decisions |
| Scott et al.196 | 2013 | Australia | Deciding when to stop: towards evidence-based deprescribing of drugs in older populations | Opinion piece/commentary | n/a | To describe the evidence base for a structured approach to deprescribing and explore the barriers that exist in routine practice |
| Sheppard et al.197 | 2018 | UK | OPtimising Treatment for MIld Systolic hypertension in the Elderly (OPTiMISE): protocol for a randomised controlled non-inferiority trial | Protocol for a randomised controlled non-inferiority trial | Participants aged ≥ 80 years, with systolic blood pressure < 150 mmHg and receiving two or more antihypertensive medications | To examine whether or not antihypertensive medication reduction is possible in older patients without significant changes in blood pressure control at follow-up |
| Sinnige et al.198 | 2016 | The Netherlands | Medication management strategy for older people with polypharmacy in general practice: a qualitative study on prescribing behaviour in primary care | Qualitative focus group study | Dutch GPs | To gain insight into GPs’ medication management strategies for patients with polypharmacy, and to explore the GPs’ perspectives and needs on decision-making support to facilitate this medication management |
| Sinnott et al.199 | 2015 | Ireland | What to give the patient who has everything? A qualitative study of prescribing for multimorbidity in primary care | Qualitative interview study | Irish GPs | To explore how GPs make decisions when prescribing for multimorbid patients, with a view to informing intervention design |
| Sinnott et al.200 | 2015 | Ireland | Improving medication management in multimorbidity: development of the MultimorbiditY COllaborative Medication Review And Decision-making (MY COMRADE) intervention using the Behaviour Change Wheel | Development of a medication review decision-making tool; systematic review and qualitative study with GPs | GPs | To develop an intervention to improve medication management in multimorbidity by GPs, within the overarching UK Medical Research Council guidance on complex interventions99 |
| Sinnott et al.201 | 2017 | Ireland | Improving medication management for patients with multimorbidity in primary care: a qualitative feasibility study of the MY COMRADE implementation intervention | Non-randomised feasibility study using a qualitative framework approach | GPs attending CPD in south-west Ireland | To assess the feasibility and acceptability of MY COMRADE by GPs |
| St Peter202 | 2015 | USA | Management of polypharmacy in dialysis patients | Opinion piece/commentary | n/a | To discuss the management of polypharmacy in dialysis patients |
| Steinman and Hanlon203 | 2010 | USA | Managing medications in clinically complex elders: ‘there’s got to be a happy medium’ | Case study | 84-year-old man with dementia with a history of atrial fibrillation, diabetes mellitus, hypertension, hyperlipidaemia, chronic kidney disease (estimated creatinine clearance of 42 ml/minute), and gastritis and gastro-oesophageal reflux disease | To describe a typical case of an older patient taking multiple medications and summarise the evidence-based literature about improving medication use and withdrawing specific drugs and drug classes. To present a systematic approach for how health professionals can assess and improve medication regimens |
| Straßner et al.204 | 2018 | Germany | German healthcare professionals’ perspective on implementing recommendations about polypharmacy in general practice: a qualitative study | Qualitative interview and focus group study | 24 GPs, four other medical specialists, one pharmacist, three nurses and six medical assistants as well as two mixed focus groups with 17 professionals | To identify determinants (hindering and facilitating factors) for the implementation of the recommendations in general practice |
| Sun et al.205 | 2019 | Canada | Exploration of home care nurse’s experiences in deprescribing of medications: a qualitative descriptive study | Qualitative focus group study | 11 home care nurses | To explore the barriers to and enablers of deprescribing from the perspective of home care nurses, as well as to conduct a scalability assessment of an educational plan to address the learning needs of home care nurses about deprescribing |
| Thomas and Killbey206 | 2011 | UK | Complex medicines management | Pilot project of multidisciplinary reviews for patients with complex needs | Four patients with complex needs | To improve the quality of care for patients receiving multiple prescribed medicines for one or more long-term condition, using a holistic, evidence-based approach |
| Townsend et al.207 | 2003 | UK | Managing multiple morbidity in mid-life: a qualitative study of attitudes to drug use | Qualitative interview study | 23 men and women aged about 50 years with four or more chronic illnesses | To examine attitudes towards drug use among middle aged respondents with high levels of chronic morbidity |
| Turner et al.208 | 2016 | Australia | What factors are important for deprescribing in Australian long-term care facilities? Perspectives of residents and health professionals | Qualitative research using nominal group technique | 11 residents/representatives, 19 GPs, 12 nurses and 14 pharmacists participated across six separate groups | To use NGT to generate then rank factors that GPs, nurses, pharmacists and residents or their representatives perceive are most important when deciding whether or not to deprescribe medications |
| Turner et al.209 | 2017 | Australia | Is my older cancer patient on too many medications? | Commentary/opinion piece | n/a | To present a six-step process for deprescribing in older patients with cancer |
| Twigg et al.210 | 2017 | UK | The UK Pharmacy Care Plan service: description, recruitment and initial views on a new community pharmacy intervention | Mixed methods using questionnaires and interviews | Pharmacists and patients | To describe the initial findings from the set up and delivery of a novel community pharmacy-based person-centred service |
| Uhl et al.211 | 2018 | Germany | Patient-perceived barriers and facilitators to the implementation of a medication review in primary care: a qualitative thematic analysis | Qualitative interview study | 31 patients (age ≥ 60 years, three or more chronic diseases, taking five or more drugs) | To gain insight into patient-perceived barriers to and facilitators of the implementation of medication review |
| van Middelaar et al.212 | 2018 | The Netherlands | Prescribing and deprescribing antihypertensive medication in older people by Dutch general practitioners: a qualitative study | Qualitative interview study | 15 GPs | To explore GPs’ routines and considerations on (de)prescribing AHM in older patients, their judgement on usability of the current guideline and needs for future support |
| van Summeren et al.84 | 2017 | The Netherlands | Outcome prioritisation tool for medication review in older patients with multimorbidity: a pilot study in general practice | Mixed-methods descriptive study | Older patients with multimorbidity (aged ≥ 69 years) with polypharmacy (five or more chronic medications) from the practices of 14 GPs | To determine proposed and observed medication changes when using an OPT during a medication review in general practice |
| Vandermause et al.213 | 2016 | USA | Preserving self: medication-taking practices and preferences of older adults with multiple chronic medical conditions | Qualitative study using interviews and assessment of diaries | 27 participants with multiple chronic conditions | To examine the experiences of older adults with multiple chronic medical conditions when a new medication was added to their existing multiple medication regimen |
| Voigt et al.214 | 2016 | Germany | Why do family doctors prescribe potentially inappropriate medication to elderly patients? | Mixed methods using 10 semistandardised content analyses of patients’ records, qualitative interviews with FPs using open questions and selected patient-specific case vignettes, and qualitative interviews with FPs’ medical assistants | Patients and FPs | To give an overview of rates of PIM prescription in the study sample of elderly multimorbid patients with polymedication in the outpatient primary care setting; to explain influencing factors on prescription of PIM; to examine knowledge and application of PRISCUS; and to understand FPs’ reasons for prescription of PIM |
| Waller et al.215 | 2005 | UK | Rational prescribing: the principles of drug selection and assessment of efficacy | Opinion piece/commentary | n/a | To provide an overview of rational prescribing |
| Weir et al.218 | 2018 | Australia | Decision-making preferences and deprescribing: perspectives of older adults and companions about their medicines | Qualitative interview study | 30 older people (aged > 75 years taking multiple medicines) and 15 companions | To explore decision-making about polypharmacy with older adults and their companions |
| Wilchesky et al.216 | 2018 | Canada | The OptimaMed intervention to reduce inappropriate medications in nursing home residents with severe dementia: results from a quasi-experimental feasibility pilot study | Quasi-experimental feasibility pilot study | 44 participating residents aged ≥ 65 years with severe dementia in three nursing homes in Quebec City, Canada | To test the feasibility of an interdisciplinary knowledge exchange intervention using a medication review guidance tool categorising medications as either ‘generally’, ‘sometimes’ or ‘exceptionally’ appropriate for nursing home residents with severe dementia |
| Williams et al.217 | 2004 | USA | The short-term effect of interdisciplinary medication review on function and cost in ambulatory elderly people | A RCT | Community-dwelling older adults taking five or more medications were assessed at baseline and 6 weeks. A medication-change intervention group of 57 elders was compared with a control group of 76 elder adults | To determine whether or not a medication review by a specialised team would promote regimen changes in elders taking multiple medications, and to measure the effect of regimen changes on monthly cost and functioning |
| Zechman et al.85 | 2019 | Switzerland | Barriers and enablers for deprescribing among older, multimorbid patients with polypharmacy: an explorative study from Switzerland | Mixed-methods interview study | Patients of a cluster-randomised study in northern Switzerland | To explore attitudes, beliefs, and concerns towards deprescribing among older, multimorbid patients with polypharmacy who chose not to pursue at least one of their GP’s offers to deprescribe |
Glossary
- Context
- The setting within which programmes and research are implemented. Examples of context can include the cultural norms and history of a community, social networks, programme infrastructure, geographic location effects, opportunities and constraints. Context can be broadly understood as any condition that triggers behavioural or emotional responses (mechanisms) in individuals affected.
- Context–mechanism–outcome configuration
- A statement, diagram or drawing that illustrates the relationship between particular features of contexts, mechanisms and outcomes.
- Mechanism
- Can be described as the underlying entities, processes or structures that are triggered by a particular context and cause outcome(s). Can be understood as being the way in which individuals respond to and reason about resources and opportunities offered by a programme, intervention or process.
- Outcome
- The impact or behaviours that result from the interaction between contexts and mechanisms.
- Programme theory
- A set of theoretical explanations or assumptions about how a programme, process or intervention is expected to work.
List of abbreviations
- AAG
- academic advisory group
- CDSS
- clinical decision support system
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CMOC
- context–mechanism–outcome configuration
- GP
- general practitioner
- MediMoL
- Medication Monitoring List
- MOOC
- massive online open course
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PICO
- population, intervention, context, outcome
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROGRESS+
- PROGnosis RESearch Strategy partneship+
- RCT
- randomised controlled trial
- SHiM
- Structured History taking of Medication use
- START
- Screening Tool to Alert doctors to the Right Treatment
- STOPP
- Screening Tool of Older Person’s Prescriptions
- TIDieR
- Template for Intervention Description and Replication
Notes
-
Outcomes and impact of deprescribing intervention by type (scoping review)
-
Detailing CMOCs developed to support programme theory refinement
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/AAFO2475).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.