Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/141/01. The contractual start date was in September 2009. The draft report began editorial review in September 2015 and was accepted for publication in February 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Robert Freeman reports personal fees from speaker fees for Astellas and Pfizer, outside the submitted work. John Norrie reports non-financial support from Health Technology Assessment (HTA) Commissioning Board and personal fees from the National Institute for Health Research (NIHR) HTA and Efficacy and Mechanism Evaluation (EME) Editorial Board, outside the submitted work. He is a member of the NIHR Journals Library Editorial Group. Andrew Elders reports a grant from the NIHR HTA programme during the conduct of the study; his institution (Glasgow Caledonian University) is due to receive payment for statistical analysis from the University of Aberdeen using funds from their NIHR grant.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Glazener et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
The PROSPECT Study (PROlapse Surgery: Pragmatic Evaluation and randomised Controlled Trials)
In 2009, the UK government National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme funded the PROSPECT Study. This monograph describes the research.
PROSPECT was a major multicentre UK randomised controlled trial (RCT) investigating the effectiveness (including safety) and cost-effectiveness of surgical treatment, primarily in terms of improvement in prolapse symptoms, in women who were having a primary or a secondary prolapse repair.
Description of the underlying health problem
Pelvic organ prolapse is the descent from its normal anatomical position of one or more of the female genital organs. Pelvic organ prolapse is caused by herniation through deficient pelvic fascia or due to weaknesses or deficiencies in the ligaments or muscles that should support the pelvic organs. There is little epidemiological research into this condition because it has a variety of presentations and they do not all cause symptoms, particularly in the early stages. 1 Commonly reported symptoms include a feeling of dragging or heaviness in the vagina, uncomfortable bulge distending the introitus, urinary symptoms (urgency and voiding difficulty), bowel symptoms, such as incomplete emptying, and sexual dysfunction.
Prevalence and natural history
Estimates of the prevalence of prolapse vary from 41% to 50% of women aged > 40 years. 2,3
It has been estimated that women have a lifetime risk of 11% of undergoing surgery for urinary incontinence (UI) or prolapse and 7% for prolapse alone. 4 The annual incidence of surgery for pelvic organ prolapse is within the range of 15–49 cases per 10,000 women-years, and it is likely to double in the next 30 years. 1,5 Little is known about the prevalence and effectiveness of different types of operations but they are notoriously prone to failure: around 30% of women undergo further operations; the mean time interval between the first and a subsequent procedure is about 12 years, and the time interval between subsequent procedures decreases with each successive repair. 4
Gynaecologists have recognised for some time that both anatomical failure of supporting pelvic structures and recurrence of prolapse after surgery are common. More recently, it has also been recognised that surgery can be followed by a greater impairment of quality of life (QoL) than the original prolapse itself (e.g. new UI after surgery). In addition, repair of one type of prolapse may predispose the women to the development of a different type of prolapse (a new or de novo prolapse) in another compartment of the vagina due to alteration in the dynamic forces within the pelvis. 4
Significance in terms of ill health and use of NHS resources
Surgery is common. In England and Wales in 2004–5, 26,947 women were admitted to hospital with a main diagnosis of female genital prolapse, and 28,297 operations were performed (some women had more than one type of prolapse operation). 6 The majority of the operations (93%) were undertaken in women who were having anterior repair (n = 8560), posterior repair (n = 5406) or both operations (n = 5654), or with a concomitant uterine prolapse (n = 6837). Only 7% were in women with vault prolapse (n = 1840). Assuming a population of about 20 million women in the age group at risk for prolapse surgery (50–85 years), the UK operation rate is currently around 14–16 women who were having prolapse operations per 10,000 per year. 6,7
The need is likely to increase because of the rising number of elderly women. It has been projected that the number of women in the age group 50–85 years (those most likely to need prolapse surgery) will increase by 1.44 million between 2012 and 2020. 6
Description of standard management
Women with prolapse may be managed conservatively with pelvic floor muscle training (PFMT) and pessaries, or with surgery. In addition they require management of associated conditions, for example lower urinary tract symptoms, such as UI or overactive bladder syndrome; bowel problems, such as constipation or faecal incontinence (FI), sexual dysfunction and oestrogen deficiency if postmenopausal.
Conservative management for women with prolapse
Although there is only one RCT to inform the use of mechanical devices (pessaries or rings), these are often used for women who are unfit for surgery or who wish to avoid surgery. They can be very efficacious, but questions remain about the best type of device, the long-term adverse effects and the use of supplementary treatment such as oestrogen. Further research is required. 8
Conservative physical treatments such as PFMT are also often recommended as first-line management. A recent update of the relevant Cochrane review9 has found some evidence supporting the use of PFMT to reduce prolapse symptoms and severity, as well as benefits for urinary and bowel symptoms.
In addition, vaginal oestrogen treatment can be used to reduce symptoms for postmenopausal women, before or after surgery. The evidence supporting its use is limited and inconclusive. 10
Surgical management for women with anterior or posterior vaginal wall prolapse
The PROSPECT Study compared surgical operations for vaginal wall pelvic organ prolapse:
-
anterior vaginal wall prolapse (urethrocele, cystocele, paravaginal defect)
-
posterior vaginal wall prolapse (enterocele, rectocele, perineal deficiency).
A woman may present with prolapse of one or both of these sites, and she may be having a primary or a secondary procedure. She may also have a concomitant uterine or vault prolapse or stress UI that requires a continence procedure. For each of these sites there are several alternative traditional surgical techniques, none of which has been properly evaluated in adequately powered multicentre RCTs. Major potential adverse effects include infection, bleeding, mesh exposure and dyspareunia, as well as failure of repair and failure to cure symptoms.
The techniques for performing anterior or posterior repair or implanting mesh or graft can vary widely between gynaecologists. These include the following.
Standard anterior and/or posterior repair
In the standard approach, the vaginal skin is opened in the midline, the fascia is separated from the skin and the fascial defect is plicated (sutured or buttressed). Any redundant vaginal skin is excised and the skin is closed.
Standard repair with mesh inlay
Over the last 10 years, gynaecologists have begun to include small pieces of mesh inlays as an extra support to the fascial defects through which the pelvic organs prolapse, analogous to the use of mesh in hernia surgery. 11 If mesh is used, it can be positioned over or under the fascial defect as a ‘mesh inlay’ and sutured in place to reinforce the tissues.
The proposed advantage of using mesh is that it will optimise surgical outcome without compromising vaginal capacity or sexual function. 12 The rationale is that it may help to reduce failure rates from breakdown of weakened tissue or failure to identify all fascial defects. 13 Although the mesh materials used may be stronger than the woman’s own fascial tissue, the indications for use and choice of materials remain controversial. 13 The extent to which mesh inlays are currently used is unknown, but recent surveys suggest that many gynaecologists are already incorporating mesh into their practice both in the UK and in the USA. 14,15 The decision to use mesh is complicated by the different types available:
-
absorbable synthetic (e.g. polyglactin)
-
absorbable biological (e.g. fascia lata, porcine dermis)
-
combined or semi-absorbable (e.g. polyglactin and polypropylene) and
-
non-absorbable (e.g. polypropylene).
There are theoretical pros and cons to each, but there is not enough evidence available to allow rigorous comparison.
Mesh insertion using a trocar (introducer device): mesh kits
Some commercial manufacturers of mesh have introduced large mesh systems, analogous to the tension-free vaginal tape (TVT) slings used in incontinence surgery. 16 These commercial devices (‘mesh kits’) are available for anterior or posterior compartments, or can be used together for both. The mesh is inserted using a trocar (introducer device). This involves blind penetration of pelvic spaces by trocars in order to thread mesh tails into positions from which they support a central mesh layer or hammock, which supports and corrects the prolapse defect. Currently available devices use non-absorbable synthetic mesh, but kits using other types of mesh (combined) have also been used.
These have been actively promoted and introduced to clinical practice without first being evaluated in rigorous independently managed RCTs. These meshes are inserted blindly using introducer devices or trocars that may damage surrounding organs or blood vessels. 17 Prospective studies have suggested that the mesh devices have been used worldwide, but it is not clear whether this is driven by gynaecological preference or commercial marketing pressure. However, clearly some women have been willing to undergo this new technology despite lack of evidence for safety or efficacy.
Evidence for the use of mesh or graft in prolapse surgery
The most recent update of the Cochrane review of surgery18 for lower compartment prolapse concludes:
The use of mesh or graft inlays at the time of anterior vaginal wall repair reduces the risk of recurrent anterior wall prolapse on examination.
The authors further add:
Anterior vaginal polypropylene mesh also reduces awareness of prolapse; however these benefits must be weighed against increased operating time, blood loss, rate of apical or posterior compartment prolapse, de novo stress urinary incontinence, and reoperation rate for mesh exposures associated with the use of polypropylene mesh.
For posterior wall repairs, the Cochrane review18 concludes:
The evidence is not supportive of any grafts at the time of posterior vaginal repair.
Repeat surgery for recurrent prolapse
There were no data on the differential effects in women who were having primary as opposed to repeat (secondary) surgery: all of the trials reported both groups of women together despite their potentially different prognoses. There is, therefore, no evidence to suggest whether or not the use of mesh (particularly non-absorbable synthetic mesh, which has the strongest mechanical strength and remains in situ indefinitely) should be reserved for more complex or recurrent prolapse. Although gynaecologists have stated that this is their belief and practice, evidence suggests that the majority (70%) of the current recipients of mesh are having their first prolapse operation. 14
An Interventional Procedures (IP) review of 503 women and a further recent case series of 289 women drew attention to the high incidence of serious adverse effects (e.g. 2.8% with damage to surrounding organs) in women who were having mesh inserted with blind introducer devices (‘mesh kits’). 17,19 Our opinion was that until benefits and risks have been properly evaluated, mesh kits using non-absorbable synthetic mesh should be reserved for more complex cases of prolapse. Therefore, in PROSPECT we limited this option to women being treated for a recurrence of prolapse in the site where previous surgery had occurred.
Current recommendations from the National Institute for Health and Care Excellence
An IP review, conducted for the National Institute for Health and Care Excellence (NICE) in 2008, investigated the use of mesh for women who were having anterior and/or posterior vaginal wall prolapse repair. 19,20 The total number of women receiving mesh in this review was 4569: mesh was inserted using an introducer device, trocar or kit in 503 of these women. 19 The IP review also included additional data from non-randomised comparative studies and case series. Using these extra data, non-absorbable synthetic mesh had the lowest failure rate compared with:
-
absorbable synthetic mesh [odds ratio (OR) adjusted for bias from study design 0.23, 95% confidence interval (CI) 0.12 to 0.44]19
-
absorbable biological mesh (OR adjusted for bias from study design 0.37, 95% CI 0.23 to 0.59). 19
On the other hand, the mesh erosion (now termed ‘exposure’) rates increased from 1% (95% CI 0.1% to 4.0%) with synthetic absorbable mesh to 6% with absorbable biological mesh to 10% with non-absorbable synthetic mesh. 19 The data were too sparse, however, for other reliable statistical analysis. There were insufficient data on women’s subjective prolapse symptoms or complications, such as infection, blood loss or dyspareunia, and none on long-term outcomes. Particular safety worries were related to the use of introducer devices (trocars) that were used for the blind insertion of mesh into intrapelvic spaces. 17
These and other findings were presented to the Interventional Procedures Advisory Committee (IPAC) in January 2008 and their guidance has now been published. 21 The committee recommended that mesh should be used only under special arrangements for clinical governance, consent and audit or research: hence the PROSPECT Study was funded to fill the evidence gap.
Decision to test alternative forms of surgery
There is not enough evidence from RCTs to guide management for women with prolapse. Additionally, the Cochrane and the IP reviews conclude that there is insufficient information about any of the surgical options to guide management of any type of pelvic organ prolapse in any population of women with prolapse.
We identified that the largest group of women are those with anterior and/or posterior prolapse, who constitute around 90% of those having prolapse surgery (including those having a concomitant hysterectomy). The evidence underlying surgery for these women was clearly inadequate, with very little evidence regarding subjective prolapse symptoms, effect on QoL and safety.
Both the Cochrane and the IP reviews18,19 identified a need for adequately powered RCTs of the use of mesh in prolapse surgery. PROSPECT comprises the largest, adequately powered and independent RCT comparing traditional prolapse operations with new methods incorporating mesh as an inlay or mesh inserted using an introducer system (mesh kit).
Questions addressed by this study
Principal objectives
To determine the effectiveness (including safety) and cost-effectiveness of surgical treatment, primarily in terms of improvement in prolapse symptoms, in women who were having anterior and/or posterior vaginal wall pelvic organ prolapse surgery, separately in two trials:
-
In women who were having a primary prolapse repair, the effects of:
-
a standard repair versus a standard repair using a non-absorbable or combined mesh inlay and
-
a standard repair versus a standard repair using a biological graft inlay.
-
-
In women who were having a repeat prolapse repair, the effects of:
-
a standard repair versus a standard repair using a non-absorbable or combined mesh inlay and
-
a standard repair versus a mesh kit procedure.
-
The two groups are being considered independently because different surgical options are considered to be appropriate for clinical reasons.
Secondary objectives
-
To determine the differential effects on other outcomes, such as urinary, sexual and bowel function, QoL, general health, need for secondary surgery and adverse effects.
-
To identify possible effect modifiers (e.g. different types of mesh, concomitant procedures, age, complex prolapse types).
-
To establish if the findings of the research, including implications for service delivery, training and introduction of mesh, are generalisable to the UK NHS.
This study assessed which of the most frequently employed techniques for the most common types of prolapse (anterior and posterior vaginal wall prolapse) are most clinically effective and safe. The study also assessed cost-effectiveness. This will guide gynaecologists in their surgical practice and purchasers in their choice of provision of health care. Given the number of prolapse procedures currently performed (28,000 annually in the UK) and the anticipated rise in need for such surgery with an ageing population (a twofold increase in the age group at risk in the next 30 years is predicted), the potential cost implications for the health service are considerable. 6
Chapter 2 Methods and practical arrangements
Study design
PROSPECT comprised two RCTs within a comprehensive cohort (CC) study. It was designed to determine the effectiveness (including safety) and cost-effectiveness of surgical treatment, primarily in terms of improvement in prolapse symptoms, in women who were having anterior and/or posterior vaginal wall pelvic organ prolapse surgery. Women who were having a primary prolapse repair and those having a secondary prolapse repair were considered independently because different surgical options were deemed to be appropriate for clinical reasons. If a woman did not receive surgery then no follow-up questionnaires were issued.
Important changes to the design after trial commencement
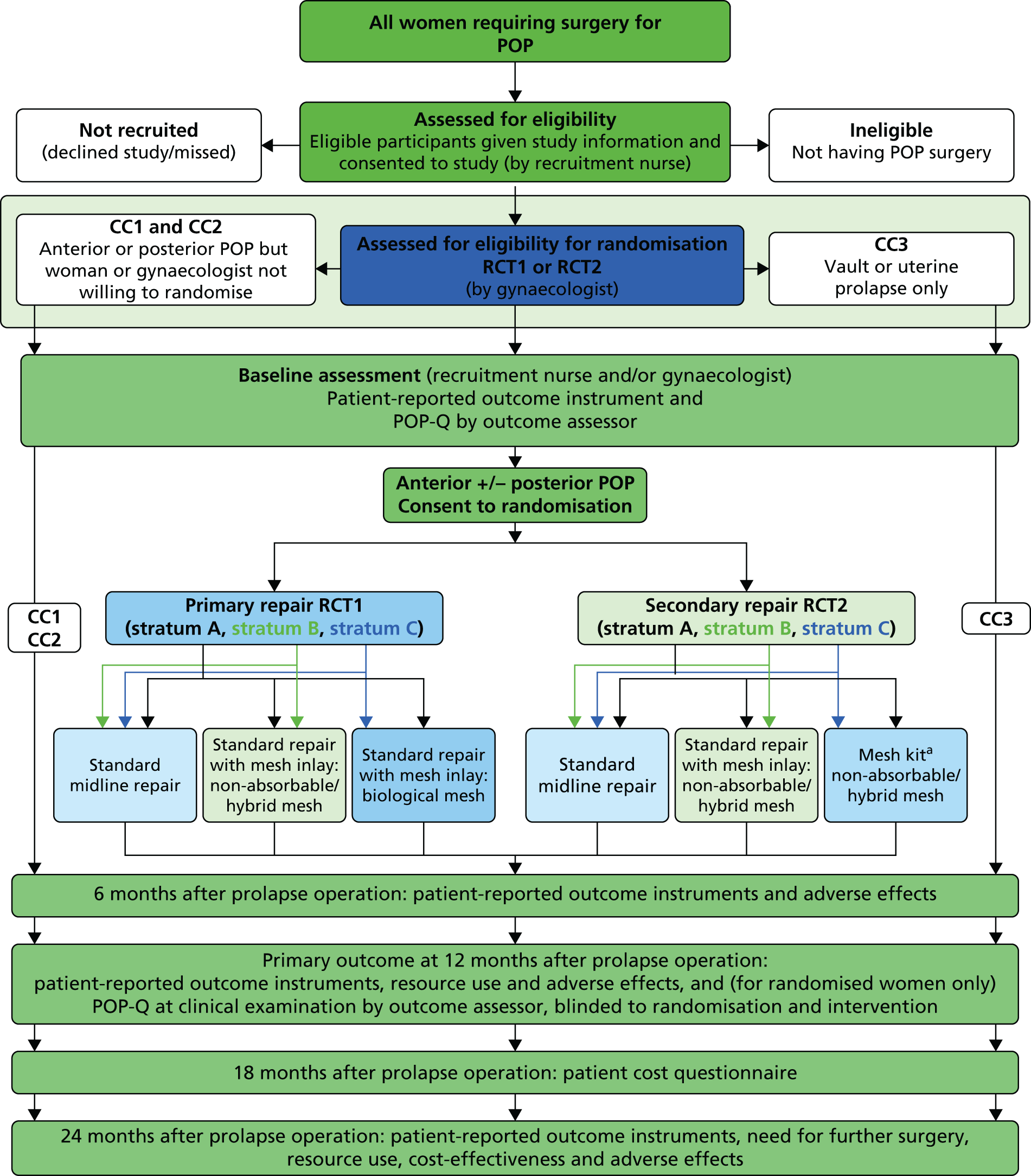
The recruitment rate to both the Primary and Secondary trials proved to be slow initially, partly because of the cost of sourcing all of the mesh types required for the study and lack of availability of certain mesh types, and partly because of some clinicians’ preference for one of the mesh types. Therefore, with the agreement of the Trial Steering Committee (TSC) and Data Monitoring Committee (DMC), a decision was made in 2010 to allow surgeons to randomise between no mesh and only one of the mesh options, creating three randomisation strata in both trials. The study design showing the comparisons options available to surgeons is shown in the flow diagram in Figure 1.
FIGURE 1.
Flow diagram of study design. a, Only gynaecologists trained in the use of mesh kits will randomise women to this option. POP, Pelvic Organ Prolapse.
Clinical centres
Both specialist urogynaecologists and general gynaecologists were eligible to take part if they had extensive experience and training in urogynaecological reconstructive surgery. To participate they had to be prepared to allow treatment allocation to be decided at random for at least a proportion of their patients: the remainder could be entered into the CC study if the patient agreed. Before participating in the trial, the surgeons had to formally choose to which comparisons they were willing to contribute.
Study population
All women under the care of a collaborating surgeon were potentially eligible for inclusion if a decision had been made to have primary or secondary pelvic organ prolapse surgery for anterior and/or posterior vaginal wall prolapse. Women undergoing concurrent hysterectomy/cervical amputation, vault surgery or continence procedures were also eligible. Only women who were unable or unwilling to give competent informed consent, or who were unable to complete study questionnaires, were deemed ineligible.
Two parallel but separate trials were conducted: one among women who were having a primary prolapse repair and the other in women who were having a secondary prolapse repair. For the purposes of PROSPECT, a secondary prolapse was defined as a recurrence of prolapse after a primary procedure, when the recurrence was in the same compartment. If the prolapse was in a different compartment and the original site did not require revision surgery, the woman was classed as having a primary repair of a de novo prolapse.
In addition, women who were unwilling or unsuitable for randomisation were eligible to be followed up using the same protocol as part of the CC study. This included women who were having uterine or vault surgery only.
Consent to participate
All women who required pelvic organ prolapse surgery were identified by a dedicated recruitment officer (RO) in each centre. A log was maintained of all of the women meeting the eligibility criterion (admission for prolapse surgery), describing reasons if they did not agree to enter the study or be randomised (see Appendix 1). Every woman was allocated a unique study number.
Every eligible woman was given a flyer containing a brief summary of the study when attending the initial clinic appointment (the fliers are reproduced in Appendix 1). The women were then given the patient information leaflet (PIL; see Appendix 1) with their admission documents (which could be during the initial clinic appointment or by separate mail, if the woman agreed). Women were given the opportunity to discuss all aspects of the study with their general practitioner (GP) and/or family members before admission, their gynaecologist, the RO, staff at preadmission clinics and/or when admitted to hospital. In addition, all documentation contained the PROSPECT Study office contact details to enable women to obtain information from the study organisers. Signed consent was obtained from each woman to participate (and, if suitable, to be randomised) and followed up after her prolapse surgery by questionnaires and an examination in Gynaecology Outpatients (the latter in randomised women only). The PIL and the consent form (see Appendix 1) both refer to the possibility of long-term follow-up, being contacted about other prolapse research and access to their NHS records for these purposes. A letter and GP information sheet were also sent to the woman’s GP (see Appendix 2). A copy of the consent form, together with a summary of the study, was filed in the woman’s hospital notes.
Women who did not wish to be randomised, or who were not suitable for randomisation, were still eligible to be followed up using the same study protocol as part of the CC study. They completed all of the study procedures and documents including follow-up, except for the clinical examination at 12 months.
Women who initially agreed to enter the study but later decided to withdraw or became unable to continue were asked for verbal consent to enable us to retain their existing data and access relevant NHS data. Women who did not agree to participate in the study (randomised or cohort) were logged anonymously along with a minimum data set of age and type of prolapse (anterior, posterior, uterine, vault; primary or secondary procedure) (see Appendix 1).
Health technologies being compared
Women were randomised to an intervention according to their surgical history (previous prolapse repair or not), the availability of the mesh (non-absorbable, biological and/or mesh kits) and the skill capacity of their operating gynaecologist (trained in mesh kit use or not). The study design is shown in the flow diagram in Figure 1.
If one of the mesh types was temporarily or permanently unavailable (owing to financial constraints) then the women could be randomised to one of the other two arms.
In addition, the expectation was that mesh kits would normally be used only for women who had been randomised to this option. If the operating gynaecologist was not trained in the use of mesh kits then the women under their care could be randomised to one of the other two arms only. Furthermore, in view of the scarcity of data about their safety and efficacy, mesh kits were used only for women who were having a secondary procedure, who have a more complex prolapse problem.
Therefore, women who were having a primary repair were randomised to:
-
standard anterior and/or posterior repair (with native tissue only) (reference technique)
-
standard anterior and/or posterior repair with a synthetic non-absorbable or hybrid mesh inlay or
-
standard anterior and/or posterior repair with biological graft inlay.
Women who were having a secondary repair were randomised to:
-
standard anterior and/or posterior repair (with native tissue only) (reference technique)
-
standard anterior and/or posterior repair with a synthetic non-absorbable or hybrid mesh inlay or
-
a mesh kit (using an introducer device/trochar) with a non-absorbable or hybrid mesh.
Treatment allocation
After entering contact details, essential baseline information and confirmation of signed consent into the internet-based PROSPECT database, the local researcher was able to randomise the woman (if appropriate) to one of the arms for which she was eligible. Randomisation was carried out as close to the time of surgery as was practical, taking into account the hospital routines and time needed for setting up the operating theatre.
Randomisation utilised the existing proven remote automated computer randomisation application at the study administrative centre in the Centre for Healthcare Randomised Trials [CHaRT, a fully registered UK Clinical Research Network clinical trials unit] in the Health Services Research Unit (HSRU), University of Aberdeen. This randomisation application was available only as an internet-based service.
Randomisation was computer allocated and stratified depending on whether a woman was having a primary or secondary repair. If not eligible for randomisation, the woman was allocated to the CC.
Primary prolapse (de novo) was defined as a prolapse in a compartment that had not been previously repaired. If the woman was having two primary procedures (i.e. both anterior and posterior vaginal wall prolapses required repair) then the randomised allocation applied to both prolapse repairs.
Secondary prolapse was defined as a recurrence of prolapse after a previous procedure, when the recurrence was in the same compartment. If the woman also required a concomitant primary repair of a de novo prolapse in a different compartment, this procedure was chosen on clinical grounds/surgeon choice (i.e. not dictated by the randomisation allocation for the secondary procedure).
If the new prolapse was in a different compartment (de novo) and the original site did not require revision surgery, the woman was classed as having a primary repair of the de novo prolapse and randomised in the Primary trial.
The allocation was computer-generated in ratios of 1 : 1 : 1 for the Primary trial and 1 : 1 : 2 for the Secondary trial. Randomisation was unbalanced in the Secondary trial in favour of mesh kits to account for the skill set of the available surgeons (not all surgeons would be trained in their use). Allocation was further minimised according to:
-
the woman’s age (< 60 years or ≥ 60 years)
-
type of prolapse being randomised (anterior, posterior or both)
-
need for a concomitant continence procedure (e.g. TVT) or not
-
need for a concomitant upper vaginal prolapse procedure (e.g. hysterectomy, cervical amputation, vault repair) or not and
-
surgeon.
Clinical management
Within the randomised comparisons, surgeons could use any mesh, graft or mesh kit, providing that any synthetic mesh was monofilament macroporous polypropylene and mesh inlays were secured with peripheral sutures. Surgeons used their mesh material of choice and followed their standard practice so that the technique that they normally used was not modified for the purposes of the trial. All of the other aspects of care were left to the discretion of the responsible surgeon. Each surgeon was asked to complete a surgical standardisation form (see Appendix 3) so that their preferred method of surgical repair could be recorded.
We did suggest, however, that the mesh or graft should be inserted under the fascial layer and secured at five points around the periphery of the inlay. If they did so, or wished to secure the inlay in another way (e.g. attach the inlay to the white line), we asked them to record the method used in the surgical standardisation form, but did not obtain information on whether or not this was actually done for individual participants.
Data collection and processing
Participant-reported outcomes were assessed by self-completed questionnaires at baseline (before surgery; see Appendix 4) and self-completed postal questionnaires at 6, 12, 18 (Participant Cost Questionnaire only) and 24 months following surgery (see Appendix 4). Where participants ticked more than one box for each question, we recorded this using the worst-case scenario. For randomised women, following one postal reminder, participants who had not returned the questionnaire were telephoned and offered the option of completing the questionnaire over the telephone. For cohort women, only a second postal reminder was issued. A number of other measures were taken to promote ongoing interest in, and commitment to, the trial, including participant newsletters and annual Christmas cards (both randomised and CC women, and collaborators at the clinical centres).
The study-specific questionnaires also included questions about care in general practice, physiotherapy and outpatient consultations related to their prolapse, as well as any complications, readmissions, reoperations and costs. Reported hospital readmissions and complications were confirmed with the clinical centre when required.
Intraoperative and postoperative data were collected by the gynaecologists, supported by ROs. This involved completing a questionnaire (see Appendix 3) at the time of surgery, providing details of the operative procedures, complications and resource use, and a short clinical questionnaire (see Appendix 3) at the 12-month outpatient review appointment, including a Pelvic Organ Prolapse Quantification (POP-Q) measurement (only randomised women).
Study outcome measures
We identified three primary outcome measures.
-
Women’s symptoms of prolapse were measured using the patient-reported Pelvic Organ Prolapse Symptom Score (POP-SS)22 at 12 months after surgery. This scale was derived from the seven questions that were judged to be most directly related to prolapse symptoms (see Appendix 4) and has been shown to reflect the range and intensity of symptoms experienced by women, as well as being responsive to change over time. 23,24 Scores were determined for each of the seven items (ranging from 0 for ‘never’ to 4 for ‘all of the time’) with an overall POP-SS out of 28. Participants who only partially completed the seven-item response schedule were assumed to have no symptoms, when no response had been given to any individual items. Women were considered to be symptomatic if their overall score was > 0.
-
QoL (condition-specific) was measured as the woman’s rating of the overall effect of prolapse symptoms on everyday life on a 0–10 visual analogue scale (VAS), for which 10 is worst.
-
The primary economic outcome measure of cost-effectiveness was the incremental cost per quality-adjusted life-years (QALYs), based on the EuroQol-5 Dimensions, 3-level version (EQ-5D-3L). 25
Other outcome measures included objective prolapse measurement; urinary, bowel and sexual symptoms [using the International Consultation on Incontinence (ICI) suite of validated questionnaires];26 intraoperative and postoperative complications, including the need for additional surgery (repeat surgery for prolapse recurrence or incontinence, and surgery required for adverse effects); cost; and cost-effectiveness.
Objective prolapse measurement
We intended that, at baseline and (for randomised women) at 12 months after surgery, women would have objective measurements of their prolapse compartments. Objective prolapse staging was carried out using the POP-Q system. 27 This measures the maximum descent of each of the three prolapse compartments (anterior, posterior and upper) relative to the hymen (at 0 cm): measurements inside the vagina are negative, whereas those outside are positive. A measure of prolapse (classified from stage 0 to 4) was determined for anterior, posterior and uterine/vault, with the leading edge of the most descended compartment used to define overall stage. An algorithm was used to ensure that POP-Q staging was correctly calculated from the component measurements of the POP-Q [Aa, Ba, C, D, Bp, Ap and total vaginal length (TVL)] in which common recording errors (e.g. Ba measurement less than Aa) were corrected or queried. If data were discrepant, they were corrected by consultation with the local hospital records to obtain additional data. If POP-Q data were missing, we accepted the surgeon’s qualitative record of stage, both overall and in individual compartments (i.e. surgeons could specify the stage without giving the POP-Q measurements).
Usually, using the classic Bump et al. 27 criteria for the POP-Q system, any measurement from –1 cm (inside the hymen) to 1 cm outside counts as stage 2. However, we further subdivided stage 2 into prolapse at the hymen or within (–1 cm to 0 cm; stage 2a or less) compared with prolapse at > 0 cm (stage 2b). 28,29 Thus, women were classified as having objective prolapse if the leading edge was at any point outside the hymen (measured at > 0 cm, stage 2b, 3 or 4).
Urinary, bowel and sexual symptoms
Symptoms related to other aspects of pelvic floor dysfunction were measured using the ICI suite of validated questionnaires. 26
Urinary incontinence was assessed using the International Consultation on Incontinence-Urinary Incontinence Short Form (ICIQ-UI-SF). Other urinary symptoms were recorded by the ICIQ-Female Lower Urinary Tract Symptoms (ICIQ-FLUTS). The latter provides subscales for filling, voiding and incontinence symptoms.
The International Consultation on Incontinence Questionnaire (ICIQ)-Bowel Symptom was not finalised when we began PROSPECT. We therefore adapted draft questions to produce a short summary of relevant bowel symptoms. We used similar questions to map on to the ROME criteria to define constipation (Table 1). 30
| ROME criteria – any two of: | Equivalent PROSPECT questions |
|---|---|
| Fewer than three bowel movements per week | Stool passing once a week or less |
| Straining | Straining most or all of the time |
| Lumpy or hard stools | Hard stools |
| Sensation of incomplete defecation | Feeling that bowel has not completely emptied most or all of the time |
| Manual manoeuvring required to defecate | Manual manoeuvre to empty bowel most or all of the time (splinting of perineum or vagina, or digital evacuation of the bowel) |
| Sensation of anorectal obstruction | No equivalent PROSPECT question |
We assessed vaginal and sexual symptoms using the International Consultation on Incontinence Questionnaire-Vaginal Symptoms (ICIQ-VS). The ICIQ-VS provides a brief and robust measure to assess the impact of vaginal symptoms and associated sexual matters on QoL and outcome of treatment. It provides subscales for vaginal symptoms, sexual matters and the overall impact of vaginal symptoms on QoL. Women were asked if they were sexually active and, if not, whether or not this was because of their vaginal or prolapse symptoms, or for another reason, including no partner. Women’s responses to this question were post-coded to ensure reliability and consistency. Data were included in the analysis of sexual outcomes for women who were sexually active or for women who were sexually inactive because of prolapse symptoms.
Safety reporting
Adverse effects were notified to the study office in a variety of ways. They could be recorded by the centre staff using the recruitment officer case report form (RO CRF; see Appendix 3) or at the time of the 12-month clinic review. Women also reported effects and readmissions in the follow-up questionnaires. If an adverse effect was suspected, it was verified if possible.
All related serious adverse effects [serious adverse events (SAEs)] and adverse effects [adverse events (AEs)] were recorded on the serious adverse event report form (see Appendix 3). Unrelated SAEs or AEs were not recorded.
Within PROSPECT, a SAE or an AE was defined as ‘related’ if it occurred as a result of a procedure required by the protocol (i.e. prolapse surgery), whether or not this procedure was the specific intervention under investigation, and whether or not it would have been administered outside the study as normal care. Signs or symptoms of the disease being studied were not considered an adverse effect.
An AE was defined as a SAE if it resulted in death, was life-threatening, required hospitalisation or prolongation of an existing admission, resulted in significant disability/incapacity or was otherwise considered medically significant by the investigator.
Adverse effects that were expected after prolapse surgery are listed below. Any AEs that were deemed to be related and serious but unexpected (i.e. not on the list below) required expedited onward reporting to the sponsor. During the conduct of PROSPECT no unexpected SAEs were reported.
Within PROSPECT the following occurrences were potentially expected:
-
Possible (expected) intraoperative occurrences associated with surgery were injury to organs or blood vessels, excess blood loss, blood transfusion, anaesthetic complications, death.
-
Possible (expected) occurrences following surgery were thrombosis, infection [urinary tract infection (UTI), sepsis, abscess], pain, urinary retention, bowel obstruction, constipation, mesh erosion, excess blood loss, haematoma, vaginal adhesions, skin tags, granulation tissue, new or persistent urinary tract symptoms, death.
Reported SAEs and AEs were further classified using the International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of complications that are related directly to the insertion of prostheses (meshes, implants, tapes) and graft in female pelvic floor surgery,31 and complications related to native tissue female pelvic floor surgery. 32
Sample size calculation
Primary trial
Pilot data showed a conservative estimate of the standard deviation (SD) of the primary participant-reported outcome POP-SS at 1 year to be eight units, and we considered a difference in means of two units to be a clinically important difference. The sample size calculation for the Primary trial was, therefore, based on a standardised effect size of 0.25. To detect a difference of 0.25 SDs with 90% power and alpha equal to 0.025 (to maintain the nominal p-value at 0.05 with tests for two comparisons), we planned to follow-up 400 women in each arm of the primary repair RCT (a total of 1200 participants). The sample size was inflated to 1450 participants, which allowed for a dropout rate of 17.5%. A trial of this size is also adequately powered to detect important differences in the economic and secondary outcomes.
Secondary trial
It was estimated that 30% of women requiring anterior and/or posterior repair would receive a secondary or subsequent repair. Therefore, during the proposed time period required for recruiting 1450 women to the primary repair RCT above, it was anticipated that approximately 620 women who were having secondary surgery would be randomised to the secondary repair RCT (assuming the same rate of eligibility and willingness to participate as in the primary repair RCT. The total expected recruitment across both trials was therefore 2070 randomised women.
Pilot data indicated that women who were having secondary repairs have a higher level of symptoms at baseline. Therefore, we considered it to be biologically plausible that these women might show a larger benefit from surgical treatment than women who were having their first repair. We therefore calculated that it would be possible to detect, with 90% power and alpha equal to 0.025, a standardised effect size of 0.38, which equates to three points on the Pelvic Organ Prolapse Symptom scale.
Avoidance of bias, including blinding
Group allocation was concealed from the woman and the ward staff, although blinding in theatre was not possible, given that this was a surgical trial. Women were not informed after their surgery of the procedure actually carried out unless they specially requested this information. Outcome assessment was largely by participant self-completed questionnaires, so avoiding interviewer bias.
Where possible, the clinical review at 12 months in outpatients was conducted by research staff who were blinded to allocation, rather than the clinical staff caring for the woman. Although women and research staff were not explicitly informed of which operation was randomly selected, examination may have revealed which operation was actually carried out.
A researcher who was blinded to allocation conducted the data collection, data entry and analysis, using study numbers only to identify women and questionnaires. In the RCTs, an intention-to-treat approach was used in all primary analyses. In addition, all analyses were clearly predefined to avoid bias (see Appendix 5).
Statistical analysis
The trial analysis was by intention to treat (all participants remained in their allocated group for analysis), giving the least biased estimate of effectiveness between interventions. Two comparisons were analysed in the primary repair RCT – standard repair compared with synthetic mesh (trial 1, combining the strata 1A and 1B; see Figure 1) and standard repair compared with biological mesh (trial 2, combining strata 1A and 1C) and three comparisons were analysed in the secondary repair RCT – standard repair compared with synthetic mesh (trial 3, combining strata 2A and 2B) and standard repair compared with mesh kit (trial 4, combining strata 2A and 2D). Study analyses were conducted according to a statistical analysis plan (SAP), using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) (see Appendix 5).
For each time point (baseline, 6, 12 and 24 months), all outcome measures are presented as summaries of descriptive statistics (mean and SD for continuous measures, and proportion for ordinal and dichotomous measures). Comparisons between randomised groups were analysed at 12 months and 24 months using general linear regression models (GLMs). POP-SS, prolapse-related QoL, EQ-5D-3L and readmissions data at 6 months were also analysed. Models were adjusted using minimisation covariates (age group, type of prolapse, concomitant continence procedure and concomitant upper compartment prolapse surgery), the equivalent baseline measure, where appropriate, and (in the primary repair trial) for randomisation stratum.
Continuous outcomes were analysed using linear mixed models, with surgeon fitted as a random effect. POP-Q stage, bowel frequency and satisfaction scales were analysed using ordinal logistic regression, and dichotomous outcomes were analysed using binary logistic regression (proportional odds models with cumulative logits). Estimates of treatment effect size were expressed as the fixed-effect solution for the mean difference (MD) in the mixed models, ORs in the ordinal models and risk ratios (RRs) in the binary models. For all estimates, 95% CIs were calculated and reported.
Subgroup analyses were carried out on the primary outcome in the primary repair RCTs (POP-SS at 1 year) to test subgroup by treatment interaction effects. Subgroups were determined a priori to be age group (< 60 years or ≥ 60 years), type of planned prolapse repair (anterior, posterior or both), planned concomitant continence procedure (yes or no), planned concomitant upper prolapse procedure (yes or no) and parity (0–2 or 3+).
The main analysis was a complete case analysis with no imputation of missing values. Sensitivity analyses, however, were carried out on the primary outcome in the primary repair RCTs (POP-SS at 1 year) to investigate the impact of missing data under various assumptions. The first sensitivity analysis used multiple imputation (MI) using fully conditional specification, which assumed the data to be missing at random. Imputed values were obtained from the generation of 10 data sets and based purely on observed values (minimisation covariates and Pelvic Organ Prolapse Symptom scale scores at baseline). Subsequent sensitivity analyses assumed data to be missing not at random, with scenarios for systematic differences between missing and observed values being examined, and whether or not this might have differed between randomised groups. These analyses adjusted the imputed values in the initial sensitivity analysis by either adding two points to the imputed Pelvic Organ Prolapse Symptom scale scores or subtracting two points. These adjustments were then repeated in one arm only, and repeated again by applying the adjustments in the other arm only. We consider two points on the Pelvic Organ Prolapse Symptom scale to be the minimum clinically important difference and hence a meaningful systematic difference to test in the sensitivity analyses. An additional sensitivity analysis was performed whereby individual unanswered Pelvic Organ Prolapse Symptom scale items were assumed to be missing (rather than assumed to be asymptomatic).
Health-economic evaluation
This section outlines the methods for the trial-based economic evaluation. The methods are applicable to both the analysis of the Primary and Secondary trial data at 1-year and 2-year follow-up. Further detailed methods regarding how the trial data are used to inform the development of a decision-analytic model for the choice of primary prolapse surgical repair, as well as detailed model methods, will be reported in the decision modelling chapter (see Chapter 9). Data were analysed at 1-year follow-up, thus following the timeline for the Primary trial outcome analysis. A further analysis was undertaken using 2-year follow-up data, which improve the information relating to recurrence/failure and the associated resource implications in terms of NHS resources consumed as well as QoL. All health-economic analyses within the RCT were based on the intention-to-treat principle. Results from the within-trial economic evaluation are presented as incremental cost-effectiveness ratios (ICERs). The primary framework of analysis for the health-economic evaluation is a cost–utility analysis, reporting results as incremental cost per QALY gained of adopting one treatment approach over another.
For all comparisons of costs and QALYs undertaken in the primary repair trial, results are based on complete case data and are presented for the following comparisons:
-
synthetic mesh repair versus standard repair
-
biological graft repair versus standard repair.
For assessments of the probability of cost-effectiveness, data are considered within a net benefit framework for complete cost and QALY pairs, and for a three-way comparison as per RCT1A (women randomised across all treatment options). For the secondary repair trial, tables of results are presented in a similar manner; however, data from all randomised women are used, not just those randomised to the three-way comparison as above. The justification of the alternative approach is to ensure best use of limited data available. For both the Primary and Secondary trial analyses, we have conducted sensitivity analysis around the choice of data used in the comparisons to explore the impact of these decisions on cost-effectiveness results.
Quality of life (quality-adjusted life-years)
The primary health-economic analysis is based on a cost–utility framework, with results reported as incremental cost per QALY gained. The purpose of a cost–utility analysis is to provide information to health-care decision-makers regarding the scarce allocation of health resources at a health-care level. It allows for a determination of value for money of one treatment approach over another and is used to guide recommendations to UK policy-makers, such as NICE.
The EQ-5D-3L25 generic QoL instrument was administered to all trial participants at baseline and at 6-month, 1-year and 2-year follow-up. The EQ-5D-3L measure divides health status into five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depressions). Each of these dimensions have three levels, so 243 possible health states exist. EQ-5D-3L responses are presented in graphical format, illustrating the percentage of respondents with any or severe problems on each health domain, split by randomised arms of the trial. The results are presented in accordance with EuroQoL guidelines. 25
The responses to the EQ-5D-3L questionnaire were valued using UK general population tariffs, based on the time trade-off technique to generate a utility score for every participant within the trial. 25 QoL data derived from the EQ-5D-3L were combined with mortality data from the trial, using the standard assumption that all participants who have died in the trial will have a utility value of 0 from the date of death to the end of follow-up. QALYs were then calculated on the basis of these assumptions, using an area beneath the curve approach, assuming linear extrapolation of utility between time points.
Resource use and costs
The perspective of the primary economic analysis is that of the NHS. A supplementary analysis presents costs from a wider patient/societal perspective. In all cases, resource use and costs relate to consultations in primary and secondary care which are related to women’s prolapse or prolapse-related symptoms.
Health services costs
The resource-use data were sourced from participant-completed questionnaires and supplemented with data that were post-coded to patient records for secondary care resource use. Post-coding was conducted by checking all reported cases of secondary care resource use against patient notes to verify reported length of stay, category of care use (so, outpatient or inpatient) and to verify that the reported use of care was for issues related to prolapse and not for some other unrelated reason. Data were obtained from the clinical centres for the price of mesh and clinical expert opinion was sought to bridge any data gaps relating to staff requirements for surgery. National average unit costs were applied to resource-use data to generate total costs to the health services. The sources of unit costs were the British National Formulary (BNF) and the NHS Business Services Authority electronic drug tariff online catalogue33 for medications resource use;34 Information Services Division (ISD) Scotland35 and NHS reference costs36 for secondary care resource-use data; and Personal and Social Services Research Unit (PSSRU) unit costs of health and social care for primary care resource-use data. 37 The costs were reported in 2013–14 UK pound sterling (£). The costs incurred in the second year of follow-up were discounted at a rate of 3.5% per annum. The sensitivity analysis explored the impact of varying the discount rate in accordance with NICE recommendations. 38
The resource-use data and costs for the health-economic analysis were broken into the following categories of NHS resource use:
-
intervention costs (including costs of completing the surgery, preparation costs and hospital resource-use costs in theatre, based on operation time, staff time and other additional treatments)
-
postoperative costs (from surgery to discharge) including time on ward, return to theatre and cost of treating any infections or complications
-
inpatient costs (cost of any follow-up operations and length of stay in hospital related to prolapse symptoms, including overnight and day-case admissions)
-
outpatient costs (including all outpatient contacts over the trial follow-up)
-
primary care costs (including GP contacts, occupational therapist, physiotherapist and nurse contacts)
-
medications and other treatments related to treating prolapse and UI symptoms.
Unit costs
Costs to the health services are estimated by combining resource-use data with unit costs of resource use. Table 2 includes a list of all unit costs used in the within-trial economic analysis, together with their source and any assumptions used to develop the unit cost used for analysis. Further details regarding calculations underpinning the unit costs presented in Table 2 are outlined in more detail in Appendix 6. Unit costs applied to the Primary and Secondary trial analyses were similar with the exception of the unit cost of mesh materials to complete the operative procedure.
| Resource-use item | Unit | Cost per unit (£) | Comments | Source |
|---|---|---|---|---|
| Synthetic mesh material | Per mesh unit | 111.09 | Average per unit cost of meshes used at all participating sites. Mean cost imputed for centres not returning data | Direct contact with sites/manufacturer price lists |
| Biological graft materials | Per mesh unit | 305.41 | Average per unit cost of meshes used at all participating sites using biological graft. Mean cost imputed for centres not returning data | Direct contact with sites/manufacturer price lists |
| Anterior mesh kits (Secondary trial only) | Per mesh kit | 645.45 | Average per unit cost of meshes used at all participating sites using anterior mesh kits. Mean cost imputed for centres not returning data | Direct contact with sites/manufacturer price lists |
| Posterior mesh kits (Secondary trial only) | Per mesh kit | 583.00 | Average per unit cost of meshes used at all participating sites using posterior mesh kits. Mean cost imputed for centres not returning data | Direct contact with sites/manufacturer price lists |
| Gynaecologist/anaesthetist time (consultant) | Per hour | 142 | If surgery was supervised, assume supervision provided by a consultant grade. Includes qualification costs | PSSRU 201437 |
| Gynaecologist/anaesthetist time (registrar) | Per hour | 124 | Includes qualification costs | PSSRU 201437 |
| Gynaecologist/anaesthetist time (associate) | Per hour | 71 | Includes qualification costs | PSSRU 201437 |
| Band 5 theatre nurse | Per hour | 100 | Including qualification costs, cost per 1 hour of patient contact. Assume three band 5 nurses present for all procedures (Dr Karen Cranfield, Aberdeen Royal Infirmary, 2015, personal communication) | PSSRU 201437 |
| Band 4 theatre nurse | Per hour | 91.59 | Per hour of client contact, including qualification costs. Assume one band 4 nurse present for duration of all procedures (Dr Karen Cranfield, personal communication) | |
| General anaesthesia | Per case | 20.60 | Based on calculation (see Appendix 6) | BNF;34 personal communication |
| Spinal anaesthesia | Per case | 1.85 | Based on calculation (see Appendix 6) | BNF;34 personal communication |
| Local anaesthesia | Per case | 0.40 | Based on calculation (see Appendix 6) | BNF;34 personal communication |
| Surgical antibiotics | Per case | 1.06 | Assume co-amoxiclav (Augmentin®; GSK, Middlesex, UK); Dr Karen Cranfield, personal communication | BNF;34 personal communication |
| Other surgical drugs | Per case | 6.45 | For general and spinal anaesthesia only; resource use provided by Dr Karen Cranfield (see Appendix 6 for detailed calculation) | BNF;34 personal communication |
| Theatre overheads | Per hour | 352.69 | Currently excludes consumables | ISD35 Scotland R140X |
| Cost of catheterisation | Per catheter | 6.25 | Assume Folysil® all-silicone catheters, female (Coloplast Ltd, Peterborough, UK); NHS EDT, April 2015 – assume no additional procedure time required if catheterised during surgery | NHS EDT33 |
| Vaginal pack | Cost per vaginal pack | 4.67 | Sorbsan packing (Aspen Medical Europe Ltd, Ashby-de-la Zouch, UK) 30 cm/2 g: £3.47 plus Hibitane obstetric cream (Derma UK, Bedfordshire, UK): £1.20 |
NHS EDT33 |
| Other treatments during admission for intervention | ||||
| Return to theatre | Per case | 814 | No data available on time in theatre for returns; conservatively assume duration was 1 hour | Direct cost, ISD35 R142 |
| Laxatives | Per pack of tablets | 3.43 | Bisacodyl 5 mg | BNF34 |
| Length of stay (gynaecology ward) | Per day | 179 | Payment by results tariff of £1433 spread over 8 days, so £179 per day | Payment by results, 2014 tariffs36 |
| Consultations with secondary and primary health-care professionals/procedures for subsequent treatment or consultations | ||||
| New prolapse procedure | Per procedure | 2331 | Weighted calculation of appropriate HRG codes for surgery for prolapse. See Appendix 6 for further details | NHS Reference Costs 2013–14 36 |
| New incontinence procedure | Per procedure | 1372.48 | Weighted average of elective and day-case procedures for HRG code M533 (introduction of TVT/TOT); see Appendix 6 for calculation details | NHS Reference Costs 2013–14 36 |
| Other readmission | Cost per admission | 853.64 (weighted average) | Weighted average of elective in patient/day-case procedures for HRG codes MA22/MA23 minimal/minor genital tract procedures: £803.81 (day case) £1207.85 (> 0 nights’ admission) See Appendix 6 for detailed calculations |
Payment by results, 2014 tariffs36 |
| Outpatient consultation (first attendance) | Per consultation | 133 | NHS reference costs | NHS Reference Costs 2013–14 36 |
| Outpatient consultation (repeat) | Per consultation | 81 | NHS reference costs | NHS Reference Costs 2013–14 36 |
| GP visit | Per visit | 46 | Per 11.7-minute consultation, including qualification costs | PSSRU 201437 |
| Practice nurse | Per visit | 13.69 | Per 15.5-minute consultation, including qualification costs | PSSRU 201437 |
| Community physiotherapist | Per visit | 23.94 | Per 30-minute consultation, including qualification costs | PSSRU 201437 |
| Hospital clinical nurse specialist | Per visit | 22.50 | Based on a per-hour cost of £90 per hour of client contact, assuming average appointment of 15 minutes’ duration | PSSRU 201437 |
| Community pharmacist | Per visit | 32.50 | Based on per-hour cost of £142, including qualification costs, and average appointment duration of 15 minutes | PSSRU 201437 |
| Accident and emergency | Per visit | 103 | Cost per visit (see Appendix 6 for more details on calculation) | NHS Reference Costs 2013–14 36 |
| Urodynamics | Per consultation | 186 | See Appendix 6 for calculation details. Based on HRG code LB42, assume outpatients | NHS Reference Costs 2013–14 36 |
| Ultrasound scan | Per visit to have scan | 52 | Diagnostic imaging in outpatients assumed. See Appendix 6 for further details | NHS Reference Costs 2013–14 36 |
| Other treatments | ||||
| Absorbent pads | Per pad – day | 0.61 | Based on average across a number of products and data reported in Fader 2008. Data inflated to present-day values. Unit costs multiplied by frequency of leakage to generate cost per woman (see Appendix 6 for more details) | Fader 2008;39 PSSRU 2014;37 HCIS inflation index |
| Per pad – night | 0.66 | |||
| Permanent/indwelling catheter | Per woman (yearly cost) | 390.52 | Based on a number of assumptions. See Appendix 6 for calculation details | NHS EDT 201533 |
| Reusable/intermittent catheter | Per woman (yearly cost) | 1816.50 | Based on a number of assumptions. See Appendix 6 for more details | NHS EDT;33 NHS Warrington40 Trust documentation for guidance of care |
| Oestrogen treatment | Per 24-applicator pack | 16.72 | Estradiol (Vagifem®; Novo Nordisk, West Sussex, UK) vaginal tablets, 10 µg, in disposable applicators. Multiplied by resource-use requirement over follow-up | BNF 201534 |
| Ring pessary | Per pessary | 19.98 | Average across EDT products (see Appendix 6 for calculation) | EDT 201533 |
| Shelf pessary | Per pessary | 21.51 | Average across EDT products (see Appendix 6 for calculation) | EDT 201533 |
| Drug treatment for bladder problems | Per 56-tablet pack | 2.92 | Assume tolterodine tartrate, generic version, to cover frequency and urgency symptoms, 2 mg twice daily dose assumed | BNF 201534 |
Intervention costs
The resource-use data required to deliver each intervention were collected prospectively for every participant in the study. The operative details were recorded at the time of surgery (e.g. time in theatre, grade of operating gynaecologist, grade of anaesthetist and grade of surgical supervision if present). The details of concomitant surgery and catheterisation were recorded and incorporated into the costing analysis. The details were sourced from data recorded on the CRFs (see Appendix 3). The data from the CRFs were supplemented with centre-specific data for the costs of mesh products. Each centre was asked to provide information on the mesh products used by each surgeon at their site for each trial intervention. The surgeon-specific data on mesh use were costed using NHS list prices, sourced from participating centres financial departments.
For some cases, we were not able to identify mesh costs directly from the participating surgeons. This resulted in some missing data for mesh costs. In such cases, we imputed mean costs of mesh calculated from those surgeons/centres that provided data. It is possible that there is heterogeneity across surgeons in terms of the size of mesh product used, or within individual surgeons, who may use different mesh sizes on a case-by-case basis. Where possible, we have costed the same (or similar) mesh sizes across different mesh products so as to avoid any bias against individual mesh products. It should be noted that the analysis does not seek to make statements about the effectiveness or cost-effectiveness of individual mesh products, but rather seeks to develop an average cost for each arm of the trial, which is relevant and generalisable to clinical practice in the UK.
When data regarding surgical resource use (particularly regarding the number of supplementary staff present during a typical surgical procedure, such as nurses and theatre assistants) were unavailable from formal records, we have made assumptions based on the clinical opinion of experts working on the trial team. When there was uncertainty in the resource-use estimates to complete the intervention, and when any assumptions were required, sensitivity analysis explored the impact of these assumptions on the total intervention cost and on the estimates of cost-effectiveness.
The purpose of the intervention costing analysis was to find an average procedure cost, based on typically used meshes at participating centres. Data on mesh usage were available from the 35 participating centres. Unit costs of mesh usage were also sourced through a separate costing exercise directly from centres, which were asked to provide NHS list prices. When data were missing for individual mesh products at centres, the average of all mesh products within that category (e.g. synthetic mesh) was assumed and applied as the unit cost. A similar approach was taken for biological graft repair.
Inpatient costs over follow-up
As length of stay is one of the secondary outcomes of the trial, we collected detailed data on inpatient length of stay in relation to both the participant’s prolapse surgery and their UI. The hospital-based costs in the immediate aftermath of the surgery (up until date of discharge of the patient) were recorded on the RO CRF (see Appendix 3), eliciting information on whether or not the patient returned to theatre for a procedure-related event within 72 hours of having their operation and if catheterisation was required in the first 10 days postoperatively. Longer-term inpatient resource-use data were collected from the participant-completed questionnaires issued at 6-month, 1-year and 2-year follow-up. When participants reported having a hospital readmission, these were checked against patient records to determine the reason for admission. Furthermore, this post-coding exercise identified any participant reporting errors (e.g. patient confused follow-up surgery with index operation; participant double-counted single admissions on both 1-year and 2-year questionnaires; participant misidentified reason for readmission). The costs of additional surgery related to prolapse and/or urinary leakage were estimated using national tariffs, as well as any other inpatient costs. The data collected from both the 1-year and the 2-year follow-ups were used to inform the economic model extrapolating resource use over the patient’s lifetime.
Outpatient costs
The participant-completed questionnaires were used to determine outpatient contacts related to the women’s prolapse symptoms over follow-up. Again, these were post-coded against patient records to check the accuracy of the data and resolve any discrepancies.
Owing to the post-coding exercises undertaken, we have a high degree of confidence in the estimates of secondary care resource use across the trial for each individual woman returning a questionnaire. Therefore, if a woman did not report a secondary care event, it was assumed that no resource use was incurred. If a woman did not return a questionnaire then data were treated as missing.
Primary care costs
Participants were asked to provide detailed information on contacts with primary care health professionals in relation to their prolapse symptoms and UI (see Appendix 4). This included visits to the GP, practice nurse, occupational therapist and physiotherapist at each follow-up time point.
For primary care resource-use questions that are left blank on a returned participant questionnaire, resource use is assumed to be zero. The reason for this is to ensure the best possible use of the available data to generate a reasonably sized complete case data set for the economic analysis. Sensitivity analysis explored the effect of multiply imputed data. As with the secondary care data, if a participant questionnaire is not returned then data are treated as missing.
Total NHS costs
The total costs from the health services perspective were calculated by summing all intervention treatment and follow-up costs related to the respective prolapse repairs for each participant in the data set. If one of the component costs was missing because of a non-returned questionnaire then that participant was dropped from the complete case analysis. If a component cost was missing for primary care consultations then these data were treated according to the assumptions outlined above. The total NHS costs and individual component costs incurred within the second year were discounted by 3.5%.
Participant- and companion-incurred costs and indirect costs, including opportunity costs of time and travel
Participant resource utilisation comprised three main elements: self-purchased health care; travel costs for making return visit(s) to NHS health care (such as petrol, public transport and parking); and time costs of travelling and attending NHS health care (such as time involved away from usual activities or work). All self-purchased health care relate to treatment purchased for the management or treatment of prolapse-related symptoms. Likewise, time and travel costs relate to time spent travelling to and attending hospital or primary care for prolapse symptoms. Estimation of travel costs required information from participants about the number of visits to, for example, their GP or physiotherapist (estimated from the health-care utilisation questions) and the unit cost of making a return journey to each type of health-care provider (from the participant time and travel cost questionnaire; see Appendix 4).
The cost of participant time was estimated in a similar manner. The participant was asked, in the participant time and travel cost questionnaire, how long they spent travelling to, and attending, their last visit to each type of health-care provider. Participants were also asked what activity they would have been undertaking (e.g. paid work, leisure, housework) had they not attended the health-care provider. They were further asked if they were accompanied by a friend or a relative. If so, their time and travel costs were also incorporated into the analysis. These data are presented in their natural units, for example hours, and also costed using standard economic conventions, using the Department of Transport estimates for the value of work and leisure time. 41 These unit time costs were then combined with the number of health-care contacts derived from the health-care utilisation questions to elicit a total time and travel cost from a patient perspective.
The data collected through the health services resource-use questionnaire were used to estimate the costs of self-purchased health care, including pads bought by the participant, prescription costs and over-the-counter medications. The cost to the participant of any self-purchased health care was collected directly within the questionnaire.
Indirect costs were defined as the production losses resulting from treatment when the participant was unable to return to work or was required to take sick leave due to her prolapse problems. The cost of days lost was estimated using the average UK gross hourly wage in the economy. When a participant’s own reported costs associated with a specific type of health service visit were missing, the mean cost for that type of visit was imputed. Participants completing the annual health resource utilisation questionnaire were asked how many days they were off work in the last 12 months as a result of prolapse symptoms or problems. Questions were asked at both 1-year and 2-year follow-up. The data were recorded as natural units and multiplied by standard economic costings as reported below (see Table 3). The total production losses due to time away from work for non-retirees as a result of prolapse symptoms were estimated and compared across treatment groups.
The unit costs applied to the participant (and companion) time, travel and indirect economic costs data are outlined in Table 3. The unit costs were based on standard economic sources and were inflated, where appropriate, to 2014 values. For the purposes of inflation, we utilised the Cochrane economics group inflation calculator application, using International Monetary Fund-reported inflation data. 44
| Activity | Unit cost (£, 2014) | Assumptions made/notes | Source of data |
|---|---|---|---|
| Unit costs applied to participant and companion travela | |||
| Cost per mile travelled by car | 0.45 per mile | HMRC-approved mileage rate (most recent data: year 2013) | HMRC42 |
| Car parking charges | Various | Specified in participant questionnaire | Participant-reported data |
| Cost of public transport fares (bus, train, taxi) | Various | Specified in participant questionnaire | Participant-reported data |
| Cost of return journey by hospital car | 18.00 per return journey | Various costs across NHS Trusts (data from South Devon publicly available and applied to all) | Torbay and South Devon NHS Foundation Trust43 |
| Cost of non-emergency patient transport service (via ambulance) | 44.65 per return journey | Not included in reference costs since 2011 (therefore indicative cost only) Note: incurred directly by PCTs, so not included in total participant cost calculation |
NHS Reference Costs 2009–1036,44,45 |
| Unit costs applied to participant and companion time | |||
| Paid work | 13.21 per hour | Based on average economic wage per week of £518, assuming 39.2-hour working week | ONS; annual survey of hours and earnings 201446 |
| Housework | 10.53 per hour | Costs of housework in the NHS (assumed annual salary of £21,000 gross; 2012 values inflated to 2014) | NHS pay review body report 201247 |
| Child care | 13.21 per hour | As paid work | ONS 201448 |
| Caring for a friend/family member | 13.21 per hour | As paid work | ONS 201448 |
| Voluntary work | 13.21 per hour | As paid work | ONS 201448 |
| Leisure activities | 6.54 per hour | Value of non-working time (2010 values inflated to 2014) | TAG data book, autumn 201341 |
| Retired | 6.54 per hour | Value of non-working time (2010 values inflated to 2014) | TAG data book, autumn 201341 |
| Unemployed | 6.54 per hour | Value of non-working time (2010 values inflated to 2014) | TAG data book, autumn 201341 |
| Ill/disabled (long term, unrelated to prolapse) | 6.54 per hour | Value of non-working time (2010 values inflated to 2014) | TAG data book, autumn 201341 |
The data on time and travel costs, participant-incurred medical costs and time away from work or usual activities (to attend medical appointments and as a result of recovery from surgery) were all summed together to generate a total participant cost. The incremental cost differences between groups from a participant perspective were estimated using the same methods outlined in the statistical analysis of economic data detailed in the following section.
Statistical analysis of economic data
The economic analysis was conducted following the intention-to-treat principle. The perspective was predominantly that of the NHS, with a supplementary wider economic and patient perspective conducted. The period of follow-up was 2 years and costs and QALYs in the second year were discounted at a rate of 3.5%. All components of costs were described with the appropriate descriptive statistics where relevant: mean and SD for continuous and count outcomes; numbers and percentages for dichotomous and categorical outcomes (e.g. numbers reporting problems on EQ-5D-3L). All analyses were conducted using Stata® version 14.1 software (StataCorp LP, College Station, TX, USA).
To investigate the potential for skewed cost data (i.e. a small proportion of participants incurring very high costs), we used GLMs, testing alternative model specifications for appropriate fit to the data. The GLM models allow for heteroscedasticity by selecting and specifying an appropriate distributional family for the data. This family offers alternative specifications to reflect the relationship between the mean and variance of the estimates under consideration. 49,50 Two diagnostic actions were performed to select the most appropriate distributional family: (1) a modified Park test, which identified two potentially viable distributional families for costs, namely Gaussian or gamma, and (2) as a check on the most appropriate model, the Akaike information criterion (AIC) was consulted, which identified a Gaussian model with an identity link as having the lowest AIC score and the most appropriate model fit. This suggests a standard ordinary least squares (OLS) model should be fitted for cost data. The next-best model fit according to the AIC criteria was a gamma regression with log link, and this was explored in sensitivity analysis. Regression models applied to cost components (such as ‘other treatments’ and ‘hospital costs’) in the analyses above are also assumed to follow the same distributional assumptions as the total cost data. A standard OLS model was also identified as the most appropriate model and applied to the analysis of incremental QALY gains. All analyses were conducted using heteroscedastic robust standard errors (SEs).
Analysis models were run to estimate the incremental effect of treatment group on costs and QALYs. Models were adjusted using minimisation covariates (age group, type of prolapse, concomitant continence procedure and concomitant upper compartment prolapse surgery), as well as surgeon and baseline EQ-5D-3L score. 51 For the Secondary trial, using all available data, analyses were further adjusted for randomised stratum. The coefficient on treatment in the respective linear OLS models is taken as the estimate of incremental costs for use in the economic evaluation. 49,50
Overall results of the cost–utility analysis are reported as incremental cost per QALY gained for different treatment arms (relative to standard repair). The cost per QALY is presented using the ICER, calculated as the coefficient of treatment effect on costs divided by the coefficient of treatment effect on QALYs from the respective linear regression models. Estimates of the ICER are then compared with the recommended willingness-to-pay (WTP) decision-making threshold in the UK, currently between £20,000 and £30,000 per QALY gained. 38
We used non-parametric bootstrapping methods to estimate 95% CIs for treatment effects on costs and QALYs, using 1000 repetitions. 52 These were further used to summarise the uncertainty surrounding the estimated ICERs, which was illustrated using:
-
Incremental scatterplots of bootstrapped repetitions for incremental costs and incremental QALY pairs for the respective mesh treatments compared with standard repair. 53,54 Presentation of the bootstrapped iterations of costs and outcomes on the cost-effectiveness plane allows the reader to see the probability of one intervention outperforming another in terms of cost-effectiveness, illustrating the probability of that said intervention falling into each quadrant of the cost-effectiveness plane being (1) less costly and more effective; (2) more costly and less effective; (3) less costly and less effective; or (4) more costly and more effective.
-
The bootstrapped estimates of treatment effect were further used to generate cost-effectiveness acceptability curves (CEACs). 54 CEACs were generated using estimates of net monetary benefit (NMB), generated using the bootstrapped replications, in accordance with the net benefit statistic given in Equation 1:
where ‘QALYs’ and ‘cost’ are the estimated total QALYs and total costs for a treatment strategy and lambda (λ) represents the ceiling ratio of a decision-maker’s WTP for a QALY gained. For the purposes of the base-case analysis, λ is set to £30,000, the upper end of the commonly accepted range of ICERs considered to offer good value for money at NICE. However, for the base-case analyses, a number of alternative threshold values presented at £0, £10,000, £20,000, £30,000 and £50,000 are explored, presented numerically within the tables and visually represented on the CEACs presented. 53,54
The study initially planned to present results as cost per woman cured for the trial-based analyses. However, it is not clear how ‘cure’ should be defined. For example, it may be subjective improvement, anatomical improvement or a change in QoL: these are not always in accord with each other. Although many women experienced improvements in their prolapse symptoms, few achieved a state of being completely symptom free. As we are unable to explicitly define cure for the clinical effectiveness analysis, it would be misleading, therefore, to do so for the economic evaluation. The greatest value to decision-makers in the UK NHS relates to an assessment of cost per QALY gained, which is the primary economic outcome and has been used as the basis of all the economic analyses.
Deterministic sensitivity analyses
Although the presentation of CEACs and scatterplots addresses the issue of sampling uncertainty in the data, other assumptions surrounding the most appropriate discount rate and analysis models undertaken may create additional uncertainty, which are not captured in the presented CEACs. Furthermore, the impact of missing data on cost-effectiveness outcomes is explored. All sensitivity analyses (other than the use of imputed data sets) were conducted using complete case 2-year follow-up data for total cost and QALY pairs.
Missing data
We have used a combination of pragmatic and statistical approaches to deal with missing data. Pragmatic approaches have been outlined through this chapter where relevant and were applied to all base-case analyses. As base analyses were conducted using complete case data for cost and QALY pairs, there was a substantial proportion of missing data. This can pose significant problems for data analysis, especially surrounding data reported using participant-completed questionnaires. Therefore, we have undertaken statistical MI of missing data as a sensitivity analysis. The imputation analysis was undertaken using Stata’s multiple imputation (MI) procedure. 55 Missing component costs (e.g. cost of primary care, outpatient care) and utility values were imputed at each questionnaire time point (6 months, 1 year and 2 years).
Components of cost data were imputed, based on linear regression models that were adjusted for minimisation variables, baseline utility and treatment allocation group. Missing utility values were imputed using predictive mean matching, accounting for the five closest estimates. Chained equations were used for the imputations. The imputation procedure predicted 10 plausible alternative imputed data sets, which was found to be sufficient to provide stable estimates. An analysis of incremental costs and outcomes was undertaken across the 10 imputed data sets and combined to generate one imputed estimate of incremental costs and QALYs.
We also explore the impact of changing the discount rate used for second-year costs and QALYs in accordance with NICE best practice recommendations, ranging the discount rate from 0% to 6% per annum. Furthermore, to ensure comparability of our economic analysis with the clinical effectiveness analyses presented in following chapters, we have conducted a secondary analysis for trial-based cost-effectiveness using data from all of the women randomised to the Primary trial arm.
All of the analysis methods for base case, uncertainty and sensitivity analyses were conducted similarly for both the Primary trial analyses (see Chapter 5) and Secondary trial analyses (see Chapter 7) unless otherwise stated. As noted at the outset, the main difference between the economic analyses across the two chapters pertains to the data considered for the base-case analysis. For the base-case Primary trial analysis, we consider RCT1A (women randomised only across the three-way comparison), whereas for the base-case Secondary trial analysis we consider all women who were having a secondary prolapse surgical repair (RCT2).
Subgroup analyses
We did not identify any additional subgroup analyses that were required to estimate cost-effectiveness for the within-trial analysis.
Decision-analytic model
Owing to the chronic nature of prolapse repair and the potential for different failure rates over time, data from the trial analysis are extrapolated over a longer-term time horizon using a Markov decision-analytic model. The data to populate the model are informed by the trial in terms of costs, utility weights, time to failure and other key parameters. Data on further analysis of trial data to populate the economic model and the modelling methods themselves are reported in Chapter 9.
Management of the study
The study office team
The study office was based at CHaRT in Aberdeen and provided day-to-day support for the clinical centres. It was responsible for all data collection (such as mailing questionnaires), follow-up, data processing and analysis. It was also responsible for randomisation and communicating with the centres about PROSPECT-specific issues. PROSPECT newsletters were developed for participants and collaborators to inform everyone of progress and maintain enthusiasm.
The PROSPECT Study office team (Aberdeen-based grant-holders and study office members) met formally at least monthly during the course of the study to ensure smooth running and trouble-shooting.
Project Management Group
The study was supervised by the Project Management Group (PMG), which consisted of the grant-holders and representatives from the study office. The PMG met, in person or by teleconference every 3 months on average.
Trial Steering Committee
The study was overseen by an independent TSC. The membership comprised four independent members (see Acknowledgements for membership details), the Chief Investigator and grant-holders. Observers or members of the host university (Aberdeen) and the funders (the NIHR HTA) were invited to attend, as were other members of the PROSPECT Study office. The committee met 11 times between August 2009 and July 2015 at approximately 6-monthly intervals, as decided by the Committee.
Data Monitoring Committee
A separate and independent DMC was convened (see Acknowledgements for membership details) and comprised four members: an academic clinician (as the independent chairperson); a gynaecologist, who was not involved in the trial; a statistician with experience of monitoring accumulating RCT data; and a consumer representative.
The members met once to agree terms of reference (August 2009) and a further five times between September 2011 and September 2014 to monitor accumulating data and oversee safety issues. During the period of recruitment to the study, interim analyses were supplied, in strict confidence, to the DMC, together with any other analyses that the committee requested. In the light of these interim analyses, the DMC would have advised the TSC if, in its view:
-
one of the methods of prolapse surgery had been proven, beyond reasonable doubt, to be different from the control (standard management) for all or some types of women (with respect to either effectiveness or unacceptable safety concerns), and
-
the evidence from the economic data was sufficient to guide a decision from health-care providers about choosing operations.
On each occasion, the DMC recommended continuation of the trial with no change of protocol. All other groups, the TSC, PMG, clinical collaborators and study office team (except the trial statistician, who supplied the confidential analyses) remained ignorant of the interim results considered by the committee.
Chapter 3 Results: all
Between January 2009 and August 2013, 4083 women were identified as potential participants in the PROSPECT Study, of which 3089 (76%) were eligible and gave their consent. The flow of women through the study is shown in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 2) in line with CONSORT recommendations. 56
FIGURE 2.
CONSORT diagram of women who were recruited to the PROSPECT Study. a, Missed [operation performed in a different hospital/Trust, private hospital or by a different consultant; research nurse not aware of the operation date because of cancellations/fast tracking/lack of communication between the research team; surgery date after the end of the study; not consented prior to operation date – research nurse not available; consent form not returned, unable to contact, inappropriate to approach (personal/medical reasons). Eligible for cohort only and trial no longer recruiting to cohort study; Other, e.g. moved away, did not attend, etc.; 339 women]. b, Reasons for ineligibility (261 women): surgery not required [e.g. no prolapse, changed mind about needing surgery (117); removed from waiting list/unfit for surgery (45); unable to give informed consent (32); unable to complete questionnaires (16); other reasons for non-recruitment (‘psychological or family problems’, ‘not clinically or medically suitable to take part in a research study’ and ‘consultant wished to decide procedure’ 32); reason not recorded (19)]. c, Reasons for non-randomisation in Primary trial (entry to CC1): ‘Clinical decision’ includes ‘wanted to use mesh’, ‘did not want to use mesh’ and ‘other clinical reason’ (379); ‘Participant decision’ includes ‘wanted mesh’, ‘did not want mesh’ ‘wanted surgeon to decide’ and ‘did not want to be randomised’ (613); ‘Other’ reasons include ‘mesh unavailable’, ‘operating surgeon not trained in mesh inlays/kits’, ‘theatre time issues’ and ‘not recorded’ (134). d, Reasons for non-randomisation in Secondary trial (entry to CC2: ‘Clinical decision’ includes ‘wanted to use mesh’, ‘did not want to use mesh’ and ‘other clinical reason’ (133); ‘Participant decision’ includes ‘wanted mesh’, ‘did not want mesh’ ‘wanted surgeon to decide’ and ‘did not want to be randomised’ (96); ‘Other’ reasons include ‘mesh unavailable’, ‘operating surgeon not trained in mesh inlays/kits’, ‘theatre time issues’ and ‘not recorded’ (12). e, Randomised in error: one woman had baseline comorbidities that made her ineligible for PROSPECT; one woman had prolapse surgery privately after declining to participate but prior to randomisation. f, Two women should have been randomised in the Secondary trial. The women remained eligible for PROSPECT and were followed up in the secondary cohort CC2. g, One woman had secondary prolapse surgery after consenting but prior to randomisation. She remained eligible for PROSPECT and is followed up in the secondary cohort CC2. N/A, not applicable.
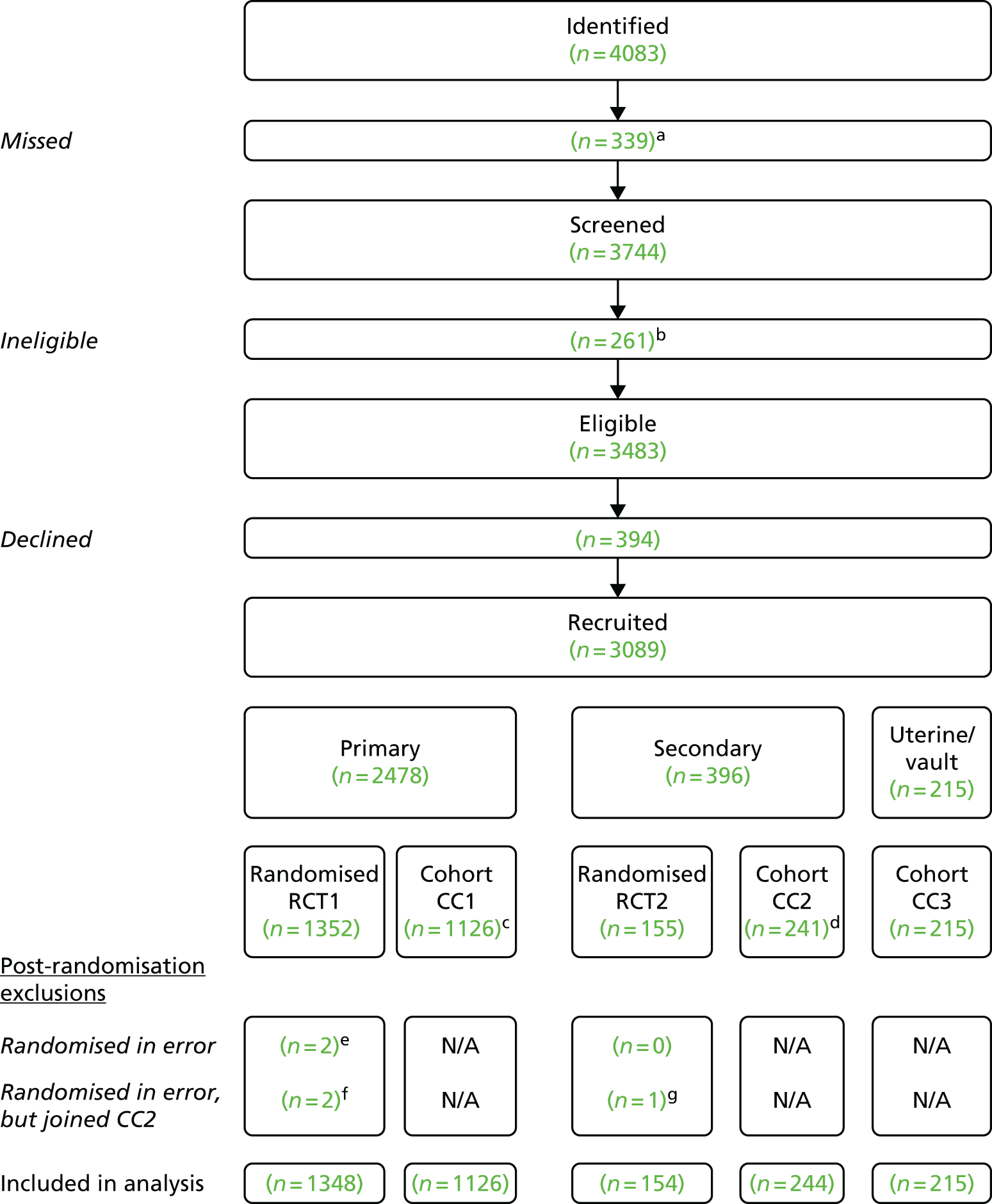
Of the 3089 women participating in the study, 2478 were recruited to the primary group (1352 randomised to the Primary trial (RCT1); 1126 to the Primary CC (CC1), 396 to the secondary group (155 to the Secondary trial (RCT2); 241 to the Secondary CC (CC2) and a further 215 women were in the third CC (CC3) if they were thought to need only uterine or vault prolapse surgery (see Figure 2). There were two post-randomisation exclusions that were not included in the study analyses (see Figure 2), leaving 3087 women analysed in the PROSPECT Study.
This chapter describes how these women were identified from the women admitted for prolapse surgery in the 37 hospitals, 35 of which recruited women. It reports the baseline differences between the comparable groups of women and their baseline characteristics up to the point of entry to the RCTs or the CCs. The subsequent findings are described in Chapter 4 (primary prolapse surgery), Chapter 6 (secondary prolapse surgery) and Chapter 8 (upper compartment prolapse surgery only: uterine and vault prolapse).
Study recruitment
As described in Chapter 2, women who attended gynaecology outpatient departments with symptomatic pelvic organ prolapse and then chose to have prolapse surgery, and women on the waiting list for prolapse surgery, were invited to participate in the PROSPECT Study. Women were asked if they were willing to be randomised to the appropriate options for their type of prolapse, and if not, they were asked to consent to follow-up by questionnaire as part of the CC. The centres and surgeons who participated in PROSPECT, the operations they offered and the numbers they recruited are listed in Table 4. The rate of recruitment is illustrated in Figure 3.
| Centre and surgeon | Primary repair | Secondary repair | Uterine/vault only | Uterine | Vault | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Synthetic mesh | Biological graft | Number randomised | Number recruited to CC1 | Synthetic mesh | Mesh kit | Number randomised | Number recruited to CC2 | Number recruited to CC3 | |||
| Aberdeen | |||||||||||
| C Bain/Hemming | ✓ | ✓ | 174 | 18 | ✓ | ✓ | 19 | 16 | 6 | 4 | 2 |
| K Cooper | ✓ | ✓ | 53 | 17 | ✓ | ✓ | 7 | 7 | 1 | 0 | 1 |
| M Abdel-Fattah | ✓ | ✗ | 8 | 3 | ✓ | ✓ | 0 | 3 | 1 | 0 | 1 |
| P Terry | ✓ | ✓ | 5 | 1 | ✓ | ✗ | 1 | 0 | 1 | 1 | 0 |
| Ayrshire & Arran | |||||||||||
| W Agur | ✓ | ✓ | 33 | 106 | ✓ | ✓ | 4 | 23 | 2 | 0 | 2 |
| D Rae | ✓ | ✗ | 5 | 1 | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 |
| Barnsley | |||||||||||
| K Farag | ✓ | ✓ | 4 | 10 | ✓ | ✗ | 1 | 1 | 0 | 0 | 0 |
| M Dass | ✗ | ✗ | N/A | 14 | ✓ | ✓ | 1 | 1 | 0 | 0 | 0 |
| Birmingham | |||||||||||
| P Toozs-Hobson | ✓ | ✗ | 35 | 35 | ✓ | ✗ | 10 | 22 | 1 | 0 | 1 |
| P Latthe | ✓ | ✗ | 1 | 6 | ✓ | ✗ | 1 | 5 | 0 | 0 | 0 |
| M Parsons | ✓ | ✗ | 5 | 7 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| Bolton | |||||||||||
| A Williams | ✓ | ✓ | 5 | 13 | ✓ | ✗ | 0 | 1 | 0 | 0 | 0 |
| P Chia | ✓ | ✓ | 3 | 5 | ✓ | ✗ | 0 | 1 | 0 | 0 | 0 |
| N Ali-Ross | ✗ | ✗ | N/A | 1 | ✗ | ✗ | N/A | 0 | 0 | 0 | 0 |
| Bradford | |||||||||||
| C Ramage | ✓ | ✓ | 60 | 25 | ✓ | ✓ | 13 | 1 | 0 | 0 | 0 |
| S Calvert | ✗ | ✗ | 0 | 37 | ✓ | ✓ | 3 | 1 | 1 | 1 | 0 |
| Brighton | |||||||||||
| S Ismail | ✓ | ✓ | 0 | 0 | ✗ | ✓ | 1 | 0 | 0 | 0 | 0 |
| Calderdale | |||||||||||
| Y Chan | ✓ | ✓ | 12 | 4 | ✓ | ✗ | 3 | 3 | 0 | 0 | 0 |
| A Bondili | ✓ | ✓ | 1 | 0 | ✓ | ✗ | 1 | 2 | 0 | 0 | 0 |
| Chester | |||||||||||
| M Ibraheim | ✓ | ✓ | 33 | 45 | ✓ | ✓ | 1 | 7 | 2 | 1 | 1 |
| L Dinardo | ✓ | ✓ | 0 | 3 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| Derby | |||||||||||
| J Dasgupta | ✓ | ✗ | 19 | 4 | ✓ | ✗ | 2 | 0 | 2 | 2 | 0 |
| V Chilaka | ✓ | ✓ | 1 | 0 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| Exeter | |||||||||||
| M Taylor | ✓ | ✓ | 14 | 21 | ✓ | ✗ | 2 | 8 | 0 | 0 | 0 |
| R Sturley | ✓ | ✓ | 11 | 14 | ✓ | ✗ | 4 | 2 | 0 | 0 | 0 |
| Harrogate | |||||||||||
| A Barnett | ✓ | ✗ | 5 | 8 | ✓ | ✓ | 1 | 1 | 0 | 0 | 0 |
| T Jackson | ✓ | ✗ | 7 | 6 | ✓ | ✓ | 1 | 0 | 0 | 0 | 0 |
| Hull | |||||||||||
| J Gandhi | ✓ | ✗ | 16 | 5 | ✓ | ✓ | 3 | 4 | 0 | 0 | 0 |
| Leicester | |||||||||||
| D Tincello | ✓ | ✗ | 31 | 6 | ✓ | ✓ | 1 | 2 | 0 | 0 | 0 |
| Luton | |||||||||||
| A Fayyad | ✓ | ✗ | 8 | 18 | ✓ | ✓ | 0 | 1 | 0 | 0 | 0 |
| Maidstone | |||||||||||
| R Connell | ✓ | ✓ | 4 | 3 | ✓ | ✓ | 3 | 1 | 0 | 0 | 0 |
| Manchester | |||||||||||
| A Smith | ✓ | ✓ | 64 | 39 | ✓ | ✓ | 11 | 14 | 130 | 25 | 105 |
| F Reid | ✓ | ✓ | 59 | 65 | ✓ | ✓ | 6 | 8 | 38 | 20 | 18 |
| K Ward | ✓ | ✓ | 0 | 0 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| Mid Yorkshire | |||||||||||
| K Fishwick | ✓ | ✓ | 37 | 24 | ✓ | ✓ | 0 | 5 | 3 | 0 | 3 |
| North Bristol | |||||||||||
| P Smith | ✗ | ✓ | 46 | 16 | ✓ | ✓ | 1 | 4 | 0 | 0 | 0 |
| North Cumbria | |||||||||||
| M Mater | ✓ | ✗ | 8 | 3 | ✗ | ✓ | 0 | 2 | 0 | 0 | 0 |
| North Devon | |||||||||||
| S Eckford | ✓ | ✗ | 33 | 54 | ✓ | ✓ | 0 | 6 | 2 | 0 | 2 |
| O Eskandar | ✓ | ✗ | 30 | 73 | ✓ | ✓ | 1 | 7 | 3 | 1 | 2 |
| Nottingham | |||||||||||
| R Parkinson | ✓ | ✗ | 0 | 1 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| P Hooper | ✓ | ✗ | 42 | 29 | ✓ | ✗ | 0 | 10 | 3 | 1 | 2 |
| M Das | ✓ | ✗ | 9 | 2 | ✓ | ✗ | 2 | 2 | 0 | 0 | 0 |
| Portsmouth | |||||||||||
| P Hogson | ✓ | ✓ | 4 | 6 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| Plymouth | |||||||||||
| R Freeman | ✓ | ✓ | 60 | 65 | ✓ | ✗ | 14 | 8 | 4 | 3 | 1 |
| L Bombieri | ✗ | ✓ | 116 | 30 | ✗ | ✗ | N/A | 5 | 1 | 1 | 0 |
| Preston | |||||||||||
| S Prashar | ✓ | ✗ | 41 | 41 | ✓ | ✓ | 7 | 6 | 0 | 0 | 0 |
| Rotherham | |||||||||||
| D Patel | ✓ | ✗ | 6 | 8 | ✓ | ✓ | 1 | 2 | 0 | 0 | 0 |
| South Devon | |||||||||||
| S Narayanan | ✓ | ✓ | 58 | 48 | ✓ | ✓ | 10 | 5 | 0 | 0 | 0 |
| South Tees | |||||||||||
| P Ballard | ✓ | ✓ | 40 | 61 | ✓ | ✓ | 5 | 13 | 3 | 2 | 1 |
| A Khunda | ✓ | ✓ | 28 | 15 | ✓ | ✓ | 4 | 1 | 1 | 1 | 0 |
| St Mary’s, London | |||||||||||
| V Khullar | ✓ | ✓ | 3 | 1 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| R Fernando | ✓ | ✓ | 0 | 0 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| A Digesu | ✓ | ✓ | 2 | 0 | ✓ | ✗ | 0 | 0 | 0 | 0 | 0 |
| Sunderland | |||||||||||
| J Chamberlain | ✓ | ✓ | 8 | 0 | ✓ | ✗ | 3 | 0 | 1 | 1 | 0 |
| Taunton | |||||||||||
| A Naguib | ✗ | ✓ | 25 | 0 | ✗ | ✗ | N/A | N/A | 1 | 1 | 0 |
| West Middlesex | |||||||||||
| M Reyad | ✓ | ✓ | 2 | 0 | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 |
| Whipps Cross, London | |||||||||||
| S Hussain | ✓ | ✓ | 6 | 3 | ✓ | ✓ | 2 | 1 | 2 | 2 | 0 |
| B Dawlatly | ✓ | ✓ | 3 | 0 | ✓ | ✓ | 0 | 1 | 1 | 1 | 0 |
| S Visvanathan | ✓ | ✓ | 1 | 0 | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 |
| Wolverhampton | |||||||||||
| A Elnaqa | ✓ | ✓ | 17 | 21 | ✓ | ✓ | 1 | 6 | 2 | 1 | 1 |
| C Cox | ✓ | ✗ | 16 | 22 | ✓ | ✓ | 3 | 4 | 1 | 0 | 1 |
| K Afifi | ✓ | ✗ | 1 | 14 | ✓ | ✓ | 0 | 3 | 1 | 0 | 1 |
| York | |||||||||||
| N Dean | ✗ | ✓ | 14 | 11 | ✗ | ✗ | N/A | 4 | 1 | 0 | 1 |
| O Adekanmi | ✗ | ✓ | 13 | 24 | ✗ | ✗ | N/A | 10 | 0 | 0 | 0 |
| A Evans | ✗ | ✓ | 3 | 14 | ✗ | ✗ | N/A | 4 | 0 | 0 | 0 |
FIGURE 3.
Recruitment graph.
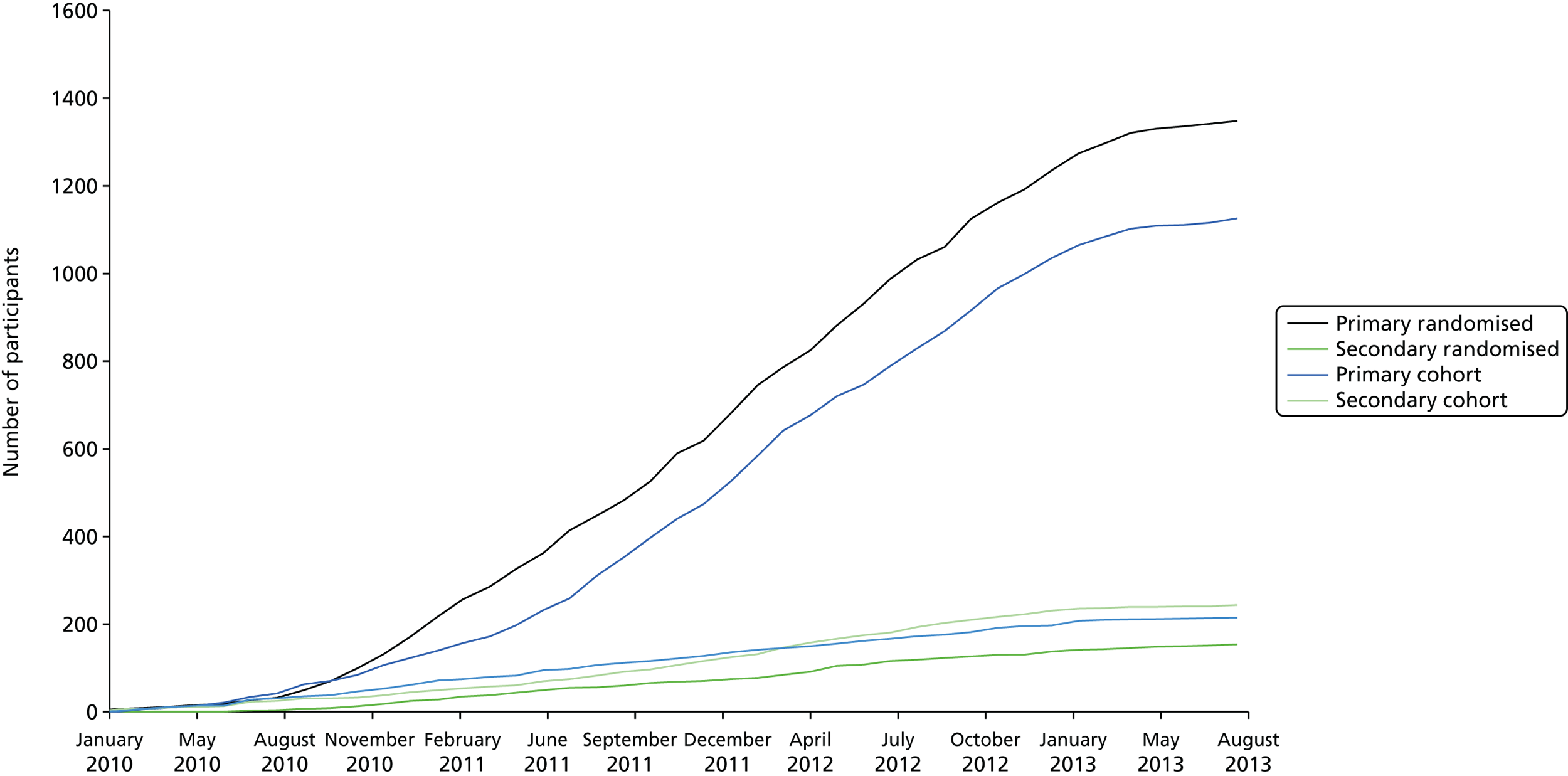
Non-recruited women
Of the 4083 women approached regarding trial participation, 994 did not enter any of the study groups because they were either missed (n = 339), ineligible (n = 261) or declined (n = 394) (see Figure 2). The 994 women who were not recruited to any part of the study are described in Figure 2. The most common reasons were ‘not interested’ (394/994; 40%), a missed opportunity to recruit the potential participant (339/994; 34%), operation cancelled because it was no longer required (117/994; 12%) or because the woman was unfit for surgery (45/994; 5%). Excluding the 339 women who were missed, and the 117 who were found not to need surgery, they represented 538 of 3627 (15%) of all of the potentially eligible women in the centres.
Age was recorded for all recruited women and for 936 of 994 (94%) of non-recruited women. The mean age of non-recruited women was 63.4 years (SD 11.9 years) n = 936, compared with 59.7 years (SD 11.0 years) n = 3089 for recruited women: the recruited women were significantly younger (p < 0.001). We could determine the primary/secondary status of 580 of the ineligible women, and only 13.8% were having further prolapse surgery – the same as the proportion in the recruited women. Therefore, the discrepancy in age was not explained by a larger proportion of women who were having further surgery among the non-recruited women.
The baseline characteristics of the 3087 women who agreed to participate in PROSPECT and were truly eligible for the study are described in Table 5. More women were randomised if they were having a primary procedure (n = 1348) than those who went into the non-randomised cohort (n = 1126), whereas for those having a secondary (repeat) procedure, fewer were randomised (n = 154) than not (n = 244). At preoperative assessment, a further 215 women were not thought to have an anterior or posterior prolapse that required surgical repair, but did have uterine or vault prolapse. These women are described and compared in detail in Chapter 8 and are not further analysed in this chapter. However, their data are provided in the tables for completeness (CC3).
| Baseline characteristic | Primary RCT: RCT1 (N = 1348 women) | Primary cohort: CC1 (N = 1126 women) | p-value | Secondary RCT: RCT2 (N = 154 women) | Secondary cohort: CC2 (N = 244 women) | p-value | Uterine/vault: CC3 (N = 215 women) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 59.5 | (10.4) | 1348 | 59.4 | (11.6) | 1126 | 0.848 | 62.2 | (9.8) | 154 | 62.1 | (10.1) | 244 | 0.934 | 60.7 | (12.2) | 215 |
| Parity (mean) | 2.7 | (1.1) | 1344 | 2.5 | (1.1) | 1115 | 0.006 | 2.7 | (1.1) | 152 | 2.5 | (1.1) | 244 | 0.197 | 2.7 | (1.4) | 215 |
| Parity (median) | 2.0 | (0–9) | 1344 | 2.0 | (0–12) | 1115 | 2.0 | (1–8) | 152 | 2.0 | (0–8) | 244 | 2.0 | (0–12) | 215 | ||
| BMI, kg/m2 (mean) | 28.6 | (4.8) | 1204 | 28.2 | (4.8) | 1023 | 0.034 | 29.1 | (4.9) | 134 | 28.7 | (4.8) | 220 | 0.496 | 27.2 | (4.3) | 202 |
| BMI, kg/m2 (median) | 27.9 | (16–49) | 1204 | 27.5 | (17–50) | 1023 | 28.1 | (19–46) | 134 | 28.2 | (19–44) | 220 | 26.8 | (19–40) | 202 | ||
| Delivery mode history | |||||||||||||||||
| Spontaneous vaginal delivery | 2.3 | (1.3) | 1320 | 2.2 | (1.2) | 1085 | 0.219 | 2.3 | (1.3) | 147 | 2.2 | (1.3) | 237 | 0.676 | 2.5 | (1.5) | 211 |
| Forceps | 0.2 | (0.5) | 1320 | 0.2 | (0.4) | 1085 | 0.120 | 0.3 | (0.5) | 147 | 0.2 | (0.5) | 237 | 0.211 | 0.2 | (0.4) | 211 |
| Breech | 0.0 | (0.2) | 1320 | 0.0 | (0.2) | 1085 | 0.388 | 0.1 | (0.2) | 147 | 0.0 | (0.2) | 237 | 0.191 | 0.0 | (0.2) | 211 |
| Elective CS | 0.1 | (0.3) | 1320 | 0.1 | (0.2) | 1085 | 0.381 | 0.0 | (0.1) | 147 | 0.0 | (0.1) | 237 | 0.802 | 0.0 | (0.2) | 211 |
| Emergency CS | 0.0 | (0.2) | 1320 | 0.0 | (0.2) | 1085 | 0.074 | 0.0 | (0.2) | 147 | 0.0 | (0.2) | 237 | 0.741 | 0.0 | (0.1) | 211 |
| Vacuum | 0.0 | (0.2) | 1320 | 0.0 | (0.1) | 1085 | 0.206 | 0.0 | (0.1) | 147 | 0.0 | (0.1) | 237 | 0.576 | 0.0 | (0.1) | 211 |
| EQ-5D | |||||||||||||||||
| Score | 0.71 | (0.24) | 1232 | 0.71 | (0.25) | 964 | 0.839 | 0.69 | (0.25) | 145 | 0.65 | (0.26) | 213 | 0.151 | 0.65 | (0.33) | 189 |
| Conservative treatment | |||||||||||||||||
| Vaginal pessary | 14.4% | 193 | 1342 | 11.8% | 131 | 1111 | 0.059 | 8.7% | 13 | 150 | 9.9% | 24 | 242 | 0.681 | 13.1% | 28 | 213 |
| Physiotherapy for POP | 26.7% | 358 | 1340 | 30.3% | 335 | 1105 | 0.049 | 35.5% | 54 | 152 | 32.1% | 76 | 237 | 0.480 | 29.7% | 62 | 209 |
| Physiotherapy for UI | 16.1% | 215 | 1338 | 17.7% | 196 | 1107 | 0.281 | 16.4% | 25 | 152 | 16.4% | 39 | 238 | 0.987 | 17.8% | 37 | 208 |
| Drugs for UI | 10.3% | 137 | 1335 | 11.8% | 130 | 1100 | 0.221 | 13.8% | 21 | 152 | 16.4% | 39 | 238 | 0.493 | 10.7% | 22 | 205 |
| Previous surgery | |||||||||||||||||
| Prolapse repair | 10.3% | 139 | 1348 | 12.2% | 137 | 1126 | 0.144 | 100.0% | 154 | 154 | 100.0% | 244 | 244 | N/A | 43.7% | 94 | 215 |
| Anterior | 5.0% | 67 | 1348 | 5.0% | 56 | 1126 | 0.997 | 81.2% | 125 | 154 | 86.1% | 210 | 244 | 0.192 | 26.5% | 57 | 215 |
| Posterior | 3.0% | 40 | 1348 | 3.6% | 40 | 1126 | 0.413 | 55.2% | 85 | 154 | 57.0% | 139 | 244 | 0.728 | 19.1% | 41 | 215 |
| Anterior and posterior | 0.0% | 0 | 1348 | 0.0% | 0 | 1126 | N/A | 36.4% | 56 | 154 | 43.0% | 105 | 244 | 0.187 | 13.0% | 28 | 215 |
| Vault | 1.5% | 20 | 1348 | 2.6% | 29 | 1126 | 0.052 | 9.7% | 15 | 154 | 14.8% | 36 | 244 | 0.145 | 11.6% | 25 | 215 |
| Unknown | 1.6% | 22 | 1348 | 2.3% | 26 | 1126 | 0.224 | 1.3% | 2 | 154 | 1.2% | 3 | 244 | 0.952 | 3.3% | 7 | 215 |
| Hysterectomy | 26.8% | 361 | 1348 | 29.8% | 336 | 1126 | 0.092 | 62.1% | 95 | 153 | 73.0% | 178 | 244 | 0.023 | 67.9% | 146 | 215 |
| Vaginal | 9.8% | 132 | 1348 | 10.7% | 121 | 1126 | 0.436 | 36.6% | 56 | 153 | 44.7% | 109 | 244 | 0.112 | 32.1% | 69 | 215 |
| Cervical amputation | 1.9% | 25 | 1348 | 2.7% | 30 | 1126 | 0.174 | 8.5% | 13 | 153 | 7.0% | 17 | 244 | 0.575 | 2.3% | 5 | 215 |
| Abdominal | 16.9% | 228 | 1348 | 18.7% | 210 | 1126 | 0.260 | 25.5% | 39 | 153 | 27.0% | 66 | 244 | 0.732 | 34.9% | 75 | 215 |
| UI surgery | 6.0% | 80 | 1341 | 7.7% | 86 | 1111 | 0.082 | 13.2% | 20 | 151 | 16.4% | 39 | 238 | 0.400 | 13.7% | 29 | 211 |
Epidemiological characteristics
There were no significant differences between the women who were having primary prolapse surgery who were randomised and those who were not randomised (RCT1 vs. CC1) or between women who were having a second or subsequent repair (RCT2 vs. CC2) according to randomisation status (see Table 5). However, those having a repeat repair were, on average, 2.6 years older than those having a primary procedure, and those having uterine or vault surgery only (CC3) were, on average, 1.2 years older than those having primary surgery.
There were also no differences between any of the groups with respect to:
-
body mass index (BMI)
-
parity (the median number of children was two)
-
delivery mode history.
Generic quality of life: EuroQol-5 Dimensions (3-level version)
There were no significant differences between randomised and cohort women who were having first or repeat surgery with respect to EQ-5D-3L scores at baseline. However, those having repeat surgery or uterine or vault operations had slightly lower (worse) scores than those having their first repair.
Previous conservative treatment
Around one-quarter to one-third of women had PFMT for prolapse symptoms, supervised by a physiotherapist, before resorting to surgery, with this being slightly more common for women who were having a repeat procedure (see Table 5). Fewer than 15% of women who were having a primary repair, and around 10% of those having a secondary repair, were currently using a vaginal pessary (ring or other type). Just over 15% of women in each group had already had supervised PFMT for UI, and 10–15% had used drug treatment for this problem in the past.
Previous surgery
From Table 5, around 10–12% of women in the primary groups were having a second anterior or posterior prolapse repair. However, these women were classed as primary because the compartment that required surgery as part of PROSPECT was the opposite to that which had previously been repaired. This is in accordance with the recommended IUGA/ICS terminology. 57 If the woman thought she had had a previous prolapse repair but it was not possible to discover in which compartment, she was classed as primary for the purpose of allocation, but this applied to only 60 women across the five groups.
Very few women who were having a primary repair had had a previous vault repair (around 2%), whereas this was more common for women who were having a repeat repair (10–15%).
Fewer than 10% of women who were having their first repair had previous concomitant continence surgery, whereas it was around 15% for those having a repeat repair, a similar proportion to those having an upper compartment procedure only.
Slightly more women had undergone previous uterine surgery (hysterectomy) in the cohorts than in the randomised groups, both for primary and secondary repairs, but this was statistically significant for only the latter (p = 0.023; see Table 5). Among women who were having their first repair, more had undergone a previous abdominal hysterectomy than a vaginal hysterectomy (or cervical amputation, which is necessarily carried out via the vagina), whereas for those having a second repair, more women had had a previous vaginal hysterectomy than abdominal. Overall, many more women who were having a repeat repair had already had a previous hysterectomy than those having their first repair (around 62–73% of the Secondary group and just under 30% of the Primary group).
Planned surgery
Although it is known that in about 20% of case the actual operation carried out differs from that planned in advance,29 PROSPECT was designed so that women would remain in the group to which they were allocated, irrespective of the actual procedure performed. In order to randomise women appropriately, taking account of minimisation criteria, gynaecologists had to specify in advance which compartments they thought would need to be repaired.
Gynaecologists planned surgery for the women based on their preoperative findings on examination. Table 6 shows that just under half of the women were expected to require an anterior repair, about one-quarter a posterior repair, and the remainder both procedures. The proportions were the same whether the procedure was primary or repeat, and whether or not the women were randomised.
| Type of surgery | Primary RCT: RCT1 (N = 1348 women) | Primary cohort: CC1 (N = 1126 women) | p-value | Secondary RCT: RCT2 (N = 154 women) | Secondary cohort: CC2 (N = 244 women) | p-value | Uterine/vault: CC3 (N = 215 women) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior repair | 44.8% | 604 | 1348 | 44.5% | 501 | 1126 | 0.876 | 46.8% | 72 | 154 | 45.9% | 112 | 244 | 0.868 | 0.0% | 0 | 215 |
| Posterior repair | 25.8% | 348 | 1348 | 26.8% | 302 | 1126 | 0.572 | 26.0% | 40 | 154 | 26.6% | 65 | 244 | 0.883 | 0.0% | 0 | 215 |
| Anterior and posterior repair | 29.4% | 396 | 1348 | 28.7% | 323 | 1126 | 0.706 | 27.3% | 42 | 154 | 27.5% | 67 | 244 | 0.968 | 0.0% | 0 | 215 |
| Upper compartment repair only | 0.0% | 0 | 1348 | 0.0% | 0 | 1126 | N/A | 0.0% | 0 | 154 | 0.0% | 0 | 244 | N/A | 100.0% | 215 | 215 |
| Concomitant prolapse surgery | |||||||||||||||||
| Vaginal hysterectomy | 34.7% | 468 | 1348 | 32.7% | 368 | 1126 | 0.286 | 13.6% | 21 | 154 | 6.6% | 16 | 244 | 0.018 | 10.2% | 22 | 215 |
| Abdominal hysterectomy | 0.1% | 1 | 1348 | 0.4% | 4 | 1126 | 0.121 | 0.6% | 1 | 154 | 0.8% | 2 | 244 | 0.848 | 2.3% | 5 | 215 |
| Cervical amputation | 1.7% | 23 | 1348 | 1.4% | 16 | 1126 | 0.571 | 0.0% | 0 | 154 | 0.8% | 2 | 244 | 0.260 | 1.9% | 4 | 215 |
| Vault repair | 15.5% | 209 | 1348 | 20.9% | 235 | 1126 | 0.001 | 22.7% | 35 | 154 | 27.0% | 66 | 244 | 0.335 | 90.7% | 195 | 215 |
| Concomitant UI surgery | 11.0% | 148 | 1348 | 13.3% | 150 | 1126 | 0.075 | 4.5% | 7 | 154 | 6.6% | 16 | 244 | 0.402 | 4.2% | 9 | 215 |
In terms of concomitant prolapse surgery, hysterectomy was planned more frequently in women in the Primary trial, whereas in the Secondary trial more women were thought to need a vault repair (see Table 6). The need for vault repair was higher in both cohort groups (although statistically significant only in the larger Primary trial), suggesting that women who might need a concomitant upper compartment procedure were less likely to be randomised. Cervical amputation was planned much less commonly (< 2% in any group). Between RCT and CC cohort groups, there was little difference in the frequency of women who were thought to need continence surgery, but the proportions were fewer in the secondary groups.
Preoperative objective measurements
Although gynaecologists were expected to use the POP-Q,27 not all did so. However, attempts were made to locate important missing data from the centres, using alternative sources such as medical notes, correspondence and asking the centre staff. The aim was to have as complete a set of prolapse staging as possible, separately in each compartment. The leading edge of the most descended compartment relative to the hymen was used for overall (POP-Q) stage.
The most common stage for women who were having an anterior or posterior repair was stage 2 (around 60%; Table 7). The majority of the remaining women were stage 3, and very few were stage 4, or indeed stage 0 or 1. Using a more strict definition of ‘leading edge of prolapse beyond the hymen (> 0 cm on POP-Q; stage 2b, 3 or 4), 61–67% of women in the Primary trial and 52–58% in the Secondary trial had an objective prolapse (see Table 7). Significantly more women had objective prolapse in the randomised arm of the Primary trial than in the primary cohort; the small excess among cohort women in the Secondary trial was not significant.
| POP-Q measurement/stage | Primary RCT: RCT1 (N = 1348 women) | Primary cohort: CC1 (N = 1126 women) | p-value | Secondary RCT: RCT2 (N = 154 women) | Secondary cohort: CC2 (N = 244 women) | p-value | Uterine/vault: CC3 (N =215 women) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| POP-Q measurement (cm) | |||||||||||||||||
| Ba (posterior edge) | 0.5 | (2.1) | 1190 | 0.4 | (2.1) | 860 | 0.792 | 0.1 | (2.0) | 143 | 0.3 | (2.1) | 201 | 0.360 | 2.0 | (2.4) | 193 |
| C (cervix/vault) | –3.4 | (3.4) | 1109 | –3.2 | (3.3) | 812 | 0.414 | –4.1 | (3.2) | 135 | –3.3 | (3.5) | 191 | 0.037 | 0.2 | (3.8) | 184 |
| Bp (posterior edge) | –0.4 | (1.9) | 1187 | –0.5 | (1.8) | 855 | 0.121 | –0.5 | (2.0) | 140 | –0.3 | (2.1) | 194 | 0.484 | 0.7 | (2.7) | 192 |
| TVL | 8.4 | (1.7) | 1090 | 8.5 | (1.8) | 809 | 0.029 | 8.2 | (2.3) | 129 | 8.0 | (1.5) | 179 | 0.422 | 8.5 | (1.7) | 159 |
| Overall POP-Q stage | |||||||||||||||||
| 0 | 0.2% | 2 | 1293 | 0.0% | 0 | 997 | < 0.001 | 0.0% | 0 | 153 | 0.4% | 1 | 227 | 0.200 | 0.0% | 0 | 203 |
| 1 | 1.0% | 13 | 1293 | 2.0% | 20 | 997 | 0.0% | 0 | 153 | 0.9% | 2 | 227 | 1.5% | 3 | 203 | ||
| 2 | 56.3% | 728 | 1293 | 61.9% | 617 | 997 | 68.0% | 104 | 153 | 60.8% | 138 | 227 | 37.4% | 76 | 203 | ||
| 3 | 39.9% | 516 | 1293 | 34.1% | 340 | 997 | 30.7% | 47 | 153 | 31.3% | 71 | 227 | 47.3% | 96 | 203 | ||
| 4 | 2.6% | 34 | 1293 | 2.0% | 20 | 997 | 1.3% | 2 | 153 | 6.6% | 15 | 227 | 13.8% | 28 | 203 | ||
| 2b, 3 or 4 | 66.9% | 825 | 1233 | 60.7% | 537 | 884 | 0.004 | 52.1% | 76 | 146 | 57.8% | 119 | 206 | 0.288 | 82.1% | 160 | 195 |
Clinical baseline data
Prolapse symptoms at baseline
The overall prolapse symptom score (POP-SS) was around 13.5 in the women who were having their first repair [with no difference between the randomised and cohort women (13.7 vs. 13.3; Table 8)] but significantly higher in those having a repeat procedure [with no difference between the randomised and cohort women (14.4 vs. 14.9)]. Using a POP-SS of 0 to indicate absence of symptoms, > 99% of women had at least one symptom. The women who were having a uterine or vault repair only (CC3) had an intermediate score, with a mean POP-SS of 14.5 (see Table 8). The latter group will be further described separately in Chapter 8.
| Symptom | Primary RCT: RCT1 (N = 1266 women) | Primary cohort: CC1 (N = 997 women) | p-value | Secondary RCT: RCT2 (N = 148 women) | Secondary cohort: CC2 (N = 221 women) | p-value | Uterine/vault: CC3 (N = 202 women) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| POP-SS at baseline | |||||||||||||||||
| Score | 13.7 | (5.9) | 1266 | 13.3 | (5.8) | 995 | 0.068 | 14.4 | (5.4) | 148 | 14.9 | (5.8) | 220 | 0.427 | 14.5 | (6.6) | 197 |
| Other measures of prolapse symptoms at baseline | |||||||||||||||||
| Symptoms (years) | 3.6 | (5.0) | 1218 | 3.6 | (5.0) | 948 | 0.868 | 2.8 | (3.3) | 142 | 3.8 | (5.3) | 205 | 0.034 | 4.3 | (5.2) | 184 |
| Bother (years) | 2.6 | (4.1) | 1170 | 2.5 | (3.4) | 916 | 0.392 | 2.4 | (3.0) | 140 | 2.9 | (4.1) | 201 | 0.203 | 3.2 | (3.7) | 177 |
| Number of women symptomatic | 99.6% | 1261 | 1266 | 99.6% | 991 | 995 | 0.979 | 100.0% | 148 | 148 | 99.5% | 219 | 220 | 0.411 | 100.0% | 197 | 197 |
| Prolapse-related QoL score | 6.6 | (2.7) | 1251 | 6.7 | (2.7) | 969 | 0.220 | 6.9 | (2.3) | 148 | 6.9 | (2.5) | 216 | 0.977 | 7.0 | (2.9) | 193 |
The most common individual prolapse symptom was ‘a feeling of something coming down from or in your vagina’, which was reported in > 90% of women, with two-thirds of women reporting this most or all of the time (Table 9). The QoL score (‘overall, how much do your prolapse symptoms interfere with your everyday life?’) ranged from 6.6 to 7.0 of 10 (see Table 8). About half of the women found the prolapse to pose hygiene problems, and about one in five needed to relieve pressure or discomfort from the prolapse using their fingers (see Table 9). Women had been symptomatic for ≥ 3 years and bothered by their symptoms for about a year less.
| Symptom | Primary RCT: RCT1 (N = 1266 women) | Primary cohort: CC1 (N = 997 women) | p-value | Secondary RCT: RCT2 (N = 148 women) | Secondary cohort: CC2 (N = 221 women) | p-value | Uterine/vault: CC3 (N = 202 women) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual prolapse symptoms | |||||||||||||||||
| SCD any | 92.8% | 1175 | 1266 | 93.9% | 934 | 995 | 0.319 | 93.9% | 139 | 148 | 94.5% | 208 | 220 | 0.799 | 97.0% | 191 | 197 |
| SCD freq. | 66.1% | 837 | 1266 | 66.5% | 662 | 995 | 0.834 | 73.0% | 108 | 148 | 71.4% | 157 | 220 | 0.736 | 77.7% | 153 | 197 |
| Pain any | 80.7% | 1022 | 1266 | 81.6% | 812 | 995 | 0.595 | 80.4% | 119 | 148 | 85.9% | 189 | 220 | 0.161 | 85.8% | 169 | 197 |
| Pain freq. | 34.4% | 436 | 1266 | 36.4% | 362 | 995 | 0.337 | 37.2% | 55 | 148 | 45.0% | 99 | 220 | 0.135 | 47.7% | 94 | 197 |
| Abdo. any | 81.1% | 1027 | 1266 | 79.2% | 788 | 995 | 0.253 | 83.8% | 124 | 148 | 85.9% | 189 | 220 | 0.575 | 80.2% | 158 | 197 |
| Abdo. freq. | 34.2% | 433 | 1266 | 33.4% | 332 | 995 | 0.677 | 37.8% | 56 | 148 | 45.0% | 99 | 220 | 0.172 | 35.0% | 69 | 197 |
| Back any | 72.9% | 923 | 1266 | 68.6% | 683 | 995 | 0.027 | 73.6% | 109 | 148 | 82.7% | 182 | 220 | 0.036 | 70.6% | 139 | 197 |
| Back freq. | 29.9% | 379 | 1266 | 25.8% | 257 | 995 | 0.031 | 35.1% | 52 | 148 | 34.1% | 75 | 220 | 0.836 | 28.9% | 57 | 197 |
| Strain blad. any | 71.3% | 903 | 1266 | 69.5% | 692 | 995 | 0.357 | 70.9% | 105 | 148 | 67.7% | 149 | 220 | 0.513 | 69.0% | 136 | 197 |
| Strain blad. freq. | 29.0% | 367 | 1266 | 26.1% | 260 | 995 | 0.132 | 27.0% | 40 | 148 | 29.5% | 65 | 220 | 0.600 | 32.5% | 64 | 197 |
| Blad. not empty any | 82.5% | 1044 | 1266 | 83.0% | 826 | 995 | 0.731 | 85.1% | 126 | 148 | 82.7% | 182 | 220 | 0.540 | 84.3% | 166 | 197 |
| Blad. not empty freq. | 38.3% | 485 | 1266 | 35.7% | 355 | 995 | 0.199 | 41.9% | 62 | 148 | 35.9% | 79 | 220 | 0.247 | 39.6% | 78 | 197 |
| Bowel not empty any | 82.5% | 1044 | 1266 | 79.0% | 786 | 995 | 0.037 | 85.1% | 126 | 148 | 85.0% | 187 | 220 | 0.972 | 80.2% | 158 | 197 |
| Bowel not empty freq. | 37.2% | 471 | 1266 | 30.8% | 306 | 995 | 0.001 | 34.5% | 51 | 148 | 43.2% | 95 | 220 | 0.094 | 31.0% | 61 | 197 |
| Actions necessitated by prolapse symptoms | |||||||||||||||||
| Fingers to ease discomfort | 20.8% | 259 | 1247 | 19.4% | 189 | 972 | 0.440 | 16.4% | 24 | 146 | 16.0% | 34 | 213 | 0.904 | 28.0% | 53 | 189 |
| Extra hygiene measures | 51.7% | 640 | 1239 | 47.9% | 465 | 970 | 0.083 | 51.7% | 74 | 143 | 56.1% | 120 | 214 | 0.421 | 58.9% | 113 | 192 |
| Fingers to help empty bladder | 3.7% | 46 | 1249 | 4.9% | 48 | 976 | 0.151 | 4.8% | 7 | 147 | 3.7% | 8 | 214 | 0.632 | 11.1% | 21 | 190 |
| Fingers to help empty bowel | 10.8% | 134 | 1244 | 11.2% | 109 | 972 | 0.741 | 5.5% | 8 | 145 | 8.3% | 18 | 217 | 0.316 | 4.7% | 9 | 192 |
| Digital evacuation of bowel | 7.5% | 93 | 1246 | 8.4% | 82 | 977 | 0.419 | 6.3% | 9 | 144 | 5.1% | 11 | 215 | 0.646 | 4.6% | 9 | 196 |
Among women having a recent repair, there was a significant difference in duration of symptoms in the cohort women (CC2, 3.8 years) compared with the randomised women (RCT2, 3.8 vs. 2.8 years; p = 0.034).
There were no other differences between any of the groups with respect to:
-
duration of prolapse symptoms (the mean duration ranged from 2.8 to 4.3 years)
-
duration of bothersome symptoms (the mean ranged from 2.4 to 3.2 years of bother)
-
mean prolapse symptom score (the mean ranged from 13.3 to 14.9 out of a maximum score of 28 on the Pelvic Organ Prolapse Symptom scale.
Urinary symptoms at baseline
Using the ICIQ-UI-SF,26 up to 80% of women reported at least some UI; however, 20–23% had more severe leakage based on a higher score (Table 10). The most common type of UI was stress UI: women were counted as symptomatic if they had the symptom ‘most or all of the time’. There were no systematic differences between the groups of women.
| Symptom | Primary RCT: RCT1 (N = 1266 questionnaires) | Primary cohort: CC1 (N = 997 questionnaires) | p-value | Secondary RCT: RCT2 (N = 148 questionnaires) | Secondary cohort: CC2 (N = 221 questionnaires) | p-value | Uterine/vault: CC3 (N = 202 questionnaires) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any incontinence | 77.5% | 979 | 1263 | 76.1% | 756 | 994 | 0.415 | 81.0% | 119 | 147 | 74.7% | 165 | 221 | 0.159 | 75.0% | 147 | 196 |
| Incontinence-related QoL score | 7.2 | (5.7) | 1251 | 7.2 | (5.8) | 974 | 0.761 | 7.6 | (5.6) | 146 | 7.0 | (5.8) | 220 | 0.761 | 7.1 | (6.0) | 192 |
| Severe incontinence | 20.5% | 257 | 1251 | 22.0% | 214 | 974 | 0.413 | 20.5% | 30 | 146 | 20.0% | 44 | 220 | 0.898 | 22.9% | 44 | 192 |
| ICIQ-UI-SF score | 3.6 | (3.4) | 1224 | 3.7 | (3.5) | 956 | 0.440 | 3.8 | (3.4) | 145 | 3.5 | (3.3) | 211 | 0.399 | 3.8 | (3.6) | 181 |
| Stress UI | 24.4% | 275 | 1125 | 25.0% | 220 | 881 | 0.786 | 19.6% | 27 | 138 | 18.8% | 36 | 192 | 0.853 | 22.2% | 38 | 171 |
| Urgency UI | 9.6% | 121 | 1255 | 10.6% | 104 | 982 | 0.459 | 10.2% | 15 | 147 | 10.7% | 23 | 215 | 0.880 | 11.9% | 23 | 194 |
| Overactive bladder | 5.8% | 72 | 1243 | 5.6% | 55 | 977 | 0.870 | 6.8% | 10 | 147 | 9.0% | 19 | 211 | 0.453 | 7.3% | 14 | 192 |
| ICIQ-FLUTS filling score | 5.3 | (2.9) | 1235 | 5.3 | (2.9) | 970 | 0.942 | 6.0 | (2.9) | 146 | 6.0 | (3.1) | 208 | 0.950 | 5.7 | (3.1) | 189 |
| ICIQ-FLUTS voiding score | 3.1 | (2.6) | 1244 | 3.1 | (2.6) | 975 | 0.850 | 3.4 | (2.7) | 146 | 3.2 | (2.8) | 212 | 0.510 | 3.4 | (2.7) | 192 |
| ICIQ-FLUTS incontinence score | 6.1 | (4.2) | 1111 | 6.1 | (4.3) | 858 | 0.754 | 6.0 | (4.1) | 136 | 6.2 | (4.3) | 190 | 0.761 | 5.7 | (4.2) | 169 |
Bowel symptoms at baseline
Using ROME58 criteria to define bowel symptoms, around one-quarter or more of the women reported constipation (Table 11). Over one-third had FI, defined as loss of solid or liquid stool, but not including loss of flatus (wind). Three-quarters of the FI was ‘passive,’ defined as ‘not accompanied by bowel urgency’. There were no systematic differences between the groups of women at baseline.
| Symptom | Primary RCT: RCT1 (N = 1266 questionnaires) | Primary cohort: CC1 (N = 997 questionnaires) | p-value | Secondary RCT: RCT2 (N = 148 questionnaires) | Secondary cohort: CC2 (N = 221 questionnaires) | p-value | Uterine/vault: CC3 (N = 202 questionnaires) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bowel frequency | |||||||||||||||||
| > 3 times a day | 5.8% | 72 | 1250 | 5.0% | 49 | 977 | 0.262 | 5.6% | 8 | 144 | 7.3% | 16 | 218 | 0.817 | 4.6% | 9 | 195 |
| 1–3 times a day | 33.9% | 424 | 1250 | 36.2% | 354 | 977 | 37.5% | 54 | 144 | 41.3% | 90 | 218 | 32.3% | 63 | 195 | ||
| About once a day | 40.2% | 502 | 1250 | 40.4% | 395 | 977 | 36.1% | 52 | 144 | 33.0% | 72 | 218 | 39.0% | 76 | 195 | ||
| Once every 2–3 days | 17.1% | 214 | 1250 | 16.6% | 162 | 977 | 16.7% | 24 | 144 | 15.6% | 34 | 218 | 20.0% | 39 | 195 | ||
| Weekly or less | 3.0% | 38 | 1250 | 1.7% | 17 | 977 | 4.2% | 6 | 144 | 2.8% | 6 | 218 | 4.1% | 8 | 195 | ||
| Constipation | 29.0% | 358 | 1236 | 24.6% | 239 | 970 | 0.023 | 25.5% | 37 | 145 | 31.0% | 66 | 213 | 0.262 | 24.0% | 46 | 192 |
| Bowel urgency | 12.0% | 150 | 1253 | 10.8% | 106 | 985 | 0.372 | 9.6% | 14 | 146 | 8.6% | 19 | 220 | 0.755 | 10.7% | 21 | 197 |
| FI (any) | 34.4% | 431 | 1252 | 33.4% | 328 | 982 | 0.612 | 39.5% | 58 | 147 | 36.4% | 80 | 220 | 0.549 | 32.3% | 63 | 195 |
| Passive FI | 73.3% | 315 | 430 | 75.8% | 248 | 327 | 0.420 | 82.8% | 48 | 58 | 81.3% | 65 | 80 | 0.820 | 71.4% | 45 | 63 |
| Active FI | 26.7% | 115 | 430 | 24.2% | 79 | 327 | 17.2% | 10 | 58 | 18.8% | 15 | 80 | 28.6% | 18 | 63 | ||
| Severe FI | 12.7% | 159 | 1252 | 11.2% | 110 | 982 | 0.280 | 11.6% | 17 | 147 | 11.4% | 25 | 220 | 0.953 | 9.2% | 18 | 195 |
| Bowel symptoms QoL score | 3.7 | (3.2) | 1220 | 3.6 | (3.4) | 964 | 0.427 | 3.8 | (3.2) | 141 | 3.6 | (3.1) | 212 | 0.745 | 3.2 | (3.3) | 195 |
Vaginal and sexual symptoms at baseline
We used the ICI-validated instruments to measure a variety of vaginal and sexual symptoms. These were common, and had important effects on QoL. Although the majority of women were not sexually active, in about 40% of women this was most often attributable to their prolapse symptoms (Table 12). Among the women who were sexually active, or whose reason for no sex life was ‘due to prolapse symptoms’, around 10% had dyspareunia at baseline. There were no systematic differences between the groups of women.
| Symptom | Primary RCT: RCT1 (N = 1266 questionnaires) | Primary cohort: CC1 (N = 997 questionnaires) | p-value | Secondary RCT: RCT2 (N = 148 questionnaires) | Secondary cohort: CC2 (N = 221 questionnaires) | p-value | Uterine/vault: CC3 (N = 202 questionnaires) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal | |||||||||||||||||
| ICIQ-VS score | 22.3 | (9.1) | 1130 | 22.4 | (9.3) | 878 | 0.787 | 22.3 | (9.3) | 134 | 23.8 | (9.6) | 188 | 0.162 | 24.2 | (10.1) | 171 |
| Vaginal symptoms QoL score | 5.1 | (3.1) | 1220 | 5.2 | (3.2) | 952 | 0.344 | 5.3 | (3.4) | 139 | 5.4 | (3.2) | 204 | 0.811 | 5.3 | (3.5) | 187 |
| Vagina too tight | 1.7% | 20 | 1193 | 2.8% | 26 | 925 | 0.076 | 2.2% | 3 | 139 | 5.6% | 11 | 197 | 0.122 | 3.2% | 6 | 185 |
| Sexual | |||||||||||||||||
| Sex life at present | 37.7% | 469 | 1243 | 38.6% | 373 | 967 | 0.686 | 38.6% | 56 | 145 | 29.9% | 64 | 214 | 0.086 | 35.4% | 69 | 195 |
| Reason for no sex life | |||||||||||||||||
| No partner | 26.9% | 208 | 774 | 27.9% | 166 | 594 | 0.711 | 36.0% | 32 | 89 | 27.3% | 41 | 150 | 0.239 | 35.7% | 45 | 126 |
| Vaginal symptoms | 4.0% | 31 | 774 | 4.7% | 28 | 594 | 1.1% | 1 | 89 | 5.3% | 8 | 150 | 3.2% | 4 | 126 | ||
| Prolapse symptoms | 42.4% | 328 | 774 | 41.8% | 248 | 594 | 36.0% | 32 | 89 | 38.7% | 58 | 150 | 43.7% | 55 | 126 | ||
| Other reason | 21.8% | 169 | 774 | 19.5% | 116 | 594 | 22.5% | 20 | 89 | 24.7% | 37 | 150 | 12.7% | 16 | 126 | ||
| Reason not given | 4.9% | 38 | 774 | 6.1% | 36 | 594 | 4.5% | 4 | 89 | 4.0% | 6 | 150 | 4.8% | 6 | 126 | ||
| Dyspareunia | 8.8% | 57 | 646 | 9.3% | 46 | 492 | 0.759 | 8.1% | 6 | 74 | 15.5% | 15 | 97 | 0.146 | 10.8% | 10 | 93 |
| ICI Sexual Matters score | 23.1 | (14.2) | 639 | 23.4 | (14.3) | 485 | 0.744 | 25.4 | (12.9) | 73 | 24.4 | (15.5) | 95 | 0.668 | 23.3 | (15.1) | 89 |
| Sex life QoL score | 6.4 | (3.4) | 743 | 6.4 | (3.2) | 575 | 0.901 | 7.4 | (2.7) | 78 | 6.8 | (3.3) | 112 | 0.214 | 6.7 | (3.4) | 114 |
Comparison between women who were having a first repair and those who were having a repeat repair
From Table 5, it might seem that around 11% of women in the primary repair groups (RCT1, CC1) had had previous prolapse surgery, but the compartment that required surgery was the opposite to that which had previously been repaired. The compartment of previous surgery was unknown in 60 women, who were therefore classified as having a first repair.
We identified a number of important demographic and clinical differences between women who were having a primary and a secondary repair. Those having a primary repair were, on average, 2.7 years older than those having a secondary procedure (59.4 years vs. 62.1; p < 0.001; see Table 5).
Among women who were having a primary repair, fewer had had a previous hysterectomy than those having a repeat repair (28% vs. 69%; p < 0.001; see Table 5), and in the repeat repair group, the previous hysterectomy was more likely to have been via the vaginal, rather than abdominal, route. Very few women who were having a primary repair had experienced a previous vault repair (2%), whereas this was more common for women who were having a second repair (13%; p < 0.001; see Table 5). Only 7% of women who were having their first repair had previously had continence surgery, whereas 15% of those having a second procedure had done so (p < 0.001; see Table 5).
Women who were having their first prolapse repair had a slightly lower (better) level of symptoms measured on the Pelvic Organ Prolapse Symptom scale (13.5) compared with those having repeat surgery (14.7; p < 0.001; see Table 8). This was reflected in a higher (better) score for the primary group on the generic QoL scale [EuroQol-5 Dimensions (EQ-5D) 0.71 vs. 0.67; p = 0.001; see Table 5]. Significantly more women who were having their first prolapse operation (64%) had a prolapse beyond the hymen (> 0 cm) than those (55%) having a repeat repair (p = 0.001; see Table 7).
Finally, more than three times as many women who were having a first repair were expected to require a concomitant vaginal hysterectomy (34% vs. 9%), and twice as many were expected to have concomitant continence surgery (12% vs. 6%), compared with those having a repeat repair, whereas women who were having a repeat repair were more likely to require a concomitant vault repair (25% vs. 18% for the primary group; see Table 6).
Summary
The women (both randomised and CC) enrolled in the PROSPECT Study represent 85% of the UK women who had prolapse surgery in the PROSPECT centres. We used strict definitions according to IUGA/ICS recommendations to categorise them and allocate them to clinically meaningful groups. In this chapter, we compared randomised and non-randomised women.
Summary of findings
The average age for a first prolapse repair was just < 60 years, with women who were having repeat (secondary) surgery being around 2.5 years older (see Table 5). Most women’s babies had been delivered vaginally. Although the mean BMI was < 30 kg/m2, 43 morbidly obese women with a BMI of ≥ 40 kg/m2 did receive surgery.
Women who were having a repeat repair were more likely to have had a previous vaginal hysterectomy, vault repair or continence surgery. This difference in clinical characteristics justified our initial decision to conduct two separate trials among women who were having a first repair (primary) and a repeat repair (secondary).
Prolapse symptoms and measurements
Most women were expected to have surgery for an anterior vaginal wall prolapse (see Table 6). The majority of women had stage 2 prolapse, whereas around one-third had stage 3 or 4 (see Table 7). When prolapse was redefined as the leading edge beyond the hymen (> 0 cm on POP-Q), around 63% of women had a protruding prolapse: this was most common among women who were randomised in the Primary trial (67% compared with both the Primary CC (61%) and the Secondary women [52% (RCT2), 58% (CC2)].
Women had a high level of prolapse symptomatology, as shown by their POP-SSs, of around 14 out of a maximum score of 28 (see Table 8). The most common symptom was a feeling of something coming down (over 90%; see Table 9). Women who were having repeat surgery were less likely to have prolapse beyond the hymen (see Table 7) but their symptom score was, on average, one point higher (worse) than those having their first repair, and their prolapse-related QoL score was significantly worse (see Table 8).
Other clinical symptoms
Over three-quarters of women had UI, and this was slight or moderate in most cases (see Table 10). Nevertheless, at least one in five women had severe urine leakage, defined using the ICI-UI SF score of ≥ 13, and most of them had stress UI (‘most or all of the time’). However, only around 10% of those with UI having a first repair, and 5% having a repeat repair, were expected to undergo continence surgery (see Table 6); on the other hand, 1 in 20, and 1 in 8, respectively, had already had previous continence surgery (see Table 5).
Around 1 in 10 women had bowel urgency or severe FI, and over one-third of women reported at least occasional faecal leakage, mostly passive (see Table 11). The pattern of bowel problems was remarkably similar in women who were having first or repeat prolapse surgery, and within these groups.
Just over one-third of the women were sexually active at baseline (see Table 12). Around 10% of them reported dyspareunia before surgery: as more women answered this question than the number professing to be sexually active, it can be inferred that some women may have refrained from intercourse because of dyspareunia or other prolapse symptoms. We allowed for this by including the women who were sexually inactive because of prolapse symptoms in the denominator for this analysis. Interestingly, the proportion with dyspareunia was similar in women who were having first or repeat prolapse surgery, around 10–15%.
In summary, there were no important clinical differences between women randomised in PROSPECT, and the comparable CC populations who were not randomised.
Strengths and weaknesses
PROSPECT is the most comprehensive study of women who were having prolapse surgery in the UK. The large number of women (over 3000), centres (35) and recruiting gynaecologists (70), and recruitment of 80% of their patients who had prolapse surgery, ensured that the findings are representative of the majority of general gynaecological practice within the NHS in the UK. The centres were a mix of secondary and tertiary referral hospitals.
We used a tightly defined classification of primary and secondary repair using international recommendations to separate our population of women. Within each group, there were few systematic differences between women who were randomised, and those who were not. On the other hand, there were clear clinical and demographic differences between women who were having a first or a repeat procedure, thus justifying our decision to analyse these groups of women in separate trials.
Our definition of secondary surgery was prespecified to refer to ‘repeat surgery in the same compartment’. This resulted in some women who had a previous repair in another compartment being classified as ‘primary’. Although we do not know the original denominator, we can calculate an approximation of the total number of women who were having any further prolapse surgery: dividing the number of women who were having any repeat repair (n = 674) by the number presenting for a first operation (2474 – 276 = 2198) suggests that the population rate of further surgery is 30.7%, very similar to that published by Olsen et al. 4 for further prolapse surgery in any compartment.
Conflict of interest
The study was not at risk of bias because it was publicly funded, and the investigators did not have personal or professional links with industry. The commercial companies which manufactured the mesh and mesh kits did not provide any funding or material in kind (such as the supply of free materials) to the centres, the surgeons or the investigators.
Choice of validated outcome measures
We chose outcome measures for PROSPECT to reflect international standards57 of reporting and ensure that the findings would be relevant to the needs of all groups that were likely to be affected by the findings, including patients, clinical staff and policy-makers. These outcomes were measured at baseline to provide values for later statistical adjustments. Our primary measure of prolapse symptoms was the subjective woman-reported prolapse symptom scale (Pelvic Organ Prolapse Symptom scale), developed and validated in a variety of populations for both research and in clinical practice. 27 This tool is relevant to women and, arguably, focused on the symptoms that led them to seek treatment.
We used validated instruments to measure secondary urinary and vaginal symptoms. 26 Similar short validated measures for bowel function were not available in the ICI suite of outcome measures when we started our study. We therefore adapted the questions used in the long ICIQ-Bowel Symptom instrument. We are currently validating them. In addition, we adapted some of these bowel function questions to approximate those advocated in the ROME consultation (see Table 1).
The objective assessment of prolapse stage was carried out using the standardised and internationally recognised POP-Q system. 27 Although the majority of women were classed as stage 2, based on the leading edge of the prolapse, some researchers have called into question whether or not this is an appropriate cut-off point for the diagnosis of prolapse. The mismatch between symptoms and objective findings is well recognised. 3,59,60 Based on this argument, Nygaard et al. 28 chose to define objective prolapse as leading edge beyond the hymen (> 0 cm) in a prolapse surgery trial. We have therefore used this cut-off point to dichotomise the ordinal POP-Q findings in the women who had individual measurements recorded at baseline (83% of all women).
Blinding of participants
Clinical baseline data were reported by women before randomisation using self-completed questionnaires. The objective assessment of prolapse stage before surgery was also carried out, as far as possible, by observers who did not have knowledge of the randomised operations, normally before a decision for surgery had been made.
Objective outcome measures
We were able to ascribe a prolapse stage to 93% of women at baseline, although only 83% had at least one recorded measurement on the POP-Q examination. It is difficult to explain why a small minority of women appeared not to have significant prolapse (stage 0 or 1). We can offer a number of suggestions. These measurements may have been recorded:
-
without the use of provocation, such as Valsalva manoeuvre or coughing, or
-
without the use of position and gravity to demonstrate the maximum descent, or
-
at a time when the prolapse was not evident (e.g. in the morning), or
-
in theatre under anaesthetic, or
-
with a pessary in place, or
-
incorrectly.
We did check that the patients’ symptoms were bothersome (according to the Pelvic Organ Prolapse Symptom scale). These women clearly requested surgery, with which their gynaecologists concurred.
During the study we placed emphasis on the importance of accurate recording of stage and compartment using the POP-Q guideline. 27 We hope that demonstration of these few apparently anomalous women will improve clinical practice with respect to selection of women for prolapse surgery, and/or their better assessment.
Conclusions and further research
The wide inclusion criteria, minimal exclusion criteria and high recruitment rate have ensured that the women studied in PROSPECT are representative of those having prolapse surgery in the UK and the clinical practice of the gynaecologists who treat them. There were clear clinical and demographic differences between women who were having a first and a repeat repair, justifying our decision to study these groups in separate trials. However, within each trial, women who were and were not randomised were broadly similar. The findings of the randomised trials in PROSPECT will therefore be generalisable to the wider population of women with prolapse.
The findings in this chapter will serve as a benchmark for future research in women with prolapse. The clinical messages regarding symptoms and clinical practice may be helpful in improving prolapse management in the UK and internationally.
Chapter 4 Results: Primary trial (randomised controlled trial 1, comprehensive cohort 1)
This chapter describes the women who were having their first anterior or posterior prolapse repair, both those randomised (RCT1) and those who were not randomised but agreed to be followed up in the CC (CC1). The baseline characteristics of the women enrolled in RCT1 and CC1 have been described and compared in Chapter 3; by and large, the populations were similar.
The flow of women through the study is shown in the CONSORT diagram (Figure 4) in line with recommendations of CONSORT. 56
FIGURE 4.
CONSORT diagram for Primary trial. a, Reasons for non-compliance with randomised allocation – standard: no prolapse surgery (4); no anterior or posterior repair (14); mesh not required (0); mesh required (5); morbidity/surgical complications (0); patient decided did not want mesh post randomisation (0); theatre not informed/wrong information given (1); mesh not available (0); not enough theatre time (0); consultant not in theatre (0); no reason given for non-compliance (1). b, Reasons for non-compliance with randomised allocation – synthetic: no prolapse surgery (4); no anterior or posterior repair (14); mesh not required (17); mesh required (1); morbidity/surgical complications (17); patient decided did not want mesh post randomisation (5); theatre not informed/wrong information given (11); mesh not available (1); not enough theatre time (2); consultant not in theatre (1); no reason given for non-compliance (11). c, Reasons for non-compliance with randomised allocation – biological: no prolapse surgery (0); no anterior or posterior repair (7); mesh not required (13); mesh required (4); morbidity/surgical complications (7); patient decided did not want mesh post-randomisation (5); theatre not informed/wrong information given (10); mesh not available (7); not enough theatre time (8); consultant not in theatre (2); no reason given for non-compliance (6). d, ‘Other surgery’ includes tape for UI, vaginal hysterectomy or suspension, cervical amputation, vault repair without anterior or posterior repair.
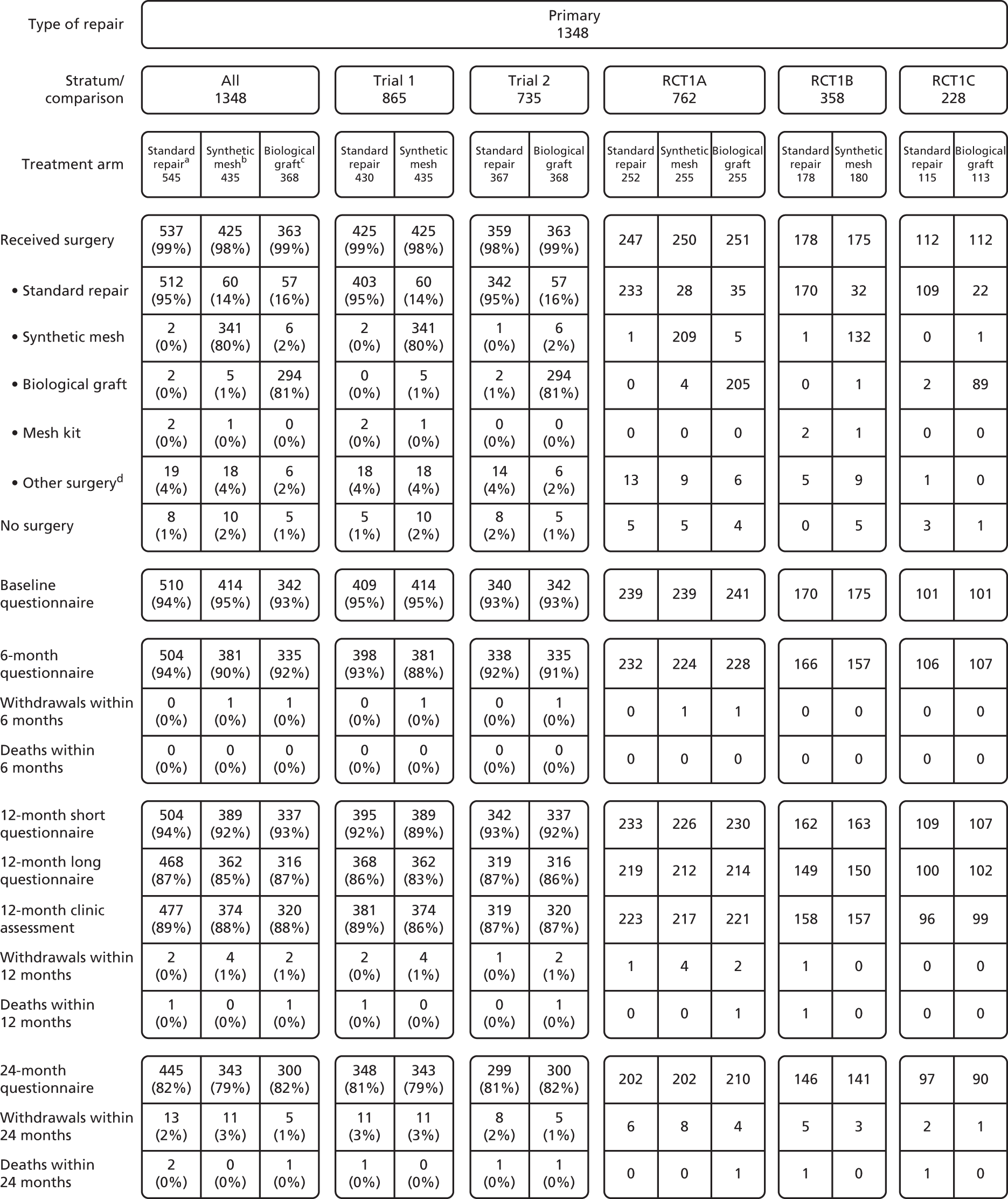
The women received surgery in 35 centres across the UK (see Table 4). Although 1348 women were randomised in total, they are further subdivided according to the panel of operations against which they were randomised. Therefore, RCT1 consists of three strata: RCT1A, for which women were randomly allocated to any of the three options for this trial; RCT1B, for which women were randomised between standard repair with no mesh and synthetic mesh inlay; and RCT1C, for which women were randomised between no mesh and a biological graft inlay.
In this chapter, the data are presented according to the strata:
-
Trial 1 Standard repair (no mesh) compared with synthetic mesh inlay [stratum 1A (three-way randomisation) and stratum 1B (two-way randomisation)], and
-
Trial 2 Standard repair (no mesh) compared with biological graft inlay [stratum 1A (three-way randomisation) and stratum 1C (two-way randomisation)].
Because the analyses were carried out separately for each trial, some women in the ‘no mesh’ group from stratum 1A are included in the standard repair arm in both trial 1 and trial 2.
Baseline comparability of randomised groups
Women’s characteristics at baseline
There were no important epidemiological or clinical differences between the randomised groups of women, including the EQ-5D-3L (trial 1, trial 2; Table 13) or between the randomised women in RCT1 and the non-randomised in CC1 (see Table 13). In this chapter, the data for the cohort women are provided in the outcome tables for comparison with the randomised groups but they have not been formally statistically compared.
| Baseline characteristic | Trial 1: standard vs. synthetic | Trial 2: standard vs. biological | CC1 (N = 1126 women) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair (N = 430 women) | Synthetic mesh (N = 435 women) | Standard repair (N = 367 women) | Biological graft (N = 368 women) | ||||||||||||
| Age (years) | 59.8 | (10.1) | 430 | 59.5 | (10.4) | 435 | 59.7 | (10.4) | 367 | 58.9 | (10.5) | 368 | 59.4 | (11.6) | 1126 |
| Parity (mean) | 2.6 | (1.1) | 429 | 2.7 | (1.2) | 433 | 2.6 | (1.1) | 367 | 2.7 | (1.1) | 367 | 2.5 | (1.1) | 1115 |
| Parity (median) | 2 | (0–8) | 429 | 2 | (0–9) | 433 | 2 | (0–8) | 367 | 2 | (1–7) | 367 | 2 | (0–12) | 1115 |
| BMI (kg/m2) (mean) | 28.6 | (4.9) | 387 | 28.8 | (4.9) | 386 | 28.5 | (4.8) | 325 | 28.5 | (4.6) | 326 | 28.2 | (4.8) | 1023 |
| BMI (kg/m2) (median) | 28 | (19–45) | 387 | 28 | (19–49) | 386 | 28 | (18–45) | 325 | 28 | (16–42) | 326 | 28 | (17–50) | 1023 |
| Delivery mode history | |||||||||||||||
| Spontaneous vaginal delivery | 2.2 | (1.3) | 421 | 2.4 | (1.3) | 425 | 2.2 | (1.3) | 358 | 2.2 | (1.2) | 360 | 2.2 | (1.2) | 1085 |
| Forceps | 0.2 | (0.5) | 421 | 0.2 | (0.5) | 425 | 0.3 | (0.5) | 358 | 0.2 | (0.5) | 360 | 0.2 | (0.4) | 1085 |
| Breech | 0.0 | (0.2) | 421 | 0.0 | (0.2) | 425 | 0.0 | (0.2) | 358 | 0.0 | (0.2) | 360 | 0.0 | (0.2) | 1085 |
| Elective caesarean | 0.1 | (0.3) | 421 | 0.1 | (0.2) | 425 | 0.1 | (0.3) | 358 | 0.0 | (0.2) | 360 | 0.1 | (0.2) | 1085 |
| Emergency caesarean | 0.0 | (0.3) | 421 | 0.1 | (0.2) | 425 | 0.0 | (0.2) | 358 | 0.0 | (0.2) | 360 | 0.0 | (0.2) | 1085 |
| Vacuum delivery | 0.0 | (0.1) | 421 | 0.0 | (0.1) | 425 | 0.0 | (0.2) | 358 | 0.0 | (0.2) | 360 | 0.0 | (0.1) | 1085 |
| EQ-5D-3L | |||||||||||||||
| Score | 0.72 | (0.24) | 398 | 0.71 | (0.23) | 406 | 0.72 | (0.24) | 330 | 0.71 | (0.25) | 329 | 0.71 | (0.25) | 964 |
| Previous conservative treatment | |||||||||||||||
| Current vaginal pessary | 14.7% | 63 | 429 | 13.1% | 57 | 434 | 17.5% | 64 | 366 | 14.0% | 51 | 364 | 11.8% | 131 | 1111 |
| Physiotherapy for prolapse | 27.0% | 116 | 429 | 30.1% | 130 | 432 | 27.6% | 101 | 366 | 25.8% | 94 | 365 | 30.3% | 335 | 1105 |
| Physiotherapy for UI | 15.5% | 66 | 427 | 17.6% | 76 | 432 | 15.1% | 55 | 365 | 15.6% | 57 | 365 | 17.7% | 196 | 1107 |
| Drugs for UI | 8.9% | 38 | 428 | 12.1% | 52 | 430 | 9.3% | 34 | 365 | 10.7% | 39 | 363 | 11.8% | 130 | 1100 |
| Previous surgery | |||||||||||||||
| Previous prolapse repair | 11.4% | 49 | 430 | 12.9% | 56 | 435 | 10.1% | 37 | 367 | 8.2% | 30 | 368 | 12.2% | 137 | 1126 |
| Anterior | 4.4% | 19 | 430 | 7.1% | 31 | 435 | 4.1% | 15 | 367 | 3.8% | 14 | 368 | 5.0% | 56 | 1126 |
| Posterior | 4.7% | 20 | 430 | 3.7% | 16 | 435 | 4.1% | 15 | 367 | 1.1% | 4 | 368 | 3.6% | 40 | 1126 |
| Anterior and posterior | 0.0% | 0 | 430 | 0.0% | 0 | 435 | 0.0% | 0 | 367 | 0.0% | 0 | 368 | 0.0% | 0 | 1126 |
| Vault | 2.1% | 9 | 430 | 1.6% | 7 | 435 | 1.9% | 7 | 367 | 1.1% | 4 | 368 | 2.6% | 29 | 1126 |
| Unknown compartment | 1.6% | 7 | 430 | 1.4% | 6 | 435 | 1.4% | 5 | 367 | 2.2% | 8 | 368 | 2.3% | 26 | 1126 |
| Hysterectomy | 23.3% | 100 | 430 | 28.7% | 125 | 435 | 25.1% | 92 | 367 | 28.8% | 106 | 368 | 29.8% | 336 | 1126 |
| Vaginal | 7.0% | 30 | 430 | 12.2% | 53 | 435 | 7.9% | 29 | 367 | 10.1% | 37 | 368 | 10.7% | 121 | 1126 |
| Cervical amputation | 2.3% | 10 | 430 | 1.6% | 7 | 435 | 2.2% | 8 | 367 | 1.9% | 7 | 368 | 2.7% | 30 | 1126 |
| Abdominal | 16.3% | 70 | 430 | 16.3% | 71 | 435 | 17.2% | 63 | 367 | 18.8% | 69 | 368 | 18.7% | 210 | 1126 |
| Continence surgery | 7.2% | 31 | 429 | 6.3% | 27 | 431 | 5.8% | 21 | 365 | 5.4% | 20 | 367 | 7.7% | 86 | 1111 |
The ages of the recruited women ranged from 24 to 90 years. The mean BMI was < 30 kg/m2 for all groups of women but 10% had a BMI of > 35 kg/m2. The majority of women were parous: only 1% had not had any deliveries. Most babies had been born by spontaneous vaginal delivery. All of the groups were comparable at baseline.
Regarding previous treatment, 13.1–17.5% were using a vaginal pessary, and 25.8–30.1% had seen a physiotherapist for prolapse symptoms, but rather fewer for UI; and around 1 in 10 had used drugs for UI. Regarding previous prolapse surgery, 8.2–12.9% had prior treatment, but these were all in the compartment opposite to the one now requiring repair. In terms of upper compartment procedures, 23.3–28.8% of women had a prior hysterectomy (more than half of those were via the abdominal route); and 5.4–7.2% had already had continence surgery.
Preoperative prolapse measurements
Women in the randomised groups in each trial were comparable in terms of the maximum descent of the three different prolapse compartments. Using qualitative descriptions of prolapse stage to supplement missing POP-Q data, > 98% of the women had prolapse stage 2 or greater before surgery. For the women who had a quantitative score measured using the POP-Q system, about two-thirds were found to have the leading edge of the prolapse outside the hymen (> 0 cm). Table 14 shows the level of prolapse in the individual compartments.
| POP-Q measurement/stage | Trial 1: standard vs. synthetic | Trial 2: standard vs. biological | CC1 (N = 1126 women) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair (N = 430 women) | Synthetic mesh (N = 435 women) | Standard repair (N = 367 women) | Biological graft (N = 368 women) | ||||||||||||
| POP-Q measurement (cm) | |||||||||||||||
| Ba (anterior edge) | 0.6 | (2.2) | 382 | 0.5 | (2.1) | 380 | 0.4 | (2.1) | 328 | 0.4 | (2.1) | 327 | 0.4 | (2.1) | 860 |
| C (cervix/vault) | –3.5 | (3.6) | 359 | –3.5 | (3.3) | 352 | –3.4 | (3.3) | 314 | –3.2 | (3.3) | 300 | –3.2 | (3.3) | 812 |
| Bp (posterior edge) | –0.3 | (2.0) | 380 | –0.5 | (1.9) | 380 | –0.4 | (1.8) | 327 | –0.3 | (1.8) | 325 | –0.5 | (1.8) | 855 |
| TVL | 8.5 | (1.4) | 357 | 8.5 | (1.7) | 359 | 8.3 | (1.5) | 292 | 8.2 | (1.7) | 292 | 8.5 | (1.8) | 809 |
| Overall POP-Q stage | |||||||||||||||
| 0 | 0.2% | 1 | 413 | 0.0% | 0 | 421 | 0.3% | 1 | 352 | 0.3% | 1 | 354 | 0.0% | 0 | 997 |
| 1 | 0.7% | 3 | 413 | 1.7% | 7 | 421 | 0.6% | 2 | 352 | 0.6% | 2 | 354 | 2.0% | 20 | 997 |
| 2 | 55.4% | 229 | 413 | 56.1% | 236 | 421 | 61.1% | 215 | 352 | 53.1% | 188 | 354 | 62.0% | 618 | 997 |
| 3 | 39.7% | 164 | 413 | 39.9% | 168 | 421 | 35.5% | 125 | 352 | 44.9% | 159 | 354 | 34.3% | 342 | 997 |
| 4 | 3.9% | 16 | 413 | 2.4% | 10 | 421 | 2.6% | 9 | 352 | 1.1% | 4 | 354 | 1.7% | 17 | 997 |
| 2b, 3 or 4 | 65.6% | 259 | 395 | 68.8% | 273 | 397 | 62.7% | 210 | 335 | 69.3% | 235 | 339 | 60.6% | 536 | 884 |
Prolapse symptoms at baseline
Women had noticed symptoms of prolapse for a mean of 3.3–3.8 years, and had been bothered for 2.4–2.8 years before surgery (Table 15). The Pelvic Organ Prolapse Symptom scale is composed of seven individual prolapse symptoms (each scored from 0 to 4, where 0 is ‘never’ and 4 is ‘all the time’; see Chapter 2). The mean POP-SS ranged from 13.7 to 13.8 out of a maximum score of 28. Almost all women (over 99%) were deemed to be symptomatic using the criterion of scoring at least 1 on the Pelvic Organ Prolapse Symptom scale (see Table 15). The most common symptom was ‘a feeling of something coming down from or in the vagina’, and over 90% of women reported this symptom at least occasionally, whereas about two-thirds had a visible prolapse outside the hymen (see Table 14).
| Symptom | Trial 1: standard vs. synthetic | Trial 2: standard vs. biological | CC1 (N = 997 women) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair (N = 409 women) | Synthetic mesh (N = 414 women) | Standard repair (N = 340 women) | Biological graft (N = 342 women) | ||||||||||||
| POP-SS at baseline | 13.7 | (6.1) | 409 | 13.7 | (5.6) | 414 | 13.8 | (6.0) | 340 | 13.7 | (5.9) | 342 | 13.3 | (5.8) | 995 |
| Individual prolapse | |||||||||||||||
| SCD any | 92.4% | 378 | 409 | 93.0% | 385 | 414 | 92.4% | 314 | 340 | 93.0% | 318 | 342 | 93.9% | 934 | 995 |
| SCD freq. | 64.5% | 264 | 409 | 66.9% | 277 | 414 | 67.1% | 228 | 340 | 65.5% | 224 | 342 | 66.5% | 662 | 995 |
| Pain any | 80.4% | 329 | 409 | 79.0% | 327 | 414 | 82.4% | 280 | 340 | 81.0% | 277 | 342 | 81.6% | 812 | 995 |
| Pain freq. | 32.8% | 134 | 409 | 34.3% | 142 | 414 | 33.2% | 113 | 340 | 36.5% | 125 | 342 | 36.4% | 362 | 995 |
| Abdo. any | 79.2% | 324 | 409 | 83.1% | 344 | 414 | 78.8% | 268 | 340 | 80.7% | 276 | 342 | 79.2% | 788 | 995 |
| Abdo. freq. | 33.3% | 136 | 409 | 35.7% | 148 | 414 | 31.8% | 108 | 340 | 33.6% | 115 | 342 | 33.4% | 332 | 995 |
| Back any | 72.9% | 298 | 409 | 74.6% | 309 | 414 | 73.8% | 251 | 340 | 70.5% | 241 | 342 | 68.6% | 683 | 995 |
| Back freq. | 28.1% | 115 | 409 | 30.4% | 126 | 414 | 28.5% | 97 | 340 | 31.0% | 106 | 342 | 25.8% | 257 | 995 |
| Strain blad. any | 70.4% | 288 | 409 | 72.0% | 298 | 414 | 69.1% | 235 | 340 | 73.4% | 251 | 342 | 69.5% | 692 | 995 |
| Strain blad. freq. | 29.8% | 122 | 409 | 28.7% | 119 | 414 | 29.1% | 99 | 340 | 28.7% | 98 | 342 | 26.1% | 260 | 995 |
| Blad. not empty any | 80.4% | 329 | 409 | 83.1% | 344 | 414 | 79.4% | 270 | 340 | 83.6% | 286 | 342 | 83.0% | 826 | 995 |
| Blad. not empty freq. | 38.6% | 158 | 409 | 37.7% | 156 | 414 | 38.8% | 132 | 340 | 38.0% | 130 | 342 | 35.7% | 355 | 995 |
| Bowel not empty any | 81.4% | 333 | 409 | 82.4% | 341 | 414 | 83.5% | 284 | 340 | 82.5% | 282 | 342 | 79.0% | 786 | 995 |
| Bowel not empty freq. | 37.9% | 155 | 409 | 34.8% | 144 | 414 | 38.8% | 132 | 340 | 38.0% | 130 | 342 | 30.8% | 306 | 995 |
| Other measures of prolapse symptoms | |||||||||||||||
| Duration of symptoms (years) | 3.8 | (5.9) | 395 | 3.3 | (4.5) | 392 | 3.8 | (5.4) | 331 | 3.7 | (4.6) | 331 | 3.6 | (5.0) | 948 |
| Duration of bother (years) | 2.8 | (4.9) | 384 | 2.4 | (3.8) | 380 | 2.5 | (3.7) | 322 | 2.7 | (3.7) | 310 | 2.5 | (3.4) | 916 |
| Symptomatic | 100.0% | 409 | 409 | 99.5% | 412 | 414 | 100.0% | 340 | 340 | 99.1% | 339 | 342 | 99.6% | 991 | 995 |
| Prolapse-related QoL score | 6.5 | (2.8) | 408 | 6.6 | (2.7) | 406 | 6.7 | (2.7) | 338 | 6.6 | (2.8) | 338 | 6.7 | (2.7) | 969 |
| Actions necessitated by prolapse symptoms | |||||||||||||||
| Fingers to ease discomfort | 23.6% | 95 | 402 | 19.7% | 80 | 406 | 23.0% | 77 | 335 | 19.5% | 66 | 339 | 19.4% | 189 | 972 |
| Extra hygiene measures | 54.0% | 217 | 402 | 51.5% | 208 | 404 | 51.5% | 172 | 334 | 49.4% | 165 | 334 | 47.9% | 465 | 970 |
| Fingers to help empty bladder | 4.5% | 18 | 403 | 2.9% | 12 | 409 | 3.6% | 12 | 336 | 4.5% | 15 | 337 | 4.9% | 48 | 976 |
| Fingers to help empty bowel | 11.1% | 45 | 407 | 11.2% | 45 | 401 | 11.8% | 40 | 339 | 9.9% | 33 | 335 | 11.2% | 109 | 972 |
| Digital evacuation of bowel | 6.9% | 28 | 407 | 8.0% | 32 | 401 | 7.4% | 25 | 337 | 7.4% | 25 | 338 | 8.4% | 82 | 977 |
As well as the women in each trial being comparable at baseline for the overall score, there were no systematic differences in any individual prolapse symptoms or other measures of the effect of prolapse on QoL or in modifying women’s behaviour to ameliorate the effects of prolapse.
Urinary symptoms at baseline
The urinary symptoms reported by women were captured using a variety of validated questionnaires and scales from the ICI Modular Questionnaire suite26 (Table 16). Around four in five women had at least some urinary leakage, and this was severe for one in five.
| Symptom | Trial 1: standard vs. synthetic | Trial 2: standard vs. biological | CC1 (N = 997 women) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair (N = 409 women) | Synthetic mesh (N = 414 women) | Standard repair (N = 340 women) | Biological graft (N = 342 women) | ||||||||||||
| Any incontinence | 76.8% | 314 | 409 | 76.5% | 315 | 412 | 76.2% | 259 | 340 | 78.0% | 266 | 341 | 76.1% | 756 | 994 |
| ICIQ-UI-SF score | 7.0 | (5.5) | 403 | 7.1 | (5.8) | 408 | 6.9 | (5.5) | 337 | 7.4 | (5.8) | 339 | 7.2 | (5.8) | 974 |
| Severe incontinence | 19.4% | 78 | 403 | 21.1% | 86 | 408 | 19.3% | 65 | 337 | 21.8% | 74 | 339 | 22.0% | 214 | 974 |
| Incontinence-related QoL score | 3.5 | (3.3) | 394 | 3.6 | (3.5) | 402 | 3.5 | (3.4) | 326 | 3.7 | (3.4) | 330 | 3.7 | (3.5) | 956 |
| Stress UI | 23.6% | 84 | 356 | 24.1% | 90 | 374 | 25.3% | 74 | 293 | 24.9% | 76 | 305 | 25.0% | 220 | 881 |
| Urgency UI | 8.9% | 36 | 406 | 10.0% | 41 | 411 | 9.2% | 31 | 338 | 10.7% | 36 | 338 | 10.6% | 104 | 982 |
| Overactive bladder | 5.3% | 21 | 399 | 5.1% | 21 | 409 | 3.9% | 13 | 333 | 8.1% | 27 | 335 | 5.6% | 55 | 977 |
| ICIQ-FLUTS filling score | 5.1 | (2.9) | 397 | 5.3 | (2.9) | 404 | 5.2 | (2.9) | 331 | 5.5 | (3.0) | 334 | 5.3 | (2.9) | 970 |
| ICIQ-FLUTS voiding score | 3.2 | (2.7) | 402 | 3.1 | (2.5) | 406 | 3.1 | (2.6) | 334 | 3.1 | (2.6) | 336 | 3.1 | (2.6) | 975 |
| ICIQ-FLUTS incontinence score | 6.1 | (4.1) | 350 | 6.0 | (4.2) | 371 | 6.2 | (4.1) | 291 | 6.4 | (4.2) | 300 | 6.1 | (4.3) | 858 |
There were no systematic differences between the women in either trial but urinary symptoms were common in women with prolapse.
Bowel symptoms at baseline
We captured a variety of bowel symptoms (Table 17). There were no systematic differences between the randomised groups in terms of frequency of bowel movements, constipation, bowel urgency or FI, or in the effect bowel symptoms had on QoL. Around 30% of the women had constipation (using the ROME30 criteria) and over one-third reported FI at least occasionally.
| Symptom | Trial 1: standard vs. synthetic | Trial 2: standard vs. biological | CC1 (N = 997 women) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair (N = 409 women) | Synthetic mesh (N = 414 women) | Standard repair (N = 340 women) | Biological graft (N = 342 women) | ||||||||||||
| Bowel frequency | |||||||||||||||
| > 3 times a day | 5.9% | 24 | 408 | 4.9% | 20 | 405 | 7.4% | 25 | 339 | 6.2% | 21 | 337 | 5.0% | 49 | 977 |
| 1–3 times a day | 33.3% | 136 | 408 | 36.3% | 147 | 405 | 32.2% | 109 | 339 | 31.8% | 107 | 337 | 36.2% | 354 | 977 |
| About once a day | 40.2% | 164 | 408 | 37.5% | 152 | 405 | 39.5% | 134 | 339 | 42.1% | 142 | 337 | 40.4% | 395 | 977 |
| Once every 2–3 days | 16.4% | 67 | 408 | 17.5% | 71 | 405 | 17.1% | 58 | 339 | 18.4% | 62 | 337 | 16.6% | 162 | 977 |
| Weekly or less | 4.2% | 17 | 408 | 3.7% | 15 | 405 | 3.8% | 13 | 339 | 1.5% | 5 | 337 | 1.7% | 17 | 977 |
| Constipation | 29.0% | 117 | 404 | 27.1% | 108 | 399 | 32.0% | 108 | 338 | 29.1% | 97 | 333 | 24.6% | 239 | 970 |
| Bowel urgency | 11.7% | 48 | 409 | 9.9% | 40 | 405 | 11.8% | 40 | 339 | 15.0% | 51 | 339 | 10.8% | 106 | 985 |
| FI (any) | 34.3% | 140 | 408 | 34.0% | 138 | 406 | 33.4% | 113 | 338 | 35.8% | 121 | 338 | 33.4% | 328 | 982 |
| Passive FI | 72.1% | 101 | 140 | 77.4% | 106 | 137 | 74.3% | 84 | 113 | 66.9% | 81 | 121 | 75.8% | 248 | 327 |
| Active FI | 27.9% | 39 | 140 | 22.6% | 31 | 137 | 25.7% | 29 | 113 | 33.1% | 40 | 121 | 24.2% | 79 | 327 |
| Severe FI | 12.5% | 51 | 408 | 11.3% | 46 | 406 | 10.7% | 36 | 338 | 15.4% | 52 | 338 | 11.2% | 110 | 982 |
| Bowel symptoms QoL score | 3.8 | (3.2) | 396 | 3.6 | (3.2) | 393 | 3.8 | (3.2) | 328 | 3.8 | (3.3) | 332 | 3.6 | (3.4) | 964 |
Vaginal and sexual symptoms at baseline
We used the validated ICIQ-VS and the ICI Sexual Matters instruments to capture aspects of vaginal and sexual function. 26 Between 59.9% and 64.9% of women were not sexually active (Table 18); around one-quarter of these women did not have sexually active partners, and the most common reason in the remainder was ‘due to their prolapse symptoms’. Around 6.6–11.4% of women who answered the question reported pain with intercourse (dyspareunia). There were no systematic differences between the randomised groups in terms of these clinical measures at baseline.
| Symptom | Trial 1: standard vs. synthetic | Trial 2: standard vs. biological | CC1 (N = 997 women) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair (N = 409 women) | Synthetic mesh (N = 414 women) | Standard repair (N = 340 women) | Biological graft (N = 342 women) | ||||||||||||
| Vaginal | |||||||||||||||
| ICIQ-VS score | 22.1 | (9.0) | 367 | 22.2 | (9.4) | 365 | 21.7 | (8.7) | 302 | 22.8 | (9.1) | 307 | 22.4 | (9.3) | 878 |
| Vaginal symptoms QoL score | 4.9 | (3.1) | 396 | 5.1 | (3.1) | 396 | 5.0 | (3.1) | 328 | 5.2 | (3.2) | 329 | 5.2 | (3.2) | 952 |
| Vagina too tight | 1.8% | 7 | 387 | 1.6% | 6 | 385 | 2.2% | 7 | 323 | 1.8% | 6 | 327 | 2.8% | 26 | 925 |
| Sexual | |||||||||||||||
| Sex life at present (yes) | 37.3% | 152 | 407 | 37.1% | 148 | 399 | 35.1% | 119 | 339 | 40.1% | 135 | 337 | 38.6% | 373 | 967 |
| Reason for no sex life | |||||||||||||||
| No partner | 23.5% | 60 | 255 | 31.1% | 78 | 251 | 24.1% | 53 | 220 | 25.2% | 51 | 202 | 27.9% | 166 | 594 |
| Vaginal symptoms | 5.1% | 13 | 255 | 2.0% | 5 | 251 | 5.9% | 13 | 220 | 4.5% | 9 | 202 | 4.7% | 28 | 594 |
| Prolapse symptoms | 42.7% | 109 | 255 | 39.4% | 99 | 251 | 43.2% | 95 | 220 | 48.0% | 97 | 202 | 41.8% | 248 | 594 |
| Other reason | 23.9% | 61 | 255 | 21.9% | 55 | 251 | 22.3% | 49 | 220 | 17.3% | 35 | 202 | 19.5% | 116 | 594 |
| Reason not given | 4.7% | 12 | 255 | 5.6% | 14 | 251 | 4.5% | 10 | 220 | 5.0% | 10 | 202 | 6.1% | 36 | 594 |
| Dyspareunia | 8.3% | 18 | 217 | 6.6% | 13 | 197 | 11.4% | 20 | 175 | 11.3% | 21 | 186 | 9.3% | 46 | 492 |
| ICI Sexual Matters score | 22.4 | (14.4) | 215 | 23.5 | (13.3) | 195 | 23.3 | (15.2) | 173 | 23.5 | (14.7) | 183 | 23.4 | (14.3) | 485 |
| Sex life QoL score | 6.4 | (3.4) | 244 | 6.5 | (3.3) | 231 | 6.4 | (3.5) | 195 | 6.5 | (3.4) | 217 | 6.4 | (3.2) | 575 |
Surgery planned before surgery and actually received during surgery
Planned operations
The most common operation (anticipated for three-quarters of women) was anterior repair, with just over half of the women planning to have a posterior repair: of these women, around 30% were having a joint procedure (Table 19). Concomitant surgery included about one-third of the women who were thought to need a vaginal hysterectomy, and a further 12.6–18.2% requiring a vault repair. Finally, 9.5–11.7% were thought to require a continence procedure. There were no differences between the women in different arms of the study (see Table 19).
| Type of surgery | Trial 1: standard vs. synthetic | Trial 2: standard vs. biological | CC1 (N = 1126) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Standard repair | Biological graft | ||||||||||||
| Number of women | N = 430 | N = 435 | N = 367 | N = 368 | |||||||||||
| Planned prolapse procedure | |||||||||||||||
| Anterior repair | 48.6% | 209 | 430 | 48.5% | 211 | 435 | 40.9% | 150 | 367 | 40.5% | 149 | 368 | 44.5% | 501 | 1126 |
| Posterior repair | 25.8% | 111 | 430 | 25.5% | 111 | 435 | 25.3% | 93 | 367 | 26.6% | 98 | 368 | 26.8% | 302 | 1126 |
| Anterior and posterior repair | 25.6% | 110 | 430 | 26.0% | 113 | 435 | 33.8% | 124 | 367 | 32.9% | 121 | 368 | 28.7% | 323 | 1126 |
| Upper compartment repair only | 0.0% | 0 | 430 | 0.0% | 0 | 435 | 0.0% | 0 | 367 | 0.0% | 0 | 368 | 0.0% | 0 | 1126 |
| Planned concomitant prolapse procedure | |||||||||||||||
| Vaginal hysterectomy | 35.8% | 154 | 430 | 32.6% | 142 | 435 | 36.5% | 134 | 367 | 33.4% | 123 | 368 | 32.7% | 368 | 1126 |
| Cervical amputation | 1.9% | 8 | 430 | 2.1% | 9 | 435 | 1.6% | 6 | 367 | 1.4% | 5 | 368 | 1.4% | 16 | 1126 |
| Abdominal hysterectomy | 0.2% | 1 | 430 | 0.0% | 0 | 435 | 0.0% | 0 | 367 | 0.0% | 0 | 368 | 0.4% | 4 | 1126 |
| Vault repair | 12.6% | 54 | 430 | 15.6% | 68 | 435 | 16.6% | 61 | 367 | 18.2% | 67 | 368 | 20.9% | 235 | 1126 |
| Continence procedure | 9.5% | 41 | 430 | 10.3% | 45 | 435 | 11.7% | 43 | 367 | 11.4% | 42 | 368 | 13.3% | 150 | 1126 |
| Number of women | N = 425 | N = 425 | N = 359 | N = 363 | N = 1104 | ||||||||||
| Surgery actually performed | |||||||||||||||
| Actual prolapse procedure | |||||||||||||||
| Anterior repair only | 43.3% | 184 | 425 | 44.0% | 187 | 425 | 36.8% | 132 | 359 | 36.4% | 132 | 363 | 40.8% | 450 | 1104 |
| Posterior repair only | 29.4% | 125 | 425 | 29.9% | 127 | 425 | 28.7% | 103 | 359 | 32.2% | 117 | 363 | 27.6% | 305 | 1104 |
| Anterior and posterior repair | 23.1% | 98 | 425 | 21.9% | 93 | 425 | 30.6% | 110 | 359 | 29.8% | 108 | 363 | 25.5% | 282 | 1104 |
| Neither | 4.2% | 18 | 425 | 4.2% | 18 | 425 | 3.9% | 14 | 359 | 1.7% | 6 | 363 | 6.1% | 67 | 1104 |
| Concomitant prolapse procedure | |||||||||||||||
| Vaginal hysterectomy | 31.8% | 135 | 425 | 24.2% | 103 | 425 | 31.2% | 112 | 359 | 26.2% | 95 | 363 | 29.8% | 329 | 1104 |
| Abdominal hysterectomy | 0.0% | 0 | 425 | 0.0% | 0 | 425 | 0.0% | 0 | 359 | 0.3% | 1 | 363 | 0.4% | 4 | 1104 |
| Cervical amputation | 2.1% | 9 | 425 | 2.8% | 12 | 425 | 2.2% | 8 | 359 | 2.5% | 9 | 363 | 0.7% | 8 | 1104 |
| Uterine suspension | 4.7% | 20 | 425 | 3.1% | 13 | 425 | 4.5% | 16 | 359 | 2.2% | 8 | 363 | 5.0% | 55 | 1104 |
| Vault repair | 11.5% | 49 | 425 | 10.1% | 43 | 425 | 10.9% | 39 | 359 | 11.3% | 41 | 363 | 13.5% | 149 | 1104 |
| Continence procedure | 10.1% | 43 | 425 | 10.6% | 45 | 425 | 9.7% | 35 | 359 | 12.1% | 44 | 363 | 12.2% | 135 | 1104 |
Surgeons could use any mesh, graft or mesh kit, providing that any synthetic mesh was monofilament macroporous polypropylene and mesh inlays were secured with peripheral sutures.
In line with expectations, most women received the surgery planned. Twenty-three women did not receive surgery at all; reasons were being unfit for surgery, change of patient’s mind, surgeon finding that the prolapse surgery was unnecessary, etc. (see Figure 4). Forty-three women did not have either an anterior or a posterior repair once anaesthetised because the surgeon did not deem it necessary for clinical reasons, and therefore they were unable to receive their randomised allocation (see Figure 4).
Surgery actually received
In both trials, more women had a vaginal hysterectomy in the standard arms than in the intervention arms, but this was not significant. It is possible that knowledge of the allocated intervention influenced the surgery actually performed. However, overall there were no substantial differences between the groups in the panel of operations carried out.
Compliance with randomised allocation
In addition to women who did not have any prolapse surgery (N = 23; see Figure 4) or did not require either an anterior or posterior repair (N = 43), and therefore could not receive their randomised allocation, six women who were randomised to standard repair received mesh (N = 2), graft (N = 2) or mesh kit (N = 2); 66 women who were randomised to synthetic mesh did not receive any (N = 60) or received a biological graft (N = 5) or mesh kit (N = 1); and 63 women who were randomised to biological graft did not receive it (N = 57) or received synthetic mesh (N = 6). In some cases, the reason for these protocol deviations were as a result of the appropriate mesh not being available in theatre or failure to inform the theatre staff in good time.
In trial 1, more women failed to receive their allocated (randomised) intervention in the synthetic mesh arm than in the control standard arm because of a clinical decision by the surgeon that mesh was not required, or because of morbidity or complications. Similarly in trial 2, more women did not receive their allocated biological graft than a standard repair because the surgeon decided that graft was or was not indicated and due to morbidity (see Figure 4).
Surgical characteristics and protocols
The majority of the operations were carried out by consultant gynaecological surgeons or specialty (staff grade) doctors (Table 20). Between 18.8% (synthetic mesh arm) and 30.5% (standard repair arm of trial 2) were undertaken by a junior doctor, but in those cases nearly 90% were supervised by a consultant. However, consultants were more likely to operate on women randomised to mesh or graft than standard repair. Around 80% of the women had a general anaesthetic, with no systematic differences between the groups.
| Surgical characteristic | Trial 1: standard vs. synthetic | Trial 2: standard vs. biological | CC1 (N = 1102 women) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair (N = 425 women) | Synthetic mesh (N = 425 women) | Standard repair (N = 359 women) | Biological graft (N = 363 women) | ||||||||||||
| Grade of gynaecologist | |||||||||||||||
| Consultant | 70.6% | 300 | 425 | 78.6% | 331 | 421 | 59.4% | 212 | 357 | 68.0% | 247 | 363 | 70.8% | 777 | 1098 |
| Specialty doctor | 7.1% | 30 | 425 | 2.6% | 11 | 421 | 10.1% | 36 | 357 | 7.2% | 26 | 363 | 12.9% | 142 | 1098 |
| Specialty doctor supervised | 69.2% | 18 | 26 | 80.0% | 8 | 10 | 76.7% | 23 | 30 | 83.3% | 20 | 24 | 69.4% | 84 | 121 |
| Registrar/junior | 22.4% | 95 | 425 | 18.8% | 79 | 421 | 30.5% | 109 | 357 | 24.8% | 90 | 363 | 16.3% | 179 | 1098 |
| Registrar/junior supervised | 87.6% | 78 | 89 | 89.7% | 70 | 78 | 87.5% | 91 | 104 | 89.7% | 78 | 87 | 82.9% | 141 | 170 |
| Prophylactic antibiotic | 92.4% | 387 | 419 | 96.9% | 409 | 422 | 91.8% | 325 | 354 | 97.2% | 351 | 361 | 95.5% | 1016 | 1064 |
| Type of anaesthetic | |||||||||||||||
| General | 80.0% | 340 | 425 | 78.5% | 332 | 423 | 84.9% | 304 | 358 | 87.1% | 316 | 363 | 84.8% | 924 | 1090 |
| Spinal | 20.7% | 88 | 425 | 22.9% | 97 | 423 | 15.6% | 56 | 358 | 15.2% | 55 | 363 | 16.3% | 178 | 1090 |
| Local | 10.8% | 46 | 425 | 11.8% | 50 | 423 | 8.7% | 31 | 358 | 6.3% | 23 | 363 | 14.0% | 153 | 1090 |
| Duration (minutes) | 78.3 | (34.6) | 412 | 84.2 | (32.0) | 412 | 84.4 | (41.6) | 352 | 88.0 | (38.6) | 355 | 82.7 | (37.0) | 1052 |
| Estimated blood loss (ml) | 132.7 | (132.4) | 394 | 160.6 | (152.7) | 387 | 135.7 | (145.7) | 331 | 146.3 | (114.0) | 326 | 138.6 | (158.2) | 974 |
| Vaginal pack inserted | 81.4% | 341 | 419 | 86.9% | 353 | 406 | 83.1% | 294 | 354 | 88.8% | 317 | 357 | 74.8% | 796 | 1064 |
| Catheter inserted | 91.7% | 387 | 422 | 96.7% | 405 | 419 | 91.3% | 327 | 358 | 96.1% | 349 | 363 | 93.4% | 1020 | 1092 |
| Suprapubic | 1.8% | 7 | 387 | 0.5% | 2 | 404 | 1.8% | 6 | 327 | 1.4% | 5 | 349 | 0.3% | 3 | 1016 |
| Urethral | 98.2% | 380 | 387 | 99.0% | 400 | 404 | 97.9% | 320 | 327 | 98.3% | 343 | 349 | 99.7% | 1013 | 1016 |
| Both | 0.0% | 0 | 387 | 0.5% | 2 | 404 | 0.3% | 1 | 327 | 0.3% | 1 | 349 | 0.0% | 0 | 1016 |
| Length of stay (days) | 2.4 | (1.5) | 423 | 2.6 | (1.5) | 419 | 2.6 | (1.6) | 356 | 2.9 | (2.7) | 363 | 2.3 | (1.6) | 1092 |
Duration of surgery was significantly longer in the mesh group (by 5.9 minutes), but not significantly longer in the graft group (3.6 minutes) (Table 21). Blood loss was higher in the mesh group but not significantly so in the graft group compared with standard repair. The mean length of stay ranged from 2.4 to 2.9 days, with no differences between the randomised groups. This time included any preoperative days if the women were admitted a day before surgery.
| Surgical characteristic | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 (N = 1104 women) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard (N = 425 women) | Synthetic (N = 425 women) | Effect size | 95% CI | p-value | Standard (N = 359 women) | Biological (N = 363 women) | Effect size | 95% CI | p-value | ||||||||||||
| Duration (minutes) | 78.3 | (34.6) | 412 | 84.2 | (32.0) | 412 | 5.91 | 2.05 to 9.76 | 0.003 | 84.4 | (41.6) | 352 | 88.0 | (38.6) | 355 | 4.29 | –0.05 to 8.62 | 0.052 | 82.7 | (37.0) | 1052 |
| Blood loss (ml) | 132.7 | (132.4) | 394 | 160.6 | (152.7) | 387 | 28.1 | 9.5 to 46.8 | 0.003 | 135.7 | (145.7) | 331 | 146.3 | (114.0) | 326 | 13.5 | –4.3 to 31.30 | 0.138 | 138.6 | (158.2) | 974 |
| Length of stay | 2.4 | (1.5) | 423 | 2.6 | (1.5) | 419 | 0.11 | –0.06 to 0.27 | 0.218 | 2.6 | (1.6) | 356 | 2.9 | (2.7) | 363 | 0.26 | –0.03 to 0.56 | 0.081 | 2.3 | (1.6) | 1092 |
Outcomes
The outcomes are compared between women within each of trial 1 and trial 2. The data for the equivalent outcomes for the cohort women are provided for comparison only but are not formally statistically compared with either trial.
Serious and related adverse effects in first and second years
The diagnoses in Table 22 are confined to those that met our definition of ‘serious’ (see Chapter 2). An adverse effect (AE) was defined as ‘serious’ (SAE) if it was related to prolapse surgery and resulted in death; was life-threatening; required hospitalisation or prolongation of an existing admission; resulted in significant disability/incapacity; or was otherwise considered medically significant by the investigator. If it did not meet the requirement for ‘serious’ then it was classed as ‘other’.
| Adverse effect | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | Cohort | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | CC1 | |||||||||||
| Intraoperative complications | |||||||||||||||||||||
| Number of women at 1 year | N = 430 | N = 435 | N = 367 | N = 368 | N = 1126 | ||||||||||||||||
| Injury to organs | 0.2% | 1 | 430 | 0.7% | 3 | 435 | 3.05 | 0.32 to 28.83 | 0.330 | 0.3% | 1 | 367 | 0.5% | 2 | 368 | 1.97 | 0.18 to 21.63 | 0.578 | 0.1% | 1 | 1126 |
| Excess blood loss | 0.5% | 2 | 430 | 0.9% | 4 | 435 | 2.12 | 0.39 to 11.41 | 0.380 | 0.3% | 1 | 367 | 0.3% | 1 | 368 | 1.02 | 0.06 to 16.23 | 0.987 | 0.2% | 2 | 1126 |
| Blood transfusion | 0.7% | 3 | 430 | 0.2% | 1 | 435 | 0.32 | 0.03 to 3.03 | 0.319 | 0.5% | 2 | 367 | 0.3% | 1 | 368 | 0.52 | 0.05 to 5.70 | 0.595 | 0.5% | 6 | 1126 |
| Anaesthetic complications | 0.2% | 1 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.3% | 1 | 367 | 0.5% | 2 | 368 | 1.99 | 0.18 to 21.82 | 0.573 | 0.6% | 7 | 1126 |
| Death | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Serious adverse effects in first year | |||||||||||||||||||||
| Thrombosis | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.3% | 1 | 368 | N/A | N/A | N/A | 0.1% | 1 | 1126 |
| Infection | 3.0% | 13 | 430 | 3.0% | 13 | 435 | 0.98 | 0.46 to 2.08 | 0.957 | 2.2% | 8 | 367 | 3.0% | 11 | 368 | 1.35 | 0.55 to 3.32 | 0.508 | 2.54% | 28 | 1126 |
| Pain | 2.3% | 10 | 430 | 2.8% | 12 | 435 | 1.18 | 0.542 to 2.69 | 0.697 | 1.4% | 5 | 367 | 1.9% | 7 | 368 | 1.43 | 0.47 to 4.42 | 0.529 | 1.4% | 16 | 1126 |
| Urinary retention | 2.3% | 10 | 430 | 1.1% | 5 | 435 | 0.48 | 0.17 to 1.40 | 0.180 | 2.7% | 10 | 367 | 2.2% | 8 | 368 | 0.81 | 0.32 to 2.01 | 0.644 | 1.2% | 13 | 1126 |
| Bowel obstruction | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Constipation | 0.2% | 1 | 430 | 0.2% | 1 | 435 | 0.99 | 0.06 to 15.66 | 0.992 | 0.3% | 1 | 367 | 0.5% | 2 | 368 | 2.02 | 0.18 to 22.07 | 0.565 | 0.4% | 4 | 1126 |
| Excess blood loss | 1.4% | 6 | 430 | 1.8% | 8 | 435 | 1.33 | 0.47 to 3.80 | 0.588 | 0.5% | 2 | 367 | 0.3% | 1 | 368 | 0.52 | 0.05 to 5.74 | 0.597 | 0.8% | 9 | 1126 |
| Vaginal adhesions | 0.2% | 1 | 430 | 0.9% | 4 | 435 | 3.24 | 0.35 to 29.78 | 0.299 | 0.8% | 3 | 367 | 1.1% | 4 | 368 | N/A | N/A | N/A | 0.7% | 8 | 1126 |
| Haematoma | 0.7% | 3 | 430 | 1.4% | 6 | 435 | 2.02 | 0.51 to 8.00 | 0.319 | 0.3% | 1 | 367 | 1.1% | 4 | 368 | 4.12 | 0.47 to 36.48 | 0.203 | 1.1% | 12 | 1126 |
| Skin tags | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.3% | 1 | 368 | N/A | N/A | N/A | 0.3% | 3 | 1126 |
| Granulation tissue | 0.2% | 1 | 430 | 0.2% | 1 | 435 | 0.99 | 0.06 to 15.75 | 0.993 | 0.3% | 1 | 367 | 0.5% | 2 | 368 | 1.96 | 0.18 to 21.53 | 0.582 | 0.0% | 0 | 1126 |
| Urinary tract symptoms | 0.0% | 0 | 430 | 0.5% | 2 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0. 2% | 2 | 1126 |
| Death | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Any serious adverse effects (excluding mesh complications) | 7.2% | 31 | 430 | 7.8% | 34 | 435 | 1.08 | 0.68 to 1.72 | 0.730 | 6.3% | 23 | 367 | 9.8% | 36 | 368 | 1.57 | 0.95 to 2.59 | 0.076 | 6.64% | 74 | 1126 |
| Serious adverse effects in second year | |||||||||||||||||||||
| Number of women at 2 years | N = 430 | N = 435 | N = 367 | N = 368 | N = 1126 | ||||||||||||||||
| Thrombosis | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Infection | 0.2% | 1 | 430 | 0.2% | 1 | 435 | 0.98 | 0.46 to 2.08 | 0.957 | 0.0% | 0 | 367 | 0.3% | 1 | 368 | N/A | N/A | N/A | 0.4% | 4 | 1126 |
| Pain | 0.5% | 2 | 430 | 0.5% | 2 | 435 | 1.014 | 0.14 to 7.11 | 0.991 | 0.3% | 1 | 367 | 0.3% | 1 | 368 | 1.00 | 0.06 to15.81 | 0.999 | 0.1% | 1 | 1126 |
| Urinary retention | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.1% | 1 | 1126 |
| Bowel obstruction | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Constipation | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Excess blood loss | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Vaginal adhesions | 0.7% | 3 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.8% | 3 | 367 | 0.8% | 3 | 368 | 1.00 | 0.20 to 4.90 | 0.997 | 0.2% | 2 | 1126 |
| Haematoma | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.1% | 1 | 1126 |
| Skin tags | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Granulation tissue | 0.2% | 1 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.3% | 1 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Urinary tract symptoms | 0.0% | 0 | 430 | 0.2% | 1 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.1% | 1 | 1126 |
| Death | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Any serious adverse effects (excluding mesh complications) | 1.4% | 6 | 430 | 0.9% | 4 | 435 | 0.66 | 0.19 to 2.30 | 0.510 | 1.1% | 4 | 367 | 1.4% | 5 | 368 | 1.25 | 0.34 to 4.60 | 0.740 | 0.6% | 7 | 1126 |
Serious non-mesh adverse effects
The proportion of women reporting a serious adverse effect related to prolapse surgery but not mesh-related ranged from 6.3% to 9.8% in the first year, and 0.9% to 1.4% in the second year (Table 22). There was no statistically significant difference between the randomised groups in either trial and the rates were similar to those observed in the cohort. Individual serious effects were rare, the most common being infection, pain and urinary retention, all of which are common after gynaecological surgery, generally of short duration and easily treated. The data from the cohort women were similar.
Other related adverse effects in first and second years
The pattern for other (non-serious) adverse effects was very similar in both trials (Table 23). The overall number of effects was similar, and there were no statistically significant differences between the randomised groups in either trial.
| Adverse effect | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| Intraoperative complications | |||||||||||||||||||||
| Number of women at 1 year | N = 430 | N = 435 | N = 367 | N = 368 | N = 1126 | ||||||||||||||||
| Injury to organs | 0.5% | 2 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.5% | 2 | 367 | 0.3% | 1 | 368 | 0.39 | 0.03 to 4.88 | 0.468 | 0.2% | 2 | 1126 |
| Excess blood loss | 0.0% | 0 | 430 | 0.2% | 1 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.3% | 1 | 368 | N/A | N/A | N/A | 0.4% | 5 | 1126 |
| Blood transfusion | 0.2% | 1 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.3% | 1 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Anaesthetic complications | 0.5% | 2 | 430 | 0.2% | 1 | 435 | 0.49 | 0.04 to 5.43 | 0.564 | 0.3% | 1 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.4% | 5 | 1126 |
| Other adverse effects in first year | |||||||||||||||||||||
| Thrombosis | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Infection | 4.0% | 17 | 430 | 1.8% | 8 | 435 | 0.47 | 0.21 to 1.07 | 0.073 | 3.3% | 12 | 367 | 2.2% | 8 | 368 | 0.66 | 0.27 to 1.60 | 0.359 | 2.7% | 30 | 1126 |
| Pain | 1.4% | 6 | 430 | 1.4% | 6 | 435 | 1.00 | 0.33 to 3.07 | 0.999 | 1.4% | 5 | 367 | 0.8% | 3 | 368 | 0.60 | 0.14 to 2.48 | 0.480 | 0.9% | 10 | 1126 |
| Urinary retention | 1.2% | 5 | 430 | 1.4% | 6 | 435 | 1.18 | 0.37 to 3.84 | 0.778 | 2.2% | 8 | 367 | 0.8% | 3 | 368 | 0.38 | 0.10 to 1.41 | 0.146 | 1.1% | 12 | 1126 |
| Bowel obstruction | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Constipation | 0.2% | 1 | 430 | 0.2% | 1 | 435 | 0.99 | 0.06 to 15.75 | 0.993 | 0.3% | 1 | 367 | 0.8% | 3 | 368 | 2.99 | 0.31 to 28.63 | 0.342 | 0.5% | 6 | 1126 |
| Excess blood loss | 0.5% | 2 | 430 | 0.2% | 1 | 435 | 0.50 | 0.05 to 5.49 | 0.571 | 0.3% | 1 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.6% | 7 | 1126 |
| Vaginal adhesions | 0.5% | 2 | 430 | 0.5% | 2 | 435 | 1.02 | 0.14 to 7.20 | 0.984 | 0.5% | 2 | 367 | 0.3% | 1 | 368 | 0.53 | 0.05 to 5.87 | 0.607 | 0.4% | 5 | 1126 |
| Haematoma | 0.2% | 1 | 430 | 0.2% | 1 | 435 | 0.98 | 0.06 to 15.63 | 0.987 | 0.0% | 0 | 367 | 0.5% | 2 | 368 | N/A | N/A | N/A | 0.2% | 2 | 1126 |
| Skin tags | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Granulation tissue | 0.5% | 2 | 430 | 0.5% | 2 | 435 | 0.99 | 0.14 to 6.99 | 0.991 | 0.5% | 2 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.3% | 3 | 1126 |
| Urinary tract symptoms | 0.0% | 0 | 430 | 0.2% | 1 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.2% | 2 | 1126 |
| Any other adverse effects (excluding mesh complications) | 8.1% | 35 | 430 | 6.4% | 28 | 435 | 0.79 | 0.49 to 1.28 | 0.340 | 7.64% | 28 | 367 | 5.2% | 19 | 368 | 0.68 | 0.39 to 1.19 | 0.175 | 6.3% | 71 | 1126 |
| Other adverse effects in second year | |||||||||||||||||||||
| Number of women at 2 years | N = 430 | N = 435 | N = 367 | N = 368 | N = 1126 | ||||||||||||||||
| Thrombosis | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Infection | 0.5% | 2 | 430 | 0.5% | 2 | 435 | 0.99 | 0.14 to 7.01 | 0.994 | 0.3% | 1 | 367 | 1.1% | 4 | 368 | 3.95 | 0.44 to 35.17 | 0.218 | 0.3% | 3 | 1126 |
| Pain | 1.2% | 5 | 430 | 0.7% | 3 | 435 | 0.61 | 0.15 to 2.54 | 0.449 | 0.5% | 2 | 367 | 0.8% | 3 | 368 | 1.49 | 0.25 to 8.83 | 0.661 | 0.2% | 2 | 1126 |
| Urinary retention | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.3% | 1 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Bowel obstruction | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 0.0% | 0 | N/A | N/A | N/A | 0.0% | 0 | 1126 | ||
| Constipation | 0.7% | 3 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.5% | 2 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.1% | 1 | 1126 |
| Excess blood loss | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.1% | 1 | 1126 |
| Vaginal adhesions | 0.9% | 4 | 430 | 0.2% | 1 | 435 | 0.24 | 0.03 to 2.17 | 0.206 | 1.4% | 5 | 367 | 0.3% | 1 | 368 | 0.21 | 0.02 to 1.77 | 0.15 | 0.0% | 0 | 1126 |
| Haematoma | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Skin tags | 0.0% | 0 | 430 | 0.0% | 0 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Granulation tissue | 0.1% | 1 | 430 | 0.2% | 1 | 435 | 0.99 | 0.06 to 15.66 | 0.992 | 0.3% | 1 | 367 | 0.0% | 0 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Urinary tract symptoms | 0.0% | 0 | 430 | 0.2% | 1 | 435 | N/A | N/A | N/A | 0.0% | 0 | 367 | 0.3% | 1 | 368 | N/A | N/A | N/A | 0.0% | 0 | 1126 |
| Any other adverse effects (excluding mesh complications) | 3.0% | 13 | 430 | 1.6% | 7 | 435 | 0.53 | 0.21 to 1.32 | 0.172 | 3.0% | 11 | 367 | 2.4% | 9 | 368 | 0.80 | 0.34 to 1.90 | 0.613 | 0.5% | 6 | 1126 |
Prolapse symptoms at 6 months, 1 year and 2 years
The women’s report of prolapse symptoms, measured using the Pelvic Organ Prolapse Symptom scale, was less than half of the preoperative level (mean score before surgery 13.7/28; at 6 months 5.0/28; at 1 year 5.4/28; at 2 years 5.2/28) and the improvement remained at 2 years (Tables 24 and 25). There were no statistically significant differences between the randomised groups in either trial 1 or trial 2 at any time point.
| Symptom | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| Six-month outcomes | |||||||||||||||||||||
| Number of women at 6 months | N = 398 | N = 381 | N = 338 | N = 335 | N = 966 | ||||||||||||||||
| POP-SS at 6 months | 4.7 | (5.4) | 398 | 5.3 | (5.1) | 380 | 0.57 | –0.12 to 1.26 | 0.104 | 5.0 | (5.5) | 338 | 4.9 | (5.5) | 335 | –0.44 | –1.23 to 0.35 | 0.275 | 4.8 | (5.1) | 959 |
| Symptomatic | 78.9% | 314 | 398 | 85.5% | 325 | 380 | 1.07 | 1.00 to 1.14 | 0.038 | 81.1% | 274 | 338 | 80.9% | 271 | 335 | 1.00 | 0.93 to 1.08 | 0.956 | 81.0% | 777 | 959 |
| Prolapse-related QoL score | 2.0 | (2.8) | 390 | 2.2 | (2.7) | 374 | 0.22 | –0.16 to 0.60 | 0.262 | 2.0 | (2.9) | 332 | 2.0 | (2.7) | 330 | –0.17 | –0.58 to 0.25 | 0.428 | 2.1 | (2.8) | 946 |
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 395 | N = 389 | N = 342 | N = 337 | N = 972 | ||||||||||||||||
| POP-SS at 1 year | 5.4 | (5.5) | 395 | 5.5 | (5.1) | 389 | 0.00 | –0.70 to 0.71 | 0.989 | 5.5 | (5.6) | 342 | 5.6 | (5.6) | 337 | –0.15 | –0.93 to 0.63 | 0.706 | 5.2 | (5.3) | 963 |
| Symptomatic | 83.0% | 328 | 395 | 84.6% | 329 | 389 | 1.01 | 0.95 to 1.08 | 0.641 | 82.7% | 283 | 342 | 81.9% | 276 | 337 | 0.99 | 0.93 to 1.06 | 0.848 | 83.5% | 804 | 963 |
| Prolapse-related QoL score | 2.0 | (2.7) | 389 | 2.2 | (2.7) | 380 | 0.13 | –0.25 to 0.51 | 0.500 | 2.2 | (2.8) | 335 | 2.4 | (2.9) | 330 | 0.13 | –0.30 to 0.56 | 0.544 | 2.3 | (2.8) | 942 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 348 | N = 343 | N = 299 | N = 300 | N = 848 | ||||||||||||||||
| POP-SS at 2 years | 4.9 | (5.1) | 347 | 5.3 | (5.1) | 342 | 0.32 | –0.39 to 1.03 | 0.372 | 4.9 | (5.1) | 298 | 5.5 | (5.7) | 299 | 0.32 | –0.48 to 1.12 | 0.430 | 5.3 | (5.1) | 833 |
| Other measures of prolapse symptoms | |||||||||||||||||||||
| Symptomatic | 81.6% | 283 | 347 | 85.1% | 291 | 342 | 1.04 | 0.97 to 1.11 | 0.296 | 81.2% | 242 | 298 | 81.9% | 245 | 299 | 0.99 | 0.92 to 1.07 | 0.846 | 86.2% | 718 | 833 |
| Prolapse-related QoL score | 1.9 | (2.5) | 335 | 2.2 | (2.6) | 329 | 0.15 | –0.23 to 0.54 | 0.435 | 2.0 | (2.5) | 290 | 2.2 | (2.8) | 291 | 0.10 | –0.33 to 0.52 | 0.662 | 2.2 | (2.7) | 811 |
| Symptom | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| Six-month outcomes | |||||||||||||||||||||
| Number of women at 6 months | N = 398 | N = 381 | N = 338 | N = 335 | N = 966 | ||||||||||||||||
| Individual prolapse symptoms | |||||||||||||||||||||
| SCD any | 30.9% | 123 | 398 | 32.9% | 125 | 380 | 1.09 | 0.90 to 1.34 | 0.377 | 29.9% | 101 | 338 | 33.7% | 113 | 335 | 1.11 | 0.88 to 1.39 | 0.376 | 30.6% | 293 | 959 |
| SCD freq. | 7.8% | 31 | 398 | 9.7% | 37 | 380 | 1.21 | 0.77 to 1.90 | 0.403 | 9.2% | 31 | 338 | 11.0% | 37 | 335 | 1.08 | 0.68 to 1.71 | 0.745 | 8.6% | 82 | 959 |
| Pain any | 25.4% | 101 | 398 | 27.4% | 104 | 380 | 1.08 | 0.86 to 1.36 | 0.509 | 25.1% | 85 | 338 | 24.2% | 81 | 335 | 0.90 | 0.69 to 1.18 | 0.452 | 25.1% | 241 | 959 |
| Pain freq. | 4.8% | 19 | 398 | 6.1% | 23 | 380 | 1.22 | 0.69 to 2.15 | 0.503 | 5.9% | 20 | 338 | 5.4% | 18 | 335 | 0.78 | 0.41 to 1.50 | 0.465 | 4.8% | 46 | 959 |
| Abdo. any | 27.4% | 109 | 398 | 35.3% | 134 | 380 | 1.22 | 0.99 to 1.50 | 0.058 | 27.5% | 93 | 338 | 34.9% | 117 | 335 | 1.13 | 0.90 to 1.41 | 0.300 | 30.9% | 296 | 959 |
| Abdo. freq. | 3.8% | 15 | 398 | 5.0% | 19 | 380 | 1.26 | 0.66 to 2.43 | 0.484 | 4.1% | 14 | 338 | 3.9% | 13 | 335 | 0.56 | 0.24 to 1.29 | 0.175 | 4.3% | 41 | 959 |
| Back any | 33.4% | 133 | 398 | 38.9% | 148 | 380 | 1.17 | 0.98 to 1.40 | 0.082 | 34.0% | 115 | 338 | 38.8% | 130 | 335 | 1.15 | 0.95 to 1.39 | 0.161 | 34.1% | 327 | 959 |
| Back freq. | 7.5% | 30 | 398 | 7.4% | 28 | 380 | 0.94 | 0.59 to 1.51 | 0.793 | 9.5% | 32 | 338 | 8.7% | 29 | 335 | 0.75 | 0.46 to 1.23 | 0.261 | 7.2% | 69 | 959 |
| Strain blad. any | 36.2% | 144 | 398 | 38.2% | 145 | 380 | 1.08 | 0.91 to 1.29 | 0.367 | 36.4% | 123 | 338 | 37.0% | 124 | 335 | 0.99 | 0.81 to 1.21 | 0.927 | 37.6% | 361 | 959 |
| Strain blad. freq. | 10.1% | 40 | 398 | 8.2% | 31 | 380 | 0.83 | 0.53 to 1.28 | 0.391 | 11.2% | 38 | 338 | 8.1% | 27 | 335 | 0.71 | 0.44 to 1.15 | 0.164 | 6.8% | 65 | 959 |
| Blad. not empty any | 52.0% | 207 | 398 | 57.6% | 219 | 380 | 1.11 | 0.98 to 1.25 | 0.100 | 53.3% | 180 | 338 | 50.1% | 168 | 335 | 0.88 | 0.76 to 1.01 | 0.073 | 55.4% | 531 | 959 |
| Blad. not empty freq. | 13.3% | 53 | 398 | 13.9% | 53 | 380 | 1.10 | 0.78 to 1.55 | 0.585 | 13.3% | 45 | 338 | 13.1% | 44 | 335 | 0.88 | 0.60 to 1.30 | 0.531 | 10.9% | 105 | 959 |
| Bowel not empty any | 60.1% | 239 | 398 | 62.1% | 236 | 380 | 1.05 | 0.94 to 1.16 | 0.382 | 61.8% | 209 | 338 | 55.2% | 185 | 335 | 0.90 | 0.80 to 1.02 | 0.101 | 58.7% | 563 | 959 |
| Bowel not empty freq. | 12.1% | 48 | 398 | 13.2% | 50 | 380 | 1.17 | 0.81 to 1.68 | 0.406 | 16.0% | 54 | 338 | 11.0% | 37 | 335 | 0.71 | 0.48 to 1.03 | 0.070 | 10.9% | 105 | 959 |
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 395 | N = 389 | N = 342 | N = 337 | N = 972 | ||||||||||||||||
| Individual prolapse symptoms | |||||||||||||||||||||
| SCD any | 36.2% | 143 | 395 | 35.5% | 138 | 389 | 0.98 | 0.82 to 1.18 | 0.849 | 34.2% | 117 | 342 | 41.5% | 140 | 337 | 1.18 | 0.97 to 1.43 | 0.103 | 34.8% | 335 | 963 |
| SCD freq. | 9.1% | 36 | 395 | 9.8% | 38 | 389 | 1.08 | 0.70 to 1.66 | 0.740 | 10.8% | 37 | 342 | 11.6% | 39 | 337 | 1.00 | 0.65 to 1.54 | 0.999 | 7.9% | 76 | 963 |
| Pain any | 25.6% | 101 | 395 | 30.6% | 119 | 389 | 1.20 | 0.96 to 1.51 | 0.103 | 25.1% | 86 | 342 | 30.6% | 103 | 337 | 1.21 | 0.94 to 1.54 | 0.132 | 28.0% | 270 | 963 |
| Pain freq. | 4.3% | 17 | 395 | 4.6% | 18 | 389 | 1.01 | 0.53 to 1.92 | 0.970 | 5.0% | 17 | 342 | 5.3% | 18 | 337 | 0.90 | 0.47 to 1.73 | 0.751 | 5.3% | 51 | 963 |
| Abdo. any | 31.6% | 125 | 395 | 35.5% | 138 | 389 | 1.07 | 0.88 to 1.30 | 0.506 | 32.2% | 110 | 342 | 33.2% | 112 | 337 | 0.99 | 0.79 to 1.23 | 0.909 | 31.7% | 305 | 963 |
| Abdo. freq. | 5.6% | 22 | 395 | 4.6% | 18 | 389 | 0.85 | 0.46 to 1.59 | 0.608 | 5.8% | 20 | 342 | 6.2% | 21 | 337 | 0.97 | 0.52 to 1.79 | 0.917 | 4.4% | 42 | 963 |
| Back any | 36.2% | 143 | 395 | 39.3% | 153 | 389 | 1.07 | 0.90 to 1.28 | 0.431 | 37.4% | 128 | 342 | 38.0% | 128 | 337 | 1.06 | 0.88 to 1.28 | 0.542 | 37.3% | 359 | 963 |
| Back freq. | 7.8% | 31 | 395 | 8.2% | 32 | 389 | 1.05 | 0.66 to 1.65 | 0.848 | 7.9% | 27 | 342 | 9.8% | 33 | 337 | 1.19 | 0.74 to 1.93 | 0.469 | 7.9% | 76 | 963 |
| Strain blad. any | 44.1% | 174 | 395 | 41.6% | 162 | 389 | 0.93 | 0.80 to 1.08 | 0.350 | 42.1% | 144 | 342 | 40.4% | 136 | 337 | 0.88 | 0.74 to 1.05 | 0.156 | 39.7% | 382 | 963 |
| Strain blad. freq. | 10.9% | 43 | 395 | 7.2% | 28 | 389 | 0.63 | 0.41 to 0.99 | 0.045 | 11.7% | 40 | 342 | 10.4% | 35 | 337 | 0.81 | 0.53 to 1.25 | 0.340 | 9.2% | 89 | 963 |
| Blad. not empty any | 56.7% | 224 | 395 | 59.9% | 233 | 389 | 1.03 | 0.92 to 1.15 | 0.585 | 55.8% | 191 | 342 | 56.7% | 191 | 337 | 0.95 | 0.84 to 1.08 | 0.471 | 57.7% | 556 | 963 |
| Blad. not empty freq. | 13.9% | 55 | 395 | 11.6% | 45 | 389 | 0.83 | 0.57 to 1.19 | 0.307 | 14.6% | 50 | 342 | 12.8% | 43 | 337 | 0.84 | 0.58 to 1.21 | 0.337 | 11.8% | 114 | 963 |
| Bowel not empty any | 63.8% | 252 | 395 | 66.6% | 259 | 389 | 1.01 | 0.92 to 1.11 | 0.771 | 62.9% | 215 | 342 | 65.3% | 220 | 337 | 1.08 | 0.97 to 1.20 | 0.153 | 61.8% | 595 | 963 |
| Bowel not empty freq. | 15.9% | 63 | 395 | 12.6% | 49 | 389 | 0.83 | 0.59 to 1.15 | 0.263 | 16.7% | 57 | 342 | 12.8% | 43 | 337 | 0.77 | 0.54 to 1.09 | 0.137 | 11.8% | 114 | 963 |
| Actions necessitated by prolapse symptoms | |||||||||||||||||||||
| Fingers to ease discomfort | 1.1% | 4 | 352 | 1.2% | 4 | 347 | 1.19 | 0.30 to 4.64 | 0.803 | 1.3% | 4 | 308 | 1.9% | 6 | 309 | 1.42 | 0.32 to 6.31 | 0.643 | 1.1% | 9 | 854 |
| Extra hygiene measures | 5.7% | 20 | 349 | 6.6% | 23 | 349 | 1.16 | 0.65 to 2.05 | 0.620 | 7.5% | 23 | 308 | 5.3% | 16 | 304 | 0.57 | 0.29 to 1.13 | 0.106 | 4.9% | 42 | 859 |
| Fingers to help empty bladder | 0.3% | 1 | 364 | 0.8% | 3 | 353 | 3.16 | 0.33 to 30.64 | 0.321 | 0.0% | 0 | 313 | 0.6% | 2 | 311 | N/A | N/A | N/A | 0.6% | 5 | 875 |
| Fingers to help empty bowel | 2.0% | 7 | 358 | 1.7% | 6 | 349 | 0.86 | 0.30 to 2.47 | 0.776 | 1.9% | 6 | 311 | 1.9% | 6 | 311 | 0.98 | 0.32 to 2.99 | 0.972 | 2.7% | 24 | 873 |
| Digital evacuation of bowel | 3.0% | 11 | 366 | 1.7% | 6 | 357 | 0.54 | 0.21 to 1.41 | 0.207 | 3.8% | 12 | 315 | 1.9% | 6 | 314 | 0.40 | 0.15 to 1.09 | 0.072 | 3.3% | 29 | 881 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 348 | N = 343 | N = 299 | N = 300 | N = 848 | ||||||||||||||||
| Individual prolapse symptoms | |||||||||||||||||||||
| SCD any | 30.5% | 106 | 347 | 33.9% | 116 | 342 | 1.06 | 0.85 to 1.32 | 0.592 | 30.5% | 91 | 298 | 40.1% | 120 | 299 | 1.26 | 1.01 to 1.58 | 0.042 | 34.9% | 291 | 833 |
| SCD freq. | 6.3% | 22 | 347 | 7.9% | 27 | 342 | 1.27 | 0.74 to 2.16 | 0.383 | 5.7% | 17 | 298 | 11.4% | 34 | 299 | 1.80 | 1.03 to 3.15 | 0.041 | 8.0% | 67 | 833 |
| Pain any | 21.3% | 74 | 347 | 28.7% | 98 | 342 | 1.32 | 1.01 to 1.71 | 0.040 | 21.8% | 65 | 298 | 27.1% | 81 | 299 | 1.23 | 0.93 to 1.64 | 0.152 | 25.3% | 211 | 833 |
| Pain freq. | 2.9% | 10 | 347 | 2.6% | 9 | 342 | 0.79 | 0.32 to 1.95 | 0.610 | 2.3% | 7 | 298 | 3.3% | 10 | 299 | 1.18 | 0.45 to 3.10 | 0.735 | 4.8% | 40 | 833 |
| Abdo. any | 30.8% | 107 | 347 | 32.5% | 111 | 342 | 0.99 | 0.80 to 1.23 | 0.943 | 30.9% | 92 | 298 | 32.1% | 96 | 299 | 1.01 | 0.80 to 1.28 | 0.921 | 33.0% | 275 | 833 |
| Abdo. freq. | 4.3% | 15 | 347 | 3.5% | 12 | 342 | 0.78 | 0.37 to 1.61 | 0.495 | 4.0% | 12 | 298 | 5.4% | 16 | 299 | 1.03 | 0.49 to 2.16 | 0.939 | 3.5% | 29 | 833 |
| Back any | 37.5% | 130 | 347 | 42.7% | 146 | 342 | 1.08 | 0.91 to 1.29 | 0.377 | 37.6% | 112 | 298 | 34.1% | 102 | 299 | 0.92 | 0.75 to 1.12 | 0.392 | 39.0% | 325 | 833 |
| Back freq. | 6.6% | 23 | 347 | 6.4% | 22 | 342 | 0.86 | 0.50 to 1.49 | 0.601 | 6.4% | 19 | 298 | 6.7% | 20 | 299 | 0.91 | 0.50 to 1.65 | 0.754 | 7.6% | 63 | 833 |
| Strain blad. any | 39.2% | 136 | 347 | 43.3% | 148 | 342 | 1.05 | 0.89 to 1.24 | 0.581 | 38.6% | 115 | 298 | 45.5% | 136 | 299 | 1.06 | 0.88 to 1.27 | 0.520 | 41.9% | 349 | 833 |
| Strain blad. freq. | 7.5% | 26 | 347 | 6.7% | 23 | 342 | 0.80 | 0.46 to 1.38 | 0.421 | 9.4% | 28 | 298 | 10.7% | 32 | 299 | 0.99 | 0.61 to 1.60 | 0.962 | 8.6% | 72 | 833 |
| Blad. not empty any | 54.8% | 190 | 347 | 62.9% | 215 | 342 | 1.14 | 1.01 to 1.28 | 0.037 | 54.4% | 162 | 298 | 58.2% | 174 | 299 | 0.99 | 0.86 to 1.13 | 0.838 | 62.3% | 519 | 833 |
| Blad. not empty freq. | 11.0% | 38 | 347 | 10.5% | 36 | 342 | 0.86 | 0.55 to 1.34 | 0.497 | 12.8% | 38 | 298 | 14.4% | 43 | 299 | 0.95 | 0.64 to 1.40 | 0.780 | 10.8% | 90 | 833 |
| Bowel not empty any | 65.1% | 226 | 347 | 67.3% | 230 | 342 | 1.00 | 0.90 to 1.10 | 0.936 | 66.4% | 198 | 298 | 65.2% | 195 | 299 | 0.97 | 0.88 to 1.09 | 0.642 | 66.7% | 556 | 833 |
| Bowel not empty freq. | 13.3% | 46 | 347 | 13.7% | 47 | 342 | 1.06 | 0.74 to 1.54 | 0.745 | 13.8% | 41 | 298 | 13.4% | 40 | 299 | 0.99 | 0.67 to 1.46 | 0.957 | 10.8% | 90 | 833 |
| Actions necessitated by prolapse symptoms | |||||||||||||||||||||
| Fingers to ease discomfort | 1.7% | 6 | 343 | 0.9% | 3 | 331 | 0.45 | 0.11 to 1.87 | 0.269 | 1.4% | 4 | 295 | 2.1% | 6 | 288 | 1.54 | 0.42 to 5.65 | 0.514 | 1.1% | 9 | 825 |
| Extra hygiene measures | 5.6% | 19 | 341 | 5.2% | 17 | 330 | 0.93 | 0.48 to 1.77 | 0.815 | 5.1% | 15 | 293 | 6.6% | 19 | 287 | 1.21 | 0.61 to 2.41 | 0.579 | 5.0% | 41 | 823 |
| Fingers to help empty bladder | 0.6% | 2 | 347 | 0.6% | 2 | 338 | 2.02 | 0.18 to 22.20 | 0.566 | 0.7% | 2 | 298 | 1.3% | 4 | 298 | 2.07 | 0.38 to 11.31 | 0.400 | 0.5% | 4 | 835 |
| Fingers to help empty bowel | 2.7% | 9 | 336 | 2.4% | 8 | 335 | 0.92 | 0.38 to 2.24 | 0.859 | 2.8% | 8 | 289 | 0.3% | 1 | 293 | 0.12 | 0.02 to 0.97 | 0.046 | 3.5% | 29 | 830 |
| Digital evacuation of bowel | 2.6% | 9 | 342 | 0.6% | 2 | 337 | 0.29 | 0.06 to 1.37 | 0.118 | 4.7% | 14 | 295 | 1.4% | 4 | 296 | 0.33 | 0.11 to 0.97 | 0.044 | 3.5% | 29 | 838 |
Specifically the primary outcome was the POP-SS at 1 year (see Table 24).
-
In trial 1, the MD in the POP-SSs for standard repair (5.4, SD 5.5) compared with synthetic mesh inlay (5.5, SD 5.1), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1B (two-way randomisation), was MD 0.00 (95% CI –0.70 to 0.71).
-
In trial 2, the MD for standard repair (5.5, SD 5.6) compared with biological graft (5.6, SD 5.6), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1C (two-way randomisation), was MD –0.15, 95% CI –0.93 to 0.63.
At 2 years, the POP-SSs remained relatively stable, still with no difference between the groups (see Table 24).
-
In trial 1, the MD in the POP-SSs for standard repair (4.9, SD 5.1) compared with synthetic mesh inlay (5.3, SD 5.1), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1B (two-way randomisation) was MD 0.32, 95% CI –0.39 to 1.03.
-
In trial 2, the MD for standard repair (4.9, SD 5.1) compared with biological graft (5.5, SD 5.7), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1C (two-way randomisation) was MD 0.32, 95% CI –0.48 to 1.12.
The lack of difference between the groups was supported by (see Tables 24 and 25):
-
data from individual prolapse symptoms (whether measured as ‘any’ or occurring ‘most or all of the time’)
-
the proportion of women who had at least one prolapse symptom (‘symptomatic’ defined as POP-SS of > 0)
-
the prolapse-related QoL score measured as the interference of prolapse symptoms with everyday life, and
-
the need to undertake extra hygiene measures or manoeuvres to ease discomfort or to assist pelvic floor functions, such as emptying the bladder or bowel.
All of these measures demonstrated significant improvements from before surgery, but no difference between the randomised groups at any time point in either trial (see Tables 24 and 25).
The improvement at 1 year was maintained at 2 years, with respect to all of the prolapse outcomes and QoL outcomes measured. However, there were still no statistically significant differences between the randomised groups in either trial. The data from the cohort women were similar (see Tables 24 and 25).
EuroQol-5 Dimensions (3-level version)
There were no statistically significant differences between the randomised groups in the generic EQ-5D-3L QoL scores at 6 months, 1 year or 2 years in either trial 1 or trial 2 (Table 26). However, the score improved from baseline levels in all the groups of women. The data from the cohort women were similar. This outcome is further explored in the section on economic outcomes in Chapter 5.
| EQ-5D-3L | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| At 6 months | |||||||||||||||||||||
| Number of women | N = 398 | N = 381 | N = 338 | N = 335 | N = 966 | ||||||||||||||||
| Score | 0.82 | (0.26) | 383 | 0.83 | (0.22) | 372 | 0.01 | –0.02 to 0.04 | 0.400 | 0.82 | (0.27) | 326 | 0.82 | (0.25) | 318 | 0.01 | –0.02 to 0.05 | 0.499 | 0.83 | (0.24) | 935 |
| At 1 year | |||||||||||||||||||||
| Number of women | N = 395 | N = 389 | N = 342 | N = 337 | N = 972 | ||||||||||||||||
| Score | 0.83 | (0.25) | 385 | 0.83 | (0.22) | 384 | 0.01 | –0.02 to 0.04 | 0.651 | 0.81 | (0.27) | 335 | 0.82 | (0.25) | 333 | 0.02 | –0.01 to 0.06 | 0.205 | 0.83 | (0.25) | 949 |
| At 2 years | |||||||||||||||||||||
| Number of women | N = 348 | N = 343 | N = 299 | N = 300 | N = 848 | ||||||||||||||||
| Score | 0.81 | (0.28) | 340 | 0.83 | (0.22) | 334 | 0.02 | –0.02 to 0.06 | 0.257 | 0.81 | (0.28) | 291 | 0.82 | (0.27) | 294 | 0.03 | –0.01 to 0.07 | 0.170 | 0.83 | (0.24) | 821 |
Specifically the EQ-5D-3L scores at 1 year were compared (see Table 26).
-
In trial 1, the MD in the EQ-5D-3L scores for standard repair (0.830) compared with synthetic mesh inlay (0.834), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1B (two-way randomisation), was MD 0.01, 95% CI –0.02 to 0.04.
-
In trial 2, the MD for standard repair (0.81) compared with biological graft (0.82), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1C (two-way randomisation), was MD 0.02, 95% CI –0.01 to 0.06.
The EQ-5D-3L scores at 2 years were virtually unchanged (see Table 26):
-
In trial 1, the MD in the EQ-5D-3L scores for standard repair (0.81) compared with synthetic mesh inlay (0.83), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1B (two-way randomisation), was MD 0.02, 95% CI –0.02 to 0.06.
-
In trial 2, the MD for standard repair (0.81) compared with biological graft (0.82), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1C (two-way randomisation), was MD 0.03, 95% CI –0.01 to 0.07.
Urinary symptoms
Detailed information on urinary symptoms was obtained at baseline, 1 year and 2 years. The proportion of women who had concomitant continence surgery ranged from 9.7% to 12.1% (see Table 19). There was an overall decrease of 10% in the proportion of women who had any UI (from around 77% to around 65%) and the proportion with severe UI more than halved (from around 20% to around 7%) at 1 year (see Tables 16 and 27).
The findings were virtually unchanged by 2 years: there did not appear to be any further recovery or deterioration in urinary symptoms over that time span. However, there was no difference between the randomised groups with respect to any of the urinary outcomes measured at 1 or 2 years in either trial (Table 27). The data from the cohort women were similar.
| Symptom | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 368 | N = 362 | N = 319 | N = 316 | N = 893 | ||||||||||||||||
| Any incontinence | 63.9% | 235 | 368 | 64.9% | 235 | 362 | 1.00 | 0.91 to 1.10 | 0.977 | 64.9% | 207 | 319 | 64.2% | 203 | 316 | 0.98 | 0.89 to 1.08 | 0.673 | 65.6% | 586 | 893 |
| ICIQ-UI-SF score | 4.1 | (4.3) | 361 | 4.4 | (4.7) | 354 | 0.29 | –0.30 to 0.88 | 0.333 | 4.4 | (4.6) | 315 | 4.1 | (4.3) | 313 | –0.44 | –1.04 to 0.15 | 0.144 | 4.4 | (4.5) | 876 |
| Severe incontinence | 5.8% | 21 | 361 | 8.2% | 29 | 354 | 1.34 | 0.79 to 2.26 | 0.274 | 8.3% | 26 | 315 | 5.4% | 17 | 313 | 0.61 | 0.33 to 1.12 | 0.110 | 7.0% | 61 | 876 |
| UI QoL score | 1.6 | (2.3) | 345 | 1.8 | (2.6) | 344 | 0.21 | –0.13 to 0.55 | 0.229 | 1.8 | (2.5) | 301 | 1.6 | (2.4) | 302 | –0.16 | –0.52 to 0.19 | 0.361 | 1.7 | (2.5) | 859 |
| Stress UI | 8.4% | 24 | 286 | 8.1% | 24 | 296 | 1.11 | 0.64 to 1.92 | 0.715 | 10.8% | 27 | 251 | 10.6% | 27 | 255 | 1.26 | 0.78 to 2.05 | 0.340 | 11.0% | 80 | 728 |
| Urgency UI | 3.3% | 12 | 366 | 5.3% | 19 | 361 | 1.59 | 0.79 to 3.22 | 0.195 | 4.1% | 13 | 318 | 2.2% | 7 | 314 | 0.47 | 0.19 to 1.14 | 0.093 | 4.5% | 40 | 891 |
| Overactive bladder | 1.4% | 5 | 363 | 2.3% | 8 | 355 | 1.69 | 0.56 to 5.08 | 0.352 | 1.9% | 6 | 315 | 0.3% | 1 | 313 | 0.16 | 0.02 to 1.31 | 0.087 | 1.8% | 16 | 883 |
| ICIQ-FLUTS filling score | 3.6 | (2.4) | 363 | 3.8 | (2.4) | 355 | 0.10 | –0.19 to 0.39 | 0.500 | 3.7 | (2.6) | 315 | 3.7 | (2.4) | 311 | –0.19 | –0.50 to 0.12 | 0.234 | 3.7 | (2.4) | 879 |
| ICIQ-FLUTS voiding score | 1.8 | (2.1) | 363 | 2.0 | (2.0) | 359 | 0.22 | –0.06 to 0.50 | 0.120 | 1.9 | (2.1) | 317 | 1.9 | (2.0) | 313 | 0.02 | –0.27 to 0.31 | 0.895 | 1.8 | (2.1) | 887 |
| ICIQ-FLUTS incontinence | 4.2 | (3.3) | 279 | 4.3 | (3.5) | 288 | 0.25 | –0.27 to 0.78 | 0.345 | 4.6 | (3.6) | 247 | 4.2 | (3.5) | 250 | –0.38 | –0.94 to 0.18 | 0.182 | 4.5 | (3.7) | 713 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 348 | N = 343 | N = 299 | N = 300 | N = 848 | ||||||||||||||||
| Any incontinence | 65.5% | 228 | 348 | 66.9% | 228 | 341 | 1.00 | 0.91 to 1.11 | 0.947 | 65.6% | 196 | 299 | 62.7% | 188 | 300 | 0.94 | 0.85 to 1.03 | 0.188 | 66.9% | 567 | 848 |
| ICIQ-UI-SF score | 4.2 | (4.4) | 343 | 4.4 | (4.3) | 334 | 0.00 | –0.59 to 0.59 | 0.998 | 4.3 | (4.5) | 294 | 4.1 | (4.4) | 297 | –0.49 | –1.11 to 0.13 | 0.121 | 4.4 | (4.7) | 829 |
| Severe incontinence | 5.5% | 19 | 343 | 6.3% | 21 | 334 | 1.01 | 0.51 to 1.99 | 0.974 | 7.1% | 21 | 294 | 6.7% | 20 | 297 | 0.80 | 0.44 to 1.46 | 0.468 | 8.6% | 71 | 829 |
| UI QoL score | 1.6 | (2.4) | 337 | 1.8 | (2.4) | 329 | –0.02 | –0.36 to 0.33 | 0.930 | 1.7 | (2.4) | 289 | 1.7 | (2.5) | 290 | –0.12 | –0.49 to 0.24 | 0.513 | 1.8 | (2.6) | 826 |
| Stress UI | 8.0% | 24 | 301 | 8.3% | 24 | 290 | 1.05 | 0.59 to 1.86 | 0.867 | 9.2% | 24 | 262 | 8.2% | 21 | 256 | 0.91 | 0.53 to 1.56 | 0.735 | 9.8% | 72 | 733 |
| Urgency UI | 3.4% | 12 | 348 | 5.3% | 18 | 339 | 1.74 | 0.74 to 4.07 | 0.205 | 3.0% | 9 | 299 | 4.3% | 13 | 299 | 1.27 | 0.55 to 2.89 | 0.576 | 5.6% | 47 | 840 |
| Overactive bladder | 1.4% | 5 | 346 | 3.0% | 10 | 338 | 1.97 | 0.69 to 5.68 | 0.207 | 1.7% | 5 | 297 | 1.7% | 5 | 296 | 0.56 | 0.16 to 1.96 | 0.367 | 2.0% | 17 | 835 |
| ICIQ-FLUTS filling score | 3.8 | (2.6) | 343 | 4.0 | (2.5) | 335 | 0.09 | –0.26 to 0.44 | 0.631 | 3.9 | (2.6) | 293 | 3.8 | (2.5) | 296 | –0.32 | –0.65 to 0.02 | 0.062 | 3.8 | (2.4) | 831 |
| ICIQ-FLUTS voiding score | 1.8 | (2.2) | 343 | 2.0 | (2.1) | 338 | 0.10 | –0.21 to 0.41 | 0.531 | 1.9 | (2.2) | 296 | 2.0 | (2.3) | 298 | –0.03 | –0.35 to 0.30 | 0.864 | 1.9 | (2.1) | 839 |
| ICIQ-FLUTS incontinence | 4.0 | (3.5) | 294 | 4.3 | (3.3) | 281 | 0.14 | –0.39 to 0.67 | 0.602 | 4.1 | (3.6) | 255 | 4.1 | (3.7) | 252 | –0.40 | –0.98 to 0.18 | 0.175 | 4.2 | (3.7) | 715 |
Bowel symptoms
Detailed information on bowel symptoms was obtained at baseline, 1 year and 2 years. Bowel frequency and urgency were largely unchanged after prolapse surgery (Table 28). However, fewer women had constipation or FI; this improvement was reflected in the bowel symptoms QoL score, which was around half of the baseline level at both 1 year and 2 years after surgery (see Tables 17 and 28). Nevertheless, there was no difference between the randomised groups with respect to any of the bowel outcomes measured at 1 year or 2 years in either trial (see Table 28). The data from the cohort women were similar.
| Symptom | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 368 | N = 362 | N = 319 | N = 316 | N = 893 | ||||||||||||||||
| Bowel frequency | |||||||||||||||||||||
| > 3 daily | 4.4% | 16 | 365 | 2.5% | 9 | 359 | 0.85 | 0.62 to 1.14 | 0.277 | 4.5% | 14 | 314 | 2.9% | 9 | 312 | 0.86 | 0.62 to 1.19 | 0.363 | 3.6% | 32 | 883 |
| 1–3 times daily | 27.9% | 102 | 365 | 30.1% | 108 | 359 | 33.4% | 105 | 314 | 32.1% | 100 | 312 | 27.4% | 242 | 883 | ||||||
| Once daily | 46.3% | 169 | 365 | 46.8% | 168 | 359 | 41.4% | 130 | 314 | 43.9% | 137 | 312 | 49.5% | 437 | 883 | ||||||
| Every 2–3 days | 18.4% | 67 | 365 | 17.8% | 64 | 359 | 18.2% | 57 | 314 | 19.2% | 60 | 312 | 17.9% | 158 | 883 | ||||||
| Weekly or less | 3.0% | 11 | 365 | 2.8% | 10 | 359 | 2.5% | 8 | 314 | 1.9% | 6 | 312 | 1.6% | 14 | 883 | ||||||
| Constipation | 14.0% | 51 | 365 | 12.6% | 45 | 357 | 1.04 | 0.73 to 1.48 | 0.811 | 14.7% | 46 | 313 | 13.5% | 42 | 310 | 0.96 | 0.66 to 1.40 | 0.835 | 14.1% | 123 | 871 |
| Bowel urgency | 8.2% | 30 | 364 | 4.7% | 17 | 359 | 0.62 | 0.36 to 1.08 | 0.091 | 7.6% | 24 | 314 | 5.1% | 16 | 314 | 0.62 | 0.34 to 1.13 | 0.118 | 6.7% | 59 | 887 |
| FI (any) | 27.9% | 102 | 365 | 25.4% | 91 | 358 | 0.92 | 0.74 to 1.13 | 0.411 | 26.6% | 84 | 316 | 24.5% | 77 | 314 | 0.92 | 0.72 to 1.17 | 0.495 | 27.1% | 240 | 885 |
| Passive FI | 74.5% | 76 | 102 | 84.6% | 77 | 91 | 76.2% | 64 | 84 | 85.7% | 66 | 77 | 80.4% | 193 | 240 | ||||||
| Active FI | 25.5% | 26 | 102 | 15.4% | 14 | 91 | 23.8% | 20 | 84 | 14.3% | 11 | 77 | 19.6% | 47 | 240 | ||||||
| Severe FI | 6.6% | 24 | 365 | 7.3% | 26 | 358 | 1.18 | 0.70 to 1.99 | 0.537 | 5.7% | 18 | 316 | 8.9% | 28 | 314 | 1.33 | 0.75 to 2.35 | 0.334 | 6.4% | 57 | 885 |
| Bowel symptoms QoL | 1.8 | (2.4) | 359 | 1.7 | (2.3) | 348 | 0.03 | –0.29 to 0.35 | 0.859 | 1.9 | (2.4) | 313 | 1.7 | (2.4) | 310 | –0.13 | –0.48 to 0.23 | 0.483 | 1.9 | (2.5) | 869 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 348 | N = 343 | N = 299 | N = 300 | N = 848 | ||||||||||||||||
| Bowel frequency | |||||||||||||||||||||
| > 3 daily | 5.0% | 17 | 343 | 4.7% | 16 | 338 | 1.03 | 0.76 to 1.40 | 0.848 | 6.1% | 18 | 296 | 3.4% | 10 | 298 | 1.30 | 0.93 to 1.81 | 0.130 | 3.1% | 26 | 843 |
| 1–3 times daily | 27.1% | 93 | 343 | 28.4% | 96 | 338 | 33.1% | 98 | 296 | 30.2% | 90 | 298 | 31.0% | 261 | 843 | ||||||
| Once daily | 48.7% | 167 | 343 | 48.8% | 165 | 338 | 40.9% | 121 | 296 | 47.0% | 140 | 298 | 48.5% | 409 | 843 | ||||||
| Every 2–3 days | 15.7% | 54 | 343 | 15.4% | 52 | 338 | 16.9% | 50 | 296 | 17.4% | 52 | 298 | 15.9% | 134 | 843 | ||||||
| Weekly or less | 3.5% | 12 | 343 | 2.7% | 9 | 338 | 3.0% | 9 | 296 | 2.0% | 6 | 298 | 1.5% | 13 | 843 | ||||||
| Constipation | 12.7% | 43 | 338 | 11.6% | 39 | 335 | 0.95 | 0.65 to 1.41 | 0.814 | 13.7% | 40 | 292 | 12.2% | 36 | 296 | 0.89 | 0.60 to 1.32 | 0.568 | 12.6% | 104 | 825 |
| Bowel urgency | 8.2% | 28 | 343 | 3.8% | 13 | 338 | 0.50 | 0.26 to 0.97 | 0.040 | 7.8% | 23 | 294 | 6.1% | 18 | 297 | 0.70 | 0.38 to 1.29 | 0.252 | 5.8% | 49 | 839 |
| FI (any) | 25.9% | 89 | 343 | 27.2% | 92 | 338 | 1.13 | 0.92 to 1.41 | 0.249 | 27.5% | 81 | 295 | 25.8% | 77 | 298 | 0.95 | 0.75 to 1.21 | 0.692 | 24.6% | 206 | 837 |
| Passive FI | 74.2% | 66 | 89 | 87.0% | 80 | 92 | 76.5% | 62 | 81 | 80.5% | 62 | 77 | 80.1% | 165 | 206 | ||||||
| Active FI | 25.8% | 23 | 89 | 13.0% | 12 | 92 | 23.5% | 19 | 81 | 19.5% | 15 | 77 | 19.9% | 41 | 206 | ||||||
| Severe FI | 7.9% | 27 | 343 | 5.0% | 17 | 338 | 0.71 | 0.40 to 1.26 | 0.245 | 6.8% | 20 | 295 | 10.1% | 30 | 298 | 1.09 | 0.64 to 1.86 | 0.747 | 7.3% | 61 | 837 |
| Bowel symptoms QoL | 1.8 | (2.6) | 333 | 1.7 | (2.3) | 337 | –0.05 | –0.40 to 0.30 | 0.767 | 1.9 | (2.5) | 288 | 1.7 | (2.4) | 294 | –0.09 | –0.45 to 0.27 | 0.623 | 1.9 | (2.6) | 823 |
Vaginal and sexual symptoms
Detailed information on vaginal and sexual symptoms was obtained at baseline, 1 year and 2 years (see Tables 18 and 29). Both the mean vaginal symptom score and the QoL decreased (improved) after prolapse surgery (Table 29).
| Symptom | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 368 | N = 362 | N = 319 | N = 316 | N = 893 | ||||||||||||||||
| Vaginal | |||||||||||||||||||||
| ICIQ-VS | 7.2 | (7.2) | 338 | 7.5 | (8.1) | 327 | 0.52 | –0.64 to 1.68 | 0.381 | 7.1 | (6.9) | 294 | 9.0 | (9.1) | 294 | 1.31 | 0.04 to 2.59 | 0.044 | 7.9 | (8.5) | 804 |
| Vaginal symptoms QoL | 1.4 | (2.3) | 346 | 1.6 | (2.4) | 343 | 0.14 | –0.20 to 0.48 | 0.432 | 1.5 | (2.3) | 301 | 1.8 | (2.6) | 306 | 0.33 | –0.06 to 0.72 | 0.098 | 1.8 | (2.6) | 847 |
| Vagina too tight | 3.2% | 11 | 349 | 2.0% | 7 | 348 | 0.55 | 0.20 to 1.45 | 0.226 | 3.0% | 9 | 305 | 3.0% | 9 | 305 | 1.00 | 0.42 to 2.41 | 0.992 | 2.8% | 24 | 850 |
| Sexual | |||||||||||||||||||||
| Sex life at present | 48.6% | 175 | 360 | 46.9% | 169 | 360 | 44.1% | 138 | 313 | 48.7% | 152 | 312 | 48.5% | 426 | 878 | ||||||
| Reason for no sex life | |||||||||||||||||||||
| No partner | 37.3% | 69 | 185 | 38.2% | 73 | 191 | 34.3% | 60 | 175 | 35.6% | 57 | 160 | 38.1% | 172 | 452 | ||||||
| Vaginal symptoms | 7.0% | 13 | 185 | 5.2% | 10 | 191 | 9.1% | 16 | 175 | 8.1% | 13 | 160 | 5.8% | 26 | 452 | ||||||
| Prolapse symptoms | 11.4% | 21 | 185 | 11.0% | 21 | 191 | 13.7% | 24 | 175 | 15.0% | 24 | 160 | 9.5% | 43 | 452 | ||||||
| Other reason | 38.4% | 71 | 185 | 38.7% | 74 | 191 | 37.1% | 65 | 175 | 32.5% | 52 | 160 | 39.4% | 178 | 452 | ||||||
| Reason not given | 5.9% | 11 | 185 | 6.8% | 13 | 191 | 5.7% | 10 | 175 | 8.8% | 14 | 160 | 7.3% | 33 | 452 | ||||||
| Dyspareunia | 4.3% | 8 | 186 | 5.2% | 9 | 173 | 1.73 | 0.52 to 5.78 | 0.373 | 6.0% | 9 | 149 | 4.8% | 8 | 165 | 1.17 | 0.43 to 3.23 | 0.758 | 5.4% | 24 | 445 |
| ICI Sexual Matters score | 11.3 | (12.9) | 180 | 11.2 | (13.1) | 173 | –0.40 | –3.27 to 2.46 | 0.781 | 12.2 | (13.3) | 144 | 10.9 | (13.1) | 163 | –2.46 | –5.60 to 0.69 | 0.126 | 12.0 | (13.9) | 439 |
| Sex life QoL score | 2.7 | (3.2) | 184 | 3.0 | (3.4) | 184 | 0.47 | –0.23 to 1.16 | 0.189 | 3.0 | (3.4) | 150 | 2.6 | (3.4) | 164 | –0.57 | –1.30 to 0.17 | 0.128 | 2.8 | (3.2) | 448 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 348 | N = 343 | N = 299 | N = 300 | N = 848 | ||||||||||||||||
| Vaginal | |||||||||||||||||||||
| ICIQ-VS | 7.0 | (7.3) | 313 | 7.3 | (7.8) | 311 | –0.18 | –1.34 to 0.98 | 0.755 | 6.8 | (6.8) | 271 | 8.1 | (8.8) | 278 | 0.36 | –0.95 to 1.67 | 0.585 | 7.4 | (8.1) | 772 |
| Vaginal symptoms QoL | 1.5 | (2.3) | 331 | 1.7 | (2.4) | 332 | 0.12 | –0.23 to 0.47 | 0.497 | 1.3 | (2.1) | 283 | 1.6 | (2.4) | 283 | 0.15 | –0.21 to 0.51 | 0.422 | 1.6 | (2.5) | 805 |
| Vagina too tight | 3.3% | 11 | 329 | 0.9% | 3 | 329 | 0.16 | 0.04 to 0.74 | 0.018 | 2.8% | 8 | 283 | 2.4% | 7 | 288 | 0.84 | 0.31 to 2.25 | 0.726 | 2.8% | 23 | 811 |
| Sexual | |||||||||||||||||||||
| Sex life at present | 47.3% | 159 | 336 | 42.2% | 139 | 329 | 42.1% | 120 | 285 | 50.3% | 147 | 292 | 47.5% | 391 | 824 | ||||||
| Reason for no sex life | |||||||||||||||||||||
| No partner | 29.9% | 53 | 177 | 34.2% | 65 | 190 | 30.3% | 50 | 165 | 34.5% | 50 | 145 | 33.3% | 144 | 433 | ||||||
| Vaginal symptoms | 6.8% | 12 | 177 | 2.6% | 5 | 190 | 8.5% | 14 | 165 | 6.2% | 9 | 145 | 6.5% | 28 | 433 | ||||||
| Prolapse symptoms | 9.0% | 16 | 177 | 8.4% | 16 | 190 | 9.7% | 16 | 165 | 9.7% | 14 | 145 | 7.4% | 32 | 433 | ||||||
| Other reason | 40.1% | 71 | 177 | 39.5% | 75 | 190 | 38.8% | 64 | 165 | 29.7% | 43 | 145 | 35.6% | 154 | 433 | ||||||
| Reason not given | 14.1% | 25 | 177 | 15.3% | 29 | 190 | 12.7% | 21 | 165 | 20.0% | 29 | 145 | 17.3% | 75 | 433 | ||||||
| Dyspareunia | 5.4% | 9 | 166 | 2.8% | 4 | 145 | 0.49 | 0.15 to 1.55 | 0.223 | 4.0% | 5 | 125 | 3.9% | 6 | 154 | 0.93 | 0.29 to 2.99 | 0.903 | 5.0% | 20 | 400 |
| ICI Sexual Matters score | 10.6 | (13.0) | 166 | 10.3 | (12.5) | 145 | –0.15 | –3.17 to 2.88 | 0.923 | 10.1 | (12.8) | 125 | 10.0 | (12.3) | 152 | –0.62 | –3.85 to 2.61 | 0.706 | 12.2 | (14.1) | 399 |
| Sex life QoL score | 2.3 | (3.0) | 164 | 2.5 | (3.1) | 151 | 0.13 | –0.59 to 0.84 | 0.728 | 2.1 | (2.8) | 126 | 2.3 | (3.0) | 157 | 0.13 | –0.59 to 0.85 | 0.728 | 2.7 | (3.2) | 402 |
More women were sexually active after surgery (increased from < 40% before to around 50% after) and fewer cited prolapse symptoms as a reason for not having a sex life (reduced from > 40% to < 15%). This was reflected in a halving of the ICI Sexual Matters score, and a greater reduction (improvement) in the sex life QoL score. The rates for dyspareunia were low both before (around 9%) and after surgery (around 5%; see Tables 18 and 29). However, there was no difference between the randomised groups with respect to any of the vaginal or sexual symptom outcomes measured. The improvements in these outcomes were maintained at 2 years, but still with no differences between the randomised groups in either trial (see Table 29). The data from the cohort women were similar.
Postoperative prolapse measurements in randomised women
A 1-year clinic review was offered to all randomised women and 88% attended. Objective measurement showed improvement in each of the three prolapse compartments (Table 30). The proportion of women with the leading prolapse edge beyond the hymen (> 0 cm) reduced substantially. Nevertheless, just under 20% of women still had residual prolapse.
| POP-Q measurement/stage | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | |||||||||
| Number of women | N = 381 | N = 374 | N = 319 | N = 319 | ||||||||||||||
| POP-Q measurement (cm) | ||||||||||||||||||
| Ba (posterior edge) | –1.3 | (1.6) | 323 | –1.3 | (1.6) | 327 | 0.06 | –0.17 to 0.29 | 0.622 | –1.3 | (1.7) | 299 | –1.2 | (1.7) | 293 | 0.12 | –0.1 to 0.4 | 0.344 |
| C (cervix/vault) | –6.0 | (2.1) | 318 | –6.0 | (2.3) | 321 | –0.03 | –0.36 to 0.31 | 0.875 | –5.8 | (1.9) | 292 | –5.7 | (2.1) | 292 | 0.15 | –0.2 to 0.5 | 0.371 |
| Bp (posterior edge) | –2.0 | (1.2) | 322 | –2.1 | (1.1) | 326 | –0.03 | –0.21 to 0.15 | 0.737 | –2.1 | (1.2) | 299 | –2.0 | (1.2) | 290 | 0.13 | –0.1 to 0.3 | 0.196 |
| TVL | 8.1 | (1.2) | 320 | 8.2 | (1.3) | 318 | 0.12 | –0.07 to 0.30 | 0.212 | 7.8 | (1.2) | 291 | 7.8 | (1.2) | 286 | 0.07 | –0.1 to 0.3 | 0.503 |
| Overall POP-Q stage | ||||||||||||||||||
| 0 | 16.4% | 56 | 341 | 14.2% | 48 | 339 | 1.11 | 0.83 to 1.47 | 0.494 | 16.7% | 51 | 305 | 14.0% | 42 | 299 | 1.26 | 0.93 to 1.71 | 0.131 |
| 1 | 31.7% | 108 | 341 | 33.3% | 113 | 339 | 31.5% | 96 | 305 | 28.4% | 85 | 299 | ||||||
| 2 | 44.9% | 153 | 341 | 46.6% | 158 | 339 | 44.3% | 135 | 305 | 48.2% | 144 | 299 | ||||||
| 3 | 6.5% | 22 | 341 | 5.6% | 19 | 339 | 6.9% | 21 | 305 | 8.4% | 25 | 299 | ||||||
| 4 | 0.6% | 2 | 341 | 0.3% | 1 | 339 | 0.7% | 2 | 305 | 1.0% | 3 | 299 | ||||||
| 2b, 3 or 4 | 13.9% | 47 | 338 | 16.1% | 54 | 336 | 1.12 | 0.79 to 1.60 | 0.519 | 15.5% | 47 | 303 | 18.1% | 54 | 298 | 1.14 | 0.80 to 1.62 | 0.471 |
Specifically, the RR for the proportion of women with residual prolapse beyond the hymen at 1 year (see Table 30) was:
-
In trial 1, in the standard repair group (13.9%) compared with synthetic mesh inlay (16.1%), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1B (two-way randomisation), RR 1.12, 95% CI 0.79 to 1.60.
-
In trial 2, in the standard repair group (15.5%) compared with biological graft (18.1%), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1C (two-way randomisation), RR 1.14, 95% CI 0.80 to 1.62.
Thus, the finding that more women appeared to have residual prolapse after mesh or graft repair than after standard repair was not statistically significant, and the difference was so small that it is not likely to be clinically significant.
Readmissions and further treatment required for failure and adverse effects at 6 months, 1 year and 2 years
When women reported that, at 6 months or later, they had been readmitted to hospital, we verified the information by enquiry from centre staff when necessary and post-coded the corrected information. If it was related to the initial prolapse surgery, a hospital readmission was automatically counted as a SAE. Surgery for recurrence of prolapse (repeat if same compartment, further surgery if in the opposite compartment), or for continence surgery, was differentiated from readmission for complications related to prolapse surgery, such as bleeding, infection and mesh removal (Table 31).
| Further treatment | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| 6-month outcomes | |||||||||||||||||||||
| Number of women at 6 months | N = 398 | N = 381 | N = 338 | N = 335 | N = 966 | ||||||||||||||||
| Readmitted (0–6 months) | 2.8% | 11a | 398 | 3.1% | 12b | 381 | 1.15 | 0.51 to 2.57 | 0.738 | 2.7% | 9c | 338 | 4.2% | 14d | 335 | 1.54 | 0.68 to 3.51 | 0.304 | 3.3% | 32e | 966 |
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 395 | N = 389 | N = 342 | N = 337 | N = 972 | ||||||||||||||||
| Readmitted (6–12 months) | 1.0% | 4f | 395 | 1.3% | 5g | 389 | 1.32 | 0.36 to 4.81 | 0.677 | 1.2% | 4h | 342 | 1.8% | 6i | 337 | 1.67 | 0.48 to 5.79 | 0.416 | 0.7% | 7j | 972 |
| New prolapse surgery | 1.5% | 6 | 395 | 3.1% | 12 | 389 | 1.99 | 0.76 to 5.24 | 0.163 | 2.0% | 7 | 342 | 3.0% | 10 | 337 | 1.44 | 0.56 to 3.73 | 0.451 | 2.7% | 26 | 972 |
| Same compartment | 0.8% | 3 | 395 | 2.1% | 8 | 389 | 2.55 | 0.68 to 9.53 | 0.163 | 1.5% | 5 | 342 | 1.5% | 5 | 337 | 0.98 | 0.29 to 3.34 | 0.976 | 0.9% | 9 | 972 |
| Different compartment | 0.8% | 3 | 395 | 1.0% | 4 | 389 | 1.35 | 0.31 to 5.96 | 0.692 | 0.6% | 2 | 342 | 1.5% | 5 | 337 | 2.50 | 0.49 to 12.74 | 0.269 | 1.7% | 17 | 972 |
| Waiting for prolapse surgery | 1.5% | 6 | 395 | 0.5% | 2 | 389 | 0.33 | 0.07 to 1.65 | 0.178 | 1.5% | 5 | 342 | 1.2% | 4 | 337 | 0.82 | 0.22 to 3.01 | 0.761 | 1.5% | 15 | 972 |
| Continence surgery | 1.3% | 5 | 395 | 0.5% | 2 | 389 | 0.40 | 0.08 to 2.04 | 0.269 | 0.6% | 2 | 342 | 2.1% | 7 | 337 | 3.49 | 0.73 to 16.66 | 0.116 | 0.9% | 9 | 972 |
| Waiting for continence surgery | 0.3% | 1 | 395 | 0.5% | 2 | 389 | 2.13 | 0.19 to 23.50 | 0.536 | 0.9% | 3 | 342 | 0.3% | 1 | 337 | 0.32 | 0.03 to 3.10 | 0.328 | 0.4% | 4 | 972 |
| Stitches removed | 1.6% | 6 | 381 | 1.1% | 4 | 371 | 0.69 | 0.20 to 2.43 | 0.565 | 2.5% | 8 | 326 | 3.7% | 12 | 322 | 1.53 | 0.64 to 3.67 | 0.344 | 2.9% | 27 | 938 |
| Any mesh complication | 0.5% | 2 | 430 | 7.4% | 32 | 435 | 0.5% | 2 | 367 | 0.5% | 2 | 368 | 0.8% | 9 | 1126 | ||||||
| Surgical removal | 0.5% | 2 | 430 | 5.3% | 23 | 435 | 0.5% | 2 | 367 | 0.3% | 1 | 368 | 0.7% | 7 | 1126 | ||||||
| Conservative treatment | 0.0% | 0 | 430 | 1.8% | 8 | 435 | 0.0% | 0 | 367 | 0.0% | 0 | 368 | 0.1% | 1 | 1126 | ||||||
| No treatment | 0.0% | 0 | 430 | 0.2% | 1 | 435 | 0.0% | 0 | 367 | 0.3% | 1 | 368 | 0.1% | 1 | 1126 | ||||||
| De novo | 0.2% | 1 | 430 | 6.2% | 27 | 435 | 0.0% | 0 | 367 | 0.0% | 0 | 368 | 0.3% | 3 | 1126 | ||||||
| Concomitant | 0.2% | 1 | 430 | 1.1% | 5 | 435 | 0.5% | 2 | 367 | 0.5% | 2 | 368 | 0.5% | 6 | 1126 | ||||||
| Treatment for prolapse at 1 year | |||||||||||||||||||||
| Medicines for prolapse | 12.9% | 50 | 387 | 17.6% | 67 | 381 | 1.36 | 0.97 to 1.89 | 0.075 | 15.5% | 52 | 336 | 18.9% | 62 | 328 | 1.23 | 0.88 to 1.71 | 0.235 | 15.6% | 149 | 956 |
| Oestrogens | 13.4% | 53 | 395 | 17.5% | 68 | 389 | 1.32 | 0.95 to 1.83 | 0.100 | 14.3% | 49 | 342 | 18.1% | 61 | 337 | 1.26 | 0.89 to 1.78 | 0.185 | 12.3% | 120 | 972 |
| Ring pessary | 3.3% | 13 | 395 | 2.1% | 8 | 389 | 0.61 | 0.26 to 1.46 | 0.269 | 2.3% | 8 | 342 | 2.1% | 7 | 337 | 0.88 | 0.32 to 2.41 | 0.811 | 2.1% | 20 | 972 |
| Shelf pessary | 2.5% | 10 | 395 | 0.8% | 3 | 389 | 0.30 | 0.08 to 1.07 | 0.063 | 1.5% | 5 | 342 | 1.5% | 5 | 337 | 0.94 | 0.28 to 3.22 | 0.925 | 1.3% | 13 | 972 |
| Physiotherapy | 6.3% | 24 | 380 | 8.6% | 32 | 370 | 1.36 | 0.82 to 2.27 | 0.232 | 6.7% | 22 | 330 | 8.2% | 27 | 328 | 1.22 | 0.71 to 2.10 | 0.462 | 8.5% | 81 | 948 |
| GP for prolapse | 23.6% | 91 | 385 | 26.5% | 100 | 378 | 1.12 | 0.88 to 1.43 | 0.341 | 27.5% | 92 | 334 | 29.1% | 95 | 327 | 1.06 | 0.83 to 1.35 | 0.638 | 24.3% | 230 | 946 |
| Practice nurse | 3.9% | 15 | 382 | 6.5% | 25 | 382 | 1.68 | 0.90 to 3.12 | 0.104 | 4.0% | 13 | 329 | 5.8% | 19 | 326 | 1.45 | 0.73 to 2.88 | 0.293 | 7.1% | 67 | 942 |
| GOPD | 29.2% | 112 | 383 | 37.1% | 138 | 372 | 1.25 | 1.02 to 1.53 | 0.031 | 29.0% | 97 | 334 | 32.9% | 108 | 328 | 1.12 | 0.89 to 1.41 | 0.338 | 32.1% | 304 | 946 |
| Treatment for urinary problems at 1 year | |||||||||||||||||||||
| Pads | 28.0% | 109 | 389 | 31.3% | 120 | 384 | 1.11 | 0.89 to 1.37 | 0.347 | 30.8% | 104 | 338 | 28.2% | 93 | 330 | 0.92 | 0.73 to 1.16 | 0.483 | 28.8% | 275 | 956 |
| Permanent catheter | 0.3% | 1 | 375 | 0.5% | 2 | 369 | 1.83 | 0.17 to 20.02 | 0.621 | 0.3% | 1 | 322 | 0.0% | 0 | 320 | N/A | N/A | N/A | 0.2% | 2 | 927 |
| Intermittent catheter | 1.1% | 4 | 372 | 1.4% | 5 | 367 | 1.24 | 0.34 to 4.56 | 0.749 | 0.9% | 3 | 318 | 1.9% | 6 | 321 | 2.05 | 0.52 to 7.98 | 0.302 | 1.4% | 13 | 927 |
| Drugs for UI | 7.1% | 28 | 395 | 8.5% | 33 | 389 | 1.18 | 0.73 to 1.90 | 0.503 | 6.7% | 23 | 342 | 8.9% | 30 | 337 | 1.36 | 0.81 to 2.29 | 0.240 | 8.5% | 83 | 972 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 348 | N = 343 | N = 299 | N = 300 | N = 848 | ||||||||||||||||
| Readmitted (12–24 months) | 0.9% | 3k | 348 | 0.0% | 0 | 343 | N/A | N/A | N/A | 0.7% | 2l | 299 | 1.3% | 4m | 300 | 1.95 | 0.36 to 10.56 | 0.437 | 0.6% | 5n | 847 |
| New prolapse surgery | 4.6% | 16 | 348 | 4.4% | 15 | 343 | 0.94 | 0.47 to 1.88 | 0.869 | 5.0% | 15 | 299 | 5.0% | 15 | 300 | 0.99 | 0.49 to 1.98 | 0.976 | 3.4% | 29 | 848 |
| Same compartment | 2.6% | 9 | 348 | 2.0% | 7 | 343 | 0.79 | 0.30 to 2.11 | 0.641 | 2.3% | 7 | 299 | 2.7% | 8 | 300 | 1.13 | 0.41 to 3.06 | 0.817 | 1.4% | 12 | 848 |
| Different compartment | 2.0% | 7 | 348 | 2.3% | 8 | 343 | 1.14 | 0.42 to 3.10 | 0.799 | 2.7% | 8 | 299 | 2.3% | 7 | 300 | 0.86 | 0.32 to 2.33 | 0.764 | 2.0% | 17 | 848 |
| Waiting for prolapse surgery | 0.9% | 3 | 348 | 0.3% | 1 | 343 | 0.34 | 0.04 to 3.27 | 0.351 | 0.7% | 2 | 299 | 0.0% | 0 | 300 | N/A | N/A | N/A | 0.1% | 1 | 848 |
| Continence surgery | 1.1% | 4 | 348 | 1.5% | 5 | 343 | 1.28 | 0.35 to 4.73 | 0.714 | 2.3% | 7 | 299 | 1.3% | 4 | 300 | 0.56 | 0.17 to 1.90 | 0.353 | 0.8% | 7 | 830 |
| Waiting for continence surgery | 0.0% | 0 | 348 | 0.0% | 0 | 343 | N/A | N/A | N/A | 0.0% | 0 | 299 | 0.0% | 0 | 300 | N/A | N/A | N/A | 0.2% | 2 | 848 |
| Stitches removed | 0.0% | 0 | 339 | 0.9% | 3 | 331 | N/A | N/A | N/A | 0.0% | 0 | 293 | 0.3% | 1 | 291 | N/A | N/A | N/A | 0.2% | 2 | 848 |
| Any mesh complication | 0.2% | 1 | 430 | 5.7% | 25 | 435 | 0.3% | 1 | 367 | 0.3% | 1 | 368 | 0.2% | 2 | 1126 | ||||||
| Surgical removal | 0.0% | 0 | 430 | 3.9% | 17 | 435 | 0.0% | 0 | 367 | 0.0% | 0 | 368 | 0.2% | 2 | 1126 | ||||||
| Conservative | 0.2% | 1 | 430 | 0.9% | 4 | 435 | 0.3% | 1 | 367 | 0.0% | 0 | 368 | 0.0% | 0 | 1126 | ||||||
| No treatment | 0.0% | 0 | 430 | 0.9% | 4 | 435 | 0.0% | 0 | 367 | 0.3% | 1 | 368 | 0.0% | 0 | 1126 | ||||||
| De novo | 0.0% | 0 | 430 | 5.3% | 23 | 435 | 0.0% | 0 | 367 | 0.0% | 0 | 368 | 0.1% | 1 | 1126 | ||||||
| Concomitant | 0.2% | 1 | 430 | 0.5% | 2 | 435 | 0.0% | 0 | 367 | 0.3% | 1 | 368 | 0.1% | 1 | 1126 | ||||||
| Treatment for prolapse at 2 years | |||||||||||||||||||||
| Medicines for prolapse | 11.1% | 38 | 341 | 11.0% | 37 | 337 | 0.95 | 0.62 to 1.44 | 0.797 | 14.6% | 43 | 295 | 12.5% | 37 | 296 | 0.85 | 0.56 to 1.27 | 0.419 | 10.6% | 88 | 834 |
| Oestrogens | 11.8% | 41 | 348 | 14.9% | 51 | 343 | 1.25 | 0.85 to 1.83 | 0.249 | 15.1% | 45 | 299 | 16.3% | 49 | 300 | 1.08 | 0.75 to 1.57 | 0.672 | 11.4% | 97 | 848 |
| Ring pessary | 3.2% | 11 | 348 | 1.7% | 6 | 343 | 0.55 | 0.21 to 1.47 | 0.235 | 2.0% | 6 | 299 | 3.3% | 10 | 300 | 1.67 | 0.62 to 4.52 | 0.311 | 2.2% | 19 | 848 |
| Shelf pessary | 1.7% | 6 | 348 | 0.9% | 3 | 343 | 0.44 | 0.11 to 1.75 | 0.242 | 2.3% | 7 | 299 | 1.7% | 5 | 300 | 0.66 | 0.21 to 2.03 | 0.468 | 1.4% | 12 | 848 |
| Physiotherapy | 5.6% | 19 | 342 | 7.5% | 25 | 333 | 1.34 | 0.75 to 2.38 | 0.324 | 5.2% | 15 | 290 | 6.8% | 20 | 293 | 1.27 | 0.67 to 2.43 | 0.461 | 6.0% | 50 | 832 |
| GP for prolapse | 13.6% | 46 | 337 | 15.5% | 52 | 335 | 1.15 | 0.80 to 1.65 | 0.459 | 12.2% | 36 | 294 | 13.2% | 38 | 288 | 1.06 | 0.70 to 1.62 | 0.775 | 14.2% | 116 | 819 |
| Practice nurse | 3.3% | 11 | 336 | 2.7% | 9 | 330 | 0.82 | 0.34 to 1.95 | 0.648 | 2.7% | 8 | 292 | 2.1% | 6 | 289 | 0.75 | 0.27 to 2.14 | 0.595 | 3.2% | 26 | 817 |
| GOPD | 15.2% | 52 | 342 | 17.4% | 58 | 333 | 1.14 | 0.81 to 1.60 | 0.461 | 15.5% | 46 | 296 | 17.7% | 52 | 293 | 1.13 | 0.79 to 1.61 | 0.519 | 13.1% | 108 | 827 |
| Treatment for urinary problems at 2 years | |||||||||||||||||||||
| Pads | 26.9% | 92 | 342 | 28.6% | 97 | 339 | 1.07 | 0.84 to 1.36 | 0.588 | 27.3% | 81 | 297 | 25.5% | 76 | 298 | 0.94 | 0.72 to 1.23 | 0.659 | 28.1% | 233 | 830 |
| Permanent catheter | 0.0% | 0 | 341 | 0.0% | 0 | 331 | N/A | N/A | N/A | 0.0% | 0 | 295 | 0.0% | 0 | 291 | N/A | N/A | N/A | 0.2% | 2 | 823 |
| Intermittent catheter | 1.2% | 4 | 339 | 1.2% | 4 | 332 | 1.00 | 0.25 to 3.93 | 0.996 | 1.4% | 4 | 293 | 1.4% | 4 | 291 | 0.97 | 0.25 to 3.82 | 0.962 | 1.6% | 13 | 826 |
| Drugs for UI | 6.6% | 23 | 348 | 9.3% | 32 | 343 | 1.39 | 0.83 to 2.31 | 0.212 | 8.7% | 26 | 299 | 8.3% | 25 | 300 | 0.94 | 0.56 to 1.59 | 0.818 | 7.4% | 63 | 848 |
Readmission (not related to mesh exposure or further surgery for prolapse or incontinence)
The overall rate of readmission was low, and there was no significant difference between the randomised groups (see Table 31). The rate in the first 6 months, ranging from 2.7% to 4.2% (see Table 31), was mostly related to adverse effects, whereas after that time the rate reduced (1.0–1.8%) and readmissions were more likely to be related to treatment failure than adverse effects. There were no statistically significant differences between the randomised groups in either trial (see Table 31).
Treatment for repeat or further prolapse
Thirty women (from all the randomised groups) reported that they had had further prolapse surgery in the first year and another 50 women had more prolapse surgery in the second year: a total of 74 women. Six women had surgery in both years. Of the 1073 randomised women who completed questionnaires at both 1 year and 2 years, 66 had further prolapse surgery, making a total further surgery rate of 6.2% (see Table 31). Similarly, 50 of 837 (6.0%) of the cohort women had undergone further prolapse surgery. Overall, there was no statistically significant difference between the randomised groups in either trial in the number of women who were having further prolapse surgery at 1 year or 2 years (see Table 31); for example:
-
In trial 1, comparing the number of women who had further prolapse surgery in the first year in the standard repair group (1.5%) with synthetic mesh inlay (3.1%), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1B (two-way randomisation), RR 1.99, 95% CI 0.76 to 5.24.
-
In trial 2, comparing the number of women who had further prolapse surgery in the first year in the standard repair group (2.0%) with biological graft (3.0%), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1C (two-way randomisation), RR 1.44, 95% CI 0.56 to 3.73.
In the second year, more women received another prolapse repair:
-
In trial 1, comparing the number of women who had further prolapse surgery in the second year in the standard repair group (4.6%) with synthetic mesh inlay (4.4%), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1B (two-way randomisation), RR 0.94, 95% CI 0.47 to 1.88.
-
In trial 2, comparing the number of women who had further prolapse surgery in the second year in the standard repair group (5.0%) with biological graft (5.0%), adjusted for baseline values and based on data only from women in stratum 1A (three-way randomisation) and stratum 1C (two-way randomisation), RR 0.99, 95% CI 0.49 to 1.98.
Few women required other treatment, such as pessaries or physiotherapy for persistent or recurrent prolapse symptoms, and there were no differences between the randomised groups in either trial regarding further use of services (also see Chapter 5). The data from the cohort women were similar.
Treatment for urinary incontinence and other bladder problems
Fourteen women had continence surgery in the first year, and a further 16 in the second year (one had continence surgery in both years): a total of 29 women. Of the 1073 randomised women who completed questionnaires at both years, 26 had continence surgery, thus a rate of 2.4%. Similarly, 15 of 837 (1.8%) of the cohort women had had repeat continence surgery. However, around 30% of women were using absorbent pads for urine leakage, and just under 10% were using drugs for urine problems, with similar proportions among the cohort women. Twelve women were using intermittent catheterisation for obstructed or incomplete voiding by 2 years, and 13 in the cohort. There were no statistically significant differences between the women in either trial, and the data from the cohort women were similar.
Treatment for mesh complications
In the synthetic mesh trial, there were 34 instances of serious adverse effects associated with mesh complications in the first year for randomised women, but only 25 women required surgery to remove part of the mesh, of whom two were in the standard group: 18 (72%) were asymptomatic and 16 (64%) had exposures of < 1 cm2 (see Table 31). One of these women had total mesh removal within 2 weeks of surgery because of severe infection causing rejection. A further eight women (see Table 31) had conservative treatment only (such as local oestrogen, cautery with silver nitrate, or antibiotics) in the first year and one needed no treatment. In the second year, 26 women had a mesh complication (see Table 31), of whom 17 had surgical removal: 13 (76%) were asymptomatic and 10 (59%) had exposures of < 1 cm2. Five received conservative treatment and another four required no treatment (see Table 31).
In the biological graft trial, four women had a mesh complication in the first year but all had concomitant synthetic mesh and only three required surgical intervention (none were symptomatic or had exposures of > 1 cm2). Two women had a mesh complication in the second year but neither required surgical treatment.
The cumulative mesh complication rates over 2 years were 2 of 430 (0.5%) for standard repair (trial 1), 46 of 435 (10.6%) for mesh inlay and 2 of 368 (0.5%) for biological graft.
Satisfaction with treatment at 1 year and 2 years
Women reported that they took around 3 months to recover, with no differences between any of the randomised groups (Table 32). Over 80% of the women were very much or much better than before surgery, and similar proportions were completely or fairly satisfied. Over 90% of women would ‘recommend the surgery to a friend’. The data were similar at 1 year and 2 years, suggesting that, on average, the positive benefits of surgery were sustained, with no statistically significant differences between the randomised groups, and the findings were similar among the cohort women (see Table 32). These data are in line with the clinical outcome data, supporting the positive benefits of prolapse surgery for the majority of women.
| Recovery/satisfaction | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | CC1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | ||||||||||||
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 368 | N = 362 | N = 319 | N = 316 | N = 893 | ||||||||||||||||
| Time to recovery (months) | 3.0 | (1.8) | 342 | 3.0 | (1.7) | 342 | 0.00 | –0.27 to 0.26 | 0.990 | 3.1 | (1.8) | 296 | 3.3 | (1.9) | 293 | 0.28 | –0.03 to 0.58 | 0.073 | 3.1 | (2.1) | 828 |
| Prolapse compared with before surgery at 1 year | |||||||||||||||||||||
| Very much better | 57.7% | 203 | 352 | 56.2% | 199 | 354 | 0.93 | 0.69 to 1.26 | 0.655 | 57.6% | 174 | 302 | 52.8% | 160 | 303 | 0.81 | 0.58 to 1.12 | 0.198 | 51.0% | 439 | 860 |
| Much better | 24.1% | 85 | 352 | 26.8% | 95 | 354 | 24.5% | 74 | 302 | 25.7% | 78 | 303 | 28.6% | 246 | 860 | ||||||
| A little better | 9.7% | 34 | 352 | 9.3% | 33 | 354 | 8.9% | 27 | 302 | 8.6% | 26 | 303 | 9.9% | 85 | 860 | ||||||
| No change | 2.8% | 10 | 352 | 3.4% | 12 | 354 | 3.6% | 11 | 302 | 7.3% | 22 | 303 | 5.6% | 48 | 860 | ||||||
| A little worse | 3.1% | 11 | 352 | 1.4% | 5 | 354 | 3.3% | 10 | 302 | 2.6% | 8 | 303 | 1.4% | 12 | 860 | ||||||
| Much worse | 2.0% | 7 | 352 | 2.5% | 9 | 354 | 1.3% | 4 | 302 | 2.3% | 7 | 303 | 2.1% | 18 | 860 | ||||||
| Very much worse | 0.6% | 2 | 352 | 0.3% | 1 | 354 | 0.7% | 2 | 302 | 0.7% | 2 | 303 | 1.4% | 12 | 860 | ||||||
| Satisfaction with surgery at 1 year | |||||||||||||||||||||
| Completely satisfied | 58.0% | 203 | 350 | 58.9% | 208 | 353 | 1.03 | 0.76 to 1.40 | 0.852 | 58.4% | 177 | 303 | 56.5% | 170 | 301 | 0.90 | 0.65 to 1.26 | 0.548 | 50.5% | 434 | 860 |
| Fairly satisfied | 30.0% | 105 | 350 | 26.1% | 92 | 353 | 28.7% | 87 | 303 | 27.2% | 82 | 301 | 34.9% | 300 | 860 | ||||||
| Fairly dissatisfied | 3.1% | 11 | 350 | 7.1% | 25 | 353 | 4.3% | 13 | 303 | 5.3% | 16 | 301 | 4.3% | 37 | 860 | ||||||
| Very dissatisfied | 5.4% | 19 | 350 | 4.8% | 17 | 353 | 5.0% | 15 | 303 | 6.6% | 20 | 301 | 6.2% | 53 | 860 | ||||||
| Not sure | 3.4% | 12 | 350 | 3.1% | 11 | 353 | 3.6% | 11 | 303 | 4.3% | 13 | 301 | 4.2% | 36 | 860 | ||||||
| Recommend to a friend | 90.2% | 312 | 346 | 90.9% | 310 | 341 | 1.01 | 0.96 to 1.06 | 0.604 | 90.2% | 267 | 296 | 90.3% | 262 | 290 | 1.01 | 0.95 to 1.07 | 0.751 | 90.3% | 758 | 839 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 348 | N = 343 | N = 299 | N = 300 | N = 848 | ||||||||||||||||
| Prolapse compared with before surgery at 2 years | |||||||||||||||||||||
| Very much better | 56.6% | 194 | 343 | 53.3% | 180 | 338 | 0.88 | 0.65 to 1.20 | 0.425 | 57.5% | 169 | 294 | 49.3% | 146 | 296 | 0.73 | 0.52 to 1.01 | 0.058 | 50.3% | 419 | 833 |
| Much better | 23.3% | 80 | 343 | 26.0% | 88 | 338 | 23.1% | 68 | 294 | 27.7% | 82 | 296 | 26.2% | 218 | 833 | ||||||
| A little better | 10.8% | 37 | 343 | 13.9% | 47 | 338 | 10.2% | 30 | 294 | 9.1% | 27 | 296 | 10.9% | 91 | 833 | ||||||
| No change | 5.2% | 18 | 343 | 3.3% | 11 | 338 | 5.1% | 15 | 294 | 5.7% | 17 | 296 | 6.5% | 54 | 833 | ||||||
| A little worse | 1.7% | 6 | 343 | 1.8% | 6 | 338 | 2.7% | 8 | 294 | 5.4% | 16 | 296 | 2.5% | 21 | 833 | ||||||
| Much worse | 0.9% | 3 | 343 | 1.2% | 4 | 338 | 0.3% | 1 | 294 | 1.0% | 3 | 296 | 2.3% | 19 | 833 | ||||||
| Very much worse | 1.5% | 5 | 343 | 0.6% | 2 | 338 | 1.0% | 3 | 294 | 1.7% | 5 | 296 | 1.3% | 11 | 833 | ||||||
| Satisfaction with surgery at 2 years | |||||||||||||||||||||
| Completely satisfied | 56.3% | 193 | 343 | 54.0% | 183 | 339 | 0.91 | 0.67 to 1.25 | 0.570 | 58.5% | 172 | 294 | 54.2% | 161 | 297 | 0.86 | 0.61 to 1.20 | 0.365 | 52.2% | 434 | 832 |
| Fairly satisfied | 28.9% | 99 | 343 | 34.2% | 116 | 339 | 28.9% | 85 | 294 | 28.3% | 84 | 297 | 30.8% | 256 | 832 | ||||||
| Fairly dissatisfied | 5.5% | 19 | 343 | 4.1% | 14 | 339 | 4.8% | 14 | 294 | 6.4% | 19 | 297 | 6.1% | 51 | 832 | ||||||
| Very dissatisfied | 5.5% | 19 | 343 | 2.7% | 9 | 339 | 4.4% | 13 | 294 | 6.7% | 20 | 297 | 6.0% | 50 | 832 | ||||||
| Not sure | 3.8% | 13 | 343 | 5.0% | 17 | 339 | 3.4% | 10 | 294 | 4.4% | 13 | 297 | 4.9% | 41 | 832 | ||||||
| Recommend to a friend | 90.5% | 304 | 336 | 89.4% | 295 | 330 | 0.98 | 0.93 to 1.03 | 0.413 | 91.3% | 262 | 287 | 87.0% | 247 | 284 | 0.95 | 0.90 to 1.01 | 0.104 | 87.3% | 705 | 808 |
Analysis by treatment received
Post hoc analysis of the primary outcome measure, the POP-Q data and a selection of key secondary outcomes for the primary repair trials (trials 1 and 2) was undertaken following discussion at the collaborators’ meetings on 17 October 2014 and 4 September 2015. This analysis compared randomised groups but used the actual type of prolapse repair that the women had received rather than the planned procedure to define the subgroups. Results are presented for all women who received prolapse surgery (Table 33), the subgroup of women who received an anterior repair only (Table 34), a posterior repair only (Table 35) and those who received both an anterior and posterior repair concomitantly (Table 36).
| All women who received prolapse surgery | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | |||||||||
| POP-SS at 12 months | 5.4 | (5.5) | 395 | 5.5 | (5.1) | 389 | 0.00 | –0.70 to 0.71 | 0.989 | 5.5 | (5.6) | 342 | 5.6 | (5.6) | 337 | –0.15 | –0.93 to 0.63 | 0.706 |
| Leading edge (2b or more) | 13.9% | 47 | 339 | 16.0% | 54 | 337 | 1.12 | 0.79 to 1.60 | 0.522 | 15.5% | 47 | 304 | 18.0% | 54 | 300 | 1.13 | 0.79 to 1.61 | 0.502 |
| Anterior edge (2b or more) | 11.2% | 38 | 339 | 12.5% | 42 | 337 | 1.19 | 0.78 to 1.81 | 0.424 | 12.5% | 38 | 304 | 15.7% | 47 | 300 | 1.28 | 0.87 to 1.89 | 0.213 |
| Posterior edge (2b or more) | 3.0% | 10 | 338 | 4.1% | 14 | 338 | 1.40 | 0.63 to 3.08 | 0.409 | 3.3% | 10 | 304 | 3.4% | 10 | 297 | 1.00 | 0.43 to 2.35 | 0.995 |
| Ba (anterior edge) | –1.3 | (1.6) | 324 | –1.3 | (1.6) | 328 | 0.05 | –0.18 to 0.28 | 0.653 | –1.3 | (1.7) | 300 | –1.2 | (1.7) | 295 | 0.12 | –0.1 to 0.4 | 0.358 |
| C (cervix/vault) | –6.0 | (2.1) | 319 | –6.0 | (2.3) | 321 | –0.01 | –0.35 to 0.32 | 0.939 | –5.8 | (1.9) | 293 | –5.7 | (2.1) | 294 | 0.15 | –0.2 to 0.5 | 0.383 |
| Bp (posterior edge) | –2.0 | (1.2) | 323 | –2.1 | (1.1) | 327 | –0.03 | –0.21 to 0.15 | 0.720 | –2.1 | (1.2) | 300 | –2.0 | (1.2) | 292 | 0.12 | –0.1 to 0.3 | 0.230 |
| Repeat prolapse surgery (any) within 12 months | 1.5% | 6 | 395 | 3.1% | 12 | 389 | 1.96 | 0.74 to 5.16 | 0.174 | 2.0% | 7 | 342 | 3.0% | 10 | 337 | 1.40 | 0.54 to 3.63 | 0.488 |
| Repeat same compartment | 0.8% | 3 | 395 | 2.1% | 8 | 389 | 2.50 | 0.67 to 9.35 | 0.172 | 1.5% | 5 | 342 | 1.5% | 5 | 337 | 0.95 | 0.28 to 3.23 | 0.933 |
| Repeat different compartment | 0.8% | 3 | 395 | 1.0% | 4 | 389 | 1.35 | 0.30 to 5.96 | 0.694 | 0.6% | 2 | 342 | 1.5% | 5 | 337 | 2.45 | 0.48 to 12.47 | 0.281 |
| New continence surgery | 1.3% | 5 | 395 | 0.5% | 2 | 389 | 0.40 | 0.08 to 2.02 | 0.265 | 0.6% | 2 | 342 | 2.1% | 7 | 337 | 3.34 | 0.70 to 15.94 | 0.130 |
| Any SAE within 12 months | 7.2% | 31 | 430 | 7.8% | 34 | 435 | 1.08 | 0.68 to 1.72 | 0.730 | 6.3% | 23 | 367 | 9.8% | 36 | 368 | 1.57 | 0.95 to 2.59 | 0.076 |
| Serious mesh exposure within 12 months | 0.5% | 2 | 430 | 7.4% | 32 | 435 | 0.5% | 2 | 367 | 0.5% | 2 | 368 | ||||||
| Women who received an anterior repair | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | |||||||||
| POP-SS at 12 months | 4.7 | (4.8) | 172 | 5.3 | (5.5) | 173 | 0.37 | –0.68 to 1.42 | 0.485 | 5.0 | (5.1) | 127 | 5.4 | (5.9) | 122 | 0.23 | –1.11 to 1.57 | 0.740 |
| Leading edge (2b or more) | 15.8% | 23 | 146 | 16.7% | 25 | 150 | 1.01 | 0.61 to 1.66 | 0.982 | 19.7% | 23 | 117 | 26.9% | 29 | 108 | 1.11 | 0.76 to 1.62 | 0.587 |
| Anterior edge (2b or more) | 12.3% | 18 | 146 | 13.3% | 20 | 150 | 0.98 | 0.53 to 1.80 | 0.944 | 15.4% | 18 | 117 | 25.9% | 28 | 108 | 1.16 | 0.90 to 1.49 | 0.256 |
| Posterior edge (2b or more) | 3.4% | 5 | 145 | 4.0% | 6 | 150 | 1.12 | 0.35 to 3.59 | 0.846 | 4.3% | 5 | 117 | 2.8% | 3 | 107 | 0.75 | 0.18 to 3.11 | 0.695 |
| Ba (anterior edge) | –1.2 | (1.7) | 139 | –1.3 | (1.6) | 147 | –0.15 | –0.49 to 0.19 | 0.381 | –1.0 | (1.7) | 115 | –0.6 | (1.8) | 108 | 0.23 | –0.2 to 0.7 | 0.271 |
| C (cervix/vault) | –6.1 | (2.1) | 136 | –6.0 | (2.4) | 143 | 0.00 | –0.51 to 0.51 | 0.997 | –6.1 | (1.7) | 113 | –5.2 | (2.4) | 108 | 0.56 | 0 to 1.1 | 0.046 |
| Bp (posterior edge) | –2.0 | (1.1) | 139 | –2.0 | (1.1) | 146 | –0.02 | –0.27 to 0.23 | 0.877 | –2.1 | (1.0) | 115 | –2.0 | (1.1) | 107 | 0.17 | –0.1 to 0.4 | 0.222 |
| Repeat prolapse surgery (any) within 12 months | 1.7% | 3 | 172 | 3.5% | 6 | 173 | 1.91 | 0.48 to 7.52 | 0.355 | 1.6% | 2 | 127 | 2.5% | 3 | 122 | 1.53 | 0.26 to 8.97 | 0.640 |
| Repeat same compartment | 1.2% | 2 | 172 | 2.9% | 5 | 173 | 2.37 | 0.47 to 12.06 | 0.299 | 1.6% | 2 | 127 | 2.5% | 3 | 122 | 1.53 | 0.26 to 8.97 | 0.640 |
| Repeat different compartment | 0.6% | 1 | 172 | 0.6% | 1 | 173 | 0.99 | 0.06 to 15.82 | 0.997 | 0.0% | 0 | 127 | 0.0% | 0 | 122 | N/A | N/A | N/A |
| New continence surgery | 1.2% | 2 | 172 | 0.6% | 1 | 173 | 0.40 | 0.08 to 2.02 | 0.265 | 0.8% | 1 | 127 | 1.6% | 2 | 122 | 2.08 | 0.19 to 22.43 | 0.546 |
| Any SAE within 12 months | 8.2% | 15 | 184 | 4.3% | 8 | 187 | 0.52 | 0.23 to 1.19 | 0.123 | 6.8% | 9 | 132 | 10.6% | 14 | 132 | 1.55 | 0.70 to 3.46 | 0.281 |
| Serious mesh exposure within 12 months | 1.1% | 2 | 184 | 7.5% | 14 | 187 | 6.75 | 1.55 to 29.41 | 0.011 | 0.8% | 1 | 132 | 0.0% | 0 | 132 | N/A | N/A | N/A |
| Women who received a posterior repair | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | |||||||||
| POP-SS at 12 months | 6.4 | (6.3) | 116 | 5.7 | (4.9) | 116 | –0.56 | –1.90 to 0.79 | 0.415 | 6.5 | (6.3) | 97 | 6.0 | (5.9) | 112 | –1.06 | –2.59 to 0.47 | 0.172 |
| Leading edge (2b or more) | 13.1% | 13 | 99 | 18.0% | 18 | 100 | 1.69 | 0.83 to 3.47 | 0.149 | 13.8% | 12 | 87 | 8.1% | 8 | 99 | 0.62 | 0.26 to 1.49 | 0.282 |
| Anterior edge (2b or more) | 10.1% | 10 | 99 | 12.0% | 12 | 100 | 1.57 | 0.69 to 3.58 | 0.283 | 10.3% | 9 | 87 | 3.0% | 3 | 99 | 0.40 | 0.11 to 1.44 | 0.160 |
| Posterior edge (2b or more) | 4.0% | 4 | 99 | 5.9% | 6 | 101 | 2.36 | 0.57 to 9.82 | 0.238 | 4.6% | 4 | 87 | 5.1% | 5 | 99 | 1.05 | 0.30 to 3.74 | 0.935 |
| Ba (anterior edge) | –1.6 | (1.5) | 95 | –1.5 | (1.6) | 96 | 0.17 | –0.32 to 0.66 | 0.490 | –1.8 | (1.6) | 86 | –1.8 | (1.3) | 97 | 0.05 | –0.4 to 0.5 | 0.807 |
| C (cervix/vault) | –6.2 | (2.1) | 95 | –6.2 | (2.2) | 95 | –0.01 | –0.65 to 0.62 | 0.967 | –6.0 | (2.0) | 86 | –6.1 | (1.5) | 97 | –0.15 | –0.7 to 0.4 | 0.612 |
| Bp (posterior edge) | –1.9 | (1.4) | 95 | –2.1 | (1.3) | 96 | –0.13 | –0.56 to 0.30 | 0.544 | –2.0 | (1.5) | 86 | –1.9 | (1.3) | 96 | 0.08 | –0.3 to 0.5 | 0.698 |
| Repeat prolapse surgery (any) within 12 months | 1.7% | 2 | 116 | 1.7% | 2 | 116 | 0.96 | 0.14 to 6.76 | 0.965 | 3.1% | 3 | 97 | 3.6% | 4 | 112 | 1.10 | 0.25 to 4.83 | 0.898 |
| Repeat same compartment | 0.0% | 0 | 116 | 0.0% | 0 | 116 | N/A | N/A | N/A | 1.0% | 1 | 97 | 0.0% | 0 | 112 | N/A | N/A | N/A |
| Repeat different compartment | 1.7% | 2 | 116 | 1.7% | 2 | 116 | 0.96 | 0.14 to 6.76 | 0.965 | 2.1% | 2 | 97 | 3.6% | 4 | 112 | 1.62 | 0.30 to 8.70 | 0.574 |
| New continence surgery | 0.0% | 0 | 116 | 0.0% | 0 | 116 | N/A | N/A | N/A | 0.0% | 0 | 97 | 1.8% | 2 | 112 | N/A | N/A | N/A |
| Any SAE within 12 months | 4.0% | 5 | 125 | 10.2% | 13 | 127 | 2.98 | 1.09 to 8.11 | 0.123 | 2.9% | 3 | 103 | 6.8% | 8 | 117 | 2.28 | 0.62 to 8.36 | 0.214 |
| Serious mesh exposure within 12 months | 0.0% | 0 | 125 | 7.1% | 9 | 127 | 1.0% | 1 | 103 | 0.9% | 1 | 117 | ||||||
| Women who received an anterior and a posterior repair | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Biological | Effect size | 95% CI | p-value | |||||||||
| POP-SS at 12 months | 5.6 | (5.9) | 90 | 5.4 | (4.9) | 86 | –0.22 | –1.79 to 1.34 | 0.777 | 5.1 | (5.3) | 105 | 5.3 | (4.9) | 98 | –0.07 | –1.33 to 1.18 | 0.908 |
| Leading edge (2b or more) | 10.3% | 8 | 78 | 11.7% | 9 | 77 | 1.53 | 0.60 to 3.88 | 0.370 | 10.3% | 9 | 87 | 19.5% | 17 | 87 | 1.79 | 0.82 to 3.91 | 0.143 |
| Anterior edge (2b or more) | 9.0% | 7 | 78 | 10.4% | 8 | 77 | 2.03 | 0.76 to 5.39 | 0.156 | 9.2% | 8 | 87 | 18.4% | 16 | 87 | 2.17 | 0.95 to 4.91 | 0.064 |
| Posterior edge (2b or more) | 1.3% | 1 | 78 | 2.6% | 2 | 77 | 1.49 | 0.14 to 15.77 | 0.743 | 1.1% | 1 | 87 | 2.4% | 2 | 85 | 1.49 | 0.15 to 15.17 | 0.737 |
| Ba (anterior edge) | –1.3 | (1.6) | 74 | –1.1 | (1.7) | 75 | 0.23 | –0.23 to 0.68 | 0.328 | –1.3 | (1.5) | 86 | –1.1 | (1.8) | 84 | 0.12 | –0.4 to 0.6 | 0.639 |
| C (cervix/vault) | –5.6 | (1.8) | 72 | –5.6 | (2.5) | 73 | –0.03 | –0.85 to 0.80 | 0.951 | –5.5 | (1.6) | 81 | –5.7 | (2.1) | 83 | –0.17 | –0.8 to 0.5 | 0.611 |
| Bp (posterior edge) | –2.2 | (1.1) | 73 | –2.1 | (1.2) | 75 | 0.02 | –0.37 to 0.40 | 0.935 | –2.3 | (1.0) | 86 | –2.2 | (1.4) | 83 | 0.01 | –0.4 to 0.4 | 0.961 |
| Repeat prolapse surgery (any) within 12 months | 1.1% | 1 | 90 | 3.5% | 3 | 86 | 2.48 | 0.27 to 23.10 | 0.424 | 1.9% | 2 | 105 | 2.0% | 2 | 98 | 1.06 | 0.15 to 7.43 | 0.954 |
| Repeat same compartment | 1.1% | 1 | 90 | 3.5% | 3 | 86 | 2.48 | 0.27 to 23.10 | 0.424 | 1.9% | 2 | 105 | 2.0% | 2 | 98 | 1.06 | 0.15 to 7.43 | 0.954 |
| Repeat different compartment | 0.0% | 0 | 90 | 0.0% | 0 | 86 | N/A | N/A | N/A | 0.0% | 0 | 105 | 0.0% | 0 | 98 | N/A | N/A | N/A |
| New continence surgery | 1.1% | 1 | 90 | 1.2% | 1 | 86 | 0.40 | 0.08 to 2.02 | 0.265 | 1.0% | 1 | 105 | 3.1% | 3 | 98 | 2.80 | 0.30 to 26.40 | 0.369 |
| Any SAE within 12 months | 10.2% | 10 | 98 | 12.9% | 12 | 93 | 1.31 | 0.60 to 2.88 | 0.494 | 10.0% | 11 | 110 | 13.0% | 14 | 108 | 1.37 | 0.65 to 2.90 | 0.412 |
| Serious mesh exposure within 12 months | 0.0% | 0 | 98 | 9.7% | 9 | 93 | 0.0% | 0 | 110 | 0.9% | 1 | 108 | ||||||
There were no significant differences in the POP-SS at 1 year between groups (standard repair vs. synthetic mesh or standard repair vs. biological graft) for any of the subgroups or for the combined group. For women who had an anterior repair only, the rate beyond the hymen for the anterior edge (POP-Q stage 2b or more) was 12.3% for standard compared with 13.3% for synthetic mesh and 15.4% for standard compared with 25.9% for biological graft. However, these differences were not significant (see Table 34). Similarly, for women who had a posterior repair only, the rate beyond the hymen for the posterior edge was 4.0% for standard compared with 5.9% for synthetic mesh, and 4.6% for standard compared with 5.1% for biological graft, and these differences were not significant (see Table 35). There were no significant differences between groups in any of the other outcome measures, except serious mesh complications.
Subgroup analysis
The results of the subgroup analyses are summarised in Table 37. There are no significant subgroup interaction effects from any of the planned subgroup analyses.
| Subgroup | Trial 1 | Trial 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Interaction test: p-value | Standard repair | Biological graft | Interaction test: p-value | |||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | |||
| Age group (years) | ||||||||||||||
| < 60 | 6.0 | 5.8 | 188 | 6.4 | 5.7 | 185 | 0.397 | 5.9 | 5.7 | 161 | 6.1 | 5.9 | 159 | 0.865 |
| ≥ 60 | 4.9 | 5.2 | 207 | 4.6 | 4.4 | 204 | 5.2 | 5.5 | 181 | 5.2 | 5.3 | 178 | ||
| Type of planned prolapse repair | ||||||||||||||
| Anterior only | 4.7 | 4.9 | 188 | 5.4 | 5.5 | 191 | 0.392 | 5.0 | 5.2 | 138 | 5.2 | 5.4 | 135 | 0.658 |
| Posterior only | 6.4 | 6.4 | 103 | 5.6 | 5.0 | 102 | 6.8 | 6.5 | 85 | 5.7 | 6.1 | 93 | ||
| Both | 5.6 | 5.6 | 104 | 5.5 | 4.7 | 96 | 5.2 | 5.2 | 119 | 6.1 | 5.3 | 109 | ||
| Planned concomitant continence procedure | ||||||||||||||
| Yes | 5.7 | 4.7 | 38 | 5.9 | 5.3 | 41 | 0.658 | 5.8 | 4.7 | 40 | 6.2 | 6.5 | 39 | 0.106 |
| No | 5.4 | 5.6 | 357 | 5.4 | 5.1 | 348 | 5.5 | 5.7 | 302 | 5.6 | 5.5 | 298 | ||
| Planned concomitant upper prolapse procedure | ||||||||||||||
| Yes | 4.6 | 4.8 | 186 | 5.0 | 5.1 | 0.323 | 4.8 | 5.1 | 175 | 5.3 | 5.4 | 162 | 0.224 | |
| No | 6.1 | 6.0 | 209 | 5.9 | 5.1 | 213 | 6.2 | 6.0 | 167 | 5.9 | 5.7 | 175 | ||
| Parity | ||||||||||||||
| Zero to two deliveries | 5.2 | 5.3 | 223 | 5.0 | 4.7 | 201 | 0.512 | 5.3 | 5.3 | 184 | 5.4 | 5.5 | 184 | 0.856 |
| Three or more deliveries | 5.6 | 5.9 | 171 | 5.9 | 5.5 | 187 | 5.8 | 5.9 | 158 | 5.9 | 5.7 | 153 | ||
Sensitivity analysis
Several sensitivity analyses were carried out on the primary outcome (POP-SS at 1 year) to examine the impact of missing data under varying assumptions and test the assumption of treating unanswered individual Pelvic Organ Prolapse Symptom scale items as asymptomatic (Table 38). In the main analysis comparing standard repair with synthetic mesh, the point estimate for the effect size was 0.00, and this estimate varied between –0.17 and 0.29 in the sensitivity analyses. In the comparison between standard repair and biological graft, the point estimate in the main analysis was –0.15, which varied between –0.19 and 0.18 in the sensitivity analyses (see Table 38). None of the sensitivity analyses showed any significant difference between groups and, therefore, the results of the sensitivity analyses are consistent with the main analysis.
| Analysis | Trial 1: standard repair vs. synthetic mesh | Trial 2: standard repair vs. biological graft | ||||
|---|---|---|---|---|---|---|
| Effect size | 95% CI | p-value | Effect size | 95% CI | p-value | |
| Main analysis | 0.00 | –0.70 to 0.71 | 0.989 | –0.15 | –0.93 to 0.63 | 0.706 |
| Assuming missing at random | 0.08 | –0.66 to 0.82 | 0.839 | 0.01 | –0.77 to 0.79 | 0.985 |
| Missing POP-SSs assumed to be 2 points higher | 0.01 | –0.76 to 0.77 | 0.985 | 0.03 | –0.79 to 0.85 | 0.950 |
| Missing POP-SSs assumed to be 2 points lower | –0.09 | –0.85 to 0.67 | 0.818 | –0.04 | –0.86 to 0.78 | 0.922 |
| Missing POP-SSs assumed to be 2 points higher in the standard repair arm only | –0.09 | –0.83 to 0.66 | 0.821 | –0.13 | –0.91 to 0.65 | 0.745 |
| Missing POP-SSs assumed to be 2 points lower in the standard repair arm only | 0.24 | –0.50 to 0.98 | 0.528 | 0.14 | –0.64 to 0.93 | 0.717 |
| Missing POP-SSs assumed to be 2 points higher in the mesh arm only | 0.29 | –0.46 to 1.03 | 0.448 | 0.18 | –0.61 to 0.96 | 0.658 |
| Missing POP-SSs assumed to be 2 points lower in the mesh arm only | –0.13 | –0.88 to 0.61 | 0.722 | –0.16 | –0.95 to 0.62 | 0.686 |
| Unanswered Pelvic Organ Prolapse Symptom scale items treated as missing | –0.17 | –0.89 to 0.56 | 0.652 | –0.19 | –1.00 to 0.62 | 0.647 |
Missing POP-SSs in the standard repair arm would need to be 11 points higher than their missing-at-random imputed values for there to be a significant benefit for synthetic mesh or missing POP-SSs would need tobe seven points higher in the synthetic mesh arm for there to be a significant benefit for standard repair. Similarly, missing POP-SSs would need to be nine points higher for standard repair for there to be a significant benefit for biological graft or missing POP-SSs would need to be eight points higher for biological graft for there to be a significant benefit for standard repair.
Discussion
Summary of findings
Effectiveness
There were no statistically significant differences at 1 year in the primary clinical outcomes after prolapse surgery using native tissue, synthetic non-absorbable mesh or biological graft material to reinforce the repair. In particular, the CI around the primary measure of women’s symptoms, the POP-SS, was smaller than the minimally important clinical difference of two,23 suggesting that it would be unlikely that there was a clinically significant difference between the groups in both trials. There were also no important differences in the secondary clinical or objective outcomes, or in the proportion of women requiring further treatment in either of the trials.
Adverse effects
The overall incidence of serious adverse effects was low, and there was no excess in the mesh or graft groups, other than mesh related, compared with the standard repair groups in either trial. Although women could have a mesh-related complication only if they received mesh, in about one-third of cases this was treated conservatively.
In the synthetic mesh trial, some women had mesh complications but most were small mesh exposures measuring < 1 cm2 and many were asymptomatic or did not require treatment. Although there was no evidence of difference in other adverse effects up to 2 years after surgery, synthetic mesh use did result in additional surgical procedures for removal of a small part of the mesh, which may be considered to be an unnecessary risk.
Only six women in the biological graft trial had mesh exposure, all in women who had concomitant procedures with synthetic mesh; three of them required surgical correction.
Cost-effectiveness
See Chapter 5.
Strengths and weaknesses
Strengths
The PROSPECT trial is the largest trial of the use of mesh or graft in prolapse surgery to date. It was powered to detect a clinically meaningful difference in the primary outcome, prolapse symptoms, in women who were having a first anterior or posterior prolapse operation. Owing to experience or availability of resources locally, some surgeons were not able to randomise between all three options, hence the comparison between standard and biological graft did not quite reach the expected sample size of 400. However, because of the high response rates we reached within 2% of our target at 1 year for the standard compared with synthetic comparison.
Generalisable because of the wide range of UK centres and gynaecologists
Women who enrolled in RCT1 and CC1 have been described and compared in Chapter 3; by and large, the populations were similar, suggesting that the findings from the randomised women will be generalisable to the larger population of women who were having prolapse surgery in the UK.
Data available separately for primary and secondary surgery
Our ability to differentiate between women who were having a first or a repeat procedure in a particular compartment ensured that our findings can be applied to the needs of specific categories of women. It is possible that women who require a repeat repair will present a greater anatomical challenge because of previous scarring and hence may require a different surgical approach (see Chapter 6).
Non-randomised cohort
Another strength was the inclusion of women who were not randomised. Data collection using the same questionnaires as the trial women demonstrated that our population was representative of the majority of women who were having prolapse surgery in the UK (see Chapter 3). Outcome data collected from the cohort women demonstrated that their outcomes were similar to those from the randomised women, thus ensuring generalisability of the findings. A further benefit was the ability to identify less common adverse effects.
Pragmatic nature of the research
One of the strengths of the trial was its pragmatic reflection of actual practice in a representative sample of UK prolapse surgeons across a large number of hospital settings, including a wide range of surgical techniques and types of mesh and graft. We did not standardise the surgical techniques used: surgeons varied in whether they placed the mesh under or over the fascia, and whether they repaired fascial defects or lateral detachment. Furthermore the operations were performed by a variety of staff, although primarily consultant surgeons. This was reflected in the range of concomitant surgery performed. Consultants were, however, more likely to operate on women who were randomised to mesh or graft than to standard repairs.
Validated outcome measures
We used validated outcome measures such as the Pelvic Organ Prolapse Symptom scale and ICI suite of instruments to measure women’s symptoms of pelvic floor dysfunction, ensuring that our findings are comparable with other trials and can be combined within a meta-analysis. 23,26 We captured a wide range of adverse effects, and made efforts to verify these from alternative sources, such as hospital records, when possible. Essential missing data were actively sought from the women. Participants, outcome assessors and data entry clerks were blinded to randomisation and, as far as possible, to treatment actually received.
Weaknesses
The limitations of our study should be acknowledged. The large number of interventions and outcomes make it likely that some differences may have occurred by chance. However, the findings from all of the outcomes were remarkably consistent, and leave little room for doubt regarding the findings of the trials.
We have presented data from women who were having a first repair in the compartment requiring surgery, although around 10% of the women had undergone a previous repair in the opposite compartment. Thus, these results do not apply to women who were having repeat surgery in that compartment, who are presented in Chapter 6.
Because of the chronic and relapsing nature of prolapse, longer follow-up is required: the average time to a repeat operation is around 12 years. 4 Although we did not identify differences in the repeat surgery rate between the groups, it is likely that 2 years is too short a time scale to provide a definitive answer. We were able to identify complications, early recurrence and need for extra treatment, however. We have commenced follow-up for at least 6 years after surgery, and also plan electronic data linkage to capture outcomes from non-responders.
Pelvic Organ Prolapse Quantification stage (controversy over dividing up Stage 2)
The POP-Q system classes measurements from –1 cm inside the hymen to 1 cm as Stage 2. 27 We and other researchers28 have arbitrarily used a measurement of > 0 cm to indicate objective failure, while recognising that women with worse findings may not have symptoms and, vice versa, women with objective ‘cure’ may still have prolapse symptoms. However, Table 30 shows that the findings would have been the same whichever stage of prolapse was chosen as the cut-off.
Women with no prolapse at baseline/relationship between symptoms and objective findings
A small number of women were classed as having stage 0 or 1 on the POP-Q system before surgery. All of these women were symptomatic, and clearly their surgeons felt that prolapse surgery was indicated despite lack of objective measurable prolapse: in some cases there was evidence that the full descent had not been measured. The lack of concordance between prolapse symptoms and objective prolapse descent has been noted previously. 62 It is still unclear why some women appeared to have surgery in the absence of measured descent; there may be a training issue.
We agree that although the primary purpose of surgery should be improvement in the woman’s symptoms, if the woman has persistent symptoms but no prolapse it is difficult to justify repeat surgery.
Comparison with other research
The PROSPECT trial has shown that, in the first 2 years after surgery, there is no benefit to women who were having their first repair either in terms of prolapse symptoms or anatomical cure from the use of synthetic mesh or biological graft to reinforce a standard anterior or posterior repair. This is in stark contrast with the conclusions of the most recent Cochrane review,18 which found both a reduction in the number of women with prolapse symptoms with synthetic mesh (29% vs. 21%, RR 1.44, 95% CI 1.15 to 1.80; six RCTs, including 930 women) and improved anatomical measurements (45% vs. 21%, RR 2.45, 95% CI 1.64 to 3.67; 11 RCTs, 1155 women).
On the other hand, our findings concur with the uncertainty of the evidence for a difference for biological grafts (RR for number of women with symptoms 1.03, 95% CI 0.61 to 1.75; 3 RCTs, 401 women; RR for objective failure 1.35, 95% CI 0.74 to 2.46; 6 RCTs, 565 women) but with narrower CIs. Given that surgical failures requiring repeat repair occur, on average, 12 years after initial surgery, longer-term follow-up is required to determine true effectiveness and other sequelae of mesh or graft insertion.
Importantly, complications from mesh insertion were similar in frequency to those reported in the Cochrane review,18 and, in general, minor and easily resolved.
Differences in concomitant operations actually carried out in both trials were observed. More women had a vaginal hysterectomy in the standard arms than in the intervention arms but these differences were not significant. It is possible that knowledge of the allocated intervention influenced the surgery actually performed (so that if the woman was randomised to mesh or graft, she was less likely to have a concomitant hysterectomy). However, overall there were no substantial differences between the groups in the panel of operations that was carried out. This emphasises the importance of taking into consideration concomitant symptoms when managing women whose primary complaint is prolapse.
Summary
In both trials, the difference between the groups, and the size of the CI to either side, was considerably less than the prespecified minimally important clinical difference of two. The finding that more women appeared to have residual prolapse after mesh or graft repair than after standard repair was not statistically significant, and the difference was so small that it is not likely to be clinically significant. We conclude that although prolapse surgery was very effective in reducing women’s prolapse symptoms, the use of synthetic mesh or biological graft did not provide additional benefit in women who were having their first repair.
Conclusions
There appears to be no benefit to women who were having their first prolapse repair from using synthetic mesh or biological graft to reinforce the repair, in terms of prolapse symptoms, anatomical cure of prolapse or non-mesh adverse effects. However, a minority of women required an additional surgical procedure to remove exposed mesh, which may be considered to be an unnecessary risk. This additional risk suggests that mesh should be used in the future only in the context of RCTs aimed at identifying benefit from modifying mesh type or insertion techniques or in defined categories of high-risk women.
Chapter 5 Health economics results: Primary trial
This chapter describes the within-trial cost–utility analysis of the randomised women who were having their first anterior or posterior prolapse repair (RCT1).
In this chapter, the data are presented as incremental cost per QALY gained over 2-year follow-up. The perspective of the analyses is primarily that of the UK NHS, with a supplementary analysis incorporating wider perspective costs, including participant travel costs, opportunity costs of time for participants and companions spent attending appointments, self-purchased health care and time off work as a result of prolapse symptoms. This latter analysis provides a wider scope on the economic consequences of prolapse surgery.
The base-case health-economic analysis is presented for complete case data for women who were randomised to the three-way comparison of standard repair, synthetic mesh and biological graft (i.e. all women randomised to RCT1A for the Primary trial (see Chapter 2 for further information). The rationale for taking this approach for the economic analysis, and for taking a slightly different base-case approach to that of the statistical analysis of effect size, is that, in order to conduct a meaningful economic analysis and follow best practice economic evaluation procedures, all treatment options need to be considered together in a single analysis. This enables the exclusion of any treatment options that may be more costly and less effective than any other treatment alternative. The approach allows for the calculation and comparison of net benefits for all treatment strategies, to determine the strategy with the greatest probability of cost-effectiveness. Data presented for the estimation of incremental costs, QALYs and ICERs throughout the chapter are thus based on women randomised to RCT1A only. A sensitivity analysis reproduces the base-case cost-effectiveness analysis utilising all of the available data across the Primary trial. This considers the impact on results of considering two-way comparisons, thus making use of all of the data that are available for analysis.
The trial-based analysis seeks to inform short-term cost-effectiveness of prolapse treatment options. Data from the three-way comparison are further used to populate a Markov cohort decision-analytic model, which explores longer-term costs and QALY implications of prolapse treatment. The methods underpinning the model and the longer-term cost-effectiveness results are presented in Chapter 9.
EuroQol-5 Dimensions (3-level version), quality-adjusted life-years
The proportion of women with any health problems reported on the EQ-5D-3L measure of generic QoL is shown in Figures 5–7. These figures present the data as reported by women across randomised groups at 6 months, 1 year and 2 years, respectively. The descriptive data in Figures 5–7 are based on all of the available data recorded. This contrasts with the economic evaluation data in later sections, which are based on complete cost and QALY pairs. The figures illustrate the percentage of women who were having any problems on each of the domains of QoL (i.e. women scoring a ‘2’ or a ‘3’). In general, a substantial proportion of women appear to have some pain or discomfort, with little difference between 6-month and 1-year follow-up. The fewest problems were experienced in self-care, with only a small proportion of women reporting any problems. A visual inspection of the graphical data does not indicate any substantial differences between the percentage of women reporting problems across the randomised groups in any of the individual dimensions of generic QoL at 6 months or 1- or 2-year time points.
FIGURE 5.
Proportion of women experiencing any problems on each EQ-5D-3L domain at 6 months. Analysis based on all of the available EQ-5D data points.

FIGURE 6.
Proportion of women experiencing any problems on each EQ-5D-3L domain at 1 year. Analysis based on all of the available EQ-5D data points.

FIGURE 7.
Proportion of women experiencing any problems on each EQ-5D-3L domain at 2 years. Analysis based on all of the available EQ-5D data points.
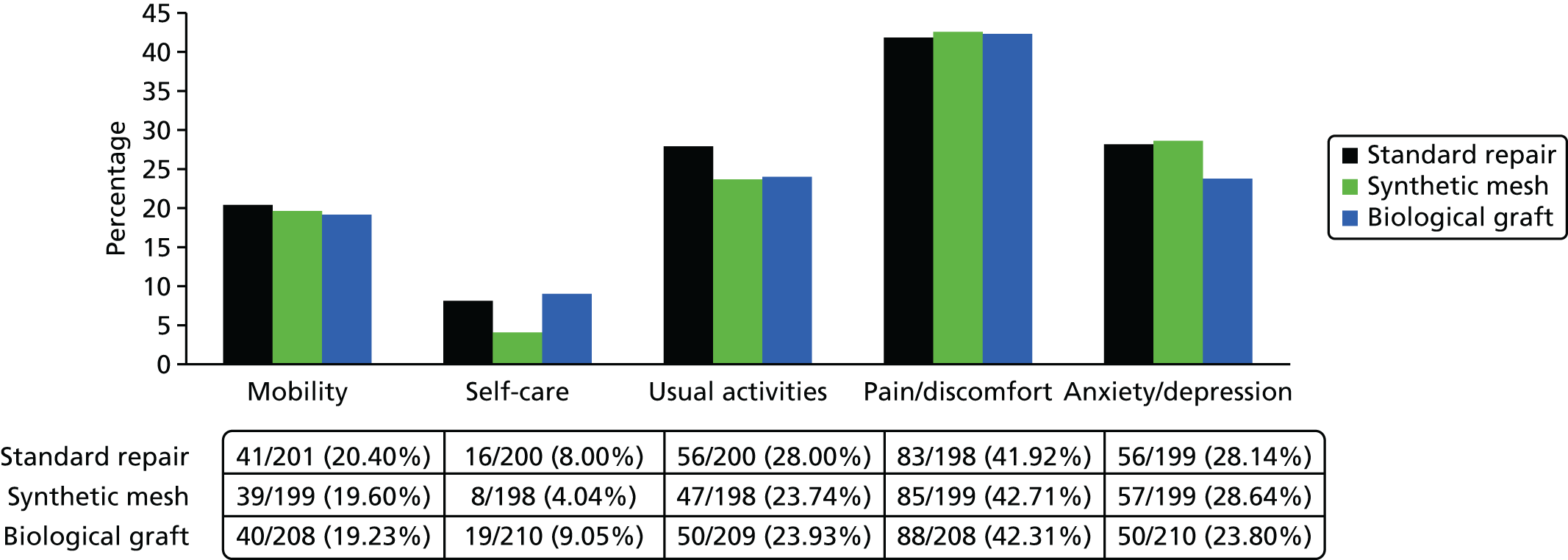
Figures 5–7 indicate the individual aspects of QoL that impact on overall utility for these women. Table 39 provides descriptive data of mean utility scores (generated using time trade-off tariffs, based on UK general population norms25) and QALYs generated by combining utilities with duration (length) of life over follow-up.
| Treatment group | Standard repair: mean (SD); n | Synthetic mesh: mean (SD); n | Biological graft: mean (SD); n |
|---|---|---|---|
| EQ-5D-3L: baseline | 0.722 (0.245); 231 | 0.711 (0.233); 234 | 0.697 (0.265); 230 |
| EQ-5D-3L: 6 months | 0.810 (0.284); 222 | 0.816 (0.229); 219 | 0.798 (0.268); 215 |
| EQ-5D-3L: 12 months | 0.816 (0.273); 227 | 0.827 (0.217); 223 | 0.809 (0.267); 228 |
| QALYs gaineda (baseline to 1 year) | 0.792(0.235); 197 | 0.808 (0.174); 196 | 0.778 (0.231); 193 |
| EQ-5D-3L: 24 months | 0.796 (0.293); 196 | 0.825 (0.230); 198 | 0.810 (0.270); 207 |
| QALYsb (baseline to 2 years) | 1.573 (0.498); 169 | 1.644 (0.302); 171 | 1.579 (0.452); 173 |
Results for incremental QALYs gained are presented comparing synthetic mesh/biological graft with standard repair for both raw differences between QALY estimates and also modelled differences. The modelled differences are based on linear regression (OLS) models, with adjustment for baseline covariates, including baseline EQ-5D-3L score, surgeon, age, BMI, concomitant continence procedure at baseline and compartment of prolapse.
Analyses were conducted using heteroscedastic robust SEs. Table 40 presents the incremental QALYs gained for each group at both 1 year and 2 years, calculated using an area-under-the-curve approach.
| QALYs | 1-year outcomes | 2-year outcomesb | ||||
|---|---|---|---|---|---|---|
| Mean (SD); n | Raw MD (vs. standard) | Adjusted MD (vs. standard); (95% CI)a | Mean (SD); n | Raw MD (vs. standard) | Adjusted MD (vs. standard); (95% CI)a | |
| Standard repair | 0.792 (0.235); 197 | – | – | 1.573 (0.498); 169 | – | – |
| Synthetic mesh | 0.808 (0.174); 196 | 0.016 | 0.0109 (–0.021 to 0.043) | 1.644 (0.302); 171 | 0.071 | 0.071 (–0.004 to 0.145) |
| Biological graft | 0.778 (0.231); 193 | –0.014 | –0.001 (–0.036 to 0.033) | 1.579 (0.452); 173 | 0.006 | 0.039 (–0.041 to 0.120) |
There were no statistically significant differences in QALYs between treatment groups over 1 year of follow-up. In terms of covariates included within the analysis model, baseline utility was the only significant predictor of overall QALYs in the model, indicating that QALYs were influenced by baseline EQ-5D-3L score. This is to be expected, given that we use an area-under-the-curve approach to calculate QALYs gained between EQ-5D-3L-reported time points, which are thus influenced by the starting point EQ-5D-3L score. Furthermore, the age of the women was not found to impact on QALY estimates or was the result impacted by type of prolapse (anterior/posterior) or whether or not the woman had a concomitant continence procedure. Overall, there is no evidence of any difference in generic QoL between randomised groups over the first year of follow-up.
There were no significant differences between the groups regarding QALYs gained at 2 years. However, the point estimates of QALYs were higher in both synthetic mesh and the biological graft groups, especially synthetic mesh, with a large proportion of the distribution predicting positive QALY gains for synthetic mesh. Nevertheless, there remains some uncertainty regarding the most beneficial treatment strategy in terms of QALYs gained from the trial-based analysis.
NHS resource use and costs
Costs to health services
Costs include intervention procedure costs, inpatient and follow-up secondary care costs, and costs of primary care services relating to the index prolapse surgery. This may include for example treatment of complications, treatment failure or increased contact with health-care professionals for prolapse-related issues. Similarly to the presentation of QALY data, the descriptive statistics and the regression analyses are based on complete case data for those women randomised across all three groups (i.e. RCT1A). The total NHS costs are calculated by multiplying resource use by the appropriate unit cost estimates outlined in Table 2 (see Chapter 2).
Intervention costs
The total costs to the NHS, based on the microcosting approach using data collected within the trial are presented in Table 41. There were no significant differences between groups in terms of staff time and length of hospitalisation costs. This is suggestive of similar operation, equipment and surgical expertise required to perform all types of procedures (whether mesh based or not). Despite substantial variation in costs of length of initial hospitalisation for a prolapse procedure, there was substantial uncertainty and no clear statistical differences between groups.
| Intervention cost table | Standard repair | Synthetic mesh | Biological graft | Incremental analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | n | Synthetic vs. standard (£): MD (95% CI)a | Biological vs. standard (£): MD (95% CI)a | |
| Mesh cost | 0 | 7 | 247 | 137 | 175 | 248 | 288 | 265 | 251 | ||
| Staff time in theatre | 920 | 428 | 252 | 963 | 373 | 255 | 922 | 361 | 255 | ||
| Cost of drugs in theatre | 24 | 8 | 252 | 24 | 8 | 255 | 25 | 7 | 255 | ||
| Cost of catheterisation | 5 | 2 | 252 | 6 | 2 | 255 | 6 | 2 | 255 | ||
| Cost of vaginal packing | 4 | 2 | 252 | 4 | 2 | 255 | 4 | 2 | 255 | ||
| Theatre overheads | 465 | 217 | 252 | 491 | 184 | 255 | 473 | 182 | 255 | ||
| Subtotal: theatre costs | 1420 | 649 | 247 | 1627 | 591 | 248 | 1718 | 595 | 251 | ||
| Costs from theatre – discharge | 1411 | 919 | 247 | 1501 | 891 | 248 | 1540 | 1083 | 251 | ||
| Total intervention costs | 2831 | 1151 | 247 | 3128 | 1042 | 248 | 3258 | 1279 | 251 | 251 (46 to 455) | 499 (265 to 732) |
The intervention costs of synthetic mesh and biological graft repairs were substantially and statistically significantly more expensive than standard midline repairs as a result of the additional cost of materials required to carry out the procedures. There was substantial variation in the price of mesh across different products within similar groups. These analyses make no statements about the effectiveness of one material relative to another, and are based on the assumption that mesh or graft material products within a category are equally effective (i.e. assuming all types of synthetic mesh are equally effective and all types of biological graft are equally effective). The variability in the price of mesh procedures across participating sites, using alternative mesh products is evident through the large SDs for mesh costs presented in Table 41.
Health services resource-use costs over trial follow-up
The additional costs of mesh procedures are combined with costs to the health services over the trial follow-up period for each treatment group and are presented in Table 42. These include all secondary care (readmissions, reoperations, visits to ward, outpatient consultations) and primary care (e.g. GP, nurse, physiotherapist) contacts with health professionals. We have taken the following approach to presentation of cost data. Each category of cost is presented for full cases within that category (e.g. hospital resource use, primary care costs). These are then summed, along with the intervention cost, for complete cases across all the categories, and presented as the total cost to the health services at 2 years. Data presented in Table 42 and for the statistical analyses are based on the three-way comparison (i.e. RCT1A).
| NHS resource use and costs | Resource use | Costs | ||||||
|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Biological graft | Standard repair: mean (SD); N | Synthetic mesh: mean (SD); N | Biological graft: mean (SD); N | Incremental analysis of costsa | ||
| Synthetic vs. standard | Biological vs. standard | |||||||
| Total intervention costs | 2831 (1151); 247 | 3128 (1042); 248 | 3258 (1279); 251 | 251 (46 to 455) | 499 (265 to 732) | |||
| 1-year data | ||||||||
| Hospital resource use (0–6 months) | ||||||||
| New prolapse procedure; n/N (%) | 1/232 (0.4%) | 3/224 (1.3%) | 2/228 (0.9%) | |||||
| New incontinence procedure; n/N (%) | 1/232 (0.4%) | 0/224 (0.0%) | 0/228 (0.0%) | |||||
| Other related readmissions; n/N (%) | 6/232 (2.6%) | 12/224 (5.4%) | 7/228 (3.1%) | |||||
| Further prolapse-related surgery within 6 months; n/N (%) | 8/232 (3.9%) | 15/224 (7.1%) | 9/228 (4.4%) | 47 (259); 232 | 92 (368); 224 | 54 (288); 228 | ||
| Outpatient visits; n/N (%) | 9/232 (3.9%) | 13/224 (5.8%) | 21/228 (9.2%) | 5 (26); 232 | 8 (31); 224 | 12 (39); 228 | ||
| Subtotal (hospital use 0–6 months) | 52 (260); 232 | 100 (368); 224 | 66 (288); 228 | 43 (–24 to 112) | –8 (–60 to 44) | |||
| Hospital resource use (6–12 months) | ||||||||
| New prolapse procedure; n/N (%) | 5/233 (2.1%) | 8/226 (3.5%) | 10/230 (4.3%) | 50 (339); 233 | 83 (432); 226 | 101 (476); 230 | ||
| New incontinence procedure; n/N (%) | 2/233 (0.9%) | 1/226 (0.4%) | 3/230 (0.9%) | 12 (127); 233 | 6 (91); 226 | 18 (156); 230 | ||
| Other related readmissions; n/N (%) | 4/233 (1.7%) | 11/226 (4.9%) | 5/230 (2.2%) | 17 (134); 233 | 52 (233); 226 | 19 (131); 230 | ||
| Outpatient visits: mean (SD) | 0.38 (0.72) | 0.62 (1.02) | 0.49 (0.80) | 45 (78); 233 | 68 (100); 226 | 56 (84); 230 | ||
| Subtotal (hospital use 6–12 months) | 124 (404); 232 | 208 (513); 226 | 194 (546); 230 | 86 (–7 to 179) | 54 (–42 to 150) | |||
| Other consultations (0–12 months) | ||||||||
| Physiotherapy: mean (SD) | 0.21 (0.94) | 0.23 (1.02) | 0.17 (0.78) | 5 (23); 232 | 6 (24); 226 | 4 (19); 230 | ||
| GP nurse: mean (SD) | 0.04 (0.23) | 0.17 (0.74) | 0.10 (0.80) | 1 (3); 233 | 2 (10); 226 | 1 (11); 230 | ||
| GP doctor: mean (SD) | 0.43 (1.07) | 0.68 (1.56) | 0.51 (1.16) | 20 (49); 233 | 31 (72); 226 | 23 (53); 229 | ||
| Other: mean (SD) | 0.04 (0.27) | 0.03 (0.20) | 0.04 (0.33) | 3 (29); 233 | 1 (8); 225 | 1 (7); 229 | ||
| Subtotal (other consultations 0–12 months) | 28 (70); 232 | 40 (86); 225 | 30 (62); 228 | 12 (–4 to 29) | 1 (–12 to 15) | |||
| Other treatments (0–12 months) | ||||||||
| Shelf pessary; n/N (%) | 4/233 (1.7%) | 3/226 (1.3%) | 3/230 (1.3%) | 1 (8); 233 | 1 (7); 226 | 1 (7); 230 | ||
| Ring pessary; n/N (%) | 5/233 (2.2%) | 6/226 (2.7%) | 5/230 (2.2%) | 1 (6); 233 | 1 (6); 226 | 1 (6); 230 | ||
| Incontinence drugs; n/N (%) | 14/233 (6.0%) | 21/226 (9.3%) | 15/230 (6.5%) | 3 (8); 233 | 4 (10); 226 | 3 (8); 230 | ||
| Oestrogen; n/N (%) | 34/233 (14.6%) | 46/226 (20.4%) | 39/230 (17.0%) | 3 (7); 233 | 4 (8); 226 | 3 (7); 230 | ||
| Intermittent catheters; n/N (%) | 3/232 (1.3%) | 4/224 (1.8%) | 6/230 (2.6%) | 23 (205); 233 | 32 (240); 226 | 47 (290); 230 | ||
| Permanent catheter; n/N (%) | 1/232 (0.4%) | 1/224 (0.5%) | 0/230 (0.0%) | 2 (26); 233 | 2 (26); 226 | 0 (0); 230 | ||
| Absorbent pads; n/N (%) | 70/233 (30.0%) | 67/226 (29.7%) | 60/230 (26.1%) | 217 (367); 233 | 225 (459); 226 | 166 (302); 230 | ||
| Other drug treatments; n/N (%) | 15/233 (6.4%) | 10/226 (4.4%) | 9/230 (3.9%) | 3 (14); 233 | 9 (94); 226 | 1 (5); 230 | ||
| Subtotal (other treatments 0–12 months) | 253 (412); 233 | 278 (546); 226 | 223 (403); 230 | –21 (–120 to 77) | –47 (–132 to 38) | |||
| Total 1-year follow-up costs | 461 (706); 222 | 622 (967); 211 | 499 (778); 219 | 112 (–64 to 288) | –4 (–160 to 151) | |||
| Total health services costs (1 year) | 3240 (1304); 222 | 3702 (1358); 211 | 3800 (1495); 219 | 438 (168 to 708) | 611 (332 to 891) | |||
| NHS resource use and costs | Resource use | Costsb | ||||||
| Standard repair | Standard repair | Standard repair | Standard repair: mean (SD); N | Synthetic mesh: mean (SD); N | Biological graft: mean (SD); N | Incremental analysis of costs | ||
| Synthetic vs. standard | Biological vs. standard | |||||||
| 2-year data | ||||||||
| Hospital resource use (12–24 months) | ||||||||
| New prolapse procedure; n/N (%) | 11/202 (5.4%) | 9/201 (4.5%) | 9/209 (4.3%) | 123 (512); 202 | 101 (467); 201 | 97 (458); 209 | ||
| New incontinence procedure; n/N (%) | 4/202 (2.0%) | 1/201 (0.5%) | 4/209 (1.9%) | 26 (185); 202 | 7 (94); 201 | 25 (182); 209 | ||
| Other related readmissions; n/N (%) | 2/202 (1.0%) | 10/201 (5.0%) | 2/209 (1.0%) | 10 (98); 202 | 52 (232); 201 | 9 (97); 209 | ||
| Outpatient visits: mean (SD) | 0.19 (0.55) | 0.23 (0.57) | 0.28 (0.66) | 18 (50); 202 | 22 (55); 200 | 26 (60); 209 | ||
| Subtotal (hospital resource use 12–24 months) | – | – | – | 177 (603); 202 | 182 (554); 200 | 158 (523); 209 | –33 (–156 to 90) | –22 (–149 to 106) |
| Other consultations (12–24 months) | ||||||||
| Physiotherapy: mean (SD) | 0.19 (1.06) | 0.25 (1.03) | 0.22 (1.00) | 4 (25); 202 | 6 (24); 202 | 5 (23); 210 | ||
| GP nurse: mean (SD) | 0.03 (0.23) | 0.03 (0.27) | 0.03 (0.22) | 0 (3); 202 | 0 (4); 202 | 0 (3); 210 | ||
| GP doctor: mean (SD) | 0.32 (2.17) | 0.40 (1.17) | 0.19 (0.63) | 14 (97); 202 | 18 (52); 202 | 9 (28); 209 | ||
| Other: mean (SD) | 0.04 (0.34) | 0.08 (0.67) | 0.03 (0.31) | 0 (3); 200 | 0 (4); 201 | 0 (6); 209 | ||
| Subtotal other consultations (12–24 months) | 20 (102); 200 | 24 (61); 201 | 15 (43); 208 | 6 (–14 to 26) | –7 (–26 to 11) | |||
| Other treatments (12–24 months) | ||||||||
| Shelf pessary | 5/202 (2.5%) | 2/202 (1.0%) | 5/210 (2.4%) | 2 (10); 202 | 1 (6); 201 | 1 (10); 209 | ||
| Ring pessary | 6/202 (3.0%) | 5/202 (2.5%) | 9/210 (4.3%) | 1 (7); 202 | 1 (6); 201 | 2 (8); 209 | ||
| Incontinence drugs | 16/202 (7.9%) | 16/202 (7.9%) | 18/210 (8.6%) | 3 (7); 202 | 3 (9); 201 | 3 (7); 209 | ||
| Oestrogen | 27/202 (13.4%) | 35/202 (17.3%) | 32/210 (15.2%) | 5 (9); 204 | 6 (10); 208 | 5 (9); 216 | ||
| Intermittent catheters | 4/201 (2.0%) | 3/201 (1.5%) | 4/210 (1.9%) | 35 (245); 202 | 26 (213); 201 | 33 (240); 210 | ||
| Permanent catheter | 0/202 (0.00%) | 0/201 (0.00%) | 0/210 (0.00%) | 0 (0); 202 | 0 (0); 201 | 0 (0); 209 | ||
| Absorbent pads | 57/201 (28.4%) | 52/201 (25.9%) | 57/209 (27.3%) | 247 (537); 202 | 191 (421); 202 | 195 (373); 210 | ||
| Other drug treatments | 10/202 (5.0%) | 8/201 (4.0%) | 6/210 (2.9%) | 2 (12); 202 | 15 (140); 201 | 0 (2); 210 | ||
| Subtotal, other treatments (12–24 months) | 293 (629); 202 | 241 (500); 201 | 229 (433); 209 | –86 (–213 to 42) | –80 (–200 to 40) | |||
| Total 2-year follow-up costs (12–24 months) | 459 (972); 200 | 439 (841); 199 | 393 (692); 207 | –91 (–294 to 112) | –84 (–275 to 107) | |||
| Total health services costs (2 years) | 3685 (1769); 194 | 4112 (1756); 184 | 4194 (1743); 196 | 363 (–32 to 758) | 565(180 to 950) | |||
At 1 year post operation, based on the data available from RCT1A, synthetic mesh and biological graft are both significantly more costly than the standard repair, with biological graft being the most expensive treatment option. Biological graft is significantly more expensive over 2 years of follow-up. There remains some weak evidence (p < 0.1) that synthetic mesh is also more costly to the health services over 2 years. These outcomes would be expected, given that the additional cost of mesh is applied to these arms in the intervention costing. It is important to conclude that there is no evidence of differences in costs of follow-up care between any of the trial interventions. Overall, over a 2-year time horizon, including intervention and follow-up health services costs, synthetic mesh is, on average, £363 more costly than standard repair (95% CI –£32 to £758) based on costs discounted at a rate of 3.5% per annum, applied to the second year of follow-up. Biological graft is estimated to be £565 more expensive than standard repair (95% CI £180 to £950). It is clear that from the perspective of NHS payers that mesh products, and in particular biological graft prolapse repairs, present significant cost outlay for provision of care.
Base-case cost-effectiveness results (NHS perspective)
Given that there are no substantial counteracting cost savings of follow-up care over the shorter-term time horizon, it is necessary to compare the additional costs of mesh repairs with any potential gains in QoL. The base-case economic (cost–utility) analysis is presented according to the regression models outlined for costs and QALYs in Chapter 2. The base-case economic analysis is presented for complete case data of cost and QALY pairs, ensuring that the joint distribution of costs and effects is not broken. As with the data presented in previous tables, all analyses are for women randomised to the three-way (RCT1A) trial comparison.
One-year cost-effectiveness results
Table 43 presents the main results of the economic analysis from a NHS perspective over a 1-year time horizon. Based on these data, at 1-year follow-up, biological graft is not a cost-effective alternative to either standard repair or synthetic mesh, as it is more costly and generates fewer QALYs. Furthermore, at 1-year follow-up, it is unlikely that synthetic mesh would offer a cost-effective treatment option, with an ICER of £35,750 per QALY gained, it is above the £20,000–30,000 threshold value of cost-effectiveness commonly accepted by UK decision-makers. Figure 8 illustrates the scatterplot of incremental costs and incremental QALYs for this analysis, showing substantial uncertainty in QALYs gained, but definitively showing that in nearly all of the simulations both mesh procedures are more expensive than standard repair. The CEAC in Figure 9 shows that at 1-year follow-up, based on the NMB, there is substantial uncertainty regarding the most cost-effective treatment strategy. On the balance of probabilities, data from the CEACs indicate that standard repair has a slightly higher probability of being the most cost-effective treatment strategy than alternative options up to a WTP of approximately £40,000 per QALY gained. Considering that decision-makers may be willing to pay £30,000 for a QALY gained, there is a 57% chance that standard repair and 40% chance that synthetic mesh are the most cost-effective treatments. There is little chance biological graft would be cost-effective. However, as the threshold of WTP for a QALY increases, the probability that synthetic mesh becomes cost-effective increases. Overall, the data do not allow one to draw clear conclusions on cost-effectiveness over a 1-year follow-up.
| Treatment | Costs: mean (SD) | Incremental costs (vs. standard)a | QALYs: mean (SD) | Incremental QALYs (vs. standard)a | Incremental cost (£) per QALY gained (vs. standard) | Probability of cost-effectiveness at alternative threshold values of WTP for a QALY gain (%)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Standard repair (n = 195) | 3216 (1301) | – | 0.790 (0.236) | – | – | 1.00 | 0.91 | 0.70 | 0.57 | 0.43 |
| Synthetic mesh (n = 195) | 3698 (1387) | 429 (161 to 697) | 0.808 (0.174) | 0.012 (–0.021 to 0.044) | 35,750 | 0.00 | 0.09 | 0.29 | 0.40 | 0.50 |
| Biological graft (n = 191) | 3823 (1500) | 600 (316 to 885) | 0.781 (0.231) | –0.001 (–0.038 to 0.036) | Dominated | 0.00 | 0.00 | 0.02 | 0.04 | 0.07 |
FIGURE 8.
Scatterplot of incremental costs and QALYs for mesh treatments compared with standard repair: 1-year follow-up data.

FIGURE 9.
Cost-effectiveness acceptability curves: 1-year follow-up data.
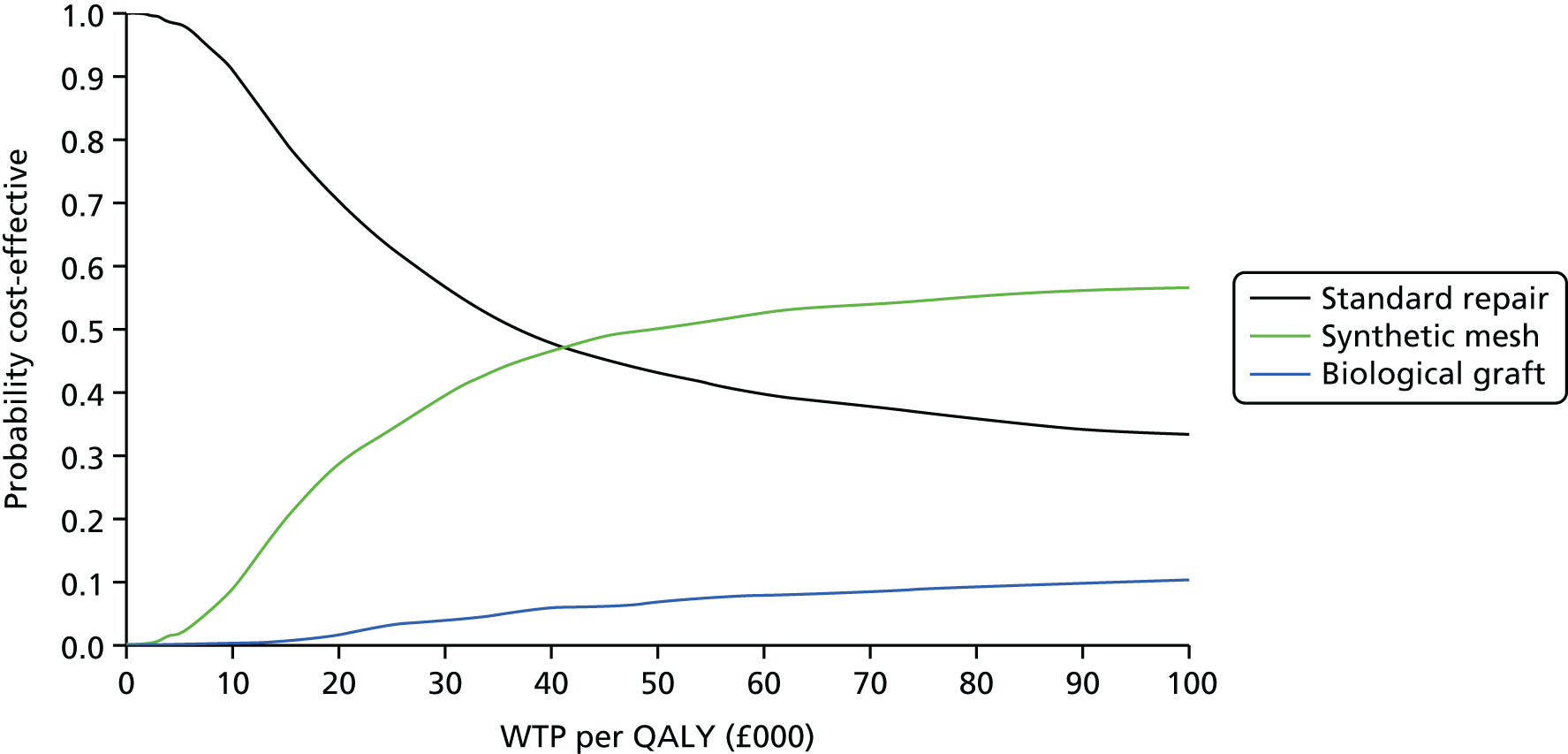
Two-year cost-effectiveness results
The results of the cost-effectiveness analysis over 2 years is presented in Table 44. Two analyses are undertaken: the base-case analysis presents complete case data, and the secondary analysis presents the results from an imputed data set. In all cases, costs and outcomes for the second year of follow-up are discounted at a rate of 3.5% in accordance with economic evaluation best practice guidelines.
| Treatment | Costs: mean (SD) | Incremental costsa,b (vs. standard) | QALYs: mean (SD) | Incremental QALYsa,b (vs. standard) | Incremental cost (£) per QALY gained (vs. standard) | Probability of cost-effectiveness at alternative threshold values of WTP for a QALY gain (%)c | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10k | £20k | £30k | £50k | ||||||
| Complete case analysis | ||||||||||
| Standard repair (n = 165) | 3664 (1777) | 1.569 (0.502) | – | 0.93 | 0.19 | 0.08 | 0.05 | 0.03 | ||
| Synthetic mesh (n = 168) | 4081 (1762) | 337 (–73 to 747) | 1.643 (0.304) | 0.075 (0.000 to 0.150) | 4493 | 0.06 | 0.75 | 0.83 | 0.84 | 0.84 |
| Biological graft (n = 170) | 4165 (1691) | 555 (156 to 954) | 1.582 (0.455) | 0.041 (–0.042 to 0.124 | 13,537 | 0.00 | 0.07 | 0.10 | 0.12 | 0.13 |
| Imputed data set analysis | ||||||||||
| Standard repair (n = 647) | 3570 (468) | 1.559 (0.297) | 0.93 | 0.69 | 0.57 | 0.52 | 0.47 | |||
| Synthetic mesh (n = 647) | 3889 (468) | 319 (–56 to 694) | 1.555 (0.297) | –0.003 (–0.068 to 0.063) | Dominated | 0.07 | 0.22 | 0.28 | 0.29 | 0.30 |
| Biological graft (n = 647) | 4098 (468) | 527 (161 to 893) | 1.554 (0.297) | –0.004 (–0.073 to 0.065) | Dominated | 0.00 | 0.09 | 0.16 | 0.20 | 0.23 |
Complete case analysis
The results of the complete case analysis show that synthetic mesh provides greater QALYs than standard repair, for an analysis in which complete case cost and QALY pairs are considered. This leads to a favourable point estimate of the ICER for synthetic mesh compared with standard repair of £4493 per QALY gained, falling well below a threshold value of WTP for a QALY gained. Biological graft repair is not cost-effective. Although its ICER compared with standard repair is just over £13,000 per QALY, it is more costly and less effective than synthetic mesh. Biological graft is therefore dominated by synthetic mesh and, based on current data, is not a cost-effective use of resources. However, this estimate should be interpreted in light of the considerable uncertainty surrounding it. Figures 10 and 11 present the scatterplot and CEACs, respectively, for the 2-year outcomes. Despite the favourable point estimate of the ICER for synthetic mesh repair, there remains some uncertainty, with an 84% probability, that synthetic mesh is the most cost-effective treatment strategy. The probability of biological repair being the preferred treatment option is substantially lower, never reaching a probability of cost-effectiveness of > 13% at threshold values of up to £50,000 per QALY gained.
FIGURE 10.
Scatterplot of incremental costs and QALYs for mesh treatments compared with standard repair: complete case analysis, 2-year follow-up data.

FIGURE 11.
Cost-effectiveness acceptability curves: complete case analysis, 2-year follow-up data.
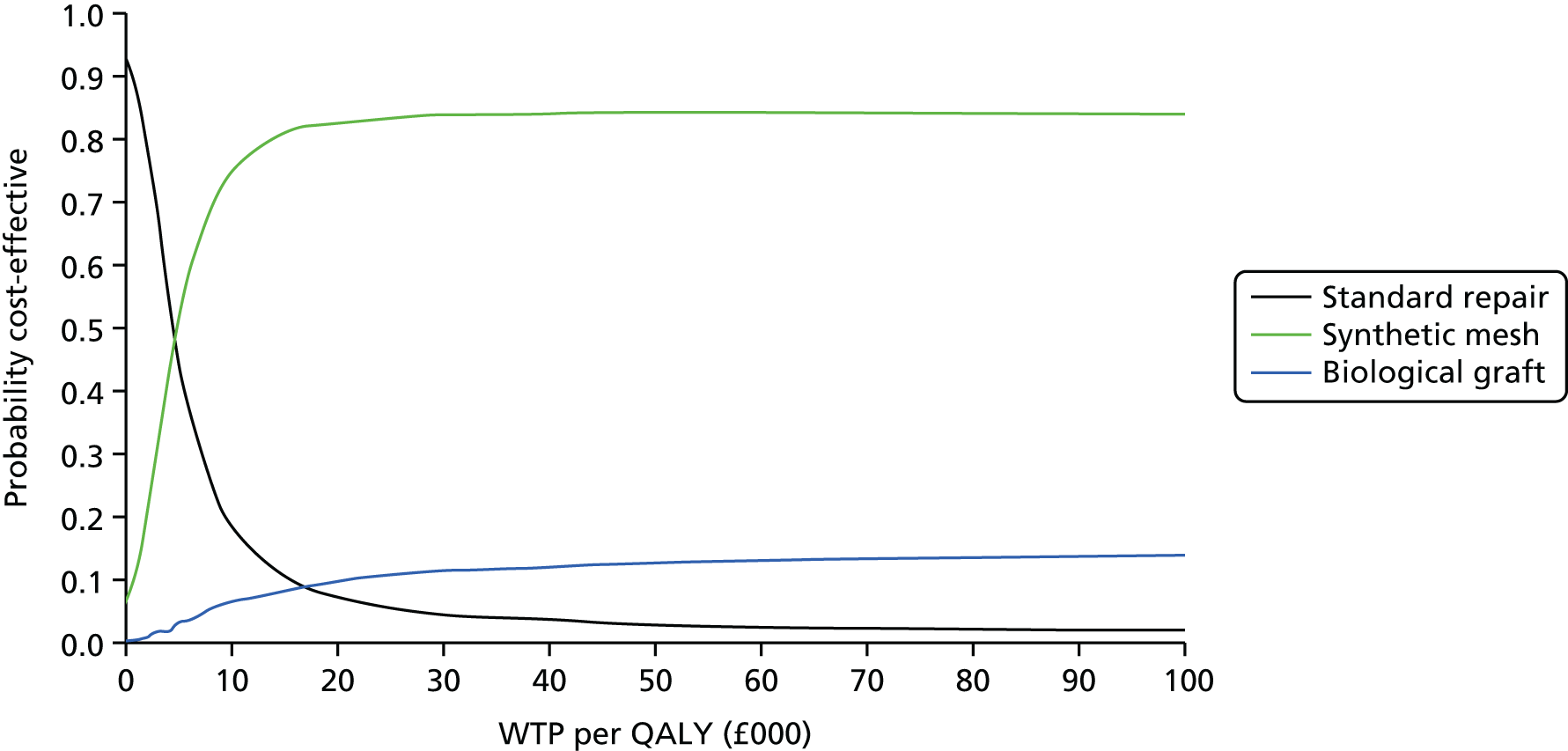
Based on the complete case analysis of 2-year cost-effectiveness outcomes, there is no definitive evidence regarding the most cost-effective treatment strategy, although synthetic mesh may offer a cost-effective alternative to standard repair, depending on the threshold of WTP for a QALY gained adopted by a decision-maker. The higher the threshold value, the more likely it is that synthetic mesh would be considered a cost-effective use of NHS resources.
Missing data
The complete case analysis results showed potential improvements in QALY gains for synthetic mesh at 2 years. However, the analysis of complete case QALY data ignored some important missing data information, with a substantial proportion of participants missing complete QALY data at each time point up to 2 years. Data completeness for QALYs at 2 years was evenly distributed across the different arms of the trials with missing data 83 of 252 (33%), 84 of 255 (33%) and 82 of 255 (32%) for standard repair, synthetic mesh and biological graft, respectively. Further analysis shows that participants suffering from failures or complications tended to report less-complete EQ-5D-3L data than those not experiencing such effects. For example, missing data for 2-year QALYs for those with severe complications was between 40% and 45% across the randomised groups. Furthermore, we investigated the mechanism of missingness of data by exploring the impact of baseline covariates on missing data. Missing data were found to differ significantly between age groups, with older women reporting more complete data. We therefore excluded the assumption that missing QALY data were missing at random. This finding, together with the extent of missingness, particularly among respondents experiencing negative health effects, is suggestive that the complete case analyses may overestimate true QALYs for women who are experiencing prolapse. Multiple imputed data analysis explores the impact of missing data on results. The analysis reported in the lower section of Table 44 imputes data based on predictive mean matching for QALYs and standard multivariate regression models for costs. Estimates of incremental costs were not found to impact on overall results. It can be seen from the results that there remains substantial uncertainty regarding the most cost-effective treatment strategy, but, based on data imputation, the potential QALY gains for mesh in the complete case analysis have been removed and all of the strategies generate broadly similar outcomes. Given that there remain no QALY differences between mesh and standard repairs in the imputed data set, mesh is no longer a viable alternative from a cost-effectiveness point of view, and both meshes are dominated by the standard repair approach. As with all of the other analyses, however, there remains substantial uncertainty surrounding the point estimates of cost-effectiveness, with a probability of cost-effectiveness of standard repair (52%), synthetic mesh (29%) and biological repair (20%) at a WTP of £30,000 per QALY gained in the imputed data set compared with 5%, 84% and 12%, respectively, in the base-case analysis.
Considering the two analyses presented for complete case and imputed data sets, there is some uncertainty regarding cost-effectiveness from the within-trial analyses. Although the within-trial analysis is informative regarding short-term cost-effectiveness, and determining drivers of uncertainty, it is likely that the true cost-effectiveness of mesh materials will not be determined by such a short time horizon, which may be insufficient to capture longer-term risk of recurrence and any associated complications. It is therefore necessary to consider longer-term outcomes of cost-effectiveness. This will be achieved by continuing the PROSPECT follow-up to a minimum of 6 years, and through the development of a decision-analytic model presented in Chapter 9. The decision model will make initial projections of longer-term cost-effectiveness, which will be validated using the longer-term trial follow-up once the data become available.
Costs directly incurred by participants and indirect costs
A further analysis was conducted incorporating both participant and indirect costs into the analysis over the 2-year study follow-up.
Table 45 reports mean costs (from a wider economic perspective) of attending primary care, outpatient appointments and inpatient admissions, respectively. We have taken the following approach to presentation of data in Table 45. Each category of cost is presented for full cases within that category (e.g. time off work due to prolapse symptoms). These are then summed together, across all the categories for all available cost data for participant and companion time and travel costs, and presented as the total participant cost at 2 years. Complete case data for participant total cost and NHS total cost are then added together to generate a wider economic cost.
| Costs | Standard repair | Synthetic mesh | Biological graft | Incremental analysisa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | n | Synthetic vs. standard: MD (95% CI) | Biological vs. standard: MD (95% CI) | |
| Time off work due to prolapse problems | 1376 | 4945 | 236 | 1207 | 3475 | 231 | 1310 | 3800 | 235 | 20 (–836 to 877) | 74 (–769 to 917) |
| Participant and companion time and travel costs (primary care appointments) | 27 | 168 | 184 | 26 | 63 | 179 | 17 | 51 | 175 | 0 (–32 to 32) | –11 (–41 to 20) |
| Participant and companion time and travel costs (outpatient appointments) | 27 | 53 | 160 | 36 | 61 | 160 | 73 | 281 | 157 | 5 (–9 to 18) | 56 (–10 to 123) |
| Participant and companion time and travel costs (inpatient appointments) | 266 | 156 | 247 | 262 | 145 | 248 | 263 | 165 | 251 | –12 (–43 to 19) | –5 (–37 to 28) |
| Self-purchased health care and medication | 3 | 30 | 235 | 4 | 26 | 228 | 16 | 165 | 234 | 0 (–7 to 7) | 16 (–10 to 42) |
| Total indirect and participant costs | 1621 | 4891 | 247 | 1432 | 3417 | 248 | 1562 | 3795 | 251 | –18 (–853 to 817) | 70 (–749 to 890) |
| Total NHS cost (2 years) | 3685 | 1769 | 194 | 4111 | 1756 | 184 | 4194 | 1743 | 196 | 337 (–73 to 747) | 555 (156 to 954) |
| Overall total NHS, participant and indirect costs | 5431 | 5795 | 194 | 5685 | 4516 | 184 | 5897 | 4582 | 196 | 143 (–1034 to 1319) | 416 (–715 to 1547) |
The analysis includes participant-incurred costs for attending their PROSPECT surgery. As women with prolapse symptoms attended a large number of consultations and appointments with the health services, the personal and economic cost was also substantial. However, there were no differences across randomised groups, and it is important to note large SDs, which indicate great uncertainty in participant time and travel costs across the groups.
Furthermore, a small proportion of women incurred direct private health-care costs or self-purchased medication. However, the majority did not and, as with the analyses above, there were no differences across groups.
Mean indirect costs of sick leave taken by participants over 2 years for reasons related to prolapse symptoms were £1376, £1207 and £1310 per woman for standard repair, synthetic mesh and biological graft, respectively. This large value reflects the fact that prolapse symptoms have a substantial impact on everyday life for women in terms of financial consequences. However, there were no differences across the randomised groups in terms of time taken as sick leave in relation to prolapse problems and symptoms. The wider economic impact is likely to be greater still if one were to consider the lost productivity of days spent at work, where bothersome symptoms interfered with women’s normal work activities but may not necessarily have required sick leave. Therefore, the estimates of true economic cost are likely to be underestimated.
Combining all of the costs of sick leave, opportunity costs of time for participants and companions to attend appointments, travel costs to attend appointments and total costs to the NHS, we can estimate a wider overall economic cost to society. This is limited, of course, to the costs considered, and the true economic costs may be much higher. Nonetheless, the analysis gives an overall impression of the most immediate wider economic costs associated with prolapse surgery and the alternative treatment options considered in the PROSPECT Study. Total economic costs were estimated as £5431, £5685 and £5897 for standard repair, synthetic mesh and biological graft, respectively. By incorporating indirect costs and economic productivity losses of time off work, it is evident that there are substantial costs to prolapse beyond the health sector with substantial impact on prolapse patients, their families and the wider economy. However, incorporating these estimates into the overall analysis greatly increases overall uncertainty, as is evident from the costs presented in Table 46, with large SDs surrounding overall economic costs.
| Treatment | Costs: mean (SD)a | Incremental costs (vs. standard)b | QALYs: mean (SD)a | Incremental QALYs (vs. standard)b | Incremental cost (£) per QALY gained (vs. standard) | Probability of cost-effectiveness at alternative threshold values of WTP for a QALY gain (%)c | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Standard repair (n = 165) | 5479 (6026) | – | 1.569 (0.502) | – | 0.43 | 0.16 | 0.07 | 0.04 | 0.03 | |
| Synthetic mesh (n = 168) | 5740 (4657) | –26 (–1302 to 1250) | 1.643 (0.304) | 0.075 (0.000 to 0.150) | Dominant | 0.45 | 0.74 | 0.82 | 0.84 | 0.85 |
| Biological graft (n = 170) | 5813 (4199) | 306 (–909 to 1521) | 1.582 (0.455) | 0.041 (–0.042 to 0.124) | 7463 | 0.12 | 0.10 | 0.11 | 0.11 | 0.13 |
Combining the indirect and participant costs with the total NHS costs does not have any substantial impact on overall findings, except to increase the uncertainty surrounding the preferred treatment option. Although the initial point estimates of incremental costs and incremental QALYs tend to favour mesh, with, on average, cost savings and QALY gains, these are meaningless unless considered in the light of the uncertainty surrounding the data. To further explore and illustrate the associated uncertainty, a scatterplot of incremental costs and effectiveness for synthetic mesh and biological graft (compared with standard repair), as well as a CEAC derived from the results of 1000 bootstrapped replicates of mean costs and QALYs are presented. Figures 12 and 13 illustrate that the probability of standard repair, synthetic mesh or biological graft being the most cost-effective treatment strategy at a threshold value of WTP for a QALY gained of £30,000 are 4%, 84% or 11%, respectively.
FIGURE 12.
Scatterplot of incremental costs and QALYs for mesh treatments vs. standard repair: 2-year follow-up, wider economic perspective.

FIGURE 13.
Cost-effectiveness acceptability curves: 2-year follow-up, wider economic perspective.
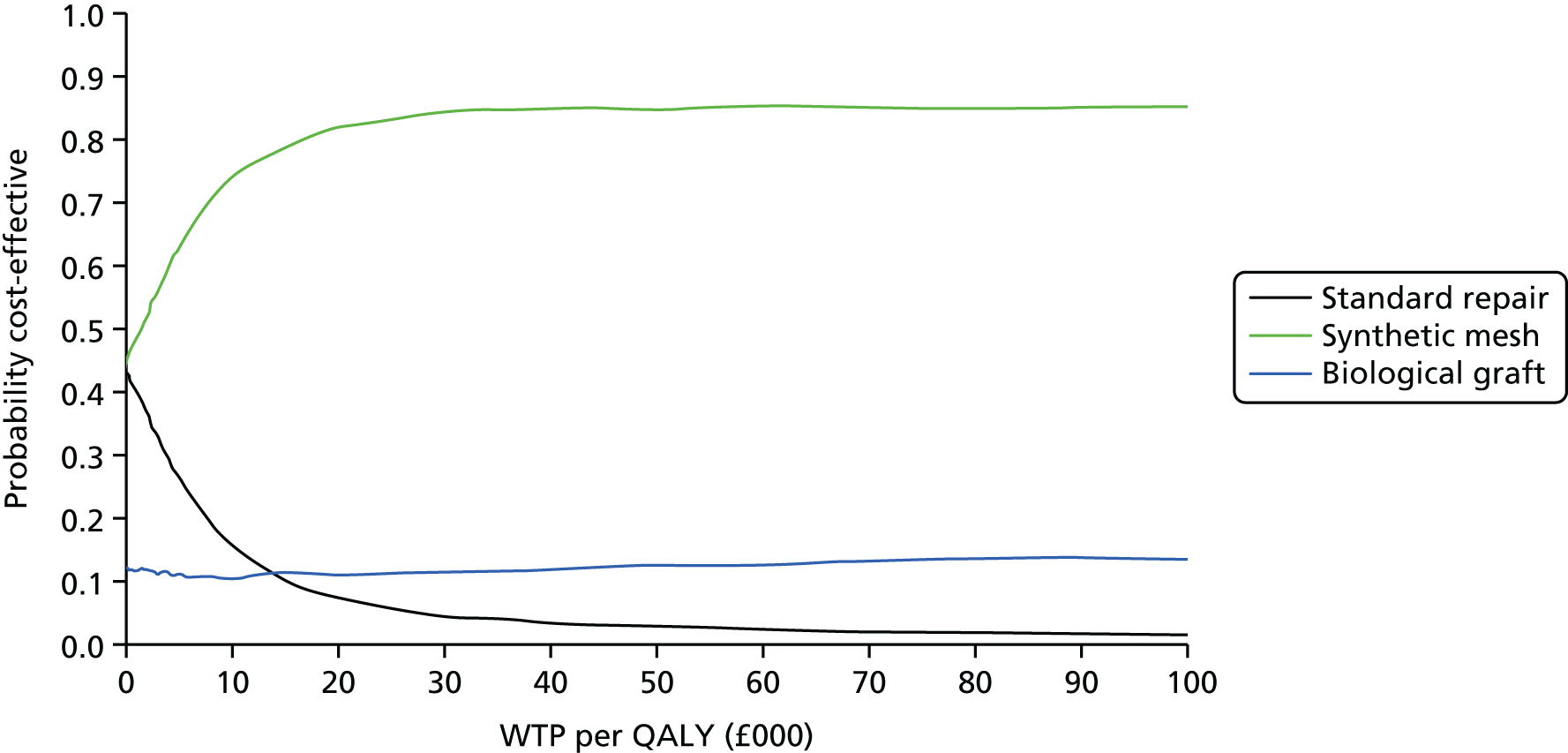
The uncertainty surrounding both the NHS- and participant-incurred costs as well as uncertain QALY gains means that there is no clearly definitive cost-effectiveness strategy, even when a wider perspective of economic costs is considered.
Deterministic sensitivity analyses
As demonstrated in the CEACs and scatterplots presented, there is substantial uncertainty driven by data variability in our analysis. Although the complete case analysis indicates that synthetic mesh may be cost-effective with approximately 80% probability at a threshold value of WTP for a QALY gain of £30,000, there remains some uncertainty, particularly in relation to missing data, with no definitively cost-effective strategy emerging. Furthermore, although CEACs and scatterplots based on bootstrapped iterations are important in presenting sampling uncertainty, they do not consider the impact of methodological assumptions, such as the discount rate or the choice of comparison used in the analysis.
A number of sensitivity analyses were carried out, as described in Chapter 2, to assess the uncertainty in our results to these data choices and assumptions, particularly around missing data for cost and QALY outcomes. The results of all deterministic sensitivity analyses undertaken are presented in Table 47. The data on the right-hand side of the table present the probability of cost-effectiveness of each strategy for each analysis undertaken, based on the net benefit statistic, for a £30,000 ceiling ratio of a decision-maker’s WTP for a QALY gained. All deterministic sensitivity analyses were carried out on the complete case data set.
| Analysis | Costs (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER (£/QALY) | P(CE) @WTP = £30,000/QALY gaina | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Biological graft | Standard repair | Synthetic mesh | Biological graft | Synthetic mesh vs. standard repair | Biological graft vs. standard repair | Synthetic mesh vs. standard repair | Biological graft vs. standard repair | Synthetic mesh vs. standard repair | Biological graft vs. standard repair | Standard repair | Synthetic mesh | Biological graft | |
| Base-case analysisb | 3664 | 4081 | 4165 | 1.569 | 1.643 | 1.582 | 337 (–73 to 747) | 555 (156 to 954) | 0.075 (–0 to 0.150) | 0.041 (–0.042 to 0.124) | 4493 | 13,537 | 0.05 | 0.84 | 0.12 |
| Costs and QALYs undiscountedb | 3680 | 4096 | 4178 | 1.596 | 1.672 | 1.610 | 335 (–81 to 751) | 553 (149 to 957) | 0.077 (0.000 to 0.153) | 0.042 (–0.042 to 0.127) | 4351 | 13,167 | 0.05 | 0.84 | 0.12 |
| Costs and QALYs discounted at 6% per annumb | 3654 | 4071 | 4156 | 1.550 | 1.624 | 1.563 | 339 (–67 to 745) | 556 (160 to 953) | 0.074 (0.000 to 0.148) | 0.040 (–0.041 to 0.122) | 4451 | 13,900 | 0.05 | 0.84 | 0.11 |
| Gamma regression models of cost, with log linkb | 3640 | 3978 | 4166 | 1.569 | 1.643 | 1.582 | 338 (–84 to 760) | 526 (123 to 928) | 0.075 (–0 to 0.151) | 0.041 (–0.043 to 0.125) | 4507 | 12,829 | 0.03 | 0.84 | 0.13 |
| Data from all Primary trial womenc | 3654 | 4029 | 4246 | 1.592 | 1.641 | 1.624 | 483 (214 to 751) | 541 (253 to 828) | 0.054 (0.004 to 0.104) | 0.056 (–0.003 to 0.115) | 8944 | 9661 | 0.02 | 0.51 | 0.47 |
Table 47 indicates that for the majority of scenario analyses undertaken, the conclusions remain unchanged, with substantial uncertainty regarding the most cost-effective treatment option. Including an analysis of all women in the Primary trial (i.e. RCT1A, RCT1B and RCT1C) gives broadly similar conclusions. Point estimates of the ICER increase but remain well below £20,000 for the comparison of synthetic mesh with standard repair. The ICER for the comparison of biological graft with standard repair falls. However, in terms of uncertainty, the probability that synthetic mesh is a cost-effective use of resources is lower for the analysis of all women (51%) than the base case (84%) at a £30,000 threshold value of WTP for a QALY gained. It is worth noting in this analysis that the probability of biological graft being the most cost-effective treatment strategy increases somewhat. This is due to a combination of factors, namely (1) higher and significant incremental costs for SM using all of the data; (2) less difference in point estimates of incremental QALYs compared with the base-case analysis. The net result is a slightly higher probability of biological graft being the most cost-effective treatment when compared against the base case. The analysis is not sufficiently different from the base case to change any of the conclusions drawn. In order to provide data for comparison with the trial-based clinical effectiveness analysis, we have presented the scatterplots and CEACs from this analysis for completeness in Figures 14 and 15. These results further illustrate the uncertainty in the 2-year data and emphasise that there is no clear cost-effective treatment strategy for prolapse repair based on trial data.
FIGURE 14.
Primary trial, RCT1: incremental scatterplot of cost-effectiveness plane – complete case analysis, 2-year outcomes, all randomised women to Primary trial.

FIGURE 15.
Primary trial, RCT1: CEAC – complete case analysis, 2-year outcomes, all randomised women to Primary trial.

Discussion
Summary of findings
For women requiring a primary prolapse surgery procedure, synthetic mesh and biological graft were both more costly procedures, driven by the cost of the mesh materials. There were no differences in costs of time or equipment to perform the respective procedures, nor were there any differences evident in terms of follow-up care required across groups. The estimated ICER for the 2-year follow-up from a NHS perspective was £4493 per QALY gained for synthetic mesh. Considering that society might be willing to pay up to £20,000 or £30,000 for a QALY gain, this result is favourable for synthetic mesh. Biological graft repair had a higher ICER, of £13,537 per QALY relative to standard repair, but was dominated (being more costly and generating fewer QALYs) compared with synthetic mesh. However, these potentially favourable ICERs for synthetic mesh should be interpreted in light of the uncertainty surrounding the results. Based on bootstrapped replications and calculated net benefit statistics, synthetic mesh had the greatest probability of cost-effectiveness at a threshold value of £30,000 WTP for a QALY gained (84%). This indicates that there is a 16% chance that one of the other treatment strategies may be the most cost-effective. There is thus some uncertainty in the data regarding the most cost-effective strategy.
A further level of uncertainty is explored through deterministic sensitivity analysis and, in particular, the impact of missing data on cost-effectiveness conclusions. Using MI models to address any bias or systematic patterns of missingness in the data indicates that mesh remains more costly but no longer generates potential QALY gains, and is thus not a cost-effective treatment strategy using imputed data sets. Overall, across the deterministic analyses undertaken, there is some uncertainty as to the cost-effectiveness of synthetic mesh ranging from 84% for the most favourable base-case analysis and falling to 51% when considering all randomised women who were having a primary repair (analysis comparable with statistical clinical effectiveness analysis), and falling further to 29% for the analysis imputing missing data.
Based on the uncertainty illustrated in the trial-based data and the potential impact of missing data on results, there is no strong evidence to suggest that synthetic mesh is a cost-effective use of resources. A complete case analysis is most favourable to synthetic mesh. Despite potentially favourable ICERs, the estimates are surrounded by considerable uncertainty and definitive conclusions cannot be drawn based on 2-year data alone.
Conclusions of the cost-effectiveness analysis are robust to changes in the model used to analyse the data. We explored a gamma family, log link regression model for costs, as both this and a normal distribution passed the modified Park’s test for distributional family. Furthermore, both models had similar AIC values, with the normal having only a slightly lower score, hence its choice for the base-case analysis. However, the conclusions remain broadly robust to the choice of analysis model for the data.
Strengths
A key strength of the study was the UK-wide multicentre design randomising women from 35 centres across the UK. This adds to the external validity and generalisability of the results UK wide. Including a full within-trial cost-effectiveness analysis is a key strength, although data may be of limited value in determining longer-term cost-effectiveness results. The main strength from the within-trial analysis is that a comprehensive microcosting approach was undertaken, further adding to the generalisability of results across participating centres. The incorporation of a wider economic perspective on costs as a secondary analysis adds value – in terms of a broader economic perspective and understanding of the non-health-care costs to women, their families and the economy – more generally of prolapse symptoms and problems. The analysis of QALYs based on EQ-5D-3L patient-level responses follows best practice methods and is another advantage, and will be informative for developing utility weights to populate the decision-analytic model reported in Chapter 9.
Limitations
Despite the strengths outlined, there were a number of limitations. First, the estimates of mesh costs are based on average prices across mesh categories and we make no statements about the cost-effectiveness of individual mesh products. This is an area requiring further research to determine if individual products provide better outcomes and more cost-effective treatment options for women. Second, there were some missing data for cost and QALY outcomes. MI of missing cost and EQ-5D-3L data were conducted. Imputation did not alter the cost estimates from the analysis, but did alter the QALY outcomes substantially, removing any potential benefit of mesh procedures. This adds further uncertainty to the trial-based economic analysis and renders it impossible to draw definitive conclusions on cost-effectiveness over a short 2-year time horizon. It should be noted that this short time horizon provides a further limitation, as it fails to address the cost and QoL impacts of any long-term complications or treatment failures and any differences in time to failure/time to experiencing serious complications following initial surgery.
Conclusion
To summarise, over 2-year follow-up, there was no strong evidence on the grounds of cost-effectiveness to recommend the adoption of either synthetic mesh or biological graft for women who were having their first prolapse procedure. There was some evidence that synthetic mesh may improve QoL outcomes but results were subject to biases associated with missing data, which may have over-represented the QALY gains and hence cost-effectiveness outcomes. The probability of cost-effectiveness varied substantially across analyses undertaken, further illustrating the uncertainty in the short-run cost-effectiveness results. It is likely, however, that the cost-effectiveness of mesh procedures will be determined over the longer term, where it will be possible to observe treatment failures, reoperations for prolapse and associated complications. As a result, Chapter 9 reports on the findings of a Markov cohort decision-analytic model, extrapolating these cost-effectiveness findings over 5 years and making initial projections of time to events such as surgery for prolapse failure and surgery for severe complications. Extended follow-up to 6 years will further provide an opportunity to validate the results of the economic modelling exercise.
Chapter 6 Results: Secondary trial (randomised controlled trial 2, comprehensive cohort 2)
This chapter describes the women who were having a repeat anterior or posterior prolapse repair, both those randomised (RCT2) and those who were not randomised but agreed to be followed up in the CC (CC2). The baseline characteristics of RCT2 and CC2 have been compared in Chapter 3; by and large, the populations were similar, suggesting that the findings from the randomised women are generalisable to the larger population of women who were having secondary prolapse repair in the UK.
The flow of women through the study is shown in the CONSORT diagram (Figure 16). The women received surgery in 35 centres across the UK (see Table 4). Although 154 women were randomised in total, they can be further subdivided according to the panel of operations offered by their surgeon. RCT2, therefore, consists of three strata: RCT2A, in which women could be randomly allocated to any of the three options for this trial; RCT2B, in which women were randomised between two options – standard repair with no mesh and synthetic mesh inlay; and RCT2D, in which they were randomised between no mesh and a mesh kit.
FIGURE 16.
CONSORT diagram for Secondary trial. a, Reasons for non-compliance with randomised allocation: standard – no prolapse surgery (0); no anterior or posterior repair (3); mesh not required (0); mesh required (3); morbidity/surgical complications (0); patient decided did not want mesh post randomisation (0); theatre not informed/wrong information given (0); mesh not available (0); not enough theatre time (0); consultant not in theatre (0); no reason given for non-compliance (1). b, Reasons for non-compliance with randomised allocation: synthetic – no prolapse surgery (0); no anterior or posterior repair (3); mesh not required (0); mesh required (0); morbidity/surgical complications (6); patient decided did not want mesh post randomisation (0); theatre not informed/wrong information given (1); mesh not available (0); not enough theatre time (1); consultant not in theatre (0); no reason given for non-compliance (3). c, Reasons for non-compliance with randomised allocation: mesh kit – no prolapse surgery (0); no anterior or posterior repair (3); mesh not required (0); mesh required (0); morbidity/surgical complications (2); patient decided did not want mesh post-randomisation (1); theatre not informed/wrong information given (0); mesh not available (7); not enough theatre time (0); consultant not in theatre (0); no reason given for non-compliance (1). d, Other surgery includes tape for UI, vaginal hysterectomy or suspension; cervical amputation; vault repair.

In this chapter, the data are presented according to the strata:
-
Trial 3 No mesh compared with synthetic mesh inlay [stratum 2A (three-way randomisation) and stratum 2B (two-way randomisation)].
-
Trial 4 No mesh compared with mesh kit [stratum 2A (three-way randomisation) and stratum 2D (two-way randomisation).
Because the analyses were carried out separately for each trial, some women in the ‘no mesh’ group from stratum 2A will be represented twice.
Baseline comparability of randomised groups
Women’s characteristics at baseline
There were no important differences between the randomised groups of women (trial 3, trial 4; Table 48) or between the randomised women in RCT2 and the non-randomised in CC2 (see Table 5). In this chapter, the data for the cohort women are provided in the outcome tables for comparison with the randomised groups, but they have not been formally statistically compared. Women who were having secondary repair were much less likely to agree to be randomised (39%) than those in the Primary trial (54%).
| Baseline characteristic | Trial 3: standard vs. synthetic | Trial 4: standard vs. mesh kit | CC2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Standard repair | Mesh kit | ||||||||||||
| Number of women | N = 55 | N = 52 | N = 25 | N = 46 | N = 244 | ||||||||||
| Age | 61.9 | (8.8) | 55 | 61.4 | (9.5) | 52 | 64.3 | (8.9) | 25 | 63.1 | (11.1) | 46 | 62.1 | (10.1) | 244 |
| Parity (mean) | 2.6 | (1.2) | 54 | 2.7 | (1.1) | 51 | 2.6 | (1.1) | 25 | 2.8 | (1.0) | 46 | 2.5 | (1.1) | 244 |
| Parity (median) | 2 | (1–6) | 54 | 3 | (1–8) | 51 | 2 | (1–6) | 25 | 3 | (1–5) | 46 | 2 | (0–8) | 244 |
| BMI (mean) | 29.5 | (5.2) | 52 | 28.6 | (4.2) | 43 | 29.0 | (4.2) | 25 | 29.1 | (5.2) | 38 | 28.7 | (4.8) | 220 |
| BMI (median) | 29 | (21–46) | 52 | 28 | (21–39) | 43 | 29 | (21–40) | 25 | 28 | (91–42) | 38 | 28 | (19–44) | 220 |
| Delivery mode history | |||||||||||||||
| Spontaneous vaginal delivery | 2.2 | (1.3) | 54 | 2.1 | (1.4) | 48 | 2.3 | (1.3) | 25 | 2.5 | (1.1) | 44 | 2.2 | (1.3) | 237 |
| Forceps | 0.2 | (0.5) | 54 | 0.4 | (0.6) | 48 | 0.2 | (0.4) | 25 | 0.1 | (0.4) | 44 | 0.2 | (0.5) | 237 |
| Breech | 0.1 | (0.3) | 54 | 0.0 | (0.2) | 48 | 0.1 | (0.3) | 25 | 0.0 | (0.2) | 44 | 0.0 | (0.2) | 237 |
| Elective caesarean | 0.0 | (0.0) | 54 | 0.0 | (0.2) | 48 | 0.0 | (0.0) | 25 | 0.0 | (0.0) | 44 | 0.0 | (0.1) | 237 |
| Emergency caesarean | 0.0 | (0.1) | 54 | 0.0 | (0.1) | 48 | 0.0 | (0.0) | 25 | 0.0 | (0.3) | 44 | 0.0 | (0.2) | 237 |
| Vacuum delivery | 0.0 | (0.1) | 54 | 0.0 | (0.1) | 48 | 0.0 | (0.2) | 25 | 0.0 | (0.0) | 44 | 0.0 | (0.1) | 237 |
| EQ-5D | |||||||||||||||
| Score | 0.64 | (0.30) | 52 | 0.75 | (0.16) | 50 | 0.76 | (0.18) | 24 | 0.69 | (0.24) | 42 | 0.65 | (0.26) | 213 |
| Previous conservative treatment | |||||||||||||||
| Current vaginal pessary | 9.3% | 5 | 54 | 3.8% | 2 | 52 | 12.5% | 3 | 24 | 14.0% | 6 | 43 | 9.9% | 24 | 242 |
| Physiotherapy for prolapse | 36.4% | 20 | 55 | 34.6% | 18 | 52 | 48.0% | 12 | 25 | 36.4% | 16 | 44 | 32.1% | 76 | 237 |
| Physiotherapy for UI | 14.5% | 8 | 55 | 13.7% | 7 | 51 | 16.0% | 4 | 25 | 22.2% | 10 | 45 | 16.4% | 39 | 238 |
| Drugs for UI | 10.9% | 6 | 55 | 11.5% | 6 | 52 | 16.0% | 4 | 25 | 18.2% | 8 | 44 | 16.4% | 39 | 238 |
| Previous surgery | |||||||||||||||
| Previous prolapse repair | 100.0% | 55 | 55 | 100.0% | 52 | 52 | 100.0% | 25 | 25 | 100.0% | 46 | 46 | 100.0% | 244 | 244 |
| Anterior | 81.8% | 45 | 55 | 71.2% | 37 | 52 | 88.0% | 22 | 25 | 91.3% | 42 | 46 | 86.1% | 210 | 244 |
| Posterior | 60.0% | 33 | 55 | 59.6% | 31 | 52 | 44.0% | 11 | 25 | 45.7% | 21 | 46 | 57.0% | 139 | 244 |
| Anterior and posterior | 41.8% | 23 | 55 | 30.8% | 16 | 52 | 32.0% | 8 | 25 | 37.0% | 17 | 46 | 43.0% | 105 | 244 |
| Vault | 7.3% | 4 | 55 | 9.6% | 5 | 52 | 0.0% | 0 | 25 | 13.0% | 6 | 46 | 14.8% | 36 | 244 |
| Unknown compartment | 1.8% | 1 | 55 | 0.0% | 0 | 52 | 4.0% | 1 | 25 | 2.2% | 1 | 46 | 1.2% | 3 | 244 |
| Hysterectomy | 68.5% | 37 | 54 | 63.5% | 33 | 52 | 2.4% | 13 | 24 | 52.2% | 24 | 46 | 73.0% | 178 | 244 |
| Vaginal | 40.7% | 22 | 54 | 38.5% | 20 | 52 | 33.3% | 8 | 24 | 28.3% | 13 | 46 | 44.7% | 109 | 244 |
| Cervical amputation | 5.6% | 3 | 54 | 9.6% | 5 | 52 | 8.3% | 2 | 24 | 10.9% | 5 | 46 | 7.0% | 17 | 244 |
| Abdominal | 27.8% | 15 | 54 | 25.0% | 13 | 52 | 20.8% | 5 | 24 | 23.9% | 11 | 46 | 27.0% | 66 | 244 |
| Continence surgery | 14.8% | 8 | 54 | 11.8% | 6 | 51 | 12.5% | 3 | 24 | 11.1% | 5 | 45 | 16.4% | 39 | 238 |
The mean ages of the randomised women ranged from 61.4 to 64.3 years (see Table 48), on average about 2.5 years older than the women who were having their first repair. The mean BMI was < 30 kg/m2 for all groups of women, but 10% had a BMI of > 35 kg/m2. The previous obstetric history was very similar to women who were having their first prolapse operation. All the women were parous. Most babies were born by spontaneous vaginal delivery.
Generic QoL was captured using the EQ-5D-3L validated tool. 25 Women in the synthetic mesh arm in trial 3 had a significantly higher (better) EQ-5D-3L score at baseline than those who were randomised to standard repair (t-test p = 0.024), whereas in trial 4 the score was lower in the mesh kit arm than the standard repair arm, but this was not significant (t-test p = 0.243; see Table 48).
Regarding previous treatment, 3.8–14.0% were using a vaginal pessary; 34.6–48.0% had already seen a physiotherapist for prolapse symptoms; rather fewer (13.7–22.2%) had seen a physiotherapist for UI; and more than 1 in 10 had used drugs for UI (see Table 48). Compared with the women who were having primary surgery, many more had already had a hysterectomy (63.5–68.5%) of which around 35% were carried out via the vaginal route, and 11.1–14.8% had already had continence surgery. All women had had previous anterior or posterior prolapse surgery and were presenting for repeat surgery in at least one of those compartments.
Preoperative prolapse measurements
Women in the randomised groups were comparable in terms of the maximum descent of the three different prolapse compartments. Using qualitative descriptions of prolapse stage to supplement missing POP-Q data, all randomised women were found to have a prolapse stage 2 or greater before surgery (Table 49). For the women who had a quantitative score measured using the POP-Q system, women were found to have the leading edge of the prolapse outside the hymen (> 0 cm) most often in the groups randomised to mesh inlay (54.0%) or mesh kit (65.9%) compared with the standard repair groups (38.9%, 50.0%, respectively).
| POP-Q measurement/stage | Trial 3: standard vs. synthetic | Trial 4: standard vs. mesh kit | CC2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Standard repair | Mesh kit | ||||||||||||
| Number of women | N = 55 | N = 52 | N = 25 | N = 46 | N = 244 | ||||||||||
| POP-Q measurement (cm) | |||||||||||||||
| Ba (anterior edge) | –0.2 | (1.8) | 54 | –0.1 | (2.0) | 48 | 0.1 | (2.1) | 24 | 0.7 | (2.1) | 40 | 0.3 | (2.1) | 201 |
| C (cervix/vault) | –4.3 | (2.5) | 52 | –4.4 | (3.2) | 45 | –3.8 | (2.3) | 22 | –3.3 | (3.9) | 37 | –3.3 | (3.5) | 191 |
| Bp (posterior edge) | –0.5 | (1.8) | 52 | –0.5 | (1.9) | 49 | –0.4 | (2.1) | 23 | –0.5 | (2.4) | 38 | –0.3 | (2.1) | 194 |
| TVL | 8.1 | (3.3) | 50 | 8.3 | (1.4) | 42 | 7.6 | (1.3) | 21 | 8.0 | (1.2) | 36 | 8.0 | (1.5) | 179 |
| Overall POP-Q stage | |||||||||||||||
| 0 | 0.0% | 0 | 55 | 0.0% | 0 | 52 | 0.0% | 0 | 25 | 0.0% | 0 | 45 | 0.4% | 1 | 227 |
| 1 | 0.0% | 0 | 55 | 0.0% | 0 | 52 | 0.0% | 0 | 25 | 0.0% | 0 | 45 | 0.9% | 2 | 227 |
| 2 | 76.4% | 42 | 55 | 71.2% | 37 | 52 | 68.0% | 17 | 25 | 55.6% | 25 | 45 | 60.8% | 138 | 227 |
| 3 | 21.8% | 12 | 55 | 28.8% | 15 | 52 | 28.0% | 7 | 25 | 42.2% | 19 | 45 | 31.3% | 71 | 227 |
| 4 | 1.8% | 1 | 55 | 0.0% | 0 | 52 | 4.0% | 1 | 25 | 2.2% | 1 | 45 | 6.6% | 15 | 227 |
| 2b, 3 or 4 | 38.9% | 21 | 54 | 54.0% | 27 | 50 | 50.0% | 12 | 24 | 65.9% | 27 | 41 | 57.8% | 119 | 206 |
Prolapse symptoms at baseline
Women had noticed symptoms of prolapse for 2.6–3.5 years in total, and had been bothered for 2.2–2.7 years before surgery (Table 50). The POP-SS is composed of seven individual prolapse symptoms (each scored from 0 to 4, where ‘0’ is never and ‘4’ is all the time; see Chapter 2). At baseline, the mean POP-SS ranged from 13.5 to 15.3 out of a maximum score of 28 (see Table 50). All women were deemed to be symptomatic using the criterion of scoring at least one on the Pelvic Organ Prolapse Symptom scale (apart from one woman in CC2). The most common symptom was ‘a feeling of something coming down from or in the vagina’ and > 90% of women reported this at least occasionally, whereas > 50% had a visible prolapse outside the hymen (see Table 50).
| Symptom | Trial 3: standard vs. synthetic | Trial 4: standard vs. mesh kit | CC2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Standard repair | Mesh kit | ||||||||||||
| Number of women | N = 54 | N = 50 | N = 24 | N = 43 | N = 221 | ||||||||||
| POP-SS | 14.3 | (5.4) | 54 | 13.7 | (5.1) | 50 | 13.5 | (5.7) | 24 | 15.3 | (5.4) | 43 | 14.9 | (5.8) | 220 |
| Other measures of prolapse symptoms | |||||||||||||||
| Duration of symptoms (years) | 2.6 | (3.1) | 53 | 2.8 | (3.9) | 49 | 3.5 | (4.2) | 24 | 3.1 | (2.6) | 39 | 3.8 | (5.3) | 205 |
| Duration of bother (years) | 2.2 | (2.9) | 52 | 2.3 | (3.6) | 48 | 2.4 | (3.9) | 24 | 2.7 | (2.5) | 39 | 2.9 | (4.1) | 201 |
| Number of women symptomatic | 100.0% | 54 | 54 | 100.0% | 50 | 50 | 100.0% | 24 | 24 | 100.0% | 43 | 43 | 99.5% | 219 | 220 |
| Prolapse-related QoL score | 6.5 | (2.6) | 54 | 7.1 | (2.3) | 50 | 5.5 | (2.9) | 24 | 7.2 | (1.9) | 43 | 6.9 | (2.5) | 216 |
| Individual prolapse symptoms | |||||||||||||||
| SCD any | 90.7% | 49 | 54 | 92.0% | 46 | 50 | 87.5% | 21 | 24 | 100.0% | 43 | 43 | 94.5% | 208 | 220 |
| SCD freq. | 68.5% | 37 | 54 | 70.0% | 35 | 50 | 58.3% | 14 | 24 | 81.4% | 35 | 43 | 71.4% | 157 | 220 |
| Pain any | 77.8% | 42 | 54 | 78.0% | 39 | 50 | 87.5% | 21 | 24 | 86.0% | 37 | 43 | 85.9% | 189 | 220 |
| Pain freq. | 31.5% | 17 | 54 | 34.0% | 17 | 50 | 29.2% | 7 | 24 | 46.5% | 20 | 43 | 45.0% | 99 | 220 |
| Abdo. any | 83.3% | 45 | 54 | 80.0% | 40 | 50 | 79.2% | 19 | 24 | 88.4% | 38 | 43 | 85.9% | 189 | 220 |
| Abdo. freq. | 35.2% | 19 | 54 | 32.0% | 16 | 50 | 37.5% | 9 | 24 | 46.5% | 20 | 43 | 45.0% | 99 | 220 |
| Back any | 77.8% | 42 | 54 | 70.0% | 35 | 50 | 83.3% | 20 | 24 | 72.1% | 31 | 43 | 82.7% | 182 | 220 |
| Back freq. | 40.7% | 22 | 54 | 32.0% | 16 | 50 | 33.3% | 8 | 24 | 30.2% | 13 | 43 | 34.1% | 75 | 220 |
| Strain blad. any | 74.1% | 40 | 54 | 62.0% | 31 | 50 | 75.0% | 18 | 24 | 76.7% | 33 | 43 | 67.7% | 149 | 220 |
| Strain blad. freq. | 22.2% | 12 | 54 | 30.0% | 15 | 50 | 20.8% | 5 | 24 | 27.9% | 12 | 43 | 29.5% | 65 | 220 |
| Blad. not empty any | 88.9% | 48 | 54 | 76.0% | 38 | 50 | 91.7% | 22 | 24 | 90.7% | 39 | 43 | 82.7% | 182 | 220 |
| Blad. not empty freq. | 42.6% | 23 | 54 | 34.0% | 17 | 50 | 37.5% | 9 | 24 | 48.8% | 21 | 43 | 35.9% | 79 | 220 |
| Bowel not empty any | 81.5% | 44 | 54 | 88.0% | 44 | 50 | 70.8% | 17 | 24 | 86.0% | 37 | 43 | 85.0% | 187 | 220 |
| Bowel not empty freq. | 35.2% | 19 | 54 | 40.0% | 20 | 50 | 25.0% | 6 | 24 | 25.6% | 11 | 43 | 43.2% | 95 | 220 |
| Actions necessitated by prolapse symptoms | |||||||||||||||
| Fingers to ease discomfort | 18.9% | 10 | 53 | 14.0% | 7 | 50 | 16.7% | 4 | 24 | 14.3% | 6 | 42 | 16.0% | 34 | 213 |
| Extra hygiene measures | 54.9% | 28 | 51 | 48.0% | 24 | 50 | 52.2% | 12 | 23 | 51.2% | 21 | 41 | 56.1% | 120 | 214 |
| Fingers to help empty bladder | 5.7% | 3 | 53 | 2.0% | 1 | 50 | 12.5% | 3 | 24 | 4.7% | 2 | 43 | 3.7% | 8 | 214 |
| Fingers to help empty bowel | 5.7% | 3 | 53 | 8.0% | 4 | 50 | 4.2% | 1 | 24 | 2.4% | 1 | 41 | 8.3% | 18 | 217 |
| Digital evacuation of bowel | 9.6% | 5 | 52 | 6.1% | 3 | 49 | 8.3% | 2 | 24 | 2.4% | 1 | 42 | 5.1% | 11 | 215 |
As well as the groups being comparable at baseline for the overall score, there were no systematic differences between the groups in any individual prolapse symptoms or other measures of the effect of prolapse on QoL or in modifying women’s behaviour to ameliorate the effects of prolapse (see Table 50).
Urinary symptoms at baseline
The urinary symptoms reported by women were captured using a variety of validated questionnaires and scales from the ICI Modular Questionnaire suite. 26 Around four in five women had at least some urinary leakage, and this was severe for one in five (Table 51).
| Symptom | Trial 3: standard vs. synthetic | Trial 4: standard vs. mesh kit | CC2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Standard repair | Mesh kit | ||||||||||||
| Number of women | N = 54 | N = 50 | N = 24 | N = 43 | N = 221 | ||||||||||
| Any incontinence | 83.0% | 44 | 53 | 76.0% | 38 | 50 | 83.3% | 20 | 24 | 83.7% | 36 | 43 | 74.7% | 165 | 221 |
| Incontinence-related QoL score | 7.3 | (5.2) | 53 | 7.2 | (5.8) | 49 | 6.2 | (4.2) | 24 | 8.4 | (5.9) | 43 | 7.0 | (5.8) | 220 |
| Severe incontinence | 15.1% | 8 | 53 | 20.4% | 10 | 49 | 8.3% | 2 | 24 | 25.6% | 11 | 43 | 20.0% | 44 | 220 |
| ICIQ-UI-SF score | 3.6 | (3.2) | 52 | 3.6 | (3.5) | 50 | 2.6 | (3.0) | 24 | 4.3 | (3.5) | 42 | 3.5 | (3.3) | 211 |
| Stress UI | 23.5% | 12 | 51 | 20.0% | 9 | 45 | 16.7% | 4 | 24 | 14.6% | 6 | 41 | 18.8% | 36 | 192 |
| Urgency UI | 7.5% | 4 | 53 | 8.0% | 4 | 50 | 4.2% | 1 | 24 | 16.3% | 7 | 43 | 10.7% | 23 | 215 |
| Overactive bladder | 3.8% | 2 | 53 | 6.0% | 3 | 50 | 4.2% | 1 | 24 | 11.6% | 5 | 43 | 9.0% | 19 | 211 |
| ICIQ-FLUTS filling score | 6.0 | (2.9) | 52 | 5.6 | (3.1) | 50 | 5.6 | (2.3) | 23 | 6.3 | (2.8) | 43 | 6.0 | (3.1) | 208 |
| ICIQ-FLUTS voiding score | 3.3 | (2.6) | 53 | 3.6 | (2.8) | 50 | 2.7 | (2.1) | 23 | 3.3 | (2.7) | 43 | 3.2 | (2.8) | 212 |
| ICIQ-FLUTS incontinence score | 5.8 | (4.0) | 49 | 5.8 | (4.2) | 45 | 4.8 | (3.2) | 23 | 6.4 | (4.3) | 41 | 6.2 | (4.3) | 190 |
There were no systematic differences between the groups but urinary symptoms were common in women with secondary prolapse (e.g. around 80% reported some UI). This emphasises the importance of taking into consideration concomitant symptoms when treating women whose primary complaint is prolapse.
Bowel symptoms at baseline
There were no systematic differences between the groups in terms of frequency of bowel movements, constipation, bowel urgency or FI, or in the effect bowel symptoms had on QoL (Table 52). Around one-quarter of the women had constipation (classified according to the ROME30 criteria). Two in five women reported FI at least occasionally, whereas around 1 in 10 had severe FI (sometimes or more often; see Table 52).
| Symptoms | Trial 3: standard vs. synthetic | Trial 4: standard vs. mesh kit | CC2 (N = 221 women) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair (N = 54 women) | Synthetic mesh (N = 50 women) | Standard repair (N = 24 women) | Mesh kit (N = 43 women) | ||||||||||||
| Bowel frequency | |||||||||||||||
| > 3 times a day | 2.2% | 1 | 45 | 5.1% | 2 | 39 | 4.8% | 1 | 21 | 4.9% | 2 | 41 | 3.2% | 6 | 188 |
| 1–3 times a day | 37.8% | 17 | 45 | 30.8% | 12 | 39 | 28.6% | 6 | 21 | 24.4% | 10 | 41 | 33.5% | 63 | 188 |
| About once a day | 35.6% | 16 | 45 | 35.9% | 14 | 39 | 42.9% | 9 | 21 | 48.8% | 20 | 41 | 45.2% | 85 | 188 |
| Once every 2–3 days | 20.0% | 9 | 45 | 23.1% | 9 | 39 | 19.0% | 4 | 21 | 17.1% | 7 | 41 | 14.9% | 28 | 188 |
| Weekly or less | 4.4% | 2 | 45 | 5.1% | 2 | 39 | 4.8% | 1 | 21 | 4.9% | 2 | 41 | 3.2% | 6 | 188 |
| Constipation | 23.1% | 12 | 52 | 36.0% | 18 | 50 | 13.0% | 3 | 23 | 14.3% | 6 | 42 | 31.0% | 66 | 213 |
| Bowel urgency | 11.3% | 6 | 53 | 12.0% | 6 | 50 | 4.3% | 1 | 23 | 4.8% | 2 | 42 | 8.6% | 19 | 220 |
| FI (any) | 37.0% | 20 | 54 | 40.0% | 20 | 50 | 37.5% | 9 | 24 | 40.5% | 17 | 42 | 36.4% | 80 | 220 |
| Passive FI | 75.0% | 15 | 20 | 80.0% | 16 | 20 | 88.9% | 8 | 9 | 94.1% | 16 | 17 | 81.3% | 65 | 80 |
| Active FI | 25.0% | 5 | 20 | 20.0% | 4 | 20 | 11.1% | 1 | 9 | 5.9% | 1 | 17 | 18.8% | 15 | 80 |
| Severe FI | 13.0% | 7 | 54 | 12.0% | 6 | 50 | 16.7% | 4 | 24 | 7.1% | 3 | 42 | 11.4% | 25 | 220 |
| Bowel symptoms QoL score | 3.4 | (3.1) | 50 | 3.8 | (3.4) | 48 | 2.9 | (3.4) | 21 | 3.9 | (2.9) | 42 | 3.6 | (3.1) | 212 |
Vaginal and sexual symptoms at baseline
We used the validated ICIQ-VS and the ICIQ Sexual Matters instruments to capture aspects of vaginal and sexual function. 26 About two-thirds of the women were not sexually active (Table 53); of these, over one-third did not have sexually active partners, and the most common reason in the remainder was attributable to their prolapse symptoms. Only six of the randomised women who answered the question reported pain with intercourse (dyspareunia) before surgery (and a further 15 in the non-randomised cohort; see Table 53). There were no systematic differences between the groups in terms of these parameters at baseline.
| Symptom | Trial 3: standard vs. synthetic | Trial 4: standard vs. mesh kit | CC2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Standard repair | Mesh kit | ||||||||||||
| Number of women | N = 54 | N = 50 | N = 24 | N = 43 | N = 221 | ||||||||||
| Vaginal | |||||||||||||||
| ICIQ-VS score | 21.1 | (10.0) | 49 | 22.2 | (9.5) | 46 | 17.5 | (9.2) | 23 | 23.4 | (7.8) | 38 | 23.8 | (9.6) | 188 |
| Vaginal symptoms QoL score | 4.9 | (3.5) | 51 | 5.4 | (3.4) | 47 | 3.9 | (3.4) | 23 | 5.7 | (3.0) | 40 | 5.4 | (3.2) | 204 |
| Vagina too tight | 3.8% | 2 | 52 | 0.0% | 0 | 47 | 0.0% | 0 | 23 | 2.6% | 1 | 39 | 5.6% | 11 | 197 |
| Sexual | |||||||||||||||
| Sex life at present (yes) | 30.2% | 16 | 53 | 52.0% | 26 | 50 | 25.0% | 6 | 24 | 34.1% | 14 | 41 | 29.9% | 64 | 214 |
| Reason for no sex life | |||||||||||||||
| No partner | 29.7% | 11 | 37 | 41.7% | 10 | 24 | 38.9% | 7 | 18 | 37.0% | 10 | 27 | 27.3% | 41 | 150 |
| Vaginal symptoms | 2.7% | 1 | 37 | 0.0% | 0 | 24 | 0.0% | 0 | 18 | 0.0% | 0 | 27 | 5.3% | 8 | 150 |
| Prolapse symptoms | 43.2% | 16 | 37 | 29.2% | 7 | 24 | 33.3% | 6 | 18 | 33.3% | 9 | 27 | 38.7% | 58 | 150 |
| Other reason | 18.9% | 7 | 37 | 25.0% | 6 | 24 | 22.2% | 4 | 18 | 25.9% | 7 | 27 | 24.7% | 37 | 150 |
| Reason not given | 5.4% | 2 | 37 | 4.2% | 1 | 24 | 5.6% | 1 | 18 | 3.7% | 1 | 27 | 4.0% | 6 | 150 |
| Dyspareunia | 4.5% | 1 | 22 | 15.6% | 5 | 32 | 0.0% | 0 | 8 | 0.0% | 0 | 20 | 15.5% | 15 | 97 |
| ICI Sexual Matters score | 23.1 | (13.7) | 21 | 25.7 | (12.5) | 32 | 20.6 | (13.8) | 7 | 27.3 | (12.8) | 20 | 24.4 | (15.5) | 95 |
| Sex life QoL score | 7.1 | (3.4) | 24 | 7.2 | (2.6) | 32 | 6.1 | (3.3) | 9 | 8.0 | (1.8) | 22 | 6.8 | (3.3) | 112 |
Surgery planned before surgery and actually received during surgery
Planned operations
The most common operation (anticipated for about half of the women) was anterior repair only, with about one-quarter planning to have a posterior repair only, whereas a further quarter were having both. Planned concomitant surgery included 8.0–21.7% of women who were thought to need a vaginal hysterectomy, and a further 19.2–28.0% requiring a vault repair. Finally, 2.2–7.3% were thought to require a continence procedure.
Surgery actually received
Most women received the surgery planned (see Figure 16 and Table 54). In trial 3, in the standard repair group, more women (27.3%) had a combined anterior/posterior repair than in the other two groups (synthetic mesh 13.7% and mesh kit 13.3%) and more had a concomitant vault repair (25.5% compared with 9.8% and 13.3%, respectively) (see Table 54). It is possible that knowledge of the allocated intervention influenced the surgery actually carried out, but the numbers were too small to draw definite conclusions.
| Type of surgery | Trial 3: standard vs. synthetic | Trial 4: standard vs. mesh kit | CC2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Standard repair | Mesh kit | ||||||||||||
| Planned surgery | |||||||||||||||
| Number of women | N = 55 | N = 52 | N = 25 | N = 46 | N = 244 | ||||||||||
| Prolapse procedure | |||||||||||||||
| Anterior repair | 43.6% | 24 | 55 | 44.2% | 23 | 52 | 56.0% | 14 | 25 | 52.2% | 24 | 46 | 45.9% | 112 | 244 |
| Posterior repair | 25.5% | 14 | 55 | 28.8% | 15 | 52 | 20.0% | 5 | 25 | 23.9% | 11 | 46 | 26.6% | 65 | 244 |
| Anterior and posterior repair | 27.3% | 15 | 55 | 13.7% | 7 | 51 | 20.0% | 5 | 25 | 13.3% | 6 | 45 | 22.1% | 53 | 240 |
| Upper compartment repair only | 0.0% | 0 | 55 | 0.0% | 0 | 52 | 0.0% | 0 | 25 | 0.0% | 0 | 46 | 0.0% | 0 | 244 |
| Concomitant prolapse procedure | |||||||||||||||
| Vaginal hysterectomy | 9.1% | 5 | 55 | 11.5% | 6 | 52 | 8.0% | 2 | 25 | 21.7% | 10 | 46 | 6.6% | 16 | 244 |
| Cervical amputation | 0.0% | 0 | 55 | 0.0% | 0 | 52 | 0.0% | 0 | 25 | 0.0% | 0 | 46 | 0.8% | 2 | 244 |
| Abdominal hysterectomy | 0.0% | 0 | 55 | 1.9% | 1 | 52 | 0.0% | 0 | 25 | 0.0% | 0 | 46 | 0.8% | 2 | 244 |
| Vault repair | 27.3% | 15 | 55 | 19.2% | 10 | 52 | 28.0% | 7 | 25 | 21.7% | 10 | 46 | 27.0% | 66 | 244 |
| Continence procedure | 7.3% | 4 | 55 | 3.8% | 2 | 52 | 4.0% | 1 | 25 | 2.2% | 1 | 46 | 6.6% | 16 | 244 |
| Surgery actually performed | |||||||||||||||
| Number of women | N = 55 | N = 51 | N = 25 | N = 45 | N = 240 | ||||||||||
| Actual prolapse procedure | |||||||||||||||
| Anterior repair only | 38.2% | 21 | 55 | 49.0% | 25 | 51 | 44.0% | 11 | 25 | 51.1% | 23 | 45 | 43.8% | 105 | 240 |
| Posterior repair only | 29.1% | 16 | 55 | 31.4% | 16 | 51 | 24.0% | 6 | 25 | 31.1% | 14 | 45 | 26.7% | 64 | 240 |
| Anterior and posterior repair | 27.3% | 15 | 55 | 13.7% | 7 | 51 | 20.0% | 5 | 25 | 13.3% | 6 | 45 | 22.1% | 53 | 240 |
| Neither | 5.5% | 3 | 55 | 5.9% | 3 | 51 | 12.0% | 3 | 25 | 4.4% | 2 | 45 | 7.5% | 18 | 240 |
| Concomitant prolapse procedure | |||||||||||||||
| Vaginal hysterectomy | 7.3% | 4 | 55 | 7.8% | 4 | 51 | 12.0% | 3 | 25 | 8.9% | 4 | 45 | 7.1% | 17 | 240 |
| Abdominal hysterectomy | 0.0% | 0 | 55 | 0.0% | 0 | 51 | 0.0% | 0 | 25 | 0.0% | 0 | 45 | 0.4% | 1 | 240 |
| Cervical amputation | 1.8% | 1 | 55 | 2.0% | 1 | 51 | 4.0% | 1 | 25 | 0.0% | 0 | 45 | 1.3% | 3 | 240 |
| Uterine suspension | 0.0% | 0 | 55 | 0.0% | 0 | 51 | 0.0% | 0 | 25 | 4.4% | 2 | 45 | 3.8% | 9 | 240 |
| Vault repair | 25.5% | 14 | 55 | 9.8% | 5 | 51 | 28.0% | 7 | 25 | 13.3% | 6 | 45 | 26.3% | 63 | 240 |
| Continence procedure | 7.3% | 4 | 55 | 3.9% | 2 | 51 | 8.0% | 2 | 25 | 0.0% | 0 | 45 | 6.7% | 16 | 240 |
Compliance with randomised allocation
Two women did not receive surgery at all (see Figure 16). Ten women were not thought to need an anterior or posterior repair once anaesthetised because the surgeon did not deem it necessary, and therefore they were unable to carry out the randomised allocation (see Figure 16).
Three women randomised to standard repair alone received a mesh inlay because the surgeon thought it was clinically indicated, but none had a mesh kit. In trial 3, a further 14/51 women failed to receive their allocated (randomised) intervention in the synthetic mesh arm: this was most often due to surgical complications. Similarly in trial 4 a further 14/45 women did not receive their allocated mesh kit, most often (n = 7) because the kit was not available on the day of surgery (see Figure 16).
Summary
Overall there were no important differences in clinical characteristics at baseline between the women randomised to the two groups in trial 3 (standard repair vs. synthetic mesh inlay) or in trial 4 (standard repair vs. mesh kit).
Outcomes
Description of surgery and operative characteristics
There were no statistically significant differences in the duration of surgery (Table 55). Blood loss was higher in the synthetic mesh group (by 36.8 ml), but not significantly so in the mesh kit group, compared with standard repair. The mean length of stay ranged from 2.4 to 3.0 days, with no differences between the randomised groups (see Table 55). This time included any preoperative days if the women were admitted a day before surgery.
| Surgical characteristic | Trial 3: standard vs. synthetic | Trial 4: standard vs. mesh kit | CC2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Synthetic mesh | Standard repair | Mesh kit | ||||||||||||
| Received surgery | N = 55 | N = 51 | N = 25 | N = 45 | N = 240 | ||||||||||
| Grade of staff | |||||||||||||||
| Consultant | 78.2% | 43 | 55 | 84.0% | 42 | 50 | 76.0% | 19 | 25 | 93.3% | 42 | 45 | 85.8% | 206 | 240 |
| Specialty doctor | 9.1% | 5 | 55 | 6.0% | 3 | 50 | 4.0% | 1 | 25 | 4.4% | 2 | 45 | 3.8% | 9 | 240 |
| Specialty doctor supervised | 100.0% | 5 | 5 | 100.0% | 3 | 3 | 100.0% | 1 | 1 | 50.0% | 1 | 2 | 57.1% | 4 | 7 |
| Registrar/junior | 12.7% | 7 | 55 | 10.0% | 5 | 50 | 20.0% | 5 | 25 | 2.2% | 1 | 45 | 10.4% | 25 | 240 |
| Registrar/junior supervised | 85.7% | 6 | 7 | 100.0% | 5 | 5 | 80.0% | 4 | 5 | 100.0% | 1 | 1 | 87.5% | 21 | 24 |
| Duration (minutes) | 70.4 | (28.7) | 55 | 74.0 | (23.7) | 48 | 66.7 | (33.5) | 25 | 76.2 | (28.3) | 45 | 82.7 | (40.8) | 234 |
| Estimated blood loss (ml) | 108.4 | (56.9) | 51 | 145.2 | (103.3) | 48 | 107.5 | (66.5) | 24 | 126.6 | (101.1) | 41 | 116.1 | (112.4) | 223 |
| Length of stay (days) | 2.6 | (2.0) | 54 | 3.0 | (2.2) | 49 | 2.4 | (1.5) | 25 | 2.8 | (1.8) | 45 | 2.6 | (1.4) | 240 |
| Type of anaesthetic | |||||||||||||||
| General | 81.8% | 45 | 55 | 82.4% | 42 | 51 | 72.0% | 18 | 25 | 84.4% | 38 | 45 | 83.1% | 197 | 237 |
| Spinal | 21.8% | 12 | 55 | 17.6% | 9 | 51 | 28.0% | 7 | 25 | 15.6% | 7 | 45 | 20.3% | 48 | 237 |
| Local | 9.1% | 5 | 55 | 2.0% | 1 | 51 | 16.0% | 4 | 25 | 11.1% | 5 | 45 | 5.1% | 12 | 237 |
| Prophylactic antibiotic | 96.4% | 53 | 55 | 94.0% | 47 | 50 | 92.0% | 23 | 25 | 91.1% | 41 | 45 | 95.2% | 220 | 231 |
| Vaginal pack inserted | 80.8% | 42 | 52 | 84.0% | 42 | 50 | 73.9% | 17 | 23 | 88.9% | 40 | 45 | 73.6% | 170 | 231 |
| Catheter inserted | 90.9% | 50 | 55 | 94.1% | 48 | 51 | 84.0% | 21 | 25 | 100.0% | 45 | 45 | 94.5% | 225 | 238 |
| Suprapubic | 0.0% | 0 | 50 | 0.0% | 0 | 48 | 4.8% | 1 | 21 | 4.4% | 2 | 45 | 2.2% | 5 | 224 |
| Urethral | 100.0% | 50 | 50 | 100.0% | 48 | 48 | 95.2% | 20 | 21 | 95.6% | 43 | 45 | 97.3% | 218 | 224 |
| Both | 0.0% | 0 | 50 | 0.0% | 0 | 48 | 0.0% | 0 | 21 | 0.0% | 0 | 45 | 0.4% | 1 | 224 |
| Surgical characteristic | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| Number of women who received surgery | N = 55 | N = 51 | N = 25 | N = 45 | N = 240 | ||||||||||||||||
| Duration of surgery (minutes) | 70.4 | (28.7) | 55 | 74.0 | (23.7) | 48 | 3.65 | –5.73 to 13.03 | 0.440 | 66.7 | (33.5) | 25 | 76.2 | (28.3) | 45 | 8.28 | –6.05 to 22.61 | 0.250 | 82.7 | (40.8) | 234 |
| Length of stay | 2.6 | (2.0) | 54 | 3.0 | (2.2) | 49 | 0.34 | –0.44 to 1.13 | 0.385 | 2.4 | (1.5) | 25 | 2.8 | (1.8) | 45 | 0.51 | –0.18 to 1.20 | 0.145 | 2.6 | (1.4) | 240 |
| Blood loss (ml) | 108.4 | (56.9) | 51 | 145.2 | (103.3) | 48 | 38.80 | 7.3 to 70.3 | 0.017 | 107.5 | (66.5) | 24 | 126.6 | (101.1) | 41 | 27.6 | –9.3 to 64.4 | 0.138 | 116.1 | (112.4) | 223 |
Serious related adverse effects in first and second years
The diagnoses in Tables 57 and 58 are confined to those that met our definition of ‘serious’ (see Chapter 2). An adverse effect (AE) was defined as ‘serious’ (SAE) if it was related to prolapse surgery and resulted in death; was life-threatening; required hospitalisation or prolongation of an existing admission; resulted in significant disability/incapacity; or was otherwise considered medically significant by the investigator. If it did not meet the requirement for ‘serious’ it was classed as ‘other’.
| Adverse effect | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| Number of women at 1 year | N = 55 | N = 52 | N = 25 | N = 46 | N = 244 | ||||||||||||||||
| Intraoperative complications | |||||||||||||||||||||
| Injury to organs | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Excess blood loss | 0.0% | 0 | 55 | 1.9% | 1 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Blood transfusion | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Anaesthetic complications | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Death | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Serious adverse effects in first year | |||||||||||||||||||||
| Thrombosis | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Infection | 5.5% | 3 | 55 | 3.8% | 2 | 52 | 0.70 | 0.12 to 3.96 | 0.686 | 4.0% | 1 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 2.9% | 7 | 244 |
| Pain | 7.3% | 4 | 55 | 1.9% | 1 | 52 | 0.28 | 0.03 to 2.35 | 0.239 | 8.0% | 2 | 25 | 2.2% | 1 | 46 | 0.20 | 0.02 to 2.04 | 0.176 | 1.2% | 3 | 244 |
| Urinary retention | 3.6% | 2 | 55 | 1.9% | 1 | 52 | 0.50 | 0.05 to 5.25 | 0.562 | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 1.2% | 3 | 244 |
| Bowel obstruction | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Constipation | 0.0% | 0 | 55 | 1.9% | 1 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Excess blood loss | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Vaginal adhesions | 0.0% | 0 | 55 | 1.9% | 1 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Haematoma | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 2.0% | 5 | 244 |
| Skin tags | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Granulation tissue | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Urinary tract symptoms | 0.0% | 0 | 55 | 1.9% | 1 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.8% | 2 | 244 |
| Death | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Any serious adverse effects (excluding mesh exposure) | 12.7% | 7 | 55 | 9.6% | 5 | 52 | 1.05 | 0.66 to 1.68 | 0.831 | 12.0% | 3 | 25 | 6.5% | 3 | 46 | 0.49 | 0.11 to 2.16 | 0.345 | 7.8% | 19 | 244 |
| Number of women at 2 years | N = 55 | N = 52 | N = 25 | N = 46 | N = 244 | ||||||||||||||||
| Serious adverse effects in second year | |||||||||||||||||||||
| Thrombosis | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Infection | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Pain | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Urinary retention | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Bowel obstruction | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Constipation | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Excess blood loss | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Vaginal adhesions | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Haematoma | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Skin tags | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Granulation tissue | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Urinary tract symptoms | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Death | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Any serious adverse effects (excluding mesh exposure) | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 4.3% | 2 | 46 | N/A | N/A | N/A | 1.2% | 3 | 244 |
| Adverse effect | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| Number of women | N = 55 | N = 52 | N = 25 | N = 46 | N = 244 | ||||||||||||||||
| Intraoperative complications | |||||||||||||||||||||
| Injury to organs | 0.0% | 0 | 55 | 1.9% | 1 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Excess blood loss | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Blood transfusion | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Anaesthetic complication | 0.0% | 0 | 55 | 1.9% | 1 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Other adverse effects in first year | |||||||||||||||||||||
| Thrombosis | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 2.5% | 0 | 244 |
| Infection | 3.6% | 2 | 55 | 7.7% | 4 | 52 | 2.12 | 0.41 to 10.87 | 0.367 | 4.0% | 1 | 25 | 2.2% | 1 | 46 | 0.61 | 0.04 to 9.08 | 0.718 | 0.8% | 6 | 244 |
| Pain | 1.8% | 1 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 2 | 244 |
| Urinary retention | 3.6% | 2 | 55 | 1.9% | 1 | 52 | 0.53 | 0.05 to 5.58 | 0.600 | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Bowel obstruction | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Constipation | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Excess blood loss | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.8% | 0 | 244 |
| Vaginal adhesions | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.4% | 2 | 244 |
| Haematoma | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 1 | 244 |
| Skin tags | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Granulation tissue | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 0 | 244 |
| Urinary tract symptoms | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 1 | 244 |
| Any other adverse effects (excluding mesh exposure) | 9.1% | 5 | 55 | 13.5% | 7 | 52 | 1.23 | 0.43 to 3.46 | 0.701 | 4.0% | 1 | 25 | 4.3% | 2 | 46 | 1.16 | 0.12 to 11.77 | 0.897 | 0.4% | 12 | 244 |
| Number of women | N = 55 | N = 52 | N = 25 | N = 46 | N = 244 | ||||||||||||||||
| Other adverse effects in second year | |||||||||||||||||||||
| Thrombosis | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Infection | 0.0% | 0 | 55 | 1.9% | 1 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Pain | 0.0% | 0 | 55 | 1.9% | 1 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Urinary retention | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Bowel obstruction | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 2.2% | 1 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Constipation | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Excess blood loss | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Vaginal adhesions | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.4% | 1 | 244 |
| Haematoma | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Skin tags | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Granulation tissue | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Urinary tract symptoms | 0.0% | 0 | 55 | 0.0% | 0 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 0.0% | 0 | 46 | N/A | N/A | N/A | 0.0% | 0 | 244 |
| Any other adverse effects (excluding mesh exposure) | 0.0% | 0 | 55 | 3.8% | 2 | 52 | N/A | N/A | N/A | 0.0% | 0 | 25 | 4.3% | 2 | 46 | N/A | N/A | N/A | 1.2% | 3 | 244 |
Non-mesh serious adverse effects in first and second years
Very few women had any serious adverse effects, excluding mesh exposure (see Table 57). The overall number of women experiencing at least one serious adverse effect during the first year after surgery ranged from 6.5% (in the mesh kit group) to 12.7% (in the standard repair group of trial 3; see Table 57). One woman in the synthetic mesh inlay group experienced excess blood loss during surgery (> 500 ml) and one in the mesh kit group required a blood transfusion. Individual serious events thereafter were rare, the most common being infection, pain and urinary retention, all of which are common after gynaecological surgery, generally of short duration and easily treated. There were no statistically significant differences between the randomised groups.
Only two women had any serious adverse effects in the second year: both were in the mesh kit group.
Other non-mesh adverse effects in first and second years
Fifteen women experienced at least one minor adverse effect in the first year and a further four in the second year (see Table 58).
There were no statistically significant differences between the randomised groups in either trial 3 or trial 4 in terms of any adverse effects (excluding mesh exposure), as there were very few events.
Prolapse symptoms at 6 months, 1 year and 2 years
The women’s report of prolapse symptoms, measured using the POP-SS at 6 months, 1 year and 2 years, was less than half of the preoperative level (mean score before surgery 14.5/28; at 6 months 5.9/28; at 1 year 6.3/28; at 2 years 5.2/28; see Tables 50 and 59). There were no differences between the randomised groups at any outcome time point.
Specifically the primary clinical outcome was the POP-SS at 1 year (Table 59).
| Symptom | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| 6-month outcomes | |||||||||||||||||||||
| Number of women at 6 months | N = 50 | N = 47 | N = 22 | N = 43 | N = 214 | ||||||||||||||||
| POP-SS at 6 months | 5.5 | (5.5) | 50 | 5.8 | (5.9) | 47 | 0.35 | –1.80 to 2.50 | 0.746 | 4.8 | (5.3) | 22 | 6.2 | (5.6) | 43 | 0.29 | –2.59 to 3.16 | 0.840 | 6.5 | (5.8) | 214 |
| Symptomatic prolapse | 76.0% | 38 | 50 | 87.2% | 41 | 47 | 1.13 | 0.91 to 1.42 | 0.273 | 77.3% | 17 | 22 | 90.7% | 39 | 43 | 1.11 | 0.73 to 1.70 | 0.613 | 15.4% | 33 | 214 |
| Prolapse-related QoL score | 1.9 | (2.9) | 49 | 2.2 | (2.9) | 46 | 0.20 | –1.00 to 1.40 | 0.743 | 1.7 | (3.2) | 22 | 2.2 | (2.6) | 43 | 0.14 | –1.50 to 1.79 | 0.861 | 87.4% | 187 | 214 |
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 49 | N = 44 | N = 21 | N = 44 | N = 216 | ||||||||||||||||
| POP-SS at 1 year | 6.6 | (6.0) | 49 | 6.1 | (6.4) | 44 | –0.41 | –2.92 to 2.11 | 0.747 | 6.6 | (5.5) | 21 | 5.9 | (5.3) | 44 | –1.21 | –4.13 to 1.72 | 0.408 | 7.2 | (5.9) | 215 |
| Symptomatic | 81.6% | 40 | 49 | 88.6% | 39 | 44 | 1.05 | 0.82 to 1.33 | 0.714 | 90.5% | 19 | 21 | 86.4% | 38 | 44 | 0.93 | 0.67 to 1.28 | 0.638 | 90.2% | 194 | 215 |
| Prolapse-related QoL score | 2.5 | (2.9) | 47 | 3.0 | (3.4) | 44 | 0.43 | –0.90 to 1.75 | 0.522 | 2.0 | (2.6) | 21 | 2.3 | (2.8) | 43 | –0.31 | –1.99 to 1.36 | 0.706 | 2.9 | (3.0) | 209 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 43 | N = 39 | N = 20 | N = 39 | N = 191 | ||||||||||||||||
| POP-SS 2 years | 4.8 | (5.0) | 43 | 5.4 | (5.5) | 39 | 0.58 | –1.68 to 2.84 | 0.607 | 3.9 | (4.4) | 20 | 5.4 | (5.3) | 39 | 0.65 | –2.20 to 3.50 | 0.642 | 7.1 | (6.1) | 189 |
| Symptomatic | 83.7% | 36 | 43 | 82.1% | 32 | 39 | 1.00 | 0.80 to 1.24 | 0.981 | 85.0% | 17 | 20 | 76.9% | 30 | 39 | 0.92 | 0.63 to 1.33 | 0.655 | 84.7% | 160 | 189 |
| Prolapse-related QoL score | 1.7 | (2.4) | 41 | 2.4 | (2.7) | 36 | 0.38 | –0.84 to 1.60 | 0.529 | 1.5 | (2.6) | 18 | 2.5 | (2.7) | 37 | 0.32 | –1.45 to 2.09 | 0.712 | 3.2 | (3.1) | 177 |
-
In trial 3, the MD in the POP-SSs for standard repair (6.6, SD 6.0) compared with synthetic mesh (6.1, SD 6.4), adjusted for baseline values and minimisation variables, and based on data only from women in stratum 2A (three-way randomisation) and stratum 2B (two-way randomisation), was –0.41 (95% CI –2.92 to 2.11).
-
In trial 4, the MD for standard repair (6.6, SD 5.5) compared with mesh kit (5.9, SD 5.3), adjusted for baseline values and minimisation variables, and based on data only from women in stratum 2A (three-way randomisation) and stratum 2D (two-way randomisation), was –1.21 (95% CI –4.13 to 1.72).
At 2 years:
-
In trial 3, the MD in the POP-SSs for standard repair (4.8, SD 5.0) compared with synthetic mesh (5.4, SD 5.5), adjusted for baseline values and minimisation variables, and based on data only from women in stratum 2A (three-way randomisation) and stratum 2B (two-way randomisation), was 0.58 (95% CI –1.68 to 2.84).
-
In trial 4, the MD for standard repair (3.9, SD 4.4) compared with mesh kit (5.4, SD 5.3), adjusted for baseline values and minimisation variables, and based on data only from women in stratum 2A (three-way randomisation) and stratum 2D (two-way randomisation), was 0.65 (95% CI –2.20 to 3.50).
Thus, the difference between the groups was small and not statistically significant. The size of the CIs included the prespecified minimal clinically important difference of three, which we considered to be clinically important for the Secondary trial.
We conclude that, although prolapse surgery was very effective in reducing women’s prolapse symptoms, the trials were not powered to rule in or out whether or not the use of synthetic mesh inlay or a mesh kit provided additional benefit for this outcome in women who were having a repeat repair.
The lack of enough evidence of a difference between the groups was similar when we analysed:
-
data from individual prolapse symptoms (whether measured as ‘any’ or occurring ‘most or all of the time’ (Table 60);
-
the proportion of women who had at least one prolapse symptom (‘symptomatic’);
-
the prolapse-related QoL score measured as the interference of prolapse symptoms on everyday life, and
-
the need to undertake extra hygiene measures or manoeuvres to ease discomfort, or to assist pelvic floor functions such as emptying the bladder or bowel (see Table 60).
| Symptom | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| Six-month outcomes | |||||||||||||||||||||
| Number of women at 6 months | N = 50 | N = 47 | N = 22 | N = 43 | N = 214 | ||||||||||||||||
| Individual prolapse symptoms | |||||||||||||||||||||
| SCD any | 36.0% | 18 | 50 | 44.7% | 21 | 47 | 1.16 | 0.71 to 1.89 | 0.546 | 40.9% | 9 | 22 | 37.2% | 16 | 43 | 0.68 | 0.33 to 1.37 | 0.276 | 0.9 | (1.2) | 214 |
| SCD freq. | 10.0% | 5 | 50 | 8.5% | 4 | 47 | 0.93 | 0.27 to 3.22 | 0.906 | 9.1% | 2 | 22 | 18.6% | 8 | 43 | 1.29 | 0.29 to 5.76 | 0.743 | 44.9% | 96 | 214 |
| Pain any | 26.0% | 13 | 50 | 36.2% | 17 | 47 | 1.28 | 0.70 to 2.33 | 0.421 | 31.8% | 7 | 22 | 34.9% | 15 | 43 | 0.98 | 0.47 to 2.06 | 0.956 | 0.8 | (1.1) | 214 |
| Pain freq. | 4.0% | 2 | 50 | 6.4% | 3 | 47 | 1.38 | 0.25 to 7.46 | 0.710 | 9.1% | 2 | 22 | 14.0% | 6 | 43 | 1.40 | 0.32 to 6.15 | 0.655 | 43.0% | 92 | 214 |
| Abdo. any | 32.0% | 16 | 50 | 29.8% | 14 | 47 | 1.01 | 0.56 to 1.81 | 0.980 | 27.3% | 6 | 22 | 37.2% | 16 | 43 | 1.07 | 0.48 to 2.40 | 0.874 | 0.8 | (1.0) | 214 |
| Abdo. freq. | 4.0% | 2 | 50 | 8.5% | 4 | 47 | 2.25 | 0.43 to 11.65 | 0.334 | 4.5% | 1 | 22 | 9.3% | 4 | 43 | 1.66 | 0.21 to 13.12 | 0.629 | 45.3% | 97 | 214 |
| Back any | 46.0% | 23 | 50 | 44.7% | 21 | 47 | 1.11 | 0.74 to 1.66 | 0.627 | 40.9% | 9 | 22 | 27.9% | 12 | 43 | 0.73 | 0.38 to 1.40 | 0.342 | 0.9 | (1.1) | 214 |
| Back freq. | 14.0% | 7 | 50 | 14.9% | 7 | 47 | 1.50 | 0.63 to 3.55 | 0.355 | 4.5% | 1 | 22 | 9.3% | 4 | 43 | 2.67 | 0.36 to 19.99 | 0.340 | 48.1% | 103 | 214 |
| Strain blad. any | 36.0% | 18 | 50 | 34.0% | 16 | 47 | 0.96 | 0.56 to 1.65 | 0.878 | 36.4% | 8 | 22 | 37.2% | 16 | 43 | 1.10 | 0.57 to 2.14 | 0.776 | 0.7 | (1.0) | 214 |
| Strain blad. freq. | 4.0% | 2 | 50 | 10.6% | 5 | 47 | 3.12 | 0.60 to 16.39 | 0.178 | 0.0% | 0 | 22 | 4.7% | 2 | 43 | N/A | N/A | N/A | 39.7% | 85 | 214 |
| Blad. not empty any | 58.0% | 29 | 50 | 48.9% | 23 | 47 | 0.87 | 0.58 to 1.29 | 0.486 | 50.0% | 11 | 22 | 67.4% | 29 | 43 | 1.30 | 0.79 to 2.16 | 0.302 | 1.1 | (1.2) | 214 |
| Blad. not empty freq. | 10.0% | 5 | 50 | 19.1% | 9 | 47 | 2.22 | 0.85 to 5.81 | 0.105 | 13.6% | 3 | 22 | 9.3% | 4 | 43 | 0.64 | 0.17 to 2.43 | 0.509 | 61.2% | 131 | 214 |
| Bowel not empty any | 54.0% | 27 | 50 | 57.4% | 27 | 47 | 1.02 | 0.73 to 1.42 | 0.905 | 45.5% | 10 | 22 | 74.4% | 32 | 43 | 1.42 | 0.86 to 2.33 | 0.173 | 1.3 | (1.2) | 214 |
| Bowel not empty freq. | 16.0% | 8 | 50 | 10.6% | 5 | 47 | 0.57 | 0.21 to 1.51 | 0.258 | 18.2% | 4 | 22 | 18.6% | 8 | 43 | 1.05 | 0.40 to 2.73 | 0.920 | 68.7% | 147 | 214 |
| Digital evacuation of bowel | |||||||||||||||||||||
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 49 | N = 44 | N = 21 | N = 44 | N = 216 | ||||||||||||||||
| Individual prolapse symptoms | |||||||||||||||||||||
| SCD any | 44.9% | 22 | 49 | 40.9% | 18 | 44 | 0.91 | 0.58 to 1.43 | 0.680 | 57.1% | 12 | 21 | 36.4% | 16 | 44 | 0.57 | 0.29 to 1.10 | 0.094 | 49.3% | 106 | 215 |
| SCD freq. | 20.4% | 10 | 49 | 11.4% | 5 | 44 | 0.52 | 0.20 to 1.35 | 0.177 | 23.8% | 5 | 21 | 6.8% | 3 | 44 | 0.24 | 0.06 to 1.00 | 0.049 | 15.3% | 33 | 215 |
| Pain any | 30.6% | 15 | 49 | 31.8% | 14 | 44 | 0.98 | 0.54 to 1.77 | 0.937 | 28.6% | 6 | 21 | 36.4% | 16 | 44 | 1.34 | 0.59 to 3.03 | 0.485 | 40.9% | 88 | 215 |
| Pain freq. | 14.3% | 7 | 49 | 9.1% | 4 | 44 | 0.64 | 0.21 to 1.94 | 0.428 | 9.5% | 2 | 21 | 6.8% | 3 | 44 | 0.72 | 0.13 to 3.96 | 0.708 | 9.3% | 20 | 215 |
| Abdo. any | 46.9% | 23 | 49 | 29.5% | 13 | 44 | 0.58 | 0.35 to 0.98 | 0.043 | 52.4% | 11 | 21 | 29.5% | 13 | 44 | 0.50 | 0.24 to 1.04 | 0.062 | 51.2% | 110 | 215 |
| Abdo. freq. | 10.2% | 5 | 49 | 6.8% | 3 | 44 | 0.87 | 0.23 to 3.30 | 0.843 | 4.8% | 1 | 21 | 6.8% | 3 | 44 | 1.08 | 0.13 to 9.25 | 0.945 | 9.3% | 20 | 215 |
| Back any | 46.9% | 23 | 49 | 29.5% | 13 | 44 | 0.70 | 0.42 to 1.16 | 0.162 | 47.6% | 10 | 21 | 34.1% | 15 | 44 | 0.79 | 0.45 to 1.40 | 0.423 | 54.0% | 116 | 215 |
| Back freq. | 18.4% | 9 | 49 | 9.1% | 4 | 44 | 0.56 | 0.19 to 1.64 | 0.292 | 14.3% | 3 | 21 | 9.1% | 4 | 44 | 0.61 | 0.17 to 2.18 | 0.450 | 11.2% | 24 | 215 |
| Strain blad. any | 40.8% | 20 | 49 | 47.7% | 21 | 44 | 1.15 | 0.74 to 1.77 | 0.533 | 33.3% | 7 | 21 | 50.0% | 22 | 44 | 1.35 | 0.71 to 2.54 | 0.361 | 42.8% | 92 | 215 |
| Strain blad. freq. | 12.2% | 6 | 49 | 18.2% | 8 | 44 | 1.43 | 0.60 to 3.43 | 0.425 | 9.5% | 2 | 21 | 4.5% | 2 | 44 | 0.43 | 0.07 to 2.76 | 0.371 | 11.2% | 24 | 215 |
| Blad. not empty any | 59.2% | 29 | 49 | 63.6% | 28 | 44 | 1.04 | 0.74 to 1.47 | 0.804 | 61.9% | 13 | 21 | 65.9% | 29 | 44 | 0.99 | 0.66 to 1.50 | 0.976 | 63.3% | 136 | 215 |
| Blad. not empty freq. | 16.3% | 8 | 49 | 18.2% | 8 | 44 | 0.97 | 0.44 to 2.14 | 0.946 | 9.5% | 2 | 21 | 11.4% | 5 | 44 | 0.66 | 0.15 to 2.92 | 0.586 | 15.8% | 34 | 215 |
| Bowel not empty any | 59.2% | 29 | 49 | 63.6% | 28 | 44 | 1.19 | 0.76 to 1.88 | 0.447 | 61.9% | 13 | 21 | 65.9% | 29 | 44 | 1.00 | 0.68 to 1.49 | 0.981 | 75.3% | 162 | 215 |
| Bowel not empty freq. | 14.3% | 7 | 49 | 13.6% | 6 | 44 | 0.95 | 0.37 to 2.45 | 0.911 | 19.0% | 4 | 21 | 18.2% | 8 | 44 | 1.43 | 0.48 to 4.23 | 0.521 | 15.8% | 34 | 215 |
| Actions necessitated by prolapse symptoms | |||||||||||||||||||||
| Fingers to ease discomfort | 0.0% | 0 | 45 | 0.0% | 0 | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 2.6% | 1 | 38 | N/A | N/A | N/A | 1.6% | 3 | 185 |
| Extra hygiene measures | 4.5% | 2 | 44 | 12.8% | 5 | 39 | 2.28 | 0.48 to 10.85 | 0.302 | 5.3% | 1 | 19 | 8.1% | 3 | 37 | 1.40 | 0.15 to 13.10 | 0.766 | 7.5% | 14 | 186 |
| Fingers to help empty bladder | 0.0% | 0 | 46 | 0.0% | 0 | 39 | N/A | N/A | N/A | 0.0% | 0 | 21 | 0.0% | 0 | 41 | N/A | N/A | N/A | 0.5% | 1 | 186 |
| Fingers to help empty bowel | 2.2% | 1 | 46 | 2.6% | 1 | 39 | 1.18 | 0.08 to 18.24 | 0.906 | 4.8% | 1 | 21 | 0.0% | 0 | 39 | N/A | N/A | N/A | 3.3% | 6 | 184 |
| Digital evacuation of bowel | 4.3% | 2 | 46 | 7.7% | 3 | 39 | 2.16 | 0.39 to 11.81 | 0.376 | 4.8% | 1 | 21 | 4.9% | 2 | 41 | 0.94 | 0.09 to 10.24 | 0.961 | 5.8% | 11 | 189 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 43 | N = 39 | N = 20 | N = 39 | N = 191 | ||||||||||||||||
| Individual prolapse symptoms | |||||||||||||||||||||
| SCD any | 30.2% | 13 | 43 | 25.6% | 10 | 39 | 0.78 | 0.38 to 1.60 | 0.497 | 25.0% | 5 | 20 | 35.9% | 14 | 39 | 1.17 | 0.47 to 2.87 | 0.739 | 43.4% | 82 | 189 |
| SCD freq. | 7.0% | 3 | 43 | 5.1% | 2 | 39 | 0.84 | 0.15 to 4.74 | 0.845 | 5.0% | 1 | 20 | 7.7% | 3 | 39 | 1.10 | 0.10 to 12.20 | 0.941 | 16.4% | 31 | 189 |
| Pain any | 25.6% | 11 | 43 | 17.9% | 7 | 39 | 0.70 | 0.31 to 1.56 | 0.382 | 30.0% | 6 | 20 | 30.8% | 12 | 39 | 0.77 | 0.34 to 1.76 | 0.539 | 41.3% | 78 | 189 |
| Pain freq. | 4.7% | 2 | 43 | 2.6% | 1 | 39 | 0.55 | 0.05 to 5.84 | 0.621 | 5.0% | 1 | 20 | 7.7% | 3 | 39 | 0.56 | 0.05 to 6.71 | 0.646 | 9.5% | 18 | 189 |
| Abdo. any | 23.3% | 10 | 43 | 28.2% | 11 | 39 | 1.16 | 0.54 to 2.52 | 0.702 | 20.0% | 4 | 20 | 33.3% | 13 | 39 | 1.34 | 0.55 to 3.27 | 0.526 | 45.0% | 85 | 189 |
| Abdo. freq. | 2.3% | 1 | 43 | 5.1% | 2 | 39 | 2.84 | 0.27 to 29.91 | 0.384 | 5.0% | 1 | 20 | 5.1% | 2 | 39 | 0.86 | 0.08 to 9.20 | 0.901 | 10.1% | 19 | 189 |
| Back any | 41.9% | 18 | 43 | 30.8% | 12 | 39 | 0.71 | 0.38 to 1.31 | 0.272 | 35.0% | 7 | 20 | 33.3% | 13 | 39 | 1.04 | 0.51 to 2.14 | 0.911 | 49.2% | 93 | 189 |
| Back freq. | 9.3% | 4 | 43 | 10.3% | 4 | 39 | 0.83 | 0.20 to 3.53 | 0.806 | 5.0% | 1 | 20 | 5.1% | 2 | 39 | 1.52 | 0.14 to 16.13 | 0.729 | 12.2% | 23 | 189 |
| Strain blad. any | 34.9% | 15 | 43 | 53.8% | 21 | 39 | 1.59 | 0.93 to 2.71 | 0.093 | 30.0% | 6 | 20 | 41.0% | 16 | 39 | 1.51 | 0.73 to 3.13 | 0.270 | 44.4% | 84 | 189 |
| Strain blad. freq. | 9.3% | 4 | 43 | 12.8% | 5 | 39 | 1.34 | 0.48 to 3.70 | 0.575 | 0.0% | 0 | 20 | 5.1% | 2 | 39 | N/A | N/A | N/A | 13.2% | 25 | 189 |
| Blad. not empty any | 55.8% | 24 | 43 | 59.0% | 23 | 39 | 1.30 | 0.90 to 1.87 | 0.158 | 65.0% | 13 | 20 | 56.4% | 22 | 39 | 0.86 | 0.57 to 1.30 | 0.467 | 63.5% | 120 | 189 |
| Blad. not empty freq. | 9.3% | 4 | 43 | 17.9% | 7 | 39 | 1.82 | 0.59 to 5.62 | 0.299 | 0.0% | 0 | 20 | 15.4% | 6 | 39 | N/A | N/A | N/A | 19.0% | 36 | 189 |
| Bowel not empty any | 55.8% | 24 | 43 | 66.7% | 26 | 39 | 1.12 | 0.76 to 1.64 | 0.579 | 45.0% | 9 | 20 | 64.1% | 25 | 39 | 1.60 | 0.84 to 3.03 | 0.152 | 72.0% | 136 | 189 |
| Bowel not empty freq. | 7.0% | 3 | 43 | 17.9% | 7 | 39 | 2.07 | 0.67 to 6.45 | 0.208 | 5.0% | 1 | 20 | 15.4% | 6 | 39 | 2.81 | 0.41 to 19.20 | 0.292 | 19.0% | 36 | 189 |
| Actions necessitated by prolapse symptoms | |||||||||||||||||||||
| Fingers to ease discomfort | 0.0% | 0 | 43 | 0.0% | 0 | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 0.0% | 0 | 38 | N/A | N/A | N/A | 1.1% | 2 | 182 |
| Extra hygiene measures | 4.7% | 2 | 43 | 10.3% | 4 | 39 | 2.44 | 0.46 to 13.09 | 0.297 | 5.0% | 1 | 20 | 5.3% | 2 | 38 | 1.09 | 0.11 to 10.88 | 0.944 | 9.9% | 18 | 182 |
| Fingers to help empty bladder | 0.0% | 0 | 43 | 0.0% | 0 | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 0.0% | 0 | 39 | N/A | N/A | N/A | 0.5% | 1 | 186 |
| Fingers to help empty bowel | 0.0% | 0 | 43 | 2.6% | 1 | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 0.0% | 0 | 37 | N/A | N/A | N/A | 3.2% | 6 | 187 |
| Digital evacuation of bowel | 0.0% | 0 | 42 | 5.1% | 2 | 39 | N/A | N/A | N/A | 0.0% | 0 | 19 | 0.0% | 0 | 39 | N/A | N/A | N/A | 7.0% | 13 | 187 |
All of these measures demonstrated significant improvements from before surgery, but not enough evidence of difference between the randomised groups in either trial at any time point.
The improvement at 1 year was maintained at 2 years, with respect to all of the prolapse and QoL outcomes measured. However, there were still no statistically significant differences between the randomised groups in either trial at 2 years. The improvement in the cohort women was similar.
EuroQol-5 Dimensions (3-level version)
There were no statistically significant differences between the randomised groups in the generic QoL scores at 6 months, 1 year or 2 years in either trial 3 or trial 4, but the sample size was too small to differentiate reliably between the groups (Table 61). However, the score improved from baseline levels in all the groups of women.
| EQ-5D-3L | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| At 6 months | |||||||||||||||||||||
| Number of women | N = 50 | N = 47 | N = 22 | N = 43 | N = 214 | ||||||||||||||||
| Score | 0.77 | (0.31) | 48 | 0.83 | (0.21) | 46 | 0.00 | –0.09 to 0.10 | 0.938 | 0.85 | (0.23) | 22 | 0.77 | (0.26) | 42 | –0.04 | –0.17 to 0.10 | 0.590 | 0.74 | (0.26) | 203 |
| At 1 year | |||||||||||||||||||||
| Number of women | N = 49 | N = 44 | N = 21 | N = 44 | N = 216 | ||||||||||||||||
| Score | 0.74 | (0.30) | 50 | 0.83 | (0.22) | 43 | 0.03 | –0.07 to 0.14 | 0.519 | 0.79 | (0.27) | 22 | 0.83 | (0.19) | 41 | 0.05 | –0.07 to 0.17 | 0.411 | 0.73 | (0.28) | 209 |
| At 2 years | |||||||||||||||||||||
| Number of women | N = 43 | N = 39 | N = 20 | N = 39 | N = 191 | ||||||||||||||||
| Score | 0.76 | (0.29) | 42 | 0.82 | (0.19) | 38 | 0.00 | –0.11 to 0.11 | 0.975 | 0.76 | (0.29) | 20 | 0.87 | (0.14) | 38 | 0.13 | 0.02 to 0.25 | 0.025 | 0.75 | (0.27) | 188 |
Specifically, the EQ-5D-3L scores at 1 year were compared (see Table 61).
-
In trial 3 the MD in the EQ-5D-3L scores for standard repair (0.74, SD 0.30) compared with synthetic mesh inlay (0.83, SD 0.22), adjusted for baseline values and, based on data only from women in stratum 2A (three-way randomisation) and stratum 2B (two-way randomisation), was 0.03 (95% CI –0.07 to 0.14).
-
In trial 4, the MD for standard repair (0.79, SD 0.27) compared with mesh kit (0.83, SD 0.19), adjusted for baseline values and, based on data only from women in stratum 2A (three-way randomisation) and stratum 2D (two-way randomisation), was 0.05 (95% CI –0.07 to 0.17).
The EQ-5D-3L scores at 2 years showed:
-
In trial 3, the MD in the EQ-5D-3L scores for standard repair (0.76, SD 0.29) compared with synthetic mesh inlay (0.82, SD 0.19), adjusted for baseline values and, based on data only from women in stratum 2A (three-way randomisation) and stratum 2B (two-way randomisation) was 0.00 (95% CI –0.11 to 0.11).
-
In trial 4, the MD for standard repair (0.76, SD 0.29) compared with mesh kit (0.87, SD 0.14), adjusted for baseline values and, based on data only from women in stratum 2A (three-way randomisation) and stratum 2D (two-way randomisation), was 0.13 (95% CI 0.02 to 0.25).
The findings from the cohort women were similar. Although there was a statistically significant difference in trial 4, with women who were having a mesh kit having a higher (better) QoL score than the standard repair group even after adjustment for baseline imbalances, it may have been a chance finding and its clinical significance is uncertain. This outcome is further explored in the section on economic outcomes in Chapter 7, and in the modelling Chapter 9.
Urinary symptoms
Detailed information on urinary symptoms was obtained at baseline, 1 year and 2 years. The proportion of women who had concomitant continence surgery ranged from 0.0% to 8.0% (see Table 54). There was a decrease of > 10% in the proportion of women who had any UI [from 81% at baseline (see Table 51) to 69% at 1 year (Table 62)] and the proportion with severe UI more than halved (from 21% to 8%). However, there was no difference between the randomised groups in either trial 3 or trial 4 with respect to any of the urinary outcomes measured.
| Symptom | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 46 | N = 39 | N = 21 | N = 41 | N = 191 | ||||||||||||||||
| Any incontinence | 69.6% | 32 | 46 | 64.1% | 25 | 39 | 0.90 | 0.63 to 1.28 | 0.566 | 57.1% | 12 | 21 | 73.2% | 30 | 41 | 1.21 | 0.83 to 1.79 | 0.322 | 72.1% | 137 | 190 |
| ICIQ-UI-SF score | 4.2 | (3.9) | 46 | 4.8 | (5.1) | 39 | 0.27 | –1.55 to 2.09 | 0.768 | 3.3 | (3.6) | 21 | 5.4 | (5.0) | 40 | 0.55 | –1.45 to 2.55 | 0.580 | 5.7 | (5.1) | 188 |
| Severe incontinence | 2.2% | 1 | 46 | 12.8% | 5 | 39 | 5.52 | 0.68 to 44.77 | 0.110 | 0.0% | 0 | 21 | 10.0% | 4 | 40 | N/A | N/A | N/A | 11.2% | 21 | 188 |
| UI QoL score | 1.7 | (2.4) | 46 | 2.4 | (3.2) | 38 | 0.36 | –0.81 to 1.53 | 0.533 | 1.2 | (2.1) | 21 | 2.2 | (2.7) | 41 | 0.30 | –1.09 to 1.68 | 0.664 | 2.4 | (2.9) | 186 |
| Stress UI | 10.8% | 4 | 37 | 9.7% | 4 | 31 | 0.89 | 0.24 to 3.38 | 0.868 | 5.9% | 1 | 17 | 17.1% | 6 | 35 | 2.76 | 0.36 to 21.35 | 0.331 | 12.5% | 20 | 160 |
| Urgency UI | 0.0% | 0 | 46 | 5.3% | 2 | 38 | N/A | N/A | N/A | 0.0% | 0 | 21 | 7.3% | 3 | 41 | N/A | N/A | N/A | 6.3% | 12 | 189 |
| Overactive bladder | 0.0% | 0 | 45 | 2.6% | 1 | 38 | N/A | N/A | N/A | 0.0% | 0 | 21 | 0.0% | 0 | 41 | N/A | N/A | N/A | 3.7% | 7 | 189 |
| ICIQ-FLUTS filling score | 3.7 | (2.4) | 45 | 4 | (2.7) | 38 | 0.41 | –0.55 to 1.37 | 0.392 | 3.9 | (3.0) | 21 | 4.1 | (2.0) | 41 | –0.09 | –1.32 to 1.15 | 0.886 | 4.6 | (2.9) | 188 |
| ICIQ-FLUTS voiding score | 2.2 | (2.3) | 46 | 2.3 | (2.1) | 39 | 0.15 | –0.76 to 1.06 | 0.744 | 2.0 | (2.3) | 21 | 2.1 | (2.2) | 41 | –0.06 | –1.23 to 1.11 | 0.916 | 2.2 | (2.4) | 188 |
| ICIQ-FLUTS incontinence | 3.9 | (3.0) | 37 | 4.5 | (3.8) | 31 | 0.22 | –1.37 to 1.81 | 0.779 | 3.2 | (3.0) | 17 | 5.6 | (4.5) | 34 | 0.58 | –1.47 to 2.63 | 0.562 | 5.2 | (3.9) | 153 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 43 | N = 39 | N = 20 | N = 39 | N = 191 | ||||||||||||||||
| Any incontinence | 60.5% | 26 | 43 | 71.8% | 28 | 39 | 1.10 | 0.75 to 1.59 | 0.634 | 65.0% | 13 | 20 | 79.5% | 31 | 39 | 1.18 | 0.87 to 1.59 | 0.292 | 71.2% | 136 | 191 |
| ICIQ-UI-SF score | 3.8 | (4.4) | 42 | 5.0 | (4.5) | 39 | 1.04 | –0.79 to 2.86 | 0.258 | 3.7 | (3.7) | 20 | 5.5 | (5.1) | 39 | 0.58 | –1.52 to 2.68 | 0.574 | 5.5 | (4.9) | 189 |
| Severe incontinence | 7.1% | 3 | 42 | 10.3% | 4 | 39 | 1.64 | 0.38 to 7.07 | 0.507 | 5.0% | 1 | 20 | 10.3% | 4 | 39 | 1.58 | 0.20 to 12.48 | 0.663 | 12.2% | 23 | 189 |
| UI QoL score | 1.3 | (2.2) | 40 | 2.1 | (2.7) | 39 | 0.60 | –0.51 to 1.70 | 0.282 | 1.1 | (1.8) | 19 | 2.2 | (3.1) | 39 | 0.32 | –1.00 to 1.65 | 0.619 | 2.3 | (2.7) | 184 |
| Stress UI | 13.9% | 5 | 36 | 11.8% | 4 | 34 | 1.35 | 0.40 to 4.55 | 0.626 | 11.8% | 2 | 17 | 10.8% | 4 | 37 | 0.86 | 0.21 to 3.54 | 0.833 | 16.4% | 27 | 165 |
| Urgency UI | 4.7% | 2 | 43 | 7.7% | 3 | 39 | 1.34 | 0.24 to 7.46 | 0.734 | 5.0% | 1 | 20 | 10.3% | 4 | 39 | 1.71 | 0.21 to 13.74 | 0.613 | 8.4% | 16 | 190 |
| Overactive bladder | 0.0% | 0 | 43 | 5.1% | 2 | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 2.6% | 1 | 39 | N/A | N/A | N/A | 1.1% | 2 | 189 |
| ICIQ-FLUTS filling score | 3.9 | (2.3) | 43 | 4.2 | (3.1) | 39 | 0.35 | –0.64 to 1.34 | 0.483 | 4.1 | (2.3) | 20 | 4.1 | (2.4) | 39 | –0.30 | –1.64 to 1.03 | 0.644 | 4.5 | (2.5) | 185 |
| ICIQ-FLUTS voiding score | 1.6 | (2.7) | 42 | 2.3 | (2.2) | 39 | 0.54 | –0.41 to 1.50 | 0.260 | 1.1 | (1.3) | 19 | 2.2 | (2.1) | 39 | 1.09 | 0.03 to 2.15 | 0.044 | 2.3 | (2.3) | 189 |
| ICIQ-FLUTS incontinence | 4.0 | (3.5) | 36 | 4.2 | (3.9) | 33 | 0.07 | –1.56 to 1.70 | 0.934 | 3.6 | (2.9) | 17 | 4.7 | (4.1) | 37 | 0.30 | –1.38 to 1.98 | 0.714 | 5.3 | (4.1) | 163 |
The findings were virtually unchanged at 2 years (see Table 62): there did not appear to be any further recovery or deterioration in urinary symptoms over that time span. The benefits of surgery, and the lack of power to differentiate between the randomised groups in either trial 3 or trial 4, were largely maintained at 2 years. The results for the cohort women were similar.
Bowel symptoms
Detailed information on bowel symptoms was obtained at baseline, 1 year and 2 years (see Tables 52 and 63). Bowel frequency and constipation were largely unchanged after prolapse surgery. However, fewer women had bowel urgency or FI, and the effect of bowel symptoms on QoL was less than before surgery (Table 63). Nevertheless, there was no difference between the randomised groups with respect to any of the bowel outcomes measured.
| Symptom | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 46 | N = 39 | N = 21 | N = 41 | N = 191 | ||||||||||||||||
| Bowel frequency | |||||||||||||||||||||
| > 3 daily | 2.2% | 1 | 45 | 5.1% | 2 | 39 | 0.76 | 0.31 to 1.88 | 0.558 | 4.8% | 1 | 21 | 4.9% | 2 | 41 | 0.51 | 0.16 to 1.62 | 0.255 | 3.2% | 6 | 188 |
| 1–3 times daily | 37.8% | 17 | 45 | 30.8% | 12 | 39 | 28.6% | 6 | 21 | 24.4% | 10 | 41 | 33.5% | 63 | 188 | ||||||
| Once daily | 35.6% | 16 | 45 | 35.9% | 14 | 39 | 42.9% | 9 | 21 | 48.8% | 20 | 41 | 45.2% | 85 | 188 | ||||||
| Every 2–3 days | 20.0% | 9 | 45 | 23.1% | 9 | 39 | 19.0% | 4 | 21 | 17.1% | 7 | 41 | 14.9% | 28 | 188 | ||||||
| Weekly or less | 4.4% | 2 | 45 | 5.1% | 2 | 39 | 4.8% | 1 | 21 | 4.9% | 2 | 41 | 3.2% | 6 | 188 | ||||||
| Constipation | 8.9% | 4 | 45 | 23.1% | 9 | 39 | 1.48 | 0.54 to 4.06 | 0.443 | 4.8% | 1 | 21 | 12.2% | 5 | 41 | 2.74 | 0.35 to 21.74 | 0.339 | 17.4% | 32 | 184 |
| Bowel urgency | 8.7% | 4 | 46 | 2.6% | 1 | 39 | 0.32 | 0.04 to 2.68 | 0.292 | 0.0% | 0 | 21 | 2.5% | 1 | 40 | N/A | N/A | N/A | 6.3% | 12 | 190 |
| FI (any) | 26.1% | 12 | 46 | 43.6% | 17 | 39 | 1.41 | 0.84 to 2.35 | 0.190 | 28.6% | 6 | 21 | 39.0% | 16 | 41 | 1.59 | 0.57 to 4.49 | 0.378 | 36.0% | 68 | 189 |
| Passive FI | 75.0% | 9 | 12 | 94.1% | 16 | 17 | 100.0% | 6 | 6 | 93.8% | 15 | 16 | 85.3% | 58 | 68 | ||||||
| Active FI | 25.0% | 3 | 12 | 5.9% | 1 | 17 | 0.0% | 0 | 6 | 6.3% | 1 | 16 | 14.7% | 10 | 68 | ||||||
| Severe FI | 10.9% | 5 | 46 | 17.9% | 7 | 39 | 1.38 | 0.48 to 3.97 | 0.545 | 9.5% | 2 | 21 | 12.2% | 5 | 41 | 1.36 | 0.30 to 6.17 | 0.690 | 8.5% | 16 | 189 |
| Bowel symptoms QoL | 1.3 | (2.1) | 46 | 1.9 | (2.3) | 37 | 0.05 | –0.83 to 0.94 | 0.903 | 1.0 | (1.5) | 20 | 2.1 | (2.6) | 41 | 0.23 | –1.19 to 1.65 | 0.741 | 2.4 | (2.7) | 188 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 43 | N = 39 | N = 20 | N = 39 | N = 191 | ||||||||||||||||
| Bowel frequency | |||||||||||||||||||||
| > 3 daily | 4.7% | 2 | 43 | 5.1% | 2 | 39 | 0.97 | 0.38 to 2.48 | 0.957 | 5.0% | 1 | 20 | 5.1% | 2 | 39 | 1.06 | 0.33 to 3.47 | 0.919 | 3.7% | 7 | 191 |
| 1–3 times daily | 32.6% | 14 | 43 | 35.9% | 14 | 39 | 35.0% | 7 | 20 | 20.5% | 8 | 39 | 36.6% | 70 | 191 | ||||||
| Once daily | 44.2% | 19 | 43 | 33.3% | 13 | 39 | 50.0% | 10 | 20 | 46.2% | 18 | 39 | 42.4% | 81 | 191 | ||||||
| Every 2–3 days | 16.3% | 7 | 43 | 20.5% | 8 | 39 | 10.0% | 2 | 20 | 25.6% | 10 | 39 | 14.7% | 28 | 191 | ||||||
| Weekly or less | 2.3% | 1 | 43 | 5.1% | 2 | 39 | 0.0% | 0 | 20 | 2.6% | 1 | 39 | 2.6% | 5 | 191 | ||||||
| Constipation | 9.5% | 4 | 42 | 10.5% | 4 | 38 | 0.99 | 0.27 to 3.62 | 0.984 | 10.5% | 2 | 19 | 12.8% | 5 | 39 | 0.60 | 0.10 to 3.64 | 0.579 | 18.9% | 36 | 190 |
| Bowel urgency | 7.0% | 3 | 43 | 13.2% | 5 | 38 | 1.73 | 0.45 to 6.71 | 0.425 | 5.0% | 1 | 20 | 7.7% | 3 | 39 | 1.50 | 0.17 to 13.44 | 0.718 | 7.4% | 14 | 189 |
| FI (any) | 27.9% | 12 | 43 | 44.7% | 17 | 38 | 1.35 | 0.74 to 2.47 | 0.326 | 30.0% | 6 | 20 | 38.5% | 15 | 39 | 1.04 | 0.47 to 2.31 | 0.918 | 37.0% | 70 | 189 |
| Passive FI | 83.3% | 10 | 12 | 70.6% | 12 | 17 | 83.3% | 5 | 6 | 80.0% | 12 | 15 | 82.9% | 58 | 70 | ||||||
| Active FI | 16.7% | 2 | 12 | 29.4% | 5 | 17 | 16.7% | 1 | 6 | 20.0% | 3 | 15 | 17.1% | 12 | 70 | ||||||
| Severe FI | 4.7% | 2 | 43 | 15.8% | 6 | 38 | 2.04 | 0.43 to 9.66 | 0.371 | 10.0% | 2 | 20 | 15.4% | 6 | 39 | 1.92 | 0.43 to 8.52 | 0.391 | 13.2% | 25 | 189 |
| Bowel symptoms QoL | 1.5 | (2.3) | 42 | 2.0 | (2.7) | 38 | –0.30 | –1.24 to 0.65 | 0.525 | 2.0 | (3.0) | 20 | 2.3 | (2.7) | 37 | –0.67 | –2.26 to 0.93 | 0.398 | 2.6 | (2.8) | 186 |
The benefits of surgery, were largely maintained at 2 years (see Table 63). The data from the cohort women were similar.
Vaginal and sexual symptoms
Detailed information on vaginal and sexual symptoms was obtained at baseline, 1 year and 2 years (see Tables 53 and 64). Both the mean vaginal symptom score and the QoL score decreased (improved) after prolapse surgery (Table 64). However, there was no difference between the randomised groups for these outcomes.
| Symptom | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | Cohort | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | CC2 | |||||||||||
| 1-year outcomes | |||||||||||||||||||||
| Number of women | N = 46 | N = 39 | N = 21 | N = 41 | N = 191 | ||||||||||||||||
| Vaginal symptoms | |||||||||||||||||||||
| ICIQ-VS | 8.3 | (7.4) | 44 | 7.9 | (8.6) | 37 | –1.29 | –4.99 to 2.42 | 0.487 | 6.7 | (6.0) | 18 | 5.8 | (4.8) | 35 | –2.82 | –6.67 to 1.02 | 0.143 | 10.1 | (9.2) | 171 |
| Vaginal symptoms QoL score | 1.9 | (2.8) | 42 | 2.0 | (2.8) | 38 | –0.11 | –1.43 to 1.22 | 0.873 | 0.6 | (0.8) | 18 | 1.8 | (2.3) | 36 | 0.97 | –0.25 to 2.18 | 0.113 | 2.2 | (2.9) | 179 |
| Vagina too tight | 7.0% | 3 | 43 | 2.7% | 1 | 37 | 0.38 | 0.04 to 3.46 | 0.390 | 0.0% | 0 | 20 | 5.3% | 2 | 38 | N/A | N/A | N/A | 6.4% | 11 | 172 |
| Sexual symptoms | |||||||||||||||||||||
| Sex life at present | 40.0% | 18 | 45 | 48.8% | 20 | 41 | 28.6% | 6 | 21 | 41.0% | 16 | 39 | 35.3% | 65 | 184 | ||||||
| Reason for no sex life | |||||||||||||||||||||
| No partner | 37.0% | 10 | 27 | 28.6% | 6 | 21 | 40.0% | 6 | 15 | 30.4% | 7 | 23 | 37.0% | 44 | 119 | ||||||
| Vaginal symptoms | 11.1% | 3 | 27 | 9.5% | 2 | 21 | 13.3% | 2 | 15 | 4.3% | 1 | 23 | 10.1% | 12 | 119 | ||||||
| Prolapse symptoms | 3.7% | 1 | 27 | 23.8% | 5 | 21 | 0.0% | 0 | 15 | 8.7% | 2 | 23 | 21.0% | 25 | 119 | ||||||
| Other reason | 40.7% | 11 | 27 | 28.6% | 6 | 21 | 33.3% | 5 | 15 | 56.5% | 13 | 23 | 28.6% | 34 | 119 | ||||||
| Reason not given | 7.4% | 2 | 27 | 9.5% | 2 | 21 | 13.3% | 2 | 15 | 0.0% | 0 | 23 | 3.4% | 4 | |||||||
| Dyspareunia | 0.0% | 0 | 18 | 13.0% | 3 | 23 | N/A | N/A | N/A | 0.0% | 0 | 6 | 5.6% | 1 | 18 | N/A | N/A | N/A | 11.8% | 9 | 76 |
| ICI Sexual Matters score | 5.4 | (9.3) | 18 | 14.3 | (15.4) | 21 | 5.10 | –5.79 to 16.00 | 0.295 | 4.0 | (6.7) | 6 | 16.9 | (15.5) | 18 | 6.58 | –14.62 to 27.77 | 0.314 | 15.7 | (16.7) | 73 |
| Sex life QoL score | 2.3 | (3.1) | 19 | 3.5 | (3.5) | 22 | 0.37 | –1.92 to 2.67 | 0.721 | 0.3 | (0.8) | 6 | 3.7 | (3.5) | 18 | 3.74 | –6.46 to 13.93 | 0.256 | 4.2 | (4.0) | 76 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women | N = 43 | N = 39 | N = 20 | N = 39 | N = 191 | ||||||||||||||||
| Vaginal symptoms | |||||||||||||||||||||
| ICIQ-VS | 7.3 | (7.6) | 39 | 7.9 | (7.8) | 37 | –0.64 | –4.56 to 3.28 | 0.742 | 6.1 | (6.2) | 17 | 7.9 | (7.4) | 36 | 0.08 | –4.91 to 5.08 | 0.973 | 10.5 | (9.3) | 169 |
| Vaginal symptoms QoL score | 1.8 | (2.8) | 40 | 1.8 | (2.4) | 39 | –0.14 | –1.40 to 1.12 | 0.824 | 1.4 | (3.2) | 18 | 1.8 | (2.5) | 35 | –0.16 | –2.09 to 1.76 | 0.864 | 2.6 | (3.0) | 181 |
| Vagina too tight | 4.9% | 2 | 41 | 7.9% | 3 | 38 | 1.71 | 0.30 to 9.59 | 0.543 | 0.0% | 0 | 18 | 2.7% | 1 | 37 | N/A | N/A | N/A | 5.2% | 9 | 174 |
| Sexual symptoms | |||||||||||||||||||||
| Sex life at present | 32.6% | 14 | 43 | 55.3% | 21 | 38 | 30.0% | 6 | 20 | 39.5% | 15 | 38 | 34.6% | 63 | 182 | ||||||
| Reason for no sex life | |||||||||||||||||||||
| No partner | 34.5% | 10 | 29 | 23.5% | 4 | 17 | 42.9% | 6 | 14 | 21.7% | 5 | 23 | 31.9% | 38 | 119 | ||||||
| Vaginal symptoms | 13.8% | 4 | 29 | 5.9% | 1 | 17 | 0.0% | 0 | 14 | 8.7% | 2 | 23 | 7.6% | 9 | 119 | ||||||
| Prolapse symptoms | 3.4% | 1 | 29 | 17.6% | 3 | 17 | 0.0% | 0 | 14 | 8.7% | 2 | 23 | 12.6% | 15 | 119 | ||||||
| Other reason | 34.5% | 10 | 29 | 35.3% | 6 | 17 | 42.9% | 6 | 14 | 52.2% | 12 | 23 | 38.7% | 46 | 119 | ||||||
| Reason not given | 13.8% | 4 | 29 | 17.6% | 3 | 17 | 14.3% | 2 | 14 | 8.7% | 2 | 23 | 9.2% | 11 | 119 | ||||||
| Dyspareunia | 0.0% | 0 | 15 | 4.5% | 1 | 22 | N/A | N/A | N/A | 0.0% | 0 | 6 | 0.0% | 0 | 16 | N/A | N/A | N/A | 10.0% | 7 | 70 |
| ICI Sexual Matters score | 8.5 | (10.8) | 15 | 13.9 | (14.0) | 22 | 6.57 | –5.47 to 18.62 | 0.255 | 2.7 | (4.1) | 6 | 12.2 | (9.8) | 16 | 5.64 | –91.28 to 102.56 | 0.595 | 18.1 | (16.3) | 70 |
| Sex life QoL score | 2.5 | (3.5) | 15 | 3.7 | (3.6) | 23 | 1.49 | –1.68 to 4.67 | 0.320 | 1.5 | (3.2) | 6 | 2.8 | (2.8) | 17 | 2.33 | –32.13 to 36.78 | 0.549 | 4.4 | (3.8) | 73 |
More women were sexually active after surgery, and many fewer cited prolapse symptoms as a reason for not having a sex life (see Table 64). This was reflected in a halving of the ICI Sexual Matters score, and an even greater reduction (improvement) in the sex life QoL score. Women in the synthetic mesh and mesh kit groups did have higher (worse) scores on each of the outcomes measured, but this did not reach statistical significance. In summary, there was no difference between the randomised groups with respect to any of the vaginal or sexual symptom outcomes measured (see Table 64) due to the small sample size resulting in a lack of power.
The benefits of surgery, and the lack of power to differentiate between the randomised groups, were largely maintained at 2 years (see Table 64). The data from the cohort women were similar.
Postoperative prolapse measurements in randomised women
A 1-year clinic review was offered to all randomised women and 83% attended. Objective measurement showed improvement in each of the three prolapse compartments. The proportion of women with the leading prolapse edge beyond the hymen (> 0 cm) reduced substantially (Table 65). There was no statistically significant difference between groups in the prolapse stage, based on POP-Q scores or clinician’s estimates of stage (see Table 65) in trial 3 (RR 0.75, 95% CI 0.33 to 1.68; p = 0.479) or in terms of failure defined as ‘leading edge of the prolapse at > 0 cm beyond the hymen on POP-Q’ (RR 0.59, 95% CI 0.18 to 1.92; p = 0.380).
| POP-Q measurement/stage | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | |||||||||
| 1-year reviews | N = 46 | N = 44 | N = 21 | N = 38 | ||||||||||||||
| POP-Q measurement (cm) | ||||||||||||||||||
| Ba (posterior edge) | –1.4 | (1.5) | 42 | –1.4 | (1.4) | 41 | –0.22 | –0.80 to 0.36 | 0.445 | –1.2 | (1.9) | 19 | –1.8 | (1.0) | 35 | –0.74 | –1.4 to –0.10 | 0.026 |
| C (cervix/vault) | –5.6 | (2.4) | 41 | –6.2 | (1.5) | 41 | –0.61 | –1.38 to 0.16 | 0.119 | –5.1 | (2.7) | 18 | –6.0 | (1.8) | 33 | –0.65 | –1.6 to 0.3 | 0.173 |
| Bp (posterior edge) | –1.8 | (1.6) | 41 | –2.2 | (1.1) | 41 | –0.49 | –1.08 to 0.10 | 0.099 | –1.9 | (1.7) | 18 | –2.2 | (0.8) | 34 | –0.38 | –1.2 to 0.4 | 0.317 |
| TVL | 7.7 | (1.2) | 43 | 7.9 | (1.4) | 42 | 0.09 | –0.47 to 0.65 | 0.746 | 7.7 | (1.0) | 19 | 8.1 | (1.2) | 33 | 0.64 | 0.1 to 1.20 | 0.028 |
| Overall POP-Q stage | ||||||||||||||||||
| 0 | 13.6% | 6 | 44 | 7.0% | 3 | 43 | 0.75 | 0.33 to 1.68 | 0.479 | 10.5% | 2 | 19 | 14.3% | 5 | 35 | 0.24 | 0.07 to 0.83 | 0.024 |
| 1 | 36.4% | 16 | 44 | 44.2% | 19 | 43 | 31.6% | 6 | 19 | 45.7% | 16 | 35 | ||||||
| 2 | 40.9% | 18 | 44 | 46.5% | 20 | 43 | 42.1% | 8 | 19 | 40.0% | 14 | 35 | ||||||
| 3 | 9.1% | 4 | 44 | 2.3% | 1 | 43 | 15.8% | 3 | 19 | 0.0% | 0 | 35 | ||||||
| 4 | 0.0% | 0 | 44 | 0.0% | 0 | 43 | 0.0% | 0 | 19 | 0.0% | 0 | 35 | ||||||
| 2b, 3 or 4 | 14.0% | 6 | 43 | 14.0% | 6 | 43 | 0.59 | 0.18 to 1.92 | 0.380 | 16.7% | 3 | 18 | 0.0% | 0 | 35 | N/A | N/A | N/A |
There was statistical evidence of a difference between groups in trial 4: women who had a standard repair were more likely to have residual prolapse than those who were randomised to mesh kit (RR 0.24, 95% CI 0.07 to 0.83; p = 0.024; see Table 65). This may have been a chance finding as a result of the small sample size. Using the strict definition of failure of ‘leading edge of the prolapse at > 0 cm beyond the hymen on POP-Q’, 3 of 18 (16.7%) of the women who had undergone standard repair had residual prolapse compared with none of 35 women after receiving mesh kit treatment in trial 4.
Further treatment required for failure or adverse effects at 6 months, 1 year and 2 years
When women reported at 6 months or later that they had been readmitted to hospital, we verified the information by enquiry from site staff when necessary and corrected the information where necessary. A hospital readmission was automatically counted as a SAE if it was related to the initial prolapse surgery. Readmission for related adverse effects such as bleeding or infection was differentiated from (a) new surgery for pelvic organ prolapse (repeat if same compartment, further if in the opposite compartment); (b) readmission for continence surgery; and (c) readmission for surgical mesh removal.
Readmission for adverse effects
The overall rate of readmission was low, and there was no significant difference between the randomised groups. The rate in the first 6 months, ranging from 0% to 6.4% (Table 66) was mostly related to adverse effects (two cases of infection in the standard arm, one case each of retention, constipation and adhesions in the mesh inlay arm), whereas after that time only one woman was readmitted (randomised to mesh inlay, for a Fenton’s operation for vaginal tightness). There were no statistically significant differences between the randomised groups at 1 year or 2 years (see Table 66).
| Further treatment | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| 6-month outcomes | |||||||||||||||||||||
| Number of women at 6 months | N = 50 | N = 47 | N = 22 | N = 43 | N = 214 | ||||||||||||||||
| Readmitted (0–6 months) | 4.0% | 2a | 50 | 6.4% | 3b | 47 | 1.76 | 0.30 to 10.37 | 0.532 | 0.0% | 0 | 22 | 0.0% | 0 | 43 | N/A | N/A | N/A | 2.8% | 6c | 214 |
| Readmitted (6–12 months) | 0.0% | 0 | 49 | 0.0% | 0 | 44 | N/A | N/A | N/A | 0.0% | 0 | 21 | 2.3% | 1d | 44 | N/A | N/A | N/A | 1.9% | 4e | 216 |
| 1-year outcomes | |||||||||||||||||||||
| Number of women at 1 year | N = 49 | N = 44 | N = 21 | N = 44 | N = 216 | ||||||||||||||||
| New prolapse surgery | 6.1% | 3 | 49 | 2.3% | 1 | 44 | 0.37 | 0.04 to 3.43 | 0.382 | 9.5% | 2 | 21 | 0.0% | 0 | 44 | N/A | N/A | N/A | 5.1% | 11 | 216 |
| Same compartment | 6.1% | 3 | 49 | 0.0% | 0 | 44 | N/A | N/A | N/A | 9.5% | 2 | 21 | 0.0% | 0 | 44 | N/A | N/A | N/A | 3.2% | 7 | 216 |
| Different compartment | 0.0% | 0 | 49 | 2.3% | 1 | 44 | N/A | N/A | N/A | 0.0% | 0 | 21 | 0.0% | 0 | 44 | N/A | N/A | N/A | 1.9% | 4 | 216 |
| Waiting for prolapse surgery | 2.0% | 1 | 49 | 2.3% | 1 | 44 | 1.06 | 0.07 to 16.23 | 0.967 | 0.0% | 0 | 21 | 2.3% | 1 | 44 | N/A | N/A | N/A | 0.9% | 2 | 216 |
| Continence surgery | 0.0% | 0 | 49 | 2.3% | 1 | 44 | N/A | N/A | N/A | 0.0% | 0 | 21 | 4.5% | 2 | 44 | N/A | N/A | N/A | 1.4% | 3 | 216 |
| Waiting for continence surgery | 0.0% | 0 | 49 | 0.0% | 0 | 44 | N/A | N/A | N/A | 0.0% | 0 | 21 | 2.3% | 1 | 44 | N/A | N/A | N/A | 1.9% | 4 | 216 |
| Stitches removed | 6.4% | 3 | 47 | 2.4% | 1 | 42 | 0.34 | 0.04 to 3.03 | 0.331 | 5.0% | 1 | 20 | 2.3% | 1 | 43 | 0.48 | 0.03 to 7.14 | 0.596 | 3.8% | 8 | 208 |
| Any mesh complication | 0.0% | 0 | 55 | 11.5% | 6 | 52 | 0.0% | 0 | 25 | 6.5% | 3 | 46 | 1. 6% | 4 | 244 | ||||||
| Surgical removal | 0.0% | 0 | 55 | 5.8% | 3 | 52 | 0.0% | 0 | 25 | 2. 2% | 1 | 46 | 1.6% | 4 | 244 | ||||||
| Conservative treatment | 0.0% | 0 | 55 | 3.8% | 2 | 52 | 0.0% | 0 | 25 | 4.3% | 2 | 46 | 0.0% | 0 | 244 | ||||||
| No treatment | 0.0% | 0 | 55 | 1.9% | 1 | 52 | 0.0% | 0 | 25 | 0.0% | 0 | 46 | 0.0% | 0 | 244 | ||||||
| De novo mesh | 0.0% | 0 | 55 | 9.6% | 5 | 52 | 0.0% | 0 | 25 | 4.3% | 2 | 46 | 0.8% | 2 | 244 | ||||||
| Concomitant, etc. mesh | 0.0% | 0 | 55 | 0.9% | 1 | 52 | 0.0% | 0 | 25 | 2.2% | 1 | 46 | 0.8% | 2 | 244 | ||||||
| Treatment for prolapse at 1 year | |||||||||||||||||||||
| Medicines for prolapse | 21.3% | 10 | 47 | 27.3% | 12 | 44 | 1.49 | 0.71 to 3.12 | 0.292 | 23.8% | 5 | 21 | 24.4% | 10 | 41 | 1.11 | 0.43 to 2.85 | 0.826 | 21.6% | 46 | 213 |
| Oestrogens | 26.5% | 13 | 49 | 34.1% | 15 | 44 | 1.34 | 0.72 to 2.46 | 0.355 | 19.0% | 4 | 21 | 27.3% | 12 | 44 | 1.43 | 0.52 to 3.92 | 0.485 | 21.3% | 46 | 216 |
| Ring pessary | 0.0% | 0 | 49 | 0.0% | 0 | 44 | N/A | N/A | N/A | 0.0% | 0 | 21 | 0.0% | 0 | 44 | N/A | N/A | N/A | 3.7% | 8 | 216 |
| Shelf pessary | 0.0% | 0 | 49 | 0.0% | 0 | 44 | N/A | N/A | N/A | 0.0% | 0 | 21 | 0.0% | 0 | 44 | N/A | N/A | N/A | 1.4% | 3 | 216 |
| Physiotherapy | 8.5% | 4 | 47 | 18.6% | 8 | 43 | 2.20 | 0.71 to 6.77 | 0.169 | 5.0% | 1 | 20 | 9.3% | 4 | 43 | 2.25 | 0.27 to 18.63 | 0.454 | 10.6% | 22 | 208 |
| GP for prolapse | 29.2% | 14 | 48 | 36.4% | 16 | 44 | 1.27 | 0.71 to 2.27 | 0.422 | 30.0% | 6 | 20 | 22.5% | 9 | 40 | 0.71 | 0.29 to 1.72 | 0.446 | 26.0% | 53 | 204 |
| Practice nurse | 4.2% | 2 | 48 | 14.0% | 6 | 43 | 4.26 | 0.83 to 22.00 | 0.083 | 5.0% | 1 | 20 | 0.0% | 0 | 40 | N/A | N/A | N/A | 10.0% | 20 | 200 |
| GOPD | 42.2% | 19 | 45 | 29.5% | 13 | 44 | 0.73 | 0.41 to 1.30 | 0.283 | 47.4% | 9 | 19 | 40.5% | 17 | 42 | 0.72 | 0.39 to 1.33 | 0.298 | 38.9% | 81 | 208 |
| Treatment for urinary problems at 1 year | |||||||||||||||||||||
| Pads | 36.7% | 18 | 49 | 36.4% | 16 | 44 | 0.89 | 0.53 to 1.50 | 0.658 | 30.0% | 6 | 20 | 31.0% | 13 | 42 | 0.88 | 0.38 to 2.04 | 0.767 | 40.3% | 85 | 211 |
| Permanent catheter | 2.4% | 1 | 42 | 0.0% | 0 | 40 | N/A | N/A | N/A | 10.5% | 2 | 19 | 0.0% | 0 | 39 | N/A | N/A | N/A | 1.0% | 2 | 200 |
| Intermittent catheter | 4.9% | 2 | 41 | 0.0% | 0 | 39 | N/A | N/A | N/A | 5.9% | 1 | 17 | 2.6% | 1 | 39 | 0.41 | 0.03 to 6.11 | 0.515 | 2.5% | 5 | 202 |
| Drugs for UI | 12.2% | 6 | 49 | 20.5% | 9 | 44 | 1.90 | 0.75 to 4.86 | 0.178 | 9.5% | 2 | 21 | 13.6% | 6 | 44 | 1.45 | 0.32 to 6.45 | 0.628 | 13.9% | 30 | 216 |
| 2-year outcomes | |||||||||||||||||||||
| Number of women at 2 years | N = 43 | N = 39 | N = 20 | N = 39 | N = 191 | ||||||||||||||||
| Readmitted (12–24 months) | 0.0% | 0 | 43 | 2.6% | 1f | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 0.0% | 0 | 39 | N/A | N/A | N/A | 1.6% | 3g | 191 |
| New prolapse surgery | 9.3% | 4 | 43 | 7.7% | 3 | 39 | 0.76 | 0.18 to 3.17 | 0.711 | 10.0% | 2 | 20 | 2.6% | 1 | 39 | 0.15 | 0.01 to 2.07 | 0.158 | 4.7% | 9 | 191 |
| Same compartment | 7.0% | 3 | 43 | 2.6% | 1 | 39 | 0.35 | 0.04 to 3.21 | 0.353 | 5.0% | 1 | 20 | 2.6% | 1 | 39 | 0.55 | 0.04 to 8.36 | 0.665 | 3.7% | 7 | 191 |
| Different compartment | 2.3% | 1 | 43 | 5.1% | 2 | 39 | 1.73 | 0.16 to 18.53 | 0.652 | 5.0% | 1 | 20 | 0.0% | 0 | 39 | N/A | N/A | N/A | 1.0% | 2 | 191 |
| Waiting for prolapse surgery | 0.0% | 0 | 43 | 2.6% | 1 | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 0.0% | 0 | 39 | N/A | N/A | N/A | 0.5% | 14 | 191 |
| Continence surgery | 0.0% | 0 | 43 | 2.6% | 1 | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 5.1% | 2 | 39 | N/A | N/A | N/A | 2.1% | 4 | 191 |
| Waiting for continence surgery | 0.0% | 0 | 43 | 0.0% | 0 | 39 | N/A | N/A | N/A | 0.0% | 0 | 20 | 0.0% | 0 | 39 | N/A | N/A | N/A | 0.0% | 0 | 191 |
| Stitches removed | 0.0% | 0 | 41 | 2.6% | 1 | 39 | N/A | N/A | N/A | 0.0% | 0 | 19 | 0.0% | 0 | 38 | N/A | N/A | N/A | 2.2% | 4 | 186 |
| Any mesh complication | 0.0% | 0 | 55 | 3.8% | 2 | 52 | 0.0% | 0 | 25 | 4.3% | 2 | 46 | 0.8% | 2 | 244 | ||||||
| Surgical removal | 0.0% | 0 | 55 | 1.9% | 1 | 52 | 0.0% | 0 | 25 | 2.2% | 1 | 46 | 0.4% | 1 | 244 | ||||||
| Conservative | 0.0% | 0 | 55 | 1.9% | 1 | 52 | 0.0% | 0 | 25 | 2.2% | 1 | 46 | 0.4% | 1 | 244 | ||||||
| No treatment | 0.0% | 0 | 55 | 0.0% | 0 | 52 | 0.0% | 0 | 25 | 0.0% | 0 | 46 | 0.0% | 0 | 244 | ||||||
| De novo mesh | 0.0% | 0 | 55 | 1.9% | 1 | 52 | 0.0% | 0 | 25 | 4.3% | 2 | 46 | 0.4% | 1 | 244 | ||||||
| Concomitant, etc. mesh | 0.0% | 0 | 55 | 1.9% | 1 | 52 | 0.0% | 0 | 25 | 0.0% | 0 | 46 | 0.4% | 1 | 244 | ||||||
| Treatment for prolapse at 2 years | |||||||||||||||||||||
| Medicines for prolapse | 11.9% | 5 | 42 | 17.9% | 7 | 39 | 1.80 | 0.61 to 5.36 | 0.288 | 10.0% | 2 | 20 | 7.7% | 3 | 39 | 0.72 | 0.13 to 3.88 | 0.701 | 16.3% | 31 | 190 |
| Oestrogens | 16.3% | 7 | 43 | 28.2% | 11 | 39 | 1.74 | 0.75 to 4.07 | 0.198 | 15.0% | 3 | 20 | 23.1% | 9 | 39 | 1.50 | 0.47 to 4.81 | 0.492 | 25.1% | 48 | 191 |
| Ring pessary | 4.7% | 2 | 43 | 2.6% | 1 | 39 | 0.61 | 0.06 to 6.33 | 0.682 | 5.0% | 1 | 20 | 2.6% | 1 | 39 | 0.56 | 0.04 to 8.28 | 0.673 | 2.6% | 5 | 191 |
| Shelf pessary | 7.0% | 3 | 43 | 2.6% | 1 | 39 | 0.41 | 0.05 to 3.66 | 0.424 | 5.0% | 1 | 20 | 0.0% | 0 | 39 | N/A | N/A | N/A | 3.1% | 6 | 191 |
| Physiotherapy | 4.8% | 2 | 42 | 10.3% | 4 | 39 | 1.97 | 0.39 to 9.99 | 0.411 | 5.0% | 1 | 20 | 5.1% | 2 | 39 | 1.16 | 0.11 to 11.96 | 0.902 | 10.2% | 19 | 187 |
| GP for prolapse | 9.5% | 4 | 42 | 18.4% | 7 | 38 | 1.72 | 0.55 to 5.40 | 0.355 | 15.8% | 3 | 19 | 10.5% | 4 | 38 | 0.77 | 0.20 to 3.00 | 0.704 | 18.2% | 34 | 187 |
| Practice nurse | 9.3% | 4 | 43 | 2.6% | 1 | 38 | 0.26 | 0.03 to 2.19 | 0.214 | 15.0% | 3 | 20 | 0.0% | 0 | 39 | N/A | N/A | N/A | 4.9% | 9 | 185 |
| GOPD | 23.3% | 10 | 43 | 26.3% | 10 | 38 | 1.08 | 0.51 to 2.31 | 0.834 | 15.0% | 3 | 20 | 12.8% | 5 | 39 | 0.84 | 0.23 to 3.07 | 0.791 | 21.3% | 40 | 188 |
| Treatment for urinary problems at 2 years | |||||||||||||||||||||
| Pads | 27.9% | 12 | 43 | 20.5% | 8 | 39 | 0.67 | 0.30 to 1.48 | 0.320 | 15.0% | 3 | 20 | 27.0% | 10 | 37 | 1.57 | 0.48 to 5.10 | 0.455 | 41.2% | 77 | 187 |
| Permanent catheter | 0.0% | 0 | 42 | 0.0% | 0 | 38 | N/A | N/A | N/A | 5.3% | 1 | 19 | 0.0% | 0 | 38 | N/A | N/A | N/A | 0.0% | 0 | 188 |
| Intermittent catheter | 2.4% | 1 | 42 | 0.0% | 0 | 38 | N/A | N/A | N/A | 5.3% | 1 | 19 | 2.6% | 1 | 38 | 0.54 | 0.04 to 7.98 | 0.655 | 1.6% | 3 | 187 |
| Drugs for UI | 14.0% | 6 | 43 | 17.9% | 7 | 39 | 1.28 | 0.45 to 3.61 | 0.644 | 5.0% | 1 | 20 | 15.4% | 6 | 39 | 3.34 | 0.44 to 25.41 | 0.243 | 13.1% | 25 | 191 |
Readmission for new prolapse surgery
Four women reported that they had had new prolapse surgery in the first year and a further eight in the second year (at least one woman in each of the randomised groups), with one woman having surgery in both years. The numbers were too few to draw conclusions (see Table 66). Complete questionnaire data were received from 122 randomised women and 185 cohort women. The repeat prolapse surgery rate was therefore 10 of 122 (8.2%) for the randomised women and 17 of 185 (9.2%) in the cohort (see Table 66).
Readmission for continence surgery
Only three randomised women had continence surgery in the first year, and another three in the second year (see Table 66). The numbers were too few to differentiate between the groups. The surgery rate was 5 of 122 (4.1%) for the randomised women. In the cohort, three women had continence surgery in the first year, and four in the second year, with one woman having continence surgery in both years. The surgery rate was 6 of 186 (3.2%) in the cohort.
Treatment for mesh complications in the Secondary trials
In the first year, none of the women in the standard group and 6/52 (11.5%) in the mesh inlay group had a mesh complication (see Table 66). There were a further 3 of 46 (6.5%) mesh complications in the mesh kit group. Three women in the mesh inlay group had surgery to remove or overlay the mesh, and one in the mesh kit group. In the second year, none of the women in the standard group and 2/52 (3.8%) in the mesh inlay group had a mesh complication, with a further 2 of 46 (4.3%) in the mesh kit group. Of these, only one of the women in the mesh inlay group and one in the mesh kit group required surgical correction of the mesh exposure.
So in total, six women required mesh surgery in the 2 years of follow-up. A further six women received conservative treatment (such as mesh trimming in outpatients, oestrogen treatment or cautery with silver nitrate) and the rest did not require any treatment (see Table 66).
The cumulative mesh complication rates over 2 years were 7 of 52 (13.5%) for mesh inlay and 4 of 46 (8.7%) for mesh kit, with no mesh exposures after standard repair.
Other treatment for prolapse symptoms
Few women required other treatment – such as pessaries or physiotherapy – for symptoms, and there was no difference between the randomised groups regarding further use of services (see also Chapter 7).
Satisfaction with treatment at 1 year and 2 years
Women reported that they took around 3 months to resume normal activities (Table 67), with no differences between the randomised groups in trial 3, but in trial 4 the 19 women in the standard arm took significantly longer than those who were randomised to a mesh kit (p = 0.011). Over 80% of the randomised women reported that they were very much or much better than before surgery at 1 year, and similar proportions were completely or fairly satisfied. At 1 year, 89.7% of randomised women reported that they would ‘recommend the surgery to a friend’. The data were similar at 1 year and 2 years (see Table 67), suggesting that, on average, the positive benefits of surgery were sustained, with no statistically significant differences between the groups in either trial. The results for the cohort women were similar (see Table 67).
| Recovery/satisfaction’ | Trial 3: standard repair vs. synthetic mesh | Trial 4: standard repair vs. mesh kit | CC2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | Synthetic | Effect size | 95% CI | p-value | Standard | Mesh kit | Effect size | 95% CI | p-value | ||||||||||||
| 1-year questionnaires | N = 46 | N = 39 | N = 21 | N = 41 | N = 191 | ||||||||||||||||
| Time to recovery (months) | 3.4 | (2.4) | 42 | 3.5 | (2.2) | 39 | 0.20 | –0.77 to 1.16 | 0.681 | 4.5 | (3.0) | 19 | 2.9 | (1.5) | 34 | –1.66 | –2.91 to –0.41 | 0.011 | 3.2 | (1.8) | 170 |
| Prolapse compared with before surgery | |||||||||||||||||||||
| Very much better | 51.2% | 22 | 43 | 55.3% | 21 | 38 | 1.18 | 0.47 to 2.95 | 0.731 | 61.1% | 11 | 18 | 46.2% | 18 | 35.4% | 64 | 181 | 0.372 | 35.4% | 64 | 181 |
| Much better | 25.6% | 11 | 43 | 26.3% | 10 | 38 | 16.7% | 3 | 18 | 41.0% | 16 | 33.7% | 61 | 181 | 33.7% | 61 | 181 | ||||
| A little better | 18.6% | 8 | 43 | 10.5% | 4 | 38 | 16.7% | 3 | 18 | 5.1% | 2 | 13.3% | 24 | 181 | 13.3% | 24 | 181 | ||||
| No change | 0.0% | 0 | 43 | 7.9% | 3 | 38 | 0.0% | 0 | 18 | 0.0% | 0 | 6.1% | 11 | 181 | 6.1% | 11 | 181 | ||||
| A little worse | 0.0% | 0 | 43 | 0.0% | 0 | 38 | 0.0% | 0 | 18 | 2.6% | 1 | 6.1% | 11 | 181 | 6.1% | 11 | 181 | ||||
| Much worse | 2.3% | 1 | 43 | 0.0% | 0 | 38 | 5.6% | 1 | 18 | 2.6% | 1 | 1.7% | 3 | 181 | 1.7% | 3 | 181 | ||||
| Very much worse | 2.3% | 1 | 43 | 0.0% | 0 | 38 | 0.0% | 0 | 18 | 2.6% | 1 | 3.9% | 7 | 181 | 3.9% | 7 | 181 | ||||
| Satisfaction with surgery | |||||||||||||||||||||
| Completely satisfied | 45.5% | 20 | 44 | 41.0% | 16 | 39 | 0.82 | 0.31 to 2.16 | 0.685 | 52.6% | 10 | 19 | 43.6% | 17 | 35.4% | 64 | 181 | 0.534 | 35.4% | 64 | 181 |
| Fairly satisfied | 38.6% | 17 | 44 | 35.9% | 14 | 39 | 36.8% | 7 | 19 | 33.3% | 13 | 43.1% | 78 | 181 | 43.1% | 78 | 181 | ||||
| Fairly dissatisfied | 9.1% | 4 | 44 | 7.7% | 3 | 39 | 5.3% | 1 | 19 | 10.3% | 4 | 7.2% | 13 | 181 | 7.2% | 13 | 181 | ||||
| Very dissatisfied | 2.3% | 1 | 44 | 2.6% | 1 | 39 | 0.0% | 0 | 19 | 2.6% | 1 | 9.4% | 17 | 181 | 9.4% | 17 | 181 | ||||
| Not sure | 4.5% | 2 | 44 | 12.8% | 5 | 39 | 5.3% | 1 | 19 | 10.3% | 4 | 5.0% | 9 | 181 | 5.0% | 9 | 181 | ||||
| Recommend to a friend | 90.5% | 38 | 42 | 86.5% | 32 | 37 | 0.95 | 0.81 to 1.12 | 0.575 | 94.4% | 17 | 18 | 91.9% | 34 | 83.8% | 145 | 173 | 0.653 | 83.8% | 145 | 173 |
| 2-year questionnaires | N = 43 | N = 39 | N = 20 | N = 39 | N = 191 | ||||||||||||||||
| Prolapse compared with before surgery | |||||||||||||||||||||
| Very much better | 47.6% | 20 | 42 | 48.7% | 19 | 39 | 1.01 | 0.41 to 2.49 | 0.974 | 45.0% | 9 | 20 | 50.0% | 19 | 38 | 1.53 | 0.47 to 4.96 | 0.478 | 37.4% | 70 | 187 |
| Much better | 33.3% | 14 | 42 | 25.6% | 10 | 39 | 40.0% | 8 | 20 | 39.5% | 15 | 38 | 28.3% | 53 | 187 | ||||||
| A little better | 9.5% | 4 | 42 | 10.3% | 4 | 39 | 0.0% | 0 | 20 | 5.3% | 2 | 38 | 17.6% | 33 | 187 | ||||||
| No change | 4.8% | 2 | 42 | 12.8% | 5 | 39 | 5.0% | 1 | 20 | 0.0% | 0 | 38 | 6.4% | 12 | 187 | ||||||
| A little worse | 2.4% | 1 | 42 | 2.6% | 1 | 39 | 5.0% | 1 | 20 | 2.6% | 1 | 38 | 3.7% | 7 | 187 | ||||||
| Much worse | 2.4% | 1 | 42 | 0.0% | 0 | 39 | 5.0% | 1 | 20 | 0.0% | 0 | 38 | 4.8% | 9 | 187 | ||||||
| Very much worse | 0.0% | 0 | 42 | 0.0% | 0 | 39 | 0.0% | 0 | 20 | 2.6% | 1 | 38 | 1.6% | 3 | 187 | ||||||
| Satisfaction with surgery | |||||||||||||||||||||
| Completely satisfied | 50.0% | 20 | 40 | 48.7% | 19 | 39 | 0.96 | 0.37 to 2.49 | 0.935 | 38.9% | 7 | 18 | 52.6% | 20 | 38 | 1.87 | 0.56 to 6.24 | 0.309 | 38.9% | 72 | 185 |
| Fairly satisfied | 37.5% | 15 | 40 | 35.9% | 14 | 39 | 55.6% | 10 | 18 | 36.8% | 14 | 38 | 34.6% | 64 | 185 | ||||||
| Fairly dissatisfied | 2.5% | 1 | 40 | 2.6% | 1 | 39 | 0.0% | 0 | 18 | 2.6% | 1 | 38 | 13.5% | 25 | 185 | ||||||
| Very dissatisfied | 2.5% | 1 | 40 | 7.7% | 3 | 39 | 5.6% | 1 | 18 | 2.6% | 1 | 38 | 9.7% | 18 | 185 | ||||||
| Not sure | 7.5% | 3 | 40 | 5.1% | 2 | 39 | 0.0% | 0 | 18 | 5.3% | 2 | 38 | 3.2% | 6 | 185 | ||||||
| Recommend to a friend | 97.5% | 39 | 40 | 82.1% | 32 | 39 | 0.83 | 0.65 to 1.06 | 0.00 | 100.0% | 18 | 18 | 97.2% | 35 | 36 | N/A | N/A | N/A | 81.6% | 142 | 174 |
These findings are in line with the clinical outcome data, supporting the positive and sustained benefits of prolapse surgery for the majority of women.
Discussion
Summary of findings
Effectiveness
There were no statistically significant differences at 1 year in the primary clinical or economic outcomes after prolapse surgery using native tissue, synthetic non-absorbable mesh or a mesh kit to reinforce the repair in either trial 3 or trial 4. However, because of the smaller numbers, these trials did not have enough statistical power to rule out any potentially true clinical differences. This was reflected in the wide CIs around the primary outcome – prolapse symptoms measured by the POP-SS at 1 year – of –0.41 (95% CI –2.92 to 2.11) in trial 3 and –1.21 (95% CI –4.13 to 1.72) in trial 4 (see Table 59). Although there were also no differences in the secondary clinical or objective outcomes, or in the proportion of women requiring further treatment in either of the trials, these analyses were not sufficiently powered to be conclusive.
Adverse effects
The overall incidence of serious adverse effects was around 10% (see Table 66), primarily pain, infection and urinary retention. Women could have a mesh-related complication only if they received mesh: the total numbers are very small. The cumulative mesh complication rates over 2 years were 7 of 52 (13.5%) for mesh inlay and 4 of 46 (8.7%) for mesh kit, with no mesh exposures after standard repair.
Cost-effectiveness
See Chapter 7.
Strengths and weaknesses
Strengths
As the women who were having a repeat repair were recruited opportunistically alongside the women who were having their first repair, the numbers were, as expected, too small to achieve statistical power for any of the outcomes. The numbers were reduced by our very strict definition of ‘repeat repair’, which required that the previous surgery had to be in the same compartment. About 10% of women who had previous surgery in the opposite compartment were therefore recruited into the Primary trial (see Chapter 4). In addition, women were less likely to be randomised than in the Primary trial (around 61% declined randomisation, further reducing the numbers).
The PROSPECT Study is rare in being one of the few RCTs to distinguish between primary and secondary surgery. Although the trial on its own was not sufficiently powered to detect a difference, this had two important implications. First, the information is now available for meta-analysis with other trials if women who were having a further repair are reported separately. If this happens, a meta-analysis in the future will shed more light on the fate of women whose surgery has already failed. It has therefore set a marker to all future trials (and indeed past ones) that prolapse surgery trials should be reported using the subgroups of primary and secondary surgery (defined as repeat in the same compartment). Second, it has made the clinical community more aware of the distinction, both in the presentation of their patients and that different treatment might be appropriate for these women.
Another one of the strengths of the Secondary trial was its pragmatic reflection of actual practice in a representative sample of UK prolapse surgeons across a large number of hospital settings. This was reflected in the range of concomitant surgery, and our ability to differentiate between women who were having a first or a repeat procedure in a particular compartment. Owing to preference or local resources available, surgeons were not all able to randomise between all three options but the analysis by strata took account of this.
Non-randomised cohort
Another strength was the inclusion of women who were not randomised. Data collection using the same questionnaires as for the randomised (trial) women demonstrated that our population was representative of the majority of women who were having prolapse surgery in the UK (see Chapter 3). Outcome data collected from those cohort women demonstrated that their outcomes were similar to those from the randomised women, thus ensuring generalisability of the findings. A further benefit was the ability to identify rare adverse effects.
Pragmatic nature of the research
Our secure and effective randomisation programme ensured that women were comparable at baseline and that concomitant surgery and other confounding variables were accounted for.
Validated outcome measures
We used validated outcome measures – such as the Pelvic Organ Prolapse Symptom scale and ICI-Q suite of instruments – to measure women’s symptoms of pelvic floor dysfunction, ensuring that our findings are comparable with other trials and can be combined in meta-analysis. We captured a wide range of adverse effects and made efforts to verify these from alternative sources when possible. Essential missing data were actively sought from the women. Participants, outcome assessors and data entry clerks were blinded to randomisation and, as far as possible, to treatment actually received.
Weaknesses
Limitations of our study should be acknowledged. The large number of interventions and outcomes make it likely that some differences may have occurred by chance. Furthermore, the smaller numbers in the Secondary trial meant that we would have been able to identify only large differences, which we did not find.
The number of women randomised to the secondary RCT was small. The trial was not designed so that a prespecified level of power should be achieved (unlike the primary RCT). Further to this, there were fewer recruits to the Secondary trial than had been anticipated as a result of our stricter definition of a secondary repair.
Because of the chronic and relapsing nature of prolapse, longer follow-up is required: the average time to a repeat operation is around 12 years. 4 Although we did not identify differences in the repeat surgery rate between the groups, it is likely that 2 years is too short a time scale to provide a definitive answer. We have commenced follow-up of the PROSPECT women for at least 6 years after surgery, and also plan electronic data linkage to capture outcomes from non-responders.
The POP-Q system classes measurements from –1 cm inside the hymen to 1 cm as stage 2. We and other researchers28 have arbitrarily used a measurement of > 0 cm to indicate objective failure, while recognising that women with worse findings may not have symptoms and vice versa (women with objective ‘cure’ may still have prolapse symptoms). However, Table 65 shows that the findings would have been the same whichever stage of prolapse was chosen as the cut-off.
Conclusions
The information from the Secondary trial has been inconclusive either in terms of symptoms or anatomical cure from the use of synthetic mesh or a mesh kit in women who were having repeat prolapse surgery. We encourage existing and future triallists to report results for primary and repeat prolapse surgery separately, so that more information can be identified for this group of women who are at even higher risk of failure than after their first repair. 4
We are unable to say whether or not mesh or a mesh kit confers benefit to women who were having a repeat prolapse repair, in terms of prolapse symptoms or anatomical cure of prolapse in the first 2 years after surgery. However, some women required an additional surgical procedure to remove exposed mesh, which may be considered to be an unnecessary risk. Long-term follow-up may show whether or not the excess risk is compensated for by a decreased need for repeat surgery in the future.
Chapter 7 Health economics results: Secondary trial
This chapter describes the results of the within-trial cost–utility analysis over 2 years of follow-up for women who were having a secondary prolapse surgical procedure. These are the results pertaining to women randomised to RCT2A (three-way comparison of mesh kits vs. mesh inlay vs. standard repair) and RCT2B (comparisons of mesh inlay vs. standard repair).
For the purposes of the Secondary trial analysis, we have chosen to present the base case for all secondary repair women receiving a randomised treatment. This approach differs from that adopted for the base-case analysis of the economic analysis for women receiving a primary prolapse repair (see Chapter 5). The rationale for taking this alternative approach for the economic analysis of the Secondary trial data is that because of the small sample size available for the Secondary trial, the approach generates the greatest power for analysis. The approach outlined here is similar to that undertaken for the statistical analysis of the clinical outcomes data. A sensitivity analysis will explore the use of a more purist approach to analysis, including data from the three-way comparison only (i.e. RCT2A).
As with the primary repair trial analysis in Chapter 5, the chapter begins with a presentation of the EQ-5D-3L and QALY results, followed by costs and cost-effectiveness, measured as incremental cost per QALY gained. The analysis perspective remains that of NHS decision-makers for the base case; however, a supplementary analysis is presented, which outlines the results of the economic analysis, incorporating a wider economic perspective (including the cost of participant and companion time and travel, participant-purchased health care and wider personal and economic costs as a result of time off work because of prolapse symptoms and problems). The chapter concludes with a range of deterministic sensitivity analyses to explore uncertainty in the results presented. Where appropriate, aggregate data from the secondary repair trial will be adapted and combined with data from the Secondary CC (CC2) to populate the secondary repair arms of the economic decision-analytic model in Chapter 9.
Generic quality-of-life outcomes (EuroQol-5 Dimensions, quality-adjusted life-years)
The proportion of women with any health problems reported on the EQ-5D-3L measure of generic QoL is shown in Figures 17–19. These figures present the data as reported by women across randomised (standard repair, mesh inlay and mesh kit) groups at 6 months, 1 year and 2 years, respectively. The descriptive data in Figures 17–19 are based on all of the available data recorded. This contrasts with the economic evaluation data in later sections, which are based on complete cost and QALY pairs. The figures illustrate the percentage of women who were having any problems on each of the domains of QoL (i.e. women scoring a ‘2’ or a ‘3’). Over half of all of the women responding to the EQ-5D-3L questionnaire report having some pain or discomfort at baseline and 1 year after surgery. There are some indications that the proportion experiencing pain and discomfort may fall after 2 years. However, because of the small sample size in the Secondary trial, it is difficult to draw any clear conclusions regarding potential differences across treatment groups and time points. Across the randomised arms, the fewest problems were experienced in self-care, with only a small proportion of women reporting any problems. A visual inspection of the graphical data appears to show some differences in the percentage of women experiencing problems; however, this variation is not consistent across time points, and there does not appear to be any systematic differences evident. Instead, the variation evident on the graph is most likely to be due to the relatively small sample size for the Secondary trial.
FIGURE 17.
Proportion of women experiencing any problems on each EQ-5D domain at 6 months. Analysis based on all of the available EQ-5D data points.
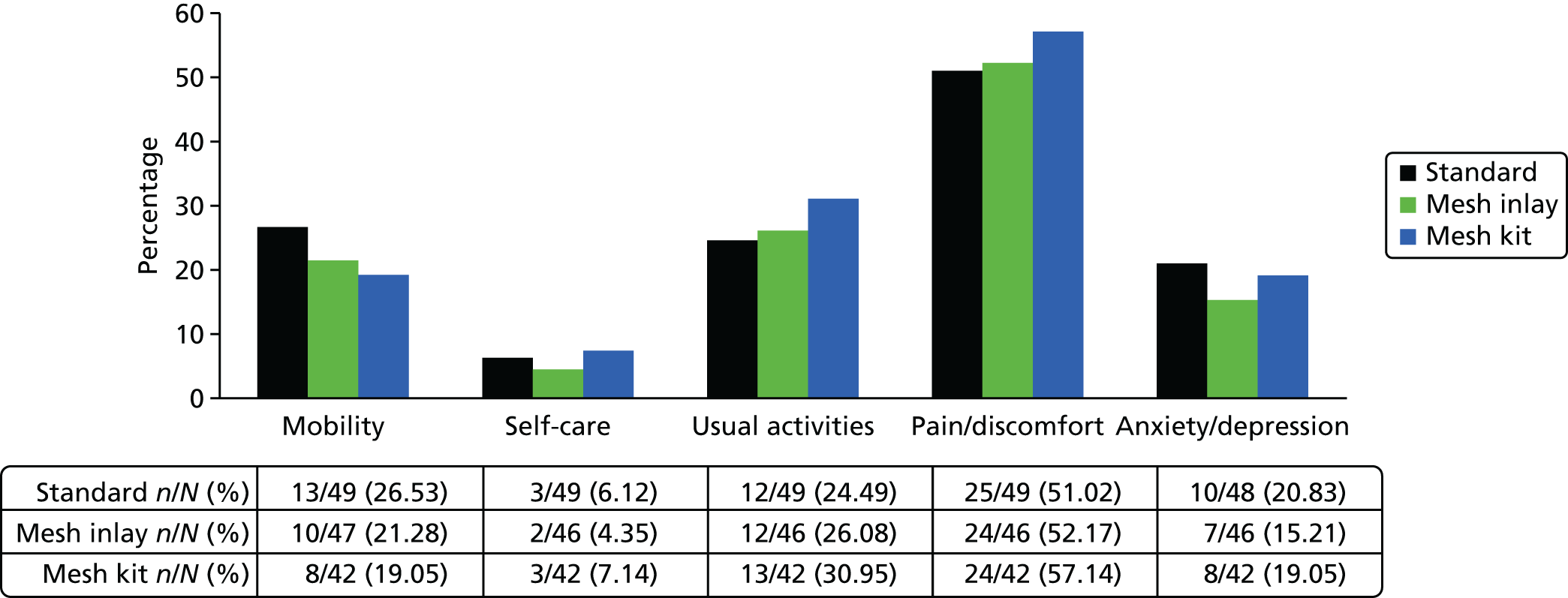
FIGURE 18.
Proportion of women experiencing any problems on each EQ-5D domain at 1 year. Analysis based on all of the available EQ-5D data points.

FIGURE 19.
Proportion of women experiencing any problems on each EQ-5D domain at 2 years. Analysis based on all of the available EQ-5D data points.
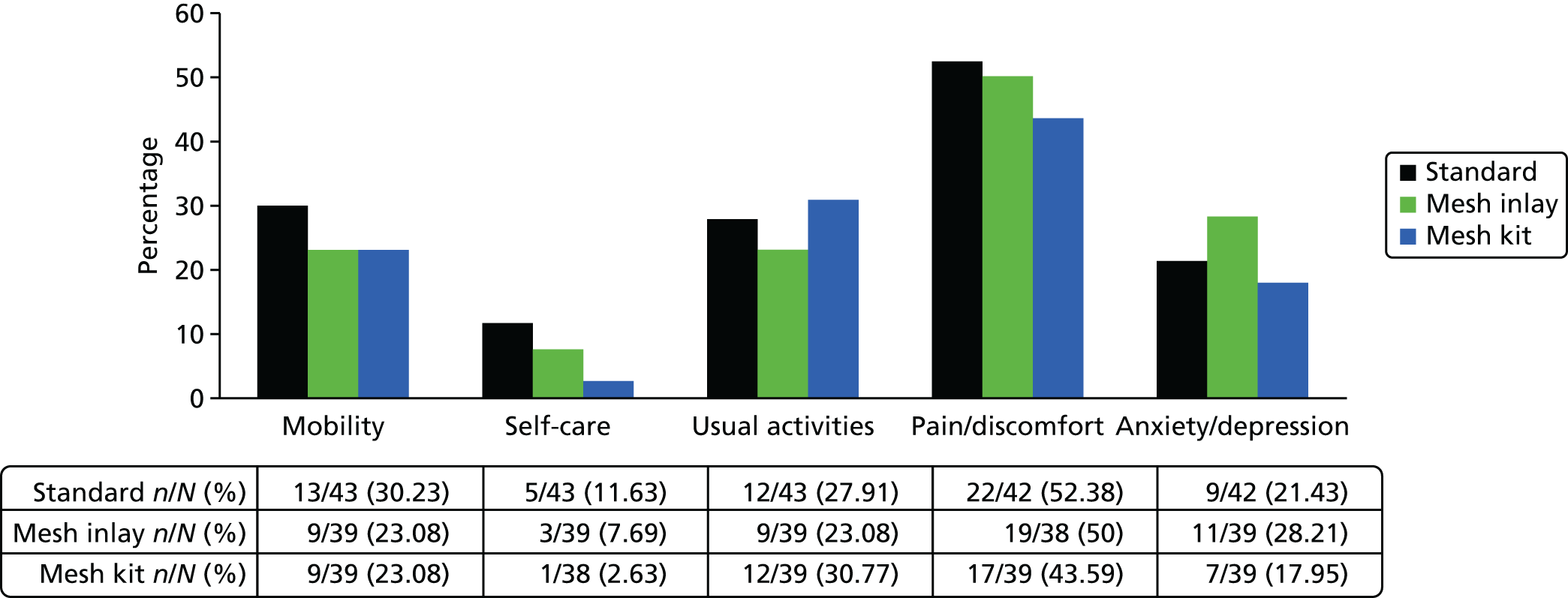
Figures 17–19 indicate the individual aspects of QoL that impact on overall utility for these women. Table 68 provides the utility weights generated using time trade-off tariffs, which use population preferences to value health outcomes on a scale for which ‘1’ represents full health and ‘0’ represents death. These utility values are used for QALY calculations. Note that in Table 68, data are presented for summary statistics for all of the randomised women to the Secondary trial (i.e. all women randomised to RCT2).
| Treatment group | Standard pair: mean (SD); n | Mesh Iay: mean (SD); n | Mesh kit: mean (SD); n |
|---|---|---|---|
| EQ-5D: baseline | 0.641 (0.296); 52 | 0.749 (0.161); 50 | 0.692 (0.238); 42 |
| EQ-5D: 6 months | 0.769 (0.306); 48 | 0.826 (0.209); 46 | 0.771 (0.263); 42 |
| EQ-5D: 12 months | 0.743 (0.304); 50 | 0.830 (0.218); 43 | 0.827 (0.194); 41 |
| QALYs gaineda (baseline to 1 year) | 0.727 (0.268); 45 | 0.816 (0.148); 42 | 0.764 (0.191); 38 |
| EQ-5D: 24 months | 0.764 (0.294); 42 | 0.818 (0.194); 38 | 0.867 (0.142); 38 |
| QALYs gaineda (baseline to 2 years) | 1.476 (0.501); 38 | 1.606 (0.332); 35 | 1.614 (0.306); 34 |
On initial visual inspection of the data, it would appear as if QALYs for the mesh inlay and mesh kit groups are substantially higher than in the standard repair group (discounted 2-year QALY = 1.476). However, these results should be considered in the light of substantial variation across secondary repair women who also reported better health outcomes on the EQ-5D-3L at baseline. It is important, therefore, to adjust QALY estimates for these baseline imbalances across groups. Table 69 presents these incremental QALYs gained as two effect sizes: the first for mesh inlay compared with standard repair and the second for mesh kit compared with standard repair. The estimates of QALYs gained are based on non-parametric bootstrapped linear regression (OLS) models, with adjustment for baseline covariates, including baseline EQ-5D-3L score, centre, age, BMI, concomitant continence procedure at baseline and compartment of prolapse. Additionally, the analysis model includes a fixed effect for randomised stratum. Analyses were conducted using heteroscedastic robust SEs.
| QALYs | Outcomes | |||||
|---|---|---|---|---|---|---|
| 1 year | 2 yeara | |||||
| Mean (SD); n | Raw MD (vs. standard) | Adj. MD (vs. standard) (95% CI)b | Mean (SD); n | Raw MD (vs. standard) | Adjusted MD (vs. standard) (95% CI)b | |
| Standard repair | 0.727 (0.268); 45 | 1.476 (0.501); 38 | ||||
| Mesh inlay | 0.816 (0.148); 42 | 0.089 | 0.011 (–0.061 to 0.084) | 1.606 (0.332); 35 | 0.13 | 0.018 (–0.149 to 0.185) |
| Mesh kit | 0.764 (0.191); 38 | 0.037 | –0.011 (–0.086 to 0.064) | 1.614 (0.306); 34 | 0.138 | 0.096 (–0.081 to 0.274) |
There were no differences in QALYs between treatment groups at 1 year or 2 years. As with the Primary trial analysis, baseline EQ-5D-3L utility score was a significant predictor of overall QALYs, thus emphasising the importance of controlling for baseline utility when estimating QALY gains or losses between treatment groups. None of the other variables included in the estimation models was found to have a significant effect on QALYs gained. Overall, there is no evidence of any difference in generic QoL or QALYs between randomised groups over the follow-up period. The lack of any significant effect is likely to be due, in part, to the small sample size recruited to RCT2. Based on current evidence, there is insufficient information to definitively determine a favourable treatment approach for secondary prolapse repair in terms of QALYs gained and further research is required to determine the repair strategy that generates greatest QALY improvement for women who were having a secondary surgical prolapse repair. Despite uncertainty regarding the most beneficial strategy in terms of QALYs gained, it is important to consider this uncertainty alongside cost implications of different surgeries within a cost-effectiveness analysis framework.
NHS resource use and costs
Costs to health services
This section outlines the results of our analysis for costs to the health services of alternative strategies for secondary prolapse repair surgery. Costs include intervention procedure costs, inpatient and follow-up secondary care costs, and costs of primary care services relating to the prolapse surgery. This may include, for example, treatment of complications, treatment failure or increased contact with health-care professionals for prolapse-related issues. The descriptive statistics and the regression analyses are based on complete case cost data for all women who were having a secondary prolapse repair (RCT2). The total NHS costs are calculated by multiplying resource use by the appropriate unit cost estimates outlined in Table 2 (see Chapter 2).
Intervention costs
Table 70 reports the total intervention costs for each of the secondary surgical prolapse repairs considered.
| Intervention costs | Standard repair | Mesh inlay | Mesh kit | Incremental analysisa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | n | Mesh inlay vs. standard (£): MD (95% CI) | Mesh kit vs. standard (£): MD (95% CI) | |
| Mesh cost | 17 | 89 | 55 | 128 | 175 | 51 | 609 | 400 | 45 | ||
| Staff time in theatre | 818 | 370 | 55 | 859 | 281 | 52 | 856 | 329 | 46 | ||
| Cost of drugs in theatre | 25 | 7 | 55 | 24 | 8 | 52 | 25 | 8 | 46 | ||
| Cost of catheterisation | 6 | 2 | 55 | 6 | 2 | 52 | 6 | 1 | 46 | ||
| Cost of vaginal packing | 4 | 2 | 55 | 4 | 2 | 52 | 4 | 2 | 46 | ||
| Theatre overheads | 414 | 169 | 55 | 435 | 133 | 52 | 448 | 164 | 46 | ||
| Subtotal: theatre costs | 1282 | 551 | 55 | 1457 | 498 | 51 | 1949 | 632 | 45 | ||
| Costs from theatre: discharge | 1508 | 1139 | 55 | 1642 | 1214 | 51 | 1573 | 1069 | 45 | ||
| Total intervention costs | 2790 | 1295 | 55 | 3099 | 1358 | 51 | 3522 | 1100 | 45 | 398 (–197 to 993) | 914 (349 to 1478) |
All surgical procedures had similar costs associated with staff requirements, time in theatre and overheads to complete the respective procedures. Regarding the cost of materials, mesh kits were the most costly, followed by mesh inlays and standard repair. Summing these components together, there was no statistically significant difference in total intervention cost between mesh inlay and standard repair. However, this is probably because of the small sample size. Owing to the substantial additional cost of mesh kit materials, the total intervention cost for mesh kits was statistically significantly higher than a standard repair. It should be noted that the cost of materials for secondary mesh interventions were highly variable across centres and suppliers, as is evident in the large SDs reported. These analyses make no statements about the effectiveness or cost-effectiveness of one mesh inlay material relative to another, or one type of mesh kit relative to another.
Health services resource-use costs over trial follow-up
The additional costs of mesh procedures (in particular mesh kits) are combined with costs to the health services over the trial follow-up period for each treatment group and are presented in Table 71. These include all secondary care (readmissions, reoperations, visits to ward, outpatient consultations) and primary care (e.g. GP, nurse, physiotherapist) contacts with health professionals. We have taken the following approach to the presentation of cost data. Each category of cost is presented for full cases within that category (e.g. hospital resource use, primary care costs). These are then summed, along with the intervention cost, for complete cases across all of the categories, and presented as the total cost to the health services at 2 years. Data presented in Table 71 and for the statistical analyses are based on all women who were randomised to receive a secondary repair surgery (i.e. all women randomised to RCT2). Owing to the small sample size, non-parametric bootstrapping is used to create CIs around MDs in costs. Regression models include a fixed effect for randomised stratum and heteroscedastic robust SEs are used for all models. The costs presented are presented in accordance with the assumptions outlined in Chapter 2 regarding handling of missing data.
| NHS resource use and costs | Resource use: n/N (%) | Costsa | ||||||
|---|---|---|---|---|---|---|---|---|
| Standard repair | Mesh inlay | Mesh kit | Standard repair: mean (SD); N | Mesh inlay: mean (SD); N | Mesh kit: mean (SD); N | Incremental analysis of costs (£)b | ||
| Mesh inlay vs. standard | Mesh kit vs. standard | |||||||
| Total intervention costs | – | – | – | 2790 (1295); 55 | 3099 (1358); 51 | 3522 (1100); 45 | 398 (–197 to 993) | 914 (349 to 1478) |
| 1-year data | ||||||||
| Hospital resource use (0–6 months) | ||||||||
| New prolapse procedure | 0/50 (0.0) | 0/47 (0.0) | 1/43 (2.3) | |||||
| New incontinence procedure | 0/50 (0.0) | 0/47 (0.0) | 0/43 (0.0) | |||||
| Other related readmissions | 2/50 (4.0) | 5/47 (10.6) | 1/43 (2.3) | |||||
| Further prolapse-related surgery within 6 months | 2/50 (4.0) | 5/47 (10.6) | 2/43 (4.7) | 40 (203); 50 | 120 (355); 47 | 54 (355); 43 | ||
| Outpatient visits: with any visitb | 4/50 (8.0) | 3/47 (6.4) | 4/43 (9.3) | 11 (36); 50 | 8 (33); 47 | 12 (39); 43 | ||
| Subtotal (hospital use 0–6 months) | – | – | – | 51 (204); 50 | 128 (354); 47 | 67 (356); 43 | 98 (–40 to 237) | 86 (–53 to 224) |
| Hospital resource use (6–12 months) | ||||||||
| New prolapse procedure: | 3/49 (6.1) | 1/44 (2.3) | 0/44 (0.0) | 143 (565); 49 | 53 (351); 44 | 0 (0); 44 | ||
| New incontinence procedure | 0/49 (0.0) | 1/44 (2.3) | 2/44 (4.5) | 0 (0); 49 | 31 (207); 44 | 62 (289); 44 | ||
| Other related readmissions | 0/49 (0.0) | 2/44 (4.5) | 2/44 (4.5) | 0 (0); 49 | 55 (255); 44 | 55 (255); 44 | ||
| Outpatient visitsb | 0.59 (1.04) | 0.66 (1.14) | 0.64 (0.97) | 67 (103); 49 | 72 (113); 44 | 67 (98); 44 | ||
| Subtotal (hospital use 6–12 months) | – | – | – | 210 (612); 49 | 211 (481); 44 | 184 (409); 44 | –38 (–290 to 213) | 11 (–243 to 264) |
| Other consultations (0–12 months) | ||||||||
| Physiotherapyb | 0.08 (0.4) | 1.11 (3.20) | 0.11 (0.54) | 2 (10); 49 | 27 (77); 44 | 3 (13); 44 | ||
| GP nurseb | 0.04 (0.29) | 0.20 (0.70) | 0 (0) | 1 (4); 49 | 3 (10); 44 | 0 (0); 44 | ||
| GP doctorb | 0.55 (1.24) | 0.77 (1.36) | 0.98 (3.75) | 25 (57); 49 | 36 (63); 44 | 45 (173); 44 | ||
| Otherb | 0.11 (0.48) | 0.05 (0.30) | 0 (0) | 6 (28); 49 | 8 (56); 44 | 0 (0); 44 | ||
| Subtotal (other consultations 0–12 months) | – | – | – | 34 (65); 49 | 73 (111); 44 | 48 (175); 44 | 41 (–3 to 84) | 44 (–49 to 137) |
| Other treatments (0–12 months) | ||||||||
| Shelf pessary | 0/49 (0.0) | 0/44 (0.0) | 0/44 (0.0) | 0 (0); 49 | 0 (0); 44 | 0 (0); 44 | ||
| Ring pessary | 0/49 (0.0) | 0/44 (0.0) | 0/44 (0.0) | 0 (0); 49 | 0 (0); 44 | 0 (0); 44 | ||
| Incontinence drugs | 6/49 (12.2) | 9/44 (20.5) | 6/44 (13.6) | 4 (10); 49 | 6 (12); 44 | 6 (11); 44 | ||
| Oestrogen | 13/49 (26.5) | 15/44 (34.1) | 12/44 (27.3) | 5 (8); 49 | 6 (9); 44 | 5 (9); 44 | ||
| Intermittent catheters | 2/49 (4.1) | 0/44 (0.0) | 1/44 (2.3) | 74 (363); 49 | 0 (0); 44 | 41 (274); 44 | ||
| Permanent catheter | 1/49 (2.0) | 0/44 (0.0) | 0/44 (0.0) | 8 (56); 49 | 0 (0); 44 | 0 (0); 44 | ||
| Absorbent pads | 18/49 (36.7) | 16/44 (36.4) | 13/44 (29.5) | 211 (296); 49 | 226 (327); 44 | 277 (465); 44 | ||
| Other drug treatments | 1/49 (2.0) | 3/44 (6.8) | 0/44 (0.0) | 1 (9); 49 | 0 (1); 44 | 0 (0); 44 | ||
| Subtotal (other treatments 0–12 months) | – | – | – | 304 (512); 49 | 238 (332); 44 | 329 (523); 44 | –71 (–288 to 147) | –18 (–256 to 221) |
| Total 1-year follow-up costs | – | – | – | 607 (913); 47 | 657 (773); 44 | 580 (684); 43 | 9 (–387 to 405) | 15 (–362 to 392) |
| Total health services costs (1 year) | – | – | – | 3423 (1596); 47 | 3675 (1787); 44 | 4104 (1376); 43 | 421 (–397 to 1239) | 996 (296 to 1697) |
| 2-year data | ||||||||
| Hospital resource use (12–24 months) | ||||||||
| New prolapse procedure | 4/43 (9.3) | 3/38 (7.9) | 1/39 (2.6) | 210 (662); 43 | 178 (615); 38 | 58 (361); 39 | ||
| New incontinence procedure | 0/43 (0.0) | 1/38 (2.6) | 2/39 (5.1) | 0 (0); 43 | 35 (215); 38 | 68 (296); 39 | ||
| Other related readmissions | 0/43 (0.0) | 2/38 (5.3) | 0/39 (0.0) | 0 (0); 43 | 51 (225); 38 | 0 (0); 39 | ||
| Outpatient visitsb | 0.21 (0.47) | 0.26 (0.55) | 0.15 (0.49) | 20 (44); 43 | 25 (54); 39 | 16 (51); 39 | ||
| Subtotal (hospital resource use 12–24 months) | – | – | – | 229 (663); 43 | 288 (683); 38 | 142 (456); 39 | –111 (–473 to 251) | –161 (–470 to 148) |
| Other consultations (12–24 months) | ||||||||
| Physiotherapya | 0.05 (0.21) | 0.64 (3.25) | 0.18 (0.85) | 1 (5); 43 | 15 (75); 39 | 4 (20); 39 | ||
| GP nursea | 0.19 (0.63) | 0(0) | 0(0) | 2 (8); 43 | 0 (0); 39 | 0 (0); 39 | ||
| GP doctora | 0.19 (0.70) | 0.31 (1.06) | 0.77 (4.33) | 8 (31); 43 | 14 (47); 39 | 34 (192); 39 | ||
| Othera | 0.05 (0.31) | 0.08 (0.35) | 0(0) | 1 (7); 42 | 1 (3); 39 | 0 (0); 39 | ||
| Subtotal other consultations (12–24 months) | – | – | – | 13 (36); 42 | 29 (99); 39 | 38 (211); 39 | 7 (–28 to 42) | –3 (–22 to 16) |
| Other treatments (12–24 months) | ||||||||
| Shelf pessary | 3/43 (7.0) | 1/39 (2.6) | 0/39 (0.0) | 4 (16); 43 | 2 (10); 39 | 0 (0); 39 | ||
| Ring pessary | 2/43 (4.7) | 1/39 (2.6) | 1/39 (2.6) | 2 (8); 43 | 1 (6); 39 | 1 (6); 39 | ||
| Incontinence drugs | 6/43 (14.0) | 7/38 (18.4) | 6/39 (15.4) | 5 (9); 43 | 7 (12); 38 | 5 (12); 39 | ||
| Oestrogen | 7/46 (15.2) | 11/40 (27.5) | 9/39 (23.1) | 7 (10); 46 | 10 (11); 40 | 8 (11); 39 | ||
| Intermittent catheters | 1/43 (2.3) | 0/38 (0.0) | 1/39 (2.6) | 41 (268); 43 | 0 (0); 38 | 45 (281); 39 | ||
| Permanent catheter | 0/43 (0.0) | 0/38 (0.0) | 0/39 (0.0) | 0 (0); 43 | 0 (0); 38 | 0 (0); 39 | ||
| Absorbent pads | 12/43 (27.9) | 8/38 (21.1) | 10/39 (25.6) | 209 (366); 43 | 141 (301); 38 | 260 (630); 39 | ||
| Other drug treatments | 2/43 (4.7) | 2/38 (5.3) | 1/39 (2.6) | 1 (6); 43 | 10 (56); 38 | 0 (1); 39 | ||
| Subtotal, other treatments (12–24 months) | – | – | – | 268 (433); 43 | 167 (307); 38 | 319 (677); 39 | 2 (–171 to 175) | 130 (–111 to 371) |
| Total 2-year follow-up costs (12–24 months) | – | – | – | 521 (738); 42 | 485 (797); 38 | 499 (895); 39 | –101 (–512 to 310) | –35 (–441 to 371) |
| Total health services costs (2 years) | – | – | – | 3815 (2019); 41 | 4051 (2098); 37 | 4495 (1739); 39 | 238 (–929 to 1405) | 873 (–27 to 1774) |
At 1 year post operation, based on the data available from the Secondary trial, mesh kits are significantly more costly than the standard repair and are the most costly of the three treatment options. There were no significant differences between mesh inlays and standard repair in 1-year total costs to the health services. The cost results are primarily driven by the additional intervention cost of mesh kit repairs as outlined in the preceding section.
At 2-year follow-up, based on the data available from RCT2, there is some (weak; p < 0.1) evidence to suggest that women who were randomised to the mesh kit group incurred greater costs than standard repair. On average, mesh kits were £873 more costly than standard repair (95% CI –£27 to £1774). These additional costs to the health services was driven almost entirely by differences in the price of the original mesh product used for the surgical procedure. This evidence, although classified as weak, is based on a very small sample size, and is indicative of the substantial extra costs associated with the mesh kit intervention. There was not enough evidence to make any statements on the differences in treatment costs for the comparison of mesh inlay with standard repair. Incremental costs are £238 (95% CI –£929 to £1405). It should be noted that this estimate of incremental health services costs is surrounded by substantial uncertainty, large SDs and wide CIs.
Base-case cost-effectiveness results (NHS perspective)
Although there are no clear differences in costs evident as a result of the uncertainty surrounding estimates of incremental costs, it is important to consider the joint uncertainty across costs and outcomes within a cost-effectiveness analysis framework. The base-case economic (cost–utility) analysis is presented according to the regression models outlined for costs and QALYs in the methods section (see Chapter 2) and the presentation of results follows a similar approach to that of the Primary trial cost-effectiveness analysis. The base-case economic analysis is presented for complete case data of cost and QALY pairs, ensuring that the joint distribution of costs and effects is not broken. As with the data presented in previous tables, all analyses are for all women who were randomised to the Secondary trial comparison (RCT2). Owing to the small sample size, alternative combinations of costs and QALYs will be explored (e.g. considering complete case cost and complete case QALY data separately).
Cost-effectiveness results
One-year cost-effectiveness results
Table 72 presents the main results of the economic analysis from a NHS perspective over a 1-year time horizon. An initial interpretation of the results suggests that, based on ICERs presented, over a 1-year time horizon, neither mesh inlays nor mesh kits would offer a cost-effective use of NHS resources, based on commonly accepted threshold values of WTP for a QALY gained, set at between £20,000 and £30,000 per QALY. Data are based on complete case analysis of cost and QALY pairs. Owing to the small sample size, the point estimates of the ICERs are based on highly uncertain data, and estimates of incremental costs and QALYs that are surrounded by very wide CIs. Therefore, point estimates of the ICER are not particularly meaningful for the Secondary trial analysis and should be interpreted in light of the uncertainty surrounding them. Based on the data presented in the tables, there is insufficient evidence to draw any clear cost-effectiveness conclusions regarding the most cost-effective secondary repair strategy. The most appropriate interpretation of the cost-effectiveness data can be made using the simulations from the bootstrapped estimates of incremental costs and QALYs, with 1000 repetitions. Figure 20 illustrates the scatterplot of incremental costs and incremental QALYs for the 1-year analysis of the Secondary trial data for mesh inlay compared with standard repair, and also for mesh kit versus standard repair, although Figure 21 shows the CEACs, illustrating the probability of each strategy being cost-effective at alternative threshold values of WTP for a QALY gained. As can be seen from these figures, there is great uncertainty surrounding the optimal strategy in terms of cost-effectiveness. Using data presented in the CEACs, the probability of the interventions being cost-effective over a 1-year time horizon are as follows: standard repair (55%), mesh inlay (39%) and mesh kits (6%), demonstrating that there is no clearly cost-effective strategy, based on the current data. The only reasonable conclusion to draw from these data is that mesh kits do not appear to be cost-effective over 1 year. This is because of the substantial additional cost of the kits themselves.
| Treatment | Costs: mean (SD)a | Incremental costs (vs. standard) | QALYs: mean (SD)a | Incremental QALYs (vs. standard) | Incremental cost (£) per QALY gained (vs. standard) | Probability of cost-effectiveness at alternative threshold values of WTP for a QALY gain (%)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10k | £20k | £30k | £50k | ||||||
| Standard repair (n = 44) | 3454 (1639) | – | 0.728 (0.272) | – | – | 0.84 | 0.74 | 0.64 | 0.55 | 0.48 |
| Mesh inlay (n = 42) | 3734 (1808) | 471 (–404 to 1346) | 0.816 (0.148) | 0.007 (–0.060 to 0.074) | 67,286 | 0.16 | 0.24 | 0.33 | 0.39 | 0.44 |
| Mesh kits (n = 38) | 4165 (1386) | 933 (200 to 1665) | 0.764 (0.191) | –0.017 (–0.086 to 0.052) | Dominated | 0.00 | 0.02 | 0.04 | 0.06 | 0.08 |
FIGURE 20.
Scatterplot of incremental costs and QALYs for mesh treatments compared with standard repair: secondary prolapse repair (1-year follow-up data).
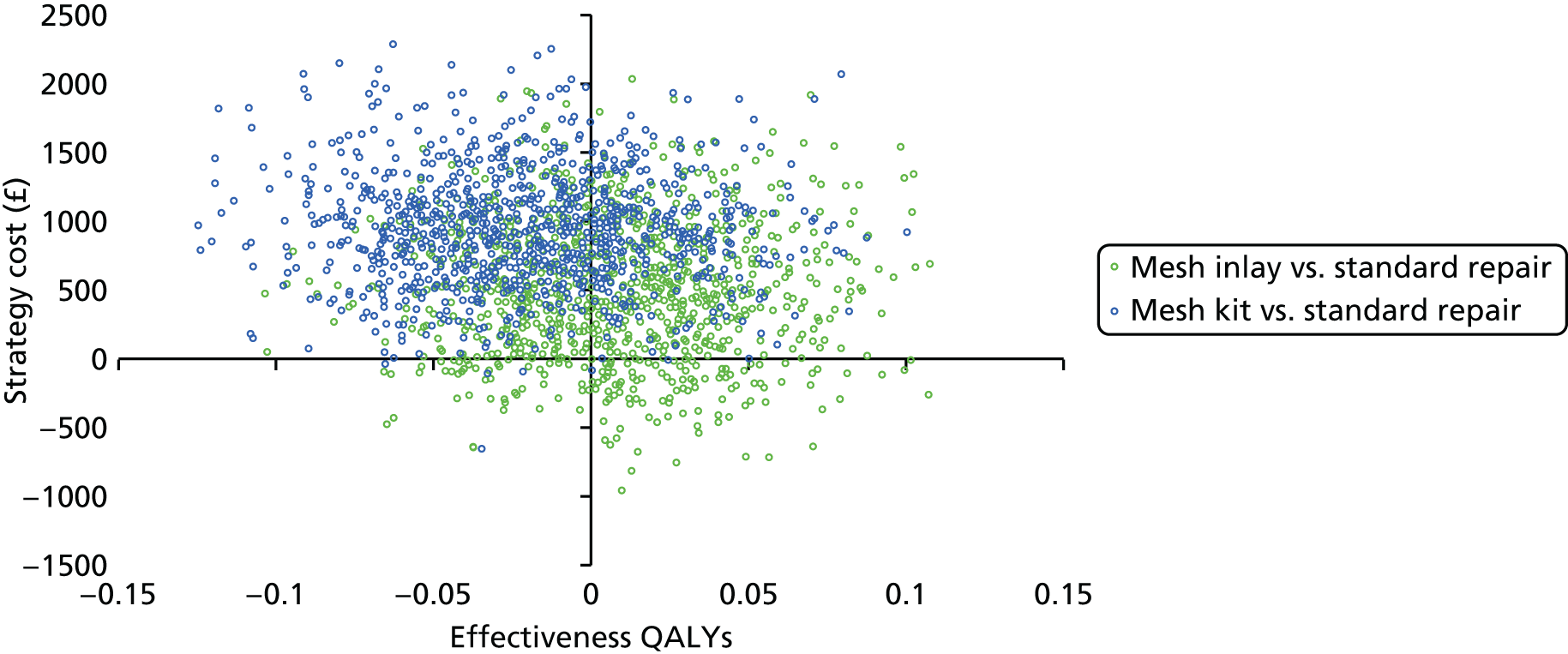
FIGURE 21.
Cost-effectiveness acceptability curves: secondary prolapse repair (1-year follow-up data).
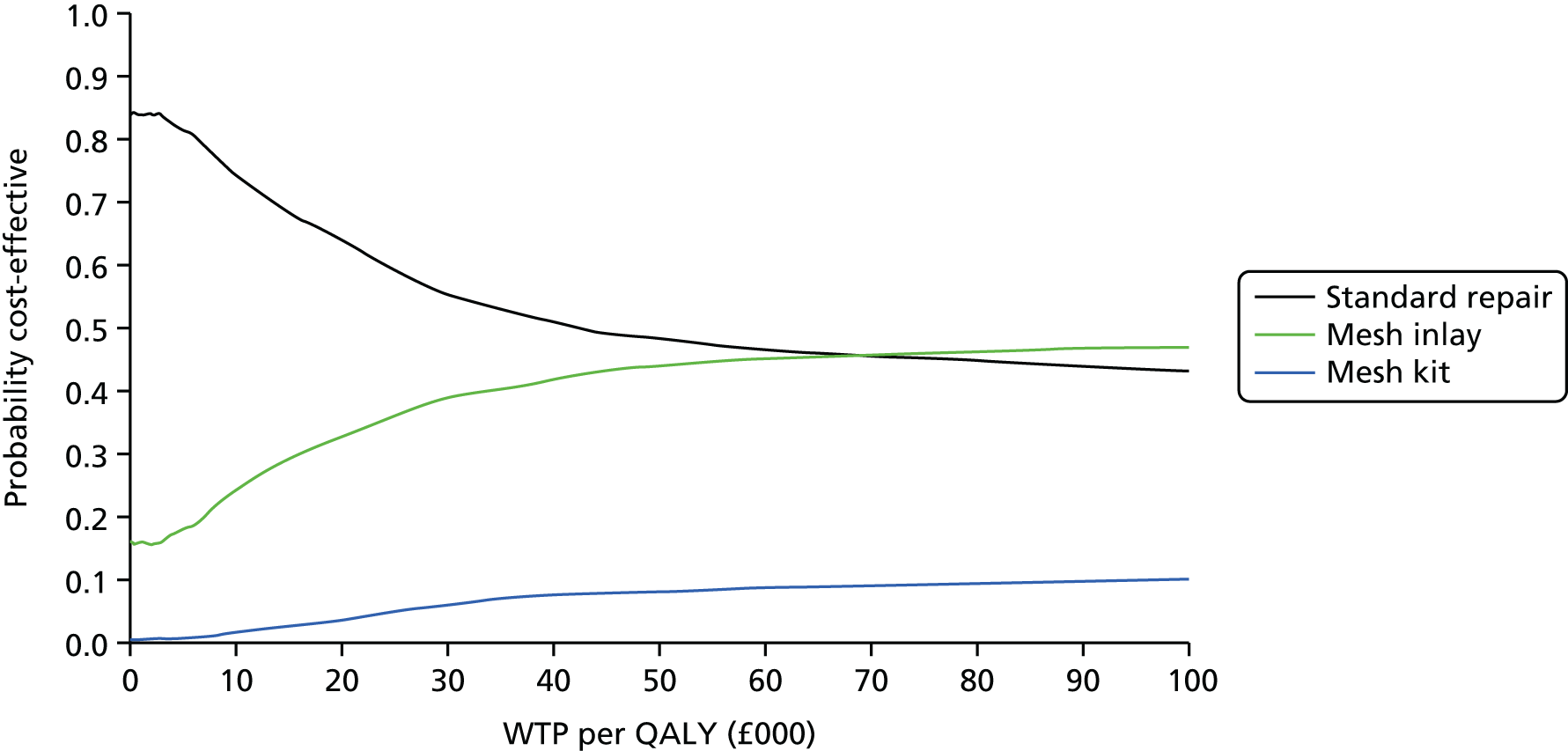
Two-year cost-effectiveness results
The results of the analysis over a 2-year time horizon, with costs and QALYs in the second year discounted at a rate of 3.5% per annum, are presented in Table 73 and Figures 22 and 23 for the base-case results, scatterplots of incremental cost-effectiveness and CEACs, respectively. Considering the same £30,000 threshold value of WTP for a QALY gained, the probability of cost-effectiveness for each treatment strategy is as follows: standard repair (32%), mesh inlay (19%) and mesh kit (49%). Based on the current data, at 2 years’ follow-up the mesh kits are the most likely to be cost-effective, followed by standard repair and mesh inlay. There is insufficient evidence, however, to clearly recommend any one treatment strategy for secondary prolapse repair, based on the data available, as none of the treatment strategies is definitively cost-effective. The figures illustrate that the estimates of incremental costs and QALYs are surrounded by considerable uncertainty as a result of the small sample size for the Secondary trial. Further research, based on larger samples, is required to definitively determine the most cost-effective strategy for women who were having a secondary prolapse repair.
| Treatment | Costs: mean (SD)a | Incremental costs (vs. standard) | QALYs: mean (SD)a | Incremental QALYs (vs. standard) | Incremental cost (£) per QALY gained (vs. standard) | Probability of cost-effectiveness at alternative threshold values of WTP for a QALY gain (%)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Standard repair (n = 36) | 3883 (2127) | 1.486 (0.493) | 0.57 | 0.45 | 0.36 | 0.32 | 0.29 | |||
| Mesh inlay (n = 34) | 4133 (2153) | 236 (–1091 to 1564) | 1.600 (0.335) | –0.023 (–0.163 to 0.118) | Dominated | 0.38 | 0.24 | 0.21 | 0.19 | 0.18 |
| Mesh kit (n = 34) | 4528 (1721) | 642 (–309 to 1592) | 1.614 (0.306) | 0.050 (–0.085 to 0.185) | 12,840 | 0.05 | 0.31 | 0.44 | 0.49 | 0.53 |
FIGURE 22.
Scatterplot of incremental costs and QALYs for mesh treatments compared with standard repair: secondary prolapse repair (2-year follow-up data).

FIGURE 23.
Cost-effectiveness acceptability curves: secondary prolapse repair (2-year follow-up data).
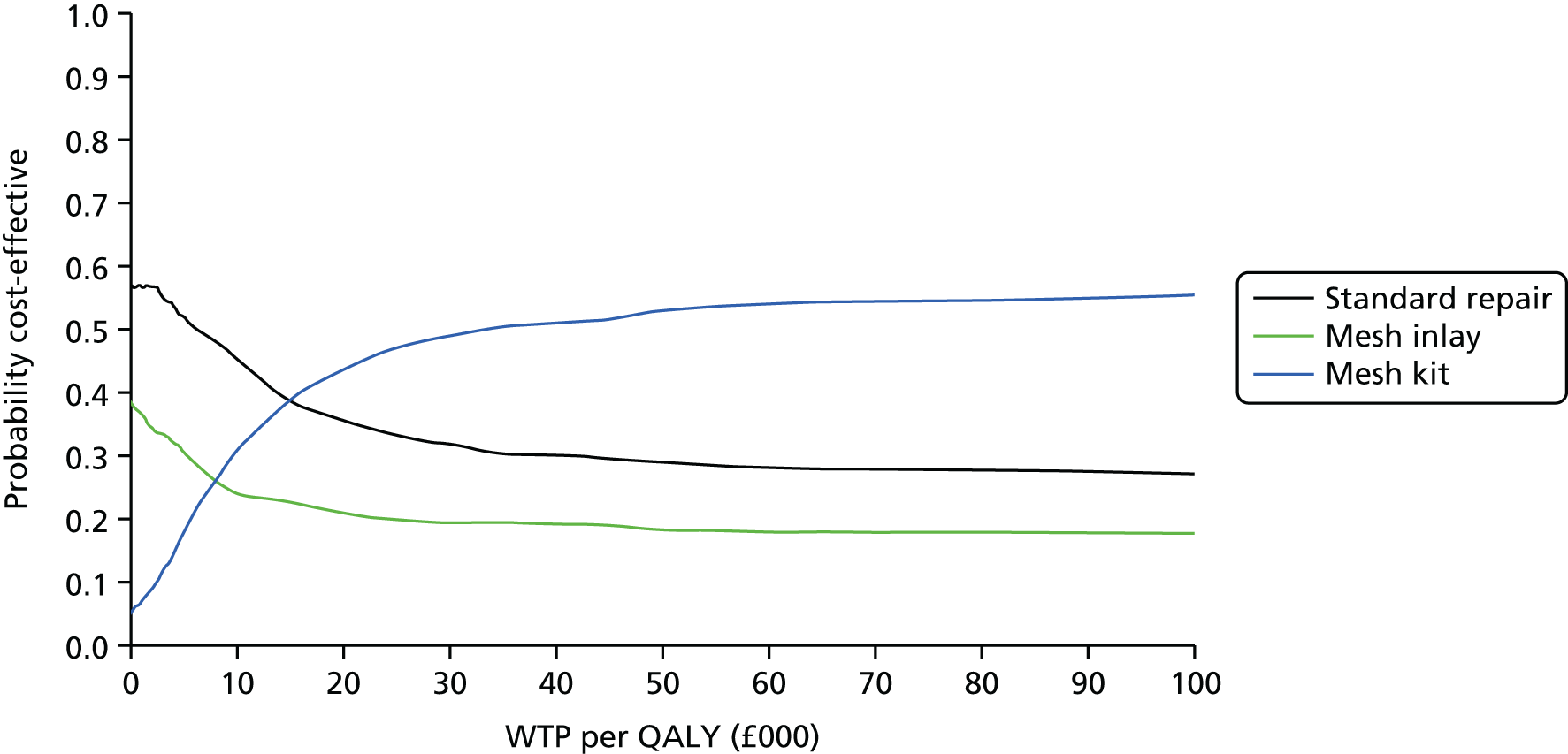
As with the Primary trial analysis, the true clinical effectiveness and cost-effectiveness of mesh materials for secondary prolapse repair cannot be determined by such a short time horizon, which may be insufficient to capture longer-term risk of recurrence and any associated complications. Longer-term data are thus required on costs and QALYs to accurately determine cost-effectiveness. Currently, the data available for secondary repairs are too sparse to populate an accurate economic modelled projection of longer-term outcomes, with too few data to develop time to effect analysis for probability of failures and serious complications. We therefore await the completion of longer-term follow-up of PROSPECT women to determine a more accurate estimate of cost-effectiveness for secondary prolapse repair.
Costs directly incurred by participants and indirect costs
A further analysis was conducted incorporating both participant and indirect costs into the analysis.
Table 74 reports mean costs (from a wider economic perspective) of attending primary care, outpatient appointments and inpatient admissions, respectively. Inpatient admissions also includes participant-incurred costs for attending their PROSPECT surgery. As well as admissions for surgery (main PROSPECT surgery and any follow-up admissions) and outpatient consultations, PROSPECT women who were having a secondary repair also experienced a large number of primary care consultations, either with GPs, practice nurses, specialist nurses or physiotherapists. As a result, the economic cost of time spent travelling to, and attending, appointments for participants and their companions (if they reported being accompanied on a visit) was substantial. However, there was no evidence of any differences across randomised groups, and it is important to note large SDs, which indicate great uncertainty in participant time and travel costs across the groups. However, one should also consider that the estimates are highly uncertain and based on very small sample sizes.
| Costs | Standard repair | Mesh inlay | Mesh kit | Incremental analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | n | Mesh inlay vs. standard: MD (95% CI) | Mesh kit vs. standard: MD (95% CI) | |
| Time off work due to prolapse problems | 707 | 2485 | 49 | 1017 | 3424 | 45 | 805 | 3244 | 44 | 424 (–1115 to 1965) | 39 (–1486 to 1564) |
| Participant and companion time and travel costs (primary care appointments) | 10 | 20 | 42 | 23 | 40 | 34 | 20 | 88 | 39 | 9 (–7 to 26) | –4 (–15 to 8) |
| Participant and companion time and travel costs (outpatient appointments) | 39 | 87 | 39 | 19 | 24 | 30 | 44 | 62 | 32 | –21 (–52 to 10) | 27 (–1 to 54) |
| Participant and companion time and travel costs (inpatient appointments) | 283 | 210 | 55 | 262 | 129 | 51 | 272 | 152 | 45 | –19 (–99 to 61) | –20 (–102 to 63) |
| Self-purchased health care and medication | 4 | 22 | 48 | 0 | 2 | 45 | 6 | 37 | 43 | –4 (–12 to 4) | –1 (–7 to 4) |
| Total indirect and participant costs | 952 | 2428 | 55 | 1186 | 3235 | 51 | 1114 | 3224 | 45 | 228 (–1120 to 1577) | 77 (–1356 to 1510) |
| Total NHS cost (2 years) | 3815 | 2019 | 41 | 4051 | 2098 | 37 | 4495 | 1739 | 39 | 238 (–929 to 1405) | 873 (–27 to 1774) |
| Overall total NHS, participant and indirect costs | 4905 | 4079 | 41 | 5572 | 4698 | 37 | 5222 | 2485 | 39 | 935 (–1347 to 3218) | 499 (–1482 to 2481) |
Furthermore, a small proportion of women incurred direct private health-care costs or self-purchased medication. However, the majority did not and there were no differences across groups.
Mean indirect costs of sick leave taken as a result of prolapse symptoms were £707, £1017 and £805 per woman for standard repair, mesh inlay and mesh kits, respectively, over the 2-year trial period. The large values reflect the fact that prolapse symptoms have a substantial impact on everyday life for women in terms of financial consequences. However, there were no differences across the randomised groups in terms of time taken as sick leave in relation to prolapse problems and symptoms. The wider economic impact is likely to be greater still if one were to consider the lost productivity of days spent at work, where bothersome symptoms interfered with women’s normal work activities but may not necessarily have required sick leave. Therefore, the estimates of true economic cost are likely to be underestimated.
Combining all of the costs of sick leave, opportunity costs of time for participants and companions to attend appointments, travel costs to attend appointments and total costs to the NHS, we can estimate a wider overall economic cost to society. This is, of course, limited to the costs considered and the true economic costs may be much higher. Nonetheless, the analysis gives an overall impression of the most immediate wider economic costs associated with prolapse surgery and the alternative treatment options considered in the PROSPECT trial. Total economic costs were estimated as £4905, £5572 and £5222 for standard repair, mesh inlay and mesh kit, respectively. There were no differences between groups either for the total economic costs or for any individual component of travel, time or productivity costs.
Incorporating indirect costs and economic productivity losses of time off work into the analysis of costs significantly increases the cost burden to society, showing that the costs of prolapse treatment go far beyond the health service implications. Incorporating these estimates into the overall cost-effectiveness, from a wider and more societal perspective of analysis, further increases the uncertainty for an analysis that was already highly uncertain due to the small sample recruited to the Secondary trial. The results of the analysis incorporating wider economic costs is presented in Table 75, with large SDs surrounding estimates of overall economic costs.
| Treatment | Costs (£): mean (SD)a | Incremental costs (£) vs. standard: MD (95% CI) | QALYs: mean (SD)a | Incremental QALYs (vs. standard): MD (95% CI) | Incremental cost (£) per QALY gained (vs. standard) (£per QALY) | Probability of cost-effectiveness at alternative threshold values of WTP for a QALY gain (%)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Standard repair (n = 36) | 3883 (2127) | 1.486 (0.493) | 0.52 | 0.42 | 0.35 | 0.33 | 0.30 | |||
| Mesh inlay (n = 34) | 4133 (2153) | 1030 (–1525 to 3586) | 1.600 (0.335) | –0.023 (–0.163 to 0.118) | Dominated | 0.14 | 0.10 | 0.11 | 0.11 | 0.12 |
| Mesh kit (n = 34) | 4528 (1721) | 293 (–1839 to 2426) | 1.614 (0.306) | 0.050 (–0.085 to 0.185) | 5860 | 0.34 | 0.48 | 0.54 | 0.56 | 0.58 |
To further explore the impact of the wider costing approach on conclusions, and in the light of the significant uncertainty, it is best to interpret the results using the scatterplot of incremental costs and effectiveness for mesh inlay and mesh kits (compared with standard repair), as well as the CEAC calculated using the net benefit statistic derived from the results of 1000 bootstrapped replicates of mean costs and QALYs. Figures 24 and 25 illustrate that the probabilities of standard repair, mesh inlay and mesh kit being the most cost-effective treatment strategy at a threshold value of WTP for a QALY gained of £30,000 are 33%, 11% and 56%, respectively.
FIGURE 24.
Scatterplot of incremental wider economic costs and QALYs for mesh treatments compared with standard repair – secondary prolapse repair (2-year follow-up).
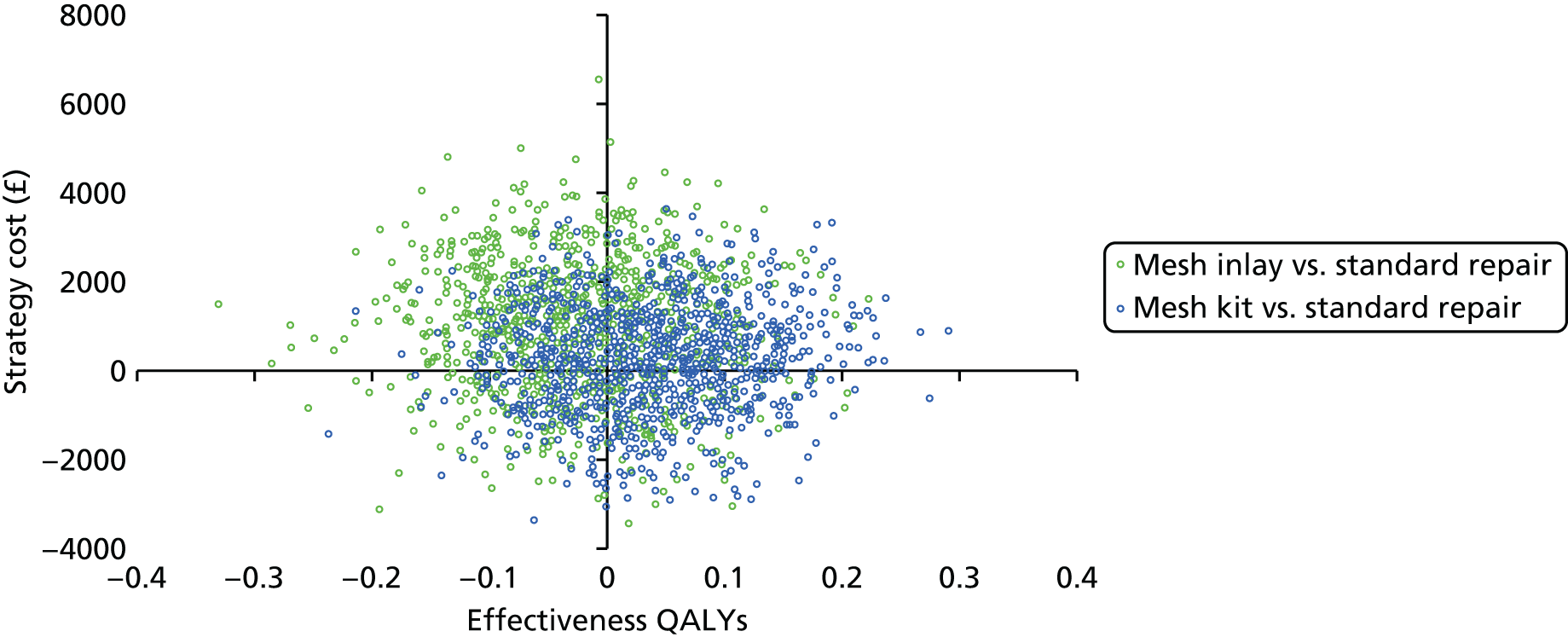
FIGURE 25.
Cost-effectiveness acceptability curves: Secondary trial – 2-year follow-up, wider economic perspective.

The uncertainty surrounding both the NHS and participant-incurred costs, as well as uncertain QALY gains, means that there is insufficient evidence to draw conclusions on cost-effectiveness from a wider economic perspective for women who were having their second prolapse repair surgery.
Deterministic sensitivity analyses
As demonstrated in the CEACs and scatterplots presented in this chapter for the Secondary trial analysis, there is substantial uncertainty driven by the small sample size of women randomised to the secondary comparison. Although CEACs and scatterplots based on bootstrapped iterations are important in presenting sampling uncertainty, they do not consider the impact of missing data, methodological assumptions such as the discount rate or the choice of comparison used in the analysis.
A number of sensitivity analyses were carried out as described in the Chapter 2 to assess the uncertainty in our results to these data choices and assumptions. Complete cost data at 2 years of follow-up were used for the sensitivity analyses. We explored a gamma family, log link regression model for costs, as both this and a normal distribution passed the modified Park’s test for distributional family. Furthermore, both models had similar AIC values, with the normal distribution having only a slightly lower score, hence its choice for the base-case analysis. However, the conclusions remain broadly robust to the choice of analysis model for the data.
Our results were also consistent across alternative discount rates applied to costs and QALYs in the second year. Overall, the ranking of treatment options based on the net benefit statistic (at a threshold value of WTP of £30,000 per QALY gained) remained unchanged for the exploration of alternative discount rates and analysis model, with mesh kits and standard repairs being slightly preferred over mesh inlay.
The base case was conducted for all women randomised to the Secondary trial. The approach differed to the primary economic analysis, as it was felt that because of the small sample size, the trade-offs of presenting a three-way comparison were outweighed by the advantages of increasing the power of the analysis as much as possible. However, we also explore the impact of re-running the analysis for the Secondary trial using data provided by only those women who were randomised to the three-way comparison (RCT2A). The choice of comparison for the data analysis was found to impact on the treatment rankings, with standard repair having the highest probability of cost-effectiveness for this analysis. However, as with all other analyses, no one treatment strategy was clearly cost-effective and substantial uncertainty surrounding estimates was illustrated.
Missing data
Missing data are likely to be particularly important for the secondary comparison given the limited sample, for which even small differences between numbers of women with missing data between groups can have a large impact on outcomes. It was not possible to impute individual resource-use item data for every category of costs, given that the number of parameters was greater than the number of observations for some imputed parameters. Therefore, imputation was undertaken at a total cost level. QALYs were based on calculation of imputed EQ-5D-3L scores at 6 months, 1 year and 2 years. The point estimates from the regressions of incremental costs and QALYs remain broadly unchanged for the comparison of mesh kit compared with standard repair. However, point estimates become more favourable to the mesh inlay comparison under the assumptions of the imputed data set. On first impression, this seems like a substantial difference in conclusions; however, when considering the width of the CIs, the conclusion that there is no evidence of any difference between the groups remains unchanged (both in terms of costs to the NHS and QALYs gained). Considering the assessment of sampling uncertainty for the imputed data set, the probability of cost-effectiveness at a threshold value of WTP for a QALY gained for each strategy is as follows: standard repair (14%), mesh inlay (56%) and mesh kit (30%). These probabilities compare with 32%, 19% and 49%, respectively, for each of the three strategies that were estimated in the base-case analysis. The probability of cost-effectiveness is higher for mesh inlay under the assumptions of the imputed data set. However, again, substantial uncertainty exists with no strategy presenting a probability of cost-effectiveness of > 60%.
The results across all of the deterministic sensitivity analyses and imputation models undertaken for the Secondary trial comparison are presented in Table 76. Data on the right-hand side of the table present the probability of cost-effectiveness of each strategy for each analysis undertaken, based on the net benefit statistic, for a £30,000 ceiling ratio of a decision-maker’s WTP for a QALY gained.
| Analysis | Costs (£)a | QALYsa | Incremental cost (£) | Incremental QALYs | ICER (£/QALY) | P(CE) @WTP = £30,000/QALY gainb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard repair | Mesh inlay | Mesh kit | Standard repair | Mesh inlay | Mesh kit | Mesh inlay vs. standard repair: MD (95% CI) | Mesh kit vs. standard repair: MD (95% CI) | Mesh inlay vs. standard repair: MD (95% CI) | Mesh kit vs. standard repair: MD (95% CI) | Mesh inlay vs. standard repair | MK vs. standard repair | Standard repair | Mesh inlay | Mesh kit | |
| Base case | 3883 | 4133 | 4528 | 1.486 | 1.600 | 1.614 | 236 (–1091 to 1564) | 642 (–309 to 1592) | –0.023 (–0.163 to 0.118) | 0.050 (–0.085 to 0.185) | Dominated | 11,560 | 0.32 | 0.19 | 0.49 |
| Costs and QALYs undiscounted | 3903 | 4152 | 4546 | 1.512 | 1.628 | 1.643 | 231 (–1107 to 1569) | 636 (–324 to 1596) | –0.023 (–0.166 to 0.120) | 0.052 (–0.085 to 0.190) | Dominated | 10,904 | 0.33 | 0.17 | 0.51 |
| Costs and QALYs discounted at 6% per annum | 3870 | 4121 | 4515 | 1.468 | 1.581 | 1.594 | 241 (–1116 to 1599) | 379 (–541 to 1299) | –0.023 (–0.161 to 0.116) | 0.056 (–0.076 to 0.188) | Dominated | 6,768 | 0.32 | 0.20 | 0.48 |
| Data from three-way comparison (RCT2A) only | 3590 | 4044 | 4586 | 1.667 | 1.597 | 1.610 | –391 (–1707 to 943) | 413 (–597 to 1413) | –0.040 (–0.234 to 0.154) | –0.017 (–0.153 to 0.120) | 9775c | Dominated | 0.47 | 0.32 | 0.20 |
| Multiple imputation of missing cost and QALY data | 4016 | 3933 | 4639 | 1.530 | 1.605 | 1.578 | –83 (–1071 to 904) | 623 (–474 to 1719) | 0.074 (–0.055 to 0.204) | 0.048 (–0.101 to 0.196) | Dominant | 12,979 | 0.14 | 0.56 | 0.30 |
| Gamma regression of cost, log link | 3886 | 4191 | 4657 | 1.486 | 1.560 | 1.614 | 222 (–1202 to 1645) | 613 (–456 to 1682) | –0.023 (–0.163 to 0.118) | 0.050 (–0.085 to 0.185) | Dominated | 12,260 | 0.37 | 0.15 | 0.49 |
Across all of the analyses undertaken, as with the base-case analysis, there was substantial uncertainty in all the estimates of incremental costs, incremental QALYs and ICERs. Conclusions should be drawn in the light of this uncertainty and not necessarily on the point estimates of the ICERs presented, which, as a result of the very wide CIs, were found to be quite unstable. In summary, there is no clear evidence that any one treatment is particularly cost-effective for the surgical repair of secondary prolapse. This conclusion was consistent across alternative analyses. There was some further uncertainty, however, regarding cost-effectiveness, depending on whether imputed data were used or not. However, across all analyses explored, none of the treatment options has a probability of cost-effectiveness of > 60% if decision-makers were willing to pay a maximum of £30,000 per QALY gained. Therefore, substantial sampling uncertainty remained for all of the analyses considered. Further research, over a longer time horizon is required to determine the long-run trade-offs between costs and QALYs, which will probably be driven primarily by any differences that may emerge over the longer-term in risk of complications and risk of prolapse surgery failure.
Discussion
Summary of main findings
For the base-case economic analysis of mesh inlays and mesh kits compared with standard repair for women requiring a secondary prolapse surgery procedure, there is evidence to suggest that mesh kits are substantially more expensive as a result of the additional cost of materials over and above standard repairs and, indeed, mesh inlays. There were no differences, however, in costs of time or equipment to perform the respective procedures, nor were there any differences evident in terms of follow-up care required across groups. There was no strong evidence that either mesh strategy provides QALY gains relative to standard repair. It should be noted that point estimates of incremental costs, incremental QALYs and hence incremental cost per QALY gained were surrounded by very wide CIs, illustrating the uncertainty surrounding the estimates. This is primarily due to a small sample size and a lack of power to estimate cost-effectiveness outcomes. As such, point estimates of the estimated ICER should be interpreted with extreme caution and in the light of the uncertainty surrounding these estimates. The best way to interpret the estimates of cost-effectiveness in the light of this uncertainty is to consider the data presented in scatterplots of incremental costs and QALYs gained and the CEACs presented. The latter suggest that, were society willing to pay up to £30,000 for a QALY gain, there are probabilities of 32%, 19% and 49% that standard repair, mesh inlays and mesh kits, respectively, are the optimal treatment strategy from a cost-effectiveness point of view. These probabilities clearly illustrate great uncertainty regarding the most cost-effective strategy. Similarly, uncertain estimates are presented for all of the deterministic sensitivity analyses undertaken and also for the exploratory imputation of missing data. Under none of the circumstances considered would any one treatment option represent a > 60% probability of being the most cost-effective option. Under these uncertain estimates, there is no evidence to draw conclusions regarding cost-effectiveness of any of the treatment options considered for secondary prolapse repair on the basis of 2-year follow-up data.
Strengths
Despite the uncertainty and the small sample size for this trial, the data presented are the only data available specifically in relation to costs and QALYs for women experiencing a secondary prolapse repair. Furthermore, the sample, although small, is the largest trial and body of evidence considered to date for secondary repairs. By following these women up over a longer time, it will be possible to gain a better picture of the longer-term trade-offs between complications and recurrences, which will probably have a heavy impact on longer-term cost-effectiveness estimates.
As with the Primary trial, a key strength of the study was the UK-wide multicentre design randomising women from 35 centres across the UK. This adds to the external validity and generalisability of the results UK-wide. Including a full within-trial cost-effectiveness analysis is a key strength, although data may be of limited value in determining longer-term cost-effectiveness results. The main strength from the within-trial analysis is that a comprehensive microcosting approach was undertaken, further adding to the generalisability of results across participating centres.
The incorporation of a wider economic perspective on costs as a secondary analysis adds value in terms of providing initial indications of the costs to women and economic costs to society of secondary prolapse symptoms and problems. The estimates generated from the time and travel cost estimates can be applied in future studies of prolapse repair, to expand the perspective of the analysis. The analysis of QALYs, based on EQ-5D-3L patient-level responses, follows best practice methods; this is another advantage, which will be useful for any future modelling exercises of secondary prolapse repair. For the purposes of this evaluation, there are insufficient numbers of observations with long enough follow-up to accurately project time to effect analysis to populate a decision-analytic model specifically for secondary prolapse repair. However, once longer-term data are available, this will be possible and thus forms a part of the longer-term plan for this project.
Limitations
The main limitation of the Secondary trial economic analysis is the small sample size available for analysis. This greatly limits the statistical power of the analysis and limits the interpretation of the results presented because of the wide CIs and unstable point estimates of incremental costs, incremental QALYs and hence ICERs.
Furthermore, as with the Primary trial, we have conducted a microcosting approach to develop intervention costs, based on data available from the trial, supplemented by contact with trial-participating surgeons to glean information on mesh materials used to conduct prolapse repairs. Although the microcosting is an advantage, it has also generated some limitations. First, the estimates of mesh costs are based on average prices across mesh categories and we make no statements about the cost-effectiveness of individual mesh products. This is an area requiring future research to determine if individual products provide better outcomes and more cost-effective treatment options for women. Second, the data are presented for average practice for each surgeon, and are not available at an individual patient level for all of the women participating in the trial.
For the Secondary trial data analysis, we have chosen to include all women who were randomised, not just those who were randomised to the three-way comparison, as we did for the Primary trial. From a cost-effectiveness perspective, sourcing data for net benefit calculations from the three-way comparison is the preferable approach to take. However, in this case, because of the small randomised sample for the Secondary trial, the advantages of a straight ‘purist’ three-way comparison are outweighed by the disadvantages of a substantial loss in already limited statistical power. Readers should note the potential limitations of this approach for cost-effectiveness analysis in a net benefit framework.
There were some missing data for cost and QALY outcomes, which are particularly problematic given the small sample size. Exploratory imputations of missing total cost and EQ-5D data were conducted, indicating wide variability in ICERs presented, although a consideration of uncertainty and the CEACs presented broadly similar results to the base-case analysis.
Furthermore, it should be noted that this short time horizon provides a further limitation, as it fails to address the cost and QoL impacts of any long-term complications or treatment failures, and any differences in time to failure/time to experiencing serious complications following initial surgery.
Conclusions
There was no clear evidence of the most cost-effective treatment strategy for secondary prolapse repair. Estimates of ICERs and cost-effectiveness were highly uncertain and should be interpreted in light of this uncertainty and the limitations outlined. It is unlikely that a 2-year follow-up is of sufficient duration to capture all of the costs and QALYs that are of importance to women and the NHS, and hence longer-term follow-up of the PROSPECT Study will be used to update the cost-effectiveness results. This will also provide an opportunity to develop more robust methods of extrapolation over a lifetime, which will enable the development of a longer-term decision-analytic model specifically for secondary prolapse repair. Unfortunately, as a result of the small sample size, there are insufficient data to develop robust or stable models of time-to-event data to develop an economic model at this time for secondary prolapse repair. Extended follow-up to 6 years will be used to rerun the cost-effectiveness analyses for secondary repairs and provide better cost-effectiveness evidence. Furthermore, longer-term data will provide more accurate estimates of failures and complications and provide an opportunity to build a model for secondary prolapse repair.
Chapter 8 Results upper compartment: uterine and vault prolapse (comprehensive cohort 3)
This chapter describes the women who, when assessed clinically before surgery, were not thought to need an anterior or posterior prolapse repair. If they had, they would have been eligible for randomisation in the mesh trials (see Chapters 4 and 5). These women had an upper compartment (uterine or vault) procedure and agreed to be followed up as part of the CC (CC3).
The flow of women through the study is shown in the CONSORT diagram (Figure 26). The women received surgery in centres across the UK (see Table 4). Although 215 women had uterine or vault surgery in total, they have been reported separately because of the fundamental clinical differences between them. The most important difference is that women who have a vault prolapse must have had a hysterectomy in the past. Therefore, in this chapter, the data are presented according to clinical presentation, statistical comparisons are not made between the groups and the information is presented and discussed separately.
FIGURE 26.
CONSORT diagram for uterine and vault groups.
It is important to realise that the total number of women in CC3 is not representative of the distribution of uterine and vault prolapse in the UK. There are an artificially high number of women who were having vault prolapse because several of the PROSPECT centres were tertiary referral centres for vault prolapse or specialised in its laparoscopic treatment. The true ratio of uterine–vault prolapse is around 4 : 1, suggesting that one in four women who have a hysterectomy go on to have a subsequent vault prolapse repair. These numbers are in line with Health Episode Statistics (HES) data, which suggest that 25% of women who were having a hysterectomy will go on to require a vault repair. 6
Interestingly, although most (but not all) vaginal hysterectomies are carried out for prolapse and most (but not all) abdominal hysterectomies for other gynaecological conditions, the number of women with prolapse was marginally greater after an abdominal hysterectomy (543/961, 56.5%) than after a vaginal hysterectomy (418/961, 43.5%; see Table 5). This must be set against the much higher number of women who were having an abdominal hysterectomy in the past (e.g. > 31,000 women who were having an abdominal hysterectomy compared with around 6500 having a vaginal hysterectomy in 2004–5). 6
Overall, around 25% of women who were having a uterine prolapse repair can expect to have a vault repair later. 6 This information is derived from online HES data, and so may underestimate total procedures, as it was based only on numbers of main operations and therefore did not count concomitant procedures.
Baseline characteristics of women who were having a uterine or a vault prolapse repair
Women who were having surgery for a uterine prolapse were, on average, 5.6 years younger than those having a vault repair (Table 77). However, compared with women who were having a uterine prolapse repair, women who were having a vault repair had a higher prolapse symptom score (POP-SS of 15.3 vs. 12.8); higher score on QoL (7.3 vs. 6.5); worse generic QoL score (EQ-5D-3L score of 0.63 vs. 0.69); more severe incontinence (23.8% vs. 21%) and prior surgery for UI (16.8% vs. 7.4%) and were more likely to have had prolapse surgery before (57.5% vs. 14.5%). They were less likely to be currently using a pessary (11.8% vs. 15.9%) or to have received physiotherapy for their prolapse symptoms (27.5% vs. 34.3%).
| Baseline characteristic | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women | N = 69 | N = 146 | ||||
| Age (years) | 56.9 | (15.2) | 69 | 62.5 | (10.1) | 146 |
| Parity (mean) | 2.6 | (1.4) | 69 | 2.8 | (1.4) | 146 |
| Parity (median) | 2.0 | (0–7) | 69 | 3.0 | (0–12) | 146 |
| BMI (kg/m2) (mean) | 27.4 | (4.3) | 64 | 27.1 | (4.3) | 138 |
| BMI (kg/m2) (median) | 27.8 | (19–36) | 64 | 26.6 | (19–40) | 138 |
| Delivery mode history | ||||||
| Spontaneous vaginal delivery | 2.3 | (1.5) | 68 | 2.6 | (1.5) | 143 |
| Forceps | 0.2 | (0.4) | 68 | 0.1 | (0.4) | 143 |
| Breech | 0.0 | (0.0) | 68 | 0.1 | (0.3) | 143 |
| Elective CS | 0.0 | (0.2) | 68 | 0.0 | (0.2) | 143 |
| Emergency CS | 0.0 | (0.0) | 68 | 0.0 | (0.1) | 143 |
| Vacuum | 0.0 | (0.0) | 68 | 0.0 | (0.1) | 143 |
| EQ-5D-3L | ||||||
| Score | 0.69 | (0.30) | 60 | 0.63 | (0.34) | 129 |
| Conservative treatment | ||||||
| Vaginal pessary | 15.9% | 11 | 69 | 11.8% | 17 | 144 |
| Physiotherapy for POP | 34.3% | 23 | 67 | 27.5% | 39 | 142 |
| Physiotherapy for UI | 17.9% | 12 | 67 | 17.7% | 25 | 141 |
| Drugs for UI | 7.7% | 5 | 65 | 12.1% | 17 | 140 |
| Previous surgery | ||||||
| Hysterectomy | 0.0% | 0 | 69 | 100.0% | 146 | 146 |
| Vaginal | 0.0% | 0 | 69 | 47.3% | 69 | 146 |
| Cervical amputation | 2.9% | 2 | 69 | 2.1% | 3 | 146 |
| Abdominal | 0.0% | 0 | 69 | 51.4% | 75 | 146 |
| UI surgery | 7.4% | 5 | 68 | 16.8% | 24 | 143 |
| Prolapse repair | 14.5% | 10 | 69 | 57.5% | 84 | 146 |
| Anterior | 10.1% | 7 | 69 | 34.2% | 50 | 146 |
| Posterior | 5.8% | 4 | 69 | 25.3% | 37 | 146 |
| Anterior plus posterior | 4.3% | 3 | 69 | 17.1% | 25 | 146 |
| Vault | 2.9% | 2 | 69 | 15.8% | 23 | 146 |
| Unknown | 1.4% | 1 | 69 | 4.1% | 6 | 146 |
Although not all of these differences were statistically significant, together they provide an impression of the qualitative differences between the populations of women with the two types of prolapse: this supports our decision not to combine or directly compare the data from the two groups. Thus, in summary, women who were having vault prolapse surgery were older, had more severe symptoms and had received more invasive treatment than those presenting with a uterine prolapse alone. For that reason, the two samples of women will be described separately in the rest of this chapter.
Uterine prolapse: women requiring a prolapse repair for descent of the uterus only
Women’s characteristics at baseline
The mean age of women presenting with a uterine prolapse alone was 56.9 years (see Table 77). This is considerably younger than the other groups of women in PROSPECT, including those with a primary or secondary anterior or posterior prolapse (see Table 5) or a vault prolapse (see Table 77). However, the groups were generally all comparable on other characteristics such as parity, BMI and delivery mode history.
About one-third of the women with a uterine prolapse had seen a physiotherapist for prolapse, and about one in eight had used a pessary for prolapse symptoms (see Table 77). One in five women had seen a physiotherapist for urine symptoms and fewer than 1 in 10 had used drugs for urinary problems.
Preoperative prolapse measurements
The leading edge of the upper compartment (point C on the POP-Q) was, on average, at or beyond the hymen (1.7 cm for uterine prolapse; Table 78). The majority of women had stage 3 or 4 prolapse, unlike those having only an anterior or posterior repair, for which the most common preoperative stage was stage 2 (see Table 7). Using a more strict definition of prolapse (leading edge > 0 cm beyond the hymen), 77.0% of women had a prolapse beyond the hymen.
| POP-Q measurement/stage | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women | N = 69 | N = 146 | ||||
| POP-Q measurement (cm) | ||||||
| Ba (anterior edge) | 2.0 | (2.3) | 60 | 2.0 | (2.4) | 133 |
| C (cervix/vault) | 1.7 | (3.1) | 51 | –0.4 | (3.9) | 133 |
| Bp (posterior edge) | 0.2 | (2.9) | 59 | 0.9 | (2.6) | 133 |
| TVL | 8.5 | (1.3) | 51 | 8.4 | (1.9) | 108 |
| Overall POP-Q stage | ||||||
| 0 | 0.0% | 0 | 64 | 0.0% | 0 | 139 |
| 1 | 4.7% | 3 | 64 | 0.0% | 0 | 139 |
| 2 | 37.5% | 24 | 64 | 37.4% | 52 | 139 |
| 3 | 43.8% | 28 | 64 | 48.9% | 68 | 139 |
| 4 | 14.1% | 9 | 64 | 13.7% | 19 | 139 |
| 2b, 3 or 4 | 77.0% | 47 | 61 | 84.3% | 113 | 134 |
Prolapse symptoms at baseline
All women had at least one prolapse symptom on the Pelvic Organ Prolapse Symptom scale, the most common of which was ‘a feeling of something coming down from or in (your) vagina’ (Table 79). Women who were having a uterine prolapse repair had a lower prolapse symptom score (POP-SS of 12.8) and less bother from their prolapse, based on their prolapse-related QoL score (6.5) than women who were having anterior or posterior repair (see Tables 15 and 50) or vault repair (see Table 79). The principal individual prolapse symptom was a feeling of ‘something coming down’. They were also less likely to need to use preventative manoeuvres or extra hygiene measures to relieve their prolapse and other symptoms of pelvic floor dysfunction.
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women | N = 65 | N = 137 | ||||
| POP-SS overall | 12.8 | (6.3) | 61 | 15.3 | (6.6) | 136 |
| Individual prolapse symptoms | ||||||
| SCD any | 98.4% | 60 | 61 | 96.3% | 131 | 136 |
| SCD freq. | 70.5% | 43 | 61 | 80.9% | 110 | 136 |
| Pain any | 83.6% | 51 | 61 | 86.8% | 118 | 136 |
| Pain freq. | 31.1% | 19 | 61 | 55.1% | 75 | 136 |
| Abdo. any | 75.4% | 46 | 61 | 82.4% | 112 | 136 |
| Abdo. freq. | 26.2% | 16 | 61 | 39.0% | 53 | 136 |
| Back any | 62.3% | 38 | 61 | 74.3% | 101 | 136 |
| Back freq. | 31.1% | 19 | 61 | 27.9% | 38 | 136 |
| Strain blad. any | 59.0% | 36 | 61 | 73.5% | 100 | 136 |
| Strain blad. freq. | 27.9% | 17 | 61 | 34.6% | 47 | 136 |
| Blad. not empty any | 82.0% | 50 | 61 | 85.3% | 116 | 136 |
| Blad. not empty freq. | 32.8% | 20 | 61 | 42.6% | 58 | 136 |
| Bowel not empty any | 68.9% | 42 | 61 | 85.3% | 116 | 136 |
| Bowel not empty freq. | 24.6% | 15 | 61 | 33.8% | 46 | 136 |
| Other measures of prolapse symptoms | ||||||
| Symptoms (years) | 5.2 | (6.3) | 57 | 3.9 | (4.5) | 127 |
| Bother (years) | 3.2 | (3.4) | 55 | 3.2 | (3.8) | 122 |
| Number of women symptomatic | 100.0% | 61 | 61 | 100.0% | 136 | 136 |
| Prolapse-related QoL score | 6.5 | (3.0) | 61 | 7.3 | (2.8) | 132 |
| Actions necessitated by prolapse symptoms | ||||||
| Fingers to ease discomfort | 17.5% | 11 | 63 | 33.3% | 42 | 126 |
| Extra hygiene measures | 49.2% | 30 | 61 | 63.4% | 83 | 131 |
| Fingers to help empty bladder | 8.2% | 5 | 61 | 12.4% | 16 | 129 |
| Fingers to help empty bowel | 1.6% | 1 | 62 | 6.2% | 8 | 130 |
| Digital evacuation of bowel | 3.2% | 2 | 63 | 5.3% | 7 | 133 |
Urinary symptoms at baseline
Based on a variety of validated measures of assessing bladder function, women with uterine prolapse were similar to the other groups of women who were having prolapse surgery on nearly every measure. In particular, UI was just as prevalent, four in five women had any incontinence, and one in five had severe symptoms (Table 80).
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women | N = 65 | N = 137 | ||||
| Any incontinence | 77.8% | 49 | 63 | 73.7% | 98 | 133 |
| ICIQ-UI-SF score | 7.1 | (5.8) | 62 | 7.1 | (6.1) | 130 |
| Severe incontinence | 21.0% | 13 | 62 | 23.8% | 31 | 130 |
| Incontinence QoL | 3.8 | (3.6) | 57 | 3.8 | (3.6) | 124 |
| Stress UI | 21.4% | 12 | 56 | 22.6% | 26 | 115 |
| Urgency UI | 9.5% | 6 | 63 | 13.0% | 17 | 131 |
| Overactive bladder | 6.3% | 4 | 63 | 7.8% | 10 | 129 |
| ICIQ-FLUTS filling score | 5.0 | (3.1) | 62 | 6.0 | (3.1) | 127 |
| ICIQ-FLUTS voiding score | 3.4 | (2.8) | 62 | 3.4 | (2.7) | 130 |
| ICIQ-FLUTS incontinence score | 5.5 | (4.1) | 56 | 5.8 | (4.3) | 113 |
Bowel symptoms at baseline
Women with uterine prolapse were similar with respect to most aspects of bowel function to those with other types of prolapse. At least one-fifth had constipation and almost one-third had FI (Table 81). Passive FI was much more common than active (FI accompanied by bowel urgency).
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women | N = 65 | N = 137 | ||||
| Bowel frequency | ||||||
| > 3 times a day | 3.3% | 2 | 61 | 5.2% | 7 | 134 |
| 1–3 times a day | 34.4% | 21 | 61 | 31.3% | 42 | 134 |
| About once a day | 34.4% | 21 | 61 | 41.0% | 55 | 134 |
| Once every 2–3 days | 21.3% | 13 | 61 | 19.4% | 26 | 134 |
| Weekly or less | 6.6% | 4 | 61 | 3.0% | 4 | 134 |
| Constipation | 21.0% | 13 | 62 | 25.4% | 33 | 130 |
| Bowel urgency | 7.9% | 5 | 63 | 11.9% | 16 | 134 |
| Any FI | 31.7% | 20 | 63 | 32.6% | 43 | 132 |
| Passive FI | 75.0% | 15 | 20 | 69.8% | 30 | 43 |
| Active FI | 25.0% | 5 | 20 | 30.2% | 13 | 43 |
| Severe FI | 4.8% | 3 | 63 | 11.4% | 15 | 132 |
| Bowel symptoms QoL | 2.9 | (3.3) | 62 | 3.4 | (3.3) | 133 |
Vaginal and sexual symptoms at baseline
About two in five women were sexually active (Table 82). For those who did have a partner, the most common reason for no sex life was their prolapse symptoms. Very few of the women (3.1%) had dyspareunia (pain with intercourse) but the numbers were small.
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women | n = 65 | n = 137 | ||||
| Vaginal | ||||||
| ICIQ-VS score | 23.6 | (9.6) | 56 | 24.5 | (10.4) | 115 |
| Vaginal symptoms QoL score | 5.1 | (3.5) | 59 | 5.4 | (3.6) | 128 |
| Vagina too tight | 3.3% | 2 | 60 | 3.2% | 4 | 125 |
| Sexual | ||||||
| Sex life at present (yes) | 41.0% | 25 | 61 | 32.8% | 44 | 134 |
| Reason for no sex life | ||||||
| No partner | 44.4% | 16 | 36 | 32.2% | 29 | 90 |
| Vaginal symptoms | 5.6% | 2 | 36 | 2.2% | 2 | 90 |
| Prolapse symptoms | 36.1% | 13 | 36 | 46.7% | 42 | 90 |
| Other reason | 8.3% | 3 | 36 | 14.4% | 13 | 90 |
| Reason not given | 5.6% | 2 | 36 | 4.4% | 4 | 90 |
| Dyspareunia | 3.1% | 1 | 32 | 14.8% | 9 | 61 |
| Sexual Matters Score | 21.1 | (14.1) | 30 | 24.4 | (15.5) | 59 |
| Sex life QoL score | 6.4 | (3.5) | 37 | 6.9 | (3.4) | 77 |
Planned surgery and surgery actually carried out
Planned surgery
To be enrolled in CC3, women were clinically assessed as not needing an anterior or posterior repair. Only three women were thought to need continence surgery (despite > 20% having severe UI; Table 83).
| Type of surgery | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women | N = 69 | N = 146 | ||||
| Planned prolapse surgery | ||||||
| Anterior repair | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Posterior repair | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Anterior and posterior repair | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Upper compartment repair only | 100.0% | 69 | 69 | 100.0% | 146 | 146 |
| Concomitant prolapse surgery | ||||||
| Vaginal hysterectomy | 31.9% | 22 | 69 | 0.0% | 0 | 146 |
| Abdominal hysterectomy | 5.8% | 4 | 69 | 0.7% | 1 | 146 |
| Cervical amputation | 4.3% | 3 | 69 | 0.7% | 1 | 146 |
| Vault repair | 72.5% | 50 | 69 | 99.3% | 145 | 146 |
| Concomitant UI surgery | 4.3% | 3 | 69 | 4.1% | 6 | 146 |
| Surgery actually received | ||||||
| Anterior repair only | 10.3% | 7 | 68 | 2.8% | 4 | 144 |
| Posterior repair only | 4.4% | 3 | 68 | 1.4% | 2 | 144 |
| Anterior and posterior repair | 2.9% | 2 | 68 | 2.1% | 3 | 144 |
| Neither | 82.4% | 56 | 68 | 93.8% | 135 | 144 |
| Vaginal hysterectomy | 29.4% | 20 | 68 | 0.0% | 0 | 144 |
| Vault repair | 14.7% | 10 | 68 | 89.6% | 129 | 144 |
| Continence surgery | 4.4% | 3 | 68 | 3.5% | 5 | 144 |
Surgery actually received
Although the women in this cohort were thought to have a uterine prolapse, only around 30% had a vaginal hysterectomy. A total of 14.7% of uterine women had a hysterectomy with a concomitant vault repair (see Table 83).
Description of surgical characteristics and protocols
The majority of women with a uterine prolapse received surgery from a consultant gynaecologist (70.6%); junior doctors were supervised in > 90% of cases (Table 84). Most women had a general anaesthetic, received prophylactic antibiotics and were in theatre for approximately 2 hours. The mean length of stay was > 2 days.
| Surgical characteristic | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women | N = 68 | N = 144 | ||||
| Grade of gynaecologist | ||||||
| Consultant | 70.6% | 48 | 68 | 78.3% | 112 | 143 |
| Specialty doctor | 0 | 68 | 1.4% | 2 | 143 | |
| Specialty doctor supervised | N/A | 0 | 0 | 100.0% | 2 | 2 |
| Registrar/junior | 29.4% | 20 | 68 | 20.3% | 29 | 143 |
| Specialty doctor supervised | 94.4% | 17 | 18 | 89.7% | 26 | 29 |
| Type of anaesthetic | ||||||
| General | 86.8% | 59 | 68 | 93.7% | 134 | 143 |
| Spinal | 14.7% | 10 | 68 | 6.3% | 9 | 143 |
| Local | 1.5% | 1 | 68 | 5.6% | 8 | 143 |
| Prophylactic antibiotic | 92.6% | 63 | 68 | 98.6% | 139 | 141 |
| Estimated blood loss (ml) | 246.9 | (353.6) | 54 | 52.9 | (62.7) | 106 |
| Duration (minutes) | 121.8 | (44.1) | 67 | 131.9 | (47.2) | 142 |
| Vaginal pack inserted | 51.5% | 34 | 66 | 15.7% | 22 | 140 |
| Catheter inserted | 98.5% | 65 | 66 | 99.3% | 142 | 143 |
| Suprapubic | 1.5% | 1 | 65 | 1.4% | 2 | 142 |
| Urethral | 98.5% | 64 | 65 | 98.6% | 140 | 142 |
| Both | 0.0% | 0 | 65 | 0.0% | 0 | 142 |
| Length of stay (days) | 2.4 | (3.3) | 68 | 1.8 | (1.5) | 143 |
Outcomes for women who were having a uterine prolapse repair
Serious and related adverse effects in first and second years
The proportion of women who had at least one serious adverse effect in the first year (excluding mesh complications) was 4.3% in the uterine group (three women had seven events; Table 85). One woman had urinary tract symptoms in the second year. Five women also had at least one non-serious adverse event in the first year (excluding mesh complications; Table 86).
| Adverse effect | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women in first year | N = 69 | N = 146 | ||||
| Intraoperative | ||||||
| Injury to organs | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
| Excess blood loss | 1.4% | 1 | 69 | 0.0% | 0 | 146 |
| Blood transfusion | 2.9% | 2 | 69 | 0.0% | 0 | 146 |
| Anaesthetic complications | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
| Death | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Postoperative | ||||||
| Thrombosis | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Infection | 1.4% | 1 | 69 | 0.0% | 0 | 146 |
| Pain | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
| Urinary retention | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Bowel obstruction | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
| Constipation | 1.4% | 1 | 69 | 0.0% | 0 | 146 |
| Excess blood loss | 2.9% | 2 | 69 | 0.0% | 0 | 146 |
| Vaginal adhesions | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Haematoma | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Skin tags | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Granulation tissue | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Urinary tract symptoms | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Death | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Number of women with any serious complication in first year | 4.3% | 3 | 69 | 2.7% | 4 | 146 |
| Number of women in second year | N = 69 | N = 146 | ||||
| Thrombosis | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Infection | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
| Pain | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
| Urinary retention | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Bowel obstruction | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Constipation | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Excess blood loss | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Vaginal adhesions | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Haematoma | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Skin tags | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Granulation tissue | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Urinary tract symptoms | 1.4% | 1 | 69 | 0.0% | 0 | 146 |
| Death | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Number of women with any serious complication in second year | 1.4% | 1 | 69 | 0.7% | 1 | 146 |
| Adverse effect | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women in first year | N = 69 | N = 146 | ||||
| Intraoperative | ||||||
| Injury to organs | 0.0% | 0 | 69 | 1.4% | 2 | 146 |
| Excess blood loss | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Blood transfusion | 1.4% | 1 | 69 | 0.0% | 0 | 146 |
| Anaesthetic complications | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Postoperative | ||||||
| Thrombosis | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Infection | 0.0% | 0 | 69 | 1.4% | 2 | 146 |
| Pain | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
| Urinary retention | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Bowel obstruction | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Constipation | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Excess blood loss | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Vaginal adhesions | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Haematoma | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Skin tags | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Granulation tissue | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Urinary tract symptoms | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Death | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Number of women with any complication in first year | 1.4% | 1 | 69 | 2.7% | 4 | 146 |
| Number of women in second year | N = 69 | N = 146 | ||||
| Thrombosis | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Infection | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
| Pain | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Urinary retention | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Bowel obstruction | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Constipation | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Excess blood loss | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Vaginal adhesions | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Haematoma | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Skin tags | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Granulation tissue | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Urinary tract symptoms | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Death | 0.0% | 0 | 69 | 0.0% | 0 | 146 |
| Number of women with any other complication in second year | 0.0% | 0 | 69 | 0.7% | 1 | 146 |
Prolapse symptoms and EuroQol-5 Dimensions (3-level version)
At 6 months, the women’s report of their prolapse symptoms, using the POP-SS (maximum score 28), fell from 12.8 (see Table 79) for the uterine group to 4.3 (Table 87). Similarly, each individual prolapse symptom also improved (Table 88), as did the prolapse-related QoL scores (Table 87).
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women at 6 months | N = 54 | N = 121 | ||||
| POP-SS at 6 months | 4.3 | (4.7) | 53 | 5.8 | (6.2) | 120 |
| Number of women symptomatic | 81.1% | 43 | 53 | 82.5% | 99 | 120 |
| Prolapse-related QoL score | 2.4 | (3.1) | 50 | 2.8 | (3.4) | 119 |
| Number of women at 1 year | N = 57 | N = 116 | ||||
| POP-SS (overall score at 1 year) | 5.2 | (5.5) | 55 | 5.5 | (5.8) | 115 |
| Number of women symptomatic | 83.6% | 46 | 55 | 80.0% | 92 | 115 |
| Prolapse-related QoL score | 2.4 | (3.2) | 53 | 2.5 | (3.3) | 113 |
| Number of women at 2 years | N = 48 | N = 104 | ||||
| POP-SS (overall score at 2 years) | 5.3 | (5.9) | 47 | 5.6 | (5.7) | 104 |
| Number of women symptomatic | 80.9% | 38 | 47 | 79.8% | 83 | 104 |
| Prolapse-related QoL score | 1.9 | (3.0) | 45 | 2.3 | (2.9) | 104 |
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women at 6 months | N = 54 | N = 121 | ||||
| SCD any | 24.5% | 13 | 53 | 30.0% | 36 | 120 |
| SCD freq. | 7.5% | 4 | 53 | 11.7% | 14 | 120 |
| Pain any | 20.8% | 11 | 53 | 25.0% | 30 | 120 |
| Pain freq. | 5.7% | 3 | 53 | 8.3% | 10 | 120 |
| Abdo any | 34.0% | 18 | 53 | 37.5% | 45 | 120 |
| Abdo freq. | 0.0% | 0 | 53 | 7.5% | 9 | 120 |
| Back any | 35.8% | 19 | 53 | 36.7% | 44 | 120 |
| Back freq. | 3.8% | 2 | 53 | 7.5% | 9 | 120 |
| Strain blad. any | 34.0% | 18 | 53 | 30.8% | 37 | 120 |
| Strain blad. freq. | 5.7% | 3 | 53 | 10.0% | 12 | 120 |
| Blad. not empty any | 49.1% | 26 | 53 | 58.3% | 70 | 120 |
| Blad. not empty freq. | 7.5% | 4 | 53 | 19.2% | 23 | 120 |
| Bowel not empty any | 50.9% | 27 | 53 | 70.8% | 85 | 120 |
| Bowel not empty freq. | 7.5% | 4 | 53 | 19.2% | 23 | 120 |
| Actions necessitated by prolapse symptoms | ||||||
| Fingers to ease discomfort | N/A | N/A | N/A | N/A | N/A | N/A |
| Extra hygiene measures | N/A | N/A | N/A | N/A | N/A | N/A |
| Fingers to help empty bladder | N/A | N/A | N/A | N/A | N/A | N/A |
| Fingers to help empty bowel | N/A | N/A | N/A | N/A | N/A | N/A |
| Digital evacuation of bowel | N/A | N/A | N/A | N/A | N/A | N/A |
| Number of women at 1 year | N = 57 | N = 116 | ||||
| POP-SS (overall score at 1 year) | 5.2 | (5.5) | 55 | 5.5 | (5.8) | 115 |
| SCD any | 29.1% | 16 | 55 | 32.2% | 37 | 115 |
| SCD freq. | 12.7% | 7 | 55 | 13.9% | 16 | 115 |
| Pain any | 30.9% | 17 | 55 | 26.1% | 30 | 115 |
| Pain freq. | 3.6% | 2 | 55 | 7.0% | 8 | 115 |
| Abdo any | 30.9% | 17 | 55 | 33.0% | 38 | 115 |
| Abdo freq. | 9.1% | 5 | 55 | 7.0% | 8 | 115 |
| Back any | 36.4% | 20 | 55 | 39.1% | 45 | 115 |
| Back frequent | 7.3% | 4 | 55 | 9.6% | 11 | 115 |
| Strain blad. any | 29.1% | 16 | 55 | 32.2% | 37 | 115 |
| Strain blad. freq. | 5.5% | 3 | 55 | 7.0% | 8 | 115 |
| Blad. not empty any | 49.1% | 27 | 55 | 56.5% | 65 | 115 |
| Blad. not empty freq. | 12.7% | 7 | 55 | 13.0% | 15 | 115 |
| Bowel not empty any | 60.0% | 33 | 55 | 66.1% | 76 | 115 |
| Bowel not empty freq. | 12.7% | 7 | 55 | 13.0% | 15 | 115 |
| Actions necessitated by prolapse symptoms | ||||||
| Fingers to ease discomfort | 2.4% | 1 | 42 | 0.0% | 0 | 110 |
| Extra hygiene measures | 7.1% | 3 | 42 | 8.4% | 9 | 107 |
| Fingers to help empty bladder | 0.0% | 0 | 46 | 0.9% | 1 | 109 |
| Fingers to help empty bowel | 0.0% | 0 | 44 | 1.9% | 2 | 108 |
| Digital evacuation of bowel | 4.3% | 2 | 46 | 4.6% | 5 | 109 |
| Number of women at 2 years | N = 48 | N = 104 | ||||
| SCD any | 38.3% | 18 | 47 | 36.5% | 38 | 104 |
| SCD freq. | 12.8% | 6 | 47 | 8.7% | 9 | 104 |
| Pain any | 21.3% | 10 | 47 | 23.1% | 24 | 104 |
| Pain freq. | 2.1% | 1 | 47 | 2.9% | 3 | 104 |
| Abdo any | 27.7% | 13 | 47 | 34.6% | 36 | 104 |
| Abdo freq. | 4.3% | 2 | 47 | 5.8% | 6 | 104 |
| Back any | 36.2% | 17 | 47 | 43.3% | 45 | 104 |
| Back frequent | 10.6% | 5 | 47 | 10.6% | 11 | 104 |
| Strain blad. any | 38.3% | 18 | 47 | 38.5% | 40 | 104 |
| Strain blad. freq. | 12.8% | 6 | 47 | 9.6% | 10 | 104 |
| Blad. not empty any | 57.4% | 27 | 47 | 55.8% | 58 | 104 |
| Blad. not empty freq. | 17.0% | 8 | 47 | 13.5% | 14 | 104 |
| Bowel not empty any | 61.7% | 29 | 47 | 69.2% | 72 | 104 |
| Bowel not empty freq. | 17.0% | 8 | 47 | 13.5% | 14 | 104 |
| Actions necessitated by prolapse symptoms | ||||||
| Fingers to ease discomfort | 0.0% | 0 | 47 | 2.0% | 2 | 101 |
| Extra hygiene measures | 10.6% | 5 | 47 | 6.9% | 7 | 101 |
| Fingers to help empty bladder | 0.0% | 0 | 48 | 2.0% | 2 | 102 |
| Fingers to help empty bowel | 2.1% | 1 | 48 | 2.0% | 2 | 102 |
| Digital evacuation of bowel | 2.1% | 1 | 48 | 2.9% | 3 | 102 |
At 1 year, this improvement was maintained (POP-SS of 5.2 for the uterine group; see Table 87). The improvement from baseline was supported by data from individual prolapse symptoms (measured as occurring ‘ever’ or ‘most or all of the time’); the proportion of women who had at least one prolapse symptom (‘symptomatic’); QoL data based on the interference of prolapse symptoms on everyday life; the generic QoL measure EQ-5D-3L; and the need to undertake extra hygiene measures or manoeuvres to assist pelvic floor functions (see Tables 88 and 89). All of these measures demonstrated significant improvements from before surgery.
| EQ-5D-3L | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women at 6 months | N = 54 | N = 121 | ||||
| Score | 0.83 | (0.22) | 54 | 0.79 | (0.27) | 115 |
| Number of women at 1 year | N = 57 | N = 116 | ||||
| Score | 0.83 | (0.26) | 57 | 0.78 | (0.27) | 112 |
| Number of women at 2 years | N = 48 | N = 104 | ||||
| Score | 0.79 | (0.28) | 48 | 0.80 | (0.31) | 104 |
The improvement at 1 year was maintained at 2 years, with respect to all the prolapse outcomes and QoL outcomes measured.
Urinary symptoms
Detailed information on urinary symptoms was obtained at baseline, 1 year and 2 years (see Tables 80 and 90). The number of women who had concomitant continence surgery was 3 of 69 in the uterine group (see Table 83).
At 1 year in the uterine group, the proportion of women who had any UI decreased from 77.8% to 65.2% (see Tables 80 and 90), and the proportion with severe UI more than halved (from 21% to 6.7%). There were similar moderate improvements in all the other measures of bladder function measured. The improvement at 1 year was maintained at 2 years, with respect to all the urinary outcomes and bladder-related QoL outcomes measured (Table 90).
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women at 1 year | N = 46 | N = 112 | ||||
| Any incontinence | 65.2% | 30 | 46 | 68.5% | 76 | 111 |
| ICIQ-UI-SF score | 4.7 | (4.4) | 45 | 5.0 | (5.1) | 108 |
| Severe incontinence | 6.7% | 3 | 45 | 8.3% | 9 | 108 |
| Incontinence-related QoL score | 2.1 | (2.8) | 45 | 2.0 | (2.7) | 106 |
| Stress UI | 17.9% | 7 | 39 | 18.3% | 17 | 93 |
| Urgency UI | 0.0% | 0 | 46 | 0.9% | 1 | 109 |
| Overactive bladder | 2.2% | 1 | 46 | 0.9% | 1 | 106 |
| ICIQ-FLUTS filling score | 3.5 | (2.4) | 46 | 4.2 | (2.3) | 106 |
| ICIQ-FLUTS voiding score | 1.5 | (1.8) | 46 | 1.7 | (2.4) | 108 |
| ICIQ-FLUTS incontinence score | 4.6 | (3.0) | 38 | 4.8 | (4.0) | 92 |
| Number of women at 2 years | N = 48 | N = 104 | ||||
| Any incontinence | 64.6% | 31 | 48 | 65.4% | 68 | 104 |
| ICIQ-UI-SF score | 4.7 | (4.8) | 46 | 4.5 | (5.0) | 103 |
| Severe incontinence | 8.7% | 4 | 46 | 7.8% | 8 | 103 |
| Incontinence-related QoL score | 1.9 | (2.6) | 46 | 1.8 | (2.7) | 98 |
| Stress UI | 17.1% | 7 | 41 | 17.2% | 15 | 87 |
| Urgency UI | 6.4% | 3 | 47 | 2.9% | 3 | 102 |
| Overactive bladder | 4.3% | 2 | 46 | 2.0% | 2 | 102 |
| ICIQ-FLUTS filling score | 4.0 | (3.0) | 46 | 4.1 | (2.5) | 102 |
| ICIQ-FLUTS voiding score | 1.7 | (2.5) | 48 | 1.7 | (2.2) | 102 |
| ICIQ-FLUTS incontinence score | 4.7 | (3.5) | 39 | 4.9 | (4.3) | 87 |
Bowel symptoms
Detailed information on bowel symptoms was obtained at baseline, 1 year and 2 years (see Tables 81 and 91). Frequency of bowel movements, constipation, bowel urgency and FI were common and largely unchanged from baseline to after prolapse surgery in the uterine group at both 1 year and 2 years (Table 91).
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women at 1 year | N = 46 | N = 112 | ||||
| Bowel frequency | ||||||
| ≥ 4 times a day | 2.2% | 1 | 46 | 0.9% | 1 | 110 |
| 1–3 times a day | 30.4% | 14 | 46 | 30.0% | 33 | 110 |
| About once a day | 43.5% | 20 | 46 | 48.2% | 53 | 110 |
| Once every 2 or 3 days | 21.7% | 10 | 46 | 16.4% | 18 | 110 |
| Weekly or less | 2.2% | 1 | 46 | 4.5% | 5 | 110 |
| Constipation | 21.7% | 10 | 46 | 17.6% | 19 | 108 |
| Bowel urgency | 2.2% | 1 | 46 | 10.8% | 12 | 111 |
| FI (any) | 23.9% | 11 | 46 | 36.0% | 40 | 111 |
| Passive FI | 100.0% | 11 | 11 | 70.0% | 28 | 40 |
| Active FI | 0.0% | 0 | 11 | 30.0% | 12 | 40 |
| Severe FI | 6.5% | 3 | 46 | 13.5% | 15 | 111 |
| Bowel symptoms QoL score | 1.8 | (3.1) | 46 | 2.3 | (2.9) | 110 |
| Number of women at 2 years | N = 48 | N = 104 | ||||
| Bowel frequency | ||||||
| ≥ 4 times a day | 0.0% | 0 | 47 | 3.9% | 4 | 103 |
| 1–3 times a day | 25.5% | 12 | 47 | 26.2% | 27 | 103 |
| About once a day | 55.3% | 26 | 47 | 47.6% | 49 | 103 |
| Once every 2 or 3 days | 17.0% | 8 | 47 | 19.4% | 20 | 103 |
| Weekly or less | 2.1% | 1 | 47 | 2.9% | 3 | 103 |
| Constipation | 10.6% | 5 | 47 | 19.2% | 20 | 104 |
| Bowel urgency | 6.3% | 3 | 48 | 3.9% | 4 | 103 |
| FI (any) | 29.2% | 14 | 48 | 37.9% | 39 | 103 |
| Passive FI | 85.7% | 12 | 14 | 87.2% | 34 | 39 |
| Active FI | 14.3% | 2 | 14 | 10.3% | 4 | 39 |
| Severe FI | 10.4% | 5 | 48 | 10.7% | 11 | 103 |
| Bowel symptoms QoL score | 2.2 | (3.2) | 46 | 2.3 | (2.9) | 101 |
Vaginal and sexual symptoms
Detailed information on vaginal and sexual symptoms was obtained at baseline, 1 year and 2 years (see Tables 82 and 92). Both the mean vaginal symptom score and the QoL score decreased (improved) after prolapse surgery, and this was maintained at 2 years (Table 92). More women were sexually active after surgery, and many fewer cited prolapse symptoms as a reason for not having a sex life (reduced from 36.1% to 12.0%). This was reflected in a more than halving of the ICI Sexual Matters score, and a reduction (improvement) to one-third of baseline levels in the sex life QoL score (see Tables 82 and 92). These improvements were maintained at 2 years.
| Symptom | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women at 1 year | N = 46 | N = 112 | ||||
| Vaginal | ||||||
| ICIQ-VS score | 8.2 | (10.5) | 41 | 7.2 | (7.8) | 97 |
| Vaginal symptoms QoL score | 2.0 | (3.2) | 43 | 1.8 | (2.7) | 105 |
| Vagina too tight | 2.3% | 1 | 43 | 1.0% | 1 | 104 |
| Sexual | ||||||
| Sex life at present | 46.8% | 22 | 47 | 36.9% | 41 | 111 |
| Reason for no sex life | ||||||
| No partner | 48.0% | 12 | 25 | 40.0% | 28 | 70 |
| Vaginal symptoms | 4.0% | 1 | 25 | 4.3% | 3 | 70 |
| Prolapse symptoms | 12.0% | 3 | 25 | 7.1% | 5 | 70 |
| Other reason | 28.0% | 7 | 25 | 42.9% | 30 | 70 |
| Reason not given | 8.0% | 2 | 25 | 5.7% | 4 | 70 |
| Dyspareunia | 8.3% | 2 | 24 | 4.7% | 2 | 43 |
| ICI Sexual Matters score | 11.0 | (14.1) | 24 | 10.8 | (12.5) | 42 |
| Sex life QoL score | 3.2 | (3.7) | 25 | 2.6 | (3.3) | 43 |
| Number of women at 2 years | N = 48 | N = 104 | ||||
| Vaginal | ||||||
| ICIQ-VS score | 7.8 | (8.2) | 46 | 7.9 | (8.6) | 96 |
| Vaginal symptoms QoL score | 1.7 | (2.6) | 46 | 1.8 | (2.8) | 98 |
| Vagina too tight | 2.1% | 1 | 48 | 2.0% | 2 | 98 |
| Sexual | ||||||
| Sex life at present | 47.9% | 23 | 48 | 43.4% | 43 | 99 |
| Reason for no sex life | ||||||
| No partner | 52.0% | 13 | 25 | 30.4% | 17 | 56 |
| Vaginal symptoms | 0.0% | 0 | 25 | 5.4% | 3 | 56 |
| Prolapse symptoms | 8.0% | 2 | 25 | 5.4% | 3 | 56 |
| Other reason | 20.0% | 5 | 25 | 44.6% | 25 | 56 |
| Reason not given | 20.0% | 5 | 25 | 14.3% | 8 | 56 |
| Dyspareunia | 8.3% | 2 | 24 | 2.3% | 1 | 44 |
| ICI Sexual Matters score | 13.3 | (13.9) | 23 | 9.5 | (11.4) | 43 |
| Sex life QoL score | 2.8 | (3.2) | 25 | 2.4 | (3.2) | 46 |
Further treatment required for failure or adverse effects at 6 months, 1 year and 2 years
When women reported, at 6 months or later, that they had been readmitted to hospital, we verified the information by enquiry from site staff when necessary and post-coded the corrected information. A hospital readmission was automatically counted as a SAE if it was related to the initial prolapse surgery. Repeat surgery for recurrence of prolapse (failure if same compartment, de novo if in the opposite compartment), or for continence surgery, was differentiated from readmission for related complications such as bleeding, infection and surgery for mesh removal.
The overall rate of readmission was low (two women in the uterine group in the first 6 months; Table 93). Admissions in the first 6 months were related to adverse effects (pain). After that time, one woman had further surgery for prolapse in a different compartment, and one in the second year (see Table 93). No women required surgery for mesh removal at any time point.
| Further treatment | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women at 6 months | N = 54 | N = 121 | ||||
| Readmitted (0–6 months)a | 3.7% | 2 | 54 | 1.7% | 2 | 121 |
| Number of women at 1 year | N = 57 | N = 116 | ||||
| Readmitted (6–12 months)b | 0.0% | 0 | 57 | 0.9% | 1 | 116 |
| New prolapse surgery | 1.8% | 1 | 57 | 4.3% | 5 | 116 |
| Same compartment | 0.0% | 0 | 57 | 1.7% | 2 | 116 |
| Different compartment | 1.8% | 1 | 57 | 2.6% | 3 | 116 |
| Waiting for prolapse surgery | 0.0% | 0 | 57 | 2.6% | 3 | 116 |
| Continence surgery | 0.0% | 0 | 57 | 0.0% | 0 | 116 |
| Waiting for continence surgery | 0.0% | 0 | 57 | 0.9% | 1 | 116 |
| Stitches removed since operation | 0.0% | 0 | 56 | 1.8% | 2 | 114 |
| Mesh complication | 0.0% | 0 | 57 | 0.0% | 0 | 116 |
| Treatment for urinary problems | ||||||
| Pads | 28.6% | 16 | 56 | 38.6% | 44 | 114 |
| Permanent catheter | 1.8% | 1 | 56 | 0.9% | 1 | 107 |
| Intermittent catheter | 3.6% | 2 | 56 | 1.8% | 2 | 109 |
| Drugs for UI | 3.5% | 2 | 57 | 10.3% | 12 | 116 |
| Treatment for prolapse | ||||||
| Medicines for prolapse | 12.5% | 7 | 56 | 20.4% | 23 | 113 |
| Oestrogens | 17.5% | 10 | 57 | 16.4% | 19 | 116 |
| Ring pessary | 1.8% | 1 | 57 | 2.6% | 3 | 116 |
| Shelf pessary | 0.0% | 0 | 57 | 0.0% | 0 | 116 |
| Physiotherapy | 12.5% | 7 | 56 | 9.1% | 10 | 110 |
| GP for prolapse | 17.5% | 10 | 57 | 21.3% | 23 | 108 |
| Practice nurse for prolapse | 8.9% | 5 | 56 | 2.7% | 3 | 110 |
| GOPD to see gynaecologist | 41.8% | 23 | 55 | 45.5% | 50 | 110 |
| Number of women at 2 years | N = 48 | N = 104 | ||||
| Readmitted (12–24 months)c | 2.1% | 1 | 48 | 0.0% | 0 | 104 |
| New prolapse surgery | 2.1% | 1 | 48 | 4.8% | 5 | 104 |
| Same compartment | 0.0% | 0 | 48 | 1.0% | 1 | 104 |
| Different compartment | 2.1% | 1 | 48 | 3.8% | 4 | 104 |
| Waiting for prolapse surgery | 0.0% | 0 | 48 | 0.0% | 0 | 104 |
| Continence surgery | 0.0% | 0 | 48 | 3.8% | 4 | 104 |
| Waiting for continence surgery | 0.0% | 0 | 48 | 0.0% | 0 | 104 |
| Stitches removed since operation | 0.0% | 0 | 46 | 1.0% | 1 | 100 |
| Mesh complication | 0.0% | 0 | 48 | 0.0% | 0 | 104 |
| Surgical removal in theatre | 0.0% | 0 | 48 | 0.0% | 0 | 104 |
| Conservative/GOPD procedure | 0.0% | 0 | 48 | 0.0% | 0 | 104 |
| Treatment for urinary problems at 2 years | ||||||
| Pads | 31.3% | 15 | 48 | 32.4% | 33 | 102 |
| Permanent catheter | 0.0% | 0 | 48 | 1.0% | 1 | 101 |
| Intermittent catheter | 0.0% | 0 | 47 | 0.0% | 0 | 102 |
| Drugs for UI | 8.3% | 4 | 48 | 10.6% | 11 | 104 |
| Treatment for prolapse in year 2 | ||||||
| Medicines for prolapse | 8.3% | 4 | 48 | 11.8% | 12 | 102 |
| Oestrogens | 8.3% | 4 | 48 | 16.3% | 17 | 104 |
| Ring pessary | 4.2% | 2 | 48 | 1.9% | 2 | 104 |
| Shelf pessary | 2.1% | 1 | 48 | 1.9% | 2 | 104 |
| Physiotherapy | 10.9% | 5 | 46 | 6.7% | 7 | 104 |
| GP for prolapse | 21.3% | 10 | 47 | 11.7% | 12 | 103 |
| Practice nurse for prolapse | 6.4% | 3 | 47 | 1.0% | 1 | 102 |
| GOPD to see gynaecologist | 21.3% | 10 | 47 | 13.6% | 14 | 103 |
Few women required other treatment – such as pessaries or physiotherapy – for symptoms.
Satisfaction with treatment at 1 year and 2 years
Although most women were better than before surgery by 1 year, around 10% (four women) were worse, with similar findings at 2 years (Table 94). This was reflected in the satisfaction rates and in the proportion of women who would recommend surgery to a friend.
| Recovery/satisfaction | Uterine | Vault | ||||
|---|---|---|---|---|---|---|
| Number of women at 1 year | N = 46 | N = 112 | ||||
| Time to recovery (months) | 2.8 | (1.5) | 40 | 3.4 | (2.2) | 101 |
| Comparison of prolapse with before surgery | ||||||
| Very much better | 65.9% | 27 | 41 | 64.7% | 66 | 102 |
| Much better | 19.5% | 8 | 41 | 18.6% | 19 | 102 |
| A little better | 4.9% | 2 | 41 | 4.9% | 5 | 102 |
| No change | 0.0% | 0 | 41 | 3.9% | 4 | 102 |
| A little worse | 4.9% | 2 | 41 | 2.9% | 3 | 102 |
| Much worse | 2.4% | 1 | 41 | 2.0% | 2 | 102 |
| Very much worse | 2.4% | 1 | 41 | 2.9% | 3 | 102 |
| Satisfaction with surgery | ||||||
| Completely satisfied | 63.4% | 26 | 41 | 57.5% | 61 | 106 |
| Fairly satisfied | 19.5% | 8 | 41 | 26.4% | 28 | 106 |
| Fairly dissatisfied | 4.9% | 2 | 41 | 6.6% | 7 | 106 |
| Very dissatisfied | 9.8% | 4 | 41 | 6.6% | 7 | 106 |
| Not sure | 2.4% | 1 | 41 | 2.8% | 3 | 106 |
| Recommend to a friend | 87.5% | 35 | 40 | 93.3% | 97 | 104 |
| Number of women at 2 years | N = 48 | N = 104 | ||||
| Comparison of prolapse with before surgery | ||||||
| Very much better | 55.3% | 26 | 47 | 59.2% | 61 | 103 |
| Much better | 14.9% | 7 | 47 | 24.3% | 25 | 103 |
| A little better | 12.8% | 6 | 47 | 6.8% | 7 | 103 |
| No change | 6.4% | 3 | 47 | 3.9% | 4 | 103 |
| A little worse | 4.3% | 2 | 47 | 2.9% | 3 | 103 |
| Much worse | 0.0% | 0 | 47 | 2.9% | 3 | 103 |
| Very much worse | 6.4% | 3 | 47 | 0.0% | 0 | 103 |
| Satisfaction with surgery | ||||||
| Completely satisfied | 56.3% | 27 | 48 | 58.3% | 60 | 103 |
| Fairly satisfied | 16.7% | 8 | 48 | 27.2% | 28 | 103 |
| Fairly dissatisfied | 8.3% | 4 | 48 | 4.9% | 5 | 103 |
| Very dissatisfied | 14.6% | 7 | 48 | 8.7% | 9 | 103 |
| Not sure | 4.2% | 2 | 48 | 1.0% | 1 | 103 |
| Recommend to a friend | 82.6% | 38 | 46 | 90.0% | 90 | 100 |
Vault prolapse: women requiring a prolapse repair for vault descent alone
Women’s characteristics at baseline
The mean age of women presenting with a vault prolapse alone was 62.5 years (see Table 77). This is older than the women who were having primary or uterine-only surgery in PROSPECT (see Table 5). However, the groups were generally all similar on other characteristics, such as parity, BMI and delivery mode history.
About one-third of the women with a vault prolapse had seen a physiotherapist, and about 1 in 10 were using a pessary for prolapse symptoms (see Table 77). One in five women had seen a physiotherapist for urine symptoms and around 1 in 10 had used drugs for urinary problems.
Preoperative prolapse measurements
The leading edge of the upper compartment (point C on the POP-Q) was, on average, just inside the hymen (–0.4 cm for vault prolapse; see Table 78). The majority of women had stage 3 or 4 prolapse, unlike those having only an anterior or posterior repair, for whom the most common preoperative stage was stage 2 (see Table 7). Using a more strict definition of prolapse (leading edge > 0 cm beyond the hymen), 84.3% of women had a prolapse beyond the hymen.
Prolapse symptoms at baseline
All women had at least one prolapse symptom on the Pelvic Organ Prolapse Symptom scale, the most common of which was ‘a feeling of something coming down from or in (your) vagina’ (see Table 79). Women who were having a vault prolapse repair had a higher prolapse symptom score (POP-SS of 15.3) and more bother from their prolapse, based on their prolapse-related QoL score (7.3) than women who were having anterior or posterior repair (see Tables 15 and 50) or uterine-only repair (see Table 79). The principal individual prolapse symptom was a feeling of ‘something coming down’. They were also more likely to need to use preventative manoeuvres or extra hygiene measures to relieve their prolapse and other symptoms of pelvic floor dysfunction (see Table 79).
Urinary symptoms at baseline
Based on a variety of validated measures of assessing bladder function, women with vault prolapse were similar to the other groups of women who were having prolapse surgery on nearly every measure. In particular, UI was just as prevalent: three in four women with any incontinence, and one in four with severe symptoms (see Table 80).
Bowel symptoms at baseline
Similarly, women with vault prolapse were similar to those with other types of prolapse with respect to most aspects of bowel function (see Table 81). About one-quarter had constipation and almost one-third had FI. Passive FI was much more common than active (FI accompanied by bowel urgency).
Vaginal and sexual symptoms at baseline
About one-third of women were sexually active (see Table 82). For those who did have a partner, the most common reason for no sex life was their prolapse symptoms. Around 15% of the women had dyspareunia (pain with intercourse) but the numbers were small.
Planned surgery and surgery actually carried out
To be enrolled in CC3, women were clinically assessed as not needing an anterior or posterior repair. Only six women were thought to need continence surgery (see Table 83), despite > 20% having severe urine incontinence.
Surgery actually received
The women in this cohort were thought to have a vault prolapse, and nearly 90% did, in fact, have a vault repair (see Table 83).
Description of surgical characteristics and protocols
The majority of women with a vault prolapse received surgery from a consultant gynaecologist (78.3%; see Table 84); if carried out by a junior doctor, the surgeon was supervised in around 90% of cases. Most women had a general anaesthetic, received prophylactic antibiotics and, on average, were in theatre for approximately 2 hours. The mean length of stay was just under 2 days.
Outcomes for women who were having a vault prolapse repair
Serious and other related adverse effects in first and second years
The proportion of women who had at least one serious adverse effect in the first year (excluding mesh complications) was 2.7% in the vault group (four women; see Table 85). One woman had serious infection and pain in the second year. No women had any incidence of mesh exposure.
Prolapse symptoms and EuroQol-5 Dimensions
At 6 months, the women’s report of their prolapse symptoms, using the POP-SS (maximum score 28), fell from 15.3 for the vault group to 5.8 (see Table 87). Similarly, each individual prolapse symptom also improved (see Table 88), as did the prolapse-related QoL scores (see Table 87).
At 1 year, this improvement was maintained (POP-SS of 5.5 for the vault group; see Table 87). The improvement from baseline was supported by data from individual prolapse symptoms (measured as occurring ‘ever’ or ‘most or all of the time’); the proportion of women who had at least one prolapse symptom (‘symptomatic’); QoL data, based on the interference of prolapse symptoms on everyday life (see Table 87); the generic QoL measure EQ-5D-3L (see Table 89); and the need to undertake extra hygiene measures or manoeuvres to assist pelvic floor functions. All of these measures demonstrated significant improvements from before surgery.
The improvement at 1 year was maintained at 2 years, with respect to all of the prolapse outcomes and QoL outcomes measured.
Urinary symptoms
Detailed information on urinary symptoms was obtained at baseline, 1 year and 2 years (see Tables 80 and 90). The number of women who had concomitant continence surgery was 5/146 in the vault group (see Table 83).
At 1 year in the vault group, the proportion of women who had any UI decreased from 73.7% to 68.5% and the proportion with severe UI more than halved (from 23.8% to 8.3%; see Tables 80 and 90). There were similar moderate improvements in all of the other measures of bladder function measured. The improvement at 1 year was maintained at 2 years, with respect to all of the urinary outcomes and bladder-related QoL outcomes measured (see Table 90).
Bowel symptoms
Detailed information on bowel symptoms was obtained at baseline, 1 year and 2 years (see Tables 81 and 91). Frequency of bowel movements, constipation, bowel urgency and FI were common, and largely unchanged from baseline to after prolapse surgery in the vault group at both 1 year and 2 years (see Table 91).
Vaginal and sexual symptoms
Detailed information on vaginal and sexual symptoms was obtained at baseline, 1 year and 2 years (see Tables 82 and 92). Both the mean vaginal symptom score and the QoL score decreased (improved) after vault prolapse surgery, and this was maintained at 2 years. More women were sexually active after surgery, and many fewer cited prolapse symptoms as a reason for not having a sex life (reduced from 46.7% to 5.4% in the second year; see Tables 82 and 92). This was reflected in a more than halving of the ICI Sexual Matters score and a reduction (improvement) to one-third of baseline levels in the sex life QoL score.
Further treatment required for failure or adverse effects at 6 months, 1 year and 2 years
When women reported, at 6 months or later, that they had been readmitted to hospital, we verified the information by enquiry from site staff when necessary and post-coded the corrected information. A hospital readmission was automatically counted as a SAE if it was related to the initial prolapse surgery. Repeat surgery for recurrence of prolapse (failure if same compartment, de novo if in the opposite compartment), or for continence surgery, was differentiated from readmission for related complications, such as bleeding, infection and surgery for mesh removal.
The overall rate of readmission was low (two women in the vault group in the first 6 months; Table 93). Admissions in the first 6 months were related to adverse effects (constipation and pain). After that time, five women had further surgery for prolapse (two in the same and three in a different compartment, and five in the second year; see Table 93). No women required surgery for mesh removal at any time point.
Few women required other treatment – such as pessaries or physiotherapy – for symptoms.
Satisfaction with treatment at 1 year and 2 years
Although most women were better than before surgery by 1 year, around 12% (12 women) were unchanged or worse, with similar findings at 2 years (see Table 94). This was reflected in the satisfaction rates, and in the proportion of women who would recommend surgery to a friend (90.0%).
Discussion
Summary of findings
This chapter has reported the findings for the cohort of women (CC3) who were not eligible for the randomisation arms of PROSPECT because they were not thought to need either an anterior or posterior repair. The women who were having vault prolapse were clearly a different population from those with uterine prolapse, based on epidemiological and clinical characteristics. They have therefore been described separately.
Chapter 9 Modelling
Little is known about the prevalence and effectiveness of different types of treatments for prolapse, except that they are prone to failure: around 30% of women undergo further operations. The mean time interval to the first secondary operation is about 12 years, and the time interval between subsequent procedures decreases with each successive repair. 4
Gynaecologists have recognised for some time that both anatomical failure of supporting pelvic structures and recurrence of prolapse after surgery are common. It has also been recognised that surgery can be followed by a greater impairment of QoL than the original prolapse itself (e.g. new UI after surgery). In addition, repair of one type of prolapse may predispose the women to the development of a different type of prolapse (a new, or de novo, prolapse) in another compartment of the vagina due to alteration in the dynamic forces within the pelvis. 4
This chapter focuses on presenting the methods and the results of a de novo economic model to guide decision-makers on the cost-effectiveness of alternative surgical procedures for primary prolapse repair.
Although the within-trial cost-effectiveness results are informative regarding short-run costs and QALYs for alternative treatments for the surgical management of primary prolapse repair, it is important to note that prolapse is a chronic condition and the effects of treatment on costs and outcomes may persist into the future. Therefore, we have developed a model to extrapolate the findings of the 2-year within-trial analysis to a longer-term period.
Modelling approach
A probabilistic Markov model was developed (Treeage Pro™ software 2014, TreeAge Software, Inc., Williamstown, MA, USA) to estimate expected values for costs and QALYs for different primary surgical prolapse repair strategies (standard repair, synthetic mesh and biological graft) over a base-case time horizon of 5 years. Results were obtained with a Monte Carlo (probabilistic or second-order) simulation of the developed Markov model with 1000 iterations. The model parameters were drawn from appropriate distributions attached to baseline data, relative effect sizes, costs and utilities. Baseline results were presented as mean costs and QALYs from the iterations, and the simulation was used to present uncertainty in modelled outcomes. Uncertainty is presented as incremental scatterplots on the cost-effectiveness plane and CEACs.
The model describes the treatment outcomes and follow-up of women who were having a primary prolapse repair. The perspective adopted is that of the UK NHS. Costs were based on 2013–14 UK pound sterling (£) values.
Model framework
The model was developed to extrapolate results of the Primary trial analysis (2-year follow-up) to a longer (5-year) time horizon. As prolapse is a chronic condition, with the potential for long-term failure and complications following surgery, a Markov state-transition decision-analytic model was used to represent these stochastic processes that evolve over time (in this case, monthly cycle lengths). The structure of a Markov model allows patients to move between defined mutually exclusive health states in a controlled manner over specified time periods. The pathways presented and transitions allowed within the model were developed in consultation with clinicians and trial collaborators.
The Markov model structure is outlined in Figure 27.
FIGURE 27.
Markov model structure: primary prolapse repair.

All women enter the model in the ‘primary prolapse repair’ health state, for which they receive surgery. This initial ‘primary prolapse repair’ surgical state is set to the duration of three monthly cycles to reflect the likely time to recovery from prolapse surgery. After their initial surgical treatment, women then move into one of four mutually exclusive health states.
-
They may enter the ‘post-prolapse surgery’ health state (defined as women who are not experiencing serious complications or requiring repeat prolapse surgery). Within this health state, some women will still experience some prolapse-related symptoms or other (non-serious) complications* and may receive treatments for this, including physiotherapy or oestrogen treatments. Others will not require any further treatment in that cycle and are considered to be ‘stable’. Women might stay in this state for the duration of the model (if they do not experience serious complications or require repeat prolapse surgery). At the end of each monthly cycle, they may transition from this state if they have serious complications, require further prolapse surgery or die.
-
Women may suffer serious complications at any point (as defined in the clinical classification of complications) following their surgery. If a woman experiences serious complications, she enters the ‘serious complications’ health state and receives treatment. Serious complications may be mesh or non-mesh related. Some will require surgery to address their complications. A woman who is experiencing serious complications might have these resolved during a single monthly cycle or might require to remain in the health state for a longer time period until the complications resolve.
-
Women might suffer a recurrence of their prolapse, which requires further repeat prolapse surgery at any time. For these women, surgery is deemed to have failed. For the purposes of this model, a failure is considered as a requirement for further prolapse surgery. Women who experience failures that are not requiring surgery remain in the post-prolapse surgery health state (see ‘1’, above). Women who were having a failure requiring surgery enter the ‘second surgery’ health state, for which they go through a similar model process as those following their first repair. A failure requiring surgical repair is considered to be any repeat prolapse surgery, whether it occurs in the same or a different compartment.
-
There is a small chance that a woman may die from natural causes. The ‘death’ state considers that women may die from any causes at any point in time and this is assumed to be all-cause mortality.
[Note: *The complications have been classified in accordance with the trial reporting of adverse effects as serious mesh complications, serious non-mesh complications, other mesh complications and other non-mesh complications. ‘Other’ complications have been defined as those that were not categorised as serious adverse effects for trial reporting, but may still have had a significant impact on quality of life and use of health services. Complications were considered ‘serious’ if they met one or more of the following criteria: the complications (1) resulted in the patient’s death, in which case they progressed straight to the death state in the model; (2) resulted in hospitalisation; (3) resulted in prolongation of an existing hospital stay; (4) lead to persistent or significant disability or incapacity; (5) were life-threatening; and (6) were considered medically significant by the trial investigator.]
Table 95 illustrates the treatment sequences for primary prolapse repair and any further repair surgery. The women receive a maximum of two further prolapse surgical repairs. Women who are still found to have prolapse failure after the third surgical procedure are assumed to proceed to containment management. This includes the use of pessaries, physiotherapy and regular outpatient consultation. The follow-up treatments are assumed to be the same for all the women, as there is no evidence to inform the specific follow-up treatments. Clinical expert opinion (Professor Cathryn Glazener, University of Aberdeen, July 2015, personal communication) indicates that women may get any of the considered options as a secondary repair (e.g. standard, mesh inlay or mesh kit). Therefore, for the purposes of the economic analysis, all of the types of surgery are grouped together for the second and third prolapse repair as an aggregate of outcomes reported for the Secondary trial (see Chapter 5).
| First treatment | Second treatment | Third treatment |
|---|---|---|
| Standard repair | Second surgery | Third surgery |
| Synthetic mesh inlay | ||
| Biological graft |
Survival analysis, based on time-to-event data, is used to guide the proportion of the modelled cohort progressing to repeat surgery health states and is outlined in more detail later in the chapter.
Women can continue moving through the states in the model for a maximum of 5 years (equivalent to 60 monthly cycles). A monthly cycle length has been chosen to reflect the time increments for which data regarding time of treatment failure and complications requiring surgery were available. This time horizon was selected as the study follows up patients for only 2 years and there are no long-term follow-up data. Costs and benefits that occur in the future were discounted following the standard practice, that is the recommended 3.5% for both costs and benefits (NICE 201338).
Summary of the key assumptions made in the economic model
Details regarding the choice of data used to populate the model together with justifications for any assumptions made are outlined throughout the remainder of this section.
Assumptions related to the structure of the model
-
The average age of women considered in the model is 59 years. This is the average age of the women who took part in the primary RCT. In the sensitivity analysis, different ages were considered.
-
The cycle length of the model is 1 month. This cycle length was chosen to take in account the time to failure that was recorded in the trial.
-
Cumulative costs and benefits are estimated for the 5-year period. In sensitivity analysis, the effect of a longer-term follow-up (10 years) was considered, although data to populate longer time to effect analysis are sparse and highly uncertain.
-
Women who are in the ‘post-prolapse repair’ health state may be stable, but may also experience prolapse-related symptoms, and may also have prolapse failure (not requiring surgery). These are defined as women using containment products and they receive a utility decrement and additional cost within the ‘post-prolapse surgery’ health state. However, they do not require or receive surgery in that state but may do so at a point in the future.
-
Women experiencing a repeat surgery are assumed to remain in the ‘second prolapse repair’ health state for a duration of 6 months. This is to allow for the time required to diagnose the failure, waiting list for treatment and a period of convalescence following surgery.
-
Women experiencing serious complications may have their complications resolved within 1 month, or a proportion will remain with longer-term unresolved complications, assumed to be 25% resolved within 1 month, 50% within 3 months, 75% within 6 months and all resolved within 1 year.
Assumptions relating to the treatments offered
-
The treatment strategies compared different initial treatments, namely standard midline repair, synthetic mesh and biological graft. Women requiring secondary repair surgery were assumed to receive the costs and outcomes associated with an aggregate of all of the secondary repairs (i.e. standard repair, mesh inlay or mesh kits). In reality, women may decide to receive specific secondary treatments that are deemed appropriate in conjunction with their clinicians.
-
In the model, women were assumed to move into the containment failure management state (which is an absorbing state as they cannot leave it) after they had three surgeries. In reality, women whose symptoms recur may continue to seek surgical treatment until their symptoms are satisfactorily resolved. However, there are no data to populate this level of detail within the model, and, as such, the impact on cost-effectiveness is likely to be small, given the low proportion of women receiving three prolapse repairs within our modelled time horizon.
-
Women are assumed to enter the containment arm of the model if they receive containment products that included medicines, oestrogens, pessaries and physiotherapy. Women may also receive these products without surgical repair, in which case they receive a utility decrement and additional cost within the ‘post-prolapse surgery’ state of the model (if they have not progressed through all three surgical repairs). This allows for the fact that women may get containment products on an ongoing basis, without surgical repair, or although they wait to see if symptoms clear up before requiring surgery.
Assumptions relating to data to populate the economic model
-
Long-term estimates of failures and complication rates were based on the extrapolation of trial data from the three-way comparison (RCT1A).
-
It was assumed that probabilities of failure and complications following a third repair surgery were equal to those following a second repair surgery.
-
The costs of the second and third surgery were based on the aggregate cost of all three secondary treatment (standard midline repair, synthetic mesh and mesh introducer kits) surgeries from the trial data, as it was considered there was an equal chance that patients could receive any of the treatments.
-
The model also took into account that some of the women did not have any further surgical treatments but may have had no surgical treatments, such as pessaries and other containment products as well as physiotherapy. Costs were attached to a proportion of the women in different cycles, as well as those in the containment state.
Estimation of model parameters
The model is parameterised using data from the three-way trial comparison (RCT1A) and relates only to women who were having a primary prolapse repair. When sufficient data were not available for individual model parameters from the trial, published and unpublished evidence in the field was consulted and, laterally, clinical expert opinion was used to populate any remaining parameters.
The methods used to assemble data followed recognised methodology, which varied according to the type of parameter, extent of uncertainty and role within the model. The modelling exercise complied with recent recommendations on good practice for modelling63 and the results are presented in terms of incremental cost per QALY gained.
Mortality parameters
As women move through the model there is a chance that they may die. This chance is based on the annual rates of age-specific all-cause mortality for women (Office for National Statistics interim life tables46). As there were no intraoperative deaths reported during the PROSPECT trial in primary repair women, we have not added any additional surgical mortality to treatments. Furthermore, as there were relatively few deaths and no difference across arms regarding mortality over follow-up, no additional mortality risk that was specifically related to prolapse was applied.
Probability parameters
The main probabilities for the model are the probabilities of developing failures and complications. Probabilities reported in the tables to follow are adjusted, as appropriate, to reflect the model cycle length of 1 month using Treeage Pro’s ‘probtoprob’ function.
Treatment failures
The probability of treatment failure is included within the model in two distinct ways. First of all there is a probability of failure, which may not require surgery and may be managed using conservative/containment techniques, as outlined above. Probabilities of conservatively managed failure are elicited directly from RCT1A of the trial for 1 year and 2 years, respectively. The longer run probability of failure requiring containment products is assumed to remain static at the 2-year probability over the remainder of the model time horizon. Table 96 presents data for the probability of failure requiring conservative treatment management. Uncertainty was incorporated surrounding the estimates by sampling from a beta distribution, for which the alpha parameter is the number of events of interest (in this case the number of women with a conservatively managed failure) in the standard repair arm. The beta parameter is given as the total number of women in the standard repair arm minus the number of women with the event of interest.
| Variable | Time point (year) | Point estimate | Alpha | Beta | Distribution applied | Source |
|---|---|---|---|---|---|---|
| Conservative managed failure (primary repair) | 1 | 0.0837 | 18 | 197 | Beta | RCT1A trial data |
| 2 | 0.0576 | 11 | 180 |
The probability of failure, managed conservatively, following a secondary repair was assumed to be equal to that following a primary repair procedure, but was not incorporated as treatment-specific estimate within the model. The probability of having a conservative failure (not requiring surgery) beyond 2 years was assumed to remain constant for the remainder of the modelled time horizon.
Second, the more serious failures are defined as those that require further surgery (i.e. a secondary prolapse repair). There is conflicting evidence on the long-term failure (requiring surgery) rates for prolapse repairs, and hence significant uncertainty regarding the transition probabilities for the model. Transition probabilities to the health state of second and, subsequently, third prolapse surgery were estimated using survival analysis of time-to-event data over 2-year follow-up for the primary (RCT1A) and secondary (RCT2) trials, respectively. As outlined previously, women who were having a third failure were assumed to be treated using conservative management for the remainder of the model beyond having a third failure.
Extrapolation of the long-term reoperation rates for time to first failure (and time to second failure from the Secondary trial data) were presented using Kaplan–Meier survival curves, using a Weibull distribution. The formula used for the survival function is given as Equation 2. Equation 3 presents the formula that was used to estimate transition probabilities for the baseline standard repair treatment:
where:
-
S(t) is the survival function representing the probability of success (i.e. not experiencing a prolapse failure).
-
tp represents the formula for calculating transition probabilities to the failure health state for each monthly cycle in the model.
-
t is the time (measured in terms of the number of cycles, for which each cycle is equivalent to 1 month).
-
λ is the constant parameter from the regression model; this is the scale parameter that shows the probability that a woman’s prolapse will recur in the next period, given the fact that she was successful in the current period.
-
γ is the shape parameter that describes the rate of change in the probability that a woman will have a recurrence of prolapse over time (i.e. that she will have failure). Shape parameters of > 1 indicate an increasing rate over time, whereas shape parameters of < 1 indicate a decreasing rate over time.
The above formula was adjusted by the appropriate hazard ratios to estimate transitions for the synthetic mesh and biological graft repair arms, respectively. Uncertainty is incorporated into the model by using the Cholesky decomposition of the covariance matrix estimated from the output of the Weibull regression models. A multinormal distribution was used to sample from the appropriate table containing Cholesky decomposition estimates multiplied by the coefficient of the log of the hazard ratios, lambda and gamma parameters. All statistical analyses of time-to-event data were estimated using Stata version 14 statistical analysis software. Using this process it was possible to estimate a hazard function using the values reported in Table 97.
| Treatment | λ (lambda) | γ (gamma) | Hazard ratio: mean (95% CI) | |
|---|---|---|---|---|
| Non-adjusted | Adjusteda | |||
| Standard midline | 0.000615 | 1.408625 | ||
| Synthetic mesh | 0.000764 | 1.408625 | 1.214 (0.598 to 2.462) | 1.075 (0.495 to 2.335) |
| Biological graft | 0.000835 | 1.408625 | 1.359 (0.681 to 2.710) | 1.164 (0.551 to 2.459) |
Figure 28 shows the shape of the Kaplan–Meier curves that were fitted to the data reported in Table 97.
FIGURE 28.
Kaplan–Meier survival estimates for surgery for prolapse failure.

Time until the first major surgical procedure for prolapse failure was estimated using the survival time regression models with a Weibull distribution specified above. The hazard ratio was 1.075 (95% CI 0.495 to 2.335) for synthetic mesh compared with standard repair, and as 1.164 (95% CI 0.551 to 2.459) for biological graft compared with standard repair, based on adjustment for all minimisation covariates included in the regression model. Without adjustment, and examining only the hazard ratios, based on the observed data directly, unadjusted hazard ratios were 1.214 (95% CI 0.598 to 2.462) and 1.359 (95% CI 0.681 to 2.710) for synthetic mesh and biological graft repairs, respectively.
Based on these data, there is no evidence of a difference between groups in terms of time to failure event at 2 years, with hazard ratios close to ‘1’ indicating near equivalence between the groups. This estimate is generated using a model specifying a Weibull distribution. In order to select the appropriate model, we undertook a two-stage process. First, we tested and rejected (p = 0.66) the proportional hazards assumption using a Cox proportional hazards model. The next step was to select an appropriate distribution for the extrapolation model. A number of potentially appropriate distributions exist. Each was evaluated independently and likelihood ratio tests reported. None of the explored distributions performed particularly well, given the few data available. Likelihood ratio tests were conducted for log-logistic, Gompertz, exponential and Weibull distributions. In reality, any of these distributions could have been chosen. We selected a Weibull because of its wide use in economic evaluation literature and its flexible mathematical characteristics were deemed appropriate for use in this analysis. [We rejected the proportional hazards assumption for the Cox proportional hazards model (p = 0.6598). We then compared the following distributions on the basis of their likelihood ratio (chi-squared) and associated p-values: (A) log-logistic (likelihood ratio 0.81; p = 0.67); (B) Gompertz (likelihood ratio 0.77; p = 0.68); (C) exponential (likelihood ratio 0.76; p = 0.68); (D) Weibull (likelihood ratio 0.68; p = 0.68).]
Estimated CIs from both the adjusted and unadjusted regressions are wide, in part as a result of the relatively small number of failures requiring surgery across all three groups. Nonetheless, there is evidence that the prolapse failure rate increases over time, but at a declining rate, yet the proportional hazards model cannot be rejected.
Transition probabilities to third prolapse surgery, that is, long-term failure (requiring surgery) following a secondary repair surgery, are estimated using data from the Secondary trial analysis (see Chapters 6 and 7). Analysis was not treatment specific and, instead, incorporated failures for all women in the Secondary trial. Methods were similar to those estimating failure following a primary surgical repair. The associated Kaplan–Meier plot for all secondary surgery repairs together is presented in Figure 29.
FIGURE 29.
Kaplan–Meier survival estimates for surgery for prolapse failure (secondary). The Kaplan–Meier curve relates to all women who were having a secondary prolapse repair regardless of treatment strategy. Data are sourced from secondary repair trials (RCT2 and RCT3).
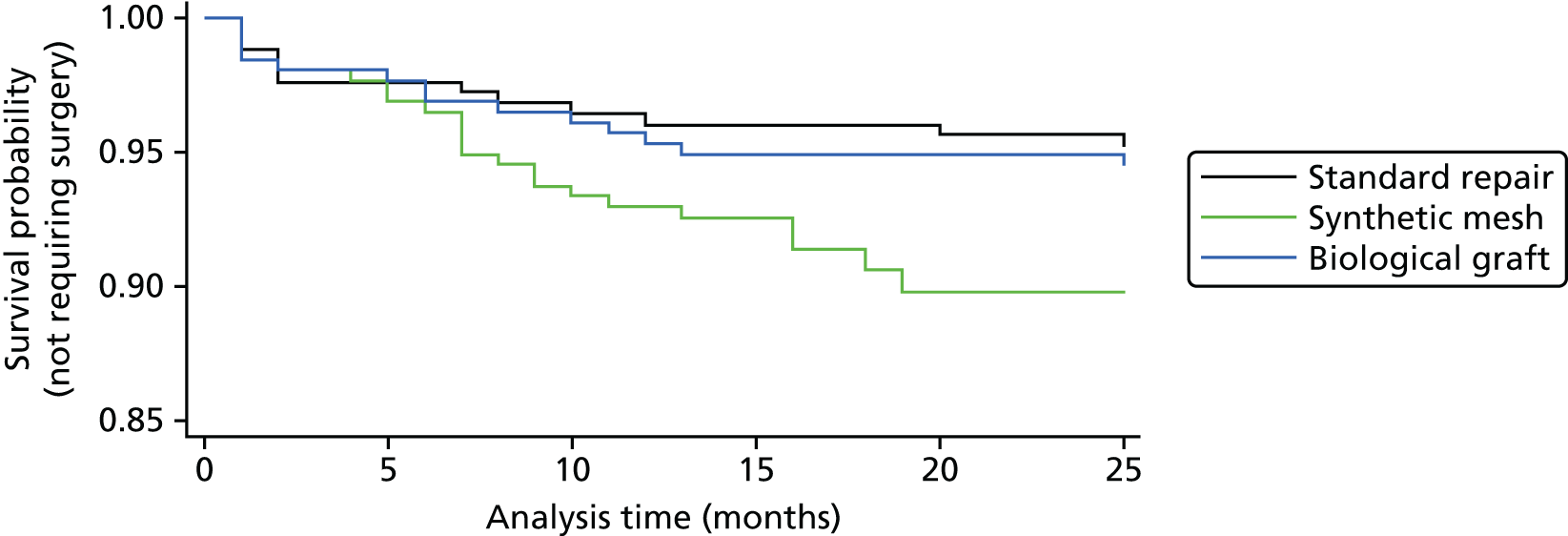
The probability of further failure, following a third surgical repair, was assumed to be equivalent to the probability of failure following a secondary repair. At this point, it was assumed that women received only containment management and entered the absorbing, containment state, at which they remained for the duration of the model with a probability of all-cause mortality.
Complications
The probabilities of complications are divided into two distinct groups, namely mesh complications and non-mesh-related complications. These are further subdivided according to whether they were classified as SAEs within the trial or other adverse effects/complications. All of the complications included in the model were related to the prolapse repair preceding their occurrence. For example, complications following a primary repair were assumed to be related to the primary repair, complications following a secondary repair were assumed to be related to the secondary repair, and so on. Data are incorporated separately for 1- and 2-year outcomes to reflect the drop in complications over time. Baseline complications are determined according to the events observed within RCT1A (primary three-way trial comparison) for the standard repair arm of the trial.
Table 98 presents data for the probability of the various estimates of complications included in the model. Uncertainty surrounding the estimates was incorporated by sampling from a beta distribution, for which the alpha parameter is the number of events of interest (in this case, the number of women with a conservatively managed failure) in the standard repair arm. The beta parameter is given as the total number of women in the standard repair arm minus the number of women with the event of interest (i.e. a complication). It should be noted that these data are based on RCT1A only, and, as such, are not directly comparable with the complications data that were reported for trial 1 in Chapter 4.
| Variable | Following surgery | Time point | Point estimate | Alpha | Beta | Distribution applied | Source |
|---|---|---|---|---|---|---|---|
| Serious mesh complications | Primary | 1 year | 0.004 | 1 | 251 | Beta | RCT1A data |
| Serious non-mesh complications | 0.063 | 15 | 237 | ||||
| Other mesh complications | 0.000 | 0.00001 | 251.99999 | ||||
| Other non-mesh complications | 0.063 | 17 | 235 | ||||
| Serious mesh complications | Primary | 2 years | 0.004 | 1 | 251 | Beta | RCT1A data |
| Serious non-mesh complications | 0.012 | 4 | 248 | ||||
| Other mesh complications | 0.000 | 0.00001 | 251.99999 | ||||
| Other non-mesh complications | 0.024 | 8 | 244 | ||||
| Serious mesh complications | Secondary | 1 year | 0.025 | 10 | 387 | Beta | RCT2, CC2 |
| Serious non-mesh complications | 0.083 | 33 | 364 | ||||
| Other mesh complications | 0.020 | 3 | 150 | ||||
| Other non-mesh complications | 0.092 | 14 | 139 | ||||
| Serious mesh complications | Secondary | 2 years | 0.007 | 1 | 152 | Beta | RCT2, CC2 |
| Serious non-mesh complications | 0.020 | 3 | 150 | RCT1A data | |||
| Other mesh complications | 0.007 | 1 | 152 | RCT2, CC2 | |||
| Other non-mesh complications | 0.033 | 5 | 148 | ||||
Beyond the trial follow-up period, because of the low numbers of complications reported at 2-year time point in the trial, we assume that the probabilities of ‘other’ complications are ‘0’ beyond 2 years. The probability of serious complications beyond 2 years is estimated using a time-to-event analysis to project rates of complications requiring surgery over the longer time horizon of the model.
Surgery for complications
The probability of complications beyond 2 years was restricted to only those women requiring surgery, as this is the most costly and serious of all complications, with the greatest impact on QoL. Owing to small numbers at 2 years, there was insufficient evidence to project differences and relative risks for each of the four categories of complications to the longer term. However, dates of surgery for women with complications (e.g. mesh removal surgery) were available and included in a time-to-event analysis. The methods and formulae used to generate model transition probabilities to the serious complications state for surgery are similar to those reported in the above section, Treatment failures. Table 99 presents the parameters and hazard ratios used for the analysis of time to first surgery for serious complications.
| Treatment | λ (lambda)a | γ (gamma)a | Hazard ratio: mean (95% CI) | |
|---|---|---|---|---|
| Non-adjusted | Adjusted | |||
| Standard midline | 0.00588 | 0.6588213 | ||
| Synthetic mesh | 0.0129 | 0.6588213 | 2.196 (1.108 to 4.353) | 2.558 (1.123 to 5.825) |
| Biological graft | 0.00681 | 0.6588213 | 1.158 (0.536 to 2.504) | 1.173 (0.450 to 3.055) |
Figure 30 presents the Kaplan–Meier curves describing the data that were used to project longer-run transition probabilities for complications requiring surgery, beyond the 2-year follow-up.
FIGURE 30.
Kaplan–Meier survival estimates for surgery for serious complications.
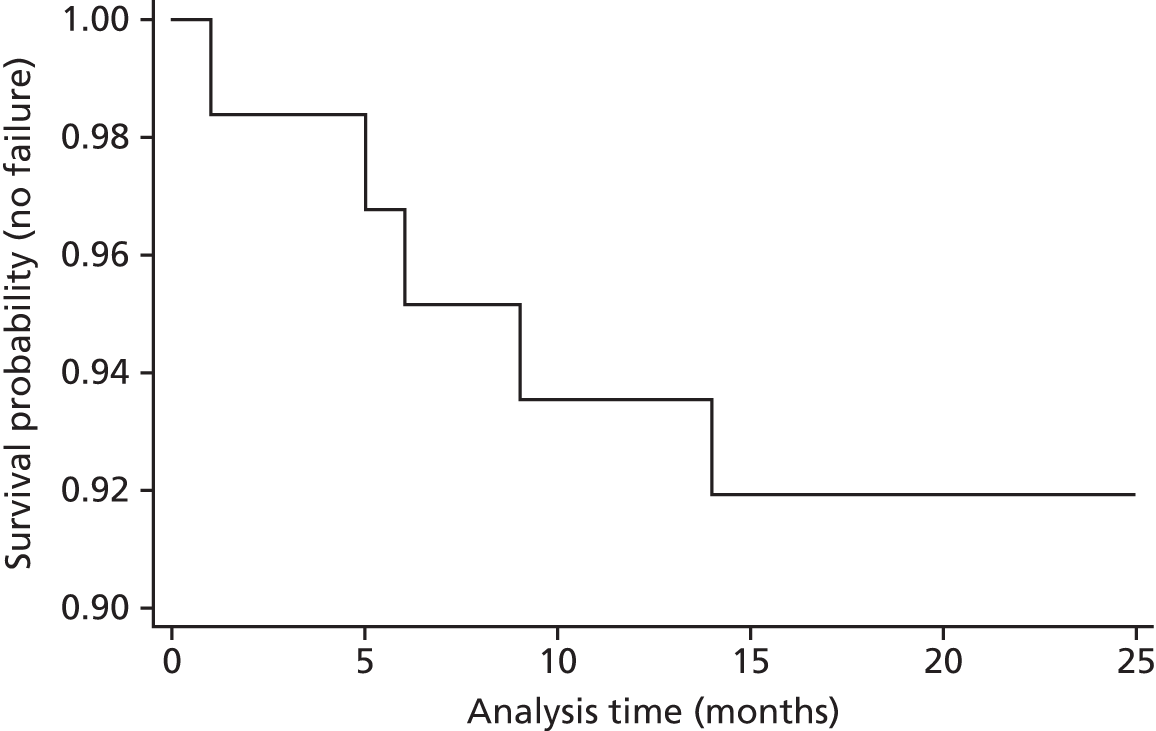
Analysis of time until the first surgical procedure for complications related to prolapse surgery estimated the hazard ratio as 2.558 (95% CI 1.123 to 5.825) for synthetic mesh compared with standard repair, and as 1.173 (95% CI 0.450 to 3.055) for biological graft compared with standard repair. Based on these data, surgery for complications occurs more often and earlier in the synthetic mesh group than in the standard repair group. This difference is driven by mesh complications in the synthetic mesh group. The estimation models for the hazard ratio are adjusted for baseline utility (QoL) and other minimisation variables, as outlined in Chapter 2. Estimating the same hazard ratios, based on raw data only, without adjustment, the hazard ratios were 2.196 (95% CI 1.108 to 4.353) and 1.158 (95% CI 0.536 to 2.504) for synthetic mesh and biological graft repairs compared with standard repair, respectively.
There were no apparent differences in complications requiring surgery between the biological graft and the standard repair groups, although CIs were wide, in part due to the relatively small number of complications requiring surgery across these two groups.
Data from the estimates of time to event are used to estimate transition probabilities in the decision-analytic model. As with the analysis of time to surgery for prolapse failure, the analysis of surgery for complications is based on Weibull regression models to project data shown at 2 years in Figure 30 over the longer-term time horizon required to populate the decision model. The decision to use a Weibull distribution followed a similar logic to that for the extrapolation of failure data. [Details of likelihood ratio tests for alternative distributional assumptions: we rejected the proportional hazards assumption for the Cox proportional hazards model (p = 0.2934). We then compared the following distributions on the basis of their likelihood ratio (chi-squared) and associated p-values: (A) log-logistic (likelihood ratio 6.48; p-value = 0.04); (C) log-normal (likelihood ratio 5.39; p-value = 0.07); (D) Gompertz (likelihood ratio 6.48; p-value = 0.04); (E) exponential (likelihood ratio 6.70; p-value = 0.04); and (F) Weibull (likelihood ratio 6.57; p-value = 0.04).] Following examination of the goodness of fit of the respective models (all of the assumed distributions performed equally well), we chose a Weibull because of its extensive use in economic evaluation literature and its flexible mathematical properties. Transition probabilities are calculated for monthly cycles of the model. A similar, but not treatment-specific, analysis was undertaken to project long-term complications for all of the women who were having surgery following a secondary repair. Data from this analysis are presented in Appendix 6. Owing to a lack of appropriate data to populate the model, we did not consider complications following a third prolapse procedure.
Relative risk parameters
For time-to-event parameters, hazard ratios were used to assign relative effect sizes to the time to experience a failure/surgery for complications. Uncertainty in these estimates was incorporated through the use of Cholesky decomposition matrices, as outlined in the Probability parameters section above. In relation to parameters based on proportions (conservative treatment for failure and non-surgical complications), absolute parameter values for synthetic mesh repair and biological graft repair were calculated by applying relative effect sizes (for synthetic mesh and biological graft vs. standard repair) to baseline probabilities. All of the relative effect sizes were incorporated into the model as point estimates of relative RRs with 95% CIs, estimated using the within-trial data as detailed in Chapters 2 and 4. Owing to a lack of data to inform longer-term extrapolation of each individual parameter beyond the trial follow-up, we have projected the probabilities of only complications requiring surgery or failures requiring surgery beyond 2 years. We rely on the time-to-event analyses described above to guide these model probabilities.
Furthermore, in instances for which baseline data may not have been available or zero events were observed in either the standard or comparison groups (e.g. serious mesh complications in the standard repair arm at 2 years were zero), raw data on probabilities in the comparator arms was used to populate the model in the place of relative risk estimates. When this is the case, it is clearly outlined in Table 100 and the probability distributions used are outlined in Table 101. Relative risk data were not applied to probabilities following a secondary surgery, as it was not possible to know whether failure of a second repair was linked to the original primary repair or the secondary repair that took place. We therefore pragmatically assumed that all of the data following secondary repair (i.e. the Secondary trial and the Secondary CC) provide the most reliable data for complications and failures following a second prolapse surgery, and these data are applied to all of the secondary repairs in the model.
| Parameter | Comparison | Time | Mean | Lower value | Upper value | Distribution applied | Mean of logs | SE of logs | Notes/sources |
|---|---|---|---|---|---|---|---|---|---|
| Serious complications | |||||||||
| Relative risk serious non-mesh complications | Standard mesh vs. standard repair | 1 year | 1.34 | 0.71 | 2.52 | Log-normal | 0.29 | 0.32 | Data from RCT1A |
| Biological graft vs. standard repair | 1.93 | 1.07 | 3.50 | 0.66 | 0.30 | ||||
| Relative risk serious mesh complications | Standard mesh vs. standard repair | 1 year | 22.52 | 3.07 | 165.28 | Log-normal | 3.11 | 1.02 | Data from RCT1A |
| Biological graft vs. standard repair | N/A | N/A | N/A | N/A | N/A | N/A | RR not calculable; see Table 101 | ||
| Relative risk serious non-mesh complications | Standard mesh vs. standard repair | 2 years | 0.66 | 0.19 | 2.30 | Log-normal | –0.42 | 0.64 | Data from RCT1A |
| Biological graft vs. standard repair | 0.49 | 0.09 | 2.66 | –0.71 | 0.86 | ||||
| Relative risk serious mesh complications | Standard mesh vs. standard repair | 2 years | 24.91 | 3.4 | 182.79 | Log-normal | 3.22 | 1.02 | Data from RCT1A |
| Biological graft vs. standard repair | 1.22 | 0.08 | 19.32 | 0.20 | 1.40 | ||||
| Other complications | |||||||||
| Relative risk other non-mesh complications | Standard mesh vs. standard repair | 1 year | 0.93 | 0.48 | 1.79 | Log-normal | –0.07 | 0.34 | Data from RCT1A |
| Biological graft vs. standard repair | 0.82 | 0.41 | 1.62 | –0.20 | 0.35 | ||||
| Relative risk other mesh complications | Standard mesh vs. standard repair | 1 year | N/A | N/A | N/A | N/A | N/A | N/A | RR not calculable; see Table 101 |
| Biological graft vs. standard repair | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Relative risk other non-mesh complications | Standard mesh vs. standard repair | 2 years | 0.63 | 0.21 | 1.88 | Log-normal | –0.46 | 0.56 | Data from RCT1A |
| Biological graft vs. standard repair | 1.1 | 0.43 | 2.81 | 0.10 | 0.48 | ||||
| Relative risk other mesh complications | Standard mesh vs. standard repair | 2 years | N/A | N/A | N/A | N/A | N/A | N/A | RR not calculable; see Table 101 |
| Biological graft vs. standard repair | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Failures requiring conservative management | |||||||||
| Relative risk failure requiring conservative management | Standard mesh vs. standard repair | 1 year | 0.85 | 0.44 | 1.64 | Log-normal | –0.16 | 0.33 | Data from RCT1A |
| Biological graft vs. standard repair | 0.84 | 0.44 | 1.62 | –0.17 | 0.33 | ||||
| Standard mesh vs. standard repair | 2 years | 1.42 | 0.68 | 2.99 | 0.35 | 0.38 | |||
| Biological graft vs. standard repair | 1.13 | 0.52 | 2.42 | 0.12 | 0.39 | ||||
| Variable | Treatment | Time point | Point estimate | Alpha | Beta | Distribution applied | Source |
|---|---|---|---|---|---|---|---|
| Serious mesh complicationsa | Biological graft | 1 year | 0 | 0 | 255 | Beta | RCT1A |
| Other mesh complications | Standard mesh | 1 year | 0.0235 | 6 | 249 | ||
| Biological graft | 0.00392 | 1 | 254 | ||||
| Standard mesh | 2 year | 0.0275 | 7 | 248 | |||
| Biological graft | 0.00392 | 1 | 254 |
Table 100 details point estimates and 95% CIs of relative effect sizes used in the model. Uncertainty surrounding the point estimates was characterised using log-normal distributions. Data used to define the distributions are presented as mean and SE of the log estimates. Table 101 details the point estimates used for data for which relative risks were not calculable. For these parameters, beta distributions were applied, based on count data as in Table 98.
Resource utilisation and cost parameters
Costs for use within the economic model are health state specific, based on health-care resource use observed from women experiencing specific events from the trial-based analysis for RCT1A. Mean (SD) costs for women who were experiencing health states were used to populate the economic model. Costs are estimated from the perspective of the UK NHS, and reported in 2014 UK pound sterling (£) values. Costs were based on resource-use data that were reported in the trials. All relevant unit costs underpinning the cost distributions can be found in Chapters 2 and 5 and Appendix 6. Costs of secondary repair surgery are based on intervention costs (microcosted) from the Secondary trial analysis. Base-case costs for secondary repairs in the model were calculated assuming an aggregate of all of the intervention costs from the Secondary trial, regardless of treatment received. Sensitivity analysis explored the impact of assuming that all of the women receive standard repair or all women receive mesh kit for the secondary repair. A further sensitivity analysis used cost estimates from the Primary trial (RCT1A) for those women experiencing a prolapse failure. The latter analysis was based on NHS unit costs, rather than microcosting approach for the base-case analysis of secondary intervention cost. Costs for health states applied to the base-case model were calculated across all of the women who were randomised to RCT1A and were not treatment specific.
The way in which costs for health states were estimated in the base-case analysis depended on whether or not dates of events were available from the trial data set. Costs for events for which no dates of event were available are applied to health states as average costs of treatments incurred over the whole trial follow-up and divided evenly across each model cycle. This includes the cost of conservatively treated failures and women who had no further symptoms (i.e. were stable) in the ‘post-prolapse surgery’ health state of the model. As many women would not have a clear cure and may still experience symptoms related to prolapse, those women in the ‘post-prolapse surgery’ health state still incurred a cost. Women categorised as being ‘stable’ in the ‘post-prolapse surgery’ health state were defined as those women not reporting conservative management treatments, not having surgery for failure and not experiencing serious adverse effects or any other prolapse-related complications.
Costs of health states for which dates of event were available (e.g. date of surgery for complications or failures) were estimated only for women reporting resource use within 4 months of the event. This allowed a more accurate reflection of resource use that was applied to the limited time in the respective health states for those who experienced complications.
Furthermore, costs included in the model were split between 1- and 2-year follow-up. The justification for this is that the costs of problems incurred over the second year of follow-up are more appropriate for longer-term extrapolation, as they do not include routine follow-up care following surgery and furthermore the patient reported costs in year 2 are unlikely to include trial-based consultations (such as 1-year prospect follow-up appointment). Costs applied to health states for each model cycle beyond 2 years were assumed to be the same as those incurred between 1- and 2-year follow-up from the trial. Adjustments were undertaken to apply costs to each monthly model cycle.
For the costs of treating serious and other complications, it was assumed that the treatment costs would be similar in terms of resource use for both mesh and non-mesh complications. This is on the basis that to be considered a serious adverse effect, women would have had a substantial contact with health-care professionals and associated treatments. Furthermore, splitting the cost regressions from the trial, based on four different categories (serious mesh, other mesh, serious non-mesh and other non-mesh complications), generated great uncertainty, as a result of small numbers of participants reporting full-cost data within each of these individual groups. Therefore, we pragmatically decided to group costs of serious complications together. A similar approach was taken for ‘other’ complications.
As a summary, by using estimates of costs from the trial-based analysis in Chapter 5, the resource utilisation and costs included in the model are a comprehensive reflection of all resource use, including:
-
the interventions, incorporating the surgery, preparations and hospital resource use in theatre, based on operation time, staff time and other additional treatments.
-
postoperative resource use (from surgery to discharge), including time on ward, return to theatre, catheterisation, cost of treating any infections, for example UTIs and any other adverse effects
-
inpatient admissions (any follow-up operations and length of stay in hospital related to prolapse symptoms, including overnight and day-case admissions)
-
outpatient attendances (including all outpatient contacts over the trial follow-up period)
-
primary care visits (including GP contacts, occupational therapist, physiotherapist and nurse contacts)
-
medications related to treating prolapse and UI symptoms.
Detailed information on how unit costs were developed within the trial are provided in Chapters 2 and 5. Costs from the data set were adapted to reflect the additional costs of experiencing prolapse-related events. These health state-specific costs are applied within the model and were assumed to follow a gamma distribution. Unit costs, together with alpha and beta parameters (where appropriate), are presented in Table 102 in accordance with the specifications outlined by the Treeage software. All costs used in the model were discounted by 3.5% per annum in line with current best practice guidance. 38
| Variable | Point estimate (£) | SE (£) | Alpha | Beta | Distribution |
|---|---|---|---|---|---|
| Cost of primary repair (standard) | 2831 | 1151 | 6.05 | 467.96 | Gamma |
| Cost of primary repair (synthetic mesh) | 3128 | 1042 | 9.01 | 347.11 | |
| Cost of primary repair (biological graft) | 3258 | 1279 | 6.49 | 502.10 | |
| Cost of further surgery for failure (all secondary repairs: base case) | 3112 | 1289 | 5.83 | 533.91 | |
| Cost of further surgery for failure (standard repair: sensitivity analysis) | 2790 | 1295 | 4.64 | 601.08 | |
| Cost of further surgery for failure (mesh inlay: sensitivity analysis) | 3099 | 1358 | 5.21 | 595.08 | |
| Cost of further surgery for failure (mesh kit: sensitivity analysis) | 3522 | 1100 | 10.25 | 343.55 | |
| Cost of further surgery for failure (sensitivity analysis based on Primary trial data) | 782 | 942 | 0.6891 | 1134.74 | |
| Cost of surgery for complications (year 1) | 1515 | 948 | 2.5539 | 593.20 | |
| Cost of surgery for complications (year 2) | 937 | 1088 | 0.7417 | 1263.33 | |
| Cost of being stable in the ‘post-prolapse surgery’ health state (year 1) | 306 | 475 | 0.42 | 737.34 | |
| Cost of being stable in the ‘post-prolapse surgery’ health state (year 2) | 251 | 524 | 0.23 | 1093.93 | |
| Cost of serious complications (mesh and non-mesh; year 1) | 1095 | 1045 | 1.098 | 997.28 | |
| Cost of serious complications (mesh and non-mesh; year 2) | 858 | 1079 | 0.63 | 1356.92 | |
| Cost of other complications (mesh and non-mesh; year 1) | 760 | 1044 | 0.53 | 1434.13 | |
| Cost of other complications (mesh and non-mesh; year 2) | 738 | 1371 | 0.29 | 2546.94 | |
| Cost of conservatively managed failures and containment (year 1) | 598 | 709 | 0.71 | 840.60 | |
| Cost of conservatively managed failures and containment (year 2) | 529 | 766 | 0.48 | 1109.18 |
Quality of life
As with the cost data reported above, utility estimates are applied to health states based on mean data for women experiencing a health event from RCT1A. For the base-case analysis, utilities are applied to health states on the assumption that all women in a health state will have equal utility. Utility weights were adjusted to reflect the model cycle length of 1 year and were combined with length of time in a health state to generate QALYs gained. QALYs were then discounted at a rate of 3.5% per annum in line with best practice guidelines. As with costs, the utility of the ‘post-prolapse surgery’ health state was estimated for those women who were not falling into any of the other health states. Rather than reflecting a health state for which everyone is well or recovered, this state is more ‘all encompassing’, picking up the QoL impact of prolapse for those not experiencing more major problems. Utility values were also estimated for the women who had complications requiring hospitalisation and had a prolapse failure, requiring reoperation as well as those who had complications that did not need hospitalisations. Furthermore, a separate utility was estimated for those women who were in the ‘post-prolapse surgery’ health state, but experienced the need for containment or conservative management of their prolapse symptoms. This value was less than for those in a failure health state, but greater than for those requiring surgery for prolapse failure.
All health state-specific utility estimates calculated on the basis of RCT1A (primary repair surgery) were also applied to women who were experiencing a similar health state following second and third repairs. This assumption implies that utilities are not impacted on by the number of previous surgeries experienced. There were insufficient data available from the Secondary trial to estimate health state-specific utilities for women who were having a second repair. However, data reported from the Primary and Secondary trial analyses overall (see Tables 39 and 68) indicate that women who were having primary and secondary repair had similar EQ-5D scores overall. It is therefore unlikely that, on the basis of currently available data, this assumption has any substantial impact on results.
Table 103 reports the point estimate of all utilities applied to health states within the model. Uncertainty in utility data is characterised and incorporated by sampling from beta distributions for the utility of each modelled health state. Alpha and beta parameters are calculated using the method-of-the-moments approach and the parameters of the distribution are presented in Table 103.
| Health state | Mean value | SE | Distribution | Alpha | Beta | Notes/sources |
|---|---|---|---|---|---|---|
| Treatment failure | 0.609 | 0.296 | Beta | 1.048 | 0.673 | RCT1A |
| Complications requiring surgery | 0.646 | 0.454 | Beta | 0.054 | 0.030 | |
| Stable in the ‘post-prolapse surgery’ health state | 0.831 | 0.248 | Beta | 1.060 | 0.215 | |
| Serious mesh complications (not requiring surgery) | 0.722 | 0.297 | Beta | 0.923 | 0.356 | |
| Serious non-mesh complications (not requiring surgery) | 0.722 | 0.297 | Beta | 0.923 | 0.356 | |
| Other mesh complications (not requiring surgery) | 0.739 | 0.314 | Beta | 0.709 | 0.250 | |
| Other non-mesh complications (not requiring surgery) | 0.739 | 0.314 | Beta | 0.709 | 0.250 | |
| Failure (conservative management) | 0.797 | 0.239 | Beta | 1.458 | 0.372 | |
| Baseline utility (standard repair) | 0.722 | 0.245 | Beta | 1.692 | 0.652 | |
| Baseline utility (synthetic mesh) | 0.711 | 0.233 | Beta | 1.980 | 0.805 | |
| Baseline utility (biological graft) | 0.697 | 0.265 | Beta | 1.399 | 0.608 | |
| Baseline utility (all combined) | 0.710 | 0.248 | Beta | 1.667 | 0.681 | |
| Additional treatment effect (synthetic mesh): sensitivity analysis only | 0.019 | 0.018 | Beta | 1.028 | 53.343 | |
| Additional treatment effect (biological graft): sensitivity analysis only | 0.006 | 0.020 | Beta | 0.091 | 14.190 |
Assessment of cost-effectiveness
Cost–utility analysis
The costs and consequences (QALYs) of the different treatment options were estimated for women with an average age of 59 years (the average age of women undergoing prolapse surgery in the primary repair trial) over a 5-year time horizon. The model generated expected values of costs and QALYs, based on a hypothetical cohort of n = 1000 women who were having a primary prolapse repair. The base-case model results were calculated using a probabilistic analysis using second-order Monte Carlo simulation with 1000 repetitions. Results from the cost-effectiveness analyses were based on mean estimates of baseline probabilities, relative effect sizes, utilities and costs that were drawn from the sampling distributions outlined in the above tables of model inputs. Expected values of costs and QALYs were estimated according to the sampled data for each treatment group (standard repair, synthetic mesh and biological graft).
The expected values of costs and QALYs were combined into a single measure of efficiency and reported as incremental costs per QALY gained. These are ratios of the differences in costs of the interventions divided by the differences in the benefits (QALYs). These data reflect the rate of return in QALYs to the quantity of resources used measured in monetary terms. The ICER is used in conjunction with an identified WTP threshold value to determine if the intervention can be judged to be cost-effective. Interventions reporting an ICER of < £20,000–30,000 per QALY gained are generally considered to offer good value for money to the NHS. Interventions that are less costly and generate greater QALYs than a comparator are the dominant treatment, and offer an even stronger case for cost-effectiveness. Results of mean probabilistic results for each treatment group are presented on the cost-effectiveness plane.
Sampling uncertainty is presented in two distinct ways. First, scatterplots of incremental costs and QALYs generated from the simulations are plotted on the incremental cost-effectiveness plane to show the differences in costs and QALYs for synthetic mesh and biological graft, respectively, compared with standard repair. This specifically shows the uncertainty in the respective quadrants of the cost-effectiveness plane.
Second, CEACs are presented alongside the incremental scatterplots. CEACs illustrate the uncertainty in cost-effectiveness outcomes caused by the combined statistical variability in the model’s parameter estimates. They show the likelihood that each individual treatment strategy is the most cost-effective use of resources at various threshold values of society’s WTP for a QALY gained. CEACs and scatterplots are presented for the base-case and all secondary analyses. Further to these illustrations of cost-effectiveness, all results (base-case and sensitivity analyses) are also accompanied by an indication of the treatment ranking in terms of the most likely treatment to be cost-effective at a threshold value of WTP of £30,000 per QALY, based on the assessment of NMB.
Deterministic sensitivity analysis
Cost-effectiveness acceptability curves illustrate the sampling uncertainty in the model parameters. However, further uncertainty may exist in the choice of data used to populate the model, the methodological or structural assumptions made or the subgroup analyses considered. Various sensitivity analysis were undertaken to explore the importance of these uncertainties and assumptions.
Parameter uncertainty
In addition to exploring sampling uncertainty in parameters, there is uncertainty regarding the appropriate choice of data that are used to populate the model.
Utilities
Alternative choices of utility weights were available from the trial data for use within the model to generate QALY gains. The base-case analysis is informed by utilities generated for health states in the trial, on the assumption that all women in a health state will experience equal utility. For example, all women suffering a failure will have a utility of 0.609 and all women who are non-symptomatic in the ‘post-prolapse surgery’ health state will experience a utility of 0.831. Sensitivity analysis explores the impact on cost-effectiveness of applying treatment-specific utilities to all model health states. This analysis is undertaken to reflect that the complete case analysis of the Primary trial indicated some additional incremental utility that was associated with synthetic mesh for all women, including those with complications or experiencing failures over and above women in the same health state for standard repair and/or biological graft. Although the estimates of treatment-specific utility are unexplained by clinical outcomes, and may be biased by substantial missing data, they are included, nonetheless, in the model for completeness. Additional treatment-specific utility is estimated for synthetic mesh and biological graft based on a GLM with adjustment for all model health states and baseline covariates from the trial. The coefficient on treatment is added to the specific health-state utility in the respective arms of the model to estimate a treatment-specific utility for each modelled health state. This reflects the unexplained additional utility, which appears to be experienced by women who are randomised to synthetic mesh in the complete case analysis of the trial-based economic evaluation. It is likely that this analysis would provide an upper bound on the likelihood of cost-effectiveness for synthetic mesh. The additional utility (see Table 103) effectively increases the utility of women in all health states for which they received mesh as the baseline treatment. This analysis should be interpreted in the light of the uncertainty in the surrounding trial data.
Costs
As with the trial-based analysis, the estimates of costs to the health services and hence cost-effectiveness of treatments are subject to uncertainty surrounding the intervention cost applied in the model. Therefore, as a sensitivity analysis, we have explored the impact of using the most costly meshes for all mesh repair and the least costly meshes for all mesh repair as plausible upper and lower bounds of the intervention costs.
Furthermore, the costs included in the base-case analysis for treatment failure are generated using the microcosting approach based on Secondary trial data for all of the treatments. A number of alternative estimates are explored in sensitivity analysis, namely assume that all women who were having a secondary repair:
-
get standard repair
-
get mesh inlay
-
get mesh kit
-
incur the costs of treatment failure within the Primary trial (based on the reference costs used to generate the costs of resource use for these women within the trial).
Structural uncertainty
As highlighted in the estimation of model probabilities, there was no reliable evidence on long-term reoperation for prolapse surgery failure. Furthermore, we curtail the model to run for only 2 years for which the most accurate data were available (i.e. the same time horizon as the trial follow-up). This allows us to validate the structure of the model and the predicted results internally against the trial data that were used to generate the model parameters.
The incidence of prolapse is likely to increase with age. The mean age of the participants in the trial was 59 years with a SD of 10 years. The costs and benefits in the model were estimated for 5 years. The sensitivity analysis explores the implications of varying the age of women at the start of treatment and the impact of adopting a longer-term period. Therefore, the starting age was changed to 49 and 69 years, and the time horizon was increased to 10 years. A further exploratory analysis is run over a 30-year time horizon. However, results should be interpreted cautiously, as estimates of time to event are highly uncertain for failures. The model will be updated with data from the 6-year follow-up of PROSPECT and the time horizon extended, based on a more comprehensive time-to-event analysis.
Methodological uncertainty
Discount rates were used at a rate of 3.5% per annum applied to costs and QALYs in the base-case analysis. Sensitivity analysis explores the impact of varying these between 0% and 6% per annum in the sensitivity analysis following best practice guidance.
Utility data in the model are based on trial results and, as such, the utility weights are specific to women suffering from prolapse. As a sensitivity analysis, these utility weights are further adjusted to reflect population norms, which are age adjusted according to the stage of the model, reflecting the fact that utility is likely to fall over time as women get older.
Results
Base-case cost-effectiveness results
The base-case results for the estimates of cost-effectiveness from the probabilistic analysis are presented with treatments ranked in ascending order of costs. Treatment strategies that are more costly and generate fewer QALYs than a comparator are excluded on the basis of absolute dominance and are not considered cost-effective. For the remaining strategies, ICERs are calculated. The treatment strategy with the highest ICER falling under the threshold value is considered the most cost-effective treatment option (note, for the purposes of interpretation, the threshold value of the ICER is considered to be < £20,000–30,000 per QALY gained). The base-case results are presented numerically in Table 104 and graphically on the cost-effectiveness plane in Figure 31.
| Strategy | Cost (£) | Incremental cost | QALYs | Incremental QALY | Incremental cost-effectiveness | Incremental cost-effectiveness (vs. standard repair) | NMB ranking (threshold = £30k) |
|---|---|---|---|---|---|---|---|
| Standard repair | 4811 | 3.753 | 0 | 0 | 1 | ||
| Synthetic mesh inlay | 5264 | 453 | 3.748 | –0.0047 | Dominated | Dominated | 3 |
| Biological graft | 5304 | 492 | 3.749 | –0.0035 | Dominated | Dominated | 2 |
FIGURE 31.
Base-case cost-effectiveness plane.
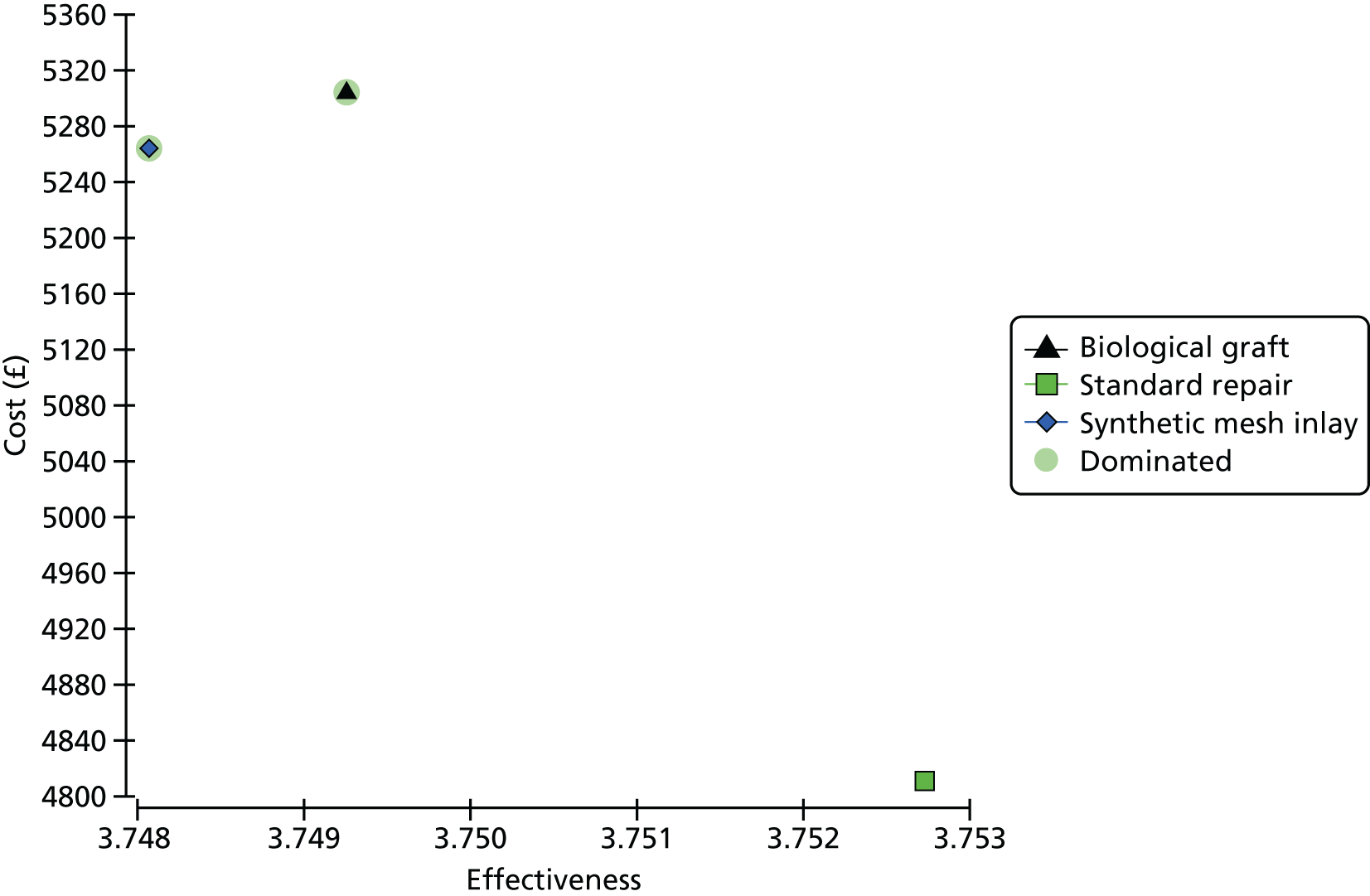
For the base-case economic analysis, standard repair is considered the most cost-effective treatment strategy, being less costly, with marginally greater QALYs gained. However, differences in QALYs are small in magnitude and results are driven primarily by the additional intervention cost of mesh. Uncertainty in the base-case results is illustrated according to the CEAC presented in Figure 32. The probabilities of cost-effectiveness for the base-case results at a threshold value of WTP of £30,000 per QALY gained are 50%, 23% and 27% for standard repair, synthetic mesh and biological graft, respectively. The results of the base-case cost-effectiveness analysis are comparable with the results of the within-trial analysis using the imputed data set in Chapter 5. The sensitivity analysis section explores a direct comparison between modelled results over 2 years, and the trial results are reported in Chapter 5.
FIGURE 32.
Cost-effectiveness acceptability curve.
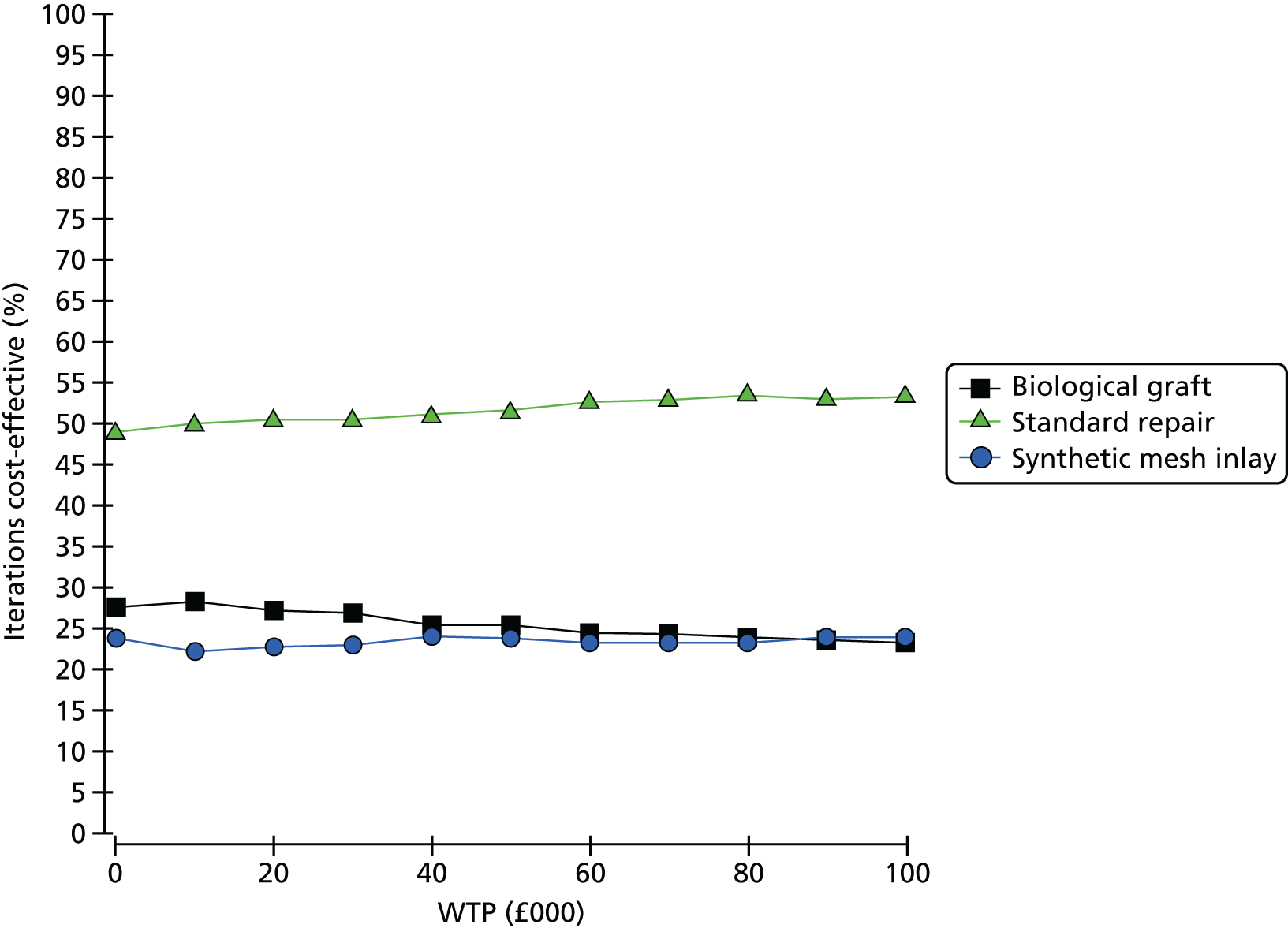
Uncertainty in the individual comparisons of synthetic mesh with standard repair and biological mesh with standard repair is presented in Figure 33 as scatterplots of the 1000 Monte Carlo simulation estimates of expected costs and QALYs for the respective treatment groups.
FIGURE 33.
Incremental cost-effectiveness for mesh repairs vs. standard repair.
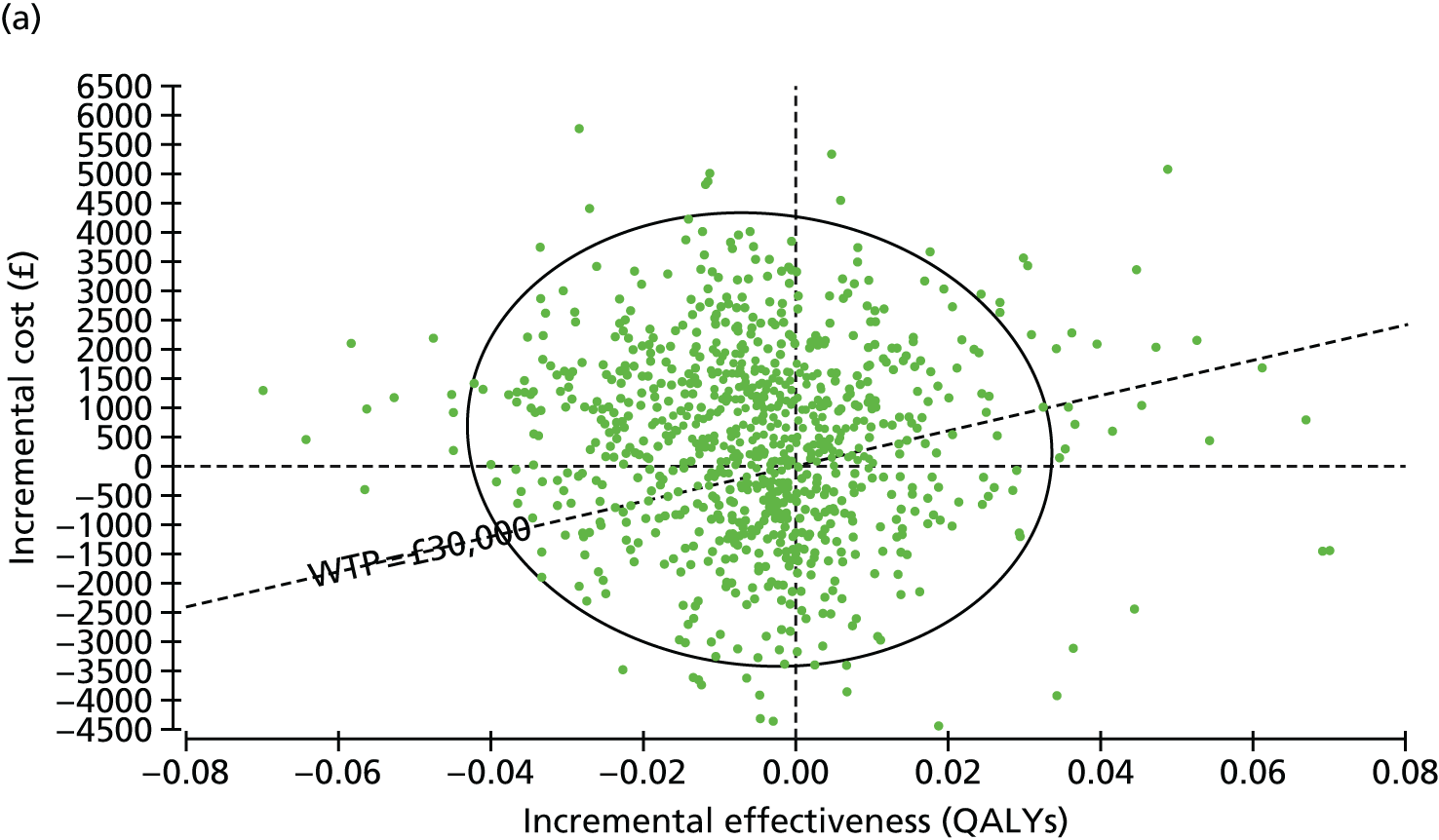

Secondary analysis
Table 105 presents the results of a secondary analysis, which details the cost-effectiveness based on treatment-specific utilities applied to the model. This analysis incorporates the coefficient of treatment effect on QALYs that are generated from GLM models, adjusting for health state in the trial-based analysis model. It essentially adds an additional utility to the synthetic mesh repair for all women in all of the health states, and is more directly comparable with the data seen in the complete case analysis of the trial outcomes reported in Chapter 5. Caution should be noted when interpreting the analysis, given that complete case trial data may have been subject to the biases of missing data. The analysis is provided for completeness. The base-case analysis model is more consistent with an imputed data set of missing utility data from the trial. Figure 34 illustrates the uncertainty in this secondary analysis using CEACs. As with the base-case analysis, a validation check will be explored in deterministic sensitivity analyses running the model over a 2-year time horizon.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness | Incremental effectiveness | Incremental cost-effectiveness | Incremental cost-effectiveness (vs. standard) | NMB ranking (threshold = £30k) |
|---|---|---|---|---|---|---|---|
| Standard repair | 4811 | 0 | 3.753 | 0 | 0 | 2 | |
| Synthetic mesh inlay | 5264 | 453 | 3.829 | 0.076 | 5933 | 5933 | 1 |
| Biological graft | 5304 | 40 | 3.769 | –0.060 | Dominated | 29,883 | 3 |
FIGURE 34.
Secondary analysis, use of treatment-specific utility.
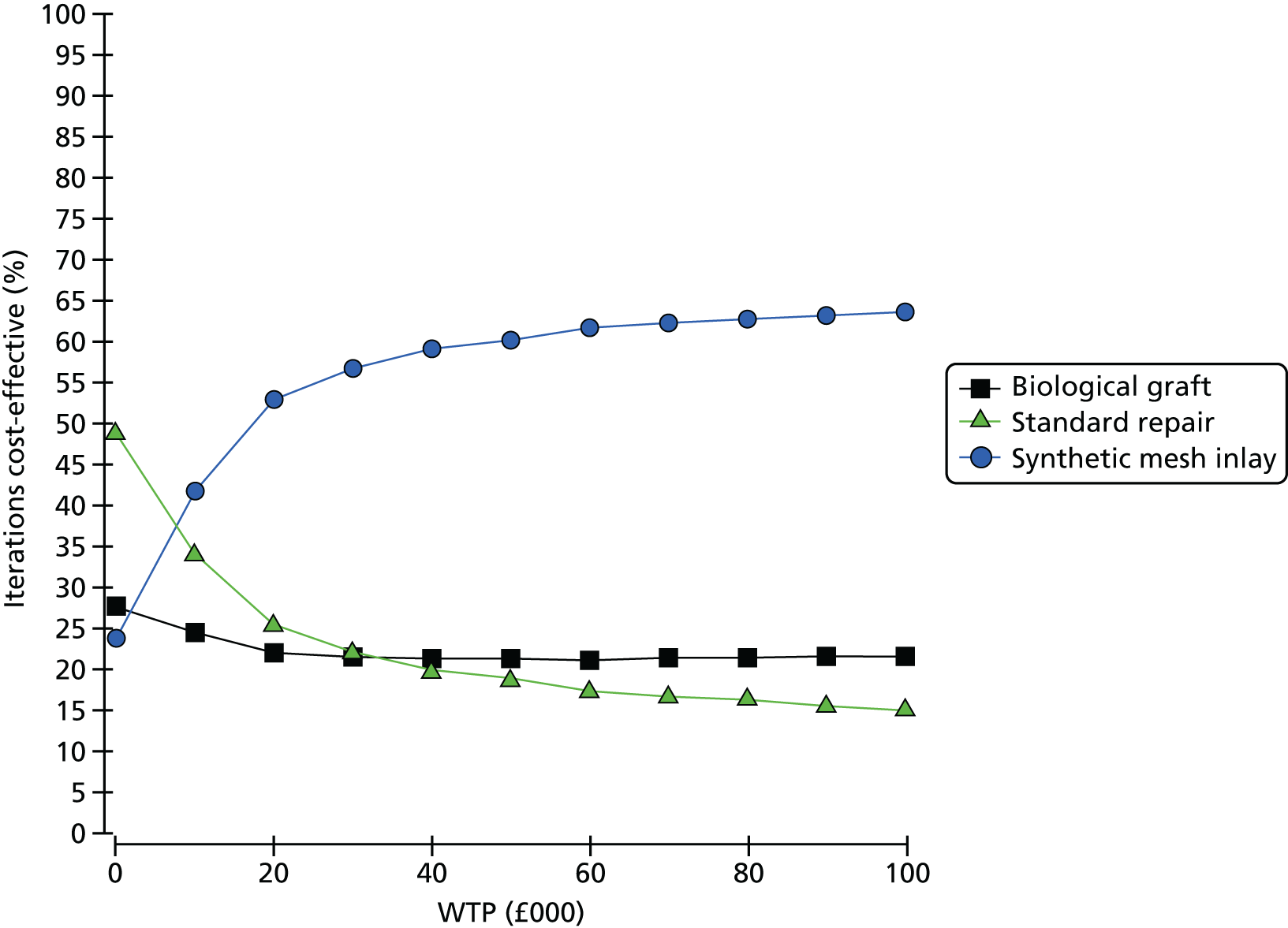
The secondary analysis including additional incremental QALYs for synthetic mesh indicates a lower ICER and higher probability of cost-effectiveness for synthetic mesh. With an ICER of < £20,000 per QALY gained, there may be an argument for adopting synthetic mesh on cost-effectiveness grounds under this analysis. However, whether or not a decision-maker would consider this as evidence of cost-effectiveness would be determined by whether or not he/she trusts the treatment-specific additional utility gained from synthetic mesh across all of the health states. This analysis may be subjected to biases of missing data that are generated in the trial-based cost-effectiveness analyses. More specifically, the base-case analysis, informed by an imputed data set addressing biases, is more likely to accurately reflect cost-effectiveness. Furthermore, there is some uncertainty in this result, as is illustrated in the CEAC in Figure 34. The probabilities of cost-effectiveness at a threshold value of WTP of £30,000 per QALY gained are 22%, 57% and 21% for standard repair, synthetic mesh and biological graft, respectively. The probability of cost-effectiveness of synthetic mesh increases with the higher the threshold value of WTP for a QALY, but never reaches > 65% probability of cost-effectiveness at thresholds up to £100,000 per QALY gained. There is thus substantial uncertainty regarding the most cost-effective treatment strategy across both the base-case and secondary analyses presented here. There is no clear evidence from either model by which to recommend either mesh repair as a cost-effective use of scarce NHS resources. The probability of biological graft being cost-effective is consistently lower than the alternative options, driven by the additional cost of providing the intervention, with no clear gain in QoL over the modelled time horizon.
Results of deterministic sensitivity analyses
The results of the full range of deterministic sensitivity analyses undertaken and described through the methods sections above are outlined in Table 106. The results are presented in a similar way to the base-case and secondary analyses outlined above. ICERs are presented incrementally based on ranked treatment options, and also compared with a common baseline comparator (standard repair). The most cost-effective treatment option, following a net benefit approach, under each scenario, is indicated in the final column of the table.
| Strategy | Cost (£) | Incremental cost (vs. standard) (£) | Efficiency | Incremental efficiency (vs. std) | Incremental cost-effectiveness | Incremental cost-effectiveness (vs. standard) | NMB ranking (threshold = £30k) |
|---|---|---|---|---|---|---|---|
| Base-case analysis | |||||||
| Standard repair | 4811 | 3.753 | 1 | ||||
| Synthetic mesh inlay | 5264 | 453 | 3.748 | –0.0047 | Dominated | Dominated | 3 |
| Biological graft | 5304 | 492 | 3.749 | –0.0035 | Dominated | Dominated | 2 |
| Validation check: running model for a 2-year time horizon (compare with imputed data set in Chapter 5) | |||||||
| Standard repair | 3596.75 | 0 | 1.584 | 0.000 | 0 | 1 | |
| Synthetic mesh inlay | 3915.92 | 319.17 | 1.582 | –0.001 | Dominated | Dominated | 3 |
| Biological graft | 4018.87 | 422.12 | 1.582 | –0.002 | Dominated | Dominated | 2 |
| Validation check: running model for a 2-year time horizon with treatment-specific utilities (compare with complete data set in Chapter 5) | |||||||
| Standard repair | 3596.75 | 0 | 1.584 | 2 | |||
| Synthetic mesh inlay | 3915.92 | 319.17 | 1.614 | 0.031 | 10,387 | 10,387 | 1 |
| Biological graft | 4018.87 | 422.12 | 1.586 | 0.003 | Dominated | 156,783 | 3 |
| Extending the model time horizon to 10 years (exploratory only) | |||||||
| Standard repair | 6720.14 | 6.808 | 1 | ||||
| Synthetic mesh inlay | 7320.80 | 600.66 | 6.798 | –0.009 | Dominated | Dominated | 3 |
| Biological graft | 7328.99 | 608.85 | 6.801 | –0.007 | Dominated | Dominated | 2 |
| Extending the model time horizon to 30 years (exploratory only) | |||||||
| Standard repair | 15055.2 | 0 | 19.584 | 0.000 | 0 | 1 | |
| Biological graft | 15843.08 | 787.88 | 19.559 | –0.026 | Dominated | Dominated | 2 |
| Synthetic mesh inlay | 15867.61 | 812.41 | 19.550 | –0.035 | Dominated | Dominated | 3 |
| Discounting at a rate of 0% per annum for costs and QALYs | |||||||
| Standard repair | 4957.18 | 0 | 4.015 | 0.000 | 0 | 0 | 1 |
| Synthetic mesh inlay | 5425.01 | 467.83 | 4.010 | –0.005 | Dominated | Dominated | 3 |
| Biological graft | 5459.01 | 501.82 | 4.011 | –0.004 | Dominated | Dominated | 2 |
| Discounting at a rate of 6% per annum for costs and QALYs | |||||||
| Standard repair | 4718.57 | 0 | 3.586 | 0.000 | 0 | 0 | 1 |
| Synthetic mesh inlay | 5161.52 | 442.95 | 3.582 | –0.004 | Dominated | Dominated | 3 |
| Biological graft | 5205.1 | 486.52 | 3.583 | –0.003 | Dominated | Dominated | 2 |
| Model start age = 49 years | |||||||
| Standard repair | 4827.72 | 3.783 | 1 | ||||
| Synthetic mesh inlay | 5281.99 | 454.26 | 3.778 | –0.005 | Dominated | Dominated | 3 |
| Biological graft | 5321.29 | 493.56 | 3.779 | –0.003 | Dominated | Dominated | 2 |
| Model start age = 69 years | |||||||
| Standard repair | 4770.14 | 3.678 | 1 | ||||
| Synthetic mesh inlay | 5218.60 | 448.46 | 3.673 | –0.005 | Dominated | Dominated | 3 |
| Biological graft | 5259.91 | 489.77 | 3.674 | –0.003 | Dominated | Dominated | 2 |
| Assuming that the utility of a conservatively managed failure is equal to that of failure requiring surgery | |||||||
| Standard repair | 4811.27 | 0 | 3.742 | 0.000 | 0 | 0 | 1 |
| Synthetic mesh inlay | 5263.87 | 452.6 | 3.735 | –0.007 | Dominated | Dominated | 3 |
| Biological graft | 5303.74 | 492.48 | 3.737 | –0.005 | Dominated | Dominated | 2 |
| Imputing a high estimate of mesh material costs (25%) | |||||||
| Standard repair | 4811.27 | 0 | 3.753 | 0.000 | 0 | 0 | 1 |
| Synthetic mesh inlay | 5298.12 | 486.85 | 3.748 | –0.005 | Dominated | Dominated | 3 |
| Biological graft | 5375.74 | 564.48 | 3.749 | –0.003 | Dominated | Dominated | 2 |
| Imputing a low estimate of mesh material costs (–25%) | |||||||
| Standard repair | 4811.27 | 0 | 3.753 | 0.000 | 0 | 0 | 1 |
| Synthetic mesh inlay | 5229.62 | 418.35 | 3.748 | –0.005 | Dominated | Dominated | 3 |
| Biological graft | 5231.74 | 420.48 | 3.749 | –0.003 | Dominated | Dominated | 2 |
Interpretation of sensitivity analysis results
Model validation checks
As a check on the face validity of our model results, we have undertaken two analyses of the model over a 2-year time horizon. Given that the trial-based follow-up was of 2 years’ duration, it is only reasonable to assume that the model would reasonably accurately reflect the data that were seen in the trial. Therefore, the model was rerun over a 2-year time horizon to determine how closely the results matched the trial analysis. It should be noted that this analysis was conducted to check model face validity results and not as a direct attempt to replicate the trial-based analyses. An analysis in which health-state utilities were not treatment specific matches the conclusions and cost-effectiveness rankings of treatments seen in the imputed data set for the Primary trial analysis. Adding an additional treatment-specific utility to the model produces rankings similar to those found in the complete case analysis. This reflects the conclusions of the trial-based analyses in which imputed data sets showed lower probabilities of mesh repairs being cost-effective than in complete case analysis. The important note to take from this analysis is that the direction of effect is the same, and conclusions remain unchanged from those estimated in the trial analysis. This is reassuring for the internal validity of the model structure. The longer-term follow-up of the trial data will provide an opportunity to validate the projections of long-term failures and complications up to 6 years of follow-up.
Other sensitivity analyses
For a range of sensitivity analyses undertaken, the base-case model conclusions remain unchanged. Based on current data and projected over 5 years, there is little chance of mesh being cost-effective. This is, in part, due to the additional cost of mesh procedures and also to the additional costs and QoL decrements that are associated with treating mesh-related complications. As a result, there would need to be a substantial fall in mesh cost and/or a substantial change in failure rates over the extended follow-up period before mesh would be considered a cost-effective use of scarce NHS resources. Despite the additional cost of biological grafts, even relative to synthetic mesh, there is roughly equal chance of either mesh being cost-effective. This is driven in the model by higher surgery rates for complications in the synthetic mesh group than with biological grafts. In order to be considered cost-effective at a WTP of £30,000 per QALY gained, the total intervention cost would need to reduce by 20% and 21% for synthetic meshes and biological grafts, respectively. The price of the mesh products would be required to fall by a substantially higher percentage in order to achieve this overall percentage reduction in total intervention cost. Furthermore, given current projections of complications over 5 years, the true failure rate for standard repair would need to be substantially greater than synthetic mesh and biological graft at 5 years for either mesh to become definitively cost-effective. In order to fully understand the cost-effectiveness of mesh, more information is required on the longer-term trade-offs between the additional risk of complications (particularly for synthetic meshes) and any longer-term differences between treatment arms regarding failure rates.
Discussion
Summary of main findings
The base-case analysis indicates that standard repair costs less and has only marginally higher QALYs than both synthetic mesh and biological graft procedures. The base-case ICER therefore indicates that standard repair is the dominant treatment strategy. These results are similar to those of the imputed data set for the within-trial analysis in Chapter 5. The cost-effectiveness results are driven primarily by the additional cost of mesh materials. Considering that decision-makers would be willing to pay up to £30,000 for a QALY gained, there are 23% and 27% chances of synthetic mesh and biological graft being considered to be cost-effective, respectively.
The secondary analysis that takes into account the treatment-specific utilities indicates a more favourable result for synthetic mesh. This analysis is most comparable with (and developed using) the complete case analysis of cost and QALY pairs from the trial. This analysis includes the additional unexplained health-related QoL that is evident in the complete case trial analysis, which increases the likelihood of synthetic mesh being cost-effective. This analysis provides a likely upper bound on the likelihood of mesh being cost-effective. Under the assumptions of this analysis, adding treatment-specific utilities to health states, synthetic mesh and biological grafts remain more expensive than standard repair. However, synthetic mesh also generates greater QALY gains. The incremental cost per QALY gained when synthetic mesh is compared with standard repair is £5933. When considering that society’s maximum WTP for a QALY gained might be £30,000, there is 22% chance that standard repair would be considered to be the most cost-effective treatment option, a 57% chance for synthetic mesh and a 21% chance for biological graft. Therefore, despite the potentially favourable ICERs, the reader’s attention is drawn to the uncertainty surrounding the most cost-effective treatment option.
These analyses indicate two plausible estimates of incremental cost per QALY gained, and each are surrounded by considerable uncertainty regarding the optimum treatment strategy for primary prolapse repair. Owing to the additional costs of synthetic mesh and biological graft repairs, there is no strong evidence from any of the considered economic models to recommend mesh as a cost-effective use of scarce NHS resources, based on 2-year data. There would need to be a substantial increase in the failure rate of standard repair over the longer term, relative to mesh, before the mesh repairs would become cost-effective.
The results of the deterministic sensitivity analysis were similar to the base-case analysis, indicating that the results were not particularly sensitive to choices around model structure, methodological assumptions or the choice of data to populate the model. The cost of standard midline repair was always less than that of both synthetic mesh and biological graft. The differences in QALYs were small throughout all the analyses undertaken with no treatment strategy offering a clearly superior outcome in terms of QALYs gained. Scatterplots of incremental QALYs show substantial uncertainty. There was also uncertainty in overall modelled costs, but to a lesser degree than QALY outcomes.
Strengths
A strength of this analysis is that it is the first analysis that reports a model that is populated using data derived from a large RCT and the model parameters – such as benefits, costs, reoperation and complication rates – were derived from the trial participants. The study also included women who were having secondary treatment and it was undertaken from a UK perspective.
Limitations
Modelling the cost-effectiveness analysis was a challenge as a result of the lack of long-term data, although the trial participants were followed up for 2 years. The second and third prolapse treatment costs and outcomes were informed by the results of the Secondary trial, which was based on women who had already had a prolapse treatment. The costs and outcomes of women who had second and third treatments were based on an aggregate of the three available treatments, as there was no difference in costs or outcomes. This was considered appropriate as the number of women in this trial was small. The model had a provision for only two follow-up surgeries, which may be considered to be an overestimation in a 5-year follow-up period. Evidence from the Secondary trial suggested that, on average, women had prolapse symptoms or are bothered by prolapse for at least 2 years before they had surgery. In addition, there were some women in the Secondary trial who had a new prolapse operation, although the numbers were quite low – about 5% – so the additional costs would be expected to be low as well.
It should be noted that in terms of comparison with the clinical trial data set, the model projections performed reasonably well. However, caution should be noted when comparing the analyses directly as the model is based on projections and assumptions from the trial data and does not exactly replicate the trial analyses. Over a 2-year time horizon, the most valid data come from the economic evaluation alongside the randomised trial. However, 2-year follow-up in itself is not particularly meaningful, and cost-effectiveness conclusions overall should be drawn on the best possible projection of longer-term costs and QALYs generated using the modelling approach.
We present results from the decision-analytic model as incremental cost per QALY gained. Given recommendations for best practice and requirements for decision-making bodies, such as NICE, cost per QALY is the most appropriate method by which to judge the value of new technologies to NHS decision-makers. We initially planned to run the model on the basis of cost per woman cured. However, given the low proportion of women completely symptom-free across the trial groups at 2 years, such an analysis would not be meaningful. Furthermore, given difficulty in reaching a consensus on what constitutes ‘cure’, we are unable to estimate the model for cost per symptom-free woman: such an analysis would lead to misleading recommendations to decision-makers. We would encourage decision-makers to draw opinions on economic value from the cost-per-QALY analyses that are presented in the decision modelling chapter (see Chapter 9).
The within-trial results highlighted the uncertainty on the costs and QALYs, and this was reflected in the modelling analysis. Several assumptions had to be made because of the lack of data. Although there were data on the number of participants who had further treatment, there were no data on the specific additional surgery the women had.
Generalisability of results
The base-case analysis results are similar to those that have been previously published;64 however, the analysis that uses treatment-specific utilities differs, as they indicate that synthetic mesh has a > 50% chance of being considered to be cost-effective at a society’s WTP threshold of between £20,000 and £30,000. However, these results have to be treated with caution, taking into account that there is great uncertainty.
Future research
The modelled results project 2-year outcomes over a longer time horizon. However, there is no information to accurately predict long-term trends and the model essentially extrapolates trends of surgery for failure and complications over time. This may or may not be an accurate reflection of true failure and complication rates. It is therefore imperative that further research from the long-term follow-up of the PROSPECT trial is included before a definitive decision on cost-effectiveness can be reached. The current model time horizon of 5 years is also too short to definitively determine cost-effectiveness, which should be estimated over an individual’s whole lifetime. However, insufficient data were available at 2 years to make this projection. Furthermore, there were insufficient data available in the literature to supplement the trial data regarding differences in failure rates and complications between the randomised arms over a longer time horizon. The PROSPECT long-term follow-up will therefore be used to validate or refute the modelled projections of failures and complications, and will provide more accurate data of time to failure/complications in order to further extend the modelled time horizon. At this point, there is likely to be a much clearer knowledge of the true longer-term trade-offs between complications and failure rates for meshes compared with standard midline repairs for women with primary prolapse repair.
Once the initial stage of longer-term follow-up has been completed, more accurate data will exist to make longer-term model projections, especially around failure and complication rates. Once 6-year follow-up is complete, we will conduct a value of information analysis to determine if evidence at this point is sufficient to make recommendations to decision-makers on cost-effectiveness grounds or if further research is worthwhile. If we determine positive expected value of perfect information at this stage, we will use expected value of partial perfect information methods to determine the model parameters that require more, longer-term data to definitively determine the most cost-effective prolapse surgery strategy for women who are having their first primary prolapse repair.
Conclusions
Over the 5-year follow-up presented in the model, depending on the utilities used, there was conflicting evidence regarding the most cost-effective treatment for women with prolapse. Although the outcome data were extrapolated to 5 years, there is still uncertainty about the long-term failure rates and complications of all the treatments for both primary and secondary prolapse. As stated in the within-trial analysis summaries, there is need for longer-term follow-up to better inform decision-makers.
Chapter 10 Overarching discussion
Summary of findings
The PROSPECT Study has shown that, compared with standard repair, there is no benefit in the first 2 years after surgery to women from the use of synthetic mesh inlay or biological graft inlay in the treatment of women who were having their first repair of an anterior or posterior pelvic organ prolapse. In the trial among women who were having a secondary (repeat) repair in either compartment, there was not enough evidence to say whether synthetic non-absorbable mesh inlay or mesh kit was any more or less effective than standard repair in the first 2 years after surgery, because the sample size was too small to be conclusive.
However, there was clear evidence that the majority of women did report improvement in their prolapse symptoms, QoL and other aspects of urinary and sexual function, whichever operation was chosen. The majority of women felt that their health was better after surgery, and this effect was sustained for at least the first 2 years after surgery.
Adverse effects were rare, other than those related to mesh exposure. Importantly, there was no excess risk in the short term to women from the more complex surgery involved in the use of mesh (e.g. extra dissection to insert and fix the mesh inlays): the incidence of ‘expected’ adverse effects, such as infection and bleeding, was similar in all of the groups of women, and infrequent.
Both synthetic mesh inlay and biological graft inlays were more costly than standard repair for women who were having their first pelvic organ prolapse repair. There was no evidence of differences between treatments in terms of use of health services over 2 years of follow-up. Despite some uncertainty in cost-effectiveness, there was no clear compelling evidence to recommend mesh as a cost-effective use of NHS resources based on the analysis at 2 years. Based on the results of a decision-analytic model to extrapolate trial data over a longer (5-year) follow-up, there was insufficient evidence to recommend either mesh repair. The results of the longer-term modelling suggest that the longer-term rate of failure of standard repair would need to increase substantially relative to synthetic mesh inlay of biological graft inlay before either may be considered cost-effective.
Strengths and limitations
Pragmatic study
One of the strengths of the PROSPECT Study was its pragmatic reflection of actual practice in a representative sample of women under the care of UK gynaecologists carrying out prolapse surgery across a large number of hospital settings. This was reflected in the range of concomitant surgery, and in our ability to differentiate between women who were having a first or a repeat repair in a particular compartment, defined using the most up-to-date recommendations for nomenclature.
Validated outcome measures
We used validated, reliable and reproducible instruments to measure outcomes that were relevant and important to women. Our primary outcome, the POP-SS, comprised seven symptoms that are commonly reported by women who have prolapse. Our findings were robust whether we used the composite score or the individual symptoms. The other measures of pelvic floor dysfunction (urinary, sexual, bladder or bowel) provided similar findings.
The use of the validated and standardised POP-Q system to objectively measure prolapse provided some external validity to the trial, in that it is the most commonly reported outcome in the other RCTs that have examined prolapse surgery. 18 We found that although post-surgery measurements were, on average, better than before, 15% of women who had undergone a primary repair and 10% of women who had a secondary repair still had a prolapse outside the hymen. This calls into question what can be defined as ‘cure’ of prolapse. We agree with Barber et al. 62 – that the important perspective in relation to ‘cure’ is the woman’s view, encapsulated in her own report of prolapse symptoms, their effect on QoL and her satisfaction with the overall outcome of surgery, rather than the physical presence of the prolapse.
The mismatch between objective POP-Q and women’s symptoms has been noted by other researchers. 3,60 Data from PROSPECT will provide a rich data source for methodological research into which outcomes matter to women, the relationship between different outcomes, the size of effect, which is important in clinical terms and hence which outcomes most truly reflects success and failure.
Use of Pelvic Organ Prolapse Quantification and redefinition of ‘failure’
The POP-Q system classes measurements from –1 cm inside the hymen to 1 cm as stage 2. 27 We and other researchers28 have pragmatically used a measurement of > 0 cm to indicate objective failure of treatment, although recognising that women with worse anatomical findings may not have symptoms and vice versa (women with objective ‘cure’ may still have prolapse symptoms). This has resulted in a division of Bump et al. ’s stage 2 into 2A (leading edge at the hymen or less, 0 or –1 cm) and 2B (leading edge beyond the hymen, > 0 cm). 27 However, the clinical findings of PROSPECT (in terms of success/failure of treatment) would have been the same whichever stage of prolapse was chosen as the cut-off.
However, we would encourage the gynaecological community to consider what level of prolapse constitutes ‘normal vaginal wall laxity’ and which level of prolapse could be considered as clinically significant. The need for surgery should depend not only on objective findings, but also on the woman’s clinical symptoms and their effect on QoL, and the chance that surgery is likely to improve them (rather than improving the anatomical appearance of the prolapse).
Trial design and conduct
We conceived the trial to use primarily a randomised controlled design for maximum scientific reliability. We designed the study to include CCs of women who were unwilling to be randomised or whose surgeons were unwilling to randomise them. This provided generalisability but also simplified the process of recruitment in the centres (in that all women were potentially eligible for one part of the study). The very high recruitment rate (88% of all eligible women) justified our approach.
We used a secure and unbiased third-party method of randomisation that utilised minimisation to take account of predictable confounding factors, such as planned concomitant surgery. Allocation was minimised according to:
-
the woman’s age (< 60 years or ≥ 60 years)
-
type of prolapse being randomised (anterior, posterior or both)
-
need for a concomitant continence procedure (e.g. TVT) or not
-
need for a concomitant upper vaginal prolapse procedure (e.g. hysterectomy, cervical amputation, vault repair) or not
-
surgeon.
Web-based randomisation and data entry further simplified the recruitment and follow-up processes.
Our high recruitment rates were matched by high retention rates (withdrawals were rare and non-response to questionnaires was around 10%). We used evidence-based retention strategies, such as newsletters and telephone calls, to maintain engagement with our participants.
Blinding of care providers and participants
It was not possible to blind the surgeon to the randomised procedure. Once in theatre, the surgeon could, and sometimes did, choose to carry out a different operation to that randomised or that which was originally planned, if this was clinically indicated. This pragmatic approach reflected real-life practice.
The women, on the other hand, were asked if they would be willing to remain blinded to the surgery to which they had been randomised, and that which was actually carried out, unless there was a clinical need to reveal this information. The majority of the women agreed to this. As the primary outcome was their self-reported prolapse symptoms by questionnaire at intervals after surgery, this would be expected to reduce the chance that they might report ‘better’ or ‘worse’ symptoms or QoL, depending on their attitude to the surgery that they actually received.
Blinding of outcome assessors and participants
Data entry from the questionnaires was carried out by staff blinded to randomisation and surgery actually conducted, using study numbers only to identify participants. The clinical examination at 1 year was carried out by staff similarly blinded, before looking at the medical notes to find out what had been done. The women were asked not to disclose any information that they knew about their surgery before they had been examined. This process was successful in 90% of cases for blinding to randomised allocation, and in 83% of cases for blinding to treatment received.
Generalisability
The inclusion of around 1500 non-randomised women in a CC had a number of advantages. By and large, the populations in the RCTs and CCs were similar for both the women who were having primary surgery and those who were having repeat surgery, suggesting that the findings from the randomised women are generalisable to the larger population of women who are having prolapse surgery in the UK. As the outcomes in the CCs were similar to those of the comparable randomised women, we conclude that the findings of the RCTs are generalisable to the whole population of women who are having prolapse surgery in the UK.
The inclusion of the CCs also increased the power of the study to identify the background rate of adverse effects in women who are having prolapse surgery. In particular, more women who were having repeat surgery entered the non-randomised group, sometimes by their own choice to have mesh and sometimes because their surgeon felt that mesh was indicated. Despite this, their outcomes were broadly similar to those of the women who were randomised.
Primary compared with secondary (repeat) surgery
When we planned PROSPECT, we found a range of views among gynaecologists about the value of mesh and its place in women who are having prolapse surgery. In general, those who were less likely to use mesh thought that it might be of more value in women who had already had a failed previous repair, as it was known that those women were even more likely to have a failed repeat repair. 4 This led to our decision to run two separate RCTs, according to whether the compartment had previous surgery (secondary) or not (primary). We did find important demographic differences between these populations of women (see Chapter 3), justifying our decision to run two trials.
Although we knew that there would be fewer women available for the Secondary trial, the numbers were further reduced by our strict definition, such that some women who had undergone prolapse surgery in the past, but in a different compartment, were deemed to be eligible for the Primary trial. In addition, the women and their surgeons were less open to randomisation, with 61% declining randomisation but being willing to enter CC2.
For these reasons, we are unable to be conclusive about the findings in the Secondary trial. In particular, our assumption that these women were more likely to require non-absorbable mesh to provide a stronger repair cannot be reliably confirmed or denied.
Analysis by strata
We found that some surgeons were not able to randomise women to all of the three arms in each trial, for reasons including personal preference, availability of mesh or training in a particular technique. As a result, the Primary trial consisted of three strata (a three-arm trial and two two-arm trials comparing standard with synthetic mesh and standard with biological graft). Similarly, the Secondary trial consisted of three strata (a three-arm trial and two two-arm trials comparing standard with synthetic mesh and standard with mesh kit). An advantage was that this allowed extra surgeons and centres to participate, thus boosting the potential population and shortening the trial.
A limitation is that, in order to calculate unbiased estimates of treatment effects, we could use only two out of three strata in any analysis (strata A + B for comparisons with synthetic mesh, strata A + C for comparisons with biological graft). However, the power of the analysis was reduced by only a modest amount because the three-arm stratum A was the largest stratum in both the Primary and Secondary RCTs and was used in every analysis.
Economic analysis
A full economic evaluation was conducted alongside the Primary and Secondary trials, following best practice guidelines for economic evaluations. Furthermore, a longer-term decision-analytic model was developed to extrapolate the trial outcomes over a longer (5-year) time horizon. The decision-analytic model projects longer-term failure and complications rates for each treatment based on the data observed in the trial. Although this allows for a longer-term assessment of cost-effectiveness, the data used to populate the model may not reflect the true longer-term rates of complications and failures. The data presented from the model are a best estimate of future cost and QALY implications for primary prolapse repair treatments based on the current data available. Longer-term follow-up data from PROSPECT will be used to validate the model structure and parameters. These data will inform an updated model, which will project cost-effectiveness results over a much longer time horizon. This will enable a more comprehensive assessment of lifetime costs and outcomes of prolapse surgery.
A limitation of the economic analysis is that there were insufficient data to model the longer-term costs and outcomes specifically relating to secondary prolapse repair. Further information is required to robustly assess any differences in long-term cost-effectiveness of mesh for women who were having a secondary prolapse repair.
Need for further research among women requiring treatment for secondary prolapse
As noted, our findings for women who were having repeat surgery were inconclusive because of the small sample size. Although we expected 3 in 10 of the women to be eligible for the secondary group, the numbers were lower because our strict definition of ‘repeat surgery’ (according to the most recent classification this is now defined as ‘in the same compartment’57) and because fewer than expected were willing to be randomised. Those women have a higher risk of failure than those having their first procedure. 4 A further RCT among women who have already had a previous failure in a particular compartment is therefore needed so that we can offer them sound evidence-based advice (see also Chapter 11).
Meaning of the study
Comparison with the most recent Cochrane review
PROSPECT has shown that, in the first 2 years after surgery, there is no benefit to women who were having their first repair either in terms of prolapse symptoms or anatomical cure from the use of synthetic mesh, biological graft or (less conclusively, because of smaller sample size) mesh kit to reinforce a standard anterior or posterior repair. This contradicts the conclusions of the most recent Cochrane review in 2013,18 which found both fewer women with prolapse symptoms with synthetic mesh (RR 1.44, 95% CI 1.15 to 1.80; nine RCTs including 930 women) compared with standard repairs and less objective prolapse (in terms of anatomical measurements: RR 2.45, 95% CI 1.64 to 3.67; 11 RCTs, 1150 women).
On the other hand, our findings concur with the uncertainty of the 2013 evidence18 for a difference for biological grafts (RR for number of women with symptoms 1.03, 95% CI 0.61 to 1.75; three RCTs, 400 women; RR for objective failure 1.35, 95% CI 0.74 to 2.46; six RCTs, 560 women) but with narrower CIs.
Comparison with other randomised controlled trials of no mesh compared with mesh use in prolapse repair
We have now updated the findings of the 2013 Cochrane review18 by adding information from a further 13 RCTs and two studies, which updated information on already included RCTs. This new systematic review now includes information from 4232 women, in addition to the current PROSPECT data from 1348 women who were having primary surgery and 154 having repeat surgery (5734 women in total; see Appendix 7).
The new message from this updated review is that, including additional evidence from the 13 new RCTs and PROSPECT, the overall result no longer favours synthetic non-absorbable mesh inlay in terms of the number of women with prolapse symptoms [(36% after standard repair vs. 30% after mesh inlay; RR at 1 year 1.17, 95% CI 1.00 to 1.37; six RCTs; 1210 women; see Figure 35); (31% compared with 28%; RR at 2 years 1.11, 95% CI 0.94 to 1.33; six RCTs, 1175 women; see Figure 36)]. However, mesh inlay still seemed to be better than standard repair in that fewer women had objective residual prolapse [(27% after standard repair vs. 11% with mesh inlay; RR at 1 year 2.79, 95% CI 1.83 to 4.26; 16 RCTs; 2360 women; see Figure 37); (44% vs. 19%; RR at 2 years 2.53, 95% CI 1.52 to 4.22, eight RCTs, 700 women; see Figure 38)]. This difference in objective findings did not translate into a greater number of women who were having further prolapse surgery by 2 years (5% after standard repair vs. 4% with mesh inlay; RR 1.23, 95% CI 0.85 to 1.79; 13 RCTs, 2238 women; see Figure 39).
In contrast, findings for mesh kits were conflicting. At 1 year, fewer women had prolapse symptoms after standard repair (35% vs. 48% after mesh kit; RR 0.73, 95% CI 0.59 to 0.90; three RCTs; 495 women; see Figure 35), but at 2 years the numbers were small and the difference was not statistically significant (RR 1.10, 95% CI 0.79 to 1.53; two RCTs; 151 women; see Figure 36). Although fewer women had symptoms at 1 year, more women had objective prolapse after standard repair (46% vs. 11% after mesh kit, RR 3.67, 95% CI 2.07 to 6.52; four RCTs, 342 women; see Figure 37) and more women required further prolapse surgery (5% vs. 1.6% after mesh kit; RR 3.65, 95% CI 1.51 to 8.86; five RCTs, 746 women; see Figure 39), although the total number of women needing surgery (n = 24) was small. These counterintuitive results underline the lack of concordance between subjective and objective prolapse outcomes.
There were no new trials testing the effects of biological grafts. The inclusion of data from the PROSPECT Primary trial (see Chapter 4) has made little difference to the findings. They show either no benefit from the use of biological grafts or marginally favour the no-graft standard repair (e.g. number of women with prolapse symptoms at 2 years; 28% vs. 35% with graft; RR 0.78, 95% CI 0.63 to 0.97; three RCTs, 737 women; see Figure 36).
It is difficult to understand why meta-analysis from numerous smaller trials should have previously demonstrated advantages from synthetic mesh in terms of symptoms and anatomical findings in contrast with the findings from PROSPECT. It may be that a single large trial that is free from risk of bias is more powerful than a meta-analysis of many smaller trials of cumulatively equal size (Professor Adrian Grant, HSRU, November 2014, personal communication) but other factors may play a role.
Women requiring repeat prolapse surgery
The previous RCTs included in the most recent Cochrane review18 rarely reported their outcome data separately according to the crucial baseline characteristic of repeat surgery. Even when they did, they identified potentially eligible women as having had ‘any previous prolapse surgery’ rather than our stricter definition of ‘in the same compartment’. Therefore, it is likely that the data from those RCTs cannot be combined with those from the secondary group in PROSPECT because of the heterogeneity in baseline classification. This is further discussed in Chapter 11.
Long-term follow-up
Given that surgical failures requiring repeat repair occur, on average, 12 years after initial surgery, longer-term follow-up is required to determine true effectiveness, cost-effectiveness and other sequelae of mesh or graft insertion. We are funded to follow PROSPECT women in the longer term (for 6 years after surgery in the first instance) to establish the true incidence of surgical failure and long-term adverse effects.
Adverse effects and mesh complications
Complications from mesh insertion were few and, in general, resolved within the first 2 years. It remains to be established if there are important long-term adverse effects from the use of mesh or if these may still be offset by a reduction in the need for repeat prolapse surgery in the long term.
Although there were no differences in outcomes related to pain or dyspareunia (see Tables 22, 23, 29, 57, 58 and 64), the effect of mesh or indeed non-mesh prolapse surgery on long-term pain requires quantitative and qualitative evaluation. This is hampered by the lack of effective outcome measures for pain. Ideally, the issue of chronic pain should now be addressed prospectively using standard definitions and allowing assessment of the degree of pain, as has also been noted in the context of hernia surgery. 65
Conclusions
In summary, there is no clear superiority of the synthetic mesh, biological graft or mesh kit over standard repair in the first 2 years after surgery. Unless there is a significant decrease in the reoperation rates for failure in the medium or long term in the mesh or graft arms relative to standard repair, it is unlikely that any type of mesh or graft is going to be cost-effective, given the excess cost over standard repair and the excess cost of treatment for the adverse effect of mesh exposure or extrusion. Long-term follow-up is now ongoing.
Chapter 11 Conclusions and recommendations
The PROSPECT Study includes the largest multicentre RCT evaluating the use of mesh in women who are having a first anterior or posterior prolapse repair, and also separately in women who are having repeat operations. It has comprehensively assessed both the clinical effectiveness and cost-effectiveness of the use of mesh within the UK NHS, and the results are generalisable to the community of women with these types of prolapse.
The 1- and 2-year results have demonstrated that there is no extra clinical benefit from mesh in women who are having their first repair in terms of prolapse symptoms or anatomical resolution of the prolapse. The incidence of adverse effects (other than mesh exposure) was similar in women who had surgery with or without mesh or graft. The findings on prolapse symptoms, anatomical prolapse and adverse effects are not conclusive in women who are having a repeat repair, as a result of the smaller sample size, but are of the same order.
Equally, however, the short-term result of the prolapse surgery studied for all of the groups was good in terms of relief of prolapse symptoms and satisfaction rates, achieved in > 80% of women, although 8% in the primary RCT and 10% in the secondary RCT had a complication that was classified as serious in the first year (excluding mesh exposure). These benefits were retained to 2 years, but long-term follow-up of the participants in PROSPECT is essential to identify the need for repeat or further prolapse surgery and the development of new adverse effects.
Implications for practice
Based on the findings of PROSPECT, there does not appear to be a reason to suggest to women who were having their first repair that mesh can improve their outcomes in the first 2 years after prolapse surgery. However, there may be yet unidentified benefits in the medium or longer time span in terms of reduced need for repeat prolapse surgery. Long-term follow-up is required to identify any potential benefit and, crucially, late presentation of adverse effects that may affect women’s health and QoL. Assessment of any longer-term trade-offs between the need for reoperation and longer-term adverse effects will ultimately inform decision-makers with regard to the most cost-effective treatment strategy.
Because of the risks of any surgery, women should be advised to first explore other effective means to manage their prolapse symptoms before resorting to surgery. These may include modification of lifestyle factors, such as obesity and heavy lifting, the use of pessaries, PFMT and other forms of exercise, and, for postmenopausal women, local oestrogen treatment.
Implications for research
The evidence consistently shows that 30% of women who have surgery for prolapse will require further prolapse surgery in either the same or a different compartment in the long term. Long-term follow-up information from PROSPECT will provide detailed prognoses for different clinical groups of women, such as those who were having a first or repeat repair, who did and did not have combined prolapse surgery in more than one compartment, and with or without concomitant surgery. The longer-term follow-up data will provide crucial evidence to more definitively determine the most cost-effective treatment strategy for primary prolapse repair.
Treatment for women with secondary prolapse
Research is required to determine whether the need for further surgery can be reduced if a first prolapse operation fails. Those women have a higher risk of failure than those having their first procedure, and had a higher symptom score before surgery, indicating worse prolapse symptoms (see Table 8). A further RCT among women who have already had a previous failure in a particular compartment would therefore be needed so that we can offer them sound evidence-based advice. However, given the results of PROSPECT to date, strong doctor/patient preferences and the current political and medico-legal climate, it is unlikely that any UK surgeons would be willing or able to ‘continue’ the Secondary trial. It may be that in other countries (such as France) where mesh is still widely used, a new trial would be feasible.
It is also possible that the long-term follow-up of PROSPECT, which includes CC women who were not randomised, will provide more detailed information on the outcomes of women who have more than one operation. Previous research has rarely focused on this group of women. Arguably, their higher level of symptoms and risk of failure might make them more tolerant of a higher risk of adverse effects if their overall outcomes can be improved.
An alternative approach would be an individual patient data meta-analysis of existing mesh surgery trials, which would require international co-operation from the triallists and participants who were randomised in earlier trials. The updated evidence (see Appendix 7) contains information from about 5700 women (of whom 1500 are from PROSPECT) who were randomised to standard repair compared with a repair plus mesh or graft. Given that approximately 30% would be having a new repair, data from 1700 women might be available, giving 850 per arm and sufficient power to detect clinically meaningful differences. However, only about two-thirds of these were trials of synthetic mesh and it is unlikely that enough triallists would be able to provide comparable data from sufficient women, given the wide variation in the methods and types of outcome reporting and how ‘secondary’ prolapse surgery has been defined in the past.
Other prolapse research
New research is required into the reasons for the poor outcomes of surgery, which may suggest specific ways to improve practice. These might include:
-
differences in surgical technique (which has been shown to vary considerably within PROSPECT)
-
training of surgeons in the most effective surgical techniques
-
better definition of the defects resulting in prolapse, including training in examination techniques, such as the POP-Q
-
better selection of women who may or may not respond well to prolapse surgery (e.g. risk factors such as younger age, obstetric history)
-
timing of that surgery in relation to the natural history of the progression of prolapse symptoms
-
the effect of prolapse surgery on concomitant symptoms of pelvic floor dysfunction, such as bladder, bowel and sexual function
-
aspects of postoperative management that may affect success, such as the need for vaginal packing
-
the need to treat prolapse in more than one compartment
-
the value of and need for concomitant continence surgery
-
the value of preoperative and postoperative PFMT
-
the value of preoperative and postoperative oestrogen treatment in postmenopausal women, and
-
whether or not women who appear to have a genetic predisposition to prolapse have a higher rate of surgical failure, and whether or not this can be modified.
In addition, research is required into means of prevention of prolapse or slowing down prolapse progression. The identification of remediable causes of prolapse, or identification of factors related to poorer outcomes (such as obesity) may suggest alternative or concomitant treatments to help women.
Research should use validated and standardised terminology, and classification of conditions. In particular, it should use outcome measures that focus on the needs of women by addressing their perception of prolapse symptoms, the effect on their QoL and avoidance of adverse effects, rather than anatomical (objective) cure.
One important outcome is pain, but effective outcome measures for pain need to be developed. Ideally, the issue of chronic pain should now be addressed prospectively using standard definitions and allowing assessment of the degree of pain, as has also been noted in the context of hernia surgery. 65
Any further research undertaken should include (where feasible and sensible to do so) comprehensive evaluation of the cost (to both the NHS and to women themselves) and QoL implications of treatment strategies on the UK NHS. When RCTs are undertaken to address questions of clinical importance, these should be accompanied by full and comprehensive economic evaluation studies. Such studies should, ideally, and where data allow, include decision-analytic modelling to explore long-term implications of treatment decisions.
Acknowledgements
The authors wish to thank the women who participated in the PROSPECT Study. We also thank Margaret MacNeil for her secretarial support and data management; Dawn McRae, Elaine Adam, Gill Murray-Dickson and Lynda Constable for their trial management support; the programming team in CHaRT, led by Gladys McPherson; other staff within CHaRT for their assistance with the trial (Seonaidh Cotton, Diana Collins, Lynn McKenzie, Sophie Halpin and Janice Cruden); Lynne Swan and Christine Dallas for recruitment and participant follow-up; Lara Kemp for additional secretarial assistance; other staff who have contributed to the economic data collection or interpretation (Luke Vale, Dr Karen Cranfield, Sabina McDonald and Paul McNamee); other staff within HSRU for providing the evidence synthesis update (see Appendix 7) – Fiona Stewart, Lynda Constable, Clare Robertson and Moira Cruickshank; Fiona Reid, Jennifer Burr and Luke Vale for their contributions to the study design; members of the PMG for their ongoing advice and support of the trial, plus the independent members of the TSC and DMC; and the staff at the recruitment sites who facilitated the recruitment, treatment and follow-up of trial participants (all listed below). Finally, we would like to thank the NIHR and the HTA programme for funding the PROSPECT Study in the first instance and for the generous continuation of funding for follow-up research.
This monograph is dedicated to the late Professor Adrian Grant. We would like to acknowledge the inspiration of Adrian Grant, who was the Director of the HSRU at the outset of this study. His vision in establishing the Cochrane Incontinence Review Group and CHaRT, the HSRU (UK Clinical Research Collaboration registered) clinical trials unit, led directly to the design and funding of PROSPECT and its success. He was a tremendous colleague, mentor and friend whose insight, support and guidance will be greatly missed.
Project Management Group
Cathryn Glazener, Christine Hemming, Kevin Cooper, Anthony Smith, Robert Freeman, Alison McDonald, Graeme MacLennan, Gladys McPherson, Suzanne Hagen, Isobel Montgomery, John Norrie, Mary Kilonzo, Dwayne Boyers, Andrew Elders, Suzanne Breeman, Margaret MacNeil, Dawn McRae, Elaine Adam, Gill Murray-Dickson, Fiona Reid, Jennifer Burr and Luke Vale.
Independent members of the Trial Steering Committee
Professor Henry Kitchener (chairperson), Ranee Thakar, Pamela Warner, Trish Emerson (2012 to present), Catherine Rodger (2010–12).
Independent members of the Data Monitoring Committee
Professor James Neilson (chairperson), Lucia Dolan, Paula Williamson and Gill Gyte.
Recruitment sites
Aberdeen: Christine Hemming/Bain,* Kevin Cooper, Peter Terry, Mohamed Abdel Fattah, Lynn Swan, Christine Dallas, Angela Allan and Christina Henderson.
Ayrshire & Arran: Wael Agur,* David Rae, Margo Henry and Danielle Gilmour.
Barnsley: Khalid Farag,* Meenakshi Dass and Michelle Reid.
Birmingham: Philip Toozs–Hobson,* Claire Burton, Pallavi Latthe, Matthew Parsons, Paula Trinham and Anna Zhao.
Bolton: Abimbola Williams,* Philip Chia, Nadia Ali-Ross, Katrina Rhead and Raksha Mistry.
Bradford: Carmel Ramage,* Sue Calvert and Anne Bowyer.
Bristol: Philip Smith,* Jenny Cloete, Leigh Morrison and Heather Wilcox.
Brighton: Sharif Ismail.*
Chester: Mofid Ibraheim,* Lorraine Dinardo, Denise Archer, Nichola Kearsley, Janet Spriggs and Hollie Devlin.
Cumbria: Mohamed Matar,* Claire Hagon and Toni Wilson.
Derby: Jay Dasgupta,* Victor Chilaka and Jill Smith.
Harrogate: Adrian Barnett,* Tracy Jackson, Rachael Worton and Caroline Bennett.
Huddersfield: Yi Ling Chan,* A Bondili and Judith Kitchingman.
Hull: Jagdish Gandhi* and Helen Bexhell.
Imperial: Vik Khullar,* Ruwan Fernando, Alex Digesu, Jenny Underwood and Anand Singh.
Leicester: Douglas Tincello,* Victoria Fowler, Carla Christie and Katie Warwick.
Luton: Abdalla Fayyad,* Victoria Bastion and Rose Ann Chin.
Manchester: Anthony Smith,* Fiona Reid, Karen Ward, Karen Rose, David Iles, Lucy Dwyer, Linda Green, Rachel Biancardi, Wilfred Kumakech and Stephanie Bateman.
Maidstone: Rowan Connell,* Susan Lord, Sharon Jones, Tracey Nolan and Rebecca Casey.
Mid Yorkshire: Kathryn Fishwick,* Tracey Lowry, Jackie Ward, Sarah Buckley and Claire Townend.
North Devon: Seumas Eckford,* Osama Eskander, Geraldine Belcher, Amanda Skinner, Lucia Stancombe and Deborah Passmore.
Nottingham: Richard Parkinson,* Paul Hooper, Mausumi Das, Rosario Hannigan, Andy Jarvis, Emma Hickman, Delia Bester and Sarah Heawood.
Plymouth: Robert Freeman,* Luigi Bombieri, Paula Brockman, Angela King, Beverley Cree and Heidi Hollands.
Portsmouth: Patrick Hogston,* Richard Parkinson, Denise Wright and Elinor Jenkins.
Preston: Sanjeev Prashar,* Pauline MacDonald, Julie Butler and Sue Flintoff.
Rotherham: Daksha Patel,* Janet Field, Rachel Walker, Mishell Cunningham, Sally Anne Pearson and Meredyth Harris.
Royal Devon & Exeter: Myles Taylor,* Rachel Sturley, Karen Brown, Sarah Irvine, Julia Halpin, Alison Potter and Caroline Renton.
South Tees: Paul Ballard,* Aethele Khunda, Colette Anderson and Julie Potts.
Sunderland: Jonathan Chamberlain,* Denise Milford and Eileen Walton.
Taunton: Adel Naguib,* Paula Hill and Libby Caudwell.
Torbay: Subramanian Narayanan,* Andrew Hall, Pauline Mercer and Barbara Finson.
West Middlesex: Fabian Imoh-Ita,* Manal Reyad, Mehari Teklay and Christine Adamson.
Whipps Cross: Sarah Hussain,* Shankar Visvanathan, Bashir Dawlatly and Zandile Maseko.
Wolverhampton: Ayman El Naqa,* Charles Cox, Khaled Afifi, Sharon Kempson and Nick Denyer.
York: Nicola Dean,* Adrian Evans, Olugbenga Adekanmi, Andy Gibson, Zoe Coleman, Thomas Smith, Susannah Howard and Amanda Lilley.
(*Principal Investigator.)
The HSRU is funded by the Chief Scientist Office of the Scottish Government Health Directorate.
Contributions of authors
Cathryn Glazener (Chief Investigator and Professor) contributed to the conception and the design of the trial, the conduct of the trial, the interpretation of the results and the writing/editing of the report.
Suzanne Breeman (Trial Manager, Triallist) was responsible for the day-to-day management of the trial, contributed to the interpretation of the data and made significant contributions to the drafting of the report.
Andrew Elders (Statistician) conducted the statistical analyses, contributed to drafting many chapters of the monograph and reviewed the final report.
Christine Hemming (Consultant Obstetrician and Gynaecologist) contributed to the conception and design of the study, the recruitment of participants and the interpretation of the data, and made significant contributions to the drafting of the report.
Kevin Cooper (Consultant Obstetrician and Gynaecologist) contributed to the conception and design of the study, the recruitment of participants and the interpretation of the data, and made significant contributions to the drafting of the report.
Robert Freeman (Consultant Obstetrician and Gynaecologist) contributed to the conception and design of the study, the recruitment of participants and the interpretation of the data, and made significant contributions to the drafting of the report.
Anthony Smith (Consultant Urogynaecologist) contributed to the conception and design of the study, the recruitment of participants and the interpretation of the data and made significant contributions to the drafting of the report.
Suzanne Hagen (Professor and Statistician) contributed to the conception and the design of the trial and the interpretation of the data and made significant contributions to the drafting of the report.
Isobel Montgomery (Patient Representative) contributed to the conception and the design of the trial, participated in trial meetings from the perspective of a patient who has undergone prolapse surgery and commented on draft chapters of the report.
Mary Kilonzo (Health Economist) contributed to the analysis of the health economics data and the drafting of the health economics chapters and commented on other chapters of the report.
Dwayne Boyers (Health Economist) contributed to the analysis of the health economics data and the drafting of the health economics chapters and commented on other chapters of the report.
Alison McDonald (Senior Trial Manager, Triallist) contributed to the design of the trial and the management of the trial and provided commentary on the draft chapters of the report.
Gladys McPherson (Senior Programmer) contributed to the design of the trial, the development of the trial database and the analysis of results.
Graeme MacLennan (Senior Statistician) contributed to the design of the trial, the conduct of the trial, the interpretation of results and the preparation of the report.
John Norrie (Professor and Director of CHaRT, Statistician and Triallist) contributed to the design of the trial, the conduct of the trial, the interpretation of results and the preparation of the report.
Publications
Glazener CMA, Breeman S, Elders A, Hemming C, Cooper KG, Freeman RM, et al. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT) [published online ahead of print 20 December 2016]. Lancet 2016.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Hunskaar S, Burgio K, Clark A, Lapitan MC, Nelson R, Sillen U, et al. Incontinence – 3rd International Consultation on Incontinence. Plymouth: Health Publication Ltd; 2005.
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol 2002;186:1160-6. http://dx.doi.org/10.1067/mob.2002.123819.
- Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol 2000;183:277-85. http://dx.doi.org/10.1067/mob.2000.107583.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997;89:501-6. http://dx.doi.org/10.1016/S0029-7844(97)00058-6.
- Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol 2001;184:1496-501. http://dx.doi.org/10.1067/mob.2001.114868.
- Office of National Statistics (ONS) . 2012-Based National Population Projections n.d. http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-uk--england-and-wales--scotland-and-northern-ireland/mid-2014/sty---overview-of-the-uk-population.html (accessed October 2016).
- Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol 1997;104:579-85. http://dx.doi.org/10.1111/j.1471-0528.1997.tb11536.x.
- Bugge C, Adams EJ, Gopinath D, Reid F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev 2013;2. http://dx.doi.org/10.1002/14651858.cd004010.pub3.
- Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst Rev 2011;12. http://dx.doi.org/10.1002/14651858.cd003882.pub4.
- Ismail SI, Bain C, Hagen S. Oestrogens for treatment or prevention of pelvic organ prolapse in postmenopausal women. Cochrane Database Syst Rev 2010;9. http://dx.doi.org/10.1002/14651858.cd007063.pub2.
- EU Hernia Trialists Collaboration . Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg 2002;235:322-32. http://dx.doi.org/10.1097/00000658-200203000-00003.
- Birch C, Fynes MM. The role of synthetic and biological prostheses in reconstructive pelvic floor surgery. Curr Opin Obstet Gynecol 2002;14:527-35. http://dx.doi.org/10.1097/00001703-200210000-00015.
- Kohli N, Miklos JR. Use of synthetic mesh and donor grafts in gynecologic surgery. Curr Womens Health Rep 2001;1:53-60.
- Jha S, Moran PA. National survey on the management of prolapse in the UK. Neurol Urodyn 2007;26:325-31. http://dx.doi.org/10.1002/nau.20331.
- Pulliam SJ, Ferzandi TR, Hota LS, Elkadry EA, Rosenblatt PL. Use of synthetic mesh in pelvic reconstructive surgery: a survey of attitudes and practice patterns of urogynecologists. Int Urogynecol J 2007;18:1405-8. http://dx.doi.org/10.1007/s00192-007-0360-6.
- Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al. Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence. Health Technol Assess 2003;7. http://dx.doi.org/10.3310/hta7210.
- Abdel-Fattah M, Ramsay I. West of Scotland Study Group . Retrospective multicentre study of the new minimally invasive mesh repair devices for pelvic organ prolapse. BJOG 2008;115:22-30. http://dx.doi.org/10.1111/j.1471-0528.2007.01558.x.
- Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 2013;4. http://dx.doi.org/10.1002/14651858.cd004014.pub5.
- Jia X, Glazener C, Mowatt G, Maclennan G, Fraser C, Burr J. Systematic Review of the Efficacy and Safety of Using Mesh or Grafts in Surgery for Anterior and Or Posterior Vaginal Wall Prolapse n.d. www.nice.org.uk/guidance/ipg267/resources/systematic-review-of-the-efficacy-and-safety-of-using-mesh-or-grafts-in-surgery-for-anterior-andor-posterior-vaginal-wall-prolapse2 (accessed June 2008).
- Jia X, Glazener C, Mowatt G, MacLennan G, Bain C, Fraser C, et al. Efficacy and safety of using mesh or grafts in surgery for anterior and/or posterior vaginal wall prolapse: systematic review and meta-analysis. BJOG 2008;115:1350-61. http://dx.doi.org/10.1111/j.1471-0528.2008.01845.x.
- National Institute for Health and Care Excellence (NICE) . Surgical Repair of Vaginal Wall Prolapse Using Mesh 2008. www.nice.org.uk/guidance/ipg267 (accessed June 2008).
- Hagen S, Glazener C, Sinclair L, Stark D, Bugge C. Psychometric properties of the pelvic organ prolapse symptom score. BJOG 2009;116:25-31. http://dx.doi.org/10.1111/j.1471-0528.2008.01903.x.
- Hagen S, Glazener C, Cook J, Herbison P, Toozs-Hobson P. Further properties of the Pelvic Organ Prolapse Symptom Score: minimally important change and test-retest reliability. Neurourol Urodyn 2010;29:1055-6.
- Madhuvrata P, Glazener C, Boachie C, Allahdin S, Bain C. A randomised controlled trial evaluating the use of polyglactin (Vicryl) mesh, polydioxanone (PDS) or polyglactin (Vicryl) sutures for pelvic organ prolapse surgery: outcomes at 2 years. J Obstet Gynaecol 2011;31:429-35. http://dx.doi.org/10.3109/01443615.2011.576282.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Abrams P, Avery K, Gardener N, Donovan J. The International Consultation on Incontinence Modular Questionnaire: www.iciq.net. J Urol 2006;175:1063-6. http://dx.doi.org/10.1016/S0022-5347(05)00348-4.
- Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10-7. http://dx.doi.org/10.1016/S0002-9378(96)70243-0.
- Nygaard I, Brubaker L, Zyczynski HM, Cundiff G, Richter H, Gantz M, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA 2013;309:2016-24. http://dx.doi.org/10.1001/jama.2013.4919.
- Fayyad A, Hill S, Gurung V, Prashar S, Smith AR. How accurate is symptomatic and clinical evaluation of prolapse prior to surgical repair?. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:1179-83. http://dx.doi.org/10.1007/s00192-007-0306-z.
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377-90. http://dx.doi.org/10.1053/j.gastro.2006.03.008.
- Haylen BT, Freeman RM, Swift SE, Cosson M, Davila GW, Deprest J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) & grafts in female pelvic floor surgery. Int Urogynecol J 2011;22:3-15. http://dx.doi.org/10.1007/s00192-010-1324-9.
- Haylen BT, Freeman RM, Lee J, Swift SE, Cosson M, Deprest J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related to native tissue female pelvic floor surgery. Int Urogynecol J 2012;23:515-26. http://dx.doi.org/10.1007/s00192-011-1659-x.
- NHS Business Services Authority (NHS BSA) . Electronic Drug Tariff n.d. www.nhsbsa.nhs.uk/924.aspx (accessed August 2015).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- ISD Scotland . Hospital Care Data Tables n.d. www.isdscotland.org/Health-Topics/Hospital-Care/Publications/data-tables.asp?id=1343#1343 (accessed February 2015).
- Department of Health . NHS Reference Costs 2013–14 n.d. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed February 2015).
- Curtis L. Unit Costs of Health and Social Care 2014 2014. www.pssru.ac.uk/project-pages/unit-costs/2014/ (accessed February 2015).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal n.d. http://publications.nice.org.uk/guide-to-the-methods-of-technology-appraisal-2013-pmg9%23close (accessed 10 February 2015).
- Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al. Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product designs. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12290.
- Warrington Clinical Commissioning Group . Continence and Catheter Care Formulary n.d. www.warringtonccg.nhs.uk/Downloads/Public%20Information/Catheter%20Care%20Formulary%20Updated%20April%2013.pdf (accessed February 2015).
- Department of Transport . Value of Time and Operating Costs: Transport Analysis Guidance (TAG) n.d. www.dft.gov.uk/webtag/documents/archive/1208/unit3.5.6.pdf (accessed February 2015).
- HM Revenue & Customs . Rates and Allowances: Travel-Mileage and Fuel Allowances n.d. www.gov.uk/government/publications/rates-and-allowances-travel-mileage-and-fuel-allowances (accessed July 2015).
- Torbay and South Devon NHS Foundation Trust . Patient Transport Services n.d. www.torbayandsouthdevon.nhs.uk/services/patient-transport-services/ (accessed July 2015).
- EPPI-Centre . Inflation Calculator n.d. http://eppi.ioe.ac.uk/costconversion/ (accessed February 2015).
- Department of Health . NHS Reference Costs 2009–10 n.d. https://www.gov.uk/government/publications/nhs-reference-costs-2009-2010 (accessed February 2015).
- Office for National Statistics . National Life Tables 1980–82 to 2011–13 n.d. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77–365199 (accessed May 2014).
- NHS Pay Review Body . Twenty-Sixth Report 2012 n.d. www.official-documents.gov.uk/document/cm82/8298/8298.pdf (accessed February 2015).
- Office for National Statistics . Earnings and Labour Productivity Data n.d. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77–222531 (accessed February 2015).
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. New York, NY: Oxford University Press; 2007.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. http://dx.doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401. http://dx.doi.org/10.1146/annurev.publhealth.23.100901.140534.
- Royston P. Multiple imputation of missing values: update of ice. Stata J 2005;5:527-36.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Toozs-Hobson P, Freeman R, Barber M, Maher C, Haylen B, Athanasiou S, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for reporting outcomes of surgical procedures for pelvic organ prolapse. Int Urogynecol J 2012;23:527-35. http://dx.doi.org/10.1007/s00192-012-1726-y.
- Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006;15:237-41.
- Gutman RE, Ford DE, Quiroz LH, Shippey SH, Handa VL. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms?. Am J Obstet Gynecol 2008;199:683.e1-7. http://dx.doi.org/10.1016/j.ajog.2008.07.028.
- Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse?. Am J Obstet Gynecol 2003;189:372-7. http://dx.doi.org/10.1067/S0002-9378(03)00698-7.
- Glazener CMA, Breeman S, Elders A, Hemming C, Cooper KG, Freeman RM, et al. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT) [published online ahead of print 20 December 2016] . Lancet 2016. http://dx.doi.org/10.1016/S0140-6736(16)31596-3.
- Barber MD, Brubaker L, Nygaard I, Wheeler TL, Schaffer J, Chen Z, et al. Defining success after surgery for pelvic organ prolapse. Obstet Gynecol 2009;114:600-9. http://dx.doi.org/10.1097/AOG.0b013e3181b2b1ae.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Jacklin P, Duckett J. A decision-analytic Markov model to compare the cost-utility of anterior repair augmented with synthetic mesh compared with non-mesh repair in women with surgically treated prolapse. BJOG 2013;120:217-23. http://dx.doi.org/10.1111/1471-0528.12028.
- McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al. Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9140.
- Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. J Obstet Gynaecol 2008;28:427-31. http://dx.doi.org/10.1080/01443610802150077.
- Robert M, Girard I, Brennand E, Tang S, Birch C, Murphy M, et al. Absorbable mesh augmentation compared with no mesh for anterior prolapse: a randomized controlled trial. Obstet Gynecol 2014;1231:288-94. http://dx.doi.org/10.1097/AOG.0000000000000105.
- Gandhi S, Goldberg RP, Kwon C, Koduri S, Beaumont JL, Abramov Y, et al. A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol 2005;192:1649-54. http://dx.doi.org/10.1016/j.ajog.2005.02.061.
- Hviid U, Hviid TV, Rudnicki M. Porcine skin collagen implants for anterior vaginal wall prolapse: a randomised prospective controlled study. Int Urogynecol J 2010;21:529-34. http://dx.doi.org/10.1007/s00192-009-1018-3.
- Meschia M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kocjancic E. Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: a multicenter, randomized study. J Urol 2007;177:192-5. http://dx.doi.org/10.1016/j.juro.2006.08.100.
- Sung VW, Rardin CR, Raker CA, Lasala CA, Myers DL. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol 2012;119:125-33. http://dx.doi.org/10.1097/AOG.0b013e31823d407e.
- Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, et al. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG 2009;116:1380-6. http://dx.doi.org/10.1111/j.1471-0528.2009.02254.x.
- De Tayrac R, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso S, et al. Comparison between trans-obturator trans-vaginal mesh and traditional anterior colporrhaphy in the treatment of anterior vaginal wall prolapse: results of a French RCT. Int Urogynecol J 2013;24:1651-61. http://dx.doi.org/10.1007/s00192-013-2075-1.
- El-Nazer MA, Gomaa IA, Ismail Madkour WA, Swidan KH, El-Etriby MA. Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study. Arch Gynecol Obstet 2012;286:965-72. http://dx.doi.org/10.1007/s00404-012-2383-6.
- Turgal M, Sivaslioglu A, Yildiz A, Dolen I. Anatomical and functional assessment of anterior colporrhaphy versus polypropylene mesh surgery in cystocele treatment. Eur J Obstet Gynecol Reproduct Biol 2013;170:555-8. http://dx.doi.org/10.1016/j.ejogrb.2013.07.014.
- Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364:1826-36. http://dx.doi.org/10.1056/NEJMoa1009521.
- Delroy CA, Castro RDA, Dias MM, Feldner PC, Bortolini MAT, Girao MJBC, et al. The use of transvaginal synthetic mesh for anterior vaginal wall prolapse repair: a randomized controlled trial. Int Urogynecol J 2013;24:1899-907. http://dx.doi.org/10.1007/s00192-013-2092-0.
- Chmielewski L, Walters MD, Weber AM, Barber MD. Reanalysis of a randomized trial of 3 techniques of anterior colporrhaphy using clinically relevant definitions of success. Am J Obstet Gynecol 2011;205:69.e1-8. http://dx.doi.org/10.1016/j.ajog.2011.03.027.
- Minassian VA, Parekh M, Poplawsky D, Gorman J, Litzy L. Randomized controlled trial comparing two procedures for anterior vaginal wall prolapse. Neurourol Urodyn 2014;33:72-7. http://dx.doi.org/10.1002/nau.22396.
- Dahlgren E, Kjolhede P. RPOP-PELVICOL Study Group . Long-term outcome of porcine skin graft in surgical treatment of recurrent pelvic organ prolapse. An open randomized controlled multicenter study. Acta Obstet Gynecol Scand 2011;90:1393-401. http://dx.doi.org/10.1111/j.1600-0412.2011.01270.x.
- Guerette NL, Aguirre O, VanDrie DM, Biller DH, Davila GW. Multi-center, randomized, prospective trial comparing anterior colporrhaphy alone to bovine pericardium collagen matrix graft reinforced anterior colporrhaphy: 12-month analysis. Abstract no. 11. Int Urogynecol J Pelvic Floor Dysf 2006;17:63-4.
- Gutman RE, Nosti PA, Sokol AI, Sokol ER, Peterson JL, Wang H, et al. Three-year outcomes of vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol 2013;122:770-7. http://dx.doi.org/10.1097/AOG.0b013e3182a49dac.
- Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol 2010;203:235.e1-8. http://dx.doi.org/10.1016/j.ajog.2010.03.030.
- Qatawneh A, Al-Kazaleh F, Saleh S, Thekrallah F, Bata M, Sumreen I, et al. Transvaginal cystocele repair using tension-free polypropylene mesh at the time of sacrospinous colpopexy for advanced uterovaginal prolapse: a prospective randomised study. Gynaecol Surg 2013;10:79-85. http://dx.doi.org/10.1007/s10397-012-0758-0.
- Tamanini JT, de Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, Girão MJ. A prospective, randomized, controlled trial of the treatment of anterior vaginal wall prolapse: medium term follow up. J Urol 2015;193:1298-304. http://dx.doi.org/10.1016/j.juro.2014.10.003.
- Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol 2001;184:1357-62. http://dx.doi.org/10.1067/mob.2001.115118.
- Feldner PC, Castro RA, Cipolotti LA, Delroy CA, Sartori MG, Girao MJ. Anterior vaginal wall prolapse: a randomized controlled trial of SIS graft versus traditional colporrhaphy. Int Urogynecol J Pelvic Floor Dysf 2010;21:1057-63. http://dx.doi.org/10.1007/s00192-010-1163-8.
- Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol 2006;195:1762-71. http://dx.doi.org/10.1016/j.ajog.2006.07.026.
- Ali S, Han HC, Lee LC. A prospective randomized trial using Gynemesh PS for the repair of anterior vaginal wall prolapse (Abstract number 292). Int Urogynecol J Pelvic Floor Dysf 2006;17.
- Dos Reis Brandão da Silveira S, Haddad JM, de Jármy-Di Bella ZI, Nastri F, Kawabata MG, da Silva Carramão S, et al. Multicenter, randomized trial comparing native vaginal tissue repair and synthetic mesh repair for genital prolapse surgical treatment. Int Urogynecol J 2015;26:335-42. http://dx.doi.org/10.1007/s00192-014-2501-z.
- Gupta B, Vaid NB, Suneja A, Guleria K, Jain S. Anterior vaginal prolapse repair: a randomised trial of traditional anterior colporrhaphy and self-tailored mesh repair. S Afr J Obstet Gynaecol 2014;20:47-50. http://dx.doi.org/10.7196/sajog.749.
- Halaska M, Maxova K, Sottner O, Svabik K, Mlcoch M, Kolarik D, et al. A multicentre randomized prospective controlled study comparing sacrospinous fixation and transvaginal mesh in the treatment of posthysterectomy vaginal vault prolapse. Am J Obstet Gynecol 2012;207:e1-7. http://dx.doi.org/10.1016/j.ajog.2012.08.016.
- Lamblin G, Van-Nieuwenhuyse A, Chabert P, Lebail-Carval K, Moret S, Mellier G. A randomized controlled trial comparing anatomical and functional outcome between vaginal colposuspension and transvaginal mesh. Int Urogynecol J 2014;25:961-70. http://dx.doi.org/10.1007/s00192-014-2344-7.
- Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 2008;111:891-8. http://dx.doi.org/10.1097/AOG.0b013e31816a2489.
- Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. Anterior colporrhaphy compared with collagen-coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. BJOG 2014;121:102-11. http://dx.doi.org/10.1111/1471-0528.12454.
- Sivaslioglu AA, Unlubilgin E, Dolen I. A randomized comparison of polypropylene mesh surgery with site-specific surgery in the treatment of cystocoele. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:467-71. http://dx.doi.org/10.1007/s00192-007-0465-y.
- Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME. Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol 2011;117:242-50. http://dx.doi.org/10.1097/AOG.0b013e318203e6a5.
- Vollebregt A, Fischer K, Gietelink D, van der Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar-guided transobturator anterior mesh. BJOG 2011;118:1518-27. http://dx.doi.org/10.1111/j.1471-0528.2011.03082.x.
- Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1337-44. http://dx.doi.org/10.1097/AOG.0b013e318237edc4.
- Thijs S, Deprest J, De Ridder D, Claerhout F, Roovers J. A randomized controlled trial of anterior colporraphy and Perigee™ as a primary surgical correction of symptomatic cystocele. Abstract no. 96. Int Urogynecol J Pelvic Floor Dysf 2010;21:142-3.
Appendix 1 Information for participants and gynaecologists
Study flyer
Clinic poster
Patient information leaflet
Surgical information sheet
Instructions to gynaecologists
Ineligible or declined form
Consent form
Appendix 2 Participant and general practitioner letters
Baseline invitation letter
General practitioner letter
Six-month letter
Six-month reminder letter 1
Six-month reminder letter 2
One-year letter
One-year reminder letter 1
One-year reminder letter 2
One-year additional letter
One-year addition reminder letter
Costs questionnaire letter
Costs questionnaire reminder letter
Two-year letter
Two-year reminder letter 1
Two-year reminder letter 2
Best contact form
First letter to best contact
Best contact reminder letter
Second letter to best contact
Appendix 3 Case report forms
Log book
Surgical assessment form
Recruitment officer case report form
Twelve-month clinic review form
Serious adverse event/death report form
Withdrawal/change of status form
Surgical standardisation form
Appendix 4 Postal questionnaires
Baseline questionnaire
Six-month questionnaire
One-year questionnaire
One-year additional questionnaire
Patient costs questionnaire
Two-year questionnaire
Appendix 5 Statistical analysis plan
Appendix 6 Health economics
Cost of anaesthesia drugs
| Anaesthesia type | Drug | Unit price (£) | Price per: | Resource use | Cost per average case (£) | Comments | Sources |
|---|---|---|---|---|---|---|---|
| General | Propofol, 1% injection | 4.18 | 20-ml ampoule | 1 ampoule | 4.18 | One ampoule will be sufficient for a standard case; Dr Karen Cranfield | BNF;34 personal communication |
| Fentanyl, 100 µg | 0.45 | 2-ml ampoule (50 µg/ml) | 1 ampoule | 0.45 | One ampoule is sufficient to treat an average cost; Dr Karen Cranfield | ||
| Morphine | 0.72 | 1-ml vial | 1 vial | 0.72 | For pain relief; Dr Karen Cranfield | ||
| Sevoflurane (volatile agent) | 123 | 250-ml bottle | 25 ml | 12.30 | Baxter UK, Berkshire, 2013 | www.baxterhealthcare.co.uk/downloads/prescribing_information/hospital_products/anaesthesia_critical_care/sevoflurane_pi.pdf | |
| Laryngeal mask | 29.50 | Box of 10 | 1 | 2.95 | Pro-act medical; laryngeal airway disposable pre-breathe; Dr Karen Cranfield | Pro-act medical; personal communication | |
| Total cost: general anaesthesia | 20.60 | Anaesthesia consumables cost per average case | |||||
| Spinal | Bupivacaine hydrochloride anhydrous injection (0.5%) | 1.45 | 4-ml ampoule | 1 ampoule | 1.45 | Resource-use requirements provided by Dr Karen Cranfield | BNF;34 personal communication |
| Lidocaine | 0.40 | 10-ml ampoule | 1 ampoule | 0.40 | |||
| Total cost: spinal anaesthesia | 1.85 | Anaesthesia consumables cost per average case | |||||
| Local | Lidocaine | 0.40 | 10-ml ampoule | 1 ampoule | 0.40 | Resource-use requirements provided by Dr Karen Cranfield | BNF;34 personal communication |
| Total cost: local anaesthesia | 0.40 | ||||||
Other surgical drugs
| Anaesthesia type | Drug | Unit price (£) | Price per: | Resource use | Cost per average case (£) | Comments | Sources |
|---|---|---|---|---|---|---|---|
| General/spinal | Cyclizine lactate injection (50 mg/ml) | 0.65 | 1-ml ampoule | 1 ampoule | 0.65 | Resource-use requirements provided by Dr Karen Cranfield | BNF;34 personal communication |
| Dexamethasone, injection | 4.80 | 2-ml vial | 1 vial | 4.80 | |||
| Ondansetron injection (2 mg/ml) | 1.00 | 2-ml ampoule | 1 ampoule | 1.00 | |||
| Total: other surgical drugs | 6.45 | ||||||
Absorbent pads for the leakage of urine
| Pads | Number | Number of days/nights39 | Total number of pads (day) | Price (2005) | Price (£) per day pad | Total number of pads (night) | Price 2005 | Per night pad | Notes/sources | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per day | Per night | 2005 | 2014 | 2005 | 2014 | |||||||
| Washable pads | 3 | 1 | 30 | 90 | 9 | 9/90 = £0.10 | 0.10/232.3 × 290.5 = 0.13 | 30 | 6 | 6/30 = £0.20 | 0.20/232.3 × 290.5 = 0.25 | Fader 2008,39 HCHS inflation index (PSSRU) |
| Pull-up pads | 3 | 1 | 30 | 90 | 78.70 | 78.70/90 = 0.87 | 0.87/232.3 × 290.5 = 1.09 | 30 | 25.50 | 25.50/30 = 0.85 | 0.85/232.3 × 290.5 = 1.06 | |
| Average cost | 0.61 | 0.66 | ||||||||||
| Answer to question on leakage | Number of times pass per day | Number of pads required (day) | Unit cost (£) of day pad | Total cost (£) of day pad per year | Number of pads (night) | Unit cost (£) of night pad | Total cost (£) of night pad per year | Total cost (£) of pads |
|---|---|---|---|---|---|---|---|---|
| Not answered/never | 1 | 0.61 | 0.61 × 1 × 365 = £222.65 | 1 | 0.66 | 0.66 × 1 × 365 = £240.90 | 463.55 | |
| Once weekly or less often | 1+ (1/week) | 0.61 | 222.65 + (1 × 52 × 0.61) = 254.37 | 1 | 0.66 | £240.90 | 495.27 | |
| Two to three times per week | 1+ (3/week) | 0.61 | 222.65 + (3 × 52 × 0.61) = 317.81 | 1 | 0.66 | £240.90 | 558.71 | |
| Daily | 2 | 0.61 | 222.65 × 2 = 445.30 | 1 | 0.66 | £240.90 | 686.20 | |
| Several times/day | 4 | 0.61 | 222.65 × 4 = 890.60 | 1 | 0.66 | £240.90 | 1131.50 | |
| All of the time | 1–6 or missing | 6 | 0.61 | 222.65 × 6 = 1335.90 | 2 | 0.66 | 481.80 | 1817.70 |
| 7–8: 8 | 9 | 0.61 | 222.65 × 9 = 2003.85 | 2 | 0.66 | 481.80 | 2485.65 | |
| 9–10: 10 | 11 | 0.61 | 222.65 × 11 = 2449.15 | 2 | 0.66 | 481.80 | 2930.93 | |
| 11–12: 12 | 13 | 0.61 | 222.65 × 13 = 2894.45 | 2 | 0.66 | 481.80 | 3376.25 | |
| 13+: 14 | 15 | 0.61 | 222.65 × 15 = 3339.75 | 2 | 0.66 | 481.80 | 3821.55 |
Indwelling catheter/permanent catheter unit cost
| Requirement | Product | Manufacturer | Pack size | Number of packs required for 1 year of treatment | Unit cost (£), 2015 tariff | Total cost (£) | Reference/notes |
|---|---|---|---|---|---|---|---|
| Sterile catheterisation insertion pack | Cath-it® (1 pack) | Richardson Healthcare, Hertfordshire, UK | 1 | 4 | 10.95 | 43.80 | NHS EDT April 2015 |
| Sterile lubricant for instillation | Optilube® sterile lubricating jelly (1 × 11-ml syringe) | Optimum Medical, Leeds, UK | 1 | 4 | 1.13 | 4.52 | |
| Indwelling catheter | Folysil® X-tra (size 14), pack size 1 | Coloplast, Peterborough, UK | 1 | 6 (four, plus two spares) | 6.25 | 37.50 | |
| Leg bags (assumes patients have continuous drainage) | Simpla® profile, 500 ml, 25-cm tube | Coloplast | 10 | 6 | 29.26 | 175.56 | |
| Catheter stabilisation device | Leg bag holder – aqua sleeve, size standard | Coloplast | 4 | 2 | 8.33 | 16.66 | |
| Night drainage bags | Single use, Prosys® leg drainage bags (2 l) | CliniSupplies, London, UK | 10 | 37 | 3.04 | 112.48 | |
| Total | 390.52 |
Intermittent/disposable or reusable catheters (assume three per day)
| Product | Manufacturer | Pack size | Number of packs required for 1 year | Unit cost (£, 2015) | Total cost (£) | Reference/notes |
|---|---|---|---|---|---|---|
| HiSlip plus® | CS Bullen Healthcare Ltd, Liverpool, UK | 30 | 37 | 32.55 | 1204.35 | |
| Advance Plus intermittent catheter | Hollister, Libertyville, IL, USA | 25 | 44 | 69.02 | 3036.88 | |
| SpeediCath® compact | Coloplast | 30 | 37 | 45.85 | 1696.45 | |
| SpeediCath | Coloplast | 30 | 37 | 44.28 | 1638.36 | |
| Hydrosil | Rochester Medical (acquired by Bard Medical), Sussex, UK | 30 | 37 | 43.19 | 1598.03 | |
| LoFric® Sense™ | Wellspect Healthcare, Stonehouse, UK | 30 | 37 | 46.62 | 1724.94 | |
| Average cost for a full year of treatment | 1816.50 |
Accident and emergency unit costs for use in the analysis
| Code | Description | HRG | HRG description | Number of attendances | National average (£) | Lower quartile (£) | Upper quartile (£) | No. of data submissions |
|---|---|---|---|---|---|---|---|---|
| T01NA | Type 01 Non-Admitted | VB09Z | Emergency Medicine, Category 1 Investigation with Category 1-2 Treatment | 3,262,747 | 103 | 84 | 121 | 139 |
Costing of urodynamics
| OPCS code | HRG | HRG description | Assumed category | Assumed discipline | National average unit cost (£, 2013–14) | Lower quartile (£) | Upper quartile (£) |
|---|---|---|---|---|---|---|---|
| M474 | LB42 -A | Dynamic studies of urinary tract; age 19+ | Outpatients | Gynaecology | 186 | 129 | 209 |
| U264 | LB42 -A | ||||||
| For use in analysis | 186 | 129 | 209 |
Costing of ultrasound
| OPCS code: | OPCS description | HRG | HRG description | Assumed category | Assumed discipline | National average unit cost (£, 2013–14) | Lower quartile (£) | Upper quartile (£) |
|---|---|---|---|---|---|---|---|---|
| M497 | High intensity focused ultrasound of bladder | LB49 – flag a | High-intensity focused ultrasound | Outpatient | Gynaecology | 166 | 170 | 170 |
| Q555 | Transvaginal ultrasound examination of female genital tract | MA23 | Lower genital tract, minor procedures, category 2 | Outpatient | Gynaecology | 160 | 123 | 191 |
| U216 | Ultrasound scan NEC | RA23 | Ultrasound scan < 20 minutes | Diagnostic imaging – outpatient | Outpatient | 52 | 38 | 62 |
| For use in analysis: | 52 | 38 | 62 |
Costs of resource use seeing health professionals
| Expert seen | Unit cost (£) | Unit cost (£), including qualification costs | Per: | Average length per case | Cost per case (£), including qualification costs | Sources/notes |
|---|---|---|---|---|---|---|
| Community pharmacist | 128 | 142 | Hour of direct clinical activities | 0.25 | 32 (35.50) | PSSRU 2014; section 9.6 – community pharmacist; no average time given (assume 15 minutes) |
Costs of repeat prolapse surgery
| OPCS code | OPCS description | HRG | HRG description | Assumed category | Assumed discipline | National average unit cost (£, 2013–14) | Lower quartile (£) | Upper quartile (£) | Weight | National average unit cost (2013–14) | Lower quartile (£) | Upper quartile (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P232/P233/ | Anterior/posterior colporrhaphy NEC | MA04D | Lower genital tract – intermediate open procedure (cc score (0–2) | Elective inpatient | N/A | 2187 | 1656 | 2340 | 0.5 | 1093.50 | 828 | 1170 |
| P236/P237/P247 | Colporrhaphy with mesh reinforcement/sacrospinous fixation | MA03D | Lower genital tract – major open procedure (cc score 0–2) | Elective inpatient | N/A | 2475 | 2075 | 2766 | 0.5 | 1237.50 | 1037.50 | 1383 |
| For use in analysis: | 2331 | 1865.50 | 2553 | |||||||||
Costs of incontinence surgical procedure (total vaginal length – transobturator tape)
| OPCS code | OPCS description | HRG | HRG description | Assumed category | Assumed discipline | National average unit cost (£, 2013–14) | Lower quartile (£) | Upper quartile (£) | Weight | National average unit cost (£, 2013–14) | Lower quartile (£) | Upper quartile (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M533/M536 | Introduction of TVT/TOT | LB51 (B) | Vaginal tape operations for UI_ (CC 0–1) | Elective inpatient | N/A | 1586 | 1293 | 1850 | 0.483 | 766.04 | 624.52 | 893.55 |
| M533/M536 | Introduction of TVT/TOT | LB51 (B) | Vaginal tape operations for UI_ (CC 0– | Day-case procedure | N/A | 1173 | 951 | 1354 | 0.517 | 606.44 | 491.67 | 700.02 |
| For use in analysis | Weighted average | 1372.48 | 1116.19 | 1593.57 | ||||||||
Costs of readmissions to hospital
| OPCS code | OPCS description | HRG | HRG description | Category | Assumed discipline | National average unit cost (£, 2013 –14) | Lower quartile (£) | Upper quartile (£) | Weight | Average cost (£) | Lower quartile (£) | Upper quartile (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P209 | Unspecified extirpation of lesion of vagina | MA23 | Minimal lower genital tract procedures | Elective inpatient | N/A | 1062 | 731 | 1369 | 0.072 | 76.46 | 52.63 | 98.57 |
| P261 | Insertion of Hodge pessary into vagina | MA23 | Minimal lower genital tract procedures | Day case | N/A | 733 | 461 | 940 | 0.584 | 428.07 | 269.22 | 548.96 |
| P271 | Evacuation of haematoma from vagina | MA22 | Minor lower genital tract procedures | Elective inpatient | N/A | 1416 | 1131 | 1722 | 0.051 | 72.22 | 57.68 | 87.82 |
| P292 | Colpotomy NEC | MA22 | Minor lower genital tract procedures | Day case | N/A | 945 | 767 | 1078 | 0.293 | 276.89 | 224.73 | 315.85 |
| For use in analysis (weighting of all) | 853.64 | 604.26 | 1051.2 | |||||||||
| Day-case weighting | 803.81 | 563.21 | 986.09 | |||||||||
| Elective inpatient weighting | 1207.85 | £895.80 | 1514.43 | |||||||||
Costs for a ring pessary
| Product | Price (£) for 1 |
|---|---|
| Bioteque America (San Jose, CA, USA) | 20.00 |
| GBUK Healthcare (Selby, UK) | 19.00 |
| Milex (Mediplus, High Wycombe, UK) | 20.94 |
| Average | 19.98 |
Costs for a shelf pessary (assume Gellhorn pessary)
| Product | Price (£) for 1 |
|---|---|
| Bioteque America | 21.50 |
| GBUK | 20.49 |
| Milex | 22.55 |
| Average | 21.51 |
Appendix 7 Evidence synthesis
Meta-analyses: prolapse surgery with no mesh compared with prolapse surgery with mesh (Fiona Stewart, Lynda Constable, Moira Cruickshank, Clare Robertson)
This appendix contains the results of meta-analyses of RCTs comparing prolapse surgery with and without mesh, updated with new RCTs published since the last Cochrane review in 201318 and with the new data from PROSPECT.
Meta-analyses have been undertaken for outcomes measured up to 1 year after surgery and for the same outcomes measured beyond the first postoperative year (usually for 2 years, with some trials following up participants for 3 years).
Within 1 year of surgery, the risk of having persistent prolapse symptoms is significantly greater for women who are undergoing surgery with mesh kit than for those without mesh (RR 0.73, 95% CI 0.59 to 0.90). There is no evidence of a difference between surgery without mesh and surgery with absorbable, biological graft or non-absorbable mesh (Figure 35). PROSPECT has contributed over half of the evidence for the graft and mesh inlay outcomes.
FIGURE 35.
Number of women with prolapse symptoms (up to 1 year after surgery). df, degrees of freedom; M-H, Mantel-Haenszel.
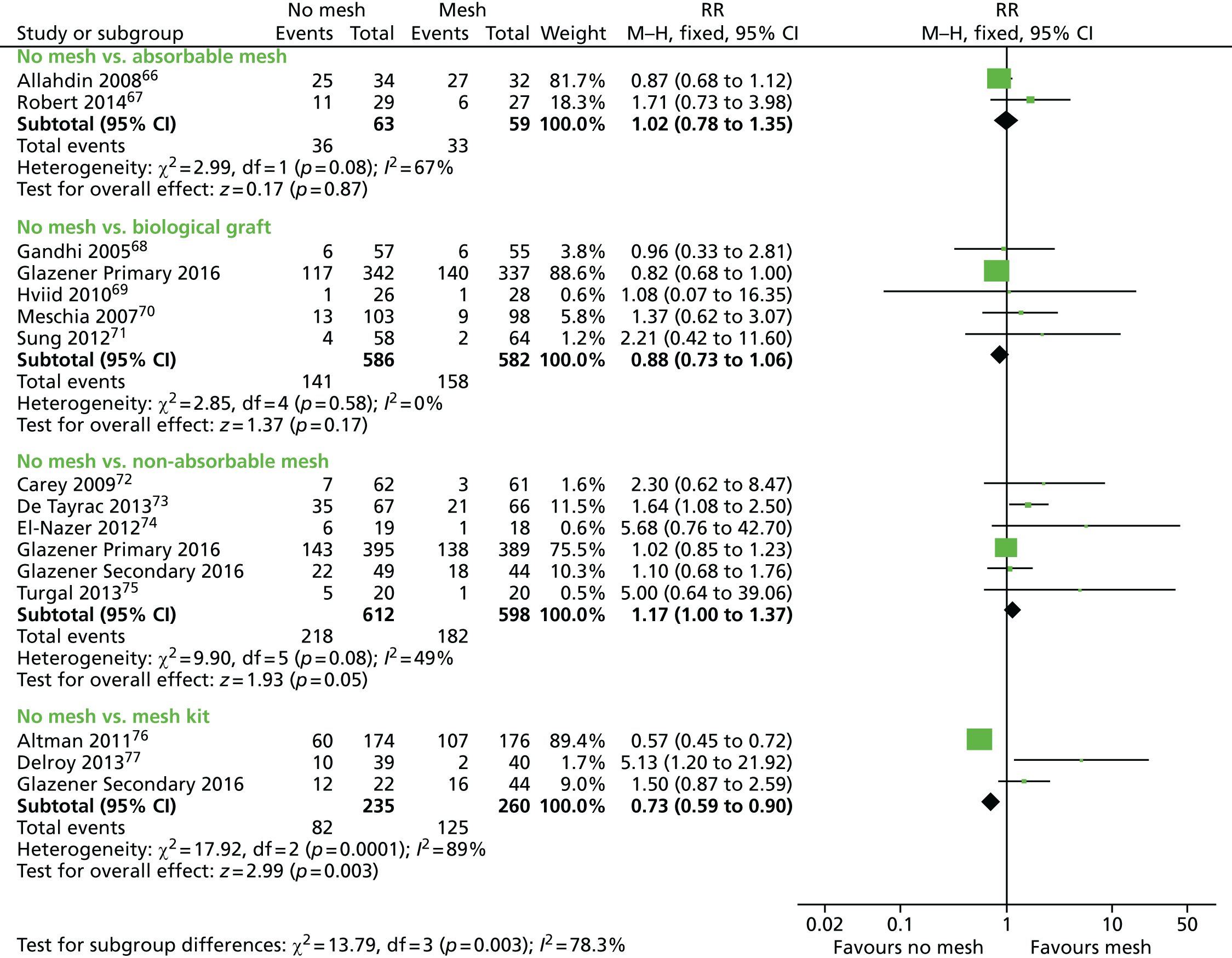
References
No mesh compared with absorbable mesh
Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. J Obstet Gynaecol 2008;28:427–31. 66
Robert M, Girard I, Brennand E, Tang S, Birch C, Murphy M, et al. Absorbable mesh augmentation compared with no mesh for anterior prolapse: a randomized controlled trial. Obstet Gynecol 2014;123:288–94. 67
No mesh compared with biological graft
Gandhi S, Goldberg RP, Kwon C, Koduri S, Beaumont JL, Abramov Y, et al. A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol 2005;192:1649–54. 68
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Hviid U, Hviid TV, Rudnicki M. Porcine skin collagen implants for anterior vaginal wall prolapse: a randomised prospective controlled study. Int Urogynecol J 2010;21:529–34. 69
Meschia M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kocjancic E. Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: a multicenter, randomized study. J Urol 2007;177:192–5. 70
Sung VW, Rardin CR, Raker CA, Lasala CA, Myers DL. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol 2012;119:125–33. 71
No mesh compared with non-absorbable mesh
Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, et al. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG 2009;116:1380–6. 72
De Tayrac R, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso S, et al. Comparison between trans-obturator trans-vaginal meshand traditional anterior colporrhaphy in the treatmentof anterior vaginal wall prolapse: results of a French RCT. Int Urogynecol J 2013;24:1651–61. 73
El-Nazer MA, Gomaa IA, Ismail Madkour WA, Swidan KH, El-Etriby MA. Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study. Arch Gynecol Obstet 2012;286:965–72. 74
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Turgal M, Sivaslioglu A, Yildiz A, Dolen I. Anatomical and functional assessment of anterior colporrhaphy versus polypropylene mesh surgery in cystocele treatment. Eur J Obstet Gynecol Reproduct Biol 2013;170:555–8. 75
No mesh compared with mesh kit
Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364:1826–36. 76
Delroy CA, Castro RDA, Dias MM, Feldner PC, Jr, Bortolini MAT, Girao MJBC, et al. The use of transvaginal synthetic mesh for anterior vaginal wall prolapse repair: a randomized controlled trial. Int Urogynecol J 2013;24:1899–907. 77
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Beyond the first postoperative year, the risk of persistent prolapse symptoms is greater with biological graft than surgery without mesh (RR 0.78, 95% CI 0.63 to 0.97). There is no evidence of a difference between surgery without mesh and surgery with absorbable or non-absorbable mesh or mesh kit (Figure 36). PROSPECT has contributed over half the evidence for the graft and mesh inlay outcomes.
FIGURE 36.
Number of women with prolapse symptoms (> 1 year after surgery). df, degrees of freedom; M-H, Mantel-Haenszel.

References
No mesh compared with absorbable mesh
Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. J Obstet Gynaecol 2008;28:427–31. 66
Chmielewski L, Walters MD, Weber AM, Barber MD. Reanalysis of a randomized trial of 3 techniques of anterior colporrhaphy using clinically relevant definitions of success. Am J Obstet Gynecol 2011;205:69.e1–8. 78
Minassian VA, Parekh M, Poplawsky D, Gorman J, Litzy L. Randomized controlled trial comparing two procedures for anterior vaginal wall prolapse. Neurourol Urodyn 2014;33:72–7. 79
No mesh compared with biological graft
Dahlgren E, Kjolhede P, RPOP-PELVICOL Study Group. Long-term outcome of porcine skin graft in surgical treatment of recurrent pelvic organ prolapse. An open randomized controlled multicenter study. Acta Obstet Gynecol Scand 2011;90:1393–401. 80
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Guerette NL, Aguirre O, VanDrie DM, Biller DH, Davila GW. Multi-center, randomized, prospective trial comparing anterior colporrhaphy alone to bovine pericardium collagen matrix graft reinforced anterior colporrhaphy: 12-month analysis. Abstract no. 11. Int Urogynecol J Pelvic Floor Dysf 2006;17(Suppl. 2):63–4. 81
No mesh compared with non-absorbable mesh
El-Nazer MA, Gomaa IA, Ismail Madkour WA, Swidan KH, El-Etriby MA. Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study. Arch Gynecol Obstet 2012;286:965–72. 74
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Gutman RE, Nosti PA, Sokol AI, Sokol ER, Peterson JL, Wang H, et al. Three-year outcomes of vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol 2013;122:770–7. 82
Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol 2010;203:235.e1–8. 83
Qatawneh A, Al-Kazaleh F, SAleh S, Thekrallah F, Bata M, Sumreen I, et al. Transvaginal cystocele repair using tension-free polypropylene mesh at the time of sacrospinous colpopexy for advanced uterovaginal prolapse: a prospective randomised study. Gynaecol Surg 2013;10:79–85. 84
No mesh compared with mesh kit
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Tamanini JT, de Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, Girão MJ. A prospective, randomized, controlled trial of the treatment of anterior vaginal wall prolapse: medium term follow up. J Urol 2015;193:1298–304. 85
Within the first postoperative year, the risk of objective failure is significantly lower for women undergoing surgery with non-absorbable mesh (RR 2.79, 95% CI 1.83 to 4.26) or mesh kit (RR 3.67, 95% CI 2.07 to 6.52). There is no evidence of a difference between surgery without mesh and surgery with absorbable mesh or biological graft (Figure 37). PROSPECT has contributed over half of the evidence for the graft and about one-third for the mesh inlay outcomes.
FIGURE 37.
Number of women with objective failure (up to 1 year after surgery). df, degrees of freedom; M-H, Mantel-Haenszel.

References
No mesh compared with absorbable mesh
Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. J Obstet Gynaecol 2008;28:427–31. 66
Minassian VA, Parekh M, Poplawsky D, Gorman J, Litzy L. Randomized controlled trial comparing two procedures for anterior vaginal wall prolapse. Neurourol Urodyn 2014;33:72–7. 79
Robert M, Girard I, Brennand E, Tang S, Birch C, Murphy M, et al. Absorbable mesh augmentation compared with no mesh for anterior prolapse: a randomized controlled trial. Obstet Gynecol 2014;1231:288–94. 67
Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol 2001;184:1357–62. 86
No mesh compared with biological graft
Feldner PC, Jr, Castro RA, Cipolotti LA, Delroy CA, Sartori MG, Girao MJ. Anterior vaginal wall prolapse: a randomized controlled trial of SIS graft versus traditional colporrhaphy. Int Urogynecol J Pelvic Floor Dysf 2010;21:1057–63. 87
Gandhi S, Goldberg RP, Kwon C, Koduri S, Beaumont JL, Abramov Y, et al. A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol 2005;192:1649–54. 68
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Guerette NL, Aguirre O, VanDrie DM, Biller DH, Davila GW. Multi-center, randomized, prospective trial comparing anterior colporrhaphy alone to bovine pericardium collagen matrix graft reinforced anterior colporrhaphy: 12-month analysis. Abstract no. 11. Int Urogynecol J Pelvic Floor Dysf 2006;17(Suppl. 2):63–4. 81
Hviid U, Hviid TV, Rudnicki M. Porcine skin collagen implants for anterior vaginal wall prolapse: a randomised prospective controlled study. Int Urogynecol J 2010;21:529–34. 69
Meschia M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kocjancic E. Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: a multicenter, randomized study. J Urol 2007;177:192–5. 70
Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol 2006;195:1762–71. 88
No mesh compared with non-absorbable mesh
Ali S, Han HC, Lee LC. A prospective randomized trial using Gynemesh PS (trademark) for the repair of anterior vaginal wall prolapse (Abstract number 292). Int Urogynecol J Pelvic Floor Dysf 2006;17(Suppl. 2):221. 89
Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, et al. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG 2009;116:1380–6. 72
De Tayrac R, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso S, et al. Comparison between trans-obturator trans-vaginal meshand traditional anterior colporrhaphy in the treatmentof anterior vaginal wall prolapse: results of a French RCT. Int Urogynecol J 2013;24:1651–61. 73
Dos Reis Brandão da Silveira S, Haddad JM, de Jármy-Di Bella ZI, Nastri F, Kawabata MG, da Silva Carramão S, et al. Multicenter, randomized trial comparing native vaginal tissue repair and synthetic mesh repair for genital prolapse surgical treatment. Int Urogynecol J 2015;26:335–42. 90
El-Nazer MA, Gomaa IA, Ismail Madkour WA, Swidan KH, El-Etriby MA. Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study. Arch Gynecol Obstet 2012;286:965–72. 74
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Gupta B, Vaid NB, Suneja A, Guleria K, Jain S. Anterior vaginal prolapse repair: a randomised trial of traditional anterior colporrhaphy and self-tailored mesh repair. S Afr J Obstet Gynaecol 2014;20:47–50. 91
Halaska M, Maxova K, Sottner O, Svabik K, Mlcoch M, Kolarik D, et al. A multicentre randomized prospective controlled study comparing sacrospinous fixation and transvaginal mesh in the treatment of posthysterectomy vaginal vault prolpase. Am J Obstet Gynecol 2012;207:e1–7. 92
Lamblin G, Van-Nieuwenhuyse A, Chabert P, Lebail-Carval K, Moret S, Mellier G. A randomized controlled trial comparing anatomical and functional outcome between vaginal colposuspension and transvaginal mesh. Int Urogynecol J 2014;25:961–70. 93
Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 2008;111:891–8. 94
Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol 2010;203:235.e1–8. 83
Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. Anterior colporrhaphy compared with collagen-coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. BJOG 2014;121:102–11. 95
Sivaslioglu AA, Unlubilgin E, Dolen I. A randomized comparison of polypropylene mesh surgery with site-specific surgery in the treatment of cystocoele. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:467–71. 96
Turgal M, Sivaslioglu A, Yildiz A, Dolen I. Anatomical and functional assessment of anterior colporrhaphy versus polypropylene mesh surgery in cystocele treatment. Eur J Obstet Gynecol Reproduct Biol 2013;170:555–8. 75
Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME. Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol 2011;117:242–50. 97
No mesh compared with mesh kit
Delroy CA, Castro Rde A, Dias MM, Feldner PC, Bortolini MA, Girão MJ, et al. The use of transvaginal synthetic mesh for anterior vaginal wall prolapse repair: a randomized controlled trial. Int Urogynecol J 2013;24:1899–907. 77
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Tamanini JT, de Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, Girão MJ. A prospective, randomized, controlled trial of the treatment of anterior vaginal wall prolapse: medium term followup. J Urol 2015;193:1298–304. 85
Vollebregt A, Fischer K, Gietelink D, van der Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar-guided transobturator anterior mesh. BJOG 2011;118:1518–27. 98
Beyond the first postoperative year, the risk of objective failure for women undergoing operations without mesh is greater than for surgery with non-absorbable mesh (RR 2.53, 95% CI 1.52 to 4.22). There is no evidence of a difference between surgery without mesh and surgery with absorbable mesh, biological graft or mesh kit (Figure 38). PROSPECT did not measure objective outcomes after the first year.
FIGURE 38.
Number of women with objective failure (> 1 year after surgery). df, degrees of freedom; M-H, Mantel-Haenszel.
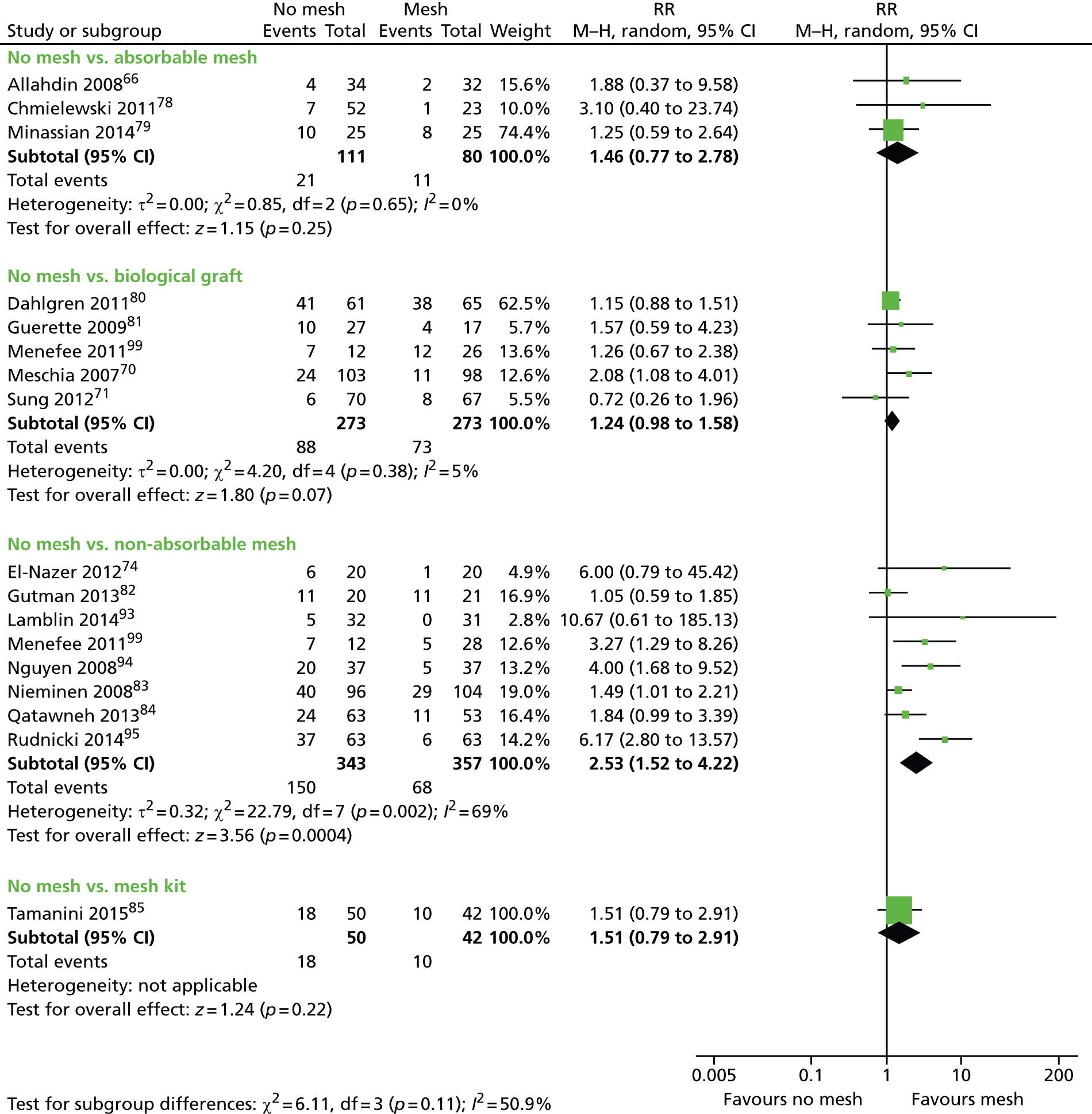
References
No mesh compared with absorbable mesh
Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. J Obstet Gynaecol 2008;28:427–31. 66
Chmielewski L, Walters MD, Weber AM, Barber MD. Reanalysis of a randomized trial of 3 techniques of anterior colporrhaphy using clinically relevant definitions of success. Am J Obstet Gynecol 2011;205:69.e1–8. 78
Minassian VA, Parekh M, Poplawsky D, Gorman J, Litzy L. Randomized controlled trial comparing two procedures for anterior vaginal wall prolapse. Neurourol Urodyn 2014;33:72–7. 79
No mesh compared with biological graft
Dahlgren E, Kjolhede P, RPOP-PELVICOL Study Group. Long-term outcome of porcine skin graft in surgical treatment of recurrent pelvic organ prolapse. An open randomized controlled multicenter study. Acta Obstet Gynecol Scand 2011;90:1393–401. 80
Guerette NL, Aguirre O, VanDrie DM, Biller DH, Davila GW. Multi-center, randomized, prospective trial comparing anterior colporrhaphy alone to bovine pericardium collagen matrix graft reinforced anterior colporrhaphy: 12-month analysis. Abstract no. 11. Int Urogynecol J Pelvic Floor Dysf 2006;17(Suppl. 2):63–4. 81
Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1337–44. 99
Meschia M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kocjancic E. Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: a multicenter, randomized study. J Urol 2007;177:192–5. 70
Sung VW, Rardin CR, Raker CA, Lasala CA, Myers DL. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol 2012;119:125–33. 71
No mesh compared with non-absorbable mesh
El-Nazer MA, Gomaa IA, Ismail Madkour WA, Swidan KH, El-Etriby MA. Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study. Arch Gynecol Obstet 2012;286:965–72. 74
Gutman RE, Nosti PA, Sokol AI, Sokol ER, Peterson JL, Wang H, et al. Three-year outcomes of vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol 2013;122:770–7. 82
Lamblin G, Van-Nieuwenhuyse A, Chabert P, Lebail-Carval K, Moret S, Mellier G. A randomized controlled trial comparing anatomical and functional outcome between vaginal colposuspension and transvaginal mesh. Int Urogynecol J 2014;25:961–70. 93
Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1337–44. 99
Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 2008;111:891–8. 94
Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol 2010;203:235.e1–8. 83
Qatawneh A, Al-Kazaleh F, Saleh S, Thekrallah F, Bata M, Sumreen I, et al. Transvaginal cystocele repair using tension-free polypropylene mesh at the time of sacrospinous colpopexy for advanced uterovaginal prolapse: a prospective randomised study. Gynaecol Surg 2013;10:79–85. 84
Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. Anterior colporrhaphy compared with collagen-coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. BJOG 2014;121:102–11. 95
No mesh compared with mesh kit
Tamanini JT, de Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, Girão MJ. A prospective, randomized, controlled trial of the treatment of anterior vaginal wall prolapse: medium term followup. J Urol 2015;193:1298–304. 85
The risk of requiring further surgery for prolapse is greater for women undergoing surgery with mesh kit than surgery without mesh (RR 3.65, 95% CI 1.51 to 8.86). There is no evidence of a difference between surgery without mesh and surgery with absorbable mesh, biological graft or non-absorbable mesh (Figure 39). PROSPECT has contributed well over half the evidence for the graft and about one-third for the mesh inlay outcomes.
FIGURE 39.
Number of women undergoing repeat prolapse surgery. df, degrees of freedom; M-H, Mantel-Haenszel.

References
No mesh compared with absorbable mesh
Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. J Obstet Gynaecol 2008;28:427–31. 66
Chmielewski L, Walters MD, Weber AM, Barber MD. Reanalysis of a randomized trial of 3 techniques of anterior colporrhaphy using clinically relevant definitions of success. Am J Obstet Gynecol 2011;205:69.e1–8. 78
No mesh compared with biological graft
Feldner PC, Jr, Castro RA, Cipolotti LA, Delroy CA, Sartori MG, Girao MJ. Anterior vaginal wall prolapse: a randomized controlled trial of SIS graft versus traditional colporrhaphy. Int Urogynecol J Pelvic Floor Dysf 2010;21:1057–63. 87
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Guerette NL, Aguirre O, VanDrie DM, Biller DH, Davila GW. Multi-center, randomized, prospective trial comparing anterior colporrhaphy alone to bovine pericardium collagen matrix graft reinforced anterior colporrhaphy: 12-month analysis. Abstract no. 11. Int Urogynecol J Pelvic Floor Dysf 2006;17(Suppl. 2):63–4. 81
Hviid U, Hviid TV, Rudnicki M. Porcine skin collagen implants for anterior vaginal wall prolapse: a randomised prospective controlled study. Int Urogynecol J 2010;21:529–34. 69
Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1337–44. 99
Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol 2006;195:1762–71. 88
No mesh compared with non-absorbable mesh
Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, et al. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG 2009;116:1380–6. 72
De Tayrac R, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso S, et al. Comparison between trans-obturator trans-vaginal meshand traditional anterior colporrhaphy in the treatmentof anterior vaginal wall prolapse: results of a French RCT. Int Urogynecol J 2013;24:1651–61. 73
Dos Reis Brandão da Silveira S, Haddad JM, de Jármy-Di Bella ZI, Nastri F, Kawabata MG, da Silva Carramão S, et al. Multicenter, randomized trial comparing native vaginal tissue repair and synthetic mesh repair for genital prolapse surgical treatment. Int Urogynecol J 2015;26:335–42. 90
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Gutman RE, Nosti PA, Sokol AI, Sokol ER, Peterson JL, Wang H, et al. Three-year outcomes of vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol 2013;122:770–7. 82
Halaska M, Maxova K, Sottner O, Svabik K, Mlcoch M, Kolarik D, et al. A multicentre randomized prospective controlled study comparing sacrospinous fixation and transvaginal mesh in the treatment of posthysterectomy vaginal vault prolpase. Am J Obstet Gynecol 2012;207:e1–7. 92
Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1337–44. 99
Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 2008;111:891–8. 94
Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol 2010;203:235.e1–8. 83
Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. Anterior colporrhaphy compared with collagen-coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. BJOG 2014;121:102–11. 95
Sivaslioglu AA, Unlubilgin E, Dolen I. A randomized comparison of polypropylene mesh surgery with site-specific surgery in the treatment of cystocoele. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:467–71. 96
Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME. Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol 2011;117:242–50. 97
No mesh compared with mesh kit
Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364:1826–36. 76
Delroy CA, Castro RDA, Dias MM, Feldner Jr PC, Bortolini MAT, Girao MJBC, et al. The use of transvaginal synthetic mesh for anterior vaginal wall prolapse repair: a randomized controlled trial. Int Urogynecol J 2013;24:1899–907. 77
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Tamanini JT, de Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, Girão MJ. A prospective, randomized, controlled trial of the treatment of anterior vaginal wall prolapse: medium term followup. J Urol 2015;193:1298–304. 85
Vollebregt A, Fischer K, Gietelink D, van der Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar-guided transobturator anterior mesh. BJOG 2011;118:1518–27. 98
There is no evidence of a difference between surgery without mesh and surgery with biological graft, non-absorbable mesh or mesh kit in terms of the risk of requiring surgery for UI (Figure 40). PROSPECT has contributed almost all of the evidence for the graft and nearly a half for the mesh inlay outcomes.
FIGURE 40.
Number of women undergoing continence surgery. df, degrees of freedom; M-H, Mantel-Haenszel.

References
No mesh compared with biological graft
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1337–44. 99
No mesh compared with non-absorbable mesh
De Tayrac R, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso S, et al. Comparison between trans-obturator trans-vaginal meshand traditional anterior colporrhaphy in the treatmentof anterior vaginal wall prolapse: results of a French RCT. Int Urogynecol J 2013;24:1651–61. 73
Dos Reis Brandão da Silveira S, Haddad JM, de Jármy-Di Bella ZI, Nastri F, Kawabata MG, da Silva Carramão S, et al. Multicenter, randomized trial comparing native vaginal tissue repair and synthetic mesh repair for genital prolapse surgical treatment. Int Urogynecol J 2015;26:335–42. 90
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Gutman RE, Nosti PA, Sokol AI, Sokol ER, Peterson JL, Wang H, et al. Three-year outcomes of vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol 2013;122:770–7. 82
Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 2008;111:891–8. 94
Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol 2010;203:235.e1–8. 83
Thijs S, Deprest J, De Ridder D, Claerhout F, Roovers J. A randomized controlled trial of anterior colporraphy and Perigee™ as a primary surgical correction of symptomatic cystocele. Abstract no. 96. Int Urogynecol J Pelvic Floor Dysf 2010;21(Suppl. 1):142–3. 100
No mesh compared with mesh kit
Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364:1826–36. 76
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Vollebregt A, Fischer K, Gietelink D, van der Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar-guided transobturator anterior mesh. BJOG 2011;118:1518–27. 98
The risk of requiring surgery for mesh exposure is significantly greater for those undergoing surgery with non-absorbable mesh (RR 0.09, 95% CI 0.05 to 0.17). However, that is expected, as the majority of the women in the no-mesh arms would not have received mesh prolapse surgery, although they might have had mesh for a concomitant operation, such as tape continence surgery or vault suspension. There is no evidence of a difference between surgery without mesh and surgery with biological graft or mesh kit (Figure 41).
FIGURE 41.
Number of women undergoing surgery for mesh exposure. df, degrees of freedom; M-H, Mantel-Haenszel.

References
No mesh compared with biological mesh
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1337–44. 99
Tamanini JT, de Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, Girão MJ. A prospective, randomized, controlled trial of the treatment of anterior vaginal wall prolapse: medium term followup. J Urol 2015;193:1298–304. 85
Vollebregt A, Fischer K, Gietelink D, van der Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar-guided transobturator anterior mesh. BJOG 2011;118:1518–27. 98
No mesh compared with non-absorbable mesh
Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, et al. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG 2009;116:1380–6. 72
De Tayrac R, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso S, et al. Comparison between trans-obturator trans-vaginal meshand traditional anterior colporrhaphy in the treatment of anterior vaginal wall prolapse: results of a French RCT. Int Urogynecol J 2013;24:1651–61. 73
El-Nazer MA, Gomaa IA, Ismail Madkour WA, Swidan KH, El-Etriby MA. Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study. Arch Gynecol Obstet 2012;286:965–72. 74
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Gutman RE, Nosti PA, Sokol AI, Sokol ER, Peterson JL, Wang H, et al. Three-year outcomes of vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol 2013;122:770–7. 82
Halaska M, Maxova K, Sottner O, Svabik K, Mlcoch M, Kolarik D, et al. A multicentre randomized prospective controlled study comparing sacrospinous fixation and transvaginal mesh in the treatment of posthysterectomy vaginal vault prolpase. Am J Obstet Gynecol 2012;207:e1–7. 92
Lamblin G, Van-Nieuwenhuyse A, Chabert P, Lebail-Carval K, Moret S, Mellier G. A randomized controlled trial comparing anatomical and functional outcome between vaginal colposuspension and transvaginal mesh. Int Urogynecol J 2014;25:961–70. 93
Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1337–44. 99
Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 2008;111:891–8. 94
Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol 2010;203:235.e1–8. 83
Qatawneh A, Al-Kazaleh F, Saleh S, Thekrallah F, Bata M, Sumreen I, Al-Mustafa M. Transvaginal cystocele repair using tension-free polypropylene mesh at the time of sacrospinous colpopexy for advanced uterovaginal prolapse: a prospective randomised study. Gynaecol Surg 2013;10:79–85. 84
Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. Anterior colporrhaphy compared with collagen-coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. BJOG 2014;121:102–11. 95
Sivaslioglu AA, Unlubilgin E, Dolen I. A randomized comparison of polypropylene mesh surgery with site-specific surgery in the treatment of cystocoele. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:467–71. 96
Thijs S, Deprest J, De Ridder D, Claerhout F, Roovers J. A randomized controlled trial of anterior colporraphy and Perigee™ as a primary surgical correction of symptomatic cystocele. Abstract no. 96. Int Urogynecol J Pelvic Floor Dysfunct 2010;21(Suppl. 1):142–3. 100
Turgal M, Sivaslioglu A, Yildiz A, Dolen I. Anatomical and functional assessment of anterior colporrhaphy versus polypropylene mesh surgery in cystocele treatment. Eur J Obstet Gynecol Reprod Biol 2013;170:555–8. 75
Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME. Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol 2011;117:242–50. 97
No mesh compared with mesh kit
Glazener 2016, Primary and Secondary – reference is to the current monograph.
Tamanini JT, de Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, Girão MJ. A prospective, randomized, controlled trial of the treatment of anterior vaginal wall prolapse: medium term followup. J Urol 2015;193:1298–304. 85
Vollebregt A, Fischer K, Gietelink D, van der Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar-guided transobturator anterior mesh. BJOG 2011;118:1518–27. 98
Appendix 8 Mega CONSORT, including comprehensive cohort 3 participants
| Type of repair | Primary | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratum/comparison | All: 1348 | Trial 1: 865 | Trial 2:735 | RCT1A: 762 | RCT1B: 358 | RCT1C: 228 | ||||||||
| Treatment arm | Standard repair: 545 | Synthetic mesh: 435 | Biological graft: 368 | Standard repair: 430 | Synthetic mesh: 435 | Standard repair: 367 | Biological graft: 368 | Standard repair: 252 | Synthetic mesh: 255 | Biological graft: 255 | Standard repair: 178 | Synthetic mesh: 180 | Standard repair: 115 | Biological graft: 113 |
| Received surgery | 537 (99%) | 425 (98%) | 363 (99%) | 425 (99%) | 425 (98%) | 359 (98%) | 363 (99%) | 247 | 250 | 251 | 178 | 175 | 112 | 112 |
| Standard repair | 512 (95%) | 60 (14%) | 57 (16%) | 403 (95%) | 60 (14%) | 342 (95%) | 57 (16%) | 233 | 28 | 35 | 170 | 32 | 109 | 22 |
| Synthetic mesh | 2 (0%) | 341 (80%) | 6 (2%) | 2 (0%) | 341 (80%) | 1 (0%) | 6 (2%) | 1 | 209 | 5 | 1 | 132 | 0 | 1 |
| Biological graft | 2 (0%) | 5 (1%) | 294 (81%) | 0 (0%) | 5 (1%) | 2 (1%) | 294 (81%) | 0 | 4 | 205 | 0 | 1 | 2 | 89 |
| Mesh kit | 2 (0%) | 1 (0%) | 0 (0%) | 2 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Other surgery | 19 (4%) | 18 (4%) | 6 (2%) | 18 (4%) | 18 (4%) | 14 (4%) | 6 (2%) | 13 | 9 | 6 | 5 | 9 | 1 | 0 |
| No surgery | 8 (1%) | 10 (2%) | 5 (1%) | 5 (1%) | 10 (2%) | 8 (2%) | 5 (1%) | 5 | 5 | 4 | 0 | 5 | 3 | 1 |
| Baseline questionnaire | 510 (94%) | 414 (95%) | 342 (93%) | 409 (95%) | 414 (95%) | 340 (93%) | 342 (93%) | 239 | 239 | 241 | 170 | 175 | 101 | 101 |
| 6-month questionnaire | 504 (94%) | 381 (90%) | 335 (92%) | 398 (93%) | 381 (88%) | 338 (92%) | 335 (91%) | 232 | 224 | 228 | 166 | 157 | 106 | 107 |
| Withdrawals within 6 months | 0 (0%) | 1 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Deaths within 6 months | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12-month short questionnaire | 504 (94%) | 389 (92%) | 337 (93%) | 395 (92%) | 389 (89%) | 342 (93%) | 337 (92%) | 233 | 226 | 230 | 162 | 163 | 109 | 107 |
| 12-month long questionnaire | 468 (87%) | 362 (85%) | 316 (87%) | 368 (86%) | 362 (83%) | 319 (87%) | 316 (86%) | 219 | 212 | 214 | 149 | 150 | 100 | 102 |
| 12-month clinic assessment | 477 (89%) | 374 (88%) | 320 (88%) | 381 (89%) | 374 (86%) | 319 (87%) | 320 (87%) | 223 | 217 | 221 | 158 | 157 | 96 | 99 |
| Withdrawals within 12 months | 2 (0%) | 4 (1%) | 2 (1%) | 2 (0%) | 4 (1%) | 1 (0%) | 2 (1%) | 1 | 4 | 2 | 1 | 0 | 0 | 0 |
| Deaths within 12 months | 1 (0%) | 0 (0%) | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | 1 (0%) | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 24-month questionnaire | 445 (82%) | 343 (79%) | 300 (82%) | 348 (81%) | 343 (79%) | 299 (81%) | 300 (82%) | 202 | 202 | 210 | 146 | 141 | 97 | 90 |
| Withdrawals within 24 months | 13 (2%) | 11 (3%) | 5 (1%) | 11 (3%) | 11 (3%) | 8 (2%) | 5 (1%) | 6 | 8 | 4 | 5 | 3 | 2 | 1 |
| Deaths within 24 months | 2 (0%) | 0 (0%) | 1 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | 1 (0%) | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Type of repair | Secondary | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratum/comparison | All: 154 | Trial 3: 107 | Trial 4: 71 | RCT3: 91 | RCT2: 59 | RCT2B: 4 | ||||||||
| Treatment arm | Standard repair: 56 | Synthetic mesh: 52 | Mesh kit: 46 | Standard repair: 55 | Synthetic mesh: 52 | Standard repair: 25 | Mesh kit: 46 | Standard repair: 24 | Synthetic mesh: 24 | Mesh kit: 43 | Standard repair: 31 | Synthetic mesh: 28 | Standard repair: 1 | Mesh kit: 3 |
| Received surgery | 56 (100%) | 51 (98%) | 45 (98%) | 55 (100%) | 51 (98%) | 25 (100%) | 45 (98%) | 24 | 24 | 42 | 31 | 27 | 1 | 3 |
| Standard repair | 49 (88%) | 9 (18%) | 4 (9%) | 49 (89%) | 9 (18%) | 20 (80%) | 4 (9%) | 20 | 3 | 3 | 29 | 6 | 0 | 1 |
| Synthetic mesh | 3 (5%) | 37 (73%) | 7 (16%) | 2 (4%) | 37 (73%) | 1 (4%) | 7 (16%) | 0 | 17 | 7 | 2 | 20 | 1 | 0 |
| Biological graft | 1 (2%) | 0 (0%) | 1 (2%) | 1 (2%) | 0 (0%) | 1 (4%) | 1 (2%) | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Mesh kit | 0 (0%) | 2 (4%) | 31 (69%) | 0 (0%) | 2 (4%) | 0 (0%) | 31 (69%) | 0 | 1 | 29 | 0 | 1 | 0 | 2 |
| Other surgery | 3 (5%) | 3 (6%) | 2 (4%) | 3 (5%) | 3 (6%) | 3 (12%) | 2 (4%) | 3 | 3 | 2 | 0 | 0 | 0 | 0 |
| No surgery | 0 (0%) | 1 (2%) | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (2%) | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Baseline questionnaire | 55 (98%) | 50 (96%) | 43 (93%) | 54 (98%) | 50 (96%) | 24 (96%) | 43 (93%) | 23 | 24 | 40 | 31 | 26 | 1 | 3 |
| 6-month questionnaire | 51 (91%) | 47 (92%) | 43 (96%) | 47 (90%) | 22 (88%) | 43 (93%) | 21 | 23 | 41 | 29 | 24 | 1 | 2 | |
| Withdrawals within 6 months | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Deaths within 6 months | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12-month short questionnaire | 50 (89%) | 44 (86%) | 44 (98%) | 49 (89%) | 44 (85%) | 21 (84%) | 44 (96%) | 20 | 21 | 41 | 29 | 23 | 1 | 3 |
| 12-month long questionnaire | 47 (84%) | 39 (76%) | 41 (91%) | 46 (84%) | 39 (75%) | 21 (84%) | 41 (89%) | 20 | 17 | 39 | 26 | 22 | 1 | 2 |
| 12-month clinic assessment | 46 (82%) | 44 (86%) | 38 (84%) | 46 (84%) | 44 (85%) | 21 (84%) | 38 (83%) | 21 | 21 | 36 | 25 | 23 | 0 | 2 |
| Withdrawals within 12 months | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Deaths within 12 months | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (4%) | 0 (0%) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24-month questionnaire | 44 (79%) | 39 (75%) | 39 (85%) | 43 (78%) | 39 (75%) | 20 (80%) | 39 (85%) | 19 | 18 | 37 | 24 | 21 | 1 | 2 |
| Withdrawals within 24 months | 1 (2%) | 3 (6%) | 2 (4%) | 1 (2%) | 3 (6%) | 0 (0%) | 2 (4%) | 0 | 3 | 2 | 1 | 0 | 0 | 0 |
| Deaths within 24 months | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (4%) | 0 (0%) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Type of repair | Primary: 1126 | Secondary: 244 | Uterine/vault: 215 | ||
|---|---|---|---|---|---|
| Stratum/comparison | Comprehensive cohort: 1585 | ||||
| Treatment arm | CC1: 1126 | CC2: 244 | CC3: 215 | Uterine: 69 | Vault: 146 |
| Received surgery | 1104 (98%) | 240 (98%) | 212 (99%) | 68 (99%) | 144 (99%) |
| Standard repair | 931 (84%) | 128 (53%) | 17 (8%) | 12 (18%) | 5 (3%) |
| Synthetic mesh | 44 (4%) | 52 (22%) | 3 (1%) | 0 (0%) | 3 (2%) |
| Biological graft | 45 (4%) | 17 (7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mesh kit | 17 (2%) | 25 (10%) | 1 (0%) | 0 (0%) | 1 (1%) |
| Other surgery | 67 (6%) | 18 (8%) | 191 (90%) | 56 (82%) | 135 (94%) |
| No surgery | 22 (2%) | 4 (2%) | 3 (1%) | 1 (1%) | 2 (1%) |
| Baseline questionnaire | 997 (89%) | 221 (91%) | 202 (94%) | 65 (94%) | 137 (94%) |
| 6-month questionnaire | 966 (88%) | 214 (89%) | 175 (83%) | 54 (79%) | 121 (84%) |
| Withdrawals within 6 months | 5 (0%) | 0 (0%) | 1 (0%) | 0 (0%) | 1 (1%) |
| Deaths within 6 months | 1 (0%) | 0 (0%) | 1 (0%) | 0 (0%) | 1 (1%) |
| 12-month short questionnaire | 972 (88%) | 216 (90%) | 173 (82%) | 57 (84%) | 116 (81%) |
| 12-month long questionnaire | 893 (81%) | 191 (80%) | 158 (75%) | 46 (68%) | 112 (78%) |
| 12-month clinic assessment | 11 (1%) | 8 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Withdrawals within 12 months | 14 (1%) | 0 (0%) | 5 (2%) | 1 (1%) | 4 (3%) |
| Deaths within 12 months | 3 (0%) | 0 (0%) | 1 (0%) | 0 (0%) | 1 (1%) |
| 24-month questionnaire | 848 (77%) | 191 (80%) | 152 (72%) | 48 (71%) | 104 (72%) |
| Withdrawals within 24 months | 32 (3%) | 6 (3%) | 11 (5%) | 3 (4%) | 8 (6%) |
| Deaths within 24 months | 3 (0%) | 1 (0%) | 4 (2%) | 2 (3%) | 2 (1%) |
Glossary
- For all negative continuous outcomes
- For example, the Pelvic Organ Prolapse Symptom Score. A positive effect size (mean difference) of > 0 favours standard repair.
- For all positive continuous outcomes
- For example, the EuroQol-5 Dimensions. A positive effect size (mean difference) of > 0 favours synthetic/biological/mesh kit.
- For all negative dichotomous outcomes
- An effect size (risk ratio) of > 1 favours standard repair.
- For all positive dichotomous outcomes
- An effect size (risk ratio) of > 1 favours synthetic/biological/mesh kit.
- Readmission
- Related to prolapse surgery (for complications). Readmission for new prolapse, incontinence or mesh complications surgery presented separately.
- Serious
- Causing death, requiring hospitalisation or prolongation of existing hospital admission, threatening life, resulting in significant incapacity or disability, or otherwise considered important by the investigator.
- How is prolapse compared with before surgery?
- Very much better = cured (1); much or a little better (or very much better) = improved or cured (< 4); no change or worse = failed (> 3).
- Satisfied with results of operation?
- Completely satisfied = cured (1); fairly satisfied = improved or cured (1 or 2); fairly or very dissatisfied = failed (3 or 4); not sure = separate category (5).
- ‘Any’ symptom Pelvic Organ Prolapse Symptom Score
- Symptom reported as ‘occasionally or more often’.
- ‘Frequent’ symptom Pelvic Organ Prolapse Symptom Score
- Symptom reported as ‘most or all of the time’.
- Pelvic Organ Prolapse Symptom Score
- Range 0–28, where 0 = no symptoms and 28 = all seven symptoms all of the time. Primary clinical outcome.
- Prolapse-related quality of life
- ‘Overall, how much do prolapse symptoms interfere with everyday life?’ Using a visual analogue scale, score range from 0 (not at all) to 10 (a great deal). Primary quality-of-life outcome.
- Symptomatic prolapse
- At least one prolapse symptom (Pelvic Organ Prolapse Symptom Score of > 0).
- Abdo. any
- ‘A heaviness or dragging feeling in your lower abdomen (tummy)?’ Any = occasionally or more.
- Abdo. freq.
- Frequent = most or all of the time.
- Back any
- ‘A heaviness or dragging feeling in your lower back?’ Any = occasionally or more.
- Back freq.
- Frequent = most or all of the time.
- Blad. not empty any
- ‘A feeling that your bladder has not emptied completely?’ Any = occasionally or more.
- Blad. not empty freq.
- Frequent = most or all of the time.
- Bowel not empty any
- ‘A feeling that your bowel has not emptied completely?’ Any = occasionally or more.
- Bowel not empty freq.
- Frequent = most or all of the time.
- Pain any
- ‘An uncomfortable feeling or pain in your vagina which is worse when standing?’ (any = occasionally or more).
- Pain freq.
- Frequent = most or all of the time.
- SCD any
- ‘A feeling of something coming down from or in your vagina?’ (any = occasionally or more).
- SCD freq.
- Frequent = most or all of the time.
- Strain blad. any
- ‘A need to strain (push) to empty your bladder?’ (any = occasionally or more).
- Strain blad. freq.
- Frequent = most or all of the time.
- Digital evacuation of bowel
- Do you have to insert a finger into your back passage to help empty stool (faeces, motion) from your bowel? (most or all of the time).
- Extra hygiene measures
- Do you have to take extra measures to ensure the prolapse does not cause personal hygiene problems? (most or all of the time).
- Fingers to ease discomfort
- Do you have to insert a finger into your vagina to push up the prolapse to ease discomfort or pain? (most or all of the time).
- Fingers to help empty bladder
- Do you have to use your fingers to push up the prolapse to help empty your bladder (pass water)? (most or all of the time).
- Fingers to help empty bowel
- Do you have to insert a finger into your vagina to help empty your bowels? (most or all of the time).
- Objective prolapse (on examination)
- Stage 2b, 3 or 4, defined as leading edge beyond the hymen (> 0 cm) when POP-Q data are available.
- Any incontinence
- Defined as ‘How often do you leak urine?’ (any frequency) and/or ‘How much urine do you usually leak?’ (whether you wear protection or not) (any amount).
- Incontinence-related quality-of-life score
- ‘Overall, how much does leaking urine interfere with your everyday life?’ Using a visual analogue scale, score range from 0 (not at all) to 10 (a great deal).
- International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form score
- Sum of responses to above three questions. Range 0 (no incontinence symptoms) to 21 (leaking all the time, a large amount and affecting quality of life = 10).
- Overactive bladder
- Nocturia twice or more; and urinary urgency ‘most or all of the time’; and urinary frequency nine or more times per day.
- Severe urinary incontinence
- International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form score of 13–21.
- Stress urinary incontinence
- ‘Does urine leak when you are physically active, exert yourself, cough or sneeze?’ (most or all of the time).
- Urgency urinary incontinence
- ‘Does urine leak before you can get to the toilet?’ (most or all of the time).
- Active faecal incontinence
- Any faecal incontinence when bowel urgency ‘most or all of the time’ is also reported.
- Bowel symptoms QoL score
- ’Overall, how much do bowel symptoms interfere with your everyday life?’ Measured using a visual analogue scale: score range from 0 (not at all) to 10 (a great deal). This could be due to any one or a combination of the above bowel symptoms.
- Bowel urgency
- ’Do you have to rush to the toilet when you need to open (move) your bowels?’ (most or all of the time).
- Constipation (ROME criteria, adapted)
- Any two of the following: stool passing once a week or less; straining most or all of the time; hard stools; bowel not feeling empty most or all of the time; manual manoeuvre to empty bowel most or all of the time.
- Faecal incontinence (any/severe)
- Faecal incontinence of solid or liquid stool: ‘Do stools (faeces, motion) leak at inappropriate time or place, or before you can get to the toilet?’ (any = occasionally or more often; severe = sometimes, most or all of the time).
- Passive faecal incontinence
- Any faecal incontinence not accompanied by bowel urgency ‘most or all of the time’.
- Dyspareunia (any, severe)
- Pain during sexual intercourse (any = a little or somewhat; severe = a lot).
- Dyspareunia at baseline
- Denominator includes number of women who were sexually active and those who did not have a sex life because of prolapse symptoms.
- International Consultation on Incontinence Questionnaire-Vaginal Symptoms score
- Combination of responses to vaginal symptom questions.
- Sex life quality of life
- ’Overall, how much do you feel that your sex life has been spoilt by vaginal symptoms?’ Measured using a visual analogue scale: score range from 0 (not at all) to 10 (a great deal).
- Vagina too tight
- ‘Do you feel that your vagina is too tight?’ (most or all of the time).
- Vaginal symptoms QoL score
- ’Overall, how much do your vaginal symptoms interfere with your everyday life?’ Measured using a visual analogue scale; score range from 0 (not at all) to 10 (a great deal).
List of abbreviations
- AE
- adverse event
- Akaike
- information criterion
- BMI
- body mass index
- BNF
- British National Formulary
- CC
- comprehensive cohort
- CEAC
- cost-effectiveness acceptability curve
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DMC
- Data Monitoring Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions (3-level version)
- FI
- faecal incontinence
- GLM
- general linear regression model
- GP
- general practitioner
- HES
- Health Episode Statistics
- HSRU
- Health Services Research Unit
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICI
- International Consultation on Incontinence
- ICIQ
- International Consultation on Incontinence Questionnaire
- ICIQ-FLUTS
- International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms
- ICIQ-UI-SF
- International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form
- ICIQ-VS
- International Consultation on Incontinence Questionnaire-Vaginal Symptoms
- ICS
- International Continence Society
- IP
- Interventional Procedures
- IPAC
- Interventional Procedures Advisory Committee
- ISD
- Information Services Division
- IUGA
- International Urogynecological Association
- MD
- mean difference
- MI
- multiple imputation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OLS
- ordinary least squares
- OR
- odds ratio
- PFMT
- pelvic floor muscle training
- PIL
- patient information leaflet
- PMG
- Project Management Group
- POP-Q
- Pelvic Organ Prolapse Quantification
- POP-SS
- Pelvic Organ Prolapse Symptom Score
- PROSPECT
- PROlapse Surgery: Pragmatic Evaluation and randomised Controlled Trials
- PSSRU
- Personal and Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RO
- recruitment officer
- RR
- risk ratio
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SE
- standard error
- TSC
- Trial Steering Committee
- TVL
- total vaginal length
- TVT
- tension-free vaginal tape
- UI
- urinary incontinence
- UTI
- urinary tract infection
- VAS
- visual analogue scale
- WTP
- willingness to pay