Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/36/37. The contractual start date was in April 2013. The draft report began editorial review in May 2018 and was accepted for publication in March 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Dias et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scaphoid fracture accounts for 90% of all carpal fractures1 and 2–7% of all fractures. 2 It is an important public health problem, as it predominantly affects young active individuals (mean age 29 years)3 in their most productive working years.
The scaphoid typically fractures when the wrist is suddenly extended either when putting the hand out to break a fall or when the palm is struck forcibly by an object.
Most fractures (64%) affect the waist of the scaphoid, but 5% affect the proximal pole (the proximal 20% of the scaphoid) and around 13.3% involve the distal part of the scaphoid. The tuberosity fractures in 18.1% of cases. Figure 1 illustrates this.
FIGURE 1.
Locations of scaphoid fractures and the proportions reported (information sourced from Garala et al. 4) Non-tuberosity fractures account for 81.9% of all scaphoid fractures and 78.2% of these involve the waist.

The scaphoid, lunate and triquetrum form the proximal carpal row, attached to each other by ligaments. These bones are subject to loads when the muscles whose tendons cross the wrist bones contract. This row acts together like a helix. When the scaphoid flexes under load, the triquetrum extends so that the middle section, the lunate, remains in a neutral and stable position. 5,6
A fracture of the scaphoid breaks this helix so that the two parts then rotate; the distal scaphoid fragment flexes under load while the remainder of the proximal carpal helix extends. After a fracture, the proximal carpal row can no longer stabilise the distal carpal row and hence the wrist. The resulting abnormal loading between the distal part of the broken scaphoid and the distal radius leads to cartilage degeneration and arthritis, while the proximal part extends, causing carpal collapse. This pattern after failure of union is called a scaphoid non-union advanced collapse (SNAC) wrist. 7
Scaphoid fractures disrupt the proximal carpal row and alter the complex mechanics of this row, thereby altering how the wrist is stabilised to permit the hand and digits to function efficiently.
About 88–90% of these fractures unite when treated initially in a plaster cast. However, 10–12% of scaphoid fractures treated with plaster cast do not unite, with a higher incidence (14–50%) in displaced fractures. 8–10 Retrograde intraosseous circulation11 jeopardises the blood circulation, particularly in the proximal scaphoid, and may explain the higher failure of union in proximal fractures. Non-union, if untreated, almost inevitably leads to arthritis, especially in the radioscaphoid joint, usually within 5 years. 12,13 This disables patients at a very young age.
Diagnosis of the fracture is usually confirmed in emergency departments on radiographs of the scaphoid taken in different projections and it is usual to obtain a ‘scaphoid series of radiographs’, which include posterior–anterior, lateral and 45° supination and pronation views, as shown in Figure 2. In addition, an elongated view of the scaphoid is obtained to avoid missing any fractures that may be obscured because of the palmar and radial inclination of the scaphoid. It is possible to have an incomplete fracture of the scaphoid and these usually heal uneventfully. 14 However, clear and bicortical fractures cause clinical concern, as these are more likely to be unstable.
FIGURE 2.
Five radiographic views of the scaphoid. (a) Posterior–anterior view, in which the fracture is just visible; (b) elongated scaphoid view, in which the fracture is ‘clear’ and ‘bicortical’; the gap could be > 1 mm and the radial margin shows a small step; (c) semisupine oblique view; (d) lateral view, through which alignment can be assessed; and (e) semiprone view, which also shows the fracture and suggests displacement.

Treatments
Getting a fracture to heal restores the integrity of the proximal carpal row and thereby the stability of the wrist. This stability restores hand function, reduces the feeling of weakness and significantly reduces the risk of carpal collapse and the resulting arthritis15 (SNAC).
The aim of treatment is to immobilise the fracture while the physiological processes of healing occur. Immobilising the fracture fragments relative to one another can be done in various ways. Movement between fracture fragments can be constrained by immobilising the injured wrist in a cast or by surgically introducing a screw across the fracture.
Fixation
Immediate surgical fixation may avoid the need to immobilise the wrist in a cast and could accelerate return to function, work and sport;16 however, this method requires the person to have an operation and be exposed to surgical risks. The fracture is fixed with a standard Conformité Européenne (CE)-marked headless screw generating compression at the fracture site but avoiding the pressure effects of the screw head on articular cartilage. This can be done either percutaneously or in open surgery. 17–19 The surgical techniques for this method are well described and are now standard. 20–22 These screws did not change during the recruitment period for this study. Some surgeons use splintage for the first few weeks after surgery.
Cast treatment
The usual treatment is immobilisation in a below-elbow cast for 6–10 weeks, followed by mobilisation. The type of below elbow cast used does not affect union rate. 8 The 10–12% of patients who develop non-union [as seen on radiographs and/or computed tomography (CT) scans] usually have urgent surgical fixation. This is the current standard non-operative pathway. 3
Current evidence
Eight randomised controlled trials (RCTs)3,18,23–28 have reported on 463 participants with undisplaced or minimally displaced fractures of the scaphoid waist who had either of these two treatments. These RCTs had small sample sizes (ranging from 25 to 88 participants) and have been systematically reviewed nine times;29–37 these reviews all commented on the low quality of the evidence.
Some studies reported that fracture fixation facilitated earlier restoration of function and return to previous activity levels, especially if a cast or splint was not used after fixation, but patients had a higher rate of complications, of between 9% and 22%, although these were usually minor. 3,18,25
It is unclear if patients who had surgical fixation of undisplaced or minimally displaced scaphoid fractures had better longer-term benefits than those treated in a cast.
The rate of union was similar between the surgical and cast treatments, with early fixation of those fractures that failed to unite. 3 Another study28 reported similar outcomes at 10 years.
Displaced fractures
A scaphoid fracture is considered displaced if there is a step or gap of ≥1 mm. 38 Angulation and rotation between fragments are more difficult to assess. A systematic review reported that non-union occurs in around 18% of displaced fractures39 and that, when treated in a cast, the relative risk of non-union between undisplaced and displaced fractures is 4.4 [95% confidence interval (CI) 2.3 to 8.7]. 39 At present, the evidence of the treatment of displaced fractures is weak and recommendations are based on case series. 40 When the displacement is > 2 mm, clinicians consider the fracture so unstable that they usually recommend early reduction and fixation.
Increase in surgical activity
Despite insufficient evidence, there is an increasing trend41 to immediately fix scaphoid fracture rather than to immobilise in a cast for 6 weeks and fix only the 10–12% that fail to unite. 3 This current trend to fix fractures may be attributed to short-term benefits, but concerns remain about the lack of evidence on the long-term benefits and additional risks of surgery, such as malunion, infection, implant-related problems and avascular necrosis.
Hospital Episode Statistics for NHS hospitals in England recorded a two-third increase in acute scaphoid fracture fixations between 2007/8 and 2009/10 (1534, 1720 and 2582 in 2007/8, 2008/9 and 2009/10, respectively), before this study was commissioned. The rate of surgical fixation42 rose very slightly from 37% to 41% from 2007/8 to 2008/9 but then increased sharply to 62% in 2009/10. This trend of an increasing intervention rate emphasised the need for this study.
Economic aspects
There is also a lack of information on the economic aspects43 of this injury and its treatment. One study used a decision-analytic model43 and utility scores were obtained from 50 medical students. This study concluded that early fixation provided more quality-adjusted life-years (QALYs) and consumed fewer economic resources than cast treatment. However, a different view has also been suggested,44,45 namely that cast treatment is economically less costly. This conclusion came from studies of both non-randomised (95 patients) and randomised evidence (52 patients), based on a comparison of direct and indirect costs in patients who had their fracture treated in a cast or had it fixed.
What do patients feel and experience?
There is little published evidence on patients’ experiences and preferences after a scaphoid fracture. Understanding our patients’ priorities helps efficient patient-centred clinical decision-making. We know little about patients’ experience of their recovery and the impact that the injury and treatment have on them. We also have a poor understanding of the issues pertinent to recruiting participants in surgical clinical trials. 46,47
Five-year review
The long-term consequences of cast immobilisation and internal fixation have not been adequately determined in RCTs. The consolidation of partial union of the fracture has not been investigated, nor has the progression of carpal malalignment or the development of arthritis. Although this report focuses on outcomes at 52 weeks, the study will investigate function, impairment and arthritis at 5 years. 48
Null hypothesis
There is no difference in the Patient-Rated Wrist Evaluation (PRWE)49 score at the 52-week follow-up between adults with a scaphoid waist fracture treated with screw fixation versus those treated with plaster cast immobilisation and with fixation of only those fractures that fail to unite.
Research question
Our aim was to determine the clinical effectiveness and cost-effectiveness of surgical fixation versus plaster cast treatment (with early fixation of 10–12% of fractures that fail to unite) of scaphoid waist fractures in adults in an adequately powered multicentre pragmatic RCT [the Scaphoid Waist Internal Fixation for Fractures Trial (SWIFFT)]48 and to qualitatively investigate patients’ experiences of their treatment and participation in the trial.
Research objectives
Our primary objective was to determine the effectiveness of surgical fixation versus non-operative plaster cast treatment (with fixation of those that fail to unite, estimated to be 10–12% of the total) of scaphoid waist fractures in adults. The outcome was assessed using the PRWE50 (a patient self-reported assessment of wrist pain and function) at 52 weeks, which was the primary end point. The PRWE was also completed at 6, 12 and 26 weeks and will be completed at 5 years. The power of the study permitted identification of a clinically meaningful difference of 6 points in the PRWE.
Our secondary objectives were to:
-
assess radiological union of the fracture at 52 weeks using radiographs and CT scans; recovery of wrist range and strength; return to work and unpaid recreational activities; and complications
-
conduct an economic analysis to investigate the cost-effectiveness of surgical fixation versus initial immobilisation in a plaster cast
-
qualitatively explore patients’ experiences of fracture and its treatment and to investigate attitudes towards, and experiences of, participating in a surgical clinical research trial
-
undertake a 5-year clinical review of all trial participants to determine the long-term consequences of cast immobilisation and internal fixation.
Chapter 2 Trial design and methods
This chapter describes the trial design and methods used to address the objectives regarding the clinical effectiveness of the health-care interventions being compared. The methods of the health economic evaluation and the nested qualitative study are described in their dedicated chapters.
Trial design
This was a multicentre, stratified [displacement present or not, with equal allocation (1 : 1)], parallel-group design conducted in England and Wales among patients aged ≥ 16 years with a clear bicortical fracture of the scaphoid waist as seen on plain radiographs. Patients were randomly assigned to either immediate surgical fixation or initial non-operative wrist immobilisation in a below-elbow cast with later surgical fixation of only those fractures that failed to unite.
Participants
The diagnosis of fracture was on standard radiographic views available at each hospital (i.e. posterior–anterior, lateral, 45° semiprone, 45° semisupine) and an elongated scaphoid view (e.g. Ziter51). If these radiographic views were not taken routinely at a participating site, we sought to obtain them after the patient had consented in the trial to confirm eligibility before randomisation.
A CT scan was also taken at baseline to compare with the CT scan at 52 weeks for the bone union assessment. The baseline CT scan was taken within 2 weeks of the patient’s injury (i.e. before randomisation) or, if that was not feasible, before surgery if the patient was allocated to surgery. It was decided that, although eligibility assessments should be based on the radiographic views, because a baseline CT scan was available, it was necessary for a member of the radiology team to confirm whether a fracture was visible or not. If the baseline CT scan was viewed before randomisation, and a member of the radiology team could not confirm to the participating site staff that there was a clear bicortical fracture of the scaphoid waist, the surgeon would decide whether or not to continue to recruit the patient. This was to prevent the potential for an immediate crossover to plaster cast if the surgeon thought there was a not a sufficiently visible fracture to operate on. If this happened after randomisation, the patient remained in the trial because there was a fracture on the series of radiographic views. This could, however, influence the surgeon’s decision to continue to operate on the patient when allocated to surgery (i.e. could lead to a crossover).
Inclusion criteria
Patients were eligible for the trial if they:
-
were skeletally mature and aged ≥ 16 years
-
presented at a participating site within 2 weeks of their injury and within which time it was feasible to have surgery
-
had a clear, unequivocal bicortical fracture of the scaphoid waist seen on a series of plain radiographs of the scaphoid that:
-
– did not involve the proximal pole (the proximal 20% of the scaphoid) and
-
– included minimally displaced fractures with a step or gap of ≤ 2 mm on any radiographic view.
-
Exclusion criteria
Patients were excluded from the trial if:
-
their fracture had > 2-mm displacement, as these fractures are likely to be unstable and require surgical intervention
-
they had a concurrent wrist fracture in the opposite limb
-
they had a trans-scaphoid perilunate dislocation
-
they had multiple injuries in the same limb
-
they lacked the mental capacity to comply with treatment or data collection
-
they were pregnant, because radiation exposure would be contraindicated
-
they were not resident in the trauma catchment area of a participating site to allow follow-up.
Setting
The trial recruited from the orthopaedic departments of 30 NHS hospitals in England and one hospital in Wales. There were three additional hospitals in England that screened for patients but did not recruit to the trial. Recruitment started in July 2013 and the final follow-up was in September 2017. All 34 participating trusts are listed in Appendix 1.
Interventions
Cast treatment followed by surgical fixation if there is confirmed non-union
The control treatment was non-operative, with wrist immobilisation in a below-elbow cast for 6–10 weeks, followed by mobilisation. The below-elbow cast could include the thumb or not, as this does not affect union rate. 8 Early CT was obtained if plain radiographs at 6–12 weeks raised any suspicion of non-union. If non-union was confirmed on radiographs and/or CT scans, urgent surgical fixation was expected to be performed and was encouraged by the trial team, which monitored this with the completion of the 6- and 12-week treatment confirmation forms (see Report Supplementary Materials 1 and 2). The surgical procedure to treat a non-union and postoperative care were similar to the surgical arm of this trial. Cast immobilisation, identification and confirmation of non-union at 6–12 weeks, and immediate surgical fixation of confirmed non-union is the current standard non-operative pathway. 3 We anticipated that most, if not all, fixation of confirmed non-union would occur by around 12 weeks.
Surgical fixation
Surgical treatment was by percutaneous or open surgical fixation depending on the surgeon’s preferred technique. Standard CE-marked headless compression screws18,52 were used to avoid the pressure effects of the screw head on articular cartilage. These are standard surgical techniques. 20–22 The type of implant used was not restricted, but the screw used was recorded (see Report Supplementary Material 3). The surgical approach or the postoperative care was not specified and was instead left to clinical discretion, as it was expected that most surgeons would use some splintage for the first few weeks after surgery. The application of a plaster cast or splint following surgery, and its duration, was recorded. At each recruiting site, it was agreed which surgeons would fix the scaphoid fractures and that surgeons would use techniques with which they were familiar to avoid learning-curve problems.
Rehabilitation
All participants randomised into the two groups received standardised, written physiotherapy advice detailing the exercises they needed to perform for rehabilitation following their injury (see Report Supplementary Material 4). All participants were advised to move their shoulder, elbow and finger joints fully within the limits of their comfort. Those participants treated in a cast performed range-of-movement exercises at the wrist as soon as their plaster cast was removed at the 6-week follow-up appointment as long as there were no concerns regarding bone union. Those participants who had the fracture fixed began their wrist exercises as soon as comfort permitted, if they did not have a plaster cast, or as soon as the cast was removed. Any other rehabilitation input beyond the written information sheet (including a formal referral to physiotherapy) was the decision of the treating clinicians. A record of any additional rehabilitation input (including the reason for referral and number of appointments) was recorded at the 52-week hospital visit.
Outcomes
The outcomes and the time points when various outcomes were assessed are described below.
Primary outcome
The primary outcome and end point for the trial was the PRWE total score at 52 weeks from randomisation. The PRWE was completed at baseline for the time before and after injury, and at 6, 12, 26 and 52 weeks, and will be completed 5 years after randomisation. The PRWE is a 15-item questionnaire that is completed by the participant. It is a brief, reliable and valid instrument for assessing wrist pain and disability. 50,53 Scoring for all the questions is on a 10-point ordered scale, ranging from ‘no pain’ or ‘no difficultly’ (0) to ‘worst ever pain’ or ‘unable to do’ (10). A total score can be computed on a scale of 0–100 (0 = no disability), as well as two non-overlapping subscales (pain and function), which are weighted equally. The PRWE score was chosen as the primary outcome because patient-reported functional outcomes are favoured for decision-making and it allows the assessment of both wrist pain and function.
Two small RCTs3,18 of patients with scaphoid fractures have demonstrated that there is little change in objective and subjective outcomes between 26 and 52 weeks. In the case of the 10–12% of patients who are treated initially in cast but do not heal, surgery should be performed between 6 and 12 weeks after randomisation. Therefore, if assessed at 26 weeks, this would leave only 14–20 weeks for healing and recovery to take place. To allow all patients the time to heal from surgery and to stabilise after any complications, 52 weeks was chosen as the primary end point.
Secondary outcomes
The secondary outcomes were health-related quality of life (HRQoL), bone union, range of movement and grip strength, return to work and unpaid recreational activities, and complications.
Health-related quality of life
Patient-Rated Wrist Evaluation
The total PRWE scores at the other time points (6, 12, and 26 weeks) and the PRWE subscale scores of pain and function were secondary outcomes.
Short Form questionnaire 12-items
The Short Form questionnaire 12-items (SF-12) is a generic patient-reported outcome measure of physical and mental health, the population norms of which have a mean of 50 points and a standard deviation of 10 points; higher scores indicate better health. 54 The SF-12 was completed at 6, 12, 26 and 52 weeks to measure the potential broader consequences of a scaphoid fracture on participants’ physical and mental health.
EuroQol
The EuroQol-5 Dimensions, three-level version (EQ-5D-3L), is a validated, generic patient-reported outcome measure covering five health domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with three response options to each domain. 55,56 The use of this non-fracture-specific instrument allows the assessment of HRQoL outcomes in the health economic analysis. The EQ-5D-3L has high validity and reliability in proximal humerus fractures57 and hip fractures. 58 It was completed at baseline and at 6, 12, 26 and 52 weeks.
Bone union
The secondary outcome of bone union59 was determined at 52 weeks (in line with the primary end point). A CT scan and a series of plain radiographs were obtained comprising posterior–anterior, lateral, 45° semiprone and 45° semisupine views and an elongated scaphoid view (e.g. Ziter-type view). Routine radiographs were also collected for bone union assessment at 6- and 12-week hospital clinics.
Union was defined as complete disappearance of the fracture line8 on radiographs and complete bridging on CT scans60,61 in comparison with those taken at baseline. Partial union was based on the proportion of the fracture plane traversed by bridging trabeculae on true sagittal and coronal multiplanar reconstructions (MPRs) of the scaphoid on CT. CT was used to determine non-union, as there is only poor to moderate inter-observer agreement (range of kappa from 0.11 to 0.53) when determining the union of a scaphoid fracture on plain radiographs. 62
Scaphoid fracture displacement was assessed on radiographs and on a CT scan. 63
Malunion was assessed on the 52-week CT scan. 64 Malunion was defined as a ratio of scaphoid height to length ≥ 0.6 in the true sagittal axis of the scaphoid, to assess any humpback deformity. 65
A limitation of this assessment of bone union, however, was that the presence of the screw to fix the fracture would unblind the observer regarding whether the participant had had an operation or not. To minimise the potential for this to introduce bias, two consultant radiologists with a special interest in musculoskeletal radiology and a consultant orthopaedic surgeon (chief investigator) interpreted the plain radiographs and CT scans independently of each other. All three met to discuss cases in which there was discordance in line with the rules defined in the standard operating procedure (see Report Supplementary Material 5). The two radiologists were both employed at participating hospital sites (Leicester and Birmingham). During the trial, they did not report on plain radiographs or CT scans of the scaphoid during clinical practice, in an attempt to maintain independence when reporting on the imaging of trial participants.
Range of movement and grip strength
The range of movement of both wrists was measured using a goniometer66 and the grip strength of both hands was measured using a calibrated Jamar dynamometer. 67–70 Both were recorded at baseline and at 6, 12 and 52 weeks (see Report Supplementary Material 6). The measurements were performed with the subject seated and their arm by their side, their elbow bent at 90° and their wrist in a neutral position for rotation. 71 Staff were advised to use the second setting on the Jamar dynamometer, except for when testing participants with large hands, when the third setting was used. 72 The Beighton joint laxity score (excluding the thumb count for the injured wrist) was recorded at baseline to measure the hypermobility of joints. 73 These assessments were standardised across participating sites using an instruction sheet (see Report Supplementary Material 7).
Return to work and unpaid recreational activities
This was established through participant self-reporting on the number of days off work and their ability to perform usual activities when at work and when performing unpaid recreational activities. This was recorded at the 6-, 12-, 26- and 52-week follow-ups.
Complications
Expected and unexpected complications were recorded at the 6-, 12- and 52-week hospital visits (see Report Supplementary Material 8). The expected complications included:
-
infection, defined as ‘surgical site infection’74
-
delayed wound healing, defined as any wound that had not healed by 2 weeks
-
complex regional pain syndrome (CRPS), defined as a puffy, painful swelling of the whole hand restricting full tuck of the fingers at 2 weeks
-
nerve events (hypoaesthesia or numbness in the territory of the palmar cutaneous branch of the median nerve, superficial division of the radial nerve or the median nerve)
-
vessel events [large (> 2 cm) haematoma in the line of the radial artery]
-
screw-related complications (protrusion of either end into the adjacent joint, fracture or bending of the screw, a radiolucent halo around any part of the screw > 1 mm, screw backing out or moving)
-
degenerative changes in the adjacent joints75
-
avascular necrosis76 of the proximal pole of the scaphoid.
In addition, the three raters reviewed the imaging at each time point for complications. This included assessing the presence (or not) of OA, the presence (or not) of screw penetration, screw lucency (none, < 1 mm or 1–2 mm) and avascular necrosis (no radiodensity, just radiodense, marked radiodensity on one view or one MPR, or marked radiodensity on more than one view or MPR).
Sample size
For surgery to justify its increased costs and the exposure to risk, it must result in greater or quicker improvement in patients’ wrist symptoms and function than non-operative management. A 6-point improvement in the PRWE score in the surgery group (compared with the controls) was chosen to be the minimally clinical important difference. The standard deviation of the PRWE score at 52 weeks was taken to be 20 points based on the PRWE user manual. 49 This figure was reported for distal radius fracture rather than scaphoid fracture at 6 months. The only published evidence for scaphoid fracture implies a standard deviation in the range of 8–10 points;28 however, this estimate was at a median of 10 years after the patient’s injury. To be conservative, a standard deviation of 20 was chosen. This gives a standard effect size of 0.3 for the 6-point PRWE difference. A superiority design was used to observe an effect size of 0.3 at 80% power using a two-sided 5% significance level requiring 350 participants in total. After allowing for 20% attrition, the recruitment target was 438 participants (219 surgery and 219 plaster cast). The estimate of attrition was expected to be realistic, given that four previous RCTs (three studies had a single centre and one study had two centres) of the treatment of scaphoid fractures had reported response rates for completion of patient-reported functional outcomes to be between 77% and 100%. 31
There were no planned interim analyses for the trial or stopping guidelines. There was, however, an internal pilot study from which the data contributed to the final analyses. The primary reason for the pilot study was to check the assumptions about the site set-up, patient recruitment and the feasibility of the trial. The independent oversight committees reviewed the progress that was made during the internal pilot and recommended that the trial continue with the planned increase in the number of sites. In the absence of a standard deviation for the primary outcome at 52 weeks, this was estimated from the participants who were recruited into the internal pilot study. This estimate corroborated the standard deviation chosen for the sample size calculation.
Recruitment
A research nurse identified patients who were potentially eligible for fracture clinics, referred from accident and emergency (A&E) departments or other sources (e.g. walk-in centres, cottage hospitals). The orthopaedic surgeon confirmed eligibility (see Report Supplementary Material 9) and invited the patient to consider joining the study. The research nurse or clinician provided the patient with an information sheet (see Report Supplementary Material 10) and answered any questions. The patient was asked to consent at that time or was offered up to 48 hours to discuss the study with family or friends before deciding whether to take part or not. When patients gave consent (see Report Supplementary Material 11), they were asked to complete a baseline form (see Report Supplementary Material 12). The site staff then contacted York Trials Unit (YTU), either by telephone or via the internet, to access the secure randomisation service. For patients who did not consent, a form was completed to record the following aspects: reasons for non-consent, patient and surgeon treatment preferences, and the agreed treatment plan (see Report Supplementary Material 13). Both patients who consented to take part in the trial [using the main trial information sheet (see Report Supplementary Material 10)] and those who did not consent to take part in the trial [using a separate information sheet (see Report Supplementary Material 14)] were invited to take part in an interview. This is explained further in Chapter 5.
Strategies for achieving adequate participant enrolment to reach the target sample size included seeking advice from a patient focus group, sharing best practice with the research nurses at participating sites, and biannual discussions with our principal investigators (PIs) at the scientific meetings of the British Society for Surgery of the Hand (BSSH). Hospital staff were provided with training about study procedures at the site initiation visits and with a trial site manual. During the trial, training sessions and reminders were communicated using e-mail bulletins, face-to-face meetings with the PIs at BSSH conferences and a training day with research nurses. In addition, the trial co-ordinators provided support and guidance to staff at participating sites (e.g. when new staff joined or replaced existing site staff) and also sought guidance from the chief investigator.
Randomisation
The randomisation sequence was based on a computer-generated randomisation algorithm provided by a remote randomisation service (telephone or online access) at YTU. The unit of randomisation was the individual patient on a 1 : 1 basis. As the non-union rate for displaced scaphoid fractures is 14%, compared with 10% for transverse undisplaced fractures,8,10,39 randomisation was stratified by the presence or not of displacement as assessed by the staff at the recruiting site. Random block sizes of 6 and 12 were used. Displacement was defined as a step or gap of 1–2 mm inclusive as seen on any radiographic view. The research nurse used the remote randomisation service to register eligible and consenting participants before computer generation of the allocation. The research nurse then informed the treating surgeon of the allocation. This ensured treatment concealment and immediate unbiased allocation.
Blinding
As the trial was pragmatic and compared surgery with initial cast treatment, blinding of participants and clinicians to treatment allocation was not possible. When possible, the treating surgeon took no part in the postoperative assessment of participants. The statistician was blind to group allocation until after data were hard locked and no further changes could be made. To minimise bias in the assessment of bone union, all radiographs and CT scans were assessed independently by two consultant musculoskeletal radiologists and a consultant orthopaedic surgeon (chief investigator). Any disagreement was resolved through discussion.
Statistical methods
Analyses were conducted using the principles of intention to treat (ITT), including all randomised participants in the groups to which they were allocated, where data were available. Analyses were conducted in Stata® v15 (StataCorp LP, College Station, TX, USA) using two-sided statistical tests assessed at the 5% significance level.
Recruitment
Site and patient recruitment was presented. The characteristics of the population of patients who were screened, ineligible and eligible, stratified by consent, were summarised. The flow of participants through the trial was presented in a Consolidated Standards of Reporting Trials (CONSORT) diagram.
Baseline characteristics of randomised participants
Baseline participant characteristics and fracture details were summarised descriptively overall and by randomised group, both ‘as randomised’ and ‘as analysed’, comparing the groups as included in the primary outcome analysis model (i.e. with full data for the baseline covariates and valid PRWE data for at least one post-randomisation time point). No formal statistical comparisons were undertaken on baseline data.
Follow-up
For each time point, the number of participant questionnaires sent and returned, with median [interquartile range (IQR)] days to completion and return, was presented by treatment group and overall. The number of questionnaires completed at home, over the telephone or in the hospital was reported. Return rates for hospital forms were tabulated by randomised group and time point.
Hospital visits
Participants were asked to attend a hospital follow-up visit at 6, 12 and 52 weeks post randomisation. Baseline participant characteristics and fracture details were summarised descriptively overall and by randomised group according to whether or not participants attended the hospital visit at each time point.
Compliance with random allocation and treatment received
The treatment received by participants in the two groups was summarised, with reasons given for any treatment crossover.
Primary outcome (Patient-Rated Wrist Evaluation) analysis
The PRWE was assessed at baseline (pre and post injury, prior to randomisation) and at 6, 12, 26 and 52 weeks post randomisation. The PRWE total score is a value between 0 and 100, where a higher score indicates worse pain and functioning. The score is computed by scoring the two subscales out of 50 (pain = sum of items 1–5; function = sum of items 6–15 divided by two) and summing them, so that pain and function problems are weighted equally. If there was up to one missing item in each subscale, then the missing item was replaced by the mean of the completed items within that subscale. 49 If two consecutive responses (between 0 and 10) to a particular item were selected, then the higher of the two (the worse response) was taken for analysis. Other ambiguous responses were treated as missing. If more than one item was missing in either subscale, then a score for that subscale, or for the total, could not be calculated.
The number and percentage of participants with valid and partial PRWE data were reported by randomised group and time point. Baseline participant characteristics and fracture details were summarised descriptively overall and by randomised group according to whether or not participants had valid PRWE outcome data at each post-randomisation time point. Total PRWE scores were summarised by time point according to whether or not participants had valid data for (1) all post-randomisation time points, (2) at least one, but not all, post-randomisation time points or (3) no post-randomisation time points. PRWE scores (subscales and total) were summarised descriptively by treatment group and overall.
Total PRWE scores were compared between the two groups using a covariance pattern, mixed-effect linear regression model incorporating all post-randomisation time points. Treatment group, time point, a treatment-by-time interaction, participant age at randomisation, baseline fracture displacement and dominance of injured hand were included as fixed effects, and participant was included as a random effect (repeated observations per participant). Fracture displacement was used as a stratification factor in the randomisation; however, there were several instances in which the wrong displacement category was used in the randomisation. Such stratification errors were identified when there was a discrepancy between the data provided at randomisation and the data recorded on the study eligibility form. If the randomisation data were incorrect, a file note was raised and the trial statistician was notified; if the data on the study eligibility form were incorrect, the form was amended. The displacement used in the randomisation was the variable included in the primary analysis, but the errors were discussed. The primary model was not adjusted for baseline total PRWE, as, in this young population, we expected pre-injury PRWE score to be low and have little variability and post-injury PRWE score to be confounded, as patients would most likely be in a plaster cast.
An unstructured covariance pattern for the correlation between the observations for a participant over time was specified in the final model based on Akaike information criterion (AIC)77 (smaller value preferred).
An estimate of the difference between treatment groups in total PRWE score was extracted from the repeated measures model for each time point, and overall, with a 95% CI and p-value. The primary end point was the treatment effect estimate at 52 weeks. Estimates for the treatment effect at other time points (6, 12 and 26 weeks) and overall served as secondary outcomes. This repeated-measures approach is more efficient and parsimonious than conducting separate linear regressions for each time point, and also allows for the ‘overall’ (across the whole 52 weeks) effect to be investigated using the same model. The analysis takes advantage of the extra information provided at, and the correlation between, all post-randomisation time points. The standard errors for treatment effects at individual time points were calculated using information from all time points and were hence more robust and accurate than standard errors calculated from separate analyses at each time point. An additional advantage of the mixed model approach is that the presence of missing data across time points does not cause a problem under the assumption that they are missing at random. For the estimates at a single time point, say at 52 weeks, it is primarily the observations at that time point that determine the treatment effect and power; however, owing to the covariance between the post-randomisation time points, greater power and precision is obtained than that from a comparison using only the 52-week data. 78
Model assumptions were checked as follows: the normality of the standardised residuals was checked using a quantile–quantile plot and homoscedasticity was assessed by means of a scatterplot of the standardised residuals against fitted values.
Sensitivity analyses
Missing data
Any response bias was partially minimised by using a mixed-effect, repeated measures model, which allows the inclusion of intermittent responders in the primary analysis. Multiple imputation by chained equations was also used to handle missing PRWE outcome data. Missing outcome and covariate data were predicted by age, fracture displacement, hand dominance and available PRWE data at other follow-up time points. A ‘burn-in’ of 10 was used and 20 imputed data sets were created. Separate linear regressions were then run on the multiply imputed data set to compare the PRWE between the two groups at 6, 12, 26 and 52 weeks, adjusting for age, displacement and dominance of injured wrist. Estimates were combined using Rubin’s rules via the ‘mi estimate’ command in Stata. 79
Handling multisite data
Participants were recruited from multiple sites. To investigate whether or not site affects the outcome, a sensitivity analysis was conducted including site as a random effect (within which participants were nested) in the model as described for the primary analysis. 80
Timing of data collection
The primary analysis model was repeated including only data collected 1 week either side of the 6-week time point, 2 weeks either side of the 12-week time point, 6 weeks either side of the 26-week time point and 8 weeks either side of the 52-week time point.
Post hoc sensitivity analysis including smoking status
Current smoking status (yes/no) was included as a covariate in the primary analysis model in a post hoc sensitivity check, as this factor was found to be imbalanced by chance at baseline between the randomised groups and thought to be associated with healing and complications. The decision to conduct this sensitivity analysis was taken after the primary analysis results were known; this decision was not prespecified in the statistical analysis plan.
Displacement and lack of fracture as assessed by independent review of baseline imaging data
Discrepancies were reported between the displacement of the fracture (< 1 mm or 1–2 mm inclusive) as judged by the treating clinician on plain radiographs and as used for the randomisation and the judgement agreed on by three independent reviewers using the baseline CT scans and radiographs. The primary analysis model was rerun including a variable indicating the level of displacement judged by the three raters instead of that on which randomisation was based.
We report the numbers of cases in which a consensus was reached between the three raters that (1) there was no fracture or (2) displacement of the fracture was > 2 mm, based on their assessment of the baseline radiographs/CT scans, as these were exclusion criteria. Separate sensitivity analyses of the primary outcome model were conducted excluding these patients.
Complier-average causal effect analysis
To account for non-compliance (surgery to plaster cast) and contamination (plaster cast to surgery), a complier-average causal effect (CACE) analysis was conducted. A two-stage least squares instrumental variable approach81,82 was used with randomised treatment as the instrumental variable (implemented using the ‘ivregress’ command in Stata) to compare PRWE scores at 52 weeks, adjusting for age, fracture displacement and hand dominance. For this analysis, it was assumed that, had they been offered surgery, participants allocated to the plaster cast group would have the same probability of non-compliance as those allocated to the surgery group; likewise, it was assumed that, had they been offered non-surgical management, participants allocated to the surgery group would have the same probability of contamination as those allocated to the plaster cast group. Finally, it was assumed that simply being offered the allocated treatment has no effect on the outcome. These assumptions were plausible under randomisation, which should balance covariates across the two groups.
Subgroup analysis
In total, three subgroup analyses were undertaken: one exploring patient treatment preferences as expressed at baseline and two exploring fracture displacement. Owing to the errors in classification of fracture displacement at randomisation, two approaches for the displacement subgroup analysis were taken: one using displacement as defined at randomisation and one using the classification given on the study eligibility forms. Total PRWE scores are summarised by randomised group and time point, stratified by the levels of the baseline factor of interest. To investigate whether the treatment effect varied across the levels of these baseline factors, the factor was included in the primary analysis model alongside an interaction between randomised treatment allocation and baseline factor, using a two-sided p-value of 0.05. Interpretation of these models was made cautiously, as the trial was not powered for interactions. 83,84
Descriptive summaries of baseline participant and fracture data and PRWE scores are presented for:
-
participants in the plaster cast arm stratified by whether or not they needed surgery owing to non-union
-
participants in the surgery arm stratified by whether or not the surgical screw used was too long and caused cartilage damage, as determined on the CT scans by the three independent raters.
There were two feasibility requirements for this trial: (1) that a CT scan was performed within 2 weeks of a patient’s injury (or before surgery if this occurred earlier) and (2) that, for patients in the surgery arm, surgery was performed within 2 weeks after presentation to A&E or another clinic. It was considered a protocol deviation if these tasks were performed outside these parameters. A linear regression for the surgery arm only investigated whether the time from injury to surgery in days was predictive of total PRWE score at 52 weeks, adjusted for age, fracture displacement and hand dominance. Descriptive summaries of baseline participant and fracture data and PRWE scores are presented for:
-
participants stratified by whether or not their CT scan was performed within 2 weeks of the injury (or before surgery if this occurred earlier)
-
participants in the surgery arm stratified by whether or not their surgery was performed outside the target 2-week period from first presenting at A&E or another clinic.
Secondary analysis
Secondary outcomes
The secondary outcomes, namely the pain and function subscales of the PRWE, the physical and mental health component summaries of the SF-12 and grip strength, were summarised descriptively for each time point by treatment group and overall, and were analysed using the same method as for the primary outcome, adjusting for the same covariates. The most appropriate choice of covariance structure was identified separately for use with each outcome, which was always an unstructured pattern.
Union
Plain radiographs at 6, 12 and 52 weeks and CT images at 52 weeks were reviewed by three independent raters at the end of the trial for union of the fracture. Union, as reviewed on CT images at 52 weeks, was measured as a percentage (0–100%) and categorised as 0% (non-union), > 0–20% (slight union), > 20–70% (partial union), > 70–100% (but not including 100%; mostly united) and 100% (complete union). The same categories of union were used for radiograph images at 6, 12 and 52 weeks. The extent of union was presented for each time point by randomised group. Participants were dichotomised at each time point as ‘probably need surgery’ (non-union and slight union) and ‘probably do not need surgery’ (partial to complete union) and analysed using a logistic regression model (52-week data only). It was originally planned that a mixed-effect logistic regression model would also be conducted to compare the treatment groups at 52 weeks, with participant as a random effect to account for the repeated measures of union at 6, 12 and 52 weeks, adjusting for age, displacement and dominance of injured wrist as fixed effects. However, this model did not converge. As an alternative sensitivity check, multiple imputation was used to impute the dichotomised union variables at 6, 12 and 52 weeks (imputation included union variables, age, displacement, dominance of injured wrist and allocation). A ‘burn-in’ of 10 was used and 20 imputed data sets were created. A logistic regression was run on the multiply imputed data set to compare union between the two groups at 52 weeks, adjusting for age, displacement and dominance of injured wrist.
The 52-week PRWE scores for patients overall and for patients who did and did not attend imaging at 52 weeks were summarised by treatment group.
Malunion
Scaphoid height and length were measured by the three independent raters of the CT images and plain radiographs. Malunion was defined using a ratio of scaphoid height to length of > 0.6. During the study, literature was published that suggested that a threshold ratio of 0.7 might be more appropriate85 than 0.6. 64 Rates of malunion at the 0.6 and 0.7 thresholds are presented overall and for each treatment group at 6, 12 and 52 weeks.
Complications
Complications were defined as medical, surgical and plaster cast related, and were assessed by clinical examination at 6, 12 and 52 weeks. The number of patients who experienced a complication of a certain type was summarised by randomised group. The presence of any medical, surgical or cast complication recorded on the complications form up to 52 weeks was analysed by logistic regression, adjusting for age, hand dominance and fracture displacement.
Cases in which two out of the three raters agreed, based on the imaging, that there was a complication were reported.
Adverse events
All serious and non-serious adverse events (AEs) were summarised by treatment group.
Agreement analysis
A descriptive analysis of agreement between the three raters was performed. Radiographs provide categorical grades of fracture, displacement and union and these were summarised by cross-tabulating the results from each pair of raters and calculating their percentage agreement. The CT scans provide continuous measures of displacement and of the percentage of union. Agreement between these continuous measures was summarised in Bland–Altman plots that compared each pair of raters, and the limits of agreement were calculated. For all numerical summaries, 95% CIs were provided.
Data management
All hospital forms, imaging compact discs and participant questionnaires were sent from and returned to YTU. A central database at YTU was used to prompt the sending out and return of participant questionnaires and hospital case report forms (CRFs). This included automated e-mail reminders sent to participating sites to help ensure the timely return of hospital CRFs.
Essential trial documentation, which individually and collectively permits evaluation of the conduct of a clinical trial and the quality of the data produced, was kept with the Trial Master File and Investigator Site Files. This documentation will be retained for a minimum of 5 years after the conclusion of the trial in accordance with Good Clinical Practice. The postal questionnaires and hospital CRFs will be stored for a minimum of 5 years after the conclusion of the trial as paper records and for a minimum of 20 years in electronic format. 86
Design of patient questionnaires and hospital case report forms
The patient questionnaires and hospital CRFs were designed using TeleForm software (version 10; Cardiff Software, Cambridge, UK). Specification CRFs were populated with variable names and appropriate scoring. To maximise data quality, when hospital CRFs were returned to YTU, key variables required for the statistical analysis were reviewed for completion and accuracy by a trial co-ordinator/research data administrator who resolved any queries with the research nurse at the site. The hospital sites were reimbursed according to a payment schedule for the completion of CRFs and provision of research imaging up to a total of £430.30. This was agreed between each trust and trial sponsor using a Clinical Trial Agreement. No checks regarding the data quality of the postal questionnaires were made on immediate return to YTU. A trial co-ordinator did, however, as a duty of care, check if a participant had responded with the extreme answers to the following: questions 4a, 4b, 6a and 6b of the SF-12 and the final EQ-5D-3L question. The trial co-ordinator also checked free-text responses to see if the participant could be at harm. When this occurred, the PI and research nurse at the recruiting site were notified by e-mail.
After this initial check, all postal questionnaires and hospital CRFs were prepared for scanning by a research data administrator using the TeleForm software. When a form would not scan, the data were manually entered. When a form was scanned, the data were then verified depending on what TeleForm identified as requiring correction. The verified data were then temporarily held in the download database and available for second checking. This involved each hard copy of all forms being compared against the entry stored in the download database and correcting the electronic data as necessary. All data were scanned, downloaded and second checked in the validate database. The automated data validation was undertaken by the data manager who applied predetermined rules to check for agreed variables to see if the data were recorded correctly. Data that had been validated were then held in the survey database and were available on a formal request from the trial statistician and health economist.
Strategies to follow up patients
Participants at the 6-, 12-, 26- and 52-week follow-ups were notified by post to expect the questionnaire (see Report Supplementary Material 15) 2 weeks before it was due. Reminder letters were sent 2 and 4 weeks after the due letter was sent out. When there was no response to reminder letters, a trial co-ordinator at YTU contacted participants by telephone, who were invited to provide answers for, as a minimum, the primary outcome (PRWE) and EQ-5D-3L. At 52 weeks only (the primary time point for the study), in addition to the telephone call at 6 weeks, a letter was sent to non-responding participants requesting that they complete the PRWE only. At 26 weeks, if participants did not attend a hospital appointment, an unconditional incentive of £5 was included with the postal questionnaire. At 52 weeks, a £40 payment was made to participants who attended the clinic. For the final 11 months of the follow-up period of the study, participants were also offered up to £40 to cover their travel expenses, or more if this was agreed with the trial team. The participants could complete the follow-up questionnaires during the clinic visits.
Various techniques were used to minimise loss to follow-up. This included regular participant newsletters, which publicised the progress of the study on the trial website (www.swifft.co.uk). A trial ‘tagline’ was placed on postal envelopes sent to participants to highlight the importance of their involvement in the research (i.e. ‘SWIFFT Study: Patients helping to improve healthcare through research’). As the return of postal questionnaires can be improved when participants are included in a prize draw,87 anyone who returned their questionnaire at 26 weeks could win an iPad® (Apple Inc., Cupertino, CA, USA) worth £500. Participants who attended their hospital clinic appointment at 52 weeks were also entered into a prize draw to win an iPad worth £500. If, at 52 weeks, it was difficult to arrange the hospital appointment, a letter was sent from the hospital to the participant along with a leaflet to encourage their attendance. Other strategies to collect follow-up data on participants who did not return their questionnaire or attend clinics included using a participating hospital’s Picture Archiving Communication System (PACS) for the local area/region to retrieve imaging of patients; accessing Summary Care Records to view participants’ addresses and/or the general practitioner (GP) they were registered with to help contact them; and asking a participant’s GP if they have had an operation on their scaphoid fracture.
A trial participant could withdraw from the study at any time for any reason, but any data collected up to that point was used in the analysis. A participant could withdraw from all data collection, or from postal questionnaire collection or hospital data collection only, which was recorded using a change in status form (see Report Supplementary Material 16).
Data management for the review of imaging
The forms used to capture assessments of the scaphoid fracture and conduct measurements on the imaging collected were created using the ‘Design’ module in Formic Fusion® software (5.5.1; Formic Limited, Middlesex, UK) and include the SWIFFT master radiograph form, SWIFFT baseline CT form and SWIFFT 52-week CT form.
Once completed by reviewers, variables within each form were checked visually for completeness and for whether or not measurements were within an expected range. Completed forms were then scanned using the Formic ‘Capture & Process’ module and responses were reviewed. The Formic scanning software flagged hand-written text and those checkboxes with no mark or more than one mark; these were manually corrected to reflect the entry on the form. The scanned image of the form was also held within the database to permit independent verification. Once exported to the ‘SWIFFT logging database’ (Microsoft Access®, Microsoft Corporation, Redmond, WA, USA), the data then went through a final electronic check to ensure all measurements were within the limits assigned by the chief investigator and to check for errors in measurements (e.g. cm instead of mm). The data were then checked for these rules in Stata v15 and potential problems were flagged and checked by each reviewer.
Data from the three reviewers were assessed and any classification conflicts on fracture displacement at baseline and state of the union at 52 weeks were identified and resolved in ‘conflict resolution meetings’ using specific forms (SWIFFT conflict CT form, SWIFFT conflict radiograph form and SWIFFT 52-week inter-observer conflict forms), which were used when conflicts were resolved. Predefined agreement rules were used to generate the final data based on all three reviewers’ assessments.
All conflicts identified were reviewed by all reviewers at face-to-face/teleconference meetings. All reviewers looked at the images together and a collective decision was made and recorded. From the 439 baseline radiographs reviewed, 106 were taken back to a conflict meeting. Of the 431 CT images reviewed at baseline, 155 were taken back to a conflict meeting to agree displacement thresholds. These baseline conflicts were reviewed over 26 meetings between September 2013 and February 2018. At 52 weeks, 297 radiographs and 292 CT scans were reviewed. All radiographs and CT scans at 52 weeks that were classified as a ‘non-union’ by any one reviewer were brought back to the conflict resolution meeting for confirmation. Of both radiographs and CT scans, only 17 were taken back to three conflict meetings to agree the state of the union.
Finally, the reviewers also agreed the cases that did not have an identifiable fracture on baseline radiographs (one) and CT scans (five). There were also a total of 52 conflicts identified on the categorical data at baseline. For baseline radiographs, 15 conflicts were identified from the categorical classification of the orientation of the fracture line. These were reviewed by all reviewers and were resolved. For baseline CT scans, 37 conflicts were identified involving the fracture line (27) and fracture location (10). All of these were reviewed at a conflict meeting in January 2018 and all were resolved.
Adverse event management
Adverse events were defined as any untoward medical occurrence in a trial participant that did not necessarily have a causal relationship with the treatment. Serious AEs (SAEs) were defined as any untoward medical occurrence that:
-
resulted in death
-
were life-threatening
-
required hospitalisation or prolongation of existing inpatient hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was a congenital anomaly or birth defect.
In addition, SAEs included any other important medical condition that, although not included in the above, may have required medical or surgical intervention to prevent one of the outcomes listed. (S)AEs related to scaphoid fracture and its treatment during the 12 months after randomisation were recorded by site PIs on a CRF (see Report Supplementary Materials 17 and 18). The trial office was notified of any SAEs within 24 hours of the PI becoming aware of them, and of any AEs within 5 days. The categorisation of causality and expectedness was confirmed by the chief investigator. AEs that were expected with this injury to the wrist and related to anaesthesia and/or surgery were infection, CRPS, screw-related complications, chondrolysis, delayed wound healing, nerve or vessel events, fracture of scaphoid tuberosity, and nausea and/or disorientation. AEs specific to the plaster cast were soft cast/broken cast that led to movement of the wrist, CRPS, pressure sores, nerve compression or pain due to a tight cast. Movement in a cast was considered untoward, as it could mean that the fracture was not properly immobilised and could result in failure to unite.
All (S)AEs were routinely reported to the Trial Management Group (TMG), Trial Steering Committee (TSC), Data Monitoring Committee (DMC) and sponsor. SAEs that were related to the research and unexpected were reported to the Research Ethics Committee (REC). The chief investigator reviewed all (S)AEs that were unresolved at initial reporting a month later. This was to ensure that adequate action had been taken. Additional reviews at 1-month intervals were conducted until the chief investigator confirmed that no further monitoring was required.
The chief investigator was also informed, by the reviewers of the radiographs/CT scans collected for the study, of any abnormalities identified. The chief investigator judged whether or not the abnormality was clinically important and could affect patient safety (e.g. a protruding screw). The need to notify the PI of the site, and whether or not to record this as an AE, was also considered. No actions or treatments were discussed with the PI.
Ethics approval and monitoring
Standard NHS cover for negligent harm was available. There was no cover for non-negligent harm.
Ethics committee approval and any changes to the project protocol
SWIFFT was approved by Derby Research Ethics Committee – East Midlands on 21 May 2013 (REC reference 13/EM/0154). NHS permission was given by the research and development department of each participating site. A summary of the changes made to the protocol since the original REC approval are listed in Appendix 2. The trial protocol was published in BMC Musculoskeletal Disorders. 48
Trial Management Group
The day-to-day management of the trial was overseen by the TMG, which met on a quarterly basis. A representative of the sponsor attended when available. These meetings monitored the progress as regards recruitment (e.g. enrolment, consent and eligibility), allocation to study groups, adherence of the trial interventions to the protocol, retention of trial participants, monitoring of (S)AEs and reasons for participant withdrawal. The review of progress was undertaken at the level of the participating site and, as necessary, feedback was given to the PI and research nurses at each site.
Trial Steering Committee
A TSC was appointed by the funding body to provide overall supervision of the trial and to advise on its continuation. Membership is listed in the Acknowledgements.
Data Monitoring Committee
The DMC was appointed by the funding body with access to the unblinded comparative data as provided by a statistician at YTU who was independent of the trial team. The DMC monitored the data and notified the TSC of whether or not there were any ethical or safety reasons why the trial should not continue. Membership is listed in the Acknowledgements.
Patient and public involvement
A patient who had a fracture of their left wrist and was treated at the sponsor site commented on the study documentation and was invited to TMG meetings. Alternatively, their opinion was sought outside these meetings. This patient also contributed to a video that was posted on the trial website, which was publicised in a newsletter to participants, to encourage completion of postal questionnaires and attendance at hospital visits. In Leicester, a group of eight individuals [six of whom had experience of a scaphoid fracture and two who had not but were typical of the patient population (i.e. male and aged under 30 years)] met to advise the TMG on strategies to maximise the retention of trial participants; these individuals were also contacted by e-mail for advice. 88 This meeting was led by the chief investigator, a senior qualitative researcher and the patient representative on the TMG. This group will also advise on the summary of the findings for trial participants and other dissemination activities. In one of our newsletters to participants, a photograph and positive feedback about their involvement in the trial was included from the participant who won the prize draw of the iPad at the 26-week follow-up. Two members of the Patient Liaison Group of the British Orthopaedic Association were independent members of the TSC and advised on strategies to maximise recruitment and retention.
Chapter 3 Clinical effectiveness results
Recruitment
Site recruitment
In our original protocol, we estimated that we would need 17 sites to recruit patients into SWIFFT. The number of sites was increased to meet the slightly lower than planned recruitment rate. Forty sites were approached to take part in SWIFFT between May 2013 and March 2015 and, in total, 34 sites were set up and opened to recruitment, the last of which commenced screening/recruitment in June 2015. Thirty-three sites screened at least one patient and 31 sites recruited at least one participant (median 10, range 1–61). Two of the three sites that did not recruit any participants screened one and two patients, respectively, one of which was eligible but non-consenting. The lead site, Leicester Royal Infirmary within the University Hospitals of Leicester NHS Trust, recruited the highest number of patients of all sites (n = 61). Table 1 presents the number of patients recruited by each site, ordered by when each site was set up to recruit (Leicester first), with sites identified at trust level. One hospital from each trust took part in SWIFFT. All sites agreed to recruit an average of one patient per month, but only two sites achieved this.
| Site | Date opened | Months open | Number recruited | Average number ofrecruits per month |
|---|---|---|---|---|
| University Hospitals of Leicester NHS Trust | 1 July 2013 | 37 | 61 | 1.6 |
| South Tees Hospitals NHS Foundation Trust | 17 August 2013 | 36 | 10 | 0.3 |
| University Hospitals Coventry and Warwickshire NHS Trust | 9 September 2013 | 35 | 24 | 0.7 |
| Nottingham University Hospitals NHS Trust | 25 September 2013 | 35 | 27 | 0.8 |
| Chelsea and Westminster Hospital NHS Foundation Trust | 19 November 2013 | 11a | 0 | 0.0 |
| Southport and Ormskirk Hospital NHS Trust | 21 November 2013 | 13a | 0 | 0.0 |
| Bolton NHS Foundation Trust | 2 December 2013 | 32 | 17 | 0.5 |
| Northumbria Healthcare NHS Foundation Trust | 6 February 2014 | 30 | 5 | 0.2 |
| Oxford University Hospitals NHS Trust | 7 February 2014 | 30 | 35 | 1.2 |
| University Hospital Southampton NHS Foundation Trust | 10 February 2014 | 30 | 17 | 0.6 |
| Royal Berkshire NHS Foundation Trust | 10 February 2014 | 30 | 9 | 0.3 |
| Salford Royal NHS Foundation Trust | 10 February 2014 | 30 | 4 | 0.1 |
| Brighton and Sussex University Hospitals NHS Trust | 26 February 2014 | 30 | 5 | 0.2 |
| Worcestershire Acute Hospitals NHS Trust | 10 March 2014 | 8a | 0 | 0.0 |
| Hampshire Hospitals NHS Foundation Trust | 17 March 2014 | 29 | 6 | 0.2 |
| Royal United Hospitals Bath NHS Trust | 17 March 2014 | 29 | 23 | 0.8 |
| Poole Hospital NHS Foundation Trust | 22 April 2014 | 28 | 5 | 0.2 |
| Royal Cornwall Hospitals NHS Trust | 9 May 2014 | 27 | 24 | 0.9 |
| Cardiff and Vale University Health Board | 12 May 2014 | 27 | 22 | 0.8 |
| Maidstone and Tunbridge Wells NHS Trust | 21 May 2014 | 27 | 5 | 0.2 |
| Taunton and Somerset NHS Foundation Trust | 1 June 2014 | 26 | 7 | 0.3 |
| University Hospitals Plymouth NHS Trust | 9 June 2014 | 26 | 20 | 0.8 |
| North West Anglia NHS Foundation Trust | 12 June 2014 | 26 | 17 | 0.7 |
| Cambridge University Hospitals NHS Foundation Trust | 16 June 2014 | 26 | 5 | 0.2 |
| The Royal Liverpool and Broadgreen University Hospitals NHS Trust | 17 June 2014 | 26 | 5 | 0.2 |
| Barts Health NHS Trust | 20 June 2014 | 26 | 24 | 0.9 |
| Gloucestershire Hospitals NHS Foundation Trust | 10 July 2014 | 25 | 15 | 0.6 |
| North Bristol NHS Trust | 1 September 2014 | 23 | 13 | 0.6 |
| University Hospitals Bristol NHS Foundation Trust | 6 October 2014 | 22 | 10 | 0.5 |
| Newcastle upon Tyne Hospitals NHS Foundation Trust | 10 November 2014 | 21 | 11 | 0.5 |
| Medway NHS Foundation Trust | 12 November 2014 | 21 | 2 | 0.1 |
| Lancashire Teaching Hospitals NHS Foundation Trust | 24 November 2014 | 21 | 1 | 0.05 |
| University Hospitals Birmingham NHS Foundation Trust | 16 February 2015 | 18 | 6 | 0.3 |
| King’s College Hospital NHS Foundation Trust | 22 June 2015 | 14 | 4 | 0.3 |
Patient recruitment
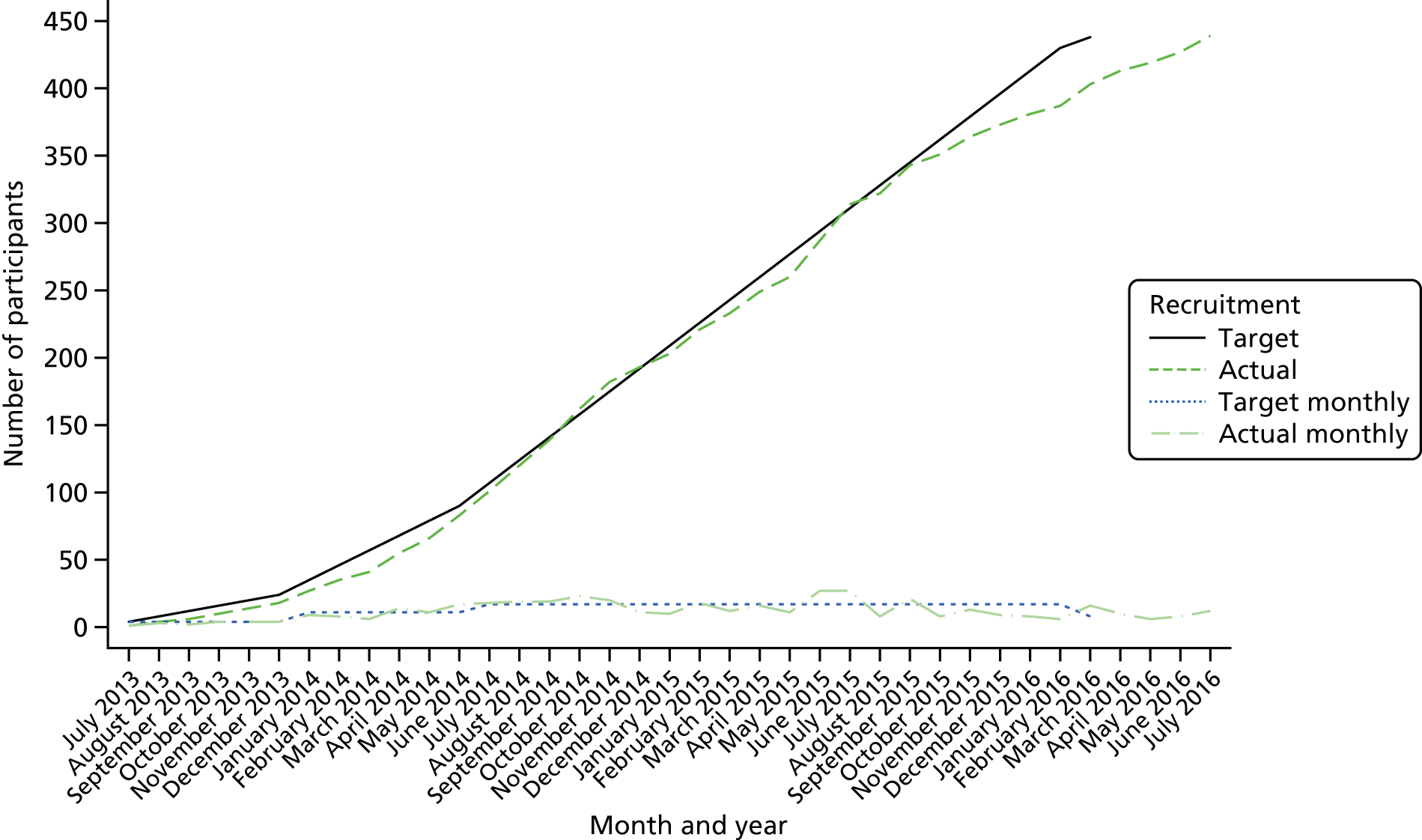
Our required sample size was 438 participants, which we aimed to achieve by the end of March 2016; however, recruitment was slightly slower than anticipated in the final few months and we agreed with the trial sponsor, the trial funder and the REC (February 2016) to extend recruitment beyond March 2016 until the 438 patients had been recruited.
The first participant was randomised on 23 July 2013. As of 31 July 2016, 439 patients had been enrolled in the trial and sites were notified that recruitment should cease; thus, patients were recruited over 37 months. Figures 3 and 4 demonstrate our final recruitment figures.
FIGURE 3.
Recruitment into SWIFFT by month.
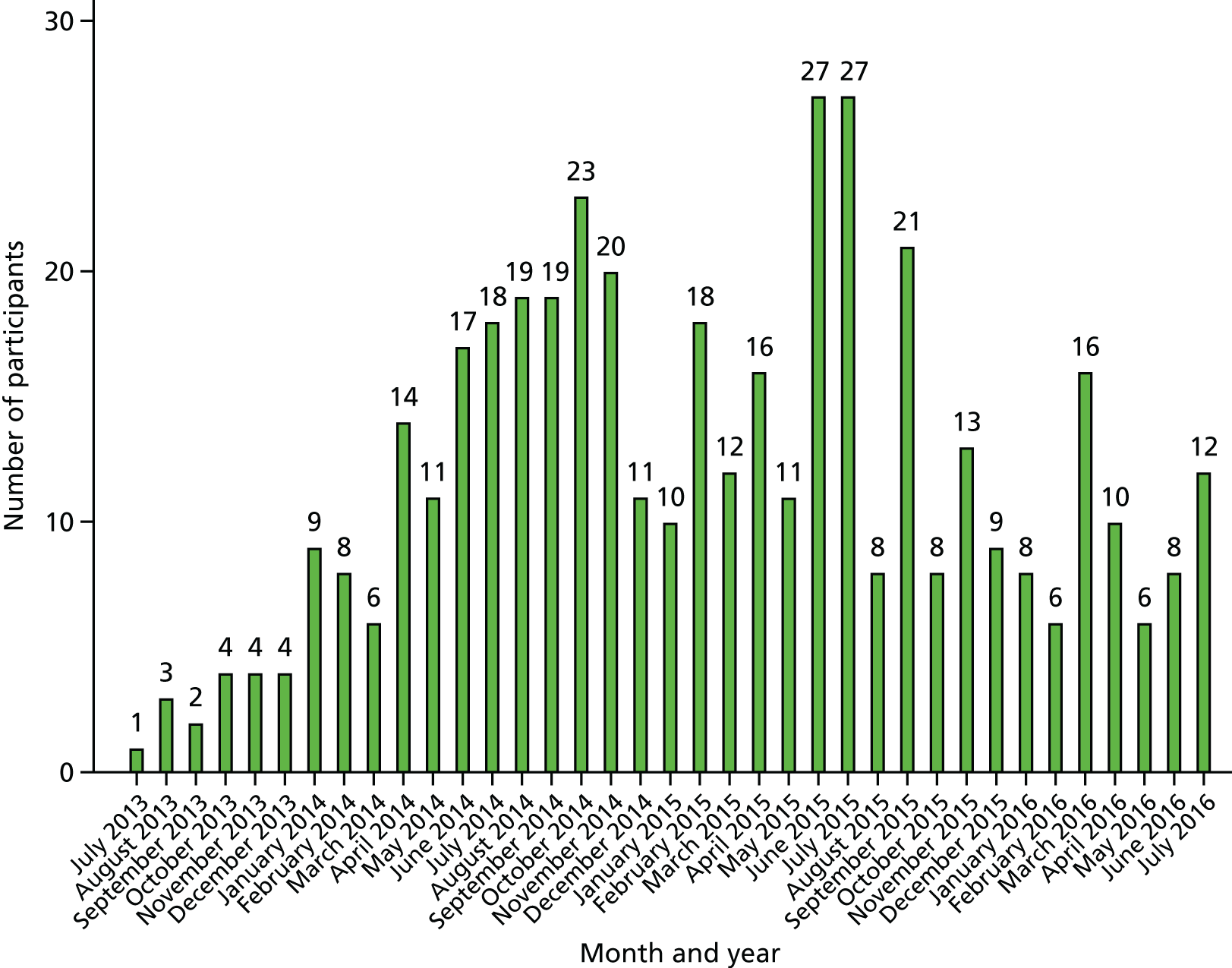
FIGURE 4.
Recruitment targets of participants into SWIFFT.
Characteristics of screened patients
A total of 1047 patients who met the inclusion criteria (aged 16 years or over and skeletally mature, presenting within 2 weeks of injury with a radiologically confirmed clear and bicortical fracture of the scaphoid waist that does not include the proximal pole) were assessed for participation in the trial from July 2013 to July 2016, of which 775 (74.0%) were eligible and 439 (41.9%) were eligible and consenting, the latter all being randomised. The number of participants screened per site ranged from 1 to 133 (median 23) and the percentage of screened participants who were eligible per site ranged from 0 to 100% (median 83.3%). Table 2 displays the characteristics of the different populations. Eligible patients tended to be younger and were more likely to be male and have a displaced fracture than ineligible patients. There were no marked differences between consenting and non-consenting patients in gender, age or time since injury; however, there was an indication that consenting patients were more likely to have a displaced fracture than non-consenting patients. The numbers of patients for whom each type of radiograph was used to determine eligibility, along with the percentage of those screened, are as follows: elongated scaphoid view (n = 910, 86.9%), posterior–anterior view (n = 1024, 97.8%), 45° semisupine view (n = 610, 58.3%), lateral view (n = 1017, 97.1%) and 45° semiprone view (n = 834, 79.7%). Screened patients had a median of four scaphoid radiographic views taken.
| Characteristic | Screened (N = 1047) | Ineligible (N = 272) | Eligible (N = 775) | |
|---|---|---|---|---|
| Non-consenting (N = 336) | Consenting (N = 439) | |||
| Gender, n (%) | ||||
| Male | 834 (79.7) | 203 (74.6) | 268 (79.8) | 363 (82.7) |
| Female | 210 (20.1) | 66 (24.3) | 68 (20.2) | 76 (17.3) |
| Missing | 3 (0.3) | 3 (1.1) | 0 (0.0) | 0 (0.0) |
| Age (years) | ||||
| n | 1040 | 266 | 335 | 439 |
| Mean (SD) | 33.7 (14.8) | 36.6 (17.5) | 32.5 (14.6) | 32.9 (12.7) |
| Median (min., max.) | 29.2 (16.0, 94.8) | 30.0 (16.2, 94.8) | 28.2 (16.0, 79.7) | 29.3 (16.1, 80.6) |
| Time since injury (days)a | ||||
| n | 1044 | 269 | 336 | 439 |
| Mean (SD) | 1.0 (1.8) | 1.2 (2.5) | 1.0 (1.5) | 0.8 (1.4) |
| Median (min., max.) | 0 (0, 14) | 0 (0, 14) | 1 (0, 9) | 1 (0, 10) |
| Displacement involvement,b n (%) | ||||
| Displacement | 342 (32.7) | 61 (22.4) | 111 (33.0) | 170 (38.7) |
| No displacement | 651 (62.2) | 160 (58.8) | 222 (66.1) | 269 (61.3) |
| Missing | 54 (5.2) | 51 (18.8) | 3 (0.9) | 0 (0.0) |
Reasons for exclusion
A total of 272 (26.0%) of the 1047 patients screened were ineligible for the trial for one or more reasons (Table 3). Twenty-nine of these should strictly never have been screened for participation in the trial, as they did not fulfil the inclusion criteria: their injury was more than 2 weeks old (n = 15), they did not have a radiologically confirmed bicortical fracture (n = 7), their fracture included the proximal pole (n = 6) or they were skeletally immature (n = 1). A further 156 patients failed at least one of the exclusion criteria, most commonly having a concurrent other injury in the same limb (n = 70). The remaining 87 were ineligible for other reasons: the site did not think it was feasible for the patient’s surgery (if allocated) to be scheduled within 2 weeks from presentation (n = 30), the patient was not approached about study (e.g. they presented at the weekend when there was no clinician available to confirm eligibility) (n = 21), the patient was deemed to be unsuitable for surgery (n = 16), the fracture was seen on radiography but not on the subsequent CT scan (n = 8) or other miscellaneous/unknown reasons (n = 12).
| Reason for ineligibility | Number of (%) ineligible patients (N = 272) |
|---|---|
| Exclusion criteria (N = 156) – reasons not mutually exclusive | |
| Fracture displaced by > 2 mm | 43 (15.8) |
| Concurrent wrist fracture in the opposite limb | 12 (4.4) |
| Trans-scaphoid perilunate dislocation | 8 (2.9) |
| Multiple injuries in the same limb | 70 (25.7) |
| Patient not a resident in the trauma centre catchment area of the participating site | 21 (7.7) |
| Previous injury or disease in the same wrist | 2 (0.7) |
| Patient lacks the mental capacity and is unable to understand the trial and instructions for treatment | 11 (4.0) |
| Patient is pregnant | 6 (2.2) |
| Other reasons (N = 116) | |
| Patient did not meet the inclusion criteria to be approached about the trial | 29 (10.7) |
| Presenting > 2 weeks after injury | 15 |
| No radiologically confirmed bicortical fracture | 7 |
| Fracture includes proximal pole | 6 |
| Aged < 16 years and/or skeletally immature | 1 |
| No fracture seen on CT scan prior to randomisation | 8 (2.9) |
| Surgery not feasible within 2 weeks of injury | 30 (11.0) |
| Patient not suitable for surgery | 16 (5.9) |
| Study not discussed with patient (e.g. patient presented at the weekend) | 21 (7.7) |
| Othera | 12 (4.4) |
Patient consent
The percentage of eligible patients who consented to take part in the trial varied in the 31 sites that recruited a patient from 13.9% to 100% (median 63.2%). A reason was provided for 325 of the 336 (96.7%) patients who did not consent to the study, despite being eligible: patient had a preference for non-operative treatment (n = 206), patient had a preference for surgery (n = 40), patient was unable to commit to follow-ups (n = 24), patient did not want to take part in research and/or was unhappy with the concept of random treatment allocation (n = 13), consent was not given in time to allow surgery (if allocated) to be conducted within 2 weeks of presentation at A&E or another clinic (n = 13), patient circumstances did not allow for surgery (if allocated) to be conducted within 2 weeks of presentation at A&E or another clinic (e.g. planned holiday or exams) (n = 7) and other miscellaneous reasons (e.g. patient trying to become pregnant, patient having multiple comorbidities, etc.) (n = 22).
Treatment preference data were collected for 320 of the 336 eligible but non-consenting patients: 30 (9.4%) had no treatment preference, 57 (17.8%) had a preference for surgery and 233 (72.8%) preferred not to have surgery. A response to the treatment that the surgeon advised the patient to have was provided for 325 patients: no surgery (n = 191, 58.8%), surgery (n = 32, 9.9%) and uncertain (n = 102, 31.4%). The agreed treatment for 328 of these patients was recorded: surgery (n = 45, 13.7%) and no surgery (n = 283, 86.3%).
In total, the median time spent obtaining consent from each participant to participate in the trial was 20 minutes. In general, approximately 10 more minutes were spent with participants who went on to be randomised than those who did not [randomised: mean [standard deviation (SD)] = 31.6 minutes (18.9 minutes), median = 30 minutes; not randomised: mean (SD) = 19.5 minutes (12.7 minutes), median = 20 minutes].
Participant flow
The flow of patients through the trial is summarised in a CONSORT flow diagram in Figure 5. Of the 439 randomised participants, 408 (92.9%) were eligible for inclusion in the primary analysis model (surgery arm: 203/219, 92.7%; non-surgery arm: 205/220, 93.2%). The trial was designed to allow for 20% attrition.
FIGURE 5.
The SWIFFT CONSORT flow diagram. Modified from Lancet, vol. 396, Dias JJ, Brealey SD, Fairhurst C, Amirfeyz R, Bhowal B, Blewitt N, et al. , Surgery versus cast immobilisation for adults with a bicortical fracture of the scaphoid waist (SWIFFT): a pragmatic, multicentre, open-label, randomised superiority trial, pp. 390–401,89 Copyright (2020), with permission from Elsevier.

Baseline characteristics of randomised participants
The 439 participants were randomised using equal allocation to surgery (n = 219) or plaster cast management (n = 220) of their fractures. The allocation of participants was stratified by fracture displacement at enrolment (< 1 mm or 1 to 2 mm inclusive). On 24 occasions, the incorrect displacement stratum was used in the randomisation: 16 participants with no fracture displacement were randomised within the displacement stratum and eight participants with displacement were randomised within the no displacement stratum [see Chapter 2, Primary outcome (Patient-Rated Wrist Evaluation) analysis]. A baseline questionnaire was not received from two participants (both allocated to the surgery group). The recruited participants were predominantly male (82.7%) and the average age was 32 years (range 16–80 years). Just over half of the participants (53.6%) had injured their left wrist and their non-dominant side (56.8%). Nearly half of the participants stated that they did not have a treatment preference at enrolment to the trial (47.6%), but, of the other 230 participants, most (84.3%) expressed a preference for surgery. This trend is substantially different from that observed in the population of eligible but non-consenting participants, who predominantly stated a preference to not receive surgery. The treatment groups as randomised and as analysed appear to be balanced on all measured baseline participant characteristics and fracture details (see Tables 4 and 5), with the exception of ethnicity, education and smoking status. Participants in the plaster cast group were more likely to have a degree or higher qualifications and were less likely to be smokers and to be white than those in the surgery group, both as randomised and as analysed. No formal statistical comparisons were conducted and so we cannot see if these differences are statistically significant, but these differences are consistent with a chance imbalance. The trial team considered these imbalances post hoc and it was decided that a sensitivity analysis would be included with an adjustment to the primary analysis model for smoking status, as this is likely to be associated with outcome. It was not felt that ethnicity and education status were likely to be associated with outcome and so no sensitivity analyses were conducted with these factors.
| Characteristic | As randomised | As analysed | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) | Surgery (N = 203) | Plaster cast (N = 205) | Total (N = 408) | |
| Gender, n (%) | ||||||
| Male | 180 (82.2) | 183 (83.2) | 363 (82.7) | 168 (82.8) | 169 (82.4) | 337 (82.6) |
| Female | 39 (17.8) | 37 (16.8) | 76 (17.3) | 35 (17.2) | 36 (17.6) | 71 (17.4) |
| Age (years) | ||||||
| n | 219 | 220 | 439 | 203 | 205 | 408 |
| Mean (SD) | 32.9 (13.2) | 32.9 (12.2) | 32.9 (12.7) | 33.2 (13.2) | 32.9 (12.4) | 33.1 (12.8) |
| Median (min., max.) | 28 (16, 80) | 29 (16, 76) | 29 (16, 80) | 29 (16, 80) | 29 (16, 76) | 29 (16, 80) |
| Ethnicity, n (%) | ||||||
| White | 205 (93.6) | 195 (88.6) | 400 (91.1) | 191 (94.1) | 180 (87.8) | 371 (90.9) |
| Black | 0 (0.0) | 5 (2.3) | 5 (1.1) | 0 (0.0) | 5 (2.4) | 5 (1.2) |
| Asian | 7 (3.2) | 10 (4.5) | 17 (3.9) | 7 (3.4) | 10 (4.9) | 17 (4.2) |
| Other | 5 (2.3) | 10 (4.5) | 15 (3.4) | 5 (2.5) | 10 (4.9) | 15 (3.7) |
| Missing | 2 (0.9) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Education, n (%) | ||||||
| No formal qualifications | 24 (11.0) | 27 (12.3) | 51 (11.6) | 22 (10.8) | 25 (12.2) | 47 (11.5) |
| Some qualifications/no degree | 151 (68.9) | 129 (58.6) | 280 (63.8) | 139 (68.5) | 120 (58.5) | 259 (63.5) |
| Degree or higher | 41 (18.7) | 64 (29.1) | 105 (23.9) | 41 (20.2) | 60 (29.3) | 101 (24.8) |
| Missing | 3 (1.4) | 0 (0.0) | 3 (0.7) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Employment status, n (%) | ||||||
| Part-time | 20 (9.1) | 18 (8.2) | 38 (8.7) | 20 (9.9) | 18 (8.8) | 38 (9.3) |
| Full-time | 127 (58.0) | 120 (54.5) | 247 (56.3) | 119 (58.6) | 111 (54.1) | 230 (56.4) |
| Self-employed | 21 (9.6) | 36 (16.4) | 57 (13.0) | 19 (9.4) | 31 (15.1) | 50 (12.3) |
| Student | 20 (9.1) | 21 (9.5) | 41 (9.3) | 19 (9.4) | 21 (10.2) | 40 (9.8) |
| Retired | 7 (3.2) | 5 (2.3) | 12 (2.7) | 7 (3.4) | 5 (2.4) | 12 (2.9) |
| Looking after family/home | 1 (0.5) | 6 (2.7) | 7 (1.6) | 0 (0.0) | 5 (2.4) | 5 (1.2) |
| Not employed but seeking work | 9 (4.1) | 5 (2.3) | 14 (3.2) | 8 (3.9) | 5 (2.4) | 13 (3.2) |
| Other | 11 (5.0) | 9 (4.1) | 20 (4.6) | 10 (4.9) | 9 (4.4) | 19 (4.7) |
| Missing | 3 (1.4) | 0 (0) | 3 (0.7) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Type of employment, n (%) | ||||||
| Unskilled manual | 25 (11.4) | 23 (10.5) | 48 (10.9) | 24 (11.8) | 20 (9.8) | 44 (10.8) |
| Skilled manual | 63 (28.8) | 60 (27.3) | 123 (28.0) | 56 (27.6) | 56 (27.3) | 112 (27.5) |
| Unskilled non-manual | 19 (8.7) | 12 (5.5) | 31 (7.1) | 19 (9.4) | 11 (5.4) | 30 (7.4) |
| Skilled non-manual | 33 (15.1) | 46 (20.9) | 79 (18) | 32 (15.8) | 44 (21.5) | 76 (18.6) |
| Professional | 20 (9.1) | 19 (8.6) | 39 (8.9) | 20 (9.9) | 17 (8.3) | 37 (9.1) |
| Other | 19 (8.7) | 30 (13.6) | 49 (11.2) | 18 (8.9) | 28 (13.7) | 46 (11.3) |
| Missing | 40 (18.3) | 30 (13.6) | 70 (15.9) | 34 (16.7) | 29 (14.1) | 63 (15.4) |
| Current smoker, n (%) | ||||||
| Yes | 73 (33.3) | 56 (25.5) | 129 (29.4) | 64 (31.5) | 50 (24.4) | 114 (27.9) |
| No | 143 (65.3) | 163 (74.1) | 306 (69.7) | 138 (68.0) | 154 (75.1) | 292 (71.6) |
| Missing | 3 (1.4) | 1 (0.5) | 4 (0.9) | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| If yes | ||||||
| Number of cigarettes per day, median (min., max.) | 10 (1, 40) | 10 (1, 30) | 10 (1, 40) | 10 (1, 40) | 10 (1, 30) | 10 (1, 40) |
| Number of years, median (min., max.) | 10 (1, 50) | 10 (1, 36) | 10 (1, 50) | 10 (1, 50) | 10 (1, 36) | 10 (1, 50) |
| Past smoker, n (%) | ||||||
| Yes | 116 (53.0) | 109 (49.5) | 225 (51.3) | 110 (54.2) | 99 (48.3) | 209 (51.2) |
| No | 85 (38.8) | 101 (45.9) | 186 (42.4) | 81 (39.9) | 96 (46.8) | 177 (43.4) |
| Missing | 18 (8.2) | 10 (4.5) | 28 (6.4) | 12 (5.9) | 10 (4.9) | 22 (5.4) |
| Diabetes, n (%) | ||||||
| Yes | 7 (3.2) | 4 (1.8) | 11 (2.5) | 6 (3.0) | 4 (2.0) | 10 (2.5) |
| No | 209 (95.4) | 216 (98.2) | 425 (96.8) | 196 (96.6) | 201 (98.0) | 397 (97.3) |
| Missing | 3 (1.4) | 0 (0.0) | 3 (0.7) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Steroid use, n (%) | ||||||
| Yes | 6 (2.7) | 4 (1.8) | 10 (2.3) | 6 (3.0) | 4 (2.0) | 10 (2.5) |
| No | 210 (95.9) | 216 (98.2) | 426 (97.0) | 196 (96.6) | 201 (98.0) | 397 (97.3) |
| Missing | 3 (1.4) | 0 (0.0) | 3 (0.7) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Characteristic | As randomised | As analysed | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) | Surgery (N = 203) | Plaster cast (N = 205) | Total (N = 408) | |
| Time since injury, daysa | ||||||
| n | 219 | 220 | 439 | 203 | 205 | 408 |
| Mean (SD) | 5.1 (3.1) | 5.3 (3.3) | 5.2 (3.2) | 4.9 (3.0) | 5.4 (3.3) | 5.2 (3.2) |
| Median (min., max.) | 5 (1, 14) | 5 (0, 14) | 5 (0, 14) | 4 (1, 14) | 5 (0, 14) | 5 (0, 14) |
| Affected wrist, n (%) | ||||||
| Left | 115 (52.5) | 118 (53.6) | 233 (53.1) | 110 (54.2) | 110 (53.7) | 220 (53.9) |
| Right | 104 (47.5) | 102 (46.4) | 206 (46.9) | 93 (45.8) | 95 (46.3) | 188 (46.1) |
| Affected wrist is dominant hand, n (%) | ||||||
| Yes | 100 (45.7) | 95 (43.2) | 195 (44.4) | 92 (45.3) | 89 (43.4) | 181 (44.4) |
| No | 117 (53.4) | 125 (56.8) | 242 (55.1) | 111 (54.7) | 116 (56.6) | 227 (55.6) |
| Missing | 2 (0.9) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Displacement (eligibility), n (%) | ||||||
| No displacement | 135 (61.6) | 134 (60.9) | 269 (61.3) | 123 (60.6) | 123 (60) | 246 (60.3) |
| Displacement | 84 (38.4) | 86 (39.1) | 170 (38.7) | 80 (39.4) | 82 (40) | 162 (39.7) |
| Displacement (randomisation), n (%) | ||||||
| No displacement | 131 (59.8) | 130 (59.1) | 261 (59.5) | 119 (58.6) | 119 (58) | 238 (58.3) |
| Displacement | 88 (40.2) | 90 (40.9) | 178 (40.5) | 84 (41.4) | 86 (42) | 170 (41.7) |
| Radiographs,b n (%) | ||||||
| Elongated scaphoid view | 209 (95.4) | 210 (95.5) | 419 (95.4) | 193 (95.1) | 195 (95.1) | 388 (95.1) |
| Posterior–anterior view | 215 (98.2) | 218 (99.1) | 433 (98.6) | 200 (98.5) | 203 (99.0) | 403 (98.8) |
| 45° semisupine view | 159 (72.6) | 166 (75.5) | 325 (74.0) | 144 (70.9) | 156 (76.1) | 300 (73.5) |
| Lateral view | 218 (99.5) | 217 (98.6) | 435 (99.1) | 203 (100.0) | 202 (98.5) | 405 (99.3) |
| 45° semiprone view | 198 (90.4) | 196 (89.1) | 394 (89.7) | 184 (90.6) | 183 (89.3) | 367 (90.0) |
| Previous wrist problems on same side, n (%) | ||||||
| Yes | 43 (19.6) | 45 (20.5) | 88 (20.0) | 43 (21.2) | 42 (20.5) | 85 (20.8) |
| No | 173 (79.0) | 173 (78.6) | 346 (78.8) | 159 (78.3) | 161 (78.5) | 320 (78.4) |
| Missing | 3 (1.4) | 2 (0.9) | 5 (1.1) | 1 (0.5) | 2 (1.0) | 3 (0.7) |
| If yes, what injury?, n (%) | ||||||
| Previous fracture | 23 (53.5) | 28 (62.2) | 51 (58.0) | 23 (53.5) | 25 (59.5) | 48 (56.5) |
| Arthritis | 2 (4.7) | 1 (2.2) | 3 (3.4) | 2 (4.7) | 1 (2.4) | 3 (3.5) |
| Ligament, tendon or nerve injury | 10 (23.3) | 8 (17.8) | 18 (20.5) | 10 (23.3) | 8 (19.1) | 18 (21.2) |
| Other | 6 (14.0) | 8 (17.8) | 14 (15.9) | 6 (14.0) | 8 (19.1) | 14 (16.5) |
| Missing | 2 (4.7) | 0 (0.0) | 2 (2.3) | 2 (4.7) | 0 (0.0) | 2 (2.4) |
| Injury mechanism, n (%) | ||||||
| Fall | ||||||
| Standing | 28 (12.8) | 29 (13.2) | 57 (13.0) | 26 (12.8) | 26 (12.7) | 52 (12.7) |
| Walking | 24 (11.0) | 24 (10.9) | 48 (10.9) | 22 (10.8) | 23 (11.2) | 45 (11.0) |
| Running | 40 (18.3) | 38 (17.3) | 78 (17.8) | 37 (18.2) | 33 (16.1) | 70 (17.2) |
| From height | 28 (12.8) | 34 (15.5) | 62 (14.1) | 26 (12.8) | 31 (15.1) | 57 (14.0) |
| From moving object | 42 (19.2) | 31 (14.1) | 73 (16.6) | 41 (20.2) | 31 (15.1) | 72 (17.6) |
| Hit on palm of hand | ||||||
| Object striking palm | 16 (7.3) | 15 (6.8) | 31 (7.1) | 15 (7.4) | 15 (7.3) | 30 (7.4) |
| Handle whipping back | 9 (4.1) | 11 (5.0) | 20 (4.6) | 8 (3.9) | 11 (5.4) | 19 (4.7) |
| Other sudden extension | 11 (5.0) | 8 (3.6) | 19 (4.3) | 11 (5.4) | 8 (3.9) | 19 (4.7) |
| Punched something | 4 (1.8) | 12 (5.5) | 16 (3.6) | 4 (2.0) | 10 (4.9) | 14 (3.4) |
| Road traffic accident | 9 (4.1) | 8 (3.6) | 17 (3.9) | 9 (4.4) | 7 (3.4) | 16 (3.9) |
| Other | 6 (2.7) | 10 (4.5) | 16 (3.6) | 4 (2.0) | 10 (4.9) | 14 (3.4) |
| Missing | 2 (0.9) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Place of injury,b n (%) | ||||||
| Sport | 88 (40.2) | 78 (35.5) | 166 (37.8) | 85 (41.9) | 72 (35.1) | 157 (38.5) |
| Home | 27 (12.3) | 43 (19.5) | 70 (15.9) | 24 (11.8) | 38 (18.5) | 62 (15.2) |
| Work | 22 (10) | 18 (8.2) | 40 (9.1) | 19 (9.4) | 17 (8.3) | 36 (8.8) |
| Road traffic accident | 26 (11.9) | 34 (15.5) | 60 (13.7) | 25 (12.3) | 33 (16.1) | 58 (14.2) |
| Public place | 49 (22.4) | 48 (21.8) | 97 (22.1) | 46 (22.7) | 46 (22.4) | 92 (22.5) |
| Other | 3 (1.4) | 0 (0) | 3 (0.7) | 3 (1.5) | 0 (0.0) | 3 (0.7) |
| Missing | 4 (1.8) | 2 (0.9) | 6 (1.4) | 3 (1.5) | 2 (1.0) | 5 (1.2) |
| Treatment preference, n (%) | ||||||
| Surgery | 93 (42.5) | 101 (45.9) | 194 (44.2) | 89 (43.8) | 96 (46.8) | 185 (45.3) |
| No surgery | 13 (5.9) | 19 (8.6) | 32 (7.3) | 11 (5.4) | 16 (7.8) | 27 (6.6) |
| No preference | 110 (50.2) | 99 (45.0) | 209 (47.6) | 102 (50.2) | 92 (44.9) | 194 (47.5) |
| Missing | 3 (1.4) | 1 (0.5) | 4 (0.9) | 1 (0.5) | 1 (0.5) | 2 (0.5) |
Follow-up
Participant questionnaires
In total, follow-up participant questionnaires were returned for 359 (81.8%), 349 (79.5%), 313 (71.3%) and 364 (82.9%) of the 439 randomised participants at 6, 12, 26 and 52 weeks, respectively (Table 6). Return rates were lower in the plaster cast group at all time points, except at 6 weeks, when they were similar between the two groups.
| Time point | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|
| 6 weeks | |||
| Sent, n (%)a | 214 (97.7) | 217 (98.6) | 431 (98.2) |
| Returned, n (%)b | 178 (83.2) | 181 (83.4) | 359 (83.3) |
| Days to complete, median (IQR) | 5 (1–11) | 2 (0–9) | 4 (0–11) |
| Days to return, median (IQR) | 13 (8–24) | 12 (5–24) | 13 (6–24) |
| Completed within 1 week, n (%)c | 119 (66.9) | 118 (65.2) | 237 (66.0) |
| Mode of completion, n (%) | |||
| Post | 99 (55.6) | 93 (51.4) | 192 (53.5) |
| In clinic | 78 (43.8) | 87 (48.1) | 165 (46.0) |
| Telephone | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| 12 weeks | |||
| Sent, n (%)a | 212 (96.8) | 217 (98.6) | 429 (97.7) |
| Returned, n (%)b | 182 (85.9) | 167 (77.0) | 349 (81.4) |
| Days to complete, median (IQR) | 7 (2–21) | 5 (0–19) | 6 (0–20) |
| Days to return, median (IQR) | 15 (8–31) | 13 (7–35) | 14 (7–33) |
| Completed within 2 weeks, n (%)c | 122 (67.0) | 110 (65.9) | 232 (66.5) |
| Mode of completion, n (%) | |||
| Post | 97 (53.3) | 77 (46.1) | 174 (49.9) |
| In clinic | 70 (38.5) | 75 (44.9) | 145 (41.6) |
| Telephone | 15 (8.2) | 15 (9.0) | 30 (8.6) |
| 26 weeks | |||
| Sent, n (%)a | 212 (96.8) | 216 (98.2) | 428 (97.5) |
| Returned, n (%)b | 163 (76.9) | 149 (69.0) | 313 (72.9) |
| Days to complete, median (IQR) | 10 (5–27) | 10 (4–30) | 10 (5–28) |
| Days to return, median (IQR) | 20 (10–38) | 19 (10–37) | 20 (10–37) |
| Completed within 6 weeks, n (%)c | 136 (83.4) | 131 (87.9) | 267 (85.6) |
| Mode of completion, n (%) | |||
| Post | 165 (92.7) | 165 (91.2) | 330 (91.9) |
| Telephone | 13 (7.3) | 16 (8.8) | 29 (8.1) |
| 52 weeks | |||
| Sent, n (%)a | 212 (96.8) | 213 (96.8) | 425 (96.8) |
| Returned, n (%)b | 186 (87.7) | 178 (83.6) | 364 (85.7) |
| Days to complete, median (IQR) | 5 (0–16) | 7 (2–26) | 6 (2–22) |
| Days to return, median (IQR) | 12 (7–33) | 17 (8–36) | 14 (7–35) |
| Completed within 8 weeks, n (%)c | 170 (91.4) | 157 (88.2) | 327 (89.8) |
| Mode of completion, n (%) | |||
| Post | 103 (57.9) | 110 (60.8) | 213 (59.3) |
| In clinic | 67 (37.6) | 56 (30.9) | 123 (34.3) |
| Telephone | 8 (4.5) | 15 (8.3) | 23 (6.4) |
The participant questionnaires were primarily returned by post (50% or more at each time point). At 6, 12 and 52 weeks, when participants were invited to attend a hospital visit, there was the opportunity to complete the questionnaire in clinic. This occurred in 46.0%, 41.6% and 34.3% of cases at weeks 6, 12 and 52, respectively. Questionnaire data were completed by telephone as a last resort for hard-to-reach participants in 84 instances.
The numbers of days from the date that the questionnaire was due (e.g. the 6-week questionnaire was due 42 days after randomisation) to the completion of the questionnaire as recorded by the patient (‘Days to complete’) and the return of the questionnaire to the YTU (‘Days to return’) are presented in Table 6. If the questionnaire was completed/returned before the due date (i.e. if it was completed during a clinic visit rather than having been posted by the YTU on the due date), the number of days was recorded as zero. Medians and IQRs are presented, as the data had a right-skewed distribution because most patients completed and returned their forms promptly. There was no obvious difference between the two groups in time to completion or time to return.
Hospital data collection forms
The return of hospital forms is summarised in Table 7. Treatment confirmation forms were collected at 6 and 12 weeks post randomisation; at least one treatment confirmation form was completed for all but one participant (who was allocated to surgery but withdrew on the day of randomisation). A complications form was completed for over 84% of the randomised participants at each of the 6-, 12- and 52-week time points. Wrist range of movement and grip strength forms were received for 389 (88.6%), 336 (76.5%) and 309 (70.4%) participants at 6, 12 and 52 weeks, respectively. At 52 weeks, there appears to be a difference in the percentage of grip and range forms returned between the two groups (surgery, 74.4%; plaster cast, 66.4%), otherwise there are no obvious differences between the two groups in the return rates of hospital forms. The return of treatment confirmation and complications forms was higher than for the grip and range forms, as completion of these forms did not require the participant to attend the hospital visit.
| Hospital form | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|
| Wrist range of movement and grip strength form, n (%) | |||
| 6 weeks | 189 (86.3) | 200 (90.9) | 389 (88.6) |
| 12 weeks | 172 (78.5) | 164 (74.6) | 336 (76.5) |
| 52 weeks | 163 (74.4) | 146 (66.4) | 309 (70.4) |
| Treatment confirmation form, n (%) | |||
| 6 weeks | 200 (91.3) | 211 (95.9) | 411 (93.6) |
| 12 weeks | 202 (92.2) | 195 (88.6) | 397 (90.4) |
| At least one | 219 (99.5) | 220 (100.0) | 438 (99.8) |
| Complications form, n (%) | |||
| 6 weeks | 197 (90.0) | 206 (93.6) | 403 (91.8) |
| 12 weeks | 181 (82.7) | 189 (85.9) | 370 (84.3) |
| 52 weeks | 199 (90.9) | 196 (89.1) | 395 (90.0) |
Patient withdrawals
Five participants (surgery, n = 2; plaster cast, n = 3) withdrew from hospital follow-up but agreed to continue completing participant questionnaires; three of these were before the 6-week visit (no hospital visits attended) and two were following the 6-week visit (12- and 26-week visits not attended). The reasons provided were work commitments (surgery, n = 1; plaster cast, n = 1), the participant moved away from the trial catchment area (surgery, n = 1), the participant was too ill to commit to extra hospital visits (plaster cast, n = 1) and the participant sought further opinions at an alternative hospital, with surgical fixation and follow-ups completed there instead of at the recruiting site (plaster cast, n = 1).
A further 14 participants were withdrawn completely from the trial; eight before the 6-week time point (surgery, n = 5; plaster cast, n = 3), two between the 6- and 12-week time points (surgery, n = 2), one between 12 and 26 weeks (plaster cast, n = 1) and three between 26 and 52 weeks (plaster cast, n = 3). The reasons for these withdrawals were the participant no longer wanted to take part in the study, for example was too busy or was sick of the questionnaires (surgery, n = 2; plaster cast, n = 3); no fracture present on the CT scan (surgery, n = 4; plaster cast, n = 1); the participant was unhappy with their treatment allocation (surgery, n = 1; plaster cast, n = 2); and the participant emigrated (plaster cast, n = 1).
Hospital visits
Baseline participant and fracture data are summarised according to whether patients attended hospital visits at 6, 12 and 52 weeks (see Appendix 3, Tables 46–51). Non-smokers and participants in employment were more likely to attend than smokers and those not in employment, respectively, at all time points. Participants with fracture displacement at baseline were more likely to attend their 6- and 52-week hospital visits, but were equally likely to attend at 12 weeks.
Compliance with random allocation and treatment received
For all but four of the randomised participants, their affected wrist was recorded as being immobilised at enrolment, largely by a plaster cast (n = 328, 74.7%), but alternatively by a splint (n = 80, 18.2%) or a backslab (n = 27, 6.2%). From the treatment confirmation and surgery forms, we were able to ascertain the further treatment received for each participant within the 12 months following randomisation.
Allocated to receive surgery
Of the 219 patients allocated to surgery, 188 (85.8%) received treatment as allocated (Table 8). Surgery took place, on average, 10.2 days (median 11 days, range 3–20 days) after injury, 9.5 days (median 10 days, range 1–20 days) after presenting at A&E or another clinic and 4.5 days (median 5 days, range 0–15 days) after randomisation. Of the 188 participants who underwent surgery, 39 (20.7%) received routine treatment (i.e. surgery and no subsequent plaster cast or splint), 141 (75%) had further routine treatment with a plaster cast or splint only after surgery (not recorded as being due to a treatment failure) and 8 (4.3%) had at least one other surgery within the 12 months (seven had one other surgery and one had two).
| Treatment pathway | Definition of pathway | n (%) | Further details |
|---|---|---|---|
| Crossover | Participant immediately switched to plaster cast following consent and randomisation, no surgery | 31 (14.2) |
|
| Routine treatment | Participant had one surgery within the 12 months from randomisation and no subsequent plaster cast and/or splint | 24 (11.0) |
|
| Treatment failure | Participant had surgery and subsequent plaster cast and/or splint due to treatment failure (e.g. poor stability from surgery) | 0 (0.0) | – |
| Further routine treatment | Participant had surgery and subsequent plaster cast and/or splint following routine practice | 156 (71.2) |
|
| Participant had index surgery but there was subsequent evidence of non-union, so was offered further surgery | 2 (0.9) |
|
|
| Participant had index surgery and received further surgery (not for non-union) | 6 (2.7) |
|
The reasons for repeat surgeries (for the seven patients with only one extra surgery within 12 months of randomisation) were removal of the screw due to (1) protrusion (n = 3), (2) experiencing pain and poor flexion of wrist (n = 1) or (3) the scaphoid screw breaching the cortex of the scaphoid at the capitate articulation owing to mispositioning of the screw (n = 1); revision surgery owing to pain/prominent metal work (n = 1); or persistent non-union (n = 1). For the participant who underwent two further surgeries, the first was to remove the screw and perform iliac bone graft/k-wire stabilisation owing to non-union of the scaphoid fracture; the second was for persistent non-union.
The remaining 31 (14.2%) participants allocated to receive surgery were treated non-surgically. It should be noted that the participant with no treatment confirmation form was allocated to the surgery group but withdrew on the day of randomisation and provided no follow-up data. However, one of the reasons provided for withdrawal was that the participant wanted conservative treatment; therefore, this patient is assumed not to have received surgery. The reasons for participants being treated non-surgically are as follows: no fracture was observed on the CT scan subsequent to randomisation (n = 10), the participant’s choice was not to have surgery (n = 9), the participant did not have a bicortical scaphoid waist fracture [n = 8; these fractures were instead described as a waist plus distal pole fracture (n = 2), a radial styloid fracture (n = 1), a unicortical fracture (n = 1), a lunate fracture (n = 1), a Y-shaped waist fracture involving the tubercle (n = 1), an associated scaphoid tubercle fracture (n = 1) and an incomplete fracture (n = 1)]; and other reasons [n = 4; the surgeon felt that surgery was inappropriate/unnecessary following a review of the CT scan but no further information is available (n = 2), no appropriate time for surgery was available for either the surgeon or the patient (n = 1) and the patient was admitted to hospital with pericarditis (n = 1)]. Of these, 30 received a plaster cast and/or splint following randomisation, while one did not receive any treatment because the treating clinician deemed that their subsequent CT scan indicated no injury (see Table 8).
Following randomisation, 41 participants did not report wearing any plaster cast/splint and 65 were reported to be wearing only a splint (for a median of 40 days post randomisation, range 4–97 days – although the upper range may not be accurate, as the final date of splint wearing was often not recorded/known and the splint may have been worn for longer than the 12-week reporting period of the treatment confirmation forms). Of the remaining 113 participants, 29 had a cast (with thumb incorporated) for a median of 21 days (range 2–60 days) following randomisation [of whom two then had a cast (with thumb free) applied for a median of 37 days] and 84 had a cast (with thumb free) for a median of 18 days (range 1–63 days) following randomisation [of whom two then had a cast (with thumb incorporated) for a median of 9 days]. Overall, 103/219 (47.0%) participants allocated to the surgery group were still in a plaster cast or splint 6 weeks post randomisation and 13 (5.9%) still had a cast or splint at 12 weeks.
Allocated to receive plaster cast intervention
Of the 220 patients allocated to not receive immediate surgical fixation, the majority (n = 195, 88.6%) were treated conservatively and did not receive surgery (Table 9), although two of these were considered for surgical fixation owing to non-union. Six (2.7%) participants immediately crossed over to surgery following randomisation with no cast applied before this, for the following reasons: participant choice (n = 4), the CT scan showed displacement (n = 1) and the radiographs were reviewed again at a later date and displacement was judged to be > 2 mm (n = 1). For these six participants, surgery took place, on average, 13.5 days (median 12 days, range 5–32 days) after injury, 12.8 days (median 11.5 days, range – 31 days) after presenting at A&E or another clinic and 8.8 days (median 8.5 days, range 0–24 days) after randomisation. None of these six participants underwent further surgery within 12 months from randomisation.
| Treatment pathway | Definition of pathway | n (%) | Further details |
|---|---|---|---|
| Crossover | Patient immediately switched to surgery following randomisation | 6 (2.7) |
|
| Routine treatment | Participant treated conservatively – no surgery | 193 (87.7) |
|
| Treatment failure | Surgery was undertaken to stabilise the fracture (before 5 weeks from randomisation). This is not a crossover because the patient did have a plaster cast applied | 1 (0.5) |
|
| Further routine treatment – surgery (after 5 weeks post randomisation) | Surgery was undertaken after 5 weeks from randomisation – not owing to a failure to unite | 1 (0.5) |
|
| Further routine treatment – surgery recommended (after 5 weeks post randomisation) as per the specified treatment pathway because of a failure to unite | Surgery was not received | 2 (0.9) |
|
| One surgery was performed within 12 months of randomisation | 16 (7.3) |
|
|
| Two or more surgeries were performed within 12 months of randomisation | 1 (0.5) |
|
One (0.5%) participant had a plaster cast applied but received surgery 29 days after randomisation owing to treatment failure, as their fracture was displacing with the plaster cast. This participant received a revision surgery to remove the screw 3 months after initial fixation.
One (0.5%) participant received surgery within 6 months of randomisation at a non-participating hospital to fix what the treating surgeon deemed to be a historic fracture. This was noted by the participant on a participant questionnaire. Limited information is available for this participant, as they withdrew from hospital follow-up at their recruiting site.
The remaining 17 (7.7%) participants received surgery as part of their expected treatment pathway when the treating surgeon judged that the bone had failed to unite with conservative treatment. Sixteen of these received only one surgery in the 12 months following randomisation and one received three surgeries (the second one for persistent non-union and the third to remove the wires from the second operation). The initial surgery took place, on average, 159.0 days (median 161 days, range 68–358 days) after injury, 157.0 days (median 156 days, range 67–358 days) after presenting at A&E or another clinic and 151.6 days (median 153 days, range 61–350 days) after randomisation. Fourteen participants met the protocol definition of the control condition by receiving early surgical fixation of their fractures following detection of persistent non-union after plaster cast management (defined here as surgery within 6 months of randomisation, although only five had their surgery within 12 weeks), while three had delayed surgical fixation, one of whom opted to attend a private hospital for their fixation, as they were told there would be a 4- to 5-month wait for surgery at the treating centre.
There were two participants who were deemed to have a suspected non-union at 12 weeks and for whom surgical fixation was recommended, but who did not receive surgery. These participants did not fully comply with the anticipated control condition for the trial. The limited information we have about these participants is as follows: for one, the operation was scheduled but then delayed and the participant self-discharged after waiting and declined all further treatment, including offers of surgery; for the second, it was the surgeon’s decision not to operate.
Following randomisation, two participants did not report wearing any plaster cast/splint and two were reported to be wearing only a splint (for a median of 44 days post randomisation). Of the remaining 216 participants, 45 had a cast (with thumb incorporated) for a median of 42 days (range 7–84 days) following randomisation [of which eight then had a cast (with thumb free) for a median of 25 days (range 14–42 days)] and 171 had a cast (with thumb free) for a median of 42 days (range 7–98 days) following randomisation [of which five then had a cast (with thumb incorporated) for a median of 44 days (range 12–69 days)]. Overall, 187/220 (85.0%) participants allocated to the plaster cast group were still in a plaster cast or splint 6 weeks post randomisation and 47 (21.4%) still had a cast or splint at 12 weeks.
Surgical fixation details
A surgery form was received for 210 of the 213 participants in the trial who received surgical fixation of their fracture (surgery group, n = 187/188, 99.5%; plaster cast group, n = 23/25, 92.0%). Surgery was performed by 102 surgeons across 30 sites. Each surgeon performed between one and six operations (median 1). Details of the operation, for initial surgical fixation procedures only, are provided in Table 10. Surgery lasted a median of 55 minutes (range 15–140 minutes) with a mean (SD) of 2 (0.9) surgeons in attendance. Most commonly, the main operating surgeon was a consultant (n = 39, 66.2%), followed by a specialist trainee (n = 49, 23.3%) and a staff grade/associate specialist (n = 22, 10.5%). When the main operating surgeon was a specialist trainee, an assisting consultant was recorded as being present in 33 of 49 (67.4%) surgeries. Acutrak® screws (Acumed LLC, Hillsboro, OR, USA) were the most frequently used type and a second screw was used for two (1.0%) participants. There were no reported intraoperative complications and most participants (n = 113, 53.8%) were treated with a plaster cast postoperatively.
| Surgery details | Surgery (N = 210) |
|---|---|
| Lead surgeon grade, n (%) | |
| Consultant | 139 (66.2) |
| Staff grade/associate specialist | 22 (10.5) |
| Specialist trainee | 49 (23.3) |
| Total number of surgeons present in theatre | |
| Mean (SD) | 2.0 (0.9) |
| Median (min., max.) | 2 (1, 9) |
| Duration of surgery, minutes (n = 197) | |
| Mean (SD) | 59.0 (23.6) |
| Median (min., max.) | 55 (15, 140) |
| Surgery type, n (%) | |
| Percutaneous | 164 (78.1) |
| Open | 43 (20.5) |
| Both | 1 (0.5) |
| Missing | 2 (1.0) |
| Incision, n (%) | |
| Palmar | 171 (81.4) |
| Dorsal | 33 (15.7) |
| Both | 2 (1.0) |
| Missing | 4 (1.9) |
| Type of screw used, n (%) | |
| Acutrak | 152 (72.4) |
| Medartis® (Basel, Switzerland) | 33 (15.7) |
| Twinfix® (Stryker Corporation, Kalamazoo, MI, USA) | 10 (4.8) |
| Headless compression screw | 10 (4.8) |
| Herbert™ (Zimmer Biomet, Warsaw, IN, USA) | 1 (0.5) |
| Missing | 4 (1.9) |
| Second screw, n (%) | |
| Yes | 2 (1.0) |
| No | 202 (96.2) |
| Missing | 6 (2.9) |
| K-wire used, n (%) | |
| Yes | 39 (18.6) |
| No | 168 (80.0) |
| Missing | 3 (1.4) |
| Placement of screw, n (%) | |
| Central position | |
| Yes | 148 (70.5) |
| No | 55 (26.2) |
| Missing | 7 (3.3) |
| Position less than perfect but acceptable | |
| Yes | 74 (35.2) |
| No | 131 (62.4) |
| Missing | 5 (2.4) |
| Uncertain bone hold | |
| Yes | 2 (1.0) |
| No | 201 (95.7) |
| Missing | 7 (3.3) |
| Intraoperative complications, n (%) | |
| Fracture around screw | 0 (0.0) |
| Nerve injury | 0 (0.0) |
| Vascular injury | 0 (0.0) |
| Tendon injury | 0 (0.0) |
| Postoperative management, n (%) | |
| Cast | 113 (53.8) |
| Bandage | 65 (31.0) |
| Splint | 31 (14.8) |
| Missing | 1 (0.5) |
| Unexpected procedure,a n (%) | 2 (1.0) |
Primary outcome (Patient-Rated Wrist Evaluation) analysis
The PRWE (pre and post injury) was assessed at baseline and at 6, 12, 26 and 52 weeks post randomisation. The PRWE total score is a value between 0 and 100, where a higher score indicates worse pain and functioning. The pain and function subscale scores are each out of a maximum of 50 (50 being the worst score). The trial was powered to detect an effect size of 0.3 (assuming a SD of 20); this is equivalent to a difference in total PRWE score of 6 points.
Primary end point analysis
There was no evidence of a difference in PRWE score between the surgery and plaster cast groups at the 52-week time point, with an adjusted mean difference of –2.1 in favour of the surgery group (95% CI –5.8 to 1.6; p = 0.27).
This result was extracted from a multilevel model in which participant was treated as a random effect and observations over time (6, 12, 26 and 52 weeks) were nested within participant. The effect of randomised treatment group was assessed while adjusting for time, group-by-time interaction, age, fracture displacement and hand dominance. The predicted means and associated 95% CIs for each group and time point are presented in Table 11 and displayed in Figure 6.
| Time point | Surgery, mean (95% CI) | Plaster cast, mean (95% CI) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| 6 weeks | 35.6 (32.6 to 38.6) | 39.8 (36.8 to 42.8) | –4.2 (–8.5 to 0.1) | 0.06 |
| 12 weeks | 21.0 (18.1 to 24.0) | 26.6 (23.6 to 29.6) | –5.6 (–9.8 to –1.4) | 0.01 |
| 26 weeks | 16.2 (13.5 to 18.9) | 16.5 (13.8 to 19.2) | –0.3 (–4.1 to 3.6) | 0.89 |
| 52 weeks | 11.9 (9.2 to 14.5) | 14.0 (11.3 to 16.6) | –2.1 (–5.8 to 1.6) | 0.27 |
| Overall | 21.3 (18.9 to 23.6) | 24.4 (22.0 to 26.7) | –3.0 (–6.3 to 0.3) | 0.07 |
FIGURE 6.
Adjusted mean PRWE scores (with 95% CIs) over time by randomised group for primary analysis. Modified from Lancet, vol. 396, Dias JJ, Brealey SD, Fairhurst C, Amirfeyz R, Bhowal B, Blewitt N, et al. , Surgery versus cast immobilisation for adults with a bicortical fracture of the scaphoid waist (SWIFFT): a pragmatic, multicentre, open-label, randomised superiority trial, pp. 390–401,89 Copyright (2020), with permission from Elsevier.
Different covariance structures were applied to the model. An unstructured pattern that models all variances and covariances separately resulted in the lowest AIC and so was used in the final model.
Diagnostics of model fit revealed that the standardised residuals demonstrated only a minor deviation from normality and were uniform against fitted values; therefore, analyses were carried out on untransformed values. Model coefficients for the covariates with 95% CIs are provided as the software output in Appendix 4 to aid understanding of the fitted model.
Valid data
The primary analysis included data from 408 patients (surgery, n = 203; plaster cast, n = 205) with a valid PRWE score for at least one follow-up time point and complete baseline covariates. A valid response is defined here as sufficient data (maximum of one missing PRWE item in each of the two subscales) to allow the calculation of the total score. The number of participants with valid PRWE data at each time point is presented by randomised group in Table 12, with reasons for missing data.
| Time point | Response type | Surgery (N = 219), n (%) | Plaster cast (N = 220), n (%) | Total (N = 439), n (%) |
|---|---|---|---|---|
| Baseline (pre injury) | Valid response | 205 (93.6) | 203 (92.3) | 408 (92.9) |
| No or partial responsea | ||||
| Partial response | 0 (0) | 2 (0.9) | 2 (0.5) | |
| All responses missing | 12 (5.5) | 15 (6.8) | 27 (6.2) | |
| Did not return questionnaire | 2 (0.9) | 0 (0.0) | 2 (0.5) | |
| Baseline (post injury) | Valid response | 213 (97.3) | 209 (95) | 422 (96.1) |
| No or partial responsea | ||||
| Partial response | 4 (1.8) | 11 (5) | 15 (3.4) | |
| All responses missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Did not return questionnaire | 2 (0.9) | 0 (0.0) | 2 (0.5) | |
| 6 weeks | Valid response | 176 (80.4) | 172 (78.2) | 348 (79.3) |
| No or partial responsea | ||||
| Partial response | 2 (0.9) | 9 (4.1) | 11 (2.5) | |
| All responses missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Did not return questionnaire | 36 (16.4) | 36 (16.4) | 72 (16.4) | |
| Withdrawn from questionnaires | 5 (2.3) | 3 (1.4) | 8 (1.8) | |
| 12 weeks | Valid response | 178 (81.3) | 163 (74.1) | 341 (77.7) |
| No or partial responsea | ||||
| Partial response | 4 (1.8) | 4 (1.8) | 8 (1.8) | |
| All responses missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Did not return questionnaire | 30 (13.7) | 50 (22.7) | 80 (18.2) | |
| Withdrawn from questionnaires | 7 (3.2) | 3 (1.4) | 10 (2.3) | |
| 26 weeks | Valid response | 156 (71.2) | 146 (66.4) | 302 (68.8) |
| No or partial responsea | ||||
| Partial response | 6 (2.7) | 3 (1.4) | 9 (2.1) | |
| All responses missing | 1 (0.5) | 0 (0.0) | 1 (0.2) | |
| Did not return questionnaire | 49 (22.4) | 67 (30.5) | 116 (26.4) | |
| Withdrawn from questionnaires | 7 (3.2) | 4 (1.8) | 11 (2.5) | |
| 52 weeks | Valid response | 186 (84.9) | 176 (80) | 362 (82.5) |
| No or partial responsea | ||||
| Partial response | 0 (0.0) | 2 (0.9) | 2 (0.5) | |
| All responses missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Did not return questionnaire | 26 (11.9) | 35 (15.9) | 61 (13.9) | |
| Withdrawn from questionnaires | 7 (3.2) | 7 (3.2) | 14 (3.2) | |
The percentage of randomised participants with valid PRWE data ranged from 68.8% (26 weeks) to 82.5% (52 weeks) for the post-randomisation time points. Overall, 408 participants (surgery, n = 203, 92.7%; plaster cast, n = 205, 93.2%) had valid PRWE data for at least one post-randomisation time point and so were included in the primary analysis model.
Demographic and injury characteristics at baseline for participants who provided valid PRWE data at each time point are presented by randomised group in Appendix 3, Tables 52–55.
Overall, 249 patients (56.7%) had a valid PRWE response at all post-randomisation follow-up time points (complete responders: surgery, n = 130, 59.4%; plaster cast, n = 119, 54.1%) and a further 159 patients (intermittent responders: surgery, n = 73, 33.3%; plaster cast, n = 86, 39.1%) had a valid PRWE response at one or more, but not all, post-randomisation time points. Table 13 provides the descriptive PRWE total scores for complete and intermittent responders and the baseline PRWE scores for those who had no valid post-randomisation PRWE data. Complete responders had, on average, better PRWE outcomes (lower mean scores) than intermittent responders pre injury and at 52 weeks post randomisation, but had similar outcomes at baseline (post injury) and at 6, 12 and 26 weeks post randomisation.
| Total PRWE score | Responders | ||
|---|---|---|---|
| Complete (n = 249) | Intermittent (n = 159) | Non (n = 31) | |
| Baseline (pre injury) | |||
| Mean (SD) | 2.7 (9.3) | 4.8 (14.7) | 1.1 (2.3) |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Min., max. | (0, 85) | (0, 90.5) | (0, 8) |
| Baseline (post injury) | |||
| Mean (SD) | 74.4 (16.8) | 72.4 (21.5) | 72.1 (16.5) |
| Median (IQR) | 77.5 (65.3–77.5) | 76.8 (62–76.8) | 74.5 (63.8–74.5) |
| Min., max. | (0, 100) | (0, 100) | (35, 96.5) |
| 6 weeks | |||
| Mean (SD) | 37.3 (19.7) | 37.0 (24.6) | – |
| Median (IQR) | 37 (22.5–37) | 30.5 (17.5–30.5) | – |
| Min., max. | (0, 85.9) | (0, 100) | – |
| 12 weeks | |||
| Mean (SD) | 22.9 (20.0) | 23.9 (23.0) | – |
| Median (IQR) | 17. (8–17.5) | 17 (5–17) | – |
| Min., max. | (0, 90) | (0, 80.5) | – |
| 26 weeks | |||
| Mean (SD) | 15.4 (17.1) | 15.3 (21.7) | – |
| Median (IQR) | 10.5 (3.5–10.5) | 5 (0–5) | – |
| Min., max. | (0, 84) | (0, 91.5) | – |
| 52 weeks | |||
| Mean (SD) | 11.7 (17.5) | 15.0 (19.6) | – |
| Median (IQR) | 4.5 (0–4.5) | 4 (0–4) | – |
| Min., max. | (0, 96) | (0, 88) | – |
Descriptive Patient-Rated Wrist Evaluation statistics
Total mean PRWE scores pre injury and at enrolment (post injury) were similar between the two groups (Table 14). Total mean PRWE scores improved (decreased) over time following injury in both groups, but were higher (worse) in the plaster cast group than in the surgery group at all post-randomisation time points except at 26 weeks. At 52 weeks, the unadjusted mean difference was –2.8 points (95% CI –6.5 to 1.0 points) favouring the surgery group.
| PRWE score | Surgery | Plaster cast | Total |
|---|---|---|---|
| Baseline (pre injury) | |||
| Pain subscale | |||
| Mean (SD) | 2.2 (6.6) | 2.5 (6.9) | 2.4 (6.7) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Min., max. | (0, 50) | (0, 41) | (0, 50) |
| Function subscale | |||
| Mean (SD) | 1.0 (5.1) | 1.1 (5.7) | 1.0 (5.4) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Min., max. | (0, 43) | (0, 49.5) | (0, 49.5) |
| Total | |||
| Mean (SD) | 3.1 (10.8) | 3.6 (11.8) | 3.4 (11.3) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Min., max. | (0, 85) | (0, 90.5) | (0, 90.5) |
| Baseline (post injury) | |||
| Pain subscale | |||
| Mean (SD) | 34.7 (10.8) | 34.3 (9.7) | 34.5 (10.2) |
| Median (IQR) | 36.0 (30.0–42.0) | 35.0 (28.4–42.0) | 36.0 (29.0–42.0) |
| Min., max. | (0, 50) | (0, 50) | (0, 50) |
| Function subscale | |||
| Mean (SD) | 39.1 (10.4) | 38.5 (10.0) | 38.8 (10.2) |
| Median (IQR) | 41.8 (34.0–47.0) | 40.5 (33.5–46.0) | 41.0 (33.5–46.5) |
| Min., max. | (0, 50) | (0, 50) | (0, 50) |
| Total | |||
| Mean (SD) | 73.9 (19.8) | 73.2 (17.4) | 73.5 (18.6) |
| Median (IQR) | 78.5 (65.5–87.5) | 76.0 (63.5–86.5) | 77.5 (64.0–87.0) |
| Min., max. | (0, 100) | (0, 100) | (0, 100) |
| 6 weeks | |||
| Pain subscale | |||
| Mean (SD) | 18.9 (10.5) | 18.4 (10.7) | 18.6 (10.6) |
| Median (IQR) | 19.0 (10.0–27.0) | 17.0 (10.0–26.0) | 18.0 (10.0–26.3) |
| Min., max. | (0, 44) | (0, 50) | (0, 50) |
| Function subscale | |||
| Mean (SD) | 16.8 (12.8) | 20.1 (12.4) | 18.5 (12.7) |
| Median (IQR) | 13.5 (6.5–25.5) | 19.0 (9.8–27.3) | 17.0 (8.0–26.5) |
| Min., max. | (0, 47) | (0, 50) | (0, 50) |
| Total | |||
| Mean (SD) | 35.7 (21.4) | 38.8 (21.0) | 37.2 (21.2) |
| Median (IQR) | 33.8 (18.8–49.0) | 38.3 (22.5–52.3) | 35.4 (19.5–51.5) |
| Min., max. | (3, 85.5) | (0, 100) | (0, 100) |
| 12 weeks | |||
| Pain subscale | |||
| Mean (SD) | 12.8 (11.0) | 14.6 (11.2) | 13.7 (11.1) |
| Median (IQR) | 10.0 (4.5–18.0) | 12.0 (6.0–21.0) | 11.0 (5.0–19.0) |
| Min., max. | (0, 45) | (0, 47) | (0, 47) |
| Function subscale | |||
| Mean (SD) | 7.9 (9.3) | 11.2 (11.5) | 9.5 (10.5) |
| Median (IQR) | 5.0 (1.0–11.0) | 7.5 (2.5–16.0) | 6.0 (1.5–13.0) |
| Min., max. | (0, 44.5) | (0, 46.5) | (0, 46.5) |
| Total | |||
| Mean (SD) | 20.7 (19.6) | 25.9 (21.8) | 23.2 (20.8) |
| Median (IQR) | 15.0 (6.0–27.0) | 20.0 (8.7–35.5) | 17.5 (7.0–31.5) |
| Min., max. | (0, 89.5) | (0, 90) | (0, 90) |
| 26 weeks | |||
| Pain subscale | |||
| Mean (SD) | 10.5 (10.6) | 9.9 (10.0) | 10.2 (10.3) |
| Median (IQR) | 7.0 (3.0–15.0) | 8.0 (2.0–13.0) | 8.0 (2.0–15.0) |
| Min., max. | (0, 43) | (0, 44) | (0, 44) |
| Function subscale | |||
| Mean (SD) | 5.3 (8.3) | 5.4 (8.7) | 5.4 (8.5) |
| Median (IQR) | 1.8 (0.0–5.8) | 2.0 (0.0–6.0) | 2.0 (0.0–6.0) |
| Min., max. | (0, 41) | (0, 47.5) | (0, 47.5) |
| Total | |||
| Mean (SD) | 15.7 (18.1) | 15.1 (17.8) | 15.4 (18.0) |
| Median (IQR) | 9.0 (3.5–20.5) | 10.5 (2.0–18.0) | 9.5 (3.0–19.0) |
| Min., max. | (0, 84) | (0, 91.5) | (0, 91.5) |
| 52 weeks | |||
| Pain subscale | |||
| Mean (SD) | 7.7 (10.1) | 9.2 (11.3) | 8.4 (10.7) |
| Median (IQR) | 4.0 (0.0–10.0) | 4.0 (0.0–14.0) | 4.0 (0.0–12.0) |
| Min., max. | (0, 42) | (0, 48) | (0, 48) |
| Function subscale | |||
| Mean (SD) | 3.7 (7.2) | 4.9 (9.0) | 4.3 (8.1) |
| Median (IQR) | 0.5 (0.0–3.5) | 0.5 (0.0–5.0) | 0.5 (0.0–4.5) |
| Min., max. | (0, 43.5) | (0, 48) | (0, 48) |
| Total | |||
| Mean (SD) | 11.4 (16.6) | 14.2 (19.8) | 12.8 (18.2) |
| Median (IQR) | 4.0 (0.0–14.0) | 4.5 (0.0–19.3) | 4.5 (0.0–16.5) |
| Min., max. | (0, 85.5) | (0, 96) | (0, 96) |
Patient-Rated Wrist Evaluation at the secondary time points
Adjusted PRWE means and group differences for the primary analysis model are presented in Table 11 and displayed in Figure 6. The analysis showed a statistically significant difference between treatment groups at week 12 (p = 0.01) and a borderline result at week 6 (p = 0.06) but not at week 26 (p = 0.89) or at the primary end point of 52 weeks (p = 0.27). There was no overall effect of treatment group (difference of 3.0 points in favour of the surgery group; p = 0.07). Although statistically significant, the mean difference observed at 12 weeks was lower than our minimum clinically important difference of 6 points, although the confidence interval does include this difference.
Sensitivity analyses
Missing data
Adjusted PRWE means and group differences for the separate linear regression analysis models, for each time point, run on the multiply imputed data set are presented in Table 15. Analyses showed very similar results to the primary analysis, that is, a statistically significant difference between treatment groups at weeks 6 (p = 0.049) and 12 (p = 0.01) but not at week 26 (p = 0.93) or the primary end point of 52 weeks (p = 0.28). The adjusted mean difference at 52 weeks was –2.0 (95% CI –5.7 to 1.6) in favour of the surgery group. Although separate linear regressions were performed for these analyses, as opposed to a repeated measures model, there is no estimate for the overall treatment effect over time.
| Time point | Surgery, mean (95% CI) | Plaster cast, mean (95% CI) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Missing dataa (N = 439; surgery, n = 219; plaster cast, n = 220) | ||||
| 6 weeks | 35.1 (32.1 to 38.1) | 39.8 (36.7 to 42.9) | –4.7 (–9.0 to –0.5) | 0.03 |
| 12 weeks | 20.7 (17.9 to 23.6) | 26.6 (23.7 to 29.5) | –5.9 (–9.9 to –1.9) | < 0.01 |
| 26 weeks | 16.1 (13.4 to 18.8) | 16.4 (13.7 to 19.2) | –0.3 (–4.2 to 3.5) | 0.87 |
| 52 weeks | 12.0 (9.3 to 14.6) | 14.1 (11.4 to 16.8) | –2.1 (–5.9 to 1.6) | 0.26 |
| Handling multisite data (N = 408; surgery, n = 203; plaster cast, n = 205) | ||||
| 6 weeks | 36.2 (32.6 to 39.8) | 40.2 (36.6 to 43.8) | –4.0 (–8.2 to 0.3) | 0.07 |
| 12 weeks | 21.6 (18.1 to 25.1) | 27.0 (23.4 to 30.6) | –5.4 (–9.5 to –1.2) | 0.01 |
| 26 weeks | 16.8 (13.5 to 20.1) | 16.9 (13.6 to 20.3) | –0.1 (–3.9 to 3.7) | 0.96 |
| 52 weeks | 12.5 (9.2 to 15.7) | 14.4 (11.1 to 17.7) | –1.9 (–5.6 to 1.8) | 0.31 |
| Overall | 21.9 (18.8 to 24.9) | 24.8 (21.7 to 27.8) | –2.8 (–6.1 to 0.4) | 0.09 |
| Timing of data collection (N = 380; surgery, n = 190; plaster cast, n = 190) | ||||
| 6 weeks | 37.3 (33.9 to 40.7) | 37.7 (34.2 to 41.2) | –0.4 (–5.3 to 4.4) | 0.86 |
| 12 weeks | 20.6 (17.5 to 23.8) | 26.4 (23.1 to 29.7) | –5.7 (–10.3 to –1.2) | 0.01 |
| 26 weeks | 15.2 (12.5 to 17.9) | 15.4 (12.7 to 18.1) | –0.2 (–4.0 to 3.6) | 0.93 |
| 52 weeks | 10.8 (8.2 to 13.3) | 13.8 (11.2 to 16.5) | –3.1 (–6.7 to 0.6) | 0.10 |
| Overall | 19.9 (17.6 to 22.2) | 22.2 (19.9 to 24.5) | –2.4 (–5.6 to 0.9) | 0.16 |
| Adjusted for smoking status (N = 406; surgery, n = 202; plaster cast, n = 204) | ||||
| 6 weeks | 35.3 (32.3 to 38.3) | 40.0 (36.9 to 43.0) | –4.7 (–9.0 to -0.4) | 0.03 |
| 12 weeks | 20.7 (17.8 to 23.7) | 26.8 (23.8 to 29.8) | –6.0 (–10.2 to –1.8) | 0.01 |
| 26 weeks | 15.9 (13.2 to 18.6) | 16.7 (14.0 to 19.5) | –0.8 (–4.7 to 3.0) | 0.67 |
| 52 weeks | 11.3 (8.8 to 13.9) | 14.2 (11.5 to 16.8) | –2.8 (–6.5 to 0.9) | 0.14 |
| Overall | 20.9 (18.6 to 23.2) | 24.6 (22.2 to 26.9) | –3.6 (–6.9 to –0.3) | 0.03 |
| Including displacement as agreed by three independent raters (N = 408; surgery, n = 203; plaster cast, n = 205) | ||||
| 6 weeks | 35.5 (32.5 to 38.5) | 39.8 (36.8 to 42.8) | –4.3 (–8.5 to –0.0) | 0.05 |
| 12 weeks | 21.0 (18.0 to 23.9) | 26.6 (23.6 to 29.6) | –5.6 (–9.8 to –1.4) | 0.01 |
| 26 weeks | 16.2 (13.6 to 18.9) | 16.5 (13.8 to 19.2) | –0.3 (–4.1 to 3.6) | 0.89 |
| 52 weeks | 11.9 (9.3 to 14.5) | 13.9 (11.3 to 16.6) | –2.1 (–5.8 to 1.6) | 0.27 |
| Overall | 21.2 (18.9 to 23.5) | 24.4 (22.0 to 26.7) | –3.1 (–6.3 to 0.2) | 0.07 |
| Excluding those with no fracture (N = 407; surgery, n = 202; plaster cast, n = 205) | ||||
| 6 weeks | 35.7 (32.6 to 38.7) | 39.8 (36.8 to 42.8) | –4.1 (–8.4 to 0.1) | 0.06 |
| 12 weeks | 21.1 (18.1 to 24.0) | 26.6 (23.6 to 29.6) | –5.5 (–9.7 to –1.3) | 0.01 |
| 26 weeks | 16.3 (13.6 to 19.0) | 16.5 (13.8 to 19.2) | –0.2 (–4.1 to 3.6) | 0.91 |
| 52 weeks | 11.9 (9.3 to 14.6) | 14.0 (11.3 to 16.6) | –2.0 (–5.8 to 1.7) | 0.29 |
| Overall | 21.3 (19.0 to 23.6) | 24.4 (22.0 to 26.7) | –3.0 (–6.3 to 0.3) | 0.08 |
| Excluding those with displacement > 2 mm (N = 383; surgery, n = 191; plaster cast, n = 192) | ||||
| 6 weeks | 35.0 (31.9 to 38.0) | 39.8 (36.7 to 42.9) | –4.8 (–9.2 to -0.5) | 0.03 |
| 12 weeks | 20.7 (17.6 to 23.7) | 26.2 (23.1 to 29.3) | –5.6 (–9.9 to –1.3) | 0.01 |
| 26 weeks | 15.7 (13.0 to 18.3) | 16.3 (13.6 to 19.0) | –0.6 (–4.4 to 3.2) | 0.76 |
| 52 weeks | 11.4 (8.8 to 13.9) | 13.7 (11.0 to 16.3) | –2.3 (–6.0 to 1.4) | 0.22 |
| Overall | 20.7 (18.4 to 23.0) | 24.1 (21.7 to 26.4) | –3.3 (–6.6 to 0.0) | 0.05 |
Handling multisite data
Adjusted PRWE means and group differences are presented in Table 15 for the analysis in which the primary outcome model also included site as a random effect (within which participants were nested). An unstructured covariance pattern was specified for this model. The analysis showed no statistically significant differences between treatment groups post randomisation, except at week 12 (p = 0.01). The adjusted mean difference at 52 weeks was –1.9 (95% CI –5.6 to 1.8). There was no overall effect of treatment group (difference of 2.8 points in favour of the surgery group; p = 0.09).
Timing of data collection
The primary analysis model was repeated including only data collected 1 week either side of the 6-week time point (surgery, n = 118; plaster cast, n = 112), 2 weeks either side of the 12-week time point (surgery, n = 121; plaster cast, n = 108), 6 weeks either side of the 26-week time point (surgery, n = 133; plaster cast, n = 128) and 8 weeks either side of the 52-week time point (surgery, n = 170; plaster cast, n = 155). A total of 380 participants were included in this analysis (190 in each group).
Adjusted PRWE means and group differences for the model as specified above are presented in Table 15. The analysis showed no statistically significant differences between treatment groups post randomisation, except at week 12 (p = 0.01). The adjusted mean difference at 52 weeks is in this population was –3.1 (95% CI –6.7 to 0.6). There was no overall effect of treatment group (difference of 2.4 points in favour of the surgery group; p = 0.16).
Post hoc sensitivity analysis including smoking status
Smoking status (yes/no) was included as a covariate in the primary analysis model in a sensitivity check, as this factor was found to be imbalanced by chance at baseline (smokers: 33% in the surgery group, 26% in the plaster cast group) and thought to be associated with poorer bone healing and complications. The analysis showed similar results to the primary analysis, that is, a statistically significant difference between treatment groups at week 12 (p = 0.01), but at not at week 26 (p = 0.67) or the primary end point of 52 weeks (p = 0.14). Week 6 was now statistically significant at the 5% level (p = 0.03). However, there was evidence of an overall effect of treatment group (difference of 3.6 points in favour of the surgery group; p = 0.03). The magnitude of the effect at 52 weeks increased compared with the primary analysis (from 2.1 to 2.8 points in favour of the surgery group) and the 95% CI includes the minimum clinically important difference of 6 points (see Table 15).
Displacement and lack of fracture as assessed by independent review of baseline imaging data
The randomisation for the trial was stratified by presence (or not) of displacement of a scaphoid fracture (< 1 mm or 1–2 mm inclusive) as seen on the plain radiographic views taken at baseline and used by the treating clinician to determine eligibility (although the discrepancies discussed in Chapter 3, Baseline characteristics of randomised participants, should be noted). This judgement of displacement is included as a covariate in the primary analysis model. The extent of fracture displacement was also subsequently assessed and agreed on by three independent raters who reviewed all available participant baseline imaging data (CT scans and radiographs) throughout the trial.
Baseline radiographic images were available and reviewed for all but one participant (in the surgery arm). Baseline CT images were available and reviewed for 431 participants (surgery, n = 214, 97.3%; plaster cast, n = 217, 99.1%). Both baseline and CT images were reviewed for 431 (98.2%) participants, radiographs were reviewed for only seven participants (1.6%) and neither were reviewed for one participant (0.2%). The maximum fracture displacement, in millimetres, observed on either the CT or the radiographic images was identified and used to categorise the participant’s fracture displacement as < 1 mm, 1–2 mm inclusive or > 2 mm. Overall, 213 (81.6%) of the 261 fractures that were deemed by the treating clinician to not be displaced at baseline were classified as not displaced (< 1 mm) on review, 39 (14.9%) were classified as displaced 1–2 mm, eight (3.1%) were classified as displaced > 2 mm and one (0.4%) was missing (see Appendix 3, Table 56). Of the 178 fractures that were deemed to be displaced (1–2 mm) by the treating clinician at baseline, 112 (62.9%) were classified as not displaced (< 1 mm) on review, 47 (26.4%) were classified as displaced 1–2 mm and 19 (10.7%) were classified as displaced > 2 mm.
The primary analysis model was rerun, replacing displacement as used in the randomisation as a covariate with the variable indicating the extent of displacement agreed by the three raters (see Table 15) providing very similar results to the primary analysis (see Table 11).
Consensus was reached between the three raters that displacement of the fracture was > 2 mm, based on their assessment of the baseline radiography/CT scans, for 27 (6.2%) randomised participants. A fracture could be seen on radiographic imaging for all but one of the 438 participants (n = 437, 99.8%) for whom these data were available and on CT imaging for 426 (98.8%) of 431 participants. In five participants no fracture could be seen on the CT scan, but in four of these participants the fracture could be seen on the radiographic images; thus, consensus was reached between the three raters that only one participant did not actually have a fracture (participant allocated to surgery group). Sensitivity analyses of the primary outcome model were conducted that excluded these participants (see Table 15).
The quality of the radiographic imaging in the participant who was deemed not to have a fracture was considered to be ‘good’. In the five cases in which a fracture could not be seen on CT images, the quality of the images was deemed to be ‘good’ in three cases and ‘fair’ in the other two.
In addition, for patients for whom raters thought there was no fracture on the CT scan, later images were reviewed for evidence of bone healing to confirm whether there had been a fracture. For the participant for whom the baseline radiographic images also indicated there was no fracture, this was confirmed in all other images. In the four others, the radiographic images indicated a fracture: one only had baseline images, so later images could not be reviewed; one had only baseline and 52-week images and, at 52 weeks, the fracture was interpreted as having united; one had baseline, 6-week and 52-week images and in all subsequent images the fracture was considered to be united; and one had only 6-week images, in which the fracture was considered to have united. However, union cannot be said to confirm that a fracture was initially present.
Complier-average causal effect analysis
The CACE analysis considers the effect of receiving early surgical fixation compared with no surgical fixation of the fracture, and as such does not account for the ‘partial’ compliers in the plaster cast group [i.e. the two participants for whom surgery was considered for non-union after a period of plaster cast management, and the three participants who received delayed surgical fixation (6 months after randomisation) of their non-united fracture]. We have considered these a pragmatic aspect of the ‘control’ condition. Six (2.7%) participants in the plaster cast group crossed over to the surgery group and received immediate surgical fixation of their fractures (contamination), while 31 (14.2%) participants in the surgery group did not receive surgery but instead were managed conservatively (non-compliance). The CACE estimate of the treatment effect with adjustment for non-compliance and contamination is a difference of –3.1 (95% CI –7.3 to 1.1; p = 0.15) in 52-week total PRWE score. This difference is in favour of the surgery group and is larger than the ITT treatment effect estimate at 52 weeks, demonstrating a greater, but not statistically significant, benefit of surgery among participants who complied with their treatment allocation. The 95% CI is –7.3 to 1.1; therefore, we cannot rule out a clinically meaningful difference or no effect.
Subgroup analysis
Patient preference for treatment
The first subgroup analysis was undertaken to test the hypothesis that participants who expressed a preference for surgery at baseline would benefit more from surgery than participants who expressed a preference against surgery or who had no particular treatment preference.
Descriptive summary statistics for the PRWE total score are presented in Appendix 3, Table 57, and displayed in Appendix 3, Figure 14, by treatment group stratified by baseline treatment preference. No notable differences between the two groups are observed at any time point within the subgroup who did not express a treatment preference at baseline. Unadjusted mean scores are lower (better) in the surgery group at all time points, except 26 weeks in the subgroup who expressed a preference for surgery. In the subgroup who preferred no surgery, unadjusted mean scores are lower (better) in the surgery group at all time points, except baseline (post injury) in the subgroup who expressed a preference for surgery, and the differences between the groups are larger at 6, 12 and 26 weeks than in the subgroup with a preference for surgery.
No significant interaction was observed between randomised allocation and treatment preference (surgery group and preference for surgery, p = 0.54; surgery group and preference for no surgery, p = 0.65). At 52 weeks, the adjusted mean difference in total PRWE score between the surgery and plaster cast groups was –0.9 (95% CI –6.0 to 4.2; p = 0.72) in the no preference subgroup, –3.0 (95% CI –8.2 to 2.2; p = 0.25) in the surgery preference subgroup and –4.2 (95% CI –17.2 to 8.9; p = 0.53) in the no-surgery preference subgroup.
Fracture displacement (randomisation)
The second subgroup analysis tested the hypothesis that patients with a displaced fracture at baseline would benefit more from surgery than those with a non-displaced fracture. The relationship between fracture displacement (as stratified on in the randomisation) and randomised group in terms of PRWE is illustrated in Appendix 3, Table 58 and Figure 15. Overall, participants with a displaced fracture tended to have higher post-randomisation PRWE scores than those with no or minimal displacement, and scores in the surgery group were lower than in the plaster cast group at 6, 12, 26 and 52 weeks for this subgroup. There was no statistically significant interaction effect (p = 0.35); at 52 weeks, the adjusted mean difference between the surgery and plaster cast groups was –0.8 (95% CI –5.4 to 3.8; p = 0.73) in the no displacement subgroup and –4.0 (95% CI –9.3 to 1.4; p = 0.15) in the displaced subgroup, both favouring the surgery group.
Fracture displacement (study eligibility form)
A third subgroup analysis tested the same hypothesis as above using displacement as recorded on the study eligibility form. The relationship between fracture displacement (as recorded on the study eligibility form) and randomised group in terms of PRWE is illustrated in Appendix 3, Table 59 and Figure 16. Overall, participants with a displaced fracture tended to have higher post-randomisation PRWE scores than those with no or minimal displacement, and scores in the surgery group were lower than in the plaster cast group at 6, 12, and 52 weeks for this subgroup. There was no statistically significant interaction effect (p = 0.70); at 52 weeks, the adjusted mean difference between the surgery and plaster cast groups was –1.6 (95% CI –6.1 to 3.0; p = 0.50) in the no displacement subgroup and –2.9 (95% CI –8.4 to 2.6; p = 0.30) in the displaced subgroup, both favouring the surgery group.
Surgery patients for whom the screw caused cartilage damage
A total of 188 (85.8%) participants allocated to the surgery group received treatment as allocated. For 142 (75.5%) of these, CT images at 52 weeks were assessed by three independent raters for surgical screw penetration. No screw penetration was observed for 49 (34.5%) participants. The extent of the penetration was measured for the remaining 93 participants (mean 1.6 mm, SD 0.95 mm, range 0.2–4.7 mm), and was categorised as minor (< 1 mm), which is unlikely to have an effect on articular cartilage (n = 25/93, 26.9%); moderate (1–2 mm inclusive), which will probably have an effect on cartilage, causing damage (n = 44, 47.3%); or severe (> 2 mm), which will definitely cause lasting damage to articular cartilage (n = 24, 25.8%). Descriptive summaries of baseline participant and fracture data and PRWE scores for these participants, stratified by whether or not the surgical screw used was too long and caused cartilage damage (none plus minor versus moderate plus severe) as determined on the CT scans, are provided in Appendix 3, Tables 58–60. Participants with displaced fractures at baseline were less likely to suffer from cartilage damage caused by the screw. At 52 weeks, those who did not have significant surgical screw protrusion tended to perform better on the PRWE (total unadjusted mean: 8.9 versus 10.8).
Plaster cast patients who required surgery for non-union
Of the 220 participants allocated to the plaster cast group, six crossed over to receive immediate surgical fixation while, of the remaining 214, 19 (8.9%) were deemed to require surgical fixation of a non-united fracture following plaster cast management (although two participants did not receive the surgery, see Allocated to receive plaster cast intervention). Descriptive summaries of baseline participant and fracture data and PRWE scores for the 214 plaster cast participants stratified by whether or not they needed surgery owing to non-union are presented in Appendix 3, Tables 60–62. Participants with displaced fractures at baseline were more likely to require surgery for non-union than those with < 1-mm displacement. Other risk factors include being female and a smoker. In addition, those that required surgery had had their injuries for slightly longer at enrolment to the trial than those who did not require surgery (median: 6 versus 4 days). At 52 weeks, those who did not require surgery for non-union tended to perform better on the PRWE (total unadjusted mean: 12.8 vs. 29.1).
Feasibility requirements
There were two feasibility requirements for this trial: (1) that a CT scan was performed within 2 weeks (14 days) of a patient’s injury (and before surgery if this occurred earlier) and (2) for patients in the surgery arm, that surgery was performed within 2 weeks of presentation to A&E or another clinic.
The majority of participants had a CT scan within 2 weeks of their injury (and before surgery if this was earlier) (surgery group, n = 216, 98.6%; plaster cast group, n = 196, 89.1%; see Appendix 3, Tables 63–65). Three participants in the surgery group did not meet this feasibility requirement: two crossed over treatment and received neither a CT scan nor surgery and one had their CT scan 15 days after their injury, but this was before their surgery which occurred 5 days later. Twenty-four participants in the plaster cast group did not meet this feasibility requirement: five received routine treatment and did not receive a CT scan; one immediately crossed over to surgery, which took place 7 days after injury, but did not receive a CT scan; and 18 had their CT scan between 15 and 47 days after injury (three of whom received surgery at a later date as part of their further routine treatment for non-union).
Among participants allocated to the surgery group, 182 (83.1%) had surgical fixation of their fracture within 14 days of presenting at A&E or another clinic. Of the remaining 37 participants, 31 did not receive surgery and six had their surgery between 15 and 20 days after presentation (see Appendix 3, Tables 63–65). A linear regression for the surgery arm indicated that time from injury to surgery in days may be predictive of PRWE score at 52 weeks, such that a 1-day delay in surgery is associated with a 0.78-point increase in PRWE score (95% CI –0.01 to 1.57; p = 0.054).
Secondary analysis
Patient-Rated Wrist Evaluation subscales: pain and function
The PRWE subscale scores for pain and function are summarised in Table 14. Adjusted PRWE pain and function subscale means and group differences are presented in Table 16 and displayed in Appendix 3, Figure 17. The pain subscale analysis included data from 409 participants (surgery, n = 203, 92.7%; plaster cast, n = 206, 93.6%) and the function analysis included data from 408 participants (surgery, n = 203, 92.7%; plaster cast, n = 205, 93.2%). The analysis of the pain subscale showed no statistically significant difference between treatment groups overall or at any individual post-randomisation time point. The adjusted mean difference at 52 weeks was –1.1 (95% CI –3.3 to 1.0) in favour of the surgery group. However, a statistically significant difference in function subscale score, favouring the surgery group, was seen at 6 and 12 weeks. This difference did not persist to 26 or 52 weeks, but there is an overall statistically significant difference between the two groups over time.
| Time point | Surgery, mean (95% CI) | Plaster cast, mean (95% CI) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| PRWE pain subscale (N = 409; surgery, n = 203; plaster cast, n = 206) | ||||
| 6 weeks | 18.8 (17.3 to 20.4) | 19.0 (17.5 to 20.5) | –0.1 (–2.3 to 2.0) | 0.89 |
| 12 weeks | 13.1 (11.5 to 14.6) | 15.0 (13.4 to 16.6) | –2.0 (–4.2 to 0.3) | 0.09 |
| 26 weeks | 11.0 (9.4 to 12.5) | 10.6 (9.0 to 12.2) | 0.4 (–1.8 to 2.6) | 0.75 |
| 52 weeks | 7.9 (6.4 to 9.5) | 9.1 (7.5 to 10.6) | –1.1 (–3.3 to 1.0) | 0.31 |
| Overall | 12.7 (11.5 to 14.0) | 13.5 (12.2 to 14.8) | –0.7 (–2.5 to 1.1) | 0.44 |
| PRWE function subscale (N = 408; surgery, n = 203; plaster cast, n = 205) | ||||
| 6 weeks | 16.7 (14.9 to 18.5) | 20.5 (18.7 to 22.3) | –3.8 (–6.3 to -1.3) | < 0.01 |
| 12 weeks | 8.1 (6.6 to 9.5) | 11.5 (10.0 to 13.0) | –3.4 (–5.6 to –1.3) | < 0.01 |
| 26 weeks | 5.4 (4.1 to 6.6) | 6.0 (4.7 to 7.3) | –0.6 (–2.4 to 1.2) | 0.52 |
| 52 weeks | 3.9 (2.7 to 5.1) | 4.9 (3.7 to 6.1) | –1.0 (–2.6 to 0.7) | 0.25 |
| Overall | 8.6 (7.5 to 9.7) | 10.8 (9.7 to 12.0) | –2.2 (–3.8 to –0.6) | 0.01 |
Short Form questionnaire 12-items: physical and mental health component scores
Mean mental component summary (MCS) and physical component summary (PCS) scores of the SF-12 improved (increased) over time following randomisation in both groups (except between 26 and 52 weeks in PCS in the plaster cast arm) (Table 17). At 52 weeks, the unadjusted mean difference in MCS was –1.1 points (95% CI –3.2 to 1.0 points) favouring the plaster cast group, but in PCS was 1.8 points (95% CI 0.2 to 3.3 points) favouring the surgery group.
| SF-12 | Surgery | Plaster cast | Total | |
|---|---|---|---|---|
| 6 weeks | ||||
| MCS | ||||
| Mean (SD) | 49.5 (11.4) | 49.3 (10.8) | 49.4 (11.1) | |
| Median (IQR) | 52.4 (41.2–58.1) | 51.3 (43.9–57.0) | 51.5 (42.5–57.3) | |
| Min., max. | (12.8, 67.8) | (16.0, 68.8) | (12.8, 68.8) | |
| PCS | ||||
| Mean (SD) | 43.7 (8.6) | 43.9 (8.1) | 43.8 (8.3) | |
| Median (IQR) | 44.0 (38.6–50.3) | 43.9 (38.0–49.9) | 43.9 (38.3–50.1) | |
| Min., max. | (21.1, 62.1) | (23.6, 63.7) | (21.1, 63.7) | |
| 12 weeks | ||||
| MCS | ||||
| Mean (SD) | 50.9 (11.1) | 51.5 (10.2) | 51.2 (10.7) | |
| Median (IQR) | 52.8 (44.7–59.4) | 53.2 (45.7–59.3) | 53.0 (45.3–59.3) | |
| Min., max. | (16.4, 67.9) | (15.6, 66.4) | (15.6, 67.9) | |
| PCS | ||||
| Mean (SD) | 49.9 (7.5) | 47.7 (8.5) | 48.9 (8.1) | |
| Median (IQR) | 51.9 (45.0–55.9) | 49.3 (42.6–53.5) | 50.2 (43.9–54.8) | |
| Min., max. | (21.5, 62.1) | (21.4, 64.6) | (21.4, 64.6) | |
| 26 weeks | ||||
| MCS | ||||
| Mean (SD) | 50.9 (12.1) | 52.2 (9.9) | 51.5 (11.1) | |
| Median (IQR) | 54.2 (46.2–58.4) | 54.4 (47.9–59.1) | 54.2 (47.5–59.1) | |
| Min., max. | (8.2, 67.9) | (11.4, 68.2) | (8.2, 68.2) | |
| PCS | ||||
| Mean (SD) | 51.8 (7.4) | 52.0 (7.6) | 51.9 (7.5) | |
| Median (IQR) | 54.5 (48.9–56.3) | 54.2 (49.5–56.7) | 54.3 (49.1–56.7) | |
| Min., max. | (25.9, 63.0) | (17.8, 62.5) | (17.8, 63.0) | |
| 52 weeks | ||||
| MCS | ||||
| Mean (SD) | 51.4 (10.1) | 52.5 (10.0) | 51.9 (10.1) | |
| Median (IQR) | 54.2 (46.8–57.6) | 55.8 (47.6–59.3) | 54.8 (47.5–58.2) | |
| Min., max. | (15.3, 64.7) | (13.0, 68.5) | (13.0, 68.5) | |
| PCS | ||||
| Mean (SD) | 53.2 (6.3) | 51.5 (8.1) | 52.3 (7.3) | |
| Median (IQR) | 55.9 (49.3–57.2) | 53.5 (48.4–56.2) | 54.8 (49.0–56.7) | |
| Min., max. | (33.6, 65.7) | (22.2, 69.4) | (22.2, 69.4) | |
| Time point | Surgery, mean (95% CI) | Plaster cast, mean (95% CI) | Difference (95% CI) | p-value |
| SF-12 MCS subscale (N = 408; surgery, n = 202; plaster cast, n = 206) | ||||
| 6 weeks | 49.7 (48.1 to 51.3) | 49.1 (47.5 to 50.7) | 0.5 (–1.7 to 2.8) | 0.63 |
| 12 weeks | 50.6 (49.0 to 52.1) | 50.7 (49.1 to 52.3) | –0.2 (–2.4 to 2.1) | 0.88 |
| 26 weeks | 51.0 (49.4 to 52.6) | 51.6 (49.9 to 53.3) | –0.6 (–3.0 to 1.7) | 0.60 |
| 52 weeks | 51.0 (49.6 to 52.5) | 52.3 (50.8 to 53.7) | –1.2 (–3.3 to 0.8) | 0.24 |
| Overall | 50.6 (49.3 to 51.8) | 50.9 (49.7 to 52.2) | –0.4 (–2.2 to 1.4) | 0.69 |
| SF-12 PCS subscale (N = 408; surgery, n = 202; plaster cast, n = 206) | ||||
| 6 weeks | 43.9 (42.7 to 45.1) | 43.4 (42.2 to 44.6) | 0.5 (–1.2 to 2.2) | 0.59 |
| 12 weeks | 49.8 (48.7 to 50.9) | 47.6 (46.5 to 48.8) | 2.2 (0.6 to 3.8) | 0.01 |
| 26 weeks | 51.6 (50.5 to 52.7) | 51.6 (50.5 to 52.8) | –0.0 (–1.6 to 1.5) | 0.95 |
| 52 weeks | 53.1 (52.1 to 54.2) | 51.5 (50.5 to 52.6) | 1.6 (0.2 to 3.1) | 0.03 |
| Overall | 49.6 (48.8 to 50.4) | 48.5 (47.7 to 49.3) | 1.1 (–0.1 to 2.2) | 0.08 |
Adjusted SF-12 means and group differences for the models are presented in Table 17 and displayed in Appendix 3, Figure 18. Both analysis models included data from 408 participants (surgery, n = 202, 92.2%; plaster cast, n = 206, 93.6%). The analysis of the MCS subscale showed no statistically significant difference between treatment groups overall or at any individual post-randomisation time point. The adjusted mean difference at 52 weeks was –1.2 (95% CI –3.3 to 0.8) in favour of the plaster cast group. However, a statistically significant difference in PCS subscale score, favouring the surgery group, was seen at 12 and 52 weeks, but not at 6 or 26 weeks or overall (p = 0.08). The adjusted mean difference at 52 weeks was 1.6 (95% CI 0.2 to 3.1) in favour of the surgery group.
Wrist range of movement and grip strength: affected wrist
Measures of wrist range of movement and grip strength for the affected wrist are presented in Table 18. Similar mean values were observed across these variables in the two groups at baseline. These were assessed at hospital visits at baseline and at 6, 12 and 52 weeks post randomisation.
| Wrist range of movement and grip strength measures | Surgery | Plaster cast | Total |
|---|---|---|---|
| Baseline | |||
| Beighton laxity score | |||
| Mean (SD) | 1.1 (2.0) | 0.9 (1.7) | 1.0 (1.8) |
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Min., max. | (0.0, 10.0) | (0.0, 8.0) | (0.0, 10.0) |
| Extension (degrees) | |||
| Mean (SD) | 32.0 (18.6) | 28.9 (17.2) | 30.4 (17.9) |
| Median (IQR) | 30.0 (20.0–42.0) | 30.0 (18.0–40.0) | 30.0 (20.0–40.0) |
| Min., max. | (0.0, 135.0) | (–15.0, 90.0) | (–15.0, 135.0) |
| Flexion (degrees) | |||
| Mean (SD) | 35.0 (25.5) | 34.9 (21.7) | 35.0 (23.6) |
| Median (IQR) | 30.0 (20.0–45.0) | 35.0 (22.0–44.0) | 32.0 (20.0–45.0) |
| Min., max. | (0.0, 160.0) | (0.0, 162.0) | (0.0, 162.0) |
| Radial deviation (degrees) | |||
| Mean (SD) | 14.3 (9.5) | 14.3 (9.6) | 14.3 (9.6) |
| Median (IQR) | 13.0 (10.0–20.0) | 14.0 (9.0–20.0) | 13.0 (9.0–20.0) |
| Min., max. | (0.0, 60.0) | (0.0, 70.0) | (0.0, 70.0) |
| Ulnar deviation (degrees) | |||
| Mean (SD) | 18.0 (10.9) | 18.6 (11.0) | 18.3 (10.9) |
| Median (IQR) | 17.0 (10.0–22.5) | 18.0 (10.0–25.0) | 18.0 (10.0–25.0) |
| Min., max. | (0.0, 70.0) | (0.0, 60.0) | (0.0, 70.0) |
| Forearm rotation supination (degrees) | |||
| Mean (SD) | 66.9 (26.7) | 63.6 (27.8) | 65.3 (27.3) |
| Median (IQR) | 75.0 (56.5–85.0) | 70.0 (50.0–85.0) | 73.0 (50.0–85.0) |
| Min., max. | (0.0, 124.0) | (–10.0, 118.0) | (–10.0, 124.0) |
| Forearm rotation pronation (degrees) | |||
| Mean (SD) | 72.2 (23.1) | 71.2 (25.0) | 71.7 (24.0) |
| Median (IQR) | 80.0 (67.5–90.0) | 80.0 (68.5–90.0) | 80.0 (68.0–90.0) |
| Min., max. | (0.0, 100.0) | (0.0, 105.0) | (0.0, 105.0) |
| Grip strength (kg) | |||
| Mean (SD) | 9.6 (10.0) | 9.8 (10.6) | 9.7 (10.3) |
| Median (IQR) | 6.0 (2.0–15.3) | 7.0 (2.0–12.7) | 6.7 (2.0–14.4) |
| Min., max. | (0.0, 61.7) | (0.0, 58.0) | (0.0, 61.7) |
| 6 weeks | |||
| Extension (degrees) | |||
| Mean (SD) | 51.0 (20.2) | 40.0 (18.3) | 45.4 (20.0) |
| Median (IQR) | 50.0 (38.0–60.0) | 40.0 (28.0–50.0) | 45.0 (30.0–56.0) |
| Min., max. | (5.0, 135.0) | (0.0, 90.0) | (0.0, 135.0) |
| Flexion (degrees) | |||
| Mean (SD) | 51.6 (28.3) | 40.1 (23.4) | 45.7 (26.5) |
| Median (IQR) | 49.0 (30.0–65.0) | 35.0 (25.0–50.0) | 40.0 (30.0–60.0) |
| Min., max. | (5.0, 162.0) | (–5.0, 158.0) | (–5.0, 162.0) |
| Radial deviation (degrees) | |||
| Mean (SD) | 21.7 (10.7) | 21.3 (12.8) | 21.5 (11.8) |
| Median (IQR) | 20.0 (15.0–28.0) | 20.0 (11.0–28.0) | 20.0 (13.0–28.0) |
| Min., max. | (0.0, 60.0) | (0.0, 70.0) | (0.0, 70.0) |
| Ulnar deviation (degrees) | |||
| Mean (SD) | 29.3 (12.1) | 23.5 (13.0) | 26.3 (12.9) |
| Median (IQR) | 30.0 (20.0–38.0) | 20.0 (15.0–30.0) | 25.0 (18.0–35.0) |
| Min., max. | (1.0, 60.0) | (0.0, 70.0) | (0.0, 70.0) |
| Forearm rotation supination (degrees) | |||
| Mean (SD) | 82.4 (15.7) | 74.9 (20.3) | 78.5 (18.6) |
| Median (IQR) | 90.0 (80.0–90.0) | 80.0 (65.0–90.0) | 85.0 (72.0–90.0) |
| Min., max. | (0.0, 131.0) | (0.0, 108.0) | (0.0, 131.0) |
| Forearm rotation pronation (degrees) | |||
| Mean (SD) | 82.8 (14.4) | 80.1 (15.5) | 81.4 (15.0) |
| Median (IQR) | 90.0 (80.0–90.0) | 85.0 (75.0–90.0) | 90.0 (80.0–90.0) |
| Min., max. | (0.0, 110.0) | (10.0, 104.0) | (0.0, 110.0) |
| Grip strength (kg) | |||
| Mean (SD) | 24.1 (12.7) | 20.1 (14.0) | 22.0 (13.5) |
| Median (IQR) | 23.3 (15.3–32.7) | 18.2 (9.3–28.7) | 20.0 (11.3–30.7) |
| Min., max. | (0.0, 77.3) | (0.0, 81.7) | (0.0, 81.7) |
| 12 weeks | |||
| Extension (degrees) | |||
| Mean (SD) | 61.1 (17.7) | 56.9 (19.5) | 59.1 (18.7) |
| Median (IQR) | 60.0 (50.0–70.0) | 55.0 (43.5–70.0) | 60.0 (45.0–70.0) |
| Min., max. | (13.0, 125.0) | (2.0, 125.0) | (2.0, 125.0) |
| Flexion (degrees) | |||
| Mean (SD) | 62.0 (23.7) | 55.3 (22.3) | 58.7 (23.2) |
| Median (IQR) | 60.0 (45.0–75.0) | 55.0 (41.0–70.0) | 58.0 (45.0–72.0) |
| Min., max. | (15.0, 144.0) | (5.0, 144.0) | (5.0, 144.0) |
| Radial deviation (degrees) | |||
| Mean (SD) | 26.1 (12.7) | 26.2 (14.5) | 26.1 (13.6) |
| Median (IQR) | 25.0 (18.0–30.0) | 23.0 (15.0–32.0) | 24.0 (18.0–30.0) |
| Min., max. | (5.0, 80.0) | (0.0, 80.0) | (0.0, 80.0) |
| Ulnar deviation (degrees) | |||
| Mean (SD) | 35.4 (12.7) | 31.6 (13.7) | 33.5 (13.3) |
| Median (IQR) | 35.0 (28.0–40.0) | 30.0 (22.0–40.0) | 31.0 (25.0–40.0) |
| Min., max. | (10.0, 80.0) | (0.0, 80.0) | (0.0, 80.0) |
| Forearm rotation supination (degrees) | |||
| Mean (SD) | 87.1 (13.8) | 82.3 (18.2) | 84.7 (16.3) |
| Median (IQR) | 90.0 (85.0–90.0) | 90.0 (80.0–90.0) | 90.0 (80.0–90.0) |
| Min., max. | (10.0, 140.0) | (0.0, 126.0) | (0.0, 140.0) |
| Forearm rotation pronation (degrees) | |||
| Mean (SD) | 86.5 (8.5) | 83.4 (13.8) | 85.0 (11.5) |
| Median (IQR) | 90.0 (85.0–90.0) | 90.0 (80.0–90.0) | 90.0 (80.0–90.0) |
| Min., max. | (26.0, 104.0) | (0.0, 120.0) | (0.0, 120.0) |
| Grip strength (kg) | |||
| Mean (SD) | 30.8 (12.5) | 28.2 (14.4) | 29.5 (13.5) |
| Median (IQR) | 29.3 (22.3–39.3) | 28.5 (18.7–37.8) | 28.7 (20.0–38.7) |
| Min., max. | (0.0, 82.0) | (0.0, 89.0) | (0.0, 89.0) |
| 52 weeks | |||
| Extension (degrees) | |||
| Mean (SD) | 68.4 (21.0) | 68.8 (15.5) | 68.6 (18.6) |
| Median (IQR) | 70.0 (56.0–80.0) | 70.0 (56.0–80.0) | 70.0 (56.0–80.0) |
| Min., max. | (15.0, 140.0) | (40.0, 115.0) | (15.0, 140.0) |
| Flexion (degrees) | |||
| Mean (SD) | 69.8 (20.3) | 68.4 (16.4) | 69.1 (18.5) |
| Median (IQR) | 70.0 (55.0–85.0) | 70.0 (60.0–80.0) | 70.0 (58.0–80.0) |
| Min., max. | (20.0, 152.0) | (22.0, 105.0) | (20.0, 152.0) |
| Radial deviation (degrees) | |||
| Mean (SD) | 32.2 (17.4) | 32.5 (14.5) | 32.4 (16.1) |
| Median (IQR) | 28.0 (20.0–40.0) | 30.0 (22.0–40.0) | 30.0 (20.0–40.0) |
| Min., max. | (6.0, 90.0) | (8.0, 80.0) | (6.0, 90.0) |
| Ulnar deviation (degrees) | |||
| Mean (SD) | 40.6 (14.8) | 39.9 (13.7) | 40.3 (14.3) |
| Median (IQR) | 40.0 (30.0–50.0) | 40.0 (30.0–49.0) | 40.0 (30.0–50.0) |
| Min., max. | (8.0, 90.0) | (12.0, 80.0) | (8.0, 90.0) |
| Forearm rotation supination (degrees) | |||
| Mean (SD) | 88.3 (13.3) | 85.2 (13.9) | 86.8 (13.6) |
| Median (IQR) | 90.0 (86.0–90.0) | 90.0 (80.0–90.0) | 90.0 (85.0–90.0) |
| Min., max. | (30.0, 136.0) | (30.0, 122.0) | (30.0, 136.0) |
| Forearm rotation pronation (degrees) | |||
| Mean (SD) | 86.8 (10.5) | 86.2 (9.5) | 86.5 (10.0) |
| Median (IQR) | 90.0 (85.0–90.0) | 90.0 (85.0–90.0) | 90.0 (85.0–90.0) |
| Min., max. | (5.0, 114.0) | (40.0, 109.0) | (5.0, 114.0) |
| Grip strength (kg) | |||
| Mean (SD) | 36.9 (12.7) | 37.4 (14.2) | 37.2 (13.4) |
| Median (IQR) | 36.2 (28.7–44.8) | 38.5 (28.7–46.2) | 37.3 (28.7–45.2) |
| Min., max. | (10.3, 109.7) | (4.7, 88.3) | (4.7, 109.7) |
Grip strength
On average, grip strength, in kilograms, increased over time in both groups and was higher in the surgery group than in the plaster cast group at all post-randomisation time points except 52 weeks.
Adjusted grip strength means and group differences are presented in Table 19 and displayed in Appendix 3, Figure 19. The analysis models included data from 407 participants (surgery, n = 201, 91.8%; plaster cast, n = 206, 93.6%) and showed a statistically significant difference between treatment groups at 6 weeks post randomisation (p < 0.001) favouring the surgery group, a borderline statistically significant result at 12 weeks (p = 0.06) and no difference at 52 weeks. The adjusted mean difference at 52 weeks was –1.0 (95% CI –3.7 to 1.8) favouring the plaster cast group.
| Time point | Surgery, mean (95% CI) | Plaster cast, mean (95% CI) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| 6 weeks | 23.8 (22.0 to 25.6) | 19.4 (17.6 to 21.2) | 4.4 (1.8 to 6.9) | 0.001 |
| 12 weeks | 30.9 (29.0 to 32.8) | 28.3 (26.4 to 30.2) | 2.6 (–0.1 to 5.3) | 0.06 |
| 52 weeks | 37.0 (35.1 to 39.0) | 38.0 (36.1 to 40.0) | –1.0 (–3.7 to 1.7) | 0.48 |
| Overall | 30.1 (28.5 to 31.7) | 27.9 (26.3 to 29.5) | 2.0 (–0.3 to 4.2) | 0.08 |
Union
Assessment of union via radiographs was possible for 188 (85.8%) participants in the surgery group and 201 (91.4%) participants in the plaster cast group at 6 weeks, of whom 13 (6.9%) and 32 (15.9%), respectively, displayed non-union or only slight union (Table 20). At week 12, radiographic images were available for assessment of union for fewer participants than at 6 weeks: 169 (77.2%) in the surgery group and 163 (74.1%) in the plaster cast arm. A lower proportion of those reviewed were graded as having non-union or slight union at this time point than at 6 weeks in both groups (surgery, n = 7, 4.1%; plaster cast, n = 23, 14.1%).
| Time pointa | Union | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|---|
| 6 weeks, n (%) | Union | 47 (21.5) | 26 (11.8) | 73 (16.6) |
| Almost full union | 81 (37.0) | 73 (33.2) | 154 (35.1) | |
| Partial union | 47 (21.5) | 70 (31.8) | 117 (26.7) | |
| Slight union | 11 (5.0) | 23 (10.5) | 34 (7.7) | |
| Non-union | 2 (0.9) | 9 (4.1) | 11 (2.5) | |
| Missing | 31 (14.2) | 19 (8.6) | 50 (11.4) | |
| 12 weeks, n (%) | Union | 102 (46.6) | 63 (28.6) | 165 (37.6) |
| Almost full union | 45 (20.5) | 44 (20.0) | 89 (20.3) | |
| Partial union | 15 (6.8) | 33 (15.0) | 48 (10.9) | |
| Slight union | 7 (3.2) | 13 (5.9) | 20 (4.6) | |
| Non-union | 0 (0.0) | 10 (4.5) | 10 (2.3) | |
| Missing | 50 (22.8) | 57 (25.9) | 107 (24.4) | |
| 52 weeks, n (%) | Union | 93 (42.5) | 72 (32.7) | 165 (37.6) |
| Almost full union | 64 (29.2) | 59 (26.8) | 123 (28) | |
| Partial union | 3 (1.4) | 10 (4.5) | 13 (3) | |
| Slight union | 3 (1.4) | 5 (2.3) | 8 (1.8) | |
| Non-union | 1 (0.5) | 4 (1.8) | 5 (1.1) | |
| Missing | 55 (25.1) | 70 (31.8) | 125 (28.5) | |
| PRWE total score,b n [mean (SD)] | Attended imaging | 161 [10.6 (15.5)] | 145 [15.0 (20.1)] | 306 [12.7 (18.0)] |
| Did not attend | 25 [16.4 (22.1)] | 31 [10.6 (17.7)] | 56 [13.2 (19.8)] |
The proportion of participants assessed for union decreased at each time point in both groups. At 52 weeks, the grading of union was based on CT images or on radiographic images when CT was not available and was assessed for 314 participants (surgery, n = 164, 74.9%; plaster cast, n = 150, 68.2%), of whom 13 (4.1%) were deemed to have non-union or only slight union of their fracture (surgery, n = 4, 2.4%; plaster cast, n = 9, 6.0%). Furthermore, in Table 20, the percentages are calculated using a denominator that includes participants with missing data. There is a substantial number of participants with missing data (28.5% overall at 52 weeks), which may underestimate the proportion of participants with union.
The 52-week PRWE scores, where available, are summarised for the two groups according to whether or not the participants had imaging available for union assessment at 52 weeks (see Table 20). Overall, there is little difference in the mean PRWE scores of those who attended and did not attend imaging (12.7 vs. 13.2, respectively); however, there are differences between the two randomised groups. In the surgery group, participants who did not attend imaging tended to have higher (worse) scores than those who did attend imaging (mean 16.4 vs. 10.6, respectively) and so were perhaps less likely to have full union of their fractures. However, the trend reverses in the plaster cast group, with those not attending imaging having lower (better) scores and so more likely to have better union.
Participants in the surgery group were less likely than those in the plaster cast group to have non-union or only slight union of their fracture at 52 weeks, but this difference was not statistically significant in a logistic regression model adjusting for age, fracture displacement and hand dominance [odds ratio (OR) 0.40, 95% CI 0.12 to 1.33; p = 0.13). Similarly, results following multiple imputation of the union variables at 6, 12 and 52 weeks indicated that the likelihood of participants having non-union or only slight union of their fracture at 52 weeks was lower for participants allocated to the surgery group than for those in the plaster cast group (OR 0.51, 95% CI 0.17 to 1.57), but this difference was not statistically significant (p = 0.24). In the surgery group, 4 out of 219 participants were deemed to have non-union or slight union at 52 weeks (1.8%), compared with 9 out of 220 in the plaster cast group (4.1%). Based on these figures, the increase in the number of participants who need to be offered surgery compared with the number offered plaster cast management (followed by fixation of fractures that fail to unite with cast immobilisation) to prevent one extra non-union or slight union at 52 weeks is 44 [number needed to treat (NNT)]. However, data are missing in over a quarter of participants, and this calculation, by default, assumes that all those in whom data are missing experience at least partial union. At the other extreme, assuming that all those in whom data are missing experience slight union or non-union, the NNT is 11. For non-union alone, the NNT is 73 and 12 in the first and second scenario, respectively.
The one participant in the plaster cast group whose fracture was assessed at 52 weeks by three raters as showing non-union underwent surgical fixation the day after randomisation, then wore a splint for 2 weeks; no concerns about non-union were raised and recorded in the treatment confirmation form at 6 or 12 weeks. One of the four participants in the plaster cast group whose fractures were assessed as showing non-union had surgery to fix non-union 3 months after randomisation, a further surgery for persistent non-union 6 months after that and surgery a month later to remove the wires from the second operation. The remaining three participants received routine treatment with a plaster cast and were not offered surgery; for two of them, this was because non-union was not suspected by the treating clinician on review of radiography, while the third did not attend for radiography at 6 or 12 weeks.
Malunion
Malunion was determined by calculating the ratio of the scaphoid height to length, using thresholds of both 0.6 and 0.7 (Table 21). By default, more participants are classified as having malunion using the 0.6 threshold than 0.7. Considering those with non-missing data only, at 6 weeks, 175 (93.6%) participants in the surgery group and 180 (90.0%) participants in the plaster cast group had malunion based on the 0.6 threshold. At 0.7, the figures are 52 (27.8%) and 51 (25.5%), respectively. Malunion at both thresholds remained reasonably steady in both groups at 6, 12 and 52 weeks, as determined from radiographic images. However, at 52 weeks, based on CT scans, the rate of malunion dramatically decreased to 60 (38.29%) participants in the surgery group and 45 (33.3%) participants in the plaster cast group at the 0.6 threshold, and to seven (4.5%) and seven (5.2%), respectively, at 0.7.
| Time point | Union, n (%) | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|---|
| 0.6 threshold | ||||
| Baseline (radiography) | No malunion | 30 (13.7) | 28 (12.7) | 58 (13.2) |
| Malunion | 182 (83.1) | 190 (86.4) | 372 (84.7) | |
| Missing | 7 (3.2) | 2 (0.9) | 9 (2.1) | |
| Baseline (CT scan) | No malunion | 154 (70.3) | 160 (72.7) | 314 (71.5) |
| Malunion | 63 (28.8) | 54 (24.5) | 117 (26.7) | |
| Missing | 2 (0.9) | 6 (2.7) | 8 (1.8) | |
| 6 weeks | No malunion | 12 (5.5) | 20 (9.1) | 32 (7.3) |
| Malunion | 175 (79.9) | 180 (81.8) | 355 (80.9) | |
| Missing | 32 (14.6) | 20 (9.1) | 52 (11.8) | |
| 12 weeks | No malunion | 10 (4.6) | 12 (5.5) | 22 (5.0) |
| Malunion | 159 (72.6) | 151 (68.6) | 310 (70.6) | |
| Missing | 50 (22.8) | 57 (25.9) | 107 (24.4) | |
| 52 weeks (radiography) | No malunion | 9 (4.1) | 13 (5.9) | 22 (5.0) |
| Malunion | 148 (67.6) | 128 (58.2) | 276 (62.9) | |
| Missing | 62 (28.3) | 79 (35.9) | 141 (32.1) | |
| 52 weeks (CT scan) | No malunion | 97 (44.3) | 90 (40.9) | 187 (42.6) |
| Malunion | 60 (27.4) | 45 (20.5) | 105 (23.9) | |
| Missing | 62 (28.3) | 85 (38.6) | 147 (33.5) | |
| 0.7 threshold | ||||
| Baseline (radiography) | No malunion | 167 (76.3) | 173 (78.6) | 340 (77.4) |
| Malunion | 45 (20.5) | 45 (20.5) | 90 (20.5) | |
| Missing | 7 (3.2) | 2 (0.9) | 9 (2.1) | |
| Baseline (CT scan) | No malunion | 214 (97.7) | 212 (96.4) | 426 (97) |
| Malunion | 3 (1.4) | 2 (0.9) | 5 (1.1) | |
| Missing | 2 (0.9) | 6 (2.7) | 8 (1.8) | |
| 6 weeks | No malunion | 135 (61.6) | 149 (67.7) | 284 (64.7) |
| Malunion | 52 (23.7) | 51 (23.2) | 103 (23.5) | |
| Missing | 32 (14.6) | 20 (9.1) | 52 (11.8) | |
| 12 weeks | No malunion | 117 (53.4) | 118 (53.6) | 235 (53.5) |
| Malunion | 52 (23.7) | 45 (20.5) | 97 (22.1) | |
| Missing | 50 (22.8) | 57 (25.9) | 107 (24.4) | |
| 52 weeks (radiography) | No malunion | 96 (43.8) | 101 (45.9) | 197 (44.9) |
| Malunion | 61 (27.9) | 40 (18.2) | 101 (23.0) | |
| Missing | 62 (28.3) | 79 (35.9) | 141 (32.1) | |
| 52 weeks (CT scan) | No malunion | 150 (68.5) | 128 (58.2) | 278 (63.3) |
| Malunion | 7 (3.2) | 7 (3.2) | 14 (3.2) | |
| Missing | 62 (28.3) | 85 (38.6) | 147 (33.5) | |
Complications
At least one surgical complication, up to and including 52 weeks post randomisation, was experienced by 31 (14.2%) participants randomised to the surgery group and by three (1.4%) participants in the plaster cast group. The most common surgical complication was screw protrusion (Table 22), reported for 10 participants in the surgery group and one in the plaster cast group at 6, 12 or 52 weeks. A nerve event (of any kind) was reported for one participant in the plaster cast group (hypoaesthesia at 52 weeks) and for 10 unique participants in the surgery group. Table 22 presents only complications that were reported for at least one participant throughout the course of the study; however, we also explicitly asked about the following surgical complications, of which none were reported: superficial division of radial nerve, vessel events and avascular necrosis.
| Surgery-related complications, n (%)a | Time point | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|---|
| Surgical site infection | 6 weeks | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 1 (0.5) | 2 (0.9) | 3 (0.7) | |
| Delayed wound healing | 6 weeks | 2 (0.9) | 0 (0.0) | 2 (0.5) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 1 (0.5) | 1 (0.5) | 2 (0.5) | |
| Regional pain syndrome | 6 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 weeks | 1 (0.5) | 0 (0.0) | 1 (0.2) | |
| 52 weeks | 1 (0.5) | 0 (0.0) | 1 (0.2) | |
| Nerve event | ||||
| Hypoaesthesia | 6 weeks | 2 (0.9) | 0 (0.0) | 2 (0.5) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 1 (0.5) | 1 (0.5) | 2 (0.5) | |
| Numbness | 6 weeks | 3 (1.4) | 0 (0.0) | 3 (0.7) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Superficial division of median nerve | 6 weeks | 3 (1.4) | 0 (0.0) | 3 (0.7) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other nerve event | 6 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 weeks | 2 (0.9) | 0 (0.0) | 2 (0.5) | |
| 52 weeks | 1 (0.5) | 0 (0.0) | 1 (0.2) | |
| Screw-related complications | ||||
| Protrusion | 6 weeks | 2 (0.9) | 0 (0.0) | 2 (0.5) |
| 12 weeks | 1 (0.5) | 0 (0.0) | 1 (0.2) | |
| 52 weeks | 7 (3.2) | 1 (0.5) | 8 (1.8) | |
| Bending | 6 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 1 (0.5) | 0 (0.0) | 1 (0.2) | |
| Radiolucent halo | 6 weeks | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 2 (0.9) | 0 (0.0) | 2 (0.5) | |
| Implant problem – movement | 6 weeks | 2 (0.9) | 0 (0.0) | 2 (0.5) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 3 (1.4) | 0 (0.0) | 3 (0.7) | |
| Otherb | 6 weeks | 3 (1.4) | 0 (0.0) | 3 (0.7) |
| 12 weeks | 2 (0.9) | 0 (0.0) | 2 (0.5) | |
| 52 weeks | 1 (0.5) | 1 (0.5) | 2 (0.5) | |
| ≥ 1 surgical complication | 6 weeks | 14 (6.4) | 0 (0.0) | 14 (3.2) |
| 12 weeks | 6 (2.7) | 0 (0.0) | 6 (1.4) | |
| 52 weeks | 15 (6.9) | 3 (1.4) | 18 (4.1) | |
| Any time point | 31 (14.2) | 3 (1.4) | 34 (7.7) | |
At least one issue relating to the plaster cast, up to and including 52 weeks, was reported for five (2.3%) participants randomised to the surgery group and for 40 (18.2%) participants in the plaster cast group (Table 23). The most commonly reported issue was that the cast caused soreness (this was generally minor rubbing of the skin), reported for three surgery participants and 23 plaster cast participants at 6 or 12 weeks.
| Plaster cast issues, n (%)a | Time point | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|---|
| Cast broken | 6 weeks | 0 (0.0) | 7 (3.2) | 7 (1.6) |
| 12 weeks | 1 (0.5) | 1 (0.5) | 1 (0.5) | |
| 52 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cast too soft | 6 weeks | 1 (0.5) | 9 (4.1) | 10 (2.3) |
| 12 weeks | 0 (0.0) | 1 (0.5) | 1 (0.2) | |
| 52 weeks | 0 (0.0) | 1 (0.5) | 1 (0.2) | |
| Cast too tight | 6 weeks | 2 (0.9) | 8 (3.6) | 10 (2.3) |
| 12 weeks | 0 (0.0) | 1 (0.5) | 1 (0.2) | |
| 52 weeks | 0 (0.0) | 2 (0.9) | 2 (0.5) | |
| Cast caused soreness | 6 weeks | 3 (1.4) | 20 (9.1) | 23 (5.2) |
| 12 weeks | 0 (0.0) | 3 (1.4) | 3 (0.7) | |
| 52 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Otherb | 6 weeks | 0 (0.0) | 5 (2.3) | 5 (1.1) |
| 12 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 52 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| One or more cast complication | 6 weeks | 5 (2.3) | 40 (18.2) | 45 (10.3) |
| 12 weeks | 1 (0.5) | 4 (1.8) | 5 (1.1) | |
| 52 weeks | 0 (0.0) | 3 (1.4) | 3 (0.7) | |
| Any time point | 6 (2.7) | 45 (20.5) | 51 (11.6) |
At least one medical complication, up to 52 weeks, was reported for four (1.8%) participants randomised to the surgery group and for five (2.3%) participants in the plaster cast group. These were either a chest infection or another issue (gastric upset, depression or tenderness of the affected wrist; Table 24). We also explicitly asked about the following medical complications, of which none were reported: myocardial infarction, stroke, deep vein thrombosis requiring treatment and pulmonary embolism requiring treatment.
| Medical complications, n (%)a | Time point | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|---|
| Chest infection | 6 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 weeks | 1 (0.5) | 2 (0.9) | 3 (0.7) | |
| 52 weeks | 4 (1.8) | 0 (0.0) | 4 (0.9) | |
| Otherb | 6 weeks | 0 (0.0) | 2 (0.9) | 2 (0.5) |
| 12 weeks | 0 (0.0) | 1 (0.5) | 1 (0.2) | |
| 52 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| One or more medical complication | 6 weeks | 0 (0.0) | 2 (0.9) | 2 (0.5) |
| 12 weeks | 1 (0.5) | 3 (1.4) | 4 (0.9) | |
| 52 weeks | 4 (1.8) | 0 (0.0) | 4 (0.9) | |
| Any time point | 4 (1.8) | 5 (2.3) | 9 (2.1) |
There was no evidence of a difference between the two groups in the likelihood of participants experiencing at least one surgical, medical or cast complication (as recorded on the complications form, with details in Tables 22–24) up to 52 weeks [surgery group, n = 39, 17.8%; plaster cast group, n = 51, 23.2% (OR 0.72, 95% CI 0.45 to 1.15; p = 0.17)]. We must be careful not to overinterpret this finding to mean that the likelihood of experiencing a surgical, cast or medical complication is similar in both groups. Cast complications were observed more frequently among the plaster cast group and surgical complications were observed more frequently among the surgery group. Complications related to the plaster cast tended to be relatively minor and would not result in ongoing problems (see Table 23). However, surgery-related complications are more severe and are more likely to have potentially long-lasting consequences for the individual (see Table 22). As an example, Table 23 shows that, in the plaster cast group, half (30 of 58 events) of the complications were due to the cast being broken, soft or tight, and, in the statistical analysis of the complications, are given the same importance as CRPS or an infection. Non-union symptoms (n = 8) were recorded as AEs or complications, even when this was an expected part of the control pathway and when non-union had been identified. There are also some discrepancies between the reporting of events on the complications form and on the AEs form. For example, the AEs form logs three participants as having CRPS, but the complications form records only two. The three raters’ review of the imaging identified further complications that were not accounted for in the AE or complications forms, particularly after surgery. This could have been influenced by whether or not the problem had been recognised, by the surgeon’s decision on whether or not this needed to be reported or by whether or not the complication was identified on imaging done solely for research purposes and hence was not available to clinicians. The logistic regression included complications from the hospital forms only and did not include those from other data sources (i.e. AE reporting or imaging).
Cases when two out of the three raters agreed that there was a complication on a review of imaging data are reported by randomised group and time point in Table 25.
| Complication observed on independent review of imaging data, n (%)a | Time point | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|---|
| Osteoarthritisb | Baseline (radiography) | N = 218 | N = 220 | N = 438 |
| 21 (9.6) | 22 (10.0) | 43 (9.8) | ||
| Baseline (CT scan) | N = 217 | N = 214 | N = 431 | |
| 22 (10.1) | 26 (12.2) | 48 (11.1) | ||
| 6 weeks | N = 188 | N = 201 | N = 389 | |
| 15 (8.0) | 21 (10.5) | 36 (9.3) | ||
| 12 weeks | N = 169 | N = 163 | N = 332 | |
| 21 (12.4) | 28 (17.2) | 49 (14.8) | ||
| 52 weeks (radiography) | N = 157 | N = 142 | N = 299 | |
| 25 (15.9) | 24 (16.9) | 49 (16.4) | ||
| 52 weeks (CT scan) | N = 157 | N = 135 | N = 292 | |
| 42 (26.8) | 36 (26.7) | 78 (26.7) | ||
| Avascular necrosisb,c | Baseline (radiography) | N = 218 | N = 220 | N = 438 |
| None | 204 (93.6) | 204 (92.7) | 408 (93.2) | |
| Just | 14 (6.4) | 15 (6.8) | 29 (6.6) | |
| Missing | 0 (0.0) | 1 (0.5) | 1 (0.2) | |
| Baseline (CT scan) | N = 217 | N = 214 | N = 431 | |
| None | 197 (90.8) | 187 (87.4) | 384 (89.1) | |
| Just | 20 (9.2) | 27 (12.6) | 47 (10.9) | |
| 6 weeks | N = 188 | N = 201 | N = 389 | |
| None | 141 (75.0) | 131 (65.2) | 272 (69.9) | |
| Just | 46 (24.5) | 61 (30.4) | 107 (27.5) | |
| Marked 1 | 1 (0.5) | 8 (4.0) | 9 (2.3) | |
| Marked > 1 | 0 (0.0) | 1 (0.5) | 1 (0.3) | |
| 12 weeks | N = 169 | N = 163 | N = 332 | |
| None | 130 (76.9) | 109 (66.9) | 239 (72.0) | |
| Just | 39 (23.1) | 51 (31.3) | 90 (27.1) | |
| Marked 1 | 0 (0.0) | 1 (0.6) | 1 (0.3) | |
| Marked > 1 | 0 (0.0) | 2 (1.2) | 2 (0.6) | |
| 52 weeks (radiography) | N = 157 | N = 142 | N = 299 | |
| None | 135 (86.0) | 110 (77.5) | 245 (81.9) | |
| Just | 22 (14.0) | 27 (19.0) | 49 (16.4) | |
| Marked 1 | 0 (0.0) | 4 (2.8) | 4 (1.3) | |
| Missing | 0 (0.0) | 1 (0.7) | 1 (0.3) | |
| 52 weeks (CT scan) | N = 157 | N = 135 | N = 292 | |
| None | 80 (51.0) | 57 (42.2) | 137 (46.9) | |
| Just | 72 (45.9) | 69 (51.1) | 141 (48.3) | |
| Marked 1 | 5 (3.2) | 5 (3.7) | 10 (3.4) | |
| Marked > 1 | 0 (0.0) | 3 (2.2) | 3 (1.0) | |
| Fragmented | 0 (0.0) | 1 (0.7) | 1 (0.3) | |
| Screw penetrationd | 6 weeks | N = 172 | N = 6 | N = 178 |
| 57 (33.1) | 1 (16.7) | 58 (32.6) | ||
| 12 weeks | N = 156 | N = 6 | N = 162 | |
| 55 (35.3) | 2 (33.3) | 57 (35.2) | ||
| 52 weeks (radiography) | N = 135 | N = 15 | N = 150 | |
| 52 (38.5) | 5 (13.3) | 57 (38.0) | ||
| 52 weeks (CT scan) | N = 136 | N = 11 | N = 147 | |
| 75 (55.1) | 8 (72.7) | 83 (56.5) | ||
| Screw lucencyd | 6 weeks | N = 172 | N = 6 | N = 178 |
| None | 139 (80.8) | 4 (66.7) | 143 (80.3) | |
| < 1 mm | 32 (18.6) | 2 (33.3) | 34 (19.1) | |
| 1–2 mm | 1 (0.6) | 0 (0.0) | 1 (0.6) | |
| 12 weeks | N = 156 | N = 6 | N = 162 | |
| None | 113 (72.4) | 6 (100.0) | 119 (73.5) | |
| < 1 mm | 41 (26.3) | 0 (0.0) | 41 (25.3) | |
| 1–2 mm | 2 (1.3) | 0 (0.0) | 2 (1.2) | |
| 52 weeks (radiography) | N = 135 | N = 15 | N = 150 | |
| None | 99 (73.3) | 7 (46.7) | 106 (70.7) | |
| < 1 mm | 32 (23.7) | 8 (53.3) | 40 (26.7) | |
| 1–2 mm | 4 (3.0) | 0 (0.0) | 4 (2.7) | |
| 52 weeks (CT scan) | N = 136 | N = 11 | N = 147 | |
| None | 108 (79.4) | 7 (63.6) | 115 (78.2) | |
| < 1mm | 22 (16.2) | 3 (27.3) | 25 (17.0) | |
| > 1mm | 4 (2.9) | 1 (9.1) | 5 (3.4) | |
| Uneven | 2 (1.5) | 0 (0.0) | 2 (1.4) |
Osteoarthritis (OA) was detected at baseline on either radiographic or CT images for 25 (11.4%) participants in the surgery group and 31 (14.1%) participants in the plaster cast group. OA was more likely to be detected on CT images than in plain radiographic views. Of these 25 participants in the surgery group, 23 had radiographic or CT imaging at 52 weeks and 17 were seen to have OA on either of these. OA was detected for a further 28 participants at 52 weeks in the surgery group. Of the 31 participants in the plaster cast group with OA at baseline, 22 had radiographic or CT imaging at 52 weeks and 20 were seen to have OA on either of these. OA was detected for a further 22 participants at 52 weeks in the plaster cast group.
Penetration of the screw (used during surgical fixation) of the radioscaphoid joint (RSJ), the scaphotrapezium joint (STJ) or another joint was reported for a total of 104 unique participants at 6, 12 or 52 weeks (surgery, n = 94, 42.9%; plaster cast, n = 10, 4.6%). At 52 weeks, the CT scans of 80 participants in the surgery group were reported to show screw penetration in one or more joints (RSJ, n = 44/80, 55.0%; STJ, n = 48/80, 60.0%; another joint, n = 19/80, 23.8%. For the plaster cast group, of which a smaller proportion underwent surgery, the CT scans of nine participants were reported to show screw penetration in one or more joints (RSJ, n = 4/9, 44.4%; STJ, n = 6/9, 66.7%; another joint, n = 0/9, 0.0%). On CT scans at 52 weeks, the maximum screw protrusion was measured and seen to be, on average, 1.7 mm (SD 0.9 mm, range 0.4–4.7 mm) and was similar in both groups. Overall, maximum screw protrusion was categorised as < 1 mm (20.7%), 1–2 mm (52.4%) or > 2 mm (26.8%).
Lucency was assessed based on each of these categories on follow-up radiographic images (worst of each view available) and the 52-week CT scan (worst of each of three MPRs available) agreed by three observers using agreement rules. This was considered only when an implant was present. In the surgery group, serious lucency (1–2 mm) was observed on radiography for only one participant at 6 weeks, for two participants (including the one at 6 weeks) at 12 weeks and for four participants (including the one at 12 weeks who did not display lucency at 6 weeks) at 52 weeks. No serious lucency was observed on radiographs for any participant in the plaster cast group.
The prevalence of avascular necrosis (which is also known as osteonecrosis) is summarised for the two groups in Table 25; prevalence appears to be similar in both groups at baseline but, at later time points, the plaster cast group has a higher proportion of more significant cases of avascular necrosis.
Adverse events
At least one non-serious AE was reported for 24 (11.0%) participants in the surgery group and for 29 (13.2%) participants in the plaster cast group (difference of –2.2%, 95% CI –8.3% to 3.9%; Table 26). Of those who experienced at least one event, most reported only one event (surgery group, n = 19, 79.2%; plaster cast group, n = 23, 79.3%) but participants reported up to three events each. In total, 30 events were reported for participants in the surgery group and 36 events were reported for participants in the plaster cast group, of which, respectively, 24 (80.0%) and four (11.1%) were related to receiving anaesthesia and/or surgery, three (3.0%) and 23 (63.9%) were related to cast treatment and three (3.0%) and nine (25.0%) were other events. Sixteen events (surgery group, n = 5, 16.7%; plaster cast group, n = 11, 30.6%) were deemed to be unexpected by the reporting clinician.
| Non-serious AEs, n (%) | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|
| Participants reporting ≥ 1 AEs | 24 (11.0) | 29 (13.2) | 53 (12.1) |
| Total number of non-serious AEs | 30 | 36 | 66 |
| Number of non-serious events per participant | |||
| 0 | 195 (89.0) | 191 (86.8) | 386 (87.9) |
| 1 | 19 (8.7) | 23 (10.5) | 42 (9.6) |
| 2 | 4 (1.8) | 5 (2.3) | 9 (2.1) |
| 3 | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| AEs related to anaesthesia and/or surgerya | |||
| Screw-related complication | 9 (30.0) | 1 (2.8) | 10 (15.2) |
| Nerve or vessel event | 4 (13.3) | 1 (2.8) | 5 (7.6) |
| Infection | 2 (6.7) | 2 (5.6) | 4 (6.1) |
| CRPS | 3 (10.0) | 0 (0.0) | 3 (4.6) |
| Symptoms consistent with non-union | 1 (3.3) | 0 (0.0) | 1 (1.5) |
| Other | 5 (16.7) | 0 (0.0) | 5 (7.6) |
| Any of the above | 24 (80.0) | 4 (11.1) | 28 (42.4) |
| AEs related to cast treatmenta | |||
| Pain related to the cast | 2 (6.7) | 6 (16.7) | 8 (12.1) |
| Symptoms consistent with non-union | 0 (0.0) | 8 (22.2) | 8 (12.1) |
| Pressure sores | 0 (0.0) | 5 (13.9) | 5 (7.6) |
| Pain due to a tight cast | 1 (3.3) | 2 (5.6) | 3 (4.6) |
| Soft cast/broken cast leading to movement of wrist | 0 (0.0) | 2 (5.6) | 2 (3.0) |
| Any of the above | 3 (3.0) | 23 (63.9) | 26 (39.4) |
| Othera | |||
| Reinjury | 2 (6.7) | 7 (19.4) | 9 (13.6) |
| Allergy to dressing | 0 (0.0) | 2 (5.6) | 2 (3.0) |
| Substance abuse | 1 (3.3) | 0 (0.0) | 3 (1.5) |
| Any of the above | 3 (3.0) | 9 (25.0) | 12 (18.2) |
| Gradingb | |||
| Mild | 22 (73.3) | 28 (77.8) | 50 (75.8) |
| Moderate | 7 (23.3) | 7 (19.4) | 14 (21.2) |
| Severe | 1 (3.3) | 0 (0.0) | 1 (1.5) |
| Missing | 0 (0.0) | 1 (2.8) | 1 (1.5) |
| Causalityb | |||
| Not related | 2 (6.7) | 8 (22.2) | 10 (15.2) |
| Unlikely to be related | 2 (6.7) | 2 (5.6) | 4 (6.1) |
| Possibly related | 10 (33.3) | 2 (5.6) | 12 (18.2) |
| Probably related | 4 (13.3) | 1 (2.8) | 5 (7.6) |
| Definitely related | 12 (40.0) | 23 (63.9) | 35 (53.0) |
| Expectednessb | |||
| Expected | 25 (83.3) | 25 (69.4) | 50 (75.8) |
| Unexpected | 5 (16.7) | 11 (30.6) | 16 (24.2) |
Three SAEs were reported among three (1.4%) participants in the surgery group (Table 27). All three were related to anaesthesia and/or surgery and two were deemed to be unexpected at the time of reporting.
| SAEs, n (%) | Surgery (N = 219) | Plaster cast (N = 220) | Total (N = 439) |
|---|---|---|---|
| Participants reporting ≥ 1 SAE | 3 (1.4) | 0 (0.0) | 0 (0.0) |
| Total number of SAEs | 3 | 0 | 0 |
| Number of serious events per participant | |||
| 0 | 216 (98.6) | 220 (100.0) | 436 (99.3) |
| 1 | 3 (1.4) | 0 (0.0) | 3 (0.7) |
| Type of eventb | |||
| Hospitalisation | 2 (66.7) | 0 (0.0) | 2 (66.7) |
| Persistent or significant disability/incapacity | 1 (33.3) | 0 (0.0) | 3 (33.3) |
| AEs related to anaesthesia and/or surgerya | |||
| Anaesthetic complication | 2 (66.7) | 0 (0.0) | 2 (66.7) |
| Symptoms consistent with non-union | 1 (33.3) | 0 (0.0) | 1 (33.3) |
| Causalityb | |||
| Definitely related | 3 (100.0) | 0 (0.0) | 3 (100.0) |
| Expectednessb | |||
| Expected | 1 (33.3) | 0 (0.0) | 1 (33.3) |
| Unexpected | 2 (66.7) | 0 (0.0) | 2 (66.7) |
| Durationb | |||
| ≤ 24 hours | 2 (66.7) | 0 (0.0) | 2 (66.7) |
| > 24 hours | 1 (33.3) | 0 (0.0) | 1 (33.3) |
Agreement analysis
Descriptive analyses of the agreement between the three independent raters on review of radiographic and CT imaging are presented in Appendix 5.
Participant use of home exercises to care for wrist
When entered into the study, participants should have been provided with written advice about home exercises to perform to care for their wrist. Participants were asked on the 12-week participant questionnaire how they found doing these exercises. Responses to these questions are provided in Appendix 3, Table 66. Of those who provided a response to the question, 151/176 (85.8%) in the surgery group and 123/155 (79.4%) in the plaster cast group reported that they found the exercises very or quite useful. Among those who performed the exercises, the exercises were reported to have been performed on a median of 41 days (range 1–168 days) in the surgery group and on a median of 35 days (range 2–137 days) in the plaster cast group. On the 52-week complication form, data were collected on whether or not the participant was referred for physiotherapy for the treatment of their wrist injury during the previous year. Approximately twice as many participants allocated to the surgery group had been referred for physiotherapy (n = 58, 26.5%) as in the plaster cast group (n = 30, 13.6%).
Participant perceptions of their wrist and treatment preference at 52 weeks
On the 52-week questionnaire, participants were asked about the state of their wrist at present in comparison with a year ago (see Appendix 3, Table 67). Of those who provided a response to the question, a very similar proportion in the two groups reported that their wrist felt much or slightly better (surgery group, n = 166/181, 91.7%; plaster cast group, n = 156/174, 89.7%). Participants were also asked, based on their experiences of the treatment received as part of the trial, if they were to again injure their wrist to the same extent as they did 52 weeks ago, which treatment they would prefer. Of those who provided a response to the question, in the surgery group, three-quarters reported that they would have surgery again (n = 137/181, 75.7%), 36 (19.9%) participants did not have a preference and eight (4.4%) participants reported that they would not have surgery. In the plaster cast group, there was a reasonably equal split among the three categories (surgery, n = 59/175, 33.7%; no preference, n = 68, 38.9%; no surgery, n = 48, 27.4%).
Chapter 4 Economic evaluation
The role of economic evaluation is to consider the relative merits of competing treatment alternatives weighed against their costs, using a consistent analytical perspective. This chapter investigates the possible impact of available treatments for adults with bicortical, minimally displaced fractures of the scaphoid waist on the health of the patient and the costs to the NHS and personal social services (PSS), in both the short and the long term.
This is achieved in two ways; first, the short-term effect on patients’ health and costs to the NHS of the treatment are considered through a within-trial analysis, using direct results of the clinical trial up to 52 weeks of follow-up. This analysis focuses on the short-term health implications of treatment and the immediate period of rehabilitation, as well as the associated costs of this care. Second, as persistent non-union of the fracture and the development of OA have potentially lifelong implications, it is necessary to consider the long-term implications of treatment. This is achieved through an extrapolated analysis, in which mathematical modelling of the expected future health of the patients is used to estimate the health and resource use implications beyond the time frame of this SWIFFT report.
Within-trial analysis: methods
A rich array of information was collected from both patients and clinicians on health and resource use during and after treatment. This within-trial analysis will consider quality of life (QoL), as reported by the EQ-5D-3L, and the level of NHS costs, estimated through the treatment received and the patient-reported estimates of interaction with the NHS, at a per patient level for the 52 weeks after randomisation into the trial (the ‘within analysis period’). This analysis was conducted using the Stata software package.
The base case is an ITT analysis. An additional scenario of per protocol is presented, whereby any patients who were deemed to cross over from one treatment allocation to another are excluded from the analysis.
Missing data are imputed for both cost and QoL components, as detailed later in this section. Relevant summary statistics and estimates of the QoL and costs associated with patients are reported both at a complete case level (i.e. dropping all cases of missing data) and after imputation. Regression analyses are also conducted on total QoL and costs in the within-trial period to consider the impact of key patient characteristics on QoL and costs, beyond the treatment allocation. The approach taken to missing data and the regression analyses conducted on QoL and costs in this chapter are consistent with those outlined for the PRWE in Chapter 2, Statistical methods.
The primary clinical justification for surgical intervention is the reduced future risk of OA and other long-term AEs that may not become evident until after the within-trial period. Therefore, considering the cost-effectiveness of the treatment options with only a within-trial time frame would be misleading. As a result, conclusions about the cost-effectiveness of the treatment options will be limited to the presentation of a within-trial point estimate incremental cost-effectiveness ratio (ICER), namely the difference in average costs between two treatment pathways divided by the difference in average QoL for the within-trial period.
Quality of life: EuroQol-5 Dimensions, three-level version
The EQ-5D-3L is a validated questionnaire to assess the generic health status or QoL of a patient, consisting of questions covering five dimensions of health: mobility, self-care, usual activity, pain/discomfort and anxiety/depression. 90 It is the most widely used means of consistently estimating the general health of a patient and is used by the National Institute for Health and Care excellence (NICE) as its preferred measure. 91 While other disease-specific questionnaires are available, such as the PRWE, they do not allow for comparison across disease areas and they cannot be used to determine the optimal allocation of limited NHS resources. A score of 0 is equivalent to death and 1 represents perfect health; negative scores are considered worse than death. Under the UK preference weighting, achievable scores range from –0.594 (worst response across all five dimensions) to 1 (best responses).
The responses to EQ-5D-3L questionnaires were collected at baseline and then at 6, 12, 26 and 52 weeks after randomisation. In this section, the combined EQ-5D-3L scores using the preference weighting are presented, such that each patient who completed a questionnaire at each time point has a single EQ-5D-3L QoL ‘score’ given to them based on their responses to the questionnaire. These scores are carried forward to the missing data analysis discussed later in this section and the within-trial analyses.
After having imputed for any missing questionnaires (see later section), the QoL scores are combined to estimate a score for the patient across the full within-trial period, using the equation below, where ti is the time in days at which questionnaire ‘i’ was conducted and QoLi is the QoL score reported at time ‘i’:
This combined QoL score is used to inform the regression analysis conducted to estimate the impact of patient characteristics, including treatment allocation, on QoL.
Resource use and unit costs
Costs to the NHS are considered in two ways: those reported by clinical staff and those reported by patients at 6, 12, 26 and 52 weeks during the trial (see the trial CRFs in Report Supplementary Material 15). As the trial did not directly seek to report the costs of care, but instead reported the frequency of various interactions and care provision, all elements reported have to be linked with an appropriate unit cost to estimate total costs. We assumed that all interactions with the NHS were captured by the trial CRFs.
Relevant resource use is reported from the time of randomisation into the trial and does not include the cost of initial presentation with the injury or the care that might have occurred prior to the randomly allocated treatment, typically immobilisation with a cast or splint. Randomisation should ensure that these initial treatment options and costs are balanced between the two groups.
For the purpose of the costing of the within-trial analysis, only those interactions reported as being related to the patient’s wrist are included. Unrelated interactions reported by the patients should not affect the incremental cost, but risk introducing bias if patients had high cost but completely unrelated health care. An investigation into the level of NHS and PSS interactions for reasons other than the wrist injury confirmed that there was no statistically significant difference between the randomised group (mean number of interactions: 5.65 for plaster immobilisation, 5.91 for surgical fixation; p = 0.828).
The unit costs associated with each component of the within-trial analysis are presented in Table 28.
| Stage | Cost item | Value (£) | Source of estimation |
|---|---|---|---|
| Treatment and imaging | Cost of casts, both the initial cast at diagnosis and additional casts | 10 |
Consistent with NICE NG3888 Assumes costs of hospital attendance, etc., are covered in patient-reported activity |
| Cost of primary surgery (patients randomised to surgical arm) | 1632 | Weighted average of adult HT44 (intermediate hand procedures for trauma, mapped from all open OPCS codes), reference costs 2015/1692 | |
| Cost of secondary surgery (repeat surgery for surgery group and surgery for plaster cast group) | 2509 | Weighted average of adult HT43 (major hand procedures for trauma, mapped from all closed OPCS codes), reference costs 2015/1692 | |
| Cost of radiography | 30 | Reference costs 2015/16, direct access plain film | |
| Cost of CT scans | 94 | Reference costs 2015/16, RD20A, CT scan of one area, without contrast, 19 years and over92 | |
| Cost of magnetic resonance imaging | 145 | Reference costs 2015/16, RD01A, magnetic resonance imaging scan of one area, without contrast, 19 years and over92 | |
| Follow-up care – from trial patient-reported questionnaires, only for those ‘about your wrist’ rather than ‘other reasons’ | GP – at practice | 37 | Based on estimates from PSSRU (11.7-minute consultation)93 |
| GP – at home | 74 | Based on estimates from PSSRU (11.4-minute consultation plus 12 minutes of travel)93 | |
| GP – by phone | 22 | Based on estimates from PSSRU (7.1-minute consultation)93 | |
| Physiotherapist – at GP’s practice | 49 | Reference costs 2015/16, A08A1 (physiotherapist, adult, one to one, community)92 | |
| Nurse – at GP’s practice | 12 | Based on estimates of duration of contact and cost per hour of face-to-face time from PSSRU93 | |
| District/community nurse | 38 | Reference costs 2015/16 (N02AF, district nurse, adult, face to face, community)92 | |
| Occupational therapist | 79 | Reference costs 2015/16 (A06A1, occupational therapist, adult, one to one, community)92 | |
| Hospital – physiotherapist | 46 | Reference costs 2015/16, WF01A, non-admitted face-to-face attendance, physiotherapy92 | |
| Hospital – occupational therapist | 58 | Reference costs 2015/16, WF01A, non-admitted face-to-face attendance, occupational therapist92 | |
| Hospital – A&E | 157 | Reference costs 2015/16, WF01B, non-admitted face-to-face attendance, first, A&E92 | |
| Hospital – fracture clinic | 110 | Reference costs 2015/16, WF01A, non-admitted face-to-face attendance, trauma and orthopaedics92 | |
| Hospital – pain management clinic | 131 | Reference costs 2015/16, WF01A, non-admitted face-to-face attendance, pain management92 | |
| Hospital – in-patient stay | 269 per day | Weighted average of reference costs 2015/16, HE41 hand fracture without intervention, excess bed-days92 |
Patient-reported costs are estimated at 6, 12, 26 and 52 weeks for the purpose of the missing data analysis, and treatment and imaging costs are estimated for the full within-trial period.
After imputation for patient-reported costs at the four time points and imaging costs, costs are combined into an estimate of the total patient-level cost for the within-trial period.
Patient-reported medications, aids and out-of-pocket costs such as private health care related to the initial injury are excluded from this analysis. In the case of medications, all reported cases were identified as being low-cost painkillers, which either cost less than the patient prescription cost or could have been purchased by patients over the counter. Aids are not routinely provided to patients on the NHS, as reflected by the trial data, and as such reflect a private cost that, alongside out-of-pocket costs, fall outside the NHS and PSS perspective taken in this chapter.
Missing data
Missing data were imputed across the patient-reported questionnaires related to QoL and resource use in addition to imaging. Using the framework described by Faria et al.,94 the missing data were assumed to be missing at random. Imputation was conducted across all nine cost and QoL variables identified as having missing data (i.e. costs at 6, 12, 26 and 52 weeks and QoL at baseline and at 6, 12, 26 and 52 weeks). Consistent with the missing data approach outlined in the PRWE analysis in the previous chapter, these missing variables were assumed to be correlated to each other as well as to treatment allocation, age at randomisation, whether or not the patient’s dominant hand was fractured and displacement of the fracture. The number of imputations was set equal to the proportion of missing values in the variable with the largest level of missingness, presented in Results in this chapter. Imputation was conducted using the ‘ICE’ multiple imputation package in Stata and reporting using the ‘MIM’ package. 95,96
Impact of lost employment and unpaid activities
In addition to considering the costs to the NHS and PSS and the QoL of patients, this within-trial analysis reports the impact of treatment allocation on days of lost employment and unpaid activities. The method of this analysis is reported in Appendix 6, Impact of lost employment and unpaid activities.
Extrapolated model: methods
As the implications, in terms of both NHS costs and patient health, of the different treatment options and associated AEs can persist beyond the 52-week follow-up of the trial, an extrapolation model is required to estimate cost-effectiveness over a lifetime. These beyond-trial implications were discussed in Chapter 1 and can be broadly categorised by the future burden of OA, SNAC and long-term pain or limited mobility that occur as a direct result of the initial injury and its treatment.
Systematic review of existing cost-effectiveness evidence
A literature review was conducted to determine if previous economic evaluations had sufficiently determined the cost-effectiveness of surgical fixation versus plaster cast immobilisation for bicortical, minimally displaced fractures of the scaphoid waist in adults. A secondary aim of the search was to determine if previous mathematical models could be adapted to estimate the long-term cost-effectiveness, and thus remove the need to construct a de novo mathematical model. Full details and results of the search strategy and results are given in Appendix 6, Systematic review of previous cost-effectiveness studies.
The review found no suitably robust cost-effectiveness analysis. Therefore, we determined that a de novo mathematical model was required to investigate the long-term cost-effectiveness of surgical fixation compared with cast immobilisation.
Cost-effectiveness analysis: analytical methods and model inputs
This section details the scope of the de novo extrapolated model and provides details on its structure, the base-case inputs and the scenarios considered.
Analytical approach
The analysis presented here uses a methodology consistent with the NICE Guide to the Methods of Technological Appraisal91 and Decision Modelling for Health Economic Evaluation. 97 In brief, real-world observations from trial data, published literature and expert opinion were used to estimate the expected lifetime health and resource use per patient for each treatment comparator.
The conventional outcomes of these models that were used to determine the cost-effectiveness of the different treatment pathways are lifetime cost, expected life-years and expected QALYs. These estimates are compared across treatment pathways by estimating the ICER. A cost-effectiveness threshold of £20,000/QALY is used as the base-case value to estimate the net health benefit (NHB) of each of the treatment options,98 calculated using the following equation:
To incorporate the uncertainty present in the estimation of such models, two approaches are used: (1) probabilistic sensitivity analysis (PSA) and (2) scenario analysis. PSA explicitly incorporates the uncertainty present in parameter estimates by using the range of values over which these estimates exist (characterised by an informative distribution), rather than single point estimates, as inputs into the mathematical models. 97
Scenario analyses are used to test the structural uncertainty of the models, with the underlying structure and sources of evidence changed to explore the impact of such uncertainty on the results of the models.
A value of information analysis explored the population expected value of perfect information (EVPI), an assessment of the benefit of resolving all uncertainty in the parameter estimates of the base-case model.
Analytical perspective
Consistent with the within-trial analysis, the full, extrapolated analysis takes as its primary perspective the health outcomes for the patient, expressed in QALYs, and the costs to the NHS, expressed in GBP at 2017 prices. A lifetime time horizon is used in the base-case analysis to reflect the full duration of impact of potential AEs including OA and SNAC. Both costs and outcomes are discounted using a 3.5% annual discount rate consistent with current guidelines. 91 The model was developed using Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA).
Decision problem
Consistent with the SWIFFT protocol, this analysis seeks to estimate the most cost-effective treatment pathway for adults presenting with an undisplaced (< 1 mm step or gap) or minimally displaced (1–2 mm inclusive) bicortical fracture of the scaphoid waist.
An important element of any analysis that seeks to determine the most cost-effective treatment is the full incorporation of all possible treatment alternatives, including those beyond the two considered in SWIFFT. A brief review of the literature, using the framework outlined in Appendix 6, Systematic review of previous cost-effectiveness studies, and discussions with the SWIFFT TMG highlighted that no active treatments beyond combinations of cast and surgical fixation were considered relevant to an NHS setting, with the only other treatment identified, pulsed electromagnetic fields, rarely used in the UK. To ensure the extrapolated model includes all possible treatment options the following four are considered (the first two are extensions of SWIFFT):
-
No treatment – all patients who experience a scaphoid waist fracture are left untreated. While unlikely to happen in practice, and considered unethical to include in SWIFFT, this is an important anchor point to ensure that all active treatments are well demonstrated to be effective and cost-effective compared with providing no intervention, and a means of model validation. Furthermore, the TMG confirmed that some patients choose to not have treatment, often not attending diagnosis or refusing the recommended treatment. This arm is therefore important for exploring the validity of patients’ decisions.
-
Cast immobilisation only – all patients are treated with cast immobilisation alone, such that patients who are identified as having non-union are not able to progress to surgery. While not incorporated into SWIFFT, this allows the analysis to test whether the addition of surgical fixation for non-union patients is cost-effective compared with casting alone. Furthermore, it represents the historic treatment option in many cases, before fixation surgery was routinely offered. In this and all other similar cases, ‘cast’ refers to all forms of non-surgical immobilisation, including casts with thumb incorporated and excluded, and splints.
-
Cast immobilisation followed by immediate surgery – in line with the SWIFFT population, patients are initially treated with casting with immediate surgical fixation soon after cast removal offered to all patients in whom non-union is detected.
-
Surgical fixation – in line with SWIFFT, patients are initially treated with surgical fixation; if primary fixation is unsuccessful (due to either implant problems or failure of union), patients are offered additional surgery.
The assumptions related to each are considered later in this chapter.
Population
The population considered in the extrapolated model is consistent with SWIFFT, as described in Chapter 2.
Inevitably, when an economic evaluation model has to use evidence from the existing literature to inform an extrapolation, there are some differences between the analysed population and that considered in the relevant literature. These are identified and discussed later in this chapter, with biases explored where possible.
Model structure
The model itself has two components: (1) a short-term decision model, which characterises the first 52 weeks after initial presentation, consistent with the SWIFFT period; and (2) a long-term element, over which the implications of the treatment and union status on long-term outcomes and costs are considered. These two components are presented separately below for clarity but are intricately linked.
The model takes as a starting point the time at which a treatment decision is made, such that patients have already experienced the injury and attended hospital for their initial assessment. As a result, the nature of the fracture is known and the patient has been identified as within this population.
Short-term decision model
Covering the first year since treatment, the short-term element closely matches the within-trial analysis by drawing directly from the findings of SWIFFT, with the additional treatment pathways of no treatment and primary cast immobilisation only. Figure 7 provides an overview of the short-term element of the model through this 52-week period, from the treatment decision to the 52-week time point, at which point patients enter the long-term ‘Markov’ model, symbolised by the ‘M’ in the figure. The key features of each treatment arm in the short-term element are as follows:
-
No treatment – after having been identified as within the population considered in this analysis, all patients receive no active treatment (i.e. neither cast nor surgery). As a result, no short-term treatment costs are incurred, but patients are assumed in the base-case analysis to never achieve union of the fracture. While it is expected that some untreated fractures would unite, as the literature provides examples of such cases,99,100 it is not possible to estimate this as a proportion of all cases and, as such, the base case assumes no cases of union. This assumption is explored in a later scenario.
-
Cast immobilisation only – primary cast immobilisation is assumed to be the only treatment available to patients, such that all are initially immobilised in a cast but, should the fracture fail to unite, no subsequent surgery is available. Treatment characteristics and rates of union are drawn directly from the SWIFFT primary cast immobilisation arm, but all those offered post-cast surgery for non-union are assumed to remain as non-unions.
-
Cast immobilisation with immediate surgery for non-union – this treatment arm is consistent with the cast-only arm, except that patients who fail to achieve union are offered surgical fixation (like in SWIFFT), a proportion of whom will opt for it. The patients who failed to achieve union with the cast and chose to not have surgery are assumed to end the short-term component while still having non-union, while patients who opted for surgical fixation can achieve union or non-union at a rate determined by SWIFFT results.
-
Primary surgery – like the surgery group of SWIFFT, patients are treated with surgical fixation as their primary treatment; many patients initially receive a cast, which is then removed and surgery is performed. After surgery, patients can achieve union or non-union. A small proportion determined by SWIFFT have multiple rounds of surgery if the primary surgery is unsuccessful or there are complications. Furthermore, most patients are provided with a cast after surgery as part of routine therapy.
All cost and QoL impacts that occur within this first year are included in the estimates presented later in this section. Mortality within the first year is not explicitly modelled, as scaphoid-related death is not expected to occur and the primary population is young, with no related or unrelated deaths observed in SWIFFT.
FIGURE 7.
Schematic of the short-term element of the economic evaluation model.

Long-term Markov model
The long-term cost and health outcomes extrapolated beyond the 52-week short-term model are estimated using two Markov models, presented in Figures 8 and 9. In a Markov model, patients reside in one out of a set of mutually exclusive health states at particular points in time. 101 During discrete time intervals, these patients can either remain in a particular health state or move to a separate health state, typically because they have experienced a particular clinical event.
FIGURE 8.
Long-term Markov model for successful union.
FIGURE 9.
Long-term Markov model for non-union.
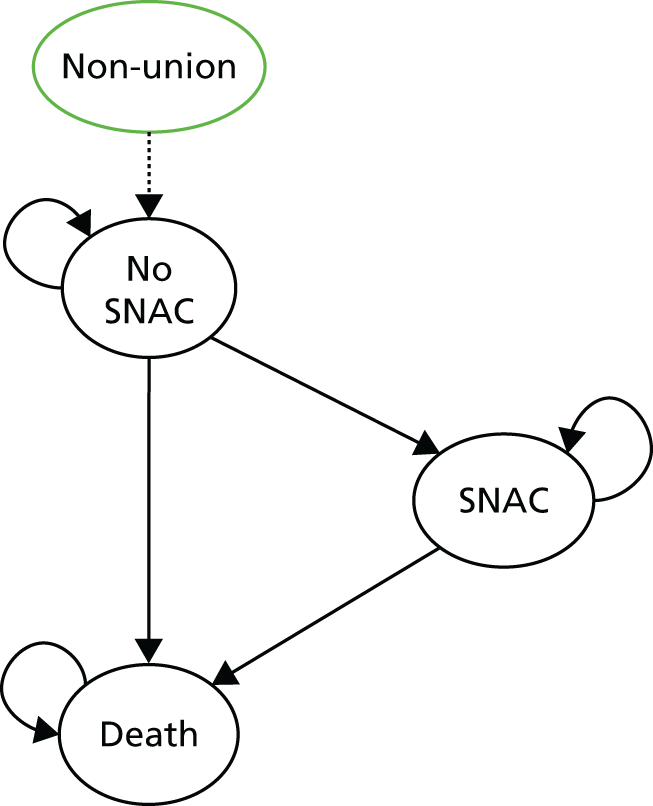
As shown in Figures 8 and 9, the Markov model that patients enter is dependent on their union status at the end of the short-term decision model, with patients who achieve union entering the model outlined in Figure 8 and non-union patients entering the model outlined in Figure 9. This is consistent with the very different long-term clinical expectations associated with the two groups, as discussed in Chapter 1, with union patients likely to regain usual health and function, but those with non-union likely to have long-term health concerns.
The treatment pathway that a patient experienced to get to their union status is informative to their rate of transition through the model. In other words, while a patient’s prior treatment is assumed to not affect the set of long-term health states they can achieve, it does affect the likelihood with which they achieve them.
Patients achieving successful union at the end of the short-term element are placed into one of two starting states in the union Markov model: ‘no OA or other AEs’ or ‘no OA but long-term AEs’. This distinction is made because of the prevailing evidence that a number of patients experience lifelong symptomatic AEs associated with the original injury and treatment that are not related to the development of OA. Specifically, Lindström and Nyström102 reported the presence of pain and weakness not associated with OA in their long-term follow-up study of patients treated with cast immobilisation. Limitations in the available evidence on the long-term transitions necessitated the assumption that these patients represent a separate group from those who will go on to develop OA.
Patients who experience permanent long-term symptomatic AEs without OA remain in this state until they die of other causes, as the treatment or related events are unlikely to cause death. We also assumed that these non-OA AEs are fixed for the patient’s remaining lifetime, with all time-limited AEs being resolved within the short-term element of the model (i.e. within a year of injury).
Patients who enter the long-term model in an AE-free state (i.e. no OA or other AEs) can similarly stay in this state until they die of other causes, or they may develop OA, which is modelled as being either symptomatic or non-symptomatic, again informed by the literature. As is discussed later, limitations in the data mean we are unable to model the potential development of OA from non-symptomatic to symptomatic, or the potential for different levels of symptomatic presentation. From each of the OA states, patients stay in that state until they die of other causes.
The dearth of evidence relating to the development and transition between AEs in this population necessitated the assumption that patients do not transit between the three AE states. As a result, the analysis assumes that patients experience at most only one lifelong AE. As there is no difference in the cost and no utility difference between the two symptomatic states, discussed later in this section, and the asymptomatic OA state is based on long-term observational data, this model assumption does not affect the result of the analysis.
In contrast, patients who end the short-term element in a non-union state are modelled based on their risk of developing SNAC, as shown in Figure 9. As discussed in Chapter 1, SNAC represents a SAE that results from non-union, whereby the proximal carpal row can no longer stabilise the distal carpal row, and hence the wrist. The resulting abnormal loading leads to carpal collapse, cartilage degeneration and arthritis. Owing to a dearth of evidence relating to the natural history of events associated with non-union, we were not able to model separate states reflecting the development of OA and other symptoms within the non-union Markov model. Patients in this Markov model enter in the ‘no SNAC’ state and throughout their lifetime face a risk of progressing to SNAC or dying from unrelated causes. As with OA in the union Markov model, SNAC is modelled as being a binary disease state, but, unlike OA, is assumed to always be symptomatic as a result of the severity of the condition. Owing to the challenging nature of treating SNAC, and the lifetime implications even if treatment is successful, patients are modelled as remaining in the SNAC state for the rest of their life if it develops. Unlike the union model, patients are not modelled as separately having non-OA-related long-term symptoms and non-symptomatic OA. This assumption is the result of the observation by Dias et al. 103 that almost all patients (9 of 10) had pain or stiffness after a mean of 2.1 years. Therefore, our base-case model assumes that all non-union patients had some QoL decrement, incorporating a range of AEs including low-grade OA and non-OA AEs.
Model inputs
Much of the evidence defining the patient cohort and the short-term element is sourced directly from SWIFFT. As SWIFFT currently has only 52 weeks of patient follow-up, all long-term model inputs are informed by the wider literature.
Consistent with SWIFFT, the base-case patient cohort is modelled as being 33 years old, with 84% being male. A patient’s age is assumed to affect only the rate of mortality, as, while there is likely to be a correlation between age and OA and other AEs, there was insufficient evidence to incorporate such a correlation. Similarly, the gender mix affects only the mortality rate.
Short- and long-term element transition estimates
The short- and long-term model transition estimates are reported in Table 29. The base-case mean estimates are reported alongside the 95% CI and the informative distribution used to inform the PSA. The distributions are reported in terms of the distribution selected, conforming to conventional approaches,97 and the parameters required to describe the distribution.
| Parameter | Base-case value (95% CI) | Distribution (informative parameters) | Source | |
|---|---|---|---|---|
| Short-term element of the model | ||||
| Probability of being identified as requiring surgery/repeat surgery after initial treatment | Cast immobilisation | 0.090 (0.005 to 0.130) | Beta (alpha 20, beta 200) | SWIFFT; 20 cases including 17 participants who had surgery plus three participants who did not have surgery at 52 weeks but had an identification of non-union |
| Surgery as initial treatment | 0.037 (0.016 to 0.065) | Beta (alpha 8, beta 221) | SWIFFT; eight participants who had secondary surgery | |
| Probability of having surgery post cast, non-union | 0.948 (0.901 to 0.981) | Beta (alpha 110, beta 6) | Review of the existing literature | |
| Probability of having repeat surgery post initial surgery, non-union or issue | 0.948 (0.901 to 0.981) | Beta (alpha 110, beta 6) | ||
| Probability of non-union after second-line surgery | 0.059 (0.002 to 0.206) | Beta (alpha 1, beta 16) | SWIFFT; 17 patients who underwent secondary surgery and one patient who had confirmed non-union | |
| Probability of having non-union after no treatment | 1 | Fixed | Assumption | |
| Long-term element of the model – union Markov model | ||||
| Probability of having long-term adverse symptoms that are not OA related | ||||
| Cast immobilisation | 0.048 (0.024 to 0.079) | Beta (alpha 11, beta 218) | Lindström and Nyström (1990)102 | |
| Surgery as last treatment provided | 0.048 (0.024 to 0.079) | Beta (alpha 11, beta 218) | Assumed to be same as cast | |
| Probability of developing OA | ||||
| Cast immobilisation |
Limiting value: 0.056 Time constant: 1.5 CI: 0.035 to 0.087 |
Exponential decay towards a limiting value, with limiting value characterised as a beta distribution (see Appendix 6, Probability of developing osteoarthritis) | Lindström and Nyström (1990);102 5.6% of 218 patients had OA after primary healing with a minimum follow-up of 7 years | |
| Surgery as initial treatment |
Limiting value: 0.056 Time constant: 1.5 CI: 0.035 to 0.087 |
Exponential decay towards a limiting value, with limiting value characterised as a beta distribution | Assumed to be same as cast | |
| Probability that developed OA is symptomatic | ||||
| Cast | 0.992 (0.918 to 1.00) | Beta (alpha 11.9, beta 0.1) | Lindström and Nyström (1990)102 | |
| Surgery | 0.992 (0.918 to 1.00) | Beta (alpha 11.9, beta 0.1) | Assumed to be same as cast | |
| Mortality from all causes | As per published tables | Fixed | ONS National Life Tables, England and Wales, 2014–16104 | |
| Long-term element of the model – non-union Markov model | ||||
| Survival function of no SNAC to SNAC |
Lambda 0.0069 Gamma 1.77 |
Weibull | Weibull function fitted to time-to-event data from Moritomo et al. (1999)105 | |
| Mortality from all causes | As per published tables | Fixed | ONS National Life Tables, England and Wales, 2014–16104 | |
The parameters required for the short-term element of the model are derived from SWIFFT, with the exception of the assumption that all untreated patients have a non-union. This assumption biases against a no-treatment scenario and, while some of the literature suggests rates of union in an untreated population,99,100 there is insufficient evidence to determine a rate of non-union in the population considered here.
The long-term Markov models are reliant on published literature until the 5-year follow-up of SWIFFT is completed. In the base-case model, the choice and frequency of the different treatment options affects a patient’s long-term health only through its ability to effect union of the fracture; this core assumption is relaxed in a series of scenarios. The model parameters for which this assumption applies are presented in Table 29.
Base-case estimates of the probability of having long-term adverse symptoms that are not OA related are drawn from the study by Lindström and Nyström,102 who reported the presence of pain and weakness not associated with OA in their long-term follow-up study of patients treated with cast immobilisation, finding that 4.8% of patients had long-term non-OA AEs.
The probability of developing OA as a result of the initial injury and treatment is modelled as an exponential decay. It is estimated by fitting an exponential function to three time points extracted from Lindström and Nyström102 (presented in more detail in Appendix 6, Probability of developing osteoarthritis): time 0, when no patients had developed related OA; year 1, when 2.6% had radiological OA; and year 7, when 5.6% had radiological OA,. We assume that no OA, which can directly be linked to the original fracture and treatment, occurs beyond 10 years. Some authors report a prevalence of OA in patients with united fractures with greater follow-up. For example, Düppe13 reported that, 36 years after the initial event, 15% had OA. However, there was no significant difference between the rate of OA in the injured and uninjured wrists assessed, suggesting that much of the identified OA was unrelated to the original fracture. While the literature does suggest that repeat intervention and surgery may exacerbate rates of OA,27 we found insufficient evidence to support this theory. Therefore, the probability of developing OA is assumed to be treatment independent, as discussed earlier. This assumption is justified by limited data on the rate of OA collected during SWIFFT, which showed no statistically significant difference in the rate of early OA between the two randomised groups.
Once developed, the probability of OA being symptomatic is modelled as a time-invariant probability applied at the point of development. In the base-case analysis, the probability is estimated from Lindström and Nyström,102 who found that all those who developed OA (12 of 229 patients) were symptomatic. Scenarios exploring the treatment independence of this parameter and alternative estimates are explored.
For the non-union patients, the probability of developing SNAC is modelled as a Weibull function fitted to time-to-event patient-level data reported by Moritomo et al. ,105 using only those patients reported as having a fracture to the middle third. The Weibull function represented the best-fitting function to the extracted Kaplan–Meier of those tried based on AIC; details of the regressions explored are presented in Appendix 6, Probability of developing scaphoid non-union advanced collapse. The Cholesky decomposition method was used to reflect uncertainty in the analysis. 97 One major limitation of the Moritomo et al. study is that it considers a symptomatic population alone, albeit one in which some non-union patients have symptoms but not radiologically detectable SNAC. Findings similar to those of Moritomo et al. can be found elsewhere in the literature; for example, Mack et al. ,12 again looking only at patients presenting symptomatically, found that arthritis is likely to develop if sufficient time passes after the time of non-union development. Similarly, Dias et al. 103 identified that after a mean review period of only 2.1years, 5 of 10 patients with non-union had signs of OA and dorsal intercalated segment instability, suggesting significant degeneration. Vender et al. 15 also conducted a retrospective radiological review of 64 participants with symptomatic non-union among an untreated population, finding a similar time to arthritis onset.
In both the union and non-union Markov models, patients are subject to a mortality risk throughout, conditional on the cohort age and gender mix alone, such that non-union, OA, SNAC and all other modelled factors were assumed to have no impact on the rate of mortality. The required estimates were drawn from Office for National Statistics National Life Tables, England and Wales, 2014–16. 104
We assume that patients are at risk of developing OA caused by the initial injury or subsequent treatment only for 10 years; this assumption is made to reduce the potential impact of conflation of OA caused by the injury with that due to other causes. In addition, we assume that all OA detected in the informative studies for 10 years after injury is a direct result of the initial injury and treatment.
In the base case, we assumed that the patient populations, treatments used and clinical definitions (e.g. union and OA) evaluated in each of the informative studies match with those analysed in this study or, where they do vary, do not introduce any bias so large as to make them uninformative or misleading. A review of the key characteristics and definitions, including all studies pertinent to the base-case and scenario analyses, is given in Appendix 6, Systematic review of previous cost-effectiveness studies. Table 69 in Appendix 6 shows that many of the characteristics appear consistent, with some limitations regarding insufficient detail, for example displacement of the original fracture. The potentially inconsistent definitions relate to symptoms, with some of the key studies considering only pain, while others consider pain and weakness.
Resource use and cost estimates
The costs used in the mathematical model are presented in Table 30. Consistent with the model structure, the costs incurred by the NHS during the first year, as described by the short-term model, are directly taken from SWIFFT, with the additional two treatment arms (no treatment and cast immobilisation only). The short-term model costs are composed of the initial cost of each treatment alongside treatment-specific costs incurred for the first year post injury and any additional treatments within that period (i.e. secondary surgery). The no-treatment option is assumed to have no costs in this first year, as no interactions with the NHS are expected until patients progress into the extrapolated model. The arm involving casting without surgery is assumed to have the same cost as the SWIFFT arm involving casting with surgery for non-union with the exclusion of any surgical interventions.
| Parameter | Base-case value (£) (95% CI) | Distribution | Source |
|---|---|---|---|
| Short-term costs modelled (first year) | |||
| Follow-up cost for cast as first treatment (not including any surgery costs) | 587 (504 to 672) | Gamma | Estimated from the trial data; includes the cost of all recasting, NHS interactions and imaging, but not surgery, using the ITT population, after imputation for missing values as detailed later |
| Cost of initial surgical treatment (not including follow-up) | 1632 | Fixed | As per the within-trial analysis (see Table 28) |
| Cost of secondary surgery (not including follow-up) | 2509 | ||
| Follow-up cost after surgery (not including any surgery costs) | 618 (551 to 648) | Gamma | Estimated from the trial data; includes the cost of all recasting, NHS interactions and imaging, but not surgery, using the ITT population, after imputation for missing values as detailed later |
| Long-term costs modelled (52 weeks to end of analysis) | |||
| Cost of diagnosis of symptomatic OA | 74 | Fixed | Assumption of two GP visits with no radiological investigation; cost drawn from Personal Social Services Research Unit, 2015 (11.7-minute consultation)93 |
| Annual cost of treating symptomatic OA | 38 (10 to 83) | Gamma | Assumption of a mean of one GP visit per year with no investigation or treatment cost (as treated with over-the-counter medication); standard error assumed to be half of the mean |
| Annual cost of treating non-OA symptomatic AEs | 38 (10 to 83) | Gamma | |
| Cost of SNAC | 2551 (695 to 5592) | Gamma | Modelled as one-off cost, assuming half of patients receive very major surgery (£3440, reference cost HN42A)92 and half receive casting, both with associated costs per year of care from trial; standard error assumed to be half of the mean |
The costs associated with the long-term model, also reported in Table 30, reflect the structure of the Markov model, shown in Figure 8, taking into consideration the occurrence of OA, symptomatic AEs that are not related to OA and events related to non-union. In the base-case union model, patients in the ‘healthy group’ of no AEs including OA, as well as those in the non-symptomatic OA group, are assumed to not be associated with any costs to the NHS, as both groups are categorised by the lack of symptoms with which they would present to an NHS setting for investigation and treatment. Similarly, the non-union base case assumes that patients in the non-SNAC state will be associated with no additional regular cost to the NHS, under the assumption that all suitable interventions would have been attempted, and thus costed, prior to the diagnosis of non-union. Once a patient progresses to SNAC, a one-off treatment cost is applied to reflect any active interventions attempted to resolve or reduce the impact of the collapse. Little evidence was identified relating to the range and frequency of treatments for SNAC. Shah and Stern106 describe highly personalised treatment of SNAC, including initial non-surgical treatment, wrist immobilisation with splints, anti-inflammatory medication and corticosteroid injections, coupled with surgical options for refractory cases. In calculating costs, we assumed that half of the patients would be treated with immobilisation, with the other half progressing to major surgery, in addition to the associated 52-week follow-up costs estimated from the trial data. A standard error equal to half of the mean SNAC cost is assumed to reflect the lack of certainty around the variable.
Health-related quality of life inputs
As with the estimation of cost for the mathematical model, the HRQoL inputs into the short-term model are determined by SWIFFT, such that patients have a QoL score for the first year of the analysis, which is estimated from the trial data described earlier in this chapter. In the base-case analysis, patients in both the no-treatment arm and the cast immobilisation only arm are assumed to have the same QoL as those in the cast plus surgery for non-union arm of SWIFFT as a conservative estimate of their QoL.
In the long-term model, the QoL impact of being in a state associated with adverse health (e.g. symptomatic OA, SNAC and non-union) is applied as decrements to the expected QoL that a ‘normal’ patient who is otherwise free of associated problems would expect to experience. These QoL norms are drawn from extensive surveys of the general public using the EQ-5D-3L by Ara and Brazier107 and are stratified by age and gender. Patients in the ‘no OA or other permanent adverse events’ category of the union Markov model are assumed to achieve this QoL norm.
Evidence on the QoL of OA and SNAC relevant to this analysis is limited and diverse. Our review of the literature identified four relevant studies: Kovács et al. ,108 Neuprez et al. ,109 Slatkowsky-Christensen et al. 110 and the economic evaluation by Davis et al. 43 The base-case analysis uses the QoL decrement of OA estimated by Slatkowsky-Christensen et al. 110 to represent the QoL of patients in the symptomatic OA, non-OA related AEs and non-union ‘no SNAC’ states under the assumption that all represent, on average, a similar level of QoL decrement. While we were unable to identify any evidence that explored the QoL impact of non-union prior to the development of SNAC, we believe this assumption is the most appropriate in the base case, as patients would not be expected to regain full QoL with non-union. This is one of the many uncertainties that will be explored during the 5-year follow-up.
In contrast, the severe state of SNAC is estimated by applying the Kovács et al. 108 QoL score as a decrement using the Slatkowsky-Christensen et al. 110 ‘healthy’ population baseline to ensure a consistent baseline. The Kovács estimate is used here, as it represents a more severe patient population, which we believe to be the best fit to a SNAC outcome. While the use of QoL scores estimated using different tools (i.e. the Short Form Six Dimension and EQ-5D-3L) is discouraged in current best guidance91 owing to risks of inconsistency, we believe that, given available evidence, this is the optimal approach. As with other parameters in the model, these are subject to PSA and exploration through sensitivity analysis.
All QoL decrements are applied for the duration of a patient’s time in each state, as, while treatment may alleviate some symptoms, the progressive nature of many of these AEs will have the opposite effect. As a result, it was not possible, given existing evidence, to model changes in the QoL within each state over time, as reflected in the structural model assumptions discussed earlier in this chapter.
To reflect the QoL implications of additional surgery, patients who required surgery as a secondary line of treatment received an additional QoL decrement equal to that observed in the first year after surgery from SWIFFT. This assumption is relaxed in a scenario.
The full set of informative QoL estimates are reported in Table 31. All uncertain QoL parameters are characterised as gamma distributions for the PSA to reflect the bounding of possible QoL scores. 97
| Parameter | Base-case value (95% CI) | Distribution | Source |
|---|---|---|---|
| Short-term HRQoL modelled (first year) | |||
| QoL for first year post cast | 0.812 (0.780 to 0.844) | Gamma | Directly drawn from the trial data, using the ITT population, after imputation for missing values as detailed later |
| QoL for first year post surgery | 0.834 (0.788 to 0.843) | Gamma | Directly drawn from the trial data, using the ITT population, after imputation for missing values as detailed later |
| QoL for first year for those who are untreated | 0.812 (0.780 to 0.844) | Gamma | Assumed to be the same as cast, as the least invasive intervention |
| Long-term HRQoL modelled (52 weeks to end of analysis), all applied as decrements to population norms107 | |||
| Union and no OA or other AEs decrement | 0 | Fixed | Assumption |
| Symptomatic OA decrement | –0.130 (–0.120 to –0.140) | Gamma | Decrement estimated from Slatkowsky-Christensen et al. (2007)110 |
| Non-OA symptomatic AEs decrement | –0.130 (–0.120 to –0.140) | Gamma | Assumed to be the same as decrement for OA |
| Non-symptomatic OA decrement | 0 | Fixed | Assumption that a lack of symptoms implies no HRQoL decrement |
| Non-union (no SNAC state) decrement | –0.130 (–0.120 to –0.140) | Gamma | Assumed to be the same as decrement for OA |
| SNAC decrement | –0.275 (–0.0689 to –0.4811) | Gamma | Modelled as being from the time of development to end of life. Estimated by comparing the response in Kovács et al. (2012)108 (0.495) to the ‘healthy’ control used in Slatkowsky-Christensen et al. (2007)110 (0.77) |
Scenario analyses
To develop the mathematical model into the form presented above, a number of simplifying assumptions and interpretations of the available evidence were necessary, as is true of all mathematical models. 97 While the assumptions made in the base-case analysis are considered to be the most reasonable, given the evidence available, it is important to test the impact of different approaches on the results of the analysis. A number of scenarios, detailed in Appendix 6, Extrapolated model scenario analyses, have been constructed to conduct these tests; as far as possible, other sources of evidence are used to inform the scenarios.
Results
Within-trial analysis: results
In this section, we present the results of the within-trial analysis. This section is structured around four different scenarios relating to an ITT or per-protocol analysis and whether a complete-case approach or after multiple imputation approach is taken. The ITT analysis is presented as the base case and the summary statistics relating to QoL and costs are reported only for this analysis. The majority of within-trial analyses results are presented in Appendix 6, Within-trial analysis results, with the focus here on the regression analyses conducted across the QoL and cost outcomes.
Regression analyses
The dependent variables for both regressions are total cost and average QoL over the within-trial period. Additional regressions are reported, adjusting for QoL at baseline (Tables 32 and 33).
| Adjusted for baseline QoL | Scenario | Regression constant | Surgical allocation | QoL at baseline | Fracture displacement | Dominant wrist injured | Age |
|---|---|---|---|---|---|---|---|
| Unadjusted | Complete case ITT | 589.96 (0.120) | 1580.27 (0.000) | N/A | 180.06 (0.490) | –16.66 (0.934) | 1.48 (0.833) |
| With MI ITT | 882.80 (0.002) | 1228.13 (0.000) | N/A | 225.47 (0.168) | 74.39 (0.622) | 1.58 (0.767) | |
| Complete case PP | 678.06 (0.072) | 1770.87 (0.000) | N/A | 128.68 (0.595) | 141.08 (0.467) | 4.27 (0.535) | |
| With MI PP | 799.81 (0.004) | 1549.14 (0.000) | N/A | 190.84 (0.252) | 86.87 (0.578) | 2.72 (0.624) | |
| Adjusted | Complete case ITT | 939.86 (0.001) | 1308.11 (0.000) | –530.67 (0.022) | 183.41 (0.223) | 32.24 (0.804) | –0.08 (0.984) |
| With MI ITT | 1160.66 (0.000) | 1294.53 (0.000) | –593.71 (0.015) | 212.60 (0.164) | 1.86 (0.989) | 0.19 (0.964) | |
| Complete case PP | 911.11 (0.002) | 1615.50 (0.000) | –519.08 (0.055) | 143.33 (0.340) | –17.04 (0.904) | 1.73 (0.692) | |
| With MI PP | 1091.85 (0.000) | 1594.40 (0.000) | –562.63 (0.036) | 173.21 (0.255) | –28.19 (0.841) | 1.39 (0.764) |
| Adjusted for baseline QoL | Scenario | Regression constant | Surgical allocation | QoL at baseline | Fracture displacement | Dominant wrist injured | Age |
|---|---|---|---|---|---|---|---|
| Unadjusted | Complete case ITT | 0.8162 (0.000) | 0.0208 (0.319) | N/A | –0.0283 (0.176) | –0.0285 (0.163) | –0.0011 (0.091) |
| With MI ITT | 0.8042 (0.000) | 0.0182 (0.304) | N/A | –0.0350 (0.047) | –0.0399 (0.027) | –0.0016 (0.010) | |
| Complete case PP | 0.8112 (0.000) | 0.0257 (0.233) | N/A | –0.0342 (0.118) | –0.0348 (0.101) | –0.0012 (0.076) | |
| With MI PP | 0.7984 (0.000) | 0.0173 (0.331) | N/A | –0.0423 (0.021) | –0.0433 (0.018) | –0.0015 (0.026) | |
| Adjusted | Complete case ITT | 0.6947 (0.000) | 0.0250 (0.289) | 0.2895 (0.000) | –0.0202 (0.371) | 0.0046 (0.826) | –0.0020 (0.020) |
| With MI ITT | 0.6733 (0.000) | 0.0158 (0.379) | 0.2732 (0.000) | –0.0261 (0.164) | 0.0229 (0.203) | –0.0020 (0.005) | |
| Complete case PP | 0.6761 (0.000) | 0.0238 (0.315) | 0.3272 (0.000) | –0.0168 (0.494) | 0.0033 (0.884) | –0.0021 (0.018) | |
| With MI PP | 0.6690 (0.000) | 0.0118 (0.505) | 0.2823 (0.000) | –0.0265 (0.175) | 0.0218 (0.227) | –0.0019 (0.009) |
The regression analyses highlight a number of interesting findings. First, as expected, across all scenarios, allocation to the surgical arm of the trial is associated with greater incremental costs and greater QoL. However, while the correlation with costs is found to be statistically significant, the correlation with QoL is not significant across any of the scenarios considered. The per-protocol analyses find a larger impact on cost and QoL, as they remove the crossover patients whose inclusion reduces the reported benefit of surgical allocation.
Also consistent to a priori expectations, increased fracture displacement [i.e. minimally (1–2 mm inclusive) compared with undisplaced (< 1 mm step or gap)] was associated with a lower QoL (statistically significant also in the two multiple imputation scenarios) and a higher cost. Age was also found to be associated with a decrease in QoL and an increase in costs, while those who had injured their non-dominant hand had increased QoL but had no consistent impact on costs.
Adjusting for baseline QoL using a regression approach had only a relatively small impact on the treatment allocation regression estimate, with no consistent direction across the different scenarios. The scale of this change, while surprising given the relative size of the incremental mean difference in baseline reported in Table 34, is due to many of the differences being already explained by the existing explanatory variables in the unadjusted regressions and the random component of the variable. The impact of QoL at baseline on the cost regression is expected to be the result of adjusting for some level of comorbidities or patients’ willingness to interact with the NHS.
| Adjusted for baseline QoL | Scenario | Incremental cost (95% CI) | Incremental QALYs (95% CI) | ICER: surgery versus cast |
|---|---|---|---|---|
| Unadjusted | Complete case ITT | £1580 (£1282 to 1879) | 0.0208 (–0.0200 to 0.0616) | £75,962/QALY |
| With MI ITT | £1228 (£1011 to 1445) | 0.0182 (–0.0165 to 0.0530) | £67,473/QALY | |
| Complete case PP | £1771 (£1490 to 2052) | 0.0257 (–0.0165 to 0.0678) | £68,910/QALY | |
| With MI PP | £1549 (£1344 to 1754) | 0.0173 (–0.0176 to 0.0522) | £89,538/QALY | |
| Adjusted | Complete case ITT | £1308 (£1063 to 1609) | 0.0250 (–0.0240 to 0.0694) | £52,320/QALY |
| With MI ITT (base case) | £1295 (£1084 to 1504) | 0.0158 (–0.0221 to 0.0570) | £81,962/QALY | |
| Complete case PP | £1616 (£1314 to 1988) | 0.0238 (–0.0247 to 0.0649) | £67,899/QALY | |
| With MI PP | £1594 (£1296 to 1961) | 0.0118 (–0.0123 to 0.0529) | £135,085/QALY |
Clearly, using the limited time scale of the within-trial analysis, the use of surgical fixation is not cost-effective, especially if the difference in baseline QoL is adjusted for. However, as discussed, such a limited timescale overlooks many of the outcomes correlated with the choice of treatment for scaphoid waist fractures, primarily the impact of non-union after this period of treatment on the incidence of arthritis, SNAC and other related AEs.
Impact of lost employment
The summary statistics of the patient-reported days of lost employment are reported in Table 35. Primarily, the table shows that the majority of patients experienced some days of lost employment in the first 6 weeks of the analysis period (with only 21.6% and 31.3% reporting no lost days over that period in the surgery and cast group, respectively), but from 12 weeks onwards most were back to full-time work (with medians of 0 for all other periods). There did, however, remain a number of patients who were forced to continue missing work because of their wrist fracture, characterised by the persistent mean number of days lost, despite close to 90% reporting no lost days. There were very few cases of patients having to miss work for all, or even most, of the period covered by the questionnaire.
| Questionnaire period | Treatment allocation | Number of responses | Mean days lost | Median days lost (95% percentile) | Percentage reporting 0 days |
|---|---|---|---|---|---|
| 6 weeks | Surgery | 148 | 12.07 | 6 (40) | 21.6 |
| Cast | 150 | 12.13 | 4 (42) | 31.3 | |
| 12 weeks | Surgery | 159 | 2.38 | 0 (21) | 76.7 |
| Cast | 145 | 3.90 | 0 (30) | 69.0 | |
| 26 weeks | Surgery | 140 | 1.34 | 0 (5) | 91.4 |
| Cast | 135 | 3.72 | 0 (32) | 88.9 | |
| 52 weeks | Surgery | 164 | 1.51 | 0 (4) | 91.5 |
| Cast | 160 | 1.94 | 0 (6) | 91.8 | |
| Total, complete case | Surgery | 102 | 16.62 | 9.5 (51) | 13.7 |
| Cast | 91 | 17.57 | 5 (67) | 28.6 |
A detailed description of this table and the estimation methods are provided in Appendix 6, Impact of lost employment and unpaid activities, which also includes details of the impact on unpaid work.
Extrapolated model: results
In this section, the results of the extrapolated mathematical model are reported. First, we report the headline cost-effectiveness results (i.e. the expected costs, QALYs and resultant ICER and NHB), alongside a consideration of how the NHB accumulates over time. We then go on to consider the decision uncertainty present in the decision model. This is reported in two ways: the probability of each of the four strategies being cost-effective at a given cost-effectiveness threshold represented by the cost-effectiveness acceptability curve (CEAC) and a series of scenario analyses exploring the impact of structural uncertainty on the result.
Base-case analysis
Table 36 reports the cost-effectiveness results for the base-case analysis in terms of each treatment option’s expected costs and QALYs, alongside a range of ICERs and the NHBs at thresholds of £20,000/QALY and £30,000/QALY.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) versus . . . | NHB at threshold of . . . | ||
|---|---|---|---|---|---|---|
| Lowest cost (cast only) | Next least effective non-dominated | £20,000/QALY | £30,000/QALY | |||
| Cast only | 836 | 18.72 | – | – | 18.68 | 18.70 |
| Cast plus surgery | 921 | 19.07 | 243 | 243 | 19.02 | 19.04 |
| No treatment | 1749 | 14.75 | Dominated | Dominated | 14.67 | 14.70 |
| Surgery | 2404 | 19.12 | 3952 | 29,660 | 19.00 | 19.04 |
The results show that, by taking a lifetime perspective, surgery is the most expensive option, followed by no treatment, cast plus surgery for non-union and finally cast only. The high cost of the surgery arm is driven by the high upfront cost of the initial treatment, in contrast to the no-treatment cost, which is the result of high future costs. These different cost accumulations are shown in Figure 10, which shows the cumulative discount costs and clearly shows the large costs in the non-treatment arm after the initial treatment period, which result from the large costs that result from the long-term implications of non-union.
FIGURE 10.
Cumulative costs.
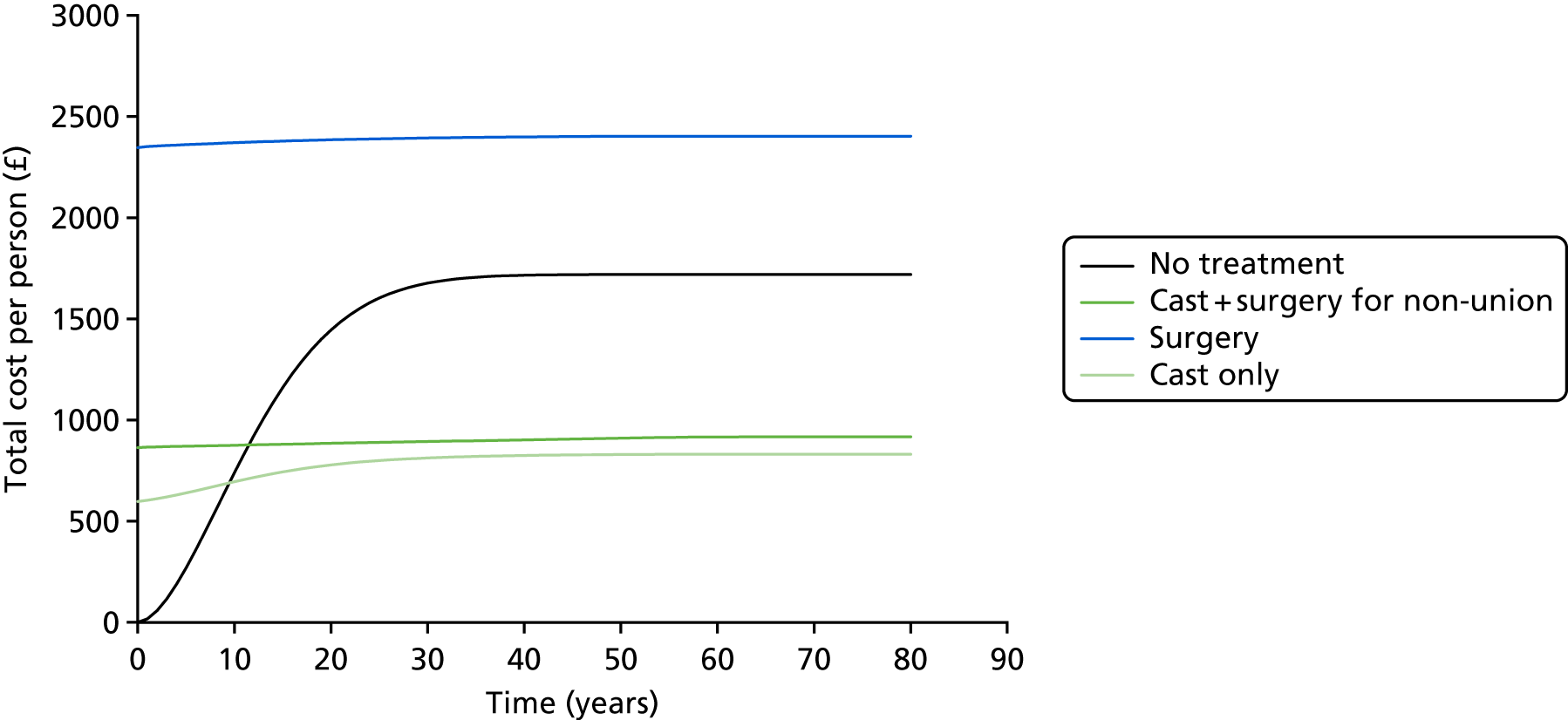
The total QALYs for the no-treatment arm are much lower than for any of the active treatment arms owing to the lifetime impact of AEs related to non-union. Surgery was found to result in the highest total QALYs, followed by cast plus surgery and cast only.
The ICERs and NHBs show that, at their expected values (i.e. not taking into account any uncertainty), cast plus surgery is the most cost-effective option, with the highest NHB at a conventional threshold of £20,000/QALY, followed by primary surgery, cast only and, finally, no treatment.
The accumulation of NHB over time can be seen in Figure 11, which shows the cumulative incremental NHB over the analysis period at a threshold of £20,000/QALY, compared with no treatment. It shows that, with the exception of the first year, all three active treatment options have a positive cumulative incremental NHB throughout. The closeness of the primary surgery and cast plus surgery lines show that there is a very small absolute difference between the two over time, with the impact of the higher upfront cost in the surgery treatment never being overcome by future health or cost gains, such that, at any time point in the analysis, cast plus surgery is the most cost-effective treatment when considering mean values at a threshold of £20,000/QALY.
FIGURE 11.
Cumulative incremental NHB in relation to no treatment (QALY).
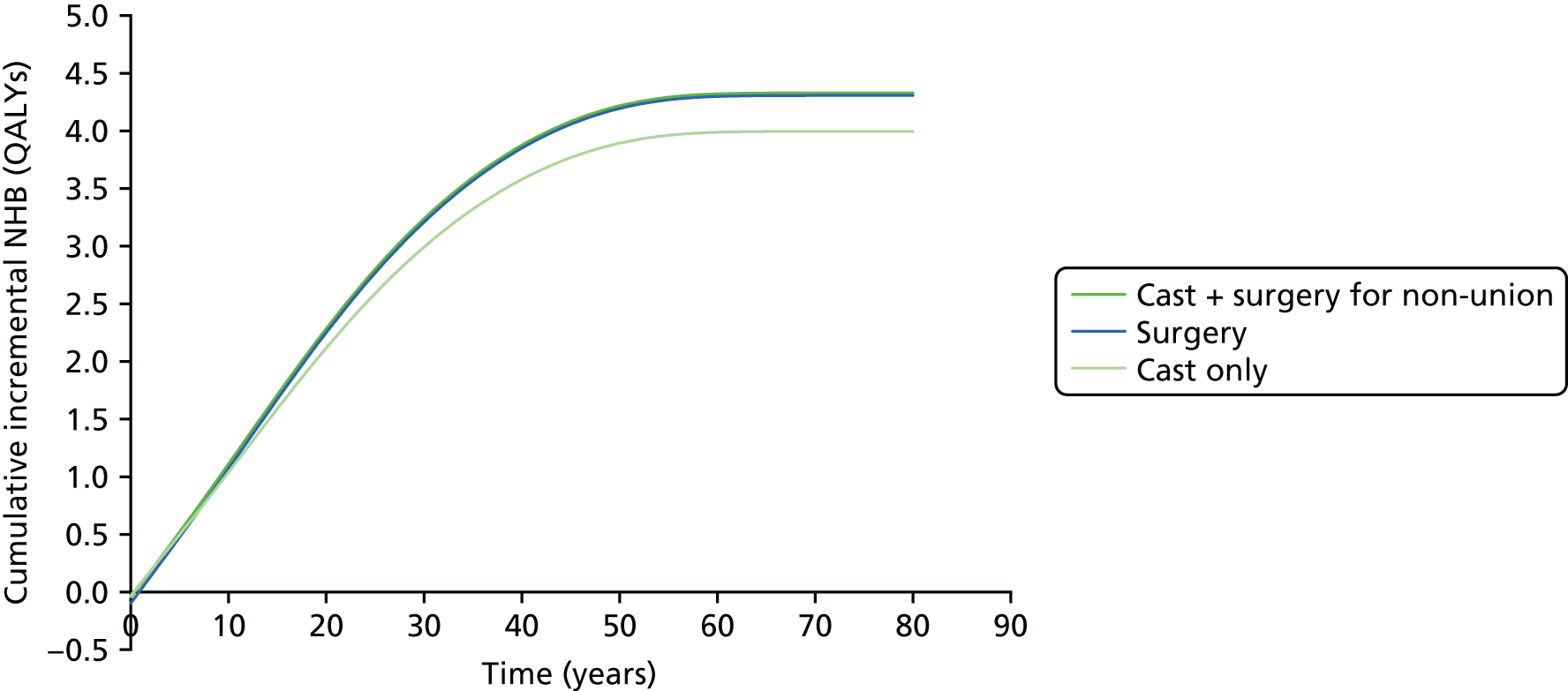
There are a number of ways of reporting the parametric uncertainty associated with the base-case analysis (i.e. how much the modelled uncertainty in each of the parameters affects our decision). Figure 12 provides the conventional CEAC, which reports the probability of each of the four arms being the most cost-effective option for a range of cost-effectiveness thresholds. The CEAC shows that, at very low threshold values, below £300/QALY, the cast-only treatment is the most cost-effective. This swaps to cast plus surgery for thresholds between £300 and 29,660/QALY, after which surgery becomes the most cost-effective for all higher thresholds. As the threshold increases to extreme values, primary surgery becomes the more cost-effective option owing to its slightly higher estimated lifetime QALYs, but there remains a large level of uncertainty in the decision.
FIGURE 12.
Cost-effectiveness acceptability curve.
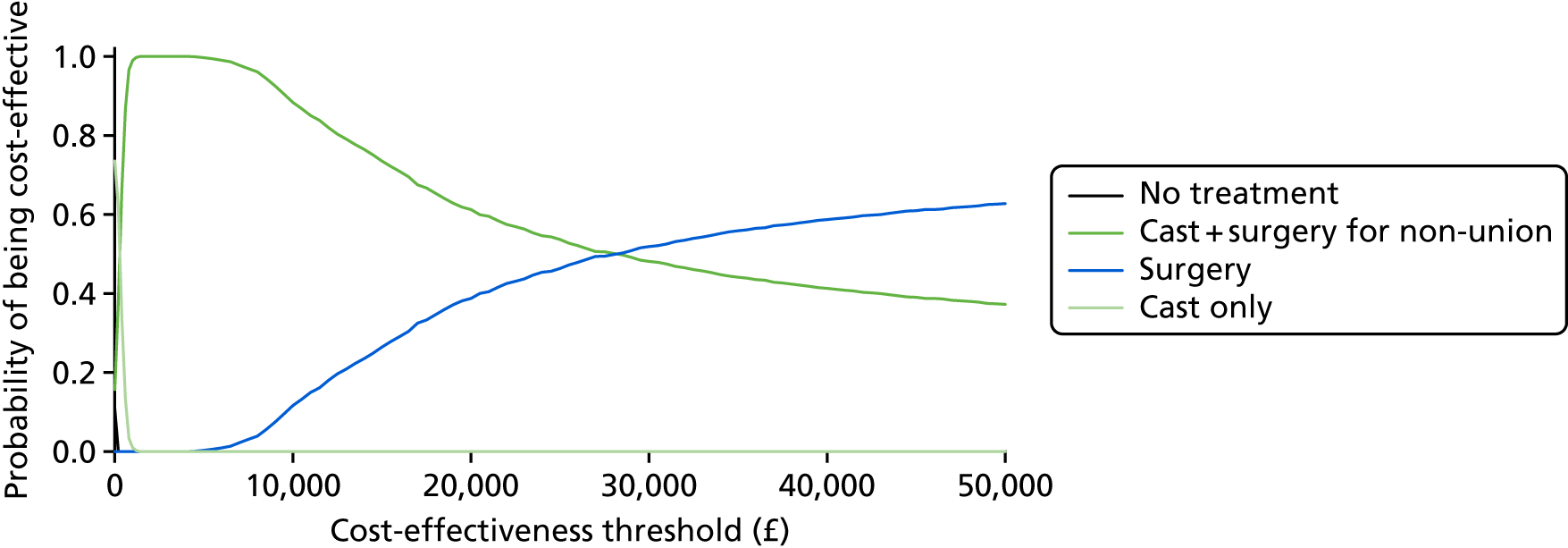
The CEAC further shows that, if a threshold of £13,000/QALY was used, as indicated by recent research,111 cast plus surgery would remain the most cost-effective option, with 79% of the simulations favouring it, with the expected incremental NHB when compared with primary surgery increasing to 0.07.
Another important factor to consider is the relative rank of each treatment option, as it is important to understand the situation when a treatment option is not the most cost-effective. Table 37 provides the probability of each treatment option being ranked at each place at a cost-effectiveness threshold of £20,000/QALY. It shows that surgery and cast plus surgery are always the top two treatment options. In addition, cast only is almost always ranked third and no treatment is always last. This distribution becomes more marked at higher threshold values. This, combined with the base-case result and CEAC, allow us to conclude that the cast plus surgery option results in the highest NHB and is the most likely to be cost-effective at a range of conventional threshold values; in addition, even when it is not the most cost-effective, it is almost always the second most cost-effective option. Table 37 also shows the large level of uncertainty in the decision at conventional thresholds, with the parametric uncertainty in our analysis resulting in two-thirds of simulations favouring cast plus surgery over initial surgical fixation.
| Rank | No treatment | Cast plus surgery for non-union | Surgery | Cast only |
|---|---|---|---|---|
| 1 | 0.00 | 0.61 | 0.39 | 0.00 |
| 2 | 0.00 | 0.39 | 0.60 | 0.01 |
| 3 | 0.00 | 0.00 | 0.01 | 0.99 |
| 4 | 1.00 | 0.00 | 0.00 | 0.00 |
Furthermore, Appendix 6, Extended model results, reports the cost-effectiveness scatterplots generated from the PSA, alongside predictions made by the model for the rate of OA and SNAC at 1, 5 and 10 years to facilitate future validation of this analysis.
Value of information analysis
In addition to considering which treatment option is the most cost-effective and the probabilities of cost-effectiveness as we have done so far, it is also important to consider the implications of that decision uncertainty. For example, if a treatment is estimated as being the most cost-effective 80% of the time but, when it is incorrect, the resultant NHB is catastrophic, it may not be deemed the best option by decision-makers until further research can be used to reduce this level of uncertainty.
To calculate the population EVPI, estimates of the population size and time frame are required. We assumed a 10-year time frame and a per annum population of 4650. The per annum population was estimated by using the 7265 total scaphoid fractures reported by Garala,4 with 64%4 estimated as being located in the waist.
The population EVPI is presented in Figure 13, showing how the value of generating additional information varies as the threshold varies. The figure shows that, at lower threshold values, there is a relatively low level of EVPI, associated with a high value of additional information, as there is a low level of uncertainty about the most cost-effective treatment option, as shown earlier in this section. However, the value of perfect information increases at an increasing rate as the threshold increases, as both the probability and the implications of an incorrect decision increase. The ‘kink’ in the curve is the point at which primary surgery becomes the treatment that is most likely to be cost-effective, but, as the level of uncertainty remains high, the value of resolving it continues to increase. One approach to interpreting population EVPI is as a framework to measure conditions under which future research is potentially cost-effective. 97
FIGURE 13.
Population EVPI across different threshold values.
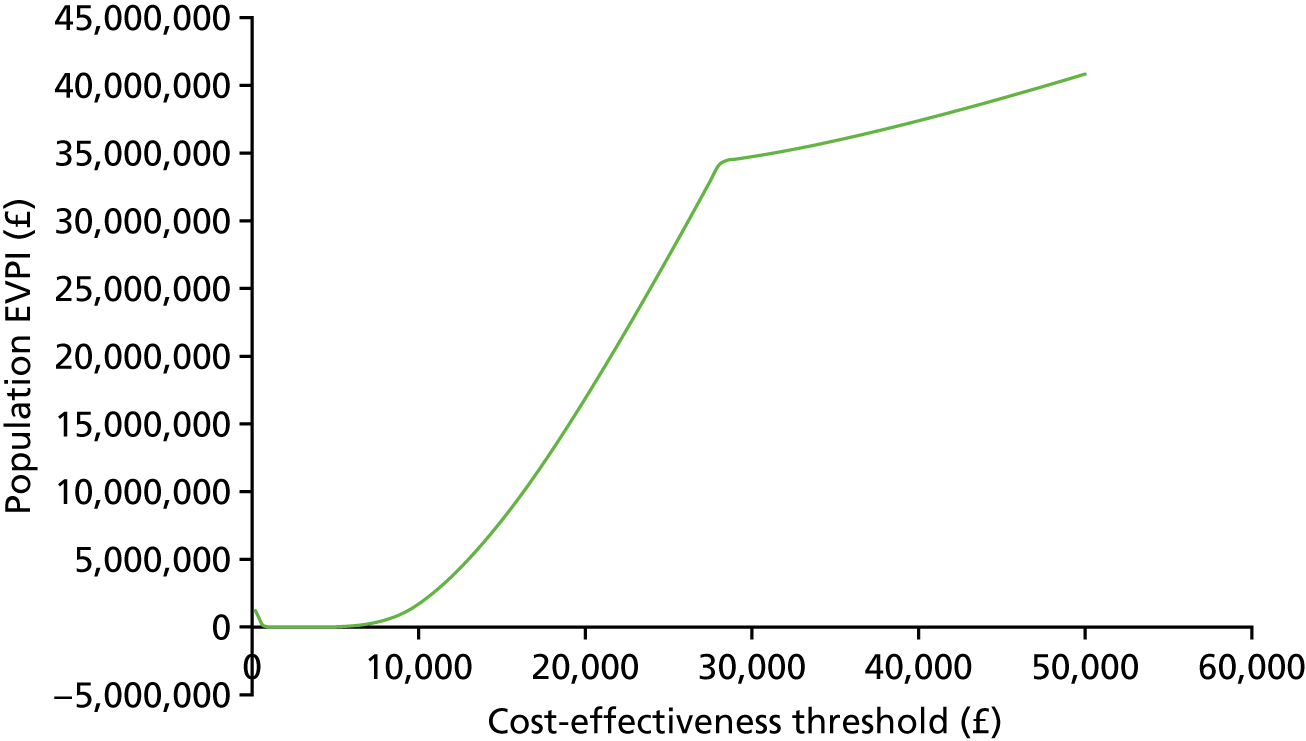
Scenario analyses
A number of scenarios were conducted to explore some of the elements of uncertainty not well captured by the PSA. The full methods and results of these scenarios are presented in Appendix 6, Extrapolated model scenario analyses. In brief, the scenarios show that the headline result of the analysis is not sensitive to the majority of parameter changes but is sensitive to changes in how union is defined (scenario 1) and the assumption made regarding what happens to the patients who failed to achieve initial union with cast immobilisation only (scenario 2). These sensitivities are because the model result is almost completely driven by the rate of non-union between the treatment options, which were very similar in SWIFFT at the 52-week follow-up.
Chapter 5 Qualitative study
Introduction
Context of the qualitative study
A number of factors suggest the value of integrating qualitative research within SWIFFT. The fact that fractures of the scaphoid waist are most common in a younger, active (and economically active) population suggests that the impact of the injury may be wide-reaching and significant. A failure rate of 10–12% for plaster cast management, the potential for complications associated with surgery and the fact that cast and surgical treatments are of different orders (one invasive, one conservative) might suggest that patients will have opinions about the appropriateness of (and a preference for) the treatment options available. Furthermore, a comparison of medical and surgical treatments creates its own challenge of trial delivery, in ensuring equipoise, managing expectations of treatment and ensuring long-term engagement with trial procedures.
This nested study will seek to generate personalised, contextualised and specific insight about participants’ experience of a scaphoid fracture and about their involvement in SWIFFT. It will generate additional insight that will complement the findings of the main trial and it will offer insight that will support the design and delivery of future surgical trials.
Aims and objectives
The aim of this study is to provide complementary, detailed and person-centred insight that will inform the interpretation of trial findings and that might support future clinical decision-making.
The study objectives were to:
-
explore participant experience of a fracture of the scaphoid waist
-
explore participant experience of treatment of a scaphoid waist fracture
-
consider preferences for treatment options and those factors that might be pertinent in this
-
consider involvement in a surgical, randomised clinical trial.
Fracture and recovery: a patient’s perspective
With the exception of hip fracture, qualitative investigation of patient experience of fracture is limited, with only a small number of papers that address injury of the hand, wrist or arm. 112–119 Despite some variation (‘wrist fracture’, ‘wrist disorder’, ‘hand injury’, ‘hand disability’, ‘finger fracture’, ‘flexor tendon surgery’), this body of work points to a number of common themes that are likely to be pertinent to a fracture of the scaphoid waist: functional ability, relationships with others, perceptions of treatment/recovery and recovery goals.
Functional ability
Functional ability was addressed in all studies. 112–119 ‘Wrist disorders’ are described as affecting all aspects of life (including domestic, recreational and economic) and spanning activities that require fine motor skills as well as those that require strength. 117 Employment was presented as an area in which limitations might have a significant impact, namely in changed roles, reliance on others and challenges to self-confidence. 112,115,119 The financial consequences of absence from work were a further potential consequence of a wrist injury. 117,118 These and other limitations might lead individuals to feel anxious, frustrated, emotionally distressed or even depressed;114,116,117,119 some authors propose a holistic approach to managing injuries (which incorporates both psychological and clinical therapy). 112,114,116,118
Watson et al. 118 are alone in their assessment that the nature and extent of limitations might vary among a heterogeneous patient population, with factors such as single-parent status and self-employment associated with an increased impact. They also propose that the time spent in a cast might be associated with how limitations are perceived – a longer period in a cast increasing the experience of limitations associated with the injury. 118
Relationships with others
Functional limitations associated with immobilised hands or wrists may lead individuals to become more reliant on others for practical help and support;117–119 husbands/wives, friends or family might all play an important role in helping an individual to maintain normal activities. 117 Such support, while generally perceived positively, might have some negative consequences for those who find a lack of independence or a reliance on others emotionally challenging. 116 Schier and Chan112 describe how social roles (as a spouse, caregiver, worker) might be fundamentally changed after a hand injury and that a ‘sense of loss’ for ‘previous social roles’ is conceivable.
A more positive perspective is offered by Chan and Spencer,114 who identify that supportive relationships can be a motivator for patients to make positive adaptive behaviours (e.g. adhering to rehabilitation programmes so as to resume parenting activities). Supportive relationships might also provide moral (rather than practical) support at clinic appointments and in gathering information about the injury and treatment. 118
Perceptions of treatment/recovery
A potential disconnect between how an injury is perceived and the complexity/duration of treatment can have consequences for adherence to rehabilitation programmes113 and for remaining optimistic about recovery. 119 The fact that a treatment might feel excessive when compared with how the injury is understood (simple, uncomplicated, minor?) offers conceptual challenges in accepting treatment and imagining recovery.
Removal of a cast was associated with ‘a sense of relief, improved function and the start of recovery’ (emphasis added);118 this demonstrates the pertinence of lay conceptions of treatment and highlights that a patient’s own sense of recovery might be at odds with their clinical recovery, in other words that perceptions of recovery might be more about regaining normality than about the clinical/physical repair of the fracture or injury. In the same study, participants describe uncertainty and anxiety about the removal of a cast, concerned by the loss of protection and uncertainty about the status of the wrist repair. 118 A lack of feedback and information about recovery is a source of anxiety for some patients,119 and elsewhere patients describe the importance of feeling confident that they are receiving the right care and feeling certain about their recovery trajectory. 118
It seems that patient understanding of injury and recovery is influential in shaping their experience of treatment and their satisfaction with it.
Recovery goals
Uncertainty about achievable levels of functionality,116 acceptance about possible longer-term limitations114 and ‘readjusting expectations and accepting limitations’119 all point to some sense of uncertainty about what long-term recovery might look like. The fact that such concerns are less common in this body of work and less present in initial clinical consultations118 reinforces the assessment that ‘wrist fracture is perceived too trivial to warrant [detailed/longer-term concern]’. 118
Amman et al. 115 suggest that patients ‘had an inner drive to strive for normality’, which mirrors a concern for ‘regaining normality’ demonstrated in orthopaedic trauma patients (which includes wrist, elbow and shoulder injuries). 120 Pre-injury wrist status might be important in establishing some sense of what this might mean,117 although, to borrow from the hip fracture literature, notions of ‘normality’ will be shaped by broader conceptions of prior health and by what is considered acceptable with regard to natural deterioration (such as that associated with ageing). 121
Summary and assessment of literature
It is evident that hand or wrist injuries can have wide-ranging impacts on functional abilities in domestic, leisure and employment environments and that such difficulties can lead to a greater reliance on other people. A lack of clarity about longer-term goals and the potential for dissonance between how injury (minor) and treatment (complex/long-term) are conceived may lead to uncertainty about recovery. A patient’s (subjective) ‘sense of recovery’ may thus be important in their level of satisfaction with treatment. The fact that subjective experience is influenced by clinical, personal, social or even economic factors suggests a wide variation in how recovery might be managed.
It should be noted that this assessment is based on limited literature and that the existing evidence base varies in scope and method, from detailed reviews of two or three interviews112,114,116 to more structured assessment of larger data sets. 117 Differences in focus (hand, wrist or finger) and reach (from one-off interviews to longitudinal studies) also make synthesis and confident extrapolation difficult. Little has been written about wrist fracture,118,119 and fracture of the scaphoid waist is not explicitly identified in these papers. Surgical treatment is not explicitly explored and an investigation of patient preference for treatment is lacking.
Randomised trials in surgery
Difficulties recruiting to randomised clinical trials are well documented,122–127 with issues of participant retention gaining increased consideration. 128–132 For clinical trials in which surgery is compared with a non-surgical treatment, these difficulties are recognised as being more pronounced,47 and McCulloch et al. 46 identify a range of factors that they consider as inhibiting surgical trial delivery. Among these are surgeons’ equipoise, patients’ equipoise, surgical learning curves, issues with blinding, life-threatening and urgent conditions, definitions (of procedures) and quality control monitoring. 46
A more general assessment highlights the relevance of contextual and specific characteristics in trial delivery; the setting of a study, the population of interest and specific procedures may all pose challenges to the appeal or completion of a study. 128,133 For SWIFFT, the younger, active male population suggests some challenge to trial delivery. Previous research has demonstrated that females are more likely to participate in research134 and that older people are more likely to fulfil their commitment to a study. 135 Insight generated in this qualitative research might provide further insight into the challenges of delivering a trial with this population and might point to factors that enhance or inhibit success.
Methods
Study design
A nested interview study was conducted to explore participants’ experience of the fracture of the scaphoid waist, its treatment and their experience (or opinion about) involvement in clinical research. This study involved both individuals recruited to the SWIFFT pragmatic RCT, as well as a small number of individuals who declined to participate in the trial. Interviews were conversational (semistructured rather than structured) and analysed using an inductive, thematic approach.
Participants
A purposive sample136 of those SWIFFT participants who indicated a willingness to be interviewed were recruited to this study. Interviewees were purposively selected to ensure that both men and women, experiencing different treatments and of different ages and occupations, were interviewed. A predefined sampling frame (Table 38) was constructed to guide participant selection and to ensure that the sample broadly reflected general patterns in the incidence of scaphoid fractures, that is, to prioritise younger people (under 30 years of age) and to prioritise male participants. The sampling frame also distinguishes between manual and non-manual occupations to enable this comparison in the analysis.
| Group | Gender (n) | Age (n) | Occupation (n) | |||
|---|---|---|---|---|---|---|
| Male | Female | Under 30 years | Over 30 years | Manual | Non-manual | |
| Surgery | 12 | 6 | 12 | 6 | 9 | 9 |
| Cast | 12 | 6 | 12 | 6 | 9 | 9 |
| Total | 24 | 12 | 24 | 12 | 18 | 18 |
Those who declined to participate in SWIFFT were also invited to take part in this interview study. This second group was recruited to support a broader consideration of patient preference for treatment, that is, to reflect that those who declined may have different attitudes about treatment from those who took part. Again, a sampling frame was constructed to prioritise interviews with male participants and with those under 30 years of age (Table 39).
| Group | Gender (n) | Age (n) | Occupation (n) | |||
|---|---|---|---|---|---|---|
| Male | Female | Under 30 years | Over 30 years | Manual | Non-manual | |
| Total | 6 | 3 | 6 | 3 | – | – |
Recruitment to this element of SWIFFT research was also informed by concerns for data saturation, that is, the point at which no new insight is generated in the undertaking of additional data collection. 137 Prior assessment has suggested that core topics and themes might be established with as few as six interviews and that more complete data saturation is commonly achieved with 10–13 interviews. 138–140 Here, 18 in-trial interviews per treatment arm were proposed as sufficient to reach data saturation and as sufficient to be confident of the interpretation of combinations such as male/cast (n = 12) or surgery/under 30 years (n = 12).
In total, 36 in-trial interviewees and nine interviews with those who declined to participate in SWIFFT were proposed.
All interviewees were separately consented for this element of the SWIFFT research.
Data collection
All participants were interviewed within 6 weeks of randomisation and those in the trial were interviewed again at 52 weeks. Earlier and later interviews covered similar topics and were organised in three parts: (1) wrist fracture and its impact, (2) treatment of fracture and (3) participation in clinical research. Interviews at 6 weeks were intended to capture an immediate response to the scaphoid injury; interviews at 52 weeks were intended to reflect on treatment options and individual recovery. At both time points, questions about clinical research were included to generate general (understanding and perceptions) and specific (to support the delivery of SWIFFT) insight.
The same interview schedule was used for all interviews at 6 weeks, with some adjustment in the prompts used and how questions were phrased for those who declined participation in the trial. Interview schedules are included here as Report Supplementary Material 19.
Where possible, interviews were undertaken face to face at a time and location convenient to the participant. In other cases, when it was the participant’s preference or when geography made a face-to-face meeting impractical, interviews were undertaken via the telephone. Interview questions and prompts were used as a guide for discussion only (rather than as a strict script)141–144 and interviewees were encouraged to develop issues that they felt were important and to introduce new topics that had not otherwise been considered. In this way, these interviews were intended to be discursive, generating personalised and contextualised accounts of injury and treatment. Therefore, the body of data generated was intended to capture the range of different individual experiences.
All interviews were digitally recorded and transcribed in full; transcripts were anonymised with all personal identifying data removed. Data were stored on a password-protected, networked drive and handled using the NVivo (version 11) software package (QSR International, Warrington, UK).
Data analysis
The discursive nature of the data generated (i.e. guided, but not restricted, by an interview schedule) suggests a thematic approach to data analysis. 137,145 Furthermore, the exploratory nature of the study would suggest an inductive, thematic approach, as described by Braun and Clarke. 146 This approach describes a systematic and structured method undertaken in a series of iterative steps. It is a form of qualitative analysis that explores data on their own terms and that prioritises the organic insight generated therein.
Interview transcripts were read and reread in an initial stage of data familiarisation. Next, open (inductive) coding was used to identify points of interest in a process of generating initial codes. This process was managed independently by a study researcher, with the qualitative study lead ensuring the consistency and validity of the coding.
In stage 3, these initial codes were merged and grouped according to topic or sentiment in the identification of themes. Here, themes and subthemes were organised within three broad topic areas that reflect the aims and objectives of the study, that is, injury, treatment and research. These early stages (1–3) were undertaken alongside data collection, with emergent findings presented to the SWIFFT TMG at its regular meetings.
In stage 4, themes were reviewed and refined to ensure their internal and external coherence. At this stage, themes were also interrogated to establish their utility in serving the aims and objectives of the research. Following this, themes were finalised in a stage entitled ‘definition and name’ and were connected to form broad narratives, providing insight into (1) the injury and its impact, (2) cast treatment, (3) surgical treatment and (4) research. Themes and thematic models were validated by the study chief investigator and other members of the SWIFFT TMG.
Braun and Clarke146 suggest that producing the report is the final stage of thematic analysis, stressing that interpretation continues as data are organised in an authored form. The final narratives (presented below) were validated by the SWIFFT TMG and author groups.
Results
Data overview and code book
A total of 64 interviews were undertaken with 49 individuals.
At 6 weeks, 36 in-trial interviews were carried out and, at 52 weeks, 19 interviews were carried out; in addition, nine interviews with individuals who declined the main trial were carried out at 6 weeks (Tables 40–42 describe the demographics of the sample). Of the 49 individuals interviewed, 36 in-trial participants were recruited at baseline, nine individuals who declined participation in SWIFFT were recruited at baseline and four in-trial participants were recruited at 52 weeks (to compensate for dropout). Of these, 35 were male (14 female) and 26 were under the age of 30 years. Approximately half of all interviews were with our key demographic: males under the age of 30 years (17/36 at 6 weeks, 9/19 at 52 weeks and 5/9 who declined participation).
| Group | Gender (n) | Age (n) | Occupation (n) | |||
|---|---|---|---|---|---|---|
| Male | Female | Under 30 years | Over 30 years | Manual | Non-manual | |
| Surgery | 13 | 4 | 9 | 8 | 7 | 10 |
| Cast | 12 | 7 | 10 | 9 | 7 | 12 |
| Total | 25 | 11 | 19 | 17 | 14 | 22 |
| Group | Gender (n) | Age (n) | Occupation (n) | |||
|---|---|---|---|---|---|---|
| Male | Female | Under 30 years | Over 30 years | Manual | Non-manual | |
| Surgery | 8 | 1 | 6 | 3 | 5 | 4 |
| Cast | 5 | 5 | 4 | 6 | 6 | 4 |
| Total | 13 | 6 | 10 | 9 | 11 | 8 |
| Group | Gender (n) | Age (n) | ||
|---|---|---|---|---|
| Male | Female | Under 30 years | Over 30 years | |
| Total | 6 | 3 | 5 | 4 |
The initial analysis was structured around three broad topics (injury, treatment and research) with codes subsequently organised within 11 core themes and 37 subthemes (Table 43). This represents stages 1–4 of the inductive thematic analysis. 146 Stages 5 and 6 are presented from the Thematic analysis section onwards.
| Topic | Theme | Subtheme |
|---|---|---|
| Injury | A minor injury? | Delayed seeking medical help |
| Not worthy of serious treatment | ||
| Practical difficulties | Driving | |
| Leisure pursuits | ||
| Washing | ||
| Domestic chores | ||
| Difficulties at work | ||
| Psychosocial difficulties | Loss of independence | |
| Mood | ||
| Relationships with others | ||
| Other difficulties | Employment | |
| Money and finances | ||
| Treatment | Plaster cast | Inconvenient and immobilising |
| Passive | ||
| Safe and natural | ||
| Preference for plaster cast | ||
| Surgery | Preference for surgery | |
| Active | ||
| Quicker recovery | ||
| Risks | ||
| Factors in preference | The need for speed – employment and money | |
| The need for speed – familial responsibilities | ||
| The need for speed – lifestyle and other events | ||
| Access to practical support | ||
| Prior clinical experience | ||
| Recovery | Return to normal | |
| Progress made | ||
| Long-term concerns | ||
| Research | Reasons for participating | Access to treatment options |
| Clinical benefits | ||
| Money as incentive | ||
| Positive assessment of research | Research processes acceptable | |
| Research nurses | ||
| Facilitators of research | ||
| Negative assessment of research | Barriers to research processes | |
| Research processes unacceptable |
Topics, themes and subthemes were consistent across the sample; data collection ceased following the addition of four young, male interviewees who were interviewed at 52 weeks only (to ensure data saturation for this key population).
Participants were recruited from 13 centres: Bath, Bolton, Cardiff, Coventry, Leicester, Newcastle, Nottingham, Oxford, Peterborough, Plymouth, Royal London, Southampton and Teesside.
Interviews varied in length from 14 to 73 minutes at 6 weeks and from 13 to 41 minutes at 52 weeks. Interviews were typically 40–45 minutes in length at 6 weeks and 20–25 minutes at 52 weeks.
Thematic analysis
The convention for identifying participants is as follows: plaster cast (P) or surgery (S) + participant ID number, gender, age (years), 6- or 52-week interview; an asterisk indicates an interview with someone who declined to participate in the main trial.
Model 1: the injury and its impact
Thematic model: fracture of the scaphoid waist is often initially considered a relatively minor injury. It can, however, have a wide-reaching impact in limiting function. Limited function may be associated with a greater reliance on other people and may have other psychosocial consequences.
An inconsequential injury?
For many, the fracture was the result of what they considered to be an inconsequential incident – a trip, a clash on the football pitch, a bang at work or falling off a bike. Few considered the injury serious and many continued what they were doing: ‘[S]trapped it up, checked I could still catch and pass the ball, and then went back on’ (P1121, male, under 30, 6 weeks); ‘I carried on working . . . I had to make the job safe before I walked away, so I worked for probably an hour after’ (S1008, male, under 30, 6 weeks); ‘oh yeah I carried on dancing, it didn’t hurt at the time’ (P1245, female, under 30, 6 weeks). Even an assessment that some damage might have been done did not necessarily mean that immediate action was taken:
I knew as soon as I’d landed, it was obvious . . . I could tell it was more than a sprain . . . I thought I’d damaged it . . . [but] I did what I needed to do, I just finished the delivery, and drove the van to where it was needed . . . by the time I got back here it was 9 o’clock [at night] . . . So I went down the hospital first thing on Saturday morning.
P1451, male, over 30, 6 weeks
In the few cases in which more immediate medical attention was sought, it was often because of multiple injuries, with the wrist commonly considered the least of these: ‘I went because of the blood gushing from my chin . . . I did my tooth, my chin, everything else as well’ (P1023, male, under 30, 6 weeks*); ‘the ladders slipped underneath me . . . it was my back . . . I didn’t know I’d hurt my wrist’ (P1282, male, under 30, 6 weeks).
For most, swelling, pain or bruising prompted them to seek medical attention within 24 hours, although for some it was a number of days before they sought any treatment: P1823 (female, under 30, 6 weeks) went to A&E 3 days after her fall and P1161 (male, over 30, 6 weeks) went to hospital 6 days after a trip. Even when seeking medical attention, some still suspected that they had only sprained their wrist and a number were surprised at the fracture diagnosis. The severity of the consequences of the injury (and the potential for surgery) were surprising to some:
I think when you initially do it, you always think, well, your wrists are nothing and it’s not until somebody sits down and explains to you just exactly how complicated a wrist or a foot or hand is that you suddenly realise, hang on a minute.
S1175, male, over 30, 52 weeks
I was like, well, it didn’t really hurt so how can it be that bad . . . [the hospital] said ‘it could be 6 to 10 weeks in a cast and then an operation’ . . . I was like ‘oh no, I don’t want any of this! I only just went ice-skating and accidentally got pushed over’ . . . I didn’t realise the severity of it, kind of thing, well, I just didn’t, I still can’t quite believe it now to be fair.
S1244, female, over 30, 6 weeks
Practical difficulties associated with the injury
Despite any initial thoughts that the fracture might be a minor injury, all interviewees described some degree of practical limitation, from work to washing and dining to driving.
The impact on leisure pursuits was telling for some: ‘the only real impact it’s had is my leisure activities, so I haven’t really felt that I’m comfortable going to play football just in case I fall again’ (P1201, male, under 30, 6 weeks); ‘my leisure time is somewhat disrupted, I’m a church bell-ringer, which involves my hands, and I can’t really do that so much now’ (P1856, male, under 30, 6 weeks); ‘I do quite a bit of cycling. I haven’t been on the bike since just because of grabbing the handlebar might be quite difficult, so I haven’t been able to do that’ (P1360, male, under 30, 6 weeks).
The impact on work was more important for others, especially when pay is affected by an absence from work: ‘[W]ell I’d just gone self-employed, so I’m not going to be earning now. I’m out of pocket in that respect’ (P1451, male, over 30, 6 weeks) and:
The worse thing was knowing that I had to have time off work . . . [I’m] classed as self-employed so . . . it was just the thought of not being able to work really . . . knowing that there’s kind of a month’s pay that’s going to be missing.
P1327, male, under 30, 6 weeks
Driving was a common area of discussion, with interviewees often concerned by advice not to drive and about what this might mean for their independence: ‘3 months of not driving for me would be 3 months of hell really . . . it would have affected my work so much, not being able to drive for 3 months’ (S2097, male, under 30, 52 weeks) and:
I live right in the middle of nowhere really, so in terms of getting the bus to work that’d be impossible . . . I was relying on my girlfriend for lifts and friends picking me up . . . But in terms of getting to places, yeah I was just really reliant on people giving me lifts, which was awful.
S1691, male, under 30, 6 weeks
Psychological and social impact of the injury
The final quotation in Practical difficulties associated with the injury highlights the fact that practical difficulties often translated into reliance on others and potential changes to relationships. Some (younger individuals, often students) described becoming more reliant on (or even moving back in with) parents or other family members. Others described being unable to contribute at home and relying on wives, husbands or partners for practical support. In response to the interviewer asking ‘[H]ow did your wife cope?’, one individual responded as follows:
With great difficulty because I was someone who was very active and I turned into someone who . . . wasn’t able just to take the mower out of the shed and mow the lawn or take my daughter to the park on the swings . . . So I couldn’t drive so it meant me not being able to take my son to sports . . . Everything was kind of left to her.
P1020, male, over 30, 52 weeks
Few suggested that this was problematic, although some indicated feeling low about the injury: ‘I need to find other things to do with my time . . . I feel really bad, I feel depressed . . .’ (S2284, male, under 30, 52 weeks); ‘I started to get very depressed and it affected me badly. I wasn’t coming out of my room’ (P1023, male, under 30, 6 weeks*); ‘Saturday was the day I felt a bit low . . . I was just a bit, like, I hate relying on everyone . . . I felt “oh my God this is crap” ’ (S1244, female, over 30, 6 weeks).
Less remarkable complaints about frustration and boredom were common: ‘So I just had to sit there and watch TV and in the end it was getting boring to the point where I was just going out for a walk randomly, just to try and get out‘ (S1008, male, under 30, 6 weeks); ‘I’ve definitely been frustrated with it yeah. Because it’s just so well so silly falling off my bike. And then having like everything, everything changes you know just because of that really’ (S1691, male, under 30, 6 weeks).
Model 2: plaster cast treatment
Thematic model: in comparison with surgery, initial treatment in a plaster cast is recognised as less risky and possibly more ‘natural’. It is, however, perceived to be more limiting and more limiting for a longer period of time. The fact that repair beneath the cast is unseen is a concern for some.
A more natural option
For some interviewees, plaster cast treatment was perceived as more natural and less risky than surgery. This assessment was of course informed by concerns about surgery – about anaesthesia, scarring, surgical error, etc. – but also seemed to reflect an attitude that there is benefit in allowing the body to heal naturally (P1986, female, over 30*; P1023, male, under 30*; P1360, male, under 30; among others).
Plaster cast was considered a convenient and straightforward option by some:
[When allocated to plaster cast treatment] I thought ‘oh flipping heck’. But now, in hindsight, I’m so pleased I had the plaster because you’ve no scar or anything. You risk infection don’t you if you have an operation? I’ve no scar or anything, it’s perfectly healed, so no, I’m quite happy, I would have the plaster again I think.
P1617, female, over 30, 52 weeks
It is pertinent to note that these assessments reflect participants whose recovery was positive and straightforward. Not all those who were interviewed experienced such outcomes and their assessment acknowledged that plaster cast treatment might be more or less attractive, given the nature and severity of the injury and different lifestyles and commitments:
I prefer non-intervention if possible . . . my upfront view was never do surgical intervention if you don’t need to . . . [cast is better] assuming that the bits are all in the right position . . . but I’m starting to wonder if I’ve done more damage [than I thought].
P1542, female, over 30, 6 weeks
And who’s to say if I was entering a different situation in a different time, I’d be like, oh I really do hope that I do get the surgery because I cannot be in a cast for more than 2 weeks, you know.
P1030, male, over 30, 6 weeks
An inconvenience
More common in the data were comments about the inconvenience of wearing a plaster cast:
The worst thing . . . restricted movement. That’s it you know. That’s all it is. That’s even worse than [the original injury and pain] . . . just being restricted from doing what you’d normally do . . .. I think just that, just being restricted you know . . . That to me, that’s the main one, just the restriction of the cast.
P1161, male, over 30, 6 weeks
When I had the cast on, being a bit limited for stuff you know. Which was a bit awkward, doing buttons up on shirts and stuff you know because it did used to hurt at the start. But because I used to do running and couldn’t really run with the cast on, that was a bit of a pain.
P1161, male, over 30, 52 weeks
An inability to work, difficulties at work and difficulties driving were all associated with the restrictive nature of the cast and, while these complaints varied from person to person, the consequences for some were significant and long term (for a few, these were still evident at 52 weeks). Feelings of being disabled and about losing independence were associated with this:
I’ve become more reliant on other people . . . In a sense I’ve learnt how to sort of let people do more things . . . I try and do what I usually do and I get annoyed ‘cause I can’t do it, I sort of just sit back and faff about and then try and sort of relinquish that duty to someone else . . . err, so I’ve become more submissive I suppose in that sense, and sort of wait for someone to help out.
P1485, male, under 30, 6 weeks
Other complaints focused on more mundane, personal, everyday activities. Some experienced difficulties going to the toilet: ‘I couldn’t flush the toilet normal, I couldn’t wipe my backside’ (P1020, male, over 30, 6 weeks). Difficulties using cutlery were mentioned: ‘[eating] would be a struggle, I can’t use a knife and fork’ (P1245, female, under 30, 6 weeks). Difficulties sleeping were reported: ‘I’m quite fidgety when I sleep, then I sleep on my side quite a bit so the arm gets in the way quite a lot’ (P1575, male, under 30, 6 weeks). In addition, issues with clothing were also mentioned: ‘it’s ruined so many clothes, the plaster rubbing up against your dress or blouse’ (P1316, female, over 30, 52 weeks).
Difficulties washing could be added to this list, with regard to both keeping the cast dry and the dexterity required to wash with one hand and/or with a non-dominant hand: ‘I’ve got one nice-smelling armpit, one less so’ (P1451, male, over 30, 6 weeks). The itching associated with a plaster cast was distracting to some, and individuals described using knives, knitting needles, pens and forks, among other things, to scratch beneath the cast. The state of the cast and the potential for it to become ‘manky’ (P1121, male, under 30, 6 weeks) was described by some who were concerned about hygiene: ‘it’s not the nicest smelling thing in the world – as much as you try wash your hands, it really does smell like cheese’ (P1485, male, under 30, 6 weeks); ‘I don’t like the fact that there’s one thing on my arm for 6 weeks you know. I’m a bit of a clean freak and it’s quite annoying’ (P1823, female, under 30, 6 weeks). Others mentioned how the cast might appear to others: ‘I don’t wear short sleeve when I’ve got the cast . . . like it’s not exactly the most pleasant looking’ (P1575, male, under 30, 6 weeks).
Uncertain recovery
Around half of those treated in a plaster cast interviewed at 52 weeks expressed some ongoing concerns about their recovery. P1020 (male, over 30, 52 weeks) described his recovery as being at 70%. Others described feeling some weakness in their wrist (P1560, female, over 30, 52 weeks; P1245, female, under 30, 52 weeks), wearing a wrist support (P1542, female, over 30, 52 weeks) and hoping for some further improvement: ‘I’m hoping that it isn’t going to be like this forever, I’m hoping that it’s going to be one of those things that’ll sort itself out one day’ (P1121, male, under 30, 52 weeks). Of those describing a more positive recovery, there was some disappointment at how they had been incapacitated: ‘because it hadn’t fully healed after the 6 weeks, I had to have it on for another 6 weeks and 12 weeks is kind of pushing it really, for how long I was to wear this thing for’ (P1807, male, under 30, 52 weeks) and:
I was probably a bit surprised with how long it took to heal . . . [when the cast came off] it wasn’t completely healed . . .. I do remember them saying it hadn’t completely healed. I don’t know, I think it was probably around 70% or something.
P1161, male, over 30, 52 weeks
A common observation was that cast treatment requires a long period for healing to take place: ‘a bit of disappointment that I was going to have to be stuck in the cast for so much longer . . . it’s going to be on for a longer time than it would have been for surgery’ (P1856, male, under 30, 6 weeks). A less common, but perhaps equally pertinent, observation was that the period in a cast is an uncertain period, a time during which improvement and outcome are unclear. These two elements combined (duration and uncertainty) might make cast treatment less attractive to some: ‘being in a cast for maybe 4 to 6 weeks and then maybe another decision being made . . . I think, you know, kind of 6 to 12 weeks of uncertainty’ (P1360, male, under 30, 6 weeks) and:
[It would be good] if you could see the healing process before the 6 weeks [when the cast is removed], because I think 6 weeks is a hell of a long time for somebody to kind of stop doing 50% of the things that they’d normally do, to then still not be any better off. It’s kind of a kick in the teeth. I mean if you was in the cast for 3 weeks and your healing [could be checked] or just to know something halfway through probably would have been a little bit better.
P1020, male, over 30, 52 weeks
Model 3: surgical treatment
Thematic model: in comparison with cast treatment, initial surgery is perceived as a more active form of treatment. The benefits of a more quickly removed cast are important to those whose personal, economic or familial circumstances exaggerate the impact of limited functionality.
A preference for surgery
The opportunity for surgical treatment was an important factor affecting the willingness of some patients to participate in the trial. These individuals felt that surgery was the right option for them. For some, this was an intuitive preference based on previous fractures; for others, it was an assessment reached through discussion with their consultant and the nurses recruiting to the trial. Speed of recovery, strength of repair and certainty of treatment were all factors in this; employment circumstances, family commitments, leisure pursuits and even holidays were also identified as being pertinent to surgery being more attractive:
My mind was already sort of going towards the surgery side anyway . . . me and a friend of mine we run an events catering business and June and July are the busiest months of the year . . . if I’d have been stuck in a cast, you know, I was able to come to work but my skill level dropped off when it comes to using knives.
S1345, male, over 30, 6 weeks
I said, to be honest, I’d just like to have the option of the operation if it will be quicker . . . . Because we were buying this house and we needed the money to be coming in . . . . I’ve got no such thing as light duties. It’s either all in or all out and I’ve had to put a few jobs off.
S1008, male, under 30, 6 weeks
A need for speed: a quicker recovery
At the most basic level, the inconvenience of a cast was a push factor for favouring surgery:
But for someone like me who is very independent and I have to work and drive and, you know, do all of these things, the thought of being in a cast for 8 to 10 weeks, maybe longer . . . that was quite scary.
S1749, female, over 30, 6 weeks
For others, a slow, delayed recovery might lead them to do more than they should, potentially aggravating or reinjuring their fracture:
[In a cast] I would have wanted to become independent again and I would have done other stuff, you know, I wouldn’t have been able to just sit around with a cast on, I would have been doing other stuff. So I would have probably have put myself at risk by wanting to become independent again.
S1658, female, over 30, 6 weeks
They said it could be 6 weeks but after those 6 weeks you could still need surgery . . . To be out of work for so long I’d then run the risk of, you know, trying to get back quicker and then ending up [hurting myself again] and being twice as long. So you know, it seemed like a smarter option . . . I did feel as though it [surgery] was probably the best treatment for the injury.
S1759, male, under 30, 52 weeks
The risk of the fracture not healing while in a cast was also present in other individuals’ assessments:
Yeah that’s what they said, if we just put the cast on it, it could end up, like, having surgery later on, so I thought I might as well have the surgery done now . . . I thought, oh well, it’s best to have the surgery done now and then avoid [more problems later].
S2097, male, under 30, 52 weeks
After 6 to 10 weeks [in a cast] you still might need an operation anyway because it might not have healed . . . I was thinking, oh no, I don’t want that, well I don’t want to go through doing that and then having the operation anyway, so that’s the other reason, I thought, well, you’re just better off going for the operation anyway.
S1244, female, over 30, 6 weeks
The fact that surgery following the failure of cast treatment was perceived to be a more complex procedure reinforced the appeal of immediate surgical treatment: better to have straightforward surgery immediately than to risk a more challenging procedure later.
A need for speed: a more active form of treatment
In coming to the decision that surgery offered a quicker and more certain recovery, many perceived that the more invasive and interventional nature of surgery offered benefits to the healing process. By this way of thinking, surgery was doing something beneficial, rather than simply waiting for healing to take place. Surgery was seen as offering a more certain treatment pathway, was considered to involve a more active repair of the wrist and was considered beneficial in kick-starting the healing process: ‘I suppose you feel [it is a] more involved healing process . . . The fact that you’re going to hospital and getting it treated rather than just waiting for it to heal in a cast’ (S1339, male, under 30, 6 weeks); ‘Yeah. I think there’s just a lot more certainty in the [surgery] treatment . . . . there’s just some sense that the surgery would just feel like something’s happening. It’s happening now, I’m going to be getting better’ (P1360, male, under 30, 6 weeks).
The insertion of a pin was intuitively appealing to some, adding strength to the fractured bone. This assessment was particularly important to those who were concerned about manual or physically demanding activities: ‘If I was to knock it again, it wasn’t just going to break because the screw would hold it in place . . . The operation would be better in my circumstances [self-employed joiner] because it will be a stronger fix’ (S1008, male, under 30, 6 weeks); ‘because it’s got a screw in it, for me, mechanically minded [motocross cyclist], it’s going to be stronger, so, I don’t know, I think that’s why I liked it a lot more’ (S1265, male, under 30, 6 weeks).
Assessment of surgical procedures
Those exposed to surgery expressed few concerns about the appropriateness of the procedure. The fact that the surgeons expressed confidence in the procedure was important, but the surgery being commonly offered within the NHS was particularly important. All were generally content with their initial recovery and those interviewed at 52 weeks (n = 9) indicated that their recovery had exceeded expectations (in both speed and completeness). Those interviewed at 52 weeks also indicated that they would recommend surgery to others:
The fact that it is pretty much back to exactly the way it was, obviously there’s just a pin in there, or a screw, whatever it is, you know, I mean there’s no loss of movement, there’s no stiffness in the movement, so I am quite surprised in the fact that I’ve had an injury like that and there’s no . . . you know, to be honest you kind of almost wouldn’t really know it had happened in a way.
S1345, male, over 30, 52 weeks
There were some minor concerns about scarring and some comments about pain following surgery, but none of these was considered a barrier to the appeal of surgery. The only barrier identified by some was a reluctance to undergo surgery under a local (rather than a general) anaesthetic:
[As regards local anaesthetic] I suppose it’s more, a bit modern and a bit . . . it just didn’t appeal to me really . . . I know that you can’t feel it or whatever and you probably couldn’t see what’s going on, I was just like ‘oh no’ and she said instantly in my face she could see how I weren’t up for that!
S1244, female, over 30, 52 weeks
They said to me at first they was going to like knock me out, but then when they got there they said they wasn’t, they was going to do it on the local anaesthetic. And that’s when I started to get a bit worried. I didn’t really want to hear it. I asked them straight away, well, what about, would I be able to see it? And they said no, they’d put a screen up. And then eventually I asked for some sedative and I had it and went straight to sleep.
S1452, male, under 30, 52 weeks
Model 4: research
Thematic model: surgical treatment made participation more appealing to some, although for others surgery (and randomisation) was a reason for not taking part in the wider study. The study burden was considered acceptable and some felt that taking part in a clinical trial might improve their clinical care.
The appeal of research
Individuals in both arms of the trial expressed similar opinions about agreeing to participate because of the possibility of being randomised to the surgical treatment arm:
I was approached to join the study and, yeah, I was fully willing to join and I thought it would probably be the best . . . I wanted surgery from the start, I know it was randomised, but I told them I wanted surgery.
S1452, male, under 30, 52 weeks
I think she explained to me that with the operation it could heal quicker I think . . . I said to her ‘Look, OK, fair enough. Whatever’s quicker’, you know, but I mean obviously it came that I’ve got to have the cast on.
P1161, male, under 30, 6 weeks
In contrast, some of those who declined the main trial expressed uncertainty about the surgical procedure. At least one trial decliner indicated feeling ‘scared of surgery’ (P1774, male, under 30, 6 weeks*); another suggested that surgery felt quite serious for a relatively minor injury:
I just felt [surgery is] a more drastic approach for something which is fairly common . . . . I was a little bit shocked, to be honest, when they offered me surgery . . . I think of surgery as something more, for more serious injuries, I think I could probably heal without and, like I say, all the side effects put me off.
P1023, male, under 30, 6 weeks*
In addition, a number of interviewees speculated that participating in a major clinical study might bring with it other benefits, with regard to either improved clinical care or simply less waiting at appointments:
You get seen quite quickly when you’re doing it . . . Actually I think you get treated a bit better when you’re doing it!
P1245, female, under 30, 52 weeks
And the other thing is, because it’s research, when it comes to the treatment, everything has to be within a certain period for that information to be relevant . . . So I thought everything will be pushed through quicker than just being on a waiting list as well.
S1008, male, under 30, 6 weeks
More commonly, participants indicated altruistic reasons (benefiting other patients, contributing to the NHS) for taking part in the trial.
Research processes
A few of those who declined to participate pointed to study burden as a factor in their decision, although for those involved in the study this was rarely commented on. More likely, interviewees would confirm their commitment to fulfilling their involvement as a point of principle: ‘you know, it’s just a mindset really and I said I would do it, so I was quite happy to go ahead with it . . . I wouldn’t have stopped’ (P1617, female, over 30, 52 weeks). Some gripes about the questionnaires (long, repetitive) and about follow-up appointments (parking, timing, etc.) were mentioned, but these were rarely described as serious problems.
Continuity of contact and support from the research nurses was commended by some and described as a key factor in their fulfilment of the study requirements:
I think that my experience of all the follow-ups and stuff was absolutely spot on. I had the same nurse that I saw the first day . . . she kept in contact with me, she told me everything I need to know, always there if I needed to speak to someone . . . I think that I was very well looked after and always kept informed and very helpful at all times.
S1452, male, over 30, 52 weeks
We’ve actually been treated really nicely and it was – obviously the research nurse that I went to in the hospital, or who I’d spoken to, she was lovely. She rings me about my appointments and stuff and . . . things like that. And I know what’s going on.
P1245, female, under 30, 6 weeks
Most interviewees indicated that they had a reasonable comprehension of randomisation, although in some cases this included notions that individual circumstances would be considered (S1008, male, under 30, 6 weeks) and that the process might be modified according to the doctor’s opinion (S1175, male, over 30, 6 weeks). The fact that both treatments were established and safe was important in accepting randomisation and, while many had an initial preference for one treatment or the other, all demonstrated a willingness to adhere to the randomised treatment. Among those who declined to take part in the main trial, an uncertainty about randomisation was the most clearly expressed reason for not wanting to take part. These individuals were unhappy with their clinical care being compromised by research procedures:
I just didn’t like the fact it was not the doctor giving me the best advice that he would normally give you . . . You know, they’d say this is what I recommend. I didn’t at all like the fact [that] . . . something else would then decide which way to do it, and I didn’t like that at all and it wasn’t what I was expecting . . . I didn’t think it’s really appropriate . . .. I’d rather have the doctor telling me what he thought I should do.
P1464, female, under 30, 6 weeks*
I personally don’t like the big question mark as to whether you’re [getting surgery or plaster cast] . . . Not with health issues, you know, I don’t like the lottery effect . . . I like to know what is happening rather than a random number out of a hat.
P1986, female, over 30, 6 weeks*
Some of those who declined to participate in the main trial indicated that being provided with more information about the benefits of surgery might have changed their opinion about taking part:
If someone then had taken time at that point and sit me down and, say, talk me through the benefits of what having a screw would give . . . I would have been extremely open to go on the trial.
P1263, male, over 30, 6 weeks*
Discussion
Summary of key results
A number of points can be made about a scaphoid waist fracture:
-
The initial assessment is often that this is a minor injury.
-
Functional limitations associated with the fracture may have impacts across domestic, employment, familial and social activities.
-
Individuals may become more reliant on others because of these limitations.
-
Individual circumstances may exaggerate or mitigate the impact of a fracture – personal, familial or other contextual circumstances (including the severity of the fracture, dominant/non-dominant hand) may influence how limitations are experienced.
A number of points can be made about how treatment options are viewed:
-
Cast treatment is recognised as conservative and less risky. It is, however, associated with an extended period of immobilisation.
-
Uncertainty about healing may exaggerate concerns about the duration of immobilisation.
-
Surgery is associated with a more active repair and is perceived as offering a speedier recovery.
-
Again, individual circumstances may inform treatment preference – if personal, familial or other contextual circumstances require a quicker recovery, surgery may be perceived as advantageous.
A number of points can be made about the delivery of this trial:
-
The potential for surgery was appealing to many of those interviewed. Conversely, among those who declined to take part in SWIFFT, concerns were expressed about surgery.
-
Trial procedures were not considered burdensome and there was recognition that trial processes may enhance navigation and experience of clinical processes.
Reflections on the results
This work contributes to the limited literature on patient experience of hand, wrist or arm injury. It reinforces and extends a number of themes in this literature – specifically, the wide-ranging functional impact of an injury,112–119 the relevance of an individual’s subjective ‘sense of recovery’117–119 and the potential for personal or contextual factors to shape these. 118 It also adds insight into how treatments are perceived and into the personal and contextual characteristics that may shape this.
Fracture of the scaphoid waist
Data generated here reinforce the fact that a wrist fracture can have far-reaching consequences117–119 and that these might exceed any initial assessment that this is a trivial injury. 118 Impacts on functional limitations112–119 were commonly reported and interviewees described (to a greater or lesser degree) limitations in all aspects of their life. Impacts in employment were commonly reported and concerns for the financial distress118 were evident when interviewees described being self-employed. Changes to domestic and employment responsibilities suggest social role changes previously described. 112
Moving in with parents, relying on others for lifts, help tying shoe laces and help cutting food all illustrate a need for practical support that has been reported previously. 117–119 They also suggest a challenge to independence that Fitzpatrick and Finlay116 identify. Complaints about boredom, frustration and depression mirror those concerns for the psychological consequences of a hand or wrist injury. 114,116,117,119
The fact that individual reports of functional limitation varied significantly – from those continuing as normal to those feeling significantly disabled – reminds us that this is a heterogeneous population. This reinforces the importance of establishing what patients deem to be normal,115,121 reflecting on pre-injury wrist function119 and considering those contextual factors that exaggerate or mediate disability. 118 In this, the data generated here take us beyond a simple comparison of men and women and manual and non-manual occupations and support the relevance of specific circumstances,118 such as occupation, familial responsibilities, access to familial (or other social) support and leisure/lifestyle pursuits (among many others).
Assessment of treatment options
The fact that surgery is often uncritically assessed as a quicker, stronger and more active repair overlooks important clinical facts, namely that casts are 90% successful, that surgery can be marked by complications (and poses a risk of non-union), that bone repair takes the same amount of time irrespective of treatment, etc. However, despite this, the appeal of surgery is clear; a number of things might help us to understand this.
First, the appeal of surgery reiterates and reinforces Watson’s118 recognition that an individual’s ‘sense of recovery’ might be distinct from the repair of the bone and actual (objective) recovery. The fact that non-union following conservative treatment (plaster cast) is possible (irrespective of how likely this is) informs a subjective judgement that the relative risks/complications of surgery are consequently less important.
In addition, the fact that, in Watson’s118 work, the removal of the plaster cast represents the ‘start of recovery’ also suggests a confusion between the removal of a cast with a more abstract sense of personal recovery. This interpretation suggests that participants perceive an initial remedial phase (either in surgery or in a cast) as preceding a phase of more active recovery, in which normal function is regained. The fact that this remedial phase takes longer in a plaster cast means that returning to normal115,120 is consequently perceived as taking longer to achieve. The fact that this overlooks the clinical reality would seem less important if we accept that understanding treatment is, in part at least, a subjective assessment.
As with functional limitations, it is pertinent to reflect that individual assessment of treatment may also be influenced by contextual factors that demand a speedier return to normality. This might help us to understand why the student who was able to return to their parental home (P1023) found surgery to be less appealing than the self-employed caterer (S1345). Again, we should reflect that our data have moved us beyond those simple dichotomies (male/female, younger/older) to recognise that the experience of, and preference for, a particular treatment will be shaped by a complex set of personal circumstances.
Concerns about clarity in treatment118 were important to interviewees. A dislike about not knowing and about uncertainty118,119 may also help us to understand why some interviewees found plaster cast treatment challenging. Surgery may offer no more certain outcomes, but the fact that this outcome might be known more quickly better satisfies our impatience and (for some at least) lessens the likelihood that impetuous behaviour may lead to further injury.
One final set of circumstances should be highlighted. Owing to participant dropout, our data are skewed towards interviews undertaken at 6 weeks. At this point, the worst aspects of cast treatment and the best attributes of surgery might be seen – on one side, participants might be bored by ongoing immobility or frustrated by a dirty plaster cast; on the other, they might be surprised by their mobility and pleased at how quickly normality has been regained. While we might expect these trends to even out over the longer term, it is unfortunate that, among those interviewed at 52 weeks, 5 of the 10 treated in a plaster cast demonstrated some form of ongoing concern.
Irrespective of this, it is evident that assessment of treatment (and consequent preference) is shaped by an individual’s own sense of recovery and that this is, in turn, informed by their personal circumstances. The fact that cast removal and a return to more normal functioning are perceived as significant thresholds perhaps helps us to understand the appeal of surgery to those interviewed here.
Recruitment and retention in surgical clinical trials
The challenge of recruiting to a surgical trial47 is demonstrated here in an almost contradictory fashion – surgery both attracted and discouraged participation. Among those interviewed, the appeal of surgery was often commented on as a motivating factor; however, for those who declined to participate in the trial, surgery was considered a reason not to take part. This, of course, points to the challenge of patient equipoise46 and might suggest that the SWIFFT population favours those eager for surgery. Other elements of the SWIFFT data will address this issue more directly.
This also places pressure on clinical equipoise46 and reinforces the importance of how a trial is introduced to a potential participant. The fact that some of those who declined to take part in SWIFFT subsequently (when given more information) suggested that they might have changed their mind demonstrates the complexity of getting this right.
The challenge of retaining young male participants134,135 is demonstrated here, with the reduction in this population exceeding that for other demographics. Among younger females, older males and older females, approximately 50% declined or were unavailable for interview at 52 weeks; for younger males, this proportion increased to 70%. We have commented on the challenge of this elsewhere,132 but it is worth reiterating that comments about a positive experience, continuity of contact and accessibility of research nurses suggest the softer-type strategies and the ‘valuing of a study’ that we have previously described. 132
Closing comments and recommendations
The insight generated here points to the importance of a patient’s subjective experience of scaphoid fracture and treatment and highlights the pertinence of their own sense of recovery in this. This has a number of implications for clinical practice:
-
It suggests that clinical consultations might include a more detailed consideration of a patient’s circumstances. We have argued here that a patient’s experience is shaped not simply by age, gender and occupation, but by a range of specific personal, familial and economic circumstances that influence how immobilisation is experienced.
-
It suggests that tailored information about a patient’s fracture and recovery might be beneficial. Here we have argued that a participant’s sense of recovery and treatment preference were often based on an incomplete (and possibly inaccurate) understanding of their injury.
-
Finally, it suggests that earlier removal of a cast, supported by the use of alternative forms of immobilisation, might facilitate a stronger sense of recovery for patients. We have argued that functional limitation was a common complaint and that cast removal was perceived to be an important threshold in this.
This study has also offered insight into the delivery of the clinical trial, specifically highlighting issues of patient and clinician equipoise. For future trials in which medical and surgical treatments are being compared, the following recommendations could be taken into account:
-
Those who recruit participants should have knowledge of all of the treatments being investigated. This may mean that research nurses are drawn from pertinent specialties and/or that participants have access to clinicians who have experience of treatments. This will lessen the potential for stereotypical comparisons (conservative/progressive, simple/complex, quicker/slower) and support a more informed commentary on treatment options.
-
All information provided should demonstrate equipoise, both in fact and in impression. It is evident here that some participants understood surgery to be a quicker solution and that this impression shaped how they experienced their recovery.
-
When there are issues with retention (such as with a young, male population), there is value in building in retention strategies from the outset. In this study, the introduction of an iPad lottery132 might have had a greater effect had it been established sooner.
The insight generated here points to a number of uncertainties, which might benefit from further investigation:
-
Further qualitative research with young, male patients could respond to the high dropout rate in this group seen in this trial. Exploring if those in this group have their own distinctive sense of recovery or display certain commonalities in treatment experience and expectation would provide insight for shaping clinical consultations. It may also help to reduce reinjury associated with impulsive or impatient behaviour that some predict.
-
Exploring the reasons for late clinical presentation of a scaphoid fracture would support awareness raising of this injury and would complement prior work that has demonstrated gendered patterns in health care-seeking behaviour. 147 Such research might challenge those initial assessments that this is an inconsequential injury and might also offer insight into whether or not late presentation is significant in expectations for recovery and treatment experience.
-
Research that considers the provision of information about the treatment of a scaphoid fracture might similarly inform clinical consultations and inform a patient’s sense of recovery. Research in this area might could follow two routes: first, the development of the content of the information and mechanisms for delivery and, second, testing whether or not such information improves the experience of, and satisfaction with, treatment.
Chapter 6 Discussion and conclusion
Outcomes
This report is based on the assessment of primary and secondary outcomes at 52 weeks (the primary time point) after 439 participants with a clear bicortical scaphoid waist fracture were randomised to have the control treatment, namely immobilisation in a plaster cast and early identification of non-union followed by urgent surgical fixation (n = 220), or to have early surgical fixation using CE-marked headless screws (n = 219).
Primary outcome
Patient-Rated Wrist Evaluation at 52 weeks
The main finding of this pragmatic trial is that there is no statistically significant difference in pain and function assessed by the PRWE at 52 weeks between participants randomised to receive initial cast treatment and those randomised to immediate fixation of clear and bicortical fractures of the scaphoid with 2 mm or less displacement. The adjusted mean difference in the total PRWE score was –2.1 in favour of those in the surgical fixation group (95% CI –5.8 to 1.6; p = 0.27). This difference is unlikely to be considered important by patients, as the CIs of the adjusted mean difference exclude the clinically relevant difference of 6 points in PRWE scores.
In the early period at 6 and 12 weeks, the difference, which favours surgery, is statistically significant and, although the mean difference is below the clinically relevant difference of 6 points, the lower CI is below –6 points in some analyses (indicating the possibility that the true treatment effect might be an increase of 6 points on the PRWE in the surgery group). Therefore, these differences are statistically significant but of borderline clinical importance. Importantly, 47% and 6% of participants in the surgery group, respectively, were still in plaster cast or a splint at 6 and 12 weeks post randomisation. This compares with 85% and 21% of patients still in plaster cast in the non-surgical group at these time points. Therefore, participants in the plaster cast group were more likely to still be in a cast and have consequential functional limitations. This is corroborated when looking at the pain and function components of the PRWE: there is no difference between groups in pain but there is a difference in function at these early time points. After 12 weeks, there is no statistically significant difference between the groups.
Sensitivity analyses of the Patient-Rated Wrist Evaluation
The sensitivity analyses using multiple imputation, checking for an influence of the recruiting site, investigating those scores that were obtained within the time-point windows, adjusting for smoking status and displacement of the fracture, and the CACE analysis all broadly support the primary analysis. Subgroup analyses of baseline treatment preference, and displacement, also support the results of the primary analysis.
Secondary outcomes of bone union, grip strength and SF-12 support the primary analysis findings.
Immobilisation of the broken scaphoid in different types of casts has been compared with immediate fixation using a headless screw in at least eight reported trials18,23–28,75 and one cohort study. 148 These studies (Table 44) used different methods of assessing function and disability [three used the Disabilities of the Arm, Shoulder and Hand questionnaire (DASH), one used the Patient Evaluation Measure (PEM) and another used PRWE; the other four studies used a variety of assessments including their own questionnaire, a satisfaction questionnaire and the modified Green and O’Brien score] and at different time points (4–144 months). The studies reported are small, with different time points, and some reviews29 and meta-analyses have also included case series. 24,44,149,150 The data from these studies have been extracted systematically and meta-analysed on numerous occasions. 29–34,36,37
| Author | Year | Participants, n | Follow-up, months | Patient-reported outcome measure used | Non-union, n | Conclusion summarised from text | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Surgery | Plaster cast | Surgery | Plaster cast | |||||
| Saedén27 | 2001 | 62 | 32 | 30 | 144 | Own questionnaire, no difference | 1 | 0 | Surgery allows early return of function and is an alternative to conservative treatment |
| Bond25 | 2001 | 25 | 11 | 14 | 25 | Satisfaction questionnaire, no difference | 0 | 0 | Surgery for undisplaced fractures resulted in faster union and return to military duty than plaster cast |
| Adolfsson23 | 2001 | 53 | 25 | 28 | 4 | None | 1 | 0 | Surgery resulted in better movement at 16 weeks but similar grip strength. Early surgery allows early mobilisation without adverse effects on fracture healing |
| Dias3 | 2005 | 88 | 44 | 44 | 12 | PEM, no difference; at 8 weeks surgery better | 0 | 2 | Plaster cast treatment, carefully assessing fracture healing with plain radiographs and CT scans after 6–8 weeks and recommending surgery at that time if non-union is confirmed, achieved fracture union in over 95% |
| Dias75 | 2008 | 88 | 44 | 44 | 93 | PEM, no difference; at 8 weeks surgery better | 0 | 2 | No medium-term difference in function or radiological outcome between surgery and plaster cast |
| Arora24 | 2007 | 47 | 23 | 24 | 6 | DASH, surgery better | 1 | 0 | Surgery for non-displaced scaphoid fractures had faster union and quicker return to work. Although surgery is more expensive, the total cost was not higher |
| Vinnars28 | 2008 | 83 | 43 | 42 | 120 | PRWE, no difference | 0 | 0 | No long-term benefit of surgery, compared with plaster cast, for acute non-displaced or minimally displaced fractures. The long-term risks of surgery should be considered |
| McQueen18 | 2008 | 60 | 30 | 30 | 12 | Green and O’Brien score, no difference; surgery better before 1 year | 1 | 4 | Surgery was associated with faster return of function, sport and work than plaster cast. Surgery had a low complication rate. All active patients should be offered surgery |
| Clementson26 | 2015 | 45 | 21 | 24 | 72 | DASH, no difference; at 6 and 10 weeks surgery better | 0 | 0 | Non-displaced and minimally displaced scaphoid waist fractures are best treated in a plaster cast. Surgery may provide improved function in the short term, but there is an increased risk of arthritis in the long term |
One study reported PRWE score at 10 years in 75 of 83 patients randomised to scaphoid cast treatment or surgical fixation with a Herbert headless screw and the mean score was 6 in each group. 28 No data were available for the 52-week time point. Two studies reported a patient-reported outcome measure score at 52 weeks. One3 used the PEM score, which ranges from 0 to 100, with 100 being worse, and reported an unadjusted mean difference of –1.3 favouring surgery, although the difference was not statistically significant. The second18 reported on 60 patients randomised to either a below-elbow cast leaving the thumb free or an Acutrak headless screw; authors used the Mayo modification of the Green and O’Brien score151 to assess function (pain and function) and impairment (range of wrist movement and grip strength) and noted that this score was 13% better (higher) in those having surgery, but this difference was not statistically significant. This finding is not reflected in the present study.
As the non-union rate for displaced fractures is 14%, compared with 10% for transverse undisplaced fractures,8,10,39 randomisation was stratified by the presence or not of displacement (< 1 mm or 1–2 mm inclusive) of a scaphoid fracture as seen on radiographs10,39 and used by the treating clinician to determine eligibility. The three raters identified displacement of the fracture > 2 mm in 27 (6.2%) participants. When these patients were excluded, the difference in PRWE score between treatment groups at 52 weeks was –2.1 favouring surgery. The difference was not significant (p = 0.07) and was below the clinically meaningful threshold.
Complications
Treating clinicians at the recruiting sites noted that 31 (14.2%) participants randomised to the surgery group experienced a complication, while the rate was much lower in the plaster cast group (n = 3, 1.4%). The review of radiographs identified penetration of joints by the screw in 94 participants in the surgery group (42.9%) compared with 10 (4.6%) participants in the plaster cast group. In half of these the screw protruded 1–2 mm into the joint and in a quarter there was significant protrusion of over 2 mm. The concern about such screw protrusion is that articular cartilage will be damaged irreversibly, leading to early degenerative arthritis in the involved joint.
Cast complications, in contrast, were minor, had no lasting consequence and were reported in six (2.7%) participants allocated to the surgery fixation group, probably reflecting the frequent changes of cast to inspect the wound and the initial application of the cast by the surgeon. Cast problems (soft, tight or broken cast, skin soreness) were reported for 45 (20.5%) participants in the plaster cast group; it is likely that the cast was applied by experienced fracture clinic staff and not changed as frequently.
Malunion of the scaphoid was assessed using the height to length ratio on radiographs and on the baseline and 52-week CT scans. ten Berg et al. 85 noted a ratio of 0.69 as the upper 95% CI of a normal population, so we used this to define malunion, rather than the ratio of 0.6 we had proposed in our protocol. At 52 weeks, using the 0.7 threshold (i.e. the ratio of the scaphoid height to length), malunion increased between baseline and 52 weeks on CT scans and the rate of malunion was similar (3.2%) in both treatment groups. The rate of malunion based on radiographs was higher and, again, tended to increase over time and to be higher in the surgery group.
Reoperations
Eight participants in the surgery group had 11 reoperations subsequent to their initial fixation; in six of these participants, the reoperations were for implant-related problems and, for the other two participants, the reoperations were for non-union, with one requiring scaphoid excision and a four-corner fusion. One participant required reoperation for persistent non-union following fixation of non-union after initial cast fixation. The overall rate of reoperation for all causes (assuming that none of those who were not followed up required reoperation) was 8 of 219 (3.7%) after early surgery and 1 of 220 (0.5%) after initial cast treatment and surgery to fix non-union.
Secondary outcomes
Union
The aim of immobilising a broken scaphoid is to get it to unite. Failure of union, if left untreated, mostly results in wrist arthritis,12,99,105 as there is abnormal loading between the distal scaphoid and the radius. 152 Therefore, an important outcome after treatment is to establish the state of fracture union on imaging. In clinical practice, union is commonly assessed on radiographs of the scaphoid in different projections. Bony continuity on all radiographic views is interpreted as trabeculae crossing the fracture line, and sometimes there may be sclerosis at the fracture line. Both of these features suggest union, especially if there are no adverse features, namely (1) a gap at the fracture line on any view, (2) progressive displacement of the fracture or (3) if implants are used, lucency around the implant, which may suggest failure of union. 59 The usual advice is that radiological union is considered to have occurred only when ‘bridging trabeculae’ are seen across the whole cross-section of the scaphoid on radiographs or a CT scan,153 with the latter being more reliable.
The bone may unite partially, with a gap seen across part of the fracture site and bony continuity identified across the remainder of the fracture. This can be identified on the scaphoid radiographic views but can also be quantified on a CT scan. 61 Partial union has been reported in around 42% of scaphoid fractures and usually consolidates in time, but the wrist may need protection for a while. 61
Non-union is the absence of radiographic signs of healing with a clear gap on radiographs on any view and confirmed on a CT scan. 153–155 A high-quality CT scan (fine cut, bone window) helps establish that the fracture has not united. An un-united scaphoid fracture will eventually lead to collapse of the carpus and degenerative OA termed as SNAC. 99
Clinically, there would be a concern if union was < 20% at 52 weeks and this would suggest the need for treatment, namely a bone graft to stabilise the fracture, although this partial union threshold has not been formally investigated. On radiographs, this extent of union is likely to be interpreted as a ‘non-union’ by the treating clinician.
The rate of non-union recorded in this study is very low for both groups. In the 188 participants who had screw fixation in the surgery group, there was one case of non-union. In the 198 participants in the plaster cast group who fully complied with the control pathway (treated in a cast and surgery to fix a non-union), there was also just one case of non-union. This study has not identified evidence that the rate of non-union and slight union is statistically significantly different between surgical fixation and cast immobilisation in an ITT logistic regression model that adjusted for age, fracture displacement and hand dominance (OR 0.40, 95% CI 0.12 to 1.33; p = 0.13). We observed this state in four participants (three with slight union and one with non-union) in the surgery group and nine participants (five with slight union and four with non-union) treated in a plaster cast. Three meta-analyses30,36,37 reported on union rates; two36,37 found no difference and one30 reported a significant difference in favour of surgery. These meta-analyses reviewed four to seven small trials comparing surgical fixation with plaster cast treatment for scaphoid fractures.
The control treatment pathway in SWIFFT was initial cast treatment with early identification of failure of union and early fixation of un-united fractures. Of the 18 participants who had surgery for early identified non-union, 15 had the surgery early (i.e. within 6 months from randomisation) and a further three were treated after 6 months. The reasons for this have been described in Chapter 3.
The union rate after early fixation of less than 20% after initial cast immobilisation is high. Four papers3,148,149,156 report prospectively collected data and present results of early identification of non-union after cast treatment and early fixation of these. One is a RCT3 and the other three are prospective comparative case series. These papers reported on a total of 166 scaphoid fractures treated in a below-elbow cast. Nineteen non-unions were identified when the cast was discontinued. Seventeen of these had early surgery and two declined the offer of surgery and had a persisting non-union at 52 weeks. Of the 17 that were fixed, all united (100%). Two retrospective studies157,158 also reported on 210 acute scaphoid fractures treated in a cast with 12 non-unions, 11 of which were fixed early and all of which united (100%). One 80-year-old man was not offered surgery. There is only one retrospective case series159 that reported on 308 acute fractures, of which 27 did not unite. Of these, 24 had fixation and 21 united; of the three that did not join (87.5% union), two were reoperated and one healed. Overall, the reported rate of union after early identification of failure to unite and fixation is very high. Our study confirms that it is unlikely that the risk of non-union following surgery for non-union after initial cast immobilisation is greater than if surgery is carried out in the first 14 days after the injury.
In SWIFFT, 18 patients in the plaster cast group were also offered and had surgery, as the clinicians treating them were concerned about the state of union after cast treatment. Only one had a persistent non-union at 52 weeks. In these patients, the total PRWE score was worse [mean 29.1 (SD 32.4) points] than in those who did not need surgery [mean 12.8 (SD 17.4) points]. This may reflect the delay in fixation of the non-union.
The numbers of scaphoid fractures we would need to fix to avoid one additional non-union or slight union is 44. All of these patients would be exposed to the risks of surgery.
Range of wrist movement
One year after the fracture, there is no difference between groups for range of wrist movement. This confirms that the treatment method does not have a measurable effect on the range of wrist movement at 52 weeks. At 6 weeks, when participants treated in a plaster cast are likely to still be immobilised, the range is worse than those left out of a cast after surgery and screw fixation. Wrist movement returns to normal and most studies that compared cast with surgery were unable to establish a difference between groups at time points later than 52 weeks.
One study36 found that there was no difference between groups after 6 months and others34,160 found no difference between groups at any time point. Another meta-analysis30 highlighted the observation that several trials24,27,28 had noted a better range of wrist movement after treatment in a plaster cast.
Grip strength
The strength of the hand, assessed using a Jamar® dynamometer (The Jamar Company, Inc., Duluth, MN, USA), was similar between groups. Grip strength, measured in kilograms, in those treated in a cast was slightly worse at 6 weeks (by around 4 kg) and 12 weeks after injury (by around 2.6 kg) but better at 52 weeks (cast 37.4 kg (SD 14.2) than in those in the surgery group [36.2 kg (SD 12.7 kg)]. The cast is usually removed between 6 and 12 weeks, so this was an expected difference at these time points.
Five meta-analyses30,31,34,36,160 have commented on the recovery of grip strength, reporting on various combinations of several small trials. 3,18,23–25,27,28 One of these36 found that patients who underwent surgery had better grip strength than those initially given a cast up to a year after surgery. The same study36 also reported that the difference between groups was greatest at 8 weeks, combining the results of two studies. 3,18 The authors of another study30 felt that they were unable to conduct a meta-analysis because of the different ways of recording grip strength. This study collated the results from seven studies3,18,23–25,27,28 in a table. The authors noted a consistent trend in that those having early surgery had better strength, but recommended caution in interpretation. Another meta-analysis found no difference between groups. 160
Return to work and unpaid activities
This study, in contrast with most previous trials, found little difference in days of lost employment. On average, the time that those treated in a cast were off work was 21.7 days, while those who had their broken scaphoid fixed were off work for 17.3 days. Of note was that the time off work was the same in the first 6 weeks and was only 12 days.
Five meta-analyses29,31,34,36,37 reviewed RCTs and reported on the time to return to work. One study29 included a prospective comparative study,149 which was not randomised. All five, extracting data from different combinations of studies, noted that patients who had their scaphoid fracture fixed returned to work quicker (range 1.6–7 weeks earlier) than those treated in a cast.
This study finds little difference in time off work, especially in the early period, and this may reflect the common practice of immobilising the operated fracture in a plaster cast or splint. One reason may be that around 77.7% were treated initially in a cast that did not include the thumb and therefore permitted early resumption of hand function. Another may be that patients may have felt more secure working in a cast. Finally, patients may have had direct reassurance regarding return to work in a cast and were encouraged to do so.
The overall days of lost unpaid activity was also similar in the two groups. There was a larger impact on lost unpaid activity in the surgery group for the 6 weeks after randomisation. For all time points after this, patients allocated to the surgical arm reported a smaller impact on unpaid activities than those in the plaster cast group, which is consistent with the findings for days of lost employment.
Short Form questionnaire 12-items
The SF-12 was completed to measure the potential broader consequences of a scaphoid fracture on the participants’ physical and mental health, along with EQ-5D-3L. The analyses of the MCS scores reassuringly showed that participants’ mental health improved in both treatment groups at each time point and overall. The between-group differences in the MCS were not statistically different at any time point, with an adjusted mean difference at 52 weeks of –1.2 points (95% CI –3.3 to 0.8 points) in favour of the plaster cast group. A statistically significant difference in the PCS score favouring the surgery group was seen at 12 and 52 weeks, but not at 6 or 26 weeks or overall (p = 0.08). The adjusted mean difference at 52 weeks was 1.6 points (95% CI 0.2 to 3.1 points) in favour of the surgery group. These findings corroborate the overall PRWE results and the PRWE function subscale, but appear inconsistent across time points and the differences are below that considered clinically meaningful (range 3.3–12.6 points) in other musculoskeletal (spine and knee) disorders. 161–164
Trial validity and minimising bias
Various measures were taken to ensure trial validity and minimise bias or to explore the potential for bias, some of which are discussed here.
The secure randomisation method helped to ensure that there was comparability in the characteristics of the two treatment groups, with the exception of ethnicity, education and smoking status, by chance. The imbalance in the number of smokers, with participants in the plaster cast group less likely to be smokers, was examined in a post hoc sensitivity analysis. Adjusting for smoking status (yes/no) found similar results to the primary analysis, except at 12 weeks, when a clinically relevant 6-point improvement in the PRWE score in favour of the surgery group could not be ruled out. In addition, at baseline, it was important that the series of five radiographic views were performed to ensure the correct diagnosis of the fracture for inclusion of a patient in the trial. Except for one view (45° semisupine), nearly all randomised patients were enrolled based on the series of radiographs taken at baseline. The three raters agreed that 6% of randomised participants had displacement of the fracture at baseline that was > 2 mm, which was a reason to exclude a patient. The raters agreed that only one participant did not have a fracture (in the surgery group). Sensitivity analyses of the primary outcome model that excluded these participants supported the findings of the primary analysis. A further sensitivity analysis also provided reassurance that the perceived threat to validity of low recruitment at sites did not affect the results on the PRWE. In addition, the subgroup analyses found participant treatment preferences at baseline; if there was a preference, it was in favour of surgery, but this did not affect the results of the PRWE at 52 weeks. However, participants with a preference for surgery or in treatment equipoise were dominant (n = 403, 91.8%), and those preferring cast treatment (n = 32, 7.3%) were under-represented. This helps, in part, to mitigate concerns that a lack of blinding can introduce bias in participant self-reported completion of the primary outcome.
Although it was not possible to blind assessing clinicians to the treatment allocations, this multicentre study with its pragmatic design had multiple clinicians assessing objective outcomes. The statisticians and health economists were presented with data only after collection and cleaning was completed and the statistician undertaking the analyses was different from the one monitoring the study data collection processes. The DMC and the TSC also provided independent advice and support throughout the conduct of this study.
To help ensure a good standard of care, surgeons were advised to use techniques with which they were familiar, which also helped to avoid learning-curve problems. The majority of operations were conducted by consultant surgeons, who were also present for the majority of operations when the main operating surgeon was a specialist trainee. Predominantly, a percutaneous approach with a palmar incision was performed, which is consistent with current practice. No intraoperative complications were reported. All participants were provided with standardised, written physiotherapy advice detailing the exercises they needed to perform. At 12 weeks, the majority of participants in both treatment groups self-reported that the written advice was quite or very useful and that they had performed home exercises.
The high rate of return of participant questionnaires at the primary end point of the trial reflects the success of the extensive measures taken to achieve the required retention rates. Critically, this included continuous engagement and advice from three involved groups (patients, research nurses and clinicians), as explained elsewhere. 132 The participant questionnaire return rates were lower in the plaster cast group than in the surgery group, except at 6 weeks, when they were similar between the two groups. The baseline characteristics of participants who completed a valid primary outcome at 52 weeks were comparable between the two groups, except for the continued difference in ethnicity, education and smoking status. Any response bias that could be introduced from these imbalances in the return rates and characteristics of responders was minimised by using a mixed-effect, repeated-measures model that included intermittent responders. Consequently, only 7% of participants were not included in the primary analysis model, with an almost identical number of participants included for each treatment group. The use of this statistical model had the benefit of increasing the statistical power of the analyses, compared with the use of a two-sample test for the sample size calculation. Multiple imputation analyses to explore the effect of these minimal missing data helped to ensure that this did not have an effect on the results of the PRWE. There were, however, missing data on imaging for nearly 30% of participants at 52 weeks. There were quite marked differences in the PRWE score in those participants who did or did not attend imaging between groups. Participants in the surgery group who attended imaging tended to have better scores than those who attended in the plaster group. This may have contributed to lower reporting of non-union in the surgery group. Furthermore, the lower than expected non-union rate reported for both groups could be attributed to the participation of largely specialist hand units in this study and a more rigorous assessment of non-union than in previous studies. 3,18,23–28 The other finding on imaging was that 43% of participants in the surgery group had penetration of the screw into the adjacent joint; this was 1 mm or greater on the 52-week CT scan in 68 participants. It was considered likely that this would have an adverse effect on the joint cartilage and this therefore probably explains the worse PRWE score in this participant group. This emphasises the need for imaging during surgery. In our study, imaging was reviewed by three independent raters, two senior radiologists and one surgeon not involved in the clinical care processes, with robust methods of identification and resolution of conflicts. The senior clinicians reviewing imaging could identify participants whose fracture was stabilised with a screw, but did not know the allocation after randomisation. Having three reviewers independently reviewing the imaging and performing measurements also helped to mitigate any bias in measurements.
Finally, a potential threat to study validity is non-compliance. This is when the treatment was not delivered as planned, which could have potentially diluted the treatment effect observed in the ITT primary analysis. Thirty-one patients (14%) in the surgery group did not have surgery and six patients (3%) in the plaster cast group immediately switched to surgery following randomisation. There were various reasons why participants did not get their allocated surgery. These included a fracture not being seen on the baseline CT scan, the surgeon deciding that the participant had a different type of fracture on further review of imaging/further imaging at baseline, and the participant changing their mind. ITT analysis was used throughout, which includes all participants in the group to which they were randomly assigned. This preserves the original random allocation and reflects the pragmatic nature of administering treatment in clinical practice. CACE analysis was also conducted to explore the potential dilution of the treatment effect of the six participants (3%) in the plaster cast group who crossed over to surgery and the 31 participants (14%) in the surgery group who were managed conservatively. The CACE analysis reproduced a difference in favour of surgery among participants who complied with their treatment that was larger than the ITT treatment effect on the total PRWE score at 52 weeks. Although it remained a non-statistically significant result and was not a clinically important treatment effect, the upper confidence limit now included the clinically important effect. In terms of further non-compliance in the control pathway, of the 17 participants in the plaster cast group who had surgery for early identified non-union, 14 had the surgery within 6 months from randomisation and three were treated after 6 months. Three of the four participants in the plaster cast group who had non-union or slight union at 52 weeks were also not offered surgery. Therefore, 6 of 21 participants in the plaster cast group did not have the expected immediate surgical fixation when there was non-union.
Applicability of results
Characteristics of the trial population
The review of the baseline characteristics of the trial participants and the fact that the three raters agreed, based on the baseline imaging, that only one participant did not actually have a fracture at enrolment helped to confirm the inclusion of appropriate participants in SWIFFT.
The application of the eligibility criteria meant that a quarter of the population of patients considered for the trial were excluded for genuine clinical reasons, predominantly because the inclusion of these patients meant that the surgeon would not be in equipoise about how to treat a patient. A further third of the population screened did not consent to take part. Therefore, just over 40% of the patients screened for participation were randomised. A comparison of the four key baseline characteristics revealed that eligible patients tended to be a few years younger than those excluded and were marginally more likely to be male and to have a displaced fracture. The characteristics of consenting and non-consenting patients were comparable, except that consenting patients were more likely to have a displaced fracture. As previously discussed, the statistical method used to analyse the primary outcome meant that only 7% of consenting participants were not included in the primary analysis. Multiple imputation analysis to explore the effect of these minimal missing data confirmed that this did not bias the treatment effect in the PRWE.
Applicability of the trial findings
The pragmatic design of SWIFFT helped to ensure that there was immediate application to the NHS. The criteria used to enrol participants in the trial were minimised as much as possible. There were also no stringent criteria regarding which surgeons could operate on participants. Those surgeons who did operate, or were present during the operation, were mostly consultants, as would be expected. The provision of standardised, written physiotherapy advice detailing the exercises that participants needed to perform may not be entirely reflective of NHS practice. It did, however, help to ensure that a good standard of practice was applied consistently across both groups, as was confirmed when participants self-reported this at 12 weeks. The follow-up clinics that were organised at 6 and 12 weeks were also consistent with routine clinical practice. The follow-up clinic at 52 weeks, which was the primary end point, was to ensure to the extent feasible that participants in both treatment groups had the time to complete the treatment pathway being delivered. The findings are also applicable to participants with both undisplaced and minimally displaced fractures, with the subgroup analysis showing that participants with a displaced fracture did not statistically significantly benefit more from surgery than those with an undisplaced fracture.
Most of the previous small trials addressing this research question involved a single centre. In contrast, SWIFFT recruited participants from 30 NHS hospitals in England and one in Wales, which reflected a variety of geographical locations, improving the generalisability of the results. There were also 95 surgeons who operated on participants. While the large number of hospitals and surgeons involved improves generalisability, there could be concerns about how the limited number of participants operated on by a single surgeon could influence patient outcome. Including the adjustment of hospital site in the primary model produced similar findings. Importantly, unlike previous RCTs, a thorough and detailed economic evaluation was undertaken to assess the relative cost-effectiveness of the two treatment options within the trial follow-up period and the lifetime implications of treatment decisions made. The primary analysis is that of the NHS and will therefore have direct applicability to informing future policy and commissioning decisions.
Cost-effectiveness of early surgery compared with initial cast treatment
Incremental cost-effectiveness ratio
The within-study analysis, an assessment of the costs associated with treatment and QoL of patients in the first year post randomisation, found that, up to 52 weeks, early fixation was not cost-effective, with an ICER of £67,473 to £135,085 per QALY. This range of values was based on the approach taken to the trial population, missing data and adjustment for baseline QoL. The most reasonable scenario is considered to be £81,962 per QALY, a scenario of an ITT population with multiple imputation for missing values and adjustment for baseline QoL.
Long-term model
Initial use of cast with immediate fixation of confirmed non-union was associated with a 61% probability of being cost-effective. The extrapolated model is driven by the relatively and absolutely small numbers of non-unions, alongside the uncertain long-term estimates.
This result was influenced by the relatively low cost of initial cast immobilisation compared with surgery and the finding that, if cast immobilisation was unsuccessful, surgical fixation was offered, accepted by almost all and then highly likely to result in uniting.
The long-term Markov model was based on data from small retrospective case series and was driven mainly by the very small difference in union rate at 52 weeks between the two groups, highlighting the considerable uncertainty surrounding the model. The uncertainty in this model will be addressed after the medium-term 5-year review, as this will help improve our assumptions and hence the confidence in such a model.
Health economic summary
The key finding is that, if initial cast immobilisation is unsuccessful but surgical intervention is offered soon after confirming non-union, this is highly likely to be the most cost-effective treatment, as it avoids the high upfront cost of fixing all broken scaphoids but still avoids a long-term non-union and the risks of both high surgical rate and poor QoL for patients who develop SNAC.
Health economics: strengths and limitations
The economic evaluation of the treatment of these types of scaphoid fractures, described in Chapter 4, was associated with a number of strengths and weaknesses.
It was the first evaluation of this area not only to provide an analysis of the cost and QoL implications of the treatments available using evidence from a RCT, but also to combine these with a long-term model, using evidence from the literature to consider the lifetime implications of treatment decisions made. The ‘within-trial’ model provided a methodologically rigorous exploration of the cost and QoL of patients in both arms of SWIFFT, including extensive missing data estimation alongside a series of regression analyses seeking to explore the role of confounding factors. The mathematical model presented extends the findings of SWIFFT by not only considering the lifetime implications of the two treatments, but also incorporating treatment scenarios of cast immobilisation only and no treatment, not considered in the trial. This allows for a complete evaluation of all possible treatment options available and the long-term implications of each. This structure, alongside the PSA and extensive scenario analyses, allowed a detailed exploration and presentation of the significant role of uncertainty in this decision problem, owing to both the small incremental differences between the outcomes of the two arms of the trial and the limited long-term evidence available in the literature.
However, as with any such evaluation, there are also weaknesses of the analysis, including those as a consequence of the available evidence, and these are detailed in Chapter 4. The primary weaknesses relate to the potential oversimplification of the mathematical model and the limited connection between the trial data and the long-term model. The insensitivity of the decision tree to the explicit role of the time lag between the observation of potential non-union after cast immobilisation and the occurrence of surgical fixation is also a weakness and, while it would not be expected to change the base-case result, it limits our ability to explore the impact of changes to such a parameter. For example, some time threshold is expected to exist at which, unless surgery occurs before it, the cast plus surgery arm becomes less cost-effective than initial surgery for all patients. Similarly, the simplistic approach to OA, AEs related to non-OA, and SNAC in the long-term model, while necessitated by the available evidence, risks incorrectly specifying the risks and benefits of each treatment arm. Finally, the use of the rate of non-union as the link between the short- and long-term models and the use of literature-based estimates of adverse event profiles, rather than estimates from the trial, make the result of the analysis highly sensitive to the rate of non-union estimates from the trial. This results in the model being highly sensitive to factors such as the assumption made about the three patients who had non-union at 52 weeks but were yet to have surgery, and the definition of slight union as indicative of non-union or not. However, given the available evidence and the level of clinical understanding, the models should represent the most robust base-case scenario.
The Markov model was limited by the assumptions made and the data available. The data were from very small case series that were historic. We have addressed the uncertainty using many scenarios but feel that the 5-year study of SWIFFT participants will help resolve much of the uncertainty in the Markov model.
Nested qualitative study: patients’ treatment preferences and their experience of treatment
The nested qualitative study explored the impact of a scaphoid fracture and patients’ experience of treatment and treatment preference. The study offers detailed, contextualised insight, with each interviewee offering a distinct and personalised narrative. These data complement the clinical and other data generated elsewhere in SWIFFT and provide an additional perspective to support the implementation of trial findings. The qualitative data generated here provide a valuable, subjective viewpoint that will aid clinicians in understanding their patients’ experiences and in shaping their discussions about clinical options.
Sense of recovery
The insight generated here highlights that a patient’s understanding of fracture and their sense of recovery is important in assessing treatment success. It also shows that an individual’s sense of recovery is shaped by specific personal, familial and economic circumstances; employment, social responsibilities or even hobbies and leisure pursuits will shape how they feel recovery is progressing. A broader consideration of a patient’s personal circumstances might shape more productive clinical consultations and might enhance the management of recovery.
Certainty
The act of plaster cast removal is an important threshold in a patient’s return to normal. Patients did not appreciate the uncertainty of the duration of immobilisation or the possible need for further treatment, even though the rate of this was very low.
Preference
A broadly positive assessment of surgery among those interviewed reflects a more general trend among those recruited to the study: of the 780 eligible patients, only 12% of those preferring cast treatments consented to take part, compared with 77% of those preferring surgery and 87% of those with no preference. Consequently, it is difficult to assess treatment preference based on the comments and insights offered here.
Disability
One interesting observation was the feeling of some wrist weakness, described by several of the women interviewed. This was experienced to an extent that some chose to use an external wrist support to help accommodate this. Some also expressed concern that this impairment could be permanent.
Return to normality while healing
Two elements of cast treatment concerned participants: the duration of immobilisation and the possibility that some participants could need further treatment (no matter how small the rate of further treatment was).
Many suggestions were made: one participant suggested that clinicians could check the state of healing halfway through immobilisation to identify failure earlier, although assessment of union is unreliable at earlier time points, especially on radiographs. 62 There was also a sense that patients regarded surgery as ‘doing something’ rather than ‘just waiting’ and regarded it as more active, with many readily assuming that the risks and failures would not happen to them. A small number of participants understood and verbalised that, if the fracture was displaced, fixing it would reduce the displacement and make it heal in a better and reduced position.
The main reflection of this chapter is that, considering that this trial confirms that the mode of initial treatment does not have a significant impact on fracture union, clinicians should focus more on the impact of treatment choices on the patient’s day-to-day life and their work. There is a clear need to focus on becoming ‘normal’ again. If external immobilisation is considered, then restoring independence and minimising the impacts on activities of daily living by using removable splints at an earlier time point in the pathway may help patients ‘recover’. This may need a careful and planned discussion at the outset when the injury is diagnosed. Another key point is the discussion about uncertainty in the recovery after both pathways, as in the control cast and fixing of non-union pathway one reoperation was required up to 52 weeks, while, among those having early surgery, 11 reoperations were required in eight participants and the surgery pathway had one significant non-salvageable consequence and a high rate of screw penetration.
Qualitative interviews: strengths and limitations
The qualitative substudy offers detailed, contextualised insight into the experiences of a fracture of the scaphoid waist, with each interviewee offering a distinct and personalised narrative about their injury and experience of treatment. These data complement the clinical and other data generated elsewhere in SWIFFT and provide an additional perspective to support the implementation of trial findings. The qualitative data generated here provide a valuable, subjective viewpoint that will aid clinicians in understanding their patients’ experiences and in shaping their discussions about clinical options.
The fact that our sample broadly achieved its purposive aim – approximately half of all interviews were with males under the age of 30 years – adds pertinence and credibility to the findings. The fact that the sample is larger than other comparable qualitative investigations of patient experience of hand, wrist or arm injury also adds credibility to the findings reached. The findings add to the current literature, with a more explicit focus on how surgical and plaster cast treatments are perceived and experienced and on those personal contextual factors (domestic, social and economic) that might shape an individual’s experience of fracture and treatment.
It is, of course, important to reflect that this substudy explores participants’ subjective experience of fracture and their subjective understanding of treatment and recovery. It reflects how they think things are or should be; this does not always, or automatically, translate into how things actually are.
It should also be noted that interviewees are to some extent self-selected – restricted mainly to those who agreed to take part in the trial – and that, among this group, participants could opt out of the interview substudy. Generalising is difficult across the different demographics included here (which includes both students and the retired) and differences in experience (both positive and negative) also make interpretation and generalisation difficult. It is perhaps, however, the taciturn and monosyllabic responses of some young men that make interpretation most difficult.
A key limitation that must be acknowledged is the fact that the attrition between the 6- and 52-week interviews exceeded that which had been predicted (50% rather than 30%) and that this limits the potential for direct comparison and for building narrative accounts of fracture and recovery. Most often, participants were not contactable – not responding to approaches (on at least three occasions) via telephone, SMS messaging or e-mail – although some respondents explicitly indicated that they did not wish to take part in a second interview. The fact that attrition was most pronounced in the young male participants is pertinent and this led to new male interviewees (n = 4) being added at the 52-week interview point. It should also be noted that older females are over-represented in the final interview sample – fewer younger females consented to the interview substudy and consequently additional older females were recruited to achieve the target for female interviewees (n = 11). The fact that these female participants were least likely to drop out means that older females are the second largest group interviewed at 52 weeks (n = 5/19).
Recommendations for future research
Evidence from eight published meta-analyses29–32,34–37 to evaluate surgical fixation compared with conservative treatments for acute undisplaced or minimally displaced scaphoid fractures is based on 416 patients. SWIFFT is therefore the largest study in the world and has doubled the existing evidence base and should be used to update the meta-analyses to further address the uncertainty and to confirm if no further trials are necessary.
The 5-year follow-up of SWIFFT participants will be important to investigate the outcome of the partial union of scaphoid fractures and whether or not these unite over time, as well as to explore the consequences of the progress of degenerative arthritis, malunion and screw problems (malposition and penetration within joints) on participants’ QoL. This long-term follow-up will further inform the areas of uncertainty in the extrapolated model.
Findings from the qualitative study suggest that, in future trials comparing medical and surgical treatments, patients should have access to a clinician who can adequately explain the treatments to both ensure balance in the presentation of information and lessen the potential for stereotypical comparisons of the treatments that could affect recruitment. Extensive retention strategies should also be included from the outset for a difficult patient population such as predominantly young males. Finally, research that considers the nature, form and delivery of information about a scaphoid fracture and its treatment might similarly inform future clinical consultations and inform a patient’s sense of recovery. This could include providing the patient with a leaflet to encourage surgical fixation when non-union occurs, explaining that surgery will help reduce the rate of future arthritis. Furthermore, detailed qualitative, longitudinal research with younger male patients with a scaphoid fracture would also be useful in exploring their sense of recovery, which may help to reduce reinjury associated with impulsive or impatient behaviour.
Conclusion
This prospective, pragmatic RCT could not identify, at the primary end point of 52 weeks, a statistically significant or clinically meaningful difference between the offer of cast treatment with early urgent fixation of non-unions and having all scaphoid fractures fixed surgically at the outset. The most cost-effective pathway is definitive cast treatment, with early fixation of only those fractures that do not unite with a cast. Patient treatment preferences following their injury reflect their desire to have a ‘sense of recovering’ and surgeons should address this at the outset.
SWIFFT provides evidence to suggest that the treatment of all undisplaced and minimally displaced scaphoid waist fractures should be in a cast, with investigations for non-union taking place at 6 to 12 weeks and all confirmed non-unions fixed immediately.
Acknowledgements
General acknowledgements
Foremost, we thank the patients participating in this trial, without whom this trial would have not been possible. In addition, a general thanks to all those people whose commitment and efforts in the design and conduct of SWIFFT allowed us to successfully complete this study and this final report.
A specific thanks to the following people who made a valuable contribution to the study but who are not named as authors: Mrs Jane Stewart (Research Fellow) and Mrs Iskra Potgieter (Research Assistant), who were employed to undertake a number of the qualitative interviews; Mr Christopher Bunce, who was responsible for the management of the imaging in the first 3 years of the trial; Mrs Elaine James, who provided comprehensive administrative support to the chief investigator and trial team; Mrs Nicola Woofenden, who provided secretarial and administrative support to the chief investigator and trial team; Dr Sarwat Shah (a trial co-ordinator), who contributed to the conduct of the trial, particularly data collection and data management; and the teams in York and Leicester, which contributed to the design of data collection forms, information systems and the management of data. A special thanks to Mr Ashley Langton, who was our patient representative on the TMG and who, throughout the study, contributed to many of the discussions on optimising participant retention and commented on various trial-related materials. We also thank the patient representative group, which we met with in Leicester and consulted with electronically about the progress of the study.
This project was funded by the NIHR Health Technology Assessment programme (project number 11/36/37).
Collaborators
Principal investigators
The following people were the PIs within this trial: Mr Rouin Amirfeyz (University Hospitals Bristol NHS Foundation Trust), Mr Bhaskar Bhowal (University Hospitals of Leicester NHS Trust), Mr Neil Blewitt (North Bristol NHS Trust), Mr Mark Brewster (University Hospitals Birmingham NHS Foundation Trust), Mr Dan Brown (The Royal Liverpool and Broadgreen University Hospitals NHS Trust), Mr Chris Coapes (South Tees Hospitals NHS Foundation Trust), Professor Tim Davies (Nottingham University Hospitals NHS Trust), Mr Livio Di Mascio (Barts Health NHS Trust), Mr Rupert Eckersley (Chelsea and Westminster Hospital NHS Foundation Trust), Ms Sue Fullilove (University Hospitals Plymouth NHS Trust), Mr Grey Giddins (Royal United Hospitals Bath NHS Trust), Mr Rodney Hammett (Taunton and Somerset NHS Foundation Trust), Ms Helen Hedley (née Whalley) (University Hospitals Coventry and Warwickshire NHS Trust), Mr Jonathon Hobby (Hampshire Hospitals NHS Foundation Trust), Mr Stephen Hodgson (Bolton NHS Foundation Trust), Mr Phillip Johnston (Cambridge University Hospitals NHS Foundation Trust), Mr Jonathan Jones (North West Anglia NHS Foundation Trust), Mr Andy Logan (Cardiff and Vale University Health Board), Mr William Loh (Southport and Ormskirk Hospital NHS Trust), Mr Andrew Mahon (University Hospitals Coventry and Warwickshire NHS Trust), Mr Andrew Mahon (Worcestershire Acute Hospitals NHS Trust), Mr Will Mason (Gloucestershire Hospitals NHS Foundation Trust), Mr Andrew McAndrew (Royal Berkshire NHS Foundation Trust), Mr Ian McNab (Oxford University Hospitals NHS Trust), Mr Lindsay Muir (Salford Royal NHS Foundation Trust), Mr James Nicholl (Maidstone and Tunbridge Wells NHS Trust), Mrs Anna Noon (Worcestershire Acute Hospitals NHS Trust), Mr Rob Poulter (Royal Cornwall Hospitals NHS Trust), Mr Roland Pratt (Northumbria Healthcare NHS Foundation Trust), Mr Zulfi Rahimtoola (Royal Berkshire NHS Foundation Trust), Mr Daniel Redfern (Lancashire Teaching Hospitals NHS Foundation Trust), Mr Simon Richards (Poole Hospital NHS Foundation Trust), Mr Bijayendra Singh (Medway NHS Foundation Trust), Mr Paul Stuart (Newcastle upon Tyne Hospitals NHS Foundation Trust), Mr Adel Tavakkolizadeh (King’s College Hospital NHS Foundation Trust), Mrs Lisa Tourret (Brighton and Sussex University Hospitals NHS Trust) and Professor David Warwick (University Hospital Southampton NHS Foundation Trust).
Research Associates/nurses at collaborating sites
The following people were the Research Associates and nurses that contributed to this trial: Benita Adams, Emma Alderman, Caroline Andrews, Manjit Attwal, Kirsten Baddick, Emily Bannister, Michelle Bardgett, Steven Barnfield, Alison Barratt, Keira Beacham, Laura Behar, Jackie Berry, Georgina Bird, Colin Borgin, Charlotte Boustead, Sarah Brannigan, Sarah Brown, Charlotte Bryant, Deborah Butcher, Shirley Caldwell, Ronald Carrera, Rebecca Casey, Shirley Cawley, Julie Chadwick, Carol Chillmaid, Aisling Clarkson, Louise Clarkson, Rachel Clarkson, Jacqueline Claydon, Cheryl Cleary, Kate Coates, Shirley Cocks, Carolyn Colvin, Samantha Connell, Caroline Cowley, Heather Cripps, Andrew Cuff, Jean Cummings, Anwen Davies, Nicki Devooght-Johnson, Maria Dibua, Jackie Dingle, Christine Dobb, James Doolan, Alison Dude, Angela Dunne, Ruby Einosas, Eunice Emeakaraoha, Susan Farmer, Abigail Ford, Anna Fouracres, Julie Foxton, Sherron Furtado, Michelle George, Kirsty Gladas, Abdulkerim Gokturk, Christina Haines, Ruth Halliday, Simone Hargreaves, Christine Herriot, Joanne Hill, Lucille Hooper, Robert Hull, Irena Hutchins, Juliet James, Patricia Jenkins, Katie Keating-Fedders, Alison Kelly, Keely Lane, Laura Latter, Maria Letts, Sophie Lewis, Candice Matthews, Kerri McGowen, Maria Mestee, Janet Mills, Alanna Milne, Natalie Mitchell, Maines Msiska, Doreen Muller, Norma Murray, Lisa Murthen, Lynne Nairn, Jessica Nightingale, Tracy Nolan, Claire Nott, Angie O’Sullivan, Susan O’Sullivan, Vicky Phelps, Mandy Porritt, Sangeetha Prasath, James Reed, Emma Reeves, Lesley Rice, Cheryl Richie, Carrie Ridley, Nicola Ridley, Gemma Ritchie, Jane Rowett Harris, Tinashe Samakomva, Helen Sankey, Yan Sims, Hansa Singh, Karen Smith, Nicola Smith, Tracy Smith, Debbie Speigal, Louise Spoors, Rosalyn Squire, Kellie Thom, Joanne Thunder, Lisa Trembath, Sylvia Turner, Mark Verlander, Yelnia Vigo, Sharon Wade, Alycon Walker, Lisa Watson, Gillian Welch, Alison Whitcher, Caroline White, Robyn Weston, Arlo Whitehouse, Laura Youds, Andrea Young, Gabbie Young and Yuhan Zhang.
Trial Steering Committee members
The following people made up the TSG: Professor Joseph Dias (chief investigator), Professor Wendy Baird, (chair), Professor Peter Burge (independent member), Dr Jonathan Cook (independent member), Mr Richard Palmer (independent member), Mr Nick Welch (independent member) and Mrs Carolyn Maloney and Dr David Hetmanski (Sponsor representatives; observing).
Data Monitoring Committee members
The following people made up the DMC: Professor Graeme MacLennan (chair), Mr Timothy Hems (independent member) and Mr Adam Watts (independent member).
Contributions of authors
Professor Joseph Dias (https://orcid.org/0000-0001-5360-4543) (Professor of Orthopaedic and Hand Surgery, chief investigator) was the lead applicant of the trial and contributed widely to the design and conduct of the trial from its inception, including ongoing development and grant application, and had responsibility for the overall management of the trial throughout its duration, co-ordinating and reviewing data collection on all imaging and overseeing analyses, in addition to writing and overseeing reports for publication.
Dr Stephen Brealey (https://orcid.org/0000-0001-9749-7014) (Research Fellow, trial manager) was a co-applicant and contributed to the design and conduct of the trial throughout the duration of the study and contributed to and commented on all drafts of the report.
Mrs Liz Cook (https://orcid.org/0000-0001-6902-0235) (Research Fellow, trial co-ordinator) contributed to the conduct and co-ordination of the trial throughout the duration of the study and contributed to and commented on all drafts of the report.
Ms Caroline Fairhurst (https://orcid.org/0000-0003-0547-462X) (Research Fellow, Statistician) led the preparation of the statistical analysis plan, conducted the final analysis and contributed to and commented on the final report.
Mr Sebastian Hinde (https://orcid.org/0000-0002-7117-4142) (Research Fellow, Health Economist) led the write-up of the chapter on the economic evaluation, developed the associated cost-effectiveness model and commented on all drafts of the report.
Dr Paul Leighton (https://orcid.org/0000-0001-5208-0274) (Senior Research Fellow) was a co-applicant and contributed to the design and conduct of the qualitative interview substudy, participated in trial management systems and contributed to and commented on all drafts of the report.
Dr Surabhi Choudhary (https://orcid.org/0000-0002-3246-5174) (Consultant Radiologist) contributed substantially to the acquisition and analysis of the data and provided input in the writing of the report.
Professor Matthew Costa (https://orcid.org/0000-0003-3644-1388) (Professor of Orthopaedic Trauma Surgery) was a co-applicant and contributed to the design and conduct of the trial throughout the duration of the study and contributed to and commented on all drafts of the report.
Professor Catherine Hewitt (https://orcid.org/0000-0002-0415-3536) (Professor of Trials and Statistics, Deputy Director of YTU) was a co-applicant and had overall responsibility for the statistical analyses of the trial data, contributed to the design and analysis of the study and commented on all drafts of the report.
Mr Stephen Hodgson (https://orcid.org/0000-0003-3607-862X) (Consultant Orthopaedic Surgeon) was a co-applicant and contributed to the design and conduct of the trial and contributed to and commented on all drafts of the report.
Dr Laura Jefferson (https://orcid.org/0000-0003-2139-3555) (Research Fellow, trial manager, trial co-ordinator) was involved during the development of trial materials, the setting up of sites and the acquisition of data in the trial and read and contributed to the trial report.
Dr Kanagaratnam Jeyapalan (https://orcid.org/0000-0002-6006-1994) (Consultant Radiologist) contributed to the design and conduct of the trial, undertook the radiological assessment and review of all images throughout the duration of the study and provided input in the writing of the report.
Miss Ada Keding (https://orcid.org/0000-0002-1182-887X) (Research Fellow, statistician) contributed to the reporting of data throughout the study, contributed to the planning and conduct of the statistical analysis and commented on all drafts of the report.
Dr Matthew Northgraves (https://orcid.org/0000-0001-9260-8643) (Research Fellow, trial co-ordinator) contributed to the day-to-day running of the trial and the ongoing development of the study protocol and commented on all drafts of the report.
Mr Jared Palmer (https://orcid.org/0000-0001-9667-8016) (Research Assistant) was responsible for the reconstruction of the imaging data to trial specification and administration of imaging measurements for integrity, conflicts, subsequent re-reviewing and preparation of imaging reports for management meetings and provided input in the writing of the report.
Professor Amar Rangan (https://orcid.org/0000-0002-5452-8578) (Professor of Orthopaedic Surgery) contributed to the conception and design of the study, revising the work for important intellectual content and the final approval of the work to be published and agrees to be accountable for all aspects of the work.
Professor Gerry Richardson (https://orcid.org/0000-0002-2360-4566) (Professor of Health Economics) was a co-applicant and contributed to the design and conduct of the health economics sections of the trial throughout the duration of the study and contributed to and commented on all drafts of the report.
Dr Nicholas Taub (https://orcid.org/0000-0002-8610-0613) (Research Fellow) was a co-applicant and statistician and contributed to the statistical aspects of the trial design throughout the duration of the study and commented on drafts, including the final draft of the report.
Dr Garry Tew (https://orcid.org/0000-0002-8610-0613) (Associate Professor, trial co-ordinator) contributed to the conduct of the trial from October 2013 to August 2015 and contributed to and commented on all drafts of the report.
Professor John Thompson (https://orcid.org/0000-0003-4819-1611) (Professor of Genetic Epidemiology) contributed to the trial design and commented on the trial conduct, particularly on the development of the statistical analysis plan, and was actively involved in the analysis of the imaging data and the writing of that section of the report.
Professor David Torgerson (https://orcid.org/0000-0002-1667-4275) (Director of YTU) helped to design the study and commented on drafts of the report, and attended project meetings.
Publications
Dias J, Brealey S, Choudhary S, Cook L, Costa M, Fairhurst C, et al. Scaphoid Waist Internal Fixation for Fractures Trial (SWIFFT) protocol: a pragmatic multi-centre randomised controlled trial of cast treatment versus surgical fixation for the treatment of bi-cortical, minimally displaced fractures of the scaphoid waist in adults. BMC Musculoskelet Disord 2016;17:1–15.
Leighton P, Brealey S, Dias J. Interventions to improve retention in a surgical, clinical trial: a pragmatic, stakeholder-driven approach. Journal of Evidence Based Medicine 2018;11:12–19.
Dias JJ, Brealey SD, Fairhurst C, Amirfeyz R, Bhowal B, Blewitt N, et al. Surgery versus cast immobilisation for adults with a bicortical fracture of the scaphoid waist (SWIFFT): a pragmatic, multicentre, open-label, randomised superiority trial. Lancet 2020;396:390–401.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review by the chief investigator.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Leslie IJ, Dickson RA. The fractured carpal scaphoid. Natural history and factors influencing outcome. J Bone Joint Surg Br 1981;63–B:225-30. https://doi.org/10.1302/0301-620X.63B2.7217146.
- Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg 1999;33:423-6. https://doi.org/10.1080/02844319950159145.
- Dias JJ, Wildin CJ, Bhowal B, Thompson JR. Should acute scaphoid fractures be fixed? A randomized controlled trial. J Bone Joint Surg Am 2005;87:2160-8. https://doi.org/10.2106/00004623-200510000-00002.
- Garala K, Taub NA, Dias JJ. The epidemiology of fractures of the scaphoid. Bone Joint J 2016;98:654-9. https://doi.org/10.1302/0301-620X.98B5.36938.
- Garcia-Elias M. Kinetic analysis of carpal stability during grip. Hand Clin 1997;13:151-8.
- Kobayashi M, Garcia-Elias M, Nagy L, Ritt MJ, An KN, Cooney WP, et al. Axial loading induces rotation of the proximal carpal row bones around unique screw-displacement axes. J Biomech 1997;30:1165-7. https://doi.org/10.1016/S0021-9290(97)00080-8.
- Berdia S, Wolfe SW. Effects of scaphoid fractures on the biomechanics of the wrist. Hand Clin 2001;17:533-40.
- Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br 1991;73:828-32. https://doi.org/10.1302/0301-620X.73B5.1894676.
- Cooney WP, Dobyns JH, Linscheid RL. Nonunion of the scaphoid: analysis of the results from bone grafting. J Hand Surg Am 1980;5:343-54. https://doi.org/10.1016/S0363-5023(80)80173-0.
- Dias JJ, Singh HP. Displaced fracture of the waist of the scaphoid. J Bone Joint Surg Br 2011;93:1433-9. https://doi.org/10.1302/0301-620X.93B11.26934.
- Gelberman RH, Menon J. The vascularity of the scaphoid bone. J Hand Surg Am 1980;5:508-13. https://doi.org/10.1016/S0363-5023(80)80087-6.
- Mack GR, Bosse MJ, Gelberman RH, Yu E. The natural history of scaphoid non-union. J Bone Joint Surg Am 1984;66:504-9. https://doi.org/10.2106/00004623-198466040-00003.
- Düppe H, Johnell O, Lundborg G, Karlsson M, Redlund-Johnell I. Long-term results of fracture of the scaphoid. A follow-up study of more than thirty years. J Bone Joint Surg Am 1994;76:249-52. https://doi.org/10.2106/00004623-199402000-00012.
- McLaughlin HL, Parkes JC. Fracture of the carpal navicular (scaphoid) bone: gradations in therapy based upon pathology. J Trauma 1969;9:311-19. https://doi.org/10.1097/00005373-196904000-00003.
- Vender MI, Watson HK, Wiener BD, Black DM. Degenerative change in symptomatic scaphoid nonunion. J Hand Surg Am 1987;12:514-19. https://doi.org/10.1016/S0363-5023(87)80198-3.
- Grewal R, King G. Percutaneous screw fixation led to faster recovery and return to work than immobilization for fractures of the waist of the scaphoid. J Bone Joint Surg Am 2008;90. https://doi.org/10.2106/JBJS.9008.EBO3.
- Jeon IH, Micic ID, Oh CW, Park BC, Kim PT. Percutaneous screw fixation for scaphoid fracture: a comparison between the dorsal and the volar approaches. J Hand Surg Am 2009;34:228-36.e1. https://doi.org/10.1016/j.jhsa.2008.10.016.
- McQueen MM, Gelbke MK, Wakefield A, Will EM, Gaebler C. Percutaneous screw fixation versus conservative treatment for fractures of the waist of the scaphoid: a prospective randomised study. J Bone Joint Surg Br 2008;90:66-71. https://doi.org/10.1302/0301-620X.90B1.19767.
- Zlotolow DA, Knutsen E, Yao J. Optimization of volar percutaneous screw fixation for scaphoid waist fractures using traction, positioning, imaging, and an angiocatheter guide. J Hand Surg Am 2011;36:916-21. https://doi.org/10.1016/j.jhsa.2011.02.017.
- Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br 1998;80:95-9. https://doi.org/10.1302/0301-620X.80B1.0800095.
- Herbert TJ. Open volar repair of acute scaphoid fractures. Hand Clin 2001;17:589-99.
- Slade JF, Jaskwhich D. Percutaneous fixation of scaphoid fractures. Hand Clin 2001;17:553-74.
- Adolfsson L, Lindau T, Arner M. Acutrak screw fixation versus cast immobilisation for undisplaced scaphoid waist fractures. J Hand Surg Br 2001;26:192-5. https://doi.org/10.1054/jhsb.2001.0558.
- Arora R, Gschwentner M, Krappinger D, Lutz M, Blauth M, Gabl M. Fixation of nondisplaced scaphoid fractures: making treatment cost effective. Prospective controlled trial. Arch Orthop Trauma Surg 2007;127:39-46. https://doi.org/10.1007/s00402-006-0229-z.
- Bond CD, Shin AY, McBride MT, Dao KD. Percutaneous screw fixation or cast immobilization for nondisplaced scaphoid fractures. J Bone Joint Surg Am 2001;83:483-8. https://doi.org/10.2106/00004623-200104000-00001.
- Clementson M, Jørgsholm P, Besjakov J, Thomsen N, Björkman A. Conservative treatment versus arthroscopic-assisted screw fixation of scaphoid waist fractures – a randomized trial with minimum 4-year follow-up. J Hand Surg Am 2015;40:1341-8. https://doi.org/10.1016/j.jhsa.2015.03.007.
- Saedén B, Törnkvist H, Ponzer S, Höglund M. Fracture of the carpal scaphoid. A prospective, randomised 12-year follow-up comparing operative and conservative treatment. J Bone Joint Surg Br 2001;83:230-4. https://doi.org/10.1302/0301-620X.83B2.0830230.
- Vinnars B, Pietreanu M, Bodestedt A, Ekenstam Fa, Gerdin B. Nonoperative compared with operative treatment of acute scaphoid fractures. A randomized clinical trial. J Bone Joint Surg Am 2008;90:1176-85. https://doi.org/10.2106/JBJS.G.00673.
- Alnaeem H, Aldekhayel S, Kanevsky J, Neel OF. A systematic review and meta-analysis examining the differences between nonsurgical management and percutaneous fixation of minimally and nondisplaced scaphoid fractures. J Hand Surg Am 2016;41:1135-44. https://doi.org/10.1016/j.jhsa.2016.08.023.
- Alshryda S, Shah A, Odak S, Al-Shryda J, Ilango B, Murali SR. Acute fractures of the scaphoid bone: Systematic review and meta-analysis. Surgeon 2012;10:218-29. https://doi.org/10.1016/j.surge.2012.03.004.
- Buijze GA, Doornberg JN, Ham JS, Ring D, Bhandari M, Poolman RW. Surgical compared with conservative treatment for acute nondisplaced or minimally displaced scaphoid fractures: a systematic review and meta-analysis of randomized controlled trials. J Bone Joint Surg Am 2010;92:1534-44. https://doi.org/10.2106/JBJS.I.01214.
- Ibrahim T, Qureshi A, Sutton AJ, Dias JJ. Surgical versus nonsurgical treatment of acute minimally displaced and undisplaced scaphoid waist fractures: pairwise and network meta-analyses of randomized controlled trials. J Hand Surg Am 2011;36:1759-68.e1. https://doi.org/10.1016/j.jhsa.2011.08.033.
- Modi CS, Nancoo T, Powers D, Ho K, Boer R, Turner SM. Operative versus nonoperative treatment of acute undisplaced and minimally displaced scaphoid waist fractures – a systematic review. Injury 2009;40:268-73. https://doi.org/10.1016/j.injury.2008.07.030.
- Shen L, Tang J, Luo C, Xie X, An Z, Zhang C. Comparison of operative and non-operative treatment of acute undisplaced or minimally-displaced scaphoid fractures: a meta-analysis of randomized controlled trials. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0125247.
- Suh N, Benson EC, Faber KJ, Macdermid J, Grewal R. Treatment of acute scaphoid fractures: a systematic review and meta-analysis. Hand 2010;5:345-53. https://doi.org/10.1007/s11552-010-9276-6.
- Symes TH, Stothard J. A systematic review of the treatment of acute fractures of the scaphoid. J Hand Surg Eur Vol 2011;36:802-10. https://doi.org/10.1177/1753193411412151.
- Yin ZG, Zhang JB, Kan SL, Wang P. Treatment of acute scaphoid fractures: systematic review and meta-analysis. Clin Orthop Relat Res 2007;460:142-51.
- Garala K, Singh H, Dias JJ, Buijze G, Jupiter JB. Scaphoid Fractures: Evidence-Based Management. St. Louis, MO: Elsevier Health Sciences; 2017.
- Singh HP, Taub N, Dias JJ. Management of displaced fractures of the waist of the scaphoid: meta-analyses of comparative studies. Injury 2012;43:933-9. https://doi.org/10.1016/j.injury.2012.02.012.
- Johnson N, Singh H, Dias JJ, Buijze G, Jupiter JB. Scaphoid Fractures: Evidence-Based Management. St Louis, MO: Elsevier Health Sciences; 2017.
- Slade JF, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg 2008;97:280-9. https://doi.org/10.1177/145749690809700402.
- NHS Digital, Hospital Episode Statistics (HES) for England . Inpatient Statistics, 2005–09. Activity in English NHS Hospitals and English NHS Commissioned Activity in the Independent Sector 2010.
- Davis EN, Chung KC, Kotsis SV, Lau FH, Vijan S. A cost/utility analysis of open reduction and internal fixation versus cast immobilization for Acute nondisplaced mid-waist scaphoid fractures. Plast Reconstr Surg 2006;117:1223-35. https://doi.org/10.1097/01.prs.0000201461.71055.83.
- Papaloizos MY, Fusetti C, Christen T, Nagy L, Wasserfallen JB. Minimally invasive fixation versus conservative treatment of undisplaced scaphoid fractures: a cost-effectiveness study. J Hand Surg Br 2004;29:116-19. https://doi.org/10.1016/j.jhsb.2003.10.009.
- Vinnars B, Ekenstam FA, Gerdin B. Comparison of direct and indirect costs of internal fixation and cast treatment in acute scaphoid fractures: a randomized trial involving 52 patients. Acta Orthop 2007;78:672-9. https://doi.org/10.1080/17453670710014383.
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448-51. https://doi.org/10.1136/bmj.324.7351.1448.
- Cook JA, Ramsay CR, Norrie J. Recruitment to publicly funded trials – are surgical trials really different?. Contemp Clin Trials 2008;29:631-4. https://doi.org/10.1016/j.cct.2008.02.005.
- Dias J, Brealey S, Choudhary S, Cook L, Costa M, Fairhurst C, et al. Scaphoid Waist Internal Fixation for Fractures Trial (SWIFFT) protocol: a pragmatic multi-centre randomised controlled trial of cast treatment versus surgical fixation for the treatment of bi-cortical, minimally displaced fractures of the scaphoid waist in adults. BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-1107-7.
- MacDermid JC. The Patient-Rated Wrist Evaluation (PRWE)© User Manual. Hamilton, ON: McMaster University; 2007.
- MacDermid JC, Turgeon T, Richards RS, Beadle M, Roth JH. Patient rating of wrist pain and disability: a reliable and valid measurement tool. J Orthop Trauma 1998;12:577-86. https://doi.org/10.1097/00005131-199811000-00009.
- Ziter FM. A modified view of the carpal navicular. Radiology 1973;108:706-7. https://doi.org/10.1148/108.3.706.
- Herbert TJ, Fisher WE. Management of the fractured scaphoid using a new bone screw. J Bone Joint Surg Br 1984;66:114-23. https://doi.org/10.1302/0301-620X.66B1.6693468.
- MacDermid JC. Development of a scale for patient rating of wrist pain and disability. J Hand Ther 1996;9:178-83. https://doi.org/10.1016/S0894-1130(96)80076-7.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Olerud P, Tidermark J, Ponzer S, Ahrengart L, Bergström G. Responsiveness of the EQ-5D in patients with proximal humeral fractures. J Shoulder Elbow Surg 2011;20:1200-6. https://doi.org/10.1016/j.jse.2011.06.010.
- Tidermark J, Bergström G, Svensson O, Törnkvist H, Ponzer S. Responsiveness of the EuroQol (EQ 5-D) and the SF-36 in elderly patients with displaced femoral neck fractures. Qual Life Res 2003;12:1069-79. https://doi.org/10.1023/A:1026193812514.
- Dias JJ. Definition of union after acute fracture and surgery for fracture nonunion of the scaphoid. J Hand Surg Br 2001;26:321-5. https://doi.org/10.1054/jhsb.2001.0596.
- Amirfeyz R, Bebbington A, Downing ND, Oni JA, Davis TR. Displaced scaphoid waist fractures: the use of a week 4 CT scan to predict the likelihood of union with nonoperative treatment. J Hand Surg Eur Vol 2011;36:498-502. https://doi.org/10.1177/1753193411403092.
- Singh HP, Forward D, Davis TR, Dawson JS, Oni JA, Downing ND. Partial union of acute scaphoid fractures. J Hand Surg Br 2005;30:440-5. https://doi.org/10.1016/j.jhsb.2005.05.007.
- Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br 1988;70:299-301. https://doi.org/10.1302/0301-620X.70B2.3346310.
- Ty JM, Lozano-Calderon S, Ring D. Computed tomography for triage of suspected scaphoid fractures. Hand 2008;3:155-8. https://doi.org/10.1007/s11552-007-9077-8.
- Forward DP, Singh HP, Dawson S, Davis TR. The clinical outcome of scaphoid fracture malunion at 1 year. J Hand Surg Eur Vol 2009;34:40-6. https://doi.org/10.1177/1753193408093327.
- Ring D, Patterson JD, Levitz S, Wang C, Jupiter JB. Both scanning plane and observer affect measurements of scaphoid deformity. J Hand Surg Am 2005;30:696-701. https://doi.org/10.1016/j.jhsa.2005.03.001.
- Leslie I. Terminology for Hand Surgery. London: International Federation of Societies for Surgery of the Hand; 1998.
- Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am 1984;9:222-6. https://doi.org/10.1016/S0363-5023(84)80146-X.
- Crosby CA, Wehbé MA, Mawr B. Hand strength: normative values. J Hand Surg Am 1994;19:665-70. https://doi.org/10.1016/0363-5023(94)90280-1.
- Hanten WP, Chen WY, Austin AA, Brooks RE, Carter HC, Law CA, et al. Maximum grip strength in normal subjects from 20 to 64 years of age. J Hand Ther 1999;12:193-200. https://doi.org/10.1016/S0894-1130(99)80046-5.
- Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 1985;66:69-74.
- Mathiowetz V, Rennells C, Donahoe L. Effect of elbow position on grip and key pinch strength. J Hand Surg Am 1985;10:694-7. https://doi.org/10.1016/S0363-5023(85)80210-0.
- Trampisch US, Franke J, Jedamzik N, Hinrichs T, Platen P. Optimal Jamar dynamometer handle position to assess maximal isometric hand grip strength in epidemiological studies. J Hand Surg Am 2012;37:2368-73. https://doi.org/10.1016/j.jhsa.2012.08.014.
- Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis 1973;32:413-18. https://doi.org/10.1136/ard.32.5.413.
- National Institute for Health and Clinical Excellence . Surgical Site Infections: Prevention and Treatment 2008.
- Dias JJ, Dhukaram V, Abhinav A, Bhowal B, Wildin CJ. Clinical and radiological outcome of cast immobilisation versus surgical treatment of acute scaphoid fractures at a mean follow-up of 93 months. J Bone Joint Surg Br 2008;90:899-905. https://doi.org/10.1302/0301-620X.90B7.20371.
- Smith ML, Bain GI, Chabrel N, Turner P, Carter C, Field J. Using computed tomography to assist with diagnosis of avascular necrosis complicating chronic scaphoid nonunion. J Hand Surg Am 2009;34:1037-43. https://doi.org/10.1016/j.jhsa.2009.02.016.
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Cont 1974;19:716-23. https://doi.org/10.1109/TAC.1974.1100705.
- Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester, West Sussex: John Wiley & Sons; 2015.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Pickering RM, Weatherall M. The analysis of continuous outcomes in multi-centre trials with small centre sizes. Stat Med 2007;26:5445-56. https://doi.org/10.1002/sim.3068.
- Theil H. Repeated Least Squares Applied to Complete Equation Systems. The Hague: Central Planning Bureau; 1953.
- Stock JH, Wright JH, Yogo M. A survey of weak instruments and weak identification in generalized method of moments. J Bus Econ Stat 2002;20:518-29. https://doi.org/10.1198/073500102288618658.
- Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010;340. https://doi.org/10.1136/bmj.c117.
- Kasenda B, Schandelmaier S, Sun X, von Elm E, You J, Blümle A, et al. Subgroup analyses in randomised controlled trials: cohort study on trial protocols and journal publications. BMJ 2014;349. https://doi.org/10.1136/bmj.g4539.
- ten Berg PW, Dobbe JG, Strackee SD, Streekstra GJ. Quantifying scaphoid malalignment based upon height-to-length ratios obtained by 3-dimensional computed tomography. J Hand Surg Am 2015;40:67-73. https://doi.org/10.1016/j.jhsa.2014.10.037.
- Medical Research Council . MRC Ethics Series – Good Research Practice: Principles and Guidelines 2012. www.mrc.ac.uk/publications/browse/good-research-practice-principles-and-guidelines/ (accessed 13 August 2017).
- Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, et al. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003821.
- National Institute for Health and Care Excellence . Fractures (Non-complex): Assessment and Management 2016.
- Dias JJ, Brealey SD, Fairhurst C, Amirfeyz R, Bhowal B, Blewitt N, et al. Surgery versus cast immobilisation for adults with a bicortical fracture of the scaphoid waist (SWIFFT): a pragmatic, multicentre, open-label, randomised superiority trial. Lancet 2020;396:390-401. https://doi.org/10.1016/S0140-6736(20)30931-4.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Department of Health and Social Care . Reference Costs 2015 to 2016 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Royston P. Multiple imputation of missing values: update of ice. Stata J 2005;5:527-36. https://doi.org/10.1177/1536867X0500500404.
- Carlin J, Galati J, Royston P. A new framework for managing and analyzing multiply imputed data in Stata. Stata J 2008;8:49-67. https://doi.org/10.1177/1536867X0800800104.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Ruby LK, Stinson J, Belsky MR. The natural history of scaphoid non-union. A review of fifty-five cases. J Bone Joint Surg Am 1985;67:428-32. https://doi.org/10.2106/00004623-198567030-00013.
- Lindstrom G, Nystrom A. Natural history of scaphoid non-union, with special reference to ‘asymptomatic’ cases. J Hand Surg Eur Vol 1992;17:697-700. https://doi.org/10.1016/0266-7681(92)90204-F.
- Drummond M, Sculpher M, Claxton K, Torrance G, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford Medical Publications; 2015.
- Lindström G, Nyström A. Incidence of post-traumatic arthrosis after primary healing of scaphoid fractures: a clinical and radiological study. J Hand Surg Br 1990;15:11-3. https://doi.org/10.1016/0266-7681(90)90041-2.
- Dias JJ, Brenkel IJ, Finlay DB. Patterns of union in fractures of the waist of the scaphoid. J Bone Joint Surg Br 1989;71:307-10. https://doi.org/10.1302/0301-620X.71B2.2925752.
- Office for National Statistics . National Life Tables, UK: 2014 to 2016 2017.
- Moritomo H, Tada K, Yoshida T, Masatomi T. The relationship between the site of nonunion of the scaphoid and scaphoid nonunion advanced collapse (SNAC). J Bone Joint Surg Br 1999;81:871-6. https://doi.org/10.1302/0301-620X.81B5.0810871.
- Shah CM, Stern PJ. Scapholunate advanced collapse (SLAC) and scaphoid nonunion advanced collapse (SNAC) wrist arthritis. Curr Rev Musculoskelet Med 2013;6:9-17. https://doi.org/10.1007/s12178-012-9149-4.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health 2011;14:539-45. https://doi.org/10.1016/j.jval.2010.10.029.
- Kovács C, Pecze M, Tihanyi Á, Kovács L, Balogh S, Bender T. The effect of sulphurous water in patients with osteoarthritis of hand. Double-blind, randomized, controlled follow-up study. Clin Rheumatol 2012;31:1437-42. https://doi.org/10.1007/s10067-012-2026-0.
- Neuprez A, Bruyère O, Maheu E, Dardenne N, Burlet N, D’Hooghe P, et al. Aesthetic discomfort in hand osteoarthritis: results from the LIège Hand Osteoarthritis Cohort (LIHOC). Arthritis Res Ther 2015;17. https://doi.org/10.1186/s13075-015-0807-y.
- Slatkowsky-Christensen B, Mowinckel P, Loge JH, Kvien TK. Health-related quality of life in women with symptomatic hand osteoarthritis: a comparison with rheumatoid arthritis patients, healthy controls, and normative data. Arthritis Rheum 2007;57:1404-9. https://doi.org/10.1002/art.23079.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Schier JS, Chan J. Changes in life roles after hand injury. J Hand Ther 2007;20:57-68. https://doi.org/10.1197/j.jht.2006.10.005.
- O’Brien L, Presnell S. Patient experience of distraction splinting for complex finger fracture dislocations. J Hand Ther 2010;23:249-60. https://doi.org/10.1016/j.jht.2010.01.002.
- Chan J, Spencer J. Adaptation to hand injury: an evolving experience. Am J Occup Ther 2004;58:128-39. https://doi.org/10.5014/ajot.58.2.128.
- Ammann B, Satink T, Andresen M. Experiencing occupations with chronic hand disability: narrative of hand-injured adults. Hand Ther 2012;17:87-94. https://doi.org/10.1177/1758998312471253.
- Fitzpatrick N, Finlay L. ‘Frustrating Disability’: the lived experience of coping with the rehabilitation phase following flexor tendon surgery. Int J Qual Stud Health Well-Being 2008;3:143-54. https://doi.org/10.1080/17482620802130407.
- Bialocerkowski AE. Difficulties associated with wrist disorders – a qualitative study. Clin Rehabil 2002;16:429-40. https://doi.org/10.1191/0269215502cr516oa.
- Watson NJ, Martin SA, Keating JL. The impact of wrist fracture, surgical repair and immobilization on patients: a qualitative study. Clin Rehabil 2018;32:841-51. https://doi.org/10.1177/0269215518754614.
- Bamford R, Walker D-M. A qualitative investigation into the rehabilitation experience of patients following wrist fracture. Hand Ther 2010;15:52-61. https://doi.org/10.1258/ht.2010.010013.
- Claydon JH, Robinson L, Aldridge SE. Patients’ perceptions of repair, rehabilitation and recovery after major orthopaedic trauma: a qualitative study. Physiotherapy 2017;103:322-9. https://doi.org/10.1016/j.physio.2015.11.002.
- Griffiths F, Mason V, Boardman F, Dennick K, Haywood K, Achten J, et al. Evaluating recovery following hip fracture: a qualitative interview study of what is important to patients. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-005406.
- Sully BG, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-166.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000496.
- Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-34.
- Treweek S, Mitchell E, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010;1. https://doi.org/10.1002/14651858.MR000013.pub4.
- McDonald A, Knight R, Campbell M, Entwistle V, Grant A, Cook J, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-9.
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11480.
- Adams M, Caffrey L, McKevitt C. Barriers and opportunities for enhancing patient recruitment and retention in clinical research: findings from an interview study in an NHS academic health science centre. Health Res Policy Syst 2015;13. https://doi.org/10.1186/1478-4505-13-8.
- Bower P, Brueton V, Gamble C, Treweek S, Smith CT, Young B, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-399.
- Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin Nurs 2010;19:227-33. https://doi.org/10.1111/j.1365-2702.2009.03041.x.
- Daykin A, Clement C, Gamble C, Kearney A, Blazeby J, Clarke M, et al. ‘Recruitment, recruitment, recruitment’ – the need for more focus on retention: a qualitative study of five trials. Trials 2018;19. https://doi.org/10.1186/s13063-018-2467-0.
- Leighton PA, Brealey SD, Dias JJ. Interventions to improve retention in a surgical, clinical trial: A pragmatic, stakeholder-driven approach. J Evid Based Med 2018;11:12-9. https://doi.org/10.1111/jebm.12271.
- Brueton VC, Stevenson F, Vale CL, Stenning SP, Tierney JF, Harding S, et al. Use of strategies to improve retention in primary care randomised trials: a qualitative study with in-depth interviews. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003835.
- Henderson M, Wight D, Nixon C, Hart G. Retaining young people in a longitudinal sexual health survey: a trial of strategies to maintain participation. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-9.
- Booker CL, Harding S, Benzeval M. A systematic review of the effect of retention methods in population-based cohort studies. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-249.
- Palys T, Given LM. The Sage Encyclopedia of Qualitative Research Methods. Los Angeles, CA: Sage; 2008.
- Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ 2000;320:114-16. https://doi.org/10.1136/bmj.320.7227.114.
- Francis JJ, Johnston M, Robertson C, Glidewell L, Entwistle V, Eccles MP, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229-45. https://doi.org/10.1080/08870440903194015.
- Baker SE, Edwards R. How Many Qualitative Interviews is Enough? Expert Voices and Early Career Reflections on Sampling and Cases in Qualitative Research. Southampton: ESRC National Centre for Research Methods; 2012.
- Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field 2006;18:59-82. https://doi.org/10.1177/1525822X05279903.
- Kvale S. Interviews: An Introduction to Qualitative Research Interviewing. London: Sage; 1996.
- Kvale S. Doing Interviews. London: Sage Publications Ltd; 2007.
- Britten N. Qualitative interviews in medical research. BMJ 1995;311:251-3. https://doi.org/10.1136/bmj.311.6999.251.
- Edwards R, Holland J. What Is Qualitative Interviewing?. London: Bloomsbury Academic; 2013.
- Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. London: Sage; 1998.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs 2005;49:616-23. https://doi.org/10.1111/j.1365-2648.2004.03331.x.
- Schädel-Höpfner M, Marent-Huber M, Sauerbier M, Pillukat T, Eisenschenk A, Siebert HR. Operative versus conservative treatment of non-displaced fractures of the scaphoid bone. Results of a controlled multicenter cohort study. Unfallchirurg 2010;113:804-13. https://doi.org/10.1007/s00113-010-1848-y.
- Inoue G, Shionoya K. Herbert screw fixation by limited access for acute fractures of the scaphoid. J Bone Joint Surg Br 1997;79:418-21. https://doi.org/10.1302/0301-620X.79B3.0790418.
- Al-Ajmi TA, Al-Faryan KH, Al-Kanaan NF, Al-Khodair AA, Al-Faryan TH, Al-Oraini MI, et al. A systematic review and meta-analysis of randomized controlled trials comparing surgical versus conservative treatments for acute undisplaced or minimally-displaced scaphoid fractures. Clin Orthop Surg 2018;10:64-73. https://doi.org/10.4055/cios.2018.10.1.64.
- Cooney WP, Linscheid RL, Dobyns JH. Triangular fibrocartilage tears. J Hand Surg Am 1994;19:143-54. https://doi.org/10.1016/0363-5023(94)90238-0.
- Moritomo H, Murase T, Oka K, Tanaka H, Yoshikawa H, Sugamoto K. Relationship between the fracture location and the kinematic pattern in scaphoid nonunion. J Hand Surg Am 2008;33:1459-68. https://doi.org/10.1016/j.jhsa.2008.05.035.
- Singh HP, Dias JJ. Scaphoid Fractures. J Bone Joint Surg Br 2011. https://pdfs.semanticscholar.org/3bb0/f91b8d03d034d7024b8b44c52c7337a84c65.pdf (accessed 15 December 2019).
- Grewal R, Suh N, Macdermid JC. Use of computed tomography to predict union and time to union in acute scaphoid fractures treated nonoperatively. J Hand Surg Am 2013;38:872-7. https://doi.org/10.1016/j.jhsa.2013.01.032.
- Grewal R, Frakash U, Osman S, McMurtry RY. A quantitative definition of scaphoid union: determining the inter-rater reliability of two techniques. J Orthop Surg Res 2013;8. https://doi.org/10.1186/1749-799X-8-28.
- Bhat M, McCarthy M, Davis TR, Oni JA, Dawson S. MRI and plain radiography in the assessment of displaced fractures of the waist of the carpal scaphoid. J Bone Joint Surg Br 2004;86:705-13. https://doi.org/10.1302/0301-620X.86B5.14374.
- Grewal R, Lutz K, MacDermid JC, Suh N. Proximal Pole Scaphoid Fractures: A Computed Tomographic Assessment of Outcomes. J Hand Surg Am 2016;41:54-8. https://doi.org/10.1016/j.jhsa.2015.10.013.
- Thorleifsson R, Karlsson J, Sigurjonsson K. Fractures of the scaphoid bone. A follow-up study. Arch Orthop Trauma Surg 1984;103:96-9. https://doi.org/10.1007/BF00389579.
- Valen B. Treatment of scaphoid fractures in a local hospital. Tidsskr Nor Laegeforen 2013;133:1079-82. https://doi.org/10.4045/tidsskr.12.0191.
- Yin ZG, Zhang JB, Kan SL, Wang XG. Diagnosing suspected scaphoid fractures: a systematic review and meta-analysis. Clin Orthop Relat Res 2010;468:723-34. https://doi.org/10.1007/s11999-009-1081-6.
- Parker SL, Mendenhall SK, Shau D, Adogwa O, Cheng JS, Anderson WN, et al. Determination of minimum clinically important difference in pain, disability, and quality of life after extension of fusion for adjacent-segment disease. J Neurosurg Spine 2012;16:61-7. https://doi.org/10.3171/2011.8.SPINE1194.
- Nwachukwu BU, Chang B, Voleti PB, Berkanish P, Cohn MR, Altchek DW, et al. Preoperative Short Form Health Survey Score Is Predictive of Return to Play and Minimal Clinically Important Difference at a Minimum 2-Year Follow-up After Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2017;45:2784-90. https://doi.org/10.1177/0363546517714472.
- Díaz-Arribas MJ, Fernández-Serrano M, Royuela A, Kovacs FM, Gallego-Izquierdo T, Ramos-Sánchez M, et al. Minimal Clinically Important Difference in Quality of Life for Patients With Low Back Pain. Spine 2017;42:1908-16. https://doi.org/10.1097/BRS.0000000000002298.
- Clement ND, MacDonald D, Simpson AH. The minimal clinically important difference in the Oxford knee score and Short Form 12 score after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2014;22:1933-9. https://doi.org/10.1007/s00167-013-2776-5.
- Office for National Statistics . Time Series: LMSB SA AWE Total Pay WE 2018.
- Hannemann PF, Essers BA, Schots JP, Dullaert K, Poeze M, Brink PR. Functional outcome and cost-effectiveness of pulsed electromagnetic fields in the treatment of acute scaphoid fractures: a cost-utility analysis. BMC Musculoskelet Disord 2015;16. https://doi.org/10.1186/s12891-015-0541-2.
- Karl JW, Swart E, Strauch RJ. Diagnosis of Occult Scaphoid Fractures: A Cost-Effectiveness Analysis. J Bone Joint Surg Am 2015;97:1860-8. https://doi.org/10.2106/JBJS.O.00099.
- Filan SL, Herbert TJ. Herbert screw fixation of scaphoid fractures. J Bone Joint Surg Br 1996;78:519-29. https://doi.org/10.1302/0301-620X.78B4.0780519.
Appendix 1 Participating trusts
-
Barts Health NHS Trust
-
Bolton NHS Foundation Trust
-
Brighton and Sussex University Hospitals NHS Trust
-
Cambridge University Hospitals NHS Foundation Trust
-
Cardiff and Vale University Health Board
-
Chelsea and Westminster Hospital NHS Foundation Trust
-
Gloucestershire Hospitals NHS Foundation Trust
-
Hampshire Hospitals NHS Foundation Trust
-
King’s College Hospital NHS Foundation Trust
-
Lancashire Teaching Hospitals NHS Foundation Trust
-
Maidstone and Tunbridge Wells NHS Trust
-
Medway NHS Foundation Trust
-
Newcastle upon Tyne Hospitals NHS Foundation Trust
-
North Bristol NHS Trust
-
Northumbria Healthcare NHS Foundation Trust
-
Nottingham University Hospitals NHS Trust
-
Oxford University Hospitals NHS Trust
-
North West Anglia NHS Foundation Trust
-
Poole Hospital NHS Foundation Trust
-
Royal Berkshire NHS Foundation Trust
-
Royal Cornwall Hospitals NHS Trust
-
Royal United Hospital Bath NHS Trust
-
Salford Royal NHS Foundation Trust
-
South Tees Hospitals NHS Foundation Trust
-
Southport and Ormskirk Hospital NHS Trust
-
Taunton and Somerset NHS Foundation Trust
-
The Royal Liverpool and Broadgreen University Hospitals NHS Trust
-
University Hospital Southampton NHS Foundation Trust
-
University Hospitals Birmingham NHS Foundation Trust
-
University Hospitals Bristol NHS Foundation Trust
-
University Hospitals Coventry and Warwickshire NHS Trust
-
University Hospitals of Leicester NHS Trust
-
University Hospitals Plymouth NHS Trust
-
Worcestershire Acute Hospitals NHS Trust.
Appendix 2 Table of amendments
| Type (non-substantial or substantial) | Approval date | Documents amended | Brief description of amendment |
|---|---|---|---|
| Non-substantial amendment 1 | 13 June 2013 |
Update to trial protocol (version 1.1, 5 June 2013) Update to main trial patient information leaflet (version 1.1, 5 June 2013) Update to main trial consent form (version 1.1, 5 June 2013) Update to linked interview consent form (version 1.1, 5 June 2013) Update to main interview consent form (version 1.1, 5 June 2013) Update to linked interview patient information leaflet (version 1.1, 5 June 2013) |
Clarification in the protocol regarding timing of baseline CT and surgery:Minor changes to patient information leaflets in response to the TSC patient representative recommendations. Consequently, the consent form was updated to reflect changes in patient information leaflet Change to consent status form to capture time to consent, as stated in the protocol |
| Non-substantial amendment 2 | 10 July 2013 | Hospital poster (version 1.1, 2 July 2013) | Update of the eligibility criteria listed on the hospital poster to match those stated in the protocol |
| Non-substantial amendment 3 | 29 August 2013 |
Update to main trial patient information leaflet (version 1.2, 16 August 2013) Update to main trial consent form (version 1.2, 16 August 2013) Update to interview consent form (version 1.2, 16 August 2013) |
Correction of Birmingham site details Addition of new participating sites Addition of potential radiation risks from radiographs/CT scans in the main trial patient information leaflet that were not previously explained |
| Non-substantial amendment 4 | 9 September 2013 | N/A | Change in PI at Liverpool site |
| Non-substantial amendment 5 | 8 October 2013 | N/A | Addition of new participating sites |
| Substantial amendment 1 | 3 December 2013 |
Update to trial protocol (version 2.0, 20 October 2013) Update to main trial patient information leaflet (version 2.0, 20 October 2013) Update to main trial consent form (version 2.0, 20 October 2013) Update to interview consent form (version 2.0, 20 October 2013) Update to hospital poster (version 2.0, 20 October 2013) Update to consent status form (version 2.0, 6 November 2013) Update to baseline form (version 2.0, 20 October 2013) |
Update to eligibility criteria in the protocol:
|
| Non-substantial amendment 6 | 14 January 2014 |
Update to trial protocol (version 2.1, 6 January 2014) Update to hospital poster (version 2.1, 6 January 2014) |
Change of PI at Maidstone and Cardiff sites Addition of new participating sites Update to the protocol about the referral pathway to fracture clinic to include points of contact other than A&E Changes to exclusion criteria in protocol:Addition of expected plaster cast AEs to protocol (soft/broken cast, pressure sores, CRPS, nerve compression, pain due to tight cast) |
| Non-substantial amendment 7 | 19 June 2014 | N/A | Change of PI at Royal London and Alexandra Hospital sites |
| Non-substantial amendment 8 | 19 August 2014 | Update to trial protocol (version 2.2, 14 August 2014) |
Minor modifications to trial protocol:Submission of correct version of consent status form Change in PI at Birmingham site |
| Substantial amendment 2 | 23 February 2015 | Update to trial protocol (version 3.0, 19 January 2015) |
Reinstatement of patients previously withdrawn owing to equivocal CT scan about the presence of a fracture:Verbal consent for qualitative substudy:Outline of patient engagement activities (newsletter, video, website, postal envelope tagline) and example documents for approval Update to statistical analysis plan:Clarification regarding collection of patient questionnaires in clinic: |
| Substantial amendment 3 | 26 November 2015 |
Update to trial protocol (version 4.0, 20 October 2015) Update to main trial consent form (version 3.0, 20 October 2015) Update to main trial patient information leaflet (version 3.0, 20 October 2015) |
Inclusion of letter and primary-outcome-only questionnaire at 6 weeks after initial 52-week follow-up questionnaire being sent Inclusion of prize draws at 26 weeks for participant completion of a returning questionnaire and at 52 weeks and 5 years for attending the hospital clinic visit Removal of adjustment on covariant ‘baseline grip strength of opposite wrist’ from analysis plan Clarification that there will be a 5-year follow-up |
| Non-substantial amendment 9 | 11 March 2016 | N/A | Extension to recruitment period |
| Non-substantial amendment 10 | 24 August 2016 | N/A | Change in PI at Royal Berkshire Hospital |
| Substantial amendment 4 | 17 October 2016 | Update to trial protocol (version 5.0, 14 September 2016) |
Permission to use e-mail to contact patients for the 52-week follow-up Inclusion of a patient letter from the hospital site to encourage attendance at the 52-week clinic appointment Payment of travel expenses to patients attending the 52-week clinic appointment Permission to retrieve imaging for participants attending other hospitals via PACS The use of the Summary Care Records to check contact details for patients who the trial team lose contact with Permission to contact the patient’s GP to check if they have had any further surgery for their scaphoid fracture Change to the categorisation of non-union, slight union, etc., to remove cases of overlap |
| Non-substantial amendment 11 | 11 May 2017 | N/A | Change in PI at Coventry site |
Appendix 3 Tables and figures
| Characteristic | Attended visit (N = 388) | Did not attend visit (N = 51) | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 189) | Plaster cast (N = 199) | Total (N = 388) | Surgery (N = 30) | Plaster cast (N = 21) | Total (N = 51) | |
| Gender, n (%) | ||||||
| Male | 154 (81.5) | 167 (83.9) | 321 (82.7) | 26 (86.7) | 16 (76.2) | 42 (82.4) |
| Female | 35 (18.5) | 32 (16.1) | 67 (17.3) | 4 (13.3) | 5 (23.8) | 9 (17.6) |
| Age, years | ||||||
| n | 189 | 199 | 388 | 30 | 21 | 51 |
| Mean (SD) | 33.2 (13.3) | 32.7 (12.4) | 32.9 (12.8) | 31.2 (12.6) | 34.8 (10.9) | 32.7 (11.9) |
| Median (min., max.) | 29 (16, 80) | 29 (16, 76) | 29 (16, 80) | 25 (18, 57) | 34 (21, 55) | 28 (18, 57) |
| Ethnicity, n (%) | ||||||
| White | 179 (94.7) | 174 (87.4) | 353 (91.0) | 26 (86.7) | 21 (100.0) | 47 (92.2) |
| Black | 0 (0.0) | 5 (2.5) | 5 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian | 7 (3.7) | 10 (5.0) | 17 (4.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 3 (1.6) | 10 (5.0) | 13 (3.4) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Education, n (%) | ||||||
| No formal qualifications | 18 (9.5) | 24 (12.1) | 42 (10.8) | 6 (20.0) | 3 (14.3) | 9 (17.6) |
| Some qualifications/no degree | 132 (69.8) | 114 (57.3) | 246 (63.4) | 19 (63.3) | 15 (71.4) | 34 (66.7) |
| Degree or higher | 38 (20.1) | 61 (30.7) | 99 (25.5) | 3 (10.0) | 3 (14.3) | 6 (11.8) |
| Missing | 1 (0.5) | 0 (0.0) | 1 (0.3) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Employment status, n (%) | ||||||
| Part-time | 20 (10.6) | 16 (8.0) | 36 (9.3) | 0 (0.0) | 2 (9.5) | 2 (3.9) |
| Full-time | 113 (59.8) | 114 (57.3) | 227 (58.5) | 14 (46.7) | 6 (28.6) | 20 (39.2) |
| Self-employed | 18 (9.5) | 26 (13.1) | 44 (11.3) | 3 (10.0) | 10 (47.6) | 13 (25.5) |
| Student | 17 (9.0) | 21 (10.6) | 38 (9.8) | 3 (10.0) | 0 (0.0) | 3 (5.9) |
| Retired | 7 (3.7) | 5 (2.5) | 12 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Looking after family/home | 0 (0.0) | 4 (2.0) | 4 (1.0) | 1 (3.3) | 2 (9.5) | 3 (5.9) |
| Not employed but seeking work | 5 (2.6) | 5 (2.5) | 10 (2.6) | 4 (13.3) | 0 (0.0) | 4 (7.8) |
| Other | 8 (4.2) | 8 (4.0) | 16 (4.1) | 3 (10.0) | 1 (4.8) | 4 (7.8) |
| Missing | 1 (0.5) | 0 (0.0) | 1 (0.3) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Type of employment, n (%) | ||||||
| Unskilled manual | 23 (12.2) | 17 (8.5) | 40 (10.3) | 2 (6.7) | 6 (28.6) | 8 (15.7) |
| Skilled manual | 55 (29.1) | 54 (27.1) | 109 (28.1) | 8 (26.7) | 6 (28.6) | 14 (27.5) |
| Unskilled non-manual | 17 (9.0) | 10 (5.0) | 27 (7.0) | 2 (6.7) | 2 (9.5) | 4 (7.8) |
| Skilled non-manual | 30 (15.9) | 44 (22.1) | 74 (19.1) | 3 (10.0) | 2 (9.5) | 5 (9.8) |
| Professional | 19 (10.1) | 15 (7.5) | 34 (8.8) | 1 (3.3) | 4 (19.0) | 5 (9.8) |
| Other | 17 (9.0) | 30 (15.1) | 47 (12.1) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Missing | 28 (14.8) | 29 (14.6) | 57 (14.7) | 12 (40.0) | 1 (4.8) | 13 (25.5) |
| Current smoker, n (%) | ||||||
| Yes | 56 (29.6) | 45 (22.6) | 101 (26.0) | 17 (56.7) | 11 (52.4) | 28 (54.9) |
| No | 132 (69.8) | 153 (76.9) | 285 (73.5) | 11 (36.7) | 10 (47.6) | 21 (41.2) |
| Missing | 1 (0.5) | 1 (0.5) | 2 (0.5) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| If yes | ||||||
| Number of cigarettes per day, median (min., max.) | 10 (1, 40) | 10 (1, 30) | 10 (1, 40) | 10 (2, 20) | 10 (5, 15) | 10 (2, 20) |
| Number of years, median (min., max.) | 10 (1, 50) | 10 (1, 36) | 10 (1, 50) | 9 (1, 44) | 10 (4, 20) | 10 (1, 44) |
| Past smoker, n (%) | ||||||
| Yes | 99 (52.4) | 94 (47.2) | 193 (49.7) | 17 (56.7) | 15 (71.4) | 32 (62.7) |
| No | 79 (41.8) | 96 (48.2) | 175 (45.1) | 6 (20.0) | 5 (23.8) | 11 (21.6) |
| Missing | 11 (5.8) | 9 (4.5) | 20 (5.2) | 7 (23.3) | 1 (4.8) | 8 (15.7) |
| Diabetes, n (%) | ||||||
| Yes | 6 (3.2) | 4 (2.0) | 10 (2.6) | 1 (3.3) | 0 (0.0) | 1 (2.0) |
| No | 182 (96.3) | 195 (98.0) | 377 (97.2) | 27 (90.0) | 21 (100.0) | 48 (94.1) |
| Missing | 1 (0.5) | 0 (0.0) | 1 (0.3) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Steroid use, n (%) | ||||||
| Yes | 5 (2.6) | 4 (2.0) | 9 (2.3) | 1 (3.3) | 0 (0.0) | 1 (2.0) |
| No | 183 (96.8) | 195 (98.0) | 378 (97.4) | 27 (90.0) | 21 (100.0) | 48 (94.1) |
| Missing | 1 (0.5) | 0 (0.0) | 1 (0.3) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Characteristic | Attended visit (N = 388) | Did not attend visit (N = 51) | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 189) | Plaster cast (N = 199) | Total (N = 388) | Surgery (N = 30) | Plaster cast (N = 21) | Total (N = 51) | |
| Time since injury, daysa | ||||||
| n | 189 | 199 | 388 | 30 | 21 | 51 |
| Mean (SD) | 4.9 (3.1) | 5.3 (3.4) | 5.1 (3.3) | 5.9 (2.7) | 5.3 (3.2) | 5.7 (2.9) |
| Median (min., max.) | 4 (1, 14) | 5 (0, 14) | 4 (0, 14) | 6 (1, 12) | 5 (1, 12) | 5 (1, 12) |
| Affected wrist, n (%) | ||||||
| Left | 105 (55.6) | 110 (55.3) | 215 (55.4) | 10 (33.3) | 8 (38.1) | 18 (35.3) |
| Right | 84 (44.4) | 89 (44.7) | 173 (44.6) | 20 (66.7) | 13 (61.9) | 33 (64.7) |
| Affected wrist is dominant hand, n (%) | ||||||
| Yes | 85 (45.0) | 83 (41.7) | 168 (43.3) | 15 (50.0) | 12 (57.1) | 27 (52.9) |
| No | 104 (55.0) | 116 (58.3) | 220 (56.7) | 13 (43.3) | 9 (42.9) | 22 (43.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Displacement (eligibility), n (%) | ||||||
| No displacement | 113 (59.8) | 117 (58.8) | 230 (59.3) | 22 (73.3) | 17 (81.0) | 39 (76.5) |
| Displacement | 76 (40.2) | 82 (41.2) | 158 (40.7) | 8 (26.7) | 4 (19.0) | 12 (23.5) |
| Displacement (randomisation), n (%) | ||||||
| No displacement | 109 (57.7) | 113 (56.8) | 222 (57.2) | 22 (73.3) | 17 (81.0) | 39 (76.5) |
| Displacement | 80 (42.3) | 86 (43.2) | 166 (42.8) | 8 (26.7) | 4 (19.0) | 12 (23.5) |
| Radiographs,b n (%) | ||||||
| Elongated scaphoid view | 180 (95.2) | 189 (95.0) | 369 (95.1) | 29 (96.7) | 21 (100.0) | 50 (98.0) |
| Posterior–anterior view | 187 (98.9) | 197 (99.0) | 384 (99.0) | 28 (93.3) | 21 (100.0) | 49 (96.1) |
| 45° semisupine view | 134 (70.9) | 151 (75.9) | 285 (73.5) | 25 (83.3) | 15 (71.4) | 40 (78.4) |
| Lateral view | 189 (100.0) | 196 (98.5) | 385 (99.2) | 29 (96.7) | 21 (100.0) | 50 (98.0) |
| 45° semiprone view | 170 (89.9) | 179 (89.9) | 349 (89.9) | 28 (93.3) | 17 (81.0) | 45 (88.2) |
| Previous wrist problems on same side, n (%) | ||||||
| Yes | 40 (21.2) | 41 (20.6) | 81 (20.9) | 3 (10.0) | 4 (19.0) | 7 (13.7) |
| No | 148 (78.3) | 158 (79.4) | 306 (78.9) | 25 (83.3) | 15 (71.4) | 40 (78.4) |
| Missing | 1 (0.5) | 0 (0.0) | 1 (0.3) | 2 (6.7) | 2 (9.5) | 4 (7.8) |
| If yes, what injury?, n (%) | ||||||
| Previous fracture | 21 (52.5) | 26 (63.4) | 47 (58.0) | 2 (66.7) | 2 (50.0) | 4 (57.1) |
| Arthritis | 1 (2.5) | 1 (2.4) | 2 (2.5) | 1 (33.3) | 0 (0.0) | 1 (14.3) |
| Ligament, tendon or nerve injury | 10 (25.0) | 7 (17.1) | 17 (21.0) | 0 (0.0) | 1 (25.0) | 1 (14.3) |
| Other | 6 (15.0) | 7 (17.1) | 13 (16.0) | 0 (0.0) | 1 (25.0) | 1 (14.3) |
| Missing | 2 (5.0) | 0 (0.0) | 2 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injury mechanism, n (%) | ||||||
| Fall – standing | 24 (12.7) | 27 (13.6) | 51 (13.1) | 4 (13.3) | 2 (9.5) | 6 (11.8) |
| Fall – walking | 20 (10.6) | 22 (11.1) | 42 (10.8) | 4 (13.3) | 2 (9.5) | 6 (11.8) |
| Fall – running | 37 (19.6) | 35 (17.6) | 72 (18.6) | 3 (10.0) | 3 (14.3) | 6 (11.8) |
| Fall – from height | 24 (12.7) | 31 (15.6) | 55 (14.2) | 4 (13.3) | 3 (14.3) | 7 (13.7) |
| Fall – from moving object | 35 (18.5) | 31 (15.6) | 66 (17.0) | 7 (23.3) | 0 (0.0) | 7 (13.7) |
| Hit on palm of hand – object striking palm | 15 (7.9) | 14 (7.0) | 29 (7.5) | 1 (3.3) | 1 (4.8) | 2 (3.9) |
| Hit on palm of hand – handle whipping back | 7 (3.7) | 10 (5.0) | 17 (4.4) | 2 (6.7) | 1 (4.8) | 3 (5.9) |
| Hit on palm of hand – other sudden extension | 10 (5.3) | 7 (3.5) | 17 (4.4) | 1 (3.3) | 1 (4.8) | 2 (3.9) |
| Punched something | 4 (2.1) | 9 (4.5) | 13 (3.4) | 0 (0.0) | 3 (14.3) | 3 (5.9) |
| Road traffic accident | 9 (4.8) | 6 (3.0) | 15 (3.9) | 0 (0.0) | 2 (9.5) | 2 (3.9) |
| Other | 4 (2.1) | 7 (3.5) | 11 (2.8) | 2 (6.7) | 3 (14.3) | 5 (9.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Place of injury,b n (%) | ||||||
| Sport | 79 (41.8) | 72 (36.2) | 151 (38.9) | 9 (30.0) | 6 (28.6) | 15 (29.4) |
| Home | 20 (10.6) | 38 (19.1) | 58 (14.9) | 7 (23.3) | 5 (23.8) | 12 (23.5) |
| Work | 18 (9.5) | 17 (8.5) | 35 (9.0) | 4 (13.3) | 1 (4.8) | 5 (9.8) |
| Road traffic accident | 24 (12.7) | 32 (16.1) | 56 (14.4) | 2 (6.7) | 2 (9.5) | 4 (7.8) |
| Public place | 44 (23.3) | 42 (21.1) | 86 (22.2) | 5 (16.7) | 6 (28.6) | 11 (21.6) |
| Other | 3 (1.6) | 0 (0.0) | 3 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (1.6) | 1 (0.5) | 4 (1.0) | 1 (3.3) | 1 (4.8) | 2 (3.9) |
| Treatment preference, n (%) | ||||||
| Surgery | 80 (42.3) | 92 (46.2) | 172 (44.3) | 13 (43.3) | 9 (42.9) | 22 (43.1) |
| No surgery | 10 (5.3) | 16 (8.0) | 26 (6.7) | 3 (10.0) | 3 (14.3) | 6 (11.8) |
| No preference | 98 (51.9) | 90 (45.2) | 188 (48.5) | 12 (40.0) | 9 (42.9) | 21 (41.2) |
| Missing | 1 (0.5) | 1 (0.5) | 2 (0.5) | 2 (6.7) | 0 (0.0) | 2 (3.9) |
| Characteristic | Attended visit (N = 338) | Did not attend visit (N = 101) | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 173) | Plaster cast (N = 165) | Total (N = 338) | Surgery (N = 46) | Plaster cast (N = 55) | Total (N = 101) | |
| Gender, n (%) | ||||||
| Male | 143 (82.7) | 134 (81.2) | 277 (82.0) | 37 (80.4) | 49 (89.1) | 86 (85.1) |
| Female | 30 (17.3) | 31 (18.8) | 61 (18.0) | 9 (19.6) | 6 (10.9) | 15 (14.9) |
| Age, years | ||||||
| n | 173 | 165 | 338 | 46 | 55 | 101 |
| Mean (SD) | 33.7 (13.5) | 34.1 (12.8) | 33.9 (13.1) | 30.0 (11.9) | 29.1 (9.6) | 29.5 (10.6) |
| Median (min., max.) | 29 (16, 80) | 31 (16, 76) | 30 (16, 80) | 26 (18, 57) | 25 (17, 55) | 25 (17, 57) |
| Ethnicity, n (%) | ||||||
| White | 163 (94.2) | 144 (87.3) | 307 (90.8) | 42 (91.3) | 51 (92.7) | 93 (92.1) |
| Black | 0 (0.0) | 3 (1.8) | 3 (0.9) | 0 (0.0) | 2 (3.6) | 2 (2.0) |
| Asian | 6 (3.5) | 8 (4.8) | 14 (4.1) | 1 (2.2) | 2 (3.6) | 3 (3.0) |
| Other | 4 (2.3) | 10 (6.1) | 14 (4.1) | 1 (2.2) | 0 (0.0) | 1 (1.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| Education, n (%) | ||||||
| No formal qualifications | 16 (9.2) | 21 (12.7) | 37 (10.9) | 8 (17.4) | 6 (10.9) | 14 (13.9) |
| Some qualifications/no degree | 120 (69.4) | 91 (55.2) | 211 (62.4) | 31 (67.4) | 38 (69.1) | 69 (68.3) |
| Degree or higher | 36 (20.8) | 53 (32.1) | 89 (26.3) | 5 (10.9) | 11 (20.0) | 16 (15.8) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| Employment status, n (%) | ||||||
| Part-time | 19 (11.0) | 15 (9.1) | 34 (10.1) | 1 (2.2) | 3 (5.5) | 4 (4.0) |
| Full-time | 106 (61.3) | 93 (56.4) | 199 (58.9) | 21 (45.7) | 27 (49.1) | 48 (47.5) |
| Self-employed | 15 (8.7) | 23 (13.9) | 38 (11.2) | 6 (13.0) | 13 (23.6) | 19 (18.8) |
| Student | 14 (8.1) | 16 (9.7) | 30 (8.9) | 6 (13.0) | 5 (9.1) | 11 (10.9) |
| Retired | 7 (4.0) | 5 (3.0) | 12 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Looking after family/home | 0 (0.0) | 4 (2.4) | 4 (1.2) | 1 (2.2) | 2 (3.6) | 3 (3.0) |
| Not employed but seeking work | 3 (1.7) | 4 (2.4) | 7 (2.1) | 6 (13.0) | 1 (1.8) | 7 (6.9) |
| Other | 8 (4.6) | 5 (3.0) | 13 (3.8) | 3 (6.5) | 4 (7.3) | 7 (6.9) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| Type of employment, n (%) | ||||||
| Unskilled manual | 19 (11.0) | 11 (6.7) | 30 (8.9) | 6 (13.0) | 12 (21.8) | 18 (17.8) |
| Skilled manual | 51 (29.5) | 44 (26.7) | 95 (28.1) | 12 (26.1) | 16 (29.1) | 28 (27.7) |
| Unskilled non-manual | 18 (10.4) | 9 (5.5) | 27 (8.0) | 1 (2.2) | 3 (5.5) | 4 (4.0) |
| Skilled non-manual | 28 (16.2) | 38 (23) | 66 (19.5) | 5 (10.9) | 8 (14.5) | 13 (12.9) |
| Professional | 17 (9.8) | 15 (9.1) | 32 (9.5) | 3 (6.5) | 4 (7.3) | 7 (6.9) |
| Other | 16 (9.2) | 23 (13.9) | 39 (11.5) | 3 (6.5) | 7 (12.7) | 10 (9.9) |
| Missing | 24 (13.9) | 25 (15.2) | 49 (14.5) | 16 (34.8) | 5 (9.1) | 21 (20.8) |
| Current smoker, n (%) | ||||||
| Yes | 49 (28.3) | 31 (18.8) | 80 (23.7) | 24 (52.2) | 25 (45.5) | 49 (48.5) |
| No | 123 (71.1) | 133 (80.6) | 256 (75.7) | 20 (43.5) | 30 (54.5) | 50 (49.5) |
| Missing | 1 (0.6) | 1 (0.6) | 2 (0.6) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| If yes | ||||||
| Number of cigarettes per day, median (min., max.) | 10 (1, 40) | 11 (1, 36) | 10 (1, 40) | 10 (1, 20) | 10 (4, 20) | 10 (1, 20) |
| Number of years, median (min., max.) | 10 (1, 50) | 10 (1, 40) | 10 (1, 50) | 10 (1, 44) | 10 (2, 20) | 10 (1, 44) |
| Past smoker, n (%) | ||||||
| Yes | 91 (52.6) | 70 (42.4) | 161 (47.6) | 25 (54.3) | 39 (70.9) | 64 (63.4) |
| No | 72 (41.6) | 88 (53.3) | 160 (47.3) | 13 (28.3) | 13 (23.6) | 26 (25.7) |
| Missing | 10 (5.8) | 7 (4.2) | 17 (5.0) | 8 (17.4) | 3 (5.5) | 11 (10.9) |
| Diabetes, n (%) | ||||||
| Yes | 6 (3.5) | 4 (2.4) | 10 (3.0) | 1 (2.2) | 0 (0.0) | 1 (1.0) |
| No | 166 (96.0) | 161 (97.6) | 327 (96.7) | 43 (93.5) | 55 (100.0) | 98 (97.0) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| Steroid use, n (%) | ||||||
| Yes | 4 (2.3) | 4 (2.4) | 8 (2.4) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| No | 168 (97.1) | 161 (97.6) | 329 (97.3) | 42 (91.3) | 55 (100.0) | 97 (96.0) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| Characteristic | Attended visit (N = 338) | Did not attend visit (N = 101) | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 173) | Plaster cast (N = 165) | Total (N = 338) | Surgery (N = 46) | Plaster cast (N = 55) | Total (N = 101) | |
| Time since injury, daysa | ||||||
| n | 173 | 165 | 338 | 46 | 55 | 101 |
| Mean (SD) | 4.8 (3.1) | 5.4 (3.5) | 5.1 (3.3) | 6.0 (3.0) | 5.2 (2.9) | 5.6 (3.0) |
| Median (min., max.) | 4 (1, 14) | 4 (0, 14) | 4 (0, 14) | 6 (1, 14) | 5 (0, 12) | 6 (0, 14) |
| Affected wrist, n (%) | ||||||
| Left | 93 (53.8) | 94 (57.0) | 187 (55.3) | 22 (47.8) | 24 (43.6) | 46 (45.5) |
| Right | 80 (46.2) | 71 (43.0) | 151 (44.7) | 24 (52.2) | 31 (56.4) | 55 (54.5) |
| Affected wrist is dominant hand, n (%) | ||||||
| Yes | 76 (43.9) | 68 (41.2) | 144 (42.6) | 24 (52.2) | 27 (49.1) | 51 (50.5) |
| No | 97 (56.1) | 97 (58.8) | 194 (57.4) | 20 (43.5) | 28 (50.9) | 48 (47.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| Displacement (eligibility), n (%) | ||||||
| No displacement | 107 (61.8) | 101 (61.2) | 208 (61.5) | 28 (60.9) | 33 (60.0) | 61 (60.4) |
| Displacement | 66 (38.2) | 64 (38.8) | 130 (38.5) | 18 (39.1) | 22 (40.0) | 40 (39.6) |
| Displacement (randomisation), n (%) | ||||||
| No displacement | 104 (60.1) | 97 (58.8) | 201 (59.5) | 27 (58.7) | 33 (60.0) | 60 (59.4) |
| Displacement | 69 (39.9) | 68 (41.2) | 137 (40.5) | 19 (41.3) | 22 (40.0) | 41 (40.6) |
| Radiographs,b n (%) | ||||||
| Elongated scaphoid view | 164 (94.8) | 158 (95.8) | 322 (95.3) | 45 (97.8) | 52 (94.5) | 97 (96.0) |
| Posterior–anterior view | 171 (98.8) | 165 (100.0) | 336 (99.4) | 44 (95.7) | 53 (96.4) | 97 (96.0) |
| 45° semisupine view | 121 (69.9) | 121 (73.3) | 242 (71.6) | 38 (82.6) | 45 (81.8) | 83 (82.2) |
| Lateral view | 173 (100.0) | 164 (99.4) | 337 (99.7) | 45 (97.8) | 53 (96.4) | 98 (97.0) |
| 45° semiprone view | 156 (90.2) | 147 (89.1) | 303 (89.6) | 42 (91.3) | 49 (89.1) | 91 (90.1) |
| Previous wrist problems on same side, n (%) | ||||||
| Yes | 35 (20.2) | 33 (20.0) | 68 (20.1) | 8 (17.4) | 12 (21.8) | 20 (19.8) |
| No | 137 (79.2) | 131 (79.4) | 268 (79.3) | 36 (78.3) | 42 (76.4) | 78 (77.2) |
| Missing | 1 (0.6) | 1 (0.6) | 2 (0.6) | 2 (4.3) | 1 (1.8) | 3 (3.0) |
| If yes, what injury?, n (%) | ||||||
| Previous fracture | 18 (51.4) | 20 (60.6) | 38 (55.9) | 5 (62.5) | 8 (66.7) | 13 (65.0) |
| Arthritis | 2 (5.7) | 1 (3.0) | 3 (4.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ligament, tendon or nerve injury | 8 (22.9) | 7 (21.2) | 15 (22.1) | 2 (25) | 1 (8.3) | 3 (15.0) |
| Other | 6 (17.1) | 5 (15.2) | 11 (16.2) | 0 (0.0) | 3 (25.0) | 3 (15.0) |
| Missing | 1 (2.9) | 0 (0.0) | 1 (1.5) | 1 (12.5) | 0 (0.0) | 1 (5.0) |
| Injury mechanism, n (%) | ||||||
| Fall – standing | 22 (12.7) | 21 (12.7) | 43 (12.7) | 6 (13.0) | 8 (14.5) | 14 (13.9) |
| Fall – walking | 20 (11.6) | 21 (12.7) | 41 (12.1) | 4 (8.7) | 3 (5.5) | 7 (6.9) |
| Fall – running | 33 (19.1) | 28 (17.0) | 61 (18.0) | 7 (15.2) | 10 (18.2) | 17 (16.8) |
| Fall – from height | 25 (14.5) | 25 (15.2) | 50 (14.8) | 3 (6.5) | 9 (16.4) | 12 (11.9) |
| Fall – from moving object | 34 (19.7) | 27 (16.4) | 61 (18.0) | 8 (17.4) | 4 (7.3) | 12 (11.9) |
| Hit on palm of hand – object striking palm | 12 (6.9) | 12 (7.3) | 24 (7.1) | 4 (8.7) | 3 (5.5) | 7 (6.9) |
| Hit on palm of hand – handle whipping back | 5 (2.9) | 8 (4.8) | 13 (3.8) | 4 (8.7) | 3 (5.5) | 7 (6.9) |
| Hit on palm of hand – other sudden extension | 9 (5.2) | 4 (2.4) | 13 (3.8) | 2 (4.3) | 4 (7.3) | 6 (5.9) |
| Punched something | 4 (2.3) | 7 (4.2) | 11 (3.3) | 0 (0.0) | 5 (9.1) | 5 (5.0) |
| Road traffic accident | 7 (4.0) | 5 (3.0) | 12 (3.6) | 2 (4.3) | 3 (5.5) | 5 (5.0) |
| Other | 2 (1.2) | 7 (4.2) | 9 (2.7) | 4 (8.7) | 3 (5.5) | 7 (6.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| Place of injury,b n (%) | ||||||
| Sport | 73 (42.2) | 60 (36.4) | 133 (39.3) | 15 (32.6) | 18 (32.7) | 33 (32.7) |
| Home | 17 (9.8) | 29 (17.6) | 46 (13.6) | 10 (21.7) | 14 (25.5) | 24 (23.8) |
| Work | 17 (9.8) | 13 (7.9) | 30 (8.9) | 5 (10.9) | 5 (9.1) | 10 (9.9) |
| Road traffic accident | 24 (13.9) | 27 (16.4) | 51 (15.1) | 2 (4.3) | 7 (12.7) | 9 (8.9) |
| Public place | 39 (22.5) | 38 (23.0) | 77 (22.8) | 10 (21.7) | 10 (18.2) | 20 (19.8) |
| Other | 2 (1.2) | 0 (0.0) | 2 (0.6) | 1 (2.2) | 0 (0.0) | 1 (1.0) |
| Missing | 3 (1.7) | 1 (0.6) | 4 (1.2) | 1 (2.2) | 1 (1.8) | 2 (2.0) |
| Treatment preference, n (%) | ||||||
| Surgery | 72 (41.6) | 76 (46.1) | 148 (43.8) | 21 (45.7) | 25 (45.5) | 46 (45.5) |
| No surgery | 10 (5.8) | 13 (7.9) | 23 (6.8) | 3 (6.5) | 6 (10.9) | 9 (8.9) |
| No preference | 90 (52.0) | 75 (45.5) | 165 (48.8) | 20 (43.5) | 24 (43.6) | 44 (43.6) |
| Missing | 1 (0.6) | 1 (0.6) | 2 (0.6) | 2 (4.3) | 0 (0.0) | 2 (2.0) |
| Characteristic | Attended visit (N = 310) | Did not attend visit (N = 129) | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 164) | Plaster cast (N = 146) | Total (N = 310) | Surgery (N = 55) | Plaster cast (N = 74) | Total (N = 129) | |
| Gender, n (%) | ||||||
| Male | 137 (83.5) | 122 (83.6) | 259 (83.5) | 43 (78.2) | 61 (82.4) | 104 (80.6) |
| Female | 27 (16.5) | 24 (16.4) | 51 (16.5) | 12 (21.8) | 13 (17.6) | 25 (19.4) |
| Age, years | ||||||
| n | 164 | 146 | 310 | 55 | 74 | 129 |
| Mean (SD) | 34.6 (13.7) | 34.6 (13.2) | 34.6 (13.4) | 27.8 (10.0) | 29.5 (9.4) | 28.7 (9.6) |
| Median (min., max.) | 31 (16, 80) | 31 (16, 76) | 31 (16, 80) | 24 (18, 57) | 26 (17, 55) | 25 (17, 57) |
| Ethnicity, n (%) | ||||||
| White | 153 (93.3) | 130 (89.0) | 283 (91.3) | 52 (94.5) | 65 (87.8) | 117 (90.7) |
| Black | 0 (0.0) | 2 (1.4) | 2 (0.6) | 0 (0.0) | 3 (4.1) | 3 (2.3) |
| Asian | 7 (4.3) | 8 (5.5) | 15 (4.8) | 0 (0.0) | 2 (2.7) | 2 (1.6) |
| Other | 4 (2.4) | 6 (4.1) | 10 (3.2) | 1 (1.8) | 4 (5.4) | 5 (3.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| Education, n (%) | ||||||
| No formal qualifications | 14 (8.5) | 21 (14.4) | 35 (11.3) | 10 (18.2) | 6 (8.1) | 16 (12.4) |
| Some qualifications/no degree | 114 (69.5) | 80 (54.8) | 194 (62.6) | 37 (67.3) | 49 (66.2) | 86 (66.7) |
| Degree or higher | 35 (21.3) | 45 (30.8) | 80 (25.8) | 6 (10.9) | 19 (25.7) | 25 (19.4) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| Employment status, n (%) | ||||||
| Part-time | 15 (9.1) | 14 (9.6) | 29 (9.4) | 5 (9.1) | 4 (5.4) | 9 (7.0) |
| Full-time | 105 (64.0) | 86 (58.9) | 191 (61.6) | 22 (40.0) | 34 (45.9) | 56 (43.4) |
| Self-employed | 14 (8.5) | 19 (13.0) | 33 (10.6) | 7 (12.7) | 17 (23.0) | 24 (18.6) |
| Student | 12 (7.3) | 11 (7.5) | 23 (7.4) | 8 (14.5) | 10 (13.5) | 18 (14.0) |
| Retired | 7 (4.3) | 5 (3.4) | 12 (3.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Looking after family/home | 0 (0.0) | 4 (2.7) | 4 (1.3) | 1 (1.8) | 2 (2.7) | 3 (2.3) |
| Not employed but seeking work | 3 (1.8) | 3 (2.1) | 6 (1.9) | 6 (10.9) | 2 (2.7) | 8 (6.2) |
| Other | 7 (4.3) | 4 (2.7) | 11 (3.5) | 4 (7.3) | 5 (6.8) | 9 (7.0) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| Type of employment, n (%) | ||||||
| Unskilled manual | 17 (10.4) | 10 (6.8) | 27 (8.7) | 8 (14.5) | 13 (17.6) | 21 (16.3) |
| Skilled manual | 48 (29.3) | 39 (26.7) | 87 (28.1) | 15 (27.3) | 21 (28.4) | 36 (27.9) |
| Unskilled non-manual | 18 (11.0) | 7 (4.8) | 25 (8.1) | 1 (1.8) | 5 (6.8) | 6 (4.7) |
| Skilled non-manual | 27 (16.5) | 37 (25.3) | 64 (20.6) | 6 (10.9) | 9 (12.2) | 15 (11.6) |
| Professional | 17 (10.4) | 13 (8.9) | 30 (9.7) | 3 (5.5) | 6 (8.1) | 9 (7.0) |
| Other | 16 (9.8) | 19 (13.0) | 35 (11.3) | 3 (5.5) | 11 (14.9) | 14 (10.9) |
| Missing | 21 (12.8) | 21 (14.4) | 42 (13.5) | 19 (34.5) | 9 (12.2) | 28 (21.7) |
| Current smoker, n (%) | ||||||
| Yes | 49 (29.9) | 27 (18.5) | 76 (24.5) | 24 (43.6) | 29 (39.2) | 53 (41.1) |
| No | 114 (69.5) | 118 (80.8) | 232 (74.8) | 29 (52.7) | 45 (60.8) | 74 (57.4) |
| Missing | 1 (0.6) | 1 (0.7) | 2 (0.6) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| If yes | ||||||
| Number of cigarettes per day, median (min., max.) | 10 (1, 40) | 10 (1, 30) | 10 (1, 40) | 10 (1, 20) | 10 (4, 25) | 10 (1, 25) |
| Number of years, median (min., max.) | 10 (1, 50) | 11 (1, 36) | 10 (1, 50) | 9 (1, 40) | 8 (2, 30) | 8 (1, 40) |
| Past smoker, n (%) | ||||||
| Yes | 86 (52.4) | 62 (42.5) | 148 (47.7) | 30 (54.5) | 47 (63.5) | 77 (59.7) |
| No | 68 (41.5) | 78 (53.4) | 146 (47.1) | 17 (30.9) | 23 (31.1) | 40 (31.0) |
| Missing | 10 (6.1) | 6 (4.1) | 16 (5.2) | 8 (14.5) | 4 (5.4) | 12 (9.3) |
| Diabetes, n (%) | ||||||
| Yes | 6 (3.7) | 4 (2.7) | 10 (3.2) | 1 (1.8) | 0 (0.0) | 1 (0.8) |
| No | 157 (95.7) | 142 (97.3) | 299 (96.5) | 52 (94.5) | 74 (100.0) | 126 (97.7) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| Steroid use, n (%) | ||||||
| Yes | 5 (3.0) | 2 (1.4) | 7 (2.3) | 1 (1.8) | 2 (2.7) | 3 (2.3) |
| No | 158 (96.3) | 144 (98.6) | 302 (97.4) | 52 (94.5) | 72 (97.3) | 124 (96.1) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| Characteristic | Attended visit (N = 310) | Did not attend visit (N = 129) | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 164) | Plaster cast (N = 146) | Total (N = 310) | Surgery (N = 55) | Plaster cast (N = 74) | Total (N = 129) | |
| Time since injury, daysa | ||||||
| n | 164 | 146 | 310 | 55 | 74 | 129 |
| Mean (SD) | 4.9 (3.1) | 5.3 (3.4) | 5.1 (3.3) | 5.7 (3.1) | 5.4 (3.2) | 5.5 (3.1) |
| Median (min., max.) | 4 (1, 14) | 4 (1, 14) | 4 (1, 14) | 5 (1, 14) | 5 (0, 14) | 5 (0, 14) |
| Affected wrist, n (%) | ||||||
| Left | 86 (52.4) | 77 (52.7) | 163 (52.6) | 29 (52.7) | 41 (55.4) | 70 (54.3) |
| Right | 78 (47.6) | 69 (47.3) | 147 (47.4) | 26 (47.3) | 33 (44.6) | 59 (45.7) |
| Affected wrist is dominant hand, n (%) | ||||||
| Yes | 73 (44.5) | 69 (47.3) | 142 (45.8) | 27 (49.1) | 26 (35.1) | 53 (41.1) |
| No | 91 (55.5) | 77 (52.7) | 168 (54.2) | 26 (47.3) | 48 (64.9) | 74 (57.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| Displacement (eligibility), n (%) | ||||||
| No displacement | 96 (58.5) | 86 (58.9) | 182 (58.7) | 39 (70.9) | 48 (64.9) | 87 (67.4) |
| Displacement | 68 (41.5) | 60 (41.1) | 128 (41.3) | 16 (29.1) | 26 (35.1) | 42 (32.6) |
| Displacement (randomisation), n (%) | ||||||
| No displacement | 94 (57.3) | 83 (56.8) | 177 (57.1) | 37 (67.3) | 47 (63.5) | 84 (65.1) |
| Displacement | 70 (42.7) | 63 (43.2) | 133 (42.9) | 18 (32.7) | 27 (36.5) | 45 (34.9) |
| Radiographs,b n (%) | ||||||
| Elongated scaphoid view | 157 (95.7) | 138 (94.5) | 295 (95.2) | 52 (94.5) | 72 (97.3) | 124 (96.1) |
| Posterior–anterior view | 161 (98.2) | 145 (99.3) | 306 (98.7) | 54 (98.2) | 73 (98.6) | 127 (98.4) |
| 45° semisupine view | 118 (72.0) | 111 (76.0) | 229 (73.9) | 41 (74.5) | 55 (74.3) | 96 (74.4) |
| Lateral view | 164 (100.0) | 144 (98.6) | 308 (99.4) | 54 (98.2) | 73 (98.6) | 127 (98.4) |
| 45° semiprone view | 148 (90.2) | 132 (90.4) | 280 (90.3) | 50 (90.9) | 64 (86.5) | 114 (88.4) |
| Previous wrist problems on same side, n (%) | ||||||
| Yes | 34 (20.7) | 30 (20.5) | 64 (20.6) | 9 (16.4) | 15 (20.3) | 24 (18.6) |
| No | 129 (78.7) | 115 (78.8) | 244 (78.7) | 44 (80.0) | 58 (78.4) | 102 (79.1) |
| Missing | 1 (0.6) | 1 (0.7) | 2 (0.6) | 2 (3.6) | 1 (1.4) | 3 (2.3) |
| If yes, what injury?, n (%) | ||||||
| Previous fracture | 16 (47.1) | 18 (60.0) | 34 (53.1) | 7 (77.8) | 10 (66.7) | 17 (70.8) |
| Arthritis | 2 (5.9) | 1 (3.3) | 3 (4.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ligament, tendon or nerve injury | 8 (23.5) | 7 (23.3) | 15 (23.4) | 2 (22.2) | 1 (6.7) | 3 (12.5) |
| Other | 6 (17.6) | 4 (13.3) | 10 (15.6) | 0 (0.0) | 4 (26.7) | 4 (16.7) |
| Missing | 2 (5.9) | 0 (0.0) | 2 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injury mechanism, n (%) | ||||||
| Fall – standing | 21 (12.8) | 19 (13.0) | 40 (12.9) | 7 (12.7) | 10 (13.5) | 17 (13.2) |
| Fall – walking | 19 (11.6) | 20 (13.7) | 39 (12.6) | 5 (9.1) | 4 (5.4) | 9 (7.0) |
| Fall – running | 32 (19.5) | 28 (19.2) | 60 (19.4) | 8 (14.5) | 10 (13.5) | 18 (14.0) |
| Fall – from height | 24 (14.6) | 20 (13.7) | 44 (14.2) | 4 (7.3) | 14 (18.9) | 18 (14.0) |
| Fall – from moving object | 32 (19.5) | 21 (14.4) | 53 (17.1) | 10 (18.2) | 10 (13.5) | 20 (15.5) |
| Hit on palm of hand – object striking palm | 10 (6.1) | 12 (8.2) | 22 (7.1) | 6 (10.9) | 3 (4.1) | 9 (7.0) |
| Hit on palm of hand – handle whipping back | 6 (3.7) | 9 (6.2) | 15 (4.8) | 3 (5.5) | 2 (2.7) | 5 (3.9) |
| Hit on palm of hand – other sudden extension | 8 (4.9) | 4 (2.7) | 12 (3.9) | 3 (5.5) | 4 (5.4) | 7 (5.4) |
| Punched something | 2 (1.2) | 6 (4.1) | 8 (2.6) | 2 (3.6) | 6 (8.1) | 8 (6.2) |
| Road traffic accident | 7 (4.3) | 3 (2.1) | 10 (3.2) | 2 (3.6) | 5 (6.8) | 7 (5.4) |
| Other | 3 (1.8) | 4 (2.7) | 7 (2.3) | 3 (5.5) | 6 (8.1) | 9 (7.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| Place of injury,b n (%) | ||||||
| Sport | 66 (40.2) | 53 (36.3) | 119 (38.4) | 22 (40.0) | 25 (33.8) | 47 (36.4) |
| Home | 20 (12.2) | 28 (19.2) | 48 (15.5) | 7 (12.7) | 15 (20.3) | 22 (17.1) |
| Work | 16 (9.8) | 13 (8.9) | 29 (9.4) | 6 (10.9) | 5 (6.8) | 11 (8.5) |
| Road traffic accident | 20 (12.2) | 22 (15.1) | 42 (13.5) | 6 (10.9) | 12 (16.2) | 18 (14.0) |
| Public place | 40 (24.4) | 31 (21.2) | 71 (22.9) | 9 (16.4) | 17 (23.0) | 26 (20.2) |
| Other | 1 (0.6) | 0 (0.0) | 1 (0.3) | 2 (3.6) | 0 (0.0) | 2 (1.6) |
| Missing | 3 (1.8) | 2 (1.4) | 5 (1.6) | 1 (1.8) | 0 (0.0) | 1 (0.8) |
| Treatment preference, n (%) | ||||||
| Surgery | 71 (43.3) | 67 (45.9) | 138 (44.5) | 22 (40.0) | 34 (45.9) | 56 (43.4) |
| No surgery | 10 (6.1) | 9 (6.2) | 19 (6.1) | 3 (5.5) | 10 (13.5) | 13 (10.1) |
| No preference | 83 (50.6) | 69 (47.3) | 152 (49.0) | 27 (49.1) | 30 (40.5) | 57 (44.2) |
| Missing | 0 (0.0) | 1 (0.7) | 1 (0.3) | 3 (5.5) | 0 (0.0) | 3 (2.3) |
| Characteristic | 6 weeks | 12 weeks | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 176) | Plaster cast (N = 172) | Total (N = 348) | Surgery (N = 178) | Plaster cast (N = 163) | Total (N = 341) | |
| Gender, n (%) | ||||||
| Male | 143 (81.3) | 141 (82.0) | 284 (81.6) | 150 (84.3) | 134 (82.2) | 284 (83.3) |
| Female | 33 (18.8) | 31 (18.0) | 64 (18.4) | 28 (15.7) | 29 (17.8) | 57 (16.7) |
| Age, years | ||||||
| n | 176 | 172 | 348 | 178 | 163 | 341 |
| Mean (SD) | 33.5 (13.3) | 33.3 (12.9) | 33.4 (13.1) | 33.4 (13.1) | 33.4 (12.8) | 33.4 (13.0) |
| Median (min., max.) | 30 (16, 80) | 30 (16, 76) | 30 (16, 80) | 30 (16, 80) | 30 (16, 76) | 30 (16, 80) |
| Ethnicity, n (%) | ||||||
| White | 166 (94.3) | 150 (87.2) | 316 (90.8) | 167 (93.8) | 143 (87.7) | 310 (90.9) |
| Black | 0 (0.0) | 5 (2.9) | 5 (1.4) | 0 (0.0) | 3 (1.8) | 3 (0.9) |
| Asian | 6 (3.4) | 7 (4.1) | 13 (3.7) | 7 (3.9) | 7 (4.3) | 14 (4.1) |
| Other | 4 (2.3) | 10 (5.8) | 14 (4.0) | 4 (2.2) | 10 (6.1) | 14 (4.1) |
| Education, n (%) | ||||||
| No formal qualifications | 19 (10.8) | 21 (12.2) | 40 (11.5) | 17 (9.6) | 21 (12.9) | 38 (11.1) |
| Some qualifications/no degree | 121 (68.8) | 95 (55.2) | 216 (62.1) | 119 (66.9) | 91 (55.8) | 210 (61.6) |
| Degree or higher | 35 (19.9) | 56 (32.6) | 91 (26.1) | 41 (23.0) | 51 (31.3) | 92 (27.0) |
| Missing | 1 (0.6) | 0 (0) | 1 (0.3) | 1 (0.6) | 0 (0.0) | 1 (0.3) |
| Employment status, n (%) | ||||||
| Part-time | 17 (9.7) | 15 (8.7) | 32 (9.2) | 18 (10.1) | 14 (8.6) | 32 (9.4) |
| Full-time | 106 (60.2) | 92 (53.5) | 198 (56.9) | 109 (61.2) | 94 (57.7) | 203 (59.5) |
| Self-employed | 15 (8.5) | 25 (14.5) | 40 (11.5) | 17 (9.6) | 21 (12.9) | 38 (11.1) |
| Student | 16 (9.1) | 19 (11.0) | 35 (10.1) | 14 (7.9) | 18 (11.0) | 32 (9.4) |
| Retired | 6 (3.4) | 5 (2.9) | 11 (3.2) | 7 (3.9) | 5 (3.1) | 12 (3.5) |
| Looking after family/home | 0 (0.0) | 5 (2.9) | 5 (1.4) | 0 (0.0) | 4 (2.5) | 4 (1.2) |
| Not employed but seeking work | 5 (2.8) | 5 (2.9) | 10 (2.9) | 6 (3.4) | 3 (1.8) | 9 (2.6) |
| Other | 10 (5.7) | 6 (3.5) | 16 (4.6) | 6 (3.4) | 4 (2.5) | 10 (2.9) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 1 (0.6) | 0 (0.0) | 1 (0.3) |
| Type of employment, n (%) | ||||||
| Unskilled manual | 20 (11.4) | 13 (7.6) | 33 (9.5) | 19 (10.7) | 12 (7.4) | 31 (9.1) |
| Skilled manual | 52 (29.5) | 49 (28.5) | 101 (29.0) | 53 (29.8) | 41 (25.2) | 94 (27.6) |
| Unskilled non-manual | 15 (8.5) | 8 (4.7) | 23 (6.6) | 14 (7.9) | 10 (6.1) | 24 (7.0) |
| Skilled non-manual | 28 (15.9) | 34 (19.8) | 62 (17.8) | 30 (16.9) | 37 (22.7) | 67 (19.6) |
| Professional | 18 (10.2) | 15 (8.7) | 33 (9.5) | 19 (10.7) | 14 (8.6) | 33 (9.7) |
| Other | 17 (9.7) | 25 (14.5) | 42 (12.1) | 15 (8.4) | 24 (14.7) | 39 (11.4) |
| Missing | 26 (14.8) | 28 (16.3) | 54 (15.5) | 28 (15.7) | 25 (15.3) | 53 (15.5) |
| Current smoker, n (%) | ||||||
| Yes | 50 (28.4) | 37 (21.5) | 87 (25.0) | 53 (29.8) | 31 (19.0) | 84 (24.6) |
| No | 126 (71.6) | 134 (77.9) | 260 (74.7) | 124 (69.7) | 131 (80.4) | 255 (74.8) |
| Missing | 0 (0.0) | 1 (0.6) | 1 (0.3) | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| If yes | ||||||
| Number of cigarettes per day, median (min., max.) | 10 (1, 40) | 10 (1, 30) | 10 (1, 40) | 10 (1, 20) | 10 (1, 30) | 10 (1, 30) |
| Number of years, median (min., max.) | 10 (1, 50) | 10 (1, 30) | 10 (1, 50) | 10 (1, 50) | 10 (1, 36) | 10 (1, 50) |
| Past smoker, n (%) | ||||||
| Yes | 95 (54) | 80 (46.5) | 175 (50.3) | 95 (53.4) | 69 (42.3) | 164 (48.1) |
| No | 73 (41.5) | 83 (48.3) | 156 (44.8) | 71 (39.9) | 87 (53.4) | 158 (46.3) |
| Missing | 8 (4.5) | 9 (5.2) | 17 (4.9) | 12 (6.7) | 7 (4.3) | 19 (5.6) |
| Diabetes, n (%) | ||||||
| Yes | 6 (3.4) | 3 (1.7) | 9 (2.6) | 6 (3.4) | 4 (2.5) | 10 (2.9) |
| No | 170 (96.6) | 169 (98.3) | 339 (97.4) | 171 (96.1) | 159 (97.5) | 330 (96.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 1 (0.3) |
| Steroid use, n (%) | ||||||
| Yes | 5 (2.8) | 3 (1.7) | 8 (2.3) | 4 (2.2) | 4 (2.5) | 8 (2.3) |
| No | 171 (97.2) | 169 (98.3) | 340 (97.7) | 173 (97.2) | 159 (97.5) | 332 (97.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 1 (0.3) |
| Characteristic | 6 weeks | 12 weeks | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 176) | Plaster cast (N = 172) | Total (N = 348) | Surgery (N = 178) | Plaster cast (N = 163) | Total (N = 341) | |
| Time since injury, daysa | ||||||
| n | 176 | 172 | 348 | 178 | 163 | 341 |
| Mean (SD) | 4.8 (3.0) | 5.4 (3.5) | 5.1 (3.3) | 4.9 (3.0) | 5.5 (3.4) | 5.2 (3.2) |
| Median (min., max.) | 4 (1, 4) | 5 (0, 14) | 4 (0, 14) | 4 (1, 14) | 5 (1, 14) | 5 (1, 14) |
| Affected wrist, n (%) | ||||||
| Left | 95 (54.0) | 96 (55.8) | 191 (54.9) | 97 (54.5) | 89 (54.6) | 186 (54.5) |
| Right | 81 (46.0) | 76 (44.2) | 157 (45.1) | 81 (45.5) | 74 (45.4) | 155 (45.5) |
| Affected wrist is dominant hand, n (%) | ||||||
| Yes | 80 (45.5) | 73 (42.4) | 153 (44) | 80 (44.9) | 69 (42.3) | 149 (43.7) |
| No | 96 (54.5) | 99 (57.6) | 195 (56) | 98 (55.1) | 94 (57.7) | 192 (56.3) |
| Displacement (eligibility), n (%) | ||||||
| No displacement | 109 (61.9) | 107 (62.2) | 216 (62.1) | 106 (59.6) | 102 (62.6) | 208 (61.0) |
| Displacement | 67 (38.1) | 65 (37.8) | 132 (37.9) | 72 (40.4) | 61 (37.4) | 133 (39.0) |
| Displacement (randomisation), n (%) | ||||||
| No displacement | 105 (59.7) | 103 (59.9) | 208 (59.8) | 104 (58.4) | 100 (61.3) | 204 (59.8) |
| Displacement | 71 (40.3) | 69 (40.1) | 140 (40.2) | 74 (41.6) | 63 (38.7) | 137 (40.2) |
| Radiographs,b n (%) | ||||||
| Elongated scaphoid view | 168 (95.5) | 163 (94.8) | 331 (95.1) | 168 (94.4) | 155 (95.1) | 323 (94.7) |
| Posterior–anterior view | 174 (98.9) | 170 (98.8) | 344 (98.9) | 175 (98.3) | 161 (98.8) | 336 (98.5) |
| 45° semisupine view | 124 (70.5) | 129 (75.0) | 253 (72.7) | 123 (69.1) | 121 (74.2) | 244 (71.6) |
| Lateral view | 176 (100.0) | 169 (98.3) | 345 (99.1) | 178 (100.0) | 160 (98.2) | 338 (99.1) |
| 45° semiprone view | 158 (89.8) | 154 (89.5) | 312 (89.7) | 161 (90.4) | 145 (89.0) | 306 (89.7) |
| Previous wrist problems on same side, n (%) | ||||||
| Yes | 36 (20.5) | 35 (20.3) | 71 (20.4) | 37 (20.8) | 35 (21.5) | 72 (21.1) |
| No | 139 (79.0) | 135 (78.5) | 274 (78.7) | 140 (78.7) | 128 (78.5) | 268 (78.6) |
| Missing | 1 (0.6) | 2 (1.2) | 3 (0.9) | 1 (0.6) | 0 (0.0) | 1 (0.3) |
| If yes, what injury?, n (%) | ||||||
| Previous fracture | 21 (58.3) | 20 (57.1) | 41 (57.7) | 20 (54.1) | 21 (60.0) | 41 (56.9) |
| Arthritis | 1 (2.8) | 1 (2.9) | 2 (2.8) | 1 (2.7) | 1 (2.9) | 2 (2.8) |
| Ligament, tendon or nerve injury | 8 (22.2) | 7 (20) | 15 (21.1) | 9 (24.3) | 8 (22.9) | 17 (23.6) |
| Other | 6 (16.7) | 7 (20) | 13 (18.3) | 6 (16.2) | 5 (14.3) | 11 (15.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.7) | 0 (0.0) | 1 (1.4) |
| Injury mechanism, n (%) | ||||||
| Fall – standing | 19 (10.8) | 23 (13.4) | 42 (12.1) | 23 (12.9) | 19 (11.7) | 42 (12.3) |
| Fall – walking | 21 (11.9) | 20 (11.6) | 41 (11.8) | 22 (12.4) | 17 (10.4) | 39 (11.4) |
| Fall – running | 32 (18.2) | 28 (16.3) | 60 (17.2) | 35 (19.7) | 28 (17.2) | 63 (18.5) |
| Fall – from height | 22 (12.5) | 28 (16.3) | 50 (14.4) | 22 (12.4) | 27 (16.6) | 49 (14.4) |
| Fall – from moving object | 37 (21.0) | 27 (15.7) | 64 (18.4) | 35 (19.7) | 29 (17.8) | 64 (18.8) |
| Hit on palm of hand – object striking palm | 14 (8.0) | 11 (6.4) | 25 (7.2) | 12 (6.7) | 13 (8.0) | 25 (7.3) |
| Hit on palm of hand – handle whipping back | 6 (3.4) | 9 (5.2) | 15 (4.3) | 5 (2.8) | 8 (4.9) | 13 (3.8) |
| Hit on palm of hand – other sudden extension | 10 (5.7) | 5 (2.9) | 15 (4.3) | 9 (5.1) | 3 (1.8) | 12 (3.5) |
| Punched something | 3 (1.7) | 7 (4.1) | 10 (2.9) | 4 (2.2) | 6 (3.7) | 10 (2.9) |
| Road traffic accident | 9 (5.1) | 6 (3.5) | 15 (4.3) | 9 (5.1) | 6 (3.7) | 15 (4.4) |
| Other | 3 (1.7) | 8 (4.7) | 11 (3.2) | 2 (1.1) | 7 (4.3) | 9 (2.6) |
| Place of injury,b n (%) | ||||||
| Sport | 74 (42.0) | 61 (35.5) | 135 (38.8) | 77 (43.3) | 61 (37.4) | 138 (40.5) |
| Home | 20 (11.4) | 31 (18.0) | 51 (14.7) | 19 (10.7) | 26 (16.0) | 45 (13.2) |
| Work | 17 (9.7) | 16 (9.3) | 33 (9.5) | 18 (10.1) | 14 (8.6) | 32 (9.4) |
| Road traffic accident | 23 (13.1) | 27 (15.7) | 50 (14.4) | 21 (11.8) | 29 (17.8) | 50 (14.7) |
| Public place | 39 (22.2) | 38 (22.1) | 77 (22.1) | 39 (21.9) | 36 (22.1) | 75 (22.0) |
| Other | 2 (1.1) | 0 (0.0) | 2 (0.6) | 2 (1.1) | 0 (0.0) | 2 (0.6) |
| Missing | 3 (1.7) | 2 (1.2) | 5 (1.4) | 3 (1.7) | 0 (0.0) | 3 (0.9) |
| Treatment preference, n (%) | ||||||
| Surgery | 75 (42.6) | 76 (44.2) | 151 (43.4) | 77 (43.3) | 75 (46.0) | 152 (44.6) |
| No surgery | 10 (5.7) | 14 (8.1) | 24 (6.9) | 10 (5.6) | 15 (9.2) | 25 (7.3) |
| No preference | 90 (51.1) | 81 (47.1) | 171 (49.1) | 91 (51.1) | 72 (44.2) | 163 (47.8) |
| Missing | 1 (0.6) | 1 (0.6) | 2 (0.6) | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| Characteristic | 26 weeks | 52 weeks | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 156) | Plaster cast (N = 146) | Total (N = 302) | Surgery (N = 186) | Plaster cast (N = 176) | Total (N = 362) | |
| Gender, n (%) | ||||||
| Male | 129 (82.7) | 119 (81.5) | 248 (82.1) | 153 (82.3) | 140 (79.5) | 293 (80.9) |
| Female | 27 (17.3) | 27 (18.5) | 54 (17.9) | 33 (17.7) | 36 (20.5) | 69 (19.1) |
| Age (years) | ||||||
| n | 156 | 146 | 302 | 186 | 176 | 362 |
| Mean (SD) | 33.2 (13.2) | 34.0 (13.3) | 33.6 (13.2) | 33.9 (13.4) | 33.9 (12.9) | 33.9 (13.2) |
| Median (min., max.) | 30 (16, 80) | 30 (16, 76) | 30 (16, 80) | 30 (16, 80) | 30 (16, 76) | 30 (16, 80) |
| Ethnicity, n (%) | ||||||
| White | 148 (94.9) | 129 (88.4) | 277 (91.7) | 175 (94.1) | 156 (88.6) | 331 (91.4) |
| Black | 0 (0) | 3 (2.1) | 3 (1) | 0 (0.0) | 3 (1.7) | 3 (0.8) |
| Asian | 6 (3.8) | 7 (4.8) | 13 (4.3) | 7 (3.8) | 9 (5.1) | 16 (4.4) |
| Other | 2 (1.3) | 7 (4.8) | 9 (3) | 4 (2.2) | 8 (4.5) | 12 (3.3) |
| Education, n (%) | ||||||
| No formal qualifications | 15 (9.6) | 18 (12.3) | 33 (10.9) | 17 (9.1) | 23 (13.1) | 40 (11.0) |
| Some qualifications/no degree | 105 (67.3) | 83 (56.8) | 188 (62.3) | 128 (68.8) | 100 (56.8) | 228 (63.0) |
| Degree or higher | 35 (22.4) | 45 (30.8) | 80 (26.5) | 40 (21.5) | 53 (30.1) | 93 (25.7) |
| Missing | 1 (0.6) | 0 (0) | 1 (0.3) | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| Employment status, n (%) | ||||||
| Part-time | 16 (10.3) | 15 (10.3) | 31 (10.3) | 19 (10.2) | 16 (9.1) | 35 (9.7) |
| Full-time | 91 (58.3) | 81 (55.5) | 172 (57) | 114 (61.3) | 98 (55.7) | 212 (58.6) |
| Self-employed | 13 (8.3) | 20 (13.7) | 33 (10.9) | 16 (8.6) | 27 (15.3) | 43 (11.9) |
| Student | 15 (9.6) | 15 (10.3) | 30 (9.9) | 16 (8.6) | 17 (9.7) | 33 (9.1) |
| Retired | 6 (3.8) | 5 (3.4) | 11 (3.6) | 7 (3.8) | 5 (2.8) | 12 (3.3) |
| Looking after family/home | 0 (0) | 4 (2.7) | 4 (1.3) | 0 (0.0) | 5 (2.8) | 5 (1.4) |
| Not employed but seeking work | 7 (4.5) | 2 (1.4) | 9 (3) | 5 (2.7) | 4 (2.3) | 9 (2.5) |
| Other | 7 (4.5) | 4 (2.7) | 11 (3.6) | 8 (4.3) | 4 (2.3) | 12 (3.3) |
| Missing | 1 (0.6) | 0 (0) | 1 (0.3) | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| Type of employment, n (%) | ||||||
| Unskilled manual | 17 (10.9) | 12 (8.2) | 29 (9.6) | 23 (12.4) | 15 (8.5) | 38 (10.5) |
| Skilled manual | 45 (28.8) | 39 (26.7) | 84 (27.8) | 51 (27.4) | 43 (24.4) | 94 (26.0) |
| Unskilled non-manual | 13 (8.3) | 9 (6.2) | 22 (7.3) | 19 (10.2) | 10 (5.7) | 29 (8.0) |
| Skilled non-manual | 26 (16.7) | 30 (20.5) | 56 (18.5) | 30 (16.1) | 42 (23.9) | 72 (19.9) |
| Professional | 15 (9.6) | 14 (9.6) | 29 (9.6) | 19 (10.2) | 17 (9.7) | 36 (9.9) |
| Other | 13 (8.3) | 19 (13) | 32 (10.6) | 18 (9.7) | 23 (13.1) | 41 (11.3) |
| Missing | 27 (17.3) | 23 (15.8) | 50 (16.6) | 26 (14.0) | 26 (14.8) | 52 (14.4) |
| Current smoker, n (%) | ||||||
| Yes | 43 (27.6) | 25 (17.1) | 68 (22.5) | 56 (30.1) | 35 (19.9) | 91 (25.1) |
| No | 112 (71.8) | 120 (82.2) | 232 (76.8) | 129 (69.4) | 140 (79.5) | 269 (74.3) |
| Missing | 1 (0.6) | 1 (0.7) | 2 (0.7) | 1 (0.5) | 1 (0.6) | 2 (0.6) |
| If yes | ||||||
| Number of cigarettes per day, median (min., max.) | 10 (1, 40) | 10 (1, 25) | 10 (1, 40) | 10 (1, 40) | 10 (1, 30) | 10 (1, 40) |
| Number of years, median (min., max.) | 10 (1, 40) | 8 (1, 36) | 10 (1, 40) | 10 (1, 50) | 10 (1, 36) | 10 (1, 50) |
| Past smoker, n (%) | ||||||
| Yes | 78 (50) | 60 (41.1) | 138 (45.7) | 98 (52.7) | 77 (43.8) | 175 (48.3) |
| No | 66 (42.3) | 80 (54.8) | 146 (48.3) | 76 (40.9) | 92 (52.3) | 168 (46.4) |
| Missing | 12 (7.7) | 6 (4.1) | 18 (6) | 12 (6.5) | 7 (4.0) | 19 (5.2) |
| Diabetes, n (%) | ||||||
| Yes | 5 (3.2) | 4 (2.7) | 9 (3) | 6 (3.2) | 4 (2.3) | 10 (2.8) |
| No | 150 (96.2) | 142 (97.3) | 292 (96.7) | 179 (96.2) | 172 (97.7) | 351 (97.0) |
| Missing | 1 (0.6) | 0 (0) | 1 (0.3) | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| Steroid use, n (%) | ||||||
| Yes | 4 (2.6) | 3 (2.1) | 7 (2.3) | 6 (3.2) | 4 (2.3) | 10 (2.8) |
| No | 151 (96.8) | 143 (97.9) | 294 (97.4) | 179 (96.2) | 172 (97.7) | 351 (97.0) |
| Missing | 1 (0.6) | 0 (0) | 1 (0.3) | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| Characteristic | 26 weeks | 52 weeks | ||||
|---|---|---|---|---|---|---|
| Surgery (N = 156) | Plaster cast (N = 146) | Total (N = 302) | Surgery (N = 186) | Plaster cast (N = 176) | Total (N = 362) | |
| Time since injury, daysa | ||||||
| n | 156 | 146 | 302 | 186 | 176 | 362 |
| Mean (SD) | 4.7 (3.0) | 5.3 (3.4) | 5.0 (3.2) | 4.8 (3.0) | 5.3 (3.4) | 5.1 (3.2) |
| Median (min., max.) | 4 (1, 13) | 4 (1, 14) | 4 (1, 14) | 4 (1, 14) | 4 (0, 14) | 4 (0, 14) |
| Affected wrist, n (%) | ||||||
| Left | 82 (52.6) | 82 (56.2) | 164 (54.3) | 101 (54.3) | 94 (53.4) | 195 (53.9) |
| Right | 74 (47.4) | 64 (43.8) | 138 (45.7) | 85 (45.7) | 82 (46.6) | 167 (46.1) |
| Affected wrist is dominant hand, n (%) | ||||||
| Yes | 72 (46.2) | 62 (42.5) | 134 (44.4) | 83 (44.6) | 78 (44.3) | 161 (44.5) |
| No | 84 (53.8) | 84 (57.5) | 168 (55.6) | 103 (55.4) | 98 (55.7) | 201 (55.5) |
| Displacement (eligibility), n (%) | ||||||
| No displacement | 96 (61.5) | 89 (61.0) | 185 (61.3) | 114 (61.3) | 106 (60.2) | 220 (60.8) |
| Displacement | 60 (38.5) | 57 (39.0) | 117 (38.7) | 72 (38.7) | 70 (39.8) | 142 (39.2) |
| Displacement (randomisation), n (%) | ||||||
| No displacement | 92 (59.0) | 88 (60.3) | 180 (59.6) | 110 (59.1) | 103 (58.5) | 213 (58.8) |
| Displacement | 64 (41.0) | 58 (39.7) | 122 (40.4) | 76 (40.9) | 73 (41.5) | 149 (41.2) |
| Radiographs,b n (%) | ||||||
| Elongated scaphoid view | 147 (94.2) | 138 (94.5) | 285 (94.4) | 177 (95.2) | 167 (94.9) | 344 (95.0) |
| Posterior–anterior view | 154 (98.7) | 145 (99.3) | 299 (99.0) | 183 (98.4) | 174 (98.9) | 357 (98.6) |
| 45° semisupine view | 109 (69.9) | 109 (74.7) | 218 (72.2) | 131 (70.4) | 131 (74.4) | 262 (72.4) |
| Lateral view | 156 (100.0) | 144 (98.6) | 300 (99.3) | 186 (100.0) | 173 (98.3) | 359 (99.2) |
| 45° semiprone view | 139 (89.1) | 133 (91.1) | 272 (90.1) | 169 (90.9) | 158 (89.8) | 327 (90.3) |
| Previous wrist problems on same side, n (%) | ||||||
| Yes | 34 (21.8) | 35 (24.0) | 69 (22.8) | 37 (19.9) | 37 (21) | 74 (20.4) |
| No | 121 (77.6) | 111 (76.0) | 232 (76.8) | 148 (79.6) | 138 (78.4) | 286 (79.0) |
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.3) | 1 (0.5) | 1 (0.6) | 2 (0.6) |
| If yes, what injury?, n (%) | ||||||
| Previous fracture | 20 (58.8) | 22 (62.9) | 42 (60.9) | 21 (56.8) | 23 (62.2) | 44 (59.5) |
| Arthritis | 2 (5.9) | 1 (2.9) | 3 (4.3) | 1 (2.7) | 1 (2.7) | 2 (2.7) |
| Ligament, tendon or nerve injury | 6 (17.6) | 8 (22.9) | 14 (20.3) | 7 (18.9) | 8 (21.6) | 15 (20.3) |
| Other | 5 (14.7) | 4 (11.4) | 9 (13.0) | 6 (16.2) | 5 (13.5) | 11 (14.9) |
| Missing | 1 (2.9) | 0 (0.0) | 1 (1.4) | 2 (5.4) | 0 (0) | 2 (2.7) |
| Injury mechanism, n (%) | ||||||
| Fall – standing | 18 (11.5) | 22 (15.1) | 40 (13.2) | 26 (14.0) | 23 (13.1) | 49 (13.5) |
| Fall – walking | 16 (10.3) | 18 (12.3) | 34 (11.3) | 20 (10.8) | 20 (11.4) | 40 (11.0) |
| Fall – running | 32 (20.5) | 26 (17.8) | 58 (19.2) | 36 (19.4) | 28 (15.9) | 64 (17.7) |
| Fall – from height | 17 (10.9) | 20 (13.7) | 37 (12.3) | 23 (12.4) | 25 (14.2) | 48 (13.3) |
| Fall – from moving object | 35 (22.4) | 26 (17.8) | 61 (20.2) | 36 (19.4) | 29 (16.5) | 65 (18.0) |
| Hit on palm of hand – object striking palm | 13 (8.3) | 8 (5.5) | 21 (7.0) | 12 (6.5) | 13 (7.4) | 25 (6.9) |
| Hit on palm of hand – handle whipping back | 7 (4.5) | 8 (5.5) | 15 (5.0) | 7 (3.8) | 10 (5.7) | 17 (4.7) |
| Hit on palm of hand – other sudden extension | 7 (4.5) | 2 (1.4) | 9 (3.0) | 10 (5.4) | 6 (3.4) | 16 (4.4) |
| Punched something | 1 (0.6) | 6 (4.1) | 7 (2.3) | 3 (1.6) | 8 (4.5) | 11 (3) |
| Road traffic accident | 8 (5.1) | 4 (2.7) | 12 (4.0) | 9 (4.8) | 6 (3.4) | 15 (4.1) |
| Other | 2 (1.3) | 6 (4.1) | 8 (2.6) | 4 (2.2) | 8 (4.5) | 12 (3.3) |
| Place of injury,b n (%) | ||||||
| Sport | 70 (44.9) | 55 (37.7) | 125 (41.4) | 76 (40.9) | 63 (35.8) | 139 (38.4) |
| Home | 16 (10.3) | 25 (17.1) | 41 (13.6) | 22 (11.8) | 34 (19.3) | 56 (15.5) |
| Work | 15 (9.6) | 12 (8.2) | 27 (8.9) | 19 (10.2) | 15 (8.5) | 34 (9.4) |
| Road traffic accident | 21 (13.5) | 23 (15.8) | 44 (14.6) | 22 (11.8) | 30 (17.0) | 52 (14.4) |
| Public place | 31 (19.9) | 34 (23.3) | 65 (21.5) | 44 (23.7) | 35 (19.9) | 79 (21.8) |
| Other | 2 (1.3) | 0 (0.0) | 2 (0.7) | 2 (1.1) | 0 (0.0) | 2 (0.6) |
| Missing | 3 (1.9) | 0 (0.0) | 3 (1.0) | 3 (1.6) | 2 (1.1) | 5 (1.4) |
| Treatment preference, n (%) | ||||||
| Surgery | 69 (44.2) | 64 (43.8) | 133 (44.0) | 82 (44.1) | 81 (46.0) | 163 (45.0) |
| No surgery | 8 (5.1) | 12 (8.2) | 20 (6.6) | 10 (5.4) | 14 (8.0) | 24 (6.6) |
| No preference | 79 (50.6) | 70 (47.9) | 149 (49.3) | 94 (50.5) | 80 (45.5) | 174 (48.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| Displacement agreed by three raters from baseline CT and radiographic images, n (%) | Surgery (N = 219) | Plaster cast (N = 220) | ||
|---|---|---|---|---|
| No displacement (N = 131) | Displacement (N = 88) | No displacement (N = 130) | Displacement (N = 90) | |
| < 1 mm | 106 (80.9) | 54 (61.4) | 107 (82.3) | 58 (64.4) |
| 1–2 mm, inclusive | 19 (14.5) | 26 (29.6) | 20 (15.4) | 21 (23.3) |
| > 2 mm | 5 (3.8) | 8 (9.1) | 3 (2.3) | 11 (12.2) |
| Missing | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Time point | Surgery | Plaster cast | Total |
|---|---|---|---|
| No preference | |||
| Pre injury | |||
| Mean (SD) | 4.5 (14.1) | 3.1 (12.1) | 3.8 (13.2) |
| Median (IQR) | 0.0 (0.0–3.0) | 0.0 (0.0–1.0) | 0.0 (0.0–2.0) |
| Min., max. | (0, 85) | (0, 80.11111) | (0, 85) |
| Baseline (post injury) | |||
| Mean (SD) | 72.9 (20.9) | 70.0 (19.7) | 71.5 (20.3) |
| Median (IQR) | 78.5 (64.0–88.0) | 73.0 (59.5–84.5) | 75.0 (62.0–86.5) |
| Min., max. | (0, 100) | (0, 100) | (0, 100) |
| 6 weeks | |||
| Mean (SD) | 33.8 (21.1) | 35.9 (19.0) | 34.8 (20.1) |
| Median (IQR) | 31.3 (18.0–46.5) | 35.5 (19.5–49.0) | 34.0 (19.0–48.0) |
| Min., max. | (3, 82) | (0, 80) | (0, 82) |
| 12 weeks | |||
| Mean (SD) | 20.3 (20.5) | 24.8 (21.5) | 22.3 (21.0) |
| Median (IQR) | 11.5 (5.5–28.5) | 20.3 (8.0–31.5) | 16.5 (6.0–30.5) |
| Min., max. | (0, 89.5) | (0, 90) | (0, 90) |
| 26 weeks | |||
| Mean (SD) | 16.1 (18.9) | 15.3 (16.7) | 15.7 (17.8) |
| Median (IQR) | 9.0 (4.0–20.0) | 10.5 (4.0–18.0) | 9.5 (4.0–18.5) |
| Min., max. | (0, 84) | (0, 77) | (0, 84) |
| 52 weeks | |||
| Mean (SD) | 12.0 (18.1) | 13.3 (18.7) | 12.6 (18.3) |
| Median (IQR) | 4.0 (0.0–13.0) | 4.8 (0.3–16.3) | 4.3 (0.0–15.0) |
| Min., max. | (0, 85.5) | (0, 80.5) | (0, 85.5) |
| Preference for surgery | |||
| Pre injury | |||
| Mean (SD) | 1.7 (5.5) | 4.4 (12.6) | 3.1 (9.9) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) |
| Min., max. | (0, 42) | (0, 90.5) | (0, 90.5) |
| Baseline (post injury) | |||
| Mean (SD) | 74.5 (19.1) | 76.9 (14.5) | 75.7 (16.9) |
| Median (IQR) | 77.8 (65.4–88.0) | 78.5 (70.0–89.5) | 78.5 (68.0–89.0) |
| Min., max. | (0, 98) | (27, 96.5) | (0, 98) |
| 6 weeks | |||
| Mean (SD) | 37.0 (21.3) | 40.7 (23.1) | 38.8 (22.2) |
| Median (IQR) | 34.5 (19.0–52.0) | 38.8 (24.3–55.3) | 37.5 (22.0–53.5) |
| Min., max. | (4, 85.5) | (0, 100) | (0, 100) |
| 12 weeks | |||
| Mean (SD) | 21.7 (19.1) | 26.9 (22.8) | 24.2 (21.1) |
| Median (IQR) | 17.0 (7.0–30.0) | 18.5 (10.0–35.5) | 18.0 (9.8–33.0) |
| Min., max. | (0, 80.5) | (0, 84.5) | (0, 84.5) |
| 26 weeks | |||
| Mean (SD) | 16.0 (18.1) | 13.7 (19.2) | 14.9 (18.6) |
| Median (IQR) | 10.0 (3.0–22.0) | 8.0 (0.0–16.7) | 9.5 (1.0–19.0) |
| Min., max. | (0, 74) | (0, 91.5) | (0, 91.5) |
| 52 weeks | |||
| Mean (SD) | 10.2 (14.4) | 15.0 (21.1) | 12.6 (18.1) |
| Median (IQR) | 4.5 (0.5–14.0) | 4.0 (0.0–24.5) | 4.5 (0.0–17.5) |
| Min., max. | (0, 64) | (0, 96) | (0, 96) |
| Preference for no surgery | |||
| Pre injury | |||
| Mean (SD) | 2.2 (5.3) | 2.3 (4.9) | 2.3 (5.0) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–3.0) | 0.0 (0.0–2.0) |
| Min., max. | (0, 17) | (0, 19.5) | (0, 19.5) |
| Baseline (post injury) | |||
| Mean (SD) | 77.1 (15.2) | 67.5 (15.2) | 71.6 (15.7) |
| Median (IQR) | 78.8 (69.8–86.0) | 68.5 (62.3–78.0) | 72.8 (64.3–81.5) |
| Min., max. | (43, 100) | (36, 94) | (36, 100) |
| 6 weeks | |||
| Mean (SD) | 42.1 (24.8) | 46.2 (17.3) | 44.5 (20.4) |
| Median (IQR) | 45.0 (23.5–54.5) | 48.5 (30.0–58.5) | 46.3 (28.5–57.8) |
| Min., max. | (4, 78.5) | (16.5, 72.5) | (4, 78.5) |
| 12 weeks | |||
| Mean (SD) | 17.5 (16.3) | 27.2 (19.0) | 23.3 (18.3) |
| Median (IQR) | 14.8 (4.5–25.0) | 24.0 (8.0–45.0) | 23.0 (8.0–43.5) |
| Min., max. | (0, 54.5) | (0, 57) | (0, 57) |
| 26 weeks | |||
| Mean (SD) | 9.1 (8.8) | 21.6 (16.4) | 16.6 (15.0) |
| Median (IQR) | 6.0 (2.5–15.0) | 16.8 (11.5–30.5) | 13.5 (5.0–24.3) |
| Min., max. | (0, 25.5) | (0, 51) | (0, 51) |
| 52 weeks | |||
| Mean (SD) | 15.8 (19.5) | 15.5 (18.7) | 15.6 (18.6) |
| Median (IQR) | 8.0 (2.0–24.5) | 9.5 (2.0–27.5) | 9.5 (2.0–26.0) |
| Min., max. | (0, 60) | (0, 66) | (0, 66) |
| Time point | Surgery | Plaster cast | Total |
|---|---|---|---|
| Fracture displaced < 1 mm | |||
| Pre injury | |||
| Mean (SD) | 3.6 (12.3) | 3.8 (12.8) | 3.7 (12.5) |
| Median (IQR) | 0.0 (0.0–1.1) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Min., max. | (0, 85) | (0, 90.5) | (0, 90.5) |
| Baseline (post injury) | |||
| Mean (SD) | 73.3 (20.4) | 73.3 (18.7) | 73.3 (19.5) |
| Median (IQR) | 78.0 (69.0–87.0) | 76.3 (64.0–87.0) | 77.5 (65.9–87.0) |
| Min., max. | (0, 98) | (0, 100) | (0, 100) |
| 6 weeks | |||
| Mean (SD) | 34.4 (21.1) | 38.8 (21.3) | 36.5 (21.2) |
| Median (IQR) | 32.5 (18.0–49.0) | 38.0 (23.0–54.5) | 35.0 (19.5–52.0) |
| Min., max. | (3, 82) | (0, 100) | (0, 100) |
| 12 weeks | |||
| Mean (SD) | 18.8 (17.7) | 23.0 (18.9) | 20.8 (18.3) |
| Median (IQR) | 13.8 (5.8–24.5) | 18.8 (9.1–30.8) | 17.0 (6.8–28.3) |
| Min., max. | (0, 73.3) | (0, 90) | (0, 90) |
| 26 weeks | |||
| Mean (SD) | 14.8 (16.8) | 13.2 (16.3) | 14.0 (16.5) |
| Median (IQR) | 9.4 (3.5–19.5) | 8.3 (0.5–16.0) | 9.1 (2.0–17.8) |
| Min., max. | (0, 84) | (0, 77) | (0, 84) |
| 52 weeks | |||
| Mean (SD) | 11.0 (17.0) | 13.0 (19.4) | 12.0 (18.2) |
| Median (IQR) | 4.3 (0.0–12.5) | 4.0 (0.0–18.0) | 4.0 (0.0–15.0) |
| Min., max. | (0, 85.5) | (0, 88) | (0, 88) |
| Fracture displaced ≥ 1 mm and ≤ 2 mm | |||
| Pre injury | |||
| Mean (SD) | 2.4 (8.0) | 3.3 (10.4) | 2.8 (9.2) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Min., max. | (0, 64.7) | (0, 76) | (0, 76) |
| Baseline (post injury) | |||
| Mean (SD) | 74.7 (18.9) | 73.1 (15.5) | 73.9 (17.3) |
| Median (IQR) | 79.5 (61.5–90.0) | 75.0 (62.9–85.0) | 76.8 (62.0–87.5) |
| Min., max. | (0, 100) | (34, 99) | (0, 100) |
| 6 weeks | |||
| Mean (SD) | 37.7 (21.8) | 38.7 (20.6) | 38.2 (21.1) |
| Median (IQR) | 34.5 (22.5–51.5) | 39.0 (19.5–50.5) | 36.0 (20.5–51.0) |
| Min., max. | (4, 85.5) | (4, 90.5) | (4, 90.5) |
| 12 weeks | |||
| Mean (SD) | 23.4 (22.0) | 30.5 (25.3) | 26.7 (23.7) |
| Median (IQR) | 16.3 (6.5–38.5) | 22.5 (8.0–49.5) | 18.0 (8.0–45.0) |
| Min., max. | (0, 89.5) | (0, 84.5) | (0, 89.5) |
| 26 weeks | |||
| Mean (SD) | 16.9 (19.9) | 18.0 (19.7) | 17.4 (19.8) |
| Median (IQR) | 8.2 (3.3–23.5) | 12.3 (4.0–26.0) | 10.5 (3.5–25.5) |
| Min., max. | (0, 74) | (0, 91.5) | (0, 91.5) |
| 52 weeks | |||
| Mean (SD) | 12.0 (16.1) | 15.8 (20.2) | 13.9 (18.3) |
| Median (IQR) | 4.0 (0.3–16.3) | 8.5 (1.0–21.5) | 6.5 (0.5–18.5) |
| Min., max. | (0, 64) | (0, 96) | (0, 96) |
| Time point | Surgery | Plaster cast | Total |
|---|---|---|---|
| Fracture displaced < 1 mm | |||
| Pre injury | |||
| Mean (SD) | 3.6 (12.3) | 3.2 (11.9) | 3.4 (12.1) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Min., max. | (0, 85) | (0, 90.5) | (0, 90.5) |
| Baseline (post injury) | |||
| Mean (SD) | 73.2 (20.1) | 72.5 (18.8) | 72.9 (19.4) |
| Median (IQR) | 78.0 (68.5–87.0) | 76.0 (63.0–87.0) | 77.0 (64.5–87.0) |
| Min., max. | (0, 98) | (0, 100) | (0, 100) |
| 6 weeks | |||
| Mean (SD) | 33.6 (20.7) | 38.2 (21.5) | 35.9 (21.2) |
| Median (IQR) | 32.0 (18.0–46.5) | 36.0 (21.5–54.5) | 34.3 (18.8–50.8) |
| Min., max. | (3, 82) | (0, 100) | (0, 100) |
| 12 weeks | |||
| Mean (SD) | 18.1 (16.9) | 22.7 (19.4) | 20.3 (18.3) |
| Median (IQR) | 13.3 (5.5–24.0) | 18.3 (8.0–31.0) | 16.0 (6.3–27.8) |
| Min., max. | (0, 73.3) | (0, 90) | (0, 90) |
| 26 weeks | |||
| Mean (SD) | 14.0 (15.1) | 13.0 (16.0) | 13.5 (15.5) |
| Median (IQR) | 9.0 (3.5–18.8) | 8.5 (0.0–16.0) | 9.0 (2.0–17.0) |
| Min., max. | (0, 60) | (0, 77) | (0, 77) |
| 52 weeks | |||
| Mean (SD) | 10.4 (15.9) | 13.3 (19.5) | 11.8 (17.8) |
| Median (IQR) | 4.0 (0.0–12.5) | 4.0 (0.0–18.5) | 4.0 (0.0–15.0) |
| Min., max. | (0, 85.5) | (0, 88) | (0, 88) |
| Fracture displaced ≥ 1 mm and ≤ 2 mm | |||
| Pre injury | |||
| Mean (SD) | 2.4 (8.0) | 4.2 (11.8) | 3.3 (10.1) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.5) | 0.0 (0.0–1.3) |
| Min., max. | (0, 64.7) | (0, 76) | (0, 76) |
| Baseline (post injury) | |||
| Mean (SD) | 74.9 (19.3) | 74.2 (14.9) | 74.6 (17.2) |
| Median (IQR) | 79.5 (61.5–90.0) | 76.3 (65.0–85.5) | 78.5 (63.0–88.0) |
| Min., max. | (0, 100) | (34, 99) | (0, 100) |
| 6 weeks | |||
| Mean (SD) | 39.1 (22.1) | 39.7 (20.2) | 39.4 (21.1) |
| Median (IQR) | 35.0 (22.5–53.5) | 39.5 (25.0–50.5) | 38.4 (23.3–51.8) |
| Min., max. | (4, 85.5) | (4, 90.5) | (4, 90.5) |
| 12 weeks | |||
| Mean (SD) | 24.6 (22.6) | 31.1 (24.6) | 27.6 (23.7) |
| Median (IQR) | 16.8 (6.8–38.8) | 23.0 (11.5–48.5) | 18.5 (9.5–45.4) |
| Min., max. | (0, 89.5) | (0, 84.5) | (0, 89.5) |
| 26 weeks | |||
| Mean (SD) | 18.4 (22.0) | 18.4 (20.1) | 18.4 (21.0) |
| Median (IQR) | 9.3 (3.3–24.5) | 12.0 (5.0–26.0) | 10.5 (3.5–25.5) |
| Min., max. | (0, 84) | (0, 91.5) | (0, 91.5) |
| 52 weeks | |||
| Mean (SD) | 13.0 (17.6) | 15.5 (20.2) | 14.2 (18.9) |
| Median (IQR) | 4.3 (0.5–17.5) | 8.8 (1.0–21.0) | 7.3 (0.5–18.5) |
| Min., max. | (0, 72.5) | (0, 96) | (0, 96) |
| Characteristic | Surgery (N = 142)a | Plaster cast (N = 214)b | ||
|---|---|---|---|---|
| No surgical screw complication (N = 74) | Surgical screw complication (N = 68) | Surgery required (N = 19) | No surgery required (N = 195) | |
| Gender, n (%) | ||||
| Male | 65 (87.8) | 58 (85.3) | 14 (73.7) | 164 (84.1) |
| Female | 9 (12.2) | 10 (14.7) | 5 (26.3) | 31 (15.9) |
| Age, years | ||||
| n | 74 | 68 | 19 | 195 |
| Mean (SD) | 33.1 (13.6) | 33.2 (13.4) | 29.8 (10.3) | 33.3 (12.4) |
| Median (min., max.) | 30 (16, 69) | 30 (16, 80) | 26 (18, 51) | 29 (16, 76) |
| Ethnicity, n (%) | ||||
| White | 69 (93.2) | 64 (94.1) | 13 (68.4) | 177 (90.8) |
| Black | 0 (0.0) | 0 (0.0) | 2 (10.5) | 2 (1.0) |
| Asian | 2 (2.7) | 3 (4.4) | 2 (10.5) | 8 (4.1) |
| Other | 3 (4.1) | 1 (1.5) | 2 (10.5) | 8 (4.1) |
| Education, n (%) | ||||
| No formal qualifications | 6 (8.1) | 3 (4.4) | 0 (0.0) | 27 (13.8) |
| Some qualifications/no degree | 47 (63.5) | 53 (77.9) | 17 (89.5) | 109 (55.9) |
| Degree or higher | 21 (28.4) | 12 (17.6) | 2 (10.5) | 59 (30.3) |
| Employment status, n (%) | ||||
| Part-time | 6 (8.1) | 7 (10.3) | 2 (10.5) | 16 (8.2) |
| Full-time | 52 (70.3) | 39 (57.4) | 9 (47.4) | 110 (56.4) |
| Self-employed | 5 (6.8) | 6 (8.8) | 2 (10.5) | 32 (16.4) |
| Student | 5 (6.8) | 6 (8.8) | 4 (21.1) | 15 (7.7) |
| Retired | 4 (5.4) | 3 (4.4) | 0 (0.0) | 5 (2.6) |
| Looking after family/home | 0 (0.0) | 0 (0.0) | 1 (5.3) | 5 (2.6) |
| Not employed but seeking work | 1 (1.4) | 2 (2.9) | 0 (0.0) | 5 (2.6) |
| Other | 0 (0.0) | 5 (7.4) | 1 (5.3) | 7 (3.6) |
| Missing | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Type of employment, n (%) | ||||
| Unskilled manual | 6 (8.1) | 8 (11.8) | 4 (21.1) | 19 (9.7) |
| Skilled manual | 22 (29.7) | 17 (25.0) | 6 (31.6) | 51 (26.2) |
| Unskilled non-manual | 3 (4.1) | 11 (16.2) | 1 (5.3) | 11 (5.6) |
| Skilled non-manual | 17 (23) | 7 (10.3) | 2 (10.5) | 43 (22.1) |
| Professional | 9 (12.2) | 8 (11.8) | 1 (5.3) | 18 (9.2) |
| Other | 9 (12.2) | 6 (8.8) | 1 (5.3) | 29 (14.9) |
| Missing | 8 (10.8) | 11 (16.2) | 4 (21.1) | 24 (12.3) |
| Current smoker, n (%) | ||||
| Yes | 21 (28.4) | 22 (32.4) | 6 (31.6) | 48 (24.6) |
| No | 52 (70.3) | 46 (67.6) | 13 (68.4) | 146 (74.9) |
| Missing | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| If yes | ||||
| Number of cigarettes per day, median (min., max.) | 10 (1, 20) | 9 (2, 20) | 10 (1, 12) | 10 (1, 30) |
| Number of years, median (min., max.) | 10 (2, 50) | 10 (4, 36) | 20 (1, 30) | 10 (1, 36) |
| Past smoker, n (%) | ||||
| Yes | 38 (51.4) | 37 (54.4) | 11 (57.9) | 94 (48.2) |
| No | 32 (43.2) | 27 (39.7) | 7 (36.8) | 92 (47.2) |
| Missing | 4 (5.4) | 4 (5.9) | 1 (5.3) | 9 (4.6) |
| Diabetes, n (%) | ||||
| Yes | 2 (2.7) | 3 (4.4) | 0 (0.0) | 4 (2.1) |
| No | 71 (95.9) | 65 (95.6) | 19 (100.0) | 191 (97.9) |
| Missing | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Steroid use, n (%) | ||||
| Yes | 3 (4.1) | 1 (1.5) | 1 (5.3) | 3 (1.5) |
| No | 70 (94.6) | 67 (98.5) | 18 (94.7) | 192 (98.5) |
| Missing | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Characteristic | Surgery (N = 142)a | Plaster cast (N = 214)b | ||
|---|---|---|---|---|
| No surgical screw complication (N = 74) | Surgical screw complication (N = 68) | Surgery required (N = 19) | No surgery required (N = 195) | |
| Time since injury, daysc | ||||
| n | 74 | 68 | 19 | 195 |
| Mean (SD) | 5.1 (3.2) | 4.6 (3.0) | 6.8 (3.5) | 5.2 (3.3) |
| Median (min., max.) | 4 (1, 14) | 4 (1, 13) | 6 (1, 14) | 4 (0, 14) |
| Affected wrist, n (%) | ||||
| Left | 37 (50.0) | 34 (50.0) | 11 (57.9) | 103 (52.8) |
| Right | 37 (50.0) | 34 (50.0) | 8 (42.1) | 92 (47.2) |
| Affected wrist is dominant hand, n (%) | ||||
| Yes | 34 (45.9) | 32 (47.1) | 8 (42.1) | 85 (43.6) |
| No | 40 (54.1) | 36 (52.9) | 11 (57.9) | 110 (56.4) |
| Displacement (eligibility), n (%) | ||||
| No displacement | 39 (52.7) | 42 (61.8) | 8 (42.1) | 123 (63.1) |
| Displacement | 35 (47.3) | 26 (38.2) | 11 (57.9) | 72 (36.9) |
| Displacement (randomisation), n (%) | ||||
| No displacement | 40 (54.1) | 41 (60.3) | 8 (42.1) | 119 (61.0) |
| Displacement | 34 (45.9) | 27 (39.7) | 11 (57.9) | 76 (39.0) |
| Radiographs,d n (%) | ||||
| Elongated scaphoid view | 71 (95.9) | 65 (95.6) | 19 (100.0) | 185 (94.9) |
| Posterior–anterior view | 74 (100.0) | 66 (97.1) | 19 (100.0) | 193 (99.0) |
| 45° semisupine view | 54 (73.0) | 45 (66.2) | 15 (78.9) | 146 (74.9) |
| Lateral view | 74 (100.0) | 68 (100.0) | 19 (100.0) | 192 (98.5) |
| 45° semiprone view | 70 (94.6) | 57 (83.8) | 15 (78.9) | 176 (90.3) |
| Previous wrist problems on same side, n (%) | ||||
| Yes | 12 (16.2) | 18 (26.5) | 6 (31.6) | 38 (19.5) |
| No | 62 (83.8) | 49 (72.1) | 12 (63.2) | 157 (80.5) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (5.3) | 0 (0.0) |
| If yes, what injury?, n (%) | ||||
| Previous fracture | 5 (41.7) | 10 (55.6) | 3 (50.0) | 24 (63.2) |
| Arthritis | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (2.6) |
| Ligament, tendon or nerve injury | 4 (33.3) | 4 (22.2) | 0 (0.0) | 8 (21.1) |
| Other | 1 (8.3) | 3 (16.7) | 3 (50.0) | 5 (13.2) |
| Missing | 1 (8.3) | 1 (5.6) | 0 (0.0) | 0 (0.0) |
| Injury mechanism, n (%) | ||||
| Fall – standing | 9 (12.2) | 9 (13.2) | 2 (10.5) | 26 (13.3) |
| Fall – walking | 6 (8.1) | 7 (10.3) | 0 (0.0) | 23 (11.8) |
| Fall – running | 11 (14.9) | 18 (26.5) | 1 (5.3) | 35 (17.9) |
| Fall – from height | 10 (13.5) | 10 (14.7) | 4 (21.1) | 29 (14.9) |
| Fall – from moving object | 19 (25.7) | 11 (16.2) | 1 (5.3) | 30 (15.4) |
| Hit on palm of hand – object striking palm | 4 (5.4) | 3 (4.4) | 3 (15.8) | 12 (6.2) |
| Hit on palm of hand – handle whipping back | 3 (4.1) | 3 (4.4) | 3 (15.8) | 8 (4.1) |
| Hit on palm of hand – other sudden extension | 5 (6.8) | 3 (4.4) | 0 (0.0) | 7 (3.6) |
| Punched something | 1 (1.4) | 1 (1.5) | 1 (5.3) | 11 (5.6) |
| Road traffic accident | 5 (6.8) | 1 (1.5) | 1 (5.3) | 7 (3.6) |
| Other | 1 (1.4) | 2 (2.9) | 3 (15.8) | 7 (3.6) |
| Place of injury,d n (%) | ||||
| Sport | 31 (41.9) | 32 (47.1) | 7 (36.8) | 68 (34.9) |
| Home | 6 (8.1) | 10 (14.7) | 2 (10.5) | 40 (20.5) |
| Work | 7 (9.5) | 4 (5.9) | 1 (5.3) | 17 (8.7) |
| Road traffic accident | 12 (16.2) | 5 (7.4) | 3 (15.8) | 30 (15.4) |
| Public place | 18 (24.3) | 16 (23.5) | 5 (26.3) | 42 (21.5) |
| Other | 0 (0.0) | 1 (1.5) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (1.4) | 1 (1.5) | 1 (5.3) | 1 (0.5) |
| Treatment preference, n (%) | ||||
| Surgery | 31 (41.9) | 25 (36.8) | 10 (52.6) | 85 (43.6) |
| No surgery | 7 (9.5) | 2 (2.9) | 1 (5.3) | 18 (9.2) |
| No preference | 36 (48.6) | 41 (60.3) | 8 (42.1) | 91 (46.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| PRWE score | Surgery (N = 142)a | Plaster cast (N = 214)b | |||
|---|---|---|---|---|---|
| No surgical screw complication (N = 74) | Surgical screw complication (N = 68) | Surgery required (N = 19) | No surgery required (N = 195) | ||
| Baseline (pre injury) | |||||
| Pain subscale | Mean (SD) | 3.5 (9.9) | 1.9 (4.6) | 1.7 (4.7) | 2.3 (6.3) |
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | |
| Min., max. | (0, 50) | (0, 24) | (0, 16.3) | (0, 39) | |
| Function subscale | Mean (SD) | 2.0 (8.2) | 0.6 (2.6) | 1.3 (4.4) | 0.9 (4.6) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | |
| Min., max. | (0, 43) | (0, 18) | (0, 18.5) | (0, 41.1) | |
| Total | Mean (SD) | 5.5 (16.9) | 2.5 (6.7) | 3.0 (9.0) | 3.1 (10.2) |
| Median (IQR) | 0.0 (0.0–2.5) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | |
| Min., max. | (0, 85) | (0, 42) | (0, 34.8) | (0, 80.1) | |
| Baseline (post injury) | |||||
| Pain subscale | Mean (SD) | 34.9 (11.4) | 33.8 (11.7) | 36.1 (8.2) | 34.0 (9.8) |
| Median (IQR) | 39.5 (30.0–43.0) | 36.0 (28.0–43.0) | 39.0 (30.0–43.0) | 35.0 (28.0–42.0) | |
| Min., max. | (0, 50) | (0, 50) | (21, 46.25) | (0, 50) | |
| Function subscale | Mean (SD) | 38.7 (10.9) | 38.2 (11.3) | 38.0 (11.2) | 38.6 (10.0) |
| Median (IQR) | 41.5 (32.5–47.0) | 41.3 (33.3–46.5) | 42.0 (32.0–46.5) | 40.3 (33.5–46.0) | |
| Min., max. | (0, 50) | (0, 50) | (8.5, 49) | (0, 50) | |
| Total | Mean (SD) | 73.8 (21.2) | 72.0 (21.2) | 74.1 (16.1) | 73.0 (17.5) |
| Median (IQR) | 79.5 (69.0–89.0) | 77.0 (59.5–87.4) | 76.6 (58.0–86.5) | 75.5 (63.8–86.3) | |
| Min., max. | (0, 98) | (0, 100) | (48.5, 94.5) | (0, 100) | |
| 6 weeks | |||||
| Pain subscale | Mean (SD) | 18.0 (10.1) | 19.1 (10.9) | 20.2 (13.1) | 18.1 (10.2) |
| Median (IQR) | 17.0 (10.0–25.0) | 19.0 (10.5–27.0) | 18.0 (10.0–22.0) | 17.0 (10.0–26.0) | |
| Min., max. | (3, 39) | (0, 44) | (6, 48) | (0, 47) | |
| Function subscale | Mean (SD) | 17.1 (13.4) | 16.2 (12.2) | 23.0 (16.2) | 19.9 (11.8) |
| Median (IQR) | 15.0 (4.3–25.8) | 12.5 (7.0–22.5) | 19.5 (9.0–31.0) | 19.0 (10.0–26.5) | |
| Min., max. | (0, 44) | (0, 47) | (4, 50) | (0, 46.5) | |
| Total | Mean (SD) | 35.1 (21.5) | 35.2 (21.2) | 43.1 (26.8) | 38.2 (19.8) |
| Median (IQR) | 33.3 (17.5–52.3) | 32.0 (19.5–46.5) | 42.5 (19.0–53.0) | 38.0 (23.0–51.5) | |
| Min., max. | (4, 77) | (3, 85.5) | (11, 98) | (0, 90.5) | |
| 12 weeks | |||||
| Pain subscale | Mean (SD) | 11.4 (10.3) | 13.7 (10.6) | 21.9 (13.0) | 13.9 (10.8) |
| Median (IQR) | 8.0 (3.0–17.0) | 10.0 (6.0–20.0) | 22.5 (14.0–34.0) | 11.0 (5.0–19.0) | |
| Min., max. | (0, 41) | (0, 45) | (0, 41) | (0, 47) | |
| Function subscale | Mean (SD) | 6.7 (8.4) | 8.7 (9.9) | 23.0 (13.3) | 10.1 (10.7) |
| Median (IQR) | 4.0 (0.5–10.0) | 4.8 (1.5–12.0) | 25.5 (12.3–31.0) | 6.5 (2.0–15.0) | |
| Min., max. | (0, 34.5) | (0, 44.5) | (0, 46.5) | (0, 45) | |
| Total | Mean (SD) | 17.8 (18.0) | 22.6 (20.1) | 45.0 (24.5) | 24.0 (20.6) |
| Median (IQR) | 13.5 (4.0–26.0) | 15.8 (8.0–36.8) | 50.5 (26.3–64.8) | 18.3 (8.3–31.0) | |
| Min., max. | (0, 71.5) | (0, 89.5) | (0, 75.5) | (0, 90) | |
| 26 weeks | |||||
| Pain subscale | Mean (SD) | 9.2 (10.0) | 10.7 (10.1) | 18.3 (10.1) | 9.3 (9.7) |
| Median (IQR) | 5.0 (2.0–13.0) | 8.0 (4.0–15.0) | 16.0 (11.0–23.0) | 7.0 (1.0–12.0) | |
| Min., max. | (0, 43) | (0, 39) | (6, 44) | (0, 44) | |
| Function subscale | Mean (SD) | 3.8 (7.2) | 5.7 (8.0) | 14.7 (13.4) | 4.6 (7.7) |
| Median (IQR) | 1.0 (0.0–3.5) | 2.0 (0.5–7.5) | 12.0 (4.0–23.0) | 1.5 (0.0–5.0) | |
| Min., max. | (0, 41) | (0, 35) | (1.5, 47.5) | (0, 40) | |
| Total | Mean (SD) | 13.1 (16.7) | 16.4 (17.7) | 33.0 (22.3) | 13.6 (16.5) |
| Median (IQR) | 6.3 (2.0–15.5) | 9.5 (4.0–20.5) | 32.0 (15.0–40.0) | 9.8 (1.0–17.0) | |
| Min., max. | (0, 84) | (0, 74) | (9, 91.5) | (0, 83) | |
| 52 weeks | |||||
| Pain subscale | Mean (SD) | 6.1 (8.9) | 7.2 (8.5) | 16.6 (16.0) | 8.5 (10.5) |
| Median (IQR) | 3.0 (0.0–9.0) | 4.0 (1.0–10.0) | 10.0 (4.0–28.0) | 4.0 (0.0–13.0) | |
| Min., max. | (0, 42) | (0, 37) | (0, 48) | (0, 47.5) | |
| Function subscale | Mean (SD) | 2.8 (6.9) | 3.6 (5.7) | 12.5 (16.7) | 4.2 (7.5) |
| Median (IQR) | 0.5 (0.0–3.0) | 1.0 (0.0–4.5) | 4.0 (0.5–16.5) | 0.5 (0.0–4.5) | |
| Min., max. | (0, 43.5) | (0, 26) | (0, 48) | (0, 33) | |
| Total | Mean (SD) | 8.9 (15.0) | 10.8 (13.9) | 29.1 (32.4) | 12.8 (17.4) |
| Median (IQR) | 3.8 (0.0–11.3) | 5.0 (1.0–14.5) | 14.0 (4.5–47.5) | 4.5 (0.0–17.8) | |
| Min., max. | (0, 85.5) | (0, 58) | (1, 96) | (0, 76.5) | |
| Characteristic | All (N = 439) | Surgery (N = 219) | ||
|---|---|---|---|---|
| CT ≤ 2 weeks after injury (N = 412) | CT > 2 weeks after injury (N = 27) | Surgery ≤ 2 weeks after A&E presentation (N = 182) | Surgery > 2 weeks after A&E presentation (N = 37) | |
| Gender, n (%) | ||||
| Male | 342 (83.0) | 21 (77.8) | 156 (85.7) | 24 (64.9) |
| Female | 70 (17.0) | 6 (22.2) | 26 (14.3) | 13 (35.1) |
| Age, years | ||||
| n | 412 | 27 | 182 | 37 |
| Mean (SD) | 32.9 (12.8) | 32.3 (11.4) | 32.2 (12.9) | 36.2 (14.2) |
| Median (min., max.) | 29 (16, 80) | 30 (17, 59) | 27 (16, 80) | 31 (17, 61) |
| Ethnicity, n (%) | ||||
| White | 377 (91.5) | 23 (85.2) | 171 (94.0) | 34 (91.9) |
| Black | 3 (0.7) | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Asian | 16 (3.9) | 1 (3.7) | 6 (3.3) | 1 (2.7) |
| Other | 15 (3.6) | 0 (0.0) | 5 (2.7) | 0 (0.0) |
| Missing | 1 (0.2) | 1 (3.7) | 0 (0.0) | 2 (5.4) |
| Education, n (%) | ||||
| No formal qualifications | 49 (11.9) | 2 (7.4) | 17 (9.3) | 7 (18.9) |
| Some qualifications/no degree | 263 (63.8) | 17 (63.0) | 126 (69.2) | 25 (67.6) |
| Degree or higher | 98 (23.8) | 7 (25.9) | 38 (20.9) | 3 (8.1) |
| Missing | 2 (0.5) | 1 (3.7) | 1 (0.5) | 2 (5.4) |
| Employment status, n (%) | ||||
| Part-time | 34 (8.3) | 4 (14.8) | 15 (8.2) | 5 (13.5) |
| Full-time | 237 (57.5) | 10 (37.0) | 108 (59.3) | 19 (51.4) |
| Self-employed | 50 (12.1) | 7 (25.9) | 16 (8.8) | 5 (13.5) |
| Student | 37 (9.0) | 4 (14.8) | 18 (9.9) | 2 (5.4) |
| Retired | 12 (2.9) | 0 (0.0) | 7 (3.8) | 0 (0.0) |
| Looking after family/home | 7 (1.7) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Not employed but seeking work | 14 (3.4) | 0 (0.0) | 7 (3.8) | 2 (5.4) |
| Other | 19 (4.6) | 1 (3.7) | 9 (4.9) | 2 (5.4) |
| Missing | 2 (0.5) | 1 (3.7) | 1 (0.5) | 2 (5.4) |
| Type of employment, n (%) | ||||
| Unskilled manual | 45 (10.9) | 3 (11.1) | 21 (11.5) | 4 (10.8) |
| Skilled manual | 115 (27.9) | 8 (29.6) | 54 (29.7) | 9 (24.3) |
| Unskilled non-manual | 29 (7.0) | 2 (7.4) | 15 (8.2) | 4 (10.8) |
| Skilled non-manual | 76 (18.4) | 3 (11.1) | 29 (15.9) | 4 (10.8) |
| Professional | 37 (9.0) | 2 (7.4) | 18 (9.9) | 2 (5.4) |
| Other | 44 (10.7) | 5 (18.5) | 14 (7.7) | 5 (13.5) |
| Missing | 66 (16.0) | 4 (14.8) | 31 (17.0) | 9 (24.3) |
| Current smoker, n (%) | ||||
| Yes | 120 (29.1) | 9 (33.3) | 56 (30.8) | 17 (45.9) |
| No | 289 (70.1) | 17 (63) | 125 (68.7) | 18 (48.6) |
| Missing | 3 (0.7) | 1 (3.7) | 1 (0.5) | 2 (5.4) |
| If yes | ||||
| Number of cigarettes per day, median (min., max.) | 10 (1, 40) | 10 (1, 25) | 10 (1, 20) | 10 (1, 40) |
| Number of years, median (min., max.) | 10 (1, 50) | 6 (1, 30) | 10 (2, 50) | 10 (1, 44) |
| Past smoker, n (%) | ||||
| Yes | 209 (50.7) | 16 (59.3) | 95 (52.2) | 21 (56.8) |
| No | 177 (43.0) | 9 (33.3) | 76 (41.8) | 9 (24.3) |
| Missing | 26 (6.3) | 2 (7.4) | 11 (6.0) | 7 (18.9) |
| Diabetes, n (%) | ||||
| Yes | 11 (2.7) | 0 (0.0) | 5 (2.7) | 2 (5.4) |
| No | 399 (96.8) | 26 (96.3) | 176 (96.7) | 33 (89.2) |
| Missing | 2 (0.5) | 1 (3.7) | 1 (0.5) | 2 (5.4) |
| Steroid use, n (%) | ||||
| Yes | 9 (2.2) | 1 (3.7) | 5 (2.7) | 1 (2.7) |
| No | 401 (97.3) | 25 (92.6) | 176 (96.7) | 34 (91.9) |
| Missing | 2 (0.5) | 1 (3.7) | 1 (0.5) | 2 (5.4) |
| Characteristic | All (N = 439) | Surgery (N = 219) | ||
|---|---|---|---|---|
| CT ≤ 2 weeks after injury (N = 412) | CT > 2 weeks after injury (N = 27) | Surgery ≤ 2 weeks after A&E presentation (N = 182) | Surgery > 2 weeks after A&E presentation (N = 37) | |
| Time since injury, daysa | ||||
| n | 412 | 27 | 182 | 37 |
| Mean (SD) | 5.2 (3.2) | 5.3 (3.3) | 4.8 (3.0) | 6.3 (3.5) |
| Median (min., max.) | 5 (0, 14) | 5 (1, 14) | 4 (1, 13) | 6 (1, 14) |
| Affected wrist, n (%) | ||||
| Left | 221 (53.6) | 12 (44.4) | 98 (53.8) | 17 (45.9) |
| Right | 191 (46.4) | 15 (55.6) | 84 (46.2) | 20 (54.1) |
| Affected wrist is dominant hand, n (%) | ||||
| Yes | 184 (44.7) | 11 (40.7) | 85 (46.7) | 15 (40.5) |
| No | 227 (55.1) | 15 (55.6) | 97 (53.3) | 20 (54.1) |
| Missing | 1 (0.2) | 1 (3.7) | 0 (0.0) | 2 (5.4) |
| Displacement (eligibility), n (%) | ||||
| No displacement | 253 (61.4) | 16 (59.3) | 111 (61.0) | 24 (64.9) |
| Displacement | 159 (38.6) | 11 (40.7) | 71 (39.0) | 13 (35.1) |
| Displacement (randomisation), n (%) | ||||
| No displacement | 247 (60.0) | 14 (51.9) | 109 (59.9) | 22 (59.5) |
| Displacement | 165 (40.0) | 13 (48.1) | 73 (40.1) | 15 (40.5) |
| Radiographs,b n (%) | ||||
| Elongated scaphoid view | 392 (95.1) | 27 (100.0) | 173 (95.1) | 36 (97.3) |
| Posterior–anterior view | 406 (98.5) | 27 (100.0) | 181 (99.5) | 34 (91.9) |
| 45° semisupine view | 305 (74.0) | 20 (74.1) | 132 (72.5) | 27 (73.0) |
| Lateral view | 408 (99.0) | 27 (100.0) | 182 (100.0) | 36 (97.3) |
| 45° semiprone view | 370 (89.8) | 24 (88.9) | 164 (90.1) | 34 (91.9) |
| Previous wrist problems on same side, n (%) | ||||
| Yes | 81 (19.7) | 7 (25.9) | 36 (19.8) | 7 (18.9) |
| No | 328 (79.6) | 18 (66.7) | 145 (79.7) | 28 (75.7) |
| Missing | 3 (0.7) | 2 (7.4) | 1 (0.5) | 2 (5.4) |
| If yes, what injury?, n (%) | ||||
| Previous fracture | 47 (58) | 4 (57.1) | 21 (58.3) | 2 (28.6) |
| Arthritis | 3 (3.7) | 0 (0) | 1 (2.8) | 1 (14.3) |
| Ligament, tendon or nerve injury | 17 (21) | 1 (14.3) | 9 (25.0) | 1 (14.3) |
| Other | 13 (16) | 1 (14.3) | 4 (11.1) | 2 (28.6) |
| Missing | 1 (1.2) | 1 (14.3) | 1 (2.8) | 1 (14.3) |
| Injury mechanism, n (%) | ||||
| Fall – standing | 53 (12.9) | 4 (14.8) | 21 (11.5) | 7 (18.9) |
| Fall – walking | 45 (10.9) | 3 (11.1) | 18 (9.9) | 6 (16.2) |
| Fall – running | 74 (18.0) | 4 (14.8) | 37 (20.3) | 3 (8.1) |
| Fall – from height | 60 (14.6) | 2 (7.4) | 25 (13.7) | 3 (8.1) |
| Fall – from moving object | 69 (16.7) | 4 (14.8) | 40 (22.0) | 2 (5.4) |
| Hit on palm of hand – object striking palm | 30 (7.3) | 1 (3.7) | 9 (4.9) | 7 (18.9) |
| Hit on palm of hand – handle whipping back | 20 (4.9) | 0 (0.0) | 9 (4.9) | 0 (0.0) |
| Hit on palm of hand – other sudden extension | 16 (3.9) | 3 (11.1) | 8 (4.4) | 3 (8.1) |
| Punched something | 15 (3.6) | 1 (3.7) | 4 (2.2) | 0 (0.0) |
| Road traffic accident | 15 (3.6) | 2 (7.4) | 7 (3.8) | 2 (5.4) |
| Other | 14 (3.4) | 2 (7.4) | 4 (2.2) | 2 (5.4) |
| Missing | 1 (0.2) | 1 (3.7) | 0 (0.0) | 2 (5.4) |
| Place of injury,b n (%) | ||||
| Sport | 156 (37.9) | 10 (37.0) | 77 (42.3) | 11 (29.7) |
| Home | 64 (15.5) | 6 (22.2) | 20 (11.0) | 7 (18.9) |
| Work | 38 (9.2) | 2 (7.4) | 14 (7.7) | 8 (21.6) |
| Road traffic accident | 57 (13.8) | 3 (11.1) | 24 (13.2) | 2 (5.4) |
| Public place | 93 (22.6) | 4 (14.8) | 43 (23.6) | 6 (16.2) |
| Other | 3 (0.7) | 0 (0.0) | 3 (1.6) | 0 (0.0) |
| Missing | 5 (1.2) | 1 (3.7) | 3 (1.6) | 1 (2.7) |
| Treatment preference, n (%) | ||||
| Surgery | 180 (43.7) | 14 (51.9) | 75 (41.2) | 18 (48.6) |
| No surgery | 29 (7.0) | 3 (11.1) | 10 (5.5) | 3 (8.1) |
| No preference | 200 (48.5) | 9 (33.3) | 96 (52.7) | 14 (37.8) |
| Missing | 3 (0.7) | 1 (3.7) | 1 (0.5) | 2 (5.4) |
| PRWE score | All (N = 439) | Surgery (N = 219) | |||
|---|---|---|---|---|---|
| CT ≤ 2 weeks after injury (N = 412) | CT > 2 weeks after injury (N = 27) | Surgery ≤ 2 weeks after A&E presentation (N = 182) | Surgery > 2 weeks after A&E presentation (N = 37) | ||
| Baseline (pre injury) | |||||
| Pain subscale | Mean (SD) | 2.3 (6.6) | 3.2 (9.3) | 2.2 (6.6) | 2.2 (6.3) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | |
| Min., max. | (0, 50) | (0, 35) | (0, 50) | (0, 32.5) | |
| Function subscale | Mean (SD) | 1.0 (5.1) | 2.4 (9.0) | 0.9 (5.0) | 1.3 (5.8) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | |
| Min., max. | (0, 49.5) | (0, 41) | (0, 43) | (0, 32.2) | |
| Total | Mean (SD) | 3.2 (10.9) | 5.7 (17.9) | 3.1 (10.7) | 3.4 (11.8) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | |
| Min., max. | (0, 90.5) | (0, 76) | (0, 85) | (0, 64.7) | |
| Baseline (post injury) | |||||
| Pain subscale | Mean (SD) | 34.5 (10.2) | 33.9 (10.8) | 34.3 (11.1) | 36.7 (8.8) |
| Median (IQR) | 36.0 (29.0–42.0) | 35.5 (25.0–43.0) | 36.0 (28.0–42.0) | 38.0 (31.0–42.0) | |
| Min., max. | (0, 50) | (8.75, 47) | (0, 50) | (2, 50) | |
| Function subscale | Mean (SD) | 39.0 (10.3) | 36.7 (9.4) | 38.7 (10.9) | 41.3 (7.6) |
| Median (IQR) | 41.5 (33.5–46.5) | 40.0 (34.5–42.5) | 42.0 (33.0–46.5) | 41.0 (37.5–48.5) | |
| Min., max. | (0, 50) | (15, 49) | (0, 50) | (20, 50) | |
| Total | Mean (SD) | 73.7 (18.6) | 71.6 (18.2) | 73.1 (20.5) | 78.0 (14.7) |
| Median (IQR) | 77.5 (64.0–87.5) | 75.5 (70.0–85.0) | 78.0 (62.0–88.0) | 79.0 (71.0–87.5) | |
| Min., max. | (0, 100) | (35, 96) | (0, 100) | (22, 100) | |
| 6 weeks | |||||
| Pain subscale | Mean (SD) | 18.5 (10.6) | 21.6 (9.3) | 18.7 (10.3) | 20.4 (12.1) |
| Median (IQR) | 18.0 (10.0–26.0) | 21.0 (14.5–28.5) | 19.0 (10.0–26.0) | 18.0 (10.0–33.0) | |
| Min., max. | (0, 50) | (6, 38) | (0, 44) | (2, 38) | |
| Function subscale | Mean (SD) | 18.3 (12.6) | 22.5 (14.6) | 16.2 (12.4) | 21.1 (15.2) |
| Median (IQR) | 16.5 (8.0–26.5) | 23.5 (14.5–32.5) | 13.0 (6.1–25.0) | 16.0 (9.5–32.0) | |
| Min., max. | (0, 50) | (0, 46) | (0, 44.5) | (0, 47) | |
| Total | Mean (SD) | 36.8 (21.1) | 45.4 (21.3) | 34.9 (20.8) | 41.5 (24.6) |
| Median (IQR) | 35.0 (19.5–51.3) | 43.3 (33.0–63.3) | 33.5 (18.5–48.5) | 39.5 (19.5–62.0) | |
| Min., max. | (0, 100) | (6, 84) | (3, 85.5) | (6, 82) | |
| 12 weeks | |||||
| Pain subscale | Mean (SD) | 13.4 (10.9) | 19.9 (13.3) | 12.6 (10.9) | 14.5 (11.6) |
| Median (IQR) | 11.0 (5.0–18.0) | 23.0 (7.5–31.5) | 9.0 (4.0–17.5) | 12.5 (5.0–18.0) | |
| Min., max. | (0, 47) | (0, 41) | (0, 45) | (0, 40) | |
| Function subscale | Mean (SD) | 9.1 (10.2) | 17.8 (14.3) | 7.7 (9.1) | 9.7 (10.2) |
| Median (IQR) | 5.5 (1.5–12.0) | 17.8 (5.0–27.5) | 4.3 (1.0–11.0) | 7.4 (1.5–12.0) | |
| Min., max. | (0, 45) | (0, 46.5) | (0, 44.5) | (0, 34) | |
| Total | Mean (SD) | 22.5 (20.3) | 37.8 (25.9) | 20.2 (19.4) | 24.2 (21.2) |
| Median (IQR) | 17.0 (7.0–30.0) | 38.8 (11.5–60.0) | 14.1 (6.0–26.8) | 18.8 (8.0–28.5) | |
| Min., max. | (0, 90) | (0, 78.5) | (0, 89.5) | (0, 73.3) | |
| 26 weeks | |||||
| Pain subscale | Mean (SD) | 10.0 (10.3) | 15.1 (9.8) | 10.1 (10.4) | 12.9 (12.4) |
| Median (IQR) | 7.0 (2.0–13.0) | 13.0 (7.0–23.0) | 7.0 (3.0–15.0) | 8.0 (2.0–24.0) | |
| Min., max. | (0, 44) | (0, 35) | (0, 43) | (0, 36) | |
| Function subscale | Mean (SD) | 5.3 (8.5) | 7.5 (8.4) | 5.0 (8.0) | 7.9 (10.5) |
| Median (IQR) | 1.5 (0.0–5.6) | 4.4 (1.5–10.5) | 1.5 (0.3–5.3) | 4.0 (0.0–13.0) | |
| Min., max. | (0, 47.5) | (0, 24) | (0, 41) | (0, 41) | |
| Total | Mean (SD) | 15.1 (18.0) | 21.0 (15.6) | 15.2 (17.9) | 19.4 (19.8) |
| Median (IQR) | 9.5 (2.5–18.5) | 15.5 (11.5–36.5) | 9.0 (3.5–19.0) | 12.0 (2.0–37.5) | |
| Min., max. | (0, 91.5) | (0, 45) | (0, 84) | (0, 55.5) | |
| 52 weeks | |||||
| Pain subscale | Mean (SD) | 8.1 (10.4) | 14.2 (14.0) | 7.5 (9.9) | 9.1 (11.4) |
| Median (IQR) | 4.0 (0.0–11.0) | 14.0 (2.0–20.0) | 4.0 (0.0–10.0) | 3.0 (0.0–17.5) | |
| Min., max. | (0, 47.5) | (0, 48) | (0, 42) | (0, 37) | |
| Function subscale | Mean (SD) | 4.0 (7.6) | 9.8 (13.4) | 3.5 (6.9) | 5.3 (8.8) |
| Median (IQR) | 0.5 (0.0–4.0) | 4.0 (0.0–14.5) | 1.0 (0.0–3.5) | 0.5 (0.0–10.3) | |
| Min., max. | (0, 44) | (0, 48) | (0, 43.5) | (0, 31) | |
| Total | Mean (SD) | 12.1 (17.5) | 24.0 (27.0) | 11.0 (16.1) | 14.4 (19.7) |
| Median (IQR) | 4.0 (0.0–15.0) | 17.5 (2.0–32.5) | 4.0 (0.5–13.5) | 4.3 (0.0–27.8) | |
| Min., max. | (0, 88) | (0, 96) | (0, 85.5) | (0, 60) | |
| Responses to returned 12-week questionnaire | Surgery (N = 182) | Plaster cast (N = 167) | Total (N = 349) |
|---|---|---|---|
| How useful did you find the written advice about home exercises for your hand and wrist?, n (%) | |||
| Very useful | 59 (32.4) | 38 (22.8) | 97 (27.8) |
| Quite useful | 92 (50.6) | 85 (50.9) | 177 (50.7) |
| Not very useful at all | 15 (8.2) | 21 (12.6) | 36 (10.3) |
| Not at all useful | 10 (5.5) | 11 (6.6) | 21 (6.0) |
| Missing | 6 (3.3) | 12 (7.2) | 18 (5.2) |
| Have you done any of these home exercises?, n (%) | |||
| Yes | 151 (83.0) | 136 (81.4) | 287 (82.2) |
| No | 25 (13.7) | 23 (13.8) | 48 (13.8) |
| Missing | 6 (3.3) | 8 (4.8) | 14 (4.0) |
| If yes, on how many days over the past 12 weeks have you done these exercises? | |||
| Mean (SD) | 41.8 (28.5) | 44.7 (28.6) | 43.1 (28.5) |
| Median (min., max.) | 35 (2, 137) | 41 (1, 168) | 38 (1, 168) |
| Would you have preferred to have a formal referral to physiotherapy for your wrist injury?, n (%) | |||
| Yes | 67 (36.8) | 56 (33.5) | 123 (35.2) |
| No | 102 (56.0) | 98 (58.7) | 200 (57.3) |
| Missing | 13 (7.1) | 13 (7.8) | 26 (7.5) |
| Responses to returned 52-week questionnaire | Surgery (N = 186) | Plaster cast (N = 178) | Total (N = 364) |
|---|---|---|---|
| Compared with 1 year ago, how is your wrist now?, n (%) | |||
| Much better now | 149 (80.1) | 139 (78.1) | 288 (79.1) |
| Slightly better now | 17 (9.1) | 17 (9.6) | 34 (9.3) |
| About the same now | 9 (4.8) | 11 (6.2) | 20 (5.5) |
| Slightly worse now | 5 (2.7) | 4 (2.3) | 9 (2.5) |
| Much worse now | 1 (0.5) | 3 (1.7) | 4 (1.1) |
| Missing | 5 (2.7) | 4 (2.3) | 9 (2.5) |
| Based on your experiences of the treatment that you received as part of this trial, if you injured your wrist today to the same extent as you did 1 year ago, which treatment would you prefer?, n (%) | |||
| No preference | 36 (19.4) | 68 (38.2) | 104 (28.6) |
| Surgery | 137 (73.7) | 59 (33.2) | 196 (53.9) |
| Not surgery | 8 (4.3) | 48 (27.0) | 56 (15.4) |
| Missing | 5 (2.7) | 3 (1.7) | 8 (2.2) |
FIGURE 14.
Unadjusted mean PRWE scores (with 95% CIs) over time by randomised group and patient treatment preference at baseline: (a) no preference; (b) preference for surgery; and (c) preference for no surgery. Modified from Lancet, vol. 396, Dias JJ, Brealey SD, Fairhurst C, Amirfeyz R, Bhowal B, Blewitt N, et al. , Surgery versus cast immobilisation for adults with a bicortical fracture of the scaphoid waist (SWIFFT): a pragmatic, multicentre, open-label, randomised superiority trial, pp. 390–401,89 Copyright (2020), with permission from Elsevier.
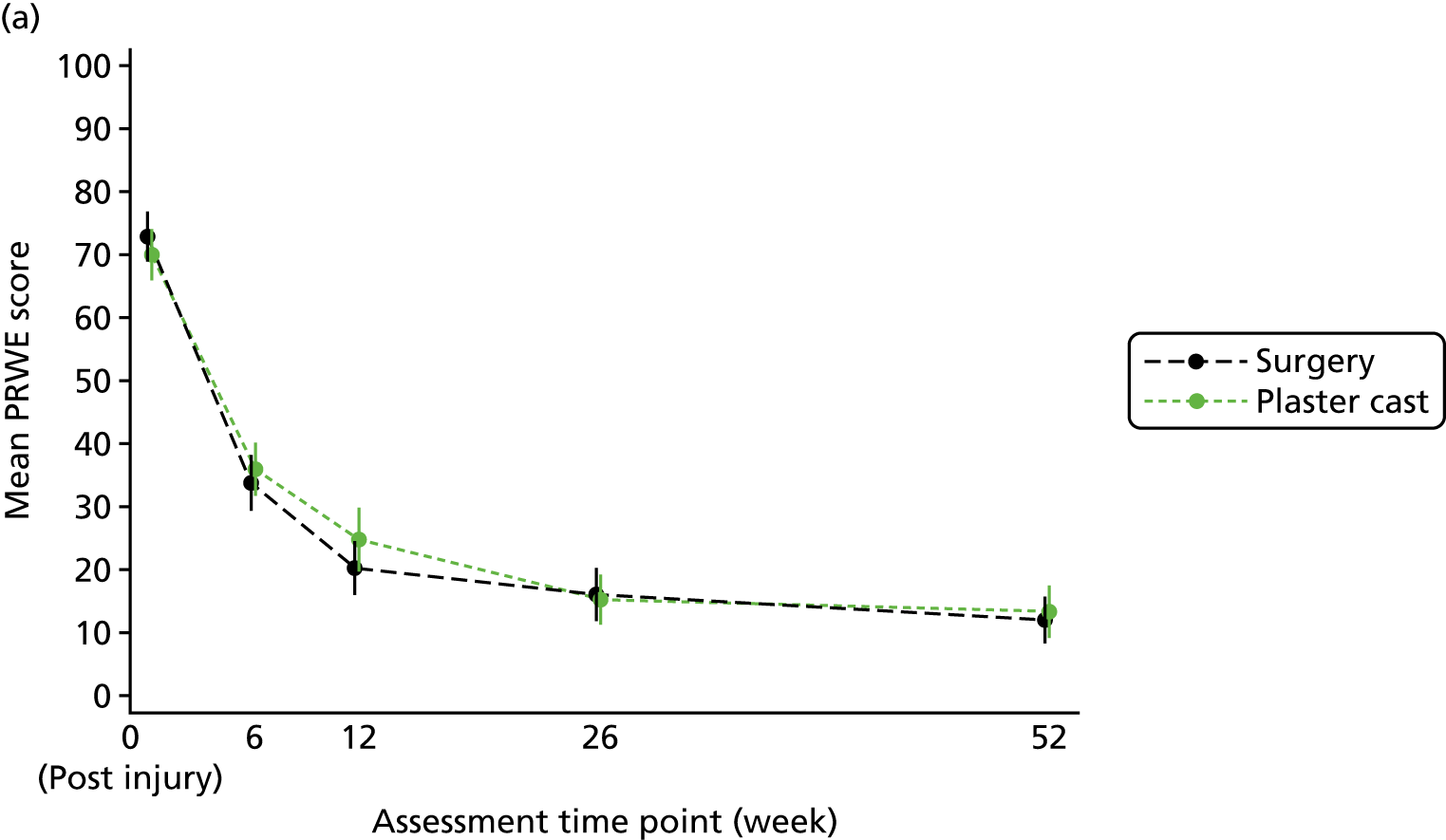

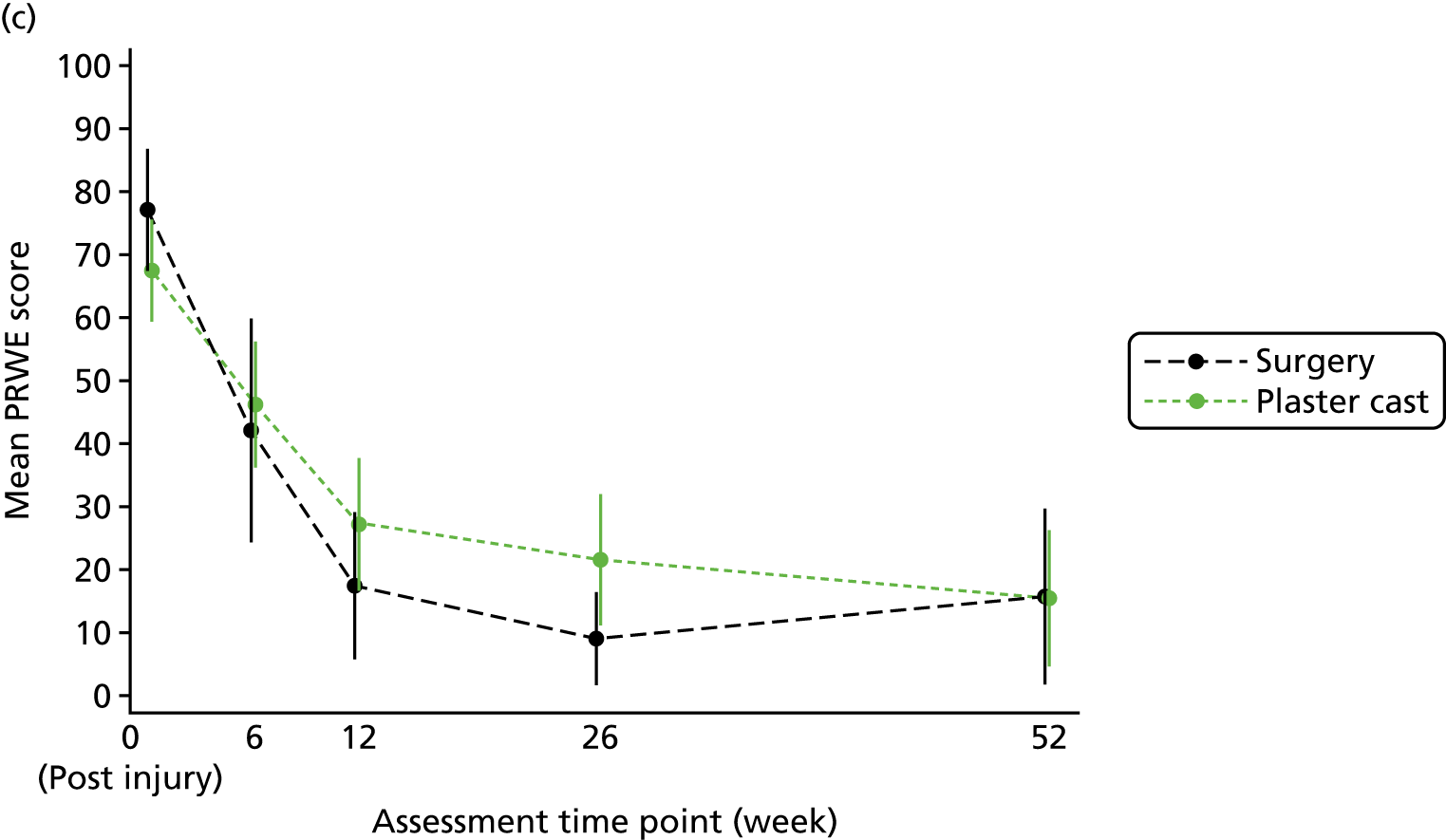
FIGURE 15.
Unadjusted mean PRWE scores (with 95% CIs) over time by randomised group and fracture displacement (as randomised) at baseline: (a) < 1 mm; and (b) ≥ 1 mm and ≤ 2 mm. Modified from Lancet, vol. 396, Dias JJ, Brealey SD, Fairhurst C, Amirfeyz R, Bhowal B, Blewitt N, et al. , Surgery versus cast immobilisation for adults with a bicortical fracture of the scaphoid waist (SWIFFT): a pragmatic, multicentre, open-label, randomised superiority trial, pp. 390–401,89 Copyright (2020), with permission from Elsevier.
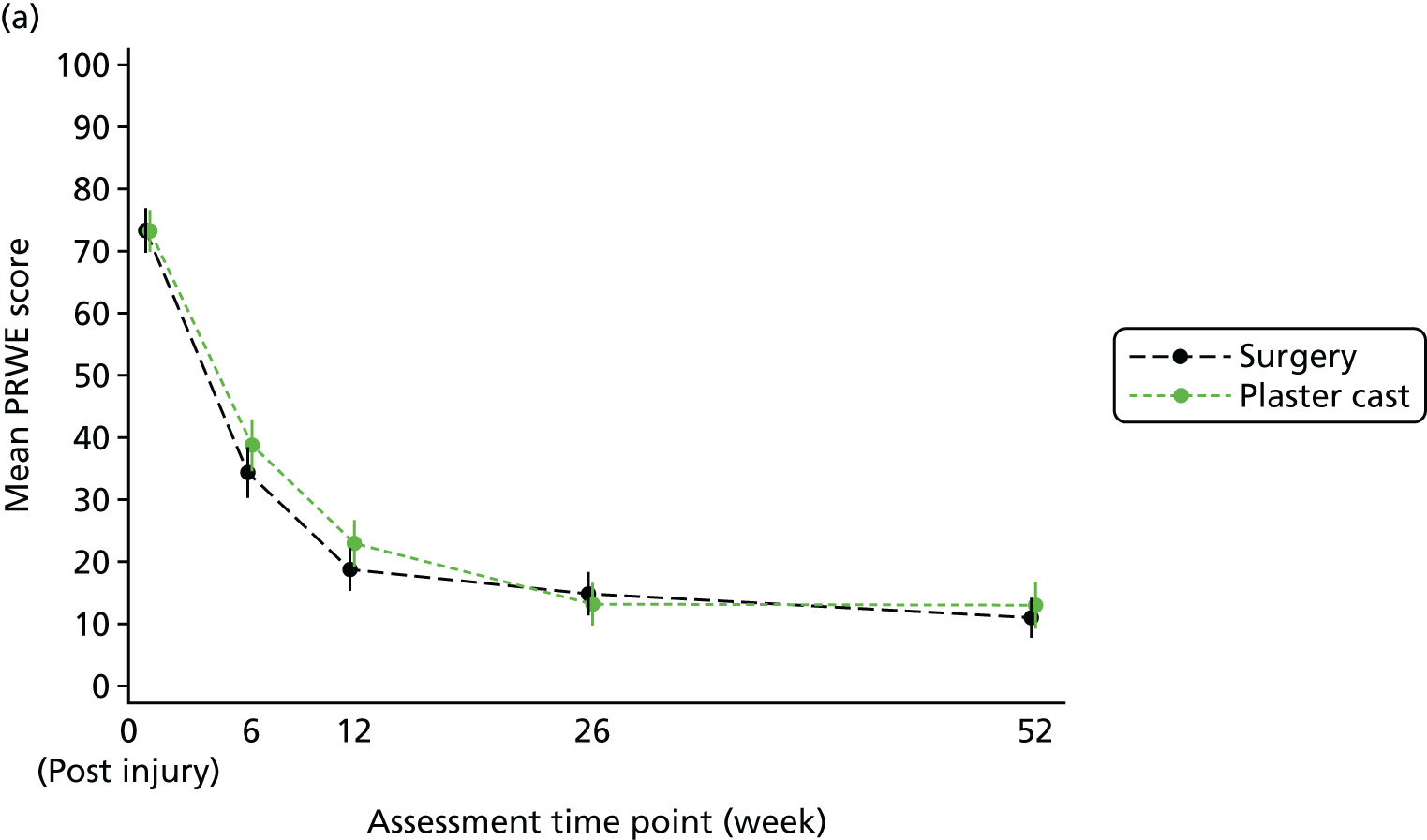

FIGURE 16.
Unadjusted mean PRWE scores (with 95% CIs) over time by randomised group and fracture displacement (as recorded on study eligibility form) at baseline: (a) < 1 mm; and (b) ≥ 1 mm and ≤ 2 mm.

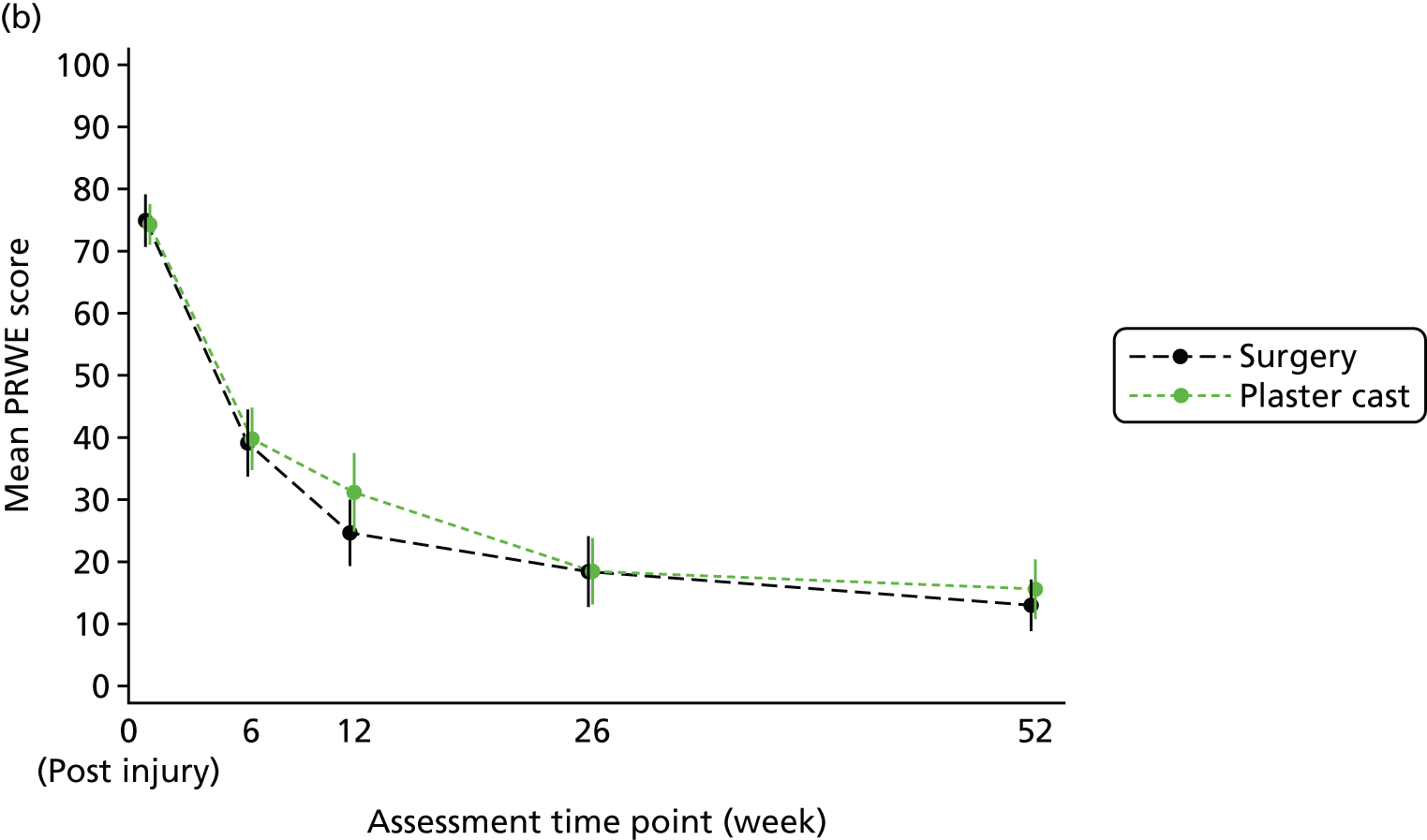
FIGURE 17.
Adjusted mean PRWE subscale scores (with 95% CIs) over time by randomised group: (a) pain subscale; and (b) function subscale.


FIGURE 18.
Adjusted mean SF-12 component subscale scores (with 95% CIs) over time by randomised group: (a) MCS; and (b) PCS.

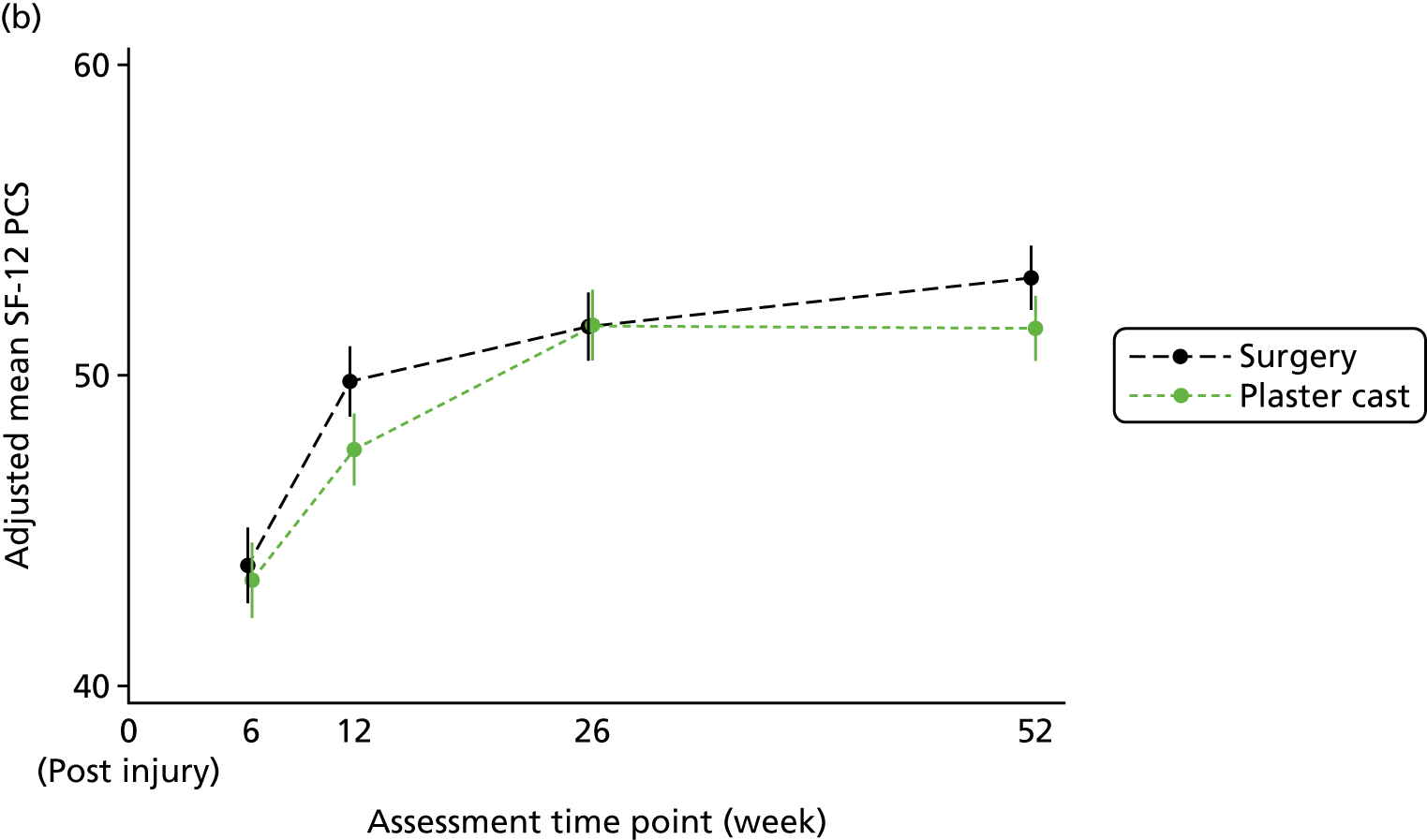
FIGURE 19.
Adjusted mean grip strength (with 95% CIs) over time by randomised group.

Appendix 4 Software output for primary analysis model

Appendix 5 Analysis of the agreement between raters on the imaging
Introduction
This report describes the agreement study that was conducted to validate the radiology assessments that were fed into the trial analysis. All radiographs and CT scans were assessed independently by three raters and, when there was disagreement, the three met to discuss the assessment and a consensus was reached. This study looks at the agreement at the first stage, that is, prior to the consensus meeting.
Methods
The assessments of the three raters were compared in pairs (i.e. A with B, A with C and B with C). When a categorical assessment was required, the measure of agreement was taken to be the percentage of radiographs or CT scans on which the two raters gave exactly the same grade. It was decided that 50% agreement was acceptable and that 80% or greater agreement was good. Percentage agreement is reported together with a 95% CI.
When a continuous assessment was required, the measure of agreement was taken to be the 95% limits of agreement and the data were displayed as a Bland–Altman plot. The limits of agreement depend on the SD of the differences between the two raters and an acceptable agreement was defined as a SD that was less than 25% of the range of the scale. A SD that was less than 10% of the range was considered good. The range was taken to be the difference between the largest and smallest measurements made by either of the two raters.
Fracture
Radiographs at baseline
Rater A versus rater B

Both raters graded 366 radiographs with 62.3% agreement (95% CI 57.3% to 67.3%).
Agreement was classified as acceptable.
Rater A versus rater C
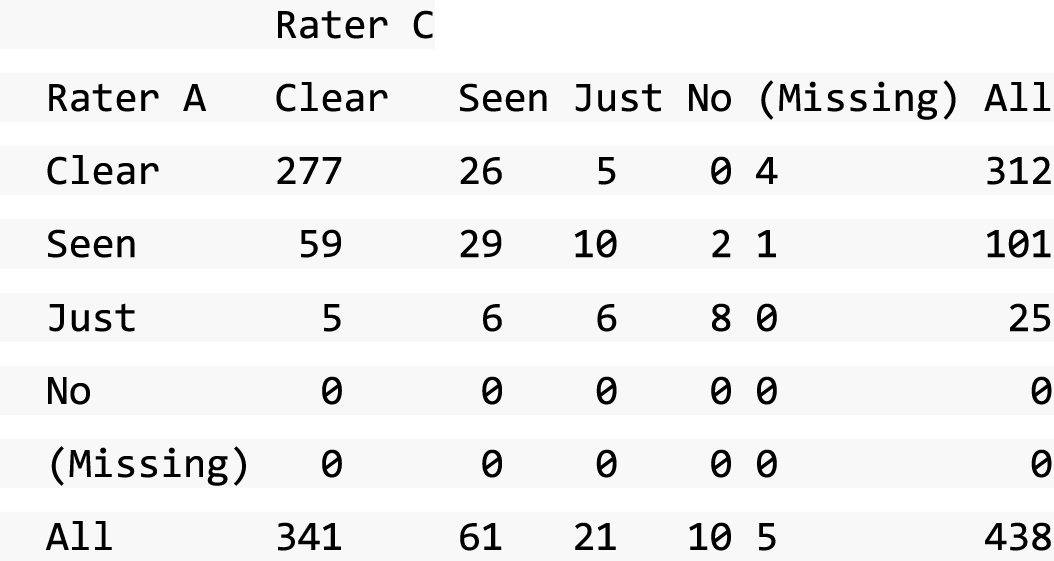
Both raters graded 433 radiographs with 72.1% agreement (95% CI 67.8% to 76.3%).
Agreement was classified as acceptable.
Rater B versus rater C

Both raters graded 365 radiographs with 59.2% agreement (95% CI 54.1% to 64.2%).
Agreement was classified as acceptable.
Computed tomography scan at baseline
Rater A versus rater B

Both raters graded 351 CT scans with 62.1% agreement (95% CI 57% to 67.2%).
Agreement was classified as acceptable.
Rater A versus rater C
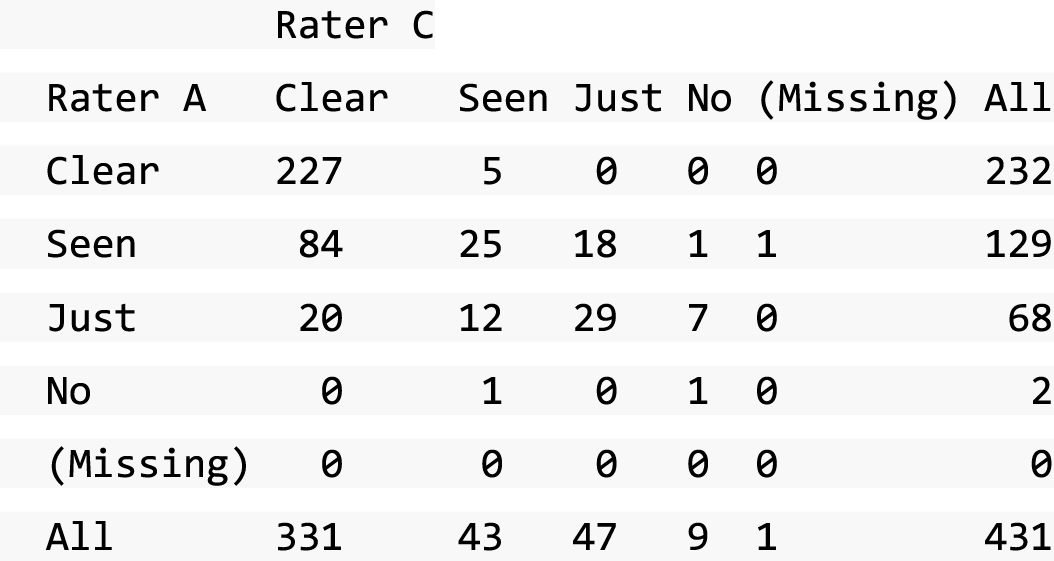
Both raters graded 430 CT scans with 65.6% agreement (95% CI 61.1% to 70.1%).
Agreement was classified as acceptable.
Rater B versus rater C

Both raters graded 350 CT scans with 61.4% agreement (95% CI 56.3% to 66.5%).
Agreement was classified as acceptable.
Displacement at baseline
The Bland–Altman plots of displacement show the difference in the measurement between two raters against their average measurement. Horizontal lines show the average difference (solid) and the 95% limits of agreement (dashed).
Gap in radiographs
Rater A versus rater B
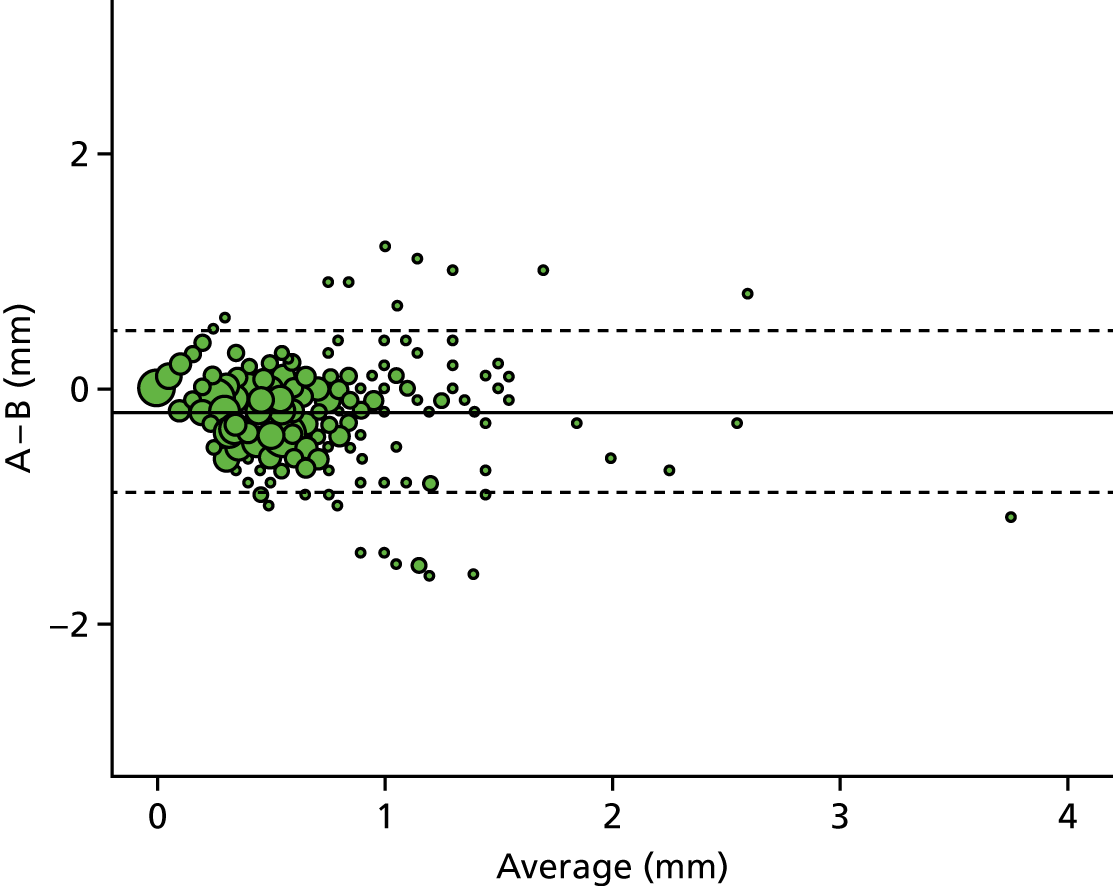
95% limits of agreement: –0.88 mm, 0.5 mm.
The SD of the differences was 0.35 mm, which is 11% of the range of 3.2 mm.
Agreement was classified as acceptable.
Rater A versus rater C

95% limits of agreement: –0.82 mm, 0.7 mm.
The SD of the differences was 0.39 mm, which is 12.2% of the range of 3.2 mm.
Agreement was classified as acceptable.
Rater B versus rater C
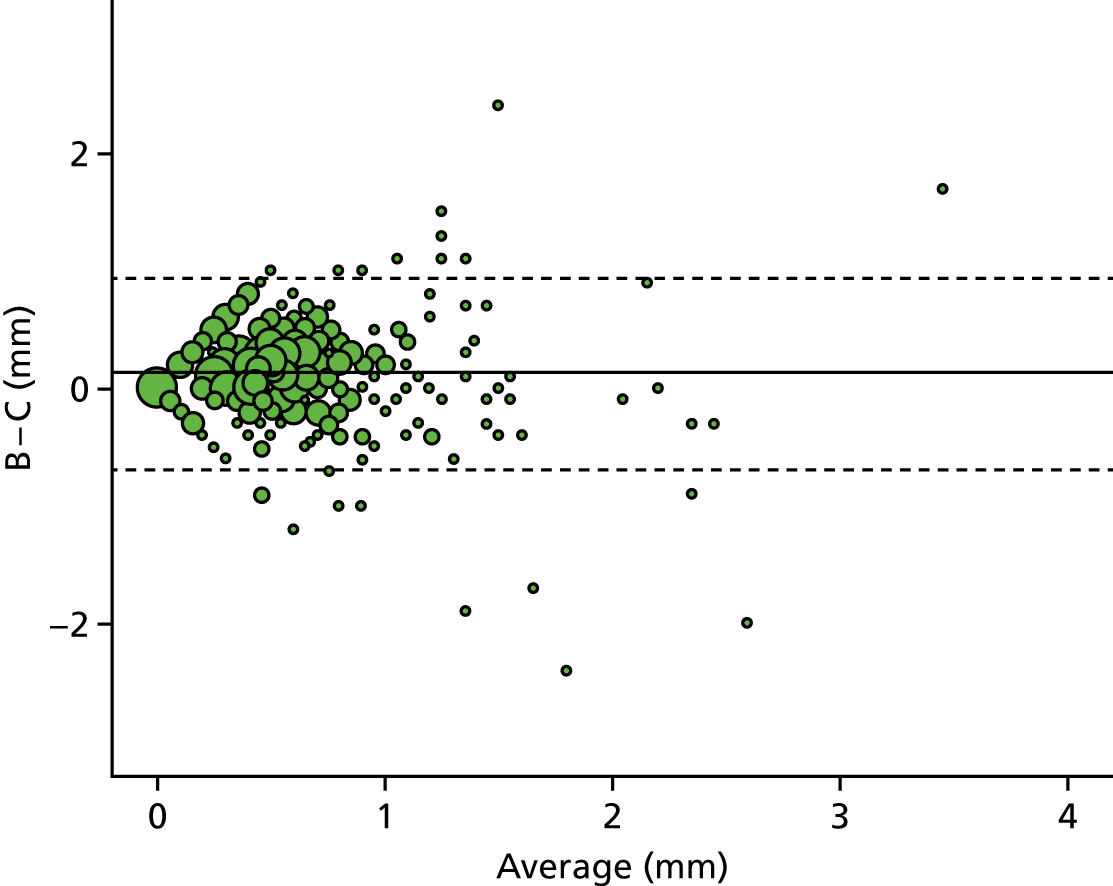
95% limits of agreement: –0.68 mm, 0.95 mm.
The SD of the differences was 0.42 mm, which is 9.7% of the range of 4.3 mm.
Agreement was classified as good.
Step in radiographs
Rater A versus rater B

95% limits of agreement: –0.8 mm, 0.69 mm.
The SD of the differences was 0.38 mm, which is 6.7% of the range of 5.7 mm.
Agreement was classified as good.
Rater A versus rater C

95% limits of agreement: –0.8 mm, 0.82 mm.
The SD of the differences was 0.41 mm, which is 7.2% of the range of 5.7 mm.
Agreement was classified as good.
Rater B versus rater C
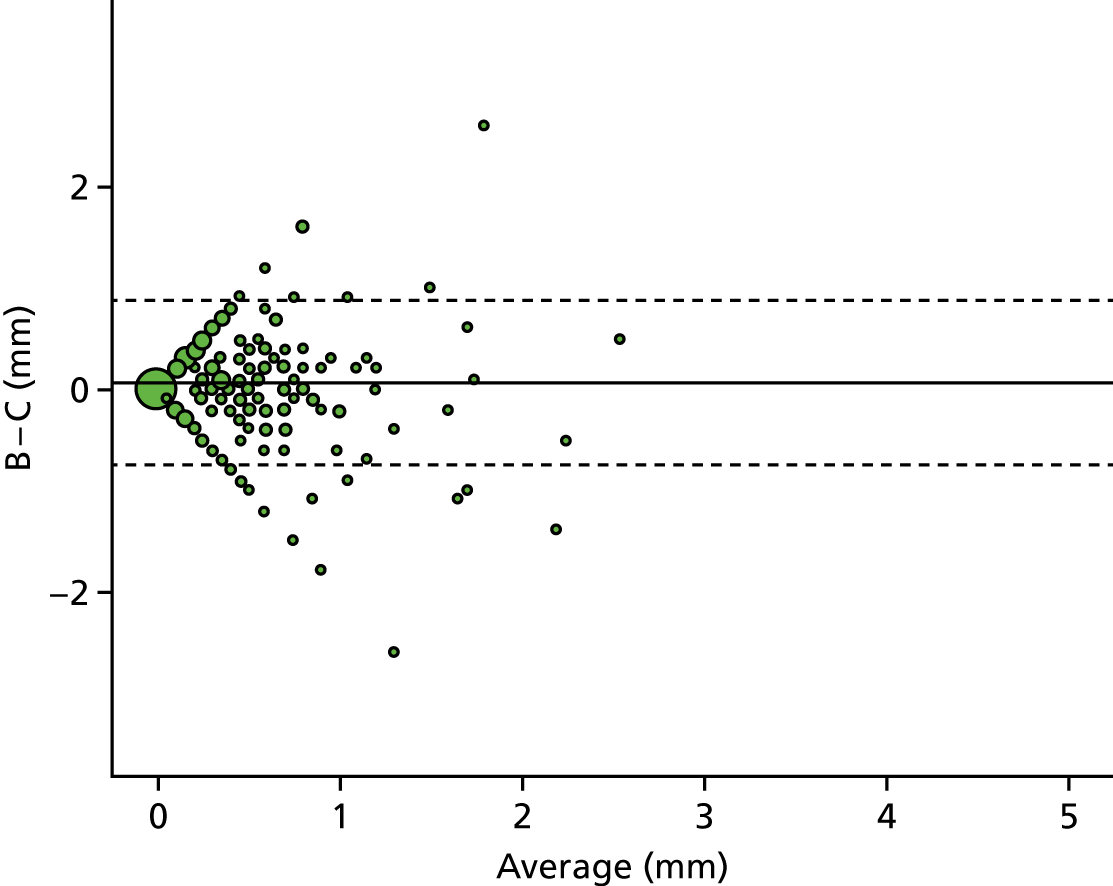
95% limits of agreement: –0.75 mm, 0.89 mm.
The SD of the differences was 0.42 mm, which is 13.5% of the range of 3.1 mm.
Agreement was classified as acceptable.
Gap in computed tomography scan: coronal plane
Rater A versus rater B
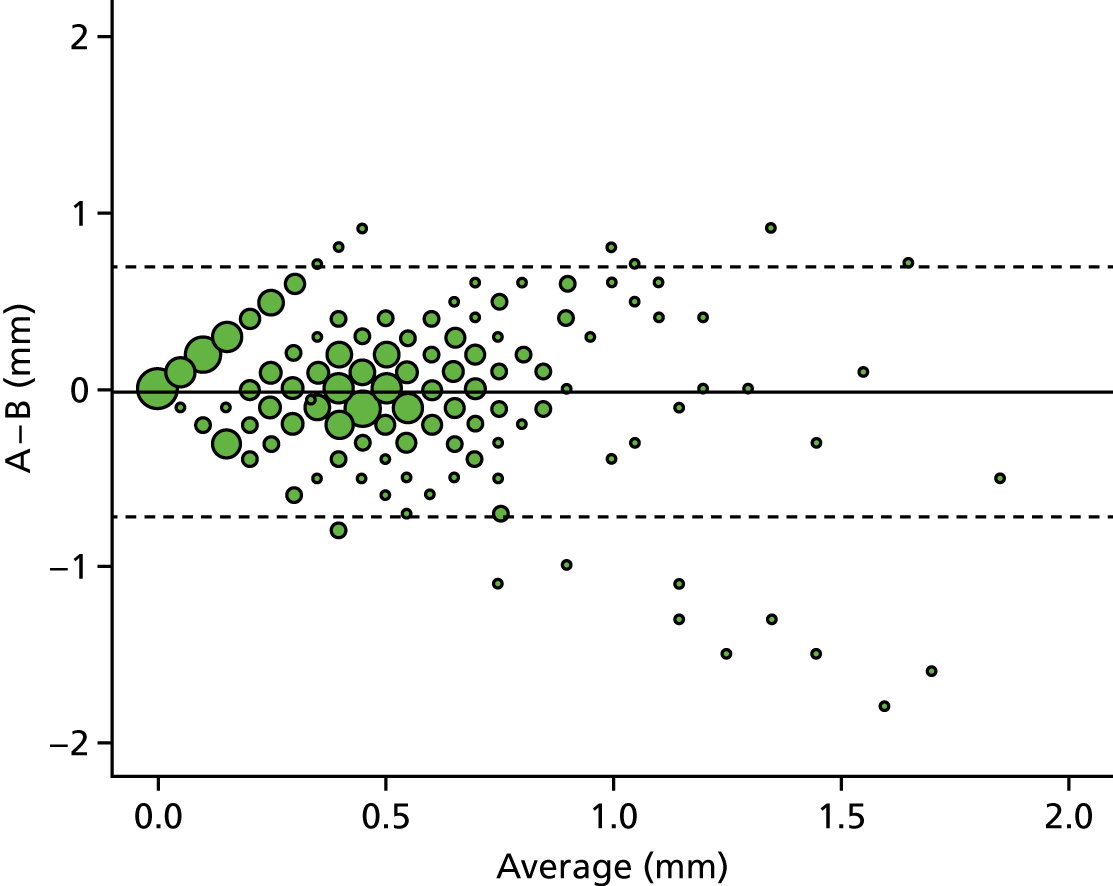
95% limits of agreement: –0.72 mm, 0.7 mm.
The SD of the differences was 0.36 mm, which is 11.3% of the range of 3.2 mm.
Agreement was classified as acceptable.
Rater A versus rater C
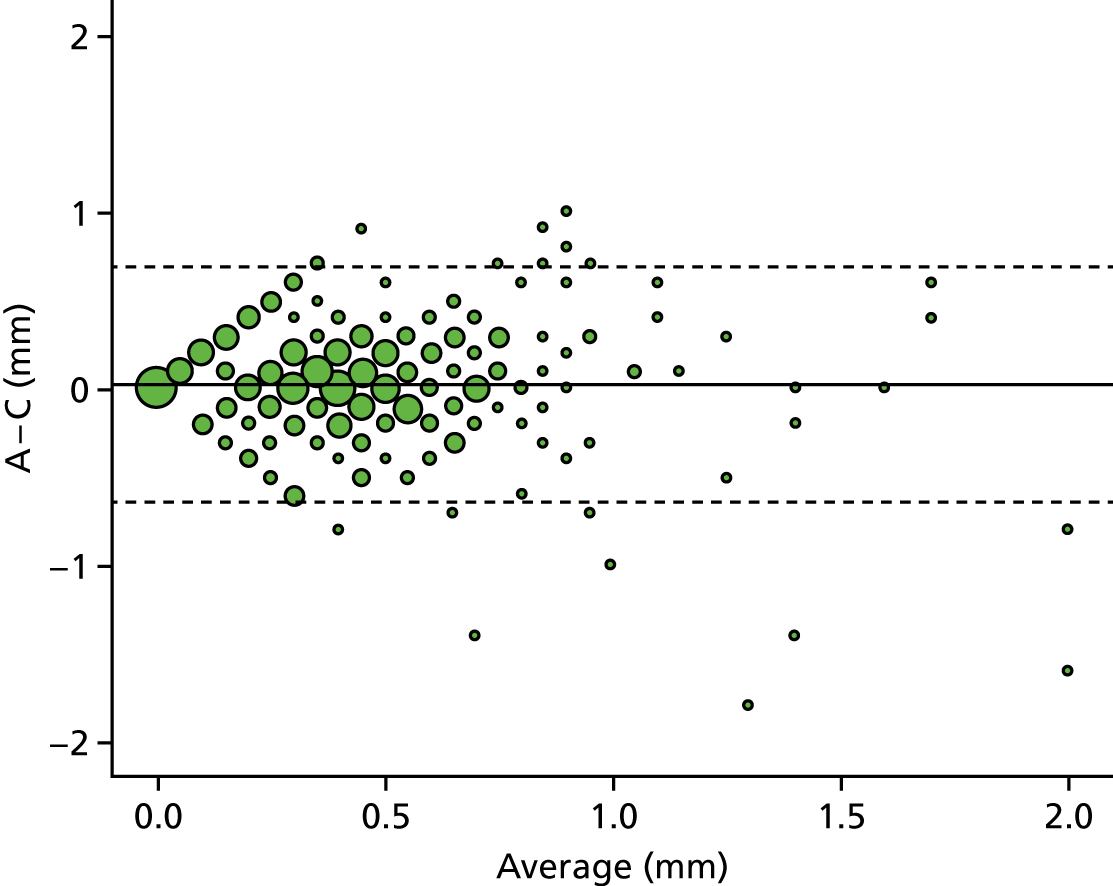
95% limits of agreement: –0.64 mm, 0.69 mm.
The SD of the differences was 0.34 mm, which is 10.6% of the range of 3.2 mm.
Agreement was classified as acceptable.
Rater B versus rater C

95% limits of agreement: –0.62 mm, 0.68 mm.
The SD of the differences was 0.33 mm, which is 9.2% of the range of 3.6 mm.
Agreement was classified as good.
Gap in computed tomography scan: sagittal plane
Rater A versus rater B

95% limits of agreement: –0.89 mm, 0.84 mm.
The SD of the differences was 0.44 mm, which is 7.9% of the range of 5.6 mm.
Agreement was classified as good.
Rater A versus rater C
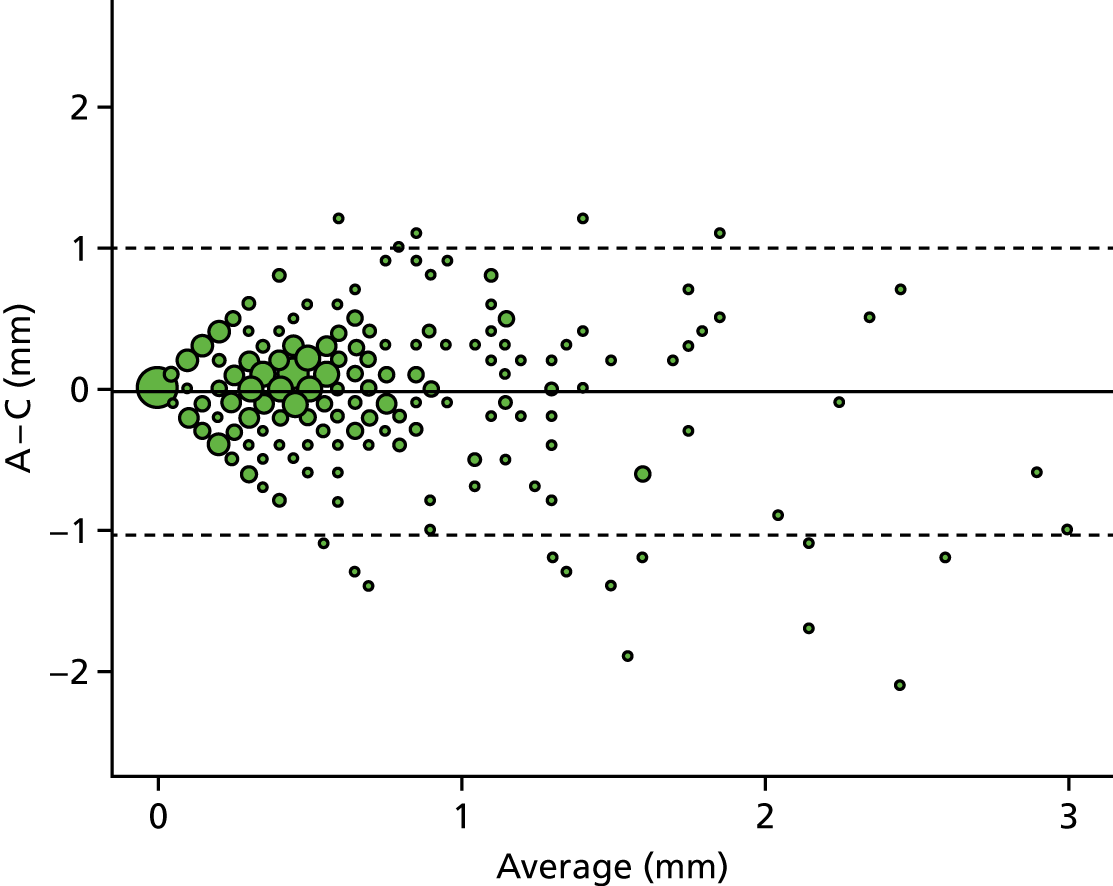
95% limits of agreement: –1.04 mm, 1 mm.
The SD of the differences was 0.52 mm, which is 9.3% of the range of 5.6 mm.
Agreement was classified as good.
Rater B versus rater C
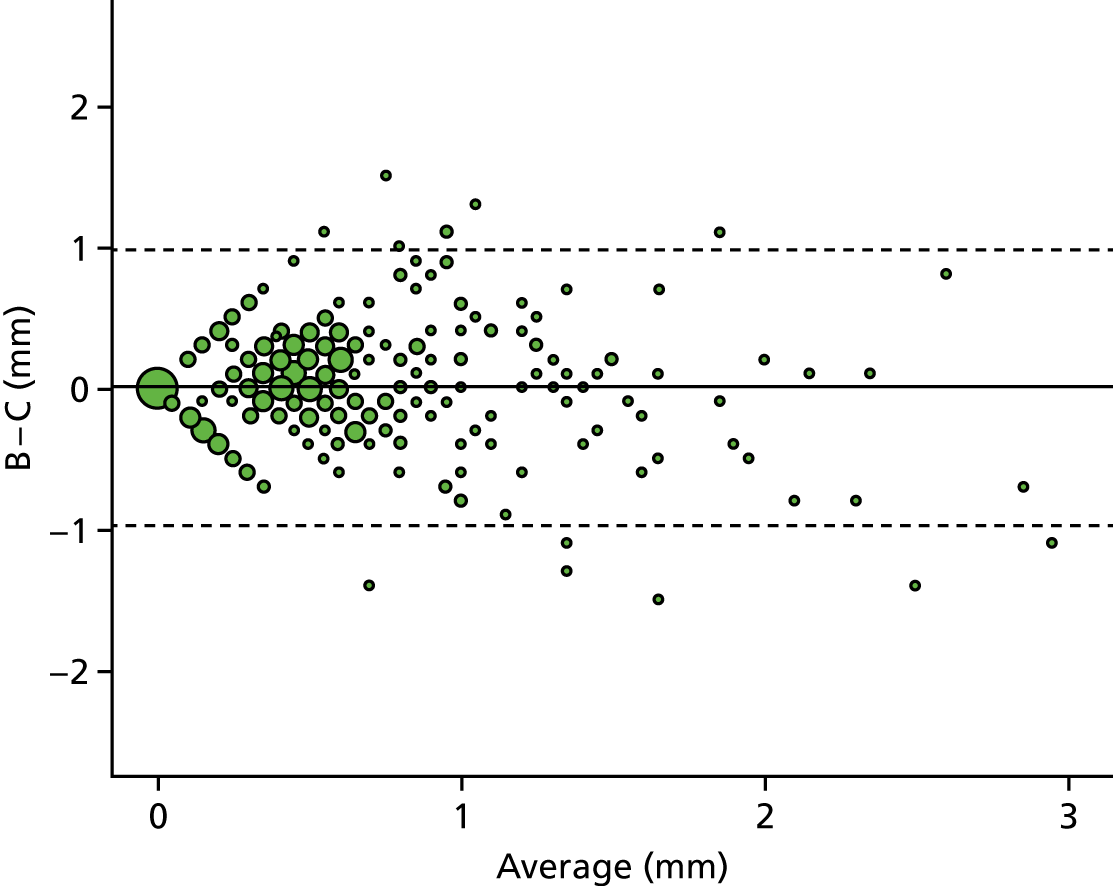
95% limits of agreement: –0.96 mm, 0.98 mm.
The SD of the differences was 0.49 mm, which is 9.9% of the range of 5 mm.
Agreement was classified as good.
Step in computed tomography scan: coronal plane
Rater A versus rater B
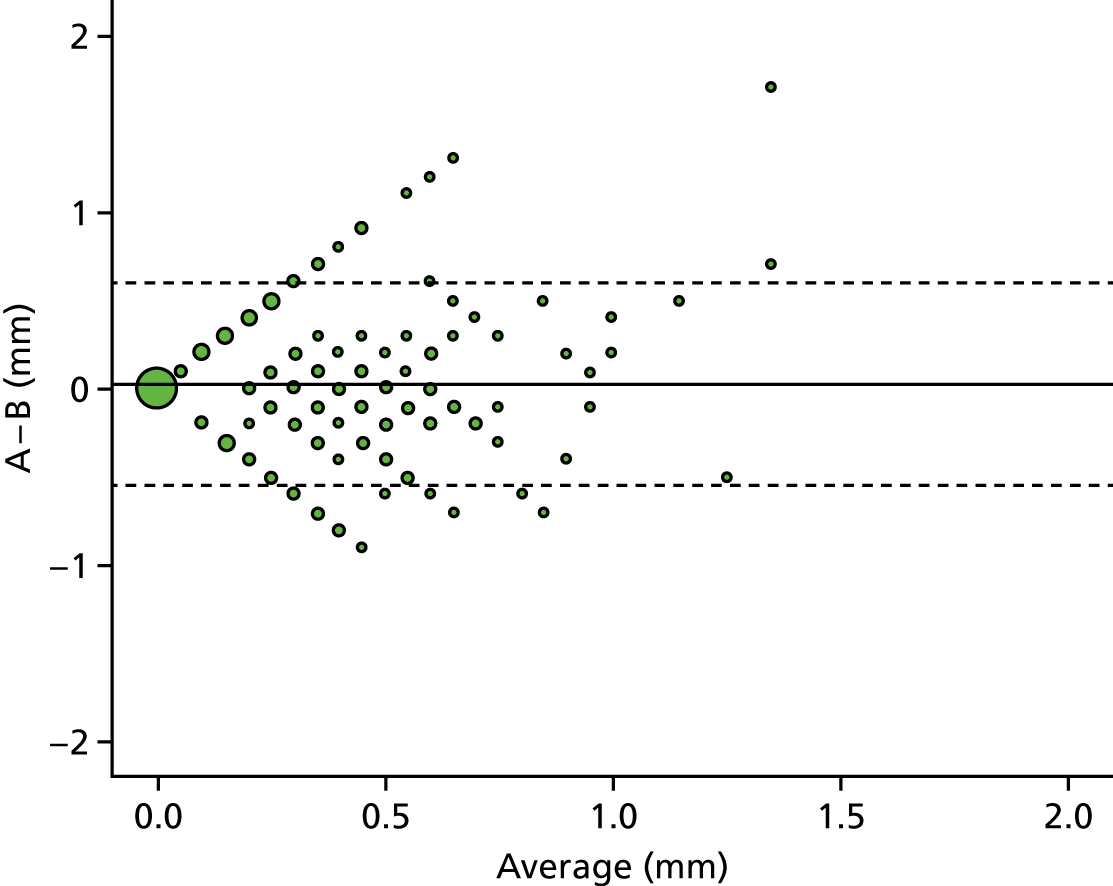
95% limits of agreement: –0.55 mm, 0.61 mm.
The SD of the differences was 0.29 mm, which is 13.4% of the range of 2.2 mm.
Agreement was classified as acceptable.
Rater A versus rater C

95% limits of agreement: –0.46 mm, 0.57 mm.
The SD of the differences was 0.26 mm, which is 11.9% of the range of 2.2 mm.
Agreement was classified as acceptable.
Rater B versus rater C

95% limits of agreement: –0.55 mm, 0.61 mm.
The SD of the differences was 0.3 mm, which is 19.7% of the range of 1.5 mm.
Agreement was classified as acceptable.
Step in computed tomography scan: sagittal plane
Rater A versus rater B

95% limits of agreement: –0.71 mm, 0.82 mm.
The SD of the differences was 0.39 mm, which is 13% of the range of 3 mm.
Agreement was classified as acceptable.
Rater A versus rater C

95% limits of agreement: –0.67 mm, 0.73 mm.
The SD of the differences was 0.36 mm, which is 11.9% of the range of 3 mm.
Agreement was classified as acceptable.
Rater B versus rater C

95% limits of agreement: –0.84 mm, 0.79 mm.
The SD of the differences was 0.41 mm, which is 12.6% of the range of 3.3 mm.
Agreement was classified as acceptable.
Union by radiographs at 52 weeks
Rater A versus rater B
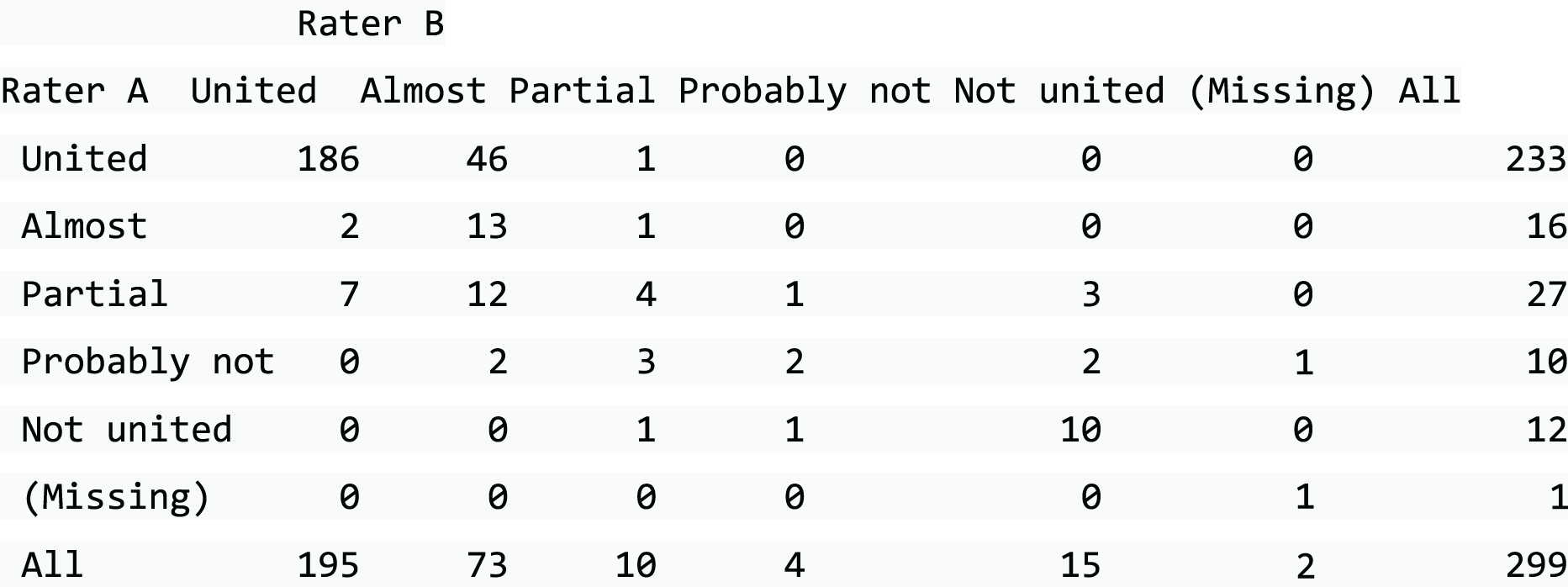
Both raters graded 297 radiographs with 72.4% agreement (95% CI 67.3% to 77.5%).
Agreement was classified as acceptable.
Rater A versus rater C
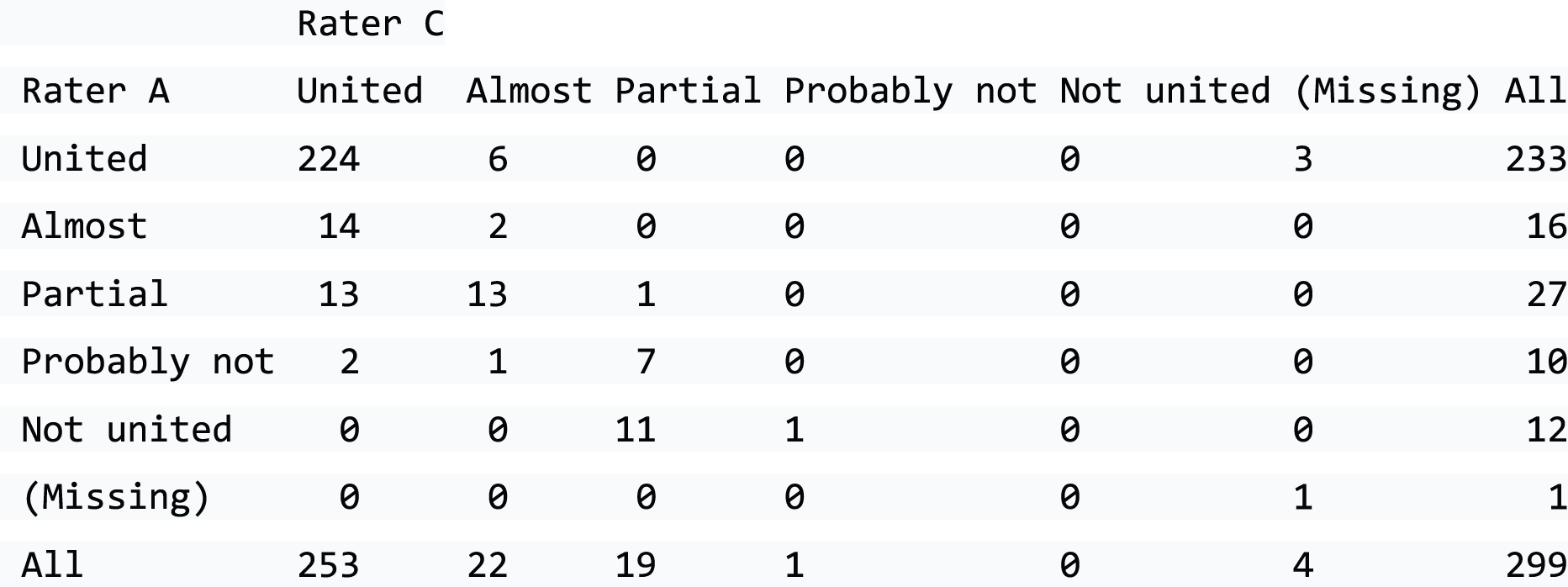
Both raters graded 295 radiographs with 76.9% agreement (95% CI 72.1% to 81.8%).
Agreement was classified as acceptable.
Rater B versus rater C

Both raters graded 294 radiographs with 68.4% agreement (95% CI 63.1% to 73.7%).
Agreement was classified as acceptable.
Percentage of union estimated from computed tomography scan at 52 weeks
Rater A versus rater B
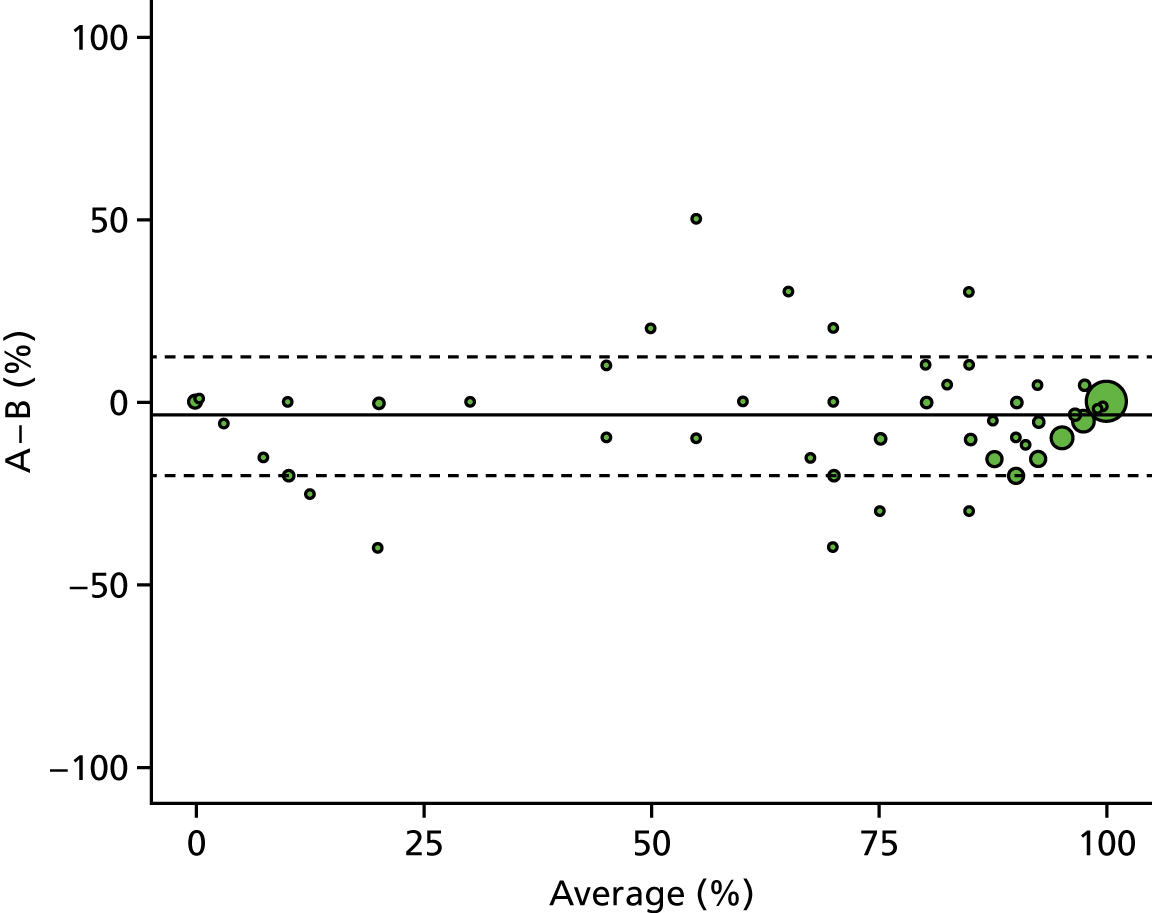
95% limits of agreement: –20.21%, 13.01%.
The SD of the differences was 8.47%, which is 8.5% of the range of 100%.
Agreement was classified as good.
Rater A versus rater C

95% limits of agreement: –27.15%, 18.7%.
The SD of the differences was 11.7%, which is 11.7% of the range of 100%.
Agreement was classified as acceptable.
Rater B versus rater C
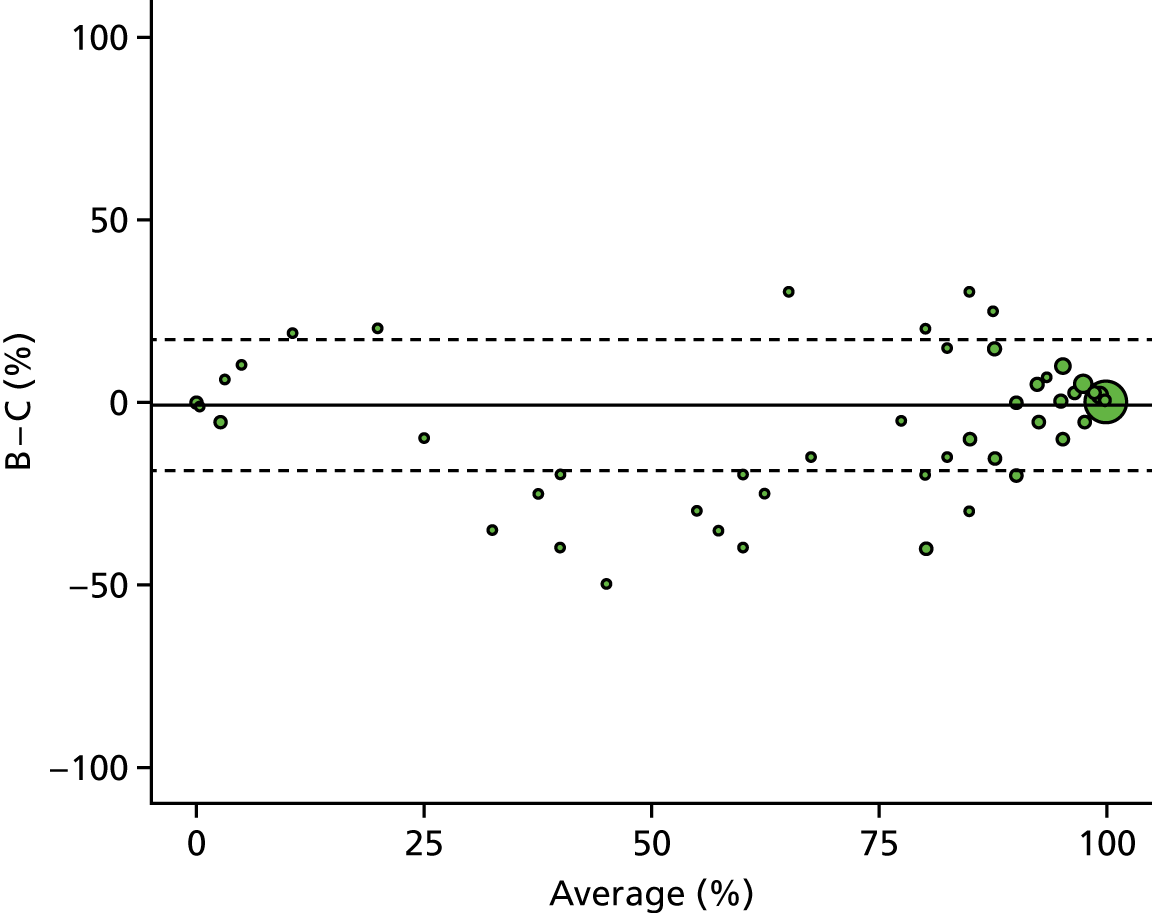
95% limits of agreement: –18.67%, 17.43%.
The SD of the differences was 9.21%, which is 9.2% of the range of 100%.
Agreement was classified as good.
Union by radiographs at 52 weeks: summary
Rater A versus rater B
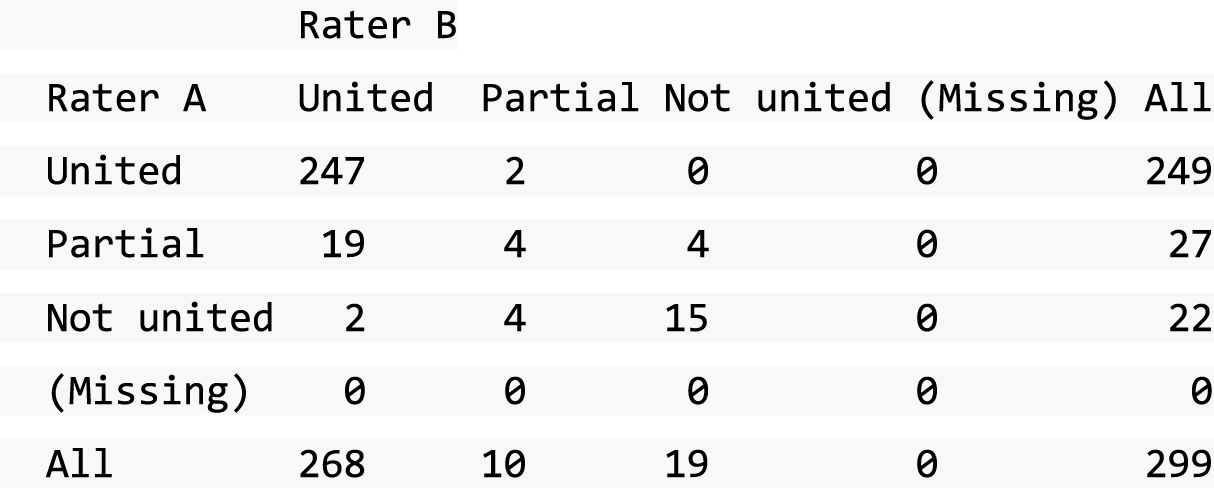
Both raters graded 297 CT scans with 89.6% agreement (95% CI 86.1% to 93%).
Agreement was classified as good.
Rater A versus rater C
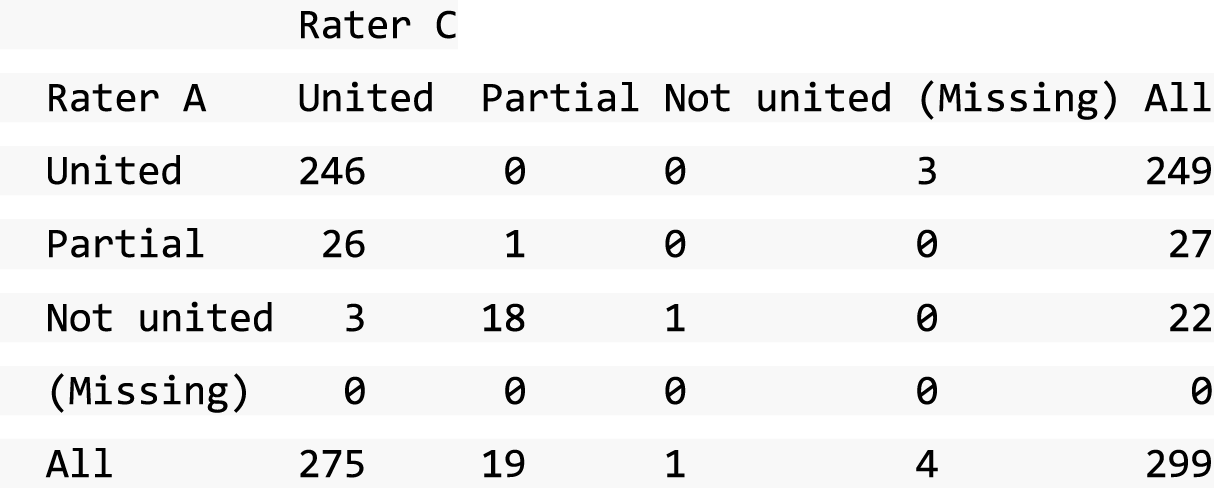
Both raters graded 295 CT scans with 84.1% agreement (95% CI 79.9% to 88.2%).
Agreement was classified as good.
Rater B versus rater C

Both raters graded 294 CT scans with 91.5% agreement (95% CI 88.3% to 94.7%).
Agreement was classified as good.
Union by radiographs at 52 weeks: views
Rater A versus rater B

Both raters graded 297 CT scans with 76.4% agreement (95% CI 71.6% to 81.3%).
Agreement was classified as acceptable.
Rater A versus rater C
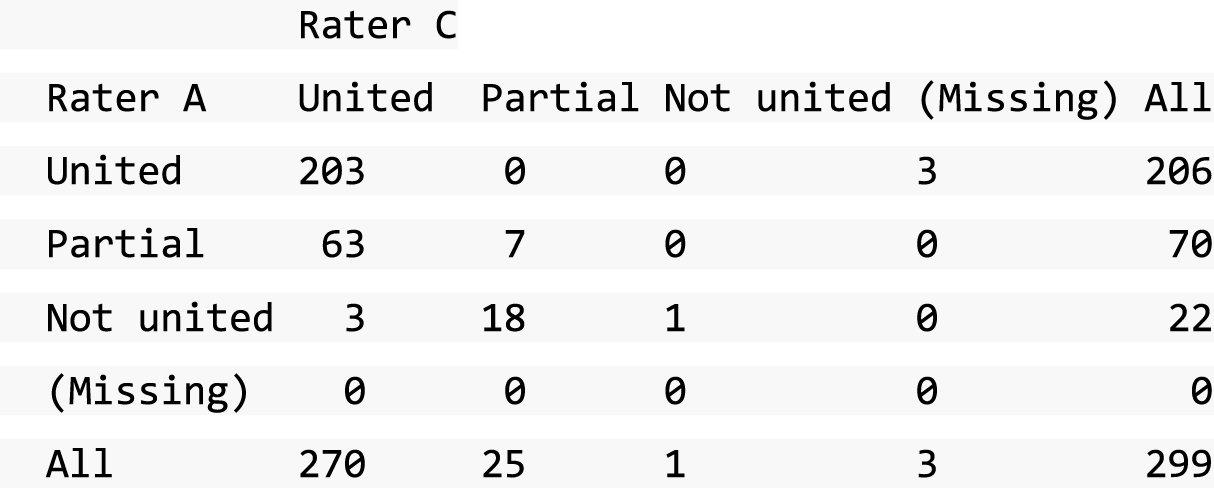
Both raters graded 295 CT scans with 71.5% agreement (95% CI 66.4% to 76.7%).
Agreement was classified as acceptable.
Rater B versus rater C
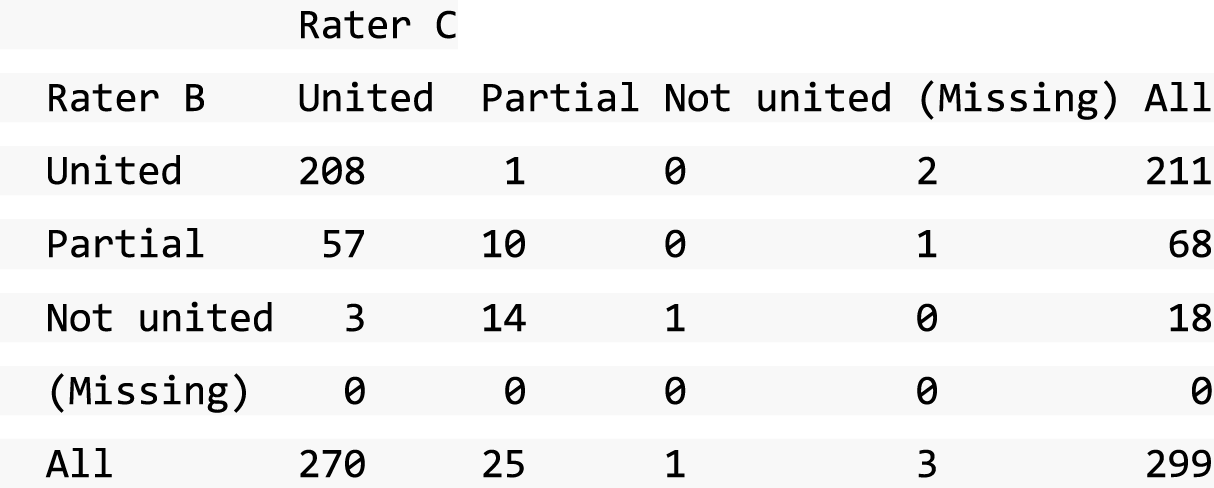
Both raters graded 294 CT scans with 74.5% agreement (95% CI 69.5% to 79.5%).
Agreement was classified as acceptable.
Union by computed tomography scan at 52 weeks: multiplanar reconstruction
Rater A versus rater B
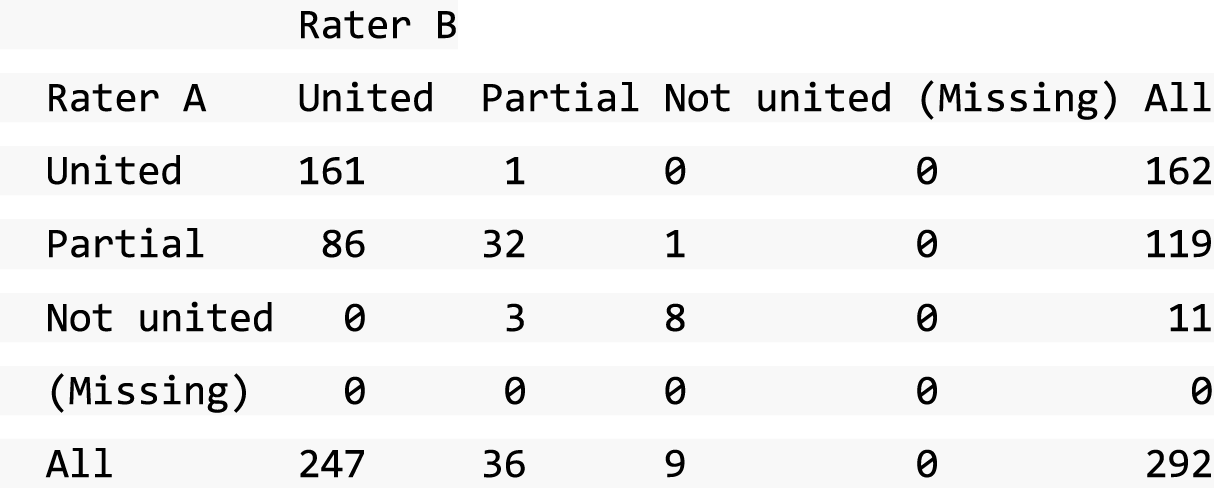
Both raters graded 292 CT scans with 68.8% agreement (95% CI 63.5% to 74.1%).
Agreement was classified as acceptable.
Rater A versus rater C

Both raters graded 289 CT scans with 76.1% agreement (95% CI 71.2% to 81%).
Agreement was classified as acceptable.
Rater B versus rater C

Both raters graded 289 CT scans with 84.4% agreement (95% CI 80.2% to 88.6%).
Agreement was classified as good.
Union by computed tomography scan at 52 weeks: estimated
Rater A versus rater B

Both raters graded 292 CT scans with 95.2% agreement (95%CI 92.8 to 97.7%).
Agreement was classified as good.
Rater A versus rater C
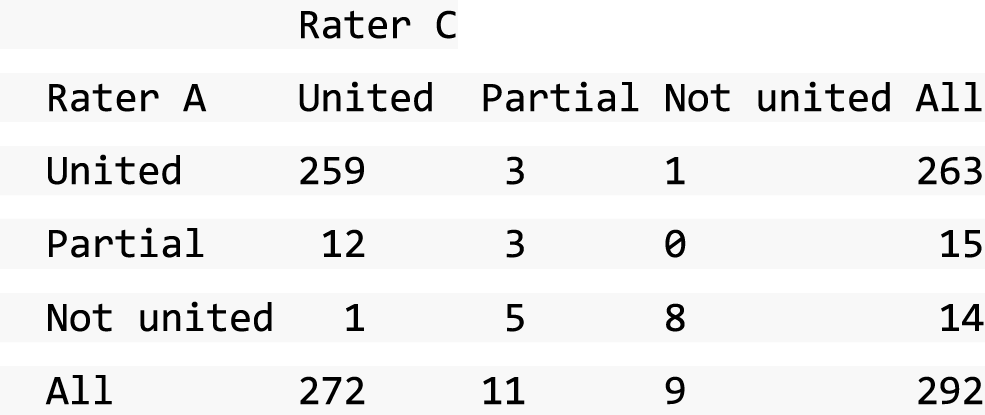
Both raters graded 292 CT scans with 92.5% agreement (95% CI 89.4% to 95.5%).
Agreement was classified as good.
Rater B versus rater C

Both raters graded 292 CT scans with 94.5% agreement (95% CI 91.9 to 97.1%).
Agreement was classified as good.
Union by computed tomography scan at 52 weeks: calculated
Rater A versus rater B
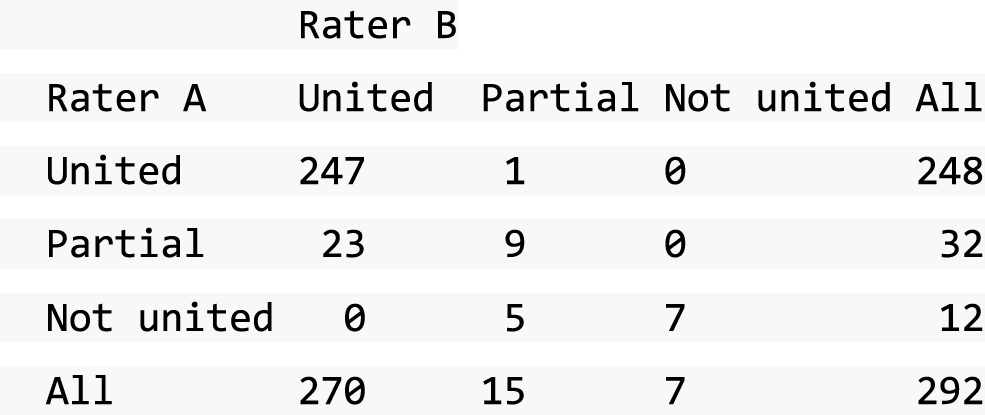
Both raters graded 292 CT scans with 90.1% agreement (95% CI 86.6% to 93.5%).
Agreement was classified as good.
Rater A versus rater C

Both raters graded 292 CT scans with 90.4% agreement (95% CI 87% to 93.8%).
Agreement was classified as good.
Rater B versus rater C
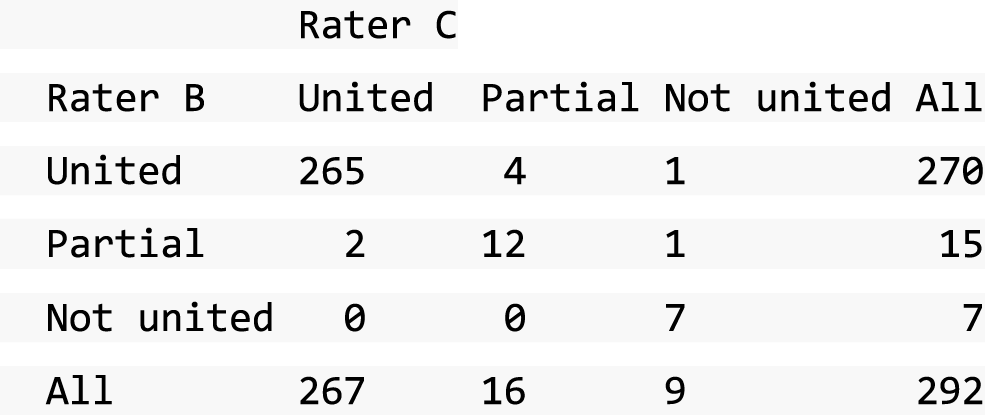
Both raters graded 292 CT scans with 97.3% agreement (95% CI 95.4% to 99.1%).
Agreement was classified as good.
Discussion
Agreement was either acceptable or good on all scales.
Appendix 6 Health economics
Impact of lost employment and unpaid activities
In addition to considering the costs to the NHS and PSS and the QoL of the patients, this within-trial analysis reports the impact of treatment allocation on days of lost employment and unpaid activities. As part of the questionnaires, patients were asked to report if they were in paid employment, how many days over the period covered by the questionnaire they missed as a result of their wrist injury and how many days of unpaid activity they lost. While such outcomes are not conventionally incorporated into an economic evaluation in a UK setting,91 they can be helpful for informing decision-makers who choose to take a broad definition of benefit (i.e. beyond patient QoL and costs to the NHS) or patients who may choose to have the surgical procedure done through private health care to reduce the number of days of work lost. Brief summary statistics are reported for the four relevant time periods, stratified by treatment allocation.
Results
Impact of lost employment
The summary statistics of the patient-reported days of lost employment are reported in Table 35. Primarily, the table shows that the majority of patients experienced some days of lost employment in the first 6 weeks of the analysis period (with only 21.6 % and 31.3% reporting no lost days over that period in the surgery and plaster cast groups, respectively), but from 12 weeks onwards most were back to full-time work (with medians of 0 for all other periods). There did, however, remain a number of patients who were forced to continue missing work as a result of their wrist, characterised by the persistent mean number of days lost, despite close to 90% reporting no lost days. Only a very small number of patients had to miss work for most if not all of the period covered by the questionnaire.
Over the entire within-trial period for the complete case analysis, surgical patients reported having lost a smaller mean number of days than patients in the plaster cast group (16.62 compared with 17.57 days, respectively) but a larger median number of days (9.5 compared with 5 days, respectively), and surgical patients also reported fewer no days of work lost (13.7% compared with 28.6%, respectively). Using the sum of the means for each time period, a larger difference is observed between the two groups, with mean lost days of employment being 17.30 and 21.69 days, respectively, suggesting a biasing impact of the inconsistent level of missing data.
This distribution is best explained by the invasive nature of the surgery, necessitating at least some days off work in more patients, as well as the large impact of a few extreme cases in which patients were absent from work for the majority of the year. These outliers appear to be more evident in the plaster cast arm, with 10 patients reporting more than 50 days off work, compared with six patients in the surgical arm. However, the high rate of missing data may be having a large biasing effect.
These findings appear inconsistent with other studies focusing on the impact of scaphoid fracture on fixation,44,45 which have found much larger differences in days of work lost, favouring surgery; however, the source of this difference is not clear.
Using estimates of the average weekly earnings from the Office for National Statistics (£512)165 allows us to crudely estimate the average societal impact of these lost days of employment, namely £1702 per person for the surgical arm and £1799 for the cast arm, a difference of £97 per person. However, it is important to note that, without an estimate of the number of days of work a person would lose if no treatment was available, it is not possible to interpret these results beyond their comparative value.
Impact on days of unpaid activity
Similar to the previous section, Table 68 reports the impact of treatment allocation on lost unpaid activity (e.g. household chores, shopping and helping others) for reasons related to the wrist injury and subsequent treatment.
| Questionnaire period | Treatment allocation | Number of responses | Mean days lost | Median days lost (95% percentile) | Percentage reporting 0 days |
|---|---|---|---|---|---|
| 6 weeks | Surgery | 167 | 12.41 | 10 (40) | 28.1 |
| Cast | 162 | 10.13 | 6 (35) | 35.8 | |
| 12 weeks | Surgery | 163 | 2.21 | 0 (18) | 83.4 |
| Cast | 151 | 5.03 | 0 (35) | 66.9 | |
| 26 weeks | Surgery | 150 | 1.77 | 0 (14) | 89.3 |
| Cast | 140 | 3.51 | 0 (25) | 85.7 | |
| 52 weeks | Surgery | 169 | 1.08 | 0 (5) | 91.7 |
| Cast | 163 | 2.73 | 0 (4) | 91.4 | |
| Total | Surgery | 118 | 16.15 | 11 (54) | 21.2 |
| Cast | 103 | 15.90 | 8 (52) | 26.2 |
The table shows that surgery had a larger impact on lost unpaid activity than plaster cast for the 6 weeks after randomisation, with larger mean and median values and fewer patients reporting no days lost. However, for all time points after this, patients allocated to the surgical arm report a smaller impact on unpaid activities than those in the plaster cast arm. As was seen in the responses to lost days of employment, the results are highly skewed, with the majority of responders reporting no impact on unpaid activity after 6 weeks but a very small number continuing to report a large number of lost days throughout the trial period.
Systematic review of previous cost-effectiveness studies
This appendix details the literature review that was conducted to determine whether or not previous economic evaluations had sufficiently determined the cost-effectiveness of surgical fixation versus plaster cast immobilisation for treatment of bicortical, minimally displaced fractures of the scaphoid waist in adults. A secondary aim of the search was to determine if previous mathematical models could be adapted to estimate the long-term cost-effectiveness of the population and thus remove the need to construct a de novo mathematical model.
Our strategy (detailed in the following section) did not specify the form of treatment required, if the evaluation considered the diagnosis or treatment of the fracture or the extent of displacement of the fracture. The strategy was submitted to Ovid MEDLINE in April 2017, with all published, in process and other non-indexed citations in any language between 1946 and April 2017 allowed. The review was conducted by a single reviewer.
Systematic search strategy
The search was conducted using Ovid MEDLINE through the University of York library on 4 April 2017.
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R), from 1946 to the present.
-
fracture fixation (17,279)
-
fracturers, bone (59,494)
-
orthopedic fixation devices (4975)
-
1 or 2 or 3 (75,839)
-
scaphoid bone (1850)
-
wrist joint (8976)
-
wrist injuries (5824)
-
5 or 6 or 7 (14,701)
-
cost–benefit analysis (70,573)
-
4 or 8 or 9 (24)
In addition to the strategy submitted to MEDLINE (detailed above), a search of the grey literature was conducted. The search consisted of iterative investigations of the literature identified using an internet search engine (Google Scholar; Google Inc., Mountain View, CA, USA), coupled with a targeted search of NICE’s website to explore any economic evaluations behind current best practice guidance.
The MEDLINE search yielded 24 hits, 16 of which were deemed relevant on review of their titles and only three of these were relevant after consideration of their abstracts. 43,166,167 The search of the grey literature identified only one fully relevant article from the NICE website and none from the internet search engine exploration. Consistent with the search strategy, the relevance of the studies was defined by the existence of some form of economic evaluation at any point of the diagnostic or treatment pathway of a fracture of the wrist region. No further limits on the type of injury or point of care were placed to ensure that all studies considering cast immobilisation versus surgical fixation were included. As a result, some of the studies deemed to be relevant considered the pre-treatment decision of how different diagnostic strategies compared, but, as these implicitly included the consequences of the treatment used, they were deemed relevant.
The four studies identified43,88,166,167 were reviewed using the Drummond Checklist for assessing the quality of economic evaluations,101 the results of which are available later in this appendix.
Of the four studies, two considered the cost-effectiveness of different diagnosis strategies for suspected scaphoid fractures. 88,167 Karl et al. 167 explored the relative cost-effectiveness of three strategies – empiric cast immobilisation for all patients followed by follow-up radiographs, immediate CT scan and immediate magnetic resonance imaging (MRI) – in a population of patients deemed to have a possible scaphoid fracture after physical examination, but with a negative initial radiograph. The authors concluded that initial advanced imaging dominated empiric casting.
Similarly, the economic evaluation that accompanied the NICE guideline (NG38)88 primarily focused on the cost-effectiveness of suspected scaphoid fractures based on imaging rather than the appropriate treatment pathway, but contains valuable insights into the pathway. This analysis considered a slightly earlier problem than Karl et al. , being prior to initial investigation. As a result, the decision problem considered whether MRI or CT scans should be used as the first-line diagnostic tool, and thus considered a broader population than Karl et al. , by considering all those with suspected scaphoid fractures rather those with indeterminate initial radiology findings. The analysis included the possibility of patients having cast immobilisation and/or surgical fixation, but these were considered to be accepted best practice depending on displacement and union, and as such no analysis of their cost-effectiveness was conducted. The evaluation concluded that the use of immediate MRI was the most cost-effective approach, dominating all other strategies except immediate CT scans.
As both Karl et al. 167 and NICE NG3888 focused on the cost-effectiveness of the diagnostic strategies prior to confirmed diagnosis and considered the treatment options to be fixed decisions, they are not directly applicable to the SWIFFT evaluation.
The study by Hannemann et al. 166 considered the cost-effectiveness of pulsed electromagnetic fields in the treatment of acute undisplaced scaphoid fractures, compared with standard cast immobilisation. While relevant to the SWIFFT evaluation as an evaluation of treatment options in scaphoid fractures, Hannemann et al. consider a less severely affected population than SWIFFT, including patients with tuberosity fractures. In addition, the study does not conduct a long-term mathematical model, instead being limited to a within-trial analysis over 52 weeks of follow-up.
Finally, Davis et al. 43 conducted an evaluation of the cost-effectiveness of open reduction and internal fixation surgery versus cast immobilisation for acute non-displaced scaphoid fractures. As a result, the study is relevant to this evaluation. The study is structured based on a decision tree model that estimates the probability of a patient experiencing one of six possible outcomes after treatment with cast immobilisation (no complications, non-union and delayed union, each with a normal and arthritis variant) and seven possible outcomes after surgical fixation (the same as the cast arm but with the addition of a risk of infection). The model estimates the cost per QALY gained of each treatment by combining the short-term cost and HRQoL of the different treatment outcomes with long-term estimates of the HRQoL implications of each outcome; no long-term costs appear to be considered despite the existence of a long-term arthritis outcome.
As reflected in the review of the Davis study presented in Table 69, there are a number of areas of the model that were difficult to fully assess. The decision tree and related characterisation of the different possible post-treatment outcomes are good, considering the potential for non-union and delayed union, infection, misplaced screws and arthritis. However, our review of the article raised a number of factors that limit the transferability of the model to the SWIFFT analysis. These factors included the lack of long-term cost associated with arthritis, a lack of clarity regarding which patients were associated with which resource use, the use of an unvalidated time trade-off questionnaire completed by 50 medical students who had not experienced the fracture, a lack of clarity with regard to how the durations and probabilities that informed the model were derived from the literature and the lack of correlation between non-union and the probability of arthritis. Furthermore, the lack of definition of non-displacement by Davis et al. makes the transferability of the model to the SWIFFT population difficult to determine.
Therefore, we determined that, in addition to the planned within-trial economic evaluation of SWIFFT, a de novo mathematical model was required to investigate the long-term cost-effectiveness of surgical fixation compared with cast immobilisation. The de novo model is expected to draw from the Davis study, but this will be determined by the results of a series of targeted parameter-specific literature reviews.
Drummond Checklist review
| Study | Was a well-defined question posed in an answerable form? | Was a comprehensive description of the competing alternatives given? | Was the effectiveness of the programmes or services established? | Were all important and relevant costs and consequences for each alternative identified? | Were costs and consequences measured accurately in appropriate physical units? | Were costs and consequences valued credibly? | Were costs and consequences adjusted for differential timing? | Was an incremental analysis of costs and consequences of alternatives performed? | Was allowance made for uncertainty in the estimates of costs and consequences? | Did the presentation and discussion of results include all issues of concern to users? |
|---|---|---|---|---|---|---|---|---|---|---|
| Davis et al. (2006)43 | Yes. There is a clear focus from both a direct and an indirect perspective | Yes. All options are considered; there is no explicit ‘do nothing’, but perhaps this is not appropriate. There is limited detail on the surgical option | Yes. Had to rely on limited RCT data. Based on a systematic review, but simple averages are taken | Partially. Short-term direct and indirect costs were well handled, but it is not clear how/if long-term costs of arthritis are modelled | Partially. Not very clear, as it is based on tariff payments, so there is no clear breakdown. Outcomes are in appropriate units | Partially. Costs are valued credibly but hard to test without trial and tariff details. Outcomes are questionable, as they are based on 50 medical students rather than patients | Unclear. There is no statement on discounting but the results would suggest it was applied | Yes | Yes, through one- and two-way scenario analyses with some conceptualisation for values chosen | There is no clear definition of non-displaced. There is good consideration of the role of non-arthritis AEs |
| Karl et al. (2015)167 | Yes. There is a clear outline of three different options and of the aim and hypothesis of the study and patients | Yes. Alternatives are well defined. It is not clear if all options are considered, as possible repeat rests are not included or discussed | Yes, through limited meta-analysis, but it focuses only on diagnostic effectiveness and a little on treatment post test | Yes. These appear to be sufficiently identified (given limitations of treatment modelling) | Yes. They appear to be appropriate | Partially, based on limited evidence in the literature, which is averaged. The approach to the cost of surgery is questionable. The utility is based on Davis, so the limitations are the same | Partially. Literature estimates are adjusted to 2014 USD. It is not clear if discounting is applied; the scale of the results would suggest not | Yes | Yes, through one- and two-way scenario analyses | Yes, with the exception of those raised elsewhere |
| Hannemann et al. (2015)166 | Yes. There is a clear outline of the aims of the analysis, although a description of the evaluation in the abstract is a little misleading, as the study is just a within-trial report | Yes. There is a good description, although it is not clear if surgery would have been an option | Yes, using linked trial evidence only | Yes. They were identified as part of the trial | Yes. The costs and health outcomes are well reported | Yes, including the costs from hospital and Dutch guidelines and QoL from trial EQ-5D, as well as some credible societal costs | N/A. It is only a 1-year timeline, but it could be argued that this should have been included | Yes | Yes. PSA is presented, but no scenario analysis | The study has limited scope, as it is only a within 1 year trial report with limited evaluation |
| NICE (2016)88 | Yes. They are clearly defined but it is a little unclear as to how the overall analysis links to the NICE guidance, so it is hard to comment on its ability to answer the primary aim | Yes. They are very well described and motivated; there is no ‘do nothing’ option, but, as it is emergency medicine, perhaps this is not a reasonable option | Partially. The effectiveness of the diagnosis is well established, but the effectiveness of the subsequent treatments (cast or surgical interventions) is not | Partially. Short-term costs are well considered but long-term costs and the consequences of different treatment options are not; however, it is hard to determine if this had an impact on the results | Yes. Costs and consequences are measured appropriately | Yes, based on a good literature search and, where no literature was available, reasonable assumptions | Yes. All costs occurred in the first year, but future QALYs were discounted appropriately | Yes | Yes, through scenario analyses | This was overall a good piece, given the limitations of the evidence, but there are concerns around the failure to justify the simplistic approach to modelling the treatment options |
| Study | Age of population at injury, years | Male to female ratio | Characteristics of original injury | OA definition | Definition of union | Surgical intervention type | Definition of symptoms |
|---|---|---|---|---|---|---|---|
| This analysis, as defined by SWIFFT (n = 439) | Mean 33 (range 16–80) | 84% | Unequivocal, bicortical, minimally displaced fracture (< 2 mm) of the scaphoid, presenting within 2 weeks of injury | Defined within-trial period by radiography and CT scan | Disappearance of the fracture line on radiographs and complete bridging on CT scans compared with those taken at baseline | As per surgical preference (open or percutaneous fixation with standard CE-marked headless compression screw) | Reported in terms of EQ-5D-3L |
| Lindström and Nyström (1990)102 (n = 229) | Range 15–76 | 83.8% | Scaphoid fractures. Fractures of the scaphoid tuberosity were excluded | One or more observations of reduced joint space, osteophytes or sclerosis of joint margins | N/A. Considers only ‘healed fractures’ (not defined) | N/A, as only cast patients | Patient-reported weakness of grip, pain related to wrist motion and at rest, and impaired range of motion |
| Saedén et al. (2001)27 (n = 61) |
29 ± 13 (surgical group) 37 ± 20 (cast group) |
84% (surgical) 73% (cast) |
Acute fractures of the scaphoid visible at first radiological examination. Fractures of the scaphoid tuberosity were excluded | Narrowing of the joint or reactive changes around it compared with the uninjured side | Radiological identified but not defined | Open fixation using Herbert screws | Symptoms reported as score out of 10 across nine questions including pain at different times, wrist movement and manual work |
| Lindstrom and Nystrom (1992)100 (n = 33) | Mean 27.9 (range 15–52) | 72.7% | Scaphoid fractures. Fractures of the scaphoid tuberosity were excluded | One or more observations of reduced joint space, osteophytes or sclerosis of joint margins | Not defined | N/A, as all untreated patients | Patient-reported pain, stiffness and weakness |
| Ruby et al. (1985)99 (n = 56) | Mean 32 (range 18–85) | 89.2% | Scaphoid fractures | At least the presence of joint narrowing, severity determined by osteophytes and intraosseous cysts | Not defined | N/A, as all untreated patients | Patient-reported pain in the wrist |
| Moritomo et al. (1999)105 (n = 33) | Mean 26.3 (range at review of all patients 13–70) | 84.5% | Limited details given other than fracture of the scaphoid | N/A | Not defined | N/A. No treatment details reported | Symptomatic population defined as seeking help for injury |
| Vender et al. (1987)15 (n = 64) | Median 22 | 86% | Scaphoid fractures | Radial styloid pointing, radioscaphoid narrowing and midcarpal joint narrowing | Displacement of the fragment cortices > 1 mm | N/A. Any patients who had previous operations were excluded | Patients who presented with clinical concerns related to the injured wrist |
Probability of developing osteoarthritis
In the extrapolated model, the probability of OA was modelled for all treatment arms as being an exponential decay towards a limiting value with the limiting value characterised as a beta distribution for the PSA. The functional form of the exponential function is:
where y∞ is the limiting value, a is a constant and τ is the time constant.
This formula is fitted to the evidence from Lindström and Nyström that, at year 1, 2.6% of patients had radiological OA and, at year 7, 5.6% had radiological OA. 102 Figure 20 shows the goodness of fit of the fitted function to the observed data, with the bounds showing the 95% CI taking account of the uncertainty of the time-limiting value.
FIGURE 20.
Goodness of fit of the exponential decay to the observed data.
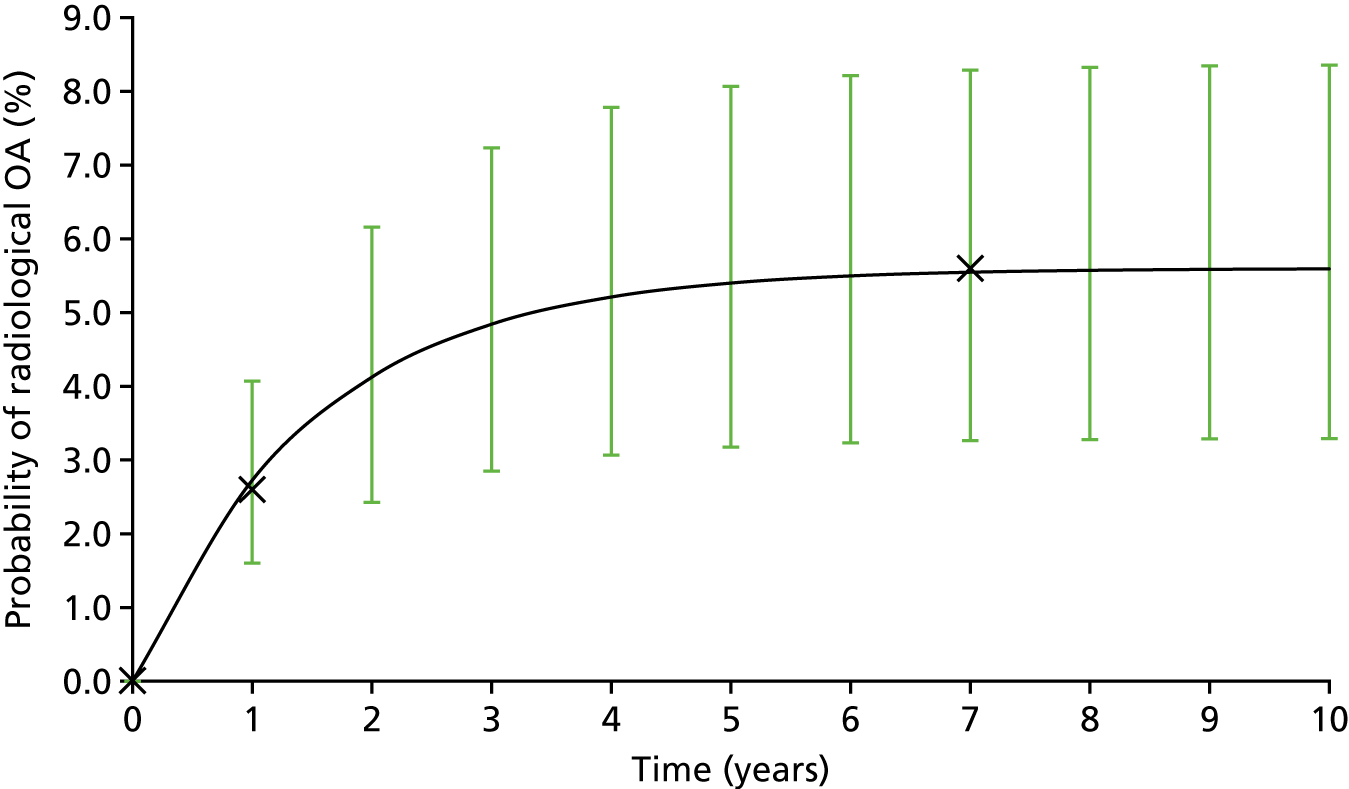
Probability of developing scaphoid non-union advanced collapse
To estimate the probability of patients with non-union developing SNAC, we conducted a survival analysis to the patient-level data reported by Moritomo et al. 105 The results of the survival analysis are reported in Table 70, which reports the AIC and Bayesian information criterion (BIC) of the seven regressions explored alongside the graphical goodness of fit of the Weibull (Figure 21), which was selected owing to having the lowest AIC and BIC.
| Regression function | AIC | BIC |
|---|---|---|
| exp | 25.12107 | 26.11681 |
| weibull | 22.51636 | 24.50783 |
| gomp | 22.79423 | 24.7857 |
| loglogistic | 24.14154 | 26.133 |
| lognormal | 23.19027 | 25.18174 |
| gamma | 24.33022 | 27.31742 |
FIGURE 21.
Weibull and Kaplan–Meier of the SNAC survival analysis.

Extrapolated model scenario analyses
To develop the mathematical model into the form presented in the previous section, a number of simplifying assumptions and interpretations of the available evidence were necessary, as is true of all mathematical models. 97 While the assumptions made in the base-case analysis are considered to be the most reasonable given the evidence available, it is important to test the impact of different approaches on the results of the analysis. The following scenarios have been constructed to conduct these tests; as far as possible, other sources of evidence are used to inform the scenarios. However, often the uncertainty is driven not by contradictory sources but by a complete lack of evidence.
Scenario 1: definition of non-union at 1 year
In the base-case analysis, we assume that the definition of non-union informative to the extrapolated model from the previous chapter includes only those patients who are classified as having a non-union at 52 weeks; therefore, all other diagnoses (union, almost full union, partial union and slight union) are categorised as union for the sake of the model. If only this ‘pure’ non-union definition is used, there is one non-union in the surgical arm and four in the plaster cast arm (three of which we define as having surgical intervention at a later time). If a broader definition is used to include the slight unions, then this increases to four in the surgical arm and nine in the plaster cast arm. The base-case assumption was made based on clinical guidance that there is no evidence to suggest that slight unions behave as non-unions; however, there is uncertainty around this. Therefore, this scenario explores the sensitivity of the model to this assumption by considering only the ‘pure’ non-unions as informative to the extrapolated model.
Scenario 2: no surgical treatment for those who fail to achieve union with cast
Our base-case analysis assumes that the three patients who failed to achieve union with cast fixation alone by 52 weeks in the cast plus surgery arm of the trial (i.e. those who did have an identified or suspected non-union after cast immobilisation but were not treated surgically) can be assumed to receive surgical intervention at a later time point. This scenario explores this assumption by assuming that these patients did not receive surgical intervention at a later date and therefore are long-term non-unions.
Scenario 3: probability of non-union after secondary surgery
The base-case analysis uses evidence from SWIFFT to estimate the probability of non-union for patients who underwent surgery after cast immobilisation (1 of 17 patients who received surgery). This scenario explores the impact of this assumption by using an estimate from Filan and Herbert168 to estimate the probability of non-union after secondary surgery (0.195, 95% CI 0.145 to 0.249).
Scenario 4: treatment-specific adverse events
The base-case analysis assumed that the choice of treatment affects only the probability of achieving union and not the subsequent risks of AE development. The base case uses the large study by Lindström and Nyström102 to inform the three parameters: the probability of having long-term non-OA AEs, the probability of developing OA and the probability that this OA is symptomatic. The major limitations of this approach are that the Lindström and Nyström102 study considered only patients treated with cast immobilisation. As observed by Saedén et al. ,27 the act of conducting surgery may result in the development of OA that may not have occurred with conservative treatment alone and may be associated with a greater level of non-OA AEs owing to the invasive nature of surgery.
This scenario explores this assumption by using less robust, treatment-specific estimates of AE rates from the literature. The primary source of evidence is the Saedén et al. 27 study, namely a small (n = 62) randomised study of cast versus Herbert screw fixation for acute scaphoid fracture. The small nature of this study and the age of the surgical procedure (patients were randomised between 1984 and 1986) were the primary reasons for it being discounted as the primary source of the base-case analysis. Furthermore, there were concerns that the results of the Saedén et al. 27 study were inconsistent with clinical expectations, as it suggests a higher overall rate of AEs in the cast immobilisation group. The use of this study changes the estimates reported in Table 29 in Chapter 4 to those reported in the condensed Table 72, with the base-case estimates reported in the second line of each row. Estimates of the probability of developing SNAC post non-union remain unchanged from the base case.
| Parameter | Base-case value (95% CI) | Distribution | Source |
|---|---|---|---|
| Long-term element of the model – union Markov model | |||
| Probability of having long-term adverse symptoms that are not OA related | |||
| Cast immobilisation |
0.188 (0.043 to 0.405) 0.048 (0.024 to 0.079) |
Beta (alpha 3, beta 13) (alpha 11, beta 218) |
Saedén et al. (2001)27 Lindström and Nyström (1990)102 |
| Surgery as last treatment provided |
0.087 (0.011 to 0.228) Assumed to be the same as cast |
Beta (alpha 2, beta 21) | Saedén et al. (2001)27 |
| Probability of developing OA | |||
| Cast immobilisation |
Limiting value: 0.308 Time constant: 1.5 CI: 0.123 to 0.527 Limiting value: 0.056 Time constant: 1.5 CI: 0.035 to 0.087 |
Exponential decay towards a limiting value, with limiting value characterised as a beta distribution |
Saedén et al. (2001)27 (only including STJs) Lindström and Nyström (1990)102 |
| Surgery as initial treatment |
Limiting value: 0.609 Time constant: 1.5 CI: 0.440 to 0.776 Assumed to be the same as cast |
Exponential decay towards a limiting value, with limiting value characterised as a beta distribution | Saedén et al. (2001)27 |
| Probability that developed OA is symptomatic | |||
| Cast |
0.750 (0.292 to 0.992) 0.992 (0.918 to 1.00) |
Beta (alpha 3, beta 1) (alpha 11.9, beta 0.1) |
Saedén et al. (2001)27 Lindström and Nyström (1990)102 |
| Surgery |
0.214 (0.050 to 0.454) Assumed same as cast |
Beta (alpha 3, beta 11) | Saedén et al. (2001)27 |
Scenario 5: additional risk of osteoarthritis development post non-union surgery
The base-case analysis assumes that there is no additional risk of developing OA as a result of multiple lines of treatment. This assumption implies that, in the case of surgery for non-union after cast immobilisation, there is no increase in the future risk of developing OA. However, Saedén et al. 27 argue that surgery that opens the STJ may result in the development of OA, but members of the TMG argue that surgery may in fact have a delaying effect on the progression of OA. This scenario will explore the sensitivity of the result to this assumption by assuming that patients undergoing surgery as a secondary treatment are, first, twice as likely to develop OA at any point than if surgery had been conducted as the primary treatment and, second, half as likely.
Scenario 6: quality of life for non-osteoarthritis permanent adverse events
The base-case model assumes that the HRQoL for non-OA permanent AEs is the same as for OA, owing to a lack of evidence. This scenario tests the impact of this assumption by testing two extreme cases:
-
the HRQoL decrement for non-OA permanent AEs is 50% less than for OA (mean decrement 0.065, 95% CI 0.045 to 0.085) such that they have a greater QoL than OA sufferers
-
the HRQoL decrement for non-OA permanent AEs is 50% greater than for OA (mean decrement 0.195, 95% CI 0.175 to 0.215) such that they have a lower QoL than OA sufferers.
A third subscenario is also considered, which attempts to separately model the role of rare but extremely impactful surgery-related AEs, such as fusion, chondrolysis and infection. While previous analyses include non-OA related AEs that are the same for both treatments (the base case) and different (scenario 1), and the quality of decrement applied is lifelong (–0.130 each year), it is possible that this underestimates the impact of a small number of high-impact and lifelong AEs that can occur after surgery. In SWIFFT, one patient was identified as having wrist fusion and reported a QoL for the within-trial period of 0.31 less than the surgical arm average. This scenario explicitly incorporates this QoL decrement occurring in 0.5% (roughly 1 in 219) of surgical patients for the rest of their lives.
Scenario 7: proportion of untreated patients who have union
The base-case analysis assumes that all patients in the untreated arm experience non-union, an assumption necessitated by the reality that it is impossible to estimate the rate in this treatment group. While, in reality, we know little about the prognosis and natural history of such a patient population, including whether any patients who did choose to not receive treatment would seek treatment later if symptoms persisted, we believe that the base case modelled provides a very important role in demonstrating the value of some form of active treatment. This scenario explores the sensitivity of the result to the assumption that all no-treatment options result in non-union by using a threshold analysis to estimate the probability that the injury fails to unite without intervention, at which point the no-treatment arm becomes the most cost-effective treatment approach. This scenario can be used if future evidence emerges to reflect the validity of the base-case assumption and therefore the merits of active treatment.
Scenario 8: probability of scaphoid non-union advanced collapse post non-union
To explore the sensitivity of the model to changes in the probability of SNAC occurring at any time point after a non-union, this scenario doubles and halves the base-case annual probabilities.
Scenario 9: proportion of patients who reject surgery post cast immobilisation
In the base-case analysis, the estimated probability of patients having surgery after a confirmed non-union after initial cast immobilisation is taken directly from SWIFFT, with 4 of 18 patients not having surgery. This scenario explores the sensitivity of the model to this parameter through a threshold analysis, identifying the rate at which patients would have to accept surgery after confirmed non-union after cast immobilisation for cast plus surgery to be the most cost-effective treatment.
Scenario 10: cost of first-line surgical fixation
The base-case analysis estimates a cost per first-line surgical fixation of £1632, as informed by the relevant reference cost. 92 However, the TMG highlighted that this reference cost was widely considered to be a large underestimate of the true cost to the hospital of providing this procedure. Attempts were made to use data collected during the trial to conduct a bottom-up costing of the procedure, but insufficient unit costs of the components and large variation in the level of reporting made it impossible to derive a meaningful estimate. This scenario therefore estimates the impact on the estimated result if the cost of surgical fixation were doubled to £3264.
Scenario 11: no difference in quality of life in first year
The base-case analysis assumes a QoL difference of 0.02 in favour of the surgical treatment arm in the first year of the model. This scenario explores the impact of incorporating the difference in baseline QoL between the two arms by setting the QoL of the two arms as the same (set to the cast value of 0.812).
Scenario 12: no quality of life decrement of non-union without scaphoid non-union advanced collapse
The base-case analysis assumes the QoL decrement for patients with long-term non-union but no SNAC is the same as that used for OA (a decrement of 0.130). This scenario tests this assumption by assuming that non-union patients without SNAC have no QoL decrement, experiencing the same QoL as the general population.
Scenario analyses results
A number of scenarios were conducted to explore some of the elements of uncertainty not well captured by the PSA, as discussed in the main report and earlier in this appendix. In this section, the results presented of the different scenario analyses are reduced to reporting the NHB at a cost-effectiveness threshold of £20,000/QALY, which gives a precise overview of which treatment option is the most cost-effective. The full list of NHBs across the scenarios are reported in Table 73.
| Scenario | QALY | |||
|---|---|---|---|---|
| Surgery | Cast plus surgery | Cast only | No treatment | |
| Base case: deterministic | 19.00 | 19.02 | 18.69 | 14.70 |
| Scenario 1: definition of non-union at 1 year | 18.99 | 19.02 | 18.58 | 14.69 |
| Scenario 2: no surgical treatment for those who fail to achieve union with cast | 19.00 | 18.98 | 18.70 | 14.69 |
| Scenario 3: probability of non-union after secondary surgery | 18.98 | 18.99 | 18.69 | 14.69 |
| Scenario 4: treatment-specific AEs from Saedén et al.27 | 18.71 | 18.40 | 18.06 | 14.69 |
| Scenario 5a: double risk of OA development post non-union surgery | 19.00 | 19.03 | 18.70 | 14.69 |
| Scenario 5b: half risk of OA development post non-union surgery | 19.01 | 19.04 | 18.68 | 14.69 |
| Scenario 6a: 50% lower QoL decrement for non-OA permanent AEs | 19.07 | 19.11 | 18.75 | 14.69 |
| Scenario 6b: 50% greater QoL decrement for non-OA permanent AEs | 18.87 | 18.90 | 18.56 | 14.69 |
| Scenario 6c: explicit inclusion of additional fusion rate | 18.97 | 19.04 | 18.68 | 14.69 |
| Scenario 7: proportion of untreated patients who have union | Would require 95.5% of untreated patients to achieve union for it to be the most cost-effective strategy | |||
| Scenario 8a: double annual probability of SNAC post non-union | 19.00 | 19.03 | 18.65 | 14.27 |
| Scenario 8b: half annual probability of SNAC post non-union | 19.01 | 19.04 | 18.75 | 15.17 |
| Scenario 9: proportion of patients who reject surgery post cast confirmed non-union | If the proportion of patients having surgery after confirmed non-union after cast immobilisation reduces from 0.948 to 0.852, primary surgery becomes cost-effective | |||
| Scenario 10: doubling of cost of first-line surgical fixation | 18.92 | 19.04 | 18.68 | 14.72 |
| Scenario 11: no difference in QoL in first year | 18.98 | 19.03 | 18.68 | 14.69 |
| Scenario 12: no QoL decrement of non-union without SNAC | 19.00 | 19.03 | 18.81 | 16.01 |
Table 73 shows that the cost-effectiveness results are sensitive to a number of key assumptions; while the headline result does not change in many of them, the incremental difference in NHB reduces in many. While scenario 4 is striking in that it results in a large change in the NHB and the resultant decision, the small scale of the Saedén et al. 27 study that informs this scenario results in very large levels of uncertainty. Furthermore, it could be argued that the population used to inform this scenario from the Saedén et al. 27 study is not indicative of those patients considered here, with the cast population indicative of a more severe subset.
Scenarios 1 and 2 highlight the sensitivity of the model to the assumptions regarding the definition of slight union patients as union or non-union, and whether the three patients who are non-union at 52 weeks in the cast arm but have not received surgery can be assumed to be offered surgery at some point (as in the base case) or will remain non-unions (as in the scenario). These scenarios show the key finding of the analysis that, if initial cast immobilisation is unsuccessful but surgical intervention is offered soon after, it is highly likely to be cost-effective, as the high upfront cost implications of conducting surgery on all patients have been avoided, but a high rate of long-term non-union and the AE risks of conducting a lot of surgical interventions are still avoided.
Scenario 4 shows that the use of the Saedén et al. 27 study to inform an estimate of treatment-specific AEs has a dramatic impact on the cost-effectiveness result. This result was flagged by the clinical advisors of this section as being contrary to clinical expectations, as surgical intervention would be expected to be associated with a greater level of AEs than cast immobilisation. However, as Saedén et al. 27 found a higher proportion of symptomatic AEs in the cast arm, the model results respond accordingly. It is likely that the Saedén et al. 27 estimate suffers from small numbers and that the QoL of those reporting symptoms in the surgical group is less than that in the cast group. However, given the evidence available, it has not been possible to incorporate such factors without resorting to speculation. The results of this scenario justify the assumption of treatment-independent AEs made in the base case and highlight the need for further research into the nature of long-term AEs in this clinical area.
Within-trial analysis results
Summary statistics: costs
This section briefly considers the values observed in terms of costs to the NHS and PSS prior to imputation for missing values. These results provide a helpful overview of the results reported by patients and prior to any assumptions being made about the nature of the missing data. The crude summary statistics and frequency histograms are reported in Table 74 and Figure 22 respectively.
As would be expected, these compete case results show high levels of costs at the earlier stage of the trial, while patients are receiving treatment (p < 0.001). With the exception of the first 6 weeks, when surgical patients will be having surgical-related reviews, the time-related costs are similar, as are imaging costs. While some surgical interventions did take place in the plaster cast arm (24 surgeries), resulting in a mean cost of £319, surgery costs are much greater in the surgery arm of the trials, as would be expected.
In contrast, the average cost resulting from cast immobilisation is actually larger in the surgical arm. We believe this to be because many patients had casts fitted and re-fitted routinely after surgery to inspect healing, whereas the majority of patients randomised to cast immobilisation required fewer re-fittings. Overall, the average costs in the surgical arm were found to be statistically significantly greater than in the plaster cast arm, in terms of both the total costs for patients with complete data across all of the cost variables and the sum of the average costs, both reported in Table 74.
| Variable | Surgery mean (n, SE) | Plaster cast mean (n, SE) |
|---|---|---|
| 6 weeks | £311 (168, £21.38) | £233 (169, £19.09) |
| 12 weeks | £125 (165, £13.60) | £118 (162, £13.29) |
| 26 weeks | £78 (153, £13.27) | £86 (140, £14.36) |
| 52 weeks | £51 (174, £11.15) | £90 (163, £28.03) |
| Imaging | £42 (169, £4.72) | £43 (170, £4.92) |
| Surgery (no missing data) | £1516 (219, £59.27) | £319 (220, £65.10) |
| Casting (no missing data) | £17 (219, £0.73) | £13 (220, £0.45) |
| Total costs, complete case | £2350 (83, £94.72) | £727 (65, £117.81) |
| Sum of average costs | £2140 | £901 |
FIGURE 22.
Histograms of patient interaction costs, complete case: (a) 6 weeks; (b) 12 weeks; (c) 26 weeks; and (d) 52 weeks.


Summary statistics: quality of life
This section briefly reports the within-trial analysis results for the QoL and cost estimates as complete case, that is, prior to any imputation for missing data. The results are reported in Table 75 and Figure 23.
These crude analyses show that patients in both treatment strategies reported bimodal, highly skewed QoL scores, with a high proportion reporting a perfect QoL score of 1, which increased with time since injury. However, there were also a small number of patients reporting very low QoL scores, including a proportion of negative scores throughout the follow-up period, indicative of some patients experiencing very poor outcomes. This distribution is typical of such injuries, with initial injury and treatment-related impacts on QoL reducing over time but some patients continuing to experience AE-related impacts. In addition, the average scores in the high 0.8 range are indicative of a ‘normal’ population of the age of the patients. 107
While Figure 23 shows a similar spread of scores throughout the within-trial period, the mean results presented in Table 74 do appear to show that the plaster cast arm has lower QoL at all time points. The complete-case average QoL over the year (i.e. adjusted for the length represented by each questionnaire as given in Chapter 2) shows a slightly greater score for the surgical patients, alongside a smaller standard error. However, this difference may be the result of differences in the baseline score, which was 0.0287 higher in the surgical than in the plaster cast arm.
| Variable | Surgery mean (n, SE) | Plaster cast mean (n, SE) |
|---|---|---|
| Baseline | 0.6260 (214, 0.0194) | 0.5973 (219, 0.0201) |
| 6 weeks | 0.7522 (174, 0.0174) | 0.7005 (179, 0.0181) |
| 12 weeks | 0.8290 (180, 0.0138) | 0.7898 (164, 0.0173) |
| 26 weeks | 0.8361 (161, 0.0177) | 0.8505 (146, 0.0183) |
| 52 weeks | 0.8820 (182, 0.0123) | 0.8551 (176, 0.0176) |
| Average QoL, complete case | 0.8317 (134, 0.0131) | 0.8140 (119, 0.0159) |
FIGURE 23.
Complete case QoL scores by time and treatment: (a) baseline; (b) 6 weeks; (c) 12 weeks; (d) 26 weeks; and (e) 52 weeks.


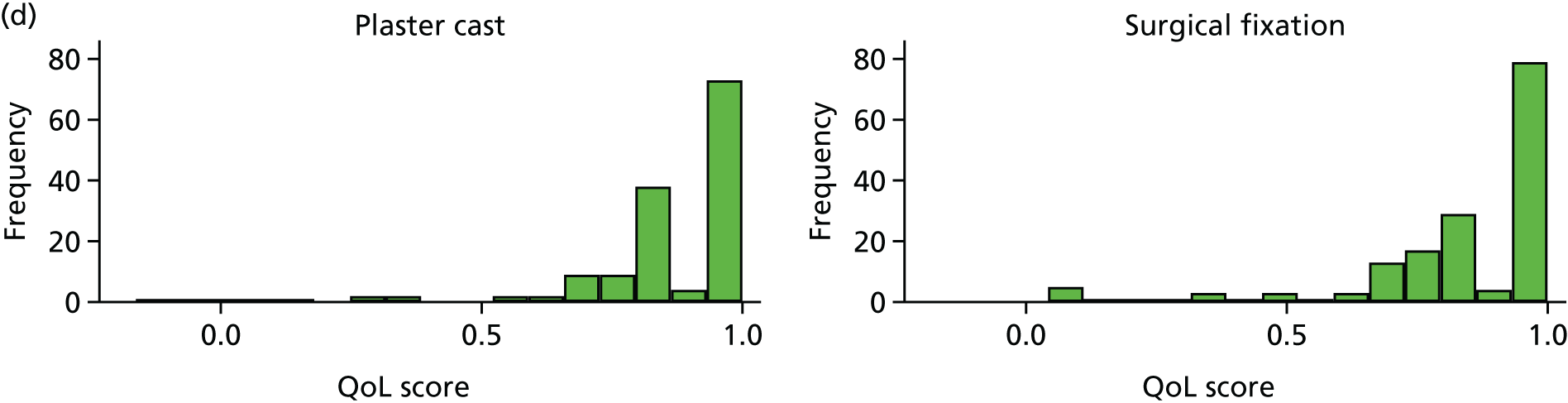

Missing data results
Missing data were found to occur across the patient-reported questionnaires related to QoL and resource use at levels reported in Table 76. Overall, the level of missing data was low, but was higher in the plaster cast arm, most likely because of a lower frequency of interaction and patient buy-in with the treatment, potentially owing to the perceived importance of the injury if cast immobilisation is deemed to be sufficient rather than the injury requiring surgical fixation.
| Variable, n (%) | Surgical fixation (N = 219) | Plaster cast (N = 220) |
|---|---|---|
| QoL score baseline | 5 (2.3) | 1 (0.5) |
| QoL score at 6 weeks | 45 (20.5) | 41 (18.6) |
| QoL score at 12 weeks | 39 (17.8) | 56 (25.5) |
| QoL score at 26 weeks | 58 (26.5) | 74 (33.6) |
| QoL score at 52 weeks | 37 (16.9) | 44 (20.0) |
| Cost at 6 weeks | 51 (23.3) | 51 (23.2) |
| Cost at 12 weeks | 54 (24.7) | 58 (26.4) |
| Cost at 26 weeks | 66 (30.1) | 80 (36.4) |
| Cost at 52 weeks | 45 (20.5) | 57 (25.9) |
| Imaging | 50 (22.8) | 50 (22.7) |
Missing data were found to be non-monotonic and patients who were missing in one period were not necessarily missing in the next. Logistic regression in the QoL and cost variables found many of the variables to be correlated with previously observed values, which, using the Faria94 framework, led to the assumption that the data were missing at random. As a result, in the multiple imputation framework, missingness is assumed to depend on all other missing variables and baseline covariates, specifically gender, whether or not the injured arm was the patient’s dominant arm, treatment allocation and age. The variables selected are consistent with those used in the previous chapter and the regression analyses presented in the next section. The imputation is run 36 times, consistent with the largest proportion of missing data observed (36.0% in costs at 26 weeks in the plaster cast arm). 94
Extended model results
Figure 24 gives scatterplots of the cost-effectiveness results first including all four treatment options and then including only the two SWIFFT options.
FIGURE 24.
Scatterplots of the cost-effectiveness results for (a) the four treatment options; and (b) only the two SWIFFT options.


Table 77 provides some of the clinical estimates of the model at three time points. The estimates allow this model to be validated as additional evidence emerges, specifically the 5-year SWIFFT update.
| Time point, years | Percentage of the initial population with union and OA who are alive | Percentage of the initial population with SNAC who are alive | ||||||
|---|---|---|---|---|---|---|---|---|
| No treatment | Cast only | Cast + surgery | Surgery | No treatment | Cast only | Cast + surgery | Surgery | |
| 1 | N/A | 2.36 | 2.58 | 2.59 | 0.69 | 0.06 | 0.00 | 0.00 |
| 5 | N/A | 4.66 | 5.08 | 5.11 | 11.34 | 1.02 | 0.10 | 0.04 |
| 10 | N/A | 4.79 | 5.23 | 5.25 | 33.53 | 3.01 | 0.31 | 0.13 |
Consolidated Health Economic Evaluation Reporting Standards checklist
| Section/item | Item number | Recommendation | Page number on which it is reported |
|---|---|---|---|
| Title and abstract | |||
| Title | 1 | Identify the study as an economic evaluation or use more specific terms such as ‘cost-effectiveness analysis’ and describe the interventions compared | 113 |
| Abstract | 2 | Provide a structured summary of objectives, perspective, setting, methods (including study design and inputs), results (including base case and uncertainty analyses) and conclusions | 3 to 4 |
| Introduction | |||
| Background and objectives | 3 | Provide an explicit statement of the broader context for the study | 28 |
| Present the study question and its relevance for health policy or practice decisions | 28 to 32 | ||
| Methods | |||
| Target population and subgroups | 4 | Describe characteristics of the base-case population and subgroups analysed, including why they were chosen | 113 and 124 |
| Setting and location | 5 | State relevant aspects of the system(s) in which the decision(s) need(s) to be made | 113 |
| Study perspective | 6 | Describe the perspective of the study and relate this to the costs being evaluated | 113 and 121 |
| Comparators | 7 | Describe the interventions or strategies being compared and state why they were chosen | 125 |
| Time horizon | 8 | State the time horizon(s) over which costs and consequences are being evaluated and say why appropriate | 124 |
| Discount rate | 9 | Report the choice of discount rate(s) used for costs and outcomes and say why appropriate | 124 |
| Choice of health outcomes | 10 | Describe what outcomes were used as the measure(s) of benefit in the evaluation and their relevance for the type of analysis performed | 113 |
| Measurement of effectiveness | 11a | Single study-based estimates: describe fully the design features of the single effectiveness study and why the single study was a sufficient source of clinical effectiveness data | 4 and elsewhere in report |
| 11b | Synthesis-based estimates: describe fully the methods used for identification of included studies and synthesis of clinical effectiveness data | 113 | |
| Measurement and valuation of preference-based outcomes | 12 | If applicable, describe the population and methods used to elicit preferences for outcomes | N/A |
| Estimating resources and costs | 13a | Single study-based economic evaluation: describe approaches used to estimate resource use associated with the alternative interventions. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | Page 113 to 121 |
| 13b | Model-based economic evaluation: describe approaches and data sources used to estimate resource use associated with model health states. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | 131 to 145 | |
| Currency, price date and conversion | 14 | Report the dates of the estimated resource quantities and unit costs. Describe methods for adjusting estimated unit costs to the year of reported costs if necessary. Describe methods for converting costs into a common currency base and the exchange rate | 124 |
| Choice of model | 15 | Describe and give reasons for the specific type of decision-analytical model used, providing a figure to show that the model structure is strongly recommended | 127 to 131 |
| Assumptions | 16 | Describe all structural or other assumptions underpinning the decision-analytical model | 127 to 131 |
| Analytical methods | 17 | Describe all analytical methods supporting the evaluation. This could include methods for dealing with skewed, missing or censored data; extrapolation methods; methods for pooling data; approaches to validate or make adjustments (such as half cycle corrections) to a model; and methods for handling population heterogeneity and uncertainty | 120 and 128 to 131 |
| Results | |||
| Study parameters | 18 | Report the values, ranges, references and, if used, probability distributions for all parameters. Report reasons or sources for distributions used to represent uncertainty where appropriate, providing a table to show that the input values are strongly recommended | 118 to 119, 135 to 137, 140 to 141 and 142 to 143 |
| Incremental costs and outcomes | 19 | For each intervention, report mean values for the main categories of estimated costs and outcomes of interest, as well as mean differences between the comparator groups. If applicable, report incremental cost-effectiveness ratios | 151 to 153 and 157 |
| Characterising uncertainty | 20a | Single study-based economic evaluation: describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate, study perspective) | 153 |
| 20b | Model-based economic evaluation: describe the effects on the results of uncertainty for all input parameters and uncertainty related to the structure of the model and assumptions | 157 to 165 | |
| Characterising heterogeneity | 21 | If applicable, report differences in costs, outcomes or cost-effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information | N/A |
| Discussion | |||
| Study findings, limitations, generalisability and current knowledge | 22 | Summarise key study findings and describe how they support the conclusions reached. Discuss limitations and the generalisability of the findings and how the findings fit with current knowledge | 165 to 167 |
| Other | |||
| Source of funding | 23 | Describe how the study was funded and the role of the funder in the identification, design, conduct and reporting of the analysis. Describe other non-monetary sources of support | 4 |
| Conflicts of interest | 24 | Describe any potential for conflicts of interest among study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with International Committee of Medical Journal Editors recommendations | 2 |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AIC
- Akaike information criterion
- BIC
- Bayesian information criterion
- BSSH
- British Society for Surgery of the Hand
- CACE
- complier-average causal effect
- CE
- Conformité Européenne
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRPS
- complex regional pain syndrome
- CT
- computed tomography
- DASH
- Disabilities of the Arm, Shoulder and Hand questionnaire
- DMC
- Data Monitoring Committee
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EVPI
- expected value of perfect information
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- MCS
- mental component summary
- MPR
- multiplanar reconstruction
- MRI
- magnetic resonance imaging
- NHB
- net health benefit
- NICE
- National Institute for Health and Care Excellence
- NNT
- number needed to treat
- OA
- osteoarthritis
- ONS
- Office for National Statistics
- OR
- odds ratio
- PACS
- Picture Archiving Communication System
- PCS
- physical component summary
- PEM
- Patient Evaluation Measure
- PI
- principal investigator
- PRWE
- Patient-Rated Wrist Evaluation
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RSJ
- radioscaphoid joint
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire 12-items
- SNAC
- scaphoid non-union advanced collapse
- STJ
- scaphotrapezium joint
- SWIFFT
- Scaphoid Waist Internal Fixation for Fractures Trial
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- YTU
- York Trials Unit
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta24520).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
