Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/130/05. The contractual start date was in May 2019. The draft report began editorial review in May 2021 and was accepted for publication in October 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research, or similar, and contains language which may offend some readers.
Permissions
Copyright statement
Copyright © 2023 Channon et al. This work was produced by Channon et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Channon et al.
Chapter 1 Introduction and background
The context of obesity in pregnancy
Prevalence and risk
More than 50% of women who gave birth in the NHS between 1 April 2016 and 31 March 2017 had a body mass index (BMI) of ≥ 25 kg/m2 (which would be classified as being in the overweight range),1 including 26.7% who had a BMI in the obese category (BMI of ≥ 30 kg/m2). Obesity levels are expected to continue to rise, with the majority of the UK population expected to be obese by 2050. 2 Obesity places women at a greater risk of experiencing complications during the antenatal, intrapartum and post-partum periods. Maternal risks associated with obesity in pregnancy include backaches, leg pain, increased fatigue, gestational diabetes, miscarriage, pre-eclampsia, thromboembolism, slow labour progress, high caesarean section rates, post-partum haemorrhage, hypertension and maternal death. 3,4
There is also a generational dimension, as described in the Foresight report,2 with an increased risk of adverse effects on the child due to biological, social and environmental factors, including child obesity,4 developing insulin resistance5 and, for girls, having polycystic ovary syndrome (PCOS). 6 With the increasing evidence of the influence of the periconceptional period on fetal growth and long-term effects on risk factors for non-communicable diseases, it is essential that services incorporate interventions not only to reduce obesity levels of women in the preconception period and maintain weight loss during pregnancy and post partum, but also to prevent this passage of risk to the next generation. 7
The Southampton Women’s Survey reported that, in their community sample, almost half of the women recruited gained excessive weight in pregnancy according to the 2009 Institute of Medicine (IOM) guidelines,8 and there are health risks associated with gestational weight gain (GWG), irrespective of pre-pregnancy weight status. 9 However, women with overweight or obesity are at greater risk of excessive GWG in early pregnancy. The development of interventions targeting women in the preconception period, with the aim of reducing GWG, may potentially reduce the risks associated with early GWG. 10
There are clear health gains from a reduction in BMI pre pregnancy. In a population-based study in Canada11 comprising 226,958 women (64% with normal weight, 20% with overweight and 12% with obesity) with singleton pregnancies, a 10% lower preconception BMI was associated with a clinically meaningful risk reduction in pre-eclampsia, gestational diabetes, preterm delivery, macrosomia and stillbirth.
Excessive GWG is associated with weight retention in the longer term,12 and women in the post-partum period describe barriers to losing post-partum weight, including depression and a lack of weight management support. 13 The cumulative effects of excessive GWG without post-partum weight loss in multiple pregnancies may, therefore, contribute to obesity. Systematic reviews of weight loss interventions (WLIs) in the post-partum period suggest that WLIs with diet and physical activity components combined or that use diet alone, but not physical activity alone, can contribute to post-partum weight loss. 14 Furthermore, post-partum interventions that have an information or communication technology component may also be effective in increasing weight loss post partum. 15 However, further research and larger, high-quality trials with longer follow-up are required.
Guidelines for health-care practitioners providing care for women before conception and during pregnancy
The risks associated with obesity in pregnancy are reflected in the National Institute for Health and Care Excellence (NICE) guidelines for health-care practitioner consultations with all women before and during a pregnancy. 16 These guidelines make lifestyle recommendations, such as stopping smoking, taking folic acid and avoiding alcohol, and they include weight management. Specific recommendations are made for consultations with women with a BMI of ≥ 30 kg/m2. Women in this group planning a pregnancy should receive information regarding the risks of obesity during pregnancy and childbirth, and advice and support to help them achieve a healthy weight prior to pregnancy, with the suggestion that losing 5–10% of their body weight would make a significant difference. Once pregnant, women with a BMI of ≥ 30 kg/m2 should be offered tailored information about their diet and exercise. Women should be told about the risks that a raised BMI poses to the pregnancy but also should be told not to diet in pregnancy and that the risks will be managed by the health-care practitioners caring for them. The monitoring of GWG during pregnancy is not advised unless there is a clinical need. These recommendations are supported by the Centre for Maternal and Child Enquiries/Royal College of Obstetricians and Gynaecologists (RCOG)17 and the RCOG’s Care of Women with Obesity in Pregnancy guidelines. 18
NICE guidance also recommends that health-care practitioners be equipped with behaviour change knowledge, skills and competencies and receive communication skills training to be able to discuss weight sensitively with women. However, unlike the IOM guidance in the USA,19 there are no UK-specific guidelines for recommended optimal weight gain in pregnancy. The FIGO (International Federation of Gynaecology and Obstetrics) guidelines stress that ‘management of obesity in pregnancy should be considered in the context of a life course approach, linking with preconception and post-partum and interconception services to prevent excess weight gain before and during pregnancy’. 20
Interventions in pregnancy
There has been a considerable focus, particularly in the last decade, on weight management interventions in pregnancy. A meta-analysis21 of individual participant data from 36 randomised controlled trials (RCTs), including 12,000 participants, demonstrated that weight management interventions targeting diet and/or weight management in women across all BMI categories have not been associated with beneficial maternal or child outcomes, with the exception of caesarean section rates. However, weight management interventions in pregnancy were associated with a small but significant reduction in GWG (–0.7 kg),21 A review of reviews by Farpour-Lambert et al. 22 demonstrated moderate- to high-quality evidence of significant reduction in several outcomes in women across all BMI categories receiving a weight management intervention targeting diet and/or physical activity. These included reductions in GWG (–1.8 to 0.7 kg), excessive GWG, caesarean section rates [relative risk (RR) 0.91–0.95] and neonatal respiratory distress syndrome rates in the child (RR 0.56). However, in women with overweight/obesity, the evidence demonstrating beneficial outcomes of weight management interventions was of low to moderate quality. There was also heterogeneity among studies; study methods, intervention settings, target population and main intervention components varied between studies and interventions.
A systematic review by Agha et al. 3 across 14 studies in pregnancy and one in the preconception period demonstrated that the techniques most commonly used in interventions effective at reducing GWG included diet and physical activity counselling, motivational counselling, weight monitoring and feedback at appointments with health-care practitioners. In a meta-analysis of 89 RCTs, the authors concluded that although there was no optimal intensity, frequency, duration and delivery method that predicted success, interventions containing a group component or a healthy eating component, no matter the intensity, were more likely to be effective. 23
The timing of the initiation of a weight management intervention is important. Excessive weight gain in the first trimester is predictive of excessive weight gain throughout pregnancy24 and gestational diabetes mellitus. 25 This means that starting an intervention in pregnancy may well be too late, as the first point of contact with a health-care practitioner in pregnancy is usually towards the end of the first trimester. Interventions most effective at reducing GWG are those initiated in early pregnancy rather than those delivered later in pregnancy,3 and in Farpour-Lambert et al. ’s22 meta-analyses investigating the effects of lifestyle interventions on GWG or post-partum weight retention, the authors concluded that weight loss prior to pregnancy is probably required to achieve both GWG goals and optimal pregnancy outcomes.
Given the limited success of interventions for obesity in pregnancy, the issue of timing and the key impact of health behaviours in the very early days of conception, attention has turned to the preconception phase as critical to improving maternal and child health. However, owing to the paucity of WLIs in the preconception stage, evidence of their overall effectiveness or of what intervention components may be beneficial is limited.
Preconception health and care
The preconception period
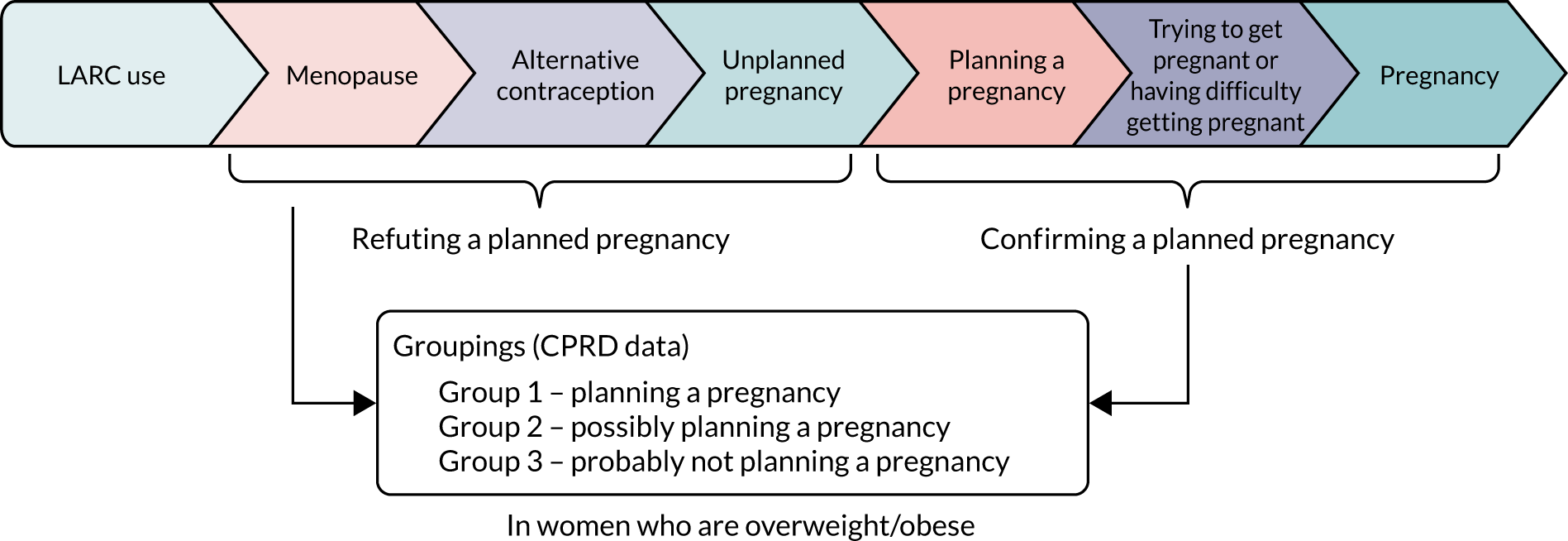
The World Health Organization defines preconception care (PCC) as:
. . . the provision of biomedical, behavioural and social health interventions to women and couples before conception occurs. Its ultimate aim is to improve maternal and child health, in both the short and long term.
As highlighted in the 2018 series on preconception health in The Lancet27–29 and by Stephenson et al. ,30 preconception is an ‘underappreciated period in the life course when health, behavioural, and environmental exposures can have far-reaching consequences, not only for pregnancy outcomes but also for health across generations’. 30 One of the complexities, critical to the clarity of focus of any programme or intervention, is defining the meaning of preconception. Stephenson et al. ,27 suggested that there were three perspectives to consider in a discussion of preconception: (1) biological perspective (the days or weeks before the embryo develops), (2) individual perspective (the weeks or months before conception for individuals who have a conscious intent to conceive) and (3) public health perspective, which includes the months or years before conception. Hill et al. 31 recognised the value of this classification but felt that it lacked enough specificity to identify the population to be defined and targeted in interventions. They proposed four perspectives, each based on a particular combination of defining attributes: reproductive age, man/woman, not pregnant, sexually active, intent to conceive. Some differences from Stephenson’s classification are that the ‘public health’ group is subdivided into those of reproductive age (public health) or younger (life course) and that the individual perspective is subdivided into those with intent and those without intent (potential preconception). Many preconception interventions target the ‘intentional preconception’ group, but this excludes those who are sexually active, who may or may not be using contraception, but have not made a conscious decision to conceive.
Healthy diet/nutrition and weight management have been identified as the top two priorities in international preconception research. 32 There are significant gaps in preconception nutritional intake internationally. 33 In the UK it is estimated that 77% of women aged 18–25 years have dietary intakes below Reference Nutrient Intake daily recommendations for iodine and that 96% of women of reproductive age have intakes of iron and folate below the daily recommendations for pregnancy. 27 The situation is not greatly improved in women who are planning a pregnancy: the results of the Southampton Women’s Survey showed that only a very small proportion of women planning a pregnancy followed the recommendations for nutrition and lifestyle in the preconception period. For example, only 2.9% of women who became pregnant [95% confidence interval (CI) 1.2% to 6.0%] complied with recommended levels of folic acid supplementation and drank four or fewer units of alcohol a week, compared with 0.66% (95% CI 0.52% to 0.82%; p < 0.001) of those women who did not become pregnant. 34
The lack of engagement with the recommendations may well be due to a lack of knowledge relating to preconception health or, potentially, beliefs relating to the behaviour, as there are indications that smoking, a more commonly known risk behaviour, does reduce before pregnancy. 35 Preconception education and counselling can improve maternal knowledge, self-efficacy and risk behaviour, but the impacts on anthropometric measures and pregnancy outcomes are less clear. 36,37 There may also be demographic differences underlying differential behavioural response to information; for example, younger preconceptional women and women with children were less likely to engage in preconception health behaviours,38 and women with higher levels of education were more likely to engage. 39,40
In England, the Preconception Partnership of key stakeholders is working to operationalise policy to ensure that PCC becomes a more integrated part of routine practice. It has proposed an annual report identifying progress on a set of core metrics for agencies, reflecting planning and preparation for pregnancy at an individual and public health level. 30 This would include improving the health environment, putting the importance of preconception health on the school curriculum and embedding it in policy initiatives. On an individual level, the goals include normalising conversations about pregnancy intentions in health care, increasing support for behaviour change to improve preconception health and training health-care providers to have conversations about preconception health.
One of the issues that is often cited as the reason for the lack of preconception health care is the number of pregnancies that are planned, often assumed to be small. In a cross-sectional survey35 of 1288 women conducted in a London maternity centre, 73% had clearly planned their pregnancy and 24% were ambivalent, with only 3% of pregnancies unplanned. These figures would suggest that planning is more common than is often assumed. There are also different types of ‘planning’, for example women with high levels of pregnancy planning who take up interventions but also those who plan but describe themselves as having poor awareness of preconception actions. 41 However, if the interventions suggested by the Preconception Partnership were put in place and preconception health conversations were more integrated into routine care, the level of positive intention and planning could become less of a barrier.
Preconception and obesity
Until very recently, few studies have targeted obesity in the preconception period; interventions in the preconception period have reported programmes for very specific populations, such as gastric bypass patients,42 those with diabetes43 or couples seeking in vitro fertilisation (IVF) treatment,44 or have been small scale. 45 Two Cochrane reviews46,47 investigating the effectiveness of preconception and antenatal health programmes and interventions in improving pregnancy outcomes or reducing weight in obese women identified no eligible studies in the preconception phase. In a systematic review48 of the impact of preconception lifestyle interventions on live birth, birthweight and pregnancy rate, six of the included studies reported participants’ weight and BMI, five of which were in the context of assisted reproduction. The Strong Healthy Women (SHW) intervention49 (a six-session preconception health course with a social cognitive approach50 to behaviour change in preconceptional and interconceptional women living in low-income rural communities) was excluded from the reviews as it was not possible to identify outcomes by BMI category (see Studies in progress).
A more recent scoping review51 of RCTs of behavioural interventions supporting women of childbearing age in the prevention and treatment of overweight and obesity identified only one intervention in the preconception period, the Prepare study protocol. 52 The Prepare trial is a pre-pregnancy intervention in the USA for women with a BMI of ≥ 27 kg/m2 identified through routine data. The intervention is based on social cognitive theory, and the intervention group received 6 months of weekly weight management calls from a health coach followed by monthly calls for 1 year. The primary outcome was GWG but with prenatal weight loss as a secondary outcome. In the results, now published,53 the women receiving the intervention lost an average of 3.5% of their weight before becoming pregnant (control arm 0.5%; p < 0.001). However, by the end of the third trimester, the GWG was the same for both groups, leading the authors to suggest that an intensive weight management approach is needed beyond preconception, and throughout pregnancy, to manage GWG.
One potential reason for the lack of evidence in relation to preconception and obesity is that weight and weight management often do not feature in preconception health programmes that have been evaluated. In a systematic review of studies identifying factors related to preconception health behaviours,38 weight status was measured in only 3 of the 24 included studies and, therefore, was not identified in the review as one of the six categories of factors. Similarly, in a scoping review of preconception health interventions, only 4 of the 29 included programmes identified weight/obesity as a risk factor to be addressed. 37 More recently, in a published trial protocol concerning the uptake of PCC in the Netherlands,54 the primary outcome was change in lifestyle behaviours (e.g. folic acid use, smoking and alcohol use) and secondary outcomes were pregnancy outcomes (e.g. miscarriage, preterm birth, gestational diabetes) and the uptake of PCC, but weight management was not included. Overall, the quantity and quality of the evidence in this field is very limited. 36
Barriers to engaging women in preconception weight loss interventions
With the rising rates of women of childbearing age who are obese,55 the development of effective preconception WLIs for women with overweight/obesity may provide an important step in reducing the health risks to mother and child. As with pregnancy,56 the preconception period may be considered a ‘teachable moment’, during which efforts may be made to positively influence women’s diet and health behaviours. However, there are barriers to overcome. As already highlighted, preconception is a difficult time to identify. ‘Planning’ a pregnancy is often declared only once the pregnancy has been confirmed; prior to this, it is something regarded by many as deeply personal and essentially private. 57 Furthermore, women may not be aware of the importance of preconception health, and they may not perceive there to be risks or that the risks are relevant to them. 45,57 Specific issues for women with overweight/obesity and who are planning a pregnancy include poor uptake of health activities, inaccurate self-categorisation of weight, unsuccessful weight loss attempts and inadequate advice regarding pre-pregnancy weight loss. 58 Often, the complex lifestyle changes required for weight loss before pregnancy are challenging to achieve, but women who recognise that they have knowledge gaps about the impact of obesity in pregnancy are keen to receive information about antenatal, intrapartum and post-partum risks. 43
The Health Survey for England (HSE)59 reports that obesity rates for women are higher in areas of highest Index of Multiple Deprivation (39%) than in the least deprived areas (22%). Evidence of obesity rates in ethnic minority groups is scarce, with the exception of the 2004 HSE. 60 These data suggest that obesity rates vary among ethnic groups, with higher rates in black and Pakistani groups and lower rates in Chinese groups than in the general population. 61 Health risks such as diabetes are observed in ethnic groups at lower rates of overweight/obesity than the white European population,62 and, therefore, reducing obesity levels in ethnic minority groups could be of increased relevance. Uptake and retention of programmes may vary in women of different ethnic backgrounds. In a process evaluation of a postnatal WLI in an ethnically diverse population, non-white British/Irish women were less likely to attend a commercial weight loss management group than white British/Irish women. The women were more likely to describe barriers to attending the sessions, such as time, access and child-care needs, than white British/Irish women. 63 Furthermore, women from ethnically diverse communities reported that they had modest or poor awareness of preconception health issues and that there was little culture of preconception preparation. 64 Therefore, cultural differences would need to be considered and addressed when targeting women from ethnic minority groups and when designing an effective intervention for ethnically diverse populations.
Health-care practitioners also experience barriers to raising weight management during preconception and pregnancy-related consultations, including lack of skills, time, financial reimbursement, sensitivity of topic and confidence in the available interventions. 65–67 Interviews with UK health-care practitioners showed that there was a low awareness of preconception health issues and confusion about responsibility for delivery of PCC. 35 Taking a broader view of health-care provision, these barriers resonate with those found in a systematic review of the barriers to and enablers of health-care practitioners in delivering behaviour change interventions (diet, physical activity, alcohol reduction, smoking cessation and weight management). 68 Four themes emerged as both barriers and enablers: (1) perceptions of the knowledge or skills needed to support patients’ behaviour change, (2) perceptions of their professional role, (3) beliefs about resources needed and (4) practitioners’ own health behaviour. Cross-disciplinary barriers included a perceived lack of time, negative attitudes towards patients and perceptions of patients’ motivation, including a lack of prioritisation of health behaviour change. 68 Training, context and attitudes towards the intervention were the enablers identified, and any programme of preconception health will need to use these to address individual and systemic barriers if provision is going to change.
Studies in progress
Several trials are in development or pre-reporting that are taking a range of approaches to weight management in the preconception period in different cultural contexts.
Strong Healthy Women was originally a six-session intervention delivered to 692 preconceptional and interconceptional women across 12 weeks in low-income and rural communities. They focused on managing stress, physical activity, nutrition (including folic acid supplementation), preventing gynaecologic infection, tobacco exposure and alcohol use (weight was not included as a focus). Their intervention adopted a social cognitive approach to behaviour change,50,69 identifying self-efficacy, motivation and intention to change as important determinants of behaviour change. Participants receiving the intervention had improved pre–post intervention outcomes relating to nutrition, physical activity and stress management compared to the control group. 70,71 At the 12-month follow-up, the intervention group had reduced weight (mean group difference of 4.33 lb), reduced BMI (mean difference of 0.75 kg/m2) and lower GWG in those who became pregnant (mean difference of 17.95 lb). 49 Despite a small sample size, these were promising results. However, the face-to-face nature of intervention delivery was considered resource-intensive and expensive by the research team, and the time investment required for participation was burdensome for some women. The study team have therefore explored a ‘smart’ version, using qualitative methods to determine which components women would be happy to receive digitally and which to retain face to face. 72
The INTER-ACT study in Belgium73 is an interpregnancy study designed to reduce pregnancy complications in women whose pregnancy weight gain exceeds the IOM-recommended levels. The lifestyle intervention incorporates an e-health application (app) and coaching delivered partially post partum and then during the following pregnancy. The women’s experience of using the app suggests that combining a personalised app with coaching is a positively received intervention.
The Jom Mama trial in Malaysia74 is a population-based pre-pregnancy intervention that recruits as couples get married. It is designed to enhance healthy dietary choices, increase exercise and manage stress. It is grounded in the theory of triadic influence and uses behaviour change counselling combined with WhatsApp chat support and an e-health habit formation app.
TOP Mums75 is a preconception intervention designed to reduce perinatal morbidity for women with a BMI of > 25 kg/m2 planning to conceive in the next year and is due to report imminently. It is a 26-week programme incorporating motivational interviewing, personalised goals and a health intervention, ‘Smarter Pregnancy’. BMI is the primary outcome from baseline to 6 weeks post partum, with GWG one of the secondary outcomes.
Other studies that will involve measurement of pre-pregnancy weight, but do not evaluate population based pre-pregnancy interventions, evaluate the quality of the preconception diet (Healthy for my Baby);76 the relationship between maternal weight and baby weight;77 reducing pre-pregnancy and pregnancy weight gain in the context of depression;78 and reducing the reoccurrence of gestational diabetes. 79
Users of long-acting reversible contraceptives
The complexities of identifying the preconception population mean that it is difficult to engage with people at the right time to have them consider taking part in a weight reduction programme to improve their preconception health. Women who use long-acting reversible contraception (LARCs) and who require removal of the device to become pregnant represent a unique group in which there is an opportunity for intervention. However, at this point in their reproductive decision-making, it may be difficult to ask women to delay conception through continued use of LARC and engage in weight loss programmes, raising pragmatic and ethical issues for both an intervention and any research study designed to establish effectiveness. A small feasibility study of an intensive WLI offered to women with a BMI of 30 kg/m2 or more attending for LARC removal80 demonstrated that some women were willing to consider delaying LARC removal for 6 months to participate. This small evidence base suggests that there may be an interest in weight loss and a willingness to delay LARC removal in relevant populations. However, with high rates of non-participation and attrition from the programme, it has not yet been established what, if anything, the nature of an acceptable intervention would be.
To answer this question, a mixed-methods approach is required, incorporating the use of routine data, qualitative data collection and analysis, and giving a central role to stakeholders. This will lead to a better understanding of the LARC pathway from an individual and population perspective and its interface with weight management. LARC users’ and health-care practitioners’ experiences of LARC services, decision-making and management of weight around pregnancy will be explored alongside their views of the ethical and methodological issues associated with the timing of informed consent and a potential preconception WLI. Health-care practitioners’ LARC practice and consultation patterns regarding LARC use and removal will be identified utilising data sets, collected routinely across the four UK nations, to compare the population across the different health-care settings as well as over time, taking into account factors such as the impact of different general practice incentives on activity and recording. 81 All of this information will be critical to consider when developing a future intervention.
Preconception weight loss as a complex intervention
A preconception weight loss programme would be described as a complex intervention in the terms of the Medical Research Council framework for the development and evaluation of complex interventions,82,83 which involve multiple interacting components in a complex context and require behaviour change on the part of practitioners and participants. The Medical Research Council framework identifies four phases in the development and evaluation of complex interventions: development, feasibility, evaluation and implementation. The Plan-it study falls into the development phase, exploring an idea for a potential intervention that could be delivered in the NHS. There are, clearly, many generic weight loss programmes that women could access in the preconception phase. The question here is whether or not it is acceptable and feasible to develop a specific preconception weight loss programme that includes a delay to a LARC removal as the entry point to the programme.
As described by O’Cathain et al. ,83 the development phase is a dynamic, iterative process that includes seeking feedback from key stakeholders on intervention ideas, reviewing evidence, drawing on theories, exploring context and undertaking some primary data collection. Through this process, the strengths of the idea and the problems can be identified and used to further refine possible intervention designs. Once an idea for an intervention has been developed that is acceptable and feasible in its formulation, it moves to the feasibility phase, when it is tested in practice, followed by an evaluation, potentially by a RCT, to establish its efficacy.
Research objectives
The aim of the Plan-it study is to establish if it is acceptable and feasible to conduct a study that asks women with overweight/obesity (BMI of ≥ 25 kg/m2) to delay removal of LARC to participate in a targeted pre-pregnancy WLI.
The study objectives are to identify:
-
the annual number of women of reproductive age (16–48 years) in the UK who request LARC removal and subsequently have a pregnancy
-
the means of identifying women with overweight/obesity at study sites who plan to have LARC removal for the purpose of planning a pregnancy and opportunities to intervene
-
suitable and acceptable interventions that can be incorporated into a preconception WLI
-
the willingness of clinicians to raise weight loss in consultations and recruit eligible women to the intervention
-
women’s views about the acceptability and feasibility of the proposed intervention
-
future potential intervention based on feasibility and acceptability to stakeholders.
Chapter 2 Methods
This chapter provides a detailed description of the study design and a general overview of the study processes. Specific methods relevant to individual study work packages (WPs) are detailed in corresponding chapters.
Study design
The Plan-it study used a concurrent mixed-methods approach, incorporating the use of routine NHS data and qualitative data collection, analysis and synthesis across WPs to address study objectives (see Chapter 1).
Work package 1 established the feasibility of defining and understanding the study population through routine data and addressed study objectives 1 and 2 (see Chapter 3):
-
to identify the annual number of women of reproductive age (16–48 years old) in the UK who request LARC removal and subsequently have a pregnancy
-
to ascertain opportunities to identify women with overweight/obesity at study sites who plan to have LARC removal for the purpose of planning a pregnancy and opportunities to intervene with a potential preconception WLI.
Work package 2 identified potentially suitable preconception/pregnancy-related WLIs and the theories underpinning them, using realist methods,84 in addition to assessing the feasibility and acceptability of a preconception WLI to stakeholders (LARC users and health-care practitioners). Study objectives 3–5 were addressed:
-
to identify suitable and acceptable programme components to be incorporated into a preconception WLI
-
to assess the willingness of health-care practitioners to raise weight loss in consultations and recruit eligible women to the intervention
-
to assess LARC users’ views on the acceptability and feasibility of the proposed intervention and of future research.
The findings from the two WPs were collated, and the barriers to and facilitators of a potential future intervention were assessed by stakeholders (see Chapter 7), to address objective 6:
-
future potential intervention based on feasibility and acceptability to stakeholders.
Work package 1: defining and understanding the population through routine data
To address study objectives 1 and 2, WP1 used routine data from Welsh sexual health clinics (SHCs) and UK general practices relating to women attending for LARC removal to:
-
understand the pattern of LARC use to identify opportunities to intervene
-
report the annual number of women in the UK requesting removal of LARC without replacing it with an alternative prescribed contraception
-
identify women, who request LARC removal and subsequently become pregnant, who would be eligible for recruitment to a WLI study
-
identify events in general practitioner (GP) and hospital records to explore time from LARC removal to conception or appointments relating to difficulties conceiving (if possible).
Comprehensive WP1 methodologies are described in Chapter 3.
Work package 2: understanding context and stakeholder views
Work package 2 was conducted in two phases. Phase 1 comprised scoping work to develop an understanding of the typical preconception pathways related to LARC use/LARC removal and weight from the perspectives of LARC users and service providers and to identify suitable weight loss and weight-related health behaviour interventions and the theories that underpin them using realist synthesis. Phase 2 focused on establishing the acceptability and feasibility of a proposed intervention.
Work package 2 phase 1: realist review – scoping suitable interventions and underlying theories
Programme theories regarding how and when health behaviour interventions in the preconception phase may function were developed, guided by the principles of scientific realism. 84 Studies of WLIs prior to and during pregnancy and relevant behaviour change interventions were identified through literature searches, including searches of systematic reviews, companion papers (e.g. qualitative studies and process evaluations) and grey literature (the search strategy is detailed in Appendix 6). These provided an understanding of how and when preconception WLIs might be delivered successfully. Barriers to and facilitators of engagement in preconception health behaviour change interventions and identified health gains or risks to health associated with the intervention were incorporated into the review. The outcome of this review was a set of context–mechanism–outcome (CMO) configurations and key components of the intervention. These were explored in stakeholder advisory groups (SAGs) as the basis of an early overall programme theory, and further developed in phase 2.
Work package 2 phase 1: understanding the preconception pathways relating to LARC
A range of qualitative methods were used to generate a detailed understanding of typical preconception pathways related to LARC use/LARC removal and weight from the perspectives of both service users (LARC users) and service providers (health-care practitioners and weight loss practitioners). LARC removal service contexts, LARC management (in the context of LARC users and health-care practitioners), the inter-relationship between discussions about overweight/obesity and family planning, feasible opportunities to intervene and potential intervention components including additional preconception health-related content were assessed. The phase comprised three components: (1) an analysis of policy documents, (2) engagement with LARC users and (3) service provider engagement (health-care and weight loss practitioners).
Analysis of policy documents
A review of policies, best practice guidelines and other clinical/advisory documents pertaining to the use (and particularly the removal) of LARCs in the UK was conducted to understand how services are expected to approach discussions of weight loss with women with overweight/obesity, how LARC treatment pathways currently operate, guidance on health behaviours prior to conception and the practical/ethical challenges to successful service delivery in current service structures, including equity of access to interventions (for detailed methodologies, see Chapter 4).
Engagement with LARC users
Qualitative surveys using closed and open-text questions were utilised to understand women’s experiences of discussing weight with health-care practitioners; how, where and when women access preconception WLIs; women’s knowledge of the risks of overweight/obesity in pregnancy; the barriers to and facilitators of the introduction of a WLI at LARC removal appointments; and the preferred components of a potential intervention. Detailed methodologies are described in Chapter 4.
Engagement with service providers (health-care and weight loss practitioners)
Qualitative surveys with health-care practitioners using closed and open-text questions focused on: PCC provision; the discussion of weight and preconception health both generally and in the specific context of LARC removal; challenges to service delivery; equity of access to interventions; and views on the potential for an intervention postponing LARC removal as part of preconception weight loss plan. Weight loss practitioners (i.e. people who support women to lose weight as part of their role) were recruited via existing contacts and a range of social media platforms. Qualitative surveys with weight loss practitioners addressed the feasibility and experiences/views on the provision of weight loss programmes in the preconception phase. Detailed methodologies are described in Chapter 4.
Summary of phase 1 findings
Information gathered in phase 1 was synthesised to describe the core components of a potential intervention, together with the contextual factors likely to be important influences on outcomes and engagement. This was refined through work with two SAGs, a LARC user SAG and a health-care practitioner SAG, recruited through the exploratory work in phase 1. The health-care practitioner SAG was run as part of a Continuing Professional Development event to incentivise health-care practitioners to attend but without disruption to clinical activity. The LARC user SAG took the form of a focus group, held remotely via Zoom (Zoom Video Communications, San Jose, CA, USA). The objectives of both SAGs were to generate the stakeholders’ views of the potential intervention components and to consider the key questions to ask participants in phase 2. Detailed methodologies are described in Chapter 6.
Phase 2: acceptability and feasibility of proposed intervention
The outputs from phase 1 were explored in phase 2, with targeted qualitative work addressing acceptability and feasibility of a potential WLI to women in the target population and to health-care practitioners. Phase 2 qualitative interview schedules were informed by the feedback from the SAGs, and the interviews further tested and refined the theories developed in phase 1. Interviews with 20 LARC users and 10 health-care practitioners were conducted over the telephone or remotely via Zoom or Microsoft Teams. Health-care practitioners were asked to explore their views regarding the type of intervention and their willingness to recruit women to a potential study. LARC users were asked to explore their views regarding the acceptability and feasibility of the potential WLI. Findings from the targeted qualitative phase 2 work were discussed at LARC user and health-care practitioner SAGs. The SAGs were conducted as a focus group remotely via Zoom or an online group discussion. The findings from phase 2 were collated, and the key design elements of a potential intervention were described.
Literature review methods
For each of the search strategies (detailed in Appendix 6), relevant papers were identified through purposive and snowball searching. A broad search strategy was developed and run in the following search engines: MEDLINE, PubMed and ScienceDirect® (Elsevier, Amsterdam, the Netherlands). Papers were restricted to English language only between set dates. Search strategies were agreed between two members of the team for each search, inclusion criteria were set and the relevance of each paper was assessed. Data to be extracted were agreed prior to analysis and a sample of data extraction was double checked by a second member of the study team. Quality was assessed and integrated into the analysis and synthesis to ensure that the findings were not overinterpreted.
Study setting and participant selection/recruitment
The study setting and participant population is described in Table 1.
| Study stage | Participant population | Setting |
|---|---|---|
| WP1 | Participants will be included in routine data sets if they met eligibility criteria | N/A |
| WP2 phase 1 | LARC users of reproductive age who self-identify as having/previously having overweight/obesity | Identified via social media/online platforms. Recruited online via Cardiff Online Surveys (formerly Bristol Online Surveys) |
| Health-care practitioners who insert or remove coils as part of their role | Identified at up to eight professional meetings. Recruited online via Cardiff Online Surveys or face to face | |
| Weight loss practitioners who support women to lose weight as part of their role | Identified via existing contacts or social media/online platforms. Recruited online via Cardiff Online Surveys | |
| WP2 end of phase 1 SAGs | LARC users of reproductive age who self-identify as having/previously having overweight/obesity | Purposively sampled from the WP2 phase 1 participant population, who had agreed to be contacted by the study team |
| Health-care practitioners who insert and/or remove coils as part of their role | Identified and recruited at a BASHH audit-focused professional meeting | |
| WP2 phase 2 interviews | LARC users of reproductive age who self-identify as having/previously having overweight/obesity | Purposively sampled from the WP2 phase 1 participant population, who had agreed to be contacted by the study team, excluding members of the phase 1 SAG |
| Health-care practitioners who insert and/or remove coils as part of their role | Purposively sampled from the WP2 phase 1 participant population, who had agreed to be contacted by the study team | |
| WP2 end of phase 2 SAGs | LARC users of reproductive age who self-identify as having/previously having overweight/obesity | Purposively sampled from the WP2 phase 1 participant population, who had agreed to be contacted by the study team |
| Health-care practitioners who insert and/or remove coils as part of their role | WP2 phase 1 participant population, who had agreed to be contacted by the study team |
Eligibility criteria
Participants were included in routine data sets if they met the eligibility criteria and in the online surveys if they self-identified as meeting the eligibility criteria. See Chapter 4 for a detailed description of LARC user and health-care practitioner eligibility criteria and Chapters 6 and 7 for relevant LARC user and health-care practitioner sampling criteria.
Informed consent
For the surveys, all inclusion and exclusion criteria were specified in publicity materials, and for the surveys, interviews and SAGs all participants were provided with a participant information sheet (PIS) and followed a consent procedure (see Chapters 4, 6 and 7).
Data management and confidentiality
All procedures for data storage, processing and management complied with the Centre for Trials Research (CTR) Standard Operating Procedures (SOPs), Clinical Practice Research Datalink (CPRD) and Public Health Wales (PHW) data-sharing agreements and the General Data Protection Regulation (GDPR),85 as appropriate. A full Data Management Plan was developed, and all data will be kept for 15 years on a Cardiff University secure server, in line with Cardiff University’s Research Governance Framework Regulations for clinical research.
The online survey was hosted by Cardiff Online Surveys (previously Bristol Online Surveys) on a Cardiff University secure server, and access was password protected. A member of the research team acted as administrator.
Hard-copy consent forms were stored in a locked filing cabinet. All electronic data, including consent/contact details, were stored in password-protected servers maintained on Cardiff University networks. All electronic identifiable data (consent, contacts forms) were stored separately from interview data. Interviews and SAGs were recorded on encrypted password-protected audio recorders, and voice files remained password protected and were accessible only to relevant members of the research team once transferred to secure Cardiff University servers. Recordings were transcribed and pseudonymised in line with the CTR SOPs. All essential documents generated by the study were kept in the study master file and/or on the electronic study master file.
Analysis
Findings from iterative literature searches were synthesised using a realist approach to evaluation. 84,86 The extracted information was summarised descriptively in explanatory accounts and then consolidated to generate potential CMO configurations to be explored in phase 2 stakeholder interviews.
Survey responses were downloaded. Responses to closed or multiple-choice questions were described. For each qualitative data collection method (open-text questions in survey responses, interviews or SAGs), responses were thematically analysed in each group of participants (LARC users, health-care practitioners and weight loss practitioners) separately. Qualitative synthesis across all interviews provided an overarching synthesis of LARC users’ and service providers’ perceptions related to the study objectives. A full qualitative analysis plan was written by the qualitative researcher and approved by the Study Management Group (SMG) and Study Steering Committee (SSC) prior to analysis taking place.
Withdrawal
For WP1, data were aggregated and anonymised, and, therefore, it was not possible to remove records once an extract had been produced. Participants in the WP2 surveys and qualitative interviews were able to withdraw at any time prior to analysis by contacting the study team. Participants in SAGs were able to withdraw at any time by leaving the SAG meeting. However, the nature of a focus group meant that any contribution thus far was unable to be withdrawn.
Ethics
Ethics approval for this study was given by the Cardiff University School of Medicine Research Ethics Committee on 30 April 2019, reference number 19/42 (see Report Supplementary Material 1).
Participant and public involvement
Participant and public involvement (PPI) representatives contributed to both the SMG and the SSC (one PPI member in each committee) and took an advisory role in study design, study progress and results dissemination.
At the end of each phase of WP2, PPI input took the form of SAGs whose role was to:
-
refine findings from WP2 phase 1 to generate potential intervention components, collaboratively with the research team, and to consider key questions to take forward in phase 2 (phase 1)
-
discuss findings from the targeted qualitative phase 2 work to describe the key design elements of a potential intervention or the reasons why a trial is currently not feasible to deliver (phase 2).
Changes to the protocol
Three changes were made to the original study protocol. (1) Phase 1 engagement with health-care practitioners via qualitative individual interviews and a qualitative survey was changed to solely the qualitative survey. This was for two reasons. First, conducting qualitative interviews during professional events proved to be impractical and, second, the study team felt that the in-depth nature of individual qualitative interviews was not required in addition to the survey at stage 1. (2) In-depth interviews with weight loss practitioners who support women to lose weight were not conducted in WP2, phase 1, as planned. This was due to a poor response rate to the phase 1 online survey and resulting limited number of participants to sample. (3) COVID-19-related restrictions led to the phase 1 LARC user SAG and the phase 2 SAGs being conducted remotely (via Zoom or Padlet) rather than face to face.
Chapter 3 Routine data work package
Introduction
Aims of the chapter
The Plan-it routine data WP used anonymised routinely collected data from SHCs in England, Wales and Scotland and the CPRD linked to the Pregnancy Register to determine the most appropriate LARC removal settings, the annual numbers of potential participants available to be recruited and an indicative time frame for recruitment.
The objectives were to set up access to anonymised data from multiple health settings to:
-
understand the pattern of LARC use (removal/insertion/in situ) to identify opportunities to intervene
-
identify women, who request LARC removal and subsequently become pregnant, who would be eligible for recruitment to a WLI study
-
report the annual number of women in the UK requesting removal of LARC without replacing it with an alternative prescribed contraception
-
identify events in GP and hospital records to explore time from LARC removal to conception or appointments relating to difficulties conceiving (if possible).
The outcomes identified were:
-
rates of women in the UK who request LARC removal and subsequently have a pregnancy from routine data
-
identification of opportunities to intervene in the preconception pathway.
Methods
Study design/setting
Routine data providers and data sets
Clinical Practice Research Datalink
The CPRD provides UK-wide individual anonymised patient GP data. Data cover > 20% of general practices in the UK and are representative of practices by country, rurality and deprivation quintiles. 87 Anonymised primary care patient data requested from CPRD include data linked to secondary care and other health-based data sets, including the Pregnancy Register. The Pregnancy Register is created by an algorithm that lists all pregnancies identified in the CPRD database. 88 For pregnancies resulting in live births, deidentified information of the linked babies in the CPRD Mother Baby Link were also provided (baby ID, baby month and year of birth, pregnancy start and end dates, gestational age of baby, and mother’s age at pregnancy). LARC-related Read codes were identified using the Read code dictionary and from the literature and reviewed by the clinical co-investigators for accuracy and inclusivity of all possible codes. Cardiff University held an Academic Risk Sharing Licence with CPRD and following Independent Scientific Advisory Committee approval, and data were made available via a Cardiff University-employed data analyst.
Sexual health clinic data
Sexual health clinic data are collected and held separately in Wales, England and Scotland. Public Health Wales holds individual-level patient data from all SHCs in Wales (from 2012), and these were made available to the study team in an aggregate format based on data requirements. Aggregated data from PHW were transferred to Cardiff University servers on agreement of data release. The required tabulations of outputs were agreed with PHW to ensure that the eligibility applied to CPRD patients was applied to the SHC data. In Scotland and England, SHC data are collated and reported by NHS National Services Scotland and NHS Digital, respectively. National statistics annual reports on sexual health service use provide open data tables that were used as a national comparison with Wales (with no formal data access requests required for these open data tables). 89,90
Study population
Clinical Practice Research Datalink
The Plan-it Study was interested in identifying women with overweight or obesity who had their LARC removed for the purpose of conception and were, therefore, eligible for a targeted pre-pregnancy WLI. The predefined study population was women of reproductive age (16–48 years inclusive) with at least one LARC event between 1 January 2009 and 31 December 2018.
Defining a LARC event
A list of LARC events was prespecified from the Clinical (medical history data entered on the GP system), Referral (referral details recorded on the GP system) and Therapy (all prescriptions issued by the GP on the GP system) data sets in the CPRD and agreed by the SMG (see Appendix 1). These were categorised as followed: LARC insertion, in situ (checks), removal. LARC event codes that belonged to males or to women who were under 16 or over 48 years old at the date of consultation, those for events before 2009 or after 2018, those with no consultation date, duplicate codes (same code recorded on the same day) or those that belonged to women who were not in the predefined study population were excluded.
Identifying women who were planning a pregnancy following LARC removal
The study population were grouped as follows:
-
Group 1 – planning a pregnancy
-
Group 2 – possibly planning a pregnancy
-
Group 3 – probably not planning a pregnancy.
Table 2 shows the algorithm used to define these groups based on the initial LARC event, a consultation code following the LARC event that either confirmed or refuted planning a pregnancy, and a confirmation of a pregnancy. Codes to refute or confirm that a pregnancy was planned are in Appendix 3.
| Group | Group description | LARC event | Consultation following LARC event (and prior to pregnancy if applicable) | Pregnancy event |
|---|---|---|---|---|
| 1 | Planning a pregnancy | LARC removal/inserted/in situ | Read code indicating a pregnancy was being planned | Yes |
| 1 | Planning a pregnancy | LARC removal/inserted/in situ | Read code indicating a pregnancy was being planned | No |
| 2 | Possibly planning a pregnancy | LARC removal/inserted/in situ | No code present to indicate that the woman was planning a pregnancy or not. No code present to indicate an unplanned pregnancy | Yes |
| 3 | Probably not planning a pregnancy | LARC removal/inserted/in situ | Code to indicate a pregnancy was not being planned (e.g. alternative contraception), menopause indication (e.g. blood tests) | Yes |
| 3 | Probably not planning a pregnancy | LARC inserted/in situ | No code present to indicate that the woman had a LARC removed or was planning/not planning a pregnancy | No |
| 3 | Probably not planning a pregnancy | LARC removal/inserted/in situ | Code to indicate a pregnancy was not being planned (e.g. alternative contraception), menopause indication (e.g. blood tests) | No |
| Not enough information | LARC removal | No code present to indicate that the woman was planning/not planning a pregnancy | No | |
Identifying a pregnancy
We requested Pregnancy Register data on the predefined population and used the estimated pregnancy start date provided as the index date at which to examine prior LARC use. The estimated pregnancy start date was estimated using the timing of the start of pregnancy (first day of their last menstrual period) and additional data from ‘Additional Clinical Details’ files. In the absence of such data, pregnancy start dates were imputed according to the type of pregnancy outcome. We excluded pregnancies that had a defined start date occurring before 2009 or after 2018, duplicate events (repeated events, overlapping events, and events that had a start date within 31 days of the previous start date).
Identifying clinical events after the LARC removal
The clinical events between the two events (LARC removal and pregnancy start date) or following the LARC event were examined to either confirm or refute that a pregnancy had been planned. These events were identified from the Clinical file in CPRD (see Appendix 2 for the list of Read codes that were prespecified and agreed by the SMG):
-
refuting a planned pregnancy – an alternative prescribed contraception (see Appendix 2, Table 23), indication of the menopause (see Appendix 3, Table 24), and an unplanned pregnancy (see Appendix 3, Table 26)
-
confirmation of a planned pregnancy – indicating trying to get pregnant, a planned pregnancy (see Appendix 3, Tables 25 and 26).
The following assumptions were agreed by the Plan-it study team in advance:
-
For those with a pregnancy, only codes that were within the 456 days (1 year and 3 months) prior to the pregnancy start date were investigated. For those with no pregnancy, only codes that were within 456 days of the LARC event were investigated.
-
If the gap between a LARC removal and LARC situ events was < 28 days, it was assumed that the woman was having a LARC check-up. These two events were combined as one rather than included twice.
-
For a woman who had the LARC in situ/insertion and LARC removal event on the same date, LARC replacement was assumed and the LARC removal event was excluded.
-
For clinical codes that appeared between a pregnancy start and end, only the planned pregnancy and unplanned pregnancy code were included.
-
LARC in situ or insertion within a week of a pregnancy start was coded as unplanned pregnancy.
-
For a woman with their first LARC code between a pregnancy start and end and after a week of a pregnancy start, the whole event was excluded.
-
For a woman who has both unplanned and planned pregnancy code between LARC removal and pregnancy start, the code that was closest to the pregnancy start was used as an indicator for grouping the woman.
-
For a woman who had both the alternative contraception and the trying/difficult to get pregnant code on the same day, the trying/difficult to get pregnant code was used as an indicator and the contraception code excluded.
-
Women with too many planned/unplanned codes were coded as Unable to code.
Body mass index
Weight and height data were selected from the ‘Additional Clinical Details’ file in CPRD (Entity Type 13 and 14). The BMI was generated following the method described in Bhaskaran et al. 92 (representativeness). Only BMIs that were recorded within 3 years of either the pregnancy start date or the last LARC event for those with no associated pregnancy were included. Appendix 5 depicts the data flow for BMI.
Sexual health clinic data
Women of reproductive age (i.e. 16–48 years old) with at least one LARC event (inserted, removed or in situ) between 1 January 2012 and 31 December 2018 were included. Data were sourced from the Sexual Health in Wales surveillance system. Data on patients who attended a SHC in Wales were recorded and coded by front-line SHC staff and then collated by PHW. Data fields of relevance to this project included date of birth (for age to be calculated), date of attendance (for year to be derived), main contraception method [CM2: implant; CM3: intrauterine device (IUD); CM4: intrauterine (IU) system], health board and deprivation quintile.
Statistical methods
For the individual anonymised data from CPRD, rates of fitting and removal of LARC in general practice were calculated and reported over time (either quarterly or annually, depending on numbers). Rates were calculated as the number of LARCs fitted and removed as a proportion of all women of reproductive age.
Trends in rates were examined by country, LARC type and, where available, attendance type (pre-booked vs. walk-in consultations). Similarly, we examined trends using the Welsh SHC data. This enabled a comparison of the case mix of women recorded for a LARC removal in general practice and those who visit a SHC by age, ethnicity, BMI and deprivation. The data from SHCs in Scotland and England were in aggregate format at NHS Sexual and Reproductive Service provider level and reported rates of fitting and removal annually. Trends in rates by age group at time of fitting or removal, change of contraception method from/to a LARC and, if recorded, BMI category and deprivation quintile were explored. These rates were compared with those arising from the SHC data in Wales. The quality of recording of BMI in all data sets was explored. Previous work in the CPRD shows that completeness of BMI has increased over time (to around 77%) and was higher in female individuals, especially those of reproductive age. 92
Analysing these data sets identified variation in the numbers, pattern and duration of use of LARCs in the different health settings, geographical areas (rural/urban) and demographic groups. It was possible to consider what opportunities (e.g. consultation types, frequency of consultations) are available to intervene in the different service delivery designs across the UK. The routine data analysis determined the most appropriate LARC removal settings, the annual numbers of potential participants available to recruit and an indicative time frame for recruitment.
For patients attending their GP for the fitting or removal of their LARC, the data allowed exploration of the duration of LARC use prior to removal and the changes to contraception use over time; through linking to the Pregnancy Register algorithm, a pregnancy episode related to the women in the cohort (estimated start of pregnancy) was flagged. Accessing these data will enable a broader understanding of the population for whom this intervention will be targeted and, potentially, identify those who had a LARC removed for the purpose of planning a pregnancy. An examination of how time to conception may differ between BMI and age categories was also possible. For women whose pregnancy was identified following a LARC removal, the natural distribution of the time between the two events (LARC end and pregnancy start) was examined to assess whether or not a rule could be applied to indicate that the pregnancy and LARC removal were associated.
Although the data between GP surgeries and SHC data could not be linked, the recording of a LARC removal in a SHC setting was explored in the GP notes. Previous work using an alternative primary care data source (The Health Improvement Network) identified that 24% of LARC-related records in primary care came from SHC letters. 93 The reporting of routine data was in accordance with RECORD (REporting of studies Conducted using Observational Routinely-collected Data). 94
Data access, linkage and cleaning methods
At the time of the project, Cardiff University held a licence with CPRD that allowed approved university staff members to access the CPRD GOLD database. The population relevant to this study was identified using the agreed code list (see Defining a LARC event). This list of patients was then linked to the Pregnancy Register (this linkage is completed by CPRD and not by Cardiff University staff). Once linked, these data sets were made available to the project staff.
Ethics
Data access requests to CPRD were reviewed by the Independent Scientific Advisory Committee (Protocol: 19_188). The CPRD has broad National Research Ethics Service Committee ethics approval for purely observational research using the primary care data and established data linkages. No further ethical review was required for this element of the project.
Results
Defining the study population in the Clinical Practice Research Datalink
The study population generation is reported in Figure 2. The study population was identified using the CPRD Patient file, which included just over 20 million patient records. Practices were excluded from the study population if the practice’s last contribution date was before 2009 or their up-to-standard date was after 2018. Practices with < 1 year between their last contribution date and their up-to-standard date (< 1 year of data available) and those not meeting the criteria in the specified years were also excluded. The following patient groups were excluded: male patients; patients of unknown or indeterminate gender; patients whose data were outside the up-to-standard date; patients whose year of birth was outside 1961–2002; patients who had died before 2009; patients registered with the practice after 2018; and patients whose current period of registration with the practice was after 2018.
FIGURE 2.
Participant flow diagram. Reproduced with permission from Channon et al. 91 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
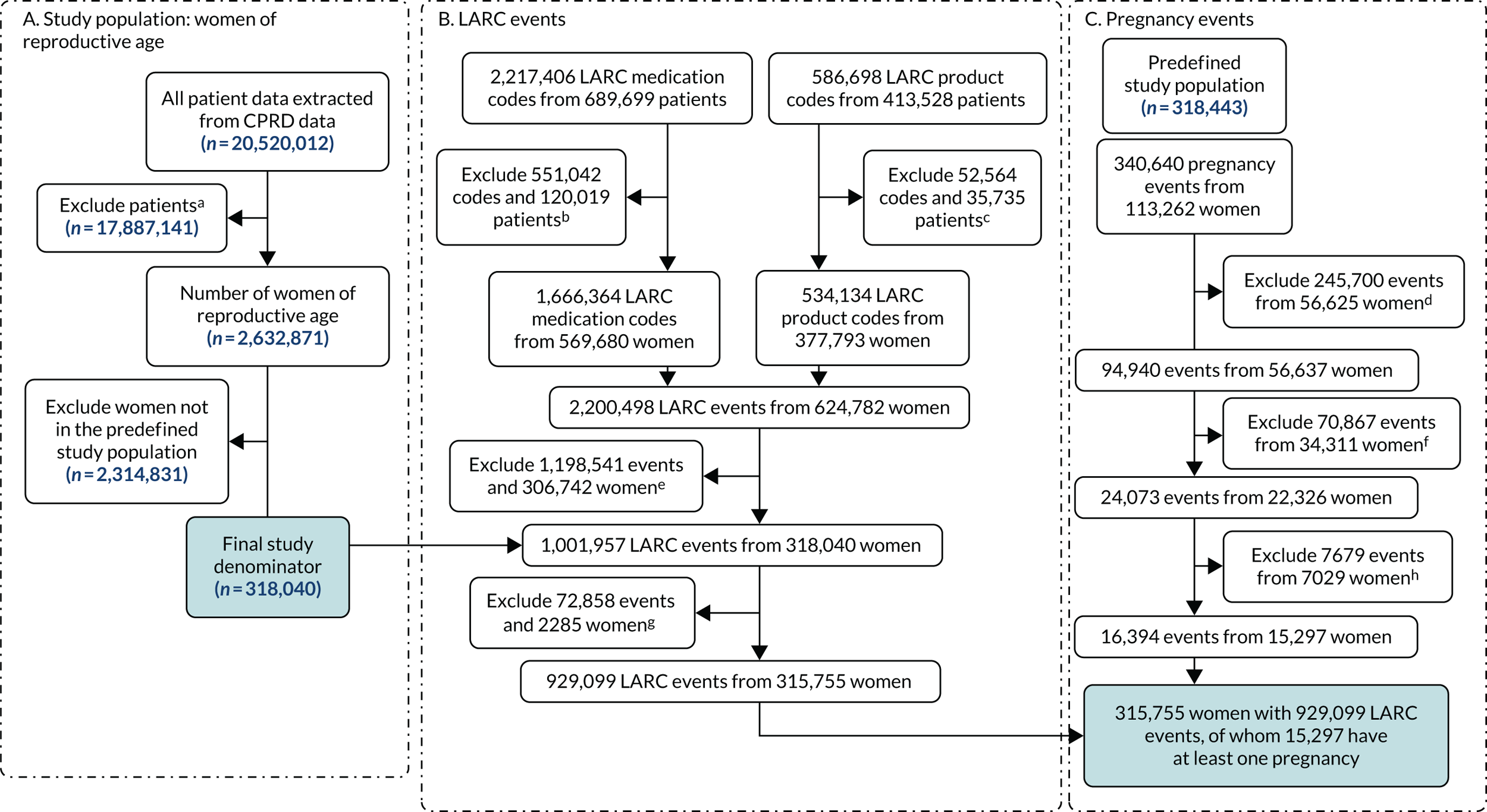

This left 2,632,871 women at reproductive age in the study denominator. However, 2,314,831 were excluded as they were not in the predefined study population (i.e. with one LARC event), leaving 318,040 eligible women of reproductive age in the study population. The rate of women at reproductive age in the study denominator was constant over the time period 2009–18 (Table 3).
| Year | Number of women aged 16–48 years (study denominator) | Number of general practices with data available | Rate of women per practice per 10,000 |
|---|---|---|---|
| 2009 | 1,413,791 | 738 | 5.22 |
| 2010 | 1,408,118 | 728 | 5.17 |
| 2011 | 1,380,332 | 713 | 5.17 |
| 2012 | 1,355,832 | 699 | 5.16 |
| 2013 | 1,330,823 | 680 | 5.11 |
| 2014 | 1,244,434 | 645 | 5.18 |
| 2015 | 1,108,991 | 580 | 5.23 |
| 2016 | 914,755 | 491 | 5.37 |
| 2017 | 812,739 | 437 | 5.38 |
| 2018 | 741,453 | 397 | 5.35 |
Objective 1: understand the pattern of LARC use (removal/insertion/in situ) to identify opportunities to intervene
Women attending general practice for a LARC-related consultation
To identify women who attended a GP surgery for a LARC event, a combination of Read and prescription codes were used. The flow chart (see Figure 2) shows that, from using these codes for LARC, 929,099 codes were present in the study population of 315,755 women. Table 4 shows these data broken down by LARC consultation type and type of LARC. Women in our study population could experience more than one code over the study period. For example, among the 127,909 women who had at least one LARC in situ code, 49,285 (39%) had more than one code recorded over the study period. Of those 269,999 women who had a LARC insertion, 182,090 (67%) had more than one LARC insertion code recorded over the study period. Of those 108,987 women who had a LARC removal, 12,615 (12%) had more than one LARC removal code recorded during the study period.
| Unique number of events | Number of women with at least one event | |
|---|---|---|
| LARC consultation typea | n = 938,161 | |
| In situ | 222,555 | 127,909 |
| Insertion | 592,402 | 269,999 |
| Removal | 123,204 | 108,987 |
| Type of LARC | n = 934,903 | |
| IUD | 411,514 | 167,008 |
| IU system | 308,058 | 131,889 |
| Implant | 215,331 | 125,497 |
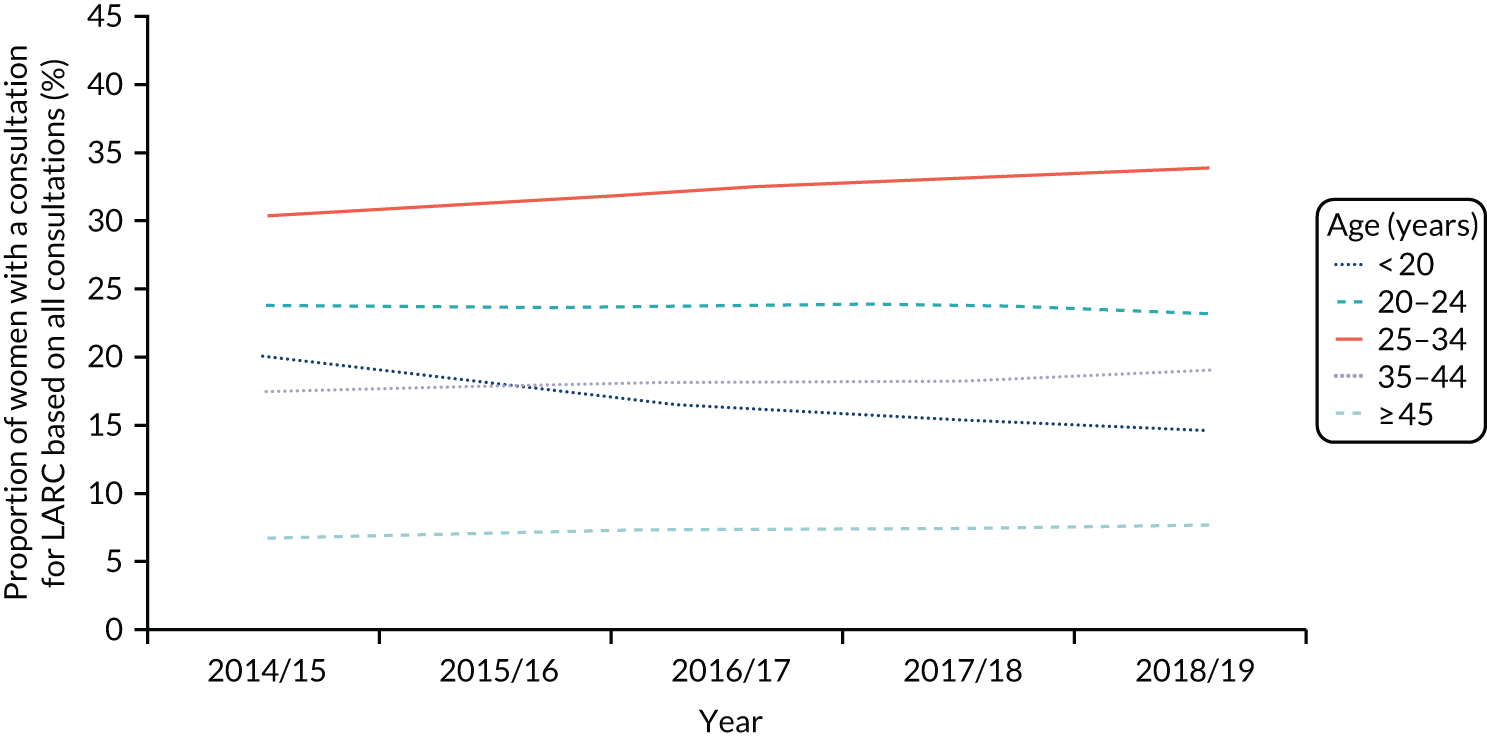
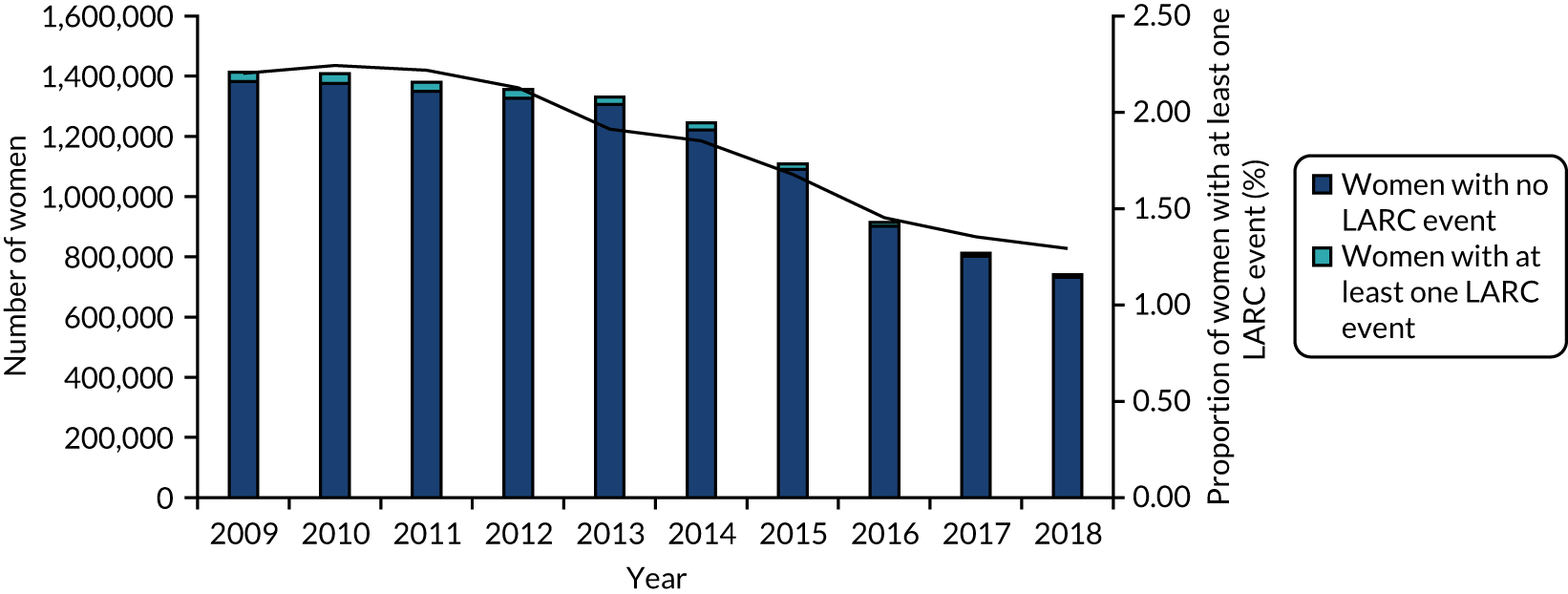
The number of women in the study population decreased over time due to the number of practices contributing to the CPRD declining; the rate of women per practice was constant (Figure 3 and Table 5). The proportion of women with one or more LARC consultations decreased over time, from 2.2% in 2009, which is approximately 42 LARC events per practice, to 1.3% in 2018, 24 LARC events per practice. By contrast, the Wales SHC data show a different picture (Figure 4); in 2018 the rate of women with SHC contact for LARC use out of contacts for any contraception was higher, at 24%.
FIGURE 3.
LARC users over time: CPRD (2009–18).
| Year | Study denominator | Study population | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women aged 16–48 years (n) | Consultations (n) | Year of first LARC consultationa | LARC consultation typeb | Type of LARC | ||||||||||||
| In situ | Insertion | Removal | IUD | IU system | Implant | |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |||
| 2009 | 1,413,791 | 2,253,647 | 61,440 | 4.3 | 31,074 | 1.4 | 65,523 | 2.9 | 11,293 | 0.5 | 58,232 | 2.6 | 27,390 | 1.2 | 21,814 | 1.0 |
| 2010 | 1,408,118 | 2,385,300 | 51,850 | 3.7 | 31,479 | 1.3 | 75,430 | 3.2 | 12,402 | 0.5 | 58,576 | 2.5 | 35,194 | 1.5 | 25,222 | 1.1 |
| 2011 | 1,380,332 | 2,474,914 | 43,456 | 3.1 | 30,540 | 1.2 | 72,290 | 2.9 | 13,891 | 0.6 | 56,355 | 2.3 | 33,977 | 1.4 | 26,207 | 1.1 |
| 2012 | 1,355,832 | 2,561,659 | 36,994 | 2.7 | 28,768 | 1.1 | 72,904 | 2.8 | 14,150 | 0.6 | 51,866 | 2.0 | 37,175 | 1.5 | 26,387 | 1.0 |
| 2013 | 1,330,823 | 2,485,337 | 32,975 | 2.5 | 25,384 | 1.0 | 71,152 | 2.9 | 14,735 | 0.6 | 46,513 | 1.9 | 38,469 | 1.5 | 26,058 | 1.0 |
| 2014 | 1,244,434 | 2,353,969 | 26,816 | 2.2 | 22,996 | 1.0 | 64,713 | 2.7 | 14,283 | 0.6 | 41,533 | 1.8 | 36,231 | 1.5 | 23,945 | 1.0 |
| 2015 | 1,108,991 | 2,149,417 | 21,344 | 1.9 | 18,558 | 0.9 | 55,091 | 2.6 | 13,410 | 0.6 | 33,673 | 1.6 | 32,266 | 1.5 | 20,780 | 1.0 |
| 2016 | 914,755 | 1,751,839 | 15,662 | 1.7 | 13,258 | 0.8 | 42,554 | 2.4 | 10,985 | 0.6 | 24,542 | 1.4 | 25,507 | 1.5 | 16,365 | 0.9 |
| 2017 | 812,739 | 1,479,553 | 13,268 | 1.6 | 10,972 | 0.7 | 38,001 | 2.6 | 9585 | 0.6 | 21,364 | 1.4 | 21,983 | 1.5 | 14,881 | 1.0 |
| 2018 | 741,453 | 1,304,630 | 11,950 | 1.6 | 9526 | 0.7 | 34,744 | 2.7 | 8470 | 0.6 | 18,860 | 1.4 | 19,866 | 1.5 | 13,672 | 1.0 |
FIGURE 4.
LARC users over time: Wales SHC data (2009–18). Note: data from 2012 to 2015 are unreliable due to under-reporting by some health boards.

To further understand LARC use in general practice and SHCs, patterns were examined by LARC consultation type (insertion, in situ, removal), type of LARC used (IUD, IU system, implant) and age group type to identify opportunities to intervene. SHC data were obtained from open-access sources, and extracts are presented in Appendix 4. LARC insertions are most frequently performed in general practice (see Figure 4) (see Appendix 3 for details of type of LARC use over time).
Objective 2: identify women requesting LARC removal who subsequently become pregnant who would be eligible to recruit to a weight loss intervention study
To identify eligible women to recruit to a WLI study, several stages need to be established (Figure 5):
-
LARC use (objective 1)
-
pregnancy within 1 year and 3 months
-
codes to refute or confirm that the pregnancy was planned
-
BMI status.
FIGURE 5.
Stages of identifying women for a WLI study.
Identifying a pregnancy
A total of 340,640 pregnancy events were identified from 113,262 (35.5% of predefined study population) women. After excluding 245,935 events that were outside the study period (before 2009 or after 2018) and duplicate entries, we have 94,705 pregnancy events from 56,637 women. A further 70,632 events were excluded, leaving 24,073 pregnancy events from 22,326 (7.0%) women in the study population.
LARC removal for the purposes of planning a pregnancy
In the 24,073 pregnancy events from 22,326 (7.0%) women in the study population, 11,381 (47.3%) conceptions occurred within the window of 1 year and 3 months (456 days), and 3090 (12.8%) occurred outside the window of 1 year and 3 months. With regard to the 411 (1.7%) pregnancy events with a removal code between pregnancy start and pregnancy end, we considered them as an unplanned pregnancy if the removal code was within 7 days after pregnancy start; otherwise, we would investigate other LARC code (if any) or exclude the pregnancy event and the removal code in between. For the 9191 (38.2%) pregnancy events with no removal code between start and end date, we included the event if the pregnancy start date was within the window of 1 year and 3 months after the last LARC in situ or LARC insertion date; otherwise, the pregnancy event would be excluded.
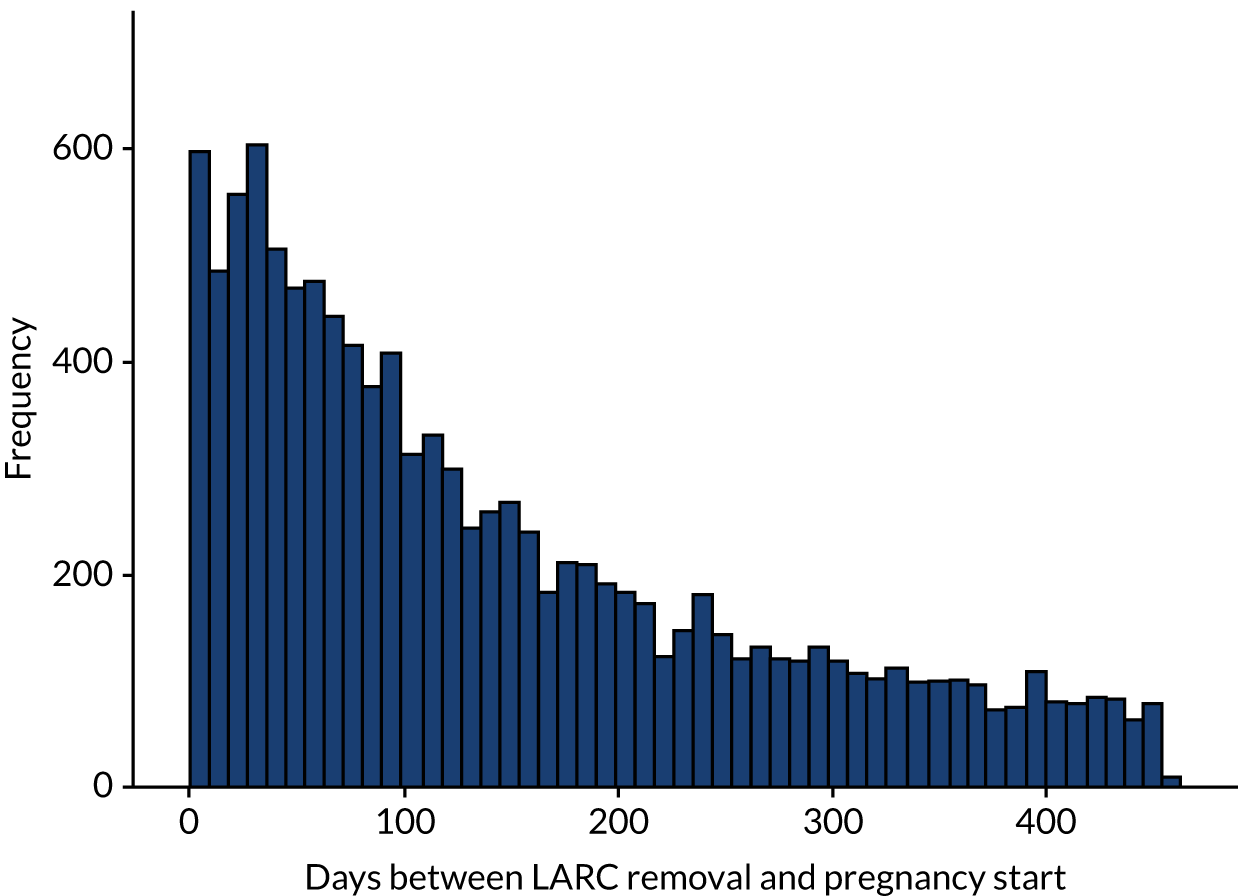
Among the 315,755 women in the study population with a LARC event, 15,297 women had at least one pregnancy, with 16,394 pregnancy events (Table 6). Of those, 4753 (29.0%) pregnancy events did not have a LARC removal code before pregnancy started and 299 (1.8%) had a removal code between pregnancy start and end. The number and percentage of the 16,394 pregnancy outcomes are described in Table 6. For the remaining 11,342 events, the median time to conception was 109 days (25th to 75th centiles = 47 to 220 days) (Figure 6).
| Pregnancy outcome | Frequency (n) | % |
|---|---|---|
| Live birth | 8910 | 54.3 |
| Stillbirth | 33 | 0.2 |
| Miscarriage | 1700 | 10.4 |
| TOP | 241 | 1.5 |
| Miscarriage or TOP or ectopic | 1231 | 7.5 |
| Ectopic | 163 | 1.0 |
| Molar | 6 | 0.0 |
| Blighted ovum | 5 | 0.0 |
| Unspecified loss | 107 | 0.7 |
| Delivery based on a third-trimester pregnancy record | 278 | 1.7 |
| Delivery based on a late-pregnancy record | 46 | 0.3 |
| Outcome unknown | 3674 | 22.4 |
| Total | 16,394 |
FIGURE 6.
Conception time for events within 456 days (n = 11,342).
We have defined 479,044 different scenarios or pathways to a pregnancy event or not among 317,684 women (note that women can have more than one scenario). The number of scenarios and number of women in each group are summarised in Table 7.
| LARC use | Read code indicating an event | Pregnancy | Group | Number of scenarios | Number of women | Age (years), mean (SD) |
|---|---|---|---|---|---|---|
| 1: LARC removal/inserted/in situ | A Read code to indicate the pregnancy was being planned (planned pregnancy code or trying/difficult to get pregnant) either between a LARC (removal/inserted/in situ) and pregnancy start, or between a pregnancy start and end | Yes | 1: planning a pregnancy | 1635 | 1616 | 29.0 (5.81) |
| 2: LARC removal/inserted/in situ | No Read code to indicate that the pregnancy was planned (planned pregnancy code or trying or difficult to get pregnant) or not planning a pregnancy (alternative contraception and menopause). No Read code to indicate that this pregnancy was unplanned (unplanned pregnancy code) | Yes | 2: possibly planning a pregnancy | 10,902 | 10,387 | 28.5 (6.06) |
| 3: LARC removal/inserted/in situ | A Read code to indicate that a pregnancy was not being planned (alternative contraception and menopause) | Yes | 3: probably not planning a pregnancy | 3851 | 3761 | 26.5 (5.98) |
| 4: LARC removal/inserted/in situ | A Read code to indicate that a pregnancy was being planned (planned pregnancy code or trying/difficult to get pregnant) | No | 1: planning a pregnancy | 4871 | 4717 | 30.6 (7.26) |
| 5: LARC removal | After a LARC removal code, no Read code or a Read code present to indicate that the woman was planning/not planning a pregnancy | No | Not enough information | 73,290 | 69,455 | 32.4 (9.03) |
| 6: LARC inserted/in situ | No code present to indicate that the woman had a LARC removed or was planning/not planning a pregnancy | No | 3: probably not planning a pregnancy | 379,495 | 277,144 | 33.0 (9.22) |
| 7: LARC removal/inserted/in situ | Code to indicate a pregnancy was not being planned (e.g. alternative contraception), menopause indication (e.g. blood tests) | No | 3: probably not planning a pregnancy | |||
| 8 | Yes | Unable to code | 6 | 6 | ||
| 9 | No | Unable to code | 59 | 59 |
Table 8 shows, for each group, the number of LARC events, the proportion with a valid BMI measurement broken down by BMI categories: ≥ 25 kg/m2 (overweight and obese), ≥ 30 kg/m2 (obese) and ≥ 40 kg/m2 (morbidly obese). Of all 474,044 LARC events with a valid BMI measurement, a small proportion (1.4%, n = 6506) were removed for planning a pregnancy, 10,902 (2.3%) were possibly planning a pregnancy and 383,346 (80.9%) were probably not having a pregnancy; 73,290 (15.5%) could not be grouped because of insufficient information. A total of 62% of women had a BMI recorded within 3 years of their LARC event, and this was similar across all groups (range 60–67%), with a 5% difference between women not planning a pregnancy and those planning a pregnancy (62% vs. 67%). Around 50% of women had a BMI of ≥ 25 kg/m2, around 28% had a BMI of ≥ 30 kg/m2 and < 5% had a BMI of ≥ 40 kg/m2. Among those planning a pregnancy, 54% had a BMI of ≥ 25 kg/m2, 28% had a BMI of ≥ 30 kg/m2 and < 5% had a BMI of ≥ 40 kg/m2.
| Group | Events, n (%) | With BMI | BMI ≥ 25 kg/m2 | BMI ≥ 30 kg/m2 | BMI ≥ 40 kg/m2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | n | % | Category | n | % | Category | n | % | Category | n | % | ||
| 1: planning a pregnancy | 6506 (1.4) | Yes | 4328 | 66.5 | < 25 | 2014 | 46.5 | < 30 | 3130 | 72.3 | < 40 | 4124 | 95.3 |
| No | 2178 | 33.5 | ≥ 25 | 2314 | 53.5 | ≥ 30 | 1198 | 27.7 | ≥ 40 | 204 | 4.7 | ||
| 2: possibly planning a pregnancy | 10,902 (2.3) | Yes | 7034 | 64.5 | < 25 | 3480 | 49.5 | < 30 | 5368 | 76.3 | < 40 | 6844 | 97.3 |
| No | 3868 | 35.5 | ≥ 25 | 3554 | 50.5 | ≥ 30 | 1666 | 23.7 | ≥ 40 | 190 | 2.7 | ||
| 3: probably not planning a pregnancy | 383,346 (80.9) | Yes | 237,155 | 61.9 | < 25 | 112,472 | 47.4 | < 30 | 176,619 | 74.5 | < 40 | 227,618 | 96.0 |
| No | 146,191 | 38.1 | ≥ 25 | 124,683 | 52.6 | ≥ 30 | 60,536 | 25.5 | ≥ 40 | 9537 | 4.0 | ||
| Not enough information | 73,290 (15.5) | Yes | 44,286 | 60.4 | < 25 | 20,629 | 46.6 | < 30 | 32,535 | 73.5 | < 40 | 423,89 | 95.7 |
| No | 29,004 | 39.6 | ≥ 25 | 23,657 | 53.4 | ≥ 30 | 11,751 | 26.5 | ≥ 40 | 1897 | 4.3 | ||
| Total | 474,044 | 292,803 (62%) | |||||||||||
Objective 3: report the annual number of women in the UK requesting removal of LARC without replacing it with an alternative prescribed contraception
For this objective, we are interested in events of LARC removal without replacement with an alternative contraception (see Appendix 2, Table 23, for alternative contraception code). Of the 123,204 LARC removal events in the study population, 24,777 were followed by an alternative contraception code (20.1%). The time between LARC removal and alternative contraception ranged from 0 to 35.84 months, with a mean of 4.38 months (standard deviation 4.76 months) and a median of 2.73 months (interquartile range 0–7.82 months) (Figure 7).
FIGURE 7.
Time between LARC removal and alternative contraception (months) (n = 24,777).
Based on this information, and without a clear cut-off point identified from clinical practice, we decided to use 4 months as a cut-off point and considered contraception events outside this window as LARC removal events without replacement with an alternative contraception. Overall, there were 14,368 (58.0%) LARC removal events without replacement with an alternative contraception (Table 9).
| Events | |
|---|---|
| LARC removal event in study population | 123,204 |
| LARC removal event with alternative contraception code | 24,777, 20.1% |
| Within 4 months: replacing with alternative contraception | 14,368, 58.0% |
| Outside 4 months: not replacing with alternative contraception | 10,409, 42.0% |
Objective 4: identify events in general practitioner and hospital records to explore time from LARC removal to conception or appointments relating to difficulties conceiving (if possible)
For these 24,073 pregnancy events, we aimed to calculate an accurate time from LARC removal to conception. We could not calculate an accurate conception time for 9602 (39.9%) pregnancy events [411 events had a LARC removal event between the estimated pregnancy start and end date; 9191 events had no LARC removal code (presumed to be because the LARC was removed elsewhere) before a pregnancy start]. For the remaining 14,471 (60.1%) valid pregnancy events following a LARC removal, the median time to conception following LARC removal was 160 days (25th to 75th centiles = 62 to 398 days) (see Figure 11). In total, 1861 (12.9%) were within the first month, 3562 (24.6%) were within the first 2 months and 5021 (34.7%) were within the first 3 months following LARC removal (Figure 8) (see Appendix 3 for the details of time to conception for women in different age and BMI categories, as well as additional analysis on further contraception patterns).
FIGURE 8.
Time from LARC removal to pregnancy start (days) (n = 14,471).

Discussion
The aim of this WP was to use routine data to determine the most appropriate LARC removal settings, the annual numbers of potential participants available to be recruited and an indicative time frame for recruitment. The records of 318,040 women of reproductive age (i.e. 16–48 years old) with at least one LARC event (where consultation type is insertion, removal or a check/in situ) between 1 January 2012 and 31 December 2018 were examined using the CPRD. Women frequently visit their general practice for LARC, and this is where insertions are most frequently performed, in particular in the 25–34 years age group. The insertion of IUDs has fallen since 2009, with an increase seen in IU systems. LARC removals were least frequently carried out in the general practice setting. No comparable open-source data from SHCs across England, Scotland and Wales were available on LARC consultation type (insertion, removals or in situ), but a mixed pattern in the type of LARC used in clinics was observed. A higher proportion of women in the 25–44 years age group consulted their GP regarding LARC use, whereas younger age groups (i.e. aged < 24 years) are more likely to attend SHCs. Across both settings, consultations for LARC were lowest in the ≥ 45 years age group. Using the Pregnancy Register, we were able to determine that only 1.4% of LARCs were removed for the purpose of planning a pregnancy, with an additional 2% when potentially planning a pregnancy; 16% could not be because of insufficient information. Among those planning a pregnancy, 54% had a BMI of ≥ 25 kg/m2, 28% had a BMI of ≥ 30 kg/m2 and < 5% had a BMI of ≥ 40 kg/m2. For valid pregnancy events following a LARC removal, the median time to conception was 160 days; 13% were within the first month and 25% within the first 2 months following LARC removal.
Our large sample size from the CPRD database improves the representativeness of our findings in terms of the British population, and its linkage with the Pregnancy Register made this work viable. We were able to examine the data from both general practices and SHCs; however, their current structure does not enable the sequential relationship between removal of LARC and subsequent pregnancy in different settings to be reliably determined. We developed comprehensive coding lists to identify nodes/stages that either confirm or refute pregnancy or the planning of pregnancy to enable women to be grouped based on whether or not the LARC was removed for pregnancy. However, the labour-intensive methods used to define women requesting LARC removal for the purpose of pregnancy, alongside the non-contemporaneous nature of the data, do not make routine data a feasible option for reliably identifying women who are in the overweight or obese category and plan to have LARC removal for the purpose of planning a pregnancy.
Chapter 4 Understanding preconception pathways relating to LARC through qualitative surveys and analysis of policy documents
Introduction
To establish the feasibility and acceptability of introducing a preconception WLI at LARC removal appointments, it is first necessary to develop an understanding of the LARC pathway from an individual perspective and its interface with weight management.
Study objectives to be addressed
Study objectives 4 and 5 are addressed directly:
-
4. to assess the willingness of health-care practitioners to raise weight loss in consultations and recruit eligible women to the intervention
-
5. to assess LARC users’ views as to the acceptability and feasibility of the proposed intervention and of future research.
The findings from this chapter will also map onto study objectives 2 and 3:
-
2. to identify opportunities to identify women with overweight/obesity who plan to have LARC removal for the purpose of planning a pregnancy and opportunities to intervene with a potential preconception WLI
-
3. to identify suitable and acceptable interventions to be incorporated into a preconception WLI.
Aims of the chapter
To answer the study objectives, the aims were to:
-
develop an understanding of how health-care services are expected to approach discussions of weight loss with women with overweight/obesity
-
develop an understanding of how LARC treatment pathways currently operate through an analysis of policy documents
-
assess current practice of how health-care practitioners approach discussions of weight loss with women with overweight or obesity and the inter-relationship between discussions about overweight/obesity and family planning through qualitative surveys with LARC users and health-care practitioners
-
assess the barriers to and facilitators of, including practical and ethical considerations, introducing weight and the option of delaying LARC removal during health-care consultations from the perspective of LARC users and health-care practitioners
-
identify feasible opportunities to intervene with a potential preconception WLI
-
assess the desired characteristics of a potential preconception WLI from the perspective of LARC users and health-care practitioners.
Methods
To address these aims, a range of qualitative methods were used, including (1) an analysis of policy documents, (2) engagement with LARC users via a qualitative survey and (3) service provider engagement (health-care practitioners and weight loss consultants) via qualitative surveys.
Analysis of policy documents
A review of policies, best practice guidelines, other clinical/advisory documents and grey literature pertaining to the use (and particularly the removal) of LARCs in the UK was conducted to meet aims A and B. Relevant data were identified via searches of websites and/or online document repositories belonging to four key organisations: Faculty of Sexual and Reproductive Healthcare of the Royal College of Obstetricians & Gynaecologists (FSRH), RCOG, NICE and British Pregnancy Advisory Service (BPAS) (the search strategy is detailed in Appendix 6). The initial results were presented to the SSC to ensure that all relevant policies had been included. A full-text analysis of included documents was conducted: key statements and/or excerpts relating to aims A and B were extracted.
Qualitative online surveys
Qualitative online surveys with LARC users and service providers (health-care practitioners and weight loss practitioners) were used to meet aims C, D, E and F.
Engagement with LARC users through qualitative surveys
An online qualitative survey (see Report Supplementary Material 2) using closed and open-text questions was utilised to understand women’s experiences of discussing weight with health-care practitioners; their knowledge of the risks of overweight/obesity in pregnancy; barriers to and facilitators of the introduction of a WLI at LARC removal appointments; feasible opportunities to intervene in the LARC pathway with a preconception WLI; and preferred components of a potential intervention. The survey for women of reproductive age who self-identify as having/previously having overweight/obesity was advertised on a range of relevant social media platforms. A range of 200–500 responses was specified, and data collection was to cease when sufficient ‘information power’ had been generated. 95
Engagement with service providers (health-care and weight loss practitioners)
Health-care practitioners who insert and/or remove coils as part of their role were identified and recruited at relevant professional events. A qualitative survey with health-care practitioners (see Report Supplementary Material 3) using closed and open-text questions focused on the discussion of weight and preconception health both generally and in the specific context of LARC removal; challenges to service delivery; barriers to and facilitators of the introduction of a WLI at LARC removal appointments; feasible opportunities to intervene in the LARC pathway with a preconception WLI; and preferred components of a potential intervention. Weight loss practitioners (people who support women to lose weight as part of their role, e.g. weight loss programme consultants, personal trainers, dieticians) were recruited via existing contacts or a range of social media platforms; a qualitative survey (see Report Supplementary Material 4) addressed their views on the provision of a WLI in the preconception phase.
Qualitative survey development
The online surveys were developed in Cardiff Online Surveys. The survey questions were developed by the research team, aligned with evidence from initial literature searches and the studies’ research questions. The surveys were piloted with contacts of the study team and signed off by the SMG, including the health-care practitioners and the study lay member.
Participant identification/selection
LARC users responded to advertisements in a broad range of targeted, relevant, online social spaces, including Facebook (Meta Platforms, Inc., Menlo Park, CA, USA), Twitter (Twitter, Inc., San Francisco, CA, USA) and Netmums (London, UK), and via Healthwise Wales (a national registry). 96
Health-care practitioners were recruited via professional events targeted at clinicians with a sexual health role, which were attended by the study team. Weight loss practitioners who support women to lose weight as part of their role were recruited via existing contacts of the study team and targeted Facebook advertisement.
Participant informed consent
On accessing the online survey, participants were taken to a page where information was provided about the study in the form of a PIS. Participants were instructed to take as much time as required to consider the information before taking part in the study. To proceed to survey questions, participants were screened by answering inclusion questions (Table 10). If participants were ineligible, they were redirected to an online page thanking them for their time. If participants were eligible and consented electronically, they were able to complete the online survey. Participants were invited to consent to future contact by the research team and also to be entered into a prize draw for £100-worth of high-street vouchers. Participants provided their contact details if applicable. There were no follow-up assessments.
| Participants | Inclusion criteria | Exclusion criteria (same for all groups) |
|---|---|---|
| LARC users | Women of reproductive age (16–48 years old) who:
|
Sufficient written English to participate in online surveys and consent to participate in the study |
| Health-care practitioners | Practitioners who insert and/or remove LARC as part of their clinical role | |
| Weight loss practitioners | Practitioners who support women to lose weight as part of their role |
For health-care practitioner surveys only, participants were recruited face to face at relevant professional events. Study team members were available to explain the study to participants and to answer any questions before the participant completed either a paper or an online version of the eligibility form, consent form and survey.
Data collection
The online surveys were hosted by Cardiff Online Survey on secure Cardiff University servers. Data were exported from Cardiff Online Surveys at the end of the data collection period in a Microsoft Excel® and Microsoft Word® (both Microsoft Corporation, Redmond, WA, USA) file, uploaded to NVivo (QSR International, Warrington, UK) and stored on secure, restricted-access folders on Cardiff University servers.
Analysis
Responses to closed questions in the survey were reported descriptively as numbers and percentages. A computer-assisted qualitative data analysis software package (NVivo) was used to manage open-text qualitative survey data. Data were subsequently analysed thematically and coded inductively. Inductive thematic analysis was considered appropriate as this approach is known to facilitate the exploration of similarities and differences across large data sets,97 allowing variation in responses within and between LARC users and health-care practitioners to be identified and explored. The six phases of thematic analysis proposed by Braun and Clarke97 were used to guide the analytic process, the results of which were discussed at regular team meetings. The LARC user and health-care practitioner data sets were coded separately during the first stage of analysis. Once each data set had been fully coded, a copy of each was merged. The resulting coding framework and data set was considered as a whole to identify key and cross-cutting themes and to highlight areas of similarity and difference across the LARC user and health-care practitioner groups.
Results
Both the policy documentation and the survey results are discussed in the context of the aims of the chapter. Where content from the surveys is quoted, the abbreviations HCP for health-care practitioner and LU for LARC user survey responses are used.
Analysis of policy documents
A total of 15 documents were included in the review: 13 were identified via the search strategy detailed in Appendix 6 and two were identified subsequently by study team members. The document types identified included FSRH clinical guidance, FSRH Clinical Effectiveness Unit-produced statements, NICE public health guidelines and clinical guidance, RCOG Green-top Guidelines, and BPAS and RCOG press releases (see Appendix 7).
Aim A: develop an understanding of how health-care services are expected to approach discussions of weight loss with women with overweight/obesity
Two main themes were identified in relation to how health-care practitioners are expected to approach discussions of weight loss with women with overweight or obesity. First, clinical encounters where preconception and/or contraception is discussed were characterised as key moments in which strategies for minimising some of the clinical risks associated with maternal obesity/overweight could and should be discussed. Specific mention of ‘obesity’ or ‘overweight’ was not typically advocated, with guidance instead referring to weight optimisation and the inclusion of general dietary and exercise-related advice:
Primary care services should ensure that all women of childbearing age have the opportunity to optimise their weight before pregnancy. Advice on weight and lifestyle should be given during preconception counselling or contraceptive consultations.
Denison et al. 98
Second, although it was not explicitly stated whether or how health-care practitioners should inform women of the underlying evidence, policy guidance aimed at health-care practitioners placed emphasis on (1) the safety of LARC use in women with overweight/obesity99 and (2) the current lack of evidence of a causal association between the use of IU contraception and weight gain. 100
The LARC removal event itself was highlighted as an opportunity for providing behavioural and lifestyle-related advice, including the need to provide dietary advice. 100
Aim B: develop an understanding of how LARC treatment pathways currently operate through analysis of policy documents
Information on how LARC treatment pathways currently operate (particularly in relation to LARC removal) was retrieved from the documents reviewed. The FSRH documents clarified that no formal training, beyond gynaecological skills and contraceptive knowledge, is required of health-care practitioners for LARC removal. 100
The need to discuss LARC removal with women was highlighted in the FSRH documents, but the guidance was minimal in scope, with very limited information on when or how such discussions should take place.
Engagement with LARC users and service providers
Study setting and participant characteristics
LARC users (n = 243) were recruited via a multipoint online recruitment strategy involving advertisements in online spaces, including boosted advertisements on Facebook, Twitter and the Netmums forum, a call for participants via Cardiff Online Surveys and distribution to potentially eligible participants via Healthwise Wales. 96 Although it is an approximation, when correlating timings of advertisements and completion of online surveys, it appears probable that the majority of participants were recruited via Healthwise Wales and Facebook (Table 11).
| Recruitment method | Estimated number of LARC users |
|---|---|
| Twitter advertisements | 0 |
| Boosted Facebook advertisements | 78 |
| Targeted Facebook ‘shares’ | 15 |
| Netmums forum | 0 |
| Call for participants via Cardiff Online Surveys | 0 |
| Healthwise Wales | 150 |
| Total | 243 |
Table 12 describes the characteristics of LARC users at the time of survey completion. All respondents had experience of using LARC: 52.7% at the time of survey completion and 47.3% in the past. The majority of LARC users self-classified as having overweight currently, while 14.8% reported having had overweight at some point in the past; 41.6% of LARC users either were pregnant currently or had been in the past and 58.4% of LARC users were planning to conceive in the future.
| Characteristic | Currently, n/% | In the past, n/% | Planning in the future, n/% |
|---|---|---|---|
| Use of LARC | 128/52.7 | 115/47.3 | N/A |
| Overweight | 207/85.2 | 36/14.8 | N/A |
| Pregnant | 12/4.9 | 89/36.6 | 142/58.4 |
Health-care practitioners (n = 100) were recruited at seven professional events by the study team (Table 13).
| Details of professional event | Number of responses | ||
|---|---|---|---|
| Event | Date | Paper completion | Electronic completion |
| FSRH/British Association for Sexual Health and HIV (BASHH) joint event (Cardiff) | 3 July 2019 | 5 | 0 |
| Mediconf event (Birmingham) | 21 September 2019 | 6 | 0 |
| Welsh Association of Sexual and Contraceptive Health | 16 November 2019 | 1 | 2 |
| Welsh Obstetrics and Gynaecology Society (Cardiff) | 11 October 2019 | 4 | 0 |
| Primary Care Women’s Health Forum (London) | 6 November 2019 | 2 | 10 |
| Faculty of Sexual and Reproductive Healthcare of the RCOG event (London) | 21–22 November 2019 | 9 | 33 |
| Totals | 27 | 45 | |
| Electronic (post event, e-mails, etc.) | 28 | ||
| Total | 100 | ||
Table 14 shows the characteristics of health-care practitioners at the time of survey completion. Most health-care practitioners were medical practitioners (79%), 18% were nursing practitioners and 3% classified their job role as ‘other’. Job role settings were split fairly evenly between primary care (46%) and SHCs (43%), with 5% at both settings and 5% at ‘other’. Most health-care practitioners had been in their job role for more than 6 years (92%), and most roles involved both LARC insertion and LARC removal (94%).
| Characteristic | Response |
|---|---|
| Job role | |
| Medical practitioner | 79/79% |
| Nursing practitioner | 18/18% |
| Other | 3/3% |
| Job role setting | |
| Primary care | 46/46% |
| Sexual health | 43/43% |
| Both | 5/5% |
| Other | 6/6% |
| Length of time in role (years) | |
| < 1 | 0/0% |
| Between 1 and 5 | 8/8% |
| Between 6 and 10 | 26/26% |
| ≥ 11 | 66/66% |
| Role in relation to LARC practice | |
| Both insertion and removal | 94/94% |
| Removal only | 3/3% |
| Did not respond | 3/3% |
| Number of women seen in relation to LARC per month | |
| < 5 | 38/38% |
| Between 6 and 10 | 23/23% |
| ≥ 11 | 38/38% |
| No response | 1/1% |
Weight loss practitioners (i.e. people who support women to lose weight as part of their role) were recruited via existing contacts or boosted Facebook advertisements. Only four weight loss practitioners completed the online surveys: two personal/fitness trainers, one dietitian/nutritionist and one life/health coach working across NHS, local authority and private organisations. All of them received referrals from the NHS, and two worked with pregnant women. In describing their clientele, all reported that they worked, on average, with one or two women per month who aimed to lose weight to experience a healthier pregnancy, and three of them (75%) also had one or two female clients per month who were trying to lose weight to increase their chances of conceiving.
Aim C: assess current practice of how health-care practitioners approach discussions of weight loss with women with overweight or obesity and the inter-relationship between discussions about overweight/obesity and family planning
Health-care practitioners and LARC users reported that weight was discussed at both general appointments and appointments related to family planning.
Conversations with health-care practitioners regarding weight at general health appointments
A total of 119 (49.2%) of LARC users reported that weight had been discussed at general health-care appointments (i.e. not specifically related to LARC removal). Weight-related discussions were more likely if the woman had a BMI of > 30 kg/m2 (61.0% compared with 32.6% for those with a BMI of < 30 kg/m2). LARC users described conversations regarding weight in many different appointment contexts with numerous health-care practitioners across primary and secondary care (‘every health-care professional’). Some health-care practitioners described introducing weight opportunistically at any health-care appointment, whereas others would be more selective, for example discussing weight only if it was relevant to the appointment.
Information provided to patients
The type of weight-related information provided in general appointments included (1) healthy lifestyle advice, (2) advice related to existing health conditions, (3) referrals for specialist weight loss support and (4) medication information.
Overall, there seemed to be little practical or psychological support available, with often very brief, unhelpful comments on weight being made; for example, ‘I was told I was overweight and to lose it. There was no help offered at all’. A few examples of positive experiences were reported, when health-care practitioners had been supportive and non-judgemental and had offered practical, long-term help.
Weight was discussed in the context of health conditions (e.g. PCOS, diabetes), but this did not seem to improve LARC users’ experiences of weight-related support. Referral to specialist support for weight loss was not commonplace, and LARC users described having to fight for the support, receiving a referral that was unsuitable for their needs or experiencing long waiting lists. Some LARC users had been prescribed medication (e.g. Orlistat) to help with weight loss.
The weight loss practitioners who completed the survey (n = 4) described the importance in preparation for pregnancy of following NHS guidelines and having a healthy lifestyle but not focusing on weight loss.
Conversations with health-care practitioners regarding weight at family planning appointments
The nature of family planning/preconception weight-related guidance and discussions varied depending on the type of health-care appointment and the health-care practitioner involved.
Information provided to LARC users included advice related to conception/fertility and contraception.
The majority of health-care practitioners (72%) said that they discussed weight during preconception discussions as part of preconception advice or when discussing fertility issues with patients. However, some health-care practitioners said that they felt that weight was not a priority in preconception advice or that this was not an appropriate time to address weight.
LARC users reported that they had received general recommendations from health-care practitioners to lose weight to increase their likelihood of conceiving but had not been given practical advice or support to do so. Infertility issues were attributed to obesity, and a reduction in BMI was a requirement for access to IVF treatment:
I have been told that I will not get any support in relation to my fertility until I have lost a considerable amount of weight to be eligible.
LU21
Discussions about weight at contraception appointments were initiated either by the health-care practitioner due to limitations on contraceptive options in women with a raised BMI or by LARC users who had concerns about weight gain resulting from the use of contraceptives (contraceptive pill or LARC).
Barriers to and facilitators of discussing weight in general health-care appointments
LARC users and health-care practitioners reported several barriers to and facilitators of discussing weight in general health-care appointments. There was a substantial overlap between these themes and those identified in relation to the discussion of weight during LARC removal. To reduce repetition, the themes are summarised in Table 15 and presented in detail in the following section in relation to the LARC removal appointment.
| Barriers | Facilitators |
|---|---|
| Sensitivity of the subject | Part of role as health-care practitioner |
| Potential vulnerability of patient (e.g. mental health) | Health-care practitioner approaching the subject sensitively |
| Lack of skills/training | Existing health-care practitioner–patient relationship |
| Risk of complaint | Patient’s right to choose and knowledge (to have the discussion/to know health risk) |
| Lack of services | |
| Lack of evidence base of risk available to share | |
| Weight not seen as relevant to the consultation agenda (including contraception) | |
| Woman not weighed in clinic so discussion based on appearance | |
| Patient’s prior experience of weight loss discussions | |
| Pragmatics of short appointment times |
Aim D: assess the barriers and facilitators, including practical and ethical considerations, to introducing weight and the option of delaying LARC removal from the perspective of LARC users and health-care practitioners
The surveys for both groups of stakeholders included specific questions about the introduction of weight as a topic during a LARC removal and the acceptability of asking/being asked to delay the LARC removal in order to take part in a WLI before pregnancy.
Many LARC users (46.8%) described feeling uncomfortable at the prospect of discussing weight at LARC removal appointments; 38.4% said that they would be comfortable and 14.8% said that they would be neither comfortable nor uncomfortable with the conversation. However, the majority of health-care practitioners said that they would feel comfortable introducing the subject of weight with patients attending for LARC removal (65%), whereas 12% reported that they would feel uncomfortable and 23% would feel neither comfortable nor uncomfortable.
In answer to the specific question about whether it would be acceptable to ask LARC users with overweight or obesity to delay LARC removal to attend a WLI prior to trying to conceive (Figure 9), 39.9% of LARC users stated that they felt it would be acceptable, compared with 29.6% of LARC users who felt that it was unacceptable and 30.5% of LARC users who were unsure. The majority of health-care practitioners (63.6%) reported that they felt that women would be willing to postpone LARC removal to take part in a preconception WLI, whereas only 17.2% of health-care practitioners felt that women would be unwilling to postpone LARC removal and 19.2% were unsure.
FIGURE 9.
Stakeholder responses to the acceptability of delaying LARC removal to lose weight.

The themes that emerged from responses to these questions are described below in the broad categories of barriers to and facilitators of introducing preconception weight and delaying LARC removal at LARC removal appointments. Many of these themes repeated or resonated with those that emerged from the LARC user and health-care practitioner responses to the questions surrounding the discussion of weight in health consultations generally.
Barriers to the introduction of preconception weight and LARC removal delay at LARC removal appointments
Barriers are detailed according to the following themes: (1) the sensitivity of weight as a topic area, (2) LARC user engagement and past experiences, (3) knowledge and beliefs of the LARC user, (4) the context of the consultation and service setting, (5) health-care practitioners’ skills, (6) the information/interventions provided and (7) ethical implications of requesting a delay in LARC removal.
Both stakeholder groups considered weight a sensitive topic and felt that conversations regarding weight at LARC removal appointments would be difficult. LARC users described that they would feel ashamed, attacked and judged for having a raised BMI if weight was discussed at LARC removal appointments; health-care practitioners were aware of the potential to upset patients and were concerned about possible repercussions:
I gained a good amount of weight on the implant, and I don’t like talking about it. I feel judged.
LU234
Following a recent complaint when I discussed weight with a patient at a contraceptive review, it has made me wary about initiating discussions.
HCP64
However, some in both groups were ambivalent, acknowledging both the discomfort with the conversation and the belief that the conversation was needed. Some LARC users appreciated the importance of the conversation and health-care practitioners’ good intentions; similarly, health-care practitioners described the necessity of discussing weight as a health risk with patients despite the potential outcomes:
Because weight is a very personal thing and it sometimes feels like an attack even though with a health professional I know it is in my best interest.
LU44
I am uncomfortable about it because it upsets women but think I it is my medical duty to mention it so try and do it as tactfully as possible.
HCP87
LARC users reported being reluctant to engage in discussions about weight loss during LARC removal appointments. Barriers included being content with their weight but also the complexity of weight loss that requires more support than a simple mention during a health-care appointment, which is ineffectual:
If there was a point. What are your aims to ask about weight? Weight isn’t as cut and dry as smoking or drinking alcohol. It’s not like you can ask ‘are you currently thinking about quitting food?’
LU5
Past experiences of weight discussions in health-care consultations could be a significant barrier to introducing the topic of weight during a LARC removal appointment. LARC users reported frustration that weight was so frequently a focus in consultations, often, in their view, inappropriately. As a result of these types of experiences, LARC users said that they avoided health care if possible, and health-care practitioners acknowledged that patients are unwilling to engage because of their previous experiences. Furthermore, LARC users reported occasions when they had attempted to seek help to lose weight but had been disappointed by the lack of support from the health-care practitioner and the quality of the care/intervention:
Almost every medical professional I have ever seen has suggested symptoms of everything from a chest infection to a sprained wrist would be improved if I lost weight.
LU4
Almost everything you go to see a health-care professional about has the opportunity for them to bring up the subject of weight. I’m rarely ill, but even if I am, I put off going to see anyone for as long as humanly possible.
LU8
I mention it to patients, feel that they are not motivated to change and get ‘fed up’ with health-care professionals telling them they are overweight when they already know they are overweight.
HCP27
Mental health issues, both related and unrelated to weight, may be a barrier to introducing the topic of weight at LARC removal appointments. Anxiety, experiences of bullying and low self-esteem were all described as barriers to engaging in conversations with health-care practitioners or WLIs. LARC users reported the potential negative impact that discussing weight could have on patients who have a history of disordered eating:
Before giving advice or recommendations on weight loss methods get to know the patient and their history better so it feels more like you’re being helped rather than being told off.
LU215
I have struggled with eating disorders in the past, and I know that any attempt at losing weight quickly turns to me starving myself for quick weight loss.
LU235
LARC users felt that weight did not map on to health as closely as was suggested by health-care practitioners and mistrusted the emphasis on BMI. Some LARC users questioned the assertions related to risk, deeming the interpretation of statistical risk exaggerated, and they wanted clearer information/statistics to be able to make an informed choice:
I know all about the risks but lots are correlation rather than causation. I have not had any complications in my pregnancy despite a high BMI . . . Don’t worry about the confounding socioeconomic factors behind those statistics I am fat so I am doomed.
LU6
LARC users believed that the LARC was a causative factor in their weight gain and wanted this to be acknowledged by health-care practitioners. One health-care practitioner reported using patients’ perception of LARC and weight gain as a way to introduce weight loss:
Sometimes a route in is in their perception of the LARC and their weight, i.e. ‘I am taking it out because I’ve gained weight’.
HCP63
LARC users, and some health-care practitioners, suggested that weight is not relevant to contraception, and that discussion of weight or health matters at a contraception appointment is not appropriate unless raised by the patient:
Client is in LARC clinic not in a weight watchers’ class or slimming clinic!
HCP43
If it was just asked off the cuff with getting contraception removed I’d be a bit taken aback. I’m not there presenting with a health complaint, so why discuss weight?
LU5
Several barriers were suggested by health-care practitioners regarding the logistics of asking women to delay LARC removal when presenting at contraception appointments. Health-care practitioners and LARC users reported long waiting times for appointments and time limits placed on appointments:
I had to wait months for an appointment to remove my implant in the first place which was difficult as I was keen to try and get pregnant – I would have been upset if I’d had to consider delaying removal further.
LU18
It would be really hard to have this kind of conversation in a very short appointment in a successful and productive way.
LU44
In my surgery, patients book in without prior counselling for a 10 min (coil) or 20min (implant) removal so time is limited. We would have to change our system.
HCP93
The reason for a patient requesting LARC removal may determine whether or not weight should be introduced during the LARC removal appointment. Participants suggested that if patients requested LARC removal for reasons other than wanting to conceive, such as side effects including heavy periods, weight gain and changes in mood, then it may not be appropriate to raise the topic of weight:
I had a coil removed for mood swings, I couldn’t have spent any longer on it. God help if a stranger had told me to leave it in and lose weight!
LU75
The majority of women for whom I remove LARC state they want removal for side effects (perceived or actual) and it is not often that I see someone that wants removal purely for pregnancy.
HCP42
Finally, LARC users reported feeling vulnerable during intimate LARC removal appointments and felt that it would be inappropriate for practitioners to instigate discussions regarding weight:
For some females it can be incredibly nerve wrecking going to have a discussion with a total stranger about birth control. Especially if it’s the coil where they are at the most intimate part of a female’s body then they start talking about the person’s weight!
LU75
Many LARC users thought that health-care practitioners lacked the required skills to deal with the sensitive topic of weight management. Similarly, health-care practitioners acknowledged that they could feel uncomfortable discussing weight with patients, particularly when providing preconception advice. Health-care practitioners wanted to be provided with guidance or accurate information regarding the risks of raised BMI in pregnancy or effective weight loss advice to enable them to have successful discussions with patients:
Making us feel crap about our choices and our bodies, is not a way to motivate anyone. Look up motivational counselling techniques. Look up life coaching techniques. How the healthcare service treats overweight and obese people is literally the opposite, with the effect that we feel crap and we get fatter.
LU8
If this HCP was someone I was only seeing as a one off. There was no follow up with the same person or I’d never met the person. No rapport or trust. I would be fairly insulted if they offered unsolicited advice.
LU5
Staff will feel uncomfortable unless they have some guidance on how and when to phrase things.
HCP87
I’d be willing to try it – but would like to have some training about how to raise weight loss anyway . . . would there be any supporting patient resources – then the patient might feel the suggestion is not coming personally from me.
HCP67
The weight status of the health-care practitioner also appeared to be a barrier to successful discussions regarding weight at LARC removal appointments. Health-care practitioners with overweight may appear hypocritical, whereas health-care practitioners with a healthy weight may appear unsympathetic and patronising:
It would be nice if the healthcare professional followed their own advice. Being out of puff and smelling of bacon is an example that rather frustrates the patient.
LU161
Some clinicians may not be keen particularly if obese themselves – practice what you preach!
HCP64
Health-care practitioners spoke about the requirement for referral pathways to effective interventions, and both stakeholder groups reported their frustration at the lack of efficacious interventions or at unsuitable interventions:
. . . unhelpful advice relating to solutions that are financially inaccessible and not accessible to single parents with limited support.
LU137
In many of the responses to the idea of delaying LARC removal to lose weight, respondents reported what can broadly be described as ethical barriers relating to personal choice, conception decision-making, impact on the care pathway and exclusion of non-LARC users.
LARC users and health-care practitioners highlighted the ethical implications of what could be interpreted as an imposition of a contraceptive decision on patients, suggesting that this should, ultimately, be the choice of the individual. Some LARC users referred to discrimination against people with overweight/obesity and said that the inference would be that ‘fat people shouldn’t breed’ (LU4). LARC users felt that state involvement in personal reproductive decisions was unacceptable. They were also concerned that people may feel pressured into agreeing to delay LARC, acknowledging that health-care practitioners, despite acting with best intentions, may be unaware of this consequence. However, health-care practitioners also acknowledged the potential to pressure patients in a ‘doctor-led approach’:
I understand that you are concerned about fat people having babies and the implications for the healthcare system but if a woman wants her coil taken out at any time for any reason that is her choice.
LU117
I would find it ethically complex to suggest to women that their freedom of choice to have their contraception removed may be compromised by their weight. I would however feel happy to discuss the options with them and share evidence about the pregnancy-outcomes in obese women.
HCP57
Because you’re denying someone the right to get pregnant due to their weight. That is a breach of their human rights.
LU180
People who are overweight are already treated like second class citizens by some professionals, this would feel like a form of eugenics unless treated very carefully.
LU23
. . . this is already happening in places, with women are reporting having been ‘refused’ to have their implant/coil removed, conditional on losing weight. That may not be what the healthcare professional thought they were saying, but that is the message women are hearing.
LU8
The complexity of decision-making surrounding trying to conceive may be a barrier to asking patients to delay LARC removal to lose weight; LARC users said that the decision to have a baby is a complex one, which may be informed by their relationship, their studies, their career choices, sibling age gaps, financial implications, etc.:
People do not lightly decide the time is right to try for a baby!?!? They will have thought long and hard about it, and undoubtedly already have concerns about weight.
LU8
Both LARC users and health-care practitioners commented on the risk of delay in older women, saying that a long delay may have implications for their chances of conceiving or lead to complications in pregnancy due to age. Additionally, women who have fertility issues may not want to delay LARC removal:
. . . may not achieve anything other than delaying pregnancy and LARC removal – so you end up with older overweight people getting pregnant.
HCP42
It was suggested that, if patients were aware that they would be asked to delay LARC removal due to their weight, this may discourage patients from having a LARC fitted in the first place, or they may go elsewhere for removal. LARC users also questioned whether a process would be in place if patients declined to attend a WLI or if they were unsuccessful at losing weight and whether this would have implications on the care they received or if they were ‘allowed’ to have a baby. Furthermore, there was an expectation that, if the risks are greater in women with overweight/obesity, then supplementary screening/care should be offered to pregnant women:
It also carries the connotation that if they do undergo a weight loss programme but don’t succeed – what happens then? . . . I feel this kind of protocol carries a lot of weight and as such needs to be undertaken very carefully and sensitively.
LU63
Being asked if I would like to discuss potential risks/additional screening in the event of falling pregnant.
LU2
Finally, it was highlighted that people may feel excluded from an intervention targeting women trying to conceive, suggesting that an effective WLI should be offered at a population level.
Facilitators of the introduction of preconception weight and LARC removal delay at LARC removal appointments
The facilitators are a mirror image of the barriers across the following themes: LARC user engagement and previous experiences, knowledge and beliefs of the LARC user, service setting/context, health-care professional and information provided/available interventions.
LARC users stated that they are accustomed to discussing weight at health-care appointments and appreciated the importance of the conversation and, therefore, despite the innate sensitivity of the topic, they would be co-operative and unlikely to be offended. Some LARC users described the prospect of delaying LARC removal as a reasonable proposal, and potentially would welcome the discussion as a motivating factor and any offers of support. Health-care practitioners also referred to patients’ attitude, wishes and motivation as facilitators of successful discussions regarding weight; if the patient has a positive attitude to weight loss, initiates the conversation or has a desire to improve their health, it allows for beneficial discussions. LARC users and health-care practitioners suggested that whether or not women find the conversation acceptable would depend on their circumstances, age, history and weight status; participants suggested that asking people to delay removal would be acceptable only if the individual had obesity and was not just ‘slightly overweight’:
I would be happy being asked to take part in a weight loss programme before trying to become pregnant. I would see it as a way to become more healthy before conceiving, which could also increase the chance of becoming pregnant if I lost weight.
LU9
Nearly two-thirds of LARC users (64.2%) said that they were aware of risks of overweight/obesity in pregnancy, and each described between 1 and 10 risks, including conception risks (difficultly conceiving), pregnancy risks (miscarriage, pre-eclampsia, failed intubations, pelvic symphysis disorder, severe pregnancy symptoms, etc.), delivery risks (shoulder dystocia, stillbirth, cesaerean section, post-partum haemorrhage, instrumental delivery) and risks to the baby (cerebral palsy, spina bifida, large or small birthweight, premature birth). A total of 15.2% of LARC users stated that they did not know any risks, 2.9% were unsure of the risks and 17.7% did not respond. LARC users thought that knowledge about or being informed of the health benefits of being a healthy weight could be a motivator to engage in a WLI. They stated that communication of the risks of obesity in pregnancy was essential to allow patients to make an informed choice and maintain autonomy. However, as discussed previously, some LARC users were sceptical about the risks of overweight/obesity in pregnancy, saying that these are exaggerated by the health-care profession:
Personally, with the current weight I am, I would be less keen to become pregnant now. I would prefer to lose some weight and get fitter – but that’s because I know how pregnancy has an impact on my body and I would want to be as prepared as possible before hand to help support my back and hips (previous pgp).
LU5
It’s in their interests, they are free to decline . . . they may be unaware of the risks. Few service user participants these days mind not smoking and not drinking alcohol in pregnancy, weight loss may simply not be on their radar.
HCP20
LARC users suggested that preconception and pregnancy may provide a window of opportunity to introduce weight loss in order to maximise fertility and improve health in pregnancy. In addition, the potential benefits to the unborn child may be a facilitator of healthy behaviours. Others described this opportunity in pragmatic terms, that is a time of increased contact with health-care practitioners and a potential willingness to discuss weight when it is considered as part of a broader preconception/healthy pregnancy package of health recommendations:
Because the healthier you are the better chance you have of getting pregnant and having a problem free pregnancy. Wouldn’t any mother want that?
LU150
LARC users believed that it was the medical professional’s role to discuss health issues such as weight with patients and expressed confidence that health-care practitioners prioritise patients’ health. They valued an ongoing relationship with health-care practitioners and continuity of care, including the practitioner’s knowledge of the patient’s existing medical conditions, as vital for successful discussions.
LARC users highlighted the importance of good communication skills; they indicated that the health-care practitioner should be sensitive, compassionate, non-judgemental and not patronising and be aware of their tone and body language to ensure that the patient is at ease during the consultation. Having adequate time was seen as a facilitator of ensuring effective communication, as was not treating the conversation like a ‘tick-box’ exercise. An open, honest discussion was important to LARC users, as was practitioners having an awareness of terminology likely to cause offence, such as ‘obese’ or ‘fat’, and an understanding of the difficulties of losing weight. LARC users suggested ways of broaching the topic, rather than giving abrupt unsolicited advice. Health-care practitioners also considered themselves potential facilitators if they approached the discussion sensitively and had a good rapport with the patient, but they also requested additional training, guidance and potentially a script to ensure that they took the right approach:
If this HCP was someone I was only seeing as a one off. There was no follow up with the same person or I’d never met the person. No rapport or trust. I would be fairly insulted if they offered unsolicited advice.
LU5
LARC users said that, instead of simply instructing the patient to lose weight, health-care practitioners should provide support for and/or practical advice about losing weight or signpost to a choice of options for effective support. Practical solutions were recommended, such as diet tips, meal planning, free gym membership, WLIs and support networks. Similarly, health-care practitioners said that they could justify initiating such conversations if they were able to offer an effective intervention. Support that had a positive focus was important to LARC users, with the discussion being in the context of supporting health goals and highlighting the benefits of weight loss, rather than just focusing on the risks. Both LARC users and health-care practitioners suggested that, to increase acceptability and reduce stigma or risking patients feeling judged or singled out, weight and healthy lifestyle should be a standard part of preconception and contraceptive discussions:
If we could provide support/refer service user participants for intervention then I think service user participants would see that and find it acceptable/understand why they have been asked.
HCP44
It will be more acceptable if offered to everyone in this scenario.
HCP8
Both LARC users and health-care practitioners thought that referring to evidence-based guidance was a facilitator of acceptable discussions and enrolment in a potential intervention:
. . . information-giving discussion including introducing some of the evidence behind the issues to help it feel objective.
LU33
Aim E: identify feasible opportunities to intervene with a potential preconception weight loss intervention
To assess the acceptability of using the LARC pathway to propose a potential preconception WLI, health-care practitioners were asked: ‘When you do think would be the best opportunity to introduce a preconception WLI to women with overweight/obesity attending for LARC removal?’. In addition, responses from LARC users regarding potentially introducing the subject of weight at LARC removal appointments mapped on to this theme. Responses from both health-care practitioners and LARC users are detailed collectively.
Prior to LARC removal
Health-care practitioners suggested that LARC removal appointments take place too late, as by that time patients have resolved to try to conceive, and that patients should be engaged in discussions regarding weight at the earliest opportunity, including at the insertion of the LARC, at smear appointments or during general contraceptive consultations, to allow optimum time for consideration of the topic.
Arranging the LARC removal appointment
LARC users suggested that advance notice that weight may be part of the discussion at LARC removal appointments would allow them to prepare. This could take the form of a discussion at the time of booking with the receptionist or service provider, a pre-assessment appointment with a nurse, or a leaflet:
Needs to be done BEFORE they attend. i.e. in pre-removal text/email/website link. When service user participants get to clinic, they want to receive treatment not delay.
HCP42
At LARC removal appointment
Although some health-care practitioners stated that the LARC removal appointment was, realistically, the only opportunity to introduce a potential preconception WLI because of logistical issues in the clinical setting, LARC users stated that this would be acceptable only if done sensitively and appropriately. Some LARC users thought that the discussion about the intervention should happen only subsequent to LARC removal in the context of optimising health for conception and pregnancy:
. . . at pre removal chat although these patients are often put into one stop clinics (chat and removal at same time).
HCP88
If you like, after you’ve done the procedure I came in for, offer me a leaflet or a follow-up telephone call about optimising my body for pregnancy.
LU8
Discussion only if wanting to get pregnant was already discussed.
LU173
At a separate appointment
LARC users suggested having a general, preconception appointment at which the benefits of weight loss would be discussed in the context of ‘preparation for pregnancy’ or a fertility preparation appointment:
If it was offered as part of a separate appointment – a holistic ‘preparation for pregnancy’ type appt.
LU18
Not at all
Some LARC users and health-care practitioners said that there would be no appropriate time to engage women in discussions about preconception weight loss because there was a lack of effective interventions:
Not at all – until we have a highly effective weight loss intervention!
HCP42
Aim E: assess the desired characteristics of a potential preconception weight loss intervention from the perspective of LARC users and health-care practitioners
Both LARC users and health-care practitioners were asked what they considered to be the most effective characteristics of a potential preconception intervention. The responses are summarised in Table 16.
| Characteristics of the intervention | Description |
|---|---|
| Accessibility | Free/low-cost, easy/immediate access |
| Duration | Defined |
| Dietary advice | Not a diet – defined as healthy eating/healthy lifestyle |
| Physical activity | Important component but unspecified |
| Psychological support | Coping mechanisms, dealing with habits, vicious cycle of overeating and dieting |
| Group-based peer support | Optional element |
| Multifaceted lifestyle advice | Broader than weight – positive frame of a healthy preparation for pregnancy |
| Signposting | To support services locally |
| Individualised | Different options to choose from (e.g. group support and physical activity) |
The majority of LARC users (87.1%) stated that a potential intervention should contain a diet/healthy eating aspect. However, the specifics of the suggested diet components varied significantly. Whereas some LARC users suggested that diet plans and dietary advice should be provided, others argued that ‘diets don’t work’ and that advice should be couched in terms of healthy eating and healthy lifestyle. Additionally, whereas some LARC users had had positive experience of commercial weight loss groups, such as Slimming World (Alfreton, UK) or Weight Watchers (WW International, New York, NY, USA), and were in favour of the inclusion of commercial weight groups in a potential intervention, some LARC users stated that commercial weight loss groups were condescending and not appropriate for an NHS setting. Very low-calorie diets were also favoured by some LARC users (27.5%) as a time-limited and goal-oriented weight loss regime; however, others described these as ‘dangerous’, especially for women with a history of disordered eating, and said that they were not appropriate for an NHS setting. LARC users also suggested referrals to dietitians as a method for receiving reliable, appropriate support.
A high proportion of LARC users (80.3%) considered physical activity advice an important component of a potential preconception WLI, with 75.1% favouring a physical activity component to the programme, including access to free community fitness centres/classes. This was also reflected in the surveys of health-care practitioners, who emphasised the importance of a combined diet and physical activity programme.
A high proportion of LARC users (71.7%) and health-care practitioners favoured the inclusion of a psychological component in the potential intervention, and referred to support to address unhealthy habits, coping mechanisms, comfort eating, societal conditioning to view unhealthy foods as treats and rewards and the vicious cycle of overeating and dieting.
Although group-based interventions were valued by 61.4% of LARC users, because of the encouragement and motivation received from other group members in a similar position and accountability to the group instructor, some LARC users were concerned about the lack of confidentiality in group settings and reflected that, for some women, the social aspect of group interventions would be intimidating and overwhelming.
LARC users suggested that the intervention should be multifaceted and bespoke, with optional components targeted to individuals. Others suggested a broad and holistic intervention related to health preconception and in pregnancy. This was echoed by health-care practitioners, who suggested the inclusion of information about other lifestyle factors, such as smoking, alcohol, drug use, immunisations and folic acid. Health-care practitioners said that any intervention would need to be culturally appropriate.
Health-care practitioners emphasised the need to fully inform patients about the risks of overweight/obesity in pregnancy. Whereas some LARC users suggested developing an intervention focused on ‘preparing for pregnancy’, others were concerned about the risk to attendees’ mental health if they attended a group intervention but were unsuccessful in conceiving:
A pregnancy related group could well cause depression in those unsuccessful service user participants so instinct says to avoid.
LU161
LARC users and health-care practitioners were questioned about the facilitators of and barriers to implementation of the intervention. The overwhelming concern from both LARC users and health-care practitioners was that the intervention should be free (whether this included a free app or free membership to a gymnasium/fitness classes) and widely accessible. Attendance at intervention sessions may also be optimised by financial incentives. Although LARC users suggested that in-person support would be most beneficial, some suggested that virtual/remote support may complement an intervention if used in addition to face-to-face contact. Barriers to the implementation of a potential preconception WLI included logistics such as timings of classes for working patients/parents and location of classes and if the patient had any medical conditions that may prevent them participating in physical activity or certain diets. The majority of health-care practitioners said that the intervention could be introduced by any health-care practitioner involved in the person’s care, including GPs, nurses and health visitors.
Conclusions
The purpose of the surveys was to develop an understanding of key stakeholders’ experiences of the interface between the LARC care pathway and weight-related discussions between health-care practitioners and LARC users. The methods were appropriate for the purpose. Data collection techniques were revised after initial attempts to conduct qualitative interviews at professional events proved to be difficult in the setting. However, the online survey responses were full and detailed and recruitment of health-care practitioners at professional events was successful.
The generalisability of the data is a limitation of this component of the study. The vast majority of health-care practitioners were medical, rather than nursing, practitioners, due to the nature of attendance at the professional events attended. However, there was balanced representation of health-care practitioners from primary care and sexual health settings and in terms of years of experience. The majority of LARC users were recruited either via boosted advertisement on Facebook or via Healthwise Wales, which may have resulted in the recruitment of highly motivated, like-minded (i.e. sharing within special interest groups) or research-knowledgeable participants. The inclusion criteria for LARC user recruitment were very specific, which was also a potential barrier; however, being mindful of these limitations, responses were highly relevant and full. The low recruitment of weight loss consultants was disappointing; only four were recruited because of limited recruitment pathways. The use of a shared coding framework across LARC users and health-care practitioners was considered appropriate, as emerging themes were very similar across LARC user and health-care practitioner groups. In addition, it allowed easy identification of when themes converged and diverged and allowed the consideration of themes during the phase 2 interviews and intervention design.
The policy review produced several findings. Encounters where PCC and/or contraception are discussed (including LARC removal appointments) are regarded by policy-makers as key opportunities for discussions of maternal weight. LARC use by women with overweight/obesity is safe, with no evidence of a link with weight gain. Training requirements for UK practitioners in relation to LARC insertion/removal are specified, but guidance on how and when LARC removal should be discussed with women is minimal.
LARC users gave a strong sense of the generic nature of weight discussions with health-care practitioners, with many reporting that weight is always discussed regardless of the context of the consultation, and also that the discussions were often based on appearance rather than arising after being weighed. The discussions about weight in health consultations, including those in relation to family planning and pregnancy, were often unhelpful and had a negative focus. In the worst cases, they were experienced as psychologically harmful and put people off going to any form of health-care consultation.
One of the key examples of the generic approach, which LARC users felt allowed no recognition of the individual, was allocation to consultant-led maternity care because of raised BMI. This caused frustration as it seemed to be an unjustified, automatic decision made without any reference to the woman’s health. This process has now been changed in the care pathway, with more personalised discussions taking place for women with a BMI of < 35 kg/m2. 101 However, given the quality of the communication described by women in this survey, it remains to be seen whether this will have reduced the sense of stigma, or potentially made it worse if there are no improvements in health-care practitioners’ communication skills around weight.
The barriers to talking about weight for both parties encompassed a full range of practical, cognitive and emotional factors, from time in clinic to beliefs about the relative unimportance of weight and the sensitivity of the topic. Few facilitators were identified apart from beliefs about the nature of the roles of the people in the dialogue (i.e. the woman’s right to knowledge and choice and the practitioners’ duty to inform). These themes were replicated in the responses to the questions about discussing weight in LARC removal appointments and the acceptability of proposing a delay in removal in order to lose weight before pregnancy. The sensitivity of the topic of weight was universally acknowledged; raising it during an intimate process such as LARC removal was seen to result in an uncomfortable combination by nearly half of the LARC users. Some health-care practitioners and LARC users were ambivalent. They recognised the importance of weight in relation to pregnancy while also being reluctant to engage in those discussions for many reasons: the sensitivity of the topic; the sense of lacking the necessary communication skills on the part of the health-care practitioners; and the futility of it, given the complexity of weight loss, the lack of support available and the lack of robust evidence (as the health-care practitioners viewed it) to support the discussion of risk and benefits. Others responded positively to the idea; they felt that many people would welcome the support to lose weight as they know that having a raised BMI presents risks to the pregnancy, and this could be a window of opportunity to introduce weight loss with a focus on the health of the baby. This would need to be introduced sensitively by a health-care practitioner with good communication skills, and, most importantly, if it were coupled with a potential programme of support, the conversation could be a positive step.
The views on the key components of a preconception WLI were many and varied, with many participants opting for a multifaceted and bespoke intervention as part of a healthy preparation for pregnancy programme.
The survey responses raised many practicalities that would need to be considered were the topic of weight to be introduced routinely as part of the LARC removal appointment, for example appointment length and health-care practitioner skills, which hypothetically could be addressed through training, the consideration of appointment schedules and so on. However, there were some more fundamental principles underlying the barriers to both the discussion of weight and to the delay of the removal that require essential changes to the potential design of any intervention at this point in the LARC pathway. These were as follows.
Timing in the context of the decision process
The decision to have a baby is often complex, thought through (also with partner), and timing is part of an emotional, social, cultural and financial web of circumstances. Although many have LARC removal for reasons other than wanting to become pregnant straight away, for example because of side effects, they would still not want to delay removal, and for those where it is a deliberate step to pregnancy, the removal appointment is considered too late.
Woman’s right to choose
The proposed intervention interweaves two complex sociopolitical issues in weight and conception, which can be captured by the concept of a ‘woman’s right to choose’, a phrase that has become synonymous with women’s reproductive rights. While there was never any intention that the intervention would remove a woman’s choices, the underlying message that some stakeholders identified was discriminatory, suggesting that women with a raised BMI should not become pregnant, and there was perceived to be a risk that women would feel pressured to participate, however unintentionally, by the health-care practitioners’ description of the option to delay the LARC removal.
Is weight part of health/family planning?
Throughout the themes is a thread of whether weight is regarded as a health/family planning issue.
In summary, several factors were important to consider when designing a potential preconception WLI and were to be explored further through the SAGs and phase 2:
-
In the discussion of the intervention, it should be clear that the woman retains the right to choose to delay or not delay the removal of the LARC. This could be achieved by timing the discussion better so that it takes place well in advance of a LARC removal appointment, or ensuring that the delay of the removal is not one of the eligibility criteria for taking part in the intervention.
-
The intervention could be offered to the general population, so that the LARC removal appointment is simply an opportunity to engage.
-
Information that is clear for all about risk should be included in referral and intervention information.
-
Health-care practitioners should be provided with training in communication skills and information regarding risks.
-
Women’s experiences with weight loss should be taken into account, as these encounters set the stage for their likelihood of engaging with any future intervention.
-
The referral and intervention should have a positive approach, focusing on the benefits of weight loss, rather than the risk of current weight.
-
The intervention should be free and available to all.
-
The intervention should be part of a healthy pregnancy approach.
Chapter 5 Realist review
Introduction
Although there is consensus that, for women with overweight or obesity, a 10% lower preconception BMI is associated with a clinically meaningful risk reduction in pregnancy outcomes, and that achieving a healthy weight prior to pregnancy is a health priority, there is little information about how best to deliver a preconception WLI for women with raised BMI. This review, following a realist approach,84 will bring together information from a range of sources to begin to develop theories about how and when health behaviour interventions in the preconception phase might work, in order to design a preconception WLI for women with raised BMI.
Overall research questions for the review
-
Preconception WLIs: what works, for whom and in what contexts?
-
What are the barriers to and facilitators of engagement in preconception health behaviour change interventions?
-
Is anything additional to be learned from WLIs during pregnancy and postnatally that could contribute to the development of a preconception WLI?
Aims of the realist review
To answer the research questions and consider any potential intervention designs, the aims were to develop an understanding of and potential theories about:
-
intervention characteristics and approaches to recruitment
-
contextual influences on the intervention
-
potential CMO configurations.
Methods
The review is guided by the principles of Pawson and Tilley’s scientific realism. 84 This approach was designed for the evaluation of complex social interventions based on a realist understanding of causation that recognises that social interventions lead to outcomes by triggering a reaction in individuals. The way in which individuals respond to interventions is understood to relate to characteristics of both the interventions themselves and the context in which the intervention is delivered. Realist research focuses on the development of explanatory theories that describe the interactions of these factors using CMO configurations. Programme theories can be developed from these CMO configurations to offer possible explanations about how the intervention works or could work and highlight facilitators of and barriers to interventions working, with a focus on theory generation (rather than testing).
Search strategies methods
In realist methodology, concept mining describes the search process across a wide range of published evidence to explore potentially relevant mechanisms and how those might be affected by context in order to help build theories. This could include reviews, intervention studies, protocols and qualitative research.
Several systematic reviews and meta-analyses related to obesity and the perinatal period have been published in recent years (see Appendix 8). The conclusions drawn in those reviews and the studies they cite were examined to identify (1) possible intervention designs and (2) relevant information on contexts, mechanisms and outcomes. Supplementary searches, based on the review research questions, were performed to ensure that more current publications were included, and to bring together additional relevant evidence to support the theory development (see Appendices 4–6).
For each search, a search strategy was developed and run, and relevant papers were identified through purposive and snowball sampling (see Appendix 6 for details of the search strategies and the papers identified).
Analysis and synthesis
A realist approach to evaluation was followed, as described by Pawson86 and implemented by other researchers as part of intervention development. 102,103 In an area of limited existing research such as preconception, a wide range of sources are used to generate theories. Following an iterative database search strategy, sources were not assessed in a traditional review approach of using a quality appraisal tool, but instead were included if they offered information that was ‘good and relevant enough’103 to maximise diversity of information. The data from the sources were transferred to data extraction spreadsheets, including a core set of descriptors such as identifiers, nature of study, setting and country, and inclusion criteria.
The relevant information from the identified sources was captured in the form of explanatory accounts (see Appendix 12). These accounts summarised the lessons that could be drawn from the source on how best to approach the problem being tackled, in this instance the design of a preconception WLI. These accounts covered a broad spectrum of approaches to allow for a comprehensive overview; some explanatory accounts were articulated in the if . . . then structure familiar from developing CMOs, but also included information from, for example, commentaries or more conceptual work from experts in the field, if those added to the depth of understanding of the contextual factors to be considered in the programme theory.
Once explanatory accounts from individual sources had been documented, they were grouped into consolidated explanatory accounts, with the original source identifier retained to ensure transparency. The first step in this process of data reduction was to identify the outcome of interest; then, we considered any information in the account that enabled us to generate dyadic links, namely mechanism–outcome or context–outcome statements, a process used by Byng et al. 104 and Pearson et al. 105 (see Appendix 13). The descriptions of interventions in academic papers were often not detailed enough to provide the depth of information to form detailed CMO configurations from just one paper but were of sufficient interest to merit inclusion, at least in the first round of grouped explanatory accounts. Once the explanatory accounts were clustered by outcome and any dyadic links were noted, the accounts could be consolidated (see Appendix 14). This was done (following Pearson’s105 method) by first looking at whether or not the account was novel. If it was, then it was entered intact into the consolidated accounts. If it was not, then it was examined to see if it challenged or refined other accounts (i.e. it added to our understanding of contexts, mechanisms or outcomes). Through this process, the consolidated explanatory accounts generated comprised both the single source explanatory accounts that offer a unique perspective and those where the initial explanatory accounts could be integrated.
The use of middle-range theories
As part of the development of programme theories to guide the intervention development, existing middle-range theories that could relate to preconception weight loss and identified barriers and facilitators were explored, as were theories that may be considered central to weight loss in pregnancy and may generalise to the preconception period. Middle-range theories are those that, on the continuum of theory development, would fall between working hypotheses (as can be generated within individual projects, often described as programme theories) and the grand theories that aim to present a unified theory of the social world. 106 They operate at a more abstract level than the programme theories, specify mechanisms by which change may occur and, therefore, enable the development of more generalisable hypotheses.
Theories were identified in three ways. First, a list of relevant theories familiar to the research team from their work in studies relating to weight loss, GWG and behaviour change was generated. Second, theories that were stated as underpinning the interventions were identified through the searches. Third, a Google Scholar (Google, Mountain View, CA, USA) search specifically focused on theory107 was conducted to identify any texts that had not been identified in relation to preconception interventions (see Appendix 6 for the search strategy).
The consolidated explanatory accounts that were developed from the research literature were then considered alongside the formal theories identified through the searches. The next step was to integrate all of the data sources to produce a theory to take forward to the next stage of development. This process is outlined in the sections below.
Results
The review results are described in two sections. First, the relevant middle-range theories are identified, with an accompanying description of each theory, and, second, the CMO configurations are identified, which, together, form the basis of a hypothesised programme theory according to which a preconception WLI could work in the general population.
Identified middle-range theories
The Google Scholar search identified two papers that described theoretical models relevant to preconception and obesity. The B’More Fit programme108 described the work of a coalition tackling obesity in a preconception population and used the social-ecological model, emphasising the multilayered nature of influence and the relationship between behaviours and the social environment. The second source included from this search was Liang et al. ,109 a review of theoretical components of guidelines for physicians. Although not specifically related to obesity, this source was thought to be relevant given the importance of obesity guidelines. In this review the theory of planned behaviour (TPB) and theoretical domains framework were most frequently used (in 16/42 and 10/42), with NPT also used (2/42).
The theories identified as of potential relevance and the source(s) used to identify these are listed in Table 17.
| Theory | Familiar to research team? | Cited by studies included in the literature review? | Theory search |
|---|---|---|---|
| COM-B/behaviour change wheel: Michie et al. 2011110 | Yes | Vesco et al. 2014,111 Hanson et al. 2017112 | No |
| NPT: May and Finch 2009113 | Yes | Liang et al. 2017109 | Yes |
| Self-determination theory: Ryan and Deci 2000114 | Yes | ||
| Self-efficacy: Bandura 1986115 | Yes | Barker et al. 2018,29 Hussein et al. 201636 | No |
| Social cognitive learning theory: Bandura 1986115 | Yes | Harrison et al. 2016,117 Liang et al. 2017,109 Poston et al. 2015,118 Hillemeier et al. 200871 | No |
| Social ecological model: Bronfenbrenner 1979119 | Yes | Truiett-Theodorson et al. 2015,108 Hanson et al. 2017,112 Hill 2021120 | Yes |
| Social practice theory: Shove et al. 2012121 | No | Barker et al. 201829 | No |
| Theoretical domains framework: Michie et al. 2005122 | Yes | Liang et al. 2017109 | Yes |
| TPB: Ajzen 199169 | Yes | Liang et al. 2017109 | Yes |
| Transtheoretical model of change: Prochaska and DiClemente 1983123 | Yes | Ockhuijsen et al. 2012,124 Hanson et al. 2017112 | No |
The lack of theory underpinning preconception interventions was identified in a review of preconception health behaviour research. 38 Many of the papers in this review either refer to interventions in broad terms, such as modifying behaviour, lifestyle modification and increasing motivation, but without references or pinpointing specific underpinning theory, or refer to constructs such as knowledge and attitudes, but often lack a definition of the construct. As a consequence, to include them would require interpretation and assumptions about potential theoretical mechanisms of action [e.g. impact of knowledge could be classified as Capability, Opportunity and Motivation to Behaviour (COM-B) self-efficacy], introducing bias and inaccuracies at this stage.
Each of the theories identified could potentially have a bearing on the review and were considered by two qualitative researchers. The four theories selected as of particular relevance and also encompassing many elements of the remaining theories were the TPB, COM-B, NPT and the social ecological model. Although areas of overlap exist between these four theories, each provides a unique structure that enables clear consideration of three central aspects of PCC programmes, namely individual behaviour change, practitioner behaviour change/embedding changes in professional practice, and relationships between individual behaviours and the social/societal contexts in which they occur.
These theories are briefly described, and their role in relation to the hypothesised programme theories is then considered in more depth.
Theory of planned behaviour69
The TPB states that whether or not a person engages in a behaviour is determined by both intention (motivation) and perceived behavioural control (PBC). The strength of a person’s intention is determined by attitudes, subjective norms and PBC.
The key constructs of the TPB are:
-
Intention – drives a person to engage in a particular behaviour; the stronger the intent (motivation), the more likely it is that the behaviour will be performed.
-
PBC – perception of how easy or difficult the behaviour is (which will often vary depending on context).
-
Attitudes – the person’s positive or negative evaluation of the behavioural beliefs, which relate to the behaviour itself and the outcomes of the behaviour.
-
Subjective norms – whether the person believes that their peers, other significant people or wider culture approve or disapprove of the behaviour (normative beliefs), combined with the individual’s motivation to comply with these groups.
-
Control beliefs – the perceived presence of facilitators or barriers that might affect a person’s ability to engage in the behaviour.
COM-B model110
The COM-B model, incorporating the behaviour change wheel, links determinants of behaviour, drawn from the theoretical domains framework, to behaviour change techniques (BCTs). The theoretical domains framework, which has 14 domains of potential determinants of behaviour,125 was originally designed to support health-care practitioners in intervention development, but its use has been extended to the health behaviour change domain, and it can, therefore, also be used to explore individual behaviour change. 126
At the centre of the wheel are the key constructs proposed in the model as at the heart of behaviour change: capability (including knowledge and skills), opportunity (including social influences and environmental context/resources) and motivation (including beliefs about capabilities and emotion). Surrounding this inner hub of the sources of behaviour are the nine intervention functions (e.g. education, incentivisation, environmental restructuring), which are encircled by the policy categories, such as guidelines, fiscal measures and service provision.
Normalisation process theory113
Normalisation process theory (NPT) focuses on how work is socially organised, and how new practices become embedded and sustained, with an emphasis on the importance of context in this normalisation process. 113 NPT describes normalisation as occurring via four generative mechanisms: coherence, cognitive participation, collective action and reflexive monitoring. 113 Each mechanism has four subprocesses (indicated in brackets):
-
Coherence relates to the process of making sense of the work involved in implementing a new intervention (differentiation, communal specification, individual specification and internalisation).
-
Cognitive participation relates to the process of working out who will be involved in the new work required (initiation, enrolment, legitimation and activation).
-
Collective action relates to understanding the process through which the new intervention is enacted, and possible constraints (interactional workability, relational integration, skill-set workability and contextual integration).
-
Reflexive monitoring refers to how people make judgments about the new intervention (systematisation, communal appraisal, individual appraisal and reconfiguration).
The social ecological theory119
Brofenbrenner’s social ecological theory (SET) is a systems model that recognises that behaviour affects and is affected by social environments and that changes that occur within social environments have the potential to influence an individual’s behaviour. The influences on behaviour are divided into levels:
-
microsystem – includes the closest relationship settings such as immediate family and social networks
-
mesosystem – includes inter-relationships in the broader personal context (e.g. school, wider peer groups)
-
exosystem – refers to influences within the larger social system, such as employment
-
macrosystem – refers to cultural beliefs and values that influence both the microsystem and the exosystem.
CMO configurations
The review process, taking information from the searches through into explanatory accounts and consolidated accounts, resulted in seven CMO configurations that together form the basis of a hypothesised programme theory by which a preconception WLI could work in the general population. Each configuration is described, citing the evidence from the literature that underpins it, followed by the fit with the middle-range theories and the stakeholder survey results. Configurations 1–6 integrate findings from these three types of sources; configuration 7 did not emerge from the literature but has been developed from the stakeholder feedback and is considered in the light of the theories.
CMO configurations:
-
Reaching out to people.
-
Recognising the diversity and wealth of the individual’s experience.
-
Build the health-care practitioners’ confidence and commitment to weight management in their practice of PCC.
-
This is something for me.
-
An intervention that is fit for purpose.
-
Building confidence and motivation.
-
Weight loss discussions should be founded on the principles of informed choice and a client-centred approach.
CMO configuration 1: reaching out to people
To achieve the widest reach of engagement (outcome), any preconception intervention with recruitment across the general population, where women have limited knowledge of the role of raised BMI in the preconception period (context), will need to be co-produced with service users with a positive health message and include a multimedia approach that is accessible in the community and on social media (mechanisms). It needs to be clear who it is for and have a range of culturally sensitive tailored messages, so that women who would be eligible to take part feel that it is relevant to them (outcomes).
Evidence from the literature
One of the central issues in addressing obesity prior to pregnancy, and PCC generally, is that there is little recognition in the wider population of its importance or personal relevance. Women without fertility issues or an existing health condition are unlikely to see PCC as relevant to them127 and do not identify with the term ‘preconception’. 128 In addition, those with a raised BMI often do not identify as overweight (70% with BMI of 25–30 kg/m2 and 18.4% with BMI of > 30 kg/m2). 129 Approximately 70% of pregnancies are planned,30,130 and a high percentage of those women (83%)131 will make at least one preconception health behaviour change. However, the least common changes will be to their dietary intake or weight. 131,132
The coproduction of the intervention and engagement strategy with service users will help to ensure that it is relevant to and resonates with potential recipients. 133 Overcoming the lack of awareness of the importance of weight in pregnancy goes well beyond educating individuals; preconception health messages need to be more widely promoted, for example in community spaces and using social media and across the age ranges so that children and young adults are aware of the messages ahead of childbearing. 29 Those broader health promotion messages about preconception health also need to resonate with different groups of people with protected characteristics to ensure that individuals at risk of marginalisation are not further excluded from health care. 134 Ideally the intervention should be delivered in both health-care locations and other settings in the community. 127 It may also be the case that an e-health intervention delivered predominantly remotely might be more engaging for many. 53,116,135 An emphasis on positive health rather than weight/risk would increase the likelihood of people expressing an interest in the intervention. 128
Fit with middle-range theories
Motivation (COM-B) is key to engagement with any behaviour change. Currently the lack of awareness of consequences of obesity (TPB) in pregnancy at an individual level and the normative beliefs and attitudes at a social level (SET) related to PCC generally but weight management in particular are all significant barriers to the success of any preconception weight management intervention. Increasing awareness of the issues at a societal and social level (SET) requires information about risk to underpin the relevance of losing weight in the preconception phase, which in turn creates a change in attitudes and societal norms (TPB). However, at an individual level, the messages need to be focused on health gain to maximise women’s sense of competence and confidence (COM-B) in order that they develop an intention to change without fearing that they are unable to make the changes necessary (TPB).
Fit with stakeholder survey
LARC users stated that they would want the discussion with the health-care practitioner to have a positive focus. The discussion should be part of supporting health goals, rather than focusing on weight alone. Participants suggested that the tone should be pleasant and not ‘lecturing’, using open questions and supportive language and highlighting the benefits rather than dwelling on risks. There was a range of knowledge and beliefs about the risks of having overweight or obesity during pregnancy. LARC users clearly understood that having obesity poses a risk to both the pregnant person and their future baby. They expressed the wish to lose weight to improve their fertility, to be in better health so their body could cope with the pregnancy and birth, and to have better outcomes for the baby with a better start in life.
CMO configuration 2: recognising the diversity and wealth of the individual’s experience
If the intervention is widely advertised, acknowledges and responds to the different individual experiences of and cultural beliefs about weight loss and health in pregnancy (context), and adopts a tailored, positive-focused approach, then it would reduce the sense of shame that women often experience when talking about weight and would maximise a sense of competence and autonomy (mechanism), thereby enhancing recruitment, engagement and retention (outcome).
Women with a raised BMI in the preconception phase will bring a vast array of experiences and beliefs that are relevant to their engagement with a potential programme. These could be about weight (loss and gain, relevance to pregnancy), diet, physical activity and other health behaviours in preconception. The intervention must include some element of tailoring at an individual level (e.g. to cultural beliefs64) and take life experiences (of weight loss and previous pregnancy in particular) into account. 29,117,134,136,137 One example of tailoring information is the mHealth intervention Smarter Pregnancy,138 which offers tailored mobile health coaching to improve awareness of the importance of nutrition and lifestyle as a part of PCC and supports women and men to make changes in these areas prior to conception.
Knowledge is unlikely to be enough to create change in itself. 38 For example, Barker et al. 29 cited the lack of impact of the social marketing campaign change4life, suggesting that individuals and communities require not only knowledge but also resources to enact change, and a purpose or meaning to provide motivation to engage with the message. There will inevitably be different levels of engagement or interest in PCC and weight loss. 41 For some, pregnancy health starts with a positive pregnancy test and there is a lack of awareness of preconception health;132 others may engage in some change in health behaviours as part of their pregnancy planning, but this is unlikely to include weight loss. 131,139 There may be some elements of a programme that could put people off. For example, physical activity needs to be carefully described and not be too onerous, as some women with a raised BMI may have little experience of formal exercise, and a lack of time for scheduled activity may also be a barrier. 140 Some programmes have been designed to target specific groups; for example, the SHW programme was offered to women in low-income communities. 49 Rather than offering a generic programme, where possible the programme should be designed with flexibility to be responsive to people’s expertise in their own life circumstances and lessons they have learned about themselves along the way.
Fit with middle-range theories
People’s experience of weight management over the course of their life, and particularly during any previous pregnancy, will have a powerful influence on their PBC and attitudes to the behaviour (TPB). Their existing knowledge and understanding of their behavioural regulation around diet and exercise (COM-B capability) as well as their social influences, such as partner/family support (COM-B opportunity; SET micro and mesosystem), will be important in the tailoring of the programme. Understanding individuals’ narratives of their experiences of weight loss and their intentions related to pregnancy weight management will be critical to retention in any preconception WLI. This would be considered part of their reflective motivation in the COM-B model, exploring their goals, beliefs about their capabilities and beliefs about consequences.
Fit with stakeholder surveys’ findings
The powerful narratives from the women who completed the survey about their experience of weight really underlined the importance of this aspect of the programme theory. Women often have considerable experience of different weight loss programmes, and they will be the expert in what has and has not worked for them in the past. Each new effort to lose weight can, in some ways, be described as a new start, but the approach needs to be tailored to factor in the woman’s knowledge, social circumstances, general health and cultural beliefs. A preconception WLI, particularly for an individual’s first pregnancy, is a unique opportunity to develop a very different frame of reference for the intervention: around their health and well-being at that particular time, but also around their baby’s health and well-being.
As well as recognising women’s experiences of weight management, this intervention will also have to overcome their experiences of sometimes very distressing interactions with health-care staff. One of the themes that came through in the survey was that discussions about weight seem to come out of the blue and lead nowhere, leaving the woman feeling punished and shamed. Although a new intervention cannot undo that experience, by making sure that there is adequate publicity, advance notice of discussions, invitations to discuss the topic rather than oversimplistic declarations about risk, and a programme on offer as part of that discussion, hopefully a more positive experience can be provided.
CMO configuration 3: build the health-care practitioners’ confidence and commitment to weight management in their practice of preconception care
Health-care practitioners identify multiple barriers to delivering PCC and discussing weight with women during consultations (context). The intervention therefore needs to include training to build practitioners’ skills and confidence in discussing weight, evidence-based guidance and an effective WLI they can refer to (mechanisms) if they are going to be willing and able to recruit women to the intervention (outcome).
It is not just the general population who do not consider weight to be a central part of PCC: weight is often not included in PCC interventions. 36,139,141 In studies exploring barriers to PCC, health-care practitioners cite time, sensitivity of topic, access to women in the preconception phase, lack of access to clinical guidance and treatments, a questionable fit with their role and perceived value. 116,124,134,135 In designing and delivering a PCC package, multiple barriers can be encountered at the individual, practitioner and organisational levels. 131,142,143 Less evidence is available related to the facilitators of delivering PCC, but training such as Healthy Conversation Skills29 has helped practitioners engage and motivate patients about nutrition and physical activity. If national evidence-based guidance and preconception checklists were developed, practitioners would feel more able to give appropriate advice. 144
Fit with middle-range theories
On an individual level, many of the aspects of CMO configuration 2 apply to the health-care practitioner as well as to the programme participants (e.g. PBC, attitudes, capability and motivation). An additional factor for the health-care practitioner is the complexity of the inter-relationship between their personal history of weight management and their professional practice. At the mesosystem level (SET), delivery of the intervention by health-care practitioners will include education (COM-B opportunity), enablement, training and modelling (COM-B motivation and capability).
All of the mechanisms specified in NPT are relevant to this intervention.
The types of considerations that will need to be taken into account in the design of the intervention are:
-
how the health-care practitioner’s role in the potential preconception WLI differs from (differentiation) or fits with (individual specification) the current PCC practice
-
how the rest of the health-care team see the potential preconception WLI and whether they have a shared understanding of it (communal specification)
-
how the health-care practitioner’s role in the potential preconception WLI relates to their previous experience of PCC or weight-related conversations (internalisation).
As has been considered for the participants, the development of the intervention and its integration into practice needs to take into account the health-care team’s current PCC and weight management approaches with regard to pregnancy. Most of the practitioners work in small teams (in sexual health or primary care), so a shared understanding of the intervention among the team will be important for its uptake. Given the evidence that this type of work has an uncertain fit with their role, this would need to be a significant part of the engagement work and, ideally, the intervention would be co-produced with a cohort of practitioners to address this adequately. It may be that a lack of engagement with the intervention from a practitioner perspective underpins many of the barriers identified to PCC implementation.
Most of the implementation of PCC will happen in individual consultations so that practitioners can reach their own decision about whether or not to implement the potential preconception WLI (initiation), but team members can play a significant role in encouraging others in the team to take part (enrolment). Understanding what they might value about a PCC programme will be important in the design (legitimisation), as will, crucially, their having the resources to deliver it (activation).
Time and workload are barriers to PCC, so a WLI would need to take place outside front-line consultations in sexual health and primary care. However, the introduction of the programme and the initial engagement of the patients will be the role of those health-care practitioners. The information about the programme, respective roles and so on would need to be clearly operationalised (interactional and skill-set workability), and the delivery of the information to patients would need to be managed with an appropriate balance of standardisation and responsiveness (relational and contextual integration).
With any new intervention, practitioners make their own decisions about its effectiveness at both an individual and a practice level (individual and communal appraisal). As part of this, there may be changes in practice over time as practitioners make judgements about and potentially reshape the intervention (reconfiguration). In an initial evaluation as part of a feasibility trial, monitoring of this would be part of the trial design in a process evaluation to try to capture both the formal and the informal appraisals of the intervention.
Fit with stakeholder survey
Many of the health-care practitioners viewed themselves as potential facilitators of a preconception WLI. They suggested that if they had the right approach, good training and a good relationship with the patient, then this would facilitate the discussion and make it more acceptable. They also referred to patients’ attitudes, wishes and levels of motivation as facilitators; if the patient is positive about losing weight, or brings it up themselves, discussing the topic is seen as more acceptable to both parties.
Barriers to engagement in a preconception WLI identified in the published literature also came through in the responses to the health-care practitioner survey: time, sensitivity of topic, access to women in the preconception phase, lack of access to clinical guidance and treatments, a questionable fit with their role and perceived value. Some practitioners referred to the fact that, although they might refer to other preconception health behaviours, such as taking folic acid, they do not include weight in those conversations. Their beliefs about the importance of weight in pregnancy, the efficacy and availability of any intervention and their low confidence in their ability to have conversations about weight may be the barriers that underpin their reluctance to engage in discussions of weight with their patients. If these barriers were addressed in the programme training with clear information about risk and a programme to refer to, then implementation might be more successful.
CMO configuration 4: this is something for me
To recruit women from the general population with a BMI of ≥ 25 kg/m2 and engage them in a WLI in the preconception phase (context), practitioners who are introducing the intervention and the patient to whom they are introducing it would need to acknowledge the principle of a preconception phase and know the patient’s weight (mechanisms) to be able to identify the intervention as relevant (outcome).
With only 29% of women whose weight falls in the BMI category of 25–30 kg/m2 identifying themselves as overweight and 18% of women with a BMI of > 30 kg/m2 not knowing that their weight would be classified as overweight/obese,129 a process would be required by which women would understand that this intervention is relevant to them. The weight of those seeking assistance with fertility would be known and highlighted as an area for change (often before they receive fertility treatment, for those with a BMI of > 30 or > 35 kg/m2, depending on the context). However, women in the general population will usually be weighed only if this is indicated by their type of contraceptive (e.g. combined oral contraceptive pill), not with all LARC. In addition to knowing their BMI, they would need to perceive preconception health as relevant, and that weight is a part of that health, if they are to engage in an intervention.
Fit with middle-range theories
In the UK, > 50% of women have overweight or obesity; therefore, in terms of social comparison across their peer groups and the wider social system (SET), women who have overweight are likely to see themselves as ‘normal’ weight. Although there are guidelines, regulations and environmental initiatives in the macrosystem (SET) that focus on reducing obesity (COM-B), currently these seem to have little impact, possibly in part because societal norms and attitudes (TPB) do not highlight the relevance of these to individuals.
Fit with stakeholder survey
LARC users reported having some knowledge about the risks of preconception obesity in relation to fertility and risks of obesity during pregnancy, which included some familiarity with the risks to the baby (size at birth and future health). However, it was not part of the survey to delineate the level of overweight to which these risks referred. The amount of weight that would trigger an invitation to delay LARC removal and take part in a preconception WLI was an important consideration to service users; an invitation to take part in a preconception WLI was considered acceptable for individuals with obesity, but not for individuals ‘slightly overweight’. Women described being upset at being engaged in a conversation about weight based purely on their appearance, but if weight measurement was seen as a standard part of a consultation about contraception, then conversations about weight could be initiated without people feeling they were being singled out.
CMO configuration 5: an intervention that is fit for purpose
Interventions that helped women identified as having a BMI in the overweight/obese range in the preconception period (context) to achieve a clinically significant weight loss of 5–10% (outcome) had multiple components including both nutritional (tailored hypocaloric diet) and psychosocial (e.g. lifestyle counselling, motivational interviewing) support over a period of several months, which allowed women to develop effective weight management techniques (mechanisms).
The majority of preconception WLIs are in the context of preparation for fertility treatment, so it could be expected that motivation would be very high among people in this group and that the findings may not be fully generalisable to our population. However, this does provide some information on the range of weight loss that women of similar age and BMI could achieve in the context of preconception. Many of these programmes incorporated some degree of meal replacement to reduce calorie intake; among those using total meal replacement, the range of average weight loss in 12–16 weeks was between 6.9% in 12 weeks145 and 13% in 16 weeks. 146 In a programme with partial meal replacement, women lost an average of 6% in 16 weeks,147 and in a tailored hypocaloric WLI without meal replacement over 12 weeks, women lost an average of 5%148 to 6.97% of their body weight. 149 When women take part in a longer intervention (e.g. 6 months), the greatest effect of the intervention takes place in the first 3 months. 150 For adults with obesity in the general population, a weight loss goal of 5–10% of body weight over a 6-month period is viewed as realistic. 151 Measuring waist circumference as well as weight was recommended, as the former is an important health indicator.
The term ‘tailored’ in relation to a WLI often appears to refer to adjusting the calorie intake to the person’s weight or pre-study calorie intake. 145,148 This was found to be an important aspect of weight management approaches in pregnancy, when moderate energy targets adjusted by weight (18–24 kcal/kg) were associated with the greatest reduction in GWG,131 possibly avoiding unrealistic targets for women with raised BMI.
Some studies include ‘lifestyle counselling’ as an intervention component, but this is insufficiently described for any potential mechanisms to be extracted. In one study, women who received motivational interviewing without a dietary or physical activity component lost an average of 7 lb in 12 weeks, suggesting that motivational interviewing is not enough on its own. Many women of reproductive age will not be prepared nutritionally for pregnancy,27 and their usual diet will be insufficient in terms of nutritional guidelines. 141 However, this can change as a result of a preconception lifestyle intervention,152 so this would contribute to the health education element of a preconception WLI.
When women agreed to delay having LARC removed for 24 weeks and completed a full meal replacement for the duration, they lost an average of 14.2% of their body weight. 80 However, this programme had a very low take-up rate and high attrition, suggesting that this approach might be too aversive for a general population. If women are offered a preconception intervention but do not delay becoming pregnant, then weight loss might not be significant in the preconception period, as some will have only very limited exposure to the intervention. 137 In the Prepare trial,53 women were recruited prior to conception and the intervention continued for up to 24 months or until the end of pregnancy, whichever was earlier. Those who received the behavioural WLI lost 3.5% of their body weight prior to conception, a significantly greater amount than those in the usual-care group. However, by the end of pregnancy there was no difference between the groups because of the significantly higher GWG in the intervention group. The authors suggest that a more intensive weight loss support needs to continue throughout pregnancy if any benefits from the preconception period are to be maintained.
It is rare that studies describing preconception WLIs explicitly identify BCTs that underpin the interventions. Two reviews153,154 of the pregnancy literature identify key BCTs (‘goals and planning’ and ‘feedback and monitoring’) as significant in reducing GWG. Clearly, in pregnancy the approach to behaviour change may differ for safety reasons (e.g. a gestational weight management intervention may not focus on weight loss) or because of barriers associated with pregnancy, such as morning sickness or physical issues, so care needs to be taken when transposing these to preconception weight loss. However, the design of a preconception WLI may utilise BCTs that may provide women with the skills to adapt these techniques at their different life stages, allowing effective weight management at the preconception, gestational and post-partum stages. We also need to consider that BCTs might operate differently for people with BMIs in different categories; in pregnancy, we know this to be the case in physical activity interventions,140 but potentially it may also be the case for other BCTs. For example, ‘feedback’ via self-weighing, which increases the effectiveness of behavioural weight programmes in the general population155 and is acceptable to women in pregnancy,156 might be experienced differently by those with a BMI of 25–30 kg/m2 compared to those with a BMI of > 35 kg/m2.
Fit with middle-range theories
Without existing evidence of a new programme’s effectiveness, it may be difficult to motivate people to engage as they will be unsure whether or not the outcomes will justify their effort (COM-B motivation). It is difficult to address this catch-22 of evidence building and engagement, but, potentially, providing information on what others have achieved on similar programmes will enhance people’s behavioural and control beliefs (TPB), and being clear about the health gains achieved with 5–10% weight loss, and the nature of the support offered, will enhance their knowledge, motivation and sense of opportunity (COM-B). This links closely with CMO configuration 6.
Fit with stakeholder survey
The surveys showed that both health-care practitioners and their patients were mindful of the lack of effective interventions available and that this acts as a limiter to their conversations. Both LARC users and health-care practitioners suggested that information about the risks of having overweight or obesity during pregnancy should be backed up by evidence. Although the research evidence may be robust, currently the risks are not presented in ways that are understandable or usable by stakeholders. If the statistics were presented in a more accessible way to highlight the importance of the healthy body during pregnancy and postnatally, then this would facilitate these discussions.
There was a high level of agreement about the key aspects of any preconception WLI across the two stakeholder groups when they were asked to describe the type of service they would like to see. This service would be an accessible preconception health programme, incorporating a combination of dietary advice, physical activity and psychological support that could be tailored using optional elements (e.g. peer support) and signposting to other accessible resources.
CMO configuration 6: building confidence and motivation
Many women’s previous experiences of weight loss efforts will have been negative, and they will know of women with a raised BMI (including themselves) who have had a healthy pregnancy and birth (context). The recruitment and approach to the intervention must build women’s (1) belief that a 5–10% weight loss can be beneficial for their quality of life, clinically significant and achievable; (2) belief that the WLI can be effective; and (3) confidence in their own ability to follow the WLI (mechanisms) if they are going to agree to take part in the intervention (outcome).
This configuration is driven largely by trying to counter the potential barriers to preconception weight loss. There is limited evidence from the general population, but, in the context of fertility treatment, where the evidence of clinical importance will have been explained and motivation will be high (e.g. with the minimum BMI eligibility criterion for accessing IVF services), the take-up of WLIs ranges widely, from 17%44 to 100%. 148 It is not possible to hypothesise the reasons for this from the methods reported, as very few studies included any process-focused evaluation. However, in one study,146 the reasons for non-participation were captured, and these included the time needed to achieve weight loss, concerns about limited success and a belief that the risks of pregnancy associated with obesity are small and manageable.
Fit with middle-range theories
Given the shortage of information on the potential mechanisms of action in preconception interventions, our initial hypotheses will need to draw on evidence from the wider population.
A systematic review of systematic reviews153 identified that the key behaviour change clusters in the efficacy of WLIs are ‘goals and planning’ and ‘feedback and monitoring’. Using the work completed by Carey et al. 157 on synthesising published data on BCTs and their mechanisms of action, the key components of an intervention cut across all of the elements of COM-B and should include (within goals and planning) self-monitoring, planning, goal-setting, a behavioural contract and a review of outcomes. In terms of feedback and monitoring, the key components would include individual feedback on outcomes, social comparison, information about health consequences and how to perform the required behaviours. A sensitive balance would be required between building beliefs in the need for weight loss (TPB attitudes and norms) and instilling confidence in the person that they have the ability to make the changes (TPB PBC).
Fit with stakeholder survey
One of the themes that came through strongly from the LARC user survey was ambivalence, which is known to be a key factor to be addressed in any behaviour change intervention and relates to the perceived importance of the change and one’s confidence in their ability to make the change. 158 Women reported knowing that they needed to talk about their weight because it was important in terms of their health but being reluctant to do so because it is a sensitive topic. Some women clearly did not want to discuss weight with health-care practitioners, whereas others reported wishing that it had been introduced and that they would have welcomed the discussion. Women gave many reasons why they did not want to have discussions about weight, but common ones were that they did not regard weight as particularly relevant in terms of their overall preconception health, that discussions were shaming without being productive (i.e. no support was offered), and that discussions were based on appearance rather than actual weight or BMI, which was experienced as judgemental and unjustified.
CMO configuration 7: weight loss discussions should be founded on the principles of informed choice and a client-centred approach
For many LARC users and health-care practitioners, discussions about weight are difficult and potentially aversive (context). A key ingredient at all stages of the discussion and any subsequent intervention is the principle of the patient’s personal choice, including information provided to make sure they can make an informed choice; all discussions need to be sensitively handled and client-led (mechanisms) so that the intervention is ethical and acceptable to both LARC user and health-care practitioner (outcome).
This configuration is driven by the findings from the LARC user survey, and so this will be described first. These components of sensitive communication, client-led conversations and informed choice were central to stakeholder responses to the proposal of delaying LARC removal in order to lose weight before conception. The woman being well informed and made aware of the risks but also maintaining a level of autonomy was important to both LARC users and health-care practitioners. LARC users identified that knowing the risks of obesity in pregnancy would enable them to make an informed choice, and both groups of stakeholders said that this information could be part of the discussion that would lead to the LARC user deciding whether to follow the advice or take part in the intervention.
Communication was important to LARC users; the manner in which the subject was introduced and discussed was key. LARC users indicated that the health-care practitioner should be sensitive, tactful, courteous, compassionate, non-judgemental and not patronising, be aware of their tone and body language and make an effort to ensure that the patient is at ease during the conversation. The discussion should also not be rushed, so that adequate time is taken to ensure that the patient is at ease and does not feel judged or blamed. Several participants mentioned honesty from the health-care practitioner, and that the practitioner should have empathy with and understanding of the difficulties in losing weight. Care should be taken in the choice of words, with some stakeholders believing that ‘obesity’ was a stigmatised word that should be avoided.
Fit with published evidence
These themes did not emerge from the published papers. For example, a scoping review of preconception health interventions139 mentions the narrowness of measured behaviours and outcomes but does not mention exploring the participants’ experiences of the communication. The evidence is obviously also limited, as only one small study80 addresses the idea of delaying LARC removal in order to lose weight before conception. This study describes the initial discussion focusing on risk but does not identify the number of women who received the information about the study, only those who expressed an interest in taking part, so the effectiveness of the recruitment strategy is unclear. It would seem that this research field is dominated by outcomes rather than process of engagement (or, at least, that is what dominates the publications), and so this is an area in which more research is needed.
Fit with middle-range theories
Informed choice can be understood in the context of both TPB and COM-B. The experience of having information sensitively provided will increase a person’s knowledge and help them consider their beliefs about the consequences of their actions and decisions (COM-B capability, TPB attitudes). Crucially, if the practitioner is genuinely in equipoise, allowing the patient to make an informed decision whether to lose weight or delay removal of a LARC, the patient will experience an enhanced sense of PBC (TPB). Whatever their decision, the patient will have stronger reflective motivation to proceed as the decision has been theirs to make.
Conclusions
This review brought together information from a range of sources to begin to develop a programme theory about how and when health behaviour interventions in the preconception phase might work, in order to design a preconception WLI for women with raised BMI. Information from published research in preconception and pregnancy was integrated, along with wider relevant health behaviour change literature, and included studies using a range of methodologies as well as expert commentaries. Four middle-range theories were selected as being of particular relevance, enabling consideration of individual behaviour change, making changes in professional practice and also the relationships between individual behaviours and the societal contexts in which they occur. From these resources, six CMO configurations were generated and explored in the context of relevant middle-range theories and the survey responses from women with lived experience and from health-care practitioners. A seventh CMO configuration was developed from the stakeholder feedback on the proposed model of intervention. These seven configurations were combined to form a potential programme theory that formed the basis of the proposed intervention to be explored in depth in the stakeholder interviews in phase 2.
Chapter 6 Work package 2: phase 1 stakeholder advisory groups
Introduction
Aim of the chapter
Work package 2 identified potentially suitable preconception/pregnancy-related WLIs and the theories underpinning them using realist methods, in addition to assessing the feasibility and acceptability of a preconception WLI to stakeholders (LARC users and health-care practitioners). The aim of this chapter is to describe the work with the SAGs, refining the intervention components and associated theory, and to consider the key questions to ask participants in phase 2.
Stakeholder Advisory Groups
Methods
The findings from the phase 1 work were synthesised to describe the core and optional components of an intervention. These findings from phase 1 were presented at two SAG meetings: a LARC user SAG and a health-care practitioner SAG. Questions to be addressed at each SAG were agreed by the study team (see Report Supplementary Material 5 and 6). The health-care practitioner SAG was held at a British Association of Sexual Health and HIV (BASHH) audit event, and the LARC user SAG was held remotely via Zoom (due to COVID-19 restrictions).
Participant identification/selection
Members of the LARC user SAG were recruited from a pool of participants who had previously consented to be contacted in the WP2 phase 1 online surveys. At the time of the invitation, this was planned as a 3-hour, face-to-face, daytime, weekend meeting, but it was changed to a meeting via Zoom due to COVID-19 restrictions. Up to 12 participants were purposively sampled to ensure diversity of representation, with attendees having a range of BMI values and reproductive histories/plans.
Members of the health-care practitioner SAG were recruited at a BASHH audit event, as a high percentage of attendees remove LARCs in their current practice.
Participant informed consent
Participants in the LARC user SAG were e-mailed the PIS (see Report Supplementary Material 7) and consent statements (see Report Supplementary Material 8) and were given sufficient time to consider the information prior to attending the SAG meeting. At the meeting, participants were given the opportunity to ask any questions and the consent statement was read aloud by a member of the research team. All participants were asked to consent verbally, and this was recorded.
The health-care practitioner SAG was included on the event agenda, and PISs (see Report Supplementary Material 9) were circulated ahead of the event by the event organisers so that practitioners could decide in advance whether or not to take part. The attending health-care practitioners were invited to attend the SAG discussion if appropriate to their professional role. The study was explained in detail to all attending health-care practitioners, and participants were asked to sign an attendance sheet providing consent for the session to be recorded.
Withdrawal
Participants in both SAGs were able to withdraw at any time by leaving the SAG meeting. However, the nature of the focus group meant that any contribution made up to that point was unable to be withdrawn.
Procedure
In both SAGs, the research team summarised the LARC users’ and health-care practitioners’ responses to the core question concerning the acceptability of asking women to delay LARC removal in order to take part in a preconception WLI and presented the potential core and optional components of the intervention developed from the phase 1 synthesis (Table 18).
| Core components | Optional components |
|---|---|
| 12- to 16-week intervention | Broader description as a preconception health programme for weight loss for pregnancy including information on folic acid, alcohol, nutrition and exercise |
| Training for practitioners on sensitive communication and introduction of the intervention | Include tailoring to fit for all – the individual session at start to identify needs/goals |
Aims:
|
Include partners |
| First contact via the GP surgery or SHC to offer women access to the intervention | Psychological support, for example monthly individual sessions of ‘health coaching’ focusing on confidence and goals |
| Include some online materials specific to health preconception | Face-to-face peer support group |
| Include resources already available, potentially with ‘referral’ (e.g. 12-week NHS weight loss online programme/NHS online dietetic provision, online exercise classes/national exercise referral scheme) | Virtual peer support (e.g. Facebook group) |
| Scales provided if none at home | Include people with BMI of 25–30 kg/m2 (‘overweight’) |
| All done face to face (i.e. no online information) | |
| All done virtually/telephone | |
| Include an exercise component |
Questions to be addressed at each SAG were agreed by the study team and used as a foundation for discussions, but prompts were used based on participant responses.
Stakeholder Advisory Group information and question guide
In the LARC user SAG, the research team described three potential preconception WLI design options, including the pros and cons of each and how each option mapped onto responses from LARC user surveys, to stimulate discussion: (1) delay LARC removal, (2) offer delay but include those who choose not to delay and (3) develop a population-based intervention for women who want to conceive in the next 1–2 years (see Report Supplementary Material 5). This was followed by an exploration of LARC users’ views on the proposed components of the programme and ideas for relevant interview questions for phase 2.
In the health-care practitioner SAG, the team focused on questions relating to the participants’ views of the proposed components of an intervention and their role in engaging women with the programme (see Report Supplementary Material 6).
Analysis
Both SAGs were recorded, anonymously transcribed and analysed thematically. Themes related to the study objectives (refinement of the intervention components and associated theories, and development of questions to ask in phase 2 participant interviews) were described.
Results of the Stakeholder Advisory Groups
Three LARC users attended the LARC user SAG meeting and 34 health-care practitioners attended the health-care practitioner SAG. The feedback from the two SAGs is summarised in four domains: (1) general response to the model of the intervention, (2) specific components of the programme, (3) barriers in practice and (4) questions to include in phase 2 interviews.
General response to the model of the intervention
In considering the general principles of the preconception WLI, the LARC user SAG results reiterated some of the ethical and practical difficulties of requiring delayed removal that had come through from the surveys. The importance of offering opportunities, rather than taking away choices, was evident. In terms of eligibility and recruitment, the option of providing information to LARC users, including the potential benefits of delayed LARC removal, but without LARC removal as a prerequisite for attendance, was favoured. Similarly, the potential for offering a preconception WLI more widely was suggested to prevent LARC users feeling singled out or penalised for making sensible choices:
I’m in that small percentage and we almost get penalised (a) for being overweight and (b) for choosing to have, to not have an unplanned pregnancy because the actual percentage of women who have babies, who have had a LARC is very small I’d imagine.
LARC user SAG P1
The health-care practitioners were generally positive about the idea of an intervention to lose weight pre conception, but there were concerns that a discussion of a delay in LARC removal could result in some women disengaging with the service and missing out on preconception advice. Both of these observations reinforced the importance of putting the principles of informed choice at the heart of the programme design (programme theory CMO 7).
The health-care practitioners reflected that their own practice around preconception advice commonly did not involve weight. This confirmed the theme from the survey that weight is a neglected area in preconception health care (CMO 3). However, the discussion also highlighted that the lack of effective interventions to refer to and the absence of a referral pathway make it much less likely that practitioners will open a discussion about weight.
Specific components of the programme
In terms of specific programme content, LARC users raised important considerations about the complexity of group support in the context of trying to conceive:
Trying for a child is very sort of sensitive in a way, you know, people are very nervous about will I get pregnant, am I able to have a baby, all those things as well, so to be put in a position that you’re sort of announcing that to a group of people that maybe you don’t know.
LARC user SAG P1
Potential difficulties with ‘remote’ weighing were highlighted; LARC users felt that accountability (to a programme facilitator) would be an important component of a WLI:
. . . if I had to just type it into a screen once a week I maybe inclined [laughs] to . . not put your numbers in or, put them in and lie, or something like that . . .
LARC user SAG P3
There were mixed views on involving partners, having a 5–10% weight reduction goal, the inclusion of people with a BMI of 25–30 kg/m2 and low-calorie meal replacements.
Barriers in practice
The ambivalence and discomfort about discussing weight that came through from the survey was reflected in the health-care practitioner SAG. Health-care practitioners recognised the potential importance of weight as a topic, but there were multiple barriers to raising it, ranging across different levels of the system from practitioners’ knowledge and beliefs about its importance in preconception health, to their sense of competence and confidence in their own abilities to have the conversation, the legitimacy of weight as a topic in their service context, their fear of patient response and the availability of resources.
Questions to be included in phase 2 interviews
The LARC users felt that it would be useful to ask the interviewees about (1) what had been helpful for them in the past, (2) details of good conversations about weight, (3) what weight loss methods had worked and (4) whether or not particular language used during recruitment could cause distress. This would enable the study team to build on people’s positive experiences. This information could then be used to inform health-care practitioners of the most effective ways to introduce the subject of weight during health-care appointments:
But I think getting people’s experience of what are the words and the language and the conversations they would want to have that . . . are there words that would be more or less triggering in a way.
LARC user SAG P1
The need for positive conversations about weight fitted with CMO 1 (incorporating co-construction and a positive focus) and CMO 2 (recognising the diversity and value of people’s lived experiences of weight management). The health-care practitioners identified the need for health-care practitioner training and discussed the impact of their own weight on their practice.
Preparation for phase 2 interviews
The discussions enriched and expanded on the themes that had been raised in the survey. Both SAGs proposed a number of questions to take forward to phase 2 interviews with LARC users and health-care practitioners.
Both SAGs reinforced ethical concerns about the delay of LARC removal being the route to accessing an intervention. As a result, it was decided that the interviews about the proposed intervention would include a discussion of delaying removal as an opportunity for patients to lose weight prior to conception, in other words as part of the informed choice that women would make at that point, with the information about BMI and risk having been provided. The need to broaden beyond the delay of LARC removal, as well as the feedback from the LARC user SAG that it would be preferable not to ‘single out’ LARC users, suggested that this could be an intervention open to many people. This was a question to take to the interviews with health-care practitioners and LARC users about the acceptability of a preconception WLI that would not have health-care practitioners as gatekeepers but would include them as one part of the pathway. There were also mixed responses to the inclusion of people with a BMI of 25–30 kg/m2, and so this was to be explored further in phase 2 interviews.
Some themes in the health-care practitioner SAG about current practice resonated with the review and led to the identification of areas for interview questions. Many of these related to the barriers at different levels of the system, including (1) the general lack of inclusion of weight loss in practitioners’ preconception thinking; (2) the lack of available WLIs to refer to; (3) the impact of their own weight on the introduction of the topic of weight into the consultation; (4) their need for training on weight, BMI and risk; and (5) the practicalities of weighing women to facilitate the discussion.
The themes specific to the LARC user SAG that required more detailed exploration in the interviews were (1) the acceptability of group support in the context of preconception weight loss and how this could be managed, (2) the best approach to self-monitoring of weight as part of the intervention and (3) the eliciting of good experiences of weight-related consultations. This last suggestion, although made by LARC users, was felt by the research team to be an important question to explore with both the LARC user and the health-care practitioner interviewees to maximise the understanding of positive conversations from both perspectives.
The feedback from the SAGs and areas highlighted for further discussion were considered in relation to the CMO configurations that emerged from the realist review and formed the basis of the topics for the phase 2 interviews to refine the intervention (Table 19).
| CMO configuration | Topics for phase 2 stakeholder interviews |
|---|---|
|
General response to the proposed programme, strategies for engagement, how to maximise diverse appeal, population based rather than just LARC users/health service referral (linking with theory 7) |
|
Successful experiences of weight loss (link with 6), language of risk and benefit, what might attract you to a particular programme |
|
What training has helped, what would help, personal barriers regarding weight (health-care provider interviews) |
|
Inclusion of people with BMI 25–30 kg/m2, views about weighing in clinic, preferred modes of intervention delivery (peer support) |
|
Inclusion of goal-setting of 5–10% weight loss, meal replacement, feedback weight, resources they use and value, potential obstacles |
|
Successful experiences of conversations and weight loss |
|
Views on the idea of including both those who delay LARC removal and those who do not. Should it be a programme offered out to the general population, with LARC removal as just one opportunity to advertise the programme? |
Chapter 7 Phase 2: acceptability and feasibility of proposed intervention
Introduction
Aim of the chapter
The aim of the chapter was to further refine outputs from the phase 1 survey and the SAGs. The potential intervention design and associated CMO configurations informed by phase 1 findings were tested with targeted qualitative interviews with LARC users and health-care practitioners.
The aims of the qualitative interviews were to assess:
-
LARC users’ views regarding the acceptability and feasibility of the potential WLI
-
health-care practitioners’ views regarding the type of intervention and their willingness to recruit women to such an intervention.
The findings from the interviews were then presented to the two SAGs for discussion to refine a final version of a proposed intervention.
Methods
LARC user and health-care practitioner qualitative interviews were conducted over the telephone or via Zoom; the LARC user SAG was held remotely via Zoom (due to COVID-19 restrictions) and the health-care practitioner SAG was conducted as a group discussion via a Padlet discussion board. Padlet was chosen primarily as it enabled asynchronous, anonymous feedback from the health-care practitioners, but it also offered flexibility of format.
Participant identification/selection
Participants in all qualitative interviews and SAGs were identified from the pool of participants who had previously consented to be contacted in the WP2 phase 1 online surveys.
Potential participants in the LARC user and health-care practitioner qualitative interviews and the LARC user SAG were contacted by a member of the study team using the person’s preferred method (e-mail/telephone) with information about the phase 2 interviews/SAG. Participants were purposively sampled to ensure a range of BMI and reproductive histories/plans (LARC users) and professional backgrounds and genders (health-care practitioner interviews). All potential participants in the health-care practitioner SAG were invited by e-mail to take part in the SAG.
Participant informed consent
Participants in the qualitative interviews and the LARC user SAG were e-mailed the PIS (see Report Supplementary Material 10–12) and consent statements (see Report Supplementary Material 13 and 14) and were given sufficient time to consider the information prior to the interview/SAG. At each interview/SAG, participants were given the opportunity to ask any questions, and consent statements were read aloud by a member of the research team. All participants were asked to consent verbally, and this was recorded.
All potential participants in the health-care practitioner SAG were e-mailed an information sheet (see Report Supplementary Material 15) and link to the Padlet discussion forum. Participants were informed that participation in the discussion was considered consent to participate.
Withdrawal
Participants were able to withdraw at any time by leaving the interview/SAG, but their contributions up to that point were unable to be withdrawn.
Procedure
Topic guides for interviews were based on the synthesis of phase 1 results, described in Table 12 (see Chapter 6; and see Report Supplementary Material 16 and 17). For the LARC user SAG, the study team presented study progress, which was followed by a discussion based on the synthesis of phase 1 results (see Report Supplementary Material 18). For the health-care practitioner SAG, a study update was provided using a combination of written text, a PowerPoint summary and a video of the chief investigator presenting the main findings. Participants were asked to respond to three questions, each focusing on a service-related topic: (1) how to contact women who use their service who might be interested in a preconception WLI, (2) how to advertise it more broadly in the community and (3) what training practitioners would want in talking about weight.
Questions to be addressed in interviews and with SAGs were agreed by the study team and used as a foundation for discussions, but prompts were used based on participant responses.
Analysis
Both SAGs were recorded, anonymously transcribed and analysed thematically. The results were further grouped and are reported according to the CMO with which they most closely align. Any repetition of the findings in phase 1 (see Chapter 6) has not been reported in detail.
Results
The results are presented in themes, according to the seven CMO configurations generated from the realist review. A summary of the themes and associated CMOs is provided in Table 20. This is followed by the refinement of the potential programme theory, a summary of the stakeholder SAGs and a description of the final potential intervention. The quotations taken from the interviews are identified as LUI or HCPI for LARC user and health-care practitioner interviews, respectively, and each is followed by an ID number.
| CMO configuration | Theme |
|---|---|
| 1: Maximising engagement | Appeal |
| Recruitment | |
| Discussion of risk | |
| 2: Recognising individuals experience | Key dimensions of previous weight loss success |
| 3: Health-care practitioners | Communication skills |
| Knowledge of risk | |
| Script | |
| 4: This is something for me | BMI eligibility |
| Weighing in clinic | |
| Mode of delivery | |
| 5: An intervention that is fit for purpose | Target weight loss |
| Weight feedback | |
| Meal replacements | |
| NHS resources | |
| Group support | |
| Challenges | |
| 6: Building confidence and motivation | Successful conversations about weight |
| Practitioners’ readiness to refer | |
| 7: Informed choice | Options and facts |
CMO 1: reaching out to people – maximising engagement
Qualitative feedback relating to CMO 1 included interviewees’ general impression of the proposed intervention and their views on the recruitment route and the approach to discussing risk.
General appeal of proposed intervention
Overall, there was a positive response to the intervention design from both LARC users and health-care practitioners. The LARC users particularly liked the free provision and the flexibility of having options that meant that the intervention would be tailored to the individual. Although the timescale of the intervention itself was generally acceptable, there was concern that it might be too short, giving less opportunity for support and conveying a ‘quick fix’ rather than a message of a lifetime commitment:
I think the danger is if you only have three meetings, from 12 to 16 weeks, then you might have people stray or it might not be enough guidance for some people. But you also don’t want to give too many appointments . . . So I think it’s a balance. Compliance might be an issue if you only do the three face to face.
LUI04
LARC users supported the idea that the intervention should have a positive, broad health focus, rather than simply focusing on weight per se, and suggested that this should be an important underlying ethos of and emphasis in the advertising:
I just think the emphasis just on weight sends out the wrong message . . . It should be more . . . a programme to help you have a healthy pregnancy when you’re trying to conceive.
LUI10
If it’s sold and marketed as an exciting, cool, forward-thinking thing to do, to plan for your pregnancy, and to plan for your life after . . . I think that could be helpful.
LUI07
Recruitment route: via the general population or health-care practitioners?
A clear majority of interviewees stated that it would be preferable to offer the intervention for self-referral (e.g. advertising on social media), rather than gatekeeping the invitation to the intervention through health-care practitioners, as this would increase reach to the target group, especially for some women with overweight/obesity who avoid appointments with health-care practitioners, as well as increasing inclusivity for all women, not just those using LARC. General promotion, such as via leaflets, may allow women to digest information prior to attending for LARC removal, allow them to discuss with partners and prevent them feeling targeted because of their appearance. The stakeholders also thought that, by allowing self-referral, participants might be more motivated because they would have more control over the process, and that the entry to the programme could be quicker and more straightforward and reduce the likelihood of an individual feeling shame:
I would do social media because not everybody goes to the doctor’s . . . you try and avoid it to be honest.
LUI13
If you’re planning that, then you would discuss that with your partner first . . . I would discuss it with him first . . .
LUI01
So it would be really good to have it out more widely wouldn’t it? . . . feel like that takes away some of the, ‘oh, the doctor had a go at me for being fat’. You come home and eat a cake [laughs] . . . Empowering people to do something about it for themselves. And having that information out there . . .
HCPI03
However, LARC users acknowledged that a health-care practitioner introducing the intervention had the advantage that health services are a more trusted source of information. In addition, existing relationships with health-care practitioners, who may know any relevant background issues/conditions, may increase engagement and reduce the risk of harm. It was suggested that combining the two recruitment methods could maximise reach in terms of people’s circumstances but also across a wider demographic:
I think because there’s so many things out there, unless it’s kind of got a bit of the professional backing, or it being delivered by somebody, you know, like that, it might not be considered as seriously . . .
LUI15
It has to be a practitioner that knows that woman and her medical history because I also suffered from postnatal depression, and around my miscarriage I had depression as well and so if you approach a woman that’s had those types of issues on top of disordered eating, weight management problems, I think you’re opening it up to a real difficult situation not just for the practitioner but also for the women.
LUI10
Discussion of risk
One of the key factors in making the decision about whether to engage with this intervention is the discussion of risk and how that message is conveyed. There were six broad themes in people’s reflections on how risk needs to be integrated into the conversation about the intervention: (1) simplify/make personal, (2) RR as part of holistic preconception approach, (3) positive health promotion, (4) facts not fear, (5) baby health and (6) fertility.
Simplify
Some practitioners talked about discussing risk in simple terms or personalising it so that the patient can understand what is being said:
We talk about risk in one in a hundred and one in a thousand, but a lot of the time people don’t seem to grasp that, they prefer percentage. It’s talking about it in normal terms and not overcomplicating things.
HCPI07
Relative risk as part of preconception health
Both groups of stakeholders said that a discussion of risk should be undertaken in relation to other risk factors (e.g. alcohol and smoking) as a way of seeing the bigger picture and also suggested easing into the discussion about weight via topics that might be more acceptable (e.g. folic acid):
If any expectant mother is serious about getting pregnant, I think you’re going to need to know the pros and cons of everything, to do with weight, smoking, alcohol, you know it’s all under the same thing.
LUI03
Positive health promotion
Some participants said that risk should not be mentioned, but that the focus should be on positive messages, offering support, and optimising health and the health of the baby, as negative messages put people off and potentially add stress:
I think it’s got to be the positive way hasn’t it? . . . You don’t want to be scaring people to death and adding to the stress. It’s a stressful time anyway.
LUI20
Facts not fear
Stakeholders suggested that couples be provided with information on risks as part of their decision-making; however, they said that this should be framed factually as medical information rather than delivered in the form of ‘shock tactics’:
I think if you sat people down and said, ‘These are the facts and these are the figures and this is the percent of actually you have a greater chance of miscarriage’, or whatever, then it might be harder hitting and hit home to people, and sometimes I think people almost shy away from that and actually it’s the truth, so maybe it needs to be said.
LUI06
Baby-focused
Many interviewees said that the health of the baby was a prime motivator, so risk factors associated with the baby might engage people more than risks to themselves:
. . . every decision I made while I was pregnant, including like the drugs during the birth were, ‘if it harms me, I don’t mind; if it harms him, it’s not happening’.
LUI17
Fertility
Participants suggested talking about the difference the target weight loss could make in terms of fertility, but said that this was a conversation to be had with a health-care practitioner.
CMO 2: recognising the diversity and wealth of individuals’ experience
All of the LARC users interviewed had experiences of weight loss attempts and had tried various approaches or programmes, which would inform their approach to any new weight loss programme. The aspects of previous weight loss plans that they found useful included (1) weekly weigh-ins (increased motivation due to accountability); (2) group support elements, including apps (although not suitable for all); (3) different apps to track progress; and (4) flexibility in order to fit in with people’s lives:
I just think physically going somewhere and knowing in a week, that actually you’re going down and step on those scales on Tuesday in front of Joe Bloggs keeps you motivated.
LUI01
So if, if you’re having a bad day and you, you know, you’d have a day when you’d eaten far more than you should have, they were very supportive and, you know, with all being there and, you know, just get back on it tomorrow.
LUI03
CMO 3: building health-care practitioners’ confidence and commitment to weight management in their practice of preconception care
Practitioners were asked about how they and their colleagues could be best supported to have discussions about preconception weight with patients in order to introduce the intervention. They felt that the communication skills required should already be integral to their role, but some suggested that training in motivational interviewing158 may be beneficial. Additional information via fact sheets or existing guidance documents and training would help overcome their lack of knowledge about some of the risks. Some thought that a script could potentially be useful for introducing the topic, and training videos and role playing using the script were suggested as ways to help practitioners become confident. Furthermore, a thorough understanding of the intervention itself would be essential for engaging patients:
I mean certainly as a GP my understanding of risk, I know it is desperately poor and my ability to articulate that and explain that in most circumstances could be better.
HCPI01
A lot of staff don’t feel confident about asking any number of things about alcohol, substance abuse . . . but what we found is that if they can be given some key strategies or phrases, they’re often happy to run with it.
HCPI10
Where it’s an awkward conversation [it] needs to be scripted. You need to have practised it.
HCPI03
I think they need to have a good idea of what the intervention was, in terms of how long it lasted, and the kind of steps that people would go through . . . when they could withdraw from it if they wanted to or not. If they became pregnant early on, what they would then do after that. And the safety of those things.
HCPI06
Practitioner weight
In the online surveys of LARC users, the issue, and potential barrier, of the practitioner’s weight was raised, and so this was explored in the stakeholder interviews. Some LARC users said that a practitioner’s weight would not make a difference to them; some, while acknowledging that it should not matter, suggested that, in reality, whether a health-care practitioner appears overweight (experienced as hypocrisy) or slim (lack of empathy) can have an impact on people’s level of engagement in discussions about weight. For health-care practitioners, the quality of the interaction was considered most important, and the weight of the health-care practitioner was irrelevant. However, some health-care practitioners described ‘uneasy’ conversations with patients because of their own weight or personal issues. The LARC users’ and practitioners’ feedback shows that the value of self-disclosure clearly varies by consultation and may be something that the practitioner has to decide on a patient-by-patient basis:
It shouldn’t because we’re not talking about their health, we’re talking about my health, but I think it does, yeah. If you’ve got somebody incredibly overweight telling you to lose weight then that’s going to be difficult, or, if you’ve got someone very underweight or, you know, very slim . . . you’d almost, I’d almost feel resentful towards them.
LUI10
You have to gauge it patient by patient. There are quite a few scenarios where it can be useful and can really get them on board with something. I think they feel like, ‘yeah, well that doctor really knows what she’s talking about, she understands, she’s been there’, type thing.
HCPI03
CMO 4: this is something for me
Three key topics in this CMO related to eligibility for and engagement with the programme where we needed feedback from stakeholders to progress the design: (1) the lower threshold of BMI for the programme, (2) the acceptability and feasibility of weighing women in clinic and (3) the preferred mode of delivery of the intervention.
Body mass index category
There were mixed views about including people with a BMI classified as overweight but not obese (25–30 kg/m2) in the intervention. The main argument in favour was prevention, to potentially reduce GWG and give a positive start to a healthy pregnancy. However, the majority of views tended towards not including women with weight in this category in the programme, with the arguments falling broadly into three overarching themes: (1) RR, (2) limited resources and (3) coherence.
Relative risk and body mass index
The use of BMI as the key eligibility criterion raised questions for some about its sensitivity as a measure of health and also the quality of differentiation between the risks at different BMI categories:
Obviously to do with muscle mass and things like that, you know. I don’t entirely agree with BMI to be honest.
LUI03
I would imagine the evidence is that if you have a BMI of 27, it probably isn’t that different in terms of pregnancy outcome to a BMI of 23. I think my instinct would be 30 and above.
HCPI08
Limited resource to be targeted
It was suggested that people with a higher BMI are the ones most in need of an intervention, and including those with a BMI of 25–30 kg/m2 would run the risk of the programme being overwhelmed. In addition, stakeholders argued that including the lower BMI group might not provide the best test of the intervention, with views differing on whether those with a higher BMI would be more or less likely to succeed.
Coherence
It was suggested that a focus on those with a BMI of ≥ 30 kg/m2 would fit better with other services (which use BMI 30 kg/m2 as a threshold) but would also make more sense to service users, creating a stronger sense of group identity. In addition, health-care practitioners would not be required to initiate much more difficult conversations with people whose weight is in the overweight BMI category but may not regard themselves as overweight, and, therefore, the potential to cause upset would be reduced.
Being weighed in clinic
The phase 1 online surveys with LARC users highlighted that LARC users found it distressing to be judged overweight and told to lose weight simply on appearance. It was, therefore, important to explore the acceptability to women of being weighed when they came to the clinic for LARC removal. Most women said that they would expect to be weighed in a clinical environment, and that measuring weight is part of routine care, but, given the negative feelings that this can evoke, it should be done privately, discreetly and with no judgement:
I think lots of procedures when you go to the GPs, you get weighed and measured for lots of standard things, and, and I think if it was just done routinely.
LUI08
I’d hate it, I hate being weighed, it’s embarrassing, especially if you’ve got a young slim nurse at the side of you . . . It’s the fear of judgement.
LUI20
When health-care practitioners were questioned about weighing patients in clinic, they also said that this can provide a good opportunity to raise the topic of weight, particularly as part of a preconception discussion, in a way that would not necessitate judging weight on appearance. However, barriers to this include time restraints and the fact that the topic may not be essential for clinical decision-making. Health-care practitioners suggested a self-weighing system, such as a machine in the waiting room, but acknowledged the need to be really sensitive to how public this would be:
It’s just such a grotesquely uncomfortable conversation for both the practitioner and the patient, that probably we do it very obliquely.
HCPI04
So I don’t weigh people unless I have to, because that’s an extra, you know, minute and a half that you don’t want to waste.
HCPI08
We just had the machine in the waiting room that they could stand on it and it just gives them a little ticket. So it does their blood pressure, their height and their weight that they can bring that in . . . Most women were happy they have to do that, yeah.
HCPI05
Mode of delivery
In designing the programme it would be important to strike the right balance of online- and face-to-face-delivered content, so the differences between modes were discussed. Positive aspects of online experiences included ease of access, no travel, the possibility of anonymity, the ability to keep track of progress and the availability of large amounts of information and ideas. However, participants described some negative aspects of online delivery, including the possibility of getting too engrossed/obsessive about one’s weight in apps without anyone else there to check. In addition, participants talked about having difficulty building relationships with people they had not met in person and missing the social aspects of in-person contact. Participants said that face-to-face delivery is easier for receiving complex information, asking questions and being given tailored explanations, and they equated this with being motivated.
Participants pointed out that there are certain things that must be done in physical meet-ups, such as being weighed by someone else or sharing food. In addition, having this in-person contact meant that there was a level of accountability that would encourage the individual to work harder between meetings. However, negative aspects of in-person groups were reported, such as the feelings that can sometimes arise from feeling judged, hearing someone else’s stories or comparing oneself with others:
You know they say these places are non-judgemental but you’re always comparing yourself against the others. Oh Sandra’s had six takeaways this week and she’s lost weight. How come I haven’t? It’s just human nature. So those weeks the app works better for me so I can have that conversation privately.
LUI09
Participants tended to appreciate having a combination of options for contact, and that there is an aspect of individual choice in this, but the key thing is to maintain the contact with the group or health coach in whatever way suits you.
COVID-19 also appeared to have had an impact on the acceptability of online/remote contact. People talked about how the months of lockdown had changed their opinions about using online groups, for example fitness classes on Zoom. They felt that this was more acceptable than it had been prior to lockdown, because more things are done virtually and people have got used to it.
CMO 5: an intervention that is fit for purpose
The questions for stakeholders that arose from this CMO concerned the acceptability of explicitly describing the 5–10% weight loss and the inclusion of meal replacements as an option. We also explored the practicalities of weight loss feedback, which resources interviewees had found useful in their weight loss experience and any obstacles they could see that had not been brought to the fore by other interview topics.
Target weight loss
There were mixed responses to the idea of a 5–10% target weight loss. The majority of the interviewees said that goals for weight loss are generally helpful because they are something to work towards, and that personalising the goal, by making it a number, would also help. Participants said that the key factor with the goal is that people perceive it as achievable, and the 5–10% proposed goal seemed to satisfy stakeholders in this regard. Some said that this goal might be low enough to encourage service users to believe that they could lose enough weight to make a difference to their health, but it could also encourage practitioners, who might feel more able to initiate the conversation with this goal in mind:
I think that would be really helpful because it breaks it down and it seems more manageable. Because if a woman knows they’re four stone overweight, that seems a massive hill to climb, doesn’t it?
HCPI09
Some participants felt that, for them, goals can be counterproductive and that the upper limit of 10% might feel too daunting for those with a very high BMI. This could potentially put people off joining the programme or result in feelings of anxiety or failure if the level of weight loss is not achieved:
It might put some people off, having a target weight, ‘cos they might feel like more of a Slimming World-type of environment. Or you might feel that you failed and that you can’t become pregnant if you don’t reach that goal.
LUI04
Participants suggested that having a goal was an individual preference, as it would work for some people but not for others. Where possible, goal-setting should be part of the individualisation of the programme; the clinical benefits of 5–10% weight loss could be given as information, but it would be the individual’s choice whether or not that would be helpful. In addition, a target could be set based on the individual’s experience of weight loss in the past rather than on theoretically achievable goals:
People are one of two aren’t they: they like goals or they don’t . . . So, I suppose it’s probably that trying to find the in between, because it’s not one-size-fits-all, is it?
HCPI02
In addition to the concern about delay of LARC removal previously discussed, LARC users talked about the need to manage expectations, for example if someone could not lose weight and felt like a failure, or if, following the delay and losing weight, the patient still had difficulty conceiving and felt let down:
[The] challenge is that expectation management of, ‘what happens if I do lose this weight but I still don’t conceive?’ Or, ‘I signed up and I didn’t manage to lose the weight and now I’m a failure’, so it’s that management of the expectations.
LUI08
How to feedback weight
The weekly weight feedback received mixed responses from LARC users. Some participants stated that they would be happy to self-report as it gives a greater sense of control over when the information is sent. However, others felt that self-reporting might lead to people not being completely honest with themselves or the person they were feeding back to, and could risk creating a situation in which people weighed themselves multiple times. Therefore, most felt that having clear evidence of time and date, via a video call, Bluetooth scales or a photograph, was the best option. Participants said that people would be committed to the programme and therefore should be expected to be honest about the weight that they send in if they self-reported:
If it was too flexible as to when you send your weight in, then at least I know I would be tempted to . . . [weigh] myself several times a day. Seven days a week and trying to find the best figure.
LUI19
On the contrary, others saying that being asked to send a photograph of the scales would be insulting as it would make them feel not trusted:
You could take photos, but then I suppose if you say, ‘oh can you submit a photo?’, it’s almost like saying I don’t trust you just to write it down . . . I think you should be able to trust a person . . . I wouldn’t have a problem with it.
LUI06
Some participants were positive about the photograph idea, as they said that they would not have to think about it or they could ask someone else to take the photograph so that they would not have to look and potentially get upset. The potential use of Bluetooth scales was popular as a way of reporting with ease and ensuring accuracy. However, some participants questioned the expense involved in these and the possibility that someone could join the intervention to receive the scales and then withdraw. Concern was expressed about the calibration of the scales if weighing was carried out at home, but interim weighing at home was seen as acceptable, given that weight would be taken at the beginning and end of the intervention. LARC users also stressed the importance of receiving a response to the weight report in order to encourage people to continue with the intervention.
Meal replacements
The discussion about meal replacements also elicited mixed responses, with many interviewees talking about both positive and negative aspects of these. Meal replacements were very much framed as a matter of personal choice. The positive aspects of meal replacements were that they offer a kick-start to weight loss, and one does not have to think about food. Reasons given for meal replacements not being included are that they are not sustainable, they do not fit well with family or social life and, once a normal pattern of eating is resumed, the person will gain weight again. A significant concern was that they did not teach people to eat healthily, for long-term health. Participants were worried that meal replacements would not be good for mental health or preparation for health in pregnancy:
. . . it’s the simplicity of it. Like, if you just do this one thing for 8 weeks, it’s almost a guarantee that at the end of it you’ll have had a little early win. And once you’ve got that under your belt, it might be a bit easier from then on. That sounds appealing.
LUI02
I guess you do risk it moving forwards into pregnancy. People thinking, ‘oh, I can just have a shake’. I don’t know what that would do to a developing baby but I mean they say they’ve got everything in them they need, which I guess is where the danger is, isn’t it?
LUI09
The consensus among LARC users was that as long as meal replacements were one option, and managed, then it would be the individual’s choice. Meal replacements should not be compulsory, and care would need to be taken to ensure that they were not the only aspect of the programme.
NHS and other resources
Participants had varying levels of experience with the resources available for losing weight. Whereas some had used resources such as Exercise on Prescription or NHS-provided Slimming World vouchers, or knew people who had, others had not heard of these NHS-supported schemes. The NHS 10-week plan was mentioned by one woman who found it helpful. There seemed to be disparities between localities, with some areas having local schemes, from weight management in the practice to motivational interviewing services, bariatric referrals, self-help schemes and so on, and other areas having very little. The health-care practitioners working in sexual health services did not have these referral options, and instead suggested that women return to their GP.
Other apps mentioned included MyFitnessPal (MyFitnessPal Inc., San Francisco, CA, USA), Strava (Strava, San Francisco, CA, USA), Couch to 5K and Mind Matters. The benefits of apps included the ability to track and keep a record of food, exercise, ideas and motivation. Exercise options included parkrun and, more unusually, Zombies, Run! (Six to Start and Naomi Alderman, London, UK).
In general, participants favoured including existing resources in the intervention, as they understood the reasoning behind not wanting to ‘reinvent the wheel’, that the existing resources are expert-led, and that using existing resources to create a bespoke programme would be flexible enough for everyone.
Group support
LARC users tended to see peer support as an important and valued aspect of weight loss. Most agreed that peer support has value in the camaraderie of knowing you are not alone. LARC users favoured different formats for the peer support: online for flexibility, text based for anonymity or distance for sensitive discussions that could be digested in one’s own time. However, some participants raised concerns around accountability and honesty in using an anonymous forum and the need for the forum to be moderated for safety:
I guess if you were doing it virtually you could choose which kind of group you wanted to be in. As obviously you don’t have to be physically nearby so I guess that would give that flexibility potentially.
LUI12
[In an online forum] anything that’s deemed to be in anyway aggressive to others or shaming or anything or belittling is, is taken down straight away. I think it’s got to be like a safe place. And, and be managed and monitored though by admins.
LUI05
Whereas some participants favoured group support, other stated that they found in-person groups, such as Slimming World, very difficult because they did not have anything in common with other members of the group and group rapport was lacking. Some participants stated that they preferred one-to-one sessions rather than group support.
Obstacles
Participants were asked if they could foresee any obstacles or challenges that we might encounter in this programme, over and above those already identified in the content-specific questions. One participant commented on the growth of distrust of medical experts and disbelief of medical advice, which might influence people’s willingness to engage with the programme:
I don’t like the emerging trend about ignoring medical experts . . . The growing, kind of body positivity movement. I am seeing a growing anti-medical thing about obesity. Cancer Research I think did a campaign either at the end of last year, the beginning of this year about obesity kind of reinforcing the link between obesity and cancer. There was a real big backlash to that. That kind of thing makes me a bit worried.
LUI02
Practitioners said that patients might not be able to fit an intervention that involved exercise classes into their work and life, and they also mentioned the differences in, for example, food and exercise facilities available in rural areas and cities.
Health-care practitioners suggested that people who are not in their first pregnancy are less likely to engage with this intervention, as they will already have had the experience and know what they are doing. They might question the evidence on risk if their previous pregnancies had resulted in healthy children, or they might just not have the time to engage with the intervention because of their busy lives as parents.
CMO 6: building confidence and motivation
Participants were asked about their experiences of successful consultations about weight to try to elicit the key ingredients for establishing strategies to build confidence and motivation.
Some LARC users said that they had never had a good conversation about weight with health-care practitioners. However, those who had done identified various aspects that had made a difference, including the attitude of the practitioner, for instance if they were non-judgemental and matter-of-fact. Some participants talked about having a good relationship with their health-care practitioner, and participants said that the experience is improved when health-care practitioners take the time to listen to the patient. One health-care practitioner said that this might be easier for nurses, who are allocated more time for consultations. Health-care practitioners felt that factual starts to the conversation might also help:
I appreciated what she said actually, she didn’t really pussy foot around . . . she didn’t shame me . . . she just said, ‘keep walking, what you’re doing, but just control maybe your portions and don’t eat as much’ [laughs]. And she was right, she was right [laughs], what can I say?
LUI05
Both groups of stakeholders felt that the conversation needed to be led by the patient, either that they introduced the topic or that it was given as an option and left to the patient to decide whether they wanted to explore it. The practitioners said that it was important to ensure that they took the position that the patient was an expert in their own condition, tailoring the discussion and acknowledging their knowledge:
At the point where you go in and say, ‘I would like some help with my weight’, that’s the point where it should be a positive experience. They should be like, ‘That’s really great you’ve come in. These are your options’.
LUI11
Happy to refer to the intervention
Health-care practitioners were also asked if, hypothetically, they would be happy to refer their patients to the programme if it was on offer. The key factors identified were having more information about the actual programme to inform patients and, in particular, that it was evidence based.
Health-care practitioners wanted to know what their role would be in the follow-up of patients who attended the WLI. Some practitioners wanted reassurance that the participant could still have their LARC removed if they decided to withdraw from the study or were unsuccessful at losing weight. Furthermore, health-care practitioners said that staying in contact with the patient, and receiving feedback regarding the outcomes so that they could continue to support the patient, would be important:
I suppose from the legalities view, you want to make all the governance is there in place, the safety. What happens after 12 to 16 weeks if they haven’t succeeded, or totally failed at it? Gone the other way even. Do we then say, ‘we’re not removing your LARC’?
HCPI02
CMO 7: weight loss discussions should be founded on the principles of informed choice and a client-centred approach
The theme of ensuring that women were given information and able to make their own choices came through several of the areas of discussion in the interview. As with the survey responses, when discussing the design, LARC users wanted it to be really clear that delay was just one option, that it was not a prerequisite for attendance and that the idea of informed choice was front and centre of the discussion. This includes presenting the risks and advantages of different decisions so that the woman can fully understand the choices being presented and is fully aware of her options. Health-care practitioners linked the discussion of risk and options with their professional responsibility and the discussions they have in relation to other procedures. The aim is to make sure that the woman can make an informed choice, fully aware of the risks and benefits:
So yes, I think, but I do think that informed choice is important and you can’t make an informed decision if you haven’t been presented with all the facts around weight and pregnancy and if you haven’t been given an option to delay with support to lose weight.
LUI04
Refinement of potential programme theory
From the results of the interviews, the programme theory for a potential preconception WLI was further refined (Figure 10).
FIGURE 10.
Refinement of a potential preconception WLI.

Phase 2 Stakeholder Advisory Groups
The LARC user SAG was attended by three members of the research team and five women, two with children and pregnant/planning to be pregnant, two planning to be pregnant in the next year and one planning to be pregnant within the next 5 years. The results are presented under the three main themes of the discussion: (1) engagement, (2) outcomes and (3) support and improving practitioner skills.
Three main points were made by the SAG with regard to maximising engagement with the programme. The advertisement of the programme would need to be wide reaching, involving physical spaces such as community pharmacies (not just online), and also be clearly identified as NHS-endorsed to be seen as trustworthy. The emerging evidence of the potential effectiveness of the intervention would need to be widely disseminated, and not just shared with clinicians, to enable women to make decisions about the suitability of the programme for them. The health coach was seen as key to retention as they would offer continuity, being the person with whom participants felt safe and connected.
In terms of engagement with the WLI, the stakeholders stressed that it would be important to consider what outcomes (not just weight loss) would be valued by service users, which could therefore be included as outcomes of any research but also reflected in the advertising. The SAG members identified the following potential benefits of importance: (1) increasing energy, (2) confidence, (3) feeling proud of yourself, (4) feeling healthier, (5) able to exercise, (6) posture and (7) less weight on joints. They also said that the critical shift, the thing that makes the difference between this and other times in a person’s life, is that preparing for family life and doing the best for your baby’s health were seen as powerful psychological motivators, far more so than thinking of one’s own health and well-being alone.
The peer support element of the proposed intervention divided opinion. Some felt that it was really important to be ‘trying to achieve something together’, whereas others felt that this was something that needed to be approached with caution, not just because they found group work unhelpful, but also because non-experts may start offering advice regarding pregnancy. In terms of training practitioners, the group reflected that it is very difficult to train people in fundamental communication skills if they do not have them already; therefore, it is important to advertise the programme so that women know to ask for it, and also to design a script for health-care practitioners to ensure that, as a minimum, the opening of the conversation is done well.
The time frame of the programme was discussed, reflecting on the recent findings from the Prepare trial in the USA,53 and it was suggested that a preconception WLI may need to support women until the end of pregnancy to counteract a rebound of preconception weight loss with greater GWG.
In summary, the LARC user SAG identified important considerations in engagement and recruitment, the centrality of the continuity of care provided by the health coach and the value of ensuring that advertising was widely disseminated not just to let people know about the programme but also to provide knowledge so that service users would be empowered to initiate the conversation with their health-care provider and suggest attending the WLI. The individuality of women’s needs around weight management means that, if possible, tailoring elements of the programme, such as the nature of provision, intensity of support and goals, would be beneficial. The programme would need to offer ‘light-touch’ support after the initial preconception programme.
Nine responses were received across the three questions in the health-care practitioner SAG. The ideas suggested for contacting women in their service included wall posters, waiting room QR codes, a link on the pre-procedure questionnaire sent for all LARC attendances, taking permission for health promotion text messages at registration, a link on the clinic website on the page for pre-pregnancy planning and also on the sexual health website. The general advertising followed similar approaches, with QR codes in toilets, gyms, and so on, but it also involved a social media campaign, including developing community champions. The themes in the responses to the training question echoed those from the interviews, of really working on improving the motivational interviewing training for practitioners and also the central importance of having an effective intervention to refer to. The biggest barrier to opening up the conversation about weight is if nowhere is offering support.
Potential intervention
The outline of a potential WLI for women in the preconception phase and the principles underlying it based on evidence and stakeholder feedback are shown in Figure 11. The WLI is framed as a healthy pregnancy programme for women with BMI of ≥ 30 kg/m2, advertised widely in the community for self-referral but also discussed by practitioners involved in family planning services when working with women of reproductive age who are considering conception in the next 12 months, including those presenting for LARC removal. For women still using contraception, the discussion with the practitioner will include the potential benefits of continuing with contraception for the next 4 months to maximise their chances of losing a clinically significant amount of weight prior to conception. Practitioners would receive clear evidence-based guidance on the benefits of reduced BMI that they could share with their patients, and would also receive training in how to introduce the topic using a loose script co-produced with service users and the research team.
FIGURE 11.
Outline of a potential preconception WLI.
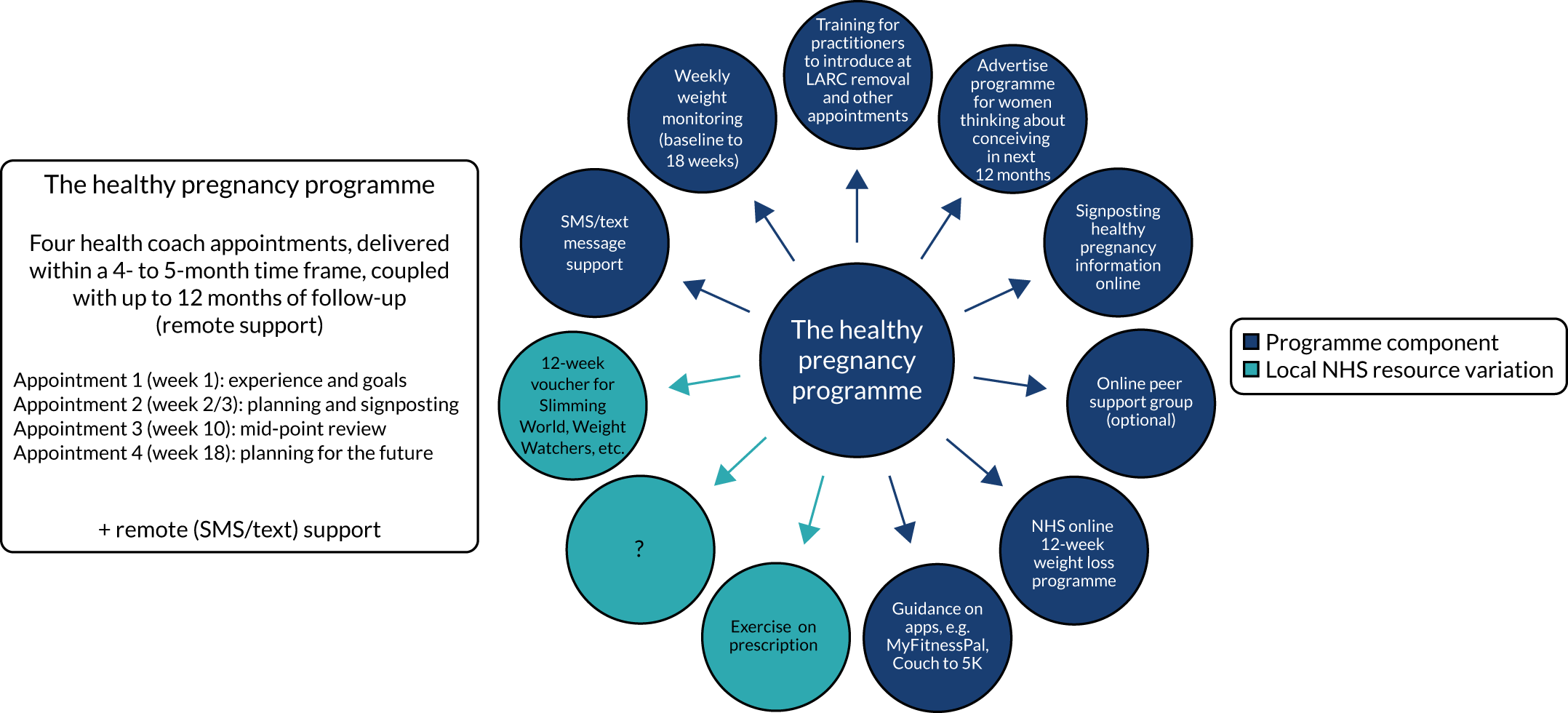
The intervention itself is a signposting intervention, delivered by a health coach who has been trained in motivational interviewing and has an understanding of the evidence-based principles of behaviour change, incorporating planning and goal-setting. With knowledge of the resources available locally and online, the health coach will support the woman to identify her own goals and understand the key components of her experience of successful and unsuccessful weight loss attempts. The information provided will focus on preparation for a healthy pregnancy and will be provided initially via an online platform and then in discussion with the health coach. Signposting to a range of interventions inherently maximises a sense of autonomy, and this will be partnered with support from the health-coach, in person and via text, introducing accountability with weight monitoring. Additional support via a group peer-support network will be offered if requested by the service user. The exact nature of the full programme will be tailored to the individual but also to the resources available locally (e.g. NHS-provided support).
Chapter 8 Discussion
The aim of the Plan-it study was to establish if it would be acceptable and feasible to conduct a study in the future that asked women with overweight/obesity to delay the removal of LARC to participate in a targeted pre-pregnancy WLI.
The main findings of the study will be summarised in relation to the study objectives; these will be followed by a broader discussion, including recommendations for further research.
Main findings
Objective 1: to identify the annual number of women of reproductive age in the UK who request LARC removal and subsequently have a pregnancy
The pathway from LARC removal to pregnancy is not easily captured. The stakeholder narratives and the CPRD routine data show that the reasons for removal are many and varied, of which planning a pregnancy is just one. The current structure of routine data collected through CPRD and SHCs does not enable the sequential relationship between removal of LARC and subsequent pregnancy to be reliably determined. The main barriers include the quality of the routine data, in particular the multiple Read codes relating to LARC use in CPRD and the very limited coding in the Sexual Health data; and the lack of connectivity between SHC and CPRD data, which, given women’s freedom to choose the service they wish to access to remove the LARC (which may well be different from the setting where it was inserted), means that it is not possible to generate a reliable timeline of LARC-related events. Having a ‘clear’ pathway in the data between removal and a pregnancy within 15 months (our assumed definition of ‘subsequent’) may well relate to a minority of the women for whom that is indeed their lived experience.
In conclusion, the relationship between LARC removal and subsequent pregnancy is not clear enough to be able to reliably answer this objective with a number. It would not be feasible, using the current routine data sets relating to LARC use and pregnancy, to reliably identify women who request a LARC removal with the intention of becoming pregnant.
Objective 2: means of identifying women at study sites who are overweight/obese and plan to have LARC removal for the purpose of planning a pregnancy and opportunities to intervene
The barriers identified in objective 1 would also hold true in relation to objective 2. In terms of BMI information, there was an overall average of 62% of women having a BMI recorded within 3 years of a LARC event. Of those, 51–54% had a BMI of ≥ 25 kg/m2, which is in line with the National Maternity and Perinatal Audit 2019,1 in which 50.4% of women giving birth had a BMI in the overweight/obese category, suggesting that there is no particular skew towards higher rates of recording of higher BMI. This rate of recording could be considered adequate for identifying women at study sites with overweight/obesity. However, the routine data barriers would impede this route in the current circumstances.
The quality of the routine data, in particular the identification of the exact purpose of the consultation, would also preclude using them to identify opportunities to intervene and introduce the topic of weight. Even if the data were more reliable, it is questionable whether using LARC-related events would be acceptable as an opportunity to intervene. When the idea of opportunities to talk about weight was explored with stakeholders, opinions ranged from feeling that weight should only ever be raised by the woman to feeling that any consultation was potentially an opportunity. Insertion was the most commonly identified LARC-related opportunity in which it felt appropriate for weight to be discussed. However, many others felt that any LARC event was a situation in which women were physically and emotionally vulnerable, and, therefore, introducing the topic of weight was not appropriate.
To conclude, from the routine data it is not possible to reliably identify women with overweight/obesity and who plan to have LARC removed for the purpose of planning a pregnancy. The acceptability of the intervention to stakeholders (described under objectives 3–5 in the following sections) would also preclude this approach to identifying opportunities to intervene.
Objective 3: suitable and acceptable interventions that can be incorporated into a pre-pregnancy weight loss intervention
The published research on preconception weight loss is dominated by studies relating to IVF that focus most commonly on hypocaloric deficit with pregnancy as the primary outcome. IVF as the context of the studies puts the emphasis on the relationship between BMI and conception, in relation to either the BMI threshold for eligibility for IVF or exploring the relationship between BMI and fertility. There is rarely any detail of support on offer or theoretical underpinning of any intervention other than the biological mechanisms relating to calorie deficit or fertility. These studies provide useful information on the level of weight loss possible with this very motivated group. However, the issue of fertility is the dominant narrative and so the studies may not offer direct guidance on the suitability of particular interventions for a broader population.
The literature on interventions in managing GWG is significantly more developed in terms of details, models, theoretical underpinning and so on. The key crossover with the preconception context is in terms of motivation for change, with our stakeholders citing the health of the baby and their own health in caring for their child as prime motivators for behaviour change at this particular point in their lives. It is useful to incorporate the evidence of the effective mechanisms underpinning intervention design in pregnancy-focused studies into a preconception WLI, with planning and feedback/monitoring being key to success. 153 Some of the barriers to WLI in pregnancy, in particular the reduction in effectiveness of physical activity interventions,117,159 would need to be considered, that is to say whether or not a preconception WLI should follow protocols closer to those of general adult or pregnancy WLIs.
The review of the literature and relevant middle-range theories using realist principles, combined with the stakeholder experiences, generated seven CMO configurations that together create a potential programme theory. A model of a signposting intervention is proposed, with focused preconception content delivered via online modules and individual sessions with a health coach. The use of a signposting model seeks to address the question of whether the intervention should be a specialist preconception WLI or essentially add bespoke content to a more generic adult WLI. The stakeholders were really supportive of the idea of not reinventing the weight loss programme wheel; there are many generic interventions available that women have used, are using or would be happy to explore. However, preconception is a special context, so the intervention requires tailoring to the preconception/pregnancy context with elements of individualised support via a health coach. This would include nutritional and physical activity information about preparation for pregnancy, motivation for change, understanding the goals in terms of clinical impact of the weight loss on perinatal health, time frames and so on. These elements came through several of the CMO configurations in the review, identifying the importance of sensitive messaging during the recruitment process so that women know that the intervention is for them (CMOs 1 and 4), that their experience (positive and negative) of weight management will be recognised (CMO 2) and that it is fit for purpose in a preconception context (CMO 5).
Objective 4: willingness of clinicians to raise weight loss in consultations with eligible women and recruit them to the intervention
The majority of clinicians reported that they were willing to raise the topic of weight in consultations, seeing discussing this as part of their role either in relation to contraceptive choices (in SHCs) or as part of general health (in primary care roles). However, practitioners reported many barriers to these discussions (in line with the published literature160,161), which ranged from the practical, in terms of time, to the sensitivity of the topic, their skills and also broader ethical concerns. These concerns included whether it was their role to discuss weight if this was not directly related to contraceptive options (e.g. if a woman’s BMI prohibited certain choices) and whether weight may be such a complex issue that a woman needed to raise the topic herself.
Over 60% of practitioners would have considered recruiting to a WLI that focused on delaying LARC removal. This would have been facilitated by information on the programme, skills training in the recruitment approach and evidence of potential effectiveness of the intervention. However, practitioners identified significant barriers that resonated with LARC users’ views, which included those to discussing weight in general (described under objective 3) but also the critical issue that the discussion was inappropriate during the LARC removal appointment. They had concerns about the impact on the woman, their professional relationship, the feasibility of recruiting in their service context with their skills/role and, generally, that weight discussions ought to be patient-initiated.
Objective 5: views of eligible women as to the acceptability and feasibility of the intervention
The views of the women who completed the survey, all of whom had experience of having a raised BMI and using LARC, were divided fairly evenly on the question of the acceptability of this WLI in its basic form, of delayed LARC removal. The argument could be made that, with nearly 40% of the respondents thinking that this would be an acceptable model of intervention, it might be worth pursuing as an option, as it may suit some people. The themes that emerged from the free-text comments, the phase 1 SAG and the interviews help to clarify the context of that ‘acceptability’. The core conditions for the intervention’s acceptability included the need for it to be approached sensitively, using a person-centred approach that respectfully acknowledged their history and difficulties with weight, their ambivalence and so on, and also in a way that puts them in control of the decision-making with a positive focus. Delaying LARC removal is not something that would be embraced enthusiastically; it is something that could potentially be tolerated with the importance of the end goal in mind. However, as well as the barriers relating to communication, there were also practical and ethical considerations, ranging from concerns about the practicalities of getting another appointment to a view that this was an unethical intervention that damaged the woman’s right to choose when she could conceive.
Across the two phases of the study, the stakeholder responses led to the conclusion that, in its basic form, an intervention comprising the delay of LARC removal in order to take part in a weight loss programme prior to conception would not be acceptable or feasible. However, including this as one option in a preconception health and weight loss programme that is designed with the key principle of informed choice at its heart would be acceptable and potentially feasible if it also followed the other aspects of the proposed programme theory. This is discussed in objective 6.
Objective 6: future potential intervention based on feasibility and acceptability to stakeholders
A potential preconception WLI has been proposed, designed as part of a healthy pregnancy programme based on feasibility and acceptability to stakeholders and informed by the hypothesised programme theory. It is based on a broad population-based recruitment approach, with individual meetings with a health coach, whose work would be founded on the principles of the COM-B model. 110 Their role would be supporting women to feel competent and confident in relation to their weight across the preconception period and pregnancy and signposting to existing programmes, supplemented by tailored online information. The proposed WLI recruitment incorporates the opportunity for discussion of options presented by LARC removal, but, in recognition of all of the ethical and pragmatic complexities of making that the sole focus, the idea of delaying removal is one potential choice and the eligibility criteria would be much wider. Short-term interventions that simply focus on a relatively brief preconception period have recently been shown53 to be unlikely to work if the aim is to impact on health and weight management throughout the pregnancy rather than, at best, in the first trimester. Therefore, although the focus of the intervention is on introducing change in a 12- to 16-week preconception period, a form of support needs to be incorporated to support women to consolidate the changes over a longer time frame.
Weight management and weight in relation to health are very complex contexts. Ranging from the highly personal to the wider population system, there are layers of meaning to weight that go well beyond the simple ‘calories in, calories out’ model that is often reflected in the interventions delivered. Similarly, LARC removal is not a straightforward procedural opportunity; it is an appointment that has been made in response to an intricate web of life choices and decisions, some of which may relate to pregnancy. Individuals’ beliefs about the health impact of weight and how it fits with other health issues is critical to their engagement with any weight intervention, and this is true for both the service user and the practitioner. One of the messages that came through from the stakeholder work was the current conceptual separation of weight from other components of a healthy pregnancy (e.g. smoking, folic acid). Similarly, there was representation in both groups of stakeholders of the view that contraception is not part of wider health and that somehow it is a separate issue. To improve preconception health, programmes need to reflect this complexity and, as with the potential programme theory described here, draw on middle-range theories that incorporate individual- and systems-level change. As identified by Bull and Willumsen162 in their review of evidence to prevent childhood obesity via the continuum of preconception, pregnancy and postnatal interventions, no single intervention will suffice; action is needed at multiple levels and a life course approach needs to be taken. Preconception programmes must incorporate both individual and societal components, underpinned by socioecological as well as individual behaviour change theories, if we are not simply going to continue making minor changes and exacerbate the social inequities that already exist in nutritional and behavioural aspects of preconception health. 27,29
The ethical considerations in delaying LARC removal as part of the original proposed intervention interlaced two complex sociopolitical issues in weight and conception, which can be captured by the concept of a woman’s right to choose. Although there was never any intention that the intervention would remove women’s choices, the underlying message that some stakeholders identified was discriminatory, suggesting that women with a raised BMI should not become pregnant, and there was perceived to be a risk that women would feel pressured to participate, however unintentionally, by the practitioner’s description of the option to delay LARC removal.
In many ways, preconception does not need a dedicated WLI. Most, if not all, general population WLIs would be suitable or could be adapted. The key differences include the unique primary motivation to succeed; the intervention can also be wrapped in preconception health provision that offers advice on a wider range of important health issues. The stakeholders seemed to feel that this would be more acceptable. However, the reason for this must be considered, along with whether it is a positive step to, in some ways, disguise the focus on weight to make it acceptable. One of the barriers to any new programme, as identified by our stakeholders, is difficulty engaging people, as they need to know that their effort will be rewarded. Being clear about the evidence (e.g. relating to health gains from a 5–10% weight loss) and also about what support will be provided would be positive steps to improving engagement.
Both groups of stakeholders recognised the need for greater awareness of the importance of weight and preconception health at a population level. It may be that investment in a public health campaign (e.g. as suggested by Barker et al. 29 and Stephenson et al. 27) has more of a lasting impact, changing the attitudes and awareness of future generations, as well as those of people who are currently of reproductive age. As identified by Hemsing et al. 37 in a review of preconception interventions, taking a more population-based approach, using gender transformative principles, would also benefit everyone (given the general health benefits of the suggested behaviour changes) and reduce the stigma experienced by women who feel solely responsible for the health of their baby.
Strengths
The main strengths of this study are the use of routine data to consider feasibility, the central involvement of key stakeholders in exploring the acceptability of the idea without incurring the expense of a feasibility pilot study, and the use of a realist-informed approach to reviewing the literature.
The work on the routine data revealed just how difficult it is to use these data to answer some questions that, on the face of it, could seem quite simple. The combination of the multiple and duplicate codes and the way in which practitioners use the codes made for a very dense data set that necessitated a lot of work to decipher. The lack of crossover between the data sets of primary care and sexual health services adds another layer of complexity, and individuals’ movements between providers of contraceptive services makes it impossible to draw firm conclusions. This was a very thorough exploration, which has established that the data sets as they currently stand could not be used as a way of identifying particular populations for this type of intervention.
The stakeholders were involved and engaged at each step of the study. The research questions were predetermined in the commission call, but our PPI partners were part of the study development, the study management and study steering groups. We had substantial engagement from women with lived experience of LARC use and raised BMI, initially from 243 women in their responses to the survey, 173 (71.2%) of whom agreed to be contacted for further involvement in the project. The survey consisted largely of open questions and open text, which meant that women could give us detailed accounts of their experiences. These informed the design of the phase 2 interviews, with input from the SAGs. The power of the women’s testimony informed all of the components of the programme theory, but, in particular, it ensured that the concept of informed choice, which did not come through in the published literature, was put at the heart of the proposed intervention.
Similarly, the project was also informed and the outcomes shaped by the engagement with practitioners. Meeting with practitioners at professional events and asking for their time in that setting was a successful and pragmatic way to engage with a large number of health-care staff. Their responses to the survey and during the interviews really brought to life the experiences of working in this area and, in particular, the barriers to any weight-related intervention. The findings resonated with and extended the research literature in the field and highlighted several areas that merit exploration in future research.
Most of the weight-related preconception interventions lacked a theoretical rationale or any form of process evaluation. The review of the literature informed by realist principles allowed the team to capture relevant work using a much more exploratory lens that highlighted these gaps and started the process of theory building alongside the stakeholder work. These components of the study will be of use in any intervention development work that follows.
Limitations
Given that COVID-19 struck half-way through the study, it was fortunate that much of the second half of the study had been designed to be delivered remotely and so the protocol did not require substantial revision. However, the pandemic did have a significant impact on the range of feedback that was available in both of the LARC user SAGs and the phase 2 practitioner SAG. Had these events been face to face as planned, the engagement and feedback would undoubtedly have been more extensive.
We designed the study to use routine data as this was thought to be the best way to answer the research questions about the size of the potential population, but unfortunately the quality of the data set was not good enough to generate the answers with any degree of reliability. The rationale for using non-NHS-based recruitment was to maximise the extent and reach of recruitment within the tight timescale of the study. However, despite our best efforts at advertising and use of social media, including costing in the advice of a communications officer from a public health organisation, the response rate to the survey from LARC users was not as high as we had hoped. This may have been because the specificity of our target group made the message difficult to convey succinctly. Those who did respond were knowledgeable about the risks related to weight and pregnancy, which suggests that they were not typical of the general population, so it may be that including recruitment via the NHS contraception and weight management clinics would have added a different perspective. We did not collect demographics in the survey because we did not want this to be a barrier to participation, but it would have been valuable information. We know that a significant proportion of the stakeholder responses came in following advertisements on Facebook and Healthwise Wales, so the cultural diversity of our group is likely to have been limited, which in turn limits the generalisability of the findings.
The engagement with practitioners also had its limitations. Despite dialogues with several commercial weight loss organisations, they were unwilling to circulate the survey to their weight loss consultants, and so our response from practitioners for whom weight loss support was part of their role was very restricted. Approaching health-care practitioners at professional events was a very successful way of engaging with them, but this could have been further enhanced by asking participants if they were willing to take part in an interview at a later date (rather than at the event, as originally planned). Recruiting through professional events also meant that the contributors tended to be those who had a lot of experience and also were from a medical background. The views of nurses and those newer to the profession were therefore less well represented. This could potentially have been mitigated by recruiting via the NHS or through the FSRH.
The search for suitable interventions focused on the research directly relevant to preconception, which we have identified as lacking in terms of theoretical principles and process evaluation. This aspect of the study could potentially have been enhanced by exploring the more population-based weight loss research, extracting key mechanisms of action and considering how these might operate in the context of preconception.
Further research
It would seem that, until very recently, this area of research was dominated by physical outcomes (i.e. weight loss, GWG, conception, etc.) rather than the woman’s experience of the intervention and the practitioner’s role in implementation. More mixed-methods studies are required with a much greater level of service user involvement. If a preconception WLI is to be successful, we need to have a much more nuanced understanding of people’s perceptions of the relationship between weight and health in pregnancy. This includes practitioners, who in their responses acknowledged that their knowledge was limited, and it is known [e.g. from the work of the Winton Centre (Cambridge, UK; URL: https://wintoncentre.maths.cam.ac.uk)] that medical practitioners’ risk communication is generally very poor. There is also the conceptual separation for stakeholders between contraception and health, which would need to be understood if SHCs were to be used for recruitment to a preconception intervention. There is clearly a need to explore not simply the practical barriers, but also how to address the attitudinal, knowledge and communication barriers that prevent these discussions taking place successfully. This needs to be a priority as, unless these barriers are reduced or removed and the quality of the communication is improved, no population-based preconception WLI based in the NHS will be feasible.
Therefore, the research priorities would be:
-
How do people of reproductive age and health-care practitioners delivering family planning and preconception services understand the role of weight in a healthy pregnancy and labour?
-
What are the best ways to discuss weight (including issues of risk) in relation to pregnancy in the preconception phase as part of routine health-care practice in order to meet the health and well-being needs of the woman and her family?
Given the low recruitment rates and extremely high attrition rates in many of the studies, the woman’s experience of preconception interventions seems to be a crucial missing piece of the evidence. Assuming that there is often extremely high motivation, for example in the studies that would enable access to fertility treatment, the low recruitment and retention rates are a surprise, but with no clear explanations, as the woman’s experience is not included in published research. Every trial should include a process evaluation to understand the barriers to and facilitators of engagement.
Conclusions
At the present time, developing an intervention that asks women with a raised BMI to delay removal of LARC to participate in a targeted preconception WLI would be neither feasible nor acceptable. However, contraception-related appointments, including those for LARC removal, do offer an opportunity to engage in discussions about preparation for pregnancy. They could be incorporated into a broader, population-based preconception programme, and one potential model of this type of programme is described. However, more development work is needed before it would be possible to progress to the feasibility and evaluation phases of the Medical Research Council framework of complex intervention development; in particular, some of the barriers to communication about weight in pregnancy must be overcome and significant improvement in the routine data sets brought about, including streamlined coding and links between services. The design of preconception WLIs needs to be informed by theories of change, reflecting the complexity of weight management at this life stage, and the profile of preconception health needs to be raised in the general population for the benefit of all.
Acknowledgements
The authors would like to thank all of the participants in the study who contributed to the survey, took part in interviews and provided valuable feedback during the SAGs.
We are very grateful for the contributions made to the study by the SSC: Dr Michelle McKinley (chairperson), Dr Rachel Rowe, Dr Victoria Moran, Dr Marion Davies (patient representative) and Dr Katie Gillies.
We would like to recognise the contribution of colleagues during the study: Lena Meister for providing administrative support for the study; Oghogho Orife, Epidemiologist, Public Health Wales, for working with us on the data from SHCs in Wales; Laura Hodges, Communications Executive in Public Health Wales, for her contribution to the social media dissemination and communication strategy for the survey; and Dr Aimee Grant for her work on the application.
The Centre for Trials Research receives funding from Health and Care Research Wales and Cancer Research UK.
Contributions of authors
Susan Channon (https://orcid.org/0000-0002-5394-1483) (Senior Research Fellow) was the chief investigator, with overall responsibility for the design, co-ordination and delivery of the study. With the co-investigators, she conceived, designed and led the original grant application. She co-facilitated the SAGs and interviews. She led on drafting report chapters and made substantial contributions to synthesis and write-up of chapters in the report, commented on drafts and outputs of the study and facilitated stakeholder activities.
Elinor Coulman (https://orcid.org/0000-0002-8854-2140) (Research Associate – Study Management) was responsible for study co-ordination, governance and team management. She led on the development and dissemination of the survey; recruited to and co-facilitated the SAGs; co-analysed the qualitative data from the surveys and interviews; co-wrote chapters in the report; and collated, reviewed and commented on the final report.
Rebecca Cannings-John (https://orcid.org/0000-0001-5235-6517) (Senior Research Fellow – Statistics) was responsible for oversight of the statistical analysis and the routine data WP; contributed to the drafting of Chapter 3; and reviewed and commented on the final report.
Josie Henley (https://orcid.org/0000-0002-2709-900X) (Research Associate – Qualitative Methods) was responsible for the qualitative components of the study. They contributed to the development of the survey and interview schedules, co-facilitated the SAGs and conducted stakeholder interviews. They co-analysed the qualitative data from the surveys and interviews; contributed to the drafting of chapters in the report; and reviewed and commented on the final report.
Mandy Lau (https://orcid.org/0000-0001-5894-570X) (Research Associate – Statistics) was responsible for conducting the statistical analysis of the routine data, led on the drafting of Chapter 3 and reviewed and commented on the final report.
Fiona Lugg-Widger (https://orcid.org/0000-0003-0029-9703) (Research Fellow – Routine Data) was responsible for oversight of the routine data WP and acquisition of the routine data; contributed to the routine data analysis and interpretation and the drafting of Chapter 3; and reviewed and commented on the final report.
Heather Strange (https://orcid.org/0000-0002-5758-8445) (Research Associate – Qualitative Methods) was responsible for overseeing the qualitative components of the study. She led on the qualitative analysis plan and policy review; contributed to the development and dissemination of the survey; co-facilitated the SAGs; and reviewed and commented on the final report.
Freya Davies (https://orcid.org/0000-0002-6956-1100) (GP and Clinical Research Fellow) contributed to the original grant application; advised on the management of the study, data analysis and interpretation; and reviewed and commented on the final report.
Julia Sanders (https://orcid.org/0000-0001-5712-9989) (Professor of Clinical Nursing and Midwifery) contributed to the original grant application; advised on the management of the study, data analysis and interpretation; and reviewed and commented on the final report.
Carolina Scherf (https://orcid.org/0000-0002-0208-6317) (Consultant in Sexual and Reproductive Health) contributed to the original grant application; advised on the management of the study, data analysis and interpretation; and reviewed and commented on the final report.
Zoë Couzens (https://orcid.org/0000-0002-2670-9611) (Principal in Public Health) contributed to the original grant application; advised on the management of the study, data analysis and interpretation; and reviewed and commented on the final report.
Leah Morantz (Patient Representative) contributed to the original grant application; advised on the survey, interviews, the management of the study, data analysis and interpretation; and reviewed and commented on the final report.
All authors contributed to the design of the final report and approved the final version.
Publication
Channon S, Coulman E, Cannings-John R, Henley J, Lau M, Lugg-Widger F, et al. The acceptability of asking women to delay removal of a long-acting reversible contraceptive to take part in a preconception weight loss programme: a mixed methods study using qualitative and routine data (Plan-it). BMC Pregnancy Childbirth 2022;22:778.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. The full trial protocol can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- National Maternity and Perinatal Audit Project Team . National Maternity and Perinatal Audit: Clinical Report 2019 2019.
- Government Office for Science . Foresight Report n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/287937/07-1184x-tackling-obesities-future-choices-report.pdf (accessed 13 September 2021).
- Agha M, Agha RA, Sandell J. Interventions to reduce and prevent obesity in pre-conceptual and pregnant women: a systematic review and meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0095132.
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16:621-38. https://doi.org/10.1111/obr.12288.
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290-6. https://doi.org/10.1542/peds.2004-1808.
- Valgeirsdottir H, Vanky E, Sundström-Poromaa I, Roos N, Løvvik TS, Stephansson O, et al. Prenatal exposures and birth indices, and subsequent risk of polycystic ovary syndrome: a national registry-based cohort study. BJOG 2019;126:244-51. https://doi.org/10.1111/1471-0528.15236.
- Jacob CM, Newell ML, Hanson M. Narrative review of reviews of preconception interventions to prevent an increased risk of obesity and non-communicable diseases in children. Obes Rev 2019;20:5-17. https://doi.org/10.1111/obr.12769.
- Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr 2010;91:1745-51. https://doi.org/10.3945/ajcn.2009.29128.
- Olander EK. Weight management in pregnancy. Nursing in Practice 2015:19-24.
- Cheney K, Berkemeier S, Sim KA, Gordon A, Black K. Prevalence and predictors of early gestational weight gain associated with obesity risk in a diverse Australian antenatal population: a cross-sectional study. BMC Pregnancy Childbirth 2017;17. https://doi.org/10.1186/s12884-017-1482-6.
- Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol 2015;125:133-43. https://doi.org/10.1097/AOG.0000000000000591.
- Amorim AR, Rössner S, Neovius M, Lourenço PM, Linné Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI?. Obesity 2007;15:1278-86. https://doi.org/10.1038/oby.2007.149.
- Christenson A, Johansson E, Reynisdottir S, Torgerson J, Hemmingsson E. Women’s perceived reasons for their excessive postpartum weight retention: a qualitative interview study. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0167731.
- Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev 2013;7. https://doi.org/10.1002/14651858.CD005627.pub3.
- Christiansen PK, Skjøth MM, Rothmann MJ, Vinter CA, Lamont RF, Draborg E. Lifestyle interventions to maternal weight loss after birth: a systematic review. Syst Rev 2019;8. https://doi.org/10.1186/s13643-019-1186-2.
- National Institute for Health and Care Excellence (NICE) . Weight Management Before, During and After Pregnancy 2010. www.nice.org.uk/guidance/ph27/resources/weight-management-before-during-and-after-pregnancy-pdf-1996242046405 (accessed 13 September 2021).
- Centre for Maternal and Child Enquiries, Royal College of Obstetricians and Gynaecologists . CMACE/RCOG/Joint/Guideline:/Management/of/Women/With/Obesity/in/Pregnancy 2010. www.rcog.org.uk/globalassets/documents/guidelines/cmacercogjointguidelinemanagementwomenobesitypregnancya.pdf (accessed 13 September 2021).
- Royal College of Obstetricians and Gynaecologists . Care of Women With Obesity in Pregnancy (Green-Top Guidelines No. 72) 2018. www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg72/ (accessed 13 September 2021).
- American College of Obstetricians and Gynecologists . Weight Gain During Pregnancy n.d. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2013/01/weight-gain-during-pregnancy (accessed 11 May 2021).
- McAuliffe FM, Killeen SL, Jacob CM, Hanson MA, Hadar E, McIntyre HD, et al. Management of prepregnancy, pregnancy, and postpartum obesity from the FIGO Pregnancy and Non-Communicable Diseases Committee: a FIGO (International Federation of Gynecology and Obstetrics) guideline. Int J Gynaecol Obstet 2020;151:16-3. https://doi.org/10.1002/ijgo.13334.
- International Weight Management in Pregnancy Collaborative Group . Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358. https://doi.org/10.1136/bmj.j3119.
- Farpour-Lambert NJ, Ells LJ, Martinez de Tejada B, Scott C. Obesity and weight gain in pregnancy and postpartum: an evidence review of lifestyle interventions to inform maternal and child health policies. Front Endocrinol 2018;9. https://doi.org/10.3389/fendo.2018.00546.
- Walker R, Bennett C, Blumfield M, Gwini S, Ma J, Wang F, et al. Attenuating pregnancy weight gain – what works and why: a systematic review and meta-analysis. Nutrients 2018;10. https://doi.org/10.3390/nu10070944.
- Tomedi LE, Simhan HN, Chang CC, McTigue KM, Bodnar LM. Gestational weight gain, early pregnancy maternal adiposity distribution, and maternal hyperglycemia. Matern Child Health J 2014;18:1265-70. https://doi.org/10.1007/s10995-013-1361-3.
- Brunner S, Stecher L, Ziebarth S, Nehring I, Rifas-Shiman SL, Sommer C, et al. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: a meta-analysis. Diabetologia 2015;58:2229-37. https://doi.org/10.1007/s00125-015-3686-5.
- World Health Organization . Maximising the Gains for Maternal and Child Health n.d. www.who.int/publications/i/item/WHO-FWC-MCA-13.02 (accessed 13 September 2021).
- Stephenson J, Heslehurst N, Hall J, Schoenaker DAJM, Hutchinson J, Cade JE, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018;391:1830-41. https://doi.org/10.1016/S0140-6736(18)30311-8.
- Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018;391:1842-52. https://doi.org/10.1016/S0140-6736(18)30312-X.
- Barker M, Dombrowski SU, Colbourn T, Fall CHD, Kriznik NM, Lawrence WT, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018;391:1853-64. https://doi.org/10.1016/S0140-6736(18)30313-1.
- Stephenson J, Vogel C, Hall J, Hutchinson J, Mann S, Duncan H, et al. Preconception health in England: a proposal for annual reporting with core metrics. Lancet 2019;393:2262-71. https://doi.org/10.1016/S0140-6736(19)30954-7.
- Hill B, Hall J, Skouteris H, Currie S. Defining preconception: exploring the concept of a preconception population. BMC Pregnancy Childbirth 2020;20. https://doi.org/10.1186/s12884-020-02973-1.
- Hill B, Skouteris H, Boyle JA, Bailey C, Walker R, Thangaratinam S, et al. Health in Preconception, Pregnancy and Postpartum Global Alliance: international network pregnancy priorities for the prevention of maternal obesity and related pregnancy and long-term complications. J Clin Med 2020;9. https://doi.org/10.3390/jcm9030822.
- Caut C, Leach M, Steel A. Dietary guideline adherence during preconception and pregnancy: a systematic review. Matern Child Nutr 2020;16. https://doi.org/10.1111/mcn.12916.
- Inskip HM, Crozier SR, Godfrey KM, Borland SE, Cooper C, Robinson SM, et al. Women’s compliance with nutrition and lifestyle recommendations before pregnancy: general population cohort study. BMJ 2009;338. https://doi.org/10.1136/bmj.b481.
- Stephenson J, Patel D, Barrett G, Howden B, Copas A, Ojukwu O, et al. How do women prepare for pregnancy? Preconception experiences of women attending antenatal services and views of health professionals. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0103085.
- Hussein N, Kai J, Qureshi N. The effects of preconception interventions on improving reproductive health and pregnancy outcomes in primary care: a systematic review. Eur J Gen Pract 2016;22:42-5. https://doi.org/10.3109/13814788.2015.1099039.
- Hemsing N, Greaves L, Poole N. Preconception health care interventions: a scoping review. Sex Reprod Healthc 2017;14:24-32. https://doi.org/10.1016/j.srhc.2017.08.004.
- Delissaint D, McKyer EL. A systematic review of factors utilized in preconception health behavior research. Health Educ Behav 2011;38:603-16. https://doi.org/10.1177/1090198110389709.
- Ezegwui HU, Dim C, Dim N, Ikeme AC. Preconception care in South Eastern Nigeria. J Obstet Gynaecol 2008;28:765-8. https://doi.org/10.1080/01443610802462647.
- Wallace M, Hurwitz B. Preconception care: who needs it, who wants it, and how should it be provided?. Br J Gen Pract 1998;48:963-6.
- Barrett G, Shawe J, Howden B, Patel D, Ojukwu O, Pandya P, et al. Why do women invest in pre-pregnancy health and care? A qualitative investigation with women attending maternity services. BMC Pregnancy Childbirth 2015;15. https://doi.org/10.1186/s12884-015-0672-3.
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753-61. https://doi.org/10.1056/NEJMoa066603.
- Cunningham J, Endacott R, Gibbons D. Communication with health professionals: the views of pregnant women with a raised BMI. Br J Midwifery 2018;26:598-604. https://doi.org/10.12968/bjom.2018.26.9.598.
- Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab 2015;100:4048-58. https://doi.org/10.1210/jc.2015-2778.
- Harden SM, Ramalingam NS, Wilson KE, Evans-Hoeker E. Informing the development and uptake of a weight management intervention for preconception: a mixed-methods investigation of patient and provider perceptions. BMC Obes 2017;4. https://doi.org/10.1186/s40608-017-0144-6.
- Opray N, Grivell RM, Deussen AR, Dodd JM. Directed preconception health programs and interventions for improving pregnancy outcomes for women who are overweight or obese. Cochrane Database Syst Rev 2015;7. https://doi.org/10.1002/14651858.CD010932.pub2.
- Furber CM, McGowan L, Bower P, Kontopantelis E, Quenby S, Lavender T. Antenatal interventions for reducing weight in obese women for improving pregnancy outcome. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD009334.pub2.
- Lan L, Harrison CL, Misso M, Hill B, Teede HJ, Mol BW, et al. Systematic review and meta-analysis of the impact of preconception lifestyle interventions on fertility, obstetric, fetal, anthropometric and metabolic outcomes in men and women. Hum Reprod 2017;32:1925-40. https://doi.org/10.1093/humrep/dex241.
- Weisman CS, Hillemeier MM, Downs DS, Feinberg ME, Chuang CH, Botti JJ, et al. Improving women’s preconceptional health: long-term effects of the Strong Healthy Women behavior change intervention in the central Pennsylvania Women’s Health Study. Womens Health Issues 2011;21:265-71. https://doi.org/10.1016/j.whi.2011.03.007.
- Bandura A, Vasta R. Annals of Child Development: Volume 6. Greenwich, CT: JAI Press; 1989.
- Hutchesson MJ, de Jonge Mulock Houwer M, Brown HM, Lim S, Moran LJ, Vincze L, et al. Supporting women of childbearing age in the prevention and treatment of overweight and obesity: a scoping review of randomized control trials of behavioral interventions. BMC Womens Health 2020;20. https://doi.org/10.1186/s12905-020-0882-3.
- LeBlanc ES, Vesco KK, Funk KL, Karanja N, Smith N, Stevens VJ. Prepare, a randomized trial to promote and evaluate weight loss among overweight and obese women planning pregnancy: study design and rationale. Contemp Clin Trials 2016;49:174-80. https://doi.org/10.1016/j.cct.2016.07.002.
- LeBlanc ES, Smith N, Vesco KK, Paul IM, Stevens VJ. Weight loss prior to pregnancy and subsequent gestation weight gain: Prepare, a randomised clinical trial. Am J Obstet Gynaecol 2021;224. https://doi.org/10.1016/j.ajog.2020.07.027.
- Maas VYF, Koster MPH, Ista E, Vanden Auweele KLH, de Bie RWA, de Smit DJ, et al. Study design of a stepped wedge cluster randomized controlled trial to evaluate the effect of a locally tailored approach for preconception care – the APROPOS-II study. BMC Public Health 2020;20. https://doi.org/10.1186/s12889-020-8329-1.
- NHS Digital . Statistics on Obesity, Physical Activity and Diet, England, 2020 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/england-2020/part-3-adult-obesity-copy (accessed 11 May 2021).
- Phelan S. Pregnancy: a ‘teachable moment’ for weight control and obesity prevention. Am J Obstet Gynecol 2010;202:135.e1-8. https://doi.org/10.1016/j.ajog.2009.06.008.
- Poels M, Koster MPH, Franx A, van Stel HF. Parental perspectives on the awareness and delivery of preconception care. BMC Pregnancy Childbirth 2017;17. https://doi.org/10.1186/s12884-017-1531-1.
- Callaway LK, O’Callaghan MJ, McIntyre HD. Barriers to addressing overweight and obesity before conception. Med J Aust 2009;191:425-8.
- NHS Digital . Health Survey for England 2019 n.d. https://files.digital.nhs.uk/EF/AB0F0C/HSE17-Adult-Child-BMI-rep-v2.pdf (accessed 11 May 2021).
- NHS Information Centre . Health Survey for England 2004: Health of Minority Ethnic Groups 2006.
- Gatineau M, Mathrani S. Ethnicity and obesity in the UK. Perspect Public Health 2011;131:159-60. https://doi.org/10.1177/1757913911412478.
- National Institute for Health and Care Excellence (NICE) . BMI: Preventing Ill Health and Premature Death in Black, Asian and Other Minority Ethnic Groups 2013. https://www.nice.org.uk/guidance/PH46 (accessed 11 May 2021).
- Taylor C, Bhavnani V, Zasada M, Ussher M, Bick D. SWAN trial team . Barriers and facilitators to uptake and retention of inner-city ethnically diverse women in a postnatal weight management intervention: a mixed-methods process evaluation within a feasibility trial in England. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-034747.
- Tuomainen H, Cross-Bardell L, Bhoday M, Qureshi N, Kai J. Opportunities and challenges for enhancing preconception health in primary care: qualitative study with women from ethnically diverse communities. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002977.
- Stotland NE, Gilbert P, Bogetz A, Harper CC, Abrams B, Gerbert B. Preventing excessive weight gain in pregnancy: how do prenatal care providers approach counseling?. J Womens Health 2010;19:807-14. https://doi.org/10.1089/jwh.2009.1462.
- Blackburn M, Stathi A, Keogh E, Eccleston C. Raising the topic of weight in general practice: perspectives of GPs and primary care nurses. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008546.
- Steel A, Lucke J, Reid R, Adams J. A systematic review of women’s and health professional’s attitudes and experience of preconception care service delivery. Fam Pract 2016;33:588-95. https://doi.org/10.1093/fampra/cmw094.
- Keyworth C, Hart J, Armitage CJ, Tully MP. What maximizes the effectiveness and implementation of technology-based interventions to support healthcare professional practice? A systematic literature review. BMC Med Inform Decis Mak 2018;18. https://doi.org/10.1186/s12911-018-0661-3.
- Ajzen I. The theory of planned behaviour. Organ Behav Hum Decision Proc 1991;50:179-211. https://doi.org/10.1016/0749-5978(91)90020-T.
- Downs DS, Feinberg M, Hillemeier MM, Weisman CS, Chase GA, Chuang CH, et al. Design of the Central Pennsylvania Women’s Health Study (CePAWHS) strong healthy women intervention: improving preconceptional health. Matern Child Health J 2009;13:18-2. https://doi.org/10.1007/s10995-008-0323-7.
- Hillemeier MM, Downs DS, Feinberg ME, Weisman CS, Chuang CH, Parrott R, et al. Improving women’s preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania women’s health study. Womens Health Issues 2008;18:87-96. https://doi.org/10.1016/j.whi.2008.07.008.
- Materia FT, Smyth JM, Heron KE, Hillemeier M, Feinberg ME, Fonzi P, et al. Preconceptional health behavior change in women with overweight and obesity: prototype for SMART strong healthy women intervention. Mhealth 2018;4. https://doi.org/10.21037/mhealth.2018.06.06.
- Bogaerts A, Bijlholt M, Mertens L, Braeken M, Jacobs B, Vandenberghe B, et al. Development and field evaluation of the INTER-ACT App, a pregnancy and interpregnancy coaching app to reduce maternal overweight and obesity: mixed methods design. JMIR Form Res 2020;4. https://doi.org/10.2196/16090.
- Skau JK, Nordin AB, Cheah JC, Ali R, Zainal R, Aris T, et al. A complex behavioural change intervention to reduce the risk of diabetes and prediabetes in the pre-conception period in Malaysia: study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1345-x.
- Timmermans YEG, van de Kant KDG, Krumeich JSM, Zimmermann LJI, Dompeling E, Kramer BW, et al. Socio-ecological determinants of lifestyle behavior of women with overweight or obesity before, during and after pregnancy: qualitative interview analysis in the Netherlands. BMC Pregnancy Childbirth 2020;20. https://doi.org/10.1186/s12884-020-2786-5.
- ClinicalTrials.gov . Healthy for My Baby – RCT of a Lifestyle Intervention for Overweight Women in Preconception n.d. https://clinicaltrials.gov/ct2/show/NCT04242069 (accessed 1 May 2021).
- Gould Rothberg BE, Magriples U, Kershaw TS, Rising SS, Ickovics JR. Gestational weight gain and subsequent postpartum weight loss among young, low-income, ethnic minority women. Am J Obstet Gynecol 2011;204:52.e1-11. https://doi.org/10.1016/j.ajog.2010.08.028.
- ClinicalTrials.gov . Preconception and Pregnancy Obesity Treatment and Prevention Among Women With a History of Depression 2017. https://clinicaltrials.gov/ct2/show/NCT03053323 (accessed 9 May 2021).
- Phelan S, Jelalian E, Coustan D, Caughey AB, Castorino K, Hagobian T, et al. Protocol for a randomized controlled trial of pre-pregnancy lifestyle intervention to reduce recurrence of gestational diabetes: Gestational Diabetes Prevention/Prevención de la Diabetes Gestacional. Trials 2021;22. https://doi.org/10.1186/s13063-021-05204-w.
- Brackenridge L, Finer N, Batterham RL, Pedram K, Ding T, Stephenson J, et al. Pre-pregnancy weight loss in women with obesity requesting removal of their intra-uterine contraceptive device in order to conceive: a pilot study of full meal replacement. Clin Obes 2018;8:244-9. https://doi.org/10.1111/cob.12252.
- Arrowsmith ME, Majeed A, Lee JT, Saxena S. Impact of pay for performance on prescribing of long-acting reversible contraception in primary care: an interrupted time series study. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0092205.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029954.
- Pawson R, Tilley N. Realistic Evaluation. London: SAGE Publications Ltd; 1997.
- General Data Protection Regulation n.d. https://gdpr-info.eu/ (accessed 11 May 2021).
- Pawson R. The Science of Evaluation: A Realist Manifesto. London: SAGE Publications Ltd; 2013.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Minassian C, Williams R, Meeraus WH, Smeeth L, Campbell OMR, Thomas SL. Methods to generate and validate a Pregnancy Register in the UK Clinical Practice Research Datalink primary care database. Pharmacoepidemiol Drug Saf 2019;28:923-33. https://doi.org/10.1002/pds.4811.
- NES Digital Service n.d. www.nationaldigitalplatform.scot (accessed May 2021).
- NHS Digital . Sexual and Reproductive Health Services, England (Contraception) 2019 20 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/sexual-and-reproductive-health-services/2019-20 (accessed 9 May 2021).
- Channon S, Coulman E, Cannings-John R, Henley J, Lau M, Lugg-Widger F, et al. The acceptability of asking women to delay removal of a long-acting reversible contraceptive to take part in a preconception weight loss programme: a mixed methods study using qualitative and routine data (Plan-it). BMC Pregnancy Childbirth 2022;22.
- Bhaskaran K, Forbes HJ, Douglas I, Leon DA, Smeeth L. Representativeness and optimal use of body mass index (BMI) in the UK Clinical Practice Research Datalink (CPRD). BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003389.
- Cea Soriano L, Wallander MA, Andersson S, Filonenko A, García Rodríguez LA. The continuation rates of long-acting reversible contraceptives in UK general practice using data from The Health Improvement Network. Pharmacoepidemiol Drug Saf 2015;24:52-8. https://doi.org/10.1002/pds.3710.
- RECORD . What Is RECORD? n.d. www.record-statement.org/ (accessed 9 April 2021).
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60. https://doi.org/10.1177/1049732315617444.
- Healthwise Wales n.d. www.healthwisewales.gov.wales (accessed 9 April 2021).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Denison FC, Aedla NR, Keag O, Hor K, Reynolds RM, Milne A, et al. Care of Women with Obesity in Pregnancy: Green-top Guideline No. 72. BJOG 2019;126:e62-e106. https://doi.org/10.1111/1471-0528.15386.
- Faculty of Sexual and Reproductive Healthcare (FSRH) . FSRH Clinical Guideline: Intrauterine Contraception 2015.
- Faculty of Sexual and Reproductive Healthcare (FSRH) Clinical Effectiveness Unit . Provision of LARC Methods to Young Women in the UK: CEU Statement 2015.
- Royal College of Obstetrician and Gyneacologists . RCOG Co-Launches New Digital Tool to Help Prepare Women for Pregnancy n.d. www.rcog.org.uk/en/news/rcog-co-launches-new-digital-tool-to-help-prepare-women-for-pregnancy/ (accessed 28 April 2021).
- Jackson SF, Kolla G. A new realistic evaluation analysis method: linked coding of context, mechanism, and outcome relationships. Am J Eval 2012;33:339-49. https://doi.org/10.1177/1098214012440030.
- Pawson R. Evidence-based Policy: A Realist Perspective. London: SAGE Publications Ltd; 2006.
- Byng R, Norman I, Redfern S. Using realistic evaluation to evaluate a practice-level intervention to improve primary healthcare for patients with long-term mental illness. Evaluation 2005;11:69-93. https://doi.org/10.1177/1356389005053198.
- Pearson M, Brand SL, Quinn C, Shaw J, Maguire M, Michie S, et al. Using realist review to inform intervention development: methodological illustration and conceptual platform for collaborative care in offender mental health. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0321-2.
- Kislov R, Pope C, Martin GP, Wilson PM. Harnessing the power of theorising in implementation science. Implement Sci 2019;14. https://doi.org/10.1186/s13012-019-0957-4.
- Booth A, Carroll C. Systematic searching for theory to inform systematic reviews: is it feasible? Is it desirable?. Health Info Libr J 2015;32:220-35. https://doi.org/10.1111/hir.12108.
- Truiett-Theodorson R, Tuck S, Bowie JV, Summers AC, Kelber-Kaye J. Building effective partnerships to improve birth outcomes by reducing obesity: the B’more Fit for healthy babies coalition of Baltimore. Eval Program Plann 2015;51:53-8. https://doi.org/10.1016/j.evalprogplan.2014.12.007.
- Liang L, Bernhardsson S, Vernooij RW, Armstrong MJ, Bussières A, Brouwers MC, et al. Use of theory to plan or evaluate guideline implementation among physicians: a scoping review. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0557-0.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Vesco KK, Karanja N, King JC, Gillman MW, Leo MC, Perrin N, et al. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: a randomized trial. Obesity 2014;22:1989-96. https://doi.org/10.1002/oby.20831.
- Hanson M, Barker M, Dodd JM, Kumanyika S, Norris S, Steegers E, et al. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol 2017;5:65-76. https://doi.org/10.1016/S2213-8587(16)30108-5.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Ryan RM, Deci EL. Intrinsic and extrinsic motivations: classic definitions and new directions. Contemp Educ Psychol 2000;25:54-67. https://doi.org/10.1006/ceps.1999.1020.
- Bandura A. Prentice-Hall Series in Social Learning Theory. Social Foundations of Thought and Action: A Social Cognitive Theory. Hoboken, NJ: Prentice-Hall; 1986.
- Redman LM, Gilmore LA, Breaux J, Thomas DM, Elkind-Hirsch K, Stewart T, et al. Effectiveness of SmartMoms, a novel eHealth intervention for management of gestational weight gain: randomized controlled pilot trial. JMIR Mhealth Uhealth 2017;5. https://doi.org/10.2196/mhealth.8228.
- Harrison CL, Brown WJ, Hayman M, Moran LJ, Redman LM. The role of physical activity in preconception, pregnancy and postpartum health. Semin Reprod Med 2016;34:e28-37. https://doi.org/10.1055/s-0036-1583530.
- Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767-77. https://doi.org/10.1016/S2213-8587(15)00227-2.
- Brofenbrenner U. The Ecology of Human Development. Cambridge, MA: Harvard University Press; 1979.
- Hill B. Expanding our understanding and use of the ecological systems theory model for the prevention of maternal obesity: a new socioecological framework. Obes Rev 2021;22. https://doi.org/10.1111/obr.13147.
- Shove E, Pantzar M, Watson M. The Dynamics of Social Practice: Everyday Life and How it Changes. London: SAGE Publications Ltd; 2012.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. on behalf of the ‘Psychological Theory’ Group . Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. https://doi.org/10.1136/qshc.2004.011155.
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-5. https://doi.org/10.1037/0022-006x.51.3.390.
- Ockhuijsen HD, Gamel CJ, van den Hoogen A, Macklon NS. Integrating preconceptional care into an IVF programme. J Adv Nurs 2012;68:1156-65. https://doi.org/10.1111/j.1365-2648.2011.05829.x.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Cowdell F, Dyson J. How is the theoretical domains framework applied to developing health behaviour interventions? A systematic search and narrative synthesis. BMC Public Health 2019;19. https://doi.org/10.1186/s12889-019-7442-5.
- van der Zee B, de Beaufort ID, Steegers EA, Denktas S. Perceptions of preconception counselling among women planning a pregnancy: a qualitative study. Fam Pract 2013;30:341-6. https://doi.org/10.1093/fampra/cms074.
- Squiers L, Mitchell EW, Levis DM, Lynch M, Dolina S, Margolis M, et al. Consumers’ perceptions of preconception health. Am J Health Promot 2013;27:10-9. https://doi.org/10.4278/ajhp.120217-QUAL-95.
- Lang AY, Hall JA, Boyle JA, Harrison CL, Teede H, Moran LJ, et al. Validation of the London Measure of Unplanned Pregnancy among pregnant Australian women. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0220774.
- Shawe J, Patel D, Joy M, Howden B, Barrett G, Stephenson J. Preparation for fatherhood: a survey of men’s preconception health knowledge and behaviour in England. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0213897.
- Goossens J, De Roose M, Van Hecke A, Goemaes R, Verhaeghe S, Beeckman D. Barriers and facilitators to the provision of preconception care by healthcare providers: a systematic review. Int J Nurs Stud 2018;87:113-30. https://doi.org/10.1016/j.ijnurstu.2018.06.009.
- Bortolus R, Oprandi NC, Morassutti FR, Marchetto L, Filippini F, Tozzi AE, et al. Why women do not ask for information on preconception health? A qualitative study. BMC Pregnancy Childbirth 2017;17. https://doi.org/10.1186/s12884-016-1198-z.
- Norris SA, Ho JC, Rashed AA, Vinding V, Skau JK, Biesma R, et al. Pre-pregnancy community-based intervention for couples in Malaysia: application of intervention mapping. BMC Public Health 2016;16. https://doi.org/10.1186/s12889-016-3827-x.
- Greenhalgh T, Clinch M, Afsar N, Choudhury Y, Sudra R, Campbell-Richards D, et al. Socio-cultural influences on the behaviour of South Asian women with diabetes in pregnancy: qualitative study using a multi-level theoretical approach. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0360-1.
- Willcox JC, van der Pligt P, Ball K, Wilkinson SA, Lappas M, McCarthy EA, et al. Views of women and health professionals on mHealth lifestyle interventions in pregnancy: a qualitative investigation. JMIR Mhealth Uhealth 2015;3. https://doi.org/10.2196/mhealth.4869.
- McBride N, Carruthers S, Hutchinson D. Reducing alcohol use during pregnancy: listening to women who drink as an intervention starting point. Glob Health Promot 2012;19:6-18. https://doi.org/10.1177/1757975912441225.
- Rönö K, Stach-Lempinen B, Eriksson JG, Pöyhönen-Alho M, Klemetti MM, Roine RP, et al. Prevention of gestational diabetes with a prepregnancy lifestyle intervention – findings from a randomized controlled trial. Int J Womens Health 2018;10:493-501. https://doi.org/10.2147/IJWH.S162061.
- Van Dijk MR, Koster MP, Rosman AN, Steegers-Theunissen RP. Opportunities of mHealth in preconception care: preferences and experiences of patients and health care providers and other involved professionals. JMIR Mhealth Uhealth 2017;5. https://doi.org/10.2196/mhealth.7834.
- Toivonen KI, Oinonen KA, Duchene KM. Preconception health behaviours: a scoping review. Prev Med 2017;96:1-15. https://doi.org/10.1016/j.ypmed.2016.11.022.
- Flannery C, Fredrix M, Olander EK, McAuliffe FM, Byrne M, Kearney PM. Effectiveness of physical activity interventions for overweight and obesity during pregnancy: a systematic review of the content of behaviour change interventions. Int J Behav Nutr Phys Act 2019;16. https://doi.org/10.1186/s12966-019-0859-5.
- Harelick L, Viola D, Tahara D. Preconception health of low socioeconomic status women: assessing knowledge and behaviors. Womens Health Issues 2011;21:272-6. https://doi.org/10.1016/j.whi.2011.03.006.
- Heslehurst N, Newham J, Maniatopoulos G, Fleetwood C, Robalino S, Rankin J. Implementation of pregnancy weight management and obesity guidelines: a meta-synthesis of healthcare professionals’ barriers and facilitators using the Theoretical Domains Framework. Obes Rev 2014;15:462-86. https://doi.org/10.1111/obr.12160.
- Keyworth C, Epton T, Goldthorpe J, Calam R, Armitage CJ. Delivering opportunistic behaviour change interventions: a systematic review of systematic reviews. Prev Sci 2020;21:319-31. https://doi.org/10.1007/s11121-020-01087-6.
- Khan NN, Boyle JA, Lang AY, Harrison CL. Preconception health attitudes and behaviours of women: a qualitative investigation. Nutrients 2019;11. https://doi.org/10.3390/nu11071490.
- Sim KA, Dezarnaulds GM, Denyer GS, Skilton MR, Caterson ID. Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: a randomized controlled trial. Clin Obes 2014;4:61-8. https://doi.org/10.1111/cob.12048.
- Rothberg A, Lanham M, Randolph J, Fowler C, Miller N, Smith Y. Feasibility of a brief, intensive weight loss intervention to improve reproductive outcomes in obese, subfertile women: a pilot study. Fertil Steril 2016;106:1212-20. https://doi.org/10.1016/j.fertnstert.2016.06.004.
- Legro RS, Dodson WC, Kunselman AR, Stetter CM, Kris-Etherton PM, Williams NI, et al. Benefit of delayed fertility therapy with preconception weight loss over immediate therapy in obese women with PCOS. J Clin Endocrinol Metab 2016;101:2658-66. https://doi.org/10.1210/jc.2016-1659.
- Becker GF, Passos EP, Moulin CC. Short-term effects of a hypocaloric diet with low glycemic index and low glycemic load on body adiposity, metabolic variables, ghrelin, leptin, and pregnancy rate in overweight and obese infertile women: a randomized controlled trial. Am J Clin Nutr 2015;102:1365-72. https://doi.org/10.3945/ajcn.115.117200.
- Espinós JJ, Polo A, Sánchez-Hernández J, Bordas R, Pares P, Martínez O, et al. Weight decrease improves live birth rates in obese women undergoing IVF: a pilot study. Reprod Biomed Online 2017;35:417-24. https://doi.org/10.1016/j.rbmo.2017.06.019.
- Mutsaerts MA, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, et al. Randomized trial of a lifestyle program in obese infertile women. N Engl J Med 2016;374:1942-53. https://doi.org/10.1056/NEJMoa1505297.
- Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts 2015;8:402-24. https://doi.org/10.1159/000442721.
- van Dammen L, Wekker V, van Oers AM, Mutsaerts MAQ, Painter RC, Zwinderman AH, et al. Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life: a randomized controlled trial. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0190662.
- McGirr C, Dombrowski S, Holmes V, McKinley M. What behaviour change techniques are associated with effectiveness of weight management interventions? A systematic review of systematic reviews. Proc Nutr Soc 2017;76. https://doi.org/10.1017/S0029665117001653.
- Hill B, Skouteris H, Fuller-Tyszkiewicz M. Interventions designed to limit gestational weight gain: a systematic review of theory and meta-analysis of intervention components. Obes Rev 2013;14:435-50. https://doi.org/10.1111/obr.12022.
- Madigan CD, Daley AJ, Kabir E, Aveyard P, Brown W. Cluster analysis of behavioural weight management strategies and associations with weight change in young women: a longitudinal analysis. Int J Obes 2015;39:1601-6. https://doi.org/10.1038/ijo.2015.116.
- Sanders J, Channon S, Cannings-John R, Coulman E, Hunter B, Paranjothy S, et al. Pregnancy and weight monitoring: a feasibility study of weight charts and midwife support. Matern Child Nutr 2020;16. https://doi.org/10.1111/mcn.12996.
- Carey RN, Connell LE, Johnston M, Rothman AJ, de Bruin M, Kelly MP, et al. Behavior change techniques and their mechanisms of action: a synthesis of links described in published intervention literature. Ann Behav Med 2019;53:693-707. https://doi.org/10.1111/mcn.12996https://doi.org/10.1093/abm/kay078.
- Miller W, Rollnick S. Motivational Interviewing. Helping People Change. New York, NY: Guildford Press; 2013.
- Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.CD007145.pub3.
- M’hamdi HI, van Voorst SF, Pinxten W, Hilhorst MT, Steegers EA. Barriers in the uptake and delivery of preconception care: exploring the views of care providers. Matern Child Health J 2017;21:21-8. https://doi.org/10.1007/s10995-016-2089-7.
- Mazza D, Chapman A, Michie S. Barriers to the implementation of preconception care guidelines as perceived by general practitioners: a qualitative study. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-36.
- Bull F, Willumsen J. Evidence to prevent childhood obesity: the continuum of preconception, pregnancy, and postnatal interventions. Obes Rev 2019;20:3-4. https://doi.org/10.1111/obr.12844.
- Joint Formulary Committee . British National Formulary 2008.
- NHS Digital . Sexual and Reproductive Health Services – England, 2014 15 to 2018 19 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/sexual-and-reproductive-health-services (accessed 2 August 2022).
- Public Health Scotland . Data Tables: Long Acting Reversible Methods of Contraception (LARC) in Scotland n.d. www.isdscotland.org/Health-Topics/Sexual-Health/Publications/data-tables2017.asp?id=2488#2488 (accessed 12 May 2021).
- Office for National Statistics . Population by Index of Multiple Deprivation Decile, England, 2001 to 2016 2018. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/008868populationbyindexofmultipledeprivationdecileengland2001to2016 (accessed 13 July 2022).
- Faculty of Sexual and Reproductive Healthcare (FSRH) Clinical Effectiveness Unit . FSRH CEU Statement: Contraception and Weight Gain 2019.
- Faculty of Sexual and Reproductive Healthcare (FSRH) . FSRH Clinical Guideline: Contraception for Women Aged over 40 Years 2017.
- Faculty of Sexual and Reproductive Healthcare (FSRH) Clinical Effectiveness Unit . Contraception for Women With Eating Disorders: CEU Statement 2018.
- Faculty of Sexual and Reproductive Healthcare (FSRH) . FSRH Clinical Guideline: Contraception After Pregnancy 2017.
- Faculty of Sexual and Reproductive Healthcare (FSRH) Clinical Effectiveness Unit . FSRH Clinical Guideline: Progestogen-Only Implant 2014.
- Faculty of Sexual and Reproductive Healthcare (FSRH) . FSRH Clinical Guideline: Overweight, Obesity and Contraception 2019.
- National Institute for Health and Care Excellence (NICE) . Long-Acting Reversible Contraception 2005.
- British Pregnancy Advisory Service . Women Cannot Control Fertility Through Contraception Alone n.d. www.bpas.org/about-our-charity/press-office/press-releases/women-cannot-control-fertility-through-contraception-alone-bpas-data-shows-1-in-4-women-having-an-abortion-were-using-most-effective-contraception/ (accessed 28 April 2021).
- Shawe J, Delbaere I, Ekstrand M, Hegaard HK, Larsson M, Mastroiacovo P, et al. Preconception care policy, guidelines, recommendations and services across six European countries: Belgium (Flanders), Denmark, Italy, the Netherlands, Sweden and the United Kingdom. Eur J Contracept Reprod Health Care 2015;20:77-8. https://doi.org/10.3109/13625187.2014.990088.
- Kothe E, Bailey C, Weiner C, Nagle C, Nowson C, Hill B, et al. An investigation of Australian midwifery curricula for obesity management and health behaviour change training. Nurse Educ Pract 2019;36:54-7. https://doi.org/10.1016/j.nepr.2019.03.003.
- Musgrave LM, Homer CSE, Kizirian NV, Gordon A. Addressing preconception behaviour change through mobile phone apps: a protocol for a systematic review and meta-analysis. Syst Rev 2019;8. https://doi.org/10.1186/s13643-019-0996-6.
- Brown HK, Mueller M, Edwards S, Mill C, Enders J, Graves L, et al. Preconception health interventions delivered in public health and community settings: a systematic review. Can J Public Health 2017;108:e388-e397. https://doi.org/10.17269/cjph.108.6029.
- Agricola E, Pandolfi E, Gonfiantini MV, Gesualdo F, Romano M, Carloni E, et al. A cohort study of a tailored web intervention for preconception care. BMC Med Inform Decis Mak 2014;14. https://doi.org/10.1186/1472-6947-14-33.
- Schwarz EB, Sobota M, Gonzales R, Gerbert B. Computerized counseling for folate knowledge and use: a randomized controlled trial. Am J Prev Med 2008;35:568-71. https://doi.org/10.1016/j.amepre.2008.06.034.
- Chan A, Pickering J, Haan E, Netting M, Burford A, Johnson A, et al. ‘Folate before pregnancy’: the impact on women and health professionals of a population-based health promotion campaign in South Australia. Med J Aust 2001;174:631-6. https://doi.org/10.5694/j.1326-5377.2001.tb143471.x.
- DeJoy SB. Pilot test of a preconception and midwifery care promotion program for college women. J Midwifery Womens Health 2014;59:523-7. https://doi.org/10.1111/jmwh.12106.
- Hussaini KS, Hamm E, Means T. Using community-based participatory mixed methods research to understand preconception health in African American communities of Arizona. Matern Child Health J 2013;17:1862-71. https://doi.org/10.1007/s10995-012-1206-5.
- Whitehill King K, Freimuth V, Lee M, Johnson-Turbes CA. The effectiveness of bundled health messages on recall. Am J Health Promot 2013;27:28-35. https://doi.org/10.4278/ajhp.120113-QUAN-27.
- Mackert M, Kim E, Guadagmo M, Donovan-Kicken E. Using Twitter for prenatal health promotion: encouraging a multivitamin habit among college-aged females. Stud Health Technol Inform 2012;182:93-103.
- Milan JE, White AA. Impact of a stage-tailored, web-based intervention on folic acid-containing multivitamin use by college women. Am J Health Promot 2010;24:388-95. https://doi.org/10.4278/ajhp.071231143.
- Wade GH, Herrman J, McBeth-Snyder L. A preconception care program for women in a college setting. MCN Am J Matern Child Nurs 2012;37:164-70. https://doi.org/10.1097/NMC.0b013e31824b59c7.
- Watson M, Watson L, Bell R, Halliday J. The increasing knowledge of the role of periconceptional folate in Victorian women of child-bearing age: follow-up of a randomized community intervention trial. Aust N Z J Public Health 2001;25:389-95. https://doi.org/10.1111/j.1467-842X.2001.tb00280.x.
- Williams P, McHenery J, McMahon A, Anderson H. Impact evaluation of a folate education campaign with and without the use of a health claim. Aust N Z J Public Health 2001;25:396-404. https://doi.org/10.1111/j.1467-842X.2001.tb00281.x.
- Beckmann MM, Widmer T, Bolton E. Does preconception care work?. Aus N Z J Obstetrics Gynaecol 2014;54:510-14. https://doi.org/10.1111/ajo.12224.
- Mutsaerts MA, Groen H, ter Bogt NC, Bolster JH, Land JA, Bemelmans WJ, et al. The LIFESTYLE study: costs and effects of a structured lifestyle program in overweight and obese subfertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomised controlled trial. BMC Womens Health 2010;10. https://doi.org/10.1186/1472-6874-10-22.
- Moran L, Tsagareli V, Norman R, Noakes M. Diet and IVF pilot study: short-term weight loss improves pregnancy rates in overweight/obese women undertaking IVF. Aust N Z J Obstet Gynaecol 2011;51:455-9. https://doi.org/10.1111/j.1479-828X.2011.01343.x.
- Barker M, Baird J, Lawrence W, Jarman M, Black C, Barnard K, et al. The Southampton Initiative for Health: a complex intervention to improve the diets and increase the physical activity levels of women from disadvantaged communities. J Health Psychol 2011;16:178-91. https://doi.org/10.1177/1359105310371397.
- Brofenbrenner U. The Ecology of Human Development. Cambridge, MA: Harvard University Press; 1979.
- Hill B, McPhie S, Fuller-Tyszkiewicz M, Gillman MW, Skouteris H. Psychological health and lifestyle management preconception and in pregnancy. Semin Reprod Med 2016;34:121-8. https://doi.org/10.1055/s-0036-1571352.
- Hillemeier MM, Domino ME, Wells R, Goyal RK, Kum HC, Cilenti D, et al. Effects of maternity care coordination on pregnancy outcomes: propensity-weighted analyses. Matern Child Health J 2015;19:121-7. https://doi.org/10.1007/s10995-014-1502-3.
- Forsum E, Brantsæter AL, Olafsdottir A, Olsen S, Thorsdottir I. Weight loss before conception: a systematic literature review. Food Nutrition Res 2013;57. https://doi.org/10.3402/fnr.v57i0.20522.
- Bogaerts AF, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van den Bergh BR. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes 2013;37:814-21. https://doi.org/10.1038/ijo.2012.162.
- Dodd JM, McPhee AJ, Turnbull D, Yelland LN, Deussen AR, Grivell RM, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on neonatal health outcomes: the LIMIT randomised trial. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0163-9.
- Dodd JM, Cramp C, Sui Z, Yelland LN, Deussen AR, Grivell RM, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0161-y.
- Dodd JM, Turnbull DA, McPhee AJ, Wittert G, Crowther CA, Robinson JS. Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: the LIMIT randomised controlled trial. BMC Pregnancy Childbirth 2011;11. https://doi.org/10.1186/1471-2393-11-79.
- Grivell RM, Yelland LN, Deussen A, Crowther CA, Dodd JM. Antenatal dietary and lifestyle advice for women who are overweight or obese and the effect on fetal growth and adiposity: the LIMIT randomised trial. BJOG 2016;123:233-43. https://doi.org/10.1111/1471-0528.13777.
- Szmeja MA, Cramp C, Grivell RM, Deussen AR, Yelland LN, Dodd JM. Use of a DVD to provide dietary and lifestyle information to pregnant women who are overweight or obese: a nested randomised trial. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/s12884-014-0409-8.
- Dodd JM, Ahmed S, Karnon J, Umberger W, Deussen AR, Tran T, et al. The cost-effectiveness of providing antenatal lifestyle advice for women who are overweight or obese: the LIMIT randomised trial. BMC Obes 2015;2. https://doi.org/10.1186/s40608-015-0046-4.
- Dodd JM, Newman A, Moran LJ, Deussen AR, Grivell RM, Yelland LN, et al. The effect of antenatal dietary and lifestyle advice for women who are overweight or obese on emotional well-being: the LIMIT randomized trial. Acta Obstet Gynecol Scand 2016;95:309-18. https://doi.org/10.1111/aogs.12832.
- Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity 2013;21:904-9. https://doi.org/10.1002/oby.20163.
- Hawkins M, Hosker M, Marcus BH, Rosal MC, Braun B, Stanek EJ, et al. A pregnancy lifestyle intervention to prevent gestational diabetes risk factors in overweight Hispanic women: a feasibility randomized controlled trial. Diabet Med 2015;32:108-15. https://doi.org/10.1111/dme.12601.
- Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, et al. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Fetal Neonatal Med 2014;27:1348-52. https://doi.org/10.3109/14767058.2013.858318.
- Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, et al. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/1471-2393-14-74.
- Flynn AC, Schneeberger C, Seed PT, Barr S, Poston L, Goff LM, et al. The Effects of the UK Pregnancies Better Eating and Activity Trial Intervention on dietary patterns in obese pregnant women participating in a pilot randomized controlled trial. Nutr Metabol Insights 2015;8:79-86. https://doi.org/10.4137/NMI.S29529.
- Poston L, Briley AL, Barr S, Bell R, Croker H, Coxon K, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth 2013;13. https://doi.org/10.1186/1471-2393-13-148.
- Gardner B, Croker H, Barr S, Briley A, Poston L, Wardle J. on behalf of the UPBEAT Trial . Psychological predictors of dietary intentions in pregnancy. J Hum Nutr Diet 2012;25:345-53. https://doi.org/10.1111/j.1365-277X.2012.01239.x.
- Maitland RA, Seed PT, Briley AL, Homsy M, Thomas S, Pasupathy D, et al. Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabet Med 2014;31:963-70. https://doi.org/10.1111/dme.12482.
- Flynn AC, Seed PT, Patel N, Barr S, Bell R, Briley AL, et al. Dietary patterns in obese pregnant women; influence of a behavioral intervention of diet and physical activity in the UPBEAT randomized controlled trial. Int J Behav Nutr Phys Act 2016;13. https://doi.org/10.1186/s12966-016-0450-2.
- Patel N, Godfrey KM, Pasupathy D, Levin J, Flynn AC, Hayes L, et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int J Obes 2017;41:1018-26. https://doi.org/10.1038/ijo.2017.44.
- Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust N Z J Obstet Gynaecol 2011;51:141-6. https://doi.org/10.1111/j.1479-828X.2010.01268.x.
- Renault KM, Nørgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O, et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol 2014;210:134.e1-9. https://doi.org/10.1016/j.ajog.2013.09.029.
- Vesco KK, Karanja N, King JC, Gillman MW, Perrin N, McEvoy C, et al. Healthy Moms, a randomized trial to promote and evaluate weight maintenance among obese pregnant women: study design and rationale. Contemp Clin Trials 2012;33:777-85. https://doi.org/10.1016/j.cct.2012.03.006.
- Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011;34:2502-7. https://doi.org/10.2337/dc11-1150.
- Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Tanvig M, Lamont RF, et al. Postpartum weight retention and breastfeeding among obese women from the randomized controlled Lifestyle in Pregnancy (LiP) trial. Acta Obstet Gynecol Scand 2014;93:794-801. https://doi.org/10.1111/aogs.12429.
- Walsh J, Mahony R, Foley M, Mc Auliffe F. A randomised control trial of low glycaemic index carbohydrate diet versus no dietary intervention in the prevention of recurrence of macrosomia. BMC Pregnancy Childbirth 2010;10. https://doi.org/10.1186/1471-2393-10-16.
- Walsh JM, McAuliffe FM. Impact of maternal nutrition on pregnancy outcome: does it matter what pregnant women eat?. Best Prac Res Clin Obstet Gynaecol 2015;29:63-78. https://doi.org/10.1016/j.bpobgyn.2014.08.003.
- Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ 2012;345. https://doi.org/10.1136/bmj.e5605.
- McGowan CA, McAuliffe FM. The influence of maternal glycaemia and dietary glycaemic index on pregnancy outcome in healthy mothers. Br J Nutr 2010;104:153-9. https://doi.org/10.1017/S0007114510000425.
- McGowan CA, Walsh JM, Byrne J, Curran S, McAuliffe FM. The influence of a low glycemic index dietary intervention on maternal dietary intake, glycemic index and gestational weight gain during pregnancy: a randomized controlled trial. Nutr J 2013;12. https://doi.org/10.1186/1475-2891-12-140.
- Walsh JM, Mahony RM, Culliton M, Foley ME, McAuliffe FM. Impact of a low glycemic index diet in pregnancy on markers of maternal and fetal metabolism and inflammation. Reprod Sci 2014;21:1378-81. https://doi.org/10.1177/1933719114525275.
- Horan MK, Donnelly JM, McGowan CA, Gibney ER, McAuliffe FM. The association between maternal nutrition and lifestyle during pregnancy and 2-year-old offspring adiposity: analysis from the ROLO study. Z Gesundh Wiss 2016;24:427-36. https://doi.org/10.1007/s10389-016-0740-9.
- Dodd JM, Deussen AR, Louise J. Optimising gestational weight gain and improving maternal and infant health outcomes through antenatal dietary, lifestyle and physical activity advice: the OPTIMISE randomised controlled trial protocol. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019583.
- Kunath J, Günther J, Rauh K, Hoffmann J, Stecher L, Rosenfeld E, et al. Effects of a lifestyle intervention during pregnancy to prevent excessive gestational weight gain in routine care – the cluster-randomised GeliS trial. BMC Med 2019;17. https://doi.org/10.1186/s12916-018-1235-z.
- Simmons D, Devlieger R, van Assche A, Jans G, Galjaard S, Corcoy R, et al. Effect of physical activity and/or healthy eating on GDM Risk: the DALI Lifestyle Study. J Clin Endocrinol Metab 2017;102:903-13. https://doi.org/10.1210/jc.2016-3455.
- van Poppel MNM, Simmons D, Devlieger R, van Assche FA, Jans G, Galjaard S, et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: the DALI randomised controlled trial. Diabetologia 2019;62:915-25. https://doi.org/10.1007/s00125-019-4842-0.
- van Poppel MN, Jelsma JGM, Simmons D, Devlieger R, Jans G, Galjaard S, et al. Mediators of lifestyle behaviour changes in obese pregnant women. secondary analyses from the DALI Lifestyle Randomised Controlled Trial. Nutrients 2019;11. https://doi.org/10.3390/nu11020311.
- Jelsma JGM, Simmons D, Gobat N, Rollnick S, Blumska K, Jans G, et al. Is a motivational interviewing based lifestyle intervention for obese pregnant women across Europe implemented as planned? Process evaluation of the DALI study. BMC Pregnancy Childbirth 2017;17. https://doi.org/10.1186/s12884-017-1471-9.
- Lifestyle Interventions for Expectant Moms . LIFE-Moms Consortium n.d. https://lifemoms.bsc.gwu.edu/ (accessed 12 May 2021).
- Poels M, van Stel HF, Franx A, Koster MPH. Actively preparing for pregnancy is associated with healthier lifestyle of women during the preconception period. Midwifery 2017;50:228-34. https://doi.org/10.1016/j.midw.2017.04.015.
- McPhie S, Skouteris H, Millar L, Olsson C, Campbell K, van der Pligt P, et al. Preconception weight management: an untapped area of women’s health. Aust J Prim Health 2017;23:61-5. https://doi.org/10.1071/PY16004.
- Duthie EA, Drew EM, Flynn KE. Patient-provider communication about gestational weight gain among nulliparous women: a qualitative study of the views of obstetricians and first-time pregnant women. BMC Pregnancy Childbirth 2013;13. https://doi.org/10.1186/1471-2393-13-231.
- Ojukwu O, Patel D, Stephenson J, Howden B, Shawe J. General practitioners’ knowledge, attitudes and views of providing preconception care: a qualitative investigation. Ups J Med Sci 2016;121:256-63. https://doi.org/10.1080/03009734.2016.1215853.
- Bombard JM, Robbins CL, Dietz PM, Valderrama AL. Preconception care: the perfect opportunity for health care providers to advise lifestyle changes for hypertensive women. Am J Health Promot 2013;27:43-9. https://doi.org/10.4278/ajhp.120109-QUAN-6.
- Cohen A, Gilman SE, Houck PR, Szanto K, Reynolds CF. Socioeconomic status and anxiety as predictors of antidepressant treatment response and suicidal ideation in older adults. Soc Psychiatry Psychiatr Epidemiol 2009;44:272-7. https://doi.org/10.1007/s00127-008-0436-8.
- Goossens J, Beeckman D, Van Hecke A, Delbaere I, Verhaeghe S. Preconception lifestyle changes in women with planned pregnancies. Midwifery 2018;56:112-20. https://doi.org/10.1016/j.midw.2017.10.004.
- Campbell F, Johnson M, Messina J, Guillaume L, Goyder E. Behavioural interventions for weight management in pregnancy: a systematic review of quantitative and qualitative data. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-491.
- Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlström PO, et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Hum Reprod 2017;32:1621-30. https://doi.org/10.1093/humrep/dex235.
- Homan G, Litt J, Norman RJ. The FAST study: Fertility ASsessment and advice Targeting lifestyle choices and behaviours: a pilot study. Hum Reprod 2012;27:2396-404. https://doi.org/10.1093/humrep/des176.
- Mahoney D. Lifestyle modification intervention among infertile overweight and obese women with polycystic ovary syndrome. J Am Assoc Nurse Pract 2014;26:301-8. https://doi.org/10.1002/2327-6924.12073.
- Montanaro C, Lacey L, Robson L, Estill A, Vukovic S. Preconception care: a technology-based model for delivery in the primary care setting supported by public health. Matern Child Health J 2019;23:1581-6. https://doi.org/10.1007/s10995-019-02806-4.
- van Dammen L, Wekker V, de Rooij SR, Mol BWJ, Groen H, Hoek A, et al. The effects of a pre-conception lifestyle intervention in women with obesity and infertility on perceived stress, mood symptoms, sleep and quality of life. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0212914.
- van Elten TM, Karsten MDA, Geelen A, Gemke RJBJ, Groen H, Hoek A, et al. Preconception lifestyle intervention reduces long term energy intake in women with obesity and infertility: a randomised controlled trial. Int J Behav Nutr Phys Act 2019;16. https://doi.org/10.1186/s12966-018-0761-6.
- Wekker V, Huvinen E, van Dammen L, Rono K, Painter RC, Zwinderman AH, et al. Long-term effects of a preconception lifestyle intervention on cardiometabolic health of overweight and obese women. Eur J Public Health 2019;29:308-14. https://doi.org/10.1093/eurpub/cky222.
- Kim LP, Koleilat M, Whaley SE. A qualitative study to examine perceptions and barriers to appropriate gestational weight gain among participants in the Special Supplemental Nutrition Program for Women Infants and Children Program. J Pregnancy 2016;2016. https://doi.org/10.1155/2016/4569742.
- Shieh C, Cullen DL, Pike C, Pressler SJ. Intervention strategies for preventing excessive gestational weight gain: systematic review and meta-analysis. Obes Rev 2018;19:1093-109. https://doi.org/10.1111/obr.12691.
- Thangaratinam S, Rogozińska E, Jolly K, Glinkowski S, Duda W, Borowiack E, et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16310.
- Willcox JC, Wilkinson SA, Lappas M, Ball K, Crawford D, McCarthy EA, et al. A mobile health intervention promoting healthy gestational weight gain for women entering pregnancy at a high body mass index: the txt4two pilot randomised controlled trial. BJOG 2017;124:1718-28. https://doi.org/10.1111/1471-0528.14552.
Appendix 1 Defining LARC events using Read codes and British National Formulary prescription codes
Inclusion criteria: women of reproductive age (16–48 years old) who had a LARC-related event (insertion, in situ or removal) between 1 January 2009 and 31 December 2018.
| MedCode | Label | LARC consultation type | Type of LARC |
|---|---|---|---|
| 8354 | [V]Coil in situ | In situ | IUD |
| 10957 | [V]Coil check | In situ | IUD |
| 45339 | [V]Coil maintenance | In situ | IUD |
| 20658 | [V]Coil check | In situ | IUD |
| 107776 | Retained intrauterine contraceptive device | In situ | IUD |
| 12029 | [V]Intrauterine contraceptive device present | In situ | IUD |
| 6306 | [V]Intrauterine contraceptive device present | In situ | IUD |
| 2145 | [V]Intrauterine contraceptive device present | In situ | IUD |
| 47908 | Intrauterine contraceptive device 6 week check | In situ | IUD |
| 22914 | Intrauterine contraceptive device annual review | In situ | IUD |
| 6265 | [V]Intrauterine contraceptive device check | In situ | IUD |
| 20557 | [V]Intrauterine contraceptive device check | In situ | IUD |
| 21515 | [V]Intrauterine contraceptive device check | In situ | IUD |
| 5917 | IUD checked – no problems | In situ | IUD |
| 225 | IUD in situ | In situ | IUD |
| 20392 | Coil follow-up administration | In situ | IUD |
| 107433 | Uterine perforation by intrauterine contraceptive device | In situ | IUD |
| 54183 | [V]Surveillance of (intrauterine) contraceptive device | In situ | IUD |
| 51242 | IUD follow-up admininstration. NOS | In situ | IUD |
| 21421 | IUD follow-up administration | In situ | IUD |
| 63924 | FP1002 due next with new IUD | In situ | IUD |
| 44917 | IUD check – 2nd call | In situ | IUD |
| 40783 | IUD check – 1st call | In situ | IUD |
| 45820 | IUD check – 3rd call | In situ | IUD |
| 13004 | IUD change due | In situ | IUD |
| 37020 | IUD – defaulted from check | In situ | IUD |
| 19950 | IUD check due | In situ | IUD |
| 445 | IUD check | In situ | IUD |
| 20538 | Mechanical complication of coil | In situ | IUD |
| 112311 | Infection associated with intrauterine contraceptive device | In situ | IUD |
| 29106 | Mechanical complication intrauterine contracep.device (IUCD) | In situ | IUD |
| 23439 | Bleeding due to intrauterine contraceptive device | In situ | IUD |
| 17735 | Mechanical complication of intrauterine contraceptive device | In situ | IUD |
| 104471 | Intrauterine contraceptive device threads seen | In situ | IUD |
| 108636 | Bleeding due to intrauterine contraceptive device | In situ | IUD |
| 30765 | IUD partially expelled | In situ | IUD |
| 7888 | IUD checked – problems | In situ | IUD |
| 5564 | IUD threads lost | In situ | IUD |
| 52230 | Intrauterine contraceptive device annual review by telephone | In situ | IUD |
| 6050 | Change of intrauterine contraceptive device | In situ | IUD |
| 88224 | Intrauterine contraceptive device fit by another GP practice | In situ | IUD |
| 22652 | Replacement of intrauterine contraceptive device | In situ | IUD |
| 95906 | Intrauterine contracep device checked by other hlth provider | In situ | IUD |
| 2738 | IUD in situ from other agency | In situ | IUD |
| 93404 | Subcutaneous contraceptive in situ | In situ | Implant |
| 26477 | Check of subcutaneous contraceptive | In situ | Implant |
| 96963 | Subcutaneous contraceptive implant palpable | In situ | Implant |
| 106241 | Contraceptive implant removal invitation | In situ | Implant |
| 98121 | Mirena coil check | In situ | IU system |
| 21114 | Coil intrauterine contraceptive device procedure | Insertion | IUD |
| 10614 | Coil contraception | Insertion | IUD |
| 24497 | [V]Reinsertion of coil | Insertion | IUD |
| 6064 | [V]Coil insertion | Insertion | IUD |
| 21365 | [V]Reinsertion of coil | Insertion | IUD |
| 17573 | IUD contraception | Insertion | IUD |
| 10754 | [V]Reinsertion of intrauterine contraceptive device | Insertion | IUD |
| 9226 | Fitting of intrauterine contraceptive device | Insertion | IUD |
| 6772 | Intrauterine contraceptive device procedure | Insertion | IUD |
| 4401 | [V]Reinsertion of intrauterine contraceptive device | Insertion | IUD |
| 31602 | [V]Reinsertion of intrauterine contraceptive device | Insertion | IUD |
| 6941 | Introduction of intrauterine contraceptive device | Insertion | IUD |
| 2144 | [V]Intrauterine contraceptive device insertion | Insertion | IUD |
| 3882 | [V]Intrauterine contraceptive device insertion | Insertion | IUD |
| 26317 | Intrauterine contraceptive device procedure NOS | Insertion | IUD |
| 17980 | [V]Intrauterine contraceptive device insertion | Insertion | IUD |
| 53523 | Other specified intrauterine contraceptive device | Insertion | IUD |
| 182 | IUD fitted | Insertion | IUD |
| 42310 | Post-coital IUD fitted | Insertion | IUD |
| 38544 | ‘Morning after’ IUD fitted | Insertion | IUD |
| 485 | IUD re-fitted | Insertion | IUD |
| 17440 | Intrauterine device procedure | Insertion | IUD |
| 40402 | Coil contraceptive claim | Insertion | IUD |
| 99571 | IUD contraceptive claim | Insertion | IUD |
| 27859 | IUD contraceptive claim | Insertion | IUD |
| 27872 | IUCD contraceptive claim | Insertion | IUD |
| 55297 | FP1002 – IUD insertion claim | Insertion | IUD |
| 103642 | Insertion of etonogestrel radio-opaque contraceptive implant | Insertion | Implant |
| 104317 | Insertion of subcutaneous contraceptive claim | Insertion | Implant |
| 22950 | Insertion of subcutaneous contraceptive | Insertion | Implant |
| 101819 | Reinsertion of subcutaneous contraceptive | Insertion | Implant |
| 70535 | Insertion of hormone into subcutaneous tissue | Insertion | Implant |
| 90157 | Replacement of hormone in subcutaneous tissue | Insertion | Implant |
| 17183 | Insertion of hormone implant | Insertion | Implant |
| 103004 | Subdermal etonogestrel implant insertion ESA | Insertion | Implant |
| 100958 | Insert subcutaneous contraceptive implnt othr healthcre prov | Insertion | Implant |
| 7255 | Introduction of Mirena coil | Insertion | IU system |
| 102368 | Intrauterine system contraception | Insertion | IU system |
| 2795 | IUD – NOS | Insertion/in situ | IUD |
| 22951 | Subcutaneous contraceptive NOS | Insertion/in situ | Implant |
| 26092 | Subcutaneous contraceptive | Insertion/in situ | Implant |
| 20424 | [V]Removal of coil | Removal | IUD |
| 18341 | [V]Removal of coil | Removal | IUD |
| 7379 | Removal of intrauterine contraceptive device NEC | Removal | IUD |
| 11561 | [V]Removal of intrauterine contraceptive device | Removal | IUD |
| 5252 | [V]Removal of intrauterine contraceptive device | Removal | IUD |
| 27929 | [V]Removal of intrauterine contraceptive device | Removal | IUD |
| 446 | IUD removed | Removal | IUD |
| 106270 | Intrauterine contraceptive device removal invitation | Removal | IUD |
| 107556 | Expulsion of intrauterine contraceptive device | Removal | IUD |
| 26476 | IUD removal awaited | Removal | IUD |
| 25680 | Removal of contraceptive coil from pouch of Douglas | Removal | IUD |
| 6225 | Removal intrauterine contracept device from pouch of Douglas | Removal | IUD |
| 32611 | Removal of displaced intrauterine contraceptive device | Removal | IUD |
| 22945 | IUD fallen out | Removal | IUD |
| 22946 | IUD expelled | Removal | IUD |
| 95476 | Intrauterine contracep device removed by other hlth provider | Removal | IUD |
| 107048 | Removal of subcut contraceptive implant using US guidance | Removal | Implant |
| 26260 | Removal of subcutaneous contraceptive | Removal | Implant |
| 104861 | Removal of subcutaneous contraceptive claim | Removal | Implant |
| 22875 | Removal of hormone implant from subcutaneous tissue | Removal | Implant |
| 71272 | Removal of hormone implant from subcutaneous tissue | Removal | Implant |
| 103620 | Removal of etonogestrel radio-opaque contraceptive implant | Removal | Implant |
| 101010 | Remov subcutaneous contraceptive implant othr healthcre prov | Removal | Implant |
| 18745 | Removal of Mirena coil | Removal | IU system |
| 109722 | Removal of intrauterine system | Removal | IU system |
British National Formulary163 chapters have been identified for inclusion (rather than specific prodcodes): chapters 7.3.2.2, 7.3.2.3 and 7.3.4.1.
| Prodcode | Product name | British National Formulary | British National Formulary chapter | Type of LARC |
|---|---|---|---|---|
| 66331 | Ancora 375 Ag intrauterine contraceptive device (RF Medical Supplies Ltd, Saint Helens, UK) | 07030400/07030450 | Contraceptive Devices/Intrauterine Contraceptive Devices | IUD |
| 66341 | Ancora 375 Cu intrauterine contraceptive device (RF Medical Supplies Ltd) | IUD | ||
| 1196 | Novagard type 6 intrauterine device (Pharmacia Ltd, Sandwich, UK) | 7030450 | Intrauterine Contraceptive Devices | IUD |
| 4904 | Gyne-T 380S intrauterine device (Janssen UK, High Wycombe, UK) | IUD | ||
| 5265 | Multiload Cu375 intrauterine contraceptive device (Organon UK, London, UK) | IUD | ||
| 5405 | Nova-T 380 intrauterine contraceptive device (Bayer plc, Reading, UK) | IUD | ||
| 5647 | T-Safe 380A QL® intrauterine contraceptive device (Williams Medical Supplies Ltd, Rhymney, UK) | IUD | ||
| 12146 | Ortho Gyne type 4a intrauterine device (Janssen UK) | IUD | ||
| 13678 | Ortho Gyne type 4b Intrauterine device T380S (Janssen UK) | IUD | ||
| 14038 | Flexi-T 300 intrauterine contraceptive device (Durbin plc, Hayes, UK) | IUD | ||
| 14295 | Flexi-T+ 380 intrauterine contraceptive device (Durbin plc) | IUD | ||
| 14959 | TT380 Slimline intrauterine contraceptive device (Durbin plc) | IUD | ||
| 18826 | Multi-Safe 375 intrauterine contraceptive device (Williams Medical Supplies Ltd) | IUD | ||
| 19143 | GyneFix intrauterine contraceptive device (Williams Medical Supplies Ltd) | IUD | ||
| 26488 | Neo-Safe T380 intrauterine contraceptive device (Williams Medical Supplies Ltd) | IUD | ||
| 33367 | Load 375 intrauterine contraceptive device (Durbin plc) | IUD | ||
| 36984 | Mini TT380 Slimline intrauterine contraceptive device (Durbin plc) | IUD | ||
| 36995 | UT380 Short intrauterine contraceptive device (Durbin plc) | IUD | ||
| 38015 | UT380 Standard intrauterine contraceptive device (Durbin plc) | IUD | ||
| 43404 | Steriload intrauterine contraceptive device (Farla Medical Ltd, London, UK) | IUD | ||
| 45739 | Optima TCu380A intrauterine contraceptive device (Farla Medical Ltd) | IUD | ||
| 49127 | Copper T380A intrauterine contraceptive device (RF Medical Supplies Ltd) | IUD | ||
| 50899 | Novaplus T 380 Ag intrauterine contraceptive device normal (RF Medical Supplies Ltd) | IUD | ||
| 51443 | Novaplus T 380 Cu intrauterine contraceptive device normal (RF Medical Supplies Ltd) | IUD | ||
| 55209 | Novaplus T 380 Cu intrauterine contraceptive device mini (RF Medical Supplies Ltd) | IUD | ||
| 56568 | Novaplus T 380 Ag intrauterine contraceptive device maxi (RF Medical Supplies Ltd) | IUD | ||
| 58198 | Novaplus T 380 Ag intrauterine contraceptive device mini (RF Medical Supplies Ltd) | IUD | ||
| 66415 | Intrauterine contraceptive device | IUD | ||
| 67254 | Mirena 20 µg/24 hours intrauterine device (Dowelhurst Ltd, Warwick, UK) | IU system | ||
| 2819 | Norplant 228 mg implant (Hoechst Marion Roussel, Kansas City, MO, USA) | 7030202 | Parenteral Progestogen-Only Contraceptives | Implant |
| 9592 | Implanon 68 mg implant (Organon UK) | Implant | ||
| 16624 | Levonorgestrel 228 mg implant | Implant | ||
| 2748 | Mirena 20 µg/24 hours intrauterine device (Bayer plc) | 06040103/07030203 | Progestogen Products Doubling (In House Use)/Intra-Uterine Progestogen-Only Device | IU system |
| 6906 | Levonorgestrel 20 µg/24 hours intrauterine device | IUD | ||
| 57359 | Mirena 20 µg/24 hours intrauterine device (Mawdsley-Brooks & Company Ltd, Salford, UK) | IU system | ||
| 60564 | Levonorgestrel 13.5 mg intrauterine device | IUD | ||
| 60632 | Jaydess 13.5 mg intrauterine device (Bayer plc) | IUD | ||
| 66047 | Levosert 20 µg/24 hours intrauterine device (Gedeon Richter UK Ltd, London, UK) | IUD | ||
| 72106 | Kyleena 19.5 mg intrauterine device (Bayer plc) | IUD | ||
| 73046 | Levonorgestrel 19.5 mg intrauterine device | IUD | ||
| 13209 | Etonogestrel 68 mg implant | Implant | ||
| 44196 | Nexplanon 68 mg implant (Merck Sharp & Dohme Ltd, London, UK) | Implant | ||
| 938 | NovaT | Other | IUD | |
| 2747 | multiload | IUD | ||
| 4904 | gyne-t | IUD | ||
| 5265 | multiload | IUD | ||
| 5405 | NovaT | IUD | ||
| 5647 | t-safe | IUD | ||
| 14038 | flexi-t | IUD | ||
| 14158 | multiload | IUD | ||
| 14295 | flexi-t | IUD | ||
| 14959 | tt380 | IUD | ||
| 18826 | multisafe | IUD | ||
| 19143 | gynefix | IUD | ||
| 21686 | gynefix | IUD | ||
| 26488 | neosafe | IUD | ||
| 33367 | load 375 | IUD | ||
| 36984 | mini tt380 | IUD | ||
| 36995 | ut380 | IUD | ||
| 38015 | ut380 | IUD | ||
| 43404 | steriload | IUD | ||
| 45739 | optima | IUD |
Appendix 2 Clinical codes
| Med code | Label |
|---|---|
| 11507 | Depot contraception |
| 29297 | Oral contracept. check administration |
| 29030 | Depot contraceptive – problem |
| 71415 | GMS3 claim – temporary contraceptive (non-IUCD) signed |
| 19267 | Oral contraceptive poisoning |
| 19496 | Withdrawal contraception |
| 56806 | [X]Other contraceptive management |
| 98936 | GMS3 claim – temporary contraceptive (non-IUCD) paid |
| 4688 | Sheath contraception |
| 25857 | GMS4 claim – contraception (non-IUCD) sent to HA |
| 96592 | Contraceptive check first letter |
| 98363 | Contraceptive check third letter |
| 103973 | Migraine induced by oestrogen contraceptive |
| 41407 | Sympto-thermal contraceptn NOS |
| 105984 | Uses contraceptive sponge |
| 91670 | Adv to GP to change patient oral contraceptive from progestog only |
| 19501 | Oral contraception NOS |
| 19500 | Contraceptive sheath NOS |
| 29034 | Transdermal contraceptive |
| 29958 | [X] Adverse reaction to unspecified oral contraceptive |
| 46842 | GMS4 claim – contraception (non-IUCD) due next visit |
| 94002 | Contraceptive registration |
| 16887 | Oral contraceptive claim |
| 100878 | Stopped using contraceptive sponge |
| 29035 | Uses sympto-thermal contracepn |
| 32976 | Hypertension induced by oral contraceptive pill |
| 5836 | Pill-oral contraceptive claim |
| 22936 | Contraceptive diaphragm |
| 60409 | [V]Contraceptive cream prescription |
| 72028 | [V]Contraceptive foam fitting |
| 6413 | [V]Contraceptive cap fitting |
| 3940 | Adverse reaction to unspecified oral contraceptive |
| 20716 | [V]Repeat prescription of oral contraceptive |
| 15806 | Post-coital contraception NOS |
| 22947 | Depot contraception stopped |
| 22940 | Depot contraceptive-no problem |
| 12989 | Contraception from other agency |
| 14830 | Contraceptive sheath |
| 19499 | Oral contraceptive started |
| 29033 | Sympto-thermal contraception |
| 106646 | Emergency contraception indicated |
| 97329 | GMS4 claim – contraception (IUCD) due with new IUCD |
| 57146 | Contraceptive sponge |
| 90951 | Advice to GP to change pt oral contraceptive from combined |
| 27523 | Headache caused by oral contraceptive pill |
| 69159 | Contraceptive sponge failure |
| 180 | Oral contraceptive prescribed |
| 22935 | Post-coital contraception NOS |
| 19508 | Oral contraceptive re-started |
| 13005 | Diaphragm contraception |
| 6255 | CAP contraception |
| 22941 | Spermicide alone contraception |
| 13003 | Depot contraceptive NOS |
| 11810 | FP1001 – contraception claim |
| 8387 | [V]Oral contraceptive prescription |
| 72078 | GMS3 claim – temporary contraceptive (IUCD) sent to HA |
| 12993 | Oral contraceptive repeat |
| 20581 | Oral contraception – problem |
| 106129 | Contraceptive sponge NOS |
| 22938 | Depot contraceptive repeated |
| 90120 | GMS4 claim – contraception (non-IUCD) paid |
| 103361 | Contraceptive sheath problem |
| 30766 | Contraceptive usage NOS |
| 17879 | [V]Repeat prescription of oral contraceptive |
| 102867 | Problem with contraception |
| 29032 | Spermicidal contraceptive |
| 6759 | Post-coital contraception |
| 41 | Contraception |
| 19506 | Depot contraceptive given |
| 110589 | Barrier contraception method |
| 6586 | Contraceptive claims |
| 71434 | GMS3 claim – temporary contraceptive (IUCD) signed |
| 13007 | Oral contraception – no problem |
| 20354 | Combined oral contraceptive |
| 61591 | GMS4 claim – contraception (IUCD) signed |
| 102367 | Uses contraception |
| 47020 | Contraceptive sheath issued |
| 69241 | GMS4 claim – contraception (non-IUCD) due |
| 113582 | GMS4 claim – contraception (IUCD) paid |
| 5666 | Oral contraception |
| 12995 | Oral contraceptive |
| 100565 | GMS4 claim – contraception (non-IUCD) forgot to claim |
| 8538 | Emergency contraception |
| 5839 | Contraceptive administration |
| 13006 | Contracep. NOS – other agency |
| 12992 | Depot contraceptive |
| 19507 | Uses contraceptive sheath |
| 41761 | GMS3 claim – temporary contraceptive (non-IUCD) sent to HA |
| 18538 | Pill contraceptive administration |
| 17291 | Oral contraceptive administration |
| 104174 | Progestogen only oral contraceptive |
| 19505 | Mini-pill: oral contraceptive |
| 37428 | [V]Repeat prescription of oral contraceptive (OC) |
| 9252 | Missed contraceptive pill |
| 20360 | Oral contraceptive changed |
| 25858 | GMS4 claim – contraception (non-IUCD) signed |
| 96063 | GMS4 claim – contraception (non-IUCD) up to date |
| 98074 | Contraceptive check second letter |
| 100069 | GMS4 claim – contraception (non-IUCD) returned unpaid |
| 68735 | GMS4 claim – contraception (IUCD) sent to HA |
| 6358 | Pill – oral contraception |
| 22944 | Spermicide + sheath contraception |
| 39563 | Stopped using sheath |
| 22943 | Uses sheath + spermicide |
| 100655 | Uses contraceptive sponge & spermicide |
| 2438 | Progestagen-only oral contraception |
| 94215 | Neuroleptic depot injection |
| 12994 | Progestagen-only pill |
| 20388 | Adverse reaction to combined oestrogens and progestogens |
| 715 | ‘Morning after’ pills given |
| 15255 | Oral contraceptive stopped |
| 102608 | UK medical eligibility criteria for contraceptive use 2009 cat 2 |
| 1848 | Contraception counselling |
| 2446 | Contraception contraindicated |
| 102610 | UK medical eligibility criteria for contraceptive use 2009 cat 3 |
| 96591 | Contraceptive check invitation |
| 109059 | Combined oral contraceptive pill contraindicated |
| 107774 | Emergency contraception declined |
| 102678 | Education for contraceptive sheath |
| 63613 | Contraceptive scheme card issued |
| 102676 | Education for postcoital contraceptive |
| 3618 | General contraceptive advice |
| 103240 | Education for contraceptive diaphragm |
| 6477 | Emergency contraception advice |
| 102957 | UK medical eligibility criteria for contraceptive use 2009 cat 4 |
| 95989 | Discussion about contraception injection |
| 47069 | [V]Unspecified contraceptive management |
| 12711 | [V]Contraceptive management |
| 102604 | Planned contraception method |
| 52109 | [V]Surveillance previously prescribed contraceptive methods |
| 102609 | UK medical eligibility criteria for contraceptive use 2009 cat 1 |
| 107837 | GMS4 claim – contraception (non-IUCD) cancelled |
| 102521 | Education for transdermal contraceptive patch |
| 108120 | Referral to contraception and sexual health service |
| 109280 | Progestogen only oral contraceptive contraindicated |
| 100652 | Contraceptive advice for patients with epilepsy |
| 19497 | Rhythm method contraception |
| 104432 | Education about missed dose of oral contraceptive |
| 26212 | Advice about progestogen only oral contraceptive |
| 101143 | Contraceptiv advice for patients with epilepsy not indicated |
| 105422 | Discussion about risks of combined oral contraception |
| 110889 | Natural contraception |
| 108552 | Education for spermicidal contraceptive |
| 2282 | Oral contraceptive advice |
| 102190 | Contraceptive advice for patients with epilepsy declined |
| 100549 | Parental consent for contraceptive treatment |
| 26039 | [V]Other specified contraceptive management |
| 102876 | UK medical eligibility criteria for contraceptive use 2009 risk |
| 269 | Total abdominal hysterectomy NEC |
| 813 | Abdominal hysterectomy and bilateral salpingoophorectomy |
| 873 | Vaginal hysterectomy |
| 1729 | Subtotal abdominal hysterectomy |
| 1830 | Abdominal hysterectomy and right salpingoopherectomy |
| 2058 | Abdominal hysterectomy and left salpingoopherectomy |
| 2448 | Abdominal hysterectomy |
| 3064 | Wertheim hysterectomy |
| 3433 | TAH – Tot abdom hysterectomy and BSO – bilat salpingophorect |
| 3666 | Hysterectomy NEC |
| 6231 | H/O: hysterectomy |
| 7411 | Vaginal hysterectomy NEC |
| 7798 | TAH – total abdom hysterectomy & bilateral salpingoophorect |
| 7949 | Abdominal hysterectomy and left salpingoophorectomy |
| 10888 | Post hysterectomy vaginal vault prolapse |
| 11662 | Abdominal hysterectomy with conservation of ovaries |
| 12910 | No smear – hysterectomy |
| 12920 | No smear – benign hysterectomy |
| 18980 | Laparoscopic hysterectomy |
| 19088 | Laparoscopic vaginal hysterectomy |
| 19182 | Radical hysterectomy |
| 23863 | Abdominal hysterectomy & bilateral salpingoophorectomy (BSO) |
| 31312 | Abdominal hysterectomy & excision of periuterine tissue NEC |
| 42949 | Vaginal hysterectomy with conservation of ovaries |
| 47215 | Ward vaginal hysterectomy |
| 49408 | Cervical smear to continue post hysterectomy |
| 52057 | Schauta radical vaginal hysterectomy |
| 54109 | Vaginal hysterectomy and excision of periuterine tissue NEC |
| 69607 | Bonney abdominal hysterectomy |
| 94490 | Total abdominal hysterectomy with conservation of ovaries |
| 94549 | Laparoscopic subtotal hysterectomy |
| 94934 | Subtotal abdominal hysterectomy with conservation of ovaries |
| 97020 | Lap assist vag hysterectomy with bilat salpingo-oophorectomy |
| 100097 | Heaney vaginal hysterectomy |
| 109060 | Subtotl abdominal hysterectomy & bilat salpingo-oophorectomy |
| 109193 | Vaginal hysterectomy and right salpingo-oophorectomy |
| 109229 | Subtotal abdominal hysterectomy & left salpingo-oophorectomy |
| 109286 | Radical hysterectomy with bilateral salpingo-oophorectomy |
| 109351 | Vaginal hysterectomy and left salpingo-oophorectomy |
| 109686 | Subtotl abdominal hysterectomy & right salpingo-oophorectomy |
| 111335 | Radical hysterectomy with conservation of ovaries |
| 168 | [V]Sterilisation |
| 2932 | [V]Post-sterilisation vasoplasty or tuboplasty |
| 3532 | H/O: sterilisation – female |
| 4178 | Laparoscopic bilateral female sterilisation |
| 6338 | [V]Admission for sterilisation |
| 6841 | Open bilateral female sterilisation |
| 6847 | Endoscopic bilateral female sterilisation |
| 7163 | Other open female sterilisation |
| 7903 | Other endoscopic female sterilisation |
| 8231 | [V]Other sterilisation |
| 12998 | Contraception: female sterilis |
| 18751 | Other laparoscopic female sterilisation |
| 43832 | Sterilising procedure |
| 48256 | [V]Reattempted sterilisation |
| 60089 | [V]Post-sterilisation tuboplasty |
| 5210 | H/O: tubal ligation |
| 9724 | [V]Admission for tubal ligation |
| 14653 | Open bilateral ligation of fallopian tubes |
| 35984 | Pomeroy open bilateral ligation of fallopian tubes |
| 55932 | Open ligation of remaining solitary fallopian tube |
| Med code | Label |
|---|---|
| 38792 | Hormone replacement therapy bleed pattern – normal |
| 62292 | Hormone replacement therapy bleed pattern – not relevant |
| 26606 | Hormone replacement therapy ongoing treatment |
| 49111 | Hormone replacement therapy bleed pattern – abnormal |
| 59447 | Hormone replacement therapy bleed pattern – no bleeding |
| 13054 | Health education – hormone replacement therapy |
| 1671 | Hormone replacement therapy |
| 337 | Hormone replacement therapy |
| 12611 | Hormone replacement therapy requested |
| 11923 | Hormone replacement therapy review |
| 50119 | Years on hormone replacement therapy |
| 52904 | [X]Other specified menopausal and perimenopausal disorders |
| 38395 | Postmenopausal osteoporosis with pathological fracture |
| 2087 | Premature menopause NOS |
| 45409 | Menopausal and postmenopausal disorder NOS |
| 9171 | Menopausal and postmenopausal disorders |
| 19954 | Artificial menopause state |
| 1583 | Postmenopausal bleeding |
| 36514 | Menopause: LH, FSH checked |
| 67495 | Post menopausal urethritis |
| 25549 | Menopausal concentration lack |
| 22074 | Menopause follow-up assessment |
| 86026 | Menopausal profile |
| 21464 | Menopause monitoring NOS |
| 17628 | Postmenopausal disorders |
| 707 | Postmenopausal atrophic vaginitis |
| 30359 | Perimenopausal atrophic vaginitis |
| 20628 | Menopause initial assessment |
| 17442 | Menopause symptoms present |
| 2702 | Menopause: bone density check |
| 4462 | H/O: post-menopausal bleeding |
| 93526 | Perimenopausal menorrhagia |
| 15436 | Post menopausal atrophic urethritis |
| 828 | Menopausal symptoms NOS |
| 94499 | Early menopause |
| 9700 | Postmenopausal osteoporosis |
| 15283 | Menopausal sleeplessness |
| 4043 | Menopausal or female climacteric state |
| 30590 | Postmenopausal state |
| 4383 | Menopause |
| 9313 | Menopause monitoring |
| 9547 | Menopausal flushing |
| 62797 | Menopause: dietary advice |
| 17051 | Menopausal arthritis |
| 46997 | Postmenopausal postcoital bleeding |
| 814 | Hot flushes – menopausal |
| 15022 | Premenopausal menorrhagia |
| 28046 | Other menopausal and postmenopausal states |
| 58681 | Menopause: sexual advice |
| 18730 | Menopausal headache |
| 21534 | Menopause: gen counselling |
| 52129 | H/O: hormone replacement (HRT) |
| 38723 | HRT: combined oestrog/progest |
| 34489 | HRT contraindicated |
| 33436 | HRT: unopposed oestrogen |
| 29275 | HRT changed |
| 26608 | HRT side-effects |
| 26607 | HRT started |
| 25078 | HRT stopped |
| 13199 | Hormone implant – HRT |
| 13198 | HRT prophylaxis |
| 73849 | [X] Adverse reaction to clonidine |
| 67676 | Clonidine poisoning |
| 26107 | Adverse reaction to clonidine |
| 11438 | Hot flushes |
| Med code | Label |
|---|---|
| 102359 | Pregnancy advice for patients with epilepsy |
| 5778 | Pregnancy advice |
| 36903 | Pregnancy advice NOS |
| 12996 | Trying to conceive |
| 10205 | Advice relating to pregnancy and fertility |
| 2949 | Seen in fertility clinic |
| 4571 | Seen in fertility clinic |
| 26088 | [V]Infertility investigation and testing |
| 9036 | Fertility counselling |
| 9983 | Treatment for infertility NOS |
| 5239 | Referral to fertility clinic |
| 33458 | Female infertility therapy |
| 1810 | Treatment for infertility |
| 9133 | Fertility investigation of female NEC |
| 2548 | Procreat/fertility counselling |
| 39295 | [V]Infertility general advice and counselling |
| 1154 | Infertility investigations NOS |
| 36458 | Other female infertility |
| 102589 | Infertility care |
| 16131 | Infertility investigation – fem |
| 41692 | Female infertility test abnormal |
| 53018 | Other female infertility NOS |
| 17756 | Female infertility test normal |
| 7246 | Subfertility |
| 25361 | Fertility counselling |
| 10445 | Advice on fertility and infertility |
| 7351 | Fertility problem |
| 33510 | [V]Procreative management |
| 100920 | Pre-conception advice for patients with epilepsy |
| 41319 | Preconception care |
| 41033 | EDC – estimated date of conception |
| 63344 | Estimated date of conception |
| 25307 | Reproductive counselling |
| 20847 | [V]IVF |
| 93810 | Other specified in vitro fertilisation (IVF) |
| 10238 | IVF |
| 52626 | In vitro fertilisation (IVF) |
| 102280 | IVF with pre-implantation for genetic diagnosis |
| 90936 | In vitro fertilisation (IVF) NOS |
| 89966 | IVF with donor sperm |
| 57000 | IVF with intracytoplasmic sperm injection (ICSI) |
| 21532 | Folic acid advice – pre pregnancy |
| 49884 | Diabetic pre-pregnancy counselling |
| 4609 | Pre-pregnancy counselling |
| 50937 | Referral to diabetes preconception counselling clinic |
| 102767 | Pre-conception advice for diabetes mellitus |
| 10761 | Pre-conception advice |
| 69751 | [V]Unspecified infertility management |
| 2957 | Infertility problem |
| 1808 | Infertility – female |
| 37047 | A/N care: H/O infertility |
| 40072 | Infertility studies |
| 30392 | Female infertility NOS |
| 26150 | [V]Other specified infertility management |
| 9938 | [V]Infertility management |
| 64063 | IVF with donor eggs |
| 97981 | IVF intracytoplasmic sperm injection (ICSI) and donor egg |
| 89716 | IVF with surrogacy |
| 20977 | Adverse reaction to clomiphene |
| 73091 | Maternity grant advice |
| 459 | Endoscopic bilateral occlusion of fallopian tubes NOS |
| 1891 | Endoscopic bilateral occlusion of fallopian tubes |
| 4935 | Open bilateral occlusion of fallopian tubes NOS |
| 17760 | Other endoscopic occlusion of fallopian tube |
| 23346 | Occlusion of vagina |
| 28161 | Endoscopic occlusion of left fallopian tube |
| 32187 | Open bilateral occlusion of fallopian tubes |
| 42543 | Unilateral occlusion of fallopian tube |
| 45721 | Other open occlusion of fallopian tube |
| 50204 | Endoscopic occlusion of right fallopian tube |
| 50773 | Other specified open bilateral occlusion of fallopian tubes |
| 55972 | Other open occlusion of fallopian tube NOS |
| 58048 | Endoscopic occlusion of remaining solitary fallopian tube |
| 59338 | Other specified endoscopic occlusion of fallopian tube |
| 61157 | Endoscopic bilateral occlusion of fallopian tubes OS |
| 64187 | Endoscopic occlusion of fallopian tube NOS |
| 68272 | Endoscopic unilateral occlusion of fallopian tubes |
| 68614 | Occlusion of cervix |
| 69724 | Other specified other open occlusion of fallopian tube |
| 95677 | In vitro fertilisation with pre-implantation for genetic diagnosis |
| 97196 | Antenatal screen shows homozygote/compound heterozygote of genetic sign |
| 97802 | Antenatal screen shows homozygote/compound heterozygote no genetic sign |
| 100785 | Antenatal screen, partner tested and no genetic risk identified |
| 102280 | IVF with pre-implantation for genetic diagnosis |
| Med code | Label | Planned/unplanned |
|---|---|---|
| 14877 | Pregnant –? planned | Planned |
| 20240 | Pregnant – planned | Planned |
| 30365 | Wanted pregnancy | Planned |
| 8767 | Open reversal of female sterilisation | Planned |
| 8844 | Open reversal of female sterilisation | Planned |
| 24538 | Endoscopic reversal of female sterilisation | Planned |
| 24657 | [V]Reversal of sterilisation | Planned |
| 40709 | Open reversal of female sterilisation NOS | Planned |
| 41343 | Laparoscopic reversal of female sterilisation | Planned |
| 56297 | Other specified endoscopic reversal of female sterilisation | Planned |
| 66719 | Endoscopic reversal of female sterilisation NOS | Planned |
| 97970 | Other specified open reversal of female sterilisation | Planned |
| 29915 | Open reversal of tubal ligation | Planned |
| 6197 | [V]Failed sterilisation NOS | Unplanned |
| 37019 | Female sterilisation failure | Unplanned |
| 15338 | Pregnancy unplanned? wanted | Unplanned |
| 7517 | Unplanned pregnancy | Unplanned |
| 30618 | Unplanned pregnancy | Unplanned |
| 14842 | Pregnant – unplanned – wanted | Unplanned |
| 15567 | Pregnant – unplanned – not wanted | Unplanned |
| 23421 | IUD failure – pregnant | Unplanned |
| 29692 | Pregnant, IUD failure | Unplanned |
| 20623 | [V]Problems related to unwanted pregnancy | Unplanned |
| 5044 | Unwanted pregnancy | Unplanned |
| 15033 | [V]Other unwanted pregnancy | Unplanned |
| 50421 | Unwanted pregnancy | Unplanned |
| 32975 | Pregnant, diaphragm failure | Unplanned |
| 14994 | Pregnant, sheath failure | Unplanned |
| 40851 | Depot contraceptive failure | Unplanned |
| 19503 | Progestogen-only pill failure | Unplanned |
Appendix 3 Routine data results
Codes to refute or confirm that the pregnancy was planned
To determine whether or not the LARC was removed for the purpose of conception, events between the two were examined to either confirm or refute (to the best of our abilities) that the pregnancy had been planned. The following clinical events were identified to confirm an intended pregnancy: trying or difficult to get pregnancy, and planning a pregnancy. The following were identified to refute a pregnancy: alternative contraception, and unplanned pregnancy and menopause (Table 27).
| Events | Unique number of events | Number of women with at least one event |
|---|---|---|
| Confirming an intended pregnancy | ||
| Trying or difficult to get pregnancy | 7494 | 6494 |
| Planning a pregnancy | 69 | 68 |
| Refuting a pregnancy | ||
| Alternative contraception | 96,134 | 78,898 |
| Unplanned pregnancy | 1232 | 1211 |
| Menopause | 5628 | 4917 |
LARC use over time
LARC insertions are most frequently performed in general practice (Figure 12). An examination of LARC prescription type over time in general practice shows that insertions for IUDs fell over time, whereas insertions for IU systems and implants increased (Figure 13). Contrast this with Scotland SHC data, which show that implants were favoured but decreased over time, with IU systems increasing (Figure 14). Similarly, in Wales, implants are most popular, with IUDs and IU systems accounting for around one-quarter of all LARC contacts (Figure 15). A higher proportion of women in the 25–44 years age group consulted their GP regarding LARC use, whereas younger age groups (under 20s and 20–24 years) were more likely to consult SHCs (Figures 16 and 17). Across both settings, consultations for LARC were lowest in the ≥ 45 years age group.
FIGURE 12.
LARC users over time by LARC consultation type: CPRD (2009–18).

FIGURE 13.
LARC users over time by LARC prescription type. (A person contacting a service for the same contraception multiple times during the year will be counted only once. The first attendance for that contraceptive is counted.) CPRD (2009–18). IU, intrauterine.
FIGURE 14.
LARC users over time by LARC prescription type. (A person contacting a service for the same contraception multiple times during the year will be counted only once. The first attendance for that contraceptive is counted.) Scotland SHC data (2009–18).
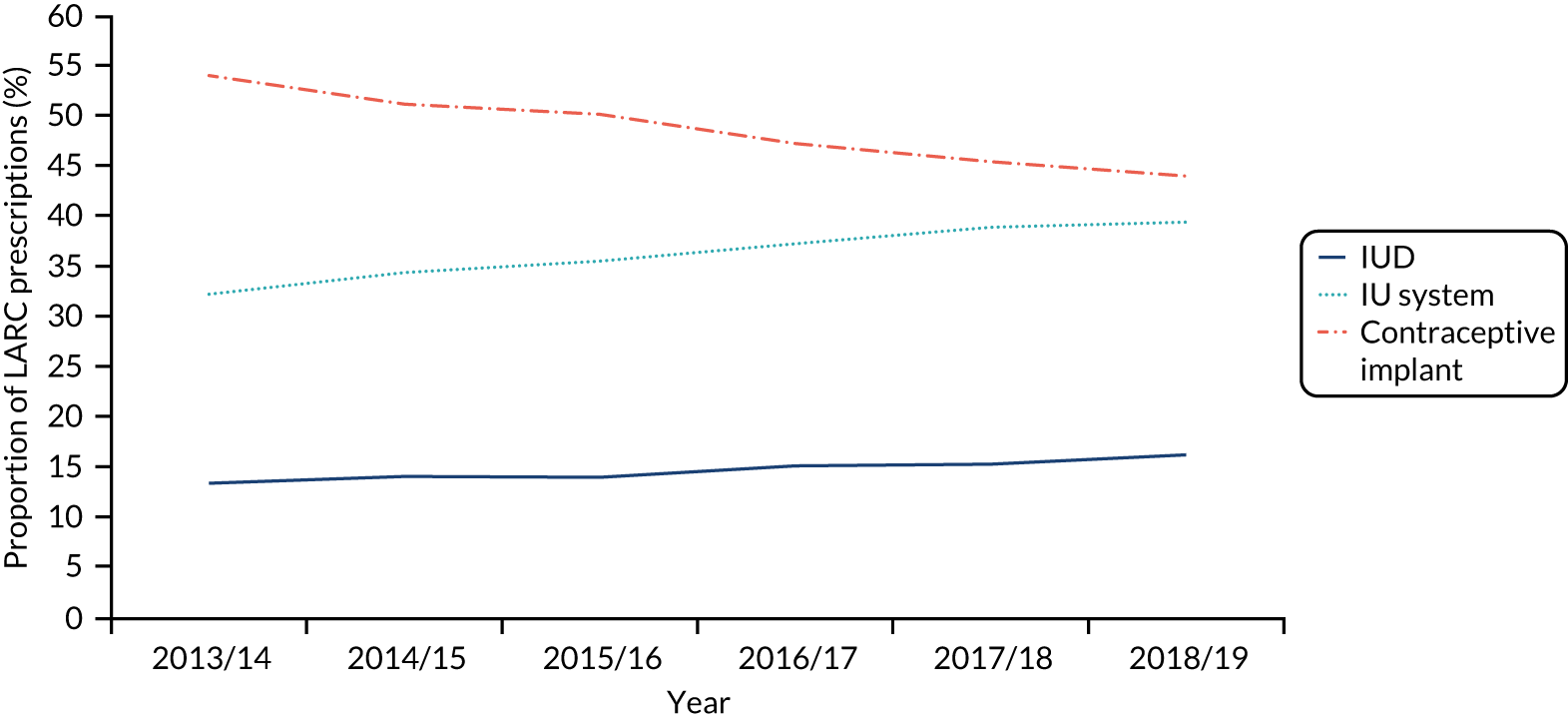
FIGURE 15.
LARC users over time by LARC prescription type. (A person contacting a service for the same contraception multiple times during the year will be counted only once. The first attendance for that contraceptive is counted.) Wales SHC data (2012–18). Note that data from 2012 to 2015 are unreliable owing to under-reporting by some health boards.
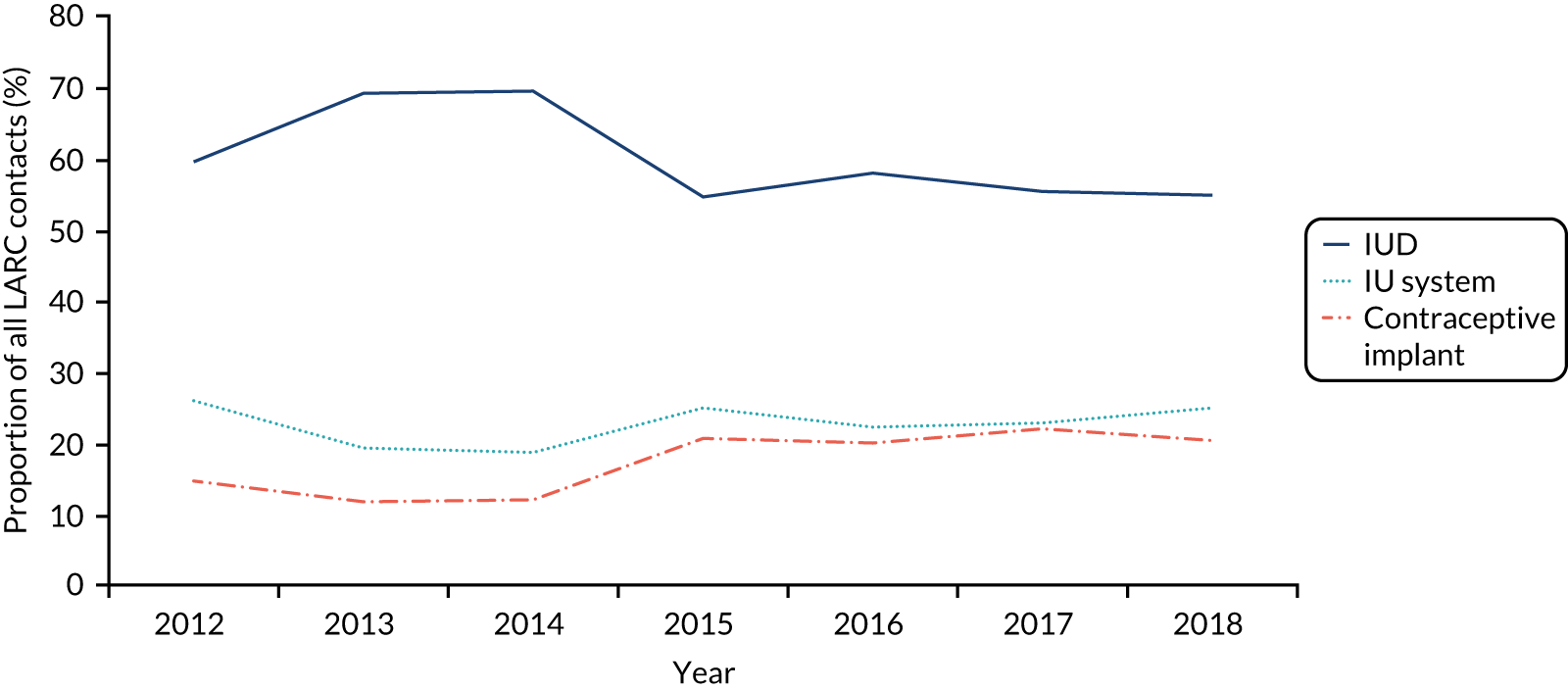
FIGURE 16.
LARC users over time by age group: CPRD (2009–18).

Time to conception for women in different age and body mass index categories
We are interested in how time to conception may differ by BMI and age categories. In total, 7469 pregnancy events (within 7224 women) have both BMI data and conception time data available. The time to conception by BMI category can be seen in Table 28. We are interested in how time to conception may differ between age category. We have split the study population by the following age categories: ≤ 28 versus ≥ 29 years and ≤ 34 versus ≥ 35 years. Age 28 years was the median age of first LARC for women with a pregnancy event, and age 34 years was the median age of first LARC for women in our study population. Additionally, we split the population according to England Sexual and Reproductive Health Services age categories (16–17, 18–19, 20–24, 25–34, 35–44 and 45–54 years). The time to conception by age category can be seen in Table 28 and Figure 18.
| Pregnancy events, n | Conception time (days), median (25th, 75th centiles) | |
|---|---|---|
| BMI category (kg/m2) | ||
| ≤ 24.99 (underweight/healthy) | 3731 | 106 (44, 218) |
| ≥ 25 (overweight) | 3738 | 113 (50, 227) |
| ≤ 29.99 (underweight/healthy/overweight) | 5699 | 107 (46, 220) |
| ≥ 30 (obesity) | 1770 | 118 (54, 231) |
| ≤ 39.99 (underweight/healthy/overweight) | 7264 | 109.5 (47, 222) |
| ≥ 40 (morbid obesity) | 205 | 130 (62, 242) |
| Age category (years) | ||
| ≤ 28a | 5911 | 112 (48, 223) |
| ≥ 29 | 5430 | 108 (47, 218) |
| ≤ 34b | 9580 | 109 (46, 217) |
| ≥ 34 | 1761 | 114 (52, 240) |
| 16–17c | 247 | 169 (75.5, 301.5) |
| 18–19 | 555 | 138 (61.5, 258.5) |
| 20–24 | 2412 | 111 (47, 220) |
| 25–34 | 6335 | 105 (44, 208.5) |
| 35–44 | 1747 | 114 (53, 240) |
| 45–54 | 17 | 154 (36, 311) |
FIGURE 18.
Time to conception by age category.
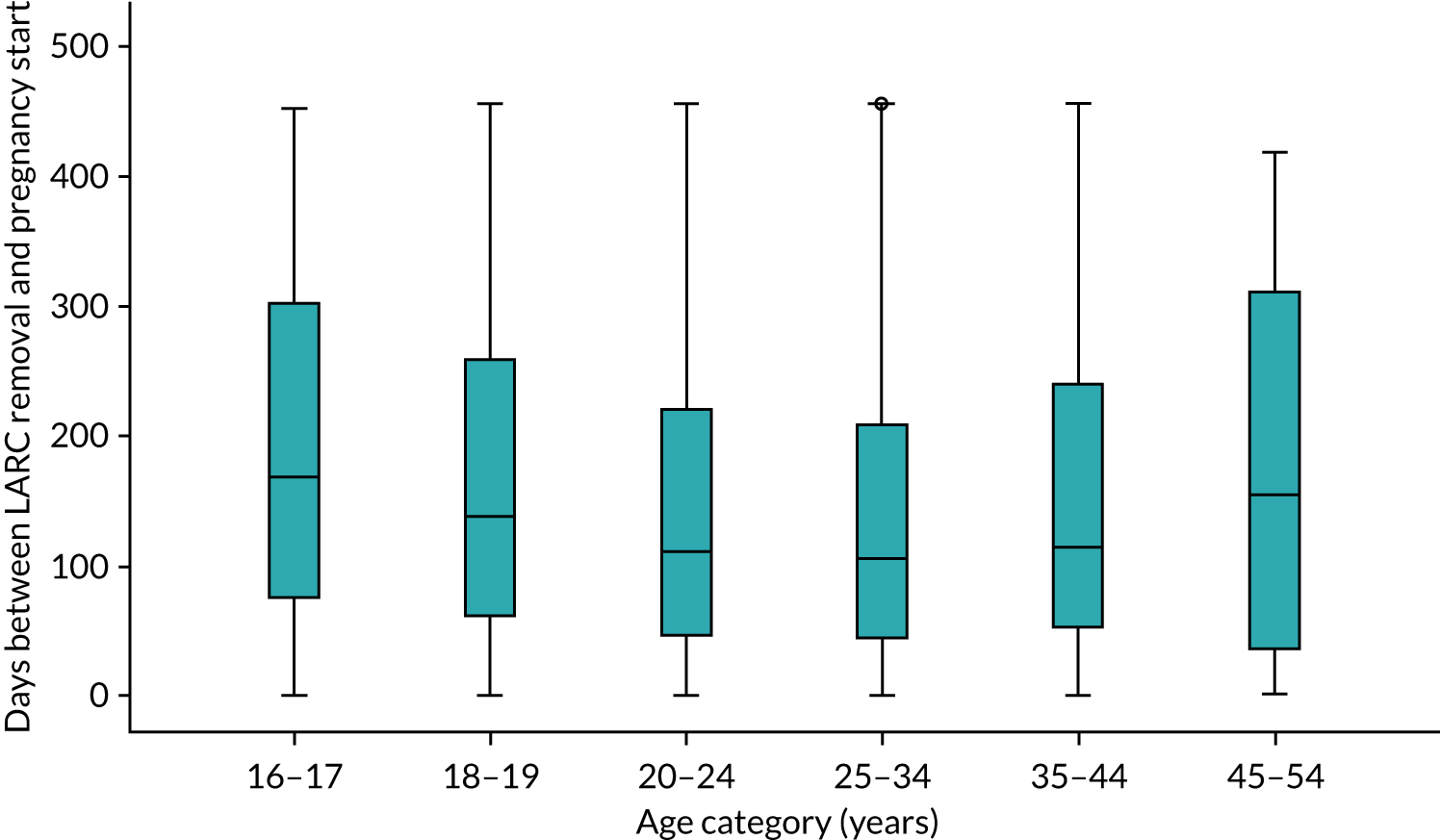
Other analysis: further contraception patterns
We are interested in further contraception patterns for women who had a LARC removed for the purpose of conception and subsequently became pregnant. After pregnancy ended, of the 16,455 pregnancy events, 1365 events (8.3%) had an alternative conception, and 956 events (5.8%) had a LARC in situ or insertion code. The median days between pregnancy end and an alternative conception was 233 days (interquartile range 7–886 days) (Figure 19), and the median days between pregnancy end and next LARC in situ or insertion event was 325 days (interquartile range 26.5–502 days) (Figure 20).
FIGURE 19.
Time between pregnancy end and an alternative conception (days) (n = 1365).

FIGURE 20.
Time between pregnancy end and next LARC in situ or insertion event (days) (n = 956). Mean 397.76 (standard deviation 459.005); n = 956.

| Population | Outcome | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| All women | Number of women aged 16–48 years | 1,413,791 | 1,408,118 | 1,380,332 | 1,355,832 | 1,330,823 | 1,244,434 | 1,108,991 | 914,755 | 812,739 | 741,453 |
| Number of GPs | 738 | 728 | 713 | 699 | 680 | 645 | 580 | 491 | 437 | 397 | |
| Average number of women per GP | 1915 | 1934 | 1935 | 1939 | 1957 | 1929 | 1912 | 1863 | 1859 | 1867 | |
| Number of BMI records | 135,526 | 131,017 | 127,482 | 121,836 | 116,660 | 102,977 | 87,948 | 71,334 | 59,858 | 52,326 | |
| Within how many women | 91,764 | 91,103 | 89,443 | 87,622 | 83,568 | 74,473 | 64,503 | 52,591 | 44,583 | 39,007 | |
| % of women with at least one BMI | 6.49 | 6.47 | 6.48 | 6.46 | 6.28 | 5.98 | 5.82 | 5.75 | 5.49 | 5.26 | |
| Women in study population | Women with at least one LARC event | 61,440 | 67,947 | 66,987 | 66,988 | 65,313 | 59,668 | 51,357 | 40,468 | 35,100 | 31,765 |
| Number of LARC in situ | 31,074 | 31,479 | 30,540 | 28,768 | 25,384 | 22,996 | 18,558 | 13,258 | 10,972 | 9526 | |
| Number of LARC insertions | 65,523 | 75,430 | 72,290 | 72,904 | 71,152 | 64,713 | 55,091 | 42,554 | 38,001 | 34,744 | |
| Number of LARC removals | 11,293 | 12,402 | 13,891 | 14,150 | 14,735 | 14,283 | 13,410 | 10,985 | 9585 | 8470 | |
| Age category (years) | |||||||||||
| 16–17 | 1846 | 2290 | 2084 | 2077 | 2011 | 1754 | 1523 | 1072 | 969 | 846 | |
| 18–19 | 2370 | 2820 | 2556 | 2780 | 2740 | 2571 | 2130 | 1534 | 1290 | 1191 | |
| 20–24 | 7509 | 8115 | 8188 | 8496 | 8650 | 7993 | 6701 | 5299 | 4438 | 3985 | |
| 25–34 | 19,457 | 18,283 | 17,763 | 18,617 | 18,580 | 16,964 | 14,706 | 12,015 | 10,632 | 9442 | |
| 35–44 | 24,399 | 19,534 | 18,346 | 17,942 | 16,851 | 15,046 | 13,356 | 10,289 | 9026 | 8377 | |
| 45–54 | 6583 | 5269 | 5754 | 6075 | 6430 | 6454 | 5866 | 5116 | 4585 | 4317 | |
| LARC type | |||||||||||
| IUD | 58,253 | 58,610 | 56,388 | 51,899 | 46,535 | 41,568 | 33,694 | 24,586 | 21,448 | 19,019 | |
| IU system | 28,266 | 36,417 | 35,150 | 38,208 | 39,396 | 37,106 | 33,053 | 25,907 | 22,243 | 20,095 | |
| Implant | 21,821 | 25,235 | 26,217 | 26,400 | 26,072 | 23,959 | 20,789 | 16,386 | 14,920 | 13,747 | |
| LARC removal without replacing with an alternative contraception within 4 months | 10,024 | 11,029 | 12,299 | 12,532 | 13,042 | 12,563 | 11,872 | 9697 | 8412 | 7366 | |
| Number of pregnancy event (start year) | 1302 | 2378 | 2582 | 2453 | 2292 | 1969 | 1542 | 989 | 705 | 182 | |
| Number of LARC removal with pregnancy for the purpose of conception (groups 1 and 2 plus pregnancy) | 1721 | 1775 | 2016 | 1813 | 1689 | 1387 | 1016 | 631 | 456 | 33 | |
| Number of LARC use for the purpose of conception (groups 1 and 2) | 2039 | 2203 | 2691 | 2543 | 2497 | 2007 | 1540 | 980 | 726 | 182 | |
| For the purpose of conception, n (%) | |||||||||||
| Without BMI | 666 (32.7) | 774 (35.1) | 877 (32.6) | 867 (34.1) | 831 (33.3) | 693 (34.5) | 557 (36.2) | 408 (41.5) | 293 (40.4) | 80 (44.0) | |
| With BMI | |||||||||||
| BMI < 24.99 kg/m2 | 725 (52.8) | 739 (51.7) | 868 (47.9) | 782 (46.7) | 797 (47.8) | 648 (49.3) | 442 (45.0) | 268 (46.9) | 187 (43.2) | 38 (37.3) | |
| BMI ≥ 25 kg/m2 | 648 (47.2) | 690 (48.3) | 946 (52.1) | 894 (53.3) | 869 (52.2) | 666 (50.7) | 541 (55.0) | 304 (53.1) | 246 (56.8) | 64 (62.7) | |
| No LARC removal code before conception – remove elsewhere | 337 | 784 | 884 | 730 | 642 | 532 | 395 | 242 | 167 | 40 | |
| Rate of women who request LARC removal and subsequently have a pregnancy (groups 1 and 2/women at reproductive age) | 0.0014 | 0.0016 | 0.0019 | 0.0019 | 0.0019 | 0.0016 | 0.0014 | 0.0011 | 0.0009 | 0.0002 | |
Appendix 4 Sexual health clinic data: open access
Scotland sexual health clinic summary table
| 2013/14 | 2014/15 | 2015/16 | 2016/17 | 2017/18 | 2018/19 | |
|---|---|---|---|---|---|---|
| Number of prescriptions | ||||||
| IUDs | 3698 | 3942 | 3893 | 4345 | 4502 | 4985 |
| Contraceptive implant | 14,823 | 14,268 | 13,899 | 13,510 | 13,297 | 13,465 |
| IU systems | 8856 | 9601 | 9859 | 10,655 | 11,392 | 12,064 |
| LARC by NHS board | ||||||
| Scotlanda | 27,377 | 27,811 | 27,651 | 28,510 | 29,191 | 30,514 |
| Ayrshire & Arran | 1754 | 1813 | 1820 | 2000 | 2046 | 2069 |
| Borders | 589 | 516 | 595 | 592 | 612 | 710 |
| Dumfries & Galloway | 440 | 474 | 467 | 592 | 645 | 737 |
| Fife | 1831 | 1849 | 1890 | 1915 | 1871 | 1819 |
| Forth Valley | 867 | 782 | 958 | 1025 | 1027 | 1013 |
| Grampian | 1500 | 1648 | 1532 | 1666 | 2071 | 2109 |
| Greater Glasgow & Clyde | 10,763 | 10,488 | 9828 | 9790 | 10,035 | 10,190 |
| Highland | 808 | 840 | 961 | 1088 | 1146 | 1218 |
| Lanarkshire | 3040 | 3374 | 3545 | 3723 | 3637 | 3874 |
| Lothian | 4031 | 4263 | 4467 | 4498 | 4550 | 5277 |
| Orkney | NA | NA | NA | NA | NA | NA |
| Shetland | NA | NA | NA | NA | NA | NA |
| Tayside | 1754 | 1764 | 1588 | 1615 | 1551 | 1489 |
| Western Isles | NA | NA | NA | NA | NA | NA |
| LARC use by age group (years) | ||||||
| < 20 | 8327 | 7936 | 7375 | 6460 | 6318 | 6749 |
| 20–24 | 10,636 | 10,481 | 10,267 | 10,476 | 10,401 | 10,571 |
| 25–29 | 9295 | 9342 | 9242 | 9619 | 9654 | 9679 |
| 30–34 | 7986 | 8009 | 8015 | 8153 | 7942 | 8208 |
| 35–39 | 6346 | 6459 | 6543 | 6954 | 7017 | 7304 |
| 40–44 | 6179 | 5970 | 5767 | 5901 | 5555 | 5852 |
| ≥ 45 | 5316 | 5512 | 5863 | 6211 | 6508 | 6792 |
| Not known | 12,261 | 12,464 | 11,943 | 11,075 | 9947 | 10,445 |
| LARC use by deprivation areab | ||||||
| 1 (most deprived) | NA | NA | 7473 | 7650 | 7342 | 6734 |
| 2 | NA | NA | 5444 | 5753 | 5599 | 5306 |
| 3 | NA | NA | 4385 | 4783 | 4670 | 4513 |
| 4 | NA | NA | 4281 | 4305 | 4212 | 4248 |
| 5 (least deprived) | NA | NA | 4289 | 4470 | 4433 | 4608 |
| Not known | NA | NA | 1779 | 1549 | 2935 | 5105 |
| Total | NA | NA | 27,651 | 28,510 | 29,191 | 30,514 |
England Sexual and Reproductive Health Services summary table
| 2014/15 | 2015/16 | 2016/17 | 2017/18 | 2018/19 | ||
|---|---|---|---|---|---|---|
| Number of prescriptions (all ages) | IUDs | 54,216 | 52,855 | 54,198 | 56,593 | 60,921 |
| Contraceptive implant | 67,666 | 70,621 | 72,229 | 74,931 | 83,369 | |
| IU systems | 138,937 | 140,019 | 134,623 | 129,760 | 136,584 | |
| Contraception-related services (excluding those aged under 16 years) | New main methoda | 244,459 | 218,291 | 211,352 | 192,441 | 189,119 |
| Change of main method | 280,508 | 280,460 | 272,779 | 265,102 | 267,074 | |
| Maintain existing main method | 930,092 | 889,076 | 825,248 | 743,033 | 711,743 | |
| Pre-contraception adviceb | 194,872 | 177,573 | 155,585 | 129,020 | 127,978 | |
| LARC by local authority (excluding those aged under 16 years between 2014/15 and 2017/18, all age at 2018/19) | England | 253,604 | 257,322 | 256,210 | 256,657 | 263,000 |
| North East region | 16,070 | 15,450 | 13,685 | 13,075 | 32,000 | |
| North West region | 45,750 | 43,675 | 40,630 | 40,950 | 30,000 | |
| Yorkshire & the Humber region | 22,140 | 19,245 | 20,845 | 23,440 | 37,000 | |
| East Midlands region | 19,165 | 17,705 | 17,830 | 18,875 | 44,000 | |
| West Midlands region | 22,900 | 22,285 | 20,420 | 22,100 | 35,000 | |
| East of England region | 17,510 | 19,060 | 16,635 | 19,150 | 37,000 | |
| London region | 51,080 | 54,055 | 54,765 | 53,130 | 36,000 | |
| South East region | 30,895 | 31,900 | 33,325 | 33,260 | 34,000 | |
| South West region | 21,915 | 22,530 | 24,300 | 22,520 | 40,000 | |
| Women using sexual and reproductive health services by age group (years) | 16–17 | 105,997 | 90,127 | 77,917 | 71,238 | 66,153 |
| 18–19 | 132,982 | 125,396 | 115,392 | 109,143 | 103,807 | |
| 20–24 | 305,978 | 298,185 | 286,336 | 276,326 | 271,202 | |
| 25–34 | 334,089 | 333,866 | 327,927 | 326,036 | 334,406 | |
| 35–44 | 154,734 | 154,166 | 147,769 | 144,958 | 153,579 | |
| 45–54 | 69,249 | 67,749 | 63,879 | 61,148 | 64,376 | |
| ≥ 55 | 11,778 | 11,582 | 10,182 | 9,574 | 11,238 | |
| LARC (IUD, IU system and implant) by age groupc (all local authorities between 2014 and 2015 and England only at 2017/18) | 16–17 | 34,177 | 30,489 | 24,404 | 24,596 | 12,733 |
| 18–19 | 43,397 | 41,119 | 36,570 | 37,047 | 19,191 | |
| 20–24 | 111,989 | 111,975 | 107,591 | 112,836 | 60,758 | |
| 25–34 | 164,616 | 168,857 | 171,967 | 178,347 | 98,643 | |
| 34–44 | 102,451 | 104,219 | 107,156 | 103,723 | 58,296 | |
| ≥ 45 | 44,399 | 46,568 | 48,787 | 46,608 | 25,871 | |
| IMD decile (women aged 13–54 years using services – percentage of population)d (all ages and including injectable contraceptive) | 1 (most deprived) | 11.46 | 10.56 | 7.73 | 8.00 | 7.89 |
| 2 | 10.61 | 8.64 | 6.75 | 6.51 | 5.99 | |
| 3 | 9.07 | 7.41 | 6.13 | 6.00 | 5.22 | |
| 4 | 7.84 | 6.26 | 5.45 | 5.16 | 4.89 | |
| 5 | 6.82 | 5.87 | 4.34 | 4.57 | 4.17 | |
| 6 | 5.99 | 5.28 | 4.66 | 4.62 | 4.17 | |
| 7 | 5.61 | 5.09 | 4.28 | 4.09 | 3.94 | |
| 8 | 5.06 | 5.33 | 4.16 | 3.93 | 3.45 | |
| 9 | 4.93 | 3.87 | 3.67 | 3.59 | 2.41 | |
| 10 (least deprived) | 4.23 | 2.95 | 2.56 | 2.40 | 2.35 | |
| Total | 7.59 | 6.50 | 5.58 | 5.04 | – |
Appendix 5 Data cleaning flow chart for body mass index
FIGURE 21.
Data cleaning flow chart for BMI.
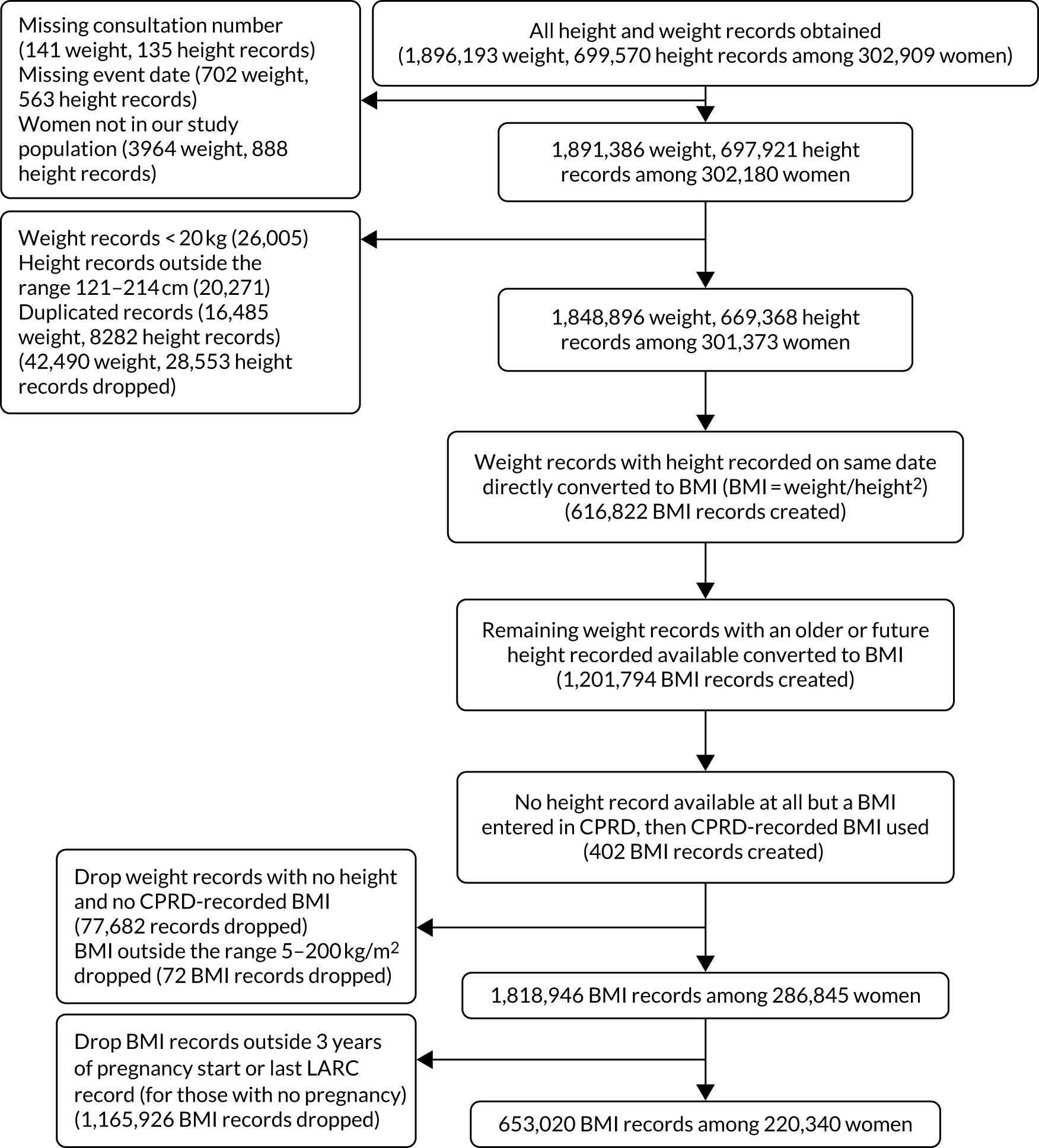
Appendix 6 Search strategies and results
Policy review
Review aims and search strategies
Aim
To identify advice, guidance and/or policy documents pertaining to use of LARCs in the UK.
Website searches targeted towards: LARC removal, management of women with obesity/overweight, diet, exercise. Specific search terms utilised: ‘LARC’, ‘removal’, ‘coil’, ‘implant’, ‘obese’, ‘obesity’, ‘overweight’, ‘weight’, ‘diet’, ‘exercise’.
Key organisations to search (websites and/or online repositories): FSRH, RCOG, NICE and BPAS.
Additional materials were identified by the study team and/or advisory group members.
Results
A total of 15 documents were identified for inclusion in the review.
| Data source: organisational website/repository or study team | Document type | Year of publication | Document title |
|---|---|---|---|
| FSRH | CEU statement167 | 2019 | ‘Contraception and weight gain’ |
| Guideline168 | 2019 | ‘Contraception for women aged over 40 years’ | |
| Clinical guidance99 | 2019 | ‘Intrauterine contraception’ | |
| CEU statement169 | 2018 | ‘Contraception for women with eating disorders’ | |
| Clinical guideline170 | 2017 | ‘Contraception after pregnancy’ | |
| CEU statement100 | 2015 | ‘Provision of LARC methods to young women in the UK’ | |
| Clinical guidance171 | 2014 | ‘Progestogen-only implant’ | |
| Guideline172 | 2019 | ‘Overweight, obesity and contraception’ | |
| RCOG | Press release101 | 2018 | ‘RCOG co-launches new digital tool to help prepare women for pregnancy’ (Tommy’s Planning for Pregnancy tool) |
| Green-top Guideline98 | 2018 | ‘Care of women with obesity in pregnancy’ | |
| NICE | Public health guideline16 | 2010 | ‘Weight management before, during and after pregnancy’ |
| Clinical guideline173 | 2005 | ‘Long-acting reversible contraception’ | |
| BPAS | Press release174 | 2017 | ‘Women cannot control fertility through contraception alone’ |
| Identified by study team | Review175 | 2014 | ‘Preconception care policy, guidelines, recommendations and services across six European countries: Belgium (Flanders), Denmark, Italy, the Netherlands, Sweden and the United Kingdom’ |
| FIGO guideline20 | 2020 | ‘Management of prepregnancy, pregnancy, and postpartum obesity from the FIGO Pregnancy and Non-Communicable Diseases Committee: a FIGO (International Federation of Gynecology and Obstetrics) guideline’ |
Barriers to and facilitators of engagement in pre-conception health behaviour change interventions and research
Review aims and search strategies
Aim
To identify studies focused on preconception health and behaviour change, including, but not specific to, diet and weight loss, and to identify key barriers to and facilitators of successful engagement in behaviour change.
A series of search strategies were designed to identify studies that addressed the following target behaviours:
-
diet
-
alcohol
-
smoking
-
folic acid
-
eating/dietary restrictions.
Date range
2008–19.
Databases searched
EMBASE™ (Elsevier, Amsterdam, the Netherlands) (via Ovid), Web of Science™ (Clarivate Analytics, Philadelphia, PA, USA).
Results
A total of eight studies were identified for inclusion in the review.
| Search strategy | EMBASE | Web of Science | Duplicates | Inclusions | Running total |
|---|---|---|---|---|---|
| “behaviour change” AND “diet” AND (“preconception” or “pre-conception”) | 6 | 4 | 2 | 8 | 8 |
| “behaviour change” AND “alcohol” AND (“preconception” or “pre-conception”) | 9 | 4 | 4 | 9 | 17 |
| “behaviour change” AND “smoking” AND (“preconception” or “pre-conception”) | 4 | 2 | 2 | 4 | 21 |
| “behaviour change” AND “folic acid” AND (“preconception” or “pre-conception”) | 3 | 2 | 1 | 4 | 25 |
| “behaviour change” AND “eating restrictions” AND (“preconception” or “pre-conception”) | 0 | 0 | 25 | ||
| “behaviour change” AND “dietary restrictions” AND (“preconception” or “pre-conception”) | 0 | 0 | 25 | ||
| Duplicates (between searches) | 8 | 17 | |||
| Studies excluded at full-text review (conference abstracts, protocols, duplicates between study searches) | 9 | 8 | |||
| Final total | n = 8 | ||||
Review of key qualitative and survey-based literature (preconception and prenatal)
A total of 18 qualitative and survey-based studies, reporting on service user and provider experiences, behaviours and perspectives on prenatal and preconception weight, were identified via the main study searches.
Review of review papers and intervention strategies
| Search aim | Search strategy | Setting | Databases searched | Inclusion criteria | Data to be extracted | Results |
|---|---|---|---|---|---|---|
| Recent preconception and obesity reviews and meta-analyses | Preconception AND obes* filter for meta-analysis/review | English-language only. High- and middle-income countries | MEDLINE, PubMed, ScienceDirect | Meta-analysis, review, date range: 2010–20 | Methods, participants, intervention characteristics, results, information relevant to CMO configurations |
8 papers included in final review Total hits, N = 139 Excluded, n = 131 Not a review, n = 20 Unavailable, n = 3 Child-focused outcomes, n = 18 Nutrition focus, n = 11 Postpartum and pregnancy n = 7 Anima/lab based n = 5 LMIC n = 8 Specific condition, n = 59 A further 13 identified via snowballing using references of papers and via other searches |
| Theories of interventions | (“logic model” OR “theory of change” OR “theory of action” OR “outcomes chain” OR “program * theory” OR “program * logic” OR “logical framework”) AND (“preconception care” OR “preconception health”) |
English language only High- and middle-income countries |
Google Scholar | Publications 2015–20 | Theory identified underpinning intervention |
14 papers included in the final review 65 identified Screen abstracts 49 not relevant/lacked adequate explanatory power to contribute to theory 11 LMIC 1 duplicate 4 read in full and 2 excluded as no theoretical component |
Review of pregnancy and weight management studies
Review aims and search strategies
Aim
To identify key effective weight management interventions in pregnancy and to summarise content and context to be considered in a preconception weight management intervention. A simple search of systematic reviews, and subsequent snowballing, was carried out to identify relevant interventions.
Date range
2010–19.
Specific search strategy
“Systematic review” AND (“intervention” OR “programme”) AND (“obesity” OR “overweight” OR “obese”) AND ((“diet” OR “physical activity” OR “exercise” OR “healthy eating” OR “lifestyle” OR “weight loss” OR “weight management” OR “healthy lifestyle” OR “weight gain”) AND (“pregnant” OR “pregnancy” OR “gestation” OR “obstetrics”)
Databases searched
PubMed.
Results
A total of 35 systematic reviews were reviewed for relevant information and key weight management in pregnancy interventions.
| Search terms | PubMed | Total hits | Excluded | Final total |
|---|---|---|---|---|
| Intervention AND (“Weight loss” OR “Weight management” OR “diet counselling” OR overweight OR obese OR obesity) AND (“Physical activity” OR exercise OR diet OR “healthy eating”) AND (pregnancy OR postnatal* Or post-natal*) AND (“Randomised controlled trail” OR “Randomized controlled trail” OR trial OR study) | 109 | 109 | 74 | 35 |
Appendix 7 Policy review results
| Organisation and website/repository searched | Document title | Document type | Year | Topic(s) of discussion | |
|---|---|---|---|---|---|
| 1 | FSRH (www.fsrh.org) | Contraception for women with eating disorders169 | CEU statement | 2018 | Weight gain associated with LARC |
| 2 | FSRH (www.fsrh.org) | Provision of LARC methods to young women in the UK100 | CEU statement | 2015 | n/a |
| 3 | FSRH (www.fsrh.org) | Intrauterine contraception99 | Clinical guidance | 2019 |
LARC removal as an opportunity to provide preconception health advice LARC removal Weight gain and LARCs |
| 4 | FSRH (www.fsrh.org) | Progestogen-only implants171 | Clinical guidance | 2014 |
Weight gain and contraception Drug interactions; LARCs and weight |
| 5 | FSRH (www.fsrh.org) | Contraception after pregnancy170 | Clinical guideline | 2017 | Obesity as a medical consideration |
| 6 | FSRH (www.fsrh.org) | Overweight, obesity and contraception172 | Guideline | 2019 |
Progesterone-only implants Anti-obesity medication and contraception Benefits and risks of combined hormonal contraception (oral, patch, transvaginal ring) Effectiveness of IUDs/LARC in women with overweight or obesity Progesterone-only injectables and weight/BMI |
| 7 | FSRH (www.fsrh.org) | Contraception for women aged over 40 years168 | Guideline | 2019 | Weight gain |
| 8 | FSRH (www.fsrh.org) | Contraception and weight gain167 | CEU statement | 2019 | Weight gain |
| 9 | Denison et al., on behalf of RCOG (www.rcog.org.uk)18 | Care of Women with Obesity in Pregnancy | Green-top Guideline (No. 72) | 2019 | PCC and maternal weight |
| 10 | RCOG (www.rcog.org.uk) | RCOG co-launches new digital tool to help prepare women for pregnancy (Tommy’s Planning for Pregnancy tool)101 | Press release | 2018 | Promotion of preconception health/healthy pregnancy advice |
| 11 | NICE (www.nice.org.uk) | Long-acting reversible contraception173 | Clinical guideline | 2005 | LARC removal |
| 12 | NICE (www.nice.org.uk) | Weight management before, during and after pregnancy16 | Public health guideline | 2010 | Pre-pregnancy planning for women with high BMI |
| 13 | BPAS (www.bpas.org) | Women cannot control fertility through contraception alone174 | Press release | 2017 | LARC failure |
| 14 |
Shawe et al. 2015175 Identified by study team/advisory group |
Preconception care policy, guidelines, recommendations and services across six European countries: Belgium (Flanders), Denmark, Italy, the Netherlands, Sweden and the UK | Review | 2014 | Scope and content of current PCC guidance in Europe |
| 15 |
McAuliffe et al. , on behalf of the International Federation of Gynaecology and Obstetrics (FIGO) Pregnancy and Non-Communicable Diseases Committee20 Identified by study team/advisory group |
Management of prepregnancy, pregnancy, and postpartum obesity | FIGO guideline | 2020 |
Impact of obesity on preconception, pregnancy and post-pregnancy care Effectiveness and applicability of interventions |
Appendix 8 Review paper results
| Authors and year | Source type | Title | Key preconception papers identified/research notes | Findings | |
|---|---|---|---|---|---|
| 1 | Hill et al. 2021120 | CR | Expanding our understanding and use of ecological systems theory model for the prevention of maternal obesity: a new socioecological framework | Theoretical model with potential to underpin intervention development (Kothe et al.176) | Considers the ecological systems theory model for maternal obesity prevention, including in the preconception phase, and the importance of moving away from an oversimplistic focus on the woman’s behaviour and responsibility for change; instead, intervention design and understanding of obesity needs to take the wider system into account |
| 2 | Hutchesson et al. 202051 | ScR | Supporting women of childbearing age in the prevention and treatment of overweight and obesity: a scoping review of randomised control trials of behavioural interventions | Only one preconception paper: the Prepare trial protocol (LeBlanc et al.52) | This scoping review identified an increasing volume of research over time undertaken to support women of childbearing age to prevent and treat overweight and obesity. Only preconception study is LeBlanc et al. protocol. Of the 87 included RCTs, 52.9% (n = 46) focused on preventing excessive GWG, 20.7% (n = 18) focused on weight loss or preventing weight retention in the post-partum period, and 16.1% (n = 14) focused on both, with the remaining studies in the general population, not related to pregnancy |
| 3 | Caut et al. 202033 | SR | Dietary guideline adherence during preconception and pregnancy: a systematic review | Importance of diet | Review of observational studies of dietary intake of men and women in preconception and pregnancy. Main findings are that usual diet is insufficient in terms of nutritional guidelines. Preconceptual and pregnant women may not be consuming enough vegetables, cereal grains, folate, iron and calcium, and may be consuming excess fat |
| 4 | Farpour-Lambert et al. 201822 | SR MA | Obesity and weight gain in pregnancy and postpartum: an evidence review of lifestyle interventions to inform maternal and child health policies | Focus on pregnancy but identifies need for preconception intervention and also some key parameters | Weight loss prior pregnancy is probably needed to achieve both GWG goals and optimal pregnancy outcomes. Weight loss objectives should be realistic (5–10% over a period of 6 months) and individualised. Structured intensive programs using cognitive–behavioural techniques in individual or group setting are effective in achieving realistic goals in an adequate time frame |
| 5 | Musgrave et al. 2019177 | SR MA | Addressing preconception behaviour change through mobile phone apps: a protocol for a systematic review and meta-analysis | When findings available they can be used to inform any app-based aspect of future intervention | This review will include trials that assess any mobile phone application that assist women of reproductive age to optimise health behaviours |
| 6 | Barker et al. 201829 | CR | Intervention strategies to improve nutrition and health behaviours before conception | Community engagement with preconception | Preconception interventions often require engagement from individuals who are unlikely to be using maternal health services. Interventions to improve health behaviours in adolescents and young adults might, therefore, need to appeal to motivations unrelated to health |
| 7 | Stephenson et al. 201827 | CR | Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health | Help to think about the stage of ‘planning’ women are at and how that relates to potential target behaviours/population-based intervention | Discussion of the concept of planning in pregnancy and whether it is linked to preconception health behaviours. Planning is more common than previously recognised and associated with a mixed pattern of health behaviours before conception. Paper proposes three definitions of the preconception period relating to embryo development and actions at individual or population level |
| 8 | Goossens et al. 2018131 | SR | Barriers and facilitators to the provision of preconception care by healthcare providers: a systematic review | Key elements to consider at provider level for any preconception intervention | Mixed-methods SR including 31 papers. Barriers were more reported than facilitators, including attitude and knowledge of provider and client. Limited resources (lack of time, tools, guidelines and reimbursement) were frequently reported at the organisational and societal levels. Conclusions: need for multilevel interventions |
| 9 | Toivonen et al. 2017139 | ScR | Preconception health behaviours: a scoping review | Identifies narrow definition of relevant preconception health behaviours | This paper briefly reviews evidence of the importance of various preconception health behaviours and examines the extent to which specific preconception health behaviours have been included in recent studies of such knowledge, behaviours and intentions. Over 40% of studies examining preconception health behaviour focused exclusively on folic acid; only 11% of all studies included male participants. Recommends including men in future research, assessing a wider variety of behaviours and consideration of intention, knowledge and behaviour |
| 10 | Brown et al. 2017178 | SR | Preconception health interventions delivered in public health and community settings: a systematic review | Agricola et al. 2014;179 Schwarz et al. 2008;180 Chan et al. 2001;181 DeJoy 2014;182 Hillemeier et al. 2008;71 Hussaini et al. 2013;183 Whitehill King et al. 2013;184 Mackert et al. 2012;185 Milan and White 2010;186 Wade et al. 2012;187 Watson et al. 2001;188 Williams et al. 2001189 | PCC interventions typically focus on medical and lifestyle determinants of preconception health and are aimed at the individual. Methodological quality of research is poor, and there is a lack of information on interventions appropriate for men and LGBTQ populations. No studies targeted broader determinants of preconception health, including mental health and environment |
| 11 | Hemsing et al. 201737 | ScR | Preconception health care interventions: a scoping review | Beckmann et al. 2014190 reporting change in BMI in 6 months prior to conception | Interventions are mostly education regarding risk and measured alcohol, caffeine and dietary changes and change in knowledge/attitudes. Only one study reports BMI change190 |
| 12 | i-WIP review of pregnancy interventions: International Weight Management in Pregnancy (i-WIP) Collaborative Group 201721 | MA | Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials | Lessons from pregnancy | Diet- and physical activity-based interventions during pregnancy reduce GWG and lower the odds of caesarean section. There is no evidence that this differs across different subgroups of women |
| 13 | Lan et al. 201748 | SR MA | Systematic review and meta-analysis of the impact of preconception lifestyle interventions on fertility, obstetric, fetal, anthropometric and metabolic outcomes in men and women | Mutsaerts et al. 2010;191 Mutsaerts et al. 2016;150 Moran et al. 2011;192 Sim et al. 2014;145 Legro et al. 201544 Identified lack of weight preconception research outside fertility treatment context | Impact of preconception lifestyle interventions on live birth, birthweight and pregnancy rate. The search returned 1802 articles and eight studies were included for analysis. Populations targeted were primarily overweight or obese subfertile women seeking reproductive assistance, with few community-based studies and none including men. MA showed greater reduction in weight and BMI with intervention |
| 14 | Hanson et al. 2017112 | CR | Interventions to prevent maternal obesity before conception, during pregnancy and post-partum | Barker et al. (Southampton initiative) 2011;193 Van Dijk 2017.138 Key theoretical models, e.g. Bronfenbrenner 1979194 | Identifies the need for an integrated approach that involves more that just primary care, taking a much wider perspective than the individual – an ecological approach to risk reduction. Much wider societal understanding on preconception health is needed. Discussion of theoretical models of behaviour change |
| 15 | Hill 2016195 | NR | Psychological health and lifestyle management preconception and in pregnancy | Importance of psychological factors | Positive role of healthful lifestyles before and during pregnancy. Factors such as psychological well-being, individual motivation for behaviour change and broader social influences are key modifiable aspects to consider in relation to changes in diet and activity. Also require system-wide changes to impact on barriers to healthful lifestyles |
| 16 | Hussein et al. 201636 | SR | The effects of preconception interventions on improving reproductive health and pregnancy outcomes in primary care: a systematic review | SHW196 intervention (includes BMI and anthropometric measures). Change to folic acid and activity but not anthropometrics | This review identified encouraging benefits of preconception education and counselling on maternal knowledge, self-efficacy and health locus of control, and risk behaviour. The effects on adverse pregnancy outcomes remain unclear |
| 17 | Opray et al. 201546 | SR | Directed preconception health programs and interventions for improving pregnancy outcomes for women who are overweight or obese | Identified lack of preconception weight research. SHW196 intervention found but excluded as unable to report outcomes by BMI category | Found no randomised controlled trials that assessed the effect of preconception health programmes and interventions in overweight and obese women with the aim of improving pregnancy outcomes |
| 18 | Muktabhant et al. 2015159 | SR | Diet or exercise, or both, for preventing excessive weight gain in pregnancy | Value of weight management combining diet and exercise in pregnancy | High-quality evidence indicates that diet or exercise, or both, during pregnancy can reduce the risk of excessive GWG, and also has other health benefits, particularly for high-risk women receiving combined diet and exercise interventions. Exercise appears to be an important part of controlling weight gain in pregnancy and more research is needed to establish safe guideline |
| 19 | Heslehurst et al. 2014142 | MS | Implementation of pregnancy weight management and obesity guidelines: a meta-synthesis of healthcare professionals’ barriers and facilitators using the theoretical domains framework | Theory-driven review of themes of health-care practitioner boundaries and facilitators with themes resonating from pregnancy | This systematic review aimed to identify the determinants of health-care professionals’ behaviours in relation to maternal obesity and weight management. Twenty-five studies were included. The domains most frequently identified included ‘knowledge’, ‘beliefs about consequences’ and ‘environmental context and resources’. Health-care professionals’ weight management practice had the most barriers compared with any other area of maternal obesity practice |
| 20 | Agha et al. 20143 | SR MA | Interventions to reduce and prevent obesity in pre-conceptual and pregnant women: a systematic review and meta-analysis | Weisman et al. 2011.49 Long-term effects of the SHW intervention | Behavioural interventions in pregnancy may be effective in reducing GWG in obese women without comorbid conditions, but not in overweight or morbidly obese women. Behavioural interventions had no effect on post-partum weight loss, gestation week of delivery and infant birth weight in overweight, obese and morbidly obese women |
| 21 | Forsum et al. 2013197 | SR | Weight loss before conception: a systematic literature review | Highlights lack of evidence; see Caut et al. 202033 | The objective of this study is to assess the effect of weight loss prior to conception in overweight or obese women on a number of health-related outcomes in mother and offspring. Concludes a lack of evidence on safe weight loss approaches pre conception |
| 22 | Dellisaint and McKyer 201138 | SR | A systematic review of factors utilised in preconception health behaviour research | Weight does not appear to be included as a risk factor. Lack of association between knowledge and behaviour change | This systematic review critically synthesises the literature focusing on factors related to preconception health behaviours among childbearing-age women. Six factors identified: alcohol, glycaemic control, physical activity, pregnancy planning and screening. Knowledge, awareness and beliefs about PCC do not lead to preconception health practice |
Appendix 9 Weight management in pregnancy key studies
| Study/lead author | Methods | Intervention | Results | Key context considerations | |
|---|---|---|---|---|---|
| 1 | Bogaerts Antenatal Lifestyle Study198 | RCT: brochure vs. lifestyle intervention vs. usual care | Antenatal lifestyle intervention based on motivational interviewing principles | Targeted lifestyle intervention programme reduces GWG and levels of anxiety |
Pre-pregnancy BMI association with anxiety Group-based intervention |
| 2 | LIMIT199–205 | RCT: diet plus activity vs. usual care | Individualised plans based on Australian recommended dietary standards plus advice on walking/incidental activity | Improved maternity dietary levels and PA during pregnancy, not sustained at 4 months post partum. No impact on other maternal outcomes or GWG | Theoretical design: informed by stage theories of health decision-making |
| 3 | HELP-her Australia206 | RCT: lifestyle programme vs. control for women at risk of GDM | Individual dietary advice and goals | Significant impact on GWG in women with overweight, not in women with obesity |
Health coach delivered intervention at antenatal visits Different impact for different baseline BMI group |
| 4 | Pregnancy Lifestyle Intervention207 | Feasibility RCT: lifestyle programme vs. control for women at risk of GDM | Targeted, culturally sensitive lifestyle intervention for Hispanic women | Improvement in diet but no impact on GWG | Culturally sensitive intervention delivered by health educators |
| 5 | Therapeutic Lifestyle Changes208 | RCT: diet and PA vs. control for women with BMI of > 25 kg/m2 | Change in eating patterns and consumption of high-GI foods | Significant effect on GWG and GDM. Improvements in diet | Intervention described as behavioural but no details; delivered by dietitian |
| 6 | UPBEAT UK118,209–215 | RCT: diet and PA for women with BMI of ≥ 30 kg/m2 | Intervention to lower GI and saturated fats | No significant effect on GWG. Improvements in PA and diet. Sustained reduction in dietary glycaemic load and saturated fat intake at 6 months post partum | Delivered in NHS combined group and individual. Differential impact associated with ethnicity. Theoretical design social cognitive theory |
| 7 | Four-step multidisciplinary approach intervention216 | RCT: four-step multidisciplinary approach vs. usual care | Intervention included dietary advice and assessment of psychological factors in eating habits | Reduced GDM and GWG | Multidisciplinary biopsychosocial intervention |
| 8 | TOP study217 | RCT: dietary advice plus problem-solving with PA vs. PA only vs. control | Intervention based on hypocaloric Mediterranean-style diet delivered by dietitian | Both interventions reduced GWG compared with controls | Dietetics delivery. Use of problem-solving, goal-setting, etc., but no theoretical aspect of design reported. Impact of PA component |
| 9 | Healthy Moms US111,218 | RCT: diet and PA intervention aimed at achieving GWG within 3% of baseline weight, plus standard care | Intervention combining individual and group sessions with dietitian focus on Dietary Approaches to Stop Hypertension, PA, behaviour change | Significant impact on GWG | Use of BCTs, but no specific theory identified. High-intensity intervention |
| 10 | Lifestyle in pregnancy (LIP)219,220 | RCT: diet and PA intervention vs. control | Individualised dietary advice and PA, including pedometer, gym membership, weekly training | Intervention reduced GWG. Increased PA during pregnancy but not sustained post partum | Tailored advice based on energy requirement goals |
| 11 | ROLO study221–227 | RCT: diet-only intervention delivered in small groups vs. routine care | Intervention delivered by dietitians in three small group sessions. Advice on healthy, low-GI diet | Intervention slowed GWG in later pregnancy, reduced overall change in insulin concentrations and improved GI dietary intake | Low intensity intervention. No PA element |
| 12 | OPTIMISE228 | RCT: individualised lifestyle advice vs. routine care on maternal and infant health outcomes for women with BMI of 18.5–24.9 kg/m2 | Intervention combines face-to-face and telephone advice using SMART goals approach | Study ongoing | Women with BMI in normal range. Individualised based on basal metabolic rate and activity level. Informed by stage theories of health decision-making |
| 13 | GeliS trial229 | Cluster RCT: lifestyle intervention vs. routine care | Intervention includes diet, moderate PA, and self-monitoring of weight gain across four sessions with range of health-care providers | No effect on GWG | Greater GWG more frequent among women with overweight and obesity. Implementation within health-care sessions is difficult |
| 14 | DALI study230–233 | Multinational RCT for women with BMI of ≥ 29 kg/m2: lifestyle intervention vs. routine care | Intervention includes tailored diet and PA across nine sessions using principles of motivational interviewing with lifestyle coach | Significantly lower GWG in intervention group but no impact on fasting glucose | Recommendations of limit to GWG. Use of motivational interviewing principles. Intervention delivery did not meet motivational interviewing fidelity |
| 15 | LIFE-MOMS234 | Consortium of seven RCTs testing different lifestyle interventions to reduce excess GWG | Intervention targeting diet, PA and behavioural strategies | Behavioural lifestyle interventions focusing on diet and PA have significant effect on reducing excess GWG | Variety of approaches can be used to support women to change behaviours to control amount of weight gain. Raises the question as to whether maternal weight gain is an appropriate outcome metric. Potentially need to explore body composition/fat mass |
Appendix 10 Barriers and facilitators
| Reference | Methodology | Study aim; health behaviour(s) addressed; intervention(s) | Results/conclusions | Key contextual influences (barriers/facilitators); limitations and/or recommendations | |
|---|---|---|---|---|---|
| 1 | Beckmann et al. 2014190 |
Design: case–control study of PCC (2010–13); statistical/quantitative study utilising routine data Participants: women attending PCC clinics, including those with subfertility and women with pre-existing health conditions that have the potential to impact pregnancy outcomes, as well as low-risk women seeking assessment and advice as to how best to maximise their chance of a healthy pregnancy outcome (n = 407). Participant consent not sought Setting: PCC services delivered at not-for-profit private tertiary maternity hospital in Brisbane, QLD, Australia Primary outcome: likelihood of ‘being healthy’ at the time of hospital maternity booking. [‘Being healthy’ defined as (1) early-pregnancy weight in a healthy range (BMI 18.5–24.9 kg/m2), (2) ceased/reduced smoking and alcohol consumption, (3) folate supplementation for at least 3 months prior to conception and first 3 months of pregnancy, (4) vaccinated against influenza, pertussis, varicella and hepatitis B, and (5) had received specialist consultation with specific aim of optimising a pre-existing health condition prior to conception] |
Aim: to evaluate whether women who receive PCC through a structured approach will be more likely to be healthy around the time of conception than women who plan their pregnancy but have not been exposed to PCC Health behaviours addressed: diet/nutrition, exercise, alcohol and smoking Intervention: 45-minute consultation with midwife addressing questions related to participants’ reproductive, medical, surgical, social and family histories; lifestyle; nutrition; home; work; and social hazards Participants received specific advice and information regarding folic acid supplementation, vaccinations, healthy eating before and during pregnancy, exercise, smoking cessation, and safe levels of alcohol intake Participants subsequently received consultation from obstetrician; positive responses elicited during midwife consultation were addressed, and examinations, investigations and referrals were completed as required |
Women in early pregnancy who attend a preconception consultation are more likely to be healthy across several domains than women who plan their pregnancy but do not attend a preconception consultation. Specifically, they are more likely to:
|
Participants weighed more, were older and experienced more recurrent miscarriages and more difficulties conceiving than the general population Possible selection bias: women who attended PCC may represent a more motivated population than women who did not attend Routinely collected data were entered manually and thus subject to data entry error This service was piloted in a tertiary maternity hospital, but most women planning a pregnancy are not high risk and PCC should be a primary health-care initiative Intervention delivered in setting not routinely accessed by general population (tertiary maternity hospital); more appropriate for use in primary health-care services |
| 2 | Cunningham et al. 201843 |
Design: qualitative/observational. Interviews conducted with study participants: standardised interview framework utilised. Interviews audio-recorded, transcribed and analysed thematically Participants: pregnant women with BMI of > 30 kg/m2 (n = 11) Setting: antenatal services located in a hospital in south-west England (January–June 2016) |
Aim: to explore the experiences of pregnant women with a raised BMI to investigate if their pregnancies were affected by their interactions with midwives and other health professionals No interventional component |
Results: Three major themes were identified:While most participants experienced the patient–midwife relationship as supportive, three reported that midwives had been embarrassed to address their weight and found the conversation difficult Pregnant women with raised BMI reported feeling ‘judged’ about their weight during communications with midwives and other health professionals; feelings of guilt and embarrassment were expressed Women reported a ‘knowledge gap’ relating to the health implications of weight and raised BMI, and the majority did not remember their midwives talking in any detail about their raised BMI or about why it might be important |
Homogeneous sample: all participants were white British and recruited from a single hospital trust Women with raised BMI in this study did not consider themselves ‘obese’ and found the term rude and offensive; alternative terms such as ‘raised BMI’ were considered more appropriate/preferable |
| 3 | Greenhalgh et al. 2015134 |
Design: qualitative Methods: narrative interviews, focus groups/group story-sharing Participants: women of Bangladeshi, Indian, Sri Lankan or Pakistani origin, with diabetes, aged 21–45 years, living in London (n = 45) Setting: diabetes and antenatal services in two deprived London boroughs |
Aim: to understand multiple influences on behaviour (and risks to metabolic health) of South Asian mothers and their unborn child, theorise how influences interact and build over time, and inform the design of culturally congruent, multilevel interventions No interventional component |
Results: participants described experiences of diabetic pregnancy as stressful, difficult to control and associated with negative symptoms, especially tiredness Participants reported that exercise and restricted diet worsened diabetes symptoms and conflicted with advice from relatives and peers Participants held beliefs that exercise during pregnancy would cause harm to the fetus and that eating would be strength-giving for mother and fetus Whereas peer advice was familiar, meaningful and morally resonant, health education advice from clinicians was usually unfamiliar and devoid of cultural meaning |
Participants cited family and culture as key influences on their understanding of pregnancy-related health Behaviour change interventions aimed at preventing and managing diabetes in South Asian women before and during pregnancy are likely to be ineffective if delivered in a sociocultural vacuum Individual education should be supplemented with community-level interventions to address the sociomaterial constraints and cultural frames within which behavioural ‘choices’ are made |
| 4 | Khan et al. 2019144 |
Design: qualitative/phenomenological. In-depth exploration of women’s attitudes and lived experiences achieved via telephone/video semistructured interviews and open-ended questions (data collected March–August 2018). Deductive and inductive thematic analysis was conducted Participants: women aged ≥ 18 years who were able to communicate in English and had recently joined or upgraded private health-care insurance cover to include obstetrics, and who were planning a pregnancy in the next 12 months (n = 7), were currently pregnant (n = 7) or had given birth in the past 12 months (n = 1) (total n = 15) Setting: private health-care services in Australia |
Aim: to understand women’s general attitudes towards preconception health and what health actions they were taking or planning to take in the lead-up to pregnancy No interventional component Health behaviours discussed: diet, physical activity, alcohol |
Participants identified healthy diet, regular physical activity, reducing alcohol intake and pre-pregnancy vitamin supplementation as important preconception health behaviours Few participants acknowledged the importance of formal preconception health checks and screening with health professionals; awareness of services was low Barriers to achieving health behaviour change included anxiety, stress and challenges obtaining reputable information Participants reported a lack of preconception information about supplementation requirements, safe foods and exercise recommendations Information source preferences included the internet or their GP |
Most participants were focused and motivated to engage in healthy preconception behaviour, signalling pregnancy intention to GP, seeking information and adopting positive preconception lifestyle behaviours Although participants were engaged, they had limited awareness of available preconception health checks and screening National preconception health guidance is currently lacking; this could usefully include preconception checklist addressing key health actions and suggested timings |
| 5 | Kothe et al. 2019176 |
Design: observational/qualitative; documentary analysis. Midwifery courses were identified (n = 568) and course outline material was obtained (n = 252) Setting: midwifery courses from 20 Australian universities Methodology: documentary data coded using Framework Analysis technique against three themes: |
Aim: to identify strengths and deficits in teaching of preconception and antenatal weight management within Australian universities’ midwifery curricula Health behaviours discussed: weight, diet, physical activity |
Results: there is a strong focus on health promotion throughout pregnancy in midwifery training in Australia. Teaching methods and skills training addressed risk identification and lifestyle management and emphasised the importance of clinical judgement and autonomous clinical practice, as well as adopting critical enquiry and sourcing reputable evidence Despite these strengths, there is little evidence that weight management advice was taught explicitly to midwifery students |
A greater emphasis on skilling midwifery students to address weight gain during pregnancy, and behavioural techniques to achieve this, is required |
| 6 | Stephenson et al. 201435 |
Design: cross-sectional questionnaire survey addressing knowledge and uptake of PCC, plus interviews with a range of health-care professionals (n = 20) Participants: pregnant women attending three maternity services in London (n = 1173), and health-care professionals working in general practice, obstetrics and gynaecology, midwifery and sexual and reproductive health |
Aim: to determine the extent to which women plan and prepare for pregnancy No interventional component Health behaviours discussed: folic acid, vitamin supplements, diet, weight, alcohol, smoking, immunisation, recreational drugs, STIs, dental checks, caffeine, stopping contraception/fertility advice |
Results: 73% had clearly planned their pregnancy, 24% were ambivalent and 3% of pregnancies were unplanned Health professional interviews indicated low awareness of preconception health issues, missed opportunities and confusion about responsibility for delivery of PCC |
Despite a high level of pregnancy planning, awareness of preconception health among women and health professionals is low, and responsibility for providing PCC is unclear. Strategies to improve awareness and uptake of pre-pregnancy health care should be identified |
| 7 | Weisman et al. 201149 |
Design: intervention evaluation utilising statistical analyses of weight-related data and qualitative interviews Intervention: SHW, a small-group behavioural intervention for preconceptional and interconceptional women designed to modify key risk factors for adverse pregnancy outcomes Participants: women in the original trial of the SHW intervention (n = 362) and who had records linked to singleton births during the 12-month follow-up period (n = 45) Setting: intervention delivered across 12 weeks via six face-to-face sessions held at community locations in low income and rural communities in central Pennsylvania, USA |
Aim: to investigate long-term (6- and 12-month) effects of SHW intervention on health-related behaviours, weight, BMI and weight gain during pregnancy |
Results: At 12-month follow-up, SHW participants were significantly more likely than controls to use a daily multivitamin with folic acid and to have lower weight and BMI The intervention’s effect on reading food labels for nutritional values dropped off between the 6- and 12-month follow-ups Among those who gave birth to singletons during the follow-up period, women who participated in the intervention had lower average pregnancy weight gain than controls Although the intervention effect was no longer significant when controlling for pre-pregnancy obesity, the adjusted means show a trend towards lower weight gain in the intervention group |
Findings strongly suggest that SHW is effective in modifying risk factors for adverse pregnancy outcomes and may help prevent weight gain during pregnancy SHW appears to help women manage weight in the months after the intervention as well as during pregnancy, and may be an effective obesity prevention strategy for women before, during and after the transition to motherhood Delivering all intervention components, activities and content in face-to-face sessions was resource-intensive and expensive, and the time investment required for participation was burdensome for some women |
| 8 | McBride et al. 2012136 |
Design: survey Participants: pregnant women receiving prenatal health care (n = 142) Setting: public health-care services in Perth, Western Australia |
Aim: to identify factors that contribute to alcohol consumption during pregnancy (which may help direct potential intervention strategies) |
Three risk groups identified: the ‘no risk’ group discontinued alcohol consumption once pregnant (33.1% of participants), the ‘low risk’ group consumed one or two drinks per week (45.8% of participants) and the ‘risky’ group consumed more than two drinks per week (21.1% of participants) Demographics: ‘low-risk’ women were more likely to have a higher education than other participants; ‘no-risk’ women were significantly more likely to be engaged in full-time home duties than other participants Women who continued to drink more likely to have done so in previous pregnancies and during the preconception period All participants reported that they would be most likely to drink in their own home or in the home of a friend Participants who continued to drink identified relaxation, social contact and taste as benefits; all groups expressed concern around the potential risk of Fetal Alcohol Syndrome (approximately one-third of women who continued to drink reported this concern) Nearly 40% of ‘high-risk’ women reported that they had received negative comments from friends, families or partners in response to their drinking, and one-third had been advised by a health professional not to drink alcohol. This group were also more likely to use other drugs, in particular tobacco and cannabis |
Conclusions: combined prevention efforts may be important to assist women in quitting multiple substances The social determinates that give rise to women’s risky use of alcohol (and other drugs) during pregnancy are likely to be complex and will therefore require a complex intervention Participatory research with women who drink while pregnant can assist in identifying potential intervention strategies that have resonance with this group, and therefore more potential for creating behaviour change Limitations: participants not representative of population at large (self-selected). Survey limited in scope; additional data collection strategies (e.g. focus groups) could identify additional data/findings |
Appendix 11 Key qualitative and survey-based literature
| Reference | Design (interventional/observational); methods; participants; setting | Research questions/aims/objectives | Results | Key contextual considerations; study limitations | |
|---|---|---|---|---|---|
| 1 | Poels et al. 2017235 |
Observational Methods: retrospective questionnaire (no qualitative component) Participants: women who received antenatal care (n = 283) Setting: primary care community midwifery practice in the Netherlands |
Objective: to assess whether or not actively preparing for pregnancy is associated with lifestyle changes during the preconception period |
A total of 56.5% (n = 160) of participants acquired preconception information for themselves in preparation for pregnancy; 24% (n = 68) received a PCC consultation from a health-care provider When compared with women who did not prepare for pregnancy, the first group was significantly more likely to make positive lifestyle changes, including ceasing alcohol consumption (adjusted OR 5.46, 95% CI 1.76 to 16.96), improving diet (adjusted OR 7.84, 95% CI 3.03 to 20.30) and using folic acid (adjusted OR 3.90, 95% CI 2.00 to 7.62) Effect sizes were larger for women who also consulted a health-care provider Key conclusions: gathering preconception information is associated with women positively changing lifestyles during the preconception period |
Self-reported, retrospective data only; study population confined to women who had recently given birth Participant sample was homogeneous and not representative of wider population (Dutch, high education level, high household income) |
| 2 | Ockhuijsen et al. 2012124 |
Interventional, with evaluative qualitative questionnaires Participants: patients (couples) on the waiting list for an IVF or ICSI treatment (n = 130); IVF clinic nurses (n = 7) Setting: a single IVF preconception clinic in a university medical centre in the Netherlands |
To understand outcomes of integrating PCC into an IVF programme by measuring nurses’ and patients’ attitudes and patients’ weight- and smoking-related behaviour |
All nurses and 101 patients (77.7%) returned completed questionnaires Patients valued increased attention to adjusting lifestyle factors with the goal to improve fertility outcomes Among participants who smoked or had a BMI of > 30 kg/m2, 30% quit smoking and 50% lost weight (mean loss: 6.1 kg) Nurses were sceptical of the value of the programme and their ability to perform their new role effectively |
Questionnaire validation limited (three expert opinions) Self-reported measurements only Small sample; patient population restricted to those on waiting list for IVF |
| 3 | McPhie et al. 2017236 |
Observational Methods: semistructured telephone interviews, thematic analysis Participants: health professionals with expertise in maternal health (n = 20) Setting: maternity services and medical clinics in Melbourne, VIC, Australia |
Aim: to identify barriers to providing preconception weight management |
Barriers facing women: lack of awareness regarding importance of preconception weight, not being provided weight management information/interventions Barriers facing health professionals: absence of implementation resources, limited access to women of childbearing age who plan to conceive, high percentage of unplanned pregnancies, time constraints in clinic, sensitivity of subject |
Participants known to researchers; risk of bias Client base of participants may not be representative of women from a wide range of backgrounds Setting (Australia) |
| 4 | Shawe et al. 2019130 |
Observational (no qualitative component) Methods: cross-sectional survey Participants: men attending antenatal care with their partners (n = 573) Setting: antenatal care clinics/maternity units in London, UK |
Research questions:
|
A total of 46.9% of participants reviewed pregnancy-related information from a variety of sources, including online, before their partner became pregnant 74% of pregnancies were planned Male ‘planners’ were more likely than other men to reduce smoking, reduce alcohol consumption and eat more healthily in preparation for pregnancy 57% of participants took no action to improve their health |
Retrospective, self-reported data only Participant demographics limited diversity; 74% of participants were white, and the majority had a high level of education (67% had a degree) and were in employment or full-time education (87%) |
| 5 | Stephenson et al. 201435 |
Observational Methods: cross-sectional questionnaire survey and telephone interviews Participants (survey): women attending maternity services in London (n = 1173) Participants (telephone interviews): doctors and nurses from a range of health-care professions (n = 20) Setting: maternity services of three north London hospitals |
Research questions:
|
Women: 73% had clearly planned pregnancy, 24% were ambivalent, and 3% of pregnancies were unplanned 51% of all women and 63% of those with a planned pregnancy took folic acid before pregnancy 21% of all women reported smoking and 61% reported drinking alcohol in the 3 months before pregnancy 48% of smokers and 41% of drinkers reduced or stopped before pregnancy The 51% of all women who reported advice from a health professional before becoming pregnant were more likely to adopt healthier behaviours before pregnancy Health professionals: barriers to providing PCC include unplanned pregnancies, lack of knowledge/interest, constrained resources, and confusion about responsibility for delivery of PCC |
Health professionals – particular setting influences ability to provide information (e.g. GPs faced particular barriers) |
| 6 | Bortolus et al. 2017132 |
Observational Methods: focus groups Participants: nulliparous and multiparous women of childbearing age (n = 14); nurses (n = 3); midwives (n = 4); obstetrics and gynaecology/paediatric consultants (n = 5) Setting: obstetrics and gynaecology, paediatrics and assisted reproduction services, Verona University Hospital, Italy |
Aim: to understand attitudes and behaviours of Italian women of childbearing age and health-care professionals regarding preconception health |
Women’s knowledge of preconception risk factors, such as pre-existing conditions, overweight/obesity and infectious disease, is poor Majority of women did not speak to a health professional before trying to become pregnant No clear professional responsibility for addressing preconception health; health-care professionals do not adopt a ‘proactive’ attitude Poor public health promotion of healthy preconception behaviour |
Small sample size Homogeneous cohort (middle class, white, educated) |
| 7 | Duthie et al. 2013237 |
Observational Methods: semistructured interviews Participants: pregnant women (third trimester) (n = 19); obstetricians (n = 7) Setting: obstetrics and gynaecology clinic at a large academic medical centre, midwestern USA |
Aim: to understand what obstetricians communicate about GWG to pregnant patients and how nulliparous patients perceive weight-related counselling from obstetricians | Four major themes identified, within which there were significant differences between the patient and health-care provider perspective
|
Limited sample (participants recruited from a single medical centre) Both obstetrician and patient participants self-selected into the study, raising the possibility that those with interest/concern related to gestational weight were more likely to enrol than others |
| 8 | Squiers et al. 2013128 |
Observational Methods: focus groups Participants: women from a range of demographic backgrounds; 18–44 years old (n = 65) Setting: Atlanta, GA, USA |
Research questions: do consumers understand behaviours that fall under the preconception health and health-care umbrella? How do consumers refer to/think about the terms ‘preconception health’ and ‘preconception care’? Is preconception health understood as a set of behaviours or a set of services? |
Women planning a pregnancy in the next 2 years had different perspectives on preconception health and health advice from women not currently planning a pregnancy Women who were not planning to get pregnant in the next 2 years reported not wanting to receive preconception health messages from a health-care provider at a routine visit Barriers to preconception health included lack of social support, a medical history of addiction and lack of awareness Participants preferred to think of preconception health behaviours as ‘promoting’ a healthy baby rather than preventing an unhealthy birth outcome Women preferred to hear preconception health messages from a health-care provider, among other channels |
No research recommendations |
| 9 | Barrett et al. 201541 |
Observational Methods: in-depth interviews (face to face or telephone), Framework Analysis Participants: pregnant and recently pregnant women (n = 20). Purposively sampled to include high and low investors in prepregnancy health and care, with variation in age, partnership status, ethnicity and pre-existing medical conditions Setting: antenatal clinic attendees, London |
Research question: why do women invest in prepregnancy health and care? |
Relatively few women received advice on healthy diet/healthy weight Three broad groups were identified: |
Sample bias towards older, well-educated women |
| 10 | Mazza et al. 2013161 |
Observational Methods: three 90-minute focus groups. Thematic analysis based on the theoretical domain framework, which describes 12 domains related to behaviour change Participants: GPs (n = 22). Purposive sampling of those working in urban and rural settings, and high and low socioeconomic settings Setting: urban and rural GP surgeries in Australia |
Research question: what are GP-perceived barriers to and enablers of delivery and uptake of PCC guidelines? |
Perceived barriers: time constraints; lack of women presenting at preconception stage; competing priorities; issues relating to the cost of and access to PCC; and the lack of resources for assisting in the delivery of PCC guidelines Perceived enablers identified by GPs included the availability of PCC checklists and patient brochures, handouts, and waiting room posters outlining the benefits and availability of PCC consultations |
Australian health-care system (hybrid funding system – some care costs met by patients) |
| 11 | Tuomainen et al. 201364 |
Observational Methods: focus groups (n = 9) and follow-up telephone interviews (n = 19) Participants: women aged 18–45 years, of Pakistani, Indian, Caribbean, African, white and mixed ethnic origin (n = 41) Setting: ethnically diverse and socially disadvantaged community settings of the UK |
Aim: to explore perceptions about preconception health and care among women from ethnically diverse communities, and identify opportunities and challenges for intervention development in primary care |
Participants had modest/poor awareness of preconception health Participants felt that preconception health could be addressed in primary care if raised within clinically ‘relevant’ consultations Challenges highlighted: little prevailing culture of preparing for pregnancy and/or pregnancies often being unplanned. For those planning pregnancy, sensitivity and maintaining secrecy when trying to conceive Participants expressed a preference for female professionals Participants felt that support was needed to engage men and to enhance access for younger people/women less disposed to general practice |
Women under 18 years old were not eligible for participation |
| 12 | Ojukwu et al. 2016238 |
Observational Participants: GPs (n = 7: four male, three female). Five GPs had experience in obstetrics and gynaecology; three had DRCOG qualifications; four were GP trainers Methods: interviews Setting: London, UK |
Aim: to examine GPs’ knowledge, attitudes and views towards preconception health and care in the general practice setting |
GPs agreed on the main issues they would discuss as part of PCC: healthy eating, being a healthy weight, folic acid supplementation, stopping smoking and reducing or abstaining from alcohol consumption Lack of GP knowledge of formal guidelines Some lack of consistency in advice given (e.g. alcohol consumption) Lack of consistency in perceived patient demand for PCC Reaching women before pregnancy was considered important, although not GPs’ responsibility (suggested role of educators/nurses) Specialist preconception services were not provided within GP surgeries; PCC was targeted at women clearly planning/with medical conditions GPs described diverse patient groups with very different health needs and significant barriers to providing care [e.g. no preparation for pregnancy, language/culture, lack of time, reluctance to provide unsolicited advice (‘nanny state’)] |
Study was conducted in London borough with disparity of wealth and large ethnic minority population; therefore, may be different from other UK areas |
| 13 | M’hamdi et al. 2017160 |
Observational Participants: HCPs who regularly provide PCC (n = 20: community midwives, n = 12; GPs, n = 3; obstetricians, n = 3; cardiologist, n = 1; gastroenterologist, n = 1) Methods: semistructured interviews Setting: the Netherlands |
Aim: to examine health-care professionals’ views of roles and responsibilities in providing PCC and identify barriers that affect delivery and/or uptake |
Multiple barriers identified:
|
Small sample size limits generalisability |
| 14 | Van der Zee et al. 2013127 |
Observational Participants: women desiring to conceive, 22–39 years of age (mean 32.8 years). N = 16. Recruited online, via ‘network of ethnic minority women’ and snowball sampling. Educational levels: low (n = 3), medium (n = 3), high (n = 10). Ethnicity/country of birth: Dutch (n = 12), Moroccan (n = 2), Surinamese (n =2) Methods: in-depth, semistructured, face-to-face interviews Setting: the Netherlands |
Aim: to explore women’s hesitancy to seek preconception counselling |
In some cases, women were disappointed or upset when a GP did not inquire further when they visited to have the coil removed or that a gynaecologist did not mention being overweight as a cause of infertility Women expressed positive attitude towards PCC in general but were hesitant about seeking PCC themselves Women seemed to regard themselves as not being in the target group for PCC Four subthemes identified (subjective norms relating to process of becoming pregnant): planning, publicity, information on fertility and artificiality |
Certain participant perceptions (e.g. artificiality) could be culturally specific. The Netherlands has a high rate of planned pregnancy when compared with other nations (85%). Limited user perspective (women only) |
| 15 | Bombard et al. 2013239 |
Design and methods: secondary analysis of large national survey (self-reported behavioural and health information) Participants: non-pregnant women aged 18–44 years with reported history of hypertension or current antihypertensive medication use (n = 2063) Setting: USA Interventional topics discussed: health-care provider advice on healthy behaviour |
Research questions: what is the prevalence of receipt of health-care provider advice among women of reproductive age with hypertension, and is receipt of advice associated with behaviour change? |
Women who received advice were more likely to report corresponding behaviour change Majority reported that a provider advised them to change eating habits (72.9%), reduce salt intake (74.6%) and exercise (82.1%) Smaller number reported being advised to reduce alcohol use (44.7%) Behaviour change: majority reported that they changed eating habits (75.5%), reduced salt intake (80.4%), exercised (70.1%) and reduced alcohol use (67.8%) |
Self-reported data only Specific population: women with hypertension history or diagnosis, awareness of health risks |
| 16 | Cohen et al. 2009240 |
Design and methods: secondary analysis of data from the US Government’s Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System telephone survey Participants: pregnant women aged 18–44 years who had completed information on attempting to lose weight for the Behavioral Risk Factor Surveillance System survey (n = 8036) Setting: USA |
Research questions: what is the prevalence of attempting to lose weight among pregnant women, and to what extent are sociodemographic and health characteristics associated with this behaviour? |
The overall proportion of pregnant women who were attempting to lose weight was 8.1% Attempting to lose weight during pregnancy was positively and significantly associated with age (35–44 years), Hispanic ethnicity, obesity, alcohol consumption and mental distress during the previous month |
Self-reported data only Data analysed collected 1996, 1998, 2000 and 2003 |
| 17 | Goossens et al. 2018241 |
Design and method: secondary data analysis of a cross-sectional study about pregnancy planning Participants: women with a planned pregnancy ending in birth (n = 430) Setting: six Flemish hospitals (Belgium) |
Objectives: (1) to study preconception lifestyle changes and associated factors in women with planned pregnancies; (2) to assess the prevalence of risk factors for adverse pregnancy outcomes in women not reporting any preconception lifestyle changes; and (3) to explore the need for and use of preconception-related advice |
Most participants (83%) reported one or more lifestyle change in preparation for pregnancy. The most commonly reported change was consumption of folic acid/multivitamins (76%) and the least common was change focused on diet and/or reducing body weight (12–18%) Nulliparous women and women with a previous miscarriage were more likely to prepare for pregnancy Multiparous women and women of lower socioeconomic status were less likely to change lifestyle before conception Experiencing financial difficulties or having a lower educational level decreased the likelihood of preparing for pregnancy 48% of participants obtained advice about preconception health (and 86% of these participants received advice from a professional caregiver) Three-quarters (77%) of the women who did not improve their lifestyle before conceiving reported one or more risk factors for adverse pregnancy outcomes |
Specificity of setting (Belgium) |
| 18 | Campbell et al. 2011242 | Design and method: systematic review of quantitative and qualitative evidence. Eleven electronic bibliographic databases were searched. Supplementary searching included reference lists of included studies and relevant review articles. A total of five controlled trials and eight qualitative studies were included in the review | Objectives: to assess effectiveness of behavioural interventions to prevent excessive weight gain in pregnancy and explore the factors that influence effectiveness |
Controlled trials: no significant difference in GWG found among participants in intervention groups compared with control groups Study design, participants and interventions varied markedly Subgroup and sensitivity analysis did not identify contextual elements that influenced intervention effectiveness Qualitative studies: three major themes emerged relating to women’s views of weight management in pregnancy:Synthesis of qualitative and quantitative results shows that some of the barriers to achieving successful weight management that women identified were not addressed by the interventions evaluated; this may have contributed to the limited effectiveness of the interventions |
Small number of studies available met inclusion criteria Many qualitative data conducted in the UK; trial data came from outside the UK: lack of alignment between contexts/cultural settings |
Appendix 12 Explanatory accounts
Abbreviations
Nature of source
CC, case–control study; FS, feasibility study; FT, feasibility trial; MA, meta-analysis, ME, methods; NR, narrative review; OC, opinion/conceptual paper; OS, observational study; PT, pilot trial; QR, qualitative review; QS, qualitative study; S, survey; ScR, scoping review; SR, systematic review.
Outcomes
BA, barriers; BC, behaviour change; FE, fertility; HM, health markers; KA, knowledge and attitudes; LT, long-term outcomes; RE, recruitment and engagement; ST, staff related; W16, weight loss in a ≤ 16 week programme; W17, weight loss in a ≥ 16 week programme.
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Barker et al. 201829 | OC | If adults are intending to get pregnant, then the intervention opportunities are to actively support preconception health, for example through text messaging, and provide practical support in an engaging way | RE | Intention | Support |
| If adults intending to get pregnant are in a specific subgroup, then they need intense, tailored support | RE | Culture | Tailoring | ||
| If adults intending to get pregnant have previously had a pregnancy, then they will be less receptive to prepregnancy input, and input needs to take previous pregnancy into account | RE | Subsequent pregnancy | Individualised | ||
| If children and young adults are engaged with health behaviour promotions before childbearing capabilities develop, then there is a better chance that they will continue with healthy behaviours in the preconception period | BC | Pre-planning | Habit formation; social movement with preparation for healthy pregnancy becoming more normalised | ||
| If adults with no immediate intention to get pregnant are informed of risk of future loss of ability to have a healthy child, then they may be more motivated by loss aversion | BC | Pre-planning | Loss aversion as motivator in response to public information campaign | ||
| If practitioners are trained in Healthy Conversation Skills, then they will be able to engage and motivate patients and clients about nutrition and PA during brief consultations | ST | Staff skills | Self-efficacy; relationship between competence and confidence of staff | ||
| If supermarkets could convey healthy preconception nutrition messages, for example next to folic acid, then it would be an opportunity to inform a lot of women | RE | Public health messages | Community messages | ||
| If interventions are delivered via digital media, then women experiencing social disadvantage are more likely to engage | RE | Comms strategy | Reach of intervention | ||
| Social marketing (e.g. change4life campaign) did not make an impact because individuals and communities require not only knowledge but also resources to enact change, and a purpose or meaning to provide motivation | BA | Resource | Social practice theory – knowledge, resource and meaning required to enact change | ||
| Becker et al. 2015148 | RCT | If women aged 18–35 years, with BMI of 25–40 kg/m2, and waiting for their first round of IVF treatment engage in 12 weeks of an individualised low-GI diet plan, then they can achieve a 5.5% weight loss | W16 |
IVF No-one refused to take part 1/16 could not comply with intervention |
Hypocaloric and low-GI individualised plan (20 kcal/kg bodyweight) |
| If women on the waiting list for IVF are invited to participate in a WLI, then there is a high take-up rate (100% in this study) | RE |
IVF No-one refused to take part 1/16 could not comply with intervention |
Motivation | ||
| If women follow too strict a weight loss regimen, then the effect on fertility is uncertainThe exact amount of energy restriction that is safe or deleterious for obese infertile women seeking IVF treatment is not clear | FE |
IVF No-one refused to take part 1/16 could not comply with intervention |
Potential risk of too few calories | ||
| Beckmann et al. 2014190 | CC | If women are motivated to attend a 90-minute session in a preconception service (including those motivated by subfertility and pre-existing conditions), then they may gain less weight prior to conception | KA | General population attending preconception clinic | Health advice |
| Brackenridge et al. 201880 | PS | If women who agree to delay having their coil removed for 24 weeks in order to lose weight are offered intensive meal replacement-based weight loss programme, then one-third of those eligible will agree to take part | RE |
BMI > 30 kg/m2, age 18–40 years Community clinic Delay IUD removal |
Hypocaloric, 966–1220 kcal/day |
| If women agree to delay having their coil removed for 24 weeks and can follow a low-energy liquid diet weight loss programme, with fortnightly 15-minute motivational support, then they will lose a clinically significant amount of weight (median BMI reduction of 14.2% for completers, 6.6% ITT of all starters) | W17 |
BMI > 30 kg/m2, age 18–40 years Community clinic Delay IUD removal |
Hypocaloric, 966–1220 kcal/day | ||
| Caut et al. 202033 | SR | Usual preconception diet is insufficient in terms of nutritional guidelines | HM | Preconception and pregnancy | Nutrition |
| Delissaint et al. 201138 | SR | Knowledge, awareness and beliefs of PCC do not lead to preconception health practice | KA | Preconception health | Need a mechanism for knowledge to be translated into change of behaviour |
| Einarsson et al. 2017243 | RCT | If participants have a high level of engagement in a 16-week intervention of very low-calorie meal replacement diet plus support every 2–3 weeks, including dietetic input, then they will experience significant weight loss of average 9.44 kg (group mean BMI was 29.8 kg/m2) | W16 |
IVF BMI 30–35 kg/m2 Nordic countries – IVF threshold is BMI of 35 kg/m2, so all women are already within range |
Hypocaloric, 12 weeks at 880 kcal/day |
| If women with BMI 30–35 kg/m2 waiting for IVF are offered 16-week very low calorie meal replacement WLI, then 77% will agree to take part | RE | Nordic countries – IVF threshold is BMI 35 kg/m2 so all women are already within range | Hypocaloric, 12 weeks at 880 kcal/day | ||
| Espinós et al. 2017149 | RCT | If women with BMI 30–40 kg/m2 waiting for IVF take part in a 12-week diet and exercise programme, then they will lose an average of 6.97% weight | W16 |
IVF BMI 30–40 kg/m2, age 18–37 years |
Reduction of calories by 500–800 per day plus exercise plus support |
| Greenhalgh et al. 2015134 | QS | If beliefs about negative effects of diet and exercise during pregnancy are addressed in a culturally relevant way, then participants are more likely to engage with an intervention | RE | South Asian women with GDM | Cultural relevance |
| If health promotion messages are not tailored to different groups of people with protected characteristics, then individuals who are understood to be particularly at risk of marginalisation will be further excluded from health care | RE | South Asian women with GDM | Inequity and marginalisation | ||
| Homan et al. 2012244 | PS | If couples undergoing fertility treatment are offered lifestyle advice designed to address lifestyle behaviours potentially influencing fertility and 4 months of weekly support, then 57% will agree to participate and 31% will take part | RE |
IVF, all BMI Targeting lifestyle rather than one behaviour such as weight |
Engagement/motivation of couples with lifestyle messages is hard to achieve even in motivated population |
| If couples undergoing fertility treatment are overweight and are motivated to receive lifestyle intervention using MI principles designed to address lifestyle behaviours potentially influencing fertility and receive weekly follow-up support telephone calls, then 47% of those who take part will lose between 1 and 5 kg in 4 months | W16 |
IVF all BMI Targeting lifestyle rather than one behaviour such as weight |
Motivation – using MI to engage with lifestyle behaviours Couples – mutual support |
||
| Hussein et al. 201636 | SR | If women receive PCC and counselling, then maternal knowledge, self-efficacy and health locus of control, and self-reported risk behaviour will improve | KA | Preconception across BMI | Information increases knowledge and changes attitudes |
| If women received preconception care and counselling, then it is unclear whether this has any effect on adverse pregnancy outcomes | KA | Preconception across BMI | The relationship between information, attitude and behaviour change is unclear | ||
| If services have designed and evaluated PCC, then weight is not included in the intervention | BA | SR across BMI | Weight is not regarded as an important aspect of PCC | ||
| Khan et al. 2019144 | QS | If the availability of pre-conception screening and advice was promoted to the general public, then anxiety would be reduced and people planning a pregnancy would engage in more healthy behaviours | RE |
Australia Preconception, pregnancy and postnatal |
Community education |
| If national evidence-based guidance and preconception checklists were developed, then clinicians would feel more able to give appropriate advice and people planning pregnancy would feel less anxious about doing the right thing | ST | National guidelines needed | |||
| LeBlanc et al. 202153 | RCT | If women take part in a remotely delivered tailored behavioural WLI, then they can lose 3.5% of their weight before conception | W17 |
USA Preconception and pregnancy |
Behavioural weight loss support |
| If women lose a significant amount of weight prior to pregnancy, then there is a risk of weight regain in pregnancy | W17 |
USA Preconception and pregnancy |
Weight loss support needs to continue into pregnancy | ||
| If women lose weight in a preconception intervention, then it is important to continue with support through pregnancy if this loss is to be maintained by the end of the third trimester of pregnancy | W17 |
USA Preconception and pregnancy |
Weight loss support needs to continue into pregnancy | ||
| Legro et al. 201544 | RCT | If women with PCOS undergoing IVF are invited to take part in a 16-week RCT delaying IVF in order to lose a goal of 7% weight, then 17% of eligible women will consent to take part | RE |
PCOS and IVF BMI 27–42 kg/m2; age 18–40 years |
Hypocaloric – individualised |
| If the intervention is tailored and includes meal replacements and weight loss medication, then participants can experience significant weight loss (between 6.2% and 6.4%) during the 16-week period | W16 |
PCOS and IVF BMI 27–42 kg/m2; age 18–40 years |
Hypocaloric (between 1200–2000 kcal/day) plus lifestyle modification, including meal replacements, weight loss medication (either sibutramine or orlistat), and increased PA | ||
| Mahoney 2014245 | PS | If women with PCOS are offered a 12-week MI intervention to help with lifestyle change to support fertility, then 22% will participate in the intervention and 16.6% will complete it | RE |
PCOS BMI > 27 kg/m2; age 18–44 years 12 weeks, six MI sessions, daily logs, etc. |
MI-based intervention |
| If individual tailored motivational interviewing is used, then participants can report behaviour change and lose an average of 7 lb in 12 weeks |
BC W16 |
PCOS BMI > 27 kg/m2; age 18–44 12 weeks, 6 MI sessions, daily logs |
MI, monitoring | ||
| Montanaro et al. 2019246 | FS | If a preconception risk assessment tool is going to be introduced into primary care services then there can be significant challenges in implementation | ST | Primary care services | Organisational challenges |
| Moran et al. 2011192 | PS | If women follow a reduced-calorie, high-protein diet, there is a meal replacement aspect and they receive lifestyle advice, then participants can lose an average of 3.8 kg in the 5- to 9-week intervention period | W16 |
IVF BMI 28–45 kg/m2; age 18–40 years |
Hypocaloric, 1200 kcal/day including 1 meal replacement and 3 contacts |
| If one-off healthy lifestyle advice is given (to control group), then participants can reduce waist circumference | HM |
IVF BMI 28–45 kg/m2; age 18–40 years |
Lifestyle advice | ||
| Mutsaerts et al. 2016150 | RCT | If women recruited to take part in a 6-month intervention prior to IVF with goal of 5–10% weight loss, then there will be a 22% discontinuation rate and 37.7% will lose ≥ 5% (43% of the completers) | W17 |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric individual – reduced by 600 calories with minimum of 1200 kcal/day Lifestyle motivational counselling Motivation of early IVF if weight loss hit 5–10% |
| Norris et al. 2016133 | OC | If stakeholders are involved at the development stage of an intervention (Jom Moma) and intervention mapping is used, then the intervention is more likely to be well received by participants | RE | Malaysia population-based intervention |
Intervention mapping Co production |
| Rönö et al. 2018137 | RCT | Recruiting women preconception (in context of GDM) is difficult, but the most effective method is by personal invitation based on risk identified through hospital registers | RE |
Gestational diabetes BMI > 30 kg/m2 |
Engagement personal |
| RCT | If participants do not delay getting pregnant while undergoing the intervention then weight loss will not be significant | BC |
Gestational diabetes BMI > 30 kg/m2 |
No delay of pregnancy so very limited exposure to intervention before conception | |
| Rothberg et al. 2016146 | FT | If women met the strict eligibility criteria for a 16-week intensive weight loss programme, then 56% would agree to be randomised | RE |
Anovulatory subfertility BMI 35–45 kg/m2; age 18–40 years Goal of 15% weight loss |
Hypocaloric VLED, 800 kcal/day for up to 12 weeks. 4-week transition to meals. Fortnightly dietetic input. PA advice |
| FT | If women were able to complete the 16-week intensive weight loss programme (n = 6), then they lost an average of 13% body weight | W16 |
Subfertility BMI 35–45 kg/m2; age 18–40 years Goal of 15% weight loss |
Hypocaloric VLED, 800 kcal/day for up to 12 weeks. Fortnightly dietetic input. PA advice | |
| Shawe et al. 2019130 | S |
Male respondents – 74% pregnancies planned If men are part of a couple planning a pregnancy, then they were more likely than other men (non-planners) to reduce smoking, reduce alcohol consumption and eat more healthily in preparation for pregnancy. However, 57% took no action to improve their health |
BC | All antenatal attenders regarding preconception behaviours | Pregnancy planning and partner involvement |
| Sim et al. 2014145 | RCT | If participants take part in a group programme, the intervention is flexible/tailored and the intervention includes support, then they can lose an average of 6.6 kg in the 12-week period | W16 |
IVF BMI 30–40 kg/m2; age 18–37 years |
Hypocaloric VLED, 6 weeks at ≈ 650 kcal/day, then 6-week ‘refeeding’ with ≈ 650 deficit calories described as individually tailored Group process as support mechanism (but not measured) |
| If invited to take part in weekly group VLED programme with tailored intervention and support RCT, then 84% agree to participate | RE |
IVF BMI 30–40 kg/m2; age 18–37 years |
Tailoring and group support | ||
| Stephenson et al. 201827 | OC | Many women of reproductive age in low-, middle-, and high-income countries will not be prepared nutritionally for pregnancy | HM | International | Nutrition |
| van Dammen et al. 2018152 | RCT | If women take part in a 6-month intervention prior to IVF with goal of 5–10% weight loss, then their cardiometabolic health and their self-reported physical quality of life improves | HM |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric and lifestyle counselling – authors suggest result mechanism is due to intrinsic motivation (of achieving a healthy pregnancy) |
| Van Dammen et al. 2019247 | RCT | If women take part in a 6-month intervention prior to IVF with goal of 5–10% weight loss, then there is no significant improvement of stress, mood, sleep quality and mental health QoL 3–8 years following participation | HM |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric and lifestyle counselling |
| van Elten et al. 2019248 | RCT | If participants lost weight in an intervention, then they will maintain a lower BMI and lower reported energy intake between 3 and 8 years following randomisation | LT |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric and lifestyle counselling Successful weight loss during the intervention predicts longer-term changes |
| RCT | If participants take part in an intervention, then their intake of unhealthy foods will be lower 3, 6 and 12 months after randomisation | HM |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric and lifestyle counselling | |
| RCT | If women take part in a 6-month intervention prior to IVF with goal of 5–10% weight loss, the largest effect of the intervention was within 3 months of randomisation. Therefore, it seems that the higher intensity of guidance in the first 3 months of the intervention programme encouraged healthy changes in diet and PA | W16 |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric and lifestyle counselling | |
| Van Dijk et al. 2017138 | QS | MHealth is a useful way of providing information but it needs to be part of an intervention that includes face to face contact and to be tailored/personalised | KA | Preconception population |
MHealth Personalised care |
| If couples engage with mHealth intervention then they are more likely to achieve positive behaviour changes and pregnancy outcomes | BC | Preconception population |
MHealth Personalised care |
||
| Weisman et al. 201149 | RCT | If women in prepregnacy and interpregnacy phase from low-income communities engage with a social cognitive programme to reduce adverse pregnancy outcomes via changing attitudes and behaviours, then their self-efficacy and intention to change health behaviours increases | KA | Non-pregnant women aged 18–35 years in low-income community setting | Social cognitive approach to behaviour change, The group format was intended to motivate women through social support from peers and the lay group facilitators |
| Wekker et al. 2019249 | RCT |
Short-term success predicts long-term success If women reach their target weight during the intervention period, then they will show improved cardiometabolic health 6 years later |
HM LT |
Preconception obesity | Achieving targets acts as longer-term motivation |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Agha et al. 20143 | SR MA | Behavioural interventions in pregnancy may be effective in reducing GWG in obese women without comorbid conditions, but not in overweight or morbidly obese women | GWG | All pregnancy | Tailoring intervention to fit with BMI classification may be critical |
| Campbell et al. 2011242 | SR | Family advice about diet in pregnancy can be a barrier to healthy eating | BA | Pregnancy | Attitudes to weight and diet in pregnancy |
| Cunningham et al. 201843 | QS | If women with BMI > 30 kg/m2 hear the term obese, then they often do not think that it applies to them | BA | Pregnant, with BMI > 30 kg/m2 | Language and knowledge |
| If women have BMI > 30 kg/m2, then raised BMI is regarded as acceptable term as it is factual | RE | Pregnant, with BMI > 30 kg/m2 | Language | ||
| Farpour-Lambert et al. 201822 | SR/MA | If women take part in an antenatal weight management intervention then there can be a reduction in the likelihood of excessive GWG and a significant reduction in longer-term post-partum weight | GWG | All pregnancy | Potential longer-term impact of antenatal weight management |
| Moderate energy targets according to weight (18–24 kcal/kg) was associated with the greatest reduction in GWG | GWG | All pregnancy | Tailor calorie targets according to weight | ||
| If women with overweight/obesity take part in a diet and exercise antenatal weight management intervention, then it reduces risk of pregnancy induced hypertension, macrosomia and neonatal RDS | HM | Pregnancy for women with overweight/obesity | Diet and exercise weight management intervention in pregnancy can reduce risks | ||
| If there are regular scheduled PA sessions, then some women find them difficult to attend | BA | All pregnancy | Barriers to PA in pregnancy | ||
| Early intervention contributes to reduced GWG | GWG | All pregnancy | Timing of intervention is important | ||
| Flannery et al. 2019140 | For overweight/obese pregnant women, knowledge about safety of PA and time are two of the barriers to taking part in PA | BA | Women with overweight/obesity in pregnancy | PA in pregnancy WLIs can be a barrier to participation | |
| Furber et al. 201347 | SR | The safety of weight loss when pregnant and obese is not substantiated | BA | Pregnancy and obesity | Further work needed on safety of weight loss in pregnancy |
| Heslehurst 2014142 | SR | If we want to change practice of managing obesity in pregnancy, then we need to address the barriers for staff in dealing with obesity, not just behaviour change for women | ST | Pregnancy and obesity | Staff – individual and organisational-level barriers to management of obesity in pregnancy |
| Hill et al. 2013154 | SR MA | Diet interventions more effective than PA interventions at reducing GWG | GWG | All pregnancy | PA interventions not as effective as diet |
| Two of the BCTs in weight management, ‘provide rewards contingent on successful behaviour’ and ‘reward and threat’, were associated with significantly less weight gain in pregnancy | GWG | All pregnancy | Important to underpin the intervention with the most effective BCTs | ||
| Hussein et al. 201636 | SR | If mobile phone apps are used in an intervention, particularly when part of multimodal intervention (i.e. a text message or app used in conjunction with another form of electronic communications), then GWG can be reduced | GWG | All pregnancy | Consider multimodal components as part of intervention |
| iWIP 201721 | MA | If women take part in weight management interventions, then there will be a modest effect on GWG but it will not improve maternal or child outcomes, except rates of caesarean sections | GWG | All pregnancy | Unclear relationship between weight management, GWG and maternal outcomes |
| Jelsma et al. 2017233 | If the WLI includes training in motivational interviewing, then only some of the practitioners will be able to deliver the intervention with fidelity |
BA ST |
Women with overweight/obesity in pregnancy | Fidelity to the intervention needs to be measured to understand impact of the intervention | |
| Kim et al. 2016250 | QS | Women are not aware of weight goals in pregnancy and receive conflicting advice from health-care practitioners | BA | All pregnancy | Lack of clarity around weight gain in pregnancy across both population and practitioners |
| Pregnancy is a time when it is acceptable to gain weight | BA | All pregnancy | Attitudes to pregnancy | ||
| McBride et al. 2012136 | S | If women drink during one pregnancy, then they are more likely to drink in subsequent pregnancy | BA | All pregnancy | Importance of engaging with woman’s experiences as context |
| McGirr et al. 2017153 | SR | ‘Goals and planning’ and ‘feedback and monitoring’ are key BCTs involved in reducing GWG | GWG | All pregnancy | Key BCTs in GWG |
| Muktabhant et al. 2015159 | MA | If women take part in an antenatal weight management intervention, then there can be a reduction in the likelihood of excessive GWG | GWG | All pregnancy | GWG can be reduced through antenatal weight management intervention |
| For overweight/obesity population, exercise interventions may not be as effective at reducing likelihood of GWG as for normal weight population | GWG | Women with overweight/obesity in pregnancy | Tailoring the intervention for the individual in particular PA | ||
| Redman et al. 2017116 | RCT | If women take part in an intervention delivered remotely, then they may be more likely to adhere to self monitoring weight than those in the face-to-face group | GWG | Women with overweight/obesity in pregnancy | Remote delivery of an intervention can have positive impact on outcomes |
| Shieh et al. 2018251 | SR MA | For overweight/obese pregnant women, healthy eating interventions had a larger effect size than PA or combined interventions | GWG | Women with overweight/obesity in pregnancy | Healthy eating is key component of a WLI in pregnancy |
| Sanders et al. 2020156 | PS | If regular weighing in pregnancy is part of an intervention, then women find this acceptable and can welcome it | GWG | All pregnancy | Self-monitoring of weight |
| Stephenson et al. 201435 | S | If an intervention is phrased as ‘eating a healthy diet’ rather than ‘being a healthy weight’, then double the number of people would be interested and engaged | RE | General population pregnancy | Communication and language |
| Thangaratinam et al. 2012252 | SR | If women who are overweight/obese take part in any type of weight management intervention, then there is a reduction in GWG | GWG | Women with overweight/obesity in pregnancy | Dietary interventions in pregnancy help reduce GWG |
| Willcox et al. 2015135 | QS | If antenatal care includes mHealth components, then women will embrace these, but health-care practitioners experience barriers to including them in the care pathway |
BA ST |
All pregnancy | MHealth can be important component of engagement and service delivery but barriers to incorporating mHealth into care pathway |
| Willcox et al. 2017253 | RCT | Interventions delivered remotely can make a significant difference to GWG for pregnant women who are overweight/obese | RE | Women with overweight/obesity in pregnancy | Remote delivery acceptable |
Appendix 13 Consolidated explanatory accounts
Abbreviations
Nature of source
CC, case study; FS, feasibility study; FT, feasibility trial; MA, meta-analysis; ME, methods; NR, narrative review; OC, opinion/conceptual paper; OS, observational study; PT, pilot trial; QR, qualitative review; QS, qualitative study; S, survey; ScR, scoping review; SR, systematic review.
Outcomes
BA, barriers; BC, behaviour change; FE, fertility; HM, health markers; KA, knowledge and attitudes; LT, long-term outcomes; RE, recruitment and engagement; ST, staff related; W16, weight loss in a ≤ 16-week programme; W17, weight loss in a ≥ 16-week programme.
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Barker et al. 201829 | OC | Social marketing (e.g. change4life campaign) did not make an impact because individuals and communities require not only knowledge but also resources to enact change, and a purpose or meaning to provide motivation | BA | Resource | Social practice theory – knowledge, resource and meaning required to enact change |
| Campbell et al. 2011242 | SR | Family advice about diet in pregnancy can be a barrier to healthy eating | BA | Pregnancy | Attitudes to weight and diet in pregnancy |
| Cunningham et al. 201843 | QS | If women with BMI > 30 kg/m2 hear the term obese, then they often do not think it applies to them | BA | Pregnant, with BMI > 30 kg/m2 | Language and knowledge |
| Farpour-Lambert et al. 201822 | SR/MA | If there are regular scheduled PA sessions, some women find them difficult to attend | BA | All pregnancy | Barriers to PA in pregnancy |
| Flannery et al. 2019140 | For overweight/obese pregnant women, knowledge about safety of PA and time are two of the barriers to taking part in PA | BA | Women with overweight/obesity in pregnancy | PA in pregnancy WLIs can be a barrier to participation | |
| Furber et al. 201347 | SR | The safety of weight loss when pregnant and obese is not substantiated | BA | Pregnancy and obesity | Further work needed on safety of weight loss in pregnancy |
| Hussein et al. 201636 | SR | If services have designed and evaluated PCC, then weight is not included in the intervention | BA | SR across BMI | Weight is not regarded as an important aspect of PCC |
| Jelsma et al. 2017233 | If the WLI includes training in motivational interviewing, then only some of the practitioners will be able to deliver the intervention with fidelity | BA | Women with overweight/obesity in pregnancy | Fidelity to the intervention needs to be measured to understand impact of the intervention | |
| Kim et al. 2016250 | QS | Women are not aware of weight goals in pregnancy and receive conflicting advice from health-care practitioners | BA | All pregnancy | Lack of clarity around weight gain in pregnancy across both population and practitioners |
| Pregnancy is a time when it is acceptable to gain weight | BA | All pregnancy | Attitudes to pregnancy | ||
| McBride et al. 2012136 | S | If women drink during one pregnancy, then they are more likely to drink in subsequent pregnancy | BA | All pregnancy | Importance of engaging with women’s experiences as context |
| Willcox et al. 2015135 | QS | If antenatal care includes mHealth components, then women will embrace these, but health-care practitioners experience barriers to including them in the care pathway | BA | All pregnancy | MHealth can be important component of engagement and service delivery but barriers in incorporating mHealth into care pathway |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Barker et al. 201829 | OC | If children and young adults are engaged with health behaviour promotions prior to childbearing capabilities developing, then there is a better chance that they will continue with healthy behaviours in the preconception period | BC | Pre-planning |
Habit formation Social movement with preparation for healthy pregnancy becoming more normalised |
| If adults with no immediate intention to get pregnant are informed of risk of future loss of ability to have a healthy child, then they may be more motivated by loss aversion | BC | Pre-planning | Loss aversion as motivator in response to public information campaign | ||
| Mahoney 2014245 | If individual tailored motivational interviewing is used, then participants can report behaviour change and lose an average of 7 lb in 12 weeks |
BC W16 |
PCOS BMI > 27 kg/m2, age 18–44 years 12 weeks, 6 MI sessions, daily logs |
MI, monitoring | |
| Rönö et al. 2018137 | RCT | If participants do not delay getting pregnant while undergoing the intervention, then weight loss will not be significant | BC |
Gestational diabetes BMI > 30 kg/m2 |
No delay of pregnancy so very limited exposure to intervention before conception |
| Shawe et al. 2019130 | S |
Male respondents – 74% pregnancies planned If men are part of a couple planning a pregnancy, then they were more likely than other men (non planners) to reduce smoking, reduce alcohol consumption and to eat more healthily in preparation for pregnancy. However, 57% took no action to improve their health |
BC | All antenatal attenders regarding preconception behaviours | Pregnancy planning and partner involvement |
| Van Dijk et al. 2017138 | QS | If couples engage with mHealth intervention, then they are more likely to achieve positive behaviour changes and pregnancy outcomes | BC | Preconception population |
MHealth Personalised care |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Becker et al. 2015148 | RCT | If women follow too strict a weight loss regimen, then the effect on fertility is uncertainThe exact amount of energy restriction that is safe or deleterious for obese infertile women seeking IVF treatment is not clear | FE |
IVF No one refused to take part 1 out of 16 could not comply with intervention |
Potential risk of too few calories |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Agha et al. 20143 | SR MA | Behavioural interventions in pregnancy may be effective in reducing GWG in obese women without comorbid conditions, but not in overweight or morbidly obese women | GWG | All pregnancy | Tailoring intervention to fit with BMI classification may be critical |
| Farpour-Lambert et al. 201822 | SR/MA | If women take part in an antenatal weight management intervention, then there can be a reduction in the likelihood of excessive GWG and a significant reduction in longer-term post-partum weight | GWG | All pregnancy | Potential longer-term impact of antenatal weight management |
| Moderate energy targets according to weight (18–24 kcal/kg) were associated with the greatest reduction in GWG | GWG | All pregnancy | Tailor calorie targets according to weight | ||
| Early intervention contributes to reduced GWG | GWG | All pregnancy | Timing of intervention is important | ||
| Hill et al. 2013154 | SR MA | Diet interventions more effective than PA interventions at reducing GWG | GWG | All pregnancy | PA interventions not as effective as diet |
| Two of the BCTs in weight management, ‘provide rewards contingent on successful behaviour’ and ‘reward and threat’, were associated with significantly less weight gain in pregnancy | GWG | All pregnancy | Important to underpin the intervention with the most effective BCTs | ||
| Hussein et al. 201636 | SR | If mobile phone apps are used in an intervention, particularly when part of multimodal intervention (i.e. a text message or app used in conjunction with another form of electronic communications), then GWG can be reduced | GWG | All pregnancy | Consider multimodal components as part of intervention |
| iWIP 201721 | MA | If women take part in weight management interventions, then there will be a modest effect on GWG but it will not improve maternal or child outcomes, except rates of caesarean sections | GWG | All pregnancy | Unclear relationship between weight management, GWG and maternal outcomes |
| McGirr et al. 2017153 | SR | ‘Goals and planning’ and ‘feedback and monitoring’ are key BCTs involved in reducing GWG | GWG | All pregnancy | Key BCTs in GWG |
| Muktabhant et al. 2015159 | MA | If women take part in an antenatal weight management intervention, then there can be a reduction in the likelihood of excessive GWG | GWG | All pregnancy | GWG can be reduced through antenatal weight management intervention |
| For overweight/obese population, exercise interventions may not be as effective at reducing likelihood of GWG as for normal weight population | GWG | Women with overweight/obesity in pregnancy | Tailoring the intervention for the individual, in particular PA | ||
| Redman et al. 2017116 | RCT | If women take part in an intervention delivered remotely, then they may be more likely to adhere to self-monitoring weight than those in the face-to-face group | GWG | Women with overweight/obesity in pregnancy | Remote delivery of an intervention can have positive impact on outcomes |
| Shieh et al. 2018251 | SR MA | For overweight/obese pregnant women, healthy eating interventions had a larger effect size than PA or combined interventions | GWG | Women with overweight/obesity in pregnancy | Healthy eating is key component of a WLI in pregnancy |
| Sanders et al. 2020156 | PS | If regular weighing in pregnancy is part of an intervention, then women find this acceptable and can welcome it | GWG | All pregnancy | Self-monitoring of weight |
| Thangaratinam et al. 2012252 | SR | If women with overweight/obesity take part in any type of weight management intervention, then there is a reduction in GWG | GWG | Women with overweight/obesity in pregnancy | Dietary interventions in pregnancy help reduce GWG |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Caut et al. 202033 | SR | Usual preconception diet is insufficient in terms of nutritional guidelines | HM | Preconception and pregnancy | Nutrition |
| Farpour-Lambert et al. 201822 | SR/MA | If women with overweight/obesity take part in a diet and exercise antenatal weight management intervention, then it reduces the risk of pregnancy induced hypertension, macrosomia and neonatal RDS | HM | Pregnancy for women with overweight/obesity | Diet and exercise weight management intervention in pregnancy can reduce risks |
| Moran et al. 2011192 | PS | If one-off healthy lifestyle advice is given (to control group), then participants can reduce waist circumference | HM |
IVF BMI 28–45 kg/m2; age 18–40 years |
Lifestyle advice |
| Stephenson et al. 201827 | OC | Many women of reproductive age in low-, middle- and high-income countries will not be prepared nutritionally for pregnancy | HM | International | Nutrition |
| van Dammen et al. 2018152 | RCT | If women take part in a 6-month intervention prior to IVF with a goal of 5–10% weight loss, then their cardiometabolic health and their self-reported physical quality of life improves | HM |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric and lifestyle counselling – authors suggest result mechanism is due to intrinsic motivation (of achieving a healthy pregnancy) |
| van Dammen et al. 2019247 | RCT | If women take part in a 6-month intervention prior to IVF with goal of 5–10% weight loss, then there is no significant improvement of stress, mood, sleep quality and mental health quality of life 3–8 years following participation | HM |
IVF BMI > 28 kg/m2; aged 18–39 years |
Hypocaloric and lifestyle counselling |
| van Elten et al. 2019248 | RCT | If participants take part in an intervention, then their intake of unhealthy foods will be lower at 3, 6 and 12 months after randomisation | HM | IVFBMI > 28 kg/m2; aged 18–39 years | Hypocaloric and lifestyle counselling |
| Wekker et al. 2019249 | RCT |
Short-term success predicts long-term success If women reach their target weight during the intervention period, then they will show improved cardiometabolic health 6 years later |
HM | Preconception obesity | Achieving targets acts as longer-term motivation |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Beckmann et al. 2014190 | CC | If women are motivated to attend a 90-minute session in a preconception service (including those motivated by subfertility and pre-existing conditions), then they may gain less weight prior to conception | KA | General population attending preconception clinic | Health advice |
| Delissaint et al. 201138 | SR | Knowledge, awareness and beliefs of PCC do not lead to preconception health practice | KA | Preconception health | Need a mechanism for knowledge to be translated into change of behaviour |
| Hussein et al. 201636 | SR | If women received preconception care and counselling, then maternal knowledge, self-efficacy and health locus of control, and self-reported risk behaviour will improve | KA | Preconception across BMI | Information increases knowledge and changes attitudes |
| If women receive PCC and counselling. then it is unclear whether this has any effect on adverse pregnancy outcomes | KA | Preconception across BMI | The relationship between information, attitude and behaviour change is unclear | ||
| Van Dijk et al. 2017138 | QS | MHealth is a useful way of providing information but it needs to be part of an intervention that includes face-to-face contact and to be tailored/personalised | KA | Preconception population |
MHealth Personalised care |
| Weisman et al. 201149 | RCT | If women in prepregnacy and interpregnacy phase from low-income communities engage with a social cognitive programme to reduce adverse pregnancy outcomes via changing attitudes and behaviours, then their self-efficacy and intention to change health behaviours increases | KA | Non-pregnant women aged 18–35 years in low-income community setting | Social cognitive approach to behaviour change. The group format was intended to motivate women through social support from peers and the lay group facilitators |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| van Elten et al. 2019248 | RCT | If participants lost weight in an intervention, then they will maintain a lower BMI and lower reported energy intake between 3 and 8 years following randomisation | LT |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric and lifestyle counselling Successful weight loss during the intervention predicts longer-term changes |
| Wekker et al. 2018249 | RCT |
Short-term success predicts long-term success If women reach their target weight during the intervention period, then they will show improved cardiometabolic health 6 years later |
LT | Preconception obesity | Achieving targets acts as longer-term motivation |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Barker et al. 201829 | OC | If adults are intending to get pregnant, then the intervention opportunities are to actively support preconception health, for example through text messaging and provide practical support in an engaging way | RE | Intention | Support |
| If adults intending to get pregnant are in a specific subgroup, then they need intense, tailored support | RE | Culture | Tailoring | ||
| If adults intending to get pregnant have previously had a pregnancy, then they will be less receptive to pre-pregnancy input and input needs to take previous pregnancy into account | RE | Subsequent pregnancy | Individualised | ||
| If supermarkets could convey healthy preconception nutrition messages (e.g. next to folic acid), then this would be an opportunity to inform a lot of women | RE | Public health messages | Community messages | ||
| If interventions are delivered via digital media, then women experiencing social disadvantage are more likely to engage | RE | Comms strategy | Reach of intervention | ||
| Becker et al. 2015148 | RCT | If women on the waiting list for IVF are invited to participate in a WLI, then there is a high take-up rate (100% in this study) | RE |
IVF No one refused to take part 1 out of 16 could not comply with intervention |
Motivation |
| Brackenridge et al. 201880 | PS | If women who agree to delay having their coil removed for 24 weeks in order to lose weight are offered an intensive meal replacement-based weight loss programme, then one-third of those eligible will agree to take part | RE |
BMI > 30 kg/m2; age 18–40 years Community clinic Delay IUD removal |
Hypocaloric, 966–1220 kcal/day |
| Cunningham et al. 201843 | QS | If women have BMI of > 30 kg/m2, then raised BMI is regarded as acceptable term as it is factual | RE | Pregnant, with BMI of > 30 kg/m2 | Language |
| Einarsson et al. 2017243 | If women with BMI of 30–35 kg/m2 waiting for IVF are offered 16-week very low-calorie meal replacement WLI, then 77% will agree to take part | RE | Nordic countries – IVF threshold is BMI of 35 kg/m2, so all women already within range | Hypocaloric, 12 weeks at 880 kcal/day | |
| Greenhalgh et al. 2015134 | QS | If beliefs about negative effects of diet and exercise during pregnancy are addressed in a culturally relevant way, then participants are more likely to engage with an intervention | RE | South Asian women with GDM | Cultural relevance |
| If health promotion messages are not tailored to different groups of people with protected characteristics, then individuals who are understood to be particularly at risk of marginalisation will be further excluded from health care | RE | South Asian women with GDM | Inequity and marginalisation | ||
| Homan et al. 2012244 | PS | If couples undergoing fertility treatment are offered lifestyle advice designed to address lifestyle behaviours potentially influencing fertility and 4 months of weekly support, then 57% will agree to participate and 31% will take part | RE |
IVF, all BMI Targeting lifestyle rather than one behaviour such as weight |
Engagement/motivation of couples with lifestyle messages is hard to achieve even in motivated population |
| Khan et al. 2019144 | QS | If the availability of preconception screening and advice was promoted to the general public, then anxiety would be reduced and people planning a pregnancy would engage in more healthy behaviours | RE |
Australia Preconception, pregnancy and postnatal |
Community education |
| Legro et al. 201544 | RCT | If women with PCOS undergoing IVF are invited to take part in a 16-week RCT delaying IVF in order to lose a goal of 7% weight, then 17% of eligible women will consent to take part | RE |
PCOS and IVF BMI 27–42 kg/m2; age 18–40 years |
Hypocaloric – individualised |
| Mahoney 2014245 | PS | If women with PCOS are offered a 12-week MI intervention to help with lifestyle change to support fertility, then 22% will participate in the intervention and 16.6% will complete it | RE |
PCOS BMI > 27 kg/m2; age 18–44 years 12 weeks, 6 MI sessions, daily logs, etc. |
MI-based intervention |
| Norris et al. 2016133 | OC | If stakeholders are involved at the development stage of an intervention (Jom Moma) and intervention mapping is used, then the intervention is more likely to be well received by participants | RE | Malaysia population-based intervention | Intervention mapping; coproduction |
| Rönö et al. 2018137 | RCT | Recruiting women pre conception (in context of GDM) is difficult, but the most effective method is by personal invitation based on risk identified through hospital registers | RE |
Gestational diabetes BMI > 30 kg/m2 |
Engagement personal |
| Rothberg et al. 2016146 | FT | If women met the strict eligibility criteria for a 16-week intensive weight loss programme, then 56% would agree to be randomised | RE |
Anovulatory subfertility BMI 35–45 kg/m2; age 18–40 years; goal of 15% weight loss |
Hypocaloric VLED. Fortnightly dietetic input. PA advice |
| Sim et al. 2014145 | RCT | If invited to take part in weekly group VLED programme with tailored intervention and support RCT, then 84% agree to participate | RE |
IVF BMI 30–40 kg/m2; age 18–37 years |
Tailoring and group support |
| Stephenson et al. 201435 | S | If an intervention is phrased ‘eating a healthy diet’ rather than ‘being a healthy weight’, then double the number of people would be interested and engaged | RE | General population pregnancy | Communication and language |
| Willcox et al. 2017253 | RCT | Interventions delivered remotely can make a significant difference to GWG for pregnant women who have overweight/obesity | RE | Overweight/obesity in pregnancy | Remote delivery acceptable |
| Author and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Barker et al. 201829 | OC | If practitioners are trained in Healthy Conversation Skills, then they will be able to engage and motivate patients and clients about nutrition and PA during brief consultations | ST | Staff skills |
Self-efficacy Relationship between competence and confidence of staff |
| Heslehurst et al. 2014142 | SR | If we want to change practice of managing obesity in pregnancy, then we need to address the barriers for staff in dealing with obesity, not just behaviour change for women | ST | Pregnancy and obesity | Staff-, individual- and organisational-level barriers to management of obesity in pregnancy |
| Jelsma et al. 2017233 | If the WLI includes training in motivational interviewing, then only some of the practitioners will be able to deliver the intervention with fidelity | ST | Women with overweight/obesity in pregnancy | Fidelity to the intervention needs to be measured to understand impact of the intervention | |
| Khan et al. 2019144 | QS | If national evidence-based guidance and preconception checklists were developed, then clinicians would feel more able to give appropriate advice and people planning pregnancy would feel less anxious about doing the right thing | ST |
Australia Preconception, pregnancy and postnatal |
National guidelines needed |
| Montanaro 2019246 | FS | If a preconception risk assessment tool is going to be introduced into primary care services, then there can be significant challenges in implementation | ST | Primary care services | Organisational challenges |
| Willcox et al. 2015135 | QS | If antenatal care includes mHealth components, then women will embrace these but health-care practitioners experience barriers to including them in the care pathway | ST | All pregnancy | MHealth can be important component of engagement and service delivery but there are barriers in incorporating mHealth into care pathway |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Becker et al. 2015148 | RCT | If women aged 18–35 years, with BMI 25–40 kg/m2 waiting for their first round of IVF treatment engage in 12 weeks of an individualised low-GI diet plan, then they can achieve a 5.5% weight loss | W16 |
IVF No one refused to take part 1/16 could not comply with intervention |
Hypocaloric and low-GI individualised plan (20 kcal/kg bodyweight) |
| Einarsson et al. 2017243 | RCT | If participants have a high level of engagement in a 16-week intervention of very low-calorie meal replacement diet plus support every 2–3 weeks, including dietetic input, then they will experience significant weight loss of average 9.44 kg (group mean BMI was 29.8 kg/m2) | W16 |
IVF BMI 30–35 kg/m2 Nordic countries’ IVF threshold is BMI 35 kg/m2, so all women were already within range |
Hypocaloric, 12 weeks at 880 kcal/day |
| Espinós et al. 2017149 | RCT | If women with BMI 30–40 kg/m2 waiting for IVF take part in a 12-week diet and exercise programme, then they will lose an average of 6.97% weight | W16 |
IVF BMI 30–40 kg/m2; age 18–37 years |
Reduction of calories by 500–800 per day plus exercise plus support |
| Homan et al. 2012244 | PS | If couples undergoing fertility treatment are overweight and are motivated to receive lifestyle intervention using MI principles designed to address lifestyle behaviours potentially influencing fertility and receive weekly follow-up support telephone calls, then 47% of those who take part will lose between 1 kg and 5 kg in 4 months | W16 |
IVF all BMI Targeting lifestyle rather than one behaviour such as weight |
Motivation – using MI to engage with lifestyle behaviours Couples – mutual support |
| Legro et al. 201544 | RCT | If the intervention is tailored and includes meal replacements and weight loss medication, then participants can experience significant weight loss (between 6.2% and 6.4%) over the 16-week period | W16 |
PCOS and IVF BMI 27–42 kg/m2; age 18–40 years |
Hypocaloric (between 1200 and 2000 kcal/day) plus lifestyle modification (including meal replacements, weight loss medication, either sibutramine or orlistat) and increased PA |
| Mahoney 2014245 | If individual tailored MI is used, then participants can report behaviour change and lose an average of 7 lb in 12 weeks | W16 |
PCOS BMI > 27 kg/m2; age 18–44 years 12 weeks, 6 MI sessions, daily logs |
MI, monitoring | |
| Moran et al. 2011192 | PS | If women follow a reduced-calorie high-protein diet, there is a meal replacement aspect and they receive lifestyle advice, then participants can lose an average of 3.8 kg in the 5- to 9-week intervention period | W16 |
IVF BMI 28–45 kg/m2; age 18–40 years |
Hypocaloric, 1200 kcal/day including one meal replacement and three contacts |
| Rothberg et al. 2016146 | FT | If women were able to complete the 16-week intensive weight loss programme (n = 6), then they lost an average of 13% body weight | W16 |
Subfertility BMI 35–45 kg/m2; age 18–40 years; goal of 15% weight loss |
Hypocaloric VLED, 800 kcal/day for up to 12 weeks. Fortnightly dietetic input. PA advice |
| Sim et al. 2014145 | RCT | If participants take part in a group programme and if the intervention is flexible/tailored and the intervention includes support, then they can lose an average of 6.6 kg in the 12-week period | W16 |
IVF BMI 30–40 kg/m2; age 18–37 years |
Hypocaloric VLED ,6 weeks at ≈ 650 kcal/day, then 6-week ‘refeeding’ with ≈ 650 deficit calories described as individually tailored Group process as support mechanism (but not measured) |
| van Elten et al. 2019248 | RCT | If women take part in a 6-month intervention prior to IVF with goal of 5–10% weight loss, then the largest effect of the intervention was within 3 months of randomisation. Therefore, it seems that the higher intensity of guidance in the first 3 months of the intervention programme encouraged healthy changes in diet and PA | W16 |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric and lifestyle counselling |
| Authors and year | Source | Explanatory account | Outcome (O), context (C), mechanism (M) | ||
|---|---|---|---|---|---|
| O | C | M | |||
| Brackenridge et al. 201880 | PS | If women agree to delay having their coil removed for 24 weeks and can follow a low-energy liquid diet weight loss programme plus fortnightly 15-minute motivational support, then they will lose a clinically significant amount of weight (median BMI reduction of 14.2% for completers, 6.6% ITT of all starters) | W17 |
BMI > 30 kg/m2; age 18–40 years Community clinic delay IUD removal |
Hypocaloric, 966–1220 kcal/day |
| LeBlanc et al. 202153 | RCT | If women take part in a remotely delivered tailored behavioural WLI, then they can lose 3.5% of their weight before conception | W17 | USA; preconception and pregnancy | Behavioural weight loss support |
| If women lose a significant amount of weight prior to pregnancy, then there is a risk of weight regain in pregnancy | W17 | USA; preconception and pregnancy | Weight loss support needs to continue into pregnancy | ||
| If women lose weight in a preconception intervention, then it is important to continue with support through pregnancy if this loss is to be maintained by the end of the third trimester of pregnancy | W17 | USA; preconception and pregnancy | Weight loss support needs to continue into pregnancy | ||
| Mutsaerts et al. 2016150 | RCT | If women are recruited to take part in a 6-month intervention prior to IVF with goal of 5–10% weight loss, then there will be a 22% discontinuation rate and 37.7% will lose ≥ 5% (43% of the completers) | W17 |
IVF BMI > 28 kg/m2; age 18–39 years |
Hypocaloric individual – reduced by 600 calories with minimum of 1200 kcal/day Lifestyle motivational counselling Motivation of early IVF if weight loss hit 5–10% |
Appendix 14 Consolidated explanatory accounts grouped by outcome
| Consolidated accounts for preconception interventions grouped by outcomes | Potential key ideas/theoretical concepts |
|---|---|
| Recruitment and engagement | |
| In the context of the general adult population | |
| If the aim of a service or research project is to maximise recruitment and engagement of people planning a pregnancy to a preconception intervention | |
Then:
|
|
| If the aim of an intervention is to target women with BMI > 25 kg/m2 | |
| Then women will need to be weighed and BMI identified, as 70.8% of those with BMI over 25 kg/m2 (and 18.4% of those with BMI > 30 kg/m2) would not identify themselves as overweight129 |
|
| In the context of people on the waiting list for fertility treatment | |
| In the context of a meal replacement intervention | |
| Behaviour change | |
| In the context of the general population: | |
| If a couple are planning a pregnancy | |
| Then the male partners were more likely than other men (non-planners) to report reducing smoking, reducing alcohol consumption and eating more healthily (in preparation for pregnancy)130 |
|
| If a woman’s partner engages with an online preconception intervention | |
| Then the woman is more likely to achieve positive behaviour changes and pregnancy outcomes138 |
|
| If the availability of pre-conception screening and advice were promoted to the general public | |
| Then anxiety would be reduced and people would engage in more healthy behaviours144 |
|
| If children and young adults are engaged with health behaviour promotions prior to childbearing capabilities developing | |
| The there is a better chance that they will continue with healthy behaviours in the preconception period29 |
|
| If adults with no immediate intention to get pregnant are informed of risk of future loss of ability to have a healthy child | |
| Then they may be more motivated by loss aversion29 |
|
| In the context of fertility treatment/PCOS | |
| If individuals or couples are motivated enough to take part in an intervention incorporating motivational interviewing focusing on lifestyle behaviours | |
| Then they will have success in making the lifestyle changes,245 increasing the likelihood of a healthy pregnancy244 |
|
| If participants take part in lifestyle counselling intervention | |
| Then their intake of unhealthy foods will be lower 3, 6 and 12 months after randomisation248 |
|
| Staff behaviour | |
| If practitioners are trained in Healthy Conversation Skills | |
| Then they will be able to engage and motivate patients and clients about nutrition and physical activity during brief consultations29 |
|
| If national evidence-based guidance and preconception checklists were developed | |
| Then clinicians would feel more able to give appropriate advice and people planning pregnancy would feel less anxious about doing the right thing144 |
|
| If a preconception risk assessment tool is going to be introduced into primary care services | |
| Then there can be significant challenges in implementation246 |
|
| Knowledge and behaviour change | |
| If an intervention just provides information and knowledge relating to preconception health | |
| Then it is unclear if it will be sufficient to impact on behaviour; there were mixed findings of reviews/studies. For example, self-report risk behaviours change but pregnancy outcomes do not36; knowledge, awareness and beliefs of preconception care do not lead to preconception health practice38; reduced weight gain190; no change in anthropomorphic results but self-report change in BMI at 12 months49 | |
| Health markers | |
| If women take part in a 6-month intervention involving hypocaloric diet and lifestyle counselling prior to IVF with goal of 5–10% weight loss | |
| Then: |
|
| If there is no intervention to improve nutrition | |
| Then most women will not meet the nutritional guidelines for pregnancy27,33 | |
| Fertility | |
| If women follow too strict a weight loss regimen | |
| Then the effect on fertility is uncertain148 | |
| Weight loss | |
| In the context of PCOS/fertility treatment | |
| If women are motivated to engage with a WLI based on a very low energy diet over 12–16 weeks | |
| Then they can lose an average of between 6.9% (12 weeks)145 and 13% (16 weeks)146 of their body weight | |
| If women are motivated to engage with an hypocaloric WLI that includes an element of meal replacement over 16 weeks | |
| Then they can lose an average of 6%44 of their body weight | |
| If women are motivated to engage with an individualised hypocaloric WLI without meal replacement (calorie intake determined by body weight) over 12 weeks | |
| Then they can lose an average of 5% (BMI 25–40 kg/m2)148 to 6.97% (BMI 30–40 kg/m2)149 of their body weight | |
| If women take part in a 6-month hypocaloric and lifestyle counselling intervention with goal of 5–10% weight loss | |
| Then the largest effect of the intervention is within 3 months of randomisation150,248 | |
| If women are motivated to attend a motivational interviewing intervention over 12–16 weeks | |
| Then they will lose between 1 and 5 kg243,245 | |
| In the context of women with raised BMI | |
| If women agree to delay having their IUD removed for 24 weeks and can follow a low energy liquid diet weight loss programme plus fortnightly 15-minute motivational support | |
| Then they will lose a clinically significant amount of weight (median BMI reduction of 14.2% for completers)80 | |
| If women take part in a remotely delivered tailored behavioural WLI | |
| Then they can lose 3.5% of their weight before conception53 | |
| Long-term outcomes | |
| If women take part in a 6-month intervention prior to IVF with goal of 5–10% weight loss | |
Then there is:
|
|
| If women take part in a remotely delivered tailored behavioural WLI | |
| Then the weight they lose pre conception may be regained during pregnancy53 | |
| Barriers | |
| If services have designed and evaluated PCC intervention | |
| Weight is not included in the intervention36 |
|
| If services/intervention are offered to women who are overweight | |
| Then 49.6% of women with raised BMI will not think it applies to them (70.8% of those with BMI of > 25 kg/m2 and 18.4% of those with BMI of > 30 kg/m2)129 |
|
| If women with a BMI over 3 kg/m2 accessing fertility treatment are invited to take part in a 16-week intensive weight loss programme | |
|
Then nearly half of those eligible declined to take part Barriers to taking part were:Barriers that led to withdrawal were disappointment at randomisation to standard-of-care therapy and inability to adhere to the programme146 |
|
| If women do not delay getting pregnant while taking part in an intervention | |
| Then weight loss will not be significant before pregnancy137 |
|
List of abbreviations
- app
- application
- BASHH
- British Association for Sexual Health and HIV
- BCT
- behaviour change technique
- BMI
- body mass index
- BPAS
- British Pregnancy Advisory Service
- CI
- confidence interval
- CMO
- context–mechanism–outcome
- COM-B
- Capability, Opportunity and Motivation to Behaviour
- CPRD
- Clinical Practice Research Datalink
- CTR
- Centre for Trials Research
- FSRH
- Faculty of Sexual and Reproductive Healthcare of the Royal College of Obstetricians & Gynaecologists
- GDPR
- General Data Protection Regulation
- GP
- general practitioner
- GWG
- gestational weight gain
- HCP
- health-care practitioner
- HCPI
- health-care practitioner interview
- HSE
- Health Survey for England
- IOM
- Institute of Medicine
- IU
- intrauterine
- IUD
- intrauterine device
- IVF
- in vitro fertilisation
- iWIP
- International Weight in Pregnancy
- LARC
- long-acting reversible contraceptive
- LU
- LARC user
- LUI
- LARC user interview
- NICE
- National Institute for Health and Care Excellence
- NPT
- normalisation process theory
- PBC
- perceived behavioural control
- PCC
- preconception care
- PCOS
- polycystic ovary syndrome
- PHW
- Public Health Wales
- PIS
- participant information sheet
- PPI
- participant and public involvement
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- RECORD
- REporting of studies Conducted using Observational Routinely-collected Data
- RR
- relative risk
- SAG
- Stakeholder Advisory Group
- SET
- social ecological theory
- SHC
- sexual health clinic
- SHW
- Strong Healthy Women
- SMG
- Study Management Group
- SOP
- Standard Operating Procedures
- SSC
- Study Steering Committee
- TPB
- theory of planned behaviour
- WLI
- weight loss intervention
- WP
- work package
Notes
-
WP2 phase 1 LARC user SAG question information
-
WP2 phase 1 HCP SAG question information
-
WP2 phase 1 LARC user SAG PIS
-
WP2 phase 1 LARC user SAG consent form
-
WP2 phase 1 HCP SAG PIS
-
WP2 phase 2 LARC user interview PIS
-
WP2 phase 2 HCP interview PIS
-
WP2 phase 2 LARC user SAG PIS
-
WP2 phase 2 LARC user SAG consent form
-
WP2 phase 2 HCP SAG PIS
-
WP2 phase 2 LARC user SAG question information
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/NKIX8285).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.