Notes
Article history
The contractual start date for this research was in February 2019. This article began editorial review in August 2022 and was accepted for publication in July 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Uthman et al. This work was produced by Uthman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Uthman et al.
Introduction
In high-income countries, such as the UK, cardiovascular disease (CVD) is one of the primary causes of morbidity and mortality. 1 Heart and circulation disorders account for a quarter of all deaths in the UK, or over 160,000 each year – an average of 460 deaths per day, or 1 every 3 minutes. 1 In the UK, around 7.6 million people (4 million men and 3.6 million women) suffer from heart or circulatory disease. The most common type of heart illness is coronary heart disease (CHD). 1 It is the leading cause of heart attack and the leading cause of death for both men and women worldwide in 2019. 1 Heart attacks cause 100,000 hospital admissions in the UK each year, or one every 5 minutes. In the UK today, around 1.4 million people have survived a heart attack and heart failure affects around 900,000 people. Strokes kill about 35,000 people in the UK every year and are the leading cause of severe disability. 1
Many of the risk factors for developing CVD are modifiable, and data suggest that a substantial percentage of the current CVD burden is either entirely or partially preventable. 2–4 Primary prevention of CVD at the community level is ideal, as the aim is to delay or avoiding the start of CVD. Several systematic reviews and meta-analyses have examined strategies for the primary prevention of CVD, but there has been no consensus regarding the strategy with the best overall results. 5,6 Some have concluded that these interventions may be successful in reducing the burden of CVD, whereas others have questioned their efficacy for CVD primary prevention. 5,6 Determining the effectiveness of various strategies for the primary prevention of CVD necessitates a systematic evaluation and synthesis of the available evidence. 7–9 This study seeks to provide such a synthesis giving an overview of existing systematic reviews. This is a relatively new strategy for summing up evidence which can help researchers synthesise evidence across interventions. This is particularly valuable when conflicting evidence has been reported in previous systematic reviews. We aim to produce a comprehensive overview of the current best evidence by identifying, analysing and synthesising the numerous published systematic reviews assessing the comparative effectiveness of different interventions for primary prevention of CVD.
This publication is part of a series of publications on ‘Determining optimal strategies for primary prevention of CVD: systematic review, network meta-analysis and cost-effectiveness review (National Institute for Health and Care Research (NIHR)/Health Technology Assessment (HTA): 17/148/05)’. Other publications in this series include:
-
Effectiveness of policies and structural interventions in reducing CVD and mortality: a systematic review of simulation-based studies.
-
Increasing comprehensiveness and reducing workload in the preparation of a systematic review of complex interventions using automated machine learning.
-
Determining optimal primary prevention interventions for major CVD events and all-cause mortality – findings from systematic review and hierarchical network meta-analysis of randomised controlled trials (RCTs).
-
How conclusive is the evidence for interventions in primary prevention of CVD: a trial sequential analysis?
-
Mind the gap! A multilevel analysis of factors associated with variation in published CVD primary prevention interventions effect estimates within and between countries.
-
Determining optimal strategies for primary prevention of CVD: systematic review of cost-effectiveness analyses in the UK.
The findings from all the workstreams, including those from the systematic review of modelling studies, will be summarised in a synopsis paper to be published alongside this series.
Methods
This systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the following number: CRD42019123940. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). 10
Review eligibility criteria
The review question guiding this overview is presented in PICOTSS format (patient, intervention, comparators, outcomes, timing, setting and study design):
Patients: The review includes adult populations (18 years and older) from population-based studies, which may target moderate/high CVD risk groups such as those with hypertension, obesity, hyperlipidaemia, type 2 diabetes or a combination of these factors. The focus of the review is on primary prevention of CVD, so we excluded trials involving individuals who have had a previous myocardial infarction (MI), stroke, revascularisation procedure coronary artery bypass grafting or percutaneous transluminal coronary angioplasty and those with angina or angiographically defined CHD. Studies with mixed populations, including both individuals with and without CVD, were included if data relevant to primary prevention could be extracted.
Intervention: Any form of intervention aimed at the primary prevention of CVD, including but not limited to drugs [lipid-lowering medications, blood pressure (BP)-lowering medications, antiplatelet agents], diet (nutritional supplements, dietary interventions), physical activity or public health (health promotion programmes, structural and policy interventions).
Comparators: Other forms of intervention (such as a minimal intervention, active intervention, concomitant intervention), placebo, usual care or no intervention control group or waiting list control.
Outcome measures: The primary outcome was all-cause mortality. Secondary outcomes were CVD-related mortality, major cardiovascular events (defined as fatal and non-fatal MI, sudden cardiac death, revascularisation, fatal and non-fatal stroke and fatal and non-fatal heart failure), CHD (fatal and non-fatal MI and sudden cardiac death, excluding silent MI) and incremental costs per quality-adjusted life-years gained reported alongside a randomised trial.
Timing: Studies of any duration.
Setting: Any setting.
Study design: Systematic reviews of RCTs. Units of randomisation could be either individuals or clusters (such as family, workplace).
Search strategy
A sensitive literature search for existing systematic reviews was developed iteratively. The process involved testing each iteration’s effectiveness in retrieving a high proportion of records for a broad cross-section of more than 70 known, relevant systematic reviews of RCTs that had been found in previous work via a variety of sources. After only a few iterations, we found that by adding a few specific medical subject heading (MeSH) and title terms we were able to find all of the known reviews. Searches were based on the concepts of prevention, CVD outcomes and systematic reviews. From inception until March 2021, we searched the Cochrane Database of Systematic Reviews (via Wiley), MEDLINE (via Ovid), EMBASE (via Ovid) and Database of Abstracts of Reviews of Effects (DARE) (via Centre for Reviews and Dissemination) databases. All searches were carried out by one of us (RC), an experienced information specialist. Appendix 1 of the supplement contains detailed search strategies. References of included studies were also checked for relevant reviews. Records were exported to EndAAFnote X9 and systematically de-duplicated.
Study selection and data extraction
All study selection, data extraction, evidence synthesis and quality assessment processes were completed by two authors separately (CN and SA). Any disagreement was handled by consensus or referral to a third investigator (OAU). We independently abstracted key participant and intervention information and reported data on prespecified outcomes using standardised data extraction templates for studies that met the inclusion criteria. For outcomes that were meta-analysed, we also retrieved pooled effect estimates. Risk ratios (RRs) or odds ratios with 95% confidence intervals (CI) were used to report dichotomous data.
Risk of bias
We independently assessed the methodological quality of each systematic review using the Assessment of Multiple SysTemAtic Reviews (AMSTAR) 2 tool. 11
Results
Study characteristics and evidence mapping
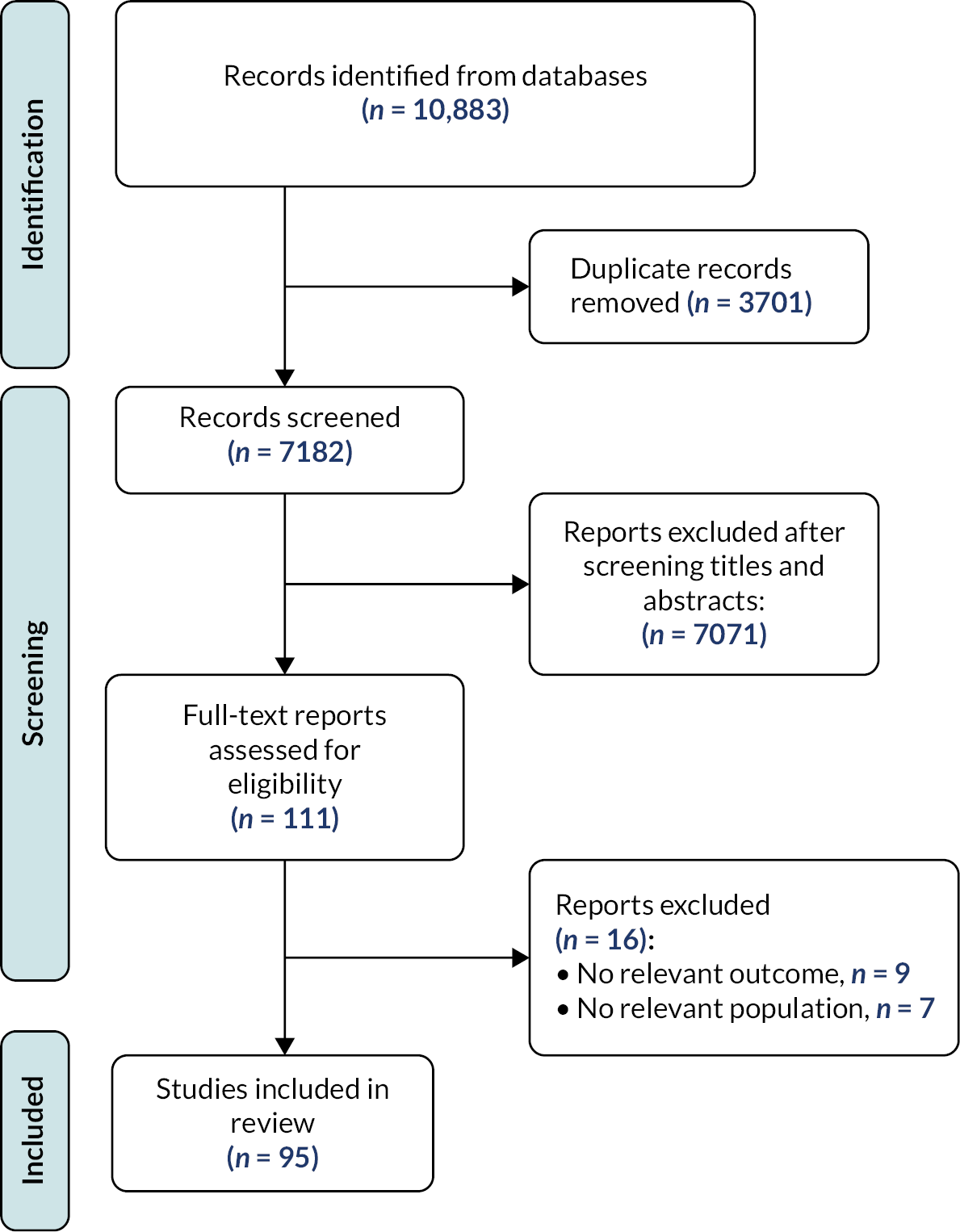
Our search identified 10,883 records, of which 111 records were evaluated as full-text articles after title and abstract screening (Figure 1). In total, we selected 95 systematic reviews for inclusion (see Appendix 2). The systematic reviews were published between 1997 and 2020. On average, the systematic reviews included 18 RCTs (range: 2–287). The largest systematic review included 287 RCTs. The number of participants included in the systematic reviews ranged from as few as 47 to as many as 963,829 (mean: 73,937). When reported, final searches for each reported review were performed between August 2014 and September 2019. Table 1 shows results from the most recent and most comprehensive systematic reviews for each intervention for major cardiovascular events, CHD events, CVD mortality and all-cause mortality.
FIGURE 1.
PRISMA study flow and selection.
| Intervention | Major cardiovascular events | CHD events | CVD mortality | All-cause mortality |
|---|---|---|---|---|
| Non-pharmacological | ||||
| Dietary | ||||
| Dietary intervention | 0.96 (0.92–1.01) | 0.91 (0.85–0.97) | 0.92 (0.86–0.99) | 0.97 (0.93–1.01) |
| Folic acid | 0.98 (0.95–1.02) | 1.03 (0.98–1.08) | 1.00 (0.98–1.02) | |
| Reduced fat | 0.84 (0.72–1.07) | 0.91 (0.77–1.07) | 0.98 (0.86–1.12) | |
| Reduced salt | 0.76 (0.57–1.01) | 0.67 (0.45–1.01) | 0.96 (0.83–1.10) | |
| Health promotion | ||||
| Digital health | 1.21 (0.58–2.54) | |||
| Multifactorial | 0.99 (0.92–1.07) | 1.00 (0.96–1.05) | ||
| Multicomponent intervention | ||||
| Diet and physical activity advice | 0.57 (0.11–3.07) | |||
| Nutritional supplements | ||||
| Beta-carotene | 1.01 (0.93–1.09) | 1.04 (0.99–1.09) | ||
| Calcium | 1.14 (0.92–1.41) | 1.04 (0.96–1.12) | ||
| Folic acid | 0.52 (0.24–1.10) | |||
| Homocysteine-Folic | 0.90 (0.81–1.00) | |||
| Multivitamin | 0.95 (0.85–1.06) | 0.91 (0.84–0.98) | 0.92 (0.87–0.98) | 0.98 (0.94–1.02) |
| Niacin | 0.66 (0.49–0.89) | 0.75 (0.59–0.96) | ||
| Omega 3 | 0.95 (0.82–1.12) | 0.85 (0.68–1.06) | 0.87 (0.73–1.03) | |
| Selenium | 1.03 (0.95–1.11) | 0.97 (0.79–1.20) | 0.97 (0.88–1.08) | |
| Vitamin A | 1.09 (0.77–1.54) | |||
| Vitamin B3 | 0.95 (0.74–1.22) | 0.93 (0.87–1.00) | 1.02 (0.93–1.12) | 1.05 (0.97–1.12) |
| Vitamin C | 0.99 (0.89–1.10) | 1.06 (0.97–1.16) | ||
| Vitamin D | 0.95 (0.95–0.98) | 0.93 (0.89–0.96) | 0.94 (0.890.99) | 0.94 (0.88–1.01) |
| Vitamin D + Calcium | 1.01 (0.95–1.07) | 0.92 (0.83–1.01) | ||
| Vitamin E | 0.97 (0.92–1.03) | 1.01 (0.98–1.04) | ||
| Pharmacological | ||||
| Antiplatelet | ||||
| Antiplatelet | 0.97 (0.96–0.99) | |||
| Aspirin | 0.90 (0.85–0.94) | 0.86 (0.79–0.92) | 0.99 (0.87–1.11) | 0.96 (0.90–1.03) |
| BP lowering | ||||
| Angiotensin-converting enzyme (ACE) inhibitors | 1.03 (1.00–1.06) | 0.95 (0.90–1.01) | 1.01 (0.97–1.05) | |
| Alpha-blockers | 1.20 (0.85–1.69) | 0.84 (0.63–1.14) | 1.04 (0.88–1.23) | |
| Angiotensin receptor blockers | 0.98 (0.93–1.02) | 1.06 (0.98–1.15) | 0.99 (0.94–1.04) | |
| Antihypertensive | 0.64 (0.53–0.76) | 0.69 (0.51–0.94) | ||
| Beta-blockers | 1.17 (1.11–1.24) | 1.03 (0.96–1.10) | 1.06 (1.01–1.12) | |
| Ca channel blockers | 0.97 (0.94–0.99) | 0.98 (0.94–1.03) | 0.97 (0.94–1.00) | |
| Diuretics | 0.97 (0.94–1.00) | 1.02 (0.97–1.09) | 1.02 (0.97–1.06) | |
| Glucose lowering | ||||
| Glucose-lowering drugs | 0.92 (0.89–0.95) | 0.92 (0.87–0.97) | 0.94 (0.90–0.98) | |
| Sodium-glucose linked transporter (SGLT) -2 inhibitors | 0.83 (0.71–0.96) | 0.86 (0.79–0.94) | 0.74 (0.67–0.81) | 0.85 (0.79–0.92) |
| Lipid lowering | ||||
| Fibrates | 0.88 (0.73–1.05) | 0.84 (0.74–0.96) | 0.86 (0.56–1.32) | 0.97 (0.074–1.26) |
| Homocysteine lowering | 0.90 (0.82–0.99) | 1.02 (0.95–1.10) | 1.01 (0.96–1.06) | |
| Statins | 0.74 (0.61–0.89) | 0.80 (0.71–0.90) | 0.85 (0.74–0.98) | 0.96 (0.88–1.04) |
| Polypills | ||||
| Polypills | 1.38 (0.91–2.10) | 1.26 (0.67–2.38) | ||
Risk of bias of included reviews
Assessment of Multiple SysTemAtic Reviews 2 rating is summarised in Appendix 3. A majority of the reviews (81 out of 95) addressed research questions and inclusion criteria using the population, intervention, comparison and outcomes (PICO) components. Just under half (42 out of 95) mentioned that the review methods were predetermined and justified any significant deviations from the protocol. Most authors (85 out of 95) clarified the chosen study designs for inclusion in their reviews.
Just over half of the authors employed a comprehensive literature search strategy (55 out of 95), conducted study selection in duplicate (67 out of 95) and performed data extraction in duplicate (68 out of 95). Only about half (51 out of 95) provided a list of excluded studies and justified their exclusions, while most (75 out of 95) described the included studies in adequate detail.
A limited number of authors (47 out of 95) utilised a satisfactory technique to assess the risk of bias (RoB) in the individual studies included in the review, and even fewer (37 out of 95) reported on the funding sources for the included studies. Only 45 authors applied appropriate methods for statistically combining results, while 42 assessed the potential impact of RoB in individual studies on the meta-analysis or other evidence synthesis results and another 42 accounted for RoB in primary studies when interpreting or discussing the review findings.
The majority of authors (84 out of 95) offered a satisfactory explanation and discussion of any observed heterogeneity in their review results. About 58 authors conducted an adequate investigation of publication bias (small study bias) and discussed its probable impact on the review results. Lastly, 75 authors reported any potential sources of conflict of interest, including any funding received for conducting their reviews.
Antiplatelets
Antiplatelets were examined in 21 systematic reviews published between 2000 and 2020. When reported, the last searches ranged from September 1997 to December 2018. Drugs examined in the reviews included antiplatelets (1 review included) and aspirin (20 reviews included). The AMSTAR ratings ranged from a score of 1 to 12 out of a possible 16. Only of 10 meta-analyses reported a beneficial effect of antiplatelets on CVD mortality. Two of 12 meta-analyses reported a beneficial effect of antiplatelets on all-cause mortality. Eight of 17 systematic reviews reported a beneficial effect of antiplatelets on major CVD events (RR ranging from 0.85 to 0.97, 95% CI ranging from 0.78 to 0.99). Five of eight systematic reviews reported a beneficial effect of antiplatelets on CHD events (RR ranging from 0.70 to 0.86, 95% CI ranging from 0.60 to 0.95).
Blood pressure lowering
Blood pressure-lowering medications were examined in three systematic reviews. When reported, the last searches ranged from January 1998 to November 2015; and these reviews were published between 2001 and 2015. The drugs examined in the reviews included angiotensin-converting enzyme (ACE) inhibitors (one review included), antihypertensives (one review included) and diuretics (one review included). The AMSTAR ratings ranged from a score of 2 to 14 out of 16. None of the reviews showed a benefit of BP lowering in primary prevention of cardiovascular events or mortality, apart from calcium channel blockers which demonstrated a reduction in major cardiovascular events (RR = 0.97, 95% CI 0.94 to 0.99).
Dietary interventions
Dietary interventions were examined in 14 systematic reviews. When reported, the last searches ranged from July 1993 to August 2019; and these reviews were published between 1997 and 2020. Interventions included different types of dietary interventions such as reduced salt (two reviews included), dietary intervention (two reviews included), nuts (one review), folic acid (one review included), green tea (one review included), Mediterranean diet (one review included), low-glycaemic-index diets (one review included), reduced fat (one review included), whole grain cereals (one review included), fibre (one review included) and garlic (one review included). The AMSTAR ratings ranged from a score of 5 to 15 of 16. The dietary interventions showed little or no significant beneficial effect of major cardiovascular events, CHD events and mortality.
Glucose-lowering medications
Glucose-lowering medications were examined in the three systematic reviews. When reported, the last searches ranged from May 2019 and January 2020; and these reviews were published between 2019 and 2020. The drugs examined in the reviews included sodium-glucose linked transporter (SGLT)-2 inhibitors (two reviews included) and glucose-lowering drugs (one review included). The AMSTAR ratings ranged from a score of 11 to 13 of 16. All glucose-lowering medications showed a significant beneficial effect on major cardiovascular events (RR = 0.92, 95% CI 0.89 to 0.95), CHD events (RR = 0.92, 95% CI 0.87 to 0.97) and mortality (RR = 0.94, 95% CI 0.90 to 0.98).
Health promotion
Health promotion interventions were examined in four systematic reviews. When reported, the last searches ranged from June 2006 to January 2014; and these reviews were published between 2010 and 2015. There were various health promotion interventions such as multifactorial interventions (two reviews included), telehealth (one review included) and digital health (one review included). The AMSTAR ratings ranged from a score of 8 to 11 of 16. The health promotion interventions showed little or no significant beneficial effect of major cardiovascular events, CHD events and mortality.
Lipid lowering
Lipid-lowering medications were examined in 25 systematic reviews. When reported, the last searches ranged from June 1996 to August 2020; and these reviews were published between 1999 and 2020. The drugs examined in the reviews included statins (20 reviews included), fibrates (4 reviews included) and homocysteine lowering (1 review included). The AMSTAR ratings ranged from a score of 1 to 16 of 16. Three of nine systematic reviews reported a beneficial effect of lipid lowering on CVD mortality (RR ranging from 0.71 to 0.89, 95% CI ranging from 0.56 to 0.98). Five of 18 systematic reviews reported a beneficial effect of lipid lowering on all-cause mortality (RR ranging from 0.66 to 0.93, 95% CI ranging from 0.49 to 0.99). Fifteen of 18 systematic reviews reported a beneficial effect of lipid lowering on major CVD events (RR ranging from 0.59 to 0.90, 95% CI ranging from 0.48 to 0.99). Eight of 10 systematic reviews reported a beneficial effect of lipid lowering on CHD events (RR ranging from 0.55 to 0.84, 95% CI ranging from 0.42 to 0.96).
Multicomponent intervention
Multicomponent interventions were examined in one systematic review. The last searches were conducted in June 2014 and published in 2015. Interventions included different types of nutrition supplements such as diet and physical activity advice (one review included). The AMSTAR rating was a score of 15 of 16. The combination of dietary intervention and physical activities showed little or no significant beneficial effect of major cardiovascular events.
Nutritional supplements
Nutrition supplements were examined in 19 systematic reviews. When reported, the last searches ranged from February 2002 to September 2019; and these reviews were published between 2004 and 2020. Interventions included different types of nutrition supplements such as multivitamin (nine reviews included), vitamin D (three reviews included), vitamin B3 (one review included), omega 3 (one review included,), omega 6 (one review included), homocysteine-folic (one review included), tomato and lycopene supplement (one review included), selenium (one review included), coenzyme q10 (one review included) and niacin (one review included). The AMSTAR ratings ranged from a score of 3 to 16 of 16.
Most of the nutritional supplements showed little or no significant beneficial effect of major cardiovascular events, CHD events and mortality.
Physical activity
Physical activity was examined in five systematic reviews. When reported, the last searches ranged from December 2013 to October 2016; and these reviews were published between 2014 and 2017. Interventions included different types of physical activity interventions such as workplace physical activity (workplace intervention that aims to boost health literacy and encourage the adoption of healthy lifestyles, with a focus on physical activity as the primary outcome) (one review included), yoga (one review included), meditation (one review included), tai chi (one review included) and physical activity (any form of health education intervention that aims to boost health literacy and encourage the adoption of healthy lifestyles, with a focus on physical activity as the primary outcome at any location) (one review included). The AMSTAR ratings ranged from a score of 14 to 16 of 16. None of the included systematic reviews reported effects of physical activities on major cardiovascular events, CHD events, CVD associated mortality or all-cause mortality.
Polypills
Polypills were examined in the two systematic reviews. When reported, the last searches ranged from December 2010 to July 2013; and these reviews were published between 2012 and 2014. There were two reviews (two reviews included). The AMSTAR ratings ranged from a score of 4 to 15 of 16. Polypills showed little or no significant beneficial effect of major cardiovascular events, CHD events and all-cause mortality.
Discussion
Main findings
This umbrella review provides information about diverse interventions for primary prevention of CVD. Our work constitutes the first comprehensive and systematic summary of diverse non-pharmacological and pharmacological interventions based on the umbrella review methodology. We included 95 systematic reviews, 41 were on various non-pharmacological interventions and 54 concerned various pharmacological interventions. Most of the reviews examined lipid-lowering interventions (n = 25) followed by antiplatelet medications (n = 21). Other reviews included nutritional supplements (n = 19), dietary interventions (n = 13) physical activity (n = 5), health promotion interventions (n = 4), BP-lowering medications (n = 3), blood glucose-lowering medications (n = 3), polypills (n = 2) and multicomponent intervention (n = 1). Out of 95 reviews analysed, most addressed research questions and inclusion criteria using PICO components and clarified their chosen study designs. Approximately half employed comprehensive literature search strategies, conducted study selection and performed data extraction in duplicate. Only a limited number of authors assessed the RoB in individual studies, applied appropriate methods for combining results and accounted for bias when interpreting findings. The majority provided explanations for heterogeneity, investigated publication bias and reported potential conflicts of interest.
We found potential promising effects, indicated by statistically significant pooled treatment effects in more than one systematic review reported for SGLT-2 inhibitors, vitamin D, dietary interventions, reduced salt, fibrates and multifactorial interventions. In addition, several systematic reviews provided evidence of the potential lack of effectiveness across more than one systematic review, such as BP-lowering medications and multivitamins.
The most comprehensive and high-quality systematic reviews of aspirin reported a 10% reduction in major cardiovascular events and a 14% reduction in CHD events; SGLT-2 inhibitors reported a 17% reduction in major cardiovascular events, a 14% reduction in CHD, a 26% in CVD associated mortality and a 15% reduction in all-cause mortality; statins reported a 26% reduction in major cardiovascular events, a 20% reduction in CHD events and a 15% in CVD associated mortality; and niacin reported an 34% reduction in major cardiovascular events and a 25% reduction in CHD events.
Results of our overview of systematic reviews can be also compared with those of previous overviews of systematic reviews of both pharmacological5 and non-pharmacological6 interventions for preventing CVD. Karmali and colleagues5 conducted an overview of systematic reviews to compare the efficacy and safety of aspirin, BP-lowering therapy, statins and tobacco cessation drugs for fatal and nonfatal atherosclerotic cardiovascular disease (ASCVD) outcomes in primary ASCVD prevention. There were 35 systematic reviews of RCTs found in a total of 1967 reports, including 15 reviews of aspirin, 4 reviews of BP-lowering medication, 12 reviews of statins and 4 reviews of cigarette cessation drugs. According to the review, high-quality data support the use of aspirin, BP-lowering treatment and statins for primary ASCVD prevention, as well as tobacco cessation medicines for smoking cessation.
Martin Ruiz and colleague6 conducted an umbrella review to determine the effectiveness of non-pharmacological interventions for prevention of CVD events and mortality in healthy adults or those at high risk of CVD. There were 24 reviews in total, with 13 of them reporting results of interest. Vitamin D supplements, increased omega 3 fatty acid consumption, Qigong and counselling or education to modify more than one cardiovascular risk factor were all found to reduce risk in a statistically meaningful way. The authors concluded that these four non-pharmacological interventions have been shown to provide a statistically significant reduction in risk of CVD events or overall mortality.
Strengths and limitations
Our study has numerous strengths. It presents a thorough, complete evaluation of the data from all published meta-analyses regarding pharmacological and non-pharmacological therapies for CVD primary prevention. First, we concentrated this evidence synthesis on systematic reviews and meta-analyses of RCTs, as RCTs provide the highest-quality evidence for determining the benefits of healthcare interventions. Second, we employed a comprehensive, transparent search approach to locate papers and a predefined procedure to guide our evidence synthesis, documenting any protocol deviations. Third, we did all-title screening, data extraction and quality evaluations in duplicate to reduce the possibility of bias during compilation of this summary. Fourth, we employed a validated instrument (the AMSTAR 2 tool) to evaluate the methodological quality of the included systematic reviews, and we used this evaluation to inform our conclusions regarding the effects of pharmacologic therapies. This methodical procedure, which includes the evaluation of study quality using standardised instruments, could serve as a model for the expedited creation of reliable guidelines.
Our umbrella review also has some limitations. We did not retrieve data from primary RCTs; thus we had to rely on the information provided by the authors of the systematic reviews we retrieved. Selection criteria, search methodologies and definitions of main prevention frequently differed across reviews, and authors of included reviews frequently employed different criteria to define primary prevention, resulting in varying numbers of trials for systematic reviews of the same intervention. In addition, primary studies that were not included in any published meta-analyses may have been omitted, and new studies that were published after the publication of each meta-analysis may have altered the results. Finally, we selected the most recent meta-analysis for each intervention that included the greatest number of primary studies. Therefore, it is possible that the chosen meta-analysis may not be that of the highest quality.
Implications for future research and policy
Because the RCTs included in each systematic review may have been undertaken in very diverse populations, the observed results may not be generalisable. More research is needed to determine whether the effect of treatment varies depending on population characteristics. The findings of this review should be interpreted with caution because the majority of studies investigating non-pharmacological interventions for primary prevention compare them to usual care, which may include recommended pharmacological treatment in higher-risk patients (e.g. statins and/or antihypertensive medications, etc.). This means that the combined effect of non-pharmacological interventions and standard pharmacological practise is being measured in these trials, rather than the effect of these interventions as an alternative to pharmacology. More research is also recommended to evaluate different intervention combinations. Identifying the most effective intervention, on the other hand, continues to be a challenge for researchers and policy-makers. There is a need for an up-to-date comprehensive evidence synthesis of all interventions to inform the NHS and UK Health Security Agency’s rational choice of a minimum set of strategies for primary prevention of CVD in order to avoid targeting relatively fewer effective interventions.
While numerous pairwise systematic reviews and meta-analyses have investigated the effectiveness of various interventions for primary prevention of CVD, no systematic review has yet comprehensively synthesised all available evidence to understand the comparative effectiveness of different drug, lifestyle and structural interventions in order to support evidence-based recommendations. Another issue is that most trials use a ‘no intervention’ control group as the comparator, which limits the usefulness of pairwise analyses in informing practical decisions about the most effective interventions. Network meta-analysis methods are designed to address this question and provide more valuable insights for policy-makers, health service commissioners and care providers when choosing between multiple intervention alternatives. Therefore, we recommend conducting an innovative network meta-analysis to better inform decision-making in primary prevention of CVD.
Patient and public involvement
Drawing on INVOLVE guidance and support for best practice, we worked closely with three dedicated patient and public involvement advisors, we welcomed guidance and support from our advisors at the preparatory phase of the project.
Conclusion
In conclusion, this study examined various interventions for the primary prevention of CVD. Antiplatelet medications showed beneficial effects in some reviews on CVD mortality, all-cause mortality and major cardiovascular events. BP-lowering medications had no significant effect on cardiovascular events or mortality, except for calcium channel blockers, which reduced major cardiovascular events. Dietary interventions had little to no significant impact on major cardiovascular events, CHD events and mortality.
Glucose-lowering medications showed a significant beneficial effect on major cardiovascular events, CHD events and mortality. Health promotion interventions had little to no significant impact on major cardiovascular events, CHD events and mortality. Lipid-lowering medications demonstrated beneficial effects on CVD mortality, all-cause mortality, major cardiovascular events and CHD events in various reviews.
Multicomponent interventions, which combined dietary intervention and physical activities, showed little to no significant impact on major cardiovascular events. Most nutritional supplements had little or no significant effect on major cardiovascular events, CHD events and mortality. Physical activity interventions did not report significant effects on major cardiovascular events, CHD events, CVD-associated mortality or all-cause mortality. Polypills had little or no significant impact on major cardiovascular events, CHD events and all-cause mortality.
Additional information
Contributions of authors
Olalekan A Uthman (https://orcid.org/0000-0002-8567-3081) (Professor, Evidence Synthesis) contributed to the protocol, study selection, data extraction, validity assessments and synthesis of the included studies. He developed the classifiers and undertook the analyses. He also contributed to the interpretation of the results and the writing of the report and had overall responsibility for the project.
Lena Al-Khudairy (https://orcid.org/0000-0003-0638-583X) (Associate Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report.
Chidozie Nduka (https://orcid.org/0000-0001-7031-5444) (Senior Research Fellow, Evidence Synthesis) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. He also contributed to the interpretation of the results and the writing of the report.
Rachel Court (https://orcid.org/0000-0002-4567-2586) (Information Specialist) contributed to the protocol development, developed the search strategies, and wrote the sections of the report relating to the literature searches. She also contributed to the protocol and interpretation of the results and commented on drafts of the report.
Jodie Enderby (https://orcid.org/0000-0002-1446-7512) (Research Associate) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report.
Seun Anjorin (https://orcid.org/0000-0003-0187-6410) (Research Associate) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. He conducted a range of searches to locate studies. He also contributed to the interpretation of the results and the writing of the report.
Hema Mistry (https://orcid.org/0000-0002-5023-1160) (Associate Professor, Health Economics) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report.
GJ Melendez-Torres (https://orcid.org/0000-0002-9823-4790) (Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. He also contributed to the interpretation of the results and the writing of the report.
Sian Taylor-Phillips (https://orcid.org/0000-0002-1841-4346) (Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report.
Aileen Clarke (https://orcid.org/0000-0001-8299-3146) (Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/GJTR5006.
Primary conflicts of interest: Aileen Clarke and Lena Al-Khudairy were supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration (ARC) West Midlands (grant number NIHR200165). Sian Taylor-Phillips was supported by a Career Development Fellowship (reference number NIHR-CDF-2016-09-018). Hema Mistry is a member of the HTA General Committee. Aileen Clarke was a member of the SRP – Cochrane Programme Grant Funding Meeting.
Data-sharing statement
No new data have been created in the preparation of this article and therefore there is nothing available for access and further sharing. All queries should be submitted to the corresponding author.
Ethics statement
This work is a systematic review of accessing, processing and analysing data that have already been published and is available to the public. As a result, no patient data were processed; and patient consent and/or registration via human research ethics committees were, therefore, not relevant.
Information governance statement
This project did not involve the handling of personal information.
Study registration details
This study is registered as PROSPERO CRD42019123940.
Funding
This article presents independent research funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme as award number 17/148/05. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
This article reports on one component of the research award Interventions for primary prevention of cardiovascular disease: umbrella review of systematic reviews. For more information about this research please view the award page (www.fundingawards.nihr.ac.uk/award/17/148/05)
About this article
The contractual start date for this research was in February 2019. This article began editorial review in August 2022 and was accepted for publication in July 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the HTA programme or the Department of Health and Social Care.
This article was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Copyright
Copyright © 2024 Uthman et al. This work was produced by Uthman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
List of abbreviations
- ASCVD
- atherosclerotic cardiovascular disease
- CHD
- coronary heart disease
- CVD
- cardiovascular disease
- MeSH
- medical subject heading
- MI
- myocardial infarction
- RoB
- risk of bias
References
- British Heart Foundation . UK Heart Disease Facts and Figures 2022. www.bhf.org.uk/what-we-do/news-from-the-bhf/contact-the-press-office/facts-and-figures#:~:text=Heart%20and%20circulatory%20diseases%20cause,men%20and%203.6%20million%20women (accessed 17 June 2022).
- Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020;395:795-808. https://doi.org/10.1016/s0140-6736(19)32008-2.
- Read SH, Wild SH. Prevention of premature cardiovascular death worldwide. Lancet 2020;395:758-60. https://doi.org/10.1016/s0140-6736(19)32034-3.
- Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321-9. https://doi.org/10.1056/NEJMoa1012848.
- Karmali KN, Lloyd-Jones DM, Berendsen MA, Goff DC, Sanghavi DM, Brown NC, et al. Drugs for primary prevention of atherosclerotic cardiovascular disease: an overview of systematic reviews. JAMA Cardiol 2016;1:341-9. https://doi.org/10.1001/jamacardio.2016.0218.
- Martin Ruiz E, de Labry AO, Epstein D. Primary prevention of cardiovascular disease: an umbrella review. An Sist Sanit Navar 2018;41:355-69. https://doi.org/10.23938/assn.0316.
- Stewart J, Addy K, Campbell S, Wilkinson P. Primary prevention of cardiovascular disease: updated review of contemporary guidance and literature. JRSM Cardiovasc Dis 2020;9. https://doi.org/10.1177/2048004020949326.
- Cho L, Davis M, Elgendy I, Epps K, Lindley KJ, Mehta PK, et al. ACC CVD Womens Committee Members . Summary of updated recommendations for primary prevention of cardiovascular disease in women: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:2602-18. https://doi.org/10.1016/j.jacc.2020.03.060.
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2019;74:1376-414. https://doi.org/10.1016/j.jacc.2019.03.009.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10. https://doi.org/10.1186/s13643-021-01626-4.
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358. https://doi.org/10.1136/bmj.j4008.
- Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2018;7. https://doi.org/10.1002/14651858.CD003177.pub3.
- Adler AJ, Taylor F, Martin N, Gottlieb S, Taylor RS, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;2014. https://doi.org/10.1002/14651858.CD009217.pub3.
- Brunner E, White I, Thorogood M, Bristow A, Curle D, Marmot M. Can dietary interventions change diet and cardiovascular risk factors? A meta-analysis of randomized controlled trials. Am J Public Health 1997;87:1415-22.
- Clar C, Al-Khudairy L, Loveman E, Kelly SA, Hartley L, Flowers N, et al. Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;7. https://doi.org/10.1002/14651858.CD004467.pub3.
- Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD009874.pub2.
- Hartley L, May MD, Loveman E, Colquitt JL, Rees K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2016;2016. https://doi.org/10.1002/14651858.CD011472.pub2.
- Hooper L, Summerbell CD, Higgins JP, Thompson RL, Capps NE, Smith GD, et al. Dietary fat intake and prevention of cardiovascular disease: systematic review. BMJ 2001;322:757-63.
- Kelly SA, Hartley L, Loveman E, Colquitt JL, Jones HM, Al-Khudairy L, et al. Whole grain cereals for the primary or secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;8. https://doi.org/10.1002/14651858.CD005051.pub3.
- Martin N, Germano R, Hartley L, Adler AJ, Rees K. Nut consumption for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2015;2015. https://doi.org/10.1002/14651858.CD011583.pub2.
- Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M, et al. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;2013. https://doi.org/10.1002/14651858.CD009825.pub2.
- Stabler SN, Tejani AM, Huynh F, Fowkes C. Garlic for the prevention of cardiovascular morbidity and mortality in hypertensive patients. Cochrane Database Syst Rev 2012;2012. https://doi.org/10.1002/14651858.CD007653.pub2.
- Taylor FC, Huffman M, Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. JAMA 2013;310:2451-2. https://doi.org/10.1001/jama.2013.281348.
- Yang HT, Lee M, Hong KS, Ovbiagele B, Saver JL. Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta-analysis of randomized controlled trials. Eur J Intern Med 2012;23:745-54. https://doi.org/10.1016/j.ejim.2012.07.004.
- Angermayr L, Melchart D, Linde K. Multifactorial lifestyle interventions in the primary and secondary prevention of cardiovascular disease and type 2 diabetes mellitus: a systematic review of randomized controlled trials. Ann Behav Med 2010;40:49-64. https://doi.org/10.1007/s12160-010-9206-4.
- Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev 2011. https://doi.org/10.1002/14651858.CD001561.pub3.
- Merriel SWD, Andrews V, Salisbury C. Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis. Prev Med 2014;64:88-95. https://doi.org/10.1016/j.ypmed.2014.04.001.
- Widmer RJ, Collins NM, Collins CS, West CP, Lerman LO, Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc 2015;90:469-80. https://doi.org/10.1016/j.mayocp.2014.12.026.
- Uthman OA, Hartley L, Rees K, Taylor F, Ebrahim S, Clarke A. Multiple risk factor interventions for primary prevention of cardiovascular disease in low- and middle-income countries. Cochrane Database Syst Rev 2015;2015. https://doi.org/10.1002/14651858.CD011163.pub2.
- Al-Khudairy L, Hartley L, Clar C, Flowers N, Hooper L, Rees K. Omega 6 fatty acids for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2015;2015. https://doi.org/10.1002/14651858.CD011094.pub2.
- Al-Khudairy L, Flowers N, Wheelhouse R, Ghannam O, Hartley L, Stranges S, et al. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;3. https://doi.org/10.1002/14651858.CD011114.pub2.
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;2012. https://doi.org/10.1002/14651858.CD007176.pub2.
- Cheng HM, Koutsidis G, Lodge JK, Ashor A, Siervo M, Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis 2017;257:100-8. https://doi.org/10.1016/j.atherosclerosis.2017.01.009.
- Flowers N, Hartley L, Todkill D, Stranges S, Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;2014. https://doi.org/10.1002/14651858.CD010405.pub2.
- Fortmann SP, Burda BU, Senger CA, Lin J, Beil T, O’Connor E, et al. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer (structured abstract). Ann Intern Med 2013.
- Hartley L, Lee MS, Kwong JS, Flowers N, Todkill D, Ernst E, et al. Qigong for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2015;2015. https://doi.org/10.1002/14651858.CD010390.pub2.
- Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev 2004;2004. https://doi.org/10.1002/14651858.CD003177.pub2.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013;61:440-6. https://doi.org/10.1016/j.jacc.2012.10.030.
- Mahmoud AN, Gad MM, Elgendy AY, Elgendy IY, Bavry AA. Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur Heart J 2019;40:607-17. https://doi.org/10.1093/eurheartj/ehy813.
- Lombardi M, Chiabrando JG, Vescovo GM, Bressi E, Del Buono MG, Carbone S, et al. Impact of different doses of omega-3 fatty acids on cardiovascular outcomes: a pairwise and network meta-analysis. Curr Atheroscler Rep 2020;22. https://doi.org/10.1007/s11883-020-00865-5.
- Cabiddu MF, Russi A, Appolloni L, Mengato D, Chiumente M. Omega-3 for the prevention of cardiovascular diseases: meta-analysis and trial-sequential analysis. Eur J Hosp Pharm 2022;29:134-8. https://doi.org/10.1136/ejhpharm-2020-002207.
- Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, et al. Korean Meta-Analysis Study Group . Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ 2013;346. https://doi.org/10.1136/bmj.f10.
- Pham DQ, Plakogiannis R. Vitamin E supplementation in cardiovascular disease and cancer prevention: part 1. Ann Pharmacother 2005;39:1870-8. https://doi.org/10.1345/aph.1G211.
- Qin X, Huo Y, Xie D, Hou F, Xu X, Wang X. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin Nutr 2013;32:722-7. https://doi.org/10.1016/j.clnu.2012.12.009.
- Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;2013. https://doi.org/10.1002/14651858.CD009671.pub2.
- Schandelmaier S, Briel M, Saccilotto R, Olu KK, Arpagaus A, Hemkens LG, et al. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst Rev 2017;6. https://doi.org/10.1002/14651858.CD009744.pub2.
- Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 2010;152:315-23. https://doi.org/10.7326/0003-4819-152-5-201003020-00010.
- Yang L, Ling W, Du Z, Chen Y, Li D, Deng S, et al. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 2017;8:684-93. https://doi.org/10.3945/an.116.014852.
- Ramôa Castro A, Oliveira NL, Ribeiro F, Oliveira J. Impact of educational interventions on primary prevention of cardiovascular disease: a systematic review with a focus on physical activity. Eur J Gen Pract 2017;23:59-68. https://doi.org/10.1080/13814788.2017.1284791.
- Hartley L, Dyakova M, Holmes J, Clarke A, Lee MS, Ernst E, et al. Yoga for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;2014. https://doi.org/10.1002/14651858.CD010072.pub2.
- Hartley L, Flowers N, Lee MS, Ernst E, Rees K. Tai chi for primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD010366.pub2.
- Reed JL, Prince SA, Elliott CG, Mullen KA, Tulloch HE, Hiremath S, et al. Impact of workplace physical activity interventions on physical activity and cardiometabolic health among working-age women: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10. https://doi.org/10.1161/CIRCOUTCOMES.116.003516.
- Antithrombotic Trialists Collaborators . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. [Erratum appears in BMJ 2002;324(7330):141]. BMJ 2002;324:71-86.
- Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol 2011;107:1796-801. https://doi.org/10.1016/j.amjcard.2011.02.325.
- Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA 2006;295:306-13. https://doi.org/10.1001/jama.295.3.306.
- Calvin AD, Aggarwal NR, Murad MH, Shi Q, Elamin MB, Geske JB, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care 2009;32:2300-6. https://doi.org/10.2337/dc09-1297.
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 2007;297:2018-24. https://doi.org/10.1001/jama.297.18.2018.
- Caldeira D, Alves M, David C, Costa J, Ferreira JJ, Pinto FJ. Aspirin in the primary prevention of cardiovascular disease on diabetic patients: systematic review and meta-analysis. Prim Care Diabetes 2020;14:213-21. https://doi.org/10.1016/j.pcd.2019.11.004.
- De Berardis G, Sacco M, Strippoli GF, Pellegrini F, Graziano G, Tognoni G, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009;339. https://doi.org/10.1136/bmj.b4531.
- Desai D, Ahmed HM, Michos ED. Preventing cardiovascular disease in patients with diabetes: use of aspirin for primary prevention. Curr Cardiol Rep 2015;17. https://doi.org/10.1007/s11886-015-0566-z.
- Eidelman RS, Hebert PR, Weisman SM, Hennekens CH. An update on aspirin in the primary prevention of cardiovascular disease. Arch Intern Med 2003;163:2006-10. https://doi.org/10.1001/archinte.163.17.2006.
- Gelbenegger G, Postula M, Pecen L, Halvorsen S, Lesiak M, Schoergenhofer C, et al. Aspirin for primary prevention of cardiovascular disease: a meta-analysis with a particular focus on subgroups. BMC Med 2019;17. https://doi.org/10.1186/s12916-019-1428-0.
- Masson G, Lobo M, Masson W, Molinero G. Aspirin in primary prevention. Meta-analysis stratified by baseline cardiovascular risk. Arch Cardiol Mex 2020;90:293-9. https://doi.org/10.24875/acm.20000267.
- Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA. Aspirin for the primary prevention of stroke and other major vascular events: meta-analysis and hypotheses. Arch Neurol 2000;57:326-32.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. [Summary for patients in Ann Intern Med. 2002 Jan 15;136(2):I55;PMID: 11928737]. Ann Intern Med 2002;136:161-72.
- Nudy M, Cooper J, Ghahramani M, Ruzieh M, Mandrola J, Foy AJ. Aspirin for primary atherosclerotic cardiovascular disease prevention as baseline risk increases: a meta-regression analysis. Am J Med 2020;133:1056-64. https://doi.org/10.1016/j.amjmed.2020.04.028.
- Raju N, Sobieraj-Teague M, Hirsh J, O’Donnell M, Eikelboom J. Effect of aspirin on mortality in the primary prevention of cardiovascular disease. Am J Med 2011;124:621-9. https://doi.org/10.1016/j.amjmed.2011.01.018.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001;85:265-71.
- Stavrakis S, Stoner JA, Azar M, Wayangankar S, Thadani U. Low-dose aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Am J Med Sci 2011;341:1-9. https://doi.org/10.1097/MAJ.0b013e3181f1fba8.
- Sutcliffe P, Connock M, Gurung T, Freeman K, Johnson S, Ngianga-Bakwin K, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0081970.
- Xie M, Shan Z, Zhang Y, Chen S, Yang W, Bao W, et al. Aspirin for primary prevention of cardiovascular events: meta-analysis of randomized controlled trials and subgroup analysis by sex and diabetes status. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0090286.
- Younis N, Williams S, Ammori B, Soran H. Role of aspirin in the primary prevention of cardiovascular disease in diabetes mellitus: a meta-analysis. Expert Opin Pharmacother 2010;11:1459-66. https://doi.org/10.1517/14656561003792538.
- Zhang C, Sun A, Zhang P, Wu C, Zhang S, Fu M, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010;87:211-8. https://doi.org/10.1016/j.diabres.2009.09.029.
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2015;387:957-67. https://doi.org/10.1016/s0140-6736(15)01225-8.
- Fretheim A, Odgaard-Jensen J, Brørs O, Madsen S, Njølstad I, Norheim OF, et al. Comparative effectiveness of antihypertensive medication for primary prevention of cardiovascular disease: systematic review and multiple treatments meta-analysis. BMC Med 2012;10. https://doi.org/10.1186/1741-7015-10-33.
- Wei CY, Quek RGW, Villa G, Gandra SR, Forbes CA, Ryder S, et al. A systematic review of cardiovascular outcomes-based cost-effectiveness analyses of lipid-lowering therapies. PharmacoEconomics 2017;35:297-318. https://doi.org/10.1007/s40273-016-0464-2.
- Zou CY, Liu XK, Sang YQ, Wang B, Liang J. Effects of SGLT2 inhibitors on cardiovascular outcomes and mortality in type 2 diabetes: a meta-analysis. Medicine 2019;98. https://doi.org/10.1097/md.0000000000018245.
- Giugliano D, Longo M, Maiorino MI, Bellastella G, Chiodini P, Solerte SB, et al. Efficacy of SGLT-2 inhibitors in older adults with diabetes: systematic review with meta-analysis of cardiovascular outcome trials. Diabetes Res Clin Pract 2020;162. https://doi.org/10.1016/j.diabres.2020.108114.
- Ghosh-Swaby OR, Goodman SG, Leiter LA, Cheng A, Connelly KA, Fitchett D, et al. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: an updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol 2020;8:418-35. https://doi.org/10.1016/s2213-8587(20)30038-3.
- Allemann S, Diem P, Egger M, Christ ER, Stettler C. Fibrates in the prevention of cardiovascular disease in patients with type 2 diabetes mellitus: meta-analysis of randomised controlled trials. Curr Med Res Opin 2006;22:617-23. https://doi.org/10.1185/030079906X89865.
- Das A, Roy B, Bandyopadhyay D, Dasgupta S, Chakraborty S, Soudant C, et al. Non-statin interventions in the prevention of cardiovascular events: sex-based meta-analysis. Prog Cardiovasc Dis 2020;63:228-32. https://doi.org/10.1016/j.pcad.2020.03.012.
- Gencer B, Marston NA, Im K, Cannon CP, Sever P, Keech A, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet 2020;396:1637-43. https://doi.org/10.1016/s0140-6736(20)32332-1.
- Berger JS, Lala A, Krantz MJ, Baker GS, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients without clinical cardiovascular disease: a meta-analysis of randomized trials. Am Heart J 2011;162:115-24.e2. https://doi.org/10.1016/j.ahj.2011.04.006.
- Bukkapatnam RN, Gabler NB, Lewis WR. Statins for primary prevention of cardiovascular mortality in women: a systematic review and meta-analysis. Prev Cardiol 2010;13:84-90. https://doi.org/10.1111/j.1751-7141.2009.00059.x.
- Chang YH, Hsieh MC, Wang CY, Lin KC, Lee YJ. Reassessing the benefits of statins in the prevention of cardiovascular disease in diabetic patients – a systematic review and meta-analysis. Rev Diabet Stud 2013;10:157-70. https://doi.org/10.1900/rds.2013.10.157.
- Chen YH, Feng B, Chen ZW. Statins for primary prevention of cardiovascular and cerebrovascular events in diabetic patients without established cardiovascular diseases: a meta-analysis. Exp Clin Endocrinol Diabetes 2012;120:116-20. https://doi.org/10.1055/s-0031-1297968.
- Loomba RS, Arora R. Prevention of cardiovascular disease utilizing fibrates – a pooled meta-analysis. Am J Ther 2010;17:e182-8. https://doi.org/10.1097/MJT.0b013e3181dcf72b.
- Major RW, Cheung CK, Gray LJ, Brunskill NJ. Statins and cardiovascular primary prevention in CKD: a meta-analysis. Clin J Am Soc Nephrol 2015;10:732-9. https://doi.org/10.2215/CJN.07460714.
- Marti-Carvajal AJ, Solà I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 2017;8. https://doi.org/10.1002/14651858.CD006612.pub5.
- Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 2008;52:1769-81. https://doi.org/10.1016/j.jacc.2008.08.039.
- Mills EJ, O’Regan C, Eyawo O, Wu P, Mills F, Berwanger O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of > 40 000 patients. Eur Heart J 2011;32:1409-15. https://doi.org/10.1093/eurheartj/ehr035.
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an intervention trial evaluating rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010;121:1069-77. https://doi.org/10.1161/CIRCULATIONAHA.109.906479.
- Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomised trials. BMJ 2000;321:983-6.
- Saha SA, Kizhakepunnur LG, Bahekar A, Arora RR. The role of fibrates in the prevention of cardiovascular disease – a pooled meta-analysis of long-term randomized placebo-controlled clinical trials. Am Heart J 2007;154:943-53. https://doi.org/10.1016/j.ahj.2007.07.011.
- Saha SA, Arora RR. Fibrates in the prevention of cardiovascular disease in patients with type 2 diabetes mellitus – a pooled meta-analysis of randomized placebo-controlled clinical trials. Int J Cardiol 2010;141:157-66. https://doi.org/10.1016/j.ijcard.2008.11.211.
- Taylor F, Huffman MD, Macedo AF, Moore THM, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013. https://doi.org/10.1002/14651858.cd004816.pub5.
- Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (Cochrane review). Am J Hypertens 2011;24:843-53. https://doi.org/10.1038/ajh.2011.115.
- Teng M, Lin L, Zhao YJ, Khoo AL, Davis BR, Yong QW, et al. Statins for primary prevention of cardiovascular disease in elderly patients: systematic review and meta-analysis. Drugs Aging 2015;32:649-61. https://doi.org/10.1007/s40266-015-0290-9.
- Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:2307-13. https://doi.org/10.1001/archinte.166.21.2307.
- Tonelli M, Lloyd A, Clement F, Conly J, Husereau D, Hemmelgarn B, et al. Alberta Kidney Disease Network . Efficacy of statins for primary prevention in people at low cardiovascular risk: a meta-analysis. CMAJ 2011;183:E1189-202. https://doi.org/10.1503/cmaj.101280.
- Warshafsky S, Packard D, Marks SJ, Sachdeva N, Terashita DM, Kaufman G, et al. Efficacy of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors for prevention of stroke. J Gen Intern Med 1999;14:763-74.
- de Vries FM, Kolthof J, Postma MJ, Denig P, Hak E. Efficacy of standard and intensive statin treatment for the secondary prevention of cardiovascular and cerebrovascular events in diabetes patients: a meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0111247.
- de Vries FM, Denig P, Pouwels KB, Postma MJ, Hak E. Primary prevention of major cardiovascular and cerebrovascular events with statins in diabetic patients: a meta-analysis. Drugs 2012;72:2365-73. https://doi.org/10.2165/11638240-000000000-00000.
- Sepanlou SG, Farzadfar F, Jafari E, Danaei G. Cardiovascular disease prevention using fixed dose pharmacotherapy in Iran: updated meta-analyses and mortality estimation. Arch Iran Med 2012;15:531-7. https://doi.org/012159/AIM.004.
- de Cates AN, Farr MRB, Wright N, Jarvis MC, Rees K, Ebrahim S, et al. Fixed-dose combination therapy for the prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD009868.pub2.
- Hartley L, Mavrodaris A, Flowers N, Ernst E, Rees K. Transcendental meditation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;2017. https://doi.org/10.1002/14651858.CD010359.pub3.
Appendix 1 Search strategy
MEDLINE (Ovid)
Search date: 14 March 2019 (note: see below for update searches).
Actual databases searched: Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions(R) < 1946 to March 13, 2019>.
Search Strategy:
--------- --------- -------- ---------- ------------- ------- --------
-
1 exp Primary Prevention/ (143046)
-
2 primary prevention.ti,ab,kf. (17517)
-
3 1 or 2 (156409)
-
4 exp Cardiovascular Diseases/ or exp Stroke/ (2253958)
-
5 (cardiovascular* or coronary* or heart* or myocardial infarction* or cardiac* or stroke* or cerebrovascular accident*).ti,ab,kf. (602893)
-
6 4 or 5 (2507942)
-
7 3 and 6 (13417)
-
8 *Cardiovascular Diseases/pc or exp *Coronary Disease/pc or exp *Myocardial Infarction/pc or exp *Heart Failure/pc or exp *Heart Arrest/pc or exp *Stroke/pc (47349)
-
9 ((prevent* or (reduc* adj risk*)) and (cardiovascular* or coronary* or heart* or myocardial infarction* or cardiac* or stroke* or cerebrovascular accident*)).ti. (20124)
-
10 7 or 8 or 9 (65565)
-
11 (metaanalys* or ‘meta analys*’ or ‘meta-analys*’).mp. (171776)
-
12 (systematic* adj2 review*).mp. (161842)
-
13 11 or 12 (261344)
-
14 10 and 13 (3434)
-
15 limit 10 to (meta analysis or ‘systematic review’) (2006)
-
16 14 or 15 (3434)
-
17 limit 16 to (comment or editorial or letter) (229)
-
18 16 not 17 (3205)
Update 23 October 2019
Actual databases searched: Ovid MEDLINE All.
Search strategy:
Re-ran search above plus…
-
19 limit 18 to ed = 20190314-20191023 (203)
-
20 limit 18 to ep = 20190314-20191023 (66)
-
21 (201903* or 201904* or 201905* or 201906* or 201907* or 201908* or 201909* or 201910*).dt,ez. (826293)
-
22 18 and 21 (93)
-
23 19 or 20 or 22 (276)
Update 3 March 2021
Actual databases searched: Ovid MEDLINE All
Search strategy:
-
19 limit 18 to ed = 20191023-20210303 (459)
-
20 limit 18 to ep = 20191023-20210303 (233)
-
21 (201910* or 201911* or 201912* or 2020* or 2021*).dt,ez. (2085624)
-
22 18 and 21 (339)
-
23 19 or 20 or 22 (578)
EMBASE (Ovid)
Search date: 14 March 2019 (note: see below for update searches)
Actual databases searched: EMBASE Classic+EMBASE < 1947 to 2019 March 13>
Search Strategy:
--------- ---------- --------- ------------ ----- --------- ----------
-
1 primary prevention/ (37798)
-
2 primary prevention.ti,ab,kw. (26632)
-
3 1 or 2 (50311)
-
4 exp cardiovascular disease/ or exp cerebrovascular accident/ (4109044)
-
5 (cardiovascular* or coronary* or heart* or myocardial infarction* or cardiac* or stroke* or cerebrovascular accident*).ti,ab,kw. (2609271)
-
6 4 or 5 (4920793)
-
7 3 and 6 (24747)
-
8 *cardiovascular disease/pc or exp *coronary artery disease/pc or exp *heart infarction/pc or *heart failure/pc or exp *heart arrest/pc or exp *cerebrovascular accident/pc (36993)
-
9 ((prevent* or (reduc* adj risk*)) and (cardiovascular* or coronary* or heart* or myocardial infarction* or cardiac* or stroke* or cerebrovascular accident*)).ti. (31542)
-
10 7 or 8 or 9 (75904)
-
11 (metaanalys* or ‘meta analys*’ or ‘meta-analys*’).mp. (252681)
-
12 (systematic* adj2 review*).mp. (271759)
-
13 11 or 12 (403426)
-
14 10 and 13 (5597)
-
15 limit 10 to (meta analysis or ‘systematic review’) (3674)
-
16 14 or 15 (5597)
-
17 limit 16 to (conference abstract or conference paper or ‘conference review’ or editorial or letter) (1043)
-
18 16 not 17 (4554)
Update 23 October 2019
Actual databases searched: EMBASE Classic+EMBASE 1947 to 2019 Week 42
Search strategy:
Re-ran search above plus…
-
19 limit 18 to dd = 20190314-20191023 (30)
-
20 limit 18 to em = 201903-201910 (67)
-
21 limit 18 to dc = 20190314-20191023 (220)
-
22 19 or 20 or 21 (287)
Update 3 March 2021
Actual databases searched: EMBASE Classic+EMBASE 1947 to 2021 Week 08
Search strategy:
Re-ran search above plus…
-
19 limit 18 to dd = 20191023-20210303 (62)
-
20 limit 18 to em = 201910-202103 (497)
-
21 limit 18 to dc = 20191023-20210303 (450)
-
22 19 or 20 or 21 (593)
Cochrane Database of Systematic Reviews (Wiley)
Date Run: 14 March 2019 (note: see below for update search)
ID Search Hits
-
MeSH descriptor: [Primary Prevention] explode all trees3804
-
‘primary prevention’:ti,ab,kw3139
-
#1 or #26141
-
MeSH descriptor: [Cardiovascular Diseases] explode all trees95561
-
MeSH descriptor: [Stroke] explode all trees8034
-
(cardiovascular* or coronary* or heart* or (myocardial next infarction*) or cardiac* or stroke* or (cerebrovascular next accident*)).ti,ab,kw1318
-
#4 or #5 or #696699
-
#3 and #7934
-
[mh ^‘cardiovascular diseases’[mj]/PC]51
-
[mh ‘coronary disease’[mj]/PC]1482
-
[mh ‘myocardial infarction’[mj]/PC]653
-
[mh ‘heart failure’[mj]/PC]260
-
[mh ‘heart arrest’[mj]/PC]197
-
[mh ‘stroke’[mj]/PC]810
-
#9 or #10 or #11 or #12 or #13 or #143278
-
((prevent* or (reduc* near/2 risk)) and (cardiovascular* or coronary* or heart* or (myocardial next infarction*) or cardiac* or stroke* or (cerebrovascular next accident*))):ti5631
-
#8 or #15 or #168568
Cochrane Reviews: 149
Update 23 October 2019
Re-ran search above plus…
Limits: with Cochrane Library publication date from Mar 2019 to Oct 2019, in Cochrane Reviews9
Update 3 March 2021
Re-ran search above. Noticed an error in line 6 (a full stop rather than a colon before the field codes). As this may have affected the final number of Cochrane Reviews retrieved in the original search and the previous update, we retrieved all Cochrane Reviews found (226) and de-duplicated in EndNote against those already found in previous searches of both the Cochrane Database of Systematic Reviews, MEDLINE and EMBASE.
Cochrane Reviews: 226
DARE (CRD)
Date searched: 14 March 2019
-
MeSH DESCRIPTOR Primary Prevention EXPLODE ALL TREES914
-
(primary prevention)1551
-
#1 OR #22001
-
MeSH DESCRIPTOR Cardiovascular Diseases EXPLODE ALL TREES10675
-
MeSH DESCRIPTOR Stroke EXPLODE ALL TREES1354
-
(cardiovascular* or coronary* or heart* or myocardial infarction* or cardiac* or stroke* or cerebrovascular accident*)11754
-
#4 OR #5 OR #615077
-
#3 AND #7602
-
MeSH DESCRIPTOR Cardiovascular Diseases WITH QUALIFIER PC475
-
MeSH DESCRIPTOR Myocardial Infarction EXPLODE ALL TREES WITH QUALIFIER PC202
-
MeSH DESCRIPTOR Heart Failure EXPLODE ALL TREES WITH QUALIFIER PC38
-
MeSH DESCRIPTOR Heart Arrest EXPLODE ALL TREES WITH QUALIFIER PC90
-
MeSH DESCRIPTOR Stroke EXPLODE ALL TREES WITH QUALIFIER PC347
-
MeSH DESCRIPTOR coronary disease EXPLODE ALL TREES WITH QUALIFIER PC286
-
#9 OR #10 OR #11 OR #12 OR #13 OR #141255
-
(((prevent* or (reduc* adj2 risk*)) and (cardiovascular* or coronary* or heart* or myocardial infarction* or cardiac* or stroke* or cerebrovascular accident*))):TI733
-
#8 OR #15 OR #161910
-
(#17) IN DARE1006
Not updated since 2015.
Appendix 2 Characteristics of included studies
| Study | Intervention | Number of RCTs | Number of participants | Last searches | AMSTAR 2 rating score |
|---|---|---|---|---|---|
| Non-pharmacological | |||||
| Dietary | |||||
| Abdelhamid (2020)12 | Dietary intervention | 82 | 162,796 | August 2019 | 14 |
| Adler (2014)13 | Reduced salt | 8 | 7284 | May 2013 | 14 |
| Brunner (1997)14 | Dietary intervention | 17 | 7141 | July 1993 | 5 |
| Clar (2017)15 | Low glycaemic index diets | 21 | 2538 | July 2016 | 16 |
| Hartley (2013)16 | Green tea | 11 | 821 | October 2012 | 15 |
| Hartley (2016)17 | Fibre | 23 | 1513 | January 2015 | 16 |
| Hooper (2001)18 | Reduced fat | 27 | 30,902 | May 1999 | 8 |
| Kelly (2017)19 | Whole grain cereals | 9 | 1414 | July 2017 | 16 |
| Martin (2015)20 | Nuts | 7 | 435 | July 2015 | 16 |
| Rees (2013)21 | Mediterranean diet | 11 | 52,044 | September 2012 | 14 |
| Stabler (2012)22 | Garlic | 1 | 47 | November 2011 | 16 |
| Taylor (2013)23 | Reduced salt | 6 | 6489 | October 2008 | 13 |
| Yang (2012)24 | Folic acid | 26 | 58,804 | May 2012 | 9 |
| Health promotion | |||||
| Angermayr (2010)25 | Multifactorial | 25 | 7703 | July 2007 | 16 |
| Ebrahim (2011)26 | Multifactorial | 55 | 163,471 | June 2006 | 11 |
| Merriel (2014)27 | Telehealth | 8 | 5106 | June 2013 | 16 |
| Widmer (2015)28 | Digital health | 2 | 1055 | January 2014 | 8 |
| Uthman (2015)29 | Diet and physical activity advice | 11 | 7310 | June 2014 | 15 |
| Nutritional supplements | |||||
| Al-Khudairy (2015)30 | Omega 6 | 4 | 660 | September 2014 | 16 |
| Al-Khudairy (2017)31 | Multivitamin | 8 | 11,445 | May 2016 | 15 |
| Bjelakovic (2011)32 | Multivitamin | 50 | 94,148 | January 2011 | 16 |
| Cheng (2017)33 | Tomato and lycopene supplement | 15 | 1197 | 16 | |
| Flowers (2014)34 | Coenzyme Q10 | 6 | 102 | December 2013 | 16 |
| Fortmann (2013)35 | Multivitamin | 26 | 27,955 | January 2013 | 16 |
| Hartley (2015)36 | Multivitamin | 1 | 60 | September 2014 | 16 |
| Hooper (2004)37 | Omega 3 | 48 | 36,913 | February 2002 | 15 |
| Lavigine (2013)38 | Niacin | 11 | 9959 | December 2011 | 5 |
| Mahmoud (2019)39 | Multivitamin | 21 | 83,291 | December 2018 | 10 |
| Marco (2020)40 | Multivitamin | 14 | 125,763 | February 2019 | 13 |
| Maria Cabiddu (2020)41 | Vitamin D | 11 | 100,609 | September 2019 | 7 |
| Myung (2013)42 | Multivitamin | 50 | 294,478 | November 2012 | 8 |
| Pham (2005)43 | Multivitamin | 8 | 104,221 | July 2005 | 3 |
| Qin (2012)44 | Homocysteine-Folic | 9 | 8234 | July 2012 | 11 |
| Rees (2013)45 | Selenium | 12 | 19,715 | October 2012 | 15 |
| Schandelmaier (2017)46 | Vitamin B3 | 23 | 39,195 | August 2016 | 14 |
| Wang (2010)47 | Vitamin D | 17 | 2988 | July 2009 | 9 |
| Yang (2019)48 | Vitamin D | 13 | 127,477 | 9 | |
| Physical activity | |||||
| Castro (2016)49 | Physical activity | 15 | 6727 | October 2016 | 14 |
| Hartley (2014)50 | Tai chi | 13 | 1520 | December 2013 | 16 |
| Hartley (2014)51 | Meditation | 4 | 430 | January 2014 | 16 |
| Hartley (2014) | Yoga | 11 | 800 | December 2013 | 16 |
| Reed (2017)52 | Workplace physical activity | 20 | 4074 | October 2014 | 16 |
| Pharmacological | |||||
| Antiplatelet | |||||
| ATC (2002)53 | Antiplatelet | 287 | 212,000 | September 1997 | 4 |
| Bartolucci (2011)54 | Aspirin | 9 | 90,000 | 1 | |
| Berger (2006)55 | Aspirin | 6 | 95 | March 2005 | 7 |
| Calvin (2009)56 | Aspirin | 9 | 89,392 | November 2008 | 12 |
| Campbell (2007)57 | Aspirin | 8 | 10,067 | February 2007 | 3 |
| Daniel Caldeira (2020)58 | Aspirin | 10 | 34,058 | November 2018 | 11 |
| De Berardis (2009)59 | Aspirin | 6 | 10,117 | November 2008 | 6 |
| Desai (2015)60 | Aspirin | 8 | 121,850 | December 2014 | 3 |
| Eidelman (2003)61 | Aspirin | 5 | 55,580 | January 2003 | 2 |
| Georg (2019)62 | Aspirin | 13 | 164,225 | November 2018 | 7 |
| Gerardo (2020)63 | Aspirin | 13 | 164,225 | December 2018 | 10 |
| Hart (2000)64 | Aspirin | 5 | 52,251 | 1 | |
| Hayden (2002)65 | Aspirin | 5 | 53,035 | January 2001 | 3 |
| Matthew (2020)66 | Aspirin | 12 | 963,829 | January 2018 | 12 |
| Raju (2011)67 | Aspirin | 9 | 100,076 | May 2010 | 12 |
| Sanmuganathan (2001)68 | Aspirin | 4 | 48,540 | January 2001 | 2 |
| Stavrakis (2011)69 | Aspirin | 7 | 7384 | November 2009 | 8 |
| Sutcliffe (2013)70 | Aspirin | 27 | 102,594 | September 2012 | 9 |
| Xie (2014)71 | Aspirin | 14 | 107,686 | December 2012 | 11 |
| Younis (2010)72 | Aspirin | 6 | 7374 | 5 | |
| Zhang (2009)73 | Aspirin | 7 | 11,618 | June 2009 | 5 |
| BP lowering | |||||
| Ettehad (2015)74 | ACE inhibitors | 124 | 613,815 | November 2015 | 14 |
| Fretheim (2012)75 | Diuretics | 25 | 10,881 | February 2011 | 9 |
| Wei (2001)76 | Antihypertensive | 4 | 10,400 | January 1998 | 2 |
| Glucose lowering | |||||
| Cai-Yan (2019)77 | SGLT-2 inhibitors | 41 | 61,076 | May 2019 | 11 |
| Dario (2020)78 | SGLT-2 inhibitors | 5 | 34,323 | January 2020 | 11 |
| Olivia (2020)79 | Glucose-lowering drugs | 30 | 225,305 | November 2019 | 13 |
| Lipid lowering | |||||
| Allemann (2006)80 | Fibrates | 8 | 12,249 | November 05 | 9 |
| Avash (2020)81 | Statins | 7 | 122,164 | November 2019 | 10 |
| Baris (2020)82 | Statins | 6 | 244,090 | August 2020 | 12 |
| Berger (2011)83 | Statins | 9 | 102,621 | January 2005 | 7 |
| Bukkapatnam (2009)84 | Statins | 6 | 21,963 | January 2009 | 6 |
| Chang (2013)85 | Statins | 22 | 137,441 | 7 | |
| Chen (2012)86 | Statins | 7 | 12,711 | January 2011 | 6 |
| Loomba (2010)87 | Fibrates | 10 | 125,763 | 5 | |
| Major (2015)88 | Statins | 6 | 8834 | September 2013 | 10 |
| Marti-Carvajal (2017)89 | Homocysteine-lowering | 15 | 71,422 | June 2017 | 16 |
| Mills (2008)90 | Statins | 19 | 63,899 | May 2008 | 8 |
| Mills (2011)91 | Statins | 10 | 41,778 | December 2010 | 8 |
| Mora (2010)92 | Statins | 5 | 13,154 | July 2009 | 3 |
| Pignone (2000)93 | Statins | 4 | 21,087 | June 1999 | 5 |
| Saha (2007)94 | Fibrates | 10 | 36,489 | July 2006 | 5 |
| Saha (2010)95 | Fibrates | 6 | 11,590 | December 2007 | 7 |
| Taylor (2013)96 | Statins | 18 | 56,934 | January 2012 | 14 |
| Taylor (2013)97 | Statins | 18 | 56,934 | May 2013 | 1 |
| Teng (2015)98 | Statins | 8 | 25,952 | August 2014 | 14 |
| Thavendiranathan (2006)99 | Statins | 7 | 42,848 | June 2005 | 6 |
| Tonelli (2011)100 | Statins | 29 | 80,711 | January 2011 | 11 |
| Warshafsky (1999)101 | Statins | 4 | 7808 | June 1996 | 7 |
| de Vries (2014)102 | Statins | 9 | 9156 | September 2013 | 8 |
| de Vries (2012)103 | Statins | 4 | 10,187 | November 2011 | 9 |
| Polypills | |||||
| Sepanlou (2012)104 | Polypills | 11 | 64,639 | December 2010 | 4 |
| de Cates (2014)105 | Polypills | 9 | 7047 | July 2013 | 15 |
Appendix 3 Assessment of multiple systematic reviews 2 rating of including studies
| Author | Did the research questions and inclusion criteria use PICO? | Explicit statement that the review methods? | Explain their selection of the study designs? | Use a comprehensive literature search strategy? | Perform study selection in duplicate? | Perform data extraction in duplicate? | Provide a list of excluded studies – reason? | Describe the included studies in adequate detail? | Assess the RoB (RoB)? | Sources of funding | Meta-analysis – use appropriate methods? | Meta-analysis – potential impact of RoB? | Account for RoB in individual studies? | Provide explanation for heterogeneity? | Publication bias likely impact? | Potential sources of conflict of interest? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adler (2014)13 | Yes | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Al-Khudairy (2015)30 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Al-Khudairy (2017)31 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Allemann (2006)80 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | Yes | No | Yes | |
| Angermayr (2010)25 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Abdelhamid (2020)12 | Yes | Partial Yes | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ATC (2002)53 | Yes | No | Yes | No | No | No | No | Partial Yes | No | No | No | No | Yes | No | Yes | |
| Avash Das (2020)81 | Yes | Yes | No | Yes | No | No | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| Baris Gencer (2020)82 | Yes | No | Yes | Yes | Yes | Yes | Partial Yes | Partial Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Bartolucci AA (2011)54 | No | No | No | No | No | No | No | Partial Yes | No | No | No | No | Yes | No | No | |
| Berger JS (2006)55 | Yes | No | Yes | No | No | No | No | Yes | No | Yes | No | No | Yes | Yes | Yes | |
| Berger JS (2011)83 | Yes | No | Yes | No | No | No | Yes | Yes | No | No | No | No | Yes | Yes | Yes | |
| Bjelakovic (2011)32 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Brunner (1997)14 | Yes | Yes | Yes | Yes | Yes | |||||||||||
| Bukkapatnam RN (2009)84 | Yes | No | Yes | Partial Yes | No | No | No | Yes | No | Yes | No | No | Yes | Yes | No | |
| Cai-Yan (2019)77 | Yes | Partial Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No |
| Calvin (2009)56 | Yes | Partial Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | |
| Campbell (2007)57 | Yes | No | Yes | Partial Yes | No | No | No | Yes | No | No | No meta-analysis | No meta-analysis | No | No | No meta-analysis | No |
| Castro (2016)49 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Chang (2013)85 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | No | No | No | No | Yes | Yes | ||
| Chen (2012)86 | Yes | No | Yes | Partial Yes | No | No | No | Partial Yes | No | No | Yes | No | No | Yes | Yes | Yes |
| Cheng (2017)33 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Clar (2017)15 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Daniel (2020)58 | Yes | No | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | No | No | Yes | No;Yes | Yes |
| Dario (2020)78 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | No | Yes | Yes | |
| De Berardis (2009)59 | Yes | No | Yes | Partial Yes | Yes | No | No | Partial Yes | No | Yes | No | No | Yes | No | Yes | |
| de Cates (2014)105 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| de Vries (2012)103 | Yes | No | Yes | Partial Yes | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes | Yes | |
| de Vries (2014)102 | Yes | No | Yes | No | Yes | Yes | Yes | Partial Yes | No | No | No | No | Yes | Yes | Yes | |
| Desai (2015)60 | Yes | Yes | Yes | Partial Yes | No | No | No | Partial Yes | No | No | No meta-analysis | No meta-analysis | No | No | No meta-analysis | |
| Ebrahim (2011)26 | Yes | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Partial Yes | No | No | Yes | Yes | Yes | Yes | |
| Eidelman (2003)61 | No | No | Yes | No | No | No | No | Partial Yes | No | No | No | No | Yes | No | No | |
| Ettehad (2015)74 | Yes | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Flowers (2014)34 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Fortmann (2013)35 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Fretheim (2012)75 | Yes | Partial Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | |
| George (2019)62 | Yes | Yes | Yes | Yes | No | No | Partial Yes | Yes | No | No | No | No | No | Yes | No | Yes |
| Gerardo (2020)63 | Yes | No | Yes | Yes | Yes | Yes | Partial Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | |
| Hart (2000)64 | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | No | |
| Hartley (2013)16 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Hartley (2014)50 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hartley (2015)36 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hartley (2016)17 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hartley (2014)51 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hartley L (2014)106 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hayden (2002)65 | No | No | No | No | No | No | Yes | Partial Yes | No | No | No | No | Yes | No | Yes | |
| Hooper (2001)18 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No | No | Yes | |
| Hooper (2004)37 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Kelly (2017)19 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Lavigine PM (2013)38 | Yes | No | Yes | No | No | No | Yes | Partial Yes | No | No | No | No | Yes | Yes | No | |
| Loomba RS (2010)87 | Yes | No | Yes | No | No | No | No | Yes | No | No | No | No | Yes | Yes | No | |
| Mahmoud Barbarawi (2019)39 | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes | Yes | Yes | |
| Major RW (2015)88 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||||
| Marco Lombardi (2020)40 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | |
| Maria Francesca Cabiddu (2020)41 | Yes | No | No | Yes | Yes | Yes | Partial Yes | Partial Yes | Partial Yes | No | Yes | No | No | Yes | No | Yes |
| Marti-Carvajal (2017)89 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Martin N (2015)20 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Matthew Nudy (2020)66 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No |
| Merriel S (2014)27 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Mills E (2008)90 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | Yes | No | No | |
| Mills EJ (2011)91 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No | No | Yes | |
| Mora S (2010)92 | No | No | No | No | No | No | No | Yes | No | No | No | No | Yes | No | Yes | |
| Myung SK (2013)42 | Yes | No | Yes | Partial Yes | Yes | No | Partial Yes | Yes | Partial Yes | Yes | No | No | Yes | Yes | Yes | |
| Olivia R Ghosh-Swaby (2020)79 | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | |
| Pham DQ (2005)43 | No | No | Yes | No | No | No | No | Yes | No | No | No meta-analysis | No meta-analysis | No | Yes | No meta-analysis | No |
| Pignone (2000)93 | No | No | Yes | No | Yes | Yes | No | Partial Yes | No | No | No | No | Yes | No | Yes | |
| Qin X (2012)44 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||
| Raju N (2011)67 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | |
| Reed (2017)52 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Rees K (2013)45 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Rees K et al. (2013)21 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Saha SA (2007)94 | No | No | No | Partial Yes | Yes | Yes | No | Yes | No | No | No | No | Yes | Yes | No | |
| Saha SA (2010)95 | Yes | No | Yes | No | No | Yes | No | Yes | No | No | No | No | Yes | Yes | Yes | |
| Sanmuganathan (2001)68 | No | No | Yes | No | No | No | No | Partial Yes | No | No | No | No | Yes | No | No | |
| Schandelmaier (2017)46 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | |
| Sepanlou SG (2012)104 | No | No | No | No | Yes | Yes | No | Partial Yes | No | No | No | No | Yes | Yes | No | |
| Stabler SN (2012)22 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Stavrakis S (2011)69 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | Yes | No | No | |
| Sutcliffe P (2013)70 | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes | No | Yes | |
| Taylor F (2013)96 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Taylor RS (2013)23 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | |
| Taylor F (2013)97 | No | No | No | No | No | No | No | No | No | No | No meta-analysis | No meta-analysis | No | No | No meta-analysis | Yes |
| Teng M (2015)98 | Yes | Yes | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Thavendiranathan P (2006)99 | Yes | No | Yes | Partial Yes | No | Yes | No | Yes | No | No | No | No | Yes | No | Yes | |
| Tonelli (2011)100 | Yes | Yes | Yes | Yes | Yes | Yes | Partial Yes | Yes | Partial Yes | No | Yes | No | Yes | Yes | Yes | |
| Uthman OA (2015)29 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Wang L (2010)47 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes | Yes | Yes | |
| Warshafsky (1999)101 | Yes | No | Yes | No | Yes | Yes | Yes | Yes | No | No | No | No | Yes | No | No | |
| Wei (2001)76 | No | No | Yes | No | No | No | No | Partial Yes | No | No | No | No | Yes | No | No | |
| Widmer R (2015)28 | No | No | Yes | Partial Yes | Yes | No | No | Partial Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
| Xie M (2014)71 | Yes | Partial Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | |
| Yang HT (2012)24 | Yes | No | Yes | Partial Yes | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes | Yes | |
| Yang Hu (2019)48 | Yes | No | Yes | Yes | No | No | No | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Younis N (2010)72 | Yes | No | Yes | Partial Yes | No | No | No | Yes | No | No | No | No | Yes | No | Yes | |
| Zhang C (2009)73 | Yes | Partial Yes | Yes | No | No | No | No | Yes | No | No | No | No | Yes | No | Yes | |
