Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number NIHR135486. The contractual start date was in February 2019. The draft report began editorial review in December 2021 and was accepted for publication in June 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Mistry et al. This work was produced by Mistry et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Mistry et al.
Introduction
Cardiovascular disease (CVD) is a term used to describe disorders that affect the heart and circulatory system, and is a common ailment in the UK. 1–3 In the UK, in 2019/20, there were over 1.8 million inpatient episodes with a major diagnosis of circulatory system disorder and almost 480,000 people were diagnosed with coronary heart disease, also known as ischaemic heart disease. 4 In 2019, the UK’s CVD mortality rate was 255 deaths per 100,000 people, with Scotland having the highest death rate of the devolved nations at 326 deaths per 100,000 people. 4 Furthermore, Scotland has the highest mortality rate for coronary heart disease, with 134 deaths per 100,000 people compared with the UK average of 108 deaths per 100,000 people. 4
Cost–utility analyses have emerged as the dominant method for guiding health-care resource allocation decisions. 5,6 The effects of therapies are quantified in quality-adjusted life-years (QALYs), which is the product of health-related quality of life (anchored at 0 for death and 1 for perfect health) and the time spent experiencing that degree of health (in years). 7 When comparing a treatment with a less effective option, incremental cost-effectiveness ratios (ICERs) are employed, and a threshold value is used to assess whether or not a treatment is cost-effective. Varying countries have different willingness-to-pay thresholds for each QALY gained. 7 The National Institute for Health and Care Excellence (NICE) (London, UK) presently reimburses new medications in the NHS based on a cost-effectiveness threshold of £20,000–30,000 for every QALY gained. 5
Numerous systematic reviews8–43 of the clinical effectiveness of different interventions for the primary prevention of CVD have been conducted; however, we are not aware of any systematic review that has investigated the cost-effectiveness of different interventions for the primary prevention of CVD. Therefore, the aim of the study was to address this research gap. The objective of this systematic review was to review cost-effectiveness analyses conducted for or within the UK NHS, including any existing models for randomised controlled trials (RCTs) assessing the cost-effectiveness of any form of intervention aimed at adults for the primary prevention of CVD (e.g. lipid-lowering medications, blood pressure-lowering medications, antiplatelet agents, nutritional supplements, dietary interventions, health promotion programmes, physical activity interventions, and structural and policy interventions). Interventions may or may not be targeted at high-risk groups.
This publication on the systematic review of cost-effectiveness analyses in the UK of optimal strategies for the primary prevention of CVD is part of a series of publications on ‘determining optimal strategies for primary prevention of cardiovascular disease’ (NIHR Journals Library reference 17/148/05). Other publications in the series are forthcoming.
The findings from all the workstreams, including those from the systematic review of economic evaluation studies, are summarised in a synopsis paper.
Methods
Information sources and search strategy
A comprehensive systematic search of the evidence for published economic evaluations, including any economic models, was performed for the following electronic databases on 13 February 2020 (see Appendix 1):
-
MEDLINE via Ovid (1946 to 12 February 2020)
-
Embase via Ovid (1947 to week 6 2020).
The search included economic-, cost- and quality of life-related terms combined with CVD and primary prevention terms, and validated UK geographic search filters developed by NICE. 44–46 In addition, we checked weekly auto-alerts from MEDLINE and Embase until 31 December 2020 for any additional studies that could be included.
Inclusion criteria
Initial scoping searches were carried out in MEDLINE in February 2020 to assess the volume and nature of evidence relating to the cost-effectiveness of interventions for the primary prevention of CVD. The scoping searches informed the development of the final search strategies for the systematic review (see Appendix 1). Owing to the high volume of studies identified in the scoping searches, as well as the need to keep the searches applicable to studies conducted for or within the UK NHS setting, the following inclusion criteria were implemented.
Study type
-
Fully published economic evaluations (including economic models) alongside a RCT.
Population
-
Adult populations (aged ≥ 18 years).
-
Interventions may or may not be targeted at groups with moderate/high risk of CVD, for example adults with hypertension, obesity, hyperlipidaemia, type 2 diabetes or a combination of these.
Intervention
-
Any form of intervention aimed at the primary prevention of CVD, including, but not limited to, drugs (e.g. lipid-lowering medications, blood pressure-lowering medications and antiplatelet agents), diet (e.g. nutritional supplements and dietary interventions), physical activity or public health (e.g. health promotion programmes and structural and policy interventions).
Comparator
-
Another form of intervention (e.g. a minimal intervention, active intervention or concomitant intervention), placebo, usual care or no intervention control group, or wait list control.
Outcomes
-
Cost–utility studies reporting outcomes as QALYs.
Setting
-
UK-based studies only.
Exclusion criteria
Studies meeting the following exclusion criteria were excluded from the review:
-
non-English-language publications
-
abstract/conference proceedings, letters and commentaries
-
studies with quality of life reported without utility or QALYs
-
studies that do not report cost per QALY.
Assessment of eligibility and data extraction
All retrieved records were collected in a specialist database (EndNote X9.3, Clarivate Analytics, Philadelphia, PA, USA) and any duplicate records were identified and removed. Two reviewers independently reviewed titles and abstracts to identify potentially relevant full-text papers for formal assessment. Full-text papers were assessed by two reviewers independently following predefined inclusion criteria (see Inclusion criteria). Discrepancies were resolved by discussion.
Independent data extraction was carried out by one reviewer using a standardised data extraction sheet in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and was then checked by a second reviewer. The following data were extracted:
-
study details (i.e. author names, title and source of publication)
-
baseline characteristics (i.e. country, study design, population, intervention, comparators and outcomes)
-
methods (i.e. study design, study population and subgroups, setting and location, type of economic analysis, study perspective, time horizon, measurement of outcomes, measurement and valuation of preference-based outcomes, resource use and unit cost data, currency and price year, discount rate and model type)
-
results [i.e. results of the base-case (incremental costs and outcomes) and sensitivity analyses]
-
discussion (i.e. study findings, limitations and generalisability)
-
other details (i.e. sources of funding and conflicts of interest).
Quality assessment
The quality of studies was assessed by one reviewer, using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist,47 and was then checked by a second reviewer. The CHEERS checklist comprises the following six dimensions: (1) title and abstract, (2) introduction, (3) methods, (4) results, (5) discussion and (6) other. Under these six dimensions, a series of questions check whether or not the criteria have been clearly reported. Any studies containing an economic model were further assessed using the framework for the quality assessment of decision analytic modelling by Philips et al. 48 The framework by Philips et al. 48 contains two main dimensions: (1) structure of the model and (2) data used to parameterise the model. Under these dimensions, several questions assess whether or not the criteria have been clearly reported.
Data synthesis
Data extracted from included studies were narratively summarised and tabulated. Findings from individual studies were compared narratively.
Results
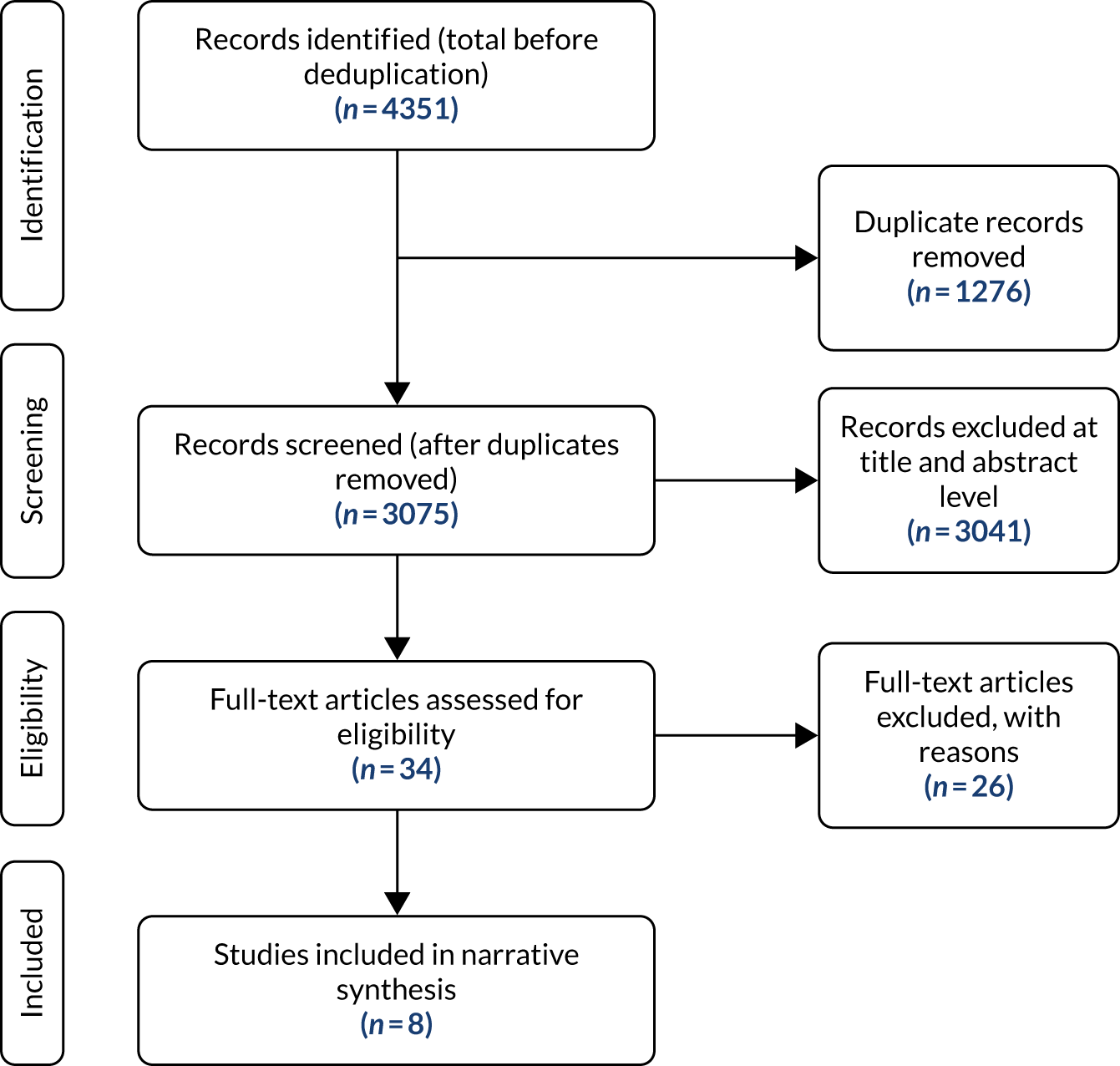
The literature search identified 4351 records through the electronic database searches. After removing duplicates, 3075 records were screened to identify potentially relevant studies. Title and abstract screening excluded 3041 records. The remaining 34 records were included for full-text assessment, after which a further 26 records were excluded, as they did not meet the formal inclusion criteria (Figure 1). The majority of studies were excluded because they were not full economic evaluations (see Appendix 2). Two further49,50 studies were identified from weekly auto-alerts and the full texts of these studies were obtained. However, both studies49,50 were excluded as they were not primary prevention studies. Eight studies51–58 met the inclusion criteria of this systematic review.
FIGURE 1.
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for cost-effectiveness studies.
Overview of included studies
Full details of the overall characteristics of included studies are provided in Table 1. In summary, four studies55–58 were conducted in the UK. The remaining four studies51–54 were part of multicountry RCTs and we have reported the results from only the UK parts of these studies (i.e. data from UK centres in multinational studies). All studies were within-clinical trial economic evaluations; however, four studies51–54 also developed an economic model using information from the clinical trial, alongside other published literature, databases and expert opinion. The majority of the populations in each study had at least one risk factor for CVD or were at high risk of CVD. Sample sizes ranged from 110 participants55 to 9098 participants. 51 In two studies,56,57 the intervention was a drug regimen (i.e. pravastatin and atorvastatin), which was compared with placebo. Two studies56,57 compared a drug treatment with another drug treatment (i.e. amlodipine-based therapy or atenolol plus atorvastatin and high adherence to atorvastatin or low adherence to atorvastatin) and three studies53,55,58 focused on interventions provided by health-care professionals that aimed to help change health behaviour or provide some form of educational support.
| Study | Setting and location | Study design | Study population | Sample size | Intervention | Comparator |
|---|---|---|---|---|---|---|
| Barton et al.55 | Five general practices in deprived communities (Liverpool, UK) | RCT. Within-clinical trial economic analysis | Patients aged ≥ 18 years with at least one risk factor for CVD (i.e. hypertension, raised cholesterol, diabetes, BMI > 30 kg/m2 or current smoker) | 72 patients in LHT group; 38 controls | In addition to what the control group received, the LHT provided patients with information, advice and support aimed at changing beliefs and behaviour. LHT support was available for 3 months, contact was made approximately every 2 weeks (six times in total), ideally via a face-to-face meeting, with additional telephone support, if required | Patients received health promotion literature, including British Heart Foundation (London, UK) patient booklets, and were asked to complete a food diary (at baseline and at 6-month follow-up) |
| Ismail et al.58 | 12 Clinical Commissioning Groups (South London, UK) | A three-arm single-blind parallel-group RCT. Within-clinical trial economic analysis | Patients aged 40–74 years with a QRISK®2 score ≥ 20.0% (the QRISK®2 indicates the probability of having a CVD event in the next 10 years) | 1742 participants: (group intervention, n = 697; individual intervention, n = 523; UC, n = 522) | Motivational interviewing enhanced with behaviour change techniques delivered by health trainers. Intervention delivered in 10 sessions over 1 year, in a group or individual format | UC consisted of referrals to locally commissioned community-based weight loss, smoking cessation and/or exercise programmes |
| Lindgren et al.51 | UK (and other European countries) | Open-label follow-up of a multicentre placebo RCT. Within-clinical trial economic model | Patients aged 40–79 years with no prior or current history of CHD, with either untreated hypertension or treated hypertension while not being treated with a statin or fibrate | 9098 UK and Ireland patients | Amlodipine-based therapy or atenolol plus atorvastatin | Amlodipine-based therapy or atenolol-based therapy plus placebo |
| Lindgren et al.52 | UK (and other European countries) | Open-label follow-up of a multicentre placebo RCT. Within-clinical trial economic model | Patients aged 40–79 years with no prior or current history of CHD, with either untreated hypertension or treated hypertension while not being treated with a statin or fibrate | 4123 patients based on adherence (high, n = 2415; low, n = 1708) | Atorvastatin: high adherence defined as > 80% of days covered | Atorvastatin: low adherence defined as < 50% of days covered |
| McConnachie et al.56 | UK | Placebo RCT. Within-clinical trial economic analysis | Men aged 45–54 years with hypercholesterolaemia who had no evidence of previous myocardial infarction | 6595 patients (placebo, n = 3293; pravastatin, n = 3302) | Pravastatin (40 mg once daily): initial 5 years of treatment | Placebo |
| Mistry et al.53 | Pairs of general practices in the UK (and six other European countries) | A matched paired cluster RCT. Within-clinical trial economic analysis and economic model | High-risk patients and their families to achieve recommended lifestyle and risk factor targets for CVD prevention in everyday clinical practice over 1 year | 2024 patients (intervention, n = 1019; UC, n = 1005) | The programme was delivered by specialist nurses, working with general practitioners and supported by software programs, educational materials and group workshops | Patients in the UC arm did not receive any form of special care |
| Raikou et al.57 | 32 centres in the UK and Ireland | RCT. Within-clinical trial economic analysis | Patients aged 40–75 years who had type 2 diabetes without a documented history of CVD (but with at least one risk factor for CVD) and without elevated LDL cholesterol. The mean age of patients was 62 years, with the majority being white (94%) and male (68%) | 2838 patients (atorvastatin, n = 1428; placebo, n = 1410) | Atorvastatin (10 mg) daily | Placebo |
| Simmons et al.54 | Two UK centres (Cambridge and Leicester, UK, and other European countries) | Pragmatic multicentre cluster-randomised parallel-group trial. Within-clinical trial economic analysis and economic model | Patients aged 40–69 years with screen-detected diabetes | 1024 participants were included in the within-clinical trial analysis and 999 participants were included in the economic model | Intensive treatment comprising screening and promotion of target-driven intensive management (i.e. medication and promotion of healthy lifestyles) of hyperglycaemia, blood pressure and cholesterol | Screening plus routine care |
Review of economic evaluation methods and results
Table 2 presents the economic evaluation methods that were conducted in the included studies. Results of cost–utility analyses were presented in the form of ICERs, whereby the ICER was the cost per QALY gained. All studies presented economic evaluations alongside a RCT. The economic model was a simple decision-analytic model in one study,54 whereas three studies51–53 used a Markov model framework. We did not class Raikou et al. 57 as using an economic model per se, as this study used a non-parametric approach to extrapolate costs and effects over the lifetime using lifetables.
| Study | Economic evaluation type | Model type | Study perspective | Time horizon | Resource use and costs | Currency (price year) | Discount rate |
|---|---|---|---|---|---|---|---|
| Barton et al.55 | CUA | N/A | NHS and Personal Social Services |
Within clinical trial: 6 months Model: N/A |
Intervention: time spent by the study team on LHT advertisement, selection, training and supervision. Each LHT (a dietitian) recorded the number of face-to-face visits with each participant, plus time taken to contact the patient, visit preparation and travel Comparator: both groups received health promotion literature and were asked to complete a food diary Other: inpatient admissions, health-care professional and voluntary group visits, and medications |
GBP (2008/9) | N/A |
| Ismail et al.58 | CUA | N/A | NR |
Within clinical trial: 12 and 24 months Model: N/A |
Intervention: time spent by staff delivering sessions, including overheads and on-costs. For the group intervention, the costs were apportioned over attendees Comparator: NR Other: inpatient care, outpatient attendances, community contacts and prescription medication |
GBP (NR) | 3.5% |
| Lindgren et al.51 | CUA | Markov model | NHS |
Within clinical trial: 3 years Model: lifetime (monthly cycles) |
Intervention: drug costs (amlodipine-based therapy or atenolol plus atorvastatin) Comparator: drug costs (amlodipine-based therapy or atenolol-based therapy plus placebo) Other: inpatient admissions, outpatient visits, medications and other health states |
Euros (2007) | 3.5% |
| Lindgren et al.52 | CUA | Markov model | NHS |
Within clinical trial: NR Model: NR |
Intervention: atorvastatin cost; dependent on adherence Comparator: NR Other: other health states |
GBP (2007) | 3.5% |
| McConnachie et al.56 | CUA | N/A | NHS |
Within clinical trial: 15 years (including follow-up) Model: N/A |
Intervention: drug costs (pravastatin) Comparator: no cost for placebo Other: hospital admissions, coronary investigations and procedures, liver function and cholesterol tests, and statin treatment |
GBP (2012) | 3.5% |
| Mistry et al.53 | CUA | Markov model | NHS |
Within clinical trial: 1 year Model: 11 years (yearly cycles) |
Intervention: EuroAction programme costs included the EuroAction nurses’ costs, training costs, production of patient educational materials and any other costs associated with implementing the programme Comparator: NR Other: primary care contacts, cardiac-related drugs, cardiac-related procedures and tests, and other health states |
GBP (2006/7) | 3.5% |
| Raikou et al.57 | CUA | No model per se, but extrapolation using a non-parametric approach and lifetables | NHS |
Within clinical trial: 4.9 years (mean follow-up) Model: lifetime |
Intervention: atorvastatin plus the use of any additional statin therapy for a cardiovascular event Comparator: the use of any additional statin therapy for a cardiovascular event Other: hospitalisations, clinic visits and tests |
GBP (2003/4) | 3.5% |
| Simmons et al.54 | CUA | Decision-analytic model | NHS |
Within clinical trial: 1–6 years Model: 10–30 years |
Intervention: material design costs; meetings with health professionals, practitioner and patient; extra patient consultations; and treatments (including prescription of cardioprotective medication and glucometers with strips) Comparator: NR Other: routine cost of treating diabetes and diabetes-related events during trial follow-up (e.g. inpatient admissions and non-inpatient costs) |
GBP (2009/10) | 3.5% |
All studies except for one58 adopted a health service (NHS) perspective. Ismail et al. 58 did not report the perspective adopted for the costs and outcomes analysis (see Table 2). The time horizon for the within-clinical trial analyses ranged from 6 months55 to 15 years,56 and one study52 did not report the time horizon for the within-clinical trial analysis. For studies that included an economic model, the time horizon varied from 10 years54 to a lifetime. 51,57 Furthermore, only two studies51,53 provided the length of the model cycle.
In terms of resource use and costs, the majority of studies detailed cost components that contributed to the intervention arm, although the resource use and costs for the comparator arm were not reported consistently (see Table 2). For example, four studies51,53,54,58 did not report any specific comparator costs. Other costs that were reported for both arms included hospital admissions, clinic visits, medications and, where appropriate for the economic model, health state costs. All studies except one51 reported the costs in Great British pounds and one study58 did not report the price year for the unit costs. Seven studies51–54,56–58 reported that both costs and benefits were discounted at 3.5% per annum. One study,55 which had a < 1-year time horizon, appropriately had no discounting of costs or outcomes.
The EuroQol-5 Dimensions instrument was used to obtain utility scores in seven studies51–55,57,58 (Table 3). Only three51,52,55 of these seven studies stated that the values that were used to calculate utility scores were obtained from the general public. One study56 obtained utility values from a previous review.
| Study | Utility measure | Whose utility values? | Results (incremental costs and outcomes) | Key SA | Authors’ conclusion |
|---|---|---|---|---|---|
| Barton et al.55 | EQ-5D completed at baseline and at 6 months | The York A1 tariff was used to assign scores to each EQ-5D health state description |
Incremental costs: £97.85 Incremental QALYs: 0.007 ICER: £14,480/QALY gained Probability of being cost-effective at £20,000/QALY: 0.395 Probability of being cost-effective at £30,000/QALY: 0.401 |
One-way SA:
|
LHTs’ provision was estimated to be cost-effective for people at risk of CVD |
| Ismail et al.58 | EQ-5D-3L completed at baseline and at 12 and 24 months | NR |
The group arm was dominated by usual care (i.e. more expensive and less effective) Individual vs. usual careIndividual vs. groupProbability of being cost-effective at £30,000/QALY |
SA around key costs:
|
Enhancing motivational interviewing with additional behaviour change techniques was not effective in reducing weight or increasing physical activity in patients with high CVD risk |
| Lindgren et al.51 | EQ-5D: not stated when administered | Values were based on a representative sample of the UK population using the EQ-5D tariff |
Atenolol-based therapy plus atorvastatin is eliminated through extended dominance Amlodipine-based therapy plus atorvastatin vs. amlodipine-based therapy aloneAmlodipine-based therapy vs. atenolol-based therapy aloneProbability of being cost-effective at €20,000/QALY |
One-way SA:
|
A combination of amlodipine-based therapy or atorvastatin appears to be cost-effective in patients with hypertension and three or more additional factors |
| Lindgren et al.52 | EQ-5D: not stated when administered | Values were based on a representative sample of the UK population using the EQ-5D tariff |
Incremental costs: £366 Incremental QALYs: 0.02 ICER: £18,300/QALY gained Probability of being cost-effective at £20,000/QALY: NR Probability of being cost-effective at £30,000/QALY: NR |
One-way SA:
|
Given the higher risk of CVD events associated with low adherence, measures to improve adherence are an important part of the prevention of CVD |
| McConnachie et al.56 | No measures. Utilities were obtained from a previous review | Not known. Utilities were obtained from a previous review |
Incremental costs: £710 Incremental QALYs: 0.136 ICER: £5221/QALY gained Probability of being cost-effective at £20,000/QALY: NR Probability of being cost-effective at £30,000/QALY: NR |
SAs:
|
Five years’ primary prevention treatment of middle-aged men with a statin significantly reduces health-care resource utilisation, is cost saving and increases QALYs |
| Mistry et al.53 | EQ-5D (during clinical trial) and utilities were obtained from a previous review | Not known. Utilities were obtained from a previous review and were based on UK population norms |
Assuming no (0-year) duration of effect of the intervention beyond the end of the clinical trial (unadjusted results) Incremental costs: £419 Incremental QALYs: 0.076 ICER: £5539/QALY gained Probability of being cost-effective at £20,000/QALY: 0.95 Probability of being cost-effective at £30,000/QALY: 0.97 |
SAs:
|
Although the study achieved healthier lifestyle changes and improvements in management of blood pressure and lipids for patients at high risk of CVD, compared with usual care, it was not possible to show, using available risk equations, which do not incorporate diet and physical activity, that the intervention reduced longer-term cardiovascular risk cost-effectively |
| Raikou et al.57 | EQ-5D scores from previous studies and quality-of-life tariffs owing to differences in non-fatal CVD event | Not stated |
Incremental costs: £2505 Incremental QALYs: 0.3871 ICER: £6471/QALY gained Probability of being cost-effective at £20,000/QALY: NR Probability of being cost-effective at £30,000/QALY: NR |
One-way SA:
|
Primary prevention of CVD with atorvastatin is a cost-effective intervention in patients with type 2 diabetes |
| Simmons et al.54 | EQ-5D. Not stated when administered in clinical trial and from previous studies | Not known. Utilities were obtained from a previous studies |
Over a 5-year time period Incremental costs: £935 Incremental QALYs: –0.0040 ICER: dominated Probability of being cost-effective at £20,000/QALY: NR Probability of being cost-effective at £30,000/QALY: NR |
One-way SA:
|
The intensive treatment was not cost-effective compared with routine care for screen-detected diabetes patients in the UK |
All studies presented their results in terms of cost per QALY gained (see Table 3). Incremental costs ranged from £97 (where the intervention cost of lay health trainer was higher than the comparator cost of no intervention)55 to £2505 (where atorvastatin daily was more expensive than the placebo comparator arm). 57 Incremental QALYs ranged from 0.0064 (where motivational interviewing enhanced with behaviour change techniques delivered by health trainers was more effective than usual care)58 to 0.3871 (where atorvastatin daily was more effective than placebo arm). 57 For the majority of studies, the ICERs were below the NICE threshold of £20,000–30,000 per QALY. 59 The exception was the study by Ismail et al. ,58 in which behaviour change techniques were delivered by health trainers in an individual format and compared with usual care. Ismail et al. 58 reported a small difference in both the incremental costs and the incremental QALYs, leading to a large ICER of –£55,313 per QALY gained. Four studies52,54,56,57 reported the probability of the intervention being cost-effective at the £20,000 and/or £30,000 willingness-to-pay threshold (or this could be deduced from the graphs on display in the articles) and four studies51,53,55,58 did not report this finding.
All studies carried out some form of sensitivity or scenario analysis, the majority of which were one-way sensitivity analyses. The main parameters that varied were costs, discount rates and time horizons. Five studies51–53,55,57 also undertook a probabilistic sensitivity analysis, namely to characterise uncertainty around key parameters.
In terms of overall results, the evaluated interventions were found to be a cost-effective use of resources, except for in two studies. 54,58 Ismail et al. 58 found that ‘enhancing motivational interviewing with additional behaviour change techniques was not effective compared with the usual care in reducing weight or increasing physical activity in those at high CVD risk’. Simmons et al. 54 found that ‘the intensive treatment was not cost-effective compared with routine care for screen-detected diabetes patients in the UK’ and, instead, the intervention was dominated by routine care (i.e. the intervention was more expensive and less effective).
Quality assessment
Table 4 presents a summary of the scores of the quality assessment of the included studies. Using the CHEERS reporting tool47 (see Appendix 3), the majority (75%) of studies fulfilled at least 20 of the 26 items. The study by Lindgren et al. 51 was the most comprehensively reported, scoring yes on 23 of the 26 items. When using the framework for the quality assessment of decision analytic modelling by Philips et al. 48 (see Appendix 4) for the studies that included an economic model, the least comprehensively reported study was by Lindgren et al. ,52 scoring yes on only 11 of the 57 items.
| Study | CHEERS checklist | Phillips et al.48 checklist | ||
|---|---|---|---|---|
| Item | Score | Item | Score | |
| Barton et al.55 | Yes | 22 | No model included | |
| No | 2 | |||
| Partial | 0 | |||
| N/A | 2 | |||
| Ismail et al.58 | Yes | 17 | No model included | |
| No | 4 | |||
| Partial | 4 | |||
| N/A | 1 | |||
| Lindgren et al.51 | Yes | 23 | Yes | 23 |
| No | 1 | No | 4 | |
| Partial | 2 | Partial | 22 | |
| N/A | 0 | Unclear | 8 | |
| N/A | 0 | |||
| Lindgren et al.52 | Yes | 18 | Yes | 11 |
| No | 3 | No | 7 | |
| Partial | 5 | Partial | 15 | |
| N/A | 0 | Unclear | 24 | |
| N/A | 0 | |||
| McConnachie et al.56 | Yes | 22 | No model included | |
| No | 1 | |||
| Partial | 2 | |||
| N/A | 1 | |||
| Mistry et al.53 | Yes | 21 | Yes | 25 |
| No | 0 | No | 3 | |
| Partial | 5 | Partial | 13 | |
| N/A | 0 | Unclear | 16 | |
| N/A | 0 | |||
| Raikou et al.57 | Yes | 20 | No model included | |
| No | 0 | |||
| Partial | 5 | |||
| N/A | 1 | |||
| Simmons et al.54 | Yes | 22 | Yes | 27 |
| No | 1 | No | 2 | |
| Partial | 3 | Partial | 14 | |
| N/A | 0 | Unclear | 10 | |
| N/A | 4 | |||
Discussion
To the best of our knowledge, this is the first systematic review investigating the cost-effectiveness of different interventions for the primary prevention of CVD. Eight studies51–58 evaluating the cost-effectiveness of interventions for the primary prevention of CVD were included in this review. The eight studies51–58 were published between 2007 and 2019. The studies focused on health promotion, lipid-lowering medicine and blood pressure-lowering medication. Seven51–53,55–58 out of eight studies found therapies that were likely to be cost-effective within NICE cost-effectiveness threshold limits. The quality of the research included in the studies was variable, although quality improved with time, which is likely because of a consensus on reporting requirements for economic evaluations. All studies51–58 included in this review, as part of our inclusion criteria, presented their findings in terms of QALYs. There was a lot of variation across the included studies51–58 in terms of the interventions, the measure of benefit, the resources used and costs, and the time horizon. However, other features, such as intervention classes, could be used to group the interventions.
Implications for practice and research
There is an ever-increasing demand for cost-effective interventions in CVD primary prevention. We found few model-based health economic analyses of interventions for primary CVD prevention conducted within the last decade, suggesting that, despite significant investment in recent years, the cost-effectiveness of the primary prevention of CVD has received little attention. A better understanding of the cost-effectiveness of the primary prevention of CVD is an essential driver of optimal resource allocation. 60 The evidence obtained by health economics analysis makes it easier to deploy highly clinically effective and cost-effective primary CVD preventative strategies on a timely basis. As a result, in the health economics evaluation of primary CVD prevention, high-quality research is essential. Future economic assessments should be undertaken and presented in accordance with best practices so that future reviews may make clear recommendations to improve health policy. 60
Patient and public involvement
Drawing on INVOLVE guidance and support for best practice, we worked closely with three dedicated patient and public involvement advisors. We invited guidance and support from our patient and public involvement advisors at the preparatory phase of the project.
Conclusion
Establishing direct comparisons and drawing firm conclusions is challenging because of the uncertainty and variation across studies. However, interventions conducted for or within the UK NHS were likely to be cost-effective in people at increased risk of CVD, compared with usual care or no intervention control.
Acknowledgements
Contributions of authors
Hema Mistry (https://orcid.org/0000-0002-5023-1160) (Associate Professor, Health Economics) contributed to the protocol, study selection, validity assessments, synthesis of the included studies, the interpretation of the results and the writing of the report, and had overall responsibility for the economic evaluation study.
Jodie Enderby (https://orcid.org/0000-0002-1446-7512) (Research Associate) contributed to the protocol, study selection, validity assessments, synthesis of the included studies, interpretation of the results and to the writing of the report.
Rachel Court (https://orcid.org/0000-0002-4567-2586) (Information Specialist) contributed to the protocol development, developed the search strategies, conducted a range of searches to locate studies, wrote the sections of the report relating to the literature searches, contributed to the protocol and interpretation of the results, and commented on drafts of the report.
Lena Al-Khudairy (https://orcid.org/0000-0003-0638-583X) (Associate Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments, synthesis of the included studies, interpretation of the results and to the writing of the report.
Chidozie Nduka (https://orcid.org/0000-0001-7031-5444) (Senior Research Fellow, Evidence Synthesis) contributed to the protocol, study selection, validity assessments, synthesis of the included studies, interpretation of the results and to the writing of the report.
GJ Melendez-Torres (https://orcid.org/0000-0002-9823-4790) (Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments, synthesis of the included studies, interpretation of the results and to the writing of the report.
Sian Taylor-Phillips (https://orcid.org/0000-0002-1841-4346) (Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments, synthesis of the included studies, interpretation of the results and to the writing of the report.
Aileen Clarke (https://orcid.org/0000-0001-8299-3146) (Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments, synthesis of the included studies, interpretation of the results and to the writing of the report.
Olalekan A Uthman (https://orcid.org/0000-0002-8567-3081) (Professor, Evidence Synthesis) contributed to the protocol, study selection, data extraction, validity assessments, synthesis of the included studies, interpretation of the results and to the writing of the report. He developed the classifiers and undertook the analyses, and had overall responsibility for the project.
Ethics statement
This work is a systematic review; it involved accessing, processing, and analysing data that has already been published and is available to the public. As a result, no patient data were processed; patient consent and/or registration via human research ethics committees were, therefore, not relevant.
Data-sharing statement
No new data have been created in the preparation of this article and, therefore, there is nothing available for access and further sharing. All queries should be submitted to the corresponding author.
Funding
This project was funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme and will be published in Health Technology Assessment. See the NIHR Journals Library website for further project information.
Article history
The research reported in this article was funded by the HTA programme under project number 17/148/05. The contractual start date was in February 2019. This article began editorial review in December 2021 and was accepted for publication in June 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and production house have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on this article document. However, they do not accept liability for damages or losses arising from material published in this article.
Disclaimer
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016;102:1945-52. https://doi.org/10.1136/heartjnl-2016-309573.
- Mayor S. Deaths from heart disease in UK fall, but prevalence is unchanged. BMJ 2016;354. https://doi.org/10.1136/bmj.i4609.
- Steel N, Ford JA, Newton JN, Davis ACJ, Vos T, Naghavi M, et al. Changes in health in the countries of the UK and 150 English local authority areas 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1647-61. https://doi.org/10.1016/S0140-6736(18)32207-4.
- Stewart C. Cardiovascular Disease in the United Kingdom (UK) – Statistics &Amp; Facts n.d. www.statista.com/topics/5003/cardiovascular-disease-in-the-uk (accessed 20 May 2022).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 1996;276:1253-8. https://doi.org/10.1001/jama.1996.03540150055031.
- Schwarzer R, Rochau U, Saverno K, Jahn B, Bornschein B, Muehlberger N, et al. Systematic overview of cost-effectiveness thresholds in ten countries across four continents. J Comp Eff Res 2015;4:485-504. https://doi.org/10.2217/cer.15.38.
- Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2018;7. https://doi.org/10.1002/14651858.CD003177.pub3.
- Abdelhamid AS, Martin N, Bridges C, Brainard JS, Wang X, Brown TJ, et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2018;7. https://doi.org/10.1002/14651858.CD012345.pub2.
- Adler AJ, Taylor F, Martin N, Gottlieb S, Taylor RS, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD009217.pub3.
- Al-Khudairy L, Flowers N, Wheelhouse R, Ghannam O, Hartley L, Stranges S, et al. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;3. https://doi.org/10.1002/14651858.CD011114.pub2.
- Al-Khudairy L, Hartley L, Clar C, Flowers N, Hooper L, Rees K. Omega 6 fatty acids for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2015;11. https://doi.org/10.1002/14651858.CD011094.pub2.
- Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in patients with diabetes mellitus. Cochrane Database Syst Rev 2010;1. https://doi.org/10.1002/14651858.CD008277.
- Bahiru E, de Cates AN, Farr MR, Jarvis MC, Palla M, Rees K, et al. Fixed-dose combination therapy for the prevention of atherosclerotic cardiovascular diseases. Cochrane Database Syst Rev 2017;3. https://doi.org/10.1002/14651858.CD009868.pub3.
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014;1. https://doi.org/10.1002/14651858.CD007470.pub3.
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;3. https://doi.org/10.1002/14651858.CD007176.pub2.
- Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev 2015;3. https://doi.org/10.1002/14651858.CD002229.pub4.
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD006103.pub2.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013;5. https://doi.org/10.1002/14651858.CD009329.pub2.
- Clar C, Al-Khudairy L, Loveman E, Kelly SA, Hartley L, Flowers N, et al. Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;7. https://doi.org/10.1002/14651858.CD004467.pub3.
- Clar C, Oseni Z, Flowers N, Keshtkar-Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev 2015;5. https://doi.org/10.1002/14651858.CD005050.pub3.
- Curioni C, André C, Veras R. Weight reduction for primary prevention of stroke in adults with overweight or obesity. Cochrane Database Syst Rev 2006;4. https://doi.org/10.1002/14651858.CD006062.pub2.
- de Cates AN, Farr MR, Wright N, Jarvis MC, Rees K, Ebrahim S, et al. Fixed-dose combination therapy for the prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD009868.pub2.
- Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD006742.pub2.
- Dyakova M, Shantikumar S, Colquitt JL, Drew CM, Sime M, MacIver J, et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2016;1. https://doi.org/10.1002/14651858.CD010411.pub2.
- Ebbert J, Montori VM, Erwin PJ, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database Syst Rev 2011;2. https://doi.org/10.1002/14651858.CD004306.pub4.
- Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev 2011;1. https://doi.org/10.1002/14651858.CD001561.pub3.
- Flowers N, Hartley L, Todkill D, Stranges S, Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD010405.pub2.
- Hartley L, Clar C, Ghannam O, Flowers N, Stranges S, Rees K. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2015;9. https://doi.org/10.1002/14651858.CD011148.pub2.
- Hartley L, Dyakova M, Holmes J, Clarke A, Lee MS, Ernst E, et al. Yoga for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;5. https://doi.org/10.1002/14651858.CD010072.pub2.
- Hartley L, Flowers N, Holmes J, Clarke A, Stranges S, Hooper L, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD009934.pub2.
- Hartley L, Flowers N, Lee MS, Ernst E, Rees K. Tai chi for primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD010366.pub2.
- Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD009874.pub2.
- Hartley L, Lee MS, Kwong JS, Flowers N, Todkill D, Ernst E, et al. Qigong for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.CD010390.pub2.
- Hartley L, Mavrodaris A, Flowers N, Ernst E, Rees K. Transcendental meditation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD010359.pub2.
- Hartley L, May MD, Loveman E, Colquitt JL, Rees K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2016;1. https://doi.org/10.1002/14651858.CD011472.pub2.
- Hemkens LG, Ewald H, Gloy VL, Arpagaus A, Olu KK, Nidorf M, et al. Colchicine for prevention of cardiovascular events. Cochrane Database Syst Rev 2016;1. https://doi.org/10.1002/14651858.CD011047.pub2.
- Hooper L, Al-Khudairy L, Abdelhamid AS, Rees K, Brainard JS, Brown TJ, et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2018;11. https://doi.org/10.1002/14651858.CD011094.pub4.
- Hooper L, Bartlett C, Davey SG, Ebrahim S. Advice to reduce dietary salt for prevention of cardiovascular disease. Cochrane Database Syst Rev 2004;1. https://doi.org/10.1002/14651858.CD003656.pub2.
- Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.CD011737.
- Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore HJ, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 2012;5. https://doi.org/10.1002/14651858.CD002137.pub3.
- Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev 2004;4. https://doi.org/10.1002/14651858.CD003177.pub2.
- Jakob T, Nordmann AJ, Schandelmaier S, Ferreira-González I, Briel M. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD009753.pub2.
- Ayiku L, Levay P, Hudson T, Craven J, Barrett E, Finnegan A, et al. The MEDLINE UK filter: development and validation of a geographic search filter to retrieve research about the UK from OVID MEDLINE. Health Info Libr J 2017;34:200-16. https://doi.org/10.1111/hir.12187.
- Ayiku L, Levay P, Hudson T, Craven J, Finnegan A, Adams R, et al. The Embase UK filter: validation of a geographic search filter to retrieve research about the UK from OVID Embase. Health Info Libr J 2019;36:121-33. https://doi.org/10.1111/hir.12252.
- Ayiku L, Levay P, Hudson T, Finnegan A. The NICE UK geographic search filters for MEDLINE and Embase (Ovid): post-development study to further evaluate precision and number-needed-to-read when retrieving UK evidence. Res Synth Methods 2020;11:669-77. https://doi.org/10.1002/jrsm.1431.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement: updated reporting guidance for health economic evaluations. Eur J Health Econ 2022;23:1309-17.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Kim LG, Wilson ECF, Davison WJ, Clark AB, Myint PK, Potter JF. Self-monitoring and management of blood pressure in patients with stroke or TIA: an economic evaluation of TEST-BP, a randomised controlled trial. Pharmacoecon Open 2020;4:511-17. https://doi.org/10.1007/s41669-020-00196-w.
- Taylor AH, Taylor RS, Ingram WM, Anokye N, Dean S, Jolly K, et al. Adding web-based behavioural support to exercise referral schemes for inactive adults with chronic health conditions: the e-coachER RCT. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24630.
- Lindgren P, Buxton M, Kahan T, Poulter NR, Dahlof B, Sever PS, et al. The lifetime cost effectiveness of amlodipine-based therapy plus atorvastatin compared with atenolol plus atorvastatin, amlodipine-based therapy alone and atenolol-based therapy alone: results from ASCOT. PharmacoEconomics 2009;27:221-30. https://doi.org/10.2165/00019053-200927030-00005.
- Lindgren P, Eriksson J, Buxton M, Kahan T, Poulter NR, Dahlöf B, et al. The economic consequences of non-adherence to lipid-lowering therapy: results from the Anglo-Scandinavian-Cardiac Outcomes Trial. Int J Clin Pract 2010;64:1228-34. https://doi.org/10.1111/j.1742-1241.2010.02445.x.
- Mistry H, Morris S, Dyer M, Kotseva K, Wood D, Buxton M. EUROACTION study group . Cost-effectiveness of a European preventive cardiology programme in primary care: a Markov modelling approach. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001029.
- Simmons RK, Borch-Johnsen K, Lauritzen T, Rutten GE, Sandbæk A, van den Donk M, et al. A randomised trial of the effect and cost-effectiveness of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with screen-detected type 2 diabetes: the Anglo-Danish-Dutch study of intensive treatment in people with screen-detected diabetes in primary care (ADDITION-Europe) study. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20640.
- Barton GR, Goodall M, Bower P, Woolf S, Capewell S, Gabbay MB. Increasing heart-health lifestyles in deprived communities: economic evaluation of lay health trainers. J Eval Clin Pract 2012;18:835-40. https://doi.org/10.1111/j.1365-2753.2011.01686.x.
- McConnachie A, Walker A, Robertson M, Marchbank L, Peacock J, Packard CJ, et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J 2014;35:290-8. https://doi.org/10.1093/eurheartj/eht232.
- Raikou M, McGuire A, Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, et al. Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia 2007;50:733-40. https://doi.org/10.1007/s00125-006-0561-4.
- Ismail K, Bayley A, Twist K, Stewart K, Ridge K, Britneff E, et al. Reducing weight and increasing physical activity in people at high risk of cardiovascular disease: a randomised controlled trial comparing the effectiveness of enhanced motivational interviewing intervention with usual care. Heart 2020;106:447-54. https://doi.org/10.1136/heartjnl-2019-315656.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. PharmacoEconomics 2008;26:733-44. https://doi.org/10.2165/00019053-200826090-00004.
- Maru S, Byrnes J, Whitty JA, Carrington MJ, Stewart S, Scuffham PA. Systematic review of model-based analyses reporting the cost-effectiveness and cost-utility of cardiovascular disease management programs. Eur J Cardiovasc Nurs 2015;14:26-33. https://doi.org/10.1177/1474515114536093.
- Briggs A, Mihaylova B, Sculpher M, Hall A, Wolstenholme J, Simoons M, et al. Cost effectiveness of perindopril in reducing cardiovascular events in patients with stable coronary artery disease using data from the EUROPA study. Heart 2007;93:1081-6. https://doi.org/10.1136/hrt.2005.086728.
- Calvert MJ, Freemantle N, Yao G, Cleland JG, Billingham L, Daubert JC, et al. Cost-effectiveness of cardiac resynchronization therapy: results from the CARE-HF trial. Eur Heart J 2005;26:2681-8. https://doi.org/10.1093/eurheartj/ehi662.
- Caro J, Klittich W. Is primary prevention with pravastatin cost-effective?. Cardiol Rev 2000;17:31-6.
- Cowie MR, Cure S, Bianic F, McGuire A, Goodall G, Tavazzi L. Cost-effectiveness of highly purified omega-3 polyunsaturated fatty acid ethyl esters in the treatment of chronic heart failure: results of Markov modelling in a UK setting. Eur J Heart Fail 2011;13:681-9. https://doi.org/10.1093/eurjhf/hfr023.
- Dalton AR, Bull RJ. Risk stratification could reduce costs in primary prevention of cardiovascular disease. BMJ 2011;343. https://doi.org/10.1136/bmj.d4913.
- De Smedt D, Annemans L, De Backer G, Kotseva K, Rydèn L, Wood D, et al. Cost-effectiveness of optimized adherence to prevention guidelines in European patients with coronary heart disease: results from the EUROASPIRE IV survey. Int J Cardiol 2018;272:20-5. https://doi.org/10.1016/j.ijcard.2018.06.104.
- De Smedt D, Kotseva K, De Bacquer D, Wood D, De Backer G, Dallongeville J, et al. Cost-effectiveness of optimizing prevention in patients with coronary heart disease: the EUROASPIRE III health economics project. Eur Heart J 2012;33:2865-72. https://doi.org/10.1093/eurheartj/ehs210.
- Fletcher K, Mant J, McManus R, Hobbs R. The Stroke Prevention Programme: a programme of research to inform optimal stroke prevention in primary care. Programme Grants Appl Res 2016;4. https://doi.org/10.3310/pgfar04030.
- Griffin SJ, Bethel MA, Holman RR, Khunti K, Wareham N, Brierley G, et al. Metformin in non-diabetic hyperglycaemia: the GLINT feasibility RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22180.
- Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the UK National Health Service perspective. Heart 2014;100:1031-6. https://doi.org/10.1136/heartjnl-2013-304598.
- Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R. Heart Protection Study Collaborative . Lifetime cost effectiveness of simvastatin in a range of risk groups and age groups derived from a randomised trial of 20,536 people. BMJ 2006;333. https://doi.org/10.1136/bmj.38993.731725.BE.
- Ismail K, Stahl D, Bayley A, Twist K, Stewart K, Ridge K, et al. Enhanced motivational interviewing for reducing weight and increasing physical activity in adults with high cardiovascular risk: the MOVE IT three-arm RCT. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23690.
- Jacobs N, Evers S, Ament A, Claes N. Cost–utility of a cardiovascular prevention program in highly educated adults: intermediate results of a randomized controlled trial. Int J Technol Assess Health Care 2010;26:11-9. https://doi.org/10.1017/S0266462309990845.
- Jones DA, Whittaker P, Rathod KS, Richards AJ, Andiapen M, Antoniou S, et al. Sodium nitrite-mediated cardioprotection in primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a cost-effectiveness analysis. J Cardiovasc Pharmacol Ther 2019;24:113-19. https://doi.org/10.1177/1074248418784940.
- Jönsson B, Buxton M, Hertzman P, Kahan T, Poulter N. Anglo-Scandinavian Cardiac Outcomes Trial Health Economics Working Group . Health economics of prevention of coronary heart disease and vascular events: a cost-effectiveness analysis based on the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). J Hum Hypertens 2001;15:53-6. https://doi.org/10.1038/sj.jhh.1001086.
- Kashef MA, Giugliano G. Legacy effect of statins: 20-year follow up of the West of Scotland Coronary Prevention Study (WOSCOPS). Glob Cardiol Sci Pract 2016;2016. https://doi.org/10.21542/gcsp.2016.35.
- Lee D, Wilson K, Akehurst R, Cowie MR, Zannad F, Krum H, et al. Cost-effectiveness of eplerenone in patients with systolic heart failure and mild symptoms. Heart 2014;100:1681-7. https://doi.org/10.1136/heartjnl-2014-305673.
- Lindgren P, Buxton M, Kahan T, Poulter NR, Dahlöf B, Sever PS, et al. Cost-effectiveness of atorvastatin for the prevention of coronary and stroke events: an economic analysis of the Anglo-Scandinavian Cardiac Outcomes Trial – lipid-lowering arm (ASCOT-LLA). Eur J Cardiovasc Prev Rehabil 2005;12:29-36. https://doi.org/10.1177/204748730501200105.
- Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111:1167-76. https://doi.org/10.1160/TH14-03-0231.
- McInnes G, Burke TA, Carides G. Cost-effectiveness of losartan-based therapy in patients with hypertension and left ventricular hypertrophy: a UK-based economic evaluation of the Losartan Intervention for Endpoint reduction in hypertension (LIFE) study. J Hum Hypertens 2006;20:51-8. https://doi.org/10.1038/sj.jhh.1001939.
- Mihaylova B, Schlackow I, Herrington W, Lozano-Kühne J, Kent S, Emberson J, et al. Cost-effectiveness of simvastatin plus ezetimibe for cardiovascular prevention in CKD: results of the Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 2016;67:576-84. https://doi.org/10.1053/j.ajkd.2015.09.020.
- Rawles J, Light J. Loss of quality adjusted days as a trial endpoint: effect of early thrombolytic treatment in suspected myocardial infarction. Grampion Region Early Anistreplase Trial (GREAT). J Epidemiol Community Health 1993;47:377-81. https://doi.org/10.1136/jech.47.5.377.
- Remak E, Manson S, Hutton J, Brasseur P, Olivier E, Gershlick A. Cost-effectiveness of the Endeavor stent in de novo native coronary artery lesions updated with contemporary data. EuroIntervention 2010;5:826-32.
- Rinciog CI, Sawyer LM, Diamantopoulos A, Elkind MSV, Reynolds M, Tsintzos SI, et al. Cost-effectiveness of an insertable cardiac monitor in a high-risk population in the UK. Open Heart 2019;6. https://doi.org/10.1136/openhrt-2019-001037.
- Thom H, West NE, Hughes V, Dyer M, Buxton M, Sharples LD, et al. Cost-effectiveness of initial stress cardiovascular MR, stress SPECT or stress echocardiography as a gate-keeper test, compared with upfront invasive coronary angiography in the investigation and management of patients with stable chest pain: mid-term outcomes from the CECaT randomised controlled trial. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003419.
- Wonderling D, McDermott C, Buxton M, Kinmonth AL, Pyke S, Thompson S, et al. Costs and cost effectiveness of cardiovascular screening and intervention: the British family heart study. BMJ 1996;312:1269-73. https://doi.org/10.1136/bmj.312.7041.1269.
Appendix 1 Search strategies for cost-effectiveness studies
Summary of bibliographic database searches
| Database | Date of search | Number of records |
|---|---|---|
| MEDLINE (Ovid) | 13 February 2020 | 1648 |
| Embase (Ovid) | 13 February 2020 | 2703 |
MEDLINE (via Ovid)
Actual database searched: Ovid MEDLINE® ALL.
Search date: 13 February 2020.
Date range searched: 1946 to 12 February 2020.
Search strategy
-
exp Primary Prevention/ (148,860)
-
primary prevention.ti,ab,kf. (18,711)
-
1 or 2 (163,009)
-
exp Cardiovascular Diseases/ (2,342,767)
-
exp Stroke/ (129,744)
-
(CVD or cardiovascular* or coronary* or heart* or myocardial* or cardiac* or stroke* or cerebrovascular or atherosclerosis or arteriosclerosis or vascular or hypertension or blood pressure or cholesterol or lipid*).ti,ab,kf. (3,176,762)
-
4 or 5 or 6 (4,133,451)
-
3 and 7 (17,743)
-
cardiovascular diseases/pc (32,957)
-
exp coronary disease/pc (20,241)
-
exp myocardial ischemia/pc (38,097)
-
exp heart failure/pc (3966)
-
exp heart arrest/pc (7169)
-
exp stroke/pc (16,705)
-
exp carotid stenosis/pc (288)
-
exp arteriosclerosis/pc (12,274)
-
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 (102,314)
-
((prevent* or reduc* or lower* or decreas* or change* or effect or effects or progression or level* or incidence) adj10 (CVD or cardiovascular* or coronary* or heart* or myocardial or cardiac* or stroke* or cerebrovascular or atherosclerosis or arteriosclerosis or vascular or hypertension or blood pressure or cholesterol or lipid*)).ti. (232,585)
-
((prevent* or reduc* or lower* or decreas* or change* or effect or effects) adj6 (CVD or cardiovascular* or coronary* or heart* or myocardial or cardiac* or stroke* or cerebrovascular or atherosclerosis or arteriosclerosis or vascular or hypertension or blood pressure or cholesterol or lipid*)).ti,ab,kf. (672,451)
-
(((prevent* or reduc* or lower* or decreas* or change* or effect or effects) adj2 (mortality or death)) and (CVD or cardiovascular* or coronary* or heart* or myocardial or cardiac* or stroke* or cerebrovascular or atherosclerosis or arteriosclerosis or vascular or hypertension or blood pressure or cholesterol or lipid*)).ti,ab,kf. (29,399)
-
8 or 17 or 18 or 19 or 20 (802,115)
-
Quality-Adjusted Life Years/ (11,825)
-
quality adjusted life year*.mp. (17,633)
-
(QALY or QALYS).mp. (10,200)
-
(utilit* adj2 (score* or value* or health)).mp. (5494)
-
(EuroQol or Euro Qol or Euro-Qol or EQ 5D* or EQ-5D* or EQ5D*).mp. (10,533)
-
(health utilities index or health-utilities-index or health-utilities index or health utility index or HUI).mp. (1753)
-
(SF 6D* or SF-6D* or SF6D* or SF-12* or SF 12* or SF 12* or short form health survey).mp. (10,403)
-
(short form 36* or SF36* or SF-36* or SF 36*).mp. (25,644)
-
Cost-Benefit Analysis/ (79,525)
-
(cost effective* or cost utilit* or cost benefit* or cost consequence*).mp. (178,346)
-
(pharmacoeconomic* or pharmaco-economic* or economic analy* or economic evaluation*).mp. (21,348)
-
(ICER* or incremental cost-effectiveness ratio*).mp. (8359)
-
(cost adj2 (evaluation* or analy* or study or studies or effective* or benefit* or utili*)).mp. (233,991)
-
(economic adj2 (evaluation* or analy* or study or studies)).ti,ab,kf. (20,353)
-
((markov or decision or economic) adj3 model*).mp. (34,645)
-
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 (307,088)
-
22 or 23 or 24 (18,532)
-
exp united kingdom/ (360,716)
-
(national health service* or nhs*).ti,ab,in. (186,480)
-
(english not ((published or publication* or translat* or written or language* or speak* or literature or citation*) adj5 english)).ti,ab. (93,720)
-
(gb or “g.b.” or britain* or (british* not “british columbia”) or uk or “u.k.” or united kingdom* or (england* not “new england”) or northern ireland* or northern irish* or scotland* or scottish* or ((wales or “south wales”) not “new south wales”) or welsh*).ti,ab,jw,in. (2,011,610)
-
(bath or “bath’s” or ((birmingham not alabama*) or (“birmingham’s” not alabama*) or bradford or “bradford’s” or brighton or “brighton’s” or bristol or “bristol’s” or carlisle* or “carlisle’s” or (cambridge not (massachusetts* or boston* or harvard*)) or (“cambridge’s” not (massachusetts* or boston* or harvard*)) or (canterbury not zealand*) or (“canterbury’s” not zealand*) or chelmsford or “chelmsford’s” or chester or “chester’s” or chichester or “chichester’s” or coventry or “coventry’s” or derby or “derby’s” or (durham not (carolina* or nc)) or (“durham’s” not (carolina* or nc)) or ely or “ely’s” or exeter or “exeter’s” or gloucester or “gloucester’s” or hereford or “hereford’s” or hull or “hull’s” or lancaster or “lancaster’s” or leeds* or leicester or “leicester’s” or (lincoln not nebraska*) or (“lincoln’s” not nebraska*) or (liverpool not (new south wales* or nsw)) or (“liverpool’s” not (new south wales* or nsw)) or ((london not (ontario* or ont or toronto*)) or (“london’s” not (ontario* or ont or toronto*)) or manchester or “manchester’s” or (newcastle not (new south wales* or nsw)) or (“newcastle’s” not (new south wales* or nsw)) or norwich or “norwich’s” or nottingham or “nottingham’s” or oxford or “oxford’s” or peterborough or “peterborough’s” or plymouth or “plymouth’s” or portsmouth or “portsmouth’s” or preston or “preston’s” or ripon or “ripon’s” or salford or “salford’s” or salisbury or “salisbury’s” or sheffield or “sheffield’s” or southampton or “southampton’s” or st albans or stoke or “stoke’s” or sunderland or “sunderland’s” or truro or “truro’s” or wakefield or “wakefield’s” or wells or westminster or “westminster’s” or winchester or “winchester’s” or wolverhampton or “wolverhampton’s” or (worcester not (massachusetts* or boston* or harvard*)) or (“worcester’s” not (massachusetts* or boston* or harvard*)) or (york not (“new york*” or ny or ontario* or ont or toronto*)) or (“york’s” not (“new york*” or ny or ontario* or ont or toronto*))))).ti,ab,in. (1,359,981)
-
(bangor or “bangor’s” or cardiff or “cardiff’s” or newport or “newport’s” or st asaph or “st asaph’s” or st davids or swansea or “swansea’s”).ti,ab,in. (53,255)
-
(aberdeen or “aberdeen’s” or dundee or “dundee’s” or edinburgh or “edinburgh’s” or glasgow or “glasgow’s” or inverness or (perth not australia*) or (“perth’s” not australia*) or stirling or “stirling’s”).ti,ab,in. (202,500)
-
(armagh or “armagh’s” or belfast or “belfast’s” or lisburn or “lisburn’s” or londonderry or “londonderry’s” or derry or “derry’s” or newry or “newry’s”).ti,ab,in. (25,051)
-
39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 (2,588,203)
-
(exp africa/or exp americas/or exp antarctic regions/or exp arctic regions/or exp asia/or exp australia/or exp oceania/) not (exp united kingdom/or europe/) (2,809,225)
-
47 not 48 (2,444,827)
-
21 and 37 and 49 (1669)
-
limit 50 to english language (1648)
Embase (Ovid)
Actual database searched: Embase Classic plus Embase.
Search date: 13 February 2020.
Date range searched: 1947 to week 6 2020.
Search strategy
-
primary prevention/ (39,911)
-
primary prevention.ti,ab,kw. (28,624)
-
1 or 2 (53,201)
-
exp cardiovascular disease/or exp cerebrovascular accident/ (4,341,232)
-
(CVD or cardiovascular* or coronary* or heart* or myocardial* or cardiac* or stroke* or cerebrovascular or atherosclerosis or arteriosclerosis or vascular or hypertension or blood pressure or cholesterol or lipid*).ti,ab,kw. (4,519,286)
-
4 or 5 (6,250,137)
-
3 and 6 (27,199)
-
*cardiovascular disease/pc or exp *coronary artery disease/pc or exp *heart infarction/pc or *heart failure/pc or exp *heart arrest/pc or exp *cerebrovascular accident/pc (38,096)
-
((prevent* or reduc* or lower* or decreas* or change* or effect or effects or progression or level* or incidence) adj10 (CVD or cardiovascular* or coronary* or heart* or myocardial or cardiac* or stroke* or cerebrovascular or atherosclerosis or arteriosclerosis or vascular or hypertension or blood pressure or cholesterol or lipid*)).ti. (322,379)
-
((prevent* or reduc* or lower* or decreas* or change* or effect or effects) adj6 (CVD or cardiovascular* or coronary* or heart* or myocardial or cardiac* or stroke* or cerebrovascular or atherosclerosis or arteriosclerosis or vascular or hypertension or blood pressure or cholesterol or lipid*)).ti,ab,kw. (971,868)
-
(((prevent* or reduc* or lower* or decreas* or change* or effect or effects) adj2 (mortality or death)) and (CVD or cardiovascular* or coronary* or heart* or myocardial or cardiac* or stroke* or cerebrovascular or atherosclerosis or arteriosclerosis or vascular or hypertension or blood pressure or cholesterol or lipid*)).ti,ab,kw. (46,849)
-
7 or 8 or 9 or 10 or 11 (1,104,950)
-
quality adjusted life year/ (25,666)
-
quality adjusted life year*.mp. (28,653)
-
(QALY or QALYS).mp. (19,438)
-
(utilit* adj2 (score* or value* or health)).mp. (9679)
-
(EuroQol or Euro Qol or Euro-Qol or EQ 5D* or EQ-5D* or EQ5D*).mp. (20,064)
-
(health utilities index or health-utilities-index or health utilities index or health utility index or HUI).mp. (3596)
-
(SF 6D* or SF-6D* or SF6D* or SF-12* or SF 12* or SF 12* or short form health survey).mp. (15,587)
-
(short form 36* or SF36* or SF-36* or SF 36*).mp. (47,782)
-
economic evaluation/ (15,337)
-
cost benefit analysis/ (83,353)
-
cost effectiveness analysis/ (147,711)
-
cost utility analysis/ (9425)
-
(cost effective* or cost utilit* or cost benefit* or cost consequence*).mp. (315,249)
-
(pharmacoeconomic* or pharmaco-economic* or economic analy* or economic evaluation*).mp. (112,824)
-
(ICER* or incremental cost-effectiveness ratio*).mp. (15,431)
-
(cost adj2 (evaluation* or analy* or study or studies or effective* or benefit* or utili*)).ti,ab,kw. (226,088)
-
(economic adj2 (evaluation* or analy* or study or studies)).ti,ab,kw. (29,362)
-
((markov or decision or economic) adj3 model*).mp. (41,674)
-
or/13-30 (497,035)
-
exp United Kingdom/ (443,429)
-
(national health service* or nhs*).ti,ab,in,ad. (336,697)
-
(english not ((published or publication* or translat* or written or language* or speak* or literature or citation*) adj5 english)).ti,ab. (43,535)
-
(gb or “g.b.” or britain* or (british* not “british columbia”) or uk or “u.k.” or united kingdom* or (england* not “new england”) or northern ireland* or northern irish* or scotland* or scottish* or ((wales or “south wales”) not “new south wales”) or welsh*).ti,ab,jx,in,ad. (3,253,878)
-
(bath or “bath’s” or ((birmingham not alabama*) or (“birmingham’s” not alabama*) or bradford or “bradford’s” or brighton or “brighton’s” or bristol or “bristol’s” or carlisle* or “carlisle’s” or (cambridge not (massachusetts* or boston* or harvard*)) or (“cambridge’s” not (massachusetts* or boston* or harvard*)) or (canterbury not zealand*) or (“canterbury’s” not zealand*) or chelmsford or “chelmsford’s” or chester or “chester’s” or chichester or “chichester’s” or coventry or “coventry’s” or derby or “derby’s” or (durham not (carolina* or nc)) or (“durham’s” not (carolina* or nc)) or ely or “ely’s” or exeter or “exeter’s” or gloucester or “gloucester’s” or hereford or “hereford’s” or hull or “hull’s” or lancaster or “lancaster’s” or leeds* or leicester or “leicester’s” or (lincoln not nebraska*) or (“lincoln’s” not nebraska*) or (liverpool not (new south wales* or nsw)) or (“liverpool’s” not (new south wales* or nsw)) or ((london not (ontario* or ont or toronto*)) or (“london’s” not (ontario* or ont or toronto*)) or manchester or “manchester’s” or (newcastle not (new south wales* or nsw)) or (“newcastle’s” not (new south wales* or nsw)) or norwich or “norwich’s” or nottingham or “nottingham’s” or oxford or “oxford’s” or peterborough or “peterborough’s” or plymouth or “plymouth’s” or portsmouth or “portsmouth’s” or preston or “preston’s” or ripon or “ripon’s” or salford or “salford’s” or salisbury or “salisbury’s” or sheffield or “sheffield’s” or southampton or “southampton’s” or st albans or stoke or “stoke’s” or sunderland or “sunderland’s” or truro or “truro’s” or wakefield or “wakefield’s” or wells or westminster or “westminster’s” or winchester or “winchester’s” or wolverhampton or “wolverhampton’s” or (worcester not (massachusetts* or boston* or harvard*)) or (“worcester’s” not (massachusetts* or boston* or harvard*)) or (york not (“new york*” or ny or ontario* or ont or toronto*)) or (“york’s” not (“new york*” or ny or ontario* or ont or toronto*))))).ti,ab,in,ad. (2,501,645)
-
(bangor or “bangor’s” or cardiff or “cardiff’s” or newport or “newport’s” or st asaph or “st asaph’s” or st davids or swansea or “swansea’s”).ti,ab,in,ad. (99,953)
-
(aberdeen or “aberdeen’s” or dundee or “dundee’s” or edinburgh or “edinburgh’s” or glasgow or “glasgow’s” or inverness or (perth not australia*) or (“perth’s” not australia*) or stirling or “stirling’s”).ti,ab,in,ad. (346,979)
-
(armagh or “armagh’s” or belfast or “belfast’s” or lisburn or “lisburn’s” or londonderry or “londonderry’s” or derry or “derry’s” or newry or “newry’s”).ti,ab,in,ad. (46,508)
-
or/32-39 (4,040,180)
-
(exp “arctic and antarctic”/or exp oceanic regions/or exp western hemisphere/or exp africa/or exp asia/) not (united kingdom/or europe/) (3,023,117)
-
40 not 41 (3,841,327)
-
12 and 31 and 42 (3512)
-
limit 43 to english language (3466)
-
limit 44 to conference abstract status (763)
-
44 not 45 (2703)
Appendix 2 Studies excluded at full-text stage
| Study | Reason for exclusion |
|---|---|
| Briggs et al.61 | Secondary prevention of CVD |
| Calvert et al.62 | Secondary prevention of CVD |
| Caro and Klittich63 | Paper not available and no abstract |
| Cowie et al.64 | Secondary prevention of CVD |
| Dalton and Bull65 | A letter |
| De Smedt et al.66 | Countries do not report separate data. Costs are in Euros |
| De Smedt et al.67 | Secondary prevention of CVD |
| Fletcher et al.68 | Model is based on a review; not a within clinical trial analysis |
| Griffin et al.69 | A full economic evaluation was not conducted, the study looked at the feasibility of conducting a cost-effectiveness analysis in a future clinical trial |
| Griffiths et al.70 | Treatment and not primary prevention |
| Mihaylova et al.71 | Secondary prevention of CVD |
| Ismail et al.72 | This is the full report; the later study was included and is the cost-effectiveness paper |
| Jacobs et al.73 | Study was conducted in Belgium |
| Jones et al.74 | Secondary prevention of CVD |
| Jönsson et al.75 | Presents only methods, there are no results |
| Kashef et al.76 | Not a full economic evaluation, no QALY data |
| Kim et al.49 | Secondary prevention of CVD |
| Lee et al.77 | Secondary prevention of CVD |
| Lindgren et al.78 | Not a full economic evaluation, no QALY data |
| Lowres et al.79 | Excluded as study conducted in Australia, but used treatment/outcomes data from a UK study |
| McInnes et al.80 | Secondary prevention of CVD |
| Mihaylova et al.81 | Secondary prevention of CVD |
| Rawles and Light82 | Not a full economic evaluation, no cost data |
| Remak et al.83 | Not primary prevention and data are pooled and not from one main trial |
| Rinciog et al.84 | Secondary prevention of CVD |
| Taylor et al.50 | Secondary prevention of CVD |
| Thom et al.85 | Secondary prevention of CVD |
| Wonderling et al.86 | Not a full economic evaluation, no QALY data |
Appendix 3 Critical appraisal of the economic evaluation studies using the CHEERS checklist
| CHEERS item | Study | |||||||
|---|---|---|---|---|---|---|---|---|
| Barton et al.55 | Ismail et al.58 | Lindgren et al.51 | Lindgren et al.52 | McConnachie et al.56 | Mistry et al.53 | Raikou et al.57 | Simmons et al.54 | |
| Title and abstract | ||||||||
| Title | Y | Y | Y | Y | Y | Y | Y | Y |
| Abstract | Y | Y | Y | Y | Y | Y | Y | Y |
| Introduction | ||||||||
| Background and objectives | Y | Y | Y | Y | Y | Y | Y | Y |
| Methods | ||||||||
| Target population and subgroups | Y | Y | Y | P | Y | P | Y | Y |
| Setting and location | Y | Y | Y | P | P | Y | Y | Y |
| Study perspective | Y | N | Y | Y | Y | Y | Y | Y |
| Comparators | Y | Y | Y | Y | Y | Y | Y | Y |
| Time horizon | Y | P | Y | N | Y | Y | Y | Y |
| Discount rate | N/A | N | Y | Y | Y | Y | Y | Y |
| Choice of health outcomes | Y | Y | Y | Y | Y | Y | Y | Y |
| Measurement of effectiveness | Y | Y | Y | Y | Y | Y | Y | Y |
| Measurement and valuation of preference-based outcomes | Y | N | Y | Y | N | P | P | P |
| Estimating resources and costs | Y | Y | Y | P | Y | Y | Y | Y |
| Currency, price date and conversion | Y | P | Y | Y | Y | Y | Y | Y |
| Choice of model | N/A | N/A | Y | Y | N/A | Y | N/A | Y |
| Assumptions | Y | Y | Y | P | Y | Y | P | P |
| Analytical methods | Y | Y | Y | P | Y | Y | P | Y |
| Results | ||||||||
| Study parameters | Y | Y | P | Y | Y | Y | P | P |
| Incremental costs and outcomes | Y | Y | Y | Y | P | Y | Y | Y |
| Characterising uncertainty | Y | P | Y | Y | Y | Y | Y | Y |
| Characterising heterogeneity | N | N | N | N | Y | P | Y | N |
| Discussion | ||||||||
| Study findings | Y | Y | Y | Y | Y | Y | Y | Y |
| Limitations | Y | Y | Y | N | Y | P | Y | Y |
| Generalisability | Y | P | P | Y | Y | P | P | Y |
| Other | ||||||||
| Source of funding | Y | Y | Y | Y | Y | Y | Y | Y |
| Conflicts of interest | N | Y | Y | Y | Y | Y | Y | Y |
| Total (n) | ||||||||
| Y | 22 | 17 | 23 | 18 | 22 | 21 | 20 | 22 |
| N | 2 | 4 | 1 | 3 | 1 | 0 | 0 | 1 |
| P | 0 | 4 | 2 | 5 | 2 | 5 | 5 | 3 |
| N/A | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
Appendix 4 Philips et al.’s quality assessment checklist for studies that included an economic model
| Philips et al.’s48 criterion | Study | |||
|---|---|---|---|---|
| Lindgren et al.52 | Lindgren et al.51 | Mistry et al.53 | Simmons et al.54 | |
| Structure | ||||
| 1. Is there a clear statement of the decision problem? | Y | Y | Y | Y |
| 2. Is the objective of the model specified and consistent with the stated decision problem? | Y | Y | Y | Y |
| 3. Is the primary decision-maker specified? | N | Y | Y | Y |
| 4. Is the perspective of the model stated clearly? | Y | Y | Y | Y |
| 5. Are the model inputs consistent with the stated perspective? | Y | Y | Y | Y |
| 6. Has the scope of the model been stated and justified? | Y | Y | Y | Y |
| 7. Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | P | Y | Y | Y |
| 8. Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | Y | Y | Y |
| 9. Are the sources of the data used to develop the structure of the model specified? | P | P | Y | Y |
| 10. Are the causal relationships described by the model structure justified appropriately? | UNC | P | P | P |
| 11. Are the structural assumptions transparent and justified? | UNC | P | P | P |
| 12. Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | UNC | P | P | P |
| 13. Is there a clear definition of the options under evaluation? | Y | Y | Y | Y |
| 14. Have all feasible and practical options been evaluated? | P | Y | Y | Y |
| 15. Is there justification for the exclusion of feasible options? | UNC | UNC | UNC | UNC |
| 16. Is the chosen model type appropriate given the decision problem and specified casual relationships within the model? | Y | Y | Y | Y |
| 17. Is the time horizon of the model sufficient to reflect all important differences between the options? | UNC | Y | Y | Y |
| 18. Are the time horizon of the model, the duration of treatment and the treatment effect described and justified? | UNC | Y | Y | Y |
| 19. Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | Y | Y | Y |
| 20. Is the cycle length defined and justified in terms of the natural history of disease? | N | Y | Y | N/A |
| Data | ||||
| 21. Are the data identification methods transparent and appropriate given the objectives of the model? | P | P | P | P |
| 22. Where choices have been made between data sources are these justified appropriately? | UNC | UNC | UNC | P |
| 23. Has particular attention been paid to identifying data for the important parameters of the model? | UNC | P | P | P |
| 24. Has the quality of the data been assessed appropriately? | UNC | UNC | UNC | UNC |
| 25. Where expert opinion has been used are the methods described and justified? | N | UNC | UNC | UNC |
| 26. Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | P | P | Y | Y |
| 27. Is the choice of baseline data described and justified? | P | P | P | Y |
| 28. Are transition probabilities calculated appropriately? | UNC | Y | Y | N/A |
| 29. Has a half-cycle correction been applied to both costs and outcomes? | N | N | N | N/A |
| 30. If not, has the omission been justified? | N | N | N | N/A |
| 31. If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | P | Y | Y | Y |
| 32. Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | UNC | P | P | Y |
| 33. Have alternative extrapolation assumptions been explored through sensitivity analysis? | P | Y | Y | Y |
| 34. Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | UNC | Y | Y | Y |
| 35. Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis | UNC | P | P | Y |
| 36. Are the costs incorporated into the model justified? | P | Y | Y | Y |
| 37. Has the source for all costs been described? | P | Y | Y | Y |
| 38. Have discount rates been described and justified given the target decision-maker? | Y | Y | Y | Y |
| 39. Are the utilities incorporated into the model appropriate? | P | P | Y | Y |
| 40. Is the source of utility weights referenced? | Y | Y | P | Y |
| 41. Are the methods of derivation for the utility weights justified? | P | P | P | P |
| 42. Have all data incorporated into the model been described and referenced in sufficient detail? | P | P | P | P |
| 43. Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate?) | UNC | UNC | UNC | UNC |
| 44. Is the process of data incorporation transparent? | UNC | UNC | UNC | UNC |
| 45. If data have been incorporated as distributions, has the choice of distributions for each parameter been described and justified? | UNC | P | UNC | UNC |
| 46. If data have been incorporated as distributions, is it clear that second order uncertainty is reflected? | UNC | P | UNC | UNC |
| 47. Have the four principal types of uncertainty been addressed? | UNC | P | UNC | UNC |
| 48. If not, has the omission of particular forms of uncertainty been justified? | UNC | N | N | N |
| 49. Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | UNC | P | UNC | P |
| 50. Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | UNC | P | UNC | UNC |
| 51. Has heterogeneity been dealt with by running the model separately for different subgroups? | N | N | P | N |
| 52. Are the methods of assessment of parameter uncertainty appropriate? | UNC | UNC | UNC | UNC |
| 53. If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | P | P | UNC | P |
| 54. Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | UNC | P | UNC | P |
| 55. Are any counterintuitive results from the model explained and justified? | UNC | P | UNC | P |
| 56. If the model has been calibrated against independent data, have any differences been explained and justified? | N | UNC | UNC | P |
| 57. Have the results been compared with those of previous models and any differences in results explained? | P | P | P | P |
| Total (n) | ||||
| Y | 11 | 23 | 25 | 27 |
| N | 7 | 4 | 3 | 2 |
| P | 15 | 22 | 13 | 14 |
| UNC | 24 | 8 | 16 | 10 |
| N/A | 0 | 0 | 0 | 4 |
List of abbreviations
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CVD
- cardiovascular disease
- ICER
- incremental cost-effectiveness ratio
- NICE
- National Institute for Health and Care Excellence
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
