Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0611-20008. The contractual start date was in July 2013. The final report began editorial review in October 2019 and was accepted for publication in April 2021. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Velikova et al. This work was produced by Velikova et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Velikova et al.
SYNOPSIS
Overview
This research programme was established to develop, implement and evaluate a system for patients to self-report adverse events (AEs) during and after cancer treatments. The programme was established in 2011 with a National Institute for Health Research (NIHR) Programme Development grant, followed in July 2013 by a grant from the NIHR Programme Grants for Applied Research programme.
The eRAPID (electronic patient self-Reporting of Adverse-events: Patient Information and aDvice) system is a secure online system that:
-
can be adapted for patients receiving chemotherapy, surgery and radiotherapy
-
allows patients to self-report AEs from home or hospital
-
can be integrated into routine care by documenting AEs in existing electronic patient records (EPRs) in real time and generating clinician notifications for severe AEs
-
provides patient advice on AE management (guiding self-management or hospital contact).
The overall aims of the eRAPID approach were to improve the safe delivery of cancer treatments and enhance patient care and experiences by integrating AE reports with timely symptom management. The intervention was developed and evaluated in the three main cancer treatment modalities: systemic cancer treatment (e.g. chemotherapy and targeted therapies), pelvic radiotherapy and upper gastrointestinal cancer surgery.
Background
Over 250,000 patients in the UK are diagnosed with cancer each year. Cancer is treated with multimodality treatments. Most patients will be treated with surgery, around 50% will have radiotherapy and increasing numbers receive systemic treatment, such as chemotherapy, monoclonal antibodies and targeted agents. 1 An estimated 1.8 million people in the UK are living with and beyond cancer. 2 Cancer has a significant financial impact on the NHS, which has an estimated expenditure on cancer services of £5.1B (2008/9). 3
Cancer treatments can cause acute and long-term AEs. An AE is any unfavourable sign, symptom or disease temporally associated with the use of a medical treatment/intervention. We use the accepted term AE to indicate side effects, symptoms and toxicities of cancer treatments.
Reporting of AEs is an essential requirement in clinical trials to provide data on treatment safety, prescribing and patient information. The standard system for AE reporting in cancer trials is the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE). 4 Originally developed for chemotherapy, CTCAE has been recommended since 2003 for reporting radiotherapy AEs and surgical complications. CTCAE relies on clinician’s AE interpretation, focusing on ‘safety’ issues rather than patient experiences. 5
In routine oncology practice, AEs are typically monitored informally and are rarely systematically documented. The UK National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report on patients dying within 30 days of systemic therapy found that in 35% of cases no chemotherapy AEs were recorded, 43% had severe AEs and 17% delayed seeking advice for at least 24 hours. 6 The Department of Health and Social Care (DHSC) recommended improvements in the quality/safety of chemotherapy services through better data collection on chemotherapy activity/outcomes and better patient information on treatment benefits/toxicities. 1,3 However, 18% of cancer patients receiving chemotherapy present to emergency services within 14 days of a scheduled hospital visit for symptom management. 2,7,8 Strategies to reduce preventable emergency admissions can reduce the cost of care. 7
In the UK, an estimated 17,000 patients are treated annually with radical chemoradiotherapy for pelvic cancers (e.g. gynaecological or lower gastrointestinal malignancies). 9 Radical radiotherapy is frequently the treatment of choice for prostate cancer. Patients typically have an intermediate/good prognosis (i.e. with a 5-year survival rate of 40–89%) but, because of the unavoidable inclusion of normal tissue in the radiotherapy treatment volume, there can be significant acute, consequential and late AEs. These include pelvic radiation disease (30%; symptoms include bowel urgency, diarrhoea, pain and urinary urgency/frequency) and altered sexual/hormonal function (30–45%). 10 These significantly impair patients’ quality of life (QoL) and increase the utilisation of health services. Effective approaches are recommended for radiotherapy-related gastrointestinal symptoms, but routine AE monitoring is necessary to identify and direct patients to specialised services. 11–14 Furthermore, the need to systematically document radiotherapy AEs is critical to the evaluation of modern technical radiotherapy [such as intensity-modulated radiotherapy and image-guided radiotherapy], which promises higher tumour control and fewer and less severe AEs. The potential benefits need to be proven; this will mainly be achieved through prospective practice audit rather than through trials.
Surgery for upper gastrointestinal cancer (e.g. pancreaticobiliary, oesophageal and gastric cancer) is centralised, with one hospital providing services within a cancer network. In the postoperative period, patients need significant specialist care, which may be unavailable at their local hospitals. They frequently call the cancer centre post discharge, with a 28-day re-admission rate between 5% and 15%. 15 Around 50% of patients are re-admitted within 12 months of surgery for a variety of reasons, including recurrent disease. 16 A recently published trial on post thoracic surgery17 suggested that automated remote monitoring of patient symptoms with alerts to clinicians can improve symptom control.
The need to monitor cancer treatment AEs by cancer clinicians is at odds with health care that relies increasingly on self-management/home-based care. Long-term follow-up of cancer patients is becoming a burden on NHS resources. 18 Remote monitoring of AEs could offer an alternative to hospital-based follow-up, ensuring that AE information is routinely collected and individual patient needs are met by appropriate advice/clinical support. Such an approach will educate patients about treatment-related AEs, support self-management and give patients timely information on when and who to contact. These aims are fundamental to improving cancer patient care. 19
Patient-reported outcome measures
Patient-reported outcome measures (PROMs) are measurements of any aspect of a patient’s health status that comes directly from the patient, without interpretation by anyone else. 20 A range of PROMs have been used as self-reporting tools for symptom monitoring, originally completed in the outpatient clinics and now electronically both in hospitals and from home (relying on patient devices, e.g. home desktop computers, laptops, tablets or smartphones). A growing body of evidence supports the measurement of symptoms, functioning and health-related-quality of life (HRQoL) in clinical trials and patient care. 21–23 Systematic reviews have found that routine use of PROMs in clinical practice improves symptom monitoring, physician–patient communication and decision-making. 24–27 Our trial in chemotherapy showed better communication, symptom control and patient well-being when oncologists used PROMs in consultations. 28 In addition, oncologists expressed the need to see severity-graded AEs in the existing PROMs system. 29
Pioneering research using CTCAE items adapted for patient self-report has shown concordance with clinician-evaluated AEs but more patient-reported data on mild AEs. 30,31 In 2009, the US National Cancer Institute commissioned an ongoing programme [the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™)]32 to adapt CTCAE criteria for patient self-reporting. The ultimate objective is for patient self-reporting to become part of the Common Terminology Criteria for Adverse Events v5.0. 33 The PRO-CTCAE has now been developed and validated and is available for wider use in clinical trials and oncology practice. 32
There is rapidly growing interest to integrate patient self-reporting of symptoms and AEs into routine clinical practice for monitoring clinical status. A growing body of evidence demonstrates that clinicians miss about half of their patients’ symptoms during treatment. 34 The consequences of missing symptoms include patient suffering owing to poor symptom control, missed treatments, emergency department visits and hospitalisations. 35
Since 2015, a number of well-designed randomised controlled trials (RCTs) outside the UK36–38 have shown that systematic monitoring of patients’ symptoms using PROMs improves patient–clinician communication, clinician awareness of symptoms, symptom management, patient satisfaction, QoL and overall survival in advanced cancers. Despite the demonstrated benefits, there are challenges in integrating patient self-reporting into practice for monitoring owing to the need to modify existing clinical processes.
Electronic and mobile reporting technology
Our research of 20 years (> 3000 patients) shows that patients can routinely complete PROMs on touch-screen computers in the clinic. 39 Patients are willing to use PROMs from home through the internet or mobile devices. 40–43 Cancer Care Ontario (Toronto, ON, Canada) has an online system for interactive symptom assessment during treatment [Interactive Symptom Assessment and Collection (ISAAC)]. 44 Mobile telephones have been used to collect patient-reported data on chemotherapy AEs in Scotland and in Oxford, UK. 45,46 A growing body of literature supports telehealth and remote monitoring as a cost-effective approach in the management of chronic conditions, but the evidence remains inconclusive. 47–49 A growing number of web platforms [e.g. HealthUnlocked (HealthUnlocked, London, UK) and PatientsLikeMe (PatientsLikeMe, Cambridge, MA, USA)] provide direct patient support without integration in routine care.
Accessibility to patient population
In January to February 2020, 96% of households in the UK had access to the internet (as reported by the Office for National Statistics). 50 In our research (NIHR Research for Patient Benefit grant PB-PG-0107-12239), patients aged > 65 years and those of lower socioeconomic status declined participation in web-based studies because of lack of internet access.
Alignment with NHS policy
The NHS White Paper (2010)51 recommends wider use of PROMs and patient experience data to put patients at the heart of the service. Our approach is in line with the NHS agenda, emphasising wider, effective use of PROMs and improved use of technology, telehealth, remote monitoring and self-care. The DHSC has recently reported results from the Whole System Demonstrators programme,52 showing that telecare/telehealth helps people manage their health. A project is underway in elective surgery collecting PROMs to evaluate NHS organisations’ performance and quality of care. 26 The DHSC recommended the introduction of an innovative quality-of-life metric to track and respond to the long-term impact of cancer. 53 The Independent Cancer Taskforce, in its strategy for England 2015–2053 and NHS Long Term Plan,54 recommended the introduction and implementation of QoL data collection for cancer survivors in England. Pilot projects are underway looking at electronic methods of patient self-reported questionnaires.
The need for applied health research
An increasing amount of evidence between 2004 and 2013 suggested that there are significant advantages in improving AE reporting in patient care (e.g. earlier detection and timely/appropriate AE management, supporting patient self-management and improving symptom control). Since 2014, further evidence has been published, from RCTs and population data, showing patient benefits from online reporting of AEs and other PROMs. 37,38 The studies confirm better symptom control, reduced visits to emergency departments and even improved survival of patients with advanced cancers. 37,38 Those recent studies were performed in the USA,36 Canada,55 Australia56 and France. 38 There is relatively limited experience of this approach in the UK,57 particularly of investigating the integration of AE online reporting in electronic records and the addition of patient online self-reporting to the existing acute oncology patient care. There is a need for the development and robust evaluation of a feasible cost-effective model for NHS cancer care. Furthermore, with the ever-increasing wider use of mobile technology, an increasing number of commercial applications (apps) (e.g. smartphone apps) are available and being used by patients and organisations without robust research on their impact on patients, families and health-care professionals (HCPs).
A NIHR-funded applied health research programme, the eRAPID research programme, was set up in 2011, and ran until 2019, to address a range of issues in online AE monitoring in oncology, applicable to the NHS, such as:
-
the introduction of a secure electronic platform with real-time integration with EPRs
-
development of a pool of items for patient AE reporting consistent with CTCAE severity grades, with an algorithm for evidence-based patient advice and clinician alerts/guidelines
-
integration of patient self-monitoring into existing care pathways, training and support for responsible staff
-
evaluation of the feasibility, patient/staff acceptability, clinical effectiveness, safety and cost-effectiveness of the eRAPID model.
An applied health research programme: eRAPID
The eRAPID research programme aimed to develop and evaluate an online system for patients to self-report AEs during and after cancer treatments. Essential features of the system were envisaged to be the following:
-
AE reporting from home/hospital using patients’ own electronic devices
-
integration in routine care by documenting AEs in existing EPRs in real time and generating alerts to HCPs for severe AEs
-
patient advice on self-management of mild AE.
The overall aims of the eRAPID approach are to improve the safe delivery of cancer treatments and enhance patient care/experiences by integrating AE reports with timely symptom management. eRAPID is expected to benefit patients (e.g. better self-management of mild AEs and earlier detection/treatment of severe AEs), clinicians (e.g. improved AE documentation and support for patient management) and the NHS (e.g. reducing costs from hospital contacts and admissions).
Programme structure/overview
The eRAPID intervention was developed and tested in the three main cancer treatment modalities:
-
systemic treatment (e.g. chemotherapy and targeted therapies)
-
pelvic radiotherapy
-
upper gastrointestinal cancer surgery.
The programme is organised into five work packages (WPs) applied across the treatment modalities:
-
WP 1 – electronic platform: implement a secure, flexible electronic platform for patient AE reporting, with real-time AE documentation in EPRs.
-
WP 2 – patients: AEs items and advice. Develop patient-reported adverse event (PRAE) items, with defined severity grades and evidence-based patient advice and alerts.
-
WP 3 – HCPs and care pathways: understand/map the patient pathways aiming to integrate eRAPID and identify key HCPs to deliver it.
-
WP 4 – feasibility pilot studies: pilot eRAPID, assessing patient and clinician feasibility/acceptability.
-
WP 5 – large-scale evaluation: a RCT to establish clinical effectiveness and cost-effectiveness in systemic treatment.
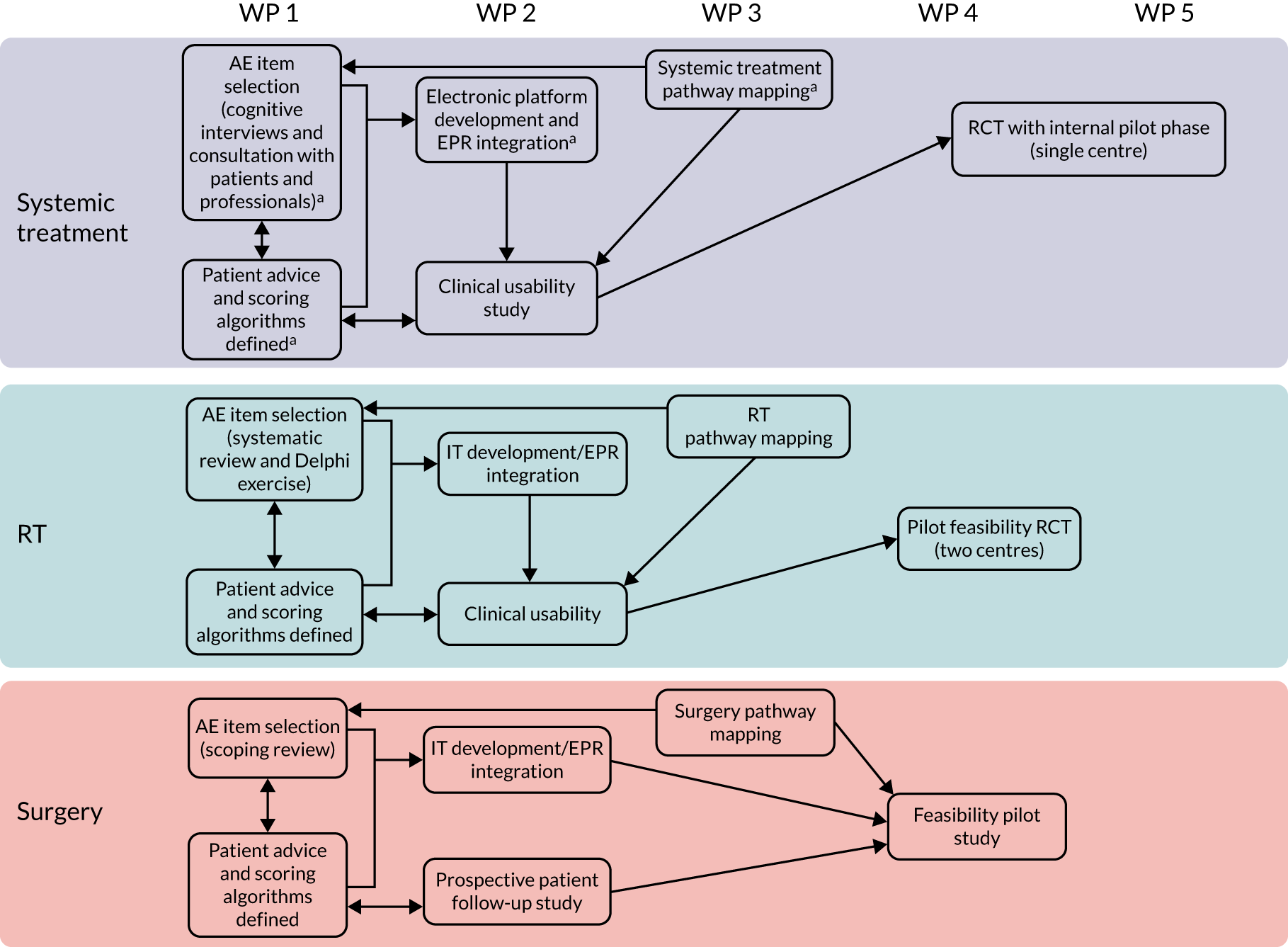
An overview of the eRAPID research programme pathways can be seen in Figure 1. Table 1 provides a more detailed description of the WPs, including changes to the original plans and completion times.
FIGURE 1.
The eRAPID research programme pathway diagram. a, Work started during the eRAPID NIHR Programme Development grant. IT, information technology; RT, radiotherapy.
| WP | Systemic treatment | Radiotherapy | Surgery |
|---|---|---|---|
| 1: Electronic platform | |||
| 2: Patient AE items and advice |
|
|
|
| 3: HCPs and care pathways |
|
|
|
| 4: Feasibility pilot studies |
|
|
|
| 5: Large-scale evaluation trial |
|
|
|
The research plan follows the Medical Research Council (MRC) framework for complex interventions and the whole-system informing self-management engagement (WISE) model, ensuring that patients are informed, HCPs are trained and response services are appropriately structured. 58,59 WPs 1–3 constitute the developmental phases of the MRC framework (identifying the active components of the eRAPID intervention, modelling process and outcomes).
The three clinical areas/treatment modalities need AE reporting for different reasons (acute setting during treatment or intermediate/long-term follow-up) and have different levels of routine use of AE reporting.
Systemic treatment
Chemotherapy (using cytotoxic drugs) remains the main systemic cancer treatment, despite significant AEs. Chemotherapy increases cure rates in high-risk, early breast and colorectal cancer following surgery, and in metastatic cancers chemotherapy provides disease and symptom control. Chemotherapy utilisation has significantly increased over a 4-year period (60% increase between 2003 and 2007, from around 40,000 to 65,000 programmes of chemotherapy per annum). 1 Chemotherapy drugs cause significant acute toxicity: neutropenia with risk of serious infections, gastrointestinal, fatigue, hair loss, and skin and neurological toxicity. In a trial of adjuvant breast cancer chemotherapy, > 60% of patients experienced at least one severe AE (grade 3 or 4). 60 Methods for detecting and recording chemotherapy AEs in routine practice are variable and often insufficient, as highlighted by the NCEPOD confidential enquiry. 6 The National Chemotherapy Advisory Group has recommended standardised processes for recording AEs/toxicities during chemotherapy and proactive targeted support services to identify problems before they become serious. 61
We aimed to implement eRAPID during systemic treatment to ensure timely detection/recording of AEs, better patient information/support, improved patient safety by encouraging self-management of mild AEs, appropriate hospital contacts and timely hospital admissions for serious AEs.
The key research questions were:
-
Is it feasible to collect routine AE data during systemic treatment from patients’ homes and in clinics?
-
Will oncology HCPs review eRAPID reports during decision-making processes?
-
Will the eRAPID intervention lead to clinical benefits (better control of AEs), increased patient safety and better patient experiences?
-
Will the eRAPID intervention be cost-effective?
WPs 1 and 2 were completed during the NIHR Programme Development grant period (2011–12). During the programme, we planned the completion of WP 3 (finalising AE items, clinical algorithm and patient advice) and the completion of the combined feasibility/pilot randomised study of the full eRAPID intervention (WP 4) followed by a single-centre RCT in patients with early breast or colorectal cancer or any stage ovarian cancer.
Changes to the original plans
Instead of performing a sequential pilot study followed by a RCT, on completion of WPs 2 and 3 we decided to carry out a usability study to test all active components of the intervention together. Afterwards, with permission from NIHR, we carried out an internal pilot in the RCT, allowing more time-efficient progress to the large-scale RCT. After the internal pilot we decided to include metastatic colorectal cancer patients as their treatment and pathway was similar to the adjuvant group. In 2017, we also included metastatic breast cancer patients in an attempt to improve recruitment and complete the RCT on time.
Radiotherapy
The focus was on two main patient groups: prostate cancer patients undergoing radical radiotherapy and patients receiving pelvic radiotherapy (radical or adjuvant) with or without concurrent chemotherapy for gynaecological or lower gastrointestinal malignancies. Prostate cancer is the most common cancer in men and patients with localised cancer have good long-term prognoses, but have to live with long-term effects of radiotherapy. About 20% of patients will have long-term gastrointestinal side effects, and 30–45% will have post-radiotherapy sexual dysfunction, but the true extent of problems is probably underestimated, because AEs are rarely systematically recorded. Patients receiving concurrent chemoradiotherapy for gynaecological or lower gastrointestinal cancer experience more severe acute AEs, which require active management and support and often limit the delivery of chemotherapy.
The late effects of radiotherapy fully develop and affect patients months or years after treatment, when centralised follow-up in specialised radiotherapy clinics is infrequent. There is a need for a feasible cost-effective model to allow remote measurement of radiotherapy AEs. Such data can help patients get appropriate specialist support. Systematic data collection will allow comparison and evaluation of new radiotherapy approaches.
The key questions were:
-
Is it feasible to collect routine AE data during clinic visits and from patients’ homes after radiotherapy?
-
Can we implement eRAPID in different clinical/treatment settings?
-
Will eRAPID and management guidelines lead to better AE control and better patient experience?
-
Can we monitor late AEs and develop predictive radiotherapy AE models?
-
Can eRAPID data be linked with radiotherapy dosimetric and to treatment set-up information for future exploratory research?
The radiotherapy work was carried out at the Leeds site (Leeds Cancer Centre, Leeds Teaching Hospitals NHS Trust, Leeds, UK) and the Manchester site (The Christie Hospital, The Christie NHS Foundation Trust, Manchester, UK), allowing assessment of eRAPID feasibility in different settings. The research plans comprised WPs 1–4. For radiotherapy, we had an opportunity to build research capacity by involving our NIHR Academic Clinical Fellowship recipient at the Leeds site (Alexandra Gilbert, clinical oncology trainee). She completed a doctoral project on the eRAPID approach during and following radiotherapy for lower gastrointestinal and gynaecological cancers and addressed key questions 4 and 5. She holds a prestigious national Cancer Research UK Clinical Trial Fellowship Award.
Changes to the original plans
In 2015, after completing WP 3, we established that the radiotherapy techniques/doses and side effects differ substantially between prostate cancer patients receiving radiotherapy only and those in the chemotherapy arms. We decided to stratify the pilot study and increase the sample size to recruit 42 intervention-arm and 42 control-arm patients in the prostate radiotherapy-only arm, and 42 intervention-arm and 42 control-arm patients in the chemoradiotherapy arm. Two websites were introduced to more closely match local practices in terms of patient information, advice and available services.
Surgery
Upper gastrointestinal cancer surgery is highly specialised and centralised, often with significant post-operative morbidity that is managed centrally, far from patients’ homes. Upper gastrointestinal cancer surgery is associated with 30% recurrence rates in the first post-operative year when patients present with increasing symptoms and decreased function. Current follow-up for upper gastrointestinal malignancies focuses on symptom assessment and functions; routine imaging is not recommended. 62,63 Models for standardised reporting of surgical morbidity during post-operative recovery and follow-up that lead to improved symptom management and patient support and earlier diagnosis of recurrence are needed.
We aimed to develop the eRAPID information technology (IT) system for surgical teams to use after hospital discharge following major cancer-related upper gastrointestinal surgery. Provision of self-reported AE data from patients’ homes may allow timely detection of problems and access to specialist care and support. It can be hypothesised that PRAE data may supplement/replace regular telephone-based post-discharge consultations and hospital visits.
The key questions were:
-
Can we integrate eRAPID in a different hospital and clinical setting?
-
Can patients complete eRAPID from home after being discharged following major surgery?
-
Does eRAPID provide added value to routine surgical follow-up (i.e. better control of symptoms/morbidity)?
-
How does eRAPID inform service organisation?
This work, comprising WPs 1–4, was carried out by the surgical research team in the Bristol site (Bristol Royal Infirmary, University Hospitals Bristol NHS Foundation Trust, Bristol, UK). The pilot study was originally planned as a prospective, randomised, parallel-arm design with repeated measures, but with an option to consider quasi-experimental iterative designs if experience during WPs 2 and 3 suggested greater suitability of an alternative approach.
Changes to the original plans
Original plans included development, pathway mapping and usability work (WPs 1–3), followed by a small randomised pilot study in the Bristol site (WP 4). In 2015, following initial work on WPs 1–3, we proposed to undertake the development and mapping work prior to performing extensive usability and feasibility testing in a larger, two-centre, non-randomised pilot study in the Bristol and Birmingham sites. A larger pilot study was considered beneficial for three reasons. First, AEs (e.g. hospital re-admission, reoperation, sepsis, pneumonia) occur in relatively fewer patients undergoing major cancer-related upper gastrointestinal surgery than in patients undergoing systemic therapy, during which toxicity is common. Second, the use of electronic methods for patients to self-report AEs following surgery in the Bristol site was novel, whereas at the Leeds site clinicians are experienced in using the online platform to collect PROMs. Therefore, we needed to fully examine how HCPs interact with the eRAPID interface and the acceptability of the eRAPID IT system and its integration into the clinical pathway before proceeding to a randomised pilot study. In 2018, a second site, Birmingham, was added to the pilot study to explore the acceptance of the intervention in a different surgical oncology setting (where electronic AE reporting and monitoring is also novel) and to reflect a wider range of services. Third, the problems with IT integration with EPRs that we experienced in the Bristol site meant that a more thorough evaluation over a longer time period would be beneficial. Consequently, we undertook a larger, two-centre pilot study to allow comprehensive exploration of the feasibility and acceptability of the eRAPID surgery system at a clinical site at which the system had not been developed. The version of eRAPID tested at the Birmingham site was not integrated within the hospital EPR system. Therefore, data from the Birmingham site enabled full exploration of integration and ease of use of the online system where EPR system integration was not viable.
Programme management
Working groups were assembled for each treatment modality (systemic treatment, radiotherapy and surgery) to provide an ongoing forum for discussion, co-ordination and decision-making. The various groups consisted of co-applicants and additional expert advisors and met every 6 weeks. A Trial Steering Committee (TSC) was created, including independent members (as per NIHR requirements) alongside local senior researchers, clinicians and a patient representative. The TSC met every 4–6 months. The TSC was chaired by David Cameron, who drew on his extensive experience in managing large clinical trials as a senior medical oncologist and former National Cancer Research Institute director. Other independent members included Janet Dunn from the University of Warwick, who provided statistical expertise in design and analysis of trials, and Sara Faithful from the University of Surrey, who has a nursing background and an interest in the development of methodology for PROMs in radiotherapy. Virginia Cucchi was the patient and public representative.
In addition, an independent Data Monitoring and Ethics Committee (DMEC) was established to monitor the safety data and ethics of the RCT in systemic therapy. The DMEC had three members [a statistician (chairperson), medical oncologist and senior research nurse] and met every 6 months.
Structure and overview of report
The eRAPID research programme was conducted in three parallel treatment strands: (1) systemic treatment (chemotherapy), (2) radiotherapy and (3) surgery, with cross-cutting WPs underpinning activities in each area. This subsequent sections of this report present the research methods and results separately for the three treatment areas. This approach offers clear insight into the main differences in patient experiences and clinical pathways between treatment modalities and how eRAPID was adapted and assessed to reflect these variations.
Electronic platform
In Electronic platform, an overview of the electronic platform that was developed to deliver the eRAPID programme is provided. The choice of technological approach is explained in the context of the wider health informatics and EPR systems in place at the cancer centres participating in the eRAPID research programme. Further IT experiences and learning points are covered in more detail in Systemic treatment, Radiotherapy and Surgery.
Systemic treatment
In Systemic treatment, the systemic (chemotherapy) strand of the eRAPID research programme is described. The systemic work was the main focus on the research programme. The development of the intervention in this setting is described along with the lessons learnt from the initial clinical usability testing. The methods and results of the single-centre, large-scale RCT, which formally assessed the impact of eRAPID on patient symptoms, QoL and clinical process outcomes, are presented. The health economic analysis is included in the evaluation of the eRAPID intervention.
Radiotherapy
Radiotherapy describes the developmental work for the eRAPID intervention in pelvic radiotherapy conducted across two sites (Leeds and Manchester), including a systematic review of patient-reported measures in early prostate cancer, patient and staff interviews, and the Delphi consensus process to select items for online reporting during pelvic radiotherapy, engaging staff and patient advisors to develop the clinical algorithms, patient advice and websites design. The two-centre pilot RCT is reported in this section as well.
Surgery
Surgery describes the iterative development and feasibility evaluation in a pilot study of the eRAPID approach to support patients following major upper gastrointestinal surgery, led by the Bristol site.
Discussion
Discussion, conclusions and recommendations provides an overview of key results in the current research and clinical environment, reflections, learning points and recommendations for future research.
Electronic platform
Overview of the eRAPID intervention and supporting technology
The aim of the eRAPID IT WP was to create a secure and practical solution to (1) remotely capture electronic PROMs from patients outside the hospital, (2) deliver immediate severity-tailored AE management advice to patients and (3) give HCPs access in real time to patient-reported symptom data in EPRs alongside clinical information. In addition, it was vital that the system was capable of implementation across multiple hospital centres. The initial design of the eRAPID IT system was established during the NIHR Programme Development grant, and was subsequently refined and expanded in the current research programme. More detail on the technological methods can be found in the published papers arising from the programme grant: (1) Warrington et al. 64 describe the evolution of our group’s approach to the collection of electronic PROMs that led to the eRAPID research programme, (2) Holch et al. 65 provide a technical overview of the eRAPID IT solution and (3) Warrington et al. 66 report the results and implications of the eRAPID clinical field usability testing (the first full test of the system in a real-life clinical setting).
In summary, the technology underpinning the eRAPID intervention predominantly encompasses three inter-related elements:
-
internet-based questionnaire software (QTool version 2; University of Leeds, Leeds, UK) for collecting PROMs from patients and delivering severity-tailored AE advice
-
intranet-based web app interface allowing the transfer and display of PROMs data in local EPRs (QStore; University of Leeds, Leeds, UK)
-
patient-facing website, with password protection, hyperlinked to access online symptom reporting (on QTool), general symptom advice, and health and lifestyle information for patients receiving cancer treatment.
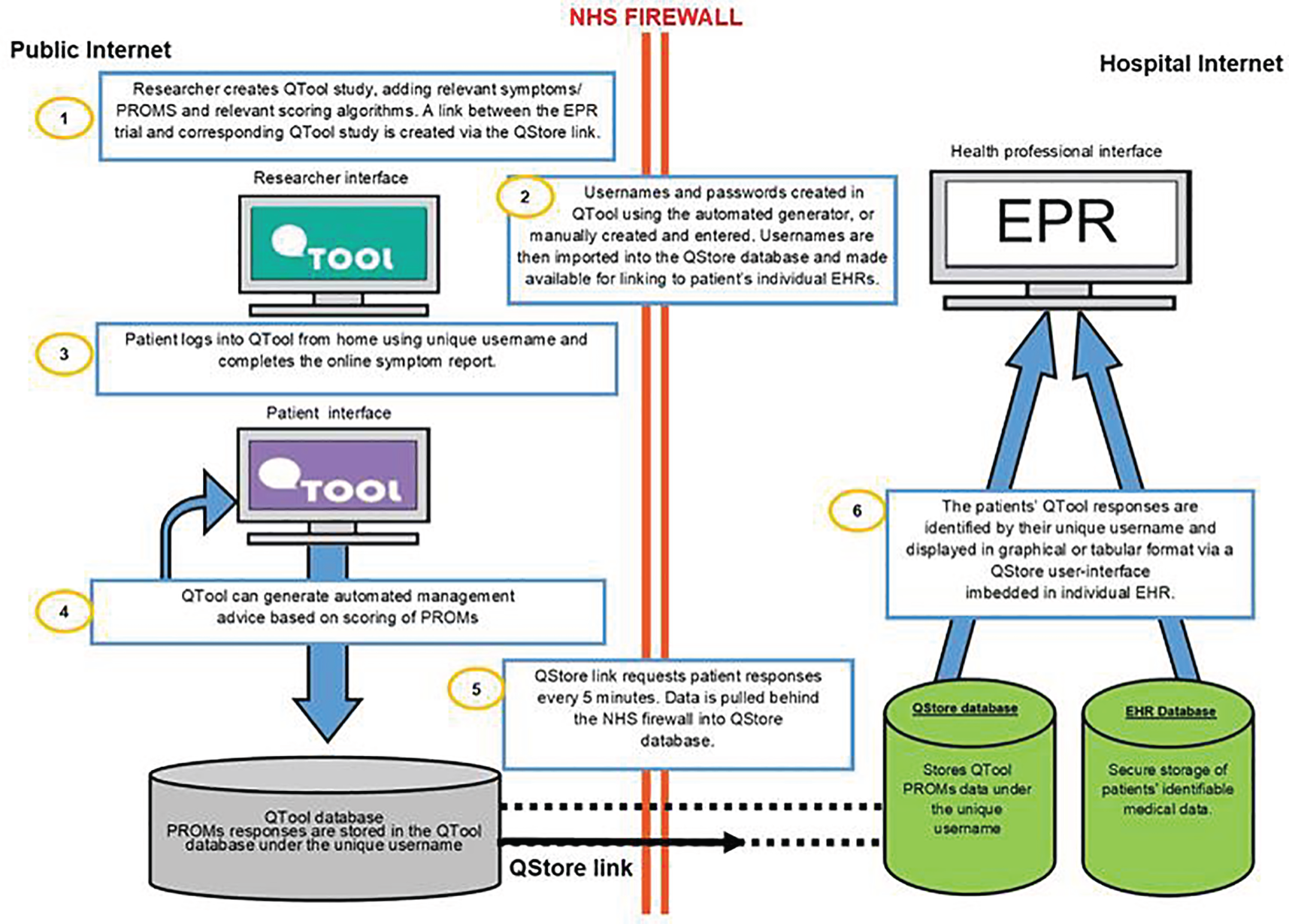
Diagrammatic summaries of the eRAPID intervention are provided in the figures in this section. Figure 2 presents a flow diagram of the eRAPID intervention processes from the perspectives of patient and staff users, and Figure 3 describes the flow of data and the underlying IT architecture.
FIGURE 2.
Flow diagram of eRAPID process for patient and staff users. Adapted with permission from Warrington et al. 66 This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/.
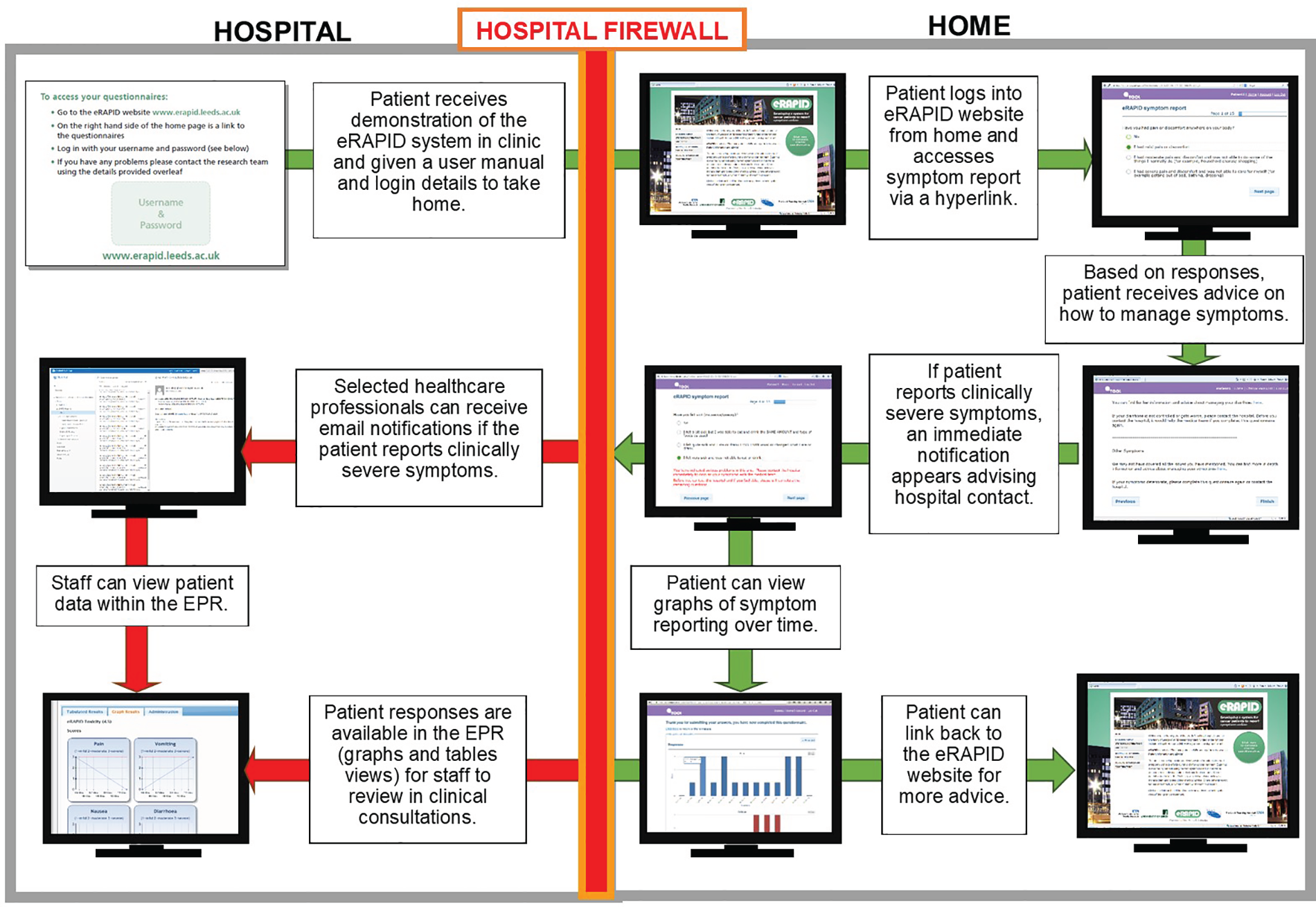
FIGURE 3.
Flow diagram of eRAPID data and underlying IT architecture. Adapted from Holch et al. 65 © 2017 European Society for Medical Oncology. Published by Elsevier Inc.
Additional screenshots providing examples of the patient and staff views of the system are shown in Figures 4 and 5.
FIGURE 4.
Screenshots of the patient-facing eRAPID website and symptom reporting in QTool. (a) The eRAPID website portal screen; (b) the eRAPID website homepage with hyperlink to QTool; (c) example symptom item in QTool; (d) example of immediate advice to contact the hospital following report of a clinically severe symptom; (e) management advice provided on completion of symptom report; and (f) graphical display of symptom report.
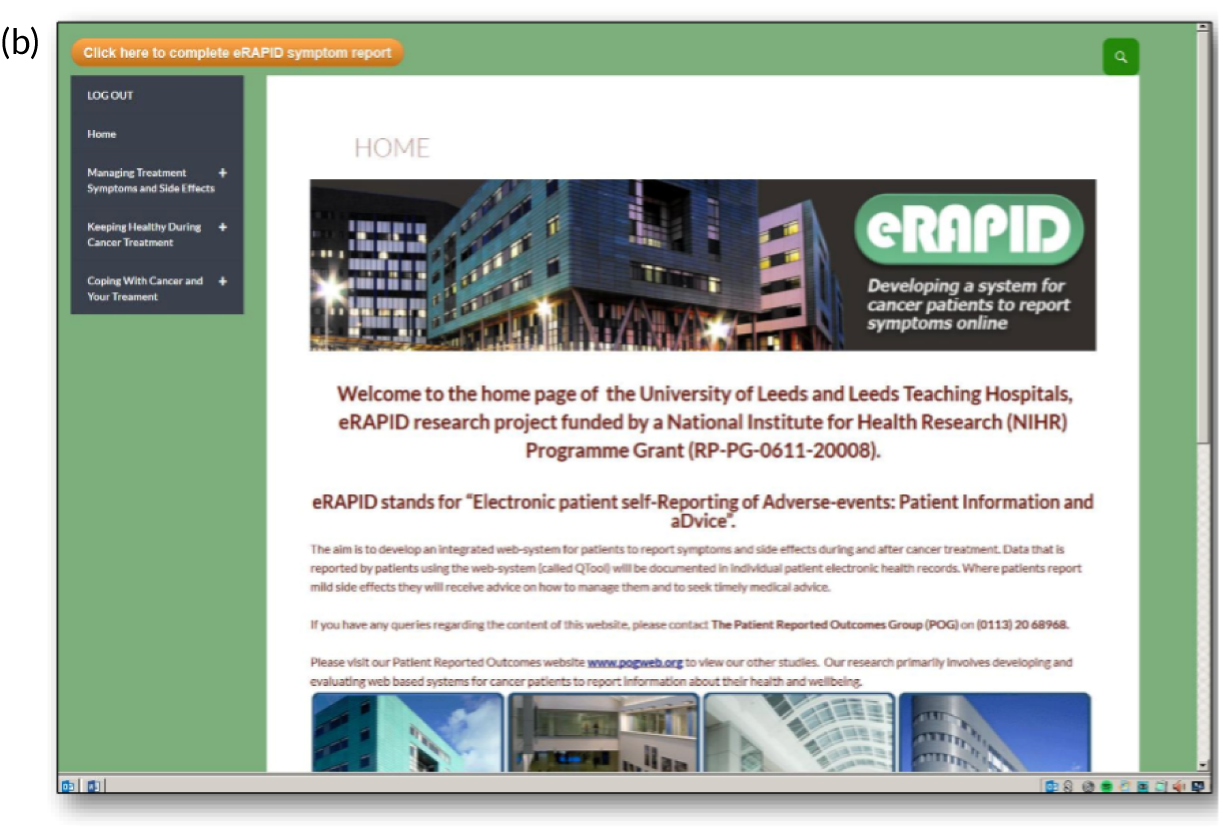
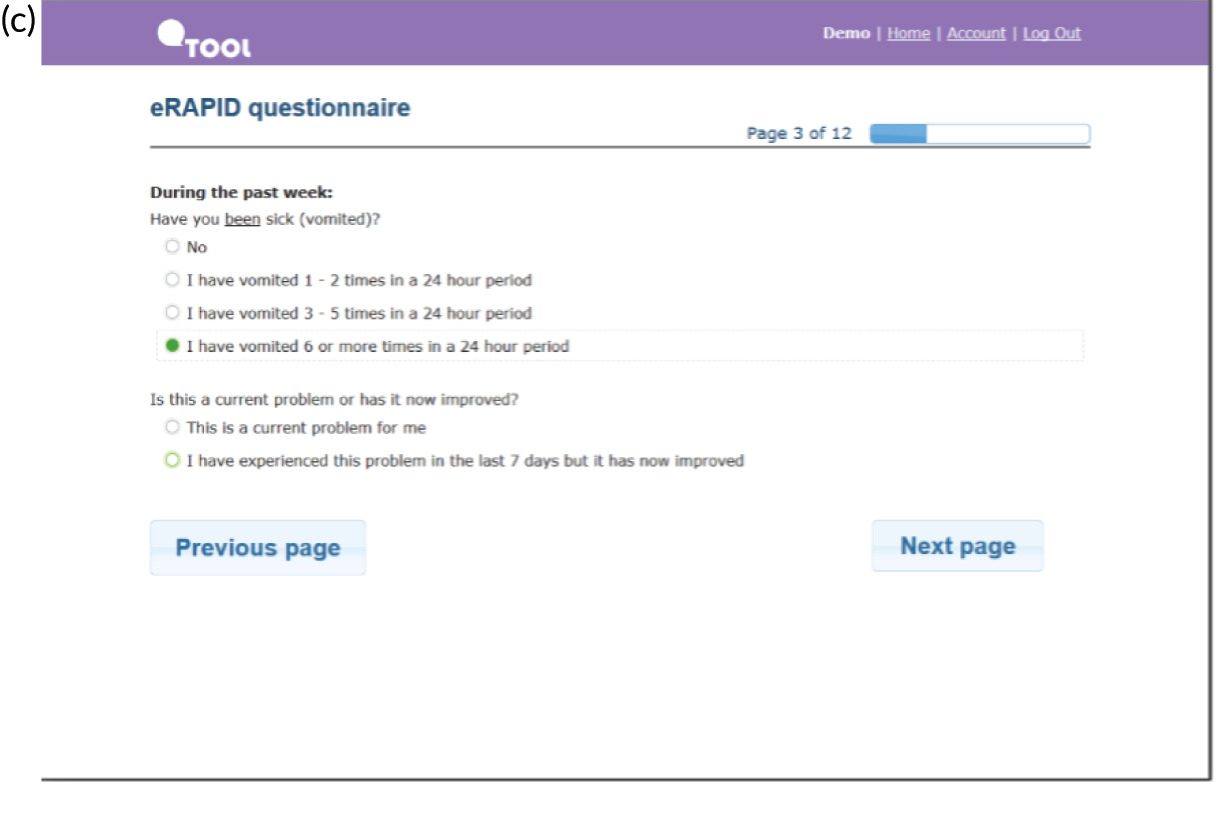
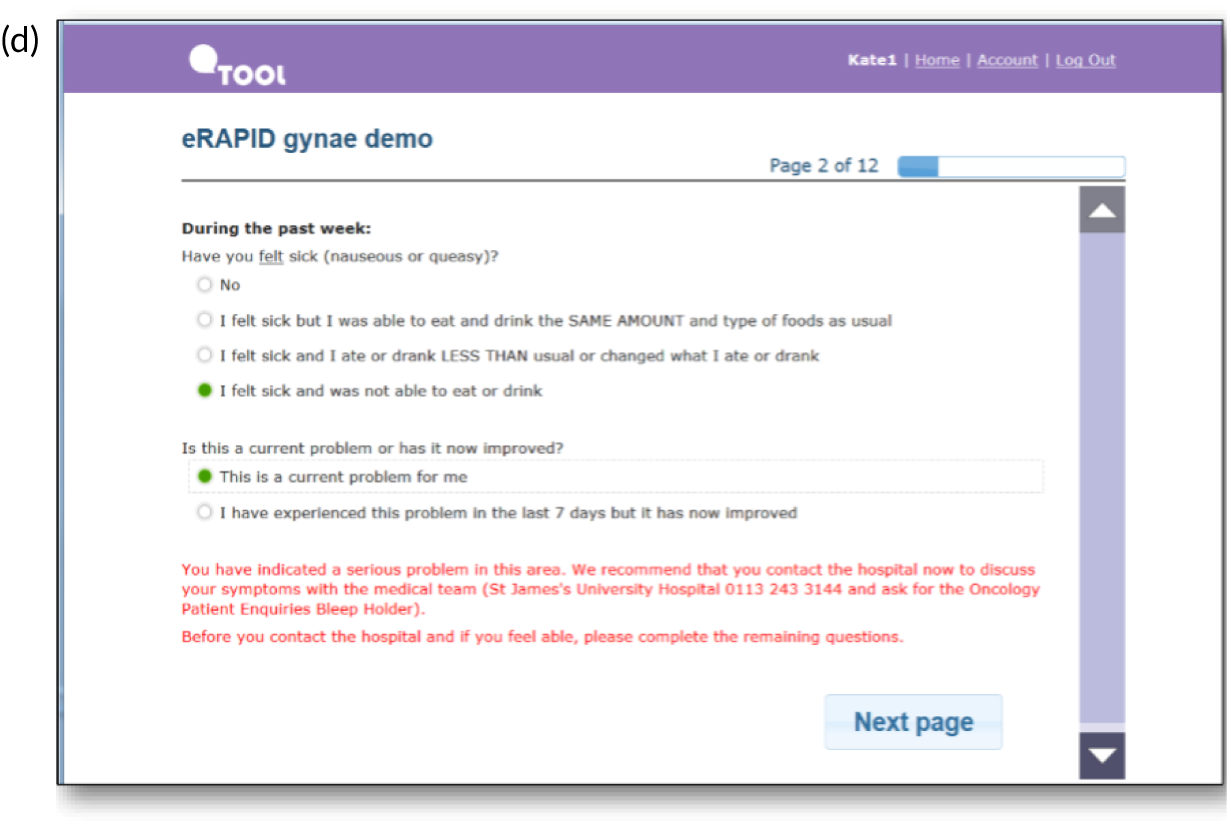
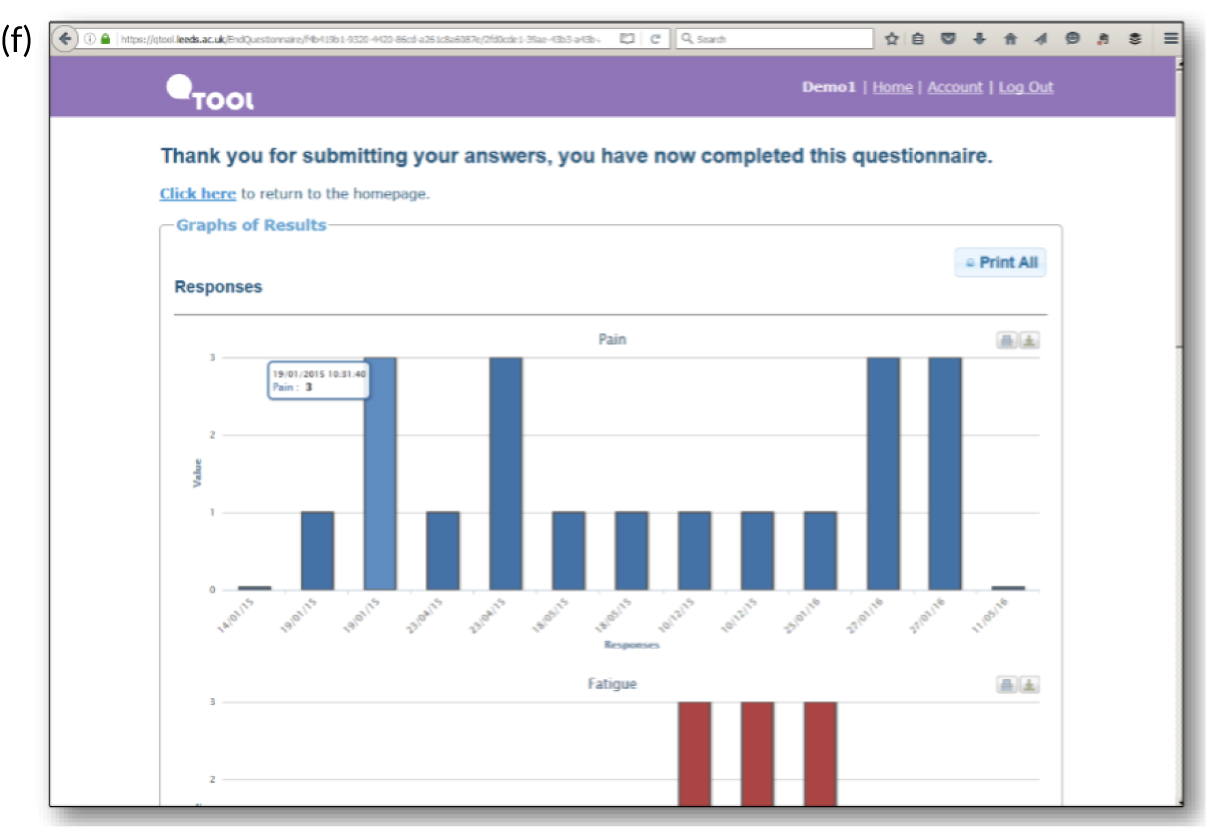
FIGURE 5.
Screenshots of clinician views of eRAPID symptom reports in EPRs. (a) Graphical display of symptom reports; and (b) tabular display of symptom reports.
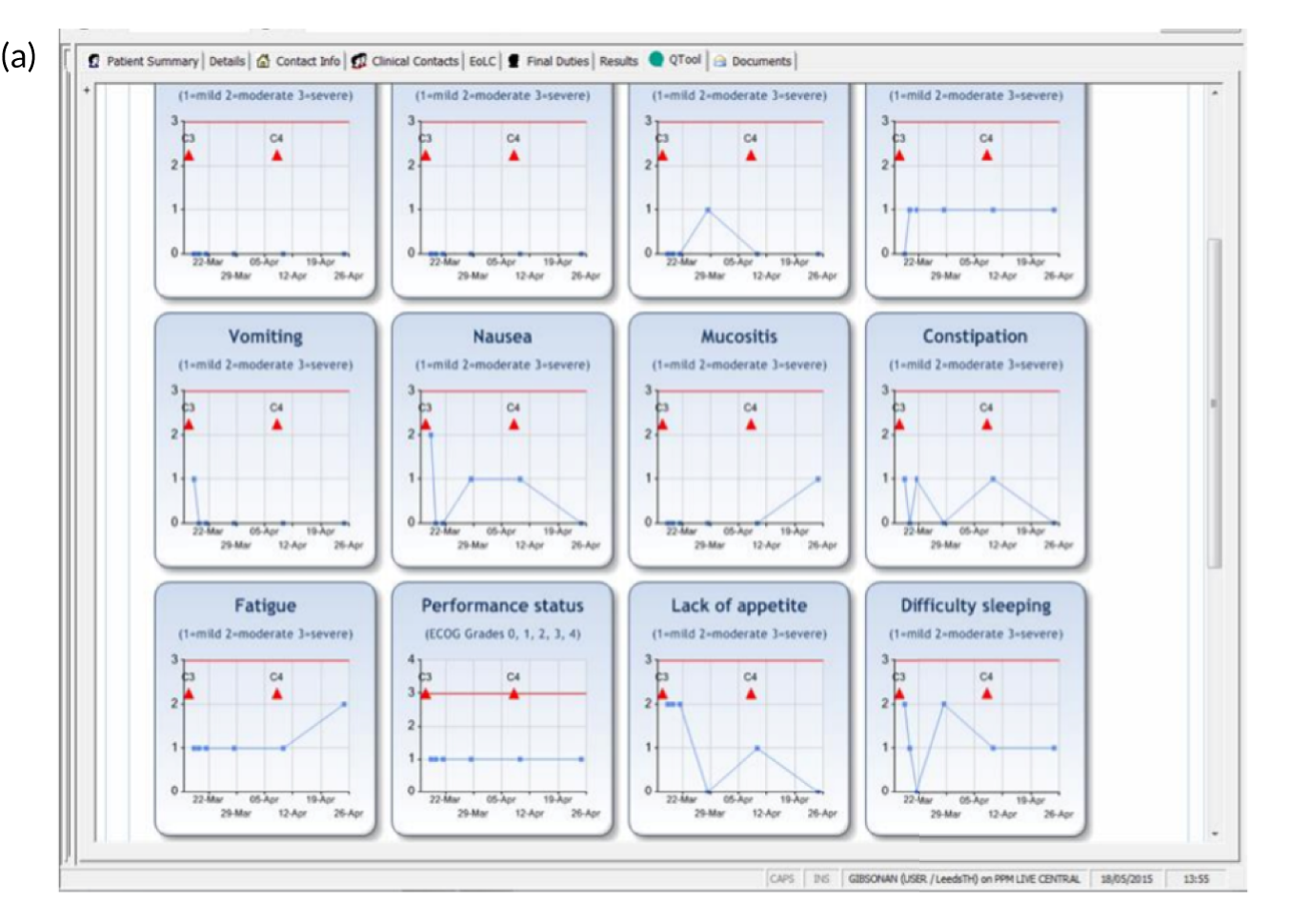

The findings from clinical usability testing provided valuable additional information through staff and patient feedback, which helped refine the intervention, including how patient symptom data were presented in EPRs. 66
Initial development work and electronic patient record integration at the Leeds site
The approach that we took to facilitate the collection and incorporation of electronic PROMs in routine care can be described as a ‘hybrid’ system,67 whereby a stand-alone PROM collection tool interfaces with, but is not fully integrated with, an existing EPR.
Software for online reporting of patient-reported outcome measures: QTool
The eRAPID IT system was designed in conjunction with QTool, an existing web-based questionnaire-building tool (developed by the private software company X-Lab Limited, with functional requirements provided by our research team). Intellectual property ownership is by University of Leeds, which also provides ongoing support for QTool governance and use in other projects. QTool had been previously commissioned by our research team for a study involving cancer survivors that demonstrated that it was possible to link PROMs to cancer registry data. 68
The QTool web app is hosted on University of Leeds servers and consists of two sites: (1) a participant site where the patient logs in using an anonymous username and password to complete symptom questionnaires that can be scheduled or left open to complete as frequently as the patient wants; and (2) an administrator site that allows researchers/clinicians to design and make live (i.e. publish online) the questionnaires along with setting up various other features such as scoring, dependencies and general maintenance. From the administrator site, researchers can also download all participant results into Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). QTool has a web service that can be used by software apps to communicate automatically and download participant results in Extensible Markup Language (XML). This web service is used by QStore to get the QTool results and match them up to relevant patients for display in the EPR.
A series of developments to QTool were necessary to meet the requirements of eRAPID. The eRAPID team worked closely with X-Lab Limited to incorporate the functionality to calculate scores and create item dependencies and question branching to facilitate the use of symptom-based severity algorithms into the symptom report. These could then be used to allow the display of automated patient advice based on the questionnaire responses.
It was identified early in the testing of QTool that the randomly generated passwords were problematic for patients (leading to mistakes and inability to log in). There was a clear need to generate patient-friendly usernames and passwords so that patients could log in to the system reliably. Our chosen approach was to create random passwords by concatenating random words from two separate ‘word banks’ and appending random numbers to the end (e.g. redcar10, bluemoon8). As a result of this approach, we had very few instances of patients unable to log in to the system. Unique usernames and passwords were generated in advance for prospective patients and entered into QTool. When patients consented to join the study, a researcher (with witness) would allocate the patient a username by linking the patient in QStore and then sending out a welcome letter.
Integration of patient-reported outcome measure reports in electronic patient records: QStore web application and QTool Response Fetcher
A key milestone of the eRAPID NIHR Programme Development grant was the successful creation of the secure anonymised interface between QTool patient symptom reports and Patient Pathway Manager (PPM; Leeds Cancer Centre, Leeds, UK), the Leeds Teaching Hospitals NHS Trust EPR system. The main challenge of this task was to maintain security of patient data in EPRs and work in accordance with the strict regulations of the national broadband network N3, used by the NHS. To allow for the display of patient symptom reports in individual patient EPRs, a link was created between QTool and PPM using a custom web service that we developed and named QStore. QStore is hosted on NHS servers, at the Leeds site on Leeds Teaching Hospitals NHS Trust server alongside PPM. The QStore app was developed to display the patient QTool responses (in both graphical and table format) almost in real time (within 3–5 minutes) in clinician-friendly views in the individual EPR (without a need to log in to other software). The app runs as an embedded web app in a host EPR system and, therefore, is reliant on custom integration. An essential part of the QStore system is the QTool Response Fetcher, a small server app that runs on a local NHS server and is designed to look for new QTool responses from patients who are registered with QStore. If the patient is found, the results are downloaded and stored on the QStore database, where they have been linked to the EPR patient identity. This app is automatically run through the Windows Task Scheduler (Microsoft Corporation, Redmond, WA, USA) and set to be run every 5 minutes, 24 hours per day, 7 days per week (24/7). In QStore there is functionality to customise how the QTool data are presented. QStore has an interface for administrators/researchers to set up QTool-linked studies, manually link from individual EPRs to the usernames created in QTool and customise the display of patient-reported data in graphical and tabulated form (see Figure 5).
The degree of integration between QStore and the host EPR system varied across the three centres dependent on the flexibility and features of the EPR system in question.
Patient reminders
Following the clinical usability testing, patients requested to be sent reminders when they are due to complete the online symptom reports. Therefore, a patient reminder system was developed that would automatically send out e-mails or short message service (SMS) text messages to patients on a pre-determined schedule. In the reminder system, a patient was automatically set to have no contact if they were flagged as having left the trial or deceased.
eRAPID website
The development of the eRAPID website was not part of the original application. The need for a website that collates all patient advice was identified when the team started developing the self-management advice. It became apparent that comprehensive advice may require more text than QTool can accommodate. Furthermore, the TSC and our patient advisors felt that it would be useful to have all relevant information on symptom management and lifestyle recommendations in one place for patients to browse, if required. Therefore, we adopted an approach whereby the website contained comprehensive information on symptom management, including relevant, locally available services. The eRAPID website was developed using WordPress (Automattic, Inc., San Francisco, CA, USA) (to allow easier modifications to the content by non-technical staff) and contains patient advice presented in a clear manner, driven by a simple menu for easy patient navigation. The website provides an entry point for patients on the eRAPID study and also contains, on the front page, a link to the QTool website, where users can log in and complete their questionnaires. The patient advice content could easily be published to the website by our researchers, with the ability to update the advice at any time, as required. Following advice from the TSC, we added password protection to allow only patients randomised to eRAPID to access the website and avoid contamination from the usual-care arm. The eRAPID intervention patients were required to log in to the eRAPID webpage before they could access the advice and complete their symptom report. The eRAPID website is hosted on University of Leeds servers, as access to those servers is logistically simpler, and at the time Leeds Teaching Hospitals NHS Trust did not have a patient-facing service.
Challenges
QTool software
QTool (version 2, hosted on University of Leeds servers) was stable and did not cause any problems during the study period. There were a few planned downtimes for routine maintenance and upgrades, but these were minimised thanks to the University of Leeds IT staff. The password generator worked reliably for the entirety of the eRAPID study. Occasionally, a patient was linked to the wrong QTool username; in such cases, the patient was unlinked in QStore by the IT manager. In 2017, QTool was upgraded to meet requirements of other clinical trials, resulting in QTool version 4. We completed eRAPID on version 2 but want to emphasise the importance of ongoing technical support for any software that is live and developing.
QStore
QStore (the integration software app) generally worked quite well. However, owing to the bespoke coupling of QStore with EPR systems, updating/upgrading an EPR system could break QStore integration, requiring considerable work by us and hospital EPR IT teams to resolve and restore the integration. QStore was hosted on secure NHS servers that the research team had no or very restricted access to. Therefore, maintenance and upgrading of the QStore software could be carried out only with the assistance of local NHS IT teams, for whom this was not a high priority.
In 2016, Leeds Teaching Hospitals NHS Trust started a phased introduction of a new EPR system, Patient Pathway Manager+ (PPM+) (Leeds Cancer Centre, Leeds, UK), to replace PPM. The team developed an adaptation of QStore to display patient symptom reports in PPM+, but PPM+ lacked the full functionality of PPM in relation to setting up clinical studies. Therefore, the study set-up and the linkage between patient identifiers (IDs) and patient usernames had to be carried out in PPM. To address this significant problem, a new integration software was developed called Patient Reported Outcomes Management Pathways Tracker (PROMPT) (University of Leeds, Leeds, UK) (see Further developments).
QTool Response Fetcher
The QTool Response Fetcher app periodically failed to run (and required manual rebooting) from the NHS server (because of network issues or server updates), which caused patient QTool results to not show up in a timely manner in EPRs. The app was moved to another, more reliable, server and subsequently continued running reliably with no further issues. During the time that Response Fetcher was unreliable, we developed a ‘response fetcher monitor alert system’ that allowed immediate notification if Response Fetcher failed.
Patient reminder application
The patient reminder app worked reliably, with a few problems related to:
-
accounts for SMS and e-mail administration running out, without a facility to notify the team to renew them
-
a bug in the reminder system that resulted in a patient receiving duplicate reminders
-
patients sometimes not receiving e-mails because of invalid e-mail addresses entered or because their client e-mail provider incorrectly categorised the eRAPID e-mails as spam.
eRAPID website
Because the eRAPID website was a public WordPress website, it was subject to some cyber attacks, and security updates were needed at times. It is important to state that no identifiable patient data were held on the website, so there was no risk of breaching patient confidentiality. Owing to the cyber attacks on WordPress websites, the University of Leeds had to change from a single-site to a multisite WordPress-hosted solution. This meant that the eRAPID site had to be taken down and redesigned as a multisite installation, which caused some disruption for patients in the study at the time (the site was offline for approximately 13 days). During this downtime, eRAPID participants still had access to the tailored advice through QTool and were able to ring the emergency hotline or their nurse, as usual.
Further developments
These challenges led the team to look for a solution that consolidates the multiple components of the integration app and meets the need for ongoing adaptation to the evolving EPR. The programme IT manager developed a new integration software, PROMPT, which has extended functionality and addresses most of the challenges outlined in Challenges.
Key functions of PROMPT include the following:
-
Easily create clinical PROMs pathways (schedules) to schedule required symptom report completions over any pre-defined time period.
-
Register patients on PROMs pathways with a unique start date.
-
Automatically generate unique usernames and passwords at the point of registration of a patient (on the EPR) with a clinical PROMs pathway.
-
Automatically manage patient reminders to complete PROMs according to the pathway schedule, with the addition of being able to automatically create printed letters (welcome letters, address labels, etc.), as well as SMS and e-mail patient communications.
-
Track patient compliance.
-
Display patient PROMs results in real time in graphic or tabular format.
-
Define PROMs thresholds, trigger notifications and show trends.
-
Combine other clinically relevant patient data, such as chemotherapy events and blood biomarkers, with the PROMs results.
-
Integration with EPR systems.
-
The Response Fetcher is integrated into the PROMPT system, running as a Windows (Microsoft Corporation, Redmond, WA, USA) service instead of as an app. This has the advantage of being a more reliable and robust solution, utilising inbuilt Windows functionality to enable specification of automatic recovery procedures on failure.
The PROMPT software contains identifiable patient data that can support patient care and, therefore, is hosted on a Leeds Teaching Hospitals NHS Trust server behind an NHS firewall. However, the PROMPT system was designed from the ground up, with the ability to run as a stand-alone web app with a secure administration login feature. It is also possible to run and maintain a single version of PROMPT across multiple hospitals, hosted centrally on a suitable cloud or NHS-approved central server (suitable for multicentre trials). A centrally hosted solution greatly improves the ability to maintain and keep the PROMPT system up to date.
The PROMPT system can work with QTool version 4, which has some new functionality and is hosted by Leeds Institute of Data Analytics (LIDA), University of Leeds, a more secure server for patient-sensitive data. PROMPT is currently used in all new research and clinical projects.
Integration of eRAPID information in the Manchester and Bristol sites
At the time of creation, the eRAPID IT approach was, to our knowledge, unique in the UK and superior in design and function to its few international counterparts. The initial work at the Leeds site, conducted during the NIHR Programme Development grant and early stages of the eRAPID research programme, delivered a proof of principle that patients could provide data for their EPRs that could be accessed by HCPs in real time. Moving forward through the eRAPID research programme, a fundamental aim was to demonstrate that the technical concept of the system could be implemented in other centres in the UK, specifically for the delivery of the multicentre eRAPID radiotherapy pilot (involving Leeds Cancer Centre and the Christie Hospital) and for the surgical work undertaken at University Hospitals Bristol NHS Foundation Trust.
From the outset we did not underestimate the practical and logistical challenges of interfacing QTool with other NHS EPR systems outside the Leeds site. We were prepared to take a flexible approach to the levels of integration with EPRs to ensure successful delivery of the overall research goals. By the end of April 2016, live integration of QStore into the EPR systems in the Manchester site [Clinical Web Portal (CWP); Christie NHS Foundation Trust, Manchester, UK] and Bristol [Medway (System C, Maidstone, UK)] was successfully achieved. The preceding years of developmental work had, as anticipated, presented a number of hurdles in driving the work forward.
eRAPID information technology in the Manchester site
Integration with electronic patient records
The eRAPID website at the Christie Hospital was developed with the same technology as at Leeds Teaching Hospitals NHS Trust (WordPress) and hosted at the University of Leeds; however, research staff at the Christie Hospital were given access to manage the content pages containing patient advice.
The version of QTool was the same as the version used at Leeds Teaching Hospitals NHS Trust (hosted at University of Leeds), except that a separate organisation was created for the Christie Hospital, and staff there were given access to manage their eRAPID questionnaires.
A bespoke version of QStore was developed for the Christie Hospital because it was required to be integrated with the local bespoke EPR system, CWP, which is owned by and managed by the Christie Hospital IT team (Christie NHS Foundation Trust). The integration was tailored specifically to work with the CWP system, which involved the Christie Hospital IT team creating a small web service that QStore could call to obtain patient demographic information directly from CWP and which was subsequently used for patient identification in QStore. QStore was hosted locally at the Christie Hospital on their development server and could be accessed and maintained only by the Christie Hospital IT team.
For managing patient reminders, research staff at the Christie Hospital were lent a University of Leeds laptop that was installed with our reminder system front end, which linked via a virtual private network to the University of Leeds server used to send out patient reminders.
Challenges
Initially, good progress was made with developing the test version of the system and early usability testing with staff. However, during 2015 and the beginning of 2016, changes in IT staff in the Manchester site caused delays in progress with making QStore live.
Once live, the system largely functioned well and worked reliably (bar some instances for downtime when the Christie Hospital servers were taken down for maintenance purposes).
One issue that could not be resolved was the identification of patients reporting symptom notifications in QStore. This became problematic because the real-time patient identification relied on a web service developed by the Christie Hospital IT team on a test database, but was not implemented on the live CWP server. This meant that the identification of patients with severe symptom notifications had to be carried out by manual look-up rather than by having the patient’s name display in QStore.
eRAPID information technology in the Bristol site
Integration with electronic patient records
The eRAPID website at the Bristol site was developed with the same technology that was used at Leeds Teaching Hospitals NHS Trust (WordPress) and hosted at the University of Leeds; however, research staff at Bristol Royal Infirmary were given access to manage the content pages containing patient advice.
The version of QTool was the same as the version used at Leeds Teaching Hospitals NHS Trust (hosted at University of Leeds) except that a separate organisation was created for the Bristol site, and staff there were given access to manage their eRAPID questionnaires.
A bespoke version of QStore was developed for the Bristol site because it needed to be integrated with Medway (a commercial EPR system); however, because Medway was supplied and managed by a third-party software company, we had very limited ways in which we could integrate QStore into Medway. The agreed web integration was via a secure, encrypted URL that required passing a unique hospital code along with patient name, NHS number and date of birth. The encryption used a unique 128-bit Advanced Encryption Standard (AES) key. QStore could not be hosted locally at Bristol Royal Infirmary; therefore, a version was hosted on a dedicated University of Leeds virtual server and managed by the University of Leeds IT team. Administration rights to directly log in to access and manage parts of QStore (such as alert reports) were given to research staff at the Bristol site.
For managing patient reminders at the Bristol site, a bespoke, stand-alone reminder app was installed on a local computer connected to the Bristol Royal Infirmary network, so staff there could set up reminders for their patients.
Challenges
At the start of the eRAPID research programme, a new release of Medway was being installed and QStore IT integration could not begin until this was complete. Significant time and input from the local eRAPID co-applicant/surgical lead was required to establish working relationships with key staff. Between April 2016 and August 2017, there were four instances of loss of integration with Medway, totalling 145 days, resulting in clinicians being unable to access eRAPID results through the EPR system. A separate ‘Administration and Report’ section in QStore was developed, allowing staff to log in directly to QStore via a secure login to administrate and see participant results and notification reports during periods of downtime. From May 2016 to March 2018, there were five instances of reminder system downtime, totalling 39 days.
Conclusions and reflections on delivering the eRAPID information technology work package
Over a period of 2 years, we successfully implemented and maintained the eRAPID IT system at the Leeds, Manchester and Bristol sites to support the delivery of the systemic RCT and pilot trials in the radiotherapy and surgical arms. Our solution was, to our knowledge, one of the first at the time to allow patients to report symptoms online (via public-facing website), which are then securely transferred in near real time and displayed in their EPR to be used for patient care. The technical solution is not difficult and was developed within 6 months. The main challenges came, as expected, from the practicalities of working with different hospital IT teams and different EPR systems across the UK. The eRAPID requirements (however minimal) were not a priority for the IT departments and individual staff in the hospital centres; therefore, a lot of effort was required to maintain working relationships and momentum on the approval and tasks needed to integrate QStore in Medway and CWP.
The full IT system for eRAPID had multiple components, the maintenance of which was dependent on different IT systems and organisations. This solution worked to a satisfactory degree but required close and staff-intensive monitoring at each centre. Having three centres with bespoke integration engines each (QStore versions) added to the complexity. In the beginning of the programme, we explored the option for a centralised solution with the Health Informatics Service at Calderdale and Huddersfield NHS Foundation Trust, but it proved too costly and unfeasible. To address these experienced challenges we developed a new, more functional, integration engine, PROMPT. PROMPT meets the current needs of this approach, but this may not be for long.
Our experience clearly shows the need for continuous development of any IT systems used for patient care to match EPR systems and to keep up with the fast pace of IT development. For example, at the start of eRAPID, the main access to the internet for the majority of the public was via laptops or desktop computers. During the programme, the use of mobile internet, tablets and smartphones became ubiquitous, and we had to adapt QTool to recognise mobile devices and automatically display the questions for a smaller screen. However, in the programme, we have not had the time and financial resources to develop an app for smartphones.
We learned how essential and difficult it is to engage hospital IT teams in the ongoing support of any system for PROMs. Our system resulted in a ‘hybrid’ integration of online PROMs reporting with EPR, and required efforts from hospital IT departments; these efforts were perceived to be additional and not a priority. If a PROMs system is fully integrated, such as a patient portal, then it will be an integral part of the EPR system and maintained as part of the EPR system. Such patient portals have been recently introduced widely in the USA, but are still rare in UK. The PROMs component of the patient portals would be less flexible in terms of allowing clinical teams to choose and easily change PROMs, as the questions require technical programming. However, from a patient perspective, these systems would be clearly linked to their care.
Systemic treatment
Background
Systemic drug treatments for cancer (e.g. chemotherapy, hormonotherapy, biological therapy and targeted agents) are associated with acute and long-term AEs. AEs are documented consistently in clinical trials; however, the routine recording of treatment side effects experienced by cancer patients is typically not well documented or easily accessible in medical records. Monitoring and documenting side effects during chemotherapy are recognised to be essential for modern cancer care, because an AE may lead to changes in drug dosage or cessation of treatment and can significantly compromise patients’ QoL. Severe AEs can escalate to hospitalisation for potentially life-threatening toxicities (e.g. neutropenic sepsis). Patients with breast, gastrointestinal or colorectal cancer and those with metastatic disease are among those most likely to have emergency admissions. Not all patients are able to understand or judge the clinical severity of particular symptoms or the appropriate care options when outside the hospital environment. Furthermore, there is sufficient evidence suggesting that clinicians often miss or underestimate patients’ symptoms during treatment. 31
It has been recognised that a structured AE-reporting system would be useful to facilitate correct documentation and grade responses, leading to tailored management for AEs. Many studies28,38,69,70 show that systematic monitoring of patients’ symptoms using patient-reported outcomes contributes to better care and improves patient–clinician communication, symptom management, QoL and overall survival. However, despite the growing evidence of patient benefits, this approach is far from being implemented in routine practice because it requires modifications to existing clinical processes. Many challenges exist, including technical and administrative challenges and challenges relating to clinical workflow, clinical engagement and engagement from management.
The eRAPID IT system was devised to address those challenges in the UK setting and the subsequent programme of research aimed to provide a rigorous scientific evaluation of potential benefits.
The overall aims of the eRAPID IT system were to improve the safe delivery of cancer treatments, enhance patient care and standardise documentation of AEs in the medical records. We expected that eRAPID would benefit both patients and HCPs. For patients, it may enable earlier symptom detection and self-management, timely admissions for serious toxicity and appropriate contacts with clinical teams. For staff, it may reduce the number of contacts, save time spent recording AEs, improve patient–professional communication and support shared decision-making.
The development and evaluation of the eRAPID approach in systemic treatment (i.e. chemotherapy sometimes combined with targeted treatments) represents the main part of the research programme, spanning and completing all five WPs. The work included the development and testing of the electronic platform; the development of the AE items with accompanying clinical algorithm and patient advice; mapping professional and care pathways; and usability testing of the full package, followed by a pragmatic RCT with an internal pilot and embedded cost-effectiveness substudy.
The key research questions were as follows:
-
Is it feasible and acceptable to collect routine AE data during systemic cancer treatment? Will patients and clinicians engage with eRAPID?
-
Will the eRAPID intervention lead to better symptom control and better patient experiences?
-
Will eRAPID improve patient self-efficacy in managing treatment-related symptoms?
-
Will eRAPID lead to earlier symptom detection, timely admissions and appropriate hospital calls?
-
Will the eRAPID intervention be cost-effective?
Changes to the original plans are described in Synopsis.
Patient and public involvement specific to eRAPID in systemic treatment
The aim of patient and public involvement (PPI) was to contribute to all aspects of the research programme from study conception to dissemination. As the PPI co-applicant, Carolyn Morris provided commentary on the grant application. Following confirmation of funding, the team introduced the programme to the Leeds Research Advisory Group (RAG). Barbara Woroncow (RAG member) volunteered to be the PPI representative, and TSC member initially with Shelley Mason, and was later replaced by Virginia Cucchi, who was recruited through an advertisement in a NIHR newsletter. All PPI members had past or ongoing experience of undergoing treatment for cancer and had worked in advisory roles in other research settings (locally and/or nationally).
Input on the application of the patient outcomes measures and the management and design of the eRAPID studies from a patient perspective has been invaluable. From the initial stages of eRAPID conception in the Programme Development Grant, we collaborated with PPI representatives on the research focus, study delivery and dissemination. To increase awareness of the research programme and to ensure engagement from local clinicians and managers, we held a 1-day launch event at Leeds General Infirmary (Leeds, UK), which was opened by our chief executive and included a presentation from our patient representative Barbara Woroncow, as well as the research team.
Over the years, the PPI group has regularly attended and contributed to the direction of the eRAPID research programme through membership of the systemic working groups (for both the systemic and the radiotherapy workstreams) and the wider eRAPID Steering Committee meetings. Carolyn Morris was an integral member of the systemic workgroup meetings throughout the programme to provide strategic guidance. In 2016, at the 20th anniversary of the Patient Centred Outcomes Research (PCOR) Group at the University of Leeds, Barbara Woroncow contributed to presentations of the programme, which were later published in a University of Leeds bulletin, and presentations were made available online. In addition, Barbara Woroncow presented an overview of the eRAPID systemic study as part of a workshop at the 2019 Health Services Research conference. 71 Carolyn Morris and Barbara Woroncow have co-authored several publications. 65,72–77
Members of our local Leeds RAG have been involved with the eRAPID research programme in terms of both general updates and feedback on the research provided at the bi-annual meetings. They have played a vital role in the development and ongoing refinement of the eRAPID intervention for systemic therapy. To ensure clarity, the wording of questionnaires was considered both by patient advocates who had undergone chemotherapy and by patient advocates who had not undergone chemotherapy. Specifically, members informed some of the key decisions made on the wording of questionnaire items and self-management advice, and improving the usability of the online platform. From all of the comments and feedback received through our initial system usability testing with RAG members, 68% led to changes to the presentation, content and readability of the patient-facing content. In addition, PPI members reviewed patient study materials, such as information sheets and eRAPID IT system user guides. Further details of RAG involvement in the eRAPID research programme throughout development and programme grants are provided in our published papers. 73,76
Overall, we feel that our approach to PPI collaboration benefited from involving representatives with experience of working in wider national research contexts and having the local insight from our RAG. We are aware that our local PPI group was very committed and supportive of the research conducted by our team; many of the members have been involved for a number of years. We have found it challenging to recruit younger members to the RAG, and many of the members have completed cancer treatment several years ago, which may influence their views and perceptions of the eRAPID approach.
Development of eRAPID intervention components
During the development of the eRAPID approach, we followed the MRC’s complex intervention guidance78 to optimise the design and acceptability of the system. The eRAPID intervention consists of active components that were developed individually and then evaluated collectively as a single, complex system. For a detailed description of the developmental work, see Report Supplementary Material 1.
Information technology system
As described in Electronic platform and one of our published papers (see Holch et al. 65), a robust, secure online system was developed that allows patients to log in with a provided username and report their symptoms remotely. The functionality of the online software allows the set-up of immediate automated advice based on clinical algorithm. Furthermore, the self-reported data are pulled behind the NHS firewall to a database linked to the electronic records, where the patient is identified and the results are displayed for the clinical team.
Mapping professional and care pathways
We performed process mapping of patient treatment pathways and interviews with HCPs and patients to identify where and how eRAPID will best fit in the clinical flow and the key HCPs to engage in the new approach. 79 The eRAPID studies started very soon after acute oncology procedures were introduced at the Leeds Cancer Centre (Leeds, UK), leading to a major restructuring of the emergency care pathways.
Selection of symptom items for patient self-reporting
During the eRAPID research programme development grant, we decided to use the CTCAE system and conducted extensive work in selecting items and converting the AE items descriptors into patient self-reporting language, preserving the severity grading. Cognitive patient interviews were performed to ensure comprehension and clinical meaningfulness of the items. For further detail, see the published paper on the cognitive interview to finalise items wording. 76
Development of severity thresholds and a clinical algorithm
A unique feature of our system is the provision of immediate automated advice to patients on how to self-manage mild symptoms and when to contact the hospital. This required set-up of severity thresholds for mild, moderate and severe symptoms, plus combinations of symptoms that may not be severe but taken together may require medical attention. We employed an iterative process, asking the oncologists to determine the symptom severity thresholds, focusing on patient safety first, but also to support patient education in symptom self-management. See Report Supplementary Material 1 for details of the clinical algorithm’s categories.
Training of patients and health-care professionals
Patient and professional engagement with the system was recognised to be an essential factor that would determine its regular use in clinical practice. We planned early and ongoing training for both groups. After patients consented and were allocated to the eRAPID intervention, a researcher gave patients a postcard with their username and password and showed them a brief demonstration version of eRAPID with example questions, automated advice and graphs of their responses. Each patient was provided with a manual for eRAPID.
At the start of the projects, we trained staff through brief presentations during regular team meetings. Once the project was running, researchers showed individual clinicians how to view patient self-reports in the electronic records and reminded them to discuss them during the consultation and note their actions if they had notifications for severe symptoms. An e-learning package for staff training was created later, which could be accessed using a hyperlink in patient records.
See Report Supplementary Material 2 and 3 for full details of the patient user guides and clinician training materials.
Usability field test of the live system in a clinical context
Once all of the components of eRAPID were individually developed, we performed usability testing of the full system in a real clinical setting (for full details see our published paper by Warrington et al. 66). We approached 12 patients with breast cancer on adjuvant chemotherapy and asked them to use eRAPID for four cycles (12 weeks). A total of 10 clinicians (oncologists and nurses) looked at the self-reports during consultations. We monitored the use of eRAPID and interviewed all participants. Patients liked the system, particularly the self-management advice and when to contact their team or the emergency number. The clinical algorithm was modified because we observed that some alerts were generated retrospectively, after the problem was addressed. We added a branching question for severe symptoms to indicate if the severe symptom is still persistent or current (or not in the past 7 days).
Randomised controlled trial with internal pilot study
The aim of the RCT with internal pilot study72 was to evaluate the potential benefits of the eRAPID IT system for patients and staff when added to usual care during cancer chemotherapy. Our hypothesis was that the eRAPID intervention can bring benefit to patients, staff and the NHS. A cost-effectiveness study was embedded in the trial design.
The specific objectives were to evaluate the impact of the intervention on patient experiences (i.e. symptom control and patient self-efficacy) and process of care (i.e. number of hospital calls and admissions) and to examine cost-effectiveness and patient and clinician engagement with the intervention. The main research questions are listed in Background.
Methods
Design
The eRAPID study was a prospective, single-centre, randomised (1 : 1), two-arm, parallel-arm study with an internal pilot phase. Full inclusion and exclusion criteria and trial procedures are described in the published protocol. 75 The sample included patient participants receiving systemic treatment (i.e. chemotherapy with or without targeted therapies) for breast, colorectal or gynaecological cancer at Leeds Cancer Centre. HCPs (i.e. senior oncologists, trainees and senior nurses) involved in the care of those patients were invited to participate. Consenting patients were randomised to either the intervention arm (i.e. eRAPID added to usual care) or the control arm (i..e. usual care). Randomisation was stratified by cancer site (breast, colorectal and/or gynaecological), sex (breast and colorectal cancer patients only) and previous chemotherapy. The eRAPID patients were asked to complete symptom questionnaires weekly, and also when experiencing symptoms, for 18 weeks. The HCPs were trained on how to access eRAPID reports through EPRs and they saw participants in both arms.
Outcome measures
The primary outcome measure was Functional Assessment in Cancer Therapy Scale – General (FACT-G) Physical Well-Being (PWB) score at 18 weeks. 80 The FACT-G PWB score was chosen as the primary outcome as it performed well in previous trials and covered the most common treatment-related symptoms and their impact on patients’ lives. We anticipated a cumulative effect of the intervention; therefore, we selected 18 weeks (i.e. end of chemotherapy) as the primary end point, and 6 and 12 weeks as the secondary end points.
The main secondary outcome was cost-effectiveness assessed using clinical process measures (i.e. acute admissions, calls to the emergency hotline and other hospital calls), use of health-care services [including general practitioner (GP) contacts, medications and personal expenses], EuroQol-5 Dimensions, five-level version (EQ-5D-5L), utility scores81 and utility scores derived from the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)82 (see Health economics analysis of eRAPID in systemic treatment). We evaluated adherence to the eRAPID intervention by patients and clinicians. Other secondary outcome measures included questionnaires on aspects of patient self-efficacy and activation [e.g. the Self-Efficacy for Managing Chronic Disease 6-Item Scale (SES),83 Cancer Behaviour Inventory–Brief (CBI-B)84 and Patient Activation Measure85 (PAM)].
To separate the possible effects of the online intervention questions from the outcome measures, patients were given the outcome questionnaires on paper to complete at home and return by post at baseline and at 6, 12 and 18 weeks post randomisation. Clinical process measures (e.g. acute admissions and calls to hospital staff) were downloaded from the electronic hospital records. A subset of participants (where feasible within the funding time frame) were also assessed at 12 months for further cost-effectiveness analysis to examine any potential longer-term impact of the intervention on QoL and clinical processes.
Statistical analysis
The sample size for the full trial was based on a patient-reported outcome measured by the FACT-G PWB subscale at 18 weeks. At 80% power and 5% significance, a sample of 176 patients per arm was necessary to detect a 2-point change in this subscale score. A 2-point change in score corresponds to a small to moderate effect size (0.3). Allowing for 30% attrition, a minimum of 252 patients per arm was required (i.e. 176/0.7), equalling 504 patients in total across the two arms.
Multivariable, multilevel, mixed-effects, repeated measures models were used to compare the differences in patient outcome scores between the treatment arms over time [using PROC MIXED in SAS® software version 9 (SAS Institute Inc., Cary, NC, USA)]. The model was adjusted for stratification factors (i.e. cancer site, sex and previous chemotherapy), time (i.e. follow-up week), treatment arm, treatment arm by time interaction, baseline PWB score and age at study entry as fixed effects. Participant and participant by time interaction were modelled as random effects (by including the ‘repeated’ statement in the analysis model).
Multiple correlation structures appropriate to the repeated measures setting were fitted, including compound symmetry, auto regressive and unstructured. The model using unstructured correlation had the lowest Akaike information criterion score (measure of model fit) and the fewest assumptions on the structure of the data. For these reasons, the unstructured correlation structure was chosen for the final model. The normality of the residuals was checked and shown to be approximately normal.
Missing data patterns were explored. Baseline covariates were found to be associated with missingness and the missing data mechanism was suggested to be missing at random. Therefore, multiple imputation by predictive mean matching was performed. The main primary analysis was performed on the imputed data, followed by sensitivity analysis using all observed data. An ad hoc exploratory subgroup analysis of patients with metastatic cancer and patients with non-metastatic cancer was performed on the imputed data. A negative binomial model was used for the analysis of the process of care measures. No adjustment for multiple testing was undertaken. Post hoc analysis was conducted to explore associations between patient adherence to online symptom reporting and clinician use of symptom data in clinical consultations. A median split was used to categorise patients as ‘adherent’ or ‘non-adherent’ and a clinician use composite score was calculated per patient from clinician feedback checklists.
Patient and staff qualitative feedback
Qualitative feedback on the eRAPID IT system was obtained from a subset of patients and clinical staff through end-of-study interviews. Additional written feedback was also received through free-text comments in (1) patient end-of-study feedback forms and (2) clinician feedback forms (completed by clinicians when reviewing online symptom reports during consultations).
Interviews were transcribed and analysed using thematic analysis. 86 Free-text comments were collated and reviewed for key content and summarised under overarching key themes. A report of the qualitative data is provided in Appendix 1, eRAPID systemic RCT qualitative findings.
Results
Internal pilot phase
Between 22 January and 23 September 2015, 134 patients were identified, of whom 25 were excluded after screening as ineligible and 87 out of 109 eligible patients consented, giving a consent rate of 80%. Only 13 participants (15%) withdrew. No significant IT problems were encountered. The research team improved the methods to gather data from the electronic records on clinical process measures (e.g. admissions and changes to treatment). The overall recruitment (consent rate of > 60% of eligible patients, recruitment rate of > 10 patients per month) and attrition (attrition rate of < 30%) targets were met and the TSC recommended progression to the main trial.
Full trial
Between 22 January 2015 and 11 June 2018, 1484 patients were assessed for eligibility; 702 were excluded pre approach and a further 92 were excluded after being approached for full eligibility assessment. From 690 fully eligible patients, 182 (26.4%) declined participation and 508 (73.6%) consented and were randomly assigned to the eRAPID intervention (n = 256) or usual care (n = 252). A total of 55 HCPs participated (16 oncologists, 25 trainees, two trust oncologists, 10 senior nurses and two pharmacists). Of these HCPs, 19 were regular team members and saw > 10 patients in the eRAPID arm; the remaining 36 were either trainee oncologists who rotated between different teams or temporary staff.
Primary outcome
For the main primary analysis on the imputed data, there were no clinical or statistical significant effects of the eRAPID intervention at 18 weeks [adjusted difference in mean FACT-G PWB score between treatment arms 0.20, 95% confidence interval (CI) –0.81 to 1.20; p = 0.6992]. There was a statistically significant effect of the eRAPID intervention at 6 weeks (adjusted difference in mean FACT-G PWB score 1.08, 95% CI 0.12 to 2.05; p = 0.0280) and 12 weeks (adjusted difference in mean FACT-G PWB score 1.01, 95% CI 0.05 to 1.98; p = 0.0395). Neither point estimate is clinically relevant (change > 2 points); however, 2 is contained in the upper end of the interval for 6 weeks, so clinical relevance cannot be ruled out.
The descriptive responder analysis of FACT-G PWB ‘change scores’ at an individual level suggests that patients receiving the eRAPID intervention experience less clinically relevant deterioration at 6 and 12 weeks and more symptom stability (symptom control).
A sensitivity analysis on the unimputed observed data yielded similar estimated differences at the follow-up time points. There was no statistically significant effect of the eRAPID intervention at 18 weeks (adjusted mean difference in mean FACT-G PWB score –0.05, 95% CI –1.08 to 0.98; p = 0.920). There was a borderline statistically significant difference at 12 weeks (adjusted mean difference in mean FACT-G PWB score 0.9, 95% CI –0.02 to 1.89; p = 0.055), but no statistically significant difference at 6 weeks.
One limitation of using imputed data sets is the inability to robustly combine type-III global effects. Because both analyses present similar results, the global effects from the unimputed data give a practical estimate for the overall effect of treatment over time. The overall effect of eRAPID intervention, compared with usual care over time, was not significant (treatment × time interaction p = 0.1233).
As expected in the model, the baseline FACT-G PWB score was highly statistically significant (p < 0.0001), as was the effect of cancer site. Both participants with gynaecological cancer (p = 0.0026) and participants with colorectal cancer (p = 0.0006) had better FACT-G PWB scores than participants with breast cancer, after adjusting for baseline score.
A pre-planned exploratory subgroup analysis was conducted on the imputed data to determine whether or not there was any evidence of heterogeneity of the intervention effect for patients with metastatic cancer and patients with non-metastatic cancer. The primary analysis was repeated separately on the two new data sets. There was no evidence of an eRAPID effect in the metastatic cancer subgroup.
In the non-metastatic cancer arm, no effect was seen at 18 weeks (adjusted difference in mean FACT-G PWB score 0.04, 95% CI –1.08 to 1.17; p = 0.9430). However, a small, statistically significant, positive effect for the eRAPID intervention was observed at 6 weeks (adjusted difference in mean FACT-G PWB score 1.45, 95% CI 0.32 to 2.58; p = 0.0112) and 12 weeks (adjusted difference in mean FACT-G PWB score 1.13, 95% CI 0.07 to 2.19; p = 0.0362). At both time points, the CIs do not rule out a clinically significant effect.
Process of care measures
There was no effect for the eRAPID intervention on admissions [estimate 0.13, standard error (SE) 0.18, Wald χ2 = 0.71, incidence rate ratio (IRR) 1.14, 95% CI 0.84 to 1.53; p = 0.4003] or calls/visits to acute oncology (estimate 0.05, SE 0.12, Wald χ2 = 0.20, IRR 1.05, 95% CI 0.84 to 1.31; p = 0.6516). No between-arm differences were found for the measures of chemotherapy delivery: dose reductions, delays, number of delivered cycles and chemotherapy discontinuation.
Adherence to the intervention
A total of 3314 online symptom reports were completed during the study. The mean number of reports per patient was 12.7 [standard deviation (SD) 12.6, median 14.0, range 0–117)]. The median compliance per patient (defined as completed/expected reports adjusted for withdrawals) was 72.2%. Compliance per week gradually decrease between week 1 (71.9%) and week 17 (56.7%; lowest compliance), and the average weekly compliance was 64.7%.
A total of 18,867 individual online symptoms were reported (as part of 3314 logins); of those, 323 (1.7%) were severe and 4342 (23.0%) were moderately severe. Emergency alerts were activated in 29 cases (of 3314 online completions; 0.9%) and serious symptoms not requiring immediate medical attention were reported on 163 occasions (4.9%). The vast majority of self-reported symptoms triggered self-management advice (2714/3314; 81.9%).
This was a pragmatic trial including all staff assessing patients during chemotherapy (i.e. 15 oncologists, 25 trainees, two staff doctors, 10 senior nurses and two pharmacists). Clinicians saw a variable number of patients (mean 14.3, SD 23.4, median 3, range 1–123). Clinician engagement was evaluated by self-reported use of the online data using a checklist completed after each consultation with a patient allocated to the eRAPID intervention. Overall, 787 forms were completed. Broadly, in about 70% of cases clinicians reported looking at the online reports, in 56% of cases they used them at least ‘somewhat’ and in 62% of cases found them at least ‘somewhat useful’. On 35–45% of occasions, the online reports were not considered at all, suggesting somewhat limited buy-in from clinicians and suboptimal intensity of the eRAPID intervention.
In an exploratory logistic regression analysis, we found that greater patient age, higher baseline FACT-G PWB score and clinician use of the data during consultation were predictors of higher levels of patient adherence (defined as ≥ 72.2% of expected completions). Furthermore, patients with high levels of adherence had significantly better FACT-G PWB scores over time (eRAPID intervention: adjusted mean FACT-G PWB score 21.7, 95% CI 21.0 to 22.5; usual care: adjusted mean FACT-G PWB score 20.2, 95% CI 19.4 to 21.0; p < 0.000) than those with lower adherence. The significant differences were seen at 12 and 18 weeks.
In a post hoc exploratory logistic regression analysis, we found that greater patient age, higher baseline FACT-G PWB score and clinician use of the data during the consultation were associated with higher levels of patient adherence (defined as ≥ 72.2% of expected completions). In a post hoc, mixed-effects model, patients allocated to the eRAPID intervention with high levels of adherence had significantly better FACT-G PWB scores over time (high level of adherence: adjusted mean FACT-G PWB score 21.7, 95% CI 21.0 to 22.5; low level of adherence: adjusted mean FACT-G PWB score 20.2, 95% CI 19.4 to 21.0; p < 0.0001) than those with lower adherence. The significant differences were seen at 12 and 18 weeks.
Patient and staff qualitative feedback
A total of 45 patients and 19 staff were interviewed, and 185 patients provided usable feedback in end-of-study questionnaires. A total of 123 staff feedback forms contained free-text comments. The qualitative findings provide vital insight into use of the eRAPID IT system and aid interpretation of the quantitative results. A summary of the results are outlined in Table 2 and a report is provided in Appendix 1, eRAPID systemic RCT qualitative findings.
| Type of feedback | Patients | Clinical staff |
|---|---|---|
| Benefits/positive feedback on the eRAPID IT system |
|
|
| Limitations/negative feedback on the eRAPID IT system |
|
|
| Recommendations for future system development and use |
|
|
From the patient feedback, it was apparent that many patients found the system easy to use and felt that it enhanced connections with the hospital – ‘. . . it’s like keeping in touch . . . without making an appointment to see anyone’ (study ID 44) – and helped guide decision-making: ‘. . . gave me and my family more confidence to manage side effects especially early on in the treatment. Gave me “permission” to contact the hospital if I was worried by side effects’ (study ID 402). Patients also mentioned that the system served to heighten personal involvement with health care: ‘felt good to record my symptoms every week – felt like I was taking an active role in my treatment’ (study ID 123).
Patients valued explicit use of their AE symptom reports by staff: ‘at clinic visits I had sometimes forgotten about some of the symptoms I had experienced over the 3-week period since my last visit, however the consultants using the system were able to raise issues raised – prescribing additional medication if necessary’ (study ID 216). Patients were disappointed when staff failed to use the data and there was evidence that this could affect intervention adherence: ‘no feedback from anyone – was expecting at least someone discussing usage of system but didn’t happen at all after using it for three times – so stopped using it’ (study ID 82). In staff feedback, many acknowledged the benefit of the eRAPID IT system and felt that it had been useful in practice, although others expressed reservations about the relevance of AE reports and the potential increase in consultation length.
Secondary patient-reported outcomes
Self-efficacy measures
Self-efficacy measures were included to evaluate a hypothesised increase in patients’ ability and confidence to manage effectively the side effects of cancer treatment by the provision of tailored information and advice. SES scores showed a statistically significant difference in least squares means at 18 weeks (p = 0.0073), with a small effect size of 0.433. Participants in the eRAPID intervention arm reported a higher average score (by 0.48, 95% CI 0.13 to 0.83) at 18 weeks than those in the control arm. There was no evidence of significant treatment effects for CBI-B or PAM score (although PAM score was included to be evaluated as a predictor of patient engagement).
Other quality-of-life measures
No significant between-arm differences were found for FACT-G or EQ-5D-5L utility scores. On the EuroQol-5 Dimensions (EQ-5D) visual analogue scale, eRAPID patients reported better overall health at 12 weeks (difference in means 3.50, 95% CI 0.35 to 6.66; p = 0.0302) and 18 weeks (difference in means 4.48, 95% CI 1.11 to 7.86; p = 0.0095), but not at 6 weeks (difference in means 1.36, 95% CI –1.66 to 4.39; p = 0.3773). EORTC QLQ-C30 summary scores suggested better symptom control at 12 weeks in the intervention arm (p = 0.0111). However, these are results from secondary outcome analyses and should be interpreted with caution.
Discussion
The primary hypothesis that eRAPID would have a significant positive impact on symptom control during chemotherapy (measured by FACT-G PWB subscale at 18 weeks) has not been confirmed. There is evidence that the addition of eRAPID to usual care brings a small statistically significant difference at the early time points of 6 and 12 weeks. Clinical relevance of the intervention to patient benefit was not confirmed at the 12 week follow-up; however, this cannot been ruled out at 6 weeks.
In addition, adding online self-reporting does not generate more NHS work (no difference in number of triage calls, admissions and hospital calls). This is further supported by the cost-effectiveness analysis below. Exploratory analysis of the intervention effect suggested that patients with early stage disease treated with (neo)-adjuvant chemotherapy for the first time benefited, whereas those with metastatic disease did not. The lack of effect in metastatic disease may mask the short-term benefit in non-metastatic cancers (6 and 12 weeks but not at 18 weeks). These are exploratory analyses, which would need to be confirmed in further research.
We found some evidence of the secondary hypothesis that the intervention will empower patients and increase their self-efficacy to manage better treatment side effects.
Overall, it appears that eRAPID intervention had an early impact, soon after starting treatment (6 and 12 weeks), with improvements in symptom/side effects control. However, towards the end of treatment at 18 weeks, the effect on symptom control is not present but there is a reported patient benefit in increased self-efficacy and perception of better overall health.
The majority of patients adhered to the weekly online reporting schedule and many reported perceived benefits from the monitoring and the provided advice. Patients with poor levels of compliance were those who were well and didn’t have many side effects to report and those who were very ill. Clinician engagement was variable. The exploratory analysis, examining the fidelity of the intervention, suggested that patient adherence was associated with clinicians explicitly discussing the online reports. Patients with higher adherence levels benefitted more than those with lower adherence levels. Therefore, both patient and clinician components of the intervention are important.
To the best of our knowledge, this is the first randomised trial in the UK evaluating online self-reporting of symptoms during cancer treatment and the first internationally to include detailed patient advice on symptom management. Other trials have focused mainly on alerting clinicians for severe symptoms. 36,38
The eRAPID intervention was added to already good usual-care practice in a large cancer centre. Acute oncology service reorganisation occurred at the start of the research programme, providing high-quality pathways for managing acute treatment toxicity, consistent patient support via a 24/7 patient hotline and streamlined admissions directly to oncology.
Strength and limitations of the trial
This was a large, pragmatic trial of a complex online intervention with a classic parallel-arm design that achieved good recruitment and retention rate. We recruited > 50% of the total chemotherapy population, despite the strict requirement for patient internet access. This confirms the generalisability of our findings.
However, it was a single-centre trial because of the complexities of setting up the secure online system integrated with EPRs. We engaged a large number of clinicians with different skills (e.g. oncologists and nurses) across several clinical teams, which is of course a strength. However, in practice, each clinician saw a relatively small number of patients allocated to the intervention and needed ongoing reminders to engage in the trial. These difficulties appear to have affected patient adherence and no doubt diluted the intervention effects.
Health economics analysis of eRAPID in systemic treatment
An economic evaluation was conducted in the RCT to estimate the cost-effectiveness of the eRAPID IT system for AE reporting from home or hospital, compared with usual care, for patients receiving systemic treatment (i.e. chemotherapy or targeted therapies) for breast, colorectal or gynaecological cancer. The analysis was guided by the recommendations of the National Institute for Health and Care Excellence (NICE). 87
Methods
The within-trial analysis evaluated the cost-effectiveness of the eRAPID IT system, compared with usual care, in patients receiving systemic treatment for breast, colorectal or gynaecological cancer in the UK. Costs, estimated from the health-care provider perspective (direct and indirect), and outcomes [quality-adjusted life-years (QALYs)] of patients randomised to use the eRAPID IT system versus usual care were compared over the 18-week time horizon of the trial. Because the time frame was < 1 year, discounting of the costs and benefits was not required. An exploratory analysis was undertaken at 12 months post randomisation, replicating the methods in the primary analysis. For further description of the methods, see Appendix 1, eRAPID health economics report.
Measurement of outcomes, resource use and costs
Quality-adjusted life-years were calculated based on patient health state utility values obtained from the EQ-5D-5L questionnaire at baseline, at 6, 12 and 18 weeks and at 12 months, which were mapped to EuroQol-5 Dimensions, three-level version (EQ-5D-3L), utility values using the van Hout mapping algorithm. 81,88–90
All health-care resource use over the trial period was collected using hospital records and patient-completed questionnaires at 6, 12 and 18 weeks and 12 months, and was converted to costs using appropriate UK unit costs. 91–93 Total costs for each patient were calculated as the sum of costs assigned for hospital services, community health and social services, medications and the intervention cost.
Adjusting for baseline imbalance
Multiple regression analysis was used to estimate differential mean QALYs and predict adjusted QALYs, controlling for utility at baseline. 94,95
Missing data
Where there were missing quality-of-life or cost follow-up data, multiple imputation methods were used to generate estimates of missing values based on the distribution of observed data, as per recommended best practices for economic evaluation alongside clinical trials. 96–98
Cost-effectiveness analysis
The primary analysis consisted of a cost–utility analysis over the 18-week period of the main trial and included adjustment for baseline variables and imputation of missing data. The incremental cost per QALY gained by patients randomised to use the eRAPID IT system compared with patients randomised to usual care was calculated, producing an incremental cost-effectiveness ratio (ICER).
Sensitivity analyses were conducted to explore the impact of assumptions made in the primary analysis and alternative perspectives for analysis.
The level of sampling uncertainty around the ICER was explored using a non-parametric bootstrap to generate 10,000 estimates of incremental costs and benefits. This was used to illustrate the probability that use of the eRAPID IT system is cost-effective at a range of cost-effectiveness threshold values. 99,100 The secondary analysis was undertaken from a societal perspective.
Results
For a description of the results, see Appendix 1, eRAPID health economics report.
Resource use and costs
Resource use over the trial and associated costs are presented in Appendix 1, eRAPID health economics report, tables 1–4. There was little difference in use of health-care resources and associated costs between arms, and multiple regression analysis indicated that the difference in total costs between arms was not statistically significant (£25.28, 95% CI –£1240.91 to £1167.69; p > 0.05).
Quality of life
Patient and carer EQ-5D-5L scores are presented in Appendix 1, eRAPID health economics report, table 5. EQ-5D-5L scores decreased over the trial period in both arms, but scores were higher at each time point in the eRAPID arm than in the usual-care arm. Multiple regression analysis indicated that there was no significant difference in total QALYs gained between arms (0.003, 95% CI –0.005 to 0.011; p < 0.05).
Missing data
The EQ-5D-5L scores were complete at all follow-up points for 349 (69%) patients. Resource use questionnaires were complete at all follow-up points for 205 (40%) patients.
Cost-effectiveness results
Cost-effectiveness results are presented in Table 3. The eRAPID arm had both the highest QALY gain and the lowest costs over the trial period. This indicates that the eRAPID IT system dominates usual care and may be preferred as the more cost-effective option.
| Treatment arm | Cost (£), mean (SD)a | Incremental cost (£)b | QALYs, mean (SD)a | Incremental QALYb | ICER (£/QALY) |
|---|---|---|---|---|---|
| Usual care | 8330.36 (435.23) | 0.255 (0.004) | eRAPID dominates | ||
| eRAPID | 8305.08 (450.5) | –25.28 | 0.259 (0.004) | 0.003 |
Bootstrapped estimates of the incremental costs and effects indicated that use of the eRAPID IT system may be a more cost-effective use of resources than usual care. However, mean differences in costs and QALYs were small and not statistically significant and, consequently, at the NICE-recommended cost-effectiveness threshold of £20,000 per QALY gained, the eRAPID IT system had a 55% probability of being cost-effective.
Results of sensitivity analyses are presented in Appendix 1, eRAPID health economics report, table 9. The results of the primary analysis were robust to all sensitivity analyses explored, with the eRAPID IT system dominating usual care in each case.
Results of the secondary analysis are presented in Appendix 1, eRAPID health economics report, table 9. This analysis shows that the eRAPID IT system has lower costs and higher QALY gains, indicating that its use may be cost-effective even when societal perspective costs are included.
Based on data collected for the subsample with 12-month follow-up data (see Appendix 1, eRAPID health economics report, table 11), the probability that eRAPID is cost-effective compared with usual care is 36%.
Discussion of health economic analysis
Principal findings
The primary within-trial cost-effectiveness analysis indicated that use of the eRAPID IT system may be more cost-effective than usual care for the management of AEs in patients receiving systemic treatment for breast, colorectal or gynaecological cancer. Higher QALY gains and lower costs were observed in the eRAPID arm than in the usual-care arm. However, these mean differences were small and not statistically significant, and the cost-effectiveness acceptability curve showed that eRAPID had only a 55% probability of being cost-effective at the NICE-recommended cost-effectiveness threshold of £20,000 per QALY gained. Nevertheless, the results were robust to all scenarios explored in the sensitivity analyses.
Secondary analysis indicated that use of the eRAPID IT system may also be more cost-effective than usual care when costs are analysed from a societal perspective, although the difference in costs remained small.
Strengths and weaknesses of the economic analysis
A strength of this analysis lies in the design of the study. The randomised controlled design enabled the collection of good-quality data for use in this within-trial cost-effectiveness analysis. In addition, the use of linked hospital data has provided more robust data and meant that some of the biases common in self-reported data have been avoided.
However, the lack of a completely connected system for all health-care records meant that self-reported data was nevertheless relied on to record use of non-hospital health-care services. Consequently, some biases from self-report may have remained. In addition, health-care resource use questionnaires were not well completed, with the number of missing resource use questionnaires increasing over the trial period. This resulted in a large proportion of patients with missing community health-care use data.
The internal pilot phase of the trial provided a valuable opportunity to review the design of the trial and the feasibility of data collection mechanisms. Following the pilot phase, some changes were made to the data collection forms (hospital resource use was no longer collected in patient case report forms because this would be collected directly from hospital records). However, this produced some inconsistencies in the data collected for patients in the pilot phase compared with in the main trial, which could be viewed as a limitation.
The exploratory analysis considered the longer-term impact of using data from a subsample with 12-month post-randomisation data, but results should be treated with caution given the small sample size and differences in patient population compared with the main trial (particularly the increased proportion of patients with metastatic cancer).
Meaning of the study
The economic analysis has followed recommended best practices for economic evaluations conducted in UK settings and consequently uses QALYs based on responses to the EQ-5D-5L as the primary outcome. The use of QALYs, preferably calculated based on the EQ-5D-5L, is mandated by NICE to ensure consistency across evidence used to inform their recommendations and guidelines. However, QALYs focus on the measurement of patient health and other important aspects, such as patient experience and satisfaction, may not be captured. Furthermore, patient responses to the EQ-5D-5L were necessarily collected at fixed time points according to pre-defined data collection schedules. However, owing to the intermittent and cyclical nature of patient health care and the potential for AEs during chemotherapy cycles, the use of fixed data collection points may mean that fluctuations in patient health are missed. 101
The results of this analysis indicate that the eRAPID IT system may be more cost-effective than usual care in the management of patients receiving systemic treatment for breast, colorectal or gynaecological cancer. However, the differences in QALYs and costs were small in real terms and were not statistically significant. Therefore, other factors (such as patient and health-care provider acceptability of the system) are also likely to be important.
Given that the cost of developing the eRAPID IT system, which was covered in earlier research grants,102 represents a sunk cost, it was not included in the cost-effectiveness analysis presented here. Instead, the intervention cost was calculated based on the costs that would apply if the system was implemented more widely, including the maintenance cost of QTool and the cost of a training manual for each patient. However, the costs included are likely to overestimate the per-patient cost because the software maintenance cost was split only between the patients in the eRAPID intervention arm, but in practice would be split across a much larger patient group. In addition, patient feedback on the training manual indicated that this resource could be simplified and shortened significantly, which would further reduce the cost in producing it. Consequently, the costs associated with the intervention may be overestimated and the resulting cost-effectiveness of the eRAPID IT system underestimated. For example, during the 1-year period between April 2019 and March 2020 there were approximately 1761 new patients with breast, gynaecological or lower gastrointestinal cancers across hospitals in Leeds, compared with 508 in the intention-to-treat population of this study. However, the maintenance costs for the system may apply to each region that implements eRAPID, with each having a different number of eligible patients across which to split the costs. Therefore, we split only by the study population to present a conservative estimate of likely costs and cost-effectiveness.
Radiotherapy
Background
New radiotherapy approaches have improved the survival of patients with cancers of the pelvic region. 103 However, this success comes at the cost of acute and late treatment-related AEs. 104 It is increasingly recognised that timely and more standardised documentation of AEs is critical to understanding the toxicity profile of modern radiotherapy techniques and to the provision of good patient-centred support services. 105 Monitoring online symptoms and AEs during systemic treatment for advanced cancer has shown improved symptom control and survival. 37,38
This purpose of the eRAPID radiotherapy work was to develop and assess the feasibility of collecting electronically reported treatment-related AEs in an acute curative pelvic radiotherapy setting, building on the eRAPID experience in systemic therapy. Typically, radiotherapy involves daily hospital attendance during treatment, but often AEs peak weeks after treatment and late effects such as bowel and urinary toxicity may develop months to years later. 106–109 In this project, we initially decided to focus on establishing and evaluating the feasibility of online symptom monitoring during and immediately after radiotherapy. If our approach is proven to be feasible, future research should extend it to longer-term monitoring for late effects (2–5 years following radiotherapy).
A developmental approach to AE item selection was undertaken because of the different treatment types, schedules and patterns of AEs in radiotherapy. The CTCAE system, originally developed for chemotherapy, does not sufficiently cover AEs pertinent to pelvic radiotherapy (e.g. bowel and urinary urgency).
The radiotherapy eRAPID development work was undertaken at two sites: Leeds and Manchester. It aimed to address the following questions:
-
Is it feasible to collect routine AE data during clinic visits and from patients’ homes after radiotherapy?
-
Can eRAPID be implemented in different clinical/treatment settings?
-
Will eRAPID monitoring and the associated patient management advice and guidelines lead to better AE control and better patient experience?
Changes to original plans
See Table 1 for an overview of the planned research activities. Some changes were made as the study progressed:
-
A systematic rather than a scoping review was conducted for PROM selection for radical prostate radiotherapy.
-
Cognitive interviews were not required because validated questionnaires were used with standard wording. However, the Delphi consensus exercise was extended to include staff, patients and patient advocates to strengthen the patient voice in item selection.
-
Because there were differing treatment and toxicity profiles of prostate (radiotherapy only) and other (chemoradiotherapy) pelvic radiotherapy groups, the planned sample size was increased and stratified accordingly at each site.
-
In the usability study, endometrial cancer patients (in addition to cervical cancer patients) were included to boost recruitment. The two cancer sites have a similar treatment trajectory and AE profile and were involved in the usability study.
-
Two tailored websites for patient advice for both the Leeds site and the Manchester site were developed to accommodate local radiotherapy practice variations.
Patient and public involvement specific to eRAPID radiotherapy system development
The aim and early involvement of PPI is described in Systemic treatment, Patient and public involvement specific to eRAPID in systemic treatment. The development of the eRAPID IT system in radiotherapy had substantial involvement from patient and public representatives. Carolyn Morris, a patient representative and eRAPID co-applicant, was a member of the workgroup that managed the radiotherapy workstream. The group met every 6 weeks and oversaw developments and guided decisions on recruitment, data collection, data analysis and interpretation. As mentioned in Systemic treatment, Patient and public involvement specific to eRAPID in systemic treatment, members of the Leeds site RAG and the equivalent PPI group at the Manchester site have been involved with the programme in terms of both general updates and feedback on the research provided at the biannual meetings. During and outside these meetings they have provided input on an equal basis with HCPs and researchers, informing the development and formatting of patient advice, website content and navigability, patient information sheets and questionnaire wording, they have suggested optimal times to use eRAPID in care pathways and they participated in beta-testing of the eRAPID IT system (including testing alerts and algorithms). Carolyn Morris is a listed co-author on several of our publications.
Clinical trials day
On an annual basis, the eRAPID research programme was presented in the Leeds Cancer Centre atrium, where there was engagement with patients, carers and members of the public about our research. This gave team members experience of talking to the local community about our research programme and gave us an opportunity to recruit new patients/carers to the RAG.
Patient adverse events and algorithm development (including self-management advice and website)
Methods
We conducted a systematic review of RCTs110 to identify instruments used to monitor acute and late AEs for prostate cancer. Subsequently, mapping exercises with clinical oncologists aimed to assess the coverage of the instruments for prostate AEs to identify the most comprehensive tools available and any AE gaps produced by adopting these tools. A Delphi exercise was conducted with staff and patients to reach consensus on the best items to fill these gaps from other validated questionnaires and tools [e.g. the EORTC QLQ C-3082 and European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Prostate Cancer Module (EORTC QLQ-PR25),111 Short Form questionnaire-36 items (SF-36),112 Expanded Prostate Cancer Index Composite (EPIC),113 eRAPID systemic items,76 Eastern Cooperative Oncology Group criteria114 and FACT-G80].
To identify AEs associated with anal, rectal, cervical, endometrial and vulval cancers and to rate their severity, patient interviews were carried out. Additional mapping exercises with clinical oncologists were also conducted to map and assess severity for these disease sites. Finally, chemoradiation items were selected from the item library developed in the eRAPID systemic therapy work programme. 76
Working with clinicians, we developed algorithms to establish symptom severity thresholds to determine appropriate severity-dependent symptom-specific management advice. To develop patient advice and information in QTool and on the patient websites, we reviewed and compiled this into patient-friendly language. We sourced advice from reputable national resources and local patient information leaflets about the management of common pelvic radiotherapy AEs. For more detail, please refer to Appendix 1, eRAPID radiotherapy Delphi paper, and Appendix 1, eRAPID radiotherapy developmental paper.
Results
The systematic review demonstrated that PROMs were rarely used for acute toxicity reporting and were largely employed for collecting data on late effects. Overall coverage of all relevant AEs could not be gained from one PROM alone and required augmentation with additional items. 76 Our mapping exercises found that the Male Pelvic Questionnaire (MPQ)115 provided the best coverage for AEs associated with prostate treatment; however, additional items were included to cover hormone and social/emotional items drawn from EPIC,113 European Organization for the Research and Treatment of Cancer (EORTC) measures82,111 and eRAPID AEs. 76 The MPQ115 and Female Pelvic Questionnaire116 together provided best coverage for anal, rectal, cervical, endometrial and vulval cancers. Additional items for chemotherapy were drawn from the eRAPID AE item library and stoma items from EORTC modules. A new item was developed for skin toxicity and mapped to the CTCAE using the eRAPID question format. The resulting questionnaire included 51 standard items, of which 25 were presented to prostate cancer patients, 29 to gynaecological cancer patients and 47 to anorectal cancer patients, covering urinary and bowel toxicity (stoma and non-stoma items), fatigue, physical limitations, sexual problems and chemotherapy-related AEs.
Professional and care pathways
Methods
Semistructured interviews were carried out to assess staff and patient views of eRAPID at both sites to map usual-care pathways for each disease site and determine the most appropriate clinical contact and time points for completion of the eRAPID questionnaire. 117,118 We asked about organisational issues, patient trajectories and algorithms for clinical decision-making and determined the patients’ points of contact during and outside office hours. For more detail, see Appendix 1, eRAPID radiotherapy developmental paper.
Results
Stakeholders interviews
A total of 48 patient interviews and nine relative interviews were carried out (across both sites) during radiotherapy (n = 14), at the end of treatment (n = 21) and re-interviewed a proportion of patients (n = 13) 6 weeks after treatment. A total of 26 key staff members (across both sites), including 10 clinical oncologists, eight nurse specialists, two clinic nurses, four radiographers and two service managers, were interviewed.
Care pathway maps were developed for patients receiving radiotherapy for prostate, cervical, anal, rectal, endometrial and vulval cancer at both sites (six in total). Analysis of the pathways determined the most appropriate clinical contacts. At the Leeds site, this was the oncology bleep holder (current treatment) and the medical team (post treatment). At the Manchester site, it was the treatment hotline (current treatment) and medical team (post treatment). The contact numbers were integrated into QTool advice.
The complexity of the symptom patterns and treatment pathways necessitated development of new design features in the online symptom report to accommodate overlap from AEs associated with different types of treatment and time dependencies across three disease sites. The resulting questionnaire had a more complex structure than that developed for systemic therapy. This higher degree of complexity was also reflected in the self-management advice. Two websites were developed, one tailored to each hospital, because there was variation in local practices.
Usability field study
Methods
At the end of the radiotherapy eRAPID development phase, a mixed-method usability field study was carried out. This aimed to test the whole system with end users (staff and patients) in a real-world clinical setting. This testing was in addition to rigorous technical testing of the eRAPID IT system by staff and patients during the study. For more detail see Appendix 1, eRAPID radiotherapy developmental paper.
Results
The usability testing took place between March 2016 and August 2016 with 10 prostate patients from the Leeds site and 10 gynaecological patients from the Manchester site, and 12 staff. All patients completed eRAPID weekly during radiotherapy treatment (4 or 5 weeks). Patients were prepared to report symptoms despite the fact that they were seeing their radiographer every day, and the length of the questionnaire was not deemed too burdensome. The symptom report was seen by patients as a useful reminder or prompt about what they should be looking out for and they felt that it helped them prepare for their consultations with their clinical team. This usability study enabled observation of staff and patient use of eRAPID in a clinical setting, and some modifications were made. Feedback from staff and patients was positive, and there was great enthusiasm for using the system. For more details see Appendix 1, eRAPID radiotherapy developmental paper.
Feasibility pilot randomised controlled trial
The aims of this randomised feasibility and pilot RCT study were to establish feasibility, recruitment and attrition rates, select a primary outcome measure for a future RCT, and refine the intervention by exploring staff and patient views. For full details see the published protocol. 74
Methods
The study employed a prospective, two-centre, randomised (1 : 1), parallel-arm trial with repeated measures and mixed-methods design. This used a basket trial design to test the eRAPID intervention in two distinct treatment arms: (1) radical radiotherapy for early prostate cancer and (2) pelvic chemoradiotherapy for lower gastrointestinal cancers (e.g. anal and rectal) and gynaecological cancers (e.g. cervical, vaginal, vulval and endometrial). Randomisation was stratified by participating study site and cancer site/treatment type (prostate cancer receiving radiotherapy with or without hormonotherapy and gynaecology/lower gastrointestinal cancers on chemoradiotherapy). Sample size was based on the recommendation for 30 patients per arm for each treatment type,119 allowing for 30% overall attrition (i.e. 42 patients per arm, 84 for each treatment type, totalling 168 patients). Participants randomised to eRAPID reported AEs from home weekly for 12 weeks, then at 18 and 24 weeks, in addition to usual care.
We measured and descriptively analysed patient-reported outcomes (validated HRQoL questionnaires: FACT-G80 and EORTC-QLQ-C30;82 measures of patient self-efficacy: SES83 and PAM;85 process of care measures: hospital records of patient contacts and admissions; and health economic measures: EQ-5D-5L81 and self-reported use of resources).
Semistructured interviews were conducted with staff and patients using eRAPID; in addition, they completed a short end-of-study questionnaire that collected numerical and free-text data about their experiences. Interviews were transcribed verbatim and subjected to thematic content analysis. 86
Ethics approval was granted by Yorkshire and the Humber – Leeds East Research Ethics Committee (reference 16/YH/0371). The trial was registered as ClinicalTrials.gov NCT02747264.
Results
Between December 2016 and June 2018, 502 patients from the Leeds and Manchester sites were screened for eligibility, 228 were approached and 167 provided informed consent (73.2%) and were randomised [eRAPID intervention arm, n = 83; usual-care arm, n = 84; 87 had prostate cancer, 46 had gynaecological cancer and 34 had lower gastrointestinal cancer; 103 (61.7%) were male; and the mean age was 61.7 years (SD 14.8 years)]. The active withdrawal rate was low: 16 out of 167 (9.6%; eRAPID intervention arm, n = 10; usual-care arm, n = 6). Patient compliance with online self-reporting was 82% of that expected in week 1, 63% in week 12 and 40% in week 24. Prostate cancer patients completed the most reports (mean 14.4 reports, range 3–27 reports) per patient and gynaecological cancer patients completed the least reports (mean 6.8 reports, range 0–15 reports). This low level of adherence was because of patients with cancers of the cervix/endometrium (11/23 patients completed none to three online reports). Non-adherent patients were younger (mean age 35.8 years) in comparison with the adherent patients in the chemoradiotherapy arm (mean age 45.2 years). The algorithms activated alerts for severe symptoms (4% for chemoradiotherapy and 0.5% for prostate cancer patients). Over 50% of patients triggered the algorithm for milder symptoms and were given self-management advice. No differences in recruitment and retention rates were seen between the two study sites. The mean number of patient calls to hospital was 0.8 (range 0–8) for both arms (calls by nurses to patients: eRAPID intervention arm, n = 1.1, range 0–15; usual-care arm, n = 0.8, range 0–9).
Return rates of outcome measures were 95.8% at baseline, 77.8% at 6 weeks and 73.7% at 24 weeks. Missing item rates were low; only 0.6–5.4% of the returned questionnaires had a large number of missing items, not allowing the calculation of the scores.
Descriptive statistical analysis of patient outcome measures suggested that patients randomised to the eRAPID intervention reported less deterioration in scores over time than patients randomised to usual care, with the biggest difference observed at 6 weeks. There was a notable difference between chemoradiotherapy and prostate cancer patients: change scores were minimal in the prostate cancer arm and larger in the chemoradiotherapy arm, suggesting that the between-arm differences are driven by the chemoradiotherapy effects.
Interviews were performed with 11 patients and four clinicians from the Leeds site, three Manchester site staff provided written feedback and 61 patients from across both sites returned the end-of-study feedback questionnaire. Themes and example quotations are summarised in Appendix 1, eRAPID radiotherapy pilot draft paper, and Appendix 1, eRAPID radiotherapy qualitative findings. Both staff and patients found the system easy to use, albeit reflecting on different system interfaces and uses. Reassurance from the information and support and monitoring of side effects was a strong positive element for patient participants. However, motivation to use the system diminished when patients felt ‘recovered’. Staff referring to and discussing the reports in consultations were a big incentive for patients, and some staff did this regularly; however, patients were not always sure if staff were using them. This was of concern because a major benefit of eRAPID for patients was its potential to inform staff of the side effects experienced. However, patients did feel supported by the intervention and felt that it connected them with the clinical team.
Staff reported finding it difficult to identify which patients were using the eRAPID IT system because eRAPID patients formed a small number in the midst of their busy clinics. Prompting was often required from the research team to remind clinicians to look at the reports. However, clinicians found eRAPID useful in preparation for their clinics and thought it would be useful to extend the follow-up period beyond 24 weeks to capture late effects.
For a more detailed description, see Appendix 1, eRAPID radiotherapy pilot draft paper.
Conclusions from the pilot randomised controlled trial
This two-centre pilot RCT of online monitoring of AEs during and after pelvic radiotherapy confirmed that recruitment was feasible and that the eRAPID intervention was acceptable to both patients and staff. A consent rate of > 70%, withdrawal rate of < 10% and rate of compliance with online completions of 60–70% met our a priori criteria in the prostate and lower gastrointestinal cancer arms. However, the eRAPID approach may not be suitable for young women aged < 40 years with lower education levels who receive intensive chemotherapy for advanced cervical cancer. Patient outcome measures suggest potential differences in the expected direction mainly in the chemoradiotherapy groups, with most impact at 6 weeks; however, this observation needs confirming in a formally powered RCT. The trialled patient outcome measures, FACT-G and EORTC QLQ-C30, showed similar properties and detected differences in the expected direction. The team had a slight preference for recommending the EORTC QLQ-C30 summary score, because most of the scales comprising the summary score (including functional and symptom scales) showed differences in the same direction.
The qualitative findings, similar to those in the systemic RCT, emphasised the importance of engaging clinicians to discuss the reported symptoms. This was seen by patients as a major incentive. Other patient benefits were acknowledged, such as personal reassurance and support for symptom management.
Conclusions for eRAPID radiotherapy study
The eRAPID radiotherapy strand successfully delivered a wide range of research activity, developing and integrating the electronic platform, identifying suitable questionnaires for self-reporting AEs, selecting item thresholds, developing advice and delivering a two-centre feasibility pilot RCT.
We showed that it is possible, with robust developmental work, to use symptom items or fully validated pelvic-specific PROMs (and additional items to cover gaps) to report treatment-related AEs in radiotherapy. We successfully delivered a systematic review identifying PROMs used in prostate cancer RCTs and conducted a Delphi consensus exercise (with staff and patients) to select the items for online reporting. Engaging patients and key clinicians, we developed item thresholds and severity-tailored patient advice applicable to local radiotherapy practice at each centre.
The methodology used for developing the eRAPID radiotherapy intervention can be recommended for future projects. Our experience confirmed again that the eRAPID approach is a complex health-care intervention that requires a high level of tailoring to the cancer site, treatment and hospital. One size does not fit all, although the proof of principle remains intact.
The pilot RCT established the feasibility and acceptability of the eRAPID approach at two leading cancer centres in the UK. We noted the similar recruitment and retention rate at both sites (at the Leeds site with a lot of eRAPID experience and at the Manchester site with minimal prior experience), which supports generalisability. We noted very high levels of adherence to weekly eRAPID reporting among the prostate cancer patients (i.e. among male and older patients) and noticeably lower levels of adherence in the young women with cervical cancer.
It is reassuring that differences in the expected direction were observed in the patient outcome measures, confirming that the selected eRAPID items and tailored advice were appropriate. Perhaps unexpected was the observation that the eRAPID intervention appears to have had an impact in patients receiving chemoradiotherapy and no detectable effect in those with prostate cancer. The observed, encouraging trends in the data will enable hypothesis generation for a future RCT. In addition, this study enabled us to identify a primary outcome measure for a future definitive trial, with the EORTC QLQ-C30 identified as the main candidate. The SES also performed well.
A limitation in the pilot RCT was the inability to integrate the eRAPID reports into MOSAIQ™ (Elekta Solutions AB, Stockholm, Sweden) radiotherapy software within the time frame of the programme; as a consequence, radiographers did not have easy access to patient self-reports. The patients told us clearly that they expected their clinician to use the AE reports and, if they did not, this was a disincentive. However, recent successful integration of the eRAPID IT system into MOSAIQ will enable radiographers to access the AE reports during a future trial.
Research recommendations
This two-centre trial has generated sufficient evidence on the feasibility and acceptability of the eRAPID IT system in radiotherapy to recommend a multicentre RCT to formally evaluate the utility in routine care during and immediately after radiotherapy. We recommend that, even when the eRAPID intervention is delivered in a multicentre setting, it has to be adapted to local radiotherapy care pathways and adhere to local patient information and support.
The importance of engaging clinicians to regularly review and act on the AE reports was highlighted; therefore, we recommend that the design of a future trial considers alternatives to the traditional parallel-arm RCT to enable clinicians to see larger number of patients with self-reports. Stepped-wedge designs or cluster randomisation may be considered.
It was challenging integrating and maintaining the bespoke IT platform across two sites with differences in infrastructure and compatibility. This model is unlikely to be sustainable for a multicentre trial. We recommend an IT set-up that allows HCPs to access patient online reports via a centrally hosted software.
Clinicians strongly recommended a longer follow-up period (of up to 5 years) to allow late-effects monitoring. However, the feasibility of longer-term monitoring will require further investigation and must explore the costs and benefits for patients because our study did not provide long-term data. Furthermore, patients indicated less willingness to engage in online reporting when they felt ‘well’.
Reflections on the work programme in radiotherapy
Although we successfully delivered the radiotherapy work across two sites, we underestimated the length of time required for both IT aspects and the adaptation of the eRAPID intervention at each site. We did not anticipate the need for two patient websites, to accommodate differences in patient advice owing to variable lengths in radiotherapy schedules and patient care pathways for managing acute toxicity. This highlights the issues with a lack of standardisation of radiotherapy processes and pathways, which will continue to be a challenge. Our results suggest that the key is to engage with key staff members and patients to enable the smooth integration of the system elsewhere.
Our study provided a proof of concept to enable staff to see the benefits of integrating patient-reported AEs in EPRs. Following the eRAPID programme, The Christie NHS Foundation Trust has started a new programme introducing routine PROMs into mainstream oncology care using a commercial software. Furthermore, the study has demonstrated a proof of principle that symptom items can be adapted for online reporting and severity thresholds can be established with clinical input.
Surgery
Background
Major surgery for cancer aims to cure patients but can have serious side effects; it is associated with a significant length of hospital stay, AE risk and a detrimental impact on patients’ QoL. Recovery (i.e. resolution of symptoms/post-operative complications) takes months. Up to 50% of patients experience resource-intensive short- or long-term complications or AEs (e.g. infection, sepsis or pneumonia) following surgery for oesophageal cancer. 120 Up to 13% of patients are re-admitted within 30 days. 121,122 During the first month, patients typically also report significantly reduced physical function123 and frequent, distressing and potentially problematic symptoms.
Enhanced recovery after surgery (ERAS) programmes are increasingly used to improve clinical outcomes and are directly associated with early discharge from hospital. 124 Although this is beneficial in many ways, it means that patients are required to deal with distressing and potentially problematic symptoms post discharge. Historically, these symptoms would have been detected and managed in hospital. 121 Some of these post-discharge problems will escalate to serious complications and require re-admission. Therefore, it is important to equip patients post discharge with the correct advice and information. Currently, ERAS protocols are not standardised125 and do not extend beyond the hospital stay. 126 Post-discharge care is fragmented between different services127 and inconsistent. 125,128 It predominantly involves costly and infrequent outpatient appointments or telephone calls from clinical nurse specialists. Provision of such care is inconsistent and services are threatened by NHS pressures. 17,129 The timing of clinic follow-up varies between hospitals, with patients often not reviewed until several weeks post discharge. 129 Detection of problems post discharge depends on the patient’s ability to recognise and distinguish between typical and clinically concerning symptoms. Barriers include patient concerns about contacting care teams outside scheduled appointments and lacking knowledge about when to seek help. 64,130 Late reporting of problems can exacerbate symptoms and delay AE detection, resulting in more severe complications, increased NHS burden, poorer outcomes and delayed adjuvant treatment. 28,131–134 Little previous research has investigated how using electronic patient reporting and monitoring of symptoms post discharge can improve post-hospital care and detect AEs.
The aim of the eRAPID surgery study was to develop and examine the feasibility of a real-time, remote electronic system to monitor patients’ symptoms and problems after discharge from hospital following cancer-related major abdominal surgery. The eRAPID surgery system was intended to be integrated into hospital EPR and provide tailored feedback to patients and clinicians based on the severity of reported symptoms.
Changes to the original research plan
Original plans for testing the eRAPID surgery system included development work and usability testing with patients and HCPs prior to a small pilot study at a single site (the Bristol site) to examine feasibility acceptability and adherence ahead of a future main trial. Changes were made to the original surgery research plan to undertake development work followed by more extensive usability and feasibility testing within a larger, two-centre, non-randomised pilot study (to include the Birmingham site). A larger usability study was considered beneficial for three reasons:
-
Patients in this surgery cohort experienced relatively fewer AEs than systemic therapy patients.
-
Patients and HCPs in the Bristol site cohort (and in surgical cohorts more generally) were relatively unfamiliar with systems for electronic reporting of patient-reported outcomes (including AEs) compared with those in the systemic cohort in the Leeds site. Therefore, we needed to examine how HCPs interact with the eRAPID interface and the acceptability of the eRAPID IT system and its integration into the clinical pathway before proceeding to a randomised pilot study. A second site (the Birmingham site) was included in the pilot study to explore the feasibility of the system as an intervention in a different surgical oncology setting (where electronic AE reporting and monitoring is also novel) and to reflect a wider range of services.
-
We experienced delays and complications related to IT integration with EPRs at the Bristol site that would benefit from more comprehensive evaluation over a longer time period. The version of eRAPID tested at the Birmingham site was not integrated in hospital EPRs. Therefore, data from the Birmingham site enabled a full exploration of integration and ease of use of the online web-based system where EPR integration was not viable. Data were analysed separately and focused primarily on examining participant recruitment, eRAPID symptom report response rates and data completeness.
Patient and public involvement specific to eRAPID surgery system development
The eRAPID surgery system was developed with input from PPI representatives throughout the study. An established local patient support group for patients and family/friends of patients affected by upper gastrointestinal cancer at the Bristol site was consulted by members of the research team. Proposals for the eRAPID surgery study were presented during a meeting attended by 15 support group members. Feedback was sought on the relevance and design of the proposed study. In addition, members were asked to complete a brief questionnaire regarding which symptoms had been the most difficult to manage during their recovery and what advice they had encountered that they considered had been the most useful. The findings from the PPI meeting highlighted the importance of including qualitative work (e.g. interviews) with patients to explore their experiences and perceptions of using the eRAPID IT system and receiving system advice and alerts, and informed the research plans to develop the eRAPID surgery system clinical algorithms that would trigger feedback and advice based on symptom report data. In addition, findings from the PPI consultation were considered at the algorithm-development stakeholder meetings.
Patient adverse event items and algorithm development135
This section contains text reproduced or adapted from Avery et al. 135 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Methods
The eRAPID surgery system was developed between February 2014 and August 2017, and the developmental work is now published. 135
Patient adverse event item development
A scoping review was conducted of existing, established, validated items from the EORTC QLQ-C30 and related modules identified for inclusion in the patient symptom report. This measurement system is appropriate for patients undergoing cancer surgery and is established and routinely used as a PROM in international trials to improve patient outcomes and care. Items of relevance to symptoms and complications experienced by patients after cancer-related major abdominal surgery (e.g. surgery for oesophageal, gastric and hepatopancreatobiliary upper gastrointestinal cancer) were selected. Face-to-face cognitive interviews with a purposively selected broad sample of upper gastrointestinal cancer surgery patients aged ≥ 18 years evaluated the suitability, acceptability and comprehension of the items selected. Participants’ thoughts during item completion were explored using verbal probes to identify problems experienced with understanding or responding to items. Sampling and data analyses proceeded iteratively until no further refinements were required.
Algorithm development
Clinical algorithms to determine when tailored feedback/advice would be triggered based on patient-reported symptoms were developed from the following:
-
Data from eRAPID patient-generated symptom report data from consecutive patients discharged from the Bristol site from 15 April 2016 to 15 August 2017 following cancer-related upper gastrointestinal surgery. Patients completed the symptom self-report at discharge (baseline), twice in the first week post discharge (days 2 and 7) and weekly thereafter for 8 weeks (although participants could complete the symptom report at additional time points if they wished, e.g. if experiencing new symptoms).
-
Data from weekly telephone interviews between participants and a study researcher/research nurse, focusing on patients’ reflections of the nature and severity of post-discharge symptoms and contact with and use of health-care services in the previous week.
-
Two stakeholder meetings with clinicians responsible for the clinical care of patients undergoing major cancer-related upper gastrointestinal surgery and a patient representative. The objective of the stakeholder meetings was to determine symptom severity thresholds to categorise which symptoms or symptom combinations would be considered clinically important/concerning at a given time point post discharge. Discussions focused on identifying bothersome or concerning (e.g. indicative of a potential AE) symptoms that may warrant advice from either a patient or a clinician perspective, and appropriate advice content. A patient participant attended stakeholder meetings to facilitate discussion of patients’ perspectives on the findings from earlier PPI work (described in Patient and public involvement specific to eRAPID surgery system development) around which symptoms patients find concerning and may warrant advice/reassurance.
-
End-of-study interviews with a purposively sampled 10% subset of participants, approximately 10 weeks post discharge. Interview topic guides were developed, which were informed by literature, analysis of nurse-led telephone consultations and study team discussion (including a professor of upper gastrointestinal surgery and cancer nurse specialist). Data were analysed in an iterative, cyclical manner as data collection proceeded to develop and refine the clinical algorithms.
Algorithms were further refined by comparing patient symptom report data and associated actions and advice triggered by the system with (1) data from the weekly telephone interviews; (2) advice and reassurance about symptoms and problems provided by a cancer nurse specialist and participants during audio-recorded routine (i.e. usual care) telephone consultations; and (3) post-discharge clinical events or outcomes of participants (e.g. re-intervention, re-admission to hospital or visit to a GP or primary health-care provider) identified from hospital EPRs, re-admission alerts and patient-reported health-care use.
Patient advice development
Three data sources informed the development of tailored patient self-management advice: (1) end-of-study interviews, (2) audio-recorded routine telephone consultations between a cancer nurse specialist and participants during the first week (at approximately days 2 and 7) post discharge and (3) a scoping review and thematic content analysis of NHS-recommended patient recovery information and patient information leaflets (conducted between September 2016 and January 2017). Relevant targeted sections of audio-recordings were transcribed verbatim. Data were analysed to identify themes relating to post-operative advice and reassurance, and options for appropriate phrasing and terminology for the self-management advice were identified from transcriptions and from NHS patient information and patient information leaflets. Draft advice was iteratively refined through discussion with the study team and clinicians involved in the stakeholder meetings.
Results
Patient adverse event item development
A total of 30 relevant items were identified from 95 potential items included in seven validated EORTC questionnaires. Five additional items were included following consultation with clinicians, resulting in a total of 35 items included in the patient symptom report. Item comprehensiveness and acceptability were verified through interviews with 18 participants (men, n = 16; mean age, 66.3 years).
Algorithm development
Some 300 patients were screened, of whom 130 (43%) were eligible and invited to participate and of whom 61 (47%) consented. A total of 59 (97%) participants (men, n = 34; mean age, 61 years) accessed the eRAPID IT system a total of 459 times, and 444 completed symptom self-reports were obtained. Weekly follow-up interviews with these 59 participants (men, n = 34; mean age, 61 years) and end-of-study interviews with seven participants (men, n = 4; mean age, 58 years) led to refinements to patient symptom report branching logic, and the addition of nine subitems to distinguish between ‘typical’ and ‘atypical’ symptoms, and eight subitems to distinguish between ongoing and resolved symptoms.
One patient representative, six nurses, two dieticians and one surgeon from four hospital sites participated in two stakeholder meetings. Findings indicated that algorithms should account for date since hospital discharge to allow more accurate evaluation of whether or not symptoms were typical and atypical (i.e. indicative of an AE) depending on the individual patient’s stage of recovery. The importance of reassurance that some symptoms are expected during recovery was also identified.
A comparison between data sets (e.g. system-triggered actions/advice, clinician consultations and clinical events/outcomes) from 27 participants (men, n = 18; mean age, 63 years) reporting clinically significant symptoms resulted in several further refinements to the patient symptom report questionnaire (e.g. addition of items to evaluate wound problems, feeding tubes and contact with HCPs) and algorithms (e.g. refinement of symptom severity thresholds and addition of subitems and branching logic) over an 8-month period of system testing.
Patient advice development
Analysis of data from 15 routine care telephone consultations between a cancer nurse specialist and eight participants (men, n = 5; mean age, 62 years) identified four themes relating to post-operative advice and reassurance around pain, other physical symptoms, diet/nutrition and managing recovery sought by participants. 135 Data from seven end-of-study participant interviews (men, n = 4; mean age, 58 years) confirmed that the self-management advice was acceptable and relevant. This was combined with data from analysis of 28 patient information leaflets identified from 16 NHS trusts and three cancer support charities to develop draft self-management advice for 22 symptoms, tailored to individual patient symptom severity. Draft advice was reviewed and refined at two stakeholder meetings.
Professional and care pathways135
This section contains text reproduced or adapted from Avery et al. 135 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Methods
To ensure that the eRAPID IT system was developed and integrated in harmony with existing care pathways and that it would optimally inform the post-discharge follow-up care of surgical patients, analysis of care pathways at the Bristol site was conducted by study researchers and the hospital cancer nurse specialist team. The most appropriate time points for eRAPID symptom report completion and the most suitable clinical contacts for patients experiencing potential AEs were identified to inform development of system-triggered feedback/advice.
Results
Analysis showed that patients are not routinely reviewed in a clinic by a surgeon until several weeks post discharge, by which time most symptoms and problems are expected to have resolved (Figure 6). Instead, the cancer nurse specialist team is responsible for telephone follow-up of patients to monitor patients’ progress with recovery and to detect problems or AEs during the early post-discharge period. Therefore, the specialist nurse team was identified as the most appropriate clinical contact for patients experiencing potential AEs, except for minor to moderate wound problems, for which GP or local community health-care teams were most appropriate. Analyses also confirmed that the most appropriate time points for patient completion of the eRAPID symptom self-report were twice in the first week then weekly for 8 weeks post discharge, to reflect the time points that the specialist nurse team typically contacts patients. The timing of follow-up contact points reflects the expected symptom profile and expected typical recovery trajectory of patients undergoing major cancer-related upper gastrointestinal surgery, where post-discharge symptoms and problems are frequent but expected to gradually improve. This contrasts with chemotherapy regimen symptom patterns. It was acknowledged, however, that it would be important for patients to have the option to complete the symptom report at additional time points (e.g. if they experienced new symptoms). Findings from the care pathway analysis were used to inform the development of the clinical algorithms and feedback/advice generated by the eRAPID IT system.
FIGURE 6.
Patient care pathway analysis. a, Macmillan Cancer Support, London, UK. BRI, Bristol Royal Infirmary; CNS, clinical nurse specialist; GOSH, Gastro/Oesophageal Support and Help Cancer Group Bristol.

Feasibility pilot study
A mixed-methods feasibility pilot study of the eRAPID surgery system was developed between August 2017 and March 2018. Two publications reporting the findings have been published;136,137 see Appendix 1, eRAPID radiotherapy developmental paper.
Methods
Following development of the eRAPID surgery system, a mixed-methods approach was undertaken to assess the system’s usability and feasibility. The study took place at two sites (the Bristol and Birmingham sites). The rationale for including a second site in which the eRAPID IT system was not accessible through EPRs is provided in Changes to original research plan.
Piloting of the eRAPID surgery system
Consecutive patients from each centre who had undergone cancer-related upper gastrointestinal surgery were recruited. Eligible patients had undergone cancer-related upper gastrointestinal surgery, were ready for hospital discharge to their home, were aged ≥ 18 years, had access to a computer/mobile device and the internet at home, and were fluent in English. Participating patients were asked to prospectively complete the symptom self-report at discharge (baseline), twice in the first week (at approximately days 2 and 7) and weekly thereafter for 8 weeks. However, participants were told that they could complete the symptom report at additional time points if they wished (e.g. if they experienced new symptoms). Data analysis was conducted separately for the two sites and focused on symptom report response rates, reasons for non-completion, data completeness, frequency and severity of symptoms reported, and any system actions generated.
Rates of participation of relevant HCPs in monitoring the shared e-mail inbox to which alerts triggered by the eRAPID IT system were sent were evaluated. Although HCPs at the Bristol site were able to access eRAPID IT system patient symptom report data through EPRs during patient post-discharge follow-up consultations (telephone and/or face-to-face clinic appointments), which either took place as part of usual care or resulted from the eRAPID IT system alert, it was not possible to monitor rates of use owing to administrative and time constraints. However, weekly interviews were undertaken with participating HCPs to explore their engagement with and perspectives of the eRAPID IT system (described in Qualitative interviews with patients and health-care professionals).
Qualitative interviews with patients and health-care professionals
Semistructured qualitative interviews with participants were conducted weekly (all participants) to coincide with eRAPID symptom self-report time points and at the end of the study (a 10% purposive subsample). Interviews explored patients’ experiences of using the system and receiving tailored feedback and/or advice to contact a health-care professional. Weekly interviews were conducted with the lead cancer nurse specialist (Bristol site only) to examine engagement with the system and perspectives on the frequency, nature and relevance of patient contact resulting from eRAPID advice/alerts. End-of-study interviews with the lead nurse and hospital dietician (Bristol site only) were also conducted to explore views towards the practicality and usefulness of the eRAPID IT system in the context of routine clinical care. Interview topic guides were developed, informed by literature, nurse-led telephone consultations and study team discussion. Interviews containing data relevant to the study objectives were transcribed verbatim (targeted transcription) and textual data analysed in accordance with the principles of thematic analysis. Emerging codes were refined through iterative discussion with the study team as analyses progressed and until thematic saturation was reached.
Technical performance
The technical functionality of the eRAPID IT system, including integration with EPRs and the number of failed logins, HCP e-mail alerts and participant text/e-mail reminders, was monitored throughout the study.
Piloting of potential outcome measures for a main trial
The timing and acceptability of potential outcome measures for use in a future definitive main trial were examined. Participants completed paper-based FACT-G and EQ-5D questionnaires at baseline, week 3 (week 4 post surgery) and week 7 (week 8 post surgery). Methods for health-care resource use data collection were also examined. Patients completed a paper-based health resource use questionnaire at the end of the study, which recorded use of prescription and non-prescription medication and other costs associated with patients’ recovery from surgery. In addition, patients were telephoned by the study researcher at weekly intervals to record the frequency and reasons for HCP (e.g. GP and community nurse) contact.
Results136,137
This section contains text reproduced or adapted from Richards et al. 136 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
This section contains text reproduced or adapted from Richards et al. 137 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Piloting of the eRAPID surgery system
Between August 2017 and March 2018 at the Bristol site, 109 patients were screened; 41 (38%) were eligible and 29 (71%) consented to participate (men, n = 19; mean age, 64.2 years). A total of 17 (25%) patients were ineligible because they did not undergo their planned surgical procedure and 15 (14%) were ineligible because they lacked a computer/mobile device/internet access. There did not appear to be any major differences between patients who did and patients who did not take part. Seven (24%) participants withdrew because they felt too tired/unwell or had experienced a prolonged re-admission to hospital. A total of 11 (55%) of the 20 Birmingham site patients screened between November 2017 and October 2018 consented to participate, of whom two (18%) withdrew. At the Bristol site, response rates to the eRAPID IT system symptom report questionnaire at key assessment time points exceeded 70% (range 72–93%), excluding day 2 to 3 post discharge (55%) when patients might have felt too unwell to access the system. The most frequent reasons for non-completion were participants starting adjuvant chemotherapy (12%) and not wanting to complete the symptom report at that time point (10%). These findings indicate that it may be beneficial to incorporate non-responses at critical time points as an indicator of potentially concerning symptoms that could trigger an alert to clinicians. At the Birmingham site, eRAPID symptom report response rates exceeded 60% at all time points (range 63–100%), excluding week 8 (response rate 50%) when participants are expected to have achieved a good level of recovery from surgery.
At the Bristol site, all four members of the upper gastrointestinal specialist team responsible for the care of patients post discharge after surgery, including three cancer nurse specialists and one dietician, agreed to participate in the study. All nurses participated in accessing and acting on alerts triggered by the eRAPID IT system and communicated to the shared e-mail account. Although exact frequencies are not available, all four team members utilised eRAPID patient symptom report data to inform their routine patient follow-up care (during nurse–patient telephone consultations and/or face-to-face clinic appointments). At the Birmingham site, all three upper gastrointestinal cancer nurse specialists in the team participated in the study and accessed and acted on eRAPID alerts. Ad hoc administrative discussions with nursing staff at the Birmingham site indicated that the clinical nurse specialist team considered the lack of integration with hospital EPRs a barrier to the usefulness of the eRAPID IT system for contributing to routine clinical care and limited their engagement with the system.
Between 30 August 2017 and 17 April 2018, 29 Bristol site participants completed the eRAPID symptom report a total of 197 times (median 9, range 1–11); of these reports, nine (5%) triggered a level-3 action (clinician alert), 72 (36%) triggered a level-2 action (advice to contact a clinician), 76 (39%) triggered a level-1 action (self-management advice) and 40 (20%) triggered no advice (Table 4). Seven (78%) of the nine level-3 complications triggering HCP feedback and patient advice to contact a HCP immediately were reported during the first 4 weeks post discharge. Potentially concerning symptoms decreased thereafter, coinciding with milder/expected symptoms. A further eight level-3 alerts triggered by baseline (i.e. pre-discharge) symptom report completion were removed from the data set following discussion with clinicians because they were considered irrelevant because the patients were still in hospital. A total of 60% (n = 43) of the 72 level-2 actions (i.e. participant advised to call a HCP) were triggered during the first 2 weeks post discharge. Just 15% (n = 11) of level-2 actions were triggered after 6 weeks post discharge. Most (n = 48, 63%) of the 76 level-1 actions (i.e. self-management advice) were triggered after 3 weeks post discharge, most commonly at weeks 4 and 5. A total of 27 (68%) of the 40 level-0 actions (i.e. no participant advice required) were triggered after 5 weeks post discharge. The findings support literature indicating that most complications and problematic/distressing symptoms occur within 30 days post surgery, with reduced symptoms from 4 weeks post discharge. 137 At the Birmingham site between 10 November 2017 and 22 November 2018, participants completed the eRAPID symptom report a total of 63 times (median 7, range 1–10); of these reports, one (2%) triggered a level-3 action, 20 (32%) triggered a level-2 action, 36 (57%) triggered a level-1 action and six (10%) triggered no advice. The pattern of timing of these actions was similar to the pattern at the Bristol site. One level-3 alert was triggered at week 3. Most (n = 14, 70%) level-2 actions were triggered in the first 3 weeks post discharge, most (n = 25, 69%) level-1 self-management advice was triggered after 3 weeks post discharge and the majority (n = 5, 83%) of level-0 feedback was triggered after 4 weeks post discharge.
| Reported symptoms and subsequent actions | Number of times symptom triggered action (N = 197), n (%) | Number of patients triggering actions at any time point (N = 29), n (%) |
|---|---|---|
| Level 3 action: alert to cancer nurse specialista | 9 (4.6) | 3 (10.3) |
| Pain | 7 (3.6) | 2 (6.9) |
| Fever and chills | 2 (1.0) | 1 (3.4) |
| Level 2 action: advised to contact HCPa | 72 (36.5) | 24 (82.8) |
| Wound problems | 32 (16.2) | 14 (48.3) |
| Appetite loss | 26 (13.2) | 12 (41.3) |
| Fever and chills | 18 (9.1) | 6 (20.7) |
| Physical function | 10 (5.1) | 8 (27.6) |
| Nausea and vomiting | 8 (4.1) | 8 (27.6) |
| Shortness of breath | 7 (3.6) | 5 (17.2) |
| Level 1 action: symptom adviceb | 76 (38.6) | 22 (75.9) |
| Fatigue | 58 (29.4) | 20 (70.0) |
| Pain | 27 (13.7) | 12 (41.3) |
| Physical function | 22 (11.2) | 10 (34.5) |
| Constipation | 20 (10.2) | 10 (34.5) |
| Nausea and vomiting | 20 (10.2) | 8 (27.6) |
| Reflux | 17 (8.6) | 8 (27.6) |
| Level 0 (no/minimal symptoms): no advice required | 40 (20.3) | 9 (31.0) |
At the Bristol site, most level-3 actions (n = 7, 78%) were generated by pain symptoms. Level-2 actions were most commonly triggered by problems with wounds (44%), appetite loss (36%), fever (24%), physical function (13%) and nausea/vomiting (11%). Level-1 actions were generated most frequently for problems with fatigue (n = 58) and/or pain (n = 27), physical function (n = 22), nausea or vomiting (n = 20) and constipation (n = 20). The eRAPID IT system ensures that patients are not overburdened with self-management by limiting advice to a maximum of the six most clinically concerning symptoms per eRAPID self-report completion. 135 Level 1 actions were triggered for a median of three (range 1–6) symptoms per level-1 action triggered.
A total of 109 weekly interviews were conducted with patient participants. Analyses indicated that some level-3 system actions had been triggered by symptoms for which the patient had already received treatment from another care team or symptoms relating to pre-existing conditions and, therefore, that no action needed to be taken by the participating cancer nurse specialist team. This indicated that further minor refinements to the level-2 and level-3 algorithms were needed to ensure that symptoms relating to underlying health conditions and relevant symptoms that are already being well-managed do not trigger HCP alerts. Corresponding weekly telephone interview data were available for 36 (50%, 17 participants) of the 72 level-2 actions triggered. In most cases (23/36, 64%), participants contacted a HCP for only new or previously unreported symptoms, in accordance with level-2 advice. In eight (35%) cases, all participants with new symptoms adhered to advice and contacted a HCP. Of these, four participants contacted a GP and four contacted the cancer nurse specialist team. This led to four participants receiving advice and/or reassurance from clinicians, three undergoing clinical interventions or investigations (e.g. appointments and blood tests) and one receiving antibiotics for a wound infection. In 15 cases (65%), participants appropriately followed advice not to contact a HCP, either because they had an upcoming clinical appointment and/or because their symptoms were already being appropriately managed. Among the 13 (36%) instances when participants did not adhere to level-2 advice, reasons for not contacting a HCP included feeling that their symptoms did not warrant reporting (e.g. because they already had an appointment scheduled, n = 6, 46%), not recalling receiving the advice (n = 6, 46%) and choosing not to contact a HCP (n = 1, 8%).
Five Bristol site participants were re-admitted to hospital for complications (fevers/infections, n = 3; nausea and vomiting, n = 1; and bile leak, n = 1). These participants completed the eRAPID symptom report a total of 14 times within 7 days prior to re-admission, resulting in two level-3 events (14%), nine level-2 events (64%) and one level-1 event (7%). This indicates that, in most cases, the eRAPID IT system produced appropriate advice regarding the symptoms reported by patients. Interviews indicated that some other participants may not have completed the symptom self-report prior to hospital re-admission because they had recognised the severity of their symptoms and instead contacted the care team without being prompted by the eRAPID IT system.
Qualitative interviews with patients and health-care professionals
Of the 173 potential weekly interviews, 109 (63%) were conducted, including at least one interview with all 29 patient participants. Of these, 35 interviews from 16 participants were selected because they contained data of relevance to the research questions, in accordance with the principles of targeted transcription. Most participants reported positively on their experience of using the eRAPID IT system. Data confirmed that patients found the system valuable and reassuring, particularly with regards to receiving confirmation that their symptoms were typical for their stage of recovery and having a method for objectively tracking progress with symptom improvement over time. Patients reported feeling empowered to take a central role in their own recovery because the system provided new and tailored symptom self-management advice and reminded them of and reinforced the guidance that they had previously received. Patients also stated that the system enabled them to overcome feelings of isolation and uncertainty post discharge136 (see Appendix 1, eRAPID radiotherapy developmental paper).
Health-care professional interviews (n = 10 nurses/dieticians) indicated that the system was a valuable adjunct to routine surgical follow-up care, particularly for interpreting the clinical significance of patients’ symptoms and progress in their individual recovery. Most system-initiated patient contact was regarded as appropriate and timely and, in some cases, directly informed patients’ subsequent clinical management. HCPs indicated that symptom self-report data collected by the system were useful to facilitate and enhance nurse–patient telephone consultations and acknowledged the value of the system to provide reassurance to patients outside ongoing nurse–patient consultations. The potential for technical problems with hospital systems (e.g. accessing EPRs) and problems with accessing computers in clinic were regarded as factors that may influence the use of the eRAPID surgery system in routine clinical practice.
Technical performance
At the start of the eRAPID research programme, a new Medway release was being installed, which delayed QStore IT integration. Establishing working relationships with key staff took time and significant input from the local eRAPID co-applicant/surgical lead. Between April 2016 and August 2017 there were four instances of loss of Medway integration, resulting in clinicians being unable to access eRAPID results through EPRs. There were two instances where integration was lost for 1 day and one instance where integration was lost for 6 days. A Medway software update resulted in the longest incident of integration loss, from 13 June 2016 to 28 October 2016. A separate ‘Administration and Report’ section in QStore was developed, allowing staff to log in directly to QStore using a secure login to administrate and see participant results and notification reports during periods of downtime. From May 2016 to March 2018, there were five instances of reminder system downtime, totalling 39 days.
Piloting of potential outcome measures for a main trial
The FACT-G and EQ-5D questionnaires were completed by 19 (66%) and 20 (69%) participants at week 4 post discharge and by 15 (52%) participants at week 8. Health resource use forms were completed by 15 (52%) participants.
Discussion
A real-time, hospital-integrated symptom-monitoring system has been developed and tested to optimise the effective management of symptoms and complications experienced by patients after hospital discharge following cancer-related surgery. At the Bristol site, initial system development included creating the symptom report questionnaire using validated items and mapping patient care and recovery pathways. The system was then successfully integrated with the EPR system at the Bristol site, and algorithms were developed to set thresholds for triggering feedback to patients/clinicians. The system has been co-developed with stakeholder input at all stages and in harmony with existing care pathways to ensure that system actions are relevant and meaningful to patients and HCPs, and to optimise the functionality and usability of the system in an NHS setting. System feedback was designed to be immediate, patient tailored and symptom dependent, developed with input from patients, patient representatives and various HCPs (e.g. cancer nurse specialists, dietitians and surgeons) and multiple data sources, including patient interviews and patient symptom report data. Feedback providing patient advice was developed from HCP–patient telephone consultations, patient interviews, hospital patient information leaflets and patient support websites.
Findings from a pilot study at two sites show that the use of the eRAPID surgery system for real-time remote monitoring of symptoms in patients recovering from cancer-related upper gastrointestinal surgery is feasible and acceptable to both patients and HCPs. This mixed-methods study evaluated system functioning, intervention adherence, data quality and completeness and patients’ and clinicians’ views on the acceptability of using the system alongside usual care. The level of engagement with the eRAPID surgery system during the pilot study was high, with good rates of patient recruitment. The proportion of patients who were ineligible because they lacked access to a computer/laptop/mobile device or the internet is in keeping with internet usage trends of UK households for people aged ≥ 65 years. 138 The number of patients declining to participate was similar to the number reported in other pilot studies and studies of surgical patients,139,140 and probably reflects how unwell patients typically feel after major cancer surgery. Similarly, the number of participants who withdrew from the study or who did not complete a symptom self-report at the earliest time point because they felt too unwell was as expected because of the frequent and severe symptoms experienced after such major surgery. Because the eRAPID surgery system has been designed to detect symptoms indicative of problems/complications that may require clinical intervention, it is important that mechanisms for monitoring patients who decline to use or feel too unwell to access the system are considered. For example, non-completion of symptom reports at key time points may be interpreted as an indicator of concerning symptoms that could trigger an alert to clinicians.
Rates of adherence to completing symptom self-reports and data completeness were good. Problematic symptoms and those indicative of AEs were detected in a timely manner, and associated actions triggered by the system, including those prompting contact with HCPs and subsequent interventions, were considered appropriate. This indicates the utility of the eRAPID surgery system to detect, capture and generate new representations of data is of relevance to both clinical management and patient self-management. Patients reported the system to be valuable, reassuring and empowering, particularly when there was uncertainty about whether or not symptoms required clinical intervention. HCPs regarded the patient-reported data as clinically informative to the prompt and timely monitoring and management of patients’ symptoms and complications. Based on findings from the pilot study, it is now recommended that the eRAPID surgery system is further evaluated in the context of a clinical effectiveness study.
A systematic review conducted by the study team of studies using electronic patient-reported outcome data systems post discharge after surgery shows that evidence of the benefit of electronic patient-reported outcome data feedback post discharge after major cancer surgery is lacking, with just one RCT identified. 141 This US study was in colorectal cancer and evaluated electronic patient-reported outcome data collection only, despite the importance of feedback and clinical system integration. The review concluded that, before wider adoption of this technology, high-quality RCT evidence is required to generate evidence to understand the effectiveness and economic impact of tailored feedback from real-time, electronic symptom monitoring on the quality of patients’ post-discharge recovery from cancer-related upper gastrointestinal surgery as an adjunct to usual care. Data from the eRAPID surgery pilot study indicate that a definitive RCT is appropriate and feasible. Planning for a full-scale RCT commenced in 2019.
Reflections on the work programme in surgery
Although clinicians at the Bristol site accessed and made use of patient symptom reports in EPRs, they did not do so for all participants or at all time points. Clinicians reported that time restraints, difficulty identifying which patients in the clinic workload were eRAPID participants and limited access to computers prevented use of patient symptom reports in EPRs in some cases.
Time and resource constraints meant that it was not possible to integrate eRAPID into the EPR system at the Birmingham site. Clinicians could access participants’ symptom reports only by logging into a secure online server using anonymised study identifiers. Findings indicated that the system can be successfully implemented in hospital sites outside that in which it was developed and with less experience of electronic capture/monitoring of patient-reported data. However, anecdotal evidence from the Birmingham site clinical nurse specialist team indicated that this lack of integration with existing hospital record systems limited clinician engagement and the usefulness of the eRAPID IT system in routine clinical care.
Pilot study data indicate that minor refinements to the system may further optimise the system’s performance. E-mail alerts generated for severe symptoms before hospital discharge were not clinically relevant and confused clinicians, indicating that this function should be removed. Similarly, a minority of the severe alerts resulted in unnecessary clinical contact. Changes to the wording of symptom report items may ensure that symptoms that have resolved or are already being treated do not trigger alerts. Participant interviews indicated that some hospital re-admissions for AEs occurred as a result of participants contacting care teams without prompting by eRAPID alerts. In these instances, participants recognised the severity of their symptoms, indicating that the eRAPID IT system may be the most beneficial for providing guidance regarding moderate/severe symptoms or instances where patients are uncertain about their symptoms’ clinical significance.
Research recommendations
Clinician feedback indicated that the eRAPID surgery system would benefit from additional tailoring according to surgical specialty (e.g. hepatobiliary surgeries) to better reflect differences in recovery symptom profiles. This could be achieved by creating tailored versions of the eRAPID IT system based on type of surgery or adjusting inclusion criteria to recruit patients who have undergone specific procedures.
The feasibility of the eRAPID surgery system has been demonstrated and evaluation of its clinical effectiveness through a multicentre RCT is recommended. A funding application to undertake a multicentre, parallel-arm RCT with an internal pilot phase was submitted in November 2019. The primary objective of this RCT will be to compare the quality of recovery over the 4 weeks post discharge following oesophagogastric cancer surgery between patients receiving tailored feedback from electronic symptom reporting and monitoring through the eRAPID IT system plus usual care and patients receiving usual care alone. The study will generate evidence about the economic impact and affordability of the eRAPID IT system in major cancer surgery patients.
Discussion, conclusions and recommendations
Summary of key findings, limitations and conclusions
During cancer treatment, patients can experience a wide range of symptoms and side effects. Typically, these are monitored and recorded by clinical staff as part of routine or acute assessments during treatment. Usual-care practices are associated with a number of limitations: for example, patients can find it challenging to recall symptoms over longer time frames and to know how best to manage problems that arise at home. Clinical staff may not accurately record all of the symptoms and issues that patients experience. Consequently, there is considerable variation in AE documentation in medical records. This 7-year programme of research set out to develop and evaluate an online approach to symptom monitoring during cancer treatment.
Overall, we successfully delivered and evaluated a new approach to online symptom monitoring in oncology. A wide range of activities was conducted across the three main cancer treatment modalities (i.e. systemic treatment, radiotherapy and surgery) covering all stages of the research process:
-
Designing and developing the IT platform and EPR integration.
-
Identifying, developing and testing the components of the intervention in collaboration with patients and HCPs (e.g. selecting symptom items for self-reporting, agreeing on clinically relevant thresholds and clinical algorithms for alerts, and preparing patient advice). Furthermore, the intervention was specifically tailored for each treatment modality and site.
-
Implementing the full intervention package (i.e. electronic system with the items, algorithm and advice) across three geographical sites.
-
Completing a feasibility study (in surgery) and randomised pilot studies (in radiotherapy and systemic treatment).
-
Completing a definitive, single-centre RCT in systemic treatment, including cost-effectiveness analysis.
An essential feature of the secure eRAPID IT system was the functionality for clinicians to view patient reports from within EPRs without the need to open and log in to an additional system. This greatly facilitated the practical clinical usefulness of the eRAPID IT system. Developing a secure system to provide such a feature was a significant achievement at the time (2012) and is still a rare occurrence in the NHS. This approach brought challenges because of the bespoke levels of integration required at each site. There is a need for significant ongoing maintenance and support from central IT departments. Patient portals that provide access to medical records can accommodate online reporting. 142,143 We believe that this will be the main approach in the future, although patient portals may have less flexibility for tailoring the questions, scoring algorithms and patient advice than the approach taken in this programme.
Although we originally anticipated that the eRAPID intervention would require tailoring to each cancer site and specific treatment, we found that the level of adaptation required to make the intervention clinically relevant and useful was much higher than expected. High-level tailoring of the content was needed for each cancer site, treatment type and study site.
To the best of our knowledge, the eRAPID intervention is one of the first internationally to include detailed severity-dependent advice to patients on how to self-manage mild/moderate symptoms and when to contact their medical team. This makes the educational and supportive effect stronger, as reflected in the observed improvement in self-efficacy. It is important to point out that the eRAPID intervention was still closely integrated in the site’s care pathway and, therefore, our studies do not provide evidence in support of the growing number of stand-alone smartphone apps for monitoring and managing cancer treatment side effects.
Working in collaboration with clinical teams and patients, we adopted different approaches to the selection of items for online symptom reporting across the three treatment modalities (either adhering to accepted CTCAE reporting systems or selecting pertinent items from validated PROMs). Thus, we provided a proof of principle that any symptom items can be adapted for online reporting and a severity threshold set-up with clinical input.
Across the feasibility study, pilot studies, RCT and the study sites, we note the highly consistent patient consent rates (> 60%) and adherence to online reporting (broadly 60–75%). These results are encouraging and confirm the feasibility and acceptability of this approach across treatments and centres.
Interviews and feedback from patient participants revealed a range of perceived benefits, such as improved connection with the hospital, reassurance that symptoms were in line with those expected, provision of valuable advice on symptom management, supporting decisions on when to contact the hospital and as a general aide memoir or diary of symptom patterns and recovery during treatment. Many patients welcome the increased levels of personal engagement the eRAPID IT system provided in their cancer care. Patients expected clinicians to discuss the online reports to support their clinical assessments and management and were dissatisfied when this did not occur.
The descriptive quantitative results from the pilot RCT in pelvic radiotherapy showed consistent positive trends in symptom and AE control, in functional domains (e.g. emotional, social and role functioning), global health/QoL and self-efficacy. The positive differences were observed early (during the first 6 weeks) and mainly in patients receiving chemoradiotherapy.
The systemic RCT primary outcome analysis did not find a benefit from the eRAPID intervention at 18 weeks, the selected primary time point at the end of chemotherapy. However, a small positive effect was observed at the secondary time points early in the treatment period (at 6 weeks and 12 weeks) and in patients treated with curative intent who are chemotherapy naive. Over 60% of patients were treated with curative intent and > 75% of them reported mild/moderate symptoms/side effects. Benefits in symptom control were seen early (at weeks 6 and 12) as patients started toxic treatments, which stabilised by week 18 in both arms. Most side effects usually occur in the first few weeks, diminishing later as chemotherapy doses are adjusted and patients learn how to handle the toxicity. The results suggest that most benefit was seen in patients who had mild/moderate symptom burden at baseline and early during treatment, who received self-management advice, who adhered to > 70% of the weekly reports and whose clinicians explicitly discussed the self-reports. Our findings extend the existing evidence from metastatic disease, demonstrating benefits on symptom control during curative neoadjuvant or adjuvant chemotherapy, in particular early in the course of treatment. Importantly, adding online self-reporting to usual care does not generate more hospital-based NHS work as measured by numbers of emergency calls, acute admissions and calls to clinical nurse specialists. Health economic analysis did not indicate a statistically significant difference in the cost of eRAPID compared with the cost of usual care. There was evidence that the intervention increases patient self-efficacy at 18 weeks as well as patient-reported overall health.
Limitations
The methods and overall findings generated from the programme should be considered in the context of a number of key limitations.
We were able to deliver WPs across the main treatment groups: systemic treatment, radiotherapy and surgery. However, we focused on certain cancer diagnoses within each of these. Consequently, results may not be generalisable to broader cancer populations. In addition, the components of the eRAPID intervention (e.g. choice of items, patient advice and symptom scoring algorithms) would require tailoring and refinement to be clinically appropriate for use with different patient and HCP groups.
The 1 : 1 RCT research methodology meant that staff saw a limited number of eRAPID intervention patients throughout the systemic treatment and radiotherapy trials. This reduced the opportunities for cancer HCPs to become familiar with incorporating patient-reported data into their patient interactions, which may have played a part in reducing the overall impact of the intervention. In addition, oncology staff saw patients across both study arms, creating the potential for contamination bias. This too may have reduced the intervention effect and could help explain the relatively limited impact of eRAPID.
Findings from the health economic analysis of the systemic RCT were limited by numbers of missing data (particularly use of resources and EQ-5D score). In addition, as software costs were spread over trial participants, only the overall cost-effectiveness may have be underestimated. It is also important to acknowledge that the collection of health economic and quality-of-life outcome data at fixed time points throughout the trial may mean that changes and fluctuations in patient health are missed.
The intervention was focused on online home reporting. With hindsight, supplementing online home reporting with opportunities for in-clinic reporting would have increased clinical visibility and patient engagement and adherence, and widened the eligibility to those without internet access. Approaches that combine these administration methods are recommended in future clinical projects.
We were unable to collect quantitative data on the extent to which the patients followed the eRAPID self-management advice. The results from patient interviews supported this explanation. Future work that explores the underlying mechanisms (at both the patient and the professional level) that lead to improved clinical management and outcomes is warranted.
We worked closely to co-design the intervention with patients and staff and collected important quantitative and qualitative data across the programme to assess the acceptability and clinical value of eRAPID. However, we recognise that our methodological approach was not formally guided by theoretical frameworks, such as normalisation process theory.
Implications for practice
Findings from this research indicate that the eRAPID intervention was acceptable to patients and staff. eRAPID was associated with better QoL and self-efficacy in patients receiving systemic treatment with curative intent, supporting future use of the intervention in this setting.
Recommendations for research
With the widespread use of smartphones, apps and mobile technology by a large proportion of the population, it is increasingly important to provide robust evidence on the benefits and potential risks of using this technology in cancer care. Research recommendations are provided for each treatment area.
In systemic anticancer treatment:
-
A meta-analysis of clinical trials of electronic symptom monitoring during chemotherapy given with curative intent is needed to examine the growing evidence in this patient group. Our trial is, to our knowledge, the largest study to suggest benefits in a population of patients treated predominantly with curative intent, adding to the growing evidence of benefits in advanced cancers. Results from another ongoing UK and European multicentre trial [electronic Symptom Management using the Advanced Symptom Management System (ASyMS) Remote Technology (eSMART)] have recently been published. 57,144
-
Further research examining, the integration of eRAPID-type interventions in newly designed care pathways (e.g. nurse- or pharmacist-led face-to-face or telephone clinics), replacing elements of traditional care rather than just adding to usual care.
-
Further research into approaches to enhance clinicians’ use of the online reports should be included. For example, in our RCT we demonstrated that that patient adherence to the intervention was associated with clinician engagement, which was variable.
-
Further studies should target the recently introduced immunotherapies and small-molecule oral therapies. These drugs have a different AE profile and the treatment is over long time periods.
-
Further research can be recommended in patients with metastatic disease. International trials suggested significant patient benefit in metastatic cancers, including better symptom management, QoL and survival. 38,70 It is important to replicate those findings in a UK oncology setting.
We have assessed the feasibility of the eRAPID intervention during acute pelvic radiotherapy treatment. In radiotherapy:
-
We recommend a multicentre trial to formally evaluate an eRAPID-type eHealth intervention during and immediately after pelvic radiotherapy.
-
Research, initially in the form of feasibility studies, is needed to evaluate online symptom reporting long term following radiotherapy to monitor morbidity and inform and develop interventions for late effects. Oncologists recognise the gap in radiotherapy evidence created by the lack of a mechanism for collecting late-effects information (at 6 months and beyond). Rapid technical developments in radiotherapy have led to new treatment approaches but we lack the methodology to capture their long-term effects.
The feasibility of the eRAPID surgery system has been demonstrated for surgical patients. In surgery:
-
Future research should focus on a formal evaluation of clinical effectiveness through a multicentre RCT.
-
Additional tailoring of eRAPID according to surgical specialties will be advisable to better reflect differences in post surgical recovery.
Finally, some methodological considerations should be contemplated:
-
We recommend designs that serve to increase the exposure of clinical staff to the intervention (e.g. 2 : 1 randomisation or quasi-experimental designs, such as cluster randomisation and stepped-wedge design).
-
Attention should be paid to optimising sustainable clinician training in the use and interpretation of patient-reported outcome data.
-
From a cost-effectiveness perspective, because the AEs of cancer treatment often persist (or worsen) for months or years after completion, a robust evidence on the cost-effectiveness of the eRAPID IT system over a longer follow-up trajectory is needed.
Acknowledgements
This large programme of research would not have been possible without the input and support of a number of people across the participating sites.
At the Leeds site, we are extremely grateful for the hard work and dedication provided by members of the PCOR team at the University of Leeds, in particular Andrea Gibson, Marie Holmes, Zoe Rogers, Sarah Dickinson, Rosemary Peacock, Beverly Clayton, Alexandra Gilbert, Simon Pini, Jeremy Dwyer, Leanne Shearsmith and Leon Bamforth. Thanks also to Colin Johnston, Clare Harley and Peter Selby and the encouragement and guidance provided by the patient representatives involved in the PCOR RAG. Thanks also to Gillian Santorelli and Faye Samy for the statistical expertise that they provided while working at Leeds Institute of Clinical Trials Research.
In addition, we received support from many colleagues at Leeds Teaching Hospitals NHS Trust, including Martin Waugh, David Fox, Foluke Ajayi, Michael Harvey, Mike Philpott, Phillip Wood, Lucy Turner, Krystina Koslowska, Martin Waugh and Donna Johnstone.
At the Manchester site, we thank Jacki Routledge for her expertise and commitment to delivering the radiotherapy work at the Christie Hospital. Thanks also to the additional support provided from Anthony Murphy, Summayyah Jogi, Megan Bunce, Julia Glennon, Katy Jenner and Ananya Choudhury.
At the Bristol site, thank you to past eRAPID team members Elaine O’Connell and Rhiannon Macefield.
Across all the participating cancer centres, the research was delivered thanks to the enthusiasm of the cancer patients and clinical staff who were involved in developmental phases and who consented to participate in the subsequent RCT and pilot studies.
We are very grateful for the expertise and time provided by additional members of our TSC and DMEC: David Cameron, Barbara Woroncow (PPI representative), Janet Dunn, Karen Turner, Dawn Teare, Sara Faithful, Penny Wright, Peter Barrett-Lee, Virginia Cucchi (PPI representative) and Shelley Mason (past PPI representative).
Finally, we would like to acknowledge Sue Keir (PPI representative) and Richard Jones, who were both eRAPID research programme co-applicants and are sadly no longer with us.
Contributions of authors
Galina Velikova (https://orcid.org/0000-0003-1899-5942) (Professor of Psycho-social and Medical Oncology, Patient Centred Outcomes Research) was programme lead and co-produced the final report.
Kate Absolom (https://orcid.org/0000-0002-5477-6643) (University Academic Fellow, Patient-Centred Outcomes Research) led the systemic treatment workstream and co-produced the final report.
Jenny Hewison (https://orcid.org/0000-0003-3026-3250) (Professor, Psychology of Healthcare) was a co-applicant on the programme grant and co-produced the final report.
Patricia Holch (https://orcid.org/0000-0002-4255-8315) (Senior Lecturer and Research Fellow, Psychology) led the radiotherapy workstream and co-produced the final report.
Lorraine Warrington (https://orcid.org/0000-0002-8389-6134) (Research Fellow, Patient Centred Outcomes Research) co-produced the final report.
Kerry Avery (https://orcid.org/0000-0001-5477-2418) (Senior Lecturer, Health Service Research) led the surgical workstream and co-produced the final report.
Hollie Richards (https://orcid.org/0000-0001-9181-7005) (Senior Research Associate, Health Service Research) co-produced the final report.
Jane Blazeby (https://orcid.org/0000-0002-3354-3330) (Professor, Surgery) was a co-applicant on the programme grant and co-produced the final report.
Bryony Dawkins (https://orcid.org/0000-0002-7038-1975) (Research Fellow, Health Economics) co-produced the final report.
Claire Hulme (https://orcid.org/0000-0003-2077-0419) (Professor, Health Economics) was a co-applicant on the programme grant and co-produced the final report.
Robert Carter (https://orcid.org/0000-0003-3399-3873) (IT Manager, Patient-Centred Outcomes Research) was the programme informatics lead and co-produced the final report.
Liz Glidewell (https://orcid.org/0000-0003-2519-2654) (Senior Lecturer, Health Sciences) was a co-applicant on the programme grant and co-produced the final report.
Ann Henry (https://orcid.org/0000-0002-5379-6618) (Associate Professor, Clinical Oncology) helped co-produce the final report.
Kevin Franks (https://orcid.org/0000-0001-9468-7947) (Consultant, Clinical Oncology) helped co-produce the final report.
Geoff Hall (https://orcid.org/0000-0002-8864-5932) (Professor of Digital Health and Cancer Medicine, Medical Oncology) was a co-applicant on the programme grant and helped co-produce the final report.
Susan Davidson (https://orcid.org/0000-0001-9435-7438) (Clinical Director, Radiotherapy) was a co-applicant on the programme grant and helped co-produce the final report.
Karen Henry (https://orcid.org/0000-0002-4527-6647) (Lead Cancer Nurse, Oncology) was a co-applicant on the programme grant and helped co-produce the final report.
Carolyn Morris (https://orcid.org/0000-0003-0334-547X) (Patient and Public Involvement) was a co-applicant on the programme grant and helped co-produce the final report.
Mark Conner (https://orcid.org/0000-0002-6229-8143) (Professor, Applied Social Psychologist) was a co-applicant on the programme grant and helped co-produce the final report.
Lucy McParland (https://orcid.org/0000-0001-9729-2561) (Statistician, Clinical Trials Research Unit) co-produced the final report.
Katrina Walker (https://orcid.org/0000-0001-6922-9100) (Medical Statistician, Clinical Trials Research Unit) co-produced the final report.
Eleanor Hudson (https://orcid.org/0000-0001-8758-7163) (Medical Statistician, Clinical Trials Research Unit) co-produced the final report.
Julia Brown (https://orcid.org/0000-0002-2719-7064) (Professor, Clinical Trials Research Unit) was a co-applicant on the programme grant and helped co-produce the final report.
Publications
Warrington L, Absolom K, Velikova G. Integrated care pathways for cancer survivors – a role for patient-reported outcome measures and health informatics. Acta Oncol 2015;54:600–8. https://doi.org/10.3109/0284186X.2014.995778
Holch P, Warrington L, Potrata B, Ziegler L, Hector C, Keding A, et al. Asking the right questions to get the right answers: using cognitive interviews to review the acceptability, comprehension and clinical meaningfulness of patient self-report adverse event items in oncology patients. Acta Oncol 2016;55:1220–6. https://doi.org/10.1080/0284186X.2016.1213878
Warrington L, Holch P, Kenyon L, Hector C, Kozlowska K, Kenny AM, et al. An audit of acute oncology services: patient experiences of admission procedures and staff utilisation of a new telephone triage system. Support Care Cancer 2016;24:5041–8. https://doi.org/10.1007/s00520-016-3370-4
Absolom K, Holch P, Warrington L, Samy F, Hulme C, Hewison J, et al. Electronic patient self-Reporting of Adverse-events: Patient Information and aDvice (eRAPID): a randomised controlled trial in systemic cancer treatment. BMC Cancer 2017;17:318. https://doi.org/10.1186/s12885-017-3303-8
Holch P, Henry AM, Davidson S, Gilbert A, Routledge J, Shearsmith L, et al. Acute and late adverse events associated with radical radiation therapy prostate cancer treatment: a systematic review of clinician and patient toxicity reporting in randomized controlled trials. Int J Radiat Oncol Biol Phys 2017;97:495–510. https://doi.org/10.1016/j.ijrobp.2016.11.008
Holch P, Warrington L, Bamforth LCA, Keding A, Ziegler LE, Absolom K, et al. Development of an integrated electronic platform for patient self-report and management of adverse events during cancer treatment. Ann Oncol 2017;28:2305–11. https://doi.org/10.1093/annonc/mdx317
Basch E, Barbera L, Kerrigan CL, Velikova G. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ Book 2018;38:122–34. https://doi.org/10.1200/EDBK_200383
Holch P, Pini S, Henry AM, Davidson S, Routledge J, Brown J, et al. eRAPID electronic patient self-Reporting of Adverse-events: Patient Information and aDvice: a pilot study protocol in pelvic radiotherapy. Pilot Feasibility Stud 2018;4:110. https://doi.org/10.1186/s40814-018-0304-6
Thanarajasingam G, Minasian LM, Baron F, Cavalli F, De Claro RA, Dueck AC, et al. Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies. Lancet Haematol 2018;5:e563–e598.
Absolom K, Gibson A, Velikova G. Engaging patients and clinicians in online reporting of adverse effects during chemotherapy for cancer: the eRAPID system (electronic patient self-Reporting of Adverse events: Patient Information and aDvice). Med Care 2019;57:S59–S65. https://doi.org/10.1097/MLR.0000000000001085
Avery KNL, Richards HS, Portal A, Reed T, Harding R, Carter R, et al. Developing a real-time electronic symptom monitoring system for patients after discharge following cancer-related surgery. BMC Cancer 2019;19:463. https://doi.org/10.1186/s12885-019-5657-6
Lindner OC, Velikova G, Stark DP. Digitally enabled patient-reported outcome measures in cancer care. Lancet Oncol 2019;20:e2.
Warrington L, Absolom K, Holch P, Gibson A, Clayton B, Velikova G. Online tool for monitoring adverse events in patients with cancer during treatment (eRAPID): field testing in a clinical setting. BMJ Open 2019;9:e025185. https://doi.org/10.1136/bmjopen-2018-025185
Warrington L, Absolom K, Conner M, Kellar I, Clayton B, Ayres M, Velikova G. Electronic systems for patients to report and manage side effects of cancer treatment: systematic review. J Med Internet Res 2019;21:e10875. https://doi.org/10.2196/10875
Richards HS, Blazeby JM, Portal A, Harding R, Reed T, Lander T, et al. A real-time electronic symptom monitoring system for patients after discharge following surgery: a pilot study in cancer-related surgery. BMC Cancer 2020;20:543. https://doi.org/10.1186/s12885-020-07027-5
Richards HS, Portal A, Absolom K, Blazeby JM, Velikova G, Avery KNL. Patient experiences of an electronic PRO tailored feedback system for symptom management following upper gastrointestinal cancer surgery. Qual Life Res 2020. https://doi.org/10.1007/s11136-020-02539-w
Absolom K, Warrington L, Hudson E, Hewison J, Morris C, Holch P, et al. Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol 2021;39:734–47. https://doi.org/10.1200/JCO.20.02015
Data-sharing statement
Requests for access to data should be addressed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Department of Health and Social Care (DHSC) . Chemotherapy Services in England: Ensuring Quality and Safety. A Report of the National Chemotherapy Advisory Group 2009. www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_104501.pdf (accessed 16 July 2020).
- Tsai SC, Liu LN, Tang ST, Chen JC, Chen ML. Cancer pain as the presenting problem in emergency departments: incidence and related factors. Support Care Cancer 2010;18:57-65. https://doi.org/10.1007/s00520-009-0630-6.
- Department of Health and Social Care (DHSC) . Improving Outcomes: A Strategy for Cancer 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123371 (accessed 22 October 2020).
- United States Department of Health and Human Services . Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 2009.
- Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med 2010;362:865-9. https://doi.org/10.1056/NEJMp0911494.
- Mort D, Lansdown M, Smith N, Protopapa K, Mason M. For Better, For Worse? A Review of the Care of Patients Who Died Within 30 Days of Receiving Systemic Anti-cancer Therapy. London: National Confidential Enquiry into Patient Outcome and Death; 2008.
- Considine J, Livingston P, Bucknall T, Botti M. A review of the role of emergency nurses in management of chemotherapy-related complications. J Clin Nurs 2009;18:2649-55. https://doi.org/10.1111/j.1365-2702.2009.02843.x.
- De Luigi A. Analysis of reasons for admission to the emergency department for cancer patients. Ann Oncol 2002;13.
- West CM, Davidson SE. Measurement tools for gastrointestinal symptoms in radiation oncology. Curr Opin Support Palliat Care 2009;3:36-40. https://doi.org/10.1097/SPC.0b013e328325d18d.
- Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol 2007;19:790-9. https://doi.org/10.1016/j.clon.2007.08.011.
- Andreyev HJ, Davidson SE, Gillespie C, Allum WH, Swarbrick E. British Society of Gastroenterology . Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 2012;61:179-92. https://doi.org/10.1136/gutjnl-2011-300563.
- Davidson SE, Trotti A, Ataman OU, Seong J, Lau FN, da Motta NW, et al. Improving the capture of adverse event data in clinical trials: the role of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 2007;69:1218-21. https://doi.org/10.1016/j.ijrobp.2007.04.054.
- Davidson SE, Burns MP, Routledge JA, Swindell R. The impact of radiotherapy for carcinoma of the cervix on sexual function assessed using the LENT SOMA scales. Radiother Oncol 2003;68:241-7. https://doi.org/10.1016/S0167-8140(03)00190-7.
- Gillespie C, Goode C, Hackett C, Andreyev HJ. The clinical needs of patients with chronic gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther 2007;26:555-63. https://doi.org/10.1111/j.1365-2036.2007.03405.x.
- Lazzarino AI, Nagpal K, Bottle A, Faiz O, Moorthy K, Aylin P. Open versus minimally invasive esophagectomy: trends of utilization and associated outcomes in England. Ann Surg 2010;252:292-8. https://doi.org/10.1097/SLA.0b013e3181dd4e8c.
- Yermilov I, Bentrem D, Sekeris E, Jain S, Maggard MA, Ko CY, et al. Re-admissions following pancreaticoduodenectomy for pancreas cancer: a population-based appraisal. Ann Surg Oncol 2009;16:554-61. https://doi.org/10.1245/s10434-008-0178-6.
- Cleeland CS, Wang XS, Shi Q, Mendoza TR, Wright SL, Berry MD, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol 2011;29:994-1000. https://doi.org/10.1200/JCO.2010.29.8315.
- NHS England . Cancer Workforce Plan 2021. www.hee.nhs.uk/sites/default/files/documents/Cancer%20Workforce%20Plan%20phase%201%20-%20Delivering%20the%20cancer%20strategy%20to%202021.pdf (accessed 9 July 2021).
- NHS Improvement . Transforming Inpatient Care Programme for Cancer Patients: The Winning Principles 2008. www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/Transforming-Inpatient-Care-The-Winning-Principles.pdf (accessed 30 August 2019).
- United States Food and Drug Administration (FDA) . Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims 2009. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf (accessed 22 October 2020).
- Bottomley A, Flechtner H, Efficace F, Vanvoorden V, Coens C, Therasse P, et al. Health related quality of life outcomes in cancer clinical trials. Eur J Cancer 2005;41:1697-709. https://doi.org/10.1016/j.ejca.2005.05.007.
- Fayers PM, Hopwood P, Harvey A, Girling DJ, Machin D, Stephens R. Quality of life assessment in clinical trials – guidelines and a checklist for protocol writers: the U.K. Medical Research Council experience. MRC Cancer Trials Office. Eur J Cancer 1997;33:20-8. https://doi.org/10.1016/S0959-8049(96)00412-1.
- Moinpour CM, Denicoff AM, Bruner DW, Kornblith AB, Land SR, O’Mara A, et al. Funding patient-reported outcomes in cancer clinical trials. J Clin Oncol 2007;25:5100-5. https://doi.org/10.1200/JCO.2007.11.5329.
- Valderas JM, Kotzeva A, Espallargues M, Guyatt G, Ferrans CE, Halyard MY, et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res 2008;17:179-93. https://doi.org/10.1007/s11136-007-9295-0.
- Espallargues M, Valderas JM, Alonso J. Provision of feedback on perceived health status to health care professionals: a systematic review of its impact. Med Care 2000;38:175-86. https://doi.org/10.1097/00005650-200002000-00007.
- Greenhalgh J, Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract 1999;5:401-16. https://doi.org/10.1046/j.1365-2753.1999.00209.x.
- Haywood K, Marshall S, Fitzpatrick R. Patient participation in the consultation process: a structured review of intervention strategies. Patient Educ Couns 2006;63:12-23. https://doi.org/10.1016/j.pec.2005.10.005.
- Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 2004;22:714-24. https://doi.org/10.1200/JCO.2004.06.078.
- Velikova G, Awad N, Coles-Gale R, Wright EP, Brown JM, Selby PJ. The clinical value of quality of life assessment in oncology practice-a qualitative study of patient and physician views. Psycho-oncology 2008;17:690-8. https://doi.org/10.1002/pon.1295.
- Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 2006;7:903-9. https://doi.org/10.1016/S1470-2045(06)70910-X.
- Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 2009;101:1624-32. https://doi.org/10.1093/jnci/djp386.
- National Cancer Institute . Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™) n.d. http://outcomes.cancer.gov/tools/pro-ctcae.html (accessed 23 June 2021).
- Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 2015;1:1051-9. https://doi.org/10.1001/jamaoncol.2015.2639.
- Kotronoulas G, Kearney N, Maguire R, Harrow A, Di Domenico D, Croy S, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32:1480-501. https://doi.org/10.1200/JCO.2013.53.5948.
- Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol 2011;29:2683-8. https://doi.org/10.1200/JCO.2010.34.2816.
- Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016;34:557-65. https://doi.org/10.1200/JCO.2015.63.0830.
- Basch E, Deal AM, Dueck AC. Patient-reported symptom monitoring during chemotherapy – reply. JAMA 2017;318:1935-6. https://doi.org/10.1001/jama.2017.14903.
- Denis F, Basch E, Septans AL, Bennouna J, Urban T, Dueck AC, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA 2019;321:306-7. https://doi.org/10.1001/jama.2018.18085.
- Wright EP, Selby PJ, Crawford M, Gillibrand A, Johnston C, Perren TJ, et al. Feasibility and compliance of automated measurement of quality of life in oncology practice. J Clin Oncol 2003;21:374-82. https://doi.org/10.1200/JCO.2003.11.044.
- Basch E, Artz D, Dulko D, Scher K, Sabbatini P, Hensley M, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol 2005;23:3552-61. https://doi.org/10.1200/JCO.2005.04.275.
- Bush N, Donaldson G, Moinpour C, Haberman M, Milliken D, Markle V, et al. Development, feasibility and compliance of a web-based system for very frequent QOL and symptom home self-assessment after hematopoietic stem cell transplantation. Qual Life Res 2005;14:77-93. https://doi.org/10.1007/s11136-004-2394-2.
- Snyder CF, Jensen R, Courtin SO, Wu AW. PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res 2009;18:793-800. https://doi.org/10.1007/s11136-009-9497-8.
- McCann L, Maguire R, Miller M, Kearney N. Patients’ perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care 2009;18:156-64. https://doi.org/10.1111/j.1365-2354.2008.00938.x.
- Maguire R, McCann L, Miller M, Kearney N. Nurse’s perceptions and experiences of using of a mobile-phone-based Advanced Symptom Management System (ASyMS) to monitor and manage chemotherapy-related toxicity. Eur J Oncol Nurs 2008;12:380-6. https://doi.org/10.1016/j.ejon.2008.04.007.
- Kearney N, McCann L, Norrie J, Taylor L, Gray P, McGee-Lennon M, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer 2009;17:437-44. https://doi.org/10.1007/s00520-008-0515-0.
- Weaver A, Young AM, Rowntree J, Townsend N, Pearson S, Smith J, et al. Application of mobile phone technology for managing chemotherapy-associated side-effects. Ann Oncol 2007;18:1887-92. https://doi.org/10.1093/annonc/mdm354.
- Kroenke K, Theobald D, Wu J, Norton K, Morrison G, Carpenter J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA 2010;304:163-71. https://doi.org/10.1001/jama.2010.944.
- Chaudhry SI, Phillips CO, Stewart SS, Riegel B, Mattera JA, Jerant AF, et al. Telemonitoring for patients with chronic heart failure: a systematic review. J Card Fail 2007;13:56-62. https://doi.org/10.1016/j.cardfail.2006.09.001.
- Darkins A, Ryan P, Kobb R, Foster L, Edmonson E, Wakefield B, et al. Care Coordination/Home Telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health 2008;14:1118-26. https://doi.org/10.1089/tmj.2008.0021.
- Office for National Statistics . Internet Access – Households and Individuals, Great Britain: 2020 n.d. www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/bulletins/internetaccesshouseholdsandindividuals/2020 (accessed 9 July 2021).
- Department of Health and Social Care (DHSC) . Equity and Excellence: Liberating the NHS 2010. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_117353 (accessed 16 July 2020).
- Department of Health and Social Care (DHSC) . Whole System Demonstrators 2012. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Healthcare/Longtermconditions/wholesystemdemonstrators/index.htm (accessed 30 August 2019).
- Cancer Research UK. Achieving World-Class Cancer Outcomes: A Strategy for England 2015–2020 n.d. www.cancerresearchuk.org/sites/default/files/achieving_world-class_cancer_outcomes_-_a_strategy_for_england_2015-2020.pdf (accessed 23 June 2021).
- NHS England . NHS Long Term Plan n.d. www.england.nhs.uk/long-term-plan/ (accessed 23 June 2021).
- Barbera L, Sutradhar R, Seow H, Mittmann N, Howell D, Earle CC, et al. The impact of routine Edmonton Symptom Assessment System (ESAS) use on overall survival in cancer patients: results of a population-based retrospective matched cohort analysis. Cancer Med 2020;9:7107-15. https://doi.org/10.1002/cam4.3374.
- Girgis A, Durcinoska I, Arnold A, Descallar J, Kaadan N, Koh ES, et al. Web-based patient-reported outcome measures for personalized treatment and care (PROMPT-Care): multicenter pragmatic nonrandomized trial. J Med Internet Res 2020;22. https://doi.org/10.2196/19685.
- Maguire R, Fox PA, McCann L, Miaskowski C, Kotronoulas G, Miller M, et al. The eSMART study protocol: a randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015016.
- Kennedy A, Chew-Graham C, Blakeman T, Bowen A, Gardner C, Protheroe J, et al. Delivering the WISE (Whole Systems Informing Self-Management Engagement) training package in primary care: learning from formative evaluation. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-7.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- Ellis P, Barrett-Lee P, Johnson L, Cameron D, Wardley A, O’Reilly S, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. Lancet 2009;373:1681-92. https://doi.org/10.1016/S0140-6736(09)60740-6.
- National Chemotherapy Advisory Group . Chemotherapy Services in England: Ensuring Quality and Safety. 2010. https://webarchive.nationalarchives.gov.uk/20130104173757/www.dh.gov.uk/en/Publicationsandstatistics/Publications/DH_104500 (accessed 9 July 2021).
- Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology . Guidelines for the management of oesophageal and gastric cancer. Gut 2011;60:1449-72. https://doi.org/10.1136/gut.2010.228254.
- Sheffield KM, Crowell KT, Lin YL, Djukom C, Goodwin JS, Riall TS. Surveillance of pancreatic cancer patients after surgical resection. Ann Surg Oncol 2012;19:1670-7. https://doi.org/10.1245/s10434-011-2152-y.
- Warrington L, Absolom K, Velikova G. Integrated care pathways for cancer survivors – a role for patient-reported outcome measures and health informatics. Acta Oncol 2015;54:600-8. https://doi.org/10.3109/0284186X.2014.995778.
- Holch P, Warrington L, Bamforth LCA, Keding A, Ziegler LE, Absolom K, et al. Development of an integrated electronic platform for patient self-report and management of adverse events during cancer treatment. Ann Oncol 2017;28:2305-11. https://doi.org/10.1093/annonc/mdx317.
- Warrington L, Absolom K, Holch P, Gibson A, Clayton B, Velikova G. Online tool for monitoring adverse events in patients with cancer during treatment (eRAPID): field testing in a clinical setting. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-025185.
- Snyder C, Wu AW. Users’ Guide to Integrating Patient-Reported Outcomes in Electronic Health Records 2017. www.pcori.org/sites/default/files/PCORI-JHU-Users-Guide-To-Integrating-Patient-Reported-Outcomes-in-Electronic-Health-Records.pdf (accessed 22 October 2020).
- Ashley L, Jones H, Thomas J, Newsham A, Downing A, Morris E, et al. Integrating patient reported outcomes with clinical cancer registry data: a feasibility study of the electronic Patient-Reported Outcomes From Cancer Survivors (ePOCS) system. J Med Internet Res 2013;15. https://doi.org/10.2196/jmir.2764.
- Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2015;33:1-9.
- Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197-8. https://doi.org/10.1001/jama.2017.7156.
- Smith A, Tsang C, Woroncow B. Improving Care With Electronic Patient-Reported Outcome Feedback to Patients and Clinicians n.d.
- Absolom K, Warrington L, Hudson E, Hewison J, Morris C, Holch P, et al. Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol 2021;39:734-47. https://doi.org/10.1200/JCO.20.02015.
- Absolom K, Holch P, Woroncow B, Wright EP, Velikova G. Beyond lip service and box ticking: how effective patient engagement is integral to the development and delivery of patient-reported outcomes. Qual Life Res 2015;24:1077-85. https://doi.org/10.1007/s11136-014-0909-z.
- Holch P, Pini S, Henry AM, Davidson S, Routledge J, Brown J, et al. eRAPID electronic patient self-Reporting of Adverse-events: Patient Information and aDvice: a pilot study protocol in pelvic radiotherapy. Pilot Feasibility Stud 2018;4. https://doi.org/10.1186/s40814-018-0304-6.
- Absolom K, Holch P, Warrington L, Samy F, Hulme C, Hewison, et al. Electronic patient self-Reporting of Adverse-events: Patient Information and aDvice (eRAPID): a randomised controlled trial in systemic cancer treatment. BMC Cancer 2017;17. https://doi.org/10.1186/s12885-017-3303-8.
- Holch P, Warrington L, Potrata B, Ziegler L, Hector C, Keding A, et al. Asking the right questions to get the right answers: using cognitive interviews to review the acceptability, comprehension and clinical meaningfulness of patient self-report adverse event items in oncology patients. Acta Oncologica 2016;55:1220-6. https://doi.org/10.1080/0284186X.2016.1213878.
- Hector C, Holch P, Warrington L, Morris C, Ziegler L, Absolom K, et al. Development of online patient-advice for the self-management of low-level chemotherapy related toxicities (eRAPID): involvement of patients and staff. Psycho-oncology 2013;22.
- Medical Research Council (MRC) . Developing and Evaluating Complex Interventions 2019. https://mrc.ukri.org/documents/pdf/complex-interventions-guidance/ (accessed 22 October 2020).
- Warrington L, Holch P, Kenyon L, Hector C, Kozlowska K, Kenny AM, et al. An audit of acute oncology services: patient experiences of admission procedures and staff utilisation of a new telephone triage system. Support Care Cancer 2016;24:5041-8. https://doi.org/10.1007/s00520-016-3370-4.
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9. https://doi.org/10.1200/JCO.1993.11.3.570.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256-62.
- Heitzmann CA, Merluzzi TV, Jean-Pierre P, Roscoe JA, Kirsh KL, Passik SD. Assessing self-efficacy for coping with cancer: development and psychometric analysis of the brief version of the Cancer Behavior Inventory (CBI-B). Psycho-Oncology 2011;20:302-12. https://doi.org/10.1002/pon.1735.
- Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918-30. https://doi.org/10.1111/j.1475-6773.2005.00438.x.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 22 October 2020).
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated October 2019) 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 22 October 2020).
- The EuroQoL Group . EuroQol – a new facility for the measurement of health-related quality-of-life. Health 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2018 2018.
- Joint Formulary Committee . British National Formulary (online) n.d. www.medicinescomplete.com (accessed March 2019).
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401. https://doi.org/10.1146/annurev.publhealth.23.100901.140534.
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. https://doi.org/10.1191/0962280202sm304ra.
- Sanghera S, Coast J. Measuring quality-adjusted life-years when health fluctuates. Value Health 2020;23:343-50. https://doi.org/10.1016/j.jval.2019.09.2753.
- National Institute for Health Research . Towards Safer Delivery and Monitoring of Cancer Treatments. Electronic Patient Self-Reporting of Adverse-Events: Patient Information and Advice (eRAPID) n.d. https://fundingawards.nihr.ac.uk/award/RP-DG-1209-10031 (accessed 23 November 2021).
- Viani GA, Viana BS, Martin JE, Rossi BT, Zuliani G, Stefano EJ. Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: a randomized clinical trial. Cancer 2016;122:2004-11. https://doi.org/10.1002/cncr.29983.
- Jackson A, Marks LB, Bentzen SM, Eisbruch A, Yorke ED, Ten Haken RK, et al. The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys 2010;76:155-60. https://doi.org/10.1016/j.ijrobp.2009.08.074.
- Andreyev HJ, Wotherspoon A, Denham JW, Hauer-Jensen M. ‘Pelvic radiation disease’: new understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol 2011;46:389-97. https://doi.org/10.3109/00365521.2010.545832.
- Adams E, Boulton MG, Horne A, Rose PW, Durrant L, Collingwood M, et al. The effects of pelvic radiotherapy on cancer survivors: symptom profile, psychological morbidity and quality of life. Clin Oncol 2014;26:10-7. https://doi.org/10.1016/j.clon.2013.08.003.
- Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. https://doi.org/10.1016/S1470-2045(12)70116-X.
- Kirwan JM, Symonds P, Green JA, Tierney J, Collingwood M, Williams CJ. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol 2003;68:217-26. https://doi.org/10.1016/S0167-8140(03)00197-X.
- Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 2009;74:1405-18. https://doi.org/10.1016/j.ijrobp.2008.10.091.
- Holch P, Henry AM, Davidson S, Gilbert A, Routledge J, Shearsmith L, et al. Acute and late adverse events associated with radical radiation therapy prostate cancer treatment: a systematic review of clinician and patient toxicity reporting in randomized controlled trials. Int J Radiat Oncol Biol Phys 2017;97:495-510. https://doi.org/10.1016/j.ijrobp.2016.11.008.
- van Andel G, Bottomley A, Fosså SD, Efficace F, Coens C, Guerif S, et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer 2008;44:2418-24. https://doi.org/10.1016/j.ejca.2008.07.030.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. https://doi.org/10.1097/00005650-199206000-00002.
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899-905. https://doi.org/10.1016/S0090-4295(00)00858-X.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. https://doi.org/10.1097/00000421-198212000-00014.
- Farnell DJ, Routledge J, Hannon R, Logue JP, Cowan RA, Wylie JP, et al. Efficacy of data capture for patient-reported toxicity following radiotherapy for prostate or cervical cancer. Eur J Cancer 2010;46:534-40. https://doi.org/10.1016/j.ejca.2009.11.017.
- Farnell DJ, Mandall P, Anandadas C, Routledge J, Burns MP, Logue JP, et al. Development of a patient-reported questionnaire for collecting toxicity data following prostate brachytherapy. Radiother Oncol 2010;97:136-42. https://doi.org/10.1016/j.radonc.2010.05.011.
- Holmes M, Holch P, Rogers Z, Dickinson S, Davidson S, Routledge J, et al. Patient and Relatives Attitudes to the Implementation of ERAPID (electronic Patient Self-Reporting of Adverse-Events: Patient Information and ADvice) During and After Pelvic Radiotherapy: A Qualitative Interview Study n.d.
- Rogers Z, Holch P, Holmes M, Davidson S, Routledge J, Henry A, et al. Healthcare Professional (HCP) Attitudes to the Implementation of ERAPID (electronic Patient Self-Reporting of Adverse-Events: Patient Information and ADvice) During and After Pelvic Radiotherapy: A Qualitative Interview Study n.d.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- National Oesophago-Gastric Cancer Audit . An Audit of the Care Received by People With Oesophago-Gastric Cancer in England and Wales: 2017 Annual Report 2017.
- Bhagat R, Bronsert MR, Juarez-Colunga E, Weyant MJ, Mitchell JD, Glebova NO, et al. Postoperative complications drive unplanned re-admissions after esophagectomy for cancer. Ann Thorac Surg 2018;105:1476-82. https://doi.org/10.1016/j.athoracsur.2017.12.024.
- Mamidanna R, Bottle A, Aylin P, Faiz O, Hanna GB. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg 2012;255:197-203. https://doi.org/10.1097/SLA.0b013e31823e39fa.
- Guinan EM, Bennett AE, Doyle SL, O’Neill L, Gannon J, Foley G, et al. Measuring the impact of oesophagectomy on physical functioning and physical activity participation: a prospective study. BMC Cancer 2019;19. https://doi.org/10.1186/s12885-019-5888-6.
- Pisarska M, Małczak P, Major P, Wysocki M, Budzyński A, Pędziwiatr M. Enhanced recovery after surgery protocol in oesophageal cancer surgery: systematic review and meta-analysis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0174382.
- Dorcaratto D, Grande L, Pera M. Enhanced recovery in gastrointestinal surgery: upper gastrointestinal surgery. Dig Surg 2013;30:70-8. https://doi.org/10.1159/000350701.
- Francis NK, Mason J, Salib E, Allanby L, Messenger D, Allison AS, et al. Factors predicting 30-day re-admission after laparoscopic colorectal cancer surgery within an enhanced recovery programme. Colorectal Dis 2015;17:O148-54. https://doi.org/10.1111/codi.13002.
- Mitchell AP, Hirsch BR, Abernethy AP. Lack of timely accrual information in oncology clinical trials: a cross-sectional analysis. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-92.
- Sibbern T, Bull Sellevold V, Steindal SA, Dale C, Watt-Watson J, Dihle A. Patients’ experiences of enhanced recovery after surgery: a systematic review of qualitative studies. J Clin Nurs 2017;26:1172-88. https://doi.org/10.1111/jocn.13456.
- Cancer Research UK . Follow Up Appointments n.d. www.cancerresearchuk.org/about-cancer/oesophageal-cancer/treatment/follow-up (accessed 13 July 2020).
- Mooney KH, Beck SL, Friedman RH, Farzanfar R, Wong B. Automated monitoring of symptoms during ambulatory chemotherapy and oncology providers’ use of the information: a randomized controlled clinical trial. Support Care Cancer 2014;22:2343-50. https://doi.org/10.1007/s00520-014-2216-1.
- Basch E, Bennett A, Pietanza MC. Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst 2011;103:1808-10. https://doi.org/10.1093/jnci/djr493.
- Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346. https://doi.org/10.1136/bmj.f167.
- Johansen MA, Berntsen GK, Schuster T, Henriksen E, Horsch A. Electronic symptom reporting between patient and provider for improved health care service quality: a systematic review of randomized controlled trials. part 2: methodological quality and effects. J Med Internet Res 2012;14. https://doi.org/10.2196/jmir.2216.
- Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol 2007;25:5121-7. https://doi.org/10.1200/JCO.2007.12.4784.
- Avery KNL, Richards HS, Portal A, Reed T, Harding R, Carter R, et al. Developing a real-time electronic symptom monitoring system for patients after discharge following cancer-related surgery. BMC Cancer 2019;19. https://doi.org/10.1186/s12885-019-5657-6.
- Richards HS, Portal A, Absolom K, Blazeby JM, Velikova G, Avery KNL. Patient experiences of an electronic PRO tailored feedback system for symptom management following upper gastrointestinal cancer surgery [published online ahead of print June 13 2020]. Qual Life Res 2020. https://doi.org/10.1007/s11136-020-02539-w.
- Richards HS, Blazeby JM, Portal A, Harding R, Reed T, Lander T, et al. A real-time electronic symptom monitoring system for patients after discharge following surgery: a pilot study in cancer-related surgery. BMC Cancer 2020;20. https://doi.org/10.1186/s12885-020-07027-5.
- Kaur G, Hutchison I, Mehanna H, Williamson P, Shaw R, Tudur Smith C. Barriers to recruitment for surgical trials in head and neck oncology: a survey of trial investigators. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002625.
- Blazeby JM. Recruiting patients into randomized clinical trials in surgery. Br J Surg 2012;99:307-8. https://doi.org/10.1002/bjs.7818.
- Pinkney TD, Calvert M, Bartlett DC, Gheorghe A, Redman V, Dowswell G, et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial). BMJ 2013;347. https://doi.org/10.1136/bmj.f4305.
- Bennet AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA: Cancer J Clin 2012;62:336-47. https://doi.org/10.3322/caac.21150.
- University Hospitals of Derby and Burton NHS Foundation Trust . Online Patient Portal n.d. www.uhdb.nhs.uk/patient-portal/ (accessed 12 September 2019).
- Imperial College Healthcare NHS Trust . Building an Online Record of Your Health. n.d. www.careinformationexchange-nwl.nhs.uk (accessed 12 September 2019).
- Maguire R, McCann L, Kotronoulas G, Kearney N, Ream E, Armes J, et al. Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART). BMJ 2021;372. https://doi.org/10.1136/bmj.n1647.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- National Institute of Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Department of Health and Social Care . NHS Reference Costs 2016 to 2017 2017. https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 23 November 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- BBC News . Q&Amp;A: NHS 111 2013. http://www.bbc.co.uk/news/health-22370621 (accessed 23 November 2021).
- University of Leeds . MyPrint Charges 2019 n.d. https://leeds.service-now.com/it?id=kb_article%26sys_id=004b00650f1e3640e50d1b2be1050e46 (accessed 13 August 2019).
- Office for National Statistics . Average Hourly Pay n.d. https://www.ethnicity-facts-figures.service.gov.uk/work-pay-and-benefits/pay-and-income/average-hourly-pay/latest (accessed 23 November 2021).
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5 D-5 L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology 2010;76:1245-50. https://doi.org/10.1016/j.urology.2010.01.027.
- Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-prostate instrument. Urology 1997;50:920-8. https://doi.org/10.1016/S0090-4295(97)00459-7.
- Esper P, Hampton JN, Smith DC, Pienta KJ. Quality-of-life evaluation in patients receiving treatment for advanced prostate cancer. Oncol Nurs Forum 1999;26:107-12.
- McHorney CA, Ware JE, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40-66. https://doi.org/10.1097/00005650-199401000-00004.
- Jisc . Online Surveys n.d. https://www.onlinesurveys.ac.uk/ (accessed 23 November 2021).
- Valentini V, Glimelius B. Rectal cancer radiotherapy: towards European consensus. Acta Oncol 2010;49:1206-16. https://doi.org/10.3109/0284186X.2010.506884.
- Rogers L, Siu SS, Luesley D, Bryant A, Dickinson HO. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev n.d.
- Rogers L, Siu SS, Luesley D, Bryant A, Dickinson HO. Adjuvant radiotherapy and chemoradiation after surgery for cervical cancer. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD007583.pub2.
- Melcher AA, Sebag-Montefiore D. Concurrent chemoradiotherapy for squamous cell carcinoma of the anus using a shrinking field radiotherapy technique without a boost. Br J Cancer 2003;88:1352-7. https://doi.org/10.1038/sj.bjc.6600913.
- Gilbert A, Sebag-Montefiore D, Davidson S, Velikova G. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol 2015;136:429-39. https://doi.org/10.1016/j.ygyno.2014.11.071.
- Gilbert A, Ziegler L, Martland M, Davidson S, Efficace F, Sebag-Montefiore D, et al. Systematic review of radiation therapy toxicity reporting in randomised controlled trials of rectal cancer: a comparison of patient-reported outcomes and clinician toxicity reporting. Int J Radiat Oncol Biol Phys 2015;92:555-67. https://doi.org/10.1016/j.ijrobp.2015.02.021.
- Office for National Statistics . Cancer Survival in England – Adults Diagnosed 2013–2017 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed.Outocmes (accessed 9 June 2020).
- Sipaviciute A, Sileika E, Burneckis A, Dulskas A. Late gastrointestinal toxicity after radiotherapy for rectal cancer: a systematic review [published online ahead of print 16 April 2020]. Int J Colorectal Dis 2020. https://doi.org/10.1007/s00384-020-03595-x.
- Howell D, Harth T, Brown J, Bennett C, Boyko S. Self-management education interventions for patients with cancer: a systematic review. Support Care Cancer 2017;25:1323-55. https://doi.org/10.1007/s00520-016-3500-z.
- Fromme EK, Holliday EB, Nail LM, Lyons KS, Hribar MR, Thomas CR. Computerized patient-reported symptom assessment in radiotherapy: a pilot randomized, controlled trial. Support Care Cancer 2016;24:1897-906. https://doi.org/10.1007/s00520-015-2983-3.
- Macnair A, Sharkey A, Le Calvez K, Walters R, Smith L, Nelson A, et al. The Trigger Project: the challenge of introducing electronic patient-reported outcome measures into a radiotherapy service. Clinic Oncol 2020;32:e76-9. https://doi.org/10.1016/j.clon.2019.09.044.
- Maguire R, Ream E, Richardson A, Connagh J, Johnston B, Kotronoulas G, et al. Development of a novel remote patient monitoring system: the advanced symptom management system for radiotherapy to improve the symptom experience of patients with lung cancer receiving radiotherapy. Cancer Nurs 2015;38:E37-47. https://doi.org/10.1097/NCC.0000000000000150.
- El Shafie, RA, Weber D, Bougatf N, Sprave T, Oetzel D, Huber PE, et al. Supportive care in radiotherapy based on a mobile app: prospective multicenter survey. JMIR Mhealth and Uhealth 2018;6. https://doi.org/10.2196/10916.
- Richards KAR, Hemphill MA. A practical guide to collaborative qualitative data analysis. J Teach Physic Educ 2018;37:225-31. https://doi.org/10.1123/jtpe.2017-0084.
Appendix 1 Reports of studies not yet published
Work programme in systemic treatment: two reports
1. eRAPID systemic randomised controlled trial qualitative findings: a summary of the qualitative findings from the eRAPID systemic randomised controlled trial
Introduction
To help to understand the value of the eRAPID system in supporting patient care during systematic cancer treatment, a qualitative substudy was embedded within the RCT. Interviews and written feedback were obtained from patients and staff to gain insight into how the intervention was received and used, and its impact on clinical management. Summarised below are the main findings from this qualitative work, which helped to underpin and provide wider context to the main trial findings.
Methods
Interview procedures
Patients: we aimed to conduct interviews with 5–10 intervention participants per cancer group (in the internal pilot and then for the main trial). A purposive sampling strategy was applied for the main trial phase. A semistructured interview schedule covered questions around experiences and views of the eRAPID system and any impact on medical encounters and care.
Staff: end-of-study interviews with up to five health professionals (specialist nurses and oncologists) from each disease group were planned. A semistructured interview explored the access to and use of the eRAPID symptom reports and the perceived value of the patient data in clinical practice.
Additional feedback
Additional feedback was obtained from patients and oncology staff via the following:
-
Patient end-of-study feedback forms (completed at 18 weeks), which included free-text items covering the good and bad things about using eRAPID, suggestions for improvement and further comments about study participation.
-
Clinician feedback forms (completed by clinicians throughout the study when reviewing online symptom reports during face-to-face patient consultations). These included free-text boxes in which staff could describe how the symptom reports were used and provide additional comments.
Analysis
Interviews
Interview recordings were transcribed verbatim, transferred to NVivo 11 (QSR International, Warrington, UK) and were analysed by a core group of eRAPID researchers. A thematic framework approach was applied. 86,145
Feedback forms
The free-text comments (from patients and staff) were collated, reviewed for key content and summarised under the overarching coding framework developed from the interview analysis.
Results
Summary of qualitative data obtained
In total, 45 patients and 19 staff were interviewed. Fifty-five members of staff participated in the trial, providing a total of 784 clinician feedback forms following consultations with eRAPID intervention patients. Data from 185 patient end-of-study feedback forms were used for analysis.
Patient perspectives of eRAPID
Patient feedback covered three key themes:
-
general acceptability and functionality of the system
-
personal value of using eRAPID
-
impact of eRAPID on clinical care.
General acceptability and functionality of the system:
-
Patients found the system to be straightforward and easy to use.
-
The e-mail/text reminders were useful triggers to help to maintain regular completion, although symptom reporting became established in the weekly routine for some participants.
-
Issues with health were a common explanation for missing report completions (e.g. tiredness, impaired cognitive functioning and hospital admission).
-
The repetitiveness of the weekly completions and advice was seen as a downside by some patients (particularly when symptoms had not changed).
-
In terms of ideas for system improvement, suggestions included developing an eRAPID app and functionality to add information and context around symptom experience.
The personal value of eRAPID was described in a number of ways:
-
Helped to maintain a connection with the hospital – ‘Felt closer to the medical system’ (ID 166).
-
Gained reassurance from the trusted advice and information provided by eRAPID to help monitor symptoms. This was in addition to confidence that patients and caregivers were taking the right action, including when to seek medical advice, particularly in the early weeks of starting treatment.
-
Empowerment gained from personal symptom tracking – . . . ‘felt like I was taking an active role in my treatment’ (ID 123).
-
It was a useful memory aid to help recall symptom experience between treatments.
One participant commented that their use of the system had been primarily driven by the fact they had agreed to take part in a study and provide information.
Impact of eRAPID on clinical care. Varied responses around the impact of eRAPID on care were provided. Some felt that their reports had led staff to better understand the patient’s experiences and that explicit reference to their data had been made to guide treatment dosages or other supportive medication:
. . . the consultants using the system were able to raise issues raised – prescribing additional medication if necessary.
ID 216
Others, however, reported being surprised and confused that symptom reports were not used at all by the medical team. This was massively off-putting for some, leading to complete dissociation with online report completion. A key recommendation that came from the patient end-of-study feedback was to improve staff engagement with the reported data.
Staff perspectives
Staff feedback largely came under three themes:
-
general acceptability and functionality of the system
-
impact of eRAPID on clinical assessments
-
perceptions of patient views of eRAPID.
General acceptability and functionality of the system. Staff found eRAPID patient-reported data easy to access within the electronic records: ‘you just have to click a button, all the information is there, so it was easy to use, readily available’ (colorectal, senior oncologist).
Both the table and the graph presentation options were considered helpful to meet different informational needs and personal preferences.
Some practical issues were reported to have influenced viewing/using the data, including not being able to access a computer in some assessment areas and lack of time.
Impact on clinical assessments. A number of positive examples described how the symptom reports had been useful:
-
helping to structure or prepare for the consultation (possibly time saving in some instances)
-
building a connection/rapport with the patient
-
helping to track symptom changes over time
-
identifying issues that would not otherwise have been discussed.
Others felt that it added time to the consultation and one staff participant mentioned that the use of the patient-reported data was a challenge to their usual clinical practice and way of doing things.
There were some criticisms that, on occasion, patients reported symptoms that were not associated with cancer or treatment and that there could be discordance between what patients reported online and what they described in person.
In addition, the RCT methodology was recognised as giving staff limited experience of seeing patients with symptom data (and made it easy to forget which patients were on the trial).
Perceptions of patient views of eRAPID. Although some professionals were aware that patients had found the eRAPID system acceptable and useful, others did remember any particular feedback. Staff identified a range of potential barriers to effectively using eRAPID routinely, including variation in patient adherence with online reporting, issues for non-English speakers and access to IT.
Conclusions
-
The qualitative aspects of the eRAPID systemic RCT provided useful additional information on how the intervention worked and was well received by patients and staff.
-
Both participant groups reported that eRAPID was easy to use/access.
-
From the patient point of view, the positives of eRAPID were that it was a trusted information and support tool and helped to maintain a link with the hospital.
-
Patients expressed a range of ways in which the system could be improved (through advances with the technology) and better use of the data (more engagement from the clinical teams).
From the staff perspective, some professionals were positive about the potential benefit of the system for assisting with consultation preparation and focusing discussions. In practice, we realise that the design of the RCT meant that some staff had limited exposure to patients allocated to the eRAPID intervention, which meant that they could forget to check for or use the patient reports.
The qualitative findings presented here are limited in a number of ways. For example:
-
Interviews and feedback from patients were gathered at the end of the 18-week study period rather than routinely throughout. This may bias or limit what was recalled about how eRAPID was used.
-
Clinicians were relied on to provide written summaries of how they had used the eRAPID data in clinical encounters. Observations or audio-recordings would have provided supplementary insight on how patient data were used in consultations.
As adoption of PROMs into current practice is becoming increasingly commonplace, it remains vital to explore how systems work in practice to ensure that they meet the needs of both patients and clinical teams. An important element of this will be to help support and train both patients and staff on methods to maximise the use and clinical value of PROMs data.
2. eRAPID health economics report
Background
An economic evaluation was conducted to estimate the cost-effectiveness of the eRAPID system for AE reporting from home or hospital compared with usual care for patients receiving systemic treatment (chemotherapy or targeted therapies) for colorectal, breast or gynaecological cancers. The analysis was guided by the recommendations of the NICE methods guide. 146
Methods
An embedded health economic evaluation was conducted alongside the eRAPID trial, consisting of a within-trial analysis evaluating the costs and benefits accruing to patients over the 18 weeks of the trial. An exploratory analysis was undertaken at 12 months post randomisation.
Aims and end points
The primary aim of this analysis was to produce estimates of the cost-effectiveness of the eRAPID system compared with usual care for patients receiving systemic treatment for colorectal, breast or gynaecological cancers. The primary end point was the cost per incremental QALY gained when using the eRAPID system compared with usual care at 18 weeks post randomisation.
Perspective and time frame
The study adopted a health-care provider perspective for the main analysis and an additional analysis was undertaken from a societal perspective. Costs (direct and indirect) and outcomes of patients randomised to the eRAPID arm of the trial were compared with those randomised to usual care over the 18-week time horizon of the trial. Given that the time frame was less than 1 year, discounting of costs and benefits was not required.
Measurement of outcomes
Health state utility values were obtained from patient responses to the EQ-5D-5L questionnaire,81,89 which was administered at baseline and 6, 12 and 18 weeks post randomisation, and at 12 months post randomisation for a subset of patients. In November 2018, NICE updated their position statement with regards the use of EQ-5D-5L in reference case analyses and recommended using a mapping function to map EQ-5D-5L responses to the EQ-5D-3L value set. 88 Consequently, patient responses to the EQ-5D-5L questionnaire were mapped to EQ-5D-3L utility values using the van Hout et al. 90 crosswalk. The utility values represent patients’ quality of life and were multiplied by duration (t) in each health state to generate QALYs, which were used as the main outcome measure for this analysis, using an area under the curve approach:
where EQ5DBaseline, EQ5D6, EQ5D12 and EQ5D18 are the EQ-5D scores at baseline, 6 weeks, 12 weeks and 18 weeks, respectively. If an individual died during the trial, we assumed that their utility value was zero from the date of death to trial end and assumed a linear transition to this value from their last completed EQ-5D.
Multivariate regression was used to analyse the difference in QALYs between treatment groups, controlling for baseline quality of life, age and gender.
Measurement of resource use and costs
All health-care resource use data were collected for the trial period of 18 weeks from randomisation using patient-completed questionnaires administered at 6, 12 and 18 weeks and 12 months, along with data obtained directly from hospital records. This included use of primary and secondary care services along with prescription medications. Patient out-of-pocket costs, including travel, non-prescription medications and other health-care-related expenses, were also collected.
Cost analysis
All use of health-care services within the trial period was converted to costs using appropriate UK unit costs estimated for the price year 2018. Unit costs were assigned to health-care resource use from the Personal Social Services Research Unit’s Unit Costs of Health and Social Care147 and the DHSC’s National Schedule of Reference Costs,148 and costs were assigned to medications using prices from the British National Formulary. 93 Unit costs of health-care resource use items used in the analysis are presented in Table 5. Patients’ use of health-care resources and total costs were calculated for the intention-to-treat population.
Total costs for each patient were calculated as the sum of costs assigned for hospital services, community health and social services, chemotherapy, hormone/targeted therapies and medications, as well as the intervention cost. The eRAPID intervention was developed before this trial and those development costs are, therefore, sunk costs and not relevant to the wider implementation of the system. The intervention cost included in this cost-effectiveness analysis, therefore, consists of the cost of the patient manual, which provided training and guidance on using the eRAPID system, and the cost of maintenance of the QTool software. Only the cost of printing the manual was included (not its development) and this was calculated based on University of Leeds printing charges. 151 A maintenance cost for the QTool software for the 18 weeks of the trial was calculated based on an annual maintenance cost of £10,000, which was divided by the number of patients in the eRAPID intervention group. Time taken off work by patients was included in the societal perspective analysis using a human capital approach and a median hourly pay of £11.31 for UK adults. 152 In the absence of additional information, patients who reported working full time were assumed to work 7.5 hours per day and patients who reported working part time were assumed to work 4 hours per day. Multivariate regression was used to analyse the difference in costs between treatment groups controlling for age and gender.
| Resource item | Location | Unit cost (£) | Source | Details |
|---|---|---|---|---|
| Community health-care services | ||||
| GP surgery | Clinic | 37.40 | PSSRU 201891 | Per-patient contact lasting 9.22 minutes |
| Home | 85.00 | (Per-patient contact lasting 9.22 minutes + average 12-minute travel time) × 4.00/minute | ||
| Telephone | 15.10 | GP-led triage, per call lasting 4 minutes | ||
| Nurse | Clinic | 10.85 | PSSRU 201891 | Per 15.5-minute consultation (PSSRU 2015),149 based on 42 per hour (PSSRU 2018)91 |
| Home | 18.05 | Consultation + 7.20 (based on 12-minute travel time) | ||
| Telephone | 7.70 | Practice nurse, nurse-led triage, per call lasting 6.56 minutes | ||
| Physiotherapist | Clinic | 57.00 | NHS Reference Costs 2017–18 92 | Clinical psychologist, band 7, per working hour |
| Home | 68.40 | Consultation + 11.40 (average 12-minute travel time) | ||
| Telephone | 22.80 | Assumeda | ||
| Psychologist | Clinic | 55.00 | NHS Reference Costs 2016–17 148 | Physiotherapist, adult, one to one |
| Home | 66.00 | Consultation + 11 (average 12-minute travel time) | ||
| Telephone | 22.00 | Assumeda | ||
| Counsellor | Clinic | 55.00 | NHS Reference Costs 2016–17 148 | Occupational therapist, adult, one to one |
| Home | 66.00 | Consultation + 11 (average 12-minute travel time) | ||
| Telephone | 22.00 | Assumeda | ||
| Hospital services | ||||
| Inpatient stay (24 hours) | 431.11 | NHS Reference Costs 2017–18 92 | Elective inpatients excess bed-days | |
| Outpatient visit | 139.59 | NHS Reference Costs 2017–18 92 | Outpatient procedures | |
| A&E | 160.32 | NHS Reference Costs 2017–18 92 | Emergency medicine | |
| Hospital consultations | ||||
| Allied health professional | ||||
| Dietetics | Telephone | 54.00 | NHS Reference Costs 2017–18 92 | Dietetics, non-admitted, non-face-to-face attendance, first |
| Gynaecological oncology | Telephone | 23.00 | NHS Reference Costs 2017–18 92 | Gynaecological oncology, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 116.00 | NHS Reference Costs 2017–18 92 | Gynaecological oncology, non-consultant led, non-admitted face-to-face attendance, first | |
| Occupational health | Telephone | 72.00 | NHS Reference Costs 2017–18 92 | Occupational therapy, non-admitted non-face-to-face attendance, first |
| Visit | 74.00 | NHS Reference Costs 2017–18 92 | Occupational therapy, non-admitted face-to-face attendance, first | |
| Speech and language therapy | Telephone | 85.00 | NHS Reference Costs 2017–18 92 | Speech and language therapy, non-admitted non-face-to-face attendance, first |
| Visit | 117.00 | NHS Reference Costs 2017–18 92 | Speech and language therapy, non-admitted face-to-face attendance, first | |
| Consultant | ||||
| Breast oncology | Telephone | 159.00 | NHS Reference Costs 2017–18 92 | Medical oncology, consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 229.00 | NHS Reference Costs 2017–18 92 | Medical oncology, consultant led, non-admitted face-to-face attendance, first | |
| Palliative medicine | Telephone | 447.00 | NHS Reference Costs 2017–18 92 | Palliative medicine, consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 330.00 | NHS Reference Costs 2017–18 92 | Palliative medicine, consultant led, non-admitted face-to-face attendance, first | |
| Nurse | ||||
| Oncology | Telephone | 30.00 | NHS Reference Costs 2017–18 92 | Medical oncology, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 162.00 | NHS Reference Costs 2017–18 92 | Medical oncology, non-consultant led, non-admitted face-to-face attendance, first | |
| Breast surgery | Telephone | 210.00 | NHS Reference Costs 2017–18 92 | Breast surgery, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 127.00 | NHS Reference Costs 2017–18 92 | Breast surgery, non-consultant led, non-admitted face-to-face attendance, first | |
| Clinical oncology | Telephone | 82.00 | NHS Reference Costs 2017–18 92 | Clinical oncology, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 126.00 | NHS Reference Costs 2017–18 92 | Clinical oncology, non-consultant led, non-admitted face-to-face attendance, first | |
| Colorectal surgery | Telephone | 82.00 | NHS Reference Costs 2017–18 92 | Colorectal surgery, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 109.00 | NHS Reference Costs 2017–18 92 | Colorectal surgery, non-consultant led, non-admitted face-to-face attendance, first | |
| Gynaecological oncology | Telephone | 23.00 | NHS Reference Costs 2017–18 92 | Gynaecological oncology, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 116.00 | NHS Reference Costs 2017–18 92 | Gynaecological oncology, non-consultant led, non-admitted face-to-face attendance, first | |
| Palliative medicine | Telephone | 214.00 | NHS Reference Costs 2017–18 92 | Palliative medicine, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 111.00 | NHS Reference Costs 2017–18 92 | Palliative medicine, non-consultant led, non-admitted face-to-face attendance, first | |
| Research | Telephone | 36.00 | NHS Reference Costs 2017–18 92 | Other specialist nursing, adult non-face to face |
| Visit | 79.00 | NHS Reference Costs 2017–18 92 | Other specialist nursing, adult face to face | |
| Surgery | Telephone | 85.00 | NHS Reference Costs 2017–18 92 | General surgery, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 107.00 | NHS Reference Costs 2017–18 92 | General surgery, non-consultant led, non-admitted face-to-face attendance, first | |
| Thoracic medicine | Telephone | 109.00 | NHS Reference Costs 2017–18 92 | Thoracic surgery, non-consultant led, non-admitted non-face-to-face attendance, first |
| Visit | 133.00 | NHS Reference Costs 2017–18 92 | Thoracic surgery, non-consultant led, non-admitted face-to-face attendance, first | |
| Adjuvant chemotherapy nurse specialist | Visit | 89.00 | NHS Reference Costs 2017–18 92 | Specialist nursing, cancer related, adult, face to face |
| Other | ||||
| Clinical nurse specialist helpline | Telephone | 35.00 | NHS Reference Costs 2017–18 92 | Specialist nursing, cancer related, adult, non-face to face |
| Leeds Cancer Centre | Telephone | 35.00 | NHS Reference Costs 2017–18 92 | Specialist nursing, cancer related, adult, non-face to face |
| Other health-care services (specified by participants) | ||||
| 111 service | 8.00 | BBC News150 | ||
| Acupuncture | 129.00 | NHS Reference Costs 2016–17 148 | Acupuncture for pain management | |
| Audiology | 48.00 | NHS Reference Costs 2016–17 148 | Audiology, non-admitted non-face to face | |
| Breast care nurse | 78.00 | NHS Reference Costs 2016–17 148 | Specialist nursing, breast care nursing/liaison, adult face to face | |
| Cancer nurse | 89.00 | NHS Reference Costs 2016–17 148 | Specialist nursing, cancer related, adult face to face | |
| Chiropodist/podiatrist | 41.00 | NHS Reference Costs 2016–17 148 | Podiatrist, tier 1, general podiatry | |
| Community mental health worker | 95.00 | NHS Reference Costs 2016–17 148 | Mental health specialist teams, IAPT, adult and elderly, per care contact | |
| Community stoma nurse | 51.00 | NHS Reference Costs 2016–17 148 | Specialist nursing, stoma care services, adult face to face | |
| Dentist | 92.00 | NHS Reference Costs 2016–17 148 | General dental service, attendance | |
| Diabetic nurse | 67.00 | NHS Reference Costs 2016–17 148 | Specialist nursing, diabetic nursing/liaison, adult face to face | |
| Dietitian | 86.00 | PSSRU 201891 | Dietitian | |
| Home care | 16.88 | PSSRU 201891 | Home care worker, based on 30-minute visit (80% of total time) + travel time (20% of total time) at 27 per hour | |
| Midwife | 56.00 | NHS Reference Costs 2016–17 148 | Community midwife, ante natal visit | |
| Paramedic | 192.00 | NHS Reference Costs 2016–17 148 | Ambulance, see and treat or refer | |
| Parkinson’s nurse | 76.00 | NHS Reference Costs 2016–17 148 | Specialist nursing, parkinson’s and alzheimer’s nursing/liaison, adult, face to face | |
| Pharmacist | 6.76 | PSSRU 201891 | Pharmacist, band 6, based on 9.22-minute consultation (assumed same as GP) at 44 per working hour | |
| Psychiatrist | 109.00 | PSSRU 201891 | Consultant: psychiatric, per working hour | |
| Social worker | 61.00 | PSSRU 201891 | Social worker, per hour of client-related work | |
| Specialist nurse | 76.00 | NHS Reference Costs 2016–17 148 | Other specialist nursing, adult face to face | |
| Specialist ophthalmic | 118.00 | NHS Reference Costs 2016–17 148 | Ophthalmology, consultant led, non-admitted face-to-face attendance, first | |
| Surveillance sigmoidoscopy | 182.00 | NHS Reference Costs 2016–17 148 | Diagnostic flexible sigmoidoscopy, aged ≥ 19 years | |
Adjusting for baseline imbalance
Given that patients’ baseline utility is likely to be correlated with their utility over the follow-up period, any imbalance in baseline utilities must be accounted for when calculating differential effects between treatment groups. 94,95 Multiple regression analysis was used to estimate differential mean QALYs controlling for utility at baseline.
Missing data
Based on the descriptive analysis of the missing data, the analysis was conducted under the assumption that the missing data were missing at random. 97 Consequently, where there was missing quality-of-life or cost follow-up data, multiple imputation methods were used to generate estimates of missing values based on the distribution of observed data, as per recommended best practices for economic evaluation alongside clinical trials. 96
When choosing the level at which to impute missing data (more or less aggregated), a balance needs to be struck between maintaining the data structure and achieving a stable imputation model. 97 Consequently, for quality-of-life data, missing EQ-5D index values were imputed at each follow-up. For costs, missing data were imputed for each follow-up at the level of total community health and social care costs, not at the unit of resource level. Missing baseline EQ-5D values were imputed using mean imputation to ensure that imputed values were independent of treatment allocation. 98 EQ-5D index values were recorded as missing if any EQ-5D items were missing for a given time point. Costs were counted as missing if all resource items on the case report form were missing.
The imputation was performed in Stata® Version 15 (StataCorp LP, College Station, TX, USA) using predictive mean matching to perform multiple imputation by chained equations. Predictive mean matching ensures that only plausible values of the missing variable are imputed as the imputed value is drawn from another individual whose predicted value is close to the predicted value of the individual with the missing observation. 97
Cost-effectiveness analysis
Primary analysis
The cost-effectiveness analysis adopted an intention-to-treat perspective for analysing and summarising the health economic trial data. The primary analysis consisted of a cost–utility analysis over the 18-week trial period and included adjustment for baseline variables and imputation of missing data. The incremental cost-per-QALY gained by patients using the eRAPID system compared with usual care was calculated, producing an ICER153 as follows:
NICE considers a cost per QALY within the range of £20,000–30,000 to be acceptable. 146 Therefore, the lower limit of this threshold (£20,000) was used to guide the analysis of cost-effectiveness.
Secondary analysis
In addition to the primary analysis conducted from the health-care provider perspective, a secondary analysis was also undertaken from the societal perspective. This analysis was also conducted over the 18-week trial period, but, in addition to the costs included in the primary analysis, the secondary analysis included costs to patients, such as travel expenses and over-the-counter medicines, and productivity losses. The secondary analysis was carried out using the same methods outlined for the primary analysis.
The exploratory analysis at 12 months replicates the methods used in the primary analysis.
Sensitivity analyses
Sensitivity analyses were conducted to explore the impact of assumptions made in the primary analysis and alternative perspectives for analysis. ICERs from each of the sensitivity analyses were compared to the main trial results to identify areas of uncertainty.
The effect of adjusting for baseline imbalance on cost-effectiveness was explored in an analysis with no adjustment for baseline differences between groups. In addition, the effect of not imputing missing data was considered in an analysis including complete cases only. The effect of using generic versus condition-specific measures of HRQoL was explored in a sensitivity analysis using EORTC QLQ-C30 to calculate QALYs rather than EQ-5D. As NICE recently updated their position statement88 advising the use of the van Hout mapping algorithm for obtaining health state utility values, the effect of this was explored in a sensitivity analysis using the EQ-5D-5L value set provided by Devlin et al. 154
An additional sensitivity analysis had been planned to explore the impact of favouring data extracted directly from hospital records by undertaking an analysis using patient reported data only. However, following the pilot phase of the trial, hospital resource use was not collected from patients, so this analysis was not possible.
Uncertainty analysis
The level of sampling uncertainty around the ICER was determined using a non-parametric bootstrap to generate 10,000 estimates of incremental costs and benefits. The bootstrapped estimates were plotted on the cost-effectiveness plane to illustrate the uncertainty surrounding the cost-effectiveness estimates. 100 A CEAC illustrating the probability that the eRAPID system is cost-effective at a range of threshold values (£0–100,000) was also constructed using the bootstrapped samples. 99
Results
Sample
Of the 508 patients recruited to the trial, 191 patients had complete resource use and EQ-5D-5L data for all follow-ups.
Resource use and costs
Table 6 shows the average resource use of patients for community health-care services in each trial arm over the 18-week duration of the trial.
Hospital resource use is presented in Table 7. Given that these data were extracted directly from hospital records and, therefore, do not relate to data collected in case report forms at each follow-up, hospital resource use is presented as a total for the trial period.
Average health-care costs over the trial period are presented in Table 8. Multiple regression analysis indicated that the difference in total costs between groups was not statistically significant (95% CI –£1240.91 to £1167.69, p > 0.05).
| Item | Location | Baseline | 6 weeks | 12 weeks | 18 weeks | ||||
|---|---|---|---|---|---|---|---|---|---|
| Usual care (n = 26) | eRAPID (n = 33) | Usual care (n = 160) | eRAPID (n = 136) | Usual care (n = 128) | eRAPID (n = 134) | Usual care (n = 114) | eRAPID (n = 108) | ||
| GP | Clinic | 1.115 (1.071) | 1.455 (1.148) | 0.825 (0.858) | 0.809 (0.985) | 0.656 (0.943) | 0.649 (0.998) | 0.623 (0.78) | 0.796 (1.174) |
| Home | 0.077 (0.272) | 0.212 (0.927) | 0.025 (0.157) | 0.022 (0.147) | 0.078 (0.409) | 0.022 (0.148) | 0.018 (0.132) | 0.037 (0.19) | |
| Telephone | 0 (0) | 0 (0) | 0.425 (0.765) | 0.603 (1.137) | 0.398 (0.934) | 0.47 (1.249) | 0.386 (0.698) | 0.426 (0.739) | |
| Nurse | Clinic | 0.462 (1.303) | 0.333 (0.736) | 0.338 (1.223) | 0.221 (0.767) | 0.344 (1.031) | 0.396 (1.377) | 0.474 (1.483) | 0.37 (1.01) |
| Home | 2.885 (8.524) | 3 (9.189) | 1.481 (2.592) | 2.051 (4.146) | 2.414 (4.56) | 1.993 (3.082) | 1 (1.829) | 1.815 (3.523) | |
| Telephone | 0 (0) | 0 (0) | 0.2 (0.853) | 0.191 (0.923) | 0.18 (1.16) | 0.09 (0.607) | 0.07 (0.318) | 0.083 (0.435) | |
| Physiotherapist | Clinic | 0.269 (0.533) | 0.091 (0.384) | 0.088 (0.468) | 0.015 (0.121) | 0.023 (0.152) | 0.015 (0.122) | 0.018 (0.132) | 0.019 (0.135) |
| Home | 0 (0) | 0 (0) | 0.013 (0.111) | 0 (0) | 0 (0) | 0 (0) | 0.026 (0.281) | 0 (0) | |
| Telephone | 0 (0) | 0 (0) | 0 (0) | 0.007 (0.086) | 0 (0) | 0.015 (0.122) | 0.009 (0.094) | 0 (0) | |
| Psychologist | Clinic | 0.115 (0.588) | 0.061 (0.242) | 0.019 (0.237) | 0.029 (0.209) | 0.008 (0.088) | 0.037 (0.285) | 0.079 (0.464) | 0.139 (1.089) |
| Home | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Telephone | 0 (0) | 0 (0) | 0.069 (0.87) | 0.015 (0.171) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Counsellor | Clinic | 0 (0) | 0 (0) | 0.038 (0.334) | 0.044 (0.295) | 0.094 (0.553) | 0.067 (0.28) | 0.105 (0.522) | 0.019 (0.135) |
| Home | 0 (0) | 0 (0) | 0.013 (0.158) | 0.007 (0.086) | 0 (0) | 0 (0) | 0.009 (0.094) | 0.019 (0.192) | |
| Telephone | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.044 (0.336) | 0 (0) | |
| Item | Usual care | eRAPID | ||
|---|---|---|---|---|
| Mean (SD), n | Minimum, maximum | Mean (SD), n | Minimum, maximum | |
| Inpatient visits | 2.757 (1.804), 115 | 1, 9 | 3 (2.526), 112 | 1, 15 |
| Inpatient days | 6.661 (6.584), 115 | 1, 44 | 8.143 (9.975), 112 | 1, 71 |
| Hospital consultation | 2.04 (1.549), 101 | 1, 8 | 2.409 (2.181), 110 | 1, 12 |
| Outgoing telephone consultation | 2.589 (2.632), 95 | 1, 20 | 2.861 (2.302), 108 | 1, 12 |
| Incoming telephone consultation | 1.676 (1.628), 34 | 1, 9 | 1.935 (1.931), 31 | 1, 10 |
| CNS helpline | 1.286 (0.488), 7 | 1, 44 | 2.375 (3.114), 8 | 1, 10 |
| Other hospital visit (assessment) | 1.813 (1.125), 107 | 1, 6 | 1.748 (1.065), 107 | 1, 7 |
| A&E | 1.071 (0.267), 14 | 1, 2 | 1 (0), 13 | 1, 1 |
| Total costs | Usual care | eRAPID | ||
|---|---|---|---|---|
| Mean (SD), n | Minimum, maximum | Mean (SD), n | Minimum, maximum | |
| Community health and social services | 259.84 (222.09), 114 | 0, 1798 | 282.01 (243.81), 97 | 0, 1314.75 |
| Hospital services | 1629.95 (2520.48), 252 | 0, 18,968.84 | 1921.24 (3546.28), 256 | 0, 31,094.81 |
| Chemotherapy | 4583.97 (2489.91), 252 | 0, 24120 | 4463.29 (2493.92), 256 | 514, 15229 |
| Hormone/targeted therapies | 1791.27 (4813.59), 252 | 0, 27,226.52 | 1548.75 (4643.52), 256 | 0, 27,226.52 |
| Prescription medications | 76.34 (140.65), 234 | 0, 1275.17 | 57.59 (77.07), 231 | 0, 525.8 |
| Intervention cost | 0 (0), 252 | 0, 0 | 15.59 (0), 256 | 15.59, 15.59 |
| Out-of-pocket costs | 313.97 (559.23), 252 | 0, 5340 | 327.4 (788.64), 256 | 0, 9200 |
| Time out of work | 1176.67 (2163.37), 250 | 0, 10,687.95 | 1029.76 (1937.39), 256 | 0, 9670.05 |
| Total cost (health-care provider) | 9044.22 (7056.23), 112 | 2522.76, 41,196.16 | 8603.34 (7901.21), 93 | 1871.53, 43,064.25 |
| Total cost (societal) | 10,976.03 (7342.09), 112 | 2895.93, 41,652.16 | 10,830.49 (8006.2), 93 | 1879.52, 43,198.25 |
Quality of life
Mean (SD) EQ-5D scores for each trial arm at each follow-up are presented in Table 9. EQ-5D scores decreased over the trial period in both arms, but scores were higher at each time point in the eRAPID arm. However, baseline scores were also slightly higher in the eRAPID arm. Multiple regression analysis indicated that the difference in QALYs gained between groups was not statistically significant (95% CI –0.004 to 0.011, p > 0.05).
| Time point | Usual care, mean (SD), n | eRAPID, mean (SD), n | ||
|---|---|---|---|---|
| EQ-5D score | Change from baseline | EQ-5D score | Change from baseline | |
| Baseline | 0.753 (0.18), 248 | 0.758 (0.185), 250 | ||
| 6 weeks | 0.752 (0.197), 226 | 0 (0.176), 224 | 0.776 (0.175), 213 | –0.001 (0.183), 209 |
| 12 weeks | 0.734 (0.18), 210 | –0.025 (0.178), 208 | 0.747 (0.192), 202 | –0.028 (0.191), 196 |
| 18 weeks | 0.708 (0.213), 202 | –0.05 (0.212), 200 | 0.739 (0.216), 189 | –0.052 (0.209), 184 |
Missing data
Complete and missing community health-care use and EQ-5D-5L data are presented in Tables 10 and 11, respectively. Missing data for hospital resource use are not presented because these data were extracted from hospital records directly and, therefore, it is assumed to be complete in the absence of information to indicate otherwise. A total of 205 (40%) patients completed resource use questionnaires for all follow-ups. A total of 349 (69%) patients had complete EQ-5D-5L scores for all follow-up.
| Time point | Usual care | eRAPID | ||
|---|---|---|---|---|
| n valid (missing) | Percentage complete | n valid (missing) | Percentage complete | |
| 6 weeks | 160 (92) | 63 | 136 (116) | 53 |
| 12 weeks | 128 (124) | 51 | 134 (118) | 52 |
| 18 weeks | 114 (138) | 45 | 108 (144) | 42 |
| Time point | Usual care | eRAPID | ||
|---|---|---|---|---|
| n valid (missing) | Percentage complete | n valid (missing) | Percentage complete | |
| Baseline | 248 (4) | 98 | 250 (6) | 98 |
| 6 weeks | 226 (26) | 90 | 213 (43) | 83 |
| 12 weeks | 210 (42) | 83 | 202 (54) | 79 |
| 18 weeks | 202 (50) | 80 | 189 (67) | 74 |
Cost-effectiveness results
Cost-effectiveness results are presented in Table 12. The eRAPID group had both the highest QALY gain over the trial period and the lowest costs. This indicates that the eRAPID system dominates usual care and may be preferred as a cost-effective option.
| Treatment group | Costa (£), mean (SD) | Incremental costb (£) | QALY,a mean (SD) | Incremental QALYb | ICER (£/QALY) |
|---|---|---|---|---|---|
| Usual care | 8330.36 (435.23) | 0.255 (0.004) | eRAPID dominates | ||
| eRAPID | 8305.08 (450.5) | –25.28 | 0.259 (0.004) | 0.003 |
Uncertainty analysis
Bootstrapped estimates of the incremental costs and incremental effects are plotted on the cost-effectiveness plane in Figure 7. This shows the joint distribution of the incremental costs and effects for patients using the eRAPID system compared with usual care. The majority of the points lie to the right of the y-axis, indicating that use of the eRAPID system is likely to increase QALYs gained. The spread of points both above and below the x-axis indicates the uncertainty around the impact of the eRAPID system on costs.
FIGURE 7.
Cost-effectiveness plane: eRAPID vs. usual care.
The probability that use of the eRAPID system is cost-effective compared with usual care is presented on the cost-effectiveness acceptability curve shown in Figure 8. Based on data collected over the 18 weeks of the main trial and a cost-effectiveness threshold of £20,000 per QALY, the eRAPID intervention has a 55% probability of being cost-effective.
FIGURE 8.
Cost-effectiveness acceptability curve: eRAPID vs. usual care.

Sensitivity analysis
The cost-effectiveness results for each scenario explored in the sensitivity analyses are presented in Table 13. The results of the primary analysis are robust to all sensitivity analyses conducted. In each case, higher QALY gains and lower costs are observed in the eRAPID group than in the usual-care group, indicating that the eRAPID system is likely to be a more cost-effective use of resources.
Secondary analysis
Results from the secondary analysis, conducted from the societal perspective, are also presented in Table 13. This shows that even when societal perspective costs, including patient out-of-pocket costs for food, travel, non-prescription medications, additional expenses and time out of work, are included in the analysis, lower costs are still observed in the eRAPID group. Although the difference in costs remains small, this difference is larger than is observed in the primary analysis, conducted from a health-care provider perspective. This indicates that the eRAPID system may have an additional impact on the wider societal costs, over and above the difference in health-care resource use. As in the primary cost-effectiveness analysis, higher QALY gains and lower costs are observed in the eRAPID group than in the usual-care group, indicating that the use of the eRAPID system may be a cost-effective use of resources even when costs are analysed from a societal perspective.
| Treatment group | Costa (£), mean (SE) | Incremental costb (£) | QALY,a mean (SE) | Incremental QALYb | ICER (£/QALY) |
|---|---|---|---|---|---|
| Sensitivity analysis: intention to treat – no adjustment for baseline | |||||
| Usual care | 8330.36 (435.23) | 0.255 (0.004) | eRAPID dominates | ||
| eRAPID | 8305.08 (450.5) | –25.28 | 0.259 (0.004) | 0.004 | |
| Sensitivity analysis: complete case | |||||
| Usual care | 11,069.05 (7400.83) | 0.254 (0.051) | eRAPID dominates | ||
| eRAPID | 10,971.14 (8242.43) | –97.90 | 0.264 (0.044) | 0.003 | |
| Sensitivity analysis: alternative measures of HRQoL – EORTC QLQ-C30 | |||||
| Usual care | 8330.60 (435.16) | 0.264 (0.003) | eRAPID dominates | ||
| eRAPID | 8307.04 (450.54) | –23.55 | 0.268 (0.003) | 0.004 | |
| Sensitivity analysis: using EQ5D-5L value set | |||||
| Usual care | 8331.48 (435.17) | 0.278 (0.003) | eRAPID dominates | ||
| eRAPID | 8307.11 (450.44) | –24.37 | 0.282 (0.003) | 0.003 | |
| Secondary analysis: societal perspective | |||||
| Usual care | 9811.67 (453.53) | 0.255 (0.004) | eRAPID dominates | ||
| eRAPID | 9662.24 (463.3) | –149.42 | 0.259 (0.004) | 0.003 | |
Exploratory analysis
Sample characteristics for the participants with 12-month post-randomisation data are shown in Table 14. For demographic variables, the samples are comparable; however, there are some differences in the clinical compositions of the patients in the main trial compared with the subsample with 12-month follow-up data, with a higher proportion of patients with colorectal cancers and a lower proportion of patient with breast cancer. The proportion of patients having previous chemotherapy is also higher in the 12-month subsample, reflecting a higher proportion of patients with metastatic disease than in the main trial.
| Characteristics | Main trial | Subsample with 12-month follow-up data | ||
|---|---|---|---|---|
| eRAPID | Usual care | eRAPID | Usual care | |
| Sample, N | 256 | 252 | 135 | 132 |
| Age at baseline (years), mean (SD); minimum, maximum | 55.95 (12.27); 22, 86 | 56.06 (11.37); 18, 79 | 56.4 (12.84); 22, 82 | 56.59 (11.62); 18, 79 |
| Gender, n (%) | ||||
| Male | 51 (19.92) | 51 (20.24) | 33 (24.44) | 29 (21.97) |
| Female | 205 (80.08) | 201 (79.76) | 102 (75.56) | 103 (78.03) |
| Weight (kg), mean (SD) | 75.01 (17.69) | 77.07 (18.68) | 77.17 (19.17) | 74.72 (16.99) |
| BMI (kg/m2), mean (SD) | 27.48 (5.95) | 28.38 (6.46) | 27.97 (6.32) | 27.72 (6.26) |
| Baseline EQ-5D score, mean (SD); n | 0.758 (0.185); 250 | 0.753 (0.180); 248 | 0.751 (0.185); 129 | 0.750 (0.191); 130 |
| Cancer site, n (%) | ||||
| Breast | 117 (45.70) | 116 (46.03) | 49 (36.30) | 51 (38.64) |
| Gynaecological: ovarian | 37 (14.45) | 41 (16.27) | 20 (14.81) | 25 (18.94) |
| Gynaecological: cervical | 6 (2.34) | 2 (0.79) | 4 (2.96) | 1 (0.76) |
| Gynaecological: endometrial | 10 (3.91) | 10 (3.97) | 7 (5.19) | 5 (3.79) |
| Colorectal | 86 (33.59) | 83 (32.94) | 55 (40.74) | 50 (37.88) |
| Had previous chemotherapy, n (%) | ||||
| Yes | 55 (21.48) | 51 (20.24) | 37 (27.41) | 34 (25.76) |
| No | 201 (78.52) | 201 (79.76) | 98 (72.59) | 98 (74.24) |
| Baseline disease status, n (%) | ||||
| Early cancer (primary and local recurrence) | 161 (62.89) | 156 (61.90) | 72 (53.33) | 71 (53.79) |
| Metastatic cancer | 95 (37.11) | 96 (38.10) | 63 (46.66) | 61 (46.21) |
Similar to the main trial analysis, the exploratory 12-month analysis (Table 15) shows small differences in costs and QALYs between the eRAPID and the usual-care groups. However, although higher QALYs gained in the eRAPID group are maintained over 12 months, costs are also higher in the eRAPID group.
| Treatment group | Costa (£), mean (SE) | Incremental costb (£) | QALY,a mean (SE) | Incremental QALYb | ICER (£/QALY) |
|---|---|---|---|---|---|
| Exploratory analysis for subsample with 12-month follow-up: intention to treat | |||||
| Usual care (n = 132) | 10,023.78 (641.66) | 0.663 (0.021) | |||
| eRAPID (n = 135) | 10,635.43 (699.15) | 611.65 | 0.673 (0.022) | 0.009 | 64,455.74 |
| Exploratory analysis for subsample with 12-month follow-up: societal perspective costs | |||||
| Usual care (n = 132) | 11,467.41 (669.27) | 0.663 (0.021) | |||
| eRAPID (n = 135) | 11,843.72 (707.83) | 376.31 | 0.673 (0.022) | 0.009 | 39,662.81 |
| Exploratory analysis for subsample with 12-month follow-up: complete case | |||||
| Usual care (n = 81) | 9923.68 (6909.49) | 0.706 (0.207) | eRAPID dominates | ||
| eRAPID (n = 69) | 9603.93 (7341.43) | –635.86 | 0.738 (0.187) | 0.015 | |
| Exploratory analysis for subsample with 12-month follow-up: EORTC QLQ-C30, intention-to-treat sample | |||||
| Usual care (n = 132) | 10,023.12 (641.84) | 0.716 (0.018) | Usual care dominates | ||
| eRAPID (n = 135) | 10,637.44 (699.67) | 614.32 | 0.707 (0.020) | –0.008 | |
| Exploratory analysis for subsample with 12-month follow-up: EORTC QLQ-C30, complete case | |||||
| Usual care (n = 77) | 9919.67 (7023.03) | 0.716 (0.018) | eRAPID dominates | ||
| eRAPID (n = 67) | 9713.63 (7406.84) | –271.144 | 0.707 (0.020) | 0.009 | |
Based on data collected for the subsample with 12-month follow-up data, the probability that eRAPID is cost-effective compared with usual care is 36%.
Discussion
Principal findings
The primary within-trial cost-effectiveness analysis indicated that the use of the eRAPID system may be more cost-effective than usual care for the management of AEs in patients receiving systemic treatment for colorectal, breast or gynaecological cancers at 18 weeks. Higher QALY gains and lower costs were observed in the eRAPID group than in the usual-care group. However, these mean differences were small and not statistically significant, and the cost-effectiveness acceptability curve showed that eRAPID had only a 55% probability of being cost-effective at the NICE-recommended cost-effectiveness threshold of £20,000 per QALY gained. Nevertheless, the results were robust to all scenarios explored in the sensitivity analyses. Secondary analyses indicated that the use of the eRAPID system may also be more cost-effective than usual care when costs are analysed from a societal perspective, although the difference in costs remained small.
Strengths and weaknesses of the economic analysis
A strength of this analysis lies in the design of the study. The randomised controlled design enabled the collection of good-quality data for use in this within-trial cost-effectiveness analysis. In addition, the use of linked hospital data has provided more robust data and meant that some of the biases with self-reported data have been avoided.
However, the lack of a completely connected system for all health-care records meant that self-reported data were still relied on to record the use of non-hospital health-care services. Consequently, some biases from self-reporting may have remained. In addition, health-care resource use questionnaires were not well completed, with the number of missing resource use questionnaires increasing over the trial period. This resulted in a large proportion of patients with missing community health-care use data.
The internal pilot phase of the trial provided a valuable opportunity to review the design of the trial and the feasibility of data collection mechanisms. Following the pilot phase, some changes to the data collection forms were made (hospital resource use was no longer collected in patient case report forms as this would be collected directly from hospital records). However, this produced some inconsistencies in the data collected for patients within the pilot phase as compared with the main trial, which could be viewed as a limitation.
The economic analysis followed recommended best practices for economic evaluations conducted in UK settings and consequently uses QALYs based on responses to the EQ-5D as the primary outcome. The use of QALYs, preferably calculated based on EQ-5D, is mandated by NICE87 to ensure consistency across evidence used to inform their recommendations and guidelines. However, QALYs focus on the measurement of patient health and other important aspects, such as patient experience, and satisfaction may not be captured. Furthermore, patient responses to the EQ-5D questionnaire were necessarily collected at fixed time points according to predefined data collection schedules. However, owing to the intermittent and cyclical nature of patient health care and the potential for AEs over chemotherapy cycles, the use of fixed data collection points may mean that fluctuations in patient health are missed. 101
Meaning of the study
The results of this analysis indicate that the eRAPID system may be more cost-effective than usual care in the management of patients receiving systemic treatment for colorectal, breast or gynaecological cancers. However, the differences in QALYs and costs were small in real terms and were not statistically significant. Therefore, other factors (such as patient and health-care provider acceptability of the system) are also likely to be important.
Given that the cost of developing the eRAPID system, which was covered in earlier research grants,102 represents a sunk cost, it was not included within the cost-effectiveness analysis presented here. Instead, the intervention cost was calculated based on the costs that would apply if the system was implemented more widely, including the maintenance cost of the QTool software and the cost of a training manual for each patient. However, the costs included are likely to overestimate the per-patient cost because the maintenance cost was split only between the patients in the eRAPID intervention group, but in practice this cost would be split across a much larger patient group. In addition, patient feedback on the training manual indicated that this resource could be simplified and shortened significantly, which would further reduce the cost in producing it. Consequently, the costs associated with the intervention may be overestimated and the resulting cost-effectiveness of the eRAPID system underestimated. For example, in the year April 2019 to March 2020 there were approximately 1761 new patients with breast, gynaecological or lower GI cancers across Leeds hospitals, as compared with 508 in the intention-to-treat population of this study. However, the maintenance costs for the system may apply to each region that implements eRAPID, with each having a different number of eligible patients across whom to split the costs. We, therefore, split only by the study population to present a conservative estimate of likely costs and cost-effectiveness.
Unanswered questions and further research
The analysis reported here provides useful insights into the costs and effects associated with the use of the eRAPID system over the trial period of 18 weeks. However, the duration of cancer treatment and the associated effects may mean that use of the system over a longer time frame would be relevant. The exploratory analysis using data from a subsample with 12-month follow-up data found small differences in costs and QALYs between the groups. However, although higher QALY gains in the eRAPID group were maintained, higher costs were also observed, driving down the probability that eRAPID is cost-effective over this time horizon and for this subsample. The exploratory results should be treated with caution given the small sample size and differences in patient population compared with the main trial (particularly the increased proportion of patients with metastatic cancer). Consequently, further research is required to provide robust evidence on the cost-effectiveness of the eRAPID system over a longer time horizon.
Work programme in radiotherapy treatment: four reports
1. eRAPID radiotherapy Delphi paper: a selection of patient-reported adverse event items for radical prostate radiotherapy patients – a Delphi consensus study with staff and patients
Introduction
Traditionally, radiotherapy AEs are reported by clinicians; however, the timing and accuracy of reporting and clinician–patient communication can be improved by asking patients to report their own symptoms via PROMs. Well-timed and appropriate documentation of AEs throughout the patient journey is essential to both understand the toxicity profile of the treatments and comprehensively support patients. 105 eRAPID is a programme to develop, evaluate and implement an online system for reporting and managing AEs during cancer treatment, developed for use with radiotherapy patients, including those receiving radical treatment to the prostate. Patients report AEs from home during and post treatment. Thus, the selection of appropriate self-report items to report treatment-related AEs was crucial to the success of the programme in capturing patient experiences.
In the preliminary work, we identified the AE and existing validated PRO questionnaires in radical prostate radiotherapy through a systematic review of RCTs, staff and patient interviews, and local clinical use. We found that PROMs were not used to report acute AEs (AEs occurring within 3 months of completion of radiotherapy) within trials and, for late AEs, the augmentation of existing questionnaires with additional items was required to provide comprehensive coverage. 110 The aim of this developmental phase was to reach consensus on appropriate items to include for online reporting of AEs using Delphi consensus methodology with an expert panel of HCPs and patients.
Method
The validated PROM questionnaires mapped against the identified AEs were MPQ,115 EPIC SF (the Expanded Prostate Cancer Index Composite Short Form),113,155 National Cancer Institute (NCI) PRO-CTCAE,33 EORTC QLQ-C30,82 EORTC QLQ-PR25,111 FACT-G,80 FACT-P (Functional Assessment of Cancer Therapy – Prostate),156,157 SF-36,112,158 eRAPID PRAE76 and Toxicity and Response Criteria of The Eastern Cooperative Oncology Group. 114 The MPQ was selected because of the comprehensive coverage of urinary and bowel symptoms. However, the MPQ did not provide full coverage and certain AEs needed more in-depth coverage (e.g. hormonal, sexual, social and emotional functioning). To enable the selection of appropriate PRO AE items to augment the MPQ, 45 participants took part in the Delphi process. This included 20 patients and 25 HCPs from St James’s Institute of Oncology in Leeds and The Christie Hospital Manchester.
Patient-reported outcome items relating to 11 symptom areas not covered by the MPQ were ranked by participants via a secure online questionnaire and survey tool (Bristol Online Surveys, now known as Online Surveys159) over a series of three rounds. Participants were sent a copy of the MPQ and advised that patients would complete the eRAPID questionnaire weekly/fortnightly. Participants were asked to rank questions corresponding to symptoms and side effects not covered in the MPQ and were asked to consider (1) how well the question reflected the symptom/side effect in question, (2) how well the questions would fit in with the MPQ and (3) the potential questionnaire burden for patients. Figure 9 below shows an example question from the survey on weight gain.
FIGURE 9.
Example question from the Delphi survey on weight gain; participants indicate preference via radio buttons and can comment in the expanding free-text box.

Typically, the number of PROM options varied from as small as two (Figure 9) to as many as six (when there were more PROM options to select from). Participants were advised to select whether some additional items should be included in the eRAPID questionnaire. Figure 10 below is a schematic representation of the process and shows which areas were put forward in each round. Where the pre-determined 55% consensus threshold was not achieved, the two highest options and a summary of free-text comments were presented back to participants to inform further selections.
FIGURE 10.
Schematic showing question areas put forward to participants on the Delphi panel in each round.

Results
Consensus was reached for 4 out of 11 areas in round 1 of the survey (range 62–77%), including weight gain, relationship/social impact, inclusion of questions on quality of orgasm (68%) and frequency of erections (56%). In round 2, consensus was achieved on the remaining seven areas, including depression, performance status and fatigue (range 55–89%). Generally, EORTC and eRAPID PRAE items were preferred owing to their conciseness and significance to patients. Interestingly, when HCPs were given feedback from patients, their subsequent selection of items converged more with the patient view (and vice versa). In round 3, weekly reporting of sexual issues was deemed excessive by all participants: 43% of patients suggested monthly reporting, and 57% of patients and 77% of HCPs recommended less frequent reporting. Table 16 below provides a summary of the results.
| Round 1 > 55% consensus (N = 45) (20 patients, 25 HCPs) | Round 2 > 55% consensus (N = 39) (18 patients, 21 HCPs) | Round 3 > 55% consensus (N = 37) (17 patients, 20 HCPs) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Item selected | Overall (%) | Patient (%) | HCP (%) | Area | Item selected | Overall (%) | Patient (%) | HCP (%) | Area | Item selected | Overall (%) | Patient (%) | HCP (%) |
| Weight gain | EORTC-PR-25 | 62.2 | 75.0 | 52.0 | Breast tenderness | EPIC | 55.3 | 52.9 | 57.1 | |||||
| Relationship/family | EORTC QLQ C-30 | 77.8 | 80.0 | 76.0 | Performance status | EORTC QLQ-C30 | 57.9 | 41.2 | 75.0 | |||||
| Social impact | EORTC QLQ C-30 | 70.5 | 75.0 | 66.7 | Fatigue | EPIC | 59.0 | 58.8 | 61.9 | |||||
|
Sexual function Should we include questions on: Orgasm? Y/N |
EPIC | 68.2 | 75.0 | 54.2 | Hot flushes | EORTC QLQ-PR25 | 71.1 | 76.5 | 70.0 | Frequency of sexual questions | Weekly | 0 | 0 | 0 |
| Frequency of erections? Y/N |
Did not reach consensus on PRO optiona Put forward to second round |
56.8 | 65.0 | 50.0 | Depression | PRAE | 78.9 | 62.5 | 90.5 | Monthly | 32.4 | 47.1 | 20.0 | |
| Sexual impact: Infertility? Y/N | 47.7 | 65.0b | 33.3 | Anxiety | PRAE | 82.1 | 70.6 | 95.2 | Other (3, 6, 12 monthly) | 67.6 | 52.9 | 80.0 | ||
| Sexual function | ||||||||||||||
| Erectiona | EPIC | 89.7 | 82.4 | 95.2 | ||||||||||
| Sexual impact | ||||||||||||||
| Masculinity | EORTC QLQ-PR25 | 82.1 | 82.4 | 81.0 | ||||||||||
Conclusion
This work has contributed to the development of patient reporting of AEs during radiotherapy treatment. It highlighted the lack of patient-reported AEs in the acute setting in prostate radiotherapy RCTs (systematic review). The MPQ was selected and supplemented with additional items, resulting in a comprehensive questionnaire covering hormonal, emotional and sexual AEs experienced by prostate cancer patients. It was determined that a baseline assessment followed by a monthly or quarterly assessment would be welcomed, and online reporting of sexual problems seemed to be acceptable to patients. Endorsed by patients and staff, the included questions are a balance of items clinically relevant to HCPs and those reflecting patient voices. The items have been utilised in the pilot study of the eRAPID intervention during pelvic radiotherapy. We recommend including patient-reported outcomes to evaluate radical radiation therapy along the treatment trajectory and advocate working towards a consistent approach to PROM assessment of radiation therapy-related AEs.
2. eRAPID radiotherapy developmental paper
Development of the eRAPID system for pelvic radiotherapy
Radiotherapy for pelvic malignancies typically involves daily attendance for treatment, often with surgery, chemotherapy and biological therapies. 160–163 These produce acute and late effects, peaking after treatment finishes (typically bowel and urinary toxicity). 106,110 To capture these late effects, radiotherapy patients need a longer and often multidisciplinary follow-up, and, for this reason, the pathways may be unclear. 164,165
Stage one: the development of the eRAPID symptom questionnaires, clinical alerts and self-management advice, mapping of patient care pathways and the integration of the system into the EPRs at both cancer centres. Clinical algorithms were developed and tested to establish symptom severity thresholds and symptom-specific management advice, including locally tailored self-management information, was developed on two specifically designed websites.
Stage two: a usability study at both the Leeds and the Christie centres was conducted and refinements were made to components of the intervention based on feedback from patients and staff. The intervention would then be ready for evaluation in the randomised pilot study.
Stage one of development of adverse event questionnaires and items for prostate, gastrointestinal and gynaecological cancers
A systematic review and mapping exercise with oncologists found that the MPQ116 was the most comprehensive PROM used in RCTs for acute and late AEs during and after radical radiotherapy for prostate cancer. A Delphi exercise with patients and staff was undertaken to reach consensus on which items from other questionnaires would augment the MPQ. Eleven items were added to cover hormonal, emotional, sexual, social and general domains. The outcome of the development work was an augmented eRAPID pelvic radiotherapy AE questionnaire fully addressing AEs associated with radiotherapy for prostate, anal, rectal, cervical, vaginal, endometrial and vulval cancer (Figure 11).
FIGURE 11.
Core questionnaire and additional items in the prostate and chemoradiation groups used to develop the eRAPID pelvic radiotherapy AE questionnaire for prostate, gastrointestinal and gynaecological cancer.

Mapping patient care pathways
We conducted 26 semistructured interviews with staff and patients from both trusts exploring views of the clinical work flow, acceptability, timing and potential utility of eRAPID. 117,118 Patient care pathway maps were developed for patients receiving radiotherapy for prostate, cervical, anal, rectal, endometrial and vulval cancer. Perceived benefits included improved communication, increased reporting of sensitive subjects, reassurance and support to self-manage symptoms.
Development of clinical algorithms
The algorithms determine the generation of suitable and timely symptom management advice according to patient-reported severity and generation of alerts to clinicians. They were developed using an iterative approach. Consultations with clinicians enabled the development of clinical priority lists for the symptoms. For each question, the patient’s response was allocated a level of 1, 2, or 3, with 3 being the most severe. Algorithms were based on the structure developed for systemic therapy.
Less-severe symptoms were mapped to scores of between 0 and 2. Three levels of advice were given (Table 17). Each item was mapped against CTCAE scores (0–3) and levels of advice (L1–3) for each disease site for each trust for use in the pilot.
| Algorithm | Severity | Advice given to patient |
|---|---|---|
| A1 | Level 3 (severe) current problems | Advice to contact hospital immediately and notification sent to hospital |
| A2 | Level 3 (severe) resolved problems | Ring hospital or mention at next appointment |
| B | Level 2 (moderate symptoms) or
|
Self-management advice provided for three or more Level 2 (moderate problems). Ring hospital or mention at next appointment |
| C | Level 1 (mild) symptoms | Self-management advice provided for each symptom |
| D | No symptoms | Patient thanked and asked to complete again next week |
New design features for radiotherapy
Radiotherapy treatment pathways are complex, which leads to a larger number of symptoms and symptom clusters elevating moderate symptoms to a more serious level, as well as late effects. New design features were developed, and greater use was made of dependencies to keep the number of items short and relevant to the patient at each time point.
Another new feature was making some levels dependent on ‘time zero’ (first treatment time point) or responses to the previous item. For example, ‘intense or excruciating dysuria’ was upgraded to level 3 for prostate patients 1 week after finishing radiotherapy.
Some items were dependent on the recording of a patient’s ‘time zero’ on the EPR (e.g. linking chemotherapy, sexual and patient understanding questions to appropriate time points in their treatment). If ‘time zero’ was inadvertently not recorded, then QTool failed to show any questions. The system was changed to present a default questionnaire (covering all symptoms), not assuming patients’ stage in treatment and defaulting to the safest option.
New features to communicate scoring were developed, including combining numerical and textual data to enable a score of 0, 1, 2, 3 or ‘declined to answer’ or ‘not applicable’ for items that provided both options.
Development of patient self-management advice
Relevant advice was obtained through a literature search of reputable national sources and local NHS patient information. The information was consolidated and streamlined using WordPress. Advice was clarified and verified through an iterative consultation process with clinical staff and the PCOR RAG. Changes were made in line with comments and recommendations received. Opinions on the websites content, structure and navigability were sought across both sites from 244 health-care staff and patients.
Self-management advice was created for > 30 low-level AEs (e.g. urinary frequency and diarrhoea). More in-depth self-management advice was hosted on two purpose-built, accessible websites.
Stage two of development usability study of the erapid system in radiotherapy
The aim of usability testing was to evaluate how the eRAPID system worked in real-world clinical settings with patients and staff at Leeds Cancer Centre and the Christie Manchester. Patient and staff feedback adapted and improved system performance and integration into standard practice prior to the pilot.
Usability testing was carried out with patients receiving radical and post-surgical radiotherapy for prostate cancer (n = 10) and patients receiving pelvic chemoradiotherapy for cervical or endometrial cancer (n = 10). Patients were given a demonstration of eRAPID, a unique username and password, a user guide and a feedback booklet to complete during the evaluation. Patients were asked to complete the symptom monitoring questionnaire (electronic PRO radiotherapy AE) each week during radiotherapy treatment: 5 weeks for cervical and endometrial patients and 4 weeks for prostate patients.
Health professional staff involved as lead clinicians for the patient were provided with an eRAPID training session and a practitioner information sheet, which included instructions on how to retrieve patient data from the EPR. If a patient reported a severe toxicity, a nominated oncologist received an e-mail alert.
Usability of eRAPID and general feedback were collected in a range of methods:
-
Patient evaluation – feedback form, semistructured interview and end-of-study system usability questionnaire.
-
Patient health-care episodes – health economic questionnaire and collection of demographic and clinical data.
-
Staff evaluation and system performance issues – interviews and ongoing feedback from oncologists and radiographers. Issues discussed and acted on at the regular radiotherapy workgroup meeting.
Results
Testing took place between March and August 2016. Twelve staff took part, including oncologists, a clinical nurse specialist and a radiographer. Ten prostate patients from Leeds and 10 gynaecological patients from the Christie participated, with a combined total of 92 completions.
Following feedback (Figure 12) and prior to the pilot, tweaks were made to the wording and timings of the, and significant changes to the, grading of some alerts.
FIGURE 12.
Type and amount of feedback collected from staff and patients during the study.

Feedback was positive and indicated that the system had value for patients. There was a high level of completion of symptom reports and the reports were not seen as burdensome. Reminders (text or e-mail) were developed for patients. Feedback from staff taking part in the usability study indicated that reminders, training and support were essential to the regular use of eRAPID, even for those staff who had had substantial involvement in the development.
Technical IT issues were encountered. Although the feasibility of developing further use of eRAPID in radiotherapy across two sites was established, there were significant issues throughout the development phase relating to the integration of IT systems, with both trust EPRs and radiotherapy-specific systems, such as MOSAIQ (radiotherapy delivery software). Issues with integration into the CWP at the Christie was a key cause of delay to the start of the recruitment for the pilot RCT.
There were limitations to the field testing. It was possible to evaluate the system using only questionnaires developed for gynaecological and prostate cancer. However, some of the lessons learned were general and applicable across the questionnaire design, irrespective of cancer site.
Conclusions
The outcome of the development work was an augmented eRAPID pelvic radiotherapy adverse event questionnaire that fully addressed AEs associated with radiotherapy for prostate, anal, rectal, cervical, vaginal, endometrial and vulval cancer. Extensive mapping of radiotherapy care pathways revealed a complex situation and required the development of new design features in the electronic questionnaire to take into the account the larger number of dependencies within pelvic radiotherapy, which encompassed the need to understand the side effects of multiple cancer types and combined systemic radiation treatment pathways.
3. eRAPID radiotherapy feasibility pilot study
Introduction
Radiotherapy in cancer care improves patient survival, but the potent multimodal regimes166 lead to significant treatment-related pelvic side effects. 167 PROMs can improve the timing and accuracy of symptom reporting,28 the timely preventative management and survival, particularly when captured electronically. 70 Self-management interventions are effective in reducing cancer symptom severity and improving QoL. 168
Patient-reported outcome measures in radiotherapy110,165 are becoming more integrated into clinical oncology practice. 169 Electronic reporting for post radiotherapy symptoms is being trialled for bowel and lung toxicity. 170,171 We are piloting the eRAPID system in pelvic radiotherapy in two cancer groups with differing treatment and side effect profiles. We aimed to determine the feasibility and acceptability of the eRAPID intervention for patients and staff. Primary end points included recruitment/attrition rates and adherence to symptom reporting. Secondary aims were to establish the number of hospital calls/admissions and aid the selection of outcome measures for a definitive trial.
Methods
Study design and participants
A prospective, randomised, two-arm parallel-group design with repeated mixed-methods measures was employed. The basket trial concept enabled testing eRAPID in two distinct treatment groups: (1) receiving radical radiotherapy for early prostate cancer and (2) receiving pelvic chemoradiotherapy for lower gastrointestinal cancers (i.e. anal, rectal) and gynaecological cancers (i.e. cervical, vaginal, vulval endometrial).
Procedures
Procedures and randomisation are described in the published protocol. 75 Figure 13 is a summary of patient and process outcome measures collected. 74
FIGURE 13.
Study diagram. BL, baseline; PAM, patient activation measure; SUS, system usability scale.
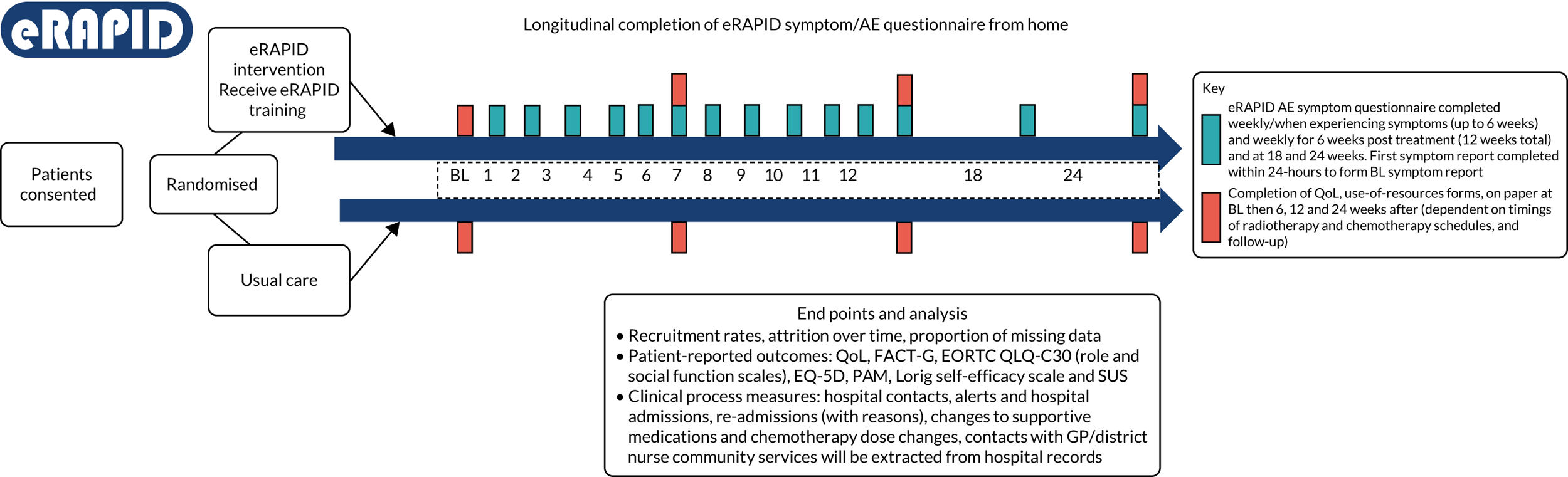
Usual care
Patients received health-care professional assessment before treatment and were given verbal and written information on AE management and contacting the hospital 24/7. Patients had regular radiographer reviews and a 6–8-week follow-up with oncologists post discharge.
eRAPID intervention
In addition to usual care, participants reported AEs weekly online (or when experiencing symptoms) for 12 weeks, and then at 18 and 24 weeks. Results were displayed in the EPRs. Immediate automated advice was generated to self-manage mild AEs or a prompt was given to contact the hospital for serious AEs, supported with more detailed advice via the eRAPID websites. Severe alerts were sent to the clinical team, monitored by senior nurses. Patients and staff were trained in using the system.
Statistical analysis
Sample size. Based on 30 patients per group for each treatment type,119 we aimed to recruit 42 patients per group, 84 in each treatment group: a total of 168. All analyses were performed by arm and treatment type using SAS® software version 9 (SAS Institute Inc., Cary, NC, USA) and SPSS version 26 [Statistical Product and Service Solutions (SPSS Inc., Chicago, IL, USA)].
Results
Recruitment, attrition, and completion rates
Over 15 months, a total of 253 patients were identified. Of the 228 fully eligible patients, 61 declined and 167 patients were consented (recruitment rate of 73.3%). A total of 103 males and 64 females took part [mean age prostate radiotherapy 70.0 (SD 7.0) years; chemoradiotherapy 52.1 (SD 15.1) years]. The chemoradiotherapy group had a lower education level and lower co-morbidity rates than the prostate group.
Sixteen patients withdrew: 10 (12.0%) from the eRAPID arm and six (7.1%) from the usual-care arm (one patient in the chemoradiotherapy usual-care arm died). Most eRAPID withdrawals (n = 8) were from the chemoradiotherapy arm (n = 7, gynaecological cancers) by 6 weeks. Three withdrawals from each treatment group were noted in the usual-care arm. Reasons for withdrawals included ‘too ill’ (n = 3), ‘too much to think about/too busy’ (n = 2), ‘being well, no symptoms’ (n = 1), ‘wanting to move on’ (n = 2) and ‘not confident using internet/prefer paper’ (n = 2).
A total of 88% of eRAPID patients and 92% of usual-care patients completed the study; completion was lower in the chemoradiotherapy arm (eRAPID, 80%; usual care, 90%) than the prostate radiotherapy arm (eRAPID, 95%; usual care, 93%). Completion of outcome measures at 24 weeks was excellent in the prostate radiotherapy arm (eRAPID, 95%; usual care, 95%) but less in the chemoradiotherapy arm (eRAPID, 63%; usual care, UC). Chemoradiotherapy patients had lower outcome completion rates across all time points (between 75% and 60%) than prostate radiotherapy patients (between 91% and 97%).
Adherence to eRAPID online symptom reports and algorithm generation
Prostate radiotherapy patients had high adherence to weekly symptom reporting but adherence was lower for chemoradiotherapy patients (Figure 14). This low adherence was because of the patients with cancers of the cervix/endometrium (11/23 patients completed 0–3 online reports). Non-adherent patients were younger (mean age 35.8 years; 9/11 were aged < 40 years) than adherent patients in the chemoradiotherapy arm (mean age 45.2 years; 4/12 were aged < 40 years).
FIGURE 14.
Adherence to eRAPID online symptom reports: proportion of QTool completions by protocol and treatment group.

The algorithms activated alerts for severe symptoms (4% for chemoradiotherapy patients and 0.5% for prostate patients) (Figure 15). Moderately severe symptoms were similar between treatments (40.5% and 38.3%, respectively) and just over 50% of reports generated self-management advice.
FIGURE 15.
Activated eRAPID algorithms during study: percentage of QTool algorithm generation by treatment group.

Clinical process measures
The mean number of calls to hospital staff in the 24 weeks was small [1.3, chemoradiotherapy arm (SD 1.9) vs. 1.6, usual care arm, (SD 2.2); 0.4, prostate radiotherapy arm (SD 1.1) vs. 0.1 usual care arm (SD 0.3)]. The mean number of unscheduled hospital visits was close to 0 in both treatment groups.
Missing items, floor and ceiling effects
The number of missing items affecting score calculations was ≤ 4.1%. No floor effects were seen. Ceiling effects were seen for EQ-5D utility score (24.1%) 28.5% of this total were in the prostate group, 6-item SES (16.9%) and the FACT-PWB (17.4%).
Data trends
Less deterioration over time was reported by participants in the eRAPID chemoradiotherapy arm than in the usual-care arm. Greater differences were seen at 6 weeks for FACT-G scores, EORTC QLQ-C30 summary scores, QLQ-C30 Global health/QoL score and EQ5D-VAS scores, remaining after adjustment for baseline scores. Figures 16 and 17 show graphically these trends over time. No trends were observed from the prostate radiotherapy arm.
FIGURE 16.
Mean EORTC QLQ-C30 Global QoL scores. Higher QLQ-C30 scores = better QoL.
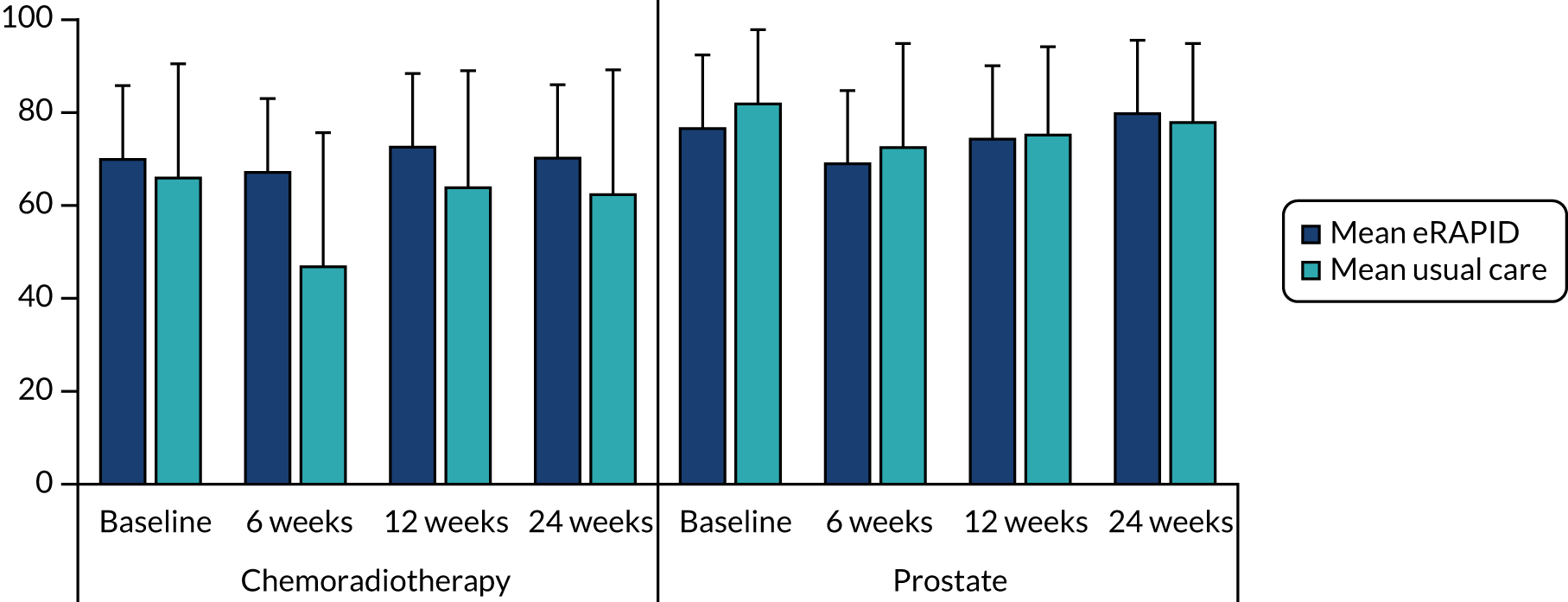
FIGURE 17.
Mean FACT-G scores. Range 0–108; high score = good function.
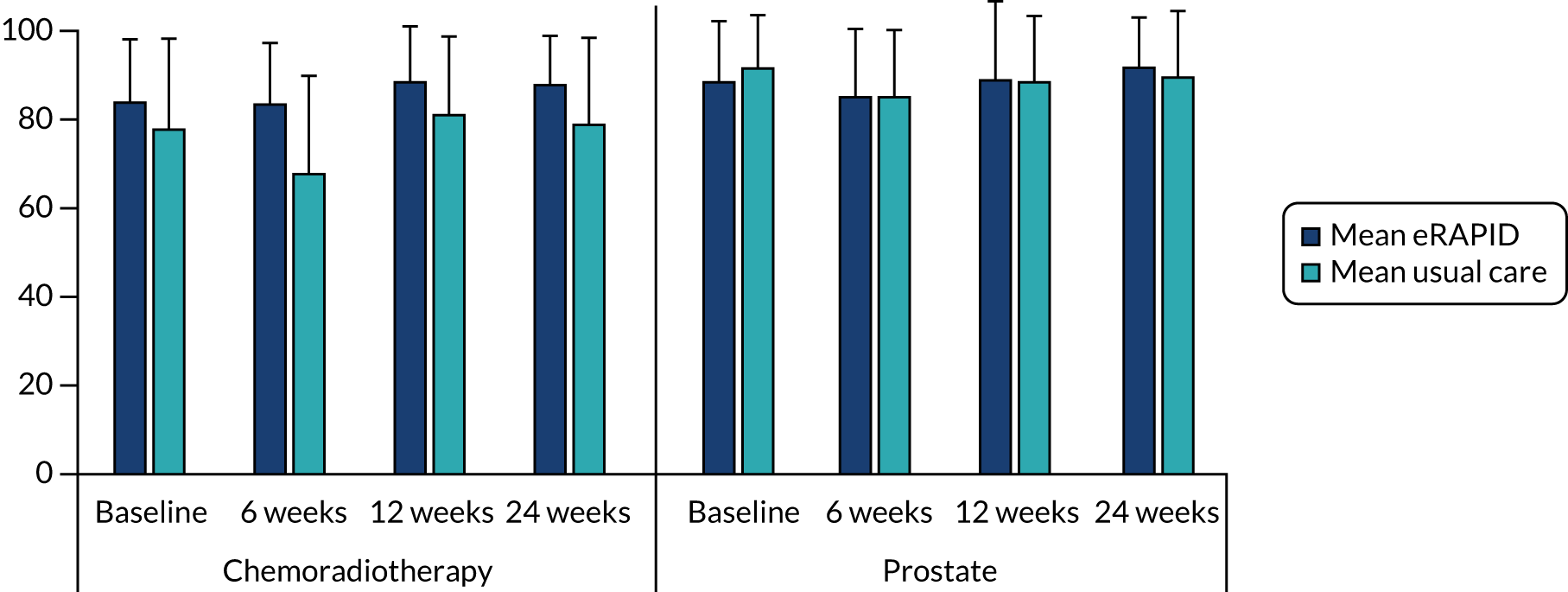
Conclusion
This pilot established the feasibility and acceptability of online symptom reporting with severity-dependent immediate advice for patients undergoing two main modalities of pelvic radiotherapy (chemoradiotherapy for lower GI cancers) and radiotherapy for early prostate cancer in two major UK cancer centres. To the best of our knowledge, this is one of the first studies in pelvic radiotherapy to add immediate severity-dependent advice to address self-reported symptoms and enable clinicians to view online reports from within EPRs.
A recruitment rate of > 70% and a withdrawal rate of < 10% was achieved. Prostate radiotherapy patients were the most consistent and prolific symptom reporters, followed by patients with anorectal cancers consistent with previous chemotherapy studies. 36,72 Younger gynaecological patients did not adhere to the online symptom reporting; they received more intensive concurrent chemoradiotherapy and had a lower level of education. Those gynaecological patients who did report had more severe and prolonged problems (e.g. bowel and urinary urgency, pain, skin reactions) than those who did not, suggesting that when they were ill they were less likely to report. These findings suggest that eRAPID may not be useful for women aged < 40 years with lower education levels receiving intensive chemotherapy for advanced cervical cancer.
eRAPID did not generate extra hospital visits or calls. Alerts for severe symptoms were low. The potential outcome measures performed well. Trends towards less symptom deterioration over time were demonstrated for eRAPID chemoradiotherapy patients (particularly at 6weeks); however, baseline score imbalances and missing data from gynaecological patients must be considered. Limited benefits were seen for prostate radiotherapy patients; however, the willingness to report and self-manage symptoms could be translated into longer-term supportive care interventions. 172
The encouraging feasibility and patient adherence findings for prostate and anorectal cancers justify further studies exploring reporting and management of persistent late effects. This study adds to evidence on clinical benefits from regular online symptom monitoring in cancer and informs future randomised trials/health services development projects.
4. eRAPID radiotherapy qualitative findings
Introduction
The eRAPID radiotherapy feasibility pilot included an embedded qualitative element. Through this work we captured the views of patients and staff around the use and value of the eRAPID system to help to understand how the intervention was received, its impact on patient care and the scope for future refinement.
Methods and analysis
Qualitative feedback was gathered from several sources:
-
End-of-study semistructured interviews with patients explored views about the practicalities of using the system, the relevance of the symptom reports and self-management advice, and the impact on their treatment or consultations.
-
Free-text comments in end-of-study patient surveys in which a series of questions explored experiences of using eRAPID and suggestions for refinement.
-
End-of-study semistructured staff interviews explored challenges and benefits of the practicalities of using eRAPID, as well as the impact on clinical work and relationship with patients.
-
Staff end-of-study feedback captured further information on the challenges and benefits of the system.
Interviews were digitally recorded and transcribed verbatim, and data were subsequently managed using NVivo 11 software. Written feedback and comments from the end-of-study feedback questionnaires were analysed for content. A core group of the eRAPID research team conducted the qualitative analysis collaboratively173 using the principles of thematic analysis. 86
Results
Eleven patients and four staff from Leeds were interviewed, three staff from the Christie provided written feedback and 61 patients from across both sites returned the end-of-study survey (Table 18).
| Feedback | Patients (n) | Staff (n) |
|---|---|---|
| Interviews | ||
| Urology | 4 | 0 |
| Anorectal | 6 | 2 |
| Gynaecological | 1 | 2 |
| Subtotal | 11 | 4 |
| Written feedback | ||
| Urology | 40 | 1 |
| Anorectal | 13 | 0 |
| Gynaecological | 8 | 2 |
| Subtotal | 61 | 3 |
| Total | 72 | 7 |
System relevance and ease of use
Patients’ perspectives
The electronic system was seen as easy to use even by participants who did not regard themselves as confident IT users. The training provided by the research team was valued, especially around how to find the login page, and some patients also required some support from relatives. Overall, the symptom items and questions were considered to be written in easy-to-follow language. Patients highlighted some difficulties in distinguishing between pre-existing conditions and side effects of treatment, or wanting more space to describe symptoms in their own words. Several patients suggested introducing more comment boxes so that patients could add in concerns that they wanted to raise with their health-care professional or to describe the complexity of their symptom that may not marry with the predetermined questions.
The items were considered relevant, corresponding with what could be experienced during the course of radiotherapy treatment. However, patients with few side effects or whose side effects had diminished at the end of treatment found that the questions were less relevant and found the completion repetitive.
Staff perspectives
Staff felt that eRAPID reports were easy to access and view in the EPRs. It was evident from staff interviews that there was limited use of eRAPID symptoms reports by staff. This was evident for the radiographers, who saw the patients every day during treatment but did not have easy access to the eRAPID patient reports. At the Leeds Cancer Centre, this was because integration with the radiotherapy-specific system MOSAIQ had not been achieved in time for the pilot study. Despite this, there was positive feedback from those staff who had been able to engage with the system. The symptom items and questions were seen as relevant and mirroring those usually asked in consultations.
Benefits of using eRAPID
Patient perspectives
Many patients felt that a primary benefit of using eRAPID was that it was a source of reassurance. Personal symptom monitoring had an educational role, particularly in developing a better knowledge of the kinds of side effects that could be experienced and by providing advice on how to deal with them. Some participants appreciated the tailored aspect of the self-management information (i.e. they were given advice relevant to their answers to their questions rather than having to read a whole booklet or website):
After the first time I used it, I thought that was really good, because obviously your symptoms change as you’re going through, so the answers that the system is giving you was sometimes different, so I found that really useful.
P0140, anorectal
Having ready access to relevant tailored information could provide a kind of security blanket. There were examples when eRAPID had directly supported early detection, clinical management of AEs and also changes in supportive medications:
Absolutely, yeah, it was, you know, spot on, the right number rang, got straight through to the right person, and then within like an hour I was in hospital.
P0125, anorectal
It could be used to bolster confidence and encourage calling the hospital, as noted by a patient who did not have anyone on hand to help with the decision about whether or not to call. Finally, it was seen as a means of providing staff with a better understanding of a patient perspective of any side effects experienced, and in this way it may benefit symptom detection.
Staff perspectives
Health-care professionals reported that eRAPID provided a useful basis for their preparation for clinic in terms of either targeting problematic symptoms or preparing supportive medications. Some staff also felt that the symptom report encouraged patients to disclose symptoms that they may not realise were clinically significant, things they may forget or were embarrassed to bring up, or issues that needed to be volunteered by the patient as they were not standard areas to explore. Staff also saw potential benefit of using eRAPID in standard care for toxicity/late effects recording and potentially enabling stratified follow-up.
Difficulties with eRAPID
Patient perspectives
Participants reported that symptom reporting required a degree of effort, especially when they were feeling unwell or tired from that day’s treatment. Furthermore, patients had to remember to do it and this could be difficult, especially when people were feeling well and/or had other priorities to deal with.
Another difficulty identified by patients was that they could not necessarily identify whether or not staff were using their symptom reports; although some staff referred to the reports or talked them through with patients, this was not usual practice.
Staff perspectives
An issue identified by staff was that the pilot eRAPID time frame was not sufficient to identify any late effects of radiotherapy, which could occur months or years after the end of treatment.
During the study, staff reported finding it difficult to remember which patients were using the eRAPID system and felt that prompts from the research team were required to remember to look at the reports. Some staff felt that if it was a part of routine care this problem may be overcome. Staff also noted that the adoption of eRAPID would need consideration around how to meet the needs of patients without computer access at home or who did not want to use, or did not feel confident in using, online systems.
Conclusions
The qualitative data collected in the eRAPID radiotherapy pilot RCT adds nuance to our understanding of the feasibility and acceptability of online approaches to patient monitoring. Both staff and patients found the system easy to use. From the patients’ perspective, the reassurance the system provided was one of the key positive elements. However, there were issues with motivation in maintaining symptom report completions over the longer term, especially when feeling well. Staff, on the other hand, felt that it would be useful to consider extending the follow-up period to capture late effects of treatment. Further attention will need to be given to the patient views of the benefit of continuing monitoring over the longer term when they may want to move on from frequent surveillance.
List of abbreviations
- 24/7
- 24 hours per day, 7 days per week
- AE
- adverse event
- app
- application
- CBI-B
- Cancer Behaviour Inventory–Brief
- CI
- confidence interval
- CTCAE
- Common Terminology Criteria for Adverse Events v4.0
- CWP
- clinical web portal
- DHSC
- Department of Health and Social Care
- DMEC
- Data Monitoring and Ethics Committee
- EORTC
- European Organization for the Research and Treatment of Cancer
- EORTC QLQ-C30
- European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire
- EORTC QLQ-PR25
- European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Prostate Cancer Module
- EPIC
- Expanded Prostate Cancer Index Composite
- EPR
- electronic patient record
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- eRAPID
- electronic patient self-Reporting of Adverse-events: Patient Information and aDvice
- ERAS
- enhanced recovery after surgery
- FACT-G
- Functional Assessment in Cancer Therapy Scale – General
- GP
- general practitioner
- HCP
- health-care professional
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ID
- identifier
- IRR
- incidence rate ratio
- IT
- information technology
- MPQ
- Male Pelvic Questionnaire
- MRC
- Medical Research Council
- NCEPOD
- National Confidential Enquiry into Patient Outcome and Death
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PAM
- Patient Activation Measure
- PCOR
- Patient Centred Outcomes Research
- PPI
- patient and public involvement
- PPM
- Patient Pathway Manager
- PPM+
- Patient Pathway Manager+
- PRAE
- patient-reported adverse event
- PRO
- patient-reported outcome
- PRO-CTCAE™
- Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events
- PROM
- patient-reported outcome measure
- PROMPT
- Patient Reported Outcomes Management Pathways Tracker
- PWB
- Physical Well-Being
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RAG
- Research Advisory Group
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SES
- Self-Efficacy for Managing Chronic Disease 6-Item Scale
- SF-36
- Short Form questionnaire-36 items
- SMS
- short message service
- TSC
- Trial Steering Committee
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/FDDE8516).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
