Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0609-10106. The contractual start date was in March 2011. The final report began editorial review in March 2015 and was accepted for publication in May 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Disclaimers
this report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Raine et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Overview of the ASCEND programme
In this chapter, we describe the background to, and rationale for, our research programme – ASCEND – the first major research programme to focus specifically on tackling socioeconomic inequalities in uptake in an organised cancer screening programme.
Background
Burden of disease and NHS context
Bowel cancer constitutes a significant public health burden in the UK. It is the fourth most common cancer (approximately 41,600 cases annually) and the second leading cause of cancer death (15,700 deaths annually). 1 Its incidence rises with age2 and early diagnosis is vital to improve outcomes: 93% of patients with early-stage disease (Dukes A) survive for 5 years, compared with 6.6% of those with late-stage (Dukes D) disease. 3
Randomised controlled trials (RCTs) have demonstrated that bowel cancer mortality can be reduced by screening using the guaiac faecal occult blood test (gFOBt). 4 Following successful pilots (the first conducted in two English health authorities and three Scottish health boards; the second conducted in the English sites only),5,6 the NHS Bowel Cancer Screening Programme (BCSP) began in England in 2006. 7 Reduction in mortality as a consequence of population screening is dependent on participation. Thus, the combined results of four international RCTs (including the UK’s Nottingham Trial8) showed that participation rates of > 50% in biennial population screening reduced mortality by 16%,9 saving up to 2500 lives a year. 10
The Nottingham Trial, which began in 1981, reported uptake of 57%, and a 13% reduction in bowel cancer mortality at 11-year follow-up. 11 Subsequently, the first and second NHS BCSP pilots reported initial and ongoing uptake of 58.5% and 51.9%, respectively. 5 Data from the first 2 years of the English NHS BCSP showed uptake of 54%. 12 Further decreases in mortality can be achieved through improvements in the population participation rate. 9,13
The NHS BCSP offers the gFOBt every 2 years to 60- to 74-year-olds. Five hubs covering England co-ordinated a call/recall programme and were also responsible for analysing the gFOBt kit samples. Further details of the screening programme are provided in Chapter 2.
Relevance to priorities and needs of the NHS
A commitment to improve health and reduce inequalities in health and health care forms the cornerstone of the government’s public health and health-care policies. 14,15 Organised screening programmes have been assumed to be superior to opportunistic screening in terms of socioeconomic equity, because they use population lists to ensure that all eligible individuals are invited and call/recall systems to avoid under- or overscreening. Direct financial barriers are also avoided in the UK because the screening programmes are run by the NHS and, therefore, individuals incur no costs either for the primary screening test or for follow-up investigations or treatment. In addition, bowel cancer screening with the home-based gFOBt kit avoids barriers associated with travelling to a medical facility or interacting with health professionals. Nonetheless, striking gradients in uptake across levels of deprivation were reported in the two pilot studies and the first 2 years of the national screening programme. 5,12,16 The initial pilot reported 61% participation in the most socially advantaged areas, falling to 37% in the most deprived areas,5,12 and this gradient persisted in the beginning of the national programme, when participation was 61% in the least deprived areas and 35% in the most deprived areas. 12
Identifying effective strategies to achieve equity in uptake is vital to avoid exacerbating inequalities in mortality. Our research programme focuses on reducing the socioeconomic gradient in uptake without compromising uptake in any socioeconomic circumstance (SEC) group. In contrast with most inequalities research, we address the gradient in uptake rather than the gap between the most and least socioeconomically advantaged, in order to take account of the stepwise relationship between SECs and health, whereby more socioeconomically advantaged individuals have better health and better access to health care. 17 Thus, the costs of inequalities are borne not only by those at the bottom of the SEC hierarchy but at every level. Policies that target the most disadvantaged subgroups only, or which aim to narrow the gap between the most and least disadvantaged, underestimate the pervasive effect across the SEC hierarchy and exclude those in need in the intermediate SEC groups. This research programme was therefore designed to focus on reducing the socioeconomic gradient in uptake, which entails designing interventions that increase uptake to a greater extent in lower than higher SEC groups. Our approach reflects the concept of proportionate universalism whereby actions are universal, but with a scale or intensity proportionate to the level of disadvantage. 18
This programme was developed to contribute directly to the following three additional national initiatives:
-
The Department of Health’s target for cancer mortality, which includes a target to reduce the inequalities gap. 19
-
The Cancer Reform Strategy,20 which began the National Cancer Equality Initiative to focus on improving collection of data to improve understanding about current inequalities, promoting research to fill knowledge gaps about inequalities, and spreading good practice.
-
The Marmot Review of Health Inequalities which highlighted the need for ‘bespoke initiatives’ to reduce the socioeconomic gradient in bowel cancer screening uptake and which championed proportionate universalism. 18
Need for research in this area and rationale for our research programme
We have previously examined whether or not the socioeconomic inequalities identified during the Bowel Cancer Screening Pilot persisted once the NHS BCSP was rolled out. We analysed national uptake for the smallest geographical unit that is routinely recorded by the NHS BCSP, namely postcode sector, each of which contains an average of 3000 addresses. We found that between October 2006 and January 2009, overall screening uptake was 53% but it varied from 61% in the least deprived quintile of postcode sectors to 35% in the most deprived quintile. 12
As further evidence of the need for this research, and as part of the ASCEND programme, we undertook a new study to evaluate the extent of and factors associated with socioeconomic-related inequality in bowel cancer screening uptake in England using individual-level data from the English Longitudinal Study of Ageing (ELSA). We used data from the fifth wave of data collection of ELSA for 1833 participants who were eligible for at least one NHS BCSP invitation. Our outcome measure was completion of a gFOBt home testing kit. We ranked the sample using a composite measure of socioeconomic status, predicted from net non-pension wealth and a number of individual-level socioeconomic and sociodemographic characteristics and plotted the cumulative uptake of bowel cancer screening against it using a concentration curve. We then derived the concentration index, which provided a measure of socioeconomic-related inequality in screening uptake. We then fitted univariate probit models for the association between a number of sociodemographic (age, gender, ethnicity, marital status, education), socioeconomic (quintiles of net non-pension wealth, housing, vehicle ownership, economic activity, social class) and health-related (self-reported general health, long-standing illness, difficulties with daily activities and using the toilet, health literacy, partner screening status) variables and the probability of screening. Variables showing significant associations were included in a multivariate model and in a decomposition analysis of the Concentration Index. 21,22 This provided a measure of socioeconomic inequality by accounting for the probability of screening and the distribution of each variable across different levels of SEC. We found a significant pro-rich gradient in screening uptake [concentration index +0.06, 95% confidence interval (CI) +0.04 to +0.09] with 41.7% of individuals in the poorest and 65.5% in the richest quintiles of predicted non-pension wealth participating in screening. Socioeconomic-related inequalities in screening were mostly explained by differences in education (19.6%), partner screening status (17.4%), disability (14.3%) and health literacy (8.7%). The findings suggest that interventions for reducing the gradient in bowel cancer screening participation should aim to increase acceptability and comprehension of the screening test among those with lower levels of literacy and emphasise the social implications of screening and its benefit not just to the individual but also to their family and friends. 23
In other work,24 we have also demonstrated that the low uptake and striking socioeconomic gradient is not seen among people with a positive gFOBt kit result who are invited for further investigation (usually colonoscopy). Overall, colonoscopy uptake is 84% with little variation between socially advantaged and disadvantaged areas (86–80%). 12 The high uptake of colonoscopy regardless of SEC indicates that addressing the gFOBt uptake gradient should improve subsequent uptake of effective treatment and, therefore, contribute to reducing inequalities in survival.
Prior to the establishment of the NHS BCSP, there was evidence that more disadvantaged patients with bowel cancer tended to present as emergency admissions and at a later disease stage25,26 and their outcomes are poorer. Furthermore the deprivation gap in survival is widening, reaching 7% for colon cancer and 9% for rectal cancer. 27 These findings further demonstrate the need to devise strategies to achieve early diagnosis among all social groups as well as reduce inequalities, if we are to improve survival across the board.
Previous research into improving uptake of cancer screening has focused primarily on factors such as ways of establishing contact. 28 Although this approach can help reach screening targets, it is unlikely to reduce inequalities and may even increase them if more socioeconomically advantaged individuals are more responsive.
A few studies have specifically addressed socioeconomic inequalities in uptake, but often by focusing on underserved groups, for example by providing community support workers. 29 Even if they are successful, these initiatives serve only one group in the population and do not address the gradient. In addition, they are often highly intensive and therefore impractical for wide-scale implementation.
Therefore, we set out to design and evaluate effective interventions which focus specifically on the socioeconomic gradient in uptake. Multiple potential causes for lower uptake of screening in more socioeconomically deprived groups include general factors (e.g. stress caused by lack of financial resources, reduced subjective life expectancy) which limit ability to engage in future-focused health protective actions30,31 and more specific factors, such as greater concern about the negative aspects of the test itself (e.g. embarrassment, contact with faecal material). 32,33 Health literacy limitations are also implicated in comprehension of the screening information materials34 and recognition of the organisation that sends the screening invitations. All these factors may have their upstream roots in the more stressful, constrained lives of people with fewer social and economic resources. 30 In addition, life stress may directly affect screening uptake if ‘passive’ barriers in the form of competing priorities reduce translation of screening intentions into action. 35 The four interventions that we tested addressed some of these barriers to uptake. We were required to design interventions that could easily be added to (rather than replace) existing invitation and information materials (which had been through formal approval procedures).
Aim and objectives
Our aim was to reduce socioeconomic inequalities in bowel cancer screening uptake in England without compromising uptake in any socioeconomic group.
In order to achieve this, we set out the following objectives:
-
to explore psychosocial and cultural determinants of low uptake of the gFOBt
-
to develop and test four theoretically based interventions to reduce the socioeconomic gradient in screening uptake
-
to undertake RCTs to evaluate the effectiveness and cost-effectiveness of each intervention within the NHS BCSP.
ASCEND workstreams
Our programme was divided into three workstreams to address our objectives.
Workstream 1
In order to explore the psychosocial and cultural determinants of low uptake of gFOBt, we conducted 18 focus groups and 16 key informant interviews. These generated rich qualitative data, which are reported in Chapters 3 and 4 and which were used to inform workstream 2.
Workstream 2
We developed and piloted four interventions designed to reduce inequalities in uptake of bowel cancer screening. Two of the interventions consisted of provision of additional information leaflets designed to meet the information preferences of individuals with lower SECs; one provided a simplified version of the screening information focusing on the ‘gist’ of the message and one provided personal narratives describing the screening experience. The third intervention added an endorsement of the programme from a familiar health professional [the individual’s general practitioner (GP)] to circumvent lack of recognition of the NHS BCSP – a strategy that has been shown to improve screening uptake in those with lower SECs. 36,37 The fourth intervention provided an enhanced ‘cue to action’, designed to help bridge the ‘intention–behaviour’ gap by briefly restating the screening offer and adding a ‘reminder’ label to the reminder letter sent to individuals who had not responded within 35 days of their initial invitation. The interventions are described individually in Chapters 5–8.
Workstream 3
Each of the four interventions developed in workstream 2 were tested in separate two-arm, cluster randomised trials targeting all individuals who were routinely invited for bowel cancer screening in the NHS BCSP in England over each study period. The RCTs took place between November 2012 and August 2013. We hypothesised that each intervention [the ‘gist’ leaflet, the ‘narrative’ leaflet, the general practice endorsement (GPE) and the enhanced reminder (ER)] would be low cost and progressively more effective at improving screening rates across increasing levels of deprivation. We also undertook a national survey of research activities and health promotion activities to ascertain usual practice during the trial period. This workstream is described in Chapters 9–11.
Workstream 4
We planned to combine the successful components from workstream 3 into a complex intervention for experimental evaluation; however, the results of the trials of the individual interventions did not justify this.
Conclusions
We draw conclusions from the study and make recommendations for future research and practice in Chapter 12.
Patient and public involvement and engagement
Our first ASCEND patient representative, Mr Fuller, contributed expertise from a patient perspective during the drafting of the original application, particularly regarding workstream 2 and the planning of the development of the interventions. Unfortunately, Mr Fuller was unable to offer further support following the grant being awarded owing to ill health. We recruited another patient representative (Mr Band), who reviewed some of the ASCEND development work (namely the narrative leaflet) and attended our Advisory Group meeting prior to the start of the national trials. In addition, we worked with individuals from a number of charity and community groups and organisations, Primary Care Research Networks and faith settings who helped us to identify specific groups of people to take part in the development stages of the study (see Chapters 3–6 for further details).
The research team also undertook engagement activities, presenting information about the study at conferences and to other groups, as well as publishing peer-reviewed papers of the study findings.
Chapter 2 Overview of Bowel Cancer Screening Programme usual practice
Introduction
The NHS BCSP, established in England in 2006, offers bowel cancer screening every 3 years to adults aged 60–69 years (inclusive) using a gFOBt kit that is completed at home. Between 2008 and 2014, the programme was gradually extended to include subjects aged 70–74 years. Primary endoscopy screening is now being added to the programme following the UK Flexible Sigmoidoscopy Screening Trial,38 with a single flexible sigmoidoscopy (FS) offered at 55 years. FS screening started after the completion of the ASCEND programme.
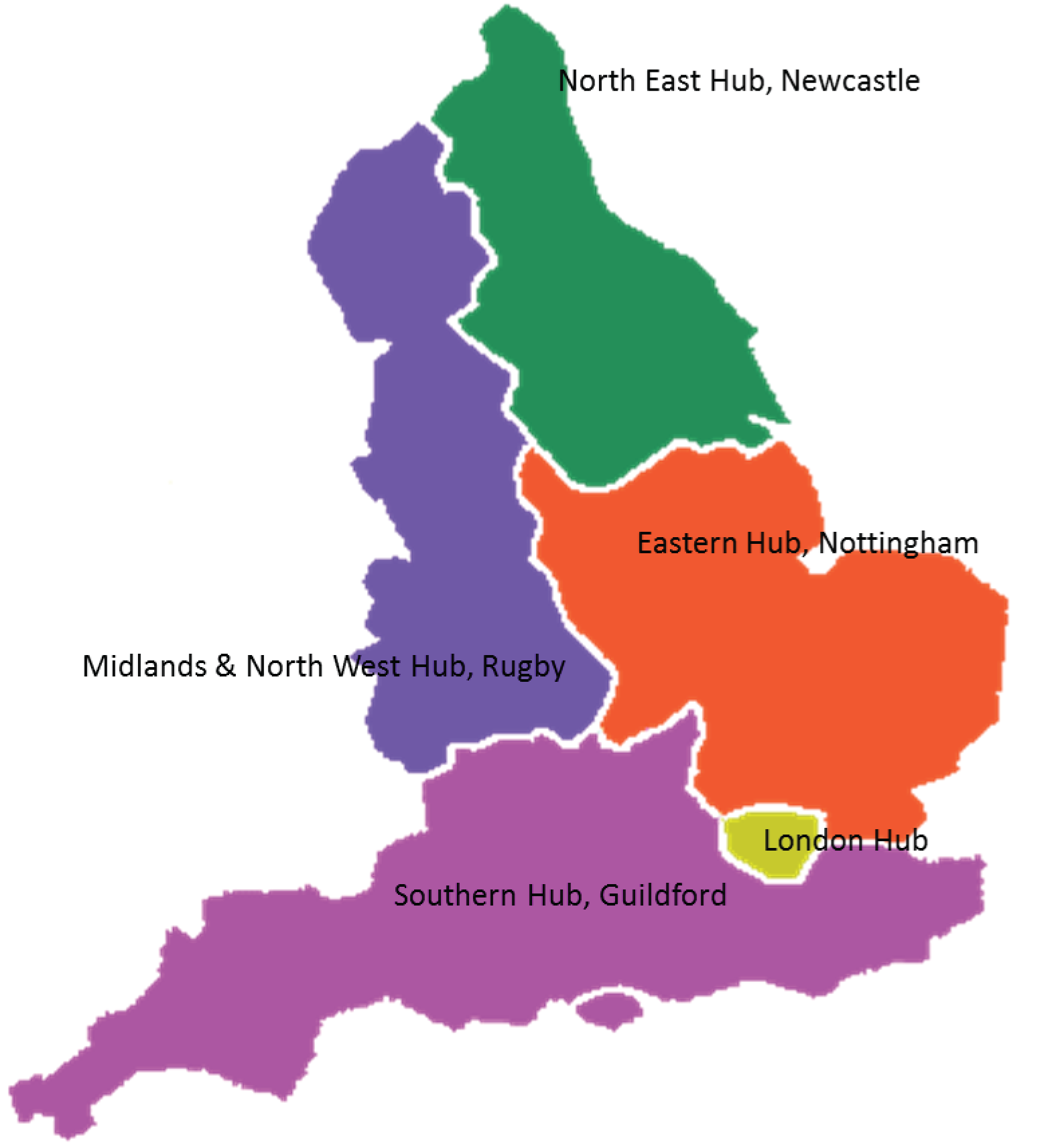
Bowel Cancer Screening Programme hubs
Five NHS BCSP hubs provide the ‘call and recall’ service for the screening programme in England: London, Southern England, Eastern England, Midlands and North West England and North East England (Figure 1). This involves the use of the Bowel Cancer Screening System (BCSS) database to invite the eligible population as their screening becomes due. To support this process, all hubs offer a helpline service to provide advice and a laboratory service to test all returned kits.
FIGURE 1.
Map of England showing the five Bowel Cancer Screening hubs.
Screening pathway
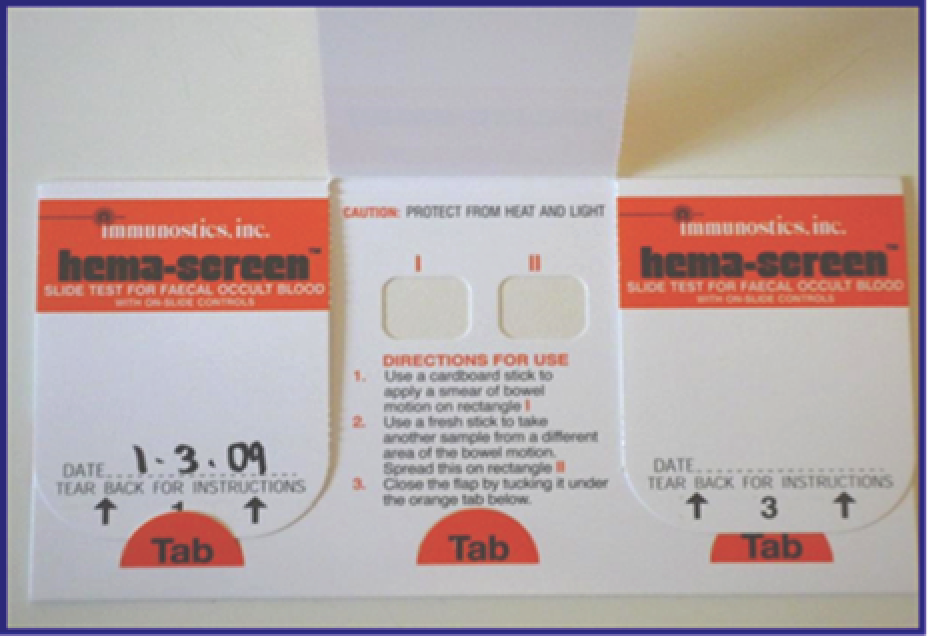
A detailed diagram of the NHS BCSP screening pathway is included in Appendix 1. In brief, each hub sends an initial invitation (‘pre-invitation’) pack to all eligible people in their area describing the programme and its objectives. The pre-invitation pack contains a letter (see the S1 letter, Appendix 2) and ‘The Facts’ booklet (see Appendix 3). A week later, the hub sends out the gFOBt kit (Figure 2) with a formal invitation letter (referred to as the S9 letter; see Appendix 4) and a leaflet explaining how to do the test (the screening instruction test kit leaflet; see Appendix 5). The test kit includes the gFOBt card, cardboard spatulas for sample collection and a reply-paid envelope in which to return the test for analysis at the NHS BCSP hub laboratory. There are three flaps on the test kit, each of which covers two sample application ‘windows’. Two tiny samples are taken from a bowel motion and spread onto each of the two windows using the cardboard spatulas provided. The flap is then sealed and dated and the process is repeated for the second and third bowel motions (using the windows under the second and third flaps, respectively). Once all six windows have been used, the test kit is returned to the laboratory for analysis. The test kit must be received by the hubs within 21 days (subjects are told 14 days, to allow time for return postage) of the first sample being taken to ensure that a valid test result can be obtained. The hubs process the completed gFOBt kits in their laboratories and send out result letters to participants within 2 weeks of receiving a completed test kit. If a test kit has not been returned within 4 weeks of the date it was sent, the hub sends out a reminder letter (referred to as the S10 letter; see Appendix 6).
FIGURE 2.
The gFOBt kit.
The possible test results for kit 1 are described in Table 1. Up to three test kits may be needed to reach a ‘definitive’ test result of ‘normal’ or ‘abnormal’. If none of the six windows is positive for blood in the faecal sample on the first test kit, the test result is normal and the participant will be invited to be screened again in 2 years’ time (if they remain within the eligible age group). If five or six windows test positive on their first kit, the result of the screening test is abnormal and the participant is offered an appointment with a specialist screening practitioner (SSP). If between one and four windows are positive on their first kit, the result of that test is ‘unclear’ and the participant will be asked to repeat the test. If one or more windows test positive on a second test kit the definitive test result is abnormal (‘weak positive’) and the participant is referred to a SSP. If no windows are positive on the second test kit, a third test kit is sent and any positive windows result in referral to SSP. Participants will be asked to repeat the test if a test was completed incorrectly (‘spoilt’) or there was a problem in the laboratory during processing (‘technical failure’).
| gFOBt kit result | Spoilt kit | ||||
|---|---|---|---|---|---|
| Normal | Unclear | Abnormal | Technical failure | ||
| Explanation | 0 positive spots | 1–4 positive spots | 5 or 6 positive spots | Technical problem in the laboratory’s processing of the kit | Unreadable test kit owing to incorrect use, out-of-date sample or no readable date |
| Action | Participants are sent a discharge letter. The letter also contains a list of the symptoms of bowel cancer to promote awareness between screening episodes and after the age of 74 years. The gFOBt is offered again in 2 years if < 75 years | Participants are sent a covering letter and another kit. If the second kit gives an abnormal or unclear result, participants are offered an appointment to see a specialist nurse. If the second kit is normal, participants are sent another kit to confirm a definitive result overall | Participants are sent a covering letter containing a clinic appointment to see a specialist nurse within 2 weeks of the gFOBt kit being processed | Participants are sent a covering letter and one further kit | Participants are sent a covering letter with an explanation of why the kit could not be processed and a replacement kit |
General practitioners are not directly involved in the delivery of the NHS BCSP but the practice receives written (or electronic) notification of the screening results for their patients including a notification of a SSP appointment and colonoscopy outcome. Practices are also informed about subjects who have not taken part in screening.
Each NHS BCSP hub provides a freephone helpline service. Helpline staff are not medically trained but have received training on common diseases of the bowel and have a sound knowledge of the screening pathway. Helpline staff provide guidance on how to complete the test kit, are able to answer queries about the appropriateness of screening (complex clinical queries are passed to the hub management team) and can reschedule clinical NHS BCSP appointments etc.
Individuals who receive an abnormal test result are invited to attend an appointment with a SSP at a local screening centre. The SSP will discuss a follow-up investigation and arrange a colonoscopy, if appropriate. There are 60 screening centres across England (between 6 and 18 per hub area). Colonoscopy is an invasive procedure that involves passing a thin, flexible tube with a tiny camera attached through the rectum and around the bowel to look directly at the lining of the large bowel. Preparation for colonoscopy requires the subject to use a self-administered laxative for thorough bowel cleansing and a sedative is sometimes used during the procedure. If polyps are found in the bowel, most can be removed painlessly using a wire loop passed down the colonoscope tube. These tissue samples are then checked for any abnormal cells that might be cancerous. On average, for every 10 people undergoing a colonoscopy following an abnormal gFOBt kit result, five will have a normal result (or non-cancerous/non-polyp abnormality), four will have a polyp (which, if removed, may prevent cancer developing) and one will have cancer.
Screening ‘episodes’
For screening participants, a screening episode refers to the period of time from when an S1 pre-invitation letter is sent to an eligible individual to when they receive a definitive normal test result or to an outcome from colonoscopy for those referred for further investigation. If a subject has not responded by returning a test kit or contacting the hub to opt out of screening within 18 weeks of the initial pre-invitation letter being sent out, the screening episode is closed and the subject will receive another invitation to take part in screening 2 years after their previous screening due date, if they are still within the age group to be screened (60–74 years).
Subjects are first invited to take part in screening within approximately 6 weeks following their 60th birthday and may then be invited to take part in screening every 2 years, depending on test results, outcomes from any follow-up and age, and regardless of whether or not they have taken part in the past. Individuals aged ≥ 75 years can request a test kit every 2 years from the NHS BCSP if they wish to be screened.
The first time an individual is invited to be screened is referred to as the prevalent screening episode (‘prevalent previous non-responders’ refers to subjects invited to be screened at least once previously but who have not participated). ‘Incident’ screening is the term used for episodes of repeat screening (i.e. in the case of subjects who have participated in screening previously).
Initiatives to promote the Bowel Cancer Screening Programme
A number of organisations promote awareness of the NHS BCSP. Clinical Commissioning Groups [and formerly primary care trusts (PCTs)] fund a variety of local initiatives to promote uptake of screening, including promotion of screening at flu vaccination clinics, placing posters in general practices and providing information stands at health fairs, general practices, libraries, conferences and hospital open days, as well as various GPE strategies and targeted recruitment of individuals not taking part in screening. The Southern Hub is piloting a new approach to increase participation by working in partnership with general practices and sending a second reminder letter using the surgery letter head and a local GP signature. The National Awareness and Early Diagnosis Initiative also co-ordinates and provides support to activities that promote the earlier diagnosis of several cancer types in England, including breast, bowel and lung cancer.
Several charities, including Beating Bowel Cancer (Teddington, UK), Bowel Cancer UK (London, UK) and Cancer Research UK (London, UK), also fund campaigns to promote uptake of bowel cancer screening.
Chapter 3 Workstream 1: focus group study
A version of this chapter has been published as Palmer et al. 32 This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/.
Introduction
In workstream 1 we explored the psychosocial and cultural determinants of low uptake of gFOBt. In this chapter, we describe how we carried out focus groups with men and women from different socioeconomic backgrounds in London and South Yorkshire in order to identify specific barriers to screening uptake.
Uptake of bowel cancer screening in South Asian communities is particularly low, at 31.9% in the Muslim community, 34.6% in the Sikh community and 43.7% in the Hindu community, compared with average uptake across the whole population (53%). 12,40–42 Low uptake of colorectal cancer (CRC) screening in the UK has continued to be identified in areas with higher ethnic diversity,5,12 and within all South Asian religiolinguistic groups even when age, deprivation and gender are adjusted for. 40,43 We therefore also explored culturally specific barriers to uptake in South Asian communities to identify additional appropriate methods which could be specifically targeted at minority ethnic groups who speak English. To do this, we carried out interviews with 16 participants acting as ‘key informants’ on behalf of their communities. This interview study is described in Chapter 4.
The current research evidence in this area includes qualitative explorations of attitudes towards bowel cancer screening. Most of these studies were conducted in the USA or report insights relating to a range of screening methods including gFOBt, colonoscopy and FS. 44–47 This limits their applicability to the UK NHS BCSP. The sparse literature focusing on gFOBt33,42,48–52 has identified reasons for non-uptake including feeling healthy and having no bowel symptoms, fear of the outcome of screening and ‘not wanting to know’, which was often linked to doubts about the value of screening to detect health problems. Difficulties in understanding the kit instructions, concerns about hygiene and storage of the kit, avoiding or delaying decision-making, intention to take part but failure to do so owing to practicalities, and the preference for a doctor to do such tests have also been identified as reasons for not completing screening. 33,42,50,51,53 An association between being invited to be screened and entry into ‘old age’ has also been identified as a factor influencing the decision to be screened. 52 The need to deal with faeces to complete the test has not been reported as a major barrier to participation in screening, with previous studies42 reporting that the majority of respondents have not tended to express disgust or reluctance to handle faecal matter. However, the material realities of the gFOBt do constitute a barrier to participation for some individuals. 42,45,50
Few qualitative studies have focused on the English NHS BCSP. Two studies carried out during the NHS BCSP pilot phase explored the acceptability of gFOBt with individuals who had not yet been invited to take part in the NHS BCSP. 42,51 Findings from these studies were therefore based on participants’ hypothetical commentaries regarding the acceptability of the programme design and screening process. Recruitment of participants with actual experience of being invited to the NHS BCSP is limited to three qualitative studies. In one, the majority of the participants had completed the gFOBt kit50 and the other two report on small samples, each restricted to one geographical area in England. 33,52 The experiences of individuals who have been invited to the NHS BCSP and have not taken part, who might be expected to offer the richest insights into screening non-uptake, have been largely absent from the research literature to date. Furthermore, the beliefs of individuals who do not take part in one round of screening but subsequently take part in another round is limited to one recent study which describes how beliefs, awareness and intention change over time. 33 These participants are of particular interest because of their potential to explain why people make different uptake decisions on separate screening rounds.
Finally, research exploring the views and experiences of ethnic minority participants to an invitation to the NHS BCSP is, with the exception of Szczepura,42 extremely limited, despite ethnic diversity in the UK population and variation in screening uptake linked to ethnicity. 12,40,54
A single study on FS screening (an invasive form of bowel cancer screening not routinely used in the NHS BCSP at the time this research was conducted) identified specific cultural barriers associated with the threats to masculinity in African Caribbean men. 55 However, it is not known whether such barriers are also present in relation to gFOBt or if there are any culturally specific issues related to gFOBt screening among African Caribbean women.
The research presented here therefore adds to the literature in two important respects. First, we explored non-uptake decisions in a socially and ethnically diverse sample of individuals with experience of non-uptake within the English NHS BCSP. Second, we included participants who made different uptake decisions on separate screening rounds. This allowed us to identify ‘tipping points’, that is, the factors that changed an individual’s mind about screening.
Aims
-
To explore psychosocial determinants of bowel cancer screening uptake with an attention to the possible differences between socioeconomic and ethnic groups.
-
To explore the reasons for participating in screening following non-participation in a previous screening round.
Objective
To understand determinants of initial non-uptake and reasons for subsequent participation in gFOBt screening using focus groups, homogeneous with respect to gender, SECs and ethnicity.
Methods
Theoretical underpinnings
Our study is rooted in the interpretivist tradition of qualitative social research and thus focuses on the subjective understandings and meanings held by the participant. 56 An attention to participants’ interpretations and meanings provides a context to their actions, from which explanations for particular actions and behaviours may be derived. 57 We used a focus group design to explore the experiences of people invited to take part in the NHS BCSP and we used the rich textual data generated therein to illuminate the meanings, interpretations and influences that underlie participants’ decisions not to undertake screening. Focus groups are of particular value when exploring reasons why people choose not to do something, or what Barbour terms ‘why not?’ questions. 58 We took a nuanced view of the data we collected, accepting that while they may reveal aspects of the actual experience of receiving an invitation to screening, they also comprise ‘accounts’ that are produced within, and shaped by, the social setting of a focus group, and in which particular behaviours are likely to be framed in certain ways. 56
Recruiting participants
To explore sociocultural differences in barriers to uptake, we purposively sampled participants from two distinct geographical areas: inner-city London and two towns in South Yorkshire (Barnsley and Doncaster). In each setting we sampled individuals from a diverse range of SECs. We aimed to recruit participants who had not taken up screening on at least one occasion. We also purposively sampled participants of African Caribbean ethnic minority background owing to the dearth of research examining ethnic minority experiences of bowel cancer screening and following the finding of a study on FS that specific cultural barriers associated with threats to masculinity were experienced by African Caribbean men. 55
Inclusion and exclusion criteria
Our inclusion criteria were:
-
individuals who had been invited to participate in bowel cancer screening but who did not take part on at least one occasion
-
individuals who had a good understanding of, and who were able to communicate in, English.
Our exclusion criteria were:
-
individuals who did not wish to provide informed consent for the study
-
individuals who had a known previous cancer diagnosis.
Focus groups benefit from being organised around the homogeneity of the participants. 58,59 This may be especially pertinent when the research topic is of a potentially sensitive nature. Therefore, we planned to establish separate focus groups for men and women and to group participants based on their ethnic and occupational backgrounds where possible.
We planned to convene seven all-female and seven all-male English-language focus groups. Groups were to be single sex owing to the sensitive and personal nature of the topic to be covered. Two of these groups (one male and one female) would comprise African Caribbean participants to ensure ethnic diversity in our sample.
We initially planned to recruit participants through general practice lists and via community settings assisted by our charity partners Age UK (London, UK), Beating Bowel Cancer and ContinYou (London, UK). However, we encountered difficulties with these approaches, which we describe below.
Difficulties in convening focus groups
Through general practice lists
We anticipated that some focus groups would be convened through general practices serving (1) relatively affluent resident populations, (2) relatively disadvantaged populations and (3) a high prevalence of African Caribbean individuals. We planned to identify appropriate practices using routinely available data on deprivation (derived from practices’ postcode) and ethnicity from the Public Health Observatories. We established relationships with research officers at the local Primary Care Research Networks in London and the South Yorkshire and Bassetlaw Bowel Screening Health Promotion Group, which agreed to assist us in recruiting participants. However, we soon found that, although general practices held information on their patients’ bowel cancer screening uptake status, it was not held on easily searchable databases; therefore, this approach would require a great deal of effort and expense in order to identify potential participants.
Through charity partners (Age UK, Beating Bowel Cancer and ContinYou)
Our three charity partners, Age UK, Beating Bowel Cancer and ContinYou, volunteered to help recruit participants via their networks and community groups; however, although each charity offered valuable insights and expertise to the research team, particularly during the initial planning stages of our research, it soon became clear that they would not be able to assist with identifying relevant individuals or convening appropriate focus groups. Age UK tend to have contact with people who were older than the screening age or who had already positively engaged with the NHS BCSP. Beating Bowel Cancer has a well-established, extensive network of community, health promotion and district nurses; however, it transpired that its contacts were mainly with younger people who had developed and overcome bowel cancer, but were too young for their cancer to have been identified through screening by the NHS BCSP. The charity ContinYou is a community learning organisation that focuses on engagement with socially excluded groups, including those with lower levels of educational attainment and poor health literacy, and minority ethnic groups. However, during the period between the grant proposal being submitted and the grant being awarded, ContinYou shifted its focus to deliver education services for young people and, therefore, contacts with individuals eligible for bowel cancer screening (aged 60–69 years old) had ceased.
Through community settings
Owing to the lack of research exploring the experiences of minority ethnic communities to bowel screening, we wished to maximise our opportunity to explore specific ethnic issues related to non-uptake, particularly among the British South Asian and African Caribbean communities. In order to achieve this we took account of previous studies which found that alternative recruitment strategies to those used to recruit white European origin participants may be required. 60,61 Therefore, we attempted to opportunistically sample people of African Caribbean origin through community settings. However, despite contacting 12 community groups (seven in London and five in South Yorkshire), all of which were enthusiastic to assist us, we were unable to recruit enough men who met our inclusion criteria. This was because very few men attended these groups and those who did were commonly older than the screening age range or had taken part in screening. We did, however, recruit a focus group comprising women of African Caribbean origin from a church-based exercise group in North London. This group was arranged without reference to occupational background.
The difficulties we faced recruiting non-white British participants through community groups, in combination with advice received from a bowel cancer improvement practitioner working with Muslim South Asian communities in Manchester, informed the decision to use key informant interviews in place of focus groups for the study exploring experience and access to bowel cancer screening among UK South Asian communities. This aspect of workstream 1 was redesigned and accepted by the National Institute for Health Research (NIHR) following peer review.
Alternative methods of recruitment
Convening focus groups through the Bowel Cancer Screening Programme
In response to the difficulties we encountered with recruitment, we approached the London NHS BCSP Hub and the North East of England NHS BCSP Hub to help with recruitment. The two hubs agreed to act as patient identification centres, enabling us to target our recruitment at participants who had not taken up screening on at least one occasion. Therefore, we identified postcodes in areas of London and South Yorkshire, within which the hubs were asked to invite people to participate in focus groups on our behalf. We did this by identifying lower-layer super output areas (LSOAs) that encompassed a range of Index of Multiple Deprivation (IMD) scores, including those in the least deprived and most deprived quintiles. 62 We then mapped LSOAs on to postcode maps, which enabled us to identify postcode sectors from which we wished to invite participants to participate in the study. We supplied hubs with these postcode sectors and they invited a total of 5100 eligible participants living in addresses in these areas, during two periods of recruitment to the study. In order to achieve our objectives, the hubs invited individuals with a variety of uptake experiences, including individuals who had never taken part in screening, individuals who had taken part in one round of screening but not in a subsequent round, and individuals who had not taken part in initial screening but then gone on to take part in a subsequent round.
We prepared focus group invitation packs that the hubs sent out to 2560 and 2540 potential participants from across areas in the first and second wave of recruitment, respectively. The pack contained a letter of invitation to the study signed by the hub director and the study chief investigator (see Appendix 7), an information sheet (see Appendix 8), a consent form (see Appendix 9) and a return stamped addressed envelope. Potential participants were invited to telephone or e-mail the researchers to further discuss the study if they wished. The researchers had contact only with participants who returned a consent form and expressed an interest in taking part in focus groups; all non-responders remained anonymous.
Using this method of recruitment through the NHS BCSP, we were successful in recruiting enough participants to establish 16 focus groups (four male and four female in London, and four male and four female in Yorkshire).
Convening a focus group through a market research recruitment agency
Finally, we approached an external market research recruitment agency to assist in the recruitment of a focus group with men of African Caribbean origin.
Overall we established 18 focus groups, of which eight were conducted in Yorkshire and 10 were conducted in London (Table 2).
| Focus group | Location | Gender | Occupation | Number of participants/ethnicity |
|---|---|---|---|---|
| FG01 | London | Male | Professional | White European, n = 11 |
| FG02 | London | Male | Non-professional | White European, n = 5 |
| FG03 | London | Female | Professional | White European, n = 7; South Asian, n = 1 |
| FG04 | London | Female | Non-professional | White European, n = 5; African Caribbean, n = 1 |
| FG05 | South Yorkshire | Male | Professional | White European, n = 7 |
| FG06 | South Yorkshire | Male | Non-professional | White European, n = 8 |
| FG07 | South Yorkshire | Female | Professional | White European, n = 7 |
| FG08 | South Yorkshire | Female | Non-professional | White European, n = 6 |
| FG09 | South Yorkshire | Male | Non-professional | White European, n = 7 |
| FG10 | South Yorkshire | Male | Professional | White European, n = 8 |
| FG11 | South Yorkshire | Female | Non-professional | White European, n = 6 |
| FG12 | South Yorkshire | Female | Professional | White European, n = 6 |
| FG13 | London | Male | Non-professional | White European, n = 6 |
| FG14 | London | Male | Professional | White European, n = 7; West African, n = 1 |
| FG15 | London | Female | Non-professional | White European, n = 4; African Caribbean, n = 2 |
| FG16 | London | Female | Professional | White European, n = 6 |
| FG17 | London | Female | Not recorded | African Caribbean, n = 9; South Asian, n = 1 |
| FG18 | London | Male | Not recorded | African Caribbean, n = 6; West African, n = 1 |
Composition of each focus group
In addition to organising separate focus groups with male and female participants, we also used participants’ occupational background to group them by broadly similar SECs.
Although we invited individuals from the least deprived and most deprived areas (as defined by IMD quintile, i.e. based on postcode), we also needed to take account of the possibility of ecological fallacy (defined as ‘an error of deduction that involves deriving conclusions about individuals solely on the basis of an analysis of group data’), in this case, IMD derived by postcode rather than individualised data63 when grouping individuals of broadly similar socioeconomic backgrounds. 64
A standard measure for occupational background was not used. Instead, we used a consensus method between two researchers to group participants by ‘professional’ or ‘non-professional’ occupational background. This pragmatic approach was taken in the spirit of the qualitative research design adopted for this study in which flexibility and responsiveness to the emerging research context are required.
The two groups comprising majority African Caribbean participants were not grouped by reference to occupational background. This information was not available owing to the recruitment method used for these focus groups.
Generation of the topic guide
We developed a topic guide to ensure that key topics were covered in each focus group (see Appendix 10). We used the existing literature to inform the questions included on the guide, including questions to explore previously unreported areas of interest with our focus group participants. Iterations of the topic guide were discussed among the team to ensure that topics of importance for the study were covered and that these questions were framed to enable detailed and unstructured responses.
Running the focus groups
The first eight groups were conducted during July 2011 and a further 10 were conducted during April 2012. We elicited participants’ availability to attend a focus group on several dates and ran the groups at the times when the majority of participants were able to attend. We conducted all the London-based focus groups at university meeting rooms, apart from the female African Caribbean group, which was conducted at the church where the group met for other purposes. We identified conference centres and community spaces in South Yorkshire at locations that would be convenient for focus group participants to reach. Each group lasted approximately 1 hour and one facilitator (CP or CvW) ran the group while another member of the research team took detailed notes (MT or SS). Each group was audio-recorded with consent from each participant. We provided refreshments and at the end of the focus groups each participant was given £20 to thank them for taking part and to cover travel expenses.
Ethics
The study received ethics approval from South East London research ethics committee five (reference 11/H0805/7) and NHS trust research governance was obtained at the relevant sites. Written informed consent was obtained from all participants.
Patient and public involvement and engagement
We collaborated with community groups to recruit potential focus group participants. In Doncaster we worked with three community centres (contact details were provided by ContinYou colleagues), an indoor bowls centre and a men’s 60+ social group, supported by AgeUK (found through our own research). ContinYou also put us in touch with three community centres in London. In addition, we approached an over-50s exercise group at a London sports centre, a ‘men’s shed’ group and an over-50s library group, inviting them to take part in our research. AgeUK worked with us in London, for example, inviting us to a black and minority ethnic elders meeting in London, where we made a contact who arranged a focus group with Caribbean women based at a church exercise group in North London. All of these groups were extremely willing to help but, unfortunately, we often found that many of the people available were outside the age bracket we were seeking and, if they were of screening age, they tended to be positive about screening.
Following the publication of our results in the British Journal of Cancer,32 we produced an information sheet summarising the findings and posted this to all participants who expressed an interest in receiving them.
Data management and analysis
After each focus group, the audio-recording was sent securely to a third-party transcription agency and transcribed verbatim. The research team members who led each focus group (CP or MT) then checked the transcripts for accuracy in comparison with the audio-recordings and written notes and removed any identifying information. Transcripts were not returned to participants for comment or correction. Audio-recordings and electronic transcripts were saved on a secure password-protected drive. Paper transcripts and written notes were kept in locked office cabinets.
An inductive analytical approach was used to generate themes from the data. During the first stage of analysis, two members of the research team (CP and MT) working with hard copies of the data descriptively coded each transcript as it was generated; this was done in a grounded way, meaning that all data were closely and repeatedly read and coded without reference to ‘a priori’ topics linked to the research questions of interest to the study. During this initial open and grounded coding stage, the researchers would meet regularly to discuss the emerging codes and areas of commonality and difference between each researcher’s codes. As further transcripts were coded, the researchers coidentified patterns of repeating or similar codes across the data, which they clustered into early themes and wrote brief descriptive summaries of each. There was little disagreement between the researchers when clustering codes into early themes; however, the labelling and descriptions of early themes were discussed and revised collaboratively to agree on wording that was acceptable to both researchers.
Following grounded coding of the first eight focus group transcripts, these data and associated codes and preliminary themes were entered in to NVivo version 10 (QSR International, Warrington, UK) to increase data manageability and retrieval. The researchers had regular meetings in which early themes and their associated data extracts were printed out and discussed with reference to the data extracts to within the theme and against other early themes. During these discussions, themes were refined and the changes saved within NVivo. The associated summaries of the early themes were also extended and refined and saved within Microsoft Office® 2010 (Microsoft Corporation, Redmond, WA, USA) documents with the associated data extracts coded within that theme. At this point, the researchers considered all thematic categories and began to jointly identify the thematic areas that were emerging to be most relevant to the research questions of interest. The researchers presented the developing themes and associated data extracts to the wider research team at frequent intervals in order to inform, when possible, the content and development of the gist, narrative, GP and ER letter interventions.
Following the identification of key themes, the researchers created a framework grid in which the data extracts illustrating each emerging key theme were inserted alongside the focus group number and participant from which they had originated. This allowed the researchers to easily identify the individual focus groups from which data extracts had been categorised and, importantly, allowed the researchers to identify the ‘spread’ of emerging key themes across the eight focus groups. This revealed that the coidentified key themes were present and repeated across the majority of the focus groups.
As the remaining 10 focus groups were completed and transcribed, the data were coded by the same two researchers using the thematic framework developed during analysis of the first eight groups. The researchers also undertook some open coding for which data did not fit into a pre-developed theme. The themes were revised and refined with reference to these data when necessary, as were the thematic summaries. The thematic framework was barely changed with the addition of the second phase of focus group data indicating that the key themes relating to gFOBt non-uptake had reached saturation. However, analysis of these data did lead to the generation of new codes relating to ‘tipping points’ to screening, which were clustered into themes following the same processes described above and saved as word documents comprising a descriptive summary below which all the relevant data extracts were saved. The identification of these new themes was due to the inclusion of participants in the second phase of focus groups who had changed from non-uptake to uptake of gFOBt on a subsequent invitation to the NHS BCSP. The framework grid of key themes and illustrative data extracts was extended with data extracts and the new ‘tipping point’ themes from focus groups 9–18. Our analysis produced detailed summaries of the key themes accompanied by extensive illustrative empirical material, which were written up for publication.
Once all focus group data had been analysed, the researchers undertook additional comparative analyses to determine if themes could be identified that were specific to focus groups clustered by gender, occupational background, ethnicity or geographical location. Only one such theme was identified, which related to the way in which participants with ‘professional occupations’ described ‘delay’ as a key component of their gFOBt non-uptake. However, in the vast majority, the key themes relating to gFOBt non-uptake were present and repeated in each focus group. The key theme relating to ‘tipping points’ to screening was present and repeated across groups 9–18 because these were the groups containing participants with experience of taking part in a subsequent screening round.
Throughout the analytical process, emerging themes and their associated illustrative quotations continued to be presented to the wider research team for discussion and to inform intervention development. The key themes were also presented to study coapplicants present at the ASCEND steering group.
Results
Participant characteristics
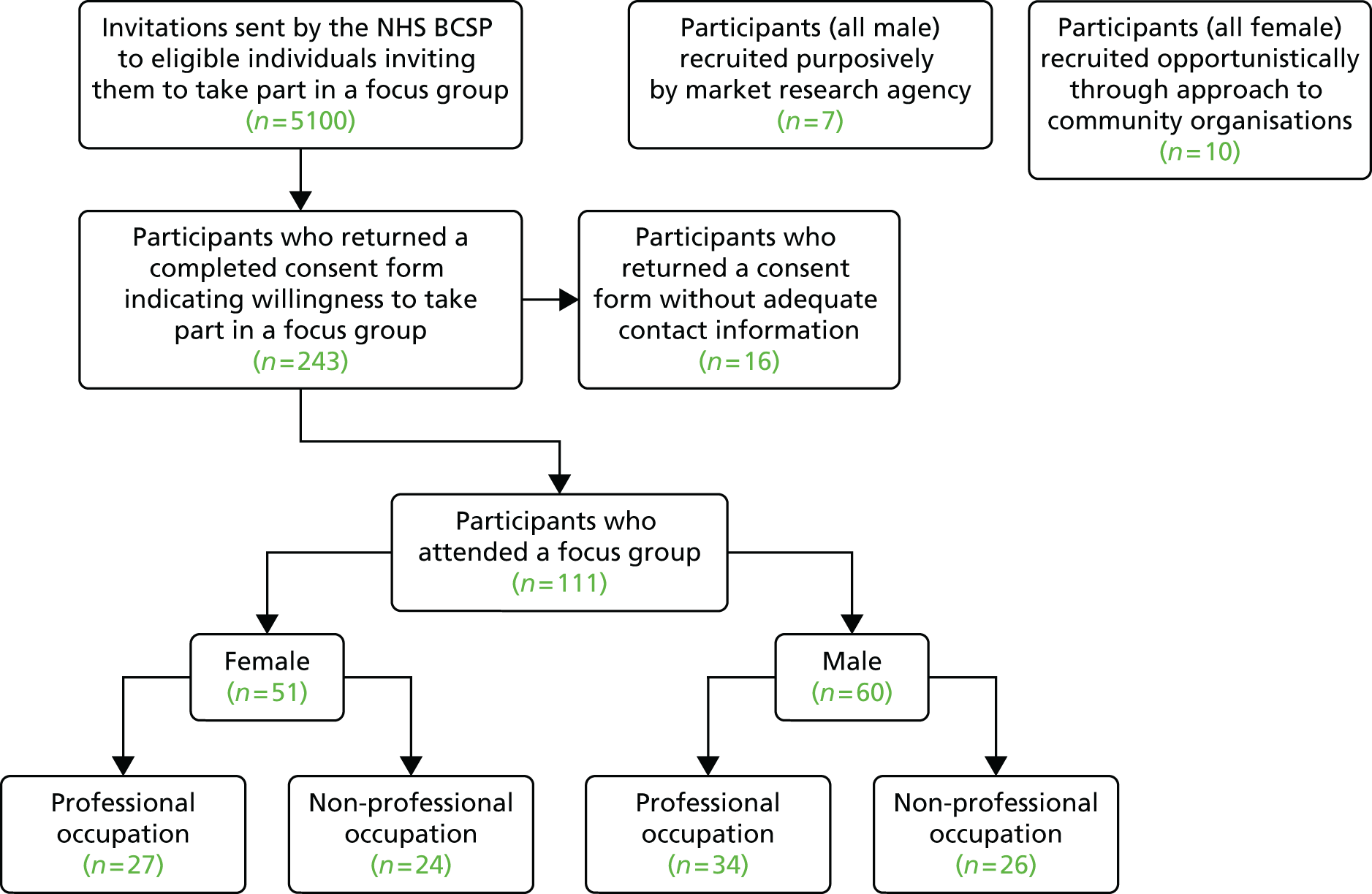
In the first wave of recruitment, 2560 participants were invited (1280 from areas served by each hub). We anticipated a 2.5% response rate, which would equate to approximately 32 responders in each hub area. We estimated that we should be able to convene two focus groups of between 6 and 12 participants in both areas with this level of response. Our response rate was actually nearer 5% and, therefore, we were able to convene four focus groups in each area. In the second wave of recruitment (and in order to achieve at least a further six focus groups), the hubs posted invitations to 2540 individuals (1270 from areas served by each hub).
As a result of these two separate waves of recruitment via the NHS BCSP, we received 243 completed consent forms from 129 men and 114 women. A total of 111 individuals subsequently attended a focus group. We intended to run 14 focus groups but were able to hold 16 owing to higher than anticipated response rates. We paid attention to ensuring the sample included a balance of men (n = 60) and women (n = 51), and individuals with reported professional (n = 61) and non-professional (n = 50) occupational backgrounds (see Table 2). Reported professional occupations included teacher, local government officer, solicitor, civil servant, nurse, dentist, journalist, artist and social worker, and non-professional occupations included sales assistant, cook, cleaner, carer, builder, miner, driver, waitress, postman and carpenter.
Recruitment using a market research company resulted in one focus group of seven primarily African Caribbean men and the approach by the research team to the north London church exercise group resulted in one focus group of 10 primarily African Caribbean women. In common with the purposively sampled focus groups, the two opportunistically recruited focus groups comprising individuals of African Caribbean origin also included participants who had and had not taken part in bowel cancer screening; however, these two groups were not organised with reference to occupational background.
In total, 18 focus groups were held with a total of 128 participants (Figure 3). The majority of participants recalled receiving invitation(s) and gFOBt kit(s) from the NHS BCSP. One hundred participants reported gFOBt non-uptake on at least one occasion, of whom 31 went on to complete the gFOBt kit when they were invited to take part in a subsequent screening round. Nine participants had not completed the gFOBt owing to ‘alternative uptake’ of bowel cancer screening, such as colonoscopy, endoscopy or gFOBt kit completion in primary or private care. Our comparative analyses found high consistency in accounts for non-uptake regardless of gender, ethnicity, occupational background or geographical location.
FIGURE 3.
Recruitment of focus group participants.
Themes
We present our findings as a series of six themes described below. To ensure anonymity, the identity of participants who provided the selected direct quotations is limited to focus group attended (i.e. FG01) and assigned participant number within this group (e.g. P1).
Themes common across non-professional and professional occupational groups
Risks posed by faeces
Participants in all focus groups explained their aversion to completing a gFOBt kit by reference to the perceived risks that collecting, storing and posting samples of faeces posed to hygiene. These risks were heightened by the requirement to complete the kit with samples from three separate bowel movements, which meant that the kit had to be stored over several days. Participants reported that the completion of the gFOBt kit threatened to physically pollute them or their environment and that they would need to go to extreme lengths to manage these perceived threats:
People’s hands have to handle this yes? You don’t know how strong germs get . . . so I don’t fancy it going through the post.
FG17P1
It’s like sort of not flushing, only worse, it’s sort of not nice . . . I wanted to scrub the bathroom down every day, so I thought it’s not worth the hassle.
FG03P5
Completion of the gFOBt kit was considered to pose serious and fundamental threats to notions of socially acceptable and proper behaviour. Participants reported discomfort at the idea of handling faeces because this was an activity that was abnormal, broached a cultural taboo or could cause embarrassment and shame:
You wouldn’t normally leave faeces in your bathroom for 3 days.
FG07P3
It’s just not the done thing is it?
No – to mess about with it.
What will happen in the bathroom with it . . . you know the whole complication of where to put it and if someone else walks in and finds it.
FG16P4
The perceived taboo of interacting with faeces was further illustrated by participants’ concerns about being ‘found out’ to have completed the gFOBt kit. Being found to have stored or posted faecal samples was believed to be potentially socially and personally damaging, in that it could reflect badly on the individual and undermine them in the eyes of others. Some participants described the requirements of the gFOBt as ‘offensive’ and ‘degrading’. The use of the term ‘degradation’ is of particular significance, because it carries ideas of personal cost in that it compromises the individual. Thus, completing the kit raised the threat of being at best embarrassed and, at worst, disgraced and discredited:
Put your poo in the post . . . I thought oh god, y’know it’s got your name on it and what if they open it.
FG15P5
I’ve had two I’ve sent them both in the dustbin. I won’t discuss it but I were a bit offended.
FG09P6
I’ve had one and I ain’t done it, I just felt degraded to tell you the truth.
FG11P5
The aversion to dealing with faeces that emerged in participants’ accounts of non-uptake is underpinned by deeply ingrained definitions of faeces as a taboo substance65 and rigid social rules surrounding how it is appropriately dealt with.
Detachment from familiar health-care settings
Participants reported discomfort with the detachment of the gFOBt from ‘usual’ health-care settings and professionals. They expressed a preference to attend a health setting such as a general practice or hospital and for ‘someone else’ to undertake the screening on their behalf:
Why don’t they send you t’doctors or hospital to have it done there? . . . if the doctor sent for me and said I want to do so and so for you I’d go, or the hospital but doing that meself, I didn’t like it at all.
FG09P6
On one level, participants linked their desire to ‘go somewhere’ such as a general practice or hospital with the avoidance of having to collect and sample their faeces. However, participants’ references to medical settings as ‘preferred’ also revealed that the invitation to the NHS BCSP was out of context and unsettling, because it required them to undertake a health procedure outside the settings in which health care is usually practised. Further linked to this was a perception of ‘self-testing’ as unusual and unexpected, particularly in comparison with other screening experiences or medical interactions where ‘a professional’ is involved in the procedure:
I thought ‘oh my god now we are asked to be doctors’.
FG16P6
I threw mine away, I’d rather have it done for me.
FG04P5
I’d rather go somewhere and have it done to be quite honest.
FG04P6
Participants emphasised that it was unusual to play an active role in a health procedure, when the norm in medical encounters was for them to be the passive ‘receiver’ of care. They also disliked the impersonal nature of home testing:
I would prefer my doctor to have some obvious interaction with me in the actual process rather than it being done with an anonymous third party.
FG01P8
By extension, it was noteworthy how many participants claimed that, had they been given an appointment to attend, or been told by their GP to complete a gFOBt kit, they would have done so. Therefore, it appeared that the detachment from clinical settings and professional roles may have reduced the perceived importance of the offer of screening:
The message that was communicated to me was that this was hardly urgent or serious because if it was they would send me off to have a clinician do it.
FG01P1
If the letter had come from my GP . . . I would have taken it more seriously.
FG18P6
The prospect of self-sampling at home inhibited rather than facilitated uptake.
The implications of knowing the screening results
The most complex theme to emerge related to the implications of knowing the screening results. Participants preferred not to be in possession of this information for several reasons. First, they commonly referred to the undesirable implications of a positive result. Thus, they expressed unwillingness to undergo the recommended procedures that may follow a positive gFOBt kit result, such as colonoscopy or bowel surgery. These participants often referred to previous experiences (their own or those of family members) of bowel investigations or treatment for gastroenterological problems and described their negative consequences:
It’s the after effects, if they do find something, that would put me off taking the test in the first place . . . it’s the colonoscopy, the treatment of the colonoscopy.
FG10P3
Non-uptake was therefore a means to protect oneself from the possible unpleasant consequences of a positive test.
Second, participants distinguished between ‘being unwell’ and ‘knowing about being unwell’. A positive screening result meant that they would need to ‘redefine’ themselves as being unwell, which they did not wish to do because they believed it was unnecessary:
If there’s something the matter with me now, and I don’t know about it, I’m fine. If somebody says I’ve got a problem, I’m going to worry about it, and I don’t want that, you know you live life as it is now and I don’t want people finding things.
FG10P6
Thus, there emerged from participants’ accounts an alternative reading of screening as an activity that, rather than maintaining good health, may actually be complicit in generating ill health. By presenting screening as a process that could undermine health and questioning the value of the knowledge offered by screening to the maintenance of good health, participants pointed out that the benefit of declining to complete the gFOBt kit allowed one to ‘get on with life’:
This is just like sticking your head down the loo and thinking you’ve got cancer all day, you know there’s a balance of how much ‘into’ things you should get.
FG03P3
To me it’s like mollycoddling yourself so everything working right, don’t mess about with yourself.
FG05P1
Finally, the possibility that screening might identify cancer and result in subsequent interventions was described by some participants as too frightening to contemplate. The knowledge offered by screening was, for some, a stressful and frightening prospect, to the extent that actively choosing not to be in possession of this information was preferable:
I’m scared, simple as that . . . it’s the test coming back positive that worries me, so I tend to ignore it and hope it goes away.
FG02P2
What’s frightening?
What actually might be discovered, what I don’t know is not there like, if you know what I mean.
Thus, some participants demonstrated an ambivalence towards, or overt rejection of, the knowledge offered by screening. Analysis of the accounts of participants who ‘didn’t want to know’ found that it was also common for such participants to describe cancer as a particularly serious and frightening diagnosis for which treatment was unpleasant and often futile. Participants’ attitude towards cancer treatment further underpinned their rejection of the knowledge offered by screening because, if there was perceived to be little benefit associated with treatment, there was little point in taking part in screening:
[A friend] went through all that chemotherapy and all that suffering it didn’t make a . . . difference.
FG08P6
Judgements of good health and low relevance of screening
Many participants believed that the gFOBt was irrelevant because they were certain that they did not have, and were unlikely to get, bowel cancer. The evidence they cited included a lack of symptoms, being physically active and having no family history of bowel cancer:
I’ve got no symptoms so I’m all right, y’know, I go to the toilet regular and y’know, I exercise and I’m fit.
FG09P1
Descriptions of being in good health were often interwoven with other themes of non-uptake.
Themes present among professional occupational groups only
Delaying uptake, leading to non-uptake
No themes emerged solely from participants with non-professional backgrounds; however, we identified one theme associated with non-uptake which was discussed only by participants with professional backgrounds. These respondents commonly described their non-uptake in terms of delay, rather than outright rejection. Participants reported that the gFOBt kit was ‘put to one side’, or ‘put in the in-tray’ implying some degree of intention to participate, but ultimately kits were not completed. Delay was often linked to descriptions of the complexity of the instructions for completing the gFOBt kit and also the time-consuming nature of kit completion:
You’ve got to really sit down and read it, you can’t, it’s not just something you can pick up and say ‘oh I’ll go and do that now’, you’ve got to study it.
FG12P4
It’s quite a long-winded, drawn out thing I just kept putting off doing it.
FG03P8
There was a common misconception among participants in all focus groups that samples had to be taken on three consecutive days. Respondents from professional backgrounds cited this rigid, 3-day ‘window’ for test completion as a cause of delay and subsequent non-completion because it was not possible to fit the test requirements in with their bowel movements or routine and lifestyle:
If I start it on one day I’ve got to remember then to do it for the next 2 days and that was a big block for me because I’m very rarely in the same place for 3 days in a row.
FG14P8
Non-uptake followed by uptake in a subsequent screening round: the power of talk – a key ‘tipping point’
Participants from all occupational backgrounds who reported that they had not initially participated in screening and had then completed the gFOBt kit in a subsequent screening round described being influenced by discussions with family members, friends and health professionals. They reported being questioned about their initial refusal to complete the test or being told outright to take part in bowel cancer screening. They also recalled supportive discussions in which their concerns about or aversions to the gFOBt kit were discussed and challenged. Participants reported that becoming aware that their partner or friends had already completed the gFOBt kit was influential. In addition, they reported that becoming aware that a family member or friend had developed bowel cancer influenced them to take part in screening:
My brother-in-law was diagnosed with bowel cancer after I’d had the first request which I totally ignored . . . my wife did [her gFOBt kit] and she got her results back which were clear, peace of mind, I thought well you silly bugger, you know, why didn’t I do [it].
FG09P4
A friend also had it and she was telling me about how she did it and I thought gosh it’s not as complex as I think.
FG16P2
Discussions in which other individuals championed participation in screening or revealed their own gFOBt kit uptake were repeatedly implicated by participants as the key tipping point to a decision to undertake screening on a subsequent occasion. Through talking with others, participants described themselves as ‘nagged’, encouraged and reassured to undertake the gFOBt. Furthermore, through talking with others and becoming aware of others’ completion of the gFOBt kit, uptake was repositioned as a normal activity. The particular power of talk appeared to normalise the unusual, unexpected and potentially taboo aspects of the gFOBt kit:
I think as well it’s a critical mass isn’t it, so you discover your friends are all doing it or whatever so then it does become a slightly normal thing to do.
FG16P1
Discussion
Our analysis of participants’ accounts has identified key themes that inform understanding of why non-uptake of bowel cancer screening occurs. Furthermore, our comparative analysis of focus group data by area (inner-city London, Doncaster and Barnsley), gender, occupational background and ethnicity found few differences in the ways in which participants accounted for non-uptake. In common with previous studies,45,50,51 we have identified the threats to hygiene posed by completion of a gFOBt kit. However, our analysis extends the understanding of the problems posed by faeces with the concept of ‘social pollution’. Taboos surrounding interactions with faeces65,66 mean that completion of a gFOBt kit may be considered ‘improper’ in addition to unpleasant. The troubling nature of faeces is therefore a key element in participants’ non-uptake of the gFOBt. In addition, we found that the requirement to undertake the gFOBt kit oneself and in one’s own home represented a detachment from familiar medical settings and roles, which, in turn, impersonalised or devalued screening as a valid health endeavour. The prospect of self-testing at home was for some unsettling rather than facilitating of uptake. This finding is unreported elsewhere in the literature.
Ambivalence about the value and benefit of the knowledge offered by screening, and of medical intervention more generally, has been identified previously. 47,50,51 Participants confidently articulated the negative implications of participation in screening and emphasised the benefits of not being in possession of such knowledge. Non-uptake was therefore presented as having protective and beneficial effects. In addition, we found that participants described delay and non-uptake of the gFOBt kit owing to its prolonged and complex nature, and identified misconceptions about correct test completion that may contribute to perceptions of complexity. We further identified the well-documented misconception that having no symptoms meant that screening was not needed. 44,47,49,50 Finally, we have identified the influence of talking with others as a tipping point to uptake of screening. We suggest that it is through talk that completion of a gFOBt kit may be ‘reformed’ as a normal and culturally appropriate activity, and that concerns about its unexpected and potentially inappropriate aspects may be alleviated.
Strengths and weaknesses
This study adds significantly to the qualitative literature exploring gFOBt non-uptake and offers unique insights relating to the NHS BCSP in England. The strength of the study lies in our grounded and inductive analysis of an extensive qualitative data set generated through focus groups with participants who had actual experience of screening non-uptake within the NHS BCSP. Moreover, this is the first study, to our knowledge, to have explored changes of uptake decision at a subsequent screening round to generate insights into the ‘tipping points’ for uptake of gFOBt. Importantly, our analysis has identified the ‘faecal factor’ to be a key element in non-uptake of screening, differing somewhat from previous studies that have found such concerns to be downplayed. 45,50,51 However, in these studies, the majority of participants had undertaken screening and, therefore, such concerns were likely to have been minimised.
Our data comprise the accounts of participants who recalled an invitation to screening, who knew they had not taken part and were, as such, able to report on the experience in a focus group. However, there were a small number of participants who exhibited little knowledge or recollection of being invited to screening. These participants illustrated that it is possible to be unaware of one’s non-uptake, possibly because the invitation to screening or purpose of the gFOBt has made little or no impression (or may never have been received). Low literacy on the part of a participant may render the entire experience of being invited to screening inaccessible and unknown67 and, thus, ‘unreportable’ in the context of a focus group. We therefore acknowledge the limitations, as well as the utility, of insights based on participants’ accounts. Finally, we acknowledge that our sample excluded participants who required care owing to dementia, stroke or learning difficulties because of challenges for such participants to take part in a focus group.
A further strength of the study is the unique method of recruitment, which was largely achieved through direct invitation from the NHS BCSP. This enabled us to ensure that our sample included a majority of individuals who had not taken part in screening. It further enabled composition of the focus groups which maximised the potential for comparative analyses of the data by reference to gender, area (inner-city London and two towns in South Yorkshire) and SECs of participants. However, we were not able to specifically recruit low-literacy adults (one of the groups we had intended to recruit via ContinYou) using this method, as we might have been able to had we been able to recruit participants through our charity partners and community settings. Indeed, inviting people by a written letter may have positively excluded low-literacy individuals.
Some participants reported a screening history that did not match the hub’s records. The hub invited to focus groups only those individuals who had not responded to a screening invitation on at least one occasion. However, a small number of participants reported never having been invited to screening or always having taken part when invited to be screened. This discrepancy may be secondary to incorrect NHS BCSP records, invitations being lost in in the post or sent to an incorrect address (addresses are supplied to the NHS BCSP by the individual’s GP), participants’ forgetfulness or unwillingness to admit to non-uptake within the focus group. In the London hubs’ experience, invitations are particularly likely to be lost in the post if individuals live in blocks of flats where the postal officer leaves all the post in the hallway of the block rather than in individual letter boxes.
In our first wave of NHS BCSP recruitment, a number of participants reported having previous experience of ‘alternative’ bowel screening through colonoscopy or gFOBt in primary or private care. These participants were positive about screening and uptake, and simply had not taken part in the NHS BCSP offer of screening because they already had recent information about their bowel cancer status. Therefore, in our second wave of recruitment we elicited whether or not participants had previous experience of ‘alternative uptake’ of bowel screening and did not invite people in this category, in order to maximise the number of participants who did not take up screening for reasons other than receiving surveillance outside the screening programme.
Previous/current occupation was used as a pragmatic indicator of SECs to allocate participants into groups with others of similar occupational background and, therefore, SECs. It is possible that organising participants by a different classification system may have produced different results. However, owing to the large number of focus groups undertaken, and the homogeneity of results across all groups regardless of occupation, it is unlikely that this would have been the case.
Conclusion/summary
Our focus group study provided insights into why people do not take part in bowel cancer screening and why people participate in one round of screening but not in another. The findings from this study were used specifically to inform the design of ASCEND intervention 4, described in Chapter 8. Although not intended to be used directly to inform the design of intervention 2 and 3, it emerged from the focus groups that learning of other people’s views and screening experiences could act as a decision ‘tipping point’ and that input from a GP would influence people’s intention to take part in screening, further emphasising the potential impact of these interventions.
Chapter 4 Workstream 1: key informant study
Introduction
In workstream 1 we explored the psychosocial and cultural determinants of low uptake of the gFOBt. In this chapter, we describe how we undertook interviews with ‘key informants’ representing South Asian Muslim, Sikh and Hindu faith communities in London, in order to explore culturally specific barriers to uptake in South Asian communities and to identify appropriate strategies to increase uptake which could be specifically targeted for minority ethnic groups who speak English.
Uptake overall within the NHS BCSP is about 56%, but this figure masks much lower rates in South Asian communities, which vary from 31.9% in the Muslim community, through 34.6% in the Sikh community to 43.7% in the Hindu community, than in ‘non-Asians’. 40–42 South Asian minority ethnic communities (comprising Indian, Pakistani and Bangladeshi groups) make up 7.5% of the population of England and Wales. 68,69 Low uptake of bowel cancer screening in the UK has continued to be identified in areas with higher ethnic diversity5,12,54 and within all South Asian religiolinguistic groups, even when age, deprivation and gender are adjusted for. 40,43 A study of participation in bowel cancer screening using FS (an internal examination undertaken by a medical professional) similarly found lower uptake by South Asian participants after socioeconomic status had been controlled for. 70 Inequalities are also present in the longer-established UK breast and cervical screening programmes. 40,43,69,71–73 Uptake across screening programmes has consistently been lower in London than in the rest of the country, and this has in part been attributed to its diverse population. 73–75
Many of the studies which have sought to explain reasons for ethnic disparities in screening uptake originate from the USA and present insights relating to black American, Hispanic, Latino and other ethnic groups. Importantly, many of the structural and financial barriers exposed in US studies do not apply to the English health-care system, in which health care is free at the point of access and bowel cancer screening is made available through a nationally organised screening programme. 44,76–78 Their findings therefore offer limited scope for comparison with the UK setting.
A number of UK studies have found that reasons for low or late uptake of breast and cervical screening by minority ethnic women include low knowledge and awareness of these cancers and related screening services;75,79–81 inaccurate screening registers;82 misconceptions about the smear test; fear; embarrassment and negative previous experience;75,81 language, literacy and administrative difficulties; cultural values and beliefs; misconceptions about perceived risk; concerns about surgery hygiene; and poor attitudes of GPs. 73,74 Reasons for non-uptake of bowel cancer screening are likely to include some of those identified for breast and cervical screening programmes, but may also relate to the way in which bowel cancer screening is offered by written invitation requiring home-based self-completion of the gFOBt, with no face-to-face contact with a medical professional.
Research exploring acceptability of the gFOBt among minority ethnic communities is limited to a single study undertaken prior to the initiation of the NHS BCSP involving participants from diverse minority ethnic groups, most of whom had not yet been invited to bowel cancer screening. 42 The study found that although people supported the principle of screening and completing a gFOBt once it had been explained to them, many participants would not respond to postal invitations without prior warning being given, preferably by trusted local sources, invitees would require support from family members to translate information materials and translated materials could pose problems owing to poor literacy in their first language and limitations of translations because of dialects spoken. 42 Two studies exploring low uptake of bowel cancer screening by FS in diverse minority ethnic groups reported low knowledge and awareness of bowel cancer, anxiety about test invasiveness, fear of cancer, language difficulties, religious sensitivities and embarrassment as barriers to participation. 55,70 However, the relevance of these findings to low uptake of gFOBt is limited owing to differences in screening test procedures. Importantly, no studies have explored explanations for the differences in gFOBt uptake identified in the Muslim, Sikh and Hindu faith communities. 42 The UK South Asian community comprises multiple ethnic groups with a diversity of religious, linguistic, cultural and geographical origins, and there have been calls for greater attentiveness to this heterogeneity in health research involving participants of South Asian origin. 83,84
Aims
-
To explore reasons for the variations in bowel cancer screening uptake between South Asian Muslim, Sikh and Hindu faith groups.
-
To explore reasons for low uptake of bowel cancer screening in all South Asian communities and identify possible methods by which uptake might be improved.
Objective
To understand reasons for low uptake of gFOBt screening by UK South Asian communities using key informant interviews with participants representing Muslim, Hindu and Sikh faith groups in London.
Methods
Theoretical and methodological underpinnings
This study used interviews with key informants to generate insights regarding acceptability and accessibility of an invitation to the NHS BCSP among a number of South Asian minority ethnic communities. The key informant approach originates within the anthropological ethnographic tradition in which members of a particular culture or community facilitate access to, and understanding of, features of their community on behalf of a researcher. Key informants are individuals who, owing to their position and immersion in a particular community, can provide information and insight regarding the experiences and needs of the community they represent. 85 Key informant interviews have been successfully used across a number of social research disciplines to generate contextually detailed and culturally informed knowledge about community members’ use (or non-use) of health services. 86 We have taken a realist view of the data generated during interviews with key informants.
Recruiting key informant participants
We aimed to recruit key informants from the three largest South Asian faith communities in the UK. Approximately half of the UK South Asian minority ethnic population lives in London,68,87 and we recruited key informants via direct approaches to 26 London-based faith and community organisations that provide services specifically to South Asian communities. Organisations were identified via internet searches and professional and personal contacts. Potential participants were contacted by e-mail or telephone, provided with information about the study and invited to participate. Key informants were purposively sampled to ensure that they held an embedded role within an organisation serving one of the three main South Asian faith communities (Islam, Hinduism and Sikhism) and that they spoke English at a level suitable for interview. A snowball technique was used to generate further contacts for possible participation.
In addition, we recruited GPs working in areas with large South Asian populations via two Comprehensive Local Research Networks (North West London and East London) and personal contacts. Populations represented by GPs comprised mixed faith, ethnic and linguistic groups. Interviews were arranged for a time and place convenient to each participant.
Final key informant participant sample
A total of 16 key informants participated in the study, of whom 12 held roles across a total of 10 community or faith organisations that served people of South Asian minority ethnic origin as follows: four informants representing the Sikh community held roles in Gurdwaras or community groups based in West London, four informants representing the Hindu community held roles in Mandirs or community groups in West and North London, four informants representing the Muslim community held roles in third-sector organisations that support the Bangladeshi community in East London and, therefore, represented a specific Muslim ethnocultural group originating from the Sylhet region of Bangladesh and the final four key informant participants were GPs serving areas with a large South Asian minority ethnic population in the London boroughs of Barnet, Tower Hamlets, Harrow and Redbridge (Table 3). Fifteen participants were of South Asian minority ethnic heritage and one was white European.
| Interview number | Organisation key informant recruited from | Area | South Asian community represented | Sex |
|---|---|---|---|---|
| 1 | Social/community group linked to Gurdwara | Southall, West London | Sikh community | Male and female |
| 2 | Mandir | Neasden, North West London | Hindu community | Female |
| 3 | Community health charity | Tower Hamlets, East London | Muslim community (Bangladeshi) | Female |
| 5 | Community centre | Tower Hamlets, East London | Muslim community (Bangladeshi) | Male |
| 6 | Community charity | Tower Hamlets, East London | Muslim community (Bangladeshi) | Female |
| 7 | Mandir | Wembley, North West London | Hindu community | Female |
| 8 | Community charity | Tower Hamlets, East London | Muslim community (Bangladeshi) | Female |
| 9 | Gurdwara | Hounslow, West London | Sikh community | Male |
| 10 | Social/community group | Barnet, North London | Hindu community | Male |
| 11 | Mandir | Southall, West London | Hindu community | Male |
| 12 | Gurdwara | Ealing, West London | Sikh community | Male |
| 13 | GP | Barnet, North London | Mixed with large Indian Gujarati-speaking population | Female |
| 14 | GP | Tower Hamlets, East London | Bangladeshi largely Sylheti-speaking population | Female |
| 15 | GP | Harrow, North West London | Mixed with large Indian Gujarati-speaking population | Female |
| 16 | GP | Redbridge, Essex | Mixed Pakistani, Indian and Bangladeshi population | Male |
Topic guide
We used a short topic guide (Box 1) to ensure coverage of relevant questions.
-
What do you think might happen when this invitation and test kit comes through the door?
-
Would it be easy for people to take part in bowel cancer screening offered in this way? (Why/why not?)
-
Why might people not take part?
-
Why do you think uptake of screening for bowel cancer is lower in South Asian communities/the (specific) South Asian community you work with?
-
(Other than what you have already described) are there any other reasons why you think uptake of bowel cancer screening is lower in South Asian communities/the (specific) South Asian community you work with?
-
Are there ways in which uptake of bowel cancer screening could be increased in South Asian communities/the (specific) South Asian community you work with? (How do you think this could be done?)
Undertaking the interviews
Between May and December 2013, semistructured face-to-face interviews were undertaken with 16 key informants. All interviews were undertaken at the organisation from which the key informant had been recruited and were conducted in English by a female researcher (CP). Interviews began with a brief explanation and presentation of the NHS BCSP invitation materials, which formed the starting point for participants’ comments and reflections about the accessibility and acceptability of an invitation to the NHS BCSP for members of the communities they represented. Interviews lasted between 25 and 40 minutes and were audio-recorded with the permission of the participant. A donation was made to the key informant participant’s organisation in recognition of their time. Donations were not made to the GP participants.
Data management and analysis
Following each interview, the audio-recording was transcribed verbatim using a professional transcription service. Transcriptions were checked and amended for accuracy against the audio-recordings and interview notes, and any identifying information was removed from the transcripts. Transcripts were not returned to participants for comment or correction.
We undertook an inductive analytical approach to generate themes from the data. 57 Each transcript was read and coded by at least two out of three team members (CP, MT and LM) and the data were initially analysed separately by reference to faith community. Authors met to generate preliminary themes using the codes and compared themes to identify commonalities and differences between data generated across the three faith communities. Key themes were then refined and defined in relation to the areas of research interest.
Ethics
Ethics approval for this study was granted by the National Research Ethics Service Committee London-Bromley (reference 11/H0805/7). NHS research and development approvals were gained for interviews undertaken with GP informants. Written informed consent was obtained from all participants.
Results
Key informants provided detailed commentaries about how their communities would be likely to respond to an invitation to take part in the NHS BCSP. Results are presented as three main themes relating to low uptake of bowel cancer screening that were described across faith groups: limitations posed by the written word, low awareness of bowel cancer and screening, and difficulties around faeces and gFOBt completion. Within each theme we also report suggestions to increase accessibility and uptake. Finally, we present reasons for low uptake specific to Muslim Bangladeshi and Sikh faith groups. Data extracts are identified by community represented and interview number (see Table 3).
Themes
Limitations posed by the written word
Unanimously, key informants described how many South Asian elders eligible for bowel cancer screening would not be able to engage with the letter and accompanying information that is sent by the NHS BCSP, because they did not read or had limited ability to read and speak in English:
They don’t speak English, they don’t read English.
Muslim community informant 8
Some of the elderly [. . .] don’t necessarily have English as their first language.
Hindu community informant 2
. . . quite a lot of the older people don’t speak English very well.
Sikh community informant 9
Key informants across faith communities reported that it was common for sons and daughters to translate and interpret written materials for older members of the family, and that support of this kind would be required for the NHS BCSP invitation materials. Informants therefore suggested that the ability of those invited to participate in screening may be heavily mediated by younger family members, who may further make their own judgements about the importance and relevance of the screening invitation:
In terms of letters and stuff they won’t read them. They’ll get the kids to read it.
Sikh community informant 9
She [my mum] said to me, there’s a letter for me from the doctor. I came and looked at it but I didn’t put that much emphasis on the importance, I didn’t encourage her to take up.
Muslim community informant 3
In recognition of the language needs of the population, the NHS BCSP offers translated materials, which are available on request by telephoning the helpline. However, calling the helpline to request a translation was perceived to require additional effort and motivation, potentially in the absence of knowledge regarding what the recipient is seeking information about:
So what kind of person would ring [the NHS BCSP helpline], it would be someone who is very motivated to want to do it. Or very interested in these letters coming through but they don’t understand it.
GP informant 14
You’ve got to have some understanding of English to be able to say ‘I’ve got this letter, what shall I do’.
Hindu community informant 2
Therefore, although translated materials were offered, informants identified that variable proficiency in reading English would make accessing these challenging and, again, would require reliance on a family member to mediate.
Indeed, the written word (whether in English or translated) as a medium for communication was repeatedly described by key informants as unappealing and lacking impact and importance within their communities; for these reasons, postal communications were often overlooked. Moreover, informants perceived that the NHS BCSP invitation letter and materials comprised too many words and too much information, even for those who do read English:
I think they would probably get lost in these words.
GP informant 16
It’s a lot to take in [. . .] It’s just too much, to be honest.
Hindu community informant 2
Although several key informants suggested there may be some value in offering translated written materials, it was noteworthy that, across all faith communities, human interaction involving face-to-face discussion, verbal descriptions and demonstrations was the favoured means of communication to effectively share information:
Our people are more visual learners [. . .] they rather see and hear before they make any decision.
Muslim community informant 6
They’ll need support, someone needs to show them what to do [. . .] it will require a conversation, because otherwise I don’t see how they would understand how to do it.
Muslim community informant 8
Somebody who is like 60 or 65, English is not their first language, they probably need some human touch where people can come and explain to them.
Hindu community informant 11
Key informants emphasised that these interactive approaches would be particularly useful within places of worship or other settings in which community members gathered communally. Approaching community members in a familiar place and at the group rather than individual level was strongly endorsed as a means of increasing the understanding and confidence of community members, enabling them to more readily engage in screening:
I think it’s coming into the community [. . .] at our Mandir it works really well because you’re capturing the audience in their home, as it were, and they feel comfortable [. . .] as long as, of course, it’s in their language as well.
Hindu community informant 2
Go into the community, showing them, telling them the importance.
Muslim community informant 3
Low awareness of cancer and screening
Informants reported that there was low awareness of bowel cancer and bowel cancer screening within their communities. This was partly linked to the limitations of written invitation materials to enable understanding as described previously. Informants suggested that participation in screening would increase if communities were given information about the purpose and value of screening in culturally accessible ways, as well as how to undertake the practical side of gFOBt kit completion:
Unless they understood how important it was, they wouldn’t do it. [. . .] you would need to tell them what the facts and figures are, why it’s important for them to do it, what the risks are.
Muslim community informant 8
Explain to them properly if you send that kit back and if you have any problem then [. . .] they will be treated in early stages they will be fine.
Sikh community informant 1
Informants also identified low awareness of cancer being potentially curable and reported that cancer was perceived to be serious, frightening and final. Informants suggested that such perceptions could undermine engagement with screening:
Cancer is one of those things that everyone regards as you can’t do anything about it, once you get it you get it, and that’s end of.
Muslim community informant 8
Most people think if they find they have the cancer, that’s it, that’s the end of the story. They’re too scared to know the word, hear the word . . .
Hindu community informant 11
Informants across faith communities suggested that owing to low awareness of screening, the invitation to be screened was likely to be perceived as having come ‘out of the blue’ and unsolicited because it comes from a national source, rather than from a local familiar person (e.g. a GP) or organisation. Informants suggested using media other than the written word, such as television or radio, to raise people’s awareness of the NHS BCSP and to prepare and familiarise them for the forthcoming invitation and gFOBt kit:
I would get something on radio and that would get the message across and then at least then they’ll be looking out for the letters.
Sikh community informant 9
So you could almost do like a description of what might happen [on Asian radio and Asian television Sky channels] and then they’d at least have an idea of what it is.
Hindu community informant 2
Informants suggested that awareness-raising should include positive information about early diagnosis and cancer curability to counteract some of the fear surrounding cancer. Informants recommended that information be provided ‘in language’ to groups within faith and community settings, incorporating demonstrations of the gFOBt kit, face-to-face explanation and the opportunity to ask questions. Indeed, informants reported health awareness activities were already taking place within a number of the community and faith settings represented.
Difficulties associated with faeces and test completion
Informants suggested that the requirement to complete the kit with samples of faeces and to store the kit over a period of days would be considered unpleasant and compromising to hygiene. They also considered that community members would require help with how to collect and sample faeces but that, because of the personal nature of the test process, younger family members would be less able to assist:
Doing something like this and having it out for 3 days with faecal matter on it is totally abhorrent to them.
GP informant 13
I see not many people doing this to be honest, especially because they don’t want to go around sneaking their own poo.
Muslim community informant 8
This is something very personal you know toilet is something you don’t dare – even dare to ask children you know ‘can you do that?’
Hindu community informant 10
Informants suggested that a simplified test procedure that required a one-off sample might help overcome some difficulties with test completion. They also suggested that community members be given the option to take the gFOBt to their GP or practice nurse to seek explanation and practical instruction.
Reasons for low uptake specific to faith groups
Offering screening using written materials in any language was described as being a particularly ineffective way to communicate with the Bangladeshi Muslim faith community owing to its largely oral (non-written) culture. The majority of this community speak Sylheti, which is rarely used in its written form, and community members were unlikely to be able to read in Bengali, meaning that written translations of screening information would be of no use:
They don’t function in a written way [. . .] written information does not give people the ability to go and do what needs doing.
GP informant 14
Most of these people, even in this age, they even don’t know how to read Bangla [Bengali].
Muslim community informant 5
Informants representing Sikh faith communities described a particular reluctance to disclose a cancer diagnosis or talk about cancer more generally. This was explained in terms of a social stigma surrounding cancer and fear of the potentially negative reactions from the wider community that may be elicited in response to cancer:
. . . within the family someone will get cancer and they don’t talk about it. It’s just a social stigma on things [. . .] they think that ‘what will other people think?’
Sikh community informant 9
People don’t want to talk, disclose their own thing. If even they do find out [they have cancer] they don’t want to tell other people.
Sikh community informant 1
Discussion
We identified common reasons that may explain ongoing low uptake of bowel cancer screening across South Asian faith groups. In all three faith communities, the delivery of bowel cancer screening using a written approach directed at the individual was considered likely to be inaccessible to a significant number of South Asian people of screening age. We also identified low awareness of the existence and purpose of bowel cancer screening and of cancer curability. In addition, there were potential difficulties with gFOBt kit completion linked to the requirement to deal with faeces and test complexity.
This study is the first, to our knowledge, to explore differences between South Asian faith groups in the reasons for low uptake of bowel cancer screening. Uptake of bowel cancer screening within England’s South Asian population is lowest in the South Asian Muslim community. 42 This may, in part, be explained by our finding that communication via written materials is particularly inappropriate for the London Bangladeshi Muslim community owing to the largely oral culture of this community. The second lowest uptake of bowel cancer screening in the South Asian population is reported among the Sikh community. 42 Our finding that reluctance to talk about cancer is linked to social stigma surrounding cancer within this community may go some way to explain the poorer uptake in this faith group.
Our findings that written invitation materials may pose particular challenges and that, even when translated, written invitation materials are of limited value owing to widespread low literacy specifically among older members of South Asian communities (to whom bowel cancer screening is offered) are in common with previous research. 42 Difficulties related to language have also been found in relation to low uptake of other screening programmes. 73–75
Although previous studies have identified reluctance to talk about cancer linked with fearful perceptions of cancer in mixed South Asian minority ethnic groups, these studies have not attempted to distinguish between faith groups regarding the specific experience and impact of social stigma surrounding cancer. 42,81
Our finding of low awareness of the purpose and value of bowel cancer screening across South Asian faith communities confirms previous findings. 42,55,73,75,81 A number of studies also found low knowledge and awareness of cancer and fearful perceptions of cancer in minority ethnic groups,42,55,70,73,75,80,81 which mirrors our finding that awareness of cancer curability was perceived to be low across South Asian faith communities. Finally, we found perceived difficulties associated with the sampling and storage of faeces in order to complete the gFOBt kit, including limitations on getting help from family members owing to the personal nature of the test, which were also experienced within the majority (white European and African Caribbean) population. 32,50,51
We identified agreement across faith communities in the preferred approaches to increase accessibility and awareness of bowel cancer screening offered by the NHS BCSP. In common with previous studies, we have identified an overwhelming preference for face-to-face, verbal and interactive approaches in order to provide information and raise awareness about the availability and purpose of bowel cancer screening. 42,74 We also identified a desire for the provision of information to take place ‘in language’ within community, faith and/or social settings;42,55,73 and the potential value of ethnic community media to publicise the NHS BCSP and gFOBt completion. 42,55 A simplified test kit and the option to seek screening guidance from the GP or practice nurse were noted as further potential strategies to increase uptake. Our findings reiterate existing evidence regarding strategies to improve accessibility and uptake of health services more generally for South Asian communities, but we have also demonstrated that specific South Asian ethnocultural groups may have distinctive features and needs.
Strengths and limitations
Interviews with key informants generated culturally and contextually informed knowledge about how an invitation to the NHS BCSP may be received within faith communities. However, limitations exist in the approach used because informants were expected to speak ‘on behalf of’ communities but may have given their own personal views on bowel cancer screening as well as reporting more general cultural issues. Furthermore, key informants are likely to have had a less detailed understanding of the NHS BCSP invitation and test completion than participants with personal experience of being invited to screening.
Although we aimed to recruit informants to ensure representation of the three main South Asian faith groups, we acknowledge that there is further diversity within each group that our sample was not able to include owing to the scope of this particular study. Specifically, we were unable to recruit participants representing the Pakistani Muslim ethnocultural group. We further acknowledge that the number of informants representing each individual faith community was small and this may have meant that further subtle differences between faith communities in their likely responses to an offer of bowel cancer screening remained unexamined. Although our study sample was limited to London, the shared ethnic and religious origins of South Asian communities across the UK are likely to mean that our findings have wider relevance. Finally, we acknowledge that the language the interviews were conducted in (English) and the gender and ethnicity of the researcher conducting the interviews (white European and female) is likely to have had an impact on the sample of informants recruited to the study and the generation of data therein.
Conclusion
Our findings identify barriers to bowel cancer screening uptake in the UK for many people within South Asian communities that persist despite being first identified over a decade ago. 42 Design and evaluation of interventions to increase accessibility and awareness of bowel cancer screening are required and should incorporate verbal and interactive approaches in the appropriate language for the target community in order to address the limitations of written correspondence. An encouraging recent intervention study tested the provision of health promotion information either face to face or by telephone through general practices with a large minority ethnic population and found that these approaches led to significant improvements in the likelihood of bowel cancer screening uptake. 88 Approaches should further be delivered within community and faith settings in partnership with representatives from these settings, and be backed up with the use of local ethnic media. Given the role of children in mediating access to health for older people across South Asian communities, it may also be beneficial to raise general and practical awareness of the NHS BCSP across all age groups. Interventions involving GPs opportunistically offering screening to low uptake groups could be explored. Design and delivery of interventions may need to be further tailored to the distinct needs of specific South Asian minority ethnic and faith groups, for example, by devising ways to tackle the social stigma associated with cancer in the Sikh community.
Reasons for low uptake of bowel cancer screening are predominantly shared across South Asian faith communities. However, specific cultural issues such as non-written culture or social stigma surrounding cancer may further explain low uptake in individual faith groups. Strategies to increase access to screening should move away from the use of written messages mailed directly to individuals. Locally targeted efforts using verbal and face-to-face approaches delivered in community settings, and backed up with the use of local ethnic media, should be developed to enable people from South Asian communities to make an informed choice about what screening can offer.
Chapter 5 Workstream 2, intervention 1: developing and piloting a ‘gist’ leaflet
Introduction
The first ASCEND intervention to be evaluated nationally in workstream 3 involved the inclusion of a ‘gist’ leaflet with the invitation letter and materials sent out by the NHS BCSP. In this chapter, we will first describe the theoretical background to this intervention and we will then report how we carried out a think-aloud study, which describes how the current NHS BCSP materials are received and how we then developed the leaflet and tested it for comprehension with people with low levels of literacy. Finally, we provide details of a pilot study that tested if the leaflet would influence people’s intention to take part in screening. The results of the national RCT of the ‘gist’ intervention are reported in Chapter 10.
Theoretical background
Fuzzy trace theory (FTT) is a theory of judgement and decision-making. 89,90 It has recently been applied to the field of medicine and health91 and is a dual-processing theory which proposes that information is encoded into memory in two parallel forms: a gist representation and a verbatim representation. Gist representations are defined as vague, qualitative concepts capturing the bottom-line meaning of information. As such, they are subjective to the individual and affected by a range of different core values. In turn, these core values are influenced by factors such as emotional state, general world view, literacy and numerical ability. In contrast, verbatim representations are precise and quantitative and said to capture the surface form of the information (i.e. they are literal). For example, an individual reading ‘The Facts’ booklet would first read about the efficacy of gFOBt screening in reducing bowel cancer deaths. A verbatim representation of this would be ‘gFOBt screening reduces my chances of dying from bowel cancer by 16%’ and a gist representation might be ‘my chances of dying would be lower if I take part in gFOBt screening’.
Gist representations are formed along a continuum, which ranges from the simplest to most complicated. People exhibit a consistent preference to use the simplest form of gist available when making a judgement or decision89,92 particularly at older ages. 93 For example, in a sample of students and physicians making judgements on the cardiac risk of nine hypothetical patients, better discriminatory decisions resulted from processing information in an ‘all-or-none’ fashion, as opposed to weighing up several details at once. 94
Fuzzy trace theory argues that by presenting information in a format more closely aligned with preferred processing styles (i.e. gist), retrieval will be improved and the cognitive burden placed on the reader will be reduced. 95 This will be particularly true for people with lower levels of health literacy and numeracy. 96–98
It has therefore been suggested that pre-formulated gist-based information may improve gist extraction, reduce cognitive burden and improve public understanding of screening. 95 As such, FTT provides an elegant theoretical model on which to base a cancer communication intervention that aims to reduce socioeconomic inequalities in screening.
Study A: a ‘think-aloud’ study
A version of the ‘think-aloud’ study has been published in Smith et al. 99 This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
In order to inform the design and content of our ‘gist’ leaflet, we carried out a ‘think-aloud’ study to provide detailed commentary on how individuals process the information contained within the NHS BCSP ‘The Facts’ booklet. We set out to identify areas of the existing booklet (‘The Facts’) that were difficult to read, confusing to the reader or detrimental to motivation in order to quantify them within a typology of utterances. Furthermore, we sought to identify additional responses to the information using a more in-depth qualitative analysis.
Methods
Participant recruitment
We recruited participants through two community organisations: Social Action for Health (SAfH) and ContinYou. Additional individuals who had previously agreed to take part in studies with the University College London (UCL) Health Behaviour Research Centre Panel were also recruited. Individuals were purposively sampled from disadvantaged groups, because of the established link between literacy100,101 and CRC screening uptake. 12 A total of 21 eligible participants were identified and were sent an information sheet and consent form to be returned to the study team via a freepost return envelope.
Inclusion and exclusion criteria
Inclusion criteria were being aged 45–60 years (i.e. before the age at which CRC screening is offered in England) and no previous diagnosis of CRC.
The exclusion criteria were not being able to speak or read English, having previously participated in the NHS BCSP and having severe cognitive impairment.
These criteria were chosen to ensure that individuals were relatively naive to the processes of CRC screening and the accompanying information materials. Three participants completed interviews but were subsequently excluded because one was illiterate and two had cognitive impairments. Participants were paid £20 for their time and travel expenses. Interviews took place in the community or in university meeting rooms.
The think-aloud method
The ‘think-aloud’ method entails the verbalisation of a person’s thoughts that would normally be silent, while enabling the individual to continue with the primary task (such as completing a puzzle, calculating a mathematical sum or reading textual information). The verbalised thoughts represent the current contents of short-term memory, providing access to cognitive processes that occur during a task. 102 A recent meta-analysis has demonstrated that the method is empirically and conceptually distinct to introspection. 103,104
Our study used a ‘marked protocol’ in which participants were prompted to make a comment every time they encountered a small red dot in the leaflet. There were a total of 66 prompts that were placed by a researcher at the end of bullet points and short paragraphs (i.e. two short sentences). When lengthy paragraphs were included (i.e. two or three longer sentences), a prompt was placed after each sentence in the paragraph. A marked protocol was used in this study as this approach has previously been shown to elicit more instances of confusion and miscomprehension, which were primary aims of the study. 105
Participants were asked to complete a brief sociodemographic questionnaire (see Appendix 11) on arrival followed by the structured interview. In line with best practice for reporting think-aloud studies,103 the statement below was read to participants prior to beginning:
In this exercise we are interested in what you think about when you read information. In order to do this I’m going to ask you to THINK ALOUD as you read through some information. What I mean by think aloud is that I want you to tell me EVERYTHING you are thinking from the time you reach a red dot. I would like you to think aloud CONSTANTLY from the time you reach a red dot until you have finished telling me what you are thinking. I don’t want you to plan out what you say or try to explain to me what you are saying. You may want to make predictions about what you are reading, rephrase what you think the text is saying, share a story that describes something in the text that you’re familiar with, remark on something in the text that is confusing, or say something else that helps you understand the text you’re reading better. Just act as if you are in the room speaking to yourself. It is most important that you keep talking. If you are silent for any long period of time I will prompt you by saying ‘please carry on thinking out loud’.
Adapted from Ericsson and Simon102 and Crain-Thoreson et al. 105
Participants were asked to practise thinking aloud on a control leaflet (‘Recycle to Save the Environment’), which contained three prompts, before going on to read ‘The Bowel Cancer Screening Programme “The Facts” Booklet’ (see Appendix 3). After participants had completed three successful utterances, they were deemed ready to participate. If they did not reach this threshold during the practice session, the procedure was explained again and they were given additional time to practise.
Sample size
When determining the sample size for think-aloud studies, it has been argued that a single test subject yields up to one-third of usability problems, and after as few as five participants most issues are identified. 106 Therefore, we aimed to recruit a sample of 15–20 participants to ensure that the aims of our study were met. Saturation (i.e. when no new themes or information was gained after several consecutive interviews) was used as the marker at which recruitment ceased.
Measures of participant characteristics
The following participant characteristics were recorded: age, gender, marital status, first language, living arrangement, employment status, education level, screening history (women only) and experience with cancer.
Data analysis
Interviews were audio-recorded and transcribed. Occasions when participants deviated from the text (i.e. failed to read the text or misspoke) were coded as reading mistakes. After this, prompted and unprompted utterances (any statements made following a passage of text) were coded. Participants were not instructed to make unprompted utterances prior to starting the interview. However, there was author consensus that unprompted utterances, when made, were not substantially different from prompted utterances. All utterances were therefore collapsed and analysed together. Analyses were performed in NVivo 9 (QSR International, Warrington, UK).
We used a mixed-methods approach to analyse the data. First, a coding framework was developed in consultation with previous literature105 and the research team (Table 4). We then performed a content analysis, with utterances allocated to at least one theme. An utterance could be coded into several themes if deemed necessary; however, when possible, multiple coding was kept to a minimum. An utterance could also be split into several sections if the participant was discussing several aspects of the text. Two of the transcripts (> 10% of the data) were second coded by an additional researcher to assess inter-rater reliability. Reliability was found to be adequate to excellent (κ = 0.5–1.0).
| Name of theme | Description |
|---|---|
| Deep processing | An inference based on the text, which goes beyond repetition |
| Rephrasing of the text, which goes beyond repetition | |
| An anecdote which explains the text | |
| Surface processing | Repetition or very near repetition of the text |
| Self-reported learning | |
| Self-reported previous knowledge | |
| Miscomprehension | Confusion about a statement |
| An incorrect statement following a passage of text | |
| Asserts that factual information is opinion | |
| Emotional (negative) | A negative reaction with at least one emotion in the sentence |
| Person mentions the information makes them feel the opposite of a positive emotion | |
| Emotional (positive) | A positive reaction with at least one emotion in the sentence |
| Person mentions the information makes them feel the opposite of a negative emotion | |
| Unanswered questions | An individual has unanswered questions following a passage |
| Layout | An individual comments on the layout of the information |
| Unnecessary information | Comments that indicate the information is unnecessary |
| Decrease motivation | An individual remarks that something in the text would be demotivating to screening participation |
| Increase motivation | An individual remarks that something in the text would be motivating to screening participation |
In addition to the content analysis, we conducted an in-depth thematic analysis to provide insight into the subthemes contained within the framework. Thematic analysis is used to identify, analyse and report patterns (themes) within data. 107 Although the majority of the comments were brief and provided little insight past surface-level meaning, a thematic analysis allowed exploration of deeper-level meanings of some comments. We used this approach because the aims of the study were to extract general perceptions about ‘The Facts’ booklet, rather than understand individual experience with the information. To increase the validity of the thematic analysis, two researchers were responsible for analysing the transcripts and identifying and categorising themes within each interview. These themes were analysed across all transcripts using the constant comparison method. 108 To increase the validity further, the wider study group was responsible for suggesting alternative themes within the data and asked to assess whether or not the suggested themes were adequately represented by the quotations.
Results
Participant characteristics
A total of 18 participants [mean age 55 years (range 48–60 years)] took part. As indicated in Table 5, the sample was mixed. Participants predominantly spoke English as a first language and were of white ethnicity: their level of education was mixed, and most had experience of cancer in some form.
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 7 (39) |
| Female | 11 (61) |
| Marital status | |
| Married/living with partner | 6 (33) |
| Single/divorced/separated | 9 (50) |
| Widowed | 3 (17) |
| English as first language | 18 (100) |
| Living arrangement | |
| Own home/mortgage | 9 (50) |
| Renting/other | 9 (50) |
| Employment | |
| Currently employed | 10 (56) |
| Unemployed/disabled or too ill to work | 6 (33) |
| Retired | 2 (11) |
| Education | |
| ≤ GCSE or O-level | 4 (22) |
| > GCSE or O-level | 14 (78) |
| Health literacy | |
| Adequate | 16 (89) |
| Inadequate/marginal | 2 (11) |
| Ethnicitya | |
| White | 15 (83) |
| Non-white | 2 (11) |
| Cancer experienceb | |
| 0 | 2 (11) |
| 1 | 13 (72) |
| 2 | 3 (17) |
| Breast screening historyc | |
| Yes | 11 (100) |
| No | 0 (0) |
| Cervical screening historyc | |
| Yes | 10 (91) |
| No | 1 (9) |
Content analysis
In the 18 interviews, 270 reading mistakes were recorded (mean 15 per person; range 0–59). The interviews yielded 776 coded utterances (mean 43.1 per person; range 8–95), which were analysed within the predetermined framework.
There was substantial variation in the types of comments made by participants (Figure 4). The comprehension theme was largely made up of comments that implied higher-level understanding (i.e. deep processing, 17.9% of all comments) or repetitions of the text and unsubstantiated self-reported knowledge (i.e. surface processing, 15.2%).
FIGURE 4.
Typology of participant utterances. Reproduced from Smith et al. 99 This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Responders were significantly more likely than non-respondents to be female [χ2(1) = 16.09; p < 0.001], older [t(4401) = 6.16; p < 0.001], from an affluent neighbourhood [χ2(1) = 115.07; p < 0.001] and be in a home with two or more invitees [χ2(1) = 4.05; p = 0.044].
Miscomprehension was less common (6.2%); however, this still amounted to 48 instances of mistakes or self-reported lack of understanding. There were a high number of comments in the emotional theme. Emotionally negative statements were three times more common than emotionally positive statements (18.0% and 5.7%, respectively). The information preferences theme suggested that people desired further information on specific aspects of the booklet (unanswered questions: 15.2%), while others suggested improvements to the style and layout of the booklet (layout: 13.1%). A minority of statements questioned the necessity of certain information that they had just read (unnecessary information: 4.8%) and utterances rarely alluded to whether the participant felt motivated (1.4%) or demotivated (2.5%) by information in the booklet.
Thematic analysis
Difficulties with numerical information
The use of numbers to convey risk information in ‘The Facts’ booklet was common, which participants often considered to be unnecessary. For example, one participant preferred to think categorically about the efficacy of screening to reduce CRC deaths (i.e. anything is better than nothing), rather than in verbatim terms (i.e. a 16% reduction):
I know we have to have . . . the evidence and that, but I think if I hadn’t done research myself . . . I would just find that got in the way really. This thing about 16%. What’s 16%? What does it mean to the person on the street? I know anything is better than nothing for reducing the risk of dying, but surely it should be a lot more percentage than that, but is it something that I want to know about?
QE, 50 years, female, degree-level education
The use of numerical information to convey the lifetime incidence of CRC as 1 in 20 led to confusion. For example, one participant overestimated the likelihood of being diagnosed with CRC as a result of an information-processing error:
That’s about, yeah, that’s one in four of the population isn’t it?
IT, 51 years, male, higher educational qualifications
The prevalence of screening outcomes proved difficult to interpret. The booklet explains that, following a gFOBt, approximately 98 out of 100 individuals will receive a normal result (no blood found), 4 out of 100 will receive an unclear result (a small amount of blood), and 2 out of 100 receive an abnormal result (blood was found, further investigation is required). However, there was confusion as to whether or not the normal prevalence figure includes those who have previously received an unclear result:
Does that equate with the 98 out of 100 in the previous paragraph? Something, somewhere doesn’t seem quite. Four people out of 100 and then we had 98 out of 100, anyway, not quite sure about that.
WW, 56 years, female, degree-level education
As with the gFOBt kit results section, colonoscopy outcomes were misinterpreted. The booklet explains that 1 person out of 10 will be diagnosed with cancer, 4 people out of 10 will have a polyp removed and 5 people out of 10 will have nothing found. In this instance, the participant appears to discount the number of people receiving a polyp diagnosis, thus overestimating the prevalence of cancer following an abnormal gFOBt kit result:
Half of people that go for these colonoscopes [sic] don’t have cancer? And the other half do? Hmm.
IT, 51 years, male, higher educational qualifications
Unfamiliar topics and complex terminology
Participants questioned whether or not it was necessary to have such a long and complex booklet to inform people about the screening programme:
This is an awful lot for people to read, this is just handed out? Hell of a lot to read isn’t it?
OU, 54 years, female, degree-level education
Comments were often made about the introduction of unfamiliar topics and scientific terminology:
A bit difficult to understand, if you’re not up to date with those kind of informations.
RT, 58 years, female, no formal qualifications
Participants argued that a leaflet that aims to provide complex and technical information would benefit from the use of vernacular language as opposed to scientific terminology:
. . . I would prefer a more high-level definition of what the bowel is actually. This just seems to provide too much detail . . .
SM, 51 years, male, degree-level education
There was also difficulty when describing the difference between the possible outcomes of a gFOBt. Despite the bold text within this paragraph describing the exact meaning of abnormal, it was easily misinterpreted as the definite identification of a malignancy or polyp:
So that’s good, it gives you all of the different results of the testing . . . normal, you’re not going to have any more tests for 2 years. If it’s unclear you have another one to make sure it’s nothing suspicious and if it’s abnormal you’ve definitely got something that needs further investigation.
CW, 56 years, female, degree-level education
This and other complex areas of the booklet were improved by the provision of summary boxes and diagrams. To improve the booklet further, it was recommend that, when technical phrases are introduced, the most familiar word should be used first and the more technical phrase included within brackets that follow:
I’m wondering sometimes with these things whether it isn’t better to have the common word before the technical, so piles (haemorrhoids), just because seeing those words that are hard to pronounce can put you off.
OU, 54 years, female, degree-level education
Emotional responses
As demonstrated by the quantitative analysis, there was a mixture of emotionally negative and positive comments. For example, some participants found the scientific explanations of cancer interesting and somewhat reassuring:
Yeah that’s interesting, I’ve never really known an awful lot about cancer, and how it spreads and what happens so that again seems to make it quite sensible and slightly not too scary. Because obviously everybody talking about cancer, everybody gets very ‘the big C’.
WW, 56 years, female, degree-level education
Despite the reassurance offered by these explanations, the colonoscopy risk information frequently led to negative emotional responses. In particular, the risk of death (1 in 10,000) led some to question why this may occur:
Oh oh that is shocking. That is shocking. I’d like to know more, now that’s been said . . . what on earth would they have had to do for that to happen – whether a heart attack, or a shock to the body or you perforate the liver or something that’s vital to keep you alive.
CW, 56 years, female, degree-level education
Others questioned the necessity of including such information, preferring instead to supply it on a ‘need to know’ basis or in a less prominent position:
I’d write it in small and I’d write it at the end . . . It wouldn’t be something massive, I don’t think it, anything put there to make people more worried about the procedure, the procedure’s complicated enough.
JS, 52 years, male, A-level education
The nature of the test was often considered to be distasteful:
Yeah I think that probably, there’s nothing else you can do about it but it is rather embarrassing and unpleasant.
BD, 56 years, female, no formal qualifications
One individual commented that the description evoked unpleasant images about the procedure that may induce aversion to participation:
OK, yeah, wipe the samples on a special card . . . I’m getting a bit unpleasant mental images of that procedure.
SM, 51 years, male, degree-level education
Summary
This think-aloud study of 18 adults who were naive to CRC screening explored how people interpret the current information booklet that is provided to invitees of the English NHS BCSP (‘The Facts’; see Appendix 3). Despite the extensive testing process the information went through,109 and its approval by the plain English campaign, our study suggests that it may not always meet the information needs of some older adults. Furthermore, this gap in understanding is not filled by health-care professional counselling, suggesting that communication inequalities may be created through the introduction of home-based organised screening programmes. 110
The study enabled us to successfully identify specific areas of ‘The Facts’ booklet that were difficult to read, confusing to the reader and detrimental to motivation to be screened. We also observed strong emotional responses to some aspects of the screening process. We were able to address some of these findings in further adaptations of the gist leaflet, with the consideration that this may aid knowledge translation and reduce the cognitive burden placed on individuals when deciding whether or not to take up the offer of CRC screening.
Designing the ‘gist’ leaflet
The Department of Health policy for screening communications endorsed in the UK cancer strategy is to ‘empower the greatest number possible . . . to make an informed choice to participate in cancer screening’ (© Crown copyright 2010; contains public sector information licensed under the Open Government Licence v3.0). 111 We therefore needed to ensure that the gist leaflet would be accommodated within this policy context. 112 The NHS BCSP specified that our leaflet must be provided in addition to, rather than instead of, the existing booklet. Although this is not the ideal situation according to FTT, it represents a compromise for using psychological theory within the constraints of an organised health-care system.
Consultation of best practice
Best practice guidelines from the fields of information design, cognitive psychology and health literacy were used to complement a theory-based approach during the design phase. 113–115
Expert input
Prior to starting the design process, we interviewed 11 SSPs to discuss their views on the development of the gist leaflet. These interviews focused on the following areas: (1) what parts of ‘The Facts’ booklet they felt members of the public found difficult to understand, (2) what information contained in ‘The Facts’ booklet they felt was essential to know prior to making a decision about bowel cancer screening participation, (3) how acceptable they found the idea of providing supplementary information to the public as part of their invitation and (4) whether or not they had any advice on ways in which to provide this information.
During development of the leaflet, members of the research team with expertise in health services research, epidemiology, public health, behavioural science, decision-making, communication and literacy provided continual feedback. This panel met frequently throughout the design process, using their specific area of expertise to make suggestions in response to public feedback.
In addition, a version of the leaflet was presented at the ASCEND advisory group meeting in September 2012. This provided an opportunity to receive feedback during the design phase and ensure that the leaflet would be acceptable to those administering it (i.e. the hub directors) as well as the target audience.
Factors considered in the design of the leaflet
The following is a description of the factors that were considered in the design of the gist leaflet.
Numerical information
Evidence suggests that attempts to encourage further understanding of risk information through the provision of numbers may be misguided. 116 The empirical evidence to support the provision of numerical information for improving medical decision-making is scarce. Furthermore, it is possible that the provision of excessive numerical information can ‘hurt rather than help’ this process. 92,117–121 Specifically, in a bowel cancer screening context, it has been shown to increase the prevalence of negative attitudes about bowel cancer screening. 122 At the same time, it is important that information is not so oversimplified that it is no longer accurate or fails to enable people to make an informed decision about screening. 123 Concerns that simplifying health information might disadvantage certain groups are alleviated by the finding that low literacy messages can improve knowledge even among more educated samples. 124
To overcome difficulties with processing numerical information, we attempted to encourage gist-based processing by providing a verbal description (i.e. gist) of the number [e.g. ‘most people (98 out of 100)’]. This approach has been used successfully in previous research. 125–127 Findings broadly indicate that comprehension of the information is improved, particularly for people with low numeracy. 128 Furthermore, the same study suggested that evaluative categories can increase deliberative processing of the numerical information. Numerical descriptors may also increase perceptions of risk and, as a result, may be more effective at altering behaviour than numerical information in isolation. 127 In line with current evidence, natural frequencies with the same denominator were used to present key numerical information. 129
Reduction of concepts
In keeping with the ‘less-is-more’ approach, the leaflet was designed to encourage gist-based processing by removing specific concepts which were deemed ambiguous in the think-aloud study. This resulted in four pages of text being used for the gist leaflet, compared with 15 pages in ‘The Facts’ booklet. An example of information that was streamlined was the role of gFOBt screening in preventing bowel cancer (by removing polyps detected at follow-up colonoscopy). This was justified because of the unconvincing evidence that gFOBt-based screening reduces bowel cancer incidence. 130 The leaflet therefore focused on the primary mechanism by which gFOBt screening works: the early detection of colorectal adenomas. A further example of streamlining was the removal of academic references from within the text to accommodate the preferences of people with low health literacy. 131
After consultation with the wider research team, a decision was reached to remove any mention of ‘unclear’ results. This decision was made because ‘unclear’ was considered confusing to the reader in the think-aloud study, without any additional benefit of its inclusion. Its removal also fits with providing information in the simplest gist format (i.e. nominal), without overlapping categories. 91
Navigation
Guidelines on the layout of health information designed for groups with low health literacy suggest providing essential information at the beginning of the text. 115 This has been shown to improve comprehension and decision-making. 121 To identify what was considered to be essential information, we searched the relevant literature to identify aspects of screening that are considered essential to make an informed decision. 112,132 We also drew on the interviews with the SSPs to inform this process.
Information that was deemed essential to making a screening decision was presented on the front page. This included (1) the prevalence of the cancer, (2) how the test works, (3) the efficacy of the test and (4) who is invited. To avoid the front page becoming too dense with information, additional essential information that could not be explained succinctly (i.e. in a single sentence) was contained in subsequent pages. This information included (1) the disadvantages of screening, (2) the possible outcomes, (3) practical aspects of screening and (4) where more information could be found.
After providing the essential information on page 1, we aimed to improve the navigability of the information by providing ‘sign-posting’ to direct the reader to the location in the leaflet where more detailed information could be found (i.e. pages 2 and 3). 133 Page 4 was devoted to ‘sign-post’ other information sources (i.e. Bowel Cancer Screening: The Facts). As such, the booklet was designed to be a cascade of information formats ranging from the simplest gist-based information through to more detailed information for those who wanted it.
Language
Health literacy, European Union and NHS guidelines suggest that vernacular rather than formal language should be used when possible in cancer communication materials. 114,115,123,134 The use of words with multiple definitions (e.g. spot) may be confusing for the reader; however, this was accounted for by testing the comprehensibility of the leaflet (described in Study B: user testing the ‘gist’ leaflet). These guidelines also recommend that information should be written in short sentences and bullet point lists. Evidence from cognitive psychology suggests that this reduces the cognitive burden of information by enabling participants to ‘chunk’ information and retain more in short-term memory. 135,136 This is particularly important for people with poor basic skills owing to the strong association between health literacy and cognitive ability. 137 Importantly, reducing the cognitive burden of information can increase subsequent recall and this is apparent for all health literacy groups. 138
Aesthetic appeal
European Union guidelines suggest that information materials should be appealing to the recipient. 134 The aim of this is to encourage engagement and processing of the information and reduce immediate defensive reactions such as avoidance. In response to the guidelines, a blue background was used because experimental evidence has demonstrated that it invokes a lower disgust response,139 which is a frequently cited barrier to bowel cancer screening participation. 50,140–142
The research team produced a version of the ‘gist leaflet’ which aimed to provide a simple summary of the essential information required to understand the offer of bowel cancer screening and to address issues around non-uptake (see Appendix 12). In order to test the comprehensibility of the leaflet, we carried out a series of user tests, described below in Study B: user testing the ‘gist’ leaflet.
Study B: user testing the ‘gist’ leaflet
A version of the user testing study has been published in Smith et al. 143 © 2013 The Authors. Published by Elsevier Ireland Ltd. Open access under CC BY license.
Methods
Recruitment of participants
We recruited 28 participants, some of whom were recruited via postal invitation through two of our charity partners, SAfH and ContinYou. SAfH is a non-governmental organisation involved in health promotion within disadvantaged areas of London and ContinYou is an adult education organisation that works with children and adults in deprived communities across the UK. We also recruited participants from the established research panel in the Health Behaviour Research Centre, Department of Epidemiology and Public Health, UCL.
By recruiting participants through community organisations, we were able to target and include the perspective of individuals who may struggle to access and use health information owing to limited health literacy and numeracy skills. A number of barriers exist to the recruitment of such individuals and we were mindful of these in our approach. 144
We used a mixed-methods, user-testing approach to assess the comprehensibility of the information leaflet. 145–147 In rounds of approximately 8–10 people at a time, we identified problems with the gist-based leaflet. Both quantitative (face-to-face administered questionnaire) and qualitative (brief semistructured interview) methods were used to achieve this purpose. Retesting assessed the impact of revisions on a new set of participants and was repeated as necessary.
Inclusion and exclusion criteria
Inclusion criteria were people aged 45–59 years (i.e. before the age at which bowel cancer screening is offered in England) and with no previous diagnosis of bowel cancer.
Exclusion criteria were not being able to speak or read English, having previously undergone bowel cancer screening and having severe cognitive impairment.
The study was approved by the UCL research ethics committee (reference 2247/002).
Process of user testing
Participants were asked to complete a brief sociodemographic questionnaire on arrival, followed by a standard health literacy assessment. They read through the gist-based leaflet for as long as they wanted and completed a researcher-led comprehension test. The participant had access to the gist-based leaflet at all times. This was followed by a brief (5- to 10-minute) semistructured interview at which participants were asked to comment on the following: overall impressions of the leaflet, use of language, order of information, use of headings, use of the word ‘poo’ within a health context, missing information, size of the print, ways to simplify the information and any other changes that they would like to see.
Measures of participant characteristics
Participants were asked to provide the following information: age, gender, marital status, first language, employment status, education level, amount of experience with written documents and whether they had a previous cancer diagnosis or knew someone else that has been diagnosed with cancer. (For the sociodemographic questionnaire, see Appendix 11.)
Health literacy was assessed using the UK version of the Test of Functional Health Literacy in Adults (UK-TOFHLA)100 which has numeracy and literacy sections. The numeracy section involves tasks relating to date and time calculation, computation of medication dosage, and patient navigation, and this section takes approximately 10 minutes to complete. The literacy section is based on the ‘cloze’ procedure. Three passages of text (instructions on how to prepare for an X-ray, eligibility for NHS prescriptions and a consent form for surgery) of increasing difficulty are given to the participant and every fifth word is missing. When a word is missing, a blank line is drawn and four possible words that could be used are provided. This section takes approximately 12 minutes to complete. A score of 100 points is calculated, with each section having a maximum score of 50 points. Scores are converted into three groups: inadequate (0–59 points), marginal (60–74 points) and adequate (75–100 points) health literacy. 148
Tested materials
The Flesch Kincaid formula149 was used to calculate the reading ease of the gist-based leaflet. Scores range from 0 to 100 points, with higher scores indicating greater reading ease. The readability scores for version 1, 2 and 3 were 82.1, 79.4 and 81, respectively. This corresponded to a US grade level of 4–5 (equivalent to age 9–10 years).
Outcome
The primary outcome was the percentage of participants correctly responding to eight true or false statements about bowel cancer and bowel cancer screening. In line with European guidelines for medicinal package testing,150 each statement had to be answered correctly by at least 80% of participants for our leaflet to be deemed legible, clear and easy to read.
Data analysis
We calculated the total number of individuals who answered each statement correctly (statement totals) as well as the mean number of statements correctly answered per participant (individual totals). Data from the semistructured interviews were digitally recorded, transcribed verbatim and analysed using thematic analysis, which is a qualitative technique for identifying patterns (themes) within data. 107 The purpose of the thematic analysis was to pin-point the particular areas of the gist-based leaflet that caused difficulties with comprehension.
Results
Participant characteristics
The majority of participants were female (75%), employed (54%), and white (54%), had a General Certificate of Secondary Education (GCSE) level of education or below (57%), were adequately literate (82%), were without a partner (68%), spoke English as a first language (75%) and had either received a cancer diagnosis themselves (11%) or knew someone that had (82%). The majority had used written documents in their current of previous employment at least some of the time (75%). As rounds progressed, more individuals had a lower level of education, marginal or inadequate health literacy scores, spoke English as a second language or were from a minority ethnic group.
Round 1
As demonstrated in Table 6, the majority of the statements were answered correctly by at least 80% of participants. However, two statements {‘The FOB [faecal occult blood] test is done at home’ (true) and ‘People with an abnormal result always have cancer’ (false)} were answered correctly by < 80% of participants. At an individual level, participants were able to answer a mean of 7.2 out of 8 statements correctly (range 5–8).
| True/false statement | Round | ||
|---|---|---|---|
| 1: correct n (%) | 2: correct n (%) | 3: correct n (%) | |
| 1. Doing the FOB test lowers the risk of dying from bowel cancer (true) | 6 (100) | 11 (100) | 11 (100) |
| 2. The FOB test is done at home (true) | 4 (67) | 10 (91) | 9 (82) |
| 3. Most people who do the FOB test will receive an abnormal result (false) | 5 (83) | 9 (82) | 9 (82) |
| 4. Only women are sent a FOB test (false) | 6 (100) | 11 (100) | 11 (100) |
| 5. Bowel cancer is a common cancer in people over 60 (true) | 6 (100) | 10 (91) | 10 (91) |
| 6. People only need to do the FOB test once in their life (false) | 6 (100) | 10 (91) | 11 (100) |
| 7. The FOB test can miss bowel cancer (true) | 6 (100) | 9 (82) | 9 (82) |
| 8. People with an abnormal result always have cancer (false) | 4 (67) | 8 (73) | 9 (82) |
In response to the threshold not being met for the statement that ‘the FOB test is done at home’, we changed the sentence ‘A FOB test kit with instructions is sent through the post’ to ‘A FOB test kit with instructions is sent through to the home’ in order to clarify where the test was completed.
More than 20% of individuals did not correctly answer the statement that an abnormal test result does not necessarily mean cancer has been found. One participant commented that:
I do wonder about the fact that if you have an abnormal test that it doesn’t necessarily indicate that you’ve got cancer. That’s inferred but it doesn’t necessarily say that.
AL, 55 years, female, degree-level education
To improve comprehension of the meaning of an abnormal result, we added the following sentence: ‘An abnormal result does not always mean cancer has been found’. Our interviews demonstrated that the language used was easy to understand for the audience:
It’s quite well set out, and it’s readable and gives you basically all the information.
WG, 58 years, female, no formal qualifications
However, further changes were identified by participants that could make it more accommodating for low literacy groups:
There were a couple of words in it that I thought might need thinking about . . . ’discuss’, I wonder whether ‘talk about’ would be more appropriate?
AL, 55 years, female, degree-level education
Changes were also made to the spacing between and within lines to improve readability.
Round 2
Nearly all statements were answered correctly by at least 80% of the participants (see Table 6); however, the statement on the meaning of an abnormal result remained problematic [‘People with an abnormal result always have cancer’ (false)]. At a participant level, a mean of 7.1 out of 8 statements were answered correctly (range 4–8).
Changes to the layout of the leaflet were made in response to difficulties with participants remembering all of the information that they had just read:
I think it’s OK, but it’s remembering what you read. If you read something and don’t remember, it doesn’t do you any benefit does it?
DW, 52 years, female, no formal qualifications
Changes included placing boxes around text that related to each subheading, reducing the number of bullet points on the final page, changing the colour of the background and increasing the size of the font on the front page to increase the readability of the text for individuals with eyesight difficulties.
It’s very clear. Maybe I would say, it could be done in more bigger letters, you know if somebody’s old or something.
SF, 51 years, female, no formal qualifications
These changes were particularly apparent on the final page, which assisted participants when searching for the correct answer to the statement that did not meet the threshold. The text relating to the statement ‘For most people, the follow-up test will show there is no bowel cancer’ was altered in an attempt to improve comprehension. Participants reported being confused about the age of eligibility for screening. To reduce this confusion, sentences discussing the age extension were removed:
That’s all clear and it’s explained further, all very simple. But this I couldn’t get [age extension]. That’s like a random statement. It’s not really backed up or [explained] why.
VY, 45 years, male, advanced high school qualifications
Participants also wanted reassurance that the test was simple, as some felt that it might be complicated and that people may be less likely to participate as a result. This resulted in changes to the text regarding the age that people are invited to screening, as well as an additional sentence highlighting ‘The FOB test is easy to do’. The title of the booklet (‘A two minute guide’) was changed to ‘The essentials’ as the former may have been perceived as intimidating by less literate and slower readers.
This is meant to be a 2-minute guide. Well people read at their own pace and you know they might think well, oh. A simple guide? Or is that being patronising . . . or the essentials?
FV, 55 years, female, degree-level education
Finally, the full title of the FOB test was added in response to comments questioning the phrase, FOB test:
I think the only thing is, FOB, what does that stand for?
WF, 58 years, male, no formal qualifications
Round 3
As demonstrated in Table 6, all statements were answered correctly at least 80% of the time. The predefined threshold was therefore met and the leaflet was considered fit for purpose. At a participant level, individuals were able to answer a mean of 7.2 out of 8 statements correctly (range 6–8).
Summary
In this study we user tested our ‘gist-based’ CRC screening information leaflet, which promotes comprehension of the screening offer. We used principles of FTT and best practice guidelines from the fields of information design, cognitive psychology and health literacy to provide accessible information about the aims, benefits and disadvantages of the English NHS BCSP. Readability scores indicated that the leaflet was suitable for individuals with low literacy (e.g. reading age of 9–10 years) and may therefore increase the accessibility of the programme to disadvantaged groups. User testing indicated that the leaflet was well comprehended in all rounds and, after three rounds of testing, the pre-defined threshold was reached and the leaflet was considered fit for purpose. The final version can be seen in Appendix 13.
Study C: a pilot study to evaluate the ‘gist’ leaflet
A version of the gist evaluation RCT has been published in Smith et al. 151 Parts of this text have been reproduced from Smith SG, Kobayashi LC, Wolf MS, Raine R, Wardle J, von Wagner C. The associations between objective numeracy and colorectal cancer screening knowledge, attitudes and defensive processing in a deprived community sample. J Health Psychol 2016;21:1665–75,151 with permission from Sage.
We used a RCT design to compare sociocognitive outcomes with the ‘gist’ leaflet as a supplement to standard information (gist + facts) and standard information alone (facts). Interactions with levels of numeracy were also examined. In keeping with FTT, we hypothesised that the ‘gist’ leaflet would increase knowledge and screening intentions and that the difference between conditions would be stronger among low-numeracy individuals.
Methods
A multicentre parallel randomised trial design was used. Participants were allocated to two groups (‘facts only’ or ‘gist + facts’) on a 1 : 1 allocation ratio.
Participants and setting
General practices in the North of England were identified. Using the IMD (a neighbourhood deprivation score based on several socioeconomic markers), three practices in deprived areas and one practice in an affluent area were recruited. IMD is a well-validated marker of socioeconomic status and is linked to bowel cancer screening uptake. 54,152,153
Inclusion and exclusion criteria
Staff at the general practices produced a list of all men and women aged between 45 years and 59.5 years. This age group would not yet have been invited to bowel cancer screening and, therefore, had no direct experience with the procedure or the information materials. GPs were invited to exclude patients who had severe cognitive impairments, were vulnerable (e.g. had had a recent diagnosis of cancer or other significant illness), were under bowel cancer surveillance or were registered as not speaking English.
Randomisation and blinding
Eligible patients at each practice were randomised to intervention or control groups, with all members of a household allocated to the same study group to limit contamination. Random number generation software (version 1.0; Random Allocation Software, developed by M Saghaei, Department of Anesthesia, Isfahan University of Medical Sciences, Isfahan, Iran) was used to generate a restricted randomisation sequence for participant group allocation. Blocking was used to ensure evenly balanced group sizes, which limits the unpredictability of randomisation; however, this bias was reduced by the use of random blocks. 154 A researcher posted the study materials from the practice. Group allocation was not concealed at any stage after the random sequence was generated; it was not possible to be blind to the group allocation at data entry or analysis stages because some questions were included for only one study group. Participants were not aware of a comparator group. Randomisation occurred prior to consent, which was assumed based on the return of a completed questionnaire.
Study groups
In the ‘facts-only’ group (control), each participant was provided with a study invitation letter from their GP, a questionnaire and an example ‘screening pack’ consisting of a NHS-marked envelope with a mock NHS screening invitation letter (watermarked ‘example’) and the standard patient information booklet (‘Bowel Cancer Screening: The Facts’). The packs were as similar as possible to a real screening invitation to increase ecological validity of the study. Reminders were sent to non-responders after approximately 3 weeks.
The ‘gist + facts’ group (intervention) were sent the same as above as well as the ‘gist’ leaflet. The questionnaire was a little longer because it also contained questions relating to the ‘gist’ leaflet (see both questionnaires, in Appendices 15 and 16).
Outcome measures
Outcome measures included level of gist knowledge, degree of intention to be screened, level of numeracy and acceptability of study materials, as well as a range of demographic characteristics (i.e. age, gender, marital status, ethnicity, employment status, level of education).
Sample size
This study aimed for a 5% difference in intention between the study groups. To detect this size of effect (Cohen’s w = 0.12), 818 respondents were needed assuming 80% power and p = 0.05.
Analysis
Respondents were compared with non-respondents using data that had been provided by the participant’s GP on gender, age, deprivation and number of people in the household, using chi-squared tests and t-tests, as appropriate.
Analysis included all individuals returning a questionnaire with primary or secondary outcome data. The extent to which participants read the assigned information materials was monitored using descriptive statistics and chi-squared tests. Study outcome variables were described using means, standard deviations (SDs) and percentages, when appropriate. Differences between intention and gist knowledge between the study groups were assessed using the chi-squared test. Independent t-tests were used to test for differences between study groups on the perceived risk and bowel cancer worry items. To investigate condition by numeracy interactions, logistic regression (LR) was used. Data were analysed using Statistical Product and Service Solutions (SPSS) version 21 (IBM Corporation, Armonk, NY, USA).
Missing data
Missing data for outcomes (except gist knowledge) were considered to be missing at random, justifying the use of pairwise deletion. Missing gist knowledge data were considered to be missing not at random if the individual had responded to at least five items out of a possible nine. Individuals who answered fewer than five items (n = 6) were excluded for all gist knowledge outcomes. Missing data for the remaining individuals were dealt with by transforming total scores to account for the number of items that participants responded to, which enabled participants with a small number of missing data to be allocated a score from 0 to 9. In total, 10.5% of the numeracy data were missing. These data were also considered to be missing not at random. We coded missing numeracy data as incorrect but sensitivity analyses were carried out, excluding individuals with missing data and yielded similar results.
Results
The study ran between July 2012 and March 2013, with questionnaires returned up to May 2013 (Figure 5). Individuals (n = 4452) were randomised by household (n = 3706), with 2216 allocated to the ‘facts’ group and 2236 to the ‘gist + facts’ group (Table 7). A total of 3631 (81.6%) individuals were sent a reminder [the ‘facts-only’ group = 1808 (81.6%); the ‘gist + facts’ group = 1823 (81.5%)] approximately 3 weeks after the initial invitation (median 22 days, range 22–41 days).
FIGURE 5.
Numbers of participants in control ‘facts-only’ and intervention ‘gist + facts’ groups.
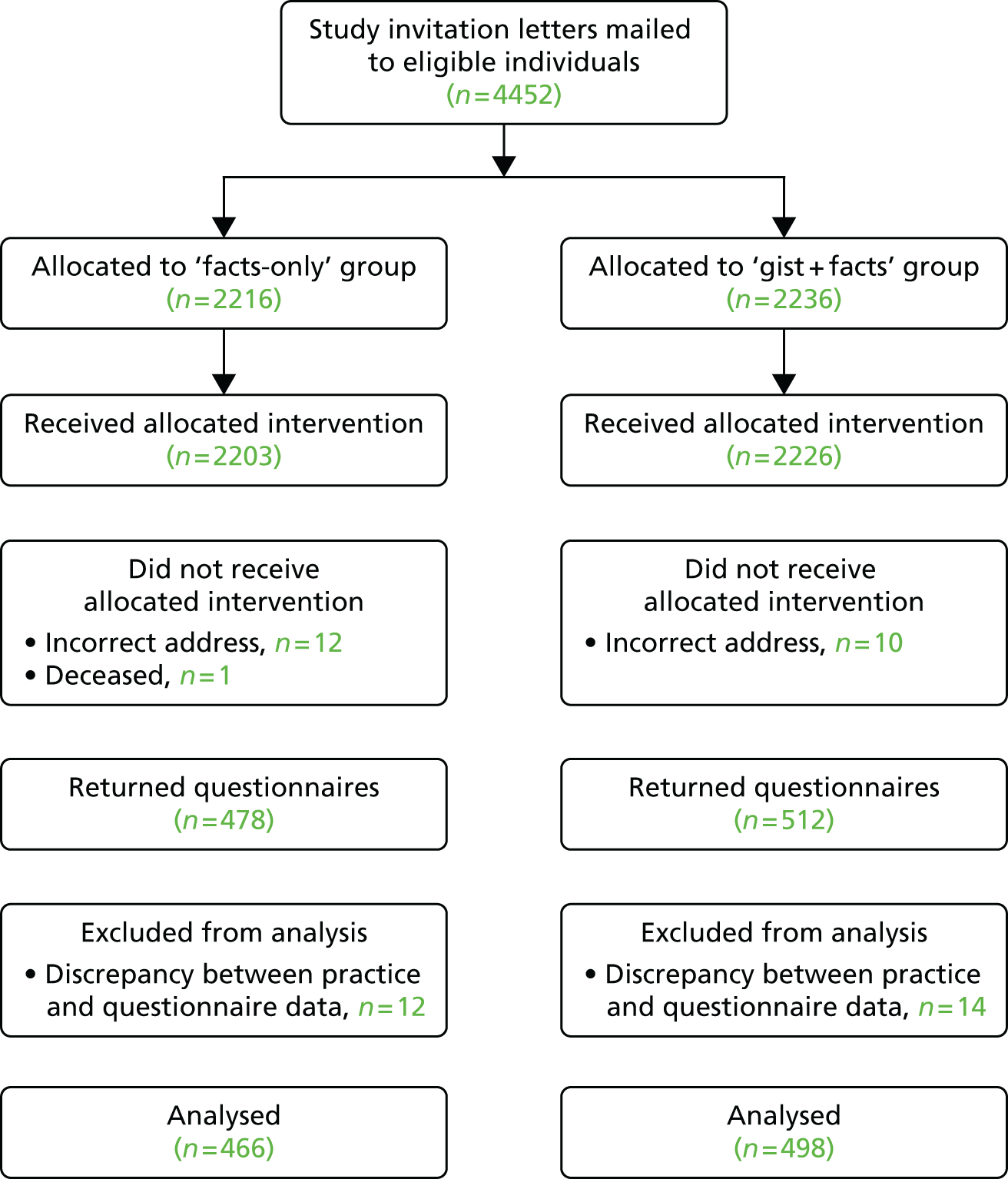
| Characteristics | All (%), n = 4452 | ‘Facts-only’ group (%), n = 2216 | ‘Gist + facts’ group (%), n = 2236 |
|---|---|---|---|
| Gender | |||
| Male | 2420 (54.4) | 1194 (53.9) | 1226 (54.8) |
| Female | 2032 (45.6) | 1022 (46.1) | 1010 (45.2) |
| Number in household | |||
| 1 | 2984 (67) | 1476 (66.6) | 1508 (67.4) |
| 2 | 1400 (31.4) | 714 (32.2) | 686 (30.7) |
| 3 | 60 (1.3) | 22 (1.0) | 38 (1.7) |
| 4 | 8 (0.2) | 4 (0.2) | 4 (0.2) |
| IMD score quintiles | |||
| 1 (low deprivation) | 996 (22.4) | 473 (21.4) | 523 (23.5) |
| 2 | 794 (17.9) | 412 (18.7) | 382 (17.1) |
| 3 | 930 (21.0) | 462 (20.9) | 468 (21.0) |
| 4 | 834 (18.8) | 420 (19.0) | 414 (18.6) |
| 5 (high deprivation) | 884 (19.9) | 441 (20.0) | 443 (19.9) |
| Age | 51.1 (4.1) | 51.2 (4.1) | 51 (4.2) |
Respondents were excluded from analysis if questionnaire data on age and gender did not match GP records (n = 26) or if the study materials were not delivered as intended (n = 23). Exclusions were approximately equal for both study groups. A sample of 4403 individuals remained and this figure was used as the denominator for calculating a response rate. A total of 1269 questionnaires were returned, of which 964 were completed and provided outcome data, giving a response rate of 21.9%. The response rate varied between the practices (Manchester, 13.0%; Liverpool a, 18.1%; Liverpool b, 19.6%; Stockport, 31.8%) and these differences were statistically significant [χ2(3) = 128.76; p < 0.001].
Questionnaire data indicated that a high proportion of participants were married (66.9%), white (83.8%) and in employment (72.2%) and had either some formal education (49.9%) or a degree-level education (36.5%) (Table 8). The sample was well distributed by gender (51.4% female) and age group (45–49 years, 32.7%; 50–54 years, 34%; 55–59 years, 33.3%). Despite the educated sample, a high proportion answered the numeracy item incorrectly (35.3%) or did not provide an answer (10.5%).
| Characteristics | n (valid %) |
|---|---|
| Gender | |
| Male | 466 (48.6) |
| Female | 493 (51.4) |
| Age (years) | |
| 45–49 | 313 (32.7) |
| 50–54 | 325 (34.0) |
| 55–59 | 319 (33.3) |
| Marital status | |
| Married | 640 (66.9) |
| Unmarried | 317 (33.1) |
| Ethnicity | |
| White | 799 (83.8) |
| Black | 42 (4.4) |
| South Asian | 58 (6.1) |
| Other | 55 (5.8) |
| Education | |
| No formal education | 128 (13.6) |
| Some formal education | 471 (49.9) |
| Undergraduate or higher | 345 (36.5) |
| Employment status | |
| Employed | 689 (72.2) |
| Unemployed | 95 (10.0) |
| Full-time homemaker | 44 (4.6) |
| Retired | 37 (3.9) |
| Student | 5 (0.5) |
| Disabled | 84 (8.8) |
| Numeracy | |
| Correct | 523 (54.3) |
| Incorrect | 340 (35.3) |
| Missing | 101 (10.5) |
Respondents had a high level of knowledge (mean 7.70, SD 1.74 out of a possible 9) and a large proportion (93.1%) were classified as having ‘adequate’ gist knowledge. Individuals in the ‘gist + facts’ group were more likely to have adequate knowledge (95.2%) than those in the ‘facts-only’ group [90.9%; χ2(1) = 6.74; p = 0.009] (Table 9). Low-numeracy individuals were less likely to have adequate knowledge [89.0% vs. 96.6% (7.6% difference) χ2(1) = 21.34; p < 0.001], but there was no significant group by numeracy level interaction [odds ratio (OR) = 0.42; p = 0.130].
| Variable | ‘Gist + facts’ group (%) | ‘Facts-only’ group (%) | Significance |
|---|---|---|---|
| Intention | 75.7 | 73.8 | χ2(1) = 0.45; p = 0.50 |
| Gist knowledge | 95.2 | 90.9 | χ2(1) = 6.74; p = 0.009 |
A large proportion of the sample said they would ‘definitely’ (74.7%) or ‘probably’ (22.9%) participate in screening and very few reported that they would ‘probably not’ (1.6%) or ‘definitely not’ (0.8%) participate. There were no significant differences between the two groups in the proportion of individuals who definitely intended to participate [χ2(1) = 0.45, p = 0.50] (see Table 9). Low-numeracy individuals were less likely to say they would ‘definitely’ participate in bowel cancer screening [71.2% vs. 77.7% (6.5%); χ2(1) = 5.40; p = 0.020], but there was no significant group by numeracy level interaction (OR = 1.02; p =0 .936).
In the whole sample, 81.7% reported reading all of the information at least once, but those with poor numeracy were less likely to have read them [74.4% vs. 88.0% (13.6% difference); χ2(1) = 29.56; p < 0.001]. There was no significant group by numeracy level interaction in terms of reading the information (OR = 1.37; p = 0.367).
The ‘gist + facts’ group were marginally less likely to have read the materials than the ‘facts-only’ group [79.7% vs. 83.9%; χ2(1) = 2.83; p = 0.093]. Within the ‘gist + facts’ group, participants were more likely to read at least some of the ‘gist’ leaflet (88.6%) than ‘The Facts’ booklet (80.5%). Figure 6 shows that participants in the ‘gist + facts’ group with low numeracy were slightly less likely to read the ‘gist’ leaflet [84.5% vs. 92.5% (8.0% difference); χ2(1) = 7.86; p = 0.005] and even less likely to read ‘The Facts’ booklet [72.2% vs. 88.5% (16.3% difference); χ2(1) = 21.07; p < 0.001]. There was also a significant difference in reading between the low- and high-numeracy groups in the ‘facts-only’ group [79.1% vs. 88.1% (9.0% difference); χ2 = 8.56; p = 0.003] (see Figure 6).
FIGURE 6.
Proportion of participants who reported reading at least some of their allocated materials by numeracy group. F only, facts only; G + F, gist + facts.
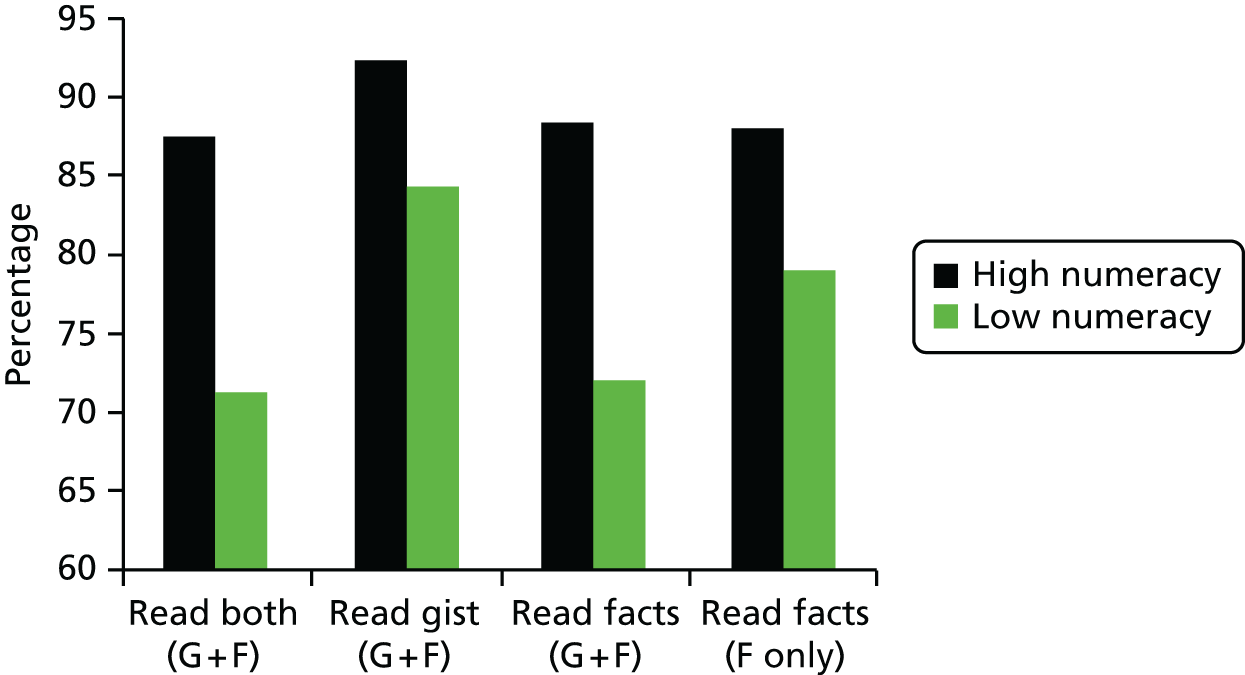
Summary
This multicentre parallel RCT found that the inclusion of a supplementary gist-based leaflet alongside the usual materials sent out with the invitation to take part in bowel cancer screening by the NHS BCSP increased knowledge but did not increase intention to participate in screening. This finding has to be tempered by the very high intention levels among the study respondents.
Patient and public involvement and engagement
At each stage of the leaflet development, we sought input from members of the public as well as from individuals specifically identified to have low health literacy.
The charities ContinYou (then specialising in working with adults with low literacy nationally) and SAfH (a London-based organisation working alongside marginalised local people and their communities in order to improve health and well-being) assisted the research team in recruiting individuals with low literacy for the development of the gist-based information leaflet.
Primary Care Research Networks in Manchester and Liverpool assisted the research team in identifying GPs situated in areas of high deprivation in order for us to undertake a RCT via a postal questionnaire survey regarding intention to participate in bowel cancer screening.
We described the development of the gist leaflet and the results of the primary care-based pilot study were presented to an advisory meeting comprising the study team, representatives from third-sector organisations and patient representatives. At the meeting, copies of the gist leaflet were also provided and additional input was given before the final version was tested in the RCT.
Summaries of the findings of the trial carried out through GPs were sent to participants who ticked a box requesting that details were sent to them.
Conclusion
Following a consideration of acceptability of current NHS BCSP information materials, our research team designed and user tested a simple gist-based leaflet, which aimed to provide information to help people make an informed decision on whether or not to accept an offer of bowel cancer screening. This gist leaflet was tested in a national RCT (intervention 1, workstream 3) in November 2012 and the results are reported in Chapter 10.
Chapter 6 Workstream 2, intervention 2: developing and piloting a ‘narrative’ leaflet
Introduction
The second ASCEND intervention to be evaluated nationally in workstream 3 involved the inclusion of a ‘narrative’ leaflet with the invitation letter and materials sent out by the NHS BCSP. Support for this intervention was obtained from the results of the focus group study described in Chapter 3. In particular, the theme ‘the power of talk’, which described how learning of other people’s experience with bowel cancer and bowel cancer screening helped normalise the screening process and ‘tip’ people into making a personal decision to take part in screening. In the focus groups, this mostly referred to experience of family and friends; however, as this was not possible in the format of a national leaflet, we attempted to simulate this effect by using the stories of other people who had undergone CRC screening. Specifically, we tried to appeal to a broad representation of people from the target age range by using a mixture of males and females as well as individuals from ethnic minority backgrounds. In addition, focus group findings concerning resistance to handling faecal matter and the implications of knowing gFOBt results were also highly pertinent.
In this chapter, we will first describe the theoretical background to this intervention. Second, we will describe how we collected the narratives for use in the leaflet and how we then developed and tested the leaflet with several different groups, including experts and lay people. Third, we provide details of a pilot study which tested if the leaflet could influence people’s beliefs about bowel cancer screening and intention to take part in screening. The results of the national RCT of the ‘narrative’ intervention are reported in Chapter 10.
Background
Cancer screening information is usually presented didactically (i.e. factual, statistical information)155 and the information material currently delivered to individuals eligible for bowel cancer screening is no exception. However, narrative based communication (i.e. stories with character and event examples) is suggested as a format more attuned to engaging various audiences. 156 Narratives are thought to reduce counter-arguing, facilitate mental imagery and provide role models of behaviour, making them particularly useful for enhancing health behaviours. 156–158
The positive influence of narratives has been highlighted within a number of health-related behaviours, including breast cancer screening, skin cancer prevention and hepatitis B vaccinations. 159–161 Intentions to take part in such health-promoting activities have been found to be higher after exposure to narrative information compared with either statistical information or no information at all. In a US-based study by Dillard et al. ,162 incorporating narratives into information about bowel cancer screening increased perceived personal risk of bowel cancer. This was a positive result in accordance with theoretical models of behaviour change such as the Extended Parallel Processing Model163 and the Health Belief Model,164 which assert that people need to consider themselves susceptible to a disease in order to ‘motivate’ preventative behaviours. In addition, the inclusion of the narrative had the additional positive effect of reducing the impact of perceived barriers which, in combination with perceived susceptibility, lead to positive behaviour change. Consequently, narratives were found to significantly increase interest in receiving a colonoscopy. 162 A more recent US study165 reported that individuals presented with a bowel cancer screening information leaflet containing a narrative were four times more likely to participate in bowel cancer screening than those given information without a narrative. The impact of narrative information with regard to beliefs about bowel cancer screening, intention to do the gFOBt and actual screening behaviour has not previously been investigated in the UK.
Accessing narrative health information is becoming easier owing to the array of online resources that are now available. Healthtalkonline166 and Cancer Genetic Storybank167 are examples of websites dedicated to using personal experiences to educate the viewer about selected health issues. However, the population we are targeting with the gFOBt may have limited use of the internet. In a recent Ofcom report on media literacy, ‘narrow’ users (i.e. use of the internet is limited) were more likely to be aged ≥ 55 years and in lower SEC groups. 168 Therefore, despite limitations of design (e.g. length and number of stories presented), the written paper form remains an important medium, particularly to the age group served by the NHS BCSP, and fits within the format of the current system.
Aims
-
To collect personal narratives from individuals who have experience of taking part in the NHS BCSP.
-
To develop a narrative leaflet, to supplement current information materials sent out by the NHS BCSP.
-
To test if the narrative leaflet had an impact on beliefs about screening, including intention to be screened, among individuals of pre-screening age.
Collecting personal ‘narratives’ of screening
For the content of the narrative leaflet, individuals (n = 20) who had previously taken part in the NHS BCSP were interviewed about their personal screening experience. Efforts were taken to ensure a mix of screening outcomes. The interviews were transcribed and then each transcript reviewed with suitable extracts highlighted for possible inclusion in the leaflet. The content of the leaflet was decided within the ASCEND research group. A draft of the leaflet was presented to the Social Marketing Team at Stirling University, Stirling, UK, and then evaluated via interviews and focus groups with pre-screening-aged men and women, and further refinements were subsequently made. Finally, we evaluated the leaflet using the Suitability and Comprehensibility Assessment of Materials questionnaire. 169 These stages of development are described in more detail below.
Participant recruitment
A variety of strategies were used to recruit individuals to take part in this interview study. A study advertisement was placed on the Beating Bowel Cancer website and Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com) asking those interested in telling their story to contact the research team. This recruitment method was unsuccessful and so promotion of the research was e-mailed directly to eligible candidates by our colleagues at Beating Bowel Cancer and we recruited six participants using this method. The study was also advertised to individuals taking part in other research studies conducted by the UCL Health Behaviour Research Centre or the UCL Department of Applied Health Research, which led to recruiting an additional 13 participants. Finally, one individual was recruited by the research team during a recruitment drive in a local library.
Inclusion and exclusion criteria
Inclusion criteria were:
-
being of screening age (60–74 years)
-
having taken part in bowel cancer screening
-
being able to speak English.
Wengraf’s Biographic Narrative Interpretive Method
The interviews followed a format described in Wengraf’s Biographic Narrative Interpretive Method170 and lasted an average of 70 minutes (range 26–124 minutes). This interview format consists of three stages:
-
The researcher asks the participant to tell their story of screening. To do this, a single question aimed at inducing narrative is asked of each participant. The single question aimed at inducing narrative used for the purposes of this research was as follows:
-
Can you please tell me your story of how you came to do the bowel cancer screening test kit and how it all turned out? Tell me the events and experiences which were important for you. Begin wherever you like. Take all the time you need. I’ll listen first, I won’t interrupt. I’ll just take some notes for after you’ve finished telling me about your experience.
-
The researcher does not ask further questions or make comment at this stage, but rather listens to the story told by the participant.
-
-
When the participant has finished telling their story, the researcher asks for more narration on relevant topics brought up by the participant (topic question aimed at inducing narrative) using the words used by the participant and in the order that the participant introduced them.
-
The researcher now has the opportunity to ask open-ended questions regarding topics of interest not spontaneously addressed by the participant and, if necessary, to seek confirmation of any points raised in the story.
Procedure
All 20 eligible participants were sent an information sheet (see Appendix 16) and consent form (see Appendix 17) to be returned to the study team via a freepost return envelope. Participants elected to be interviewed either in their own home (n = 7), in UCL offices (n = 7) or over the telephone (n = 6). Each participant completed a demographic question sheet (see Appendix 18) on the day of their interview and, at the end of the interview, was reminded of the possibility of including photographs of participants in the leaflet. If agreed, the researcher took photos of the participant and a further consent form for the use of the photo by UCL was signed (see Appendix 19). At the end of each interview, the participant was offered £20 for their time and towards any travel expenses incurred. Each interview was transcribed verbatim, anonymised and a copy sent to the participant for their review. A study debriefing form (see Appendix 20) was sent with the transcript. A total of 19 out of 20 participants accepted the invitation to review their transcript.
Sample size
We aimed to interview 16–24 people, varying by gender, ethnicity, SECs and screening outcome.
Measures of participant characteristics
The following participant characteristics were recorded: age, gender, marital status, living arrangement, car ownership, employment status, education level, ethnic background and religion.
Participant characteristics
The participants comprised 12 females and eight males with an average age of 66.5 years (range 60–73 years). Out of the 20 participants, eight had been diagnosed with bowel cancer through the NHS BCSP, three had received a normal gFOBt kit result and nine had benign polyps removed during colonoscopy. Table 10 gives further characteristics of the participants.
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 8 (40) |
| Female | 12 (60) |
| Marital status | |
| Married/living with partner | 12 (60) |
| Single/divorced/separated | 5 (25) |
| Widowed | 3 (15) |
| Living arrangement | |
| Own home/mortgage | 18 (90) |
| Renting/other | 2 (10) |
| Employment | |
| Currently employed/self-employed | 4 (20) |
| Retired | 16 (80) |
| Educationa | |
| No formal qualifications | 6 (30) |
| A levels or Highers/vocational qualifications/other pre-degree qualifications | 8 (40) |
| Degree | 4 (20) |
| Ethnicityb | |
| White | 14 (70) |
| Non-white | 3 (15) |
| Other | 2 (10) |
| Religionb | |
| None | 3 (15) |
| Christian (Catholic or Church of England) | 11 (55) |
| Other | 5 (25) |
Designing the narrative leaflet
We developed the narrative leaflet according to the principles set out by the Department of Health’s Improving Outcomes: A Strategy for Cancer111 described in Chapter 5. As was the case for the first ASCEND intervention, the ‘gist’ leaflet, the ‘narrative’ leaflet was provided in addition to, rather than instead of, the existing booklet. This was to ensure that every individual continued to have access to information necessary for making an informed choice about bowel cancer screening participation. 112
Our aim was to ensure that the narrative leaflet presented a variety of credible messengers with whom our target population could identify, but without being overwhelmed by the amount of material provided. The leaflet went through many iterations during the design process. Below, we summarise four iterations of the leaflet that represent the main design changes, along with summaries of specific input for each design and present the final leaflet used in the intervention.
Managing interview data
Interviews were audio-recorded and transcribed. The narratives contained in the transcripts were reduced for review in two ways: (1) four researchers independently highlighted pertinent quotations within each narrative, which were then grouped together into topic themes (e.g. early diagnosis) and (2) each narrative was individually summarised with key quotations included to illustrate points made. Suitable quotations and summarised stories were selected through discussions with the research team and applied to various iterations of the leaflet design.
Iteration 1
The first version of the leaflet was a trifold. We chose this format to make it stand out more from the standard information booklet ‘The Facts’ (see Appendix 3). Following the leaflet proposal outlined in the original research plan, the initial design included the summarised narratives of four participants, selected to ensure variation in gender, ethnicity and screening experience (see Appendix 21). However, following extensive discussion, it became clear that the leaflet was too text-heavy. Subsequently, a decision was made not to focus on individual stories but rather to illustrate the ‘story’ of the bowel cancer screening process with a variety of quotations. Telling the ‘story of screening’ allowed us to highlight key issues involved in the decision to accept screening, such as the importance of early detection, the relative ease of doing the test and the fact that most people receive a normal result, using the words of real people who have taken part in the programme. This format also allowed more people to be represented in the leaflet, increasing opportunities for the reader to identify with the ‘characters’ and further illustrating the normality of receiving and completing the screening test.
Iteration 2
We presented this second version of the leaflet (see Appendix 22) to a team of experts in the Institute for Social Marketing at the University of Stirling, Stirling, UK. Table 11 lists feedback from the Institute for Social Marketing team which was discussed with the study team and the outcome agreed.
| Topic | Comments, tips and suggestions | Outcome |
|---|---|---|
| Layout | People like diagrams and would help make layout ‘more eye catching’ | Diagrams to explain bowel cancer and information related to FAQs were already available in ‘The Facts’ booklet |
| Could include a section on FAQs | Focus groups to discuss layout and style were arranged | |
| Colour and font style should be assessed with focus groups | ||
| Inclusion of logos | Add the NHS logo, a ‘trusted’ logo, on the front of the leaflet | The NIHR logo, which incorporates ‘NHS’, was added to the front of the leaflet and the UCL logo kept out |
| Charity logos would have impact only if very relevant to the reader | ||
| A University logo, in their experience, did not tend to make an impact | ||
| Quotations | Keeping character within the quotations was deemed important | The leaflet was a national and not a local intervention and so the language used had to be modified to suit a varied UK audience. Some character in quotations was therefore lost |
| Quality over quantity (i.e. one convincing quotation is better than three short ‘average’ quotations) | One key quotation was selected for each point to be made | |
| Photographs | A ‘disconnection’ between the photographs and quotations was felt | Photographs were matched to quotations and integrated into main text Photos of participants in their homes or in the street were taken where possible. Some participants provided their own photographs |
| Fewer but larger, more naturalistic, photos were recommended (i.e. something that places them in the real world/community) |
Iteration 3
The resulting leaflet (see Appendix 23) was tested with a sample of 45- to 63-year-olds, during three telephone interviews and two focus groups. The participants who took part in telephone interviews (two male and one female) had previously participated in a research study within the UCL Health Behaviour Research Centre and had indicated interest in further research opportunities. The participants who took part in two focus groups (N = 10: male, n = 4; female, n = 6) were recruited through the Camden Carers Association, a local charity group that had previously assisted with recruitment to research studies. All participants bar one (telephone interview; male) had not yet been invited for bowel cancer screening.
Telephone interviewees felt that the leaflet was ‘reassuring’, with genuine photos and a good selection of comments that they could identify with. Each commented that the title was not clear and should include ‘bowel cancer screening’. Other minor comments relating to font, colour and wording were also taken into consideration. When there were conflicting comments, the research team reached a consensus on the preferred option.
The focus groups were more critical of the leaflet. Suggestions included having more information about what bowel cancer is, diagrams of the bowel, information about the numbers of people who survive bowel cancer, a number to call for more information and a link to a website at which more personal stories could be accessed. Many suggestions were considered by the research team to be covered by ‘The Facts’ booklet and, therefore, were not necessary or possible for this supplementary leaflet. Although the addition of a website was an interesting idea, its development and maintenance was felt to be outside the scope of this research project. Minor details relating to colour and font were considered and, when possible, accommodated, but the most notable change made following the focus groups was the removal of the map from the front cover. The majority of focus group participants found the map confusing and misleading. A table with details of suggestions from the focus groups and interviews is presented in Appendix 24.
Iteration 4
This next version (see Appendix 25) was sent to all 20 participants who had been interviewed about their screening experience and subsequently provided content for the leaflet. Telephone interviews with 14 of the participants (six were not available and did not reply to the invitation to contact the researchers directly) gave each person an opportunity to discuss how they had been represented in the leaflet. All were pleased with the final result and were happy with how they had been portrayed.
In addition, this version of the leaflet was presented at the ASCEND Advisory Group meeting in September 2012. This provided an opportunity to receive feedback during the design phase and to ensure that the leaflet would be acceptable to those administering it (i.e. the hub directors) as well as the target audience. Positive feedback resulted in no further changes being made to the leaflet.
Suitability and comprehensibility assessment of materials
Finally, four researchers in the UCL Health Behaviour Research Group and a Health Promotion Specialist working in North West London evaluated the leaflet using the Suitability and Comprehensibility Assessment of Materials questionnaire. 169 Completion of this questionnaire involves assigning a series of scores to the following aspects of the leaflet: literacy demand; numeracy; graphic material; layout and typography; and learning, stimulation and motivation. Scores are given as a percentage and a minimum score of 40% is required for the leaflet to be considered adequate for use. The overall score for this leaflet was 86%, deeming the leaflet to be of a ‘superior standard’ (> 70%).
A pilot study to evaluate the ‘narrative’ leaflet
A version of this study has been published in McGregor et al. 171 © 2015 McGregor et al. ; licensee BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
A RCT was conducted to test the hypothesis that the inclusion of a narrative information leaflet would lead to a positive shift in beliefs about bowel cancer screening, beliefs that have previously been found to be predictive of health intention and behaviour.
The Extended Parallel Process Model163 predicts that positive behaviour change in response to a health threat (i.e. cancer) will occur if there is a high level of perceived susceptibility to the disease, in addition to a strong belief that the proposed behaviour can be successfully carried out and will successfully reduce the threat. The narrative leaflet was designed with this in mind and, therefore, the presentation of various people who had already successfully completed the test as well as selected quotations that illustrated surprise at a diagnosis and relief at having cancer found and treated early were anticipated to increase self-efficacy for test completion, perceived personal risk of getting bowel cancer and perceived response efficacy of the test.
The Health Belief Model164 also suggests that, for a behaviour to be elicited, perceived barriers to the behaviour should be low and the perceived benefits high. We included specific quotations in the narrative leaflet to counter-argue known test barriers (i.e. disgust with test procedure and the belief that screening is only for those with symptoms),172,173 hypothesising that endorsement of such barriers would decrease and quotations portraying reassurance following participation would help enhance the perceived benefit of peace of mind from doing the test. 174
Methods
Design and study population
Adults registered at one of three GPs in England (two in London, one in rural North West England) were invited to take part in this study. An age restriction of between 45 and 59.5 years was applied to ensure that participants had not previously taken part in the NHS BCSP, but were approaching the age of screening eligibility. Exclusion criteria included having had a recent diagnosis of cancer or currently undergoing bowel surveillance. A total of 4125 eligible adults were identified and, using random allocation software,175 were randomly assigned to one of two groups: ‘standard information only’ (SI) (n = 2067) and ‘standard information + narrative leaflet’ (SI + narrative) (n = 2058). To minimise crossover effects, eligible invitees were clustered according to household before being randomised, to ensure that cohabiting individuals were assigned to the same group.
Required sample size
The sample size calculation was based on the results reported in an article considering the impact of narrative information on breast cancer screening attitudes and intentions. 159 Assuming α = 0.05 and power (1–β) = 0.90, the number of participants needed to find a significant between-group difference in intention in the current study was 684 (342 per group). A low response rate was anticipated and, therefore, approximately 4000 people were invited to take part.
Procedure and materials
Each eligible participant was sent a covering letter along with an information pack, a questionnaire and a freepost return envelope. The covering letter explained the purpose, process and voluntary nature of the study, and was signed by a staff member from the individual’s general practice. The information pack resembled the official invitation sent out as part of the NHS BCSP in England comprising a NHS-branded envelope containing a sample screening invitation letter and the standard information booklet published by the NHS BCSP (‘Bowel Cancer Screening: The Facts’, see Appendix 3). For those randomised to the SI + narrative group, the information pack also contained a copy of the narrative leaflet developed for this study, entitled ‘Bowel Cancer Screening: People’s Stories’ (see Appendix 25). A statement explaining that this was a mock NHS BCSP invitation letter was affirmed on the covering letter and on the front cover of the questionnaire. The questionnaire also included a statement to confirm that consent to participate was presumed on its completion and return.
Individuals who did not respond within 4 weeks of the initial invitation received a reminder letter, again signed by their general practice. A further copy of the questionnaire, information pack and freepost return envelope were included with the reminder letter.
Data collection took place between June 2012 and January 2013. This study was granted ethics approval by the National Research Ethics Service Committee, North East – Northern and Yorkshire and is a registered trial (ISRCTN74502911).
Measures
The questionnaire booklet contained questions adapted from published studies that addressed beliefs thought to influence uptake of bowel cancer screening: perceived risk, self-efficacy, test response efficacy, anticipated disgust with the procedure, symptoms as a prerequisite to screening, peace of mind and intention. 162,172,174,176–178
An introductory question was asked to encourage or remind the participant to read the information material sent to them prior to beginning the questionnaire: ‘Have you read the orange booklet, “Bowel Cancer Screening: The Facts” found inside the NHS envelope?’. The intervention group were also asked the same question in relation to the narrative leaflet. For both questions, responses were on a four-point scale ranging from ‘no’ to ‘I have read it all more than once’.
A single item was used to measure perceived risk: ‘If I never do the FOB screening test, I would feel very vulnerable to bowel cancer’. 162 A five-point response scale from ‘strongly disagree’ to ‘strongly agree’ was provided.
Single items with a four-point response scale from ‘strongly disagree’ to ‘strongly agree’ were used to measure self-efficacy (‘I would be confident that I could do the FOB test correctly’176), response efficacy (‘Doing the FOB test would reduce my chances of dying from bowel cancer’177), anticipated disgust (‘Doing the FOB test would be disgusting’172), symptom absence (‘I would only do the FOB test if I had symptoms of bowel cancer’172) and anticipated peace of mind (‘Doing the FOB test would give me peace of mind’174).
Future intention to participate in bowel cancer screening was measured by a single item: ‘Imagine you have just turned 60 and have received the bowel screening test kit (FOB test kit) in the post. Doing the test involves taking small amounts of your stool (poo) on three different days and putting them on the FOB test kit. Realistically speaking, how likely are you to do this?’. 178 Responses were recorded on a four-point scale ranging from ‘definitely not’ to ‘yes, definitely’.
Demographic information
Respondents were asked to give their age, gender and ethnicity. A composite score for socioeconomic deprivation was calculated using three questions on current living arrangements (1 point for not owning a home), education (1 point for having no formal qualifications) and car ownership (1 point for not owning a car). Scores ranged from 0 (least deprived) to 3 (most deprived). 179
Data analysis
To analyse the data, SPSS version 20.0 (IBM Corporation, Armonk, NY, USA) was used. Between-group comparisons were conducted using independent-samples t-tests. A mediation analysis addressed the impact that the bowel cancer screening beliefs had on the group and intention relationship. Mediation was conducted using INDIRECT, a SPSS macro. See Appendix 26 for a correlation matrix of each belief.
Results
A total of 1256 people (SI = 606, SI + narrative = 650) returned a completed questionnaire (30.5% response). Those who provided age information inconsistent with general practice records (n = 35) were removed from the analysis. Therefore, the final sample consisted of questionnaire data from 1221 participants (SI, n = 590; SI + narrative, n = 631). The two groups were similar in terms of sociodemographic characteristics (Table 12). Of those who answered the introductory question(s), the majority self-reported that they had read at least some of the information materials provided (SI, 96%; SI + N, 94%; ‘The Facts’ booklet and narrative leaflet, 90%).
| Characteristic | SI, N = 590 | SI + N, N = 631 |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 51.94 (4.31) | 51.80 (4.16) |
| Missing | – | – |
| Gender, n (%) | ||
| Female | 334 (56.6) | 353 (55.9) |
| Male | 256 (43.4) | 278 (44.1) |
| Missing | – | – |
| Ethnicity, n (%) | ||
| White | 513 (86.9) | 547 (86.7) |
| Non-white | 75 (12.8) | 82 (13.0) |
| Missing | 2 (0.3) | 2 (0.3) |
| Socioeconomic deprivation score, n (%) | ||
| 0 (least deprived) | 326 (55.3) | 362 (57.4) |
| 1 | 145 (24.6) | 147 (23.3) |
| 2 | 71 (12.0) | 77 (12.2) |
| 3 (most deprived) | 19 (3.2) | 16 (2.5) |
| Missing | 29 (4.9) | 29 (4.6) |
t-tests
The results of the t-tests are presented in Tables 13–15. The group that received the additional narrative leaflet perceived themselves to be at higher risk of bowel cancer if they did not participate in bowel cancer screening (p = 0.045) and were more likely to believe that doing the screening test would reduce their chances of dying from bowel cancer (p = 0.008) (see Tables 13 and 15, respectively). In addition, the group with the narrative leaflet were less likely to perceive doing the test as disgusting (p = 0.007) and more likely to believe that doing the test would provide them with peace of mind (p = 0.002) (see Table 15).
| Belief | Group | n | Response (%) | Results | |||||
|---|---|---|---|---|---|---|---|---|---|
| Strongly disagree | Disagree | Not sure | Agree | Strongly agree | Mean (SD) | t-test | |||
| Perceived risk | SI | 583 | 4.3 | 23.2 | 35.7 | 29.2 | 7.7 | 3.13 (0.99) | t(1208) = –2.00; p = 0.045 |
| SI + N | 628 | 5.3 | 20.3 | 30.0 | 33.5 | 11.0 | 3.25 (1.06) | ||
| Belief | Group | n | Response (%) | Results | ||||
|---|---|---|---|---|---|---|---|---|
| Strongly disagree | Disagree | Agree | Strongly agree | Mean (SD) | t-test | |||
| Self-efficacy | SI | 584 | 0.7 | 2.9 | 62.7 | 33.7 | 3.29 (0.55) | t(1208) = –1.92; p = 0.055 |
| SI + N | 628 | 0.8 | 2.4 | 57.2 | 39.6 | 3.36 (0.57) | ||
| Response efficacy | SI | 582 | 2.2 | 8.1 | 60.0 | 29.7 | 3.17 (0.66) | t(1201) = –2.64; p = 0.008 |
| SI + N | 627 | 2.1 | 5.9 | 54.7 | 37.3 | 3.27 (0.66) | ||
| Anticipated disgust | SI | 584 | 28.9 | 52.2 | 15.9 | 2.9 | 1.92 (0.75) | t(1204) = 2.69; p = 0.007 |
| SI + N | 622 | 34.1 | 52.3 | 11.7 | 1.9 | 1.81 (0.71) | ||
| Symptom absence | SI | 577 | 44.0 | 49.0 | 4.2 | 2.8 | 1.67 (0.70) | t(1196) = 1.39; p = 0.165 |
| SI + N | 621 | 49.9 | 43.2 | 3.9 | 3.1 | 1.60 (0.71) | ||
| Peace of mind | SI | 582 | 1.0 | 6.7 | 61.0 | 31.3 | 3.22 (0.61) | t(1199) = –3.15; p = 0.002 |
| SI + N | 626 | 1.0 | 4.5 | 54.6 | 39.9 | 3.33 (0.61) | ||
| Belief | Group | n | Response (%) | Results | ||||
|---|---|---|---|---|---|---|---|---|
| Definitely not | Probably not | Yes, probably | Yes, definitely | Mean (SD) | t-test | |||
| Intention | SI | 582 | 0.3 | 3.6 | 27.5 | 68.6 | 3.64 (0.57) | t(1208) = –1.99; p = 0.048 |
| SI + N | 628 | 1.6 | 1.8 | 24.0 | 73.6 | 3.71 (0.53) | ||
The group who received the additional narrative leaflet also showed a significantly stronger intention to take part in screening in the future [mean (SD) = 3.71 (0.53) vs. 3.64 (0.57); t(1208) = –1.98; p = 0.048]. Indeed, the proportion of participants who indicated that they ‘definitely’ intended to take part in screening in the future was 5% higher in the group who received the additional narrative leaflet (74% vs. 69%) (see Table 13).
Both groups generally agreed that they were confident to complete the gFOBt kit and correctly disagreed that the test was only for those who had symptoms; however, there was no statistically significant between-group difference on the strength of either belief (p > 0.05).
Mediation analysis
A mediation analysis was conducted to help explain how the narrative affected intentions by modifying specific beliefs about screening. The direct effect of intervention on each belief, including intention, and of each belief on intention was confirmed with multiple regression. The parallel multiple mediator model is presented in Table 10. The model was based on a sample of 1106 people (SI, n = 529; SI + N, n = 577) and accounted for 29.8% of the variance in intention.
Age, gender, ethnicity and socioeconomic deprivation were included as control variables in the model. Older age was associated with stronger intention [β = 0.008, t(1095) = 2.55; p = 0.014], women were more likely to intend to do the bowel cancer screening test than men [β = 0.066, t(1095) = 2.34; p = 0.019] and a higher socioeconomic deprivation level (i.e. more deprived) was associated with lower intention [β = –0.061, t(1095) = –3.43; p < 0.001]. There was no association between ethnicity and intention (p = 0.135). The relationship between group and beliefs, beliefs and intention, and group and intention were retained when controlling for gender, age, deprivation and ethnicity. The direct effects are presented in Figure 7 and Table 16.
FIGURE 7.
Parallel multiple mediator model showing relationships between group, intention and beliefs. Unstandardised beta coefficients show direct effects. R2 = 0.2978, n = 1106. a, p < 0.05; b, p < 0.001; c, p < 0.01. Reproduced from McGregor et al. 171 © 2015 McGregor et al. ; licensee BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
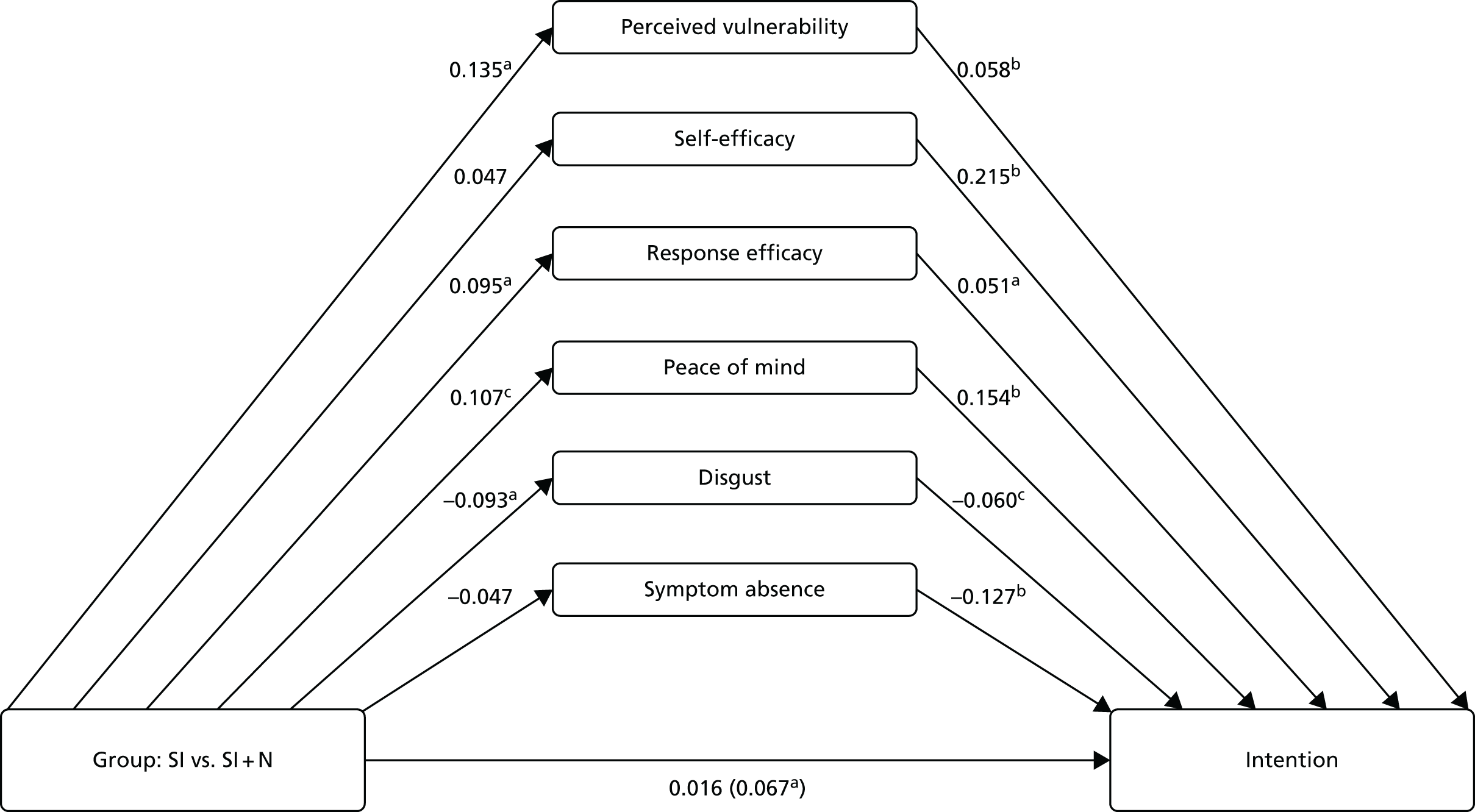
| Variable | βa | SE | t b | p-value |
|---|---|---|---|---|
| Direct effect of group on each belief | ||||
| Perceived vulnerability | 0.135 | 0.062 | 20.19 | 0.029 |
| Self-efficacy | 0.047 | 0.033 | 10.44 | 0.150 |
| Response efficacy | 0.095 | 0.040 | 20.38 | 0.017 |
| Anticipated disgust | –0.093 | 0.044 | –20.13 | 0.034 |
| Symptom absence | –0.047 | 0.041 | –10.14 | 0.254 |
| Peace of mind | 0.107 | 0.036 | 20.97 | 0.003 |
| Direct effect of each belief on intentionc | ||||
| Perceived vulnerability | 0.058 | 0.014 | 40.04 | 0.000 |
| Self-efficacy | 0.215 | 0.030 | 70.24 | 0.000 |
| Response efficacy | 0.051 | 0.024 | 20.13 | 0.033 |
| Anticipated disgust | –0.060 | 0.021 | –20.93 | 0.004 |
| Symptom absence | –0.127 | 0.022 | –50.67 | 0.000 |
| Peace of mind | 0.154 | 0.028 | 50.44 | 0.000 |
| Total effect of group on intention | ||||
| Group | 0.067 | 0.032 | 20.08 | 0.038 |
| Direct effect of group on intentiond | ||||
| Group | 0.016 | 0.028 | 0.578 | 0.564 |
Importantly, the direct effect of group on intention reduced and became non-significant when the belief variables were controlled for, indicating that the leaflet affected intention through beliefs (see Figure 7 and Table 16).
Indirect effects were assessed using bias-corrected bootstrap CIs. Perceived peace of mind, vulnerability to bowel cancer, anticipated disgust and perceived test efficacy all mediated the effect of group on intention. The SI + N group had stronger intentions to be screened as a result of their tendency to, in order of strength of influence, (1) more strongly believe that screening will provide peace of mind (β = 0.0167, 95% CI 0.0061 to 0.0319), (2) perceive themselves as more vulnerable to bowel cancer if they do not have screening (β = 0.008, 95% CI 0.0014 to 0.0181), (3) consider the screening test as less disgusting (β = 0.006, 95% CI 0.0007 to 0.0143) and (4) more strongly believe that screening would reduce their chances of dying from bowel cancer (β = 0.005, 95% CI 0.0004 to 0.0136) than the SI group. Examining the CIs for each mediator contrast showed that the indirect effect of peace of mind was significantly stronger than the indirect effect of response efficacy (β = –0.012, 95% CI –0.0275 to –0.0009). No other significant contrasts were found, suggesting that each of the other mediator indirect effects were of comparable strength.
Discussion
The addition of a narrative leaflet to standard information material had a positive effect on a number of beliefs about bowel cancer screening, which, in turn, significantly increased respondents’ intention to participate in the screening programme.
The mediation analysis found that the narrative leaflet influenced intention to be screened predominantly through its ability to strengthen the benefit of anticipated peace of mind from screening, enhance feelings of vulnerability to bowel cancer without screening, reduce perceived disgust with the procedure, and enhance the belief that screening could reduce your chance of dying from bowel cancer.
Although intention to be screened may not directly lead to screening behaviour (the ‘intention–behaviour gap’), it remains an important prerequisite. Previous research has found that lower perceived barriers and higher perceived benefits of screening are predictive of both intention to be screened and screening behaviour itself180 and, therefore, the narrative leaflet’s ability to positively manipulate factors addressing a selection of benefits and barriers suggest potential for an influence on screening behaviour.
A limitation of this study is the percentage of people who returned completed questionnaires (30.4%). This may have subsequently added bias to the results. Within the SI group, a high percentage of respondents (96%) said they would ‘probably’ or ‘definitely’ do the gFOBt and the variation of socioeconomic deprivation was limited within groups; therefore, it could be suggested that the study failed to recruit the very people we hoped the narrative leaflet would influence. Indeed, the socioeconomic deprivation measure used may have overestimated the number of people in London within the more deprived groups; the selected measure was itself a limitation because London is not a fair domestic representation of the UK, with car and home ownership rates lower than in the rest of England due to circumstances not necessarily linked to deprivation. 181,182
A reason for the low participation rate may have been the subject matter. The leaflet was designed to help reduce barriers to screening but the initial invitation cover letter introducing the topic of bowels (a topic of conversation often regarded as socially unacceptable)183 may have put people off participating any further, with the result that the mock NHS invitation envelope may not have been opened and the leaflet not seen. Following on from this is the response burden associated with participation [i.e. reading a 16-page booklet (‘The Facts’) and then completing and returning a questionnaire]. The multiple tasks involved may have deterred participation, not only with respect to the length of time needed to participate but also the additional onus on reading material. Although the leaflet fitted the format of the NHS BCSP, additional reading material may not be an effective way of engaging people, especially those with low literacy levels.
The salience of the topic being investigated is also considered an important determinant of response rates. 184 Bowel cancer screening is currently offered to people aged 60–74 years as part of the NHS BCSP, yet we purposively wanted to obtain the views of those naive to screening and, therefore, invited people aged 45–59.5 years. As a result, it may be that the relevance of the topic to the invited individual was low and, therefore, participation was not considered a priority.
Despite the limitation of a low response rate, this study has shown that the use of a paper-based narrative leaflet, tailored only to the broad characteristics of the eligible population (i.e. men and women aged > 60 years), has the potential to positively impact beliefs about bowel cancer screening and intention to be screened.
Within a real-world situation, the NHS envelope containing the screening invitation and information material will be delivered directly, with no additional research tasks requested, and to people within the eligible age bracket. Consequently, more people are likely to engage with the narrative leaflet when it is sent as part of the bowel screening programme and, therefore, it has the potential to heighten intention to be screened at a more pertinent point in the decision-making process. As the leaflet was found to enhance selected benefits and reduce selected barriers to screening, factors previously found to be predictive not only of intention but also behaviour,180 a positive impact on screening uptake looks promising. Further research in workstream 3 will assess the impact of the leaflet on actual behaviour.
Patient and public involvement and engagement
We were assisted by a number of different organisations in recruiting research participants to take part in the narrative interviews. Some were identified following their response to a request advertised by Beating Bowel Cancer, the UK’s leading charity working to promote awareness of bowel cancer. AgeUK invited us to attend a local forum to promote the study but interested members were not found to be eligible for participation. In addition, one participant was recruited when the researchers attended a health promotion event at a local library. Verbal promotion of recruitment for the narrative interviews was also conducted by the research team whenever opportunities arose within the public domain (e.g. when recruiting for workstream 1).
A local carers centre recruited members of the public to take part in two focus groups in order to provide feedback on the content and style of the narrative leaflet that was produced using the narrative interviews. An additional three telephone interviews were undertaken with individuals recruited through ContinYou (a charity working with adults with low literacy) and Groundwork (an organisation working in the most disadvantaged areas of the UK, helping to create better environments to improve people’s prospects and to promote environmentally responsible ways of living and working). The final draft of the leaflet was then reviewed by those who had taken part in the original narrative interviews (including our patient representative coapplicant).
The research team worked with primary care research networks in Cumbria and London in order to identify general practices willing to assist with recruitment of participants to take part in a small RCT concerned with assessing levels of intention to take part in bowel cancer screening.
We presented a summary of the development of the leaflet and the results of the primary care-based pilot study to an advisory meeting consisting of the study team, representatives from third-sector organisations and patient representatives. At the meeting, copies of the leaflet were also provided and additional input was given, which informed the final version used in the RCT.
A summary of the pilot study results has been sent to those who completed a questionnaire and added their contact details for the stated purpose of receiving a copy of the results. A similar summary has also been sent to those who provided the narratives for the developed leaflet.
Summary
We interviewed a variety of individuals (n = 20) with real experiences of taking part in the NHS BCSP and used these rich accounts to design a ‘narrative’ leaflet. We revised and refined the leaflet following feedback from a number of expert groups and focus groups with the general public and then evaluated its impact on beliefs about screening in a small RCT with individuals of pre-screening age (Figure 8). Following the positive results from this small RCT on screening intention, the leaflet was then tested in a national RCT (intervention 2, workstream 3) in March 2013 and the results are reported in Chapter 10.
FIGURE 8.
Flow chart showing development of narrative leaflet. SAM + CAM, suitability assessment of materials and comprehensibility assessment of materials.
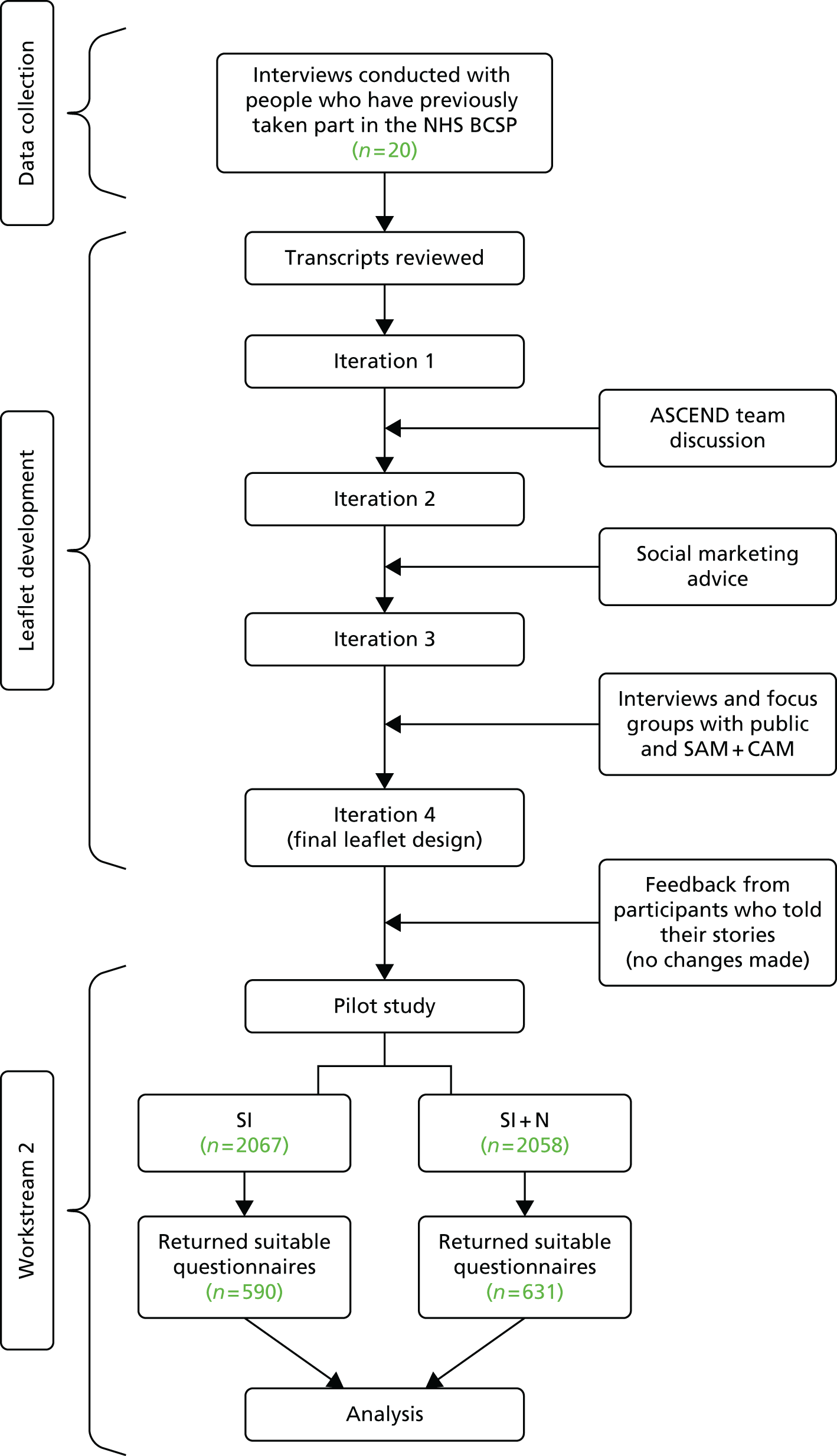
Chapter 7 Workstream 2, intervention 3: developing the general practice endorsement intervention
Background
The results of workstream 1 highlighted the perceived detachment of the NHS BCSP from familiar health-care settings and professionals; however, it has been demonstrated that a screening invitation from a credible and trusted source, such as a GP, is more likely to be accepted185,186 and may have a specific role in addressing low uptake in disadvantaged populations. 187 Including the GP’s signature on the screening invitation has been found to increase gFOBt uptake, but such active participation is costly and time-consuming. 188 A large Australian RCT (n = 2400) with 95% power to detect a difference in participation rates found that a screening invitation naming the relevant general practice was almost as effective at increasing uptake as having the letter signed by the GP. 36 In a study by Cole et al. ,36 letters with GPE achieved a 6% higher participation rate than the regular screening invitation, although the trial lacked the power to detect socioeconomic differences across groups. There is evidence to suggest that endorsement by a GP might reduce the socioeconomic gradient in screening uptake. One study explored predictors of participation via different methods of invitation for bowel cancer screening and reported that, when deciding whether or not to take part in screening, participants with lower education relied more on their GP’s advice than written information accompanying a mailed screening invitation. 189 This suggests that a letter including endorsement by a GP could potentially encourage participation in this population.
The NHS BCSP operates independently from general practices and, although GPs are notified about their patients’ screening results or if their patients have not taken up the offer of screening, there is no additional GP involvement. Reports from the NHS BCSP pilot studies, which included telephone interviews and a focus group with individuals who had not responded to the invitation to be screened, indicate that the perceived anonymity of the NHS BCSP pre-invitation and the lack of involvement of the GP are significant barriers to uptake. 190 However, GPs are generally positive about the NHS BCSP. 190,191 The British Medical Association also recommends that doctors should be more involved in research and interventions, particularly those aimed at reducing the impact of social determinants on health. 192
Therefore, the third ASCEND intervention tested in a national RCT was the addition of a GPE statement appearing on the NHS BCSP pre-invitation (S1) letter. This was the first RCT to examine the effectiveness of a simple endorsement by an individual’s general practice in the NHS BCSP invitation letter at a national level. Adding the practice name to the pre-invitation (S1) letter was considered to have minimal cost once GPs had agreed to their practice name being used. Some PCTs at the time of the development of this intervention were introducing unevaluated GP interventions as part of their strategy to achieve the Department of Health target for reducing cancer mortality, demonstrating that a simple practice endorsement was likely to be welcomed by users and commissioners.
Development of the general practice endorsement pre-invitation (S1) letter
Information provided by workstream 1 focus groups
During the workstream 1 focus groups, which explored reasons for non-uptake of bowel cancer screening (see Chapter 3), many participants indicated their belief that GP involvement would be a facilitator of their taking part in bowel cancer screening; indeed actual or perceived GP support for the NHS BCSP appeared to carry considerable power:
If the NHS wants me to do something, the only way to achieve it is to have my GP [XXX] tell me to do it.
FG01 P1
I think if I had got it from my GP just saying your annual check is due I think that would have far greater impact and almost be an obligation to do it.
FG01 P8
The lack of GP involvement in the NHS BCSP current pre-invitation process was reported by some participants to contribute to it being regarded as an arm’s-length, ‘anonymous’ or unimportant process. It appears that the lack of GP involvement in the current NHS BCSP pre-invitation approach contributes to its interpretation by some as depersonalised and lacking in personal relevance. Several participants mentioned that GP involvement would make the screening pre-invitation more personal and one even suggested potential wording for the intervention:
. . . if it came from the GP rather than some anonymous NHS office or whatever, perhaps the letter that goes out could say, ‘Your doctor has recommended that . . .’ or something like that . . .
FG01 P3
These initial impressions provided evidence that including an endorsement by the general practice in the current pre-invitation (S1) letter had the potential to increase participation in the eligible NHS BCSP population.
Primary Care Advisory Group
A Primary Care Advisory Group (PCAG) including academic experts in screening and GPs working in advantaged and disadvantaged areas was convened to aid the development of an effective GPE. This group comprised five GPs, a practice manager, a NHS BCSP hub director and two clinical academics.
The PCAG helped to design the content of the GPE and various wordings were suggested and discussed. Finally, ‘Your GP Practice [insert general practice name] supports the Bowel Cancer Screening Programme’ was agreed to be an effective endorsement, which could easily be incorporated into the screening pre-invitation. During the PCAG meetings it became clear that the main difficulty with incorporating the endorsement was working within the restrictions of the original format of the NHS BCSP pre-invitation (S1) letter because the main body of the pre-invitation text could not be changed and space was limited. Therefore, the endorsement needed to be short enough in length, once the general practice name had been added, to fit onto a maximum of two lines. With these constraints in mind, the PCAG advised that the endorsement should appear as a bold, stand-alone statement within a greyed out placeholder at the top of the pre-invitation (S1) letter.
Practicalities of adding the general practice endorsement intervention statement to the pre-invitation (S1) letter
Once the content and the format of the endorsement had been agreed by the research team, it was sent to colleagues at the Health and Social Care Information Centre (HSCIC) responsible for the BCSS software for formatting in preparation for implementation in the trials. We were informed by HSCIC that the GPE placeholder in the format suggested by the PCAG could be incorporated into the screening pre-invitation letter as long as the general practice name did not exceed 40 characters. As the general practice name would be inserted automatically when letters were generated by the BCSS, they would appear on the pre-invitation (S1) letter as they did when the practice became active on the system (i.e. not accounting for any potential change to the practice name after that time). Permission to add the GPE to the screening pre-invitation by willing general practices for the duration of the ASCEND programme was granted by Professor Julietta Patnick, CBE (Commander of the Most Excellent Order of the British Empire) (Director of the National Health Service Cancer Screening Programmes). The final version of the GPE pre-invitation (S1) letter can be found in Appendix 27.
Recruitment of general practices to endorse the screening offer
To prepare for this intervention it was necessary to seek permission from all current general practices with screening-eligible participants in England (n = 8184) for the NHS BCSP to automatically add their practice name to the pre-invitation (S1) letter received by their patients.
Previous research has not focused on general practices serving disadvantaged populations that tend to have lower levels of engagement in national initiatives. 193–195 In order to establish the best way to approach practices across England, we consulted the PCAG, which provided us with helpful insights into the best way to recruit practices to endorse the pre-invitation. The five NHS BCSP hub directors also provided feedback on recruitment materials developed by the research team.
We anticipated that a modest incentive would engage GPs, especially those from more disadvantaged areas. Effective incentives include realistic information on potential additional consultations about the NHS BCSP made to the general practice (these were found to be marginal in the English NHS BCSP pilot191), feedback on uptake and positivity rates, comparisons with regional and national benchmarks, and provision of promotional material for waiting rooms. The PCAG suggested that offering GPs information about the outcome of the RCT in their area would be an incentive that could realistically be provided by the hubs within their existing capacity.
Recruitment materials
The PCAG was consulted regarding the development of the general practice recruitment materials. The group suggested the recruitment materials should comprise an invitation letter with an example of the GPE pre-invitation (S1) letter on the reverse, along with an additional information sheet.
The recruitment letter was to be sent from the NHS BCSP hub directors to the general practices in the areas that they covered. The aim of the invitation was to provide information about the potential effectiveness of endorsement by the general practice on bowel cancer screening uptake and to outline what would be involved. It was agreed that the recruitment letter would need to be short, concise and have easy, multiple response options (i.e. post, e-mail or telephone). It was also agreed that it should incorporate an incentive for GPs to take part, in the form of feedback of how the GPE affected the screening uptake of their patients (see Appendix 28).
The NHS BCSP supplied the research team with the list of all current general practices in the BCSS with individuals who were eligible for gFOBt screening in England. It became apparent early on that the NHS Prescription Services (from which the BCSS derives general practice information) truncates practice names before they are added to the BCSS. There was concern among the research team that if the truncated name were to be used on the endorsed pre-invitation, people receiving the invitations might not recognise it or might become confused by the shortened practice name, and that it would look unprofessional. Therefore, the research team reviewed the Prescription Services general practice database and chose the most appropriate name from the registered practice details to include in the general practice invitation letter. Practices were able to indicate their preferred practice name to the research team as long as the general practice name did not exceed 40 characters.
It was agreed that the information sheet would emphasise the importance of bowel cancer screening, the effect of GPE on uptake and what would be expected of general practices if they did agree for their practice name to be used (see Appendix 29).
The most important aim of the invitation materials was to ensure that GPs and practice managers understood that endorsing the programme would involve very little or no extra work for them. Rather, they were simply giving their permission for their practice name to be added automatically to the NHS BCSP pre-invitation (S1) letters sent out to their patients (see Appendix 27). Once drafted, the PCAG and a further 10 GPs (who had not been involved in the development process) evaluated the draft recruitment materials. These groups considered the content of the invitation materials to be satisfactory and provided helpful guidance with respect to the recipient of the materials, suggesting that the invitation letter should be addressed to the practice manager as well as the lead GP, together with feedback relating to minor wording and formatting issues.
Pilot study
Following further refinement of the materials, the research team conducted a pilot study to test the materials on a sample of 250 general practices representing all socioeconomic areas in England. Each of the 250 general practices was sent the recruitment materials by post and then received a follow-up telephone call from the ASCEND research team within 1 week. The aim of the follow-up call was to ascertain whether or not the materials had been received, whether or not members of the practice had discussed the invitation, if they had any comments about the invitation, if their practice name appeared correctly in the placeholder, how often the practice takes part in research and the number of GPs working at the practice. Practices that did not respond within 4 weeks were sent a reminder letter, with a second reminder at 8 weeks if no response had been recorded. A total of 78% of pilot practices agreed to endorse the NHS BCSP (more than double the anticipated response of 30%, based on previous experience of general practice recruitment to research studies). 196
National recruitment
National recruitment involved sending invitations to the remaining general practices in England that were not involved in the pilot study described above (n = 7934). The recruitment of practices took place over 36 weeks and was completed approximately 2 weeks before the intervention was scheduled to take place.
As with the pilot, practices were sent the invitation letter and information sheet followed by two postal reminders (including the information sheet) after 4 and 8 weeks, as necessary. A third reminder letter was sent at 18 weeks from the initial invitation, without an information sheet, and included an enhanced statement on letters sent to practices located in more deprived postcode areas (quintiles 4 or 5).
The decision to send a third reminder letter was based on the response to the initial invitation and subsequent reminder letters. By the time the second reminder letter had been sent out to practices, a trend appeared to be emerging in the type of practices responding. As seen in Figure 9, practices in more deprived areas were less likely to respond.
FIGURE 9.
Distribution of general practices across IMD quintile (based on practice postcode). Q5 represents the most deprived areas. A total of 1% of practices were not assigned to a quintile owing to lack of data for their practice postcode in the National Statistics Postcode Directory 2007. 197
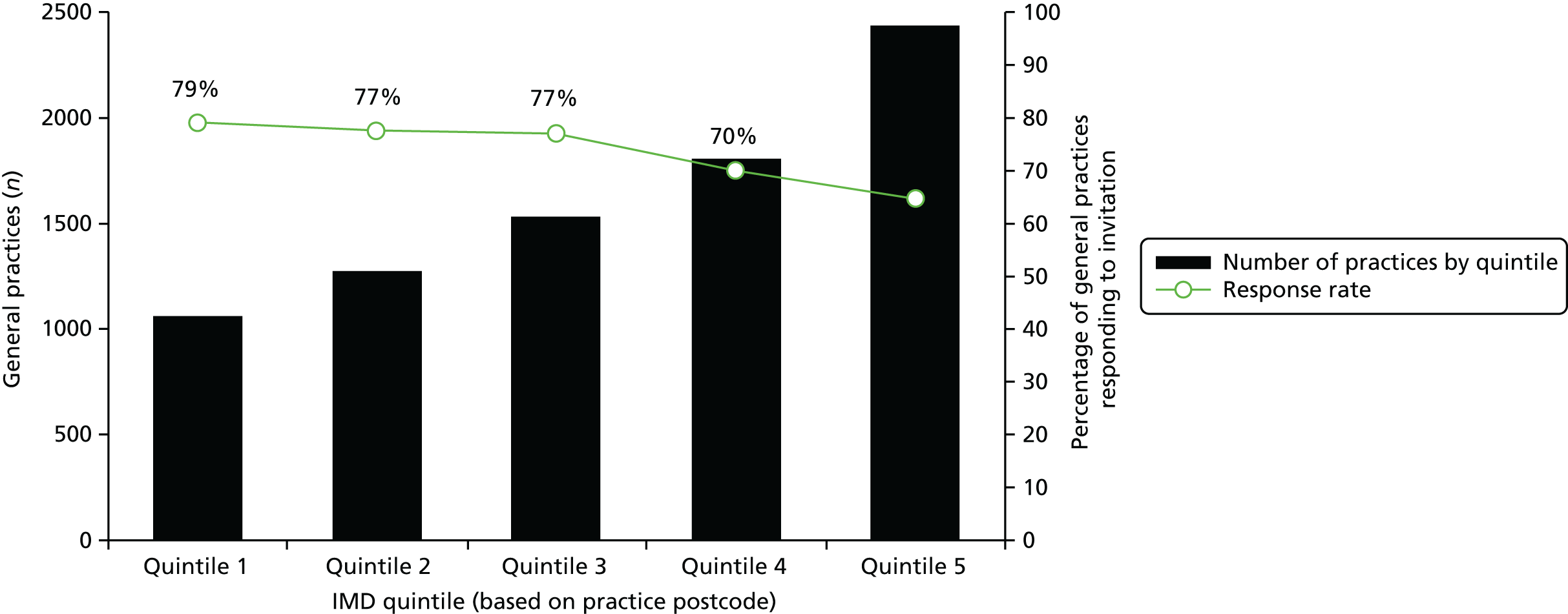
Previous research has shown that GPs serving patients in more deprived areas are less optimistic about bringing about behavioural change in their patients, which could explain the gradient observed here. 198 To address this, two versions of the third reminder (without an information sheet) were sent out to remaining practices in the UK. This time, practices in deprived areas (quintiles 4 and 5) received the following additional message:
We are particularly keen to include practices that look after patients from more deprived areas because those people are less likely to take part in screening.
Results
Response
The number of general practices that granted permission to use their practice names in this ASCEND intervention exceeded our expectation. It was anticipated that 30% of general practices across all IMD quintiles would give permission to use their names in screening pre-invitations. The final number of general practices agreeing to take part in the intervention was 6480, the breakdown of which can be seen in Table 17.
| Status | Invitation letter | First reminder letter | Second reminder letter | Third reminder letter |
|---|---|---|---|---|
| Invited, n | 8142a | 4983 | 3123 | 2082 |
| Refused, n | 8 | 7 | 3 | 0 |
| Agreed, n | 3295 | 1837 | 881 | 467 |
| Agreement by letter (%) | 40.5 | 36.9 | 28.2 | 22.4 |
| Cumulative agreement (%) | 40.5 | 63.0 | 73.9 | 79.6 |
Between the start of recruitment and the RCT, 42 practices were excluded from the general practice list. A total of 40 practices had registered as closed, one practice was registered as a prison prescribing cost centre and one practice was registered as a walk-in centre, neither of which should have been included in the initial recruitment list.
Screening-eligible individuals registered with practices that were either excluded (n = 42), did not respond to our request (n = 1644) or actively expressed their disapproval of our study (n = 18) were not randomised to receive this intervention.
Including the enhanced statement on the third reminder letters sent to practices located in more deprived postcodes showed little change in practice response and the response was lower than after sending the previous reminder letters (Table 18). Furthermore, the response rate from practices in quintiles 4 and 5 was still lower than that from practices in quintiles 1, 2 and 3.
| Status | Third reminder letter (Q1–Q3) | Third reminder letter, enhanced (Q4–Q5) | Total |
|---|---|---|---|
| Invited, n | 776 | 1306 | 2082 |
| Agreed, n | 190 | 277 | 467 |
| Agreement by letter (%) | 24.5 | 21.2 |
Patient and public involvement and engagement
A PCAG comprising professionals including GPs, a practice manager and coapplicants was convened, to assist with the development of the materials used to recruit GPs to endorse the programme and the wording of the endorsement itself. The GPs and practice manager were recruited with help from the Primary Care Research Network for Greater London and through contacts made during the course of the ASCEND programme.
A summary of uptake rates of general practices agreeing to take part in the GPE trial was sent to all general practices that had requested this information during their recruitment to the study.
Summary
This intervention was powered on a very conservative estimate that only 30% of practices would agree to endorse the NHS BCSP and, therefore, we were pleasantly surprised to find that the majority of general practices agreed to do so (80%). The overwhelming response from general practices shows their positive support towards the NHS BCSP.
The high uptake from general practices could be because no additional work was involved in order to take part, as practices simply had to agree that their name could be used on the screening invitation letters. Unlike other research in the UK involving GPE letters, practices did not have to provide an electronic signature or letterhead in order to take part. 37,199
Recruitment letters were sent on NHS BCSP hub letterhead and signed by hub directors, which could have influenced practice response more than a letter from the ASCEND research team. Hub directors already sign all correspondence from the NHS BCSP sent to GPs in their region regarding their patients’ screening pathways.
One invitation and three reminder letters were sent to general practices over a 5-month period, which could have encouraged practices that were too busy or indecisive about responding to earlier letters.
The RCT of the GPE intervention took place in June 2013. Further details are provided in Chapters 9–11.
Chapter 8 Workstream 2, intervention 4: development of the enhanced reminder intervention
This intervention is similar to the GPE intervention described in Chapter 7 in that it also involved a small change being made to a current NHS BCSP letter: the reminder (S10) letter (see Appendix 6).
Background
The value of sending reminders has been well established. A recent meta-analysis of interventions to increase uptake in cancer screening reviewed six separate studies that compared mailing a letter with mailing a letter plus a postal reminder. 196 In all six studies, results were in favour of using mailed reminder letters,196 consistently showing that reminder letters significantly improve uptake. Importantly, research on breast screening attendance has suggested that reminders (telephone or letter) may be helpful in increasing uptake in low-income women,200 particularly if the content of the reminder message is personally relevant and addresses barriers to screening participation, which are known to be socially graded.
Socially graded barriers to cancer screening include lack of awareness of bowel cancer and lack of perceived benefits of bowel cancer screening. There is a strong socioeconomic gradient in cancer awareness. People with a low level of education and a low household income are more likely to have lower awareness of cancer and of the increasing risk of cancer with age. 201–203 This may be particularly true for bowel cancer which, among other cancers, tends to be under-reported in the media relative to the population burden, as shown by a recent content analysis of UK media coverage. 204 Recent evidence has also demonstrated that low awareness of bowel cancer is significantly more prevalent among more deprived groups. 204
In addition to the lack of familiarity with the disease, there is also strong evidence that lower SECs are associated with lack of perceived value of cancer screening. Evidence from the UK FS trial showed that individuals from lower socioeconomic groups tend to perceive the barriers to screening to be higher and the benefits of screening to be lower than do individuals from higher socioeconomic groups. 205 Therefore, for the fourth intervention of the ASCEND study, we developed an ER letter which aimed to increase the personal relevance of the reminder letter by using an additional message to target low awareness of bowel cancer and reinforce the value of cancer screening.
Development of the enhanced reminder letter
We developed the specific messages of the ER letter, consistent with the findings from the workstream 1 focus group concerning beliefs about lack of susceptibility to bowel cancer and an associated failure to understand the potential benefits of screening. In addition, we added a set of reminder-related questions as part of the focus group study in workstream 1. When participants in the focus groups were asked what they thought of the current NHS BCSP reminder (S10) letter, the consensus was that the reminder letter made little impact or impression on them:
So you remember getting a letter – what did you think when you got that letter through?
It didn’t, nothing, I just thought, I’ve not done it [laughs] I’m not doing it, no.
Participants had little recall of receiving the letter:
OK, does anyone else remember getting a reminder letter?
No I never got anything.
Don’t think I did.
When participants did recall receiving the reminder letter, they described putting it to one side or discarding it:
I hadn’t done it, and I still haven’t – got into one of my piles, that’s the trouble and I don’t know which pile it’s in.
FG03 P2
I binned it straightaway, binned it straightaway.
FG06 P7
Bowel Cancer Screening Programme reminder letter helpline monitoring
We examined data from workstream 1 focus groups relating to issues with the usual reminder letter (S10) (see Appendix 6). These issues were itemised, along with other known common issues raised at the reminder stage, and listed in a helpline pro forma (see Appendix 30) to collect feedback on calls made to NHS BCSP hubs about the usual reminder (S10) letter. Owing to the high volume of calls made to the hubs each day, the pro forma needed to be quick and easy to complete so as not to have a negative impact on the helpline workload. Short written instructions on how to complete the pro forma were also designed. These requested helpline assistants to simply note the date of the call and select a tick box option to record the nature of the call (e.g. ‘did not receive the kit’). A free-text box was also provided for any further information. The calls to the hub would remain completely confidential and anonymous (a footnote was added to the pro forma requesting assistants to refrain from adding any identifying information about the caller).
The initial version of the pro forma was sent to the hub director and hub helpline staff in the North East NHS BCSP Hub for evaluation. Feedback was given in a teleconference between the research team and the hub director and hub helpline staff, during which minor formatting suggestions to improve the ease of data collection were advised. Once these changes were accommodated, the North East Hub agreed to pilot the pro forma for 1 week and return the completed forms to the ASCEND research team. Once the completed forms were returned, we made some minor amendments to the form to strengthen the link with the specific aim of the ASCEND project, which was to elicit more detail on the potential misapprehensions the public may have about the current reminder letter. We addressed this by adding an additional column for recording queries about the content of the letter, with a prompt for the helpline assistant to elaborate further in the ‘additional comments’ column. We also emphasised this aim as part of the general introduction provided with the pro forma.
The second version of the pro forma (see Appendix 32) was then used to monitor calls in the North East Hub for 4 weeks. After the first week of monitoring, hub staff reported that the form was easy and not too burdensome for the helpline staff to complete and no further changes were made to the pro forma. We asked the North East Hub staff to return all forms to the ASCEND research team once data collection was complete.
Staff at the Midlands and North West NHS BCSP Hub also agreed to use the helpline pro forma to monitor calls made to their hub for 1 month (from 4 July 2011 to 25 July 2011) and return their completed forms to the ASCEND team. The delay between monitoring in the North East Hub and Midlands and North West Hub was due to the requirement to train helpline staff in using the pro forma and to ensure that the monitoring took place at a time convenient to the hub.
Once the forms had been returned, we then undertook a frequency analysis of issues relating to the reminder (S1/S10) letter recorded in both hubs over a 1-month period. From 894 phone calls made to the hubs relating to the reminder letter, the majority referred to not having received the original screening invitation/test kit (30%) or mislaying the original screening invitation/test kit (22%). These data were used to inform the development of the ER intervention.
Practicalities of adding text to the reminder letter
We were aware from the development of the GPE invitation letter (see Chapter 7) that we would be restricted by the format of the usual reminder letter. In common with the invitation letter, we were advised by the NHS BCSP that the main body of text on the usual reminder letter could not be changed. Therefore, before developing the wording for the ER, we contacted colleagues at the HSCIC to ensure that the enhancement we developed would fit into the usual letter without disrupting the format. We were granted space for a one-line ‘reminder’ banner at the top of the letter and an additional five-line paragraph at the bottom of the letter.
Following this, the first ER prototype was developed. Input was provided by the research team and after some minor adjustments it was presented to four more focus group participants from workstream 1. The feedback from these groups referred mainly to recommendations on how best to format the ER messages. The consensus was that the messages needed to stand out more in order to attract people’s attention:
Yeah, I think a lot of that should be bolder, so it stands out . . .
FG15 P2
. . . put in a nice big box and so you see it and you think wow, I need to do that.
FG15 P2
Just in bold print or something.
FG16 P1
We made amendments to the final version of the ER letter following this feedback and this version (see Appendix 32) was sent to HSCIC for programming and formatting in preparation for the implementation of this intervention. The results of the trial are reported in Chapter 10.
Chapter 9 Workstream 3 randomised controlled trials: introduction and methods
Introduction
In workstream 2 we developed and tested four intervention strategies designed to target known barriers to uptake in lower socioeconomic groups (see Chapters 5–8). These were (1) ‘gist’ information, a leaflet summarising the key screening information in language suited to respondents with lower health literacy; (2) ‘narrative’ information, a leaflet describing the experiences of people who had taken part in screening; (3) a GPE added to the screening invitation letter; and (4) enhancing the reminder letter by reiterating the screening offer: ER. In workstream 3, we conducted national RCTs for each of these interventions. This chapter describes how the RCTs were carried out.
Methods
Interventions
We were required to ensure that the interventions added to, rather than replaced, the current invitation system in the NHS BCSP, described in Chapter 2. Thus, the first two interventions involved the addition of ‘gist’ and ‘narrative’ information leaflets to screening invitation letters (S1). The remaining two interventions involved modifications to current letters produced by the BCSS. These were a GPE banner added to the invitation letter (S1) and ER intervention added to the reminder letter (S10). Figure 10 shows the stages at which the interventions were to run within the screening programme. For examples of the usual invitation letter (S1) and usual reminder letter (S10) (see Appendices 3 and 7, respectively).
FIGURE 10.
The ASCEND study interventions and the NHS BCSP timeline.
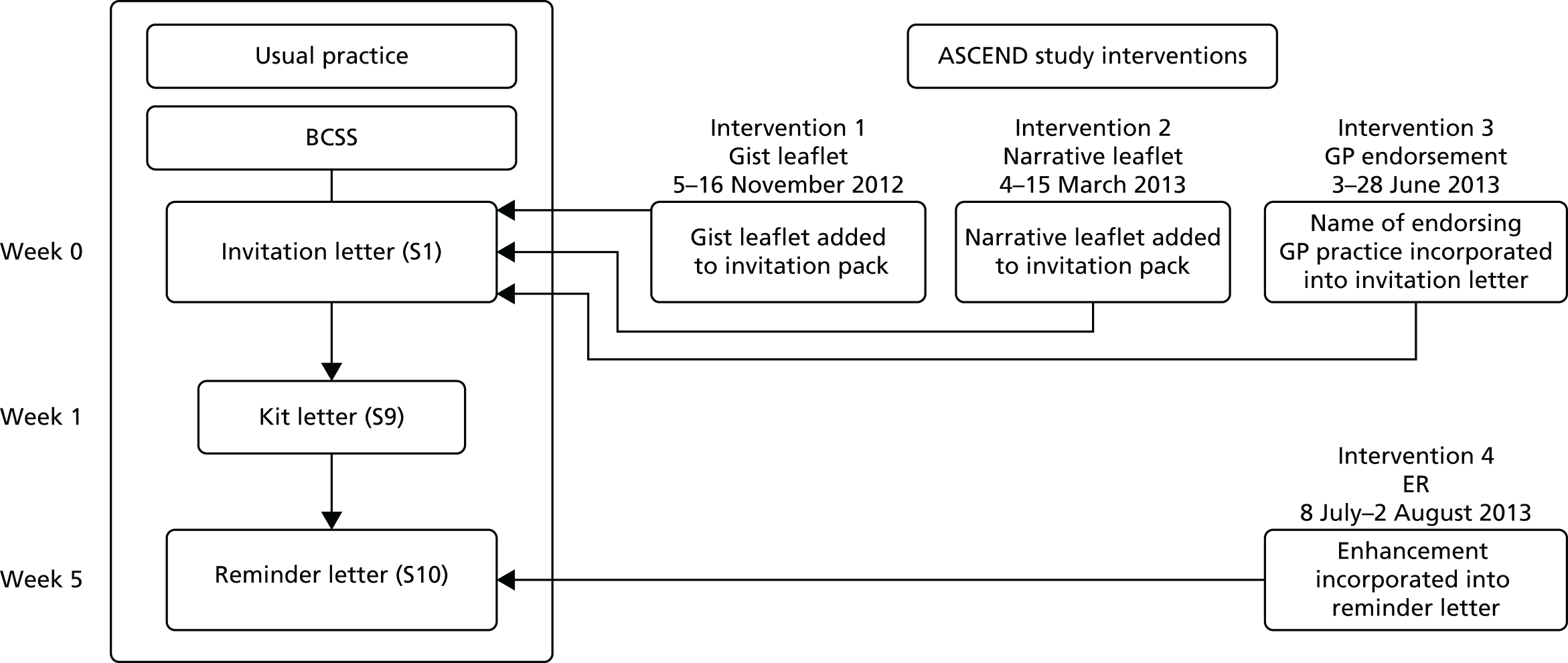
Usual practice in the Bowel Cancer Screening Programme
Men and women aged between 60 and 74 years in England and registered with a GP are invited to be screened for bowel cancer and are sent a gFOBt kit through the post to complete at home. Subjects are invited every 2 years through their regional NHS BCSP hub, which also analyses the tests, sends out test results and contacts diagnostic Screening Centres in its region to arrange for further investigation of subjects with positive gFOBts. On their screening due date, hubs send eligible subjects an invitation letter (S1) that explains that they are due for screening and to expect a gFOBt kit shortly. The kit letter (S9) is posted to them 1 week later and includes a cover letter, information booklet and a gFOBt kit. If after 4 weeks they have not responded, a reminder letter (S10) is posted.
Intervention 1: ‘gist’ information leaflet
The leaflet (see Appendix 13) was pre-printed and included with all the S1 invitation letters on predetermined randomisation dates (see Appendix 33). The trial was run in all five NHS BCSP hubs between 5 and 16 November 2012. The trial took place on 10 working days within that period with an assumption that all the hubs would send S1 invitation letters to their population on every day during that period, resulting in 50 randomised day clusters in each of the five hubs: 25 with usual S1 letters and 25 with the ‘gist’ information leaflet added.
Intervention 2: ‘narrative’ information leaflet
This leaflet (see Appendix 25) was pre-printed and included with all the S1 invitation letters on predetermined randomisation dates. The trial was run in all five NHS BCSP hubs on 10 working days between 4 and 15 March 2013. As with intervention 1, the trial took place on the assumption that all hubs would send S1 invitation letters to their population each day during that period, resulting in 50 randomised day clusters in each of the five hubs: 25 with usual S1 letters and 25 with the ‘narrative’ information leaflet added.
Intervention 3: general practitioner endorsement of invitation (S1) letter
In the months preceding the GPE intervention, we contacted all general practices in England (n = 8184) to ask if they would give permission to allow their practice name to be used on screening invitations during the 4-week study period (recruitment of general practices is described in detail in Chapter 7). Eighty per cent of general practices agreed to take part in the study (Figure 11).
FIGURE 11.
Flow chart showing the possible statuses for intervention 3.
The research team worked closely with HSCIC, the organisation responsible for the design and maintenance of the BCSS. The system identifies the population eligible for screening in each of the five hubs and the hubs are responsible for creating all the letters that are sent to the screening participants and their GPs. For the purpose of our study, HSCIC modified the BCSS to enable selection of invitees belonging to general practices which agreed to endorse the NHS BCSP prior to creation of each invitation letter (S1). In addition, the BCSS took into account the dates when GPE invitations needed to be produced by any of the five hubs. The schema of processes involved within the BCSS and the hubs are shown in Figure 12.
FIGURE 12.
Intervention 3 (GPE) within the BCSS. CFH, Connecting for Health.

The GPE trial ran for 20 working days in each hub, resulting in 10 clusters of invitees receiving an endorsed invitation letter and 10 control clusters in each hub. The intervention took place between 3 and 28 June 2013, and intervention 4 was designed to run 5 weeks after the start of intervention 3 so as to include all non-responding participants.
Intervention 4: enhanced reminder letter
This trial ran for 20 working days in each hub, resulting in 10 clusters of invitees receiving the ER on random days within the trial period. This intervention took place 5 weeks from the start of intervention 3 (GPE), between 8 July and 2 August 2013, in all the hubs in order to achieve the factorial design as shown in Figure 13. This design allowed us to examine the effect of the ER alone and its effect subsequent to GPE. The schema of processes for the ER within the BCSS and the hubs is shown in Figure 14.
FIGURE 13.
Factorial design of interventions 3 (GPE) and 4 (ER).
FIGURE 14.
Intervention 4 (ER) within the BCSS.
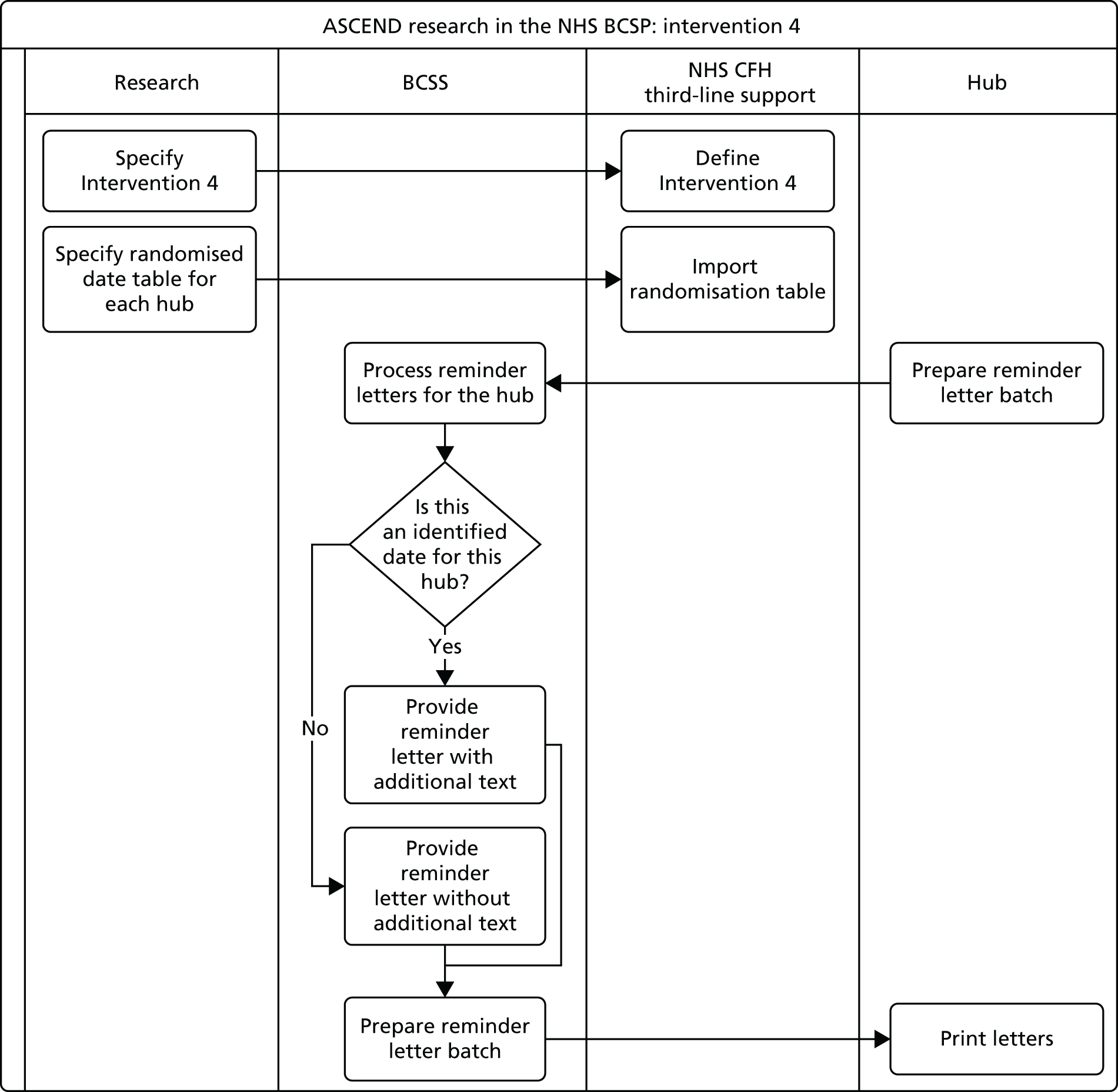
Comparators
Interventions 1 and 2 compared the effectiveness of an additional leaflet (gist or narrative) added to the materials posted with the S1 letter with usual NHS BCSP practice.
Interventions 3 and 4 compared the effectiveness of the GPE banner on the S1 letter and the enhanced text on the S10 reminder letter with usual practice (see Figure 13).
Randomisation
Cluster randomisation was used in all four trials. Individuals who were routinely invited for screening by the NHS BCSP in England were allocated to receive an intervention on randomly selected days within the pre-specified time period. In consultation with all NHS BCSP hubs, we identified several periods in the calendar which would enable us to run our trials outside major public holidays and also to allow temporal separation of the interventions, when necessary. Two weeks before the start of each intervention, the randomisation sequence for each NHS BCSP hub was generated by a statistician. For each set of random numbers, days were allocated to the intervention materials (standard information leaflet + gist or narrative in trials 1 and 2; GPE invitation in trial 3; ER letter in trial 4) or usual care (standard information leaflet for trials 1 and 2, standard invitation/reminder letters for trials 3 and 4), based on whether the number was above or below the median of the random numbers. The BCSS is designed to generate batches of invitations by screening centre. Because the number of daily invitations to each screening centre population is quite variable, we expected some imbalance in the numbers receiving intervention versus control interventions in some hubs. Therefore, in addition to randomising by day, the hubs ensured that letter batches were generated for each screening centre every day during each intervention.
For interventions 1 and 2, each of the NHS BCSP hubs received a table with dates on which their population should receive the leaflet interventions. These tables with randomisation dates were also given to HSCIC (the organisation responsible for the BCSS) and to REAL Digital International (Croydon, UK), the company that distributes invitations for three of the NHS BCSP hubs (London, Southern and Eastern). The other two hubs print all the materials ‘in-house’.
For interventions 3 and 4, cluster randomisation was undertaken directly by the BCSS. HSCIC was given randomisation dates for each hub and these were programmed into the BCSS to specify which days would generate intervention letters. As a further measure of quality assurance, randomisation schedules were not provided to the hubs and were instead sent only to HSCIC. Hubs were effectively ‘blind’ to the randomisation schedule and reported back to confirm whether or not the intervention was included on the S1 letter every day, which the Trial Office then checked against the randomisation schedule. Randomisation schedules were supplied to the HSCIC 2 weeks in advance of the trial.
Blinding
Blinding was not possible but the possibility of biasing participation was minimal owing to the lack of direct contact with participants. Individuals were unaware of a comparator condition unless a member of their household received an invitation during the study period that contained different information materials.
Eligible population
All men and women aged 60–74 years who are registered with a GP are eligible to be screened for bowel cancer in England and were therefore eligible to be included in our trials. Invited individuals could contact their NHS BCSP hub and opt out of the current screening episode or choose to be ‘ceased’ from the screening programme if they wish. ‘Ceased’ subjects, if ceased prior to their screening due date, would not be invited to be screened.
Because the screening programme started in 2006, many of the eligible population had been invited and/or had participated in previous rounds of screening.
Consent
Consent forms were not required in this study because the interventions took place as part of subjects’ usual communication from the NHS BCSP. The activities of the NHS BCSP are covered by National Information Governance Board approval with regard to the handling of patient-identifiable data (reference PIAG 1–08(a)/2003).
Exclusion criteria
We had two exclusion criteria:
-
In intervention 3 (GPE invitation letters) we randomised people eligible to receive this intervention only if they were registered with practices which had agreed to endorse the NHS BCSP. However, owing to the intensive approach we took to contact all general practices in England (see Chapter 7), permission to link the screening invitation to the practice address was granted by 6480 out of the 8142 practices (80%). If no permission was granted, individuals attending that practice were excluded from the GPE trial.
-
Participants requesting translation of materials were excluded from the analysis (see Translation of materials into other languages).
Outcomes
Primary outcome
Screening uptake was defined as the return of a gFOBt kit within 18 weeks of the invitation that led to a ‘definitive’ test result of either ‘normal’ (i.e. no further investigation required) or ‘abnormal’ (i.e. requiring referral for further testing, usually colonoscopy) by the date of data extraction (18 weeks after the last day of the intervention).
Index of Multiple Deprivation
The English Indices of Deprivation 2010206 use 38 separate indicators, organised across seven distinct domains (income, employment, health and disability, education skills and training, barriers to housing and other services, crime, and living environment). These are combined, using appropriate weights, to calculate the IMD. This is an overall measure of multiple deprivation experienced by people living in an area and is calculated for every LSOA in England. Each LSOA covers approximately 1500 individuals. The IMD can be used to rank every LSOA in England according to their relative level of deprivation. We used IMD quintile because of its demonstrated ability to explain socioeconomic variations in bowel cancer screening uptake at the LSOA level. 12 IMD is freely available and widely accepted and used, enabling direct comparison of our results with other studies.
Secondary outcomes
Our secondary outcomes were:
-
time taken to return gFOBt kit by IMD quintile
-
proportion of spoilt kits and their relationship to IMD quintile
-
proportion of non-delivered kits by IMD quintile
-
incremental cost per screening invitation
-
incremental cost per screening invitation, both by IMD quintile and overall
-
all of the above outcomes analysed using other socioeconomic variables.
Statistics
Sample size calculations
The sample size calculations were based on achieving a reduction in the socioeconomic gradient measured as an increasingly larger percentage point increase in uptake in increasingly more deprived quintiles. We anticipated that this would be accompanied by an improvement in mean overall uptake of screening, although this was not necessary to the sample size calculation.
Randomised controlled trials of each intervention were powered assuming that there would be a similar proportional effect in each NHS BCSP hub. Instead of positing an absolute effect (which may differ across hubs, given their different demographic characteristics), we assumed a fixed proportional effect. Because different hubs have different underlying uptake rates, we did not assume generalisability of absolute uptake rates or of absolute effects of the intervention on uptake rates as is common in clinical trial interpretation. Instead, we made the less sweeping assumption that proportional effects of interventions on uptake rates within specific IMD quintiles would be comparable across hubs.
Calculations of effects of interventions 1 and 2
We estimated an average increase of 3%, based on increasing uptake by 5% in the lowest IMD quintile (most socially disadvantaged group) and 1% in the highest quintile; giving an overall 1%–2%–3%–4%–5% difference by quintile (1% change in the least deprived group, 2% in the next least deprived, etc.). This estimate was drawn from the outcomes that are considered feasible in research aiming to increase screening uptake. 207 It would result in 35,175 more people being screened per year (11,366 in the lowest IMD quintile and 1932 in the highest). These numbers were calculated before the NHS BCSP had been extended to individuals aged up to and including 74 years in every hub. However, this had been achieved by the time the RCTs were carried out and so we expected the actual numbers of screened individuals to be higher than originally calculated.
We based our sample size calculations on the ability to detect a difference in the parameter b, where the logit of the overall participation rate = a + bx (participation in each IMD quintile). This can be re-expressed as a comparison of two proportions where each proportion is a weighted average of the within-quintile uptake rates. 208 The analytical results of the sample size calculations were confirmed by computer simulations.
Because the NHS BCSP hubs vary in the size of the population they serve, baseline uptake rates and socioeconomic profile, we calculated the required sample size for each hub separately. Thus, for 90% power to detect as significant a change in the gradient conferring as a 5-percentage-point increase in lowest quintile uptake versus a 1-percentage-point increase in the highest quintile, the estimated numbers required per group (intervention and control) overall in all hubs combined were 13,500, 12,200, 11,700, 5400 and 4500 if we assumed that all participants had the composition of Midlands and North West, London, North East, Eastern, and Southern Hubs, respectively. We used the maximum of the calculated sample sizes, that for the Midlands and North West, as a failsafe option. This meant that, whatever the socioeconomic composition and underlying uptake rate of the hub or combination of hubs in any given RCT, the study size would be adequate. Thus, each RCT needed a total of 13,500 participants per arm across all hubs. However, as we randomised by day with an average of approximately 3000 letters sent per hub per day, we needed to increase this by the variance inflation factor:
where VIF is the variance inflation factor, c is the coefficient of variation between days with respect to number of invitations and r is the intraclass correlation coefficient of uptake levels by day. From data supplied by the hubs, c was estimated as 0.42 and r as 0.0002, provided the duration was of the order of 1 month, so that there was no serious seasonal variation. Thus, we needed to multiply the study sizes by 1.7. We therefore aimed to achieve 23,000 per arm or 46,000 in total for each RCT to detect a 1%–2%–3%–4%–5% difference in uptake in the least to most deprived IMD quintile (90% power; p < 0.05).
Bearing in mind that a total of 60–70,000 invitations are sent out nationally in a typical working week, this would mean that the required sample size could be obtained within a working week (5 days). However, the number of clusters would be small. We therefore used 2 weeks’ (10 days’) invitations for each of the narrative and gist interventions. This resulted in the study being overpowered, but achieved 40–50 day/hub clusters, which conferred confidence of avoiding inadvertent bias, for example owing to one large but aberrant day/hub cluster.
Calculations for interventions 3 and 4
The same fundamental assumptions were made as above. In addition, for the GPE intervention, with all the hubs participating and all general practices participating, we would require the same period and study size as for the narrative and the gist interventions. However, assuming 30% general practice participation, in order to recruit 46,000, we would need to run the trial for close to 3 weeks (15 days). As a failsafe measure, we allowed 4 full working weeks (20 days) to achieve approximately 40,000 subjects per arm and a total of 100 day/hub clusters. In addition, for the ER and in order to be conservative, we doubled the time required, assuming that around 50% of individuals need reminders and that the ER has only the same effect on those receiving it as any other intervention applied to the entire invited population. This could theoretically have been achieved in 2 weeks but, as a failsafe, we ran the trial for 4 weeks, which would amount to approximately 60,000 reminders and a total of 50 day/hub clusters per arm.
Data analyses
We undertook a descriptive analysis of sociodemographic characteristics in the two arms across each intervention and analysed uptake differences by LR. Although randomisation should ensure comparability, the analysis was performed with and without adjustment for age, sex, screening round and hub. 209 The question of whether or not the intervention had a greater impact on uptake in the lower SEC groups was assessed by a test of interaction between trial arm and IMD quintile in the LR analysis. Analyses were also performed with conservative variance estimation in order to take account of the cluster randomisation. We also used hierarchical LR to account for heterogeneities (i.e. owing to varying policies and procedures in PCTs). We adjusted for screening episode status and checked for heterogeneity of effects between incident and prevalent screens. The use of the maximum sample size from the calculation above also enabled subgroup analyses by sex, age, hub and incident versus prevalent screening episode. A secondary analysis of time taken to return the gFOBt kit by IMD quintile was examined using the log-rank method. 210 Analyses were performed on an intention-to-treat basis using SAS v9.3 (SAS Institute Inc., Cary, NC, USA) and Stata v12.1 (StataCorp LP, College Station, TX, USA).
Definitions of variables
Interventions
For each intervention, the term SI refers to the standard NHS BCSP invitation information posted (the control group for each intervention).
Index of Multiple Deprivation quintiles
Individuals were categorised by IMD quintile based on the ranking of LSOAs using overall IMD score:206
-
least deprived (0–8.49)
-
second quintile (8.50–13.79)
-
third quintile (13.80–21.35)
-
fourth quintile (21.36–34.17)
-
most deprived (34.18–87.80).
Bowel Cancer Screening Programme hub codes
The hubs are referred to below using the NHS BCSP-specific hub codes:
-
BCS01: Midlands and North West
-
BCS02: Southern
-
BCS03: London
-
BCS04: North East
-
BCS05: Eastern.
Screening episode
Individuals were categorised by screening according to the following three subgroups:
-
‘Prevalent first-time invitees’ (i.e. subjects invited to be screened for the first time)
-
‘Prevalent previous non-responders’ (i.e. subjects invited to be screened at least once previously, who have never responded)
-
‘Incident’ (i.e. subjects screened previously).
Adequately screened
An individual was defined as ‘adequately screened’ if they returned a gFOBt kit within 18 weeks of the invitation that led to a ‘definitive’ test result of either ‘normal’ (i.e. no further investigation required) or ‘abnormal’ (i.e. requiring referral for further testing, usually colonoscopy) by the date of data extraction (18 weeks after the last day of the intervention).
Local and concurrent initiatives
It was unfeasible to use hierarchical LR to account for heterogeneities. Instead we attempted to identify all research initiatives and health promotion activities between October 2012 and October 2013. This covered the period directly before, during and directly after the ASCEND trials. In order to identify these initiatives we contacted specific key informants from a wide range of national and regional organisations, as well as a number of opportunistic contacts. More detailed methods and our results are reported in Appendix 34.
Translation of materials into other languages
Text on the back of the usual invitation letter appears in 13 languages, inviting individuals to ask an English-speaking person to call the telephone helpline on their behalf to request the written materials in the language required. ‘The Facts’ leaflet is available in 21 languages. 7 Data on the number of calls made to the telephone helpline with requests for written information in another language were collected routinely by only one hub.
We intended to translate each of the ASCEND interventions into all of the languages currently offered by the NHS BCSP. This would require including a code within the invitation letter which callers would give to the telephone helpline assistant at the hub so the hub would know whether to send translations of the intervention materials or the usual materials. However, during the development of the interventions, the NHS BCSP hub directors informed us that they believed that very few requests for translated materials would be made during the intervention periods (10 days for narrative and ‘gist’ leaflets; 20 days each for GPE and ER). To confirm this, each hub monitored telephone requests for translated materials over 10 days. Four hubs undertook this exercise during March 2012 to reflect the period in which one of the interventions (the narrative leaflet) was scheduled to take place in 2013. The fifth hub (Midlands and North West) provided us with the data it collected over 23 days in March 2011.
Very few requests for translated materials were made during the monitoring periods (Table 19). It was therefore agreed between the research team and the funders that paying for translations was not an efficient use of resources.
| Language requested | NHS BCSP hub | Total requests in each language | ||||
|---|---|---|---|---|---|---|
| Londona | Southerna | Easterna | North Easta | Midlands and North Westb | ||
| Arabic | 1 | 0 | 0 | 0 | 0 | 1 |
| Bengali | 2 | 1 | 0 | 0 | 1 | 4 |
| Czechc | 0 | 0 | 1 | 0 | 0 | 1 |
| Farsi | 1 | 0 | 0 | 0 | 0 | 1 |
| French | 0 | 0 | 0 | 0 | 1 | 1 |
| Gujarati | 1 | 1 | 3 | 0 | 0 | 5 |
| Hindi | 0 | 0 | 1 | 0 | 0 | 1 |
| Italian | 1 | 0 | 0 | 0 | 0 | 1 |
| Nepalesed | 0 | 2 | 0 | 0 | 0 | 2 |
| Polish | 0 | 0 | 0 | 0 | 1 | 1 |
| Punjabi | 0 | 0 | 0 | 1 | 12 | 13 |
| Traditional Form Chinese | 3 | 0 | 1 | 0 | 2 | 6 |
| Turkish | 0 | 0 | 1 | 0 | 0 | 1 |
| Urdu | 0 | 0 | 2 | 2 | 2 | 6 |
| Welshc | 0 | 0 | 0 | 0 | 1 | 1 |
| Total | 9 | 4 | 9 | 3 | 20 | 45 |
Measuring costs
We calculated the extra costs incurred by each intervention in terms of the incremental cost per screening invitation. Originally as part of workstream 3 we planned to calculate the incremental cost per screening invitation of each intervention taking a lifetime time horizon and including the costs of each intervention, screening costs and costs of diagnosing and managing bowel cancer from the perspective of the NHS and Personal Social Services. The costs were to be considered alongside the primary outcomes of the RCT in a cost–consequences analysis framework to identify which interventions should be combined in the RCT of the complex intervention to be tested in a fourth workstream. Owing to the RCT results (see Chapter 10), workstream 4 was not funded and the resourcing for the health economic analysis was limited. Hence, the economic analysis was reduced to a simple calculation of intervention costs. As the intervention costs were expected to be extremely small, the reviewers argued that we did not need to undertake a more comprehensive economic analysis in workstream 3. Note that since the plans for the economic analysis were curtailed, we used the limited resources available to instead undertake a new study evaluating individual-level factors affecting inequality in bowel cancer screening uptake.
All costs were based on actual costs incurred during the study and valued using market prices. For the gist and narrative interventions, extra costs were incurred when printing and transporting the additional leaflets. Average marginal costs per person screened were calculated by dividing the total additional costs by the number of people receiving each intervention in the two trials. For the GPE and the ER, costs were incurred when modifying the NHS BCSP information technology (IT) system to incorporate the GPE as part of the standard invitation and the ER as part of the reminder letter. No additional intervention costs were incurred.
Ethics
Ethics approval
Ethics approval was obtained from the UK National Research Ethics Service, London, Harrow Ethics Committee, Reference number 12/LO/1396, prior to commencement of the study. Local Ethics Committee approval was not required as this was a national trial incorporated within the NHS BCSP. Site approval was obtained at each of the NHS BCSP hubs.
Risks for trial participants
Risks to individuals associated with the ASCEND programme were not any higher than the risks associated with a usual screening invitation from the NHS BCSP. During workstreams 1 and 2, the ASCEND project gained input from patient representatives and charities who provided advice on the design and approach taken by the study and the development of interventions. No concerns were raised.
Clinical trial documentation
In accordance with UCL Records Management Policy, the UCL Joint Research Office and the European Union Good Clinical Practice Directive 2005/28/EC, all primary research data will be retained for a minimum of 20 years following completion of the study.
Data management
A data analyst at the NHS BCSP Southern Hub working on behalf of all NHS BCSP hubs designed and piloted the data extraction algorithms. The raw data were extracted by HSCIC from BCSS and given to the data analyst at the Southern Hub to clean and anonymise by replacing BCSS pseudoanonymised identifiers with project-specific identifiers that cannot be cross-referenced to any other BCSS data sets. The IMD score was supplied in lieu of postcode.
The Southern Hub specified the data to be extracted by HSCIC on behalf of the ASCEND research team. Data were extracted using direct Search and Query Language queries on the BCSS with the additional use of Oracle Business Intelligence Enterprise Edition bespoke reports. Patient-identifiable information included practice, IMD score (derived from postcode) and year of birth.
Data transfer between HSCIC and the Southern Hub was carried out via an encrypted network connection (NHS.NET) or NHS secure file transfer (SFT).
The raw data were then loaded into an encrypted Oracle database at the Southern Hub and algorithms were written to process and anonymise the data into the format required by the ASCEND academic statisticians. Any permutations of geodemographic variables that could lead to potentially identifiable patient information were subject to further levels of anonymisation at this stage.
Although anonymised, data transfer between Southern Hub and the ASCEND research team used password-protected files and SFT protocol. The ASCEND research team kept the data secure and did not attempt to reverse engineer, link the data to other data sets or use the data for any purpose other than the ASCEND project. The project adhered to the NHS Cancer Screening Programmes Confidentiality and Disclosure Policy.
The data were extracted from the BCSS 18 weeks following the last intervention date for interventions 1 and 2 in March and July, respectively, and in November 2013 for interventions 3 and 4.
Quality control and quality assurance
Process evaluation
Prior to the start of any of the interventions the trial managers visited all NHS BCSP hubs and conducted semistructured interviews with hub directors and managers. The principle objective of these interviews was to identify organisational and resource-related barriers to successful roll-out of each intervention.
Particular note was taken of any adverse effects (e.g. excessive extra workload) that could make implementation of any of the interventions unfeasible. With an increase in gFOBt uptake we expected an increase in the workload in the hubs and in associated screening centres. In addition, we expected that general practices may experience an increase in their workload following intervention 3 (e.g. an increase in patients asking questions about the screening invitation).
It was expected that subjects, screening centres and general practices would communicate their experience to the NHS BCSP helpline and so we asked the hubs to record all queries associated with the interventions while the trials were taking place (and for up to 18 weeks afterwards to ensure all queries were included).
The hubs reported no substantial organisational or resource-related barriers and no increase in helpline queries. A summary of the queries received is described in the process evaluation report in Appendix 35.
Evaluation of intervention fidelity
In order to ensure fidelity of the interventions, the trial managers established and piloted quality check methods at each hub to ascertain whether or not the appropriate materials were sent to eligible people on particular days. In agreement with all the hubs, a standard operating procedure (SOP) was also put in place prior to the start of each trial.
In addition, hubs were to approach a random subsample of 100 recipients of each intervention 2 weeks after receipt of the information materials. However, at the time of the inventions, this exercise was not feasible owing to additional workload incurred by hubs in setting up the new FS screening programme, which started after the completion of the ASCEND programme. Instead, a more extensive quality assurance process was implemented as an objective and less intrusive assessment of fidelity, described in Interventions 1 and 2 and Interventions 3 and 4.
Interventions 1 and 2
These two interventions required inclusion of an additional information leaflet with each S1 invitation letter on pre-specified random dates. Randomisation tables were provided to each hub and also to REAL Digital International, the company that prints and distributes NHS BCSP letters for the Eastern, Southern and London Hubs.
The quality checks for interventions 1 and 2 included adding one ‘monitor’ letter with each batch of letters the hubs produced during the trial. Monitor letters were addressed to the imperial trial office in order to check that the contents of letters corresponded to the randomisation schedule for the hub. In addition, hubs also undertook an internal quality assurance measure to include photographic evidence of the content of the letters each day at the start of the packing processing, as well as signing off a logbook spreadsheet to note when the intervention was included.
Interventions 3 and 4
These two interventions required insertion of the name of the individual’s general practice into the S1 letter or additional text into the S10 letter. This involved implementing changes on the BCSS. We conducted extensive consultations with HSCIC, whose programmers applied the necessary changes to the BCSS to ensure that GPE letters and ER letters were generated on pre-specified random dates. Changes to the BCSS included addition of a variable that indicated whether a subject had received an intervention or was a part of the control cluster.
The research team provided HSCIC with details of all the general practices that had agreed to endorse the NHS BCSP and randomisation dates for each hub.
The process diagrams for the GPE and ER letters are detailed in Figures 12–14.
In order to ensure fidelity, and similarly to the first two interventions, a SOP was produced for each hub to follow and was monitored closely by the trial managers during the intervention periods. The quality checks included a logbook designed to enable the hub staff to report back on whether or not intervention banners were present on their daily S1 and S10 letter batches. Hub staff sent copies of the completed logbooks on a daily basis to the trial office as evidence of the appropriate randomisation for each hub.
The trial managers produced a post-trial report after each intervention on the effectiveness in adherence to the SOPs and feedback from the hubs.
Monitoring and auditing
An Independent Data Monitoring and Ethics Committee was established to monitor the progress of the trial. Data were supplied to the Committee and meetings took place on the following dates: 28 January 2013, 12 July 2013 and 11 April 2014.
An advisory group was also established and included all ASCEND coapplicants and a number of external experts. The advisory group met formally in September 2012 and also provided expert input on an individual basis as required throughout the study period.
The chief investigator was responsible for the day-to-day monitoring and management of the study. The UCL Hospital/UCL/Royal Free Joint Research Office, on behalf of UCL as sponsor, monitors and conducts random audits on a selection of studies in its clinical research portfolio each year. Monitoring and auditing was conducted in accordance with the Department of Health Research Governance Framework for Health and Social Care (and the second edition of this document was published in April 2005)211 and in accordance with the sponsor’s monitoring and audit policies and procedures.
Chapter 10 Workstream 3: the ASCEND national randomised controlled trial results
Introduction
Chapter 9 described how the four RCTs were carried out, and in this chapter we present the results from the four trials. The gist and narrative trials were run over 10 consecutive days in November 2012 and March 2013, respectively. The GPE and ER RCTs were run over 20 consecutive days in June 2013 and July–August 2013, respectively.
Findings
Trial sample sizes were as follows: gist (n = 163,525), narrative (n = 150,417), GPE (n = 265,434) and ER (n = 168,480); Table 20.
| Cluster randomisation nationally by hub and day: Eastern, London, North East, Midlands and North West and Southern | Length of trial (weekdays) | Trial sample size (number of clusters) | Dates of trials |
|---|---|---|---|
| Intervention 1: gist leaflet | 10 weekdays | 163,525 (50 clusters) | 5–16 November 2012 |
| Intervention 2: narrative leaflet | 10 weekdays | 150,417 (50 clusters) | 4–15 March 2013 |
| Intervention 3: GPE | 20 weekdays | 265,434 (98 clusters) | 3–28 June 2013 |
| Intervention 4: ER | 20 weekdays | 168,480 (99 clusters) | 8 July–2 August 2013 |
Interventions 1 and 2 each comprised 50 clusters, as planned. Interventions 3 and 4 were supposed to each comprise 100 clusters; however, owing to protocol violations whereby the incorrect randomisation was employed on three occasions, intervention 3 ended up comprising 98 clusters and intervention 4 comprised 99 clusters.
Overview of results for all trials
Tabular data will be presented in the following order below:
-
baseline data for each trial and each arm (see Tables 21–24 for gist, narrative, GPE and ER, respectively)
-
numbers and percentages adequately screened for each trial and trial arm by sex, hub, screening episode type and age (see Tables 25–28, respectively)
-
numbers and percentages adequately screened for each trial, trial arm by SEC quintile (see Tables 29–32, respectively)
-
LR results for each trial by sex, hub, screening episode type and age (see Tables 33–36, respectively)
-
LR results for each trial by SEC quintile (see Tables 37–40, respectively)
-
test results for heterogeneity of intervention effect by screening episode type for each intervention for each trial (see Table 41)
-
times taken to return kits for each trial by SEC quintile (see Tables 42–45, respectively)
-
diagnostic outcomes by arm for each trial (see Tables 46–49, respectively).
We report an overview of the results in this subsection and brief summaries of main results trial by trial in the following four subsections.
Tables 21–24 show the baseline characteristics age sex, SECs, hub and screening episode type (incident, prevalent first invitation or prevalent previous non-responder), of subjects in each trial, by trial arm. These demonstrate that the baseline characteristics were well balanced in each trial. In BCS05, the intervention group was slightly larger than the control group in two trials (gist and GPE) and slightly smaller than the control group in the other two trials. In BCS02, the control group was slightly larger in the GPE RCT. In all cases, these imbalances were expected because of the process of randomising by day and hub, since numbers of invitations sent varied by hub and day. In all four trials the proportion of individuals screened decreased as deprivation increased in both arms. Individuals were categorised by IMD quintiles based on the national distribution of scores, rather than by the distribution of scores in our sample (i.e. not 20% in each quintile).
| Variablesa | SI + gist (N = 84,421) | SI (N = 79,104) |
|---|---|---|
| Age at invite (years), median (range) | 66 (59–74) | 66 (59–74) |
| IMD deprivation score, median (range) | 14.9 (0.5–87.8) | 14.8 (0.5–87.8) |
| Sex, % (n) | ||
| Female | 51.2 (43,195) | 51.4 (40,671) |
| Male | 48.8 (41,226) | 48.6 (38,433) |
| SEC quintile, % (n) | ||
| Least deprived (0–8.49) | 22.6 (19,055) | 23.5 (18,554) |
| Second quintile (8.50–13.79) | 23.5 (19,787) | 23.2 (18,295) |
| Third quintile (13.80–21.35) | 21.7 (18,320) | 20.3 (15,993) |
| Fourth quintile (21.36–34.17) | 17.5 (14,747) | 17.1 (13,469) |
| Most deprived (34.18–87.80) | 14.7 (12,374) | 16.0 (12,660) |
| Missing | 138 | 133 |
| Hub, % (n) | ||
| BCS01 | 26.6 (22,469) | 30.8 (24,369) |
| BCS02 | 24.5 (20,651) | 26.6 (21,004) |
| BCS03 | 8.8 (7416) | 8.4 (6636) |
| BCS04 | 16.1 (13,614) | 16.3 (12,858) |
| BCS05 | 24.0 (20,271) | 18.0 (14,237) |
| Screening episode, % (n) | ||
| Incident | 53.3 (45,019) | 53.3 (42,143) |
| Prevalent first-time invitees | 15.4 (13,034) | 15.7 (12,410) |
| Prevalent previous non-responders | 31.2 (26,368) | 31.0 (24,551) |
| Variablesa | SI + narrative (N = 73,722) | SI (N = 76,695) |
|---|---|---|
| Age at invite (years), median (range) | 65 (59–74) | 65 (59–74) |
| IMD deprivation score, median (range) | 15.1 (0.5–87.8) | 15.1 (0.5–87.8) |
| Sex, % (n) | ||
| Female | 51.5 (37,937) | 51.0 (39,086) |
| Male | 48.5 (35,785) | 49.0 (37,609) |
| SEC quintile, % (n) | ||
| Least deprived (0–8.49) | 23.2 (17,027) | 22.3 (17,073) |
| Second quintile (8.50–13.79) | 22.5 (16,517) | 23.1 (17,675) |
| Third quintile (13.80–21.35) | 20.8 (15,287) | 21.1 (16,161) |
| Fourth quintile (21.36–34.17) | 17.6 (12,897) | 17.5 (13,385) |
| Most deprived (34.18–87.80) | 16.0 (11,722) | 15.9 (12,127) |
| Missing | 272 | 274 |
| Hub, % (n) | ||
| BCS01 | 29.1 (21,421) | 27.5 (21,118) |
| BCS02 | 28.0 (20,667) | 21.8 (16,723) |
| BCS03 | 11.5 (8509) | 11.5 (8795) |
| BCS04 | 17.7 (13,053) | 16.8 (12,900) |
| BCS05 | 13.7 (10,072) | 22.4 (17,159) |
| Screening episode, % (n) | ||
| Incident | 49.1 (36,232) | 53.8 (41,293) |
| Prevalent first-time invitees | 20.7 (15,281) | 16.3 (12,510) |
| Prevalent previous non-responders | 30.1 (22,209) | 29.8 (22,892) |
| Variablesa | SI + GPE (N = 131,423) | SI (N = 134,011) |
|---|---|---|
| Age at invite (years), median (range) | 65 (59–74) | 65 (59–74) |
| IMD deprivation score, median (range) | 14.7 (0.5–87.8) | 14.6 (0.5–87.8) |
| Sex, % (n) | ||
| Female | 51.0 (66,986) | 51.2 (68,591) |
| Male | 49.0 (64,437) | 48.8 (65,420) |
| SEC quintile, % (n) | ||
| Least deprived (0–8.49) | 23.1 (30,350) | 23.4 (31,381) |
| Second quintile (8.50–13.79) | 23.6 (30,952) | 23.4 (31,340) |
| Third quintile (13.80–21.35) | 21.3 (27,950) | 21.0 (28,181) |
| Fourth quintile (21.36–34.17) | 17.1 (22,450) | 17.2 (23,007) |
| Most deprived (34.18–87.80) | 14.6 (19,174) | 14.6 (19,540) |
| Missing | 547 | 562 |
| Hub, % (n) | ||
| BCS01 | 27.4 (35,993) | 25.8 (34,598) |
| BCS02 | 24.2 (31,760) | 30.3 (40,550) |
| BCS03 | 9.0 (11,818) | 9.9 (13,255) |
| BCS04 | 16.2 (21,272) | 16.0 (21,439) |
| BCS05 | 23.3 (30,580) | 18.0 (24,169) |
| Screening episode, % (n) | ||
| Incident | 52.3 (68,695) | 52.3 (70,134) |
| Prevalent first time invitees | 17.0 (22,287) | 17.6 (23,582) |
| Prevalent previous non-responders | 30.8 (40,441) | 30.1 (40,295) |
| Variablesa | SI + ER (N = 78,067) | SI (N = 90,413) |
|---|---|---|
| Age at invite (years), median (range) | 65 (59–74) | 64 (59–74) |
| IMD deprivation score, median (range) | 16.4 (0.5–87.8) | 16.2 (0.5–87.8) |
| Sex, % (n) | ||
| Female | 48.4 (37,747) | 48.2 (43,574) |
| Male | 51.6 (40,320) | 51.8 (46,839) |
| SEC quintile, % (n) | ||
| Least deprived (0–8.49) | 20.4 (15,933) | 20.9 (18,928) |
| Second quintile (8.50–13.79) | 21.3 (16,594) | 21.5 (19,446) |
| Third quintile (13.80–21.35) | 20.6 (16,092) | 20.2 (18,286) |
| Fourth quintile (21.36–34.17) | 18.8 (14,679) | 18.6 (16,853) |
| Most deprived (34.18–87.80) | 18.5 (14,441) | 18.2 (16,489) |
| Missing | 328 | 411 |
| Hub, % (n) | ||
| BCS01 | 28.2 (22,051) | 28.2 (25,490) |
| BCS02 | 24.5 (19,131) | 25.6 (23,107) |
| BCS03 | 13.8 (10,809) | 11.5 (10,385) |
| BCS04 | 15.7 (12,291) | 14.2 (12,796) |
| BCS05 | 17.7 (13,785) | 20.6 (18,635) |
| Screening episode, % (n) | ||
| Incident | 30.4 (23,722) | 28.6 (25,813) |
| Prevalent first time invitees | 18.6 (14,483) | 23.5 (21,271) |
| Prevalent previous non-responders | 51.1 (39,862) | 47.9 (43,329) |
Tables 25–28 summarise the number of individuals in each trial arm who returned a gFOBt kit (or kits) that led to a ‘definitive’ screening test result within 18 weeks of being sent the routine screening invitation (i.e. who were adequately screened). Thus, overall uptake was 57.4% for gist, 57.7% for narrative, 57.9% for GPE and 25.4% for the ER. Once again, the distribution of adequately screened individuals was broadly similar in both arms of each trial. However, the proportion of adequately screened individuals decreased as the deprivation score increased in both arms (Tables 29–32). For example, in the gist trial, the proportion decreased from 65.8% for the least deprived to 43.0% for the most deprived in the intervention arm and from 65.6% for the least deprived to 42.0% for the most deprived in the control arm (see Table 29).
| Variables | SI + gist (N = 48,653) | SI (N = 45,290) |
|---|---|---|
| Sex, % (n) | ||
| Female | 59.2 (25,585) | 59.1 (24,017) |
| Male | 56.0 (23,068) | 55.4 (21,273) |
| Hub, % (n) | ||
| BCS01 | 54.9 (12,336) | 54.6 (13,297) |
| BCS02 | 59.0 (12,177) | 61.9 (12,991) |
| BCS03 | 55.0 (4078) | 55.2 (3665) |
| BCS04 | 58.2 (7918) | 57.4 (7382) |
| BCS05 | 59.9 (12,144) | 55.9 (7955) |
| Screening episode, % (n) | ||
| Incident | 85.2 (38,351) | 85.0 (35,830) |
| Prevalent first-time invitees | 49.6 (6466) | 48.2 (5981) |
| Prevalent previous non-responders | 14.5 (3836) | 14.2 (3479) |
| Age (years) | ||
| 60–64 | 54.9 (19,727) | 54.2 (18,200) |
| 65–69 | 60.8 (18,657) | 61.1 (17,346) |
| 70+ | 57.7 (10,269) | 56.9 (9744) |
| Total | 57.6 (48,653) | 57.3 (45,290) |
| Variables | SI + narrative (N = 41,822) | SI (N = 44,904) |
|---|---|---|
| Sex, % (n) | ||
| Female | 59.3 (22,499) | 60.9 (23,811) |
| Male | 54.0 (19,323) | 56.1 (21,093) |
| Hub, % (n) | ||
| BCS01 | 53.4 (11,439) | 57.6 (12,163) |
| BCS02 | 61.5 (12,712) | 60.2 (10,069) |
| BCS03 | 45.4 (3864) | 49.2 (4327) |
| BCS04 | 59.1 (7716) | 60.8 (7837) |
| BCS05 | 60.5 (6091) | 61.2 (10,508) |
| Screening episode, % (n) | ||
| Incident | 85.6 (31,031) | 85.7 (35,389) |
| Prevalent first-time invitees | 50.2 (7678) | 49.8 (6231) |
| Prevalent previous non-responders | 14.0 (3113) | 14.3 (3284) |
| Age (years) | ||
| 60–64 | 53.3 (18,264) | 55.2 (19,014) |
| 65–69 | 60.9 (14,673) | 62.4 (16,673) |
| 70+ | 57.9 (8885) | 59.2 (9217) |
| Total | 56.7 (41,822) | 58.5 (44,904) |
| Variables | SI + GPE (N = 76,520) | SI (N = 77,122) |
|---|---|---|
| Sex, % (n) | ||
| Female | 60.8 (40,707) | 60.2 (41,290) |
| Male | 55.6 (35,813) | 54.8 (35,832) |
| Hub, % (n) | ||
| BCS01 | 55.2 (19,869) | 55.4 (19,150) |
| BCS02 | 62.7 (19,915) | 60.3 (24,437) |
| BCS03 | 49.5 (5850) | 48.2 (6385) |
| BCS04 | 59.8 (12,710) | 58.9 (12,631) |
| BCS05 | 59.4 (18,176) | 60.1 (14,519) |
| Screening episode, % (n) | ||
| Incident | 86.4 (59,380) | 85.7 (60,119) |
| Prevalent first-time invitees | 51.4 (11,465) | 49.4 (11,646) |
| Prevalent previous non-responders | 14.0 (5675) | 13.3 (5357) |
| Age (years) | ||
| 60–64 | 55.9 (33,331) | 54.8 (33,480) |
| 65–69 | 61.0 (27,382) | 60.5 (27,466) |
| 70+ | 58.7 (15,807) | 58.8 (16,176) |
| Total | 58.2 (76,520) | 57.5 (77,122) |
| Variables | SI + ER (N = 20,166) | SI (N = 22,712) |
|---|---|---|
| Sex, % (n) | ||
| Female | 27.2 (10,267) | 26.4 (11,511) |
| Male | 24.5 (9899) | 23.9 (11,201) |
| Hub, % (n) | ||
| BCS01 | 23.7 (5231) | 23.1 (5899) |
| BCS02 | 30.5 (5827) | 29.4 (6795) |
| BCS03 | 22.6 (2444) | 21.1 (2196) |
| BCS04 | 23.7 (2911) | 22.2 (2836) |
| BCS05 | 27.2 (3753) | 26.8 (4986) |
| Screening episode, % (n) | ||
| Incident | 59.2 (14,033) | 58.1 (14,985) |
| Prevalent first-time invitees | 25.8 (3739) | 25.4 (5398) |
| Prevalent previous non-responders | 6.0 (2394) | 5.4 (2329) |
| Age (years) | ||
| 60–64 | 26.7 (10,251) | 26.1 (12,229) |
| 65–69 | 26.8 (6674) | 24.8 (6898) |
| 70+ | 21.9 (3241) | 22.6 (3585) |
| Total | 25.8 (20,166) | 25.1 (22,712) |
| Variable | SI + gist (N = 84,421), % (n) | SI (N = 79,104), % (n) |
|---|---|---|
| Adequately screened | 57.6 (48,653) | 57.3 (45,290) |
| First quintile (least deprived) | 65.8 (12,547) | 65.6 (12,178) |
| Second quintile | 62.2 (12,305) | 62.4 (11,412) |
| Third quintile | 58.6 (10,732) | 58.4 (9335) |
| Fourth quintile | 52.0 (7663) | 51.9 (6987) |
| Fifth quintile (most deprived) | 43.0 (5322) | 42.0 (5316) |
| Variable | SI + narrative (N = 73,722), % (n) | SI (N = 76,695), % (n) |
|---|---|---|
| Adequately screened | 56.7 (41,822) | 58.5 (44,904) |
| First quintile (least deprived) | 64.6 (11,005) | 66.8 (11,411) |
| Second quintile | 62.1 (10,253) | 62.7 (11,080) |
| Third quintile | 58.3 (8911) | 59.4 (9601) |
| Fourth quintile | 50.7 (6535) | 52.9 (7083) |
| Fifth quintile (most deprived) | 42.4 (4966) | 46.0 (5580) |
| Variable | SI + GPE (N = 131,423), % (n) | SI (N = 134,011), % (n) |
|---|---|---|
| Adequately screened | 58.2 (76,520) | 57.5 (77,122) |
| First quintile (least deprived) | 65.2 (19,792) | 66.0 (20,716) |
| Second quintile | 63.1 (19,530) | 62.6 (19,604) |
| Third quintile | 59.3 (16,571) | 58.0 (16,336) |
| Fourth quintile | 53.0 (11,902) | 51.5 (11,839) |
| Fifth quintile (most deprived) | 44.0 (8433) | 42.6 (8324) |
| Variable | SI + ER (N = 78,067), % (n) | SI (N = 90,413), % (n) |
|---|---|---|
| Adequately screened | 25.8 (20,166) | 25.1 (22,712) |
| First quintile (least deprived) | 34.7 (5522) | 34.9 (6601) |
| Second quintile | 30.8 (5107) | 29.7 (5782) |
| Third quintile | 26.8 (4316) | 25.0 (4578) |
| Fourth quintile | 21.1 (3104) | 20.4 (3436) |
| Fifth quintile (most deprived) | 14.1 (2040) | 13.3 (2198) |
Univariate analyses (Tables 33–36) demonstrate the lack of significant association between screening uptake and intervention in any of the four trials in subgroups of screening episode, age, sex and hub. Analyses were performed with conservative variance estimation to take account of the cluster randomisation result in wider CIs. The GPE intervention increased the odds of uptake by 6% among incident responders (p = 0.045; see Table 35). In addition, an interaction with deprivation score was seen for incident responders to the ER (p = 0.054; see Table 36).
| Gist intervention | Univariate OR | Univariate OR with conservative variance | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | p-value for interaction with IMD score | |
| Hub | |||||
| BCS01 | 1.01 (0.98 to 1.05) | 0.46 | 1.01 (0.83 to 1.24) | 0.90 | 0.10 |
| BCS02 | 0.89 (0.85 to 0.92) | < 0.001 | 0.89 (0.84 to 0.94) | < 0.001 | 0.93 |
| BCS03 | 0.99 (0.93 to 1.06) | 0.78 | 0.99 (0.64 to 1.52) | 0.97 | < 0.01 |
| BCS04 | 1.03 (0.98 to 1.08) | 0.22 | 1.03 (0.89 to 1.19) | 0.68 | 0.09 |
| BCS05 | 1.18 (1.13 to 1.23) | < 0.001 | 1.18 (0.97 to 1.43) | 0.09 | 0.58 |
| Sex | |||||
| Female | 1.01 (0.98 to 1.04) | 0.60 | 1.01 (0.91 to 1.12) | 0.89 | 0.78 |
| Male | 1.02 (1.00 to 1.05) | 0.09 | 1.02 (0.92 to 1.14) | 0.65 | 0.33 |
| Screening episode | |||||
| Incident | 1.01 (0.98 to 1.05) | 0.49 | 1.01 (0.95 to 1.08) | 0.67 | 0.38 |
| Prevalent first-time invitees | 1.06 (1.01 to 1.11) | 0.02 | 1.06 (0.96 to 1.16) | 0.23 | 0.13 |
| Prevalent previous non-responders | 1.03 (0.98 to 1.08) | 0.23 | 1.03 (0.94 to 1.13) | 0.50 | 0.09 |
| Age (years) | |||||
| < 65 | 1.03 (1.00 to 1.06) | 0.05 | 1.03 (0.94 to 1.13) | 0.52 | 0.86 |
| 65–69 | 0.98 (0.95 to 1.02) | 0.36 | 0.98 (0.85 to 1.13) | 0.83 | 0.47 |
| 70 + | 1.04 (0.99 to 1.08) | 0.11 | 1.04 (0.90 to 1.19) | 0.64 | 0.46 |
| Total | 1.02 (1.00 to 1.04) | 0.12 | 1.02 (0.92 to 1.13) | 0.77 | 0.48 |
| Narrative intervention | Univariate OR | Univariate OR with conservative variance | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | p-value for interaction with IMD score | |
| Hub | |||||
| BCS01 | 0.84 (0.81 to 0.88) | < 0.001 | 0.84 (0.67 to 1.06) | 0.14 | 0.68 |
| BCS02 | 1.06 (1.01 to 1.10) | 0.01 | 1.06 (0.89 to 1.25) | 0.52 | 0.78 |
| BCS03 | 0.86 (0.81 to 0.91) | < 0.001 | 0.86 (0.68 to 1.08) | 0.20 | 0.67 |
| BCS04 | 0.93 (0.89 to 0.98) | < 0.01 | 0.93 (0.81 to 1.07) | 0.34 | 0.25 |
| BCS05 | 0.97 (0.92 to 1.02) | 0.21 | 0.97 (0.80 to 1.18) | 0.75 | 0.74 |
| Sex | |||||
| Female | 0.93 (0.91 to 0.96) | < 0.001 | 0.93 (0.81 to 1.07) | 0.34 | 0.47 |
| Male | 0.92 (0.89 to 0.95) | < 0.001 | 0.92 (0.81 to 1.05) | 0.20 | 0.20 |
| Screening episode | |||||
| Incident | 1.00 (0.96 to 1.04) | 0.82 | 1.00 (0.92 to 1.08) | 0.91 | 0.46 |
| Prevalent first-time invitees | 1.02 (0.97 to 1.07) | 0.47 | 1.02 (0.91 to 1.14) | 0.77 | 0.26 |
| Prevalent previous non-responders | 0.97 (0.92 to 1.03) | 0.32 | 0.97 (0.88 to 1.07) | 0.58 | 0.18 |
| Age (years) | |||||
| < 65 | 0.92 (0.90 to 0.95) | < 0.001 | 0.92 (0.82 to 1.05) | 0.21 | 0.11 |
| 65–69 | 0.94 (0.90 to 0.97) | < 0.001 | 0.94 (0.79 to 1.11) | 0.45 | 0.88 |
| 70+ | 0.95 (0.90 to 0.99) | 0.02 | 0.95 (0.82 to 1.09) | 0.43 | 0.17 |
| Total | 0.93 (0.91 to 0.95) | < 0.001 | 0.93 (0.81 to 1.06) | 0.27 | 0.27 |
| GPE intervention | Univariate OR | Univariate OR with conservative variance | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | p-value for interaction with IMD score | |
| Hub | |||||
| BCS01 | 0.99 (0.96 to 1.02) | 0.69 | 0.99 (0.90 to 1.10) | 0.91 | 0.42 |
| BCS02 | 1.11 (1.08 to 1.14) | < 0.001 | 1.11 (0.99 to 1.24) | 0.07 | 0.01 |
| BCS03 | 1.05 (1.00 to 1.11) | 0.035 | 1.05 (0.88 to 1.26) | 0.56 | 0.95 |
| BCS04 | 1.04 (1.00 to 1.08) | 0.08 | 1.04 (0.94 to 1.14) | 0.47 | 0.31 |
| BCS05 | 0.97 (0.94 to 1.01) | 0.132 | 0.97 (0.84 to 1.13) | 0.73 | 0.25 |
| Sex | |||||
| Female | 1.02 (1.00 to 1.05) | 0.031 | 1.02 (0.94 to 1.12) | 0.58 | 0.22 |
| Male | 1.03 (1.01 to 1.06) | 0.003 | 1.03 (0.96 to 1.12) | 0.40 | 0.13 |
| Screening episode | |||||
| Incident | 1.06 (1.03 to 1.09) | < 0.001 | 1.06 (1.00 to 1.13) | 0.045 | 0.68 |
| Prevalent first-time invitees | 1.09 (1.05 to 1.13) | < 0.001 | 1.09 (1.01 to 1.16) | 0.02 | 0.44 |
| Prevalent previous non-responders | 1.06 (1.02 to 1.11) | 0.002 | 1.06 (1.00 to 1.13) | 0.055 | 0.22 |
| Age (years) | |||||
| < 65 | 1.05 (1.02 to 1.07) | < 0.001 | 1.05 (0.98 to 1.12) | 0.20 | 0.06 |
| 65–69 | 1.02 (1.00 to 1.05) | 0.09 | 1.02 (0.92 to 1.13) | 0.66 | 0.55 |
| 70+ | 0.99 (0.96 to 1.03) | 0.71 | 0.99 (0.89 to 1.10) | 0.90 | 0.32 |
| Total | 1.03 (1.01 to 1.04) | 0.001 | 1.03 (0.95 to 1.11) | 0.49 | 0.11 |
| Univariate OR | Univariate OR with conservative variance | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | p-value for interaction with IMD score | |
| Hub | |||||
| BCS01 | 1.03 (0.99 to 1.08) | 0.14 | 1.03 (0.96 to 1.11) | 0.38 | 0.99 |
| BCS02 | 1.05 (1.01 to 1.10) | 0.02 | 1.05 (0.92 to 1.20) | 0.44 | 0.001 |
| BCS03 | 1.09 (1.02 to 1.16) | 0.01 | 1.09 (0.93 to 1.28) | 0.29 | 0.90 |
| BCS04 | 1.09 (1.03 to 1.16) | 0.004 | 1.09 (0.97 to 1.22) | 0.14 | 0.73 |
| BCS05 | 1.02 (0.97 to 1.08) | 0.35 | 1.02 (0.84 to 1.25) | 0.81 | 0.98 |
| Sex | |||||
| Female | 1.04 (1.01 to 1.07) | 0.012 | 1.04 (0.95 to 1.14) | 0.41 | 0.37 |
| Male | 1.04 (1.00 to 1.07) | 0.03 | 1.04 (0.95 to 1.13) | 0.45 | 0.24 |
| Screening episode | |||||
| Incident | 1.05 (1.01 to 1.08) | 0.013 | 1.05 (0.97 to 1.12) | 0.21 | 0.05 |
| Prevalent first-time invitees | 1.02 (0.98 to 1.07) | 0.35 | 1.02 (0.95 to 1.10) | 0.51 | 0.12 |
| Prevalent previous non-responders | 1.12 (1.06 to 1.19) | < 0.001 | 1.12 (1.03 to 1.23) | 0.008 | 0.43 |
| Age (years) | |||||
| < 65 | 1.03 (1.00 to 1.06) | 0.07 | 1.03 (0.96 to 1.11) | 0.44 | 0.06 |
| 65–69 | 1.11 (1.07 to 1.15) | < 0.001 | 1.11 (0.99 to 1.25) | 0.08 | 0.62 |
| 70+ | 0.96 (0.91 to 1.01) | 0.13 | 0.96 (0.83 to 1.10) | 0.56 | 0.79 |
| Total | 1.04 (1.02 to 1.06) | 0.001 | 1.04 (0.95 to 1.14) | 0.42 | 0.21 |
Fully adjusting for screening episode, age, sex and hub (Tables 37–40) confirmed that the gist and narrative interventions did not have a significant effect on uptake overall. The GPE invitation and ER both increased the odds of uptake by 7% (p < 0.0001 and p = 0.001 in a fully adjusted model; see Tables 39 and 40). GPE also had a stronger effect in the more deprived IMD quintiles but the interaction was not significant (p = 0.5). However, the ER trial showed a significant interaction with deprivation (p = 0.005), with a stronger effect in the most deprived quintile (OR = 1.11, 95% CI 1.04 to 1.20; p = 0.003) than in the least deprived quintile (OR = 1.00, 95% CI 0.94 to 1.06; p = 0.98).
| Variable | Univariate (gist) | Univariate (gist) with conservative variance | Multivariatea with conservative variance | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| IMD quintile | ||||||
| First quintile (least deprived) | 1.01 (0.97 to 1.05) | 0.67 | 1.01 (0.92 to 1.11) | 0.85 | 1.06 (1.01 to 1.11) | 0.02 |
| Second quintile | 0.99 (0.95 to 1.03) | 0.70 | 0.99 (0.91 to 1.09) | 0.86 | 1.02 (0.97 to 1.07) | 0.50 |
| Third quintile | 1.01 (0.97 to 1.05) | 0.69 | 1.01 (0.90 to 1.13) | 0.88 | 1.00 (0.94 to 1.08) | 0.92 |
| Fourth quintile | 1.00 (0.96 to 1.05) | 0.88 | 1.00 (0.91 to 1.11) | 0.94 | 1.01 (0.94 to 1.08) | 0.86 |
| Fifth quintile (most deprived) | 1.04 (0.99 to 1.10) | 0.10 | 1.04 (0.92 to 1.18) | 0.50 | 1.04 (0.96 to 1.12) | 0.37 |
| Total | 1.02 (1.00 to 1.04) | 0.12 | 1.02 (0.92 to 1.13) | 0.77 | 1.03 (0.99 to 1.06) | 0.15 |
| Variable | Univariate (narrative) | Univariate (narrative) with conservative variance | Multivariatea with conservative variance | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| IMD quintile | ||||||
| First quintile (least deprived) | 0.91 (0.87 to 0.95) | < 0.001 | 0.91 (0.81 to 1.01) | 0.08 | 0.98 (0.93 to 1.04) | 0.57 |
| Second quintile | 0.97 (0.93 to 1.02) | 0.24 | 0.97 (0.88 to 1.08) | 0.62 | 1.00 (0.94 to 1.06) | 0.91 |
| Third quintile | 0.95 (0.91 to 1.00) | 0.04 | 0.95 (0.85 to 1.08) | 0.46 | 1.05 (0.97 to 1.13) | 0.24 |
| Fourth quintile | 0.91 (0.87 to 0.96) | < 0.001 | 0.91 (0.78 to 1.06) | 0.25 | 1.00 (0.94 to 1.06) | 0.95 |
| Fifth quintile (most deprived) | 0.86 (0.82 to 0.91) | < 0.001 | 0.86 (0.74 to 1.00) | 0.05 | 0.92 (0.86 to 0.98) | 0.02 |
| Total | 0.93 (0.91 to 0.95) | < 0.001 | 0.93 (0.81 to 1.06) | 0.27 | 1.00 (0.96 to 1.03) | 0.80 |
| Variable | Univariate (GPE) | Univariate (GPE) with conservative variance | Multivariatea with conservative variance | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| IMD quintile | ||||||
| First quintile (least deprived) | 0.97 (0.93 to 1.00) | 0.04 | 0.97 (0.88 to 1.05) | 0.43 | 1.04 (0.99 to 1.08) | 0.08 |
| Second quintile | 1.02 (0.99 to 1.06) | 0.16 | 1.02 (0.95 to 1.10) | 0.54 | 1.06 (1.02 to 1.10) | 0.004 |
| Third quintile | 1.06 (1.02 to 1.09) | 0.002 | 1.06 (0.98 to 1.14) | 0.16 | 1.08 (1.03 to 1.13) | 0.001 |
| Fourth quintile | 1.06 (1.03 to 1.10) | 0.001 | 1.06 (0.98 to 1.16) | 0.15 | 1.09 (1.04 to 1.15) | 0.001 |
| Fifth quintile (most deprived) | 1.06 (1.02 to 1.10) | 0.01 | 1.06 (0.97 to 1.15) | 0.19 | 1.07 (1.01 to 1.13) | 0.02 |
| Total | 1.03 (1.01 to 1.04) | < 0.001 | 1.03 (0.95 to 1.11) | 0.49 | 1.07 (1.04 to 1.10) | < 0.0001 |
| Variable | Univariate (ER) | Univariate (ER) with conservative variance | Multivariatea with conservative variance | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| IMD quintile | ||||||
| First quintile (least deprived) | 0.99 (0.95 to 1.04) | 0.67 | 0.99 (0.90 to 1.09) | 0.85 | 1.00 (0.94 to 1.06) | 0.98 |
| Second quintile | 1.05 (1.00 to 1.10) | 0.03 | 1.05 (0.97 to 1.14) | 0.21 | 1.04 (0.98 to 1.11) | 0.20 |
| Third quintile | 1.10 (1.05 to 1.15) | < 0.001 | 1.10 (1.02 to 1.18) | 0.02 | 1.13 (1.06 to 1.20) | < 0.001 |
| Fourth quintile | 1.05 (0.99 to 1.11) | 0.10 | 1.05 (0.96 to 1.14) | 0.29 | 1.09 (1.02 to 1.17) | 0.009 |
| Fifth quintile (most deprived) | 1.07 (1.00 to 1.14) | 0.04 | 1.07 (0.97 to 1.18) | 0.19 | 1.11 (1.04 to 1.20) | 0.003 |
| Total | 1.04 (1.02 to 1.06) | 0.001 | 1.04 (0.95 to 1.14) | 0.42 | 1.07 (1.03 to 1.11) | 0.001 |
Multivariate analyses including an interaction term between intervention arm and screening episode showed no significant interaction for any of the four interventions (Table 41).
| Intervention | Test for heterogeneity |
|---|---|
| 1 (gist) | p = 0.75 |
| 2 (narrative) | p = 0.84 |
| 3 (GPE) | p = 0.96 |
| 4 (ER) | p = 0.10 |
Median times (ranges) to return the kits following the date the S1 letter was sent are shown in Tables 42–44 and median times (ranges) to return the kits following the date of the S9 reminder letter being sent are shown in Table 45. These times are broadly similar for the gist, narrative and GPE interventions, and approximately double the time to return a kit after a reminder letter. They are also similar in intervention and control arms and across IMD quintiles.
| Variable | SI + gist, median (range) | SI, median (range) |
|---|---|---|
| Time taken to return the gFOBt kit in days | 23 (12–126) | 22 (11–126) |
| First quintile (least deprived) | 24 (12–126) | 22 (12–126) |
| Second quintile | 23 (12–126) | 22 (12–126) |
| Third quintile | 23 (12–126) | 22 (12–126) |
| Fourth quintile | 23 (13–126) | 21 (11–126) |
| Fifth quintile (most deprived) | 21 (12–126) | 21 (12–126) |
| Variable | SI + narrative, median (range) | SI, median (range) |
|---|---|---|
| Time taken to return the gFOBt kit in days | 26 (11–126) | 26 (10–126) |
| First quintile (least deprived) | 26 (13–126) | 27 (13–126) |
| Second quintile | 26 (11–126) | 27 (13–125) |
| Third quintile | 25 (11–126) | 26 (12–125) |
| Fourth quintile | 25 (13–125) | 26 (10–126) |
| Fifth quintile (most deprived) | 24 (13–126) | 26 (12–126) |
| Variable | SI + GPE, median (range) | SI, median (range) |
|---|---|---|
| Time taken to return the gFOBt kit in days | 22 (8–126) | 23 (11–126) |
| First quintile (least deprived) | 23 (12–126) | 24 (11–126) |
| Second quintile | 23 (12–126) | 24 (13–126) |
| Third quintile | 22 (11–126) | 23 (11–126) |
| Fourth quintile | 22 (8–126) | 22 (13–126) |
| Fifth quintile (most deprived) | 21 (12–126) | 22 (12–125) |
| Variable | SI + ER, median (range) | SI, median (range) |
|---|---|---|
| Time taken to return the gFOBt kit in days | 11 (–4 to 89) | 11 (0–89) |
| First quintile (least deprived) | 11 (–4 to 89) | 11 (0–89) |
| Second quintile | 11 (–4 to 89) | 11 (0–89) |
| Third quintile | 11 (–4 to 89) | 11(0–89) |
| Fourth quintile | 11 (–4 to 89) | 11 (0–89) |
| Fifth quintile (most deprived) | 11 (–2 to 89) | 11 (0–89) |
Follow-up appointments at screening centres were arranged and diagnostic outcomes are known for individuals who had a definitive abnormal screening result. Tables 46–49 demonstrate that the proportions of individuals with known outcomes, and the distribution of diagnoses, are similar across trials and trial arms.
| Variable | SI + gist (N = 911), % (n) | SI (N = 792), % (n) |
|---|---|---|
| Diagnostic outcome known | 81.7 (744) | 79.9 (633) |
| Abnormal (including abnormal no histology) | 25.9 (236) | 24.2 (192) |
| Cancer detected | 5.2 (47) | 6.6 (52) |
| High-risk adenoma | 8.2 (75) | 7.1 (56) |
| Intermediate-risk adenoma | 12.8 (117) | 11.1 (88) |
| Low-risk adenoma | 15.5 (141) | 15.8 (125) |
| Normal (no abnormalities found) | 14.1 (128) | 15.2 (120) |
| Diagnostic outcome unknown | 18.3 (167) | 20.1 (159) |
| Variable | SI + narrative (N = 675), % (n) | SI (N = 688), % (n) |
|---|---|---|
| Diagnostic outcome known | 81.2 (548) | 79.5 (547) |
| Abnormal (including abnormal no histology) | 23.0 (155) | 22.2 (153) |
| Cancer detected | 6.5 (44) | 7.1 (49) |
| High-risk adenoma | 9.3 (63) | 7.7 (53) |
| Intermediate-risk adenoma | 12.6 (85) | 10.9 (75) |
| Low-risk adenoma | 17.8 (120) | 17.2 (118) |
| Normal (no abnormalities found) | 12.0 (81) | 14.4 (99) |
| Diagnostic outcome unknown | 18.8 (127) | 20.5 (141) |
| Variable | SI + GPE (N = 1458), % (n) | SI (N = 1396), % (n) |
|---|---|---|
| Diagnostic outcome known | 82.2 (1198) | 82.7 (1154) |
| Abnormal (including abnormal no histology) | 25.3 (369) | 25.8 (360) |
| Cancer detected | 7.3 (106) | 6.3 (88) |
| High-risk adenoma | 7.4 (108) | 8.1 (113) |
| Intermediate-risk adenoma | 11.4 (167) | 12.4 (173) |
| Low-risk adenoma | 16.2 (237) | 15.7 (219) |
| Normal (no abnormalities found) | 14.4 (211) | 14.4 (201) |
| Diagnostic outcome unknown | 17.8 (260) | 17.3 (242) |
| Variable | SI + ER (N = 399), % (n) | SI (N = 435), % (n) |
|---|---|---|
| Diagnostic outcome known | 79.4 (317) | 76.6 (333) |
| Abnormal (including abnormal no histology) | 24.8 (99) | 20.7 (90) |
| Cancer detected | 7.3 (29) | 8.0 (35) |
| High-risk adenoma | 5.5 (22) | 6.9 (30) |
| Intermediate-risk adenoma | 11.0 (44) | 12.9 (56) |
| Low-risk adenoma | 14.8 (59) | 16.5 (72) |
| Normal (no abnormalities found) | 16.0 (64) | 11.5 (50) |
| Diagnostic outcome unknown | 20.6 (82) | 23.4 (102) |
Summary of results of the gist trial
Overall rates of being adequately screened were 57.6% in the intervention arm and 57.3% in the control arm. This difference was not significant, either in the standard analysis (OR = 1.02, 95% CI 1.00 to 1.04; p = 0.12) or in the multivariate adjusted conservative variance analysis (OR = 1.03, 95% CI 0.99 to 1.06; p = 0.15). There was no significant heterogeneity of the intervention effect by SECs, with ORs in the multivariate adjusted conservative variance analysis of 1.06 (95% CI 1.01 to 1.11) in the least deprived quintile and 1.04 (95% CI 0.96 to 1.12) in the most deprived quintile. The only significant intervention effect was observed in the least deprived quintile and only in the multivariate adjusted conservative variance analysis (p = 0.02).
Summary of results of the narrative trial
Overall rates of being adequately screened were 56.7% in the intervention arm and 58.5% in the control arm. This difference was significant in the standard analysis (OR = 0.93, 95% CI 0.91 to 0.95; p < 0.001) but not in the multivariate adjusted conservative variance analysis (OR = 1.00, 95% CI 0.96 to 1.03; p = 0.80). There was no significant heterogeneity of the intervention effect by SECs, with ORs in the multivariate adjusted conservative variance analysis of 0.98 (95% CI 0.93 to 1.04) in the least deprived quintile and 0.92 (95% CI 0.86 to 0.98) in the most deprived quintile, the latter being the only significant result (p = 0.02) in subgroups of SECs.
Summary of results of the general practice endorsement trial
Overall rates of being adequately screened were 58.2% in the intervention arm and 57.5% in the control arm. This was significant in both the standard univariate analysis (OR = 1.03, 95% CI 1.01 to 1.04; p < 0.001) and the multivariate adjusted conservative variance analysis (OR = 1.06, 95% CI 1.04 to 1.10; p < 0.001). There was a suggestion of a greater effect in more deprived quintiles with an OR of 1.04 (95% CI 0.99 to 1.08) in the least deprived quintile and an OR of 1.07 (95% CI 1.01 to 1.13) in the most deprived, both in the multivariate adjusted conservative variance analysis, but this heterogeneity was not significant (p = 0.5). As noted above (see Chapter 9, Comparators), there was overlap between the study populations of the GPE and ER trials. Table 50 shows the status of subjects by the two trials, cross-tabulated. This shows that, in the GPE trial, a larger proportion of the intervention group than of the control group were randomised. However, the unadjusted OR for participation within 4 weeks associated with GPE (before the reminder could have been received) was 1.06, which was higher than the 1.03 observed for participation overall.
| ER trial status | GPE trial population | Total | ||
|---|---|---|---|---|
| Usual letter, n | GPE letter, n | Not in GPE trial, n | ||
| Not in ER trial | 74,200 | 66,502 | 140,702 | |
| Control in ER trial | 37,880 | 30,259 | 22,274 | 90,413 |
| ER intervention in ER trial | 21,931 | 34,662 | 21,474 | 78,067 |
| Total | 134,011 | 131,423 | 43,748 | 309,182 |
Summary of results of the enhanced reminder trial
Overall rates of being adequately screened were 25.8% in the intervention arm and 25.1% in the control arm. This difference was statistically significant in both the univariate standard analysis (OR = 1.04, 95% CI 1.02 to 1.06; p = 0.001) and the multivariate adjusted conservative variance analysis (OR = 1.07, 95% CI 1.03 to 1.11; p = 0.001). There was a significantly stronger effect of the intervention in more deprived quintiles (p = 0.005). In the multivariate adjusted conservative variance analysis, the OR associated with the intervention in the least deprived quintile was 1.00 (95% CI 0.94 to 1.06) and in the most deprived quintile, was 1.11 (95% CI 1.04 to 1.20). The effect of the intervention in the multivariate adjusted conservative variance analysis was significant in all deprivation quintiles except for the least deprived.
Concurrent initiatives
We identified 65 research initiatives and 101 health promotion activities, of which 14% and 59%, respectively, were localised and specific to bowel cancer screening uptake. Of these, three research initiatives and 27 health promotion activities occurred within a 3-month time frame of each of the ASCEND interventions.
Measuring costs
For the gist and narrative interventions costs of £3106 and £3887, respectively, were incurred for printing and transporting the additional leaflets. These costs were incurred for screening 88,421 and 73,722 participants, and the average incremental cost per screening invitation of providing the interventions was £0.04 and £0.05, respectively.
Using the results from the individual RCTs, it is possible to estimate the potential number of additional cancers and polyps that might be detected if GPE and the ER were implemented nationally across England. We calculated average marginal effects of GPE and the ER from the RCTs and used these to predict their impact on the detection of colorectal adenomas and cancer in the NHS BCSP.
With regard to GPE, a 7% increase in the odds of screening all participants, as found in the multivariable analysis of the trial data, was associated with predictive margins (adjusted average probabilities of uptake) of 0.584 (95% CI 0.581 to 0.586) in the intervention group and 0.574 (95% CI 0.571 to 0.577) in the control group. This translates into a 1.7% relative increase in the probability of screening among all participants (0.584/0.574) and a 1-percentage-point absolute increase (0.584–0.574; the average marginal effect). Although this appears to be a relatively small effect, in absolute terms the impact would be large if it was rolled out nationally given the population size. In the 2013/14 fiscal year, the number invited for screening in the NHS BCSP in England was 3,976,616 (Bowel Cancer Screening System National. National Fiscal Summary 12 June 2015. Claire Nickerson, Public Health England, 2015, personal communication). An average marginal effect of 1% (i.e. 0.010) suggests that if GPE were implemented nationally, then 39,766 extra people each year would be screened. In 2013/14, the positivity rate among the screened population was 1.84% (Bowel Cancer Screening System National. National Fiscal Summary 12 June 2015. Claire Nickerson, Public Health England, 2015, personal communication). Evidence suggests that 83% of people with a positive test result attend a SSP clinic and undergo further investigation212 and, among those who go on to have further investigations, 10.1% will have a CRC and 27.2% will have colorectal adenomatous polyps classed as medium or high risk that require further investigation. 212 Hence, if GPE was implemented nationally this could detect up to an additional 165 people (39,766 × 0.0184 × 0.83 × 0.272) with polyps classed as high or intermediate risk and 61 people (39,766 × 0.0184 × 0.83 × 0.101) with a CRC each year.
For the ER there was also a 7% increase in the odds of screening, this time achieved among those randomised in the ER trial, that is, receiving a reminder letter. This was associated with predictive margins (adjusted average probabilities of uptake) of 0.259 (95% CI 0.255 to 0.265) in the intervention group and 0.250 (95% CI 0.248 to 0.253) in the control group. This implies a 3.6% relative increase in the probability of screening (0.259/0.250) and a 0.9-percentage-point absolute increase (0.259 minus 0.250; the average marginal effect). In the 2013/14 fiscal year, the number of reminder (S10) letters sent in the NHS BCSP in England was 2,144,277 (Bowel Cancer Screening System National. National Fiscal Summary 12 June 2015. Claire Nickerson, Public Health England, 2015, personal communication). An average marginal effect of 0.9% (i.e. 0.009) suggests that if the ER were implemented nationally, then 19,298 extra people each year might be screened. We assume as above that the positivity rate among the screened population was 1.84% in 2013/14, that 83% of people with a positive test result attend a SSP clinic and undergo further investigations and that among those who go on to have further investigations, 10.1% will have a CRC and 27.2% will have colorectal adenomatous polyps classed as medium or high risk requiring further investigation. On this basis, if the ER was implemented nationally it might detect up to an additional 80 people (19,298 × 0.0184 × 0.83 × 0.272) with polyps classed as high or intermediate risk and 30 people (19,298 × 0.0184 × 0.83 × 0.101) with a CRC in England each year.
Chapter 11 Workstream 3 randomised controlled trials: discussion
Introduction
The results presented in Chapter 10 demonstrate that the gist and narrative trials showed no effect on the gradient in uptake (interactions with deprivation quintile: p > 0.05) or overall uptake (gist: OR = 1.03, 95% CI 0.99 to 1.06; p = 0.15; narrative: OR = 1.00, 95% CI 0.96 to 1.03; p = 0.80). In the GPE trial, there was no effect on the gradient but an increase in overall uptake (OR = 1.07, 95% CI 1.04 to 1.10; p < 0.0001). The ER trial showed a significant interaction with deprivation (p < 0.05) with a stronger effect in the most deprived (OR = 1.11, 95% CI 1.04 to 1.20; p = 0.003) than the least deprived quintile (OR = 1.00, 95% CI 0.94 to 1.06; p = 0.98) and higher overall uptake (OR = 1.07, 95% CI 1.03 to 1.11; p < 0.001).
Interpretation
Reducing socioeconomic inequalities in cancer mortality is a priority worldwide. Cancer screening is a major part of a combined effort to bring diagnoses forward to an earlier, more treatable, stage. However, even in the UK, where screening does not incur any cost to the individual, uptake is consistently lower in more socioeconomically deprived groups. 213,214 Together, our four trials constitute the largest evaluation of interventions designed specifically to reduce inequalities in uptake within an organised screening programme. An important strength of the ASCEND trial was that the trials were powered to measure the impact of interventions on SEC gradient in the total eligible population rather than just focusing on disadvantaged groups. Our interventions therefore had the potential to reach a much larger number of non-participants. Use of routinely collected data enabled us to include the whole study population with the exception of a very small group of individuals without IMD scores for their postcodes (see Appendix 36) in our analysis. The lack of attrition accelerates the potential for implementation of these interventions. Each intervention also had a well-established rationale and empirical background, and followed a structured, comprehensive development process. However, only the ER demonstrated a reduction in the SEC gradient in uptake. The gist and narrative interventions showed no effect at all on uptake, while GPE increased uptake overall but did not modify the SEC gradient. None of our interventions promoted an earlier response.
The gist and narrative leaflets were designed to make the screening offer more visible to people with lower literacy skills on the basis of extensive evidence that lower SECs are associated with lower literacy. Both leaflets demonstrated this potential when their effect was evaluated on the basis of knowledge and attitudes, with additional evidence that the narrative leaflet increased intention to attend screening. 171,215 However, in the trials, neither of the leaflets increased bowel cancer screening uptake, either in lower SEC groups or overall. One possible explanation for the discrepancy from the pilot data is the emerging body of evidence indicating that the determinants of intention may differ from the determinants of action and perhaps we influenced intentions rather than actions. 35 Another possible explanation relates to the fact that both leaflets had to be added to the existing invitation/information, rather than being provided as an alternative (because the existing materials are approved nationally to ensure appropriate provision of essential information). Consequently, although the new leaflets were designed to be simple, they nonetheless increased the total mass of written material that participants received and this may have undermined the goal of making the screening offer more visible. We also did not have the resources to translate the leaflets into different languages, which may have limited their impact in more ethnically diverse urban regions, although we saw no hub-specific effects.
A specific issue with the narrative leaflet was that we tried to address negative beliefs about being diagnosed with bowel cancer by presenting two case studies of patients who had been diagnosed early and reported their support of the programme and the relief associated with having their lives saved. However, we acknowledge that these examples may inadvertently work against this effect by reinforcing the link between screening and cancer. This may have overridden the positive messages in the leaflet. Although the pilot study did indicate that the leaflet was associated with increased screening intentions, we were able to observe this effect only among responders to the questionnaire. Our national trial of GPE significantly increased uptake overall. The effect size was smaller than in many previous studies,36,196,216,217 but this was probably because most of those had used letters directly from the GP or had an individual GP’s signature on the letter. In the NHS BCSP, all invitation letters come from the hubs; therefore, the only practical option was to include a banner noting that the individual’s general practice supported the programme, but without individual GPs’ names or signatures. It would not be surprising if this diluted the impact. However, given the exceptionally high level of agreement by general practices to endorse the NHS BCSP, the small one-off cost to modify the standard invitation letter within the NHS BCSP IT system (to adjust for any recent changes in practice name) and the zero marginal cost per person screened, we recommend that the NHS BCSP consider adding GPE to the screening invitation letter.
Because previous studies had shown effects of GPE in low-income groups (e.g. Ahmed et al. 218), we had hypothesised that it might have a stronger effect in lower than in higher SEC groups. No previous study had been powered to examine effects on the SEC gradient and the size of the present trial provides a definitive negative result, at least with the format of GPE that was used.
The one intervention that reduced the SEC gradient was the ER. Our aim was to offer anyone who may not have engaged with the original materials an additional opportunity to see the screening offer. Unlike the gist and narrative interventions, which tried to achieve this with an additional leaflet, the ‘enhancement’ was incorporated into the one-page reminder letter. The information pack which contained this letter did not include any of the other usual information materials (e.g. ‘The Facts’ booklet) and was therefore much thinner than the invitation letter used in the other trials. It is likely that this increased its visibility and impact. Although the change in the gradient was small (as was the effect on overall uptake), this intervention was also virtually ‘cost free’ and, therefore, offers a practical way for the screening programme to achieve a reduction in the SEC gradient.
The ASCEND programme inevitably had limitations in terms of the range and types of interventions that were tested. People from socioeconomically deprived backgrounds are likely to be struggling with multiple social and economic challenges and this could make it difficult for them to prioritise cancer screening, but these ‘upstream’ issues are never going to be addressed by minor variations in the format of the screening offer. Similar observations have also been made in other NHS BCSPs where addressing psychosocial variables through written materials has also failed. 196 Nonetheless, we believe that ensuring that the screening offer is not only mailed to all eligible adults, but is equally accessible to respondents at all levels of literacy, is an obligation of the NHS screening programmes. We also did not address broader attitudes to cancer outcomes that could be important. For example, cancer fatalism is known to be higher in lower SEC groups and has been shown to be associated with later stage of diagnosis;219 however, fatalism is not easily modified with simple written materials. There were also other downstream barriers that we did not address, of which the most well established is ‘faecal aversion’. The gFOBt is widely regarded as unpleasant and some individuals are likely to be more averse to contact with faecal material than others,220 although there is no existing evidence that this barrier is socially graded. In contrast, the need to store the gFOBt kit between samples could be a socioeconomically graded barrier because it requires privacy and privacy may be limited for people living in less advantaged circumstances. In the context of the ASCEND study, we were unable to identify ways of addressing this barrier, but it is possible that if the NHS BCSP implements the faecal immunochemical test (FIT), which typically requires only one sample, inequalities in participation would be reduced. Use of FIT has recently been shown to reduce the SEC gradient in uptake (compared with gFOBt) as part of an evaluation of FIT in the Scottish NHS BCSP. 221
One issue that should be considered is whether or not individuals who did not take up the offer are making a ‘rational’ decision (e.g. judging that screening would not offer benefit given their personal circumstances). The lower perceived life expectancy in lower SEC groups222 has been put forward as one explanation for differential engagement in activities that produce benefit only in the distant future. 31 Contrary to this view is evidence that ‘non-attenders’ often express interest in screening once they have discussed it in a one-to-one setting,223 suggesting that, when the information is presented verbally with opportunities to clarify, screening is widely perceived to be advantageous. In addition, inequalities in breast screening uptake (involving women of a similar age) are much lower than for bowel cancer screening in women, with an absolute difference from the least to most deprived IMD quintile of only 10% in one study,213 suggesting that screening per se is not the problem.
Although the over-riding message of this study is the challenge of shifting the gradient in screening participation within the context of a national screening programme, the research design in which strategies to be tested are embedded in routine programme delivery provides a model for future research. There may also be scope to improve the impact of the interventions. The gist and narrative leaflets may have been ‘swamped’ among other elements of the mailing. One alternative could be to supply the necessary screening information in smaller ‘bites’ by integrating additional communication points into the screening pathway. There may also be scope for additional reminder letters. Given that successive reminders would, through a process of elimination, target an increasingly deprived population, this should also reduce the uptake gradient. More controversially, it may be timely to consider adding direct contact with health professionals or trained lay experts to present the case for screening and support informed decision-making for some population groups. ‘Patient navigation’ has been shown to increase uptake across a number of cancer screening programmes and has been designed specifically with low literacy and socially disadvantaged groups in mind. 223
In conclusion, three out of four trials of interventions aimed at tackling inequalities in screening uptake failed to reduce the SEC gradient. An ER letter was the only strategy to significantly reduce the gradient, while GPE increased overall uptake. Given their minimal cost, these interventions could be implemented immediately to support the enhanced and equitable delivery of cancer screening within the NHS BCSP. The results of these trials are testament to the difficulty of modifying inequalities in screening within an organised programme, but they highlight the importance of continuing to research effective strategies to achieve equity in early diagnosis of cancer.
Comparison with other studies
The effectiveness of the ER letter in reducing the socioeconomic gradient in uptake of a public health intervention is significant because such an effect has rarely (if ever) been demonstrated. Cesar Victora’s ‘inverse equity hypothesis’, which predicts that new public health interventions initially reach those of higher socioeconomic status and only later affect the poor when the affluent have achieved new minimum achievable levels for morbidity, has been confirmed internationally. 224 Our results are therefore highly noteworthy.
The results of the ER could be explained in relation to insights gained from our own qualitative work (workstream 1), previous evidence and health behaviour theory. For example, there was strong evidence that reminders significantly increase uptake of cancer screening. 203,225 However, workstream 1 focus group participants consistently reported not recognising the letter. The text at the top of the letter was therefore designed to clearly state the purpose and may have increased its personal relevance (‘A reminder to you’). The additional text at the bottom of the letter aimed to further increase the urgency of the letter by addressing well-established predictors of CRC screening uptake. The first sentence clearly stated the importance of CRC screening as a way to reduce risk of CRC. Perceived risk has been extensively studied as a predictor of cancer screening. 226–228 Importantly, the message was designed to be simple and direct about the increased risk of CRC without using complex statistical information following the sample principles underlying the development of the gist leaflet and thereby increasing its effectiveness among low-literate individuals. 143 The second sentence addressed response efficacy (i.e. the belief that screening is effective), which is a key construct in the Health Belief Model, which has been found useful in trying to predict CRC screening uptake. 229 Third, we reduced perceived barriers to screening by affirming that ‘it is never too late to screen’ and increased self-efficacy by offering guidance about how to seek further guidance.
Future research should focus on which of these aspects had the strongest impact, particularly among socioeconomically deprived individuals.
The gist RCT was the first trial of information materials guided by FTT. Several studies have shown that people, particularly older adults, have a preference for extracting gist-like representations from health information. 93,94 However, there have been no trials of whether or not materials developed with these preferences in mind have an impact on screening knowledge or behaviour. Our null results add to the mixed evidence for behaviour change interventions using educational materials. 230 Three trials of mailed materials focusing on barriers to screening uptake reported an increase in uptake: one in FS screening231 and two in gFOBt screening. 178,232 Two other trials observed no effect37,233 and one saw reduced uptake with mailed materials. 234 A recent US-based study also failed to demonstrate a statistically significant increase in uptake associated with adding an educational pamphlet, video and simplified gFOBt kit instruction sheet to usual care materials. 235
The narrative RCT was the first trial to incorporate personal quotations and stories regarding the NHS BCSP experience into information material. Presenting information in a narrative format is becoming a popular form of health communication and has shown encouraging results in the science literature. 156,158 Dillard et al. 162 found that by integrating a short narrative to non-narrative factual information, the anticipated impact of barriers to screening (via colonoscopy) was reduced, while perceived personal risk and intention to have a colonoscopy were enhanced. 162 The inclusion of a bowel cancer screening narrative has also been found to increase colonoscopy attendance fourfold when compared with providing non-narrative information only. 165 Until now, no research has focused on narratives in relation to screening via gFOBt. The evaluation/pilot trial of the current narrative leaflet indicated an increase in intention and positive outcomes on factors predictive of screening intention, including enhanced personal risk and reduced impact of disgust as a barrier. 171 However, within the RCT, this intention did not translate to an increase in screening attendance, again adding to the mixed evidence available on the effectiveness of health education interventions. Another reason why both gist and narrative leaflets did not show the promised effect is that the development of such interventions is limited to a highly selected sample of people willing to participate in research. This was true of both studies pre-testing the effectiveness of our leaflets despite a concerted effort to develop links with community organisations and by purposefully selecting primary care practices serving deprived areas.
The GPE intervention was successful in gaining approval from four out of five general practices, which is an unprecedented general practice participation rate compared with other studies, allowing us to randomise over 260,000 individuals from 6480 general practices. By comparison, the largest study of GPE to date randomised 2400 individuals. 36 Importantly, this large sample size allowed us to be the first study to address the question of whether or not GPE that has been shown to positively influence uptake also has the ability to reduce the socioeconomic gradient. The ability to conduct a study of GPE on such a large scale meant that we had to curtail the requirements on general practices. Furthermore, integrating the GPE in an existing letter severely reduced the number of characters used in the message. Both of these issues could have adversely affected the strength and visibility of the message.
Taken together, these results indicate that despite a strong theoretical background and extensive pre-testing, the design of effective behavioural interventions is challenging.
Methodological strengths
Among the strengths of our trial were its national coverage, substantial power to detect small differences in uptake, and an intervention, which, if effective, could easily and cost-effectively be incorporated into the existing NHS BCSP. We used novel, innovative methods to develop a gist leaflet that enhanced comprehension and carried out extensive user testing and piloting to demonstrate its accessibility to adults with basic literacy skills. Similarly, the narrative leaflet was designed by drawing on the expertise of colleagues in social marketing, health psychology and public health promotion. The final layout and content were established through focus groups and one-to-one interviews with members of the public, with subsequent piloting in the community confirming its acceptability.
Research limitations
Unlike the narrative and the gist pilot, it was not possible to pre-test the impact of the GPE and the ER interventions. In the case of the GPE, the main issue to be addressed during workstream 2 was that of recruiting general practices. In addition, our ability to manipulate the length and content of the message was severely limited by requirements stipulated by the NHS BCSP. In the case of the ER letter it was impossible to pre-test its impact on a population outside the screening programme. It is therefore difficult to ascertain the degree to which people perceived these interventions (e.g. whether or not they noticed them and accepted or rejected their respective messages). Given that all of these interventions were run in a routine screening programme, we were also prevented from conducting any further evaluation of the interventions among people who had been part of the interventions. Doing so would have required seeking consent from individuals and would have been dominated by individuals who had participated in screening.
These trials had limitations. The NHS BCSP hub directors were clear that parallel RCTs and individual randomisation of subjects for all interventions could not be performed for feasibility reasons. Therefore, cluster randomised trials were adopted as the strongest alternative. Cluster randomised trials can have compromised statistical efficiency owing to clustering, which increases the sample size required to achieve appropriate power. 236 This was addressed in our sample size calculation and our target was surpassed in all trials.
A further limitation is the possibility that the study groups became contaminated, such that individuals in the control groups were exposed to the interventions. Although this limitation is noted, it would also have applied to a parallel randomised trial.
Although it is possible that localised initiatives may have affected uptake rates in specific regions, it is considered unlikely that they would have confounded the ASCEND intervention results overall, primarily because it is improbable that concurrent interventions would occur on the same alternate days as each intervention.
In addition, our findings may have been influenced by other factors operating within a cluster affecting the outcome. We surveyed the number and location of health promotion activities occurring during the period of the trials, but we cannot rule out the possibility that these reduced the effectiveness of the interventions.
Finally, our study did not include evaluation of the sociopsychological determinants of the behaviour of individuals during the decision-making period. This explains the limitations outlined above, such as our focus on behavioural intentions rather than actions and our inability to obtain an in-depth and comprehensive understanding of the determinants of social variations in decision-making processes in real time. It is possible that ethnographic case studies of everyday practices through sustained observation within different sociocultural contexts in addition to semistructured interviews conducted over the decision-making time period could yield important insights. However, it should also be recognised that the completion of the screening test (and, therefore, the final decision-making step) takes place in the privacy of one’s home (making observation and interview data more difficult and resource intensive to obtain) and that frank interview responses might be influenced by sociocultural norms on the appropriateness of discussing an intimate topic. Furthermore, observation and explicit exploration of decision-making (which might otherwise be exclusively implicit) could influence the decision made.
Patient and public involvement
Our Patient Representative, Mr Roger Band, reviewed and commented on the results of the all the trials.
Conclusion
The four largest trials of interventions aimed at reducing socioeconomic inequalities in screening uptake within an organised NHS BCSP failed to substantially affect the gradient. However, the ability of the simple reminder message to achieve the hypothesised reduction in inequalities is a highly notable achievement that has been rarely (if ever) documented in the published literature. Furthermore, the effectiveness of gist and narrative information leaflets when used alone are unknown and should be explored as part of alternative communication opportunities.
These results highlight the difficulty of modifying inequalities in screening uptake through easily implementable, cost-effective, interventions that respect informed decision-making.
Chapter 12 Conclusions and recommendations for future research
Socioeconomic inequalities in health and health care are widespread internationally. Research is usually designed to improve access to, and uptake of, health care and health outcomes among socially disadvantaged groups. To our knowledge, this is the first national research programme to focus on the entire population in order to improve uptake across the whole socioeconomic gradient. By developing four theoretically based interventions that aimed to increase the uptake of bowel cancer screening across the socioeconomic gradient, we hope to ultimately reduce inequalities in cancer outcomes. Here we summarise our key findings and their implications for future research and practice. These results are equally applicable to the FIT, which the NHS BCSP have begun to introduce as a pilot in 2014.
-
The national RCT of the ER (see Chapter 10) demonstrated a significant effect on both the social gradient in uptake and uptake overall. Given the extremely low marginal cost of this intervention, the findings suggest that implementation by the NHS BCSP would be beneficial.
-
Our national trial of GPE significantly increased uptake overall (although it did not reduce the social gradient in uptake) (see Chapter 10). Given the exceptionally high level of agreement by general practices to endorse the NHS BCSP, the small one-off cost to modify the standard invitation letter within the NHS BCSP IT system (to adjust for any recent changes in practice name) and the zero marginal cost per person screened, the evidence suggests that the NHS BCSP could usefully consider adding GPE to the screening invitation letter.
-
Enhanced and explicit involvement of familiar health-care settings and professionals should be further investigated, with an emphasis on tailoring NHS involvement to the specific needs of defined sociocultural groups. The impact of such initiatives on the social gradient and ethnic differences in uptake should be examined.
-
The evidence suggests that policies aimed at reducing the gradient in bowel cancer screening participation could include strategies aimed at increasing acceptability and comprehension of the screening test among those with lower levels of literacy. This is supported by the strong relationship between health literacy and SECs. The pilot of the gist leaflet in workstream 2 demonstrated that improving the accessibility of screening information to individuals with low health literacy may improve their comprehension of the decision, but additional intervention may be required to have an impact on screening behaviour. This observation was supported by the null effect of the gist leaflet on screening behaviour in workstream 3. One alternative approach suggested from data in workstream 1 is patient navigation, a method involving a trained health professional offering one-to-one support to help address individual barriers to screening. A patient navigation trial has been carried out by members of our team to promote uptake of FS in the UK screening programme. 237 Effective interventions should also emphasise the social implications of screening and its benefit not just to the individual but to their family and friends.
-
Our exploration of why non-participants subsequently undergo screening (see Chapter 3) found that talking increased awareness of screening uptake among peers and significant others and was key to overcoming objections and to subsequent screening participation. Our results suggest that initiatives such as the advertising campaign in Greater Manchester to normalise open discussions about bowel cancer screening should be designed and evaluated (Audrey Howarth Greater Manchester Bowel Cancer Screening Health Improvement Team, 2012, personal communication).
-
Reasons for low CRC screening uptake shared across South Asian faith groups were limitations posed by the written word, low awareness of CRC and screening, and difficulties with handling faeces and gFOBt completion. In addition, we identified that written materials are particularly inappropriate for the Sylheti-speaking Bangladeshi Muslim community, because they are unlikely to be able to read either Bengali or English. Sikh participants described a social stigma surrounding cancer which may hinder engagement with screening. A preference for information to be delivered verbally within a familiar community setting was described across faith groups. To increase accessibility to CRC screening in South Asian communities, screening information should be delivered via local ethnic media and face to face within community groups and faith settings.
-
The gist and narrative leaflets were designed to make the screening offer more visible to people with lower literacy skills on the basis of extensive evidence that social disadvantage is associated with lower literacy. The gist leaflet improved comprehension compared with standard information alone (see Chapter 5) and the narrative leaflet led to stronger screening intentions than the standard information (see Chapter 6). However, there was no significant increase in uptake when examined in combination with the nationally approved standard materials. This may be because the determinants of action differ from the determinants of intention, and we influenced intentions rather than actions. In addition, although the new leaflets were designed to provide simple information, they nonetheless increased the total mass of written material that participants received and this may have undermined the goal of making the screening offer more visible. The leaflets supplemented rather than replaced the existing NHS BCSP information booklet because this was a requirement of the NHS National Cancer Screening Programme. One way forward could be to allow RCT testing of the leaflets in comparison to existing materials. An alternative approach could be to supply the necessary screening information in smaller ‘bites’ by integrating additional ‘communication points’ into the screening pathway.
-
We demonstrated that it is possible to successfully engage GPs in applied health research. Nationally, 80% of general practices agreed to participate in the RCT of GPE in the NHS BCSP (see Chapter 7). This exceptionally high response rate was achieved by adopting the following practices, all of which have implications for future research requiring GP collaboration: recruitment letters were sent by NHS BCSP hub directors rather than from the ASCEND research team, GPs had to do no more than agree that their practice name could be used on the screening invitation letters, and we sent up to three reminder letters at approximately monthly intervals.
-
Our research programme ASCEND was a highly successful example of experts from academia and from the health service working effectively across traditional boundaries to create new knowledge to improve the delivery of health services. We also demonstrated that, although studies of health care delivery typically rely on observational and quasi-experimental methods, it is possible to use a randomised design without adding substantially to the cost or difficulty of the study. We have also provided an example of applying a randomised design without loss of external validity, thereby offering an unparalleled ability to provide credible evidence of the impact of ER and GPE and potentially accelerating the pace for their widespread implementation.
-
In the future, further development of the randomised design should be considered, involving ‘realist’ RCTs. 238 These could first explore decision-making processes leading to change and then test how intervention effects vary with context and among different sociocultural groups. Such RCTs could be orientated towards building and validating theories about how interventions interact with context to produce outcomes. They would comprise an important extension of existing pragmatic methods and enhance our understanding of sociocultural variations in screening uptake.
Research recommendations are summarised in Box 2. They are equally applicable to gFOBt and to FIT.
-
The co-design and experimental ‘realist’ evaluation of socioculturally appropriate bowel cancer screening information interventions tailored to defined ethnic and social groups.
-
Research is required which evaluates the appropriateness and cost-effectiveness of:
-
verbal (rather than written) information
-
enhanced involvement of familiar NHS settings and professionals
-
targeted translation and literacy assistance methods
-
additional reminders (including the use of text messaging technology).
-
-
The use of an action research methodology, in which researchers work alongside and ‘within’ a defined community to codevelop a solution to a problem (i.e. low screening uptake within South Asian communities) may be a valuable route to the development of culturally appropriate interventions for non-English-language speakers.
-
Exploration of the reasons for lower screening uptake among the sick and disabled, followed by the co-design and testing of interventions to enhance informed decision-making in these groups.
-
National ‘realist’ experimental evaluations of the gist and narrative leaflets in comparison with existing NHS BCSP information materials.
-
Design and experimental evaluation (in ‘realist’ RCTs) of initiatives to normalise open discussions about bowel cancer screening.
-
Further exploration of the ER to better understand the individual contribution of its different components.
Acknowledgements
We dedicate this report to Professor Jane Wardle (1950–2015).
We are most grateful to the members of the public across England who participated in the developmental stages of this research and acknowledge their important contribution to the development of the study interventions. We are grateful for the help provided by Local Research Networks in London, Cumbria, Liverpool and Manchester for their assistance with recruitment and to Dr Gianluca Baio for helping to put together the original grant application.
Contributions of authors
Rosalind Raine, Wendy Atkin and Jane Wardle conceived the idea for this study and led all components of workstreams 1, 2 and 3, respectively.
Rosalind Raine led the research team (Jane Wardle, Wendy Atkin, Stephen Duffy, Stephen Morris, Christian Von Wagner, Allan Hackshaw, Stephen P Halloran, Sandra Rainbow, Samuel G Smith, Graham Handley, Richard Logan, Neil Stubbs, Austin Obichere, Roger Band) which designed the successful application for funding. As the Chief Investigator and guarantor, Rosalind Raine managed the study overall and has final responsibility for the analysis and report content.
Christian von Wagner, Ines Kralj-Hans, Lesley McGregor, Cecily Palmer, Samuel G Smith, Mary Thomas, Rosemary Howe and Gemma Vart contributed to study design, review of the literature, recruitment of participants, data collection, analysis, interpretation and drafting of the chapters relating to the research component that they were involved with across workstreams.
Rosalind Raine, Christian von Wagner, Lesley McGregor, Cecily Palmer, Samuel G Smith, Mary Thomas and Rosemary Howe prepared the first draft of this report.
Stephen Duffy and Allan Hackshaw collaborated in the funding application and were responsible for the statistical aspects of the study, including involvement in the study design, analysis and interpretation.
Nicholas Counsell and Sue Moss conducted statistical analyses (workstream 3).
Roger Band provided guidance from a patient perspective throughout the research programme.
Samuel G Smith, Stephen P Halloran, Neil Stubbs, Graham Handley, Richard Logan, Sandra Rainbow, Austin Obichere and Stephen Smith provided NHS BCSP-specific and clinical expertise throughout the research programme.
Julia Snowball prepared the downloaded trial data.
Stephen Morris and Francesca Solmi conducted the health economic analyses (workstream 3).
All authors contributed to subsequent drafts, approved the final draft and contributed to analytic design, interpretation and drafting of this report.
Publications and presentations (up to March 2015)
Publications
Solmi F, von Wagner C, Kobayashi LC, Raine R, Wardle J, Morris S. Decomposing socio-economic inequality in colorectal cancer screening uptake in England. Soc Sci Med 2015;134:76–86.
McGregor LM, von Wagner C, Vart G, Yuen WC, Raine R, Wardle J, et al. The impact of supplementary narrative-based information on colorectal cancer screening beliefs and intention. BMC Cancer 2015;15:162.
Smith SG, Kobayashi LC, Wolf MS, Raine R, Wardle J, von Wagner C. The associations between objective numeracy and colorectal cancer screening knowledge, attitudes and defensive processing in a deprived community sample. J Health Psychol 2016;21:1665–75.
Smith SG, Raine R, Obichere A, Wolf MS, Wardle J, von Wagner C. The effect of a supplementary (‘gist-based’) information leaflet on colorectal cancer knowledge and screening intention: a randomized controlled trial. J Behav Med 2015;38:261–72.
Palmer CK, Thomas MC, von Wagner C, Raine R. Reasons for non-uptake and subsequent participation in the NHS Bowel Cancer Screening Programme: a qualitative study. Br J Cancer 2014;110:1705–11.
Smith SG, Wolf MS, Obichere A, Raine R, Wardle J, von Wagner C. The development and testing of a brief (‘gist-based’) supplementary colorectal cancer screening information leaflet. Patient Educ Couns 2013;93:619–25.
Smith S, Vart G, Wolf S, Obichere A, Baker H, Raine R, et al. How do people interpret information about colorectal cancer screening? Health Expect 2015;18:703–14.
Morris S, Baio G, Kendall E, von Wagner C, Wardle J, Atkin W, et al. Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: a retrospective analysis of the NHS Bowel Cancer Screening Programme. Br J Cancer 2012;107:765–71.
Brentnall AR, Duffy SW, Baio G, Raine R. Strategy for power calculation for interactions: application to a trial of interventions to improve uptake of bowel cancer screening. Contemp Clin Trials 2012;33:213–217.
Von Wagner C, Baio G, Snowball J, Raine R, Morris S, Atkin W, et al. Inequalities in participation in an organised national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol 2011;40:712–8.
Presentations
Smith S. Examining the Effect of a ‘Gist-based’ Colorectal Cancer Screening Information Leaflet: A Multi-centre Parallel Randomised Controlled Trial. Society for Behavioural Medicine, Philadelphia, PA, April 2014.
McGregor LM. The Impact of Narratives on Beliefs About Bowel Cancer Screening in England. Division of Health Psychology annual conference, York, September 2014.
Von Wagner C. Decision-making and Participation in Bowel Cancer Screening: Challenges and Interventions for Low Health Literate Individuals (Symposium).12th International Conference on Communication in Healthcare, Amsterdam, September 2014.
Thomas MC. Exploring Reasons for Low Uptake of Bowel Cancer Screening within South Asian Communities in London. UK Society for Behavioural Medicine, Nottingham, December 2014.
Palmer CK. An Overview of the ASCEND Study and a Focus on the Qualitative Work re Focus Groups with Non-responders. Bowel Cancer Screening Programme health improvement team for Greater Manchester, Bolton, 2013.
McGregor L. Psychologically Informed Public Health Campaigns – Reducing the Social Gradient in Bowel Cancer Screening. Cross-Professional Conference: Psychologically minded services and interventions in physical healthcare: new pathways to better patient outcomes, Belfast, March 2013.
McGregor L. The Psychological Impact of a Bowel Cancer Diagnosis Through Screening: An Interpretative Phenomenological Analysis. Division of Health Psychology annual conference, Brighton, September 2013.
Smith S. Examining the Effect of a brief (‘gist-based’) Supplementary Colorectal Cancer Screening Information Leaflet: A Multi-centre Parallel Randomised Controlled Trial. UK Society for Behavioural Medicine, Oxford, December 2013.
Raine R. An Overview and Achievements to Date of the ASCEND Study. Bowel Cancer Screening Programme Southern Hub’s Conference, Guildford, 2012.
Palmer CK. Accounting for Non-uptake Of Bowel Cancer Screening: A Qualitative Exploration. Society for Social Medicine, London, September 2012.
Smith S. Perceptions of the English NHS Bowel Cancer Screening Programme Information Materials: A Think-Aloud Study. National Cancer Research Institute (NCRI) Conference, Liverpool, 2012.
Smith S. Perceptions of the English NHS Bowel Cancer Screening Programme Information Materials: A Think-Aloud Study. UK Health Literacy Conference, Southampton, May 2012, and Division of Health Psychology Annual Conference, Liverpool, September 2012.
McGregor L. Narrative Leaflet Dissemination and Feedback Workshop. Institute for Social Marketing, University of Stirling, May 2012.
Von Wagner C. Introduction to the ASCEND Study. London Quality Assurance Annual Bowel Cancer Screening Study Day, September 2011, and the Bowel Cancer Screening Programme Southern Hub’s Annual Conference, Guildford, November 2011.
Data sharing statement
All available data can be obtained from the corresponding author, Professor Rosalind Raine.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Cancer Research UK . Bowel Cancer Incidence Statistics 2014. www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/ (accessed March 2015).
- Office for National Statistics . Cancer Statistics Registrations: Registrations of Cancer Diagnosed in 2006 2010. www.statistics.gov.uk/statbase/Product.asp?vlnk=8843 (accessed March 2015).
- National Cancer Intelligence Network . Colorectal Cancer Survival by Stage 2009. www.ncin.org.uk/publications/data_briefings/colorectal_cancer_survival_by_stage (accessed March 2015).
- Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ 1998;317:559-65. http://dx.doi.org/10.1136/bmj.317.7158.559.
- Weller D, Coleman D, Robertson R, Butler P, Melia J, Campbell C, et al. The UK colorectal cancer screening pilot: results of the second round of screening in England. Br J Cancer 2007;97:1601-5. http://dx.doi.org/10.1038/sj.bjc.6604089.
- UK Colorectal Cancer Screening Pilot Group . Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ 2004;329:133-8. http://dx.doi.org/10.1136/bmj.38153.491887.7C.
- Public Health England . NHS Bowel Cancer Screening Programme n.d. www.cancerscreening.nhs.uk/bowel/publications/the-facts-other-languages.html (accessed March 2015).
- Hardcastle JD, Farrands PA, Balfour TW, Chamberlain J, Amar SS, Sheldon MG. Controlled trial of faecal occult blood testing in the detection of colorectal cancer. Lancet 1983;322:1-4. http://dx.doi.org/10.1016/S0140-6736(83)90001-6.
- Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 2008;103:1541-9. http://dx.doi.org/10.1111/j.1572-0241.2008.01875.x.
- Parkin DM, Tappenden P, Olsen AH, Patnick J, Sasieni P. Predicting the impact of the screening programme for colorectal cancer in the UK. J Med Screen 2008;15:163-74. http://dx.doi.org/10.1258/jms.2008.008024.
- Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut 2002;50:840-4. http://dx.doi.org/10.1136/gut.50.6.840.
- von Wagner C, Baio G, Raine R, Snowball J, Morris S, Atkin W, et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol 2011;40:712-18. http://dx.doi.org/10.1093/ije/dyr008.
- Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA 2000;284:1954-61. http://dx.doi.org/10.1001/jama.284.15.1954.
- Tackling Health Inequalities: Cross Cutting Review. London: Department of Health; 2002.
- Choosing Health – Making Healthy Choices Easier. London: Department of Health; 2004.
- Whynes DK, Frew EJ, Manghan CM, Scholefield JH, Hardcastle JD. Colorectal cancer, screening and survival: the influence of socio-economic deprivation. Public Health 2003;117:389-95. http://dx.doi.org/10.1016/S0033-3506(03)00146-X.
- Graham H. Tackling inequalities in health in England: remedying health disadvantages, narrowing health gaps or reducing health gradients?. J Soc Pol 2004;33:115-31. http://dx.doi.org/10.1017/S0047279403007220.
- Marmot M. Fair Society, Healthy Lives: The Marmot Review: Strategic Review of Health Inequalities in England Post-2010 2010.
- The King’s Fund . The NHS in England: The Operating Framework for 2006 2007 2006. www.kingsfund.org.uk/sites/files/kf/field/field_publication_file/briefing-nhs-in-england-operating-framework-2006–7-may06.pdf (accessed March 2015).
- Department of Health . Cancer Reform Strategy 2007. www.nhs.uk/NHSEngland/NSF/Documents/Cancer%20Reform%20Strategy.pdf (accessed March 2015).
- Kakwani N, Wagstaff A, van Doorslaer E. Socioeconomic inequalities in health: measurement, computation and statistical inference. J Econom 1997;77:87-104. http://dx.doi.org/10.1016/S0304-4076(96)01807-6.
- Wagstaff A, van Doorslaer E, Watanabe N. On decomposing the causes of health sector inequalities, with an application to malnutrition inequalities in Vietnam. J Econom 2003;112:219-27. http://dx.doi.org/10.1016/S0304-4076(02)00161-6.
- Solmi F, Von Wagner C, Kobayashi LC, Raine R, Wardle J, Morris S. Decomposing socio-economic inequality in colorectal cancer screening uptake in England. Soc Sci Med 2015;134:76-8. http://dx.doi.org/10.1016/j.socscimed.2015.04.010.
- Morris S, Baio G, Kendall E, von Wagner C, Wardle J, Atkin W, et al. Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: a retrospective analysis of the NHS Bowel Cancer Screening Programme. Br J Cancer 2012;107:765-71. http://dx.doi.org/10.1038/bjc.2012.303.
- Smith JJ, Tilney HS, Heriot AG, Darzi AW, Forbes H, Thompson MR, et al. Social deprivation and outcomes in colorectal cancer. Br J Surg 2006;93:1123-31. http://dx.doi.org/10.1002/bjs.5357.
- Raine R, Wong W, Scholes S, Ashton C, Obichere A, Ambler G. Social variations in access to hospital care for patients with colorectal, breast, and lung cancer between 1999 and 2006: retrospective analysis of hospital episode statistics. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.b5479.
- Coleman MP, Rachet B, Woods LM, Mitry E, Riga M, Cooper N, et al. Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br J Cancer 2004;90:1367-73. http://dx.doi.org/10.1038/sj.bjc.6601696.
- Bonfill X, Marzo M, Pladevall M, Martí J, Emparanza JI. Strategies for increasing women participation in community breast cancer screening. Cochrane Database Syst Rev 2001;1. http://dx.doi.org/10.1002/14651858.cd002943.
- Chiu L. Inequalities of Access to Cancer Screening: A Literature Review. Sheffield: NHS Cancer Screening Programmes; 2003.
- Haushofer J, Fehr E. On the psychology of poverty. Science 2014;344:862-7. http://dx.doi.org/10.1126/science.1232491.
- Pepper GV, Nettle D. Out of control mortality matters: the effect of perceived uncontrollable mortality risk on a health-related decision. Peer J 2014;2. http://dx.doi.org/10.7717/peerj.459.
- Palmer CK, Thomas MC, von Wagner C, Raine R. Reasons for non-uptake and subsequent participation in the NHS Bowel Cancer Screening Programme: a qualitative study. Br J Cancer 2014;110:1705-11. http://dx.doi.org/10.1038/bjc.2014.125.
- Hall NJ, Rubin GP, Dobson C, Weller D, Wardle J, Ritchie M, et al. Attitudes and beliefs of non-participants in a population-based screening programme for colorectal cancer. Health Expect 2015;18:1645-57. http://dx.doi.org/10.1111/hex.12157.
- von Wagner C, Semmler C, Good A, Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: The role of information processing. Patient Educ Couns 2009;75:352-7. http://dx.doi.org/10.1016/j.pec.2009.03.015.
- Power E, Van Jaarsveld CH, McCaffery K, Miles A, Atkin W, Wardle J. Understanding intentions and action in colorectal cancer screening. Ann Behav Med 2008;35:285-94. http://dx.doi.org/10.1007/s12160-008-9034-y.
- Cole SR, Young GP, Byrne D, Guy JR, Morcom J. Participation in screening for colorectal cancer based on a faecal occult blood test is improved by endorsement by the primary care practitioner. J Med Screen 2002;9:147-52. http://dx.doi.org/10.1136/jms.9.4.147.
- Hewitson P, Ward AM, Heneghan C, Halloran SP, Mant D. Primary care endorsement letter and a patient leaflet to improve participation in colorectal cancer screening: results of a factorial randomised trial. Br J Cancer 2011;105:475-80. http://dx.doi.org/10.1038/bjc.2011.255.
- Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624-33. http://dx.doi.org/10.1016/S0140-6736(10)60551-X.
- Information for Primary Care. London: NHS; 2012.
- Szczepura A, Price C, Gumber A. Breast and bowel cancer screening uptake patterns over 15 years for UK south Asian ethnic minority populations, corrected for differences in socio-demographic characteristics. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-346.
- Alexander FWD. Evaluation of the UK Colorectal Cancer Screening Pilot. Edinburgh: NHS; 2003.
- Szczepura A, Johnson M, Orbell S, Gumber A, O’Sullivan I, Clay D, et al. Ethnicity: UK Colorectal Cancer Screening Pilot: Final Report 2003. http://webarchive.nationalarchives.gov.uk/20150506150512/http://www.cancerscreening.nhs.uk/bowel/ethnicity-finalreport.pdf (accessed March 2015).
- Price CL, Szczepura AK, Gumber AK, Patnick J. Comparison of breast and bowel cancer screening uptake patterns in a common cohort of South Asian women in England. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-103.
- Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health 2000;25:263-78. http://dx.doi.org/10.1023/A:1005104406934.
- Weitzman ER, Zapka J, Estabrook B, Goins KV. Risk and reluctance: understanding impediments to colorectal cancer screening. Prev Med 2001;32:502-13. http://dx.doi.org/10.1006/pmed.2001.0838.
- Wackerbarth SB, Peters JC, Haist SA. “Do we really need all that equipment?”: factors influencing colorectal cancer screening decisions. Qual Health Res 2005;15:539-54. http://dx.doi.org/10.1177/1049732304273759.
- McCaffery K, Borril J, Williamson S, Taylor T, Sutton S, Atkin W, et al. Declining the offer of flexible sigmoidoscopy screening for bowel cancer: a qualitative investigation of the decision-making process. Soc Sci Med 2001;53:679-91. http://dx.doi.org/10.1016/S0277-9536(00)00375-0.
- Aubin-Auger I, Mercier A, Lebeau JP, Baumann L, Peremans L, Van Royen P. Obstacles to colorectal screening in general practice: a qualitative study of GPs and patients. Fam Pract 2011;28:670-6. http://dx.doi.org/10.1093/fampra/cmr020.
- Clavarino AM, Janda M, Hughes KL, Del Mar C, Tong S, Stanton WR, et al. The view from two sides: a qualitative study of community and medical perspectives on screening for colorectal cancer using FOBT. Prev Med 2004;39:482-90. http://dx.doi.org/10.1016/j.ypmed.2004.05.015.
- Chapple A, Ziebland S, Hewitson P, McPherson A. What affects the uptake of screening for bowel cancer using a faecal occult blood test (FOBt): a qualitative study. Soc Sci Med 2008;66:2425-35. http://dx.doi.org/10.1016/j.socscimed.2008.02.009.
- O’Sullivan I, Orbell S. Self-sampling in screening to reduce mortality from colorectal cancer: a qualitative exploration of the decision to complete a faecal occult blood test (FOBT). J Med Screen 2004;11:16-22. http://dx.doi.org/10.1258/096914104772950709.
- Ekberg M, Callender M, Hamer H, Rogers S. Exploring the decision to participate in the National Health Service Bowel Cancer Screening Programme. Eur J Cancer Prev 2014;23:391-7. http://dx.doi.org/10.1097/CEJ.0000000000000007.
- Dent OF, Bartrop R, Goulston KJ, Chapuis PH. Participation in faecal occult blood screening for colorectal cancer. Soc Sci Med 1983;17:17-23. http://dx.doi.org/10.1016/0277-9536(83)90074-6.
- von Wagner C, Good A, Wright D, Rachet B, Obichere A, Bloom S, et al. Inequalities in colorectal cancer screening participation in the first round of the national screening programme in England. Br J Cancer 2009;101:60-3. http://dx.doi.org/10.1038/sj.bjc.6605392.
- Austin KL, Power E, Solarin I, Atkin WS, Wardle J, Robb KA. Perceived barriers to flexible sigmoidoscopy screening for colorectal cancer among UK ethnic minority groups: a qualitative study. J Med Screen 2009;16:174-9. http://dx.doi.org/10.1258/jms.2009.009080.
- Bourgeault IDR, de Vries R. The SAGE Handbook of Qualitative Methods in Health Research. London: Sage; 2010.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Barbour R, Bourgeault IDR, de Vries R. The Sage Handbook of Qualitative Methods in Health Research. London: Sage; 2010.
- Bloor MFJ, Thomas M, Robson K. Focus Groups in Social Research. London: Sage; 2001.
- McLean CA, Campbell CM. Locating research informants in a multi-ethnic community: ethnic identities, social networks and recruitment methods. Ethn Health 2003;8:41-6. http://dx.doi.org/10.1080/13557850303558.
- Twamley KPS, Macfarlane A, Harding S, Ahmed S, Mirsky J. Recruiting UK-born ethnic minority women for qualitative health research – lessons learned from a study of maternity care. Res Policy and Planning 2009;27:25-38.
- Office for National Statistics . Neighbourhood Statistics 2011. www.neighbourhood.statistics.gov.uk/dissemination/ (accessed March 2015).
- Miller RL, Brewer J. The A-Z of Social Research. London: Sage Publications Ltd; 2003.
- Craig P, Forbes J. Social position and health: are old and new occupational classifications interchangeable?. J Biosoc Sci 2005;37:89-106. http://dx.doi.org/10.1017/S0021932003006424.
- Thompson A. ‘Sometimes, I think I might say too much’: dark secrets and the performance of inflammatory bowel disease. Symb Interact 2013;36:21-39. http://dx.doi.org/10.1002/symb.50.
- Lawton J. Contemporary hospice care: the sequestration of the unbounded body and ‘dirty dying’. Sociol Health Ill 1998;20:121-43. http://dx.doi.org/10.1111/1467-9566.00094.
- Baker DW, Parker RM, Williams MV, Pitkin K, Parikh NS, Coates W, et al. The health care experience of patients with low literacy. Arch Fam Med 1996;5:329-34. http://dx.doi.org/10.1001/archfami.5.6.329.
- Office for National Statistics . Ethnicity and National Identity in England and Wales 2011 2012. www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11 (accessed March 2015).
- Szczepura A. Access to health care for ethnic minority populations. Postgrad Med J 2005;81:141-7. http://dx.doi.org/10.1136/pgmj.2004.026237.
- Robb KA, Solarin I, Power E, Atkin W, Wardle J. Attitudes to colorectal cancer screening among ethnic minority groups in the UK. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-34.
- Webb R, Richardson J, Esmail A, Pickles A. Uptake for cervical screening by ethnicity and place-of-birth: a population-based cross-sectional study. J Public Health 2004;26:293-6. http://dx.doi.org/10.1093/pubmed/fdh128.
- Moser K, Patnick J, Beral V. Inequalities in reported use of breast and cervical screening in Great Britain: analysis of cross sectional survey data. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b2025.
- Thomas VN, Saleem T, Abraham R. Barriers to effective uptake of cancer screening among black and minority ethnic groups. Int J Palliat Nurs 2005;11. http://dx.doi.org/10.12968/ijpn.2005.11.11.20096.
- Naish J, Brown J, Denton B. Intercultural consultations: investigation of factors that deter non-English speaking women from attending their general practitioners for cervical screening. BMJ 1994;309:1126-8.
- Box V. Cervical screening: the knowledge and opinions of black and minority ethnic women and of health advocates in East London. Health Educ J 1998;57:3-15. http://dx.doi.org/10.1177/001789699805700102.
- Brouse CBC, Wolf R, Shmukler C, Neugut A, Shea S. Barriers to colorectal cancer screening with fecal occult blood testing in a predominantly minority urban population: a qualitative study. Am J Public Health 2003;93:1268-71. http://dx.doi.org/10.2105/AJPH.93.8.1268.
- Guessous I, Dash C, Lapin P, Doroshenk M, Smith RA, Klabunde CN. National Colorectal Cancer Roundtable Screening Among the 65 Plus Task Group . Colorectal cancer screening barriers and facilitators in older persons. Prev Med 2010;50:3-10. http://dx.doi.org/10.1016/j.ypmed.2009.12.005.
- Griffith KA, Passmore SR, Smith D, Wenzel J. African Americans with a family history of colorectal cancer: barriers and facilitators to screening. Oncol Nurs Forum 2012;39:299-306. http://dx.doi.org/10.1188/12.ONF.299-306.
- Botha JL, Manku-Scott TK, Moledina F, Williams A. Indirect discrimination and breast screening. Ethn Dis 1993;3:189-95.
- Scanlon KWA. Breast cancer awareness in Britain: are there differences based on ethnicity?. Divers Health Soc Care 2005;2:211-21.
- Karbani G, Lim JN, Hewison J, Atkin K, Horgan K, Lansdown M, et al. Culture, attitude and knowledge about breast cancer and preventive measures: a qualitative study of South Asian breast cancer patients in the UK. Asian Pac J Cancer Prev 2011;12:1619-26.
- Hoare T. Breast screening and ethnic minorities. Br J Cancer Suppl 1996;29:S38-41.
- Bhopal R. Glossary of terms relating to ethnicity and race: for reflection and debate. J Epidemiol Community Health 2004;58:441-5. http://dx.doi.org/10.1136/jech.2003.013466.
- Bhopal RS, Phillimore P, Kohli HS. Inappropriate use of the term ‘Asian’: an obstacle to ethnicity and health research. J Public Health Med 1991;13:244-6.
- Krysik JFJ. Research for Effective Social Work Practice. New York, NY: Routledge; 2010.
- Marshall MN. The key informant technique. Fam Pract 1996;13:92-7. http://dx.doi.org/10.1093/fampra/13.1.92.
- Bahl V. Improving access and quality for ethnic minority women. Womens Health Issues 2001;11:348-54. http://dx.doi.org/10.1016/S1049-3867(01)00121-9.
- Shankleman J, Massat NJ, Khagram L, Ariyanayagam S, Garner A, Khatoon S, et al. Evaluation of a service intervention to improve awareness and uptake of bowel cancer screening in ethnically-diverse areas. Br J Cancer 2014;111:1440-7. http://dx.doi.org/10.1038/bjc.2014.363.
- Reyna VF, Brainerd CJ. Fuzzy-trace theory: an interim synthesis. Learn Individ Differ 1995;7:1-75. http://dx.doi.org/10.1016/1041-6080(95)90031-4.
- Reyna VF. How people make decisions that involve risk: a dual-processes approach. Curr Dir Psychol Sci 2004;13:60-6. http://dx.doi.org/10.1111/j.0963-7214.2004.00275.x.
- Reyna VF. Theories of medical decision making and health: an evidence-based approach. Med Decis Making 2008;28:829-33. http://dx.doi.org/10.1177/0272989X08327069.
- Reyna VF, Brainerd CJ. Fuzzy-trace Theory and framing effects in choice: gist extraction, truncation, and conversion. J Behav Decis Making 1991;4:249-62. http://dx.doi.org/10.1002/bdm.3960040403.
- Reyna VF. Risk perception and communication in vaccination decisions: a fuzzy-trace theory approach. Vaccine 2012;30:3790-7. http://dx.doi.org/10.1016/j.vaccine.2011.11.070.
- Reyna VF, Lloyd FJ. Physician decision making and cardiac risk: effects of knowledge, risk perception, risk tolerance, and fuzzy processing. J Exp Psychol Appl 2006;12:179-95. http://dx.doi.org/10.1037/1076-898X.12.3.179.
- Elwyn G, Stiel M, Durand MA, Boivin J. The design of patient decision support interventions: addressing the theory-practice gap. J Eval Clin Pract 2011;17:565-74. http://dx.doi.org/10.1111/j.1365-2753.2010.01517.x.
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97-107. http://dx.doi.org/10.7326/0003-4819-155-2-201107190-00005.
- Smith SG, von Wagner C, McGregor LM, Curtis LM, Wilson EA, Serper M, et al. The influence of health literacy on comprehension of a colonoscopy preparation information leaflet. Dis Colon Rectum 2012;55:1074-80. http://dx.doi.org/10.1097/DCR.0b013e31826359ac.
- von Wagner C, Steptoe A, Wolf MS, Wardle J. Health literacy and health actions: a review and a framework from health psychology. Health Educ Behav 2009;36:860-77. http://dx.doi.org/10.1177/1090198108322819.
- Smith SG, Vart G, Wolf MS, Obichere A, Baker HJ, Raine R, et al. How do people interpret information about colorectal cancer screening: observations from a think-aloud study. Health Expect 2015;18:703-14. http://dx.doi.org/10.1111/hex.12117.
- von Wagner C, Knight K, Steptoe A, Wardle J. Functional health literacy and health-promoting behaviour in a national sample of British adults. J Epidemiol Community Health 2007;61:1086-90. http://dx.doi.org/10.1136/jech.2006.053967.
- Boxell EM, Smith SG, Morris M, Kummer S, Rowlands G, Waller J, et al. Increasing awareness of gynecological cancer symptoms and reducing barriers to medical help seeking: does health literacy play a role?. J Health Commun 2012;17:265-79. http://dx.doi.org/10.1080/10810730.2012.712617.
- Ericsson KA, Simon HA. Protocol Analysis: Verbal Reports as Data. Cambridge, MA: MIT Press; 1993.
- Fox MC, Ericsson KA, Best R. Do procedures for verbal reporting of thinking have to be reactive? A meta-analysis and recommendations for best reporting methods. Psychol Bull 2011;137:316-44. http://dx.doi.org/10.1037/a0021663.
- Ericsson KA, Fox MC. Thinking aloud is not a form of introspection but a qualitatively different methodology: reply to Schooler (2011). Psychol Bull 2011;137:351-4. http://dx.doi.org/10.1037/a0022388.
- Crain-Thoreson C, Lipman MZ, McClendon-Magnuson D. Windows on comprehension: reading comprehension processes as revealed by two think-aloud procedures. J Educ Psychol 1997;89:579-91. http://dx.doi.org/10.1037/0022-0663.89.4.579.
- Nielsen J. Estimating the number of subjects needed for a thinking aloud test. Int J Hum-Comput St 1994;41:385-97. http://dx.doi.org/10.1006/ijhc.1994.1065.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Strauss A. Qualitative Analysis for Social Scientists. Cambridge: Cambridge University Press; 1987.
- Woodrow C, Watson E, Rozmovits L, Parker R, Austoker J. Public perceptions of communicating information about bowel cancer screening. Health Expect 2008;11:16-25. http://dx.doi.org/10.1111/j.1369-7625.2007.00474.x.
- Viswanath K. Science and society: the communications revolution and cancer control. Nat Rev Cancer 2005;5:828-35. http://dx.doi.org/10.1038/nrc1718.
- Department of Health . Improving Outcomes: A Strategy for Cancer 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123371 (accessed March 2015).
- General Medical Council . Consent: Patients and Doctors Making Decisions Together 2008. www.gmc-uk.org/static/documents/content/Consent_-_English_0911.pdf (accessed March 2015).
- Abraham C, Kools M. Writing Health Communication: An Evidence-based Guide. London: Sage Publications Ltd; 2011.
- DeWalt DA, Callahan LF, Hawk VH, Broucksou KA, Hink A, Rudd R, et al. Health Literacy Universal Precautions Toolkit. Chapel Hill, NC: North Carolina Network Consortium, University of North Carolina; 2010.
- McCaffery K, Sheridam S, Nutbeam D, Clayman M, Kelly-Blake K, Rovner M, et al. 2012 Update of the International Patient Decision Aids Standards (IPDAS) Collaborations Background Document. IPDAS Collaboration; 2012.
- Campaign PE. How to Write in Plain English 2009. www.plainenglish.co.uk/files/howto.pdf (accessed March 2015).
- Peters E, Klein W, Kaufman A, Meilleur L, Dixon A. More is not always better: intuitions about effective public policy can lead to unintended consequences. Soc Issues Policy Rev 2013;7:114-48. http://dx.doi.org/10.1111/j.1751-2409.2012.01045.x.
- Hibbard JH, Peters E. Supporting informed consumer health care decisions: data presentation approaches that facilitate the use of information in choice. Annu Rev Public Health 2003;24:413-33. http://dx.doi.org/10.1146/annurev.publhealth.24.100901.141005.
- Schwartz PH. Questioning the quantitative imperative: decision aids, prevention, and the ethics of disclosure. Hastings Cent Rep 2011;41:30-9. http://dx.doi.org/10.1353/hcr.2011.0029.
- Zikmund-Fisher BJ, Fagerlin A, Ubel PA. A demonstration of “less can be more” in risk graphics. Med Decis Making 2010;30:661-71. http://dx.doi.org/10.1177/0272989X10364244.
- Peters E, Dieckmann N, Dixon A, Hibbard JH, Mertz CK. Less is more in presenting quality information to consumers. Med Care Res Rev 2007;64:169-90. http://dx.doi.org/10.1177/10775587070640020301.
- Miles A, Rodrigues V, Sevdalis N. The effect of information about false negative and false positive rates on people’s attitudes towards colorectal cancer screening using faecal occult blood testing (FOBt). Patient Educ Couns 2013;93:342-9. http://dx.doi.org/10.1016/j.pec.2013.06.010.
- Ramirez AJ, Forbes L. Approach to Developing Information about NHS Cancer Screening Programmes 2012. www.informedchoiceaboutcancerscreening.org/wp-content/uploads/2012/04/Approach-toinformed-choice-about-cancer-screening.pdf (accessed March 2015).
- Smith SW, Hitt R, Nazione S, Russell J, Silk K, Atkin CK. The effects of heuristic cues, motivation, and ability on systematic processing of information about breast cancer environmental factors. J Health Commun 2013;18:845-65. http://dx.doi.org/10.1080/10810730.2013.768722.
- Berry DC, Hochhauser M. Verbal labels can triple perceived risk in clinical trials. Drug Inf J 2006;40:249-58. http://dx.doi.org/10.1177/009286150604000302.
- Knapp P, Gardner PH, Raynor DK, Woolf E, McMillan B. Perceived risk of tamoxifen side effects: a study of the use of absolute frequencies or frequency bands, with or without verbal descriptors. Patient Educ Couns 2010;79:267-71. http://dx.doi.org/10.1016/j.pec.2009.10.002.
- Zikmund-Fisher BJ, Fagerlin A, Keeton K, Ubel PA. Does labeling prenatal screening test results as negative or positive affect a woman’s responses?. Am J Obstet Gynecol 2007;197. http://dx.doi.org/10.1016/j.ajog.2007.03.076.
- Peters E, Dieckmann NF, Västfjäll D, Mertz CK, Slovic P, Hibbard JH. Bringing meaning to numbers: the impact of evaluative categories on decisions. J Exp Psychol Appl 2009;15:213-27. http://dx.doi.org/10.1037/a0016978.
- Galesic M, Garcia-Retamero R. Statistical numeracy for health: a cross-cultural comparison with probabilistic national samples. Arch Intern Med 2010;170:462-8. http://dx.doi.org/10.1001/archinternmed.2009.481.
- Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut 2012;61:1036-40. http://dx.doi.org/10.1136/gutjnl-2011-300774.
- Smith SK, Trevena L, Nutbeam D, Barratt A, McCaffery KJ. Information needs and preferences of low and high literacy consumers for decisions about colorectal cancer screening: utilizing a linguistic model. Health Expect 2008;11:123-36. http://dx.doi.org/10.1111/j.1369-7625.2008.00489.x.
- Smith SK, Barratt A, Trevena L, Simpson JM, Jansen J, McCaffery KJ. A theoretical framework for measuring knowledge in screening decision aid trials. Patient Educ Couns 2012;89:330-6. http://dx.doi.org/10.1016/j.pec.2012.07.009.
- Dickinson D, Raynor DK, Duman M. Patient information leaflets for medicines: using consumer testing to determine the most effective design. Patient Educ Couns 2001;43:147-59. http://dx.doi.org/10.1016/S0738-3991(00)00156-7.
- Austoker J, Giordano L, Hewitson P, Villain P. International Agency for Research on Cancer . European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition – Communication. Endoscopy 2012;44:164-85.
- Wilson EA, Wolf MS, Curtis LM, Clayman ML, Cameron KA, Eigen KV, et al. Literacy, cognitive ability, and the retention of health-related information about colorectal cancer screening. J Health Commun 2010;15:116-25. http://dx.doi.org/10.1080/10810730.2010.499984.
- Wolf MS, Wilson EA, Rapp DN, Waite KR, Bocchini MV, Davis TC, et al. Literacy and learning in health care. Pediatrics 2009;124:275-81. http://dx.doi.org/10.1542/peds.2009-1162C.
- Wolf MS, Curtis LM, Wilson EA, Revelle W, Waite KR, Smith SG, et al. Literacy, cognitive function, and health: results of the LitCog study. J Gen Intern Med 2012;27:1300-7. http://dx.doi.org/10.1007/s11606-012-2079-4.
- Freed E, Long D, Rodriguez T, Franks P, Kravitz RL, Jerant A. The effects of two health information texts on patient recognition memory: a randomized controlled trial. Patient Educ Couns 2013;92:260-5. http://dx.doi.org/10.1016/j.pec.2013.03.008.
- Curtis V, Aunger R, Rabie T. Evidence that disgust evolved to protect from risk of disease. Proc Biol Sci 2004;271:131-3. http://dx.doi.org/10.1098/rsbl.2003.0144.
- Dolan NC, Ferreira MR, Davis TC, Fitzgibbon ML, Rademaker A, Liu D, et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: does literacy make a difference?. J Clin Oncol 2004;22:2617-22. http://dx.doi.org/10.1200/JCO.2004.10.149.
- Reynolds LM, Consedine NS, Pizarro DA, Bissett IP. Disgust and behavioral avoidance in colorectal cancer screening and treatment: a systematic review and research agenda. Cancer Nurs 2013;36:122-30. http://dx.doi.org/10.1097/NCC.0b013e31826a4b1b.
- von Wagner C, Good A, Smith SG, Wardle J. Responses to procedural information about colorectal cancer screening using faecal occult blood testing: the role of consideration of future consequences. Health Expect 2012;15:176-86. http://dx.doi.org/10.1111/j.1369-7625.2011.00675.x.
- Smith SG, Wolf MS, Obichere A, Raine R, Wardle J, von Wagner C. The development and testing of a brief (‘gist-based’) supplementary colorectal cancer screening information leaflet. Patient Educ Couns 2013;93:619-25. http://dx.doi.org/10.1016/j.pec.2013.08.013.
- Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer 2008;112:228-42. http://dx.doi.org/10.1002/cncr.23157.
- Knapp P, Raynor DK, Silcock J, Parkinson B. Can user testing of a clinical trial patient information sheet make it fit-for-purpose? – a randomized controlled trial. BMC Med 2011;9. http://dx.doi.org/10.1186/1741-7015-9-89.
- Knapp P, Raynor DK, Silcock J, Parkinson B. Performance-based readability testing of participant information for a Phase 3 IVF trial. Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-79.
- Raynor DK, Knapp P, Silcock J, Parkinson B, Feeney K. ‘User-testing’ as a method for testing the fitness-for-purpose of written medicine information. Patient Educ Couns 2011;83:404-10. http://dx.doi.org/10.1016/j.pec.2011.03.016.
- Parker RM, Baker DW, Williams MV, Nurss JR. The test of functional health literacy in adults: a new instrument for measuring patients’ literacy skills. J Gen Intern Med 1995;10:537-41. http://dx.doi.org/10.1007/BF02640361.
- Kincaid JP. Derivation of New Readability Formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy Enlisted Personnel. Springfield, VA: U.S. Department of Commerce; 1975.
- European Commission . Draft Guideline on the Readability of the Label and Package Leaflet of Medicinal Products for Human Use 2006. http://ec.europa.eu/health/files/pharmacos/docs/doc2006/09_2006/readability_consultation_2006_09_25_en.pdf (accessed March 2015).
- Smith SG, Kobayashi LC, Wolf MS, Raine R, Wardle J, von Wagner C. The associations between objective numeracy and colorectal cancer screening knowledge, attitudes and defensive processing in a deprived community sample. J Health Psychol 2016;21:1665-75. http://dx.doi.org/10.1177/1359105314560919.
- Robb K, Wardle J, Stubbings S, Ramirez A, Austoker J, Macleod U, et al. Ethnic disparities in knowledge of cancer screening programmes in the UK. J Med Screen 2010;17:125-31. http://dx.doi.org/10.1258/jms.2010.009112.
- von Wagner C, Good A, Whitaker KL, Wardle J. Psychosocial determinants of socioeconomic inequalities in cancer screening participation: a conceptual framework. Epidemiol Rev 2011;33:135-47. http://dx.doi.org/10.1093/epirev/mxq018.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c869.
- Hinyard LJ, Kreuter MW. Using narrative communication as a tool for health behavior change: a conceptual, theoretical, and empirical overview. Health Educ Behav 2007;34:777-92. http://dx.doi.org/10.1177/1090198106291963.
- Green MC. Narratives and Cancer Communication. J Commun 2006;56:S163-S83. http://dx.doi.org/10.1111/j.1460-2466.2006.00288.x.
- Kreuter MW, Green MC, Cappella JN, Slater MD, Wise ME, Storey D, et al. Narrative communication in cancer prevention and control: a framework to guide research and application. Ann Behav Med 2007;33:221-35. http://dx.doi.org/10.1007/BF02879904.
- Miller-Day M, Hecht ML. Narrative means to preventative ends: a narrative engagement framework for designing prevention interventions. Health Commun 2013;28:657-70. http://dx.doi.org/10.1080/10410236.2012.762861.
- Kreuter MW, Holmes K, Alcaraz K, Kalesan B, Rath S, Richert M, et al. Comparing narrative and informational videos to increase mammography in low-income African American women. Patient Educ Couns 2010;81:S6-14. http://dx.doi.org/10.1016/j.pec.2010.09.008.
- Lemal M, Van den Bulck J. Testing the effectiveness of a skin cancer narrative in promoting positive health behavior: a pilot study. Prev Med 2010;51:178-81. http://dx.doi.org/10.1016/j.ypmed.2010.04.019.
- de Wit JB, Das E, Vet R. What works best: objective statistics or a personal testimonial? An assessment of the persuasive effects of different types of message evidence on risk perception. Health Psychol 2008;27:110-15. http://dx.doi.org/10.1037/0278-6133.27.1.110.
- Dillard AJ, Fagerlin A, Dal Cin S, Zikmund-Fisher BJ, Ubel PA. Narratives that address affective forecasting errors reduce perceived barriers to colorectal cancer screening. Soc Sci Med 2010;71:45-52. http://dx.doi.org/10.1016/j.socscimed.2010.02.038.
- Witte K. Putting the fear back into fear appeals: the extended parallel process model. Commun Monogr 1992;59:329-49. http://dx.doi.org/10.1080/03637759209376276.
- Rosenstock IM. Why people use health services. Milbank Q 1966;44:94-124. http://dx.doi.org/10.2307/3348967.
- Jensen JD, King AJ, Carcioppolo N, Krakow M, Samadder NJ, Morgan S. Comparing tailored and narrative worksite interventions at increasing colonoscopy adherence in adults 50-75: a randomized controlled trial. Soc Sci Med 2014;104:31-40. http://dx.doi.org/10.1016/j.socscimed.2013.12.003.
- DIPex . Healthtalk.Org 2015. www.healthtalk.org/ (accessed 12 February 2015).
- Cancer Genetics StoryBank . Cancer Genetics StoryBank 2015. www.cancergeneticsstorybank.co.uk/storybank.htm (accessed 12 February 2015).
- Ofcom . Adults’ Media Use and Attitudes Report 2014. http://stakeholders.ofcom.org.uk/market-data-research/other/research-publications/adults/adults-media-lit-14/ (accessed March 2015).
- Helitzer D, Hollis C, Cotner J, Oestreicher N. Health literacy demands of written health information materials: an assessment of cervical cancer prevention materials. Cancer Control 2009;16:70-8.
- Wengraf T. Qualitative Research Interviewing: Semi-structured, Biographical and Narrative Methods. London: SAGE; 2004.
- McGregor LM, von Wagner C, Vart G, Yuen WC, Raine R, Wardle J, et al. The impact of supplementary narrative-based information on colorectal cancer screening beliefs and intention. BMC Cancer 2015;15. http://dx.doi.org/10.1186/s12885-015-1167-3.
- Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med 2010;38:508-16. http://dx.doi.org/10.1016/j.amepre.2010.01.021.
- van Dam L, Korfage IJ, Kuipers EJ, Hol L, van Roon AH, Reijerink JC, et al. What influences the decision to participate in colorectal cancer screening with faecal occult blood testing and sigmoidoscopy?. Eur J Cancer 2013;49:2321-30. http://dx.doi.org/10.1016/j.ejca.2013.03.007.
- Robb KA, Power E, Atkin W, Wardle J. Ethnic differences in participation in flexible sigmoidoscopy screening in the UK. J Med Screen 2008;15:130-6. http://dx.doi.org/10.1258/jms.2008.007112.
- Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol 2004;4. http://dx.doi.org/10.1186/1471-2288-4-26.
- Flight IH, Wilson CJ, McGillivray J, Myers RE. Cross-cultural validation of the preventive health model for colorectal cancer screening: an Australian study. Health Educ Behav 2010;37:724-36. http://dx.doi.org/10.1177/1090198110364107.
- Rawl S, Champion V, Menon U, Loehrer PJ, Vance GH, Skinner CS. Validation of scales to measure benefits of and barriers to colorectal cancer screening. J Psychosoc Oncol 2001;19:47-63. http://dx.doi.org/10.1300/J077v19n03_05.
- Wardle J, Williamson S, McCafferty K, Sutton S, Taylor T, Edwards R, et al. Increasing attendance at colorectal cancer screening: testing the efficacy of a mailed, psychoeducational intervention in a community sample of older adults. Health Psychol 2003;22:99-105. http://dx.doi.org/10.1037/0278-6133.22.1.99.
- Wardle J, McCaffery K, Nadel M, Atkin W. Socioeconomic differences in cancer screening participation: comparing cognitive and psychosocial explanations. Soc Sci Med 2004;59:249-61. http://dx.doi.org/10.1016/j.socscimed.2003.10.030.
- Gregory TA, Wilson C, Duncan A, Turnbull D, Cole SR, Young G. Demographic, social cognitive and social ecological predictors of intention and participation in screening for colorectal cancer. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-38.
- Census Information Scheme . 2011 Census Snapshot: Car and Van Availability 2014. http://data.london.gov.uk/census/themes/households/ (accessed March 2015).
- Census Information Scheme . Trends in Housing Tenure 2013. http://data.london.gov.uk/census/themes/housing/ (accessed March 2015).
- Reeder AI. “It’s a small price to pay for life”: faecal occult blood test (FOBT) screening for colorectal cancer, perceived barriers and facilitators. N Z Med J 2011;124:11-7.
- Moghaddam MF, Mousavi A. Prediction and control of response rate to surveys. Amer J Math Management Sci 2009;29:337-70. http://dx.doi.org/10.1080/01966324.2009.10737763.
- Bankhead C, Richards SH, Peters TJ, Sharp DJ, Hobbs FD, Brown J, et al. Improving attendance for breast screening among recent non-attenders: a randomised controlled trial of two interventions in primary care. J Med Screen 2001;8:99-105. http://dx.doi.org/10.1136/jms.8.2.99.
- Giveon S, Kahan E. Patient adherence to family practitioners’ recommendations for breast cancer screening: a historical cohort study. Fam Pract 2000;17:42-5. http://dx.doi.org/10.1093/fampra/17.1.42.
- Bell TS, Branston LK, Newcombe RG, Barton GR. Interventions to improve uptake of breast screening in inner city Cardiff general practices with ethnic minority lists. Ethn Health 1999;4:277-84. http://dx.doi.org/10.1080/13557859998056.
- Federici A, Giorgi Rossi P, Bartolozzi F, Farchi S, Borgia P, Guastcchi G. The role of GPs in increasing compliance to colorectal cancer screening: a randomised controlled trial (Italy). Cancer Causes Control 2006;17:45-52. http://dx.doi.org/10.1007/s10552-005-0380-9.
- Senore C, Armaroli P, Silvani M, Andreoni B, Bisanti L, Marai L, et al. Comparing different strategies for colorectal cancer screening in Italy: predictors of patients’ participation. Am J Gastroenterol 2010;105:188-98. http://dx.doi.org/10.1038/ajg.2009.583.
- Weller D, Moss S, Melia J, Coleman D, Worth A, Campbell C. Evaluation of the 3rd Round of the English Bowel Cancer Screening Pilot 2009. http://webarchive.nationalarchives.gov.uk/20150506150512/http://www.cancerscreening.nhs.uk/bowel/pilot.html (accessed February 2017).
- Woodrow C, Rozmovits L, Hewitson P, Rose P, Austoker J, Watson E. Bowel cancer screening in England: a qualitative study of GPs’ attitudes and information needs. BMC Fam Pract 2006;7. http://dx.doi.org/10.1186/1471-2296-7-53.
- British Medical Association . Social Determinants of Health –What Doctors Can Do 2006. www.bma.org.uk/images/socialdeterminantshealth_tcm41-209805.pdf (accessed March 2015).
- Mackay D, Sutton M, Watt G. Deprivation and volunteering by general practices: cross sectional analysis of a national primary care system. BMJ 2005;331:1449-51. http://dx.doi.org/10.1136/bmj.331.7530.1449.
- Asthana S, Gibson A. Deprivation, demography, and the distribution of general practice: challenging the conventional wisdom of inverse care. Br J Gen Pract 2008;58:720-6. http://dx.doi.org/10.3399/bjgp08X342372.
- Mercer SW, Watt GC. The inverse care law: clinical primary care encounters in deprived and affluent areas of Scotland. Ann Fam Med 2007;5:503-10. http://dx.doi.org/10.1370/afm.778.
- Camilloni L, Ferroni E, Cendales BJ, Pezzarossi A, Furnari G, Borgia P, et al. Methods to increase participation in organised screening programs: a systematic review. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-464.
- Office for National Statistics . National Statistics Postcode Directory 2007. webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/guide-method/geography/products/postcode-directories/-nspp-/index.html (accessed March 2015).
- Furler J, Stewart A, Sims J, Naccarella L. Patient social and economic circumstances – GP perceptions and their influence on management. Aust Fam Physician 2005;34:189-92.
- Damery S, Smith S, Clements A, Holder R, Nichols L, Draper H, et al. Evaluating the effectiveness of GP endorsement on increasing participation in the NHS Bowel Cancer Screening Programme in England: study protocol for a randomized controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-18.
- Chambers JA, O’Carroll RE, Cook A, Cavanagh J, Archibald D, Millar R. A pilot telephone intervention to increase uptake of breast cancer screening in socially deprived areas in Scotland (TELBRECS): study protocol for a randomised controlled trial. BMC Public Health 2014;14. http://dx.doi.org/10.1186/1471-2458-14-824.
- Hvidberg L, Pedersen AF, Wulff CN, Vedsted P. Cancer awareness and socio-economic position: results from a population-based study in Denmark. BMC Cancer 2014;14. http://dx.doi.org/10.1186/1471-2407-14-581.
- Wagner TH. The effectiveness of mailed patient reminders on mammography screening: a meta-analysis. Am J Prev Med 1998;14:64-70. http://dx.doi.org/10.1016/S0749-3797(97)00003-2.
- Mayer JA, Lewis EC, Slymen DJ, Dullum J, Kurata H, Holbrook A, et al. Patient reminder letters to promote annual mammograms: a randomized controlled trial. Prev Med 2000;31:315-22. http://dx.doi.org/10.1006/pmed.2000.0718.
- Power E, Simon A, Juszczyk D, Hiom S, Wardle J. Assessing awareness of colorectal cancer symptoms: measure development and results from a population survey in the UK. BMC Cancer 2011;11. http://dx.doi.org/10.1186/1471-2407-11-366.
- Whitaker KL, Good A, Miles A, Robb K, Wardle J, von Wagner C. Socioeconomic inequalities in colorectal cancer screening uptake: does time perspective play a role?. Health Psychol 2011;30:702-9. http://dx.doi.org/10.1037/a0023941.
- Department for Communities and Local Government . Indices of Deprivation 2010 2011. http://webarchive.nationalarchives.gov.uk/20120919132719/http://www.communities.gov.uk/communities/research/indicesdeprivation/deprivation10/ (accessed March 2015).
- Halloran SP, Launoy G, Zappa M. International Agency for Research on Cancer . European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition – Faecal occult blood testing. Endoscopy 2012;44:E65-87.
- Brentnall AR, Duffy SW, Baio G, Raine R. Strategy for power calculation for interactions: application to a trial of interventions to improve uptake of bowel cancer screening. Contemp Clin Trials 2012;33:213-17. http://dx.doi.org/10.1016/j.cct.2011.09.021.
- Spiegelhalter DJ. Monitoring clinical performance: a commentary. J Thorac Cardiovasc Surg 2004;128:820-2. http://dx.doi.org/10.1016/j.jtcvs.2004.03.024.
- Clayton D, Hills M. Statistical Models in Epidemiology. Oxford: Oxford University Press; 1993.
- Research Governance Framework for Health and Social Care. London: The Stationery Office; 2005.
- Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C. English Bowel Cancer Screening Evaluation Committee . Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439-46. http://dx.doi.org/10.1136/gutjnl-2011-300843.
- Maheswaran R, Pearson T, Jordan H, Black D. Socioeconomic deprivation, travel distance, location of service, and uptake of breast cancer screening in North Derbyshire, UK. J Epidemiol Community Health 2006;60:208-12. http://dx.doi.org/10.1136/jech.200X.038398.
- Bang JY, Yadegarfar G, Soljak M, Majeed A. Primary care factors associated with cervical screening coverage in England. J Public Health 2012;34:532-8. http://dx.doi.org/10.1093/pubmed/fds019.
- Smith SG, Raine R, Obichere A, Wolf MS, Wardle J, von Wagner C. The effect of a supplementary (’gist-based’) information leaflet on colorectal cancer knowledge and screening intention: a randomized controlled trial. J Behav Med 2015;38:261-72. http://dx.doi.org/10.1007/s10865-014-9596-z.
- Zajac IT, Whibley AH, Cole SR, Byrne D, Guy J, Morcom J, et al. Endorsement by the primary care practitioner consistently improves participation in screening for colorectal cancer: a longitudinal analysis. J Med Screen 2010;17:19-24. http://dx.doi.org/10.1258/jms.2010.009101.
- Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J Med Screen 2007;14:73-5. http://dx.doi.org/10.1258/096914107781261927.
- Ahmed NU, Haber G, Semenya KA, Hargreaves MK. Randomized controlled trial of mammography intervention in insured very low-income women. Cancer Epidemiol Biomarkers Prev 2010;19:1790-8. http://dx.doi.org/10.1158/1055-9965.EPI-10-0141.
- Lyratzopoulos G, Liu MP, Abel GA, Wardle J, Keating NL. The association between fatalistic beliefs and late stage at diagnosis of lung and colorectal cancer. Cancer Epidemiol Biomarkers Prev 2015;24:720-6. http://dx.doi.org/10.1158/1055-9965.EPI-14-0969.
- Reynolds LM, McCambridge SA, Bissett IP, Consedine NS. Trait and state disgust: an experimental investigation of disgust and avoidance in colorectal cancer decision scenarios. Health Psychol 2014;33:1495-506. http://dx.doi.org/10.1037/hea0000023.
- Digby J, McDonald PJ, Strachan JA, Libby G, Steele RJ, Fraser CG. Use of a faecal immunochemical test narrows current gaps in uptake for sex, age and deprivation in a bowel cancer screening programme. J Med Screen 2013;20:80-5. http://dx.doi.org/10.1177/0969141313497197.
- Wardle J, Steptoe A. Socioeconomic differences in attitudes and beliefs about healthy lifestyles. J Epidemiol Community Health 2003;57:440-3. http://dx.doi.org/10.1136/jech.57.6.440.
- Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin 2011;61:237-49. http://dx.doi.org/10.3322/caac.20111.
- Victora CG, Vaughan JP, Barros FC, Silva AC, Tomasi E. Explaining trends in inequities: evidence from Brazilian child health studies. Lancet 2000;356:1093-8. http://dx.doi.org/10.1016/S0140-6736(00)02741-0.
- Baron RC, Rimer BK, Breslow RA, Coates RJ, Kerner J, Melillo S, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med 2008;35:34-55. http://dx.doi.org/10.1016/j.amepre.2008.04.002.
- Vernon SW, Myers RE, Tilley BC, Li S. Factors associated with perceived risk in automotive employees at increased risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2001;10:35-43.
- Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst 1997;89:1406-22. http://dx.doi.org/10.1093/jnci/89.19.1406.
- Robb KA, Miles A, Wardle J. Subjective and objective risk of colorectal cancer (UK). Cancer Causes Control 2004;15:21-5. http://dx.doi.org/10.1023/B:CACO.0000016567.82368.6c.
- Kiviniemi MT, Bennett A, Zaiter M, Marshall JR. Individual-level factors in colorectal cancer screening: a review of the literature on the relation of individual-level health behavior constructs and screening behavior. Psychooncology 2011;20:1023-33. http://dx.doi.org/10.1002/pon.1865.
- Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med 2010;152:668-76. http://dx.doi.org/10.7326/0003-4819-152-10-201005180-00239.
- Rawl SM, Skinner CS, Perkins SM, Springston J, Wang HL, Russell KM, et al. Computer-delivered tailored intervention improves colon cancer screening knowledge and health beliefs of African-Americans. Health Educ Res 2012;27:868-85. http://dx.doi.org/10.1093/her/cys094.
- Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med 2012;43:97-118. http://dx.doi.org/10.1016/j.amepre.2012.04.009.
- Nichols S, Koch E, Lallemand RC, Heald RJ, Izzard L, Machin D, et al. Randomised trial of compliance with screening for colorectal cancer. Br Med J 1986;293:107-10. http://dx.doi.org/10.1136/bmj.293.6539.107.
- Lo SH, Good A, Sheeran P, Baio G, Rainbow S, Vart G, et al. Preformulated implementation intentions to promote colorectal cancer screening: a cluster-randomized trial. Health Psychol 2014;33:998-1002. http://dx.doi.org/10.1037/a0033507.
- Zapka JG, Lemon SC, Puleo E, Estabrook B, Luckmann R, Erban S. Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Ann Intern Med 2004;141:683-92. http://dx.doi.org/10.7326/0003-4819-141-9-200411020-00009.
- Campbell MJ. Challenges of cluster randomized trials. J Comp Eff Res 2014;3:271-81. http://dx.doi.org/10.2217/cer.14.21.
- McGregor L, Skrobanski H, Miller H, Ritchie M, Berkman L, Morris S, et al. Using Specialist Screening Practitioners (SSPs) to increase uptake of the bowel scope (flexible sigmoidoscopy) screening programme: a study protocol for a feasibility single stage phase II trial. Pilot Feas Stud 2016;2. http://dx.doi.org/10.1186/s40814-016-0093-8.
- Bonell C, Fletcher A, Morton M, Lorenc T, Moore L. Realist randomised controlled trials: a new approach to evaluating complex public health interventions. Soc Sci Med 2012;75:2299-306. http://dx.doi.org/10.1016/j.socscimed.2012.08.032.
Appendix 1 The Bowel Cancer Screening Programme screening pathway
The screening pathway
Appendix 2 The Bowel Cancer Screening Programme S1 Letter
Appendix 3 The Bowel Cancer Screening Programme ‘The Facts’ Booklet
This appendix contains public sector information licensed under the Open Government Licence v3.0.
Appendix 4 The Bowel Cancer Screening Programme S9 letter
Appendix 5 The Bowel Cancer Screening Programme ‘How to do the kit’ leaflet
Appendix 6 The Bowel Cancer Screening Programme S10 letter
Appendix 7 Focus group invitation letter
Appendix 8 Focus group participant information sheet
Appendix 9 Focus group consent form
Appendix 10 Focus group topic guide
Appendix 11 Think-aloud sociodemographic questionnaire
Appendix 12 Early version of the ‘gist’ leaflet
Appendix 13 Intervention 1: the ‘gist’ leaflet
Appendix 14 Gist pilot study questionnaire: control group
Appendix 15 Gist pilot study questionnaire: intervention group
Appendix 16 Narrative interview study participant information sheet
Appendix 17 Narrative interview study consent form
Appendix 18 Narrative interview study demographic question sheet
Appendix 19 Narrative interview study additional consent form for photographs
Appendix 20 Narrative interview study debrief form
Appendix 21 ‘Narrative’ leaflet: iteration 1
Appendix 22 ‘Narrative’ leaflet: iteration 2
Appendix 23 ‘Narrative’ leaflet: iteration 3
Appendix 24 Feedback from focus groups and interviews about the narrative leaflet
| ID | Comment | Proposed action for next iteration | Supported |
|---|---|---|---|
| TI1 | Hyacinth’s quote is irrelevant (‘a waste of space’) from a man’s perspective | Will keep the reference to breast cancer as it will mean something to females, and perhaps those with female relatives | |
| Didn’t like use of word ‘test’ in statement related to Monica. By this stage it is an ‘investigation’, not a test | Will change the wording | ||
| Monica’s quote: ‘It wasn’t a bad procedure’ is not liked. Would prefer to be told exactly what procedure would involve | Possibly take this part out. TI2 felt her quotations were too long anyway | ||
| Roger’s quote: would have also put that it helps to be organised | Decided against adding this as not mentioned within Roger’s narrative | ||
| Should add detail such as people should not wipe their bottom and use that for test | Decided this level of detail was not suitable for the leaflet. Need to minimise barrier of disgust | ||
| Judith’s story: liked the use of the word ‘control’ here. Thought it was ‘very good’. Wondered if ‘taking control’ could be added to the front page | Control will be incorporated into new title suggestions, to be discussed | ||
| Front page: OK but didn’t jump out. Doesn’t say anything about bowel cancer | The title will be changed to make ‘bowel cancer screening’ more obvious | TI2, TI3 | |
| Front page: a lot of wasted space due to little photos | Will consider when redesigning front page | ||
| Leaflet looks ‘a little bit amateurish’ but enjoyed looking at the inside of it | Medical illustrators can help us make it look more professional and leaflet will be printed on better quality paper for the study | ||
| Doesn’t like the colour green. Suggests a ‘nice, sunny, yellowy, orange colour’ | The suggested colour may make it too much like the facts booklet. Yellow on white paper may not be suitable for reading | ||
| Back page: difficult to read. Has to stop and focus on reading it. Green colour could be why | Will make the green slightly lighter and try making font bold or larger | TI2 | |
| TI2 | Font could be a bit bigger on back page | Will make the green slightly lighter and try making font bold or larger | |
| Title leaves you ‘wondering what it’s all about’. Needs to mention bowel cancer so it is ‘more explanatory’ | The title will be changed to make ‘bowel cancer screening’ more obvious | TI1, TI3 | |
| Monica’s quote is too long | Will shorten it by taking the comment on ‘procedure’ out | ||
| Black font on green boxes might be better than navy | Will try this but feel it is introducing another colour and best to keep to 2. Will try enlarging or bold first | ||
| Font could be bigger in green boxes | Will try lightening the green colour first in the hope the font stands out more | ||
| NHS logo reassures people it’s free but could be put in the middle to make it more obvious | Definitely keep the logo | ||
| TI3 | The title of ‘shared experiences’ seems ‘slightly misleading’: possible suggests people know each other | Title will be changed | |
| Needs a more relevant headline | Title will be changed | TI1, TI2 | |
| Could put NIHR/NHS logo on the back as well, in space above development sentence | Worry this would make it look like the NHS developed the leaflet | ||
| FG1 | Colour is too hard on the eye | The colour will be lightened | |
| One participant colour blind so can’t see green | Had not really considered this. Google suggests blue and yellow are good colours but not yellow on white | ||
| Needs more information about bowel cancer | As the leaflet will accompany the facts booklet we can ignore the need for facts for the moment | ||
| Bullet points of facts in leaflet to replace some stories | This information will be available in ‘The Facts’ booklet | ||
| Have a phrase on the front such as ‘Don’t put me down, read me first’ of ‘I could save your life’ | Do not want to make the leaflet appear more important than ‘The Facts’ booklet | FG2 | |
| Have an image of the kit on the leaflet | As the standard information provided by the screening programme does not include a picture of the test, we will not add one in the leaflet | ||
| Better to have a minimum of words rather than a maximum (sentence on front) | Will revise front cover | ||
| ‘Shared experiences’ could be about anything. Make more specific | Will add bowel cancer screening to the title | TI1, TI2, TI3 | |
| More cultures need to be represented: mostly white people and no Chinese person | Will continue to look for pictures but difficult to find people of ethnic minorities who will agree to have photo in leaflet. Stock photos enquire a fee | ||
| Map is confusing: do Scotland and Wales have their own programme or do they not have screening? | Will take map out completely | FG2 | |
| Wants% of people who make a full recovery | This will be in ‘The Facts’ booklet | ||
| Bigger font and smaller pictures | Do not want to make font bigger as does not work given the size of the page | ||
| Front could be bolder – front needs to stick out more | Will work on this | ||
| Flimsy, should be on better paper | Will be painted on better paper once final version confirmed | TI1 | |
| Facts booklet on back doesn’t do anything | Will keep it. Need to refer to facts booklet in some way | ||
| The picture of the bowel in the facts booklet could go in leaflet. Basically want diagrams. Body diagram on front supported | Already in the facts. Do not need to duplicate | ||
| Red suggested as colour but most agreed a pastel green better | Will ask Medical Illustrators to advise on a lighter shade of green | ||
| Too many boxes – not easy on the eye | Lighter green might help | ||
| Font bigger for helpline | Decided to take the helpline number out as this is already in ‘The Facts’ booklet. Do not want to discourage review of the booklet | ||
| Black font on green would be better than navy | Looks too harsh. If paler green used this should have desired effect | TI2 | |
| FG2 | Point out young people can get it too | Not appropriate for this leaflet | |
| Add web address and ‘24 hour’ hotline | This does not need to be included as this information is available in ‘The Facts’ booklet. Do not want to discourage review of the booklet | FG1 | |
| Can the test kit fit in letter box? | Having a photo of kit could help dispel such fears but as ‘The Facts’ booklet does not include a picture of the test kit, we should not add it | ||
| Can a website address be added that leads people to read more stories | Not possible to set up and maintain such a website | ||
| Put NHS on envelope – not a circular. Urgency in reading it (envelope with narrative) | We have no say over this BCSP envelope but for the GP pilot study supports the use of the NIHR logo | ||
| Put examples of other reasons why abnormal results | Already text heavy so we will leave it out | ||
| Add a message to indicate urgency | Not sure how to deal with this. Should not appear more important than ‘The Facts’ booklet | FG1 | |
| Question why Scotland and Wales are not on the map? | Map will be taken out | FG1 |
Appendix 25 Intervention 2: the ‘narrative’ leaflet
Appendix 26 Narrative pilot study: correlation matrix of measured beliefs
| Beliefs | PV | SE | RE | AD | SA | PM | I |
|---|---|---|---|---|---|---|---|
| Perceived vulnerability | 1 | ||||||
| Self-efficacy | 0.115* | 1 | |||||
| Response efficacy | 0.231* | 0.338* | 1 | ||||
| Anticipated disgust | –0.089* | –0.299* | –0.133* | 1 | |||
| Symptom absence | –0.106* | –0.358* | –0.222* | 0.276* | 1 | ||
| Peace of mind | 0.331* | 0.421* | 0.457* | –0.193* | –0.261* | 1 | |
| Intention | 0.233* | 0.395* | 0.289* | –0.251* | –0.339* | 0.379* | 1 |
Appendix 27 Intervention 3: general practice endorsement S1 pre-invitation letter
Appendix 28 General practitioner recruitment materials: letter
Appendix 29 General practitioner recruitment materials: information sheet
Appendix 30 Pro forma used to record calls to the Bowel Cancer Screening Programme about the reminder letter (version 1)
Appendix 31 Intervention 4: the enhanced reminder S10 letter
Appendix 32 Pro forma used to record calls to the Bowel Cancer Screening Programme about the reminder letter (version 2)
Appendix 33 Randomisation tables for all randomised controlled trials
| Trial date | NHS BCSP hub | ||||
|---|---|---|---|---|---|
| Midlands and North West | North East | Southern | London | Eastern | |
| Monday 5 November | Usual | Gist Leaflet | Usual | Usual | Gist Leaflet |
| Tuesday 6 November | Gist Leaflet | Gist Leaflet | Usual | Usual | Usual |
| Wednesday 7 November | Usual | Usual | Usual | Usual | Usual |
| Thursday 8 November | Gist Leaflet | Usual | Gist Leaflet | Gist Leaflet | Usual |
| Friday 9 November | Gist Leaflet | Gist Leaflet | Gist Leaflet | Gist Leaflet | Gist Leaflet |
| Saturday 10 November | N/A | N/A | N/A | N/A | N/A |
| Sunday 11 November | N/A | N/A | N/A | N/A | N/A |
| Monday 12 November | Usual | Usual | Gist Leaflet | Gist Leaflet | Usual |
| Tuesday 13 November | Gist Leaflet | Gist Leaflet | Usual | Usual | Usual |
| Wednesday 14 November | Usual | Usual | Gist Leaflet | Gist Leaflet | Gist Leaflet |
| Thursday 15 November | Usual | Gist Leaflet | Usual | Usual | Gist Leaflet |
| Friday 16 November | Gist Leaflet | Usual | Gist Leaflet | Gist Leaflet | Gist Leaflet |
| Trial date | NHS BCSP hub | ||||
|---|---|---|---|---|---|
| Midlands and North West | North East | Southern | London | Eastern | |
| Monday 4 March | Narrative Leaflet | Narrative Leaflet | Usual | Narrative Leaflet | Narrative Leaflet |
| Tuesday 5 March | Narrative Leaflet | Narrative Leaflet | Usual | Narrative Leaflet | Narrative Leaflet |
| Wednesday 6 March | Usual | Narrative Leaflet | Narrative Leaflet | Usual | Usual |
| Thursday 7 March | Usual | Usual | Usual | Usual | Usual |
| Friday 8 March | Usual | Usual | Usual | Narrative Leaflet | Narrative Leaflet |
| Saturday 9 March | N/A | N/A | N/A | N/A | N/A |
| Sunday 10 March | N/A | N/A | N/A | N/A | N/A |
| Monday 11 March | Narrative Leaflet | Narrative Leaflet | Usual | Narrative Leaflet | Usual |
| Tuesday 12 March | Narrative Leaflet | Usual | Narrative Leaflet | Usual | Narrative Leaflet |
| Wednesday 13 March | Narrative Leaflet | Usual | Narrative Leaflet | Usual | Usual |
| Thursday 14 March | Usual | Usual | Narrative Leaflet | Usual | Usual |
| Friday 15 March | Usual | Narrative Leaflet | Narrative Leaflet | Narrative Leaflet | Narrative Leaflet |
| Trial date | NHS BCSP hub | ||||
|---|---|---|---|---|---|
| Midlands and North West | North East | Southern | London | Eastern | |
| Monday 3 June | Usual | Usual | Usual | Usual | Usual |
| Tuesday 4 June | GPE | Usual | GPE | GPE | GPE |
| Wednesday 5 June | Usual | GPE | GPE | Usual | GPE |
| Thursday 6 June | Usual | GPE | Usual | Usual | Usual |
| Friday 7 June | GPE | Usual | Usual | GPE | GPE |
| Saturday/Sunday | N/A | N/A | N/A | N/A | N/A |
| Monday 10 June | GPE | Usual | Usual | Usual | GPE |
| Tuesday 11 June | GPE | GPE | GPE | GPE | GPE |
| Wednesday 12 June | Usual | Usual | Usual | Usual | Usual |
| Thursday 13 June | GPE | GPE | GPE | GPE | GPE |
| Friday 14 June | GPE | GPE | Usual | GPE | Usual |
| Saturday/Sunday | N/A | N/A | N/A | N/A | N/A |
| Monday 17 June | GPE | GPE | Usual | GPE | GPE |
| Tuesday 18 June | GPE | Usual | GPE | GPE | Usual |
| Wednesday 19 June | GPE | Usual | GPE | GPE | Usual |
| Thursday 20 June | GPE | GPE | GPE | Usual | Usual |
| Friday 21 June | Usual | GPE | Usual | Usual | GPE |
| Saturday/Sunday | N/A | N/A | N/A | N/A | N/A |
| Monday 24 June | Usual | Usual | GPE | Usual | GPE |
| Tuesday 25 June | Usual | Usual | Usual | Usual | GPE |
| Wednesday 26 June | Usual | GPE | GPE | GPE | Usual |
| Thursday 27 June | Usual | Usual | GPE | Usual | Usual |
| Friday 28 June | Usual | GPE | Usual | GPE | Usual |
| Trial date | NHS BCSP hub | ||||
|---|---|---|---|---|---|
| Midlands and North West | North East | Southern | London | Eastern | |
| Monday 8 July | ER | ER | ER | Usual | ER |
| Tuesday 9 July | ER | ER | Usual | Usual | Usual |
| Wednesday 10 July | Usual | Usual | ER | Usual | Usual |
| Thursday 11 July | ER | Usual | Usual | Usual | Usual |
| Friday 12 July | Usual | ER | ER | Usual | ER |
| Saturday/Sunday | N/A | N/A | N/A | N/A | N/A |
| Monday 15 July | ER | Usual | Usual | ER | ER |
| Tuesday 16 July | Usual | ER | ER | ER | ER |
| Wednesday 17 July | Usual | Usual | Usual | ER | ER |
| Thursday 18 July | Usual | Usual | Usual | Usual | Usual |
| Friday 19 July | ER | Usual | Usual | ER | Usual |
| Saturday/Sunday | N/A | N/A | N/A | N/A | N/A |
| Monday 22 July | ER | ER | ER | ER | ER |
| Tuesday 23 July | Usual | Usual | Usual | ER | ER |
| Wednesday 24 July | Usual | ER | Usual | Usual | Usual |
| Thursday 25 July | ER | ER | ER | ER | ER |
| Friday 26 July | Usual | Usual | ER | ER | Usual |
| Saturday/Sunday | N/A | N/A | N/A | N/A | N/A |
| Monday 29 July | ER | Usual | ER | ER | ER |
| Tuesday 30 July | ER | ER | Usual | Usual | Usual |
| Wednesday 31 July | ER | ER | ER | Usual | ER |
| Thursday 1 August | Usual | Usual | Usual | Usual | Usual |
| Friday 2 August | Usual | ER | ER | ER | Usual |
Appendix 34 Concurrent initiatives report
Appendix 35 Process evaluation report
Aim: the primary objective of the process evaluation was to monitor any adverse effects (i.e. excessive extra workload for the NHS BCSP helpline that could make the future implementation of any of the interventions unfeasible).
Pre-interventions: prior to the start of any of the interventions, two teleconferences were held between the ASCEND team, hub directors and managers. The primary aim of the meetings was to establish how the ASCEND team could better support the hubs during the running of the interventions. These included discussions about hub staff requirements and monitoring any adverse impact on the NHS BCSP helpline during the interventions.
One of the main points that arose from these meetings was the requirement for hub staff to have a comprehensive understanding of the ASCEND interventions to enable them to respond to helpline enquiries adequately. It was agreed that it would be beneficial for representatives from the ASCEND team to visit each of the hubs and deliver information about each of the interventions and a question-and-answer session for hub staff.
A helpline pro forma for staff to record any calls made to the NHS BCSP specifically relating to each intervention during the course of each trial was designed. In addition, a list of potential frequently asked questions (FAQs) was also created. The pro forma and FAQs belonging to the first intervention to be trialled (the essentials leaflet) was distributed prior to the teleconferences. It was expressed during these meetings that instructions on how to complete the pro forma should be made part of the staff training.
Once the staff training had been delivered, amendments incorporating feedback from the hubs were made to both the helpline pro forma and the FAQs. Finalised materials relating to each specific intervention were then distributed to the hubs before they were due to be trialled, together with a list of contact numbers should they require any assistance once the trials commenced.
Post interventions: 1 month after each of the interventions had been trialled, an e-mail was sent to hubs requesting that any completed helpline pro formas be returned to the ASCEND team so that any adverse impact on the helpline could be monitored. Five calls (two Southern Hubs/three Eastern Hubs) were reported that related specifically to the GPE letter:
Referenced that GP thought she should have screening and that she would like to if she was due.
Subject very concerned, wants to know which GP passed her details on, just recovered from breast cancer.
Subject read out the GP endorsement at the top of the letter, I asked her if she wished to take part, she said yes.
Asked if invitation was from GP. Advised that it was from us but endorsed by GP.
Thought that GP was inviting into a separate screening programme as previously been invited by the programme. Didn’t want to be included into two separate screening programmes.
No calls were reported in relation to the gist, narrative or ER interventions.
Appendix 36 Flow charts of participants for all four ASCEND trials
FIGURE 15.
Flow of participants through the gist trial.
FIGURE 16.
Flow of participants through the narrative trial.

FIGURE 17.
Flow of participants through the GPE trial.
FIGURE 18.
Flow of participants through the ER trial.
List of abbreviations
- BCSP
- NHS Bowel Cancer Screening Programme
- BCSS
- Bowel Cancer Screening System
- CI
- confidence interval
- CRC
- colorectal cancer
- ELSA
- English Longitudinal Study of Ageing
- ER
- enhanced reminder
- FAQ
- frequently asked question
- FIT
- faecal immunochemical test
- FOB
- faecal occult blood
- FS
- flexible sigmoidoscopy
- FTT
- fuzzy trace theory
- gFOBt
- guaiac faecal occult blood test
- GP
- general practitioner
- GPE
- general practice endorsement
- HSCIC
- Health and Social Care Information Centre
- IMD
- Index of Multiple Deprivation
- IT
- information technology
- LR
- logistic regression
- LSOA
- lower-layer super output area
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PCAG
- Primary Care Advisory Group
- PCT
- primary care trust
- RCT
- randomised controlled trial
- SAfH
- Social Action for Health
- SD
- standard deviation
- SEC
- socioeconomic circumstance
- SFT
- secure file transfer
- SI
- standard information only
- SOP
- standard operating procedure
- SPSS
- Statistical Product and Service Solutions
- SSP
- specialist screening practitioner
- UCL
- University College London