Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/57/46. The contractual start date was in June 2012. The draft report began editorial review in March 2016 and was accepted for publication in July 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Pasquale Giordano reports personal fees, from THD UK, outside the submitted work, and Paul Skaife is a member of the National Institute for Health Research Health Technology Assessment Elective and Emergency Specialist Care (EESC) panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Haemorrhoidal tissue, which forms the ‘anal cushions’, is a normal component of the anal canal and is composed predominantly of vascular tissue, supported by smooth muscle and connective tissue. Haemorrhoids result from enlargement of the haemorrhoidal plexus and pathological changes in the anal cushions. They are common, affecting as many as 1 in 3 of the population. 1
Approximately 23,000 haemorrhoidal operations were carried out in England in 2004–5,2 and the prevalence may be even higher in professionally active people. Repeated visits to hospital for therapy represent a significant disruption to the personal and working lives for this population in particular.
Treatment is dictated by the degree of symptoms and the degree of prolapse, and ranges from dietary advice – to rubber band ligation (RBL) in the outpatient department – to an operation under general or regional anaesthetic. Although RBL is cheap, it has a high recurrence rate, and patients often require further visits to the outpatient department for repeat banding before exploring surgical options. 3 Although there are some variations [such as LigaSure® (Covidien – Medtronic, Minneapolis, MN, USA) haemorrhoidectomy], surgery is commonly traditional ‘open’ haemorrhoidectomy (OH) or a stapled haemorrhoidopexy (SH); both require an anaesthetic. OH is associated with considerable postoperative discomfort, sometimes necessitating an overnight hospital stay and a delay in return to normal activity, but has a low recurrence rate; SH has a slightly higher recurrence rate, but is carried out as a day case and patients return to normal activity more quickly. 4 An alternative treatment is haemorrhoidal artery ligation (HAL), which also requires an anaesthetic, but is thought to enable even quicker return to normal activity. Recurrence rates are reportedly similar to SH, but complication rates are lower. 5
There are substantial data in the literature concerning the efficacy and safety of RBL, including multiple comparisons with other interventions. 6–12 Recurrence varies from 11% to > 50%. This broad range probably reflects the definition of recurrence (patient symptoms or clinical appearance), the grade of haemorrhoids treated (grade I, no prolapse; grade II, spontaneously reducible prolapse; grade III, prolapse requiring manual reduction; and grade IV, unreducible prolapse), the number of treatments and/or the intensity and length of follow-up. In most studies, the incidence of recurrence is > 30% and appears greatest for grade III haemorrhoids. Pain is common for a few hours following RBL and occasionally patients experience pain so severe as to require admission to hospital (around 1%3), bleeding (3–4%, sometimes necessitating further treatment9) and vasovagal symptoms (3%13). There have also been rare incidences of blood transfusion3,11,14–18 and severe pelvic sepsis, with a few instances leading to death. 13 Recurrences can be treated by re-banding or by surgical intervention.
Although HAL requires an anaesthetic, evidence suggests a recovery similar to RBL, but an effectiveness that approaches the more intensive surgical options. The substantial data concerning effectiveness include four systematic reviews,5,19–21 11 randomised controlled trials (RCTs),22–32 seven non-randomised trials33–39 and > 60 case series. An overview has been carried out by the National Institute for Health and Clinical Excellence (NICE), which concludes that current evidence shows it to be a safe alternative to OH or SH;40 this is summarised below.
-
In terms of efficacy, studies with > 1 year follow-up suggest bleeding, pain on defecation, and prolapse (surrogates of recurrent symptoms) in 10%, 9% and 11% of patients, respectively.
-
Regarding safety, postoperative haemorrhage requiring intervention (readmission, transfusion, reoperation or correction of coagulopathy) was reported in < 1.2%, haemorrhoidal thrombosis was seen in < 3.5% of patients and fissure formation in < 2.1%.
-
The data from the three RCTs comparing HAL with SH and OH are difficult to combine, but efficacy seems similar for all of the procedures, with OH perhaps being superior in treating prolapse, although it is unclear if a ‘pexy’ stitch was used in the HAL cases to reduce prolapse. OH appears to lead to the most postoperative pain and longest recovery. There are conflicting results as to whether or not the HAL technique results in less pain compared with SH. Complications were also more frequent in the OH group, but occurred at a similar frequency when SH and HAL were used.
Rationale
Both of the systematic reviews and the NICE overview highlight the lack of good-quality data as evidence for the advantages of the technique; most data are from case series. Even the numerous RCTs have significant methodological drawbacks that make them subject to selection, performance, attrition and detection bias. Indeed, none of the studies is powered to reach any meaningful conclusion. There are no existing RCTs that compare HAL with RBL, although there is now one non-randomised comparison with small numbers of patients. 41
Research objectives
The HubBLe study aimed to establish the clinical effectiveness and cost-effectiveness of HAL compared with conventional RBL in the treatment of people with symptomatic second- or third-degree (grade II or grade III) haemorrhoids.
The primary objective was to compare patient-reported symptom recurrence at 12 months following the procedure.
The secondary objectives were to compare postoperative:
-
symptom severity score (adapted from Nyström et al. 42)
-
health-related quality of life [HRQoL; using the European Quality of Life-5 Dimensions (EQ-5D)43]
-
continence (using the validated Vaizey incontinence score44)
-
pain [using a 10-cm visual analogue scale (VAS)]
-
surgical complications
-
need for further treatment
-
clinical appearance of haemorrhoids at proctoscopy following recurrence
-
health-care costs
-
cost-effectiveness.
Text reproduction
The findings of this trial have been published in The Lancet and as such there is reproduced text on pp. 24–26 of The Lancet publication. 45
Chapter 2 Methods
This report is concordant with the Consolidated Standards of Reporting Trials (CONSORT) statement (2010). 46
Trial design
We undertook a multicentre, parallel-group RCT of HAL compared with conventional RBL in the treatment of people with symptomatic second- or third-degree (grade II or grade III) haemorrhoids in 17 NHS Trusts in the UK. Participants were individually randomised to HAL or RBL, in equal proportion at all centres.
Important changes to methods after trial commencement
Recruitment commenced on 12 November 2012, and, following this, in response to early observations, a number of changes were made to the protocol (see Appendix 1) and trial methods. Between the initial Research Ethics Committee (REC) approval and study commencement, there were two substantial amendments clarifying the serious adverse event (SAE) reporting and the expected adverse events. The protocol was published in BMC Gastroenterology in October 2012. 47
In October 2012 (substantial amendment 3; protocol version 4.0), a change was made to the eligibility criteria to exclude patients with hypercoagulability disorders [in addition to those on warfarin or clopidogrel bisulfate (clopidogrel)] following a request from one of the Principal Investigators during a site set-up visit.
In January 2013 (substantial amendment 6; protocol version 5.0), the baseline data collection was changed to the day of surgery, rather than at randomisation. Sites and treatment arms differed in the time between randomisation and treatment, and this change was made to standardise the data collection as much as possible. This also standardised the time between the baseline and the 12-month follow-up, as the follow-up time point was based on the date of trial treatment.
In March 2013 (substantial amendment 7; protocol version 6.0), we removed one inclusion criterion and replaced it with three exclusion criteria for clarification. The inclusion criterion ‘Either presenting for the first time or after failure of one banding’ at sites had caused some confusion at sites and it was also decided during the review of this criterion that patients with some historical treatments for haemorrhoids could be included.
The study team, the sponsor and the Trial Steering Committee (TSC) discussed the windows in which treatment is clinically relevant, and agreed that there should be a difference between surgical and non-surgical treatment for haemorrhoids (and that dietary advice would not exclude patients). The amended criteria clarified that all previous surgery for haemorrhoids and more than one injection treatment or banding in the previous 3 years would lead to exclusion. For non-surgical treatments, > 3 years was considered a historical event and therefore not relevant to the current research. As one previous banding was allowed within the protocol, this was also the case for injection treatments, as they were both non-surgical; one previous course of injection treatment would not mean that patients were excluded.
In April 2013 (substantial amendment 8; protocol version 7.0), we added ‘patients will have at least 24 hours to decide whether to take part’ to the protocol, in order to provide clarification, as it was not explicitly stated previously. The 24-hour period was an appropriate time frame for the study population and intervention.
A pre-randomisation questionnaire was introduced in July 2013 (substantial amendment 9; protocol version 8.0). The Data Monitoring and Ethics Committee (DMEC) suggested that patient-reported outcomes can be affected by the knowledge of their allocation. As baseline data collection took place on the day of surgery (following Substantial Amendment 6), the majority of patients knew their allocation by this point; the concern was that perceived pain and quality of life (QoL) may differ between the groups due to expectation bias at this time,48 even though no procedure had yet taken place. The senior trial statistician reported that the early data did indeed support this hypothesis, in particular with higher self-reported symptoms in the HAL arm. As a result of this, the protocol was amended to incorporate a questionnaire to be completed before randomisation.
To improve the questionnaire completion rates at the 12-month follow-up, we introduced a prize draw for participants who returned the questionnaires in February 2014 (substantial amendment 7; no change to protocol). We completed three draws of £50 each.
Owing to the long waiting times for the HAL procedure, the dropout rate prior to the procedure was higher than expected. To account for this attrition, in April 2014 (substantial amendment 12; no change to protocol) the recruitment target was increased to 370 in order to achieve the sample size of 350 prior to treatment.
Participants and eligibility criteria
The trial was coordinated from the Clinical Trials Research Unit (CTRU) in the Sheffield School of Health and Related Research (ScHARR). Delegated study staff located at individual centres identified and consented potential participants.
The target population was patients referred to collaborating centres for treatment of haemorrhoids.
Potential participants fell into three groups:
-
Patients presenting to the surgical outpatient clinic (SOPC) with symptomatic haemorrhoids that did not require further tests: this group was identified by the clinical team from the general practitioner (GP) referral letter and a patient information sheet was sent to them prior to their clinic appointment. If they were willing to participate then they were consented and randomised when they attended the appointment.
-
Patients presenting to the SOPC with symptomatic haemorrhoids that required further tests to exclude other diagnoses: this group were identified by the clinician at the clinic appointment and given a patient information sheet. They underwent the necessary outpatient tests (usually endoscopy) and, if negative (i.e. the symptoms are due to haemorrhoids), they were contacted by the research nurse prior to attending their follow-up clinic appointment. They were then randomised and consented when they reattended the clinic.
-
Patients who returned to SOPC following one unsuccessful RBL: they were identified by the clinician at their first clinic appointment (when they have RBL) and given a patient information sheet. They were contacted prior to a follow-up appointment (usually 6 weeks after treatment) by a research nurse. If they remained symptomatic and were willing to participate, they were consented and randomised when they reattended.
All potential participants had a minimum of 24 hours in which to decide whether or not they wished to take part. Patients with investigations excluding pathologies other than haemorrhoids, and all of those who had undergone RBL, were contacted by the research nurse before the planned follow-up clinic to ascertain whether or not they met entry criteria and were interested in entering the trial. They would then be seen by the consultant and research nurse in clinic where recruitment and randomisation took place.
Potential participants were invited to take part in the trial if they were aged ≥ 18 years and had symptomatic second- or third-degree haemorrhoids.
Patients were excluded if they met any of the following criteria:
-
have had previous surgery for haemorrhoids (at any time)
-
have had more than one injection treatment for haemorrhoids in the past 3 years
-
have had more than one RBL procedure in the past 3 years
-
with known perianal sepsis, inflammatory bowel disease, colorectal malignancy, pre-existing sphincter injury
-
with an immunodeficiency
-
unable to have general or spinal anaesthetic
-
currently taking warfarin, clopidogrel or have any other hypercoagulability condition
-
pregnant women
-
unable to give full informed consent (this may be due to mental capacity or language barriers)
-
previously randomised to this trial.
Settings and locations where the data were collected
The CTRU coordinated the follow-up and data collection in collaboration with centres. Participant study data were collected and recorded on study-specific case report forms (CRFs) by centre research staff, and then entered on to a remote web-based data capture system at site. To improve the rate of completion, research nurses could complete the participant questionnaires with the participants at the visit or over the telephone, or the participants could return the questionnaires by post to the centres at all time points. Patient questionnaires, at 12 months, could also be completed online and could be returned to the CTRU by participants for data entry.
Interventions
Participants were randomised to receive either RBL or HAL. Both interventions are established and well-documented procedures, which are considered standard care by NICE. 40,49
Conventional RBL uses a device that allows a rubber band to be applied to each haemorrhoid via a proctoscope. The device used across sites was a suction device (various manufacturers), but bands can be applied using a forceps ligator. This rubber band constricts the blood supply causing it to become ischaemic before being sloughed approximately 1–2 weeks later. The resultant fibrosis reduces any element of haemorrhoidal prolapse that may have been present. This is a very commonly performed procedure in all SOPCs; figures from an audit of current practice at Sheffield Teaching Hospitals (STH) highlighted that prior to the trial over 20 such procedures were carried out every week. The procedure is a basic surgical skill with which all senior staff are familiar and competent in performing. All surgeons involved in the study performed this procedure routinely.
Haemorrhoidal artery ligation uses a proctoscope that is modified to incorporate a Doppler transducer. There are two types of equipment in common use: the HALO (haemorrhoidal artery ligation operation) device [Agency for Medical Innovations (AMI) HAL Doppler system, CJ Medical, Truro, UK] and the THD (transanal haemorrhoidal dearterialisation) device (THD Lab, Correggio, Italy). Both devices operate on the same principle, essentially enabling accurate detection of the haemorrhoidal arteries feeding the haemorrhoidal cushions. Targeted ligation of the vessels with a suture reduces haemorrhoidal engorgement. When combined with a ‘pexy’ suture, both bleeding and haemorrhoidal prolapse are addressed. All of the surgeons participating in the trial ensured that the need for a pexy suture was routinely assessed and recorded. The procedure is simple, uses existing surgical skills and has a short learning curve, with the manufacturers recommending at least five mentored cases before independently practising. All of the surgeons involved in the study had completed this training prior to the start and, in addition, had carried out more than five procedures prior to delivering the study treatment.
Trial procedures were carried out as soon as possible after randomisation. RBL is a brief procedure, which, in many cases, can be carried out at the initial clinic visit. By contrast, HAL is an invasive procedure, usually performed under general anaesthetic, and requires a theatre admission, which may be some considerable time after assessment. The HubBle trial defined ‘baseline’ as being the date of procedure, and ‘recurrence at 1 year’ as being from this date onwards.
Outcomes
Measurement of outcomes
Following consent but prior to randomisation, a participant-completed questionnaire was administered which included questions relating to pain, symptoms, EQ-5D-5L (European Quality of Life-5 Dimensions, 5-level version), continence and use of pain medications. The research nurse completed a patient assessment form, which included demographics and details of the haemorrhoids, including previous treatment(s).
The same participant questionnaire was repeated at baseline, defined as the day of the procedure; in addition, the procedure details CRF was completed by the consultant or the research nurse.
Short-term outcome assessments were completed by participants either via return of a postal questionnaire or a telephone call with the research nurse at days 1, 7 and 21 post procedure. This covered EQ-5D-5L, pain and use of pain medication.
Data were collected 6 weeks following the procedure and again at 12 months following the procedure in order to establish which patients required further treatment for recurrent symptoms or complications. Participants attended a 6-week clinic visit following the intervention at which they were asked details regarding GP visits, hospital attendance or further treatment since the procedure; the baseline questionnaire was also repeated at this time. A clinical assessment was recorded wherein complications, SAEs and further treatment (planned or completed) were reported; the haemorrhoidal grade was recorded when it was clinically assessed. The clinician was also asked whether or not, in his/her opinion, the patient’s symptoms were improved.
Twelve-month data were collected from three sources. Details of further procedures were obtained from hospitals (by reviewing hospital records and asking the patients’ consultants), by contacting the patients’ GPs and, finally, by questioning the patient via telephone interview or postal questionnaire at 12 months.
Primary outcome measure
The main research question of the study was to ascertain the 1-year incidence of recurrence following HAL or RBL. The primary outcome was defined as the proportion of patients with recurrent haemorrhoids at 12 months post procedure, as derived from the patient’s self-reported assessment in combination with GP and hospital records.
The trial was a pragmatic design with a dichotomous outcome. As no validated patient-reported symptom score exists, we based our definition of recurrence on Shanmugam et al. ’s systematic review12 definition:
1. Cured or improved: Symptom free or mild residual symptoms but not requiring further treatment at the end of study period; or, 2. Unchanged or worse: No symptom improvement and requiring further intervention or suffered complication or deterioration of symptoms.
This study simplified Shanmugam’s criteria12 into the following question, asked at 12 months by a research nurse: ‘At the moment, do you feel your symptoms from your haemorrhoids are (1) cured or improved compared with before starting treatment or (2) unchanged or worse compared with before starting treatment?’
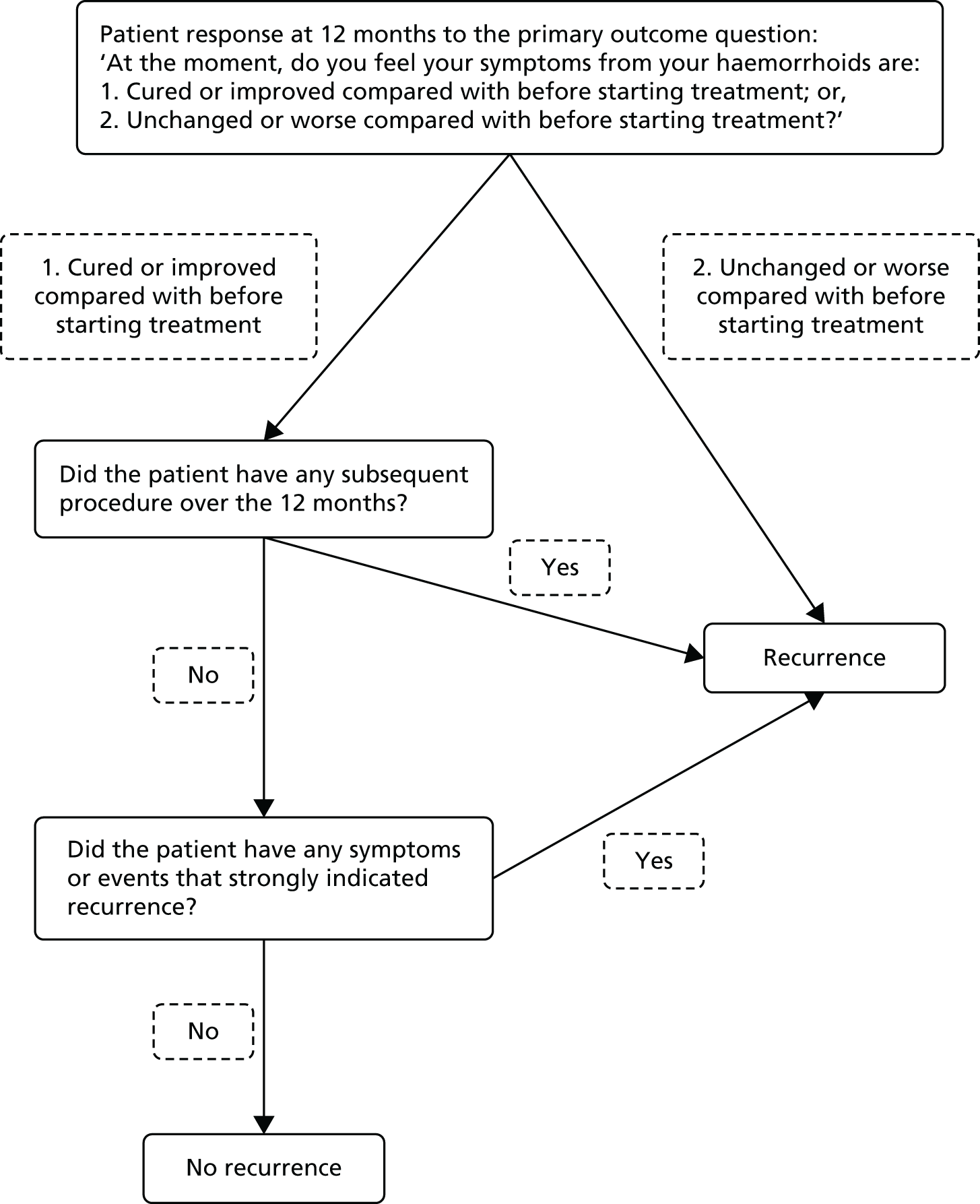
Patients were considered to have recurrent haemorrhoids when any of the following were recorded (as shown in Figure 1):
-
‘Unchanged or worse compared with before starting treatment’ at 12 months, as reported by the patient, or
-
Any subsequent procedure (RBL, HAL, THD, haemorrhoidectomy, haemorrhoidopexy, haemorrhoidal injection or other relevant procedure) over the 12 months, or
-
Presence of any symptoms or events that strongly indicated recurrent haemorrhoids [among patients not meeting (i) or (ii), as adjudicated by two trial investigators (JT, SB) who were blinded to allocated treatment].
FIGURE 1.
Decision tree for recurrence at 1 year.
Secondary outcome measures
Secondary end points seek to identify which treatment – HAL or RBL – is the most cost-effective, which is the least painful procedure with the fewest complications, and which has the greatest effect on the patient’s QoL. Secondary end points are therefore:
-
Persistence of significant symptoms defined analogously to the 12-month outcome.
-
Symptom severity score (adapted from Nyström et al. 42). This score is the sum of the scores from all five questions and is therefore a number on a nominal scale in the range of 0 to 15; where an increase in number is an increase in symptoms.
-
HRQoL using the EQ-5D-5L. 43 A summary index with a maximum score of 1 can be derived from the five dimensions by conversion using a table of scores. The maximum score of 1 indicates the best health state, by contrast with the scores of the individual questions, where higher scores indicate more severe or frequent problems.
-
Continence using the validated Vaizey incontinence score. 44 The Vaizey incontinence score is simply the sum of the scores from all of the seven questions, and is also a number on a nominal scale in the range of 0–24, for which an increase in number indicates more severe incontinence.
-
Pain using a 10-cm VAS, for which ‘0’ is ‘no pain’ and ‘10’ is ‘worst imaginable pain’.
-
Surgical complications.
-
Need for further treatment.
-
Clinical appearance of haemorrhoids at proctoscopy following persistent symptoms at 6 weeks.
-
Health-care costs/cost-effectiveness/quality-adjusted life-years (QALYs).
The schedule for collecting these end points is shown in Table 1.
| Assessment instrument | Time of assessment | ||||||
|---|---|---|---|---|---|---|---|
| Randomisation | Pre-surgery (baseline) | 1 day | 7 days | 21 days | 6 weeks | 1 year | |
| EQ-5D–5L | ○ | ○ | ● | ● | ● | ○ | ● |
| Visual analogue pain score | ○ | ○ | ● | ● | ● | ○ | |
| Vaizey incontinence score | ○ | ○ | ● | ||||
| Haemorrhoids symptom score | ○ | ○ | ○ | ● | |||
| Complications review interview | ○ | ●a | |||||
| Health and social care resource-use data | ○ | ●a | |||||
| Further treatment questionnaire | ○ | ●a | |||||
| Recurrence (primary outcome) | ●a | ||||||
| Clinical appearance at proctoscopy (when applicable) | ○ | ||||||
Sample size
Assuming that the proportion of patients who experience recurrence following RBL is 30% and following HAL is 15%, the sample size calculated to detect a difference in recurrence rates with an odds ratio (OR) of 2 with 80% power and 5% significance was 121 individuals per group. In order to account for any between-surgeon variation and loss to follow-up, this was increased to 175 per group.
This increase was based on the conservative assumption that there would be 14 surgeons in the trial (one per centre, although the number of sites was increased) and intraclass correlation (ICC) of 2.5% in keeping with typical ICCs observed by Ukoumunne et al. 50 However, it was considered likely that each site would have a minimum of two surgeons, in which case the power to detect this difference was 85% (90% power if there was no between-surgeon variation). Because the surgical procedure was well developed and standardised, ICC was expected to be virtually zero and the proposed sample size was hoped to have closer to 90% power.
The impact of loss to follow-up was minimal for the primary end point (haemorrhoidal recurrence at 12 months). Patients who did not complete their 12-month follow-up still had their hospital notes reviewed, and their GP was contacted to ascertain whether or not any complications or operative procedures were recorded. The only dropout expected was if the patient should die, move out of the area or have no traceable patient notes; we anticipated this would be less than the 5% that we allowed for in this patient population (a previous study of RBL that used only clinical follow-up reported a 1-year loss to follow-up of 10%51).
Explanation of any interim analyses and stopping guidelines
No formal interim analyses for efficacy were carried out. However, the safety of the treatments was assessed by an independent DMEC, the members of which convened annually to review SAEs experienced by study participants.
Randomisation and blinding
Randomisation was undertaken using the CTRU’s web-based randomisation system. After consent, participants were individually randomised, in a 1 : 1 ratio, to either HAL or RBL by a member of the research team at site. To ensure equal allocation across centres, randomisation was stratified by centre using permuted blocks of random sizes 2, 4 and 6. Allocation concealment was achieved by ensuring that the participant identifier was entered, following which the allocation was revealed; no member of the study team had access to the randomisation schedule during recruitment. The study was open label, with no blinding of participants, clinicians or research staff attempted.
Statistical methods
Analysis populations
The primary analyses were intention-to-treat (ITT) analyses in which individuals were analysed according to the treatment to which they were randomised, regardless of whether or not they underwent their allocated surgery. Secondary analyses were undertaken on a per protocol (PP) basis, which was restricted to those individuals who complied with the protocol. The categories of non-compliance were eligibility, missed windows, consent and treatment issues, which included patients who did not receive their allocated treatment. Safety summaries were reported, based both on randomised and actual treatment where these differed.
Analysis of 1-year recurrence (primary outcome)
The primary outcome was the recurrence of haemorrhoids at 1 year, as defined above (see Primary outcome measure). Analyses were undertaken using a random intercept logistic regression model in which the covariates were treatment allocation, gender, age at surgery and history of previous intervention as fixed effects; the surgeon was included as a random effect. Further sensitivity analyses assessed whether or not other baseline characteristics [including symptom score, EQ-5D-5L and body mass index (BMI)] altered the strength of the treatment effect and/or appeared to modify the treatment effect. ‘Goodness of fit’ of the logistic regression model was assessed using the Hosmer–Lemeshow test. 52 ICC coefficients were calculated to summarise the clustering that may exist around surgeons.
Secondary outcomes
Persistent significant symptoms at 6 weeks
Recurrence at 6 weeks was analysed in the same manner as the primary outcome. Unlike the 12-month recurrence, no blind review was incorporated, as common symptoms (e.g. severe pain or bleeding) may be due to the procedure rather than the haemorrhoids.
Haemorrhoid symptom severity score
The severity of haemorrhoidal symptoms was compared using a generalised least squares regression mixed model using the same covariates as the primary outcome. The prespecified analysis did not include baseline symptoms as a covariate because of the concerns noted below (see Important Changes to Methods after Trial Commencement), but sensitivity analyses were also undertaken, which adjusted for severity at randomisation (when available) and at baseline, and the average of the two. The difference in symptom severity was compared separately for the 6-week and 12-month time points.
European Quality of Life-5 Dimensions (5-level version) status
Health utility was assessed by the EQ-5D-5L questionnaire using the Value Set for England normative data. 53 EQ-5D-5L was assessed at days 1, 7 and 21. Longitudinal analyses of EQ-5D-5L were conducted as part of the economic evaluation described below (see Health-economic methods).
Vaizey faecal incontinence score
Incontinence was assessed using the Vaizey inventory and analysed in the same manner as haemorrhoidal symptoms.
Pain
Pain was measured using a 10-cm VAS. The VAS scores for HAL and RBL were compared at each time point using the same methods as the haemorrhoid symptom severity. The VAS was collected at randomisation, baseline, days 1, 7 and 21, and, finally, at 6 weeks.
Safety outcomes
Complications
The secondary outcome of procedural complications (adverse events such as pain from thromboses, bleeding requiring consultation, fissuring of the anal canal) elicited during the complications review interview, or from the patient notes at 6 weeks and 1 year post surgery, was compared between the two groups at each time point using Poisson regression.
Serious adverse events
An adverse event is recorded as ‘serious’ if it is an untoward occurrence that:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
consists of a congenital anomaly or birth defect, or
-
is otherwise considered medically significant by the investigator.
Serious adverse events were reported as the number of events, and the number and percentage of participants experiencing each event. Each SAE was classified as systemic complication; urinary retention; pelvic sepsis; or other.
Further treatment
The incidence of the need for further treatment, either surgical or medical, was recorded.
Clinical appearance at proctoscopy (if persistent significant symptoms)
Data were recorded for patients who underwent examination at 6 weeks. This included the clinical assessment of grade and the change from baseline.
Additional/post hoc analyses
Additional, unplanned analyses were undertaken to address questions that arose during the trial progression. These were undertaken in a similar manner to the analyses described above, with adjusted analyses using the same covariates unless otherwise stated.
General considerations
All analyses were undertaken on an ITT basis unless otherwise stated. All confidence intervals (CIs) were two-sided 95% intervals, and all statistical hypotheses were two-sided tests. Continuous measures were summarised as mean (standard deviation, SD) or median (interquartile range, IQR) as appropriate to the distribution of the data. Categorical data were summarised as numbers and percentages.
Health-economic methods
Background
We collected data as part of the trial that allowed us to conduct a full economic evaluation. The main economic analysis focused on estimating the incremental cost per QALY of HAL compared with RBL over the 12-month follow-up period of the trial. We have also presented results in terms of the incremental cost per recurrence avoided.
Patients were asked to complete the EQ-5D-5L instrument at pre-randomisation, pre-surgery (baseline) and subsequent time points following the treatment. The UK population tariffs were then used to calculate QALYs for each patient. 54 EQ-5D has been applied in previous studies in this area55 and appears to be sensitive to changes in patient outcomes. Pain was likely to be one of the main symptoms in which we might expect the treatments to differ and this is well reflected in the EQ-5D-5L instrument.
Overview
Aim
The aim of the economic evaluation was to assess the cost-effectiveness of HAL compared with RBL in terms of incremental cost per QALYs over 12 months’ follow-up.
Methods
Perspective
The economic evaluation took a NHS and Personal Social Services perspective as per NICE recommendations. 49 The estimated resource use covers the period in which a patient is in hospital, post discharge and primary care services, including costs related to recurrence of haemorrhoids.
Method of economic evaluation
We conducted a cost–utility analysis (CUA) as the main method of economic evaluation, for which the outcome was expressed in QALYs. We then carried out a cost-effectiveness analysis (CEA) for which the outcome was expressed in terms of incremental cost per recurrence avoided with HAL compared with RBL. All health-economic analyses were conducted on an ITT basis, including all of the patients randomised to each group. All analyses were conducted using Stata version 13 (StataCorp LP, College Station, TX, USA).
Outcomes
The EQ-5D-5L was used for collecting QoL data at seven time points: pre-randomisation, baseline, 1 day, 7 days, 21 days, 6 weeks and 12 months. The EQ-5D is a generic standardised instrument for measuring HRQoL. The EQ-5D-5L is a five-level version of the EQ-5D, which was recently launched to improve sensitivity of the descriptive system while keeping the same structure of the original instrument [European Quality of Life-5 Dimensions, 3-level version (EQ-5D-3L)]. 53 The EQ-5D-5L utility scores we applied were obtained using recently published tariffs based on the UK general public. 54 The individual patient-level QALYs were calculated from EQ-5D-5L scores at baseline and subsequent follow-up time points for 1 year using the area under the curve method. Discounting was not used for the calculated QALYs in the main analyses, as it was carried out for a 1-year time horizon. We examined the relationship between EQ-5D-5L and symptom scores to check the appropriateness of using this instrument in this setting.
Costs
The costing approach followed the standard stages used in economic evaluation and involves identification of resource use, measurement and valuation. 56
Resource use
Identification of resource use
We identified a range of resource use based on different CRFs and questionnaires used for collecting relevant data at different time points of the trial follow-up. These include the procedure details, clinical assessment at 6 weeks, consultant questionnaire at year, GP questionnaire at 1 year, and participant questionnaire at 1-year time points. The resource use identified is categorised in the following three domains:
Resource use during rubber band ligation procedure
-
Procedure event.
-
Procedural complications.
-
Postprocedural complications.
-
Hospital admissions.
-
Medication on discharge.
Resource use during haemorrhoidal artery ligation procedure
-
Anaesthetic.
-
Procedure event.
-
Intraoperative complications.
-
Hospital admissions.
-
Postoperative complications.
-
Medication on discharge.
Post discharge
-
Outpatient treatments.
-
Surgical treatments.
-
Emergency admissions.
-
Contact with health professionals.
-
Further treatments and medications.
-
Recurrence treatments.
Measurement of resources
All events relating to resource use identified were recorded throughout the trial using different data collection forms at different time points. These were the procedure details form at day 0, clinical assessment form at 6 weeks, consultant questionnaire at 1 year, and GP questionnaire at 1 year. The instruments used for measuring resource use within the trial are provided in Appendix 2. For the HAL procedure event, resource use included the type of anaesthetic used, grade of operating surgeon, whether or not the procedure was supervised by a consultant, timing for surgery, and overall time spent in the operating theatre. Detailed medications prescribed on discharge or as further treatment were also recorded.
Data on hospital admissions and type of admissions were recorded, and data on the durations of admissions were measured based on the NHS average estimates for some events, such as non-elective emergency admissions. Visits for health-care professionals including consultants, GPs and general practice nurses were recorded. However, the duration of each visit was not recorded and therefore average estimates were used based on the NHS or the Personal Social Services Research Unit (PSSRU), University of Kent approaches, where relevant. 57
Valuation of resources
Costs were estimated in UK pound sterling based on the financial year 2014–15. Unit costs were applied for each resource-use event at the individual patient level to calculate the total cost of resource use over a 12 months’ time horizon.
Unit costs
Procedure event unit cost
The unit cost for a RBL procedure event was estimated as an outpatient procedure, and was obtained from the National schedule of NHS reference costs for 2014–15. 58 Admissions unit costs were also obtained from the NHS reference costs. 58 For a HAL procedure, a microcosting approach was applied, based on cost per minute in procedure, recovery time and theatre overhead per minute. The cost per minute in procedure was based on the actual time spent by clinical staff operating and supervising the procedure and the PSSRU staff unit cost. The unit costs for the surgical kits used in the HAL procedure were obtained from the NHS supply system. Blood transfusion unit cost was obtained from the blood transfusion costing statement issued by NICE. 59 Unit costs for medications prescribed on discharge were calculated using the cost of generic drugs from the British National Formulary (BNF) 2015. 60
Post discharge unit cost
The unit costs for surgical treatments following recurrence of haemorrhoids were obtained from different sources. The cost for SH procedure was taken from McKenzie et al. 55 and adjusted for inflation. Unit costs for excisional haemorrhoidectomy (EH) and RBL in the theatre procedures were estimated using the NHS reference costs 2014–15. 58 The unit costs for repeated RBL and HAL procedures were calculated using the average cost for participants within the HubBLe trial. For contacts with health-care professionals, the PSSRU unit costs were applied.
All unit costs used in the economic analyses are summarised in Tables 2–4.
| Event | Description | Unit cost (£) | Source | Note |
|---|---|---|---|---|
| Procedure cost | RBL procedure | 109.00 | UK NHS reference costs 2014–15 58 | Outpatient procedure |
| Blood transfusion | 170.14 | NICE 201559 | Blood transfusion costing statement | |
| Hospital admission | Inpatient bed-day | 303.00 | UK NHS reference costs 2014–15 58 | – |
| Medication prescribed post procedure | Paracetamol | 1.27 | BNF 201560 | 500 mg, 32-tablet pack |
| Co-codamol | 6.73 | BNF 201560 | 30/500 mg, 100-tablet pack | |
| Codeine | 1.23 | BNF 201560 | 15 mg, 28-tablet pack | |
| NSAID | 3.50 | BNF 201560 | Ibuprofen 200 mg, 84-tablet pack | |
| Tramadol | 14.10 | BNF 201560 | 100 mg, 30-tablet pack | |
| Laxative | 3.82 | BNF 201560 | Bisacodyl 5 mg, 100-tablet pack | |
| Antibiotic | 5.03 | BNF 201560 | Augmentina 375 mg, 21-tablet pack |
| Event | Description | Unit cost (£) | Source | Note |
|---|---|---|---|---|
| Anaesthetic | General and local anaesthetic | 100.08 | UK NHS reference costs 2014–15 58 | |
| Spinal anaesthetic | 200.00 | |||
| HAL procedure | Consultant cost per minute | 2.30 | PSSRU 201557 | Includes costs of qualifications |
| Associate specialist cost per minute | 2.13 | PSSRU 201557 | Includes costs of qualifications | |
| Surgical trainee cost per minute | 2.13 | PSSRU 201557 | Includes costs of qualifications | |
| Fellow cost per minute | 2.13 | PSSRU 201557 | Includes costs of qualifications | |
| Specialist nurse cost per minute | 1.52 | PSSRU 201557 | Includes costs of qualifications | |
| Research nurse cost per minute | 1.52 | PSSRU 201557 | Includes costs of qualifications | |
| Registrar cost per minute | 1.20 | PSSRU 201557 | Includes costs of qualifications | |
| Scrub nurse cost per minute | 1.52 | PSSRU 201557 | Includes costs of qualifications | |
| Cost per minute in recovery | 0.41 | McKenzie 200955 | Adjusted for inflation | |
| Cost per minute for theatre overheads | 13.74 | McKenzie 200955 | Adjusted for inflation | |
| Operating event | Outpatient procedure | 109.00 | PSSRU 201557 | |
| Surgical kit for HAL procedure | 432.00 | NHS Supply system | ||
| Excision of skin tags | 109.00 | UK NHS reference costs 2014–15 58 | Outpatient procedure | |
| Procedure cost | Cost of HAL surgery (used in sensitivity analysis) | 1128.00 | UK NHS reference costs 2014–15 58 | Intermediate anal procedure (FZ22B-E) |
| Hospital admission | Inpatient bed-day | 303.00 | UK NHS reference costs 2014–15 58 | |
| Need for blood transfusion | 170.14 | NICE 201559 | Blood transfusion costing statement | |
| Medication on discharge | Paracetamol | 1.27 | BNF 201560 | 500 mg, 32-tablet pack |
| Co-codamol | 6.73 | BNF 201560 | 30/500 mg, 100-tablet pack | |
| Codeine | 1.23 | BNF 201560 | 15 mg, 28-tablet pack | |
| NSAID | 3.50 | BNF 201560 | Ibuprofen 200 mg, 84-tablet pack | |
| Tramadol | 14.10 | BNF 201560 | 100 mg, 30-tablet pack | |
| Laxative | 3.82 | BNF 201560 | Bisacodyl 5 mg, 100-tablet pack | |
| Antibiotic | 5.03 | BNF 201560 | Augmentin 375 mg, 21-tablet pack | |
| GTN paste | 39.30 | BNF 201560 | GTN ointment 0.4%, 30 g | |
| Diltiazem paste | 73.83 | BNF 201560 | 2% diltiazem cream |
| Event | Description | Unit cost (£) | Source | Note |
|---|---|---|---|---|
| Outpatient treatment | Outpatient visit | 114.00 | UK NHS reference costs 2014–15 58 | |
| Injection sclerotherapy | 4.79 | BNF 201560 | Phenol 5% injection, 5-ml ampoule | |
| EH | 1508.72 | UK NHS reference costs 2014–15 58 | FZ22E (elective) | |
| SH | 2478.42 | McKenzie 200955 | Adjusted for inflation | |
| RBL (in theatre) | 1338.45 | UK NHS reference costs 2014–15 58 | FZ22E (elective) | |
| Other elective procedure | 1338.45 | UK NHS reference costs 2014–15 58 | FZ23A | |
| Emergency admissions | Emergency admission for symptoms related to RBL/HAL | 1565.00 | UK NHS reference costs 2014–15 58 | Non-elective |
| Blood transfusion | 170.14 | NICE 201559 | Blood transfusion costing statement | |
| Emergency operation | 2761.00 | UK NHS reference costs 2014–15 58 | FZ23A | |
| Contact with health professionals | GP visit | 46.00 | PSSRU 201557 | |
| Nurse visit (GP practice) | 13.70 | PSSRU 201557 | Based on 15.5 minutes per visit | |
| Consultant visit | 114.00 | UK NHS reference costs 2014–15 58 | ||
| Further treatments | GTN paste | 39.30 | BNF 201560 | GTN ointment 0.4%, 30 g |
| Diltiazem paste | 73.83 | BNF 201560 | 2% Diltiazem cream | |
| Recurrence treatment costs | Proctoscopy at 6 weeks’ assessments | 10.99 | ||
| RBL after recurrence | 523.16 | Mean RBL cost within HubBLe trial | ||
| Admissions with complications | 1565.00 | UK NHS reference costs 2014–15 58 | Non-elective |
Outcomes
Recurrence is the primary outcome for this study (please refer to Primary Outcome Measure, above, for details). For the economic evaluation, the difference in the number of recurrence between the intervention group (HAL) and the control group (RBL) was used as an outcome for assessing the cost-effectiveness in terms of cost per recurrence avoided.
Analysis
The base-case analysis was based on imputed data, whereas complete-case analysis was conducted as a sensitivity analysis. The economic analysis involves CUA as a primary analysis, and a CEA was performed as a secondary analysis. Both analyses involve the estimation of differential costs and differential outcomes.
A subgroup analysis was performed for patients with new haemorrhoids and patients with recurrence following RBL before randomisation. Different sensitivity analyses were performed to address uncertainty associated with the estimates from our base-case analysis.
Resource use
Resource-use data were used for costing the different clinical events and service use at individual patient level. Where RBL or HAL procedures are repeated following recurrence after the initial procedure within this trial, the mean level of resource use within the HubBLe trial was applied.
Difference in costs
The mean total costs and the differences in mean total costs between HAL and RBL groups were analysed using complete-case analysis and descriptively reported alongside the numbers of complete cases, their percentage from the total participants per group, SDs and CIs. The mean costs for each group of resource-use items were similarly reported in tabular format.
Missing data
Missing data may lead to misleading estimates of within-trial CEA, and in this context complete-case analysis is undesirable, as it would reduce the sample size and may affect the power of the study. 61 To check the patterns of missing data, we performed two types of descriptive analyses of missing data: (1) number of missing data by treatment group to assess whether or not missing data differ by group; and (2) missing data patterns to determine whether or not data are missing for all items or individual items of utility scores and resource-use items over the trial follow-up. 61 The multiple imputation chained equation (MICE) method with predictive mean matching was utilised for imputing missing values of costs, QALYs and baseline utility. 61 Age, gender, grade of haemorrhoids, site code and randomisation group were used as imputation variables in this model. The number of imputations specified in this model was 53, based on the highest percentage of missing data for the variables of interest (baseline utility, QALYs and total cost). 61 The imputation was performed per randomisation arm, for all imputed variables, except baseline utility, for which imputation was performed across all observations. This model allowed the prediction of missing values from the posterior distribution of missing observations, given the values of observed data from the imputation variables.
Cost–utility analysis
Cost–utility analysis was performed as the primary economic analysis for estimating the cost-effectiveness of HAL compared with RBL. A seemingly unrelated regression (SUR) model was fitted for estimating differential costs and differential QALYs, while controlling for imbalance in baseline utility and taking into account the correlation between cost and QALYs. Controlling for imbalance in baseline utility is recommended as good practice for within-trial CEA. 62 The fitted SUR model also provided the estimation of full variance–covariance matrix which was further used for addressing uncertainty using the parametric method. 63 In addition to the advantage of controlling for heterogeneity between patients (such as an imbalance in baseline utility) offered by traditional ordinary least squares (OLS) regression, SUR offers two additional advantages: it allows (1) the use of different sets of covariates for cost and effectiveness (QALYs) to assess their joint impact on cost-effectiveness simultaneously; and (2) the testing of joint hypothesis regarding the important coefficients (difference in costs and QALYs) across the two regression equations. 63
The incremental cost-effectiveness ratio (ICER) was estimated from the SUR regression output, based on analysis of imputed data for the base case. The ICER is then compared with NICE cost-effectiveness threshold range of £20,000–30,000 per QALY gained.
To address uncertainty around our CUA estimates, a number of approaches were used based on the parametric method. We used five key parameters from the SUR regression output and conducted a fully parametric CUA. These parameters were difference in mean QALYs, standard error (SE) of the mean differential QALYs, difference in mean costs, SE of mean differential costs, and covariance between costs and QALYs. From these parameters, we produced three different graphs: the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness confidence ellipses and the net benefit line with CIs. The higher bound of NICE cost-effectiveness threshold of £30,000 per QALY was used as a standard decision rule in this analysis for illustrative purposes. The cost-effectiveness threshold was varied from £0 to £140,000 for addressing uncertainty associated with the cost–utility estimates across different levels of willingness to pay.
Combined CEAC curves were generated from the subgroup analysis for patients with new haemorrhoids and patients with recurrence following RBL to allow for easy comparisons. We also conducted an additional analysis by controlling for the grade of haemorrhoids in addition to baseline utility within the SUR model. This allowed us to assess the effect of the grade of haemorrhoids on our CUA in terms of differential cost, differential QALYs and the ICER.
Cost-effectiveness analysis
The CEA followed the standard approach for which the total difference in costs was divided by the difference in recurrence to generate the incremental cost per recurrence avoided. However, it should be noted that treatments differed following recurrence, and these were also captured by our costing approach and constitute a key part within the primary CUA.
Sensitivity analyses
We performed a number of sensitivity analyses to assess the robustness of our estimates and address some issues, which might be associated with our costing approach and unit costs applied in the base-case analysis. The main sensitivity analysis was based on using the NHS reference costs for HAL procedure rather than the microcosting approach. In this scenario, HAL was considered as a day case intermediate anal procedure and the cost associated with this Healthcare Resource Group (HRG) was applied. This was assumed to include all of the other procedure-related costs, including the anaesthetic cost, the surgical kits, staff time and theatre overhead cost as per the HRG costing approach. A similar imputation model and SUR were used for estimating differential cost, differential QALYs and the ICER for this scenario of sensitivity analysis. We also produced a CEAC curve using the same methods applied in our base-case analysis.
An additional sensitivity analysis was undertaken by controlling for the grade of haemorrhoids, in addition to baseline utility, using the same regression model. This was carried out to assess the interaction effect of the grade of haemorrhoids on our CUA. The results from this form of sensitivity analysis are presented in terms of ICER.
We also carried out a sensitivity analysis by conducting another scenario of CUA using complete cases. A similar SUR model was run for estimating differential costs, differential QALYs and the ICER based on complete cases only. The results of this analysis were reported in a tabular format.
Another sensitivity analysis was conducted by estimating a utility decrement for each subsequent procedure performed during the trial follow-up. As the EQ-5D-5L questionnaire was completed at particular follow-up time points that did not coincide with any follow-up procedures, estimated QALY decrements were applied. The QALY decrements following subsequent HAL or RBL procedures were estimated using mean utility scores from the trial at baseline, day 1 and day 7. For SH and EH utility decrements were taken from a published UK study. 2 Utility scores during the recovery period of 0.756 for EH and 0.767 for SH were used for estimating QALY decrements. 2 QALY decrements were applied at the individual patient-level data and a regression analysis was run using the same model specification in our base-case analysis.
Long-term cost-effectiveness
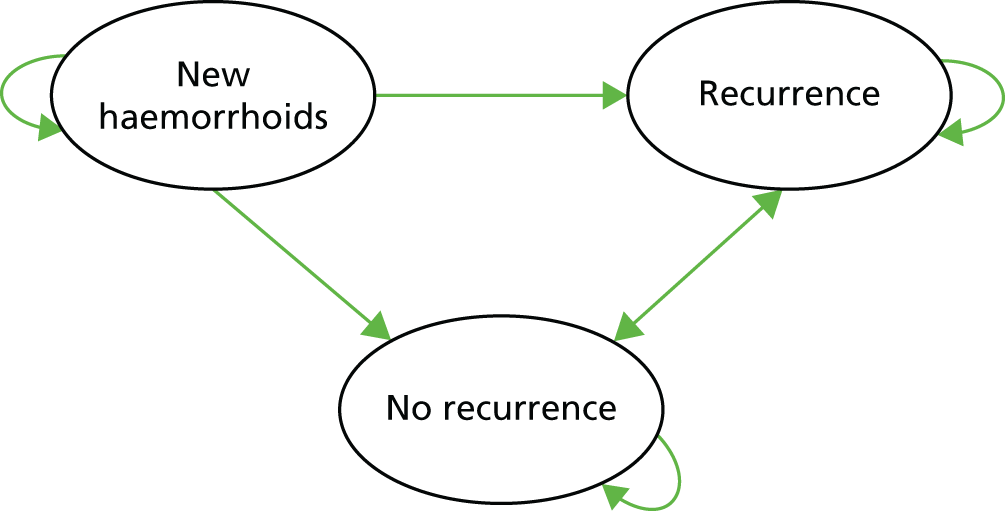
A long-term extrapolation analysis was undertaken to assess the cost-effectiveness of HAL compared with RBL beyond the trial time horizon. The cost and utility data reported at 1-year follow-up within the trial were used in combination with external data to estimate longer-term costs and QALYs. A time horizon of 3 years beyond the trial was chosen for this analysis. This choice of time horizon was driven by data from external studies for which recurrence data were available over this follow-up time. We constructed a three-health states Markov model for extrapolating within-trial cost–utility analyses to long-term cost-effectiveness. To maintain consistency with the trial analyses, health states were chosen based on the primary outcome of the trial – recurrence. Heath states included were new haemorrhoids, recurrence and no recurrence. The extrapolation model structure is shown in Figure 2.
FIGURE 2.
Extrapolation model structure.
Regression analysis was used to estimate costs and utilities from the trial data using a similar SUR model described earlier, with recurrence at baseline added as a covariate. This allowed us to estimate costs and utilities by health state. We assumed that costs and outcomes follow the same pattern observed within the trial for the purpose of these extrapolation analyses. For instance, the mean costs and QALYs for patients with recurrence following RBL after 1 year and treated with further procedures were assumed to be same. Costs and health benefits (utilities) occur at different time points within the long-term time horizon of this analysis. We used the UK Treasury discount rate of 3.5% per year for discounting all future costs and QALYs as recommended by NICE. 49
The annual probability of recurrence for years 1–3 beyond the trial were estimated from two studies3,37 that have reported long-term recurrence estimates. For RBL, the annual probability of recurrence was calculated from a retrospective study3 that reported 4 years’ follow-up (n = 805). For HAL, the probability of recurrence was calculated at 5-year follow-up in a study37 that assessed the long-term success rates for treating patients with grade II and III haemorrhoids following Doppler-guided HAL (n = 100). 37 The mean total costs over four years, per patient, within each group (HAL and RBL) were calculated based on the annual probabilities of transition between health states. The mean total QALYs over the same period were similarly estimated and adjusted for recurrence. The long-term ICER was then estimated from the model and is reported below (see Long-term cost-effectiveness).
A probabilistic sensitivity analysis (PSA) was performed to address the uncertainty around parameter estimates from external sources (i.e. long-term recurrence rates). The PSA was run on 1000 Monte Carlo simulations. The distribution of costs and QALYs used in the PSA simulations were estimated from the regression analysis on the trial data.
Patient and public involvement
We were committed to involving service users at each stage of our research, from design to dissemination. From our patient and public consultation event, we identified an individual who was willing to join the grant application team. This person attended the TSC meetings in order to provide input into study oversight and have commented on the report, particularly the plain language summary, and the patient view regarding the choice of treatment. We also had input from service users with regards to the study design, including some of the patient questionnaires and the length of follow-up involved, and sought the TSC member’s opinion on study documents submitted to the REC, both initially and relating to protocol amendments.
Chapter 3 Results
Recruitment and participant flow
Participants who were randomly assigned, received intended treatment and were analysed for the primary outcome
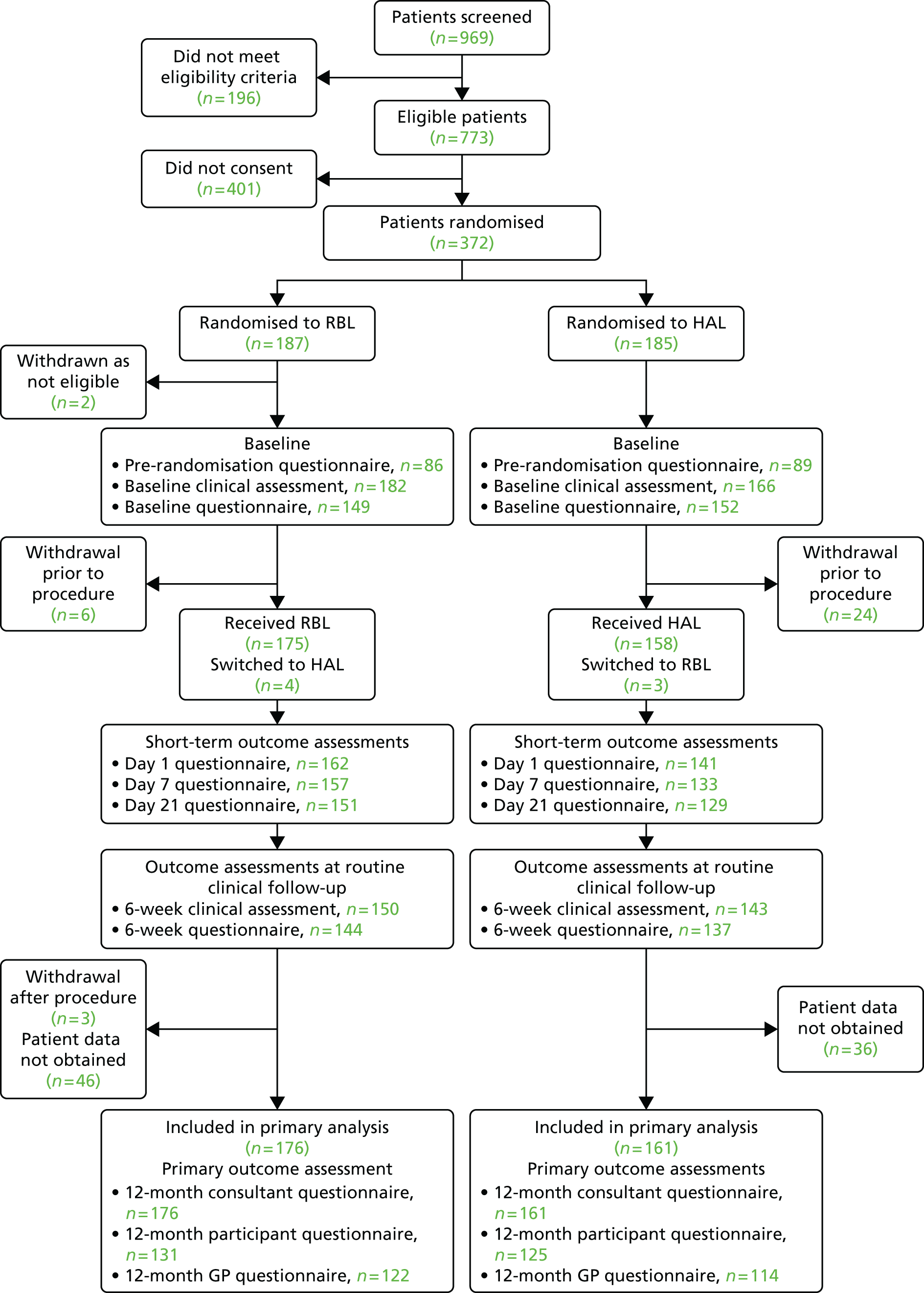
Recruitment to the trial is represented in Figure 3. A total of 372 participants were randomly assigned to receive RBL or HAL; 187 patients were allocated to receive RBL, and 185 were allocated to receive HAL. Two of these participants (both randomised to RBL) were removed from the trial completely, as they were ineligible at the time of consent; therefore, a total of 370 participants were entered into the trial. In total, 969 patients were screened for entry into the trial and the reasons for non-recruitment are shown in Table 5.
FIGURE 3.
Recruitment graph.

| Reason | Frequency |
|---|---|
| Patient preference | 251 |
| Patient preference for RBL | 128 |
| Patient preference for HAL | 70 |
| Patient preference for other surgery | 5 |
| Patient preference for immediate treatment | 3 |
| Patient preference related to general anaesthetic | 6 |
| Patient did not want any intervention or treatment | 39 |
| Clinical decision | 41 |
| Patient did not attend appointment/uncontactable | 26 |
| Patient unsure or declined (no further reason given) | 29 |
| Other reason | 12 |
| Unknown | 42 |
| Total | 401 |
Patient preference was the main reason for non-consent by patients, with 128 patients opting for RBL and 70 patients opting for HAL.
Losses and exclusions after randomisation
A total of 340 participants received treatment as part of the trial and the participant flow is shown in Figure 4; reasons for withdrawal during the trial are provided in Table 6. Primary outcome data were collected for 337 participants (161 HAL and 176 RBL): 256 patient questionnaires were returned at 12 months; 236 GP forms were returned at 12 months, and 337 consultant forms were completed at 12 months. There were 183 participants where all 3 of the 12 months forms were completed/returned.
FIGURE 4.
Flow chart summarising the numbers of each type of questionnaire completed throughout the trial.
| Reason for withdrawal | Treatment group | |
|---|---|---|
| HAL (n = 24) | RBL (n = 11) | |
| Prior to procedure | ||
| Found to be ineligible during baseline assessments | 0 | 2 |
| Participant withdrew consent | 15 | 3 |
| Lost to follow-up prior to procedure | 6 | 2 |
| Symptoms resolved | 2 | 1 |
| Ineligible at time of procedure | 1 | 0 |
| After procedure | ||
| Participant withdrew consent | 0 | 3 |
Dates defining the periods of recruitment and follow-up
The first site to open to recruitment was STH NHS Foundation Trust on 9 September 2012, and recruitment finished at all sites on 6 May 2014. Follow-up was due to end 1 year after the end of recruitment but to allow for the delay in receiving the trial treatment we extended this period and the follow-up was completed at sites on 28 August 2015.
Table 7 shows the duration between randomisation and treatment (waiting time) by treatment group for the trial. These data do not include individuals who withdrew prior to treatment; 24 participants withdrew prior to the procedure in the HAL group, compared with six (eligible) participants in the RBL group. In the RBL group, 114 of 179 (63.4%) participants received treatment on the same day as they were randomised (0 days), whereas none of the HAL group participants was treated on the same day. Although the maximum waiting time is greater for RBL, the mean and median shows that waiting times are higher for HAL.
| Treatment group | Waiting time (days) | |||
|---|---|---|---|---|
| Minimum | Maximum | Mean | Median | |
| HAL | 2 | 276 | 75.9 | 62 |
| RBL | 0 | 413 | 20.3 | 0 |
Baseline data
The characteristics of the participants included in the final analysis is shown in Table 8. The groups appear balanced at baseline in regards to gender, age, BMI, the grade of haemorrhoids and previous treatment for haemorrhoids.
| Demographic | Value | Treatment group | |
|---|---|---|---|
| RBL (n = 176) | HAL (n = 161) | ||
| Gender | Male (%) | 99 (56) | 85 (53) |
| Female (%) | 77 (44) | 76 (47) | |
| Age | Mean (SD) | 49.0 (12.9) | 48.5 (13.5) |
| Median (IQR) | 50.5 (38.5–58.0) | 49.0 (38.0–60.0) | |
| Range | 21.9–79.3 | 20.2–74.6 | |
| BMI | Mean (SD) | 28.0 (5.5) | 28.2 (7.1) |
| Median (IQR) | 27.0 (24.4–31.7) | 26.8 (24.1–30.0) | |
| Range | 17.4–44.9 | 18.8–67.4 | |
| Grade | II (%) | 115 (65) | 92 (57) |
| III (%) | 60 (34) | 68 (42) | |
| Not recorded (%) | 1 (0.6) | 1 (0.6) | |
| Previous treatment | No (%) | 124 (70) | 124 (77) |
| Yes (%) | 52 (30) | 36 (22) | |
| Not recorded | 0 | 1 (0.6) | |
| Grade of surgeon undertaking procedure | Consultant or equivalent (%) | 148 (84) | 121 (75) |
| Junior trainee, supervised by consultant (%) | 6 (3) | 34 (21) | |
| Junior trainee, not supervised by consultant (%) | 22 (13) | 6 (4) | |
The majority of the procedures were conducted by consultant or equivalent (clinician accredited for independent practice, including a clinical nurse specialist), or supervised by a consultant (87% for RBL and 96% for HAL). There were a few cases that were carried out by a trainee doctor. However, these trainees met our pre-existing competencies.
Numbers analysed
The ITT population, in which patients were analysed by their original assigned groups, included all 161 participants in the HAL arm and 176 in the RBL arm for whom recurrence data were available from either the patient, clinician or GP. Of these, the patient-completed questionnaire was returned for 125 and 131 participants in the HAL and RBL groups, respectively. The number of participants included in each analysis is provided for each measure in this section.
Outcomes and estimation
Recurrence (primary outcome)
The overall proportion of participants with a recurrence at 12 months was 49% in the RBL arm compared with 30% in the HAL arm (absolute difference 19.6%; adjusted OR 2.23, 95% CI 1.42 to 3.51; p = 0.0005). The breakdown of recurrences, overall and by criterion, is presented in Table 9. Self-reported recurrence rates were almost identical, with 29% of respondents in both arms stating that they believed that their haemorrhoids had either improved or been cured (adjusted OR for self-reported recurrence = 1.06, 95% CI 0.60 to 1.85; p = 0.85). The increased recurrence associated with RBL is mainly attributable to the high rate of additional procedures undertaken following the initial procedure [33%, compared with 14% in the HAL group (adjusted OR for further procedure = 2.86, 95% CI 1.65 to 4.93; p < 0.001)]. A further three (2%) participants in the RBL arm were considered to have symptoms that were consistent with recurrent haemorrhoids following blind review. In two cases the participants were recorded as possibly requiring further treatment at their 6-week visit but were subsequently lost to follow-up; a third patient had been hospitalised twice for excessive bleeding, but had not undergone treatment.
| Recurrence | Treatment group | |
|---|---|---|
| RBL (n = 176) | HAL (n = 161) | |
| Overall recurrence (%) | 87 (49) | 48 (30) |
| Criteria for recurrencea (%) | ||
| Self-reported recurrence (%) | 37 (29b) | 34 (29b) |
| Subsequent procedure for haemorrhoids (%) | 57 (33) | 23 (14) |
| RBL | 31 (18) | 14 (9) |
| HAL | 23 (13) | 7 (4) |
| EH | 4 (2) | 7 (4) |
| SH | 2 (1) | 1 (1) |
| From blinded review (%) | 3 (2) | 0 |
Sensitivity analyses were undertaken in which alternative covariates (EQ-5D-5L at randomisation or preoperative, BMI at randomisation, and grade of haemorrhoids at randomisation) were adjusted for; doing so yielded reasonable consistency in the ORs (range 2.05–2.81), all of which remained statistically significant. The PP analysis again provided a similar OR (2.21, 95% CI 1.38 to 3.54; p = 0.001).
Of the baseline covariates assessed, none had a statistically significant association with recurrence. Recurrences were more common, however, among participants who were male, had grade III haemorrhoids, had undergone previous treatment and had higher symptom scores.
Among the 80 participants who required a further intervention, the majority of participants underwent a single procedure. In most cases this was RBL, as described in Table 9, although some variation was noted across centres as described in Table 10: as the primary interest is to document second-line treatment, the treatment groups here refer to treatment, as received, as opposed to ‘as randomised’, which was reported previously. As RBL is a brief procedure with (relatively) minimal inconvenience to the patient, it could be argued that a second-line RBL is not itself indicative of a recurrence because the initial haemorrhoids remain incompletely treated. Consequently, an additional (and unplanned) analysis investigated the extent to which recurrence differed if follow-up RBL were not considered a recurrence. In total, 45 participants (31 in the RBL arm, 11 in HAL) underwent RBL as follow-up procedure. Of the 31 patients who underwent repeat RBL, six considered their haemorrhoids to be unchanged or worse at 1 year; three underwent further procedures; and one was considered a recurrence based on blind review. In the HAL arm, 8 of the 11 participants who were undergoing subsequent RBL considered their haemorrhoids to be unchanged or worse at 1 year and two participants underwent further procedures. Thus, if subsequent RBL were not considered a recurrence, the number with recurrent haemorrhoids is 66 (37.5%) in the RBL arm and 44 (27.3%) in the HAL arm (adjusted OR 1.53, 95% CI 0.96 to 2.44; p = 0.071). If a single HAL is compared with multiple RBLs the number with recurrent haemorrhoids is 66 (37.5%) in the RBL arm and 48 (30%) in the HAL arm (adjusted OR 1.35, 95% CI 0.85–2.15; p = 0.20).
| Site | Treatment group | |||||||
|---|---|---|---|---|---|---|---|---|
| RBL (N = 175) | HAL (N = 162) | |||||||
| n | RBL | HAL | Haemorrhoidectomy | n | RBL | HAL | Haemorrhoidectomy | |
| Sheffield | 39 | 8 | 12 | 0 | 34 | 3 | 0 | 0 |
| Birmingham | 34 | 8 | 0 | 2 | 31 | 5 | 2 | 2 |
| Oxford | 28 | 4 | 2 | 1 | 29 | 2 | 4 | 3 |
| Liverpool | 21 | 3 | 2 | 0 | 23 | 2 | 2 | 0 |
| North Tees | 13 | 2 | 0 | 1 | 10 | 1 | 0 | 0 |
| Other centres | 40 | 6 | 6 | 2 | 35 | 1 | 0 | 3 |
Persistent significant symptoms at 6 weeks
At 6 weeks, data were available for 150 participants in the RBL arm and 143 in the HAL arm: 43 (24%) participants in the RBL arm reported their haemorrhoids as being unchanged or worse compared with 12 (7%) in the HAL arm; additionally, one participant in each arm had subsequently undergone RBL, thus resulting in the overall number of patients with persistent symptoms being 44 (29%) compared with 13 (9%) (adjusted OR 4.35, 95% CI 2.19 to 8.65; p < 0.001).
Haemorrhoid symptom severity score
The haemorrhoid symptom severity scores are summarised in Table 11 and displayed graphically in Figure 5: higher scores indicate more severe symptoms. At 6 weeks, haemorrhoids symptom severity scores were higher in the RBL group, indicating that short-term symptoms were less pronounced following HAL. The mean (SD) scores were 4.0 (3.5) in the RBL group and 3.0 (3.1) in the HAL group, with an adjusted mean difference of 1.0 (95% CI 0.3 to 1.8; p = 0.010). No difference was apparent at 1 year, with the mean (SD) being 3.6 (3.2) for RBL and 3.6 (3.3) for HAL (adjusted difference = 0.0, 95% CI –0.8 to 0.8; p = 0.98).
FIGURE 5.
Haemorrhoid symptom severity scores. Note that the area of data points is proportional to the number of participants with that value. Vertical lines represent median and IQR.

| Time point | Treatment group | Difference (95% CI); p-value | |
|---|---|---|---|
| RBL | HAL | ||
| Randomisation | |||
| n | 83 | 80 | |
| Mean (SD) | 6.5 (3.2) | 6.4 (3.4) | |
| Median (IQR) | 6.0 (4.0–8.5) | 6.0 (4.0–9.0) | |
| Baseline | |||
| n | 146 | 150 | |
| Mean (SD) | 6.5 (3.3) | 6.4 (3.0) | |
| Median (IQR) | 6.0 (4.0–8.0) | 6.0 (4.0–8.5) | |
| 6 weeks | |||
| n | 142 | 137 | 1.0 (0.3 to 1.8); 0.01 |
| Mean (SD) | 4.0 (3.5) | 3.0 (3.1) | |
| Median (IQR) | 3.0 (1.0–6.0) | 2.0 (1.0–5.0) | |
| 12 months | |||
| n | 131 | 123 | 0.0 (–0.8 to 0.8); 0.98 |
| Mean (SD) | 3.6 (3.2) | 3.6 (3.3) | |
| Median (IQR) | 3.0 (1.0–5.0) | 3.0 (1.0–5.2) | |
A further (post hoc) analysis looked at the proportion of participants whose symptom score was either ‘0’ or ‘1’, as this corresponds to the definition used by Nyström et al. 42 These numbers are shown in Table 12. The proportions are consistent with the previous analysis, with a greater proportion of participants in the HAL arm reporting either a score of 0 or 1 at 6 weeks.
| Time point | Treatment group | OR (95% CI); p-value | |
|---|---|---|---|
| RBL | HAL | ||
| Randomisation | 2/83 (2%) | 4/80 (5%) | |
| Baseline | 2/146 (1%) | 5/150 (3%) | |
| 6 weeks | 44/142 (31%) | 52/137 (38%) | 0.73 (0.44 to 1.22); 0.23 |
| 1 year | 35/131 (27%) | 38/123 (31%) | 0.79 (0.46 to 1.38); 0.42 |
European Quality of Life-5 Dimensions (5-level version)
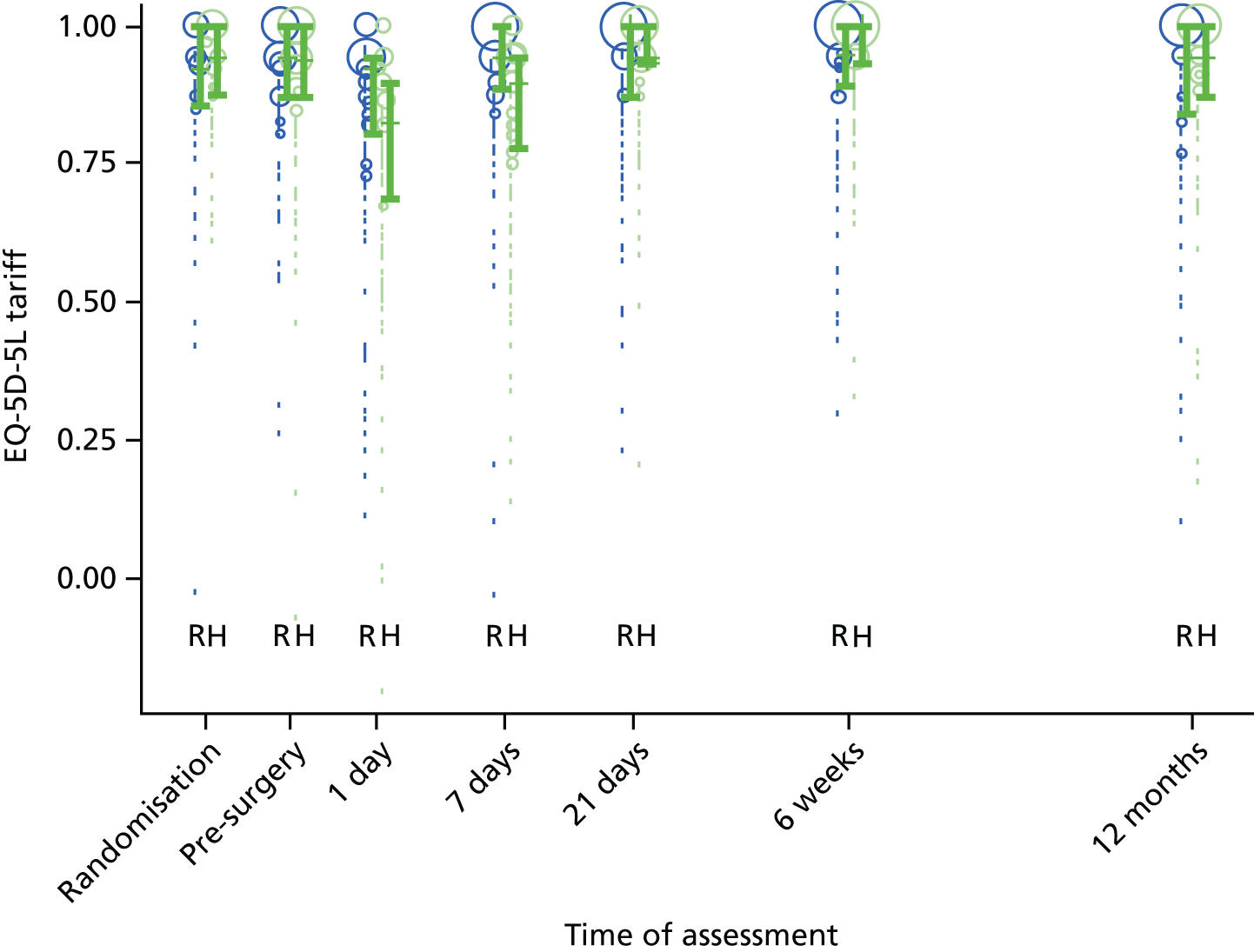
The EQ-5D-5L health tariff is summarised in Table 13 and Figure 6: higher figures indicate a better health state. Prior to procedure, the mean health utility was around 0.9 in both groups but declined at days 1 and 7 in the HAL group. For RBL the mean (SD) at 1 day was 0.84 (0.19) and at 7 days 0.92 (0.15); in other words, health state was reduced for the first day but had reverted back at 1 week. By contrast, the mean health state for HAL had not returned to baseline values by 7 days, with the mean (SD) being 0.76 (0.22) and 0.83 (0.18) at 1 and 7 days, respectively. The adjusted mean differences were 0.08 (95% CI 0.04 to 0.13; p < 0.001) at 1 day and 0.08 (95% CI 0.05 to 0.12; p < 0.001) at 7 days. The two arms were nearly similar with no statistical differences (and above baseline values) at all time points from day 21 onwards.
The EQ-5D-5L inventory was also used to derive the health state used in QALYs that are reported within the health-economic analysis in the following section.
FIGURE 6.
The EQ-5D-5L. Note that the area of data points is proportional to the number of participants with that value. Vertical lines represent median and IQR.
| Time point | Treatment group | Difference (95% CI); p-value | |
|---|---|---|---|
| RBL | HAL | ||
| Randomisation | |||
| n | 86 | 89 | |
| Mean (SD) | 0.89 (0.16) | 0.92 (0.09) | |
| Median (IQR) | 0.92 (0.86–1.00) | 0.94 (0.88–1.00) | |
| Baseline | |||
| n | 149 | 152 | |
| Mean (SD) | 0.90 (0.12) | 0.89 (0.15) | |
| Median (IQR) | 0.94 (0.87–1.00) | 0.94 (0.87–1.00) | |
| 1 day | |||
| n | 162 | 141 | 0.08 (0.04 to 0.13); < 0.001 |
| Mean (SD) | 0.84 (0.19) | 0.76 (0.22) | |
| Median (IQR) | 0.94 (0.87–0.94) | 0.82 (0.69–0.90) | |
| 7 days | |||
| n | 157 | 133 | 0.08 (0.05 to 0.12); < 0.001 |
| Mean (SD) | 0.92 (0.15) | 0.83 (0.18) | |
| Median (IQR) | 0.94 (0.89–1.00) | 0.90 (0.78–0.94) | |
| 21 days | |||
| n | 151 | 129 | –0.01 (–0.04 to 0.02): 0.35 |
| Mean (SD) | 0.92 (0.14) | 0.93 (0.11) | |
| Median (IQR) | 1.00 (0.87–1.00) | 0.94 (0.93–1.00) | |
| 6 weeks | |||
| n | 144 | 137 | –0.02 (–0.05 to 0.01): 0.12 |
| Mean (SD) | 0.92 (0.13) | 0.94 (0.11) | |
| Median (IQR) | 0.95 (0.89 to 1.00) | 1.00 (0.93 to 1.00) | |
| 1 year | |||
| N | 128 | 123 | –0.02 (–0.06 to 0.03): 0.46 |
| Mean (SD) | 0.89 (0.17) | 0.91 (0.16) | |
| Median (IQR) | 0.94 (0.84 to 1.00) | 0.94 (0.87 to 1.00) | |
Vaizey faecal incontinence score
Vaizey faecal incontinence scores are provided in Table 14 and Figure 7; higher scores indicate more severe incontinence. No between–group differences were noted in the Vaizey faecal incontinence scores. An improvement of around 1 unit was noted in both arms at 6 weeks, with a difference between arms of –0.1 (95% CI –1.3 to 1.0; p = 0.86). The improvement was maintained at 1 year, with a difference of –0.5 (95% CI –1.8 to 0.7; p = 0.38). A summary of these findings is presented in Table 14 and Figure 7.
| Time point | Treatment group | Difference (95% CI); p-value | |
|---|---|---|---|
| RBL | HAL | ||
| Randomisation | |||
| n | 84 | 88 | |
| Mean (SD) | 6.0 (5.3) | 4.9 (4.6) | |
| Median (IQR) | 4.0 (1.0–10.0) | 4.0 (2.0–7.5) | |
| Baseline | |||
| n | 142 | 147 | |
| Mean (SD) | 5.9 (5.2) | 5.3 (4.8) | |
| Median (IQR) | 4.0 (1.0–9.0) | 4.0 (2.0–8.0) | |
| 6 weeks | |||
| n | 137 | 132 | –0.1 (–1.3 to 1.0); 0.86 |
| Mean (SD) | 4.0 (4.5) | 4.1 (4.9) | |
| Median (IQR) | 3.0 (0.0–6.0) | 3.0 (0.0–6.0) | |
| 1 year | |||
| n | 118 | 107 | –0.5 (–1.8 to 0.7); 0.38 |
| Mean (SD) | 4.0 (4.7) | 4.5 (4.5) | |
| Median (IQR) | 2.0 (0.0–6.0) | 3.0 (1.0 –7.0) | |
FIGURE 7.
Vaizey faecal incontinence score. Note that the area of data points is proportional to the number of participants with that value. Vertical lines represent median and IQR.

Pain
Haemorrhoidal pain was assessed by asking the patient to rate his/her pain related to haemorrhoids as of today and over the last week. Pain today was asked at baseline and again at days 1, 7 and 21, and, finally, at 6 weeks. Pain over the last week was asked at days 7 and 21, and, finally, at 6 weeks. Both of these are summarised in Tables 15 and 16 and Figures 8 and 9; a score of ‘0’ = ’no pain’, whereas a score of ‘10’ = ’worst imaginable pain’. The change in pain as recorded by VAS is shown in Table 17. Pain was increased in the HAL group at one and seven days after the procedure, but the groups were similar at day 21 and at 6 weeks.
| Time point | Treatment group | Difference (95% CI); p-value | |
|---|---|---|---|
| RBL | HAL | ||
| Randomisation | |||
| n | 84 | 89 | |
| Mean (SD) | 2.4 (2.6) | 2.3 (2.5) | |
| Median (IQR) | 1.0 (0.0–4.0) | 1.0 (0.0–3.3) | |
| Baseline | |||
| n | 148 | 150 | |
| Mean (SD) | 2.2 (2.5) | 2.5 (2.7) | |
| Median (IQR) | 1.0 (0.0–4.0) | 1.0 (0.0–5.0) | |
| 1 day | |||
| n | 162 | 140 | –1.2 (–1.8 to –0.5); < 0.001 |
| Mean (SD) | 3.4 (2.8) | 4.6 (2.8) | |
| Median (IQR) | 3.0 (1.0–5.2) | 5.0 (2.0–7.0) | |
| 7 days | |||
| n | 157 | 133 | –1.5 (–2.0 to –1.0); < 0.001 |
| Mean (SD) | 1.6 (2.3) | 3.1 (2.4) | |
| Median (IQR) | 1.0 (0.0–2.0) | 3.0 (1.0–5.0) | |
| 21 days | |||
| n | 151 | 129 | –0.1 (–0.6 to 0.3); 0.44 |
| Mean (SD) | 1.3 (2.0) | 1.4 (1.9) | |
| Median (IQR) | 0.0 (0.0–2.0) | 1.0 (0.0–2.0) | |
| 6 weeks | |||
| n | 144 | 137 | 0.2 (–0.2 to 0.7); 0.32 |
| Mean (SD) | 1.2 (2.1) | 1.0 (1.8) | |
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) | |
| Time point | Treatment group | Difference (95% CI); p-value | |
|---|---|---|---|
| RBL | HAL | ||
| 7 days | |||
| n | 157 | 133 | –2.3 (–3.0 to –1.6); < 0.001 |
| Mean (SD) | 3.9 (3.2) | 6.3 (2.9) | |
| Median (IQR) | 3.0 (1.0–7.0) | 7.0 (4.0–9.0) | |
| 21 days | |||
| n | 151 | 129 | –0.3 (–0.9 to 0.4); 0.38 |
| Mean (SD) | 2.1 (2.7) | 2.4 (2.5) | |
| Median (IQR) | 1.0 (0.0–4.0) | 2.0 (0.0–3.0) | |
| 6 weeks | |||
| n | 144 | 137 | 0.2 (–0.4 to 0.7); 0.56 |
| Mean (SD) | 1.7 (2.6) | 1.6 (2.3) | |
| Median (IQR) | 0.0 (0.0–3.0) | 0.0 (0.0–2.0) | |
FIGURE 8.
Visual analogue scale pain scores: pain today.

FIGURE 9.
Visual analogue scale: pain over the last 7 days. Note that the area of data points is proportional to the number of participants with that value. Vertical lines represent median and IQR.

| Time point | Treatment group | Difference (95% CI); p-value | |
|---|---|---|---|
| RBL | HAL | ||
| 1 day | |||
| n | 160 | 136 | –1.0 (–1.6 to –0.3); 0.007 |
| Mean (SD) | 1.1 (3.1) | 2.1 (3.0) | |
| Median (IQR) | 1 (0 to 3) | 2 (0 to 4) | |
| More painful | 90 (56%) | 97 (71%) | |
| Same | 34 (21%) | 18 (13%) | |
| Less painful | 36 (23%) | 21 (15%) | |
| 7 days | |||
| n | 155 | 129 | –1.4 (–2.1 to –0.7); < 0.0001 |
| Mean (SD) | –0.7 (2.9) | 0.7 (2.8) | |
| Median (IQR) | 0 (–2 to 0) | 1 (0 to 2) | |
| More painful | 38 (25%) | 72 (56%) | |
| Same | 48 (31%) | 25 (19%) | |
| Less painful | 69 (45%) | 32 (25%) | |
| 21 days | |||
| n | 149 | 127 | –0.1 (–0.7 to –0.6); 0.880 |
| Mean (SD) | –1.0 (2.9) | –1.0 (2.8) | |
| Median (IQR) | –1 (–2 to 0) | 0 (–2 to 0) | |
| More painful | 25 (17%) | 26 (20%) | |
| Same | 49 (33%) | 45 (35%) | |
| Less painful | 75 (50%) | 56 (44%) | |
| 6 weeks | |||
| n | 142 | 132 | 0.3 (–0.4 to 0.9); 0.410 |
| Mean (SD) | –1.0 (2.9) | –1.3 (2.6) | |
| Median (IQR) | 0 (–2 to 0) | –1 (–3 to 0) | |
| More painful | 30 (21%) | 16 (12%) | |
| Same | 46 (32%) | 48 (36%) | |
| Less painful | 66 (46%) | 68 (51%) | |
Clinical appearance of haemorrhoids at proctoscopy
Proctoscopy was conducted at the 6-week visit only if the patient reported continuing symptoms; proctoscopy was conducted more often in the RBL group. The data in Table 18 show that, in the majority of patients in whom proctoscopy was conducted, the grade had reduced at 6 weeks, with a one-grade increase observed in only four patients in the RBL group and none in the HAL group.
| Grade | Treatment group | |
|---|---|---|
| RBL (n = 48) | HAL (n = 27) | |
| At week 6 | ||
| Grade I | 9 | 12 |
| Grade II | 27 | 12 |
| Grade III | 12 | 3 |
| Change from baseline to 6 weeks | ||
| Grade III → I | 5 | 4 |
| Grade II → I or III → II | 17 | 12 |
| Unchanged | 25 | 11 |
| Grade II → III | 4 | 0 |
All important harms or unintended effects in each group
A total of 15 individuals reported SAEs. One patient (in the RBL arm) experienced several episodes of bleeding following the procedure: further investigations revealed that the patient had a rectal tumour. This SAE was classified as pre-existing and is not included in Table 19. Of the remaining 14 SAEs, 12 followed HAL and two followed RBL. One of the participants had been randomised to the RBL arm but was switched over to HAL, but the summaries in Table 19 are based on the treatment actually received and include all treated participants. All 14 events were expected.
| SAE | Treatment group | |
|---|---|---|
| RBL (n = 178) | HAL (n = 162) | |
| Any SAE | 2 (1%) | 12 (7%) |
| Systemic complication | 0 | 3 (2%) |
| Urinary retention | 0 | 2 (1%) |
| Pelvic sepsis | 0 | 1 (< 1%) |
| Other | 2 (1%) | 6 (4%) |
Other complications, which were not classified as ‘serious’, included fissures, which occurred in 3 out of 143 (2%) of the HAL group and 0 out of 150 in the RBL group. There were no cases of fistula formation or anal stenosis in either group. At 6 weeks, 10 out of 143 (7%) of the HAL group and 10 out of 150 (7%) of the RBL group had evidence of anal skin tags.
Post hoc analyses
Some post hoc analyses have been discussed in previous sections [see Recurrence (primary outcome) regarding repeat RBL and recurrence, and see Haemorrhoid Symptom Severity Score’ regarding Nyström’s use of ‘0’ or ‘1’ on the symptoms scale being a signal of cure].
Pre-randomisation questionnaire
As previously mentioned, the baseline was originally measured prior to procedure as opposed to at randomisation. Following a recommendation from the DMEC, the protocol was subsequently amended to allow collection at both time points. The rationale for so doing was that self-reported severity may differ once the allocated treatment is known, thereby introducing an expectation bias. Specifically, participants randomised to the more intensive HAL surgery may rationalise their planned treatment by reporting higher levels of pain and discomfort than those randomised to receive RBL. Furthermore, it is possible that symptoms may change over time, which would also cause imbalance in pre-treatment values, as waiting time for HAL was longer than for RBL.
The agreement between pre-randomisation and pre-treatment values were therefore investigated among patients for whom data were available at both time points. The following are presented for each self-reported measure:
-
The Bland–Altman plot for agreement within each treatment arm, together with the overall mean difference and 95% limits of agreement (also known as a 95% reference interval). The plot is the difference between pre-treatment and pre-randomisation (i.e. the change between the two) plotted against the average of the two. The 95% limits of agreement (also known as reference limits) for the difference were derived based on a normal distribution and quantify how closely the pre-randomisation and pre-treatment value agree. The average is plotted on the horizontal axis, which allows assessment of whether or not (dis)agreement varies according to severity.
-
The difference between pre-treatment and pre-randomisation values against time elapsed between the two. The reference limits were the same as those produced for the Bland–Altman plot. Plotting the difference against elapsed time between the two measures allows an assessment of whether or not (dis)agreement increases with the time elapsed between the two measurements.
The agreement is displayed visually for each method in Figures 10–13. The difference and 95% limits of agreement are also summarised in Table 20, along with the difference between the two means and the ratio of their variances.
Overall, the extent of agreement was generally similar regardless of the severity. For the VAS pain score, there was some indication that the pre-randomisation and pre-procedure time points were in closer agreement in the HAL arm than the RBL arm, as demonstrated by the ratio of the two variances (2.63; F-test p < 0.001). The same was also true for incontinence, as estimated by the Vaizey tool, where the difference between randomisation and procedure again tended to be greater in the HAL arm than the RBL arm (ratio of variances 3.14; p < 0.001). On the other hand, the two values differed less in the HAL arm for the EQ-5D ‘thermometer’ health state (ratio of variances 0.48; p = 0.004), although this may reflect a handful of participants with outlying values. There was no indication of any systematic change (i.e. no consistent deterioration or spontaneous resolution between randomisation and procedure) on any measure.
| Measure | Treatment group, mean difference (95% agreement limits) | Difference in means (p-value) | Ratio of variances (p-value) | |
|---|---|---|---|---|
| HAL | RBL | |||
| Haemorrhoid symptom score | 0.0 (–3.0 to 3.0) | 0.1 (–3.0 to 3.1) | –0.1 (0.823) | 0. 96 (0.864) |
| EQ-5D-5L | –0.01 (–0.13 to 0.11) | –0.00 (–0.12 to 0.11) | –0.01 (0.508) | 1.11 (0.691) |
| Vaizey faecal incontinence score | –0.1 (–5.6 to 5.3) | 0.4 (–2.7 to 3.5) | –0.5 (0.231) | 3.14 (< 0.001) |
| VAS pain | 0.2 (–3.8 to 4.2) | –0.0 (–2.5 to 2.4) | 0.2 (0.479) | 2.63 (< 0.001) |
FIGURE 10.
Agreement of haemorrhoid symptom severity scores at randomisation and pre-procedure. Bland–Altman graphs are given for (a) HAL and (b) RBL. Difference between the measures in relation to elapsed time between the two is given for (c) HAL and (d) RBL.
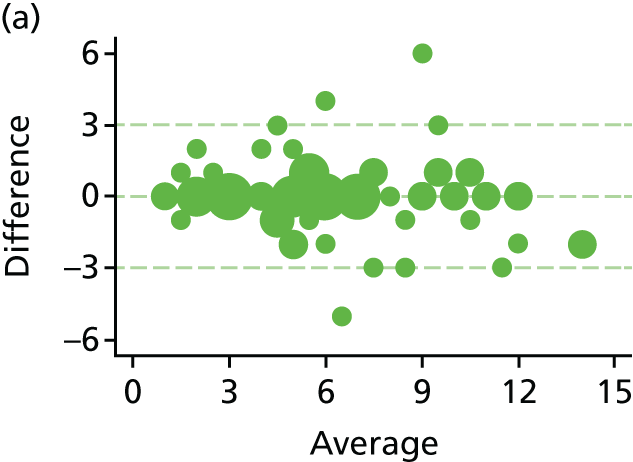
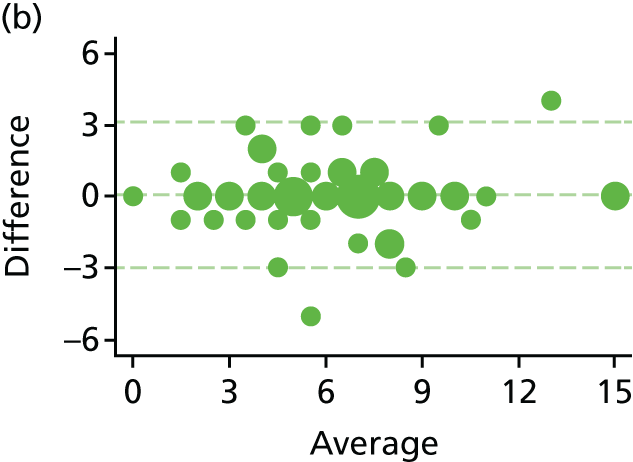

FIGURE 11.
Agreement of EQ-5D-5L scores at randomisation and pre-procedure. Bland–Altman graphs are given for (a) HAL and (b) RBL. Difference between the measures in relation to elapsed time between the two is given for (c) HAL and (d) RBL.
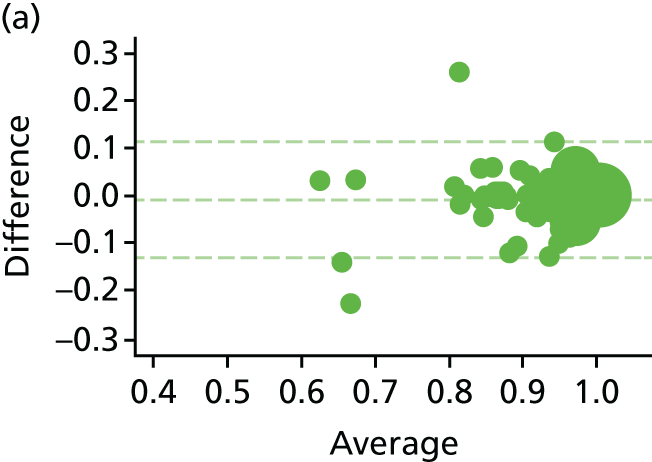
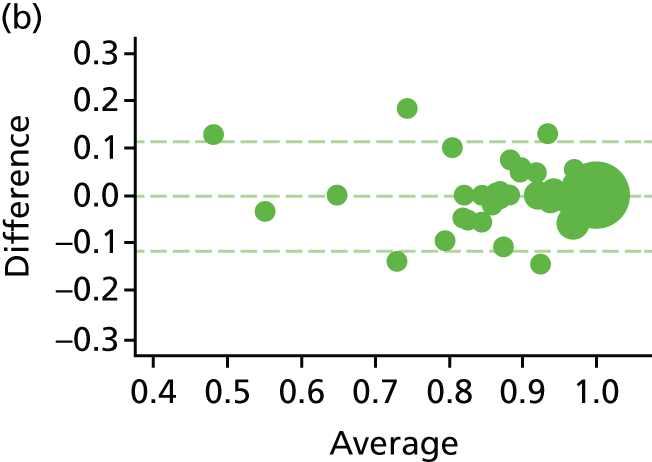
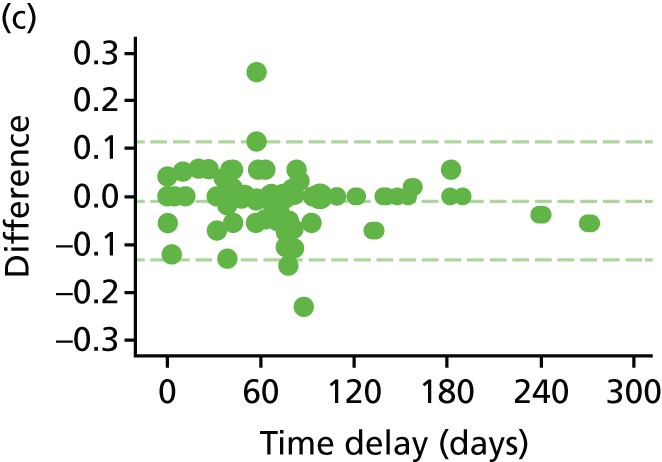

FIGURE 12.
Agreement of Vaizey scores at randomisation and pre-procedure. Bland–Altman graphs are given for (a) HAL and (b) RBL. Difference between the measures in relation to elapsed time between the two is given for (c) HAL and (d) RBL.
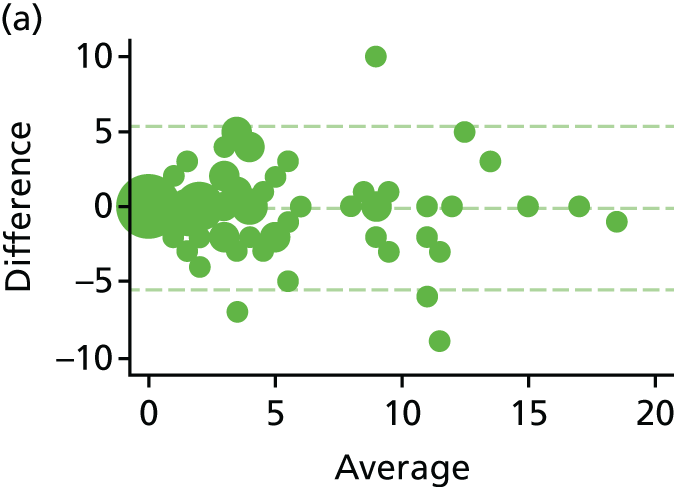

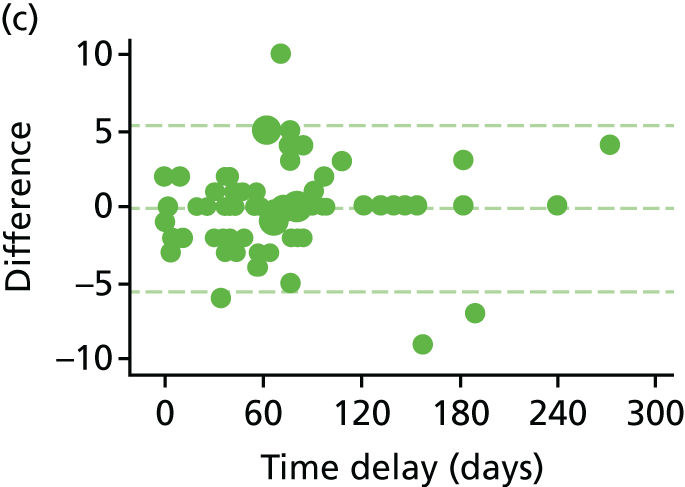

FIGURE 13.
Agreement of VAS pain scores at randomisation and pre-procedure. Bland–Altman graphs are given for (a) HAL and (b) RBL. Difference between the measures in relation to elapsed time between the two is given for (c) HAL and (d) RBL.
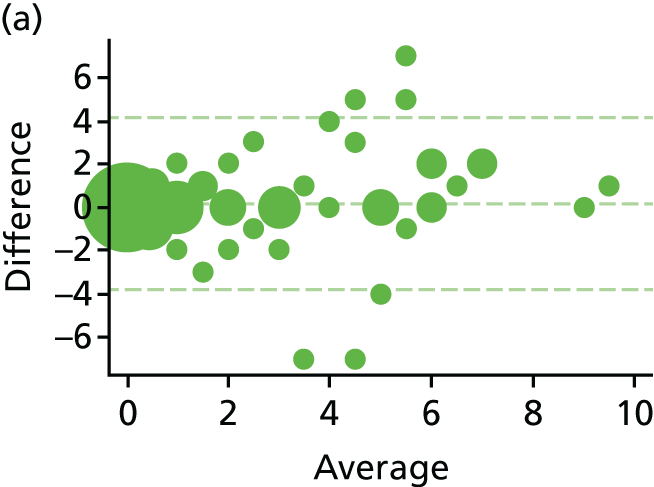



Haemorrhoidal artery ligation device
Among participants undergoing HAL, the preferred surgical device appeared to be determined by site rather than participant characteristics. Five sites (n = 73) used the AMI HALO device on all participants, with the remaining 12 sites using the THD device in 87 cases and the HALO device in two. There was no significant difference in outcomes between the types of device, although fewer recurrences were seen in those in the THD arm (26% vs. 35%; adjusted OR 0.65, 95% CI 0.33 to 1.29; p = 0.22). Symptoms and pain were also similar for the two procedures, data are shown in Table 21. The Vaizey faecal incontinence score was higher for THD than HALO at 6 weeks (mean scores 5.2 vs. 3.2) and 1 year (means 5.6 vs. 3.5), but also prior to procedure (means scores 6.3 vs. 4.8). As a consequence, the magnitude of the difference at 6 weeks (mean difference = 2.0, 95% CI 0.5 to 3.6; p = 0.01) was greater than the change from baseline (mean difference incorporating an adjustment for baseline = 1.3, 95% CI –0.3 to 2.8; p = 0.11).
| Measure: time point | Device | THD vs. HALO | |
|---|---|---|---|
| HALO | THD | ||
| n (%) with recurrence | n (%) with recurrence | OR (95% CI); p-value | |
| Recurrence | |||
| 6 weeks | 5 (7) | 8 (11) | 2.23 (0.57 to 8.76); 0.250 |
| 1 year | 26 (35) | 23 (26) | 0.65 (0.33 to 1.29); 0.221 |
| Mean (SD) | Mean (SD) | Mean difference (95% CI); p-value | |
| Haemorrhoid Symptom Severity Score | |||
| 6 weeks | 2.9 (2.7) | 3.1 (3.3) | 0.5 (–0.9 to 1.8); 0.493 |
| 1 year | 3.5 (2.9) | 3.8 (3.6) | 0.0 (–1.1 to 1.2); 0.945 |
| Pain (VAS) | |||
| 1 day | 4.7 (2.9) | 4.7 (2.9) | 0.0 (–0.9 to 0.9); 0.957 |
| 7 days | 3.3 (2.5) | 2.9 (2.3) | –0.3 (–1.1 to 0.5); 0.415 |
| 21 days | 1.5 (2.0) | 1.4 (1.9) | –0.2 (–1.0 to 0.6); 0.586 |
| 6 weeks | 0.9 (1.6) | 1.0 (2.0) | 0.1 (–0.5 to 0.8); 0.697 |
| EQ-5D-5L | |||
| 1 day | 0.78 (0.21) | 0.73 (0.25) | –0.05 (–0.12 to 0.02); 0.131 |
| 7 days | 0.83 (0.20) | 0.83 (0.15) | 0.00 (–0.06 to 0.05); 0.996 |
| 21 days | 0.93 (0.10) | 0.92 (0.13) | –0.00 (–0.05 to 0.04); 0.889 |
| 6 weeks | 0.94 (0.13) | 0.94 (0.09) | 0.00 (–0.04 to 0.05); 0.829 |
| 1 year | 0.92 (0.16) | 0.90 (0.15) | –0.02 (–0.07 to 0.04); 0.575 |
| Vaizey faecal incontinence symptom score | |||
| 6 weeks | 3.2 (4.2) | 5.2 (5.6) | 2.0 (0.5 to 3.6); 0.010 1.3 (–0.3 to 2.8); 0.110a |
| 1 year | 3.5 (3.9) | 5.6 (5.2) | 1.0 (–1.0 to 3.0); 0.304 |
Chapter 4 Health-economic results
Data completeness
Over the 12 months trial data collection period, data were missing for some participants for three key parameters used in the CEA: baseline utility, QALYs and total costs. The extent of missing data is presented in Table 22. The results of our descriptive analyses checks for missing data shows that the number of missing data is broadly similar across arms. For instance, the percentages of total costs missing were found to be 44.9% and 46.5% for RBL and HAL, respectively. These assessments were then used to inform the imputation model.
| Parameter | Treatment group, missing data: n (%)a | Difference in % missing | All participants (N = 372): n (%)a | |
|---|---|---|---|---|
| RBL (n = 187) | HAL (n = 185) | |||
| Baseline utility | 38 (20.3) | 33 (17.8) | –2.5 | 71 (19.1) |
| QALYs | 102 (–54.5) | 93 (50.3) | –4.3 | 195 (52.4) |
| Total cost | 84 (44.9) | 86 (46.5) | 1.6 | 170 (45.7) |
Costs
The mean total costs and their SD, skewness and CIs are reported descriptively, by group, in Table 23. These results are based on complete cases only and no imputation was performed at this stage. Similarly, the costs for each group of itemised resource use are presented in Table 24. The numbers of complete cases used for estimating the mean costs for each item and their percentages from the total number of participants per group are also reported. Figures in Table 23 are for complete cases for every category (no missing for all cost categories), whereas figures in Table 24 are the complete cases within each category. There is limited value of calculating and interpreting differences in mean costs at the level shown in Table 24 because of missing data and non-adjustment for patient covariates at this level. The adjusted mean difference in costs for HAL compared with RBL was estimated using regression analysis and reported within the CUA results (see Cost–utility analysis results, below). However, Table 24 shows the share of costs between different items, where the mean cost of further HAL procedure represents the highest share for the RBL group (£203.76), whereas the mean procedure cost is at the top for the HAL group (£732.56). The cost data are right-skewed, with only a few patients incurring very high costs in both arms of the trial. The histograms of cost data distribution for each group are shown in Figures 14 and 15 for RBL and HAL, respectively.
| Treatment group | n | Mean costs (£) | SE | Skewness | 95% CI |
|---|---|---|---|---|---|
| RBL | 103 | 708.67 | 95.4 | 2.33 | 109 to 6000 |
| HAL | 99 | 1766.52 | 101.5 | 2.57 | 776 to 7014 |
| Type of resource use | Treatment group | |||||
|---|---|---|---|---|---|---|
| RBL | HAL | |||||
| Mean costs (£) | SD | n (%) | Mean costs (£) | SD | n (%) | |
| Medications | 2.04 | 8.4 | 187 (100) | 7.59 | 8.4 | 185 (100) |
| RBL procedure | 109.00 | 0.0 | 187 (100) | 0.00 | 0.0 | 185 (100) |
| Excisional tag removal | 0.00 | 0.0 | 187 (100) | 10.02 | 31.6 | 185 (100) |
| HAL procedure | 0.00 | 0.0 | 179 (96) | 732.56 | 299.7 | 151 (82) |
| Admissions for surgery | 0.00 | 0.0 | 187 (100) | 23.01 | 80.5 | 158 (85) |
| Proctoscopy | 5.02 | 5.5 | 149 (80) | 4.71 | 5.5 | 140 (76) |
| Other elective procedure | 0.00 | 0.0 | 187 (100) | 7.23 | 98.4 | 185 (100) |
| Post-discharge admissions | 41.73 | 253.0 | 150 (80) | 65.66 | 314.9 | 143 (77) |
| Other procedures | 0.00 | 0.0 | 150 (80) | 0.76 | 9.1 | 143 (77) |
| Repeated RBL | 82.45 | 276.3 | 187 (100) | 32.05 | 148.0 | 185 (100) |
| Further HAL | 203.76 | 587.6 | 187 (100) | 18.72 | 179.6 | 185 (100) |
| EH | 16.14 | 155.6 | 187 (100) | 24.47 | 191.1 | 185 (100) |
| SH | 26.51 | 255.6 | 187 (100) | 0.00 | 0.0 | 185 (100) |
| RBL in theatre | 21.47 | 218.4 | 187 (100) | 14.47 | 138.8 | 185 (100) |
| Admissions in 1 year | 17.78 | 166.4 | 176 (94) | 68.04 | 320.1 | 161 (87) |
| Emergency procedure | 29.53 | 284.8 | 187 (100) | 29.85 | 286.3 | 185 (100) |
| Consultant visits | 88.59 | 131.2 | 175 (94) | 86.39 | 151.2 | 161 (87) |
| GP visits | 10.93 | 27.8 | 122 (65) | 16.54 | 35.2 | 114 (62) |
| Nurse visits | 0.56 | 6.2 | 122 (65) | 0.85 | 3.8 | 113 (61) |
FIGURE 14.
Distribution of cost data: RBL.
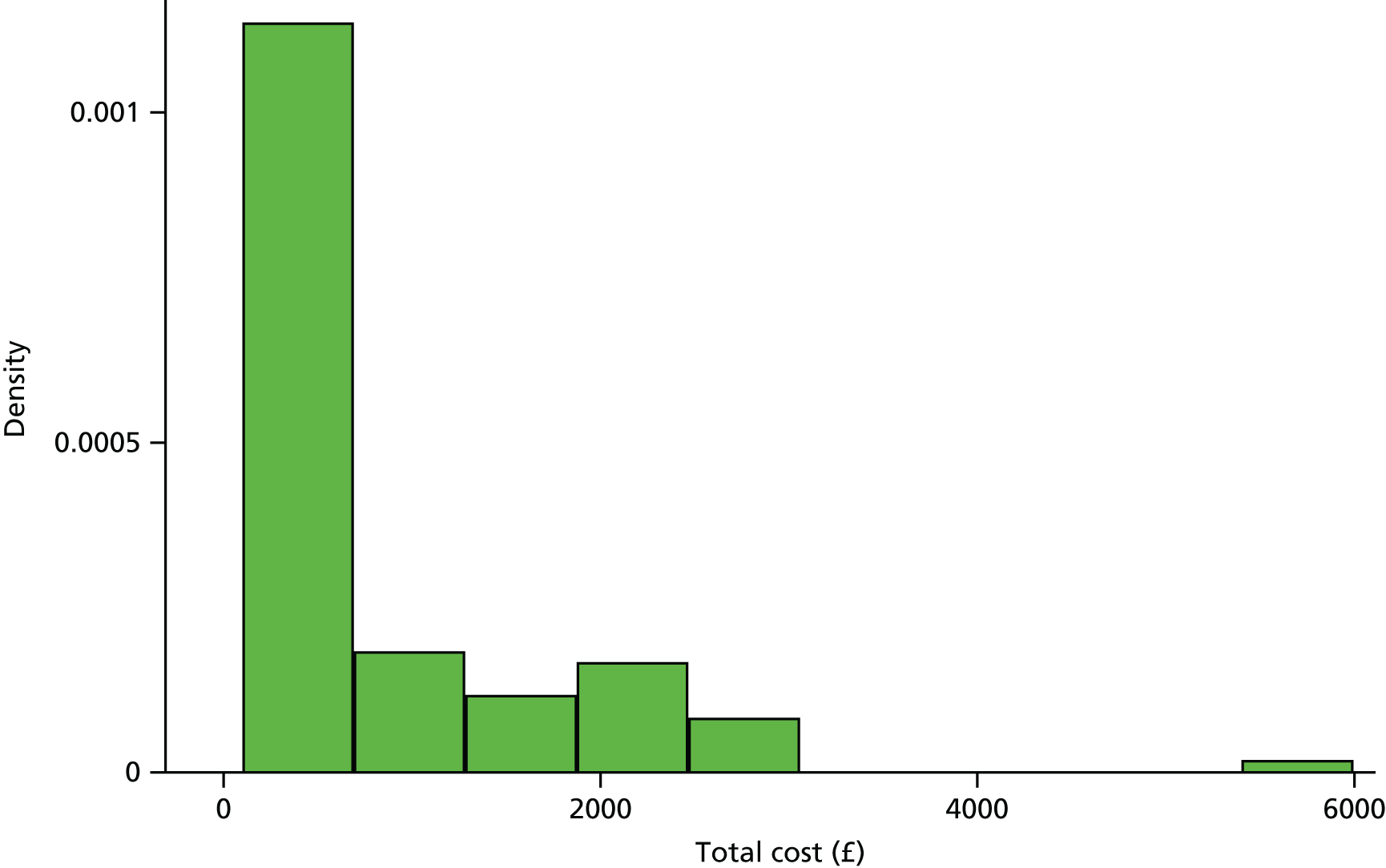
FIGURE 15.
Distribution of cost data: HAL.
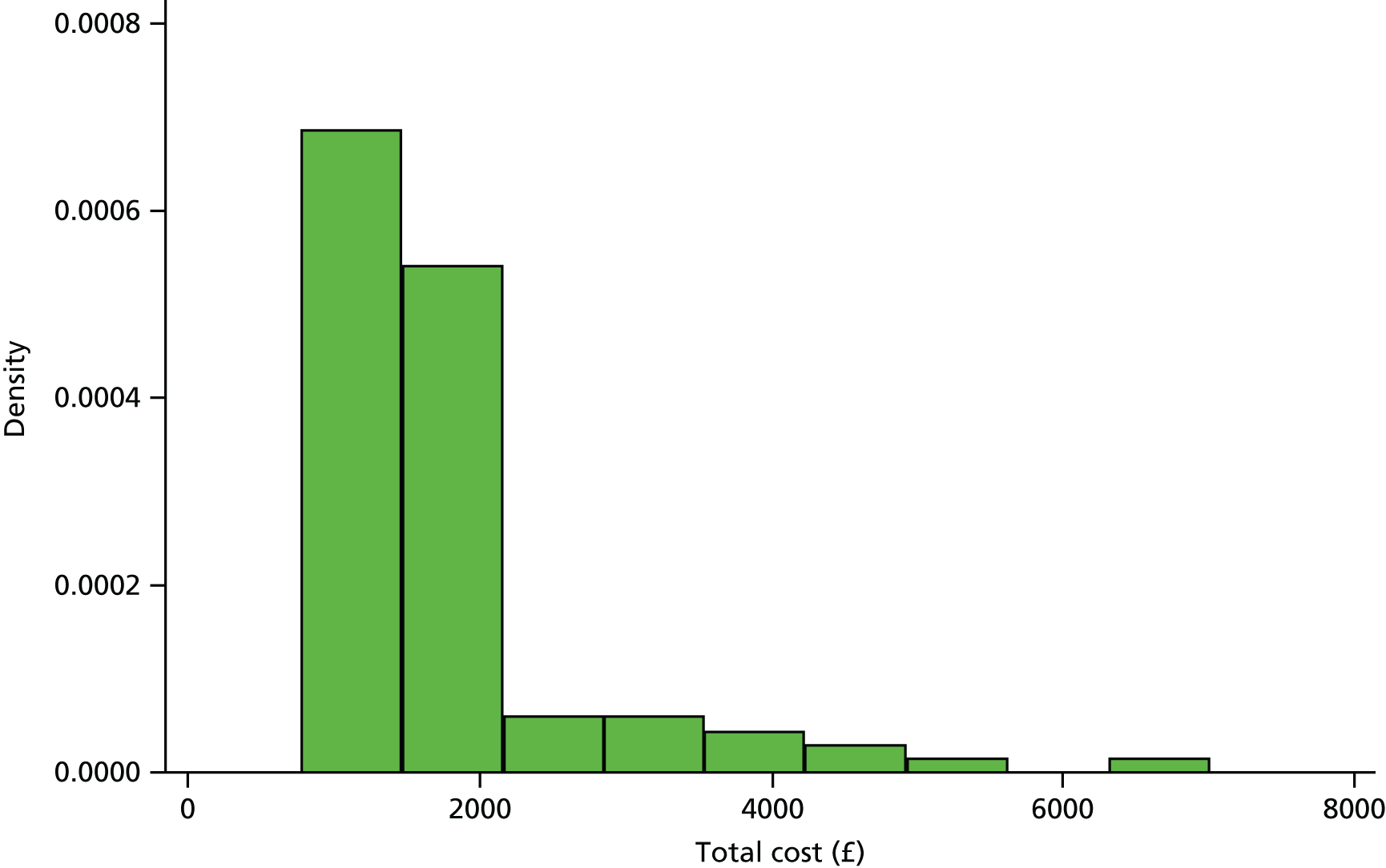
Outcomes
We examined the relationship between EQ-5D-5L and the haemorrhoids symptom score in order to validate the use of EQ-5D-5L as an outcome measure in the economic evaluation. Figure 16 plots the mean symptom scores and mean EQ-5D-5L utility scores for all participants and visits for both the observed data, and the fit from a simple linear regression (controlling for age). The plot shows that symptom score is closely related to EQ-5D-5L, with a unit increase on the 15-point symptom score corresponding to an approximate 0.02 reduction in the EQ-5D-5L.
FIGURE 16.
Symptom score vs. EQ-5D-5L.
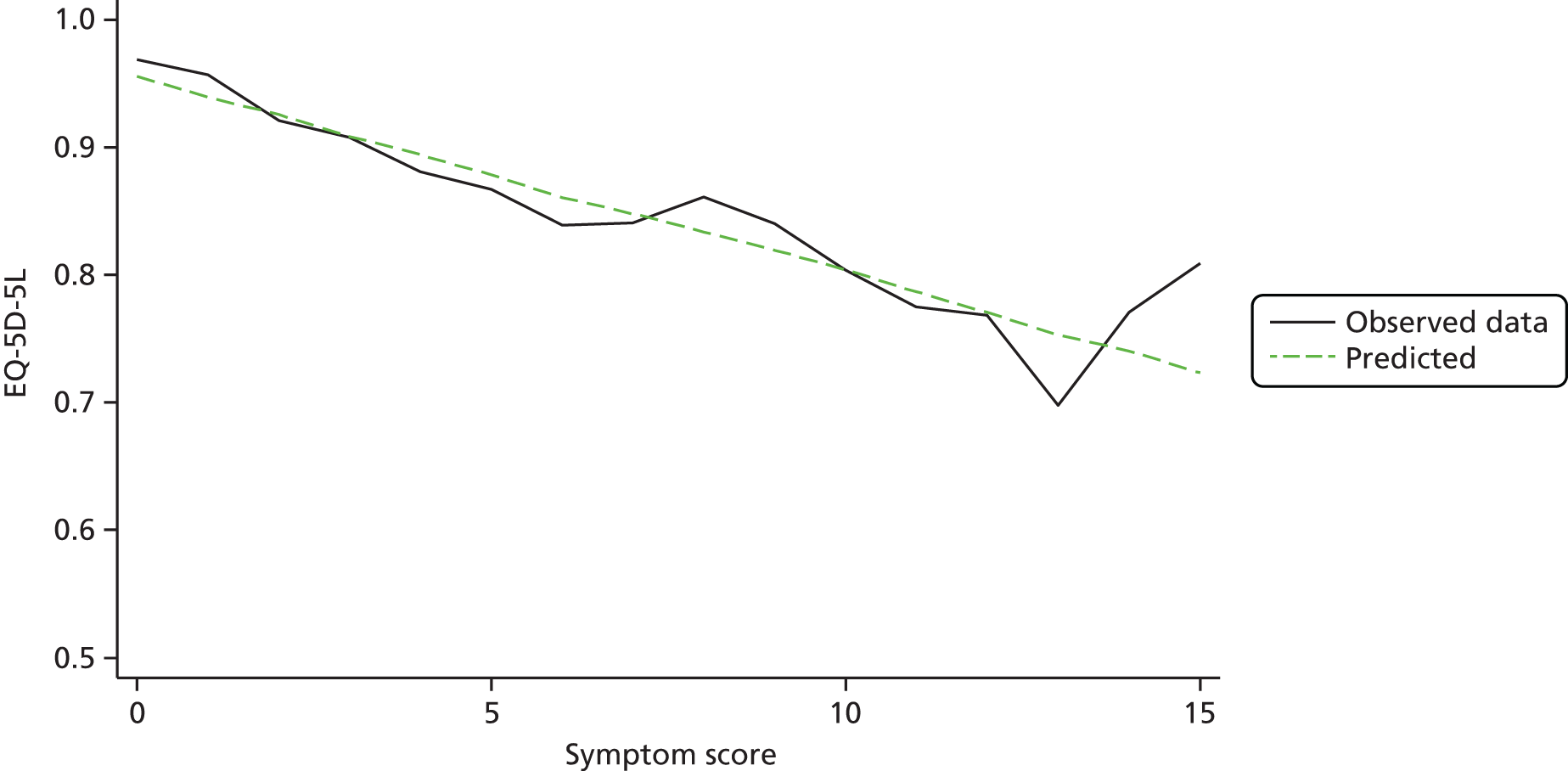
The mean baseline utility and QALYs for RBL and HAL and mean differences with their SDs and CIs are reported in Table 25. The trend of EQ-5D-5L score baseline and subsequent follow-up over 12 months’ time horizon (1 day, 1 week, 3 weeks, 6 weeks and 12 months) for both RBL and HAL for complete cases is shown in Figure 17. The EQ-5D-5L scores for each time points are descriptively reported above [see European Quality of Life-5 Dimensions (5-level version)].
| Parameter | Treatment group | Difference in means | t | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBL | HAL | |||||||||||
| n | Mean | SD | Min. | Max. | n | Mean | SD | Min. | Max. | |||
| Baseline utility | 149 | 0.90 | 0.12 | 0.26 | 1 | 152 | 0.89 | 0.15 | –0.07 | 1 | –0.01 | –0.617 |
| QALYs | 85 | 0.91 | 0.01 | 0.88 | 0.93 | 92 | 0.91 | 0.01 | 0.88 | 0.94 | 0.004 | 0.180 |
FIGURE 17.
Mean EQ-5D-5L scores over 12 months’ follow-up.
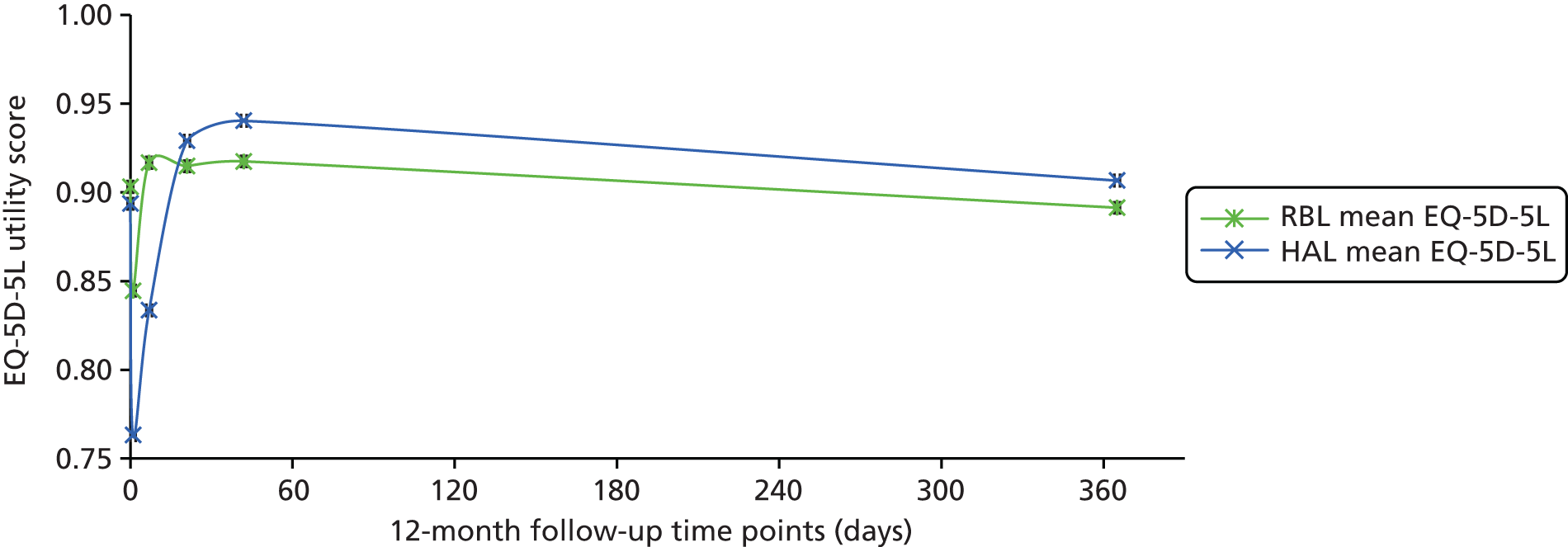
Cost–utility analysis results
The ICER estimate from our primary analysis, which was based on imputed data, was £104,427 per QALY gained. The estimated differential costs, and differential QALYs and their SE, p-value and CIs are reported in Table 26. The mean difference in total cost is £1027 (95% CI £782 to £1272) for the HAL group compared with RBL (p < 0.001). However, the difference in QALYs – controlling for baseline utility – is very small (0.01, 95% CI –0.02 to 0.04) and not statistically significant (p = 0.49). The mean total cost of HAL was £1750 (95% CI £1333 to £2167) compared with £723 (95% CI £551 to £896) for RBL based on imputed data. The correlation of the residuals in the costs and effectiveness (QALY) equations from the SUR regression was 0.1063 and not statistically significant (p = 0.246). The full regression results are provided in Appendix 3.
| Outcome | Differential mean | SE | t | p-value | 95% CI |
|---|---|---|---|---|---|
| Costs (£) | 1027 | 124 | 8.25 | < 0.001 | 782 to 1272 |
| QALYsa | 0.01 | 0.014 | 0.69 | 0.489 | –0.02 to 0.04 |
The CEAC curve generated from the parametric analysis, applied on imputed data, is presented in Figure 18. This graph shows the probability that the HAL procedure is cost-effective under a range of values for the cost-effectiveness threshold. At £20,000 per QALY threshold, HAL has zero probability of being cost-effective; at £30,000 threshold it has 0.05 probability of cost-effectiveness.
FIGURE 18.
Cost-effectiveness acceptability curve showing the probability that HAL is cost-effective given different values of cost-effectiveness threshold.
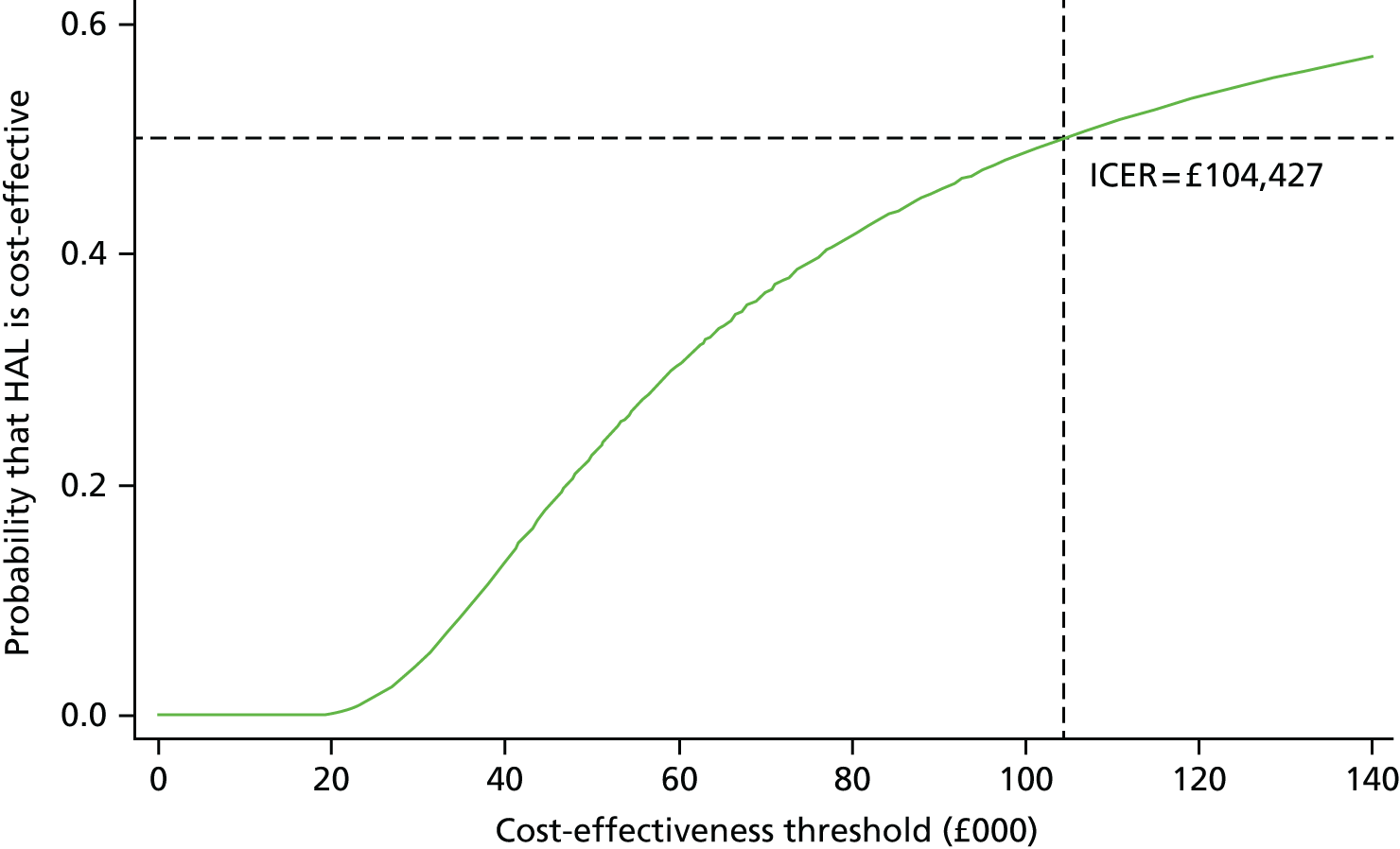
The confidence ellipses graph shown in Figure 19 represents the ICER point estimate in the cost-effectiveness plane, with 50%, 75% and 95% CIs around the point estimate. As can be seen, the ICER point estimate falls in the north-east quadrant of the cost-effectiveness plane, suggesting that HAL is more costly and more effective than RBL procedure. However, the incremental QALY is very small and the confidence ellipses cross the vertical zero line, indicating that we are less confident that incremental QALY is positive.
FIGURE 19.
Cost-effectiveness confidence ellipses illustrating the ICER point estimate and CIs.
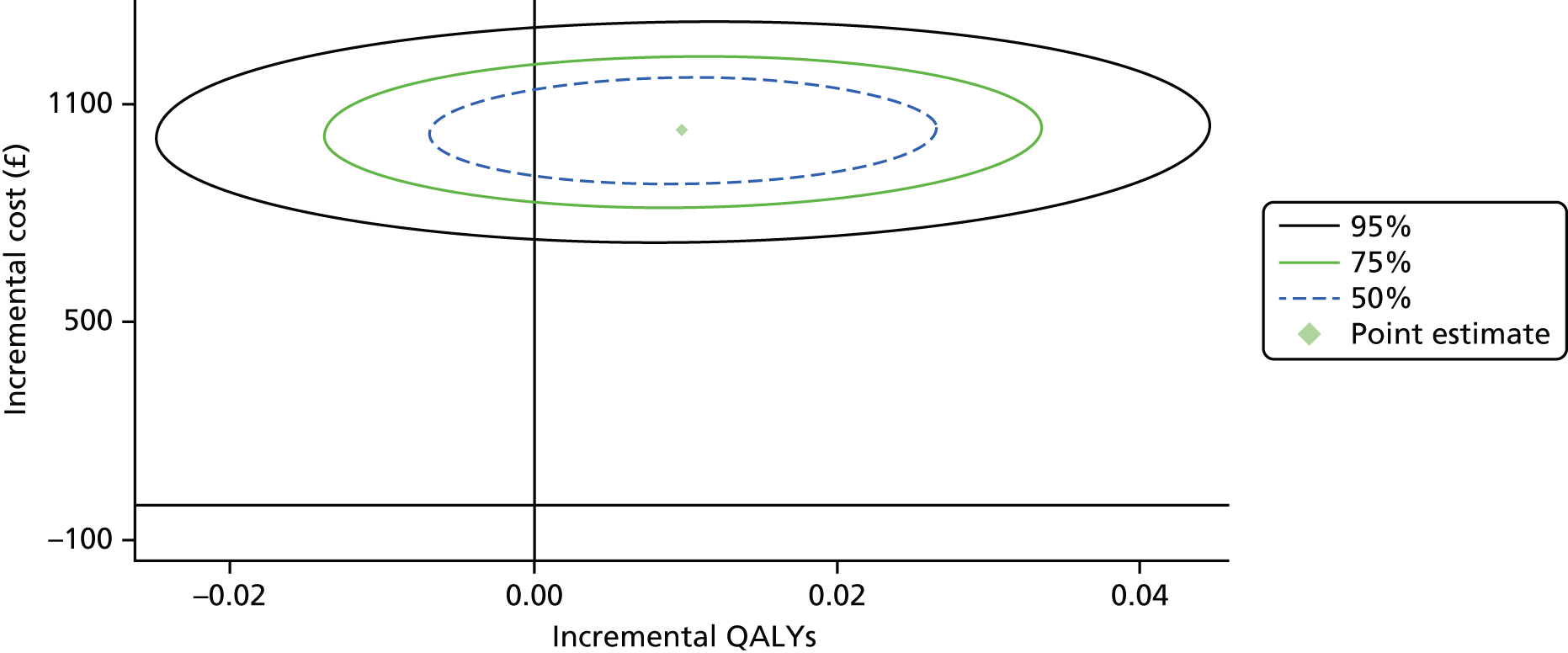
Figure 20 illustrates the incremental net monetary benefit (NMB) line with CI, which shows the same information around uncertainty in a different way. In this NMB approach, the incremental QALYs were converted into monetary terms using the upper bound of NICE cost-effectiveness threshold of £30,000 per QALY. As shown in Figure 20, if the policy-maker is willing to pay £104,427 per QALY gained from introducing the HAL procedure then the NMB would be zero, with similar degrees of uncertainty around this estimate to be either a positive or a negative value.
FIGURE 20.
Net monetary benefit line with CIs at different values of cost-effectiveness threshold. Difference in mean costs = 1027; difference in mean QALYs = 0.01; SE of the mean differential costs = 124; SE of the mean differential QALYs =0.014; covariance between costs and QALYs = 0.0443.
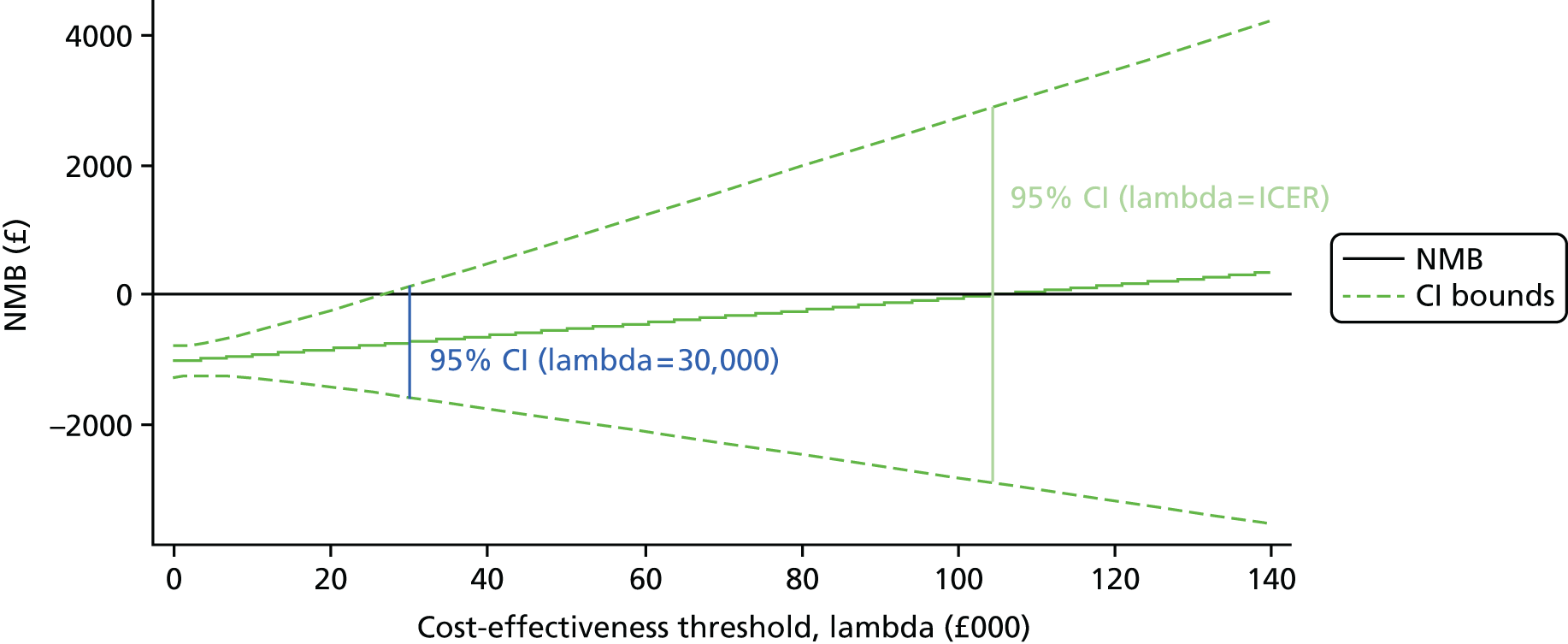
Result of subgroup analysis
Results from the subgroup analysis performed on (1) new patients and (2) patients with recurrence following RBL are presented in Figure 21. The ICER estimated for the subgroup of patients with recurrence following RBL before randomisation is higher than for new patients by £156,987. This result suggests that the generated QALY gains are more costly for patients with recurrence following RBL (before randomisation) than for new patients. This is mainly driven by a smaller difference in QALYs for patients with recurrence. For patients with recurrence following RBL, the mean difference in costs was £1091 (95% CI £623 to £1558; p < 0.001). The mean difference in QALYs was 0.004 (95% CI –0.049 to 0.058; p = 0.870). For new patients, the mean difference in costs was £1008 (95% CI £729 to £1286; p < 0.001). The mean difference in QALYs was 0.011 (95% CI –0.020 to 0.042); p = 0.479. See Appendix 3 for the full regression results.
FIGURE 21.
Subgroup analysis illustrated by combined CEAC curves.

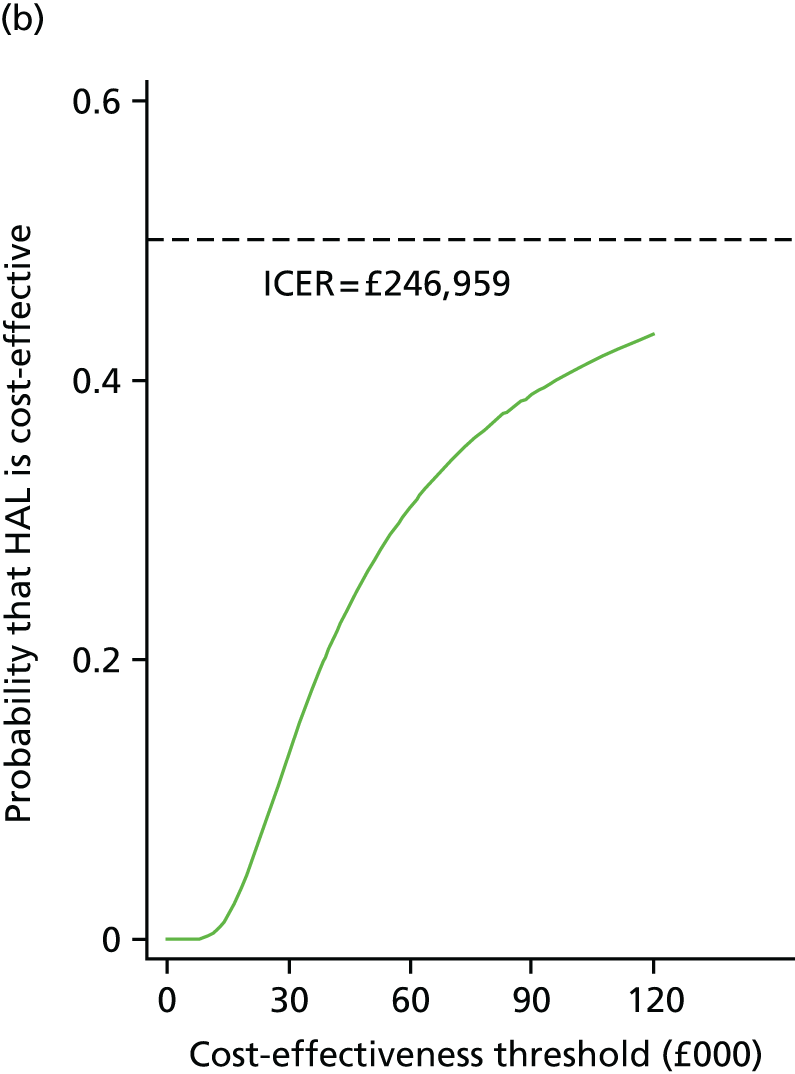
Cost-effectiveness results
The incremental cost per recurrence avoided is estimated to be £4882 (95% CI £3628 to £6135). This result, based on a derived estimate, shows that RBL groups had 87 (49.43%) recurrences compared with 48 (30%) recurrences for HAL groups [for details on clinical effectiveness, see Recurrence (Primary Outcome), above]. When a sensitivity analysis based on recurrence from the consultants’ questionnaire was completed for 1-year follow-up, the estimates of recurrence were 45 (25.6%), for the RBL group recurrences compared with 15 (9.4%) recurrences for HAL group. The incremental cost per recurrence avoided was estimated to be £6346 (95% CI £4417 to £7975) when recurrence reported by the consultant was considered.
Sensitivity analysis
Using the NHS reference cost for the HAL procedure, rather than the microcosting approach, made a difference of £48,052 per QALY to the base-case ICER estimate. The ICER for this scenario was estimated to be £152,479 per QALY, compared with £104,427 per QALY as estimated in our base-case analysis. However, the cost-effectiveness results remained similar: HAL is highly unlikely to be cost-effective at a maximum threshold of £30,000 per QALY. The results from this sensitivity analysis are presented in Figure 22. The regression results from this sensitivity analysis show that the mean difference in costs was £1498 (95% CI £1262 to £1735; p < 0.001). The mean difference in QALYs was 0.01 (95% CI –0.018 to 0.038; p = 0.489). The full regression results for all sensitivity analyses and model specifications are provided in Appendix 3.
FIGURE 22.
Sensitivity analysis using the NHS reference cost for the HAL procedure.

Our sensitivity analysis, when controlling for the grade of haemorrhoids, resulted in an ICER of £108,478 per QALY. This result shows the interaction with cost-effectiveness of HAL compared with RBL is conditional on grade III haemorrhoids. The regression results from this sensitivity analysis show that the mean difference in costs was £999 (95% CI £760 to £1239; p-value < 0.001). The mean difference in QALYs was 0.009 (95% CI –0.019 to 0.037; p = 0.518).
The CUA estimates from complete-case analysis using SUR model and controlling for baseline utility are presented in Table 27. The ICER estimate for this scenario is £90,688 per QALY gained. The correlation between cost and QALYs based on the Breusch–Pagan test of independence is reported in Table 27: this shows that cost and QALYs are positively correlated in this study, although this correlation was not statistically significant.
| Outcome | Differential mean | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Costs (£) | 1073 | 190 | 5.63 | < 0.001 | 700 to 1447 |
| QALYsa | 0.01 | 0.015 | 0.68 | 0.495 | –0.02 to 0.04 |
The results from the sensitivity analysis applying an estimated utility decrement for each subsequent procedure did not materially change the cost-effectiveness result. From this analysis, the mean difference in cost was £1030 (95% CI £760 to £1300; p < 0.001). The mean difference in QALYs was 0.008 (95% CI –0.020 to 0.036; p = 0.562), leading to an ICER of £125,076 per QALY gained. See Appendix 3 for full regression results.
Long-term cost-effectiveness
Results from the long-term CUA for HAL compared with RBL over a 4-year time horizon generated incremental costs of £1117 and incremental QALYs of 0.051, leading to an ICER of £21,887. Table 28 shows the deterministic cost-effectiveness result for the long-term extrapolation analysis. This result indicates that HAL remains not cost-effective at a £20,000 per QALY threshold.
| Outcome | Treatment group | Mean difference | ICER (£) | |
|---|---|---|---|---|
| RBL | HAL | |||
| Costs (£) | 1205 | 2322 | 1117 | |
| QALYs | 3.4807 | 3.5317 | 0.0510 | 21,887 |
The PSAs resulted in a mean difference in cost of £1125 for HAL compared with RBL, and mean difference in QALYs of 0.052, and, when combined together, led to an ICER of 21,798 per QALY gained. The probability of HAL being cost-effective at a £20,000 threshold from the long-term analysis was 0.34. Figure 23 shows the joint distribution and the uncertainty around costs and QALYs estimated from the extrapolation analyses. Each point represents a possible ICER estimate, with 1000 points estimated from the PSA simulations. There are high levels of uncertainty around both costs and QALYs, with QALYs being the most uncertain estimates. Therefore, the long-term results should be interpreted with caution. This was mainly driven by the quality of evidence on long-term recurrence, particularly for HAL.
FIGURE 23.
Uncertainties in the mean difference in costs and QALYs from long-term CEA.
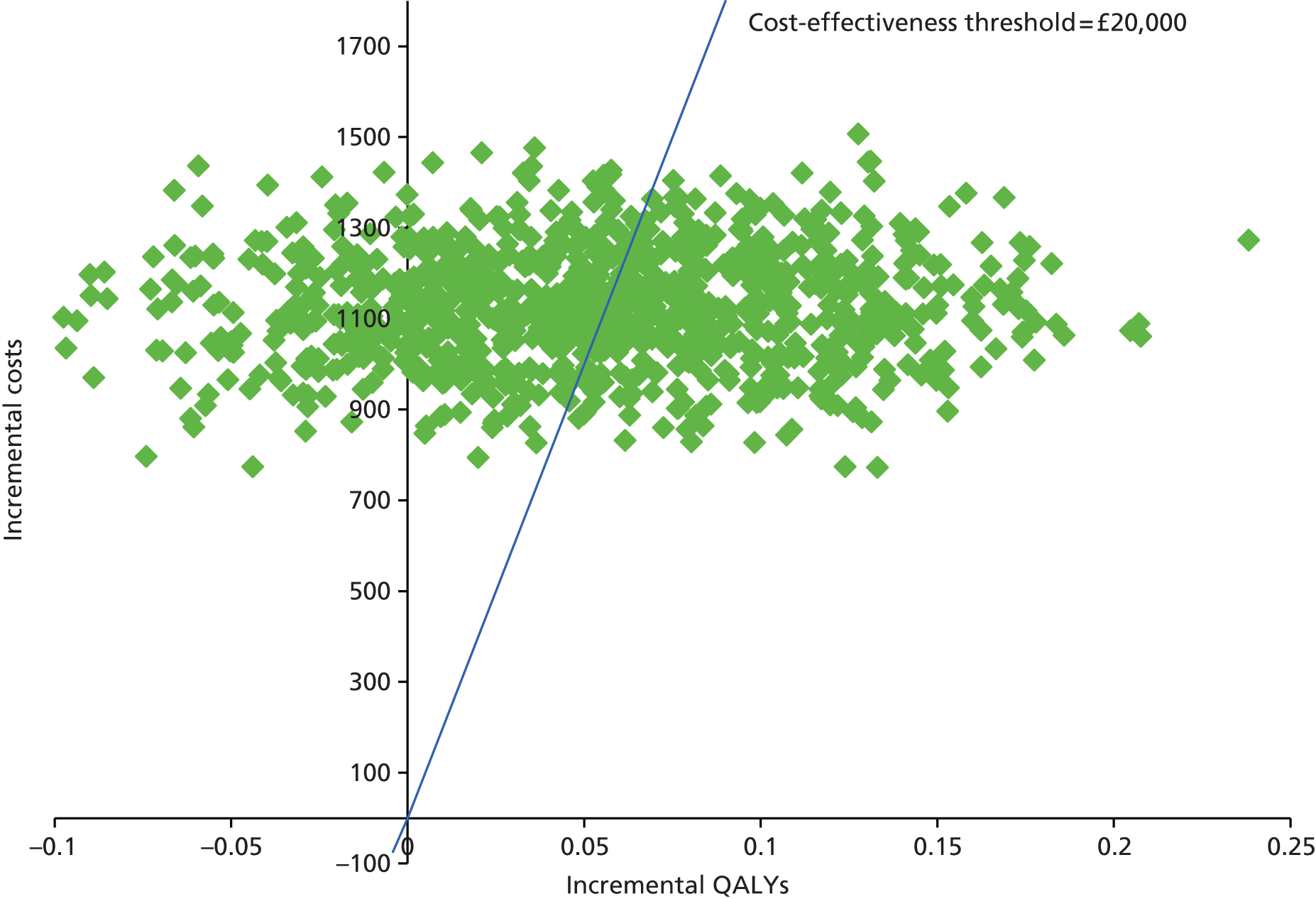
Summary
The main findings from the within-trial CUA suggests that the HAL procedure is highly unlikely to be cost-effective compared with RBL. In the base-case results, the difference in mean total costs was £1027 higher for HAL than RBL. QALYs were higher for HAL but the difference was very small (0.01), resulting in an ICER of £104,427 per additional QALY.
The base-case CEA suggests that the incremental cost per recurrence avoided was estimated £4882. In a sensitivity analysis scenario using the derived recurrence, the incremental cost per recurrence avoided was estimated to be £6346.
Chapter 5 Discussion
Main findings
Primary outcome: recurrence
Haemorrhoidal disease is a benign condition and, as such, treatment is primarily aimed at addressing the patient’s symptoms. In the absence of a validated symptom scoring system, we felt that the most important determinant of treatment success was patient-reported outcome. This was supported by a robust patient and public involvement (PPI) exercise carried out during the design of the trial. Clinical experience tells us that the physical appearance of the anal cushions post-haemorrhoid treatment correlates poorly with patients’ symptoms, meaning that anorectal visualisation is not a reliable surrogate of success. We therefore used a simple, dichotomised definition of recurrence as described by Shanmugam et al. 6,51 and measured this at 12 months post intervention. Using this patient-reported outcome alone, there was no difference in recurrence between the groups at 12 months. However, a number of patients (particularly those who underwent RBL) had undergone a further intervention for haemorrhoid symptoms within the 12 months, indicating that any symptomatic relief had been achieved only by further corrective procedures. Classing these patients as recurrence, the rate for HAL was significantly lower than for RBL (30% vs. 49%; p = 0.001) at 12 months.
The overall level of recurrence following HAL was higher than the pooled rates reported in two systematic reviews, which were 11%19 and 17.5%. 5 However, it should be noted that the recurrence rate among the constituent studies varied considerably and ranged up to 60%. 64 Similarly, reported recurrence rates for RBL also vary widely in the literature, ranging from 11% to > 50%. 6–11 This range can be attributed to many factors, but the most pertinent is probably the difficulty in defining exactly what constitutes recurrence. Some studies rely on clinical examination and patient-reported symptoms,22,25,27,51,64 whereas others rely solely on symptomatology. 24,32 When considering symptoms only, some investigators consider a patient to be recurrence free when they have no perianal symptoms at all, whereas others consider this simply an improvement. A classic example of variation dependent on definition is provided by one study64 reporting a very high recurrence rate of 60% at 30 months after a HAL procedure. However, the investigators report that, at the same time point, 86% of patients described a significant improvement in symptoms.
We adopted the more pragmatic approach6 described above in which the patient’s perception of symptomatic improvement was combined with the requirement that no further procedures were required in order to achieve a non-recurrence at 12 months. There were some practical hurdles to overcome when obtaining these data: a patient may have indicated that he/she was cured but could have forgotten about a consult or further treatment in the 12-month period since initial surgery, or a patient may have indicated recurrence when the ‘recurrence’ related to a residual skin tag65 or an alternative diagnosis clearly documented by GP or consultant records. To address these issues, we used a combination of patient-reported assessment with GP and hospital consultant perception. A decision tree was developed and followed independently by two assessors who were blinded to the intervention, with consensus reached on any areas of disagreement. This ensured fidelity and we felt this resulted in the most accurate assessment of true recurrence that was possible.
Other factors that influence the recurrence rate include the duration of follow-up and the pre-treatment haemorrhoidal grade. We chose to assess recurrence at 12 months because published data suggest that most HAL and RBL recurrences occur within 1 year. 26 Several studies have shown that using HAL for higher-grade haemorrhoids or significant prolapse adversely affects the recurrence rate;19,37,41,66,67 they also conclude that HAL is perhaps most effective for grade II and early grade III disease. We therefore limited our trial to this most clinically relevant patient population. As RBL is also most effective for this stage of disease, it was chosen as our comparator (see below).
Linked with recurrence is the need for further treatment. This was required in 31% of the RBL group and 15% of the HAL group. Eighteen per cent of the RBL group required a required further banding, the vast majority needing only one further procedure. It is common practice to repeat banding as part of a continuum of treatment in some patients with persistent symptoms, and it could be considered that these patients were actually treatment successes if by the end of the year they were cured or improved. Given that RBL is a quick, minimally invasive office procedure in comparison with HAL, it seems clinically relevant and reflective of actual practice to consider it as a ‘course’ of treatment as opposed to a single intervention. Including these patients as treatment success resulted in a reduction of the recurrence rate for the RBL arm from 49% to 37.5%, which was not statistically different from the HAL arm.
Secondary outcomes
Recurrence at 6 weeks
When we examined persistent significant symptoms at 6 weeks (i.e. immediate failure of treatment), the rates were significantly lower than at 1 year in both groups (HAL 9% vs. 30%; RBL 29% vs. 49%); 13 out of 143 (9%) patients in the HAL group and 44 out of 150 (29%) patients in the RBL group (p < 0.001) had persistent symptoms at this time point. The large increase in numbers of recurrences, in both groups after 1 year, suggests significant symptom deterioration. It is not clear when the deterioration ends. A prolonged follow-up would be relevant to see if further attrition continues, although current data suggest that most HAL recurrences occur within the year. 26
Symptom severity score
Rubber band ligation and HAL both appeared to improve symptoms, with the improvement at 6 weeks being larger for HAL than RBL. The mean improvements on the 15-point haemorrhoidal symptom severity questionnaire were 3.4 points for HAL and 2.5 for RBL at 6 weeks, with an adjusted mean difference of 1.0 (95% 0.3 to 1.8; p = 0.01). Patient’s self-assessed reported symptoms remained lower at 1 year, but the difference between groups was not maintained at this time point.
The improvement after both interventions was more modest than the one reported in the Nyström study,42 for which a difference of about 6 points was observed in patients who were undergoing both haemorrhoidectomy and SH. However, in this study the preoperative score was higher than in our study, reflecting their criteria being grade III or IV haemorrhoids.
Applying Nyström et al. ’s42 definition of cure as 0–1 point on the haemorrhoid symptom severity score, there was no difference between the two interventions at either 6 weeks or 1 year. Indeed it is striking how few patients reported a 0–1 symptom score despite considering themselves to be cured or improved according to our primary outcome measure (about one-third from each group). This suggests that many patients are not concerned with a certain level of persistent symptoms related to haemorrhoids, and may suggest that symptom severity scores need to take into account further information. This is consistent with a trial68 from general practice, in France, for which targeted questionnaires were given to all patients who were attending over a 2.5-day period. Only 2% were seen primarily with anal symptoms but the targeted questionnaire suggested that 14% had potential haemorrhoidal symptoms. Perhaps a more lenient cut-off point is required. It should be noted that the design of the trial means that assessment at 1 year did not take into account the fact that some patients required a repeat procedure to achieve this level of relief.
Health-related quality of life
As mentioned previously, haemorrhoidal disease is a benign condition and, as such, intervention is essentially aimed at improving QoL, making this is an important indicator of success. This is particularly pertinent as haemorrhoids can affect a relatively young, professionally active population.
Our results suggest that the majority of patients had an improvement in their QoL above baseline after intervention but that improvement was the same for both interventions after day 21. Before this date there was a difference in favour of RBL, probably related to the fact that the HAL procedure was more painful and this pain lasted longer. Although no long-term difference was seen between the two groups, it is important to note that both procedures did result in an improved QoL score. In other words, both interventions appear worthwhile from a QoL perspective. The alternative management option of simply reassuring that there is no sinister cause to patients’ symptoms and giving general lifestyle advice (as many have poor bowel habit) may not be appropriate. 69
Continence
A common symptom of haemorrhoidal disease, particularly when prolapse is present, is persistent soiling. Anal tone maintains continence and the majority of this resting tone comes from the action of the internal (and some external) sphincter. However, muscle tone alone is insufficient to provide a complete hermetic seal around the anal canal and there is therefore a contribution from the haemorrhoidal tissue. It follows that disruption of this tissue in the form of anal prolapse will lead to inefficiencies in this hermetic seal and leakage of faecal matter. This registers as varying levels of faecal incontinence and soiling.
Our results are consistent with this: at baseline the majority of participants from both groups had either no incontinence or very mild incontinence. Correction of the prolapse would be expected to result in a reduction in soiling and hence Vaizey score. This was, indeed, the case, in that there was a small reduction in the mean score of 1–2 points after both interventions. There was no difference in continence score between the two interventions.
From the literature, a small proportion of patients has reported incontinence following both procedures. The mechanism of this worsening incontinence is not clear. Pre-existing sphincter compromise may often exist with haemorrhoidal disease, particularly prolapse. It may be that, in these patients, the contribution form the haemorrhoids towards anal tone is essential for continence. Reduction of the haemorrhoidal bulk unmasks the pre-existing anal sphincter dysfunction. Whatever the reason, the proportion of patients who deteriorated after intervention was similar in both groups.
Pain
Our data indicate that patients report increased pain following both procedures (56% RBL vs. 71% HAL). For RBL this pain was usually of low intensity (median VAS score on day 1: 3.0) and resolved rapidly to below baseline (median VAS score day on 7: 1.0). About 50% of patients required analgesics for the first few days after treatment. For HAL, the pain was significantly greater, with moderate pain at day 1 (median VAS score: 5.0) and mild pain on day 7 post procedure (median VAS score: 3.0). Pain had resolved in almost all patients by the 3-week assessment (median VAS score: RBL 0.0 vs. HAL 1.0). Analgesia was required by the majority after a HAL procedure on a daily basis for the first week but tailed off, such that at 3 weeks three-quarters of patients had stopped taking medication.
Combining and comparing results from published studies regarding pain following HAL is difficult, as measures of pain are, by definition, subjective, are often vague and vary significantly in both wording and outcome. Estimates of the incidence of pain, at least in the first postoperative day, vary from 15% to 18.5%. 5,19 No indication is given as to the degree of pain, presumably explaining the inconsistency with our results, for which significantly more patients experienced at least a degree of discomfort and only 6% had no pain at all. There are a few RCTs for which VAS scores are utilised in a similar fashion to our trial: collecting VAS scores on various days after the procedure. One RCT24 comparing HAL with EH showed that for patients who were undergoing HAL, the mean scores were 5.5 on day 1, 2.5 on day 7, and 0 from day 14 onwards. Another RCT,23 comparing HAL with SH, showed mean VAS scores of 3.1 on day 1, 1.6 on day 7, and 0.2 on day 21. A third trial,30 comparing THD with SH, showed VAS scores of 2 on day 7, 1 on day 14, and 0.5 on day 21. All of these trials involved mucopexy. This may be the most painful part of the procedure. One trial that did not include mucopexy reported a mean VAS score of 5 on day 1, dropping to a mean of 3 by day 7. 32 Summarising these findings suggests most patients have moderate pain in the first few days after surgery, but that this pain recedes to minimal or no pain by 1–3 weeks. This is consistent with our results.
Anal pain is the most common complication after RBL. Mild pain is experienced by at least 25–50% of patients for the first 48 hours after banding,15,70–74 which is also consistent with our results. The pain may be reduced by the concomitant use of local anaesthetic. 73 This was not routinely performed in our trial but may have significant clinical advantages as well as influencing the health-economic aspect of the treatment; about 25% of patients are reluctant to return to work, or to usual activities, on the day of the procedure17,71,72 and 1% may be hospitalised due to the pain. 17
Surgical complications
The incidences of SAEs from both interventions were low (12 in HAL group vs. 2 in RBL group). Two patients from the RBL group (2%) required hospitalisation (one for severe pain and one for bleeding). This is particularly relevant as the procedure is usually carried out in the outpatient department, often with minimal consent. A similar incidence of complications and hospitalisation has been highlighted elsewhere: one study17 suggested immediate complications occurred in two-thirds of all patients. Most complications were related to pain, as in our trial, and 1% were hospitalised.
There is a vogue for some hospitals to arrange for the RBL procedure to be carried out in a day case theatre environment or during a separate, planned outpatient appointment, allowing for a formal consent process to take place. Day case admission may have avoided the need for hospitalisation in only one patient in our trial, who was complaining of immediate severe pain post procedure. At the very least, a formal consent process would have made clear to the patient the potential for these complications. However, there are significant cost implications.
Septic complications were seen in only one case in our series, but have been described extensively in the literature. 13,75 Sepsis may be local, such as perianal abscess formation, or even retroperitoneal sepsis8,76–78 and Fournier’s gangrene79 described after RBL. Rarely the sepsis can be distant; pyogenic liver abscess formation80–82 and endocarditis83 have been described after RBL, whereas brain abscess has been described after HAL. 84 This reflects the transient bacteraemia that occurs in a small proportion of patients after haemorrhoidal procedures. 13 None of these rare complications was seen in our series and we do not recommend the routine use of antibiotic prophylaxis.
We saw significant bleeding (requiring admission with or without transfusion) in two patients (1.2%) after HAL and one patient (0.6%) after RBL. From the literature, bleeding occurs in around 4–5% of patients after a HAL procedure,5,19 but only 0.15% of these were significant haemorrhages requiring blood transfusion. 5 The incidence of bleeding after RBL is 1–2.8%. 3,77 Significant bleeding requiring admission or intervention is more difficult to analyse, but pooling of the data available suggests an incidence of 0.7%,3,11,14–18 Bleeding is more common if patients are taking anti-platelet agents, non-steroidal anti-inflammatory drugs (NSAIDs) or anticoagulants. 3 These patients were excluded in our trial.
Urinary retention was seen in only two patients, both in the HAL group. Urinary retention has been described after both HAL and RBL, with a reported incidence of 0.7% after HAL5 and about 0.3% after RBL. 3,11,14,15,17,18,83 Urinary retention is dependent on the severity of disease and degree of surgery, the analgesia requirement and pre-existing bladder outlet compromise. 85–87 In addition, day case treatment may reduce the incidence. 88 Both cases of urinary retention in our trial had HAL combined with significant mucopexy (more than five mucopexy sutures).
Other complications of HAL reported in the literature include thrombosis in 1.5–6.7%5,19 and fissure in 0.8–10.3%. 5,19 Fissure was seen in 2% of our group. The incidence of thrombosis after RBL is < 1.5%3,14 and fissure occurs in < 0.2%. 3,11,14,15,17,18,83 Vasovagal faints have been reported as a common occurrence after RBL (incidence 15%). 17 We saw one case each (0.5%) of perianal haematoma and vasovagal syncope, all in the RBL group; no fissures were reported following RBL. The low incidence of vasovagal syncope in our trial remains unexplained.
Clinical appearance of haemorrhoids at proctoscopy following recurrence
The data on clinical appearance at proctoscopy were limited, as most patients did not have re-examination. Those who were re-examined tended to be the patients who complained of persistent symptoms. Almost all of these patients had clinical assessment of unchanged or improved grade of haemorrhoids with only four (8%) from the RBL group being assessed as worse (increased prolapse, grade II–III piles).
Although increased swelling or haematoma as a result of the RBL intervention may explain the deterioration in this small group, this appears unlikely 6 weeks after the intervention. The time of day at which the assessment takes place may have an influence. Assessment later in the day may explain increased engorgement and therefore size. Another possibility is incorrect assessment at baseline. Grade II haemorrhoids are sometimes misclassified as grade III. 89
Health-care costs and cost-effectiveness
The health-care cost analysis is striking. In the base-case results, HAL is around £1000 more expensive. As there is little difference in overall HRQoL between the two procedures, the ICER per additional QALY is very high and significantly exceeds the cost-effectiveness threshold of £20,000–30,000 per QALY. Even if a difference in recurrence is assumed (and this is not definite – see above), the cost per recurrence avoided is at least £5000. Essentially, HAL is highly unlikely to be cost-effective in terms of incremental cost per QALY.
Results of the subgroup analysis in patients with recurrence following RBL and new patients were broadly consistent with the overall population. The interaction effect of the grade of haemorrhoids resulted in an ICER of £108,478 per additional QALY for HAL conditional on patients with grade III piles.
In all scenarios of sensitivity analyses, the ICER remained > £100,000, indicating the robustness of our base-case results and suggesting that HAL is highly unlikely to be cost-effective. Extrapolating the CUA beyond the trial suggested a lower ICER of £21,887 per QALY, and HAL remained not cost-effective at £20,000 threshold. However, this estimate is highly uncertain because of the scarcity of good-quality evidence on long-term recurrence and utilities, particularly for HAL.
Additional data on health-care costs from other literature are sparse for this condition. Cost analysis has been carried out in one trial51 comparing SH with RBL: the cost of RBL was estimated at £252, with the cost of SH being substantially higher and unlikely to be considered cost-effective at 1 year. However, the authors did assume that the cost of increased recurrence with RBL would mean that the difference in cost would fall over a longer time horizon. This may be true for HAL, although the relative low cost of RBL makes this unlikely. It is probably more cost-effective for patients to return for repeated RBL if recurrence occurs.
Another study55 from the literature reported a trial-based CEA comparing SH with RBL for grade II symptomatic circumferential haemorrhoids, which is comparable with our study. The study55 found that the mean cost for SH was £1483 higher than for RBL, and generated a negative difference in QALYs (–0.014), which was not statistically significant. Interestingly, this study55 found that RBL is associated with higher recurrence rate than SH, with an estimated incremental cost of £4945 per recurrence avoided. The study55 concluded that SH is highly unlikely to be cost-effective compared with RBL. This conclusion is broadly similar to our findings based on the HubBLe data and, therefore, HAL is also highly unlikely to be cost-effective compared with RBL.
Strengths and weaknesses
Generalisability
Among the strengths of this study was the pragmatic, multicentre design using a mix of NHS district general hospitals and teaching hospitals across the UK, which ensures that the results are generalisable to all patients seeking treatment for grade II/III haemorrhoids. The number of subjects recruited was such that there can be considerable confidence in the conclusions drawn from the data.
Definition of recurrence
The difficulties with definition of recurrence have already been highlighted earlier and clearly alluded to in the literature. 90 We feel that the significant lengths to which we have gone to carefully define recurrence should be regarded as a strength. Our data provide a clinically meaningful result of the true incidence of recurrence that is primarily patient reported, but has taken into account clinician-derived data of further consultations and procedures. Clinician reporting alone is likely to be misleading, but at the same time must be taken into account when it comes to differentiating the symptoms related to haemorrhoids and other conditions that may co-exist (skin tags, fissures, etc.) that may lead to a false diagnosis of recurrence.
Changes from baseline data
The original design of our trial incorporated a baseline assessment of symptoms at the date of randomisation. It became clear soon after the start of the trial that there was a discrepancy between the two arms of the trial, in that many patients randomised to the RBL group had their procedure almost immediately after randomisation whereas the HAL group often had to wait a significant period on a waiting list before surgery could be carried out. It is possible that symptomatology would have changed during that period. Certainly, deterioration could have occurred, but also improvement. Recognition of the disease issue and the impending thought of surgery may have resulted in an improved lifestyle, which, in turn, may have improved symptoms (see above). The issue of surgical waiting time delay has been noted before in surgical trials,91 with a suggestion that the delay does not definitely alter data. To ensure that baseline data were collected consistently at a time close to intervention, we recollected the baseline data in those patients who were randomised to the HAL arm. Interestingly, although individual scores changed between randomisation and the actual procedure, the population average remained similar, suggesting that either could have been used as a plausible baseline value.
Justification of eligibility criteria
There are numerous therapies available for treatment of haemorrhoids, ranging from simple conservative therapies through to outpatient treatment and various surgical options. A consensus is emerging that a tailored approach is required for treatment of haemorrhoids based on grade. 92–94 Early-grade haemorrhoids can be effectively treated conservatively with diet,95,96 alteration in lifestyle94 and, perhaps, medical therapy,38,97,98 whereas at the other end of the spectrum, grade IV circumferentially prolapsed piles may respond only to haemorrhoidectomy or SH. The eTHoS trial99 hopes to provide data concerning the optimal procedure for the more advanced haemorrhoids.
Our main aim was to assess the effectiveness of HAL in the most clinically relevant scenario and compare with the most appropriate control. HAL appears to be most effective for treatment of grade II and early grade III piles92 for which non-surgical treatment is also often utilised. These non-surgical methods include RBL, injection sclerotherapy, cryotherapy, infrared coagulation, laser therapy and diathermy coagulation. Of all of these non-surgical procedures, RBL seems to be the best in terms of compliance, long-term efficacy and side effects. 100 Therefore, participants with grade II or early grade III haemorrhoids were selected as investigation group and RBL was selected as our comparator.
The consensus concerning a tailored approach regarding the treatment of haemorrhoids is difficult to confirm from the evidence available, as most published meta-analyses involve standard pairwise trials comparing only two surgical treatments directly rather than all available treatments at once. A novel method of network analysis published by Simillis et al. 101 allows simultaneous comparison of all surgical treatments. It is unfortunate that this meta-analysis has focused on treatment of only grade III and IV haemorrhoids. The addition of data from the HubBLe trial to the existing data may allow a similar network meta-analysis for treatment of earlier-grade haemorrhoids.
Haemorrhoidal grading systems
We used the Goligher grading system for haemorrhoids,102 as it is the most widely used grading system. This system has been criticised by many, particularly with regard to the definition of grade IV haemorrhoids. Goligher’s original description of grade IV haemorrhoids102 could include either skin tags or an external haemorrhoid component alone or in addition to a mucosal prolapse that needs manual positioning. There is less ambiguity with regard to grade II haemorrhoids, and grade III haemorrhoids are patient reported (i.e. the need to manually reduce). However, we have to accept that the definition of early grade III haemorrhoids has an element of ambiguity. From a pragmatic point of view, the eligibility of a patient for the trial was decided by the surgeon, who had to decide if the grade III piles were potentially treatable by the interventions prescribed.
Learning curve
The HAL procedure appears simple and easy to learn, and most investigators have assumed this to be the case. 22,103,104 However, there are no good data on the learning curve and the steps that are required to achieve competence. The existence of a learning curve has been alluded to. Pucher et al. 19 pointed out that many case series include the particular centre’s initial experience and describe a poorer outcome in the early patients. 66,105 Our trial required that surgeons had to have carried out at least five mentored cases and an additional five procedures prior to delivering the study intervention. This was based on manufacturers’ recommendations, with no data to indicate that this was adequate to have reached the top of the learning curve for competence. HAL was performed by a clinician who was accredited for independent practice or supervised by a consultant in 94% of the cases in the trial, and all procedures were completed by clinicians who met the above criteria.
If we were to be more robust in our requirement for a satisfactory level of competence we would have had to assess reproducibility. Possible assessments could include placing the stitch adequately to achieve absence of the Doppler signal. However, Denoya et al. 25 describe the Doppler signal not changing in almost half of the vessels ligated in their study. Reproducibility could relate to detection of all branches of the superior haemorrhoidal artery. Ratto et al. 106 and Denoya et al. 25 identified at least six arteries in all patients. Six ligations were certainly not carried out in all of our participants. An interesting analysis would be to see if the number of ligations correlates with the incidence of recurrence.
To add to the difficulties in assessment of reproducibility that is dependent on efficacy of detection and effective ligation of all branches of the superior haemorrhoidal artery, recent scepticism has focused on the validity of de-arterialisation. Autopsy data indicate that additional branches of the superior rectal artery are not detected even with a Doppler probe. 107 Two RCTs29,32 have randomised patients to HAL with or without Doppler probe guidance; both suggested similar results at 1-year follow-up.
Variation in intervention
Haemorrhoidal artery ligation
Haemorrhoidal artery ligation can be carried out using two equipment devices, both utilising the same principles. We allowed use of both devices within the trial, with sites invariably preferring the same device on all of their patients. There were no differences in outcomes between the different devices.
Our study included mucopexy as the standard of care. It is widely accepted that this part of the procedure produces more pain than simple artery ligation. A suture placed too near the dentate line or incorporating too much rectal tissue can result in increased pain and prolonged recovery. Again, there are few data in the literature to verify this finding, but some surgeons leave out this part of the procedure; others include it, as they claim it reduces the recurrence rate. However, data are lacking; only one previous study30 has compared patients undergoing mucopexy to those with only artery ligation and showed no difference in end points but numbers were small. An interesting analysis would be to see if the number of mucopexy ligations correlates with the incidence of recurrence as well as the intensity of pain after surgery.
As alluded to in the learning curve discussion, some trials have suggested that the Doppler part of the procedure is unnecessary. 29,32 This conclusion remains controversial. One of the trials did not include mucopexy in either procedure arm and had short-term follow-up. Both had relatively low numbers of participants. Nevertheless, this may be a factor that could significantly reduce costs and should perhaps be a subject for future research.
Rubber band ligation
There are various potential variations in practice for the procedure of RBL. The procedure was originally described by Blaisdell108 and modified by Baron using a forceps device. 109 More recently, and in the case of all of our recruiting centres, a suction device is used. Various suction banding devices can be used – some including a single firing, others delivering multiple bands. One small RCT110 has suggested that suction banding is superior in terms of pain and bleeding.
Combination of RBL and injection sclerotherapy has been suggested as a more effective method of treatment than RBL alone,111,112 but remains unproven in a well-designed trial.
The use of concomitant local anaesthetic has already been mentioned in the discussion about pain. Other variations in practice include the number of bands placed at each haemorrhoidal column. Again, no robust data exist to suggest that either technique is superior.
Variation between interventions
There was no blinding of participants or site staff in the trial as a result of the HAL procedure requiring general anaesthetic. Although equipoise was not measured in patients or clinicians, we believe equipoise was communicated to potential participants, as we produced a video for recruiting staff, highlighting the issues of randomisation and equipoise in relation to informed consent.
Symptom severity score
At the time of design of this trial, there were no validated scoring systems for haemorrhoids. The nearest we could come to such a system was that designed by Nyström et al. ,42 who altered a validated scoring system used for assessing colopouch function after rectal resection. The score has allowed a numerical assessment of the effect of both interventions, and results have complimented the dichotomous primary outcome. However, the score is not validated. For trials such as this, for which there is a poly-symptomatic condition and treatment effect must be guided by all of the patient’s symptoms and their respective influence on QoL, there is a need for a validated score. Subsequent to our study starting, such a score, known as the Sodergren score, has been developed and validated using a small population of patients. 113 Further assessment of validity is required and data from our trial may allow further development in this area.
Length of follow-up
We chose ‘1 year’ as the time for our primary outcome, as evidence suggests that most recurrences occur within the first year. However, there is definite evidence of continued recurrences with time for both procedures3,27 and from our own data, with the striking deterioration seen at 1 year assessment compared with 6 weeks. For RBL, a further 20% may recur at 3 years, even if repeat RBL is carried out. The numbers may be similar for HAL. 37 The need for a longer-term follow-up for this subgroup is essential for further information regarding effectiveness and health economics. Modelling to assess ongoing costs and to see if HAL becomes cost-effective with time is planned but requires at least a further year of data to give more reliable predicted outcomes.
One other aspect related to follow-up is the issue of repeat banding. Our original protocol did not incorporate the analysis of HAL compared with repeat banding. The timing of this repeat banding was therefore not recorded. It is likely that most repeat procedures were carried out at, or soon after, the 6-week follow-up. However, a proportion of these were carried out later. For these patients the subsequent review at 1 year from the index procedure may not be long enough from the last banding episode to allow for confidence in the true level of recurrence.
Rubber band ligation as a course of therapy
In retrospect, an alternative design could have been considered in which HAL is compared with a ‘course’ of RBL. Such a course is difficult to define. Is it simply two episodes of RBL? Is it RBL, repeated only if there has been some response? Is it RBL ad infinitum? Is it RBL until the patient or clinician calls a halt and alternative procedures are requested? Design of such a trial is simple if the course is defined as two episodes of RBL, perhaps 6 weeks apart, with follow-up for 1 year after the last RBL. Designing a trial to evaluate alternative treatment strategies is difficult. The current study has allowed the post hoc analysis of repeat RBL to be assessed while maintaining a feasible and pragmatic design.
Meaning of the study and implications for clinicians or policy-makers
Haemorrhoidal artery ligation is a more invasive procedure than RBL, requiring admission to hospital, longer waiting times, an anaesthetic and a more prolonged recovery. When offered an intervention for haemorrhoids, most patients would choose a more invasive procedure only if it were significantly more likely to achieve cure. Our data suggest that higher cure is true; the recurrence rate after HAL is significantly less than RBL and symptoms are more likely to be reduced. However, the recurrence rate for both procedures is high and may be considered by many patients as too high. Our data suggest that the HAL procedure in particular is not as effective as the current literature indicates. Similarly, recurrence after one episode of RBL is very likely. Many patients will undergo further interventions within the year and over one-quarter will still not consider themselves improved or cured. Given this level of recurrence, alternative procedures may be appropriate. However, a vigorous assessment of efficacy similar to this trial would be required in order to fairly compare these alternatives, be they conservative therapy or surgical intervention. The eTHoS trial99 may provide this information for SH and haemorrhoidectomy.
Waiting times deserve an additional comment. It takes substantially longer for a HAL procedure to be carried out. Although not recorded, during the period of waiting for a HAL, patients will continue with symptoms whilst a large proportion of those in the RBL group would have had relief and improved QoL.
Despite this gloomy primary outcome data, it is important to state that symptom severity score, continence score and QoL improved after both interventions, suggesting that both interventions are beneficial to patients.
Would most patients who accept an intervention therefore choose HAL as it is the most effective procedure? The patient representative for the trial does not think that HAL appears to be the most effective procedure, and would opt for HAL only if it had much lower recurrence. Patients are likely to be influenced by other secondary factors, such as complications and QoL. Neither of these outcomes was different between interventions. Short-term pain was significantly higher in the HAL group, although the pain suffered after intervention tended to be mild to moderate and resolved within 1–3 weeks. Our trial also showed that many patients had to wait longer for a HAL, with most receiving RBL on the day of consult. Furthermore, of the screened patients who did not enter the trial, 128 opted for the RBL procedure and 70 for the HAL procedure. Given these factors, it could be argued that many patients would choose RBL, over HAL, as they would be inclined to ‘take their chances’ with a simple and quick outpatient procedure, without general anaesthetic, returning for a repeat procedure after a few months to years if recurrence occurred. A repeat RBL would be a simple and palatable option. Indeed many clinicians would consider RBL to consist of a ‘course’ of treatment rather than a single procedure, illustrated by the fact that it is common practice for many patients to be routinely followed up some weeks later, when repeat banding can easily be carried out. If those requiring repeat RBL to achieve control at 1 year are included as treatment successes then the difference between the two groups becomes insignificant.
Of course there is another factor that may influence the patient choice of HAL over RBL, and that is the long-term incidence of recurrence. Very few long-term data exist for RBL but the data that are available suggest a significant deterioration with time. 3 Some long-term trials on HAL also suggest significant attrition. 37 All trials on long-term data have significant methodological issues as well as significant ambiguity regarding the definition of recurrence. Our own data suggest significant deterioration in both groups over the year. It remains to be seen whether this attrition persists. HAL also appeared to improve symptoms more rapidly than RBL, although this is somewhat offset in practice by the aforementioned differential waiting time: RBL is a straightforward procedure that was usually done on the same day as randomisation, whereas the median waiting time for HAL was 60 days.
One further finding in our trial that has implications for both clinicians, and possibly commissioners, is the fact that two patients were hospitalised with complications after RBL. RBL is a procedure that is often carried out in the outpatient department at the time of initial consult: 20% of our recruiting centres follow this policy. Therefore, there is potentially no formal consent process and potentially inadequate immediate follow-up as the patient is usually discharged from the department. Some recruiting centres delay RBL, inviting the patient back to a formal day case theatre setting allowing for adequate informed consent and monitoring of potential complications. This would appear to be best practice, but does incur increased management costs.
Finally, it should be noted that there are massive cost implications for the HAL in these patients. RBL is relatively cheap, even if the patient returns to a day case setting for repeat banding.
Recommendations for future research
This trial adds to the growing body of evidence that proposes various surgical interventions for grade II and II haemorrhoids. Between them, the HubBLe and eTHoS trials include the four principle treatments that are used in this patient population: RBL, HAL, SH and EH. Pooling of these data sets with other data sets that are already available101 may provide the opportunity to conduct an individual patient data (IPD) meta-analysis (network meta-analysis) to allow a comparison of all four interventions and will allow surgeons to tailor treatment options to the needs of individual patients. Considering the significant disease burden of haemorrhoids, this has the potential to provide powerful results. Further to this, a model-based economic evaluation should be conducted, synthesising all of the available evidence.
We have discussed the difficulties in defining recurrence in haemorrhoidal disease and the lack of a validated scoring system. The wealth of data available from this trial can now be analysed to enable such a system to be proposed and validated. This will provide future investigators with a robust and standardised tool with which to compare treatments and allow comparison between studies.
Our data have demonstrated that recurrence may increase with time. Further follow-up of the patient cohort would add to the results available regarding long-term effectiveness and allow an updated economic analysis. We feel that data collection at 2 and 3 years post intervention would provide clinically relevant information to aid surgeons and patients in treatment selection.
Other possible analyses of these data include correlation of the incidence of recurrence and the number of arterial branches ligated, as well as the number of mucopexy sutures. In addition, correlation with the degree of mucopexy and the intensity of pain would be helpful for clinical prediction of outcome.
Chapter 6 Conclusions
Haemorrhoidal artery ligation is a more clinically effective procedure than a single RBL intervention. However, if HAL is compared with repeat RBL then the procedures become equivalent in terms of recurrence. Similarly, symptom severity score, complications, QoL and continence score were no different between interventions, and patients had more pain in the early postoperative period after a HAL procedure. The HAL procedure is significantly more expensive than RBL and not cost-effective in terms of cost per QALY.
Acknowledgements
We gratefully acknowledge the hard work, support and advice from the following:
Participant screening and data collection: Michael Agyemang (Sheffield Teaching Hospitals NHS Foundation Trust); Amanda Davies, Manni Narewal and Tracy Henn (University Hospitals Birmingham NHS Foundation Trust); Simona Fourie and Fiona Morrison (Oxford University Hospitals NHS Trust); Daniela Shackcloth, Mercy Mbuyongha and Shirley Cooper (Aintree University Hospital NHS Foundation Trust); Deborah Wilson (North Tees and Hartlepool NHS Foundation Trust); Salvatore Lamberti (Barts Health NHS Trust); Richard Rye and Mary Flowerdew (Brighton and Sussex University Hospitals NHS Trust); Jane Hunt and Colin Barrett (North Devon Healthcare NHS Trust); Jo Brown and Helen Thompson (Cambridge University Hospitals NHS Foundation Trust); Pooja Datt (North West London Hospitals NHS Trust); Lisa Aitken and Mcdonald Mupudzi (University Hospital Southampton NHS Foundation Trust); Kathleen MacLeod (NHS Highland); Polly May and Carrie Colvin (Poole Hospital NHS Foundation Trust); Dina Bell (NHS Lanarkshire); Rachel Carson (Royal Berkshire NHS Foundation Trust); Melloney Allnut (Imperial NHS Trust); Deborah Butcher (Peterborough and Stamford Hospitals NHS Foundation Trust); Jonathan Trickett (Principal Investigator); and Vicky Frost (Ashford and St Peter’s Hospitals NHS Foundation Trust).
Study monitoring: Jack Cotter and Lizzie Swaby, CTRU, ScHARR, University of Sheffield, Sheffield, UK.
Data management: Amanda Loban, Kirsty Pemberton and Emily Turton, CTRU, ScHARR, University of Sheffield, Sheffield, UK.
Administrative and clerical support: Helen Wakefield and Heather Dakin, CTRU, ScHARR, University of Sheffield, Sheffield, UK.
We offer special thanks to the members of our two oversight committees, the TSC and the DMEC, as listed below.
The HubBLe Research Team
Trial Management Group
Mr Steven Brown (Sheffield Teaching Hospitals NHS Foundation Trust); Mr Jim Tiernan (Leeds Teaching Hospitals NHS Trust); Ms Katie Biggs (Sheffield CTRU, University of Sheffield); Dr Daniel Hind (Sheffield CTRU, University of Sheffield); Mr Mike Bradburn (Sheffield CTRU, University of Sheffield); and Mr Neil Shephard (Sheffield CTRU, University of Sheffield). Representatives from each participating NHS Trust (either the Principal Investigator or Research Nurse) were invited.
Trial Steering Committee
Professor Jonathan Lund (Derby Hospitals NHS Foundation Trust); Mr Patrick Dobbs (Sheffield Teaching Hospitals NHS Foundation Trust); Mr Jeff Garner (The Rotherham NHS Foundation Trust); and Mr Jeff Williams (Patient Representative).
Data Monitoring and Ethics Committee
Dr Jonathan Cook (University of Oxford); Mr Dermot Burke (Leeds Teaching Hospitals NHS Trust); and Mr Baljit Singh (University Hospitals Leicester).
Co-applicants
Mr Jim Tiernan (Leeds Teaching Hospitals NHS Trust); Dr Daniel Hind (Sheffield CTRU, University of Sheffield); Professor Angus Watson (Raigmore Hospital, Inverness); Professor Allan Wailoo (ScHARR, University of Sheffield); Mr Mike Bradburn (Sheffield CTRU, University of Sheffield); and Mr Neil Shephard (Sheffield CTRU, University of Sheffield).
Local principal investigators
Mr Steven Brown (Sheffield Teaching Hospitals NHS Foundation Trust); Mr Simon Radley (University Hospitals Birmingham NHS Foundation Trust); Mr Oliver Jones (Oxford University Hospitals NHS Trust); Mr Paul Skaife (Aintree University Hospital NHS Foundation Trust); Mr Anil Agarwal (North Tees and Hartlepool NHS Foundation Trust); Mr Pasquale Giordano (Barts Health NHS Trust); Mr Marc Lamah (Brighton and Sussex University Hospitals NHS Trust); Mr Mark Cartmell (Northern Devon Healthcare NHS Trust); Mr Justin Davies (Cambridge University Hospitals NHS Foundation Trust); Mr Omar Faiz (North West London Hospitals NHS Trust); Ms Karen Nugent (University Hospital Southampton NHS Foundation Trust); Professor Angus Watson (NHS Highland); Mr Andrew Clarke (Poole Hospital NHS Foundation Trust); Professor Angus MacDonald (NHS Lanarkshire); Mr Philip Conaghan (Royal Berkshire NHS Foundation Trust); Mr Paul Ziprin (Imperial College Healthcare NHS Trust); Mr Rohit Makhija (Peterborough and Stamford Hospitals NHS Foundation Trust); and Mr Jonathan Trickett (Ashford and St Peter’s Hospitals NHS Foundation Trust).
Contributions of authors
The following authors produced the first draft of the report:
Mr Steven Brown (Consultant Surgeon)
Ms Katie Biggs (Trial Manager)
Mr Neil Shephard (Statistician)
Mr Mike Bradburn (Senior Statistician)
Mr Abualbishr Alshreef (Health Economist)
Miss Lizzie Swaby (Research Assistant).
The following authors conceived of, or designed, the work:
Mr Steven Brown (Consultant Surgeon)
Mr Jim Tiernan (Registrar in General Surgery)
Dr Daniel Hind (CTRU Assistant Director)
Mr Neil Shephard (Statistician)
Mr Mike Bradburn (Senior Statistician)
Professor Allan Wailoo (Senior Health Economist)
Professor Angus Watson (Consultant Surgeon).
The following authors were involved in the acquisition of data for the work:
Mr Steven Brown (Consultant Surgeon)
Ms Katie Biggs (Trial Manager)
Miss Lizzie Swaby (Research Assistant)
Professor Angus Watson (Consultant Surgeon)
Mr Simon Radley (Consultant Surgeon)
Mr Oliver Jones (Consultant Surgeon)
Mr Paul Skaife (Consultant Surgeon)
Mr Anil Agarwal (Consultant Surgeon)
Mr Pasquale Giordano (Consultant Surgeon)
Mr Marc Lamah (Consultant Surgeon)
Mr Mark Cartmell (Consultant Surgeon)
Mr Justin Davies (Consultant Surgeon)
Mr Omar Faiz (Consultant Surgeon)
Ms Karen Nugent (Consultant Surgeon)
Mr Andrew Clarke (Consultant Surgeon)
Professor Angus MacDonald (Consultant Surgeon)
Mr Philip Conaghan (Consultant Surgeon)
Mr Paul Ziprin (Consultant Surgeon)
Mr Rohit Makhija (Consultant Surgeon).
The following authors were involved in the analysis of data:
Mr Steve Brown (Consultant Surgeon)
Mr Jim Tiernan (Registrar in General Surgery)
Mr Neil Shephard (Statistician)
Mr Mike Bradburn (Senior Statistician)
Professor Allan Wailoo (Senior Health Economist)
Mr Abualbishr Alshreef (Health Economist).
All of the authors:
-
were involved in the interpretation of data for the work and revised the work critically for important intellectual content
-
were involved in the final approval of the version to be published
-
agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Publications
Brown SR, Tiernan JP, Watson AJ, Biggs K, Shephard N, Wailoo AJ, et al. Haemorrhoidal artery ligation versus rubber band ligation for the management of symptomatic second-degree and third-degree haemorrhoids (HubBLe): a multicentre, open-label, randomised controlled trial. Lancet 2016;388:356–64.
Brown SR. Hubble trial: time to stick to basics for treatment of haemorrhoids? [published online ahead of print October 3 2016]. Tech Coloprotcol 2016.
Brown SR, Watson A. Comments to ‘Rubber band ligation versus excisional haemorrhoidectomy for haemorrhoids’. Tech Coloproctol 2016;20:659–61.
Data sharing statement
Requests for patient-level data and statistical code should be made to the corresponding author and will be considered by the HubBLe trial management group who, although specific consent for data sharing was not obtained, will release data on a case-by-case basis following the principles for sharing patient-level data as described by Smith et al. 114 The presented data do not contain any direct identifiers; we will minimise indirect identifiers and remove free-text data to minimise the risk of identification.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Loder PB, Kamm MA, Nicholls RJ, Phillips RKS. Haemorrhoids: pathology, pathophysiology and aetiology. Br J Surg 1994;81:946-54. http://dx.doi.org/10.1002/bjs.1800810707.
- Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al. Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12080.
- Iyer VS, Shrier I, Gordon PH. Long-term outcome of rubber band ligation for symptomatic primary and recurrent internal hemorrhoids. Dis Colon Rectum 2004;47:1364-70. http://dx.doi.org/10.1007/s10350-004-0591-2.
- Nisar PJ, Acheson AG, Neal KR, Scholefield JH. Stapled hemorrhoidopexy compared with conventional hemorrhoidectomy: systematic review of randomized, controlled trials. Dis Colon Rectum 2004;47:1837-45. http://dx.doi.org/10.1007/s10350-004-0679-8.
- Giordano P, Overton J, Madeddu F, Zaman S, Gravante G. Transanal hemorrhoidal dearterialization: a systematic review. Dis Colon Rectum 2009;52:1665-71. http://dx.doi.org/10.1007/DCR.0b013e3181af50f4.
- Shanmugam V, Hakeem A, Campbell KL, Rabindranath KS, Steele RJC, Thaha MA, et al. Rubber band ligation versus excisional haemorrhoidectomy for haemorrhoids. Cochrane Database Syst Rev 2005;1. http://dx.doi.org/10.1002/14651858.CD005034.pub2.
- Peng BC, Jayne DG, Ho YH. Randomized trial of rubber band ligation vs. stapled hemorrhoidectomy for prolapsed piles. Dis Colon Rectum 2003;46:291-7. http://dx.doi.org/10.1097/01.DCR.0000049484.40711.12.
- Shemesh EI, Kodner IJ, Fry RD, Neufeld DM. Severe complication of rubber band ligation of internal hemorrhoids. Dis Colon Rectum 1987;30:199-200. http://dx.doi.org/10.1007/BF02554339.
- Komborozos VA, Skrekas GJ, Pissiotis CA. Rubber band ligation of symptomatic internal hemorrhoids: results of 500 cases. Dig Surg 2000;17:71-6. http://dx.doi.org/10.1159/000018803.
- Stonelake PS, Hendrickse CW. Modern treatment for internal haemorrhoids. Rubber band ligation is effective and efficient. BMJ 1997;315.
- Forlini A, Manzelli A, Quaresima S, Forlini M. Long-term result after rubber band ligation for haemorrhoids. Int J Colorectal Dis 2009;24:1007-10. http://dx.doi.org/10.1007/s00384-009-0698-y.
- Shanmugam V, Thaha MA, Rabindranath KS, Campbell KL, Steele RJ, Loudon MA. Systematic review of randomized trials comparing rubber band ligation with excisional haemorrhoidectomy. Br J Surg 2005;92:1481-7. http://dx.doi.org/10.1002/bjs.5185.
- McCloud JM, Jameson JS, Scott AN. Life-threatening sepsis following treatment for haemorrhoids: a systematic review. Colorectal Dis 2006;8:748-55. http://dx.doi.org/10.1111/j.1463-1318.2006.01028.x.
- Bat L, Melzer E, Koler M, Dreznick Z, Shemesh E. Complications of rubber band ligation of symptomatic internal hemorrhoids. Dis Colon Rectum 1993;36:287-90. http://dx.doi.org/10.1007/BF02053512.
- Lau WY, Chow HP, Poon GP, Wong SH. Rubber band ligation of three primary hemorrhoids in a single session. A safe and effective procedure. Dis Colon Rectum 1982;25:336-9. http://dx.doi.org/10.1007/BF02553609.
- Jeffery PJ, Ritchie SM, Miller W, Hawley PR. The treatment of haemorrhoids by rubber band ligation at St Mark’s hospital. Postgrad Med J 1980;56:847-9. http://dx.doi.org/10.1136/pgmj.56.662.847.
- Kumar N, Paulvannan S, Billings PJ. Rubber band ligation of haemorrhoids in the out-patient clinic. Ann R Coll Surg Engl 2002;84:172-4.
- El Nakeeb AM, Fikry AA, Omar WH, Fouda EM, El Metwally TA, Ghazy HE, et al. Rubber band ligation for 750 cases of symptomatic hemorrhoids out of 2200 cases. World J Gastroenterol 2008;14:6525-30. http://dx.doi.org/10.3748/wjg.14.6525.
- Pucher PH, Sodergren MH, Lord AC, Darzi A, Ziprin P. Clinical outcome following Doppler-guided haemorrhoidal artery ligation: a systematic review. Colorectal Dis 2013;15:e284-94. http://dx.doi.org/10.1111/codi.12205.
- Liu H, Yang C, Chen B, Wu J, He H. Clinical outcomes of Doppler-guided haemorrhoidal artery ligation: a meta-analysis. Int J Clin Exp Med 2015;8:4932-9.
- Sajid MS, Parampalli U, Whitehouse P, Sains P, McFall MR, Baig MK. A systematic review comparing transanal haemorrhoidal de-arterialisation to stapled haemorrhoidopexy in the management of haemorrhoidal disease. Tech Coloproctol 2012;16:1-8. http://dx.doi.org/10.1007/s10151-011-0796-z.
- Bursics A, Morvay K, Kupcsulik P, Flautner L. Comparison of early and 1-year follow-up results of conventional hemorrhoidectomy and hemorrhoid artery ligation: a randomized study. Int J Colorectal Dis 2004;19:176-80. http://dx.doi.org/10.1007/s00384-003-0517-9.
- Festen S, van Hoogstraten MJ, van Geloven AA, Gerhards MF. Treatment of grade III and IV haemorrhoidal disease with PPH or THD. A randomized trial on postoperative complications and short-term results. Int J Colorectal Dis 2009;24:1401-5. http://dx.doi.org/10.1007/s00384-009-0803-2.
- De Nardi P, Capretti G, Corsaro A, Staudacher C. A prospective, randomized trial comparing the short- and long-term results of Doppler-guided transanal hemorrhoid dearterialization with mucopexy versus excision hemorrhoidectomy for grade III hemorrhoids. Dis Colon Rectum 2014;57:348-53. http://dx.doi.org/10.1097/DCR.0000000000000085.
- Denoya PI, Fakhoury M, Chang K, Fakhoury J, Bergamaschi R. Dearterialization with mucopexy versus haemorrhoidectomy for grade III or IV haemorrhoids: short-term results of a double-blind randomized controlled trial. Colorectal Dis 2013;15:1281-8. http://dx.doi.org/10.1111/codi.12303.
- Denoya P, Tam J, Bergamaschi R. Hemorrhoidal dearterialization with mucopexy versus hemorrhoidectomy: 3-year follow-up assessment of a randomized controlled trial. J Visc Surg 2014;151:329-30. http://dx.doi.org/10.1007/s10151-014-1219-8.
- Elmér SE, Nygren JO, Lenander CE. A randomized trial of transanal hemorrhoidal dearterialization with anopexy compared with open hemorrhoidectomy in the treatment of hemorrhoids. Dis Colon Rectum 2013;56:484-90. http://dx.doi.org/10.1097/DCR.0b013e31827a8567.
- Gupta PJ, Heda PS, Kalaskar S. Randomized controlled study between suture ligation and radio wave ablation and suture ligation of grade III symptomatic hemorrhoidal disease. Int J Colorectal Dis 2009;24:455-60. http://dx.doi.org/10.1007/s00384-008-0579-9.
- Gupta PJ, Kalaskar S, Taori S, Heda PS. Doppler-guided hemorrhoidal artery ligation does not offer any advantage over suture ligation of grade 3 symptomatic hemorrhoids. Tech Coloproctol 2011;15:439-44. http://dx.doi.org/10.1007/s10151-011-0780-7.
- Infantino A, Altomare DF, Bottini C, Bonanno M, Mancini S. Prospective randomized multicentre study comparing stapler haemorrhoidopexy with Doppler-guided transanal haemorrhoid dearterialization for third-degree haemorrhoids. Color Dis 2012;14:205-11. http://dx.doi.org/10.1111/j.1463-1318.2011.02628.x.
- Khafagy W, El Nakeeb A, Fouda E, Omar W, Elhak NG, Farid M, et al. Conventional haemorrhoidectomy, stapled haemorrhoidectomy, Doppler guided haemorrhoidectomy artery ligation; post operative pain and anorectal manometric assessment. Hepatogastroenterology 2009;56:1010-15.
- Schuurman JP, Borel Rinkes IH, Go PM. Hemorrhoidal artery ligation procedure with or without Doppler transducer in grade II and III hemorrhoidal disease: a blinded randomized clinical trial. Ann Surg 2012;255:840-5. http://dx.doi.org/10.1097/SLA.0b013e31824e2bb5.
- Hajdarevic B, Slaku J, Pandza H, Salihefendic N, Hadziahmetovic Z. Application of simple digital methods in the treatment of hemorrhoid disease. Stud Health Technol Inform 2009;150:433-7.
- Popov V, Zhivkov E, Tokov P, Arabadzhieva E, Tasev V, Taseva A, et al. Advantages of transanal hemorrhoidal dearterialization as compared to other surgical techniques for the treatment of hemorrhoidal disease. Khirurgiia (Sofia) 2013;3:4-7.
- Tsang YP, Fok KLB, Cheung YSH, Li KWM, Tang CN. Comparison of transanal haemorrhoidal dearterialisation and stapled haemorrhoidopexy in management of haemorrhoidal disease: a retrospective study and literature review. Tech Coloproctol 2014;18:1017-22. http://dx.doi.org/10.1007/s10151-014-1170-8.
- Tsunoda A, Kiyasu Y, Fujii W, Kano N. Comparison of the early results of transanal hemorrhoidal dearterialization and hemorrhoidectomy using an ultrasonic scalpel. Surg Today 2015;45:175-80. http://dx.doi.org/10.1007/s00595-014-0885-5.
- Avital S, Inbar R, Karin E, Greenberg R. Five-year follow-up of Doppler-guided hemorrhoidal artery ligation. Tech Coloproctol 2012;16:61-5. http://dx.doi.org/10.1007/s10151-011-0801-6.
- Pol RA, van der Zwet WC, Kaijser M, Schattenkerk ME, Eddes EH. Comparison of Doppler-guided haemorrhoidal artery ligation without mucopexy and rubber band ligation for haemorrhoids. Arab J Gastroenterol 2011;12:189-93. http://dx.doi.org/10.1016/j.ajg.2011.11.001.
- Theodoropoulos GE, Sevrisarianos N, Papaconstantinou J, Panoussopoulos SG, Dardamanis D, Stamopoulos P, et al. Doppler-guided haemorrhoidal artery ligation, rectoanal repair, sutured haemorrhoidopexy and minimal mucocutaneous excision for grades III-IV haemorrhoids: a multicenter prospective study of safety and efficacy. Colorectal Dis 2010;12:125-34. http://dx.doi.org/10.1111/j.1463-1318.2008.01739.x.
- Haemorrhoidal Artery Ligation. London: NICE; 2010.
- Spyridakis M, Christodoulidis G, Symeonidis D, Dimas D, Diamantis A, Polychronopoulou E, et al. Outcomes of Doppler-guided hemorrhoid artery ligation: analysis of 90 consecutive patients. Tech Coloproctol 2011;15:21-4. http://dx.doi.org/10.1007/s10151-011-0727-z.
- Nyström PO, Qvist N, Raahave D, Lindsey I, Mortensen N. Randomized clinical trial of symptom control after stapled anopexy or diathermy excision for haemorrhoid prolapse. Br J Surg 2010;97:167-76. http://dx.doi.org/10.1002/bjs.6804.
- The EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut 1999;44:77-80. http://dx.doi.org/10.1136/gut.44.1.77.
- Brown SR, Tiernan JP, Watson AJ, Biggs K, Shephard N, Wailoo AJ, et al. Haemorrhoidal artery ligation versus rubber band ligation for the management of symptomatic second-degree and third-degree haemorrhoids (HubBLe): a multicentre , open-label , randomised controlled trial. Lancet 2016;6736:1-9. http://dx.doi.org/10.1016/s0140-6736(16)30584-0.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010;115:1063-70. http://dx.doi.org/10.1097/AOG.0b013e3181d9d421.
- Tiernan J, Hind D, Watson A, Wailoo AJ, Bradburn M, Shephard N, et al. The HubBLe trial: haemorrhoidal artery ligation (HAL) versus rubber band ligation (RBL) for haemorrhoids. BMC Gastroenterol 2012;12. http://dx.doi.org/10.1186/1471-230X-12-153.
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359:696-700. http://dx.doi.org/10.1016/S0140-6736(02)07816-9.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technol Assess 1999;3.
- Shanmugam V, Muthukumarasamy G, Cook JA, Vale L, Watson AJM, Loudon MA. Randomized controlled trial comparing rubber band ligation with stapled haemorrhoidopexy for Grade II circumferential haemorrhoids: long-term results. Colorectal Dis 2010;12:579-86. http://dx.doi.org/10.1111/j.1463-1318.2009.01841.x.
- Hosmer DWJ, Lemeshow S. Goodness of fit tests for the multiple logistic regression model. Commun Stat Theor 1980;9:1043-69. http://dx.doi.org/10.1080/03610928008827941.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. http://dx.doi.org/10.1007/s11136-011-9903-x.
- Devlin N, Shah K, Mulhern B, Van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. London: Office of Health Economics; 2016.
- McKenzie L, de Verteuil R, Cook J, Shanmugam V, Loudon M, Watson AJ, et al. Economic evaluation of the treatment of grade II haemorrhoids: a comparison of stapled haemorrhoidopexy and rubber band ligation. Colorectal Dis 2009;12:587-93. http://dx.doi.org/10.1111/j.1463-1318.2009.01889.x.
- Drummond M, Brandt A, Luce B, Rovira J. Standardizing methodologies for economic evaluation in health care. Practice, problems, and potential. Int J Technol Assess Health Care 1993;9:26-3. http://dx.doi.org/10.1017/S0266462300003007.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- UK NHS Reference Costs 2014–15. London: DH; 2015.
- Costing Statement: Blood Transfusion Implementing the NICE Guideline on Blood Transfusion. London: NICE; 2015.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157-70. http://dx.doi.org/10.1007/s40273-014-0193-3.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. http://dx.doi.org/10.1002/hec.843.
- Wilkerson PM, Strbac M, Reece-Smith H, Middleton SB. Doppler-guided haemorrhoidal artery ligation: long-term outcome and patient satisfaction. Colorectal Dis 2009;11:394-400. http://dx.doi.org/10.1111/j.1463-1318.2008.01602.x.
- Gao XH, Fu CG, Nabieu PF. Residual skin tags following procedure for prolapse and hemorrhoids: differentiation from recurrence. World J Surg 2010;34:344-52. http://dx.doi.org/10.1007/s00268-009-0295-9.
- Walega P, Romaniszyn M, Kenig J, Herman R, Nowak W. Doppler-guided hemorrhoid artery ligation with Recto-Anal-Repair modification: functional evaluation and safety assessment of a new minimally invasive method of treatment of advanced hemorrhoidal disease. S World J 2012. http://dx.doi.org/10.1100/2012/324040.
- Faucheron JL, Gangner Y. Doppler-guided hemorrhoidal artery ligation for the treatment of symptomatic hemorrhoids: early and three-year follow-up results in 100 consecutive patients. Dis Colon Rectum 2008;51:945-9. http://dx.doi.org/10.1007/s10350-008-9201-z.
- Abramowitz L, Benabderrahmane M, Pospait D, Philip J, Laouénan C. The prevalence of proctological symptoms amongst patients who see general practitioners in France. Eur J Gen Pract 2014;20:301-6. http://dx.doi.org/10.3109/13814788.2014.899578.
- Johannsson HO, Graf W, Påhlman L. Bowel habits in hemorrhoid patients and normal subjects. Am J Gastroenterol 2005;100:401-6. http://dx.doi.org/10.1111/j.1572-0241.2005.40195.x.
- Lee HH, Spencer RJ, Beart RW. Multiple hemorrhoidal bandings in a single session. Dis Colon Rectum 1994;37:37-41. http://dx.doi.org/10.1007/BF02047212.
- Hardwick RH, Durdey P. Should rubber band ligation of haemorrhoids be performed at the initial outpatient visit?. Ann R Coll Surg Engl 1994;76:185-7.
- Altomare DF. Transanal dearterialization with targeted mucopexy is effective for advanced haemorrhoids – a clear classification is needed. Colorectal Dis 2014;16. http://dx.doi.org/10.1111/codi.12690.
- Sajid MS, Bhatti MI, Caswell J, Sains P, Baig MK. Local anaesthetic infiltration for the rubber band ligation of early symptomatic haemorrhoids: a systematic review and meta-analysis. Updates Surg 2015;67:3-9. http://dx.doi.org/10.1007/s13304-015-0286-3.
- Patel S, Shahzad G, Rizvon K, Subramani K, Viswanathan P, Mustacchia P. Rectal ulcers and massive bleeding after hemorrhoidal band ligation while on aspirin. World J Clin Cases 2014;2:86-9. http://dx.doi.org/10.12998/wjcc.v2.i4.86.
- Guy RJ, Seow-Choen F. Septic complications after treatment of haemorrhoids. Br J Surg 2003;90:147-56. http://dx.doi.org/10.1002/bjs.4008.
- Russell TR, Donohue JH. Hemorrhoidal banding. A warning. Dis Colon Rectum 1985;28:291-3. http://dx.doi.org/10.1007/BF02560424.
- Wechter DG, Luna GK. An unusual complication of rubber band ligation of hemorrhoids. Dis Colon Rectum 1987;30:137-40. http://dx.doi.org/10.1007/BF02554954.
- Sim HL, Tan KY, Poon PL, Cheng A, Mak K. Life-threatening perineal sepsis after rubber band ligation of haemorrhoids. Tech Coloproctol 2009;13:161-4. http://dx.doi.org/10.1007/s10151-008-0435-5.
- Subramaniam D, Hureibi K, Zia K, Uheba M. The development of Fournier’s gangrene following rubber band ligation of haemorrhoids. BMJ Case Rep 2013;28. http://dx.doi.org/10.1136/bcr-2013-201474.
- Ku JJ, Marfan M, Wall D. Pyogenic liver abscess after haemorrhoidal banding. ANZ J Surg 2005;75:828-30. http://dx.doi.org/10.1111/j.1445-2197.2005.03322.x.
- Parker R, Gul R, Bucknall V, Bowley D, Karandikar S. Double jeopardy: pyogenic liver abscess and massive secondary rectal haemorrhage after rubber band ligation of haemorrhoids. Colorectal Dis 2011;13. http://dx.doi.org/10.1111/j.1463-1318.2010.02387.x.
- Xu M, Russell M, Lin A, Yoo J. Pyogenic liver abscess as a complication of internal hemorrhoid banding. Am Surg 2014;80:E36-7.
- Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM, Altman DG, et al. Evaluation and stages of surgical innovations. Lancet 2009;374:1089-96. http://dx.doi.org/10.1016/S0140-6736(09)61083-7.
- Berkel AE, Witte ME, Koop R, Hendrix MG, Klaase JM. Brain abscess after transanal hemorrhoidal dearterialization: a case report. Case Rep Gastroenterol 2013;7:208-13. http://dx.doi.org/10.1159/000351817.
- Toyonaga T, Matsushima M, Sogawa N, Jiang SF, Matsumura N, Shimojima Y, et al. Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int J Colorectal Dis 2006;21:676-82. http://dx.doi.org/10.1007/s00384-005-0077-2.
- Zaheer S, Reilly WT, Pemberton JH, Ilstrup D. Urinary retention after operations for benign anorectal diseases. Dis Colon Rectum 1998;41:696-704. http://dx.doi.org/10.1007/BF02236255.
- Kau YC, Lee YH, Li JY, Chen C, Wong SY, Wong TK. Epidural anesthesia does not increase the incidences of urinary retention and hesitancy in micturition after ambulatory hemorrhoidectomy. Acta Anaesthesiol Sin 2003;41:61-4.
- Hoff SD, Bailey HR, Butts DR, Max E, Smith KW, Zamora LF, et al. Ambulatory surgical hemorrhoidectomy – a solution to postoperative urinary retention?. Dis Colon Rectum 1994;37:1242-4. http://dx.doi.org/10.1007/BF02257789.
- Lunniss PJ, Mann CV. Classification of internal haemorrhoids: a discussion paper. Colorectal Dis 2004;6:226-32. http://dx.doi.org/10.1111/j.1463-1318.2004.00590.x.
- Gerjy R, Lindhoff-Larson A, Nyström PO. Grade of prolapse and symptoms of haemorrhoids are poorly correlated: result of a classification algorithm in 270 patients. Colorectal Dis 2008;10:694-700. http://dx.doi.org/10.1111/j.1463-1318.2008.01498.x.
- Cook J, MacLennan G, Murray D, Price A, Fitzpatrick R, Carr A, et al. Impact of Timing of Follow-up Upon Outcome in the TOPKAT Trial n.d.:57-8. http://dx.doi.org/10.1186/1745-6215-16-s2-o33.
- Yeo D, Tan KY. Hemorrhoidectomy – making sense of the surgical options. World J Gastroenterol 2014;20:16976-83. http://dx.doi.org/10.3748/wjg.v20.i45.16976.
- Ross NP, Hildebrand DR, Tiernan JP, Brown SR, Watson AJ. Haemorrhoids: 21st-century management. Colorectal Dis 2012;14:917-19. http://dx.doi.org/10.1111/j.1463-1318.2012.03086.x.
- Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol 2015;21:9245-52. http://dx.doi.org/10.3748/wjg.v21.i31.9245.
- Alonso-Coello P, Mills E, Heels-Ansdell D, López-Yarto M, Zhou Q, Johanson JF, et al. Fiber for the treatment of hemorrhoids complications: a systematic review and meta-analysis. Am J Gastroenterol 2006;101:181-8. http://dx.doi.org/10.1111/j.1572-0241.2005.00359.x.
- Perez-Miranda M, Gomez-Cedenilla A, León-Colombo T, Pajares J, Mate-Jimenez J. Effect of fiber supplements on internal bleeding hemorrhoids. Hepatogastroenterology 1996;43:1504-7.
- Ho YH, Tan M, Seow-Choen F. Micronized purified flavonidic fraction compared favorably with rubber band ligation and fiber alone in the management of bleeding hemorrhoids: randomized controlled trial. Dis Colon Rectum 2000;43:66-9. http://dx.doi.org/10.1007/BF02237246.
- Misra MC, Parshad R. Randomised clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br J Surg 2000;87:868-72. http://dx.doi.org/10.1046/j.1365-2168.2000.01448.x.
- Watson AJ, Bruhn H, MacLeod K, McDonald A, McPherson G, Kilonzo M, et al. A pragmatic, multicentre, randomised controlled trial comparing stapled haemorrhoidopexy to traditional excisional surgery for haemorrhoidal disease (eTHoS): study protocol for a randomised controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-439.
- MacRae HM, McLeod RS. Comparison of hemorrhoidal treatment modalities. A meta-analysis. Dis Colon Rectum 1995;38:687-94. http://dx.doi.org/10.1007/BF02048023.
- Simillis C, Thoukididou SN, Slesser AAP, Rasheed S, Tan E, Tekkis PP. Systematic review and network meta-analysis comparing clinical outcomes and effectiveness of surgical treatments for haemorrhoids. Br J Surg 2015;102:1603-18. http://dx.doi.org/10.1002/bjs.9913.
- Goligher JC. Advances in proctology. Practitioner 1964;193:526-32.
- Sohn N, Aronoff JS, Cohen FS, Weinstein MA. Transanal hemorrhoidal dearterialization is an alternative to operative hemorrhoidectomy. Am J Surg 2001;182:515-19. http://dx.doi.org/10.1016/S0002-9610(01)00759-0.
- Greenberg R, Karin E, Avital S, Skornick Y, Werbin N. First 100 cases with Doppler-guided hemorrhoidal artery ligation. Dis Colon Rectum 2006;49:485-9. http://dx.doi.org/10.1007/s10350-005-0281-8.
- Szmulowicz UM, Gurland B, Garofalo T, Zutshi M. Doppler-guided hemorrhoidal artery ligation: the experience of a single institution. J Gastrointest Surg 2011;15:803-8. http://dx.doi.org/10.1007/s11605-011-1460-7.
- Ratto C, Donisi L, Parello A, Litta F, Zaccone G, De Simone V. ‘Distal Doppler-guided dearterialization’ is highly effective in treating haemorrhoids by transanal haemorrhoidal dearterialization. Colorectal Dis 2012;14:e786-9. http://dx.doi.org/10.1111/j.1463-1318.2012.03146.x.
- Aigner F, Bodner G, Conrad F, Mbaka G, Kreczy A, Fritsch H. The superior rectal artery and its branching pattern with regard to its clinical influence on ligation techniques for internal hemorrhoids. Am J Surg 2004;187:102-8. http://dx.doi.org/10.1016/j.amjsurg.2002.11.003.
- Blaisdell PC. Office ligation of internal hemorrhoids. Am J Surg 1958;96:401-4. http://dx.doi.org/10.1016/0002-9610(58)90933-4.
- Barron J. Office ligation treatment of hemorrhoids. Dis Colon Rectum 1963;6:109-13. http://dx.doi.org/10.1007/BF02633461.
- Ramzisham AR, Sagap I, Nadeson S, Ali IM, Hasni MJ. Prospective randomized clinical trial on suction elastic band ligator versus forceps ligator in the treatment of haemorrhoids. Asian J Surg 2005;28:241-5. http://dx.doi.org/10.1016/S1015-9584(09)60353-5.
- Kanellos I, Goulimaris I, Christoforidis E, Kelpis T, Betsis D. A comparison of the simultaneous application of sclerotherapy and rubber band ligation, with sclerotherapy and rubber band ligation applied separately, for the treatment of haemorrhoids: a prospective randomized trial. Colorectal Dis 2003;5:133-8. http://dx.doi.org/10.1046/j.1463-1318.2003.00395.x.
- Chew SS, Marshall L, Kalish L, Tham J, Grieve DA, Douglas PR, et al. Short-term and long-term results of combined sclerotherapy and rubber band ligation of hemorrhoids and mucosal prolapse. Dis Colon Rectum 2003;46:1232-7. http://dx.doi.org/10.1097/01.DCR.0000080170.02812.8F.
- Pucher PH, Qurashi M, Howell AM, Faiz O, Ziprin P, Darzi A, et al. Development and validation of a symptom-based severity score for haemorrhoidal disease: the Sodergren score. Colorectal Dis 2015;17:612-18. http://dx.doi.org/10.1111/codi.12903.
- Smith CT, Hopkins C, Sydes M, Woolfall K, Clarke M, Murray G, et al. Good practice principles for sharing individual participant data from publicly funded clinical trials. Trials 2015;16. http://dx.doi.org/10.1186/1745-6215-16-S2-O1.
Appendix 1 Changes to protocol
| Changes to protocol | Progress report | Date | Approved by |
|---|---|---|---|
| Protocol version 2.0: adverse events were clarified in the protocol and participant information sheet | 1 (1 December 2012) | 30 July 2012 | NRES Committee Yorkshire & The Humber – South Yorkshire |
| Protocol version 3.0: clarified serious adverse event reporting to state that only related adverse events and serious adverse events will be reported | 1 (1 December 2012) | 30 August 2012 | |
| Protocol version 4.0: a change was made to the eligibility criteria to exclude patients with hypercoagulability disorders (in addition to those on warfarin or clopidogrel) | 1 (1 December 2012) | 24 October 2012 | |
| Protocol version 5.0: the baseline data collection was changed to the day of surgery, rather than at randomisation | 2 (1 June 2013) | 7 February 2013 | |
| Protocol version 6.0: the inclusion criteria ‘Either presenting for the first time or after one failure of RBL’ was replaced by three exclusion criteria for clarification: Patients that have had previous surgery for haemorrhoids (at any time); patients that have had more than one injection treatment for haemorrhoids in the past 3 years; and patients that have had more than one RBL procedure in the past 3 years | 2 (1 June 2013) | 25 March 2013 | |
| Protocol version 7.0: the sentence ‘patients will have at least 24 hours to decide whether to take part’ was added to the protocol, to provide clarification | 2 (1 June 2013) | 30 April 2013 | |
| Protocol version 8.0: a pre-randomisation questionnaire was added to the data collection schedule for participants | 3 (1 December 2013) | 5 July 2013 |
Appendix 2 Data collection tools
Screening assessment
Pre-randomisation questionnaire
Clinical assessment: baseline
Participant questionnaire: baseline
Procedure details
Participant questionnaire: 1 day
Participant questionnaire: 7 days
Participant questionnaire: 21 days
Participant questionnaire: 6 weeks
Clinical assessment: 6 weeks
Participant questionnaire: 1 year
Consultant questionnaire: 1 year
GP questionnaire: 1 year
Serious adverse event form
Study completion/discontinuation
Appendix 3 Health-economic analyses: full regression results
Base–case analysis: seemingly unrelated regression: imputed data
Subgroup analysis: recurrence following rubber band ligation
Subgroup analysis: new patients
Sensitivity analysis: using the NHS reference cost for the haemorrhoidal artery ligation procedure rather than the microcosting approach
Sensitivity analysis: controlling for the grade of haemorrhoids
Sensitivity analysis: complete-case analysis
Sensitivity analysis: applying utility decrements for each subsequent procedure
List of abbreviations
- AMI
- Agency for Medical Innovations
- BMI
- body mass index
- BNF
- British National Formulary
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CRF
- case report form
- CTRU
- Clinical Trials Research Unit
- CUA
- cost–utility analysis
- DMEC
- Data Monitoring and Ethics Committee
- EH
- excisional haemorrhoidectomy
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-5L
- European Quality of Life-5 Dimensions (5-level version)
- GP
- general practitioner
- HAL
- haemorrhoidal artery ligation
- HALO
- haemorrhoidal artery ligation operation
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICC
- intraclass correlation
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NICE
- National Institute for Health and Clinical Excellence
- NMB
- net monetary benefit
- NSAID
- non-steroidal anti-inflammatory drug
- OH
- open haemorrhoidectomy
- OR
- odds ratio
- PP
- per protocol
- PPI
- patient and public involvement
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RBL
- rubber band ligation
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- ScHARR
- Sheffield School of Health and Related Research
- SD
- standard deviation
- SE
- standard error
- SH
- stapled haemorrhoidopexy
- SOPC
- surgical outpatient clinic
- STH
- Sheffield Teaching Hospitals
- SUR
- seemingly unrelated regression
- THD
- transanal haemorrhoidal dearterialisation
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale