Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/65/01. The contractual start date was in June 2013. The draft report began editorial review in June 2016 and was accepted for publication in October 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sandra Lawton reports receiving an honorarium from Thornton & Ross and Bayer for educational activities outside the submitted work. Hywel C Williams is Director of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme. Andrew Gribbin reports salary support from Clinical Research Network and non-financial support from Nottingham Clinical Trials Unit, outside the submitted work. Sara J Brown reports grants from the Wellcome Trust Senior Fellowship in Clinical Research (106865/Z/15/Z) during the conduct of the study and personal fees from the American Academy of Asthma Allergy and Immunology outside the submitted work, and has patent GB 1602011.7 pending (outside the submitted work). Sara J Brown holds a Wellcome Trust Senior Fellowship in Clinical Research (106865/Z/15/Z). Tracey Sach reports grants from the NIHR HTA programme and grants from the NIHR Career Development Fellowship during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Thomas et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Atopic eczema (AE) (also known as atopic dermatitis or eczema) is a chronic, itchy, inflammatory skin condition that is common throughout the world. 1 Childhood AE has a substantial impact on the quality of life of both children and their families. 2–4 Standard treatment options for AE focus on topical medications: emollients, with the addition of topical corticosteroids and topical calcineurin inhibitors, tailored according to the severity of the AE. 5 Although most cases of AE can be successfully treated with topical medications, many parents express inconvenience and/or concern in using these preparations and are keen to identify new ways of managing the symptoms of AE using non-pharmacological approaches. 6
Clothing may play a role in either soothing or exacerbating AE symptoms and patients are commonly advised to avoid wool because of its tendency to worsen itch and to use cotton or fine-weave materials next to the skin. 7 Specialist clothing is now available on prescription in the UK in a variety of forms, including sericin-free silk, viscose and silver-impregnated fabrics. 8 The therapeutic silk garments included in this study are available on prescription in the UK, at a cost ranging from £66 to £155 per set of top and leggings (2014/15 prices). 8 These garments are claimed to be beneficial for the management of AE, as they can help to regulate the humidity and temperature of the surface of the skin, are smooth in texture and may reduce skin damage from scratching. Some products have antimicrobial properties that may help to reduce the bacterial load on the skin, which may be important in AE. 9 However, the evidence from randomised controlled trials (RCTs) supporting the use of silk garments is limited. 10,11
To identify RCTs published prior to the CLOTHES trial, we searched the Global Resource of Eczema Trials database. 12 At the time of starting the CLOTHES trial, 14 small RCTs assessing the effects of therapeutic clothing had been published: three RCTs investigated silk clothing [DermaSilkTM (AlPreTec Srl, San Donà di Piave, Italy)];13–15 two investigated silver-coated textiles;16,17 three investigated cellulose seaweed fibres with silver;18–20 one investigated cellulose;21 one investigated an anion textile;22 two investigated types of ethylene vinyl alcohol fibre;23,24 one investigated borage oil-coated garments;25 and one investigated cotton and synthetic fibres. 26 Since the start of the trial, an additional study on chitosan-coated textiles has been published. 27
The three previously published silk clothing RCTs are summarised in Table 1 (for further details of all trials of therapeutic clothing for AE, see Appendix 1).
| Reference | Duration (months) | Participants | Interventions | Main results | Comments on study design and interpretation |
|---|---|---|---|---|---|
| Koller et al. (2007)13 | 3 | 22 children with mild to moderate AE (unclear how many included in analysis) Within-person trial |
Intervention A: DermaSilk arm tubes (with antimicrobial coating). Worn all day on one arm for 3 months Intervention B: silk (without antimicrobial coating) arm tubes worn all day for 2 weeks, followed by cotton arm tubes for the remaining time in the trial Concurrent medication: emollients and antihistamines were permitted, but not topical corticosteroids |
No difference in local SCORAD of DermaSilk group compared with cotton group at week 2 [median (quartile 1–quartile 3)] [7.5 (6–9) vs. 8 (6.25–9.75); p = 0.274] Significant reduction of local SCORAD index in the DermaSilk-covered arm observed after 4, 8 and 12 weeks in comparison with cotton-covered arm [4 weeks: 6.5 (5–8) vs. 8 (7–9; p < 0.002; 8 weeks: 6 (5.25–7.75) vs. 8 (7–9); p < 0.0001; and 12 weeks: 6 (5–6) vs. 8 (7.25–10); p < 0.0001] |
|
| Stinco et al. (2008)14 | 1 | 30 children and adults with AE (26 analysed) Within-person trial |
Intervention A: DermaSilk (knitted fabric sleeves with bonded antimicrobial AEGIS AEM 5772/5) Intervention B: knitted silk fabric sleeves without antimicrobial finish Both interventions were worn all night and all day. One change per day, for 28 days |
No difference between groups in mean local SCORAD at 7 and 14 days. At 21 days and 28 days, mean local SCORAD of the DermaSilk group was better than the unmodified silk group (p = 0.02 and p ≤ 0.0001, respectively; confidence interval for difference in means not given). Difference of mean local SCORAD between groups over whole study was significant [mean 10.05 (SD ±9.22); p < 0.0001] No difference in mean pruritus values at day 7. At 14, 21 and 28 days, mean value of pruritus in DermaSilk group was better than unmodified silk group (p = 0.03, p = 0.01 and p ≤ 0.0001, respectively) |
|
| Fontanini et al. (2013)15 | 24 | 22 infants aged 4–18 months (20 analysed) Parallel-group trial |
Group A (n = 9): DermaSilk long-sleeved top and trousers Group B (n = 11): cotton clothing Both interventions were to be worn every day for 24 months, except during the summer and on very hot days in other seasons Both groups also received antimite mattresses, pillows and mometasone furoate for management of flares |
Topical corticosteroid use was significantly lower in the DermaSilk group [median 0.07 (interquartile range 0.05–0.09) tubes/month] than in the cotton group [0.17 (0.09–0.33) tubes/month] (p = 0.006) All parents in the DermaSilk group were satisfied with outcome (regarding itching reduction), compared with five (45%) in the cotton group |
|
In view of the limited evidence for silk garments in AE, the UK National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme issued a funding call in 2011 and, subsequently, commissioned the CLOTHing for the relief of Eczema Symptoms (CLOTHES) Trial.
Objectives
Primary objective
-
To assess whether or not the addition of silk therapeutic garments to standard AE care reduces AE severity in children with moderate to severe disease over a period of 6 months.
Secondary objectives
-
To estimate the within-trial cost-effectiveness of silk therapeutic garments from a NHS and wider (family and employer) perspective.
-
To explore parent/guardian and child views on and experiences of using silk garments and factors that might influence the use of these garments in everyday life.
-
To examine prescribers’ and commissioners’ views on the use of silk garments for the management of AE.
Role of the funder
The study was funded by the NIHR HTA programme. Espère Healthcare Ltd (UK and Ireland distributor for DermaSilk) and DreamSkinTM Health Ltd (Hatfield, UK) donated the garments. The NIHR had input into trial design through peer review of the funding proposal and the garment companies provided advice in defining how the intervention should be used. Neither of the clothing companies had a role in data collection, analysis or interpretation or writing of the report. However, both had sight of the report prior to publication and had the opportunity to comment. The corresponding author had full access to all the data and had final responsibility for the decision to submit.
Chapter 2 Methods
Extracts of text, figures and tables throughout this report have been published in Thomas KS, Bradshaw LE, Sach TH, Batchelor JM, Lawton S, Harrison EF, et al. Silk garments plus standard care for treating eczema in children: a randomised controlled observer-blind pragmatic trial (CLOTHES TRIAL). PLOS Med 2017; in press. https://doi.org/10.1371/journal.pmed.1002280. 28 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Trial design
The CLOTHES trial was a multicentre, parallel-group, observer-blind, pragmatic RCT of 6 months’ duration, followed by a 2-month observational period (Figure 1). Children aged 1–15 years with moderate to severe AE were randomised (1 : 1) to receive either silk garments plus standard AE care or standard AE care alone. The primary outcome was assessed by research nurses blinded to the treatment allocation at baseline, 2, 4 and 6 months.
FIGURE 1.
CLOTHES trial flow chart.
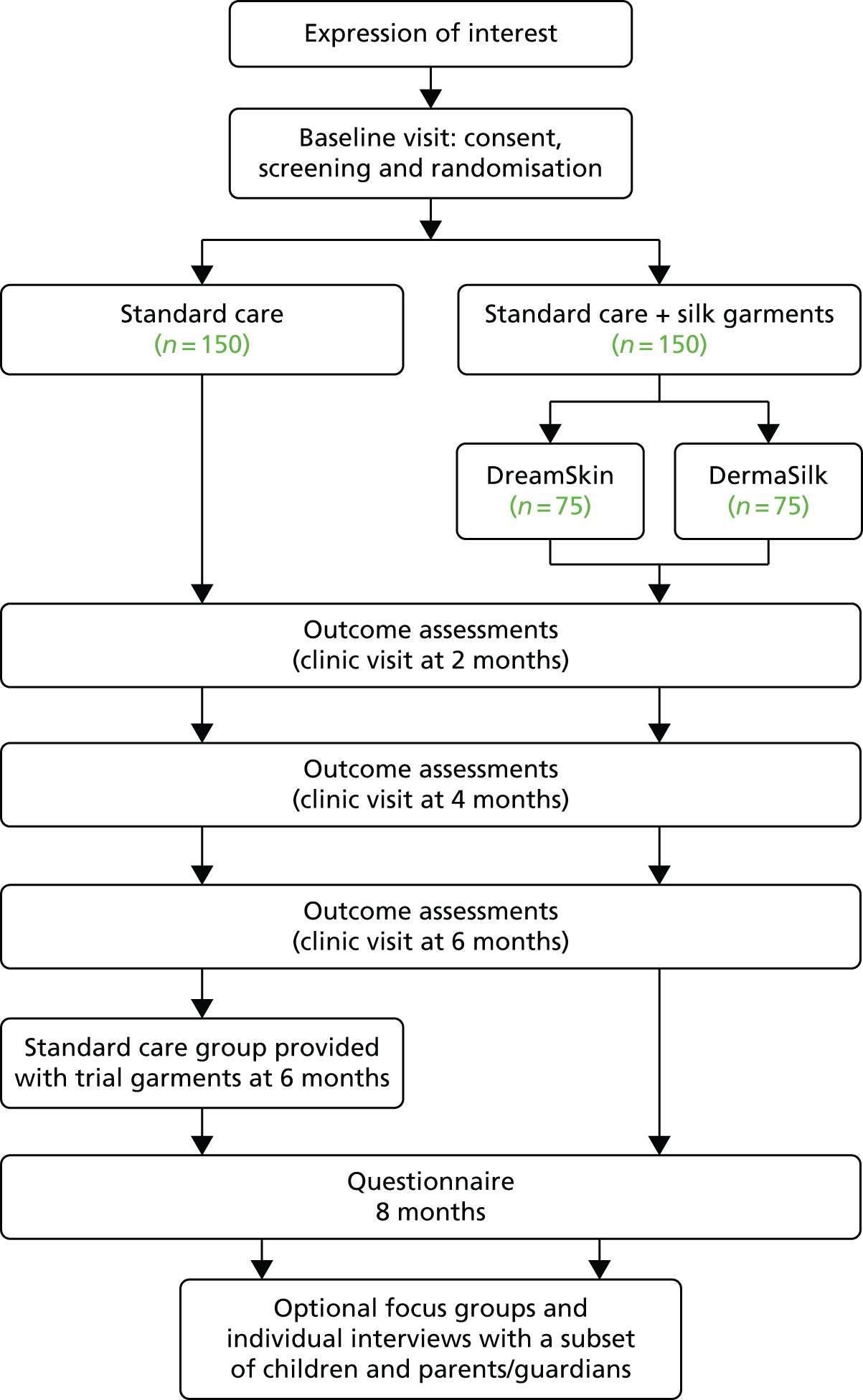
Participants randomised to silk garments were further randomised to receive one of the two brands of garments used in the trial (DermaSilk or DreamSkin). Two products were used in the trial in order to improve the generalisability of the trial findings and to avoid commercial advantage to one particular company.
Participants allocated to the standard care group were given the silk garments after the primary outcome had been recorded at 6 months and used the garments for the remaining 2-month observational period. This was done in order to minimise loss to follow-up and potential contamination in the standard care group.
The trial included a nested qualitative evaluation, health economic analysis and subgroup analysis based on presence or absence of loss-of-function mutations in the gene encoding filaggrin (FLG). This was performed because loss-of-function mutations in FLG are known to increase the risk of eczema and it is possible that they affect response to silk clothing.
During the first 6 months of trial recruitment, an internal pilot was conducted to assess ability to recruit, adherence to the intervention and retention in the trial.
The study was approved by Health Research Authority East Midlands – Nottingham 1 Research Ethics Committee (reference number 13/EM/0255) and the local research and development department for each participating centre prior to recruitment commencing at that site. The trial was registered on Current Controlled Trials prior to start of recruitment (ISRCTN77261365 11 October 2013).
Recruiting centres
Recruitment took place in five UK centres: Nottingham University Hospitals NHS Trust, Royal Free London NHS Foundation Trust, Cambridge University Hospitals NHS Foundation Trust, Portsmouth Hospitals NHS Trust and Isle of Wight NHS Trust.
Participants were identified through secondary and primary care, or by self-referral in response to adverts placed in local media, in the community and online. Potential participants were identified when they attended a secondary care clinic or by responding to invitation letters and patient information sheets that were sent to parents of children identified from secondary care clinic lists. A parent information sheet and three separate age-appropriate child information sheets (for children aged 0–5, 6–10 and 11–15 years) were used in the study (see Appendices 2–5).
A number of press releases were issued at the start of the trial. Posters and flyers were displayed in recruiting centres and research nurses also took an active role in advertising the trial in the community by placing posters and flyers in local schools, shops and community centres. The trial was promoted online by the National Eczema Society and the Nottingham Support Group for Carers of Children with Eczema and adverts were also posted in relevant web forums using ethics-approved text. If parents were interested, either they contacted the recruiting centres directly or they enquired at the trial co-ordinating centre and their details were passed on to the relevant recruiting centre.
General practice surgeries and other hospitals local to the recruiting centres were used as patient identification centres by displaying trial posters and flyers.
Participants
Children were considered for entry into the trial if the following inclusion criteria were met:
-
they were aged 1–15 years at baseline
-
they had a diagnosis of AE according to the UK Working Party’s diagnostic criteria29 and a score of ≥ 9 on the Nottingham Eczema Severity Score,30 denoting moderate or severe AE over the preceding 12 months
-
they had at least one area of active AE on a part of the body that would be covered by the silk garments
-
they were resident within travelling distance of a recruiting centre.
In addition, children were not entered into the trial if any of the following exclusions applied:
-
they had taken systemic medication (e.g. ciclosporin, oral corticosteroids) or received light therapy for AE in the preceding 3 months
-
they had used wet/dry wraps at least five times in the last month
-
they had started a new medication or treatment regimen that may affect AE in the last month
-
they were currently using silk garments for their AE and were unwilling to stop during the trial
-
they were currently taking part in another clinical trial
-
they had expressed a wish not to take part in the trial.
Only one child was enrolled per family; if more than one child in a family was eligible, the decision as to which child would be involved was made by the parents and children concerned.
Informed consent
Written informed consent was obtained from the parent/guardian of each participant at the baseline visit, prior to any trial procedures being carried out. In addition, assent was provided by the children if they wanted to. Consent to take part in the genetic study (FLG genotyping), and for samples to be stored and used for potential future research, was included as optional.
Interventions
Silk garments
The silk garments used in the study are licensed as a medical device with a Conformité Européenne (CE) mark for use in AE, denoting that they comply with European Union legislation and safety requirements. They are 100% silk garments made from antimicrobially protected, knitted, sericin-free silk. Sericin is removed from the silk fibres during manufacturing because it is a protein that coats the outside of the fibres and has the potential to cause allergic reactions.
Two products were chosen for inclusion in the trial (DermaSilk and DreamSkin), as these were the two brands available on prescription in the UK at the time of trial design. Distribution of the intervention to participants was handled from the co-ordinating centre, where a stock of garments across a range of sizes in both brands was maintained. Participants received three sets of garments (long-sleeved vest and leggings or long-sleeved body suits and leggings, depending on the age of the child) and were instructed to wear the garments as often as possible during the day and at night, either as underwear or as pyjamas (Figure 2). Three sets were provided to allow for the washing and rotation of garments. The child’s height at randomisation was used to determine the correct size of garments, which were posted out to participants as soon as possible after randomisation. On receipt of the garments, participants were instructed to try on one set to check that they fitted correctly and then confirm this with the co-ordinating centre. Standardised care instructions were provided on a paper insert included in the garment package (Box 1) and instructions were also replicated in the participant diary.
FIGURE 2.
Garments being worn.

Please wear the garments as often as possible, both during the day and at night (either as underwear or as pyjamas).
Moisturising creams should be applied thinly to the skin (just enough for the skin to glisten) and should be applied a few minutes before putting on the clothing to allow the creams to be absorbed into the skin.
How do I care for the garments?You will be given three sets of garments during the trial. This will allow one set to be in use, one in the wash and one spare. We recommend that you use all three sets within 1 week, rotating frequently.
To machine wash: wash at up to 40 °C using your usual mild non-biological detergent. The fibres of the garment are quite delicate and washing the garment inside a pillowcase on a delicate cycle will protect it during the wash. If possible, lay the garment flat to dry.
To hand wash: place in hand-hot water containing your usual mild non-biological detergent and agitate by hand for a few minutes. Rinse well with plenty of warm, clean water and squeeze dry. Do not wring. If possible, lay the garment flat to dry.
Other important pointsPlease do not use bleach. Make sure there are no bleaching agents in your detergent [such as Vanish (Reckitt Benckiser, Slough, UK)].
Please do not use fabric softeners.
Please do not tumble dry.
Any reduction in garment length is likely to be a result of a tightening of the knit. A cool steam iron can be used to restore the shape of a garment that appears to have shrunk.
Garments were replaced as required during the 6-month RCT (if they were worn out, were lost or no longer fitted the child). If replacement garments were required, the participants returned the worn garments to the co-ordinating centre with a completed garment request form and new garments were sent out. After the 6-month RCT period was over, garments were not replaced.
Standard care
All participants continued with their standard AE care in line with National Institute for Health and Care Excellence (NICE) guidance,5 including regular emollient use, avoidance of irritants and topical corticosteroids (or calcineurin inhibitors) for controlling inflammation. Participants were asked not to change their standard AE treatment for the duration of the trial unless medically warranted.
Participants who frequently used wet- or dry-wrap dressings for their AE were excluded, but occasional use of wet or dry wraps was monitored but not prohibited.
If a research nurse suspected that the AE had become infected, participants were advised to contact their normal medical team for confirmation of diagnosis and subsequent treatment.
Outcomes
Details of derivations for outcomes can be found in the statistical analysis plan (see Appendix 6).
Primary outcome
The primary outcome of AE severity measured using the objective Eczema Area and Severity Index (EASI)31 was assessed at baseline and at 2, 4 and 6 months. Baseline EASI score was used as a covariate in the analysis model. EASI score was assessed by trained research nurses who were blinded to treatment allocation. EASI score was chosen as the primary outcome as it is a validated scale recommended as the core outcome instrument for AE signs. 32 EASI involves an evaluation of four AE signs [erythema (redness), excoriation (scratching), oedema/papulation (swelling and fluid in the skin) and lichenification (thickening of the skin)] and an assessment of percentage area affected by eczema in four body regions (head and neck, upper limbs, trunk and lower limbs). EASI score ranges from 0 to 72, with higher scores representing more severe disease.
All research nurses received training in the use of EASI (using standardised training photographs and assessment of patients with AE by two independent assessors until concordance was reached). Resources were provided to assist in assessing the signs and body surface area (see Appendix 7).
Participants were assessed by the same research nurse at all time points in order to minimise interobserver variability.
Secondary outcomes
-
Global assessment of AE by research nurses [Investigator Global Assessment (IGA)] and by participants [Patient Global Assessment (PGA)] at baseline and at 2, 4 and 6 months, using a six-point scale (clear, almost clear, mild, moderate, severe and very severe).
-
Self-reported AE symptoms using the recommended core outcome instrument,33 the Patient Oriented Eczema Measure (POEM), which captures frequency of itch, sleep loss, bleeding, weeping/oozing, cracking, flaking and dryness. 34 It has a range from 0 to 28, with higher scores representing more severe disease. POEM scores were collected weekly using an online questionnaire for the first 6 months and once again at 8 months. Obtaining self-reported eczema severity every week for 6 months was used to capture long-term control of flares as well as self-reported eczema symptoms.
-
Three-Item Severity (TIS) scale35 at baseline and at 2, 4 and 6 months, assessed by research nurses at a single representative body site (defined as the most bothersome patch of AE that was covered by the garments). The selected representative body site did not have to be the same at each visit. The TIS measures three clinical signs (erythema, oedema/papulation and excoriation) and the total score ranges from 0 to 9, with higher scores representing more severe disease. Given the importance of an objective measure to capture eczema severity in this observer-blind trial, it was felt that a second validated eczema severity scale was warranted.
-
Use of AE treatments: number of days of use of topical steroids, topical calcineurin inhibitors, emollients and wet/dry wrapping, assessed weekly throughout the trial. At each visit, research nurses assessed change in AE treatment regimen and categorised as no change, neutral change, reduction or escalation.
-
Health-related quality of life at baseline and at 6 months from the perspectives of the family [Dermatitis Family Impact (DFI)],36 the main carer [EuroQoL-5 Dimensions-3 Levels (EQ-5D-3L)]37 and the child [Atopic Dermatitis Quality of Life (ADQoL) preference-based index38 and Child Health Utility-9 Dimensions (CHU-9D)39 in those aged ≥ 5 years].
-
Durability of the garments, adherence and acceptability of use (as assessed by children and parents/carers). Adherence was collected weekly, and information on durability and acceptability was captured at 6 and 8 months in the participant questionnaires. Sticker charts were provided for children to record how many days/nights the garments had been worn for the intervention group and how many days/nights they had been in the study for the standard care group (see Appendix 8). These were intended to help keep children engaged in the study and to assist in completing the adherence data in the weekly questionnaires.
-
Health resource use for treatment of AE throughout the trial: health-care visits, inpatient stays, medications, tests, personal items for AE and time off work or school.
Safety outcomes
Skin infections requiring antibiotic or antiviral treatment self-reported by parents and serious adverse events related to AE (hospitalisation as a result of AE) were recorded.
Tertiary outcomes
Although it was assumed that the different brands of garments were similar, the effects of receiving different brands of garments were also explored. Another additional exploratory analysis was conducted based on AE severity scores in areas covered by the garments (body and limbs) compared with areas uncovered by the garments (head and neck). All tertiary analyses were considered exploratory.
Data collection
Trial data generated by all centres were entered by research nurses directly into a web-based MACRO database (MACRO 4 version 3800, Elsevier, London, UK), maintained by the Nottingham Clinical Trials Unit (CTU). Access to the trial database was controlled by user logins and research nurses could enter/edit data for their site only. Paper worksheets were provided for research nurses to record data during the clinic visit (see Appendix 9) and were transcribed after the visit. Participant questionnaires completed at clinic visits were transcribed by the research nurses into the trial database. Data entry was checked against the paper record for 100% of the primary outcome and for a 10% sample of all data.
Participants were provided with a diary booklet in which they were encouraged to record all health-care visits for eczema, eczema prescriptions, purchases for AE and time off work/school because of AE (see Appendix 10). The diary was reviewed by the research nurse at each clinic appointment and used as an aide memoir to complete the relevant sections of the trial database.
Missing and/or ambiguous data were queried with research nurses and resolved whenever possible.
Weekly questionnaires were completed by the participant online or in paper format (see Appendix 11) and sent to the Nottingham CTU for data entry on the bespoke in-house system. The preference for paper or online questionnaires was recorded at baseline. Participants completing online questionnaires were emailed a unique web link to the questionnaire each week on the day completion was due. A further reminder e-mail was sent at the beginning of day 3 if the questionnaire had not been completed. Links remained active until the end of day 3, after which time the week’s entry was classed as missing. Participants who failed to complete the weekly questionnaire for ≥ 3 weeks in a row were contacted by the Nottingham CTU and encouraged to complete the questionnaires.
For the week 24 (6-month) (see Appendix 12) and week 32 (8-month) (see Appendix 13) questionnaires, online submission remained open for 14 and 7 days, respectively, in order to ensure maximum data completion at the primary end point and end of trial. For these time points, non-responders were contacted by telephone and a paper copy of the questionnaire was sent by post if required.
Sample size
Three hundred participants provided 90% power, at the 5% significance level (two-tailed), to detect a difference of around 3 points between the groups in mean EASI scores. Although this between-group difference is approximately half the published minimum clinically important difference for EASI that was suggested from one study in adults,40 we wanted to be sure that a clinically important difference to patients was not missed as a result of our focus on an objective outcome for the primary outcome. Sample size was based on repeated measures analysis of covariance, assuming a standard deviation (SD) of 13, a correlation between EASI scores at different time points of 0.6 and loss to follow-up of 10%.
Stopping rules and discontinuation
An internal pilot RCT was conducted over the first 6 months of trial recruitment to ensure delivery of the trial to time and target. Pre-defined stop/go criteria were assessed by the Trial Steering Group at 6 months as outlined in Table 2. Target recruitment for the RCT phase was ≥ 75 participants.
| Criteria to be assessed at 6 months of recruitment | Proposed action |
|---|---|
| ≥ 90% of target recruitment and retention | Continue with main trial as planned |
| 70–89% of target recruitment and retention | Continue with main trial, implement strategies for improvement |
| 50–69% of target recruitment and retention | Urgent measures required, discuss plans with Trial Steering Committee and NIHR HTA |
| < 50% of target recruitment and retention | Stop trial unless good reason for delay and rectifiable solution can be readily implemented |
Adherence to wearing the clothing was defined as a trigger for concern if participants reported using the clothing < 50% of the time.
Randomisation and blinding
Randomisation was stratified by recruiting hospital and by participant’s age: < 2, 2–5 or > 5 years. A computer-generated pseudo-random code with random permuted blocks of randomly varying size (2, 4 or 6) was created by the Nottingham CTU, in accordance with their standard operating procedure, and held on a secure University of Nottingham server. Research staff at sites were not aware of the block sizes. Participants were further randomised to one of the two silk garment brands (DermaSilk or DreamSkin) using a computer-generated pseudo-random code with random permuted blocks of randomly varying size, stratified by allocated group.
Research nurses accessed the randomisation website by means of a remote, internet-based randomisation system developed and maintained by the Nottingham CTU. Access was controlled by unique user logins. The sequence of treatment allocations was concealed until interventions had all been assigned and recruitment and data collection were complete. Study statisticians were blinded to treatment allocations until the database was locked.
After each allocation, the randomisation system notified staff at the Nottingham CTU, who then sent a letter confirming the treatment allocation to the participant (along with the silk garments as necessary). Staff at the Nottingham CTU removed branding labels from the garments and repackaged them in plain trial packaging before sending so that participants were not aware of which brand of garments they had received.
Although it was not possible to blind participants to their treatment allocation, efforts were made to minimise expectation bias by emphasising in the trial documents that the evidence supporting the use of silk garments for AE was limited and that it was not yet known if such garments offered any benefit over standard care. Participant-facing study documents also avoided the use of value-laden terms such as ‘specialist’ or ‘therapeutic’ garments.
In order to preserve blinding of the research nurses, participants were reminded in the study literature and in their clinic appointment letters/texts not to wear the garments when they attended the clinic nor to mention the garments when talking to the research nurses. Additionally, children were sent cards, both to thank them for their participation and remind them not to disclose to the research nurse whether or not they had been given the garments. All questions relating to the acceptability and use of the garments were completed by either postal or online questionnaires, and telephone and e-mail contact with participants was made by staff from the Nottingham CTU whenever possible. If the research nurses became unblinded, this was recorded. Full details of blinding arrangements are summarised in Table 3.
| Role in trial | Blinding status | Comments |
|---|---|---|
| Participants | Not blinded | Not possible to blind participants, efforts made to minimise expectation bias |
| Research nurses and principal investigators | Blinded | Participants were reminded in their clinic appointment letters not to wear the clothing when attending the clinic or to mention the clothing in any way when talking to the research nurses |
| Trial staff at the Nottingham CTU | Not blinded | Acted as the main point of contact for participants wishing to contact the research team, packaged and posted the clothing to the participants according to the randomisation schedule, and provided general advice |
| Statistician | Blinded | Statistician finalised the statistical analysis plan prior to revealing the treatment codes |
FLG genotype analysis
If participants gave written informed consent to collect a saliva sample, these samples were collected using SalivaGeneTM Collection Module II (Stratec Biomedical Systems, Birkenfeld, Germany), or SalivaGeneTM Buccal Swab (Stratec Biomedical Systems, Birkenfeld, Germany) if children were unable to spit into the container. After collection, samples were packaged by research nurses and posted to the University of Dundee, UK, where deoxyribonucleic acid (DNA) extraction was performed.
Samples were tested for the six most prevalent FLG loss-of-function mutations in the white European population as previously reported: R501X, 2282del4, R2447X, S3247X, 3702delG and 3673delC. 41 Only participants of white European ethnicity were included in the FLG genotype subgroup analysis because FLG mutations are known to be ethnically specific. Individuals in whom the four most prevalent mutations (R501X, 2282del4, R2447X and S3247X) were successfully genotyped were categorised as FLG wild type (none of the prevalent mutations was identified; these individuals constituted the control cohort), FLG heterozygotes (carrying one FLG null mutation) or FLG homozygotes or compound heterozygotes (individuals carrying two FLG null mutations).
Statistical methods
Analyses are detailed in the statistical analysis plan (see Appendix 6), which was finalised prior to database lock and release of treatment allocation codes for analysis. All analyses were carried out using Stata 13.1 (StataCorp LP, College Station, TX, USA). The main approach to analysis was modified intention to treat, that is, analysis according to randomised group regardless of adherence to allocation and including only participants who provided outcome data at follow-up. Estimates of the intervention effect are presented with 95% confidence intervals (CIs) and p-values.
All outcomes collected at the 2-monthly clinic visits were summarised by time point and treatment group. All outcomes collected from the weekly questionnaires were summarised by week and treatment group. Correlation matrices between outcomes at the 2-monthly clinic visits are given in Appendix 14 (see Table 53) and between POEM scores at 8, 16 and 24 weeks are given in Appendix 15 (see Table 55). All regression models included the randomisation stratification variables of recruiting site and age as covariates and also included baseline scores (if measured). Adjusted differences in means for the intervention group compared with the standard care group are presented for continuous outcomes, and adjusted risk differences and relative risks for binary outcomes.
Preliminary analyses
Descriptive statistics of demographic and clinical measures were used to examine balance between the randomised arms at baseline.
Primary outcome
The primary analysis used a multilevel model with observations at 2, 4 and 6 months, nested within participants, and included participants in whom EASI was assessed at least once at follow-up. The model assumed that missing EASI scores were missing at random given the observed data. The model used a random intercept and slope at the participant level, with an unstructured covariance matrix for these random effects. Diagnostic plots to check the normality of the residuals from the fixed part of the model, homogeneity of the variance of the residuals and the normality of the random effects when the model was initially fitted indicated that the assumptions for the multilevel model were not met. The score was therefore log-transformed for analysis and the effect of the trial garments is presented as a ratio of geometric means. 42,43 This ratio was back-transformed to the original EASI scale to facilitate interpretation of findings.
The effect of trial garments on AE severity changing over the study period was explored by including an interaction term between group and time point in the model. There was no evidence of a differential effect over time, so a single treatment effect has been reported that averages the treatment effect over all time points.
Sensitivity analyses for the primary outcome were performed:
-
To adjust for variables that had an observed imbalance between the groups at baseline.
-
Using multiple imputation (by chained equations) for missing outcome data.
-
To explore the impact of adherence in wearing the garments on the primary outcome by estimating the complier average causal effect (CACE) at 6 months using instrumental variable regression methods. This analysis aims to provide an unbiased estimate of the treatment effect among compliers, defined as participants who would comply with their allocation regardless of the treatment arm to which they were randomised. Estimates are presented for two measures of compliance:
-
binary compliance, defined as participants who wore the trial garments for at least 50% of the days or 50% of the nights
-
continuous compliance, defined as each additional 10% of time that the garments were worn. This was calculated by summing the number of days and nights that the trial garments were reported to be worn, then dividing by the total number of days and nights in questionnaires completed about garment wear.
-
A planned subgroup analysis based on the presence or absence of loss-of-function mutations in FLG was conducted by adding an interaction term between allocated treatment and FLG genotype (none, one or two FLG null mutations) to the primary analysis model.
Secondary outcomes
The global assessment scores (IGA and PGA) were dichotomised into ‘clear, almost clear or mild AE’ versus ‘moderate, severe or very severe AE’, and analysed using generalised estimating equations to allow estimation of risk difference and risk ratio. The TIS score was analysed using the multilevel model framework as outlined above for the primary outcome (not transformed). For the global assessment scores and the TIS score, the effect of the trial garments changing over the study period (2-, 4- and 6-month visits) was explored by including an interaction term between group and time point in the models. There was no evidence of a differential effect over time for any outcomes, so a single treatment effect per outcome has been reported that averages the treatment effect over all time points.
For each participant from the weekly questionnaire data, the mean of their weekly POEM scores between week 1 and week 24 and the percentage of days that topical treatments were used were calculated. The participant mean POEM scores and percentage of days that topical steroids were used were analysed using a linear model weighted according to the number of weekly questionnaires completed.
Quality-of-life outcomes at 6 months were analysed using linear models. Changes to treatment regimen were based on whether or not a participant had reported any treatment escalation over the 6-month RCT period and analysed using a generalised linear model. Skin infections were analysed using negative binomial regression.
Adherence to wearing the trial garments was summarised using the percentage of days and nights that the study garments were worn. Participants were classified as adherent if they wore the trial garments for at least 50% of the days or 50% of the nights. This was done for participants who completed at least half (12/24) of the weekly questionnaires. Sensitivity analyses explored adherence for all participants by making different assumptions about garment wear during periods in which the questionnaire was not completed. Adherence to wearing the trial garments was explored descriptively according to age group and baseline eczema severity.
Serious adverse events, durability and acceptability of use of the garments and information from the follow-up questionnaire at 8 months were summarised descriptively.
Tertiary outcomes
The primary analysis assumed that the effect of the different brands of garments was similar, but the impact of garment brand on AE severity was explored in a tertiary analysis. AE severity according to brand was explored by adding a term for garment brand to the primary analysis model for the EASI described above. AE symptoms according to brand were explored by comparing POEM scores after 2 months of garment wear (baseline and 2 months for the intervention group and 6 and 8 months for the standard care group).
During the study there was a supply problem with one of the garment suppliers (DreamSkin), which meant that the randomised schedule was not followed during this time and participants received the alternative brand (DermaSilk). Any participants randomised to the intervention group during a time period that DreamSkin garments of the required size were out of stock were not included in the tertiary analysis by brand of garments. Similarly, any participants in the standard care group who completed their 6-month visit during a period when DreamSkin garments of the required size were out of stock were not included.
Adherence and acceptability of the garments at 6 and 8 months were summarised descriptively by allocated group and allocated garment brand.
Additional analyses
On completion of the pre-planned analyses, and following concerns that the baseline EASI scores appeared lower than might be expected for children with moderate to severe eczema, an additional post hoc analysis was conducted to explore the interaction between baseline severity and treatment group. This was conducted by adding an interaction term between allocated group and baseline EASI score (log-transformed and continuous) to the primary analysis model.
Summary of changes to the protocol
The full protocol and statistical analysis plan are available on the CLOTHES website (www.nottingham.ac.uk/CLOTHES). Changes to the protocol initiated after the start of recruitment included an increase in the number of FLG genotype mutations to be included in the genetic analysis (two additional mutations were added: 3702delG and 3673delC) and addition of details of the nested qualitative evaluation.
Chapter 3 Results: clinical findings
Recruitment and follow-up
Recruitment to the study took place between 26 November 2013 and 5 May 2015 (Figure 3). During this time, 922 children were assessed for eligibility and 300 were subsequently randomised (Figure 4). Eighty-nine children were randomised within the first 6 months of recruitment, meeting the target of 75 participants as specified for the internal pilot phase.
FIGURE 3.
Cumulative recruitment.
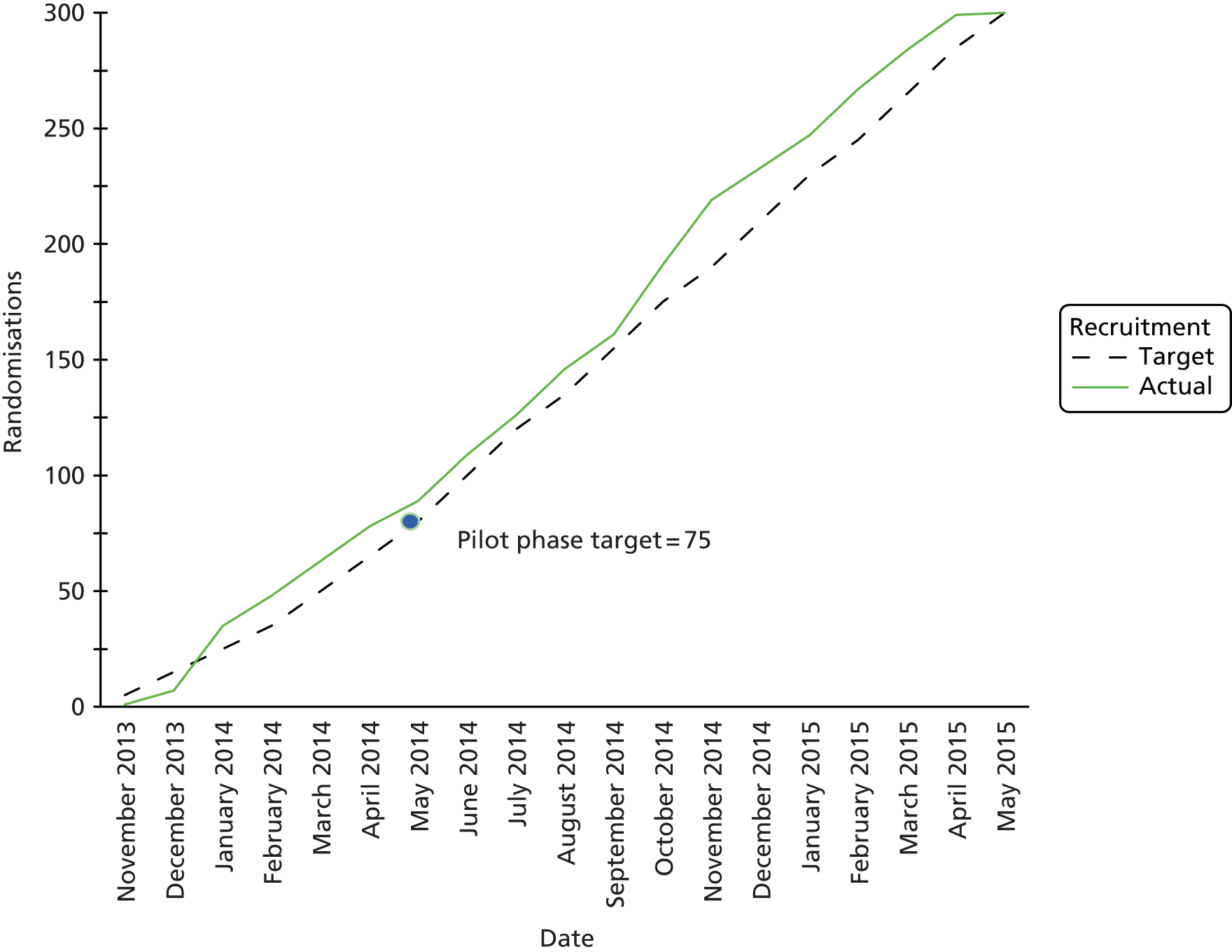
FIGURE 4.
Participant flow through the study.
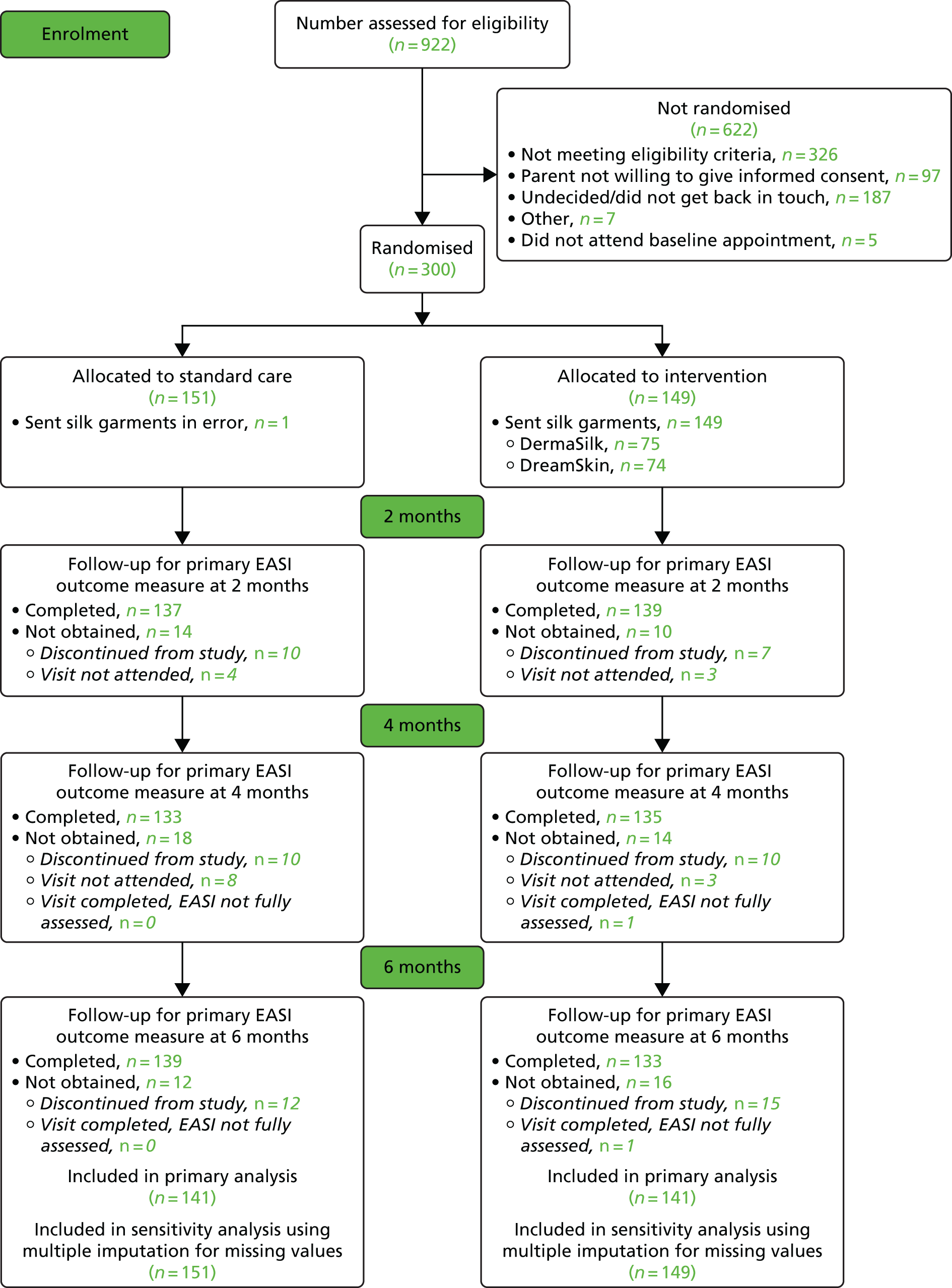
Attendance at follow-up visits was ≥ 90% for all clinic visits. In both groups, 129 (85%) attended all three follow-up visits. The same nurse performed the outcome assessments for all study visits for all but four participants.
The primary analysis included 141 participants in each group (participants were included if the primary outcome was assessed at least once after baseline) (see Figure 4).
In the case of the weekly online questionnaires (24 questionnaires over 6 months), 127 out of 151 (84%) participants in the standard care group and 126 out of 149 (85%) participants in the intervention group completed 12 or more. The median number completed was 22 (25th to 75th centile, 17 to 24) in both groups. The number of participants completing the questionnaire each week was very similar in the standard care and intervention groups (Figure 5).
FIGURE 5.
Percentage questionnaire completion by week and group.

Baseline data
Participants
The mean age of participants was 5 years (SD 3.6); 58% were male and 79% were of white ethnicity (Table 4). The majority (72%) had previously been treated in secondary care for their AE, 72% had moderate or severe AE (based on IGA scores at baseline) (Table 5) and 37% were reported to use a potent or very potent steroid as their main steroid (Table 6).
| Characteristic | Standard care (N = 151) | Intervention (N = 149) | Total (N = 300) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 5 (3.6) | 5.1 (3.7) | 5.1 (3.6) |
| Median (25th, 75th centile) | 4 (2, 8) | 4 (2, 7) | 4 (2, 7.5) |
| Minimum, maximum | 1, 14 | 1, 15 | 1, 15 |
| 1–4, n (%) | 86 (57) | 77 (52) | 163 (54) |
| 5–11, n (%) | 57 (38) | 62 (42) | 119 (40) |
| 12–15, n (%) | 8 (5) | 10 (7) | 18 (6) |
| Sex, n (%) | |||
| Male | 82 (54) | 92 (62) | 174 (58) |
| Female | 69 (46) | 57 (38) | 126 (42) |
| Ethnicity, n (%) | |||
| White | 123 (81) | 114 (77) | 237 (79) |
| Indian | 5 (3) | 2 (1) | 7 (2) |
| Pakistani | 3 (2) | 3 (2) | 6 (2) |
| Bangladeshi | 0 | 2 (1) | 2 (1) |
| Black Caribbean | 1 (1) | 2 (1) | 3 (1) |
| Black African | 3 (2) | 4 (3) | 7 (2) |
| Black (other) | 2 (1) | 0 | 2 (1) |
| Chinese | 1 (1) | 3 (2) | 4 (1) |
| Other Asian (non-Chinese) | 0 | 4 (3) | 4 (1) |
| Mixed race | 12 (8) | 13 (9) | 25 (8) |
| Other | 1 (1) | 2 (1) | 3 (1) |
| History of atopy, n (%) | |||
| Asthma | 57 (38) | 46 (31) | 103 (34) |
| Allergic rhinitis | 60 (40) | 56 (38) | 116 (39) |
| Food allergy | 80 (53) | 68 (46) | 148 (49) |
| Anaphylaxis | 23 (15) | 23 (15) | 46 (15) |
| Type of AE, n (%) | |||
| Discoid | 19 (13) | 17 (11) | 36 (12) |
| Flexural | 144 (95) | 147 (99) | 291 (97) |
| Location of AE, n (%) | |||
| Head and neck | 115 (76) | 120 (81) | 235 (78) |
| Hands and wrists | 116 (77) | 108 (72) | 224 (75) |
| Feet and ankles | 100 (66) | 96 (64) | 196 (65) |
| Limbs | 151 (100) | 149 (100) | 300 (100) |
| Trunk | 128 (85) | 122 (82) | 250 (83) |
| Previous medical care, n (%) | |||
| No previous treatment | – | – | – |
| GP only | 41 (27) | 40 (27) | 81 (27) |
| GP and in secondary care | 110 (73) | 109 (73) | 219 (73) |
| Severity assessment | Standard care (N = 151) | Intervention (N = 149) | Total (N = 300) |
|---|---|---|---|
| EASIa | |||
| Mean (SD) | 9.6 (7.8) | 11.4 (10.6) | 10.5 (9.3) |
| Median (25th, 75th centile) | 7.3 (4.2, 12) | 7 (4.1, 15.4) | 7.2 (4.1, 13.7) |
| Min., max. | 1.1, 41.1 | 1, 47 | 1, 47 |
| TISb | |||
| Mean (SD) | 4.9 (1.8) | 4.9 (1.8) | 4.9 (1.8) |
| Median (25th, 75th centile) | 5 (4, 6) | 5 (3, 6) | 5 (4, 6) |
| Min., max. | 1, 9 | 1, 9 | 1, 9 |
| Nottingham Eczema Severity Scorec | |||
| Mean (SD) | 13.1 (1.6) | 13.2 (1.7) | 13.1 (1.6) |
| Median (25th, 75th centile) | 13 (12, 14) | 13 (12, 15) | 13 (12, 14) |
| Min., max. | 9, 15 | 9, 15 | 9, 15 |
| Moderate AE (9–11), n (%) | 28 (19) | 30 (20) | 58 (19) |
| Severe AE (12–15), n (%) | 123 (81) | 119 (80) | 242 (81) |
| IGA, n (%) | |||
| Almost clear | 4 (3) | 2 (1) | 6 (2) |
| Mild | 39 (26) | 39 (26) | 78 (26) |
| Moderate | 77 (51) | 67 (45) | 144 (48) |
| Severe | 30 (20) | 36 (24) | 66 (22) |
| Very severe | 1 (1) | 5 (3) | 6 (2) |
| PGA, n (%) | |||
| Almost clear | 5 (3) | 6 (4) | 11 (4) |
| Mild | 33 (22) | 45 (30) | 78 (26) |
| Moderate | 83 (55) | 67 (45) | 150 (50) |
| Severe | 27 (18) | 25 (17) | 52 (17) |
| Very severe | 3 (2) | 6 (4) | 9 (3) |
| PGA completed by, n (%) | |||
| Parent/guardian | 129 (85) | 125 (84) | 254 (85) |
| Child | 22 (15) | 24 (16) | 46 (15) |
| POEMd | |||
| Mean (SD) | 16.6 (4.8) | 17.3 (5.8) | 17 (5.4) |
| Median (25th, 75th centile) | 16 (13, 20) | 17 (13, 21) | 17 (13, 20) |
| Min., max. | 4, 28 | 4, 28 | 4, 28 |
| POEM completed by, n (%) | |||
| Parent/guardian | 128 (85) | 122 (82) | 250 (83) |
| Child | 23 (15) | 27 (18) | 50 (17) |
| Medication usage | Standard care (N = 151), n (%) | Intervention (N = 149), n (%) | Total (N = 300), n (%) |
|---|---|---|---|
| Used emollient within the last month | 150 (99) | 146 (98) | 296 (99) |
| Consistency of main emollient | |||
| Light | 13 (9) | 6 (4) | 19 (6) |
| Creamy | 53 (35) | 57 (38) | 110 (37) |
| Greasy | 20 (13) | 21 (14) | 41 (14) |
| Very greasy | 64 (42) | 62 (42) | 126 (42) |
| Topical steroid used within the last month | 136 (90) | 130 (87) | 266 (89) |
| Potency of main steroid | |||
| Mild | 40 (26) | 34 (23) | 74 (25) |
| Moderate | 43 (28) | 40 (27) | 83 (28) |
| Potent | 51 (34) | 53 (36) | 104 (35) |
| Very potent | 2 (1) | 3 (2) | 5 (2) |
| Calcineurin inhibitors used within the last month | 14 (9) | 15 (10) | 29 (10) |
| Strength of main calcineurin inhibitor | |||
| Mild | 9 (6) | 8 (5) | 17 (6) |
| Moderate | 4 (3) | 4 (3) | 8 (3) |
| Strong | 1 (1) | 3 (2) | 4 (1) |
| Use of wet/dry wraps in the past month | |||
| No | 138 (91) | 135 (91) | 273 (91) |
| Yes | 13 (9) | 14 (9) | 27 (9) |
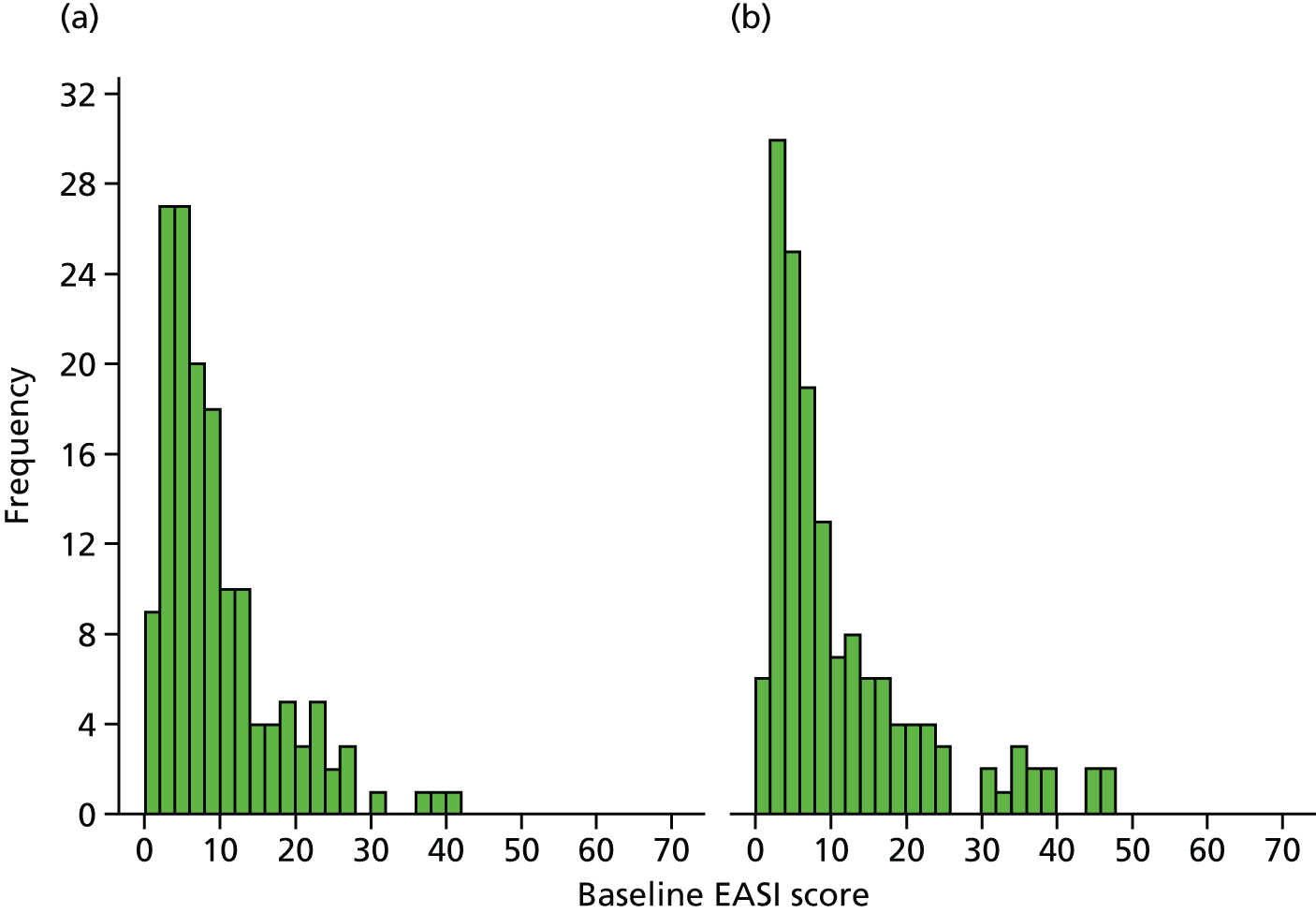
The demographic and AE characteristics were well balanced at baseline, although there were slightly more boys in the intervention group than in the standard care group, and parent-reported history of asthma and food allergy was higher in the standard care group than in the intervention group (see Table 4). The mean EASI score was slightly higher in the intervention group than in the standard care group, as more children had a baseline EASI score of > 30 (intervention, 14 participants; standard care, 4 participants; Figure 6); however, the median and interquartile ranges were similar between the groups (see Table 5). Other AE severity measures were similar between the two groups apart from the PGA scores [a greater proportion of participants in the standard care group rated their AE as moderate, severe or very severe than the intervention group (75% vs. 66%) (see Table 5)]. Health-related quality of life in the two groups was similar at baseline (Table 7).
FIGURE 6.
Histogram of baseline EASI scores by group. (a) Standard care; and (b) intervention group.
| Quality-of-life measure | Standard care (N = 151) | Intervention (N = 149) | Total (N = 300) |
|---|---|---|---|
| DFIa | |||
| Mean (SD) | 12.0 (6.3) | 12.4 (6.6) | 12.2 (6.4) |
| Median (25th, 75th centile) | 11 (7, 15) | 12 (7, 16) | 11 (7, 16) |
| Min., max. | 0, 29 | 0, 30 | 0, 30 |
| Health state from EQ-5D-3L for parentb,c | |||
| Mean (SD) | 79.5 (17.5) | 77.3 (18.2) | 78.4 (17.9) |
| Median (25th, 75th centile) | 80 (72, 90) | 80 (70, 90) | 80 (70, 90) |
| Min., max. | 8, 100 | 8, 100 | 8, 100 |
| Utility from EQ-5D-3L for parentb,d | |||
| Mean (SD) | 0.8983 (0.1612) | 0.9018 (0.1710) | 0.9 (0.1658) |
| Median (25th, 75th centile) | 1 (0.812, 1) | 1 (0.812, 1) | 1 (0.812, 1) |
| Min., max. | –0.016, 1 | 0.101, 1 | –0.016, 1 |
| n | 151 | 147 | 298 |
| CHU-9Db,e | |||
| Mean (SD) | 0.8292 (0.1263) | 0.8386 (0.1115) | 0.8341 (0.1184) |
| Median (25th, 75th centile) | 0.849 (0.7853, 0.9058) | 0.8561 (0.7524, 0.92) | 0.8503 (0.7637, 0.9189) |
| Min., max. | 0.4661, 1 | 0.5584, 1 | 0.4661, 1 |
| n | 64 | 70 | 134 |
| Completed by (n) | |||
| Parent/guardian | 17 | 23 | 40 |
| Child | 47 | 47 | 94 |
| ADQoLf | |||
| Mean (SD) | 0.6952 (0.13) | 0.6883 (0.1409) | 0.6918 (0.1354) |
| Median (25th, 75th centile) | 0.744 (0.648, 0.768) | 0.744 (0.634, 0.768) | 0.744 (0.648, 0.768) |
| Min., max. | 0.356, 0.841 | 0.356, 0.841 | 0.356, 0.841 |
| n | 151 | 149 | 300 |
| Completed by [n (%)] | |||
| Parent/guardian | 104 (69) | 102 (68) | 206 (69) |
| Child | 47 (31) | 47 (32) | 94 (31) |
FLG genotype
Table 8 shows the FLG genotyping results for participants of white ethnicity. Samples from 219 participants were tested for the six most prevalent FLG loss-of-function mutations in the white European population: R501X, 2282del4, R2447X, S3247X, 3702delG and 3673delC. Genotyping methods for the four most prevalent genotypes (R501X, 2282del4, R2447X and S3247X) were largely successful (n = 217 were included in the analysis), but the genotyping methods used for 3703delG and 3673delC were unsuccessful for 24 participants (11% of samples tested) owing to the suboptimal quality and quantity of DNA obtained from paediatric saliva samples.
| Genotype status | Standard care (N = 123), n (%) | Intervention (N = 114), n (%) | Total (N = 237), n (%) |
|---|---|---|---|
| Informed consent provided for genetic study | |||
| No | 6 (5) | 5 (4) | 11 (5) |
| Yes | 117 (95) | 109 (96) | 226 (95) |
| If yes, saliva sample collected | |||
| Noa | 1 (1) | 5 (4) | 6 (3) |
| Yes | 116 (94) | 104 (91) | 220 (93) |
| Result obtainable on FLG mutation from sampleb | |||
| No | 1 (1) | 1 (1) | 2 (1) |
| Yes | 115 (93) | 102 (89) | 217 (92) |
| Sample not received by Dundee | 0 (0) | 1 (1) | 1 (0.4) |
| Result not obtainable for each mutation tested | |||
| R501X | 0 | 0 | 0 |
| 2282del4 | 1 | 0 | 1 |
| R2447X | 1 | 1 | 2 |
| S3247X | 1 | 0 | 1 |
| 3702delG | 9 | 15 | 24 |
| 3673delC | 9 | 15 | 24 |
| FLG genotype (using mutations R501X, 2282del4, R2447X and S3247X) | |||
| No mutations | 72 (59) | 71 (62) | 143 (60) |
| One FLG null mutation | 31 (25) | 20 (18) | 51 (22) |
| Two FLG null mutations | 12 (10) | 11 (10) | 23 (10) |
| Not known | 8 (7) | 12 (11) | 20 (8) |
In total, 217 participants of white European ethnicity were categorised as FLG wild type (individuals in whom none of the prevalent mutations was identified), FLG heterozygotes (carrying one FLG null mutation) and FLG homozygotes or compound heterozygotes (individuals carrying two FLG null mutations) for the four most prevalent mutations (R501X, 2282del4, R2447X and S3247X). Of these, 74 participants had at least one mutation.
Adherence to intervention
All participants in the intervention group were sent the silk garments, on average, 1 day after randomisation. One participant allocated to the standard care group was sent the silk garments in error, but was included in the analysis according to randomised allocation (see Figure 4).
Adherence in wearing the garments was high: 102 out of 124 (82%) participants wore the clothes for ≥ 50% of the time (see Table 9). The garments were worn more often at night than during the day (median 81% of nights and 34% days) (Table 9 and Figure 7). The mean number of times that the garments were worn remained fairly constant throughout the study period (see Figure 7). Adherence to wearing the garments was not associated with age or eczema severity at baseline (correlation coefficients 0.003 to 0.20; Table 10). Sensitivity analyses for adherence according to questionnaire completion are shown in Table 9.
| Adherence | Main analysis (participants with ≥ 12 questionnaires completed) (N = 124) | Sensitivity analysis 1b,c (N = 149) | Sensitivity analysis 2b,d (N = 149) |
|---|---|---|---|
| Proportion of nights that garments were worn for at least some of the night, median (25th, 75th centile) | 80.7 (56.8, 95.9) | 74.4 (52.1, 94.8) | 61.5 (32.9, 87) |
| Percentage of days that clothing was worn for at least some of the day, median (25th, 75th centile) | 34.1 (9.8, 75.9) | 28.6 (3.7, 74.3) | 19.3 (2.5, 63.4) |
| Adherence to wearing trial garments, n (%) | |||
| Adherente | 102 (82) | 117 (79) | 87 (58) |
| Worn for at least 50% of days and 50% of nights | 50 (40) | 54 (36) | 45 (30) |
| Worn for at least 50% of days only | – | 1 (1) | – |
| Worn for at least 50% of nights only | 52 (42) | 62 (42) | 42 (28) |
| Not adherent (wore clothing for < 50% of the time) | 22 (18) | 32 (21) | 62 (42) |
FIGURE 7.
Mean number of days/nights trial garments worn each week.

| Variable | Percentage of days that clothing was worn for at least some of the day (n = 124) | Percentage of nights that clothing was worn for at least some of the night (n = 124) |
|---|---|---|
| Age | 0.003 | 0.20 |
| Baseline EASI score | –0.03 | 0.03 |
| Baseline POEM score | 0.08 | 0.13 |
Contamination
Only six participants in the standard care group reported wearing silk clothing during the 6-month study period.
Durability of the garments
Information about the number of garments and replacement garments sent out by the co-ordinating centre during the trial is presented in Chapter 4. This section presents information reported by parents on the 6-month questionnaire about the condition of the trial garments. Just over half of parents reported that at least one garment (top or leggings) could no longer be worn (Table 11). Children aged 4 years or under were more likely than older children to require replacement garments, as they outgrew the garments over the 6-month study period. Just over one-third of responders at 6 months reported that garments could no longer be worn as they had worn out or torn.
| Condition of garments at 6 months | Age (years) | |||
|---|---|---|---|---|
| 1–4 (N = 63) | 5–11 (N = 51) | 12–15 (N = 7) | Intervention (N = 121) | |
| At least one garment no longer able to be worn at 6 months, n/N (%) | 41/61 (67) | 18/46 (39) | 1/5 (20) | 60/112 (54) |
| Reasons that garments can no longer be worn, n | ||||
| Too small | 22 | 6 | 0 | 28 |
| Worn out/torn | 26 | 14 | 1 | 41 |
| Lost | 3 | 2 | 0 | 5 |
| Other | 6 | 2 | 0 | 8 |
Acceptability of use of silk clothing
At 6 months, 85 out of 121 (70%) participants reported being satisfied or very satisfied with the garments (95% CI 61% to 78%) and 89 out of 121 (74%) participants were either happy or very happy to wear the garments (95% CI 64% to 81%).
Blinding
Blinding appeared to be successful. Research nurses remained blinded to treatment allocation for 289 out of 300 (96%) participants. Unblinding occurred for three participants in the standard care group and eight participants in the intervention group. This unblinding was first reported at 2 months for one participant in the standard care group and seven participants in the intervention group and at the 4-month visit for all other participants.
Unblinding mainly occurred as a result of the child or parents saying that they had or had not received the garments. Unblinding occurred for two participants because they wore the garments to the assessment visit.
Primary outcome: Eczema Area and Severity Index
Primary analysis
Mean AE severity based on EASI scores improved in both groups during the 6-month follow-up period; however, there was no clinically important difference between the groups in the nurse-assessed EASI scores (Table 12 and Figure 8). Averaged over the 2-, 4- and 6-month follow-up visits, the ratio of geometric mean EASI scores was 0.95 (95% CI 0.85 to 1.07; p = 0.43). This CI is equivalent to a difference of approximately 1.5-point improvement to 0.5 points worse for the intervention group, compared with the standard care group in the original EASI scale units.
| Allocated group | Baseline | 2 months | 4 months | 6 months | Adjusted ratio of geometric means (95% CI); p-value |
|---|---|---|---|---|---|
| Standard care | |||||
| n | 151 | 137 | 133 | 139 | |
| Median | 7.3 | 5.3 | 4.3 | 4.2 | |
| 25th, 75th centile | 4.2, 12 | 2.5, 10.5 | 2.1, 10 | 2, 9.2 | |
| Geometric mean | 8.4 | 6.6 | 6.0 | 5.4 | |
| Intervention | |||||
| n | 149 | 139 | 135 | 133 | 0.95 (0.85 to 1.07); 0.43a |
| Median | 7 | 4.9 | 4.1 | 4 | |
| 25th, 75th centile | 4.1, 15.4 | 2.2, 9.9 | 2.2, 9.4 | 1.9, 7.9 | |
| Geometric mean | 9.2 | 6.4 | 5.8 | 5.4 | |
FIGURE 8.
Primary outcome: geometric mean EASI scores with 95% CIs.

Arithmetic means of the EASI scores and log-transformed EASI scores for each group and time point are given in Appendix 14 (see Table 52).
Sensitivity analyses for the primary outcome
Sex, history of food allergy and history of asthma were added as covariates into the analysis model because of baseline imbalance. This estimate of the ratio of the geometric mean was the same as for the primary analysis (0.95, 95% CI 0.85 to 1.07).
Multiple imputation of missing EASI data, assuming that scores were missing at random, gave a very similar result to the primary analysis (Table 13). When it was assumed that missing scores were systematically worse in the standard care group, the 95% CI for the geometric mean was equivalent to scores of 2 points lower to 0.1 points higher for the intervention group than for the standard care group.
| Sensitivity assumptions | Adjusted ratio of geometric means (95% CI) |
|---|---|
| Assuming that missing EASI scores are MAR | 0.93 (0.83 to 1.05) |
| Assuming missing EASI scores are missing not at random | |
| Favouring intervention group | |
| Assuming that missing EASI scores are 3 points higher (worse) than under MAR in the standard care group and assuming MAR in intervention group | 0.89 (0.80 to 1.01) |
| Favouring standard care group | |
| Assuming that missing EASI scores are 3 points higher than under MAR in the intervention group and assuming MAR in standard care group | 0.97 (0.86 to 1.09) |
Further exploratory analysis of the EASI scores for areas covered by the garments (body and limbs) and areas uncovered by the garments (head and neck) can be found in Appendix 16.
Causal effect of adherence with wearing trial garments on primary outcome
The CACE was estimated using the EASI scores at 6 months for participants who completed 12 or more questionnaires (standard care, n = 127; intervention, n = 124). Table 14 presents the CACE estimate based on a binary definition of adherence of wearing the trial garments for at least 50% of the days or 50% of the nights and the CACE estimate for each additional 10% of the time the garments were worn. The intention-to-treat estimate for participants included in the CACE analysis is also presented using the EASI scores at 6 months for comparison (analysis according to randomised group).
| Estimate | n | Adjusted ratio of geometric means (95% CI) |
|---|---|---|
| ITT at 6 monthsa | 243 | 1.026 (0.87 to 1.21) |
| CACE: binary – garments worn for at least 50% of days or 50% of the nightsb | 243 | 1.031 (0.85 to 1.25) |
| CACE: each additional 10% of time garments wornb,c | 243 | 1.004 (0.977 to 1.032) |
The intention-to-treat and CACE estimate based on wearing the garments for at least 50% of the days or 50% of the nights are similar (see Table 14) as a result of 82% of intervention participants satisfying this definition (see Table 9). The ratio of geometric means for all comparisons is greater than 1, favouring the standard care group. The CACE estimate for each additional 10% of time that garments were worn suggests that eczema severity (EASI scores) did not improve with greater amounts of garment wear. A further summary table using all data at 6 months is shown in Appendix 17.
Subgroup analysis for primary outcome according to FLG status
Eczema Area and Severity Index scores according to group and FLG status (none, one or two FLG null mutations) for participants of white ethnicity are shown in Table 15 and Figure 9. Participants with FLG gene mutations were no more likely to benefit from the silk clothing than participants without a mutation (p-value for interaction effect 0.47).
| Subgroup and allocated group | Baseline | 2 months | 4 months | 6 months | Adjusted subgroup-specific ratio of geometric means (95% CI)a | Adjusted interaction effectb (95% CI)a |
|---|---|---|---|---|---|---|
| FLG wild type: no mutations (+/+) | ||||||
| Standard care | ||||||
| n | 72 | 67 | 65 | 69 | ||
| Median (25th, 75th centile) | 6.2 (3.9, 10.7) | 4.5 (2.4, 9.0) | 3.2 (2.1, 9.9) | 3.3 (1.8, 6.8) | ||
| Geometric mean | 7.7 | 6.1 | 5.5 | 4.8 | ||
| Intervention | ||||||
| n | 71 | 67 | 67 | 67 | ||
| Median (25th, 75th centile) | 5.4 (3.3, 13.8) | 4.3 (2.1, 10.3) | 3.8 (2.2, 8.4) | 4.0 (2.3, 9.9) | ||
| Geometric mean | 8.1 | 6.4 | 5.7 | 6.1 | ||
| Analysis | 1.04 (0.89 to 1.21) | |||||
| One FLG null mutation (+/–) | ||||||
| Standard care | ||||||
| n | 31 | 28 | 27 | 29 | ||
| Median (25th, 75th centile) | 8.0 (3.8, 12.0) | 5.3 (2.9, 11.4) | 4.6 (2.7, 8.6) | 4.4 (1.6, 10.7) | ||
| Geometric mean | 8.5 | 7.1 | 6.5 | 5.4 | ||
| Intervention | ||||||
| n | 20 | 19 | 19 | 19 | ||
| Median (25th, 75th centile) | 8.7 (5.0, 15.7) | 6.1 (3.0, 8.4) | 4.4 (2.2, 9.5) | 4.0 (1.9, 8.0) | ||
| Geometric mean | 10.1 | 6.9 | 5.5 | 5.2 | ||
| Analysis | 0.87 (0.67 to 1.14) | 0.84 (0.61 to 1.15) | ||||
| Two FLG null mutations (–/–) | ||||||
| Standard care | ||||||
| n | 12 | 11 | 12 | 12 | ||
| Median (25th, 75th centile) | 17.9 (7.7, 23.4) | 10.7 (3.8, 23.6) | 10.8 (3.6, 16.1) | 9.9 (4.1, 14.3) | ||
| Geometric mean | 13.7 | 10.3 | 9.3 | 9.9 | ||
| Intervention | ||||||
| n | 11 | 11 | 9 | 9 | ||
| Median (25th, 75th centile) | 12.4 (8.6, 16.6) | 6.6 (5.4, 16.8) | 9.3 (5.3, 23.4) | 7.4 (2.6, 16.5) | ||
| Geometric mean | 13.0 | 8.9 | 10.2 | 7.8 | ||
| Analysis | 0.89 (0.60 to 1.30) | 0.85 (0.56 to 1.29) | ||||
FIGURE 9.
Geometric mean EASI scores by group and FLG status. FLG +/+ denotes no mutations; FLG +/– denotes one FLG null mutation; and FLG –/– denotes two FLG null mutations.

Post hoc subgroup analysis for primary outcome according to baseline eczema severity
Eczema Area and Severity Index scores according to group and baseline eczema severity (almost clear or mild EASI scores and moderate or severe EASI scores)44 are shown in Table 16. There was no evidence that the clothing was more or less effective depending on the severity of eczema at baseline.
| Subgroup and allocated group | Baseline | 2 months | 4 months | 6 months | Adjusted subgroup-specific ratio of geometric means (95% CI) |
|---|---|---|---|---|---|
| Almost clear/mild baseline EASI scores (1 to 7) | |||||
| Standard care | |||||
| n | 73 | 66 | 65 | 68 | |
| Median (25th, 75th centile) | 4 (2.6, 5.5) | 2.9 (1.8, 4.8) | 2.7 (1.5, 4) | 2.3 (1.4, 4.3) | |
| Geometric mean | 4.8 | 4.2 | 3.7 | 3.5 | |
| Intervention | |||||
| n | 75 | 72 | 72 | 70 | 0.95 (0.81 to 1.12) |
| Median (25th, 75th centile) | 4.1 (2.8, 5.5) | 2.8 (1.6, 4.3) | 2.7 (1.6, 4.3) | 2.5 (1.2, 4.2) | |
| Geometric mean | 4.9 | 3.8 | 3.7 | 3.7 | |
| Moderate/severe baseline EASI scores (7.1–50) | |||||
| Standard care | |||||
| n | 78 | 71 | 68 | 71 | |
| Median (25th, 75th centile) | 12 (8.8, 18.8) | 9.2 (5.2, 15.8) | 9.4 (4.7, 16.4) | 7.7 (4.1, 12.3) | |
| Geometric mean | 14.2 | 10.0 | 9.5 | 8.2 | |
| Intervention | |||||
| n | 74 | 67 | 63 | 63 | 0.95 (0.81 to 1.12) |
| Median (25th, 75th centile) | 15.4 (10, 23.1) | 9.6 (5.6, 19.6) | 8 (4, 17.3) | 6.9 (3.9, 13.8) | |
| Geometric mean | 17.3 | 11.3 | 9.5 | 8.3 | |
Secondary outcomes
Global assessment of atopic eczema
The proportion of participants with a nurse-assessed IGA of AE of moderate severity or worse decreased in both groups during the follow-up period, but there was no difference between the two groups: relative risk 0.98 (95% CI 0.82 to 1.12; p = 0.63; Table 17).
| Outcome and allocated group | Baseline, n/N (%) | 2 months, n/N (%) | 4 months, n/N (%) | 6 months, n/N (%) | Adjusted risk difference (95% CI); p-value | Adjusted relative risk (95% CI); p-value |
|---|---|---|---|---|---|---|
| IGA | ||||||
| Standard care | 108/151 (72) | 72/137 (53) | 63/133 (47) | 56/139 (40) | –0.1% (–9.3% to 6.3%); 0.70 | 0.98 (0.82 to 1.12); 0.63 |
| Intervention | 108/149 (72) | 71/139 (51) | 60/136 (44) | 58/134 (43) | ||
| PGA | ||||||
| Standard care | 113/151 (75) | 82/137 (60) | 72/133 (54) | 60/139 (43) | –10.1% (–18.3% to –2.0%); 0.01 | 0.83 (0.70 to 0.98); 0.03 |
| Intervention | 98/149 (66) | 62/139 (45) | 56/135 (41) | 51/134 (38) | ||
In contrast, for the participant-rated IGA, fewer participants rated their AE as moderately severe or worse in the intervention group than standard care: relative risk 0.83 (95% CI 0.70 to 0.98; p = 0.03; see Table 17).
Self-reported atopic eczema symptoms using the Patient Oriented Eczema Measure
Mean weekly POEM scores by group are shown in Figure 10. The mean of the participants’ mean weekly POEM scores over the 6-month study was 2.8 points lower in the intervention group than in the standard care group (95% CI –3.9 to –1.8; p < 0.001; Table 18). There was a more obvious separation of the groups in the first 3 months of the trial than in the final 3 months.
FIGURE 10.
Mean weekly patient reported symptoms (POEM scores) with 95% CI. Baseline POEM scores (16.6 standard care; 17.3 intervention): data not shown on graph as scores were collected in clinic rather than by online questionnaire.

| POEM scores | Standard care (n = 147), mean (SD) | Intervention (n = 145), mean (SD) | Adjusted difference in means (95% CI); p-value |
|---|---|---|---|
| POEM score at baseline clinic visit | 16.6 (4.8) | 17.3 (5.8) | |
| Participant mean of weekly POEM score during the 6-month RCT | 14.2 (5.5) | 11.6 (5.6) | –2.8 (–3.9 to –1.8); < 0.001 |
Three-Item Severity scale
The mean TIS scores improved in both groups during the follow-up period. No between-group differences were observed: difference in means 0.09 (95% CI –0.22 to 0.40; p = 0.57; Table 19).
| Allocated group | Baseline | 2 months | 4 months | 6 months | Adjusted difference in means (95% CI); p-value |
|---|---|---|---|---|---|
| Standard care | |||||
| n | 151 | 137 | 133 | 139 | |
| Mean (SD) | 4.9 (1.8) | 4 (1.9) | 4.1 (2.2) | 3.7 (1.9) | |
| Intervention | |||||
| n | 149 | 139 | 136 | 134 | 0.09 (–0.22 to 0.40); 0.57 |
| Mean (SD) | 4.9 (1.8) | 4.1 (2) | 4.1 (2.1) | 3.7 (2) | |
Use of atopic eczema treatments
The percentage of days during the study that emollients, topical corticosteroids, calcineurin inhibitors and wet/dry wraps were used is shown in Table 20.
| Frequency of medication use | Standard care (n = 147), mean (SD) | Intervention (n = 145), mean (SD) | Adjusted difference in means (95% CI); p-value |
|---|---|---|---|
| Percentage of days topical steroids used | 44.1 (28.2) | 39.3 (27.8) | –3.7 (–9.6 to 2.3); 0.23 |
| Percentage of days emollients used | 88.4 (20.1) | 86.0 (22.1) | |
| Percentage of days calcineurin inhibitors used | 5.8 (15.9) | 5.7 (16.3) | |
| Percentage of days wet/dry wraps used | 5.2 (17.1) | 3.1 (12.5) |
The mean percentage of topical corticosteroid use was slightly less in the intervention group than the standard care group, equivalent to using topical corticosteroids on 6 days fewer over the 24 weeks (95% CI equivalent to using steroids for between 16 days fewer and 4 days more). The mean frequency of usage was similar in the two groups for the other topical treatments. Details of the amount of topical corticosteroid prescribed over the 6-month trial are summarised in Chapter 4, Table 30.
The potency of participants’ main topical corticosteroid was similar in the two groups at 6 months (Figure 11).
FIGURE 11.
Potency of main steroid at baseline and follow-up.
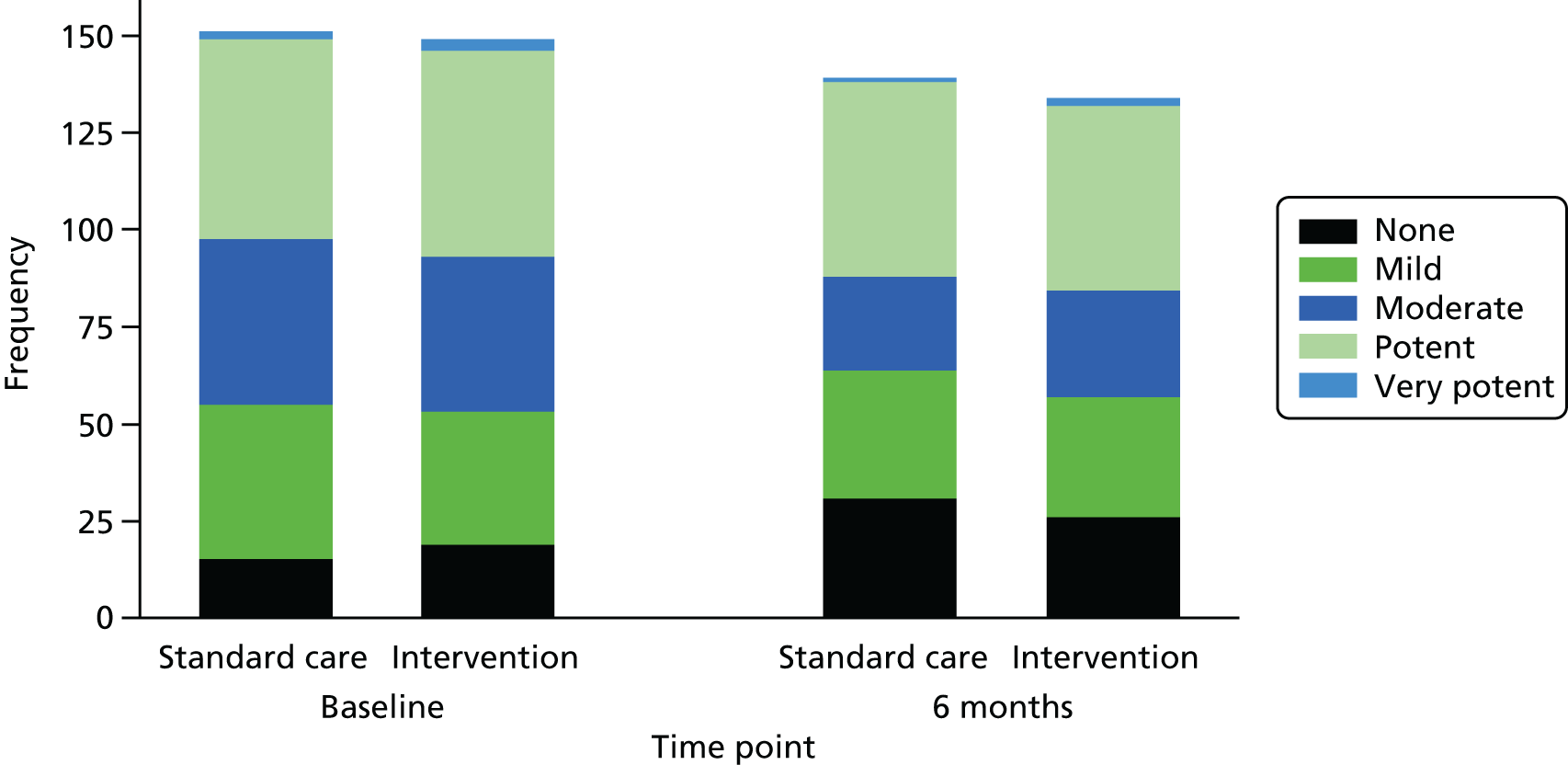
Changes in AE treatments during the trial are shown in Table 21. There were no differences between the groups in the percentage of participants who escalated their AE treatment between baseline and 6 months, although participants in the standard care group were more likely to have escalated treatment within the first 2 months of the study.
| Change in medication use | Standard care (N = 151), n (%) | Intervention (N = 149), n (%) | Adjusted risk difference (95% CI); p-value | Adjusted relative risk (95% CI); p-value |
|---|---|---|---|---|
| Between baseline and 2 months | ||||
| Treatment escalation | 34 (25) | 15 (11) | ||
| Neutral change | 18 (13) | 13 (9) | ||
| No change | 81 (59) | 105 (76) | ||
| Treatment reduction | 4 (3) | 6 (4) | ||
| n | 137 | 139 | ||
| Between 2 and 4 months | ||||
| Treatment escalation | 16 (12) | 16 (12) | ||
| Neutral change | 17 (13) | 8 (6) | ||
| No change | 96 (72) | 107 (79) | ||
| Treatment reduction | 4 (3) | 5 (4) | ||
| n | 133 | 136 | ||
| Between 4 and 6 months | ||||
| Treatment escalation | 16 (12) | 16 (12) | ||
| Neutral change | 10 (7) | 15 (11) | ||
| No change | 105 (76) | 93 (69) | ||
| Treatment reduction | 8 (6) | 10 (7) | ||
| n | 139 | 134 | ||
| Any treatment escalation between baseline and 6 monthsa | 50 (36) | 42 (30) | –5.3% (–16.3% to 5.7%); 0.34 | 0.87 (0.62 to 1.22); 0.43 |
Health-related quality of life
Health-related quality-of-life outcomes for the DFI, EQ-5D-3L, ADQoL and CHU-9D are shown in Table 22. There were no differences between any of these quality-of-life outcomes between the two groups. The difference in means were all close to 0 and favoured the intervention group for DFI, EQ-5D-3L and ADQoL, and favoured the standard care group for CHU-9D.
| Quality-of-life outcome and allocated group | Baseline | 6 months | Adjusted difference in means (95% CI); p-value |
|---|---|---|---|
| DFI | |||
| Standard care | –0.8 (–2.1 to 0.4); 0.18 | ||
| n | 151 | 138 | |
| Mean (SD) | 12.0 (6.3) | 8.6 (6.8) | |
| Intervention | |||
| n | 149 | 133 | |
| Mean (SD) | 12.4 (6.6) | 7.6 (6.1) | |
| ADQoL | |||
| Standard care | 0.0260 (–0.0018 to 0.0539); 0.07 | ||
| n | 151 | 139 | |
| Mean (SD) | 0.6952 (0.1300) | 0.7292 (0.1308) | |
| Intervention | |||
| n | 149 | 134 | |
| Mean (SD) | 0.6883 (0.1409) | 0.7515 (0.1273) | |
| CHU-9D (aged ≥ 5 years only) | |||
| Standard care | –0.0243 (–0.0584 to 0.0098); 0.16 | ||
| n | 64 | 67 | |
| Mean (SD) | 0.8292 (0.1263) | 0.8828 (0.1059) | |
| Intervention | |||
| n | 70 | 65 | |
| Mean (SD) | 0.8386 (0.1115) | 0.8677 (0.1114) | |
| EQ-5D-3L index for parents health-related quality of life | |||
| Standard care | 0.0115 (–0.0185 to 0.0415); 0.45 | ||
| n | 151 | 138 | |
| Mean (SD) | 0.8983 (0.1612) | 0.9107 (0.1529) | |
| Intervention | |||
| n | 147 | 134 | |
| Mean (SD) | 0.9018 (0.1710) | 0.9184 (0.1564) | |
Safety outcomes
The number of participants reporting a skin infection was similar in the two groups (Table 23).
| Safety outcomes | Standard care (n = 141) | Intervention (n = 142) | Adjusted relative risk (95% CI); p-value |
|---|---|---|---|
| Any skin infection during 6-month RCT, n (%)a,b | 39 (28) | 36 (25) | 0.89 (0.54 to 1.47); 0.66 |
| Number of skin infections per participant | |||
| Median (25th, 75th centile) | 1 (1, 2) | 1 (1, 2) | |
| Min., max. | 1, 5 | 1, 8 | |
| n | 39 | 36 | |
| Number of inpatient stays per participant because of AE, n (%)a,c | |||
| 0 | 139 (99) | 138 (97) | |
| 1 | 1 (1) | 2 (1) | |
| 2 | 1 (1) | 2 (1) | |
| ≥ 3 | 0 | 0 | |
| Total number of nights in hospital because of AE | |||
| Mean (SD) | 2.5 (2.1) | 2.8 (1.7) | |
| Median (25th, 75th centile) | 2.5 (1, 4) | 2.5 (1.5, 4) | |
| Min., max. | 1, 4 | 1, 5 | |
| n | 2 | 4 | |
Four participants in the intervention group and two in the standard care group had a hospital inpatient stay because of AE. The two hospital inpatient stays required by one participant in the intervention group were classified as potentially related to trial treatment by the medical monitor.
Open follow-up period
The questionnaire at 8 months was completed by 111 participants (74%) in the standard care group and 116 participants (78%) in the intervention group.
The frequency with which the clothing was worn during the open follow-up period (when all participants received the garments) is shown in Table 24. Just under half of the responders reported that the garments were worn for all or most of the time for the days and/or nights between 6 and 8 months.
| Qualitative feedback | Standard care (N = 111), n (%) | Intervention (N = 116), n (%) | Total (N = 227), n (%) |
|---|---|---|---|
| Frequency clothing worn during the follow-up period (6–8 months) | |||
| Never | 8 (7) | 17 (15) | 25 (11) |
| Rarely | 20 (18) | 18 (16) | 38 (17) |
| Some of the time | 26 (23) | 27 (23) | 53 (23) |
| All/most of the time (days only) | 3 (3) | 2 (2) | 5 (2) |
| All/most of the time (nights only) | 42 (38) | 25 (22) | 67 (30) |
| All/most of the time (days and nights) | 7 (6) | 24 (21) | 31 (14) |
| Not answered | 5 (5) | 3 (3) | 8 (4) |
| Feel that AE improved because of trial clothing | |||
| Yesa | 25 (23) | 57 (49) | 82 (36) |
| No | 28 (25) | 27 (23) | 55 (24) |
| Not sure | 49 (44) | 28 (24) | 77 (34) |
| Not answered | 9 (8) | 4 (3) | 13 (6) |
| Would ask GP to prescribe clothing | |||
| Yesb | 48 (43) | 61 (53) | 109 (48) |
| No | 32 (29) | 31 (27) | 63 (28) |
| Not sure | 22 (20) | 20 (17) | 42 (19) |
| Not answered | 9 (8) | 4 (3) | 13 (6) |
| Have asked GP to prescribe clothing | |||
| Yes | 5 (5) | 9 (8) | 14 (6) |
| No | 94 (85) | 103 (89) | 197 (87) |
| Not answered | 12 (11) | 4 (3) | 16 (7) |
| GP prescribed clothing | |||
| Yes | 3 | 5 | 8 |
| No | 2 | 4 | 6 |
| Reason GP did not prescribe clothingc | |||
| Too expensive | 1 | 4 | 5 |
| No evidence of efficacy | 1 | 4 | 5 |
| Not available in postcode | 1 | – | 1 |
| Bought silk clothing for AE during the study | |||
| Yes | 3 | 3 | 6 |
Overall, 135 out of 227 participants (59%, 95% CI 53% to 66%) were satisfied or very satisfied with the clothing at 8 months, and 139 out of 227 participants (61%, 95% CI 54% to 68%) were happy or very happy to wear the clothing.
Opinions of trial clothing at 8 months are shown in Table 24. Just over one-third of respondents thought that their/their child’s AE had improved as a result of wearing the trial garments, with a similar proportion responding that they were not sure. Just under half of respondents would ask their general practitioner (GP) to prescribe the garments. Only 14 responders had asked their GP to prescribe the clothing and six responders reported purchasing silk clothing during the trial.
Change in POEM scores and topical treatment usage between 6 and 8 months is shown in Table 25 for the standard care group and in Table 26 for the intervention group. POEM scores decreased slightly in the standard care group (who had been sent the silk garments at 6 months) between 6 and 8 months. POEM scores were similar at 8 months to the scores at 6 months for the intervention group. Topical treatment usage at 8 months was similar to that at 6 months in both groups.
| Outcomes | 6 months | 8 months (N = 111) | Change from 6 months (N = 105) | 95% CI | |
|---|---|---|---|---|---|
| All participants (N = 121) | Completed questionnaire at 8 months (N = 105) | ||||
| POEM | |||||
| Mean (SD) | 13.2 (6.7) | 13.2 (6.5) | 11.8 (7.4) | –1.6 (6.5) | –2.8 to –0.4 |
| n | 120 | 104 | 111 | 104 | |
| Number of days emollients used in previous week | |||||
| Mean (SD) | 6 (1.9) | 5.9 (1.9) | 6 (2) | 0 (1.7) | –0.4 to 0.3 |
| n | 119 | 104 | 111 | 104 | |
| Number of days topical corticosteroids used in previous week | |||||
| Mean (SD) | 2.9 (2.6) | 2.9 (2.6) | 3 (2.6) | 0 (2.1) | –0.4 to 0.4 |
| n | 118 | 103 | 110 | 103 | |
| Topical calcineurin inhibitors used in previous week, n/N (%) | 10/117 (9) | 8/103 (8) | 7/109 (6) | ||
| Topical wet/dry wraps used in previous week, n/N (%) | 10/117 (9) | 9/102 (9) | 8/108 (7) | ||
| Outcomes | 6 months | 8 months (N = 116) | Change from 6 months (N = 112) | 95% CI | |
|---|---|---|---|---|---|
| All participants (N = 121) | Completed questionnaire at 8 months (N = 112) | ||||
| POEM | |||||
| Mean (SD) | 11.3 (7.2) | 11.5 (7.2) | 11.1 (6.8) | –0.4 (4.6) | –1.2 to 0.5 |
| n | 121 | 112 | 114 | 110 | |
| Number of days emollients used in previous week | |||||
| Mean (SD) | 6.2 (1.9) | 6.3 (1.6) | 6.2 (1.8) | 0 (1.7) | –0.4 to 0.3 |
| n | 120 | 111 | 111 | 106 | |
| Number of days topical corticosteroids used in previous week | |||||
| Mean (SD) | 3 (2.5) | 3 (2.6) | 2.8 (2.4) | –0.2 (2.4) | –0.7 to 0.2 |
| n | 120 | 111 | 112 | 107 | |
| Topical calcineurin inhibitors used in previous week, n/N (%) | 14/119 (12) | 13/110 (12) | 12/108 (11) | ||
| Topical wet/dry wraps used in previous week, n/N (%) | 7/119 (6) | 7/110 (6) | 8/108 (7) | ||
Tertiary outcomes
Brand of garments
Figure 12 shows the numbers of participants randomised to the two brands of clothing. Garments were required during a DreamSkin out-of-stock period for 19 participants randomised to the intervention group (eight randomised to DermaSilk and 11 to DreamSkin) and 26 participants at 6 months who were allocated to the standard care group (nine randomised to DermaSilk and 17 to DreamSkin). These participants are not included in the analysis according to brand of clothing.
FIGURE 12.
Randomisation to brands of garments. a, Four participants in the intervention group randomised to DreamSkin initially received DreamSkin garments but were issued DermaSilk replacements as the required size of DreamSkin clothing was out of stock when the replacements were required; b, 12 participants in the standard care group were not sent any clothing at 6 months because they did not attend the 6-month visit; however, two of these participants completed the questionnaire at 8 months; c, clothing supply at 6 months was delayed for five of these participants so that they could receive DreamSkin garments.
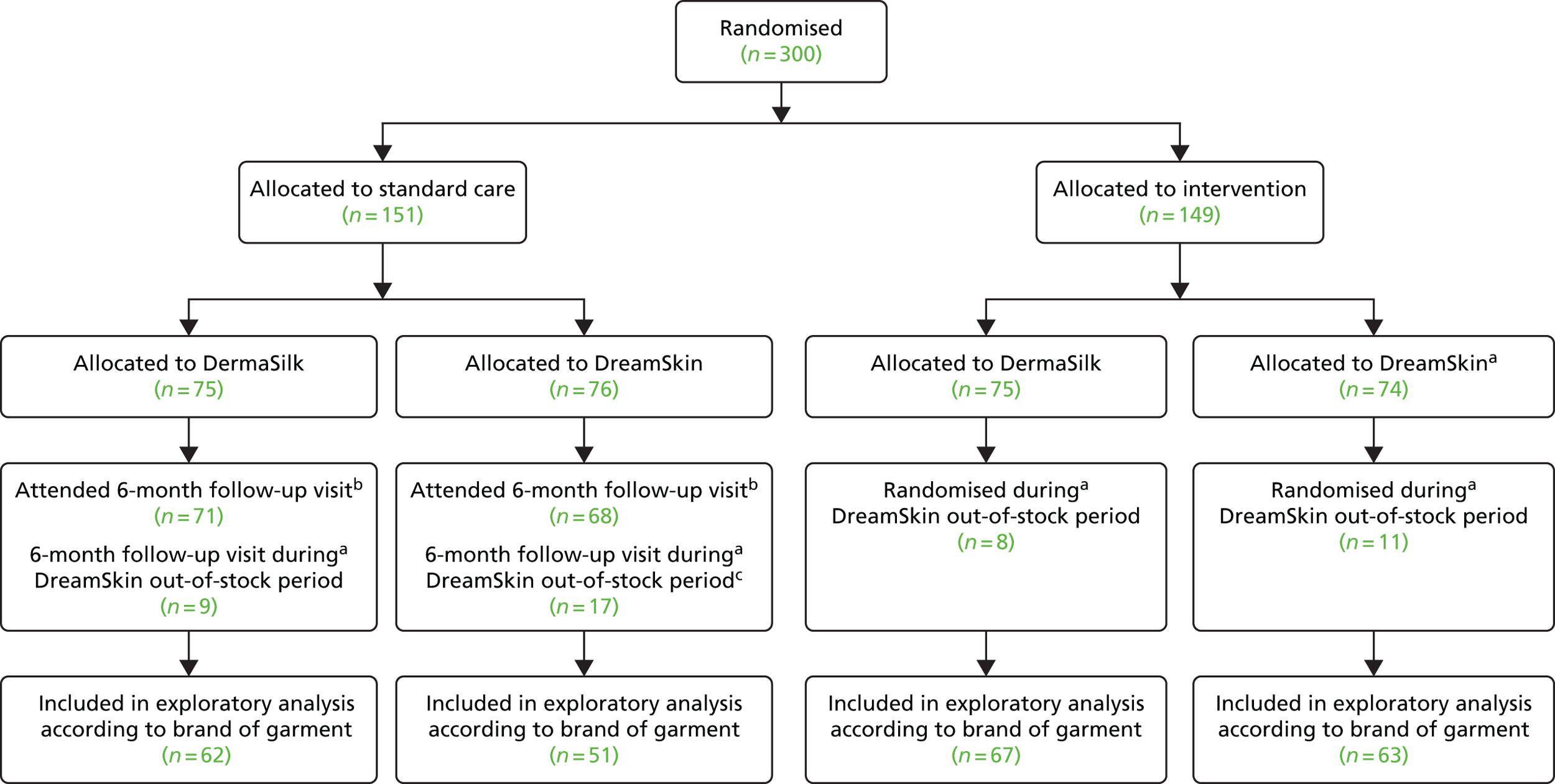
The EASI scores during the 6-month RCT were similar according to garment brand (Figure 13). There was no differential effect of the garments according to brand (ratio of geometric means for DermaSilk vs. DreamSkin was 0.98, 95% CI 0.83 to 1.16). Adherence, satisfaction and the child being happy to wear the clothing were all similar at 6 months regardless of brand (Table 27). Further comparisons of the brands using data from the 8-month questionnaires can be found in Appendix 18.
FIGURE 13.
Geometric mean EASI scores by brand of garment.
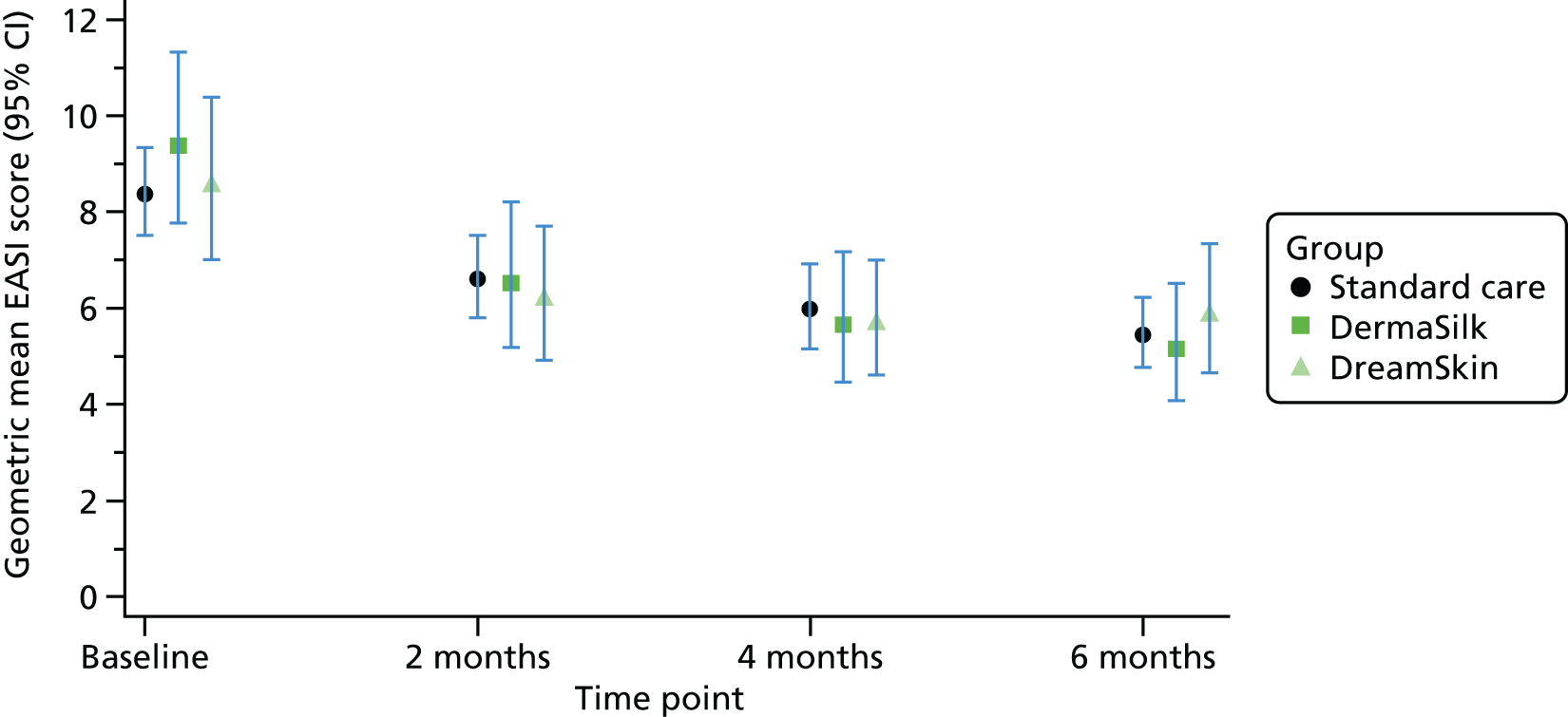
| Feedback on clothing | DermaSilk | DreamSkin |
|---|---|---|
| Adherencea | ||
| ≥ 12 weekly questionnaires completed on clothing wear (n) | 56 | 54 |
| Percentage of nights that clothing was worn for at least some of the night, median (25th, 75th centile) | 80.7 (60.8, 93.9) | 73.4 (50.7, 95.7) |
| Percentage of days that clothing was worn for at least some of the day, median (25th, 75th centile) | 29.8 (8.7, 74.8) | 31.6 (6.8, 70.2) |
| Adherence to wearing trial clothing, n (%) | ||
| Not adherent | 9 (16) | 12 (22) |
| Adherentb | 47 (84) | 42 (78) |
| Worn for at least 50% of days and nights | 21 (38) | 18 (33) |
| Worn for at least 50% of days only | – | – |
| Worn for at least 50% of nights only | 26 (46) | 24 (44) |
| Acceptabilityc | ||
| 6-month questionnaire completed (n) | 54 | 52 |
| Satisfied/very satisfied with the clothing overall, n (%) | 41 (76) | 32 (62) |
| Child happy/very happy to wear clothing, n (%) | 43 (80) | 35 (67) |
Chapter 4 Health economic evaluation
Introduction
Childhood eczema has been shown to have an impact on health-related quality of life similar to that of other common childhood conditions, such as asthma and diabetes. 3 Eczema also has a substantial cost impact on society and the individual families affected; for example, the total annual UK cost of eczema in children aged ≤ 5 years is estimated to be £70.6M (or £120.19 per child) (inflated from 1996 to 2015 price year),45 of which 64% was accounted for by NHS health-care costs. 46 A further UK study looking at patients of all ages estimated the total annual cost of eczema for the UK to be in the order of £726.7M, of which £195.3M was incurred by the NHS, £464.1M by the patients and £67.2M by society in terms of lost working days (inflated from 1995 to 2015 price year;45 original price year not reported but most likely to be 1995). 47
Core eczema treatment involves the regular use of emollients and topical corticosteroid creams. Children with more severe eczema may also need to occasionally use topical antibiotics, oral antibiotics, wet wraps, oral antihistamines, systemic immunosuppressive agents (such as ciclosporin or methotrexate) and special dietary products. 5
Silk therapeutic garments have been available as a prescription on the NHS since 2008. In that year the net cost of silk garments in the UK was £168.779 for 5507 items. 48–51 Since this time, the cost of prescribing silk garments has risen to ≥ £2M for 81,797 prescription items (for all indications) (Figure 14). Silk garments are also available for private purchase by any individual who has the means and willingness to pay for them.
FIGURE 14.
Net cost of silk garments prescribed in the UK (2008–15).
At the time this research was commissioned, there were only two companies supplying silk garments to the UK NHS (DermaSilk and DreamSkin). In 2012, a third company (SkinniesTM, Dermacea Ltd, Stourbridge, UK) had products prescribed via the NHS. In 2015, the net ingredient cost (per quantity) of a set of silk garments to the NHS varied from £66.02 for a DreamSkin bodysuit and leggings for a child aged 12–18 months to £155.47 for a DermaSilk top and bottom set (size: adult, small). As the qualitative work (see Chapter 5) undertaken alongside the trial reveals, commissioners thought that the garments were expensive and were uncertain about how many garments would need to be prescribed owing to a lack of knowledge about the quality and lifespan of the garments. For such items to be cost-effective for the NHS, they need to have clinical benefit that is either sufficiently large to justify the cost of the garments [i.e. have an incremental cost-effectiveness ratio (ICER) that is below the cost-effectiveness threshold] and/or lead to cost savings to the NHS via potential reduced consultations with health professionals or reduced prescriptions of other medications for eczema.
Prior to this trial, there was no scientific evidence about the cost-effectiveness of silk garments for the treatment of moderate to severe eczema. As a result, the national health-care system in the UK has been prescribing something for which there is not a strong evidence base demonstrating either clinical effectiveness or cost-effectiveness. One of the aims of the economic component of this trial was to assess if silk garments for eczema represent value for money for the NHS and, thus, whether or not the NHS should be funding this intervention.
This chapter presents the economic evaluation, which was conducted alongside the trial as planned in the original research in order to estimate the mean incremental cost and mean quality-adjusted life-year (QALY) change (per patient) with silk garments and standard care, compared with standard care without silk garments.
Methods
Aims and perspective
The aim of the economic evaluation was to estimate the within-trial cost-effectiveness of silk therapeutic clothing plus standard care compared with standard care alone from a NHS perspective in the base case, and from a NHS and wider (family and employer) perspective in secondary analyses. Personal Social Services costs were not explicitly asked about, as the clinical team felt that these were unlikely to be relevant to those with childhood eczema.
Two forms of economic evaluation were used, cost–utility analysis and cost-effectiveness analysis, in order to enable comparisons with non-eczema interventions and other eczema interventions, respectively.
The economic evaluation adhered to published and well-accepted guidelines for the economic evaluation of health-care interventions, as appropriate. 52–54
Resource use: identification, measurement and valuation
The range of resource use and costs captured was in keeping with the chosen perspective. Three categories of resource use were identified as important to capture. These were intervention resource use, other health resource use and wider family/employer costs. All resources were costed at 2014/15 price year levels in UK pounds sterling.
Intervention resource use (silk garments)
Intervention resource use was measured during the trial using an inventory that recorded number, type and size of garments issued to and returned by participants. At baseline each participant was issued with the equivalent of three sets of pyjamas (tops and leggings) or leggings and a bodysuit for younger children.
Garments were removed from their original packaging and repackaged in trial packaging. The inventory in some cases recorded tops and bottoms as individual items when in reality we expect that they had actually come from a single pyjama set, which would be cheaper than the two items separately. In valuing the resources, the base case thereby assumed that all those issued between the ages of 3 and 12 years (the age range within which a pyjama set is available) were from a pyjama set as this is what is most likely to be prescribed in routine care.
Some garments were returned to be exchanged for a different size. Where one of these sets had been tried on for size, these could not re-enter stock and had to be disposed of (as would be the case if the NHS prescribed them and they did not fit); these items were included in the cost. However, if items were returned unused such that they could be sent to another participant, this was recorded and these items were not included in the cost, to avoid double counting. Some participants needed replacement garments during the 6-month trial period because of a child’s growth or wear and tear. The issuing of such garments was recorded in the inventory and included in the intervention cost. In valuing the silk garments, the cost was not annuitised to take account of a lifespan longer than 6 months as data collected within the trial suggest that a lifespan of 6 months for the garments is a reasonable assumption to make.
In the base case, the silk garments were valued using the net ingredient cost per quantity from the Prescription Cost Analysis 2015 published by the Health and Social Care information Centre. 55 However, this is not the only method available for costing prescriptions. In the sensitivity analysis, an alternative approach based on the NHS Business Services Authority actual cost formula (obtained from the NHS Business Services Authority website) was used. 56 The actual cost is based on the net ingredient cost minus the national average discount percentage (where the average discount was 7.43% based on March 2015 data) plus payment for consumables, containers and a professional fee (although in the case of silk garments, payments for consumables and containers are not incurred). 57
In the trial the silk garments were posted to participants, but this does not reflect how the garments are distributed in the NHS, where patients would collect their prescription from a pharmacy. As a result we did not include the cost of posting silk garments in the trial.
Other health resource use
Health resource use beyond the intervention cost was recorded by the participant or a parent on the weekly diary card and recorded by the research nurse at each of the study visits (baseline, 2, 4 and 6 months). To aid memory, an online questionnaire prompted participants to complete their diary if a health-care professional was visited, or a prescription issued, for eczema in the last week. Participants were asked to record only those resources consumed as a result of the child’s eczema; all items reported by parents were included. This included health-care visits to primary care professionals (number of appointments to GP and practice nurse), secondary care visits (number of outpatient visits, nights in inpatient care, and accident and emergency) and prescriptions [including topical corticosteroids, topical calcineurin inhibitors, emollients (including bath emollients), wet/dry wraps, antibiotics/antivirals for skin infections and other eczema-related prescriptions].
These resource items were valued using published national sources of unit costs in UK pounds sterling for the 2014/15 price year. 55,58,59 The individual items of resource use and their unit cost can be seen in Table 28.
| Resource item | Unit cost (£) | Source |
|---|---|---|
| Intervention: silk therapeutic garments | ||
| Base case: prescription cost analysis approach (per set) | 66.02–155.49 | HSCIC55 |
| Sensitivity analysis: tariff approach (per set) | 62.83–145.02 | NHSBSA57 |
| Primary health care | ||
| GP (per surgery consultation) | 37.00 | PSSRU58 |
| GP (per telephone consultation) | 22.00 | PSSRU58 |
| GP (per consultation out of hours) | 68.91 | PSSRU58 |
| Practice nurse (per consultation) | 12.14 | PSSRU58 |
| Community eczema nurse (per consultation) | 38.00 | PSSRU58 |
| Community nurse (per consultation) | 38.00 | PSSRU58 |
| Pharmacist (per contact) | 14.67 | PSSRU58 |
| Health visitor (per contact) | 54.00 | PSSRU58 |
| Nutritionist (per telephone contact) | 82.66 | DH59 |
| Homeopathic visit | 57.00 | a |
| Blood test (per test) | 9.07 | DH59 |
| Influenza vaccination (per vaccine and nurse time) | 30.14 | HSCIC55/PSSRU58 |
| Secondary health care | ||
| A&E (per visit) | 93.00 | PSSRU58 |
| Outpatient first visit (dermatology, per consultation) | 128.40 | DH59 |
| Consultant eczema nurse (per consultation) | 161.03 | DH59 |
| Eczema nurse (per telephone contact) | 42.57 | DH59 |
| Paediatric assessment | 299.51 | DH59 |
| Inpatient stay for skin disorder without intervention (one night) | 1185.48 | DH59 |
| Inpatient stay for skin disorder without intervention (three nights) | 1756.10 | DH59 |
| Inpatient stay for skin disorder without intervention (four nights) | 2267.63 | DH59 |
| Patch test (per test) | 91.81 | DH59 |
| Medications | ||
| Various | Various | HSCIC55 |
| Wider family/employer costs | ||
| Gross mean hourly wage for all employee jobs in UK | 15.27 | Annual Survey of Hours and Earnings 60 |
| Out-of-pocket costs | Various | As reported by parents |
Primary care resource use items were valued using the published Personal Social Services Research Unit health and social care unit costs. 58 The unit cost for GPs assumed that the per patient contact lasted 12 minutes for a face-to-face appointment and 7 minutes for a telephone consultation, including direct care staff cost and qualification cost. The practice nurse visit assumed a face-to-face contact lasted 16 minutes. NHS homeopathic appointments were assumed to have taken place in primary care. Consultations with a pharmacist were assumed to have lasted 20 minutes and the unit cost of a community pharmacist was assumed to have been the same as that of a hospital pharmacist. The cost of a nutritionist or dietitian appointment was taken from the NHS reference costs59 for community health services dietitian.
Unit costs for secondary care resource use items were largely sourced from the NHS reference costs for 2015. 59 Short inpatient stays (one night) were assumed to be for skin disorders without interventions, with a Complications and Comorbidities (CC) score of 0–1; medium stays (three nights) were assumed to be for skin disorders without interventions, with a CC score of 2–5; and longer stays (four nights) were assumed to be for skin disorders without interventions, with a CC score of 6–9. Patch tests were assumed to be standard patch tests for children aged ≤ 12 years. Accident and emergency visits were assumed to be non-admitted, with category 2 investigation and category 3 treatment. The unit cost per consultation with a consultant eczema nurse was assumed to be the same as for ‘other specialist nursing, child, face to face’ (currency code N29CF) under community health services. 59
Wider family/employer costs
The resource items recorded in this category reflect a more societal perspective. They include the additional out-of-pocket costs incurred by the family and productivity costs to employers of time taken off work as a result of parents’ caring for their child with eczema.
Out-of-pocket costs incurred by the family
In our previous NIHR HTA-funded eczema trial,61 only 33% of families taking part in the trial reported any out-of-pocket costs incurred as a result of their child’s eczema. The rate of reporting seemed low and, therefore, to try and improve reporting of such costs, in this trial we undertook to further develop the question eliciting this information. We informed this development by using the out-of-pocket data we did manage to collect in the Softened Water Eczema Trial (SWET). We categorised the types of out-of-pocket costs reported and developed a table of examples to help families understand the types of things that may be relevant. The range of items included over-the-counter purchases, special clothing, laundry and bedding, special foods, equipment and travel costs for appointments.
The information provided and the wording of the question eliciting this information can be seen in Appendix 10. Respondents were asked to place a monetary value on the additional cost incurred as a result of eczema. For instance, if they bought a more expensive washing detergent because it was ‘skin kind’, they were asked to state the amount over and above that which they would have paid for a normal washing detergent.
Productivity costs
In addition, families were asked to record time off work and school as a result of eczema. Parents’ time off work was valued using the mean gross hourly wage rate for all employee jobs in the UK, as reported in the Annual Survey of Hours and Earnings in 2015, because we did not ask respondents to report their personal earnings. 60 This approach is known as the ‘human capital approach’ and assumes that a person’s productivity is equal to their wage rate to place a maximum cost on their time off work. This is not the only available approach to costing lost productivity and research has shown that different approaches can lead to different estimates that may impact on the conclusion reached about cost-effectiveness;62 we therefore also report the actual time lost. Time taken off school as a result of eczema is reported in hours and minutes and is not valued in monetary terms because of the lack of evidence about the cost of lost schooling.
Measurement of outcomes and quality-adjusted life-years
The main economic analysis is the cost–utility analysis, whereby effectiveness is measured in terms of QALYs for the child. This will be presented as the base case because it enables decision-makers to compare the value for money afforded by this intervention with that for other conditions. It also enables us to clearly differentiate the results of the cost–utility analysis based on the ADQoL from the other cost–utility analyses based on the CHU-9D and the parental EQ-5D-3L.
In the base-case analysis, utility was measured in all children using the disease-specific ADQoL. 38 The ADQoL consists of four binary choice questions,38 in answer to which respondents were asked to indicate whether they agreed more with the statement on the left or on the right:
-
you cannot join in some activities with other children/you are not limited in joining in activities with other children
-
you are very moody/you are not very moody
-
you cannot be comforted/you are quite settled
-
you sleep badly most nights/generally, you sleep very well.
The ADQoL was completed by parental proxy for children aged < 7 years and by self-report in those aged ≥ 7 years. The developer of the ADQoL used standard gamble methods to estimate utility values for the 16 health states described by the instrument. This involved asking adult participants to imagine that they were 10 years old and would be in the health state described for the rest of their life. The utility values generated ranged from 0.356 to 0.841. 38
The primary measure of effectiveness for the cost-effectiveness analysis was the difference in the proportions achieving treatment success at 6 months – defined as those with at least a 50% improvement compared with baseline on the primary outcome measure, EASI. 31 Secondary analysis was conducted using continuous data from the DFI, where scores range from 0 (no impact on family life, best score) to 30 (maximum impact on family score, worst score). 36
In addition, the main carer was asked to record their own utility using the EQ-5D-3L in order to see if the intervention impacted on parental quality of life (e.g. through sleep loss). The EQ-5D-3L is a generic preference-based health-related quality-of-life instrument with five dimensions, each of which has three levels. 37 The UK tariff was valued using time trade-off methods with a sample of the general adult population. Utility values on the EQ-5D-3L range from –0.594 to 1. 63 As there is no guidance to the best method for analysing adult utility data in combination with data on the child’s utility,64 we present a cost per QALY for the child as the base case and a cost per QALY per carer in secondary analyses, such that we do not combine the two sets of utility in a single analysis. It should be noted that the cost per QALY analysis for the main carer excludes any QALYs gained by the child with eczema and also probably underestimates the impact on the wider family in cases where a child with eczema lives with more than one adult, and in cases where they have siblings.
All utility instruments were measured at baseline and 6 months, and used to estimate QALYs for the trial period by using linear interpolation and area under the curve with and without baseline adjustment,65 and adjustment by centre and age. The total area under the curve (without baseline adjustment) was measured as:
to reflect the 6-month time frame. The primary cost–utility analysis reports the incremental cost per QALY based on the ADQoL because children of all ages, or their main carer, were asked to complete this instrument. Secondary analyses will report the cost per QALY based on the main carer’s EQ-5D-3L values separately. Previous work has not explored the ability of the EQ-5D-3L to detect impacts on carers’ quality of life for this condition.
Statistical analysis and analysis of uncertainty
Neither costs nor benefits were discounted,53 reflecting the 6-month time horizon of the analysis. A complete-case analysis approach was undertaken, with participants included only if they had complete cost and effect data at each time point.
In line with the statistical analyses, the primary analysis was conducted using the principles of intention to treat: all participants with data at baseline and follow-up were included, regardless of adherence to the allocated intervention. If > 10% of participants had missing data, then imputation of missing values was to be conducted as a sensitivity analysis.
The economic evaluation is a ‘within-trial analysis’. This means that costs and benefits were only evaluated for the trial follow-up period (6 months). Costs and outcomes in both arms of the study were estimated using the methods described in Measurement of outcomes and quality-adjusted life-years. This information on costs and benefits was used to conduct a complete-case incremental economic analysis comparing the silk garments in addition to standard care with standard care alone. This was completed for both the cost-effectiveness and cost–utility analyses. Conclusions are based on the estimated results. ICERs were calculated using accepted methodology. 52,53
The statistical analysis estimated both the unadjusted and adjusted estimates, where the latter controlled for any differences in baseline characteristics (i.e. the cost regression adjusted for baseline costs, age and recruiting centre, whereas the QALY regression adjusted for baseline utility, age and recruiting centre). All adjusted analyses used a regression-based approach (seemingly unrelated regression equations)66 to estimate incremental costs and QALYs with the exception of the cost-effectiveness analysis for EASI. As EASI was analysed as a binary variable (coded 1 for treatment success and 0 otherwise), generalised linear models, assuming costs and effects were independent, were employed. The cost regression used the Poisson family and identify link function, whereas the effects regression used the binomial family and identify link function.
As cost data were skewed, we used non-parametric bootstrapping to estimate adjusted mean (95% CI) incremental cost and mean (95% CI) incremental QALY estimates. Bootstrapping was also used to estimate cost-effectiveness acceptability curves;67,68 these show the probability that each of the intervention groups is the most cost-effective option at different monetary valuations of the outcome variable. A range of ceiling ratio (or willingness to pay per QALY) values were tested, including the £20,000 and £30,000 per QALY thresholds used by NICE in cost–utility calculations. 69
Sensitivity analysis tests the robustness of results in the face of any uncertainties. 53 It also improves the generalisability of results by indicating what could happen with different values of a parameter. Two areas of uncertainty were considered worth exploring. The first was the cost of the silk therapeutic garments, because 46% of total costs from a NHS perspective were accounted for by the cost of the silk garments in the study. Instead of using the Health and Social Care Information Centre Prescription Cost Analysis net ingredient cost per item, we reran the analysis using unit costs for silk garments based on the NHS Business Services Authority actual cost formula to estimate the actual cost to the NHS:56
In particular, this approach takes account of the average discount enjoyed by the NHS when purchasing prescription items. As payment for consumables and out-of-pocket expenses is not relevant for silk garments, we did not include these but did include the 90p fee pharmacists receive for dispensing. We did not do this for all prescription items as medication costs were not significantly different between the two treatment arms.
Second, as there is uncertainty about how best to capture utility in children, we included a second generic preference-based instrument, the CHU-9D,39 at baseline and 6 months in order to compare the results with those gained using the disease-specific ADQoL. 38 The generic health-related quality-of-life instrument, CHU-9D,39 was used only in children aged ≥ 5 years at baseline. The CHU-9D consists of nine dimensions (worry, sadness, pain, tired, annoyed, school work/homework, sleep, daily routine, ability to join in activities) using a recall period of today or last night, dependent on the question; each dimension has five levels. 39 The wording of the dimensions and levels resulted from qualitative work with children and young people. It can be self-completed by children and young people aged 7–17 years with a proxy version for parents to complete for children aged < 7 years. At the time the CLOTHES trial was designed, the CHU-9D had not been used with the under-fives and so we did not use it with those aged < 5 years. Additional guidance now exists to help parental proxies complete the CHU-9D for this age range (personal communication with K Stevens, University of Sheffield, 2014). The CHU-9D was valued by the UK general adult population using standard gamble methods and utility using this instrument can range between 0.33 and 1. 39 In this study, the CHU-9D was self-completed by children and young people aged ≥ 7 years and parental proxy completed for 5- and 6-year-olds. QALYs for the trial period based on the CHU-9D were estimated using the same methods described for estimating QALYs based on the ADQoL and main carer EQ-5D-3L (see Measurement of outcomes and quality-adjusted life-years).
Some of the subgroup and sensitivity analyses included in the original health economic analysis plan (see Appendix 19) were not conducted for the following reasons:
-
imputation of missing values, as missing values were < 10%
-
per-protocol analysis adjusting for adherence; sensitivity analyses in Chapter 3, Causal effect of adherence with wearing trial garments on primary outcome showed no evidence of a causal effect of adherence with wearing trial garments on the primary outcome.
-
subgroup analysis based on impairment in skin barrier function (FLG genotype), as subgroup analysis of the primary outcome suggested no differential effect
-
resource use data collected in the observational period (6–8 months) were not included, as there was no overall effect at 6 months.
All statistical analysis was undertaken in Stata 14.
Results
The base-case cost–utility analysis included all participants with complete resource use and ADQoL data at baseline and 6 months (n = 273: 134 in the silk garment plus standard care arm and 139 in standard care alone arm). Of the total 300 study participants, 27 (9%) were not included in the base-case economic evaluation because they had discontinued the study or had data missing. In the base case, 58.2% of participants were male and 80.2% were white, and the average age was 5 years.
Baseline health-care costs for eczema over the preceding 4 weeks were £35.60 (SD £69.46) per participant in the silk garment arm and £34.82 (SD £69.14) in the standard care arm (mean difference £0.78, 95% CI –£15.74 to £17.30).
Baseline utility of the child participants (as measured using the ADQoL) was a mean of 0.6879 (SD 0.1418) per participant in the silk garment arm and 0.6959 (0.1288) per participant in the standard care arm (mean difference –0.0081, 95% CI –0.0404 to 0.0241) (Table 29).
| Outcome | Intervention (n = 134), mean (SD) | Standard care (n = 139), mean (SD) | Mean difference (95% CI)a |
|---|---|---|---|
| Health outcomes | |||
| Utility (ADQoL) | |||
| Baseline | 0.6879 (0.1418) | 0.6959 (0.1288) | –0.0081 (–0.0404 to 0.0241) |
| 6 months | 0.7515 (0.1273) | 0.7292 (0.1308) | 0.0224 (–0.0084 to 0.0531) |
| QALYs | |||
| > 6 months | 0.3598 (0.0561) | 0.3563 (0.0562) | 0.0036 (–0.0098 to 0.0169); 0.0064 (–0.0004 to 0.0133) |
| Costs | |||
| Garments | 318.52 (136.60) | 0.00 (0.00) | 318.52 (295.71 to 341.33) |
| Primary care visits | 36.52 (57.74) | 47.01 (73.71) | –10.49 (–26.30 to 5.33) |
| Secondary care visits | 213.09 (604.47) | 153.00 (327.13) | 60.09 (–55.16 to 175.34) |
| Prescriptions | 119.82 (244.67) | 120.86 (243.81) | –1.04 (–105.92 to 203.05) |
| Total health-care costs (excluding garments) | 369.43 (805.88) | 320.86 (446.13) | 48.57 (–105.92 to 203.05) |
| Total health-care costs (including garments) | 687.96 (809.27) | 320.86 (446.13) | 367.09 (212.12 to 522.07); 364.94 (217.47 to 512.42) |
| Patient additional out-of-pocket costs | 65.00 (166.75) | 54.96 (128.75) | 10.04 (–25.38 to 45.46) |
| Productivity costs | 45.78 (120.61) | 27.29 (77.86) | 18.49 (–5.61 to 42.59) |
| Total costs (NHS, patient and employer) | 798.73 (970.99) | 403.11 (524.10) | 395.62 (210.60 to 580.64); 392.98 (216.44 to 569.53) |
Resource use and costs
Intervention resource use and costs
In the 6-month period, the mean number of sets of garments (tops and leggings) per participant was 4.15 (minimum 3, maximum 9.5). Sixty-one (45.5%) intervention participants received replacement garments over the 6 months [each participant received, on average, 1.1 extra sets (minimum 0, maximum 6.5)]. The associated mean cost of silk garments, including initial and replacement garments, was £318.52 (SD £136.60; minimum–maximum £198.06–£1167.15) per participant in the base case (see Table 31).
Other health resource use and costs
Resource use and costs for all resource items are given in Tables 30 and 31. When intervention use was combined with other health resource use, the adjusted mean incremental cost per participant was £364.94 (95% CI £217.47 to £512.42) for those who received silk garments compared with those who did not in the base case (see Table 31). The difference in total costs between groups reflects the cost of the intervention; other NHS costs were not significantly different between groups (£48.57 higher per participant, on average, in the intervention group, 95% CI –£105.92 to £203.05).
| Resource use item | Intervention (n = 134), mean (SD) | Standard care (n = 139), mean (SD) | Mean difference (95% CI) |
|---|---|---|---|
| Intervention | |||
| Silk therapeutic garments (number provided over 6 months) | 4.15 (1.55) | 0.00 (0.00) | 4.15 (3.88 to 4.41) |
| Primary health care | |||
| GP (per surgery consultation) | 0.78 (1.08) | 1.06 (1.74) | –0.29 (–0.64 to 0.06) |
| GP (per telephone consultation) | 0.01 (0.09) | 0.01 (0.12) | –0.007 (–0.03 to 0.02) |
| GP (per consultation out of hours) | 0.00 (0.00) | 0.01 (0.06) | –0.01 (–0.02 to 0.01) |
| Practice nurse (per consultation) | 0.13 (0.40) | 0.07 (0.35) | 0.06 (–0.03 to 0.15) |
| Community eczema nurse (per consultation) | 0.03 (0.27) | 0.01 (0.08) | 0.02 (–0.02 to 0.7) |
| Community nurse (per consultation at home) | 0.01 (0.09) | 0.00 (0.00) | 0.01 (–0.01 to 0.02) |
| Pharmacist (per contact) | 0.00 (0.00) | 0.01 (0.08) | –0.01 (–0.02 to 0.01) |
| Health visitor (per contact) | 0.00 (0.00) | 0.01 (0.09) | –0.01 (–0.3 to 0.01) |
| Nutritionist (per telephone contact) | 0.00 (0.00) | 0.01 (0.08) | –0.01 (–0.02 to 0.01) |
| Homeopathic (per visit) | 0.01 (0.17) | 0.07 (0.55) | –0.06 (–0.15 to 0.04) |
| Blood test (per test) | 0.01 (0.09) | 0.01 (0.17) | –0.007 (–0.04 to 0.03) |
| Influenza vaccination | 0.00 (0.00) | 0.01 (0.06) | –0.01 (–0.02 to 0.01) |
| Total number of primary care visits | 0.98 (1.17) | 1.28 (1.87) | –0.30 (–0.68 to 0.07) |
| Secondary health care | |||
| A&E (per visit) | 0.01 (0.17) | 0.01 (0.12) | 0.001 (–0.03 to 0.04) |
| Outpatient first visit (dermatology, per consultation) | 1.04 (1.69) | 0.83 (1.50) | 0.21 (–0.17 to 0.59) |
| Dermatology consultation (per telephone call or e-mail contact) | 0.01 (0.17) | 0.01 (0.11) | 0.001 (–0.03 to 0.04) |
| Consultant eczema nurse (per telephone consultation) | 0.00 (0.00) | 0.01 (0.08) | –0.01 (–0.02 to 0.01) |
| Eczema nurse (per telephone contact) | 0.00 (0.00) | 0.01 (0.08) | –0.01 (–0.02 to 0.01) |
| Paediatric assessment unit | 0.00 (0.00) | 0.02 (0.19) | –0.02 (–0.5 to 0.01) |
| Children’s ward (number of visits) | 0.04 (0.36) | 0.03 (0.34) | 0.01 (–0.07 to 0.09) |
| Inpatient stay for skin disorder without intervention | 0.04 (0.27) | 0.02 (0.19) | 0.02 (–0.03 to 0.08) |
| Patch test (per test) | 0.00 (0.00) | 0.02 (0.25) | –0.2 (–0.06 to 0.02) |
| Total number of secondary care visits | 1.16 (2.12) | 0.98 (1.65) | 0.18 (–0.27 to 0.63) |
| Total number of health-care visits | 2.13 (2.79) | 2.26 (2.55) | –0.12 (–0.76 to 0.51) |
| Medications | |||
| Prescription items (number) | 12.56 (17.97) | 12.60 (13.90) | –0.04 (–3.86 to 3.78) |
| Topical corticosteroid (g) | 139.03 (212.49) | 169.03 (295.14) | –30.00 (–91.47 to 31.47) |
| Resource use item | Intervention (n = 134), mean (SD) | Standard care (n = 139), mean (SD) | Mean difference (95% CI) |
|---|---|---|---|
| Intervention resource use | |||
| Silk therapeutic garments (including replacements) (base case) | 318.52 (136.60) | 0.00 (0.00) | 318.52 (295.71 to 341.33) |
| Primary health care | |||
| GP (surgery consultation) | 28.72 (39.98) | 39.40 (64.43) | –10.68 (–23.51 to 2.15) |
| GP (telephone consultation) | 0.16 (1.90) | 0.32 (2.63) | –0.15 (–0.70 to 0.40) |
| GP (consultation out of hours) | 0.00 (0.00) | 0.50 (5.84) | –0.50 (–1.49 to 0.50) |
| Practice nurse | 1.63 (4.89) | 0.87 (4.30) | 0.76 (–0.34 to 1.85) |
| Community eczema nurse | 4.81 (43.89) | 1.16 (13.66) | 3.65 (–4.04 to 11.34) |
| Community nurse | 0.28 (3.28) | 0.00 (0.00) | 0.28 (–0.26 to 0.83) |
| Pharmacist | 0.00 (0.00) | 0.11 (1.24) | –0.11 (–0.32 to 0.11) |
| Health visitor | 0.00 (0.00) | 0.78 (6.45) | –0.78 (–1.87 to 0.32) |
| Nutritionist (telephone contact) | 0.00 (0.00) | 0.59 (7.01) | –0.59 (–1.78 to 0.60) |
| Homeopathic visit | 0.85 (9.85) | 4.10 (31.18) | –3.25 (–8.80 to 2.30) |
| Blood test | 0.07 (0.78) | 0.13 (1.54) | –0.06 (–0.36 to 0.23) |
| Influenza vaccination | 0.00 (0.00) | 0.22 (2.56) | –0.22 (–0.65 to 0.22) |
| Total primary health-care costs | 36.52 (57.74) | 47.01 (73.71) | –10.49 (–26.30 to 5.33) |
| Secondary health care | |||
| A&E | 1.39 (16.07) | 1.34 (11.12) | 0.05 (–3.23 to 3.33) |
| Outpatients first visit (dermatology consultation) | 134.15 (217.53) | 107.15 (192.82) | 27.00 (–21.94 to 75.93) |
| Dermatologist consultant (telephone/e-mail consultation) | 0.96 (11.09) | 0.92 (7.67) | 0.03 (–2.23 to 2.30) |
| Consultant eczema nurse (telephone consultation) | 0.00 (0.00) | 0.21 (2.46) | –0.21 (–0.63 to 0.21) |
| Eczema nurse (telephone contact) | 0.00 (0.00) | 1.16 (13.66) | –1.16 (–3.48 to 1.16) |
| Paediatric assessment unit | 0.00 (0.00) | 6.46 (56.64) | –6.46 (–16.10 to 3.17) |
| Children’s ward (observation with no overnight stay) | 11.18 (106.49) | 8.62 (101.62) | 2.56 (–22.24 to 27.35) |
| Inpatient stay for skin disorder without intervention | 65.42 (401.7) | 24.84 (216.39) | 40.57 (–35.94 to 117.08) |
| Patch test | 0.00 (0.00) | 1.98 (23.36) | –1.98 (–5.96 to 1.99) |
| Total secondary health-care costs | 213.09 (604.47) | 153.00 (327.13) | 60.09 (–55.16 to 175.34) |
| Total prescription costs | 119.82 (244.67) | 120.86 (243.81) | –1.04 (–59.25 to 57.18) |
| Mean total health-care costs without silk garments | 369.43 (805.88) | 320.86 (446.13) | 48.57 (–105.92 to 203.05) |
| Mean total health-care costs with silk garments | 687.96 (809.27) | 320.86 (446.13) | 367.09 (212.12 to 522.07) |
| Wider societal costs | |||
| Patient additional out-of-pocket costs | 65.00 (166.75) | 54.96 (128.75) | 10.04 (–25.38 to 45.46) |
| Productivity costs | 45.78 (120.61) | 27.29 (77.86) | 18.49 (–5.61 to 42.59) |
| Total costs (NHS, patient and employer) | 798.73 (970.99) | 403.11 (524.10) | 395.62 (210.60 to 580.64) |
Productivity costs
On average, parents/carers took off 3.00 (SD 7.90) hours from paid employment in the silk garment arm and 1.79 (SD 5.10) hours in the standard care arm (mean difference 1.21 hours, 95% CI –0.37 to 2.79 hours) as a result of taking care of their child with eczema. Employing a human capital approach, and using a national published gross mean hourly wage rate for men and women of £15.27, resulted in mean estimates of lost productivity of £45.78 (SD £120.61) in the silk garment arm and £27.29 (SD £77.86) in the standard care arm (mean unadjusted difference £18.49, 95% CI –£5.61 to £42.59).
Time off school or nursery
Participants in the silk garment arm missed, on average, 4.17 (SD 10.41) hours off school or nursery as a result of their eczema in the 6-month trial period, compared with an average of 3.57 (SD 8.15) hours in the standard care arm (mean unadjusted difference 0.60 hours, 95% CI –1.62 to 2.82 hours).
Out-of-pocket costs incurred by the family
Families paid out of pocket for an average of 10.66 (SD 21.75) items as a result of their child’s eczema in the silk garment arm, compared with an average of 9.80 (SD 21.74) items in the standard care arm, over the 6-month trial period (mean difference 0.86, 95% CI –4.33 to 6.04). On average, the additional out-of-pocket costs incurred for these items were £65.00 (SD £166.75) in the silk garment arm and £54.96 (SD £128.75) in the standard care arm over the 6 months (mean unadjusted difference £10.04, 95% CI –£25.38 to £45.46).
Base-case cost–utility analysis
Base-case cost–utility analysis from a NHS perspective
The adjusted mean difference in QALYs per participant was 0.0064 (95% CI –0.0004 to 0.0133) (see Table 29 for the key findings from the base-case analysis). Combined with adjusted mean cost, the adjusted mean incremental cost per QALY was £56,811 (Figures 15 and 16; see also Table 29), suggesting that silk garments for moderate to severe eczema are not cost-effective within currently accepted thresholds. At a willingness to pay of £30,000 per QALY, the probability of silk garments being cost-effective was 12.13%.
FIGURE 15.
Incremental cost-effectiveness plane for the base-case analysis (ADQoL).
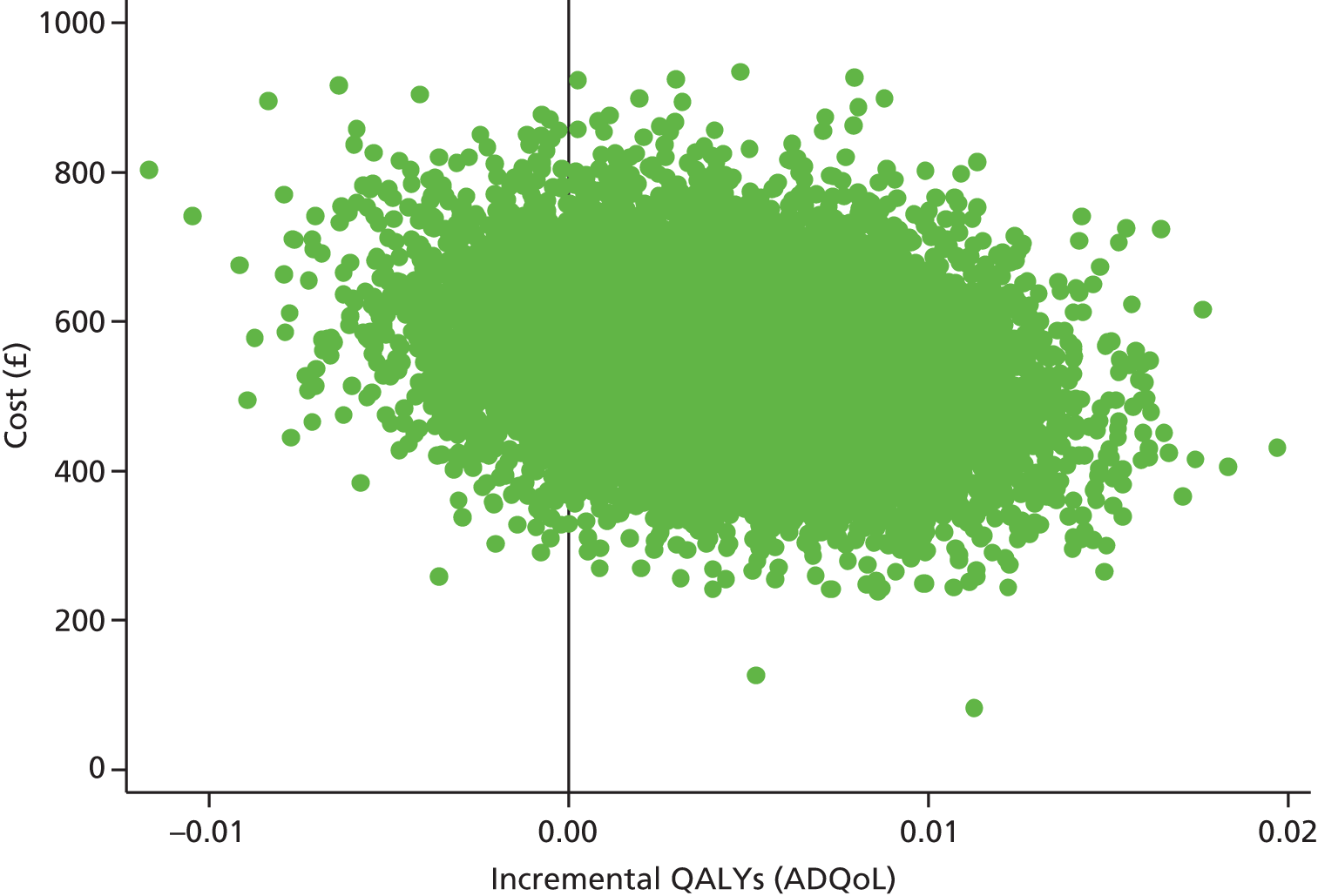
FIGURE 16.
Cost-effectiveness acceptability curves for the intervention (solid line) and standard care group (dashed line): base-case adjusted costs and ADQoL utility scores. NICE threshold for willingness to pay per QALY = £20,000–30,000. 69
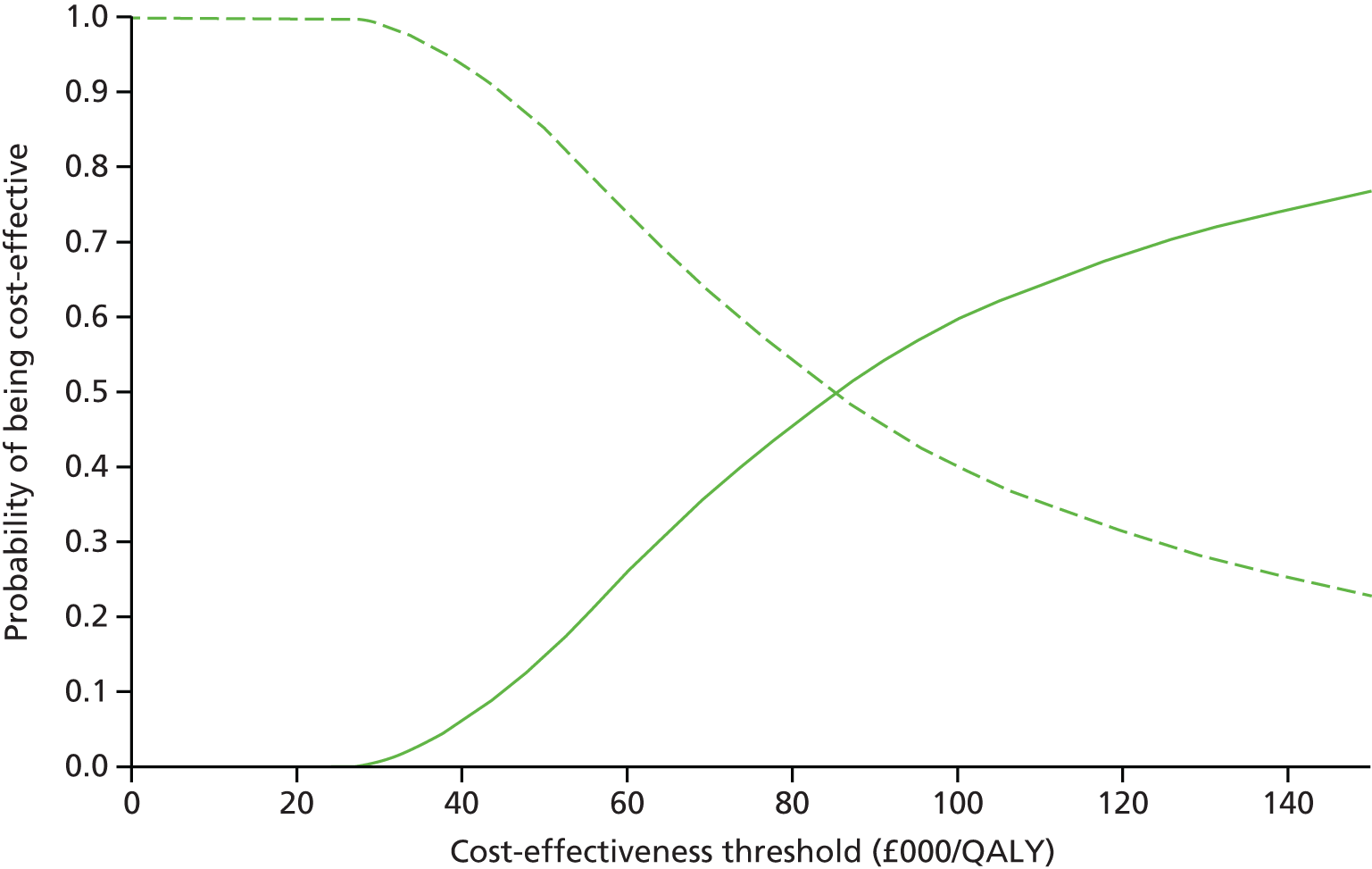
Cost–utility analysis taking a NHS and family/employer perspective
In a separate analysis, taking a NHS and family/employer perspective, the unadjusted mean cost per patient for the silk garment group was £798.73 (SD £970.99), compared with £403.11 (SD £524.10) in the control group. The adjusted mean incremental cost per participant was £392.98 (95% CI £216.44 to £569.53) for those who received silk garments compared with those who did not. The adjusted mean difference in QALYs per participant was the same as in the previous analysis taking a NHS perspective only, 0.0064 (95% CI –0.0004 to 0.0132), such that the incremental cost per QALY taking a wider perspective was £61,385.
Cost-effectiveness analysis
Eczema Area and Severity Index
The key results for the cost-effectiveness analysis using proportion of participants achieving a 50% improvement on the EASI as the measure of outcome are shown in Table 32. The incremental cost per additional person treated successfully (defined as a reduction in EASI of at least 50% compared with baseline) was £10,425.67.
| Analysis | n per arm (intervention; control) | Adjusted incremental health-care costs (including garments) (95% CI) | Adjusted incremental NHS/family/productivity costs (including garments) (95% CI) | Adjusted incremental effectiveness (95% CI) | ICER for NHS perspective | ICER for NHS/family and employer perspective |
|---|---|---|---|---|---|---|
| Base case: cost/ADQoL | 134; 139 | £364.94 (£217.47 to £512.42) | £392.98 (£216.44 to £569.53) | 0.0064 (–0.0004 to 0.0132) | £56,811 per QALY | £61,385 per QALY |
| Cost/EASIa | 133; 139 | £336.14 (£330.86 to £341.42) | £361.30 (£355.51 to £367.10) | 0.0322 (–0.0823 to 0.1468) | £10,426 per additional successful person treated | £11,206 per additional successful person treated |
| Cost/DFI | 133; 138 | £354.00 (£204.42 to £503.58) | £383.03 (£204.37 to £561.69) | –0.81 (–2.01 to 0.39) | £435 per one-point improvement on the DFI | £471 per one-point improvement on the DFI |
| Cost/main carer EQ-5D-3L | 132; 138 | £369.76 (£216.02 to £523.51) | £401.35 (£217.61 to £585.09) | 0.0029 (–0.0045 to 0.0102) | £251,849 per QALY | £273,530 per QALY |
| Tariff cost/ADQoL | 134; 139 | £346.46 (£199.08 to £493.84) | £374.50 (£198.04 to £550.95) | 0.0064 (–0.0004 to 0.0133) | £53,989 per QALY | £58,488 per QALY |
| Cost/CHU-9D | 61; 61 | £593.50 (£338.57 to £848.43) | £667.89 (£357.08 to £978.70) | –0.0061 (–0.0142 to 0.0021) | Dominated | Dominated |
Dermatitis Family Impact
The key results for the cost-effectiveness analysis using change in the DFI instrument as the measure of outcome are shown in Table 32. The incremental cost for every 1-point improvement on the DFI scale was £435.46.
Cost–utility analysis for main carer EuroQoL-5 Dimensions-3 Levels
The main results for the cost–utility analysis based on main carer quality of life, as measured using the EQ-5D-3L, are shown in Table 32. The adjusted mean incremental cost was £369.76 (95% CI £216.02 to £523.51) and the adjusted mean incremental QALY gain was 0.0029 (95% CI –0.0045 to 0.0102) (Figure 17), giving an ICER of £251,849 per QALY. At a willingness to pay of £30,000 per QALY, the probability of silk garments being cost-effective was 1.31%.
FIGURE 17.
Incremental cost-effectiveness plane (main carer EQ-5D-3L).
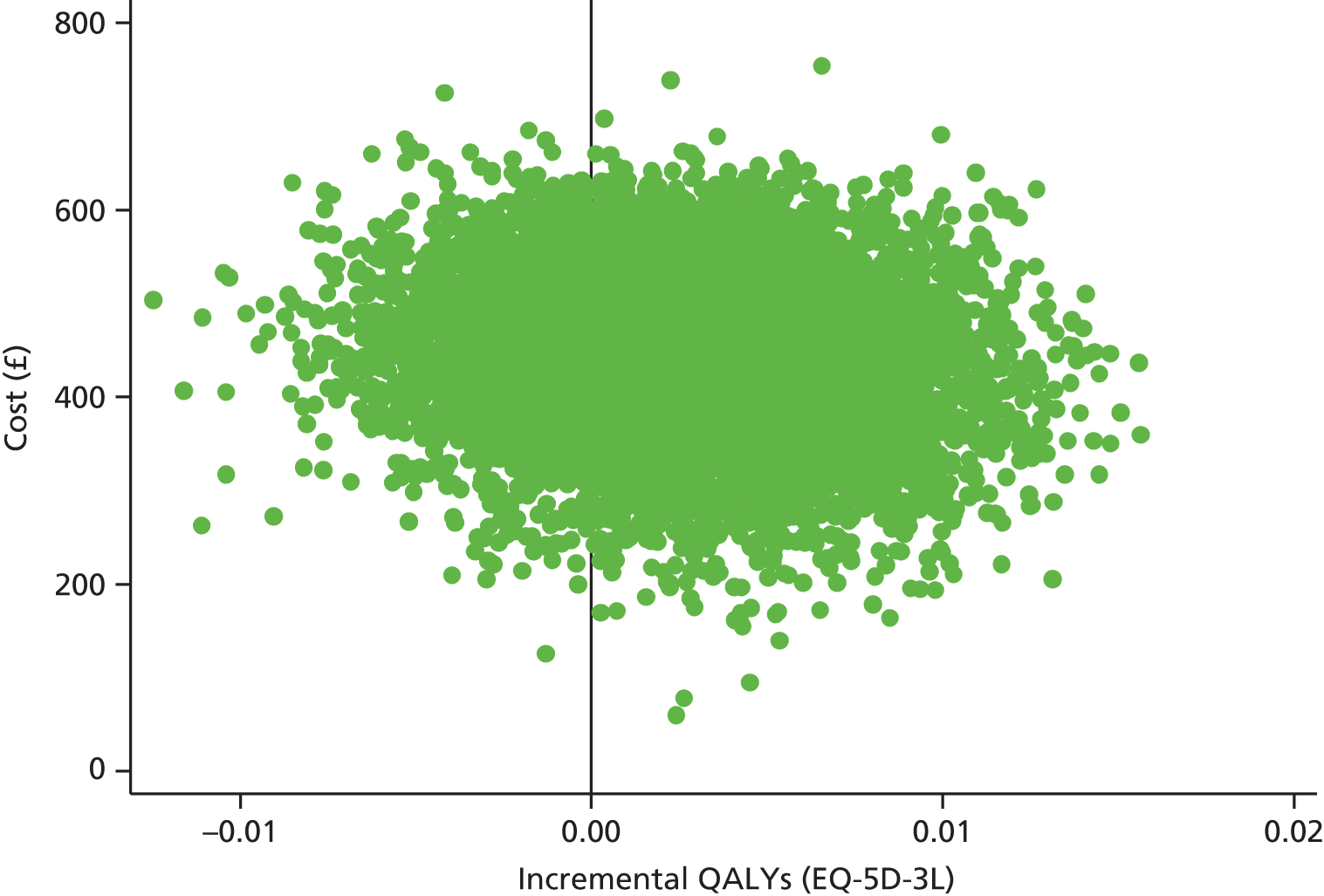
Uncertainty and sensitivity analysis
Alternative source of unit costs for the silk garments
To test the impact of taking into account the average discount enjoyed by the NHS, we used an alternative approach based on the NHS Business Services Authority formula to estimate the actual cost to the NHS. Using the March 2015 tariff data, where the average discount was 7.43%, the consumables fee £0.0124 per prescription item, the pharmacists’ professional fee £0.90 per prescription item, the analysis was re-run (see Table 32). This approach reduced the cost of silk garments, but at £53,989 per QALY the estimated incremental cost per QALY was still above the accepted NICE threshold value, such that silk garments would still not be considered to offer value for money to the NHS under this approach. At a willingness to pay of £30,000 per QALY, the probability of silk garments being cost-effective was 10.51%.
Alternative outcome measure: Child Health Utility-9 Dimensions analysis
For the analysis using the CHU-9D instead of the ADQoL to estimate child utility scores, standard care dominates as silk garments were both more expensive and less effective (in terms of QALYs measured using the CHU-9D for utility) (Figure 18 and see Table 32) than standard care. The probability of silk garments being cost-effective at a willingness-to-pay threshold of £30,000 per QALY was 0.06%.
FIGURE 18.
Incremental cost-effectiveness plane (CHU-9D).

Discussion and conclusion
Main findings
This is the first economic evaluation of silk therapeutic garment use in children with moderate to severe eczema. The economic analysis extends the clinical analysis to show that silk garments in addition to standard care are unlikely to represent value for money for the NHS. In terms of the costs, the additional costs of providing silk garments to intervention participants were not recouped through cost savings from lower use of wider health-care resource items nor from families in terms of reduced out-of-pocket costs or from employers in terms of reduced time off work by parents caring for their child with eczema. In terms of outcomes (when looking at patient-assessed health-related quality of life), although the ADQoL showed a very small, non-significant, positive benefit in terms of QALYs in favour of silk garments, this was not sufficient to outweigh the higher costs of providing silk garments. The CHU-9D showed a very small non-significant decrease in QALYs (suggesting worse health) in the intervention arm. When coupled with the higher costs, this suggests that silk garments do not represent value for money for the NHS. The small, non-statistically significant, differences found when estimating QALYs (using the different instruments) suggest that the difference between intervention and usual care groups is negligible and may have been due to chance.
The result of this within-trial cost-effectiveness study also provides an indication of the cost of treating moderate to severe eczema to the NHS. Over a 6-month period, health-care costs were in the region of £345 (SD £647.40) per child (using 2014/15 costs and excluding the cost of silk garments). In addition, the reported personal costs and time lost from work/school as a result of eczema were considerable and, provide important data to inform studies on the societal impact of this condition.
Strengths and weaknesses
Given the clinical result, it could have been argued that an economic evaluation was unnecessary. However, resource use and quality-of-life data were collected alongside the trial, and these results provide useful data to inform future studies and decisions regarding health commissioning.
The study did not explicitly ask families to record how much time they spent applying treatments for their child’s eczema. Were a treatment to be effective at reducing disease severity, it could conceivably reduce the amount of time a parent or child spends applying/taking medications and it might be important to capture this effect from a family perspective in future studies.
The study attempted to measure QALYs for the main carer in addition to the child. Our approach had limitations because children may have also lived with another parent or carer in addition to siblings, and we did not seek to capture the health-related quality-of-life effects of these extended family members. It is unclear how best to capture the wider impacts of eczema within a family in an economic evaluation. Further research looking at whose QALYs to capture, how to aggregate QALYs estimated for patients, carers and siblings, and how to present such results is needed. 64 However, given that this study found no difference in effect between treatment arms, the results are unlikely to be sensitive to the assumptions made in relation to such ‘beyond the patient’ effects. 64
Conclusion
This economic evaluation shows that in children with moderate to severe eczema, silk garments do not offer value for money to the NHS.
Chapter 5 Nested qualitative study
The value of using mixed qualitative and quantitative research methods in trials is increasingly being acknowledged,70 particularly when investigating complex health-related topics. 71 In the CLOTHES trial we have been mindful that the qualitative work is more than an adjunct to the main trial; it has been used to elucidate a deep understanding of the ‘what’, ‘why’ and ‘how come’ of participants’ beliefs and behaviours. 72 This nested qualitative study was conducted by colleagues at the University of Hull, who were not aware of the treatment allocation of the children involved. The results of this nested qualitative study were collected and analysed separately. The results were not revealed to the rest of the trial team until the data collection and analysis of the main trial results were complete, at which point the results of the qualitative study were used to inform interpretation of the trial findings.
It is now widely accepted that when children are the likely end users of a product under investigation, researchers should consult them directly, rather than depending on second-hand reports from adults. 73 In this chapter we have used the term eczema rather than AE in order to reflect the language used by the parents and children.
The purpose of the qualitative component of the CLOTHES trial was to:
-
qualitatively examine participants’ experiences of using silk garments for the treatment of eczema
-
examine barriers and motivators to prescribing silk garments from the perspectives of clinicians and commissioners.
Specific objectives were to:
-
explore factors that might influence the use of silk garments in everyday life
-
examine parent and child views on the feasibility and acceptability of using silk garments
-
explore parent and child experiences of using silk garments
-
examine barriers and motivators to prescribing silk garments from the perspectives of clinicians and commissioners.
This report is divided into three sections:
-
children’s focus groups and interviews
-
parent interviews and focus groups
-
clinician and commissioner interviews.
For each of the three elements, a generic qualitative research method was used. 74 The research team comprised three nurse researchers, all of whom were aware of their own potential impact on the study. To ensure rigour, the following steps were taken: preconceived beliefs were acknowledged prior to data collection; each researcher used a reflective log to record their own thoughts; researcher understandings were checked with participants at the end of each episode of data collection; and analysis was conducted independently and then as a team.
The study was approved by Nottingham Health Research Authority East Midlands – Nottingham 1 Research Ethics Committee (13/EM/0255). Ethical guidelines on research with children were followed. 75 All participants gave written or verbal consent or assent depending on their age and on whether the data collection was in person or over the telephone.
Views of children in the CLOTHES trial
Data collection
Interviews and focus groups with children were completed from February 2015 to May 2015. Children were recruited using a convenience sampling strategy76,77 via the study research nurses. They represented all of the recruiting centres and had all completed the trial before participating. Ten semistructured, audio-taped face-to-face or telephone interviews were conducted by the researcher (EW). The children in the interview group ranged in age from 9 to 15 years. There were three focus groups in total, comprising two groups of 7- and 8-year-olds, each with two participants, and a further group of 5- and 6-year-olds, with four participants. Parents and siblings accompanied the children during data collection according to age and personal preference. The demographic details of the children are presented in Appendix 20 (see Table 59). The researcher (EW) used a wide range of developmentally appropriate, child-friendly techniques, such as drawing, collage, photography, storytelling, stickers and a puppet. The activities were adapted to the needs and interests of individual children. The resources chosen were sex and ethnicity neutral. The activities were selected with sensitivity; for example, the use of stickers and foam people allowed children to illustrate their point without the added pressure of having to be a ‘good drawer’. Care was taken to enable children to express their views through whatever medium they preferred. A broad topic guide was used as a basis for interactions with the children (see Appendix 20). This was shared with the children so that they understood what would happen next. At the end of each interview and focus group the researcher summed up her understanding of the responses and checked with each child that this was correct.
All data collection focused on finding out from children what it is like to have eczema, what they thought of the silk garments and of being in the trial, and whether or not other children with eczema should be given the garments. All interviews were audio-recorded and transcribed in full. Photographs were taken of artefacts produced during the sessions, as most children wanted to take these home. The children enjoyed taking photographs of what they had made and while they were doing this the researcher used the opportunity to verify her understanding of their thoughts. This was documented alongside the photographs in preparation for data analysis.
Data analysis
The analysis of the data required a sound understanding of children’s physical, cognitive, emotional and social development. This contributed to ensuring that the analysis reflected the children’s voices as faithfully as possible. Interpretation of drawings, collages and craft work was undertaken with the children at each stage.
The data from the interviews and focus groups were analysed using the three methods of holistic, selective and detailed data analysis of Van Manen:78 (1) data were viewed as a whole, (2) phrases or illustrations that seemed to represent the experience under study were identified and (3) written data were reviewed line by line in order to identify themes. 79 The aim was to be attentive to the voices of children and recognise subtle significations. 80 This was achieved through prolonged and intensive engagement with the totality of the data.
Data analysis yielded six key themes: (1) living with eczema, (2) expectations of the garments, (3) wearing ‘silks’ (a term often used by the children to describe their silk garments), (4) did they help?, (5) thoughts about the garments and (6) being part of the study. A description and analysis of each theme, together with supporting illustrations, is provided with a tabulation of each theme containing exemplar data extracts.
Theme 1: living with eczema
This theme comprises two subthemes: (1) health aspects of living with eczema and (2) social and emotional implications of living with eczema (Table 33).
| Health | Social and emotional |
|---|---|
| Itchy and hot, hands and feet sore. Eczema keeps me awake at night | It makes me feel sad and grumpy |
| My skin on my back feels like fire, like rocket fire | It’s . . . like poo. I itch all the time and it’s just not fair |
| It hurts a lot . . . when water touches it, it burns | I beat myself up for it because like I really want to get rid of it |
| You can't do as many things as you want to and even the sports you want to do, you can't do it because it irritates or makes it worse | |
| People say I am ugly |
Health aspects of living with eczema
All children portrayed a negative view of living with eczema; they particularly suffered with itching, heat, and flaking and dry skin, all in different areas of their bodies and with differing severity. A picture by an 8-year-old boy depicts how burning, red and sore the eczema on his back felt (Figure 19). Many said that eczema affected their sleep at night and that this impacted on their daytime activities, particularly school and play.
FIGURE 19.
Photograph showing depiction of eczema by an 8-year-old boy.

Social and emotional implications of eczema
Several children spoke of the emotional effect that eczema had on their lives, including frustration with the condition and how it affected their general well-being. The children spoke of how eczema affects their ability to fit in with their peer group and the limiting effects the condition could have; for example, most did not like exposing their skin to others and did not enjoy sporting activities for this reason.
Theme 2: expectations of the garments
This theme has three subthemes: (1) use of other treatments, (2) garments and (3) hopes. Many participants had preconceptions about the garments (Table 34).
| Use of other treatments | Garments | Hopes |
|---|---|---|
| Still have to use cream | [Silks] made me feel hotter, I thought it would make me cooler | I was a bit dubious because nothing’s ever really worked that well for me |
| During the day we use the silk suits, put the cream on underneath | I had a lovely silk long-sleeved top and it was really comfy to wear, very easy, forgot you had it on, and I think that was what I thought it would be. If it had been pure silk and not with all of these holes . . . that would have worked for me but . . . it was a bit like a washing machine bag | I had all these expectations built up . . . I was really hopeful as well. I was really willing to wear them to start with, and then I got them for the start and everything just turned negative for me |
| I have to put cream on every day | If the trousers had been proper silk and that skin-colour tights, I’d have definitely worn them a lot longer because I think that would have really helped me | I just put them on because I think they’re going to help |
| I was a bit confused because I thought it was meant to be really good and I thought it was meant to be really soft . . . I wasn’t too happy with it |
Use of other treatments
One particularly strongly held belief was that the silk garments would mean that other treatments, especially creams, would no longer be needed or would at least be reduced. This was also an expectation that parents had, and so it became a shared belief, and was a significant factor for some children agreeing to take part in the trial. Children were disappointed when this proved not to be the case. In fact, for some, cream use actually increased when using the garments.
Garments
Children also had ideas about what the garments would be like; because the garments were silk, the childen were expecting a soft and luxurious product, and many were surprised by the ‘roughness’ of the material. This reinforces the need for practitioners to be mindful of children’s cognitive understanding when ascribing labels to products. A number of children had expected the silks to be cooling, but for many the effect was the opposite, with the garments making them hot and uncomfortable.
Hopes
Many of the children were excited to try the garments and had very high hopes that they would really help their eczema. Others were much more cautious, having previously tried so many treatments that they thought of as failures.
Theme 3: wearing silk garments
This theme recounts when the children chose to wear the silk garments. There are two subthemes: (1) day versus night and (2) school versus home, leisure and play (Table 35).
| Day vs. night | School vs. home, leisure and play |
|---|---|
| Always wear them at night | I wore them to school but not PE [physical education] days . . . people would laugh |
| During the day we use the silk suits, put the cream on underneath, then put all the suits on . . . and that sort of stops my clothes rubbing against my skin. Wearing the tights day and night | Poor fit trousers so not worn to school/don’t fit under tights |
| I prefer to wear them during the day rather than the night because the silk is really comforting on my skin | One of the problems was that at school – because they’re quite strict on uniform – that’s a problem |
| Wearing them at night because some people ask like what’s that and it’s a bit annoying | Yes I wore them to school but it was a bit weird when like everyone was like ‘What is that?’ |
| I wear them mainly at night | I did the upper body one once at school . . . but everyone kept on asking about it and I just didn’t like it |
| I usually just wear them at home | |
| I wouldn’t wear them at a sleepover just because I would find that really embarrassing, because no offence, it didn’t really look that good |
Day versus night
Figure 20 is an example of how a child conveyed the times at which he wore the garments. Few children of any age wore their silks during the day; a majority of the children preferred to wear them at night and only when at home.
FIGURE 20.
Photograph showing a child’s depiction of times garments were worn.

School versus home, leisure and play
Children reported not wanting peers to see the garments for fear of attracting unwelcome questions or comments. There was a seasonal element to garment wear, particularly not wanting the garments to be visible when wearing shorts and t-shirts in the summer. There were many issues with being able to wear them for school, ranging from fit, uniform-requirements, physical education issues and the reactions of other school children. Peer groups and friendships were very important for all children; only those with very secure friendships felt that they could openly speak about their eczema and the garments.
Theme 4: did they help?
This theme has three subthemes: (1) getting better, (2) getting worse and (3) no difference but liked them anyway. There was a real mixture of perceived success of the garments, ranging from a perceived complete cessation of eczema to no effect at all, to worsening of symptoms (Table 36).
| Getting better | Getting worse | No difference but liked them anyway |
|---|---|---|
| It made it feel better but the crusty is still there | I stopped wearing them as soon as I realised they were making [my] eczema worse. It helped the cream absorb better but it did make it all inflamed and more itchy | It was comfy. Top and bottoms were smooth |
| Better, the itch goes away | It kind of got worse. It just got more dry and really red | Feels nice on my skin |
| At night I got to sleep through for the first time. It made me feel good inside | [At] night, it wakes you up because it is scraping at my skin and because you have got loose bits of dead skin . . . it catches and it rips it | They feel nice, I like wearing them. They . . . helped me be less annoyed |
| It’s kind of helping the eczema go away. Less sore |
Getting better
Some children reported that the silks had improved their sleep, whereas others felt that the silks had made them more comfortable in bed but had not improved their eczema per se. Improved sleep was linked with better well-being at school and at home the following day. A few children stated that the clothing improved their mood.
Getting worse
Other children were disappointed in the effect of the garments, which affected their self-esteem. Some children suggested that it was their own fault the garments had not worked for them.
No difference but liked them anyway
Some children liked wearing the garments, even if they had no perceptible effect on their eczema. This was predominantly because they were comfortable, although some parents reported that the garments made their child feel special. Figure 21 shows what an 8-year-old girl thought other children should know about what was ‘good’ and ‘bad’ about the silks. The blue-stamped areas represent where the garments were hot and uncomfortable.
FIGURE 21.
Photograph of an 8-year-old girl’s thoughts on garments.

Theme 5: thoughts about the garments
This theme comprises three subthemes: (1) quality and cost, (2) fit and fabric, and (3) design and appearance (Table 37).
| Quality and cost | Fit and fabric | Design and appearance |
|---|---|---|
| Looked like a trial clothing rather than a purchase | ‘Random sizing’ poor fit. Uncomfortable – too tight | [Didn’t like] that you could see through them |
| I’ve got massive holes gaping wide | I would make them a bit tighter, problem with the elastic, and they bunch at the bottom | It’s not . . . that private to be wearing something basically see-through . . . like wearing cling film |
| Get hot . . . and they can tear | I just found them really, really uncomfortable. They were tight. There wasn’t the give in them | Gap between trouser and top untreated and elastic waistband itchy |
| I think the best thing about them is that they are 100% silk and they don’t have any . . . elastic | Kind of transparent – needed to wear pants under | No good for summer – long sleeves |
| We didn’t think they were going to be as expensive | They look really grubby | |
| They are not very pretty |
Quality and cost
Children were remarkably aware of cost and quality issues. Many thought that the garments were quite poor quality in fit, appearance, design or the fabric itself, especially given the cost. They were disappointed in the shape, size and colour. The cost was an issue for further purchase for a few, with older children (and their parents) feeling that the garments should be available on prescription from a GP.
Fit and fabric
Many felt that the sizing and fit, especially of the trousers, were poor. Children were aware that the garments did not always wash well, turning baggy and grey, and affecting fit over time as illustrated in Figure 22. Most children used the garments with creams, which made the silks sticky and oily, and, in some cases, smelly. Some felt the texture was rough and actually irritated their skin more; some also felt that the silks actually made them hotter.
FIGURE 22.
Child’s illustration of garments over time.

Design and appearance
The fact that the silks were see-through was an issue for many; this limited their use to the home, and many children had to wear another layer of clothing over the silks. At night, the garments tended to be worn under pyjamas or onesies. Issues of fit, appearance and smell compounded children’s sense of being ‘different’ from their peers. Children commented that, although three sets of garments were provided, these did not reflect sex differences in relation to puberty nor the normal varied physical growth spurts experienced at this time.
Children suggested a range of improvements to the garments, including using smoother and more closely woven fabric, resembling ‘proper’ silk. Some wanted the garments to be tighter, but others preferred the looser fit. A few thought that different colour options would be good, especially the youngest and oldest children. In terms of design, a ‘onesie’ was seen as a positive option, as were short-sleeved tops and shorts for summer. Others felt that additional protection for hands and feet would be beneficial, as these areas were not covered by the trial garments (except for those aged < 2 years) and were often troublesome. Children suggested that a few changes would make the garments more wearable in the daytime provided that this was under normal clothing, including school uniform, in the colder winter months. All except one participant felt that they would recommend other children to try the garments, even if they had not been particularly successful personally. Many of the children recognised that everyone’s experience of eczema was different and that they would respond differently to different treatments, and they seemed keen to ensure that other children did not miss any opportunity to improve their eczema.
Theme 6: being part of the study
This theme comprised two subthemes: (1) helping others and (2) the research process (Table 38).
| Helping others | The research process |
|---|---|
| Happy to know I am helping everyone in the world, who has eczema probably in the world | It felt quite good because I was doing it in a trial |
| It felt like I was helping the other people who had it as well so then they got some research. It felt quite good | [The focus group was] lots of fun it gives you a chance to talk to others about your feelings . . . you get to do lots of fun stuff at the end! |
| I didn’t mind wearing them because I knew it was for a trial to see if it could help people with eczema |
Helping others
Many of the children were really pleased to be taking part in the trial. Indeed, the majority of children demonstrated a significant level of altruism, wanting to be part of the trial particularly if it would help other children.
The research process
All children relished the opportunity to be with others with eczema and to talk about their experiences. Many were disappointed that the data collection was a one-off event. Most enjoyed the whole research process, and younger children valued the small gifts they received and enjoyed charting their progress. A few of the older children reported that they would ‘give the garments another try’, having had the opportunity to talk about them with the researcher.
Children engaged readily in the interviews and focus groups. Older children appreciated the chance to discuss their experience of eczema and the garment trial. In addition to the activities outlined in previous sections, all children were encouraged to feed back to the researcher throughout the process. All children in focus groups and face-to-face interviews chose to ‘mark’ the researcher’s work in a similar way to their experience of their schoolwork being marked by a teacher. The researcher’s understanding and performance, including whether or not she had asked all of the pertinent questions and understood their answers, were assessed by the children ‘ticking’ the work with carefully selected coloured pens.
Children took home all their artwork and checked the final photographs for use by the researcher, which gave them a sense of achievement and accomplishment. Children were asked how the findings of the project would best be communicated: most advocated either posters or a website, which they felt should be fun and incorporate activities. Figure 23 shows a dissemination poster designed in one of the focus groups.
FIGURE 23.
Dissemination poster designed by a child.

Child participants presented a range of views about the silk garments. All had high hopes when they entered the trial and, with few exceptions, their expectations were not realised. Some found the garments comfortable but many did not like the look, feel and fit of them. Even those children of a relatively young age were aware that the garments were expensive. Patterns of use varied, but most children reported wearing the garments fairly regularly at night. Although the children tended to report a limited effect on their skin condition, they almost universally recommended that other children should ‘give the garments a try’, perhaps exemplifying their experience of trying different treatment options and recognition of individual differences and needs.
Views of parents of children taking part in the CLOTHES trial
Data collection
A series of in-person focus groups and telephone interviews with the parents of children in the trial was conducted between November 2014 and August 2015. A convenience sample of parents was recruited via trial information leaflets and invitations, and with the support of the research nurses. 76,77 All participants had reached the end of the trial prior to participating and they represented all recruiting centres. In total, 28 mothers and five fathers participated, representing 11% of all trial participants. The age range of their children was 2–14 years and the sex split was 19 boys and 13 girls; 17 of the children were allocated to the standard care group and 15 were allocated to the intervention group. The demographic details are summarised in Appendix 20 (see Table 60).
The researcher (FC) conducted the semistructured focus groups and telephone interviews using a prepared topic guide (see Appendix 20). The four focus groups had between two and four participants and lasted between 42 and 95 minutes. Telephone interviews lasted between 18 and 50 minutes. All data were audio-taped and transcribed verbatim.
Data analysis
Data were analysed using the five-stage Framework Analysis process. 81 The five stages comprise (1) familiarisation with the data through reading full transcripts; (2) development of a theoretical framework through identification of recurring and important themes; (3) indexing and pilot charting; (4) summarising data in an analytical framework; and (5) synthesising data by mapping and interpreting. In line with usual qualitative research practice, the researcher completed a reflective account and this was used to inform the analysis process. Stages 1–3 of the framework are illustrated in detail in Appendix 20 and stages 4 and 5 are documented below. The analysis process yielded four key themes: (1) despair and hope, (2) fit, durability and care, (3) perceived impact of the garments and (4) engaging in the trial. A total of 13 subthemes were identified. A description of each theme is provided below, and this includes a tabulation of each theme and subtheme with exemplar data extracts.
Theme 1: despair and hope
The theme concerning despair and hope comprises four subthemes: (1) treatments, (2) adjustments, (3) quality of life and (4) hopes for the trial (Table 39).
| Treatments | Adjustments | Quality of life | Hopes for the trial |
|---|---|---|---|
| We’ve probably tried every cream that can be prescribed | We have really invested heavily into our property . . . we invested quite a lot in everything to help her with her eczema | It’s really bad head to toe at the moment it’s gone mental . . . we can’t get control of it at all . . . it’s a bit of a nightmare | Really I was hoping, you know, praying that it was going to be the answer |
| Medication wise he’s used everything under the sun literally | Limited soft toys, changing all his bedding, washing his bedding at a higher temperature | It has affected all of us, all of our lives, totally | I would do anything for my daughter not to have eczema . . . I would pay anything if it meant she didn’t have to suffer . . . I am desperate now, I am at my wits’ end now . . . I’m praying that someone somewhere can do something for her |
| She’s on a rigmarole of steroids, protopics and emollients and there’s a whole bathing regime . . . basically we’ve never really won the battle . . . the only thing that ever might keep it at bay is really strong steroid cream and even that stings . . . I’ve got real issues covering her in steroids | I wouldn’t let her go to anyone else’s [friend’s houses] | It doesn’t really, really affect his life too much. He’s just used to being itchy and uncomfortable | Trying to look for the miracle cure really |
| [Steroids] we don’t like to use them too much for too long they just thin the skin | [At school] she has a space she can go where she can keep her cream, she’s got a health plan | She just wants to be normal, she doesn’t want to stand out in any way | But you would love to have the magic answer . . . that’s why you just try anything. You’re always searching for answers aren’t you |
| Time consuming doing all the creams and treatments and if she doesn’t want to do them | He lives with it, he just seems to get on with it . . . there are times when he’s had to come home from school, sometimes he’ll take a shower and go back in again | When he was scratching at his most he would be red, he would be bleeding, he would get frustrated, but it never stopped him doing anything . . . I think it’s more down to our attitude because we didn’t want him to stop doing things because he had this illness | At first we thought . . . brilliant, brilliant, we’ve got something, it’s kind of what we’ve been looking for |
| Hated being creamed . . . would run away |
Treatments
Participants presented differing accounts of the various treatments used through the course of their children’s eczema. Virtually all had used emollients, often working their way through the ‘full gamut’ of products to find one that suited their child, and topical steroids of different potencies. Smaller numbers had been prescribed wet wraps, oral steroids, protopics, antihistamines or bleach baths, or offered photochemotherapy. Complementary therapies and specialised clothing (non-silk) had been used by some. Parents appeared to relish having the opportunity to have time to recount the ups and downs of their child’s eczema treatment; for many it was a long and challenging attempt to find the best regimen. In the focus groups there was a tangible sense of relief that their stories were typical of others. Parents tended to minimise the use of topical steroids because of concerns about side effects and long-term impact on the skin condition. They also described the development of ‘immunity’ to treatments, which resulted in the need for periodic changes of medication. Faith in the medical profession was variable, with some parents recounting excellent experiences and others less so, particularly those receiving only primary care services. There appeared to be different approaches to treatment escalation across study sites. A small number of parents felt the need to stop treatments for a day or two before a medical consultation to ensure that the doctor saw the eczema at its worst and so would take the problem more seriously.
Adjustments
Adjustments to life had been made in a number of ways and to varying degrees that did not always obviously correlate with the described disease severity. At one extreme, one parent who received Disability Living Allowance had employed a carer to accompany her child to playgroup to apply emollients and change his nappy every hour. This mother had prepared an individual ladder of care to guide the actions of the carer and this had allowed the mother to continue with her own job and ensure that her child lived as normal a life as was possible. Many parents made adjustments such as buying only cotton clothing for their children, using natural fibre bedding, often laundered very frequently, and avoiding soft toys. Many children had sensitivities or allergies; most of these had been medically diagnosed, but some were based on the observations of parents. This led to modifications to lifestyle and diet that caused varying levels of disruption; these ranged from accepted changes, such as replacing carpets with wooden flooring, to constantly monitoring diet and living in fear of anaphylaxis. It was notable that some parents and children had managed to adapt effectively even to seemingly major lifestyle changes. For some parents, eczema care, more than quality of education, influenced the choice of their child’s pre-school and school. They generally reported that care in the early years was excellent, but provision from Year 1 upwards was much more unpredictable; this provoked concern or even fear in some parents.
Quality of life
Eczema and the treatment required had a mixed impact on the quality of life of both children and their families. Every parent mentioned the scourge of itch and the disruption of sleep, with a few reporting that the whole family was disturbed by the child with eczema during the night. Listening to some parents, it was evident that they were struggling with their perceived inability to care for their child. They reported the challenges of applying topical medication regularly, in terms of both the child disliking application and the time taken to apply it. Several spoke of having tried ‘everything’ and conveyed their feelings of despair each time another treatment failed. Others appeared to feel this burden less and again this was not necessarily related to the described disease severity. Parents of children from the age of 5 years upwards talked about concerns regarding self-consciousness; some had deliberately encouraged their children to simply explain their condition to others and this had generally proved to be an effective strategy.
Hopes for the trial
Parents had a range of hopes for the trial. A few had very low expectations, but many had reached a point of desperation and were hoping for a ‘miracle cure’.
Theme 2: fit, durability and care
Participants expressed a range of views on the garments encompassing the subthemes of (1) look, fit and feel, (2) durability and (3) laundry care. Many parents and children awaited the arrival of garments with a sense of optimistic anticipation. Positive comments on the aesthetics of the garments were not forthcoming. Some suggested that they would be more attractive to children if they were coloured or decorated; however, they understood the hazards of introducing dyes to the fabric (Table 40).
| Look, fit and feel | Durability | Laundry care |
|---|---|---|
| [Daughter said] they’re just horrible . . . ugh, they’re just vile | They started off really nice . . . might as well have been putting dish rags on him by the end of it, they had holes in them | Mine fell apart because I was washing them so much |
| To be honest they are very clinical looking aren’t they, they are very basic | They start off like holes and they’d ladder round the edge almost | I couldn’t get the stains out |
| They weren’t tight enough . . . they’re a little bit baggy . . . in some places they were tight and in other places, the arms and that, were loose | Fabric’s just really . . . it just pulls apart . . . the material just disintegrates | [Three sets] . . . nowhere near enough, you’re always washing |
| The trousers won’t stay up so that’s why I had to do the top up under, to keep the trousers up | I cut the feet out because of the holes, they used to get holes in the toes where he used to scratch | [He wore them] as often as I could . . . unless I got behind in washing . . . it was all the time basically |
| He’s quite a skinny, tall lad but yes, they fitted quite well | They were getting trashed, you know, little boys outside | Three sets make sense but I could have done with a couple of extra . . . they get muddy or the odd explosive nappy |
| Actually after 6 months they still felt as they did at the beginning . . . they were still soft | They’ve been quite hard wearing | Three garments were fine, because he always had at least one fresh pair at any stage . . . they didn’t deteriorate at all |
| They didn’t feel right . . . they felt twisted . . . felt really awkward | They wore very, very quickly . . . and in the end we had holes in them and they were all pulled and grey | They went from white to grubby yellow-grey at the end of it |
| He thought they were really comfortable | . . . they have shrunk quite significantly . . . he’ll wear his underpants over the top of it . . . because it doesn’t stay up | I’d probably have three sets so I didn’t have to do the washing every day |
| She calls it her scratchy clothes | . . . they are totally worn . . . I think they are very badly made . . . they just tore from the seams . . . we sewed them up and we did everything we could to the last set | [Washing] was a struggle . . . I would only want her to wear them like one set for the day and one set for the night . . . because she shed so much skin |
| She didn’t find them particularly comfortable . . . they stuck really badly to her skin . . . it was like putting on clothes when you are wet | They are a funny sort of shape after washing . . . the tops go sort wide and the trousers were a bit shorter | |
| Because it was silk he could feel the coolness on his skin |
Look, fit and feel
Fit was an issue for many participants. On the whole, the garments fitted reasonably well for the youngest children. The exception to this was the styling of the neck, which was so loose on some that it slipped over their shoulders and gave easy access for scratching; some parents resorted to partially sewing up the neck seams. Garments for older children often bore no relation to their size or age range (despite actual height measurements being used to guide size selection); this was rectified by provision of alternative sizes by the trial team. There was marked uncertainty about how the garments should fit, although several parents commented that this information was provided. A snug fit was the preference for some, as this was viewed as an effective way to keep topical medications on the skin. Others favoured a looser fit on the assumption that this would make the garments cooler and more comfortable to wear.
‘Soft’ was a frequently used descriptor of the garments, although some parents were surprised by the texture as they had expected a feel more akin to normal silk. Parents who returned garments for replacement noticed that the texture of fabric was not consistent and, although some reported that softness was maintained over time, others suggested that the garments quite quickly became ‘crispy’ and ‘rough’. A few reported that their children found the garments scratchy and that they had a tendency to stick to their skin.
Durability
The issue of durability was raised regularly, seemingly more so for younger children, particularly boys. Although a few parents reported that the garments had lasted for the full 6 months of the trial, more recounted signs of wear and tear occurring after only a few days. Specifically, the garments were prone to the fabric pulling away from the seams, laddering and developing holes in areas of persistent scratching. This was managed by either returning the garments for replacement or, by some, creative methods of repair including the removal of the feet of the garments and the concoction of new garments from parts of damaged ones.
Laundry care
Parents adopted habits for washing the garments ranging from daily hand-washing using non-biological products to washing them every 2–3 days in a mixed family load. It appeared that washing was not a problem for parents who already laundered small loads separately, but it was burdensome to others and sometimes resulted in children missing wear for brief periods. The garments’ quick drying time was universally commended. There were reports of shrinkage after washing and some reported gradual discolouration over time. Many parents found that three sets of garments was insufficient; this was predominantly, but not exclusively, the case among younger children and those wearing them both day and night.
Theme 3: perceived impact of the garments
As seen in theme 2, parents had differing views on the look, feel and fit of the garments and this, combined with their commitment to being in the trial (subtheme 2 of theme 4), influenced patterns of usage, which forms the first subtheme of theme 3. Subsequent subthemes are the effect of the garments and intentions regarding continued use post trial (Table 41).
| Patterns of usage | Effect of the garments | Continued use post trial |
|---|---|---|
| He started asking for it ‘cos I think they kept him quite cool | We noticed a difference very quickly . . . within the first month . . . we don’t know if that’s why it’s cleared up or are we consciously putting the cream on more often | So the silks have actually retired to the drawer now . . . they are getting a bit small and tight now I think I just need to throw away my love affair with them and forget about them, which is a shame |
| . . . she wore them at school . . . [friends] just asked her what they was and she just told them | It made an immediate impact . . . this is the first time in 8 years that we’ve had a full nights sleep from him and this has absolutely 100% broken it [itch–scratch cycle], do you know this is the thing I’ve been waiting for, it’s brilliant . . . once we got the silk clothing, literally, it was like the light switched off | I wouldn’t recommend the clothes to anyone based on my experience . . . very, very disappointed |
| He was quite excited about [wearing the garments] we told him they were special clothes, his ‘whites’ | A little bit better but not massively better | I’ve since bought them, I buy them now, because they do work for us |
| . . . she didn’t do very well wearing them . . . because it hurt so much to pull them off . . . in the night when she does her damage the most and claws at herself then by the morning it’s stuck to the wounds she’s made . . . I just thought I can’t do this, it’s not worth getting her stressed out trying to get the garment on | I wouldn’t say they medically helped her and made it better, they just make her more comfortable and more bearable with the itching and stuff | We were looking at buying it . . . the cost of it! So I thought I can afford to buy a couple of pairs . . . I asked them to prescribe a couple of pairs and they did |
| She felt embarrassed because she said they were too see-through | I think it has contributed but it didn’t have an instant effect . . . it’s hard to pinpoint one thing | Would you ever consider buying them . . . no, not worth the money, no . . . if I could get them on prescription I’d definitely have them |
| She didn’t actually like wearing them . . . she had just come out of hospital and she had wet wraps . . . because they were white she thought I was going to wet wrap her . . . she got a bit wary of them | Well I think her skin improved, but I have to say that I couldn’t say that was because of the silk, but I do think . . . I think it has helped . . . I can’t 100% say that the clothing had definitely cured and fixed it, I think it has aided the whole process | If I had to pay . . . I probably wouldn’t invest in them . . . if the doctor would prescribe them, then yes, I’d definitely have some |
| To be honest they made no difference at all to her eczema | Yes, even if I had to pay for them I think I would in the long run, just for his comfort | |
| I thought that was just too expensive . . . I would probably say I would not because of the cost |
Patterns of usage
Patterns of usage varied enormously, from children wearing the garments virtually 24 hours per day throughout the trial, to the other extreme where one teenage girl could be persuaded to wear them for one night only. Parents reported differing thoughts from children about their family and friends seeing them in the garments; this appeared to be loosely related to developmental stage. Younger children quite enjoyed the attention of having something different, whereas older children preferred to wear them only at home, often covered with other clothing, partly because of their see-through nature. Most children wore the garments each night and a smaller proportion chose to wear them in the daytime. Garment usage is summarised in Appendix 20.
Effect of the garments
The reported effect of garments was wide ranging and not necessarily linked to frequency or consistency of wear. A minority stated that the garments had had a significant positive impact on skin condition. The most frequently cited benefits were comfort and coolness, sometimes leading to improved quality and quantity of sleep and providing an effective barrier to scratching. A few were very disappointed to find that the garments had no impact whatsoever.
Generally, those parents who reported improvements in their child’s skin condition or quality of life found it difficult to assess whether or not this was wholly a result of garment usage. Parents often suggested that the garments were just one element of a complex mixture of influencing factors. Other reasons for improvement in the eczema were suggested as being the usual waxing and waning nature of eczema, seasonal change, holidays in the sun and greater concordance with other treatments prompted by weekly reporting for the trial.
Continued use of garments
Parents were generally equivocal about continued use of the garments after the trial. A minority stated that they already had or definitely intended to buy further garments; for some this was because of a tangible improvement in the skin condition, whereas for others it was predominantly for comfort. Several parents had investigated the cost of garments and found this prohibitive, particularly because they were so quickly damaged or outgrown. In some cases parents continued to use garments that had become very tatty and in need of repair or were too small to the absolute end of useful life. A few parents considered requesting the garments on prescription, but did not have high hopes of success; although one parent had managed to obtain a GP prescription.
Theme 4: engaging in the trial
Participants were forthcoming about their engagement with the trial. This theme comprises three subthemes: (1) experience of participation, (2) commitment to the trial and (3) important outcome measures (Table 42).
| Experience of participation | Commitment | Important outcome measures |
|---|---|---|
| I think the way the trial was run is fantastic . . . it was always made very, very easy for us. The nurses were always so lovely with her | I was incredibly compliant to the enth degree | How his general well-being is, within himself |
| He loved getting the card . . . they made it special, a fun thing rather than a chore . . . he loved the fact that when we came here he couldn’t tell the lady | I busted a . . . because I thought if I’m doing this I’m doing it properly . . . I wanted it to be my magic cure | Well for me, it was just being avoiding having to use the steroid cream. That would be the ultimate goal |
| It is good to be involved and learn whilst the research has actually been taking place . . . we have really been excited to have been part of that | She was too embarrassed to wear them to school, so I didn’t overstress her . . . I don’t get into battles with her | A bit calmer and not be so itchy |
| I was always glad of the reminder email . . . it was good, it wasn’t too much . . . 5 minutes | Well we sort of discussed it with him and said look, there is really no point in saying you are going to do the trial if you are not going to be prepared to wear it all the time . . . he was quite on board with that | One hope really was for his eczema to get better and reduce the reliance on the steroid creams . . . you don’t want to keep putting toxic chemicals all over your child do you? |
| We had to send them back [and were told] we can’t send you some more until we’ve received the old ones back . . . and for us they were working so well I didn’t want him not to have them | The more we can invest in research, I think it’s the better. And if I can help that, then that benefits everybody doesn’t it? | Itching and flares |
| It’s easier to kind of forget about it . . . it only takes a few minutes . . . it’s more about remembering to fill in the form | I’m curious about this . . . I guess I’m quite scientifically minded so I wanted it to be a good result | How he looks |
| Going to the appointments was fine. Obviously I was able to pick times and days what suited me so that was fine | I just felt we were trying to make a difference so I didn’t mind . . . I realised that in order to maybe get better results from the study you would need to talk to people and it is like only a few minutes out of my time |
Experience of participation
Experiences of participation in the trial were almost universally positive. Parents were complimentary about the organisation of the process. In particular, they commented on the quality of information provided, the ease of communication with the trial team when required and the friendliness of the research nurses. The only minor negative was the need to return damaged or outgrown garments before new ones could be issued, sometimes leading to a break in wear for a day or two. All found the questionnaires and diaries quick and easy to complete, and appreciated the e-mail reminders. Several commented that the completion of the questionnaires had been useful in prompting more regular use of usual eczema treatments. Three issues were raised by a minority of parents. First, online questionnaires were available for only a finite time and this meant that they were occasionally missed. Second, in some cases the worst areas of eczema were on the hands and face, which were not covered by the clothing; parents suggested that this could lead to an unfairly negative evaluation. Third, parents thought that they were repeating themselves each week and that this may not be helpful. Older children sometimes completed information with parents and some parents were pleased to keep a personal copy of progress through the trial. Appointments were routinely made at convenient times and several parents were particularly grateful for home visits by the research nurses. Younger children were reported as having enjoyed being in the trial, particularly liking the sticker charts (see Appendix 8) and gifts. One boy objected to being undressed to be examined. Parents of older children reported that they were quite content to take part. Unsurprisingly, both parents and children allocated to the standard care group felt a great sense of disappointment on hearing their treatment allocation.
Commitment to the trial
Commitment to the trial revealed some insights into individual beliefs about levels of participation and about future recruitment, specifically to nested qualitative studies. All parents agreed that it was important to complete questionnaires and diaries. However, views on wearing the trial garments varied from wholehearted commitment to others who, not unreasonably, left the choice to their child. Parents who spoke of their engagement in the qualitative study reported that this opportunity was revealed right at the end of the study, and that this may have been detrimental to recruitment as some parents may have considered that the trial had already finished for them. Telephone calls from the research nurses who already had a relationship with parents were by far the most effective method of recruiting to focus groups and interviews. Some parents who were willing to take part struggled with the time and location of the focus groups, despite the variety of times and places offered. Those who did participate offered two major reasons for this: (1) they tended either to feel a sense of duty to give something back having been involved in the study or (2) they had some knowledge or interest in research and could therefore see the value of their contribution.
Important outcome measures
Parents unexpectedly found it difficult to talk about outcome measures that were important to them. On discussion, the most important success factors identified were comfort, improved sleep and general well-being, and reduced itching. Reduced medication usage, specifically steroids, was important to a fair proportion of parents. A few parents mentioned appearance and even less reduction in flares or disease severity per se.
Overall, the 34 participants in these focus groups and interviews presented mixed views on the usefulness of the silk garments. Many had struggled for years to effectively care for their child’s eczema and had tried an array of treatments, both prescribed and over the counter. Only a few had tried any type of garments. Most had very high hopes for the garments and were particularly enthusiastic about this non-pharmacological intervention. Patterns of wear varied enormously, as did views on the fit and durability of the garments. Many reported that the garments were of poor quality. Although a few parents reported unequivocal success in using the garments, many more were more circumspect about their impact, suggesting that they may have been helpful as part of a broader treatment regimen. In common with clinician and commissioners, parents had little knowledge about the silk garments and many were dubious about whether or not they represented value for money, particularly if they were purchased rather than prescribed.
Views of clinicians and commissioners
Data collection
Interviews with clinicians and commissioners in England were completed from June 2014 to January 2015. A purposive76,77 sample of participants was recruited via advertisements on health professional and dermatology websites, and snowballing. This approach yielded a range of dermatology specialist and primary care generalist participants from across the country. Some had dual roles, for example GP and commissioner, so they have been categorised by their stated primary role, and included dermatology specialist nurses (n = 9), dermatologists (n = 4), GPs (n = 3), pharmacists (n = 3) and health-care commissioners (n = 2). All clinicians had a minimum of 5 years’ experience of caring for people with eczema. Demographic information about the 21 participants is summarised in Appendix 20 (see Table 63). Semistructured telephone interviews using an interview guide (see Appendix 20) were conducted by the researcher (FC) lasting from 9 to 24 minutes. Interviews were audio-recorded and transcribed verbatim.
Data analysis
Data were analysed using the five-stage Framework Analysis process, described in Data analysis, and the researcher’s reflective account was used to inform the analysis process. Stages 1–3 of the framework are illustrated in detail in Appendix 20 and stages 4 and 5 are documented below. The analysis process yielded four key themes: (1) knowledge base, (2) reasons to use silk garments, (3) reasons for not using silk garments and (4) outcome measures. A total of 14 subthemes were identified. A description and analysis of each theme is provided below, and this includes a tabulation of each theme and subtheme with exemplar data extracts.
Findings
Theme 1: knowledge base
The theme concerning knowledge base comprises three subthemes: (1) lack of evidence base, (2) information from manufacturers and (3) treatment protocols. Participants presented differing views on the level of evidence available and the quality of evidence they required prior to prescribing silk garments (Table 43).
| Lack of evidence base | Information from manufacturers | Treatment protocols |
|---|---|---|
| There isn’t actually much evidence out there | I had contact with a rep[resentative] some while ago and was given some leaflets | There is a description of an appropriate place in treatment |
| There’s not enough big studies . . . to convince prescribers | I’ve got some of the manufacturers’ product information | A lot of CCGs [Clinical Commissioning Groups] will have issued guidance on the prescribing of silk products |
| But because there isn’t any evidence doesn’t mean that something doesn’t work | Rep[resentative]s have visited and given us evidence | We would expect them to go through all the NHS treatments first |
| I’m not keen until research evidence has been proven | You don’t just take the rep[resentative]’s word for it |
Lack of evidence base
The majority agreed that there was a significant lack of high-quality evidence and reported that until this was produced they would not consider prescription of silk garments. There was universal agreement on the need for the CLOTHES RCT, and many participants indicated that the outcome of this trial and of subsequent studies would influence their future practice. Participants noted that many treatments currently used are underpinned by very limited research evidence, citing the example of wet wraps, which are commonly used in childhood eczema. It was noted that lack of empirical evidence does not mean that a treatment does not work.
Information from manufacturers
A very limited number of participants were aware that more than one brand of silk garments is available, and most had received information from only one company representative; several could name individual representatives, but were hazy in their recollection of the product name. Views on the value of manufacturer information varied. Several participants stated that it was limited and potentially biased and that they largely discounted it; some suggested that, as this was virtually all that was available, it should be considered. Others took a more pragmatic view and were willing to base their treatment decision on this imperfect information combined with clinical need and personal experience.
Treatment protocols and guidelines
Treatment protocols and guidelines were raised predominantly by commissioners and GPs, some of whom stated that there were clear protocols in their local area. However, it was evident that the existence of such guidance was patchy, which led to a ‘postcode lottery’ on prescribing practice. The majority of participants implied the need for robust protocols that provide clear information about when and in what circumstances silk garments should be prescribed. Clarity about who should prescribe and in what quantity was also considered essential to ensure equity of provision. On the whole, participants favoured silk garments being part of a clear ladder of treatment and something to be used when other treatment options had been exhausted. This subtheme is allied to the first subtheme of theme 2 (failure of other treatment regimens); however, this subtheme refers to population-wide protocols, whereas failure of other treatment regimens applies more to decision-making in relation to individual patients.
Theme 2: reasons to use silk garments
Participants cited a number of reasons that silk garments may be used as a treatment option, and this theme comprises four subthemes: (1) failure of other treatment regimens, (2) greater concordance, (3) avoiding referral to secondary care and (4) cost-effectiveness. Around one-third of participants had prescribed, or recommended prescription of, silk garments in practice, but none claimed to be an expert in the use of these products or prescribed them on a regular basis (Table 44).
| Failure of other treatment regimens | Greater concordance | Avoiding referral to secondary care | Cost-effectiveness |
|---|---|---|---|
| It’s not a first-line treatment | If they were motivated because they liked something . . . then I would certainly carry on recommending it | If I saw a person was struggling . . . even to the point of considering secondary care referral . . . I’d probably feel confident in considering that prescription | It might potentially be a cost-effective option . . . don’t say cheaper |
| At the moment silk would be the last option | The goal of eczema treatments to give patient as much control as possible | I mean . . . it costs enough to refer them (to secondary care) | When they use the silk garments they need less steroid creams and obviously emollients |
| Once we’ve exhausted the usual treatments of emollients and topical steroids | It certainly gets rid of the wet wrap business | The most attractive place to use it is an alternative to secondary care referral | We are sometimes short-sighted in the way we look at costs . . . we should look at overall costs |
| It’s like a last resort | Compliance is obviously the biggy isn’t it . . . we do have some patients with cupboards full of stuff that hasn’t been used | . . . they don’t need as many visits . . . | |
| I tend to reserve it for the most severe cases | It’s a cost saving in the long run | ||
| More likely to prescribe if I can say we have tried this and it didn’t work |
Failure of other treatment regimens
All participants agreed that silk garments were not a first-line treatment, but rather that they were considered to be a ‘last resort’ for children who had already used emollients, topical steroids and sometimes also specialist cotton clothing and wet wrapping.
Greater concordance
Participants highlighted the challenges of treatment adherence with traditional eczema regimens. Several mentioned the acceptability and ease of use of silk, and proposed that this may lead to greater concordance with treatment plans.
Avoiding referral to secondary care
Participants, particularly nurses, suggested that, based on their clinical experience, silk garments had a value for children who were ‘hot’, ‘miserable’ and ‘itchy’ with their eczema, and that these symptoms were more likely than an objective measure of eczema severity to lead them to prescribe silk garments. An alternative reason for prescription, more commonly mentioned by GPs, was avoidance of costly referral to secondary care.
Cost-effectiveness
Some were able to quote the cost of both silk garments and secondary care referral, and considered that the choice of silk garments may be more cost-effective. In these cases cost alone was the dominant feature of decision-making. The price of silk garments was raised on many occasions. Some participants took a literal view of cost per item and this is discussed in more detail in the second subtheme of theme 3. In this subtheme participants took a far broader view. They acknowledged that the silk garments were ‘expensive’, but when set within a context of potential reduction in use of topical medication, alternative clothing, wet wraps and medical consultations, participants proposed that they could be a cost-effective option. In this group, participants also took into account the impact that these products may have on the health and well-being of both the child with eczema and his or her family.
Theme 3: reasons for not using silk garments
As seen in theme 2, many practitioners suggested that there was a place for silk garments in the armoury of childhood eczema care, but equally they were cautious and balanced this view with a number of reasons why these products should not be used at present. Theme 3 was generated from the following subthemes: (1) lack of familiarity or experience, (2) cost, (3) contentious prescription and (4) quality of product (Table 45).
| Lack of familiarity/experience | Cost | Contentious prescription | Quality of product |
|---|---|---|---|
| We haven’t used them a lot . . . so we haven’t got a lot of experience | We don’t want to see a sort of explosion [of prescriptions] . . . because that’s to no-ones benefit | Well there’s absolutely no doubt, this is a primary care prescribing thing | Within a couple of washes very dirty, look grey and have gone very baggy |
| As a GP many of us would struggle to have the time and the expertise | I mean it’s not that massive . . . it would be a manageable cost | More for secondary care I feel to prescribe it | Rep[resentative] says ‘oh they’ll wash for about a year’ . . . their knees get very thin and sometimes they only last about half of that time |
| I would never prescribe them by myself | It would worry me . . . we’re going to get inundated wanting all these little garments | Should be initiated by dermatology clinics and then obviously continued by GPs | They rip very easily |
| The problem with GPs initiating this is that you get creep prescribing . . . it escalates | Patients should be clinically assessed . . . and then it’s down to accountability and clinical confidence | Impractical in terms of current construction and surprisingly undesirable for teenage . . . patients | |
| Very good idea but practically rather expensive | Some GPs will and some GPs won’t [prescribe] | They get discoloured [parents] didn’t like the look of them | |
| My fear would be that I prescribed some very expensive item and they did not use it and it’s just a waste | There has to be some regulation here because pushy parents are going to get what they want |
Lack of familiarity or experience
All participants stated that they were not particularly familiar with silk garments and none prescribed them regularly. Some GPs believed that they would never have sufficient expertise or confidence to prescribe them, but a small number suggested that nurses, either dermatology specialist nurses or practice nurses, would be better placed to be experts in practice.
Cost
Silk garments were generally perceived as an expensive treatment option. The estimates of cost per set of top and leggings varied from around £40 to £100. The assumed cost of the garments, together with lack of familiarity, led to robust beliefs about responsibility for prescription.
Contentious prescription
There was a balance of views that prescriptions should be either from primary or secondary care, or be initiated in secondary care and continued by GPs. There was uncertainty from some participants about whether or not they were ‘allowed’ to prescribe these garments. Discussion of nurse prescribing was scant, and nurse participants generally reported that they advised medical colleagues to prescribe rather than undertaking the process themselves. Views on who should prescribe did not necessarily correlate with the participant’s job role; so, for example, not all GPs suggested that secondary care should prescribe and vice versa. A minority of participants suggested that the key factor in successful prescription was competent and thorough clinical assessment, preferably by a clinician who provides the most dermatology care for the patient. Commissioner and GP participants had concerns that if GPs began to prescribe silk garments this may open the floodgates to a widespread, costly and ineffective prescribing practice.
In practice, most prescriptions involved secondary care providers writing to GPs recommending silk garments; however, such requests were fairly regularly rejected on personal and unpredictable whim, rather than a clear clinical reason. Concerns were expressed that this could lead to treatment inequity, as ‘pushy’, ‘middle-class’ parents were more likely to persist with requests until they were met, whereas less affluent and less vocal parents were likely to give up.
Quality of product
The few participants who were relatively familiar with silk garments commented that there were quality issues, particularly considering the expense. They suggested that the garments did not wear well and that they looked unattractive and ‘grubby’ after several washes. A few participants reported that they were not acceptable to patients, particularly older children, and that if not used would lead to valuable resources being wasted; clearly, this argument could be applied to any treatment.
Theme 4: outcome measures
Participants acknowledged the need to measure outcomes of all treatments; however, the value they placed on different measures was notable. Theme 4 comprises three subthemes: (1) existing measures, (2) clinical improvement and (3) patient/parent reports (Table 46).
| Existing measures | Clinical improvement | Patient/parent reports |
|---|---|---|
| I assess . . . using a child or infant DLQI | Clinical improvement . . . a little bit hard to quantify | What the parent or child thinks . . . a description of severity |
| Eczema severity . . . we can do some kind of scoring tool | It’s clinical judgement . . . you’d be assessing social factors as well | In reality on the ground I would just say how have they improved? Mums and dads know pretty promptly whether it has |
| I suppose initially we would carry out an EASI score and a DLQI, that’s kind of like a measurable thing . . . we’d probably reassess every 3–4 months | Reduction in use of other treatments such as emollients and steroids | I would probably go by what the parents say |
| POEM . . . It’s easier to understand | Amount of flare ups they’re having | If the parent thinks it’s helping I’m okay with that |
| POEM is nice because you can share it with the patient | Are they sleeping better, are they feeling more comfortable, are they less itchy, scratching less | All the ones that had used them have had very positive results |
| You want two measures . . . quality of life and severity of the eczema . . . there must be some sort of graded . . . | The child is more relaxed because they’re sleeping and they’re not scratching all the time | Certainly the ones who’ve used them regularly do find them beneficial |
| Much more settled, that there’s not as many flares, that it’s not got infected | The parents said they thought they had helped | |
| How much distress the family suffers . . . you can’t necessarily gauge that by looking at the skin |
Existing measures
Participants generally talked in the abstract about what they considered to be best practice in measuring outcomes. None used an objective measure of disease severity, although one participant suggested that the use of EASI31 would be useful. Others raised the importance of patient/parent-based symptom or experience measures. Specifically, they advocated POEM34 and the Dermatology Life Quality Index (including child and family measures). No participants had used POEM with this patient group and a very small number had used formal quality-of-life measures. A few participants alluded to the need for measures but were not familiar with those already available.
Clinical improvement
Subjective measures of practitioner-assessed clinical improvement were much more commonly suggested. The constituents of clinical improvement included the child being more ‘settled’ and ‘comfortable’, and specifically less ‘hot’, and experiencing improved sleep and a reduction in itching, scratching and episodes of infection. A reduction in the use of topical medication was also used as a measure of effective treatment. Several participants suggested that parent and patient reports of improvement were every bit as valuable as clinicians’ views, and some stated that these alone would be sufficient for them to advise the continued use of silk clothing. The impact on family as well as children was particularly highlighted by nurse participants.
Patient/parent reports
The few participants who had prescribed silk garments reported positive, but not overwhelming, feedback from parents. They reported that children found the garments ‘cooling’ and that they did not adhere to the skin and could be used even in the presence of infection.
Overall, the 21 participants in this study had limited experience in the use of silk garments for childhood eczema, and anecdotally this is a fairly typical picture across the country. It was stated that there is a dearth of evidence about the effectiveness of these garments, a position that participants were keen to see rectified through the CLOTHES trial and subsequent studies. On the whole, silk garments were perceived as expensive, although when put into a wider context many participants suggested that they may provide value for money. The prescribing of silk garments was contentious, with participants holding firm beliefs about whether this should be the responsibility of primary or secondary care. There was consensus that use of these products should be monitored and evaluated, although there was little agreement on the preferred outcome measures to be used.
Conclusion
In this nested qualitative study children and parent participants have provided insights that correlate closely with the quantitative results in that there was some limited improvement in eczema for some children, but the hoped-for ‘miracle cure’ did not transpire. On the whole, clinicians and commissioners had limited knowledge and experience and were reluctant to prescribe garments that they perceived as costly and lacking in robust evidence of effectiveness. Collectively, the qualitative component of the CLOTHES trial illustrates a very mixed picture of knowledge, beliefs and experiences of using the silk garments.
Limitations
There are two limitations to this nested study. First, participants were essentially self-selecting and may therefore not be representative of the trial cohort. Second, the recruitment of children from recruitment centres in prescribed age bands was difficult and we would have preferred to recruit more children. We considered recruiting to wider age bands for the focus groups, but made the decision not to do this as it would have compromised the value of using age-appropriate activities to enable children to convey their thoughts and feelings.
Key learning points
Implications for interpretation of trial results
-
Children’s responses were particularly helpful in providing additional detail on possible reasons for non-adherence in wearing the garments.
-
Parents and children portrayed mixed views on the garments. Few, if any, reported the longed-for ‘miracle cure’ and there was a significant sense of disappointment in relation to effectiveness and the quality, fit and durability of garments.
-
Some parents found the weekly questionnaires useful in prompting more regular use of their child’s usual eczema care.
-
Clinicians and commissioners were generally equivocal about the use of silk garments. Most wanted a robust evidence base to inform treatment decisions and were eager to see the results of the CLOTHES trial.
-
Results of the qualitative studies were in line with the quantitative trial data, suggesting that important differences between the groups had not been missed.
Added value of qualitative studies within trials
-
The added value of the qualitative work is that it has provided a deeper, richer and more detailed understanding of the ‘what’, ‘why’ and ‘how come’ underpinning participant’s beliefs and behaviours. It has uncovered understandings, which help to inform interpretation of the results of the study.
-
Parents who participated were universally positive about having an opportunity to talk with a researcher who was interested in their child’s condition, but, perhaps more importantly, focus group participants relished meeting other parents in the same situation.
-
Likewise, children wanted to talk with the researcher and were very able to express their views with clarity, given age-appropriate means of communication.
Lessons for conduct of nested qualitative studies
-
Recruitment to qualitative studies nested within RCTs can be challenging. Research nurses who had personal contact with participants were crucial to successful recruitment. Participants in some centres were geographically widespread and the ages of children completing the trial at a time suitable for involvement in the focus groups meant that many participants were unable to take part.
-
Early awareness of the forthcoming focus groups may help to boost recruitment.
-
Child data added a valuable dimension to our understanding of childhood eczema and the use of silk garments. However, these were time-consuming to collect as there was a need for pre- and post ‘playtime’. Recruitment was particularly challenging, as the groups were age banded to ensure the appropriateness of data collection methods.
Chapter 6 Involvement of patients and the public
Public and patient involvement (PPI) in research has been strongly encouraged for many years,82 but, until recently, the evaluation of PPI activities and the impact that they may have on the design, conduct and delivery of clinical trials has been limited. 83–85
In this chapter, we aim to summarise the breadth and depth of PPI involvement that has taken place throughout the lifetime of the CLOTHES trial, and to share our experiences in documenting the likely impact of this activity.
Aims
-
To evaluate the impact of PPI on the design, conduct and dissemination of the NIHR HTA-funded CLOTHES trial.
-
To add to the literature on PPI and its potential impact on research.
Methods
This report synthesises various strands of activity that have taken place over a period of many years, spanning October 2009 until now. Diverse methodologies have been employed according to the stage of the research and the types of PPI input required. In line with INVOLVE guidance,82 specific ethical approval was not required for the majority of the described engagement activities.
Throughout the study, we adopted the eight core principles framework identified by Telford et al. ,86 as outlined in Table 47. Documentation of impacts throughout the stages of the research from prioritisation of the topic through to dissemination of the findings has been presented using the framework proposed by the Public Involvement Impact Assessment Framework Study Group87 and reported according to the Guidance for Reporting Involvement of Patients and Public guidelines. 85
| Core principles | How evidenced in CLOTHES trial |
|---|---|
| Principle 1: the roles of the consumers are agreed between the researchers and the consumers involved in the research | The role of the PPI representatives was documented in the funding application, protocol and final report |
| Principle 2: researchers budget appropriately for the costs of the consumer involvement in research | PPI costs were included in the budget (0.6–1.6% of total budget) and PPI representatives were reimbursed for their travel and child care (and in some instances, their time) |
| Principle 3: researchers respect the differing skills, knowledge and experience of consumers | Contribution of PPI representatives’ skills, knowledge and experience has been included in research reports and papers |
| Principle 4: consumers are offered training and personal support to enable them to be involved in research | Support and training in research methodology and understanding of key terminology was provided by the Centre of Evidence-Based Dermatology’s Patient Panel, and by a dedicated member of staff who is responsible for co-ordination of PPI and engagement activities within the research group |
| Principle 5: researchers ensure that they have the necessary skills to involve consumers in the research process | Researchers involved in the design and conduct of the CLOTHES trial attended the Centre of Evidence-Based Dermatology’s Patient Panel training events and were encouraged to attend conferences and workshops addressing the importance of PPI involvement in research |
| Principle 6: consumers are involved in decisions about how participants are both recruited and kept informed about the progress of the research | PPI representatives involved in meetings to discuss trial design and conduct and were involved in amending paperwork in response to potential difficulties with recruitment. PPI representatives were involved in writing and communicating updates about the trial and helping with trial updates via social media and websites |
| Principle 7: consumer involvement is described in research reports | PPI contribution and analysis of its impact included in the final report and written up as a separate paper |
| Principle 8: research findings are available to consumers, in formats and in language that they can easily understand | Trial disseminated widely with the help of PPI representatives, including lay summaries, contributions to patient support group newsletters, social media, websites and podcasts |
For the purposes of this report, we define PPI as being inclusive of all relevant stakeholders and users of the research. As well as focusing primarily on involvement of patients, their carers and the general public, we also include examples of involvement with other key stakeholders including health-care professionals, commissioners, providers of health information, guideline writers and researchers.
Patient and public information activities were logged on an ongoing basis throughout the trial using a dedicated PPI log that all members of the trial team had access to.
Results
Contextual factors relating to patient and public information involvement
Many factors contributed to ensuring strong PPI in the CLOTHES trial.
Funding body
The CLOTHES trial was funded by the NIHR HTA programme. This public funding body was one of the first in the world to recognise the importance of PPI, both at an organisation level (with PPI members on funding panels and PPI reviewers) and through the research projects that it funds. This strong endorsement by the funding bodies meant that PPI was embedded within the prioritisation process in identifying the topic area for research, encouraged the involvement of a patient representative as a co-applicant on the grant, and allowed the inclusion of costs and time to facilitate PPI input.
Research group
The trial was developed by a research group with a strong track record of conducting AE research (thus allowing ready access to patient partners and relevant networks, to facilitate speedy engagement with users). This group had organisational structures in place prior to starting the CLOTHES trial, including an established patient panel whose members receive training and support through face-to-face workshops, newsletters and attendance at relevant training courses/conferences. The panel is supported by a dedicated member of staff (PPI manager) who maintains regular newsletter communication with members of the panel and signposts members to upcoming projects requiring PPI input.
Sponsor organisation
The School of Medicine at the University of Nottingham places strategic importance on the involvement of patients and the public in both teaching and research activities across the school. It funds a PPI co-ordinator post to support researchers in developing PPI initiatives and runs regular training workshops to ensure that researchers have the necessary skills to engage effectively with PPI partners.
Patient support groups
The research team had strong pre-existing links with two active patient support groups (National Eczema Society and the Nottingham Support Group for Carers of Children with Eczema). Both support groups were very familiar with our research activity, had participated in the James Lind Alliance Eczema Priority Setting Partnership6 and were keen to support the trial.
Stages of research and opportunities for patient and public information impact
A summary of the stages of research that provided opportunities for PPI engagement are summarised in Table 48, along with details of the PPI activities and their impact.
| Stage of the research | Methods used in CLOTHES trial | Measures of impact |
|---|---|---|
| Research agenda setting |
|
|
| Research design and delivery |
|
PPI input informed multiple trial design decisions including:
|
| Ethics and oversight |
|
|
| Recruitment |
|
|
| Data collection |
|
|
| Data analysis and interpretation of results |
|
|
| Writing up |
|
|
| Dissemination |
|
|
| Time and cost |
|
|
Research agenda setting
The CLOTHES trial was developed in response to a commissioned call by the NIHR HTA. This pre-dated our formal James Lind Alliance Priority Setting Partnership, in which patients and health-care professionals identified the most important areas of treatment uncertainty. 6 Nevertheless, results of the Priority Setting Partnership confirmed patients’ strong interest in non-pharmacological interventions for the treatment of AE.
Trial design
In order to inform trial design from an early stage (pre-funding), we conducted an online patient survey (Survey MonkeyTM, SurveyMonkey, Palo Alto, CA, USA) that was designed with the assistance of PPI members of our patient panel. The survey consisted of 25 questions, took approximately 10–15 minutes to complete and was open from January to April 2012. Links to the survey were distributed via the National Eczema Society and the Nottingham Support Group for Carers of Children with Eczema [which has a Twitter (Twitter, Inc., San Francisco, CA, USA) following of 5600] to patient participants of the James Lind Alliance Priority Setting Partnership, to AE patients who had taken part in previous AE trials conducted at the Centre of Evidence Based Dermatology, and through social media and other personal and professional networks.
Participants were asked to complete the survey if they were a parent of a child with AE. A total of 475 parents completed the survey [296 (62%) completed all 25 questions]. All responders were included in the analysis regardless of completeness of individual questions.
The survey confirmed that having AE influenced parental choice of clothing for their children (69% quite a lot, 26% a little and 5% not at all) and 293 out of 454 (65%) participants said that they had bought special clothing because of their child’s AE. Factors parents considered important when choosing clothing to be used next to the skin were natural fibres (85%), avoidance of wool or scratchy fabrics (79%), softness (56%), cost (43%), fit (42%) and ease of washing (41%).
Specific issues relating to trial design and the impact of PPI input are summarised in Table 49.
| Design issue | Why decision required PPI input | PPI feedback | Design decision |
|---|---|---|---|
| Eligibility criteria | |||
| Should age of participants be limited to children aged ≤ 5 years? |
|
48% of children who expressed an interest in taking part in a trial of specialist clothing were aged > 6 years (35% were aged 6–11 years and 13% aged 12–16 years) | Eligibility criteria were broadened to include all children up to the age of 15 years in order to be as inclusive and generalisable as possible |
| Should patients who routinely use wet wraps be excluded? |
|
Of those currently using wet wraps (n = 89), 46% used wet wraps < 10 times in the previous 6 months, but 38% used them > 30 times in the last 6 months | Exclusion criteria were defined to exclude participants who used wet wraps very frequently (≥ 5 times in the previous month) – equivalent to 30 times in the last 6 months |
| Definition of intervention | |||
| How should clothing be prescribed for use (wear 24 hours per day, night-time only, or as preferred)? |
|
Of 269 responders, 52% said their child would be willing to wear the clothing only at night, while 46% said that their child was willing to wear the clothing day and night; 19% would wear the clothing during the day, but not during PE/games lessons | Children were asked to wear the clothing as often as possible, and as a minimum every night |
| Trial design: impact on trial delivery | |||
| Should participants allocated to standard care receive silk clothing after the randomised allocation follow-up is complete? |
|
Of 298 responses, only 59% said they would still be willing to take part if they were allocated to standard care | Trial design amended to allow participants allocated to standard care group to receive the silk clothing after 6-month primary outcome assessment was complete |
| Trial analysis | |||
| How much would parents be willing to pay for specialist clothing? |
|
|
|
Trial delivery and recruitment
Following publication of a press release announcing the start of recruitment into the CLOTHES trial, the trial team received considerable interest from media outlets including television, radio, newspapers and magazines. Having access to PPI partners who were willing to give interviews about the impact that AE has on their lives ensured that the news stories were widely distributed and engaging. In total, three parents, two children with AE and a representative from the National Eczema Society gave media interviews (Figure 24).
FIGURE 24.
Patient and public involvement co-applicant Amina being interviewed with her son Tahmid about the trial for BBC local news. BBC, British Broadcasting Corporation.

As a result of this media coverage, the trial team received 492 expressions of interest from potential participants in the space of 3 months. This resulted in a surge in recruitment at the start of the trial and meant that the trial was consistently ahead of recruitment targets throughout the study (Figure 25).
FIGURE 25.
Recruitment graph for the CLOTHES trial showing impact of initial media interest.

Interpretation of results
The nested qualitative study involving health-care commissioners, health-care providers and participants of the trial was extremely useful in providing additional contextual information to aid interpretation of the trial result, in guiding how best to disseminate the trial results to ensure speedy uptake and in helping to understand potential barriers to the use of silk clothing in children with eczema (see Chapter 5).
Reflections from our patient and public involvement partners
As a member of the CEBD [Centre for Evidence Based Dermatology] panel I was part of the initial priority-setting research which led to this trial and have felt privileged to be given the opportunity to play such an active role in this research, which is close to my heart.
Early mainstream media interest in this trial has made me to feel more involved than I may have had in other projects, making it a pleasant and rewarding experience. Throughout the research process I feel my contribution has been valued and treated with professionalism and the team have made a conscious decision to drive inclusive behaviours through the use of ‘simple’ plain English versus technical jargon.
Amina (PPI co-applicant)
As an attendee at the original Centre of Evidence Based Dermatology Patient Panel where the concept for the CLOTHES trial was first aired, I have been delighted to be involved at various stages of this well-run trial. I know the result will be of use and interest to the followers of the Nottingham Support Group for Carers of Children with which I am involved.
Amanda (member of CEBD patient panel and main contact for Nottingham Support Group for Carers of Children with Eczema)
As the lay member of the Trial Steering Committee I did not have as active a role as the other PPI members, but took part in all the meetings and kept up to date with the progress of the trial. I also liaised with the National Eczema Society and took part in the CLOTHES Collaborators meeting in June 2015. I am impressed with the interest in non-pharmacological interventions for the treatment of AE shown by the patients and carers in this trial and with the PPI input at all stages of the trial.
Rosemary (PPI member of Trial Steering Committee)
Discussion
This chapter documents the breadth and diversity of PPI experience that has contributed to the success of the CLOTHES trial. PPI was an integral element throughout the research life cycle of this national, multicentre RCT. It involved engagement with both children and their families, thus presenting additional challenges and opportunities for engagement. We have demonstrated how the contribution of PPI to this study has resulted in measurable impact, and hope to have inspired future researchers to think creatively about how best to engage with patient partners and the wider community to improve research.
By necessity, we have worked extremely closely with some of our patient partners and these individuals have now become skilled researchers in their own right. This phenomenon of ‘professional patients’ has been documented previously90,91 and requires awareness of the shifting roles of members of the team as time progresses. We sought to limit the impact of this effect by reaching out through social media to as many patients as possible at crucial times; to ensure that diverse views were captured (in particular through online surveys and the nested qualitative study). Wider engagement is often particularly important while setting research priorities, in finalising the trial design, and during dissemination and implementation of the results.
Documenting evidence of impact of PPI is a constant challenge and one that requires a systematic approach involving all members of the research team. It is the responsibility of the lead researcher to ensure that all members of the trial team understand the importance of PPI and how to record it. The documentation of negative impacts of PPI remains a particular challenge, and may well require different methods to elicit feedback and documentation of such effects.
Involvement in teleconference conversations that involve multiple people can often be particularly challenging for new PPI partners. Special efforts should be made by the chairperson of such meetings to ensure that PPI partners feel supported and able to voice their opinions freely. 92 It is often advisable to provide specific training prior to participation in such meetings, or to have a pre-meeting conversation with PPI members so that they have an opportunity to clarify the proposed agenda items and to ask any questions that they might feel uncomfortable in asking in the wider group.
Conclusion
The NIHR-funded CLOTHES trial was a successful trial for many reasons. Having strong PPI embedded within an environment that values PPI input was just one aspect of a strong multidisciplinary team with experienced CTU support. However, the benefits of PPI far outweighed any potential negative impacts and contributed in diverse ways throughout the lifetime of the project.
Chapter 7 Experiences of working with clothing suppliers
Background
Collaboration between academia and industry in delivering clinical research has been given high priority within the UK’s research agenda. 93 The advantages of collaboration can be multifaceted, bringing mutual benefits to all parties. However, working together with industry partners to deliver publicly funded research can also be challenging as a result of the differing expectations and priorities.
Industry partners often have varying levels of engagement with the research process, depending on the needs of the individual project. Having previously collaborated with industry to successfully deliver the NIHR HTA-funded SWET,61 the CLOTHES trial team sought to draw on lessons from this previous experience in establishing a successful collaborative relationship.
Delivery of the CLOTHES trial was supported by two silk garment suppliers (Espère Healthcare Ltd, UK distributor of DermaSilk, and DreamSkin Health, manufacturer of DreamSkin). These two companies donated the silk garments for use in the trial free of charge. This donation was made on the basis that the companies had no input into the trial design, conduct, analysis or interpretation of the trial results (other than to inform logistics of trial delivery, and to advise on appropriate use and care instructions for the garments).
This chapter describes the role that the clothing suppliers played in ensuring the success of the CLOTHES trial and reflects on lessons learned for future trials.
Priorities and responsibilities
Working with industry can highlight differences in culture between the private and public sector, as the needs and drivers for both parties are necessarily different. For the public sector, the primary aim is to provide better quality evidence to inform medical decision-making and health commissioning, as well as academic drivers ensuring the need to publish in high-impact scientific journals. For the private section, any commercial venture clearly relies on making a financial profit to pay their employees and to satisfy shareholders.
For the companies involved in supplying garments for the CLOTHES trial, there were many benefits of being involved in such a high-profile, publicly funded clinical trial. However, there were also considerable financial risks, should the study show that the products were not effective.
For the trial team, the benefits of having the garments donated from the two participating companies meant that trial set-up was expedited with regard to negotiation of treatment costs with participating NHS trusts. Under the terms of research funding within the UK, these costs are not covered from within the research budget and are negotiated with participating health-care providers prior to starting the trial. The main risk for the trial team was the potential for perceived lack of independence in conducting and reporting the trial, and potential risks over continued supply of the interventional product throughout the trial.
We sought to build a working relationship between the trial team and the clothing suppliers that was fair to all parties, and ensured that any perceived or actual influence from the clothing suppliers was minimised (Box 2). This approach was taken in order to protect the core principle of research independence and to ensure validity of the results. To achieve this, a number of processes were put into place, based on the trial team’s previous experience and the recommendations laid out in Maskell et al. 94 (Table 50).
Responsibilities of the clothing suppliers were to:
-
supply silk garments for the trial
-
provide replacement garments for any worn-out or outgrown garments
-
provide guidance to the trial development team on appropriate use of the garments
-
establish a system for supplying garments as required
-
advertise support for the trial in a responsible manner.
Responsibilities of the CLOTHES trial team were to:
-
design and conduct the trial according to a pre-defined and registered protocol
-
secure ethics and R&D approvals as needed, and to conduct the trial in line with local and national governance requirements
-
pre-define the statistical analysis plan before locking the data set
-
maintain independence and clinical equipoise in relation to the trial results
-
clearly communicate with clothing suppliers over progress of the trial and the rationale for design and analysis decisions.
R&D, research and development.
| Recommendation | Implementation within the CLOTHES trial |
|---|---|
| Provision of regular written reports for the industry partner |
|
| Continual monitoring and prompt resolution of concerns |
|
| Basic research practices education for industry partners |
|
| Minimisation of industry partner contact with participants |
|
| Clear roles and responsibilities of all stakeholders |
|
| Clarify and have a clear understanding of roles and responsibilities of research governance departments and ethics committees to avoid confusion and potential disagreements |
|
| Communication through an independent third party if possiblea |
|
| Engage with multiple partners to ensure generalisability of findings and to limit commercial advantage to any one companya |
|
Involvement of the clothing suppliers in trial design and set-up
The original NIHR HTA commissioning brief did not specify any particular brand of silk garment to be used in the trial. At the time of trial design, two CE-marked brands of commercially produced silk garments were available on the NHS formulary (DermaSilk and DreamSkin). The suppliers of these two brands agreed to support the trial and supplied a letter of agreement to donate the garments to support the funding application.
At the trial design stage, engagement with industry representatives was most helpful in four key aspects:
-
Helping to secure NIHR funding. Having letters of support from each of the clothing suppliers was helpful in demonstrating to the funders (NIHR HTA) that the clothing suppliers were fully committed to the trial.
-
Informing correct use of intervention. Advising on appropriate use of the silk garments (e.g. washing instructions, concurrent use of AE medications, number of garments required, best ways of measuring the size of the child to ensure the correct size of garments).
-
Facilitating site set-up. Having donations of the garments meant that our planned recruiting sites were opened quickly and with minimal difficulties in negotiating NHS treatment costs. However, one potential recruiting centre was unable to open as a result of unwillingness on the part of the NHS trust to agree to the low risk of paying for the silk garments, should the clothing companies be unable to fulfil their offer of donation.
-
Trial conduct and logistics of supply. The trial team worked closely with company representatives to establish appropriate systems for ensuring delivery of the product in a timely manner, as well as minimising the requirement to hold multiple sizes and styles of garments at the co-ordinating centre.
Involvement of clothing suppliers during the trial
Trial logistics
Both clothing suppliers were supportive and were responsive in addressing questions or concerns about use of the silk garments. They worked closely with the trial co-ordinators at the Nottingham CTU to ensure efficient supply of the garments and development of an audit trail.
The central team at the CTU acted as the distribution hub for all trial garments to increase efficiency of stock supply, to maintain blinding of local site staff and to act as an intermediary between participants and the clothing companies (in the event that questions arose regarding the clothing). A detailed inventory of stock was kept so that supplies could be ordered weekly, providing accountability of garment usage and serving as a reordering system. Any returned stock (because of ill fit, excess wear or child growth) was returned to the manufacturers for them to see how the clothing held up with regular use.
Although three sets of garments were supplied to all participants, the need for replacement garments during each participant’s 6 months’ involvement in the trial was underestimated during our planning phase. This presented an increased financial burden on the companies, which remained supportive and supplied the additional garments after discussions with the trial team about why they were required. In this regard, it was helpful to work with two companies, so that the financial burden could be shared.
Having two companies involved in the study was also particularly beneficial when one of the companies experienced supply problems partway through the recruitment period. Each supplier remained supportive of the trial, doing all they could to allow the trial to continue. The company that experienced stock shortages kept back reserves of their dwindling stock for the trial, leaving them with fewer to sell on the market. The other company provided cover for their commercial competitor, providing more garments to the trial than originally intended.
Independence from the trial team
As outlined in Table 50, we attempted to replicate the model of engagement with commercial companies through an independent third party, as was successfully achieved in our previous NIHR HTA-funded SWET. To achieve this, we asked a representative of the lead NHS trust and the local lead for the comprehensive research network to be independent arbiters between the companies and the Trial Management Group. However, despite initial agreement from all parties, this approach did not work well in reality. This was largely because the nature of the ordering and stock control system meant that it was necessary for the trial management team to have weekly contact with representatives from the clothing suppliers. As a good working relationship developed, the majority of trial communication inevitably involved the trial manager. Nevertheless, this situation ensured that any concerns were resolved quickly, which was beneficial for delivery of the trial, and direct contact with the rest of the Trial Management Group (including the chief investigator) was kept to a minimum.
Training in trial design and interpretation
Given the importance of this trial to the financial concerns of both companies, we strove to ensure that the rationale for our trial design was clearly explained before, during and after the trial, so that the clothing suppliers had a good understanding of the trial design and conduct. In particular, we explained our approach to controlling for bias and the implications of running a pragmatic RCT.
As outlined in Table 50, we conducted face-to-face meetings to discuss these issues prior to start of recruitment, and again prior to data analysis, to ensure that the clothing suppliers understood the reasoning behind trial design decisions. In particular, these meetings focused on explaining the following key aspects:
-
what a pragmatic trial is and implications for the design of the trial
-
importance of the primary outcome and the need for an objective primary outcome
-
interpreting unblinded trials
-
understanding p-values and CIs
-
individual versus group mean effects
-
the need for transparent reporting of results
-
timelines and availability of the findings
-
ownership of the data and write-up of study results.
Involvement of clothing suppliers after completion of the trial
Given that the CLOTHES trial failed to find any clear benefit of silk clothing for improving eczema severity, it is perhaps not surprising that the final dissemination stage of the project has also been the most challenging to manage. Both suppliers are passionate about their products and about the potential health benefits that the garments could bring to patients. Their passion is reflected in the significant investment both suppliers made in the trial. This belief in their product based on earlier, smaller, sponsored, studies and testimonies from satisfied customers undoubtedly led to an expectation, both implicit and explicit, that the trial would show the garments to be beneficial.
The need to ensure academic independence until the data had been fully analysed and interpreted, and the trial report had been subject to peer scrutiny was a difficult balance to achieve in a manner that was acceptable to all parties. Our two clothing suppliers were understandably eager to receive the trial results and to ask questions, but it was important that we did this in a transparent and accountable way, and without allowing them an opportunity to change any of the pre-planned analyses or the interpretation of the results. Both companies received a copy of the full report after it had been through peer review, but prior to publication. The companies provided reflections to the trial team that were incorporated into the report at the team’s discretion. The feedback included notification that a mistake had been made in applying the unit costs of the garments in the health economic analysis, which resulted in the analysis being corrected and re-run prior to publication. Other minor amendments to the report at this stage are listed in Appendix 21.
Of particular concern to the companies was the desire to explore the results specific to their individual brands (in addition to the combined results). This was potentially problematic as the trial was not powered to look for such comparisons, although those allocated to receive the intervention were randomised to the two brands, which allowed exploration of brand effects in tertiary analysis.
Discussion
This chapter has provided brief reflections on our experiences of delivering a large, independent trial with support from clothing suppliers in the form of donation of the trial intervention. This co-operative partnership has brought many benefits, but also challenges, particularly as the commercial needs of the companies are at odds with the overall findings of this trial. A summary of our key reflections on completion of the CLOTHES trial is presented in Box 3.
-
Ensure roles and responsibilities are documented for both parties from the outset – particularly in relation to continuity of supply, when and how the results will be shared, and intellectual property.
-
Ensure sign-off of the trial protocol by all stakeholders prior to commencing the trial.
-
Be aware of the benefits and potential difficulties of using multiple brands to represent one randomised intervention.
-
Early engagement with suppliers to establish ‘how to use their product’ can help to avoid claims of inappropriate use at a later date.
-
If planning a pragmatic clinical trial to establish effectiveness of an intervention in normal practice, special efforts should be made to ensure that all industry colleagues understand the concept of a pragmatic trial (sometimes referred to as a comparative effectiveness trial), and the implications for trial analysis and interpretation.
-
Keep detailed notes of what is discussed during meetings – this can be helpful in preventing debate at the end of the study as to what was agreed and when.
Despite our best efforts to maintain independence between the trial team and the companies involved, the need to work together over the past 3 years has meant that a relatively close relationship has been established for some members of the team (especially those at the co-ordinating centre). However, contact with research nurses and investigators at recruiting centres was successfully limited.
Our agreements with the clothing suppliers took the form of letters of agreement to supply the garments. At the time, this was felt to be sufficient to document the agreement between the University of Nottingham and the clothing suppliers. However, a formal contract that outlined key responsibilities for all parties, defined expectations and clarified ownership of intellectual property may have been useful, and we would recommend this approach for future trials.
For the CLOTHES trial, there were benefits of working with two suppliers:
-
better generalisability of study results
-
security of supply (not reliant on a single supplier for the success of the trial)
-
reduced financial burden for individual companies.
However, for non-drug trials, where the comparability of the interventions may be less clear than in drug trials, there is also a risk to the trial integrity, particularly if different products, which were assumed to be functionally similar, prove to be different in terms of treatment response. Thankfully, for the CLOTHES trial, this was not the case and the ability to generalise from one branded product to a class of products is helpful for informing clinical decision-making.
Alternative models of industry involvement in academic trials are available and these may warrant further consideration. In particular, our relationship with the companies may have been very different had the garments been supplied at a reduced cost rather than free of charge. However, in such a scenario, the delays in negotiating NHS treatment costs with each participating site would still have been present, potentially delaying the start of the trial and choice of recruiting centres. We would be pleased to see further consideration of appropriate funding models to address excess treatment costs within the UK funding landscape, as this currently represents a considerable source of delay and wasted resource.
Chapter 8 Discussion
Main findings
This trial found no evidence of any clinical or economic benefit of using silk garments compared with standard care in children with moderate to severe AE.
There were no differences between the treatment groups for any of the blinded outcomes. Furthermore, the 95% CIs around the primary efficacy estimates were narrow, suggesting that a clinically important treatment effect is unlikely to have been missed. Sensitivity analyses (imputing missing values, adjusting for baseline imbalances and exploring the impact of adherence in wearing the garments) supported the primary analysis.
Subgroup analysis based on FLG genotype showed no evidence of differential treatment response in children with an inherited impairment in the skin barrier function. A post hoc analysis exploring the impact of baseline severity on the primary outcome also showed no effect, suggesting that children with more severe disease were unlikely to benefit from the clothing more than children with milder disease.
It is possible that silk garments could prove beneficial in the absence of a change in disease severity if the garments resulted in a sparing effect on topical corticosteroid use. However, the proportion of days on which topical corticosteroids or calcineurin inhibitors were used did not differ between the groups.
The intervention garments are marketed as possessing antimicrobial properties, but this trial found no evidence to suggest a reduction in the number of skin infections in those using the garments compared with those randomised to standard care alone.
Of the seven unblinded secondary outcomes, only POEM and PGA showed differences in favour of the silk garments. However, the observed differences in POEM and PGA were small, making them unlikely to be clinically meaningful for patients. 40 These effects appeared to be most prominent during the first 3 months of the trial, when belief in the effectiveness of the garments was most likely to influence responses. It is possible that these effects occurred by chance, as many secondary outcome variables were assessed. However, a previous AE trial of non-pharmacological interventions has reported similar differences between blinded and unblinded outcomes. 61 The nested qualitative study highlighted the high hopes that both children and parents placed on the trial intervention from the outset and would suggest that risk of bias for participant-reported outcomes is likely to be high in a trial of this kind.
Relevance to existing literature
There have been no further RCTs on the use of silk garments for AE since the CLOTHES trial began (search updated 13 April 2016) and meta-analysis of the four existing silk trials (including CLOTHES) is not possible because of the heterogeneity of designs. Additional brands of silk garments have since become available for use in AE (e.g. Skinnies), but these have not been formally evaluated in RCTs.
At the time of commissioning this research (2011), £840.272 was spent on prescriptions for silk garments in England alone. By 2015, this amount had risen to £2,039,575 per annum.
Strengths and limitations
This was an adequately powered, independent RCT, with high follow-up rates and good adherence to the trial intervention. The trial placed special emphasis on objective outcome measures in order to minimise detection bias and the pragmatic study design meant that use of silk garments was evaluated as they might be used in normal practice with mixed patterns of adherence.
It is possible that our emphasis on objective AE severity outcomes meant that some important potential benefits were not captured in the primary analysis. Other factors, such as improvements in quality of life or a reduction in symptoms (especially itch and sleep loss, as recorded in POEM), may be important drivers in determining whether or not parents choose to purchase silk garments for their children. However, the magnitude of any such benefits was small, and we found no evidence of improved quality of life among trial participants using a range of validated quality-of-life scales.
It is also possible that treatment effects were masked by enhanced adherence to standard AE care, which may have resulted from participating in a study such as this in which AE activity and treatment usage were monitored weekly.
It is also possible that the effects of silk garments are best realised during a period of AE flare rather than wearing the garments all the time (day and night). Daily use of the garments in the CLOTHES trial could have led to more rapid degradation of the product than might have been seen if the garments were worn occasionally when the AE was at its worst.
Generalisability
The study has good external validity as it was pragmatic in design, recruited children with a range of AE severities and reflected normal clinical practice in the UK. Participants were able to use existing AE treatments alongside their allocated trial intervention, as would be the case if used in practice.
Participants were recruited from five UK centres covering a range of urban and rural settings. The mix of ethnic groups was broadly representative of that in the UK.
This trial included children with moderate to severe AE on the grounds that these were the patients most likely to receive silk garments from their health-care providers. However, some children in the trial had milder disease on the day of recruitment as AE severity was assessed over the previous year for eligibility. Although AE is most common in children aged < 5 years, we included children of all ages to improve the relevance of the study results to all children with AE.
Chapter 9 Conclusion
Main conclusions
This is the first large, independent trial to have evaluated silk garments for the management of AE, and the nested economic evaluation and qualitative studies support the conclusion that use of silk garments is unlikely to be cost-effective for health providers.
It is hoped that these trial results provide health commissioners with a better evidence base on which to make informed decisions about the use of silk garments for AE.
Implications for clinical practice
-
Although patients are keen to identify non-pharmacological interventions to help in the management of AE, it would appear from this trial that silk garments provide false hope for the majority of patients and high costs for health-care providers.
-
In a world where health-care resources are finite, the use of silk therapeutic garments for the management of AE appears to represent poor value for money. Whether or not parents feel that the small benefits identified in some of the secondary outcomes are sufficient to justify purchasing these garments for themselves is something for individuals to consider on a case-by-case basis.
Implications for research
-
Although this trial proved negative, patient interest in the role of non-pharmacological interventions for AE remains high, and priority areas for future research have been identified in a James Lind Alliance Priority Setting Partnership. 6 Prioritised topics relating to non-pharmacological interventions include:
-
What role might food allergy tests play in the management of AE.
-
What is the best psychological treatment for itching/scratching in AE?
-
Which is the best way for people with AE to wash: frequency of washing, water temperature or bath versus shower?
-
What are the best and safest natural products to apply to the skin for AE?
-
How much does avoidance of irritants and allergens help people with AE?
-
What is the role of diet in treating AE: exclusion diets and nutritional supplements?
-
Which is more effective in the management of AE: education programmes, GP care, nurse-led care, dermatologist-led care or multidisciplinary care?
-
-
The CLOTHES trial has evaluated just one of many types of garments that have been purported to be effective in the management of AE. Some, such as silver-impregnated fabrics, are available on prescription and are currently recommended in clinical guidelines in some countries, whereas other fabrics and products are still experimental; none have been well evaluated.
It remains entirely possible that wearing soft, smooth fibres next to the skin can prove soothing for AE patients, and further trials of other fabric types are needed.
As with many areas of research, outcomes remain a challenge for the evaluation of AE treatments. Although the HOME initiative95 has made much progress with regard to development of a core outcome set for AE,96 further work is still required in establishing how best to capture long-term control and quality of life in AE trials. In this respect, it is important for researchers throughout the world to work together and to share data sets, such as the CLOTHES data set, to allow further validation of outcomes and testing of their performance in different clinical settings and types of patients.
Acknowledgements
We would like to thank all the children and families who participated in the trial.
The initial development of the CLOTHES trial was funded by NIHR under its Programme Grants for Applied Research programme (RP-PG-0407-10177).
Clothing used in the study was donated by Espère Healthcare Ltd (UK and Ireland distributor for DermaSilk, AlPreTec SrL. Italy) and DreamSkin Health Ltd.
The trial was sponsored by the University of Nottingham, was co-ordinated from the Nottingham CTU and was supported by the NIHR Clinical Research Network and the UK Dermatology Clinical Trials Network.
Contributors to the CLOTHES trial:
-
Independent Trial Steering Committee: David Paige (chairperson), consultant paediatric dermatologist, Barts Health NHS Trust; Nick Francis, GP/senior clinical research fellow, Cardiff University; Caroline O’Leary, senior statistician, IMS Health; Rosemary Humphreys, patient representative
-
Nottingham CTU: Lelia Duley, Director of Nottingham CTU and advisor on trial design; Andrew Jadowski, trial administrator; Jennifer White, trial co-ordinator; Sarah Walker, data co-ordinator; Tessa Clarke, senior trials manager; Trish Hepburn, senior medical statistician; Justin Fenty, senior statistician; Lucinda Murphy, data manager; Daniel Simpkins, information technology (IT) and data manager; Chris Rumsey, IT programmer
-
Other support staff at recruiting centres: Sharon McCready, research nurse lead; Rachel Watson, clinical trials assistant; Gill Glasbey, research study co-ordinator
-
Contributors to the qualitative study: Rachel Harding, paediatric nurse; Jo Aspland, research associate
-
Professor Irwin McLean, Ms Linda Campbell and Ms Stephanie MacCallum for DNA extraction and genotyping of saliva samples
-
Antony Colles, Norwich CTU senior data programmer (Norwich Medical School, University of East Anglia), for assisting with formatting data for analysis
-
Members of Centre of Evidence Based Dermatology’s patient panel and people who responded to our online survey to inform the trial design
-
National Eczema Society and Nottingham Eczema Support Group for Carers of Children with Eczema for advertising the trial
-
Dr Joanne Chalmers and Mrs Tessa Clarke for assistance in developing the trial funding proposal and protocol
-
Dr Natasha Rogers, Dr Joanne Chalmers, Ms Shelley Dowey, Dr Carron Layfield and Ms Margaret McPhee for help with study set-up, dissemination and publicity for the trial.
Funding
The NIHR had input into trial design through peer review of the funding proposal, and the garment companies provided advice in defining how the intervention should be used. Neither of the funders had a role in data collection, data analysis, data interpretation or writing of the report. Both saw the report prior to publication and provided feedback that was considered for inclusion by the trial team (see Appendix 21).
The study was developed with support from the UK Dermatology Clinical Trials Network (DCTN). The UK DCTN is grateful to the British Association of Dermatologists and the University of Nottingham for financial support of the Network.
Contributions of authors
Kim S Thomas (Professor of Applied Dermatology Research and Co-Director of the Centre of Evidence Based Dermatology) contributed to the conception and design of the study, trial oversight (as chief investigator), interpretation of the data, and drafted and critically reviewed the report.
Lucy E Bradshaw (Medical Statistician) contributed to the design of the study, was responsible for the statistical analysis plan, carried out the statistical analyses, contributed to interpretation of the data, and drafted and critically reviewed the report.
Tracey H Sach (Reader in Health Economics) contributed to the design of the study, carried out the health economics analyses, contributed to interpretation of the data, and drafted and critically reviewed the report.
Fiona Cowdell (Reader in Wellbeing in Long-Term Conditions) contributed to the design of the study, was the lead for the nested qualitative evaluation, contributed to interpretation of the data, and drafted and critically reviewed the report.
Jonathan M Batchelor (Consultant Dermatologist) contributed to the design of the study and interpretation of the data, and drafted and critically reviewed the report.
Sandra Lawton (OBE, Nurse Consultant) contributed to the design of the study and interpretation of the data and critically reviewed the report.
Eleanor F Harrison (Clinical Trial Manager) managed the trial, contributed to interpretation of the data, and drafted and critically reviewed the report.
Rachel H Haines (Clinical Trial Manager) managed the trial, contributed to interpretation of the data, and drafted and critically reviewed the report.
Amina Ahmed (patient representative) contributed to the design of the study and interpretation of the data, and critically reviewed the report.
Taraneh Dean (Dean of the Science Faculty) contributed to the design of the study, was responsible for oversight of the trial at the Isle of Wight centre (as principal investigator), contributed to interpretation of the data and critically reviewed the report.
Nigel P Burrows (Consultant Dermatologist) contributed to the design of the study, was responsible for oversight of the trial at the Cambridge centre (as principal investigator), contributed to interpretation of the data and critically reviewed the report.
Ian Pollock (Consultant Paediatrician and Paediatric Allergist) contributed to the design of the study, was responsible for oversight of the trial at the London centre (as principal investigator), contributed to interpretation of the data and critically reviewed the report.
Hannah K Buckley (Consultant Paediatrician) was responsible for oversight of the trial at the Portsmouth centre (as principal investigator), contributed to interpretation of the data and critically reviewed the report.
Hywel C Williams (Professor of Dermato-Epidemiology and Co-Director of the Centre of Evidence-Based Dermatology) contributed to the design of the study, was responsible for oversight of the trial at the Nottingham centre (as principal investigator), contributed to interpretation of the data and critically reviewed the report.
Joanne Llewellyn (Research Nurse) recruited participants and conducted assessments, contributed to interpretation of the data and critically reviewed the report.
Clare Crang (Research Nurse) recruited participants and conducted assessments, contributed to interpretation of the data and critically reviewed the report.
Jane D Grundy (Research Nurse) recruited participants and conducted assessments, contributed to interpretation of the data and critically reviewed the report.
Juliet Guiness (Research Nurse) recruited participants and conducted assessments, contributed to interpretation of the data and critically reviewed the report.
Andrew Gribbin (Research Nurse) recruited participants and conducted assessments, contributed to interpretation of the data and critically reviewed the report.
Eileen V Wake (Paediatric Nurse Lecturer) contributed to the data collection, analysis and interpretation of the nested qualitative evaluation, and drafted and critically reviewed the report.
Eleanor J Mitchell (Senior Trial Manager) contributed to the design of the study, contributed to interpretation of the data and critically reviewed the report.
Sara J Brown (Professor of Molecular and Genetic Dermatology) contributed to the design of the study, contributed to interpretation of the data, and drafted and critically reviewed the report.
Alan A Montgomery (Professor of Medical Statistics and Clinical Trials) contributed to the design of the study, was responsible for the statistical analysis plan, carried out the statistical analyses, contributed to interpretation of the data and critically reviewed the report.
Publications
Harrison EF, Haines RH, Cowdell F, Sach TH, Dean T, Pollock I, et al. A multi-centre, parallel group superiority trial of silk therapeutic clothing compared to standard care for the management of eczema in children (CLOTHES trial): study protocol for a randomised controlled trial. Trials 2015;16:390.
Thomas KS, Bradshaw L, Montgommery AA, Francis NA, Ridd MJ, Santer M. Using existing trial data to inform the development of core outcome sets and improve efficiencies in research. Trials 2015;16(Suppl. 2):O74.
Thomas K, Burrows N, CLOTHES trial Working Group. Preliminary results of the CLOTHES trial: a randomised controlled trial of silk clothing for the management of eczema in children. Pediatr Dermatol 2016;33(Suppl. 1):19.
Thomas KS, Bradshaw LE, Sach TH, Batchelor JM, Lawton S, Harrison EF, et al. Silk garments plus standard care compared with standard care for treating eczema in children: a randomised controlled observer-blind pragmatic trial (CLOTHES Trial). PLOS Med 2017; in press. https://doi.org/10.1371/journal.pmed.1002280
Data sharing statement
Non-identifying data will be made available, as appropriate, for research purposes following an application to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. ISAAC Phase Three Study Group . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124:1251-8.e23. http://dx.doi.org/10.1016/j.jaci.2009.10.009.
- Meltzer LJ, Moore M. Sleep disruptions in parents of children and adolescents with chronic illnesses: prevalence, causes, and consequences. J Pediatr Psychol 2008;33:279-91. http://dx.doi.org/10.1093/jpepsy/jsm118.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 2006;60:984-92. http://dx.doi.org/10.1111/j.1742-1241.2006.01047.x.
- Paller AS, McAlister RO, Doyle JJ, Jackson A. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr 2002;41:323-32. https://doi.org/10.1177/000992280204100505.
- NICE Clinical Guideline 57: Management of Atopic Eczema in Children From Birth up to the Age of 12 Years. London: Department of Health; 2007.
- Batchelor JM, Ridd MJ, Clarke T, Ahmed A, Cox M, Crowe S, et al. The Eczema Priority Setting Partnership: a collaboration between patients, carers, clinicians and researchers to identify and prioritize important research questions for the treatment of eczema. Br J Dermatol 2013;168:577-82. http://dx.doi.org/10.1111/bjd.12040.
- National Eczema Society . Itching and Scratching n.d. www.eczema.org/itching-scratching (accessed March 2016).
- British National Formulary (Online). London: BMJ Group and Pharmaceutical Press; n.d.
- Totté JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol 2016;175:687-95. http://dx.doi.org/10.1111/bjd.14566.
- Vlachou C, Thomas KS, Williams HC. A case report and critical appraisal of the literature on the use of DermaSilk in children with atopic dermatitis. Clin Exp Dermatol 2009;34:e901-3. http://dx.doi.org/10.1111/j.1365-2230.2009.03672.x.
- Lopes C, Silva D, Delgado L, Correia O, Moreira A. Functional textiles for atopic dermatitis: a systematic review and meta-analysis. Pediatr Allergy Immunol 2013;24:603-13. http://dx.doi.org/10.1111/pai.12111.
- The Global Resource of Eczema Trials . Centre of Evidence Based Dermatology n.d. www.greatdatabase.org.uk (accessed 20 May 2016).
- Koller DY, Halmerbauer G, Böck A, Engstler G. Action of a silk fabric treated with AEGIS in children with atopic dermatitis: a 3-month trial. Pediatr Allergy Immunol 2007;18:335-8. http://dx.doi.org/10.1111/j.1399-3038.2006.00511.x.
- Stinco G, Piccirillo F, Valent F. A randomized double-blind study to investigate the clinical efficacy of adding a non-migrating antimicrobial to a special silk fabric in the treatment of atopic dermatitis. Dermatology 2008;217:191-5. http://dx.doi.org/10.1159/000141648.
- Fontanini C, Berti I, Monasta L, Longo G. DermaSilk in long-term control of infantile atopic dermatitis: a double blind randomized controlled trial. G Ital Dermatol Venereol 2013;148:293-7.
- Gauger A, Fischer S, Mempel M, Schaefer T, Foelster-Holst R, Abeck D, et al. Efficacy and functionality of silver-coated textiles in patients with atopic eczema. J Eur Acad Dermatol Venereol 2006;20:534-41. http://dx.doi.org/10.1111/j.1468-3083.2006.01526.x.
- Juenger M, Ladwig A, Staecker S, Arnold A, Kramer A, Daeschlein G, et al. Efficacy and safety of silver textile in the treatment of atopic dermatitis (AD). Curr Med Res Opin 2006;22:739-50. https://doi.org/10.1185/030079906X99990.
- Araújo CP, Gomes J, Vieira AP, Ventura F, Fernandes JC, Brito C. A proposal for the use of new silver-seaweed-cotton fibers in the treatment of atopic dermatitis. Cutan Ocul Toxicol 2013;32:268-74. http://dx.doi.org/10.3109/15569527.2013.775655.
- Park KY, Jang WS, Yang GW, Rho YH, Kim BJ, Mun SK, et al. A pilot study of silver-loaded cellulose fabric with incorporated seaweed for the treatment of atopic dermatitis. Clin Exp Dermatol 2012;37:512-15. http://dx.doi.org/10.1111/j.1365-2230.2011.04273.x.
- Fluhr JW, Breternitz M, Kowatzki D, Bauer A, Bossert J, Elsner P, et al. Silver-loaded seaweed-based cellulosic fiber improves epidermal skin physiology in atopic dermatitis: safety assessment, mode of action and controlled, randomized single-blinded exploratory in vivo study. Exp Dermatol 2010;19:e9-15. http://dx.doi.org/10.1111/j.1600-0625.2009.00943.x.
- Love WE, Nedorost ST. Fabric preferences of atopic dermatitis patients. Dermatitis 2009;20:29-33.
- Kim SH, Hwang SH, Hong SK, Seo JK, Sung HS, Park SW, et al. The clinical efficacy, safety and functionality of anion textile in the treatment of atopic dermatitis. Ann Dermatol 2012;24:438-43. http://dx.doi.org/10.5021/ad.2012.24.4.438.
- Ozawa M. Effect of underwear made from MEDIELE on skin barrier function of atopic dermatitis patients in winter season. Skin Res 2008;7.
- Yokoyama Y, Kimata H, Mitarai S, Hirano S, Shirakawa T. Ethylene vinyl alcohol (EVOH) fiber compared to cotton underwear in the treatment of childhood atopic dermatitis: a double-blind randomized study. Indian Pediatr 2009;46:611-14.
- Kanehara S, Ohtani T, Uede K, Furukawa F. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled clinical trial. J Dermatol 2007;34:811-15. http://dx.doi.org/10.1111/j.1346-8138.2007.00391.x.
- Diepgen TL, Stäbler A, Hornstein OP. Textile intolerance in atopic eczema – a controlled clinical study. Z Hautkr 1990;65:907-10.
- Lopes C, Soares J, Tavaria F, Duarte A, Correia O, Sokhatska O, et al. Chitosan coated textiles may improve atopic dermatitis severity by modulating skin staphylococcal profile: a randomized controlled trial. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0142844.
- Thomas KS, Bradshaw LE, Sach TH, Batchelor JM, Lawton S, Harrison EF, et al. Silk garments plus standard care for treating eczema in children: a randomised controlled observer-blind pragmatic trial (CLOTHES TRIAL). PLOS Med 2017. https://doi.org/10.1371/journal.pmed.1002280.
- Williams H, Burney P, Hay R, Archer C, Shipley M, Hunter J, et al. The UK working party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994;131:383-96. https://doi.org/10.1111/j.1365-2133.1994.tb08530.x.
- Emerson RM, Charman CR, Williams HC. The Nottingham Eczema Severity Score: preliminary refinement of the Rajka and Langeland grading. Br J Dermatol 2000;142:288-97. https://doi.org/10.1046/j.1365-2133.2000.03300.x.
- Barbier N, Paul C, Luger T, Allen R, De Prost Y, Papp K, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol 2004;150:96-102. https://doi.org/10.1111/j.1365-2133.2004.05696.x.
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134:800-7. http://dx.doi.org/10.1016/j.jaci.2014.07.043.
- Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012;67:1111-17. http://dx.doi.org/10.1111/j.1398-9995.2012.02874.x.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-19. http://dx.doi.org/10.1001/archderm.140.12.1513.
- Wolkerstorfer A, de Waard van der Spek FB, Glazenburg EJ, Mulder PG, Oranje AP. Scoring the severity of atopic dermatitis: three item severity score as a rough system for daily practice and as a pre-screening tool for studies. Acta Derm Venereol 1999;79:356-9. https://doi.org/10.1080/000155599750010256.
- Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. Br J Dermatol 1998;138:107-13. https://doi.org/10.1046/j.1365-2133.1998.02034.x.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Stevens KJ, Brazier JE, McKenna SP, Doward LC, Cork MJ. The development of a preference-based measure of health in children with atopic dermatitis. Br J Dermatol 2005;153:372-7. http://dx.doi.org/10.1111/j.1365-2133.2005.06736.x.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. http://dx.doi.org/10.2165/11599120-000000000-00000.
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67:99-106. http://dx.doi.org/10.1111/j.1398-9995.2011.02719.x.
- Harrison EF, Haines RH, Cowdell F, Sach TH, Dean T, Pollock I, et al. A multi-centre, parallel group superiority trial of silk therapeutic clothing compared to standard care for the management of eczema in children (CLOTHES trial): study protocol for a randomised controlled trial. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0921-9.
- Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ 1996;312. https://doi.org/10.1136/bmj.312.7038.1079.
- Bland JM, Altman DG. The use of transformation when comparing two means. BMJ 1996;312. https://doi.org/10.1136/bmj.312.7039.1153.
- Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 2015;172:1353-7. http://dx.doi.org/10.1111/bjd.13662.
- Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy 2010;6:51-9. https://doi.org/10.1332/174426410X482999.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children?. Br J Dermatol 2001;144:514-22. https://doi.org/10.1046/j.1365-2133.2001.04077.x.
- Herd RM, Tidman MJ, Prescott RJ, Hunter JA. The cost of atopic eczema. Br J Dermatol 1996;135:20-3. https://doi.org/10.1111/j.1365-2133.1996.tb03601.x.
- Health and Social Care Information Centre . Prescription Cost Analysis 2008 2009. https://data.gov.uk/dataset/prescription-cost-analysis-england (accessed 9 June 2016).
- NHS Wales Shared Services Partnership . Prescription Cost Analysis 2008 2009. http://gov.wales/statistics-and-research/prescriptions-dispensed-community/?lang=en (accessed 9 June 2016).
- HSC Business Services Organisation . Prescription Cost Analysis 2008 2009. www.hscbusiness.hscni.net/services/1806.htm (accessed 9 June 2016).
- ISD Scotland . Prescription Cost Analysis for 2008 2009. www.isdscotlandarchive.scot.nhs.uk/isd/2241.html (accessed 13 June 2016).
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II – an ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. http://dx.doi.org/10.1016/j.jval.2015.02.001.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-80.
- Health and Social Care Information Centre . Prescription Cost Analysis 2015 2016. https://data.gov.uk/dataset/prescription-cost-analysis-england (accessed 9 June 2016).
- NHS Business Services Authority . FAQs by GPs n.d. www.nhsbsa.nhs.uk/documents/prescriptionservices/gp_faqsver4.doc (accessed 5 January 2016).
- NHS Business Services Authority . Drug Tariff March 2015 2015. www.ppa.org.uk/edt/March_2015/mindex.htm (accessed 10 June 2016).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Department of Health . National Schedule of Reference Costs 2014–15 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 22 April 2016).
- Office for National Statistics . Annual Survey of Hours and Earnings, 2015 2015. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/index.html (accessed 5 January 2016).
- Thomas KS, Dean T, O’Leary C, Sach TH, Koller K, Frost A, et al. A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1000395.
- Sach TH, Whynes DK. Measuring indirect costs: is there a problem?. Appl Health Econ Health Policy 2003;2:135-9.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Al-Janabi H, Flynn TN, Coast J. QALYs and carers. PharmacoEconomics 2011;29:1015-23. http://dx.doi.org/10.2165/11593940-000000000-00000.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. http://dx.doi.org/10.1002/hec.843.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. http://dx.doi.org/10.1111/j.1524-4733.2008.00358.x.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. https://doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- NICE . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/article/PMG9/chapter/Foreword (accessed 24 July 2015).
- Snowdon C. Qualitative and mixed methods research in trials. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-1084-4.
- Guetterman TC, Fetters MD, Creswell JW. Integrating quantitative and qualitative results in health science mixed methods research through joint displays. Ann Fam Med 2015;13:554-61. http://dx.doi.org/10.1370/afm.1865.
- Nelson PA. Getting under the skin: qualitative methods in dermatology research. Br J Dermatol 2015;172:841-3. http://dx.doi.org/10.1111/bjd.13720.
- Tisdall EKM. The challenge and challenging of childhood studies? Learning from disability studies and research with disabled children. Child Soc 2012;26:181-91. https://doi.org/10.1111/j.1099-0860.2012.00431.x.
- Smith J, Bekker H, Cheater F. Theoretical versus pragmatic design in qualitative research. Nurse Res 2011;18:39-51. http://dx.doi.org/10.7748/nr2011.01.18.2.39.c8283.
- ERIC . Ethical Research Involving Children 2016. http://childethics.com (accessed 1 June 2016).
- Barratt MJ, Ferris JA, Lenton S. Hidden populations, online purposive sampling, and external validity taking off the blindfold. Field Methods 2015;27:3-21. https://doi.org/10.1177/1525822X14526838.
- Emerson RW. Convenience sampling, random sampling, and snowball sampling: how does sampling affect the validity of research?. J Vis Impair Blind 2015;109.
- Van Manen M. Researching Lived Experience: Human Science for an Action Sensitive Pedagogy. London: Althouse Press; 1997.
- Polit DF, Beck CT. Essentials of Nursing Research: Appraising Evidence for Nursing Practice. Philadelphia, PA: Lippincott Williams and Wilkins; 2014.
- Van Manen M. Phenomenology of practice. Phenomenol Pract 2007;1:11-30.
- Ward DJ, Furber C, Tierney S, Swallow V. Using framework analysis in nursing research: a worked example. J Adv Nurs 2013;69:2423-31. http://dx.doi.org/10.1111/jan.12127.
- NIHR . INVOLVE 2015. www.invo.org.uk (accessed 17 March 2016).
- Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy 2002;61:213-36. https://doi.org/10.1016/S0168-8510(01)00214-7.
- Brett J, Staniszewska S, Mockford C, Seers K, Herron-Marx S, Bayliss H. The PIRICOM Study: A Systematic Review of the Conceptualisation, Measurement, Impact and Outcomes of Patients and Public Involvement in Health and Social Care Research. London: United Kingdom Clinical Research Collaboration; 2010.
- Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care 2011;27:391-9. http://dx.doi.org/10.1017/S0266462311000481.
- Telford R, Boote JD, Cooper CL. What does it mean to involve consumers successfully in NHS research? A consensus study. Health Expect 2004;7:209-20. http://dx.doi.org/10.1111/j.1369-7625.2004.00278.x.
- Popay J, Collins M. PiiAF. The Public Involvement Impact Assessment Framework Guidance 2014. http://piiaf.org.uk/documents/piiaf-guidance-jan14.pdf (accessed November 2016).
- PiiAF . Database – Methods and Tools to Assess Impacts: Signposting Resource to Published Case Examples of Methods and Tools 2013. http://piiaf.org.uk/documents/impacts-database.pdf (accessed 10 June 2016).
- Centre of Evidence Based Dermatology . Clothing for the Relief of Eczema Symptoms (CLOTHES) n.d. www.nottingham.ac.uk/CLOTHES (accessed January 2017).
- Cleemput I, Christiaens W, Kohn L, Léonard C, Daue F, Denis A. Acceptability and perceived benefits and risks of public and patient involvement in health care policy: a Delphi survey in belgian stakeholders. Value Health 2015;18:477-83. http://dx.doi.org/10.1016/j.jval.2014.12.015.
- Gamble C, Dudley L, Allam A, Bell P, Buck D, Goodare H, et al. An evidence base to optimise methods for involving patient and public contributors in clinical trials: a mixed-methods study. Health Serv Deliv Res 2015;3.
- Williams HC, Chalmers JR. How to teleconference effectively. Br J Dermatol 2015;173:806-10. http://dx.doi.org/10.1111/bjd.13952.
- NIHR . NIHR Clinical Research Network n.d. www.nihr.ac.uk/industry/about-the-nihr-clinical-research-network.htm (accessed 7 June 2016).
- Maskell J, Newcombe P, Martin G, Kimble R. Conducting a paediatric multi-centre RCT with an industry partner: challenges and lessons learned. J Paediatr Child Health 2012;48:974-7. http://dx.doi.org/10.1111/j.1440-1754.2012.02510.x.
- HOME . Harmonising Outcome Measures for Eczema (HOME) n.d. www.homeforeczema.org (accessed January 2017).
- Chalmers JR, Schmitt J, Apfelbacher C, Dohil M, Eichenfield LF, Simpson EL, et al. Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). Br J Dermatol 2014;171:1318-25. http://dx.doi.org/10.1111/bjd.13237.
Appendix 1 Additional studies on therapeutic garments
| Type of clothing | Reference | Participants | Interventions | Main results |
|---|---|---|---|---|
| Silver textile | Juenger et al. (2006)17 | 30 | Group 1 (n = 10): silver textile undergarments Group 2 (n = 10): silver-free textile undergarments Group 3 (n = 10): prednicarbate ointment For phase 1 (days 1–14) the three interventions were applied. For phase 2 (days 14–28) all groups wore undergarments made with silver textile. In phase 3 (days 28–56) all treatments were withdrawn except for prednicarbate ointment |
Median SCORAD at 14 days was 29.9 in group 1, 48.2 in group 2 and 24.0 in group 3 Change in scores between day 1 and 14 in groups 1 and 3 differed significantly compared with the change in score in group 2 between day 1 and 14 (p = 0.03 and p = 0.14, respectively) |
| Silver textile | Gauger et al. (2006)16 | 68 (57 analysed) | Group A (n = 37): silver-coated textile consisting of micromesh material (82% polyamide, 18% lycra) with woven silver filaments with a silver content of 20% in total Group B (n = 31): placebo pure cotton textile of equal size Both interventions worn day and night next to the skin (except for consultations) for 2 weeks |
Reduction in SCORAD index after 2 weeks: 27.4% in silver group and 16.3% in placebo. No significant differences between the two groups |
| Cellulose fibres with seaweed enriched with silver ions | Araújo et al. (2013)18 | 19 | Group A (n = 12): clothing made with a biofunctional textile consisting of 70% cotton fibres, 20% cellulose fibres with algae extracts and 10% silver-activated algal cellulose fibres. The textile contains about 6000 p.p.m. (0.6%) silver Group B (n = 7): 100% cotton clothing, woven similarly to the trial textile From day 0 the clothes were worn continuously 24 hours a day until day 7 when intermittent use of the clothing started. The clothes were worn only at night until the end of the study (day 90) |
In group A at 90 days, mean SCORAD was 24.0 (SD ±12.5) compared with 24.2 (SD ±12) in the cotton clothing group, with no difference between the groups (p = 0.97) |
| Cellulose seaweed fabric with silver | Park et al. (2012)19 | 14 (12 analysed) | Garments (top and leggings) consisting of two parts: one half made from silver-loaded cellulose fabric made from seaweed (SkinDoctor®, Ventex Co. Ltd, Korea), and the other half 100% cotton, so participants were exposed to both interventions at the same time Garments worn during the day and night for 4 weeks |
SCORAD decreased from 30.8 (SD ±8.4) to 19.5 (SD ±6.3) on Skin Doctor side, and from 30.7 (SD ±8.8) to 25.33 (SD ±8.2) on cotton side after 4 weeks (p < 0.001, 95% CI 3.60 to 8.43) |
| Cellulose seaweed fabric with silver | Fluhr et al. (2010)20 | 37 | Group A: silver-loaded seaweed-based cellulose fibre garments (SeaCell® Active, SeaCell GmbH, Rudostadt, Germany) (n = 19) Group B: cotton garments (n = 18) Long-sleeved t-shirts worn for 8 weeks |
Change in Staphylococcus aureus colonisation after 8 weeks was 10 participants in silver group compared with four participants in the cotton group (p = 0.0120) |
| Cellulose fibre | Love and Nedorost (2009)21 | 15 with AE and 15 control (27 analysed) | Intervention A: cellulosic fibre (lyocell, Lenzing AG, Lenzing, Austria) garments Intervention B: 100% cotton garments Short sleeved t-shirts, long-sleeved pyjama tops and trousers and bedding were provided. Participants were randomised to use either the lyocell or cotton for 1 week. Following this, all used their normal clothes for a 1-week wash-out period and then crossed over to the other intervention for 1 week |
Atopic participants’ average itch score was lower during the week they used lyocell (2.08) than the week they wore cotton (2.67). Both lyocell and cotton produced a lower itch score then usual clothing (3.42) |
| Anion textile | Kim et al. (2012)22 | 52 (44 analysed) | Intervention A (n = 30): undergarments made from an anion textile (constructed from pure polyester filaments containing nanosized, fine-crusted tourmaline powder) Intervention B (n = 22): undergarments made from pure cotton Participants were instructed to wear the undergarments at all times during the 4-week study period |
Mean SCORAD decreased from 47.2 (SD ±14.0) to 36.1 (SD ±16.5) in the anion group, and from 41.8 (SD ±16.3) to 37.7 (SD ±17.2) in cotton group at 4 weeks. A significant difference was detected between the groups (p = 0.03) |
| Ethylene vinyl alcohol (EVOH) fibre | Ozawa et al. (2008)23 | 30 | Group A: EVOH copolymer fibre underwear (MEDIELE®) Group B: Cotton underwear Both groups wore the intervention for 4 weeks, and then crossed over to the other intervention for 4 weeks |
No difference in local SCORAD between groups |
| EVOH fibre | Yokoyama et al. (2009)24 | 21 | Group A (n = 10): EVOH fibre underwear (short sleeved) Group B (n = 11): cotton underwear Followed up for 4 weeks |
Mean SCORAD decreased from 22.1 (SD 19.1) to 10.7 (SD 12.1) in EVOH group, and from 21.4 (SD 17.0) to 15.1 (SD 14.3) in the cotton group at follow-up |
| Borage oil-coated cotton | Kanehara et al. (2007)25 | 32 | Group A: cotton undershirt coated with borage oil (n = 16) Group B: non-coated cotton undershirts (n = 16) Garments worn for 2 weeks |
In the borage oil group, itch improved from 1.44 (SD ±0.51) to 0.94 (SD ±0.57) and erythema from 0.81 (SD ±0.83) to 0.31 (SD ±0.48) after 2 weeks. No changes observed for papules, erosion, scaling or lichenification in borage oil group. No differences in clinical symptoms observed in the non-coated cotton group after 2 weeks |
| Chitosan-coated cotton | Lopes et al. (2015)27 | 78 (69 analysed) | Group A: chitosan-coated cotton (n = 43) Group B: cotton (n = 35) Long sleeved pyjama tops and trousers worn at night for 8 weeks |
SCORAD improved in both groups after 8 weeks: 44.2 (95% CI 34.5 to 53.9) to 29.4 (95% CI 21.4 to 37.4) in chitosan group and 41.4 (95% CI 34.3 to 48.6) to 25.7 (95% CI 18.3 to 33.1) in cotton group, with no significant difference observed between the groups |
| Cotton and synthetic fibres | Diepgen et al. (1990)26 | 86 participants (55 with AD and 31 healthy controls) | Intervention A: cotton shirts Interventions B–D: shirts made of synthetics with different fibre structure |
Intensity of itching/discomfort with synthetic shirts higher in patients with AD. Cotton shirts were best tolerated |
Search strategies used to identify studies
-
Global Resource for Eczema Trials (GREAT) database was searched on 18 May 2016. The full search strategy used for the GREAT database can be found at www.greatdatabase.org.uk/GD4/Home/Strategy.php (accessed 18 May 2016).
-
PubMed (www.ncbi.nlm.nih.gov/pubmed) was searched on 11 May 2016 using the following terms: (‘dermatitis, atopic’ [MeSH Terms] OR ‘eczema’ [MeSH Terms] OR ‘eczema’ [All Fields] OR ‘atopic dermatitis’ [All Fields] OR ‘neurodermatitis’ [All Fields]) AND (clothes OR clothing OR ‘clothing’ [MeSH Terms] OR fabric OR fabrics OR textile OR textiles OR ‘textiles’ [MeSH Terms] OR silk OR ‘silk’ [MeSH Terms] OR garment OR garments).
Appendix 2 Parent/guardian information sheet
Appendix 3 Child information sheet (for age 1–5 years)
Appendix 4 Child information sheet (for age 6–10 years)
Appendix 5 Child information sheet (for age 11–15 years)
Appendix 6 Statistical analysis plan
Appendix 7 Eczema Area and Severity Index resources for research nurses
Appendix 8 Participant sticker charts (intervention and control)
Appendix 9 Case report form worksheet
Appendix 10 Participant diary
Appendix 11 Weekly participant questionnaire
Appendix 12 Participant week 24 (6-month) questionnaire
Appendix 13 Participant week 32 (8-month) questionnaire
Appendix 14 Additional data on outcomes collected at clinic visits to inform future sample size calculations
| Allocated group | Baseline | 2 months | 4 months | 6 months |
|---|---|---|---|---|
| Standard care | ||||
| n | 151 | 137 | 133 | 139 |
| Mean (SD) | 9.6 (7.8) | 7.8 (7.2) | 7.7 (8.7) | 6.5 (6.4) |
| Log-transformed mean (SD) | 2.13 (0.68) | 1.89 (0.77) | 1.79 (0.86) | 1.70 (0.80) |
| Intervention | ||||
| n | 149 | 139 | 135 | 133 |
| Mean (SD) | 11.4 (10.6) | 8.8 (10.6) | 7.7 (10.1) | 7.3 (10) |
| Log-transformed mean (SD) | 2.22 (0.76) | 1.86 (0.88) | 1.75 (0.86) | 1.69 (0.87) |
| Follow-up (months) | Baseline | 2 months | 4 months |
|---|---|---|---|
| EASI | |||
| 2 | 0.71 (n = 276) | – | |
| 4 | 0.65 (n = 268) | 0.79 (n = 265) | – |
| 6 | 0.62 (n = 272) | 0.70 (n = 266) | 0.77 (n = 261) |
| TIS | |||
| 2 | 0.51 (n = 276) | – | |
| 4 | 0.49 (n = 269) | 0.51 (n = 265) | – |
| 6 | 0.42 (n = 273) | 0.45 (n = 266) | 0.62 (n = 262) |
Appendix 15 Additional data on weekly Patient Oriented Eczema Measure scores
| Week | Standard care (N = 151) | Intervention (N = 149) |
|---|---|---|
| Week 1 | ||
| Mean (SD) | 15.8 (5.6) | 15 (6.0) |
| n | 134 | 131 |
| Week 2 | ||
| Mean (SD) | 15.7 (6.1) | 13.2 (6.8) |
| n | 127 | 125 |
| Week 3 | ||
| Mean (SD) | 15.8 (6.4) | 13 (6.1) |
| n | 128 | 125 |
| Week 4 | ||
| Mean (SD) | 16.1 (6.4) | 11.8 (6.5) |
| n | 129 | 122 |
| Week 5 | ||
| Mean (SD) | 15.4 (6.5) | 11.7 (7.1) |
| n | 124 | 123 |
| Week 6 | ||
| Mean (SD) | 15 (6.8) | 11.7 (6.8) |
| n | 122 | 120 |
| Week 7 | ||
| Mean (SD) | 14.6 (6.4) | 12.2 (7.1) |
| n | 115 | 125 |
| Week 8 | ||
| Mean (SD) | 15.1 (6.7) | 12 (6.6) |
| n | 117 | 121 |
| Week 9 | ||
| Mean (SD) | 14.2 (6.7) | 11.4 (6.5) |
| n | 114 | 123 |
| Week 10 | ||
| Mean (SD) | 14.6 (6.3) | 10.9 (6.2) |
| n | 123 | 116 |
| Week 11 | ||
| Mean (SD) | 14.1 (6.9) | 10.6 (6.7) |
| n | 118 | 121 |
| Week 12 | ||
| Mean (SD) | 13.4 (6.7) | 11.5 (7) |
| n | 113 | 122 |
| Week 13 | ||
| Mean (SD) | 13.7 (6.7) | 11.5 (7.1) |
| n | 116 | 115 |
| Week 14 | ||
| Mean (SD) | 13.8 (7) | 11.7 (7.1) |
| n | 116 | 119 |
| Week 15 | ||
| Mean (SD) | 12.9 (6.8) | 11.6 (6.8) |
| n | 110 | 116 |
| Week 16 | ||
| Mean (SD) | 13.3 (7.2) | 10.9 (6.6) |
| n | 114 | 111 |
| Week 17 | ||
| Mean (SD) | 13.4 (7.2) | 11.2 (6.6) |
| n | 110 | 107 |
| Week 18 | ||
| Mean (SD) | 13.4 (6.7) | 11.4 (7.6) |
| n | 114 | 112 |
| Week 19 | ||
| Mean (SD) | 13.6 (6.6) | 10.4 (6.8) |
| n | 110 | 114 |
| Week 20 | ||
| Mean (SD) | 12.4 (6.6) | 10.8 (7) |
| n | 107 | 114 |
| Week 21 | ||
| Mean (SD) | 13.5 (6.5) | 10.7 (6.5) |
| n | 100 | 110 |
| Week 22 | ||
| Mean (SD) | 12.6 (7) | 11 (7.2) |
| n | 108 | 109 |
| Week 23 | ||
| Mean (SD) | 13.6 (6.7) | 10.3 (7) |
| n | 90 | 104 |
| Week 24 | ||
| Mean (SD) | 13.4 (6.5) | 11.4 (7.4) |
| n | 61a | 71a |
| Follow-up (weeks) | Baseline | 8 weeks | 16 weeks |
|---|---|---|---|
| 8 | 0.47 (n = 238) | – | |
| 16 | 0.39 (n = 225) | 0.64 (n = 202) | – |
| 24 | 0.35 (n = 241) | 0.55 (n = 208) | 0.64 (n = 211) |
Appendix 16 Eczema severity according to coverage of garments
The EASI score is calculated based on the severity of AE on the head and neck, upper limbs, trunk and lower limbs. The trial garments, however, did not cover the head and neck. Therefore, an exploratory analysis was conducted for the EASI scores for the head and neck only and the EASI scores for the other body areas combined.
Figure 26 shows that EASI scores for the body areas covered by the garments were similar in the standard care and intervention group at each follow-up visit, and EASI scores for the head and neck were similar in each group at each follow-up visit.
FIGURE 26.
Eczema Area and Severity Index body region scores according to coverage of garments, by group.

Body areas covered by garments are upper limbs, trunk and lower limbs. All scores above range between 0 and 72. Scores for the body areas covered by the garments (upper limbs, trunk and lower limbs) are combined and weighted based on the child’s age at randomisation (as for the calculation of the EASI total score and rescaled in order that the sum of the weights for the covered body areas was 1).
Appendix 17 Causal effect of adherence with wearing trial garments
This section presents CACE estimates for all participants with EASI scores at 6 months, based on the sensitivity analysis for garment wear for periods when questionnaires were not completed (Table 56).
| Estimate | n | Adjusted ratio of geometric means (95% CI) |
|---|---|---|
| ITT at 6 monthsa | 272 | 0.982 (0.844 to 1.144) |
| CACE: binary – garments worn for at least 50% of days or 50% of the nightsb | 272 | |
| Assuming that garments worn for the same proportion of time when questionnaires not completed as when completed | 0.978 (0.815 to 1.175) | |
| Assuming that garments not worn when questionnaires not completed | 0.973 (0.769 to 1.230) | |
| CACE: each additional 10% of time garments wornb,c | 272 | |
| Assuming that garments worn for the same proportion of time when questionnaires not completed as when completed | 0.997 (0.971 to 1.024) | |
| Assuming that garments not worn when questionnaires not completed | 0.996 (0.966 to 1.027) |
Appendix 18 Further exploratory analysis according to brand of garment
In addition to exploration of the primary outcome by brand (as summarised in the main report), we also explored the impact of brand of garments on self-reported symptoms (POEM). For this analysis, we used data from baseline and 2 months (for the intervention group), and 6 and 8 months (for the standard care group), in order to maximise the available data.
Results are shown in Table 57. There was no difference in mean POEM scores 2 months after receiving the clothing for DreamSkin compared with DermaSilk (difference in means –0.57, 95% CI –2.35 to 1.21; n = 187), using a linear regression model adjusting for POEM score prior to receiving the clothing, site, age and treatment group.
| Self-reported symptoms | Standard care | Intervention | ||
|---|---|---|---|---|
| DermaSilk (N = 62) | DreamSkin (N = 51) | DermaSilk (N = 67) | DreamSkin (N = 63) | |
| Prior to receiving garmentsa | ||||
| Mean (SD) | 13.3 (7.2) | 13.2 (6.2) | 18.1 (6.1) | 16.1 (5.6) |
| Median (25th, 75th centile) | 12 (8, 19) | 11.5 (9, 18) | 18 (13, 23) | 16 (12, 19) |
| n | 53 | 42 | 67 | 63 |
| 2 months after receiving garmentsb | ||||
| Mean (SD) | 11.8 (7.6) | 12.3 (7.2) | 12.6 (6.2) | 11.2 (6.5) |
| Median (25th, 75th centile) | 11 (5.5, 18.5) | 12 (7, 17) | 13 (8, 18) | 10 (6, 16) |
| n | 48 | 39 | 58 | 49 |
Adherence to and acceptability of the garments at 8 months by allocated group and allocated garment brand are shown in Table 58.
| Adherence and acceptability | Standard care, n (%) | Intervention, n (%) | ||
|---|---|---|---|---|
| DermaSilk (N = 48) | DreamSkin (N = 39) | DermaSilk (N = 52) | DreamSkin (N = 51) | |
| Frequency clothing worn during the follow-up period | ||||
| Never | 2 (4) | 2 (5) | 6 (12) | 11 (22) |
| Rarely | 4 (8) | 11 (28) | 6 (12) | 11 (22) |
| Some of the time | 12 (25) | 10 (26) | 14 (27) | 8 (16) |
| All/most of the time (days only) | 1 (2) | – | 1 (2) | 1 (2) |
| All/most of the time (nights only) | 24 (50) | 10 (26) | 11 (21) | 11 (22) |
| All/most of the time (days and nights) | 2 (4) | 5 (13) | 12 (23) | 8 (16) |
| Not answered | 3 (6) | 1 (3) | 2 (4) | 1 (2) |
| Satisfaction with the clothing overall | ||||
| Very dissatisfied | – | 1 (3) | 2 (4) | 4 (8) |
| Dissatisfied | 3 (6) | 6 (15) | 2 (4) | 5 (10) |
| Neither | 11 (23) | 12 (31) | 12 (23) | 11 (22) |
| Satisfied | 19 (40) | 14 (36) | 18 (35) | 17 (33) |
| Very satisfied | 12 (25) | 4 (10) | 16 (31) | 13 (25) |
| Not answered | 3 (6) | 2 (5) | 2 (4) | 1 (2) |
| Child happy to wear clothing | ||||
| Very unhappy | – | 2 (5) | 2 (4) | 6 (12) |
| Unhappy | 7 (15) | 6 (15) | 2 (4) | 2 (4) |
| Neither | 10 (21) | 9 (23) | 10 (19) | 9 (18) |
| Happy | 16 (33) | 12 (31) | 18 (35) | 14 (27) |
| Very happy | 12 (25) | 9 (23) | 18 (35) | 19 (37) |
| Not answered | 3 (6) | 1 (3) | 2 (4) | 1 (2) |
Appendix 19 Health economics analysis plan
Appendix 20 Nested qualitative study: further information
Children data analysis
| Child characteristics | Site |
|---|---|
| Focus group | |
| Girl, age (years) | |
| 5–6 | Nottingham |
| 5–6 | Nottingham |
| 5–6 | Nottingham |
| 7–8 | London |
| 7–8 | Cambridge |
| Boy, age (years) | |
| 5–6 | Nottingham |
| 7–8 | London |
| 7–8 | Cambridge |
| Face-to-face interview | |
| Girl, age (years) | |
| 9 | Isle of Wight |
| 9 | Isle of Wight |
| 15 | Isle of Wight |
| 12 | Cambridge |
| 9 | Isle of Wight |
| Boy, age (years) | |
| 11 | Isle of Wight |
| 9 | Isle of Wight |
| 10 | Cambridge |
| Telephone interview | |
| Girl, age (years) | |
| 9 | Cambridge |
| 10 | Cambridge |
| Boy, age (years) | |
| 13 | Cambridge |
Child interview and focus group topic guide
-
Grand tour question:
-
Tell me a bit about your eczema, what it’s like living with it?
-
-
Mini tour questions:
-
How have you got on with the special clothing?
-
How much did you wear the clothing (day/night/away from home)?
-
What was it like wearing the clothing (skin condition, comments from others)?
-
-
Example questions:
-
Can you tell me about any differences you have noticed (skin condition/well-being)?
-
-
Experience questions:
-
Were there particular things you liked or did not like about using the special garments?
-
Parent data analysis
| Sex of child (age, years) | Parent | Site | Reported usage |
|---|---|---|---|
| Standard carea | |||
| Girl (14)b | Father | Nottingham | Occasional nights |
| Girl (9) | Mother | Portsmouth | Every day and night |
| Girl (6) | Mother | Isle of Wight | Most nights |
| Girl (14) | Mother | Isle of Wight | One night |
| Girl (12) | Mother | Isle of Wight | Very occasional nights |
| Girl (2) | Mother | London | Twice |
| Girl (4) | Mother | London | Every night |
| Girl (8) | Mother | London | Most nights and 1 or 2 days |
| Boy (11) | Father | London | Most nights and occasional days |
| Boy (5) | Mother | London | 1 or 2 nights |
| Boy (8) | Father | London | Every night and weekend days |
| Girl (9) | Mother | Cambridge | Top virtually every day and night, leggings some nights |
| Boy (2) | Father | Cambridge | Every night |
| Boy (4) | Mother | Nottingham | Every night |
| Boy (5) | Mother | Isle of Wight | Every day and night except PE day |
| Girl (10) | Mother | Nottingham | Every night |
| Girl (5) | Mother | Cambridge | Every night |
| Intervention | |||
| Boy (2) | Mother | Portsmouth | Every night |
| Boy (2) | Mother | Isle of Wight | Every day and night |
| Boy (2) | Mother | Isle of Wight | Day and night, less over time |
| Boy (4) | Motherc | Isle of Wight | Virtually every day and night |
| Boy (4) | Fatherc | Isle of Wight | Virtually every day and night |
| Boy (11) | Mother | Isle of Wight | Day and night, approximately 50% |
| Boy (2) | Mother | Isle of Wight | Virtually every day and night |
| Girl (6) | Mother | London | Every night |
| Boy (3) | Mother | London | Every night and leggings only everyday |
| Boy (2) | Mother | Cambridge | Every night |
| Boy (5) | Mother | Cambridge | Every night and occasional daytime at the weekend |
| Girl (3) | Mother | Nottingham | Every day and night |
| Boy (13) | Mother | Nottingham | Every day and night except PE days |
| Boy (10) | Mother | Isle of Wight | Every day and night, less in the day when hot |
| Boy (4) | Mother | London | Every day and night |
| Boy (7) | Mother | London | Every night |
Parent interview and focus group topic guide
-
Grand tour question:
-
Tell me a bit about the eczema, what it’s like living with it?
-
-
Mini tour questions:
-
How have you got on with the special clothing?
-
How much did you/your child wear the clothing (day/night/away from home)?
-
What was it like wearing the clothing (skin condition, comments from others)?
-
How did you get on with looking after the garments (washing, size)?
-
-
Example questions:
-
Can you tell me about any differences you have noticed (skin condition/behaviour/well-being)?
-
-
Experience questions:
-
Were there particular things you or your child liked or did not like about using the silk garments?
-
How would you feel about continuing to use the special clothing (what things might make you continue or make you stop, barriers to use)?
-
What makes you think that the garments have worked or not worked?
-
Stages 1–3 of parent data analysis
Stage 1
All transcripts were read in full to ensure immersion in the detail of the data as a whole. Transcripts were annotated with key emergent themes.
Stage 2
The recurring key themes and subthemes from the initial readings of the transcripts are presented in Table 61.
| Key themes | Subthemes |
|---|---|
| Desperation | Tried everything |
| Impact on quality of life | |
| Allergies | |
| Fear of some treatments | |
| Hope for improvement | |
| Trial garments | Fit and look |
| Wear and tear | |
| Washing | |
| Effect of the garments | Amount of wear |
| Difference to skin condition | |
| Difference to well-being | |
| Being in the trial | Motivation |
| Inconvenience |
Stage 3
The draft framework presented in stage 2 was applied to the transcripts and data were coded and annotated, similarities and differences in data were identified and recurring themes and subthemes were refined, combined and developed. A refined framework was developed in which repetition across themes was removed and data clearly fitted with one theme only (Table 62).
| Key themes | Subthemes |
|---|---|
| Despair and hope | Treatments |
| Adjustments | |
| Quality of life | |
| Hopes for the trial | |
| Fit, durability and care | Look, feel and fit |
| Durability | |
| Laundry care | |
| Perceived impact of the garments | Patterns of use |
| Effect of garments | |
| Continued use post trial | |
| Engaging in the trial | Experience of participation |
| Commitment | |
| Important outcome measures |
Clinician and commissioner data analysis
| Job role | Region |
|---|---|
| Community dermatology specialist nurse | Northwest |
| Consultant dermatologist | Midlands |
| Consultant dermatologist | Southeast |
| Commissioning pharmacist | Midlands |
| GP with special interest | Midlands |
| GP | Central south |
| Dermatology clinical nurse specialist | Northeast |
| Dermatology support nurse – primary care | Northwest |
| Lead dermatology clinical nurse specialist – community | Northwest |
| Paediatric dermatology specialist nurse – primary care | Midlands |
| Paediatric dermatology clinical nurse specialist – secondary care | Central south |
| Pharmacist | Northwest |
| GP (specialist) | Midlands |
| Clinical commissioning group prescribing lead (GP) | Northeast |
| Consultant dermatologist | Northeast |
| Community pharmacist | Midlands |
| Clinical commissioning group prescribing lead (GP) | Northeast |
| Paediatric dermatology clinical nurse specialist | Central south |
| Dermatology clinical nurse specialist | Midlands |
| Dermatology specialty doctor | Northeast |
| Dermatology specialist nurse | Central south |
Clinician and commissioner interview guide
-
What is your experience of prescribing silk garments for children with eczema?
-
What makes you select this line of treatment?
-
Are there any barriers to you enabling children to use these garments?
-
Perceived cost.
-
Lack of evidence.
-
Durability of the garments.
-
-
Do you think it is a reasonable expectation that GPs should prescribe silk garments?
-
If no, who do you think is the most appropriate person to prescribe these garments?
-
Do you think they should be prescribed at all? Can you explain the reason for this?
Stages 1–3 of clinician and commissioner data analysis
Stage 1
All transcripts were read in full to ensure immersion in the detail of the data as a whole. Transcripts were annotated with key emergent themes.
Stage 2
The recurring key themes and subthemes from the initial reading of transcripts are presented in Table 64.
| Key themes | Subthemes |
|---|---|
| Knowledge and evidence base | Lack of knowledge |
| Lack of evidence base | |
| Using silk garments | Unclear indications for use |
| Top of ladder of treatment options | |
| Quality of product | |
| Prescription and expected outcomes | Rarely prescribed |
| Who should prescribe | |
| Subjective and objective outcome measures |
Stage 3
The draft framework presented in stage 2 was applied to the transcripts and data were coded and annotated, similarities and differences in data were identified and recurring themes and subthemes were refined, combined and developed. A refined framework was developed in which repetition across themes was removed and data clearly fitted with one theme only (Table 65).
| Key themes | Subthemes |
|---|---|
| Knowledge base | Lack of evidence base |
| Information from manufacturers | |
| Treatments protocols | |
| Reasons to use silk garments | Failure of other treatment regimens |
| Greater concordance | |
| Avoiding referral to secondary care | |
| Cost-effectiveness | |
| Reasons for not using silk garments | Lack of familiarity/experience |
| Cost | |
| Contentious prescription | |
| Quality of product | |
| Outcome measures | Existing measures |
| Clinical improvement | |
| Patient reports |
Appendix 21 Summary of amendments to report after the funding body review stage
| Summary of change | Revised wording |
|---|---|
| Plain English summary amended | Details of adherence in wearing the clothing added |
| Implication for practice amended | Removed ‘Clinical commissioners can now be encouraged to make informed decisions on the basis of these robust trial findings’ |
| Strengths and limitations amended | Added further mention of the independent nature of the trial and its pragmatic nature in reflecting normal patterns of adherence |
| Summary of change | Revised wording |
|---|---|
| Health economic analysis updated to correct an error in how the garments were costed | All figures updated throughout the report with revised cost-effectiveness analysis. Clarification added that unit costs refer to sets of garments, rather than individual items |
| Conclusion amended to remove reference to this being the first independent trial | Revised wording in abstract: ‘This trial adds to the evidence base to guide clinical decision-making’ |
| Clarified that prescription data showing an increase in silk prescribing by the NHS over the last 5 years is for all indications, not just for eczema | ‘All indications’ added where relevant |
| Potential confusion around our definition of adherence was clarified. Clarification was added relating to the CACE analysis demonstrating the likely impact of adherence in wearing the garments on the trial results | Methods and Results amended to clarify our definition of non-adherence, and CACE analysis expanded to aid understanding of how to interpret these findings |
| Details of the amount of topical corticosteroid used have been added (based on prescription data) | Added to Table 30 |
| Removed potentially inflammatory statements | Removed from Discussion: ‘Were the UK NHS to stop prescribing such items, millions of pounds could be saved each year, which could then be better invested into more effective treatments’ |
| Clarified that the nested qualitative study was conducted by colleagues at the University of Hull and results were not disclosed to the trial team until after data analysis was complete | Details added to Chapter 5 |
| Reference to previous small studies being sponsored by the silk manufacturers removed | Chapter 7, Involvement of clothing suppliers after completion of the trial section amended |
| Discussion updated to address concerns about interpretation of study results with regard to inclusion of participants with mild disease at baseline and the impact of adherence in wearing the garments | Discussion updated to reiterate the relevant sensitivity and subgroup analyses, and recognition that children with all severities of AE (as assessed by EASI at baseline) were included |
List of abbreviations
- ADQoL
- Atopic Dermatitis Quality of Life
- AE
- atopic eczema
- CACE
- complier average causal effect
- CC
- Complications and Comorbidities
- CE
- Conformité Européenne
- CHU-9D
- Child Health Utility-9 Dimensions
- CI
- confidence interval
- CLOTHES
- CLOTHing for the relief of Eczema Symptoms
- CTU
- Clinical Trials Unit
- DFI
- Dermatitis Family Impact
- DNA
- deoxyribonucleic acid
- EASI
- Eczema Area and Severity Index
- EQ-5D-3L
- EuroQoL-5 Dimensions-3 Levels
- FLG
- filaggrin
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IGA
- Investigator Global Assessment
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PGA
- Patient Global Assessment
- POEM
- Patient Oriented Eczema Measure
- PPI
- public and patient involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SWET
- Softened Water Eczema Trial
- TIS
- Three-Item Severity