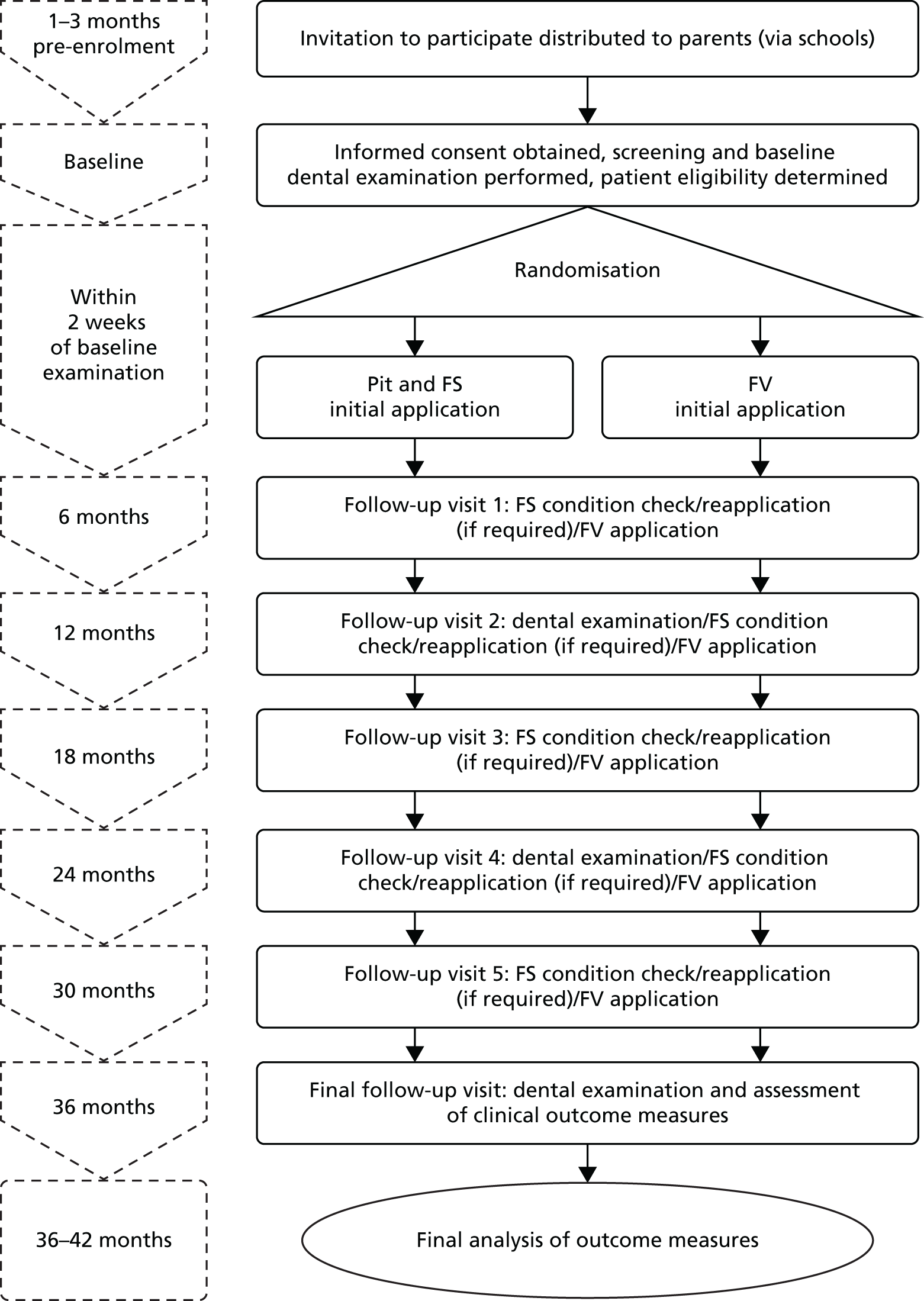
Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/104/04. The contractual start date was in April 2011. The draft report began editorial review in April 2016 and was accepted for publication in November 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Chestnutt et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction, background and objectives
Introduction
Dental caries (tooth decay) is among the most common diseases to affect humankind. In 2010, it was estimated that 35% of the global population, that is, 2.4 billion people, had untreated caries in their permanent dentition. 1 The NHS in England spends around £3.4B per year on dental services, and it is estimated that the private market accounts for a further £2.3B annually. 2
Dental caries results from the metabolism of sugars by bacteria that are normally resident in the oral cavity. The acids produced cause the demineralisation (breakdown) of the tooth surface. Initially, the caries lesion is confined to the dental enamel. In its early stages, the disease process can be halted or even reversed by a process known as remineralisation. This is facilitated by the presence of fluoride at the interface between the tooth surface and the overlying biofilm of the dental plaque.
Untreated, the disease process continues to involve the underlying dentine and eventually the dental pulp becomes inflamed, resulting in pain: toothache. Once the dentine is involved, the tooth requires a restoration to halt caries progression. Ultimately, an inflamed pulp will die and a dental abscess may result. Resolution will require either root-filling or the extraction of the tooth.
The past four decades have seen great improvement in oral health in England and Wales. In 1973, the mean number of decayed, missing, filled teeth in permanent dentition (DMFT) in 15-year-olds was 8.4. 3 By 2013 this had fallen to 1.4. 4 This improvement is widely attributed to the introduction and the widespread use of fluoride-containing toothpaste in the 1970s. However, improvements in oral health have not been uniformly distributed across the population.
Children at risk of dental caries
Mean population values of dental caries prevalence mask an important fact. Similar to many chronic, lifestyle-associated diseases, caries prevalence is markedly linked to social and economic deprivation,5,6 and the prevalence is markedly skewed. At the age of 5 years, the prevalence of dental decay in children resident in the most deprived localities is more than twice that of children living in the least deprived communities. As oral health has improved, dental caries has become concentrated in children from poorer backgrounds.
Teeth at risk of dental caries
Individual teeth differ in their susceptibility to dental decay. This largely reflects the fact that dental plaque (oral biofilm) is more likely to form in specific areas. One such area is the occlusal (or biting) surface of molar teeth. This fissured surface predisposes teeth to oral bacteria multiplying in the depths of the fissures and forming an environment favourable to the fermentation of sugars into acid.
The first permanent molars (FPMs) erupt at the back of the mouth around 6 years of age. The occlusal surface of these teeth is particularly prone to dental caries, often within a short period of eruption into the mouth. Management of occlusal caries on permanent molars has proven to be a great challenge to the dental profession. 7 Occlusal caries accounts for the majority of affected tooth surfaces in adolescents and adults. 8–11
It is, therefore, apparent that preventing dental caries on the occlusal surfaces of FPMs is a key objective in preventative dental care. These surfaces represent the surfaces most susceptible to decay and are present in susceptible children. Actions to prevent and arrest dental caries, targeted at these surfaces, have the potential to significantly improve oral health and to address inequalities.
Preventative dental technologies
There are two preventative dental technologies that have the potential to be targeted specifically at occlusal surfaces of FPMs: pit and fissure sealant and fluoride varnish (FV). The terms ‘pit and fissure sealant’ and ‘fissure sealant’ (FS) refer to the same material. Unless referring to the term used specifically by a referenced source, the term FS is used throughout this report.
Pit and fissure sealant
Pit and fissure sealant contains a bisphenol A and glycidyl methacrylate (bis-GMA) resin, which hardens on exposure to light. It is applied to the tooth surface using acid-etch technology. FS prevents dental caries by physically obliterating the pit and fissure system that has great potential to harbour cariogenic organisms. Eliminating the pit and fissure system removes this ecological niche and reduces the initiation of caries in this highly susceptible site. First developed in the 1960s, FS is an established technology and is widely used in clinical practice.
Numerous studies have investigated the clinical effectiveness of FS. A 2013 Cochrane systematic review12 of sealants for preventing dental decay in the permanent teeth concluded that, in 12 trials in which resin-based sealants were compared with no sealant controls, the sealed teeth were significantly less likely to be carious at 2 years’ follow-up [odds ratio (OR) 0.12, 95% confidence interval (CI) 0.07 to 0.19], although five of these trials were conducted in the 1970s, when caries prevalence was higher than it is now. The authors of the review concluded that the application of sealants was recommended to prevent and control dental caries. They found that the application of FS to occlusal surfaces of permanent molars was clinically effective when compared with unsealed control teeth up to 48 months but, beyond that time, effectiveness was uncertain. They further concluded that, although sealing was effective in high-risk children, evidence in other circumstances is scarce, and they were unable to achieve a primary objective of determining the effectiveness of sealants in relation to the baseline prevalence of dental caries in the population. 12
Fluoride varnish
The caries-preventative effect of fluoride has been recognised for > 100 years. Evidence arising from ecological studies in the early twentieth century linked the presence of fluoride in the public water supply to reduced caries prevalence in the population. 13 Subsequently, fluoride has been incorporated into a range of caries-preventative products for both professional use and self-use.
Fluoride primarily exerts cariostatic properties by a topical effect. 14 The presence of fluoride at the tooth surface prevents demineralisation of the dental enamel and encourages remineralisation.
Fluoride-containing varnishes were developed in the 1960s. They contained fluoride at a much higher concentration [22,600 parts per million (p.p.m.)] than that present in water fluoridation programmes (0.5–1 p.p.m. fluoride) or toothpaste (1000–1500 p.p.m fluoride). FVs were developed to prolong the contact of fluoride ions with the tooth surface, thereby enhancing their cariostatic effect.
The most widely used FV preparation in the UK is Duraphat® varnish [Colgate-Palmolive (UK) Ltd, Manchester, UK], marketed by Colgate®. This product contains 5% of sodium fluoride (22,600 p.p.m. of fluoride). Owing to the high fluoride content, the product is licensed as a prescription-only medicine and can be prescribed only by a medical or dental professional. FV is designed for application by a dental professional to specific at-risk tooth surfaces or early caries lesions. The recommended frequency of application is 2–4 times per year.
The clinical effectiveness of FV has been the subject of a recent Cochrane systematic review. 15 The authors identified 13 studies that compared FV with a placebo or no treatment, and concluded that the pooled decayed, missing, filled tooth surfaces in permanent dentition (DMFS)-prevented fraction was 43% (95% CI 30% to 57%; p > 0.0001). However, the reviewers reported that the quality of the evidence was moderate, as it mainly included studies that were at a high risk of bias with considerable heterogeneity. 15
Fissure sealant versus fluoride varnish
From the above reviews it is evident that both FS and FV are effective caries preventative agents. The question arises, therefore, as to which is the more effective, particularly in relation to the prevention of dental caries in FPMs.
Ahovuo-Saloranta et al. 16 have recently published a Cochrane systematic review on the relative effectiveness of FS versus FV. This updates a previous version of the review published in 2010. 17 The review identified four trials that had compared resin-based FS with FV. Two of these four studies, involving 358 children, suggested that, compared with FV, FS prevented more caries in FPMs at 2-year follow-up. The pooled OR was 0.65 (95% CI 0.50 to 0.94; p = 0.02). The authors stated that the body of evidence was assessed as low. Ahovuo-Saloranta et al. 16 concluded that the conclusion of the updated review remained the same as the last update in 2010, that is, as a result of the limited number of data available it is was not possible to draw clear conclusions about possible differences in effectiveness for preventing or controlling dental caries on occlusal surfaces of permanent molars.
The importance of establishing the relative clinical effectiveness, cost-effectiveness and patient preferences in relation to fissure sealant or fluoride varnish
Although the relative clinical effectiveness of FS and FV is important, a number of other factors should also be considered when choosing between these technologies.
Application and equipment
The application of FS requires the use of the acid-etch technique to achieve adhesion of the sealant to the tooth surface. Adequate moisture control is known to be a critical component of achieving a satisfactory bond. 18 This means that sealants can reasonably be applied only in the confines of a ‘dental surgery’, be that fixed or mobile. The use of a ‘3-in-1’ water/air spray is required, along with adequate aspirating facilities.
In contrast, the application of FV is much less technique sensitive and does not require the degree of specialist equipment needed for sealant placement. FV can simply be painted onto teeth using a small brush. Moisture control using cotton wool rolls or pads is sufficient. As a result, FV can be applied in a school medical room or other private location and does not necessarily need to be done within a clinic or traditional health-care setting.
Personnel required for placement
Whereas FS can be applied only by a dentist, dental therapist or dental hygienist in the UK, FV can be applied by an appropriately trained dental nurse. These factors raise the question of the relative costs associated with providing either FS or FV. Although the equipment and personnel costs required to provide FS are potentially higher, these need to be offset against the requirement for FV to be applied every 6 months and the single application and subsequent periodic check-up/top-up required for FS.
Patient acceptability
A further consideration is the relative acceptability of FS and FV to patients. At the age of 6 years, children may have limited experience of dental care. The more technically involved procedure of FS application may pose a greater challenge to an anxious or nervous child than the apparently more straightforward application of FV.
Approaches to improve oral health
There are three main approaches to improving oral health: a whole-population approach, a targeted-population approach and a high-risk individual approach. 19
The Designed to Smile programme in Wales, part of the Welsh Government’s National Oral Health Improvement Programme, takes a targeted population approach. There are several elements to the Designed to Smile programme, one of which is a FS scheme. This work is delivered in schools via the Community Dental Service (CDS) using mobile dental clinics (MDCs). This setting provided the backdrop to the current study. To ascertain whether FS or FV is the more effective technology in preventing occlusal caries in FPMs is of importance to the NHS. This was recognised in the call by the National Institute for Health Research Health Technology Assessment Board, which commissioned the research reported here. In addition to clinical effectiveness, cost-effectiveness is of interest. As discussed above, FV requires less time than FS and can be applied by staff with less training. However, although FV requires twice-yearly application, FS require only periodic checking and maintenance. It is therefore possible that the technologies will differ in their costs and, thus, in their cost-effectiveness.
Finally, the preference of children and their parents for FS or FV merits investigation. The less invasive nature of FV placement compared with FS placement means that examination of the relative acceptability of these treatments is required.
The aims and objectives of the trial were, therefore, as follows. 20
Trial aims and objectives
Trial aim
The overall aim of the study was to identify and compare the relative clinical effectiveness and cost-effectiveness of two established technologies, FS and FV, for the prevention of dental caries in FPM teeth in children aged 6 or 7 years.
Trial objectives
Primary objective
The primary objective of this trial was to compare the clinical effectiveness of FS and FV in preventing dental caries in FPMs in 6- and 7-year-olds, as determined by the:
-
proportion of children developing new caries on any one of up to four treated FPMs
-
number of treated FPM teeth that are caries free at 36 months.
Secondary objectives
The secondary objectives of this trial were to:
-
establish the costs and budget impact of FS and FV delivered in a community/school setting and the relative cost-effectiveness of these technologies
-
examine the impact of FS and FV on children and their parents/carers in terms of quality of life (QoL) and treatment acceptability measures
-
examine the implementation of treatment in a community setting with respect to the experience of children, parents, schools and clinicians.
Chapter 2 Methods
Trial design
The study was a Phase IV randomised, allocation-blinded, two-arm, parallel-group trial. The participants were randomised on a 1 : 1 allocation ratio to receive one of:
-
resin-based FS
-
FV.
There were no significant changes to the trial methodology after trial commencement. With the exception of some minor changes to improve the return rate of follow-up questionnaires, the trial was conducted as per the original protocol. 20
Approvals obtained
The Research Ethics Committee (REC) for Wales 3 (formerly known as the REC for Wales) approved the study on 15 February 2011 (reference 11/MRE09/6). The details of the REC and the NHS Research and Development department approvals and clinical trials authorisation from the Medicines and Healthcare products Regulatory Agency (MHRA) are provided in Appendices 1–3.
Participants
The target population were children aged 6 or 7 years attending primary schools in designated Communities First areas; the Welsh Government has identified these localities as areas of social and economic deprivation. All children in such schools are deemed to be at high risk of caries, according to Public Health England and the Scottish Intercollegiate Guidelines Network, and qualify for FS/FV application. 21,22
Participant selection and eligibility criteria
Children were considered eligible to join the trial if they met all of the inclusion criteria and none of the exclusion criteria. 20 It was possible to assess whether or not children met some of these criteria prior to a clinical dental examination; however, for other eligibility criteria, examination by a dentist was required.
Inclusion criteria
Children were eligible for inclusion if:
-
they were aged 6 or 7 years and attended the schools participating in the current Cardiff and Vale University Health Board Designed to Smile programme
-
the person with parental responsibility has provided written informed consent
-
they had at least one fully erupted FPM free of caries into dentine.
Exclusion criteria
Children were ineligible for inclusion if:
-
their medical history precluded inclusion [i.e. those with a history of hospitalisation for asthma, severe allergies or allergy to Elastoplast (Beiersdorf AG, Hamburg, Germany), which was determined from a medical history form (MHF) that was completed by parents]
-
they had a known sensitivity to colophony, or any of the product ingredients (e.g. methylacrylate in FS, determined from a MHF that was completed by parents)
-
they had any abnormality of the lips, face or soft tissues of the mouth that would cause discomfort in the provision of FS/FV
-
they were currently participating in another clinical trial involving an investigational medicinal product (determined from a MHF that was completed by parents)
-
they showed obvious signs of systemic illness (e.g. colds, influenza, chickenpox; determined at baseline examination).
Recruitment into the trial
Number of participants
It was planned to recruit 920 participants, who would be randomised equally (n = 460 per arm) to the two technologies (FS and FV) investigated in this trial. The statistical justification for this number is discussed below.
Recruitment of schools
Eligible schools involved in the Cardiff and Vale University Health Board (UHB) CDS Designed to Smile programme were invited to participate in the trial. The trial management team visited those schools that expressed an interest in participating to discuss the practical and logistical aspects of the trial. When applicable, agreement to participate in the trial was documented.
Recruitment of participants
In line with the procedures for informing parents of children of the existing Designed to Smile programme, teachers distributed invitation packs to children for them to take home to their parents.
The following documents were included in the invitation packs:
-
participant information sheet (PIS) for parents (see Appendix 4)
-
MHF (see Appendix 5)
-
informed consent form (ICF) (see Appendix 6).
Participant information sheet for parents
The PIS was developed in conjunction with a parents’ group and approved by the REC (detailed in Design of study materials and strategy for maximising questionnaire follow-up rates). The PIS contained all of the necessary information for a parent or legal guardian to make an informed choice about their child’s participation in the trial, as detailed in section 4.8 of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice. 23
Medical history
The medical history of potential participating children was ascertained by asking their parents or legal guardian to complete a MHF. Data were collected that specifically related to allergies, asthma (including whether or not this had resulted in hospitalisation) and sensitivity to constituents of either FS or FV, and if the child was currently participating in a clinical trial involving an investigational medicinal product. Whether or not the child was registered with a general dental practitioner was also ascertained.
Following a child’s enrolment into the trial, any changes to their relevant medical history (as described above) was established via a brief MHF (see Appendix 7), which was sent to parents in advance of the annual clinical dental examination at 12 and 24 months. Parents were instructed to complete and return the MHF to the dental team only if there had been a change in their child’s relevant medical history.
Informed consent
So that the trial could be conducted as pragmatically as possible, the consent process from the existing CDS FS programme was utilised, in that consent was provided remotely by parents completing consent forms at home and returning them to the school via their children. The consent rates for the existing FS programme are usually > 70% (depending on the school); therefore, effectively communicating the concept of the trial to parents without negatively affecting recruitment was key to the success of the trial. Given the setting and the low-risk nature of the trial, obtaining consent remotely was successfully justified to the REC.
The PIS included in the Seal or Varnish? (SoV) invitation pack introduced the trial to the parents and asked them to take the time to read the PIS before deciding whether or not they wanted their child to participate. Parents were also informed of the option for their child to enter the existing Designed to Smile programme instead of the trial. Explicit in the PIS was the opportunity to discuss any aspect of the trial with a member of the trial team over the telephone. A dedicated telephone number was provided for this purpose, with the provision for parents to leave a telephone number and e-mail address to be contacted by a member of the trial team at the next available opportunity. Parents were also asked to discuss the trial with their child.
After considering the information provided in the SoV invitation pack, and discussing the trial with a member of the trial team (if required), parents who wanted their child to participate were asked to sign and date the ICF (see Appendix 6). They were also asked to confirm on the ICF that they had been given the opportunity to discuss the trial with a member of the trial team or, alternatively, if they did not require any further information, to make a decision regarding their child’s participation.
Children returned the completed ICF and MHF forms to their class teacher. On receipt of the ICF and MHF, a designated member of the SoV MDC team liaised with an appropriate member of school staff to verify that the name and signature on the ICF were in agreement with the school’s records for the person with parental responsibility for that child.
Interventions
Study setting
The study interventions took place in the MDC, which visited schools in areas of high social and economic deprivation in the Cardiff and Cwm Taf Health Board areas in South Wales, UK. The MDCs were operated by the CDS, Cardiff and Vale UHB, under the Designed to Smile programme,24 which was a national oral health improvement programme.
Technologies evaluated
The two technologies evaluated in this trial (FS and FV) are well established and have been used routinely for several decades to prevent dental caries, as discussed in Chapter 1. Eligible participants were randomised to receive either FS or FV and remained on the intervention to which they were randomised for the duration of the study.
Pit and fissure sealant
The FS used was Delton® Light Curing Opaque Pit and Fissure Sealant (Dentsply Ltd, Stonehouse, UK; CE0086). This is one of the most commonly used FS brands in the UK. FS was supplied as 2.7-ml bottles for multiple applications and applied topically as a thin layer to the occlusal surface of eligible FPMs. The standard clinical protocol, as described by the product manufacturers, was used to apply the FS (Box 1). The full details of the clinical protocol for FS application are given in Appendix 8.
-
Prior to sealing, if necessary, teeth are cleaned with a toothbrush.
-
The tooth is isolated using dry guards/cotton wool rolls and saliva ejectors to aid moisture control.
-
The tooth surface is dried.
-
The etchant is applied to all the pits and fissures and a few millimetres beyond the final margin of the sealant for 30–60 seconds in accordance with the manufacturer’s instructions.
-
The etchant material is washed from the tooth for at least 30 seconds and the tooth dried until it has a chalky, frosted appearance. Teeth that fail to etch properly are re-etched for a further 20 seconds. Great care is taken to avoid salivary contamination, as there is agreement that moisture contamination at this stage of the process is the most common cause of sealant failure.
-
The sealant material is applied to the occlusal surface and cured by shining a polymerisation light on the tooth surface for at least 20 seconds.
-
The sealant is evaluated visually and tactically and an attempt is made to dislodge it with a probe. Any deficiencies in the material are repaired to ensure an intact sealant surface.
The initial application of FS took place within 2 weeks of the baseline dental examination and was performed by a suitably qualified and trained dental hygienist. In the case of partially erupted molars, sealant was applied if sufficient tooth surface was available. This situation was most common in the case of upper molars. The same two dental hygienists provided treatments throughout the trial using two MDCs.
The condition of the FS was re-examined at 6, 12, 18, 24 and 30 months. FS was reapplied if the existing sealant had become detached or if occlusal coverage was considered insufficient as a result of either further eruption of the tooth or part of the sealant becoming lost.
All applications of FS were documented on treatment record forms, which captured the date, batch number, participant identification (ID) number and number of teeth (one to four) treated.
Fluoride varnish
The FV used for evaluation in the study was Duraphat 50 mg/ml dental suspension (PL 00049/0042), equivalent to 22,600 p.p.m. fluoride. This is the most commonly used FV brand in the UK. FV was supplied in 10-ml tubes for multiple applications and applied topically as a thin layer to the pits, fissures and smooth surfaces of eligible FPMs. As per the Duraphat summary of product characteristics (see Appendix 9), the dosage per single application did not exceed 0.4 ml. The standard clinical protocol was used to apply the FV, as described in Box 2.
-
Prior to FV application, teeth are cleaned with a toothbrush if necessary.
-
The tooth is isolated using a cotton roll in the buccal sulcus.
-
The tooth is dried using a cotton roll.
-
A thin layer of FV is applied to the pits, fissures and smooth surfaces of FPMs using a disposable microbrush.
-
The cotton roll is removed from the buccal sulcus.
-
This procedure is repeated for all FPMs included in the trial.
-
The child is advised not to eat or drink for 30 minutes and not to brush their teeth for 4 hours after application.
The initial application of FV occurred within 2 weeks of the baseline dental examination and was performed by a qualified and trained dental hygienist in accordance with the conventional clinical protocol established by the CDS (see Box 2). FV was reapplied at 6, 12, 18, 24 and 30 months, that is, on six occasions at 6-monthly intervals in the course of the trial. The full details of the clinical protocol for FV application are given in Appendix 10.
All applications of FV were documented on treatment record forms, which captured the date, batch number, participant ID number and number of teeth (one to four) treated.
Clinical assessments
Participant eligibility
The demographic and medical history information collected from the parents was reviewed to determine if the child met the inclusion/exclusion criteria prior to a clinical examination. Children considered eligible underwent a baseline clinical examination to determine their eligibility, based on the clinical inclusion/exclusion criteria detailed in Inclusion criteria and Exclusion criteria.
Clinical dental examination
Study participants were examined in the MDC while supine under a standard overhead dental clinical light, using a plane dental mirror and a ball-ended probe. The probe was used only to remove debris and to determine surface texture. It was not used to probe for cavitation. Teeth were not dried prior to clinical dental examination. Gross debris was removed using a toothbrush.
Caries assessment using International Caries Detection and Assessment System
Caries status was assessed and recorded at baseline, for all children considered eligible, by trained and calibrated dentists using conventional diagnostic caries criteria at the d1/D1–d6/D6 level in accordance with nationally recognised diagnostic criteria. 25 Data were recorded using charts specifically designed for the collection of International Caries Detection and Assessment System (ICDAS) dental codes26 (see Appendix 11). An assessment of molar incisor hypomineralisation (MIH) was also carried out.
Further clinical examination, including ICDAS caries assessment, was performed at 12-month intervals for 36 months. Experienced community dental officers undertook the clinical dental examinations. A total of six officers were used during the study, with one examiner involved in all years of the project.
Training and calibration
An intensive training and calibration exercise was undertaken in advance of each round of annual clinical examinations. Prior to each calibration exercise, the examiners were asked to complete the online e-learning package developed by the ICDAS team. 26 At each calibration event, the examiner team received an interactive lecture to review the ICDAS caries criteria. The calibration exercise was based on projected images of 74 extracted primary teeth. The gold standard was determined by Professor Chris Deery (a trained and calibrated ICDAS examiner), who provided the images. At each calibration exercise, the images were projected under standardised conditions and compared with the gold standard examiner. The caries code (the second digit of the ICDAS code) was recoded as 0, ‘free of caries into dentine’ (caries code 0–3), and 4, ‘caries at ICDAS level 4 or higher‘, that is, ‘caries into dentine’ (caries code 4–6). Calibration was based on these categorisations at the ‘caries into dentine’ level.
As part of the annual caries assessment, approximately 5% of study participants were re-examined to determine intra-examiner reproducibility. The details of the analysis of ICDAS caries data are provided in Statistical methods.
Nomenclature for recording dental caries in the Seal or Varnish? trial
In the SoV trial dental caries was recorded using ICDAS. 26 As there is potential for confusion between the different codes and thresholds used to describe dental caries when using ICDAS and previous caries indices/scoring systems, Table 1 describes the terms used to define thresholds and levels of dental caries experience.
| Terms used in this study (designation) | ICDAS caries codes | Traditional caries scores, (e.g. BASCD, WHO) | Notes |
|---|---|---|---|
| Caries free | 00 | Sound | Describes the condition as free of either enamel or dentine caries |
| Free of caries into dentine | 00, 01, 02 and 03 | Sound, D1 and D2 | This is the status traditionally regarded as ‘caries-free’. This is the principal diagnostic level used for both primary and secondary outcomes in the study |
| Enamel caries (d1–3/D1–3) | 01, 02 and 03 | D1 and D2 | Caries lesions limited to enamel |
| Dentine caries (d4–6/D4–6) | 04, 05 and 06 | D3, both cavitated and non-cavitated | Caries lesions involving dentine, also referred to as obvious dental decay |
In this study, dental caries status is described using the following principal terms: caries free, free of caries into dentine, enamel caries and dentine caries.
Treatment acceptability assessment
Treatment acceptability was assessed in three ways. A modified version of the Delighted–Terrible Faces (DTF) scale27,28 was used with children to determine participant acceptability (see Appendix 12). During the clinical placement of the technologies under investigation, the treating dental hygienist and dental nurse recorded the following indicators of participant acceptability and adverse outcomes: vomiting, crying, gagging, excessive arm/leg movements and other signs of distress. 29 As part of the process evaluation, children and their parents from each trial arm were interviewed in order to compare their experiences of FS and FV treatment (see Chapter 5).
Caries risk-related habits
For children deemed eligible to participate in the trial, information relating to the caries risk-related habits was obtained via a dental health questionnaire (DHQ) (see Appendix 13) sent to parents at baseline and at 12, 24 and 36 months post treatment.
The questionnaire asked about tooth brushing frequency, if the child brushed on their own or with parental assistance, the type of toothpaste used and the quantity of toothpaste dispensed to the toothbrush. An enquiry was also made as to the age at which tooth brushing started. The use of mouthwash, fluoride drops and fluoride tablets was determined, as was any previous application of FV by the child’s own dentist. Attendance at a dentist outside the Designed to Smile programme was ascertained, as was the frequency of dental attendance. Parents were asked about lifetime residency in South Wales. The annual questionnaire also collected data on dietary habits, with an emphasis on the frequency of the consumption of sugar-rich food and drinks. The remaining questions enquired about the use of dental services, which informed the economic analysis of the trial.
Dental care during the trial
Parents were asked for details of their child’s dentist. The principal investigator wrote to the dentist and informed them that the child was participating in the SoV trial. Dentists were asked to refrain from providing FS or FV treatment for the duration of the study. For children who were patients of the CDS, a flag was placed on their dental record to indicate that they were participants in the SoV study and therefore should not receive FS or FV treatments.
Children and their parents continued with their usual oral hygiene regime, details of which were gathered via the annual questionnaire detailed in Caries risk-related habits.
Data collection regime
The data collection regime is shown in Table 2.
| Data type | Prior to baseline evaluation | Baseline evaluation | Randomisation and initial FS/FV application | Follow-up period (months) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | 30 | 36 | ||||
| Clinical data | |||||||||
| Medical/dental history/demographics | ✗ | ✗ | ✗ | ||||||
| Eligibility (inclusion/exclusion criteria) | ✗ | ✗ | |||||||
| Caries risk-related habits | ✗ | ✗ | ✗ | ✗ | |||||
| ICDAS caries assessment | ✗ | ✗ | ✗ | ✗ | |||||
| Pre-treatment assessmenta | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Adverse event assessment | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Health economics data | |||||||||
| NHS resource usage interviews | ✗ | ✗ | ✗ | ✗ | |||||
| Parental Resource Questionnaire | ✗ | ✗ | ✗ | ✗ | |||||
| School Resource Questionnaire | ✗ | ✗ | ✗ | ✗ | |||||
| Health-related quality of life (CHU-9D) | ✗ | ✗ | ✗ | ||||||
| Treatment acceptability and process evaluation data | |||||||||
| Observational scaleb | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| DTF scale | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Interviews with children | ✗ | ✗ | |||||||
| Interviews with parents | ✗ | ✗ | |||||||
| Questionnaires/interviews with schools | ✗ | ✗ | |||||||
| Interviews with dental teamc | ✗ | ✗ | ✗ | ✗ | |||||
| Interviews with non-consenting parents | ✗ | ✗ | ✗ | ||||||
| Interviews with non-responding parent | ✗ | ✗ | ✗ | ||||||
| Interviews with withdrawing parents | d | d | d | d | d | d | d | ||
Trial outcomes
Outcome measures for dental caries
The primary outcome measure was the development of dental caries on FPMs at 36 months. This was recorded as:
-
the proportion of children developing new caries in dentine on any one of up to four treated FPMs at 36 months
-
the number of treated FPM teeth that were free of caries into dentine at 36 months.
Outcome measures for health economic analysis
Indicators of clinical effectiveness were used alongside costs to estimate the relative cost-effectiveness of FS and FV.
Two additional outcome measures were used for the cost-effectiveness analysis. These were used to estimate incremental costs per quality-adjusted life-year (QALY) estimates.
Health-related quality of life: Child Health Utility Index 9D
A generic preference-based measure of health-related quality of life (HRQoL), the Child Health Utility Index 9D (CHU-9D),30 was used to determine HRQoL. Designed specifically for use with children, the CHU-9D consists of a set of nine questions (dimensions), with five levels of responses available per question. It has a recall period of today/last night and is intended to be self-completed by the child (see Appendix 14).
Quality-adjusted tooth-years
Utility values were measured as quality-adjusted tooth-years (QATYs),31,32 which is the production of additional years of life (tooth-year) of each tooth adjusted for the quality of the tooth. An unrestored tooth has a QATY equal to 1 in the year it was restoration free, and a restored, crowned or root canal-treated tooth has a QATY of less than perfect (i.e. < 1) in the year that it was restored and in subsequent years. The QATY for an extracted tooth is equal to 0 in that year and in subsequent years. Data to inform the calculation of QATYs were derived from the clinical data collected.
Sample size
Data from a previous cohort study among primary school children under the care of the Cardiff and Vale UHB CDS were used to derive the caries incidence in children (mean age 6.5 years) with at least one erupted FPM. 33 Overall, 40% of children had caries in one or more of their FPMs by the age of 10 years. Based on recent Cochrane reviews, it is estimated that FV would reduce the 3-year incidence from 40% to 30% in this population,15 whereas FS would reduce it further to 20%. 12 For an individually randomised trial at a power of 80% with a significance level of 5%, at least 313 children per group were required for a comparison of caries incidence of 20% versus 30% at 36-month follow-up.
Randomisation
The randomisation of participants was stratified by school and balanced for sex and primary dentition baseline caries levels using minimisation in a 1 : 1 ratio for treatments. A random component was added to the minimisation algorithm,34 such that it was not completely deterministic. The algorithm that allocated children to the study arm minimised imbalance with respect to sex and baseline caries levels, with a probability of 0.8 reducing the predictability of allocation. 35
Sequence generation
Randomisation was carried out in the South East Wales Trials Unit (SEWTU) using lists of pupil sex and caries chart data collected by the CDS from each school they visited for the screening examination. Eligible children were randomised using the minimisation algorithm. Allocation lists were produced and provided to the CDS, with a 2-week window before the CDS returned to the school for the baseline treatments.
Allocation concealment mechanism
All randomisation and allocation lists were produced by SEWTU independently of the recruiting and examining personnel in the CDS.
Implementation
The CDS carried out an initial screening examination at all schools. Data from these visits were provided to SEWTU and used to produce the allocation lists for the CDS. Allocations were provided to the CDS prior to each return school visit, ready for the children’s baseline treatment.
Blinding
The physical nature of the technologies under test limited the scope for blinding. Both the participant and the dental hygienist were aware of the treatment provided. The dentist undertaking the clinical dental examinations at baseline and at 12, 24 and 36 months was not informed of the arm to which the participant had been randomised. However, the presence or absence of FS at assessment would obviously indicate the likely treatment received.
Statistical methods
All comparative analyses were carried out on an intention-to-treat basis (without imputation). The primary outcome (D4–6MFT) was analysed using a logistic regression model to compare arms. The results for binary outcomes are presented as unadjusted and adjusted ORs for the FV arm compared with the FS arm (the reference arm). All models were adjusted for the randomisation balancing variables, sex and baseline caries, in the primary dentition. Baseline caries (decayed, missing, filled teeth in primary dentition, i.e. D4–6MFT) was categorised as none, one or two, or three or more. The number of FPMs per child in the trial was also added to the models as a covariate but removed if non-significant. As the intervention was carried out within schools, a two-level logistic model was used to determine if there was any significant clustering by school. If clustering was found to be negligible, the primary analysis outcome was taken to be the single-level model. Intraclass correlation coefficients (ICCs) are given in tables or text for all two- and three-level models. For subgroup analyses, the appropriate addition of main effects and interaction terms was made to the primary model, as was the addition of any covariate effects.
For categorical outcomes with multiple categories, ordinal regression was fitted. The test of parallel lines was conducted to check that the assumption of proportional odds was satisfied. Count data were analysed using Poisson and negative binomial regression models. When the data were found to be overdispersed (greater variance than might be expected in a Poisson distribution), the negative binomial regression model was taken as the better-fitting model. When an inflation of zeros in the data was found, zero-inflated Poisson regression and zero-inflated negative binomial regression models were employed. The Akaike information criterion was used to determine the best-fitting model. The results were presented as adjusted incidence rate ratios in the FV arm compared with the FS arm.
For continuous outcomes, a linear regression model was fitted after transformation to normality, if required. When a natural log transformation was employed, the results were interpreted as percentage difference between the arms. When baseline measurements were taken into consideration, an analysis of covariance was used, with baseline measurement as a covariate. This model extends the usual logistic regression model to include correlated interim outcome data. It allows the main treatment effects to be adjusted for changes over time and for differences between time points to be examined. Differential changes over time in caries outcome between trial arms can be examined through the inclusion of a treatment × time interaction term.
When data were collected over several time points, a repeated measures model (using a generalised logistic mixed model) was used with time points (12, 24 and 36 months). For tooth- and surface-level binary outcomes, two- and three-level logistic models were used when appropriate. An interaction term was used in the three-level models to investigate any differential effect of treatment on occlusal versus non-occlusal surfaces. For all primary and secondary outcomes, 95% CIs and p-values are presented.
Primary outcome calculation
The primary outcome was the proportion of children experiencing caries into dentine at ICDAS level 4–6 on any one of up to four FPMs in the trial at 36 months. The D4–6MFT variable was calculated (and converted to a binary outcome) from the full caries charts of those children attending the 36-month examination and included only those FPMs in the trial. FPMs that were already sealed, carious into dentine, filled or affected by post-eruptive breakdown (PEB) at baseline were excluded from the trial.
Secondary outcomes calculation
Secondary caries models at child, tooth and surface levels were as follows:
-
the number of FPMs remaining free of caries into dentine per child for those FPMs included in the trial (child-level ordinal analysis)
-
the caries status of the FPMs (treated or untreated caries code 4, 5 or 6) included in the trial (tooth-level binary analysis)
-
the number of surfaces on each FPM remaining dentine caries free per tooth within child for those FPMs included in the trial (tooth-level ordinal analysis)
-
the caries status of treated or untreated caries on each surface of each FPM, caries code 4, 5 or 6 (surface-level binary analysis)
-
the binary outcome of caries occurrence on occlusal versus non-occlusal surfaces of each FPM (surface-level binary analysis with an interaction term for surface type × arm).
The primary analysis was undertaken using 12- and 24-month follow-up time points (child-level binary analyses). In addition, an investigation of the treatment differences on the earlier stages of caries development (enamel caries) was carried out using level 1, 2 and 3 of the ICDAS classification of caries at 36 months (child-level binary analysis). One additional post hoc analysis was carried out as a surface-level analysis of the binary outcome of all caries (ICDAS 1–6) with an interaction term for surface type to investigate occlusal versus non-occlusal surface interaction with treatment.
Treatment time
The treatment times at each time point were examined and found to be non-normally distributed. A natural log-transformation of the treatment time data was performed and a linear regression analysis was used to compare arms. Treatment effects are, therefore, interpreted as percentage difference between arms. The regression model was adjusted for the randomisation balancing variables sex and baseline caries.
Sealant retention
The sealant status at each treatment visit and the retention at each clinical examination were tabulated.
Molar incisor hypomineralisation
The presence and severity of MIH in the FPMs was tabulated at each dental examination, and the prevalence was reported by treatment arm. Severe MIH presenting with PEB at baseline excluded a FPM from the study.
Secondary analysis of primary outcomes
Additional important covariates were collected via the DHQ. The variables relating to tooth brushing frequency, toothpaste, mouthwash, fluoride tablets and fluoride drops were utilised in the primary model if there were ≥ 10 percentage points of difference between the binary categories. The variables relating to tooth brushing, amount of toothpaste and FV/gel applied were utilised in the primary model if there were ≥ 20 percentage points of difference between the binary categories. The following categorisations were used for covariates added to the primary analysis:
-
frequency of tooth brushing – ‘twice or more a day’ versus ‘once or less a day’
-
toothpaste type – ‘family toothpaste’ versus ‘children’s toothpaste or other’
-
amount of toothpaste – ‘smear, pea or never’ versus ‘cover bristles’
-
additional fluoride intake – ‘any mouthwash, drops or tablets or gel’ versus ‘none’.
Global oral hygiene regimen
A combined global rating scale of fluoride use and tooth brushing regimen was created as a summed score of the binary categories for tooth brushing frequency (twice a day); who carries out tooth brushing (adult or observed); toothpaste type (family toothpaste); amount of toothpaste (cover bristles); and additional fluoride (any yes response). This resulted in a score of 0–6, which was collapsed into four categories (0–1.49, 1.5–2.49, 2.5–3.49 and 3.5–6).
Length of time tooth brushing
The age of a child in months at baseline minus the age at which they started tooth brushing (in months) was converted to years (divided by 12). The distribution for length of time spent tooth brushing had slight negative skew but was entered into a linear regression untransformed.
Cariogenic score
The cariogenic score was calculated by summating the seven cariogenic items (scored 0–5) asked as part of the diet question in the DHQ (these items were c, d, e, g, h, k and m). At least four of these items had to be completed for a score to be calculated; if three or fewer of these items were ticked, the sugar intake score was set to missing. Following summation, the score was divided by (five times the number of completed items) and then multiplied by 100.
School and child deprivation
From the parental questionnaire, the child’s postcode was used to calculate the Welsh Index of Multiple Deprivation (WIMD) 2011. 36 The WIMD is categorised as quintiles and these were compared with the population of Wales as a whole. For analyses, the WIMD was trichotomised into categories 1–3, 4 or 5. The school postcode was coded in the same way and categorised as quintile for school-level analyses. School size was also tested in these two-level regression models.
Socioeconomic group
Parental occupation had five categories: ‘higher managerial, administrative and professional occupations’, ‘intermediate occupations’, ‘routine and manual occupations’, ‘unemployed’ and ‘homemaker, carer, student or grandparent’. These categories were based on those described by the Office for National Statistics. 37
Sensitivity analyses specific to primary outcomes
-
Adjustment of the primary analysis for all treatments within the specified time window, 4 weeks either side of the scheduled treatment due date.
-
The efficacy of the number of FV treatments on the primary outcome was estimated in a way that preserves randomisation using complier average causal effect (CACE) modelling. As-treated or per-protocol analyses have been widely used as secondary analyses if the effect of treatments when actually received is of interest. However, these secondary analysis methods may yield seriously biased estimates of treatment effects. Given the questionable validity of these analyses, statistical methods such as CACE estimation have been developed to better estimate treatment efficacy, taking into account non-compliance. The efficacy of the number of treatments on the primary outcome is estimated in a way that preserves randomisation. The structural mean model (SMM) approach for CACE was implemented here by fitting an instrumental variables regression. Applications of SMMs were developed to adjust for adherence in trials with active treatments and an untreated control arm. Although SoV has two active treatments, we have assumed, for the purposes of the CACE analysis, that the sealant is a one-off standard treatment comparable with a control arm, and so treatment visits were set to a constant zero. SoV also has a binary outcome, whereas CACE is more usually applied to linear outcomes. Hence, the standard instrumental variables regression in Stata® (version 13, StataCorp LP, College Station, TX, USA) was applied with the addition of robust standard errors. Adherence in the FV arm was defined as the number of FV treatment visits that a child attended (the instrumental variable) and the outputted estimate is interpreted as the efficacy per visit. A small, non-significant efficacy estimate indicates little effect of additional treatment visits and negligible adherence effects in the trial. Future work beyond the scope of this report will take these methods further and adjust for compliance, as well as for predictors of compliance in both arms.
-
The SMM approach was implemented by fitting an instrumental variables regression. 38 Adherence was defined as the number of FV treatment visits that a child attended.
Subgroup analyses specific to primary outcome
Appropriate interaction terms were entered into the primary regression analysis in order to conduct prespecified subgroup analyses: cohort, baseline caries group. The trial was not powered to detect significant interactions and, as such, these analyses are exploratory only. ORs and 95% CIs were reported.
Imputation
Imputation was to be considered after an examination of the number of missing primary outcome data and the likely mechanism for missing data. If the primary outcome was deemed to be missing completely at random and the proportion of data missing for reasons other than moving out of the SoV area was less than 10%, then imputation would not be performed and a compete case analysis would be carried out to estimate an unbiased treatment effect. If differential dropout was observed between arms, this would indicate a missing at random or missing not at random mechanism. Mixed modelling is appropriate if the data are missing at random, whereas multiple imputation and associated sensitivity analyses may be more appropriate if the missing data were determined to be missing not at random.
Reliability
Calibration of the dental examiners was carried out each year of the study, and kappa scores were calculated for reliability. At each clinical examination, a 5% sample was re-examined and caries charts were completed a second time.
Statistical software employed
All analyses were carried out in IBM SPSS Statistics version 20 (IBM Corporation, Armonk, NY, USA), except the zero-inflated Poisson regression, zero-inflated negative binomial regression and ivregress (CACE) models, which were carried out in Stata.
Assessment of adverse effects
During the course of the study, the occurrence of any serious adverse events or serious adverse reactions was ascertained and recorded using the study serious adverse events form (see Appendix 15).
As per the Design to Smile programme, the clinical staff involved in the delivery of the intervention monitored participants during and immediately following the administration of FS or FV, and were available to manage any adverse events or serious adverse reactions in accordance with current clinical practice.
Parents were asked to tell the CDS team if their child experienced any dental or medical problems during the 48 hours after each treatment.
Design of study materials and strategy for maximising questionnaire follow-up rates
Participant information sheet
As described in section 4.8 of the ICH Guideline for Good Clinical Practice,23 in order that research participants (or their legal guardians) are able to make an informed decision on whether or not to take part in a clinical trial, all relevant information must be provided to the participants in such a way that it is as non-technical and understandable as possible.
With this in mind, it was essential that all participant materials (including the PIS) were developed taking into consideration the diverse literacy levels of the target population (i.e. parents of children in Communities First areas).
To achieve this, the PIS was developed in consultation with a parents’ group in Newport. This group is in a Communities First area of South Wales but one that is not served by the Cardiff and Vale CDS; therefore, it was not within the catchment area of the trial.
After the initial draft by the SoV project team, the PIS and consent form were reviewed by the parents’ group, and the following points that they made were taken into account for subsequent revisions.
-
All of the parents agreed that the overall amount of information/text presented was too extensive and would be very likely to discourage them from thoroughly reading the information sheet (if they read it at all). Furthermore, they felt that the tone of the wording needed to be made more ‘positive’. Once the parents had had time to digest the information and understand the study, they agreed that it was a ‘positive’ thing and would want their children to participant if a similar study was run in their area.
-
Parents felt that having separate information sheets for parents and children was unnecessary, and that it would be easier for parents to be given one simplified information sheet, which they could use to discuss the study with their child. Parents felt that the wording used in the information sheet for children was much more accessible and that the one simplified information sheet should contain wording more similar to this.
-
Parents showed a strong aversion to the word ‘research’ being used (the image of children being used as ‘guinea pigs’ was expressed by several parents). They felt that ‘evaluation’ or ‘assessment’ conveyed the same concept in a more acceptable and understandable way.
-
The use of cartoons/graphics/photographs was encouraged.
-
Parents felt that the duplication of wording in the information sheet and the consent form was unnecessary, and that cross-referencing should be used to help the parents understand better which items on the consent form referred to which parts of the PIS.
Despite considering the recommendations of the parents’ group, we were mindful of the guidance provided in section 4.8.10 of ICH topic E 6 (Guideline for Good Clinical Practice23), and tried to convey to those in the group that a minimum level of information was required when developing an information sheet for a clinical trial of an investigational medicinal product (CTIMP). The revised PIS was therefore intended to represent a risk-based approach to the information provided to parents for a CTIMP.
In addition to ensuring that the content of the PIS was suitable, the visual appeal of the document was further developed by a graphic designer, incorporating the child-friendly logos and colour schemes of the CDS.
Follow-up questionnaires: development and return rate
Information relating to caries risk-related habits and parental resource utilisation was obtained from parents via a single combined DHQ (see Appendix 13) at baseline and at 12, 24 and 36 months. This was accompanied (at the 12-, 24- and 36-month time points only) by the CHU-9D (see Appendix 14), both of which were distributed to participants’ homes at the required time points.
The initial return rates of these questionnaires were lower than expected. The initial follow-up strategy allowed for one follow-up telephone call 2 weeks after the questionnaires were handed out. However, unanswered calls and inactive numbers made this strategy ineffective.
Initially, a £5 shopping voucher was included with the questionnaires in an effort to boost return rates, but responses remained poor, with an overall return rate of 53%. A consultation exercise followed with parents of children at a primary school in a Communities First area not involved in the trial. Between this consultation and ideas developed by the trial management group, the following changes to the strategy for follow-up of questionnaires were made.
-
The questionnaire pack was to be given to the child at treatment/examination.
-
A trial-branded giveaway (pen, eraser or fridge magnet) was included.
-
A text message reminder was sent if the questionnaire was not returned 1 week after distribution.
-
A second copy of the questionnaire was sent to participants’ homes if the original was not received 2 weeks after distribution.
-
A telephone call was made directly to parents to remind them/aid with questionnaire completion (DHQ only) and return.
-
Participants were informed that all returned questionnaires gained entry to a prize draw for an iPad® (Apple Inc., Cupertino, CA, USA) at each time point.
-
The questionnaires were reformatted to increase their visual appeal, and were designed to be consistent with the PIS and ICF, using the colours and graphics of the Designed to Smile programme.
Patient and public involvement
Patient and public involvement (PPI) in the SoV trial has been utilised in two main ways. The first has been described in previous sections regarding the development of the study materials with the help of members of a parents’ group in Newport. This PPI input was pivotal in producing user-friendly materials, contributing to the successful recruitment to target. Without this detailed input, we would not have been able to justify the carefully judged brevity and clarity of the PIS to the REC.
The second major use of PPI was in the redesign of participant-facing materials, as described in Design of study materials and strategy for maximising questionnaire follow-up rates. This helped to boost the return rate of follow-up questionnaires, thereby increasing our overall data collection.
Patient and public involvement was maintained throughout the trial via lay member representation on the Trial Steering Committee (TSC). Our TSC lay member is a member of the Involving People network, a Welsh Government-funded organisation that promotes PPI in research across Wales. The contribution of this member to TSC meetings was invaluable, bringing a lay perspective to deliberations.
Health economic assessment
A health economic evaluation was conducted alongside the trial. The evaluation set out to:
-
estimate the costs of providing FS versus FV and the outcomes for the NHS, children and their families and education in order to consider a partial societal perspective
-
establish the relative cost-effectiveness of FS versus FV
-
calculate the budget impact on the NHS of FS and FV delivered in a community/school setting.
The costs associated with the interventions for each trial participant were collected and summarised into the following categories:
-
Implementation costs of the interventions.
-
Health-care utilisation costs associated with travel or caregiving/time of work for families.
-
Costs associated with the schools, for example as a result of child absence. Published unit costs were used or, when unavailable, local financial records were used to value resources in monetary terms using 2015 as the price year.
A series of within-trial incremental cost-effectiveness ratios (ICERs) were calculated (based on a NHS perspective) to estimate the cost per:
-
caries increment avoided
-
QALY, using the CHU-9D to derive health utilities
-
QATY as an exploratory analysis.
Post-trial modelling was conducted to estimate the longer-term cost-effectiveness of the interventions. One-way sensitivity analysis was undertaken to assess the impact on changes to individual parameters on the base-case results with probabilistic sensitivity analysis undertaken to estimate the joint uncertainty around parameters. A budget impact analysis was undertaken to estimate the impact on NHS budgets in delivering the interventions. A detailed report on the health economic methods is provided in Chapter 4.
Treatment acceptability assessment and process evaluation
Treatment acceptability was assessed in three ways:
-
acceptability scales were completed by clinical staff
-
acceptability scales were also completed by the children participating in the trial
-
qualitative interviews were conducted with a subsample of children.
The treatment acceptability scales and the qualitative interviews are described in Chapter 5.
Chapter 3 Clinical results
This chapter describes the clinical findings of the trial.
Introduction
The chapter begins with a description of the number of children randomised to the trial, and a Consolidated Standards of Reporting Trials (CONSORT) diagram39 describes participant flow through the trial. The baseline characteristics of the participants are described in relation to trial arm and to those starting, completing and failing to complete the trial. Next, potential confounding factors such as fluoride exposure, diet and dental attendance are reported. Participation and retention data are also described.
The main clinical results of the study in relation to the primary outcome measure, the proportion of children developing caries into dentine on any treated FPM, are reported. The impact of baseline caries experience, school attended, deprivation status and trial cohort is examined, before the impact of oral hygiene habits, diet and dental attendance is analysed.
The second main trial outcome, the number of teeth developing dentine caries (D4–6MFT) at 36 months, is reported next at both tooth and tooth-surface level. Fidelity is then described using CACE analysis. Compliance with treatment windows is also reported.
The effect of including enamel caries (D1–6MFT) in the analysis is then described. This is followed by examining the impact of the FS and FV treatments on all permanent teeth, not only FPMs. Trial outcomes at 12 and 24 months are reported to illustrate the shorter-term impact of the trial interventions. Finally, the following data are presented: sealant retention, examiner reproducibility, adverse effects and treatment time.
Participant screening for participation in the trial
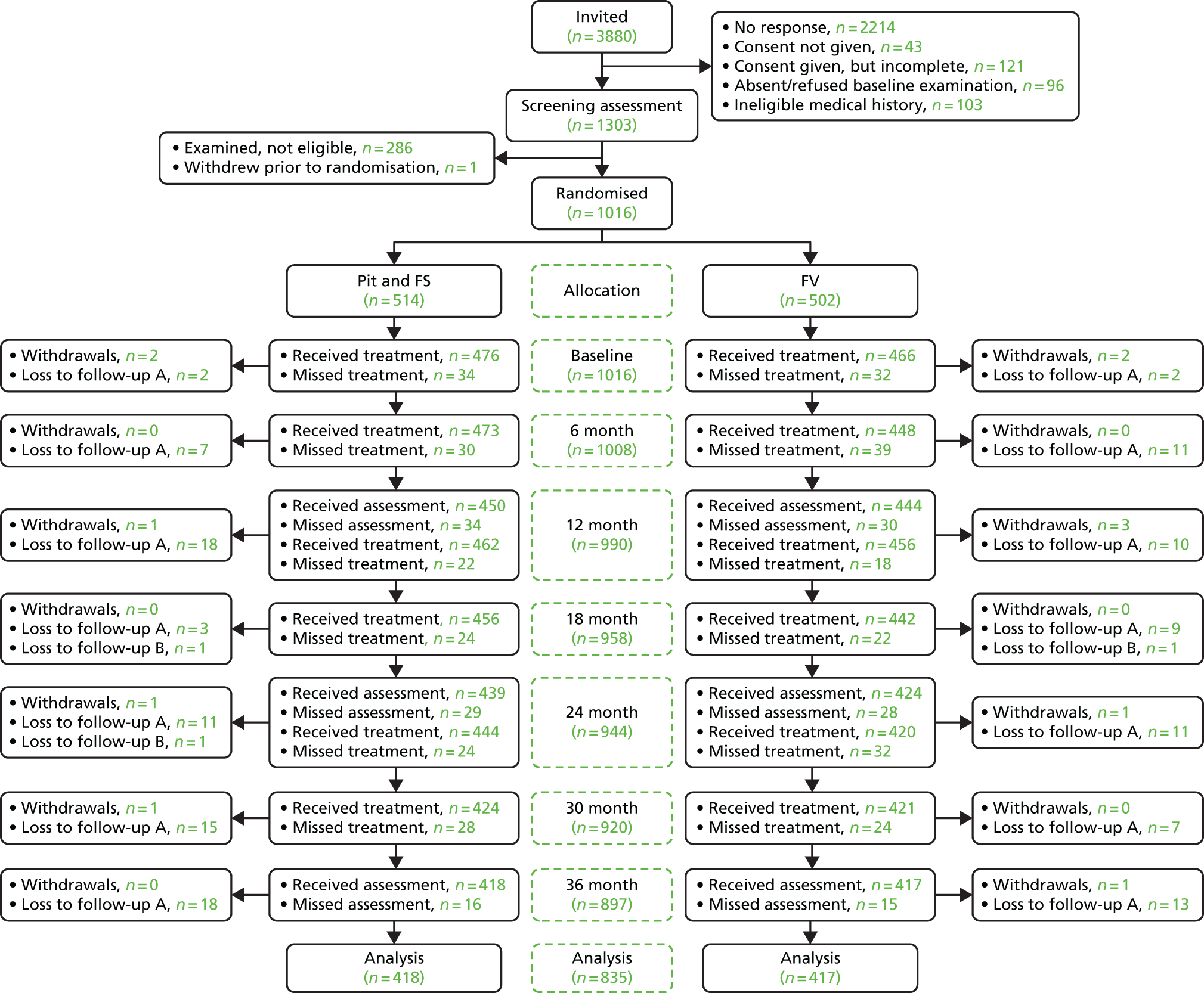
In total, 1303 children for whom parental consent had been obtained were screened for participation in the trial (Figure 2). Of these, 1016 were deemed eligible for inclusion, but one participant subsequently withdrew consent for participation and for the use of any of their data. At screening, 287 children were excluded. The participants were recruited in two cohorts between October and January in the school years 2011/12 and 2012/13.
FIGURE 2.
The CONSORT diagram of participant flow through the trial. One participant from the FV arm subsequently withdrew consent to use all data. Loss to follow-up A, moved to a non-participating school; loss to follow-up B, change in medical circumstances prevented further participation in the trial.
Participant randomisation
Participants were randomised to receive FS (514 children) or FV (501 children), as described in Chapter 2.
Characteristics of included participants at baseline
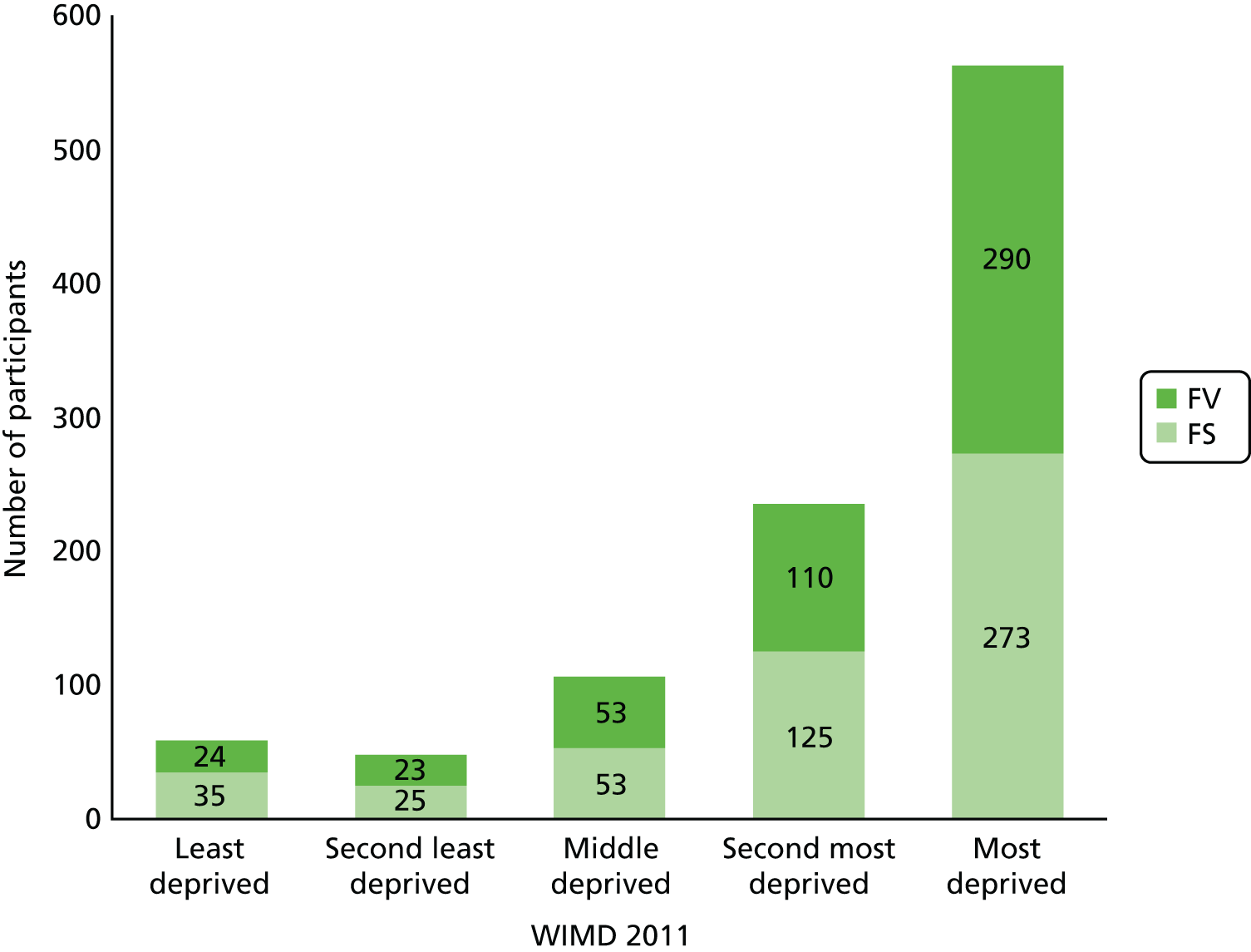
The characteristics of the study participants who were randomised at baseline are shown in Table 3. There were no apparent differences between trial arms in sex or in the proportion of children with caries experience in their primary dentition. The high prevalence of dental caries experienced in the primary dentition at baseline (54.1%) reflects the fact that the programme was specifically targeted at schools in areas of high social and economic deprivation. As shown in Figure 3, 78.6% of children randomised lived in the bottom two quintiles of deprivation in Wales. Within quintiles of deprivation, the distribution of children across trial arms was broadly similar.
| Characteristic | Intervention arm | Total, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Children randomised | 514 (50.6) | 501 (49.4) | 1015 (100) |
| Sex | |||
| Male | 237 (46.1) | 235 (46.9) | 472 (46.5) |
| Female | 277 (53.9) | 266 (53.1) | 543 (53.5) |
| Children with dentine caries in the primary dentition (d4–6) | 286 (55.6) | 266 (53.1) | 552 (54.1) |
| Children with dentine caries in the primary dentition (d4–6mft) | 342 (66.5) | 339 (67.7) | 681 (67.1) |
| Children with untreated dentine caries in any FPM (D4–6) | 22 (4.3) | 23 (4.6) | 45 (4.4) |
| Children with dentine caries in any FPM (D4–6MFT) | 27 (5.3) | 31 (6.2) | 58 (5.7) |
| d4–6mft, mean (SD) | 3.2 (3.4) | 3.2 (3.3) | 3.2 (3.3) |
| d1–6mft, mean (SD) | 4.6 (3.8) | 4.6 (3.7) | 4.6 (3.7) |
| d4–6mfs, mean (SD) | 8.9 (12.3) | 9.6 (12.4) | 9.3 (12.3) |
| d1–6mfs, mean (SD) | 11.0 (12.9) | 11.6 (12.9) | 11.3 (12.9) |
FIGURE 3.
Social and economic profile of trial participants at baseline by trial arm and by quintile of WIMD (2011).
The characteristics at baseline of the 835 children who completed the trial and of the 180 children who either were lost to follow-up or withdrew are shown in Tables 4 and 5. There were no marked differences in the baseline characteristics of either of these groups in relation to dental caries experience at baseline.
| Characteristic | Intervention arm | Total, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Children completing the trial | 418 (50.1) | 417 (49.9) | 835 (100) |
| Sex | |||
| Male | 193 (46.2) | 196 (47.0) | 389 (46.6) |
| Female | 225 (53.8) | 221 (53.0) | 446 (53.4) |
| Children with dentine caries in the primary dentition (d4–6) | 228 (54.5) | 219 (52.5) | 447 (53.5) |
| Children with dentine caries in the primary dentition (d4–6mft) | 271 (64.8) | 280 (67.1) | 551 (66.0) |
| Children with untreated dentine caries in any FPM (D4–6) | 16 (3.8) | 17 (4.1) | 33 (4.0) |
| Children with dentine caries in any FPM (D4–6MFT) | 18 (4.3) | 24 (5.8) | 42 (5.0) |
| d4–6mft, mean (SD) | 3.1 (3.4) | 3.2 (3.3) | 3.2 (3.4) |
| d1–6mft, mean (SD) | 4.5 (3.7) | 4.6 (3.7) | 4.5 (3.7) |
| d4–6mfs, mean (SD) | 8.8 (12.4) | 9.7 (12.7) | 9.3 (12.5) |
| d1–6mfs, mean (SD) | 10.9 (12.9) | 11.7 (13.2) | 11.3 (13.1) |
| Characteristic | Intervention arm | Total, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Children randomised | 96 (53.3) | 84 (46.7) | 180 (100) |
| Sex | |||
| Male | 44 (45.8) | 39 (46.4) | 83 (46.1) |
| Female | 52 (54.2) | 45 (53.6) | 97 (53.9) |
| Children with dentine caries in the primary dentition (d4–6) | 58 (60.4) | 47 (56.0) | 105 (58.3) |
| Children with dentine caries in the primary dentition (d4–6mft) | 71 (74.0) | 59 (70.2) | 130 (72.2) |
| Children with untreated dentine caries in any FPM (D4–6) | 6 (6.2) | 6 (7.1) | 12 (6.7) |
| Children with dentine caries in any FPM (D4–6MFT) | 9 (9.4) | 7 (8.3) | 16 (8.9) |
| d4–6mft, mean (SD) | 3.4 (3.3) | 3.3 (3.1) | 3.4 (3.2) |
| d1–6mft, mean (SD) | 4.8 (3.9) | 4.7 (3.4) | 4.8 (3.6) |
| d4–6mfs, mean (SD) | 9.5 (11.8) | 9.2 (11.2) | 9.4 (11.5) |
| d1–6mfs, mean (SD) | 11.7 (12.9) | 11.3 (11.5) | 11.5 (12.3) |
Characteristics of first permanent molars in children randomised to the trial
The status of the FPMs by quadrant is shown in Table 6. From this, it is apparent that the prevalence of decay into dentine was very low at baseline, the D4–6MFT ranging from 1.2% of upper-right permanent first molars to 2.9% of lower-right FPMs. A greater proportion of upper FPMs (9.2–9.4%) were unerupted than lower FPMs (4.7–5.9%). The percentage of erupted (or partially erupted) FPMs that were free of caries into dentine was recorded as 89.7% in the upper-right quadrant and 93.5% in the lower-right quadrant.
| Status | FPM | |||
|---|---|---|---|---|
| UR (N = 1015), n (%) | UL (N = 1015), n (%) | LR (N = 1015), n (%) | LL (N = 1015), n (%) | |
| Unerupted | 93 (9.2) | 95 (9.4) | 60 (5.9) | 48 (4.7) |
| D1–3T | 114 (11.2) | 120 (11.8) | 109 (10.7) | 107 (10.5) |
| D4–6T | 11 (1.1) | 10 (1.0) | 24 (2.4) | 12 (1.2) |
| MT | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| FT | 1 (0.1) | 5 (0.5) | 5 (0.5) | 5 (0.5) |
| D1–3MFT | 115 (11.3) | 125 (12.4) | 114 (11.2) | 112 (11.0) |
| D4–6MFT | 12 (1.2) | 15 (1.5) | 29 (2.9) | 17 (1.7) |
| D1–3S | 115 (11.3) | 123 (12.1) | 114 (11.2) | 111 (10.9) |
| D4–6S | 11 (1.1) | 10 (1.0) | 24 (2.4) | 12 (1.2) |
| MS | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| FS | 1 (0.1) | 6 (0.6) | 5 (0.5) | 5 (0.5) |
| D1–3MFS | 116 (11.4) | 128 (12.6) | 118 (11.6) | 116 (11.4) |
| D4–6MFS | 12 (1.2) | 15 (1.5) | 29 (2.9) | 17 (1.7) |
| Free of caries (D1–6MFT = 0) | 796 (78.4) | 785 (77.3) | 817 (80.5) | 843 (83.1) |
| Free of caries into dentine (D4–6MFT = 0) | 910 (89.7) | 905 (89.2) | 926 (91.3) | 950 (93.5) |
With 1015 children randomised to participate in the trial and assessed at baseline, the overall number of FPMs was 4060. In total, 161 (4%) of these were excluded at baseline because of the presence of FS (71 teeth), caries into dentine (56 teeth), filled teeth (17 teeth) and teeth with PEB (17 teeth) (Table 7).
| FPM | Total (N = 4060) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UR (N = 1015) | UL (N = 1015) | LR (N = 1015) | LL (N = 1015) | |||||||
| n | % | n | % | n | % | n | % | n | % | |
| Teeth included | 978 | 96.4 | 983 | 96.8 | 962 | 94.8 | 976 | 96.2 | 3899 | 96.0 |
| Teeth excluded | 37 | 3.6 | 32 | 32.2 | 53 | 5.2 | 39 | 3.8 | 161 | 4.0 |
| Reason for exclusion (n, % excluded) | ||||||||||
| Sealed | 20 | 54.1 | 14 | 43.8 | 20 | 37.7 | 17 | 43.6 | 71 | 44.1 |
| Filled | 1 | 2.7 | 6 | 18.8 | 5 | 9.4 | 5 | 12.8 | 17 | 10.5 |
| Caries at into dentine (D4–6) | 11 | 29.7 | 9 | 28.1 | 24 | 45.3 | 12 | 30.8 | 56 | 34.9 |
| PEBa | 5 | 13.5 | 3 | 9.4 | 4 | 7.5 | 5 | 12.8 | 17 | 10.5 |
Baseline characteristics: fluoride exposure, diet and dental attendance
A questionnaire distributed to the participants’ parents was used to collect information on exposure to fluoride, dietary intake and dental attendance. At the baseline examination, 597 questionnaires were returned, representing 58.8% of the study participants.
Fluoride exposure
From Table 8, it is apparent that the arms were reasonably well matched in terms of reported exposure to fluoride outside the trial interventions. Reported tooth brushing frequency was higher in the FV group, with 74.6% claiming twice-daily brushing, compared with 68.5% in the sealant group.
| Variable | Scoring | FS (N = 314), n (%) | FV (N = 283), n (%) | Total (N = 597), n (%) |
|---|---|---|---|---|
| Frequency of tooth brushing | Never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Less than once a day | 4 (1.3) | 4 (1.4) | 8 (1.4) | |
| Once a day | 77 (25.0) | 48 (17.4) | 125 (21.4) | |
| Twice a day | 221 (68.5) | 206 (74.6) | 417 (71.4) | |
| More than twice a day | 16 (5.2) | 18 (6.5) | 34 (5.8) | |
| Toothpaste type | Family toothpaste | 168 (55.3) | 134 (48.9) | 302 (52.2) |
| Children’s toothpaste | 131 (43.1) | 133 (48.5) | 264 (45.7) | |
| Other | 5 (1.6) | 7 (2.6) | 12 (2.1) | |
| Amount of toothpaste | A smear | 24 (7.8) | 23 (8.3) | 47 (8.0) |
| A pea-sized amount | 250 (81.4) | 222 (80.1) | 472 (80.8) | |
| Cover the bristles | 33 (10.7) | 32 (11.6) | 65 (11.1) | |
| Use of mouthwash | 97 (32.1) | 82 (30.3) | 179 (31.2) | |
| Use of fluoride tablets | 2 (0.9) | 0 (0.0) | 2 (0.5) | |
| Use of fluoride drops | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Use of FV | 51 (16.6) | 35 (12.6) | 86 (14.7) | |
| Lifetime resident in South Wales | 238 (90.8) | 215 (91.9) | 453 (91.3) | |
A greater proportion (55.3%) of parents in the FS arm than in the FV arm (48.9%) reported that their child used family toothpaste to clean their teeth. The amount of toothpaste used on toothbrushes was broadly similar between the trial arms. In both trial arms, 91% of the participants were lifetime residents of non-fluoridated South Wales.
Global oral hygiene regimen
The combined global rating scale of fluoride use and tooth brushing regimen, calculated as described in Chapter 2, is shown in Table 9. This shows that the global oral hygiene score increased in both trial arms from baseline to 12 months and was marginally higher in the FV arm than in the FS arm at 12, 24 and 36 months.
| Intervention | Overall | |||||
|---|---|---|---|---|---|---|
| FS | FV | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Global oral hygiene regimen | ||||||
| Baseline | 309 | 2.50 (1.083) | 280 | 2.47 (1.018) | 589 | 2.49 (1.052) |
| 12 months | 296 | 2.72 (1.136) | 270 | 2.81 (1.183) | 566 | 2.76 (1.159) |
| 24 months | 312 | 2.71 (1.204) | 292 | 2.78 (1.130) | 604 | 2.74 (1.169) |
| 36 months | 269 | 2.70 (1.035) | 254 | 2.83 (1.090) | 523 | 2.76 (1.063) |
Diet
Reported dietary intake at baseline is shown in Table 10 and summarised in Table 11.
| Variable | Scoring | Intervention arm | Total, n (%) | |
|---|---|---|---|---|
| FS, n (%) | FV, n (%) | |||
| Milk | Never and up to 4–6 times per week | 102 (33.6) | 93 (34.1) | 195 (33.8) |
| 1 per day to 2–3 times per day | 202 (66.4) | 180 (65.9) | 382 (66.2) | |
| Water | Never and up to 4–6 times per week | 88 (29.4) | 69 (25.3) | 157 (27.4) |
| 1 per day to 2–3 times per day | 211 (70.6) | 204 (74.7) | 415 (72.6) | |
| Fizzy drinks | Never and up to 4–6 times per week | 264 (88.6) | 233 (87.3) | 497 (88.0) |
| 1 per day to 2–3 times per day | 34 (11.4) | 34 (12.7) | 68 (12.0) | |
| Squash | Never and up to 4–6 times per week | 174 (60.0) | 135 (51.5) | 309 (56.0) |
| 1 per day to 2–3 times per day | 116 (40.0) | 127 (48.5) | 243 (44.0) | |
| Fruit juice | Never and up to 4–6 times per week | 210 (72.2) | 186 (70.3) | 395 (71.3) |
| 1 per day to 2–3 times per day | 81 (27.8) | 78 (29.7) | 159 (28.7) | |
| Diet/light drinks | Never and up to 4–6 times per week | 202 (69.9) | 167 (63.5) | 369 (66.8) |
| 1 per day to 2–3 times per day | 87 (30.1) | 96 (36.5) | 183 (33.2) | |
| Sweets/confectionery | Never and up to 4–6 times per week | 236 (77.4) | 214 (79.6) | 450 (78.4) |
| 1 per day to 2–3 times per day | 69 (22.6) | 55 (20.4) | 124 (21.6) | |
| Chocolate | Never and up to 4–6 times per week | 242 (79.1) | 214 (79.3) | 456 (79.2) |
| 1 per day to 2–3 times per day | 64 (20.9) | 56 (20.7) | 120 (20.8) | |
| Crisps | Never and up to 4–6 times per week | 235 (76.5) | 205 (75.9) | 440 (76.3) |
| 1 per day to 2–3 times per day | 72 (23.5) | 65 (24.1) | 137 (23.7) | |
| Fruit | Never and up to 4–6 times per week | 93 (30.5) | 67 (24.6) | 160 (27.7) |
| 1 per day to 2–3 times per day | 212 (69.5) | 205 (75.4) | 417 (72.3) | |
| Biscuits and cakes | Never and up to 4–6 times per week | 231 (75.7) | 210 (76.9) | 441 (76.3) |
| 1 per day to 2–3 times per day | 74 (24.3) | 63 (23.1) | 137 (23.7) | |
| High-fibre cereals | Never and up to 4–6 times per week | 189 (62.0) | 159 (58.5) | 348 (60.3) |
| 1 per day to 2–3 times per day | 116 (38.0) | 113 (41.5) | 229 (39.7) | |
| Other cereals | Never and up to 4–6 times per week | 220 (73.1) | 192 (71.1) | 412 (72.2) |
| 1 per day to 2–3 times per day | 81 (26.9) | 78 (28.9) | 159 (27.8) | |
| Intervention arm | Overall | |||||
|---|---|---|---|---|---|---|
| FS | FV | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Cariogenic score | ||||||
| Baseline | 307 | 45.079 (15.3528) | 275 | 46.188 (14.3435) | 582 | 45.603 (14.8820) |
| 12 months | 299 | 44.997 (16.3388) | 268 | 43.996 (14.5916) | 567 | 44.524 (15.5320) |
| 24 months | 308 | 43.929 (14.2771) | 289 | 43.053 (14.1149) | 597 | 43.505 (14.1936) |
| 36 months | 266 | 42.097 (14.1395) | 254 | 42.980 (14.5780) | 520 | 42.528 (14.3483) |
Cariogenic score
The cariogenic score, derived as described in Chapter 2, gives a summary measure of exposure to cariogenic dietary items and is reported in Table 11. This shows that, although the score reduced between the baseline and the 12-month examinations, it remained relatively constant thereafter and differed little between the intervention arms.
Dental attendance
On the topic of dental attendance, 92.6% of children in the FS arm and 90.9% of children in the FV arm were reported as being regular dental attenders, with the remainder described by their parents as attending only when they were in pain.
Participation and retention
Of the 1016 children randomised to the trial, 835 (82.2%) underwent a final clinical examination at 36 months. The number completing the FS arm was 418 and the number completing the FV arm was 417. The most common reason that children did not complete the trial was that they had moved away from the area or to a school that was not participating in the trial; this was reported as lost to follow-up (see Figure 2). The number of children who withdrew from the trial was five in the FS arm and seven in the FV arm.
It can be seen from the CONSORT diagram (see Figure 2) that the proportion of missing primary outcome in each arm is evenly distributed by reason. Only 21 out of 514 (4.1%) participants in the FS arm and 22 out of 502 (4.4%) participants in the FV arm were a result of absence on the day of the final examination or study withdrawal. Missing data attributable to participants moving out of the SoV area and a change in medical circumstances were deemed to be unrelated to the treatments and, therefore, a completely random mechanism. Moreover, the number of missing data that may possibly be related to treatment was very low; therefore, imputation was not carried out and a complete case analysis is appropriate. Estimates or treatment effect from these analyses will be unbiased with regard to missing data.
The number of children who missed treatment at each of the 6-month intervals was small; for example, at the 12-month treatment session, 22 out of 484 (4.5%) missed treatment in the FS arm, and the corresponding figure in the FV arm was 18 out of 474 (3.8%). This was because the children had been absent from school on the day the MDC visited. As shown in Table 12, in both arms of the study 95% of participants received the treatment on five or six of the six scheduled treatment visits, indicating extremely high fidelity in the study.
| Treatment visits attended | Intervention arm, n (%) | |
|---|---|---|
| FS (N = 418) | FV (N = 417) | |
| 0 | 0 (0) | 0 (0) |
| 1 | 1 (0.2) | 0 (0) |
| 2 | 0 (0) | 1 (0.2) |
| 3 | 2 (0.5) | 4 (1.0) |
| 4 | 16 (3.8) | 15 (3.6) |
| 5 | 92 (22.0) | 96 (23.0) |
| 6 | 307 (73.4) | 301 (72.2) |
At the final assessment (36 months), the clinical team visited the schools on a second occasion to pick up any absentees. Of the 866 children remaining in the trial and eligible for clinical assessment, 835 (94.6%) were examined.
Participation rates across the trial by arm and by assessment and treatment visits are shown in Figure 4. It is clear that, although there was a gradual loss of participants throughout the trial, the loss was equal between the arms, and numbers of children remaining, at 418 and 417, were well in excess of the required 313 per arm as determined in the power calculation (see Chapter 2, Sample size).
FIGURE 4.
Participation rates across the trial by arms and by assessment and treatment visits.

Clinical results
The proportion of children developing caries into dentine, having a restoration or extraction on at least one first permanent molar at 36 months
The primary outcome measure of the trial was the proportion of children developing new caries into dentine on any one of up to four treated FPMs (Table 13 and Figure 5). The proportion of children with dentine caries (D4–6MFT) on any one of their FPMs was compared between those who had sealant and those who had FV.
| Intervention arm, n (%) | Total (N = 835), n (%) | ||
|---|---|---|---|
| FS (N = 418) | FV (N = 417) | ||
| No dentine caries on any FPM | 336 (80.4) | 344 (82.5) | 680 (81.4) |
| Dentine caries (D4–6MFT) on at least one FPM | 82 (19.6) | 73 (17.5) | 155 (18.6) |
FIGURE 5.
The proportion of children with dentine caries (D4–6MFT) on any FPM in the trial at 36-month follow-up by trial arm.

The proportion of children who developed dentine caries (D4–6MFT) on at least one FPM at 36 months was broadly similar in the FS (19.6%) and the FV (17.5%) arms.
The OR for developing caries in the FV arm (when compared with the FS arm) was 0.87 (95% CI 0.61 to 1.23) in the unadjusted model, that is, children who received FV were slightly less likely to develop caries than those who received FS, but the difference was not statistically significant (Table 14).
| Unadjusted OR (95% CI) | p-value | Adjusteda OR (95% CI) | p-value | |
|---|---|---|---|---|
| FS | Reference | Reference | ||
| FV | 0.87 (0.61 to 1.23) | 0.433 | 0.84 (0.59 to 1.21) | 0.351 |
| Baseline caries prevalence | ||||
| Primary dentition free of caries into dentine | Reference | |||
| 1–2 primary teeth with caries into dentine | 1.58 (0.83 to 3.00) | 0.165 | ||
| ≥ 3 primary teeth with caries into dentine | 4.57 (2.83 to 7.39) | < 0.001 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.06 (0.74 to 1.52) | 0.756 | ||
As sex and baseline caries prevalence were used to balance the randomisation, an adjusted model was also performed, and this was taken as the primary analysis. The OR for developing caries in the FV arm was 0.84 (95% CI 0.59 to 1.21) in the adjusted model.
In summary, the final model (see Table 14) shows that there was no significant difference in the proportion of children with dentine caries (D4–6MFT) on any FPM in the trial at 36 months regardless of whether the children received FS or FV. Children who had more than three carious primary teeth at baseline were significantly more likely to develop caries into dentine on a FPM at 36 months. There was no difference between the proportion of boys and girls developing caries into dentine on at least one FPM.
The influence of treating dental hygienist
Two trained experienced dental hygienists delivered the intervention. When the treating hygienist at baseline was added to the single-level primary analysis as a factor, this was not significant (p = 0.981) and did not change the primary outcome. When the primary outcome was modelled adjusted for treating hygienist using a two-level logistic regression model, the outcome was unchanged compared with the unadjusted result. The ICC for hygienist was zero, and so there was no clustering by staff delivering the intervention.
The influence of school attended
To determine if there was any clustering in the primary outcome at school level, a two-level multilevel logistic regression was carried out. The results from the two-level model did not differ from the primary model to any significant degree (Table 15) and school was dropped as level for subsequent analyses. The ICC for school was 0.029 in the unadjusted model and 0.024 in the adjusted model.
| Unadjusted OR (95% CI) | p-value | Adjusteda OR (95% CI) | p-value | |
|---|---|---|---|---|
| FS | Reference | Reference | ||
| FV | 0.87 (0.61 to 1.23) | 0.426 | 0.84 (0.59 to 1.21) | 0.358 |
| Baseline caries | ||||
| Primary dentition free of caries into dentine | Reference | |||
| 1–2 primary teeth with caries into dentine | 1.61 (0.84 to 3.07) | 0.150 | ||
| ≥ 3 primary teeth with caries into dentine | 4.56 (2.81 to 7.40) | < 0.001 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.06 (0.74 to 1.53) | 0.747 | ||
The influence of cohort and baseline caries experience
To examine the influence of two prespecified subgroups on the primary outcome, an exploratory analysis was undertaken. The children were recruited to the trial in two cohorts (see Chapters 2 and 6) over two school years. The purpose of this analysis was to examine whether or not there were significant differences in the primary outcome measures between the trial cohorts. The second analysis examined the effect of caries in the primary dentition at the baseline examination on subsequent development of caries on FPMs. The number of children developing D4–6MFT at 36 months related to the trial cohort and to the number of decayed primary teeth at baseline is shown in Table 16.
| Intervention arm, n (%) | Total (N = 835), n (%) | ||
|---|---|---|---|
| FS (N = 418) | FV (N = 417) | ||
| No dentine caries in FPMs at 36 months | |||
| Cohort 1 | 173 (51.5) | 180 (52.3) | 353 (51.9) |
| Cohort 2 | 163 (48.5) | 164 (47.7) | 327 (48.1) |
| At least one FPM with dentine caries at 36 months | |||
| Cohort 1 | 41 (50.0) | 46 (63.0) | 87 (56.1) |
| Cohort 2 | 41 (50.0) | 27 (37.0) | 68 (43.9) |
| Total | |||
| Cohort 1 | 214 (51.2) | 226 (54.2) | 440 (52.7) |
| Cohort 2 | 204 (48.8) | 191 (45.8) | 395 (47.3) |
| No dentine caries in FPMs at 36 months | |||
| Baseline caries | |||
| Primary dentition free of caries into dentine | 130 (38.7) | 131 (38.1) | 261 (38.4) |
| 1–2 primary teeth with caries into dentine | 68 (20.2) | 69 (20.1) | 137 (20.1) |
| ≥ 3 primary teeth with caries into dentine | 138 (41.1) | 144 (41.9) | 282 (41.5) |
| At least one FPM with dentine caries at 36 months | |||
| Baseline caries | |||
| Primary dentition free of caries into dentine | 17 (20.7) | 6 (8.2) | 23 (14.8) |
| 1–2 primary teeth with caries into dentine | 8 (9.8) | 11 (15.1) | 19 (12.3) |
| ≥ 3 primary teeth with caries into dentine | 57 (69.5) | 56 (76.7) | 113 (72.9) |
| Total | |||
| Baseline caries | |||
| Primary dentition free of caries into dentine | 147 (35.2) | 137 (32.9) | 284 (34.0) |
| 1–2 primary teeth with caries into dentine | 76 (18.2) | 80 (19.2) | 156 (18.7) |
| ≥ 3 primary teeth with caries into dentine | 195 (46.7) | 200 (48.0) | 395 (47.3) |
Children in cohort 2 experienced less diseased teeth. Children in the FV arm with dentine caries on one or two primary teeth experienced more dentine caries in their FPMs than those in the FS arm. Those receiving FV who had more than three primary teeth with dentine caries were also marginally more likely to experience dentine caries in their FPMs than those receiving FS (48% vs. 46.7%).
The interaction between cohort and intervention arm is reported in Table 17. From this it is apparent that the interaction of arm and cohort is not statistically significant.
| Adjusteda OR (95% CI) | p-value | |
|---|---|---|
| FS | Reference | |
| FV | 1.07 (0.66 to 1.74) | 0.790 |
| Baseline caries | ||
| Primary dentition free of caries into dentine | Reference | |
| 1–2 primary teeth with caries into dentine | 1.62 (0.85 to 3.09) | 0.140 |
| ≥ 3 primary teeth with caries into dentine | 4.60 (2.84 to 7.44) | < 0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.04 (0.73 to 1.50) | 0.824 |
| Cohort 1 | Reference | |
| Cohort 2 | 1.19 (0.72 to 1.96) | 0.500 |
| Reference | ||
| Cohort 2 × FV arm | 0.58 (0.28 to 1.21) | 0.147 |
The interaction of baseline caries risk with trial arm does not quite reach conventional levels of statistical significance, as shown in Table 18. Those children treated with FV who had more caries at baseline in their primary dentition were more likely to develop caries at 36 months than those who received FS. However, caution should be used for the interpretation of these effects, as the subgroup sizes for baseline caries risk were small.
| Adjusteda OR (95% CI) | p-value | |
|---|---|---|
| FS | Reference | |
| FV | 0.35 (0.13 to 0.92) | 0.032 |
| Baseline caries | ||
| Primary dentition free of caries into dentine | Reference | |
| 1–2 primary teeth with caries into dentine | 0.90 (0.37 to 2.19) | 0.811 |
| ≥ 3 primary teeth with caries into dentine | 3.16 (1.75 to 5.72) | < 0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.05 (0.73 to 1.50) | 0.808 |
| Reference | ||
| FV × 1–2 primary teeth with caries into dentine | 3.89 (0.99 to 15.28) | 0.051 |
| FV × ≥ 3 primary teeth with caries into dentine | 2.69 (0.93 to 7.72) | 0.067 |
Secondary analysis for the primary outcome: proportion of children developing dentine caries (D4–6MFT) on any first permanent molar at 36 months
To determine the impact of potential confounding factors, a covariate analysis of the primary outcome was undertaken from two perspectives: child level (Table 19) and school level (Table 20).
| Covariate | Total N | FS or FV | Adjusteda OR (95% CI) | p-value |
|---|---|---|---|---|
| Global oral hygiene regimen | FS | Reference | ||
| 493 | FV | 0.90 (0.55 to 1.45) | 0.652 | |
| Additional fluoride | FS | Reference | ||
| 471 | FV | 0.93 (0.57 to 1.50) | 0.753 | |
| Cariogenic global score | FS | Reference | ||
| 487 | FV | 0.85 (0.53 to 1.39) | 0.526 | |
| Socioeconomic group | FS | Reference | ||
| 460 | FV | 0.85 (0.51 to 1.41) | 0.524 | |
| Frequency of tooth brushing | FS | Reference | ||
| 489 | FV | 0.97 (0.59 to 1.59) | 0.904 | |
| Toothpaste type | FS | Reference | ||
| 484 | FV | 0.88 (0.54 to 1.44) | 0.616 | |
| Length of time brushing tooth | FS | Reference | ||
| 467 | FV | 0.99 (0.55 to 1.47) | 0.666 | |
| WIMD child | FS | Reference | ||
| 834 | FV | 0.85 (0.59 to 1.22) | 0.371 |
| Covariate | n | FS or FV | Adjusteda OR (95% CI) | p-value | ICC |
|---|---|---|---|---|---|
| School size | 835 | FS | Reference | ||
| FV | 0.85 (0.59 to 1.22) | 0.364 | 0.022 | ||
| WIMD school | 835 | FS | Reference | ||
| FV | 0.84 (0.58 to 1.21) | 0.348 | 0.032 |
The numbers for the child-level covariate analyses were substantially lower than those for the primary outcome because of questionnaire non-responses. The only covariates that were significantly associated with outcome were frequency of tooth brushing (OR 0.36, 95% CI 0.21 to 0.60) and toothpaste type (OR 0.45, 95% CI 0.27 to 0.75). Those brushing twice or more a day were less likely to develop caries on their FPMs than those brushing once or less a day. Those using children’s or other toothpaste were less likely to develop caries on their FPMs at 36 months. None of the covariates altered the main effect for arm.
Two further potential influencing factors on the primary outcome were examined: the size of the school, in terms of the number of children enrolled, and the socioeconomic level of the locality in which the school was situated, as determined by the WIMD. 36
When modelled using a two-level logistic model of child within school, neither school size nor the WIMD of the locality in which the school was located was significantly associated with the caries outcome at 36 months, and including them did not alter the main effect of intervention arm.
The effect of fissure sealant and fluoride varnish on caries prevention by tooth
The proportion of first permanent molar teeth caries-free at 36 months
The analyses reported above relate to the proportion of children developing new caries on any one of up to four treated FPMs, that is, a child-level outcome. The second main objective in this clinical trial was:
-
the number of treated FPM teeth that were caries free at 36 months.
In both the FS and the FV arms of the trial, 120 FPM teeth (7.5% of all teeth) developed caries into dentine, required a restoration or were extracted (Table 21). A multilevel model (Table 22), adjusted for the number of decayed primary teeth at baseline and sex, confirmed that the difference between children receiving FS and children receiving FV was not statistically significant (OR 0.97, 95% CI 0.73 to 1.28; p = 0.83). A preliminary analysis showed that there was no significant differences between quadrants (upper right, upper left, lower right and lower left), but quadrant was retained in the model as a cluster level (ICC = 0.12).
| Intervention arm, n (%) | Total (N = 3205), n (%) | ||
|---|---|---|---|
| FS (N = 1609) | FV (N = 1596) | ||
| No caries | 1489 (92.5) | 1476 (92.5) | 2965 (92.5) |
| Caries on FPM surface | 120 (7.5) | 120 (7.5) | 240 (7.5) |
| Unadjusted OR (95% CI) | p-value | Adjusteda OR (95% CI) | p-value | |
|---|---|---|---|---|
| FS | Reference | Reference | ||
| FV | 1.03 (0.79 to 1.35) | 0.825 | 0.97 (0.73 to 1.28) | 0.830 |
| Baseline caries | ||||
| Primary dentition free of caries into dentine | Reference | |||
| 1–2 primary teeth with caries into dentine | 1.57 (0.90 to 2.74) | 0.111 | ||
| ≥ 3 primary teeth with caries into dentine | 5.66 (3.76 to 8.51) | < 0.001 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.00 (0.75 to 1.32) | 0.972 | ||
This means that there was no difference in the proportion of FPM teeth developing decay into dentine between the arms of the trial.
A further analysis examined the severity of caries attack as determined by the number of teeth becoming carious per child. The proportion of children with dentine caries (D4–6MFT) at 36 months by the number of FPMs by intervention arm is shown in Table 23. The proportion of FPMs per child developing dentine caries differed marginally between the intervention arms.
| Intervention arm, n (%) | Total (N = 835), n (%) | ||
|---|---|---|---|
| FS (N = 418) | FV (N = 417) | ||
| No caries on any FPM | 336 (80.4) | 344 (82.5) | 680 (81.4) |
| Caries on one FPM | 53 (12.7) | 43 (10.3) | 96 (11.5) |
| Caries on two FPM | 22 (5.3) | 17 (4.1) | 39 (4.7) |
| Caries on three FPM | 5 (1.2) | 9 (2.2) | 14 (1.7) |
| Caries on four FPM | 2 (0.5) | 4 (1.0) | 6 (0.7) |
The significance of the difference between the intervention arms was tested using ordinal regression modelling (Table 24). Those children with three or four FPMs with caries into dentine were grouped together. A marginally smaller proportion of children who received FV developed caries into dentine in their FPMs at 36 months, although the difference from the FS arm was not statistically significant (OR 0.87, 95% CI 0.64 to 1.20).
| Unadjusted OR (95% CI) | p-value | Adjusteda OR (95% CI) | p-value | |
|---|---|---|---|---|
| FS | Reference | Reference | ||
| FV | 0.89 (0.65 to 1.22) | 0.465 | 0.87 (0.64 to 1.20) | 0.399 |
| Threshold | ||||
| 0 to 1 | 11.31 (7.16 to 17.86) | < 0.001 | ||
| 1 to 2 | 32.38 (19.60 to 55.51) | < 0.001 | ||
| 2 to 3 | 98.87 (53.37 to 183.14) | < 0.001 | ||
| Baseline caries | ||||
| Primary dentition free of caries into dentine | Reference | |||
| 1–2 primary teeth with caries into dentine | 1.54 (0.84 to 2.83) | 0.167 | ||
| ≥ 3 primary teeth with caries into dentine | 4.05 (2.59 to 6.35) | < 0.001 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.03 (0.75 to 1.41) | 0.866 | ||
‘Threshold’ represents the response variable in the ordered logistic regression.
The threshold estimates are the cut-off points that differentiate between ordered categories of the outcome. The OR for trial arm is not statistically significant (and no different from the logistic model), indicating no difference between the arms for the ordinal outcome of number of FPMs with caries per child.
The effect of fissure sealant and fluoride varnish by tooth surfaces
Because of its mode of action, FS can afford protection only to the occlusal tooth surface. By contrast, FV can afford protection to the occlusal and the mesial, buccal, lingual and distal (smooth) tooth surfaces. It was, therefore, important to determine if there was a difference between the intervention arms in the number of tooth surfaces affected.
Looking first at the proportion of tooth surfaces that developed caries into dentine or that were restored or extracted because of caries at 36 months (Table 25), the number of such surfaces affected was 169 (2.1%) in the FS arm and 181 (2.3%) in the FV arm.
| Status | Intervention arm, n (%) | Total (N = 16,016), n (%) | |
|---|---|---|---|
| FS (N = 8041) | FV (N = 7975) | ||
| Free of caries into dentine | 7872 (97.9) | 7794 (97.7) | 15,666 (97.8) |
| D4–6MFS | 169 (2.1) | 181 (2.3) | 350 (2.2) |
Table 26 reports a multilevel model that examines the proportion of tooth surfaces affected by dentine caries (D4–6MFS) within quadrant within child. There was no significant difference between the intervention arms in the number of FPM tooth surfaces developing caries (OR 1.06, 95% CI 0.84 to 1.33; p = 0.619).
| Unadjusted OR (95% CI) | p-value | Adjusteda OR (95% CI) | p-value | |
|---|---|---|---|---|
| FS | Reference | Reference | ||
| FV | 1.17 (0.93 to 1.46) | 0.177 | 1.06 (0.84 to 1.33) | 0.619 |
| Baseline caries | ||||
| Primary dentition free of caries into dentine | Reference | |||
| 1–2 primary teeth with caries into dentine | 1.41 (0.86 to 2.33) | 0.173 | ||
| ≥ 3 primary teeth with caries into dentine | 6.50 (4.56 to 9.27) | < 0.001 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.38 (1.09 to 1.74) | 0.007 | ||
Table 27 further reports on the severity of caries experience on the FPMs, as represented by the number of surfaces with caries into dentine as a proportion of the total number of surfaces affected. This shows the severity of caries experience within the trial arms.
| Intervention arm, n (%) | Total (N = 835), n (%) | ||
|---|---|---|---|
| FS (N = 418) | FV (N = 417) | ||
| D4–6MFS = 0 | 336 (80.4) | 344 (82.5) | 680 (81.4) |
| 1 with caries | 46 (11.0) | 36 (8.6) | 82 (9.8) |
| 2 with caries | 14 (3.3) | 17 (4.1) | 31 (3.7) |
| 3 with caries | 11 (2.6) | 7 (1.7) | 18 (2.2) |
| 4 with caries | 3 (0.7) | 6 (1.4) | 9 (1.1) |
| 5 with caries | 4 (1.0) | 4 (1.0) | 8 (1.0) |
| 6 with caries | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| 7 with caries | 2 (0.5) | 0 | 2 (0.2) |
| 10 with caries | 1 (0.2) | 0 | 1 (0.1) |
| 20 with caries | 0 | 2 (0.5) | 2 (0.2) |
Ordinal regression modelling was undertaken on the number of FPM surfaces per child developing caries. Those children with between 5 and 20 affected surfaces were combined into one group, owing to the small numbers in these categories. As shown in Table 28, there was no statistically significant difference between those receiving FS and those receiving FV, the model having been adjusted to account for the number of surfaces with caries per child (OR 0.87, 95% CI 0.64 to 1.19; p = 0.385), the number of decayed primary teeth at baseline and sex.
| Unadjusted OR (95% CI) | p-value | Adjusteda OR (95% CI) | p-value | |
|---|---|---|---|---|
| FS | Reference | Reference | ||
| FV | 0.89 (0.65 to 1.22) | 0.456 | 0.87 (0.64 to 1.19) | 0.385 |
| Threshold 0 to 1 | 11.38 (7.20 to 17.98) | < 0.001 | ||
| 1 to 2 | 26.00 (15.93 to 42.42) | < 0.001 | ||
| 2 to 3 | 46.43 (27.37 to 78.75) | < 0.001 | ||
| 3 to 4 | 82.56 (45.76 to 148.94) | < 0.001 | ||
| 4 to 5 | 133.16 (63.37 to 259.34) | < 0.001 | ||
| Baseline caries | ||||
| Primary dentition free of caries into dentine | Reference | |||
| 1–2 primary teeth with caries into dentine | 1.54 (0.83 to 2.82) | 0.166 | ||
| ≥ 3 primary teeth with caries into dentine | 4.05 (2.58 to 6.34) | < 0.001 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.05 (0.76 to 1.43) | 0.782 |
This means that there was no difference in the severity of the caries experience between the FS- and FV-treated children.
Caries experience on occlusal and non-occlusal surfaces
Although there was no difference between arms in the proportion or the total number of tooth surfaces affected by dentine caries, or in the severity of attack, it was necessary to check if there was a difference in treatment effect by type of surface. Table 29 shows the distribution of caries-affected occlusal and non-occlusal surfaces by trial arm. Although, overall, a significantly greater proportion of occlusal surfaces than smooth surfaces had developed caries into dentine at 36 months (6.4% vs. 1.1%), it is clear that the proportion of occlusal surfaces affected by dentine caries was remarkably similar in the FS and FV arms of the trial, at 6.5% and 6.3%, respectively. Minimal differences between the intervention arms in the proportion of smooth surfaces of FPMs developing caries were observed at 36 months (1.0% vs. 1.3%). A multilevel model that accounted for quadrant and surface-level clustering, adjusted to account for caries prevalence in the primary dentition at baseline, sex, surface type and surface by arm interactions, is reported in Table 30. As previously, children with three or more affected primary teeth at baseline were significantly more likely to develop dentine caries on a FPM by 36 months and, overall, there was a significant difference between the proportion of occlusal surfaces developing dentine caries and the proportion of non-occlusal surfaces developing dentine caries (OR 7.72, 95% CI 5.57 to 10.69). However, no significant difference was observed between the trial arms for the main effect or for the interaction of arm with surface type, indicating no differential effect of treatment on occlusal and non-occlusal surfaces.
| Status | Intervention arm, n/N (%) | Total (N = 16,016) | |
|---|---|---|---|
| FS (N = 8041) | FV (N = 7975) | ||
| Caries on non-occlusal surface | 64/6432 (1.0) | 81/6380 (1.3) | 145/12,812 (1.1) |
| Caries on occlusal surface | 105/1609 (6.5) | 100/1595 (6.3) | 205/3204 (6.4) |
| Adjusteda OR (95% CI) | p-value | |
|---|---|---|
| FS | Reference | |
| FV | 1.25 (0.89 to 1.77) | 0.202 |
| Baseline caries | ||
| Primary dentition free of caries into dentine | Reference | |
| 1–2 primary teeth with caries into dentine | 1.42 (0.86 to 2.36) | 0.174 |
| ≥ 3 primary teeth with caries into dentine | 6.96 (4.85 to 9.98) | < 0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.38 (1.10 to 1.76) | 0.007 |
| Surface type | ||
| Non-occlusal | Reference | |
| Occlusal | 7.72 (5.57 to 10.69) | < 0.001 |
| Surface by arm interaction | Reference | |
| (FV × occlusal surface) | 0.75 (0.48 to 1.18) | 0.217 |
Complier average causal effect analysis
In a trial such as that reported here, compliance with the intended treatment regimen is an important consideration. Children participating in the trial were scheduled to be seen by the dental hygienist every 6 months, meaning a maximum of six times (at 0, 6, 12, 18, 24 and 30 months) during the course of the trial. The number of treatment visits attended by each child who also underwent a final clinical examination at 36 months is shown in Table 31. It is apparent that fidelity in this trial was excellent, with in excess of 70% of children attending all six treatment visits and 95% of children in the FS and FV arms of the trial attending at least five treatment visits. The association of treatment visits and final outcome by trial arm is shown in Table 31.
| Treatment visits | Intervention arm, n (%) | |||
|---|---|---|---|---|
| FS | FV | |||
| No FPM with D4–6MFT (N = 336) | At least one FPM with D4–6MFT (N = 82) | No FPM with D4–6MFT (N = 344) | At least one FPM with D4–6MFT (N = 73) | |
| 1 | 1 (0.3) | 0 (0) | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 (1.4) |
| 3 | 1 (0.3) | 1 (1.4) | 4 (1.2) | 0 |
| 4 | 13 (3.9) | 3 (3.7) | 11 (3.2) | 4 (5.5) |
| 5 | 75 (22.3) | 17 (20.7) | 80 (23.3) | 16 (21.9) |
| 6 | 246 (73.2) | 61 (74.4) | 249 (72.4) | 52 (71.2) |
From Table 31, it is clear that there was little association between the number of treatment visits and the development of caries in either trial arm.
To determine the effects of adherence to the scheduled number of treatment visits, a CACE analysis was carried out (Table 32). As FS was the standard treatment in the Designed to Smile programme prior to the implementation of this trial, a CACE analysis was carried out, assuming FS as the ‘control’ with treatment visits set to a constant (zero) and FV as the active treatment. In this way it can be established if the number of FV treatment visits had any significant impact on the main treatment effect.
| Efficacy coefficient (95% CI) | p-value | |
|---|---|---|
| Number of FV treatment visits | –0.005 (–0.014 to 0.004) | 0.303 |
| OR (95% CI) | p-value | |
| Baseline caries | ||
| Primary dentition free of caries into dentine | Reference | |
| 1–2 primary teeth with caries into dentine | 1.37 (0.97 to 1.10) | 0.310 |
| ≥ 3 primary teeth with caries into dentine | 1.22 (1.16 to 1.30) | < 0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.00 (0.95 to 1.05) | 0.961 |
The coefficient for the number of treatment visits for FV was non-significant, indicating that increasing the number of visits does not have an impact on the proportion of children experiencing caries on their FPMs. The efficacy per visit was –0.005, indicating only a small decrease in the proportion of children with caries on any FPM for additional FV visits. This small effect is attributable to the high proportion of children attending at least five visits out of a maximum of six.
From this, it is concluded that adherence to the treatment protocol was not an influencing factor in the results obtained at 36 months.
Adherence to the prespecified treatment window
The study protocol dictated that treatment visits should be scheduled within a 4-week window either side of the due 6-month treatment schedule. Adherence to this schedule is shown in Table 33. Overall, 71.6% of treatments were within the treatment window throughout the trial, and a further 26.1% were outside the schedule on only one occasion, a further indicator of the fidelity of the trial.
| Number of treatments outside window | Intervention arm, n (%) | Total (N = 835), n (%) | |
|---|---|---|---|
| FS (N = 418) | FV (N = 417) | ||
| 0 | 302 (72.2) | 296 (71.0) | 598 (71.6) |
| 1 | 106 (25.4) | 112 (26.9) | 218 (26.1) |
| 2 | 7 (1.7) | 5 (1.2) | 12 (1.4) |
| 3 | 1 (0.2) | 3 (0.7) | 4 (0.5) |
| 4 | 2 (0.5) | 0 | 2 (0.2) |
| 5 | 0 | 1 (0.2) | 1 (0.1) |
To test for the effect of adherence to the treatment window, a binary indicator was added to the primary model that categorised the participants as having received all of their treatment visits within the time window stated in the study protocol or having had at least one visit outside the treatment window. The likelihood of developing D4–6MFT on any FPM was lower for those who received at least one treatment outside the study treatment window (OR 0.66, 0.43 to 1.00; p = 0.052). Although this effect was of borderline significance, the main effect of FS versus FV remained unchanged compared with the primary analysis (Table 34).
| Adjusteda OR (95% CI) | p-value | |
|---|---|---|
| FS | Reference | |
| FV | 0.84 (0.58 to 1.21) | 0.341 |
| Baseline caries | ||
| Primary dentition free of caries into dentine | Reference | |
| 1–2 primary teeth with caries into dentine | 1.61 (0.84 to 3.06) | 0.140 |
| ≥ 3 primary teeth with caries into dentine | 4.61 (2.85 to 7.45) | < 0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.05 (0.73 to 1.50) | 0.811 |
| All treatment within window | Reference | |
| At least one outside window | 0.66 (0.43 to 1.00) | 0.052 |
This demonstrates that any deviation from the scheduled treatment window did not impact on the overall results of the trial.
The effect of including enamel caries on trial outcome
The analysis reported in Complier average causal effect analysis at the D4–6MFT level is in line with conventional reporting of dental clinical trials. In this study, dental caries was recorded using the ICDAS. 26 This index includes the recording of enamel caries. This section reports the effects of including enamel caries in the trial outcome data.
Table 35 shows the proportion of children developing enamel and dentine caries at the D1–6MFT level at 36 months. The proportion of children who developed dental caries was higher in the FS arm (77.3%) than in the FV arm (67.6%). Adjusting for the number of decayed primary teeth at baseline and sex, a significant treatment effect between arms was apparent (Table 36). The OR for developing caries when enamel caries was included in the outcome measure was significant (OR 0.56, 95% CI 0.41 to 0.78), indicating a possible protective effect of FV for enamel caries lesions. This suggests that, although there was no difference between the trial arms in terms of caries into dentine prevented, the same does not hold true when enamel caries is included in the outcome measure. A significantly greater proportion of children did not develop either dentine or enamel caries on any FPM included in the trial when treated with FV (32.4% vs. 22.7%).
| Intervention arm, n (%) | Total (N = 835), n (%) | ||
|---|---|---|---|
| FS (N = 418) | FV (N = 417) | ||
| No caries on any FPM | 95 (22.7) | 135 (32.4) | 230 (27.5) |
| Caries at D1–6MFT level on at least one FPM | 323 (77.3) | 282 (67.6) | 605 (72.5) |
| Unadjusted OR (95% CI) | p-value | Adjusteda OR (95% CI) | p-value | |
|---|---|---|---|---|
| FS | Reference | Reference | ||
| FV | 0.61 (0.45 to 0.84) | 0.002 | 0.56 (0.41 to 0.78) | 0.001 |
| Baseline caries | ||||
| Primary dentition free of caries into dentine | Reference | |||
| 1–2 primary teeth with caries into dentine | 2.37 (1.54 to 3.64) | < 0.001 | ||
| ≥ 3 primary teeth with caries into dentine | 5.12 (3.54 to 7.40) | < 0.001 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 0.81 (0.58 to 1.11) | 0.187 | ||
The consequences of including enamel caries were further explored to examine the effect by occlusal versus non-occlusal surfaces. In the FS arm of the trial, 6.7% of non-occlusal surfaces developed caries at the D1–6MFS level, compared with 5.6% in the FV arm. On occlusal surfaces, the figures for the development of either enamel or dentine caries were 39.2% and 40.5% in the case of FS and FV, respectively (Table 37).
| Intervention arm, n/N (%) | Total (N = 16,016), n/N (%) | ||
|---|---|---|---|
| FS (N = 8041) | FV (N = 7975) | ||
| Caries on non-occlusal surface | 429/6432 (6.7) | 360/6380 (5.6) | 789/12812 (6.2) |
| Caries on occlusal surface | 631/1609 (39.2) | 646/1595 (40.5) | 1277/3204 (39.9) |
A multilevel model confirmed a statistically significant difference in favour of the FV-treated surfaces. The clinical significance of this finding is unclear. It possibly shows FV to be protective for early-phase caries compared with FS (Table 38). However, this does not translate into a protective effect for dentine caries compared with FS.
| Adjusteda OR (95% CI) | p-value | |
|---|---|---|
| FS | Reference | |
| FV | 0.80 (0.68 to 0.93) | 0.004 |
| Baseline caries | ||
| Primary dentition free of caries into dentine | Reference | |
| 1–2 primary teeth with caries into dentine | 2.00 (1.69 to 2.36) | < 0.001 |
| ≥ 3 primary teeth with caries into dentine | 4.37 (3.82 to 4.99) | < 0.001 |
| Sex | ||
| Female | Reference | |
| Male | 0.92 (0.83 to 1.03) | 0.148 |
| Surface type | ||
| Non-occlusal | Reference | |
| Occlusal | 19.44 (5.78 to 65.43) | < 0.001 |
| Surface by arm interaction | Reference | |
| (Varnish × occlusal surface) | 1.29 (1.04 to 1.60) | 0.019 |
To further explore the effect of including enamel caries, analyses were conducted for enamel and dentine caries at the D2–6MFT and D3–6MFT levels. Using both of these caries levels as the cut-off point for determining the clinical outcome, children in the FV arm were significantly less likely to develop caries experience on at least one FPM than those in the FS group: D2–6MFT (OR 0.65, 95% CI 0.49 to 0.88) and D3–6MFT (OR 0.66, 95% CI 0.48 to 0.90).
Whole-mouth caries experience at 36 months
Although the caries-preventative effect of FS is limited to the occlusal surface of the treated tooth, it is possible that, by elevating the general intraoral fluoride concentration, there may be a ‘wash-over’ effect as a result of applying FV to the FPMs. To examine this possible difference between the treatments, the caries increment on all permanent teeth present at the 36-month assessment was examined.
Table 39 shows the proportion of children with caries (D4–6MFT) on any permanent tooth at 36 months.
| Number of permanent teeth with D4–6MFT | Intervention arm, n (%) | |
|---|---|---|
| FS (N = 418) | FV (N = 417) | |
| 0 | 326 (78.0) | 334 (80.1) |
| 1 | 56 (13.4) | 42 (10.1) |
| 2 | 27 (6.5) | 26 (6.2) |
| 3 | 5 (1.2) | 11 (2.6) |
| 4 | 3 (0.7) | 4 (1.0) |
| 6 | 1 (0.2) | 0 |
To examine the difference in the number of affected teeth, Poisson and binomial distribution modelling were carried out. Furthermore, to account for the excess of non-affected individuals, zero inflated models were conducted to improve model fit. The results are shown in Table 40. No significant differences were observed between intervention arms. This means that no wash-over effect was observed. A similar analysis was carried out on the D4–6MFS scores. No significant difference was observed between FS- and FV-treated children.
| Adjusteda incidence rate ratio (95% CI) | p-value | Akaike information criterion | |
|---|---|---|---|
| Poisson | 0.94 (0.74 to 1.20) | 0.624 | 1171 |
| Negative binomial | 0.93 (0.82 to 1.46) | 0.549 | 1104 |
| Zero inflated Poisson | 1.01 (0.76 to 1.35) | 0.920 | 1091 |
| Zero inflated negative binomial | 1.01 (0.75 to 1.35) | 0.924 | 1093 |
The effect of trial interventions at 12 and 24 months
All of the analyses reported above relate to the clinical outcome at 36 months, the a priori time period agreed for the determination of the primary clinical outcome. Although no significant difference was observed between FS and FV at 36 months, the question arises as to whether the outcome would have been different had the trial terminated at 12 or 24 months.
At the 12-month examination, 36 (8%) children who had received FS experienced dental decay in at least one FPM at the D4–6MFT level, compared with 37 (8.3%) of those treated with FV. By 24 months, the numbers similarly affected had increased to 61 (13.9%) and 63 (14.9%) in the FS and FV arms of the trial, respectively (Table 41).
| Time point (months), n (%) | ||||||
|---|---|---|---|---|---|---|
| 12 | 24 | 36 | ||||
| FS (N = 450) | FV (N = 444) | FS (N = 439) | FV (N = 424) | FS (N = 418) | FV (N = 417) | |
| No caries on any FPM | 414 (92.0) | 407 (91.7) | 378 (86.1) | 361 (85.1) | 336 (80.4) | 344 (82.5) |
| Caries on at least one FPM | 36 (8.0) | 37 (8.3) | 61 (13.9) | 63 (14.9) | 82 (19.6) | 73 (17.5) |
A repeated measures analysis was carried out using the proportion of children experiencing caries D4–6MFT on any FPM at 12, 24 and 36 months. Although the proportion of children experiencing caries was significantly higher at 24 months than at 12 months and at 36 months than at 12 months, there were no significant differential effects of treatment by time, and the interaction effect was dropped from the model (Table 42).
| Treatment effect (sealant is reference) | Examination (time point, months) (12 months is reference) | |||||
|---|---|---|---|---|---|---|
| 24 | 36 | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Proportion with caries at ICDAS 4–6 | 0.96 (0.76 to 1.21) | 0.878 | 1.92 (1.41 to 2.62) | < 0.001 | 2.64 (1.95 to 3.56) | < 0.001 |
It can, therefore, be concluded that there was no statistically significant difference between the treatments under investigation at any of the annual examinations.
Sealant retention
The effectiveness of FS is influenced by retention,40 and complete sealants are known to be more effective than partial sealants. 41
In this trial, children were examined at 6-month intervals and any deficient sealants were repaired. Policy was to seal teeth as soon as sufficient tooth surface had erupted, so, particularly in the case of upper molars, teeth were on occasion sealed over two visits as the tooth erupted. The status of the sealants as recorded by the dental hygienists is shown in Table 43.
| Time point (months), n (%) | |||||
|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | 30 | |
| UR intact | 198 (44.7) | 269 (68.3) | 298 (76.4) | 300 (76.7) | 275 (74.5) |
| Partial | 111 (25.1) | 70 (17.8) | 72 (18.5) | 78 (19.9) | 86 (23.3) |
| Lost | 2 (0.5) | 5 (1.3) | 2 (0.5) | 7 (1.8) | 2 (0.5) |
| Tooth unerupted | 40 (9.0) | 7 (1.8) | 3 (0.8) | 2 (0.5) | 1 (0.3) |
| Tooth extracted | 0 | 0 | 0 | 0 | 0 |
| Tooth previously unerupted | 92 (20.8) | 43 (10.9) | 16 (4.1) | 4 (1.0) | 5 (1.4) |
| N | 443 | 394 | 391 | 391 | 369 |
| Missing | 9 | 6 | 1 | ||
| UL intact | 172 (40.2) | 259 (64.3) | 298 (74.5) | 285 (72.5) | 275 (73.7) |
| Partial | 130 (30.4) | 90 (22.3) | 82 (20.5) | 93 (23.7) | 84 (22.5) |
| Lost | 4 (0.9) | 10 (2.5) | 5 (1.2) | 10 (2.5) | 9 (2.4) |
| Tooth unerupted | 22 (5.1) | 6 (1.5) | 0 | 1 (0.3) | 1 (0.3) |
| Tooth extracted | 0 | 0 | 0 | 0 | 1 (0.3) |
| Tooth previously unerupted | 100 (23.4) | 38 (9.4) | 15 (3.8) | 4 (1.0) | 3 (0.8) |
| N | 428 | 403 | 398 | 393 | 373 |
| Missing | 2 | 7 | 5 | 2 | 2 |
| LR intact | 274 (62.3) | 311 (78.1) | 339 (85.8) | 350 (88.4) | 324 (88.0) |
| Partial | 33 (7.5) | 22 (5.5) | 23 (5.8) | 20 (5.1) | 32 (8.7) |
| Lost | 1 (0.2) | 1 (0.3) | 2 (0.5) | 5 (1.3) | 2 (0.5) |
| Tooth unerupted | 41 (9.3) | 16 (4.0) | 10 (2.5) | 3 (0.8) | 2 (0.5) |
| Tooth extracted | 0 | 0 | 0 | 0 | 0 |
| Tooth previously unerupted | 91 (20.7) | 48 (12.1) | 21 (5.3) | 18 (4.5) | 8 (2.2) |
| N | 440 | 398 | 395 | 396 | 368 |
| Missing | 6 | 7 | 1 | 3 | |
| LL intact | 262 (59.4) | 314 (78.1) | 346 (87.2) | 347 (87.8) | 340 (91.4) |
| Partial | 30 (6.8) | 24 (6.0) | 17 (4.3) | 28 (7.1) | 19 (5.1) |
| Lost | 4 (0.9) | 2 (0.5) | 2 (0.5) | 4 (1.0) | 3 (0.8) |
| Tooth unerupted | 32 (7.3) | 16 (4.0) | 9 (2.3) | 5 (1.3) | 2 (0.5) |
| Tooth extracted | 0 | 0 | 0 | 0 | 0 |
| Tooth previously unerupted | 113 (25.6) | 46 (11.4) | 23 (5.8) | 11 (2.8) | 8 (2.2) |
| N | 441 | 402 | 397 | 395 | 372 |
| Missing | 8 | 8 | 4 | 2 | |
Examiner reproducibility
The kappa statistics for agreement with the gold standard examiner are shown in Table 44. Based on Landis and Koch,42 all of the examiners show substantial agreement (0.69–0.8) or excellent agreement (0.81–1) throughout the study. Calibration was at the d/D0–3:d/D4–6 level.
| Assessment | Examiner | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 2011 | 0.754 | 0.819 | 0.749 | |||
| 2012 | 0.830 | 0.883 | 0.914 | |||
| 2013 | 0.754 | 0.915 | 0.768 | |||
| 2014 | 0.938 | 0.801 | 0.690 | |||
| 2015 | 0.931 | 0.740 | ||||
To determine intra-examiner reproducibility, 5% of participants were re-examined. The resultant kappa values (DMFT sound surfaces excluded) are shown in Table 45.
| Assessment | Examiner | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 2011 | 0.929 | 0.908 | 0.818 | |||
| 2012 | 0.939 | 0.928 | 0.657 | |||
| 2013 | 0.925 | 0.946 | 0.909 | |||
| 2014 | 0.8500 | 0.960 | 0.830 | |||
| 2015 | 0.972 | |||||
Treatment time
The time taken to provide the FS and FV treatments, recorded as time spent in the dental chair by each child at the baseline and 30-month examinations, is recorded in Table 46.
| Intervention arm | Overall | |||||
|---|---|---|---|---|---|---|
| FS | FV | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Baseline | 467 | 533.0 (189.90) | 463 | 190.5 (57.68) | 930 | 362.5 (221.58) |
| 30 months | 424 | 130.0 (119.75) | 418 | 151.1 (46.38) | 842 | 140.5 (91.60) |
At baseline, the mean treatment time was higher for the FS arm than for the FV arm, whereas at 30 months the mean treatment time was slightly lower for the FS arm. This is because, as the trial progressed, fewer FSs needed to be placed in the FS arm, although some time was needed to repair FS when deficient. At baseline, 1301 FSs were placed, whereas at 12 months only 313 top-up treatments were performed. By 24 months, the number of top-up treatments had dropped to 178, and this dropped further to 130 at the 30-month time point, which was the final treatment.
Data were transformed using a natural log for regression analyses and, hence, effects are interpreted as percentage difference between arms.
At both time points, the addition of school as a level in a two-level regression (model 1) does not change the effect compared with the single-level model (model 2). At baseline, times were significantly lower in the FV arm, but at 30 months the difference was in the opposite direction (Table 47). These findings are in line with clinical expectations. At baseline, it takes much longer to place the FS because of the more involved clinical nature of the procedure, compared with the less complex method of FV placement.
| Effect (percentage difference) (95% CI) | p-value | ICC | |
|---|---|---|---|
| Baseline, model 1 | 0.37 (0.36 to 0.39) | < 0.001 | 0.142 |
| Baseline, model 2 | 0.37 (0.35 to 0.39) | < 0.001 | n/a |
| 30 months, model 1 | 1.66 (1.52 to 1.80) | < 0.001 | 0.117 |
| 30 months, model 2 | 1.64 (1.50 to 1.79) | < 0.001 | n/a |
Adverse effects
Throughout the trial adverse effects either during treatment or in the period thereafter were recorded as described in Chapter 2. For the entire duration of the trial, no adverse effects were reported.
Summary
This chapter has reported the clinical findings of the trial. The main findings are as follows.
-
Children were recruited to the trial in two cohorts over successive school years and followed for 36 months. Of the 514 and 502 children randomised to the FS and FV treatments, respectively, 418 and 417 remained at 36 months [more than the 313 required per trial arm, as detailed in the power calculation (see Chapter 2)]. The majority of those not completing the trial moved to a non-participating school.
-
There were no significant differences in participant characteristics between trial arms at baseline in those randomised, those who withdrew or were lost to follow-up and in those who completed the trial.
-
The OR of developing caries in the FV arm compared with the FS arm was 0.87 (95% CI 0.61 to 1.23) in the unadjusted model, that is, the proportion of children who received FV who were likely to develop caries into dentine was lower than the proportion of those developing dentine caries in the FS arm, but this difference was not statistically significant. As sex and baseline caries risk group were used to balance the randomisation, an adjusted model was also performed and was taken as the primary analysis. The OR for developing caries in the FV arm was 0.84 (95% CI 0.59 to 1.21) in the adjusted model.
-
Adjusting for baseline caries experience in the primary dentition, school attended (size and deprivation score) did not affect the primary outcome of the trial.
-
None of the covariates examined (fluoride use, oral hygiene regime, diet or deprivation) made any difference to the primary outcome of no significant difference between trial arms.
-
A subsequent analysis examining differences between intervention arms at tooth level did not show any difference in the number of teeth developing decay into dentine between the arms of the trial.
-
Examining the outcome at tooth-surface level showed that, although a significantly greater proportion of caries experience occurred on occlusal surfaces, there were no significant differences by trial arm.
-
Fidelity in the trial was excellent, with 95% of the children receiving treatment at five or six of the six scheduled treatment sessions over the 36 months. Overall, 71.6% of treatments were within the treatment window throughout the trial, and a further 26.1% were outside the schedule on only one occasion.
-
As per the trial protocol, and in line with convention, decay into dentine, as a cavity, restoration or tooth extracted because of caries, was taken as the caries level for the primary reported outcome. As the ICDAS was used to record caries, the effect of including enamel caries in the analysis was also determined. This resulted in the only analysis to suggest a significant difference between trial arms. The percentage of children in the FV arm of the study who developed decay at the D1–6MFT level was 67.6%, compared with 77.3% of FS-treated children (adjusted OR 0.56, 95% CI 0.41 to 0.78).
-
There was no evidence of any ‘wash-over’ effect on caries prevention in those treated with FV.
-
There were no significant differences in caries outcome between trial intervention arms at the 12- or 24-month time points.
Chapter 4 reports on the economic aspects of the trial.
Chapter 4 Health economic evaluation: methods and results
Introduction
The recently updated Cochrane review16 highlighted the scarcity and clinical diversity of data, making it difficult to draw clear conclusions on the effectiveness of FS versus FV in preventing dental caries. This review did not undertake a review of the relative cost-effectiveness of these two technologies and neither could any comparable economic evaluations be found in a rapid review of the health economics literature conducted as part of the health economic analysis plan. This chapter reports on the methods and results of the economic evaluation conducted alongside the trial. The objectives were to establish the:
-
costs and budget impact of FS and FV delivered in a community/school setting
-
relative cost-effectiveness of these technologies.
Health economic methods for the within-trial analysis
Assessment of costs
Costs were considered from the perspective of the NHS (i.e. direct health-care costs associated with the interventions) and from a partial societal perspective that considered the costs to the family and school (education sector).
The intervention costs were obtained from discussion with the trial team and MDC staff and from data collected as part of the trial case report forms. Research costs (such as the costs of the calibration training or costs of storing the technologies to be compliant with MHRA guidelines) were not included. With the technologies for both interventions being delivered in an established MDC, the costs of the clinic in the base case (e.g. costs of the van, running costs or equipment costs) were not included, as these are equivalent. However, to illustrate the costs associated with the delivery of the technologies within a MDC setting, we reported a separate cost with the MDC included.
The main intervention costs were (1) acquisition costs for FS and FV and (2) costs associated with the delivery of the technologies (including the clinical assessments). The costs of the FS and FV were taken from the trial protocol, that is, it was assumed that a standard dosage was applied to all trial participants and, when required, the costs of any reapplication were also calculated when they were recorded. The costs of delivery were calculated from ‘time in chair’ information contained within the case report form for each participant visit. The skill mix and grade of staff involved in the visit were obtained from the clinical staff providing the interventions. In addition, any specific materials or equipment used in the application of the FS or FV were obtained from the clinical staff. These costs were aggregated and an average intervention cost per participant in each trial arm was calculated (Table 48).
| Basis of estimate | Unit cost (£) | Per hour (£) | Data sources |
|---|---|---|---|
| Dentistry staff band 3 × 1 | – | 9.20 | NHS Staff Agenda for Change43 |
| Dentistry staff band 4 × 1 | – | 10.58 | NHS Staff Agenda for Change43 |
| Dentistry staff band 5 × 0.65 | – | 12.62 | NHS Staff Agenda for Change43 |
| Administration staff (band 3) × 1 | – | 9.20 | NHS Staff Agenda for Change43 |
| Administration staff (band 4) × 1 | – | 10.58 | NHS Staff Agenda for Change43 |
| FV (Duraphat) | 2.00 | – | Dental suppliera |
| Sealant (Delton) | 2.50 | – | Dental suppliera |
| Clinical disposables (e.g. gloves, cotton wool, bib, etc.) | 2.50 | – | Dental suppliera |
| Sterilisation costs | 1.75 | – | Cardiff and Vale UHBb |
Information about the other health-care visits associated with dental-related problems for each trial participant was obtained from a parent resource utilisation questionnaire (the DHQ; see Appendix 13). This captured information on dental health-care visits (e.g. to the family dentist), other health-care contacts (e.g. general practitioner or hospital) as a result of dental health problems and any prescriptions (e.g. painkillers or antibiotics) for dental health problems. As it would have been implausible to ask the parent/carer precise information about which tooth was affected, information was collected from the parent/carer on all dental health visits and events related to his or her child. The DHQ was completed by the main parent/carer at baseline and at 12, 24 and 36 months.
Additional costs incurred by the family were also captured in the DHQ. This included any travel time and time off work/other activities associated with a health-care contact. The cost of caring for the child was also considered as a result of a dental health problem (e.g. day off work to care for the child). The main parent/carer was asked to estimate the time the child had off school as a result of a dental health problem.
Cost associated with travel time (using published government mileage rates) and time off work to attend health-care appointments with the child or the time taken to care for a child off school was calculated using a human capital approach. The value of work as well as leisure time was estimated by applying hourly rates, according to the parents’ professions, to the time expended because of dental health problems during the trial period. Hourly rates of household/domestic help were used to cost the time of unemployed parents.
To capture the costs to the school, a series of questions were given to the head teacher as part of the process evaluation. These questions asked the head teacher to estimate the time the child had away from school activities in order to receive the interventions, any time required by the teacher or other support staff (e.g. to settle a child back into the classroom) and any other costs incurred by the school to support (e.g. administration time).
Prior to commencement of the trial, the DHQ, alongside the CHU-9D, was piloted for comprehensiveness, ease of completion, recall and accuracy in a primary school in South Wales, which was not included in the trial, with a similar sociodemographic to the trial population. A protocol was developed (available from the chapter author on request), with ethics approval given by Swansea University School of Human and Health Sciences prior to the pilot evaluation. Of 30 children who were given a pack to take home, six responses were obtained (20% response rate). All parents completed a postal questionnaire, with all responses indicating that their child had no difficulties understanding or completing the CHU-9D. In addition, the PPI representative on the TSC reviewed both questionnaires to check for readability and comprehension.
When possible, unit costs for the UK were applied [e.g. Personal and Social Services Research Unit,44 British National Formulary (BNF),45 NHS reference costs46] to increase generalisability. However, to ensure that the skill mix of the MDC was fully considered in the analysis, local salary scales from NHS Wales47 were obtained, and these were fully agreed with the trial team prior to analysis. These are reported in Table 48.
A summary of the other resource items, unit costs and sources is given in Table 49.
| Health service resource utilisation | Unit cost (£) | Source |
|---|---|---|
| Dentist | 110 | Average weighted NHS Reference Cost 2014/15,46 M01a-3b |
| Socioeconomic classification wages per hour | Various | Office for National Statistics, 201548 |
| Cost to schools attributed to absence | Various | GOV.uka |
| Medicines | Various | BNF, 201545 |
The intervention costs and health-care utilisation costs were summated to give the total cost per participant for each arm (NHS costs), with the wider costs to the family and school reported separately (see Table 54).
Valuation of costs
All data were valued in monetary terms (£), and the price year of 2015 was applied to costs (representing the end point of the trial). As follow-up was beyond 12 months, discounting of 3.5% was applied, in line with the National Institute for Health and Care Excellence (NICE) reference case for health technologies57 for the base-case costs. All costs were summarised into the different cost categories, with means and standard deviations (SDs) produced. A total cost (means and 95% CIs) was calculated for each trial arm, with differences reported using a Levene’s test for equality t-test and with statistical significance accepted at the 5% level. When missing data existed, a simple weighted average cost based on known cost histories based on accepted methods was used. 49
To take into account the possible impact of skewness in cost data, the distribution of costs for normality were examined, as was the impact of removing extreme outliers from the analysis. When required, the sensitivity of the results was checked by considering appropriate transformation. 50
Outcomes used in the health economic analysis
Caries avoided
The data from the main trial were used to calculate the percentage of dentine caries (D4–6MFT) avoided at 36 months.
Quality-adjusted life-years using the Child Health Utility Index 9D
The CHU-9D is a child-specific generic preference-based measure of HRQoL. It consists of a descriptive system of nine questions and a set of preference weights based on five levels with a recall period of today/last night. The CHU-9D allows the generation of utility values for each health state to enable the calculation of QALYs. 51,52
The CHU-9D was originally intended for use in a population aged 7–11 years. Prior to commencement of the trial, the original developer (Dr Katherine Stevens) was contacted to discuss whether or not it could be used in a younger age group, and there were no major concerns with regard to the trial’s slightly younger age group. The trial team were mindful of the trial population and the potential variance in ability to self-complete the CHU-9D. The CHU-9D was piloted in a non-participating school in South Wales, with a debriefing interview completed by the parent/carer to assess any problems in its use. No issues were identified. Since the commencement of the trial, further studies have reported the use of the CHU-9D in a comparable age group to the trial population at study entry. 53,54
The CHU-9D was delivered to the child’s home address with instructions given to complete at 12, 24 and 36 months. The instruction given was that either the child should self-complete or that the parent/carer would read the questions aloud to the child who would give his or her response. Owing to the practicalities of the trial, it was deemed unfeasible to collect the CHU-9D from the child, for example in school. Thus, the administration of the CHU-9D in this context may have resulted in under-/over-estimation of QoL, as it was not possible to verify whether or not this had been reported by the child himself/herself. However, this is expected to be similar across both study arms. The CHU-9D scores were computed for each time point and the QALY was calculated to obtain the difference in QALYs between the two trial arms at 36 months. In accordance with good practice, appropriate methods were explored to take into account missing data on the CHU-9D and subsequent QALY calculation.
Quality-adjusted tooth-year
Published utilities and clinical data used to derive the tooth-year were derived in order to generate a QATY. A QATY will estimate the production of additional years of life (tooth-year) of each tooth adjusted for the quality of that tooth. An unrestored tooth has a QATY of 1 in the year it was restoration free, although a restored, crowned or root canal-treated tooth has a QATY of less than perfect (i.e. less than 1) in the year that it was restored and in subsequent years. The QATY for an extracted tooth is equal to 0 in that year and subsequent years.
Although the QATY is seen as potentially useful in providing a more dental-specific analogy to the QALY, it has had limited use within the literature because of the limitations of being compared across different interventions and the lack of robust methodological work in deriving suitable utilities in order to populate the ‘Q’ element of the QATY. Owing to the lack of resources to undertake necessary methodological work to derive precise estimates of dental-specific utilities in children, this was intended to be an exploratory analysis only. Thus, only a one-way sensitivity analysis was undertaken.
The trial data were used to provide a series of health states associated with the tooth state of the four index teeth in the trial, based on the caries status. Published utilities were assigned from the published utilities from the Fyffe and Kay55 study and from an economic analysis on the cost-effectiveness of oral health programmes56 and were agreed with the trial team (Table 50).
| State | Utility | Comments |
|---|---|---|
| Sound tooth or sound and sealed tooth (completely caries free) | 1 | As per Fyfe and Kay55 |
| Unerupted tooth | 1 | As per Fyfe and Kay55 |
| Tooth with enamel caries (D1–3) either sealed or unsealed | 0.95 | A tooth in this condition can be ‘healed’ or the lesion stopped from progressing, so although in the early disease stages and not as desirable as a totally sound tooth, this state is more desirable than a filled tooth. Fyfe and Kay have no utility for this state |
| Filled tooth (FT) | 0.90 | As per Fyfe and Kay55 |
| Tooth with dentine caries (D4–6) | 0.81 | D4–6 is the equivalent to Fyfe and Kay’s55 decayed and non-painful posterior tooth |
| Extracted tooth | 0 | As per Fyfe and Kay55 |
A combined ‘FPM utility’ from the scores using the mean of the four tooth state utilities is as follows:
Analysis
Within-trial analysis
A series of incremental cost-effectiveness analyses were planned: (1) an incremental cost per caries avoided at 36 months and (2) an incremental cost per QALY (cost–utility). As part of a secondary analysis, costs and QATYs were examined. If one technology was both more effective and less costly, it would be considered to dominate the other technology and, as such, an ICER would not be reported. When there was no such dominance, an ICER would be calculated.
Univariate sensitivity analyses for all ICERs were recalculated after changing the value of a range of parameters individually to examine the uncertainty around the ICER (Table 51).
| Parameter | Intervention arm (£) | Analysis | Justification/source | |||||
|---|---|---|---|---|---|---|---|---|
| FS | FV | |||||||
| Base case | Lower range | Upper range | Base case | Lower range | Upper range | |||
| Costs (£) | ||||||||
| Staff costs of delivering the technology | 74.12 | 51.88 | 96.36 | 64.16 | 44.91 | 83.41 | BI | ± 30% from trial data |
| One-off application of FS | 14.24 | 9.97 | 18.51 | – | – | – | BI | ± 30% from trial data |
| FV application in school by dental hygienist | – | – | – | 11.48 | 8.04 | 14.92 | BI | ± 30% from trial data |
| Delivery within school with MDC | 95.73 | 67.01 | 124.45 | 86.33 | 60.43 | 112.23 | BI | ± 30% from trial data |
| Health-care resource usage for dental health problems (95% CI) | –68.13 (–5.63 to –130.63) | ICER | Based on 95% CIs from trial data | |||||
| Clinical effects | ||||||||
| Caries avoided (95% CI) | 0.021 (–0.0317 to 0.0738) | ICER | Based on 95% CIs from trial data | |||||
| Health outcomes | ||||||||
| Changes in QALY estimates (95% CI) | 0.002 (–0.010 to 0.005) | ICER | Based on 95% CIs from trial data | |||||
| Changes in QATY estimates (95% CI) | 0.007 (–0.068 to 0.011) | ICER | Based on 95% CIs from trial data | |||||
A probabilistic sensitivity analysis undertaken for the cost–utility analysis (CUA), with changes to the values of all chosen parameters (usually within the 95% CI or a reasonable, defined range), calculated the probability that the intervention was cost-effective in accordance with commonly accepted norms (£20,000–30,000 per incremental QALY gained). 57 Uncertainty associated with the individual parameters was considered.
Bootstrapping was used to construct data sets of the same trial population size by sampling at random with replacement over 1000 times. Cost-effectiveness acceptability curves (CEACs) were produced.
Budget impact analysis
A budget impact analysis to compare the costs to the NHS of FS versus FV was undertaken using the estimations of costs of the two interventions derived from the trial. The total budget impact for all patients within the trial population was calculated and examined the potential cost saving on a per-patient basis based on being treated with FS versus FV within the current intervention mix (based on the MDC). The impact to NHS budgets was estimated at 3 years. No discounting was applied to the budget impact analysis.
Model-based analysis
Deterministic sensitivity analyses were conducted to estimate the worst- and best-case scenarios of the budget impact of FS versus FV by using the lower and upper costs derived from the trial. The different scenarios that could impact on NHS budgets were (1) the cost of the intervention using a best-case/worst-case scenario; (2) the inclusion of the costs of the MDC; (3) the delivery of FV by a community dental nurse in a ‘classroom’ setting (i.e. not requiring a MDC but reflecting the potential for this technology to be delivered in a single application with minimal equipment/resources); and (4) the application of FV by a dental hygienist alone within a classroom/school medical room setting).
To assess the longer-term cost-effectiveness of FS versus FV in preventing dental caries in children, post-trial modelling was undertaken. As no suitable model could be identified from the literature search for adaptation, a de novo decision-analytic model was constructed. A UK NHS and Personal Social Services perspective was adopted in the analysis, in line with NICE methodological recommendations. 57 In the base case, costs and benefits were discounted at a rate of 3.5%.
Model structure
A Markov cohort simulation model, written in Microsoft Excel® 2013, version 15 (Microsoft Corporation, Redmond, WA, USA), was developed, with a 3-month time cycle employed in order to reflect an appropriate time point for caries progression to occur. Half-cycle corrections were applied. The time horizon of 5 and 10 years was chosen as sufficient to reflect potential changes in the change of progression of dental caries as a result of FS and FV in children up until the onset of adulthood. Although a lifetime horizon was considered, the lack of suitable evidence to make plausible assumptions about the longer-term effects of FS and FV resulted in an amendment to the original protocol plan. This was agreed with the trial team.
For FS and FV utilisation, Markov modelling allows for the construction of an arbitrary complex chain of events that represents the natural history of sealant retention, caries formation and their associated health states. 58 A sealed tooth may lose its seal and remain sound, may be resealed or may transition to caries. Caries may be restored. A restored tooth may develop further caries and this may finally lead to the tooth being lost. Varnished teeth represented the second intervention arm. A varnished tooth may develop caries, after which the pathway is the same as that for a tooth that develops caries after losing its seal. A lost/removed tooth was the absorbing state in the model.
A model structure was developed outlining the disease pathways and validated with the trial team before the model was built in Microsoft Excel. An outline of the model structure is given in Figure 6.
FIGURE 6.
Markov state diagram: outline of modelled intervention pathways for FS vs. FV. The states are represented by ovals and possible transitions between states are represented by arrows.

Key model assumptions
The model assumed that the population was the same as the trial population in terms of baseline characteristics at entry into the model. Children would be eligible to receive FS and FV as specified in the trial. After trial completion, children would no longer be in the Designed to Smile programme (i.e. they would have entered secondary school). It was assumed that all children would be entitled to NHS dental care until the age of 16 or 17 years, which would represent the ages of children up until the 10-year time horizon. 59
We assumed that children would continue to receive their original intervention (i.e. they would not receive both interventions) and that the same products would be applied in a similar way to that in the trial. The standard of application and the efficacy of the staff administering the intervention would be commensurate with the trial, as would the accuracy in diagnosing and appropriately treating the tooth. For the base case, we assumed that children would visit a NHS community dentist and receive the intervention in accordance with standard practice, including, when required, the reapplication of FV or resealing of FS.
The trial permitted the child’s tooth to be assessed to ensure that the FPMs were sufficiently erupted to be treated, as part of the early detection of caries risk provided by the MDC. The same dental recall periods were used within the first 5 years of the model, that is, periods of 6 months, as the child would still be in the Designed to Smile programme. Between 5 and 10 years, it would be assumed that the recall period would be based on an individual risk-based assessment for each child and thus it would be problematic to provide a precise estimate of the time interval. NICE guidance60 recommended that the shortest interval for oral health reviews for all patients should be 3 months, and, for patients < 18 years old, the longest interval should be 12 months. The health economic analysis undertaken for this guideline used Markov-based modelling to assess the cost-effectiveness of different dental recall periods, emphasising that, with considerable uncertainty around model parameters (particularly clinical efficacy), conclusions about the relative cost-effectiveness of different recall times could not be drawn.
On the basis of the trial outcomes, and as agreed with the trial team, it was assumed that all children attended a dental visit every 6 months. This was assumed to be the same for both trial arms.
Within this model, no assumptions were made regarding the accuracy of clinical assessments or the impact of different clinical reversal rates and it was assumed that a similar reversal rate could be achieved as that in the trial results on caries progression. The possible impact of dental health problems (e.g. caries progression in other teeth) or other oral health interventions on the subsequent health states of the FPMs was not considered.
Base-case inputs
As this was a model developed from the SoV trial, when possible and appropriate, the clinical evidence from the trial, alongside the costs and outcomes used in the within-trial health economic analysis, were used to provide data inputs. The FS was the base case, with incremental cost-effectiveness reported for FV compared with FS.
Although the trial provided estimates of the incidence of caries at baseline and the rate of caries progression as a result of the two technologies up until 36 months’ follow-up, it was problematic to estimate precise estimates of progression beyond the trial duration.
The strongest clinical evidence would be derived from the updated Cochrane review that evaluated the relative clinical effectiveness of FS versus FV (and FS + FV vs. FV alone) for preventing dental caries in the occlusal surfaces of permanent teeth of children and adolescents. 16 From this review, only one study provided longer-term outcome data,61 which was assessed to have both an unclear risk of bias because no information was provided at 48 months, and a high risk of bias as only 33% of trial participants were included at 9 years’ follow-up. This trial was also assessed as having a high risk of bias for four key domains (outcome assessment, incomplete outcome data, selective reporting and baseline comparability). The model was therefore constrained by using evidence from the trial, and discussions with the clinical trial team were used to make assumptions, when necessary. All data inputs underwent full validation by the trial team, and uncertainty was considered within the sensitivity analysis.
Clinical data
The trial data were used to inform the probability of the different progression into caries through the model.
Costs
It was assumed that modelled patients accrued costs associated with any assessment and intervention and that they continued to receive interventions to prevent/manage caries progression in the FPMs. The costs reflected a NHS and Personal and Social Services perspective, that is, drug costs, intervention costs and any other resource use that may be required (e.g. adverse events).
The same costing approach was used from the within-trial analysis of costs, valued in monetary (£) terms at 2015 prices from unit costs from published sources, for example BNF, Personal and Social Services Research Unit and NHS reference costs and, when applicable, local financial records from Cardiff and Vale UHB. Tables 48 and 49 reported the costs utilised in the model.
Health utilities
The following estimates of QoL were used (Table 52), using the trial data to estimate utility decrements.
| Value | |
|---|---|
| QoL weight for one cycle of sealed tooth | 0.002 |
| QoL weight for one cycle of lost seal | 0.002 |
| QoL weight for one cycle of varnished tooth | 1E–12 |
| QoL weight for one cycle with dental caries | 0.05 |
| QoL weight for one cycle of restored tooth affected by dental caries | 0.05 |
One-way sensitivity analysis
Table 51 presented the range of parameter estimates applied to the comparison of FS with FV in the one-way sensitivity analysis of the CUA, and these were applied to the model.
Probabilistic sensitivity analysis
A probabilistic sensitivity analysis was performed to test the robustness of the modelling conclusions in the face of uncertainty surrounding the choice of modelling inputs. Parameter values were varied within a reasonable range in each of 1000 runs and the results averaged across runs. Costs were sampled from gamma distributions, utilities from beta distributions, and rates and probabilities from log-normal or beta distributions. A CEAC was generated to depict the probability of the intervention being cost-effective at different willingness-to-pay (WTP) thresholds.
Results: within-trial analysis
Intervention costs
The interventions costs for FS versus FV are shown in Table 53. The overall intervention costs were aggregated across all the treatment and clinical assessment time points, as per the trial protocol. For the base case, the overall mean costs were lower for FV than for FS (£32,146.50 vs. £38,098.71, respectively). These were mainly attributed to the difference in time receiving treatment (in chair) between the two treatments. The overall time in chair across the trial period was 24 minutes for FS and 16 minutes for FV; even taking into account the reapplication of FV over the trial period, this cost of treatment remained lower (£74.12 per FS patient vs. £64.16 per FV patient). When the costs of the MDC were added, this increased the mean per-patient cost in each arm, but the incremental difference was small (£95.73 vs. £86.33 for FS vs. FV, respectively).
| Basis of estimate | Unit cost (£) | Per hour (£) | Per full treatment (FS = 24 minutes), (£) | Per full treatment (FV = 16 minutes), (£) | Data sources |
|---|---|---|---|---|---|
| Dentistry staff band 3 × 1 | 9.20 | 3.68 | 2.45 | NHS Staff Agenda for Change43 | |
| Dentistry staff band 4 × 1 | 10.58 | 4.23 | 2.82 | NHS Staff Agenda for Change43 | |
| Dentistry staff band 5 × 0.65 | 12.62 | 5.05 | 3.37 | NHS Staff Agenda for Change43 | |
| Administration staff (band 3) × 1 | 9.20 | 3.68 | 2.45 | NHS Staff Agenda for Change43 | |
| Administration staff (band 4) × 1 | 10.58 | 4.23 | 2.82 | NHS Staff Agenda for Change43 | |
| FV (Duraphat) | 2.00 | 12.00 | Dental suppliera | ||
| FS (Delton) | 2.50 | 15.00 | Dental suppliera | ||
| Clinical disposables (e.g. gloves, cotton wool, bib, etc.) | 2.50 | 22.50 | 22.50 | Dental suppliera | |
| Sterilisation costs | 1.75 | 15.75 | 15.75 | Cardiff and Vale UHBb | |
| Intervention costs total | 38,098.71 | 32,146.50 | – | ||
| Number of eligible pupils | 514 | 501 | – | ||
| Cost per eligible pupil | 74.12 | 64.16 | – |
Dental visits
The DHQ was used to estimate the resource use over the 3 years based on all available data for the two interventions. Children in the FV arm had, on average, fewer visits to the dentist (outside the MDC) than children in the FS arm (3.5 vs. 3.9, respectively). However, there were small differences in the reasons documented for these visits, with more children in the FS arm than in the FV arm attending for ‘check-ups’ (3 vs. 2.7, respectively), which equates to a difference of £28 per patient across the arms. The estimated mean cost per patient associated with additional dental visits in the FS was £426, compared with £368 in the FV (£58.17, 95% CI –£4.33 to £120.67). The results are shown in Table 54.
| Resource | Intervention arm | Difference | 95% CI of the difference | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FS | FV | ||||||||||||
| Mean | n | SD | Sum | Mean | n | SD | Sum | Mean | SD | Sum | |||
| Number of appointments per patient | 3.9 | 514 | 3.3 | 2024 | 3.5 | 501 | 3.3 | 1772 | –0.4 | –0.1 | –252 | 0.40 (–0.01 to 0.81) | 0.053 |
| Cost of appointments per patient | £425 | 514 | £523 | £218,680 | £367 | 501 | £490 | £183,920 | –£58 | –£32 | –£34,760 | 58.34 (–4.14 to 120.82) | 0.067 |
| Number of painkillers administered per patient | 0.07 | 514 | 0.52 | 37 | 0.13 | 501 | 0.64 | 67.00 | 0.06 | 0.12 | 30.00 | –0.06 (–0.13 to 0.01) | 0.090 |
| Cost of painkillers administered per patient | £0.01 | 514 | £0.10 | £7 | £0.03 | 501 | £0.12 | £13 | £0.01 | £0.02 | £6 | –0.01 (–0.03 to 0.00) | 0.090 |
| Number of antibiotics administered per patient | 0.11 | 514 | 0.50 | 54 | 0.17 | 501 | 0.71 | 84 | 0.06 | 0.20 | 30 | –0.06 (–0.14 to 0.01) | 0.104 |
| Cost of antibiotics administered per patient | £0.26 | 514 | £1.26 | £135 | £0.42 | 501 | £1.77 | £210 | £0.16 | £0.51 | £75 | –0.16 (–0.35 to 0.03) | 0.104 |
| Combined prescription costs per patient | £0.28 | 514 | £1.28 | £142 | £0.44 | 501 | £1.85 | £223 | £0.17 | £0.57 | £81 | –0.17 (–0.36 to 0.03) | 0.091 |
| Total cost to the NHS | £426 | 514 | £523 | £218,822 | £368 | 501 | £491 | £184,143 | –£58.17 | –£32.34 | –£34,679 | 58.17 (–4.33 to 120.67) | 0.068 |
| Cost of time off work to parent/carer | £29 | 514 | £27 | £14,799 | £25 | 501 | £26 | £12,508 | –£4 | –£1 | –£2291 | 3.83 (0.55 to 7.10) | 0.022 |
| Total cost to parent/carer | £29 | 514 | £27 | £14,799 | £25 | 501 | £26 | £12,508 | –£4 | –£1 | –£2291 | 3.83 (0.55 to 7.10) | 0.022 |
| Total combined NHS and parent/carer costs attributable to dental appointments alongside intervention | £455 | 514 | £542 | £233,621 | £393 | 501 | £508 | £196,650 | –£62 | –£33.27 | –£36,970 | 62.00 (–2.75 to 126.75) | 0.061 |
| Intervention cost per patient | £74.12 | 514 | – | £38,098.71 | £64.16 | 501 | – | £32,146.50 | –£9.96 | – | – | – | – |
| Total cost to the NHS and intervention costs | £500 | 514 | £523 | £256,920 | £432 | 501 | £491 | £216,287 | –£68 | –£32.34 | –£40,633 | –68.13 (5.63 to 130.63) | 0.033 |
| Overall costs (including intervention costs) | £529 | 514 | £542 | £271,718 | £457 | 501 | £508 | £228,794 | –£71.96 | –£33.27 | –£42,924 | –71.96 (7.21 to 136.71) | 0.029 |
An important cost driver to assess differences between the two technologies would be the treatment required as a result of caries within the FPMs, for example the cost of filling or removing the index tooth. One of the limitations of the DHQ is that it would not be precise enough to estimate direct costs attributed to the FPMs. Thus, the clinical trial data were examined to give a more accurate estimation of the costs of treating caries, assigning a cost to each event based on the unit cost of treatment. These were used as the primary source of data for these key cost drivers, with the DHQ providing information of other dental health-related events.
With regard to hospital visits (e.g. hospital-based dental outpatients) and hospital admissions associated with dental health problems, there were no recorded events across both treatment groups. No other health-care attendances were recorded (e.g. accident and emergency department attendance or out-of-hours service).
Prescription costs
Parents were asked to estimate the prescriptions given by a dentist or other health-care professional given to treat dental health problems during the trial. On examining the data, there were some anomalies noted, insofar as a prescription was recorded but not the prescribing health-care professional (e.g. dentist or general practitioner). This was discussed by the trial team and it was assumed that a community dentist would have prescribed. Painkillers and antibiotics were the only medications prescribed. It was assumed this was done as part of a dental visit already counted.
The estimated mean per-patient cost for medications prescribed in the FS arm was £0.28, compared with £0.44 in the FV arm (–£0.17, 95% CI –£0.36 to £0.03). This was seen as a non-statistically significant difference (see Table 53; p = 0.091).
The base-case analysis shows that the children randomised to the FS arm accumulated more costs, overall, than children in the FV arm. Table 54 also shows the comparison of total mean health-care costs between the two technologies. These results were used in the subsequent cost-effectiveness analyses and budget impact analysis.
Costs to the family
The costs to the family were estimated from the DHQ. With regard to over-the-counter medications, the total costs were £12.54 for FS versus £13.49 for FV. In terms of carer time as a result of taking time off to attend dental health appointment with the participating child or time to look after the child, the mean per-carer cost was estimated to be £29 versus £25 for FS versus FV, respectively. Table 54 shows the costs to the family regarding the costs resulting from time off.
Cost of hosting mobile dental clinic to the school
The schools questionnaire was returned by 30% of the participating schools. In their responses, all schools stated that there were negligible costs associated with the school’s participation related to the MDC, with little disruption to classroom time for the teacher or administrative tasks. Thus, the costs to the school of hosting the service were not formally analysed.
Overall NHS costs
Table 54 shows the health-care contacts across the trial arms from the NHS perspective. Non-statistically significant differences were seen in the contacts, that is, £425 versus £367 (FS vs. FV, respectively), with a difference of £58.17 (95% CI –£4.33 to £120.67; p = 0.068).
Total combined NHS and family costs
Table 54 shows the health-care contacts across the trial arms from the perspective of the NHS and a partial societal (family) perspective. Statistically significant differences were seen in the ‘total cost of time off work’ contacts, that is, £28.79 versus £24.97 (FS vs. FV, respectively), a difference of £3.83 (95% CI £0.55 to £7.10; p = 0.022).
The costs of the two technologies showed small but statistically significant differences; the mean cost to the NHS (including intervention costs) per child was £500 for FS, compared with £432 for FV, a difference of £68.13 (95% CI £5.63 to £130.63; p = 0.033) in favour of FV.
When costs to the family were included (with NHS costs), the costs were £529 for FS and £457 for FV, with a difference of £71.96 (95% CI £7.21 to £136.71; p = 0.029) in favour of FV.
Health outcomes
Dentine caries avoided at 36 months
The data presented in Table 13 were used to estimate the cost of each 1% reduction in the proportion of children who at 36 months had developed dental cares.
Health utilities and quality-adjusted life-years
The mean utility values derived from the CHU-9D at each of the follow-up points were similar (p-values greater than 0.05) for both technologies (Table 55). In the FS arm, the mean utility value (and resultant QALY) was 0.928 at 12 months and this increased to 0.933 at 36 months. In the FV arm, the mean utility value (and resultant QALY) was 0.931 at 12 months and this increased to 0.933 at 36 months.
| Treatment allocation | n | Mean (SD) | 95% CI of the difference | p-value |
|---|---|---|---|---|
| CHU-9D 12 months | ||||
| FS | 262 | 0.928 (0.072) | –0.003 (–0.015 to 0.009) | 0.624 |
| FV | 248 | 0.931 (0.061) | ||
| CHU-9D 24 months | ||||
| FS | 272 | 0.926 (0.074) | –0.002 (–0.015 to 0.010) | 0.720 |
| FV | 252 | 0.928 (0.069) | ||
| CHU-9D 36 months | ||||
| FS | 236 | 0.933 (0.066) | 0.000 (–0.012 to 0.013) | 0.956 |
| FV | 219 | 0.933 (0.066) | ||
The effects of these very small changes in the CHU-9D (both within and across the two arms) are reflected in the negligible utilities lost/gained and subsequent QALYs from 12 to 36 months. Overall, there was a small and non-statistically significant difference in QALYs at 36 months in the FV arm (0.931) compared with the FS arm (0.929) (difference 0.002, 95% CI –0.010 to 0.005). However, the very small difference and the 95% CIs indicate that these data should be treated with some caution (Table 56). In effect, there is a non-statistically significant difference and negligible clinically significant difference in QALYs, which was a similar result to the clinical outcomes reported in the trial.
| Time period | Intervention arm | QALY difference | |||
|---|---|---|---|---|---|
| FS | FV | ||||
| CHU-9D score | Change over time | CHU-9D score | Change over time | ||
| 12 months | 0.928 | – | 0.931 | – | – |
| 24 months | 0.926 | –0.001 | 0.928 | –0.001 | – |
| 36 months | 0.933 | 0.004 | 0.933 | 0.002 | – |
| QALY gain at 3 years | – | 0.003 | – | 0.001 | 0.002 (–0.010 to 0.005) |
Tooth utilities and quality-adjusted tooth-years
Table 57 reports the tooth utilities associated with the two technologies and QATY differences. The mean tooth utility at 36 months was 0.932 in the FS arm and 0.925 in the FV arm. The overall QATY difference was 0.007 in favour of the FS arm, a small and non-significant numerical difference. The clinical significance of this result is negligible and thus should be regarded with caution.
| Intervention | QATY difference | |||
|---|---|---|---|---|
| FS | FV | |||
| Tooth | Tooth utility | Tooth | Tooth utility | |
| UR6 | 0.925 | UR6 | 0.937 | – |
| UL6 | 0.927 | UL6 | 0.938 | – |
| LR6 | 0.933 | LR6 | 0.906 | – |
| LL6 | 0.944 | LL6 | 0.920 | – |
| Overall FPM utility | 0.932 | Overall FPM utility | 0.925 | 0.007 (–0.068 to 0.011) |
Cost-effectiveness analysis
Incremental cost per child with dentine caries avoided
The base case of the analysis for the incremental cost per child with dentine caries avoided is presented in Table 58.
| Parameter | Incremental cost of intervention (£) | Proportion of children without dentine caries | ICER (using % dentine caries free scores) |
|---|---|---|---|
| Base case | –68.13 (–5.63 to –130.63) | 0.021 (–0.0317 to 0.0738) | FV is dominanta |
| Upper 95% bound of net cost | –130.63 | 0.0738 | FV is dominanta |
| Upper 95% bound caries free (%) | |||
| Upper 95% bound of net cost | –130.63 | –0.0317 | FV is dominanta |
| Lower 5% bound of caries free (%) | |||
| Lower 5% bound of net cost | –5.63 | –0.0317 | FV is dominanta |
| Lower 5% bound of caries free (%) | |||
| Lower 5% bound of net cost | –5.63 | 0.0738 | FV is dominanta |
| Upper 95% bound caries free (%) |
With very small numerical differences in dental caries between the two trial arms, the ICER computed that FV was dominant over FS. However, appropriate caution should be exercised in using and interpreting the term ‘dominant’ in the context of these results.
Sensitivity analysis
As shown in Table 58, the results remained consistent when adjusted around the upper and lower range (95% CI) for costs and outcome. The CEAC for the incremental cost per child with dentine caries avoided is shown in Figure 7. Although there is no formal threshold, this illustrates the probability of FV being cost-effective at different monetary WTP thresholds.
FIGURE 7.
The CEAC for incremental cost per child with dentine caries avoided.

Incremental cost per quality-adjusted life-year
Table 59 shows the incremental cost per QALY.
| Parameter | Incremental cost of intervention (£) | Incremental CHU-9D utility | ICER (using CHU-9D utility scores) |
|---|---|---|---|
| Base case | –68.13 (–5.63 to –130.63) | 0.002 (–0.010 to 0.005) | FV is dominanta |
| Upper 95% bound of net cost | –130.63 | 0.005 | FV is dominanta |
| Upper 95% bound of net utility | |||
| Upper 95% bound of net cost | –130.63 | –0.010 | FV is dominanta |
| Lower 5% bound of net utility | |||
| Lower 5% bound of net cost | –5.63 | –0.010 | FV is dominanta |
| Lower 5% bound of net utility | |||
| Lower 5% bound of net cost | –5.63 | 0.005 | FV is dominanta |
| Upper 5% bound of net utility |
Figure 8 presents the CEAC.
FIGURE 8.
The CEAC (CUA).
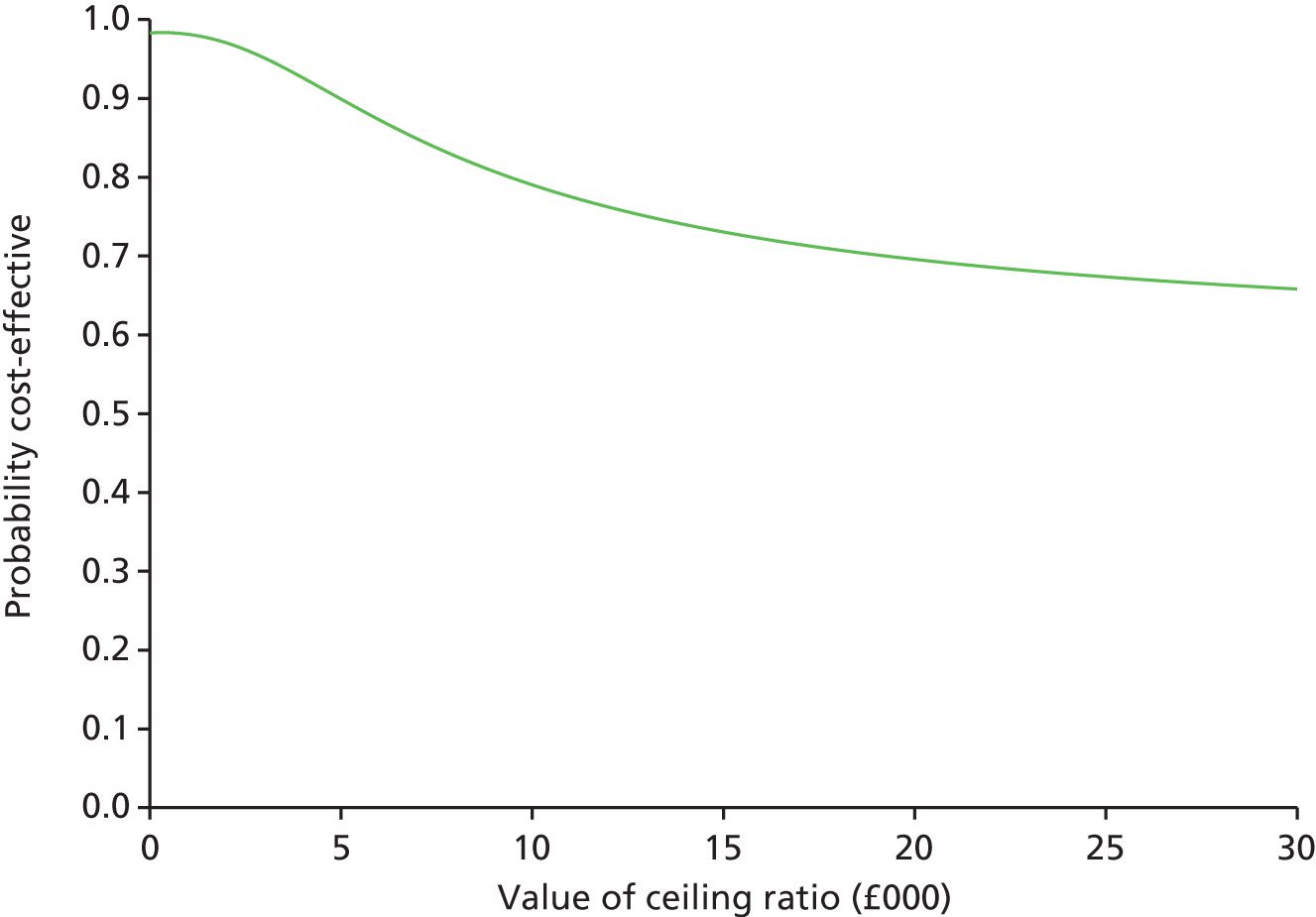
For the cost per QALY, the probability of FV being cost-effective is 70% at a NICE societal WTP threshold of £20,000 per additional QALY.
Incremental cost per quality-adjusted tooth-year
The base case of the analysis of the incremental cost per QATY is presented in Table 60. It can be seen that, in comparison with FV, FS generated slightly more QATYs. However, when the ICER calculation is performed, FV was dominant.
| Parameter | Incremental cost of intervention (£) | Incremental tooth utility | ICER (using tooth utility scores) |
|---|---|---|---|
| Base case | –68.13 (–5.63 to –130.63) | 0.007 (–0.068 to 0.011) | FV is dominanta |
| Upper 95% bound of net cost | –130.63 | 0.011 | FV is dominanta |
| Upper 95% bound of net utility | |||
| Upper 95% bound of net cost | –130.63 | –0.068 | FV is dominanta |
| Lower 5% bound of net utility | |||
| Lower 5% bound of net cost | –5.63 | –0.068 | FV is dominanta |
| Lower 5% bound of net utility | |||
| Lower 5% bound of net cost | –5.63 | 0.011 | FV is dominanta |
| Upper 5% bound of net utility |
This is a result consistent with the previous ICERs, and the same caution is applied in the interpretation of these results. This remained consistent when a one-way sensitivity analysis was performed. As this was an exploratory analysis, no CEAC is presented.
Budget impact analysis
The budget impact analysis (Table 61) was calculated on the trial population patients at 3 years (n = 1015). The total cost of FS treatment was £38,098 and the total cost of FV treatment was calculated at £32,144 (or £74.12 per patient in the FS arm and £64.16 per patient in the FV arm). The NHS costs associated with dental appointments (other than those administered in the intervention) totalled £218,822 for the FS arm and £184,143 for the FV arm. When the costs of implementation and the resource usage costs are combined and compared between arms, there is a –£35,021 cost-saving difference in favour of FV over the trial period. The results show that there is an overall per-patient cost saving of £68.13 (95% CI £5.63 to £130.63; p = 0.033) in favour of the FV arm compared with the FS arm.
| Baseline | Intervention arm | |
|---|---|---|
| FS | FV | |
| Cost of implementation (£) | 38,098 | 32,144 |
| Number of patients | 514 | 501 |
| Cost of implementation per patient (£) | 74.12 | 64.16 |
| Total cost of dental appointments (£) | 218,822 | 184,143 |
| Mean cost of dental appointments per patient (SD) | 425.7 (523.0) | 367.5 (490.6) |
| Difference (95% CI) | –58.17 (–4.33 to 120.67) | |
| Overall cost differences between the interventions (£) | –35,021 | |
| Cost saving per patient (£) | –68.13 | |
Sensitivity analysis of the budget impact analysis
Deterministic sensitivity analyses were conducted to estimate the worst-case and best-case scenarios of the budget impact of FS versus FV by using the lower and upper costs derived from the trial. To examine different scenarios that could impact on NHS budgets, three additional scenarios were explored: (1) the budget impact when the costs of the MDC are included, (2) the budget impact of a community dental nurse delivering FV in a ‘classroom’ setting (i.e. not requiring a MDC but reflecting potential for this technology to be delivered in a single application with minimal equipment/resources), and (3) the budget impact when a dental hygienist alone applies FV within a classroom/school medical room setting.
Table 62 shows the different cost scenarios for the intervention over a 3-year period as seen in the trial. It can be seen that the deterministic sensitivity analysis is unchanged in most of the modelled scenarios, that is, FV is dominant and is seen to be the optimal strategy. However, when the best-case cost scenario of £51.88 for FS is compared with the worst-case cost scenario of £83.41 for FV, FS technology dominates. That FS could cost as little as this in a real-life setting is questionable, and so caution should be exercised with regard to this. Further work would be required to explore the plausibility of this scenario.
| Parameter | Intervention arm | Budget impact | |
|---|---|---|---|
| FS | FV | ||
| Base case (lower ranges, upper ranges) | 74.12 (51.88, 96.36) | 64.16 (44.91, 83.41) | FV is dominant |
| Upper range FS | 96.36 | 83.41 | FV is dominant |
| Upper range FV | |||
| Upper range FS | 96.36 | 44.91 | FV is dominant |
| Lower range FV | |||
| Lower range FS | 51.88 | 44.91 | FV is dominant |
| Lower range FV | |||
| Lower range FS | 51.88 | 83.41 | FS is dominant |
| Upper range FV | |||
Table 63 shows the different cost scenarios for the intervention to be administered if the costs of the MDC are included. It can be seen that the deterministic sensitivity analysis is unchanged in most of the modelled scenarios, that is, FV is dominant and is seen to be the optimal strategy. However, when the best-case cost scenario of £67.01 for FS is compared with the worst-case cost scenario of £112.23 for FV, FS dominates. The plausibility of this scenario reflecting a real-life cost needs further discussion.
| Parameter | Intervention arm | Budget impact | |
|---|---|---|---|
| FS | FV | ||
| Base case (lower ranges, upper ranges) | 95.73 (67.01, 124.45) | 86.33 (60.43, 112.23) | FV is dominant |
| Upper range FS | 124.45 | 112.23 | FV is dominant |
| Upper range FV | |||
| Upper range FS | 124.45 | 60.43 | FV is dominant |
| Lower range FV | |||
| Lower range FS | 67.01 | 60.43 | FV is dominant |
| Lower range FV | |||
| Lower range FS | 67.01 | 112.23 | FS is dominant |
| Upper range FV | |||
Table 64 represents the analysis of one FV application by a dental nurse in the school. It can be seen that, by reducing the costs of the FV technology, it becomes dominant and is seen to be the optimal strategy across all the scenarios. The plausibility of this scenario reflecting a real-life cost would need further investigation.
| Parameter | Intervention arm | Budget impact | |
|---|---|---|---|
| FS | FV | ||
| Base case (lower ranges, upper ranges) | 74.12 (51.88, 96.36) | 11.48 (8.04, 14.92) | FV is dominant |
| Upper range FS | 96.36 | 14.92 | FV is dominant |
| Upper range FV | |||
| Upper range FS | 96.36 | 8.04 | FV is dominant |
| Lower range FV | |||
| Lower range FS | 51.88 | 8.04 | FV is dominant |
| Lower range FV | |||
| Lower range FS | 51.88 | 14.92 | FV is dominant |
| Upper range FV | |||
Finally, Table 65 represents the analysis of one FV application by a dental hygienist in the school. Again, it can be seen that, by reducing the costs of the FV technology, it becomes dominant and is seen to be the optimal strategy across all of the scenarios. Again, the plausibility of this scenario reflecting a real-life cost would need further investigation.
| Parameter | Intervention arm | Budget impact | |
|---|---|---|---|
| FS | FV | ||
| Base case (lower ranges, upper ranges) | 74.12 (51.88, 96.36) | 10.91 (7.64, 14.18) | FV is dominant |
| Upper range FS | 96.36 | 14.18 | FV is dominant |
| Upper range FV | |||
| Upper range FS | 96.36 | 7.64 | FV is dominant |
| Lower range FV | |||
| Lower range FS | 51.88 | 7.64 | FV is dominant |
| Lower range FV | |||
| Lower range FS | 51.88 | 14.18 | FV is dominant |
| Upper range FV | |||
Additional scenario analysis
To provide further illustration of the practical implications of the findings, an additional scenario analysis was undertaken to provide a hypothetical illustration of the time savings that could be achieved in delivering FS versus FV. As shown in the results, one of the resources attributed to costs is the ‘time in chair’ and another is the skill mix of staff that would be needed to deliver the technologies and how these findings can be used to optimise capacity and the number of children treated within the service. The ideal service configuration of preventative dental health for a population of children at high risk of caries is informed by translating the time savings gained from using FV with a dental care team that, compared with FS, needs to be less highly trained and does not include a qualified dentist. Table 66 presents an illustrative scenario (using time to treat each child gathered from the trial) in which, compared with FS, using FV allows 93 more children to be treated within the same time (assuming a day’s session, based on the school working day of 9 a.m. to 3 p.m., at a school that has this number of children).
| Intervention arm | Difference | ||
|---|---|---|---|
| FS | FV | ||
| Average time of one application of FV (minutes) | – | 2.7 | – |
| Average time per patient for one-off treatment of FS (with no top-ups) | 8.9 | – | – |
| Number of minutes in a session (9 a.m. to 3 p.m.) | 360 | 360 | – |
| Theoretical number of patients to be seen per session | 40 | 133 | 93 |
Post-trial modelling
Table 67 reports the estimations of the incremental cost per QALY at 5 and 10 years. These base-case results estimate that FV continues to be dominant over FS because of lower costs and slightly more QALY gains.
| Cost (£) | Incremental cost (£) | QALY | Incremental QALY | ICER | |||
|---|---|---|---|---|---|---|---|
| FS | FV | FS | FV | ||||
| 5 years | 1055 | 663 | 391 | 0.03 | 0.15 | –0.12 | FV is dominant |
| 10 years | 1580 | 822 | 0.05 | 0.31 | –0.26 | FV is dominant | |
The results of the univariate sensitivity analysis estimated that FV remained dominant.
Figures 9 and 10 show the CEACs generated for 5 and 10 years, respectively. They show that, at a NICE societal WTP threshold of < £20,000 per QALY gained, FV is 99% (at 5 years) and 96% (at 10 years) likely to be cost-effective.
FIGURE 9.
The CEACs for a 5-year time horizon.

FIGURE 10.
The CEACs for a 10-year time horizon.
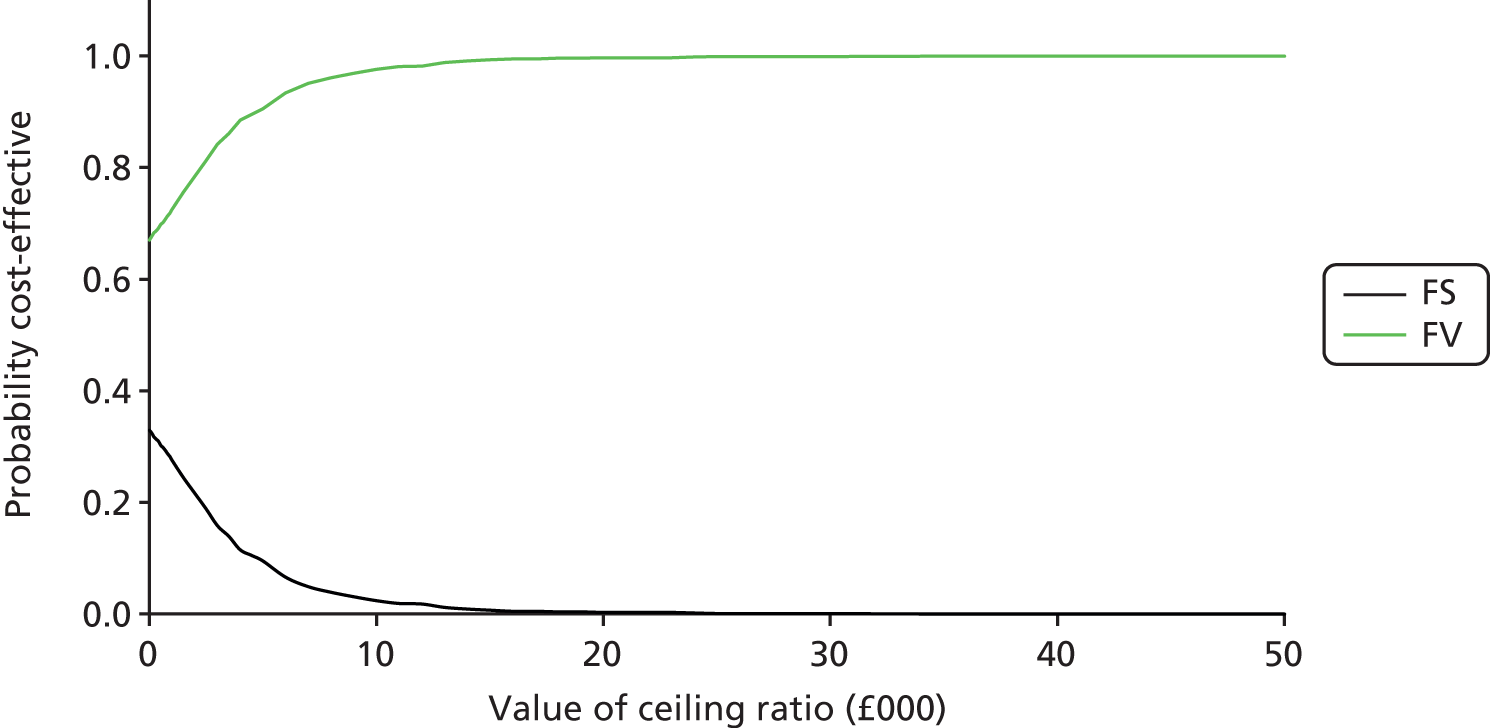
Summary of health economic results
This chapter has reported the health economic methods and findings of the trial. The main findings are as follows.
-
It is feasible to undertake a comprehensive health economic evaluation alongside a randomised controlled trial of preventative health technologies within in the context of a MDC setting.
-
The intervention costs of the two technologies were £74.12 (FS) and £64.16 (FV) per child over the course of the trial.
-
The costs of the two technologies showed small but statistically significant differences between arms; the mean cost to the NHS (including intervention costs) per child was £500 for FS compared with £432 for FV, a difference of £68.13 (95% CI £5.63 to £130.63; p = 0.033) in favour of FV.
-
When a partial societal perspective was included (with intervention costs), the mean cost per child was £529 for FS and £457 for FV, a difference of £71.96 (95% CI £7.21 to £136.71; p = 0.029) in favour of FV.
-
The budget impact analysis at 3 years showed that FV had a cost saving of £68.13 (95% CI £5.63 to £130.63; p = 0.033) compared with FS. In addition, when three different scenarios were examined, the results did not change and FV remained dominant.
-
As numerical differences in outcomes were non-significant, the ICER was very sensitive to very small numerical differences in outcomes. For all outcomes, differences between FS and FV were neither statistically significant nor clinically important, and so the ICER calculation should be treated with appropriate caution. However, results remained consistent across all three outcomes used in the health economic analysis, that is, FV was less costly than FS, with similar outcomes achieved.
-
The CEAC showed that there was a 70% probability of FV being cost-effective at a societal WTP threshold of £20,000 per QALY.
-
The results of model-based analysis to estimate the incremental cost per QALY over longer-term horizons were consistent with the results of the in-trial analysis, with FV being associated with lower costs and small QALY gains achieved, leading to FV being dominant in the ICER calculation. The CEACs showed that there was a 99% (over 5 years) and 96% (over 10 years) probability of FV being cost-effective within a societal WTP threshold of £20,000 per QALY. However, there are a number of uncertainties in the model, particularly the dearth of evidence on longer-term costs and outcomes associated with FV and FS within a preventative dental health context.
Chapter 5 describes the process evaluation.
Chapter 5 Acceptability of the Seal or Varnish? intervention and trial
Introduction
An important element of the clinical trial was determining the acceptability of the interventions provided, and the setting in which they were being provided, from the perspective of the participating children, their parents, schools/school staff and clinical staff. In addition, parents’ decisions and perceptions of their child participating or not participating in the trial were investigated. A mixed-methods approach was used.
Treatment acceptability was assessed in three ways:
-
Acceptability scales were completed by clinical staff.
-
Acceptability scales also were completed by the children participating in the trial.
-
Qualitative interviews were conducted with a subsample of children.
A longitudinal process evaluation for the trial was conducted to assess how the FS/FV intervention was implemented for children in a school-based setting in a socioeconomically deprived area and to facilitate the interpretation of outcome effects to establish what works for whom and in what circumstances. The process evaluation focused on implementation and acceptability, as these were factors likely to influence the effectiveness of the trial, in addition to treatment efficacy. The research questions addressed and the mode of data collection are shown in Table 68.
| Research question | Data collection method | Year of study in which data were collected | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Acceptability: are FV and FS treatments acceptable to children aged 6–10 years, what factors influence acceptability and are there differences between trial arms? Includes: Did the children display signs of distress during the treatment? Did the children feel happy or sad after the treatment was completed? |
Hygienist/nurse observation | ✗ | ✗ | ✗ |
| DTF scale | ✗ | ✗ | ✗ | |
| Child interviews | ✗ | ✗ | ||
| Parent interviews | ✗ | ✗ | ||
| Clinician interviews | ✗ | ✗ | ✗ | |
| Implementation: is the delivery of this type of preventative treatment acceptable to children, their parents, clinicians and the schools where the treatments take place; what are the factors affecting implementation of treatment in a school setting? | Child interviews | ✗ | ✗ | |
| Parent interviews | ✗ | ✗ | ||
| Clinician interviews | ✗ | ✗ | ✗ | |
| School staff questionnaire | ✗ | |||
| School staff interviews | ✗ | |||
| What factors affect whether parents consent or do not consent to take part in an intervention and trial such as SoV? | Parent interviews | ✗ | ✗ | |
| Parent questionnaire | ✗ | |||
| School staff questionnaire | ✗ | |||
| School staff interviews | ✗ | |||
| Does treatment with FV or FS affect (self-reported) subsequent oral health behaviour, and, if so, are there differences between trial arms? | Child interviews | ✗ | ✗ | |
| Parent interviews | ✗ | ✗ | ||
| What is the effect of the trial on recruitment or acceptability to parents, dental staff and schools? | Parent interviews | ✗ | ✗ | |
| Clinician interviews | ✗ | ✗ | ✗ | |
| School staff interviews | ✗ | |||
The methods used were, briefly, the completion of acceptability scales by treating clinicians and the participating children, interviews conducted with participating children and their parents, interviews with head teachers of schools participating in the study and interviews with CDS staff delivering treatment. Parents who were not participating in the trial were also interviewed. In addition, a school staff questionnaire was sent to schools, and a short questionnaire about reasons for non-participation was included in the second recruitment mailing to parents for completion by parents who did not want to take part in SoV.
The methodology used is described in detail in Methodology. Findings on the acceptability of the intervention to children, parents, staff and schools are then reported. Any changes in oral health behaviour were also noted, to assess whether or not there were differences between trial arms that might affect outcomes. Reasons for parents consenting or not consenting for the children to participate in SoV are then described.
Methodology
Data collection
Acceptability as determined by observed signs of distress during treatment
An observation scale (see Appendix 16) was completed independently by the dental hygienist and dental nurse for each child immediately following application of the FS or FV. This scale recorded five items of children’s behaviour: vomiting, crying, gagging, excessive arm/leg movements and other signs of distress. 29
Acceptability as reported by participants immediately following treatment
A DTF scale27,28 was used to assess the acceptability of the treatment for children, both for overall acceptability (how they felt during treatment and how they would feel if they had to have the treatment the following day) and for three specific aspects of treatment: taste, length of treatment and feeling sick (see Appendix 12). As there are few scales designed for acceptability of dental treatment, and as none was available for very young children, the DTF scale was selected, but it was adapted by graphic artists to enhance visual appeal, as the original scale was designed for slightly older children. The DTF scale was completed by children in the MDC immediately following the initial application of FS/FV and at each follow-up visit. Answers were given on a five-point Likert scale, on which children could mark a picture of a face, which ranged from smiling to sad, to indicate how they felt about different aspects of treatment. The protocol for completion of the scale was for the child to sit apart from other children, to avoid children discussing or influencing each other’s answers.
Interviews with children
School sampling
A subsample of schools participating in the trial was selected for acceptability interviews with children. All schools were in Communities First areas, which were characterised by deprivation; however, within this category there was a range of deprivation levels in schools. As the impact of preventative dental interventions in deprived areas was an area of interest for the study, the impact of level of deprivation was also of interest. 5,6 Schools were selected from the highest and lowest deprivation quartiles of schools in the SoV trial, measured by the percentage of pupils in a school receiving free school meals (FSMs). Data were used from the StatsWales website of the Welsh Assembly Government,62 and the measure used was the percentage of 5- to 15- year-olds eligible for FSMs in 2010. The overall range of children receiving FSMs in participating SoV schools was 3–75%. The high-deprivation quartile schools had an average FSM rate of 58%, while the low-deprivation quartile schools had an average FSM rate of 18%. In the UK, the proportion of children receiving FSMs averages 15% across all schools, while the Welsh average is 18%. The sampled ‘low-deprivation schools’ in the SoV trial were, therefore, similar to the Welsh average, while the high-deprivation schools within the SoV trial had much higher levels of children receiving FSMs, at 58%, more than three times the average level of FSMs in Wales.
In year 1 of the study, schools with more study participants (either larger schools or smaller schools with high study consent rates) in each selected quartile were sampled for interviews. Schools were selected in this way to allow for large enough groups of children in each school to be paired by trial arm and also by children’s choice of interview partner (see below). When numbers did not reach 24 for each trial arm, additional schools were added until at least 24 children had been interviewed in each trial arm. Because of problems contacting parents to gain consent for follow-up interviews in year 3, additional schools were sampled in year 3 to increase the sample size. The attempts to recruit for interview were stopped when around 30 children and parents had been interviewed. As respondents were providing similar answers and no new data were emerging from subsequent interviews, it was concluded that data saturation had been reached. Four schools were sampled in year 1 of the study; seven schools (including the four from year 1) were sampled for follow-up in year 3.
Child interview pairings
Children were interviewed in pairs, or occasionally singly or in threes, so that they would feel more comfortable in the interview environment. 63 The intention was also to facilitate the collection of more, and better-quality, data. Teachers and classroom assistants, and sometimes head teachers and school administrators, assisted with pairing children. Lists of children (identified by unique ID study number) participating in treatment for each school were divided by trial arm and, whenever possible, children from the same trial arm were interviewed together in order that children would be able to report on the same type of treatment in an interview. Within the trial arm groups, children were given the option to choose their interview partner (or teachers were asked to pair children with a friend). In some cases, the requirements of teachers or classroom activities also dictated which children would accompany each other, particularly when a year group was split over two or three classrooms. Originally, children were also to be purposively sampled from each trial arm for a range of acceptability scores, and children in each trial arm with similar acceptability scores were to be interviewed together, to ensure a maximum difference of acceptability across the sample. However, this proved impossible, as the sample sizes for each trial arm in each school were too small to divide the children into further groups; it would have disrupted classes too much to create a very rigid list of pairings of children, and it would have prevented children from being able to choose their interview partner.
Child interviews
Face-to-face paired interviews were conducted with children in a school setting ≤ 2 weeks after their first treatment and ≤ 2 weeks after their final treatment, to ensure that children’s recall of their experience of treatment was reasonably accurate. Most children were interviewed within a few days of their treatment. Interviews lasted for around 15–20 minutes each and most children appeared relaxed and confident in interviews. Written parental consent to interview children was included in the main study consent; in addition, a reminder telephone call was made to parents in years 1 and 3 of the study to confirm consent before the researcher visited schools. Interviews were undertaken by one of the coauthors (SMT) and took place in spare classrooms, head teacher offices, staff rooms, school libraries and school halls. All interviews took place where school staff or other children could not hear the content of an interview (although, to comply with safeguarding requirements, all spaces were also visible) and it was explained to the interviewed children that the data collected were confidential. Assent to be interviewed was also verbally checked with children at the beginning of the interview. The interview topics included acceptability of treatment and the treatment setting, social influences on attitudes towards treatment and treatment settings (such as prior experience of dental treatment), changes in the child’s oral hygiene behaviour and perceptions of treatment impact. See Appendix 17 for the children’s interview schedule. Fifty children were interviewed in year 1 of the study. Thirty-two follow-up interviews were conducted with children in year 3.
Methodological issues in child interviews
The children, especially in year 1 of the study when they were aged 6 or 7 years and at a relatively early developmental and educational stage, were limited in what they were able to report in interviews. Not all of the children were able to understand or to answer all of the questions posed in the interviews, and when the children were unable to answer a question, further probing questions were used in a very limited way to avoid causing anxiety. Several common limitations emerged across the group. First, some of the children’s concepts of time were distorted; for example, one child in the final year of the study (when the children were 8 or 9 years old and the study was in its third year) thought that he had been receiving the treatment for about 8 years and then corrected himself and said it was only about 5 or 6 years. Most children in the first year of the study struggled with describing behaviour before and after a past event (e.g. ‘have you changed how you brush your teeth since your last visit to the dental van?’). Their awareness of factors such as family and friends’ dental habits was also limited, so social influences on their attitudes towards dental treatment were not always clear. They were not always able to be clear about the FS/FV treatment visits to the MDC, other visits to a MDC (e.g. examination visits for the study were also held in the MDC) and visits to a community dentist in town, especially in year 1 of the study. For example, children occasionally described a dental examination visit or having their teeth removed rather than a FS/FV treatment visit. They were also sometimes unable to consolidate or clearly articulate their feelings about an aspect of treatment, giving contradictory answers in interviews; this is not uncommon in qualitative interviews, but it occurred to a greater degree in the child interviews. Some children may have contradicted themselves in order to agree with their interview partner. When contradictory comments were made, data are not reported, as the child’s preferences or views were not clear. Therefore, the number of unambiguous answers for each question or theme (such as whether or not children liked the taste of the treatment) reported here is lower than the number of children interviewed.
Interviews with participating parents
The interviews with parents were conducted by telephone 4–6 weeks after their child’s first and last treatments, in years 1 and 3 of the study. Parents were interviewed after their children had been interviewed.
The interview schedule was piloted with parents at a local community centre in South Wales in an area that was not part of the trial. The following comments were received.
-
The term ‘interview’ was perceived as intimidating. Parents preferred the terms ‘courtesy call’ or ‘chat’. However, because there was a need to be clear to study participants that data were going to be collected and analysed, the term ‘interview’ was retained, but simpler language was used when parents were contacted. For example, parents were told that the purpose of the interview was to find out what they thought about SoV.
-
Similarly, parents thought that ‘recorded’ sounded less ominous than ‘tape-recorded’, because ‘recorded’ sounded similar to the phrase ‘your call may be recorded for training purposes’, which people were used to hearing.
-
Parents also suggested clarifying that interviews would take place by telephone; they said that this would encourage more parents to consent, as it was perceived as easier to participate in a telephone interview than in a face-to-face interview.
-
Parents also made recommendations about recruiting other parents, suggesting that schools could be more involved in this. They suggested using posters with pictures to remind parents about the study and to increase trust because recruitment would be through the school. They also suggested enabling parents to collect and return forms directly to the school rather than by a postbox, especially to class teachers, as they are particularly trusted. Using plain English on posters and advertising, and clarifying that assistance from staff was available for parents with low literacy/English language skills, were also suggested as ways to increase accessibility.
Schools were not asked to assist further with recruitment as there were concerns about the research burden. Nevertheless, some schools did assist with recruiting. For example, one school inserted a reminder about study recruitment into the school newsletter, and one head teacher spoke to parents as a group about the trial, as he thought that the parents would not understand the paperwork.
Forty-nine parents were interviewed in year 1 of the study and 30 parents were interviewed in year 3. The aim of the interviews in year 3 was to follow up all interviewees from year 1; attempts were made to follow up as many children and parents from year 1 as possible, even if the child and parent could not be interviewed. The most common reason for not following up a child or parent was that the parent had changed their telephone number or did not answer the telephone despite several calls (the initial telephone call was partly to reconfirm consent, so if the parent was not contactable then their child was not interviewed). Another problem was the poor coverage of mobile phone reception in some areas of the South Wales valleys (many parents did not have landlines). Furthermore, telephone calls from the university were routed through the university switchboard; this resulted in the recipients of calls not being able to identify the caller number, which probably resulted in some parents not answering. A mobile phone was not a viable alternative because it could not be used in conjunction with a voice recorder for the interviews, and the reception/quality of the line tended to be poor. Three parents were followed up after the school was contacted for an up-to-date telephone number (permission to do this had been obtained in the study consent forms). Secondary reasons for not following up were that a few children were absent on the day of the interview at the school or had been withdrawn from the trial. As a result of the absent children and the various problems recontacting parents, follow-up in year 3 of the study with some parents and children was not possible. The intention was to interview the same pairs of parents and children in years 1 and 3; this was achieved for 17 child–parent pairs. To increase the overall number of interviews, additional schools were sampled and children and parents from these schools were also interviewed.
The interviews with parents were relatively short, lasting approximately 10–15 minutes, owing to parents’ brief answers and often busy schedules; on some occasions, during the interviews, young children could be heard in the background. The interview topics included the acceptability of the treatment and the treatment setting, social influences on attitudes towards treatment and treatment settings (such as prior experience of dental treatment), changes in the child’s oral hygiene behaviour, the perceptions of treatment impact and the acceptability and impact of the trial processes. See Appendix 18 for the parent interview schedule.
Questionnaire and interviews with school staff
In year 1, a questionnaire was sent by e-mail to all 65 schools participating in the study. A second e-mail was sent to non-responding schools and, if required, further contact was made by sending postal questionnaires to any schools that had still not responded. The questionnaire included both closed and open questions about schools’ experiences of the implementation of SoV (both the treatment programme and the trial) and acceptability of the intervention. Twenty-four questionnaires (37%) were returned.
In the third year of the trial, at the end of the school year, the 24 schools that had returned a questionnaire in year 1 were contacted by telephone to request their participation in an interview; those that agreed were sent consent forms to complete. Of the 24 schools contacted, five agreed to take part in an interview, and four interviews were actually conducted (one was cancelled because of sickness). Three interviewees were head teachers and one was a class teacher. The interviewees were asked about the impact of the trial and treatment delivery in the school, contextual factors that affected the feasibility and acceptability of delivering preventative treatment in a school setting, the acceptability of treatments and the recruitment of hard-to-reach parents for interventions. The interview schedule is in Appendix 19. The original intention had been to purposively sample schools based on their questionnaire responses; however, as only 24 questionnaires were returned in year 1, and as the sample of teachers or head teachers willing or able to spare time for an interview was likely to be only a fraction of this figure, all 24 schools were contacted so that the number of staff interviewed would not be too small.
Interviews with community dental unit staff
The dental team delivering the treatment was interviewed annually; this took place at the end of the school year. These interviews were conducted face to face at the CDS offices in Cardiff. Nine staff were interviewed in the first year and 12 staff were interviewed in both the second and the third years. This group included hygienists, dental nurses and the staff members who collected children from the classrooms. The staff makeup of the groups was largely the same each time, but there were occasional omissions or additions because of staff leaving or joining, or because a staff member was unavailable (e.g. through sickness) on the days of the interviews. The interviews explored the feasibility and acceptability of delivery in a school setting, perceptions of acceptability of the treatment for children, child co-operation and perceptions of parent recruitment issues. The interview schedule is in Appendix 20.
Questionnaire and interviews with non-participating parents
For the first and second cohorts, the interviews with parents who did not participate in the trial were conducted after the trial recruitment periods had ended; this was to avoid any confusion or perceptions of harassment about recruitment to the trial itself rather than to a research interview. Two recruitment methods were used; the intention was to recruit up to 20 parents by each method. In the first method, if parents had not responded to the initial SoV recruitment attempt, they were sent information about the trial a second time. This information included a consent form for a telephone interview to discuss their reasons for non-participation, and a one-page questionnaire about their reasons, for those parents who did not wish to participate in the SoV study but were willing to take part in research about non-participation. The parents were invited to return the questionnaire, the consent form or both if they did not wish to participate in the trial. Those who returned the consent form were followed up with a telephone interview.
The second recruitment method was speaking to parents who had not returned any paperwork for the SoV trial itself and had also not returned the questionnaire or consent form for the interview about non-participation. This was done to collect data from parents who may have been willing to take part in the research but were unlikely to respond via a written invitation. Direct contact, including face to face, is likely to be effective for recruitment for interviews for hard-to-reach groups that have difficulty with literacy or paperwork and has the added benefit that a researcher visiting schools in person can familiarise people with a study and reduce anxiety about particular elements. It was also expected that teacher and school staff support for an intervention would have a positive influence on recruitment rates. 64 Seven schools were visited to recruit this group of parents and these were sampled by identifying schools that had lower than average recruitment rates for SoV, although very small schools were excluded as there were too few potential interviewees. The initial plan was to recruit parents from a parents’ evening at a sample of schools, at which teachers and school staff would introduce the researcher to groups of parents and request participation in a research interview, but the research team was advised by several schools that it would be difficult because of how busy these events are and that parents would have limited time for interviews. Therefore, a second approach was devised.
In this second approach, a researcher waited with the year 2 class teacher by the exit door at the end of the school day, a time when a direct handover of the child to the parent or carer is required. The researcher and teacher/teaching assistant handed out information sheets directly to parents. This helped to associate the researcher with the school and with the class teacher, which it was hoped would build trust, and meant that the researcher was available for parents to ask any questions about the research. The researcher did not approach parents, except to give them the information sheet, to avoid parents feeling harassed to take part. This recruitment was conducted after the first child dental examination to establish trial eligibility had been carried out, so there was no possibility that this approach could be identified with trial recruitment, which had finished by this point. The information sheet made it clear that the trial recruitment had finished and that the current recruitment was for a research interview only, to find out why some parents decided not to take part in dental studies and programmes to inform future interventions. Information sheets were given only to parents who had not returned any paperwork to the trial. Parents were told that the researcher would be in the school (accessible via the school reception desk) one day during the following week, when parents could drop in to do an interview if they wanted. The interviews were set up on a ‘drop-in’ basis to make it easy and flexible for parents, rather than arranging an appointment time, and to further avoid parents feeling harassed to take part in the research. Year 2 class teachers were asked to remind parents about the interviews the day before. The information sheet also included the researcher’s telephone number in case parents had any queries about the interviews. This recruitment was carried out in years 1 and 2 of the study (for cohorts 1 and 2), and for cohort 2 an incentive of a £10 shopping voucher was offered for a completed interview.
Parents who withdrew their children from the trial were also sent a letter inviting them for a telephone interview about their reasons for deciding to no longer take part. The target for the study was to recruit up to 20 withdrawing parents and to compare data by trial arm.
The results of these recruitment efforts and the data collected on reasons for non-participation are discussed in detail in Findings.
Data analysis
Treatment acceptability as measured by observed signs of distress
A ‘signs of distress’ scale independently measured by both the dental nurse and dental hygienist was calculated as the mean of the valid item responses (scored 1 for yes and 0 for no; see Appendix 16). When three or more of the items were missing, the score was set as ‘missing’.
Treatment acceptability as determined using the Delighted–Terrible Faces scale
Child-rated treatment acceptability at each treatment visit used the DTF scale. Faces were scored 1–5 (happy to sad) and a mean score calculated over the five items. The distribution of this score precluded linear regression analysis and the results were categorised into a binary outcome as follows (scores 1–2.49 as ‘happy’ and scores 2.5–5 as ‘sad’). When three or more of the items were missing, the DTF score was set as missing.
The determination of acceptability between intervention arms of the trial is reported as unadjusted and adjusted ORs, calculated as described in Chapter 2.
Qualitative data
All interviews were digitally recorded and fully transcribed. Transcriptions were then uploaded to NVivo version 9 (QSR International, Warrington, UK) and analysis was conducted in three stages. First, data were analysed by means of framework analysis, using NVivo version 9 software. 65 Framework analysis is a method that organises responses by respondent and interview content, and allows analysis by case or theme so that they can be compared easily. 65 The research questions and interview schedule questions formed the initial categories for the framework; any additional categories for other themes that emerged from the data were added to the framework. For example, children tended to discuss not only the FS or FV treatment itself, but also the chair, protective glasses, air/water sprays and so on, and so these were recorded as separate factors affecting acceptability. Separate frameworks were produced for each trial arm, interviewee group (child, parent, CDS staff, school staff), time point (first, second and third year of trial) and school deprivation level (high/low). This was to facilitate reporting by each interviewee group and comparison between trial arms, deprivation level and across time. Most of the categories in each framework were similar (e.g. all interviewees were asked about treatment acceptability), but a few varied (e.g. only the CDS staff framework had a thematic category for child co-operation). Each category in each of the frameworks was summarised in a short list of bullet points in summary tables; this enabled a summary of each theme to be produced, but also contained sufficient detail about variance between responses to avoid bias in the reporting.
The qualitative data analysis took a postpositivist approach (compared with the constructivist paradigm that is often adopted in qualitative research). This was primarily because the research questions for the process evaluation were very specific to particular aspects of the treatment and setting, rather than being an exploratory or in-depth study of behaviours. Furthermore, the child and parent interviews were relatively short, and answers tended to be brief and to the point. The children were too young to provide very elaborate answers and parents were often very busy (young children or infants were frequently heard in the background of telephone interviews). The data collected therefore matched all interview schedule categories very closely and did not elaborate beyond brief facts or opinions. This made a framework analysis appropriate for analysing this type of data, rather than the multilevel interpretive coding that would be appropriate for more in-depth data. For the same reason, only one researcher (SMT) analysed the data. Reflexivity in terms of the interview schedule or interpretation of the data was also not conducted, as the interpretative nature of data analysis is not emphasised in postpositivist research. However, potential influences on the data were noted when appropriate (e.g. a probable social desirability bias in reporting oral hygiene and dietary behaviours, as noted in Impact of treatment and trial on oral health behaviour).
In the second stage of analysis, the framework summaries were compared by trial arm, deprivation level and time points (years 1 and 3 of the trial).
Findings
Overall acceptability of FS/FV treatment in a school setting was high for children, CDS staff, school staff and parents. Factors influencing acceptability were largely non-FS/non-FV aspects of treatment or aspects of the wider treatment or school setting. These are discussed separately in the following sections. First, the acceptability is described as it was observed during treatment and reported by the children immediately post treatment. Next, the acceptability of the actual FS/FV treatments for children is reported, before global acceptability for children is reported, which often depended on wider aspects of the dental treatment. The section after this describes the acceptability of the treatment setting to children, parents, CDS staff and schools. Finally, the impact of the SoV trial on oral hygiene behaviour and the reasons for participation and non-participation in SoV are discussed.
Acceptability of treatment
Signs of distress
Immediately after treatment in the MDC, the dental hygienist and the dental nurse recorded signs of adverse behaviour during FS or FV placement. Observations during the baseline examination are reported in Tables 69 and 70. The equivalent data at the 30-month treatment visit are shown in Tables 71 and 72.
| Behaviour | Intervention arm | Total (N = 932), n (%) | |
|---|---|---|---|
| FS (N = 468), n (%) | FV (N = 464), n (%) | ||
| Vomiting | 4 (0.9) | 3 (0.7) | 7 (0.8) |
| Gagging | 39 (8.4) | 16 (3.5) | 55 (6.0) |
| Crying | 16 (3.5) | 3 (0.7) | 19 (2.1) |
| Excessive arm movements | 22 (4.8) | 8 (1.7) | 30 (3.3) |
| Excessive leg movements | 2 (0.4) | 5 (1.1) | 7 (0.8) |
| Other signs of distress | 67 (14.6) | 20 (4.4) | 87 (9.5) |
| Behaviour | Intervention arm | Total (N = 931), n (%) | |
|---|---|---|---|
| FS (N = 467), n (%) | FV (N = 464), n (%) | ||
| Vomiting | 2 (0.4) | 1 (0.2) | 3 (0.3) |
| Gagging | 35 (7.6) | 13 (2.8) | 48 (5.2) |
| Crying | 17 (3.7) | 4 (0.9) | 21 (2.3) |
| Excessive arm movements | 24 (5.2) | 12 (2.6) | 36 (3.9) |
| Excessive leg movements | 12 (2.6) | 3 (0.7) | 15 (1.6) |
| Other signs of distress | 52 (11.4) | 20 (4.4) | 72 (7.9) |
| Behaviour | Intervention arm | Total, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Vomiting | 0 (0) | 0 (0) | 0 (0) |
| Gagging | 6 (1.4) | 7 (1.7) | 13 (1.5) |
| Crying | 3 (0.7) | 2 (0.5) | 5 (0.6) |
| Excessive arm movements | 0 (0) | 2 (0.5) | 2 (0.2) |
| Excessive leg movements | 0 (0) | 0 (0) | 0 (0) |
| Other signs of distress | 7 (1.7) | 12 (2.9) | 19 (2.3) |
| Behaviour | Intervention arm | Total, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Vomiting | 0 (0) | 1 (0.2) | 1 (0.1) |
| Gagging | 4 (1.0) | 10 (2.4) | 14 (1.7) |
| Crying | 3 (0.7) | 3 (0.7) | 6 (0.7) |
| Excessive arm movements | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Excessive leg movements | 0 (0) | 0 (0) | 0 (0) |
| Other signs of distress | 8 (1.9) | 14 (3.3) | 22 (2.6) |
Overall, the proportion of children displaying adverse behaviours or showing signs of distress during treatment at baseline, as observed by the dental hygienist, was small: 97 of the 468 children in the sealant treatment arm and 40 out of 464 children in the FV arm of the study. Children in the FS arm were more likely to gag and to cry. There was good agreement between the observations made by the dental hygienist and the accompanying dental nurse, although the nurses were more likely to observe and report excess movement of arms and legs during treatment.
To facilitate analysis, the adverse outcomes over the course of the trial as observed by the dental hygienist and the dental nurse were dichotomised (any adverse outcome vs. no adverse outcomes recorded) for analysis (Tables 73 and 74). The behaviours observed differed across the intervention arms of the study. At baseline, 21% of participants displayed some form of adverse behaviour, but this fell steadily at subsequent treatment visits up to the final treatment visit at 30 months, at which only 2.6% of the participants were observed to have some form of adverse behaviour during treatment. In contrast, the proportion of children showing signs of adverse behaviour during the application of FV remained more constant in the course of the trial, ranging from 8.7% at baseline to 3.8% and 4.1% at the 24- and 30-month visits, respectively. During the trial, increased familiarity with the treatment process and increased ability to cope as the children got older are probable influences on these findings.
| Time point | Intervention arm | Overall, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Dichotomised hygienist-rated adverse outcome scale (yes) | |||
| Baseline | 97 (21.0) | 40 (8.7) | 137 (14.9) |
| 6 months | 72 (15.4) | 35 (7.9) | 107 (11.7) |
| 12 months | 40 (8.7) | 24 (5.3) | 64 (7.0) |
| 18 months | 20 (4.4) | 23 (5.2) | 43 (4.8) |
| 24 months | 18 (4.1) | 16 (3.8) | 34 (3.9) |
| 30 months | 11 (2.6) | 17 (4.1) | 28 (3.3) |
| Time point | Intervention arm | Overall, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Dichotomised nurse-rated adverse outcome scale (yes) | |||
| Baseline | 92 (20.0) | 36 (7.8) | 128 (13.9) |
| 6 months | 56 (12.0) | 32 (7.2) | 88 (9.7) |
| 12 months | 23 (5.0) | 24 (5.3) | 47 (5.2) |
| 18 months | 16 (3.5) | 26 (5.9) | 42 (4.7) |
| 24 months | 15 (3.4) | 15 (3.6) | 30 (3.5) |
| 30 months | 14 (3.3) | 25 (5.9) | 39 (4.6) |
The difference between trial arms at baseline as observed by the dental hygienists at the baseline examination was subject to two-level and single-level logistic regression (Tables 75 and 76).
| Adverse outcome | Intervention arm | Overall, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| No | 365 (79.0) | 420 (91.3) | 785 (85.1) |
| Yes | 97 (21.0) | 40 (8.7) | 137 (14.9) |
| Model | ORa | 95% CI | p-value | ICC |
|---|---|---|---|---|
| Model 1 | 0.337 | 0.22 to 0.51 | < 0.001 | 0.152 |
| Model 2 | 0.357 | 0.24 to 0.53 | < 0.001 | n/a |
Model 1 is a two-level logistic model of child within school, whereas model 2 is a single child-level model for the dichotomised adverse outcome scale. It can be seen that the addition of school as a level does not alter the main conclusion that a significantly higher proportion of children in the FS arm experienced adverse outcomes at baseline. Both models adjust for the balancing variables from the randomisation (sex and baseline caries).
A similar analysis was of the behaviours observed by the treating dental hygienist conducted at the 30-month treatment visit (Table 77). Slightly more adverse events were recorded in the FV arm at 30 months, although the number of events was low in both intervention arms.
| Adverse outcome | Intervention arm | Overall, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| No | 411 (97.4) | 401 (95.9) | 412 (96.7) |
| Yes | 11 (2.6) | 17 (4.1) | 28 (3.3) |
Again, model 1 is a two-level logistic model of child within school, whereas model 2 is a single child-level model for the dichotomised adverse outcome scale. It can be seen that the addition of school as a level did not alter the main conclusion that the proportion of children experiencing adverse outcomes at 30 months was slightly higher in the FV arm than in the FS arm, but the difference was not significant (Table 78). Both models adjust for the balancing variables from the randomisation (sex and baseline caries).
| Model | ORa | 95% CI | p-value | ICC |
|---|---|---|---|---|
| Model 1 | 1.63 | 0.75 to 3.55 | 0.221 | 0.087 |
| Model 2 | 1.59 | 0.74 to 3.45 | 0.238 | n/a |
Acceptability of fissure sealant/fluoride varnish treatment for children
Participants’ perception of treatment acceptability
Treatment acceptability was assessed from the child’s perspective through a DTF scale (see Appendix 12), completed by all children in the MDC immediately following the initial application of FS/FV and at each follow-up visit. The scores recorded at the baseline treatment visit are recorded in Table 79. It is apparent that time and taste were the dominant factors influencing acceptability. To facilitate analysis of the trends observed over the course of the trial, the data were dichotomised to most happy/happy versus neither happy nor sad/sad/most sad.
| Score | Intervention arm | Total (N = 926), n (%) | |
|---|---|---|---|
| FS (N = 463), n (%) | FV (N = 463), n (%) | ||
| How did you feel during the treatment? | |||
| 1 – most happy | 245 (53.1) | 319 (69.0) | 564 (61.1) |
| 2 | 97 (21.0) | 56 (12.1) | 153 (16.6) |
| 3 | 77 (16.7) | 59 (12.8) | 136 (14.7) |
| 4 | 22 (4.8) | 22 (4.8) | 44 (4.8) |
| 5 – most sad | 20 (4.3) | 6 (1.3) | 26 (2.8) |
| If you had to have the treatment tomorrow how would you feel? | |||
| 1 – most happy | 180 (39.1) | 212 (46.0) | 392 (42.6) |
| 2 | 104 (22.4) | 116 (25.2) | 217 (23.6) |
| 3 | 85 (18.5) | 62 (13.4) | 147 (16.0) |
| 4 | 43 (9.3) | 43 (9.3) | 86 (9.3) |
| 5 – most sad | 51 (11.1) | 28 (6.1) | 79 (8.6) |
| How do you feel about the time it took to have the treatment? | |||
| 1 – most happy | 161 (35.4) | 273 (59.2) | 434 (47.4) |
| 2 | 65 (14.3) | 86 (18.7) | 151 (16.5) |
| 3 | 95 (20.9) | 56 (12.1) | 151 (16.5) |
| 4 | 64 (14.1) | 24 (5.2) | 88 (9.6) |
| 5 – most sad | 70 (15.4) | 22 (4.8) | 92 (10.0) |
| How did you feel about the taste of the treatment? | |||
| 1 – most happy | 75 (16.5) | 184 (39.8) | 259 (28.3) |
| 2 | 39 (8.6) | 48 (10.4) | 87 (9.5) |
| 3 | 47 (10.4) | 53 (11.5) | 100 (10.9) |
| 4 | 116 (25.6) | 78 (16.9) | 194 (21.2) |
| 5 – most sad | 177 (39.0) | 99 (21.4) | 276 (30.1) |
| Did the treatment make you feel like you wanted to be sick? | |||
| 1 – most happy | 280 (61.5) | 299 (64.7) | 579 (63.1) |
| 2 | 22 (4.8) | 26 (5.6) | 48 (5.2) |
| 3 | 35 (7.7) | 43 (9.3) | 78 (8.5) |
| 4 | 53 (11.6) | 42 (9.1) | 95 (10.4) |
| 5 – most sad | 65 (14.3) | 52 (11.3) | 117 (12.8) |
The proportion of children who opted for the neither happy nor sad face, the sad face or the very sad face at baseline to describe their feelings on completion of treatment was much higher in the FS group than in the FV group (49.2% vs. 27.1%). This proportion reduced during the course of the study so that by the 30-month examination, only 16.8% of the FS-treated group reported that they were neutral or sad immediately following treatment. This contrasts with the findings in the FV arm, in which there was a gradual increase in the proportion of children opting for the neutral or sad options on the DTF scale between baseline and 30 months (Table 80).
| Time point | Intervention arm | Overall, n (%) | |
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Dichotomised modified DTF scale (‘neither happy nor sad, sad or very sad’) | |||
| Baseline | 224 (49.2) | 125 (27.1) | 349 (38.1) |
| 6 months | 131 (28.7) | 134 (30.1) | 265 (29.4) |
| 12 months | 92 (21.2) | 148 (32.7) | 240 (27.1) |
| 18 months | 77 (17.1) | 140 (31.9) | 217 (24.4) |
| 24 months | 81 (18.7) | 159 (37.9) | 240 (28.1) |
| 30 months | 71 (16.8) | 171 (40.7) | 242 (28.7) |
Two-level and single-level logistic regression models were constructed to determine the difference between intervention arms at baseline and at 30 months (Tables 81–84).
| Intervention arm | Overall, n (%) | ||
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Very happy or happy | 231 (50.8) | 337 (72.9) | 568 (61.9) |
| Neither happy nor sad, sad or very sad | 224 (49.2) | 125 (27.1) | 349 (38.1) |
| Model | ORa | 95% CI | p-value | ICC |
|---|---|---|---|---|
| Model 1 | 0.38 | 0.29 to 0.50 | < 0.001 | 0.012 |
| Model 2 | 0.38 | 0.29 to 0.50 | < 0.001 | n/a |
| Intervention arm | Overall, n (%) | ||
|---|---|---|---|
| FS, n (%) | FV, n (%) | ||
| Very happy or happy | 351 (83.2) | 249 (59.3) | 600 (71.3) |
| Neither happy nor sad, sad or very sad | 71 (16.8) | 171 (40.7) | 242 (28.7) |
| Model | ORa | 95% CI | p-value | ICC |
|---|---|---|---|---|
| Model 1 | 3.63 | 2.60 to 5.07 | < 0.001 | 0.073 |
| Model 2 | 3.44 | 2.19 to 4.75 | < 0.001 | n/a |
Model 1 is a two-level logistic model of child within school, whereas model 2 is a single child-level model for the DTF scale. It can be seen that the addition of school as a level does not alter the main conclusion that the proportion of children reporting that they were happy or very happy at baseline was significantly higher in the FV arm than in the FS arm, and that the proportion of children reporting that they were happy or very happy at 30 months was significantly higher in the FS arm than in the FV arm. Both models adjust for the balancing variables from the randomisation (sex and baseline caries).
From the above, it is obvious that the acceptability of the technologies under test as judged by the children immediately post treatment was different at different time points in the trial. There are two main issues:
-
At baseline, the children in the FV arm were significantly happier than those in the FS arm, this situation being reversed at the final treatment at 30 months.
-
Those receiving FS became happier as the trial progressed; however, in contrast, in the FV arm there was a modest decrease in the number of children who chose a happy face over the course of the trial.
These findings are in line with what might be expected from a clinical perspective. As the FSs were applied at the beginning of the study as soon as the FPMs erupted, the treatment was more invasive and intense and, as described in Chapter 3, took longer. The children receiving FV, however, had to experience treatment of approximately the same intensity throughout the study.
Qualitative findings on treatment acceptability
In addition to the quantitative determination of treatment acceptability, treatment acceptability formed a major component of the qualitative investigations. In the interviews, the children largely talked about the actual FV or FS treatment in terms of taste. FV was variously described as ‘banana jam/gel/paste’, tasting like ‘candy floss’, ‘oranges’ or ‘apple’, being ‘burny’ and tasting ‘a bit funny and a bit nice’. FS was described as tasting like water, tasting like ‘sour apples’ and being ‘very horrible’ or ‘disgusting’ (although some children liked the taste). Children in year 1 of the study tended to think of the treatment as a ‘banana gel/paste’ and, as children were not always aware that there were two different treatments, children in both arms talked about a banana flavour. Because CDS staff were aware that not all children liked banana flavour and that this might make them reluctant to co-operate with further treatment, they referred to a ‘fruity taste’ when talking to children. In the final year interviews, children discussed different fruit flavours of the treatment, and some children thought that different flavoured treatments were used or wanted to receive a different flavoured ‘gel’ on each visit.
Children’s opinions were mixed in terms of whether or not they liked the taste; some had mild like/dislike, whereas others had stronger positive or negative reactions:
All right, so you both don’t like the taste of it? Is it just a little bit disgusting or is it really awful?
Really awful.
It’s really awful.
Paired child interview, one FS arm and one FV arm, year 3
And what do you think about having a dental van coming to the school?
I like it, I feel happy I do.
I feel happy too.
And why do you like it and why do you feel happy?
Because when like you have this banana stuff.
Aw yeah, it tastes really nice.
It tastes nice.
Is that what you like about it?
Yeah.
Paired child interview, FV arm, year 3
I think the banana gel is a bit bleurgh.
Paired child interview, FV arm, year 3
It’s not the best taste but it’s not the worst taste.
Paired child interview, FS arm, year 3
Many children said that their experience of the treatment and of visiting the MDC was good, and said they felt ‘OK’ or ‘fine’ about it, even if they did not like the taste:
So can you tell me what you thought about going to the dentist when you went?
It was quite fun.
It was quite fun and it tasted disgusting.
OK [to child 2] you thought it was disgusting and [to child 1] you thought it was quite fun.
I thought it was fun.
It was fine but it tasted horrible.
Paired child interview, FS arm, year 1
Several clinical staff members thought that children did not always complete the DTF form in a way that was internally consistent or consistent with what they expressed to staff during their treatment visit; this may be because of the number of different factors driving acceptability for children, as reflected in the previous quotation.
In year 1 of the study, in the FS arm, 13 of the 16 children who made clear statements about the taste of treatment made negative comments about the taste, whereas in the FV arm children’s reactions to the taste were more evenly spread. However, children in the FV arm who did not like the taste were more likely to express negative feelings about having to have more treatment in the future, and half of these children said nothing positive about the treatment experience at all. This may indicate that, although children were less likely to dislike the FV, those who did dislike it had stronger reactions to the taste of the treatment than did children in the FS arm. However, these results should be interpreted with caution, as the sample sizes were small.
In year 3 of the study, there were far fewer very negative comments about having more treatment than there had been in year 1. Children in the FV arm again made a fairly even spread of comments about the taste, from positive to negative, whereas FS children were more likely to make ‘middling’ assessments. This may be because some FS arm children would not have been having further treatment at this stage, unless the sealant needed to be reapplied. Two of the FS children thought that the process was quick, and two others in this group mentioned the treatment visits not always being the same. Of the children who made negative comments in the FV arm, all mentioned the taste and/or feeling sick, although only one said that they would be nervous about going back for more treatment because of this. There were no differences by deprivation level.
In the final year of the study, no child interviewed reported very negative overall attitudes towards having treatment, even though some children still reported not liking the taste. For example, one FS child in year 3 said that they would be excited to go for treatment again, but also said that the taste was really awful. Overall, most children were generally tolerant about having FS/FV treatment by this stage:
When you have to go and have your treatment in the dental van, what do you think about that?
It was all right but the taste was a little bit not that nice.
Not that nice.
It tasted like bananas.
So you didn’t really like the taste but apart from that, it was all right you said?
Yeah.
It was OK. And what about you [to child 2]? What do you think?
The taste was not that nice and it tasted like oranges.
It tasted like oranges. OK. So not that nice but apart from the taste, how was it having to go and have the treatment?
Not that bad.
Paired child interview, FV arm, year 3
The qualitative data on treatment acceptability are similar to the findings from the DTF scales and co-operation scales, in that the acceptability of FS treatment was low overall in year 1 but increased over time.
Other aspects of treatment were mentioned with less frequency than taste. Children very rarely commented on the length of treatment unless they were prompted and seemed unconcerned by this aspect of treatment. Perceptions of length of treatment depended more on the individual child than on the trial arm. Children were more likely to comment on the time taken in terms of not liking to wait in dentist waiting rooms (which they reported happened more commonly in community dentist offices than in the MDC as part of the SoV trial). A very small number of children mentioned the texture of their teeth afterwards as being sticky (FV) or ‘bumpy/dotty’ (FS). A few children mentioned feeling sick or vomiting: one child in the FV arm and two in the FS arm mentioned feeling or being sick in year 1 and two children in the FV arm in year 3 mentioned feeling sick. Most children did not notice discoloration, and the few who did were not concerned by this. When asked whether or not their teeth had changed after treatment, some children thought that the treatment had made their teeth stronger, whiter, cleaner or ‘fresh’. When children commented on changes in their teeth, this tended to be in relation to normal changes such as their adult teeth appearing, rather than any changes attributable to FS/FV.
Parental reports of treatment acceptability
Parent accounts of child acceptability corroborated child reports. Parents said that children found it novel and exciting to visit the MDC (known colloquially as ‘the van’) and that they liked various aspects of treatment such as being with their friends and being given stickers:
Has he ever said anything about the taste or about how long it takes or anything like that?
He said it’s banana taste.
Does he like that or does he not like that?
He likes that.
He likes the taste, OK. And does he say anything else about the van or going with his friends or anything like that?
He just says he likes going on the van and having a sticker.
And he likes the sticker, do you know what he likes about going on the van?
I think it’s the chair, because they let him play with the chair.
Parent interview, FS arm, year 3
Many parents said that their children did not say much about the treatment but would have complained if they had found the treatment very objectionable. A small number of children had reported to their parents that they did not like the taste or another aspect of treatment (e.g. that it was ‘gluey’) but, again, acceptability was often based on more than one factor:
She said that it tasted horrible.
OK.
That was it really just it didn’t taste very nice but she was delighted because she had stickers [laughs] you know I was like ‘What was it like, what did they do?’ and she said ‘They put something in my mouth and it didn’t taste very nice’.
Parent interview, FS arm, year 1
In year 1, children either did not say anything about the treatment to their parents or said that it tasted funny or that they did not like the taste. In year 3, children still either said little or reported that they did not like the treatment, but children at this stage also mentioned to parents aspects of the treatment that they liked. There were no differences between trial arms or by deprivation level.
Parents in the high-deprivation schools in the first year of the study were most likely to report discoloration of teeth from the treatment, in both trial arms. In the final year of the study, a very small number (n = 3) of parents in the FV arm reported discolouration. If parents did notice discoloration, they were not always sure that this was because of the treatment; other explanations were that it might be because of their child’s age or inadequate brushing. No parent was concerned about discoloration, and perceptions did not differ by trial arm.
A few parents thought that their child’s teeth were whiter or shinier; some were not sure about changes but expressed a hope that the child’s teeth were stronger or more protected because of the treatment received.
School staff reports of treatment acceptability
Staff reported that the MDC and CDS service was well liked in schools by children; one head teacher commented that usually children were disappointed if they were not called to attend. One school staff member commented that if children did not like an aspect of treatment they forgot about it soon after returning to the classroom.
Community Dental Service staff reports of treatment acceptability
Throughout the study, CDS staff had mixed opinions about which treatment was more acceptable to children. They observed varied reactions to the treatment and different aspects of treatment that were more or less popular:
The children react differently to be honest. Some of them love the varnish or some of the hate the varnish, some of them play up because their friends are there or whatever, they have a different reaction and then some of them are good with the sealants as well.
CDS staff interview, year 2
Community Dental Service staff commented that children were generally tolerant and well behaved, regardless of whether they liked the treatment.
In year 1, more staff thought that FV was better liked by children because it was quicker and did not involve cotton wool rolls, and because some children did not like the taste of the FS fixer. In year 2, the staff commented in more detail about the pros and cons of both treatments: FV was quicker and easier to apply, but it was also sticky, could cause nausea and had a lingering taste, whereas children could rinse after FS treatment; if children gagged easily it meant that applying FS could be difficult, and children sometimes found it difficult to keep their mouth open for a long time, but FS usually required only check-ups rather than reapplication. They also mentioned that FV was easier than FS for a difficult child as it was the quicker treatment. Staff used various tactics to encourage children, such as bringing nervous children back later in the day to retry treatment, treating a nervous child first (so that the child did not have to wait) or last (so that the child could observe other children receiving treatment), and sitting a child further upright to reduce gagging.
Staff in years 2 and 3 also said that children were very accepting of treatment and that refusals were rare, but they did note that acceptability still varied and that some children liked the treatment or taste and some did not. By this point, some children remembered not liking the treatment and could react negatively when attending again, which could then affect the other children they were with. One staff member thought that children from more deprived areas reacted more negatively when coming back for treatment. However, some FS children were disappointed that they did not receive any treatment when other children did.
Various CDS staff comments mirrored what children reported. One staff member commented that children receiving FS disliked the cotton wool rolls more than they disliked the length of treatment. A number of staff members reported that whether or not children found the treatment acceptable was largely driven by taste, and also that some children thought the treatment was too quick and would have liked more time out of the classroom. Staff also commented that children liked different aspects of treatment, particularly laughing at each other wearing the protective glasses; preferring to be with their friends and being less nervous if they were accompanied; and enjoying the novelty and excitement of being on the MDC:
Oh they love coming on the van. They love it. They always ask who drives it and where do people, some, some think that they sleep there overnight and it turns into a caravan but they love it, they love coming on. What’s this drawer for, you know, yeah, they love coming on.
CDS staff interview, year 2
The CDS staff members’ comments that children were chattier and asked more questions in year 3 of the study also corroborated some children’s reports that they felt more confident now that they were older.
Global intervention acceptability for children
Turning to the information analysed from the interviews with the participating children, the primary finding on the overall acceptability of FS/FV treatment for children was that there were many factors that contributed to whether or not children found the treatment acceptable. Most of these factors were related not to the FS/FV aspects of the MDC visit but rather to more general aspects of treatment or to the context in which the treatment was delivered.
Most children commented that ‘going to the van’ was ‘fun’, ‘brilliant’ and ‘good’ and that it was exciting to go there (the ‘van’ is the term children used to describe the MDC). Receiving stickers was a very popular aspect of MDC visit, and three children mentioned that they liked the CDS staff. When comparing a visit to the MDC with a visit to a family/community dentist, most children also expressed their views in relation to factors beyond the actual treatment received, such as being given stickers, the speed of the visit, which visit was warmer and the van being fun. Some children also mentioned that they liked having their teeth looked after or protected:
I like the dentist because they look after your teeth and keep them clean.
Child interview, FV arm, year 1
Fourteen children in year 1 of the study mentioned feeling scared or nervous about the treatment; of these, 12 said that being with friends (children normally attended the MDC in groups of three) made them less nervous or feel safer. In year 3 of the study, fewer children mentioned feeling nervous, and in the low-deprivation schools four children commented that they felt more confident about being at the dentist now that they were older and more used to the treatment visits:
We did both say we were nervous when we went, when we were.
Yeah, when we were younger, ‘cos it was our first time.
Yeah ‘cos it was less.
We were like . . . not confident.
Yeah.
‘Cos we were just too young to go on our own, not like on our own like.
You were quite young when you started though.
Yeah, I was talking about before.
Because you’re like quite scared, because you haven’t been to the dentist oftenly[sic].
Then you might need . . .
So when you get older and older and . . .
You’ll be more confident.
Yeah.
Paired child interview, one FS arm and one FV arm, year 3
Although the older children generally felt more relaxed and confident about a visit to the MDC, they were also more aware of what was going on around them. In year 3 of the study, a small number of children reported that the examination made them feel anxious because they did not understand the tooth-numbering terminology the CDS hygienist used and were worried that there might be something wrong with their teeth:
They say stuff like numbers and stuff and we don’t really know what that means.
You don’t know what that means, OK.
So like, they could be saying that our teeth are really bad or really good but we don’t know.
Like A2 and A3 and that.
Yeah.
Yeah.
AC.
Sounds funny doesn’t it?
Yeah.
Yeah.
So would you like to know more, you’d like to understand what they’re saying?
Yeah.
Paired child interview, one FS arm and one FV arm, year 3
Children also frequently mentioned generic aspects of the dental treatment they liked, particularly the moving dental chair and protective (and often coloured) glasses. Feelings about the three-in-one syringe were mixed, as some children found it unpleasant but some liked it because it was ‘ticklish’. The cotton wool rolls were the least popular aspect of treatment and a few children expressed a dislike of having their teeth ‘scraped’/’poked’. There were no differences in preferences between the FS and FV trial arms. Children’s responses to what they thought about the overall treatment often centred on these wider factors rather than on the FS/FV itself:
So can you tell me, what did you think about going to the dentist earlier this week?
It was good, I liked it.
It was good . . .
It was fun.
And you thought it was fun [to child 2], OK. And what did you like about it? [Pause.] Or are you not sure?
Because you got to sit on the special chair.
The special chair, a magic chair?
Yeah.
What was special about it? Why was it a good chair?
Because it can move.
Is that fun?
Yeah, and I like the glasses.
What do you like about the glasses?
Because they protect our eyes and face.
They protect your eyes. Are they a special colour?
White.
White?
Yeah.
OK. Is there anything else that you liked about going?
Yeah, I was excited.
You were excited . . .
And because you got to go in the van.
Oh right, what do you like about the van?
Because um [pause] can’t remember.
You’re not sure, that’s OK. But it’s fun going in the van?
Yeah.
Paired child interview, FV arm, year 1
Taste was the main aspect of the actual treatment acceptability mentioned by children, but it was one of several factors:
If somebody said you’ve got to go and have that same treatment done again tomorrow, how would you feel about that?
I wouldn’t mind.
You wouldn’t mind [to child 1] and how about you [to child 2]?
It would be OK.
It would be OK. So it wouldn’t be fantastic but it would be OK?
It would be fantastic.
Why would it be fantastic?
Because I love the banana gel.
Is that the best thing about going?
No.
What’s the best thing about going?
Getting the sticker.
Paired child interview, FV arm, year 3
A small number of children were so enthusiastic about the treatment visits that they responded (unprompted) that they would be happy to have the treatment done all day/every day:
If the van came back tomorrow and you had to have it done all over again, how would you feel about that?
It don’t matter, I’ll have it every day.
OK. Why would you have it every day?
Because the chair is fun.
Is that your favourite thing?
Yeah.
Paired child interview, FV arm, year 1
Children in both trial arms mentioned similar acceptability factors in year 1 and year 3 of the study, such as liking being with their friends, treatment being good for their teeth, and liking the stickers and the moving chair, and mixed attitudes towards different aspects of the treatment, such as the three-in-one syringe:
And what do you think about going there for the treatment?
I think it’s really good idea.
Mm hm.
And what’s good about it?
It’s like, we can get to wear some glasses, we can get a sticker, stuff like that.
Mm hm.
We don’t get bad teeth when we’re older.
Paired child interview, FV arm, year 3
In year 3 of the study, children reported that some people in their class liked treatment and some did not. However, most children in year 3 were more likely to report that they were ‘fine’ or happy/excited to go for treatment, even if they did not like all aspects of it.
Acceptability of treatment setting
Acceptability of treatment setting for children
To elicit information about the acceptability of the FS/FV treatment, children were asked if they preferred being in class or leaving class to visit the dentist at the MDC. In year 1 of the study, most children did not express a clear preference or were indifferent. Children who did express a preference gave various reasons: they thought that it was important to get dental care, they did not like doing schoolwork/they liked missing class, or they liked going to the MDC. One child said that they preferred being in class because they liked their teacher. There were no differences between the FS and FV trial arms.
In the final year of the study, children were more aware of having to catch up on schoolwork if they missed part of a class to visit the MDC, although this was not a major problem, as children mentioned receiving help from their friends or from the teacher to do this. The main reason that children preferred staying in the classroom was avoiding having to catch up on work later. Two children were not sure about their preference; three children said that, if the lesson was mathematics, they would prefer to go to the dentist.
Acceptability of treatment setting for parents
Several parents thought that their children behaved better, made less fuss or were less nervous about seeing a dental clinician in a school environment:
He will do it in school.
So he’s a bit better in school?
Yeah, as the teachers are there.
The teachers are around?
Yeah yeah.
And you’re not?
That’s right he’s a good boy in school see [laughs] it’s an awful thing to say but it’s true.
Right, so is it just mainly because of the teachers and because it’s a school environment that he behaves better?
He’s never naughty in school.
Parent interview, year 1
Parents did not mind their children missing some class time to attend the MDC. Some pointed out that, as the children were young, missing a small amount of time was not significant. A few also pointed out that the children missed less class time going to the MDC than they would if they went to a community dentist. Parents also appreciated not having to escort children to the appointments and commented that this was very convenient for them. A small number commented that the intervention was good for children who would not otherwise see a dental professional.
Acceptability of treatment setting for school staff
In response to the questionnaire in year 1, the majority of schools indicated that hosting SoV in their school had been straightforward, the programme ran smoothly with minimal disruption to the school, the information received was easy to understand and SoV was similar to existing dental programmes running in schools. CDS communication and co-ordination was good and both CDS and schools were flexible (e.g. in response to each other’s timetables). Six also said that it was interesting to be part of a study and two said that they were pleased to offer it to their children. One head teacher mentioned not knowing details of the study, which would have been helpful in explaining the intervention in response to parent queries. One support teacher had had problems with not being aware that the vans were coming and this causing disruption. One class teacher said that some children did not like the taste of the treatment and that staff had to comfort them when the vans arrived the next time. The only other problems were taking time to explain the study to parents (four respondents mentioned this), explaining the study to teachers in the school (two respondents) and finding the time to respond to requests for data (four respondents). Some schools distributed letters for the study, although some also actively reminded parents to return them, by telephoning or texting parents, reminding them in person and by displaying a poster (in one case). Eleven schools agreed that SoV fitted in with other health programmes or initiatives they were involved in; the most common were tooth-brushing programme and the Healthy Schools scheme. Some schools were unsure whether or not SoV had changed dental behaviour, several thought it made children more aware of the importance of looking after their teeth, although another respondent commented that they did various dental programmes so it was hard to know the effect of SoV on behaviour.
Responses in telephone interviews with school staff (mainly school heads) in year 3 were similar to the questionnaire findings. Two school staff mentioned minor disruption to classes when children left to visit the MDC and a small amount of extra paperwork but said that this was not a problem. Overall, visits to the MDC were not perceived as a problem because they were quick and efficient, and schools were very used to their visits:
Very, very minimal impact really. I mean they come in they do their stuff and off they go again.
School staff interview, year 3
The preventative programme was also perceived as meaning that children would miss less school than they would visiting a community dentist for dental problems later on:
Well I’m hoping, from the information that was provided when I took over the class room is that it was going to protect the children’s teeth, that there was going to be less cavities, less absences with them disappearing for full days when they have teeth removed and teeth repaired because they’re going to be better protected.
School staff interview, year 3
One school head also commented that the service was very child-friendly, and, because of this, the children were not stressed and their visit to the MDC did not disrupt the school day too much.
School staff were positive about having the CDS service in schools and regarded it as fitting in with their promoting health agenda and also helping them engage with families, which was seen as part of their remit:
What do you think about having the dental clinic on the school grounds?
Oh absolutely fine.
Mm hm.
Absolutely fine. I think it was nice for the parents to see.
Mm hm. Why is that?
Well, because I think a lot of them find dentists, doctors, even crossing the threshold of the school quite intimidating.
Mm hm.
It’s made it more accessible for the community.
School head interview, year 3
School staff thought that SoV, along with other dental programmes, had improved children’s awareness of dental health and oral hygiene.
Feasibility and acceptability of treatment setting for Community Dental Unit staff
Community Dental Service staff found the treatment setting unproblematic on the whole, largely because SoV was integrated with the Designed to Smile dental programme for children in South Wales, which already used MDCs to deliver dental treatment to school children and typically ran very smoothly. CDS staff reported that being part of the SoV trial had created extra paperwork and that this took some extra time, especially early in the trial when staff were still getting used to it but that they were generally positive about being part of the trial and reported no significant impact on how treatment was delivered compared with other CDS services. A small number of staff mentioned that they had little time with each child, which could be a disadvantage as chatting to a child could reduce anxiety sometimes.
Minor difficulties noted included parking a MDC in tight spaces or driving one in bad weather, and occasionally school administrators (the main liaison persons for the CDS in schools) complaining about paperwork or not being as helpful as they might be. One SoV staff member commented that because of the various dental programmes running in schools, each with their own paperwork, schools could get confused between them sometimes. However, most school administrators were helpful; for example, one would assist SoV with recruitment if numbers were low and another would text reminders to parents. Overall, CDS staff found schools very helpful and found that they valued CDSs. CDS staff reciprocated, being careful to organise themselves around the school timetable and events to minimise disruption. Two CDS staff members thought that schools in the more deprived areas were particularly welcoming to the MDC.
Community Dental Service staff also mentioned several aspects of the school setting that made treating children easier, including children being more likely to turn up for appointments:
In the schools we’ve got a captive audience.
Mm.
In the clinic we have horrendous, horrendous DNA [did not attend] rates.
CDS staff member, year 3 of study
The CDS staff were also able to treat larger numbers of children more quickly in a school setting.
Impact of treatment and trial on oral health behaviour
As discussed in Chapter 3, none of the potentially confounding covariates affected treatment outcome by trial intervention arm (see Table 19). In the qualitative element of the study, children and parents were asked about changes in oral health behaviour as a result of participating in the trial. Both child and parent interviewees tended to describe low and declining levels of child sugar and fizzy drink consumption:
Well, I know it’s really bad for my teeth and that’s why I stopped ‘cos I don’t want like my teeth to rot and stuff.
Yes, you stopped what?
I stopped drinking fizzy drinks and eating sweets . . . I’ve stopped chocolate and sweet stuff.
And what made you change?
Cos my mum told me to stop it and I never used to listen to her and now everybody is saying it’s bad for your teeth.
Mm.
And so I don’t eat it any more.
Interview, year 3
Some children also reported starting to use mouthwash or brushing their teeth more frequently. One child commented that they brushed their teeth more ‘to make them grow’. A small number of parents also said that they monitored their child’s brushing more closely. There were no differences by trial arm (the sample size was very small to make comparisons in any case, as some children found the concept of change over time difficult to grasp). Some parents and children reported improving the child’s diet or tooth-brushing habits because of the input from SoV, sometimes just by acting as a reminder to look after the child’s teeth. However, there is likely to be a social desirability bias in the data collected, especially as some parents assumed that the researcher was a dental practitioner (several requests were made by parents to inspect children’s or parents’ teeth during the study; parents were advised to see a dental practitioner).
Reasons for participating or not participating in Seal or Varnish?
Reasons for participation
Most parents were not fully aware of the details of the treatment or study but were participating to generally benefit their children’s teeth and to feel that they had done as much as possible to promote their child’s dental health:
I’m thinking myself there’s every chance now that I’ve given her the best chance to look after her teeth then and she may not have decay.
Parent interview, year 3
Most made the decision about participation themselves, although some also consulted their spouse or the child as well. A small number checked with their regular dentist first. Some parents were able to recall that it was a protective treatment that was put on teeth, although not all remembered that it was treating only molars or that there were two different treatments being used. Some thought that SoV included dental education. Randomisation to trial arm was not always understood: one parent thought that their child might receive no treatment, and another thought that their child was ‘lucky’ to be picked for treatment. Few knew how long the study lasted, and many asked about this during research interviews. A few parents had very little understanding or had completely forgotten the details of the study:
Do you understand what the study is about?
Not really no.
OK, is there anything, if you had to tell a friend about what Seal or Varnish? was what would you say?
Well to be honest I don’t really know a lot about it.
Parent interview, year 1
However, parents trusted the school and several said that they participated partly because of this:
It was with the school I was quite happy because it was recognised through the school.
Parent interview, year 1
I trust the school with everything.
Parent interview, year 1
Several parents also gave accounts of family members’ dental histories and experience of FS/FV, either their own or that of the participating child or another of their children, both positive and negative, and gave this as a reason for participation. Some parents liked that the programme would mean that their children would have dental check-ups more regularly (even if they were already having 6-monthly check-ups at a community dentist) because problems would get picked up more quickly.
A small number of parents (both participating and non-participating) also mentioned the value of doing research and were also willing to participate for this reason (although it was not a primary reason).
Reasons for non-participation
Nineteen parents returned a consent form (including in the second trial recruitment package sent home with children) to be interviewed about non-participation. When contacted by telephone, four said that they were taking part in the trial or had signed up, so no interview was attempted. Five did not answer the telephone or the number was wrong, two had a level of English that was too limited for an interview, two said that they were not participating because they were moving house, one was too busy to do an interview because of family illness and one said that they did not want to do an interview and also commented that they did not participate because they already had a dentist in town. On subsequent cross-checking of the 19 consent forms with trial records, it was found that only 8 of the 19 ‘non-consenter’ parents had not given consent. Six had given consent but were ineligible and five were actually in the trial. It is likely that a majority of parents had completed all the forms their child had brought home from school without checking the content of the form thoroughly and did not realise that the consent forms for non-participation interviews were different from the trial forms and completed and returned all the forms they received. CDS staff reported that this was common in parents receiving paperwork about other dental programmes. Hence, the contacts made with parents who returned these forms did not yield any data on reasons for non-participation, except that parents had not always understood the paperwork they received or completed it correctly.
Twelve parents returned the questionnaire about reasons for non-participation (parents could give multiple responses, and so the number of responses is > 12).
The responses indicate positive and negative reasons for non-participation. Some parents did not participate because they just wanted to continue with existing dental care, some were unsure about SoV’s benefit or acceptability to the child, and some were unwilling to take part because it was also a research study (Table 85).
| Response | Frequency of responses |
|---|---|
| I wanted my child to continue with their current/normal dental treatment at a local dental surgery | 7 |
| I did not understand what the study would involve | 3 |
| I did not want to be interviewed for the study | 3 |
| I wanted my child to continue their normal treatment from the MDC and did not want them to receive anything different | 2 |
| My child did not want the treatment and I did not want to make them have it | 2 |
| I was afraid my child might lose their place at the local NHS dentist if they went somewhere else for treatment | 1 |
| I wasn’t confident that the treatment would be good for my child | 1 |
| I was worried about what a dental examination might find in my child’s teeth | 1 |
| I do not like the school being involved in or knowing too much about my child’s health care | 1 |
| I do not like taking part in research | 1 |
| I did not like being unable to choose which treatment my child received in the study | 1 |
| I did not want to have to fill out questionnaires for the study | 1 |
| Other: ‘My child don’t like the fact that he is not able to rinse his mouth after brushing in class, even though I know its fine, but he does not like it. Funny really.’ | |
The questionnaire also asked about dental care and tooth-brushing habits, to determine whether children with poor dental habits were more or less likely to take part in this type of intervention. According to their parents, three children brushed their teeth at least twice a day, four children brushed their teeth once a day and one child did so less frequently than once a day. All parents said that their child visited a dental surgery regularly.
Face-to-face recruitment at the school handover at the end of the day, through distributing an invitation to participate in a research interview, did not work well for the hardest to reach non-participating parents (those who had never responded to any of the study recruitment materials they had received). This recruitment effort was a second measure to try to collect data on why some parents did not participate in the SoV intervention or study. During recruitment for non-participants at handover time at the end of the school day, no parents queried the researcher, although one or two made more general queries about dental services available in relation to specific problems that their child was having. These parents were given the telephone number for the CDS for information about appropriate dental services.
Five parents across three schools gave consent to be interviewed. One decided not to participate in SoV because they were moving house but would have liked to otherwise and had attended the interview primarily to find out about their own dental treatment. One was participating in the trial and two thought that they had returned consent forms. One of these parents was familiar with FS because their child’s older sibling had had this treatment, and they thought that they had consented and so did not know why they were not taking part. Three did not remember receiving any information about SoV but were generally positive about school dental programmes. Two commented that they thought that their child had probably lost the letter sent out from the school and that this had happened with school letters before: ‘sometimes she makes paper aeroplanes out of it’. None expressed any negative feelings about taking part in a trial. Therefore, this set of interviews yielded no data on reasons for non-participation, as parents were generally positive about the dental programme, except that, again, that there was confusion about or difficulties with completing the paperwork. This recruitment effort was eventually abandoned, as these five interviews yielded few useful data and had required 14 school visits (two visits each to seven schools) to achieve. The addition of a shopping voucher as an incentive in year 2 increased the participation rate only very marginally.
The overall finding from the recruitment efforts and results from speaking to these ‘non-participating’ parents was that there was a lack of understanding of and engagement with the paperwork in various respects. In the initial piloting of the materials, the paperwork such as the information sheet and consent form was found to be very long, even though it had been shortened as much as possible and a graphic design company had produced a very friendly format. Despite this, none of this group of parents could articulate what the study was about in full or mentioned the comparative aspect of SoV, indicating that the paperwork had not communicated the study to parents clearly or that the information had not been retained. One had understood that it was to ‘seal their teeth’ but thought that it was to address existing holes in children’s teeth and one thought that the SoV programme also handed out toothbrushes. Another thought that it addressed tooth grinding and that it was a student research project. No negative attitudes towards the SoV intervention or trial were expressed.
In interviews, both school and CDS staff discussed similar problems with contacting and recruiting parents for programmes, and commented that they did not always get paperwork back from parents whatever the programme. School and CDS staff thought that parents with little English would also have problems, especially with long forms; of the five non-participating parents recruited for interview through school visits, one had no English (a friend attended the interview to translate) and another spoke English as a second language. CDS staff also mentioned that some parents had problems completing paperwork because of literacy problems. One parent commented they would have preferred to receive information about SoV face to face rather than in a ‘big booklet’. School staff thought that some parents needed face-to-face support in order to understand the study and to complete recruitment forms correctly, and so would sometimes assist parents with filling in paperwork. One school staff member commented that it would have been helpful for teachers to know more about SoV so that they could have answered parents’ queries about it. Some of the difficulty was also a result of the volume of paperwork and participation in other dental programmes running in the school, leading parents to think that they were already taking part. CDS staff reported that schools often found it a challenge themselves to keep track of the paperwork from different programmes operating in the school, including several dental programmes. School and CDS staff also commented that parents who were already engaged with health and dental services were another group that was less likely to participate because they might not see a need beyond their existing dental visits. One parent commented to the researcher in the playground that their child already saw a dentist and they did not want to use up additional resources unnecessarily. Other families who tended not to engage, according to school staff, were those who were nervous of school environments or who had chaotic lives or work/other commitments that were difficult to juggle. Some school staff thought that parents needed reminders in person from school staff to sign up for programmes. School staff sometimes made an effort to recruit those families, in person, that they thought might be particularly in need of services, either to explain letters or to discuss any potential barriers.
Summary
The work reported in this chapter set out to answer a series of questions (see Table 68) that related to the acceptability of technologies under test and the setting in which they were being provided.
The first question related to the acceptability of the FV and FS treatments to the children, what factors influenced acceptability and if there were any possible differences between the trial arms.
Overall, the acceptability of the treatments was very high, as judged by the fact that very few children refused treatments or discontinued in the trial because of the lack of acceptability of the treatments (see Chapter 3). When judging acceptability from the perspective of both the parents and the children, it was apparent that this was influenced to a large degree by the wider factors rather than the actual treatments being provided. This means that acceptability needs to be understood as a broad measure, as contextual factors can make a significant difference to acceptability for children, parents and schools.
From the perspective of the children, the novelty of dental treatment in the MDC and MDC aspects such as the provision of reward stickers both seemed to feature as highly in the issues raised in the qualitative interviews as issues around the treatments provided. When pressed specifically on the treatments, the taste of the interventions featured highly in the narratives.
There were significant differences in the acceptability of the FS and FV treatments with the preference changing over the trial period. However, the more involved nature of the FS treatment in the early visits, compared with the constant application of the FV at every treatment visit, is reflected in the results.
The second question concerned implementation, whether or not the delivery of this type of preventative treatment was acceptable to children, their parents, clinicians and the schools in which the treatments took place and the factors affecting implementation of treatment in a school setting.
The answer to this question has to be yes, and it is clear that in deprived areas in which encouraging at-risk children to attend services is key, this work has shown that the school context can be an effective delivery organisation for preventative services. In the qualitative interviews, parents reported trust in the school and convenience of access as important factors in utilising the service.
The child-friendly nature of the setting, the longer-term and ongoing relationship of the CDS with the participating schools and the convenience of attending in school were all factors affecting the acceptability of the treatment setting for parents, teachers and CDS staff.
The favourable reports from the school staff on the implementation of the study and the minimal disruption to the running of the school caused by the CDS team visiting are positive features of the feedback received.
The third question concerned the factors that affect whether or not parents consent to take part in an intervention and trial such as SoV.
A clear and definitive answer to this question was not forthcoming, primarily because of the difficultly in recruiting non-consenting parents. Those who did not want/were insufficiently organised to consent to the trial were similarly difficult to consent to an interview about non-consenting. Trust in the CDS and particularly in the school’s judgement about facilitating the trial were raised as important determinants of a positive decision by parents to allow their children to participate in the trial. Tailored communication and assistance with understanding materials and completing forms could help those with low literacy or levels of English to access trial and intervention information to a greater degree.
The fourth question concerned whether or not treatment with FV or FS affects (self-reported) subsequent oral health behaviour, and if so whether or not there differences between trial arms.
In the main clinical analysis of the study (see Table 13) and qualitative analysis, there were no significant differences between trial intervention arms related to tooth brushing, diet or other oral health-related habits. In the qualitative interviews, some parents reported that the fact that their child was participating in the trial heightened their awareness of dental issues, but it is clear from the quantitative analysis that these parameters did not impact significantly on trial outcomes.
What is the effect of the trial on recruitment or acceptability to parents, dental staff and schools?
The reasons offered by parents for consenting their children to the trial included the perceived benefits. However, it is clear that, on occasion, parents were not entirely clear about or could not fully recall the purpose of the trial, again resorting to trust in the school and familiarity with the CDS.
The main trial findings and conclusions are discussed in Chapter 6.
Chapter 6 Discussion and conclusions
Main trial findings
Clinical results
This trial found that the proportion of children who developed dentine caries or had a restoration or tooth extracted because of caries (D4–6MFT) on any FPM after 36 months did not differ significantly between those treated with FS and those treated with FV (OR 0.84, 95% CI 0.59 to 1.21; p = 0.351). Similarly, when the proportion of teeth, the number of teeth or the number of tooth surfaces was studied as the outcome measure, no significant difference was observed between treatments. However, across all of these measures, marginally fewer teeth were affected in the FV-treated children.
This study was commissioned because the relative effectiveness of FS and FV was unknown. Cochrane systematic reviews published just before17 this study started and immediately before it finished16 came to the conclusion that, when comparing resin-based FS and FV application, there is some evidence to suggest that FS is more effective. However, these studies reported that it is not possible to draw definitive conclusions based on the four previous studies to have compared resin-based FS and FV. The authors attributed this to scarce and clinically diverse data, and to the low quality of previously reported studies.
What other studies found
There have been four previous studies that have investigated whether resin-based FS or FV is the more clinically effective. 61,66–68 In their 2016 review of these studies, Ahovuo-Saloranta et al. 16 said that it was not possible to reach conclusions about whether to apply FS or FV to the occlusal surfaces of permanent molars to best effect. The clinical outcomes from the current trial suggest that there is no significant difference in the clinical outcome, irrespective of whether FS or FV is used.
In this study, care was taken to ensure that potential confounding factors were accounted for in the analyses. These were adjusted to account for the deprivation score of the locality in which the schools were located, the size of the school and individually reported oral hygiene regime, diet and dental attendance. None impacted on the clinical finding of no significant difference between the FS and FV treatments in terms of clinical effectiveness.
It is, therefore, necessary to take into account cost and patient acceptability considerations, as discussed in Health economics and Treatment acceptability.
Other clinical findings
Beyond the primary and main clinical outcomes, some additional clinical parameters were explored.
Surfaces protected
Clearly, the only surfaces that could be protected by the FS were the occlusal surfaces, although the FV had the potential to protect both the occlusal and the non-occlusal surfaces. When diagnosed at the caries into dentine (D4–6MFT) level, there was, as would be expected, a significant difference in the proportion of occlusal surfaces that experienced decay compared with non-occlusal surfaces. However, no significant differences were observed between treatments in relation to the protection afforded to occlusal and non-occlusal surfaces.
The effect of including enamel caries
All of the primary outcome data were based on the traditional caries detection threshold: caries into dentine. In this study, the use of the ICDAS caries scoring system made data available at a lower diagnostic threshold, thereby enabling an analysis that included both enamel and dentine caries. This was the only analysis that suggested a significant difference in outcome between the FS and FV arms of the trial in favour of the FV-treated children. The inclusion of the D1–3lesions resulted in a significantly lower proportion of caries lesions in the FV arm than in the FS arm, a finding that may well reflect the caries-protective effect of FV on smooth surfaces. However, this effect is negated by the overall finding of no difference in the proportion of children or the number of teeth or tooth surfaces that developed D4–6MFT lesions, the primary outcome measure.
‘Wash-over’ effect
It is also possible that a ‘wash-over’ effect was observed in the FV arm. In other words, the raised intra-oral fluoride environment associated with the FV treatment may have exerted an effect on teeth distant from the FPMs. However, this proved not to be the case, and no significant differences were observed between treatments when whole-mouth D4–6MFT scores were analysed.
Trial duration
This trial took the traditional approach to the duration of caries clinical trials of 36 months’ follow-up in the absence of radiographic examination. The question arose of whether or not a difference in primary outcome would have been observed with a trial of shorter duration. It was not possible at either the 12-month or the 24-month clinical assessments to demonstrate any clinically significant difference between the FS and FV treatments.
Failure to prevent dental caries
It should be noted that, irrespective of treatment received, around one in five children developed dental caries that had progressed into dentine on at least one FPM. This probably reflects the high caries risk status of the children participating in the trial.
Health economics
The health economics analysis indicated that, within the context of a MDC setting and under the trial conditions, FV cost less than FS, even taking into account the repeated application of FV over the trial period. A similar picture is shown when the costs to the patient and family are included. Although there is no obvious single cost driver that accounts for this cost differential, it appears that the FV arm had a consistent and cumulative pattern of fewer costs in intervention and health-care utilisation and, subsequently, in costs attributed to the family.
When the costs are translated into estimating the impact of delivering FS or FV on NHS budgets, this estimates that FV results in a cost saving of £68.13 per child treated compared with FS.
The clinical findings showed no statistically significant or clinically meaningful differences across the majority of primary and secondary outcomes (including health utilities). In such a scenario, the health economic analysis inevitably becomes a cost-minimisation analysis. Thus, the cost differences and subsequent budget impact could be seen as one of the key findings of the economic analysis. In our sensitivity analysis of the budget impact, changing individual parameters estimated that there could be different results. This further examination of a range of scenarios was undertaken because of the need to fully consider the potential impact of delivering the intervention in a different ‘real-world’ clinical context rather than just under the trial conditions.
With such small and non-statistically significant differences in outcomes, any ICERs become extremely sensitive to small changes in numerical values and should be treated with appropriate caution.
The base-case results show that because numerical differences were very small and not statistically significant, the cost-effectiveness results estimate that, in terms of the cost per child with dentine caries avoided, and for cost per QALY, FV dominates FS. This result (and interpretation) should be treated with caution, particularly in the CUA, given the very small QALY differences that would be highly unlikely to translate into meaningful benefits to children. In terms of the cost per QATY analysis, there was a very small difference in effect in favour of FS, but, when the ICER was estimated, FV dominates FS. As outlined, the cost-per-QATY analysis should be treated as exploratory.
The CEAC for the CUA shows that, if the NICE guidelines are followed, there would be a 70% probability of FV being at a NICE societal WTP threshold of £20,000 per additional QALY and, as such, FV could be considered cost-effective.
An exploratory model-based analysis undertaken to look at the potential longer-term cost-effectiveness of FS and FV was consistent with the within-trial analysis.
Overall, even taking into account the need for further examination of the impact of missing data, the analysis shows that FV costs less than FS and could result in moderate cost savings to the NHS if it was delivered as the preferred treatment in the prevention of caries in the FPMs of children at high risk of dental caries.
Although the health economic evaluation has focused on reporting the relative cost-effectiveness and budget impact of FS versus FV, understanding the practical implications is important. As shown in Table 66, there are hypothetical savings to be achieved, and an estimated additional 93 children could be treated if FV was used rather than FS.
However, such a scenario is too simplistic. Further consideration would be needed to ascertain how this would impact on the service. The MDC is despatched to specific schools with different population sizes, so, although in theory greater child throughput could be achieved, this would be dependent on the number of children available to treat during one session and other logistical issues involved in organising and delivering the MDC service. Other MDC factors would need to be taken into account (e.g. delivering FV may not necessarily require the current vehicle and staff mix). Although the trial showed that FV is less costly than FS, the trial may not reflect real practice in terms of the ability to deliver FS as a one-off treatment compared with repeated applications of FV. Although the scenario analysis reported in Table 17 of our findings suggests that in such a context FS would be seen as less costly, the clinical effectiveness results achieved were under the trial conditions and, thus, appropriate caution must be placed on any interpretation that the same clinical benefits could be achieved in a different treatment protocol. The importance of child and school factors (such as turnaround of children from the classroom, having to fit with a school timetable and children not wanting the FV applied) would also need to be fully considered.
An important question to address next would be ‘what does this mean in practice in terms of service design, capacity and planning?’. There is an opportunity to take this research further by applying the trial results to an expansion of the health economic analysis to allow the modelling of plausible scenarios to support decision-makers and the dental clinical community, translating the findings from this trial into optimal models of service delivery for preventative dental health in children.
Strengths and limitations of the health economic analysis
This health economic analysis has been undertaken as part of a rigorous randomised trial to assess the clinical effectiveness and cost-effectiveness of FS and FV delivered within a MDC. Data were collected prospectively alongside the trial with the economic evaluation, which was an integral component of the trial design from the outset. It also benefits from reporting a budget impact analysis. Any changes to funding arrangements in health care over the next 5 years and the impact of any decision on local and national NHS dental budgets can now be informed by our findings. However, some limitations are acknowledged, particularly in relation to undertaking health economic evaluations in this context, and should be fully considered.
Consideration of the longer-term impact of fissure sealant versus fluoride varnish in preventative dental health programmes
Exploration of the longer-term costs and outcomes of the two technologies was an important consideration in developing the health economic analysis, given that this was done as a preventative intervention to improve the longer-term dental health of children who are at high risk of caries. A Markov model was built and validated with the trial clinicians as commensurate with the expected caries pathway as a result of having preventative treatment with FS or FV. The results show that FV remains dominant over FS, with this result remaining reasonably robust in the sensitivity analysis. However, caution must be exercised when interpreting these results.
The model-based analysis beyond the trial period was significantly hampered by the lack of evidence of the longer-term costs and outcomes in order to populate the model. The Cochrane review16 highlighted the lack of data available to compare FS and FV applications; only one study provided any data beyond our trial period,61 but this was limited by several biases, particularly the fact that only a limited number of children were followed up in the longer term. Only informed assumptions could be made about the longer-term costs and outcomes of FS versus FV based on clinical opinions from the trial team, informed by the trial results. Although it could be argued that, in the absence of good-quality evidence, the feasibility of modelling outside the trial period is questionable, this has been done to ascertain the framework for a model-based economic analysis and, importantly, the evidence that would be needed to populate the model. In the absence of data from previous trials, undertaking further follow-up of the trial cohort could provide an excellent opportunity to formally address the question of the longer-term clinical effectiveness and cost-effectiveness of FS versus FV.
Choosing and using appropriate health outcomes to inform the health economic analysis
An important issue that has arisen from this study is the need to give thorough attention to the outcomes of interest for the health economic analysis. A plethora of literature has examined oral health outcomes in the adult population but, in comparison, the evidence base for children is sparse. The strength of our evaluation is that we choose three outcomes that we expected to provide a comprehensive picture of costs and outcomes of the two technologies to support decision-making, both from a UK NHS decision-making perspective and in the clinical dental community.
Providing an estimation of the incremental cost per QALY is seen as the cornerstone of demonstrating cost-effectiveness within a UK NHS context. For example, the NICE reference case for health technology assessment57 highlights the inclusion of a QALY as its health outcome of choice. When the trial was originally conceptualised, a pragmatic decision had to be made on which measure to use, given the paucity of measures available for children and, in particular, the lack of evidence in the utility of generic HRQoL measures. We decided, after discussion with the developer and following recommendations made by the Health Technology Assessment funding panel, to use the CHU-9D, even though it had only just been published and its utility in a dental health context was still unknown. CHU-9D score was not measured at baseline; however, as this was a randomised trial, it is reasonable to assume that any differences between the two groups at that point were due to chance. The inability to consider utility curves over outcome time points only, rather than including baseline, could be seen to result in a reduction in power, but, given the preventative nature of this study (i.e. the study was not missing assessing utility during an episode of illness) and the fact that there were assessments of utility on multiple occasions, this is unlikely to be a major issue.
A study published since the start of the trial suggests that CHU-9D can be used in children aged 6 or 7 years. 53 That work, which considered the psychometric performance of the CHU-9D versus the EuroQol-5 Dimensions Youth version, suggested that children at this age can understand and complete the measure. This supported our own informal and limited evaluation of the CHU-9D that was undertaken prior to the start of the trial. The CHU-9D was assessed in the context of a child dental community clinic in New Zealand54 and was found to have the potential to capture HRQoL across different caries experience, showing that those with no apparent caries had a higher mean score than those with caries (0.88 vs. 0.87, SD 0.10), although the difference was small. Interestingly, this cross-sectional sample had lower HRQoL scores than our trial sample, although it is difficult to assess whether or not the patient populations are comparable. In terms of QALYs generated, this study reported that the difference in QALYs between those with and those without caries was 0.12. This is a fairly moderate difference, but it could suggest the potential of the CHU-9D to identify small but important changes in QALYs as a result of caries. Because of the lack of clinical differences in caries between our trial arms, we were unable to detect differences in utilities and QALYs. Subsequently, Foster Page et al. 69 undertook a randomised controlled trial to further assess the CHU-9D in terms of its responsiveness to changing components of the decayed, missing, filled tooth surfaces in primary dentition (dmfs) and DMFS scores in children aged 6–10 years over a 1-year period. They found that the mean CHU-9D improved from 0.88 to 0.9 from baseline to follow-up, with no significant difference found between caries status and CHU-9D. Again, CHU-9D scores from their study are lower than those in the present trial and lower than those in other studies, such as the 0.86 reported in the Canaway and Frew study,53 indicating that, as in this study, the impact of children receiving dental care under trial conditions cannot be ruled out. Similar to the later Foster Page et al. study,69 this trial suggests that the CHU-9D is insufficiently responsive to changes in caries experience.
An important question is ‘what could be done to improve measures aimed at capturing HRQoL in relation to this patient population?’. Whether the focus should be on generic HRQoL measures or on condition-specific measures is the subject of much debate, and it is important also to consider the context of this trial within a preventative/public health context. For example, the NICE public health guidance70 recognises the possible challenges of undertaking a CUA, and other approaches such as a cost–consequence analysis could be considered, although CUA is advocated (along with the need for HRQoL measures that can derive appropriate utilities) to ensure comparability across other parts of NICE.
The authors suggest that patient-reported outcome measures (including HRQoL) for use in a dental public health context should have a stronger conceptual and methodological basis. Understanding children’s preferences for treatment, the properties of measures and the association that these have with (1) dental-related health and (2) HRQoL warrants careful attention. Moreover, understanding and capturing the utility/disutility associated with caries experience over time will assist in ensuring longer-term examination of the cost-effectiveness of different strategies.
The cost-per-QATY analysis undertaken in this trial allows the consideration of a dental health state utility, which has been suggested as a more meaningful measure in the context of dental health. Although the QATY has been reported in studies,31,71 there has been little ‘methodological movement’ since the original work. 55,72 In this trial, published utilities were used alongside clinical opinion from the trial team to attribute a utility to (1) each individual tooth and (2) a composite QATY drawing on other studies31 and preliminary work undertaken as part of a NICE public health guideline. 56 The present analysis suggested that QATY gain was greater with FS than with FV, suggesting that FS could potentially be cost-effective. The results should be viewed with appropriate caution, given the approach taken to derive the number of QATYs. QATY gain appears to be a potentially useful dental health outcome, and further work is recommended to improve the underpinning work to derive suitable utilities for calculation of QATYs.
The importance of collaboration between health economists and the dental health research and clinical community will be instrumental to improving the quality of economic evaluations within preventative dental health.
Treatment acceptability
That so few participants withdrew from the study (as opposed to simply moving to a non-participating school) is in itself evidence that both treatments are acceptable. That is not a novel finding. These treatments have been in routine clinical use since the 1960s and have been received by a great many children around the world.
However, there are some interesting observations from the process evaluation reported in Chapter 5 that are likely to be of value in considering the future provision of these technologies in community programmes. Evaluation using the DTF scale correlated with the intensity of the treatment, with those receiving FV being significantly happier at baseline and this situation being reversed at the 30-month treatment session. This presumably reflects the fact that at baseline the FS-treated children had to spend longer in the dental chair than those receiving the FV treatment. The FS recipients were also subject to the use of three-in-one syringes and aspirators; in contrast, in the FV arm, treatment consisted of the simple act of placing cotton wool rolls in the mouth and painting the FV onto included FPMs. At the final treatment session, children in the FS needed treatment only if a FS had been lost, was judged to be incomplete or required top-up. In contrast, in the FV arm the time required in the chair was similar to that required for the baseline examination.
The feature of both treatments that was most remarked upon during the interviews was taste. This is interesting because in clinical teaching dental professionals, perhaps using ‘tell–show–do’ techniques, are taught to explain the sensations and experiences to a child before treatment. Clearly, an explanation of what may be expected in terms of taste when treating children is an important element of preparing them for treatment.
However, it is clear from the qualitative work carried out in this study that it is not so much the treatments themselves that children remember but rather the wider issues surrounding the treatments. Reward stickers, the movement of the dental chair (the chair was routinely reclined to allow children to have both the dental clinical examination and the treatment) and the coloured protective glasses that the children were required to wear all featured highly in their narratives of the experience. The novelty of the MDC visiting the school and the opportunity to have a break from the routine of daily life in the classroom also featured as a factor affecting acceptability. It was, however, interesting that in the later stages of the trial, when the children were aged 9 or 10 years, time away from the classroom and having to catch up on work did become a minor issue for some. In the course of the trial, the children would have visited the MDC on up to 10 separate occasions (four dental examinations and six treatment visits), so by the end it would have been a fairly routine experience. These findings about the broad range of factors that contributed to the acceptability of treatment for children indicate that delivery via a child-friendly service in a school setting is an important consideration for implementing this type of preventative dental treatment.
As this trial was based on the existing Designed to Smile programme, it is likely that the initial visit may not have been the child’s first experience of the MDC. Consent to participate in the trial was, however, considerably lower than the normal uptake of treatment offered by the CDS. The reason for this is unclear. Participating and non-participating parents who were interviewed were almost universally positive about the intervention, and reasons for non-participation are therefore likely to be a result of factors other than acceptability. A small number of parents appear to have not participated because their children were already receiving similar treatment and they wanted to continue with this. A more common reason for non-participation appears to be the difficulty of communicating effectively with parents via written information delivered through schools in areas of socioeconomic deprivation. Obviously it is necessary to ensure that parents and carers are adequately informed before making a decision about their child’s participation in a clinical trial and in particular in a CTIMP. The trial team worked hard with our PPI partners to ensure that the information was provided in as non-threatening and user-friendly a way as possible, for example by incorporating the logos and colours of the usual Designed to Smile programme patient-facing materials.
In the interviews exploring participation in the trial and in the piloting of materials, some interviewees discussed difficulties in English or literacy that may have affected the ability of parents to read and complete paperwork for the trial. This is likely to be a factor in participation rates in other areas of socioeconomic disadvantage or areas where there is a high proportion of residents whose first language is not English. A considerable amount of information was required to be offered to the parents and carers of potential participants. This would have been in addition to other paperwork that schools send home to parents; some interviewees mentioned that the volume of paperwork could also be a barrier for some parents, either because they did not keep track of it all or because there was too much to read. The information on details of the trial that had to be included in the paperwork for SoV may have contributed to this.
Trial fidelity
In any clinical trial, adherence to the clinical protocol (fidelity) is an important consideration. Aspects of this are now discussed.
Number of treatments received
In the course of the trial, participants were scheduled to receive treatment on six occasions. Clearly, the number of treatments received, whether application of FV at each visit or check-up or, if necessary, ‘top-up’ of the FS, is important. More than 70% of children attended all six treatment visits, and 95% of children in the FS and FV arms of the trial attended at least five treatment visits, which is excellent adherence to the scheduled treatment protocol. The CACE analysis demonstrated that such a high level of compliance means that adherence to the treatment protocol was not an influencing factor in the results obtained at 36 months. To obtain such a level of compliance reflects the relationship between the CDS team, the schools and their parents.
The CACE analysis was performed as a sensitivity analysis in order to explore the effect of attendance for treatment on treatment efficacy. A CACE analysis is preferable to a per protocol analysis, as it respects the trial randomisation, but is usually more suited to trials with two active arms rather than trials with one active trial arm and one control arm. Hence in SoV, the FS arm was selected as the ‘control’ treatment for the CACE analysis, as ideally it is a one-off treatment whereas FV needs to be applied regularly for full effectiveness. It was also the current standard treatment for children in these schools. The results indicated that, in fact, there was very little variation in treatment adherence, with the majority of children attending either five or all six FV treatments. As such, the CACE analysis indicated only slight gains in treatment efficacy with additional visits. In a wider context, this means that, for FV to be effective in the community as a caries prevention tool, treatment adherence needs to be ensured.
Treatment window
The six treatment appointments were scheduled at 6-month intervals over the course of the trial. Owing to the practicalities of scheduling visits of the MDC to schools to accommodate holidays and acknowledging that there were days when it was not convenient for the schools to host the MDC and so forth, it was agreed at the outset that a treatment window of 4 weeks either side of the treatment due date would not compromise the integrity of treatment delivery and would reflect the practical nature of a pragmatic clinical trial such as SoV. Over the course of the trial, adherence to this schedule was good. Overall, in 71.6% of schools, all treatment visits were within the treatment window, and in a further 26.1% of schools, only one of the six treatment visits was outside the schedule. This further indicates the fidelity of the trial.
Fissure sealant retention
The level of FS retention in this study was very high. This is because the study participants were seen at 6-monthly intervals and FS was ‘topped up’ if deficient. Furthermore, in the case of the maxillary FPMs, the teeth were sealed if part of the mesial–occlusal pit and fissure was erupted and the distal aspect was then sealed at a subsequent 6-month visit. At the 30-month treatment visit, only a small proportion of previously sealed teeth were reported as being ‘lost’, with 74% of upper teeth and 88–91% of lower teeth reported as ‘partial’, that is, sealant did not cover the whole of the occlusal surface.
Crossover
One deviation from protocol was observed. At the 12-month treatment visit, one child who had been randomised to the FV arm received FS. This was logged and the child continued in the trial in the arm to which they were randomised.
Examiner reproducibility
Throughout the trial, efforts were made to ensure that the dentists undertaking the clinical examinations were appropriately trained and calibrated. A professor of paediatric dentistry who was involved in the development of the ICDAS index trained the examiners at baseline and a second professor of paediatric dentistry undertook the annual training and calibration exercise before each round of examination began. The kappa scores achieved indicated that all examiners demonstrated a high level of intra-examiner reproducibility, and that inter-examiner reproducibility was also high.
Study considerations
From the issues previously discussed, the authors are of the view that this was a very robust and well-conducted study. The trial protocol was rigidly adhered to and compliance was excellent, as were follow-up and completion rates. Fidelity was as high as could possibly be expected. The trial team are content that there was nothing that they would have done differently in answering the question proposed at outset. Some aspects of the trial are considered further here.
Study numbers
The caries risk status of the participants is an important consideration. The SoV trial was set in areas of social and economic deprivation and more than half of the participants had decay into dentine in their primary dentition at the start of the study. The finding that approaching one in five of the participants experienced dental caries in at least one FPM at 36 months, across trial arms, makes clear that the study population was appropriate and typical of those whom the technologies tested are designed to benefit. The consistent finding from the regression analysis of caries experience in the primary dentition at baseline being a significant predictor of caries in the permanent dentition is as would be expected. 73 This of course did not affect the conclusions about differences in the relative caries-protective effect of FS and FV.
It is clear that the randomisation and stratification procedure employed was very successful and that the trial arms were well balanced at baseline.
The trial was more than adequately powered. This arose for the following reason. Calculations based on a previous study in South Wales and estimates of treatment effectiveness suggested that 313 participants were required per arm. The original intention was to recruit all participants in the same school year. However, this effectively meant recruiting all children in the period September–December in the first school term in order that the 6-monthly treatment visits could be accommodated. By December just over 500 children had been recruited, too few to satisfy the power calculations. With the funder’s support, recruitment was extended to a second school year. This resulted in two study cohorts, each of which was followed for 36 months. There was no observed cohort effect on the clinical outcome.
In planning the study, allowance was made for an 8% withdrawal per annum. In the event, many fewer children were lost from the study, with the most common reason for failure to complete the study being moving away from the area or to a different school that was not participating in the trial. When children moved to a participating school they continued in the trial. Recruitment to the trial in the second cohort proceeded at a much quicker pace than in the first year and so the study over-recruited by around 100 participants. The view was taken that, having invited the parents of the children to participate in the study, it would have been problematic to refuse the opportunity to participate to already consented children.
This, together with the much lower than expected dropout rate, meant that in both trial arms the number of children completing was in excess of the numbers required to satisfy the power calculation.
Questionnaire return rate
Information to inform oral health-related habits and the health economics utility scores was collected via a self-complete questionnaire. The efforts taken to maximise the return rate are described in Chapter 2. The revised follow-up strategy was found to boost return of the follow-up questionnaires, with the overall return rate of the DHQ rising to 60% for the whole trial.
Clearly, this limited the number of participants who were available for the secondary analyses, which were dependent on information on covariates gained via the questionnaire. However, there was no evidence of a difference in return rates between treatment arms. It should be remembered that this trial was conducted in Community First areas, which are areas designated by the Welsh Government as being of high social and economic deprivation. A response rate of 60% is probably as good as can be expected in this setting.
Adverse events
Care was taken in recruitment to the trial to exclude any participants with a known history of allergy to colophony, a constituent of the FV, and also those with severe asthma, that is, those who had ever been admitted to hospital overnight for the treatment of asthma. No adverse events were observed or reported in relation to the use of either FS or FV, and it is concluded that these treatments are suitable for use in children aged between 6 and 10 years.
Implications for health care
This is the largest ever study to address the question of the clinical effectiveness and cost-effectiveness of FS and FV. The very high standard to which this work was conducted and the excellent fidelity obtained mean that the findings reported here are robust.
Given that the previous systematic reviews of the clinical effectiveness of the technologies tested suggest that FS may be clinically superior, the results obtained in this trial are perhaps contrary to what many paediatric and public health dentists would have expected.
The SoV trial has clearly shown that, although at 36 months fewer than one in five children had developed decay into dentine in their FPMs, there was no clinically important difference in the proportion of children developing decay (D4–6MFT) on any FPM whether treated with FS or FV. That leads to the question of costs.
The heath economic analysis suggests that the costs favour FV. The budget impact analysis at 3 years showed that FV resulted in a cost saving of £68.13 per participant over the 3-year period compared with FS.
Both treatments are acceptable and the qualitative work undertaken has shown that factors other than the treatments themselves are uppermost in the children’s recollection of having received the treatments. The very low numbers of children stopping treatment is further proof of the acceptability of FS and FV when placed in the context of a community dental programme using MDCs. This type of intervention delivered in a school setting was also found to have high acceptability for parents and school staff.
Implications for research
There are number of research questions and issues that arise from this work.
The findings from this robust study will add to the evidence base of the Cochrane review of relative effectiveness of FS and FV. The Cochrane review should consequently be updated. 16
In the trial, the FS was applied when the FPMs first erupted and was maintained throughout the trial and the FV was applied at 6-monthly intervals. A key question, given the results obtained, and now that active treatment intervention has been discontinued, is whether or not the caries-protective effects of FS and FV will remain the same over the coming years. Presumably, the FS will remain for some months and years to come, at least in the depths of the pits and fissures. As a result, a difference in the preventative effects may become apparent after, say, a further 2 or 3 years. It would be useful to re-examine the participants in the school in 2017/18 in order to determine the effectiveness of the treatments after 5 and 6 years.
Logistics and cost limited this trial to a comparison between FS and FV. A third option that was considered by the research team was the inclusion of a third arm applying both FV and FS; this would have proven cost prohibitive. Whether or not such an approach is merited in future research is debatable.
In relation to trial fidelity, to our knowledge, this work is the first to apply CACE analysis in a dental trial. More work to understand this technique in the context of dental clinical trials with two active treatments would be advantageous.
Regarding the health economic element of the work, an important question is what could be done to improve measures aimed at capturing HRQoL in relation to this patient population. Whether the focus should be on generic HRQoL measures or condition-specific measures is a subject of debate, and it is also important to consider the context of this trial within a preventative/public health context. For example, the NICE public health guidance70 recognises the possible challenges of undertaking a CUA and that other approaches such as cost–consequence analysis could be considered, although CUA is advocated (along with the need for HRQoL measures that can derive appropriate utilities) to ensure comparability with other parts of NICE.
We suggest that there is a need for a greater conceptual and methodological basis to patient-related outcome measures (including HRQoL) for use within a dental public health context. Understanding children’s preferences for treatment, the properties of measures and the association that these have on (1) dental-related health and (2) HRQoL warrants careful attention. Moreover, understanding and capturing the utility/disutility associated with caries experience over time will assist in ensuring longer-term examination of the cost-effectiveness of different strategies.
The cost per QATY analysis undertaken in this trial allows the consideration of a dental health state utility, which has been suggested as a more meaningful measure in the context of dental health. There has been little ‘methodological movement’ since the original work. 55 In this trial, we used published utilities and sought clinical consensus to (1) attribute a utility to each individual tooth and (2) create a composite QATY drawing based upon other studies31 and preliminary work undertaken as part of a NICE public health guideline. 70 The present analysis suggested that FS derived more QATY gain than FV, suggesting that this could result in FS being potentially cost-effective. However, it is important that appropriate caution is exercised when considering these results, given the approach taken to derive the QATY. However, the QATY appears to be a potential useful dental health outcome and further work is recommended to improve the underpinning work to derive suitable utilities for the calculation of the QATY.
The importance of collaboration between health economists and the dental health research and clinical community will be instrumental to improve the quality and subsequently drive up the quality of economic evaluations within preventative dental health.
Conclusions
The findings of this trial demonstrate that in community oral health programmes targeted at children at high caries risk, the application of FV as a caries preventative measure will result in caries prevention that is not significantly different from that obtained by applying and maintaining FS after 36 months. There is a cost saving of £68.13 per child treated, using FV compared with the application of FS over this time period. Both treatments are acceptable to children aged 6–10 years; perceptions of undergoing treatment are influenced by aspects of treatment (especially taste) but also by wider factors associated with a child-friendly MDC. Acceptability to parents and to schools was also high.
Acknowledgements
The study team wishes to acknowledge grateful thanks to the schools, parents, carers and children who consented to participate in this study.
Contributions of authors
The following named authors contributed to the development of the research question and study design, study implementation (including members of the trial management group), analysis and/or interpretation of data and submission of the final report. Details of specific individual roles are below.
Ivor Gordon Chestnutt (Professor and Honorary Consultant in Dental Public Health; Co-Chief Investigator, lead applicant and the guarantor for the study) led the development of the research proposal, was the principal link between the research and clinical teams and led the final report preparation and submission.
Simon Hutchings (Trial Manager, December 2010 to December 2014) prepared the trial protocol, led permissions approvals and was a link between the Clinical Trial Unit and CDS.
Rebecca Playle (Senior Lecturer in Medical Statistics; Coapplicant and Senior Trial Statistician) led the development of the statistical plan and the analysis of the trial.
Sarah Morgan-Trimmer (Research Associate) was responsible for the qualitative element of the process evaluation.
Deborah Fitzsimmons (Professor of Health Economics; Coapplicant and Lead Health Economist) was responsible for the health economic analysis.
Nadine Aawar (Senior Trial Manager, April 2015 to March 2016) was responsible for trial governance-related issues.
Lianna Angel (Data Manager) was responsible for data management.
Sharron Derrick (CDS Manager, April 2014 to March 2016) liaised with schools and co-ordinated the clinical teams.
Cheney Drew (Trial Manager, April 2015 to March 2016) carried out trial management and was a link between stakeholders and regulatory bodies.
Ceri Hoddell (Senior Dental Officer; Coapplicant and Principal Investigator, Cardiff and Vale UHB and Clinical Advisor) was responsible for the oversight of clinical sites.
Kerenza Hood (Professor of Medical Statistics; Coapplicant and Head of the Clinical Trials Unit) contributed to trial design and was a statistical advisor.
Ioan Humphreys (Research Officer) was responsible for the Health Economics Analysis.
Nigel Kirby (Administrative Assistant, April 2011to June 2012) was responsible for administration during trial set-up, baseline and 6-month treatments.
Tin Man Mandy Lau (Junior Statistician) assisted with the statistical analysis.
Catherine Lisles (Data Manager) was responsible for data management and interim trial management duties.
Maria Zeta Morgan (Senior Lecturer in Dental Public Health) was responsible for the quantitative aspects of patient acceptability.
Simon Murphy (Professor in Public Health Improvement) was responsible for the oversight of quantitative elements of the work.
Jacqueline Nuttall (Senior Trial Manager, April 2011 to June 2013) was responsible for trial governance-related issues.
Kateryna Onishchenko (Research Assistant) was responsible for the Health Economics and developed the health economic model.
Ceri Phillips (Professor of Health Economics) was a health economics advisor.
Timothy Pickles (Statistician) was responsible for statistical analysis.
Charlotte Scoble (Administrative Assistant, July 2012 to March 2016) acted as administrative assistant in support of the trial.
Julia Townson (Senior Trial Manager, July 2013 to April 2015) was responsible for trial governance-related issues.
Beverley Withers (CDS Manager, April 2011 to March 2016) liaised with schools and co-ordinated the clinical teams.
Barbara Lesley Chadwick (Professor and Honorary Consultant in Dental Public Health; Co-Chief Investigator and guarantor for the study) led the clinical components of the research proposal and was responsible for the training and calibration of the study clinicians.
Study protocol
The protocol for this study has been published20 and is available at www.biomedcentral.com/1472-6831/12/51.
Trial Steering Committee membership
Professor Lorna MacPherson (Chairperson), Professor of Dental Public Health at the University of Glasgow.
David Thomas, Chief Dental Officer at the Welsh Government.
Dr Tanya Walsh, Lecturer in Dental Statistics at the University of Manchester.
Mrs Yasmine Pike, Service User Representative.
Independent Data Monitoring Committee membership
Professor Helen Worthington (Chairperson), Professor of Evidence Based Care at the University of Manchester.
Professor Chris Deery, Professor of Paediatric Dentistry at the University of Sheffield.
Mrs Nicola Williams, Primary School Head Teacher at Deri Primary School.
Data sharing statement
We shall make data available to the scientific community with as few restrictions as feasible, while retaining exclusive use until the publication of major outputs. Data will be available via the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res 2015;94:650-8. http://dx.doi.org/10.1177/0022034515573272.
- Improving Dental Care and Oral Health – A Call to Action. London: NHS England; 2014.
- Todd JE. Childrens’ Dental Health in England and Wales 1973. London: HMSO; 1975.
- Pitts N, Chadwick B, Anderson T. Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children: England, Wales and Northern Ireland. Leeds: Health and Social Care Information Centre; 2015.
- Locker D. Deprivation and oral health: a review. Community Dent Oral Epidemiol 2000;28:161-9. https://doi.org/10.1034/j.1600-0528.2000.280301.x.
- Watt RG, Listl S, Peres M, Heilmann A. Social Inequalities in Oral Health: From Evidence to Action 2016. www.icohirp.com/monograph.html (accessed 9 April 2016).
- Carvalho JC. Caries process on occlusal surfaces: evolving evidence and understanding. Caries Res 2014;48:339-46. https://doi.org/10.1159/000356307.
- Carvalho JC, Van Nieuwenhuysen JP, D’Hoore W. The decline in dental caries among Belgian children between 1983 and 1998. Community Dent Oral Epidemiol 2001;29:55-61. https://doi.org/10.1034/j.1600-0528.2001.00009.x.
- Chestnutt IG, Schafer F, Jacobson AP, Stephen KW. Incremental susceptibility of individual tooth surfaces to dental caries in Scottish adolescents. Community Dent Oral Epidemiol 1996;24:11-6. https://doi.org/10.1111/j.1600-0528.1996.tb00804.x.
- Hopcraft MS, Morgan MV. Pattern of dental caries experience on tooth surfaces in an adult population. Community Dent Oral Epidemiol 2006;34:174-83. https://doi.org/10.1111/j.1600-0528.2006.00270.x.
- Marthaler TM. Changes in dental caries 1953–2003. Caries Res 2004;38:173-81. http://dx.doi.org/10.1159/000077752.
- Ahovuo-Saloranta A, Forss H, Walsh T, Hiiri A, Nordblad A, Makela M, et al. Sealants for preventing dental decay in the permanent teeth. Cochrane Database Syst Rev 2013;3. https://doi.org/10.1002/14651858.cd001830.pub4.
- Murray JJ, Rugg-Gunn AJ, Jenkins GN. Fluorides in Caries Prevention. Oxford: Butterworth-Heinemann; 1991.
- Featherstone JD, Doméjean S. The role of remineralizing and anticaries agents in caries management. Adv Dent Res 2012;24:28-31. http://dx.doi.org/10.1177/0022034512452885.
- Marinho VCC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2013;7. https://doi.org/10.1002/14651858.cd002279.pub2.
- Ahovuo-Saloranta A, Forss H, Hiiri A, Nordblad A, Mäkelä M. Pit and fissure sealants versus fluoride varnishes for preventing dental decay in the permanent teeth of children and adolescents. Cochrane Database Syst Rev 2016;1. http://dx.doi.org/10.1002/14651858.CD003067.pub4.
- Hiiri A, Ahovuo-Saloranta A, Nordblad A, Mäkelä M. Pit and fissure sealants versus fluoride varnishes for preventing dental decay in children and adolescents. Cochrane Database Syst Rev 2010;3. http://dx.doi.org/10.1002/14651858.CD003067.pub3.
- Schlueter N, Klimek J, Ganss C. Efficacy of a moisture-tolerant material for fissure sealing: a prospective randomised clinical trial. Clin Oral Investig 2013;17:711-16. http://dx.doi.org/10.1007/s00784-012-0740-2.
- Chestnutt IG. Dental Public Health at a Glance. Oxford: Wiley Blackwell; 2016.
- Chestnutt IG, Chadwick BL, Hutchings S, Playle R, Pickles T, Lisles C, et al. Protocol for ‘Seal or Varnish?’ (SoV) trial: a randomised controlled trial to measure the relative cost and effectiveness of pit and fissure sealants and fluoride varnish in preventing dental decay. BMC Oral Health 2012;12. http://dx.doi.org/10.1186/1472-6831-12-51.
- Public Health England . Delivering Better Oral Health: An Evidence-Based Toolkit for Prevention. Third Edition 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/367563/DBOHv32014OCTMainDocument_3.pdf (accessed 9 April 2016).
- SIGN 138 Dental Interventions to Prevent Caries in Children – A National Clinical Guideline. Edinburgh: Scottish Intercollegiate Guidelines Network; 2014.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . Guideline for Good Clinical Practice 1996. www.ich.org/products/guidelines/efficacy/efficacy-single/article/good-clinical-practice.html (accessed 9 April 2016).
- Welsh Government . Designed to Smile 2016. www.designedtosmile.co.uk/home.html (accessed 8 March 2016).
- Pitts N. ‘ICDAS’ – an international system for caries detection and assessment being developed to facilitate caries epidemiology, research and appropriate clinical management. Community Dent Health 2004;21:193-8.
- International Caries Detection and Assessment System . International Caries Detection and Assessment System 2016. www.icdas.org (accessed 9 April 2016).
- Andrews F, Withey S. Social Indicators of Well-Being: American’s Perceptions of Life Quality. New York, NY: Plenum Press; 1976.
- Marshman Z, Hall MJ. Oral health research with children. Int J Paediatr Dent 2008;18:235-42. https://doi.org/10.1111/j.1365-263X.2008.00922.x.
- Hawkins R, Noble J, Locker D, Wiebe D, Murray H, Wiebe P, et al. A comparison of the costs and patient acceptability of professionally applied topical fluoride foam and varnish. J Public Health Dent 2004;64:106-10. https://doi.org/10.1111/j.1752-7325.2004.tb02736.x.
- Stevens K. Developing a descriptive system for a new preference-based measure of health-related quality of life for children. Qual Life Res 2009;18:1105-13. http://dx.doi.org/10.1007/s11136-009-9524-9.
- Bhuridej P, Kuthy RA, Flach SD, Heller KE, Dawson DV, Kanellis MJ, et al. Four-year cost-utility analyses of sealed and nonsealed first permanent molars in Iowa Medicaid-enrolled children. J Public Health Dent 2007;67:191-8. https://doi.org/10.1111/j.1752-7325.2007.00025.x.
- Birch S. Measuring dental health: improvements on the DMF index. Community Dent Health 1986;3:303-11.
- Treasure ET, Jones RJ, Chadwick BL, Playle R. Children’s cohort study: oral health in fluoridated and non-fluoridated areas. J Dent Res 2005;84.
- Altman DG, Bland JM. Treatment allocation by minimisation. BMJ 2005;330. https://doi.org/10.1136/bmj.330.7495.843.
- Brown S, Thorpe H, Hawkins K, Brown J. Minimization – reducing predictability for multi-centre trials whilst retaining balance within centre. Stat Med 2005;24:3715-27. http://dx.doi.org/10.1002/sim.2391.
- Welsh Index of Multiple Deprivation. Cardiff: Welsh Government; 2011.
- Standard Occupational Classification 2010 – Volume 2: The Coding Index. London: Office for National Statistics; 2010.
- Fischer K, Goetghebeur E, Vrijens B, White IR. A structural mean model to allow for noncompliance in a randomized trial comparing 2 active treatments. Biostatistics 2011;12:247-57. https://doi.org/10.1093/biostatistics/kxq053.
- Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Mejàre I, Lingström P, Petersson LG, Holm AK, Twetman S, Källestål C, et al. Caries-preventive effect of fissure sealants: a systematic review. Acta Odontol Scand 2003;61:321-30. https://doi.org/10.1080/00016350310007581.
- Chestnutt IG, Schäfer F, Jacobson AP, Stephen KW. The prevalence and effectiveness of fissure sealants in Scottish adolescents. Br Dent J 1994;177:125-9. https://doi.org/10.1038/sj.bdj.4808525.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- NHS Health Education England . Agenda for Change – Pay Rates n.d. www.healthcareers.nhs.uk/about/careers-nhs/nhs-pay-and-benefits/agenda-change-pay-rates (accessed 9 April 2016).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care 2015 2015. www.pssru.ac.uk/project-pages/unit-costs/2015 (accessed 9 April 2016).
- British National Formulary (online). London: BMJ Group and Pharmaceutical Press; n.d.
- NHS Reference Costs 2014/2015. London: Department of Health; 2015.
- Health in Wales . Pay &Amp; Conditions n.d. www.wales.nhs.uk/nhswalesaboutus/workingfornhswales/payconditions (accessed 9 April 2016).
- Office for National Statistics . Annual Survey of Hours and Earnings: 2015 Provisional Results n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2015provisionalresults (accessed 9 April 2016).
- Young TA. Estimating mean total costs in the presence of censoring: a comparative assessment of methods. PharmacoEconomics 2005;23:1229-42. https://doi.org/10.2165/00019053-200523120-00007.
- Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Economics 2011;20:897-916. https://doi.org/10.1002/hec.1653.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. http://dx.doi.org/10.2165/11599120-000000000-00000.
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. http://dx.doi.org/10.2165/11587350-000000000-00000.
- Canaway AG, Frew EJ. Measuring preference-based quality of life in children aged 6-7 years: a comparison of the performance of the CHU-9D and EQ-5D-Y – the WAVES pilot study. Qual Life Res 2013;22:173-83. http://dx.doi.org/10.1007/s11136-012-0119-5.
- Page LA, Thomson WM, Marshman Z, Stevens KJ. The potential of the Child Health Utility 9D Index as an outcome measure for child dental health. BMC Oral Health 2014;14. http://dx.doi.org/10.1186/1472-6831-14-90.
- Fyffe HE, Kay EJ. Assessment of dental health state utilities. Community Dent Oral Epidemiol 1992;20:269-73. https://doi.org/10.1111/j.1600-0528.1992.tb01697.x.
- Claxton L, Taylor M, Jenks M, Filby A. RX058: Economic Analysis of Oral Health Improvement Programmes and Interventions. Draft Report to the NICE Public Health Advisory Committee. York: University of York; 2014.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Quiñonez RB, Downs SM, Shugars D, Christensen J, Vann WF. Assessing cost-effectiveness of sealant placement in children. J Public Health Dent 2005;65:82-9. https://doi.org/10.1111/j.1752-7325.2005.tb02791.x.
- NHS Choices . NHS Dental Charges Explained 2016. www.nhs.uk/NHSEngland/AboutNHSservices/dentists/Pages/nhs-dental-charges.aspx (accessed 9 April 2016).
- Dental Checks: Intervals Between Oral Health Reviews. London: NICE; 2004.
- Bravo M, Montero J, Bravo JJ, Baca P, Llodra JC. Sealant and fluoride varnish in caries: a randomized trial. J Dent Res 2005;84:1138-43. https://doi.org/10.1177/154405910508401209.
- Welsh Government . Pupils Eligible for Free School Meals by Local Authority, Region and Year 2011. https://statswales.wales.gov.uk/Catalogue/Education-and-Skills/Schools-and-Teachers/Schools-Census/Pupil-Level-Annual-School-Census/Provision-of-Meals-and-Milk/pupilseligibleforfreeschoolmeals-by-localauthorityregion-year (accessed 9 April 2016).
- Highet G. Cannabis and smoking research: interviewing young people in self-selected friendship pairs. Health Educ Res 2003;18:108-18. https://doi.org/10.1093/her/18.1.108.
- Harrington KF, Binkley D, Reynolds KD, Duvall RC, Copeland JR, Franklin F, et al. Recruitment issues in school-based research: lessons learned from the High 5 Alabama Project. J Sch Health 1997;67:415-21. https://doi.org/10.1111/j.1746-1561.1997.tb01287.x.
- Ritchie J, Spencer L, O’Connor W, Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Scientists and Researchers. London: Sage; 2013.
- Salem K, Shahsavari F, Kazemnejad E, Poorhabibi Z. Pit and fissure sealant versus fluoride varnish in prevention of occlusal caries. J3D 2014;2:37-4.
- Liu BY, Lo ECM, Chu CH, Lin HC. Randomized trial on fluorides and sealants for fissure caries prevention. J Dent Res 2012;91:753-8. https://doi.org/10.1177/0022034512452278.
- Raadal M, Laegreid O, Laegreid KV, Hveem H, Korsgaard EK, Wangen K. Fissure sealing of permanent first molars in children receiving a high standard of prophylactic care. Community Dent Oral Epidemiol 1984;12:65-8. https://doi.org/10.1111/j.1600-0528.1984.tb01414.x.
- Foster Page LA, Beckett DM, Cameron CM, Thomson WM. Can the Child Health Utility 9D measure be useful in oral health research?. Int J Paediatr Dent 2015;25:349-57. https://doi.org/10.1111/ipd.12177.
- Methods for the Development of NICE Public Health Guidance (Third Edition. NICE: London; 2012.
- Zitzmann NU, Krastl G, Weiger R, Kühl S, Sendi P. Cost-effectiveness of anterior implants versus fixed dental prostheses. J Dent Res 2013;92:183-8. http://dx.doi.org/10.1177/0022034513504927.
- Fyffe HE, Nuttall NM. Decision processes in the management of dental disease. Part 1: QALYs, QATYs and dental health state utilities. Dental Update 1995;22:67-71.
- Li Y, Wang W. Predicting caries in permanent teeth from caries in primary teeth: an eight-year cohort study. J Dent Res 2002;81:561-6. https://doi.org/10.1177/154405910208100812.
- Pine CM, Pitts NB, Nugent ZJ. British Association for the Study of Community Dentistry (BASCD) guidance on the statistical aspects of training and calibration of examiners for surveys of child dental health. A BASCD coordinated dental epidemiology programme quality standard. Community Dent Health 1997;14:18-29.
- International Caries Detection and Assessment System (ICDAS) Coordinating Committee 2005. www.icdasfoundation.dk (accessed 9 April 2016).
Appendix 1 Research Ethics Committee approval
Appendix 2 Clinical trials authorisation: Medicines and Healthcare products Regulatory Agency
Appendix 3 Cardiff and Vale University Health Board Research and Development letter of approval
Appendix 4 Participant information sheet for parents (version 2.1)
Appendix 5 Medical history form (version 1.1)
Appendix 6 Informed consent form (version 2.1)
Appendix 7 Medical history follow-up form (version 1.2)
Appendix 8 Cardiff and Vale University Health Board Community Dental Service clinical protocol for application of Delton® Pit and Fissure Sealant
Appendix 9 Duraphat® summary of product characteristics
Appendix 10 Cardiff and Vale University Health Board Community Dental Service clinical protocol for application of Duraphat® fluoride varnish
Appendix 11 International Caries Detection and Assessment System training and calibration protocol
Appendix 12 Delighted–Terrible Faces scale
Appendix 13 Dental health questionnaire (version 2.2)
Appendix 14 Child Health Utility Index 9D questionnaire
Appendix 15 Serious adverse event form
Appendix 16 Dental hygienist observation scale
Appendix 17 Children interview schedule
Appendix 18 Participating parents interview schedule
Appendix 19 School/head teachers interview schedule
Appendix 20 Community dental staff interview schedule
Appendix 21 Non-participating parents interview schedule
Appendix 22 Schools questionnaire
Appendix 23 Non-participating parents questionnaire
Appendix 24 Participant withdrawal form (version 2.1)
Appendix 25 Dental nurse observation scale (version 1.0)
Appendix 26 F00 study eligibility case report form (version 2.3)
Appendix 27 C01 caries follow-up case report form (version 2.5)
Appendix 28 C02 caries re-examination case report form (version 2.6)
Appendix 29 F1S sealant baseline treatment record case report form (version 2.3)
Appendix 30 F1V varnish treatment record form (version 2.4)
Appendix 31 FTP treatment plan (version 1.2)
Appendix 32 F2S sealant follow-up treatment record case report form (version 2.4)
Appendix 33 Letter to school (version 1.0)
Appendix 34 Baseline examination follow-up letter (version 1.1)
Appendix 35 Clinical examination follow-up letter (version 2.0)
Appendix 36 Consent follow-up letter (LR) (version 1.0)
Appendix 37 Consent follow-up letter (UE) (version 1.0)
Appendix 38 Interim visits follow-up letter (version 2.0)
Appendix 39 Interim visits missed treatment follow-up letter (version 2.0)
Appendix 40 Annual examination follow-up letter (parent guardian) (version 1.0)
Appendix 41 Annual examination follow-up letter (dental practitioner) (version 1.0)
Appendix 42 Letter to child’s dentist withdrawal (version 1.0)
Appendix 43 Participant newsletter issue 1
Appendix 44 Participant newsletter issue 2
Appendix 45 Participant newsletter issue 3
List of abbreviations
- BNF
- British National Formulary
- CACE
- complier average causal effect
- CDS
- Community Dental Service
- CEAC
- cost-effectiveness acceptability curve
- CHU-9D
- Child Health Utility Index 9D
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CTIMP
- clinical trial of an investigational medicinal product
- CUA
- cost–utility analysis
- DHQ
- dental health questionnaire
- DMFS
- decayed, missing, filled tooth surfaces in permanent dentition
- DMFT
- decayed, missing, filled teeth in permanent dentition
- DTF
- Delighted–Terrible Faces scale
- FPM
- first permanent molar
- FS
- fissure sealant
- FSM
- free school meal
- FV
- fluoride varnish
- HRQoL
- health-related quality of life
- ICC
- intraclass correlation coefficient
- ICDAS
- International Caries Detection and Assessment System
- ICER
- incremental cost-effectiveness ratio
- ICF
- informed consent form
- ICH
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- ID
- identification
- MDC
- mobile dental clinic
- MHF
- medical history form
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MIH
- molar incisor hypomineralisation
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- p.p.m.
- parts per million
- PEB
- post-eruptive breakdown
- PIS
- participant information sheet
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QATY
- quality-adjusted tooth-year
- QoL
- quality of life
- REC
- Research Ethics Committee
- SD
- standard deviation
- SEWTU
- South East Wales Trials Unit
- SMM
- structural mean model
- SoV
- Seal or Varnish?
- TSC
- Trial Steering Committee
- UHB
- University Health Board
- WIMD
- Welsh Index of Multiple Deprivation
- WTP
- willingness to pay