Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR128829. The contractual start date was in January 2020. The draft manuscript began editorial review in April 2022 and was accepted for publication in November 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Hazelton et al. This work was produced by Hazelton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Hazelton et al.
Chapter 1 Introduction
The Perceptual disorders after stroke Intervention Evidence Review (PIONEER) final report describes the project’s three activities of scoping review, Cochrane systematic review and integration and priority setting with a stakeholder group. Chapter 1 provides background information on perception and the project with Chapter 2 giving an overview of the PIONEER project structure. Chapters 3, 4 and 6 outline the scoping, Cochrane Review and stakeholder engagement methodologies with Chapters 5, 7, 8 and 9 reporting the results. The final chapters summarise our findings and how much confidence we have in those findings (see Chapter 10), comparing them to the existing literature, considering the project’s strengths and limitations, and our recommendations for clinical practice and research (see Chapter 11). Sections of the report are reproduced from the PIONEER protocol1 which is available to download from the National Institute for Health and Care Research (NIHR) journals library, with associated published versions of the work referenced throughout.
Perception
What is perception?
Perception is the brain’s ability to integrate and interpret information detected by the different sensory systems including hearing (auditory), taste (gustatory), touch (tactile), smell (olfactory), somatosensory and visual systems. Somatosensation is a mixed sense, relating to temperature, pressure, vibration and body position (proprioception); some consider touch a component of this also. Perception involves multiple steps in processing sensory information: organising, assigning meaning and creating an understandable representation of the sensory landscape. 2 Perception is an umbrella term for various abilities, that are both successive and interactive. 3 In vision, for example, perceptual abilities range from perceiving simple physical characteristics in a scene, such as shape and colour, to ‘higher’ level skills including recognition and visuoconceptual processing. 4
PIONEER definition of perception
Detailed, working definitions of perception are a challenge, with variations in the scope and components included: consensus remains elusive. The delineation between perception and sensation, attention and cognition is one aspect of this – sensation and perception can be conflated, but perception can also be considered one of several cognitive abilities. 2,5 Conceptual differences often vary by disciplinary background, theoretical approach, research methodology and geographic location, and these also vary with time. The lack of an agreed definition of perception has been a challenge encountered by previous reviews of interventions for perceptual disorders after stroke. 6
Working with our Lived Experience and Clinical Expert stakeholder groups we explored the issue and developed a feasible, working definition for our project [see sections Stakeholder activities (what happened) and Types of participants: defining perception]. We used the World Health Organization’s (WHO) International Classification of Functioning, Disability and Health (ICF) definition of perception – the ‘specific mental functions of recognizing and interpreting sensory stimuli’7,8 (ICF code b156). This definition (1) provides a very clear distinction between perception and other closely related functions of sensation and cognition; (2) is applicable to all senses and not just vision; and (3) is internationally accepted. We excluded sensory disorders (ICF code b2) and disorders of attention (ICF code b140, encompassing visual neglect), which have a separate evidence base. 9,10
Using this definition meant automatic exclusion of a range of functions (‘consciousness, orientation, attention, memory; language; seeing; hearing; and additional sensory functions'). 7,8 We therefore excluded disorders relating to (1) sensation (ICF code b2; including visual field loss) and (2) attention (ICF code b140, encompassing visual neglect), which have been classified as ‘perceptual’ in prior research. While we recognise both are closely associated with perception, we considered each to be inherently different from it: sensation arises ‘earlier’ in the process of acquiring and processing visual information and it relates to detecting the presence of sensory information while attention is the ability to attend to sensory information. In addition, sensory and attentional disorders will present and will often be assessed and treated differently from perceptual ones. Further, both sensory and attentional deficits have existing, separate evidence bases. 9,10 Thus, a review that focuses on perception as defined by WHO provides an evidence base that is unique, and maximally clinically relevant.
Perceptual disorder aetiology
Perceptual disorders can arise from a range of conditions. Neurological aetiologies are prevalent, with perceptual disorders associated with sudden onset conditions of stroke or head trauma, and also neurological disease, including meningitis, Parkinson’s disease,11 Alzheimer’s and Lewy-body dementia. 12,13 In children, developmental disorders, including cerebral palsy,14 can affect perception, while increasingly research suggests that processing of sensory information, especially relating to vision and touch15 may be impacted among people with autism. 16 Psychiatric conditions13 including schizophrenia and depression are associated with visual, taste and smell disorders. 11
The PIONEER project focuses on perceptual disorders occurring because of stroke.
Perceptual impairment in stroke
Nature, incidence and prevalence
An estimated 100,000 people living in the UK have a stroke each year,17,18 a number forecasted to rise by 59% over the next two decades. 19 Over 1.2 million UK adults live with the long term-consequences of their stroke. 20 The average age of an individual with stroke ranges from 71 to 78 years, but around one-quarter of strokes now occur in a person of working age. 17 Overall, the presentation of stroke-related perceptual disorders and natural recovery is poorly understood. 21 While significant improvement may occur the first 6 months after onset22 longer-term prevalence data are highly variable. Available data suggest a wide range of prevalence figures, from 5% to 75% (see below). Using examples from somatosensation, we estimate that around one-fifth, or 240,000 stroke survivors in the UK may have a perceptual disorder. 23,24
Information on the incidence, prevalence and natural history of perceptual disorders after stroke has been neglected in research. We are aware however that deficits can affect a broad range of perceptual skills relating to an isolated sense, or alongside disorders in other sensory modalities.
Visual perceptual disorders
Visual perceptual disorders have perhaps been studied in greatest depth. Prevalence rates show much variation: disorders are self-reported by 5.2% of stroke survivors at the acute stage. 25 However, objective assessment suggests that visual perceptual disorders have a prevalence of 69% 1 month after stroke and in 74% at 2 years post stroke. 26 Deficits in recognition (agnosia) include visual objects, body parts, faces and non-verbal expressions. Spatial perceptual difficulties affect depth perception, location judgement and impair perception of motion. Stroke survivors may experience difficulties organising or integrating visual information, with a complex scene presenting difficulties for the stroke survivor’s identification of specific components, differentiation of foreground from background and deciding which parts belong together. 2,4,5
Hearing perception disorders
Hearing perception deficits may include difficulty with locating sounds, recognising auditory patterns, discriminating of speech from non-speech sounds, temporal aspects of auditory information (integration, resolution, ordering) and difficulty processing competing acoustic signals. 27 There are limited data on the prevalence of auditory perceptual deficits after stroke, as hearing is not routinely assessed; one case-controlled study of peripheral and central hearing loss reported a prevalence of 40% among younger (18- to 60-year-old) stroke survivors. 28
Smell and taste disorders
Stroke is also associated with perceptual disorders that present as smell dysfunction29,30 or taste impairment. 31 Almost one-third of stroke survivors may have some loss of taste, with 6% experiencing lateralised impairment of taste 1 week after stroke. 32 Smell dysfunction has been noted in 43% of stroke survivors a year after stroke, with odour perception reduced (29.7%) or absent (10.8%). 33
Somatosensation disorders
Somatosensation refers to sensation arising from the skin, muscles or joints,34 and includes perception of pressure, vibration, temperature and proprioception (kinaesthesia, joint position, movement, action and location). Stroke can cause deficits in one or a combination of these perceptual areas. Somatosensory impairment occurs in 34–63% of stroke survivors in the early phase (average 15 days post stroke), varying with the area of the body tested. 22
Touch disorders
Perception of touch is frequently impaired after stroke, reduced by up to 85% on the contralesional side in the acute and subacute phases;22,35,36 in the first 3 weeks up to 89% of stroke survivors can be affected,22 estimated to fall to 33% in the longer term. 35 Deficits can impair stroke survivors’ tactile recognition, including discrimination of texture, shape and length, and object recognition. 22,36
Impact of perceptual disorders after stroke
Perceptual disorders will reduce an individual’s ability to understand their environment and respond appropriately to it; for example, stroke survivors with visual perceptual disorders may not recognise family members, while spatial difficulties may cause disorientation and anxiety in busy environments, leading to reluctance to leave the home. 37 Visual perceptual dysfunction is associated with reduced abilities in activities of daily living (ADLs),38 greater disability, poorer quality of life (QoL)39 and can predict self-care difficulties. 40
Auditory perceptual disorders impact on listening skills and are likely to contribute to poorer auditory comprehension and communication abilities. Stroke assessments and interventions are typically based on healthcare professionals’ spoken instruction. Stroke survivors that have trouble communicating will also experience difficulties in diagnosis, and rehabilitation participation. 28,41,42
Taste dysfunction can lead to subjective unpleasantness when eating, impaired appetite, dietary changes, malnutrition and weight loss. Stroke survivors who are malnourished have poorer outcomes and require longer hospital admissions. 43,44 The inability to smell negatively impacts on eating, social communication and safety (e.g. detecting a gas leak). 45
Altered perception of the various components of somatosensation can lead to poorer performance of motor tasks, particularly control of fine motor skills in the hand (such as grip control, touch, pressure, proprioception),46 greater risk of accidents and injuries such as scalds and burns (temperature), increased incidence of falls28 (proprioception, learned non-use of limbs)47 and is linked with poor recovery of motor function and reduced independence in ADLs. 48 Those affected by altered somatosensory perception have greater activity limitations and longer hospital stays. 49
While there is evidence of the effect of perceptual impairments on stroke survivors’ rehabilitation outcomes and ability in everyday tasks, there is very limited exploration of the lived experience of such disorders, across the different senses. Stroke survivors and carers may well not recognise or understand that a problem they experience is due to impaired perception, and such find it a ‘puzzling and disabling.’50 Where perceptual disorders are not assessed and/or identified (see Poor documentation and variability) the nature of the issue can be mistakenly attributed to disorders of communication, memory, balance or motor skills. Where stroke survivors are aware of their perceptual impairment(s), they can have difficulty articulating their experiences of this51 but can detail the extra time and effort needed to accomplish tasks ‘You just have to be so methodical, so slow and it takes me forever to do stuff’52 and associated frustration. In addition, there are a range of emotional consequences: despair, anger, changes in self-confidence, feelings of worthlessness, vulnerability and changes in personal identity. 52 A number of online resources exist to inform and support carers and stroke survivors affected. 53,54
Interventions for perceptual disorders after stroke
The current literature offers some proposed interventions for the management of perceptual disorders after stroke: common to all six sensory areas are screening and assessment interventions to enable timely and accurate diagnosis. 28,33,55–57 Treatment approaches are primarily rehabilitative, aiming to compensate for the loss of function, but these vary depending on the sense affected and the nature of the dysfunction.
Therapeutic approaches to visual disorders may include sensory stimulation (visuo‐perceptual tasks),58,59 functional training (everyday tasks)60 and strategy training (alternate strategies to achieve goals) including the use of other senses to do so. 61–63 More recent interventions have used computer-based virtual reality training,64,65 incorporated visual and auditory feedback,66 or used transcranial direct current stimulation (tDCS) to stimulate the brain. 67 For auditory perception disorders, approaches may include environmental modifications, assistive listening devices,68 development of compensatory strategies or auditory training, which aims to improve the affected auditory process(es) through challenging listening tasks. 69
Few stroke-related olfactory and gustatory dysfunction treatments are reported:70 Pharmacological approaches have been considered,71 as well as referral to a dietitian for advice. 72 For impaired touch perception, interventions have focused on the upper limb, and include retaining sensory recognition and discrimination using specialist equipment. 73 Interventions for somatosensory perceptual deficit vary dependent on the specific function targeted but may include courses of sensory retraining using a range of stimuli,74 or targeted physiotherapy, which may incorporate robotic or highly specialised equipment. 75
Current services for perceptual impairment after stroke
Poor documentation and variability
Descriptions of current screening, assessment, treatment and referral pathways for stroke survivors with perceptual disorders are limited and variable. As perceptual impairments can affect all six senses, where services exist, they may be delivered by one of several healthcare professions (HCPs), including occupational therapists (OTs), physiotherapists, doctors, psychologists, orthoptists, audiologists and ear, nose and throat (ENT) services. HCPs and members of the public may be unaware of the range of perceptual impairments across hearing, smell, somatosensation, taste, touch and vision that may present after stroke and disorders may go unreported, under diagnosed or untreated. 43,76–78 Where UK service data are available, provision for visual disorders varies greatly76,79,80 with lack of standardised tests and procedures. 81 There are several barriers to effective service delivery, one of which is an evidence base on which to base treatment decisions. 79,80,82,83
Limited research informing clinical guidelines
Guidelines highlight the paucity of perceptual disorder intervention research on which to base clinical recommendations. 3,84,85 UK stroke clinical guidelines for adults (which are in update) refer to perceptual disorders, but not all sensory modalities are mentioned: three consider vision, one considers sensation (appearing to include tactile perception), and none make recommendations on hearing, taste or smell dysfunction. 3,84,85 The Royal College of Physicians (RCP) clinical guideline for stroke is the most comprehensive but focuses solely on visual perception, and agnosia (impaired object recognition), a specific visual disorder. The existing guidelines based their recommendations on a historic Cochrane Review6 which found no evidence of benefits for perceptual disorders after stroke.
Orthoptists’ clinical guidelines refer only to visual agnosia and hallucinations (recommending the provision of information) and the evidence underpinning these recommendations is unclear. 86 Paediatric stroke guidelines noted the absence of relevant research evidence and recommend the assessment of vision and hearing. Specialist support services and functional impacts, and tactile stimulation was also suggested for children with altered upper limb sensation. 87 These best practice recommendations were based on the opinion of the guideline development group.
Existing reviews of interventions for perceptual impairments in stroke
A comprehensive review of the evidence relating to all perceptual disorders after stroke has not been conducted to date. The reviews relevant to this topic have limitations as they may:
-
Include interventions for non-perceptual deficits, such as visual perceptual disorder reviews which include attentional deficits88 and those of touch and somatosensation which include sensory impairments. 89
-
Relate to a small subset of perceptual impairments within one sense, rather than considering all perceptual disorders relating to the sense as a whole. 90
-
Have a clear focus on exploring interventions for visual disorders, to exclusion of other senses.
-
Include non-stroke populations, making clinical interpretation challenging. 6
The need for this project
Across the six senses there are many interventions targeting perceptual disorders after stroke. Reviews of the evidence to date are limited. A lack of evidence and the resulting evidence uncertainties mean that stroke and rehabilitation clinical guidelines are unable to provide clinicians with evidence-based recommendations. Clinicians, aware of the limitations imposed on clinical practice by a lack of intervention research,62 have called for research to support both assessment methods and treatment approaches. 63 Perceptual disorders are likely to have an important impact on stroke survivors: identifying effective interventions for such impairments is a research priority in stroke rehabilitation and long-term care for stroke survivors, carers and clinicians. 91–93
The PIONEER study aimed to review the evidence of interventions for perceptual disorders following stroke, to highlight evidence of benefits, research gaps and future research priorities.
Aims and objectives
We aimed to identify, review and synthesise the evidence relating to interventions for the management of perceptual disorders following stroke. The three objectives were:
-
to identify all research (published and unpublished) relating to interventions for perceptual disorders after stroke, giving a comprehensive overview of the scope and nature of that evidence, and using this to highlight evidence gaps
-
to synthesise high-quality randomised controlled trial (RCT) evidence of the clinical and cost-effectiveness of interventions for perceptual disorders and appraise the quality of that evidence
-
to understand the implications of our findings for stroke survivors and HCPs working in this area and to determine future research priorities.
Chapter 2 Introduction
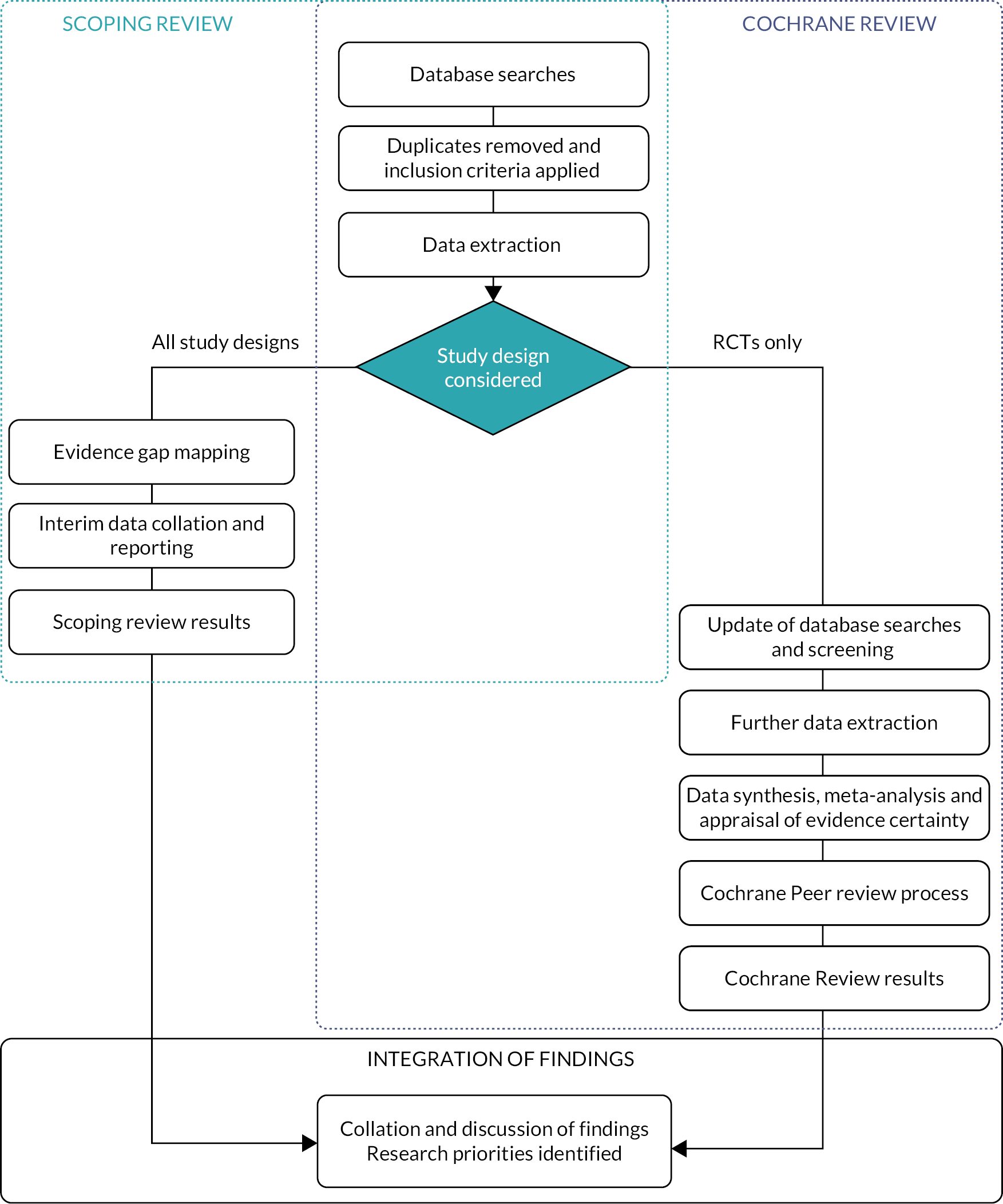
This chapter provides an overview of the PIONEER project, consisting of a scoping review, revision and expansion of a Cochrane Review and an integration and priority setting process (Figure 1). Active stakeholder involvement was integral to the project.
FIGURE 1.
Flow chart of the three project components.
Stakeholder involvement approach
To support involvement and coproduction of the PIONEER project we used a multifaceted approach, to maximise the quality, relevance and accessibility. The methods are detailed in Chapter 3, and involved:
-
coproduction with co-applicant (DJN) who has lived experience of perceptual problems after stroke;
-
Lived Experience Group: five volunteers with personal experience of stroke and associated perceptual problems or were a parent or carer of someone affected;
-
Clinical Expert Group: four clinicians with a range of expertise in relevant specialities that complimented the clinical expertise of the research team.
The project structure/overview
Systematic scoping review with evidence gap mapping
We undertook a scoping review of the literature to identify all evidence relating to interventions for perceptual disorders after stroke (see Aims and objectives). Scoping reviews map a broad field of literature, an approach ideally suited to this objective, given the variety of perceptual problems occurring post stroke, and the wide range of potential interventions.
Adhering to published guidance we followed a six-stage scoping review framework, including thorough searching and broad study design inclusion criteria. 94 We searched the relevant research comprehensively, working alongside our stakeholder groups and information specialist (JC) to develop a rigorous exploration of the existing literature. 95 We included all interventions, all participant age groups, settings, study designs (including quantitative and qualitative methods) and outcomes relating to interventions for perceptual disorders. Results were tabulated and summarised narratively. Details of the systematic scoping review methods (see Chapter 4) and results (see Chapter 5) are provided later.
Cochrane systematic review
We undertook a Cochrane systematic review and meta-analysis, exploring RCT evidence of the effectiveness of interventions for perceptual disorders after stroke (Objective 2). Cochrane Review methodologies provide the highest quality approach for synthesis of evidence intervention effectiveness. 96 We revised, expanded and updated an existing Cochrane Review6 published in 2011. We narrowed the review’s participant eligibility criteria to focus on stroke participant populations while expanded the intervention eligibility criteria to include all treatment approaches.
We identified, appraised and synthesised the relevant evidence from RCTs to determine where sufficient evidence exists of the benefits of a specific intervention for a perceptual disorder. We used the Template for Intervention Description and Replication (TIDieR) guidelines to maximise the clarity of our intervention data extraction and reporting97 and the grading quality of evidence and strength of recommendations (GRADE) approach to appraise evidence certainty. 98 Details of the Cochrane systematic review methods and results are provided in Chapters 6 and 7.
Integration and priority setting
We explored the implications of our scoping and Cochrane systematic review findings relating to interventions for people with perceptual disorders after stroke and the HCPs working with them (Objective 3). We aimed to maximise the relevance and applicability of the synthesised evidence to clinical practice and future research, and to identify any barriers to the uptake of that evidence. 99
Working with our Clinical Expert and Lived Experience Groups we interpreted our findings in the context of current clinical practice and stroke survivor experiences. 100 Using structured methods of involvement101 and priority setting,102 we agreed on the implications for clinical care in relation to: (1) stroke survivors and their carers; (2) HCPs providing care; and (3) policy-makers. We also prioritised the research gaps identified and developed recommendations for future research. Details of the methods and results are provided in Chapters 3 and 8.
Chapter 3 Introduction
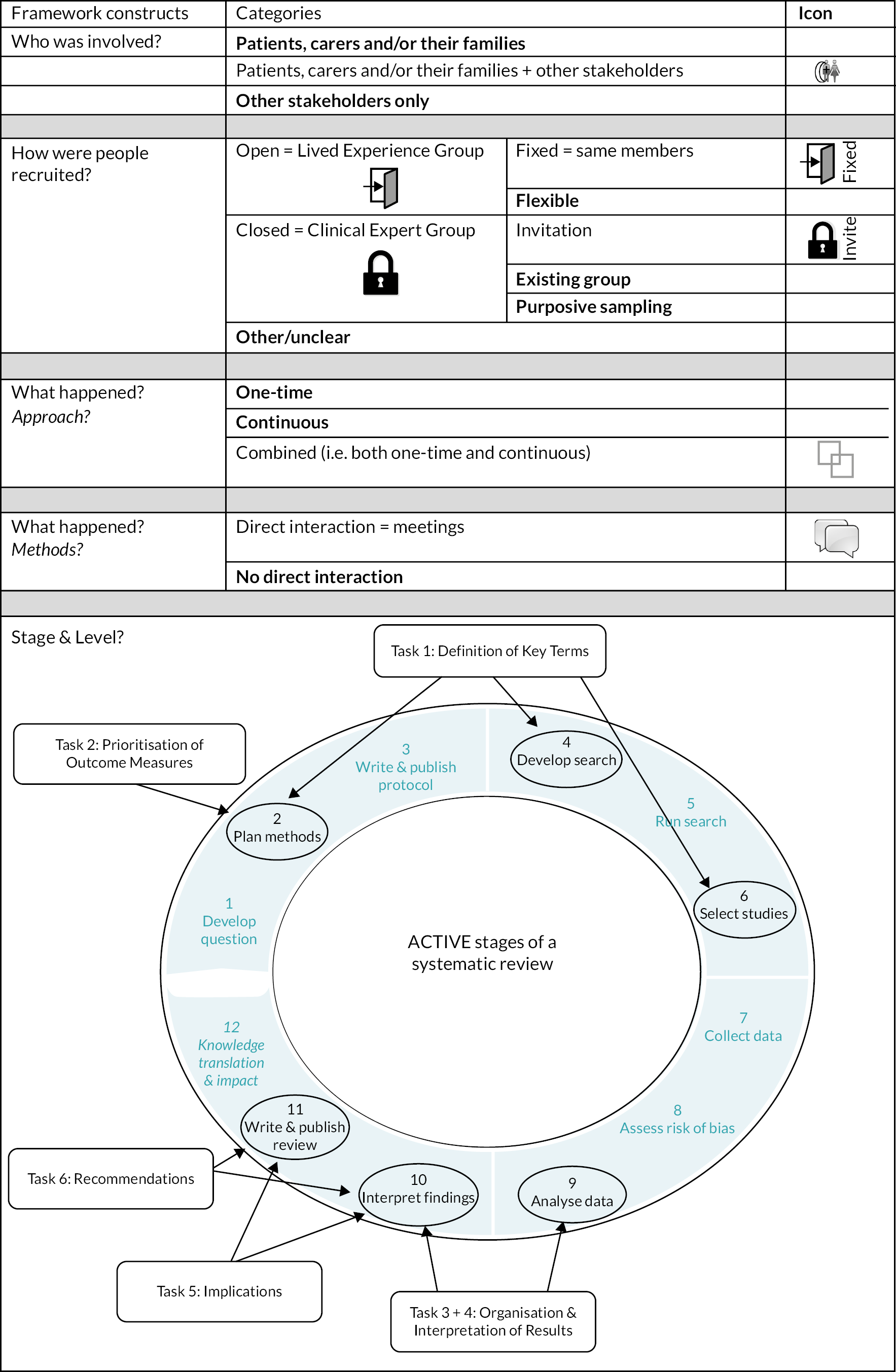
Chapter 3 details the stakeholder involvement throughout the PIONEER project; our approach, recruitment strategy, tasks undertaken, the level of stroke survivor, carer and HCP involvement and an evaluation of the stakeholder involvement impact. The chapter is structured based on the Authors and Consumers Together Impacting on eVidence (ACTIVE) framework103,104 and five stakeholder involvement in systematic reviews constructs: (1) who was involved, (2) how they were recruited, (3) when they were involved, (4) their level of involvement and (5) what happened. The methods involved in each stakeholder activity are also given, with contributions arising from our stakeholder involvement reported in relevant sections of Chapters 4, 5, 7 and 8; the impact of their contributions is described in Chapter 9.
Stakeholder involvement approach
To facilitate the contribution of a range of perspectives, experiences and knowledge, we used multifaceted stakeholder involvement including (1) a stroke survivor co-applicant, (2) a Lived Experience Group and (3) a Clinical Expert Group.
A co-applicant with lived experience of a stroke-related perceptual disorder (DJN) supported coproduction from project initiation: he was involved in the planning and conduct of all stages. His experience of systematic reviews relating to perceptual problems6 made an important contribution to the coproduction of the PIONEER scoping and Cochrane systematic reviews. People with experience of perceptual problems after stroke participated in a Lived Experience Group, and HCPs participated in a Clinical Expert Group. Both groups used a structured involvement approach based on (1) the ACTIVE framework,103,104 (2) the Involving People resources105 and (3) stakeholder involvement approaches used in previous systematic reviews. 101
Ethical approval and consent
United Kingdom guidance indicates that ethical approval is not required for stakeholder involvement activities106; however, as we planned to digitally record, store and report contributions made, we considered that seeking ethical approval was good practice. Glasgow Caledonian University’s School of Health and Life Sciences Nursing Department Research Ethics Committee granted approval (HLS/NCH/19/021). Written consent for the recording and reporting of anonymised data was obtained from stakeholders prior to the first meeting. Verbal consent for the digital recordings was given at the start of meetings. Data were anonymised and written-up, with electronic data stored securely.
Stakeholder training
We provided essential training, including an introduction to evidence-based practice and systematic reviews, to all those involved. We also signposted members to relevant online training on specific topics, for example Cochrane Review training, as the need arose. Individualised training sessions, coaching and mentoring were also available but not requested.
Stakeholder payment
The Lived Experience Group members were offered payment for their time to attend meetings and review of documents at NIHR-INVOLVE recommended rates. Payment for eligible expenses, such as travel, was also met.
PIONEER stakeholder involvement methods
Who was involved?
The Lived Experience Group were individuals aged 18 years or over, with personal experience of stroke-related perceptual problems, or as the parent or carer of a stroke survivor with perceptual problems.
The Clinical Expert Group were HCPs with expert knowledge of at least one sense addressed in this project. Clinical expertise was sought to complement that of the clinical research team, ensuring input relating to all senses, ages and healthcare settings, as well as a range of geographic locations.
How were people recruited?
Lived Experience Group
The opportunity to participate was advertised via our established stroke research stakeholder group (NMAHP Research Unit Stroke Research Advisory Group), and NIHR INVOLVE website [www.PeopleInResearch.org (accessed September 2022)]. Recruitment was based on an opt-in strategy, and replies were considered as consent to contact. Telephone conversations between the lead researcher (CH) and those who replied explored the individual’s experience of stroke and perceptual problems and the project’s terms of involvement. Volunteers who met the predetermined group profile and were interested in participating joined the group, until five members were identified.
Clinical Expert Group
We sought four HCPs through existing networks and recent publications, with expertise in visual perception, hearing perception, taste and smell and paediatric specialisms. We invited them to participate by e-mail.
When were they involved?
Stakeholder involvement can occur at any stage of a systematic review. 107 We used two involvement approaches: stakeholders participated in planned meetings at set time points, and informal communication occurring as needed throughout the review.
Planned meetings: these were held in person or online to inform decision-making for: planning methods, search development, selecting studies, analysing data, interpreting findings and writing the review (Figure 2). Stakeholders met virtually or in person at least every 3 months.
Informal communication: we provided regular updates to, and gained input from, the stakeholder groups throughout the project via e-mail or online updates. Individual stakeholders were available for consultation throughout and were contacted as required; for example, specific clinical experts were consulted regarding a study’s eligibility criteria during the study selection stage. Group members were invited to comment on draft abstracts, lay summaries and evidence gap maps via e-mail.
Level of involvement
We sought different levels of stakeholder involvement across the project:
-
Controlling aspects of the review process – we pre-planned that several decisions would be made by the stakeholder group working in partnership with the research team. For example, the stakeholders controlled decisions about the outcomes of interest.
-
Influencing the review activities and outputs – the stakeholders had a role in the review process and an opportunity to directly influence the review but stopping short of final decision-making control. For example, the stakeholders influenced the wording of statements relating to clinical implications.
-
Contributing throughout the review – stakeholders had the opportunity to take part in meetings, respond to e-mails and provide input which could indirectly impact on decisions made within the review.
We recorded group members’ involvement and impact at each review stage. We also asked stakeholders to describe their perception of involvement for each of their activities. We have reported involvement using the GRIPP2 tool. 108
Stakeholder activities (what happened)
Meetings adhered to the key principles of research coproduction, creating an environment that recognised everyone’s contributions and in which people worked together to achieve a shared understanding. 109 Ground rules were agreed at the start of meetings which highlighted respect, inclusivity and joint ownership of decisions. We used practical techniques that facilitated participants’ input (experienced facilitators, a timekeeper) and ‘devolved’ some decisions to the participants, ensuring that they had control over decision-making. 101
We sought stakeholders’ input on six planned review-related activities (see Figure 2):
-
definition of key terms
-
outcome measurement identification and prioritisation
-
interpretation of the scoping review results
-
interpretation of the Cochrane systematic review results
-
identifying clinical implications of the review findings
-
determining research gaps and priorities (see Report Supplementary Materials 1–8).
Activity 1: definition of key terms
We anticipated that operationalising the term ‘perception’ would be a challenge (see section Perception). The Lived Experience, Clinical Expert Groups and the research team sought a consensus agreement on a definition of perception and associated terminology and how these could be applied within the review process.
A full-day, face-to-face decision-making meeting was held at the project start (see Report Supplementary Material 1). Meeting participants were sent the WHO ICF perception definition (see PIONEER definition of perception) and draft definitions of key terms (such as visual, auditory, tactile, olfactory). Using a structured, facilitated discussion,110 participants considered each proposed definition before reaching a consensus definition using voting. 101 Discussions relating to disorders that were complex in nature, or their categorisation as a perceptual disorder was unclear, led to a range of a priori inclusion or exclusion decisions being developed. For each included sense, members recorded on flip charts all perceptual disorders that they were aware of, using clinical or lay terminology. Moving cards containing key terms created a potential taxonomy, which was captured by photographs. Definitions determined the project scope and the inclusion and exclusion criteria while the list of perceptual disorders informed our literature search strategy.
Activity 2: outcome measure identification and prioritisation
A prioritised list of outcome measurements for inclusion in the Cochrane systematic review was coproduced with the stakeholder groups. The impact of perceptual problems on life after stroke was explored by the Lived Experience Group during a videoconference, and impacts were listed (see Report Supplementary Material 2). E-mail correspondence finalised the generated list, with similar impacts grouped into ‘outcome measurement categories’ in collaboration with the research team and with reference to existing reviews. 6,9,111 The stakeholder groups and research team ranked the outcome measurement categories from most to least important. Rankings were pooled to form a shared list (see Report Supplementary Material 3), and the results informed the selection of outcome measurements included in the scoping and Cochrane systematic reviews.
Activity 3: organisation and interpretation of scoping review results
The stakeholders and the research team attended two videoconferences to discuss interpretation of the scoping review findings. Written review finding summaries were circulated by e-mail prior to meetings, which included presentations by researchers. Facilitated discussions were digitally recorded (see Report Supplementary Material 4).
Activity 4: organisation and interpretation of Cochrane Review results
The stakeholders and the research team attended a 2-hour videoconference to discuss interpretation of the Cochrane systematic review findings. The meeting included an introductory ‘What is a Cochrane Review?’ presentation, an evidence overview of the Cochrane Review data, followed by the systematic review results relating to hearing, taste, smell, touch, somatosensation and vision. Stakeholders shared their thoughts on the results for each sense during a facilitated discussion. The meeting concluded with a results overview and the stakeholders suggested what they considered was the overall result and implications. Meeting notes were supplemented with discussion, and captured using digital audio-recording (see Report Supplementary Material 5).
Activity 5: clinical implications
The stakeholders contributed to the project’s clinical implications based on the scoping review and Cochrane systematic review findings. They considered the findings from the perspectives of (1) stroke survivors and carers, (2) HCPs and (3) policy-makers. The stakeholders and the research team attended a 2-hour videoconference. Stakeholders received a written summary of the scoping and Cochrane systematic review results prior to the meeting, with review findings presented at the meeting. The Lived Experience Group discussed the results and the implications for stroke survivors and carers. The Clinical Expert Group and researchers considered the implications for HCPs. After regrouping, the meeting participants shared the key implications identified and agreed a list of implications for policy-makers. Notes taken during the meeting were supplemented with key discussion points captured in the digital audio-recording (see Report Supplementary Material 6).
Activity 6: research recommendations
Stakeholders sought consensus on the top research priorities for perceptual problems after stroke. During a 2-hour videoconference meeting, they generated a list of research gaps and questions relating to perceptual problems after stroke (see Report Supplementary Material 7). The research gaps were circulated by e-mail, providing an opportunity to contribute further. Questions relating to the broad topic of perceptual problems after stroke were then ranked by stakeholders from most to least important (see Report Supplementary Material 8).
Evaluation of impact of stakeholder involvement
After each meeting we asked stakeholders to provide their views about (1) what they felt they had contributed to and (2) how it impacted on the review. Throughout the project, we also documented any input from group members relating to project changes in response to their involvement.
A final meeting with the Lived Experience Group explored their views on involvement. They were provided with feedback from the research team on the impact of their involvement on the project and were asked to reflect on this and to discuss the impact that they considered they had made at different stages of the review process. They were asked what aspects of their involvement they felt had gone well, and what aspects they would change.
Summary
Our stakeholder involvement approach supported coproduction of six key tasks: defining key terms, prioritising outcome measures, interpreting the scoping and Cochrane Review results, agreeing clinical implications and identifying and prioritising research recommendations. This was supplemented by the less formal process of e-mail communication and input as needed. The results and outcomes of specific tasks are detailed in the relevant chapters; our evaluation of the impact of stakeholder involvement is presented in Chapter 9.
Chapter 4 Scoping review methods
Overview
This chapter details the rigorous methods used to scope and identify all research evidence (published and unpublished) relating to interventions for perceptual disorders after stroke, giving a comprehensive overview of the breadth and nature of that evidence, and using this to highlight evidence gaps. We present the scoping review framework used, the study inclusion criteria, the search and data organisation methods used. The data synthesis approach, interpretation of the findings and evidence gap mapping methods are also detailed below. This review has been published in the journal Stroke. 112
Introduction
Scoping reviews scope and systematically map the literature in an area that may not have previously been reviewed113 such as stroke-related perceptual disorders. This scoping review aimed to identify all available evidence relating to interventions for the management of perceptual disorders following stroke, providing an overview of the range, number and type of interventions, supporting the development of evidence gaps.
We employed Arksey and O’Malley’s six-stage scoping review framework94,114,115 to ensure rigour and transparency:
-
identifying the research question
-
identifying the relevant studies
-
study selection
-
charting the data (data extraction)
-
collating, summarising and reporting
-
consultation.
Two framework stages were augmented to maximise the relevance and accessibility of our results. Evidence gap mapping was added to stage 5 to enable visualisation of the evidence summaries and gaps identified116 and the ‘consultation’ process involving stroke survivors, carers and clinical experts occurred throughout the review, rather than only at the end. The scoping (and systematic review) protocol was registered with the PROSPERO database CRD42019160270, as well as published online1 and our reporting was supported by the relevant reporting guidelines. 117
Criteria for considering studies for this review
The study inclusion and exclusion criteria are given in Table 1 with definitions provided in Box 1.
| Study | Inclusion | Exclusion |
|---|---|---|
| Design |
|
|
| Participants |
|
|
| Intervention |
|
|
| Comparator |
|
|
| Outcomes |
|
|
Prioritised outcomes of interest:
|
-
Hearing (auditory) – processing and understanding auditory (hearing) information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret auditory information.
-
Smell (olfactory) – processing and understanding smell (olfactory) information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret olfactory information.
-
Touch (tactile) – processing and understanding touch (tactile) information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret tactile information.
-
Somatosensation [temperature, pressure and body position (proprioception/kinaesthesia)] – processing and understanding somatosensory information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret somatosensory information.
-
Taste (gustatory) – processing and understanding taste (gustatory) information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret gustatory information.
-
Vision – processing and understanding visual information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret visual information.
Types of participants: defining perception
The definition of perception was determined via discussion and voting with our stakeholder groups [see Stakeholder activities (what happened), Activity 1 and Report Supplementary Material 1]. Members agreed to use the WHO ICF definition of perception ‘specific mental functions of recognizing and interpreting sensory without amendment or addition stimuli’, with the creation of a lay definition of ‘processing and understanding information from the senses’ to assist with clearly communicating findings. Members discussed, created and voted to accept definitions of the individual senses included (see Box 1). It was decided that although touch could be considered a component of somatosensation this would be treated as distinct, to support the accessibility of the findings since touch is one of the ‘traditional’ five senses.
Discussion between the research team and stakeholders led to a priori decisions relating to the inclusion of specific disorders that were either complex in nature or their categorisation as a perceptual disorder was unclear. These covered Pusher syndrome (a disorder of perception of body position)118 (included), hallucinations (included); balance, vestibular disorders and neglect (excluded). Studies that combined stroke and non-stroke populations were included and coded to indicate they were a mixed population (see Participants included). Studies that combined perceptual impairments with other disorders such as sensory or cognitive impairments were included and coded to indicate this (as perceptual, perceptual-sensory, perceptual-cognitive, mixed or unclear if the exact perceptual impairment could not be identified).
Types of interventions
Where interventions addressed perceptual disorders across more than one sense (e.g. smell and taste), these were included and coded to indicate the mixed grouping.
Types of outcome measures
Seventeen outcomes of interest were identified in collaboration with our stakeholder groups [see Stakeholder activities (what happened), Activity 2 and Report Supplementary Material 2]. These were prioritised by stakeholders and the research teams (see Table 1).
Search methods for identification of studies
We comprehensively searched the literature to identify all relevant studies across all six senses: the terms used were based on prior searches6 and the disorders identified by the research and stakeholder teams [see Stakeholder activities (what happened), Activity 1 and Report Supplementary Material 1]. Search terms were drafted and refined by a stroke-specific information specialist (JC) and peer reviewed using current standards119 (see Appendix 1 for MEDLINE search terms and Report Supplementary Material 9 for full searches).
Electronic searches
Electronic bibliographic databases and clinical trial registers were searched from inception (unless otherwise indicated) to 7 February 2020 including the Cochrane Stroke Group Register, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library (2020, Issue 1), MEDLINE (Ovid; from 1996) and EMBASE (Ovid; from 1980) (see Report Supplementary Material 9).
Searching other resources
We also conducted an extensive search for published and unpublished studies, via:
-
OpenGrey [www.opengrey.eu/ (accessed September 2022)]
-
Grey Matters: a practical tool for searching health-related grey literature [www.cadth.ca/grey-matters-practical-tool-searching-health-related-grey-literature (accessed September 2022)]
-
Google Scholar [https://scholar.google.com/ (accessed September 2022)]
-
NIHR Clinical Research Network [www.nihr.ac.uk/explore-nihr/support/clinical-research-network.htm (accessed September 2022)]
-
Physiotherapy Evidence Database (PEDro) [https://pedro.org.au/ (accessed September 2022)]
-
OTseeker [www.otseeker.com/ (accessed September 2022)]
-
PROSPERO International prospective register of systematic reviews [www.crd.york.ac.uk/prospero/ (accessed September 2022)].
National and international guidelines, government, HCP, relevant charities and patient support organisations websites were searched. Research, professional associations, foundations and experts in the field were also contacted (see Report Supplementary Material 10). An initial database search identified few paediatric studies. In consultation with a paediatric specialist (LD) and reflecting the iterative nature of scoping review searches, further sources specific to children were searched (see Report Supplementary Material 10). Forward citation tracking using Science Citation Index and Google Scholar was also completed (last searched 24 November 2020) together with searching reference lists of included studies.
Selection of studies
Applying selection criteria
Searches were imported into EndNote software (v8) and de-deduplicated. 120 Titles were screened for inclusion with ineligible titles or duplicates excluded (KMcG). Potentially relevant abstracts were independently screened using Covidence systematic review management system (KMcG, CH). Based on the inclusion criteria, abstracts were independently categorised as ‘relevant’, ‘irrelevant’ or ‘unsure’. Studies ranked as irrelevant by both reviewers were excluded. The full text of the remaining studies was retrieved and assessed independently (KMcG, CH). Where disagreement occurred, or a study was categorised as unsure, an expert in that sensory area was consulted. Exclusion reasons were recorded and reported. 117
Screening of grey literature or studies identified during supplementary searches was conducted by one researcher (KMcG, PC or CH) and key identifiers were entered into Microsoft Excel®. Details were checked by a second researcher.
Perception terminology and decision-making
Perceptual terminology is complex (see PIONEER definition of perception) and specific challenges we encountered in determining whether a study population had a perceptual disorder included:
-
the lack of universal agreement on a definition of perception and the need to determine authors’ precise meaning, and delineation between disorders of perception, sensation, attention and cognition
-
differences in terminology between the senses, populations (adult and paediatric) and disciplinary fields.
Challenges were compounded by poor reporting of the disorder – both whether a study population had a perceptual disorder and whether an intervention addressed a perceptual disorder. Reviewers considered all relevant and available information, including reported details on type and location of stroke, the tools used to assess perceptual function and theoretical frameworks underpinning intervention design when distinguishing perceptual from other disorders. The key phrase ‘distinguish, discriminate, recognise and interpret’ from our coproduced definition [see Stakeholder activities (what happened)] supported the determination of whether a disorder reported related to perception or sensation: specifically, we sought evidence of processing of sensory information as opposed to simply detecting the presence or absence of sensory stimulation. Our research team and Clinical Expert Group specialists provided valuable third reviewer input as required in applying our inclusion criteria.
Data charting and categorising
Data extraction forms
Data extraction forms were developed in Microsoft Excel, based on the TIDieR checklist97 and forms used in prior complex evidence syntheses. Data extraction forms were independently piloted (CH, KMcG) on five studies with different research designs, populations and interventions. Completed forms were then discussed and refined to ensure that all relevant data were extracted in an efficient manner. Data charting was completed (KMcG) and cross-checked (CH). Where studies included mixed populations, stroke-specific data were extracted where possible.
Data items extracted
We extracted the following data for each included study:
-
Demographics: author, year of publication, type of publication, country.
-
Design and methods: aim, design, number of recruitment sites, stakeholder involvement, participant numbers, withdrawals or lost to follow-up.
-
Participant characteristics: age, sex (% female), stroke severity measurement, stroke type, hemisphere affected, other stroke-related impairment, time since stroke, inclusion of non-stroke survivors (n).
-
Perceptual disorder: sense, diagnosis.
-
Intervention and comparator characteristics: details extracted using the TIDieR checklist,97 including number of interventions in the study, intervention approach, theory supporting the intervention, materials used, reporting of intervention procedure, who provided the intervention, mode of delivery, location, duration, single/multiple sessions, adaptation, other interventions tested, if usual care was provided.
-
Assessed outcomes: outcome measures/tools for each eligible outcome; other outcomes specified and reported and data collection time points.
-
Additional qualitative data: we planned to extract any descriptive themes relating to intervention effect/impact, costs or implementation, including the name and description of the content and meaning. 121
Intervention categorisation
Selected data were categorised using drop-down menus within the data extraction form, to facilitate interpretation and maximise the clarity of reporting. Intervention categorisation was based on the underlying therapeutic approach and used an established approach. 32,33
Interventions were categorised as:
-
pharmacological – relating to the use of drugs
-
surgical – relating to suture, incision, excision, manipulation or other invasive procedure that usually, but not always, requires local, regional or general anaesthesia122
-
non-invasive brain stimulation (NIBS) – technologies and techniques used to modulate the excitability of the brain via transcranial stimulation (such as tDCS)123
-
rehabilitation – designed to optimise functional ability and reduce disability in individuals with health conditions, in interaction with their environment. 124 Rehabilitation interventions were subcategorised as:
-
restitution (direct training of the impaired function)
-
compensation (via training to use a spared function)
-
substitution (use of an external device or modification)125
-
mixed (a combination of the above approaches).
-
Categorisation was undertaken by a researcher (KMcG), checked by a second (CH) with expert input as required (DG, SMH). See Report Supplementary Material 11 for full list of data categorisation options.
Critical appraisal of individual sources of evidence
In keeping with scoping review guidelines,117 we undertook no critical appraisal of included study quality, reflecting the scoping review aim of identifying and mapping available evidence.
Data synthesis
Collating and summarising the results
We collated the evidence identified in the scoping review into tables using the extracted and charted data. 94,95 Data were organised by study characteristics (design, year, continent), population (side of stroke, duration of stroke, sense affected, age, sex) and intervention (using TIDieR-based descriptors including approach, materials, who delivered, modality, location, duration, number of sessions, tailoring). The frequency of outcome measures across included studies was also tabulated. We created graphs and numeric summaries. Comparisons explored the nature and breadth of the findings, and identified key areas where data were poorly reported or absent. Results were discussed with the Lived Experience Group [see Stakeholder activities (what happened)].
Reporting the results
We created narrative data summaries94 based on the following:
-
The number and characteristics of included studies, study design, year of publication and country of origin.
-
The participant population, including the details of their stroke and the senses affected.
-
Intervention summaries were created reflecting the key TIDieR97 checklist categories and grouped according to the sense affected.
-
The nature and number of outcomes in each category were presented, noting gaps.
Interactive evidence gap mapping
A series of interactive evidence gap maps116,126,127 provided a simple and accessible visual summary of the evidence using Tableau and Evidence for Policy and Practice (EPPI)-mapper software packages. Maps were shared with the Lived Experience Group to elicit their opinions, identify the most important information and how best to organise the information. Group members preferred a 2 × 2 matrix (in EPPI-mapper), which showed the relationship between the volume of evidence for an intervention category and the associated study outcomes. Group members recommended that the maps should be uncluttered, but sufficiently detailed to provide a legacy database, supporting future perceptual disorder research. Evidence gaps were highlighted.
Interpreting the findings
Our Clinical Expert and Lived Experience Groups informed our interpretation of the scoping review findings via online discussion [see Stakeholder activities (what happened) and Report Supplementary Material 4].
Summary
The scoping review aimed to identify all published and unpublished research evidence relating to interventions for perceptual disorders after stroke and to provide a comprehensive overview of the range, focus and nature of that evidence and in turn to highlight any evidence gaps. We used a systematic six-stage scoping review process to ensure rigour. Perception and perceptual disorder definitions were agreed with our stakeholders. Our peer-reviewed search involved bibliographic databases and grey literature, with search terms created by an information specialist. Identified titles were screened by a reviewer, potentially eligible abstracts and full texts were independently screened by two reviewers, with topic-specialist input as needed. Data on study, participants, interventions and outcomes were extracted and categorised using Excel sheets. Data were tabulated and numerical and narratively summarised. Evidence maps displayed our findings and highlighted evidence gaps.
Chapter 5 Scoping review results
Introduction
In this chapter we report the scoping review findings, which aimed to provide a comprehensive overview of the scope, nature and gaps in the evidence relating to interventions for perceptual disorders.
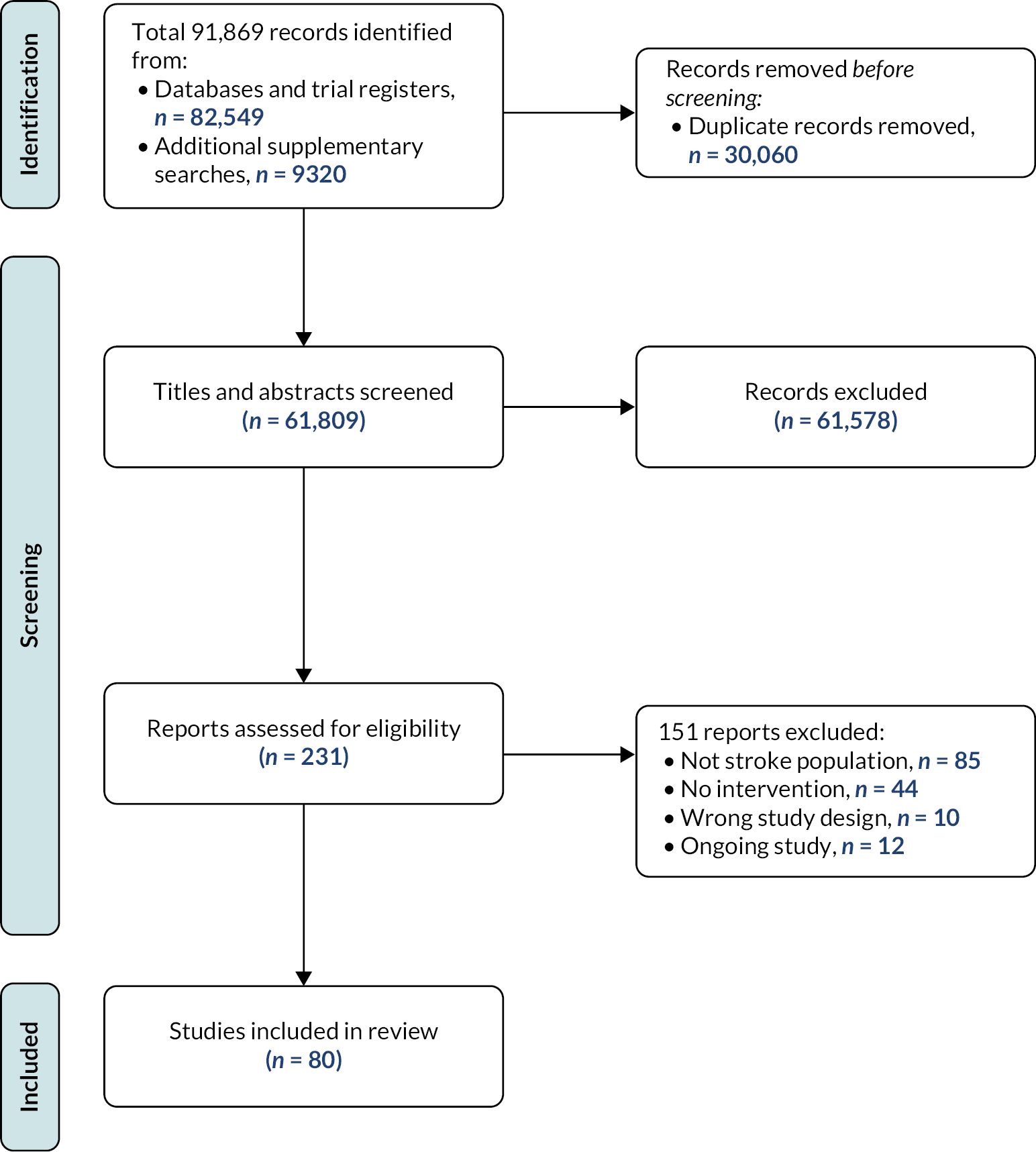
Scoping review search results
Of 91,869 identified records, we removed duplicates, studies that did not meet inclusion criteria such as those based on a non-stroke population, no perceptual disorder intervention and ineligible design. We also excluded ongoing studies (Figure 3). A total of 80 relevant studies were identified (see Appendix 2).
FIGURE 3.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis128 diagram for scoping review literature identification. Note: figure is based on one published in Stroke. 112
Reproduced with permission from Hazelton et al. 112 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
Included studies
Design and location
Included studies were predominantly case reports (45%, 36/80) or RCTs (27.5%, 22/80) but also included N-of-1 designs (11.2%, 9/80), cohort studies (11.3%, 9/80) and controlled trials (2.5%, 2/80). No qualitative studies were included. Studies were conducted in Asia (33.8%, 27/80), Europe (32.5%, 26/80), North America (18.8%, 15/80), Australia (8.8%, 7/80) and South America (3.8%, 3/80). The setting for two studies (2.5%, 2/80) was not reported.
Recruitment
Few (41.2%, 33/80) reported recruitment data, perhaps reflecting the prevalence of single-participant studies (43.7%, 35/80). Only 5% (4/80) described recruitment over multiple sites.
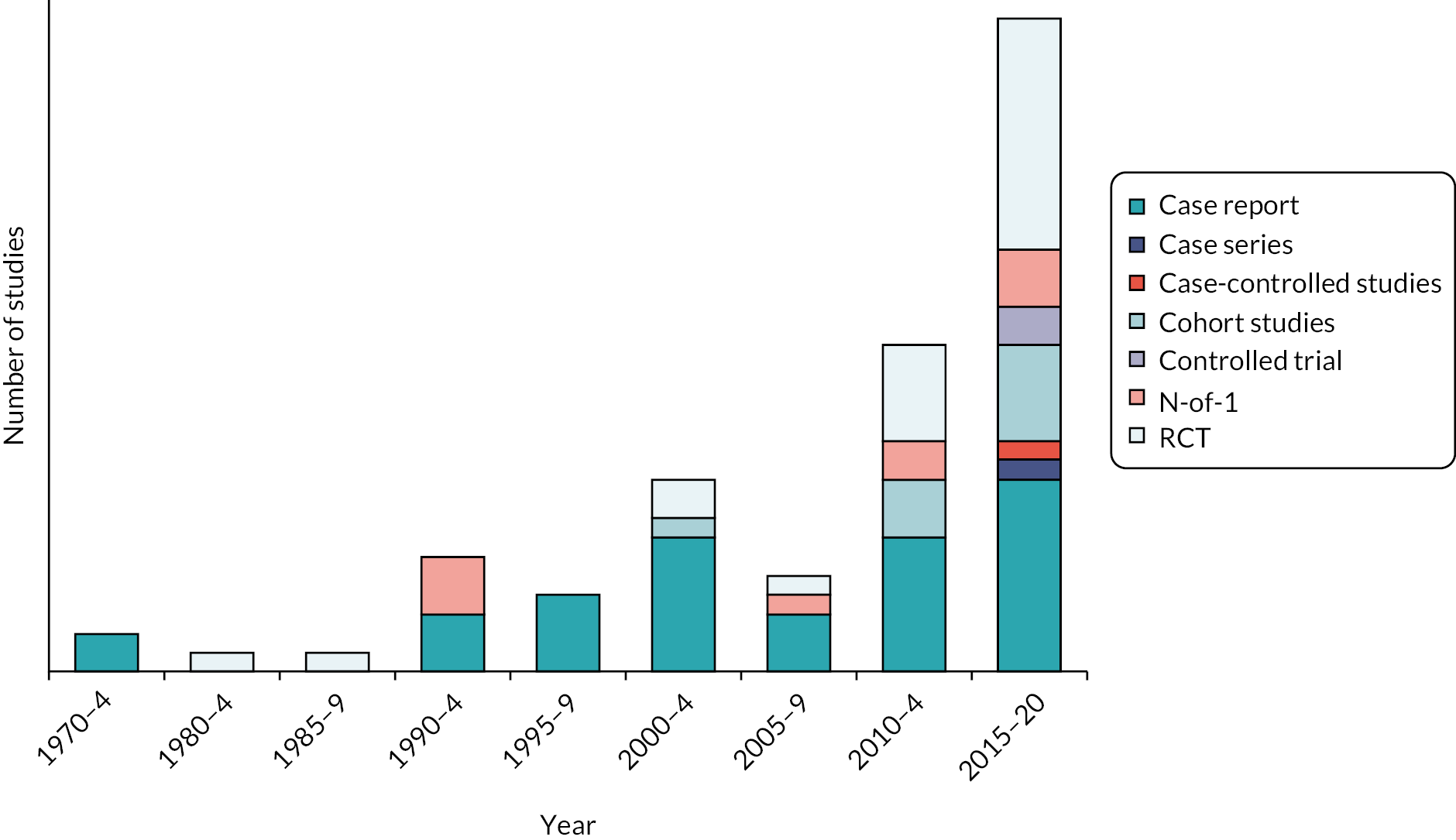
Age of data
All 80 studies were published between 1973 and 2020: 34 (42.5%) in the last 5 years and 51 (63.8%) in the last 10 years. Most RCTs (12/22, 54.5%) were published between 2015 and 2020 (Figure 4).
FIGURE 4.
Studies included in the scoping review: study design and year of publication. Note: figure is based on one published in Stroke. 112
Reproduced with permission from Hazelton et al. 112 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
Attrition
Where reported, participant withdrawal and loss to follow-up was low; 28 (35%) reported no withdrawal, 3 (3.8%) reported 1 withdrawal, 2 (2.5%) reported 2 withdrawals, 1 (1.3%) reported 4 withdrawals and 1 (1.3%) reported 7 withdrawals.
Stroke survivor or family involvement in research
Stroke survivor or carer involvement was not reported in any studies’ design or implementation.
Participants included
We included 80 studies (n = 922) in the scoping review. Eight studies recruited mixed participant populations: four recruited stroke survivors with and without perceptual disorders; four recruited participants with perceptual issues following stroke and other aetiologies. We were able to extract the data specific to those with perceptual disorder following stroke from five studies (n = 29), but this was not possible for data from three studies (n = 24) were more problematic. The scoping review data reflect information from 893 participants, of which 869 (97.3%) were stroke survivors with stroke-related perceptual disorders.
The largest sample size was 80 participants [median = 3.5, interquartile range (IQR) 1–16.5]. Most participants were recruited to RCTs (70.5%, 630/893). Thirty-five studies reported on only one participant.
Age
Most participants were adults with 53.8% (43/80 studies) 18 to 65 years, and 31.3% > 65 years (25/80 studies); 5 children (6.3%; < 18 years) were included in five individual case studies.
Sex
Fewer females were represented within included studies, with mean percentage across the studies of 34.8%. This was not observed to alter by age of the data set.
Stroke severity, lesion and concurrent impairments
Few studies reported stroke severity (16.3%, 13/80). Right hemisphere lesions were common [39/80 studies (48.8%) recruited > 60% participants with right-sided lesions]. This was most apparent for studies of somatosensory deficits with 64.2% studies having > 60% participants with right-sided lesions. Concurrent stroke-related impairments were noted in 51.3% (41/80) of studies; however, this was not reported in 49.3% (39/80) of studies.
Time since stroke
Stroke survivors were recruited across the stroke trajectory: acute (15.6%, 139/893), subacute (38.1%, 340/893) and the chronic phases (35.6%, 318/893).
Diagnosis
Perceptual disorders were diagnosed using standardised tests (79.2%, 707/893), clinical assessments (see Included studies; 47/893) or both (2.1%, 19/893). For 26 participants (2.9%) their diagnosis was based on a combined clinical assessment and self-report. Two further participants’ perceptual disorder diagnosis was based on self-report (0.2%) while the method of diagnosis was unreported for 92 participants (10.3%, 92/893).
Perceptual disorders
Perceptual disorders described in the included studies were primarily visual (42.5%, 34/80) and somatosensory disorders (35%, 28/80). Few studies focused on auditory (8.7%, 7/80) or tactile (7.5%, 6/80) perceptual disorders. Only one study focused on taste and smell disorders, along with four other studies addressing a ‘mixed perceptual disorders’ category (6.2%, 5/80). The nature of the study design varied by sense addressed (Figure 5).
FIGURE 5.
Scoping review findings by study design and sense (%). Note: figure is based on one published in Stroke. 112
Reproduced with permission from Hazelton et al. 112 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
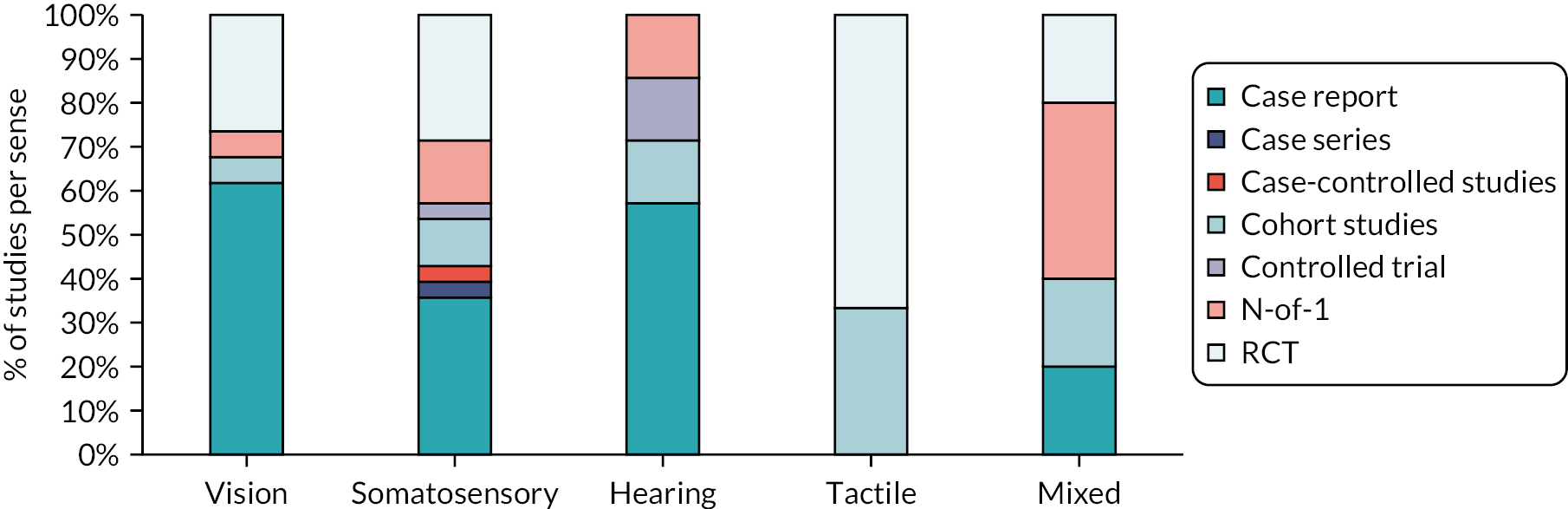
Studies conducted in Asia had a focus on somatosensation disorders (13/27, 48.1%); almost half of these studies were RCTs (six RCTs). In contrast, European studies most often described visual disorders (12/26, 46.1%) and frequently using case reports (seven case reports). Most somatosensory studies identified were recently published 71.4% (20/28) since 2015.
Visual perception
Stroke survivors with visual perceptual disorders accounted for 40% (357/893) of participants included in this review, across 34 studies (42.5%, 34/80); disorders included visual–spatial deficit (3.9%, 35/893), visual hallucination (including Charles Bonnet syndrome) (0.9%, 8/893), visual agnosia (0.3%, 3/893) or ‘other’ visual perceptual disorders (4.1%, 37/893). The largest group of participants were classed as experiencing a non-specific ‘visual perceptual deficit’ (30.6%, 274/893), which could include those with a mix of several perceptual issues, or diagnosis was based on perceptual test score, for example Motor-Free Visual Perception test (MVPT)129 (Figure 6) that did not specify the nature of the disorder detected.
FIGURE 6.
Included participants: by sense (inner ring) and perceptual disorder (outer ring). Note: figure is based on one published in Stroke. 112
Reproduced with permission from Hazelton et al. 112 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
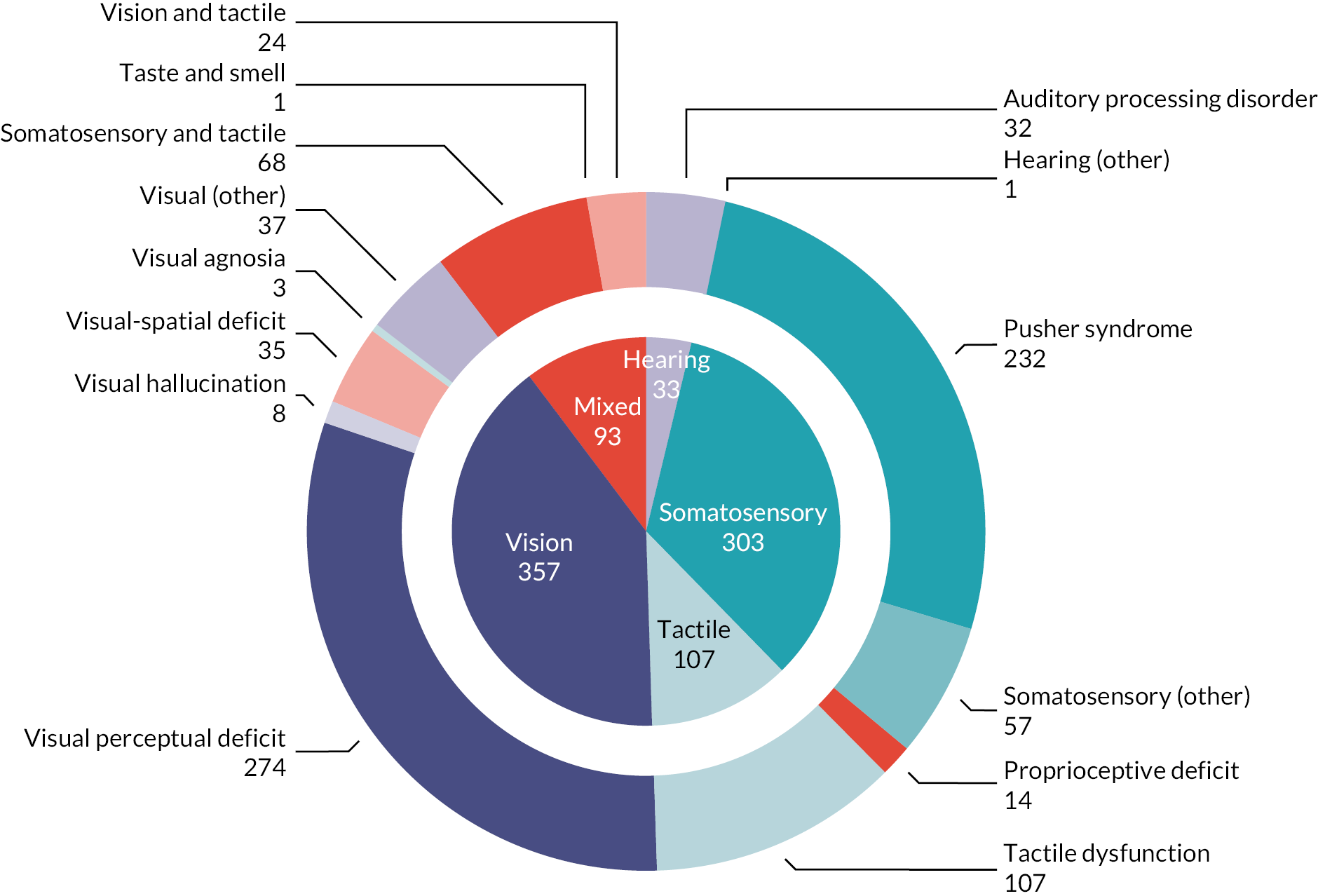
Somatosensation
Somatosensation deficits were reported by a third of participants (33.9%, 303/893) across 28 studies (35%, 28/80). Disorders included Pusher syndrome (26%, 232/893), proprioceptive deficits (1.6%, 14/893) or were categorised as ‘other’ somatosensory disorder (6.4%, 57/893).
Auditory perception
Few studies (8.7%, 7/80) described participants with an auditory perceptual disorder. These were classed as either an auditory processing disorder (3.6%, 32/893) and/or hearing ‘other’ (0.1%, 1/893).
Tactile perception
All participants were reported to experience a form of general tactile dysfunction (12%, 107/893) across six studies (7.5%, 6/80).
Mixed perceptual disorders
Studies that recruited participants with perceptual disorders of two or more senses included participants with mixed tactile-somatosensory disorder (7.6%, 68/893), mixed taste–smell disorder (0.1%, 1/893) or mixed vision–tactile disorder (2.7%, 24/893).
Interventions
We identified 93 interventions across 80 studies. Rehabilitation interventions were common (83.9%, 78/93 interventions) and further classified as: restitution, substitution, compensation or a mixed rehabilitation approach (see Intervention categorisation). No surgical or assessment-based interventions were identified.
Nature of interventions
Intervention materials included technology based, HCP led and those using specialist equipment.
Technology-based interventions
Interventions that used technological devices such machinery, computers and robotic devices supported 28 interventions (30.1%) including electrical stimulation, vibration, computer games/software, gaming devices (e.g. WiiFit®) and robotic devices (e.g. Lokomat®). Rationale was provided for all but two (7%) interventions. The procedures used were reported for all but one intervention (3.6%) while access to intervention materials was reported in 20 studies (71.4%, 20/28).
Healthcare professional-led interventions
Thirty HCP-led interventions were described (32.3%, 30/93), commonly physiotherapists (36.7%, 11/30) or OTs (16.7%, 5/30). Example interventions include route training, training in ADLs, exercise provision and postural training. HCP-led interventions primarily addressed somatosensory or vision perceptual disorders but also included one auditory study. Rationale (90.0%, 27/30), procedural information (93.3%, 28/30) and information on how to access the intervention materials (66.6%, 20/30) were provided for most interventions.
Specialist equipment
Some tactile, somatosensory and mixed perceptual disorders interventions (14%, 13/93) used specialist equipment such as balance boards, tactile discrimination grids or sponges. All provide procedural details and an intervention rationale, with information on how to access materials reported for all but two interventions (84.6%, 11/13).
Mode of delivery
Most interventions were delivered one to one (81.7%, 76/93), with few self-managed (4.3%, 4/93) or delivered to a group (3.2%, 3/93). These details were unavailable for nine interventions (9.7%, 9/93) or were unclear (1.1%, 1/93).
Location
Interventions were delivered in a hospital inpatient (37.6%, 35/93), in or outpatient (21.5%, 20/93), or outpatient basis (1.1%, 1/93). Only three were home-based (3.2%, 3/93). A third of interventions did not report this detail (33.3%, 31/94).
Frequency
Interventions were often delivered over multiple sessions (72%, 67/93). Eight were delivered in a single session (8.5%, 8/93). For 17 interventions (18.3%) these details were unreported or unclear (1.1%, 1/93).
Duration
The included interventions lasted < 1 week (15.1%, 14/93), 1–4 weeks (30.1%, 28/93); 1–3 months (10.8%, 10/93) or > 3 months (4.3%, 4/93). Several studies did not describe the duration (34.4%, 32/93) or it was unclear (5.4%, 5/93).
Modification or tailoring of interventions
Information on tailoring of interventions to participants or any intervention modifications were not reported by approximately half the interventions identified (54.3%, 51/93). Tailoring (usually to initial ability or in relation to progress) was described for 12 interventions (12.8%) with a further 23 interventions (24.5%) stating there was tailoring, but no details were provided. For seven interventions, this information was unclear (7.4%).
Intervention fidelity
Most interventions did not refer to any fidelity measures (96.8%, 90/93) or the information provided was unclear (1%, 1/93). Three interventions described plans to measure intervention fidelity (3%, 3/93).
Some intervention details were rarely reported, and we found no report of the intervention procedure (10.8%, 11/93), provider (52.7%, 49/93) or duration (34.4%, 32/93). Most described an intervention rationale (76.4%, 71/93). Some described the delivery of experimental interventions alongside usual care (22.6%, 21/93), but this was not always reported (73.1%, 68/93).
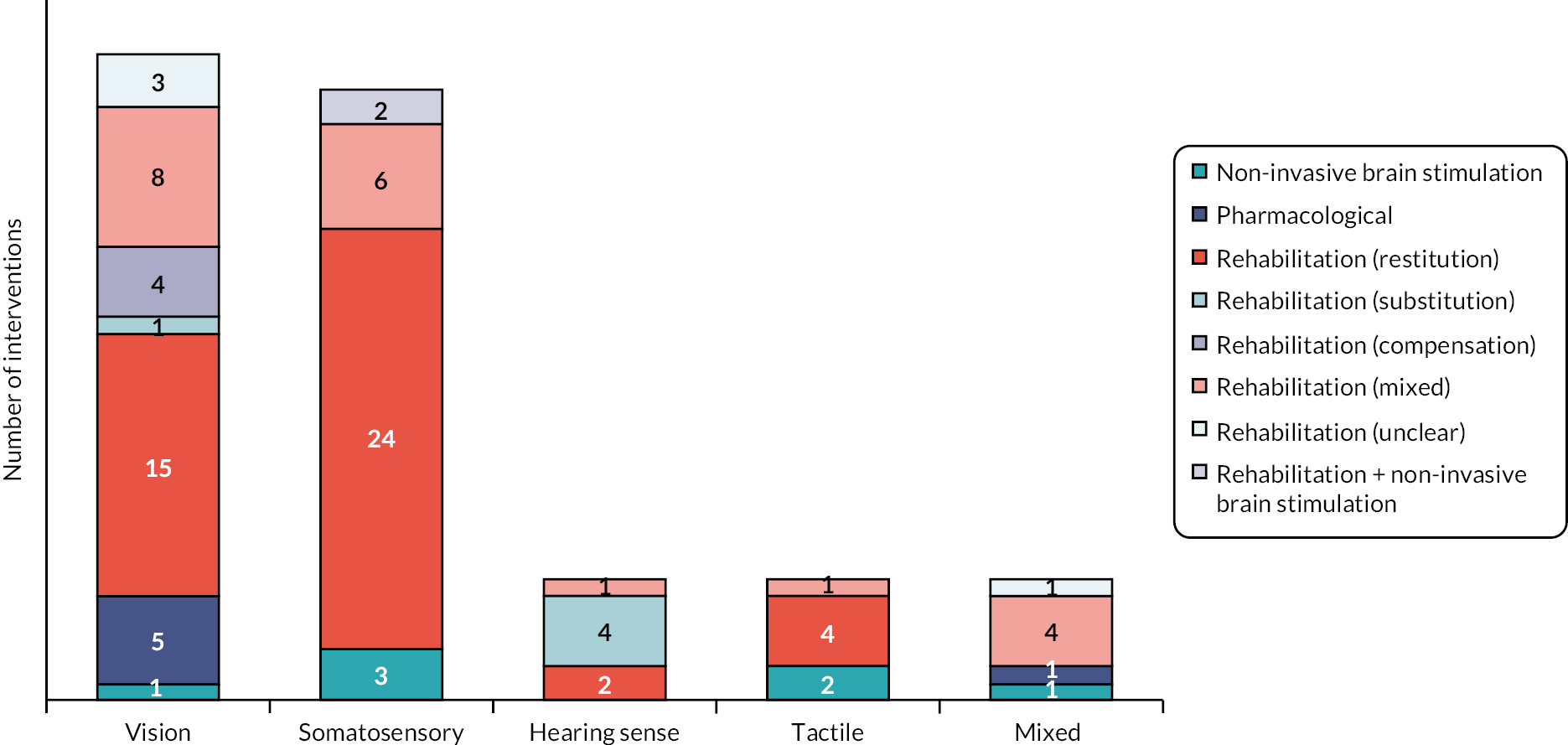
Interventions targeting perceptual disorders
Interventions addressed five perceptual disorder domains: visual (39.7%, 37/93), somatosensory (30.1%, 28/93), hearing (5.7%; 7/93), tactile (7.5%; 7/93) and mixed perceptual disorders (5.7%, 7/93; Figure 7).
FIGURE 7.
Perceptual disorder interventions by approach and sense. Note: figure is based on one published in Stroke. 112
Reproduced with permission from Hazelton et al. 112 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
Visual perception disorder interventions
Of 37 visual perceptual disorder interventions, 31 had a rehabilitation focus (83.8%, 31/37), including restitution (48.4%, 15/31), mixed (25.8.1%, 8/31), compensation (12.9%, 4/31) and substitution interventions (3.2%, 1/31). Other intervention approaches were unclear (9.7%, 3/31) (Table 2).
| Study ID (author, year) | Population | Stroke | Intervention | Delivery | Session details | Duration |
|---|---|---|---|---|---|---|
| Design Country | Number Age (years) (1) (2) refer to participant groups within studies |
Time post stroke %R hemisphere | Approach Description | Materials Who How Where |
Length Frequency Number of sessions |
/52 (weeks) /365 (days) /24 (hours) |
| Disorder addressed: Charles Bonnet syndrome | ||||||
| Chen 2011130 CR Taiwan |
N: 1 Age: 70 |
NR 81–100%R |
Pharmacological Quetiapine, then aripiprazole |
Pharmacological Unclear/ 1-1 In/outpatient |
5 mg Daily 21 |
3/52 21/365 |
| Nakagawa 1999131 CR China |
N: 1 Age: 70 |
< 1 0–20%R |
Pharmacological Dobutamine |
Pharmacological Unclear 1-1 Inpatient |
5 µg/kg/minutes NR NR |
NR NR |
| Nguyen 2011132 CR USA |
N: 1 Age: 75 |
< 1 NR |
Pharmacological Haloperidol |
Pharmacological Medic 1-1 NR |
NR Nightly NR |
NR NR |
| Roberts-Woodbury 2016133 CR NR |
N: 1 Age: 69 |
1–6 NR |
Pharmacological Risperidone |
Pharmacological Unclear NR Inpatient |
NR NR NR |
NR NR |
| Disorder: other visual hallucination | ||||||
| Cogan 1973134 CR USA |
N: 1 Age: 72 |
NR 81–100%R |
Pharmacological Librium |
Pharmacological Unclear NR NR |
NR NR NR |
NR NR |
| Flint 2005135 CR USA |
N: 1 Age: 64 |
NR 0–20%R |
Rehab (substitution) Cardboard mask covering left side of glasses |
Spec equipment Other Self-delivery NR |
NR NR NR |
Unclear NR |
| Poetter 2012136 CR USA |
N: 1 Age: 63 |
1–6 81–100%R |
Rehab (unclear) Cognitive rehabilitation for neglect |
NR NR NR Inpatient |
NR NR NR |
NR NR |
| Rafique 2016137 CR Canada |
N: 1 Age: 30 |
> 6 months 81–100%R |
NIBS rTMS using 70 mm diameter figure-of-eight coil and 1 Hz pulse at 85% of maximum output |
NIBS NR 1-1 NR |
30 minutes Daily 5 |
1/52 2.5/24 |
| Disorder: visual agnosia | ||||||
| Brunsdon 2017138 CR Australia |
N: 1 Age: 6 |
> 6 81–100%R |
Rehab (compensation) Verbally mediated topographical orientation and route training |
HCP-led Teacher 1-1 School |
Unclear Unclear Unclear |
12/52 Unclear |
| Tanemura 1999139 CR Japan |
N: 1 Age: 56 |
1–6 0–20%R |
Rehab (restitution and compensation) Practical activities including sketching, wood carving, mosaic work and fishing |
HCP-led NR 1-1 Inpatient |
NR NR NR |
NR NR |
| Zihl 2000 (4)140 CR Germany |
N: 1 NR |
NR 0–20%R |
Rehab (restitution and compensation) Stepwise training, including training of letter and feature recognition |
Tech-based NR 1-1 Inpatient |
45 minutes (2–4 per day) Unclear Unclear |
NR NR |
| Disorder: visual perceptual deficit | ||||||
| Mcdowell 2019141 CR New Zealand |
N: 1 Age: 16 |
> 6 0–20%R |
Rehab (compensation) Detailed tutorial; strategy training including and emotional strategies |
Other (Info) Other Self-delivery Home |
NR NR NR |
NR NR |
| Gottlieb 1991142 CR USA |
N: 1 Age: 80 |
< 1 0–20%R |
Rehab (compensation) Intentional blink, gives temporary clarity |
Other Other Self-delivery In/outpatient |
NR NR NR |
NR NR |
| Burr 1970143 CR Australia |
N: 1 Age: 74 |
1–6 81–100%R |
Rehab (restitution and compensation) Training in ADLs via CCTV training footage |
HCP-led OT 1-1 In/outpatient |
NR NR Unclear |
3/52 NR |
| Cho 201566 RCT South Korea |
N: 27 Mean: (1) 62.9 (SD 7.2) (2) 63.6 (SD 9.3) |
> 6 61–80%R |
Rehab (restitution) Neurofeedback training, using computer-based games |
Tech-based NR 1-1 Inpatient |
30 minutes 5× week 30 |
6/52 15/24 |
| Choi 2018144 RCT South Korea |
N: 28 Median: (1) 49.5 (IQR 2.3) (2) 51.0 (IQR 13.8) |
> 6 61–80%R |
Rehab (restitution) WiiFit training using Balance Board |
Tech-based PT 1-1 NR |
30 minutes 5× week 30 |
6/52 15/24 |
| Dutton 2017145 CR NR |
N: 1 Age: 9 |
NR 0–20%R |
Rehab (restitution and compensation) Training to detect, orient to and grasp visual stimuli to enlarge attentional visual field |
NR NR NR NR |
Half-day 5× week 5 |
NR NR |
| Edmans 1991146 N-of-1 England |
N: 4 Range: 54–65 |
1-6 0–20%R |
Rehab (restitution) Training in ADL-type tasks |
HCP-led OT 1-1 In/outpatient |
45 minutes 3× week 12–21 |
4–7/52 9–16/24 |
| Edmans 2000 60 RCT England |
N: 80 Mean: (1) 69.8 (SD 9.1) (2) 67.9 (SD 11.4) |
> 6 41–60%R |
Rehab (restitution) ‘Transfer of training’ rehabilitation |
HCP-led OT 1-1 Inpatient |
2.5 hours Unclear Unclear |
6/52 15/24 |
| Rehab (compensation) ‘Functional approach’ rehabilitation |
HCP-led OT 1-1 Inpatient |
2.5 hours Unclear Unclear |
6/52 15/24 |
|||
| Jo 2012 Cohort South Korea |
N: 17 NR |
> 6 61–80%R |
Rehab (restitution) Computerised cognitive rehabilitation program |
Tech-based OT 1-1 Inpatient |
30 minutes 3× week 12 |
4/52 6/24 |
| Kang 2009147 RCT South Korea |
N: 16 Mean: (1) 59.5 (SD 10.7) (2) 62.5 (SD 9.6) |
1–6 81–100%R |
Rehab (restitution) Computerised visual perception rehabilitation |
Tech-based OT 1-1 Inpatient |
30 minutes 3 × week 12 |
4/52 6/24 |
| Rehab (restitution) Computer-based cognitive rehabilitation program |
Tech-based OT 1-1 Inpatient |
30 minutes 3× week 12 |
4/52 6/24 |
|||
| Kim 2011148 RCT South Korea |
N: 30 Mean: (1) 70.7 (SD 6.6) (2) 71.4 (SD 5.2) |
1-6 NR |
Rehab (restitution and compensation) Dynavision wall-mounted board user strikes when illuminated |
Tech-based NR 1-1 In/outpatient |
30 minutes 3× week 12 |
4/52 6/24 |
| Zihl 2000 (3)149 CR Germany |
N: 3 Range: 58–61 |
0–20%R | Rehab (restitution) Eye movement training on slides/computer screen |
Tech-based NR NR NR |
45 minutes 3–4 per day Unclear |
Unclear Unclear |
| Lincoln 1985150 RCT England |
N: 33 Mean: 50.1 (SD 15.1) |
1–6 41–60%R |
Rehab (restitution) Perceptual training tasks |
HCP-led OT 1-1 Inpatient |
60 minutes 4× week 16 |
4/52 16/24 |
| Zihl 2000 (1)151 CR Germany |
N: 1 Age: 53 |
> 6 0–20%R |
Rehab (restitution) Computer-based hue discrimination training |
Tech-based NR NR NR |
NR NR NR |
NR NR |
| Park 2015152 RCT South Korea |
N: 30 Mean: (1) 64.7 (SD 8.9) (2) 65.2 (SD 8.0) |
1–6 NR |
Rehab (restitution) Computer training including visual perception, attention, memory and orientation |
Tech-based NR 1-1 In/outpatient |
30 minutes 5× week 20 |
4/52 10/24 |
| O’Hare 1998153 CR Scotland |
N: 1 Age: 8 |
> 6 0–20%R |
Rehab (mixed) Educational orthography with specialist reading software |
Tech-based NR 1-1 Other (home/school) |
NR NR NR |
NR NR |
| Disorder: visual–spatial deficit | ||||||
| Chen 2012154 RCT USA |
N: 11 Mean: (1) 73.8 (8.8) (2) 74.0 (8.4) |
1–6 81–100%R |
Rehab (restitution) Global processing training using Rey–Osterrieth figure |
HCP-led NR 1-1 Inpatient |
90 minutes Once 1 |
1/365 1.5/24 |
| Rehab (restitution) Rote repetition training using Rey–Osterrieth figure |
HCP-led NR 1-1 Inpatient |
90 minutes Once 1 |
1/365 1.5/24 |
|||
| Funk 2013155 Cohort Germany |
N: 13 Range: 23–60 |
1 to > 6 81–100%R |
Rehab (restitution) Line presentation on computer screen with visual feedback |
Tech-based NR 1-1 NR |
NR 3× week 11 |
4/52 NR |
| Zihl 2000 (2)156 CR Germany |
N: 1 Age: 48 |
> 6 0–20%R |
Rehab (restitution) Five-stage process progressing from table-top to PC activities |
Tech-based NR NR NR |
NR NR NR |
NR NR |
| Towle 1990157 N-of-1 England |
N: 10 NR |
NR 81–100%R |
Rehab (unclear) Practicing perceptual tasks |
NR Other (therapist) Group Inpatient |
60 minutes 3× week 24 |
8/52 24/24 |
| Disorder: visual – other | ||||||
| Gillen 2003158 CR Scotland |
N: 1 Age: 10 |
> 6 0–20%R |
Rehab (mixed) Adaptive compensatory approach to use strengths and abilities to compensate perceptual problem |
HCP-led NR 1-1 Home |
NR NR NR |
NR NR |
| Weinburg 1982159 RCT USA |
N: 35 Mean: (1) 64.2 (SD 9.0) (2) 66.8 (SD 9.8) |
1–6 81–100%R |
Rehab (restitution and compensation) Training to anchor attention and eye movements |
HCP-led NR 1-1 In/outpatient |
60 minutes 5× week 20 |
4/52 20/24 |
| Zaharia-Pushkash 2010160 CR Moldova |
N: 1 Age: 67 |
NR 81–100%R |
Rehab (unclear) Unspecified rehabilitation |
NR NR NR NR |
NR NR NR |
NR NR |
Restitution approaches used technology (66.7%, 10/15; including interactive computer-based training of visual skills) and HCP-led interventions (33.3%, 5/15) including teaching compensatory skills in real-world simulation tasks, training of eye movements or completion of perceptual-based tasks. Compensatory approaches included intentional blinking, route and strategy training while substitution interventions included covering of one side of glasses with cardboard. One study used NIBS (Repetitive Transcranial Magnetic Stimulation – rTMS) for visual hallucination.
Pharmacological interventions identified (e.g. haloperidol, Librium and risperidone) targeted visual hallucinations and were described in single case reports with limited intervention details. Generally, visual perceptual disorder studies lacked details on the intervention provider, location, intensity and duration.
Five case reports described mixed rehabilitation interventions for children with a visual perceptual disorder including specialist reading software and the development of strategies to overcome difficulties. Two interventions used a compensatory approach (route training and tutorial-based sessions). Where locations were reported they included school, home (using self-delivered tutorials) and inpatient settings.
Somatosensory disorder interventions
Four of the 35 interventions identified in the scoping review targeted somatosensory disorders and were categorised as rehabilitation (restitution 68.6%, 24/35; mixed 17.1%, 6/35), NIBS (8.6%, 3/35) and rehabilitation + NIBS (5.7%, 2/35) (Table 3). Most were HCP-led (48.6%, 17/35) and included postural control or reach training with weight-shifting exercises. Technology-based interventions (25.7%, 9/35) included robot-assisted gait training using the Lokomat device, postural training devices or gaming such as Nintendo Wii®. Interventions were predominantly delivered on a one-to-one basis (91.4%, 32/35), in an inpatient hospital setting (51.4%, 18/35), for 1 month or less (71.4%, 25/35).
| Study ID (author, year) | Population | Stroke | Intervention | Delivery | Session details | Duration/52 (weeks) |
|---|---|---|---|---|---|---|
| Design Country |
Number Age (years) (1) (2) refer to participant groups within studies |
Time post stroke %R hemisphere |
Approach Description |
Materials Who How Where |
Length Frequency Number of sessions |
/365 (days) /24 (hours) |
| Disorder: proprioceptive deficit | ||||||
| Ko 2018161 CC South Korea |
N: 14 Mean: 65.2 (SD 7.8) |
< 1 41–60%R |
Rehab (restitution) Frankel’s exercises |
HCP-led PT 1-1 In/outpatient |
15 minutes 5× week 15 |
3/52 3.45/24 |
| Disorder: Pusher syndrome | ||||||
| An 201975 RCT South Korea |
N: 14 Mean: (1) 59.3 (SD 4.6) (2) 64.4 (SD 7.5) |
< 1 81–100%R |
Rehab (restitution) Game-based postural vertical training using whole-body tilt equipment |
Tech-based NR 1-1 Inpatient |
30 minutes td 5× week 30 |
3/52 15/24 |
| Rehab (restitution) Conventional postural vertical training using posture control training exercises |
HCP-led NR 1-1 Inpatient |
30 minutes td 5× week 30 |
3/52 15/24 |
|||
| An 2020162 RCT South Korea |
N: 30 Mean: (1) 60.5 (SD6.0) (2) 64.7 (SD6.9) |
< 1 61–80%R |
Rehab (restitution) Whole-body tilting postural training using a Spine Balance 3D |
Tech-based PT 1-1 Inpatient |
30 minutes td 5× week 30 |
3/52 15/24 |
| Rehab (restitution) General postural training using visual feedback and weight shifting |
HCP-led PT 1-1 Inpatient |
30 minutes td 5× week 30 |
3/52 15/24 |
|||
| Bergmann 2018163 RCT Germany |
N: 38 Mean: (1) 72 (SD 9) (2) 71 (SD 10) |
1–6 61–80%R |
Rehab (restitution and substitution) Robot-assisted gait training with Lokomat |
Tech-based NR 1-1 Inpatient |
60 minutes 5× week 8–10 |
2/52 8–10/24 |
| Rehab (restitution) Postural control training including sensory feedback |
HCP-led PT 1-1 Inpatient |
60 minutes 5× week 8–10 |
2/52 8–10/24 |
|||
| Broetz 2004164 Cohort Germany |
N: 8 Median: 63 (range: 51–79) |
< 1 81–100%R |
Rehab (restitution) Physiotherapy with visual feedback to demonstrate body orientation |
HCP-led NR 1-1 Inpatient |
30 minutes 6× week Unclear |
Unclear Unclear |
| Freitas 2017165 CR Brazil |
N: 1 Age: 62.5 |
> 6 81–100%R |
Rehab (restitution) Mirror therapy using balance and reach training |
HCP-led NR 1-1 Outpatient |
50 minutes 3× week 13 |
5/52 12.5/24 |
| Fujino 2016166 N-of-1 Japan |
N: 3 Age: unclear |
< 1 NR |
Rehab (restitution) Relaxation therapy in prone position using treatment table |
HCP-led NR 1-1 In/outpatient |
10 minutes Daily 6 |
6/365 1/24 |
| Fujino 2019167 N-of-1 Japan |
N: 2 Age: 69–75 |
< 1 81–100%R |
Rehab (restitution and substitution) Electromyography-guided electrical stimulation therapy |
Tech-based NR 1-1 NR |
65 minutes Twice Unclear |
2/365 2/24 |
| Gillespie 2019168 CR USA |
N: 1 Age: 58 |
< 1 81–100%R |
Rehab (restitution and substitution) Standing frame |
HCP-led PT Group Inpatient |
Unclear Unclear Unclear |
18/52 6.5/24 (380 total minutes) |
| Jahn 2017169 CR Germany |
N: 1 Age: 81 |
1–6 81–100%R |
Rehab (restitution and substitution) Spacecurl: suspension device with 3D rotation |
Spec equipment PT 1-1 Inpatient |
30 minutes 3× week 12 |
4/52 6/24 |
| Jang 2018170 CR South Korea |
N: 1 Age: 67 |
< 1 81–100%R |
Rehab (restitution) Rehabilitative therapy with movement therapy and somatosensory stimulation |
NR NR 1-1 Inpatient |
Unclear 5× week 80 |
16/52 Unclear |
| Jokelainen 2000171 CR Finland |
N: 1 Age: 78 |
< 1 81–100%R |
Rehab (restitution) Occupational therapy and physiotherapy rehabilitation programme |
HCP-led PT 1-1 Inpatient |
Unclear 5× week Unclear |
Unclear Unclear |
| Kim 2016172 Other South Korea |
N: 10 Mean: (1) 63.1 (SD 12.3) (2) 62.4 (SD 14.9) |
1–6 41–60%R |
Rehab (restitution and substitution) Virtual reality visual feedback during Lokomat training |
Tech-based NR 1-1 In/outpatient |
30 minutes td 5× week 40 |
4/52 20/24 |
| Lee 2017173 N-of-1 South Korea |
N: 3 Range: 58–65 |
1–6 NR |
Rehab (restitution) Postural vertical training with/without visual feedback |
HCP-led NR 1-1 Inpatient |
60 minutes 3× week 18 |
6/52 18/24 |
| Menghetti 2009174 CR Brazil |
N: 1 Age: 78 |
NR NR |
Rehab (restitution) Aquatic physiotherapy using Bad Ragaz and Halliwick methods |
HCP-led PT 1-1 Other (teaching clinic) |
60 minutes 2×week 16 |
8/52 16/24 |
| Mikolajewska 2012175 CR Poland |
N: 1 Age: 72 |
1–6 81–100%R |
Rehab (restitution) Contraversive Pusher syndrome therapy including visual cues |
HCP-led PT 1-1 NR |
Unclear Unclear 10 |
2/52 Unclear |
| Pardo 2019176 CS USA |
N: 5 Range: 42–76 |
< 1 61–80%R |
Rehab (restitution) Physiotherapy rehabilitation programme |
HCP-led PT 1-1 Inpatient |
90 minutes 5× week 19 average |
4/52 28.5/24 |
| Scheets 2007177 CR USA |
N: 1 Age: 76 |
NR 81–100%R |
Rehab (restitution) Physiotherapy rehabilitation programme |
HCP-led PT 1-1 Inpatient |
25–45 minutes Daily 14 |
2/52 7/24 |
| Voos 2011178 CR Brazil |
N: 1 Age: 65 |
> 6 81–100%R |
Rehab (restitution) Physiotherapy including sensory stimulation, motor training and sensorimotor integration |
HCP-led NR Unclear Home |
60 minutes 2× week 48 |
24/52 48/24 |
| Wang 2016179 RCT China |
N: 25 NR |
NR NR |
Rehab (restitution) Visual feedback via a dynamic and static balance/motion control system and balance board |
Spec equipment PT Group In/outpatient |
30 minutes 5× week 15 |
3/52 7.5/24 |
| Rehab (restitution) Core stability training using exercises |
Spec equipment PT 1-1 In/outpatient |
2 hours 5× week 5 |
1/52 10/24 |
|||
| Rehab (restitution) Visual feedback and core stability exercises combined |
HCP-led PT 1-1 In/outpatient |
Unclear Unclear Unclear |
Unclear Unclear |
|||
| Yang 2015180 RCT Taiwan |
N: 12 Mean: (1) 62.4 (SD 12.9) (2) 57.6 (SD 17.3) |
1–6 61–80%R |
Rehab (restitution) Computer-generated interactive visual feedback training with Nintendo Wii balance board |
Tech-based PT 1-1 NR |
40 minutes 3× week 9 |
3/52 6/24 |
| Rehab (restitution) Mirror visual feedback training |
HCP-led PT 1-1 NR |
40 minutes 3× week 9 |
3/52 6/24 |
|||
| Yun 2018181 RCT South Korea |
N: 36 Mean: (1) 63.6 (SD 8.3) (2) 64.3 (SD 8.4) |
1–6 0–20%R |
Rehab (restitution and substitution) Robot-assisted gait training with Lokomat |
Tech-based NR 1-1 In/outpatient |
30 minutes 5× week 15 |
3/52 7.5/24 |
| Babyar 2018182 Cohort USA |
N: 10 Range: 54–87 |
< 1 81–100%R |
NIBS tDCS |
NIBS NR 1-1 NR |
15 minutes Once 1 |
1/365 |
| NIBS Galvanic vestibular stimulation |
NIBS NR 1-1 NR |
15 minutes Once 1 |
1/365 | |||
| Krewer 2013183 RCT Germany |
N: 25 Range: 55–80 |
> 6 81–100%R |
NIBS and rehabilitation (restitution and substitution) Galvanic vestibular stimulation with exoskeleton-assisted locomotion and physiotherapy |
NIBS NR 1-1 Inpatient |
20 minutes Unclear Unclear |
Unclear Unclear |
| Nakamura 2014184 N-of-1 Japan |
N: 2 Range: 83–86 |
1–6 81–100%R |
NIBS and rehabilitation (restitution and substitution) Galvanic vestibular stimulation with occupational therapy and physiotherapy |
NIBS NR 1-1 Inpatient |
20 minutes 5 days/week 10 with stimulation, 10 without |
4/52 20/365 |
| Disorder: somatosensory other | ||||||
| Colombo 2015185 CR Italy |
N: 1 Age: 40 |
> 6 0–20%R |
Rehab (restitution and substitution) 2-DOF elbow/shoulder manipulator and 1-DOF wrist manipulator |
Tech-based NR 1-1 Inpatient |
Unclear 2× per day Unclear |
3.5/52 |
| Jamal 2020186 Cohort France |
N: 32 Mean: 60.9(SD 10) |
> 6 41–60%R |
Rehab (restitution) Repetitive neck muscle vibration |
Tech-based Researcher 1-1 NR |
10 minutes Unclear |
2/52 Unclear |
| Koo 2018187 RCT South Korea |
N: 24 Mean: (1) 58.7 (SD 3.4) (2) 52.4 (SD 3.2) |
< 1 41–60%R |
NIBS tDCS |
NIBS Researcher 1-1 Inpatient |
20 minutes Unclear Unclear |
2/52 Unclear |
Hearing perception disorder interventions
Seven interventions targeted hearing perceptual disorders, all of which adopted a rehabilitative approach (see Table 4). They were categorised as restitution (28.6%, 2/7), substitution (57.1%, 4/7) or mixed (14.3%, 1/7). Interventions were primarily technology-based (e.g. hearing aids 71.4%, 5/7) but also included HCP-led phonological therapy (14.3%, 1/7). The details of one intervention were unavailable (14.3%, 1/7). Where reported, all interventions were conducted on an individual basis (71.4%, 5/7) in a hospital setting (in/outpatient, 57.1%, 4/7) with one intervention (personal frequency modulated system) self-delivered at home (14.3%, 1/7) (Table 4).
| Study ID (author, year) | Population | Stroke | Intervention | Delivery | Session detail | Duration |
|---|---|---|---|---|---|---|
| Design Country |
Number Age (years) (1) (2) refer to participant groups within studies |
Time post stroke %R hemisphere |
Approach Description |
Materials Who How Where |
Length Frequency Number of sessions |
/52 (weeks) /365 (days) /24 (hours) |
| Sense: hearing Disorder: auditory processing disorder |
||||||
| Fifer 1993188 CR USA |
N: 1 Age: 60 |
< 1 81–100%R |
Rehab (substitution) Wireless behind the ear contralateral routing of the signal hearing aid |
Tech-based NR 1-1 NR |
NR NR NR |
8/52 NR |
| Koohi 2017 (1)68 Cohort England |
N: 9 Range: 24–78 |
1+ 41–60%R |
Rehab (substitution) Speech in noise testing in sound-attenuating chamber with/without FM system |
Tech-based NR 1-1 In/outpatient |
NR NR NR |
NR NR |
| Koohi 2017 (2)189 CT England |
N: 9 Range: 24–78 |
1+ 41–60%R |
Rehab (substitution) Personal frequency modulated systems (Phonak iSense Micro receiver and Zoom link transmitter) |
Tech-based Other Self-delivery Home |
6 hours Daily 70 |
10/52 420/24 |
| Papathanasiou 1998190 CR England |
N: 1 Age: 75 |
< 1 0–20%R |
Rehab (restitution) Auditory discrimination of minimal pairs |
NR Other 1-1 Inpatient |
NR NR NR |
NR NR |
| Woolf 2014191 N-of-1 England |
N: 11 Range: 44–81 |
> 6 NR |
Rehab (restitution and compensation) Phonological and semantic-phonological therapy |
HCP-led Other 1-1 NR |
60 minutes 2× week 12 |
6/52 12/24 |
| Zgaljardic 2013192 CR USA |
N: 1 Age: 39 |
1–6 0–20%R |
Rehab (substitution) Augmentative and alternative communication devices |
Tech-based NR NR In/outpatient |
NR NR NR |
10/52 NR |
| Sense: hearing Disorder: hearing other |
||||||
| Fechtelpeter 1990193 CR Germany |
N: 1 Age: 41 |
NR 0–20%R |
Rehab (restitution) Sound identification (everyday noises) and matching to cards |
Tech-based NR 1-1 In/outpatient |
NR NR 7 (Phase 1) and 10 (Phase 2) |
NR NR |
| Sense: mixed senses Disorder: tactile and somatosensory (proprioceptive) deficit |
||||||
| Carey 1993194 N-of-1 Australia |
N: 8 Range: 34–75 |
1–6 61–80%R |
Rehab (restitution and compensation) Tactile discrimination and wrist proprioception training |
Spec equipment NR 1-1 NR |
NR NR NR |
NR NR |
| Carey 2005195 N-of-1 Australia |
N: 5 Range: 44–60 |
1–6 0–20%R |
Rehab (restitution and compensation) Transfer of training using texture grids, fabrics and proprioception stimuli |
Spec equipment NR 1-1 NR |
50 minutes 3× week NR |
NR NR |
| N: 5 Range: 47–88 |
1–6 41–60%R |
Rehab (restitution and compensation) Stimulus-specific training of sensory discrimination, stimulus generalisation of sensory discrimination |
Spec equipment NR 1-1 NR |
NR NR NR |
NR NR |
|
| Carey 201174 RCT Australia |
N: 50 Mean: (1) 61.0 (SD 12.8) (2) 61.0 (SD 14.4) |
> 6 41–60%R |
Rehab (restitution and compensation) Sensory discrimination training using texture discrimination, limb sense and object recognition |
Spec equipment NR 1-1 NR |
60 minutes 3× week 10 |
3–4/52 10/24 |
| Rehab (unclear) Non-specific repeated exposure to tactile stimuli via grasping |
Spec equipment NR 1-1 NR |
60 minutes 3× week 10 |
3–4/52 10/24 |
|||
| Sense: mixed senses Disorder: taste and smell hallucination |
||||||
| Hayashi 2004196 CR Japan |
N: 1 Age: 55 |
< 1 81–100%R |
Pharmacological Carbamazepine Valproate |
Pharmacological Unclear 1-1 NR |
NR NR NR |
NR NR |
| Sense: mixed senses Disorder: visual and tactile disorder |
||||||
| Oppenlaender 2015197 Cohort Germany |
N: 24 Median: 64 (range 42–84) |
1–6 81–100%R |
NIBS Galvanic vestibular stimulation |
NIBS Researcher 1-1 NR |
20 minutes NR 2 |
1/52 < 1/24 |
| Sense: touch Disorder: tactile dysfunction |
||||||
| Carey 2016198 Cohort Australia |
N: 11 Range: 40–79 |
NR 21–40%R |
Rehab (restitution and compensation) Touch discrimination intervention: use of three texture grids with varying stimulus difficulty Explore and discriminate the odd texture |
Spec equipment NR 1-1 NR |
45–60 minutes 3× week 18 |
6/52 13.5–18/24 |
| Enders 2013199 Cohort USA |
N: 10 Mean: 60 (SD 9) |
NR NR |
Rehab (restitution) Vibrotactile noise: monofilament and two-point discrimination with and without noise |
Tech-based NR 1-1 NR |
2 hours (noise 1 minute) Once 1 |
1/52 2/24 |
| Fujimoto 2016200 RCT Japan |
N: 8 Mean: 61.6 (SD 9.0) |
> 6 61–80%R |
NIBS tDCS with tactile stimuli |
NIBS NR 1-1 NR |
15 minutes Once 1 |
NR < 1/24 |
| Kim 2015201 RCT South Korea |
N: 30 Mean: (1) 54.7 (SD 3.1) (2) 59.4 (SD 8.6) (3) 56.4 (SD 11.9) |
> 6 21–40%R |
Rehab (restitution) Pressure sense perception training on stable surface |
Spec equipment PT 1-1 In/outpatient |
30 minutes 3× week 12 |
4/52 6/24 |
| Rehab (restitution) Pressure sense perception training on unstable surface |
Spec equipment PT 1-1 In/outpatient |
30 minutes 3× week 12 |
4/52 6/24 |
|||
| Kitisomprayoonkul202 2012 RCT Thailand |
N: 20 Mean: (1) 54.7 (SD 8.6) (2) 58.0 (SD 11.9) |
< 1 NR |
NIBS tDCS |
NIBS NR 1-1 In/outpatient |
20 minutes Once 1 |
1/52 |
| Morioka 2003203 RCT Japan |
N: 28 Mean: (1) 61.3 (SD 11.0) (2) 62.6 (SD 13.3) |
1–6 61–80%R |
Rehab (restitution) Hardness discrimination exercise: discriminate hardness of sponge rubber placed under foot |
Spec equipment NR 1-1 Inpatient |
NR 5× week 10 |
2/52 NR |
Tactile perception disorder interventions
We identified seven interventions targeting tactile perception disorders which often involved a rehabilitation approach: restitution (57.1%, 4/7) or NIBS (28.6%, 2/7). Interventions included tDCS (28.6%, 2/7) or specialised equipment (57.1%, 4/7) such as texture grids, object recognition or sensory discrimination equipment. One intervention was technology-based (14.3%, 1/7) delivering vibrotactile noise. Physiotherapists provided two interventions (28.6%, 2/7) but most providers were unreported (71.4%, 5/7). All interventions were delivered on an individual basis.
Mixed perception disorder interventions
Studies that recruited stroke survivors with perceptual disorders across more than one sense described seven interventions to address tactile and proprioceptive deficits (71.4%, 5/7) including specialised tactile and proprioceptive training equipment, such as texture grids and proprioceptive stimuli. Interventions were delivered on an individual basis, but few other details were available such as provider, location or duration. One study (14.3%, 1/7) explored the use of NIBS for a visual and tactile perceptual disorder, while another pharmacological study addressed complex taste/smell hallucinations by prescribing carbamazepine and valproate; no other details were provided.
Outcome measurements
The studies identified in the scoping review reported perceptual function (75%, 60/80), motor/sensorimotor (40%, 32/80), ADLs (22.5%, 18/80) and sensation outcome measurement (15%, 12/80) (Table 5). We also noted a few language-based outcome measures, which had not featured in our prioritised list. Other outcomes of interest such as discharge destination, economic outcomes, feasibility and acceptability, impact on family, friends and carers, impact on rehabilitation, measures of education and development, psychological well-being and mental health, QoL, social activity and participation were not reported.
| Outcome category | Hearing | Somato- sensation |
Tactile | Vision | Mixed | Total |
|---|---|---|---|---|---|---|
| Perceptual function | 4 | 24 | 6 | 22 | 4 | 60 |
| Motor/sensorimotor ability | 21 | 2 | 9 | 32 | ||
| ADLs | 1 | 12 | 5 | 18 | ||
| Sensation | 4 | 2 | 6 | 12 | ||
| Cognition | 1 | 8 | 9 | |||
| Mobility, navigation and safety | 3 | 1 | 1 | 5 | ||
| Neurological function | 2 | 1 | 1 | 4 | ||
| Language | 2 | 2 | 4 | |||
| Adverse events | 1 | 1 | 1 | 3 | ||
| EADLs | 1 | 1 | 2 | |||
| Attention | 2 | 2 |
Outcomes were measured at various time points; immediately post intervention (38.8%, 31/80); ≤ 1 month post intervention (11.3%, 9/80); 1–3 months post intervention (11.3%, 9/80); > 3 months post intervention (12/80; 15.0%).
Mapping of data
Interactive visual maps provided a visual overview of the availability of evidence across the six sensory domains and intervention approach. These are available from www2.gcu.ac.uk/hls/PIONEERmap.html (accessed November 2022) (see example in Figure 8). Data were stratified by age (child vs. adult). The bubble size reflects the magnitude of the evidence (larger indicates more); the relevant abstracts will then be visible (where available) if the bubble is clicked. The empty cells reflect the evidence gaps.
FIGURE 8.
Interactive evidence map.

The Lived-Experience Group feedback on the maps was positive
… it’s probably the one of the first charts that I have understood quite easily. I’ve been able to see it all and it’s very simple. … here you can see immediately that where the gaps are…I think that’s really, really good. I love that. It’s just very disheartening to see that nobody is doing anything about hearing, smell and taste.
Lived Experience Group member
Interpretation of scoping review results
The Lived Experience and Clinical Expert stakeholders discussed the scoping review results [see Stakeholder activities (what happened), Activity 3 and Report Supplementary Material 4] and raised the following issues:
-
There has been little high-quality research relating to perceptual problems after stroke, especially when compared to the amount and quality of research into other stroke-related impairments.
-
The lack of high-quality research in this area demonstrated a lack of awareness and understanding about perceptual problems after stroke, and that this was associated with a lack of support and information for stroke survivors and carers.
-
It is important to be aware that long-term recovery from perceptual problems is possible, rather than just in the first few months, and research should reflect this.
-
The high number of case reports identified in the scoping review may reflect need for interventions to be tailored to individual patients. The resultant heterogeneity/complexity in intervention approach and thus lack of standardised interventions may make research in this field challenging and may impact on the development of research relating to perceptual problems.
-
Better reporting would facilitate learning from research, including from individual case reports.
-
Most studies delivered interventions in hospital settings, but there is a need for research into interventions/strategies to support people living at home.
-
There were no qualitative studies which explored stroke survivor or carer experience, and no reported stakeholder involvement in studies. These were important gaps.
-
There had been more research relating to visual and somatosensory perceptual problems than for other senses (hearing, tactile, taste or smell). Stakeholders felt that this may reflect a lack of awareness of problems relating to the other senses.
Summary
Our scoping review identified 80 studies (1970–2020) that typically used a case report or RCT design to predominantly investigate visual or somatosensory perceptual disorders. Interventions (n = 93) were often rehabilitative in approach and focused on improvements in the impaired function. Many interventions included direct training by an HCP, technology-based devices or specialist equipment. Interventions were often hospital-based, lasting up to 4 weeks with few reporting longer-term outcomes. Perceptual and motor/sensorimotor outcomes were most reported.
Important evidence gaps were identified, including a paucity of evidence relating to interventions addressing hearing, taste, touch and smell perceptual disorders post stroke and interventions developed to address perceptual disorders experienced by children. Key participant and intervention details were often unreported. In addition, qualitative studies exploring the experience of perceptual interventions and involvement of stroke survivors and carers as stakeholders were not identified, with a clear focus on quantitative designs.
Chapter 6 Cochrane systematic review methods
Introduction
This chapter describes the Cochrane systematic review methods used to synthesise and appraise high-quality RCT evidence of the clinical and cost-effectiveness of interventions for perceptual impairments after stroke. The findings of the systematic review are reported in Chapter 7. The review has been published in the CDSR. 204
Our Cochrane systematic review ‘Interventions for perceptual disorders following stroke’ is an expanded update of a previous Cochrane systematic review from 2011 ‘Non‐pharmacological interventions for perceptual disorders following stroke and other adult‐acquired, non‐progressive brain injury’. 6 Following approval by the Cochrane Stroke Group (personal communication 20 November 2019), we made several a priori revisions and adjustments to the methods, as follows:
-
Participant eligibility criteria: our Cochrane Review focused on stroke populations. The 2011 review’s inclusion criteria included participants with ‘other adult-acquired, non-progressive brain injury’.
-
Intervention eligibility criteria: we included all healthcare interventions (including pharmacological). The 2011 review6 included non-pharmacological approaches only.
-
Search strategy: we updated and expanded the search terms to cover a broad range of perceptual impairments across several senses: hearing, smell, somatosensory, taste, tactile and vision. The search informing the 2011 review was last updated in August 2009 and focused on visual perceptual disorders.
-
Use GRADE98 and TIDieR97: reflecting systematic review methodological advances, we used TIDieR checklist headings to support intervention data extraction and description and rated the certainty of evidence synthesis using GRADE.
-
Primary and secondary outcomes: the outcomes considered in our review reflected stakeholders’ outcome priorities.
-
Title: we also adjusted the review title to reflect the changes above to ‘Interventions for perceptual disorders following stroke’.
Our scoping review sought evidence related to stroke survivors of any age but identified no RCTs relevant to a paediatric population (see Visual perception disorder interventions), a finding supported by an earlier guideline. 87 Thus, our Cochrane Review focused on adult participants.
Research questions
Our systematic review considered three research questions:
-
Are interventions for perceptual deficits after stroke more effective than control, placebo, standard care or no intervention?
-
Is one intervention for perceptual deficits after stroke more effective than another intervention?
-
Are interventions more effective at improving outcomes in stroke survivors with specific demographic variables?
Criteria for considering studies for this review
The Cochrane systematic review inclusion criteria mirror those of the scoping review (see Chapter 4, Criteria for considering studies for this review), with three changes:
-
Types of studies: we included RCT designs only. 205 In the case of crossover RCTs, we analysed data from baseline to the point of crossover. We included RCTs in which a comparison was made between an active treatment group that received an intervention for a perceptual disorder, to a group that received (1) no treatment, (2) a control intervention (placebo, standard care, attention control) (Table 6) or (3) an alternative perceptual intervention.
-
Types of participants: we included only adult participants (18 years and older) with impaired perception following stroke.
-
Outcome measurements: in comparison to the outcome measurements, we extracted in the scoping review (see Chapter 4, Criteria for considering studies for this review), we took forward a selection of these in the Cochrane Review (see Outcome measures).
| Comparator | Definition |
|---|---|
| No treatment | Where no intervention targeting the perceptual disorder(s) was received by the comparison group, compared to the active intervention group (e.g. a waiting list control group with delayed treatment until after the intervention period) |
| Placebo | An intervention that appears similar to, but omits a key therapeutic element of, the perceptual intervention or procedure under investigation (also described as ‘sham’ interventions)206 |
| Standard care (usual or conventional care) | An intervention that reflects the usual care in that region and at that time point before the trial start for a given perceptual disorder (Faltinsen 2019)206 |
| Attention control | An intervention that provides similar levels of trial contact and professional attention, and confers benefits of trial participation and expectations in the absence of active intervention components207–209 |
Outcome measures
The outcome measures included in this review reflect those prioritised by our stakeholders [see Stakeholder activities (what happened) and Types of outcome measures]. The full list of outcomes was discussed by the research team to select those of most relevance to the Cochrane Review questions, considering Cochrane reporting guidance, clinical practice and data availability [based on the scoping review (see Outcome measurements)]. We selected a primary outcome measure (see Primary outcome) and five secondary outcome measures (see Secondary outcomes); this did not include any outcomes of feasibility or cost-effectiveness.
Primary outcome
Activities of daily living post intervention (‘immediate’ time point) was our primary outcome. We also extracted ADLs at a 3-month post-intervention follow-up point.
We included any validated standardised ADLs measure such as the Barthel Index,210 Functional Independence Measure,211 Modified Rankin Scale,212 Katz Index of Activities of Daily Living,213 Assessment of Motor and Process Skills214 and Rehabilitation Activities Profile. 215
Where data on more than one ADLs outcome measurement instrument were available in a RCT, we extracted and analysed the measure occurring earliest in the above list.
Secondary outcomes
-
EADLs as captured by outcome measurement instruments, for example Frenchay Activities Index. 216
-
QoL:
-
Psychological well-being and mental health:
-
Perceptual function, for example Rivermead Perceptual Assessment Battery. 221
-
Adverse events, for example fall, death, fatigue, accident rates.
Data were extracted for the time point immediately after intervention delivery; measures available at follow-up time points were recorded, but data not extracted.
For further details and examples of outcome measure tools, see Report Supplementary Material 12.
Search methods for identification of studies
The initial stages of the search strategy were described earlier (see Chapter 4, Search methods for identification of studies); all RCTs included in the scoping review were taken forward to the study selection stage (see Study selection). This was followed by two further search procedures:
-
To ensure identification of RCTs published since the scoping review’s last search date, we updated our electronic searches, with the addition of a RCT study design filter to reflect narrower Cochrane Review inclusion criteria [last search 9 August 2021; Appendix 3 for example search (MEDLINE); Report Supplementary Material 13 for full searches].
-
We reviewed RCTs from the 2011 review. 6
Reference lists of included RCTs were searched and forward citation tracking (Science Citation Index) and Google Scholar was completed for all included RCTs.
Data collection
Study selection
Once database searches were updated, duplicates were excluded, as were titles unrelated to stroke and perception (KMcG/KT). Two researchers independently considered the abstracts for remaining records (CH, KMcG/KT), excluding any that did not meet the inclusion criteria. Full texts for all potentially relevant studies were independently assessed by two reviewers (CH, KMcG/KT), as well as full-text reports of all RCTs identified in the scoping review and the historical Cochrane Review6 were appraised. Disagreements relating to abstract or full-text inclusions were resolved through discussion, involving a third reviewer or relevant clinical specialist stakeholder where required. Reasons for exclusion were recorded.
Data extraction and management
Two piloted data extraction forms were used containing the data and categorisation variables (see Data charting and categorising) and detailing comparison(s) and outcomes measurement data. One reviewer extracted the data, which was checked by a second (KMc/KT/CH). For RCTs identified subsequent to the scoping review, the data were extracted directly into Revman (KT) and independently checked by a second reviewer (CH): The variables extracted were:
-
Study: country, setting, year, design, number of centres, number of randomised groups classified as (active intervention/no treatment/control).
-
Methods: randomisation method, prospective power calculation, use of intention‐to‐treat analysis, recruitment, dropouts.
-
Participant: inclusion criteria, exclusion criteria, number, age, sex, stroke details (type, time since stroke, hemisphere affected, severity), perceptual disorder, sense(s) affected and method of diagnosis, severity, presence of other stroke-related impairment.
-
Intervention: intervention approach (rehabilitation/NIBS/pharmacological/surgery/assessment and screening). Rehabilitation interventions subclassification (restoration, compensation or restitution, or a combination; see Intervention categorisation). Invention materials, procedures, provider, mode, location, session and duration details, tailoring and modification. 97
-
Outcomes: outcome measurement instrument, measurement taken, time point and final value scores. Dichotomous data: participant numbers who experienced the event in each group. Continuous data: means and standard deviations (SDs) by group.
Two review researchers (CH/KMcG/KT) independently classified all intervention approaches, seeking input from a third (DG/SH) where differences were unresolved through discussion.
For RCTs that randomised a mixed stroke/non-stroke or perceptual disorder/non-perceptual disorder subpopulations, we planned to extract the stroke-specific data wherever possible. Where this was unavailable, we used mixed population data if > 80% were stroke survivors with a perceptual disorder. We planned to conduct sensitivity analyses to investigate the effect of including these data.
Where data were unavailable, we calculated it from the available values. 222 For all outcomes, we recorded any significance test, t, f, p values and direction of findings. If a RCT provided data on more than one of the primary outcome measurement instruments, we extracted them in the order described above (see Primary outcome). Where a RCT reported more than one outcome measurement instrument relating to a single outcome previously unidentified, data on the measurement instrument’s validity and reliability were sought and compared, in consultation with a clinical expert in the relevant topic area, to choose the order of preference. This process did not consider the volume of data captured.
Data analysis
Risk of bias
Using the Cochrane ROB-1 tool we considered risk of bias. 223 Two reviewers (CH/KT) independently appraised included RCTs, grading the following risks:
Random sequence generation (selection bias): The method used to generate the random sequence was noted and judged whether it should produce comparable groups.
Allocation concealment (selection bias): The method used to conceal the allocation sequence was noted, and we considered whether intervention allocation could have been foreseen before or during enrolment.
Blinding (performance bias, detection bias): The measures used to mask RCT participants, researchers and outcome assessors from knowledge of allocation was noted, alongside any data on the measure’s effectiveness, and used to determine the degree to which blinding was achieved.
Incomplete outcome data (attrition bias): The completeness of data for each main outcome was noted, including attrition and exclusions from analyses, participant numbers in each intervention group (compared with total randomised), reasons for attrition or exclusions and any re-inclusion in analyses.
Selective reporting (reporting bias): The outcome measurement instruments, and the reported data were compared to identify missing data. Trial protocols were used where possible.
Other bias: We noted any other concerns about bias.
Risks of bias were categorised as high, low or unclear, with the reasons for decisions reported. Judgements for performance bias and detection bias were combined for reporting. If these differed, then the decision protocol was that (1) if low and unclear the overall category was labelled as unclear and (2) if high and low/unclear the overall category was considered as having a high risk of bias.
Data synthesis
The pre-specified comparisons for disorders in each sense (hearing, smell, somatosensation, tactile, taste and vision) were:
-
active intervention for perceptual impairment versus no treatment;
-
active intervention for perceptual impairment versus control (attention control/standard care/placebo).
We stratified the analysis according to intervention approach category (rehabilitation/NIBS/surgery/pharmacology/assessment and screening).
We also compared active interventions directly, where it was considered meaningful to do so. Decisions to do so were made following discussions among the research team, using tabulated data on the relevant RCTs.
Subgroup analysis and investigation of heterogeneity
We planned to consider a priori subgroup analyses where 10 or more RCTs were included in a single analysis,224 based on:
-
Treatment approach: rehabilitation, NIBS, surgery, pharmacology, assessment and screening.
-
Participants: age (younger 18–65 compared to older adults > 65); male versus female.
-
Stroke severity, time since stroke.
Sensitivity analysis
We planned sensitivity analyses on the primary outcome to explore the risk of bias and publication type (peer-reviewed publication, or other publication types such as conference abstracts, or unpublished reports). Where possible, we planned to explore the effect of selectively including RCTs which were at ‘high’ or ‘unclear’ risk.
Measures of treatment effect
Review Manager software (RevMan 5.4) supported the statistical analyses to determine treatment effects, using a random-effects model throughout. For dichotomous variables we planned to calculate a Peto odds ratio with 95% confidence intervals (CIs); for continuous data calculated the mean difference (MD) (for measurements in the same scale) and standardised MDs (for measurements in different scales) and 95% CI. We treated ADLs and other ordinal scales as continuous outcomes (an accepted meta‐analytic technique for ordinal outcome data is not yet available). Where a lower value indicated a better outcome on a measurement instrument, we multiplied the reported values by −1, so that a higher value was indicative of a better outcome across all analyses. We extracted final-value scores for analysis. If RCTs reported change-from-baseline values and the baseline value was available, we calculated the final value. If RCTs reported change values and the baseline value was not available, we used these data in meta‐analyses but planned sensitivity analyses to investigate the effect of including the data.
Unit of analysis
Where RCTs had more than one eligible active intervention group within the same comparison (against a control, placebo, standard care or no treatment group), we divided the control group data between the multiple pairwise comparisons to ensure there was no double counting of participants within any one analysis.
Heterogeneity assessment
Statistical heterogeneity was calculated using the I2 statistic; based on Cochrane guidance222 we used the I2 value to grade the level of heterogeneity:
-
I2 of 0 represents no heterogeneity.
-
0 < I2 < 30 may represent some heterogeneity.
-
30 ≤ I2 < 50 may represent moderate heterogeneity.
-
50 ≤ I2 < 75 may represent substantial heterogeneity.
-
75 ≤ I2 may represent considerable heterogeneity.
We explored individual RCT characteristics to identify potential sources of heterogeneity.
Reporting bias assessment
We compared the availability of outcomes of interest with those reported in the included RCTs and noted where an outcome measurement was described but not reported or data were unavailable for analysis (see Risk of bias). We planned to examine publication bias using a funnel plot where ≥ 10 or more RCTs were identified and reported a single outcome. 225
Assessment of the certainty of evidence
Confidence in the cumulative evidence for each meta-synthesis was independently judged by two reviewers (CH/ATB)226,227 based on the following criteria.
Risk of bias: we judged the risk of bias for each RCT using the Cochrane ROB tool (see Risk of bias). 228
Imprecision: we considered the number of studies/participants (up to two downgrades), whether the 95% CI excluded no effect (one downgrade) and presence of wide CIs (one downgrade). 229
Inconsistency: we considered overlap of CIs (one downgrade) plus the results of tests of heterogeneity (one downgrade if I2 ≥ 50%). 230
Indirectness: we considered the demographic details of the included populations (one downgrade), the nature of the interventions (one downgrade) and the outcomes measured (one downgrade). 231
Publication bias: where evidence of publication bias was identified, we planned to downgrade the evidence. 232
Beginning with a default grade of high quality, one downgrade reduced the level of evidence to moderate quality, two downgrades meant reduced it to low quality and three or more downgrades reduced it to very low quality. We used the following definitions of evidence:
-
High quality: very confident that the true effect lies close to that of the effect estimate.
-
Moderate quality: moderately confident that the true effect is close to the effect estimate, but it is possible that there is a substantial difference.
-
Low quality: limited confidence in the effect estimate; there may be a substantial difference between the effect estimate and the true effect.
-
Very low quality: little confidence in the effect estimate; it is likely that there is a substantial difference between the effect estimate and the true effect.
Summary of findings
We summarised results and our judgements of quality of evidence for comparisons of interventions with no treatment or control, for outcomes of ADLs, extended activities of daily living (EADLs), QoL, psychological well-being and mental health, perception and adverse events. We created a summary of findings tables for each sense addressed by included RCTs.
Interpreting the findings
Our Clinical Expert and Lived Experience Groups informed our interpretation of the Cochrane Review findings [see Stakeholder activities (what happened) and Report Supplementary Material 5].
Cochrane Review submission and peer review
The updated and expanded Cochrane systematic review was submitted to the Cochrane Stroke Editorial Group in keeping with Cochrane Library publication protocols where it underwent peer review233 by clinical experts, researchers and external stakeholders to ensure adherence to the appropriate Cochrane Review standards. 222
Summary
This Cochrane Review revision and expansion aimed to synthesise the RCT evidence relating to the effectiveness of interventions for perceptual disorders after stroke. Subsequent to the scoping review, it used similar rigorous data search and selection process, but with narrower inclusion criteria, limiting to RCTs, adult participants and six outcomes. We explored the comparison of active interventions to (1) no treatment, (2) control and (3) other active interventions for perceptual disorders in six senses, conducting meta-analyses where possible. Risk of bias was assessed using Cochrane tools for included RCT, and the confidence in evidence judged using GRADE criteria for each comparison. We worked with stakeholders to discuss and agree the meaning of the results.
Chapter 7 Cochrane systematic review results
Introduction
In this chapter, the results of the Cochrane systematic review and synthesis of the RCT evidence relating to the benefits of interventions for perceptual disorders following stroke are presented.
Results of the systematic search
The original scoping review search (see Chapter 5) identified 27 trials (including four ongoing). New records were identified in our updated search; screening of titles and abstracts highlighted 77 full texts to be considered, in addition to 10 records from the 2011 Cochrane Review. 6 Of 113 full texts considered, we included 18 RCTs, with further studies ongoing (n = 11) or awaiting additional information prior to assessment (n = 11) (Figure 9).
FIGURE 9.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis128 for Cochrane Review literature identification.
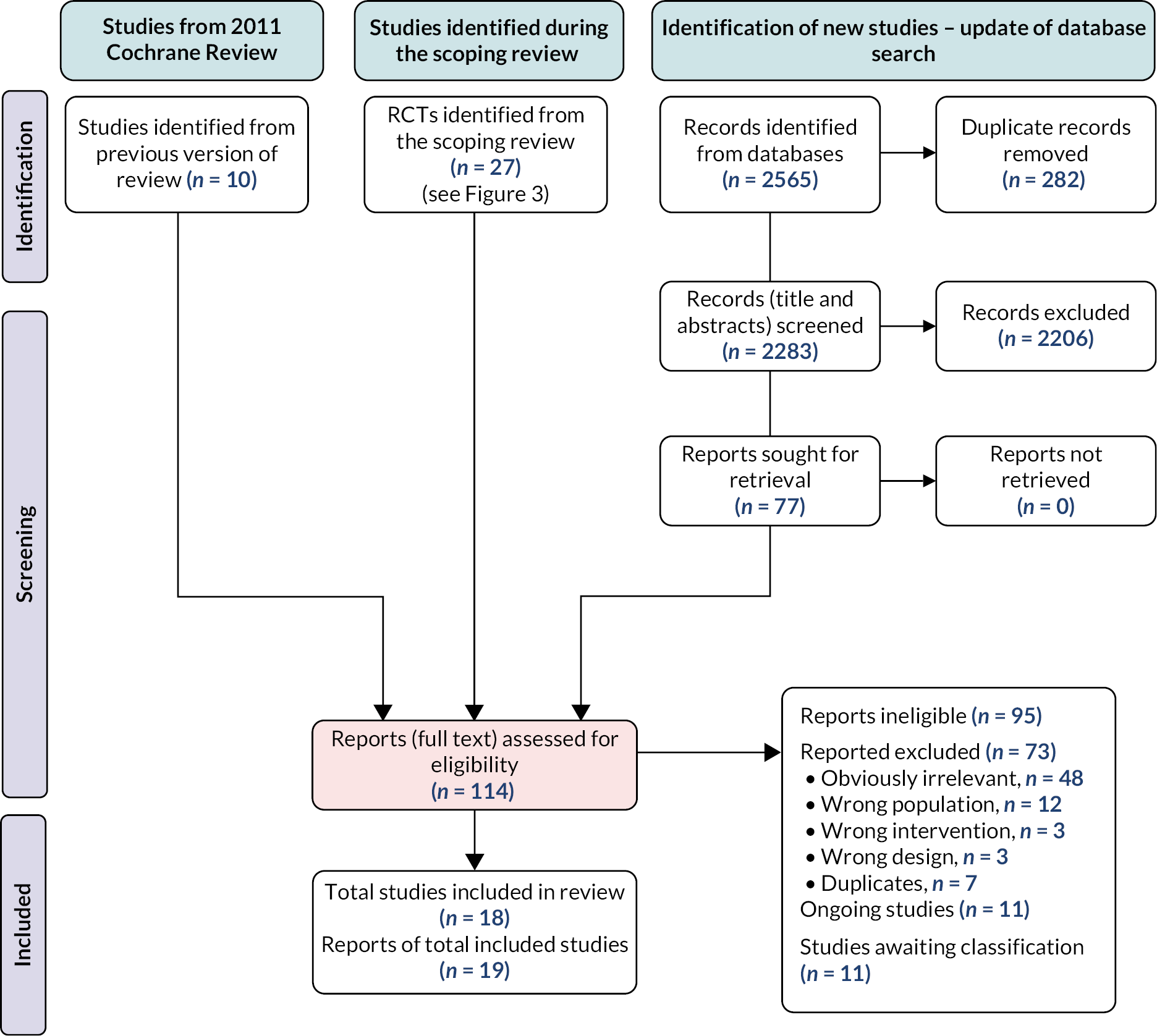
Included studies and population
We included 18 RCTs (n = 541; see Appendix 4 and Report Supplementary Material 14). Trials were conducted across seven countries in Asia, Australia, Europe and North America. Recruitment details were often absent or limited. Most RCTs (14/18, 77.8%) recruited from a single site, with three recruiting across two to six sites. 74,154,234 Trials took place in hospital or medical facilities; none were community based. One RCT used a crossover design. 235
Included RCTs randomised between 11 and 80 (mean 29.9, SD 15.9) participants. Dropout details, including the participant numbers lost during intervention delivery or follow-up, are given in Report Supplementary Material 15. Of the 541 participants included, 535 (98.9%) were stroke survivors:60,66,74,75,144,150,152,154,162,163,180,181,187,201,234–237 17 RCTs recruited stroke survivors only, but Lincoln 1985150 included 6/33 participants with head injury. Stroke duration onset varied, from approximately 19 days187 to 4.3 years. 236 The mean participant age varied from 48.8150 to 75.5 years,234 and RCTs included participants with haemorrhagic and ischaemic stroke.
Included perceptual disorders and interventions
The review included 18 RCTs that evaluated interventions for tactile, somatosensation, visual and mixed perception disorders. No interventions for disorders of hearing, taste or smell perception were identified.
Thirty-two interventions were identified and represented two approaches (1) NIBS,187 and (2) rehabilitation approaches; compensation (1 intervention),60 restitution (25 interventions),60,66,75,144,150,152,154,162,163,180,181,201,234–237 restitution combined with another rehabilitative approach (4 interventions),74,163,181,235 unclear (1 intervention). 74
The 18 included studies made 20 relevant comparisons:
-
three intervention versus no treatment comparisons
-
three interventions versus control (placebo/sham/control) comparisons
-
Fourteen intervention 1 versus intervention 2 comparisons.
Somatosensory perception dysfunction
Population: One hundred and ninety-six participants in seven RCTs. 75,162,163,180,181,187,234 Somatosensory perception disorders included Pusher syndrome (five RCTs), diagnosed using the Burke Lateropulsion Scale75,181 and Scale of Contraversive Pushing,75,163,180 and less specific disorders of somatosensation, diagnosed via practical assessments, such as pinprick and tight touch tests. 187,234 For analysis, we split these into ‘Pusher syndrome’ or ‘not Pusher syndrome’. Further population information is given in Report Supplementary Material 16.
Interventions:
-
Rehabilitation (restitution) (10 interventions) – game-based vertical posture training (n = 2),75,162 standard posture training (n = 2),75,162 conventional physiotherapy (for Pusher syndrome) (n = 2),163,234 physiotherapy + sensorimotor training,234 physiotherapy + motor training,234 computerised interactive visual feedback training (WiiFit),180 mirror feedback training. 180
-
Rehabilitation (restitution and substitution) (n = 2 studies) – robot-assisted gait training. 163,181
-
NIBS (one intervention) – tDCS. 187
Materials and procedures: One NIBS intervention used relevant equipment to deliver tDCS stimulation to the appropriate hemisphere and region. 187 All other interventions used rehabilitative approaches: for Pusher syndrome therapy these could be grouped into either (1) game-based postural training, with supporting equipment, or (2) conventional physiotherapy for Pusher syndrome; for non-pusher disorders one RCT explored sensory-motor training focusing on sense discrimination, and table-top ‘motor therapy’.
Game-based postural training used one of three named interventions: WiiFit (computer-based exercises and balance board),180 Lokomat (computer-based exercises, supportive harness and treadmill)163,181 and Spine Balance 3D (computer-based exercises and whole-body tilt apparatus). 75,162 Each was used to provide physical therapy, with participants asked to achieve a set body position or movement in response to the computerised exercises, and in relation to any positional change caused by the associated equipment.
Conventional physiotherapy for Pusher syndrome often involved postural training and weight shifting, using visual cues in the room to regulate posture, alongside verbal feedback from the therapist. Materials used were often unclear but included a chair for seated exercises and a mirror for feedback. 75,162,163,180,181
Sensory-motor training used the SENSe approach with three sensory discrimination tasks: texture discrimination, limb position sense and object recognition. 234 Materials in these tasks included different textures (fabric, wallpaper, plastic and sandpaper), different objects of varying shape, size and materials. A range of exercises were used, such as smoothing out fabric while appreciating the texture, moving the limb to a specific position and arranging bottles in order of weight. Motor therapy used tabletop games such as chess (with clear cognitive and attentional demand), with a set programme of upper limb exercises used to improve gross movement and dexterity. 234
Delivery: All interventions were delivered one to one, in a hospital setting. A physiotherapist delivered 5 of 13 interventions (which for 1 RCT specified that they had ‘more than 5 years’ experience’)162,180,181 while the remaining providers were not reported, or unclear.
Schedule and duration: There was similarity in the schedule and duration of the rehabilitation interventions. Sessions typically lasted 30–60 minutes per day, 3–5 times per week, for a duration of 2–4 weeks in total. In contrast, NIBS was delivered for 20 minutes per day for 10 days. 187
Tailoring and modification: Tailoring of interventions varied; seven interventions did not report tailoring. 75,162,163,180,187,234 Others reported that exercises were tailored to the participant’s ability before training began75,162,181,234 or that the difficulty level was altered relative to performance; for example the ‘the speed and range of trunk movement’ was increased or exercises were ‘changing from sitting to standing.’162 Detailed intervention descriptions are given in Report Supplementary Material 14. Intervention modifications were not reported by any RCT.
Tactile perception dysfunction
Population: Seventy participants with tactile perceptual dysfunction, in three RCTs. 201,235,236 Diagnostic tools included the revised Nottingham Sensory Assessment (rNSA)235 and Semmes Weinstein monofilament201,236 (see Report Supplementary Material 16).
Interventions:
-
Four rehabilitation (restitution) interventions – pressure sense perception training on stable surface,201 pressure sense perception training on unstable surface,201 hand exercises (without glove)235 and a vibrating glove ‘VTS Glove.’236
-
One rehabilitation (restitution and substitution) intervention – robot glove-based hand exercises. 235
Materials and procedures: Interventions were of two main types – pressure sense training involving exercises on a stable or unstable surface,201 and hand exercises, either with or without a glove to assist. 235,236 With pressure sense training, participants stood on either a stable foam block, or on an unstable balance pad. They were asked to shift weight to their affected side, and pressure in the heel was measured to ensure a desired level was reached. 201 Hand exercises included a range of passive range of motion tasks, and task-based activities and games. The addition of a robotic glove was used to detect movement and provide a simultaneous display of performance on a computer screen, as well as providing sensory stimulation. 235 A different, vibrating, glove was used to provide stimulation to skin on the palm and fingers; it did not require any exercises. 236
Delivery: Interventions were delivered by physiotherapists (two interventions201), OTs (two interventions235) in one-to-one sessions in a hospital setting (four interventions201,235), with the vibrating glove used by participants themselves at home (one intervention236).
Schedule and duration: Varied from 30-minute sessions, 3 days per week201 to 3-hour sessions, 7 days per week. 236 Total duration ranged from 4 to 8 weeks.
Tailoring and modification: Tailoring to participants’ ability was not reported for two interventions. 235,236 Pressure sense training was tailored to participants’ heel pressure, and allowed progression to a more difficult stage when a suitable pressure level was achieved,201 and robot-assisted hand exercise settings were adjusted to participants’ ability. 235 Detailed intervention descriptions are given in Report Supplementary Material 14. No modifications of the interventions during the RCT were reported.
Visual perception dysfunction
Population: Two hundred and twenty-five participants, in seven RCTs. 60,66,144,150,152,154,237 Visual perception disorders were diagnosed using specialised tests such as the MVPT or Rivermead Perceptual Assessment Battery,144,237 cognitive tests that include visual perception subsections (Modified Mental State Examination),66,152 and a complex figure-drawing test to test for visual memory deficit. 154
Interventions:
-
Eleven rehabilitation (restitution) interventions – image drawing – global processing training,154 image drawing – rote repetition training,154 neurofeedback (NFB) training,66 WiiFit virtual reality training (WVRT),144 general balance training,144 transfer of training perceptual treatment,60 computerised visual perception rehabilitation with motion tracking,237 computer-based cognitive rehabilitation program,237 OT-led perceptual training,150 computer-based cognitive rehabilitation training,152 conventional cognitive rehabilitation. 152
-
One rehabilitation (compensation) intervention – functional perceptual treatment. 60
Materials and procedures: All interventions used a rehabilitation approach and grouped into five main types: paper-based tasks, OT-led task-based training, physical interventions, cognitive and perceptual exercises and neurofeedback training.
Three interventions used paper-based tasks: in two, participants traced then reproduced a complex figure (Rey–Osterrieth Complex Figure) either as a whole figure or broken down into its component parts154; the third used ‘conventional cognitive rehabilitation’, the exact nature of which was not clearly stated. 152 Three interventions used an OT approach, training visual perceptual skills using functional and task-based training. 60,150 Although not well described, this training included simple perceptual activities such as stick length sorting, colour matching and parquetry mosaic tasks. One RCT explored physical interventions for visual perceptual disorders alongside balance disturbance. 144 WiiFit with balance board training used a range of activities to stimulate interest and motivation, such as simulated tightrope walking and slalom, and that encouraged multidirectional weight shifting. Balance training used a balance board, with the participant asked to shift weight, using a mirror for feedback. One approach used computerised exercises – these frequently were called ‘cognitive’ in nature, but had a clear focus on improving visual perceptual skills, including object recognition, object constancy, figure-ground organisation, visual discrimination and visual organisation. 152,237
Delivery: Delivery was poorly reported for visual perceptual disorder interventions. Where reported, a physiotherapist (one intervention144) and OT (three interventions235,237) delivered interventions on a one-to-one basis, in hospital settings.
Schedule and duration: Two interventions (involving paper-based repetition training) were delivered in a single 90-minute session. 154 For the other interventions, sessions typically lasted 30 minutes, 3–5 times per week, for 4–6 weeks.
Tailoring and modification: Tailoring was either not reported or was unclear for nine interventions; in three others, the intervention exercises and difficulty were based on the participant’s perceptual ability. 150,152 No intervention modifications were reported.
One RCT (50 participants) explored a mixed perceptual disorder, addressing tactile-somatosensory disorder. Mixed-sensory categories were not included in our analysis plan and were thus excluded.
Outcome measures
Our primary outcome, ADLs was measured by seven RCTs, using the Modified Barthel,60,235,237 Korean Modified Barthel75,162,181,187 and Edmans ADLs Index. 60
The frequency of reporting secondary outcomes varied; perception (11 RCTs), adverse events (6 RCTs), mobility and navigation (4 RCTs) and EADLs (1 RCT). No RCTs reported measures of social activity and participation, QoL, or psychological well-being and mental health outcomes (see Report Supplementary Material 17).
Six RCTs reported adverse events. It was unclear whether the data reported described the number of participants experiencing an adverse event or the number of adverse events experienced across the RCT population. We chose to present this narratively.
All five RCTs evaluating interventions for Pusher syndrome used severity outcome measures (Burke Lateropulsion Scale, Scale for Contraversive Pushing). We extracted, analysed and presented this data, as it provided treatment effectiveness information relevant to clinical practice.
Excluded studies
We excluded 73 studies with reasons provided for 18 where decisions were more difficult (see Report Supplementary Material 18). The main reason for exclusion was the absence of a perceptual disorder diagnosis.
Ongoing studies
We identified 11 ongoing RCTs of interventions, and in most cases these could be related to disorders of perception in specific senses: smell (1 RCT), somatosensation (7 RCTs) and visual perception (2 RCTs)238–246 (see Report Supplementary Material 19).
Studies awaiting classification
Eleven RCTs identified are awaiting classification159,172,202,203,247–252 (see Report Supplementary Material 20). As we could not determine if they met inclusion criteria these were not included; their status will be reconsidered at the point that any updates of this Cochrane Review are conducted.
Risk of bias in included studies
We evaluated included RCTs risk of bias (see Risk of bias) and provided an overview of these risks in Figure 10 (individual study risk of bias data is in Report Supplementary Material 14, risk of bias summary is given in Report Supplementary Material 21).
FIGURE 10.
Risk of bias graph: judgements on each methodological quality item presented as percentages across all included studies. Note: this figure is based on one published in CDSR. 204
Reproduced with permission from Hazelton et al. 204 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
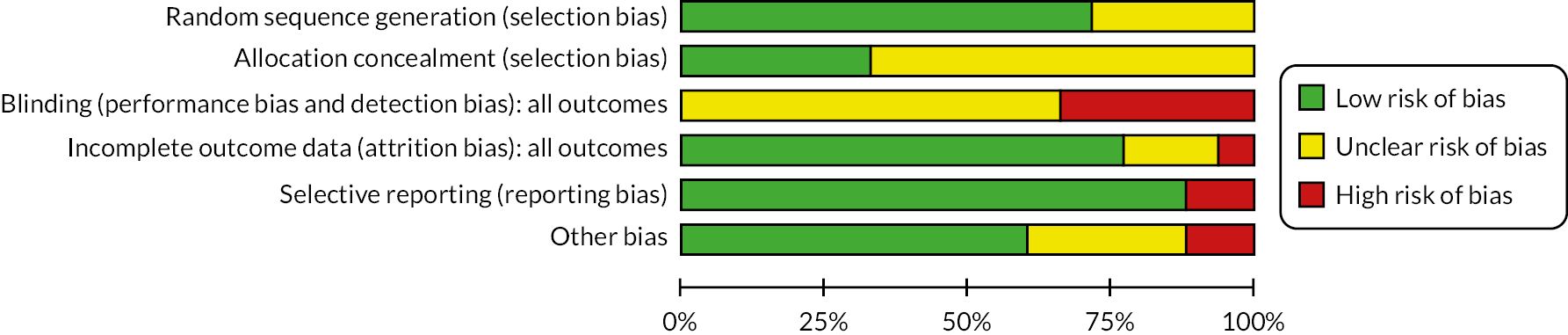
Randomisation and concealment of allocation
All included RCTs described random sequence generation, but the method used was unclear for five RCTs. 66,75,144,150,236 The concealment of allocation was clearly stated by six RCTs,60,74,154,162,163,234 including sealed, opaque envelopes or a sealed box.
Blinding
In complex rehabilitation RCTs, adequate blinding of clinicians delivering and participants receiving an intervention is a significant challenge; no RCTs were assessed as having a low risk of bias. Blinding of outcome assessors was reported by 13 RCTs but was unclear for 5 RCTs. 66,75,152,181,201 Six RCTs were judged as having a high risk of bias. 60,74,144,154,234,237
Incomplete outcome data
Three RCTs were judged as unclear66,150,154 in their reporting of attrition bias with the remaining RCTs either including all participants in their analyses or accounting for all participants (with reasons given for dropout) and an intention-to-treat analysis conducted. One RCT234 had a high risk of bias as three participants were excluded from primary and follow-up analysis.
Selective reporting
For 16 RCTs, outcome measures were well reported. Only one RCT provided partial data for three outcomes234 and a further RCT74 failed to provide data for secondary outcomes.
Other potential sources of bias
Two RCTs had a high risk of bias. In one,163 several participants had severe cognitive deficits and the authors stated these deficits might have influenced the participants’ response to the intervention, although there were no data to support this intervention response. In a second,234 the mean age of the experimental group was significantly higher than the control group, and with more right hemispheric lesions.
Comparability of groups at baseline was adequate for 11 RCTs,60,74,144,154,162,180,181,187,201,235,237 while 5 RCTs were judged unclear. This was due to a lack of reporting of the baseline characteristics of participants66,152,236 or due to difficulties ‘securing homogeneity’ of participants, the nature of which was not fully explained. 75 For one,150 there was a change to eligibility criteria part-way through the RCTs to address slow recruitment: the RCT included those left hemisphere strokes, subarachnoid haemorrhage and head injury. It is unclear what interim analyses were undertaken and what the decision-making process was for continuation, adaptation and eventual stopping of the RCT.
Effects of interventions
Hearing
There were no RCTs of interventions for hearing perceptual disorders.
Smell
There were no RCTs addressing smell perceptual disorders.
Somatosensation
We identified seven relevant RCTs: five recruited people with Pusher syndrome75,162,163,180,181 and two included participants without Pusher syndrome187,234 (Table 7).
| Patient or population: Stroke survivors with somatosensory perception disorders Settings: Any Intervention: Rehabilitation for Pushers or Rehabilitation for non-Pushers Comparison: No treatment or control |
|||||
|
Outcome
(at end of intervention period) |
Comparison |
Relative effect
(95% CI) |
n (RCTs) | GRADE | Comments |
| ADLs – rehabilitation for non-Pushers |
Active intervention vs. no treatment | – | no studies | – | |
| Active intervention vs. control |
MD 10.08 (−2.47 to 22.63) |
24(1) (Koo 2018)187 | Very lowa,b,c | No difference between intervention and control | |
| EADLs | Active intervention vs. no t)reatment or vs. control | – | No studies | – | |
| QoL – mobility and navigation – rehabilitation for non-Pushers |
Active intervention vs. no treatment | – | No studies | – | |
| Active intervention vs. control |
MD 0.50 (−0.38 to 1.38) |
24 (1) study (Koo 2018)187 | Very lowa,b | No difference between intervention and control | |
| Psychological well-being and mental health | Active intervention vs. no treatment or vs. control | – | No studies | – | |
| Perception | Active intervention vs. no treatment | – | No studies | – | |
| Active intervention vs. control | Insufficient detail to allow analysis | 24 (1) (Koo 2018)187) |
– | ||
| Adverse events | Active intervention vs. no treatment | – | No studies | – | |
| Active intervention vs. control | Insufficient detail to allow analysis | 24 (1) (Koo 2018)187 | Authors stated that ‘all the participants completed the stimulation sessions successfully without complaining about any discomfort during the procedure’ | ||
Intervention versus no treatment or control
Pusher syndrome
No RCTs.
Not Pusher syndrome
One RCT,187 comparing tDCS to sham treatment.
Activities of daily living
-
ADLs measured using the Korean Modified Barthel Index.
-
Analysis showed no difference between active intervention and control (MD 10.08, 95% CI −2.47 to 22.63, p = 0.12); the evidence was of very low certainty.
FIGURE 11.
Comparison of intervention vs. no treatment or control for somatosensory disorders. Outcome – ADLs. Note: All forest plots in this chapter are taken from the review published in CDSR. 204
Reproduced with permission from Hazelton et al. 204 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
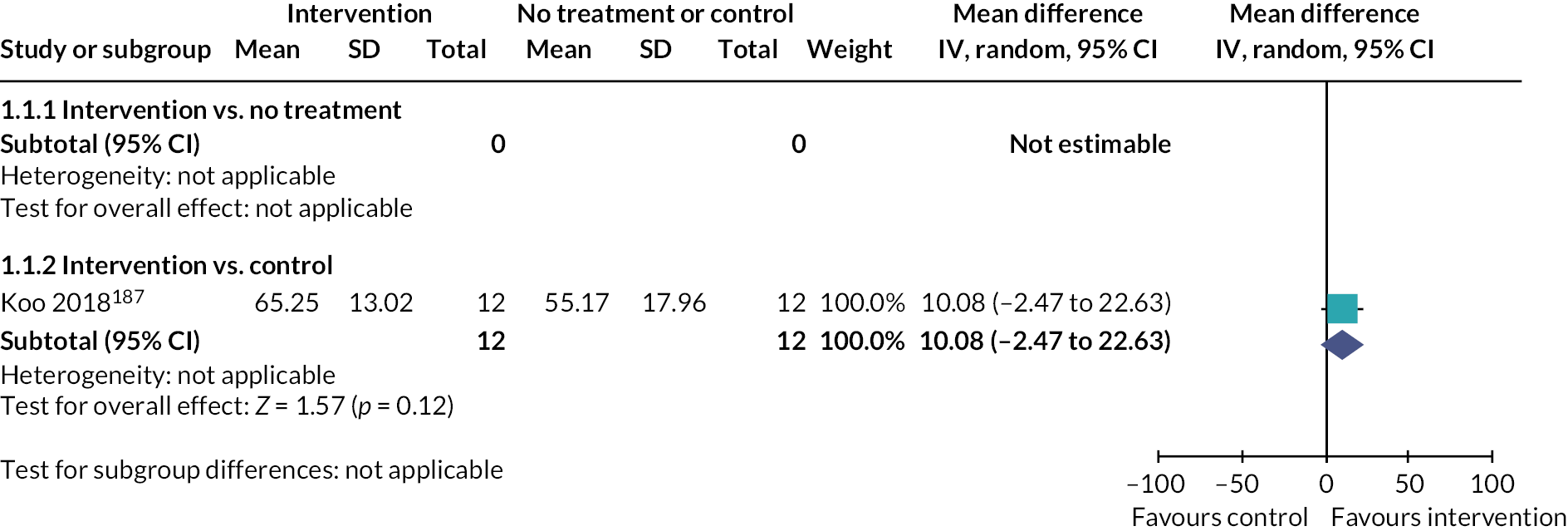
Mobility
-
Mobility assessed using the Functional Ambulation Category.
-
Analysis showed no difference between active intervention and control (MD 0.50, 95% CI −0.38 to 1.38, p = 0.27); the evidence was judged to be of very low certainty.
FIGURE 12.
Comparison of intervention vs. no treatment or control for somatosensory disorders. Outcome – mobility and navigation.
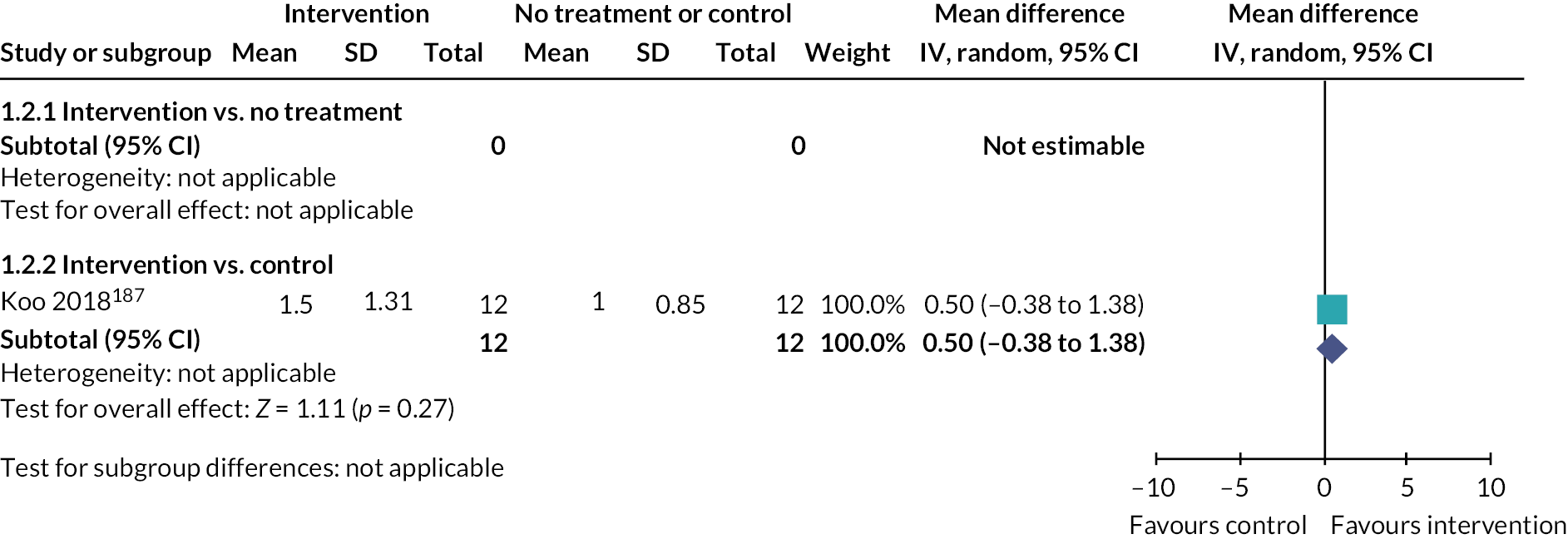
Perception
-
One RCT (24 participants). 187
-
Measured somatosensory perception (modified Nottingham Sensory Assessment).
-
Summary data were unreported (subscales only) and could not be included in analysis.
Adverse events
-
One RCT187 (24 participants).
-
Stated that ‘all the participants completed the stimulation sessions successfully without complaining about any discomfort during the procedure’.
Intervention 1 versus intervention 2
Pusher syndrome
Five RCTs addressed this comparison. 75,162,163,180,181 All RCTs compared an intervention training posture or/and movement using equipment such as balance boards, treadmills or harnesses alongside computerised to conventional physiotherapy for Pusher syndrome. Interventions in both groups were considered similar and the data were combined in meta-analyses.
Activities of daily living
-
ADLs assessed using the Korean Modified Barthel index.
-
Intervention 1 (computerised balance and movement training) was more beneficial than Intervention 2 (‘standard’ Pusher syndrome physiotherapy) [MD 10.19 (4.94 to 15.44), p = 0.0001]; there was no heterogeneity (I² = 0%) and evidence was of very low certainty.
FIGURE 13.
Comparison of active intervention 1 vs. active intervention 2 for somatosensory perception disorder. Outcome – ADLs.
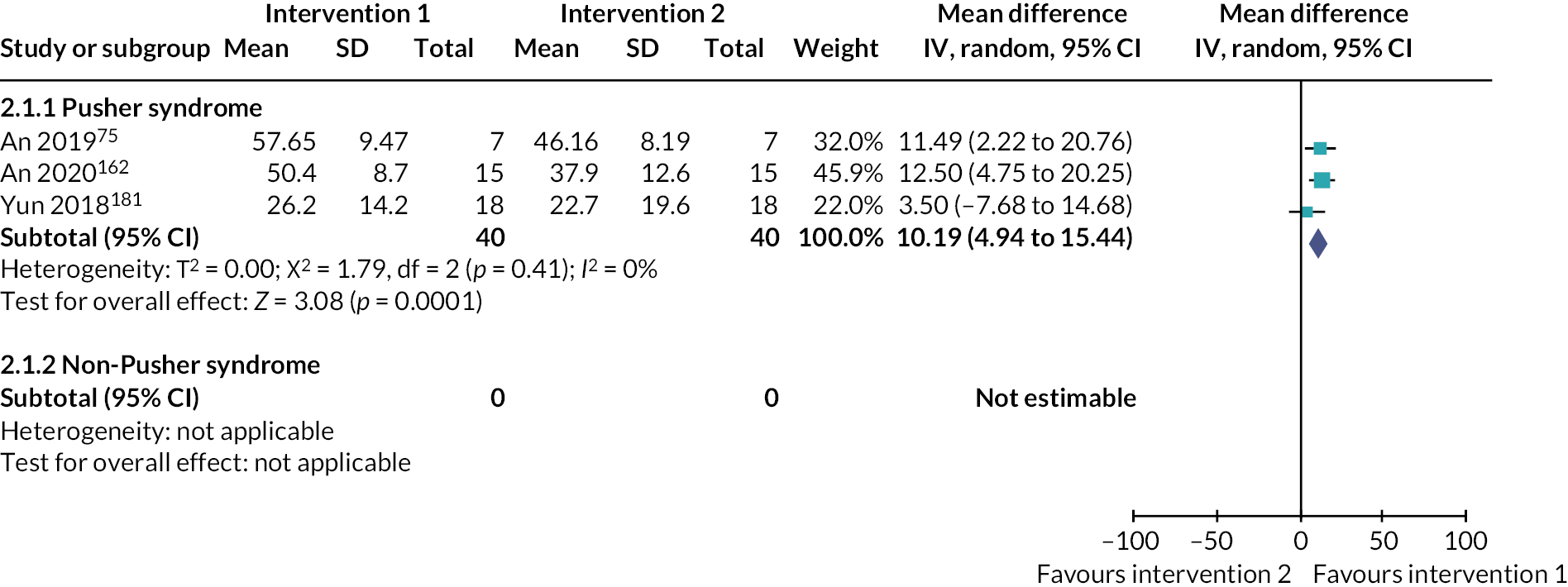
Mobility
-
Mobility assessed using the Performance Oriented Mobility Assessment – Balance (POMA-B) outcome measure.
-
Analysis showed no evidence of a difference between Interventions 1 and 2 [MD 1.00 (−1.51 to 3.51)]. Evidence was judged to be of very low certainty.
FIGURE 14.
Comparison of active intervention 1 vs. active intervention 2 for somatosensory perception disorder. Outcome – mobility and navigation.
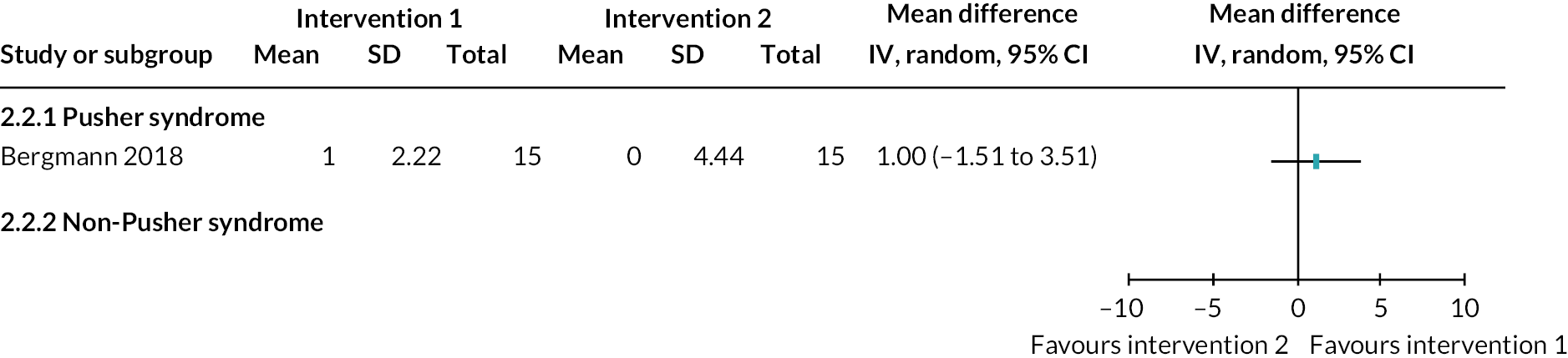
Perception
-
Assessed perception via subjective visual vertical test.
-
There was no evidence of a difference between intervention 1 and 2 [standardized mean difference – SMD 0.52 (−0.21 to 1.25)]. Evidence was judged to be of very low certainty.
FIGURE 15.
Comparison of active intervention 1 vs. active intervention 2 for somatosensory perception disorder. Outcome – perception.
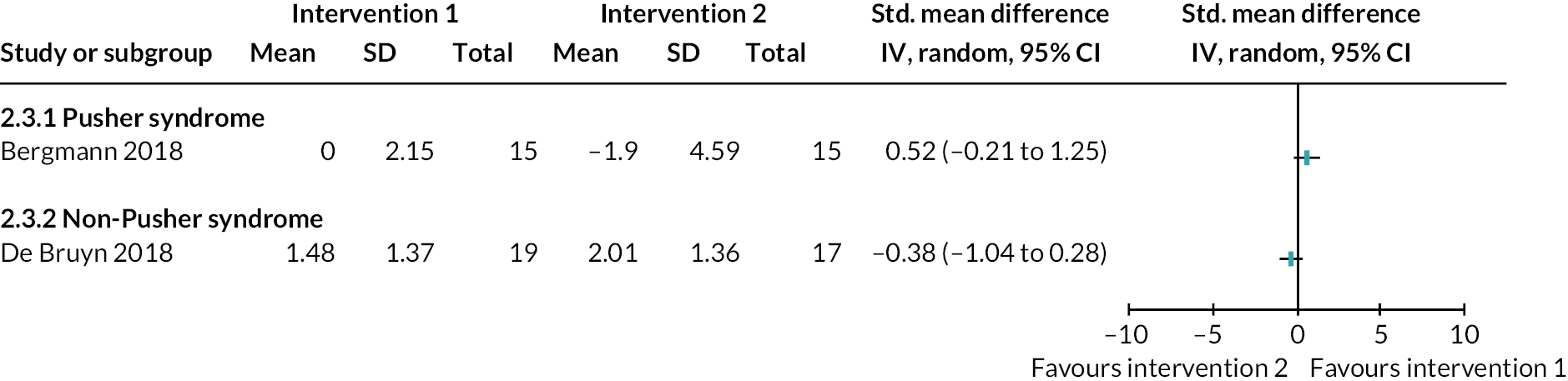
Pusher syndrome outcomes
-
Four RCTs (86 participants); Figure 16.
-
Pusher syndrome severity assessed using the Burke Lateropulsion Scale75,162 or the Scale of Contraversive Pushing. 163,180
-
Analysis showed a tendency for intervention 1 (computerised balance and movement training) to be more beneficial than intervention 2 (standard Pusher syndrome physiotherapy) (SMD 1.03, 95% CI 0.33 to 1.73), p = 0.004). Results may suggest substantial heterogeneity (I² = 50%), and the evidence was judged to be of very low certainty.
FIGURE 16.
Comparison of active intervention 1 vs. active intervention 2 for somatosensory perception disorder. Outcome – Pusher syndrome outcomes.
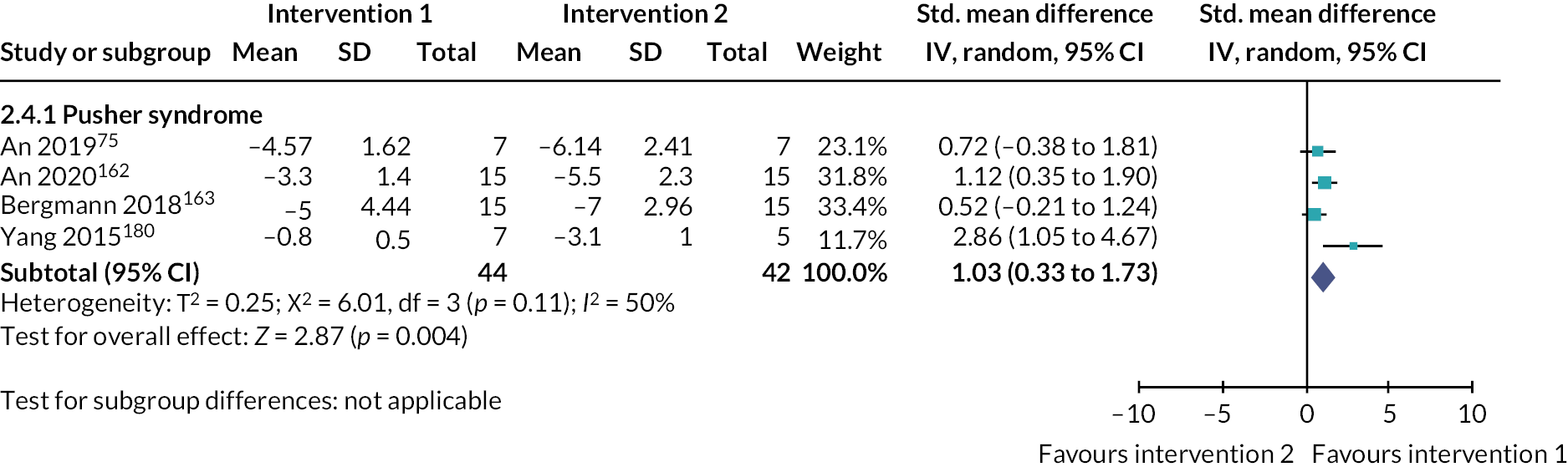
Not Pusher syndrome
One RCT234 addressed this comparison, comparing the SENSe intervention, consisting of sensorimotor therapy and sensory discrimination tasks, with cognitive table-top games and motor exercises.
Tactile
We identified three relevant RCTs. 201,235,236 Data contributed to analysis by Kim (2015)201 and Lee (2021)235 (Table 8).
| Rehabilitation interventions compared to no treatment or control for tactile perception disorders | |||||
|---|---|---|---|---|---|
| Patient or population: Stroke survivors with tactile perception disorders Settings: Any Intervention: Rehabilitation Comparison: No treatment or control |
|||||
|
Outcome
(at end of intervention period) |
Comparison |
Relative effect
(95% CI) |
n (RCTs) | GRADE | comments |
| ADLs | Active intervention vs. no treatment or vs. control | – | No studies | – | |
| EADLs | Active intervention vs. no treatment or vs. control | – | No studies | – | |
| QoL – mobility and navigation | Active intervention vs. no treatment |
MD 6.50 (−4.81 to 17.81) |
30 (2) Kim 2015 (stable),201 Kim 2015 (unstable)201 | Insufficient evidencea,b,c,d,e,f | A number of methodological concerns led to the judgement that there was insufficient evidence to support a conclusion based on these data |
| Active intervention vs. control | – | No studies | – | ||
| Psychological well-being and mental health | Active intervention vs. no treatment or vs. control | – | No studies | – | |
| Perception | Active intervention vs. no treatment |
MD 4.64 (3.06 to 6.22) |
30 (2) – Kim 2015 (stable),201 Kim 2015 (unstable)201 | Very lowa,b,f | Favours intervention |
| Active intervention vs. control | – | No studies | – | ||
Intervention versus no treatment or control
One RCT compared two active interventions with no treatment. 201
Quality of life – navigation and mobility
-
One RCT with two relevant comparisons (30 participants);201 Figure 17.
-
Mobility and navigation were measured using the timed-up-and-go measure.
-
Analysis showed no difference between active intervention and no treatment (MD 6.50, 95% CI −4.81 to 17.81, p = 0.26). Results may suggest substantial heterogeneity (I2 = 50%). Due to a number of methodological concerns, it was judged there was insufficient evidence to support a conclusion based on these data.
FIGURE 17.
Intervention vs. no treatment or control for tactile perception disorder. Outcome – mobility and navigation.
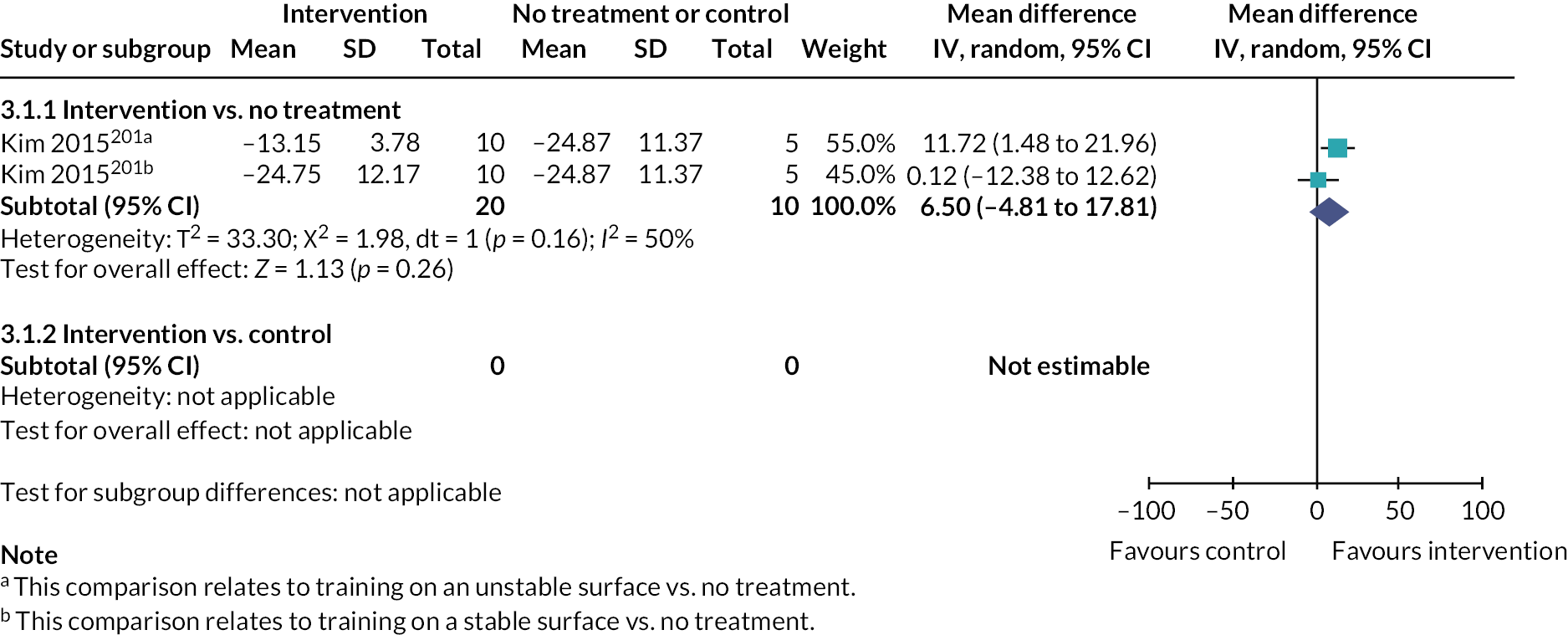
Perception
-
One RCT with two relevant comparisons (30 participants);201 Figure 18.
-
Perception was assessed using a dynamometer-based measure of proprioception.
-
Analysis showed that a tendency for active intervention was more beneficial than no treatment (MD 4.64, 95% CI 3.06 to 6.22, p ≤ 0.00001). Results showed no heterogeneity (I2 = 0%), and the evidence was judged to be of very low certainty.
FIGURE 18.
Intervention vs. no treatment or control for tactile perception disorder. Outcome – perception.
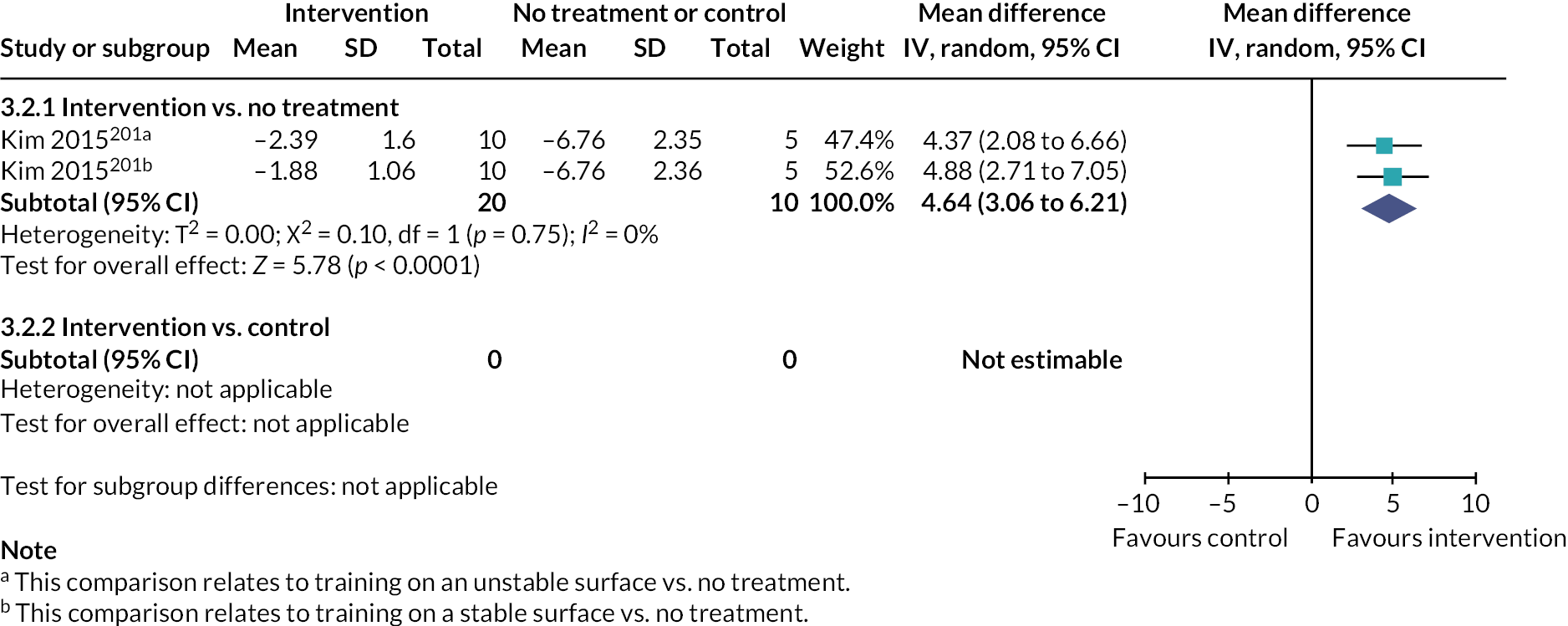
Active intervention 1 versus active intervention 2
Two RCTs addressed this comparison: one235 compared robot gloved-based hand exercises to hand exercises alone, and another201 compared pressure sense perception training on a stable surface to an unstable one (balance board). As the interventions were quite different, data were not combined in meta-analysis.
Activities of daily living
-
ADLs were assessed using the Modified Barthel Index.
-
This RCT demonstrated no difference between the interventions (MD −0.41, 95% CI −12.31 to 11.49). The evidence was considered to be of very low certainty.
FIGURE 19.
Active intervention 1 vs. active intervention for tactile perception disorder. Outcome – ADLs.
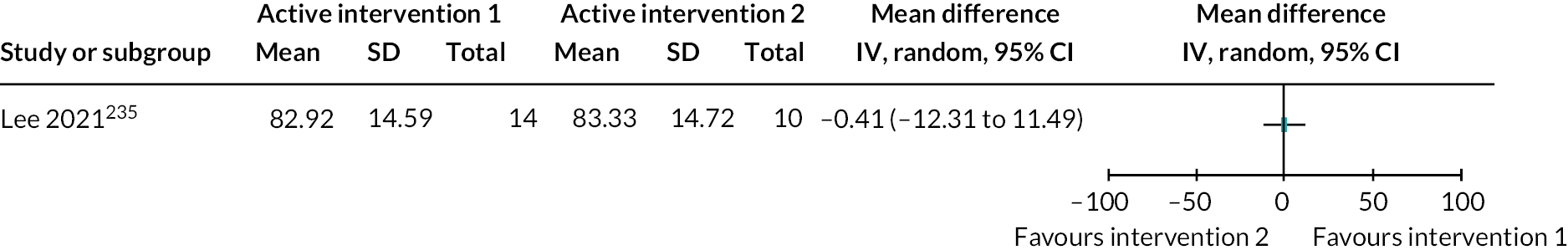
Quality of life – navigation and mobility
-
Mobility and navigation were assessed using the timed-up-and-go measure.
-
This RCT provided evidence that intervention 2 (training on the unstable balance board) was more beneficial than intervention 1 (training on the stable balance board) (MD −11.60, 95% CI −19.50 to −3.70). The evidence was judged to be of very low certainty.
FIGURE 20.
Active intervention 1 vs. active intervention for tactile perception disorder. Outcome – mobility and navigation.
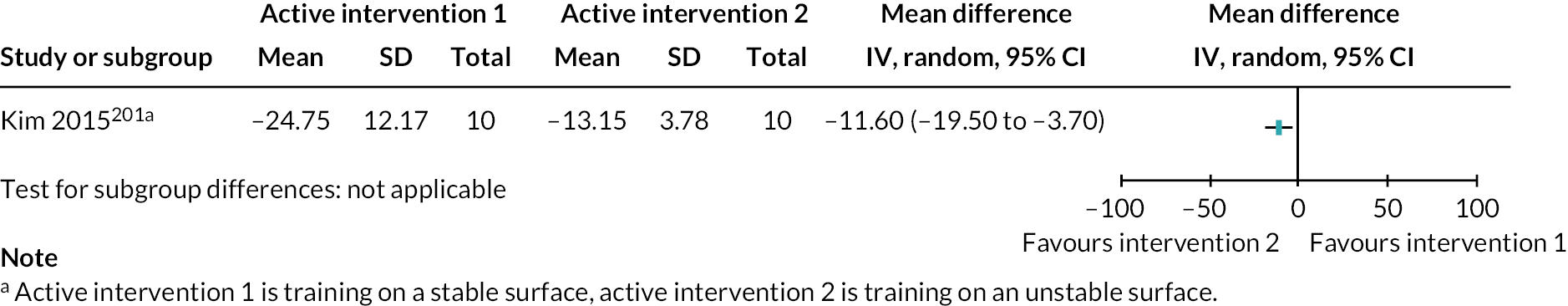
Perception
-
One assessed proprioception using a dynamometer and the other used the kinaesthetic subtest of the rNSA.
-
Analysis did not combine data for these two RCTs: individually neither RCT provided evidence of a difference between interventions 1 and 2.
FIGURE 21.
Active intervention 1 vs. active intervention for tactile perception disorder. Outcome – perception.
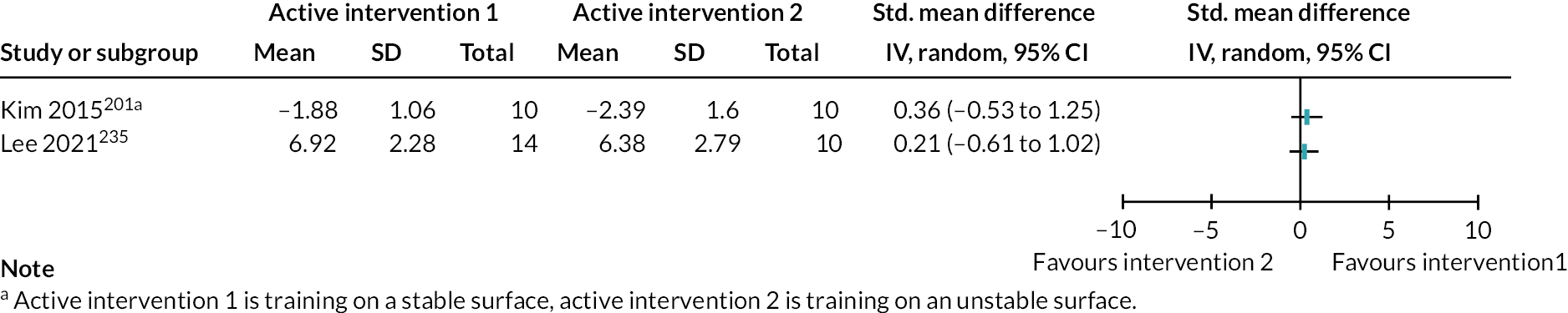
Adverse events
One RCT235 collected data on adverse events, reporting ‘no safety concerns or adverse events’.
Taste
No RCTs of interventions addressing taste perception disorder were identified.
Vision
There were seven relevant RCTs60,66,144,150,152,154,236 (Table 9).
| Rehabilitation interventions compared to no treatment or control for vision perception disorders | |||||
|---|---|---|---|---|---|
| Patient or population: Stroke survivors with vision perception disorders Settings: Any Intervention: Rehabilitation Comparison: No treatment or control |
|||||
|
Outcome
(at end of intervention period) |
Comparison |
Relative effect
(95% CI) |
n (RCT) | GRADE | Comments |
| ADLs | Active intervention vs. no treatment or vs. control | – | No studies | – | |
| EADLs (Analysis 5.1) |
Active intervention vs. no treatment | – | No studies | – | |
| Active intervention vs. control | MD 0.94 (−1.60 to 3.48) |
33 (1) Lincoln 1985150 | Very lowa,b | No difference between groups | |
| QoL – social and participation/QoL/mobility and navigation | – | No studies | – | ||
| Psychological well-being and mental health | – | No studies | – | ||
| Perception | Active intervention vs. no treatment | MD −1.75 (−5.39 to 1.89) |
27 (1) Cho 201566 | Very lowa,b,c | No difference between groups |
| Active intervention vs. control | – | No studies | – | ||
| Adverse events | Active intervention vs. no treatment or vs. control | – | No studies reported adverse events | – | |
Intervention versus no treatment or control
One RCT compared intervention (neurofeedback training) with no treatment,66 and one RCT compared intervention (perceptual training) with control (conventional (not perceptual) therapy). 150
EADLs
-
ADLs were assessed using the Rivermead ADLs scale.
-
Analysis showed there was no evidence of a difference between active intervention and control (MD 0.94, 95% CI –1.60 to 3.48, p = 0.47). The evidence was judged to be of very low certainty.
FIGURE 22.
Intervention vs. no treatment or control for visual perception impairment. Outcome – EADLs.
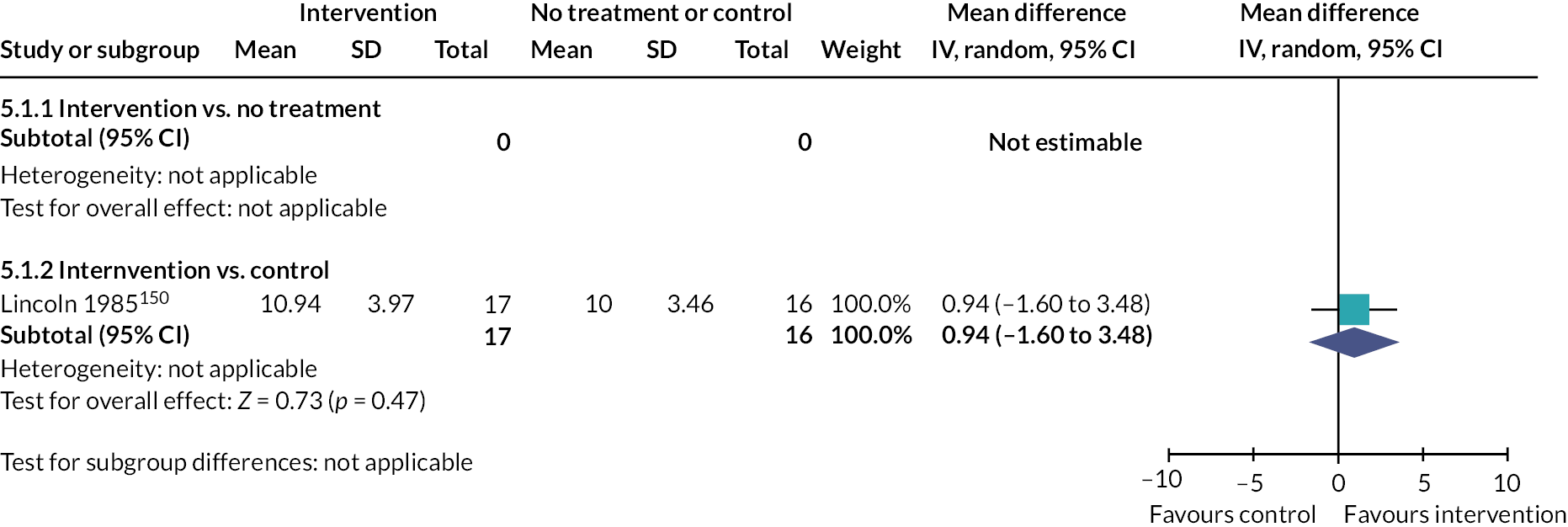
Perception
-
Perceptual outcomes assessed using the MVPT. Another RCT150 measured perception using the Rivermead Perceptual Assessment Battery: as only subscale scores for the Rivermead Perceptual Assessment Battery were reported, the data were not included in the analysis.
-
Analysis showed no difference between active intervention and no treatment (MD −1.75, 95% CI −5.39 to 1.89, p = 0.35). The evidence was judged to be of very low certainty.
FIGURE 23.
Intervention vs. no treatment or control for visual perception impairment. Outcome – perception.
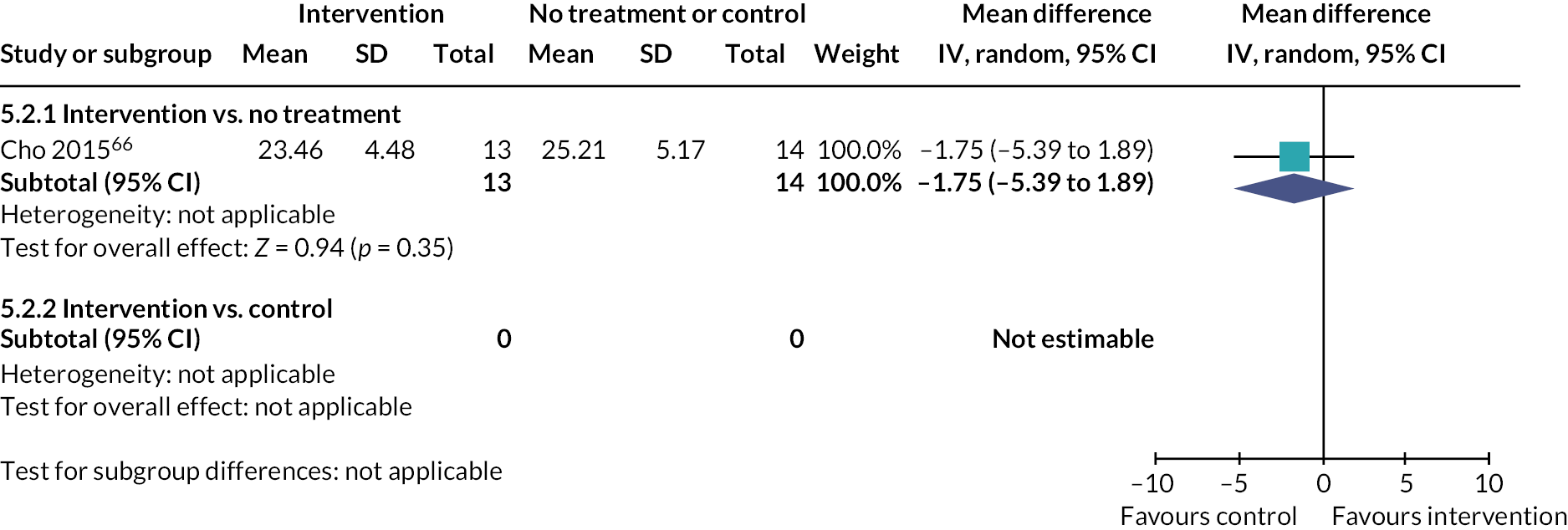
Active intervention 1 versus active intervention 2
Five RCTs addressed this comparison. 60,144,152,154,237 The interventions were dissimilar, including OT-led training in practical tasks, paper-based repetition exercises and computer-based games; statistical pooling of data was inappropriate.
Activities of daily living
-
ADLs were assessed using the Modified Barthel Index.
-
Data were not combined due to differences in the interventions. The evidence from each included RCT was judged to be of very low certainty.
FIGURE 24.
Active intervention 1 vs. active intervention for visual perception impairment. Outcome – ADLs.
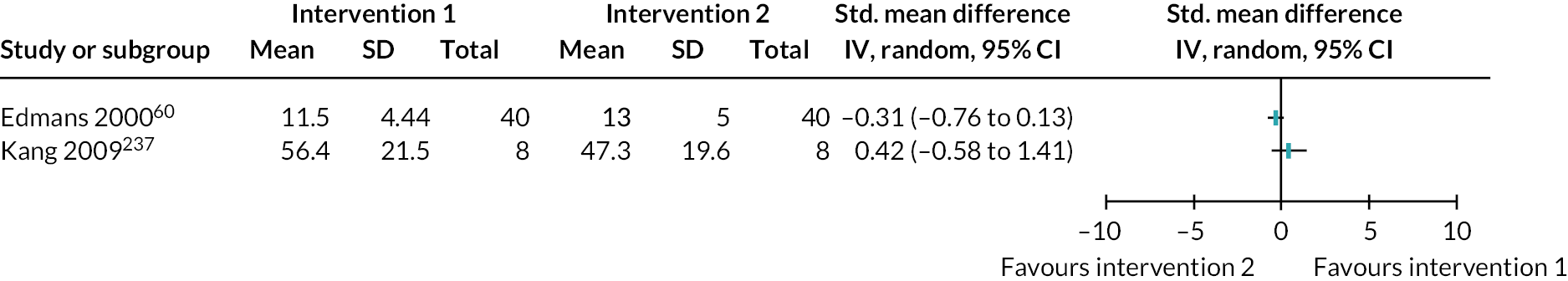
Quality of life – mobility and navigation
-
10-m walking test used to assess mobility. This compared with the WVRT versus general balance training for visual perceptual disorders.
-
This RCT showed no evidence of a difference between the two interventions (MD −0.12, 95% CI −13.62 to 13.38); evidence was judged to be of very low certainty.
FIGURE 25.
Active intervention 1 vs. active intervention for visual perception impairment. Outcome – mobility and navigation.
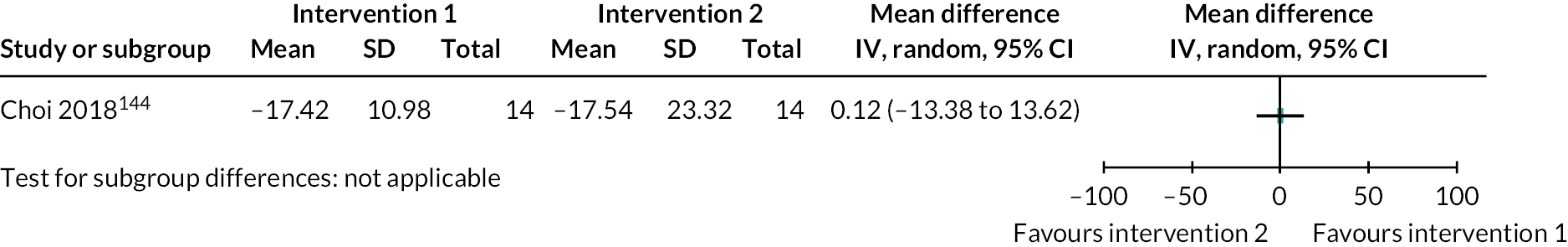
Perception
-
A range of perception outcomes were used: Modified Taylor Complex Figure,154 MVPT,60,144,152,237 and Rivermead Perceptual Assessment Battery. 60
-
Data were not pooled due to differences in the interventions but were displayed as standardised MDs. The evidence from each included RCT was judged to be of very low certainty.
FIGURE 26.
Active intervention 1 vs. active intervention for visual perception impairment. Outcome – perception.
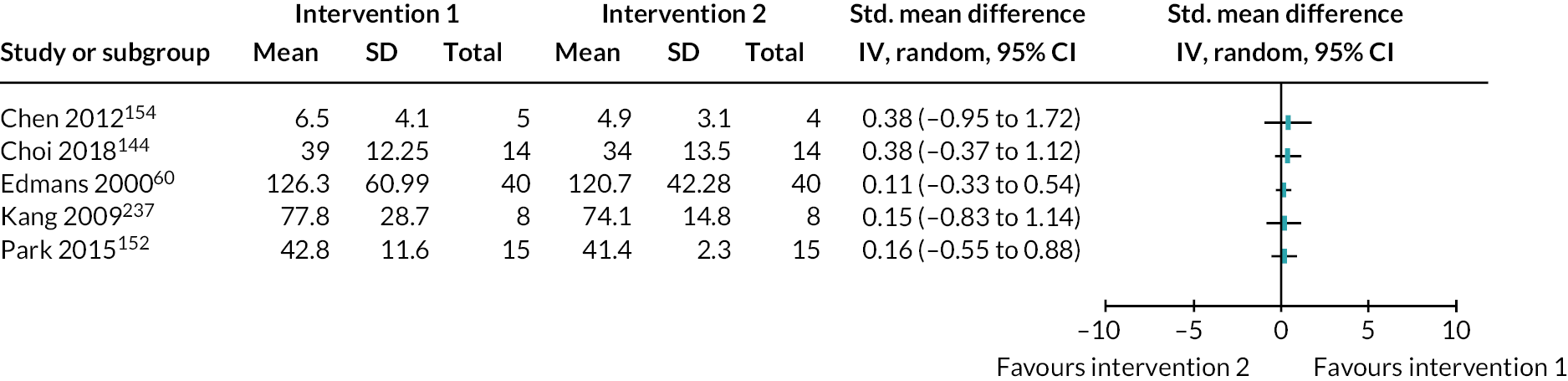
Interpretation of Cochrane Review results
Stakeholders reflected on the implications of the Cochrane systematic review findings in relation to the senses [see Stakeholder activities (what happened), Activity 4]. They expressed disappointment at the lack of RCTs and suggested that the interventions evaluated in the included RCTs did not reflect ‘real-world’ experience. They encouraged collaboration between clinicians and researchers, highlighting its importance in identifying and evaluating clinical practice for people with perception problems. Details of the key issues are provided in Report Supplementary Material 5.
Summary
This chapter presents the results of the Cochrane systematic review update, exploring the effectiveness of interventions for perceptual disorders in stroke. We identified 18 eligible RCTs, involving 539 participants. Trials addressed tactile (three RCTs, n = 70), somatosensory (seven RCTs, n = 194) and visual perception disorders (seven RCTs, n = 225), with one (n = 50) addressing a mixed population. There were no RCTs addressing hearing, smell or taste perceptual dysfunction.
There was insufficient evidence to determine the effectiveness of any one intervention for any sensory modality, nor the effect of one intervention relative to another. The quality of evidence was either low or very low across comparisons. Analysis was limited by the low number of included RCTs and participants, the small number comparing active treatment to no treatment or control, and the limited use of ADLs as an outcome measurement, our primary outcome measure. Our confidence in this evidence is considered in Chapter 10.
Chapter 8 Integration and priority setting
Introduction
In this chapter, we describe the clinical and research implications arising from integration of the scoping and Cochrane systematic review findings and input from our Lived Experience and Clinical Expert Groups. Together with the stakeholder group, we considered the findings of the scoping review (see Chapter 5) and Cochrane systematic review (see Chapter 7) in relation to their experiences of stroke-related perceptual disorders. We wanted to maximise the usefulness and application of these findings to current stroke clinical practice and future research activities.
Researchers and stakeholders shared their understanding of the findings in a facilitated discussion [see Stakeholder activities (what happened), Activity 5] to draft the implications for (1) stroke survivors and carers and (2) clinicians and policy-makers [see Stakeholder activities (what happened) and Report Supplementary Material 6]. The stakeholder group and research team also sought consensus on the top priorities for future research relating to perceptual problems after stroke, via a group discussion followed by an individual ranking process [see Stakeholder activities (what happened), Activity 6 and Report Supplementary Materials 7 and 8].
Implications for stroke survivors and carers
Stakeholders with lived experience (n = 3) expressed disappointment at the lack of evidence underpinning stroke-related perceptual disorder interventions and highlighted the need to:
-
Provide more information to stroke survivors about any perceptual disorders they have. They also recommended that this information be shared with a family member/carer to support the stroke survivor, facilitating their understanding and information retention.
-
Improve awareness and understanding of perceptual disorders following stroke among the public and HCPs involved in stroke care. This was considered particularly important for younger stroke survivors, who may not receive the same level of post-stroke support.
-
Provide regular, one-to-one support, tailored to the needs of the individual with perceptual disorders after stroke; opportunities for stroke survivor and carers to meet and talk with others affected could help address the associated psychological and emotional impacts.
In addition, the members highlighted that while most recovery occurs in the first few months post stroke, that progress can continue over much longer time periods.
Implications for clinicians
Members of the Clinical Expert Group and research team (n = 6) identified seven broad clinical implications relating to stroke survivors with perceptual disorders (see Report Supplementary Material 6 for sense-specific suggestions made).
Healthcare professionals should:
-
Be aware that perception may change following a stroke.
-
Ask about perception in their healthcare assessment appointments.
-
Ask about the everyday impacts of any perceptual disorders.
-
Be open about the lack of evidence underpinning interventions (being clear that this does not mean that nothing works).
-
Consider use of routine or simple interventions, rather than focusing only on interventions identified in the reviews, for example if hearing perception problems, write things down (where appropriate given other abilities).
-
Approach a patient holistically, considering perception in relation to their other abilities and/or disorders and in relation to individual’s goals, when choosing an intervention. For example, if a patient’s perceptual disorder is primarily causing practical difficulties in everyday life, the intervention chosen may be different from someone in which the perceptual disorder is primarily causing psychological distress.
-
Develop their knowledge about interventions for stroke survivors with perceptual disorders after stroke.
Implications for policy-makers
The stakeholder group and researchers discussed the implications for policy-makers. There was considerable overlap between what was considered important for policy-makers, and the implications for clinicians (see Implications for clinicians) relating to the need for person-centred care, awareness, education, information, screening/assessment and an enhanced evidence base (see Report Supplementary Material 6 for full list). The following points were noted as of specific importance to policy-makers:
-
Interventions for perceptual disorders after stroke are a specialist area requiring adequate numbers of appropriately trained clinical and support staff.
-
Where stroke survivors with perceptual problems are inadequately supported, there will likely be associated healthcare, societal and personal economic impacts.
-
International stroke clinical guidelines should address the assessment of and interventions for perceptual disorders after stroke regardless of whether current evidence levels are sufficient to inform practice. Guideline developers should consider qualitative evidence, including patient stories and best practice statements.
-
Stroke survivors with perceptual disorders who are independent may still require services that provide practical care and support.
Research priorities
Thirteen members of the Lived Experience Group, Clinical Expert Group and research teams discussed and agreed nine research gaps relating to perceptual disorders in stroke. In addition, sense-specific research questions were drafted.
The nine research gaps were ranked. Three Lived Experience Group, four Clinical Expert Group and eight researchers participated. These individual rankings (minimum possible score = 1, representing highest priority) were summed to create a prioritised list (Table 10).
| Rank | Research gap |
|---|---|
| 1 | Explore the lived experiences of stroke survivors and carers
|
| 2 | Enhanced assessment of perceptual problems following stroke
|
| 3 | Pragmatic exploration of interventions, which reflects the experiences and needs of stroke survivors, and clinicians’ perspectives
|
| 4 | Establish current practice for perceptual disorders after stroke
|
| 5 | Establish the prevalence of perceptual problems following stroke
|
| 6 | Explore current care delivery and pathways, across NHS, social care and charities
|
| 7 | Explore the impact of perceptual impairment on the family and carers
|
| 8 | Establish the best ways of providing education, and ensuring adequate knowledge and understanding of professionals (including those working in health and social care, and in charity/third sector organisations) |
| 9 | Research to establish a clear definition of perception This may include work to determine clear definitions and names (terms) for perceptual disorders |
See Report Supplementary Material 7 for sense-specific research recommendations (7 vision; 10 somatosensory; 9 touch/tactile, 11 taste and smell and 8 hearing), and Report Supplementary Material 8 for the research priorities wording and summed scores.
Summary
Working in partnership with our Lived Experience and Clinical Expert Groups, we integrated the findings from the scoping review (see Chapter 5) and the Cochrane systematic review (see Chapter 7) to coproduce the clinical implications for stroke survivors, clinicians and policymakers. We also coproduced and prioritised the research gaps with the top research priority identified as the need to explore the lived experience of stroke survivors with perceptual disorders, and their carers.
Chapter 9 The impact of stakeholder involvement
Introduction
This chapter considers the impact of stakeholder contributions to the PIONEER project, providing an overview of involvement activities, how these impacted on the project and the level of involvement achieved. We reflect on strengths and weaknesses of our approach.
Who was involved?
Our Lived Experience Group included two stroke survivors with perceptual disorders after stroke and three carers of stroke survivors with perceptual disorders: one carer left in month 22/24 of the project. Our Clinical Expert Group included two experts in visual perception, one in hearing, and one in taste and smell. This expertise was supplemented by Research Team members with clinical expertise in stroke and or perceptual problems including touch and somatosensory perception.
Stakeholder activities
The Lived Experience and Clinical Expert Group members contributed to decisions across six activities [see Stakeholder activities (what happened)] with the results of these activities reported in the relevant sections (see Types of participants: defining perception, Types of outcome measures, Interpretation of scoping review results, Interpretation of Cochrane Review results, Implications for stroke survivors and carers, Implications for clinicians, Implications for policy-makers and Research priorities; an overview of these activities is given in Table 11).
| Activity | Stage of review | Who took part (n=) | ||
|---|---|---|---|---|
| Lived Experience Group | Clinical Expert Group | Research team | ||
| One-time involvement activities | ||||
| 1. Definitions of key terminology | 2. Plan methods 4. Develop search 6. Select studies |
2 | 3 | 9 |
| 2. Outcome measure prioritisation | 2. Plan methods | 5 | 3 | 8 |
| 3. Interpretation of scoping review results |
9. Analyse data 10. Interpret findings |
5 | 4 | 7 |
| 4. Interpretation of Cochrane Review results | 9. Analyse data 10. Interpret findings |
4 | 1 | 11 |
| 5. Clinical implications | 10. Interpret findings 11. Write and publish review |
2 | 2 | 8 |
| 6. Research recommendations | 10. Interpret findings 11. Write and publish review |
3 | 4 | 11 |
| Continuous involvement | ||||
| Project oversight | All stages | 5 | 4 | 11 |
Evaluating stakeholder involvement
We aimed to capture the nature and level of involvement from the stakeholders’ perspectives (see Evaluation of impact of stakeholder involvement); these were collated and researchers graded the level of involvement. 103
Definitions of key terms (level of involvement: controlling)
Lived Experience Group members described the terms used to define perception as ‘complicated jargon’, saying that ‘it was like a complex vocabulary … that’s not really something I’ve come across…’.
Outputs included an agreed definition of perception (‘specific mental functions of recognizing and interpreting sensory stimuli’), lay definition of perception (‘processing and understanding information from the senses’), definitions of included senses, inclusion criteria in relation to specific (complex) disorders and detailed list of perceptual disorders.
Impact on the project: Definitions agreed by the stakeholders were applied throughout the review, including in the search strategy, selection of studies, data synthesis and interpretation of findings. Lists of disorders were used to inform the search strategy.
Despite the development of a lay definition of perception, definitions of each individual sense, provision of a glossary for each project activity, and a concerted effort by the research team to use simple language throughout, Lived Experience Group members repeatedly found it challenging to recall and to understand many of the terms when they were used throughout the project.
Outcome measure prioritisation (level of involvement: influencing and controlling)
There was general agreement between Lived Experience Group, Clinical Expert Group and researchers on the ‘Top 10’ most important outcome measures. However, there were variations in the specific rankings, with stakeholders placing greater priority on social activity and participation. The inclusion of outcome measures within the scoping review and the selection of outcomes for the Cochrane systematic review provided clear evidence that the stakeholders had an impact on the reviews; the use of specific outcomes within the review is summarised in Table 12.
| Prioritised outcome measures | Scoping review | Cochrane Review |
|---|---|---|
| 1. Performance in ADLs | ✓ | Primary outcome |
| 2. EADLs | ✓ | Secondary outcome |
| 3. Social activities and participation | ✓ | (Incorporated into QoL outcome) |
| 4. Psychological well-being and mental health | ✓ | Secondary outcome [(1) stroke survivors, and (2) family, friends and carers] |
| 5. QoL | ✓ | Secondary outcome [covering (1) QoL scales, (2) social activities and participation scales, (3) mobility, navigation and safety scales] |
| 6. Mobility navigation and safety | ✓ | (Incorporated into QoL outcome) |
| 7. Sensation, cognition, motor ability, attention | ✓ | Noted, but not analysed |
| 8. Impact on rehabilitation | ✓ | |
| 9. Perceptual function | ✓ | Secondary outcome |
| 10. Impact on family, friends and carers | ✓ | (Incorporated into psychological well-being and mental health outcome) |
| 11. Paediatric – measures of development, education | ✓ | |
| 12. Discharge destination | ✓ | |
| 13. Feasibility, acceptability, etc. | ✓ | |
| 14. Adverse events | ✓ | Secondary outcome |
| 15. Compensation using other skills | ✓ | |
| 16. Neurological function | ✓ | |
| 17. Economic outcomes | ✓ |
Outputs: List of impact of perceptual impairment on daily life, prioritised list of outcome measures.
Impact of activities: A prioritised list of outcome measures was agreed. This directly informed the selection of outcome measures for inclusion in the scoping and Cochrane Reviews.
Stakeholders were pleased with their contribution to outcome measurement prioritisation ‘It’s good news that what we’re seeing is embedded in what’s been done’. They queried whether the reviews sufficiently addressed the emotional impact of living with perceptual problems after stroke: ‘I’m not sure that we explored the emotional impact, as much as perhaps we needed to, because it is very important….’.
Interpretation of review findings, implications and research recommendations (level of involvement: contributing, influencing and controlling)
Stakeholders expressed disappointment at the small number of studies, evaluating interventions which did not reflect ‘real-world’ experiences. They urged collaboration between clinicians and researchers to identify and evaluate clinical practice for patients with perception problems.
Outputs: Interpretation of the meaning of the scoping review and Cochrane Review findings, list of clinical implications, prioritised list of research gaps.
Impact of activities: The stakeholders raised several key issues relating to the meaning of the scoping review and Cochrane Review results. These points influenced the writing of the review findings and discussion. The stakeholders agreed lists of implications which have been incorporated into the findings from this project and reached a shared consensus on a prioritised list of research gaps (see Chapter 8).
Few stakeholder feedback forms were returned after these activities, perhaps due to frequency of project meetings and time commitments which may have been exacerbated by pandemic-related challenges (see Limitations). Despite the lack of feedback forms, stakeholders spoke passionately about the importance and potential impact of their input:
I really would like to think that something would come out of this study, in terms of just getting basic things at the beginning when somebody has a stroke …
Members of the Lived Experience Group emphasised that getting these ‘basic things’ right for people with perceptual disorders after stroke could have a big impact on an individual’s life, such as early assessment and diagnosis facilitating access to the right support, for example a bus pass for someone with visual issues struggling with mobility and transport.
Reflections on involvement
The Lived Experience Group reported that they found their involvement:
-
Interesting: ‘I think is interesting to find out … listening to the data, what the results are, and what have you, compared to … myself’.
-
Rewarding: ‘for me, it’s always rewarding’.
-
Relevant: ‘I don’t think for one moment we’ve had any topics which hadn’t been relevant to the project’.
-
Supportive: ‘It’s good to hear other people’s experience’.
-
Educational: ‘I opened my mind to not only the impairments of how it’s affected me and my family, but others’.
Challenges were described due mainly to the online meeting format:
-
Practical problems relating to stroke impairment: ‘I did have some problems with some of the charts, and the way that some of the information was laid out and screen. … That was quite difficult for me. And obviously that’s because [of] my vision,…. ’
-
Lack of face-to-face interaction: ‘had it been non-COVID times it would have been much better, as we’d actually have been able to meet. Well, Zoom, and the like types of meetings are good, they’re not the same as face-to-face interaction. ’;
-
‘….it’s been a bit of really hard battle to actually do anything and everything was online … you don’t get that personal touch … you don’t pick them up on the body language. You know, for me, it’s difficult. … But, face to face I’m happy with’.
In addition, the complexity of perception terminology posed an ongoing issue: ‘I’ve tried to simplify things a bit, and I’ve struggled with the way that certain things have been worded…’. In relation to the complexity of the concept of ‘perception’, the research team reflected that it may have been beneficial to have started each meeting with a reminder of the agreed lay definitions.
When asked what they would change in order to improve their experiences of involvement, stakeholders made a number of suggestions, many of which related to the perceived benefits of face-to-face meetings:
-
Making sure people know one another: ‘when we meet face to face … I think it also helps people to really understand who’s part of the group, I’m still struggling to understand and remember who’s who, because the group’s been quite big. And, I think when you don’t know people… It can be quite difficult to try and speak’.
-
Building on this, stakeholders suggested that it may be helpful to have a ‘pre-meeting of patient and public involvement (PPI) members … without too many academics being around’, to allow stakeholders to share experiences.
-
Support for online meetings: ‘some of us aren’t very computer literate…’
-
Clear explanation of why stakeholder involvement is required: someone could perhaps … say ‘look, we can’t do this without you. And the reason we can’t do this without you is because we don’t have any experiences. We need you to lead us’.
-
More regular and detailed feedback after each task: ‘it would be helpful for us who contributed PPI to know how we have actually influenced your research and how we have, perhaps, enlightened you to things that you weren’t aware of. We get so little feedback at times’.
Summary
Our multifaceted stakeholder approach involved a co-applicant with lived experience, a group of people with lived experience and HCPs with clinical expertise relating to stroke-related perceptual problems. These stakeholders coproduced the review including having control and influence over some specific aspects. Areas of the review in which stakeholders had a demonstrable impact included: the key terminology, outcome measures and recommendations for research; interpretation of findings and clinical implications of the results. Stakeholders perceived their involvement as interesting, rewarding, relevant, supportive and educational.
Chapter 10 Confidence in evidence
Introduction
In this chapter, we summarise the findings of the PIONEER project across the scoping review, the Cochrane systematic review revision, expansion and update and research prioritisation process. We consider the methodological quality of the two reviews, including the risk of bias in our project design, delivery or interpretation of findings and confidence in our findings.
Scoping review
Our review scoped the breadth and nature of intervention research to date relating to perceptual disorders after stroke. We identified 80 studies (893 participants); these primarily explored visual (n = 357) or somatosensory (n = 303) perceptual problems, frequently using a case report (36/80) or RCT (2/80) design. The most frequent intervention approach was rehabilitative, often targeting restitution of ability in the impaired function. Pharmacological and NIBS interventions were also identified. Interventions involved training by an HCP, as well as the use of technical or robotic devices, and other specialist equipment. Interventions were most frequently delivered in hospital, for up to 4 weeks, involving one-to-one training with a therapist (as opposed to group or self-delivery techniques). Perceptual and motor/sensorimotor skills were the most common outcomes reported. Priority outcomes, such as ADLs and the impact on social participation, psychological well-being and mental health were rarely reported. Few studies captured outcomes beyond initial post-training effects. Clear evidence gaps were highlighted (see Figure 8 and Table 13).
| Scoping review: 80 studies, 893 participants | ||||||
|---|---|---|---|---|---|---|
| Perceptual disorders | Vision n = 357 (40%) |
Somatosensation n = 303 (33.9%) |
Hearing n = 33 (3.5%) |
Tactile n = 107 (11.9%) |
Mixed n = 93 (10.4%) |
|
| Visual–spatial deficit 35 (10%) Visual hallucination 8 (2%) Visual agnosia 3 (1%) ‘Other’ visual perceptual disorder 37 (10%) Unspecified 274 (77%) |
Pusher syndrome 232 (77%) Proprioceptive deficits 14 (5%) ‘Other’ somatosensory deficit 57 (19%) |
Auditory processing disorder 32 (97%) ‘Other’ hearing disorder 1 (3%) |
Tactile dysfunction 107 (100%) |
Tactile-somatosensory disorder 68 (73%) Taste–smell disorder 1 (1%) Vision–tactile disorder 24 (26%) |
||
| Interventions = 37 | Interventions = 35 | Interventions = 7 | Interventions = 7 | Interventions = 7 | ||
| Interventions descriptors (most frequent response) |
Class
Materials Who delivered |
Rehab (restit) 15 (41%) HCP-led 12 (32%) NR 18 (49%) |
Rehab (restit) 24 (69%) HCP-led 17 (49%) NR 17 (49%) |
Rehab (subst) 4 (57%) Technology 5 (71%) NR 4 (57%) |
Rehab (restit) 4 (57%) Special. equip 4 (57%) NR 5 (71%) |
Rehab (mixed) 4 (57%) Special. equip 5 (71%) NR 5 (71%) |
|
Mode
Location |
One-to-one 25 (68%) Hospital inpatient 15 (41%) |
One-to-one 32 (91%) Hospital inpatient 18 (51%) |
One-to-one 5 (71%) Hospital (unclear) 3 (43%) |
One-to-one 7 (100%) Hospital (unclear) 3 (43%)/NR 3 (43%) |
One-to-one 7 (100%) NR 7 (100%) |
|
| Overall duration | NR 19 (51%) | < 1 month 17 (49%) | 1–3 months 3 (43%) | < 1 month 2 (29%)/ NR 22 (29%) |
NR 5 (71%) | |
| Cochrane systematic review: 18 RCTs, 535 stroke survivors, 20 comparisons | ||||||
| Studies | Visual | Somatosensation | Hearing | Tactile | Taste or smell or mixed | |
| 7 RCTs (n = 225) | 7 RCTs (n = 196) | No RCTs | 3 RCTs (n = 70) | No RCTs | ||
| Interventions | Interventions 11 Restitution 1 Restitution-compensation |
Interventions 10 Restitution 2 Restitution-substitution 1 NIBS |
No RCTs | Interventions 4 Restitution 1 Restitution-substitution |
No RCTs | |
| Meta-analysis (ADLs outcome) | Intervention vs. no treatment | No RCTs | No RCTs | No RCTs | No RCTs | No RCTs |
| Intervention vs. control | No RCTs | MD 10.08 (−2.47 to 22.63) 1 RCT; n = 24 non-Pushers syndrome very lowa,b,c |
No RCTs | No RCTs | No RCTs | |
Cochrane systematic review
The Cochrane Review examined the effectiveness of stroke-related perceptual disorder interventions. Based on 18 included RCTs (n = 541), 3 RCTs examined interventions for tactile disorders (n = 70), 1 examined interventions for somatosensory disorders (n = 194), 7 considered interventions for visual perception disorders (n = 225), 1 investigated mixed tactile–somatosensory disorders (n = 50). Interventions for hearing, smell or taste perceptual dysfunction were not identified (see Table 13).
Interventions for somatosensation perception disorders
We found low-certainty evidence of no difference between interventions and control on measures of ADLs, navigation and mobility. We found low-certainty evidence of no difference between two interventions for somatosensory perception dysfunction (not Pusher syndrome) on perception outcomes. For Pusher syndrome, we found low-certainty evidence of no difference between game-based posture training and standard physiotherapy for measures of mobility and navigation and perception. We also found low-certainty evidence suggesting that game-based posture training for Pusher syndrome may be more beneficial than standard physiotherapy for improving ADLs and measures of Pusher syndrome severity (see Table 13).
Interventions for touch perception disorders
We found low-certainty evidence that there is no difference between intervention and no treatment for navigation and mobility outcomes, but there may be a beneficial effect of active intervention on perceptual function. Evidence relating to one intervention versus another was varied, and insufficient to draw generalisable conclusions (see Table 13).
Interventions for visual perception disorders
We found low-certainty evidence of no difference between intervention and no treatment on measures of perception; there was no difference between active intervention and control for measures of EADLs. We identified some data for outcomes of ADLs, navigation and mobility and perception from RCTs comparing one intervention to another. Due to differences in the interventions and comparisons, the data were not statistically pooled, and it was not possible to draw generalisable conclusions (see Table 13).
Overall, there is insufficient evidence to determine the effectiveness of any one intervention for any sensory modality, nor the effect of one intervention relative to another.
Prioritisation process
The stakeholder groups and research team collectively generated the research priorities relating to perceptual disorders after stroke. The top five research priorities highlighted the need for further research to (1) explore the lived experience of people with stroke-related perceptual disorders, (2) improve assessments of stroke-related perceptual disorders, (3) explore interventions in a way that reflects real-world needs, (4) explore clinical practice to address perceptual disorders following stroke and (5) establish the prevalence of perceptual disorders after stroke (see Chapter 8).
Methodological quality of the research identified
The research literature relating to perceptual disorders was reviewed using a scoping review followed by a Cochrane systematic review. Using recognised scoping review methodology,94 a detailed search strategy identified a broad range of research designs, perceptual disorders, interventions, outcomes and healthcare settings. Neither a research quality filter nor an appraisal of research quality was applied to the 80 studies identified. Instead, the high proportion of single-participant studies, the small number (27.5%) of randomised controlled RCTs and the absence of reported stakeholder input were highlighted.
The subsequent Cochrane systematic review included a formal quality appraisal of 18 RCTs identified. Random sequence generation was unclear for five RCTs with concealment of allocation was unclear for two-thirds of the included RCTs. Blinding of outcome assessors was at a high risk of bias or unclear bias in all RCTs. Attrition and reporting bias was low but sample sizes were small (see Figure 10 and Report Supplementary Materials 14 and 21). Thus, the RCTs contributing to the Cochrane systematic review and meta-analysis were at high risk of bias.
Meta-synthesis and risk of bias
With reference to the ROBIS tool253 we considered to what degree our scoping and Cochrane systematic reviews were at risk of systematic limitations resulting in a risk of bias and the extent to which these may have impacted on our conclusions.
Study eligibility criteria – selection biases
The PIONEER sequential review process had two research questions and different eligibility criteria. Conducted consecutively, the scoping review used very broad studies inclusion criteria to provide an overview of the topic area (see Chapter 4) while the Cochrane systematic review narrowed these selection criteria to focus on the RCT data (see Chapter 6). Both reviews were based on a thorough and up-to-date systematic search strategy developed by an information specialist and informed by research and clinical experts. Contact was made with leaders in the field and forward citation tracking was also used. Between the scoping and Cochrane Review, the search was updated to ensure that no emerging trials were omitted between the scoping review’s last search date and the start of the Cochrane Review process.
In the complex context of perceptual disorder research, we drew on existing definitions of perception (WHO)7,8 and working with our stakeholder groups, and these were expanded and operationalised to create subcategories by sense (visual, auditory, tactile, somatosensory, smell and taste) to support the planned reviews (see Chapter 3). We acknowledge that alternative definitions and categorisations could have emerged and in turn could have impacted on the identification of studies and the inclusion of additional or alternative research in the reviews. However, our consensus use of WHO definition7,8 was agreed by people with lived experience of perceptual disorders after stroke, and by multidisciplinary healthcare and research groups and thus were ideally suited to address our research questions. This ensured that all study eligibility decisions were consistently made across reviews, and thus unlikely to have introduced systematic limitations.
Both reviews used unambiguous, appropriate a priori eligibility criteria with no restrictions on the language, date or location of the study. The scoping review applied no restrictions to study design, perceptual disorders, interventions or outcome data considered eligible, reflecting the nature of the scoping review objectives. Qualitative study designs were eligible, but none were identified.
The Cochrane systematic review added three additional restrictions to the eligibility criteria used by the scoping review; only RCT data were included reflecting the Cochrane Review research questions, only adult participants were included, and a smaller number of outcome measures were included.
Both reviews focused on participants with stroke-related perceptual disorders; studies with a population where at least 80% had stroke-related perceptual disorders were also included. The scoping review included eight such mixed-sample studies [24 participants (2.6%) without a perceptual disorder or non-stroke-related perceptual problems]. Almost all (98.9%) of Cochrane Review participants experienced stroke-related perceptual disorders – one study included six participants with perceptual disorders following head injury. Throughout, we accepted the primary research diagnosis of perceptual disorders following stroke.
Data identification and selection – availability bias
In conjunction with a trained information specialist (JC) and informed by the stakeholder groups, we undertook detailed and exhaustive search of published and grey literature electronic databases (including hand searching) and forward/backward citation tracking (see Appendix 1 and Report Supplementary Material 9) to identify all relevant research to inform the scoping and subsequent Cochrane Review. The strength of this approach was the initial broad, inclusive, comprehensive search strategy supporting the scoping review. This was later narrowed using a RCT methodological filter to inform the Cochrane systematic review (see Appendix 3 and Report Supplementary Material 13). Application of methodological filters is known to increase the risk of omitting relevant studies,254,255 but our initial broader scoping review search guarded against this risk. All searches used free-text and subject index terms, reflecting the population, disorder and intervention. While it is possible that relevant research remained unidentified, we made every effort to identify it and include it in our reviews.
The data identified research conducted across the world, across disciplines and languages, over publication time points and stroke chronicity and across various subject topic areas and countries. Data eligibility criteria were applied by independent members of the research team at abstract and full-text stage. Grey literature was screened by a single member of the research team with decisions carefully checked by a second team member. Where disagreements were unresolved through discussion, a third team member or a Clinical Expert Group member was consulted.
Data collection and appraisal
Our structured Excel-based data extraction tool, underpinning the scoping review and Cochrane systematic reviews was independently piloted by two reviewers; the data extracted were compared and tool discussed and refined before it was used. All potentially relevant data were extracted. Data were extracted by one researcher and carefully checked by a second. Where clinical details were ambiguous, we involved a member of the Clinical Expert Group in decisions. Data synthesis, extraction items and categorisation used reflected published definitions. For RCTs, the data were extracted and directly entered into RevMan but were carefully checked by a second reviewer. The Cochrane Review risk of bias appraisal was undertaken by two independent researchers.
Synthesis and findings
Data restrictions
The scoping review and Cochrane systematic review methodology supported a broad and inclusive review of the stroke-related perceptual disorder literature, but the data extraction was restricted to that reported in the literature. Research teams were only contacted where it was not possible to determine inclusion based on the published data. The subsequent Cochrane Review methods included some data gathered via direct contact with the included trialists (2 of the 18 included trials data reflect information gathered from unpublished communications) (see Chapter 7). One trial reported only partial outcome data234 (MOCA, nine-hole peg test and functional magnetic resonance imaging) and a second trial described collection of secondary outcomes, but data were not available. 74
Eleven trials were classified as awaiting classification. Due to a lack of clarity of reporting it was not possible to confirm that studies met inclusion criteria in relation to the disorder (n = 6), method (including randomisation process) (n = 2) or more than one inclusion criteria (n = 3). In each case we attempted to contact the author but received no reply.
Publication bias
Funnel plot analysis to assess the risk of publication bias is not typical in scoping review methodology, and there were insufficient data within the Cochrane Review to support this analysis (see Chapter 7). Generally, identified data sets were based on small sample sizes; predominately single-participant within the scoping review and only two RCTs randomising ≥ 50 participants, raising some questions about the quality of the data available.
Age of data sets
Of the data sets included within the scoping review, 63.7% (51/80) were published since 2010 with 34 (42.5%) of these published since 2015. Most trials (13 RCTs) included in the Cochrane Review were published in the last 5 years (2015–21); the remaining 5 were published between 2012 and 1985 and included the 2 largest trials in the review (n > 50).
Origin of data sets
The data sets included in the scoping and Cochrane systematic reviews reflect international research efforts. Research identified in the scoping review emerged from Asia, Europe, North America, South America and Australia. Within the Cochrane systematic review, the trials were conducted in Australia, Belgium, Germany, South Korea, Taiwan, UK and USA.
Data synthesis
Scoping review data were classified and organised by the perceptual disorder addressed and intervention assessed (subclassified by approaches used). Categories were pre-specified and agreed with the stakeholder groups as were the outcome measures of interest in the review. This was a complex task – difficulties in categorising interventions (especially where there was lack of reporting) were addressed by having a third reviewer, who was provided with clear definitions of intervention approaches, categorise all interventions. Further specialist input relating to Pusher syndrome interventions was provided by a somatosensory expert. Data were tabulated and presented using standard Word or Excel tables and an interactive visual map which were positively received by the Lived Experience Group members. The Cochrane Review interventions were organised in a similar manner, by perceptual disorder and intervention approach (with rehabilitation approaches subclassified).
In the Cochrane Review we followed pre-specified analyses as described in our protocol, registered with PROSPERO (CRD42019160270) and NIHR’s website [https://fundingawards.nihr.ac.uk/award/NIHR128829 (accessed November 2022)] and pooled trial data that compared active interventions with no treatment/control/alternative intervention. We used a random effects model (RevMan 5.4) to support statistical analysis. Our planned sensitivity analyses included exploration of the use of a fixed-effects model, but the lack of data prevented this and other planned sensitivity and subgroup analyses.
The populations represented in the scoping and Cochrane Reviews spanned the clinical trajectory of stroke-related perceptual disorders (acute to chronic) and we sought (but did not always identify) a wide range of perceptual disorders, interventions and outcomes. A small number of participants included in both the scoping and the Cochrane Reviews were recruited to mixed study populations; they did not have perceptual disorders or their perceptual disorder was not stroke-related. Given the overall lack of data availability and overlap of the available data, it is highly unlikely that an alternative approach to data synthesis would alter our findings.
Across the included trials, however, biases were evident in the RCTs, particularly in relation to the randomisation process, blinding and inadequate outcome reporting (described above). Most biases were related to inadequate reporting, though a third of trials were considered at high risk of detection bias due to an absence of outcome assessor blinding.
Reporting the results
The scoping review data were identified and tabulated using pre-specified scoping review framework94,114,115 and a recognised classification system8,124,125 (see Chapter 4). Included information was presented in an accessible format, by study design, population and intervention component, profiled in graphs and numeric summaries. We compared the scope and levels of evidence and research gaps. Similarly, the Cochrane systematic review reported the findings in keeping with the Cochrane methodological requirements,222 adhering to a pre-specified and accessible protocol, and profiling the identified trial data by comparison, intervention versus (1) no treatment, (2) control/placebo or sham and (3) another intervention.
Quality of evidence
No measure of quality was applied to the scoping review identified data (see Chapter 5). A judgement of the quality of evidence included within the Cochrane was made using the GRADE approach and found the evidence from meta‐analyses to be of very low certainty (see Chapter 7) due primarily to risk of bias, imprecision and indirectness.
A rigorous two-stage review approach, however, enabled a broad scope of the literature followed by a Cochrane systematic review and meta-analysis where possible, of the trial data. This ensured that the most comprehensive and methodologically rigorous approach to data synthesis was carried out. The lack of consensus on definitions and outcomes to date was particularly striking, impacting in turn on the quality and disparities across the emerging evidence. Coproduced definitions of perceptual disorders, the senses addressed and outcomes considered most important to people with perceptual disorders, their families and their HCPs, underpinned our review methodologies. The trials identified reflect the recently emerging international research focus on perceptual disorders after stroke, the historical lack of research activity (particularly large-scale studies) and the many research gaps in the senses that we considered. These challenges were unrelated to our pre-specified review methodologies and ensured that our research team did not introduce biases during the data synthesis approaches.
Heterogeneity and inconsistencies
Across both reviews we identified data availability issues: the high number of studies involving a single participant, small sample sizes in group studies, the lack of RCTs and the gaps in availability of hearing, smell and taste perceptual disorders intervention research. With limited data to date, between study variation and inconsistency, clinical or statistical heterogeneity were less apparent.
Missing data/missing completely at random
In the scoping review we did not contact authors for any missing data; data extracted were based on the information contacted in published articles. Where data were missing from RCTs remained unavailable, we used presented data to calculate the missing data using standard methods. 222
Sensitivity analyses
Our scoping review did not include any formal meta-analysis or sensitivity analysis of the data identified. Sensitivity analyses using the ADLs primary outcome data were planned in the context of RCTs identified in the Cochrane systematic review: high/low risk of selection bias, performance bias, detection bias, attrition bias, reporting bias and ‘other’ sources of bias. The paucity of data and limited overlap in the available data prevented any planned sensitivity analysis. Only RCTs were eligible for inclusion in the Cochrane systematic review.
Summary
The evidence base informing interventions for perceptual disorders after stroke is very limited and what was identified and available is focused on a selective group of perceptual disorders, with typically small sample sizes and non-RCT study designs. Our PIONEER study used a two-stage review approach: a broad scoping review followed by the narrower eligibility criteria of the Cochrane Review (see Chapters 4 and 6). Our review methodologies made every effort to reduce the risk of bias in the review and meta-analysis processes, reducing the risk of selection and availability of meta-bias. Biases were evident in the primary research data. The paucity of data limited our planned examination of heterogeneity, sensitivity and subgroup analyses. Our reviews suggest the topic of perceptual disorders after stroke has been neglected in the literature to date, with the more recent emergence of small pilot RCTs in the last 5 years addressing selected perceptual disorders.
Chapter 11 Discussion and conclusion
Introduction
In this chapter we discuss the findings of the PIONEER project in relation to existing literature, consider the strengths and limitations of these findings, and their implications for researchers and clinicians.
Summary of findings
The PIONEER scoping review mapped the available evidence base relating to interventions for perceptual disorders after stroke in adults and children (see Chapter 5), finding few reports of interventions for hearing, taste, touch or smell perceptual disorders. Research reports described single case studies and, to a lesser extent, RCTs. The 80 studies identified focused primarily on rehabilitation interventions for visual and somatosensory perceptual disorders after stroke. Descriptions of participants and interventions were incomplete and qualitative data on stroke survivors’ experience of perceptual disorders and interventions were absent.
Our Cochrane systematic review update explored evidence of the effectiveness of interventions for perceptual disorders after stroke (see Chapter 7). We compared interventions to no treatment, or a control group that received a placebo, standard care or an attention control. We measured benefit based on participants’ ADLs. No RCT evidence on the treatment of hearing, smell or taste perceptual disorders following stroke was identified. While there was some evidence relating to interventions for somatosensation, touch and visual perceptual disorders, there was not enough to support any one intervention.
The Lived Experience and Clinical Expert Groups were disappointed in the quality and quantity of evidence informing the treatment of people with perceptual disorders after stroke. The groups identified key clinical implications including the provision of information about perceptual disorders to stroke survivors, their family and carers, the need to improve awareness, the importance of early, accurate diagnosis and having a holistic approach to care provision. The stakeholders generated research priorities relating to definitions, assessment, impacts, interventions, services and HCP training relating to stroke-related perceptual disorders. The breadth of future research required reflects the current paucity of research into this topic area.
Previous perceptual disorder reviews
Our Cochrane systematic review significantly expanded the scope of a pre-existing review6 to include all interventions targeting six sensory domains, while ensuring it focused on a stroke aetiology. While we identified 18 RCTs compared to the 6 RCTs included in the 2011 review, our conclusions are broadly similar, as clinical intervention remains poorly supported by high-quality RCTs.
Several additional Cochrane Reviews address related disorders such as sensory and sensorimotor function,9,256 visual neglect or attention10 and stroke-related cognitive disorders:111,257,258 these identified limited RCT evidence to support clear conclusions on intervention effectiveness. Nevertheless, these syntheses are a source of additional information for clinicians. Additional, non-Cochrane intervention reviews have potential relevance for disorders of visual perception,88,259 and touch and somatosensation. 47,89,260,261
Jutai (2003), in their narrative summary relating to perceptual impairment, neglect and apraxia [six RCTs and two cohort studies (n = 373)], concluded that there was strong evidence that transfer of training improved perceptual function. However, our RCT meta-analysis findings have resulted in a different conclusion. A series of systematic reviews (and updates) including literature up to 2014 included ‘visuospatial functioning’ – focusing on visual neglect (10/13 studies), making no recommendations relevant to perceptual disorders as defined in our review. 262 Hanna’s review259 of vision interventions included perception, but again mainly focused on neglect, with studies included in that review falling outside of our inclusion criteria.
Four reviews considered interventions relating to touch and somatosensation disorders after stroke. A 2009 paper on retraining sensation after stroke,89 updated in 2019260 to include 38 RCTs (1093 participants) with meta-analysis of data (13 comparisons). The updated review defined sensation as the ability to ‘detect and discriminate objects and textures, know where our body is in space (proprioception) and accurately perceive and discriminate sensations of pain, temperature, pressure, and vibration’, and explored sensory retraining [passive (externally applied stimulation), active (sensory retraining of graded re-education) or a hybrid]. They found limited data, with some evidence to support passive sensory techniques, but the effect of active techniques was unclear. Similarly, a review of 13 RCTs addressed ‘sensory registration, perception, or discrimination’, impairment specific to the upper limb, with a focus on outcome measures of sensation. 47 Specific sensory inclusion criteria were unclear in this review, and inclusion appeared to relate to the presence of hemiplegia. They found insufficient evidence to reach conclusions about the effects of included interventions. Clear differences exist in the populations included in these reviews and our Cochrane systematic review including the (1) nature of the intervention, (2) body part considered and (3) nature of the disorder addressed. Our review inclusion criteria required a clear diagnosis of a perceptual disorder after stroke, which was not the focus nor criteria of prior reviews, resulting in limited overlap of included studies and comparability of findings. A further review of 10 studies (n = 122) on interventions for Pusher syndrome, classified interventions as robot-assisted gait training, visual feedback, galvanic vestibular stimulation and physiotherapy interventions. 261 They noted early evidence of intervention effectiveness but urged caution in the interpretation due to the small sample sizes and lack of methodological rigor, as also noted in this work. All touch and somatosensation reviews that we identified make similar recommendations for more research in this field.
Additional systematic reviews of interventions for hearing,77,263 taste and smell11 perceptual disorders following a stroke are lacking with most reviews identified providing a high-level summary of the diagnostic challenges and the impact of perceptual disorders, mirroring the evidence gaps that we identified in our Cochrane Review.
Previous research prioritisation activities
We identified stroke-related perceptual disorders research priorities. The James Lind Alliance, working at a national level with priority setting partnerships of clinicians, patients and carers to ‘identify and prioritise evidence uncertainties’, has identified research priorities in topics relevant to the scope of this work. In 2011 the first stroke-related JLA PSP top 10 identified ‘What are the best ways to improve cognition after stroke?’ as the top priority, with its definition of cognition including perception; in addition, treatments for visual problems were priority number five. 264 The more recent JLA stroke priority setting work (2021) did not include visual or other perceptual-related disorders, though research relating to the prevalence of long-term impacts and associated interventions (#6), support for carers (#8) and what are stroke survivors’ experiences of care (#10) were prioritised. A recent Swedish study265 ranked ‘cognition and memory function’ as priority 6, with visual problems as number 8. The World Stroke Organization recommendations are not specific to any one post-stroke disorder and rank the need to ‘standardize … poststroke rehabilitation based on best evidence’ as third most important, behind better acute treatment and stroke prevention. 266
There are priority setting activities relating to individual senses:
Vision: A research priority setting activity relating to sight loss and vision presented 12 main research priorities but again, none related to perception, though one neuro-ophthalmology-specific subset93 noted two stroke-related priorities: What rehabilitation or treatment methods are most effective? (#4)’ and ‘What is the most effective way to assess vision in patients with neurological visual impairment?’(#5).
Hearing: One JLA Top 10 relates mild-to-moderate hearing loss, but appears to focus on sensory loss, not perceptual impairments. 267
Taste and smell: A priority setting exercise is underway, but has not yet completed its findings. 268
While there are areas of overlap, our research prioritisation process is not fully represented by existing findings.
Stakeholder input and relevance
It is important to consider to what extent the identified research was pragmatic in nature,269 reflecting the ‘real-world’ experiences of stroke survivors, carers and families, and the delivery of care by HCPs.
No included studies reported the involvement of stakeholders (stroke survivor or clinical) in intervention development or study design. Other points where a lack of coherence between the research studies and real world were:
-
Setting: Most studies were conducted in hospitals, whereas most rehabilitation is now provided after discharge, in a community setting.
-
Flexibility (delivery): There was a predominance of one-to-one delivery, requiring input from a clinician throughout; such methods are potentially less feasible in a community setting, and in the context of increasing self-delivery models for stroke care.
-
Organisation: Several interventions, such as robotics or vibrating gloves, were novel and of questionable relevance to current practice. Clinicians suggested that simpler or more frequently used interventions were not adequately examined within the research.
-
Flexibility: The absence of research into intervention implementation, including feasibility, and economic outcomes.
-
Primary analysis: Research was wholly quantitative in nature, with no data gathered on stroke survivors or clinicians’ experiences or the acceptability of interventions.
-
Primary outcome: The most frequently reported outcome measures were those of perceptual function. The outcomes of greatest importance to stroke survivors, measures that reflected transfer of training effects to everyday life (e.g. ADLs, EADLs, social activities and participation, psychological well-being and mental health, QoL) were not gathered alongside those of perceptual function.
-
Follow-up: There was also limited assessment of outcomes beyond the end of intervention delivery, with which the longevity of effects could be explored. Similar concerns are seen in other areas of stroke rehabilitation research, reflecting wider challenges in this area of rehabilitation. 6 There is a set of core outcomes and measures for visual disorders in stroke created with stroke survivor representatives;270 while it focuses on sensory and ocular motor disorders, the guidance on functional visual assessment is useful in this field also.
Potentially some of the limitations in study relevance could have been identified and addressed with greater stakeholder involvement. The benefits of stakeholder involvement are well recognised271 specifically in enhancing the relevance, implementation and impact of research.
This project involved stakeholder involvement throughout (see Chapters 3 and 8). The Clinical Expert Group and Lived Experience Group impacted on the project design (Chapter 9), specifically the definition of perception, delineation of disorders, deciding the outcome measures and organising the data syntheses (see Chapters 5 and 7). They have contributed to interpreting the results, implications and recommendations for clinicians, stroke survivors, policy-makers.
Equality, diversity and inclusion
Equality, diversity and inclusion in the context of this study
Key factors known to be associated with underrepresentation in stroke research (especially trials) include female sex,272 ethnic minority background, age (> 80, < 18)273 and having stroke-related impairments in cognition274 and language. 275 In our reviews we sought to extract (and categorise) data on age, sex and concurrent impairments to both describe the population and identify areas of limited inclusion. Age and sex data were generally well described (see Participants included). We grouped age data into < 18, 18–65 and > 65 years old; 31.3% (25/80) studies had participants aged > 65 years suggested good representation of older stroke survivors; however, as this was not specific to those aged > 80 we cannot comment on any lack of representation in this group. We noted a lack of studies relating to children and under-representation of women. We did not collect data on race/ethnic background; on consideration this may have been a lost opportunity to explore this important issue.
We also collected data on whether studies reported any involvement of stroke survivors or carers in the design and conduct of their study (PPI): no studies reporting any PPI (see Stroke survivor or family involvement in research), possibly suggesting of a lack of consideration of stroke survivor’s experiences and priorities within perception interventions research.
Accessibility and Inclusivity when presenting results
We were aware of potential barriers to access arising from the complexity and unfamiliarity of perception and perceptual impairment language. We simplified wording in documents, provided glossaries of research and topic-specific terms and provided reminders of the meaning of key terms each time we met. Members of the Lived Experience Group helped to create a lay definition of perception that would be widely understood, and reviewed and edited all Plain Language Summaries for project publications, alongside input from a speech and language therapist research team member familiar with creating accessible versions of research information, to support accessibility. As some members of the Lived Experience Group had visual problems, we used clear print guidance throughout to guide the choice of font layout, use of colour and contrast, etc.
Research team
Our multidisciplinary research team reflected a range of topic and research methodology expertise to support the conduct of the study to a high standard (see Equality, diversity and inclusion in the context of this study).
Our involvement of stroke survivors, carers and clinicians was extensive, and is discussed in Stakeholder input and relevance.
Strengths
Positionality
The core research team included multidisciplinary researchers with clinical expertise in optometry, audiology, physiotherapy and speech and language therapy, and neuropsychology (adult and paediatric) many of whom are stroke rehabilitation specialists and clinical academics in stroke care. All but one (SH) were based in Scotland. The core group’s methodological expertise includes extensive systematic review and data synthesis methodologies (Cochrane, mixed methods, network meta-analyses), complex interventions, priority setting, reducing research waste and maximising collaborative research efforts to the benefit of people after stroke. The core research team worked closely with the Lived Experience and Clinical Expert Groups [see Stakeholder activities (what happened)] who contributed their expertise from the broader perspectives of England (four members), international (one member) and clinical areas of audiology, ENT and visual neuropsychology.
Methodological rigour
We worked to the highest methodological standards. We built upon existing definitions to develop a clear, specific, working definition of perception at project outset and agreed our inclusion criteria in relation to the senses and specific disorders: given the complexity of this topic, this gave clear scope and practical guidance for succeeding activities. The WHO ICF8 definition underpinned our working definitions and was applied to all senses. This helped us address the challenge of perceptual terminology and reporting, with inconsistent terminology for similar conditions within and between senses, participant populations and disciplinary fields as well as lack of clarity on the precise nature of disorders. Our definition also informed our broad search strategy to identify published and non-published research, from both key electronic databases and a range of other sources including the grey literature. While we recognise that others may develop alternative approaches, our definitions and framework were feasible and were consistently applied across our reviews and supported the reporting of our findings. Thus, these definitions offer a transparent and replicable approach to support future perceptual disorder rehabilitation research.
We reported to the best practice and methodological and reporting guidelines wherever possible including TiDIER,97 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA),128 PRISMA-A, PRISMA-ScR,117 GRIPP 2,108 Cochrane Review quality appraisal process for both studies (risk of bias)223 and comparisons (GRADE),226,227 ROBIS,253 and Cochrane methodological guidance for data synthesis. 222
As noted, prior research has often explored perception in conjunction with sensory, cognitive or attentional deficits. Our very clear focus on perceptual disorders alone is therefore unique. It has offered the opportunity to closely examine the research on this specific set of disorders, and we hope this provides a distinctive contribution to clinical care, enabling HCPs to better focus on and address perception-specific disorders.
Stakeholder involvement
We involved stakeholders from project inception and throughout the study (see Chapter 3), using current UK guidance and an established framework to plan and describe involvement. 103
We acknowledge that our stakeholder discussions were typically based on a small number of participants and somewhat subjective, meaning that discussions (and rankings) could have been biased by the experiences of participants and thus not reflective of a national or international viewpoint. Our involvement of stakeholders in the clinical and research implication sections brings substantial strength, reflecting the views of many people with lived experience of perceptual problems, and of HCPs addressing their care.
Limitations
Lack of evidence
Stroke is the third leading cause of disability worldwide, and a growing number of stroke survivors are living with long-term disabilities, including perceptual disorders. The limited research activity relating to perceptual disorder interventions is important, as is the limited quality of the research identified (see Chapter 7). Consequently, there is a significant absence of high-quality evidence informing the provision of stroke care.
The reason for the lack of research in this area is not clear. The Lived Experience Group described how the considerable impact of their perceptual disorders was, for some, only appreciated months after their return home, when access to stroke rehabilitation was limited. An alternative interpretation may be that insight into such disorders may only emerge as other rehabilitation gains are made. Clinical experts suggested that people with severe stroke may have perceptual impairments masked by other conditions such as poor cognition or aphasia which in turn preclude formal assessments and limit self-report. Thus, clinicians may be unaware that a perceptual disorder is present, and why few research studies address the topic. Timely assessment, information provision and treatment should be important.
Our scoping review employed a broad and rigorous search of electronic databases and grey literature, adopting a comprehensive definition of perceptual disorders. Despite these efforts, due to the complex nature of the topic and terminology, some relevant papers may have been missed. In the absence of a universally accepted intervention categorisation, we utilised an existing method to support categorisation consistency, relevant to perceptual disorder research. 125 but which may not necessarily directly align with other categorisation approaches. 276 As a scoping review, we did not conduct quality appraisal and thus comment on quality or generalisability of study findings was not possible. Similarly, we failed to identify qualitative studies that focused on perceptual disorders after stroke; we recognise that some relevant data may have been available in more generic reports of stroke impact reports which may not have been identified in our comprehensive search of the literature. We are aware of one potentially relevant qualitative report published after our scoping review was completed,277 and of ongoing qualitative work in this field. 278
Study methodology
The scoping review identified a predominance of single subject and RCT designs, the former describing highly personalised treatments, making clinical relevance and validity difficult to establish. While RCTs can be a high-quality design approach, we found risk of bias concern in most RCTs included in our review. Small RCTs are typically insufficiently powered to generate confidence in conclusions and overlap in the small trials we identified was rare. A co-ordinated and systematic approach to enquiry in this research field is required and our scoping and Cochrane systematic review offer an important foundation on which to build this evidence base.
Reporting limitations
The TiDIER checklist,97 available since 2014, aims to support the replication of treatment, as reporting gaps have clear consequences for intervention implementation. We sought to extract the relevant intervention data using the TiDIER checklist, where possible describing the rationale, materials, procedures, intervention provider, mode of delivery, location, dosage and any modification of the intervention. Such details were not commonly available (see Interventions). Forty per cent (34/80) of the perceptual disorder intervention studies included in our scoping review were published after 2014 and thus intervention reporting could have benefited from use of this checklist.
Representation of senses
Evidence to inform interventions for some senses was lacking; interventions for hearing, taste and smell perceptual disorders and descriptions of their impact on life after stroke were not commonly reported. 28,43–45 Training and guidelines on this topic are limited3; however, recent care pathway guidance279,280 on the assessment and management of visual disorders after stroke gives greater impetus to improving care and the research needed to underpin this.
Paediatric populations
The paediatric population are particularly under-researched in relation to stroke-related perceptual disorders (see Visual perception disorder interventions), which reflects the generally limited paediatric stroke rehabilitation evidence base. 281 The paediatric and adult stroke populations are different. We lack agreement about the nature and extent282 of natural recovery following stroke among the paediatric population (due to neurodevelopmental plasticity);283 and where extensive recovery is expected, the need for management interventions is reduced. Greater evidence on the persistence of perceptual disorders and factors affected with this is a key area to inform further research for a paediatric population. Similarly, interventions designed for adult populations may not engage or suit paediatric populations leading to adherence challenges. Thus, paediatric perceptual disorder studies should be developed and conducted to inform their care and rehabilitation.
Impact of the COVID-19 pandemic
Conducted between January 2020 and December 2021, the project was subject to the impacts of the COVID-19 pandemic and associated work-related restrictions. We experienced delays in the literature identification and retrieval of full texts due to the temporary closure of the British Library. Our core research group had prior experience of videoconferencing and pivoted to this format with relative ease, but this was not the case for our Lived Experience Group. Shifting to online methods changed the nature of our meetings and altered communication quality and contribution. Members described difficulties in finding their place in the group, taking in the information presented, speaking naturally and using the technology. Clinical colleagues simultaneously experienced significant clinical demands on their time (such as emergency planning, redeployment, training requirements and supporting their teams or professional bodies with information provision and synthesis). Academic colleagues shifted to home-based working, online teaching and caring activities. As the project and pandemic progressed, demands altered and we are grateful for our clinical, academic and research colleagues’ continued contribution and our funder’s support over a very challenging period of time.
Clinical implications
Working with our Lived Experience Group and Clinical Expert Group we agreed the implications for stroke survivors, clinicians and policy-makers (see Chapter 8, Implications for stroke survivors and carers, Implications for clinicians, and Implications for policy-makers). Overall, the coproduced clinical implications recommend a greater awareness of, assessment for, and information provision relating to stroke-related perceptual disorders, and recommended a holistic approach to intervention and support.
While the evidence was insufficient to support intervention decisions, the scoping review provides a source of information with evidence maps supporting the accessibility of the results, and primary research links if required. The overall Cochrane Review finding indicated that there was insufficient evidence to support any intervention benefits impaired perception after stroke. However, a lack of evidence is not evidence of a lack of effectiveness and the limited studies identified may inform relevant best practice statements and stimulate research in this topic area.
Research implications
Across both reviews, we highlighted the small number of studies (in some cases with methodological limitations) evaluating interventions for perceptual disorders after stroke. Together with our Lived Experience and Clinical Expert Groups we developed the research priorities relating to perceptual disorders in stroke. Future research should develop a better understanding of the experiences of stroke survivors with perceptual problems, their assessment, treatment and current services. Undertaking this work, it will also be essential to establish with greater clarity the incidence, prevalence and natural history of perceptual problems due to stroke, including where this occurs alongside other stroke-related impairments.
Under-researched populations
Most of the research identified in the scoping review related to adults and thus there is a need to address perceptual disorders experienced by children and young people. Similarly, further research on hearing, taste and smell perception disorders is required, particularly to establish the incidence, natural history, prevalence and impact of these.
In developing this research field, guidance on the development of stroke rehabilitation interventions and trials of their effectiveness are available to support the exploration of the mechanisms of action, and questions relating to dosage and target population,276,284. In addition, full consideration should be given to the context of care, and feasibility, sustainability and economic factors affecting intervention delivery; pragmatic designs should be considered in order to maximise the clinical relevance and applicability of emerging findings.
Involving stakeholders’ priorities
It is important that future research considers the needs of key stakeholders: stroke survivors, carers and families affected by perceptual disorders, and clinicians providing care. Researchers should aim to use a structured process to identify and engage stakeholders as fully as possible, ensuring that their priorities are considered and outcomes of importance addressed with interventions reflecting current practice in community-based settings.
Clarity and completeness of reporting
We suggest that researchers use relevant international reporting standards97,108,128 for populations, interventions and specific study designs, etc. and fully report key aspects of their research including
-
Sufficiently detailed theoretical rationale for, and description of, the interventions to allow implementation into clinical practice and research replication.
-
Detailed diagnostic information on individuals’ perceptual problems, given the heterogeneity in perceptual problems in terms of type, severity and likely impact on everyday function.
-
Recording whether participants have concurrent impairments, including more than one perceptual impairment and/or other stroke and non-stroke relating impairments.
We further suggest the development of standardised terminology for perceptual disorders, to aid clarity or reporting and understanding for researchers, clinicians and stroke survivors, across all the senses. This could further help improve awareness, multidisciplinary identification and intervention for those affected.
Recommendations relating to randomised controlled trial design
Additional recommendations arising from the Cochrane Review and relating specifically to RCTs are:
-
Provide a standard care control group, carefully documenting the content and amount of standard care, which can be highly variable.
-
Endeavour to reduce risk of bias through rigorous methodology and reporting, for example ensure allocation concealment, attempt and report masking of outcome assessors, report all loss to follow‐up and results from all outcome measures, control for other biases.
-
Have the statistical power to answer clinically important questions about long‐term functional outcomes.
-
Include relevant outcomes, including economic analysis.
-
Adopt an intention-to-treat approach to measurement of outcomes in all individuals as well as to analysis of measured outcomes by treatment group.
-
Investigate real-world interventions, rather than solely novel technologies.
Conclusion
Healthcare professionals lack sufficient evidence to inform clinical interventions for visual, somatosensation, touch, hearing, smell and taste perception disorders after stroke. The available evidence shows limited evidence of rigorous development and testing of interventions, and a preponderance of case report, and small-scale RCTs. We encourage the use of intervention development and reporting guidance, and the involvement of stakeholders to maximise the rigor, relevance and validity of future studies. Our priorities for future research include exploring the prevalence and impact of perceptual disorders, improving assessment, and intervention research, that reflects the reality of current care contexts and population variation.
Additional information
Contributions of authors
Christine Hazelton (https://orcid.org/0000-0002-9554-4750) (Research Fellow, Optometrist, Stroke Rehabilitation) was a principal investigator and led the conceptualisation of the application, methodological design as well as data curation, formal analysis, project administration, supervision, creation of resources and visualisation, validation of results, writing original drafts and reviewing and editing the report. She co-led the acquisition of funding.
Alex Todhunter-Brown (https://orcid.org/0000-0003-4941-7985) (Senior Research Fellow, Systematic Review Specialist) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation through stakeholder engagement, formal analysis, supervision, creation of resources and visualisation, validation of results, writing original drafts and reviewing and editing the report.
Pauline Campbell (https://orcid.org/0000-0002-5879-5526) (Research Fellow, Evidence Synthesis) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, formal analysis, data curation, creation of resources and visualisation, validation of results, writing original drafts and reviewing and editing the report.
Katie Thomson (https://orcid.org/0000-0002-2558-2559) (Research Associate, Occupational Therapy Lecturer) was involved in the conducting of the investigation, formal analysis, data curation, creation of resources and visualisation, validation of results, writing original drafts and reviewing and editing the report.
Donald J Nicolson (https://orcid.org/0000-0003-4190-6434) (Lived Experience, Health Services Research) was involved in the conceptualisation of this application including methodological design and funding acquisition, conducted the investigation through stakeholder engagement events and verified results.
Kris McGill (https://orcid.org/0000-0002-0307-1440) (Research Associate, Stroke Rehabilitation) conducted the investigation through literature screening and data extraction (including data curation), verified results and edited the report.
Charlie SY Chung (https://orcid.org/0000-0003-0280-7935) (Occupational Therapist, Rehabilitation Manager) was involved in the conceptualisation of this application including methodological design and funding acquisition, conducted the investigation through stakeholder engagement events, verified results and edited the report.
Liam Dorris (https://orcid.org/0000-0002-9502-3154) (Consultant Paediatric Neuropsychologist, Paediatrics) was involved in the conceptualisation of this application including methodological design and funding acquisition, conducted the investigation through stakeholder engagement events, verified results and edited the report.
David C Gillespie (https://orcid.org/0000-0003-1325-9727) (Consultant Neuropsychologist, Neurosciences) was involved in the conceptualisation of this application including methodological design and funding acquisition, conducted the investigation through literature screening and stakeholder engagement events, verified results and edited the report.
Susan M Hunter (https://orcid.org/0000-0001-8844-6848) (Reader, Physiotherapist, Neurological Rehabilitation) was involved in the conceptualisation of this application including methodological design and funding acquisition, conducted the investigation through literature screening and stakeholder engagement events, verified results and edited the report.
Linda J Williams (https://orcid.org/0000-0002-6317-1718) (Research Fellow, Statistician) was involved in the conceptualisation of this application including methodological design and funding acquisition, conducted the investigation through formal analysis and stakeholder engagement events, verified results and edited the report.
Marian C Brady (https://orcid.org/0000-0002-4589-7021) (Professor of Stroke Care and Rehabilitation, Stroke rehabilitation; Speech and Language Therapy) was a principal investigator and was involved in the conceptualisation of the application, methodological design and funding acquisition as well as formal analysis, validation of results, writing original drafts and reviewing and editing the report.
Acknowledgements
We would like to acknowledge the invaluable contribution of our clinical and lived-experience stakeholders. This included Graham Esson, Rosalind Jack, Professor Carl Philpott, Dr Gera de Haan, Dr Christine Johnson and Dr Kathleen Vancleef. We would like to thank Joshua Cheyne for his assistance with the search process, Dr Julie Duncan Miller for her guidance when screening studies and Dr Hyo-Jong Kim (Department of Rehabilitation Medicine, Seoul National University Bundang Hospital, Korea) for translation. We also thank the Cochrane Stroke Editorial Group for their peer review and support in publishing the Cochrane Systematic Review, and Stroke for their invitation to submit our scoping review for publication.
Data-sharing statement
The data can be obtained from the corresponding author upon request.
Ethics statement
This project involved secondary research with stakeholder involvement. No ethical approval is required for secondary research. We sought ethical approval for the stakeholder involvement activities as this is good practice where participants’ data are recorded and used. The was approved by Glasgow Caledonian University’s School of Health and Life Sciences Nursing Department Research Ethics Committee (HLS/NCH/19/021) on 16 January 2020.
Information governance statement
Glasgow Caledonian University is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, Glasgow Caledonian University is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.gcu.ac.uk/aboutgcu/universitygovernance/data-protection.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/WGJT3471.
Primary conflicts of interest: Christine Hazelton has declared personal funding from the Stroke Association, UK via a non-clinical Lectureship (SA L-NC 20\100003) and grant funding from NIHR Research for Patient Benefit programme (PB-PG-0418-20040), and Fight for Sight/Stroke Association. Alex Todhunter-Brown is (joint) co-ordinating editor for Cochrane Stroke. Funding to support this role is provided by the Scottish Government’s Chief Scientist Office. Pauline Campbell has declared funding from NIHR (NIHR128829; NIHR128470; NIHR132895) and Chief Scientist Office (Scotland; HIPS/21/03). Katie Thomson has declared an honorarium and travel expenses for the Occupational Therapy Show, consultancy fees from NHS Fife and a Global Challenges Networking Grant. Donald J Nicolson has declared being employed for an SME Medical Device Company (Metix Medical) for 2 years (May 2019–July 2021) as the Medical Science Liaison, winning an Innovate UK award for the Business-led innovation in response to global disruption (de minimis) funding application stream. This evaluated the utility of a prototype high-acuity monitoring device. He was the grant holder and project lead and drew a salary from this project. He is also an employee of Healthcare Improvement. Scotland since July 2021 and is attached to the SIGN team. In 2018 he received travel expenses to present at the Association for Borderlands Studies World Conference. Charlie Chung has declared membership of the Cochrane Stroke Group and funding from the Chief Scientist Office (Scotland). Marian C Brady has declared grant funding from the Chief Scientist Office, NIHR Research for Patient Benefit programme (NIHR200739) and the Health and Social Care Delivery Research (NIHR132895) programme, the Tavistock Trust for Aphasia, NHS Greater Glasgow and Clyde Endowment Fund, Academy of Medical Sciences and the British Aphasiology Society. She has also declared travel and accommodation support from the New Zealand Speech and Language Therapist’s Association to attend the Speech Pathology Australia National Conference. She is also a member of the Data Monitoring Committee for the NIHR PhEAST trial (NIHR132016), and is chair of the Virtual International Stroke Trials Archive-Rehabilitation as well as the Collaboration of Aphasia Trialists and the International Association of Communication Science and Disorders. She is a committee member of the Aphasia Alliance (UK) and the UK Aphasia Advisory Committee. She is an editorial board member (associate editor) of both the Cochrane Stroke Editorial Group and Aphasiology and board member of the International Association of Communication Sciences and Disorders. All other authors have declared no competing interests.
Publications
Hazelton C, McGill K, Campbell P, Todhunter-Brown A, Thomson K, Nicolson DJ, et al. Perceptual disorders after stroke: a scoping review of interventions. Stroke 2022;53:1772–87. https://doi.org/10.1161/STROKEAHA.121.035671
Hazelton C, Thomson K, Todhunter-Brown A, Campbell P, Chung CS, Dorris L, et al. Interventions for perceptual disorders following stroke. Cochrane Database Syst Rev 2022;11:CD007039. https://doi.org/10.1002/14651858.CD007039.pub3
Hazelton C, Campbell P, Chung C, Dorris L, Gillespie D, Hunter D, et al. Perceptual disorders after stroke InterventiON EvidencE Review (PIONEER): a scoping review and Cochrane Review revision and expansion. Study Protocol (version 1.1). NIHR Health Technology Journals 2021:1–34.
Conference presentations
Hazelton C, McGill K, Pollock A, Campbell P, Chung C, et al. Interventions for Visual Perceptual Disorder after Stroke: A Scoping Review. Oral Presentation – Dutch Congress of Rehabilitation Medicine, 2020.
Hazelton C, McGill K, Pollock A, Campbell P, Thomson K, et al. Interventions for Perceptual Disorders Following Stroke. Oral Presentation – Occupational Therapy Show, 2021.
Hazelton C, McGill K, Pollock A, Campbell P, Thomson K, et al. Perceptual disorders after stroke Intervention Evidence Review (PIONEER): a scoping review. Eur Stroke J 2021;6:3–513. https://doi.org/10.1177/23969873211034932
Hazelton C, McGill K, Pollock A, Campbell P, Thomson K, et al. Perceptual Disorders after Stroke: A Scoping Review of Interventions with Evidence Gap Mapping. Oral Presentation – Ageing Well with Stroke Conference, 2021.
Hazelton C, McGill K, Pollock A, Campbell, Chung C, et al. Perceptual Disorders after Stroke: A Scoping Review of Interventions with Evidence Gap Mapping. Oral Presentation – UK Stroke Forum, 2020.
Hazelton C, Thomson K, McGill K, Pollock A, Campbell P, Chung C, et al. Interventions for Perceptual Disorders after Stroke: What is the Research to Date? Oral Presentation – UK Stroke Forum, 2021.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Hazelton C, Campbell P, Chung C, Dorris L, Gillespie D, Hunter D, et al. Perceptual disorders after stroke InterventiON EvidencE Review (PIONEER): a scoping review and Cochrane Review revision and expansion. Study Protocol (version 1.1). NIHR Health Technol J 2021;2021:1-34.
- Matlin M. Sensation and Perception. Boston: Allyn and Bacon Inc; 1988.
- Royal College of Physicians . National Clinical Guidelines for Stroke 2016. www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines (accessed 3 February 2022).
- Lezak M, Howieson D, Bigler E, Tranel D. Neuropsychological Assessment. New York: Oxford University Press; 2012.
- Wolfe J, Kluender K, Levi D. Sensation and Perception. Sunderland: Sinauer Associates; 2012.
- Bowen A, Knapp P, Gillespie D, Nicolson DJ, Vail A. Non-pharmacological interventions for perceptual disorders following stroke and other adult-acquired, non-progressive brain injury. Cochrane Database Syst Rev 2011;2011. https://doi.org/10.1002/14651858.CD007039.pub2.
- World Health Organization . The ICF Browser 2010. https://apps.who.int/classifications/icfbrowser/ (accessed 6 September 2022).
- World Health Organization . International Classification of Functioning, Disability and Health: ICF 2001.
- Pollock A, Hazelton C, Rowe FJ, Jonuscheit S, Kernohan A, Angilley J, et al. Interventions for visual field defects in people with stroke. Cochrane Database Syst Rev 2019;5. https://doi.org/10.1002/14651858.CD008388.pub3.
- Longley V, Hazelton C, Heal C, Pollock A, Woodward-Nutt K, Mitchell C, et al. Non‐pharmacological interventions for spatial neglect or inattention following stroke and other non‐progressive brain injury. Cochrane Database Syst Rev 2021;7.
- Hummel T, Landis BN, Huttenbrink KB. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg 2011;10. https://doi.org/10.3205/cto000077.
- Li X, Rastogi P, Gibbons JA, Chaudhury S. Visuo-cognitive skill deficits in Alzheimer’s disease and Lewy body disease: a comparative analysis. Ann Indian Acad Neurol 2014;17:12-8. https://doi.org/10.4103/0972-2327.128530.
- Ffytche DH, Blom JD, Catani M. Disorders of visual perception. J Neurol Neurosurg Psychiatry 2010;81:1280-7. https://doi.org/10.1136/jnnp.2008.171348.
- Ego A, Lidzba K, Brovedani P, Belmonti V, Gonzalez-Monge S, Boudia B, et al. Visual-perceptual impairment in children with cerebral palsy: a systematic review. Dev Med Child Neurol 2015;57:46-51. https://doi.org/10.1111/dmcn.12687.
- Cascio C, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey KA, et al. Tactile perception in adults with autism: a multidimensional psychophysical study. J Autism Dev Disord 2008;38:127-37. https://doi.org/10.1007/s10803-007-0370-8.
- Chung S, Son JW. Visual perception in autism spectrum disorder: a review of neuroimaging studies. Soa Chongsonyon Chongsin Uihak 2020;31:105-20. https://doi.org/10.5765/jkacap.200018.
- Royal College of Physicians. Sentinel Stroke National Audit Programme (SSNAP) . National Clinical Audit Annual Results Portfolio March 2016–April 2017 n.d. www.strokeaudit.org/results/Clinical-audit/Clinical-CCG-LHB-LCG.aspx (accessed 4 February 2022).
- NHS National Services Scotland . Scottish Stroke Care Audit, Scottish Stroke Improvement Programme Report 2017. www.strokeaudit.scot.nhs.uk/Publications/docs/2017-07-11-SCCA-Report.pdf (accessed 4 February 2022).
- Patel A, Berdunov V, King D, Quayyum Z, Wittenberg R, Knapp M. Executive Summary Part 2: Burden of Stroke in the Next 20 Years and Potential Returns from Increased Spending on Research 2017. www.stroke.org.uk/sites/default/files/costs_of_stroke_in_the_uk_report_-executive_summary_part_2.pdf (accessed 4 February 2022).
- Department of Health . Quality and Outcomes Framework (QOF) Achievement Data 2015 16 2016. http://bit.ly/2hQNsMB (accessed 4 February 2022).
- Winward CE, Halligan PW, Wade DT. Somatosensory recovery: a longitudinal study of the first 6 months after unilateral stroke. Disabil Rehabil 2007;29:293-9. https://doi.org/10.1080/09638280600756489.
- Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehabil 2008;22:758-67. https://doi.org/10.1177/0269215508090674.
- Gorst T, Rogers A, Morrison SC, Cramp M, Paton J, Freeman J, et al. The prevalence, distribution, and functional importance of lower limb somatosensory impairments in chronic stroke survivors: a cross sectional observational study. Disabil Rehabil 2019;41:2443-50. https://doi.org/10.1080/09638288.2018.1468932.
- Meyer S, De Bruyn N, Lafosse C, Van Dijk M, Michielsen M, Thijs L, et al. Somatosensory impairments in the upper limb poststroke: distribution and association with motor function and visuospatial neglect. Neurorehabil Neural Repair 2016;30:731-42. https://doi.org/10.1177/1545968315624779.
- Rowe FJ, Hepworth LR, Howard C, Hanna KL, Cheyne CP, Currie J. High incidence and prevalence of visual problems after acute stroke: an epidemiology study with implications for service delivery. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0213035.
- Edmans J, Lincoln N. The recovery of perceptual problems after stroke and the impact on daily life. Clin Rehabil 1991;5:301-9.
- Bellis T, Bellis J. Handbook of Clinical Neurology. Amsterdam, The Netherlands: Elsevier B.V.; 2015.
- Koohi N, Vickers DA, Lakshmanan R, Chandrashekar H, Werring DJ, Warren JD, et al. Hearing characteristics of stroke patients: prevalence and characteristics of hearing impairment and auditory processing disorders in stroke patients. J Am Acad Audiol 2017;28:491-505. https://doi.org/10.3766/jaaa.15139.
- Karpa MJ, Gopinath B, Rochtchina E, Cumming RG, Sue CM, . Prevalence and neurodegenerative or other associations with olfactory impairment in an older community. J Aging Health 2010;22:154-68. https://doi.org/10.1177/0898264309353066.
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA 2002;288:2307-12. https://doi.org/10.1001/jama.288.18.2307.
- Liu G, Zong G, Doty RL, Sun Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-013246.
- Heckmann JG, Stössel C, Lang CJG, Neundörfer B, Tomandl B, Hummel T. Taste disorders in acute stroke: a prospective observational study on taste disorders in 102 stroke patients. Stroke 2005;36:1690-4. https://doi.org/10.1161/01.STR.0000173174.79773.d3.
- Wehling E, Naess H, Wollschlaeger D, Hofstad H, Bramerson A, Bende M, et al. Olfactory dysfunction in chronic stroke patients. BMC Neurol 2015;15. https://doi.org/10.1186/s12883-015-0463-5.
- Meyer S, Karttunen AH, Thijs V, Feys H, Verheyden G. How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity, and participation problems after stroke? A systematic review. Phys Ther 2014;94:1220-31. https://doi.org/10.2522/ptj.20130271.
- Bowden JL, Lin GG, McNulty PA. The prevalence and magnitude of impaired cutaneous sensation across the hand in the chronic period post-stroke. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0104153.
- Carey LM, Matyas TA. Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. J Rehabil Med 2011;43:257-63. https://doi.org/10.2340/16501977-0662.
- Rowe FJ. Stroke survivors’ views and experiences on impact of visual impairment. Brain Behav 2017;7. https://doi.org/10.1002/brb3.778.
- Titus MN, Gall NG, Yerxa EJ, Roberson TA, Mack W. Correlation of perceptual performance and activities of daily living in stroke patients. Am J Occup Ther 1991;45:410-8. https://doi.org/10.5014/ajot.45.5.410.
- Ali M, Hazelton C, Lyden P, Pollock A, Brady M. Recovery from poststroke visual impairment: evidence from a clinical trials resource. Neurorehabil Neural Repair 2013;27:133-41. https://doi.org/10.1177/1545968312454683.
- Bernspång B, Asplund K, Eriksson S, Fugl-Meyer AR. Motor and perceptual impairments in acute stroke patients: effects on self-care ability. Stroke 1987;18:1081-6. https://doi.org/10.1161/01.str.18.6.1081.
- Bersano A, Burgio F, Gattinoni M, Candelise L. Aphasia burden to hospitalised acute stroke patients: need for an early rehabilitation programme. Int J Stroke 2009;4:443-7. http://dx.doi.org/10.1111/j.1747-4949.2009.00349.x.
- Dickey L, Kagan A, Lindsay MP, Fang J, Rowland A, Black S. Incidence and profile of inpatient stroke-induced aphasia in Ontario, Canada. Arch Phys Med Rehabil 2010;91:196-202. https://doi.org/10.1016/j.apmr.2009.09.020.
- Dutta TM, Josiah AF, Cronin CA, Wittenberg GF, Cole JW. Altered taste and stroke: a case report and literature review. Top Stroke Rehabil 2013;20:78-86. https://doi.org/10.1310/tsr2001-78.
- Dávalos A, Ricart W, Gonzalez-Huix F, Soler S, Marrugat J, Molins A, et al. Effect of malnutrition after acute stroke on clinical outcome. Stroke 1996;27:1028-32. https://doi.org/10.1161/01.str.27.6.1028.
- Green TL, McGregor LD, King KM. Smell and taste dysfunction following minor stroke: a case report. Can J Neurosci Nurs 2008;30:10-3.
- Hunter SM, Crome P. Hand function and stroke. Rev Clin Gerontol 2002;12:68-81. https://doi.org/10.1017/s0959259802012194.
- Doyle S, Bennett S, Fasoli SE, McKenna KT. Interventions for sensory impairment in the upper limb after stroke. Cochrane Database Syst Rev 2010;12. https://doi.org/10.1002/14651858.CD006331.pub2.
- Tyson SF, Hanley M, Chillala J, Selley AB, Tallis RC. Sensory loss in hospital-admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehabil Neural Repair 2008;22:166-72. https://doi.org/10.1177/1545968307305523.
- Sommerfeld DK, von Arbin MH. The impact of somatosensory function on activity performance and length of hospital stay in geriatric patients with stroke. Clin Rehabil 2004;18:149-55. https://doi.org/10.1191/0269215504cr710oa.
- Surya N, Someshwar H. Rehabilitation in perceptual disorders in stroke patients. SVOA Neurol 2020;1:1-9.
- Connell LA, McMahon NE, Adams N. Stroke survivors’ experiences of somatosensory impairment after stroke: an interpretative phenomenological analysis. Physiotherapy 2014;100:150-5. https://doi.org/10.1016/j.physio.2013.09.003.
- Doyle SD, Bennett S, Dudgeon B. Upper limb post-stroke sensory impairments: the survivor’s experience. Disabil Rehabil 2014;36:993-1000. https://doi.org/10.3109/09638288.2013.825649.
- Stroke4Carers . Perceptual Problems 2022. www.stroke4carers.org/?p=349 (accessed 6 September 2022).
- Stroke Foundation . Vision and Senses 2022. https://enableme.org.au/resources/vision-and-senses (accessed 6 September 2022).
- Carey LM, Oke LE, Matyas TA. Impaired touch discrimination after stroke: a quantitative test. Neurorehabil Neural Repair 1987;11:219-32.
- Koohi N, Vickers DA, Utoomprurkporn N, Werring DJ, Bamiou DE. A hearing screening protocol for stroke patients: an exploratory study. Front Neurol 2019;10. https://doi.org/10.3389/fneur.2019.00842.
- de Vries SM, Heutink J, Melis-Dankers BJM, Vrijling ACL, Cornelissen FW, Tucha O. Screening of visual perceptual disorders following acquired brain injury: a Delphi study. Appl Neuropsychol Adult 2018;25:197-209. http://dx.doi.org/10.1080/23279095.2016.1275636.
- Hajek V, Kates M, Donnelly R, McGree S. The effect of visuo-spatial training in patients with right hemisphere stroke. Can J Rehabil 1993;6:175-86.
- Taylor MM, Schaeffer JN, Blumenthal FS, Grisell JL. Perceptual training in patients with left hemiplegia. Arch Phys Med Rehabil 1971;52:163-9.
- Edmans JA, Webster J, Lincoln NB. A comparison of two approaches in the treatment of perceptual problems after stroke. Clin Rehabil 2000;14:230-43. https://doi.org/10.1191/026921500673333145.
- Dirette DK, Hinojosa J, Carnevale GJ. Comparison of remedial and compensatory interventions for adults with acquired brain injuries. J Head Trauma Rehabil 1999;14:595-601. https://doi.org/10.1097/00001199-199912000-00008.
- Mazer BL, Sofer S, Korner-Bitensky N, Gelinas I, Hanley J, Wood-Dauphinee S. Effectiveness of a visual attention retraining program on the driving performance of clients with stroke. Arch Phys Med Rehabil 2003;84:541-50. https://doi.org/10.1053/apmr.2003.50085.
- Lueck AH, Dutton GN. Vision and the Brain: Understanding Cerebral Visual Impairment in Children. New York: AFB Press; 2015.
- Kang S, Kim D, Seo K, . A computerized visual perception rehabilitation programme with interative computer interface using motion tracking technology – a randomized controlled, single-blind, pilot clinical trial. Clin Rehabil 2009;35:434-44.
- Kihoon J, Yu J, Jung J. Effects of virtual reality-based rehabilitation on upper extremity function and visual perception in stroke patients: a randomized controlled trial. J Phys Ther Sci 2012;24:1205-8.
- Cho HY, Kim K, Lee B, Jung J. The effect of neurofeedback on a brain wave and visual perception in stroke: a randomised controlled trial. J Phys Ther Sci 2015;27:673-6. https://doi.org/10.1589/jpts.27.673.
- Kim KU, Kim SH, An TG. Effect of transcranial direct current stimulation on visual perception function and performance capability of activities of daily living in stroke patients. J Phys Ther Sci 2016;28:2572-5.
- Koohi N, Vickers D, Chandrashekar H, Tsang B, Werring D, Bamiou DE. Auditory rehabilitation after stroke: treatment of auditory processing disorders in stroke patients with personal frequency-modulated (FM) systems. Disabil Rehabil 2017;39:586-93. https://doi.org/10.3109/09638288.2016.1152608.
- Weihing J, Chermak GD, Musiek FE. Auditory training for central auditory processing disorder. Semin Hear 2015;36:199-215. https://doi.org/10.1055/s-0035-1564458.
- Heckmann JG, Heckmann SM, Lang CJ, Hummel T. Neurological aspects of taste disorders. Arch Neurol 2003;60:667-71. https://doi.org/10.1001/archneur.60.5.667.
- Henkin RI, Schecter PJ, Friedewald WT, Demets DL, Raff M. A double blind study of the effects of zinc sulfate on taste and smell dysfunction. Am J Med Sci 1976;272:285-99. https://doi.org/10.1097/00000441-197611000-00006.
- The Stroke Association . Sensory Problems 2018. www.stroke.org.uk/effects-of-stroke/physical-effects-of-stroke/sensory-problems (accessed 3 February 2022).
- Cahill LS, Lannin NA, Mak-Yuen YYK, Turville ML, Carey LM. Changing practice in the assessment and treatment of somatosensory loss in stroke survivors: protocol for a knowledge translation study. BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-2829-z.
- Carey L, Macdonell R, Matyas TA. SENSe: study of the effectiveness of neurorehabilitation on sensation: a randomized controlled trial. Neurorehabil Neural Repair 2011;25:304-13. https://doi.org/10.1177/1545968310397705.
- An CM, Roh JS, Kim TH, Choi HS, Choi KH, Kim GM. Effects of game-based postural vertical training on pusher behavior, postural control, and activity of daily living in patients with acute stroke: a pilot study. Phys Ther Korea 2019;26:57-66. https://doi.org/10.12674/ptk.2019.26.3.057.
- Jones SA, Shinton RA. Improving outcome in stroke patients with visual problems. Age Ageing 2006;35:560-5. https://doi.org/10.1093/ageing/afl074.
- Bamiou D. Handbook of Clinical Neurology. London: Elsevier; 2015.
- Hazelton C, Pollock A, Taylor A, Davis B, Walsh G, Brady MC. A qualitative exploration of the effect of visual field loss on daily life in home-dwelling stroke survivors. Clin Rehabil 2019;33:1264-73. https://doi.org/10.1177/0269215519837580.
- Rowe FJ, Walker M, Rockliffe J, Pollock A, Noonan C, Howard C, et al. Care provision for poststroke visual impairment. J Stroke Cerebrovasc Dis 2015;24:1131-44. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.12.035.
- Rowe FJ, Walker M, Rockliffe J, Pollock A, Noonan C, Howard C, et al. Care Provision and Unmet Need for Post Stroke Visual Impairment. Final Report. London: Thomas Pocklington Trust; 2014.
- Colwell MJ, Demeyere N, Vancleef K. Visual perceptual deficit screening in stroke survivors: evaluation of current practice in the United Kingdom and Republic of Ireland. Disabil Rehabil 2021;44:6620-32. https://doi.org/10.1080/09638288.2021.1970246.
- Pollock A, Hazelton C, Brady M. Visual problems after stroke: a survey of current practice by occupational therapists working in UK stroke inpatient settings. Top Stroke Rehabil 2011;18:643-51. https://doi.org/10.1310/tsr18s01-643.
- Pollock A, Hazelton C, Brady M. Orthoptic assessment and management of patients with stroke in Scotland. Br Ir Orthopt J 2011;8:36-42.
- National Institute for Health and Care Excellence (NICE) . Stroke Rehabilitation in Adults 2013. www.nice.org.uk/guidance/cg162 (accessed 3 February 2022).
- Scottish Intercollegiate Guidelines Network . Sign 118: Management of Patients With Stroke: Rehabilitation, Prevention and Management of Complications, and Discharge Planning. A National Clinical Guideline 2010. www.sign.ac.uk/media/1056/sign118.pdf (accessed 3 February 2022).
- British and Irish Orthoptic Society . British and Irish Orthoptic Society (BIOS) Orthoptic Stroke Neuro Rehab Care Pathway 2015. www.orthoptics.org.uk/resources/clinical-advisory-group/stroke-and-neuro-rehabilitation/ (accessed 3 February 2022).
- Royal College of Paediatrics and Child Health . Stroke in Childhood – Clinical Guideline for Diagnosis, Management and Rehabilitation 2017. www.rcpch.ac.uk/resources/stroke-in-childhood-clinical-guideline (accessed 3 February 2022).
- Jutai JW, Bhogal SK, Foley NC, Bayley M, Teasell RW, Speechley MR. Treatment of visual perceptual disorders post stroke. Top Stroke Rehabil 2003;10:77-106. https://doi.org/10.1310/07BE-5E1N-735J-1C6U.
- Schabrun SM, Hillier S. Evidence for the retraining of sensation after stroke: a systematic review. Clin Rehabil 2009;23:27-39. https://doi.org/10.1177/0269215508098897.
- Teasell RW, Salter K, Cotoi A, . Perceptual Disorders [need more information]. London; 2018.
- Pollock A, St George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurol 2012;11. https://doi.org/10.1016/S1474-4422(12)70029-7.
- The Stroke Association . Shaping Stroke Research to Rebuild Lives. The Stroke Priority Setting Partnership Results for Investment (June 2021) 2021. www.stroke.org.uk/sites/default/files/research/stroke_priority_setting_partnership_full_report.pdf (accessed 25 March 2022).
- James Lind Alliance . Neuro-Ophthalmology Top 10 2022.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soci Res Methodol 2005;8:19-32. https://doi.org/10.1080/1364557032000119616.
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-69.
- Useem J, Brennan A, LaValley M, Vickery M, Ameli O, Reinen N, et al. Systematic differences between Cochrane and non-Cochrane meta-analyses on the same topic: a matched pair analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0144980.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- Camden C, Shikako-Thomas K, Nguyen T, Graham E, Thomas A, Sprung J, et al. Engaging stakeholders in rehabilitation research: a scoping review of strategies used in partnerships and evaluation of impacts. Disabil Rehabil 2015;37:1390-400. http://dx.doi.org/10.3109/09638288.2014.963705.
- Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Stakeholder involvement in systematic reviews: a protocol for a systematic review of methods, outcomes and effects. Res Involv Engagem 2017;3. https://doi.org/10.1186/s40900-017-0060-4.
- Pollock A, Campbell P, Baer G, Choo PL, Morris J, Forster A. User involvement in a Cochrane systematic review: using structured methods to enhance the clinical relevance, usefulness and usability of a systematic review update. Syst Rev 2015;4. https://doi.org/10.1186/s13643-015-0023-5.
- Manera K, Hanson C, Gutman T, Tong A. Handbook of Research Methods in Health Social Sciences. Singapore: Springer; 2019.
- Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Development of the ACTIVE framework to describe stakeholder involvement in systematic reviews. J Health Serv Res Policy 2019;24:245-55. https://doi.org/10.1177/1355819619841647.
- Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Stakeholder involvement in systematic reviews: a scoping review. Syst Rev 2018;7. https://doi.org/10.1186/s13643-018-0852-0.
- Pollock A, Morley R, Watts C. Involving People: A Learning Resource for Systematic Review Authors. UK: Cochrane Collaboration; 2022.
- INVOLVE . Public Involvement in Research and Research Ethics Committee Review 2016. www.invo.org.uk/wp-content/uploads/2016/05/HRA-INVOLVE-updated-statement-2016.pdf (accessed 25 March 2022).
- Cochrane Collaboration . Cochrane Review Ecosystem 2016. http://community.cochrane.org/review-production/cochrane-review-ecosystem (accessed 26 January 2022).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Hickey G, Brearley S, Coldham T, Denegri S, Green G, Staniszewska S, et al. Guidance on Co-producing a Research Project. Southampton: INVOLVE; 2018.
- Patton MQ. Qualitative Research and Evaluation Methods. Saint Paul, MN: SAGE Publications Ltd; 2002.
- Chung CSY, Pollock A, Campbell T, Durward BR, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane Database Syst Rev 2013;2013. https://doi.org/10.1002/14651858.CD008391.pub2.
- Hazelton C, McGill K, Campbell P, Todhunter-Brown A, Thomson K, Nicolson DJ, et al. Perceptual disorders after stroke: a scoping review of interventions. Stroke 2022;53:1772-87. https://doi.org/10.1161/STROKEAHA.121.035671.
- Mays N, Roberts E, Popay J. Methods for Studying the Delivery and Organisation of Health Services. London; 2001.
- Daudt HML, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-48.
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141-6. https://doi.org/10.1097/XEB.0000000000000050.
- Snilstveit B, Vojtkova M, Bhavsar A, Stevenson J, Gaarder M. Evidence & gap maps: a tool for promoting evidence informed policy and strategic research agendas. J Clin Epidemiol 2016;79:120-9. https://doi.org/10.1016/j.jclinepi.2016.05.015.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467-73. https://doi.org/10.7326/M18-0850.
- Karnath HO, Broetz D. Understanding and treating ‘pusher syndrome’. Phys Ther 2003;83:1119-25.
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40-6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
- Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016;104:240-3. https://doi.org/10.3163/1536-5050.104.3.014.
- Phillipson L, Hammond A. More than talking. Int J Qual Methods 2018;17. https://doi.org/10.1177/1609406918782784.
- Debas HT, Gosselin R, Thind A. Disease Control Priorities in Developing Countries. New York: Oxford University Press; 2006.
- Boes AD, Kelly MS, Trapp NT, Stern AP, Press DZ, Pascual-Leone A. Noninvasive brain stimulation: challenges and opportunities for a new clinical specialty. J Neuropsychiatry Clin Neurosci 2018;30:173-9. https://doi.org/10.1176/appi.neuropsych.17110262.
- World Health Organization . Rehabilitation 2021. www.who.int/news-room/fact-sheets/detail/rehabilitation (accessed 28 March 2022).
- Kerkhoff G. Neurovisual rehabilitation: recent developments and future directions. J Neurol Neurosurg Psychiatry 2000;68:691-706.
- Hetrick SE, Parker AG, Callahan P, Purcell R. Evidence mapping: illustrating an emerging methodology to improve evidence-based practice in youth mental health. J Eval Clin Pract 2010;16:1025-30. https://doi.org/10.1111/j.1365-2753.2008.01112.x.
- Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev 2016;5. https://doi.org/10.1186/s13643-016-0204-x.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372. https://doi.org/10.1136/bmj.n71.
- Colarusso RP, Hammill DD. The Motor Free Visual Perception Test (MVPT-3). Navato, CA: Academic Therapy Publications; 2003.
- Chen CC, Liu HC. Low-dose aripiprazole resolved complex hallucinations in the left visual field after right occipital infarction (Charles Bonnet syndrome). Psychogeriatrics 2011;11:116-8. https://doi.org/10.1111/j.1479-8301.2010.00353.x.
- Nakagawa N, Akai F, Niiyama K, Asai T, Tanada M. [A case of peduncular hallucination after aneurysmal subarachnoid hemorrhage]. No To Shinkei 1999;51:65-8.
- Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc 2011;59:761-2. https://doi.org/10.1111/j.1532-5415.2011.03363.x.
- Roberts-Woodbury J, Herrington H. Visual hallucinations after a stroke. J Am Geriatr Soc 2016;64.
- Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1973;188:139-50. http://dx.doi.org/10.1007/BF00407835.
- Flint AC, Loh JP, Brust JC. Vivid visual hallucinations from occipital lobe infarction. Neurology 2005;65. https://doi.org/10.1212/01.wnl.0000180350.58985.5f.
- Poetter CE, Vyas BB, Stewart JT, Haley JA. An unusual case of poststroke hallucinations. J Am Geriatr Soc 2012;60:165-6. https://doi.org/10.1111/j.1532-5415.2011.03709.x.
- Rafique SA, Richards JR, Steeves JKE. rTMS reduces cortical imbalance associated with visual hallucinations after occipital stroke. Neurology 2016;87:1493-500. https://doi.org/10.1212/WNL.0000000000003180.
- Brunsdon R, Nickels L, Coltheart M, Joy P. Assessment and treatment of childhood topographical disorientation: a case study. Neuropsychol Rehabil 2007;17:53-94. https://doi.org/10.1080/09602010600562575.
- Tanemura R. Awareness in apraxia and agnosia. Top Stroke Rehabil 1999;6:33-42. http://dx.doi.org/10.1310/U9KU-M4X8-DMRQ-9TR7.
- Zihl J. Rehabilitation of Visual Disorders After Brain Injury. Hove, UK: Psychology Press; 2000.
- McDowell N, Dutton GN. Hemianopia and features of Balint syndrome following occipital Lobe hemorrhage: identification and patient understanding have aided functional improvement years after onset. Case Rep Ophthalmol Med 2019;2019. https://doi.org/10.1155/2019/3864572.
- Gottlieb D, Calvanio R, Levine DN. Reappearance of the visual percept after intentional blinking in a patient with Balint’s syndrome. J Clin Neuroophthalmol 1991;11:62-5.
- Burr M, Hazen B. The use of television in the rehabilitation of stroke patients with perceptual difficulties. Aust Occup Ther J 1972;19:19-22. https://doi.org/10.1111/j.1440-1630.1972.tb00524.x.
- Choi D, Choi W, Lee S. Influence of Nintendo Wii Fit balance game on visual perception, postural balance, and walking in stroke survivors: a pilot randomized clinical trial. Games Health J 2018;7:377-84. https://doi.org/10.1089/g4h.2017.0126.
- Dutton GN, Chokron S, Little S, McDowell N. Posterior parietal visual dysfunction: an explanatory review. Vision Dev Rehabil 2017;3:10-22. https://doi.org/10.31707/VDR2017.3.1.p10.
- Edmans JA, Lincoln NB. Treatment of visual perceptual deficits after stroke: single case studies on four patients with right hemiplegia. Br J Occup Ther 1991;54:139-44.
- Kang SH, Kim DK, Seo KM, Choi KN, Yoo JY, Sung SY, et al. A computerized visual perception rehabilitation programme with interactive computer interface using motion tracking technology – a randomized controlled, single-blinded, pilot clinical trial study. Clin Rehabil 2009;23:434-44.
- Kim EJ, Lee KE, Lee KL, Kim HG, Yoon YH, Jeon SY, et al. Change of visual perception in geriatric strokes after visuomotor coordination training. J Korean Acad Rehab Med 2011;35:174-9.
- Zihl J. Rehabilitation of Visual Disorders After Brain Injury. Hove, UK: Psychology Press; 2000.
- Lincoln NB, Whiting SE, Cockburn J, Bhavnani G. An evaluation of perceptual retraining. Int Rehabil Med 1985;7:99-101. https://doi.org/10.3109/03790798509166132.
- Zihl J. Rehabilitation of Visual Disorders After Brain Injury. Hove, UK: Psychology Press; 2000.
- Park JH, Park JH. The effects of a Korean computer-based cognitive rehabilitation program on cognitive function and visual perception ability of patients with acute stroke. J Phys Ther Sci 2015;27:2577-9. https://doi.org/10.1589/jpts.27.2577.
- O’Hare AE, Dutton GN, Green D, Coull R. Evolution of a form of pure alexia without agraphia in a child sustaining occipital lobe infarction at 2 1/2 years. Dev Med Child Neurol 1998;40:417-20.
- Chen P, Hartman AJ, Priscilla Galarza C, DeLuca J. Global processing training to improve visuospatial memory deficits after right-brain stroke. Arch Clin Neuropsychol 2012;27:891-905. https://doi.org/10.1093/arclin/acs089.
- Funk J, Finke K, Reinhart S, Kardinal M, Utz KS, Rosenthal A, et al. Effects of feedback-based visual line-orientation discrimination training for visuospatial disorders after stroke. Neurorehabil Neural Repair 2013;27:142-52. https://doi.org/10.1177/1545968312457826.
- Zihl J. Rehabilitation of Visual Disorders After Brain Injury. Hove, UK: Psychology Press; 2000.
- Towle D, Edmans JA, Lincoln NB. An evaluation of a group treatment programme for stroke patients with perceptual deficits. Int J Rehabil Res 1990;13:328-35. https://doi.org/10.1097/00004356-199012000-00007.
- Gillen JA, Dutton GN. Balint’s syndrome in a 10-year-old male. Dev Med Child Neurol 2003;45:349-52. https://doi.org/10.1017/s0012162203000641.
- Weinberg J, Piasetsky E, Diller L, Gordon W. Treating perceptual organization deficits in nonneglecting RBD stroke patients. J Clin Neuropsychol 1982;4:59-75. https://doi.org/10.1080/01688638208401117.
- Zaharia-Pushkash O, Oleg P, Rodica V, Andrei U. Posterior cerebral artery stroke with Balint’s syndrome and severe cognitive impairment: clinical and neuroimaging correlation. Int J Stroke 2010;5:365-6.
- Ko EJ, Chun MH, Kim DY, Kang Y, Lee SJ, Yi JH, et al. Frenkel’s exercise on lower limb sensation and balance in subacute ischemic stroke patients with impaired proprioception. Neurol Asia 2018;23:217-24.
- An CM, Ko MH, Kim DH, Kim GW. Effect of postural training using a whole-body tilt apparatus in subacute stroke patients with lateropulsion: a single-blinded randomized controlled trial. Ann Phys Rehabil Med 2021;64. https://doi.org/10.1016/j.rehab.2020.05.001.
- Bergmann J, Krewer C, Jahn K, Müller F. Robot-assisted gait training to reduce pusher behavior: a randomized controlled trial. Neurology 2018;91:e1319-27. https://doi.org/10.1212/WNL.0000000000006276.
- Broetz D, Johannsen L, Karnath HO. Time course of ‘pusher syndrome’ under visual feedback treatment. Physiother Res Int 2004;9:138-43. https://doi.org/10.1002/pri.314.
- Freitas ACM, Bezerra LAP, de Oliveira PCA, Freitas LM, da Silva SR, de Cirne GN, et al. Evaluation of the effectiveness of mirror therapy in Pusher syndrome and hemineglect in post-stroke patients. Fisioter Bras 2017;18:362-8.
- Fujino Y, Amimoto K, Sugimoto S, Fukata K, Inoue M, Takahashi H, et al. Prone positioning reduces severe pushing behavior: three case studies. J Phys Ther Sci 2016;28:2690-3. https://doi.org/10.1589/jpts.28.2690.
- Fujino Y, Takahashi H, Fukata K, Inoue M, Shida K, Matsuda T, et al. Electromyography-guided electrical stimulation therapy for patients with pusher behavior: a case series. NeuroRehabilitation 2019;45:537-45. https://doi.org/10.3233/NRE-192911.
- Gillespie J, Callender L, Driver S. Usefulness of a standing frame to improve contraversive pushing in a patient post-stroke in inpatient rehabilitation. Proc (Bayl Univ Med Cent) 2019;32:440-2. https://doi.org/10.1080/08998280.2019.1593763.
- Jahn K, Müller F, Koenig E, Krewer C, Tillmann S, Bergmann J. Rehabilitation of verticality perception using a new training method. J Neurol 2017;264:26-7. https://doi.org/10.1007/s00415-017-8435-x.
- Jang SH, Lee HD. Recovery of an injured medial lemniscus with concurrent recovery of pusher syndrome in a stroke patient: a case report. Medicine (Baltimore) 2018;97. https://doi.org/10.1097/MD.0000000000010963.
- Jokelainen L, Jokelainen M. Työntöoireyhtymä. Duodecim 2000;116:144-7.
- Kim MS. Effect of robot assisted rehabilitation based on visual feedback in post stroke pusher syndrome. J Korea Acad Ind Cooper Soc 2016;17:562-8. https://doi.org/10.5762/kais.2016.17.10.562.
- Lee JT, Chon SC. Does the addition of visual feedback improve postural vertical training in the patients with pusher syndrome after stroke?. J Korean Soc Phys Med 2017;12:33-42.
- Meneghetti CHZ, Basqueira C, Fioramonte C, Ferracini Júnior LC. Influência da fisioterapia aquática no controle de tronco na síndrome de pusher: estudo de caso. Fisioterapia E Pesquisa 2009;16:269-73. https://doi.org/10.1590/s1809-29502009000300014.
- Mikołajewska E. Posterior pusher syndrome – case report. Cent Eur J Med 2012;7:354-7.
- Pardo V, Galen S. Treatment interventions for pusher syndrome: a case series. NeuroRehabilitation 2019;44:131-40. https://doi.org/10.3233/NRE-182549.
- Scheets PL, Sahrmann SA, Norton BJ. Use of movement system diagnoses in the management of patients with neuromuscular conditions: a multiple-patient case report. Phys Ther 2007;87:654-69. https://doi.org/10.2522/ptj.20050349.
- Voos MC, Oliveira TP, Piemonte MEP. Diretrizes para avaliação e tratamento fisioterapêutico da Síndrome de Pusher: estudo de caso. Fisioterapia E Pesquisa 2011;18:323-8. https://doi.org/10.1590/s1809-29502011000400005.
- Wang D, Lin J, Liu X. Effects of visual feedback and core stability training program on post-stroke Pusher syndrome: a pilot randomized controlled study. Chin J Rehab Med 2016;31:426-9.
- Yang YR, Chen YH, Chang HC, Chan RC, Wei SH, Wang RY. Effects of interactive visual feedback training on post-stroke pusher syndrome: a pilot randomized controlled study. Clin Rehabil 2015;29:987-93. https://doi.org/10.1177/0269215514564898.
- Yun N, Joo MC, Kim SC, Kim MS. Robot-assisted gait training effectively improved lateropulsion in subacute stroke patients: a single-blinded randomized controlled trial. Eur J Phys Rehabil Med 2018;54:827-36. https://doi.org/10.23736/S1973-9087.18.05077-3.
- Babyar S, Santos T, Will-Lemos T, Mazin S, Edwards D, Reding M. Sinusoidal transcranial direct current versus galvanic vestibular stimulation for treatment of lateropulsion poststroke. J Stroke Cerebrovasc Dis 2018;27:3621-5. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.08.034.
- Krewer C, Rieß K, Bergmann J, Müller F, Jahn K, Koenig E. Immediate effectiveness of single-session therapeutic interventions in pusher behaviour. Gait Posture 2013;37:246-50. https://doi.org/10.1016/j.gaitpost.2012.07.014.
- Nakamura J, Kita Y, Yuda T, Ikuno K, Okada Y, Shomoto K. Effects of galvanic vestibular stimulation combined with physical therapy on pusher behavior in stroke patients: a case series. NeuroRehabilitation 2014;35:31-7. https://doi.org/10.3233/NRE-141094.
- Colombo R, Sterpi I, Mazzone A, Delconte C, Pisano F. Improving proprioceptive deficits after stroke through robot-assisted training of the upper limb: a pilot case report study. Neurocase 2016;22:191-200. https://doi.org/10.1080/13554794.2015.1109667.
- Jamal K, Leplaideur S, Chochina L, Moulinet-Raillon A, Senal N, Bonan I. The long-lasting effects of repetitive neck muscle vibration on postural disturbances in standing position in chronic patients. Neurophysiol Clin 2017;47:341-2. https://doi.org/10.1016/j.neucli.2017.10.014.
- Koo WR, Jang BH, Kim CR. Effects of anodal transcranial direct current stimulation on somatosensory recovery after stroke: a randomized controlled trial. Am J Phys Med Rehabil 2018;97:507-13. https://doi.org/10.1097/PHM.0000000000000910.
- Fifer RC. Insular stroke causing unilateral auditory processing disorder: case report. J Am Acad Audiol 1993;4:364-9.
- Koohi N, Vickers D, Warren J, Werring D, Bamiou DE. Long-term use benefits of personal frequency-modulated systems for speech in noise perception in patients with stroke with auditory processing deficits: a non-randomised controlled trial study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013003.
- Papathanasiou I, Macfarlane S, Heron C. A case of verbal auditory agnosia: missing the word. missing the sound. Int J Lang Commun Disord 1998;33:214-7. https://doi.org/10.3109/13682829809179425.
- Woolf C, Panton A, Rosen S, Best W, Marshall J. Therapy for auditory processing impairment in aphasia: an evaluation of two approaches. Aphasiology 2014;28:1481-505. https://doi.org/10.1080/02687038.2014.931921.
- Zgaljardic D, Yancy S, Burton V, Masel B. Auditory agnosia and Post-Acute Brain Injury Rehabilitation (PABIR): a case report. J Head Trauma Rehabil 2013;28:E35-6.
- Fechtelpeter A, Göddenhenrich S, Huber W, Springer L. [Approaches to therapy of auditory agnosia]. Folia Phoniatr (Basel) 1990;42:83-97.
- Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Arch Phys Med Rehabil 1993;74:602-11. https://doi.org/10.1016/0003-9993(93)90158-7.
- Carey LM, Matyas TA. Training of somatosensory discrimination after stroke: facilitation of stimulus generalization. Am J Phys Med Rehabil 2005;84:428-42. https://doi.org/10.1097/01.phm.0000159971.12096.7f.
- Hayashi R. Olfactory illusions and hallucinations after right temporal hemorrhage. Eur Neurol 2004;51:240-1. https://doi.org/10.1159/000078551.
- Oppenländer K, Utz KS, Reinhart S, Keller I, Kerkhoff G, Schaadt AK. Subliminal galvanic-vestibular stimulation recalibrates the distorted visual and tactile subjective vertical in right-sided stroke. Neuropsychologia 2015;74:178-83. https://doi.org/10.1016/j.neuropsychologia.2015.03.004.
- Carey LM, Abbott DF, Lamp G, Puce A, Seitz RJ, Donnan GA. Same intervention-different reorganization: the impact of lesion location on training-facilitated somatosensory recovery after stroke. Neurorehabil Neural Repair 2016;30:988-1000. https://doi.org/10.1177/1545968316653836.
- Enders LR, Hur P, Johnson MJ, Seo NJ. Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance. J Neuroeng Rehabil 2013;10. https://doi.org/10.1186/1743-0003-10-105.
- Fujimoto S, Kon N, Otaka Y, Yamaguchi T, Nakayama T, Kondo K, et al. Transcranial direct current stimulation over the primary and secondary somatosensory cortices transiently improves tactile spatial discrimination in stroke patients. Front Neurosci 2016;10. https://doi.org/10.3389/fnins.2016.00128.
- Kim B, Bang D, Shin W. Effects of pressure sense perception training on unstable surface on somatosensory, balance and gait function in patients with stroke. J Korean Soc Phys Med 2015;10:19-27. https://doi.org/10.13066/kspm.2015.10.3.19.
- Kitisomprayoonkul W. Poster 66 transcranial direct current stimulation improves hand sensation in acute stroke. Arch Phys Med Rehabil 2012;93. https://doi.org/10.1016/j.apmr.2012.08.101.
- Morioka S, Yagi F. Effects of perceptual learning exercises on standing balance using a hardness discrimination task in hemiplegic patients following stroke: a randomized controlled pilot trial. Clin Rehabil 2003;17:600-7. https://doi.org/10.1191/0269215503cr654oa.
- Hazelton C, Thomson K, Todhunter-Brown A, Campbell P, Chung CS, Dorris L, et al. Interventions for perceptual disorders following stroke. Cochrane Database Syst Rev 2022;11. https://doi.org/10.1002/14651858.CD007039.pub3.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (v5.1.0) [Updated March 2011]. Cochrane Collaboration; 2011.
- Faltinsen E, Todorovac A, Hróbjartsson A, Gluud C, Kongerslev MT, Simonsen E, et al. Placebo, usual care and wait-list interventions for all mental health disorders. Cochrane Database Syst Rev 2019;1. https://doi.org/10.1002/14651858.MR000050.
- Kazdin AE. Research Design in Clinical Psychology. New York: Harper and Row; 1980.
- Brady MC, Godwin J, Kelly H, Enderby P, Elders A, Campbell P. Attention control comparisons with SLT for people with aphasia following stroke: methodological concerns raised following a systematic review. Clin Rehabil 2018;32:1383-95. https://doi.org/10.1177/0269215518780487.
- Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a ‘trial effect’. J Clin Epidemiol 2001;54:217-24. https://doi.org/10.1016/s0895-4356(00)00305-x.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965;14:61-5.
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. Advances in Clinical Rehabilitation. New York: Springer; 1987.
- Rankin J. Cerebral vascular accidents in patients over the age of 60 II. Prognosis. Scott Med J 1957;2:200-15. https://doi.org/10.1177/003693305700200504.
- Katz S. Assessing self-maintenance: activities of daily living, mobility, and instruments of daily living. J Am Geriatr Soc 1983;31:721-7.
- Fisher AG. Objective Measurement: Theory into Practice. Norwood: Ablex; 1994.
- Lankhorst G, Jelles F, Van Bennekom C. Rehabilitation Activities Profile: Manual and Description. Amsterdam: VU University Press; 1996.
- Holbrook M, Skilbeck CE. An activities index for use with stroke patients. Age Ageing 1983;12:166-70. https://doi.org/10.1093/ageing/12.2.166.
- Balestroni G, Bertolotti G. EuroQol-5D (EQ-5D): an instrument for measuring quality of life. Monaldi Arch Chest Dis 2012;78:155-9. https://doi.org/10.4081/monaldi.2012.121.
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud 1991;13:50-4. https://doi.org/10.3109/03790799109166684.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Robinson BC. Validation of a caregiver strain index. J Gerontol 1983;38:344-8. https://doi.org/10.1093/geronj/38.3.344.
- Whiting S, Lincoln NB, Bhavnani G, Cockburn J. The Rivermead Perceptual Assessment Battery. Windsor: NFER-Nelson; 1985.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022) 2022. www.training.cochrane.org/handbook.
- Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Deeks JJ, Higgins JP, Altman DG. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020). Cochrane Collaboration; 2020.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. https://doi.org/10.1136/bmj.315.7109.629.
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. https://doi.org/10.1016/j.jclinepi.2010.07.015.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 2011;64:407-15. https://doi.org/10.1016/j.jclinepi.2010.07.017.
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol 2011;64:1283-93. https://doi.org/10.1016/j.jclinepi.2011.01.012.
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE Working Group . GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol 2011;64:1294-302. http://dx.doi.org/10.1016/j.jclinepi.2011.03.017.
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE Working Group . GRADE guidelines: 8. Rating the quality of evidence – indirectness. J Clin Epidemiol 2011;64:1303-10. https://doi.org/10.1016/j.jclinepi.2011.04.014.
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence – publication bias. J Clin Epidemiol 2011;64:1277-82. https://doi.org/10.1016/j.jclinepi.2011.01.011.
- Cochrane Editorial and Publishing Policy Resource . Peer Review Policy 2022. https://documentation.cochrane.org/display/EPPR/Peer+review+policy (accessed 28 March 2022).
- De Bruyn N, Saenen L, Thijs L, Van Gils A, Ceulemans E, Essers B, et al. Sensorimotor vs. motor upper limb therapy for patients with motor and somatosensory deficits: a randomized controlled trial in the early rehabilitation phase after stroke. Front Neurol 2020;11. https://doi.org/10.3389/fneur.2020.597666.
- Lee HC, Kuo FL, Lin YN, Liou TH, Lin JC, Huang SW. Effects of robot-assisted rehabilitation on hand function of people with stroke: a randomized, crossover-controlled, assessor-blinded study. Am J Occup Ther 2021;75. https://doi.org/10.5014/ajot.2021.038232.
- Seim CE, Wolf SL, Starner TE. Wearable vibrotactile stimulation for upper extremity rehabilitation in chronic stroke: clinical feasibility trial using the VTS Glove. J Neuroeng Rehabil 2021;18. https://doi.org/10.1186/s12984-021-00813-7.
- Kang SH, Kim DK, Seo KM, Choi KN, Yoo JY, Sung SY, et al. A computerized visual perception rehabilitation programme with interactive computer interface using motion tracking technology – a randomized controlled, single-blinded, pilot clinical trial study. Clin Rehabil 2009;23:434-44. https://doi.org/10.1177/0269215508101732.
- DRKS00021654 . Effects of End-Effector Controlled Gait Training Compared to Balance Training Onpostural Stability, Walking Ability and Subjective Perception of Visual Verticality (SVV) in Non-Ambulatory Patients With Left-Sided Neglect 2020. https://trialsearch.who.int/Trial2.aspx?TrialID=DRKS00021654 (accessed 27 March 2022).
- NCT02524015 . Novel Treatment for Pusher Syndrome Using Physical Therapy 2015. https://clinicaltrials.gov/ct2/show/NCT02524015 (accessed 27 March 2022).
- NCT03154138 . Prismatic Adaptation for Rehabilitation of Postural Imbalance After Stroke (PEQUIE) 2017. https://clinicaltrials.gov/ct2/show/NCT03154138 (accessed 27 March 2022).
- NCT03888326 . Robot and TDCS Based Proprioceptive Rehabilitation After Stroke (RoboStim) 2018. https://clinicaltrials.gov/ct2/show/NCT03888326 (accessed 27 March 2022).
- NCT03991390 . Effectiveness of Balance Exercise Program for Stroke Patients With Pusher Syndrome 2019. https://clinicaltrials.gov/ct2/show/NCT03991390 (accessed 27 March 2022).
- NCT04490655 . Active Somatosensory Exercise for Chronic Stroke (ActSens) 2020. https://clinicaltrials.gov/ct2/show/NCT04490655 (accessed 27 March 2022).
- NCT04703218 . Re-Education of Olfactory Disorders After a Cerebral Vascular Accident in Adults 2021. https://clinicaltrials.gov/ct2/show/NCT04703218 (accessed 27 March 2022).
- NCT04818073 . Determinants of the Effectiveness of Robot-Assisted Hand Movement Training 2021. https://clinicaltrials.gov/ct2/show/NCT04818073 (accessed 27 March 2022).
- NCT04911738 . VIrtual Reality Glasses Use to Improve Lateropulsion and the Post-Stroke Postural Vertical (VIRGIL) 2021. https://clinicaltrials.gov/ct2/show/NCT04911738 (accessed 27 March 2022).
- Chiu EC, Chi FC. Effect of home-based activities of daily living (ADL) on cognition and visual perception in patients with stroke: a randomized controlled pilot study. Am J Occup Ther 2020;74. https://doi.org/10.5014/ajot.2020.74S1-PO3404.
- Kim DH, Kim KH, Lee SM. The effects of virtual reality training with upper limb sensory exercise stimulation on the AROM of upper limb joints, function, and concentration in chronic stroke patients. Physikalische Medizin, Rehabilitationsmedizin, Kurortmedizin 2019;30:86-94. https://doi.org/10.1055/a-0917-4604.
- Koval’chuk VV. [An influence of mexidol on the restoration of neurological deficit, increase of social adaptation and removal of neglect and push syndromes in stroke patients]. Zh Nevrol Psikhiatr Im S S Korsakova 2011;111:52-7.
- Leer WB. Block Design Training with Stroke Patients: A Study on the Effects of Cognitive Retraining on Improving Certain Activities of Daily Living Skills. Michigan: Michigan State University; 1984.
- Matz K, Teuschl Y, Eckhardt R, Herbst A, Dachenhausen A, Brainin M. Cognitive training in patients with first lacunar stroke – a randomized pilot trial for the prevention of post-stroke cognitive decline. Cerebrovasc Dis 2007;23.
- Muffel T, Shih PC, Kalloch B, Sehm B. P187 costs and benefits: complex effects of unilateral and bilateral tDCS over M1 on the kinematics of sensorimotor function in chronic stroke. Clin Neurophysiol 2020:e120-e1.
- Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS group . ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225-34. https://doi.org/10.1016/j.jclinepi.2015.06.005.
- Jenkins M. Evaluation of methodological search filters–a review. Health Info Libr J 2004;21:148-63. https://doi.org/10.1111/j.1471-1842.2004.00511.x.
- Lefebvre C, Glanville J, Beale S, Boachie C, Duffy S, Fraser C, et al. Assessing the performance of methodological search filters to improve the efficiency of evidence information retrieval: five literature reviews and a qualitative study. Health Technol Assess 2017;21:1-148. https://doi.org/10.3310/hta21690.
- Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, Langhorne P, et al. Interventions for disorders of eye movement in patients with stroke. Cochrane Database Syst Rev 2011.
- West C, Bowen A, Hesketh A, Vail A. Interventions for motor apraxia following stroke. Cochrane Database Syst Rev 2008;2008. https://doi.org/10.1002/14651858.CD004132.pub2.
- Hoffmann T, Bennett S, Koh CL, McKenna KT. Occupational therapy for cognitive impairment in stroke patients. Cochrane Database Syst Rev 2010;2010. https://doi.org/10.1002/14651858.CD006430.pub2.
- Hanna KL, Hepworth LR, Rowe FJ. The treatment methods for post-stroke visual impairment: a systematic review. Brain Behav 2017;7. https://doi.org/10.1002/brb3.682.
- Serrada I, Hordacre B, Hillier SL. Does sensory retraining improve sensation and sensorimotor function following stroke: a systematic review and meta-analysis. Front Neurosci 2019;13. https://doi.org/10.3389/fnins.2019.00402.
- Thanaya SAP, Mardhika PE. Therapeutic approaches for pusher syndrome after a stroke: a literature review. Intisari Sains Medis 2019;10. https://doi.org/10.15562/ism.v10i2.507.
- Cicerone KD, Goldin Y, Ganci K, Rosenbaum A, Wethe JV, Langenbahn DM, et al. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil 2019;100:1515-33. https://doi.org/10.1016/j.apmr.2019.02.011.
- Hausler R, Levine RA. Auditory dysfunction in stroke. Acta Otolaryngol 2000;120:689-703. https://doi.org/10.1080/000164800750000207.
- James Lind Alliance . Life After Stroke Top 10 2011. www.jla.nihr.ac.uk/priority-setting-partnerships/life-after-stroke/top-10-priorities/ (accessed 29 March 2022).
- Rudberg AS, Berge E, Laska AC, Jutterstrom S, Nasman P, Sunnerhagen KS, et al. Stroke survivors’ priorities for research related to life after stroke. Top Stroke Rehabil 2021;28:153-8. https://doi.org/10.1080/10749357.2020.1789829.
- Sacco RL, Sandercock P, Endres M, Feigin V, Pandian J, Shinohara Y, et al. Review and prioritization of stroke research recommendations to address the mission of the World Stroke Organization: a call to action from the WSO Research Committee. Int J Stroke 2015;10:4-9. https://doi.org/10.1111/ijs.12625.
- James Lind Alliance . Mild to Moderate Hearing Loss Top 10 2022. www.jla.nihr.ac.uk/priority-setting-partnerships/mild-to-moderate-hearing-loss/top-10-priorities/ (accessed 28 March 2022).
- Alliance JL. Smell and Taste Disorders. UK: James Lind Alliance; 2022.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- Rowe FJ, Hepworth LR, Kirkham JJ. Development of core outcome sets and core outcome measures for central visual impairment, visual field loss and ocular motility disorders due to stroke: a Delphi and consensus study. BMJ Open 2022;12. https://doi.org/10.1136/bmjopen-2021-056792.
- Hoddinott P, Pollock A, O’Cathain A, Boyer I, Taylor J, MacDonald C, et al. How to incorporate patient and public perspectives into the design and conduct of research. F1000Res 2018;7. http://dx.doi.org/10.12688/f1000research.15162.1.
- Carcel C, Harris K, Peters SAE, Sandset EC, Balicki G, Bushnell CD, et al. Representation of women in stroke clinical trials: a review of 281 trials involving more than 500,000 participants. Neurology 2021;97:e1768-74. https://doi.org/10.1212/WNL.0000000000012767.
- Silver JK, Flores LE, Mondriguez Gonzalez A, Frontera WR. An analysis of the inclusion of women, older individuals, and racial/ethnic minorities in rehabilitation clinical trials. Am J Phys Med Rehabil 2021;100:546-54. https://doi.org/10.1097/PHM.0000000000001750.
- Nelson MLA, McKellar KA, Yi J, Kelloway L, Munce S, Cott C, et al. Stroke rehabilitation evidence and comorbidity: a systematic scoping review of randomized controlled trials. Top Stroke Rehabil 2017;24:374-80. https://doi.org/10.1080/10749357.2017.1282412.
- Brady MC, Fredrick A, Williams B. People with aphasia: capacity to consent, research participation and intervention inequalities. Int J Stroke 2013;8:193-6. http://dx.doi.org/10.1111/j.1747-4949.2012.00900.x.
- Bernhardt J, Hayward KS, Dancause N, Lannin NA, Ward NS, Nudo RJ, et al. A stroke recovery trial development framework: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair 2019;33:959-69. https://doi.org/10.1177/1545968319888642.
- Cahill LS, Carey LM, Mak-Yuen Y, McCluskey A, Neilson C, O’Connor DA, et al. Factors influencing allied health professionals’ implementation of upper limb sensory rehabilitation for stroke survivors: a qualitative study to inform knowledge translation. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2020-042879.
- Ryan C, Hazelton C, Lawrence M, Kidd L. UK Stroke Supplement: 212: What are the unmet needs of stroke survivors with visual impairment? A qualitative exploration and prioritisation project. Int J Stroke 2021;16:9-37. https://doi.org/10.1177/17474930211059996.
- Rowe FJ, Hepworth LR, Howard C, Hanna KL, Helliwell B. Developing a stroke-vision care pathway: a consensus study. Disabil Rehabil 2022;44:487-95. https://doi.org/10.1080/09638288.2020.1768302.
- Scottish Government . A Progressive Stroke Pathway 2022. www.gov.scot/binaries/content/documents/govscot/publications/independent-report/2022/03/progressive-stroke-pathway/documents/progressive-stroke-pathway/progressive-stroke-pathway/govscot%3Adocument/progressive-stroke-pathway.pdf (accessed 30 March 2022).
- Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, et al. American Heart Association Stroke Council and Council on Cardiovascular and Stroke Nursing . Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke 2019;50:e51-96. https://doi.org/10.1161/STR.0000000000000183.
- Anderson V, Darling S, Mackay M, Monagle P, Greenham M, Cooper A, et al. Cognitive resilience following paediatric stroke: biological and environmental predictors. Eur J Paediatr Neurol 2020;25:52-8. https://doi.org/10.1016/j.ejpn.2019.11.011.
- Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain 2011;134:2197-221. https://doi.org/10.1093/brain/awr103.
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374. https://doi.org/10.1136/bmj.n2061.
- Cheyne J. University of Edinburgh. College of Medicine and Veterinary Medicine. Cochrane Stroke Group; 2020.
- Jo K, Yu J, Jung J. Effects of virtual reality-based rehabilitation on upper extremity function and visual perception in stroke patients: a randomized control trial. J Phys Ther Sci 2012;24:1205-8. https://doi.org/10.1589/jpts.24.1205.
Appendix 1 Search terms – MEDLINE search strategy
Search methods and strategies for this review were written by the Cochrane Stroke Group’s Information Specialist (JDC). The ‘Search methods for identification of studies’ and corresponding search strategies reported here are written based on the review’s research question, inclusion criteria in close consultation with the review team drawing on the Information Specialist’s subject expertise. The following search hedge/filter is often used as a basis for constructing search strategies and is adapted where necessary.
The search strategies were adapted from the following reference. 285
-
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp ‘intracranial embolism and thrombosis’/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/ or carotid stenosis/ or exp carotid artery injuries/ or intracranial arterial diseases/ or cerebral arterial diseases/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or exp carotid arteries/ or endarterectomy, carotid/
-
(stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).ti,ab
-
((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral arter$ or MCA$ or anterior circulation or posterior circulation or basilar arter$ or vertebral arter$ or space-occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).ti,ab
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).ti,ab
-
or/1-4
-
exp perceptual disorders/ or exp perception/
-
hearing disorders/ or hearing loss/ or deafness/ or hearing loss, central/ or hearing loss, sudden/ or hyperacusis/ or olfaction disorders/ or exp somatosensory disorders/ or exp taste disorders/ or vision disorders/ or alice in wonderland syndrome/ or amblyopia/ or blindness/ or blindness, cortical/ or color vision defects/ or diplopia/ or hemianopsia/ or photophobia/ or scotoma/ or vision, low/
-
(percept$ adj3 (impair$ or problem$ or abilit$ or deficit$ or distortion$ or defect$ or disabilit$ or disturbance$ or disorder$ or discriminat$ or deaf$)).ti,ab
-
(agnosis or agnosia or prosopagnosia or prosophthalmia or Alice in Wonderland syndrome or Todd syndrome or all?esthesia$ or syn?esthesia$ or hypoesthesia or hyperesthesia).ti,ab
-
sensation/ or hearing/ or smell/ or taste/ or touch/ or vision, ocular/ or color vision/ or exp mesopic vision/ or night vision/
-
(somatosensory$ or (sensor$ adj3 (input$ or stimul$ or deficit$ or distortion$ or defect$ or disabilit$ or disturbance$ or disorder$ or discriminat$ or processing or percept$ or hallucination$ or feedback or discriminat$ or dysfunction$ or recogn$ or interpretation)) or somatosognosia or asomatognosia or somatoparaphrenia or (body adj3 (schema or orientation))).ti,ab
-
exp Proprioception/
-
(propriocep$ or (kin?esthetic adj3 (percept$ or discriminat$))).ti,ab
-
((odo?r$ or smell$ or olfact$ or scent$ or aroma or flavo?r) adj3 (memory or acuity or function$ or percept$ or perceive$ or discriminat$ or distinguish$ or recept$ or sensitiv$ or hedonics or deprivation or hallucinat$)).ti,ab
-
(anosmia or anodmia or anosmy or Kallmann syndrome or dysosmia or hyposmia or hyposphresia or phantosmia or par?osmia or ageusia or hypogeusia or dysgeusia or troposmia or euosmia or cacosmia or malodour or superosmia).ti,ab
-
(ageusia or dysgeusia or parageusia or phantogeusia or hypogeusia or amblygeustia or hypogeusesthesia or hyp?esthesia or superosmia or phantosmia or parosmia or troposmia or euosmia or cacosmia or dysosmia or hypergeusia or phantogeusia or hyperosmia or hyposmia).ti,ab
-
((gustat$ or tast$) adj3 (acuity or percept$ or perceive$ or discriminat$ or distinguish$ or recept$ or sensitiv$ or hallucination$ or abnormalit$ or distortion$ or disturbance$ or anomal$ or loss or an?esthesia or absence or phantom)).ti,ab
-
(((speech or speak$ or voice or spoken or acoustic or audio or auditory or sound or pitch or prosody or binaural or phoneme) adj3 (percept$ or processing or stimul$ or distinguish$ or discriminat$)) or hyperacusis or misophonia or phonophobia or sonophobia or amusia or King Kopetsky syndrome).ti,ab
-
(amblyop? or aniseikonia or oscillopsia or xanthopsia or d?plop$ or polyop$ or metamorphopsia or m?cropsia or ((vision or visual or visual?percept$ or visuo?spatial or visuo?construct$ or ocular or optokinetic or optic$ or oculomotor spatial) adj3 (illusion or blurry or overload or double or percept$ or perceive$ or discriminat$ or distinguish$ or recept$ or sensitiv$ or hallucination$ or abnormalit$ or distortion$ or disturbance$ or anomal$ or disorientation or allachethesia or deficit$ or defect$ or disabilit$ or disorder$ or processing or dysfunction$ or recogn$ or interpretation or analysis or comprehension)) or stereoillusion or kakopsia or kalopsia or pelopsia or archromatopsia or akinetopsia or telopsia or stereopsis or palinopsia or teleopsia or simultanagnosia).ti,ab
-
(entomopia or palinopsia or asteropsis or strabismus or Anton syndrome or Balint syndrome or blindsight or achromatopsia or hyperchromatosis or ((facial or face) adj3 intermetamorphosis) or (visual adj3 anoneria)).ti,ab
-
((figure or shape or orientation or form or colo?r or textur$ or crowding or contour or object or face or faces) adj3 recogn$).ti,ab
-
(astereognosia or stereognosis or astereognosis or paraesthesia or hypersensitivity or ((tactile or haptic$ or touch) adj3 (stimul$ or memory or acuity or sens$ or percept$ or processing or stimul$ or distinguish$ or discriminat$ or anisotropy or locali?ation))).ti,ab
-
or/6-22
-
5 and 23
Appendix 2 Studies included in the scoping review
| No. | Study ID | Reference |
|---|---|---|
| 1 | An 201975 | An C, Roh J, Kim T, Choi H, Choi K, Gyoung-mo Kim. Visual and somatosensory integration processing is needed to reduce pusher behavior (PB) and improve postural control in hemiplegic patients with acute stroke. Phys Ther Korea 2019;26:57–66. |
| 2 | An 2020162 | An CM, Ko MH, Kim D hyun, Kim GW. Effect of postural training using a whole-body tilt apparatus in subacute stroke patients with lateropulsion: A single-blinded randomized controlled trial. Ann Phys Rehabil Med 2020. https://doi.org/10.1016/j.rehab.2020.05.001 |
| 3 | Babyar 2018182 | Babyar S, Santos T, Will-Lemos T, Mazin S, Edwards D, Reding M. Sinusoidal transcranial direct current versus galvanic vestibular stimulation for treatment of lateropulsion poststroke. J Stroke Cerebrovasc Dis 2018;27:3621–5. |
| 4 | Bergmann 2018163 | Bergmann J, Krewer C, Jahn K, Muller F. Robot-assisted gait training to reduce pusher behavior A randomized controlled trial. Neurology 2018;91:E1319–27. |
| 5 | Broetz 2004164 | Broetz D, Johannsen L, Karnath HO. Time course of ‘pusher syndrome’ under visual feedback treatment. Physiother Res Int 2004;9:138–43. |
| 6 | Brunsdon 2007138 | Brunsdon R, Nickels L, Coltheart M, Joy P. Assessment and treatment of childhood topographical disorientation: a case study. Neuropsychol Rehabil 2007;17:53–94. |
| 7 | Burr 1972143 | Burr M, Hazen B. The use of television in the rehabilitation of stroke patients with perceptual difficulties. AOTJ 1972;Jan–Mar:19–22. |
| 8 | Carey 1993194 | Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Arch Phys Med Rehabil 1993;74:602–11. |
| 9 | Carey 2005195 | Carey LM, Matyas TA. Training of somatosensory discrimination after stroke: facilitation of stimulus generalization. Am J Phys Med Rehabil 2005;84:428–42. |
| 10 | Carey 201174 | Carey L, Macdonell R, Matyas TA. SENSe: Study of the effectiveness of neurorehabilitation on sensation: a randomized controlled trial. Neurorehabil Neural Repair 2011;25:304–13. |
| 11 | Carey 2016198 | Carey LM, Abbott DF, Lamp G, Puce A, Seitz RJ, Donnan GA. Same intervention-different reorganization: the impact of lesion location on training-facilitated somatosensory recovery after stroke. Neurorehabil Neural Repair 2016;30:988–1000. |
| 12 | Chen 2011130 | Chen CC, Liu HC. Low-dose aripiprazole resolved complex hallucinations in the left visual field after right occipital infarction (Charles Bonnet syndrome). Psychogeriatrics 2011;11:116–8. |
| 13 | Chen 2012154 | Chen P, Hartman AJ, Priscilla Galarza C, DeLuca J. Global processing training to improve visuospatial memory deficits after right-brain stroke. Arch Clin Neuropsychol 2012;27:891–905. |
| 14 | Cho 201566 | Cho HY, Kim K, Lee B, Jung J. The effect of neurofeedback on a brain wave and visual perception in stroke: a randomized control trial. J Phys Ther Sci 2015;27:673–6. |
| 15 | Choi 2018144 | Choi D, Choi W, Lee S. Influence of Nintendo Wii Fit balance game on visual perception, postural balance, and walking in stroke survivors: a pilot randomized clinical trial. Games Health J 2018;7:377–84. |
| 16 | Cogan 1973134 | Cogan DG. Visual hallucinations as release phenomena. Albr von Graefes Arch für Klin und Exp Ophthalmol 1973;188:139–50. |
| 17 | Colombo 2016185 | Colombo R, Sterpi I, Mazzone A, Delconte C, Pisano F. Improving proprioceptive deficits after stroke through robot-assisted training of the upper limb: a pilot case report study. Neurocase 2016;22:191–200. |
| 18 | Dutton 2017145 | Dutton GN, Chokron S, Little S, McDowell N. Posterior parietal visual dysfunction: an explanatory review. Vis Dev Rehabil 2017;3:10–22. |
| 19 | Edmans 1991146 | Edmans JA, Lincoln NB. Treatment of visual perceptual deficits after stroke: single case studies on four patients with right hemiplegia. Br J Occup Ther 1991;54:139–44. |
| 20 | Edmans 200060 | Edmans JA, Webster J, Lincoln NB. A comparison of two approaches in the treatment of perceptual problems after stroke. Clin Rehabil 2000 [cited 2019 May 11];14:230–43. |
| 21 | Enders 2013199 | Enders LR, Hur P, Johnson MJ, Seo NJ. Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance. J Neuroeng Rehabil 2013;10:1–8. |
| 22 | Fechtelpeter 1990193 | Fechtelpeter A, Goddenhenrich S, Huber W, Springer L. Ansatze Therapie von auditiver Agnosie. Folia Phoniatr 1990;42:83–97. |
| 23 | Fifer 1993188 | Fifer RC. Insular stroke causing unilateral auditory processing disorder: case report. J Am Acad Audiol 1993;4:364–369 |
| 24 | Flint 2005135 | Flint AC, Loh JP, Brust JCM. Vivid visual hallucinations from occipital lobe infarction. Neurology. 2005;65:754–756 |
| 25 | Freitas 2017165 | Freitas ACM, Bezerra LAP, Oliveira PCA de, Freitas LM, Silva SR da, Cirne GN de M, Cacho R de O. Evaluation of the effectiveness of mirror therapy in Pusher syndrome and hemineglect in post-stroke patients. Fisioter Bras 2017;18:362–8. |
| 26 | Fujimoto 2016200 | Fujimoto S, Kon N, Otaka Y, Yamaguchi T, Nakayama T, Kondo K, Ragert P, Tanaka S. Transcranial direct current stimulation over the primary and secondary somatosensory cortices transiently improves tactile spatial discrimination in stroke patients. Front Neurosci 2016;10:1–9. |
| 27 | Fujino 2016166 | Fujino Y, Amimoto K, Sugimoto S, Fukata K, Inoue M, Takahashi H, Makita S. Prone positioning reduces severe pushing behavior: three case studies. J Phys Ther Sci 2016;28:2690–2693. |
| 28 | Fujino 2019167 | Fujino Y, Takahashi H, Fukata K, Inoue M, Shida K, Matsuda T, Makita S, Amimoto K. Electromyography-guided electrical stimulation therapy for patients with pusher behavior: a case series. NeuroRehabilitation 2019;45:537–45. |
| 29 | Funk 2013155 | Funk J, Finke K, Reinhart S, Kardinal M, Utz KS, Rosenthal A, Kuhn C, Müller H, Kerkhoff G. Effects of feedback-based visual line-orientation discrimination training for visuospatial disorders after stroke. Neurorehabil Neural Repair 2013;27:142–52. |
| 30 | Gillen 2003158 | Gillen JA, Dutton GN. Balint’s syndrome in a 10-year-old male: a case report. Dev Med Child Neurol 2003;45:349–52. |
| 31 | Gillespie 2019168 | Gillespie J, Callender L, Driver S. Usefulness of a standing frame to improve contraversive pushing in a patient post-stroke in inpatient rehabilitation. Baylor Univ Med Cent Proc 2019;32:440–2. |
| 32 | Gottlieb 1991142 | Gottlieb D, Calvanio R, Levine D. Reappearance of the visual percept after intentional blinking in a patient with Balint’s syndrome. J Clin Neuroophthalmol 1991;11:62–5. |
| 33 | Hayashi 2004196 | Hayashi R. Olfactory illusions and hallucinations after right temporal hemorrhage. Eur Neurol 2004;51:240–1. |
| 34 | Jahn 2017169 | Jahn K, Müller F, Koenig E, Krewer C, Tillmann S, Bergmann J. Rehabilitation of verticality perception using a new training method. J Neurol 2017;264:26–7. |
| 35 | Jamal 2017186 | Jamal K, Leplaideur S, Rousseau C, Chochina L, Moulinet-Raillon A, Senal N, Bonan I. The long-lasting effects of repetitive neck muscle vibration on postural disturbances in standing position in chronic patients. Neurophysiol Clin 2017;47:341–2. |
| 36 | Jang 2018170 | Jang SH, Lee H Do. Recovery of an injured medial lemniscus with concurrent recovery of pusher syndrome in a stroke patient: a case report. Med 2018;97:21–3. |
| 37 | Jo 2012 286 | Jo K, Yu J, Jung J. Effects of virtual reality-based rehabilitation on upper extremity function and visual perception in stroke patients: a randomized control trial. J Phys Ther Sci 2012;24:1205–8. |
| 38 | Jokelainen 2000171 | Jokelainen L, Jokelainen M. Työntöoireyhtymä. Duodecim 2000;116:144–7. |
| 39 | Kang 2009147 | Kang SH, Kim DK, Seo KM, Choi KN, Yoo JY, Sung SY, Park HJ. A computerized visual perception rehabilitation programme with interactive computer interface using motion tracking technology – a randomized controlled, single-blinded, pilot clinical trial study. Clin Rehabil 2009;23:434–44. |
| 40 | Kim 2011148 | Kim EJ, Lee KE, Lee KL, Kim HG, Yoon Y, Jeon SY, Yu JA. Change of visual perception in geriatric strokes after visuomotor coordination training. Ann Rehabil Med 2011;35:174–9. |
| 41 | Kim 2015201 | Kim B, Bang D, Shin W. Effects of pressure sense perception training on unstable surface on somatosensory, balance and gait function in patients with stroke. J Korean Soc Phys Med 2015;10:19–27. |
| 42 | Kim 2016172 | Kim MS. Effect of robot assisted rehabilitation based on visual feedback in post stroke Pusher syndrome. J Korea Acad Coop Soc 2016;17:562–8. |
| 43 | Kitisomprayoonkul 2012202 | Kitisomprayoonkul W. Transcranial direct current stimulation improves hand sensation in acute stroke. Arch Phys Med Rehabil 2012;93:e33. |
| 44 | Ko 2018161 | Ko EJ, Chun MH, Kim DY, Kang Y, Lee SJ, Yi JH, Chang MC, Lee SY. Frenkel’s exercise on lower limb sensation and balance in subacute ischemic stroke patients with impaired proprioception. Neurol Asia 2018;23:217–24. |
| 45 | Koo 2018187 | Koo WR, Jang BH, Kim CR. Effects of anodal transcranial direct current stimulation on somatosensory recovery after stroke. Am J Phys Med Rehabil 2018;97:507–13. |
| 46 | Koohi 2017 (1)68 | Koohi N, Vickers D, Chandrashekar H, Tsang B, Werring D, Bamiou DE. Auditory rehabilitation after stroke: treatment of auditory processing disorders in stroke patients with personal frequency-modulated (FM) systems. Disabil Rehabil 2017;39:586–93. |
| 47 | Koohi 2017 (2)189 | Koohi N, Vickers D, Warren J, Werring D, Bamiou DE. Long-term use benefits of personal frequency-modulated systems for speech in noise perception in patients with stroke with auditory processing deficits: a non-randomised controlled trial study. BMJ Open 2017;7:1–7. |
| 48 | Krewer 2013183 | Krewer C, Rieß K, Bergmann J, Müller F, Jahn K, Koenig E. Immediate effectiveness of single-session therapeutic interventions in pusher behaviour. Gait Posture 2013;37:246–50. |
| 49 | Lee 2017173 | Lee JT, Chon SC. Does the addition of visual feedback improve postural vertical training in the patients with Pusher syndrome after stroke? J Korean Soc Phys Med 2017;12:33–42. |
| 50 | Lincoln 1985150 | Lincoln NB, Whiting SE, Cockburn J, Bhavnani G. An evaluation of perceptual retraining. Disabil Rehabil 1985;7:99–101. |
| 51 | McDowell 2019141 | McDowell N, Dutton GN. Hemianopia and features of Bálint syndrome following occipital lobe hemorrhage: identification and patient understanding have aided functional improvement years after onset. Case Rep Ophthalmol Med 2019;1–7. |
| 52 | Meneghetti 2009174 | Meneghetti CHZ, Basqueira C, Fioramonte C, Ferracini Júnior LC. Influence of hydrotherapy on trunk control in the pusher syndrome: case report. Fisioter e Pesqui 2009;16:269–273. |
| 53 | Mikołajewska 2012175 | Mikołajewska E. Posterior pusher syndrome – case report. Cent Eur J Med 2012;7:354–7. |
| 54 | Morioka 2003203 | Morioka S, Yagi F. Effects of perceptual learning exercises on standing balance using a hardness discrimination task in hemiplegic patients following stroke: a randomized controlled pilot trial. Clin Rehabil 2003;17:600–7. |
| 55 | Nakagawa 1999131 | Nakagawa N, Akai F, Niiyyama K, Asai T, Tanada M. A case of peduncular hallucination after aneurysmal subarachnoid hemorrhage. No To Shinkei 1999;51:65–8. |
| 56 | Nakamura 2014184 | Nakamura J, Kita Y, Yuda T, Ikuno K, Okada Y, Shomoto K. Effects of galvanic vestibular stimulation combined with physical therapy on pusher behavior in stroke patients: a case series. NeuroRehabilitation 2014;35:31–7. |
| 57 | Nguyen 2011132 | Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc 2011;59:761–2. |
| 58 | O’Hare 1998153 | O’Hare AE, Dutton GN, Green D, Coull R. Evolution of a form of pure alexia without agraphia in a child sustaining occipital lobe infarction at 2 1/4 years. Dev Med Child Neurol 1998;40:417–20. |
| 59 | Oppenlander 2015197 | Oppenlander K, Utz KS, Reinhart S, Keller I, Kerkhoff G. Subliminal galvanic-vestibular stimulation recalibrates the distorted visual and tactile subjective vertical in right-sided stroke. Neuropsychologia 2015;74:178–83. |
| 60 | Papathanasiou 1998190 | Papathanasiou I, Macfarlane S, Heron C. A case of verbal auditory agnosia: missing the word … missing the sound... Int J Lang Commun Disord 1998;33:214–7. |
| 61 | Pardo 2019176 | Pardo V, Galen S. Treatment interventions for pusher syndrome: a case series. NeuroRehabilitation 2019;44:131–40. |
| 62 | Park 2015152 | Park JH, Park JH. The effects of a Korean computer-based cognitive rehabilitation program on cognitive function and visual perception ability of patients with acute stroke. J Phys Ther Sci 2015;27:2577–9. |
| 63 | Poetter 2008136 | Poetter CE, Vyas BB, Stewart JT, Haley JA. An unusual case of post-stroke hallucinations J Am Geriatr Soc 2008;56:181–3. |
| 64 | Rafique 2016137 | Rafique SA, Richards JR, Steeves JKE. rTMS reduces cortical imbalance associated with visual hallucinations after occipital stroke. Neurology 2016;87:1493–500. |
| 65 | Roberts-Woodbury 2016133 | Roberts-Woodbury. Visual hallucinations after a stroke. J Am Geriatr Soc 2016;64:s159. |
| 66 | Scheets 2007177 | Scheets PL, Sahrmann SA, Norton BJ. Use of movement system diagnoses in the management of patients with neuromuscular conditions: a multiple-patient case report. Phys Ther 2007;87:654–69. |
| 67 | Tanemura 1999139 | Tanemura R. Awareness in apraxia and agnosia. Top Stroke Rehabil 1999;6:33–42. |
| 68 | Towle 1990157 | Towle D, Edmans JA, Lincoln NB. An evaluation of a group treatment programme for stroke patients with perceptual deficits. Int J Rehabil Res 1990;13:328–335. |
| 69 | Voos 2011178 | Voos MC, Oliveira T de P, Piemonte MEP. Guidelines for assessment and physical therapy treatment in Pusher’s syndrome: case report. Fisioter e Pesqui 2011;18:323–8. |
| 70 | Wang 2016179 | Wang D, Lin J, Liu X. Effects of visual feedback and core stability training program on post-stroke Pusher syndrome: a pilot randomized controlled study. Chinese J Rehab Med 2016;31:426–9. |
| 71 | Weinberg 1982159 | Weinberg J, Piasetsky E, Diller L, Gordon W. Treating perceptual organization deficits in nonneglecting RBD stroke patients*. J Clin Neuropsychol 1982;4:59–75. |
| 72 | Woolf 2014191 | Woolf C, Panton A, Rosen S, Best W, Marshall J. Therapy for auditory processing impairment in aphasia: an evaluation of two approaches. Aphasiology 2014;28:1481–505. |
| 73 | Yang 2015180 | Yang YR, Chen YH, Chang HC, Chan RC, Wei SH, Wang RY. Effects of interactive visual feedback training on post-stroke pusher syndrome: a pilot randomized controlled study. Clin Rehabil 2015;29:987–93. |
| 74 | Yun 2018181 | Yun N, Joo MC, Kim SC, Kim MS. Robot-assisted gait training effectively improved lateropulsion in subacute stroke patients: a single-blinded randomized controlled trial. Eur J Phys Rehabil Med 2018;54:827–36. |
| 75 | Zaharia-Pushkash 2010160 | Zaharia-Pushkash O, Oleg P, Rodica V, Andrei U. Posterior cerebral artery stroke with Balint’s syndrome and severe cognitive impairment: clinical and neuroimaging correlation. Int J Stroke 2010;5:365–6. |
| 76 | Zgaljardic 2013192 | Zgaljardic D, Yancy S, Burton V, Masel B. Auditory agnosia and Post-Acute Brain Injury Rehabilitation (PABIR): a case report. J Head Trauma Rehabil 2013;28:E35–6. |
| 77 | Zihl 2000 (1) 151 | Zihl J. Visual agnosia. In Rehabilitation of V isual D isorders after B rain Injury. Hove, UK: Psychology Press; 2000. pp. 136–50. |
| 78 | Zihl 2000 (2)156 | Zihl J. Visual agnosia: Balint’s syndrome and its treatment. In Rehabilitation of Visual Disorders after Brain Injury. Hove: 2000. p. 122–31. |
| 79 | Zihl 2000 (3)149 | Zihl J. Disorders in space perception. In Rehabilitation of Visual Disorders after Brain Injury. Hove, UK: Psychology Press; 2000. pp. 110–22. |
| 80 | Zihl 2000 (4)140 | Zihl J. Colour vision deficits. In Rehabilitation of Visual Disorders after Brain Injury. Hove: Psychology Press; 2000. pp. 102–6. |
Appendix 3 Search terms – MEDLINE search strategy for the Cochrane Review
Search methods and strategies for this review were written by the Cochrane Stroke Group’s Information Specialist (JDC). The ‘Search methods for identification of studies’ and corresponding search strategies reported here are written based on the review’s research question, inclusion criteria in close consultation with the review team drawing on the Information Specialist’s subject expertise. The following search hedge/filter is often used as a basis for constructing search strategies and is adapted where necessary.
The search strategies were adapted from the following reference. 285
-
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp ‘intracranial embolism and thrombosis’/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/ or carotid stenosis/ or exp carotid artery injuries/ or intracranial arterial diseases/ or cerebral arterial diseases/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or exp carotid arteries/ or endarterectomy, carotid/
-
(stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).ti,ab.
-
((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral arter$ or MCA$ or anterior circulation or posterior circulation or basilar arter$ or vertebral arter$ or space-occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).ti,ab.
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).ti,ab.
-
or/1-4
-
exp perceptual disorders/ or exp perception/
-
hearing disorders/ or hearing loss/ or deafness/ or hearing loss, central/ or hearing loss, sudden/ or hyperacusis/ or olfaction disorders/ or exp somatosensory disorders/ or exp taste disorders/ or vision disorders/ or alice in wonderland syndrome/ or amblyopia/ or blindness/ or blindness, cortical/ or color vision defects/ or diplopia/ or hemianopsia/ or photophobia/ or scotoma/ or vision, low/
-
(percept$ adj3 (impair$ or problem$ or abilit$ or deficit$ or distortion$ or defect$ or disabilit$ or disturbance$ or disorder$ or discriminat$ or deaf$)).ti,ab.
-
(agnosis or agnosia or prosopagnosia or prosophthalmia or Alice in Wonderland syndrome or Todd syndrome or all?esthesia$ or syn?esthesia$ or hypoesthesia or hyperesthesia).ti,ab.
-
sensation/ or hearing/ or smell/ or taste/ or touch/ or vision, ocular/ or color vision/ or exp mesopic vision/ or night vision/
-
(somatosensory$ or (sensor$ adj3 (input$ or stimul$ or deficit$ or distortion$ or defect$ or disabilit$ or disturbance$ or disorder$ or discriminat$ or processing or percept$ or hallucination$ or feedback or discriminat$ or dysfunction$ or recogn$ or interpretation)) or somatosognosia or asomatognosia or somatoparaphrenia or (body adj3 (schema or orientation))).ti,ab.
-
exp Proprioception/
-
(propriocep$ or (kin?esthetic adj3 (percept$ or discriminat$))).ti,ab.
-
((odo?r$ or smell$ or olfact$ or scent$ or aroma or flavo?r) adj3 (memory or acuity or function$ or percept$ or perceive$ or discriminat$ or distinguish$ or recept$ or sensitiv$ or hedonics or deprivation or hallucinat$)).ti,ab.
-
(anosmia or anodmia or anosmy or Kallmann syndrome or dysosmia or hyposmia or hyposphresia or phantosmia or par?osmia or ageusia or hypogeusia or dysgeusia or troposmia or euosmia or cacosmia or malodour or superosmia).ti,ab.
-
(ageusia or dysgeusia or parageusia or phantogeusia or hypogeusia or amblygeustia or hypogeusesthesia or hyp?esthesia or superosmia or phantosmia or parosmia or troposmia or euosmia or cacosmia or dysosmia or hypergeusia or phantogeusia or hyperosmia or hyposmia).ti,ab.
-
((gustat$ or tast$) adj3 (acuity or percept$ or perceive$ or discriminat$ or distinguish$ or recept$ or sensitiv$ or hallucination$ or abnormalit$ or distortion$ or disturbance$ or anomal$ or loss or an?esthesia or absence or phantom)).ti,ab.
-
(((speech or speak$ or voice or spoken or acoustic or audio or auditory or sound or pitch or prosody or binaural or phoneme) adj3 (percept$ or processing or stimul$ or distinguish$ or discriminat$)) or hyperacusis or misophonia or phonophobia or sonophobia or amusia or King Kopetsky syndrome).ti,ab.
-
(amblyop? or aniseikonia or oscillopsia or xanthopsia or d?plop$ or polyop$ or metamorphopsia or m?cropsia or ((vision or visual or visual?percept$ or visuo?spatial or visuo?construct$ or ocular or optokinetic or optic$ or oculomotor spatial) adj3 (illusion or blurry or overload or double or percept$ or perceive$ or discriminat$ or distinguish$ or recept$ or sensitiv$ or hallucination$ or abnormalit$ or distortion$ or disturbance$ or anomal$ or disorientation or allachethesia or deficit$ or defect$ or disabilit$ or disorder$ or processing or dysfunction$ or recogn$ or interpretation or analysis or comprehension)) or stereoillusion or kakopsia or kalopsia or pelopsia or archromatopsia or akinetopsia or telopsia or stereopsis or palinopsia or teleopsia or simultanagnosia).ti,ab.
-
(entomopia or palinopsia or asteropsis or strabismus or Anton syndrome or Balint syndrome or blindsight or achromatopsia or hyperchromatosis or ((facial or face) adj3 intermetamorphosis) or (visual adj3 anoneria)).ti,ab.
-
((figure or shape or orientation or form or colo?r or textur$ or crowding or contour or object or face or faces) adj3 recogn$).ti,ab.
-
(astereognosia or stereognosis or astereognosis or paraesthesia or hypersensitivity or ((tactile or haptic$ or touch) adj3 (stimul$ or memory or acuity or sens$ or percept$ or processing or stimul$ or distinguish$ or discriminat$ or anisotropy or locali?ation))).ti,ab.
-
or/6-22
-
5 and 23
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
clinical trials as topic.sh.
-
random$.ab.
-
trial.ti.
-
or/25-31
-
exp animals/not humans.sh.
-
32 not 33
-
34 and 24
Appendix 4 Key details of included randomised controlled trials
| An 2019 75 | |
| Participants | Sense: Somatosensation (Pusher syndrome) n = 14 |
| Comparison: | Intervention 1 vs. Intervention 2 |
| Interventions |
Intervention 1: Game-based vertical posture training Rehabilitation (restitution) Intervention 2: Standard vertical posture training Rehabilitation (restitution) |
| Outcome measures |
ADLs: Korean Modified Barthel Index Perception: Burke Lateropulsion Scale Motor: Postural Assessment Scale for Stroke, Balance posture ratio Timing: Immediately post intervention |
| An 2020 162 | |
| Participants | Sense: Somatosensation n = 30 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Whole-body tilting postural training (n = 15) Rehabilitation (restitution) Intervention 2: General postural training (n = 15) Rehabilitation (restitution) |
| Outcome measures | ADLs: Korean Modified Barthel Index Adverse events: Number of events Motor (including balance): Fugl-Meyer Motor Assessment-Lower Extremity Berg Balance Scale, Postural Assessment Scale for Stroke Others: Burke Lateropulsion Scale Timing: Immediately after intervention |
| Bergmann 2018 163 | |
| Participants | Sense: Somatosensation n = 38 |
| Comparison | Intervention 1 vs. active treatment 2 |
| Interventions | Intervention 1: Robot-assisted gait training Rehabilitation (restitution and substitution) Intervention 2: Non-robotic physiotherapy Rehabilitation (restitution) |
| Outcomes | Mobility and Navigation: Performance Orientated Mobility Assessment, Functional Ambulation Classification Perception: Subjective Visual Vertical Other: Scale for Contraversive Pushing, Burke Lateropulsion Scale |
| Carey 2011 74 | |
| Participants | Sense: Mixed (tactile and somatosensory) n = 50 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Sensory discrimination training Rehabilitation (restitution and compensation) Intervention 2: Exposure to tactile stimuli Rehabilitation (unclear) |
| Outcomes | Perception: Standardised somatosensory deficit [composite of texture discrimination (FMT), limb position sense (WPST) and tactile object recognition (fTORT)] Adverse Events: Numbers affected Timing: Immediately after intervention (and time points after partial crossover) |
| Chen 2012 154 | |
| Participants | Sense: Vision n = 11 |
| Comparison | Intervention 1 vs. active treatment 2 |
| Interventions | Intervention 1: Image drawing – global processing training Rehabilitation (restitution) Intervention 2: Image drawing – rote repetition training Rehabilitation (restitution) |
| Outcomes | Perception: Rey–Osterrieth Complex Figure, Modified Taylor Complex Figure, Medical College of Georgia Complex Figures 1 and 2 Timing: Immediately post intervention, 2 weeks, 4 weeks |
| Cho 2015 66 | |
| Participants | Sense: Vision n = unclear – 27 ‘eventually completed the intervention and testing’ |
| Comparison | Intervention vs. no intervention |
| Interventions | Intervention: NFB training Rehabilitation (restitution) |
| Outcomes | Perception: MVPT Other: Brain waves – electroencephalography (EEG) Timing: Immediately post intervention |
| Choi 2108 144 | |
| Participants | Sens: vision. Study also addresses postural balance and walking n = 28 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: WVRT Rehabilitation (restitution) Intervention 2: General balance training Classification of intervention: Rehabilitation (restitution) |
| Outcomes | Perception: MVPT Motor: Berg Balance Scale Mobility and Navigation: 10 m Walking Test, Timed up and Go Timing: 1 week after intervention, 8-week follow-up |
| De Bruyn 2018 60 | |
| Participants | Sense(s) addressed: Somatosensory function n = 30 |
| Comparison: | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Sensorimotor group in addition to conventional rehabilitation Rehabilitation (restitution) Intervention 2: Motor group in addition to conventional rehabilitation Rehabilitation (restitution) |
| Outcomes | Perception: Erasmus modified Nottingham sensory assessment, Perceptual Threshold of Touch, Texture Discrimination Test, Wrist Position Sense Test, Functional Tactile Object Recognition Test Adverse events: Number Motor: Action Research Arm Test, Fugl-Meyer Upper Extremity, Stroke Upper Limb Capacity Scale |
| Edmans 2000 60 | |
| Participants | Sense(s) addressed: Vision n = 80 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Transfer of training perceptual treatment Classification of intervention: Rehabilitation (restitution) Intervention 2: Functional perceptual treatment Rehabilitation (compensation) |
| Outcomes | ADLs: Barthel ADLs Index, Edmans ADLs Index Perception: Rivermead Perceptual Assessment Battery Motor: Rivermead Motor Assessment Gross Function Scale Other: Length of stay, OT attendances, OT treatment time Timing: Immediately after treatment |
| Kang 2009 237 | |
| Participants | Sense(s) addressed: Vision n = 16 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Computerised visual perception rehabilitation with motion tracking Rehabilitation (restitution) Intervention 2: Computer-based cognitive rehabilitation program Rehabilitation (restitution) |
| Outcomes | ADLs: Modified Barthel Index Perception: MVPT Cognition: Modified Mental State Examination Other: Interest in intervention questionnaire Timing: Immediately after intervention |
| Kim 2015 201 | |
| Participants | Sense(s) addressed: Tactile n = unclear, but data for 30 participants was analysed |
| Comparison | Intervention 1 vs. Intervention 2 vs. no treatment (across 3 arms) |
| Interventions | Intervention 1: Pressure sense perception training on stable surface Rehabilitation (restitution) Intervention 2: Pressure sense perception training on unstable surface Rehabilitation (restitution) |
| Outcomes | Mobility: 10-m test, timed up and go Perception: Pressure error (dynamometer) Motor: Balancia, Functional Reach test Timing: Immediately after intervention (implied) |
| Koo 2018 187 | |
| Participants | Sense(s) addressed: Somatosensation n = 24 |
| Comparison | Intervention vs. control |
| Interventions | Intervention: Anodal tDCS NIBS Control: Sham stimulation |
| Outcomes | ADLs: Korean version of Modified Barthel Index Mobility and Navigation: Functional Ambulation Category Perception: Erasmus MC modifications to the rNSA, Stereognosis Subscale Adverse events: Number Motor: Manual Function Test, Brunnstrom Classification Sensory: Semmes–Weinstein monofilament examination |
| Lee 2021 235 | |
| Participants | Sense(s) addressed: Somatosensation n = 25 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Robot-assisted therapy Rehabilitation (restitution and substitution) Intervention 2: Conventional therapy Rehabilitation (restitution) |
| Outcomes | ADLs: Modified Barthel Index Perception: rNSA Kinaesthetic subtest Adverse events: Number Motor: Fugl-Meyer Assessment, grip dynamometer, Box and Block Test Sensory: Semmes–Weinstein hand monofilament, Other: Surface electromyography Timing: Immediately after intervention |
| Lincoln 1985 150 | |
| Participants | Sense(s) addressed: Vision n = 33 |
| Comparison | Intervention vs. control |
| Interventions | Intervention: Perceptual Training Rehabilitation (restitution) Control: Conventional therapy |
| Outcomes | ADLs: Rivermead ADLs scale Perception: Rivermead Perceptual Assessment battery Timing: Immediately after intervention |
| Park 2015 152 | |
| Participants | Sense(s) addressed: Vision n = 30 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Computer-based cognitive rehabilitation training (CoTras) Rehabilitation (restitution) Intervention 2: Conventional cognitive rehabilitation Rehabilitation (restitution) |
| Outcomes | Perception: MVPT Cognition: Lowenstein Occupational Therapy Cognitive Assessment Timing: Immediately after intervention |
| Seim 2021 236 | |
| Participants | Sense(s) addressed: Tactile n = 16 |
| Comparison | Intervention vs. control |
| Interventions | Intervention: Vibrotactile stimulation Glove Rehabilitation (restitution) Control: Sham |
| Outcomes | Motor: Voluntary angular range of motion Sensory: Semmes–Weinstein Monofilament Exam Other: Modified Ashworth Scale |
| Yang 2015 180 | |
| Participants | Sense(s) addressed: Somatosensation n = 12 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Computer-generated interactive visual feedback training Rehabilitation (restitution) Intervention 2: Mirror visual feedback training Rehabilitation (restitution) |
| Outcomes | Adverse events: Number Motor: Berg Balance Scale, Fugl-Meyer Assessment Other: Scale for Contraversive Pushing |
| Yun 2018 181 | |
| Participants | Sense(s) addressed: Somatosensation n = 38 |
| Comparison | Intervention 1 vs. Intervention 2 |
| Interventions | Intervention 1: Robot-assisted gait training Rehabilitation (restitution and substitution) Intervention 2: Conventional physical therapy Rehabilitation (restitution) |
| Outcomes | ADLs: Korean version of Modified Barthel Index Motor: Berg Balance Scale, Fugl-Meyer Assessment Adverse Events: Number Other: Burke Lateropulsion Scale, Postural Assessment for Stroke, Somatosensory Evoked Potentials |
Glossary
- a priori
- Reported in advance.
- Adverse event
- Any untoward and unintended response to an intervention.
- Auditory (hearing)
- Processing and understanding auditory (hearing) information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret auditory information.
- Auditory processing disorder
- A hearing problem that affects how the brain interprets sound rather than how sound is carried through the ear to the brain.
- Bias
- An error or deviation in results or inferences from the truth. In health care, the main types of bias arise from systematic differences in groups compared, the care provided, exposure to other factors, withdrawals/exclusions of participants or outcomes assessed. Reviews may show bias by reporting a subset of relevant data.
- Brain stimulation
- Inhibiting or activating the brain through electricity.
- Case-controlled study
- A retrospective study that looks at two groups (one with and one without an outcome) to assess if there is a difference between the groups.
- Case report
- A study that describes and interprets an individual case, often written in the form of a detailed story.
- Case series
- A descriptive study that follows a group of patients who are undergoing the same treatment over a certain period of time.
- Charles Bonnet syndrome
- Hallucinations caused by loss of vision. The hallucinations may be simple patterns, or detailed images of events, people or places.
- Cochrane Review
- A Cochrane Review is a systematic review of research in health care and health policy that is published in the Cochrane Database of Systematic Reviews.
- Cognitive
- Skills such as reasoning, memory or attention.
- Cohort study
- One where a group of participants are all given the same treatment, with measures before and after treatment to explore any changes that have occurred.
- Comparison
- An intervention (i.e. active control) or placebo, used as a reference in a clinical trial.
- Compensatory intervention
- Training the use of an undamaged function, using it to help compensate for the one that has been affected.
- Confidence intervals
- The range of values the true value lies within.
- Dichotomous variables
- One of two possible values.
- Evidence map
- Results in a user-friendly (often visual) format (web).
- Gustatory (taste)
- Processing and understanding gustatory (taste) information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret gustatory information.
- Haemorrhagic (stroke)
- Bleeding into or within the brain.
- Heterogeneity
- Variability among studies.
- Homogeneity
- Similarity between studies.
- Intervention
- The treatment used.
- Ischaemic (stroke)
- When the normal blood supply to part of your brain is cut off.
- Meta-analysis
- Combining data from multiple independent studies. May be undertaken in evidence syntheses.
- Modality (mode)
- How an intervention is provided.
- N-of-1
- (Also called a single-patient trial) is a study design which focuses on each individual participant, rather than grouping the results from all the participants together.
- Nominal group technique
- A structured method that encourages contributions from all group members and facilitates agreement or decision-making.
- Non-invasive brain stimulation
- Use of probes carefully placed on the skull to alter brain activity.
- Olfactory (smell)
- The sense of smell. Processing and understanding olfactory (smell) information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret olfactory information.
- Outcome measure
- A component of a participant’s functional status after an intervention has been applied, that is used to measure the effectiveness of an intervention.
- Perception
- Perception is the ability of the brain to interpret and integrate information detected by the different sensory systems; also Specific mental functions of recognising and interpreting sensory stimuli; also Processing and understanding information from the senses.
- Peto odds ratio
- A method for pooling odds ratios.
- Pharmacological interventions
- Drug treatment.
- Placebo
- An inactive intervention to compare its effects with those of an active intervention. Placebos are used in clinical trials to blind people to their treatment allocation. Placebos should be indistinguishable from the active intervention to ensure adequate blinding.
- Proprioceptive deficits
- A problem with perception or awareness of the position and movement of the body.
- Pusher syndrome
- Is characterised by leaning and active pushing towards the paralysed side, affecting their posture and possibly leading to instability and loss of balance.
- Randomised controlled trial
- A study design that randomly assigns participants into an experimental group, which receives the treatment, or a control group, which does not. It is considered to provide the most reliable evidence on the effectiveness of interventions.
- Restitution intervention
- Direct training of impaired function, to try and recover this.
- Scoping review
- Exploratory projects that systematically map the literature available on a topic, identifying key concepts, theories, sources of evidence and gaps in the research.
- Sensitivity analysis
- A repeat of the primary analysis or meta-analysis, substituting alternative decisions or ranges of values for decisions that were arbitrary or unclear.
- Somatosensation
- Processing and understanding somatosensory information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret somatosensory information (includes proprioception).
- Somatosensory deficit
- A difficulty with being able to process or understand mental functions of pressure, temperature or body position.
- Substitution intervention
- Using external devices or adaptation of environment to help the person cope better.
- Systematic review
- A review of a clearly formulated question that uses explicit methods to identify, select and critically appraise relevant research, and to collect and analyse data from the studies. Statistical methods (meta-analysis) may or may not be used to analyse and summarise the results of the included studies.
- Tactile (touch)
- Tactile is described as understanding information from the skin. Processing and understanding tactile information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret tactile information.
- Taxonomy
- System used to name and organise concepts.
- Vision
- Processing and understanding visual (vision) information. This may include the mental functions of being able to distinguish, discriminate, recognise and interpret visual information.
- Visual agnosia
- A condition in which a person can see but cannot recognise or interpret visual information, for example an inability to name or describe the use of an object placed in front of you when just looking at it.
- Visual hallucinations
- Hallucinations involving visual stimuli.
- Visual perceptual deficits
- Having difficulties with the processing or understanding the mental functions of being able to distinguish, discriminate, recognise and interpret visual information.
- Visual–spatial deficits
- Having difficulties with processing or understanding where items are in space.
List of abbreviations
- ACTIVE framework
- Authors and Consumers Together Impacting on eVidencE framework
- ADLs
- activities of daily living
- EADLs
- extended activities of daily living
- ENT
- ear, nose and throat
- GRADE
- grading quality of evidence and strength of recommendations
- HCP
- healthcare professional
- ICF
- International Classification of Functioning, Disability and Health
- MD
- mean difference
- MVPT
- Motor-Free Visual Perception Test
- NFB
- neurofeedback
- NIBS
- non-invasive brain stimulation
- NIHR
- National Institute for Health and Care Research
- NMAHP
- Nursing, Midwifery and Allied Health Professionals
- OT
- occupational therapist
- PIONEER
- perceptual disorders after stroke Intervention Evidence Review
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- QoL
- quality of life
- RCP
- Royal College of Physicians
- RCT
- randomised controlled trial
- rNSA
- revised Nottingham Sensory Assessment
- rTMS
- repetitive transcranial magnetic stimulation
- tDCS
- transcranial direct current stimulation
- TIDieR
- Template for Intervention Description and Replication
- WHO
- World Health Organization
- WVRT
- WiiFit virtual reality training
Notes
-
Stakeholder Activity 2a: Outcome measure identification and prioritisation – explore impact of perceptual problems on life after stroke
-
Stakeholder Activity 2b: Outcome measure identification and prioritisation – ranking the priority of different outcome domains
-
Stakeholder Activity 3: Organisation and interpretation of scoping review results
-
Stakeholder Activity 4: Organisation and interpretation of Cochrane review results
-
Risk of bias summary: Judgements on each methodological quality item for each included study
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/WGJT3471).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.