Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1272. The contractual start date was in August 2007. The final report began editorial review in January 2015 and was accepted for publication in November 2015. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Melanie Davies has acted as consultant, advisory board member and speaker for Novo Nordisk, Sanofi-Aventis, Eli Lilly and Company, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca and Janssen, and as a speaker for Mitsubishi Tanabe Pharma Corporation. She has received grants in support of investigator and investigator-initiated trials from Novo Nordisk, Sanofi-Aventis and Eli Lilly and Company. She has received grants and support from the National Institute for Health Research (NIHR) during the conduct of this study. Alastair Gray reports grants from NIHR during the conduct of the study. Kamlesh Khunti reports that he has acted as a consultant and speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Eli Lilly and Company and Merck Sharp & Dohme. He has received grants in support of investigator and investigator-initiated trials from Novartis, Novo Nordisk, Sanofi-Aventis, Eli Lilly and Company, Pfizer, Boehringer Ingelheim and Merck Sharp & Dohme. Kamlesh Khunti has received funds for research, honoraria for speaking at meetings and has served on advisory boards for Eli Lilly and Company, Sanofi-Aventis, Merck Sharp & Dohme and Novo Nordisk.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Davies et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Type 2 diabetes mellitus (T2DM) is a chronic and debilitating disease characterised by elevated blood glucose through insulin resistance, relative impairment of insulin secretion and increased hepatic glucose output. In the short term, the symptoms of T2DM are associated with a reduced quality of life, whereas in the longer term the disease may lead to serious complications such as cardiovascular disease (CVD), blindness, renal failure and amputation. 1 The life expectancy of individuals with T2DM may be shortened by as much as 10 years, with up to 75% of individuals dying of CVD. 2,3
The prevalence of T2DM has risen so steeply over the past few decades that it is now commonly referred to as an epidemic, and elevated blood glucose levels are currently estimated to be the third leading modifiable cause of mortality globally. 4 Currently, diabetes mellitus accounts for 5–13% of total health-care spending across low- to high-income regions of the globe;5 in the UK, diabetes mellitus currently accounts for approximately 10% of the total health-resource expenditure, which is projected to increase to around 17% in 2035/36. 6 The vast majority of the burden of diabetes mellitus is attributable to the T2DM form of the disease. 6
This devastating health-care burden has necessitated a shift in focus from traditional health-care models focused on treatment to those that incorporate pathways and systems for prevention. International and national health-care organisations now recognise the importance of developing targeted approaches to prevention through research, health-care recommendations and policy. In the UK, the NHS Health Check programme (formally vascular checks) was formed to address this need and is aimed at screening all individuals aged between 40 and 75 years for vascular and metabolic disease risk and treating high-risk individuals accordingly. 7 However, changes to policy have tended to precede programmes of research focused on developing and evaluating prevention pathways in the real world; therefore, there has been a lack of evidence-based tools and programmes that are suitable for implementation into routine primary care and that are available to commissioners. Our programme of work was designed to address this limitation and to develop robust evidence-based tools and systems for identifying and intervening in those with a high risk of T2DM.
Here, we highlight the background to our work with a specific focus on approaches used for identifying those with a high risk of T2DM and considerations of how to prevent T2DM with lifestyle intervention.
Identification
Type 2 diabetes mellitus is at one end of a continuous glucose control spectrum, with normal glucose control at the other. In between these two extremes there is a clinically important and much-researched state in which glucose levels are elevated but not over the threshold for diagnosis of T2DM. This state of glucose control has historically been termed prediabetes mellitus (PDM), impaired glucose regulation (IGR) or intermediate hyperglycaemia. Individuals with these elevated glucose levels are significantly more likely to develop T2DM than those with normal blood glucose.
The tests that can be used to identify those at high risk of T2DM include the oral glucose tolerance test (OGTT) and, more recently, glycated haemoglobin (HbA1c). There are usually two blood measurements taken for an OGTT: fasting plasma glucose (which is the glucose measured after 12 hours of fasting before glucose is taken) and the 2-hour post-challenge plasma glucose (which is measured 2 hours after the glucose is taken). The World Health Organization (WHO) defines impaired glucose tolerance (IGT) as a 2-hour post-glucose reading of > 7.8 mmol/l but < 11.1 mmol/l, whereas impaired fasting glucose (IFG) is defined as fasting plasma glucose concentration of > 6.1 mmol/l but < 7 mmol/l. 8 IFG and IGT can occur as isolated, mutually exclusive conditions or together. Estimates of progression to T2DM within 1 year suggest that those with isolated IGT have over five times the risk, those with isolated IFG have seven times the risk and those with both IGT and IFG have over 12 times the risk than normoglycemic individuals. 9 Both the terms PDM and IGR are commonly used to describe the presence of IFG and/or IGT as defined by the WHO.
In 2011, after the start of this work, WHO revised the criteria for the diagnosis of T2DM to include the use of HbA1c. 10 This precipitated a shift in clinical practice, with the use of OGTT in the diagnosis of T2DM being gradually phased out and HbA1c becoming the dominant method of classification. As it is potentially burdensome and confusing to define categories on a continuous glucose spectrum with different measures, this change necessitated a discussion around the definition of an HbA1c-defined PDM category analogous to that of IGT or IFG. Although the WHO found insufficient evidence for the use of HbA1c in the definition of PDM, statements from the American Diabetes Association (ADA) and an international expert committee recommended that HbA1c be used to signify a high-risk state at levels of between 5.7% and 6.4%, and 6.0% and 6.4%, respectively. 11,12 The National Institute for Health and Care Excellence (NICE) have since adopted the recommendation of 6.0% to 6.4% as an alternative to fasting or 2-hour glucose in the identification of PDM. 13 Follow-up studies have shown similar rates of progression to diabetes mellitus from the HbA1c-defined prediabetic state as seen for IFG. 14
In most countries, around 15% of adults have PDM based on WHO criteria;15 this figure rises in some minority populations and with age. For example, in elderly populations up to 50% of individuals are estimated to have PDM. 16 Of those with PDM, an estimated 4–12% develop T2DM per year, with the highest rates seen among those with both IGT and IFG. 15,17 Evidence from prospective studies suggests that approximately 25–40% of individuals with PDM go on to develop diabetes mellitus over a 3- to 8-year period, and as many as 70% will eventually develop T2DM over the course of their lifetime. 17
Along with an increased risk of T2DM, the risk of CVD and premature mortality is also elevated in individuals with PDM. 15,18 For example, those with PDM have been shown to be 50% more likely to die of CVD than people with normal blood glucose control. 19 Interestingly, as the prevalence of PDM is four times greater than T2DM, there are likely to be more premature deaths attributable to PDM than to diabetes mellitus. 19,20
Research published by Diabetes UK proposed that, although the terms IGR, IFG and IGT may be useful when talking to health-care professionals, the term ‘prediabetes’ was found by focus groups to be preferable when talking to the public. It was noted that people identified with this term and felt that it adequately portrayed the seriousness of the condition and its future risk. 21,22 Recent recommendations suggest that individuals with either IGT, IFG or elevated HbA1c be referred to as ‘persons at high risk of T2DM’; however, at the onset of this study the term PDM was commonly used and thus has been adopted throughout this report. The term PDM is used to refer interchangeably to IGT, IFG and/or elevated HbA1c, according to any recommended definition. 12,13,23 Throughout this report we shall use the term PDM to include IGT-, IFG- or HbA1c-identified high risk of T2DM.
Regardless of the invasive biochemical test used to define risk status, universal screening for T2DM risk status is problematic for several important reasons. First, screening tests are relatively expensive and there is limited appetite in an era of restricted health-care budgets for screening apparently healthy individuals for disease risk. This is consistent with a review of the evidence commissioned by the Health Technology Assessment programme, which concluded that screening for T2DM meets most of the National Screening Committees’ key criteria, although it fails on several, including a lack of adequate staffing and facilities. 24 In addition to cost and resource, there is high variation in the risk of developing both T2DM and CVD across categories of PDM, regardless of the assessment method used. For instance, data from the Finnish Diabetes Prevention Study (DPS) showed that the risk of T2DM in those with IGT more than doubled with the presence of other readily identifiable risk factors. 25 It is also known that the risk of CVD increases linearly with increasing levels of dysglycaemia, and there is no distinct threshold that justifies the use of distinct risk categories. 26 Given these factors, there has been much international focus on developing pragmatic systematic approaches for identifying and stratifying individuals with an elevated risk of T2DM for referral into diabetes-prevention initiatives. These have primarily focused around the use of risk-score technology.
Risk scores
Risk scores use non-invasive determinants of T2DM to estimate or rank risk. Work conducted in Finland in the 1990s led to the development of the seminal Finnish Diabetes Risk Score (FINDRISC), which uses weighted scores from eight risk characteristics [age, body mass index (BMI), waist circumference, physical activity levels, fruit/vegetable intake, antihypertensive medication, previous history of high glucose and family history of T2DM] to calculate an overall risk profile for developing T2DM. 27 FINDRISC has been shown to have good sensitivity (≈0.8) and specificity (≈0.8) at predicting the 10-year absolute risk of T2DM in a white European population. 27 FINDRISC is now commonly used internationally within research and clinical care contexts. However, there is a recognised need to tailor and validate risk score technology according to local circumstances and population characteristics. 28 For example, in a society in which blood glucose levels are not routinely measured, asking participants about previous blood glucose levels is redundant. Furthermore, the ethnic makeup and distribution of risk factors, such as BMI, differ markedly between populations, which affects the weighting that each factor receives to maximise risk score accuracy. Furthermore, there is a need to distinguish between self-assessment risk scores, such as FINDRISC, and automated practice-based risk scores which are designed to run on routine care databases to enable health-care professionals to easily and quickly identify diabetes mellitus risk within their registered population. This grant directly funded the development and validation of a practice-based risk score designed to rank individuals for the risk of undiagnosed T2DM and PDM based on factors routinely coded within primary care (see Chapter 3). This work was further developed into a freely available piece of software available to all general practices nationally (see Chapter 9). In addition, we secured additional funding from Diabetes UK to develop and validate a self-assessment risk score specific to the UK (see Chapter 9).
Two-stepped approach
Diabetes mellitus risk scores, predominantly those based on FINDRISC, are now routinely used within many health-care contexts internationally. However, an emerging consensus has moved towards a two-stage approach whereby risk scores are used to identify moderate- to high-risk individuals and blood tests are then employed to confirm risk status and check for undiagnosed T2DM. 29 We have also shown that this approach is the most cost-effective method of identifying those at high risk of T2DM. 30 Following a comprehensive review and analysis of the clinical effectiveness and cost-effectiveness of different screening strategies to identify T2DM risk status, NICE supported the use of a stepped algorithm involving risk-score technology to identify individuals ranking above the 50th percentile of risk followed by a fasting or HbA1c blood test to confirm their risk status. 13 Although NICE guidance on the prevention of T2DM in high-risk populations was published after this programme grant was awarded, we nevertheless employed a two-stepped approach using the practice-based risk score developed (see Chapter 3) followed by an OGTT; those confirmed to have PDM were included in the randomised controlled trial (RCT) (see Chapter 5).
Prevention programmes
Clinical trials have unequivocally demonstrated that lifestyle interventions reduce the risk of progressing to T2DM by 40–60% in those with PDM, specifically IGT. 31 For example, the Finnish DPS found that the risk of T2DM was reduced by 58% in those with an intensive lifestyle intervention compared with usual care over a 3-year period. 32 Identical findings were reported for Diabetes Prevention Program (DPP) conducted in the USA. 33 Similar and consistent results have been observed in many different and diverse countries including India,34 Japan35 and China. 36 Lifestyle interventions aimed at the prevention of T2DM have been based on promoting moderate- to vigorous-intensity physical activity, generally 150 minutes per week, and a healthy diet aimed at weight maintenance for normal-weight individuals or weight loss for overweight or obese individuals. For example, DPS had five intervention goals: (1) a reduction in body weight of ≥ 5%; (2) < 30% of energy intake derived from fat; (3) < 10% of energy intake derived from saturated fat; (4) at least 15 g of fibre per 1000 kcal; and (5) at least 30 minutes of moderate-intensity physical activity per day. 32 Interestingly, there was not a single case of T2DM over the 3-year study period in those who achieved four of these goals. 32
Successful lifestyle-change programmes have also been shown to have so-called legacy effects whereby the effect persists well after the active intervention has ceased. DPS, DPP and the Chinese Da Qing diabetes mellitus prevention study all found sustained reductions in the incidence of T2DM relative to the control group after 7–20 years of follow-up. 37–39 These findings suggest that once individuals are enabled to successfully change and self-regulate their lifestyle behaviours, benefits can be sustained long after active lifestyle interventions have ceased.
Economic modelling studies have consistently demonstrated that lifestyle-based interventions are likely to be cost-effective and may even be cost saving in some populations. 40 When considering the whole process from screening to treatment, Gillies et al. 41 estimated that screening for T2DM and PDM followed by tailored treatment to each group was more cost-effective than screening for T2DM alone in the UK, with lifestyle interventions being more cost-effective than pharmaceutical therapy for prevention [£6242 vs. £7023 per quality-adjusted life-year (QALY) gained].
The consistent clinical effectiveness and cost-effectiveness of lifestyle interventions aimed at decreasing the risk of T2DM is unsurprising given that unhealthy lifestyle practices associated with modern ‘obesogenic’ environments are the primary causal factor for T2DM. The prevalence of T2DM has been estimated to have increased by a factor of six over the past couple of centuries, ruling out genetic change as a direct causal factor. 42 Although there are some genetic factors that increase the risk of T2DM, they can be expressed only in combination with unhealthy modern environments. Given the centrality of lifestyle factors in the pathophysiology of T2DM, and considering the strong evidence of efficacy for lifestyle intervention, the promotion of lifestyle change is central to the prevention of T2DM. This has been recognised by NICE, which recommends that those identified as being at high risk of T2DM should be referred to a lifestyle intervention before pharmaceutical agents, such as metformin (e.g. Glucophage, Merck), are considered. 13
Translating lifestyle research into practice
Despite strong evidence for the clinical effectiveness and cost-effectiveness of lifestyle intervention in the prevention of T2DM, there has been a large translational gap between clinical trial evidence and implementation into routine clinical care. This is predominantly attributable to the resource-intensive nature of the lifestyle interventions tested within clinical trials. For example, the lifestyle intervention within the DPP involved 16 lengthy one-to-one counselling sessions, followed by in-person one-to-one contact at least once every 2 months and additional group-based sessions four times annually. 33 If this level of intervention was directly translated into routine care within the UK to those with PDM, it would require an estimated additional 150 million consultations per year, clearly a level that would be unachievable in even the most highly funded and resourced health-care system. Therefore, the emphasis needs to be shifted from maximising behaviour change and resources within the context of a clinical trial to examining the minimum level of intensity and resource allocation needed to produce meaningful clinical effects in routine care. In addition, there are important considerations relating to how new interventions become embedded within routine care and gain universal access. Professor Ann Albright, Director of the Division of Diabetes Translation within the Centers for Disease Control and Prevention, identified six distinct steps from basic science to national distribution, termed the continuum of translation, which are needed achieve the universal implementation of diabetes mellitus prevention. 43 More recently, Schwarz et al. 29 identified six key areas of focus when implementing diabetes mellitus prevention programmes: (1) intervention cost; (2) training and expertise of intervention providers; (3) uptake to both screening and intervention; (4) ensuring the sustainability of funding and support within health-care and political arenas; (5) developing quality management across intervention providers; and (6) using and improving technology to support the behaviour change of both patients and health-care professionals.
Finland and the USA have been at the forefront of integrating diabetes mellitus prevention programmes into health-care and community settings. In Finland, tailored lifestyle interventions offered to those classified as being at a high risk of T2DM were based on the goals of DPS but delivered in a less-intensive format of four to eight group-based education sessions. 44 In the USA, lifestyle intervention has focused on a community-based programme run through Young Men’s Christian Association (YMCA) facilities, which consists of 16 1-hour group-based sessions delivered by trained quality-assured lifestyle coaches. 45 Numerous smaller-scale diabetes mellitus prevention programmes, largely based on group education and intervention approaches, have also been developed and evaluated in the USA. 46 Internationally, European-wide diabetes mellitus prevention guidance and tools for health-care professionals have also been developed and published. 47 As discussed later (see Chapter 2), those diabetes mellitus prevention programmes that have been tailored to, and implemented in, routine care or community settings internationally have continued to demonstrate meaningful changes to some markers of health status, such as BMI.
In the UK, group-based approaches to promoting self-management and behaviour change, in the form of structured education, are already an integral and established part of many disease-management pathways, particularly T2DM. For example, the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) structured education programme developed by our group has been shown through a multicentre trial to improve CVD risk profiles, reduce depression, enhance smoking cessation and promote health behaviour change, including weight loss, in those with T2DM, while being highly cost-effective, with a cost per QALY gained of £2092. 48,49 DESMOND is now the most widely implemented self-management programme for T2DM and is currently part of routine diabetes mellitus pathways within half of all clinical commissioning groups (CCGs) nationally (www.desmond-project.org.uk/). Given that the infrastructure for delivering structured education as part of routine diabetes mellitus pathways within primary care is already established in the UK, there was a recognised opportunity to harness this approach for prevention. This is important because it does not require primary care organisation to develop and implement new programmes or systems of care; rather, existing pathways and programmes could be rolled backwards to incorporate the prevention as well as the management of T2DM. Early work undertaken by our group demonstrated the potential efficacy of structured education combined with pedometer use at promoting physical activity in those with PDM50,51 (see Chapter 9), and NICE subsequently went on to provide detailed recommendations for the content and format of lifestyle interventions aimed at the prevention of T2DM that were consistent with group-based structured education. 13
One of the central aims of our programme was to incorporate the lifestyle goals from clinical trials, including diet, weight management and physical activity, into a structured education programme that was suitable for implementation within routine care and, once developed, to evaluate its clinical effectiveness and cost-effectiveness.
Summary
In conclusion, the prevention of T2DM is a recognised national and international health-care priority. However, in order to make prevention a reality rather than an aspiration within primary care, there is an urgent need to develop methods and pathways that can facilitate the identification of those with PDM and provide methods of delivering lifestyle intervention that are both evidence-based and suitable for mass implementation within the existing health-care infrastructure. Our programme grant was aimed at addressing this unmet need within the context of primary care in the UK.
The main objectives of the programme grant were:
-
to develop and validate a risk score to identify those who require diagnostic testing to identify undiagnosed T2DM and those at high risk of future T2DM and CVD in a multiethnic population
-
to use this risk score to identify and engage those at highest risk of T2DM and offer them a lifestyle self-management programme with the aim of reducing the risk of progression to T2DM and reducing cardiovascular risk
-
to pilot and test a lifestyle self-management programme based on group care, targeting the five key areas, using information currently collated from the European Union-funded Diabetes in Europe Prevention using Lifestyle, physical Activity and Nutritional intervention (DEPLAN) project
-
to develop a training and quality-assurance programme for community-based health trainers, who may include health-care professionals, to deliver the initial programme and provide ongoing support to those at highest risk of T2DM
-
to evaluate the lifestyle self-management programme and its cost-effectiveness
-
to explore how a two-stage screening programme and prevention intervention can be implemented in primary care.
Chapter 2 Systematic review
This chapter presents a systematic review and meta-analysis of the current evidence on the effectiveness of pragmatic lifestyle interventions for the prevention of T2DM. The content is based on a previously published review conducted by our research group52 which identified studies up to July 2012 [reproduced under the terms of the Creative Commons Attribution-NonCommerical-NoDerivative Works 3.0 Unported License (CC BY-NC-ND) and courtesy of the ADA]. For this report, the review has been updated and includes published evidence up to August 2014.
Introduction
A major opportunity exists to drastically reduce the incidence of T2DM, a disease that has a huge impact on patients and health-care systems worldwide. Large, high-quality clinical trials31,33,39 show that relatively modest changes in diet and physical activity reduce the incidence of T2DM by > 50% for people with PDM. Indeed, within-trial data show that the rate of progression to T2DM after 7 years of follow-up was reduced to almost zero for people who had succeeded in making five modest lifestyle changes. 39 The main drivers of diabetes mellitus prevention appear to be weight loss and physical activity. 53,54 However, a substantial challenge remains in translating these findings into routine clinical practice. The intensive and prohibitively expensive interventions used in clinical trials to ensure lifestyle change need to be translated into practical, affordable interventions that are deliverable in real-world health-care systems and that, nevertheless, retain a reasonable degree of effectiveness. 29
Since the publication of the original diabetes mellitus prevention clinical trials between 1996 and 2001, a number of translational or ‘real-world’ diabetes mellitus prevention programmes55,56 have aimed to translate the evidence. 32–34,36 A meta-analysis of the evidence on translational interventions was published in 2010,55 although this review excluded 15 studies that were conducted in non-health-care settings. A more recent meta-analysis was published in 2012. 46 However, the authors focused only on translation of evidence from the US DPP and also included studies where up to half of the population already had diabetes mellitus. Other systematic reviews of diabetes mellitus prevention interventions have either not included a meta-analysis54,56–60 or not focused on translational studies. 31,54,57,58,61–65 Overall, the systematic reviews conducted to date indicate that real-world diabetes mellitus prevention programmes vary widely in their effectiveness, although most produce lower levels of weight loss than the more intensive interventions used in the clinical efficacy trials. 55
To consolidate the evidence we undertook a systematic review of studies, considering the effectiveness of translational interventions for prevention of T2DM in high-risk populations. The primary aim was to conduct a meta-analysis of the effectiveness of pragmatic interventions on weight loss. If sufficient data were available, a secondary aim was to consider other diabetes mellitus risk factors using similar methods.
Methods
Search strategy and study selection
We included experimental and observational studies that considered the effectiveness of a lifestyle intervention (diet and/or exercise) alone or compared with control, for which the stated aim of the intervention was diabetes mellitus risk reduction or prevention of T2DM and the focus of the study was to translate evidence from previous diabetes mellitus efficacy trials into routine health care or a community setting. For studies to be eligible for inclusion, we required them to include adults (aged ≥ 18 years) identified as being at high risk of developing T2DM (e.g. obese, sedentary lifestyle, family history of diabetes, older age, metabolic syndrome, PDM or elevated diabetes mellitus risk score);13 have a minimum follow-up of 52 weeks; and have an outcome relating to diabetes mellitus risk, as measured by a change in body composition or a change in glycaemic control, or report progression to diabetes mellitus (incidence or prevalence). The focus of the review was primary prevention; therefore, we excluded trials where > 10% of the population had established diabetes. We included only studies published in the English language and as full-length articles.
We searched EMBASE, MEDLINE and The Cochrane Library (issue 10, 2014) using a combination of Medical Subject Headings terms and keywords which were tailored to individual bibliographic databases. We restricted searches to articles published after January 1998; the starting point of 1998 was chosen to facilitate the identification of studies that were informed by or translating evidence from previous diabetes mellitus prevention efficacy trials. 32–34,36 In order to avoid missing papers the final search strategy included only terms related to the intervention and the study design. An example search strategy (MEDLINE) is outlined in Appendix 1. We combined the results of an initial search and an updated supplementary search that together identified papers up to the end of August 2014.
Two reviewers independently assessed abstracts and titles for eligibility and retrieved potentially relevant articles, with differences resolved by a third reviewer where necessary. Where studies appeared to meet all the inclusion criteria, but data were incomplete, we contacted authors for additional data and/or clarification. In an attempt to identify further papers not identified through electronic searching, we examined the reference lists of included papers and relevant reviews.
Data extraction and quality assessment
Data were extracted by one reviewer, and a second reviewer subsequently checked for consistency. We extracted data on sample size, population demographics, intervention details and length of follow-up. Where available, we recorded outcome data for the mean change from baseline to 12 months’ follow-up for the following outcomes: weight, BMI, waist circumference, fasting glucose, 2-hour glucose, HbA1c, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, systolic blood pressure (BP) and diastolic BP. Incidence of T2DM was also recorded. We retrieved all papers relating to a particular study, including those on design and methodology (if reported separately), and any supplementary online material.
We assessed the quality of selected studies according to the UK’s NICE quality appraisal checklist for quantitative intervention studies. 66 The checklist includes criteria for assessing the internal and external validity of experimental and observational quantitative studies (RCTs, non-RCTs, single-arm before-and-after studies) and allows assignment of an overall quality grade (categories ++, + or –).
Coding of intervention content
We coded intervention content (see Appendices 2 and 3) in relation to the recommendations for lifestyle interventions for the prevention of diabetes mellitus provided by both the Development and Implementation of a European Guideline and Training Standards for Diabetes prevention (IMAGE) project47 and NICE. 13 Where a study intervention was inadequately described we requested further details from the authors. If available information was insufficient to allow coding we coded data as missing; where an intervention appeared to be well described but a particular component (e.g. engaging social support) was not mentioned or could not be inferred from other text, we assumed that the component was not used.
Data synthesis and analysis
We converted all values reported in imperial units into metric units. Capillary blood glucose values were converted to plasma equivalent values. 67 If studies did not directly report the mean and standard deviation (SD) for change from baseline to 12 months for the outcomes of interest, they were calculated. We calculated the mean change by subtracting the baseline mean value from the mean at 12 months. We calculated the SD from reported p-values or confidence intervals (CIs), as recommended by Cochrane. 68 In instances in which data were reported by subgroup, combined effect sizes and SDs were estimated using the formula advocated by Cochrane. 68 Where data were insufficient to allow calculation of the SD, we imputed values for each outcome based on the correlation estimates from those studies that reported them; for weight the correlation used in these imputations was 0.95. 69–73
Weight change was chosen as the primary outcome owing to the high number of studies reporting this outcome above others, such as those relating to glycaemic control or progression to T2DM. This is most likely to be attributable to the nature of the translational interventions, which are predominantly based on large-scale intensive diabetes mellitus prevention programmes that are founded on core goals which specifically target weight loss. In addition, studies were predominantly of ≤ 12 months’ duration, which is arguably too short a period to fully assess the effect of an intervention on progression to T2DM. For the primary outcome of interest (weight), we conducted a meta-analysis to examine the pooled effect size (change from baseline to 12 months) where data were available. Owing to the uncontrolled nature of translational interventions, the majority of included studies were single-arm before-and-after studies. In order to prevent exclusion of substantial evidence, only intervention arms were included in the meta-analysis to maximise the data available for analysis. We conducted similar analyses for the secondary outcomes of interest. We performed sensitivity analyses for the primary outcome, weight, where we restricted the analysis to RCTs only. Additional sensitivity analyses comparing intervention and control arms in RCTs only were performed for the primary outcome.
We assessed publication bias using Egger’s test and heterogeneity using the I2 statistic. Owing to high levels of heterogeneity we used random-effects models throughout to calculate effect sizes. We performed all analyses in Stata version 12.1 (StatCorp, College Station, TX, USA).
Results
Identification of studies
Results relating to the identification and selection of eligible trials are summarised in Figure 1. Searches yielded 8492 citations, and 5196 unique titles and/or abstracts were screened for eligibility. Following full-text retrieval of 167 potentially relevant papers, 20 additional papers were identified from reference lists, making a total of 187. Authors for 14 studies were then contacted in order to clarify eligibility criteria and/or for additional outcome data. Replies were received for 13 studies, 10 of which were subsequently included in the 29 studies50,62,69–95 (42 papers50,51,62,69–107) that met the review criteria.
FIGURE 1.
Flow chart of selection of studies from search to final inclusion. DM, diabetes mellitus; SR, systematic review.
Summary of included studies
The 29 studies included in the systematic review are summarised in Table 1. Study interventions included either a dietary intervention or a physical activity intervention, or both. Standard/brief advice on diet and/or exercise was considered to be comparable with usual care and not judged to be an active intervention. One study focused solely on the effectiveness of a physical activity intervention,50 three combined dietary intervention and a supervised exercise programme,82,92,95 and 25 studies considered the effectiveness of a combined dietary and physical activity intervention. Fourteen of the studies were RCTs, 12 were single-arm before-and-after studies and the remaining studies included a matched cohort, a prospective cohort and a non-RCT. All papers were published within the past 11 years.
| First author and year | Study design | Study name | Definition of high risk of T2DM | Focus of intervention(s) | Number recruited overall (and by group) | Number of study groups | Follow-up (months) | Setting | Country | Ethnicity (%) | Age, years (mean) | Male (%) | BMI, kg/m2 (mean) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absetz et al., 200774 (and 200996) | Single-arm before and after | GOAL | Aged 50–65 years; any risk factor from obesity, ↑ BP, ↑ plasma glucose, ↑ lipids; FINDRISC score of ≥ 12 | Lifestyle (diet and exercise) | 352 | 1 | 12 and 36 | Primary care | Finland | NR | 58 (F); 59 (M) | 25 | 33 (F); 32 (M) |
| Ackermann et al., 200875 (and 201197) | RCT | DEPLOY | BMI ≥ 24 kg/m2; ADA diabetes mellitus risk score of ≥ 10; CBG random (110–199 mg/dl) or fasting (100–199 mg/dl) | Lifestyle (diet and exercise) | 92 | 2 | 12 | Community (YMCA) | USA | 82% white; 3% Hispanic; 12% African American; 5% other | 58 | 45 | 31 |
| Almeida et al., 201076 | Matched cohort | KPCO | Existing IFG (110–125 mg/dl) identified from medical records | Lifestyle (diet and exercise) | 1640 (1520 data available) | 2 | 12 | Integrated health-care organisation | USA | NR | 55 | 47 | 30 |
| Boltri et al., 200877 | Single-arm before and after | DPP in faith-based setting | ADA diabetes mellitus risk score of ≥ 10; CBG fasting (100–125 mg/dl) | Lifestyle (diet and supervised exercise) | 8 | 1 | 12 | Community (church) | USA | African American community | 52a | 42a | 32 |
| Costa et al., 201278 | Prospective cohort | DE-PLAN Spain | FINDRISC score of ≥ 14 or 2-hour OGTT (≥ 7.8 mmol/l and < 11.1 mmol/l) | Lifestyle (diet and exercise) | 552 (219 + 333) | 2 | Median 4.2 years | Primary care | Spain | White European | 62 | 32 | 31 |
| Davis-Smith et al., 200779 | Single-arm before and after | NR | ADA diabetes mellitus risk score of ≥ 10; CBG fasting (100–125mg/dl) | Lifestyle (diet and exercise) | 11 | 1 | 12 | Community (church) | USA | African American community | NR | 27 | 36b |
| Faridi et al., 201062 | Non-RCT | PREDICT | One or more risk factor from BMI of ≥ 25 kg/m2, FH diabetes, gestational diabetes | Lifestyle (diet and exercise) | 146 | 2 | 12 | Community (church) | USA | African American 100% | NR | 32 | 33 |
| Gilis-Januszewska et al., 201169 | Single-arm before and after | DE-PLAN Poland | FINDRISC score of ≥ 14 | Lifestyle (diet and exercise, optional supervised sessions) | 175 | 1 | 12 | Primary care | Poland | NR | NR | 22 | 32 |
| Janus et al., 201293 | RCT | pMDPS | Aged 50–75 years; AUSDRISK score of ≥ 15 | Lifestyle (diet and exercise) | 92 (49 + 43) | 2 | 12 | Community/primary care | Australia | 100% non-Aboriginal/Torres Strait Islander | ≈ 65 | 34 | ≈ 31 |
| Kanaya et al., 201294 | RCT | Live Well, Be Well | Moderate/high diabetes mellitus risk score and CBG fasting (106–160 mg/dl) | Lifestyle (diet and exercise) | 238 (119 + 119) | 2 | 12 | Community | USA | 20% African American; 20% non-Hispanic white; 32% Latino; 14% Asian; 14% other | ≈ 56 | 36 | ≈ 30 |
| Katula et al., 201180 (and 2013)107 | RCT | HELP-PD | BMI of ≥ 25 kg/m2 < 40 kg/m2 and CBG random; FPG (95–125 mg/dl) | Lifestyle (diet and exercise) | 301 (151 + 150) | 2 | 12, 18 and 24 | Community various venues | USA | 74% white; 25% African American; 1% other | 58 | 43 | 33 |
| Kramer et al., 200970 | Single-arm before and after | GLB 2005–8 | BMI of ≥ 25 kg/m2 and metabolic syndrome or CBG fasting (100–125 mg/dl) | Lifestyle (diet and exercise) | 42 | 1 | 12 | Primary care and university-based support centre | USA | White 100% | 57 | 21 | 35 |
| Kramer et al., 201281 | Single-arm before and after | GLB 2009 | Fasting glucose of 100–125mg/dl | Lifestyle (diet and exercise) | 60 (31 + 29) | 2 | 12 | Community (YMCA) and university | USA | 90% Caucasian | 55 | 35 | ≈ 36 |
| Kulzer et al., 200971 | RCT | PREDIAS | FINDRISC score of ≥ 10 or assessed as ↑ risk of diabetes mellitus by primary care physician | Lifestyle (diet and exercise) | 182 (91 + 91) | 2 | 12 | Outpatient setting | Germany | NR | 56 | 57 | 32 |
| Laatikainen et al., 200772 (and 201298) | Single-arm before and after | GGT study | FINDRISC score of ≥ 12 | Lifestyle (diet and exercise) | 311 | 1 | 12 | Primary care | Australia | NR | 57 | 28 | 34 |
| Ma et al., 201392 (Ma et al., 2009106 and Xiao et al., 2013105) | RCT | E-LITE | BMI of ≥ 25 kg/m2 and fasting plasma glucose of 100–125 mg/dl or metabolic syndrome | Lifestyle (diet and exercise; supervised exercise for one group)b | 241 (79 + 81 + 81) | 3 | 15 and 24 | Primary care | USA | 78% non-Hispanic white; 17% Asian/Pacific Islander | 53 | 53 | 32 |
| Makrilakis et al., 201073 | Single-arm before and after | DE-PLAN Greece | FINDRISC score of ≥ 15 | Lifestyle (diet and exercise) | 191 | 1 | 12 | Primary care, workplace | Greece | NR | 56 | 40 | 32 |
| Mensink et al., 200382,99 (Roumen et al., 2008102 and 2011103) | RCT | SLIM study | Aged > 40 years and FH diabetes mellitus or BMI of ≥ 25 kg/m2; IGT (OGTT 2-hour glucose ≥ 7.8 and< 12.5) and FPG of < 7.8 | Lifestyle (diet and supervised exercise) | 114 (55 + 59) | 2 | 12, 24, 36, 48 (Roumen) | Unclear | Netherlands | White Caucasian | 57 | 56 | 30 |
| Nilsen et al., 201183 | RCT | APHRODITE study | FINDRISC score of ≥ 9 | Lifestyle (diet and exercise) | 213 (104 + 109) | 2 | 18 | Primary care | Norway | NR | 47 | 50 | 37 |
| Ockene et al., 201284 | RCT | Lawrence Latino DPP | BMI of ≥ 24 kg/m2; > 30% increased likelihood of diabetes mellitus over next 7.5 years from validated risk algorithm | Lifestyle (diet and exercise) | 312 (150 + 162) | 2 | 12 | Community, family health centre | USA | 60% Dominican; 40% Puerto Rican | 52 | 26 | 34 |
| Parikh, 201085 | RCT | Project HEED | BMI of ≥ 25 kg/m2 and PDM; CBG fasting of < 126 mg/dl and 2-hour CBG following 75 g glucose | Lifestyle (diet and exercise) | 99 (50 + 49) | 2 | 12 | Community various venues | USA | 89% Hispanic; 9% African American | 48 | 15 | 32 |
| Payne et al., 200886 | Single-arm before and after | NR | Aged ≥ 45 years (or aged ≥ 35 years Aboriginal, Torres Strait Islanders, Pacific Islanders, Indian, Chinese) and BMI ≥ 30 kg/m2 and/or ↑ BP; existing CVD, PCOS, gestational diabetes; first-degree FH diabetes; IGT or IFG | Lifestyle (diet and exercise programme) | 122 (62 + 60) | 2 | 12 | Outpatient facility | Australia | NR | 53 | 22 | 35 |
| Penn et al., 200987 | RCT | NR | BMI of > 25 kg/m2 and aged > 40 years; IGT (OGTT 2-hour glucose of ≥ 7.8 and < 11.1) | Lifestyle (diet and exercise) | 102 (51 + 51) | 2 | 12 and 3.1 years mean | Outpatient setting | UK | NR | 57 | 40 | 34 |
| Penn et al., 201395 | Single-arm before and after | NR | Aged 45–65 years, and FINDRISC score of 11–20 or > 20 if GP confirms no diabetes mellitus | Lifestyle (diet and supervised exercise) | 218 | 1 | 12 | Community and leisure centres | UK | NR | 54 | 31 | 34 |
| Ruggiero et al., 201188 | Single-arm before and after | NR | BMI of ≥ 24.9 kg/m2 | Lifestyle (diet and exercise) | 69 | 1 | 12 | Community various venues | USA | Hispanic | 38 | 7 | 31 |
| Saaristo et al., 201089 (Rautio et al., 2011100 and 2012101) | Single-arm before and after | FIN-D2D | FINDRISC score of ≥ 15 or IFG or IGT or CVD event or gestational diabetes | Lifestyle (diet and exercise) | 2798 | 1 | 12 | Primary care | Finland | NR | 54 | 49 | ≈ 31 |
| Sakane et al., 201190 | RCT | NR | IGT identified as follows: IFG of ≥ 5.6 and < 7.0; random PG (≥ 7.8 < 11.1 within 2 hours of meal) or (≥ 6.1 and < 7.8, ≥ 2 hours after meal); IGT | Lifestyle (diet and exercise) | 296 (146 + 150) | 2 | 12 and 36 | Various: primary care, workplace, collaborative centre | Japan | NR | 51 | 51 | 25 |
| Vermunt et al., 201291 (and 2011104) | RCT | NR | FINDRISC score of ≥ 13 | Lifestyle (diet and exercise) | 925 (479 + 446) | 2 | 18, 30 | Primary care | Netherlands | NR | NR | NR | ≈ 29 |
| Yates et al., 200950 (and 201151) | RCT | PREPARE | BMI of ≥ 25 (23 for SAs); screened detected IGT | Lifestyle (exercise) | 98 (33 + 31 + 34) | 3 | 12, 24 | Outpatient setting | UK | 75%c white; 24% SA; 1% black | 65c | 66c | 29.2c |
Studies were conducted in the USA (n = 13), Australia (n = 3), Europe (n = 12) and Japan (n = 1); however, ethnicity was poorly reported. The number of people who were enrolled into the intervention arms in individual studies ranged from 8 to > 2700, with 26 studies including at least 50 participants. The criteria used, alone or in combination, to identify high risk included: elevated BMI, elevated diabetes mellitus risk score [FINDRISC,108 ADA,27 the Australian type 2 diabetes risk assessment tool (AUSRISK)109], raised random, fasting or 2-hour glucose (finger prick or venous sample); older age; ethnicity; family history of diabetes mellitus; previous medical history of CVD, polycystic ovary syndrome, gestational diabetes, metabolic syndrome, elevated BP or lipids. Length of follow-up ranged from 12 months to approximately 4 years. The mean age and BMI of participants ranged from 38 to 65 years and from 25 to 37 kg/m2, respectively, and the proportion of males ranged from 7% to 66%.
Outcome data for change in weight were available for 28/29 studies (not Costa et al. 78); 25 of 29 studies reported weight at 12 months (see Appendix 4). Additional 12-month data reported for 26 studies (Appendices 4 and 5) included change in BMI (20 studies), waist size (18), fasting glucose (17), 2-hour glucose (11), HbA1c (7), total cholesterol (14), LDL (9), HDL (14), triglycerides (12), systolic BP (15), diastolic BP (12) and the incidence of diabetes mellitus after 12 months (9). Outcome data for change in physical activity and diet were poorly reported. Overall, considerable heterogeneity was evident between studies in relation to several key characteristics including the setting, population, criteria used to identify diabetes mellitus risk, interventions and follow-up.
Study quality
Most studies achieved a ‘high quality’ grading for internal validity (28/29). However, details relating to the source/eligible population and area and the selected participants were less well reported; only 13 studies achieved a high quality score for external validity. (For a breakdown of study quality, see Appendix 6.)
Scoring of intervention content
Details of coding scores for study interventions are presented in Appendix 3. A total of 14 of the 28 intervention groups included in the main meta-analysis attained an overall score of ≥ 9 out of a possible 12, in relation to meeting NICE guideline recommendations; 21 scored ≥ 7. For IMAGE guideline recommendations, an overall score of ≥ 5 out of a possible 6 was achieved by 13 study groups.
Meta-analysis
Twenty-five studies involving 5785 participants (estimated 36% male) were included in the meta-analysis for mean weight change at 12 months. One study was excluded from the primary meta-analysis, as weight change was not recorded as a study outcome. 78 Three were excluded from all analyses as one study reported only 15-month data,92 and two were excluded as they reported only 18-month data. 83,91 Two studies included in the meta-analysis had two intervention arms,50,81 meaning that 27 study groups were analysed.
The pooled result of the meta-analysis (Figure 2) shows that lifestyle interventions resulted in a mean weight loss of 2.31 kg (95% CI –2.87 to –1.76 kg; I2 = 92.9%).
FIGURE 2.
Forest plot showing mean weight change in each study and the overall pooled estimate. Adapted from Dunkley et al. 52 under Creative Commons public licence 3.0, https://creativecommons.org/licenses/by/3.0/. Additional studies (Janus et al. ,93 Kanaya et al. 94 and Penn et al. 95) have been added; therefore, the subtotal line and overall line in the plots have changed, as they now include additional data. Boxes and horizontal lines represent mean weight change and 95% CI for each study. Size of box is proportional to weight of that study result. Diamonds represent the 95% CI for pooled estimates of effect and are centred on pooled mean weight change. CPC, carbohydrate reduction and hunger focus post core; TPC, traditional post core.

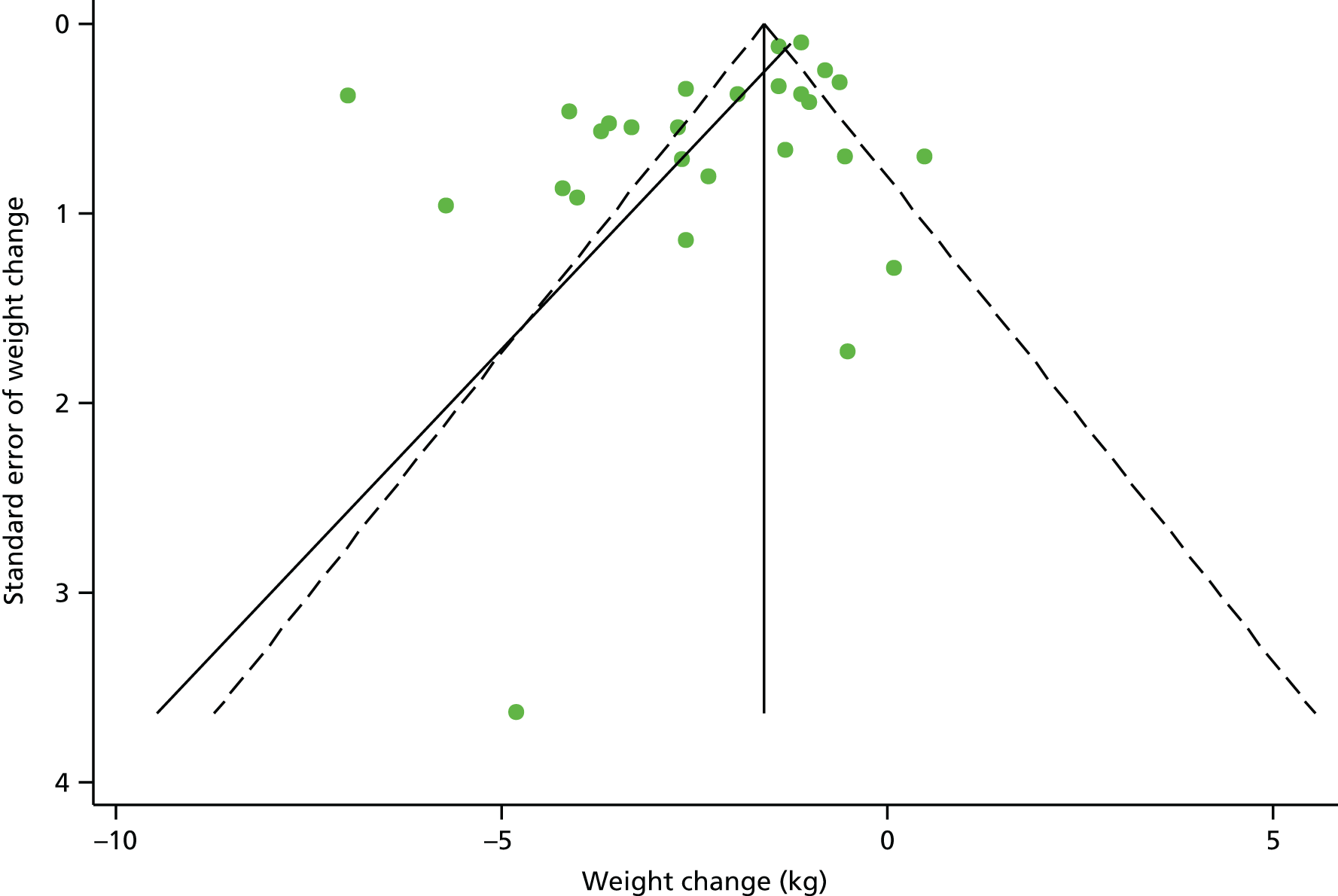
Sensitivity analysis, restricted to RCTs only, indicated a mean weight change (–2.5 kg, 95% CI –3.8 to –1.2 kg) that is similar to the overall result. Additional analysis comparing the difference in weight lost between the treatment and control arms, for RCTs only, suggests that, on average, the intervention arm lost an extra –1.79 kg (95% CI –2.78 kg to –0.80 kg; p < 0.001). Furthermore, sensitivity analyses that included studies scoring ++ for external validity demonstrated a slightly greater weight loss in higher-quality studies (–2.8 kg, 95% CI –4.1 to –1.5 kg). However, there was some evidence of publication bias (p = 0.033, Egger’s test; see Figure 3 for funnel plot).
FIGURE 3.
Funnel plot with pseudo 95% confidence limits assessing publication bias for the primary outcome weight change.
All other outcomes showed an improvement at 12 months (Table 2), with all of these reaching statistical significance with the exception of HDL cholesterol. The pooled result for the 19 studies (20 study groups) that reported BMI demonstrates that lifestyle interventions resulted in a mean decrease in BMI of 0.98 kg/m2 (95% CI –1.28 to –0.68 kg/m2; I2 = 95.2%). A collective decrease in waist circumference of 3.36 cm (95% CI –4.33 to –2.39 cm; I2 = 97.5%) was found across the studies that reported the measure (n = 18). The pooled result for the studies that conveyed HbA1c percentages (n = 8) indicated that lifestyle intervention corresponded to a 0.11% (95% CI –0.19% to –0.03%; I2 = 97.1%) decrease in HbA1c. Significant reductions in fasting glucose of 0.10 mmol/l (95% CI –0.18 to –0.02 mmol/l; I2 = 86.0%) and 0.36 mmol/l (95% CI –0.66 to –0.06 mmol/l; I2 = 92.8%) in 2-hour glucose were suggested for lifestyle intervention. Total cholesterol was reported for 16 study groups, for which the pooled result of the direct pairwise meta-analysis indicated a 0.18 mmol/l (95% CI –0.23 to –0.13 mmol/l; I2 = 39.8%) mean decrease in total cholesterol at 12 months for those in receipt of lifestyle intervention. A slightly smaller reduction in LDL cholesterol of 0.15 mmol/l (95% CI –0.22 to –0.07 mmol/l; I2 = 66.0%) was demonstrated for the intervention groups by the pooled result for the meta-analysis including 10 study groups. The pooled result for HDL cholesterol indicated a 0.02 mmol/l (95% CI –0.002 to 0.04 mmol/l; I2 = 92.8%) increase for the intervention groups across 16 study groups; however, this was not a statistically significant finding. The overall effect of lifestyle intervention on triglycerides showed a 0.1 mmol/l (95% CI –0.18 to –0.01 mmol/l; I2 = 99.2%) decrease in triglycerides measurement spanning 14 study groups. Significant combined effects of intervention on BP were demonstrated, with a decrease in systolic BP of 4.02 mmHg (95% CI –5.66 to –2.37 mmHg; I2 = 77.1%) over 16 study groups and 3.88 mmHg (95% CI –5.24 to 2.52 mmHg; I2 = 83.7%) in diastolic BP across 12 study groups. Across the nine studies that reported incident diabetes mellitus, the pooled incidence rate was 35 cases per 1000 person-years (95% CI 24 to 53 cases per 1000 person-years), which gives the number needed to treat as 29.
| Outcome | Number of study groups | Pooled effect | 95% CI | p-value | I 2 | Publication bias p-value |
|---|---|---|---|---|---|---|
| Weight (kg) | 27 | –2.31 | –2.87 to –1.76 | < 0.001 | 92.9% | 0.033 |
| BMI (kg/m2) | 20 | –0.98 | –1.28 to –0.68 | < 0.001 | 95.2% | 0.067 |
| Waist circumference (cm) | 20 | –3.36 | –4.33 to –2.39 | < 0.001 | 97.5% | 0.136 |
| HbA1c (%) | 8 | –0.11 | –0.19 to –0.03 | 0.009 | 86.7% | 0.961 |
| Fasting glucose (mmol/l) | 19 | –0.10 | –0.18 to –0.02 | 0.014 | 86.0% | 0.344 |
| 2-hour glucose (mmol/l) | 12 | –0.36 | –0.66 to –0.06 | 0.018 | 92.8% | 0.156 |
| Total cholesterol (mmol/l) | 16 | –0.18 | –0.23 to –0.13 | < 0.001 | 39.8% | 0.776 |
| LDL cholesterol (mmol/l) | 10 | –0.15 | –0.22 to –0.07 | < 0.001 | 66.0% | 0.278 |
| HDL cholesterol (mmol/l) | 16 | 0.02 | –0.002 to 0.04 | 0.082 | 92.8% | 0.931 |
| Triglycerides (mmol/l) | 14 | –0.10 | –0.18 to –0.01 | 0.022 | 99.2% | 0.585 |
| Systolic BP (mmHg) | 16 | –4.02 | –5.66 to –2.37 | < 0.001 | 77.1% | 0.018 |
| Diastolic BP (mmHg) | 12 | –3.88 | –5.24 to –2.52 | < 0.001 | 83.7% | 0.005 |
| Incident diabetes mellitus (per 1000 person-years)a | 9 | 35.3 | 23.6 to 52.7 | < 0.001 | 79.5% | 0.117 |
High levels of heterogeneity were demonstrated for all secondary outcomes. Further significant evidence of publication bias was apparent for the reporting of systolic (p = 0.018) and diastolic (p = 0.005) BP outcomes via Egger’s test. No other significant evidence of publication bias was detected.
Discussion
The 25 translational diabetes mellitus prevention programmes included in our meta-analysis significantly reduced weight in their intervention arms by a mean 2.3 kg at 12 months’ follow up. Where data were available, we found significant reductions in other diabetes mellitus and cardiovascular risk factors, including blood glucose, BP and some cholesterol measures. The pooled diabetes mellitus incidence rate in the intervention arms was 35 per 1000 person-years (number needed to treat 29). Outcome data on changes in the key lifestyle behaviour targets (physical activity and diet) were poorly reported.
Relationship to other literature
The mean level of weight loss achieved was around a half to one-third of the levels reported at the same time point within the intervention arms of clinical efficacy trials, such as the US DPP (≈6.7 kg) and the Finnish DPS (≈4.2 kg). 32,33 This is consistent with the findings of a meta-analytic systematic review published in 2010 by Cardona-Morrell et al. 55 which identified a mean net weight loss after 12 months of 1.82 kg (95% CI –2.7 to –0.99 kg). Cardona-Morrell et al. 55 interpreted the lower level of weight loss and a lack of significant differences in fasting plasma glucose and 2-hour glucose as meaning that the interventions ‘appear to be of limited clinical benefit’. Our view is that, despite the drop-off in intervention effectiveness in translational studies, the level of weight loss found in our analysis is still likely to have a clinically meaningful effect on diabetes mellitus incidence. This is based on data from the US DPP study which show that each kilogram of mean weight loss is associated with a reduction of approximately 16% in future diabetes mellitus incidence. 53 Furthermore, a recent meta-analysis, which included studies without an intervention in order to look at natural diabetes mellitus progression rates in high-risk individuals, found that progression rates to diabetes mellitus from IFG, IGT and both were 47, 56 and 76 per 1000 person-years, respectively. 14 The rate of 35 per 1000 person-years that we found suggests that the real-world lifestyle interventions studied here did lower diabetes mellitus progression rates.
For our review, the mean proportion of weight lost (%) at 12 months’ follow-up was –2.6%. This amount was slightly lower than was demonstrated by a recent meta-analysis conducted by Ali et al. ,46 which considered translational studies aimed at populations with existing diabetes mellitus (≤ 50%) or at high future risk. They found a mean weight loss of −4.1% (95% CI −5.9% to −2.4%) after at least 9 months of follow-up. 2 This difference may in part be due to a lower mean BMI at baseline in studies included in our review than in studies in the Ali et al. 46 review (range 25–36 kg/m2 and 31–40 kg/m2, respectively), and a slightly longer follow-up period (12 months vs. ≥ 9 months). In addition, their review focused on interventions based only on the US DPP, whereas we considered a broader set of interventions.
Changes in the four key dietary and physical activity targets (≤ 30% energy from fat; ≤ 10% energy from saturated fat; fibre ≥ 15 g/1000 kcal; ≥ 30 minutes moderate physical activity daily) have also been shown to have independent effects on diabetes mellitus risk reduction, irrespective of weight loss. 53 However, few of the studies we examined provided data on dietary intake or physical activity, so we cannot be sure whether diabetes mellitus prevention in these studies is driven by increased physical activity, dietary change or both.
Strengths and limitations
This study is novel in that it provides an updated meta-analysis of a global set of lifestyle interventions for diabetes mellitus prevention. Our study used comprehensive search criteria and focused on establishing the utility of pragmatic attempts to achieve diabetes mellitus prevention in real-world service delivery settings.
The study is limited in that there were insufficient data to analyse outcomes beyond 12 months; our findings may not translate into long-term therapeutic value owing to uncertainty around sustaining outcomes, such as weight loss, in the longer term. 110 Furthermore, results in individual studies were not always reported on an intention-to-treat (ITT) basis, leading to a probable overestimation of effect sizes.
Owing to the nature of pragmatic implementation studies, which include a number of uncontrolled studies, our analysis was restricted to intervention arms only; however, sensitivity analysis, restricted to RCTs only, indicated a mean weight change (–2.5 kg, 95% CI –3.8 to –1.2 kg) that is similar to the overall result. Additional sensitivity analysis restricted to RCTs showed that intervention arms lost 1.79 kg (95% CI –2.78 to –0.80 kg) more weight than control arms. This does suggest that the true intervention effect is smaller than suggested by the analysis restricted to intervention arms only.
Weight change was chosen as the primary outcome, as the majority of studies reported this outcome as opposed to other measures such as changes in glucose measures or progression to T2DM. Progression to T2DM would have been the preferable outcome to analyse diabetes mellitus risk reduction; however, as most studies were restricted to a 12-month follow-up, it is questionable whether or not this is a suitable period of time to fully evaluate the effect of intervention on the proportion of individuals who progress to T2DM. Although fasting and 2-hour glucose outcomes were reasonably well reported among studies, HbA1c was the least reported. This is most likely to be a result of the fact that the WHO began recommending the use of HbA1c as a T2DM diagnostic tool only in 2011, whereas many studies in this review predate this introduction. 50,62,69–80,82,85–87,89,96,97,99,102,106
Unpublished literature was not considered for inclusion in this review, leading to potential selection bias. Further bias may have also been introduced via the decision to limit studies to English-language studies only.
The results of Egger’s test for publication bias indicated evidence of publication bias for the primary outcome mean weight change, as well as for mean change in systolic and diastolic BP outcomes. However, the test has low power to detect publication bias for outcomes with few studies. In addition, when there are high levels of between-study heterogeneity, as is the case for all outcomes in this review, Egger’s test may not detect publication bias if present.
Implications for practice
Our review suggests that pragmatic lifestyle interventions are effective at promoting weight loss and that they could potentially lead to a reduced risk of developing diabetes mellitus and CVD in the future. However, the difficulties in translating this evidence into practice and in delivering guideline-based interventions need to be overcome. The ability to implement these findings in practice may be further hampered by a lack of resource for service provision, the design of efficient risk identification systems, and engagement of politicians and health-care organisations in funding national diabetes mellitus prevention programmes. Diabetes mellitus prevention strategies require substantial up-front investment to accrue longer-term benefits. 29
Future directions
More research is needed to examine the longer-term clinical effectiveness and cost-effectiveness of pragmatic lifestyle interventions for diabetes mellitus prevention, including diabetes mellitus incidence as well as weight-loss outcomes. The practical value of diabetes mellitus prevention interventions would be much clearer if we had data on longer-term outcomes. Research is also needed to identify the role of different types of physical activity and dietary changes,54,111 and ways to increase effectiveness without increasing cost. Possible approaches might include the use of larger group sizes and the substitution or supplementation of intervention techniques using self-delivered formats (e.g. internet, smartphone or workbook). 112
Summary
Overall, the interventions were effective, but there was wide variation in effectiveness. More research is needed to establish optimal strategies for maximising both cost-effectiveness and longer-term maintenance of the lifestyle changes that these programmes can achieve.
Chapter 3 Developing the risk score
This chapter was based on previously published data, reproduced with kind permission from Springer Science+Business Media: Diabetologia, Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multi-ethnic UK community setting, vol. 55, 2012, pp. 959–66, Gray LJ, Davies MJ, Hiles S, Taub NA, Webb DR, Srinivasan BT, Khunti K, excerpts of text, tables 1, 2 and 3, and figures 1 and 2 (please note that minor edits have been made, with the permission of the authors, for consistency). 113
Introduction
Risk scores are a way of stratifying a population for targeted screening. They use data from risk factors to calculate an individual’s score; a higher score reflects higher risk. Risk scores can be applied either to an individual as a questionnaire (these scores generally require only data from non-invasive risk factors, which would be known by members of the public) or to a population. Population risk scores are usually developed for use in primary care where a piece of software is used to calculate the score for everyone listed on the electronic medical records using routinely stored data. Screening invitations can then be sent to those at the highest risk.
Over the past decade, a plethora of risk scores have been developed and validated for detecting those at risk of T2DM. One of the first risk scores developed in this field was the FINDRISC score. This risk score was developed for use in Finland; it is questionnaire based and designed to be completed by members of the public to detect those at risk of developing T2DM in the future. 27 It includes eight questions relating to age, BMI, waist circumference, BP, history of high blood glucose, family history of diabetes mellitus, physical activity and consumption of vegetables, fruits or berries. This score has been shown to have acceptable levels of discrimination and, since its development in 2003, it has been validated for use in Greece,114 Bulgaria,115 Italy,116 Spain117 and Sweden. 118 It was decided not to validate this score for use in this project for a number of reasons. First, the FINDRISC was not developed for detecting those with existing undiagnosed PDM (IFG and/or IGT) and T2DM. Second, it is well reported that risk scores that have been developed for a particular population tend to have low validity when used on another. In addition, the FINDRISC does not include ethnicity, which is an important risk factor when assessing risk in a multiethnic population such as in the UK. 28,119,120 Third, the questionnaire nature of this risk score and the inclusion of patient-specific risk factors that would not be available routinely in primary care meant that this risk score could not be implemented in primary care for population-based stratification.
The Cambridge Diabetes Risk Score (CDRS) addresses some, but not all, of these issues. 121 This score was developed to detect undiagnosed T2DM and it collects data on age, sex, BMI, steroid and antihypertensive medication, and family and smoking history. This score would be suitable for use in primary care but it does not detect current undiagnosed PDM and it does not reflect the higher incidence of T2DM in those from black and minority ethnic (BME) groups. The FINDRISC identifies people who are at risk of developing T2DM in the next 10 years, with the CDRS detecting current undiagnosed T2DM only. To date, there is no evidence base for intervening in such a group for the prevention of T2DM. The evidence from the large pivotal trials for preventing T2DM is in people with IGT. 31 The ultimate aim of this programme of work is to develop and test a pragmatic intervention, taking the learning of the previous trials, delivered in a UK primary care setting. Therefore, we wished to identify people who have PDM rather than those at risk of developing diabetes mellitus in the future. Hence, it was decided to derive and statistically validate a new risk score that detects PDM/T2DM for use in a multiethnic population using data from two existing population-based screening studies from Leicester and Leicestershire. 122,123
The development and validation of the Leicester Practice Risk Score (LPRS) had three phases. Initially, a pilot score was developed and validated, and tested in two general practices (phase one). The aim of the pilot phase was not so much to assess the performance of a risk score per se, but to test the feasibility of a risk-score approach for identifying people with PDM in primary care. Owing to the milestones required for the programme of work, this feasibility testing needed to be completed before the final data set from the large population-based screening study was ready for analysis. A very simple pragmatic score was therefore derived to enable this approach to screening to be tested. Reporting of details of how this score was derived is outside the scope of this report. Following this pilot, complete data from a large-scale population-based screening study [Anglo–Danish–Dutch study of Intensive Treatment In people with screen detected diabetes in primary care (ADDITION)] became available; therefore, the score was redeveloped based on the learning from the pilot study. This score was subsequently used to identify those at high risk for screening within Let’s Prevent (phase two). Following this, the score was updated based on subsequent improvements in data completeness in primary care and the addition of HbA1c to the diagnostic criteria for T2DM (phase three). Given that this score is published and used in clinical practice, full details of the development and validation are given for the final updated score.
Data sets
Data sets from two existing closely related screening studies were used throughout all three phases, that is, ‘Screening Those At Risk’ (STAR) and ADDITION. These are described briefly below and their shared methodology is outlined in the final section.
Screening Those At Risk
The STAR study aimed to identify the prevalence of PDM and undiagnosed T2DM in those with at least one recognised risk factor for diabetes mellitus. Between 2002 and 2004, 3225 individuals aged 40–75 years inclusive (25–75 years for those with South Asian, Afro-Caribbean and other ethnicity owing to the reported higher risk of T2DM) with at least one risk factor for T2DM were invited for screening from 17 general practices. Risk factors for inclusion into the study included a documented clinical history of coronary heart disease, hypertension, dyslipidaemia, cerebrovascular disease or peripheral vascular disease, previous history of IGT, gestational diabetes, polycystic ovary syndrome in those with a BMI of > 25 kg/m2, a first-degree relative with T2DM or BMI of > 25 kg/m2, and current or ex-smokers. Full details of the methodology and results are published. 122
Anglo–Danish–Dutch study of Intensive Treatment In people with screen detected diabetes in primary care-Leicester
This study has been described in detail elsewhere. 123 In summary, ADDITION-Leicester invited a randomly selected 30,950 people aged 40–75 years (25–75 years if non-white, although those aged 25–40 years are excluded from these analyses) without diagnosed diabetes mellitus from 20 practices from Leicester and the surrounding county for screening between 2004 and 2008; 6749 individuals attended screening (response rate 22%). All 6749 participants underwent an OGTT and, therefore, people with PDM and previously undiagnosed T2DM were identified. Those found to have undiagnosed T2DM were included in a RCT of intensive treatment versus standard care;124 data from this trial are not included in this analysis. The analysis is based solely on the cross-sectional screening data and, therefore, includes people identified with normal glucose, PDM and T2DM.
Shared protocols
In both studies all screened participants received an OGTT using 75g of glucose, and had biomedical and anthropometric measurements taken by a trained member of research staff, which included data such as medical history, medication, BMI, BP, and a self-completed questionnaire. The questionnaire collected data on smoking status, alcohol consumption, occupational status, ethnicity, physical activity, the FINDRISC score and a number of scales to measure domains such as well-being and anxiety.
All participants were diagnosed with screen-detected IFG, IGT and T2DM according to WHO 1999 criteria,8 with PDM referring to the composite of IGT and/or IFG. HbA1c was collected for all participants at baseline.
Anthropometric measurements were performed by trained staff following standard operating procedures, with height being measured to the nearest 0.1 cm using a rigid stadiometer (Seca, Hamburg, Germany) and weight in light indoor clothing measured to the nearest 0.1 kg with a Seca scale (Seca UK, Birmingham, UK). BMI was defined as weight in kilograms divided by height in metres squared (kg/m2). Waist circumference was measured at the mid-point between the lower costal margin and the level of the anterior superior iliac crest to the nearest 0.1 cm.
Data sets used for development and statistical validation of the risk scores
The use of each data set across the three phases is outlined in Table 3. Given the larger sample size and population-based approach, the ADDITION data set is preferable for the development of a risk score, with STAR then being used for temporal (i.e. evaluation on external data from the same centre) validation. Owing to the unavailability of the ADDITION study data in a format suitable for analysis in late 2007 when the pilot study was commenced, the development of the initial risk score was divided into two phases. In phase one, a pilot risk score was developed using data from the STAR study, specifically for use in the pilot screening study. Temporal validation using the ADDITION study data set was carried out retrospectively. In phase two, the risk score for use in the Let’s Prevent study was developed. Its design is based on analysis of the ADDITION study data set, which, being larger than the STAR data set, allows greater sensitivity to the possible predictive values of potential risk factors. The same approach was used when the risk score was updated in 2010.
| Risk score | Phase | STAR | ADDITION |
|---|---|---|---|
| Pilot risk score | Development | ✓ | |
| Validation | Temporal | ||
| Initial LPRS | Development | ✓ | |
| Validation | Temporal | ||
| Updated LPRS | Development | ✓ | |
| Validation | Temporal |
The characteristics of those included in the two data sets are given in Table 4. The mean age in the ADDITION-Leicester data was 57.3 years, with 48% being male. Three-quarters of the cohort were white European, with 23.5% of other ethnicity (of which the majority were South Asian, 91%). Of the 6390 people aged ≥ 40 years screened as part of the ADDITION study, 927 (14.5) were found to have PDM and 206 (3.2) had undiagnosed T2DM based on an OGTT, which rises to 485 (7.6%) when including HbA1c in the diagnostic criteria. The STAR data set had similar characteristics but with slightly more people reporting that they were smokers (25% vs. 14%).
| Variable | ADDITION (n = 6390) | STAR (n = 3004) |
|---|---|---|
| Age (years), mean (SD) | 57.3 (9.6) | 56.7 (9.8) |
| Sex male, n (%) | 3046 (47.7) | 1383 (46.1) |
| Ethnicity | ||
| White European, n (%) | 4688 (75.8) | 2138 (73.7) |
| Other, n (%) | 1499 (24.3) | 763 (26.3) |
| Weight (kg), mean (SD) | 78.3 (16.0) | 77.9 (16.0) |
| BMI (kg/m2), mean (SD) | 28.1 (5.0) | 28.2 (5.2) |
| Waist circumference (cm), mean (SD) | 94.2 (13.1) | 95.6 (13.0) |
| Systolic BP (mmHg), mean (SD) | 137.9 (19.4) | 134.0 (20.5) |
| Diastolic BP (mmHg), mean (SD) | 85.6 (10.6) | 80.4 (10.8) |
| Current smoker, n (%) | 891 (13.9) | 762 (25.4) |
| HbA1c (%), mean (SD) | 5.7 (0.6) | 5.8 (0.7) |
| HbA1c (mmol/mol), mean (SD) | 39 (17) | 40 (7) |
| Cholesterol (mmol/l), mean (SD) | 5.6 (1.1) | 5.4 (1.0) |
| LDL (mmol/l), mean (SD) | 3.5 (0.9) | 3.4 (0.9) |
| HDL (mmol/l), mean (SD) | 1.4 (0.4) | 1.3 (0.5) |
| PDM, n (%) | 927 (14.5) | 407 (12.6) |
| T2DM (OGTT), %, mean (SD) | 206 (3.2) | 92 (3.1) |
| T2DM (OGTT or HbA1c), %, mean (SD) | 485 (7.6) | 367 (11.4) |
Statistical methods
The purpose of the risk scores was to identify those at greatest risk of glucose intolerance, defined as those with either T2DM or PDM (which includes IFG and/or IGT) who, up until screening with an OGTT, had been undiagnosed. All of the scores developed and validated as part of this project used similar methodology. To avoid repetition this is detailed below. Where differences occurred, these are also summarised.
Development
Variables considered
The variables to be considered for inclusion in the score are limited to those that are included in the ‘typical’ general practice database with a good level of reliability and completeness. The consensus is that the following items satisfy these conditions: age, sex, BMI, ethnicity (white European or other), family history (of type 1 or type 2 diabetes mellitus), smoking status (current smoker or ex or non), prescribed antihypertensives, statins or steroids, history of CVD (myocardial infarction, stroke, heart valve disease, atrial fibrillation, angina, angioplasty or peripheral vascular disease) and deprivation [measured using the Index of Multiple Deprivation (IMD) calculated from the individual’s postcode]. This pool of variables assessed covers the majority of those included in previously developed screening tools and screening guidelines. 125,126
Modelling
All modelling was carried out in Stata (version 11.1) using logistic regression with the composite of IGR [defined as IFG or IGT on OGTT (not including HbA1c 6.0–6.4 at this stage)] or T2DM [OGTT or HbA1c ≥ 6.5% (48 mmol/mol)] versus normal as the dependent variable. A staged approach to variable selection was taken. First, we assessed the association of each variable and the outcome independently (PDM/T2DM). Those that were significantly (p < 0.05) associated with the outcome were then assessed in combination and those that became non-significant when adjusted for other variables in the model were removed. This process was then repeated. Each combination of variables was compared in terms of the area under the receiver operating characteristic (ROC) curve, with the aim of maximising this. The effect of adding each previously excluded variable into the model was assessed to make sure that no potentially important variables were missed; again, their significance and effect on the ROC was assessed. Once a final model was established we assessed all possible two-way interactions and the addition of polynomial terms, although we acknowledged that we would have limited power to explore these. The importance of introducing functional polynomial terms was also assessed using the Akaike information criterion. 127 Throughout the analysis, missing data were not imputed and analysis was carried out on a complete-case basis.
The updated risk score (described in phase three) also included HbA1c of ≥ 6.5% in the definition of T2DM, given that HbA1c was recommend as a diagnostic tool by WHO in 2011. 10 HbA1c was not used in the definition of PDM as, although using a range of 6.0–6.4% has been recommended for identifying those at high risk of developing diabetes mellitus in the future,12 WHO concluded that there was insufficient evidence for classifying PDM using HbA1c. 10
Creating a scoring system
Once a final model has been developed a risk score needs to be devised from this. For the pilot score a crude, easy-to-calculate score was developed (see Phase one: pilot risk score results for details). For the initial and updated risk scores, the scores were derived by summing each of the β coefficients from the best fitting model.
Once a score had been devised, the discrimination of the score was assessed using the area under the ROC curve. Calibration was assessed using the Hosmer–Lemeshow statistic,128 the Brier score129 and a calibration plot of estimated prevalence of PDM and T2DM grouped by the predicted probability.
Statistical validation
Each of the scores developed were temporally validated (see Table 3 for data sets used). Each score was validated against the outcome for which it was developed. For the updated risk score, temporal validation was carried out using six different outcomes that reflect how the score would be used in clinical practice (i.e. one method of diagnosis will be chosen): (1) T2DM diagnosed using OGTT; (2) T2DM diagnosed using HbA1c; (3) PDM defined as IGT or IFG on OGTT; (4) HbA1c between 6.0% and 6.4%; (5) T2DM or PDM on OGTT; and (6) HbA1c of ≥ 6.0%.
The ROC curve was plotted for each outcome and the area under the curve was calculated. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratio for a positive test (LR+) and likelihood ratio for a negative test (LR−) with 95% CI were calculated, comparing each cut point on the score to the outcome.
The results of each of the risk scores developed and validated are presented below (see Results). The pilot risk score and initial risk score are described briefly with full details of the updated practice risk score given along with details of the development of a piece of software to run this risk score in general practices.
Phase one: pilot risk-score results
The risk score developed to conduct the screening pilot in primary care was derived using the STAR data set. The final model is given in Table 5.
| Variable | Beta coefficient | 95% CI | p-value |
|---|---|---|---|
| Age (years) | 0.038 | 0.027 to 0.050 | < 0.001 |
| BMI (kg/m2) | 0.081 | 0.063 to 0.099 | < 0.001 |
| Sex (female relative to male) | 0.373 | 0.179 to 0.568 | < 0.001 |
From the coefficients of the final model a crude pilot risk score formula was defined as the sum of:
-
person’s age (in years)
-
twice their BMI (in kg/m2)
-
10 if they are male (no change if female).
A simplified score was preferred for the pilot study, as this would be easily calculated by researchers; the score for the main study was calculated using a piece of software and, therefore, the simplification of the relative weightings of the score components was not needed.
Using the score to identify the 10% most at risk for invitation to further screening identified 132 people with PDM in the STAR data set and 41 people with T2DM, representing a sensitivity of 20.1% and a specificity of 92.4%. Increasing the threshold so that the top 28% of people at risk were invited for screening increases the sensitivity to 43.6% and reduces the specificity to 75.2%.
Results from the pilot screening study
Methods
Two primary practices were identified to test the pilot risk score in order to assess how effectively the tool could be used to identify those at highest risk.
Two contrasting practices were selected for the pilot screening study. Melton Mowbray is a large rural practice comprising a practice population of 36,000 with 20 general practitioners (GPs); 99% of the practice population are Caucasian and 829 patients were listed on the diabetes mellitus register (2.3% of the total practice). Spinney Hill, in contrast, is a large, inner-city practice with seven GPs comprising a total practice population of 16,000 patients, 98% of whom are of South Asian ethnicity. A total of 8% (1311) of patients were listed on the practice register for diabetes mellitus. The two practices used in the pilot study were selected from those that already had ethical permission for ongoing screening as part of the ADDITION study, but in which screening had not commenced. For this reason, recruitment of the practices was not necessary; in the Let’s Prevent study, practices would first need to be recruited to take part in the study.
For the pilot study, information needed for the risk score was obtained from Egton Medical Information Systems [(EMIS) EMIS Health, Leeds, UK] data searches. It was expected that all the general practices in the study area would be using the EMIS computer system. From the practice list data the risk score was calculated and, separately for each practice, used to classify the individuals in descending order of PDM/T2DM risk.
In the pilot study the individuals were then sent letters of invitation (in batches of 200) to take part in the study by their GP; this invitation included a questionnaire (which asked four basic questions, such as whether or not the individual was taking part in any other studies) and a reply slip together with a stamped, addressed envelope. Recruitment was stopped (for reasons of practicality) at the risk score of 125 in both practices. Individuals were invited according to their risk score. Individuals who had not replied were sent a reminder letter. A mobile clinic located on a double-decker bus was used for the majority of the pilot screening owing to its convenience and accessibility, although a small number of screening sessions for participants living in the Leicester area were held at the Leicester General Hospital.
Those individuals who had agreed to take part in the pilot study were sent information on the date, time and place of their appointment as well as instructions that from the previous midnight they should eat nothing and drink only water. The surgery sessions were held only in the morning. Participants were given a telephone reminder a day or two before their session and this had the effect of cutting the number of ‘no-shows’ down from 46% in ADDITION to almost zero. Overall, there tended to be more no-shows from the inner-city Leicester practice than from the practice located in the nearby market town.
Results
In total, 2168 people were found to be at high risk and invited to be screened. A total of 686 people gave a positive reply, representing 31.6% of those invited. At the time of analysis, 264 of those who gave a positive response had been screened (38.5%). Therefore, 12.2% of those originally invited provided data for the pilot screening study.
Baseline characteristics of the 264 participants screened in the pilot study are shown in Table 6. This sample was predominantly (73%) male, somewhat older than those included in the STAR and ADDITION studies, with a mean age of 64.5 years, but with a similar proportion of South Asians and participants with similar mean BMIs. The differences in characteristics between the diagnostic groups are generally as would be expected.
| Variable | Overall | Normal | PDM | T2DM | PDM/T2DM |
|---|---|---|---|---|---|
| n (%) | 264 | 209 (79.2) | 40 (15.2) | 15 (5.7) | 55 (20.8) |
| Male (%) | 73.1 | 73.7 | 77.5 | 53.3 | 70.9 |
| Median age, years (IQR) | 64.5 (59.0–68.9) | 64.0 (58.2–68.8) | 66.4 (60.5–69.3) | 65.2 (59.1–69.8) | 65.4 (60.4–69.5) |
| South Asian ethnicity (%) | 29.2 | 27.7 | 27.5 | 53.3 | 34.6 |
| Median BMI, kg/m2 (IQR) | 29.0 (26.4–33.0) | 28.7 (26.3–32.0) | 29.9 (26.8–36.6) | 34.0 (30.1–41.8) | 30.7 (27.4–38.2) |
| HbA1c, median (IQR) | 5.7 (5.5–6.0) | 5.7 (5.4–5.9) | 6.0 (5.7–6.3) | 7.5 (6.7–9.4) | 6.2 (5.8–6.7) |
Overall, 20.8% of those screened had either PDM (15.2%) or T2DM (5.7%). This was slightly higher than the percentage found in the ADDITION-Leicester population-based screening programme (19.3%). It was anticipated that this difference could be increased further with the refinement of the risk score using the full ADDITION-Leicester data set. Of those found with PDM, the majority had isolated IGT (72.5%).
Overall, the pilot study showed that it was feasible to run a risk score using practice data stored in the EMIS system, to invite people by postal mail to come forward for screening, to get a reasonable response rate to the invitation, to screen those who attended, and to detect people with previously undiagnosed PDM or T2DM.
Phase two: initial Leicester Practice Risk Score results
Phase one showed that it was feasible to use a risk-score approach to identify people with undiagnosed PDM and T2DM in primary care. The next phase was to develop the risk score for use in the main Let’s Prevent study and to assess its validity using the STAR study data set that had been used for the development of the pilot risk score. The starting point for the development of this risk score was different from that for the pilot. The initial plan was to recruit 20 patients from each practice; data on ethnicity would be available in terms of proportions of the main ethnic groups at the practice level but not available from individuals. It became apparent that in order to fulfil the study aim of recruiting those eligible patients judged to be at highest risk of conversion to T2DM, a more efficient sampling plan would be to invite patients for an OGTT, starting from those with the highest risk score and moving down in order of score to a common level across all the practices that would result in the required study sample size. The number of patients invited for OGTT would, therefore, reflect the size and general risk levels of practices, and it would be valid to include a proportion of patients from ethnic minority groups when deriving the risk score.
From the modelling results (Table 7), the initial risk score was defined for the study as:
Risk score = 0.0407 × age (years)
+ 0.296 (if male, no change if female)
+ 0.934 (ethnicity, as practice proportion of South Asians)
+ 0.0859 × BMI (kg/m2)
+ 0.440 (if family history of diabetes mellitus, no change otherwise)
+ 0.374 (if on antihypertensive medication, no change otherwise).
| Variable | Beta coefficient | 95% CI | p-value |
|---|---|---|---|
| Age (years) | 0.041 | 0.031 to 0.051 | < 0.001 |
| BMI (kg/m2) | 0.086 | 0.071 to 0.101 | < 0.0001 |
| Sex (female relative to male) | 0.296 | 0.136 to 0.456 | < 0.0001 |
| Ethnicity, as practice proportion of SAs | 0.934 | 0.689 to 1.178 | < 0.0001 |
| Family history of DM | 0.440 | 0.277 to 0.604 | < 0.0001 |
| Antihypertensive treatment | 0.374 | 0.184 to 0.564 | < 0.0001 |
Statistical validation of the initial risk score was carried out by examining its performance temporally on the STAR data and, additionally, by comparing its discrimination with other standard risk scores and the pilot risk score with respect to the area under the ROC curve. This was carried out for the total sample, and then separately for the South Asian and white European cohorts. Table 8 shows that the ROC area under the curve (AUC) of the initial risk score had better discrimination than either the FINDRISC or the CDRS in both of the data sets on which it was tested; however, in common with the other risk scores, it performed worse on the South Asian subset in the STAR study sample.
| Risk score | Subgroup | ROC AUC | 95% CI |
|---|---|---|---|
| Initial risk score | Total | 68.1 | 65.7 to 70.4 |
| WE only | 71.4 | 68.5 to 74.3 | |
| SA only | 66.5 | 62.6 to 70.5 | |
| Pilot risk score | Total | 66.1 | 63.7 to 68.6 |
| WE only | 70.0 | 67.1 to 72.9 | |
| SA only | 65.4 | 61.3 to 69.4 | |
| FINDRISC | Total | 65.2 | 62.8 to 67.6 |
| WE only | 66.4 | 63.4 to 69.3 | |
| SA only | 63.4 | 59.2 to 67.6 | |
| CDRS | Total | 64.1 | 61.6 to 66.6 |
| WE only | 66.0 | 63.0 to 69.1 | |
| SA only | 64.4 | 60.3 to 68.5 |
Using the initial risk score to identify the 10% most at risk for invitation to further screening gave a sensitivity of 19.2% with a specificity of 89.3%; the sensitivity is increased to 46% if the top 25% of at-risk participants are screened.
Phase three: updated Leicester Practice Risk Score results
Given the poor reporting of ethnicity in primary care, the initial score used practice-level ethnicity as a proxy for individual-level ethnicity. This may overinflate the score of white Europeans living in areas with a large South Asian population and vice versa. Recording of ethnicity has since been included in the Quality and Outcomes Framework,130 which has significantly improved the level of completeness for individual-level ethnicity, with > 90% of UK practices now recording ethnicity for all newly registered patients. 131 In addition, HbA1c has been used to diagnose T2DM since 2011. Therefore, it was decided to develop another score that would include individual-level ethnicity and define T2DM using OGTT or HbA1c to reflect these important changes to clinical practice. Although this score was developed de novo (given the change in outcome and the definition of an important predictor), the same methodology as used to develop the initial score was employed. As this score is now used in practice, a more thorough description of the development and validation is given. The subsequent section outlines the development of the LPRS software for use in primary care for running this updated risk score.
Table 9 shows the model-building process. Of the variables considered for inclusion, prescription of steroids and statins, smoking status, history of CVD and deprivation were excluded from the final model based on their association with PDM/T2DM.
| Variable | Number with data | OR (95% CI) | p-value | Taken forward to next stage |
|---|---|---|---|---|
| Independent associations, each risk factor included separately | ||||
| Age | 6378 | 1.04 (1.03 to 1.04) | < 0.0001 | ✗ |
| Sex | 6378 | 1.12 (0.99 to 1.26) | 0.05 | ✗ |
| BMI | 6157 | 1.08 (1.07 to 1.10) | < 0.0001 | ✗ |
| Ethnicity | 6175 | 1.67 (1.45 to 1.91) | < 0.0001 | ✗ |
| Family history | 6378 | 1.29 (1.14 to 1.46) | < 0.0001 | ✗ |
| Smoking status | 6141 | 0.71 (0.58 to 0.86) | < 0.0001 | ✗ |
| Antihypertensives | 6378 | 1.99 (1.75 to 2.27) | < 0.0001 | ✗ |
| Statins | 6378 | 1.76 (1.49 to 2.09) | < 0.0001 | ✗ |
| Steroids | 6378 | 1.16 (0.89 to 1.50) | 0.28 | |
| History of CVD | 6378 | 1.28 (1.08 to 1.52) | 0.004 | ✗ |
| Deprivation | 6125 | 1.01 (1.00 to 1.01) | < 0.0001 | ✗ |
| All significant risk factors from phase one included in one model | ||||
| Age | 5867 | 1.04 (1.03 to 1.05) | < 0.0001 | ✗ |
| Sex | 5867 | 1.21 (1.05 to 1.39) | 0.01 | ✗ |
| BMI | 5867 | 1.08 (1.07 to 1.10) | < 0.0001 | ✗ |
| Ethnicity | 5867 | 2.01 (1.70 to 2.38) | < 0.0001 | ✗ |
| Family history | 5867 | 1.66 (1.44 to 1.91) | < 0.0001 | ✗ |
| Smoking status | 5867 | 0.94 (0.76 to 1.16) | 0.55 | |
| Antihypertensives | 5867 | 1.67 (1.42 to 1.96) | < 0.0001 | ✗ |
| Statins | 5867 | 1.32 (1.06 to 1.63) | 0.01 | ✗ |
| History of CVD | 5867 | 0.84 (0.68 to 1.03) | 0.10 | |
| Deprivation | 5867 | 1.00 (1.00 to 1.01) | 0.08 | |
| All significant risk factors from phase two included in one model | ||||
| Age | 6143 | 1.04 (1.03 to 1.05) | < 0.0001 | ✗ |
| Sex | 6143 | 1.19 (1.04 to 1.36) | 0.01 | ✗ |
| BMI | 6143 | 1.09 (1.07 to 1.10) | < 0.0001 | ✗ |
| Ethnicity | 6143 | 2.13 (1.82 to 2.48) | < 0.0001 | ✗ |
| Family history | 6143 | 1.61 (1.40 to 1.85) | < 0.0001 | ✗ |
| Antihypertensives | 6143 | 1.65 (1.41 to 1.93) | < 0.0001 | ✗ |
| Statins | 6143 | 1.19 (0.98 to 1.45) | 0.08 | |
| Score now includes age, sex, BMI, ethnicity, family history and antihypertensives (next stage: adding the excluded variables one by one to see if they are now important when adjusted for other factors in the model) | ||||
| Steroids | 6143 | 1.08 (0.82 to 1.43) | 0.57 | |
| Smoking status | 6099 | 0.93 (0.76 to 1.15) | 0.52 | |
| History of CVD | 6143 | 0.91 (0.75 to 1.11) | 0.36 | |
| Deprivation | 5911 | 1.00 (1.00 to 1.00) | 0.09 | |
Table 10 shows the final model produced. Age, sex (male vs. female), BMI, ethnicity (‘other’ vs. white European), antihypertensive therapy (yes vs. no) and family history of diabetes (any type, yes vs. no) were all found to be significant predictors of PDM or T2DM both when modelled separately and together. Adding other variables did not improve the area under the ROC curve. There were no statistically significant two-way interactions, assessing significance at the 1% level, because of the high number of comparisons. Polynomial terms were considered for age and BMI but this did not improve the fit of the model. The area under the ROC curve for the final model was 70.1 (95% CI 68.4 to 71.7). Figure 4 shows the observed vs. the estimated prevalence of PDM and T2DM grouped by the predicted probability. This shows overall good agreement between the observed and predicted estimates. This is reflected in the result of the Hosmer–Lemeshow test based on 10 groups (χ2 = 2.4, p = 0.97) and a Brier score of 0.15.
| Variable | Coefficient | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|
| Age (per year increase) | 0.0408359 | 1.04 | 1.03 to 1.05 | < 0.0001 |
| Male | 0.1839942 | 1.20 | 1.05 to 1.37 | 0.01 |
| BMI (per kg/m2 increase) | 0.0820698 | 1.09 | 1.07 to 1.10 | < 0.0001 |
| South Asian/other BME | 0.7565977 | 2.13 | 1.83 to 2.49 | < 0.0001 |
| Prescribed antihypertensives | 0.5498978 | 1.73 | 1.50 to 2.01 | < 0.0001 |
| Family history of diabetes mellitus | 0.4770517 | 1.61 | 1.40 to 1.85 | < 0.0001 |
| ROC AUC: 70.1 (95% CI 68.4 to 71.7) | ||||
| Hosmer–Lemeshow test: χ2 = 2.4, p = 0.97 | ||||
FIGURE 4.
Comparison of the observed vs. the estimated prevalence of PDM or T2DM grouped by decile of predicted probability of PDM or T2DM. Adapted with kind permission from Springer Science+Business Media. Diabetologia, Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting, vol. 55, 2012, pp. 959–66, Gray LJ, Davies MJ, Hiles S, Taub NA, Webb DR, Srinivasan BT, Khunti K, figure 1. 113

The performance of the score in differentiating between those who had PDM or T2DM diagnosed using either an OGTT or HbA1c and those who had normal glucose tolerance in the temporal data set is shown in Table 11 and Figure 5. The score can be used in two ways: either by setting the sensitivity to a certain level or by deciding what percentage of the general practice to invite for further testing. If using an OGTT for diagnosis, then 50% of a general practice would need to be invited for testing to detect T2DM with 80% sensitivity, this is raised slightly to 54% being invited if using HbA1c. To retain 80% sensitivity for the PDM outcomes, the percentage invited would need to be increased to 60% if using an OGTT and 66% for an HbA1c between 6.0% (42 mmol/mol) and 6.4% (46 mmol/mol). Inviting the top 10% for testing, 9% of these would have T2DM using an OGTT [PPV 8.9% (95% CI 5.8%, 12.8%)] and 26% would have PDM [PPV 25.9% (95% CI 20.9%, 31.4%)]. Using HbA1c increases the PPV to 19% for T2DM [PPV 18.6% (95% CI 14.2%, 23.7%)] and 28% for an HbA1c between 6.0% and 6.4% [PPV 28.3% (95% CI 23.1%, 34.0%)]. If screening for both T2DM and PDM using an OGTT, inviting the top 10% for further testing gives a sensitivity of 17%. The high NPV (81.3%) suggests that this cut point is good for ruling out disease.
| Condition | Sensitivity 80% | Sensitivity 75% | Sensitivity 70% | Top 10% | Top 20% | Top 30% |
|---|---|---|---|---|---|---|
| T2DM OGTT | ||||||
| Invited for further testing | 50.4 | 41.9 | 39.1 | 10.0 | 20.0 | 30.0 |
| Sensitivity | 80.7 (72.1 to 87.37) | 75.2 (66.0 to 83.0) | 69.7 (60.2 to 78.2) | 22.9 (15.4 to 32.0) | 40.4 (31.1 to 50.2) | 56.9 (47.0 to 66.3) |
| Specificity | 50.8 (48.9 to 52.6) | 59.4 (57.5 to 61.2) | 62.1 (60.3 to 64.0) | 90.6 (89.4 to 91.6) | 80.9 (79.4 to 82.4) | 71.2 (69.5 to 72.9) |
| PPV | 6.2 (5.0 to 7.5) | 6.9 (5.5 to 8.5) | 6.9 (5.5 to 8.5) | 8.9 (5.8 to 12.8) | 7.8 (5.7 to 10.3) | 7.3 (5.7 to 9.3) |
| NPV | 98.5 (97.7 to 99.1) | 98.4 (97.6 to 98.9) | 98.1 (97.3 to 98.7) | 96.7 (95.9 to 97.4) | 97.1 (96.4 to 97.8) | 97.6 (96.9 to 98.3) |
| LR+ | 1.6 (1.5 to 1.8) | 1.9 (1.6 to 2.1) | 1.8 (1.6 to 2.1) | 2.4 (1.7 to 3.5) | 2.1 (1.7 to 2.7) | 2.0 (1.7 to 2.4) |
| LR– | 0.4 (0.3 to 0.6) | 0.4 (0.3 to 0.6) | 0.5 (0.4 to 0.6) | 0.9 (0.8 to 0.9) | 0.7 (0.6 to 0.9) | 0.6 (0.5 to 0.8) |
| T2DM HbA1c ≥ 6.5% | ||||||
| Invited for further testing | 53.8 | 48.7 | 44.9 | 10.0 | 20.0 | 30.0 |
| Sensitivity | 80.6 (74.8 to 85.6) | 75.2 (69.0 to 80.8) | 69.8 (63.3 to 75.8) | 23.4 (18.0 to 29.6) | 39.6 (33.2 to 46.4) | 54.5 (47.7 to 61.2) |
| Specificity | 48.5 (46.6 to 50.5) | 53.6 (51.6 to 55.5) | 57.2 (55.3 to 59.2) | 91.2 (90.0 to 92.3) | 81.8 (80.2 to 83.3) | 72.2 (70.4 to 73.9) |
| PPV | 11.9 (10.3 to 13.6) | 12.3 (10.6 to 14.1) | 12.3 (10.6 to 14.3) | 18.6 (14.2 to 23.7) | 15.8 (12.9 to 19.1) | 14.5 (12.1 to 17.0) |
| NPV | 96.7 (95.5 to 97.6) | 96.2 (95.0 to 97.1) | 95.7 (94.5 to 96.6) | 93.2 (92.2 to 94.2) | 94.0 (93.0 to 95.0) | 94.8 (93.8 to 95.8) |
| LR+ | 1.6 (1.4 to 1.7) | 1.6 (1.5 to 1.8) | 1.6 (1.5 to 1.8) | 2.7 (2.0 to 3.5) | 2.2 (1.8 to 2.6) | 2.0 (1.7 to 2.2) |
| LR– | 0.4 (0.3 to 0.5) | 0.5 (0.4 to 0.6) | 0.5 (0.4 to 0.6) | 0.8 (0.7 to 0.9) | 0.7 (0.7 to 0.8) | 0.6 (0.5 to 0.7) |
| PDM OGTT | ||||||
| Invited for further testing | 60.0 | 54.9 | 50.5 | 10.0 | 20.0 | 30.0 |
| Sensitivity | 80.0 (76.1 to 83.6) | 75.1 (70.9 to 79.0) | 70.2 (65.8 to 74.3) | 15.7 (12.5 to 19.3) | 33.7 (29.4 to 38.2) | 49.1 (44.5 to 53.8) |
| Specificity | 44.0 (41.9 to 46.0) | 49.1 (47.0 to 51.1) | 53.4 (51.4 to 55.5) | 91.2 (89.9 to 92.3) | 82.8 (81.2 to 84.3) | 73.9 (72.1 to 75.7) |
| PPV | 22.0 (20.0 to 24.0) | 22.5 (20.5 to 24.7) | 22.9 (20.7 to 25.2) | 25.9 (20.9 to 31.4) | 27.8 (24.2 to 31.7) | 27.1 (24.1 to 30.2) |
| NPV | 91.8 (90.0 to 93.3) | 90.9 (20.5 to 24.7) | 90.1 (88.4 to 91.6) | 84.6 (83.1 to 86.0) | 86.4 (84.9 to 87.8) | 88.1 (86.6 to 89.5) |
| LR+ | 1.4 (1.4 to 1.5) | 1.5 (1.4 to 1.6) | 1.5 (1.4 to 1.6) | 1.8 (1.4 to 2.3) | 2.0 (1.7 to 2.3) | 1.9 (1.7 to 2.1) |
| LR– | 0.5 (0.4 to 0.5) | 0.5 (0.4 to 0.6) | 0.6 (0.5 to 0.6) | 0.9 (0.8 to 1.0) | 0.8 (0.7 to 0.9) | 0.7 (0.6 to 0.8) |
| HbA1c 6.0–6.4% | ||||||
| Invited for further testing | 65.9 | 61.2 | 56.1 | 10.0 | 20.0 | 30.0 |
| Sensitivity | 80.0 (76.5 to 83.2) | 75.0 (71.2 to 78.4) | 70.1 (66.2 to 73.8) | 13.7 (11.0 to 16.8) | 28.7 (25.0 to 32.6) | 42.6 (38.5 to 46.8) |
| Specificity | 37.8 (35.7 to 39.8) | 42.3 (40.2 to 44.4) | 47.6 (45.5 to 49.7) | 91.0 (89.7 to 92.2) | 82.4 (80.7 to 83.9) | 73.4 (71.5 to 75.2) |
| PPV | 25.0 (23.0 to 27.0) | 25.2 (23.1 to 27.3) | 25.7 (23.6 to 27.9) | 28.3 (23.1 to 34.0) | 29.6 (25.9 to 33.6) | 29.3 (26.2 to 32.5) |
| NPV | 87.9 (85.7 to 89.9) | 86.7 (84.5 to 88.7) | 86.0 (83.9 to 87.9) | 80.3 (78.7 to 81.8) | 81.7 (80.0 to 83.3) | 83.2 (81.4 to 84.8) |
| LR+ | 1.3 (1.2 to 1.4) | 1.3 (1.2 to 1.4) | 1.3 (1.2 to 1.4) | 1.5 (1.2 to 2.0) | 1.6 (1.4 to 1.9) | 1.6 (1.4 to 1.8) |
| LR– | 0.5 (0.4 to 0.6) | 0.6 (0.5 to 0.7) | 0.6 (0.5 to 0.7) | 0.9 (0.9 to 1.0) | 0.9 (0.8 to 0.9) | 0.8 (0.7 to 0.8) |
| T2DM or PDM (OGTT) | ||||||
| Invited for further testing | 58.8 | 53.2 | 48.6 | 10.0 | 20.0 | 30.0 |
| Sensitivity | 80.0 (76.5 to 83.2) | 74.6 (70.8 to 78.1) | 70.1 (66.2 to 73.8) | 17.0 (14.1 to 20.4) | 35.0 (31.1 to 39.0) | 50.6 (46.4 to 54.8) |
| Specificity | 46.6 (44.5 to 48.7) | 52.2 (50.2 to 54.3) | 56.9 (54.8 to 58.9) | 91.8 (90.6 to 92.9) | 83.9 (82.3 to 85.4) | 75.4 (73.6 to 77.2) |
| PPV | 27.6 (25.5 to 29.9) | 28.5 (26.2 to 30.8) | 29.3 (26.9 to 31.8) | 34.8 (29.2 to 40.6) | 35.6 (31.7 to 39.7) | 34.4 (31.2 to 37.8) |
| NPV | 90.1 (88.3 to 91.8) | 89.0 (87.2 to 90.6) | 88.2 (86.4 to 89.8) | 81.3 (79.7 to 82.8) | 83.5 (81.9 to 85.0) | 85.7 (84.1 to 87.2) |
| LR+ | 1.5 (1.4 to 1.6) | 1.6 (1.4 to 1.7) | 1.6 (1.5 to 1.7) | 2.1 (1.7 to 2.6) | 2.2 (1.9 to 2.5) | 2.1 (1.9 to 2.3) |
| LR– | 0.4 (0.4 to 0.5) | 0.5 (0.4 to 0.6) | 0.5 (0.5 to 0.6) | 0.9 (0.9 to 0.9) | 0.8 (0.7 to 0.8) | 0.7 (0.6 to 0.7) |
| T2DM or PDM (HbA1c) | ||||||
| Invited for further testing | 64.4 | 57.3 | 52.0 | 10.0 | 20.0 | 30.0 |
| Sensitivity | 80.1 (77.1 to 82.8) | 74.9 (71.7 to 77.9) | 70.1 (66.8 to 73.3) | 16.4 (13.9 to 19.2) | 31.7 (28.5 to 35.1) | 45.9 (42.4 to 49.5) |
| Specificity | 41.9 (39.7 to 44.1) | 49.8 (47.5 to 52.0) | 55.3 (53.0 to 57.4) | 92.6 (91.4 to 93.7) | 84.8 (83.2 to 86.3) | 76.4 (74.5 to 78.3) |
| PPV | 35.4 (33.2 to 37.7) | 37.3 (34.9 to 39.7) | 38.4 (35.9 to 41.0) | 47.0 (41.0 to 53.0) | 45.4 (41.2 to 49.7) | 43.7 (40.3 to 47.2) |
| NPV | 84.1 (81.6 to 86.3) | 83.3 (81.0 to 85.3) | 82.3 (80.1 to 84.3) | 73.6 (71.8 to 75.3) | 75.7 (73.9 to 77.5) | 78.0 (76.1 to 79.8) |
| LR+ | 1.4 (1.3 to 1.5) | 1.5 (1.4 to 1.6) | 1.6 (1.5 to 1.7) | 2.2 (1.8 to 2.8) | 2.1 (1.8 to 2.4) | 2.0 (1.8 to 2.2) |
| LR– | 0.5 (0.4 to 0.6) | 0.5 (0.4 to 0.6) | 0.5 (0.5 to 0.6) | 0.9 (0.9 to 0.9) | 0.8 (0.7 to 0.8) | 0.7 (0.7 to 0.8) |
FIGURE 5.
Receiver operating characteristic curve for T2DM, PDM and T2DM or PDM using the OGTT and HbA1c. OGTT: (a) area under ROC curve = 70.6%; (b) area under ROC curve = 66.3%; and (c) area under ROC curve = 68.5%. HbA1c: (d) area under ROC curve = 69.4%; (e) area under ROC curve = 62.2%; and (f) area under ROC curve = 66.7%. Reproduced with kind permission from Springer Science + Business Media: Diabetologia, Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting, vol. 55, 2012, pp. 959–66, Gray LJ, Davies MJ, Hiles S, Taub NA, Webb DR, Srinivasan BT, Khunti K, figure 2. 113

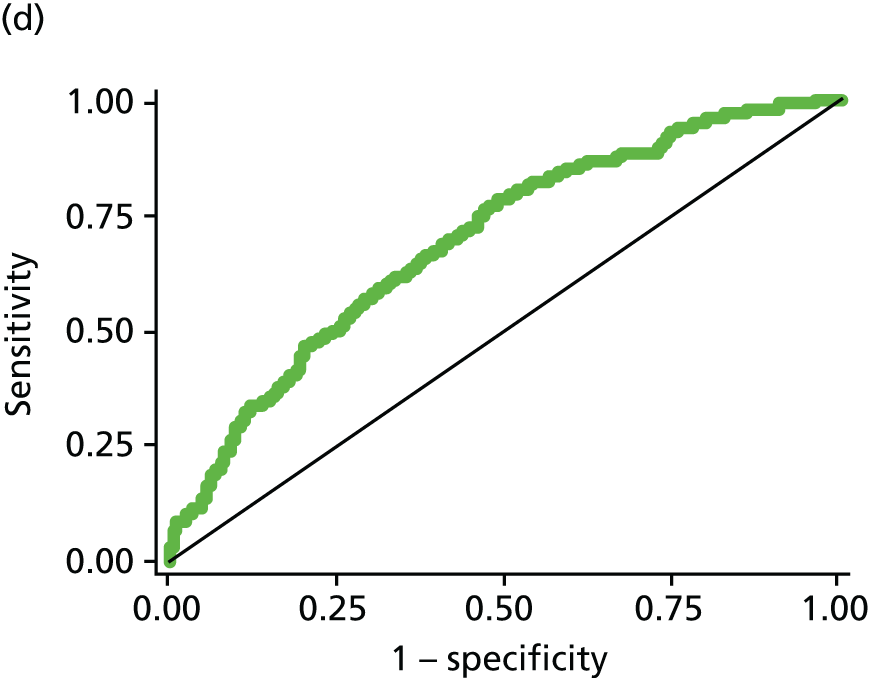
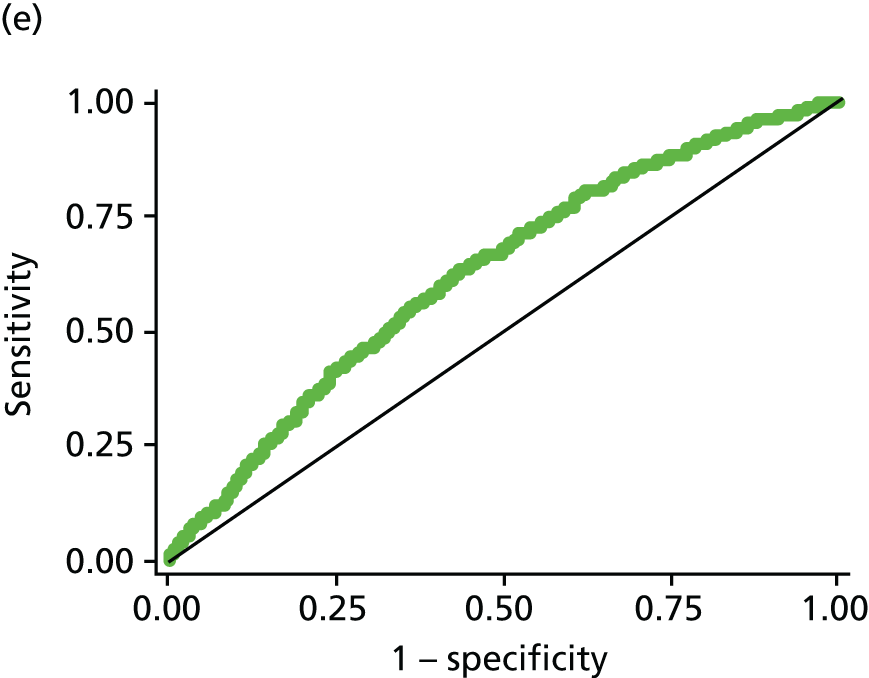
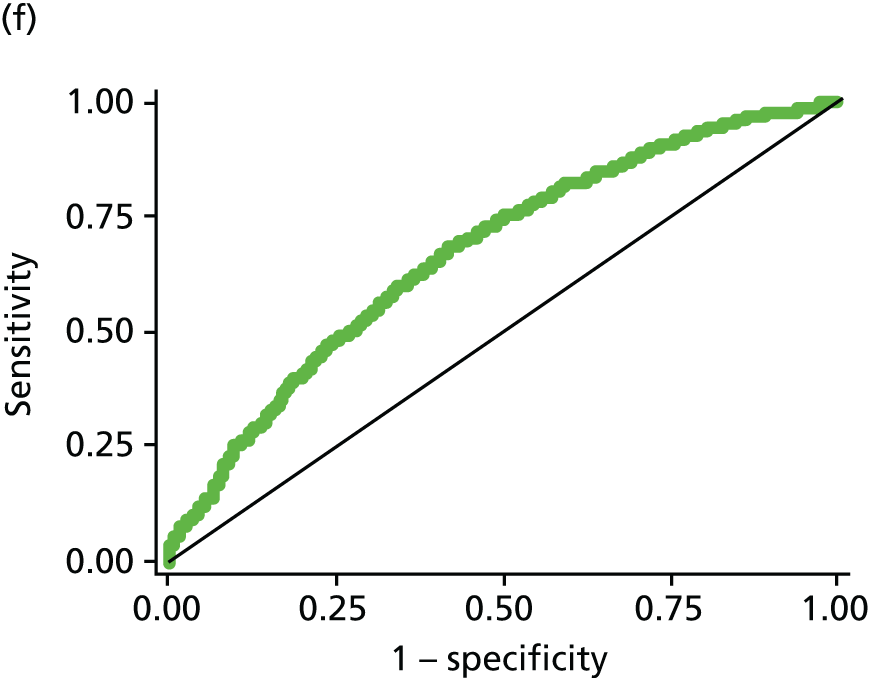
Development of the Leicester Practice Risk Score software
It is well reported that many risk scores are not used in practice. One reason for this may be because little thought is given to implementing them at the development stage. 132 To enable widespread use of the LPRS we developed a piece of software which uses existing medical records within primary care to calculate the LPRS for each patient within a practice population aged 40–75 years, having excluded people with known diabetes mellitus, the terminally ill and those with coded gestational diabetes (as they are already identified as being at higher risk and it is not necessary to screen them). When developing the software it came to light that many patients will have been screened; as it is unnecessary to rescreen these people, the software analyses existing OGTT/glucose/HbA1c data. This process also identifies any people with ‘missed’ diabetes mellitus, that is, people with glucose results in the diabetes mellitus range who have not been coded as diagnosed with diabetes mellitus. The output is presented in a single Microsoft Excel® version 10 (Microsoft Corporation, Redmond, WA, USA) spreadsheet that can be used to check records and recall patients for screening for diabetes mellitus. This software can be downloaded from http://leicesterdiabetescentre.org.uk/The-Leicester-Diabetes-Risk-Score.
Discussion
We have developed a simple and sensitive automated screening tool for use in multiethnic populations that will enable primary care practitioners to rank individuals by their risk of having undiagnosed PDM or T2DM and therefore allow targeting of screening resources. Ranking people by risk allows flexibility in the screening strategy chosen; practices can choose to hone in to the top of the list and invite fewer people for screening for a bigger ‘hit’ rate or, if resources allow, to widen their inclusion criteria, giving greater sensitivity at the offset of the specificity.
Although some existing scores have been validated against HbA1c,133,134 the updated score is the first to be developed that incorporates the new WHO diagnostic criteria into the outcome. Previous work has shown that different cohorts are detected using either an OGTT or a HbA1c to diagnose T2DM. 135 Previously developed scores may now miss people who meet the new diagnostic criteria. This is also the first computer based score developed in a multiethnic population within the UK to identify prevalent disease. The Cambridge risk score was designed to identify undiagnosed diabetes only and does not adjust for ethnicity. 121 Although not taken into account in the original score, a post hoc study using data from both Caribbean and South Asian populations showed that using alternative ethnic specific cut points could give acceptable levels of prediction for undiagnosed hyperglycaemia in these groups, but that further work needed to be carried out to refine these. 136 The multivariable risk score to predict the 10-year risk of acquiring T2DM (QDScore) predicts the 10-year risk of developing diabetes mellitus and includes similar variables to both the CDRS and scores developed here, but with the addition of deprivation and CVD (both of these were found not to improve the fit of models produced). 137 Compared with the Cambridge Risk score, the QDScore showed greater levels of discrimination, but only detects incident disease. In addition, the algorithm to compute the risk score has not been published and cannot be used to detect PDM. Other scores, including the Leicester Self Assessment score and the FINDRISC score, have been developed, which rely on the person at risk completing a questionnaire themselves and attending the GP practice. 27,138 The score developed here may increase the uptake to screening invitation by removing the need for people to calculate their own risk.
Although the score was developed using high fidelity data from a randomly selected population who all received an OGTT, there are a number of limitations to be taken into account when applying the score. First, the cross-sectional nature of the data limits the score to detecting prevalent undiagnosed disease. The score cannot, therefore, be used to estimate the risk of future disease, although detecting PDM will identify a high-risk group that is likely to develop T2DM in the future. Although this could be viewed as a limitation, screening strategies may want to focus on those who have current undetected disease as a priority. In addition, those scores predicting incident disease may give biased estimates, as those variables that are included in the score are also those that prompt testing. Future work will look at validating the score on a prospective data set. Second, only 22% of those invited for screening in the ADDITION-Leicester study attended. Although this is similar to other studies in similar populations139 and reflects the difficulty in recruiting a multiethnic urban population with wide variations in socioeconomic status into research studies, this may have affected the representativeness of the data that the score has been derived from. For example, those screened were slightly older than those invited. 140 It is difficult to predict the possible implications of the response rate to the initial study on the score produced. Reassuringly, the score contains a similar set of variables to other comparable risk scores. 27,121 Future work will further validate the score on other population-based data sets. There are some limitations with the analysis performed to derive the risk scores. We used a variable selection procedure based on the statistical significance of their association with the outcome to initially select variables for potential inclusion into the models produced. This is not the recommended approach to variable selection and can increase the risk of excluding important variables that become important only after adjustment for other variables. 141 We did, however, reassess each variable excluded in this manner to see if their inclusion in the final model would improve the discrimination of the model. Although the approach taken may have been suboptimal, the variables included in the model are similar to those included in previous scores and the score was shown to work adequately when tested in a temporal data set. There is a hierarchy of data sets to be used for evaluating a risk scores validity: (1) internal; (2) temporal (using an external data set from the same centre); and (3) external (using a truly external data set from a different centre). 142 Here we have used a temporal data set; although these data incorporate an independent population, the data set was run to the same standard operating procedures as the ADDITION study and within the same centre. Future work should focus on assessing the updated risk score in an external data set. The method of dealing with missing covariate data could also have been improved. Here risk scores were developed on complete-case data; in hindsight, a better way to deal with missing data would be to use multiple imputation. 143 Studies have shown that risk scores produced using complete-case data may be biased and could produce scores that perform poorly when used in clinical practice. 125 Future work should consider the robustness of the scores produced to missing data. Finally, the score was developed using data from Leicester (UK). The ethnic makeup of this area means that the ethnicity component of the score is based on data from South Asian participants (mostly of Indian descent). Although there were participants included from other ethnicities in both data sets (such as Chinese, Caribbean and African), there were insufficient data to model separate scores for each ethnicity. South Asians are known to have a high level of risk,144 and, therefore, assuming the same level of risk for all BME groups, may overestimate risk for some, but this was thought to be preferential to underestimating risk or estimating risk based on insufficient data.
Summary
In summary, we have developed a valid and sensitive score for identifying those at the highest risk of prevalent PDM or T2DM within a multiethnic UK population. Using an automated tool is simple to implement and can be used to target screening approaches in a cost-effective manner. For example, in the UK, this tool could be used to complement the NHS Health Check programme as the score has been developed using data that are reflective of the inclusion criteria of the health checks. The results from the screening study using the initial version of the risk score, which incorporated practice-level ethnicity data, are reported in Chapter 6.
Chapter 4 Developing the intervention
This chapter uses excerpts from Troughton et al. ,145 reproduced under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited, and Gray et al. ,146 reproduced courtesy of BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Drafting a curriculum
Research shows that lifestyle modification in people with PDM can significantly reduce the risk of developing T2DM. Both the DPP and the DPS demonstrated that, in participants with PDM, a programme addressing weight loss, diet and physical activity was able to reduce progression to T2DM by 58%. 32,33 Although these results were certainly very encouraging, their lack of transferability from research into practice was a valid concern. Many of the programmes called for resource-intensive interventions, which would simply be unachievable in our current health-care system. For example, the DPP saw participants have, on average, 20 individual counselling sessions over the course of 4 years. 33 Many countries would, therefore, be unable to replicate the intervention owing to such a huge demand on resources. It was noted, therefore, that there was an urgent need to develop a prevention programme that was effective, economically viable and could easily be implemented into a real-world UK health-care setting.
Previous pilot work prior to National Institute for Health Research grant
Early development work to establish a prevention programme that focused on key lifestyle targets and that was suitable for those with PDM commenced in 2006 prior to the successful National Institute for Health Research (NIHR) grant award.
Key findings from a qualitative study in subjects with PDM22 showed that many had no prior understanding of PDM and their risk of CVD, felt confused by the diagnosis and wanted clarification of what was meant by being at ‘high risk’. All wanted to know why they had developed PDM. Many recognised that lifestyle changes could delay progression to T2DM but were not confident that these were achievable. Many felt that they did not know what to change in their lifestyle. Although many received an education booklet at the time of diagnosis, they felt that the advice was too general.
Prior to the successful NIHR grant application, the full results of the DESMOND RCT were being finalised. Initial pilot work had already demonstrated significant improvements in outcomes (e.g. HbA1c, lipids, BP), changes in illness beliefs and increase in physical activity,147 thereby suggesting that any prevention programme developed for PDM could benefit from using a similar approach.
Early development work tested the format and content of a 3-hour prevention session developed by our group. Thirty-four people with PDM (including 10 from a South Asian population) attended four groups. Feedback showed that all felt that the programme helped them to improve their understanding of PDM, including the role of diet and exercise. All participants agreed that they intended to become more physically active and eat a healthier diet. Practical sessions were valued, and personalising risk was deemed helpful.
Exploratory quantitative evaluation showed significantly increased self-efficacy in making lifestyle changes, perceived control and perceived knowledge, as well as a significant decrease in perceived symptom load and consequences. Ten subjects provided pedometer information and reported an increase in both daily step count (by 1690 steps per day) and energy expenditure [2635 metabolic equivalent of task (MET) minutes per week]. These changes showed that the initial programme was setting the necessary preconditions for successful behaviour regulation and change. Qualitative interviews carried out by telephone revealed that many would attend extra sessions on food choices and activity and, therefore, a 6-hour session model was considered for our current format.
Following on from this exploratory work came the Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme,148 which was a RCT funded by Diabetes UK. A total of 103 individuals were recruited, of whom 30% were from a South Asian ethnic background. It was found that when participants were given structured education that enabled them to set realistic personalised step-per-day goals and to self-monitor their physical activity behaviour with a pedometer, their physical activity levels increased significantly (p < 0.05), by 2600 steps per day (equivalent to around 26 minutes per day of walking activity) and their 2-hour glucose levels decreased by 1.4 mmol/l. A decrease in 2-hour glucose of this magnitude has been associated with around a 60% reduction in the relative risk of developing T2DM. Furthermore, significant improvements in both fasting and 2-hour glucose levels, and increases in physical activity, remained even at 12 months’ follow up. 50 This study therefore suggests that structured education that targets participants’ perceptions of PDM, highlights the importance of physical activity and promotes self-management skills can substantially reduce the risk of developing T2DM. This study provided a foundation on which to build a structured education group intervention to initiate behaviour change and improve glucose tolerance in those with PDM in a UK setting.
Pilot work supported by National Institute for Health Research Grant 1272
The Let’s Prevent programme was developed by a core multidisciplinary team, in collaboration with the DESMOND collaborative and the National Physical Activity Centre in Loughborough (Collaboration in the PREPARE study). 50
In July 2007, a working party gathered to develop the Let’s Prevent intervention. Using the findings from previous pilot work, the intervention was compiled in line with best practice. The programme was based on the fundamentals of the DESMOND programme. DESMOND is currently the only education programme that fully meets the NICE guidelines for structured education in diabetes149 and its RCT demonstrated its clinical effectiveness in improving weight, smoking behaviours and illness beliefs. 48 The DESMOND model is based on an empowerment philosophy that sees the participant as capable and responsible for his or her own health decisions and behaviours. 150 The DESMOND model is also underpinned by adult learning theory and psychological models of learning. These include Leventhal’s common sense model,151 the dual process theory152 and social learning theory. 153
The Let’s Prevent intervention was designed as a group educational programme with a written curriculum suitable for the broadest range of participants, to be deliverable in a community setting for ease of access for patients and to have the potential to be integrated into routine care in the future. As per the DESMOND model, the programme was approximately 6 hours in duration, deliverable in either one full day or as two half-day equivalents. It was designed to be facilitated by two trained health-care professionals (educators) to a group of 5–12 participants with PDM, who had the option of bringing an accompanying person.
The session content was developed by a core multidisciplinary team, founded on a sound evidence base and guided by the following:
-
a review of the literature surrounding nutrition, exercise and educational principles
-
pragmatic decision-making on best practice where the evidence base was ambiguous, lacking or conflicting
-
the philosophy of DESMOND, which calls for non-directive methods of education to be used. It recognises that individuals with PDM have insight and expertise in relation to their own food choices.
The DPP and DPS informed the key messages regarding food. The key food goals within the curriculum were:
-
sustained weight reduction of > 5% of body weight
-
moderate reduction in total fat intake to < 30% of energy intake
-
lower saturated fat intake to < 10% of energy intake
-
higher fibre intake of > 15 g per 1000 kcal.
The key physical activity messages were taken from the PREPARE programme. 148 The PREPARE study demonstrated that pedometer use was an effective means of improving glucose tolerance in those with PDM when incorporated as part of an educational programme. 50,154 As in the PREPARE study, Let’s Prevent participants were given a pedometer to encourage regular goal-setting and to facilitate monitoring of their physical activity levels. The physical activity target was for participants to increase their daily step count by 4500 steps, which is equivalent to approximately 45 minutes of walking. It was suggested that this goal be broken down into smaller, more achievable, goals, such as increasing steps by 500 a day every fortnight, in order to provide sufficient time for participants to adjust to their new level of activity.
In addition, participants were given a handbook containing a summary of the key messages and a plethora of other useful resources to help further integrate their knowledge. There was a health profile sheet, which displays a continuum of risk for the following risk factors: fasting blood glucose, 2-hour blood glucose, BP, total cholesterol, HDL cholesterol, BMI, waist circumference and pedometer counts (steps). Glucose targets were adapted from the WHO diagnostic criteria for diabetes;155 BP, cholesterol, BMI and waist circumference were taken from the Department of Health document on cardiovascular risk assessment;156 and step count targets were adapted from those proposed by Tudor-Locke and Bassett. 157 Participants plot their biomedical and anthropometric data along continuums to build up a personalised picture of their risk profiles. They subsequently complete an action plan targeting their selected area of behaviour change to help them to achieve their goals. Tape measures were also provided to encourage regular monitoring.
The broad curriculum content for Let’s Prevent is detailed in Tables 12 and 13.
| Session 1 | Theory | Sample activity | Duration (minutes) |
|---|---|---|---|
| Introduction | – | – | 10 |
| Patient story | CSM | Participants asked to tell their story about how they discovered they had PDM and their current knowledge of PDM | 30 |
| Professional story | CSM, DPT | Uses participants’ stories to support them in learning how the body regulates glucose | 50 |
| Taking control 1: weight management | CSM, DPT, SLT | Uses participants’ stories to support them in discovering how weight/waist affects PDM (provides knowledge and skills for food choices to control weight) | 30 |
| Physical activity | CSM, DPT, SLT | Uses participants’ stories to support them in discovering how physical activity affects PDM (provides knowledge and skills for activity choices to manage PDM) | 40 |
| How am I doing? | SLT | Participants reflect on what issues have come up in the programme so far | 5 |
| Session 2 | Theory | Sample activity | Duration (minutes) |
|---|---|---|---|
| Reflections | SLT | Participants reflect on issues that have arisen in the programme so far | 10 |
| Professional story | CSM | Uses participants’ stories to support them in discovering how other risk factors (e.g. high BP and cholesterol) affect PDM and the development of complications | 30 |
| Taking control 2: food choices – focus on fats | DPT, SLT | Provide knowledge and skills for food choices to reduce risk factors | 50 |
| Self-management plan | SLT | Participants supported in developing their self-management plans | 30 |
| Questions | CSM | Check that all questions raised by participants throughout the programme have been answered and understood | 40 |
| What happens next? | SLT | Follow up care outlines | 5 |
The Medical Research Council (MRC) Framework for Complex Interventions to Improve Health has internationally recognised criteria to inform the development and evaluation of any lifestyle intervention programmes. 158 The MRC guidance recommends that all interventions undertake a process of development, feasibility and piloting, evaluation and implementation. In order to develop the Let’s Prevent programme, the methodology previously used in the development of the DESMOND programme was used. 159,160 This methodology encompasses a pragmatic iterative approach, similar to an ‘audit cycle’, whereby data are collected and analysed to obtain feedback and suggestions from key stakeholders, including patient and public involvement, health-care providers and facilitators (trainers, educators and interpreters). Each cycle involves identifying potential modifications that could be made; piloting these changes and collecting both qualitative and quantitative data, analysing the data and reflecting on; their implications; and making refinements and adjustments. The cycle is repeated until the pilot stage suggests that the intervention is fit for purpose (Figure 6).
FIGURE 6.
The cycle of programme development.
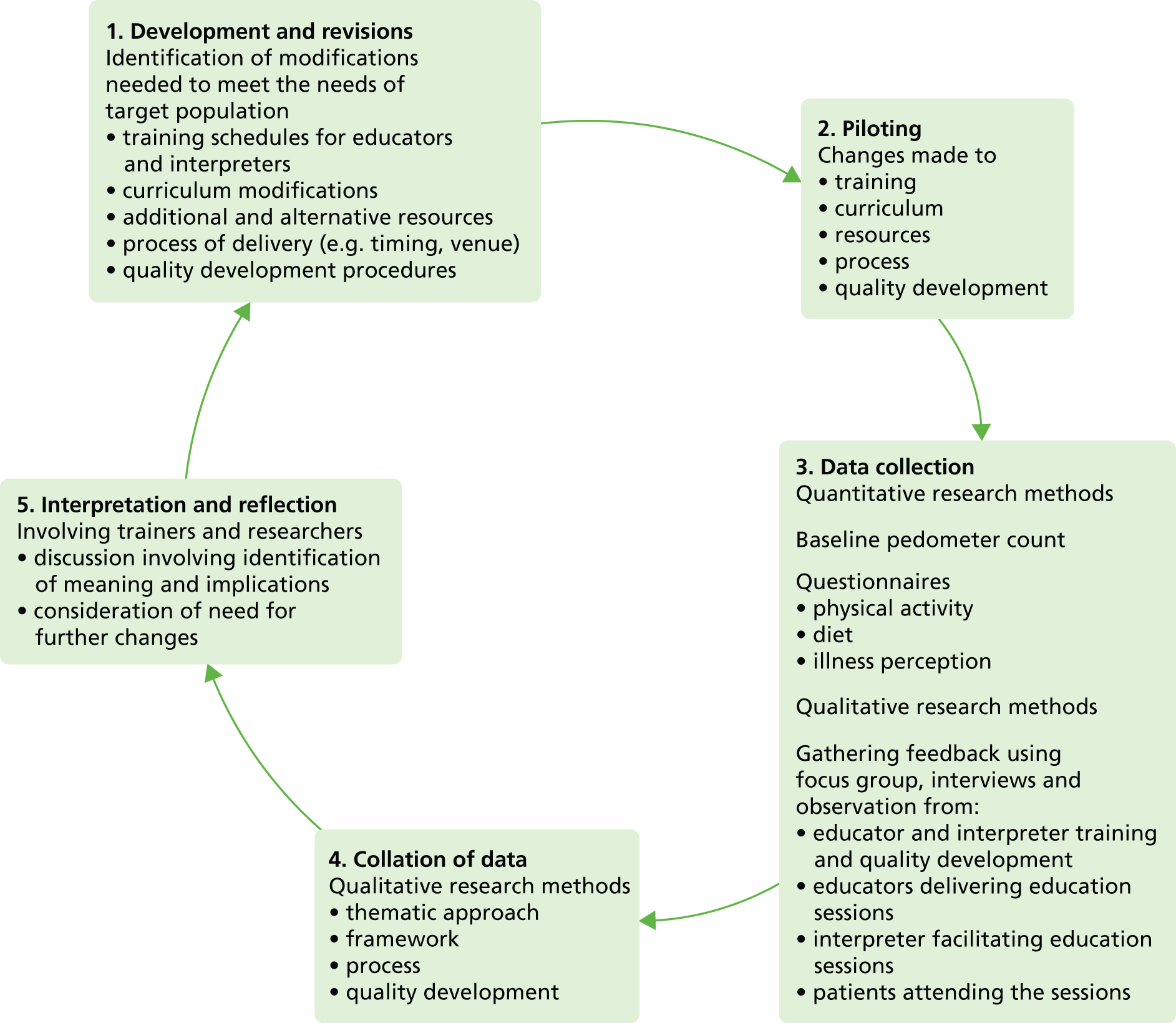
First phase of the pilot of the Let’s Prevent programme (October–November 2007)
Recruitment
During screening at a medical practice in Leicestershire, 83 individuals with English as their first language were identified as having been diagnosed with PDM in the past 12 months. An invitation letter (see Appendix 7) was sent to all 83 individuals inviting them to take part in the pilot study, which was followed by a telephone call 1 week later. A total of 38 individuals (46% of those identified) responded favourably to the initial invitation, although 35 actually attended the pilot programme (42%). The most common reasons for not wanting to attend were being unable to take time off work and ill health. The average age of those who attended was 65.83 ± 6.56 years, and 34% were female. On average, participants were significantly older in the group that responded positively [mean 65.34 years, standard error (SE) 1.06 years] than in the group that did not respond or responded negatively (mean 0.11 years, SE 1.33 years, t (81) = 2.996; p < 0.05). There was a significant association between participants’ sex and whether or not they responded positively (p < 0.05). The odds ratio indicates that participants were more likely to attend if they were male.
Three pilot Let’s Prevent programmes were delivered: two in the half-day format (group 1: n = 11; group 2: n = 11), and one full-day version (group 3: n = 13). The interventions were delivered by two trained educators.
Qualitative data collection
Qualitative data were gathered to ascertain both participants’ and educators’ experiences of the programme. Data were collected via observation, telephone and face-to-face interviews, and focus groups. To inform and facilitate the qualitative data collection process, flexible topic guides (see Appendices 8–10) were developed by trainers and qualitative researchers. Some additional lines of questioning were also suggested by educators. Interviews and focus groups were audio-recorded and observations were recorded using written notes.
Measurements
Baseline measurements were taken in the week prior to participants receiving the intervention, and follow-up measurements were taken 2 weeks after participants had attended their final programme.
Physical activity
Participants who took part in the pilot study received a SW200 pedometer (Yamax Corporation, Tokyo, Japan) and log sheet in the post 1 week prior to the intervention. These pedometers were one of the most widely used pedometers in clinical research at the time of the study and have been proven to have good reliability and validity. 161 Participants were instructed to wear the pedometer for 7 consecutive days prior to attending the pilot programme and to record the number of steps they took each day on the log sheet. In order to ensure that the pedometer was worn correctly, participants were provided with clear written instructions and a diagram depicting correct pedometer placement. The participants were also given a contact number in case any further assistance was required. Participants were asked to keep wearing the pedometer for 2 weeks after attending the pilot programme and to continue recording their daily step counts. At the end of the 2-week period participants returned their log sheet along with their follow-up questionnaire to the research centre. For the purposes of this study, at least 3 valid days of data were required per week; a valid day constituted at least 12 hours of collected data.
Physical activity was also measured by the International Physical Activity Questionnaire (IPAQ) (last 7-day short form) and was reported in MET-minutes/week derived from vigorous intensity, moderate intensity, and walking activity. The IPAQ has been found to be a valid and reliable measure of physical activity. 162
Illness perceptions
Illness perceptions were measured by the validated Brief Illness Perception Questionnaire. 163
Diet
Food intake was measured using the Dietary Instrument for Nutritional Education (DINE) food frequency questionnaire. The DINE food frequency questionnaire was designed to be a quick and easy way of measuring fibre, total fat and unsaturated fat intake in primary care. 164 As dietary fat and fibre intake are the dietary variables that are most likely to influence glucose control and the development of diabetes,165 the DINE food frequency questionnaire was considered an ideal instrument for use in the pilot study.
Statistical analysis
It was recognised that our sample was not powered to give meaningful outcomes but would give us some guidance on the direction of the results, so we undertook some data analysis using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) software. Paired sample t-tests were used to test for within-group differences between the pre- and post-programme measurements if the data were normally distributed and homogeneity of variance was assumed. Non-parametric data were analysed using Wilcoxon’s signed ranks test.
Qualitative analysis
Transcriptions or detailed notes from the interviews, focus groups and observations were reviewed to achieve familiarisation. A thematic approach was adopted, bearing in mind the aim of informing the next phase of the development process. Analysis of the data involved the use of framework charts166 to summarise and organise the information collected, followed by reflection and interpretation.
Quantitative results
Baseline and follow-up results are detailed in Table 14.
| Variable | Number of complete data sets | Baseline value | Follow-up median | Significance (two-tailed t-test) |
|---|---|---|---|---|
| Perceived effect of IGT (consequences)a | 23 | 1 | 3 | 0.01 |
| Timeline associated with IGTa | 22 | 4 | 3 | 0.30 |
| Perceived control over IGTa | 23 | 5 | 7 | 0.08 |
| Perceived response efficacy of lifestyle change at treating IGTa | 35 | 9 | 10 | 0.04 |
| Perceived symptom loada | 25 | 0 | 0 | 0.19 |
| Concern at having IGTa | 23 | 9 | 10 | 0.90 |
| IGT coherence (knowledge)a | 25 | 5 | 7 | < 0.01 |
| Emotional representationsa | 25 | 1 | 1 | 0.45 |
| Total fibre score | 25 | 28 | 37 | < 0.01 |
| Total fat score | 24 | 19 | 18 | 0.21 |
| Total unsaturated fat | 23 | 9 | 10 | 0.13 |
| Total walking activity (MET minutes/week) | 24 | 891 | 1386 | 0.01 |
| Overall moderate- to vigorous-intensity physical activity (MET minutes/week) | 23 | 2376 | 2772 | 0.99 |
| Pedometer counts (steps per day) | 14 | 5500 | 4700 | 0.97 |
Reassuringly, there was an increase in self-reported fibre intake and a decrease in self-reported total fat intake. Participants also reported an increase in perceived knowledge of PDM, perceived knowledge of the consequences of PDM and an increase in the perceived effectiveness of lifestyle change in controlling/treating their PDM status. Although there was an increase in self-reported walking activity, as measured by the IPAQ questionnaire, there was no difference between baseline and follow-up pedometer counts.
Qualitative findings
Sixteen participants were interviewed, including representatives from each of the three educational sessions, using topic guides (see Appendices 8–10).
Understanding of prediabetes mellitus
All the participants conveyed that they were motivated to attend the education sessions to find out information about their condition and what they could do to prevent progression to T2DM. The findings suggested that after attending the course they understood the concept of insulin resistance and how it is affected by physical activity and diet.
If doors are open then insulin can get through to cells and it’s important to have exercise.
Overall, a feeling of empowerment and motivation to do something about their condition was expressed by patients.
I find it helpful because I like to know about things, then you can face them better and deal with them better.
Ball is in our court; it’s up to us now.
Food games
The type of fats game and the food continuum section of the curriculum proved to be insightful and provided participants with the necessary information and tools to bring about changes to their diets. It was noted that some patients had already made changes prior to attending the sessions. Some of the reasons behind the decision to make these changes included taking part in screening and receiving the diagnosis of PDM, other health conditions and an increased level of personal awareness about the importance of a healthy lifestyle. Nevertheless, these participants still benefited from the sessions, describing them as helpful, informative and useful for reinforcing what they already knew.
I learnt some surprising things about foods . . . I found that was helpful.
The findings suggested that all participants had made changes to their diets after attending the sessions.
We are trying to think the last time we had cake.
Physical activity games
Although participants sometimes had difficulty in recalling the details of specific physical activity games, it was clear from the feedback that they had gleaned key messages. These included increasing their levels of activity in terms of the number of steps, duration and breaking up activity into smaller sized chunks. Among some patients, the findings indicated that they had also thought about the type of activities that they currently undertake and the fact that some of these, such as housework and gardening, could be increased to help contribute to their daily activity targets.
Ongoing use of resources
Attitudes to the continued use of the pedometer were mixed. Of those who said that they would continue to use the pedometer, the view was that it was a useful tool to assess how they were doing on a daily basis and that it helped them to improve their physical activity levels. Other reasons cited were that it gave them some control, provided encouragement and enabled them to monitor their progress and set goals. Those who indicated that they were not likely to continue using their pedometers said that they were already achieving the levels of activity required on a daily basis.
We’ve been doing walking since we went on the course which I have found very helpful and the weight is still going down.
Key messages
When asked what key messages they had taken from the course or what they would tell a friend about what they had learnt, participants described these as losing weight, eating properly (in particular reducing saturated fat) and looking at types of food eaten. Others spoke of the need to exercise and to read labels carefully when shopping, as they can sometimes be misleading.
Discussion
The analysis and reflections on qualitative findings indicated that, overall, there was a good level of acceptability and effectiveness for the education sessions. These sessions had resulted in patients having an increased understanding of the nature and implications of their diagnosis, and participants had also assimilated key messages about lifestyle changes with regard to diet and physical activity. The findings also helped to identify where modifications were required to further refine the training, curriculum and resources.
This pilot study demonstrated that the prevention programme was successful at targeting several important illness perceptions and health behaviours. The substantial increase in perceived knowledge of PDM is important because work from our research group has shown that changes in perceived knowledge of PDM are closely linked to health behaviour change in the longer term. 164 The increase in the perceived effectiveness of lifestyle change at treating/controlling PDM is also important because it suggests that participants developed positive outcome expectations associated with engaging in health behaviour change as a result of the intervention. Outcome expectations surrounding treatment strategies have been shown to be linked to adherence to treatments, quality of glycaemic control and general quality of life in individuals with T2DM. 167–169
Despite participants reporting an increase in walking activity, overall pedometer counts remained unchanged after the intervention. This is in contrast to a more comprehensive RCT conducted by our research group testing the efficacy of structured education at increasing physical activity levels in individuals with IGT. Data from this trial found that physical activity, as measured by a sealed pedometer, significantly increased by 2600 steps. 148 Wearing an unsealed pedometer and keeping a daily steps-per-day log has been shown to result in a substantial short-term increase in ambulatory activity, even when no further instruction is given;168 therefore, the reactivity effect of wearing a pedometer and keeping a steps-per-day log in the week prior to the educational programme may have masked any intervention effect.
Engaging participants by telephone prior to the course was felt to contribute to the good uptake of participants attending the programme. The results gave positive feedback for the feasibility of recruiting participants to a prevention programme in the general population, as demonstrated by the positive recruitment rate.
Refinement of the Let’s Prevent programme
Following the feedback from the first pilot phase changes were made to the curriculum. Revisions were made to the risk factor and complication section and some changes were made to the pre-course materials that participants received. The physical activity section was simplified and educators all received further training. Advice was sought from the PREPARE study team to see how the physical activity messages could be enhanced and the curriculum was modified to incorporate these recommendations. New educational resources were also developed. To overcome the problem of patients changing their physical activity levels before the intervention, it was decided that for the RCT sealed pedometers would be issued to collect baseline data. Patients would then be issued with an unmasked pedometer for personal recording when they attended the Let’s Prevent programme.
Second phase of the pilot of the Let’s Prevent programme (March–April 2008)
The main purpose of the repilot was to check the changes to the curriculum, resources and retraining of educators. The quantitative and qualitative methodologies used were those adopted in the first pilot. Changes were made to the topic guides used for collecting qualitative data to reflect our primary interest in evaluating the impact and effectiveness of the modifications made in response to the first pilot.
Recruitment
During screening at a medical practice in Leicestershire, 26 individuals with IGT who had English as their main language were identified. A letter of invitation to take part in the pilot study was sent out to all 26 individuals.
Of these, 14 individuals (54%) responded positively to the letter, of whom six (23% of those initially invited) actually attended the pilot programme. The main reason for declining was being unable to attend on the pilot date. The average age of those who attended was 59.16 ± 10.94 years and 83% were male. On average, participants were older in the group that did not respond or responded negatively (mean 63.82 years, SE 2.31 years) than in the group that responded positively (mean 60.42 years, SE 2.74 years). However, this difference was not significant [t(27) = –0.949; p > 0.05].
Intervention
The refined Let’s Prevent programme was delivered as one 6-hour programme (group 1: n = 6). Owing to the positive outcomes of the first phase pilot, it was considered that repiloting with a single group would be adequate to address the aims of this second pilot.
Quantitative results
Although the pilot numbers were too small to detect meaningful outcomes, an analysis was conducted to provide an early indication of direction. Baseline and follow-up results are detailed in Table 15.
| Variable | Number of complete data sets | Baseline value | Follow-up median | Significance (two-tailed t-test) |
|---|---|---|---|---|
| Perceived effect of IGT (consequences)a | 5 | 1 | 2 | 0.29 |
| Timeline associated with IGTa | 4 | 2 | 4.5 | 0.29 |
| Perceived control over IGTa | 5 | 5 | 7 | 0.16 |
| Perceived response efficacy of lifestyle change at treating IGTa | 5 | 9 | 10 | 0.59 |
| Perceived symptom loada | 5 | 0.5 | 2 | 0.14 |
| Concern at having IGTa | 5 | 6 | 8 | 0.68 |
| IGT coherence (knowledge)a | 5 | 1 | 6 | 0.10 |
| Emotional representationsa | 5 | 1.5 | 5 | 0.20 |
| Total fibre score | 5 | 36 | 52 | 0.09 |
| Total fat score | 5 | 41 | 26 | 0.05 |
| Total unsaturated fat | 5 | 10 | 10 | 1.00 |
| Total walking activity (MET minutes/week) | 5 | 1307 | 4158 | 0.07 |
| Overall moderate- to vigorous-intensity physical activity (MET minutes/week) | 5 | 3755 | 8303 | 0.10 |
| Pedometer counts (steps per day) | 5 | 5831 | 8555 | < 0.01 |
Participants’ self-reported walking activity, total energy expenditure and fibre intake all improved post intervention; however, these differences were non-significant. Pedometer counts and self-reported total fat intake were found to improve significantly post intervention and, although the small sample size means that results cannot be generalised, it was nevertheless a pleasing indication of the success of the programme.
Qualitative findings
Four out of the six patients who attended the education session were interviewed by telephone using a topic guide (see Appendices 8–10).
Understanding of diagnosis and insulin resistance
A general level of awareness of participants’ diagnostic tests was obtained through attending screening and reading leaflets that accompanied the screening session. However, attending the course further helped their knowledge, and participants illustrated how it was used to bring about changes to their diet and lifestyles.
Avoiding things that look all right but are not particularly good for you, like processed foods.
With the exception of one patient, who had read her patient leaflets prior to attending the course, all had difficulties in remembering the term ‘insulin resistance’. However, when prompted through the use of the term ‘rusty locks’, it was clear that they had understood the explanations extremely well and found it an interesting way of explaining the concept, as illustrated by the following comment.
Yeah, that was quite an interesting bit actually the way she was explaining, um you may have quite a bit of insulin in the blood but if the locking mechanism is not working the cell . . . It was not about being able to produce enough insulin . . . it was about getting it into the cells from that point of view to me it was very informative.
Key messages
Overall, patients felt that the key message was about addressing their lifestyle in relation to their diets and exercise, particularly in relation to the amount and type of fats consumed, including hydrogenated fat, and the amount and intensity of exercise. For two of the patients, key messages also included understanding their risk of developing diabetes mellitus in the future.
If you don’t change and carry on willy nilly, you will probably end up with type 2 [diabetes].
And the more you can do you can either offset it or you know prolong the onset. I thing we all pretty much understood that you know we’re not diabetic and we’re not guaranteed to become diabetic.
Physical activity
Participants’ understanding and knowledge of the impact of physical activity on their condition was evident among all the patients. They also discussed in more specific terms the number of steps required for walking; using the stairs instead of lifts; walking instead of using the car; duration of activity; and being able to break down activities into chunks of 10 and 20 minutes. All of the patients were physically active prior to attending the course, through either their work or their hobbies, and stressed that their key focus was to ensure they continued to maintain or increase their activity levels.
I have taken on board that exercise will help.
I am actually yeah I mean on my afternoon shifts I decided to walk to work rather than take the car ’cos I don’t have to be in till . . . I can walk home if it’s a nice night as well.
Ongoing use of resources
All but one of the participants said that they would carry on using the pedometer. Their comments showed that the pedometer was used as a motivational tool.
I wear it all the time. I get quite a thrill to see how many steps I done in a day so you know I compared day against day and task against task you know so yeah that’s encouraging.
The one patient who will not be using the pedometer in the future felt that, although it gave him ‘an interesting snapshot’ of his activity levels, he is aware of his normal level of activity on a daily basis and, therefore, felt that it was not necessary to use a recording device.
Action plans
All of the patients were committed to an action plan that would help them to reduce their BP and/or cholesterol. They were all confident about their ability to continue with the changes that they were making in their lives. Their commitment to achieving their goals through measures such as reducing fat and taking/maintaining exercise was very evident.
I am really hoping not to let it slip . . . want to carry on and achieve more goals.
Summary
The results provided positive feedback on the feasibility of recruiting participants to a prevention programme in the general population, as demonstrated by our positive recruitment rate. Overall, the pilot study provided encouraging evidence for the effectiveness of the Let’s Prevent programme at targeting illness perception and promoting health behaviour change.
Following the implementation of the second cycle of qualitative feedback, it was felt that the process had led to the development of an educational programme that was fit for purpose and ready to be delivered to people from an English-speaking population with a diagnosis of PDM. Although some minor modifications to the education sessions were suggested during the second cycle of revisions and piloting, it was felt that further repiloting would not be required after making the necessary changes. The effectiveness of this education package now required formal evaluation, which would form the basis of the RCT.
Drafting a curriculum for black and minority ethnic participants
Although T2DM is a significant health problem for the general population, there is a higher risk of T2DM and premature heart disease for individuals from South Asian backgrounds. 170 Therefore, it was imperative to develop a culturally informed and appropriate intervention for this at-risk population, whose dietary practices and frequently reported low levels of physical activity contribute to the onset of T2DM. 144 At the time of developing the Let’s Prevent programme, studies investigating the prevention of diabetes mellitus in migrant populations in the UK were lacking. Given that NICE guidelines state that those identified as having a moderate or high risk of developing T2DM should be offered culturally appropriate information or support in a range of formats and languages, including structured education, in order to assist them to make lifestyle changes,13 it was considered necessary to design and tailor the Let’s Prevent programme to meet the needs of ethnically diverse communities.
Previous qualitative research159,160 helped to modify the DESMOND module for people newly diagnosed with diabetes mellitus from BME groups living in the UK. Although this work did include some work with African and African Caribbean populations, the main focus of the research centred on providing education that met the language, literacy and cultural needs of the South Asian population with T2DM. It appeared that the previous methodology used was helpful in the process of modifying the programme to be relevant to the South Asian community, and so owing to its previous success, the same methodology was employed to inform the development of a Let’s Prevent (BME) curriculum. The Let’s Prevent (BME) programme was developed to address the specific needs of the South Asian population living in the UK, many of whom do not speak English. The key educational messages remained the same as in the standard Let’s Prevent programme; however, minor changes were made to ensure that the food and activity messages were relevant to the South Asian culture. The main change was the identification and development of additional teaching resources (images and models) that reduced reliance on written formats, and that could potentially facilitate delivery to those for whom English is not a first language. Educational resources were developed specifically to take into account the language, literacy and cultural needs of people from a South Asian background with PDM. Many of the images and other visual resources were derived from those already developed for the DESMOND newly diagnosed module, with some additions, such as an image for insulin resistance and supplementary materials to address the increased emphasis on physical activity. As with the generic programme, participants were enabled to assess their personal risk by filling in a personal health profile. The targets were adapted to be appropriate for the South Asian population.
Based on the DESMOND model for BME groups, the programme was designed to be delivered as four sessions of 3 hours, facilitated by two trained health-care professionals and two trained interpreters. Previous work has demonstrated that using interpreters adds considerably to the delivery time and effectively doubles the length of time required per session. 171 The outline curriculum for the BME programme is shown in Table 16.
| Session | Theory | Sample activity | Duration, educator time + interpreter time (minutes) |
|---|---|---|---|
| Session 1 | |||
| Introduction | – | – | 10 + 10 |
| Patient story | CSM | Participants are asked to tell their story about how they discovered that they had PDM and their current knowledge of PDM | 30 + 30 |
| Professional story | CSM, DPT | Uses participants’ stories to support them in learning how the body regulates glucose | 45 + 45 |
| How am I doing? | SLT | Participants reflect on issues that have arisen in the programme so far | 5 + 5 |
| Session 2 | |||
| Reflections | SLT | Participants reflect on issues that have arisen in the programme so far | 10 + 10 |
| Taking control 1: weight management | CSM, DPT, SLT | Uses participants’ stories to support them in discovering how weight/waist affects PDM (provides knowledge and skills for food choices to control weight) | 30 + 30 |
| Physical activity | CSM, DPT, SLT | Uses participants’ stories to support them in discovering how physical activity affects PDM (provides knowledge and skills for activity choices to manage PDM) | 40 + 40 |
| How am I doing? | SLT | Participants reflect on issues that have arisen during the programme so far | 5 + 5 |
| Session 3 | |||
| Reflections | SLT | Participants reflect on issues that have arisen in the programme so far | 10 + 10 |
| Professional story | CSM | Uses participants’ stories to support them in discovering how other risk factors (e.g. high BP and cholesterol) affect PDM and the development of complications | 45 + 45 |
| Taking control 2: food choices – focus on fats | DPT, SLT | Provides knowledge and skills for food choices to reduce risk factors | 20 + 20 |
| How am I doing? | SLT | Participants reflect on issues that have arisen in the programme so far | 5 + 5 |
| Session 4 | |||
| Reflections | SLT | Participants reflect on issues that have arisen in the programme so far | 10 + 10 |
| Taking control 2: food choices – focus on fats | DPT, SLT | Provides knowledge and skills for food choices to reduce risk factors | 30 + 30 |
| Self-management plan | SLT | Participants supported in developing their self-management plans | 30 + 30 |
| Questions | CSM | Checks that all questions raised by participants throughout the programme have been answered and understood | 10 + 10 |
| What happens next? | SLT | Follow-up care outlines | 5 + 5 |
Piloting and refining of the Let’s Prevent (black and minority ethnic) programme
The methodology used to develop and refine the BME programme mirrored that used for the generic programme, as described earlier in this report. This involved an iterative process including the use of qualitative and quantitative data collection. Owing to the small sample size, we recognised that there would be limitations in undertaking statistical analysis, which would not be sufficiently powered to accurately detect differences. Nevertheless, it was felt that undertaking such an analysis would give an indication of the direction of results, and statistical analyses were performed for this reason. Topic guides were adapted to reflect any additional areas of interest relevant to the target population (see Appendices 8–10) and interpreters were included in the stakeholder groups from whom qualitative data were collected.
Recruitment
During screening at a Leicester medical practice, 29 individuals from a South Asian ethnic background, for whom English was not their first language and with PDM that had been diagnosed in the past 12 months were identified. A letter of invitation to take part in the pilot study was sent out to all 29 of these individuals, followed 1 week later by a telephone call from a Gujarati-speaking administrator. Twenty-one individuals responded positively to the initial invitation (72% of all identified), of whom 14 attended the pilot programme (48%). The main stated reason for not wanting to take part was being unable to attend on the dates specified. The average age of those who attended was 53.36 ± 7.39 years and 57% were female. On average, participants were older in the group that did not respond or than responded negatively (mean 60.88 years, SE 4.47 years) than in the group that responded positively (mean 53.24 years, SE 1.83 years). However, this difference was not significant [t(27) = –1.58; p > 0.05].
Intervention
The Let’s Prevent BME programme was delivered over 4 half-days in two groups (group 1: n = 9; group 2: n = 5). All participants in both groups attended all four of the half-day sessions. The 6-hour programme was delivered by two educators and two interpreters who were trained to deliver the Let’s Prevent BME curriculum.
Quantitative results
Although the sample is too small to provide sufficient power for conclusive statistical analysis, the trend in results are detailed in Table 17.
| Variable | Number of complete data sets | Baseline value | Follow-up median | Significance (two-tailed t-test) |
|---|---|---|---|---|
| Perceived effect of IGT (consequences)a | 8 | 3 | 4 | 0.10 |
| Timeline associated with IGTa | 8 | 5 | 3.5 | 0.10 |
| Perceived control over IGTa | 8 | 7 | 7 | 0.75 |
| Perceived response efficacy of lifestyle change at treating IGTa | 8 | 8 | 8 | 0.58 |
| Perceived symptom loada | 8 | 2 | 1 | 0.07 |
| Concern at having IGTa | 8 | 7 | 6 | 0.23 |
| IGT coherence (knowledge)a | 8 | 5 | 7 | 0.35 |
| Emotional representationsa | 8 | 5.5 | 3.5 | 0.06 |
| Total fibre score | 8 | 35 | 33 | 0.69 |
| Total fat score | 8 | 21 | 14 | 0.25 |
| Total unsaturated fat | 8 | 9 | 9 | 0.90 |
| Total walking activity (MET minutes/week) | 8 | 742 | 742 | 0.73 |
| Overall moderate- to vigorous-intensity physical activity (MET minutes/week) | 8 | 1001 | 2235 | 0.13 |
| Pedometer counts (steps per day) | 5 | 4981 | 7753 | < 0.01 |
The quantitative results from this study were limited by the fact that only around half of the participants adequately filled out and returned their questionnaire; consequently, only limited conclusions can be drawn from this pilot study. Nevertheless, the intervention resulted in a large reduction in feelings of depression and anxiety (emotional representations) resulting from a diagnosis of PDM. This may have important implications in terms of how individuals self-manage their PDM in the future. The improvement in pedometer counts proved to be highly significant, with an average increase of 2772 steps per day. Although we recognise that the small sample size had a limit on the generalisability of the findings, this certainly appeared to be a very positive trend.
Qualitative findings
There were two focus groups held after completion of the final education session for the two groups of patients. A total of 12 people participated, three of whom were relatives of patients. The first focus group was conducted in Gujarati and English and the second in English only.
Understanding of prediabetes mellitus
The comments received from all the patients suggested that they were more confident about their understanding and knowledge of PDM than before the course. Their comments also showed that they felt empowered and motivated to do something about it.
Well, I understand that you are at risk and when you are diabetic there’s a lot of things that will affect your lifestyle and I need to change that. After coming to these courses . . . I am really going to try because I don’t want to become a diabetic myself.
Those who came to the BME sessions had been motivated to attend to find out information about their condition and what they could do to prevent progression to T2DM, and they appeared to have gained an understanding of insulin resistance.
Locks cannot get any sugar energy so it’s like having a rusted lock. You need to be more active to open this lock, get exercise and diet and everything.
When asked what had been learnt through the food continuum game, the overall comments in one group were about portion sizes, using less oil, increasing fibre and eating more fruit and less fried food. In both groups, comments about the food sections of the curriculum included the contribution of the sessions to their understanding of fats in their diet. This included dispelling myths about sunflower oil, which is commonly used in this community and is widely perceived as containing no cholesterol.
A lot of our people think that sunflower oil is good for you.
Although some South Asian foods were included in the BME version of the food continuum, one of the focus groups highlighted that this game should be made even more relevant to Asian diets to help people to relate more directly to the messages that were being given.
I was a little bit disappointed. I thought they would have more examples of Asian foods because we all eat a lot of Asian foods. If they couldn’t get Asian foods then they could have got pictures, you know.
. . . message is the strongest if people can relate to it themselves, if there is a high Asian population.
We are 100% Asian and that is who you are targeting here.
Physical activity games
When asked about the physical activity games, all participants had difficulty in recalling individual games but they had assimilated key messages including those related to types of activity such as walking, gardening and housework.
. . . it’s about it being vigorous.
Ongoing use of resources
All participants in one group felt that they would continue to use their pedometer, for a number of different reasons, including monitoring their progress, helping to establish a routine, encouragement and giving them some control. Patients in the other group had used their pedometers and worked out their average levels of activity, and most felt that they would be able to increase their levels without using a pedometer. Only one person in this group said that they would continue to use it to help with motivation.
Interpretation for education sessions
The overall view was that it would be useful to have a choice of sessions for South Asian patients. The BME sessions were attended by some fluent English-speaking South Asian patients, who indicated that they would have preferred to have sessions delivered in English only, since interpretation lengthened sessions and consequently increased the time that they needed to take off work. Those who spoke English less fluently said that they had benefited from having interpretation, as it aided their understanding and also helped to enable them to respond to questions. The use of images for this group, with verbal explanations from educators and interpreters, had also helped reinforce participants’ understanding of what was being said.
Style of delivery
A question about the educators’ style of delivery was asked when obtaining feedback from those who had attended the BME sessions. Responses suggested that they had found this excellent and that they had felt very comfortable about asking questions during the sessions. Very positive views were expressed about the non-didactic DESMOND style of education.
Telling you is more like college education. People like us, even though we know the language, this was something new that came into our lives. We need to be in the primary education level. This is what DESMOND was doing, telling you with pictures as well as educating your mind and making you more aware.
Discussion
The analysis of and reflections on qualitative findings indicated that, overall, there was a good level of acceptability and effectiveness of the BME version of the Let’s Prevent programme. The findings also identified where modifications were required to further refine the training, curriculum and resources to meet the needs of the BME population. The pilot specifically highlighted the fact that there are a group of patients from the BME community who want to attend a programme that is culturally relevant to them but that is delivered without interpreters.
The study yielded a high positive response rate (72%), which serves to highlight the merits of engaging participants by means of a telephone call from someone from the BME population with the appropriate language skills. The pilot study also suggested that the percentage of eligible participants willing to attend the prevention programme may be increased by running evening/weekend sessions, as the programme was limited to weekday sessions. Piloting also highlighted the need to provide culturally and linguistically appropriate resources.
Refinements to the black and minority ethnic programme and repilot
Key changes implemented to education sessions for the second cycle of the pilot included the following.
Curriculum and resources
-
Physical activity section revised and simplified.
-
Inclusion of more food models of Asian fruits and vegetables to promote positive messages and more Asian foods overall (e.g. chapattis, chickpeas) in order to promote more active discussion.
-
Facilitation notes and prompts were added to promote links between certain sections of the curriculum, to reinforce key messages, to promote continuity and to increase clarity.
-
Revision of the presentation of risk factors and complications.
-
Some changes to pre-course materials given to participants.
-
Amendment of the action planning section for the BME curriculum.
-
Organisational issues, for example, storage space for resources for education sessions.
Additional format for black and minority ethnic session
It was agreed to pilot a culturally appropriate education session for English-speaking South Asians without interpreters. It was decided to pilot one programme delivered without interpreters as two 3-hour sessions, as per the general Let’s Prevent format, but with BME food and activity sessions from the Let’s Prevent BME programme.
Second phase of pilot of the Let’s Prevent (black and minority ethnic) programme (March–April 2008)
As with the repiloting of the standard (general population) programme, the main purpose of the BME repilot was to check the changes to the curriculum, resources and retraining of educators. The methodology used was the same as for the first pilot, with topic guide modifications to reflect our primary interest in evaluating the changes made after the first cycle of piloting (Appendices 8–10).
Recruitment
During screening at two Leicester practices, 40 individuals were identified with IGT from a South Asian ethnic background, for whom English was not necessarily their main language. A letter of invitation to take part in this pilot study was sent out to all 40 of these individuals, and this was followed 1 week later by a telephone call from a Gujarati-speaking administrator. Twenty individuals (50% of those invited) responded positively to the invitation, of whom 10 (25% of those originally invited) attended the pilot programme. The main reason for not wanting to take part was being unable to make the pilot dates. The average age of those who attended was 57.40 ± 12.03 years and 60% were female. On average, participants were older in the group that did not respond or responded negatively (mean 60.75 years, SE 3.02 years) than in the group that responded positively (mean 56.35 years, SE 2.50 years).
Intervention
The diabetes mellitus prevention structured educational programme developed by our research group specifically for South Asian individuals was delivered in two groups. The first consisted of 4 half-days in one group (group 1: n = 4). This 12-hour programme was delivered by two educators who were trained to deliver the BME curriculum of the prevention programme. Each group-1 session was attended and interpreted by trained Guajarati interpreters. The group-2 programme (n = 6) was delivered over 2 half-days. The 6-hour programme was delivered by two trained educators, but participants did not require an interpreter.
Quantitative results
Table 18 shows the quantitative results. Owing to the limited sample size, no firm conclusions can be drawn; however, fibre intake, pedometer counts and perceived knowledge all significantly increased. Furthermore, there was a trend for an increase in self-reported walking activity and energy expenditure.
| Variable | Number of complete data sets | Baseline value | Follow-up median | Significance (two-tailed t-test) |
|---|---|---|---|---|
| Perceived effect of IGT (consequences)a | 9 | 1.5 | 2 | 1.00 |
| Timeline associated with IGTa | 9 | 3 | 4 | 0.48 |
| Perceived control over IGTa | 9 | 8 | 8 | 0.40 |
| Perceived response efficacy of lifestyle change at treating IGTa | 9 | 8 | 10 | 0.29 |
| Perceived symptom loada | 9 | 1 | 0 | 0.36 |
| Concern at having IGTa | 9 | 8 | 5 | 0.59 |
| IGT coherence (knowledge)a | 9 | 8 | 10 | 0.03 |
| Emotional representationsa | 9 | 3 | 2 | 0.46 |
| Total fibre score | 9 | 29 | 44 | < 0.01 |
| Total fat score | 9 | 15 | 15 | 0.33 |
| Total unsaturated fat | 9 | 10 | 10 | 0.31 |
| Total walking activity (MET minutes/week) | 10 | 693 | 891 | 0.45 |
| Overall moderate-to-vigorous physical activity (MET minutes/week) | 10 | 1040 | 3651 | 0.14 |
| Pedometer counts (steps per day) | 9 | 6592 | 8526 | 0.02 |
Qualitative findings
A focus group was held with seven South Asian participants who attended the education sessions delivered in English without interpreters, but with culturally appropriate resources. The sessions were received very positively by all those who gave feedback.
They done [sic] it nicely.
The inclusion of additional South Asian food models in response to feedback from the first pilot appeared to have assisted with engagement.
They have done it very, very well, even with our food.
Patients expressed surprise on hearing about fats and their influence on the development of diabetes mellitus, as illustrated by the following comments:
I did not realise about oil and fats – all causes it [diabetes].
If I stop eating sugar but carry on eating fatty food I will still get it [diabetes].
Participants particularly valued the contribution of food games, as they perceived a contribution of Asian diets to the development of diabetes mellitus.
Our food is totally different that is the biggest issue, we have oily foods and we fry.
Patients thought that sessions delivered in English for South Asian patients would be really helpful for younger people from these communities for their ‘future lives’. The value placed on the sessions was reflected in the view that more people from South Asian communities should be encouraged to attend this type of programme to obtain the benefits of what they had learned.
Just be careful for yourself and take care with diet, do much more exercise.
Summary
The results provide positive feedback for the feasibility of recruiting participants to a lifestyle management programme in the BME population, as demonstrated by our positive recruitment rate. Overall, the pilot study provided encouraging evidence for the effectiveness of a prevention programme at targeting illness perception and promoting health behaviour change in a multiethnic community.
The comments from patients about the food games were very positive and affirmed that the changes made to the curriculum were acceptable. Following the implementation of the second cycle of qualitative feedback, it was felt that the methods used had led to the development of a model for the provision of fit-for-purpose education for people with a diagnosis of PDM from a BME population, specifically targeted at the South Asian population. As with the general population module, it was felt that further repiloting would not be required after making the minor modifications suggested during the repiloting and that the effectiveness of this education package now required formal evaluation.
Development of a quality-development programme
Quality development is a requirement of the NICE criteria for educational interventions. 149 The DESMOND Training and Quality Development programme was an initiative in health-care professional training, which was awarded the Health Service Journal Award 2007. The Training and Quality Development programme was originally developed by a task group of the DESMOND collaborative (a multidisciplinary, multiorganisational group of health-care professionals, academics and people with diabetes mellitus) to support the clinical trial of DESMOND. Through involving educators, trainers and patients in the development process, the programme created training, mentorship and continued professional development that was uniquely integrated with the patient education programme being delivered. This method of quality development has been piloted and now used successfully for the DESMOND newly diagnosed module. Therefore, this training and quality-assurance model was deemed to be transferable to the Let’s Prevent programme.
A quality-development programme was developed for the Let’s Prevent programme to ensure that the educational intervention was delivered in a standardised way, to ensure that core content and learning outcomes were achieved and to ensure that educator behaviour was closely allied to the programme’s philosophies and learning theories (Figure 7).
FIGURE 7.
Quality-development pathway. DOS, DESMOND observation sheet; DOT, DESMOND observer tool; QD, quality development.
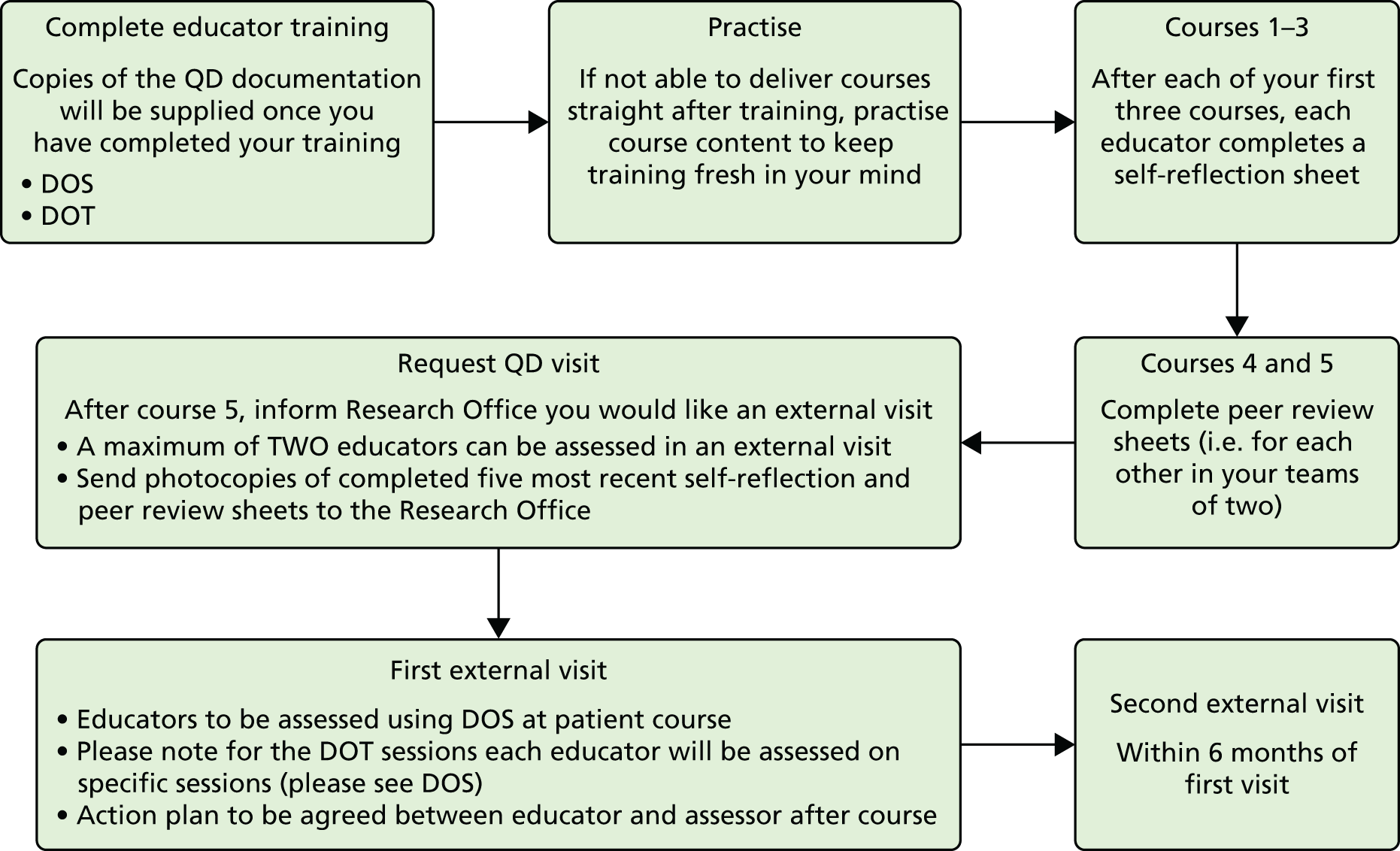
The quality-development programme consists of both internal and external processes. The internal processes encourage educators to reflect on their practice using self-reflection sheets and peer-reflection sheets (see Appendix 11), providing educators with a feedback tool for personal development. The external component of the quality-development programme is an adapted DESMOND observation sheet, used to check content and process indicators of observable educator behaviours, and an adapted DESMOND observer tool, used to assess the interaction of the educator and the group (see Appendix 12). These tools can be used by educators to identify areas for personal development and also by external trained assessors to provide quantitative data. These data provide a mechanism by which to accredit educators and inform the ongoing development of the training programmes.
Development of an educator training programme
Despite the existence of many self-management education programmes in the UK for those living with long-term conditions, current evidence suggests that these programmes have variable outcomes. One possible reason, highlighted by NICE in 2003, is the variable nature of the delivery of the programmes by health-care professionals, especially given that none has clearly described the education in terms of skills, attitudes and behaviours demonstrated by the educators, without which monitoring for internal consistency cannot take place. 149 The training programme for educators was developed by a core multidisciplinary team, in partnership with the DESMOND collaborative. The DESMOND collaborative were approached as they have proven to have national success at developing training programmes for educators.
The ‘Train the Educator’ programme for the Let’s Prevent initiative aimed to familiarise educators with the content and resources of the Let’s Prevent curriculum. Adequate training enables educators to become confident and competent to deliver the programme in accordance with its learning theories and philosophy. The training programme was delivered by trainers in a style that reflects the learning theories and philosophy underpinning the Let’s Prevent programme.
The ‘Train the Educator’ programme was designed to allow some flexibility in the way theory and practical sessions are delivered by the trainers. The dual-processing approach ensured that knowledge and skills were elicited rather than taught didactically, and this was further developed through experiential exercises, reflection and debate. A core element of training was to model sessions to trainees who take on the role of participants. By enacting a participant’s perspective, they were simultaneously experiencing the programme and learning from the observation of specific behaviours portrayed by the trainers. This process was interspersed with opportunities for trainees to practice delivering the curriculum in a safe environment, and it also allowed time for reflection, which enabled participants to review their own skills. The process was supported with a ‘Train the Educator’ manual, a curriculum and other resources provided for trainers to retain, read and reinforce their learning.
Piloting the training
The trainers delivering the training programme were experienced in developing and delivering ‘Train the Educator’ programmes as part of a national training strategy group. Five health-care professionals (one dietitian, one practice nurse, two research nurses and one diabetes mellitus specialist nurse) were recruited to be trained as educators and to deliver the intervention for the pilots. Educators attended training in October 2007.
The initial training day was evaluated by qualitative researchers, who obtained data from both trainers and educators using focus groups, interviews and written observational notes (see Appendices 13–16). In addition, data were obtained from a formal feedback session in which educators and interpreters shared their experiences and reflections with the Let’s Prevent research and development (R&D) team. Feedback was invited in relation to the training day and the implementation of the pilot education sessions.
An analysis of the data involved the use of framework charts166 to summarise and organise the information collected. Subsequently, meetings between the members of the R&D team (including the co-ordinator, trainers and qualitative researchers) were held to reflect on the key issues that had emerged and to consider the implications for the refinement of the training programme.
Educators generally gave very positive feedback about the training, with typical comments being:
It was great to be part of something new.
Good to work with a variety of educators and have the opportunity to work with new people.
The support of trainers made us welcome.
DESMOND values were shown in the delivery of training.
Constructive suggestions for improving the training programme were also extracted from the collated qualitative feedback. These included:
-
more practice modelling sessions would be beneficial where curriculum changes have occurred
-
information about the resources that patients receive prior to the course and action taken would be useful for educators
-
2 days of training would have been better than one long day
-
pre-course reading would have been useful.
Refinements to the training programme
Based on feedback, the training programme and resources were reviewed and the changes needed were identified. These included:
-
modifying the training programme to be a 2-day programme
-
including more modelling sessions
-
producing pre-course reading material.
In response to the feedback obtained and the modifications identified, an additional training day was organised to address the needs of the educators who had already undergone the 1-day training as part of the first pilot.
Future needs
The following organisational needs were also identified for implementation prior to or as part of the planned RCT:
-
more ‘Train the Educator’ programmes would be required in preparation for the RCT
-
it was identified that more educators would need to be trained in order to meet the demands of the study
-
it would be necessary to implement a quality-development process.
Development of the training programme for educators and interpreters to deliver Let’s Prevent (black and minority ethnic)
As per the standard training, the training programme for educators and interpreters for Let’s Prevent (BME) was developed by a core multidisciplinary team, in partnership with the DESMOND collaborative.
It was decided that the ‘Train the Educator and Interpreter’ programme would involve educators already trained to deliver the general population module. For these educators it would take the form of one additional day of training, which would also be attended by interpreters. The aim of this day was to familiarise educators and interpreters with the content of and resources for the Let’s Prevent (BME) curriculum. It was felt that the majority of the programme could be delivered to both educators and interpreters concurrently, but that some time was required to enable interpreters to become familiar with the learning theories and philosophies of the programme, to ensure that when interpretation was delivered, it was in a style that reflects the learning theories and philosophies of Let’s Prevent.
The training programme included opportunities for educators and interpreters to ‘have a go’ at working together to deliver the curriculum in a safe environment and time for reflection to allow participants to review their own skills. As new resources had been developed to ensure that the programme was not reliant on written words, educators and interpreters had an opportunity to practice using some of the pictorial stickers designed to replace writing on the flip charts and the other visual resources. The process was supported by a ‘Train the Educator’ manual, a curriculum and other resources for trainers to retain, read and reinforce their learning.
Piloting the training programme
The trainers delivering the training programme were experienced in developing and delivering ‘Train the Educator’ programmes as part of a national training strategy group. Five educators (one dietitian, one practice nurse, two research nurses and one diabetes mellitus specialist nurse) were recruited to be trained as educators and to deliver the intervention for the pilots. Three trained interpreters were recruited to be trained as interpreters for the Let’s Prevent BME programme. The initial training day in November 2007 was evaluated by the qualitative researchers using methods described for the general population module training. This included the collection of data from interpreters and a second dedicated feedback day to elicit responses on the BME training and initial piloting. The training programme was positively evaluated by the educators, and interpreters.
I enjoyed all the day especially cultural awareness session.
Educator
For my own professional development – it was a learning curve.
Educator
From an interpreter point of view, all sessions with various professionals were good.
Interpreter
Specific comments and suggestions for improving the training programme extracted from the feedback from educators, interpreters, and trainers included:
-
more practice modelling sessions would be beneficial where the curriculum differs from the PDM curriculum
-
information about resources that patients receive prior to the course and action taken would be useful for educators
-
more background information about South Asian diets and methods of cooking for educators would have been useful
-
DESMOND philosophy training was seen as very useful by interpreters
-
more background information on research evidence on PDM for educators would be welcomed
-
the cultural awareness session and practice working with interpreters were positively received.
Refinements to the training programme
Based on feedback, the training programme and resources were reviewed. Modifications needed were identified as including:
-
more modelling sessions for educators and interpreters to work together
-
pre-course reading materials
-
signposting to sources of cultural awareness/competency information.
Prior to the second pilot, these needs were addressed during the additional 1-day training session organised as described in relation to training for the generic (general population) programme.
Future needs
The organisational needs that were highlighted reflected those identified for the generic population programme, namely:
-
more ‘Train the Educator and Interpreter’ programmes will be required in preparation for the RCT
-
it will be necessary for more educators to be trained as trainers to meet the demands of the study
-
quality-development process for educators and interpreters will need to be implemented
-
we are analysing an externally led cultural awareness programme for our Diabetes Research Group.
Summary
Overall, in terms of the Let’s Prevent intervention, both the qualitative and quantitative data revealed a positive change in health behaviours (mainly increased walking and physical activity levels) and beliefs and knowledge related to the prevention of T2DM. The BME adaptations were well received, with individuals seeming to gain value from having culturally appropriate resources. The recruitment strategy also proved feasible. Regular feedback was used throughout with regard to the ‘Train the Trainer’ programme and the Let’s Prevent intervention itself. This allowed for maximum input from a variety of stakeholders. In accordance with the MRC recommendations, the development team took into consideration the suggested improvements and made the necessary amendments. Finally, the intervention was considered fit for purpose and ready to be tested in a RCT.
Chapter 5 The randomised controlled trial protocol
This chapter uses excerpts from Gray et al. ,146 reproduced courtesy of BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Aims and objectives
The aim of the Let’s Prevent study was to ascertain if a pragmatic, structured education programme focused on lifestyle modification and behaviour change, in combination with regular telephone support, was able to prevent the development of T2DM in people identified as having PDM through a two-stage screening process within a primary care setting, and to assess if such an intervention was cost-effective.
The objectives of the study were to:
-
provide a robust identification strategy for those at high risk of diabetes mellitus
-
provide an appropriately structured education programme to target lifestyle modification and behaviour change
-
provide a culturally sensitive structured education programme for the BME community
-
provide a support and motivational maintenance programme for the duration of the study
-
evaluate the programme to provide a low-cost solution for practical health improvement.
The study had two phases. The first phase was a screening phase which identified people at risk of PDM/T2DM through the use of a screening tool that had been developed and validated for use within primary care. For further details of the development of the screening tool, see Chapter 3. The second phase took participants who had been screened and found to have PDM and recruited them into a cluster RCT, designed to test the effectiveness of a structured education programme in preventing progression to T2DM. This chapter will outline the methodology of the two phases.
Phase one: screening for prediabetes mellitus
Identification of those at high risk of prediabetes mellitus/type 2 diabetes mellitus
All participating GP practices received a ‘practice pack’ which provided general information and contact numbers for the study. All practices had an induction visit from the project lead and research assistant who provided training and support. The LPRS (see Chapter 3), which used practice-level ethnicity as a proxy for individual ethnicity, was used to identify those at high risk of PDM/T2DM using data routinely stored on individual GP practice computer databases.
The LPRS was calculated as follows:
LPRS = 0.0407 × age (years)
+ 0.296 (if male; no change if female)
+ 0.934 (ethnicity, as practice proportion of South Asian)
+ 0.0859 × BMI (kg/m2)
+ 0.440 (if family history of diabetes mellitus; no change otherwise)
+ 0.374 (if on antihypertensive medication; no change otherwise)
Before the risk tool was applied to a practice database, the quality of the data completion was assessed. If BMI data were found to be recorded in < 40% of those registered, practices were asked to increase this before the risk tool was used. The risk score was calculated for all those listed in participating practice. The practice list was then ranked by risk score, with those with the highest scores having the highest risk. 146
The top 10% of patients with the highest score were invited for screening initially. This 10% limit could be increased to generate further invitations and increase inclusion in the study if required. Where the top 10% of the risk score identified fewer than 500 patients, all patients within the top 10% were invited. Where the number of eligible patients identified in the top 10% was > 500, the first 500 patients within the top 10% were invited. 146 If the response rate to initial invitations was sufficient, a second mailing of invitations was not conducted. This was based on past findings where the positive response rate to the first mailing had been 17% and to the second had been approximately 7%.
If the response rate to the first mailing was insufficient, a further mailing was carried out. In practices in which all participants in the top 10% were mailed, the second mailing was to those who had not responded to the initial mailing. In practices in which only the first 500 patients within the top 10% received the initial mailing, a second mailing was directed at any patients from the top 10% who were not mailed previously.
A computer programme was written to automate this process, which produced an Excel spreadsheet listing all those registered in the practice and their risk scores in descending order. This programme is now freely available for UK practices to download and use (see http://leicesterdiabetescentre.org.uk/The-Leicester-Diabetes-Risk-Score).
The invitation included a patient information sheet and a reply sheet, so patients could register their interest in taking part in the study (see Appendices 17 and 18). A self-addressed envelope was provided for returning slips. Patients were also given the number of a dedicated telephone line to contact if they were interested and/or required further information. Written informed consent was taken from all participants and participants were informed that they were able to withdraw from the study at any time.
Inclusion/exclusion criteria
Participants were invited for screening if they fulfilled the following criteria:
-
high risk according to the LPRS
-
aged 40–75 years if white European, or 25–75 years if South Asian.
Participants were excluded from the screening study if they were/had:
-
unable to give informed consent
-
pregnant or lactating
-
established diabetes
-
a terminal illness
-
required an interpreter for a language other than Gujarati.
The differential included age ranges for white European and South Asian participants were chosen to reflect the fact that South Asians, as well as being at higher risk than their white European counterparts, also develop the disease at an earlier age. The Southall and Brent Revisited study showed that people from BME communities were diagnosed, on average, 5 years earlier than white Europeans. 172 Therefore, any prevention initiative should begin at an earlier age for these groups; this also reflects NICE guidance for screening those at risk of T2DM. 126
Baseline screening visit
All participants who had been identified by the LPRS as being at high risk for PDM/T2DM and who had accepted the invitation for screening were given an appointment to attend a local clinic (see Appendix 19). Following an informed consent process, a number of clinical assessments and measurements were performed (see Appendix 20 for an example of a consent form).
Oral glucose tolerance test
An OGTT was performed at baseline. Participants were sent instructions for the OGTT along with their appointment date and time, which requested that they fast from midnight the night before their OGTT (see Appendix 19). A baseline set of bloods was taken before the participant was given a glucose load of 75 g in the form of Lucozade (Lucozade Ribena Suntory Ltd, Uxbridge, UK) (410 ml for 70 kcal/100 ml Lucozade). Timing of the 2-hour interval was taken from the start of the Lucozade drink. A post-load glucose sample was taken at 120 minutes. All participants who were identified as having PDM took part in the RCT (see Appendices 21–24). The OGTT was repeated at 12, 24 and 36 months to check the participant’s diabetes mellitus status. Participants who did not consent at baseline to an OGTT were not entered into the study on the grounds that their diabetes mellitus status could not be assessed. These individuals were referred back to their GP for standard care.
If a participant had an OGTT result in the range for diabetes mellitus at screening they were recalled as soon as possible for a second, confirmatory test (see Appendix 25). In accordance with WHO criteria, diabetes mellitus was defined as a fasting blood glucose ≥ 7.0 mmol/l and/or 2-hour plasma glucose of ≥ 11.1 mmol/l. If a participant was found to have diabetes mellitus at screening, they were excluded from the study and were referred back to their GP for commencement of standard diabetes mellitus care (see Appendices 26 and 27).
Venous blood tests
Blood tests were collected at baseline and repeated at 12, 24 and 36 months (Table 19).
| Timing | Biochemical parameter | Collection method |
|---|---|---|
| Fasting samples | Fasting glucose | 2.7 ml of fluoride |
| Lipidsa | 4.7 ml of serum gel | |
| HbA1ca | 2.7 ml of EDTA | |
| LFTs | Included in 4.7 ml of serum gel | |
| Biomarkers | 2 × 9 ml of EDTA and 9 ml of serum gel | |
| 120 minutes | Glucose | 2.7 ml of fluoride |
| Whole genetic blood | 9 ml of EDTA |
This amounted to a total of 49 ml of blood for the baseline, 12-, 24- and 36-month blood tests, and 8 ml for the 6-month test.
In-study assessments
In-study assessments were carried out alongside the blood tests at baseline and at 6, 12, 24 and 36 months post randomisation. Details of all clinical assessment and timelines are summarised in Table 20. (For examples of the case report form and patient questionnaire, see Appendices 28 and 29. )
| Measurements | Time point (months) | ||||
|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | 36 | |
| Clinical assessment | |||||
| Medical history | ✗ | ✗ | ✗ | ✗ | |
| Medication history | ✗ | ✗ | ✗ | ✗ | |
| Physical exam | ✗ | ✗ | ✗ | ✗ | |
| Cardiovascular risk score | ✗ | ✗ | ✗ | ✗ | ✗ |
| Presence of metabolic syndrome | ✗ | ✗ | ✗ | ✗ | ✗ |
| Anthropometric | |||||
| 3 × BP | ✗ | ✗ | ✗ | ✗ | ✗ |
| Height | ✗ | ✗ | |||
| Weight | ✗ | ✗ | ✗ | ✗ | ✗ |
| Waist circumference | ✗ | ✗ | ✗ | ✗ | ✗ |
| Blood tests | |||||
| OGTT | ✗ | ✗ | ✗ | ✗ | |
| HbA1c | ✗ | ✗ | ✗ | ✗ | ✗ |
| Lipids | ✗ | ✗ | ✗ | ✗ | ✗ |
| Urea and electrolytes | ✗ | ✗ | ✗ | ✗ | |
| LFT | ✗ | ✗ | ✗ | ✗ | |
| Questionnaires and lifestyle measuresa | |||||
| IPAQ (short form) | ✗ | ✗ | ✗ | ✗ | |
| DINE | ✗ | ✗ | ✗ | ✗ | ✗ |
| BIPQ | ✗ | ✗ | ✗ | ✗ | ✗ |
| HADS | ✗ | ✗ | ✗ | ✗ | ✗ |
| 15D | ✗ | ✗ | ✗ | ✗ | ✗ |
| 7-day step count | ✗ | ✗ | ✗ | ✗ | ✗ |
| Urine sample | ✗ | ✗ | ✗ | ✗ | |
| EQ-5D | ✗ | ||||
Body mass index was calculated after body weight and height were measured. Weight was measured in light clothing without shoes. Height was measured at baseline and 36 months only. Waist circumference was measured with a soft tape on standing participants, mid-way between the lowest rib and iliac crest. Hip circumference was measured over the widest part of the gluteal region and the waist-to-hip ratio calculated. Three BP recordings were obtained from the arm of the participant in a sitting position after 5 minutes of rest, with at least 2 minutes between readings, and the mean value of the second and third readings was calculated, discounting the first. All clinical measurements were carried out as detailed by Diabetes Research Network standard operating procedures. Seven-day step count was assessed by giving all study participants a sealed piezoelectric pedometer (NL-800). Participants were asked to wear the pedometer fitted to their trunks (placed on right anterior axillary line) for 7 consecutive days during waking hours (see Appendix 30). Participants were provided with a stamped addressed envelope to return the pedometers to the study co-ordinators.
Study questionnaires
A health and dietary questionnaire was completed by the participant with assistance from a trained nurse or research assistant at baseline and at 6, 12, 24 and 36 months (see Appendix 29 for the 36-month questionnaire). The questionnaires covered several areas, as summarised in Table 21.
| Questionnaire topic/instrument | Area of assessment |
|---|---|
| Basic demographic details | Smoking status, alcohol consumption, occupation, ethnicity |
| Medical and medication history | Previous/current medical and medication history |
| The validated DINE164 | Dietary fat and fibre intake |
| The validated 15D173 | Quality of life |
| The validated HADS174 | Depression and anxiety relating to diagnosis of condition and the care provided thereafter |
| The validated BIPQ163 | Cognitive and emotional representations of illness |
| The validated IPAQ (short form)162 | Health-related physical activity |
| The validated EQ-5D175 | Quality of life |
Participants also self-reported on two questions concerning sleeping pattern (‘How many hours’ sleep did you get last night?’ and ‘On average, how many hours do you sleep in 24 hours?’). This was assessed at baseline, 12, 24 and 36 months. These data were included, as there is accumulating evidence for an association between short sleep time (< 6 hours per 24 hours) and long sleep time (≥ 10 hours per 24 hours) and metabolic dysfunction (i.e. T2DM). 176 Furthermore, lifestyle intervention trials have reported improved sleep hygiene and reduced risk of developing T2DM. 177
Justification for biomarkers
Biomarkers of inflammation confer an increased risk of diabetes mellitus and CVD. The development of robust, inexpensive commercial assays will allow these potentially powerful predictors of common diseases to be utilised in clinical practice. Prospective studies in at-risk populations are invaluable testing grounds for such markers and simultaneously provide insight into pathogenesis, which may ultimately lead to novel therapeutic approaches.
Despite a growing body of research, the physiological relevance of adipokines for the development of insulin resistance and atheroma has not yet been established. Low adiponectin concentrations are associated with a greater risk of diabetes mellitus and myocardial infarction, and data from animal and human studies suggest an insulin-sensitising, anti-inflammatory role for this protein. It is especially relevant to focus further adipokine studies on groups at risk of diabetes mellitus and vascular disease.
Adiocytokines, such as tumour necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), leptin and adiponectin, have been shown to predict the risk of developing T2DM and CVD and are thought to be directly involved in the pathogenesis of these chronic diseases. 178–180 Circulating levels of adipocytokines are predominantly influenced by levels of adiposity and have been proposed as an important meditating link between obesity and chronic disease. 181 Data from our research group and others have also shown that moderate- to vigorous-intensity physical activity, including walking activity, is associated with adipocytokines, independent of body fat mass. 182,183 However, evidence for the link between health behaviour change, adipocytokines and incidence of T2DM is lacking from RCTs. Therefore, the inclusion of these biomarkers as secondary outcomes in our study was deemed important to further our current understanding of the mechanisms linking health behaviour change to the risk of developing T2DM.
Blood sampling was also conducted to allow the exploration of the role of vitamin D status in T2DM. Vitamin D deficiency has consistently been linked to poor glycaemic control;184 however, evidence is lacking from prospective studies. Therefore, taking these samples for future analysis will improve our understanding of a potentially important causal factor in the increasing prevalence of T2DM. This is particularly important for populations from a South Asian ethnic background who are known to have a high prevalence of vitamin D deficiency185 and a high risk of developing T2DM compared with white Europeans. 186
Plasma vitamin C was also measured as a biomarker of fruit and vegetable intake. Diets characterised by a high fruit and vegetable content have been associated with improvements in glucose control;187,188 therefore, we wanted a valid measure of fruit and vegetable intake. The term ‘fruit and vegetables’ covers a wide range of food groups which differ between and even within different cultures,189 thus making dietary assessment difficult. However, as well as reflecting short-term intake of vitamin C,190 plasma vitamin C has consistently been shown to be correlated with habitual reported intake of fruit and vegetables. 191 The use of vitamin C as a biomarker had the added benefit of not relying on self-reported food intake, which can often be subjective and inaccurate. 192
It has previously been demonstrated that recommendations to increase fruit and vegetable intake can produce an increase in vitamin C levels,193,194 and participants in the intervention arm of the study were encouraged to increase their fruit and vegetable intake. Therefore, the use of plasma vitamin C as a biomarker not only provided information about differences in intake between subjects at baseline but was also a useful tool that demonstrated whether or not subjects in either control or the intervention groups altered their fruit and vegetable intake after enrolment in the study.
Oxidative stress occurs when an imbalance between pro-oxidants and antioxidants occur in a biological system, and it is widely accepted that oxidative stress functions as a precursor in the development and progression of T2DM. 195 It is proposed that the accelerated complications and increased risk of CHD seen in T2DM is attributable to the presence of oxidative stress. Subjects with diabetes mellitus show both an increase in levels of free radicals, substances that promote oxidative damage and a decrease in antioxidants. 196 Antioxidants are substances that prevent or delay oxidation. 197 Increasing fruit and vegetable intake may beneficially increase essential antioxidant levels and thus reduce oxidative stress. It has been demonstrated that participants who consume two to three portions of fruit daily have lower levels of lipid peroxidation. 196 Plasma and urinary F2-isoprostanes are established biomarkers of lipid peroxidation in vivo. 198 Therefore, we also measured urinary F2-isoprostanes and, for this purpose, a urine sample was taken at baseline and at 12, 24 and 36 months. This allowed for the assessment of oxidative stress in relation to fruit and vegetable intake as well as glucose tolerance.
The biomarkers measured were:
-
inflammatory biomarkers (adipocytokine array)
-
adiponectin, leptin, resistin, IL-6, TNF-α, plasminogen activator inhibitor-1
-
-
inflammatory biomarkers (non-adipocytokine array)
-
high-sensitivity C-reactive protein, TNF-α, IL-6, plasminogen activator inhibitor-1
-
-
insulin resistance (homeostatic model assessment – insulin resistance)
-
insulin
-
-
vitamin D status
-
25-hydroxyvitamin D, calcium, phosphate
-
-
vitamin C status
-
plasma vitamin C.
-
These samples have not yet been analysed and, therefore, the data from these samples are not included in this report.
Justification for genetic study and samples
It would be interesting to study the associations and interactions of nutrition, physical activity, obesity and genes in the development, or lack of, of T2DM. The genetic assessments were focused on genes for which there is biological plausibility for interaction. The choice of genes and polymorphisms of interest was decided by an experienced group of researchers. All consenting participants were genotyped for genetic variants in key genes and data were analysed for gene–lifestyle interaction.
The demonstration of differential responses to lifestyle change by genotype not only would provide greater aetiological understanding but would also present the opportunity to investigate possibilities to use genotypic data in risk stratification and the identification of individuals who have the potential to benefit most from targeted lifestyle modification. In addition, improved understanding of gene–lifestyle interaction and diabetes mellitus risk would allow for the investigation of the policy implications for governments and industry.
Participants who did not wish to take part in the genetic study could opt out by indicating their preference on the consent form. This did not affect their participation in the study to any degree. Participants were informed that they could withdraw their consent to the storage and use of their genetic sample at any time. Samples were identification number (ID)-coded at the point of collection. Participants were asked to sign a specific consent form for allowing a sample to be taken for genetic analysis. The samples are retained at the University Hospitals of Leicester in a locked freezer at –80 °C, where they will be stored for 10 years. They were batched and sent for analysis at suitable times during the study. After 10 years the samples will be sent to a national officially recognised ‘tissue bank’ for future research if they have not already been used.
Outcome measures
The primary outcome of the screening phase was the proportion of people detected with PDM or T2DM using the LPRS (PPV). Secondary outcomes included the response rate to the invitation to screening. Those with PDM took part in phase two, the diabetes mellitus prevention cluster RCT.
Phase two: diabetes mellitus prevention cluster randomised controlled trial study design
Phase two was a cluster RCT providing a structured intervention for people with PDM. Randomisation was at practice level to negate contamination between individual participants. The practices were randomly assigned 1 : 1 to either the standard core or intervention arm by a researcher who was independent of the study team, using stratification by list size (< 6000, ≥ 6000) and ethnicity (percentage South Asian < 21%, ≥ 21%; median level of per cent of South Asian in the ADDITION-Leicester study123). Although randomisation occurred prior to recruitment, practices and participants were informed of their allocation in the results letters after the screening/baseline measurements were complete to avoid selection bias. Phase two was designed to adhere to internationally recognised criteria for developing complex interventions and for undertaking and reporting cluster RCTs. 158
Participants within the standard core practices were managed by nationally regarded ‘standard care’ guidelines for the condition, which consisted of participants being given an information booklet and general lifestyle advice by their GP or practice nurse. The booklet gave information on risk factors for T2DM and indicated how dietary and lifestyle changes and increased physical activity can be used to prevent progression of the disease. The booklet gave information in accordance with Leventhal’s common sense model,151 to address the causes, consequences, identity, control/treatment and timeline for participants with PDM.
Participants in the intervention practices were given the same written information as the control group and were also invited to attend the ‘Let’s Prevent’ programme, which was a 6-hour structured group education session. In addition, they received a telephone call every 3 months from nursing staff who were trained to offer ongoing support in behaviour change and to encourage participants to achieve their individual goals. Finally, each participant within the intervention arm was invited to attend a 3-hour refresher session once per year. This session was designed to reinforce the key messages from the programme, and allowed participants to re-examine their personalised risk profiles and set new goals/update their action plans if necessary.
The intervention
The structured group education programme was named Let’s Prevent and was a modified version of the DESMOND programme. It was based on published work performed locally for people with PDM. Let’s Prevent educational sessions consisted of one full-day session (6 hours) or two half-day sessions (3 hours each). In the case of the BME groups in which English was not readily spoken, the sessions were delivered as four 3-hour sessions by educators and interpreters (who had also undergone training). The programme followed a detailed written curriculum (see Chapter 4).
Outcome measures
The primary outcome was progression to T2DM at 3 years in people with screen-detected PDM.
The main secondary outcomes included:
-
changes in participant’s glucose levels: HbA1c, blood glucose levels fasting and post-glucose load
-
change in cardiovascular risk as calculated by the Framingham risk calculator
-
7-day step count
-
presence of metabolic syndrome as defined by the National Cholesterol Education Program Adult Treatment Panel III
-
cost-effectiveness of the intervention (for a full description see Chapter 8)
Inclusion/exclusion criteria
Participants were included in the RCT if they were:
-
diagnosed with PDM
-
aged 40–75 years if of white European ethnicity, or aged 25–75 years if of South Asian ethnicity
-
able to attend group education sessions.
Participants were excluded from the study if they:
-
were unable to give consent
-
were unable to attend group education sessions
-
were diagnosed with diabetes mellitus at screening
-
required an interpreter for a language other than Gujarati.
Sample size
It was calculated that 748 participants would need to be recruited to participate in the study: 374 in the intensive arm and 374 in the control arm, giving 280 per group after allowing for dropout. A substantial proportion, estimated at ≈30%, was predicted to be from BME groups. Recruitment took place from July 2009 to June 2011.
Assuming a 3-year cumulative conversion rate to T2DM of 35% in the control group, an intraclass correlation of 0.05, an average of 17 participants per practice and a dropout rate of 20%, we calculated that we would need 374 participants per group to detect a 40% risk reduction in the intervention group, that is, data from 44 practices, with 80% power at the 5% significance level. One secondary outcome was the percentage of participants in each group with a 10-year CVD risk > 20% at the end of 3 years. It was estimated that 55% of participants would have a CV risk > 20%. To detect a difference between the two groups of 20% of points in the proportion of participants with a 10-year risk of > 20% with 80% power and two alpha of 5%, and an intrapractice correlation coefficient of 0.0551, the required sample size was calculated to be 180 in the two groups.
Clinical assessments and measures
The clinical assessments and measurements that were taken at baseline during phase one of the trial were repeated at various time points during the RCT (Table 20 and Figure 8). Follow-up assessments occurred at 6, 12, 24 and 36 months post-baseline visit.
FIGURE 8.
Let’s Prevent study flow chart. Adapted from Gray et al. ,146 figure 1, and reproduced courtesy of Biomed Central under Creative Commons public licence 4.0, http://creativecommons.org/licenses/by/4.0/.
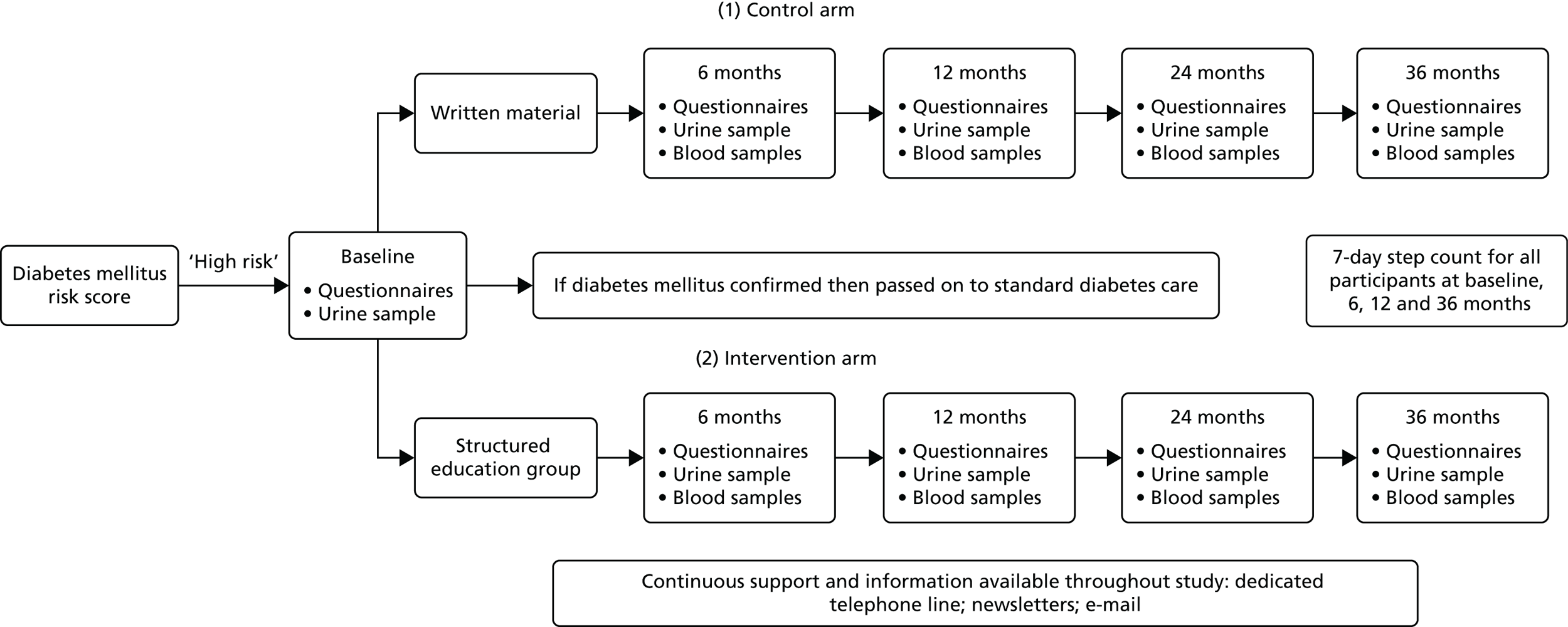
Participation in the study consisted of:
-
baseline screening visit with questionnaires, urine sample, OGTT, blood samples and clinical assessments (approximately 3 hours)
-
6-month review with questionnaires, blood sampling, body weight and BP measurements (approximately 1 hour)
-
first year review with questionnaires, urine sample, OGTT and blood sampling (approximately 3 hours)
-
second year review with questionnaires, urine sample, OGTT and blood sampling (approximately 3 hours)
-
third year review with questionnaires, urine sample, OGTT and blood sampling (approximately 3 hours).
Participants in the intervention arm additionally received the following:
-
a structured education session within 1 month of the initial visit (1 × 6-hour session; 2 × 3-hour generic course; 4 × 3-hour session for a BME group with interpreters)
-
an additional group refresher programme (3 hours) each year for those in the intervention arm, to consolidate previous learning and address any issues that had arisen
-
a telephone call every 3 months from a nurse trained in motivational techniques, to offer support and encouragement to participants in the intervention arm
-
continuous support network, available via telephone and e-mail.
Management of those found to have type 2 diabetes mellitus during the randomised controlled trial
Diagnosis of diabetes mellitus was made in accordance with WHO criteria/guidelines. 8 In all subjects without active symptoms of diabetes mellitus in whom the initial OGTT showed diabetes mellitus, participants were recalled for a second OGTT to confirm diagnosis. Furthermore, after a protocol amendment in January 2013, those who had HbA1c of ≥ 6.5%, regardless of their fasting and 2-hour blood glucose values, were also called back for a confirmatory HbA1c test. Therefore, T2DM was diagnosed using only an OGTT prior to January 2013, and with either an OGTT or a HbA1c test post January 2013 to follow the updated WHO diagnostic criteria for T2DM. 10 Progression to T2DM was the primary outcome, and participants who were identified at follow-up as having T2DM were retained in the trial. Both participants and their GPs were informed of the results of their tests. Participants remained in the study to complete the questionnaires and other biomedical data; however, they did not undertake any further OGTT. All study results were sent to the subjects’ GPs in the form of standard letters, and participants were referred back to their GPs for commencement of diabetes mellitus care. If a participant was diagnosed with T2DM by their GP between follow-up appointments, and they informed the study team, this was confirmed via blood tests taken when participants next attended for follow up. In these circumstances, participants were again excluded from any further OGTT, but their questionnaire and other biomedical data were still collected.
Study personnel and sites
The study was coordinated by the research team based at University Hospitals of Leicester (Leicester Royal Infirmary and Leicester General Hospital). Education sessions and clinical assessments took place at suitable locations within the community to ensure minimum disruption and travel for participants. General practices were approached individually and invited to take part by the primary care research network.
Data storage
Data were recorded directly on to individual participant data collection sheets. These source documents were located in an on-site locked filing cabinet for secure storage along with the questionnaires filled in by the participants. Laboratory reports that have checked and signed off by a nurse and a physician have been retained within the participant’s pack, and were entered onto an access-controlled database on daily basis. Self-evaluation data taken from questionnaires and structured contact report data were entered in batches during the course of the study. Monitoring of the data collection occurred at regular intervals with all data collection activities made in accordance with research governance and International Conference on Harmonisation Good Clinical Practice guidelines.
Data analysis
The study was reported in accordance with the internationally recognised Consolidated Standards of Reporting Trials199 statement for the reporting of RCTs. A statistical analysis plan was written and agreed by the principal investigator before the analysis began. Practice- and participant-level characteristics were compared by intervention group, using either means (SD) or medians (interquartile range) for continuous variables and counts and percentages for nominal variables. Cluster randomisation gives balance with respect to cluster-level covariates but can lead to imbalance in participant-level covariates, and, therefore, differences between the intervention groups were assessed using t-tests and chi-squared tests.
The primary outcome, progression to T2DM, was analysed on an ITT basis, as data were available for all participants. The event rate per 1000 person years was calculated by intervention group. Cox proportional hazards models with intervention group as a covariate were fitted, practices were assumed to have the same frailty (i.e. a shared frailty model was fitted). Hazard ratios (HRs) along with their 95% CIs were presented. The analysis was repeated excluding those from the intervention group who did not attend the education sessions (per-protocol). We had, in addition, assessed the dose–response relationship between level of attendance and the primary outcome; this is an exploratory unplanned analyses which should be viewed as hypothesis-generating.
For all secondary outcomes, those who developed T2DM during the study had their last value from before their diagnosis carried forward for the remainder of the study. This method was used in the previously published PREPARE study. All secondary outcomes were analysed using generalised estimating equation models with an exchangeable correlation structure, which adjusted for clustering. For binary outcomes we used a logit link with a binomial distribution for the outcome, and for continuous outcomes we used an identity link with a normal distribution. The analysis was conducted at each time point. The missing outcomes were not replaced and we derived an average of continuous outcomes over time. This procedure measures the cumulative effect of the intervention and has the maximum number of participants.
A sensitivity analysis was carried out for the main secondary outcomes. The analysis was repeated: (1) excluding those from the intervention group who did not attend the education sessions (per protocol); and (2) imputing any missing values using multiple imputation (ITT).
Subgroup analyses were performed for the primary outcome by PDM status (IGT, IFG, IGT and IFG, HbA1c 6.0–6.4%); age group (≤ 65 years, > 65 years); BMI (normal, overweight/obese); and risk score (≤ 6.04, > 6.04).
Adjustments were not made for multiple testing. All the results from planned analyses are reported and small p-values are interpreted taking into account the overall pattern of the results. Statistical significance was set at 5%. All analysis was conducted using Stata version 13.
Cost-effectiveness
A within-trial cost-effectiveness analysis was also conducted on the data to evaluate the cost-effectiveness of the Let’s Prevent intervention compared with the standard care arm (see Chapter 8). Cost-effectiveness was measured by the difference in costs divided by the difference in effects. Data were collected from the participants via a case record form, and an economic questionnaire was introduced through the study and given, at least, at patients’ 36-month clinic appointment. Cost-effectiveness was determined by: (1) incidence of T2DM at 36 months, in line with the primary outcome; and (2) QALYs gained. Quality of life was assessed using the health-state descriptive system (15D) questionnaire at baseline and all follow-up points, and the European Quality of Life-5 Dimensions (EQ-5D) instrument was also employed as part of the economic questionnaire. Base-case analysis for QALYs used the EQ-5D results and mapped 15D to EQ-5D scores for the periods without EQ-5D data. Costs included all costs associated with delivering the Let’s Prevent intervention, and all medications and health-care contacts. Missing data in costs and effects were addressed using multiple imputation techniques.
Ethics issues
Main research ethics committee approval and University Hospitals of Leicester Trust R&D approval were sought before commencement of the study. This ensured that all ethics and indemnity issues were adequately dealt with. Primary care approvals were obtained for Leicester City Primary Care Trust (PCT) and Leicestershire County and Rutland PCT. The study was adopted by the South East Midlands Diabetes Research Network, and also the East Midlands and South Yorkshire Primary Care Research Network.
An internal steering committee was established to oversee all activities required to determine safe and effective conduct of the study, and to recommend conclusion of the trial when significant benefits or risks developed, or in the case of the trial needing to be concluded early. The committee met on a regular basis to review data and discuss; however, no issues were identified.
Participants were sent a participant information sheet (see Appendix 18) to read before they registered their interest in taking part in the study by returning a response slip from the introduction letter or telephoning the dedicated study telephone number. This allowed adequate time for the participant to consider participation and actively seek involvement. An appointment was made for the participant to discuss the study and ask any questions that they may have had. A glucose tolerance test and other study assessments were then completed after informed consent had been obtained. All participants signed a consent form (see Appendix 20) to take part in the study. The consenting procedure was undertaken by research nurses and/or a suitably trained researcher before performing any study procedures. Training and assessment of non-medical health-care professionals was provided by the trust’s R&D office.
Data were collected on to case report forms (see Appendices 28 and 29). All data were entered into a restricted-access structured query language server database, held on the PCTs’ servers. This database was protected and access was given to a minimal number of users to deal with participant confidentiality issues. All study records were kept in a locked filing cabinet or room with limited access.
Additional consent was obtained for the taking and storage of samples for future genetic investigation, as well as for follow-up qualitative interviews. These specific requirements were not a condition of entry into the study, and participants could still be involved in the study if they did not wish to partake in the genetic or qualitative components.
Participants who had provided consent to allow the research team to follow up their health status after the study had finished will have their health status checked using sources that include general practice data, secondary care data, and Office for National Statistics mortality data. Data extraction from GP practice databases will be conducted using a Morbidity Query Information Export Syntax search based on participants’ NHS numbers. Short- and long-term outcomes such as weight, BMI, BP, glucose, lipids, CVD and the development of diabetes mellitus will be extracted. The help of the CCGs will be sought to obtain mortality and hospitals admission data using participant’s NHS numbers. The data will then be stored at the University Hospitals of Leicester and will be analysed independently by a statistician at the Leicester Diabetes Centre who will not have any participant identifiable data.
Confidentiality of data was maintained through the coding of participant data and the safe storage of paper and electronic data and audio tapes (which will be labelled with coded ID only).
Summary
The RCT was one of the first of its kind undertaken in the UK to look at the long-term effectiveness of a structured education programme focused on a lifestyle intervention in those patients identified through a risk-screening tool as being at high risk of T2DM. The Let’s Prevent study offered a unique and complete package, consisting of both screening and a subsequent intervention. Such an approach is in accordance with other recommendations from research, such as the IMAGE project. 47
National Institute for Health and Care Excellence guidelines around the prevention of diabetes mellitus also call for the use of lifestyle interventions as an integral part of any diabetes mellitus prevention pathway. 200 Although there has been much evidence to support the use of lifestyle interventions in improving health behaviours, and empirical evidence for both the clinical effectiveness and cost-effectiveness of such interventions have already been established, there is a lack of translational research in this domain. Let’s Prevent specifically targeted implementation within a routine health-care setting and with a multiethnic population for maximum transferability of results.
Chapter 6 Screening results
This chapter is based on a subset of results reproduced with kind permission from Springer Science+Business Media: Diabetologia, Implementation of the automated Leicester Practice Risk Score in two diabetes prevention trials provides a high yield of people with abnormal glucose tolerance, vol. 55, 2012, pp. 3238–44, Gray LJ, Khunti K, Edwardson C, Goldby S, Henson J, Morris DH, Sheppard D, Webb D, Williams S, Yates T, Davies MJ, excerpts of text, tables 1–3. 201
Introduction
As previously described (see Chapter 3), the LPRS was developed as a pragmatic way of screening primary care databases for those with PDM or undiagnosed T2DM for invitation to screening in a multiethnic UK population. Many risk scores have been developed and validated for identifying those at risk of T2DM,125,132,202 but the majority of these have not been used in practice and, therefore, there is a lack of evidence regarding their use in real-life settings. In addition, the majority of risk scores used in practice to date have identified those at risk of developing diabetes mellitus in the next 10 years rather than those with current PDM or undiagnosed T2DM. A recent systematic review203 found that 18 risk scores had been developed specifically for detecting PDM and undiagnosed T2DM and, of these, only three had published evidence of use in clinical practice. Noble et al. 132 concluded that, although much work had been done to develop risk scores for diabetes mellitus, most of these were rarely used; the authors suggested that the reasons for this may include the fact that they require data that are not routinely recorded or that scores were developed without a specific user or clear use in mind. The aim of this chapter is to report the prevalence of PDM and undiagnosed T2DM found when using a two-step screening strategy involving a risk score compared with a population-based approach.
Methods
The first phase of the Let’s Prevent study involved using the LPRS to identify people at risk of PDM and T2DM for invitation to a screening session where they received an OGTT. Those found to have undiagnosed T2DM were referred back to their GP; those with PDM were included in the cluster randomised prevention trial. For the full methodology of this first phase see Chapter 5.
Statistical methods
The primary outcome of the screening phase was the proportion of people detected with PDM or T2DM. Participants were categorised in accordance with WHO 1999 criteria. 8 Anyone who had an OGTT result in the diabetes mellitus range was recalled for a confirmatory test. In this study PDM was defined as IFG and/or IGT. IFG was defined as a fasting blood glucose concentration of between 6.1 and 6.9 mmol/l inclusive and IGT as a 2-hour blood glucose concentration of between 7.8 and 11 mmol/l inclusive.
Although HbA1c was not used for diagnosis in this study, we also report data on the following categories using HbA1c: 6.0–6.4% (42–47 mmol/mol), ≥ 6.5% (48 mmol/mol) and ≥ 6.0% (42 mmol/mol), and those suggested by the ADA, namely 5.7–6.4% (38–47 mmol/mol) and ≥ 5.7% (38 mmol/mol). 204 In addition, we assessed the yield when using a fasting blood glucose concentration of 5.5–6.9 mmol/l to define IFG, as recommended by NICE. 126 We also assessed the response rate to the invitation to screening.
The response rates and prevalence were compared with those found in the ADDITION-Leicester study. 123 As previously described (see Chapter 3), ADDITION-Leicester used a population-based screening approach in the same locality as Let’s Prevent. Random samples of 40- to 75-year-olds (25- to 75-year-olds for those of South Asian ethnicity) from 20 general practices were invited for an OGTT.
Means (SDs) and counts (%) were used to summarise the data. Differences between the screening approaches were assessed using the t-test for continuous variables and the chi-squared test for categorical variables. The prevalence (PPV) of each glucose disorder was calculated. All statistical tests are two-sided and p < 0.05 reflects statistical significance throughout.
Results
A total of 17,972 people from 44 practices were identified as high-risk in the Let’s Prevent Study; 19.2% of those invited attended screening. No difference in response rate was found between Let’s Prevent, where a risk score approach was used, and the population-based approach used in ADDITION (22.0%, p = 0.88). In total 3449 participants were screened (Table 22). The mean age of participants was 63.2 years, 60.8% were male, and the majority were of white European ethnicity (86.7%). Data were not available for those who did not attend screening.
| Characteristic | Let’s Prevent | ADDITION-Leicester | p-value |
|---|---|---|---|
| Number included | 3449 | 6479 | |
| Age (years), mean (SD) | 63.2 (8.1) | 56.1 (10.8) | < 0.0001 |
| Sex (male), n (%) | 2098 (60.8) | 3221 (47.7) | < 0.0001 |
| Ethnicity, n (%)a | |||
| White European | 2989 (86.7) | 4688 (71.7) | < 0.0001 |
| Chinese | 2 (0.1) | 8 (0.1) | |
| Caribbean/African | 72 (2.1) | 129 (2.0) | |
| South Asian | 368 (10.7) | 1684 (25.7) | |
| Mixed ethnicity | 15 (0.4) | 33 (0.5) | |
| Weight (kg), mean (SD) | 92.3 (17.9) | 78.0 (16.1) | < 0.0001 |
| BMI (kg/m2), mean (SD) | 32.4 (5.7) | 28.1 (5.0) | < 0.0001 |
| Waist circumference (cm), mean (SD) | 108.8 (12.9) | 93.9 (13.2) | < 0.0001 |
| Males | 110.5 (12.1) | 98.6 (11.5) | < 0.0001 |
| Females | 106.1 (13.5) | 89.7 (13.2) | < 0.0001 |
| Systolic BP (mmHg), mean (SD) | 145.3 (19.1) | 137.0 (19.6) | < 0.0001 |
| Diastolic BP (mmHg), mean (SD) | 86.1 (10.6) | 85.4 (10.6) | 0.01 |
| Total cholesterol (mmol/l), mean (SD) | 5.1 (1.0) | 5.5 (1.1) | < 0.0001 |
| LDL (mmol/l), mean (SD) | 3.0 (0.9) | 3.5 (0.9) | < 0.0001 |
| HDL (mmol/l), mean (SD) | 1.4 (0.4) | 1.4 (0.4) | 0.99 |
| Fasting glucose (mmol/l), mean (SD) | 5.3 (0.8) | 5.2 (0.9) | < 0.0001 |
| 2-hour glucose (mmol/l), mean (SD) | 6.7 (2.7) | 6.1 (2.5) | < 0.0001 |
| HbA1c (%/mmol/mol), mean (SD) | 5.9/40 (0.5) | 5.7/38 (0.6) | < 0.0001 |
| Antihypertensive therapy, n (%) | 1939 (56.2) | 1532 (22.7) | < 0.0001 |
| Statin therapy, n (%) | 1215 (38.0) | 737 (10.9) | < 0.0001 |
| Current smoker, n (%) | 275 (8.0) | 956 (14.2) | < 0.0001 |
Compared with ADDITION-Leicester, those screened using the risk score approach were significantly older, more likely to be male, heavier, had higher BP, glucose levels and HbA1c, and were more likely to be taking lipid-lowering and antihypertensive medications. Higher levels of total cholesterol were observed in ADDITION-Leicester, which is probably a reflection of the lower treatment levels with satins in this group (see Table 22).
Overall, 30.1% of those screened had abnormal glucose tolerance when assessed using an OGTT (Table 23). A total of 25.5% of participants were found to have PDM, of whom, the majority were people with IGT (22.4%); 4.5% were found to have undiagnosed T2DM. Using a fasting glucose level of 5.5–6.9 mmol/l to define PDM, as suggested by NICE, led to significantly more cases being identified (36.4% vs. 25.5%). Across all categories of glucose disorder, more cases were picked up when using a risk score approach than had been found in a study using a population-based screening approach.
| Glycaemic category | Let’s Prevent | ADDITION-Leicester |
|---|---|---|
| Fasting glucose 5.5–6.9 mmol/l | 36.4 (34.8 to 38.0) | 22.2 (21.2 to 23.2) |
| IFG | 7.8 (6.9 to 8.7) | 5.0 (4.5 to 5.6) |
| IGT | 22.4 (21.0 to 23.8) | 13.3 (12.4 to 14.1) |
| PDM | 25.5 (24.1 to 27.0) | 16.1 (15.2 to 17.0) |
| T2DM | 4.5 (3.8 to 5.2) | 3.2 (2.8 to 3.6) |
| Any abnormal glucose tolerance | 30.1 (28.5 to 31.6) | 19.3 (18.3 to 20.2) |
Using HbA1c gave higher prevalence across all categories (Table 24). A total of 45.0% of participants had an HbA1c of ≥ 6.0% (42 mmol/mol). Again, higher rates were seen in Let’s Prevent, which utilised a two-step approach, than in the population-based approach. Using the cut-off points suggested by the ADA204 significantly increased the yield across both screening approaches; for example, 75% of those screened using the two-step approach had a HbA1c of ≥ 5.7% (38 mmol/mol).
| Glycaemic category | Let’s Prevent | ADDITION-Leicester |
|---|---|---|
| HbA1c 6.0%–6.4% (42–47 mmol/mol) | 35.0 (33.4 to 36.6) | 17.5 (16.6 to 18.4) |
| HbA1c ≥ 6.5% (48 mmol/mol) | 10.0 (8.9 to 11.0) | 5.2 (4.7 to 5.8) |
| HbA1c ≥ 6.0% (42 mmol/mol) | 45.0 (43.3 to 46.6) | 22.8 (21.8 to 23.8) |
| HbA1c 5.7%–6.4% (38–47 mmol/mol) | 65.0 (63.4 to 66.6) | 55.3 (54.1 to 56.5) |
| HbA1c ≥ 5.7% (38 mmol/mol) | 75.0 (73.5 to 76.4) | 60.6 (59.4 to 61.7) |
Discussion
Finding effective methods to prevent T2DM is a public health priority, and given the unequivocal success of prevention programmes, there is an increasing need to develop tools in order to identify high-risk individuals who may benefit from these programmes. This study has shown a higher ‘hit rate’ when using a two-step screening approach than when using population screening, and this is in line with previous studies. 205 This approach has also been shown to be cost-effective in modelling studies. 30
Interestingly, although the yield was increased, the uptake to the screening invitation was low and comparable to that found in ADDITION-Leicester, a population-based screening programme carried out in the same area. 123 This finding suggests that informing people that they are at high risk of diabetes mellitus does not increase attendance to screening compared with a generic invitation. This is in contrast to other studies that have shown that risk stratification increases attendance. 205 There are many possible explanations for this. First, the low response rate may be attributable to people not wanting to take part in the clinical trial, rather than the screening. The NHS Health Check programme, which is not part of a research project, has seen uptake rates of around twice the level that we have reported here. 7,206 Second, everyone received an OGTT. The OGTT is costly, time-consuming and inconvenient. 24 Both patients and health-care professionals have reported that the OGTT is a barrier to attending screening. 207 The ADDITION-Europe study used a variety of screening methods to establish a cohort with screen-detected T2DM. 124 Centres that used a three-stepped approach combining a risk score, followed by a random blood glucose test, followed by an OGTT had a significantly greater uptake than centres that used a one-step approach using the OGTT only. 205 Third, both studies have been carried out in Leicester, UK, and the ethnic makeup of this locality may have had an impact on the response to screening. The area has a high prevalence of ethnic minority groups [ranging from 49% in the city of Leicester to 8% in the county of Leicestershire (2011 census; www.ethnicity.ac.uk/medialibrary/briefings/localdynamicsofdiversity/geographies-of-diversity-in-leicestershire.pdf) (accessed 21 July 2016)]. 208 Lower uptake to screening in those of South Asian ethnicity has consistently been observed. 122,123,140 This problem is amplified by the known increased risk of T2DM in this community. 140 The method of risk communication used may also affect uptake, and further investigation into this is warranted. Finally, the method of risk communication used was minimal; potential participants were told that there are risk factors for diabetes mellitus, some of which are modifiable, and that their medical records suggest that they might be at high risk. Informing people of their absolute risk of diabetes mellitus and potential benefits of early diagnosis and intervention may have increased uptake to screening.
In 2011, WHO recommended that the diagnostic criteria for T2DM be revised to include those with a HbA1c of ≥ 6.5% (48 mmol/mol). 8,10 WHO found insufficient evidence to classify PDM using HbA1c;10 however, an international expert panel and ADA have suggested that ranges of 6.0% (42 mmol/mol) to 6.4% (46 mmol/mol) and 5.7% (38 mmol/mol) to 6.0% (42 mmol/mol) can be considered. 204 Based on the ADA criteria, up to two-thirds of the population could fall into this category, which would overwhelm prevention initiatives. However, moving away from the OGTT has the potential to increase uptake to screening.
Let’s Prevent invited the top 10% most at-risk patients within a practice for screening. NICE suggest that the top 50% be invited for further testing. 200 Lowering the cut-off point will give potentially lower PPVs than seen here, as the prevalence of disease will be reduced in a lower-risk group. The benefit of using a practice-based risk score for screening is that GP practices can decide where to set the cut-off point based on the resources available. The LPRS is the first tool developed for use in GP practices that includes PDM in the conditions detected. Therefore, there are limited data with which to compare these results. Alongside the development of the risk score, we have also developed a piece of software that enables primary care to integrate this score into routine clinical practice. Using this tool optimises the high-quality data stored in general practice. 209 The LPRS software is freely available for use across the UK and can be downloaded from http://leicesterdiabetescentre.org.uk/The-Leicester-Diabetes-Risk-Score.
There are a number of limitations that must be considered. First, only those at high risk were invited for screening. Therefore, we are unable to assess sensitivity, specificity and the NPV of the score in this setting. Second, the low response rate, although similar to other studies in similar populations140 and which reflects the difficulty in recruiting a multiethnic urban population with wide variations in social economic status into research studies, may have affected the representativeness and generalisability of the data. Owing to ethical constraints, we were not able to collect data on those who were invited but did not attend screening; therefore, we are unable to compare the characteristics of those who attended with those who did not. These data were collected in the ADDITION-Leicester study and, overall, those who attended were older and more likely to be female. 140 Finally, the score used here utilised the percentage of South Asians within the practice as a proxy for individual ethnicity. Since the conception of these studies, the recording of ethnicity within general practice has improved and the updated version of the LPRS includes individuals’ ethnicity (see Chapter 3). Therefore, some South Asian participants may have received a falsely reduced score if they belonged to a practice with a high white European population and vice versa.
Summary
In summary, using a risk score to identify those at high risk of PDM/T2DM identifies a high previously undiagnosed yield using either an OGTT or HbA1c for diagnosis. Previous population-based screening programmes using universal OGTT have had lower yields. The LPRS is an inexpensive and simple way of targeting screening programmes at those at the highest risk of PDM/T2DM.
Chapter 7 Randomised controlled trial results
This chapter is based on results reprinted from Preventive Medicine, vol. 84, Melanie J Davies, Laura J Gray, Jacqui Troughton, Alastair Gray, Jaakko Tuomilehto, Azhar Farooqi, Kamlesh Khunti, Thomas Yates, A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let’s Prevent Diabetes cluster randomised controlled trial, pp. 48–56, 2016, with permission from Elsevier. 210
Introduction
As previously described in detail (see Chapter 5), the second phase of the Let’s Prevent Study was a cluster RCT in all those found to have PDM, comparing a T2DM prevention programme with standard care. The trial recruited from practices and participants from July 2009 to June 2011. Participants were followed up at 6, 12, 24 and 36 months post baseline. The primary end point was the development of T2DM during the 36-month follow-up. The aim of this chapter is to report the results of the RCT.
Results
Overall, 44 practices were recruited and randomised: 21 to the standard care group and 23 to the intervention group. Screening took place in all 44 practices, and all those found to have PDM were then included in the RCT. At one small practice (list size = 1650), no eligible participants were identified in phase one; therefore, only 43 practices are included in the RCT. The included practices ranged in size from 1470 to 24,000 patients. Six practices were recruited from areas with large South Asian populations (intervention = 4 practices, standard care = 2 practices). The median number of participants recruited per practice was 23 in the standard care arm and 17 in the intervention arm; the number of participants recruited per practice ranged from 2 to 49 (Table 25). Figure 9 shows the flow of participants through the trial. In total, 880 participants were found to have screen-detected PDM and were therefore included in the RCT (433 in the standard care arm, 447 in the intervention arm). At 36 months, 75% (n = 333) of the intervention group were still being followed up compared with 79% (n = 340) in the standard care arm (p = 0.43). Of those who were included in practices randomised to the intervention arm, 101 (22.6%) did not attend the initial education session and, therefore, are excluded from any per-protocol analysis (see Table 27). In total, 130 (29.1%) participants in the intervention arm attended all three education sessions (see Development of type 2 diabetes mellitus by level of attendance). Those who did not attend the initial session were not invited to the refresher sessions but they continued to be followed up.
| Variable | Standard care | Intervention |
|---|---|---|
| Individual level | ||
| Number of participants | 433 | 447 |
| Age, years | 63.9 (7.9) | 63.9 (7.6) |
| Male, n (%) | 278 (64.2) | 282 (63.1) |
| White European, n (%) | 363 (84.3) | 377 (84.5) |
| Deprivation, median (IQR) | 10.1 (6.3–18.1) | 13.4 (8.4–24.4)a |
| Current smoker, n (%) | 22 (5.1) | 38 (8.5)a |
| Prescribed statins, n (%) | 171 (43.3) | 184 (44.2) |
| Prescribed antihypertensives, n (%) | 270 (62.4) | 275 (61.5) |
| History CVD, n (%) | 78 (18.0) | 75 (16.8) |
| HbA1c (%) | 6.1 (0.4) | 6.1 (0.4) |
| HbA1c (mmol/mol) | 42.8 (4.6) | 43.2 (4.7) |
| Total cholesterol (mmol/l) | 5.1 (1.1) | 5.0 (1.0) |
| HDL cholesterol (mmol/l) | 1.4 (0.5) | 1.4 (0.5) |
| LDL cholesterol (mmol/l) | 3.0 (0.9) | 3.0 (0.9) |
| Triglycerides (mmol/l) | 1.7 (1.0) | 1.7 (0.9) |
| Systolic BP (mmHg) | 147.7 (17.7) | 147.9 (20.7) |
| Diastolic BP (mmHg) | 86.2 (10.6) | 86.6 (11.0) |
| Heart rate (b.p.m.) | 69.1 (12.1) | 68.3 (13.1) |
| Weight (kg) | 94.4 (18.9) | 89.9 (16.6)a |
| BMI (kg/m2) | 33.1 (5.8) | 32.0 (5.2)a |
| Waist circumference (cm) | 111.3 (13.2) | 108.0 (12.4)a |
| Average steps per day | 6308.12 (3094.44) | 6137.97 (2791.02) |
| IFG only, n (%) | 51 (11.8) | 57 (12.8) |
| IGT only, n (%) | 308 (71.1) | 301 (67.3) |
| IFG and IGT, n (%) | 74 (17.1) | 89 (19.9) |
| Cluster level | ||
| Number of practices | 20b | 23 |
| Median participants per practice (IQR) | 23 (8–34) | 17 (7–30) |
| Range participants per practice | 2–41 | 3–49 |
| Median practice size (IQR) | 6932 (4008–10,069) | 5429 (3356–8780) |
| High South Asian population, n (%) | 2 (10.0) | 4 (17.4) |
FIGURE 9.
Flow of practices and participants through the trial. Reprinted from Preventive Medicine, vol. 84, Melanie J Davies, Laura J Gray, Jacqui Troughton, Alastair Gray, Jaakko Tuomilehto, Azhar Farooqi, Kamlesh Khunti, Thomas Yates, A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let's Prevent Diabetes cluster randomised controlled trial, pp. 48–56, 2016, figure 1, with permission from Elsevier. 210
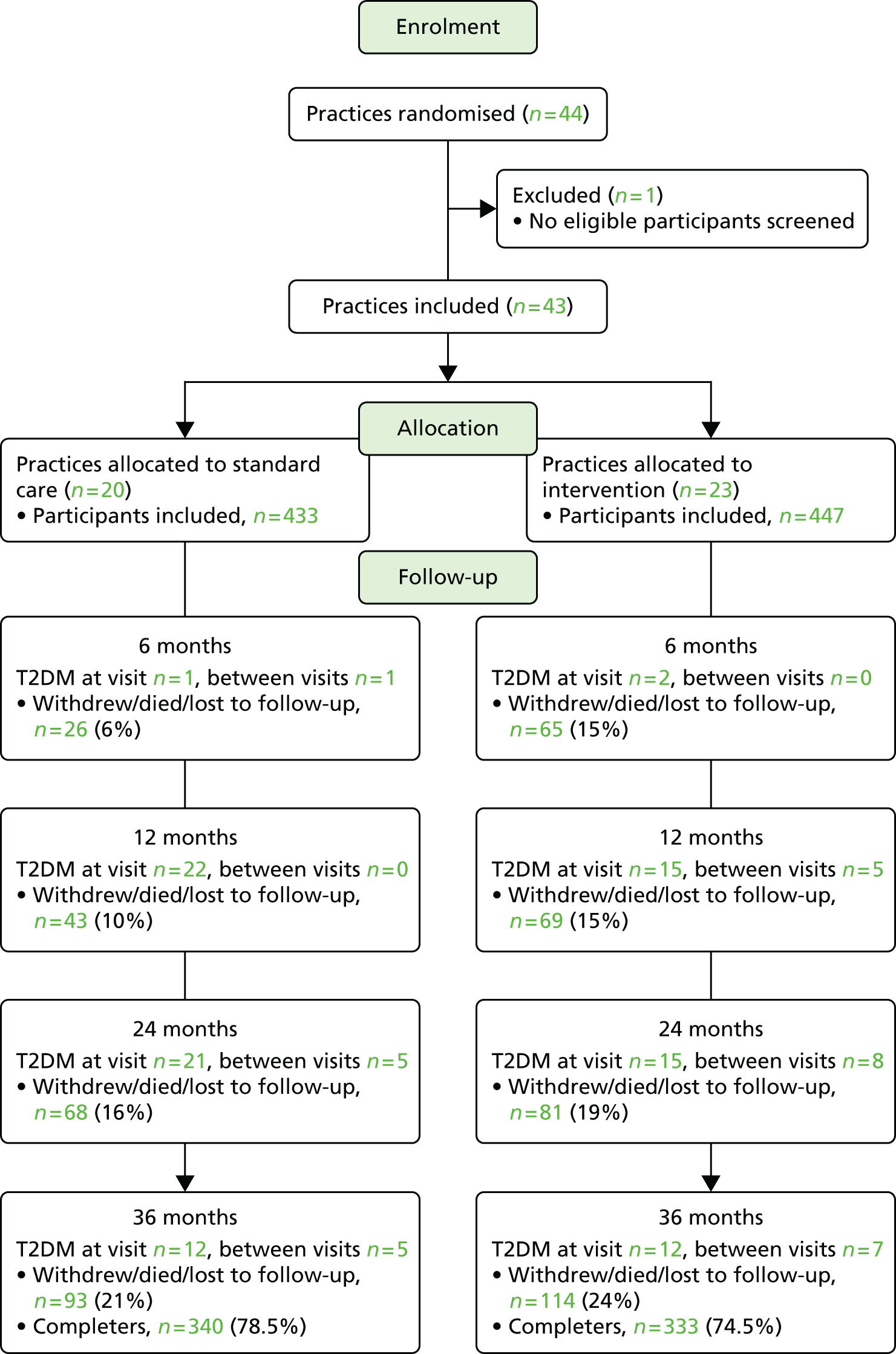
Table 25 shows the baseline characteristics of those included in the trial by randomisation. A higher level of deprivation was seen in the intervention group; this was measured using the IMD. The proportion of current smokers was significantly higher in the standard care group than in the intervention group (5.1% vs. 8.5%). The mean (SD) weight, BMI and waist circumference were also significantly higher in the standard care group than in the intervention group.
Development of type 2 diabetes mellitus
Overall, 131 participants developed T2DM over the course of the trial; this equates to 60.32 events per 1000 person-years (95% CI 50.82 to 71.58 events per 1000 person-years) (Table 26 and Figure 10). Lower event rates were seen in the intervention group than in the control group (57.60 events per 1000 person-years and 63.16 events per 1000 person-years, respectively). The HR showed a 26% reduced risk of developing T2DM in the intervention arm compared with standard care, but this did not reach statistical significance (p = 0.18). In a secondary, per-protocol analysis, the treatment effect was greater (35% reduction) when excluding those who did not attend the education programme in the intervention arm; this was also non-significant (p = 0.07). The difference in the event rates is accentuated in the per-protocol analysis (53.04 vs. 63.16, respectively).
| Analysis type | Standard care | Intervention | HR | 95% CI | p-value |
|---|---|---|---|---|---|
| ITTa | 0.74 | 0.48 to 1.14 | 0.18 | ||
| Events, n (%) | 67 (15.5) | 64 (14.3) | |||
| Rate per 1000 person-years (95% CI) | 63.16 (49.71 to 80.24) | 57.60 (45.09 to 73.59) | |||
| Per protocol | 0.65 | 0.41 to 1.03 | 0.07 | ||
| Events, n (%) | 67 (15.5) | 51 (14.7) | |||
| Rate per 1000 person-years (95% CI) | 63.16 (49.71 to 80.24) | 53.04 (40.31 to 69.80) |
FIGURE 10.
Kaplan–Meier survival curves by intervention. Green data relate to the standard care group and blue data relate to the intervention group. Trt, treatment group.
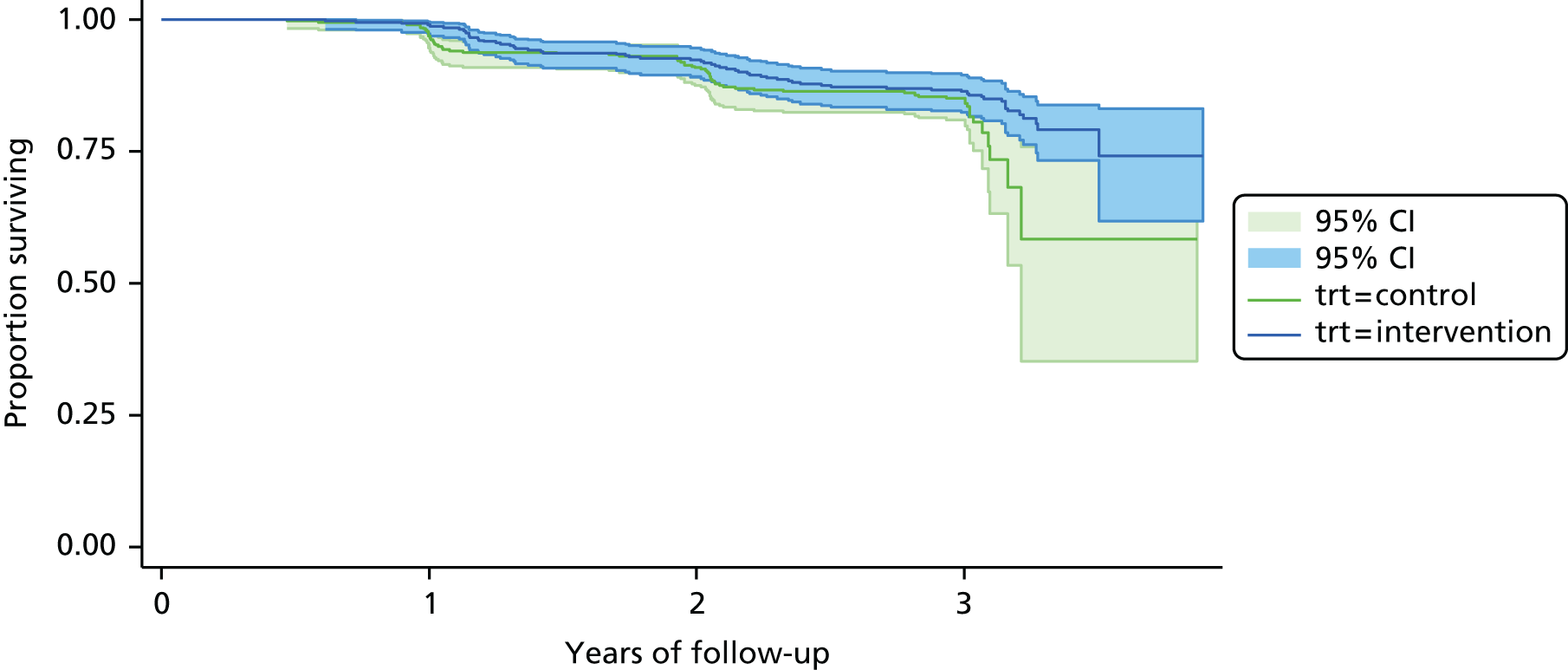
Development of type 2 diabetes mellitus by level of attendance
In addition to the pre-specified analysis of the primary outcome data by ITT and the per-protocol analysis excluding those who did not attend the first education session, we have also assessed the dose–response relationship between attendance and the development of T2DM. Of the 447 participants included in the intervention group, 77.4% attended the initial education session. Only those attending this session were invited to the refresher sessions. At 12 months, 45.6% attended the refresher and 38.5% attended at 24 months. In total, 130 participants attended all the education sessions, with 248 attending the initial session plus at least one refresher. Table 27 shows the primary outcome analysed by the level of attendance. There was a dose–response relationship with increasing attendance associated with greater reduction in the progression to T2DM in the intervention group compared with standard care, ranging from a 35% reduction in those attending the initial session to an 88% reduction in the subset who attend all sessions.
| Attendance | Intervention (n = 447), n (%) | HR (95% CI) | p-value |
|---|---|---|---|
| Initial educationa | 346 (77.4) | 0.65 (0.41 to 1.03) | 0.07 |
| 12-month refresher | 204 (45.6) | – | – |
| 24-month refresher | 174 (38.5) | – | – |
| Attended initial plus minimum of one refresher | 248 (55.5) | 0.38 (0.23 to 0.62) | < 0.0001 |
| Attended all sessions | 130 (29.1) | 0.12 (0.05 to 0.28) | < 0.0001 |
Biomedical outcomes
Table 28 shows a summary of the mean difference between the intervention and control group adjusted for cluster and baseline value at each time point. A full table of results with summary statistics at each time point is given in Appendix 31. Across both groups improvements were seen for many of the biomedical outcomes assessed. A statistically significant reduction of 0.06% in HbA1c was seen in the intervention group compared with the standard care group when the analysis was conducted across all time points. This seemed to be driven by an initial greater drop in HbA1c at 6 months (–0.06% vs. 0.01%). Significant reductions in LDL cholesterol were seen at 12 months and overall. For all other outcomes, apart from systolic BP, a greater reduction was seen in the intervention group than the standard care group at 36 months but none of these reached statistical significance.
| Outcome | Time point, months (95% CI) | Overall (95% CI) | |||
|---|---|---|---|---|---|
| 6 | 12 | 24 | 36 | ||
| Fasting glucose | NR | 0.001 (–0.10 to 0.10) | –0.06 (–0.16 to 0.04) | –0.05 (–0.18 to 0.07) | 0.0004 (–0.10 to 0.10) |
| 2-hour glucose | NR | 0.08 (–0.23 to 0.39) | –0.07 (–0.37 to 0.22) | –0.14 (–0.46 to 0.18) | –0.03 (–0.28 to 0.22) |
| HbA1c (%) | –0.07 (–0.12 to –0.01)* | –0.04 (–0.10 to 0.02) | –0.10 (–0.20 to –0.004)* | –0.07 (–0.18 to 0.04) | –0.06 (–0.11 to –0.01)* |
| Total cholesterol (mmol/l) | –0.06 (–0.18 to 0.05) | –0.07 (–0.16 to 0.02) | –0.02 (–0.12 to 0.08) | –0.11 (–0.23 to 0.02) | –0.06 (–0.14 to 0.01) |
| HDL cholesterol (mmol/l) | 0.003 (–0.05 to 0.06) | –0.01 (–0.07 to 0.05) | 0.004 (–0.06 to 0.07) | –0.02 (–0.08 to 0.05) | 0.01 (–0.04 to 0.05) |
| LDL cholesterol (mmol/l) | –0.06 (–0.15 to 0.04) | –0.10 (–0.18 to –0.02)* | –0.02 (–0.09 to 0.05) | –0.09 (–0.19 to 0.01) | –0.08 (–0.15 to –0.01)* |
| Triglyceride (mmol/l) | –0.01 (–0.16 to 0.14) | 0.05 (–0.05 to 0.15) | –0.05 (–0.15 to 0.05) | –0.06 (–0.17 to 0.06) | –0.001 (–0.08 to 0.08) |
| Body weight (kg) | –0.10 (–0.72 to 0.51) | –0.27 (–1.17 to 0.63) | –0.49 (–1.48 to 0.50) | –0.26 (–1.17 to 0.65) | –0.10 (–0.85 to 0.66) |
| BMI (kg/m2) | –0.03 (–0.24 to 0.19) | –0.11 (–0.42 to 0.21) | –0.14 (–0.50 to 0.21) | –0.05 (–0.38 to 0.27) | –0.02 (–0.28 to 0.25) |
| Waist circumference (cm) | –0.91 (–2.03 to 0.20) | –0.11 (–1.37 to 1.15) | –0.82 (–2.03 to 0.40) | –0.79 (–1.73 to 0.14) | –0.45 (–1.32 to 0.42) |
| Systolic BP (mmHg) | 1.17 (–1.45 to 3.79) | 1.22 (–0.85 to 3.30) | –1.26 (–3.79 to 1.28) | 0.55 (–2.09 to 3.19) | 0.81 (–0.97 to 2.60) |
| Diastolic BP (mmHg) | –0.22 (–1.90 to 1.46) | 0.80 (–0.66 to 2.26) | –0.37 (–1.92 to 1.19) | –0.49 (–2.15 to 1.17) | 0.24 (–0.82 to 1.30) |
| Heart rate (b.p.m.) | –1.31 (–2.90 to 0.28) | –0.61 (–1.84 to 0.61) | –0.68 (–2.00 to 0.65) | –0.52 (–1.83 to 0.78) | –0.66 (–1.58 to 0.27) |
Cardiovascular risk and metabolic syndrome
No differences were seen between the intervention groups at any of the time points for either CVD or CHD 10-year risk, the proportion with CVD risk > 20%, or the presence of the metabolic syndrome (see Appendix 31 for data).
Psychosocial and lifestyle outcomes
Table 29 shows a summary of the results for the psychosocial and lifestyle outcomes (the full results can be found in Appendix 31). Greater improvements were seen in illness perceptions, quality of life and anxiety in the intervention group than in the standard care group, all of which were significant when assessing the mean difference over time.
| Outcomes | Time point, months (95% CI) | Overall (95% CI) | |||
|---|---|---|---|---|---|
| 6 | 12 | 24 | 36 | ||
| Illness perception scorea | –1.46 (–3.13 to 0.21) | –2.06 (–4.03 to –0.09)* | –2.47 (–4.16 to –0.78)** | –1.16 (–2.69 to 0.37) | –1.61 (–2.92 to –0.30)* |
| Quality of lifeb | 0.01 (–0.001 to 0.01) | 0.01 (–0.002 to 0.02) | 0.01 (–0.002 to 0.02) | 0.02 (0.01 to 0.03)** | 0.01 (0.001 to 0.02)* |
| Anxiety scorec | –0.21 (–0.57 to 0.15) | –0.40 (–0.77 to –0.03)* | –0.09 (–0.40 to 0.21) | –0.11 (–0.44 to 0.23) | –0.28 (–0.54 to –0.02)* |
| Depression scorec | –0.08 (–0.42 to 0.26) | –0.34 (–0.81 to 0.14) | –0.09 (–0.45 to 0.27) | –0.05 (–0.44 to 0.35) | –0.21 (–0.57 to 0.16) |
| Diet | |||||
| Fibre intake | –1.69 (–4.68 to 1.29) | 0.97 (–1.27 to 3.21) | –1.64 (–4.68 to 1.39) | 1.53 (–0.94 to 4.00) | –1.01 (–3.11 to 1.08) |
| Fat intake | –1.41 (–4.60 to 1.77) | 0.45 (–2.62 to 3.51) | –0.55 (–4.04 to 2.95) | –3.60 (–7.52 to 0.31) | –0.72 (–2.92 to 1.48) |
| Unsaturated fat intake | 0.18 (–0.12 to 0.48) | 0.32 (0.05 to 0.58)* | 0.50 (0.24 to 0.76)*** | 0.38 (0.12 to 0.63)** | 0.33 (0.15 to 0.51)*** |
| Subjective physical activity | |||||
| Walking METs | 87.68 (–254.26 to 429.63) | 200.55 (–90.68 to 491.76) | 143.18 (–133.40 to 419.75) | –20.03 (–287.00 to 246.93) | 159.60 (–72.89 to 392.10) |
| Moderate METs | 123.90 (–189.44 to 437.24) | 102.18 (–64.53 to 268.88) | 128.16 (–67.06 to 323.37) | 28.65 (–180.84 to 238.14) | 144.18 (–25.98 to 314.33) |
| Vigorous METs | 154.34 (–174.69 to 483.38) | 173.82 (–116.09 to 463.73) | 214.85 (–95.51 to 525.21) | –7.14 (–279.77 to 265.49) | 160.05 (–52.07 to 372.17) |
| Total METs | 352.71 (–570.24 to 1275.65) | 447.31 (–220.84 to 1115.46) | 415.06 (–234.47 to 1064.59) | –19.82 (–568.05 to 528.41) | 428.37 (–175.19 to 1031.93) |
| Sitting time (minutes) | –27.26 (–63.34 to 8.83) | –25.94 (–49.95 to –1.92)* | –38.96 (–66.15 to –11.78)** | –20.15 (–43.91 to 3.60) | –26.29 (–45.26 to –7.32)** |
| Objective physical activity | |||||
| Average steps | 591.38 (63.61 to 1119.16)* | 551.76 (117.27 to 986.25)* | 466.30 (–65.50 to 998.10) | 535.76 (12.71 to 1058.81)* | 498.15 (162.10 to 834.20)** |
| Sleep | |||||
| Hours slept last night | NR | 0.04 (–0.15 to 0.22) | –0.05 (–0.18 to 0.09) | –0.10 (–0.26 to 0.06) | –0.05 (–0.18 to 0.08) |
| Average hours asleep in 24 hours | NR | 0.10 (–0.16 to 0.35) | –0.03 (–0.23 to 0.17) | 0.11 (–0.06 to 0.27) | 0.01 (–0.16 to 0.18) |
The diet data were collected via a food frequency questionnaire; overall, there was a lower rate of completion for these data than for the other questionnaire-based data collected; for example, 34% of participants had the total fibre intake score missing at one or more time points. Overall, no change was seen in either fibre or fat intake. At 12, 24 and 36 months and overall, a statistically significant increase in the intake of unsaturated fat was reported.
Although no differences between the intervention and standard care group were seen in the self-reported levels of activity, a significant reduction of around 30 minutes per day in time spent sitting was seen in the intervention group at 12 and 24 months and overall, compared with the standard care group. This was complemented by an increase in objectively measured average daily step count in the intervention group of 450–600 steps per day at all time points, with a significant effect seen at 6, 12 and 36 months and overall.
No change in sleeping behaviour was reported.
Sensitivity analyses
Table 30 shows the results of the sensitivity analysis. Here the analysis was repeated for the main secondary outcomes in two alternative data sets: (1) the per-protocol cohort, which excluded those who did not attend the education; and (2) the ITT cohort, which used multiple imputation to impute any missing data and therefore included all participants. The data are reassessed at 12 and 36 months only. Overall, the ITT analysis did not change the interpretation of the results for any of the secondary outcomes assessed. For the glucose outcomes at 36 months, significant reductions were seen for fasting glucose, 2-hour glucose and HbA1c when those not attending education were excluded. At 36 months a difference in HbA1c between the groups of –0.11% was seen, compared with –0.07% in the complete-case analysis. The per-protocol analysis had no effect on analysis of the CHD and CVD risk scores. Interestingly, although still not statistically significant, the effect on metabolic syndrome was reversed in the per-protocol analysis, with a protective effect being reported. The increase in step count in the intervention group compared with the standard care group was retained in both reanalyses, but with an increase in the effect seen in the intervention group in the per-protocol analysis (777 vs. 550 steps per day at 12 months and 634 vs. 535 steps per day at 36 months).
| Outcomea | Complete case (95% CI) | Per protocol (95% CI) | ITT (95% CI) | |||
|---|---|---|---|---|---|---|
| 12 months | 36 months | 12 months | 36 months | 12 months | 36 months | |
| Fasting glucose | 0.001 (–0.10 to 0.10) | –0.05 (–0.18 to 0.07) | 0.03 (–0.14 to 0.08) | –0.12 (–0.23 to –0.01)* | 0.02 (–0.09 to 0.13) | –0.02 (–0.13 to 0.08) |
| 2-hour glucose | 0.08 (–0.23 to 0.39) | –0.14 (–0.46 to 0.18) | 0.03 (–0.30 to 0.36) | –0.35 (–0.61 to –0.09)** | 0.10 (–0.22 to 0.42) | –0.10 (–0.45 to 0.25) |
| HbA1c, % | –0.04 (–0.10 to 0.02) | –0.07 (–0.18 to 0.04) | –0.04 (–0.10 to 0.02) | –0.11 (–0.21 to –0.01)* | –0.02 (–0.08 to 0.04) | –0.07 (–0.17 to 0.04) |
| CHD 10-year risk | –0.001 (–0.01 to 0.01) | 0.004 (–0.007 to 0.01) | –0.0002 (–0.01 to 0.01) | 0.005 (–0.01 to 0.02) | –0.004 (–0.01 to 0.01) | –0.004 (–0.02 to 0.01) |
| CVD 10-year risk | 0.003 (–0.01 to 0.01) | 0.01 (–0.004 to 0.02) | 0.003 (–0.01 to 0.02) | 0.01 (–0.003 to 0.02) | 0.001 (–0.01 to 0.02) | –0.0001 (–0.02 to 0.02) |
| Metabolic syndrome | 1.05 (0.78 to 1.43) | 1.10 (0.83 to 1.46) | 0.74 (0.52 to 1.05) | 0.77 (0.56 to 1.04) | 1.05 (0.78 to 1.43) | 1.10 (0.83 to 1.46) |
| Average steps per day | 551.76 (117.27 to 986.25)* | 535.76 (12.71 to 1058.81)* | 777.48 (336.66 to 1218.31)** | 634.27 (141.94 to 2665.56)* | 576.47 (110.37 to 1042.56)* | 469.52 (29.47 to 909.57)* |
Subgroup analyses
Table 31 shows the primary outcome assessed within specific subgroups. No significant associations between intervention and the development of T2DM were seen across all subgroups. There was a trend towards a greater increase in the non-significant reduction in the intervention group than in the standard care group in those with isolated IFG and those with both IFG and IGT.
| Subgroup | Number of events | HR (95% CI) | |
|---|---|---|---|
| Standard care | Intervention | ||
| PDM | |||
| IGT alone | 34 | 32 | 0.79 (0.45 to 1.38) |
| IFG alone | 7 | 6 | 0.52 (0.15 to 1.83) |
| IGT and IFG | 26 | 26 | 0.51 (0.22 to 1.16) |
| HbA1c 6.0–6.4% | 36 | 27 | 0.65 (0.38 to 1.12) |
| Age (years) | |||
| ≤ 65 | 40 | 45 | 1.19 (0.63 to 2.24)a |
| > 65 | 27 | 19 | 0.62 (0.36 to 1.06)a |
| BMI (kg/m2) | |||
| Normal | 1 | 1 | 0.59 (0.04 to 9.51) |
| Overweight/obese | 66 | 63 | 0.76 (0.50 to 1.16) |
| Risk score | |||
| ≤ 6.04 | 26 | 29 | 0.99 (0.46 to 2.10)a |
| > 6.04 | 41 | 35 | 1.05 (0.69 to 1.59)a |
Discussion
We have shown that a pragmatic diabetes mellitus prevention programme, aimed at those with PDM identified through a two-stage screening process, can lead to statistically significant improvements in HbA1c, LDL cholesterol, psychosocial well-being, sedentary time and step count up to 3 years post the initial education programme. The primary outcome of the study was reduction in the progression to T2DM; although non-significant, a large treatment effect of around a 25% reduction was seen in those practices randomised to the education. The treatment effect seems to be related to the level of attendance, with higher attendance being associated with greater reduction in progression to T2DM. This was reflected in the glucose levels recorded, with significant drops seen in fasting glucose, 2-hour glucose and HbA1c when excluding those who did not attend the education sessions.
Although not as high a reduction in T2DM as seen in more resource-intensive prevention programmes, the reduction in the progression of T2DM seen here is comparable to other pragmatic diabetes mellitus prevention programmes, such as the Indian programme. In the Indian programme, a reduction in T2DM progression of 28.5% was seen in those receiving a lifestyle modification programme. 34 A 58% reduction in T2DM (HR 0.4, 95% CI 0.3 to 0.7) was seen in the Finnish prevention programme, but this involved a significantly higher amount of contact time in those receiving the intervention. 32 Those in the intervention arm of the Finnish study received seven one-to-one sessions with a nutritionist during the first year and then one session every 3 months for the remainder of the study and were offered supervised, progressive, individually tailored circuit-type moderate-intensity resistance training sessions free of charge. 32 In a similar manner, the American DPP study intervention involved 16 lengthy one-to-one counselling sessions, followed by in-person one-to-one contact at least once every 2 months and additional group-based sessions four times annually; this also led to a 58% reduction in T2DM (95% CI 48% to 66%). 33 Such intensive prevention programmes are not achievable in the current UK health-care setting. In contrast, the Let’s Prevent programme consists of a 6-hour initial group course followed by a 1-hour refresher session each year (i.e. a total of 8 hours in group-based sessions over 3 years).
An interesting finding was the intervention attendance was strongly and linearly related to effectiveness; those who attended all three group-based contacts (initial Let’s Prevent programme and both annual follow-on sessions) had an 88% reduction in the risk of T2DM. Although total contact length and frequency have consistently been shown to be related to intervention effectiveness,52 this is the first study to show the strength of this relationship over the longer term and with face-to-face contacts separated by 12 months. This finding suggests that the weak effectiveness of the intervention overall was primarily driven by non-attendance rather than by ineffective interventional components. In total, 77% of participants attended the initial Let’s Prevent structured education programme, with 56% attending at least one follow-on session and 29% attending all three face-to-face group contacts. This demonstrates the difficulty in securing multiple contacts for behavioural interventions within a primary health-care setting and has implications for future prevention programmes. More research is needed to establish optimal methods of increasing uptake to lifestyle interventions within primary care, particularly around maximising adherence to follow-on support after the initial intervention.
Event rates reported in this study, that is, 57.60 events per 1000 person-years in the intervention arm compared with 63.16 events per 1000 person-years in the standard care arm, are consistent with a recent meta-analysis of prospective cohort studies in those with PDM which reported rates ranging from 35.54 per 1000 person-years in those with IFG up to 70.36 per 1000 person-years in those with both IGT and IFG. 14 The event rates reported here are also similar to DPP, which reported 48 per 1000 person-years in the lifestyle intervention group. 33 However, the progression rates in our study are substantially lower than other RCTs employing annual OGTTs in Europe and India, where rates of between 230 and 550 per 1000 person-years under control conditions and rates of between 110 and 393 per 1000 person-years after lifestyle interventions have been reported. 32,34 The sample size for the Let’s Prevent study was based on these European and Indian data and used a conversion rate of 35%. This lower event rate will have affected our power to detect a difference in the primary outcome.
As seen in other trials of a similar design to Let’s Prevent,48,121 improvements in many outcomes were seen in both the intervention and the control groups, although to a greater extent in those receiving the intervention. It could be that informing people that they have PDM is motivational in terms of improving one’s lifestyle or that those in the control group sought medical advice on their condition. Anecdotally, we were also aware of GPs initiating metformin in those included in the control group, even though this is not standard care for those with PDM. In addition, the NICE guidance around identifying and intervening in those at risk of T2DM was published during the RCT, which could have affected the results by increasing the interest in PDM and the prevention of T2DM in primary care. 126
Let’s Prevent was a cluster RCT; trials of this design have inherent issues compared with individually randomised studies. The study was powered based on a cluster size of 17 participants per practice,146 and the sample size was not inflated for variation in cluster size. Differences in cluster size can reduce statistical power. 211 Here the cluster sizes ranged from 2 to 49 (representing a coefficient of variation of 0.69). This was a pragmatic trial and we recruited practices of a range of sizes which led to this large variation in practice size. If this variation had been incorporated into the sample size the total sample size could have been increased by up to 42%, with the design effect increasing from 1.8 to 2.2. 212 This issue with study power could have led to the finding of no significance for the primary outcome. Interestingly, when the study is analysed ignoring the clustering, a significant effect is seen (p = 0.05). Another common issue with cluster randomised studies is that, although balance in practice-level characteristics is seen, balance in participant-level characteristics cannot be ensured. Here differences were seen in deprivation, smoking status and adiposity, with the intervention group more likely to be current smokers and have higher weight, BMI and waist circumference, suggesting a worse risk profile in the intervention group. Again this has been seen in other similar studies, such as DESMOND, where a difference in HbA1c was seen at baseline,48 and these differences could have affected the power of the study and the results seen; however, when adjusting for smoking status and BMI the interpretation of the data was not changed.
The Let’s Prevent curriculum addressed both diet and physical activity. Significant improvements in both reducing sitting time and step counts were seen. The deleterious effects of sedentary time have recently been reported, with studies showing an increased risk of diabetes mellitus, metabolic syndrome, CVD and mortality in those who sit for long durations. 212,213 Here we see a reduction of around 30 minutes per day; although small and self-reported, this is complemented by objective pedometer data showing an increase in daily step count of 500 steps across the duration of the study. This equates to an increase of around 5 minutes of purposeful walking per day, or 35 minutes per week. This is a similar increase to that found in the Early Activity in Diabetes (ACTID) diet and physical activity intervention for those with newly diagnosed T2DM, which was also run in a primary care setting. Early ACTID achieved a 5.6-minute increase in moderate/vigorous activity in the intervention group compared with the control group. 214 This is a smaller increase than was seen in the PREPARE study on which the Let’s Prevent programme was based. 50 PREPARE saw a 2000-step increase, but was a much smaller study (n = 98) which focused solely on increasing physical activity. To see a dilution effect when an intervention is implemented on a large scale is not uncommon,52 with other, similar, large-scale studies showing no effect on physical activity outcomes. 215
A lower rate of completion was seen for the food frequency questionnaire than for the other self-reported data collected. Increased intake of unsaturated fats was seen with a trend towards decreased fat intake; this might suggest that participants in the intervention group were making healthier food choices or this may be a chance finding. The results also show a trend towards decreased fibre intake in the intervention group, which goes against what is recommend in the education programme. Future research should attempt to incorporate objective measures of diet quality, which could include biomarkers such as circulating vitamin C levels.
Benefits of the education also extended to the psychosocial well-being of the participants, with decreases in anxiety and increases in quality of life and illness perception reported. This is in line with other education programmes; for example, a RCT of the DESMOND programme for those with newly diagnosed T2DM based on the same philosophy as Let’s Prevent saw long-lasting improvements in well-being. 216
Even with the inherent issues of cluster randomised trials and the impact that the variable cluster size and lower progression rate had on the power of the study, we have still managed to show positive findings from a relatively low-resource group intervention. The pragmatic nature of this relatively low-resource intervention, the fact that it was based on a programme that is now delivered as part of routine care for those with established T2DM (DESMOND) and the fact that the study was carried out in primary care are some of the key strengths of this RCT. The study also followed up participants over 3 years, thus providing data for the longer-term effects of such a programme. The NHS Health Check programme and the guidance from NICE to use a two-stage screening programme to identify those at risk of diabetes mellitus means that the number of people identified with PDM is on the rise. 13 Of the 53,799 people screened as part of the Health Check programme in Leicester City CCG, 2675 (5.0%) had PDM. 217 In addition, it is well reported that the use of HbA1c identifies a much larger disease burden than the OGTT. 135 We know that progression to diabetes mellitus can be prevented in those with PDM,31 but there are few evidence-based interventions that meet the NICE recommended standards which to refer those with PDM. Let’s Prevent could meet this pressing need, and Chapter 9 discusses pathways to implementation of such programmes.
The interpretation of this study should reflect the generalisability of the findings. The sample for the RCT was defined by the two-stage screening programme carried out in phase one of the study. Of those at high risk of having PDM who were invited for screening, only 19% attended. Although this is similar to other studies in similar populations139 and reflects the difficulty in recruiting a multiethnic urban population with wide variations in socioeconomic status into research studies, this may have affected the representativeness of the data. There were lower attendance rates among those from South Asian populations. However, as the screening was part of a research project in which people are asked to consent, provide data, etc., we would expect to see higher rates of uptake in a non-research setting. For example, the NHS Health Check programme has a 40% uptake rate. 206 A full discussion of the uptake to screening is given in Chapter 6. Future studies should look at methods for increasing the uptake to screening, particularly in hard-to-reach groups. All those screened and found to have PDM were included in the RCT. Of those attending practices in the intervention arm, 23% did not attend the initial education session. These participants continued to be followed up and, therefore, the treatment effect seen is representative of what would be expected in clinical practice. The Let’s Prevent programme was offered in two modalities, either as a 1-day (6-hour) programme or as two 3-hour sessions. A BME version of the programme delivered in Gujarati using translators was also offered. With T2DM becoming less a disease of older people and moving into younger populations, the need to deliver prevention interventions flexibly with optimal methods of ensuring continued engagement and adherence, including alternative approaches such as mobile health (mHealth) and electronic health (eHealth) solutions, should form the basis of taking this research further to increase participation. There is also a growing interest in the use of incentives for increasing uptake to interventions that promote health.
Overall, the Let’s Prevent RCT has shown that a relatively low-resource, pragmatic programme fit for implementation in the UK NHS can lead to a reduction in T2DM and improved biomedical and psychosocial outcomes. Future research should focus on increasing attendance to such programmes, which may require offering courses in formats not currently available, such as web-based formats. Chapter 8 assesses the cost-effectiveness of the intervention.
Chapter 8 Cost-effectiveness analysis (within trial)
This chapter uses excerpts from Leal et al. ,218 Cost-effectiveness of a pragmatic structured education intervention for the prevention of type 2 diabetes: economic evaluation of data from the Let’s Prevent Diabetes cluster-randomised controlled trial. BMJ Open 2017;7:e013592. This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Introduction
In the UK, diabetes mellitus accounts for approximately 10% of the total health-care expenditure, which is projected to increase to around 17% in 2035/366 as the prevalence of T2DM continues to rise. The Let’s Prevent programme is a pragmatic structured education and lifestyle modification intervention (centred on diet and exercise) for T2DM prevention within primary care pathways. The Let’s Prevent programme is based on the DESMOND programme and targets lifestyle behaviour change among individuals with PDM, using simple, non-technical language and visual aids. The DESMOND programme is the first national education programme for people with T2DM to meet NICE criteria149 and was shown to be clinically effective and cost-effective. 48,49
We used data from the Let’s Prevent trial to carry out a within-trial cost-effectiveness analysis of the structured education intervention within primary care. We estimated the incremental cost–utility analysis for the Let’s Prevent programme, with the difference in costs and in effects calculated in relation to the standard care group. The study perspective was that of the NHS, and we considered costs to the health-care service. We captured changes in life expectancy and quality of life using the QALY as the effectiveness measure.
Methods
The trial methodology is described in detail in Chapter 5. Here, only the elements specific to the cost-effectiveness analysis will be given.
Patients randomised to the standard care group received a booklet detailing information on risk factors for T2DM and how physical activity and lifestyle change can be used to prevent or delay the disease. Patients randomised to the intervention group received the same booklet as the standard care arm but were invited to attend an initial 6-hour structured education programme, three monthly nursing support telephone calls, and a 3-hour structured education update programme at 12 and 24 months. Both arms of the trial received follow-up sessions at the same time points and the same data were collected.
Economic data collection
Data were collected in the trial at baseline and at 6, 12, 24 and 36 months. These included general participant characteristics, trial-relevant blood test results, the 15D quality-of-life instrument, and participant-reported medication use and medical history. A further economic questionnaire incorporating the EQ-5D instrument and participant-reported economic outcomes was introduced halfway through the study (May 2012; trial started in December 2009 and ended in July 2014) to gather additional data relevant to economic analysis. The economic questionnaire was sent to 22 participants (2.5%) at 12 months, 408 participants (46%) at 24 months and 617 participants (70%) at 36 months.
Utilities
Utility data were available in the form of the 15D instrument at all time points (main questionnaire) and the EQ-5D instrument (economic questionnaire) at later time points. EQ-5D utility levels were generated using the EuroQol EQ-5D social tariff, estimated from a representative sample of the UK population. 219
Given the preference for EQ-5D data in health economics, we used a published regression equation220 to map 15D utility scores into estimated EQ-5D utilities when 15D but no EQ-5D data were available. Mapped utility scores were capped at a value of 1. As a supplementary exercise, reported as part of a sensitivity analysis, we also used the available pairs of 15D utility scores and EQ-5D utilities from the trial (participant and time point matched, n = 764) to generate a within-trial regression equation to map from 15D utility scores to EQ-5D utilities. In the base-case analysis, reported EQ-5D scores and mapped EQ-5D scores were merged (with preference given to the former when both were available) to maximise utility data prior to imputation.
Quality-adjusted life-years were calculated as the mean of the utility scores at the start and end of the year, or as the mean of the start, end and 6-month point in the case of the first year. These were then summed for each participant to obtain cumulative QALYs over the trial period, equivalent to an AUC calculation.
Intervention costs
The cost of the Let’s Prevent intervention was estimated by the trial team and was included in our cost-effectiveness analysis. The intervention cost (£200.37) was the total cost of providing the initial intervention, refreshers and support over the 3-year trial period (Table 32). One-off costs, such as educator training and teaching materials, were also included in the intervention cost calculation. These were not spread beyond the trial period, as this would have involved making an assumption about the lifespan of training and materials and may have been seen as favouring the intervention. Other trial-related costs (clinical tests, questionnaires, etc.) were not included in the analysis as they did not differ between the trial groups and would not exist outside the trial environment. The intervention cost was divided into yearly costs based on the year in which the relevant expenditure was required.
| One-off costs of intervention | Total costs (£) |
|---|---|
| Educator costs: attendance at initial training | 350.00 |
| Educator costs: preparation time to deliver initial curriculum | 175.00 |
| Venue costs: initial educator training | 600.00 |
| Educator costs: attendance at refresher session training | 175.00 |
| Educator costs: preparation time to deliver refresher sessions | 175.00 |
| Venue costs: educator refresher training | 200.00 |
| Resources/food models | 45.00 |
| Training materials: education curriculums (one English and one BME per educator) | 50.00 |
| Training materials: education resources per set | 282.00 |
| Total intervention | 2052.00 |
| Total per patient (447 patients) | 4.60 |
| Initial educational intervention | Cost per patient (£) |
| Administrative time | 5.44 |
| Co-ordinator time | 3.65 |
| Delivery F1 format session (8 hours, two educators) | 40.00 |
| Participant handbook | 3.20 |
| Pedometer | 10.00 |
| Refreshments | 1.00 |
| Paperwork/letters | 1.00 |
| Course materials (consumables) | 0.95 |
| Venue hire cost | 6.00 |
| Total | 71.25 |
| Education: yearly refresher session | Cost per patient (£) |
| Administrative time | 5.44 |
| Co-ordinator time | 3.65 |
| Delivery F3 format session (4 hours, one educator) | 15.00 |
| Refreshments | 1.00 |
| Paperwork/letters | 1.00 |
| Course materials (consumables) | 0.95 |
| Venue hire cost | 6.00 |
| Total | 33.05 |
| Motivational calls | Cost per patient (£) |
| Nurse time: four calls per year, 30 minutes each | 19.48 |
| Total | 19.48 |
| Total cost assuming: 10 people attending F1-format English speaking sessions, yearly refresher sessions (2 years) and motivational calls (four per year for 3 years) | |
| Grand total | 200.37 |
Health-care costs
Information on health-care use was recorded via participant self-reports in an economic questionnaire administered at 12-, 24- and 36-month follow-up points. Participants were asked to recall how many times over the previous 12 months they had seen their GP (in practice or at home), a practice nurse (practice or home), other health workers (practice or home) or a private practitioner; visited accident and emergency (A&E) or a hospital outpatient department; been admitted to day hospital or been admitted to inpatient care, and, if admitted, the number of nights spent in hospital. They were also asked to recall how much they had spent on medications over the previous 12 months, their travel costs for all health-care visits, their employment status and number of days off work sick, any lost earnings owing to ill health or health-care visits, and, if so, the estimated cost of this.
In the main questionnaire participants were screened yearly for major trial-relevant comorbidities such as myocardial infarction, stroke, peripheral vascular disease or atrial fibrillation, with the year of diagnosis recorded if present. This allowed calculation of the yearly incidence of several major conditions. However, only a limited number of conditions were recorded and there was no valid way of relating these comorbidities to the reported yearly health-care self-reported resource use. Therefore, to avoid double counting, these data were not costed independently or added to the economic analysis. Instead, health-care costs were estimated based on the self-reported questionnaire data.
If a participant had responded to any part of the economic questionnaire, missing entries in that response were recoded as zero. This was done owing to the high proportion of partially completed questionnaires, with greater degrees of missing data being apparent in questions to which a lower percentage of positive responses would be expected. Missing entries were kept as missing if the participant had not responded to any other sections of the questionnaire, or if the questionnaire had not been sent out.
Costs per health-care contact in a primary care setting were calculated for the majority of visit types using unit costs obtained from standard national sources. 221 All costs used national averages, with qualification costs and direct care costs included where applicable. (For all unit costs, see Table 49.) GP visit costs were £45 for practice visits (patient contact of 11.7 minutes) and £114 for home visits (visit time 23.4 minutes). Nurse costs were £13.40 for a practice visit (assuming an hourly cost of £40 per hour with an average contact duration of 15.5 minutes) and £70 for a home visit (assuming £70 per hour of home visiting, with 5.6 patient contacts per day on average and 69% of an 8-hour day spent on home visits). Other health-care worker costs could not be accurately assessed owing to the broadness of the category and, therefore, nurse unit costs were used as a surrogate. Costs per health-care contact in a hospital setting were costed using NHS reference costs for 2012/13. 222 Therefore, an outpatient visit cost was valued as £108 using a weighted mean of all outpatient visit types and their relative frequency; an A&E visit cost was valued at £114.86 (weighted mean of type 1 A&E visits not resulting in admission), and a day hospital visit cost was valued at £692, again calculated using a weighted mean of all day procedures and their relative frequency.
Inpatient costs were estimated using NHS reference costs for 2013/13. 222 A weighted mean cost (incorporating all bed-days for elective and non-elective admissions and their relative frequency) was calculated for inpatient admissions (£1758) and inpatient days (£581). Two different approaches were explored when valuing inpatient stays: (1) in terms of number of inpatient admissions (£1758) and (2) in terms of total number of days in hospital (£581). Furthermore, a composite variable was generated after identifying several instances in which the patient reported answers that were not logically consistent. For example, if a patient reported one or more admissions without reporting any days spent in hospital, a hospital days value was generated using a one-admission-to-5-days conversion rate (the within-trial average based on complete data for number of days in hospital per hospital admission). The converse was also applied when patients reported days in hospital but no admissions.
When dealing with remaining missing data, rather than imputing values for each category of resource use and then calculating health-care costs, all the above health-care costs were grouped into non-inpatient costs (GP, nurse, other health workers, A&E, outpatients, day hospital) and inpatient costs (overnight hospital admission), and missing values for these two categories were then imputed.
Medication costs
Medication use was collected from participants (assisted by health-care staff) in the trial questionnaire in the form of a yes/no/unknown categorisation at each time point for major trial-relevant medication categories (angiotensin-converting enzyme inhibitors, aspirin, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, statins, fibrates, thyroid medications, vitamins and steroids). For each of these medication categories a free-text entry was provided, where the name of the medication taken was recorded, although this was not always done. Any medication not falling into one of these categories was recorded in free-text format but not categorised. Participant self-reported medication costs were also recorded in the economic questionnaire, but owing to the imprecision and incompleteness of this information and uncertainty as to what it actually measured, this was not used in the analysis.
To value participant medication, we obtained unit costs for the categorised and free-text entry medications separately. Medications not available on prescription were not included. For the trial-relevant medication categories, costs were generated using weighted average medication unit costs for the relevant category from prescription cost analysis data on national prescribing volumes and costs. 223 An assumption of one tablet per day was made except where clearly inappropriate: for instance, for inhaled/intranasal/topical medications a use of one unit/month was assumed; for antibiotics a 7-day course was assumed; and for bisphosphonates once weekly administration was assumed. A coding of ‘unknown’ was treated as ‘no’ for the purposes of analysis, as this was felt to be most reflective of clinical reality. For the ‘other’ medications category, all unique word entries at 12, 24 and 36 months were extracted and assigned to author-generated categories. We then calculated weighted average medication costs for these categories (e.g. antidepressants, antiepileptics, non-steroidal anti-inflammatory drugs); medications that did not easily fit into a major category were assigned into a generic category and a standard weighted average medication cost was applied (using the mean of all previously estimated medication categories). Medications already included in the yes/no/unknown categorisation were not included in the other medication cost calculation. Using these methods, there was no evidence of any significant difference in medication costs at baseline between the intervention and control arms.
Statistical analysis
We performed a within-trial economic analysis, with total health-care costs and QALYs gained per patient calculated for the 36 months of the trial period in the intervention and standard care groups. Health-care costs consisted of medication and non-inpatient costs (GP, nurse, other health workers, A&E, outpatients, day hospital). The high proportions of missing data in several variables made the use of complete-case analysis unreliable and prone to potential bias. The degree of missing data was highest in the economic questionnaire (EQ-5D, inpatient and non-inpatient data) with 98%, 62% and 41% of data missing at 12, 24 and 36 months, respectively (see Table 50). In the main trial questionnaire, the proportion of missing data varied between variables and time points (21–45% missing data for 15D utilities; 19–41% for medication use data).
Inpatient costs were not included in the multiple imputation and subsequent analysis, because the degree of missing data was particularly high and because the available data displayed logical inconsistencies in the reported answers (e.g. patients reporting zero hospital admissions overnight but spending 2 days in hospital). We corrected for these logical inconsistencies using a 1 : 5 conversion rate where appropriate for ‘admissions: days in hospital’ (based on the within-trial average of 5 days spent in hospital per admission reported), but despite this correction inpatient costs could not be reliably imputed and resulted in heavily distorted data. Therefore, inpatient costs were not included in the primary analysis but are reported in the sensitivity analysis to demonstrate these problems.
Non-inpatient costs at 12 months were not imputed, as the proportion of missing data (98%) was too high to result in a valid imputation exercise. Therefore, we used non-inpatient costs at 24 months as an approximation of 12-month costs. This was done post multiple imputation.
Multiple imputations were performed using a chained model with 60 iterations to account for the high proportion of missing data in this trial. Multiple imputation replaced each missing value with a set of m plausible values to generate 60 replacement values (m = 60) for each of the missing cells in these data sets, using multiple linear regression models with the baseline complete covariates age, sex, BMI and practice code. The imputed variables were merged EQ-5D (reported + mapped) at 0, 6, 12, 24 and 36 months, non-inpatient costs at 24 and 36 months, and medication costs at 12, 24 and 36 months.
We accounted for the effects of clustering at practice level using a mixed-effects model with a random-effects component, at the level of practice code (Stata command Xtmixed). A covariate was deemed to be statistically significant if p < 0.05. Utilities were adjusted for baseline differences between the intervention and control groups in all subsequent analysis, using analysis of covariance. Statistical analysis was performed using Stata version 13.
Finally, all costs and QALYs were discounted to present values at a 3.5% annual rate. We calculated an incremental cost-effectiveness ratio (ICER) by dividing the mean cost difference between intervention and control groups by the mean QALY difference. We report the probability that the intervention is the most cost-effective option at a threshold of £20,000 per QALY gained using the net benefit framework and Fieller’s theorem.
Results
Utilities
Mean utility scores are reported in Tables 33 and 34. 15D utilities were available at all time points, with the percentage of missing data ranging between 20% at baseline and 45% at 36 months. EQ-5D utilities are reported at 12, 24 and 36 months with only minimal 12-month data available (n = 20). Mapped EQ-5D uses a published 15D to EQ-5D mapping equation (see Methods) to predict EQ-5D utilities using 15D data. We report the mean utilities of the merged EQ-5D variable pre and post multiple imputation (see Tables 33 and 34). This is shown graphically in Figures 11–13. A significant difference in 15D and in EQ-5D utility levels at baseline is evident, with slightly higher quality of life in the control group.
| Instrument and time point | Intervention | Control | ||
|---|---|---|---|---|
| Mean (SD) | Cases, n | Mean (SD) | Cases, n | |
| 15D (reported) | ||||
| Baseline | 0.872 (0.110) | 358 | 0.892 (0.921) | 337 |
| 6 months | 0.886 (0.097) | 283 | 0.893 (0.093) | 317 |
| 12 months | 0.890 (0.092) | 300 | 0.890 (0.097) | 314 |
| 24 months | 0.877 (0.101) | 264 | 0.883 (0.097) | 265 |
| 36 months | 0.884 (0.101) | 238 | 0.876 (0.097) | 249 |
| EQ-5D (reported)a | ||||
| 12 months | 0.835 (0.197) | 16 | 0.817 (0.174) | 4 |
| 24 months | 0.771 (0.237) | 178 | 0.800 (0.188) | 195 |
| 36 months | 0.797 (0.218) | 287 | 0.783 (0.223) | 292 |
| EQ-5D (mapped from 15D) | ||||
| Baseline | 0.783 (0.205) | 358 | 0.820 (0.171) | 337 |
| 6 months | 0.809 (0.180) | 283 | 0.822 (0.172) | 317 |
| 12 months | 0.816 (0.169) | 300 | 0.816 (0.179) | 314 |
| 24 months | 0.791 (0.186) | 264 | 0.802 (0.180) | 265 |
| 36 months | 0.804 (0.187) | 238 | 0.789 (0.179) | 249 |
| EQ-5D (reported + mapped) | ||||
| Baseline | 0.783 (0.205) | 358 | 0.820 (0.171) | 337 |
| 6 months | 0.809 (0.180) | 283 | 0.822 (0.172) | 317 |
| 12 months | 0.817 (0.170) | 304 | 0.817 (0.177) | 314 |
| 24 months | 0.783 (0.220) | 296 | 0.804 (0.188) | 303 |
| 36 months | 0.791 (0.215) | 261 | 0.784 (0.208) | 276 |
| Multiple imputation | Intervention (SE) | Control (SE) | Difference |
|---|---|---|---|
| EQ-5D (reported + mapped) | |||
| Baseline | 0.778 (0.010) | 0.819 (0.009) | –0.041 (p = 0.002) |
| 6 months | 0.796 (0.010) | 0.814 (0.009) | –0.018 (p = 0.164) |
| 12 months | 0.801 (0.009) | 0.815 (0.009) | –0.013 (p = 0.308) |
| 24 months | 0.776 (0.012) | 0.802 (0.010) | –0.027 (p = 0.095) |
| 36 months | 0.774 (0.013) | 0.779 (0.012) | –0.005 (p = 0.788) |
| Total QALYsa | 2.356 (0.029) | 2.414 (0.025) | –0.0585 (p = 0.130) |
FIGURE 11.
Complete-case mean utility scores for merged EQ-5D utility (reported + mapped) by intervention and control group at different follow-up time points. Error bars indicate CIs. Ctrl, control; Int, intervention.
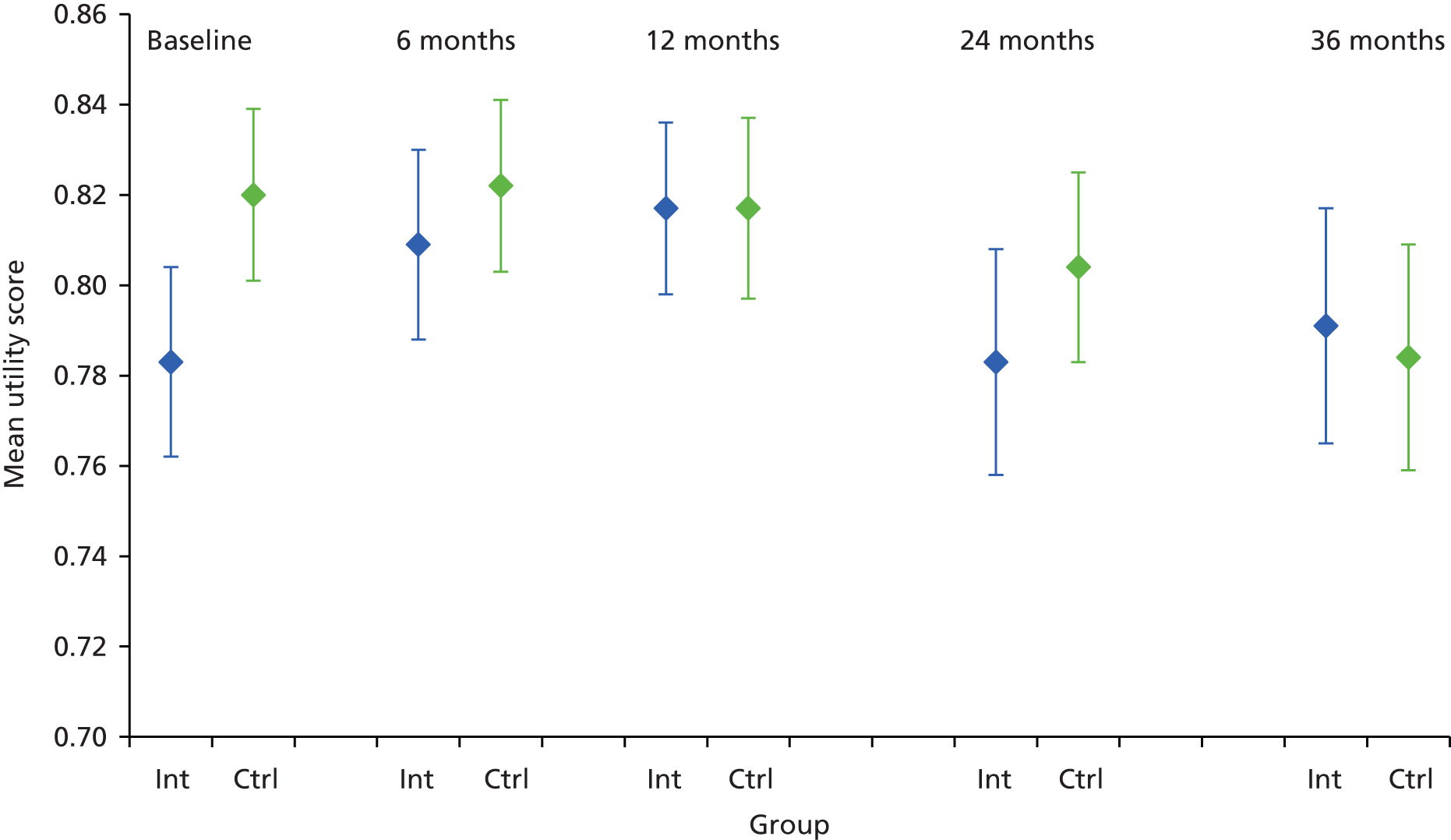
FIGURE 12.
Mean utility scores after multiple imputation for merged EQ-5D utility (reported + mapped) by intervention and control group at different time points. Error bars indicate CIs. Ctrl, control; Int, intervention. Reproduced from Leal et al. ,218 figure 1, Cost-effectiveness of a pragmatic structured education intervention for the prevention of type 2 diabetes: economic evaluation of data from the Let’s Prevent Diabetes cluster-randomised controlled trial. BMJ Open 2017;7:e013592. This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.

FIGURE 13.
Difference in mean utility scores between intervention and control groups at difference time points for different instruments. Positive values indicate higher utility in intervention group. MI reports the mean utility scores following multiple imputation.
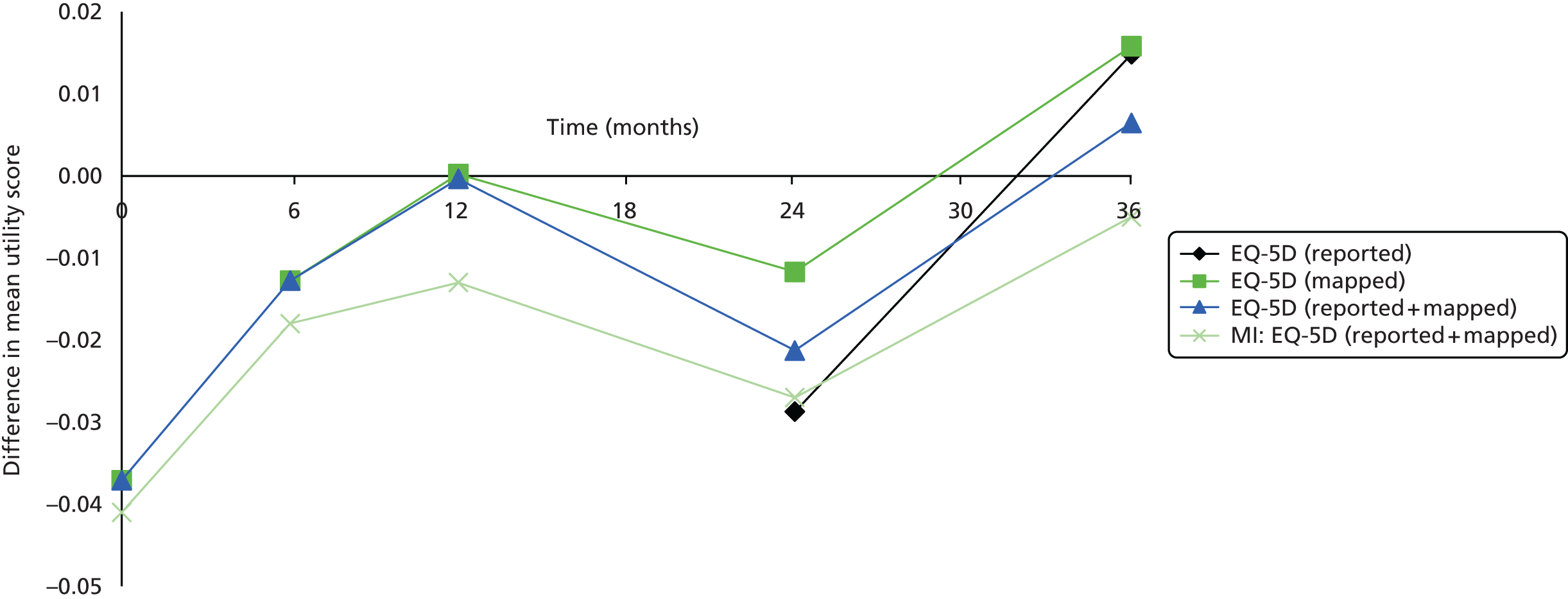
Resource use
Resource use data are reported in Table 35. Medication use is reported as the average number of medications taken per participant. The difference in number of medications reported is statistically significant at 36 months, with greater medication use reported by participants in the control group. Non-inpatient contacts are broadly similar between intervention and control groups and between time points. For inpatient resource use, the intervention group consistently reported higher hospital use in both number of admissions and number of days, although this difference is not statistically significant at any point.
| Category | Intervention (n = 447) | Control (n = 433) | ||||
|---|---|---|---|---|---|---|
| 12 months (SD) | 24 months (SD) | 36 months (SD) | 12 months (SD) | 24 months (SD) | 36 months (SD) | |
| Medication use | 3.2 (2.2) | 3.3 (2.2) | 3.2*(2.1) | 3.3 (2.1) | 3.4 (2.1) | 3.7* (2.2) |
| Non-inpatient contacts | n = 17 | n = 194 | n = 295 | n = 5 | n = 204 | n = 302 |
| GP (practice) | 2.59 (1.9) | 3.5 (3.4) | 3.0 (3.3) | 4.00 (6.7) | 3.3 (3.8) | 3.2 (3.6) |
| GP (home) | 0 (0) | 0.1 (0.7) | 0.1 (0.6) | 0 (0) | 0.1 (0.6) | 0.01 (0.1) |
| Nurse (practice) | 2.00 (1.5) | 2.0 (2.3) | 2.2 (2.8) | 3.4 (4.0) | 2.5 (4.9) | 2.0 (2.3) |
| Nurse (home) | 0 (0) | 0.1 (0.8) | 0.1 (0.9) | 0 (0) | 0.3 (2.1) | 0.01 (0.1) |
| HCW (practice) | 0.59 (2.0) | 0.3 (1.6) | 0.2 (0.9) | 0.20 (0.4) | 0.2 (0.8) | 0.3 (0.9) |
| HCW (home) | 0 (0) | 0.04 (0.4) | 0.01 (0.2) | 0 (0) | 0.01 (0.1) | 0 (0) |
| A&E | 0.12 (0.3) | 0.1 (0.4) | 0.2 (0.6) | 0.40 (0.9) | 0.2 (0.4) | 0.3 (1.3) |
| Outpatient | 0.59 (1.2) | 0.9 (1.9) | 1.1 (3.1) | 0.80 (0.8) | 0.8 (1.8) | 1.2 (3.7) |
| Day hospital | 0.06 (0.2) | 0.2 (0.6) | 0.1 (0.5) | 0.60 (0.9) | 0.1 (0.5) | 0.1 (0.4) |
| Inpatient contacts | n = 17 | n = 194 | n = 295 | n = 5 | n = 204 | n = 302 |
| Admissions overnight | 0.24 (0.6) | 0.19 (0.6) | 0.18 (0.7) | 0.80 (1.8) | 0.15 (0.6) | 0.15 (0.5) |
| Days in hospital | 0.89 (2.4) | 0.78 (4.0) | 0.69 (2.9) | 0.40 (0.9) | 0.76 (3.8) | 0.66 (3.1) |
Costs
Health-care costs are reported in Table 36 adopting a complete-case analysis and following multiple imputation. Medication and non-hospital costs were found to be lower in the intervention arm, albeit not significant.
| Category | Intervention | Control | ||
|---|---|---|---|---|
| Mean (SD) | Cases | Mean (SD) | Cases | |
| Intervention cost (£)a | ||||
| Year 1 | 93.53 | 447 | 0 | 433 |
| Year 2 | 53.42 | 447 | 0 | 433 |
| Year 3 | 53.42 | 447 | 0 | 433 |
| Medication costs (£) | ||||
| Baselineb | 108.15 (127) | 443 | 102.94 (94) | 419 |
| 12 months | 118.86 (132) | 361 | 126.15 (142) | 359 |
| 24 months | 123.73 (102) | 269 | 120.20 (95) | 294 |
| 36 months | 129.14 (147) | 258 | 136.22 (109) | 259 |
| Total medication costc | 347.88 (19) | 189 | 354.36 (18) | 204 |
| Non-inpatient costs (£) | ||||
| 12 months | 268.90 (267) | 17 | 775.78 (710) | 5 |
| 24 months | 444.92 (632) | 194 | 413.61 (621) | 204 |
| 36 months | 411.12 (631) | 295 | 428.54 (623) | 302 |
| Inpatient admission costs (£) | ||||
| 12 months | 413.65 (989) | 17 | 1406.40 (3145) | 5 |
| 24 months | 326.22 (1023) | 194 | 262.84 (1081) | 204 |
| 36 months | 321.80 (1256) | 295 | 256.13 (915) | 302 |
| Multiple imputation | ||||
| Category | Intervention (SE) | Control (SE) | Difference | |
| Medication costs (£) | ||||
| 12 months | 120.28 (6.9) | 123.82 (7.0) | –3.55 (p = 0.72) | |
| 24 months | 129.07 (5.6) | 123.82 (5.4) | 5.25 (p = 0.50) | |
| 36 months | 127.80 (7.5) | 138.47 (6.0) | –10.66 (p = 0.28) | |
| Non-inpatient costs (£) | ||||
| 12 monthsd | 442.03 (47) | 437.32 (92) | 4.71 (p = 0.97) | |
| 24 months | 442.03 (47) | 437.32 (92) | 4.71 (p = 0.97) | |
| 36 months | 417.78 (36) | 435.56 (35) | –17.78 (p = 0.73) | |
| Inpatient admission costs | ||||
| 12 months | –e | –e | –e | |
| 24 months | –e | –e | –e | |
| 36 months | –e | –e | –e | |
| Total medication cost (£)f | 377.16 (17) | 386.11 (15) | –8.96 (p = 0.70) | |
| Total non-inpatient cost (£)f | 1301.84 (113) | 1310.19 (194) | –8.35 (p = 0.97) | |
| Total intervention cost (£)f | 200.37 | 0 | ||
Cost-effectiveness analysis
The results of the cost-effectiveness analysis post multiple imputation are reported in Table 37, with a complete-case analysis included for comparison. In our analysis, the costs of the intervention group were found to be £168 higher than the control group over 3 years after accounting for clustering. The majority of this difference is accounted for by the intervention cost.
| Outcome | Complete (N = 225)a | Imputed (N = 880) | ||
|---|---|---|---|---|
| Intervention (n = 102) | Control (n = 123) | Intervention (n = 447) | Control (n = 433) | |
| Costs, £b | Medication, intervention | Non-inpatient, medication, intervention | ||
| Year 1 | 192 (9.3) | 109 (8.4) | 656 (49) | 561 (93) |
| Year 2 | 154 (9.5) | 106 (8.0) | 603 (47) | 542 (89) |
| Year 3 | 143 (8.9) | 112 (7.7) | 560 (36) | 536 (34) |
| QALYsb | ||||
| Year 1 | 0.835 (0.015) | 0.830 (0.013) | 0.793 (0.009) | 0.816 (0.008) |
| Year 2 | 0.800 (0.014) | 0.796 (0.013) | 0.762 (0.010) | 0.781 (0.008) |
| Year 3 | 0.759 (0.015) | 0.760 (0.014) | 0.723 (0.011) | 0.738 (0.010) |
| Total costs, £ | 489 (26) | 326 (21) | 1818 (114) | 1639 (192) |
| Total QALYs | 2.394 (0.042) | 2.386 (0.038) | 2.278 (0.028) | 2.334 (0.024) |
| Difference: QALY (unadjusted); 95% CI | 0.0087 (0.057); –0.103 to 0.120 | –0.0567 (0.04); –0.130 to 0.016 | ||
| Difference: QALY (adjusted for baseline); 95% CIc | 0.0349 (0.031) (–0.0257 to 0.0955) | 0.0389 (0.02); –0.004 to 0.082 | ||
| Difference: costs; 95% CI (£) | 162 (33); 97 to 228 | 179 (239); –296.52 to 655.03 | ||
| Cluster adjusted | ||||
| Difference: QALYc (adjusted); 95% CI | 0.0349 (0.031); –0.0250 to 0.0948 | 0.0461 (0.03); –0.0171 to 0.109 | ||
| Difference: costs; 95% CI (£) | 171 (41); 91 to 252 | 168 (285); –395.36 to 731.63 | ||
| ICER (£) | 4906 | 3643 | ||
| Probability cost-effective at £20,000 | 0.80 | 0.86 | ||
The costs as reported in the complete-case analysis (n = 225) are lower, as these do not include non-inpatient costs which were omitted owing to the fact that the high level of missing data would significantly reduce the number of cases available for analysis. However, the difference in costs after accounting for clustering is similar (£171) to the imputation analysis.
A cost-effectiveness acceptability curve is reported in Figure 14, showing the probability that the intervention is the most cost-effective option at difference values of willingness to pay per QALY. The Let’s Prevent intervention was found to result in a net gain of 0.046 QALYs over 3 years at an overall cost of £168 per patient, with an ICER of £3643 and a probability of 0.86 of being cost-effective at a willingness-to-pay threshold of £20,000.
FIGURE 14.
Cost-effectiveness acceptability curve showing the probability that the Let’s Prevent intervention is cost-effective for different ceilings of willingness to pay. Reproduced from Leal et al. ,218 figure 2, Cost-effectiveness of a pragmatic structured education intervention for the prevention of type 2 diabetes: economic evaluation of data from the Let’s Prevent Diabetes cluster-randomised controlled trial. BMJ Open 2017;7:e013592. This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
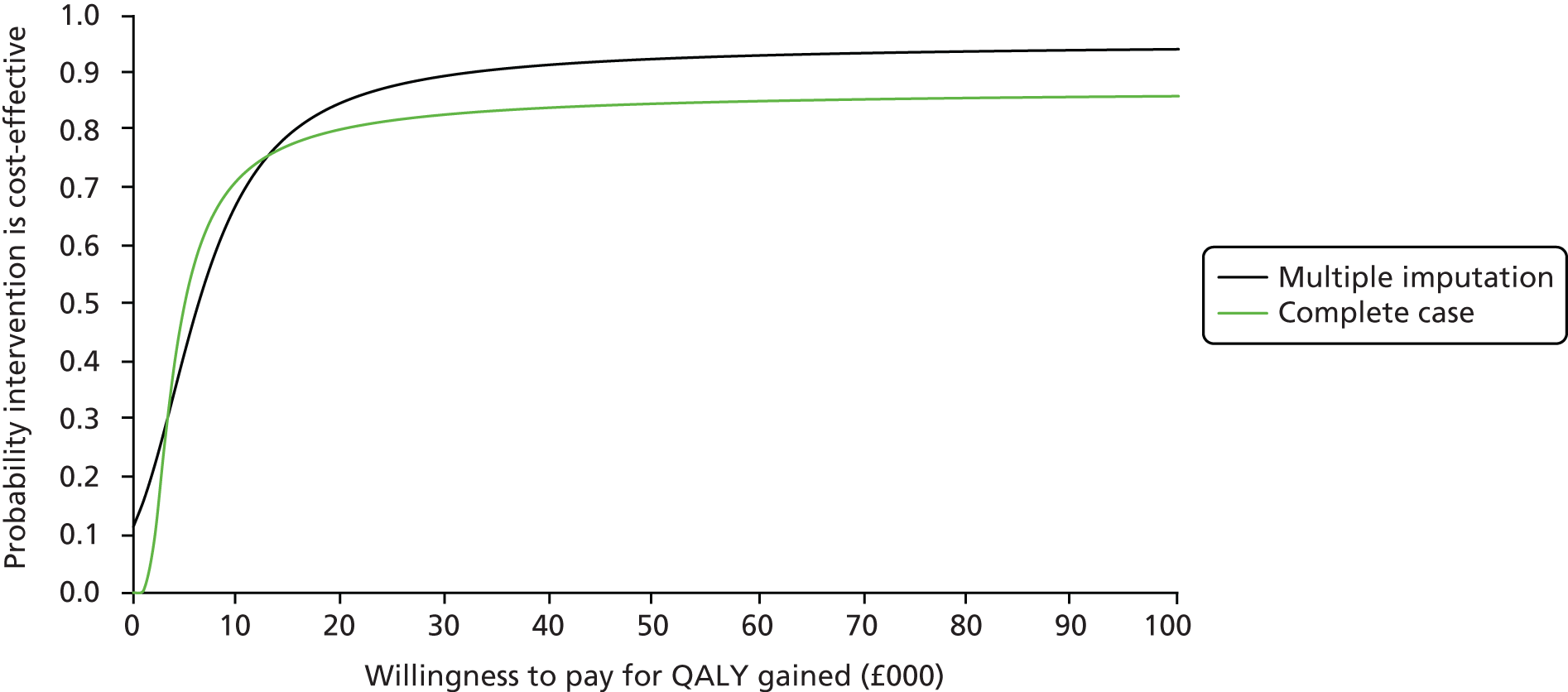
Sensitivity analysis
A sensitivity analysis was performed owing to the structural uncertainty surrounding the number of assumptions made in the analysis. This resulted in different estimates of costs and QALYs and resulting cost-effectiveness (Table 38), but in all scenarios the intervention remained cost-effective at a threshold of £20,000 per QALY.
| Scenario | Difference: total QALY, mean (SD); 95% CI | Difference: total cost (£); mean (SD); 95% CI | ICER (£) | Probability cost-effective at £20,000 |
|---|---|---|---|---|
| Estimation of difference in costs and effects without adjusting for clustering | 0.037 (0.022); –0.006 to 0.080 | 179 (239); –294 to 652 | 4845 | 0.87 |
| Multiple imputation performed without practice code as one of the baseline covariates and estimation of difference in costs and effects without adjusting for clustering | 0.029 (0.022); –0.015 to 0.072 | 225 (142); –53 to 503 | 7891 | 0.76 |
| 15D utility scores used to generate QALYs | 0.040* (0.016); 0.0078 to 0.072 | 168 (352); –532 to 869 | 4240 | 0.90 |
| 15D score used; non-inpatient costs not used | 0.039* (0.017); 0.0058 to 0.071 | 187 (25); 138 to 236 | 4848 | 0.96 |
| Mapping equation from 15D to EQ-5D estimated using trial data | 0.036 (0.029); –0.020 to 0.092 | 141 (234); –320 to 604 | 3977 | 0.82 |
| Hospital admission costs included | 0.042 (0.031); –0.019 to 0.10 | 137 (546); –945 to 1218 | 3290 | 0.80 |
| Hospital admission costs included and estimation of difference in costs and effects without adjusting for clustering | 0.029 (0.021); –0.012 to 0.071 | 500 (321); –131 to 1130 | 16,978 | 0.56 |
In our base-case analysis we adjusted for the cluster nature of the trial when performing multiple imputations and in our estimation of the difference in costs and QALYs between the options evaluated. By not adjusting for the cluster nature of the trial in these ways, the ICER increased to £7891 with a reduced probability that the intervention is cost-effective.
The majority of assumptions and missing data in this analysis concern the use of the economic questionnaire which was introduced part way through the trial, resulting in a lack of usable data at 12 months and high proportions of missing data at 24 and 36 months. Using only data from the main trial questionnaire (15D score, medication costs, intervention costs) and disregarding the economic questionnaire, the net gain in QALYs (0.039) was similar to the primary analysis using EQ-5D data (0.046). The net difference in total costs was also similar (£187), as in both scenarios the bulk of the cost difference between groups is a result of the intervention cost.
Inpatient costs were not included in the primary analysis owing to the limited and inconsistent data available. Including inpatient costs in the analysis results in markedly different results depending on whether or not clustering was adjusted for. Without adjusting for clustering, an ICER of £16,978 is estimated compared with an ICER of £3290 when adjusting for clustering.
Cost per case of diabetes mellitus prevented
At the end of the Let’s Prevent trial, 85.7% of patients in the intervention group had not developed T2DM, compared with 84.5% in the control group. Adjusting for clustering, the intervention group had an absolute risk reduction of 0.6% (95% CI –4.9% to 6.1%) for developing T2DM by the end of the trial. This equates to a cost per T2DM case prevented of £28,589, but it should be borne in mind that the trial found no statistically significant difference in this outcome measure.
Discussion
We have estimated the cost-effectiveness of the Let’s Prevent intervention, a structured lifestyle modification programme, using QALYs as our outcome measure. We have found that the intervention is likely to be cost-effective at a willingness-to-pay threshold of £20,000 per QALY gained.
The Let’s Prevent study is the first structured education- and lifestyle-based diabetes mellitus prevention RCT in participants with PDM states. Therefore, there are no directly comparable studies. However, previous studies have examined the cost-effectiveness of structured education and lifestyle interventions in patients with T2DM. The most relevant to our study is the DESMOND intervention, which delivered a structured education and lifestyle intervention (on which the Let’s Prevent intervention is based) and which was found to be cost-effective49 in its study population of patients with established T2DM, with an ICER of £5387 per QALY gained. Notably, the ‘real world’ costs of DESMOND are reported to be considerably less expensive, resulting in a ‘real world’ ICER of £2092. Although not included in this report, the ‘real word’ costs for the Let’s Prevent intervention would be significantly lower than the within-trial costs reported here. In practice, there would be economies of scale in terms of both man power and resources required. For example, the one-off costs incurred (educator training, food models, venue costs for training and training materials) would be used over a much longer period than in the trial, bringing the cost per patient down. In addition, in the trial educators were paid to run courses; in practice, the programme would be a commissioned service and existing members of staff might work in a different way to deliver the intervention. Under most such scenarios it could be anticipated that the intervention would be even more cost-effective when implemented in clinical practice than in a trial setting.
The strengths of the present analysis are that it is based on randomised data from a broad-based (43 GP practices) multiethnic patient population with a good length of follow-up (3 years) and relatively low patient drop-out rate. Data were collected on both quality of life and resource use simultaneously and at different time points.
The major limitations of this cost-effectiveness analysis relate to the degree of missing data and the reliability and validity of the data available. We attempted to compensate for these limitations by making several assumptions in our primary analysis and then exploring these assumptions in our sensitivity analysis. For such a cost-effectiveness analysis, the ideal data set would have consisted of EQ-5D utility scores at all time points, hospital use data recorded through case report forms and GP records of medications and appointments. In this study we had access to EQ-5D data at later time points, patient-reported yearly medications at all time points, and patient-reported hospital and health-care use at later time points.
In this study the EQ-5D instrument was introduced through the economic questionnaire part way through the study and, therefore, the majority of our quality-of-life data were in the form of the 15D instrument, although a significant number of EQ-5D data had been collected at later time points. Given the preference for EQ-5D in health economics, our goal was to use all reported EQ-5D data available and to estimate EQ-5D scores using 15D data if available, to reduce the number of data requiring multiple imputation. In our primary analysis we used a published regression equation to achieve this. As demonstrated in our sensitivity analysis, irrespective of the instrument or composite instrument used to estimate QALYs, we consistently demonstrated a greater increase in QALYs in the intervention than in the control group, once differences in baseline utility had been accounted for. Although the 15D instrument is not as well validated as the EQ-5D instrument, in this study it formed our most reliable and complete single data source on quality of life. Using only data from the 15D instrument, the net gain in QALYs in the intervention group was similar to our primary analysis using composite EQ-5D-based data. The difference noted in baseline utility may relate to the cluster randomised nature of this trial at the level of GP practices. Furthermore, we noted a significantly higher IMD score in the intervention group than in the standard care group at baseline.
Medication use data in this study were collected through the main trial questionnaire and the data set was comparatively complete. Although several assumptions were made in estimating medication costs, these were applied to both intervention and control groups, and the impact on difference in costs between the groups is likely to be minimal, although the accuracy of the absolute values for each group are likely to be less robust. We found no statistically significant difference between the intervention and control groups in medication cost. We did not include hospital data in our primary analysis owing to the limited number of data available, which were of questionable validity. When included in the multiple imputation models as part of the sensitivity analysis, inpatient admission data exerted a disproportionate effect on the ICER, with the net effect being markedly skewed depending on whether or not clustering was accounted for. This was thought to be a reflection of the relatively small sample of the inpatient data available, the imprecision with which it was recorded by patients and the high cost of inpatient admissions, such that the degrees of random variation that would be expected when handling data of a smaller sample would exert a dramatic effect on overall cost.
The volume of missing data was significant, ranging from 20% to 56% in the data that were used for the primary analysis. Some of the missing data relate to the introduction of the economic questionnaire half-way through the study. The remainder of the missing data is accounted for by attrition in patient response to questionnaires over time. To account for this level of missing data, we used multiple imputation, a validated technique224 for handling missing data in RCTs, which is superior to complete-case analysis when there are significant degrees of missing data. We were unable to use multiple imputation on 12-month non-inpatient data, as the degree of missing data (> 95%) did not allow for a workable imputation model. Therefore, we used 24-month estimates as a ‘best guess’ for 12-month data. Given the similarity in non-inpatient data, both between intervention and control groups, and between time points, the effects of this assumption on the analysis were minimal.
In order to account for the clustering in this trial we used a mixed-effects model to analyse data post multiple imputation. We also included the cluster code in the imputation itself as a baseline explanatory variable, although this does not correct the data for clustering but rather uses GP practice as one of the variables (in addition to age, sex, BMI, and quality of life and economic indices) to generate replacement values for missing data, without exerting any effect on complete data. Analysing cluster-randomised data in the presence of missing data is not straightforward and future research might fruitfully explore the performance of different methods of doing so.
A further limitation of this study is that the time horizon of the analysis was restricted to 3 years. A longer duration of follow-up might be expected to yield greater quality-of-life benefits for the intervention arm, thus further improving the cost-effectiveness of the intervention. Longer-term economic modelling was originally proposed in the study design, but not undertaken for two reasons. First, the primary within-trial analysis showed that the intervention was cost-effective (£3643 per QALY gained) over the 3-year follow-up period. We considered it highly likely that any extrapolation would simply make the intervention even more cost-effective and would not qualitatively change the conclusion. Second, the original intention to conduct some extrapolation was based on the assumption that the trial might produce differences in numbers with diagnosed diabetes mellitus, and the long-term outcomes of these differences could then be propagated by modelling. In fact, the trial did not find a statistically significant difference in the proportion of patients developing diabetes mellitus, in any between-group differences for CVD or CHD 10-year risk, in the proportion with a CVD risk > 20%, or in any risk factor other than a very small 0.06% difference in HbA1c. The outcomes were driven by the quality-of-life differences, which would not have been propagated by either of these models. However, additional follow-up of patients in the study would be very desirable to assess whether or not quality-of-life differences are maintained and whether any significant differences emerge in risk factors or in the development of diabetes mellitus.
Summary
This study has provided a reliable estimate of the costs of the Let’s Prevent intervention and broad-based estimates of the outcomes of the intervention (QALYs and health-care costs). Even taking into account the issues caused by missing data, the results appear robust across different assumptions, thereby indicating that the Let’s Prevent intervention is cost-effective using quality of life as our outcome measure. Owing to the modest number of cases of T2DM prevented, this study has not attempted to quantify the additional long-term benefits that this would provide.
Chapter 9 Implementation, impact and added value
Along with the primary research-oriented outputs detailed in earlier chapters, we undertook diverse associated activity which substantially contributed to the national infrastructure needed to ensure rapid implementation and dissemination of the outputs developed through this grant. This included the implementation of pathways, resources and processes for the prevention of T2DM within primary care, forming partnerships with industry and charities to extend the reach and uptake of developed materials, and contributing to national guidance. This chapter highlights these associated activities and provides a case study of how NIHR programme grants can link to the wider national agenda to maximise value for money.
Implementation pathways, resources and processes
Our programme grant coincided with the first round of NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) funding. Several investigators on the programme grant were centrally involved in the successful Leicestershire, Northamptonshire and Rutland CLAHRC, particularly in the organisation and leadership of the prevention and early detection themes. These twin pillars of NIHR-funded infrastructure afforded by CLAHRC and our programme grant supported a dedicated programme of work that was focused on developing clinically effective and feasible prevention pathways within primary care.
The initiation of the NHS Health Check programme in 2009 provided the first clear national-level policy for the prevention of vascular disease within primary care. 7 The prevention of T2DM through the identification of at-risk individuals and the provision of lifestyle change programmes was at the heart of the original business case and economic modelling that supported the creation of the NHS Health Check programme and remains a fundamental target of the policy. 225 However, there was no clear framework or mechanism for how diabetes mellitus prevention pathways could be co-opted into current primary care systems. We therefore set out to overcome this limitation by working with commissioners, GPs, practice nurses and members of the public.
After an initial scoping exercise, we centred this work around the DESMOND collaborative (see www.desmond-project.org.uk/). Since 2003, NICE has recommended the use of structured education in the management of diabetes mellitus;149 all individuals with T2DM are recommended to receive some form of structured education to aid their self-management and to promote lifestyle change at the time of diagnosis. DESMOND is one of the most prominent structured education programmes available to commissioning organisations nationally and the only programme to undergo a multicentre cluster RCT to quantify clinical effectiveness and cost-effectiveness. 48,49 As such, it is the most widely commissioned T2DM education programme in the UK, with over half of all CCGs (formally PCTs) having commissioned it for use within their diabetes mellitus care pathways. With the advent of the NHS Health Check programme, proactive regions expressed an interest in working with us to develop the systems needed to extend the delivery of structured education through DESMOND into the prevention of T2DM.
In 2010 work began with three early implementation sites to develop and pilot resources and pathways for extending structured education into the prevention of T2DM. At the time, the ‘Walking Away from Diabetes’ programme was the only structured education programme aimed at the prevention of T2DM to be underpinned by RCT evidence of efficacy. 50,51,226 Walking Away (originally called the PREPARE programme) acted as a precursor to, and informed the content of, the Let’s Prevent programme (see Chapter 4). Given that in 2010 Walking Away was already fully developed through CLAHRC, and considering the underpinning evidence of efficacy, it was chosen as the initial test-case structured education programme to inform our national implementation pilot work. However, the developed pathways are not specific to any one programme and can be utilised by other programmes, including Let’s Prevent.
Implementation pilot sites
The three initial pilot sites were Cumbria, Brighton and County Cork, Republic of Ireland. Cumbria Partnership NHS Foundation Trust had successfully embedded DESMOND into their diabetes mellitus care pathway and were planning on using opportunistic screening through the NHS Health Check programme to identify and refer at-risk individuals into a diabetes mellitus prevention programme. Brighton PCT had recently commissioned DESMOND and had a coexisting cohort of individuals within primary care who were identified and coded with IFG or IGT. There was local commitment for supporting and developing a pathway for these participants with referral into a lifestyle intervention. Finally, County Cork, Ireland, had secured funding to run a community-based model of prevention, which included the identification of at-risk individuals through the promotion of the Leicester self-assessment risk score and community campaigning. High-risk individuals were then able to request or seek referral into a lifestyle intervention. The sites provided three different test cases which would enable generalisability of developed approaches to future sites.
All three sites were visited by our team for initiation visits with relevant stakeholders. A detailed plan of implementation was than drawn up for each site. A cohort of DESMOND educators from each site were provided with one full day of vocational training to enable them to deliver Walking Away in addition to their DESMOND training. Training and subsequent quality-assurance models were based on those developed by the programme grant (see Chapter 4). The trained cohort of DESMOND educators and commissioners then met with our team several months later to discuss progress, to share successes and failures in terms of referral pathways and attendance rates, to highlight planned revisions to their pathways and to provide feedback on how the programme content and delivery could be improved. Finally, our team conducted a final site visit, which included undertaking focus groups with participants who had been through the prevention pathway from identification to referral through to attendance on the Walking Away programme. The focus groups were used to understand user-level perceptions of the referral pathway and programme content. This work provided a blueprint for the implementation of structured education in the prevention of T2DM which rapidly expanded to include differential models of training for DESMOND or non-DESMOND sites and registered or non-registered health-care professionals.
To date, our pathway has been commissioned in over 15 regional locations in the UK, from Edinburgh to Essex; in two regional locations in the Republic of Ireland; and in Gibraltar, with ongoing plans for implementation in Australia. We have worked with our implementation partners to evaluate specific elements of the pathway. For example, the implementation in Cumbria was estimated to cost as little as £30 per patient referred. Up until mid-2014, over 3000 individuals have been referred to and attended the Walking Away programme in the Cumbria site alone. A recent evaluation of the pathway in the Wigston and Oadby area of Leicestershire reported highly positive feedback from participants and demonstrated significant changes to behaviour 6 months after attending the Walking Away programme to levels that were consistent to changes reported in the original RCT (for report see Appendix 33).
Leicester Prevention Pathway
Each element of our prevention pathway developed in the above work, and directly informed through the programme grant detailed in earlier chapters, has been combined to form the Leicester Prevention Pathway. The elements and resources available within the Leicester Prevention Pathway are highlighted in Table 39.
| Pathway area | Tool name | Description | Freely available? | Available through commissioning? |
|---|---|---|---|---|
| The burden | Estimating your at-risk population | A spreadsheet tool that estimates the number of individuals within a given CCG who have undiagnosed T2DM or who are at risk of T2DM. Undiagnosed and at-risk prevalence categories can be tailored to assessment method, including HbA1c. In addition, the tool will estimate the projected annual incidence rates of T2DM from the at-risk category if usual care is maintained. Data are based on Leicester-ADDITION,9 a population-based T2DM screening study and tailored to individual CCG size and ethnic make-up | In process | NA |
| Risk identification | Algorithm | Detailed clinical algorithms for the identification of undiagnosed T2DM or PDM based on NICE guidance. These tools provide a step-by-step approach to risk identification and are designed to support decision-making within primary care. An additional algorithm was developed to support the implementation of risk identification within Boots (Boots UK, Ltd, Nottingham, UK) pharmacies (described further in the next section) | ✓ | NA |
| Practice risk score | Freely available tool which can be downloaded onto GP practice computers and used to rank all adults aged 40–75 years for the risk of T2DM; based on the risk score detailed in Chapter 3. This tool allows GP practices to quickly and efficiently identify who they should target for further screening and/or referral (see www.leicesterdiabetescentre.org.uk/Leicester_Practice_Risk_Score-5905.html) | ✓ | NA | |
| Self-assessment risk score | Self-assessment risk score to complement the practice-based risk score. Additional funding secured from Diabetes UK was used to develop a paper-based risk score which enables individuals to calculate their own risk, helps raise awareness and promotes self-referral. The risk score was developed with input from service users and translated into prominent minority languages. The score also exists in a web-based platform developed by Diabetes UK (see http://riskscore.diabetes.org.uk/2013) | ✓ | NA | |
| Information booklet | Are You at Risk of Type 2 Diabetes?: information booklet | A patient booklet that provides information on how the risk of T2DM is identified, what this means for the individual, and how the risk can be modified with lifestyle change. The booklet was designed in response to an identified clinical need for accurate and easy-to-disseminate patient information following identification of a high-risk status | ✓ | NA |
| Lifestyle intervention | Walking Away from Diabetes | A 3-hour structured education focused on increasing walking activity in the prevention of T2DM with an evidence-based, theory-driven written curriculum and educator training and quality-assurance pathways. As of November 2014, Walking Away has been commissioned in over 15 regions throughout the UK as well as in the Republic of Ireland, Gibraltar and Australia. The programme includes a host of supporting material for GP practices, including standardised referral letters for patients, coding guidelines and evaluation forms. A commissioning pack is available on request | ✗ | ✓ |
| Let’s Prevent Diabetes | A 6-hour structured education programme focusing on weight management, diet and physical activity (see Chapter 4). The programme will be available to commissioners from 2015 onwards | ✗ | ✓ |
Links with and dissemination through industrial partners
The work around the development of risk scores and risk identification algorithms supported by the programme grant (see Chapter 3) enabled significant collaboration and partnership with charity and industry partners, resulting in substantially increased reach and dissemination to the general population. In 2009 we were commissioned by the charity Diabetes UK to develop a self-assessment risk score for use by the general population. This work supported the development and validation of a scoring protocol tailored to information gained through self-assessment rather than practice records. 138 The self-assessment score and practice risk score contain similar variables and used the same development and validation analysis plan, but have been developed with their specific use in mind. The practice risk score is calculated by the LPRS software and therefore has a more sophisticated equation for calculating the score which gives greater discrimination; the self-assessment score is calculated by hand and is therefore a crude score with integer points given to categorised variables. The self-assessment additionally asks about waist circumference; at the time of developing the practice risk score this was not routinely collected in primary care and, therefore, has not been included in the practice risk score. Developing risk scores with a specific use in mind and therefore tailored to the requirements of the potential future use is recommended to those developing risk scores but has rarely been considered in the development of other risk scores. 132 This has been highlighted as a possible reason for the limited uptake and use in clinical practice of the many risk scores developed internationally for detecting those at risk of diabetes mellitus. 132
Both scores are recommended by NICE for the identification of those at risk. 126 Below is an outline of the implementation of both scores which has been achieved partly through the collaboration with charitable partners.
The practice risk score is freely available for download to be used in practices across the entire country (http://leicesterdiabetescentre.org.uk/The-Leicester-Diabetes-Risk-Score). This was highlighted in the September 2014 newsletter of the Royal College of General Physicians which is distributed to over 50,000 GPs nationally. We are also working with Diabetes UK and medical record software providers to incorporate the practice risk score into one or more of the most commonly used electronic medical records software providers. This will enable GPs to use the risk score without the need for downloading and installing it.
The self-assessment score has been completed by over 650,000 members of the public on the Diabetes UK website (http://riskscore.diabetes.org.uk/2013). The self-assessment score is also available in all Boots (Boots UK, Ltd, Nottingham, UK) and Tesco (Tesco PLC, Welwyn Garden City, UK) pharmacies across the country. We have also worked with Boots to integrate HbA1c point-of-care testing alongside the risk score. This is currently being piloted in local stores. The risk score is also available on the Tesco (www.diabetes.org.uk/Tesco/Know-your-risk/Diabetes-UK-Online-Risk-Score/), Boots (www.boots.com/en/Pharmacy-Health/Health-information/Type-2-Diabetes-Find-out-your-risk/), NHS Direct (www.nhs.uk/Tools/Pages/Diabetes.aspx) and BUPA (the British United Provident Association Ltd, London, UK; www.bupa.co.uk/individuals/health-information/tools-calculators/hi-hra-diabetes) websites.
Diabetes UK has used the self-assessment score at local road shows since 2011. In 2012 (latest available figures) they carried out 20,911 risk assessments and referred 10,945 visitors, of whom 3700 were at high risk of having or developing T2DM in the next 10 years. In a survey conducted in 2011, of those who were in these higher-risk categories, 69% had been to their general practice or intended to go. Recall of the risk factors for T2DM and how to reduce risk was high among visitors 2 months after attending the road show. Evaluation of the use of the self-assessment score within Diabetes UK activities showed that after being risk assessed, 41% had started to eat more healthily and a further 44% intended to; 33% had increased their physical activity levels and a further 43% intended to; and 44% of those referred to their GP had been to their GP to seek a test. Bridget Turner, Diabetes UK, 2013, personal communication, says:
The diabetes risk score plays an important part in encouraging more people to take greater notice of their health and their lifestyles – helping to tackle the growing public health challenge of Type 2 diabetes and working towards earlier diagnosis and prevention.
Diabetes UK has embarked on a programme of awareness-raising within BME communities by recruiting ‘Community Champions’ – volunteers who are trained to deliver healthy lifestyle and diabetes mellitus awareness messages through information stands and talks at community centres, places of worship and at festivals and events. Over 100 champions have been trained in London, and the programme is being rolled out in four other towns across England. Some champions have been trained to undertake the risk assessments.
The self-assessment score was featured in the Embarrassing Bodies television programme,227 reaching around 2 million viewers. The risk scores were awarded gold at the national Quality in Care Diabetes Awards 2011 for the best early detection/screening initiative.
National policy and guidance
The work undertaken through the programme grant and associated work has significantly informed national policy and guidance around the prevention of T2DM. The principal investigator (Professor Melanie Davies) and a coinvestigator (Professor Kamlesh Khunti) were involved as a panel member and chair, respectively, in the NICE Programme Development Group that developed new guidance for the prevention of T2DM in high-risk populations. The published risk scores developed through the programme grant were included in the guidance and expert testimony involving unpublished data from the screening and structured education phases of the programme grant, including the importance of standardising educator training and quality-assurance pathways, substantially informed guidance on the content and structure of lifestyle intervention programmes. The finalised NICE guidance was published in 2012. 13
In addition to NICE, work supported through the programme grant contributed to The Handbook of Vascular Risk Assessment, Risk Reduction and Risk Management (both the original 2008 version and updated 2012 version228) and our work in South Asian populations significantly informed guidance issued by the South Asian Health Foundation for diabetes mellitus research priorities in British South Asians. 229
Summary
The programme grant has supported a host of associated activity that significantly adds value to the primary research objectives. By developing a referral pathway and freely available materials and tools, supporting the commissioning of our various prevention programmes, working with charity and industry partners and through contributing to NICE guidance we have ensured that the research supported by the programme grant has significantly advanced methods of prevention within primary care. We feel that we have improved individual patient health within the lifetime of the 5-year funding window.
Chapter 10 Conclusions
This report represents a comprehensive body of work spanning many years, which complements recent NICE guidance and has the potential for substantial improved patient outcomes through the prevention of T2DM. This chapter pulls together the work presented in earlier chapters to summarise the main findings, suggest implications for practice, make recommendations of areas of future research and discuss dissemination activities and plans.
Main findings and outputs from this programme grant
-
We developed and validated a risk score for detecting undiagnosed PDM/T2DM in a multiethnic UK population using data from existing medical records. We have also developed a freely available piece of software which allows GPs nationally to use this risk score for screening for PDM/T2DM.
-
We developed a group education programme for the prevention of diabetes mellitus (Let’s Prevent) in those with PDM suitable for roll-out within a NHS setting. The programme consists of an initial 6-hour course followed by yearly refresher sessions with motivational telephone calls throughout. The programme was extensively piloted and then tested in a large-scale cluster RCT.
-
We identified 17,972 people at high risk of PDM/T2DM from 44 general practices using the risk score and invited them for screening. In total, 3449 participants were screened, of whom 30% had PDM/T2DM. The rates of glucose abnormalities detected were significantly higher than those previously reported when using a population-based approach. The 880 people identified with PDM were included in the RCT.
-
We conducted a cluster randomised trial; 44 GP practices were randomly assigned to receive either the Let’s Prevent structured education programme or standard care. Participants were followed up for 3 years and the primary outcome was progression to T2DM. We found a non-significant 25% reduction in the progression to T2DM in those receiving the education compared with the standard care group; this was increased to 35% when excluding those who did not attend. Positive findings were also seen for HbA1c, LDL cholesterol, psychosocial well-being, sedentary time and step count up to 3 years post the initial education programme.
-
Intervention effectiveness increased in a linear manner with increasing face-to-face contact; those who attended Let’s Prevent and both group-based follow-on support sessions (at 12 and 24 months) had a statistically significant 88% reduction in their risk of T2DM.
-
We conducted a cost-effectiveness analysis and showed that the Let’s Prevent intervention is highly cost-effective. The costs for the intervention group were found to be £168 higher over 3 years after accounting for clustering than for the standard care group; the majority of this difference is accounted for by the intervention cost. The Let’s Prevent intervention was found to result in a net gain of 0.046 QALYs over 3 years at an overall cost of £168 per patient, with an ICER of £3643 and a probability of being cost-effective of 0.86 at a willingness-to-pay threshold of £20,000.
-
Alongside this work we have developed a referral pathway and freely available materials and tools, supporting the commissioning of our various prevention programmes. Through working with charity and industry partners and contributing to NICE guidance, we have ensured that the research supported by the programme grant has significantly advanced methods of prevention within primary care.
Implications for practice
The evidence presented here suggests that a two-stage screening programme utilising a non-invasive risk score followed by a blood test is feasible in primary care and identifies people with previously undiagnosed PDM/T2DM with a high level of discrimination and calibration. Those found to have PDM can then be enrolled into a prevention programme. The evidence here suggests that Let’s Prevent offers a clinically effective and cost-effective option for use in the NHS. Let’s Prevent is based on the DESMOND programme, which has been successfully commissioned across the UK. This gives an existing infrastructure which could be used to roll out this programme with scale and at pace. Team members were actively involved in the development of the NICE guidelines on the identification of those at high risk of diabetes mellitus. The programme of work presented here meets those guidelines and gives an infrastructure for how they can be met. The risk score is already available for use and is being used around the country. We aim to make the self-management structured education intervention also available quickly for CCGs to commission.
Research recommendations
A number of areas for further research emerge from this large body of work.
-
The risk score was developed using a large data set from Leicester and Leicestershire. The ethnic make-up of this area means that the increased risk weighting for being from a BME group was dominated by those from a South Asian background. In other areas, other ethnic groups are predominant. Further research is warranted to assess whether or not the current BME coefficient within the risk score is valid for other ethnic groups.
-
Only 19% of those invited to be screened actually attended. This may reflect the fact that participants were also being asked to take part in a research study. The NHS Health Check programme sees uptake rates of around 40%, which, although still low, are double those seen in research. Research is needed into methods for increasing the uptake to screening. This could include providing screening services outside primary care (i.e. pharmacy, community events), enhancing risk communication to patients in the screening invitation, increasing opportunistic referral from GPs through the use of prompting tools and using GP- and patient-level incentives to increase uptake.
-
The Let’s Prevent structured education intervention was offered as either a 6-hour programme held on 1 day or as two 3-hour sessions held on different days. Around 23% of those in practices randomised to the intervention arm did not attend the initial education session, with only 29% of those in the intervention group attending offered face-to-face contacts (i.e. initial care session plus both refresher sessions). Offering prevention programmes in alternative modalities may increase attendance, particularly for follow-on support after initial face-to-face approaches. With near-universal mobile phone ownership and three-quarters of the population now having daily access to the internet, eHealth and mHealth interventions are increasingly being used to promote lifestyle intervention. 230,231 The integration of such technological platforms into traditional behaviour change interventions may help to maximise engagement and better reflect changing societal norms around communication and feedback. Further research is therefore needed to investigate the efficacy of integrating mHealth and eHealth approaches with traditional interventions such as Let’s Prevent.
-
Pedometers were a key part of Let’s Prevent; they were identified as important in the development of the programme and have previously been shown to be an effective interventional tool in the prevention of T2DM. 232 Research is needed to investigate whether or not the provision of a pedometer with brief counselling and promotional materials could be used to promote physical activity behaviour change in those with PDM who are unable or unwilling to attend Let’s Prevent and to investigate the acceptability of pedometer use in different ethnic populations.
-
The Let’s Prevent structured education intervention was adapted for people from a South Asian background. The programme was delivered via translators and the food models and games were tailored to be culturally appropriate. Using the same model of adaptation, the programme could be adapted for other minority groups, for example for those from Chinese or Afro-Caribbean backgrounds.
-
The RCT followed up participants for 3 years. A natural extension to the programme of work would be to follow up these participants over a longer period, such as 10 years. Such a follow-up could be used to see if the event rate difference between the two groups in terms of T2DM increases over a longer duration, and we could also assess other outcomes such as CVD. Finally, Chapter 8 assessed the costs over the duration of the study; using modelling approaches, projected future costs savings could be assessed over a 10-year time horizon.
Dissemination activities and plans
In consultation with the Leicester Diabetes Focus Group, The Leicester Centre for Ethnic Health and our public and patient involvement lead (Kenneth Jones), we shall disseminate the findings of the Let’s Prevent study though a public event. All participants and GPs who took part in the study will be invited. At the event the main findings will be announced and there will be an opportunity for people to ask questions and speak to members of the study team. For those who are not able to attend, we shall also produce a newsletter.
In terms of academic dissemination, many of the work streams reported here have already been published in high-impact journals. 52,113,145,146,201,210 The cost-effectiveness analysis will also be published and presented both locally, nationally and internationally. Locally, this will be achieved through our existing collaborations with NIHR CLARHC East Midlands and the East Midlands Academic Health Sciences Network. We presented the Leicester Diabetes Prevention Pathway at the Academic Health Sciences Network Diabetes Innovation Exchange in January 2015. National and international dissemination was achieved by presenting the results at key conferences, such as Diabetes UK and the International Diabetes Federation Congress. 233–235
Summary
The results of this programme of work show that it is feasible to identify those with PDM in a primary care setting and to intervene to reduce their incidence of T2DM and promote healthy lifestyle options, using a group programme that is both clinically effective and cost-effective. The programme of work completed mirrors NICE guidelines and achieves their recommendations to meet a previously unmet need in the UK.
Acknowledgements
Contributions of authors
Professor Melanie J Davies (Professor of Diabetes Medicine) was the principal investigator for the trial, contributed methodological and practical advice to all components of the research programme and contributed to publications arising from the research.
Dr Laura J Gray (Senior Lecturer of Population and Public Health Sciences) conducted the statistical analyses and authored chapters of the manuscript.
Dr Dariush Ahrabian (Foundation Year 2 Doctor, Public Health rotation) conducted the review of health economics and cost-effectiveness and coauthored a chapter in the manuscript.
Dr Marian Carey (Director, DESMOND Research) was a coapplicant, assisted with the development of the education programme and reviewed the final manuscript.
Professor Azhar Farooqi (GP and Visiting Professor to Diabetes Research Centre) was a coapplicant and provided practical advice, particularly with regard to implementation in primary care.
Professor Alastair Gray (Professor of Health Economics) conducted the review of health economics and cost-effectiveness and coauthored a chapter in the manuscript.
Miss Stephanie Goldby (Senior Trial Manager) was responsible for the management of the trial and reviewed the final manuscript.
Mrs Sian Hill (Trial Manager) was the co-ordinator for the study.
Dr Kenneth Jones (GP and public and patient involvement representative) was a coapplicant and reviewed chapters of final report.
Dr Jose Leal (Senior Researcher) conducted the review of health economics and cost-effectiveness and coauthored a chapter in the manuscript.
Miss Kathryn Realf (Research Associate) authored chapters of the manuscript and prepared the final manuscript for publication.
Professor Timothy Skinner (Professor of Psychological and Clinical Sciences) was a coapplicant and contributed to the initial design of the study and to the education programme.
Mrs Bernie Stribling (Director, DESMOND programme) was a coapplicant and provided practical advice as regards the implementation of structured education into primary care.
Mrs Jacqui Troughton (Dietitian, Advanced Practitioner in Diabetes) led on the development of the educational intervention and training programmes for educators, the quality assurance of educational intervention and the piloting of the intervention.
Dr Thomas Yates (Senior Lecturer of Physical Activity, Sedentary Behaviour and Health) contributed to the development of the physical activity component of the educational intervention and authored chapters of the manuscript.
Professor Kamlesh Khunti (Professor of Primary Care Diabetes and Vascular Medicine) was a coapplicant, contributed methodological and practical advice to all components of the research programme and contributed to publications arising from the research.
The Let’s Prevent Diabetes team
Keith Abrams, University of Leicester, Leicester, UK.
Sayjal Amin, University Hospitals of Leicester, Leicester, UK.
Mary Bancroft, Hockley Farm Medical Practice, Leicester, UK.
Janette Barnett, University Hospitals of Leicester, Leicester, UK.
Hannah Berkeley, University Hospitals of Leicester, Leicester, UK.
Danielle Bodicoat, University of Leicester, Leicester, UK.
Michael Bonar, University Hospitals of Leicester, Leicester, UK.
Louise Boyles, University Hospitals of Leicester, Leicester, UK.
Paul Bray, University Hospitals of Leicester, Leicester, UK.
Nichola Cairns, University Hospitals of Leicester, Leicester, UK.
Sandra Campbell, University Hospitals of Leicester, Leicester, UK.
Patrice Carter, University of Leicester, Leicester, UK.
Sudesna Chatterjee, University Hospitals of Leicester, Leicester, UK.
Pauline Cowling, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
Carolyn Currie, University Hospitals of Leicester, Leicester, UK.
Heather Daly, University Hospitals of Leicester, Leicester, UK.
Alison Dunkley, University of Leicester, Leicester, UK.
Sue Enright, University Hospitals of Leicester, Leicester, UK.
Geri Gray, University Hospitals of Leicester, Leicester, UK.
Colin Greaves, University of Exeter Medical School, Exeter, UK.
Joe Henson, University Hospitals of Leicester, Leicester, UK.
Stephen Hiles, University Hospitals of Leicester, Leicester, UK.
Jayne Hill, University Hospitals of Leicester, Leicester, UK.
Hannah Holdsworth, University Hospitals of Leicester, Leicester, UK.
Rosie Horne, University of Leicester, Leicester, UK.
Zin Zin Htike, University Hospitals of Leicester, Leicester, UK.
Shenaz Jamal, University Hospitals of Leicester, Leicester, UK.
Janet Jarvis, University Hospitals of Leicester, Leicester, UK.
Carolyn Johnson, University Hospitals of Leicester, Leicester, UK.
Janet Jones, University Hospitals of Leicester, Leicester, UK.
Sabera Khan, University Hospitals of Leicester, Leicester, UK.
Anita Khulpateea, University Hospitals of Leicester, Leicester, UK.
Judith Leonard, University Hospitals of Leicester, Leicester, UK.
Hamidreza Mani, University Hospitals of Leicester, Leicester, UK.
Lorraine Martin-Stacey, University Hospitals of Leicester, Leicester, UK.
Val Morgan, University Hospitals of Leicester, Leicester, UK.
Frances Morris, University Hospitals of Leicester, Leicester, UK.
Samiul Mostafa, University Hospitals of Leicester, Leicester, UK.
Alison Northern, University Hospitals of Leicester, Leicester, UK.
Kayleigh O’Brien, University Hospitals of Leicester, Leicester, UK.
Hersha Patel, University Hospitals of Leicester, Leicester, UK.
Naina Patel, University of Leicester, Leicester, UK.
Rachel Plummer, University Hospitals of Leicester, Leicester, UK.
Sheila Porter, University Hospitals of Leicester, Leicester, UK.
Mo Radia, University Hospitals of Leicester, Leicester, UK.
Dean Richmond, University Hospitals of Leicester, Leicester, UK.
Clare Russell, University of Leicester, Leicester, UK.
Rebecca Saker, University Hospitals of Leicester, Leicester, UK.
Jane Sennet, University Hospitals of Leicester, Leicester, UK.
David Sheppard, Saffron Group Practice, Leicester, UK.
Rebecca Spong, University of Leicester, Leicester, UK.
Margaret Stone, University of Leicester, Leicester, UK.
Nick Taub, University of Leicester, Leicester, UK.
David Webb, University of Leicester, Leicester, UK.
Emma Wilmott, University Hospitals of Leicester, Leicester, UK.
Carolina Wilson, University Hospitals of Leicester, Leicester, UK.
Panna Wilson, University Hospitals of Leicester, Leicester, UK.
Publications
Gray LJ, Davies MJ, Hiles S, Taub NA, Webb DR, Srinivasan BT, et al. Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting. Diabetologia 2012;55:959–66.
Gray LJ, Khunti K, Edwardson C, Goldby S, Henson J, Morris DH, et al. Implementation of the automated Leicester Practice Risk Score in two diabetes prevention trials provides a high yield of people with abnormal glucose tolerance. Diabetologia 2012;55:3238–44.
Gray LJ, Khunti K, Williams S, Goldby S, Troughton J, Yates T, et al. Let’s Prevent Diabetes: study protocol for a cluster randomised controlled trial of an educational intervention in a multi-ethnic UK population with screen detected impaired glucose regulation. Cardiovasc Diabetol 2012;11:56.
Carter P, Gray LJ, Talbot D, Morris DH, Khunti K, Davies MJ. Fruit and vegetable intake and the association with glucose parameters: a cross-sectional analysis of the Let’s Prevent Diabetes Study. Eur J Clin Nutr 2013;67:12–17.
Yates T, Henson J, Khunti K, Morris DH, Edwardson C, Brady E, Davies MJ. Effect of physical activity measurement type on the association between walking activity and glucose regulation in a high-risk population recruited from primary care. Int J Epidemiol 2013;42:533–40.
Dunkley A, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations. A systematic review and meta-analysis. Diabetes Care 2014;37:922–33.
Gray LJ, Khunti K, Wilmot EG, Yates T, Davies MJ. External validation of two diabetes risk scores in a young UK South Asian population. Diabetes Res Clin Pract 2014;104:451–8.
Troughton J, Chatterjee S, Hill SE, Daly H, Martin Stacy L, Stone MA, et al. Development of a lifestyle intervention using the MRC framework for diabetes prevention in people with impaired glucose regulation. J Public Health 2015:1–9.
Davies MJ, Gray LJ, Troughton J, Gray A, Tuomilehto J, Farooqi A, et al. A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let’s Prevent Diabetes cluster randomised controlled trial. Prevent Med 2016;84:48–56.
Leal J, Ahrabian D, Davies MJ, Gray LJ, Khunti K, Yates T, Gray AM. Cost-effectiveness of a pragmatic structured education intervention for the prevention of type 2 diabetes: economic evaluation of data from the Let’s Prevent Diabetes cluster-randomised controlled trial. BMJ Open 2017;7:e013592.
Data sharing statement
Data sharing requests should be made to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Massi-Benedetti M. The cost of diabetes in Europe-Type II: the CODE-2 study. Diabetologia 2002;45:1-4. http://dx.doi.org/10.1007/s00125-002-0860-3.
- Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ 2001;322:1389-93. http://dx.doi.org/10.1136/bmj.322.7299.1389.
- Davies M, Tringham J, Troughton J, Khunti KK. Prevention of type 2 diabetes mellitus. A review of the evidence and its application in a UK setting. Diabet Med 2004;21:403-14. http://dx.doi.org/10.1111/j.1464-5491.2004.01176.x.
- Mortality and Burden of Disease Attributable to Selected Major Risk Factors. Geneva: WHO; 2009.
- The Economic Impacts of Diabetes. Brussels: International Diabetes Federation; 2010.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. http://dx.doi.org/10.1111/j.1464-5491.2012.03698.x.
- NHS . NHS Health Check n.d. www.healthcheck.nhs.uk/ (accessed 21 July 2016).
- Definition, Diagnosis, and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: WHO; 1999.
- Gerstein H, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305-12. http://dx.doi.org/10.1016/j.diabres.2007.05.004.
- Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Geneva: WHO; 2011.
- American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:62-9. http://dx.doi.org/10.2337/dc10-S062.
- The International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327-34. http://dx.doi.org/10.2337/dc09-9033.
- Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk. London: NICE; 2012.
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013;56:1489-93. http://dx.doi.org/10.1007/s00125-013-2902-4.
- Definition and Diagnoses of Diabetes Mellitus and Intermediate Hyperglycemia. Geneva: WHO; 2006.
- Valensi P, Schwarz E, Hall M, Felton AM, Maldonato A, Mathieu C, et al. Pre-diabetes essential action: a European perspective. Diabetes Metab 2005;31:606-20. http://dx.doi.org/10.1016/S1262-3636(07)70239-2.
- Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279-90. http://dx.doi.org/10.1016/S0140-6736(12)60283-9.
- Santaguida PL, Balion C, Hunt D, Morrison K, Gerstein H, Raina P, et al. Diagnosis, Prognosis and Treatment of Impaired Glucose Tolerance and Impaired Fasting Glucose. Evidence Reports/Technology Assessments No. 128. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- Pekkanen J, Tuomilehto J, Qiao Q, Jousilanti P, Lindström J. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999;354:617-21. http://dx.doi.org/10.1016/S0140-6736(98)12131-1.
- International Diabetes Federation . Factsheet: Impaired Glucose Tolerance (IGT) n.d. www.idf.org/fact-sheets/impaired-glucose-tolerance (accessed 14 November 2014).
- Prediabetes: Preventing the Type 2 diabetes Epidemic. London: Diabetes UK; 2009.
- Troughton J, Jarvis J, Skinner TC, Robertson N, Khunti K, Davies M. Waiting for diabetes: perceptions of people with pre-diabetes: a qualitative study. Patient Educ Couns 2008;72:88-93. http://dx.doi.org/10.1016/j.pec.2008.01.026.
- American Diabetes Association . Summary of Revisions for the 2010 Clinical Practice Recommendations. Diabetes Care 2010;33. http://dx.doi.org/10.2337/dc10-S003.
- Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11170.
- Lindström J, Peltonen M, Eriksson JG, Aunola S, Hämäläinen H, Ilanne-Parikka P, et al. Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31:857-62. http://dx.doi.org/10.2337/dc07-2162.
- Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999;22:233-40. http://dx.doi.org/10.2337/diacare.22.2.233.
- Lindström J, Tuomilehto J. The Diabetes Risk Score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725-31. http://dx.doi.org/10.2337/diacare.26.3.725.
- Glümer C, Vistisen D, Borch-Johnsen K, Colagiuri S. DETECT-2 Collaboration . Risk scores for type 2 diabetes can be applied in some populations but not all. Diabetes Care 2006;29:410-14. http://dx.doi.org/10.2337/diacare.29.02.06.dc05-0945.
- Schwarz PE, Greaves C, Lindström J, Yates T, Davies MJ. Nonpharmacological intervention for diabetes mellitus prevention in populations: where do we stand?. Nat Rev Endocrinol 2012;8:363-73. http://dx.doi.org/10.1038/nrendo.2011.232.
- Khunti K, Gillies CL, Taub NA, Mostafa SA, Hiles SL, Abrams KR, et al. A comparison of cost per case detected of screening strategies for type 2 diabetes and impaired glucose regulation: modelling study. Diabetes Res Clin Pract 2012;97:505-13. http://dx.doi.org/10.1016/j.diabres.2012.03.009.
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39063.689375.55.
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50. http://dx.doi.org/10.1056/NEJM200105033441801.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-40. http://dx.doi.org/10.1056/NEJMoa012512.
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289-97. http://dx.doi.org/10.1007/s00125-005-0097-z.
- Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152-62. http://dx.doi.org/10.1016/j.diabres.2004.06.010.
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537-44. http://dx.doi.org/10.2337/diacare.20.4.537.
- Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, . Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677-86. http://dx.doi.org/10.1016/S0140-6736(09)61457-4.
- Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783-9. http://dx.doi.org/10.1016/S0140-6736(08)60766-7.
- Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673-9. http://dx.doi.org/10.1016/S0140-6736(06)69701-8.
- Urbanski P, Wolf A, Herman WH. Cost-Effectiveness Issues of Diabetes Prevention and Treatment n.d. https://dpg-storage.s3.amazonaws.com/dce/resources/cost_effective.pdf (accessed 14 November 2014).
- Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost-effectiveness analysis. BMJ 2008;336:1180-5. http://dx.doi.org/10.1136/bmj.39545.585289.25.
- Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol 2000;88:774-87.
- Albright A. Navigating the sea of diabetes. Diabetes Spectrum 2009;22:38-42. http://dx.doi.org/10.2337/diaspect.22.1.38.
- Saaristo T, Peltonen M, Keinanen-Kiukaanniemi S, Vanhala M, Saltevo J, Niskanen L, et al. National type 2 diabetes prevention programme in Finland: FIN-D2D. Int J Circumpolar Health 2007;66:101-12. http://dx.doi.org/10.3402/ijch.v66i2.18239.
- Green LW, Brancati F, Albright A. Primary Prevention of Diabetes Working Group . Primary prevention of type 2 diabetes: integrative public health and primary care opportunities, challenges and strategies. Fam Pract 2011;29:13-2. http://dx.doi.org/10.1093/fampra/cmr126.
- Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program?. Health Aff (Millwood 2012;31:67-75. http://dx.doi.org/10.1377/hlthaff.2011.1009.
- Paulweber B, Valensi P, Lindstrom J, Lalic NM, Greaves CJ, McKee M, et al. A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res 2010;42:3-36. http://dx.doi.org/10.1055/s-0029-1240928.
- Davies M, Heller S, Skinner T, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. http://dx.doi.org/10.1136/bmj.39474.922025.BE.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost-effectiveness analysis. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4093.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education programme aimed at promoting walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care 2009;32:1404-10. http://dx.doi.org/10.2337/dc09-0130.
- Yates T, Davies M, Sehmi S, Gorely T, Khunti K. The Prediabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme study: are improvements in glucose regulation sustained at two years?. Diabet Med 2011;28:1268-71. http://dx.doi.org/10.1111/j.1464-5491.2011.03357.x.
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care 2014;37:922-33. http://dx.doi.org/10.2337/dc13-2195.
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102-7. http://dx.doi.org/10.2337/dc06-0560.
- Yates T, Khunti K, Bull F, Gorely T, Davies MJ. The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia 2007;50:1116-26. http://dx.doi.org/10.1007/s00125-007-0638-8.
- Cardona-Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-653.
- Johnson M, Jones R, Freeman C, Woods HB, Gillett M, Goyder E, et al. Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence. Diabet Med 2013;30:3-15. http://dx.doi.org/10.1111/dme.12018.
- Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: A systematic review of randomized controlled trials. Diabetes Res Clin Pract 2011;91:1-12. http://dx.doi.org/10.1016/j.diabres.2010.06.030.
- Gillett M, Royle P, Snaith A, Scotland G, Poobalan A, Imamura M, et al. Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: a systematic review and economic evaluation. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16330.
- Taylor J, Cottrell C, Chatterton H, Hill J, Hughes R, Wohlgemuth C, et al. Identifying risk and preventing progression to Type 2 diabetes in vulnerable and disadvantaged adults: a pragmatic review. Diabet Med 2013;30:16-25. http://dx.doi.org/10.1111/dme.12027.
- Whittemore R. A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med 2011;1:480-91. http://dx.doi.org/10.1007/s13142-011-0062-y.
- Angermayr L, Melchart D, Linde K. Multifactorial lifestyle interventions in the primary and secondary prevention of cardiovascular disease and type 2 diabetes mellitus: a systematic review of randomized controlled trials. Ann Behav Med 2010;40:49-64. http://dx.doi.org/10.1007/s12160-010-9206-4.
- Faridi Z, Shuval K, Njike VY, Katz JA, Jennings G, Williams M, et al. Partners reducing effects of diabetes (PREDICT): a diabetes prevention physical activity and dietary intervention through African-American churches. Health Educ Res 2010;25:306-15. http://dx.doi.org/10.1093/her/cyp005.
- Nield L, Summerbell CD, Hooper L, Whittaker V, Moore H. Dietary advice for the prevention of type 2 diabetes mellitus in adults. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.cd005102.pub2.
- Orozco LJ, Buchleitner AM, Gimenez-Perez G, Roqué I, Figuls M, Richter B, et al. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.cd003054.pub3.
- Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2005;28:2780-6. http://dx.doi.org/10.2337/diacare.28.11.2780.
- Methods for the Development of NICE Public Health Guidance. London: NICE; 2012.
- Burnett RW, D’Orazio P, Fogh-Andersen N, Kuwa K, Külpmann WR, Larsson L, et al. IFCC recommendation on reporting results for blood glucose. Clin Chim Acta 2001;307:205-9. http://dx.doi.org/10.1016/S0009-8981(01)00431-4.
- Higgins JPT, Green SE. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 2011. www.cochrane-handbook.org (accessed 31 May 2016).
- Gilis-Januszewska A, Szybinski Z, Kissimova-Skarbek K, Piwonska-Solska B, Pach D, Topor-Madry R, et al. Prevention of type 2 diabetes by lifestyle intervention in primary health care setting in Poland: Diabetes in Europe Prevention using Lifestyle, physical Activity and Nutritional intervention (DE-PLAN) project. Br J Diabetes Vasc Dis 2011;11:198-203. http://dx.doi.org/10.1177/1474651411412429.
- Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med 2009;37:505-11. http://dx.doi.org/10.1016/j.amepre.2009.07.020.
- Kulzer B, Hermanns N, Gorges D, Schwarz P, Haak T. Prevention of Diabetes Self-Management Program (PREDIAS): effects on weight, metabolic risk factors, and behavioral outcomes. Diabetes Care 2009;32:1143-6. http://dx.doi.org/10.2337/dc08-2141.
- Laatikainen T, Dunbar J, Chapman A, Kilkkinen A, Vartiainen E, Heistaro S, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) diabetes prevention project. BMC Public Health 2007;7. http://dx.doi.org/10.1186/1471-2458-7-249.
- Makrilakis K, Liatis S, Grammatikou S, Perrea D, Katsilambros N. Implementation and effectiveness of the first community lifestyle intervention programme to prevent type 2 diabetes in Greece. The DE-PLAN study. Diabet Med 2010;27:459-65. http://dx.doi.org/10.1111/j.1464-5491.2010.02918.x.
- Absetz P, Valve R, Oldenburg B, Heinonen H, Nissinen A, Fogelholm M, et al. Type 2 diabetes prevention in the ‘real world’. Diabetes Care 2007;30:2465-70. http://dx.doi.org/10.2337/dc07-0171.
- Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: the DEPLOY pilot study. Am J Prev Med 2008;35:357-63. http://dx.doi.org/10.1016/j.amepre.2008.06.035.
- Almeida FA, Shetterly S, Smith-Ray RL, Estabrooks PA. Reach and effectiveness of a weight loss intervention in patients with prediabetes in Colorado. Prev Chronic Dis 2010;7.
- Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: results of translational research. J Public Health Manag Pract 2008;14:29-32. http://dx.doi.org/10.1097/01.PHH.0000303410.66485.91.
- Costa B, Barrio F, Cabré JJ, Piñol JL, Cos X, Solé C, et al. Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention. Diabetologia 2012;55:1319-28. http://dx.doi.org/10.1007/s00125-012-2492-6.
- Davis-Smith YM, Boltri JM, Seale JP, Shellenberger S, Blalock T, Tobin B, et al. Implementing a diabetes prevention program in a rural African-American church. J Natl Med Assoc 2007;99.
- Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS, et al. One-year results of a community-based translation of the Diabetes Prevention Programme. Diabetes Care 2011;34:1451-57. http://dx.doi.org/10.2337/dc10-2115.
- Kramer MK, Venditti EM, Semler LN, Kriska AM, Miller RG, Orchard TJ. Long-term strategies for diabetes prevention: evaluation of the group lifestyle balance post-core sessions focusing on carbohydrate and hunger management. J Diabetes Metab 2012;S2. http://dx.doi.org/10.4172/2155-6156.s2-006.
- Mensink M, Blaak EE, Corpeleijn E, Saris WH, de Bruin TW, Feskens EJ. Lifestyle intervention according to general recommendations improves glucose tolerance. Obes Res 2003;11:1588-96. http://dx.doi.org/10.1038/oby.2003.211.
- Nilsen V, Bakke P, Gallefoss F. Effects of lifestyle intervention in persons at risk for type 2 diabetes mellitus – results from a randomised, controlled trial. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-893.
- Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health 2012;102:336-42. http://dx.doi.org/10.2105/AJPH.2011.300357.
- Parikh P. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health 2010;100:232-9. http://dx.doi.org/10.2105/AJPH.2009.170910.
- Payne WR, Walsh KJ, Harvey JT, Livy MF, McKenzie KJ, Donaldson A, et al. Effect of a low-resource-intensive lifestyle modification program incorporating gymnasium-based and home-based resistance training on type 2 diabetes risk in Australian adults. Diabetes Care 2008;31:2244-50. http://dx.doi.org/10.2337/dc08-0152.
- Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC, et al. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health 2009;9. http://dx.doi.org/10.1186/1471-2458-9-342.
- Ruggiero L, Oros S, Choi YK. Community-based translation of the diabetes prevention program’s lifestyle intervention in an underserved Latino population. Diabetes Educ 2011;37:564-72. http://dx.doi.org/10.1177/0145721711411107.
- Saaristo T, Moilanen L, Korpi-Hyövälti E, Vanhala M, Saltevo J, Niskanen L, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146-51. http://dx.doi.org/10.2337/dc10-0410.
- Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tsuzaki K, et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-40.
- Vermunt PWA, Milder IEJ, Wielaard F, de Vries JH, Baan CA, van Oers JA, et al. A lifestyle intervention to reduce type 2 diabetes risk in Dutch primary care: 2.5-year results of a randomized controlled trial. Diabet Med 2012;29:e223-31. http://dx.doi.org/10.1111/j.1464-5491.2012.03648.x.
- Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med 2013;173:113-21. http://dx.doi.org/10.1001/2013.jamainternmed.987.
- Janus ED, Best JD, Davis-Lameloise N, Philpot B, Hernan A, Bennett CM, et al. Scaling-up from an implementation trial to state-wide coverage: results from the preliminary Melbourne Diabetes Prevention Study. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-152.
- Kanaya AM, Santoyo-Olsson J, Gregorich S, Grossman M, Moore T, Stewart AL. The Live Well, Be Well Study: a community-based, translational lifestyle program to lower diabetes risk factors in ethnic minority and lower-socioeconomic status adults. Am J Public Health 2012;102:1551-58. http://dx.doi.org/10.2105/AJPH.2011.300456.
- Penn L, Ryan V, White M. Feasibility, acceptability and outcomes at a 12-month follow-up of a novel community-based intervention to prevent type 2 diabetes in adults at high risk: mixed methods pilot study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-003585.
- Absetz P, Oldenburg B, Hankonen N, Valve R, Heinonen H, Nissinen A, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL Lifestyle Implementation Trial. Diabetes Care 2009;32:1418-20. http://dx.doi.org/10.2337/dc09-0039.
- Ackermann RT, Finch EA, Caffrey HM, Lipscomb ER, Hays LM, Saha C. Long-term effects of a community-based lifestyle intervention to prevent type 2 diabetes: the DEPLOY extension pilot study. Chronic Illn 2011;7:279-90. http://dx.doi.org/10.1177/1742395311407532.
- Laatikainen T, Philpot B, Hankonen N, Sippola R, Dunbar JA, Absetz P, et al. Predicting changes in lifestyle and clinical outcomes in preventing diabetes: the Greater Green Triangle Diabetes Prevention Project. Prev Med 2012;54:157-61. http://dx.doi.org/10.1016/j.ypmed.2011.12.015.
- Mensink M, Feskens EJM, Saris WHM, De Bruin TW, Blaak EE. Study on Lifestyle Intervention and Impaired Glucose Tolerance Maastricht (SLIM): preliminary results after one year. Int J Obes Relat Metab Disord 2003;27:377-84. http://dx.doi.org/10.1038/sj.ijo.0802249.
- Rautio N, Jokelainen J, Oksa H, Saaristo T, Peltonen M, Niskanen L, et al. Socioeconomic position and effectiveness of lifestyle intervention in prevention of type 2 diabetes: one-year follow-up of the FIN-D2D project. Scand J Public Health 2011;39:561-70. http://dx.doi.org/10.1177/1403494811408482.
- Rautio N, Jokelainen J, Oksa H, Saaristo T, Peltonen M, Puolijoki H, et al. Family history of diabetes and effectiveness of lifestyle counselling on the cardio-metabolic risk profile in individuals at high risk of type 2 diabetes: 1-year follow-up of the FIN-D2D project. Diabet Med 2012;29:207-11. http://dx.doi.org/10.1111/j.1464-5491.2011.03388.x.
- Roumen C, Corpeleijn E, Feskens EJM, Mensink M, Saris WH, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabet Med 2008;25:597-605. http://dx.doi.org/10.1111/j.1464-5491.2008.02417.x.
- Roumen C, Feskens EJM, Corpeleijn E, Mensink M, Saris WH, Blaak EE. Predictors of lifestyle intervention outcome and dropout: the SLIM study. Eur J Clin Nutr 2011;65:1141-7. http://dx.doi.org/10.1038/ejcn.2011.74.
- Vermunt PWA, Milder IEJ, Wielaard F, de Vries JH, van Oers HA, Westert GP. Lifestyle counseling for type 2 diabetes risk reduction in Dutch primary care: results of the APHRODITE study after 0.5 and 1.5 years. Diabetes Care 2011;34:1919-25. http://dx.doi.org/10.2337/dc10-2293.
- Xiao L, Yank V, Wilson S, Lavori PW, Ma J. Two-year weight-loss maintenance in primary care-based Diabetes Prevention Program lifestyle interventions. Nutr Diabetes 2013;3. http://dx.doi.org/10.1038/nutd.2013.17.
- Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE): a randomized controlled trial. BMC Fam Pract 2009;10. http://dx.doi.org/10.1186/1471-2296-10-71.
- Katula JA, Vitolins MZ, Morgan TM, Lawlor MS, Blackwell CS, Isom SP, et al. The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med 2013;44:324-32. http://dx.doi.org/10.1016/j.amepre.2012.12.015.
- Herman WH, Smith PJ, Thompson TJ, Engelgau MM, Aubert RE. A new and simple questionnaire to identify people at increased risk for undiagnosed diabetes. Diabetes Care 1995;18:382-87. http://dx.doi.org/10.2337/diacare.18.3.382.
- Chen L, Magliano DJ, Balkau B, Colagiuri S, Zimmet PZ, Tonkin AM, et al. AUSDRISK: an Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med J Aus 2010;192.
- Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med 2007;147:41-50. http://dx.doi.org/10.7326/0003-4819-147-1-200707030-00007.
- Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4229.
- Reed V, Schifferdecker K, Rezaee M, O’Connor S, Larson RJ. The effect of computers for weight loss: a systematic review and meta-analysis of randomized trials. J Gen Intern Med 2012;27:99-108. http://dx.doi.org/10.1007/s11606-011-1803-9.
- Gray LJ, Davies MJ, Hiles S, Taub NA, Webb DR, Srinivasan BT, et al. Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting. Diabetologia 2012;55:959-66. http://dx.doi.org/10.1007/s00125-011-2432-x.
- Makrilakis K, Liatis S, Grammatikou S, Perrea D, Stathi C, Tsiligros P, et al. Validation of the Finnish diabetes risk score (FINDRISC) questionnaire for screening for undiagnosed type 2 diabetes, dysglycaemia and the metabolic syndrome in Greece. Diabetes Metab 2011;37:144-51. http://dx.doi.org/10.1016/j.diabet.2010.09.006.
- Tankova T, Chakarova N, Atanassova I, Dakovska L. Evaluation of the Finnish Diabetes Risk Score as a screening tool for impaired fasting glucose, impaired glucose tolerance and undetected diabetes. Diabetes Res Clin Pract 2011;92:46-52. http://dx.doi.org/10.1016/j.diabres.2010.12.020.
- Franciosi M, De Berardis G, Rossi MCE, Sacco M, Belfiglio M, Pellegrini F, et al. Use of the diabetes risk score for opportunistic screening of undiagnosed diabetes and impaired glucose tolerance: The IGLOO (Impaired Glucose Tolerance and Long-Term Outcomes Observational) study. Diabetes Care 2005;28:1187-94. http://dx.doi.org/10.2337/diacare.28.5.1187.
- Soriguer F, Valdés S, Tapia MJ, Esteva I, Ruiz de Adana MS, Almaraz MC, et al. Validation of the FINDRISC (FINnish Diabetes RIsk SCore) for prediction of the risk of type 2 diabetes in a population of southern Spain. Pizarra Study. Med Clin (Barc) 2012;138:371-6. http://dx.doi.org/10.1016/j.medcli.2011.05.025.
- Hellgren MI, Petzold M, Björkelund C, Wedel H, Jansson PA, Lindblad U. Feasibility of the FINDRISC questionnaire to identify individuals with impaired glucose tolerance in Swedish primary care. A cross-sectional population-based study. Diabet Med 2012;29:1501-5. http://dx.doi.org/10.1111/j.1464-5491.2012.03664.x.
- Chien K, Cai T, Hsu H, Su T, Chang W, Chen M, et al. A prediction model for type 2 diabetes risk among Chinese people. Diabetologia 2009;52:443-50. http://dx.doi.org/10.1007/s00125-008-1232-4.
- Al-Lawati JA, Tuomilehto J. Diabetes risk score in Oman: a tool to identify prevalent type 2 diabetes among Arabs of the Middle East. Diabetes Res Clin Pract 2007;77:438-44. http://dx.doi.org/10.1016/j.diabres.2007.01.013.
- Griffin SJ, Little PS, Hales CN, Kinmonth AL, Wareham NJ. Diabetes risk score: towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev 2000;16:164-71. http://dx.doi.org/10.1002/1520-7560(200005/06)16:3<164::AID-DMRR103>3.0.CO;2-R.
- Gray LJ, Tringham J, Davies MJ, Webb DR, Jarvis J, Skinner TC, et al. Screening for type 2 diabetes in a multiethnic setting using known risk factors to identify those at high risk: a cross-sectional study. J Vasc Health Risk Manag 2010;6:837-42. http://dx.doi.org/10.2147/VHRM.S12504.
- Webb DR, Khunti K, Srinivasan B, Gray LJ, Taub N, Campbell S, et al. Rationale and design of the ADDITION-Leicester study, a systematic screening programme and randomised controlled trial of multi-factorial cardiovascular risk intervention in people with type 2 diabetes mellitus detected by screening. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-16.
- Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbæk A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 2011;378:156-67. http://dx.doi.org/10.1016/S0140-6736(11)60698-3.
- Collins GS, Mallett S, Omar O, Yu LM. Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med 2011;9. http://dx.doi.org/10.1186/1741-7015-9-103.
- Chatterton H, Younger T, Fischer A, Khunti K. Programme Development Group. Risk identification and interventions to prevent type 2 diabetes in adults at high risk: summary of NICE guidance. BMJ 2012;12. http://dx.doi.org/10.1136/bmj.e4624.
- Akaike H. A new look at the statistical model identification. IEEE Transact Automatic Control 1974;19:716-23. http://dx.doi.org/10.1109/TAC.1974.1100705.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: Wiley; 2000.
- Steyerberg EW, Vickers AJ, Cook NR. Assessing the performance of prediction models: a framework for some traditional and novel measures. Epidemiology 2010;21:128-38. http://dx.doi.org/10.1097/EDE.0b013e3181c30fb2.
- Mathur R, Bhaskaran K, Chaturvedi N, Leon DA, vanStaa T, Grundy E, et al. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health 2013;36:684-92. http://dx.doi.org/10.1093/pubmed/fdt116.
- Jamie G. QOF Database: Records 21 2012. www.gpcontract.co.uk/faq/about-site/ (accessed 31 May 2016).
- Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d7163.
- Thomas C, Hypponen E, Power C. Type 2 diabetes mellitus in midlife estimated from the Cambridge Risk Score and body mass index. Arch Intern Med 2006;166:682-8. http://dx.doi.org/10.1001/archinte.166.6.682.
- Schwarz PEH, Li J, Relmann M, Schutte AE, Bergmann A, Hanefeld M, et al. The Finnish Diabetes Risk Score is associated with insulin resistance and progression towards type 2 diabetes. J Clin Endocrinol Metab 2009;94:920-26. http://dx.doi.org/10.1210/jc.2007-2427.
- Mostafa SA, Davies MJ, Webb D, Gray LJ, Srinivasan BT, Jarvis J, et al. The potential impact of using glycated haemoglobin, HbA1c, as the preferred diagnostic tool for type 2 diabetes mellitus. Diabet Med 2010;72:762-9. http://dx.doi.org/10.1111/j.1464-5491.2010.03015.x.
- Spijkerman AMW, Yuyun MF, Griffin SJ, Dekker JM, Nijpels G, Wareham NJ, et al. The performance of a risk score as a screening test for undiagnosed hyperglycemia in ethnic minority groups. Data from the 1999 health survey for England. Diabetes Care 2004;27:116-22. http://dx.doi.org/10.2337/diacare.27.1.116.
- Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ 2009;388. http://dx.doi.org/10.1136/bmj.b880.
- Gray LJ, Taub N, Khunti K, Gardiner E, Hiles S, Webb DR, et al. The Leicester Risk Assessment score for detecting undiagnosed type 2 diabetes and impaired glucose regulation for use in a multiethnic UK setting. Diabet Med 2010;27:887-95. http://dx.doi.org/10.1111/j.1464-5491.2010.03037.x.
- Aujla N, Eborall H, Stone M, Shirley S, Scawn N, Kemp I, et al. Barriers to practice and patient recruitment to primary care based diabetes screening. Diabetic Med 2010;27.
- Webb DR, Gray LJ, Khunti K, Srinivasan B, Taub N, Campbell S, et al. Screening for diabetes using an oral glucose tolerance test within a western multi-ethnic population identifies modifiable cardiovascular risk: the ADDITION-Leicester study. Diabetologia 2011;54:2237-46. http://dx.doi.org/10.1007/s00125-011-2189-2.
- Steyerberg W. Clinical Prediction Models. New York, NY: Springer; 2009.
- Altman DG, Royston P. What do we mean by validating a prognostic model?. Stat Med 2000;19:453-73. http://dx.doi.org/10.1002/(SICI)1097-0258(20000229)19:4<453::AID-SIM350>3.0.CO;2-5.
- Vergouwe Y, Royston P, Moons KGM, Altman DG. Development and validation of a prediction model with missing predictor data: a practical approach. J Clin Epidemiol 2010;63:205-14. http://dx.doi.org/10.1016/j.jclinepi.2009.03.017.
- Gholap N, Davies MJ, Patel K, Sattar N, Khunti K. Type 2 diabetes and cardiovascular disease in South Asians. Prim Care Diabetes 2011;5:45-56. http://dx.doi.org/10.1016/j.pcd.2010.08.002.
- Troughton J, Chatterjee S, Hill SE, Daly H, Martin Stacy L, Stone MA, et al. Development of a lifestyle intervention using the MRC framework for diabetes prevention in people with impaired glucose regulation. J Public Health (Oxf) 2016;38:493-501. http://dx.doi.org/10.1093/pubmed/fdv110.
- Gray LJ, Khunti K, Williams S, Goldby S, Troughton J, Yates T, et al. Let’s Prevent Diabetes: study protocol for a cluster randomised controlled trial of an educational intervention in a multi-ethnic UK population with screen detected impaired glucose regulation. Cardiovasc Diabetol 2012;11. http://dx.doi.org/10.1186/1475-2840-11-56.
- Davies MJ, Heller S, Khunti K, Skinner TC. The DESMOND (Diabetes Education and Self-Management for Ongoing and Newly Diagnosed) programme: from pilot to randomised controlled trial in a study of structured group education for people newly diagnosed with type 2 diabetes mellitus. Diabet Med 2005;22.
- Yates T, Khunti K, Bull F, Gorely T, Mandalia P, Davies MJ. Three-month follow up data from the PREPARE (Pre-diabetes Risk Education and Physical Activity Recmmendation and Encouragement) programme study. Diab Med 2008;25.
- Guidance on the Use of Patient Education Models for Diabetes (Technology Appraisal 60). London: NICE; 2003.
- Anderson RM, Funnell MM. Patient empowerment: reflections on the challenge of fostering the adoption of a new paradigm. Patient Educ Couns 2005;57:153-7. http://dx.doi.org/10.1016/j.pec.2004.05.008.
- Leventhal H, Meyer D, Nerenz D. The Common-Sense Representation of Illness Danger. Contributions to Medical Psychology. New York, NY: Pergamon; 1980.
- Chaiken S, Wood W, Eagly AH, Higgins ET, Kruglanski A. Social Psychology: Handbook of Basic Mechanisms and Processes. New York, NY: Guildford Press; 1996.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. http://dx.doi.org/10.1037/0033-295X.84.2.191.
- Yates T, Davies MJ, Gorely T, Talbot D, Bull F, Sattar N, et al. The effect of increased ambulatory activity on markers of chronic low-grade inflammation: evidence from the PREPARE programme randomized controlled trial. Diab Med 2010;27:1256-63. http://dx.doi.org/10.1111/j.1464-5491.2010.03091.x.
- Alberti KGM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53. http://dx.doi.org/10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S.
- The Handbook for Vascular Risk Assessment, Risk Reduction and Risk Management. London: UK National Screening Committee; 2008.
- Tudor-Locke C, Bassett DR. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34:1-8. http://dx.doi.org/10.2165/00007256-200434010-00001.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Stone MA, Patel N, Amin S, Daly M, Martin-Stacey L, Troughton J, et al. Transferring research into practice: an update on DESMOND diabetes education for South Asians. Diabetes Med 2008;25.
- Stone MA, Patel N, Drake L, Gayle C. Cultural awareness in diabetes education. Pract Nurs 2006;17:621-5. http://dx.doi.org/10.12968/pnur.2006.17.12.22416.
- Schneider PL, Crouter SE, Lukajic O, Bassett DR. Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc 2003;35:1779-84. http://dx.doi.org/10.1249/01.MSS.0000089342.96098.C4.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. http://dx.doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-37. http://dx.doi.org/10.1016/j.jpsychores.2005.10.020.
- Roe L, Strong C, Whiteside C, Neil A, Mant D. Dietary intervention in primary care: validity of the DINE method for diet assessment. Fam Pract 1994;11:375-81. http://dx.doi.org/10.1093/fampra/11.4.375.
- Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J, et al. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr 2004;7:147-65. http://dx.doi.org/10.1079/PHN2003586.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analysing Qualitative Data. London: Routledge; 1994.
- Stenstrom U, Wikby A, Anderson P, Ryden O. Relationship between locus of control beliefs and metabolic control in insulin-dependent diabetes mellitus. Br J Health Psychol 1998;3. http://dx.doi.org/10.1111/j.2044-8287.1998.tb00552.x.
- Hampson S, Glasgow R, Strycker L. Beliefs versus feelings: a comparison of personal models and depression for predicting multiple outcomes in diabetes. Br J Health Psychol 2000;5:27-40. http://dx.doi.org/10.1348/135910700168748.
- Watkins KW, Connell CM, Fitzgerald JT, Klem L, Hickey T, Ingersoll-Dayton B, et al. Effect of adults’ self-regulation of diabetes on quality-of-life outcomes. Diabetes Care 2000;23:1511-15. http://dx.doi.org/10.2337/diacare.23.10.1511.
- Gholap NN, Mehta RL, Ng L, Davies MJ, Khunti K, Squire IB. Is admission blood glucose concentration a more powerful predictor of mortality after myocardial infarction than diabetes diagnosis? A retrospective cohort study. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2012-001596.
- Stone M, Patel N, Daly H, Martin-Stacey L, Amin S, Carey ME, et al. Using qualitative research methods to inform the development of a modified version of a patient education module for non-English speakers with type 2 diabetes: experiences from an action research project on two South Asian populations in the UK. Diversity Health Soc Care 2008;5:199-206.
- Tillin T, Hughes AD, Godsland IF, Whincup P, Forouhi NG, Welsh P, et al. Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: the Southall and Brent Revisited (SABRE) cohort. Diabetes Care 2012;36:383-93. http://dx.doi.org/10.2337/dc12-0544.
- Sintonen H, Pekurinen M, Walker SR, Rosser RM. Quality of Life Assessment: Key Issues in the 1990s. Dordrecht: Kluwer Academic Publishers; 1993.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 2006;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Gusi N, Olivares PR, Rajendram R, Preedy VR, Watson RR. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer; 2010.
- Ganswisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Sleep duration as a risk factor for Diabetes incidence in a larger US sample. Sleep 2007;30:1667-73.
- Tuomilehto H, Peltonen M, Partinen M, Lavigne G, Eriksson JG, Herder C, et al. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care 2009;32:1965-71. http://dx.doi.org/10.2337/dc08-1980.
- Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system?. Diabetologia 1998;41:1241-8. http://dx.doi.org/10.1007/s001250051058.
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135-43. http://dx.doi.org/10.1161/hc0902.104353.
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821-30. http://dx.doi.org/10.1172/JCI200319451.
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005;96:939-49. http://dx.doi.org/10.1161/01.RES.0000163635.62927.34.
- Yates T, Davies M, Brady E, Webb D, Gorely T, Bull F, et al. Walking and inflammatory markers in individuals screened for type 2 diabetes. Prev Med 2008;47:417-21. http://dx.doi.org/10.1016/j.ypmed.2008.06.015.
- Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C. ATTICA Study . The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: The ATTICA study. Prev Med 2005;40:432-7. http://dx.doi.org/10.1016/j.ypmed.2004.07.010.
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017-29. http://dx.doi.org/10.1210/jc.2007-0298.
- Iqbal SJ, Kaddam I, Wassif W, Nichol F, Walls J. Continuing clinically severe vitamin D deficiency in asians in the UK (Leicester). Postgrad Med J 1994;70:708-14. http://dx.doi.org/10.1136/pgmj.70.828.708.
- Barnett AH, Dixon AN, Bellary S, Hanif MW, O’Hare JP, Raymond NT, et al. Type 2 diabetes and cardiovascular risk in the UK south Asian community. Diabetologia 2006;49:2234-46. http://dx.doi.org/10.1007/s00125-006-0325-1.
- Giugliano D, Esposito K. Mediterranean diet and metabolic diseases. Curr Opin Lipidol 2008;19:63-8. http://dx.doi.org/10.1097/mol.0b013e3282f2fa4d.
- Lindeberg S, Jonsson T, Granfeldt Y, Borgstrand E, Soffman J, Sjöström K, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia 2007;50:1795-807. http://dx.doi.org/10.1007/s00125-007-0716-y.
- Pomerleau J, Lock K, McKee M, Altmann DR. The challenge of measuring global fruit and vegetable intake. J Nutr 2004;134:1175-80.
- Sargeant LA, Wareham NJ, Bingham SA, Day NE, Luben RN, Oakes S, et al. Vitamin C and hyperglycemia in the European Prospective Investigation into Cancer-Norfolk (EPIC-Norfolk) Study. A population-based study. Diabetes Care 2000;23:726-32. http://dx.doi.org/10.2337/diacare.23.6.726.
- Block G, Norkus E, Hudes M, Mandel S, Helzlsouer K. Which plasma antioxidants are most related to fruit and vegetable consumption?. Am J Epidemiol 2001;154:1113-18. http://dx.doi.org/10.1093/aje/154.12.1113.
- McKeown NM, Day NE, Welch AA, Runswick SA, Luben RN, Mulligan AA, et al. Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am J Clin Nutr 2001;74:188-96.
- Zino S, Skeaff M, Williams S, Mann J. Randomised controlled trial of effect of fruit and vegetable consumption on plasma concentrations of lipids and antioxidants. BMJ 1997;314:1787-91. http://dx.doi.org/10.1136/bmj.314.7097.1787.
- van het Hof KH, Tijburg LB, Pietrzik K, Weststrate JA. Influence of feeding different vegetables on plasma levels of carotenoids, folate and vitamin C. Effect of disruption of the vegetable matrix. Br J Nutr 1999;82:203-12.
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress and antioxidants: a review. J Biochem Molec Toxicol 2003;17:24-38. http://dx.doi.org/10.1002/jbt.10058.
- Dierckx N, Horvath G, van Gils V, Vertommen J, van de Vliet J, De Leeuw I, et al. Oxidative stress status in patients with diabetes mellitus: relationship to diet. Eur J Clinical Nutr 2003;57:999-1008. http://dx.doi.org/10.1038/sj.ejcn.1601635.
- Halliwell B, Gutteridge JMC. Antioxidant Defences: Endogenous and Diet Derived. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 2007.
- Higdon JV, Frei B. Obesity and oxidative stress, a direct link to CVD?. Arterioscler Thromb Vasc Biol 2003;23:365-7. http://dx.doi.org/10.1161/01.ATV.0000063608.43095.E2.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. http://dx.doi.org/10.1016/S0140-6736(00)04337-3.
- Public Health Guidence 38, Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk. London: NICE; 2012.
- Gray LJ, Khunti K, Edwardson C, Goldby S, Henson J, Morris DH, et al. Implementation of the automated Leicester Practice Risk Score in two diabetes prevention trials provides a high yield of people with abnormal glucose tolerance. Diabetologia 2012;55:3238-44. http://dx.doi.org/10.1007/s00125-012-2725-8.
- Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev 2011;33:46-62. http://dx.doi.org/10.1093/epirev/mxq019.
- Barber SR, Davies MJ, Khunti K, Gray LJ. Risk assessment tools for detecting those with pre-diabetes: a systematic review. Diabetes Res Clin Pract 2014;105:1-13. http://dx.doi.org/10.1016/j.diabres.2014.03.007.
- American Diabetes Association . Standards of Medical Care in Diabetes – 2010. Diabetes Care 2010;33:511-61.
- van den Donk M, Sandbaek A, Borch-Johnsen K, Lauritzen T, Simmons RK, Wareham NJ, et al. Screening for type 2 diabetes. Lessons from the ADDITION-Europe study. Diabet Med 2011;28:1416-24. http://dx.doi.org/10.1111/j.1464-5491.2011.03365.x.
- Dalton ARH, Bottle A, Okoro C, Majeed A, Millett C. Uptake of the NHS Health Checks programme in a deprived, culturally diverse setting: cross-sectional study. J Public Health 2011;33:422-9. http://dx.doi.org/10.1093/pubmed/fdr034.
- Eborall H, Stone M, Aujla N, Taub N, Davies M, Khunti K. Influences on the uptake of diabetes screening: a qualitative study in primary care. Br J Gen Pract 2012;62:e204-11. http://dx.doi.org/10.3399/bjgp12X630106.
- Geographies of Diversity in Leicestershire. Manchester: Centre on Dynamics of Ethnicity; 2013.
- de Lusignan S, van Weel C. The use of routinely collected computer data for research in primary care: opportunities and challenges. Fam Pract 2006;23:253-63. http://dx.doi.org/10.1093/fampra/cmi106.
- Davies MJ, Gray LJ, Troughton J, Gray A, Tuomilehto J, Farooqi A, et al. A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let’s Prevent Diabetes cluster randomised controlled trial. Prevent Med 2016;84:48-56. http://dx.doi.org/10.1016/j.ypmed.2015.12.012.
- Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol 2006;35:1292-300. http://dx.doi.org/10.1093/ije/dyl129.
- Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 2012;55:2895-905. http://dx.doi.org/10.1007/s00125-012-2677-z.
- Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0034916.
- Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the early ACTID randomised controlled trial. Lancet 2011;378:129-39. http://dx.doi.org/10.1016/S0140-6736(11)60442-X.
- Kinmonth AL, Wareham NJ, Hardeman W, Sutton S, Prevost AT, Fanshawe T, et al. Efficacy of a theory-based behavioural intervention to increase physical activity in an at-risk group in primary care (ProActive UK): a randomised trial. Lancet 2008;371:41-8. http://dx.doi.org/10.1016/S0140-6736(08)60070-7.
- Khunti K, Gray L, Skinner T, Campbell M, Carey M, Cradock S, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2333.
- Carter P, Bodicoat DH, Davies MJ, Ashra N, Riley D, Joshi N, et al. A retrospective evaluation of the NHS Health Check Programme in a multi-ethnic population. J Public Health (Oxf) 2016;38:534-42. http://dx.doi.org/10.1093/pubmed/fdv115.
- Leal J, Ahrabian D, Davies MJ, Gray LJ, Khunti K, Yates T, et al. Cost-effectiveness of a pragmatic structured education intervention for the prevention of type 2 diabetes: economic evaluation of data from the Let's Prevent Diabetes cluster-randomised controlled trial. BMJ Open 2017;7. http://dx.doi.org/10.1136/bmjopen-2016-013592.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Richardson J, Iezzi A, Khan MA, Maxwell A. Validity and reliability of the Assessment of Quality of Life (AQoL)–8D multi-attribute utility instrument. Patient 2014;7:85-96. http://dx.doi.org/10.1007/s40271-013-0036-x.
- Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- National Schedule of Reference Costs: Spell Costs. London: Department of Health; 2013.
- Prescription Cost Analysis, England – 2013. Leeds: Health and Social Care Information Centre; 2013.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157-70. http://dx.doi.org/10.1007/s40273-014-0193-3.
- Economic Modelling for Vascular Checks. London: Department of Health; 2008.
- Yates T, Davies MJ, Henson J, Troughton J, Edwardson C, Gray LJ, et al. Walking away from type 2 diabetes: trial protocol of a cluster randomized controlled trial evaluating a structured education programme in those at high risk of developing type 2 diabetes. BMC Public Health 2012;13. http://dx.doi.org/10.1186/1471-2296-13-46.
- Embarrassing Bodies. London; 2011.
- Davies M, Khunti K, Webb D, Mostafa S, Gholap N, Crasto W, et al. Updating the Handbook for Vascular Risk Assessment, Risk Reduction and Risk Management. Leicester: UK National Screening Committee and University of Leicester; 2012.
- Davies M, Yates T, Khunti K. Prevention of Type 2 Diabetes. Research Priorities for Diabetes in British South Asians. Birmingham: Diabetes UK/South Asian Health Foundation; 2009.
- Khokhar B, Jones J, Ronksley PE, Armstrong MJ, Caird J, Rabi D. Effectiveness of mobile electronic devices in weight loss among overweight and obese populations: a systematic review and meta-analysis. BMC Obes 2014;1. http://dx.doi.org/10.1186/s40608-014-0022-4.
- Hutchesson MJ, Rollo ME, Krukowski R, Ells L, Harvey J, Morgan PJ, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev 2015;16:376-92. http://dx.doi.org/10.1111/obr.12268.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296-304. http://dx.doi.org/10.1001/jama.298.19.2296.
- Davies MJ, Gray LJ, Goldby S, Hill S, Yates T, Khunti K. A Community-Based Primary Prevention Programme for Type 2 Diabetes in the UK: A Cluster Randomised Controlled Trial. American Diabetes Association, Boston, MA, USA, 5–9 June 2015. Diabetes 2015;64.
- Davies MJ, Gray LJ, Ahrabian D, Leal J, Troughton J, Gray A, et al. A Community Based Primary Prevention Programme for Type 2 Diabetes in the UK: A Cluster Randomised Controlled Trial n.d.
- Davies MJ. Diabetes Prevention in the Real World – Translating RCT into Action n.d.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-42.
Appendix 1 Systematic review search strategy (MEDLINE)
Example search strategy: MEDLINE.
-
Aerobic train$.tw.
-
Behav$ Modif$.tw.
-
Behav$ therap$.tw.
-
Cognitive$ therap$.tw.
-
counsel$.ti.
-
Health$ Educ$.tw.
-
Health$ Promot$.tw.
-
Health$ behav$.tw.
-
Educat$ program$.tw.
-
Patient Educ$.tw.
-
(Diet$ adj2 Intervention$).tw.
-
(Diet$ adj2 Modif$).tw.
-
Food habit$.tw.
-
(Health$ adj2 Eating).tw.
-
(Nutrition$ adj2 Counselling).tw.
-
(Nutrition$ adj2 Therap$).tw.
-
(Exercis$ adj2 intervention$).tw.
-
Physical Exercise.tw.
-
(Exercis$ adj2 therap$).tw.
-
Physical endurance.tw.
-
Physical education.tw.
-
Physical Fitness.tw.
-
Physical Activit$.tw.
-
Physical Train$.tw.
-
Resistance Train$.tw.
-
Strength Train$.tw.
-
(Lifestyle adj2 advice).tw.
-
(Lifestyle adj2 Guid$).tw.
-
(Lifestyle adj2 Modif$).tw.
-
Lifestyle Program$.tw.
-
Weight control$.tw.
-
Weight Train$.tw.
-
Weight reduc$.tw.
-
Weight loss program$.tw.
-
weight loss.tw.
-
(Weight adj loss adj program$).tw.
-
(lifestyle adj2 intervention).tw.
-
Sport$.tw.
-
walk$.tw.
-
jog$.tw.
-
swim$.tw.
-
cycle$.tw.
-
Bicycle$.tw.
-
exp Health Promotion/
-
exp Program Evaluation/
-
exp Patient Education as Topic/
-
exp Diet Therapy/
-
exp Nutrition Therapy/
-
exp Exercise Therapy/
-
exp Diet, Reducing/
-
(diabet$ adj4 lessen$).tw.
-
(diabet$ adj5 (reduc$ adj4 risk$)).ti,ab.
-
(diabet$ adj4 (lower$ adj5 incidence$)).ti,ab.
-
(diabet$ adj4 (decreas$ adj5 risk$)).ti,ab.
-
(diabet$ adj4 (reduc$ adj5 incidence$)).ti,ab.
-
(diabet$ adj4 (decreas$ adj5 incidence$)).ti,ab.
-
(diabet$ adj4 (lower$ adj5 risk$)).ti,ab.
-
(diabet$ adj4 (delay$ adj5 onset$)).ti,ab.
-
(diabet$ adj4 (reduc$ adj5 onset$)).ti,ab.
-
(diabet$ adj4 (reduc$ adj5 progress$)).ti,ab.
-
(diabet$ adj4 (decreas$ adj5 onset$)).ti,ab.
-
(risk$ adj4 develop$ adj4 diabet$).ti.
-
(reduc$ adj4 develop$ adj4 diabet$).ti,ab.
-
(decreas$ adj4 develop$ adj4 diabet$).ti,ab.
-
(diabet$ adj4 prevent$).tw.
-
(diabet$ adj4 reduc$).tw.
-
(diabet$ adj4 decreas$).tw.
-
(diabet$ adj4 lower$).tw.
-
(diabet$ adj4 lessen$).tw.
-
(diabet$ adj4 (reduc$ adj5 prevalence)).ti,ab.
-
(Diabet$ adj4 (decreas$ adj5 progress$)).ti,ab.
-
(diabet$ adj4 (lessen$ adj5 prevalence)).ti,ab.
-
(diabet$ adj4 (decreas$ adj5 prevalence)).ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50
-
51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73
-
Diabetes Mellitus, Type 2/pc [Prevention & Control]
-
exp Exercise/
-
exp Diet/
-
77 or 78
-
76 and 79
-
74 and 75
-
OBSERVATIONAL.ti,ab.
-
RCT.ti,ab.
-
(RANDOMI$4 adj CONTROL adj TRIAL$).ti,ab.
-
Experimental studies.ti,ab.
-
(QUASI adj EXPERIMENTAL).ti,ab.
-
TRIAL$.ti,ab.
-
Time-series.ti,ab.
-
Cross-sectional.ti,ab.
-
Cross-sectional studies.ti,ab.
-
longitudinal study.ti,ab.
-
Clinical trial.ti,ab.
-
randomized.ab.
-
placebo.ab.
-
dt.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
(Before adj2 after).ab.
-
Cohort analy$.ab.
-
exp cohort studies/
-
(cohort adj (study or studies)).ab.
-
(follow up adj (study or studies)).ab.
-
Retrospective.ab.
-
82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104
-
80 or 81
-
105 and 106
-
animal/ not (animal/ and human/)
-
107 not 108
-
limit 109 to english language
-
limit 110 to yr=2012-current
Appendix 2 Coding of intervention content
| Component | Coding |
|---|---|
| 1. Aim to promote changes in both diet and physical activity | Yes/no (1,0) |
| 2. Use established, well-defined behaviour change techniques (e.g. specific goal-setting, relapse prevention, self-monitoring, motivational interviewing, prompting self-talk, prompting practice, individual tailoring, time management) | Yes/no (1,0). Yes is scored if, as well as basic information provision, it includes ≥ 3 techniques from table 14 in the IMAGE guideline (which provides definitions used by NICE and other reviewers), or from a recognised taxonomy of behaviour change techniques (Michie et al.236) |
| 3. Work with participants to engage social support for the planned behaviour change (i.e. engage important others such as family, friends and colleagues) | Yes/no (1,0). Yes is scored if participants are encouraged to identify and seek social support outside the group (i.e. in their day-to-day lives). Encouraging social support within the group in a group-based intervention is not sufficient to code yes |
| 4. Maximise the frequency or number of contacts with participants (within the resources available) | High/medium/low (2,1,0), based on median split of total number of contacts Structured physical activity (e.g. gym-based exercise) sessions that were offered have not been counted, as they are assumed not to involve a substantial interactive component. Written contacts (newsletters, etc.) were not counted |
| 5. Use a coherent set of ‘self-regulatory’ intervention techniques (specific goal-setting, ideally with coping planning, also known as ‘relapse prevention’); prompting self-monitoring; providing feedback on performance; problem-solving; review of behavioural goals) | Yes/no (1,0). Yes is scored if the intervention includes goal setting, self-monitoring (of outcomes or behaviours) and at least one other self-regulation technique [providing feedback on performance, problem-solving (relapse prevention), revising action plans in the light of performance] |
| 6. Use a group size of 10–15. This recommendation is designed to balance cost and effectiveness, rather than to be an exact specified range, so we coded for ‘a group size of no more than 15’ (the point at which effectiveness is expected to be diminished) | Yes/no (1,0). If a range was reported for group size (e.g. groups of 15–20), the mid-point of the range was used for coding purposes If individual (one-to-one) intervention was used, then a yes is coded (1 case) |
| 7. Provide at least 16 hours of contact time over the first 18 months | Yes/no (1,0). Contact time is assumed to be 1 hour per group session if session length is not stated (one case) or 10 minutes for a telephone contact (two cases), 30 minutes for an individual counselling session (one case) and 15 minutes for a GP visit (one case) |
| 8. Ensure programmes adopt a person-centred, empathy-building approach | Yes/no (1,0). Coded as yes if it is explicitly stated that a person-centred, empathy-building or empowerment theory-based approach was used throughout, or if motivational interviewing or other empathy-building techniques are specified |
| 9. Allow time between sessions, spreading them over a period of 9–18 months | Yes/no (1,0) |
| 10. Information provision: to raise awareness of the benefits of and types of lifestyle changes needed | Yes/no (1,0) |
| 11. Exploration and reinforcement of participants’ reasons for wanting to change and their confidence about making changes | Yes/no (1,0) |
| 12. Gradual building of confidence (self-efficacy) by starting with achievable and sustainable short-term goals and setting of graded tasks | Yes/no (1,0) |
| 13. Use a logical sequence of intervention methods (e.g. motivation, action-planning, maintenance) | Yes/no (1,0) |
| Total IMAGE guidance score | Possible maximum score of 6 points: 1 point for each yes for items 1, 2, 3 and 5. For item 4, score 2 points for a high amount of contact, 1 point for a medium amount |
| Total NICE guidance score | Possible maximum score of 12 points: IMAGE score (as above but without item 4, which overlaps with item 7) plus 1 point for each yes for items 6–13 |
| 14. Intervention fidelity checking | We also coded whether or not the developers used specific methods to check intervention fidelity (e.g. monitoring the first four sessions and giving formative feedback) |
Appendix 3 Coding scores for study interventions
| Coding details | Main reference (first author, year) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absetz, 200996 | Ackermann, 200875 | Almeida, 201076 | Boltri, 200877 | Costa, 201278 | Davis-Smith, 200779 | Faridi, 201062 | Gilis-Januszewska, 201169 | Janus, 201293 | Kanaya, 201294 | Katula, 201180 | Kramer, 200970 | Kramer, 201281 | Kramer, 201281 | Kulzer, 200971 | Laatikainen, 201298 | Makrilakis, 201073 | Mensink, 200382 | Ockene, 201284 | Parikh, 201085 | Payne, 200886 | Penn, 200987 | Penn, 201395 | Ruggerio, 201188 | Saaristo, 201089 | Sakane, 201190 | Yates, 200950 | Yates, 200950 | |
| Study name | GOAL trial | DEPLOY | KPCO | DPP in faith-based setting | DEPLAN Spain | PREDICT | DEPLAN Poland | pMDPS | Live Well, Be Well | HELP-PD | GLB 2005–8 | GLB 2009 CPC | GLB 2009 TPC | PREDIAS | GGT study | DEPLAN Greece | SLIM study | Lawrence Latino DPP | Project HEED | Payne et al.86 | Penn et al.87 | Penn et al.95 | Ruggerio et al.88 | FIN-D2D | Sakane et al.90 | PREPARE | PREPARE + pedometer | |
| Criteria for coding intervention content | ||||||||||||||||||||||||||||
| 1. Diet and physical activity | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 2. Established techniques | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ✗ | ✗ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Engage social support | 0 | 1 | 0 | 1 | 1 | ✗ | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | ✗ | ✗ | ✗ | 0 | 0 | 0 | 1 | 1 | 0 | ✗ | 0 | 0 |
| 4. Maximised the frequency or number of contacts | 0 | 2 | 0 | 2 | 1 | 0 | ✗ | 2 | 0 | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 2 | 1 | 0 | 0 | 0 |
| Number of contacts in 1 year (total number if different) | 6 | 23 | 1 | 16 | 10 | 6 | ✗ | 16 | 6 | 19 | 41 (65) | 12 | 21 | 21 | 12 | 6 | 6 | 5 (13) | 16 | 8 | 13 | 8 (24) | 3 | 22 | 8 | 6 (10) | 3 | 3 |
| Number of physical activity sessions in 1 year | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 78 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 24 | 1 | 17 | 0 | 0 | 0 | 0 | 0 |
| 5. Self-regulatory intervention techniques | 1 | 1 | 0 | 1 | 1 | ✗ | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ✗ | ✗ | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 6. Group size ≤ 15 | 1 | 1 | 0 | 1 | 1 | 1 | ✗ | 1 | ✗ | ✗ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ✗ | 1 | 0 | 1 | 0 | 1 | 1 | ✗ | 1 | 1 |
| 7. Contact time ≥ 16 hours | 0 | 1 | 0 | 1 | 0 | 0 | ✗ | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 8. Person centred, empathy-building approach | 1 | 0 | 0 | 0 | 1 | ✗ | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | ✗ | ✗ | 1 | 1 | 1 | 1 | 0 | 0 | 1 | ✗ | 1 | 1 |
| 9. Sessions spread | 0 | 1 | 0 | 0 | 1 | 0 | ✗ | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | ✗ | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| 10. Information provision | 1 | 1 | 1 | 1 | 1 | ✗ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11. Exploration and reinforcement of motivation | 1 | 1 | 1 | 1 | 1 | ✗ | 0 | 1 | ✗ | 0 | 1 | 1 | 1 | 1 | 1 | 1 | ✗ | ✗ | 1 | 0 | 1 | 1 | 0 | ✗ | 1 | ✗ | 0 | 0 |
| 12. Building of confidence (self-efficacy) | 1 | 1 | 0 | 1 | 0 | ✗ | 0 | 1 | ✗ | 1 | 1 | 1 | 1 | 1 | 0 | 1 | ✗ | ✗ | 1 | 0 | 1 | 1 | 1 | ✗ | 1 | ✗ | 1 | 1 |
| 13. Logical sequence of intervention methods | 1 | 1 | 0 | 1 | 1 | ✗ | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ✗ | ✗ | ✗ | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| Total NICE score | 9 | 11 | 4 | 10 | 10 | 3 | 4 | 11 | 4 | 8 | 11 | 9 | 11 | 11 | 11 | 9 | 4 | 4 | 7 | 7 | 10 | 10 | 8 | 9 | 7 | 5 | 7 | 7 |
| NICE score without imputation | 9 | 11 | 4 | 10 | 10 | ✗ | ✗ | 11 | ✗ | ✗ | 11 | 9 | 11 | 11 | 11 | 9 | ✗ | ✗ | ✗ | 7 | 10 | 10 | 8 | ✗ | 7 | ✗ | 7 | 7 |
| Total IMAGE score | 3 | 6 | 2 | 6 | 5 | 2 | 3 | 6 | 3 | 5 | 6 | 5 | 6 | 6 | 5 | 3 | 1 | 1 | 5 | 4 | 5 | 4 | 4 | 6 | 3 | 3 | 2 | 2 |
| IMAGE score without imputation | 3 | 6 | 2 | 6 | 5 | ✗ | ✗ | 6 | 3 | 5 | 6 | 5 | 6 | 6 | 5 | 3 | ✗ | ✗ | ✗ | 4 | 5 | 4 | 4 | 6 | 3 | ✗ | 2 | 2 |
| 14. Intervention fidelity checking | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
Appendix 4 Mean change (baseline to 12 months) in outcomes for body composition and glycaemic control
| Main reference (first author, year) | Weight, kg | BMI, kg/m2 | Waist, cm | HbA1c, % (mmol/mol) | Fasting glucose, mmol/l | 2-hour glucose, mmol/l | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | ± | SD | n | Mean | ± | SD | n | Mean | ± | SD | n | Mean | ± | SD | n | Mean | ± | SD | n | Mean | ± | SD | |
| Absetz, 200996 | 312 | –0.8 | ± | 4.5 | 312 | –0.3 | ± | 1.6 | 312 | –1.6 | ± | 4.8 | – | – | – | – | 312 | 0.1 | ± | 0.6 | 312 | 0.1 | ± | 1.7 |
| Ackermann, 200875 | 29 | –5.7 | ± | 5.2 | 29 | –2.1 | ± | 2.1 | – | – | – | – | 29 | –0.1 | ± | 0.4 | – | – | – | – | – | – | – | – |
| (–1.1 | ± | 4.4) | ||||||||||||||||||||||
| Almeida, 201076 | 760 | –1.4 | ± | 3.5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Boltri, 200877 | 8 | –0.5 | ± | 4.9 | 8 | –0.2 | ± | 2 | – | – | – | – | – | – | – | – | 8 | –0.4 | ± | 0.2 | – | – | – | – |
| Costa, 201278 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Davis-Smith, 200779 | 10 | –4.8 | ± | 11.5 | 10 | –1.9 | ± | – | – | – | – | – | – | – | – | – | 10 | –0.55 | ± | 0.5 | – | – | – | – |
| Faridi, 201062 | 83 | 0.1 | ± | 11.8 | 83 | –0.63 | ± | 6.72 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Gilis-Januszewska, 201169 | 175 | –1.92 | ± | 5.01 | 175 | –0.69 | ± | 1.9 | 175 | –3.26 | ± | 6.11 | – | – | – | – | 175 | 0.11 | ± | 0.72 | 175 | 0.31 | ± | 2.35 |
| Janus, 201293 | 38 | –2.65 | ± | 4.44 | 38 | –0.98 | ± | 160.27 | 38 | –7.45 | ± | 7.09 | 37 | 0.08 | ± | 0.43 | 37 | –0.03 | ± | 0.36 | 36 | –0.11 | ± | 1.8 |
| Kanaya, 201294 | 113 | –0.61 | ± | 3.4 | – | – | – | – | 113 | –0.06 | ± | 4.68 | – | – | – | – | 113 | –0.88 | ± | 10.84 | – | – | – | – |
| Katula, 201180 | 135 | –7.0 | ± | 4.5 | 135 | –2.29 | ± | 1.2 | 135 | –5.61 | ± | 2.3 | – | – | – | – | 135 | –0.25 | ± | 0.6 | – | – | – | – |
| Kramer, 200970 | 42 | –4.2 | ± | 5.7 | 42 | –1.6 | ± | 2.1 | 42 | –7.1 | ± | 6.1 | – | – | – | – | – | – | – | – | – | – | – | – |
| Kramer, 2012 (CPC)81 | 29 | –4 | ± | 5 | 29 | –1.5 | ± | 1.7 | 29 | –5.59 | ± | 2.5 | 27 | –0.16 | ± | 0.3 | 27 | –0.29 | ± | 0.6 | – | – | – | – |
| (–1.7 | ± | 3.3) | ||||||||||||||||||||||
| Kramer, 2012 (TPC)81 | 31 | –2.6 | ± | 6.4 | 31 | –0.9 | ± | 1.5 | 31 | –4.32 | ± | 3 | 31 | –0.1 | ± | 0.2 | 31 | –0.05 | ± | 0.5 | – | – | – | – |
| (–1.1 | ± | 2.2) | ||||||||||||||||||||||
| Kulzer, 200971 | 91 | –3.6 | ± | 5.1 | 91 | –1.3 | ± | 1.7 | 91 | –4.1 | ± | 6 | 91 | 0 | ± | 0.3 | 91 | –0.27 | ± | 0.7 | 91 | –0.46 | ± | 1.89 |
| (0.0 | ± | 3.3) | ||||||||||||||||||||||
| Laatikainen, 201298 | 221 | –2.6 | ± | 5.2 | 237 | –0.93 | ± | 1.9 | 220 | –4.3 | ± | 5.3 | – | – | – | – | 221 | –0.1 | ± | 0.5 | 232 | –0.6 | ± | 1.7 |
| Makrilakis, 201073 | 125 | –1 | ± | 4.7 | 125 | –0.5 | ± | 2.1 | 125 | –0.3 | ± | 6.8 | – | – | – | – | 125 | –0.15 | ± | 0.69 | 125 | 0.03 | ± | 1.85 |
| Mensink, 200382 | 47 | –2.7 | ± | 3.8 | 47 | –0.9 | ± | 1.4 | 47 | –3.5 | ± | 3.4 | 47 | –0.2 | ± | 0.7 | 47 | –0.1 | ± | 0.7 | 47 | –0.8 | ± | 0.3 |
| (–2.2 | ± | 7.7) | ||||||||||||||||||||||
| Ockene, 201284 | 147 | –1.1 | ± | 4.6 | 147 | –0.4 | ± | 1.6 | – | – | – | – | 147 | –0.1 | ± | 0.3 | 147 | 0.03 | ± | 0.7 | – | – | – | – |
| (–1.1 | ± | 3.3) | ||||||||||||||||||||||
| Parikh, 201085 | 35 | –3.3 | ± | 3.3 | – | – | – | – | 35 | –3.3 | ± | 6.6 | 35 | –0.3 | ± | 0.2 | 35 | 0.62 | ± | 0.8 | 35 | 0.19 | ± | 2.1 |
| (–3.3 | ± | 2.2) | ||||||||||||||||||||||
| Payne, 200886 | 122 | –4.1 | ± | 5.2 | 122 | –1.46 | ± | 2 | 120 | –4.68 | ± | 6.8 | – | – | – | – | 122 | –0.15 | ± | 0.5 | 118 | –0.34 | ± | 1.4 |
| Penn, 200987 | 39 | –2.3 | ± | 5.1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Penn, 201395 | 134 | –3.7 | ± | 6.7 | 134 | –1.29 | ± | 2.84 | 134 | –6.45 | ± | 4.00 | – | – | – | – | – | – | – | – | – | – | – | – |
| Ruggerio, 201188 | 57 | –1.3 | ± | 5.1 | 57 | –0.5 | ± | 2.03 | 55 | –3.5 | ± | 6.2 | – | – | – | – | – | – | – | – | – | – | – | – |
| Saaristo, 201089 | 2798 | –1.1 | ± | 5.6 | 2786 | –0.4 | ± | 1.9 | 2709 | –1.3 | ± | 5.6 | – | – | – | – | – | – | – | – | – | – | – | – |
| Sakane, 201190 | 146 | –1.4 | ± | 4.1 | 123 | –0.6 | ± | 1 | 123 | –1.7 | ± | 2.2 | – | – | – | – | 123 | –0.1 | ± | 0.6 | 123 | –1.2 | ± | 1.8 |
| Yates, 2009 (PREPARE + pedometer)50 | 29 | 0.49 | ± | 3.8 | – | – | – | – | 29 | 0.5 | ± | 3.8 | – | – | – | – | 29 | –0.2 | ± | 0.5 | 29 | –1.75 | ± | 2.2 |
| Yates, 2009 (PREPARE)50 | 29 | –0.54 | ± | 3.8 | – | – | – | – | 29 | –0.5 | ± | 3.7 | – | – | – | – | 29 | –0.03 | ± | 0.4 | 29 | 0.19 | ± | 1.7 |
Appendix 5 Mean change (baseline to 12 months) in outcomes for lipids, blood pressure and incident diabetes
| Main reference (first author, year) | Total cholesterol | LDL | HDL | Triglycerides | Systolic BP | Diastolic BP | T2DM (n/1000 person-years) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | ± | SD | n | Mean | ± | SD | n | Mean | ± | SD | n | Mean | ± | SD | n | Mean | ± | SD | n | Mean | ± | SD | ||
| Absetz, 200996 | 312 | –0.1 | ± | 0.9 | – | – | – | – | 312 | 0 | ± | 0.3 | 312 | –0.07 | ± | 0.63 | – | – | – | – | – | – | – | – | 28.4 |
| Ackermann, 200875 | 29 | –0.35 | ± | 0.8 | – | – | – | – | 29 | 0.05 | ± | 0.2 | – | – | – | – | 29 | –1.6 | ± | 15.7 | – | – | – | – | – |
| Almeida, 201076 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Boltri, 200877 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 8 | –8 | ± | 12.9 | 8 | –8 | ± | 6.4 | – |
| Costa, 201278 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 46.0 |
| Davis-Smith, 200779 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 10 | –13 | ± | 11.6 | 10 | –19 | ± | 10.2 | – |
| Faridi, 201062 | 83 | –0.62 | ± | 6.84 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Gilis-Januszewska, 201169 | 175 | –0.23 | ± | 1.16 | – | – | – | – | 175 | 0 | ± | 0.32 | 175 | –0.13 | ± | 1.14 | 175 | –2.07 | ± | 14.4 | 175 | –1.96 | ± | 9.01 | – |
| Janus, 201293 | 37 | –0.09 | ± | 0.73 | 37 | –0.12 | ± | 0.67 | 37 | 0.07 | ± | 0.18 | 37 | –0.09 | ± | 0.49 | 38 | –6.55 | ± | 14.73 | 38 | 0.7 | ± | 9.55 | – |
| Kanaya, 201294 | – | – | – | – | 113 | –0.15 | ± | 0.64 | 113 | 0.08 | ± | 0.21 | 113 | –0.02 | ± | 0.85 | 113 | 0.34 | ± | 14.67 | – | – | – | – | – |
| Katula, 201180 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Kramer, 200970 | 41 | –0.17 | ± | 0.57 | – | – | – | – | 41 | 0.07 | ± | 0.21 | – | – | – | – | 38 | –13 | ± | 18 | 38 | –4.3 | ± | 8.1 | – |
| Kramer, 2012 (CPC)81 | 26 | 0.15 | ± | 1 | 26 | 0.22 | ± | 0.9 | 26 | –0.01 | ± | 0.22 | 26 | –0.08 | ± | 0.02 | 21 | –4.2 | ± | 12 | 21 | –5.9 | ± | 10.6 | – |
| Kramer, 2012 (TPC)81 | 27 | –0.08 | ± | 0.9 | 27 | 0.13 | ± | 0.8 | 27 | –0.08 | ± | 0.3 | 27 | –0.29 | ± | 0.02 | 27 | –8.4 | ± | 17.6 | 27 | –8.5 | ± | 11.2 | – |
| Kulzer, 200971 | 91 | –0.26 | ± | 0.92 | – | – | – | – | 91 | –0.03 | ± | 0.18 | 91 | –0.4 | ± | 1.54 | 91 | –4.6 | ± | 19.1 | 91 | –4.4 | ± | 11.7 | – |
| Laatikainen, 201298 | 221 | –0.3 | ± | 0.9 | 229 | –0.25 | ± | 0.7 | 221 | 0.1 | ± | 0.2 | 221 | –0.2 | ± | 0.8 | 236 | –1.01 | ± | 12.5 | 220 | –2.3 | ± | 9.4 | – |
| Makrilakis, 201073 | 125 | –0.37 | ± | 0.99 | 125 | –0.39 | ± | 0.91 | 125 | 0 | ± | 0.07 | 125 | 0.03 | ± | 0.68 | 125 | –6 | ± | - | 125 | 1 | ± | – | – |
| Mensink, 200382 | 40 | 0 | ± | 0.6 | 40 | 0.01 | ± | 0.5 | 40 | –0.04 | ± | 0.1 | 40 | –0.01 | ± | 0.5 | – | – | – | – | – | – | – | – | 60.6 |
| Ockene, 201284 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 12.9 |
| Parikh, 201085 | – | – | – | – | 35 | 0.03 | ± | 0.9 | – | – | – | – | – | – | – | – | 35 | –1 | ± | 13 | 35 | –2 | ± | 9 | 360.0 |
| Payne, 200886 | 120 | –0.23 | ± | 0.7 | 98 | –0.21 | ± | 0.8 | 101 | 0.02 | ± | 0.2 | 120 | –0.17 | ± | 0.6 | 119 | –10.5 | ± | 19.2 | 119 | –4.03 | ± | 10.5 | 8.2 |
| Penn, 200987 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 32.7 |
| Penn, 201395 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ruggerio, 201188 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Saaristo, 201089 | 2480 | –0.18 | ± | 0.82 | 2395 | –0.18 | ± | 0.76 | 2453 | 0.03 | ± | 0.28 | 2443 | –0.06 | ± | 0.83 | 2748 | –1.54 | ± | 14.81 | 2748 | –1.57 | ± | 8.57 | 58.9 |
| Sakane, 201190 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 25.3 |
| Yates, 2009 (PREPARE + pedometer)50 | 29 | –0.04 | ± | 0.81 | – | – | – | – | 29 | –0.03 | ± | 0.2 | 29 | 0.03 | ± | 0.6 | 29 | –0.4 | ± | 13.3 | – | – | – | – | – |
| Yates, 2009 (PREPARE)50 | 29 | –0.02 | ± | 0.55 | – | – | – | – | 29 | 0 | ± | 0.2 | 29 | 0.08 | ± | 0.7 | 29 | –2.5 | ± | 16.3 | – | – | – | – | – |
Appendix 6 Study quality
| Checklist criteria | Main reference (first author, year) | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absetz, 200996 | Ackermann, 200875 | Almeida, 201076 | Boltri, 200877 | Costa, 201278 | Davis-Smith, 200779 | Faridi, 201062 | Gilis-Januszewska, 201169 | Janus, 201293 | Kanaya, 201294 | Katula, 201180 | Kramer, 200970 | Kramer, 201281 | Kulzer, 200971 | Laatikainen, 201298 | Ma, 201392 | Makrilakis, 201073 | Mensink, 200382 | Nilsen, 201183 | Ockene, 201284 | Parikh, 201085 | Payne, 200886 | Penn, 200987 | Penn, 201395 | Ruggerio, 201188 | Saaristo, 201089 | Sakane, 201190 | Vermunt, 2011104 | Yates, 200950 | ||
| 1.1 | Source population or area well described | + | ++ | + | ++ | + | ++ | ++ | ++ | + | ++ | + | ++ | ++ | + | + | + | ++ | ++ | + | ++ | ++ | ++ | + | + | ++ | + | + | ++ | + |
| 1.2 | Eligible population or area representative | ++ | ++ | + | + | ++ | + | + | + | ++ | ++ | ++ | + | + | + | + | + | + | + | ++ | ++ | ++ | ++ | + | + | ++ | ++ | + | ++ | + |
| 1.3 | Selected participants or areas representative | ++ | ++ | ++ | + | ++ | ++ | + | + | ++ | + | ++ | + | + | + | ++ | + | + | ++ | + | + | ++ | + | ++ | + | + | + | ++ | ++ | + |
| 2.1 | Allocation: selection bias minimised | NA | + | NR | NA | + | NA | + | NA | ++ | ++ | ++ | NA | + | ++ | NA | + | NA | ++ | ++ | ++ | ++ | NA | ++ | NA | NA | NA | + | + | ++ |
| 2.2 | Interventions (and comparisons) well described and appropriate | ++ | ++ | + | ++ | ++ | + | + | ++ | + | + | ++ | ++ | ++ | ++ | ++ | ++ | + | + | + | ++ | ++ | ++ | ++ | ++ | + | ++ | + | + | ++ |
| 2.3 | Allocation concealed | NA | NA | NA | NA | − | NA | NA | NA | ++ | ++ | NA | NA | + | ++ | NA | ++ | NA | ++ | ++ | NR | NA | NA | ++ | NA | NA | NA | + | NA | ++ |
| 2.4 | Participants and/or investigators blinded | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 2.5 | Exposure to intervention and comparison adequate | NA | ++ | NR | NA | NR | NA | + | NA | ++ | ++ | ++ | NA | ++ | + | NA | + | NA | ++ | ++ | + | ++ | NA | ++ | NA | NA | NA | ++ | ++ | ++ |
| 2.6 | Contamination acceptably low | NA | ++ | NR | NA | ++ | NA | ++ | NA | ++ | ++ | ++ | NA | ++ | ++ | NA | ++ | NA | ++ | ++ | ++ | ++ | NA | ++ | NA | NA | NA | ++ | ++ | ++ |
| 2.7 | Other interventions similar in groups | NA | ++ | NR | NA | ++ | NA | ++ | NA | ++ | + | ++ | NA | ++ | + | NA | + | NA | ++ | ++ | ++ | ++ | NA | ++ | NA | NA | NA | ++ | ++ | ++ |
| 2.8 | All participants accounted for at study conclusion | ++ | + | ++ | ++ | + | ++ | + | ++ | + | ++ | ++ | + | ++ | + | + | ++ | + | + | ++ | ++ | + | ++ | + | + | ++ | NA | ++ | ++ | ++ |
| 2.9 | Setting reflects usual UK practice | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | + | + | ++ | ++ | + | + | ++ | ++ | ++ | ++ | ++ | + |
| 2.10 | Intervention or control reflects usual UK practice | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | + | ++ | ++ | + | + | ++ | + | ++ | + | ++ | ++ | ++ | + | + | + | ++ | ++ | ++ | ++ | ++ |
| 3.1 | Outcome measures reliable | ++ | + | + | + | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | + | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ |
| 3.2 | Outcome measures complete | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | + | + | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | + | ++ | ++ | + | + | ++ | ++ | ++ | ++ | ++ |
| 3.3 | All important outcomes assessed | ++ | ++ | + | ++ | + | ++ | + | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | + | ++ | ++ | ++ | ++ | ++ |
| 3.4 | Outcomes relevant | ++ | ++ | NA | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 3.5 | Similar follow-up times in groups | NA | ++ | ++ | NA | ++ | NA | ++ | NA | ++ | ++ | ++ | NA | ++ | ++ | NA | ++ | NA | ++ | ++ | ++ | ++ | NA | ++ | NA | NA | NA | ++ | ++ | ++ |
| 3.6 | Follow-up time meaningful | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 4.1 | Groups similar at baseline | NA | ++ | NR | NA | ++ | NA | + | NA | + | ++ | ++ | NA | ++ | ++ | NA | ++ | NA | ++ | ++ | ++ | ++ | NA | ++ | NA | NA | NA | ++ | ++ | ++ |
| 4.2 | ITT analysis conducted | NR | + | NR | ++ | ++ | ++ | + | ++ | + | ++ | + | ++ | ++ | ++ | + | ++ | + | ++ | + | + | ++ | ++ | ++ | + | + | NR | ++ | + | + |
| 4.3 | Study sufficiently powered | NR | NR | NR | NR | ++ | NR | ++ | NR | NR | ++ | ++ | ++ | ++ | ++ | + | ++ | NR | + | ++ | NR | NR | ++ | NR | NR | NR | NR | ++ | ++ | ++ |
| 4.4 | Estimates of effect size given or calculable | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 4.5 | Analytical methods appropriate | ++ | ++ | ++ | + | ++ | + | ++ | ++ | + | + | ++ | ++ | + | + | + | ++ | ++ | ++ | + | ++ | + | + | + | ++ | ++ | ++ | + | ++ | ++ |
| 4.6 | Precision of intervention effects given or calculable | ++ | ++ | ++ | + | ++ | + | ++ | ++ | + | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ |
| 5.1 | Study results internally valid (i.e. unbiased) | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 5.2 | Findings generalisable to source population (i.e. externally valid) | ++ | ++ | + | + | ++ | ++ | + | + | ++ | ++ | ++ | + | + | + | + | + | + | ++ | + | ++ | ++ | ++ | + | + | ++ | + | + | ++ | + |
Appendix 7 Invitation letter
Appendix 8 Topic guide for telephone interviews
Topic guide for Melton patients attending pre-diabetes mellitus DESMOND sessions (brief version for telephone interviews)
Pre-course
-
Before you came on the course, did you receive the following information:
-
Preparing for pre-diabetes DESMOND?
-
A pedometer?
-
In this booklet did you find the instructions for using the pedometer?
Were you able to read it before the course started?
-
How helpful did you find the booklet?
Probe reasons: in what ways
-
How helpful were the instructions for the use of the pedometer and log book?
-
Did you use the pedometer as instructed in the booklet or not?
Probe: reasons for either response.
-
When you were told that you were at a pre-diabetes stage, how did you feel about that?
-
Did you understand what that meant or not?
After attending the course
-
After attending the course do you feel as if you know more about pre-diabetes than you did before?
-
Do you remember the educators talking about insulin resistance?
-
What do you remember about that? (Ask fluidly and say this is not a test.) Did you know about that before?
-
I would just like to ask what key message/s you have taken away from these education sessions.
-
After coming on a course like this, people like to make changes to their diet and lifestyle but can find it difficult.
If you were being very honest with yourself, are you likely to make changes after attending these?
Probe:
-
Did you find it helpful with making changes? In what ways?
-
Did you find it unhelpful with making changes? In what ways?
-
Can you remember any of the games?
-
Do you remember the following physical activity games?
-
Health benefits [e.g. reducing cholesterol and the number of minutes of activity (30 minutes = improves cholesterol and BP)]
-
What was the key message from that game to you?
-
Activity continuum (showed activity cards with low and moderate intensity exercises)
What was the key message from that game to you?
-
The 45-minute game (showed e.g. minutes of walking, vacuuming for 10 minutes and mowing the lawn for 10 minutes = 30 minutes)
What was the key message from that game to you?
-
Pedometer game (walking at moderate intensity for 10 minutes is equivalent to 1000 steps)
What was the key message from that game to you?
-
Are you likely or unlikely to use the pedometer after attending the course? Explore reasons given see if he/she likely/unlikely to follow guidance.
-
Are you likely to make an activity action plan after attending the course? Explore reasons given see if he/she likely/unlikely to follow guidance.
-
Some people have said that going on a course like this, has helped their confidence in making changes with their diet and lifestyle.
Do you feel the same way?
If so, what helped you feel this way about your diet?
If so, what helped you feel this way about your lifestyle?
-
If you had a choice, when you were booked onto the course, which of the following would you have preferred?
-
Course delivered with interpreters.
-
Course delivered in English only with South Asian food resources.
-
Course delivered in English without South Asian food resources.
-
-
If you had a choice of which of the following would you prefer?
-
Two half days.
-
Four sessions like these you have attended.
-
-
How did you find the interpretation, useful or not useful? Why?
-
Did you find having the stickers/images useful or not useful?
-
How useful were the food resources in helping you understand South Asian diets?
Appendix 9 Topic guides for black and minority ethnic focus groups
Topic guide for Wesley Hall patients attending pre-diabetes Diabetes Education and Self-Management for Ongoing and Newly Diagnosed sessions (focus groups)
Pre-course
-
Before you came onto to the course, what information did you receive?
Prompt:
-
Preparing for pre-diabetes DESMOND leaflets.
-
A pedometer.
-
In this booklet did you find the instructions for using the pedometer?
Probe: Were you able to read it before the course started?
How helpful did you find the information in the leaflets?
-
How helpful were the instructions for the use of the pedometer and log book?
-
Did you use the pedometer as instructed in the booklet or not?
Probe: reasons for either response.
-
Did you understand what being at pre-diabetes meant or not?
After attending the course
-
After attending the course what do you understand about pre-diabetes?
Probe: Anything else?
-
Do you remember the educators talking about insulin resistance?
-
What do you remember about that, (ask fluidly and say this is not a test). Probe: Did you know about that before?
How helpful was that you?
Food activities
-
Can you remember any of the food activities?
Probe: Which ones?
Why?
Key message you took away for yourself?
Prompt if not remembered:
-
100 calorie game.
-
Oily fats.
-
Food continuum.
-
How useful were the food activities in helping you understand South Asian diets?
Probe: Was there anything else that you would have liked to know about that was not covered or was it covered?
Physical activities
-
Can you remember any of the physical activities that were discussed?
Probe: Which ones can you remember?
-
I am going to take you through each of the physical activity sections that were discussed. With each of them I would like you tell me what the key messages/learning you have taken away?
-
Health benefits [e.g. reducing cholesterol and the number of minutes of activity (30 minutes = improves cholesterol and BP)].
-
What was the key message from that game to you?
-
Activity continuum (showed activity cards with low- and moderate- intensity exercises).
What was the key message from that game to you?
-
The 45-minute game (showed e.g. minutes of walking, vacuuming for 10 minutes and mowing the lawn for 10 minutes = 30 minutes).
What was the key message from that game to you?
-
Pedometer game (walking at moderate intensity for 10 minutes is equivalent to 1000 steps).
What was the key message from that game to you?
-
Are you likely or unlikely to use the pedometer after attending the course? Explore reasons given see if he/she likely/unlikely to follow guidance.
-
Are you likely to make an activity action plan after attending the course? Explore reasons given see if he/she likely/unlikely to follow guidance.
-
If you were being very honest with yourself, are you likely make changes in your diet and lifestyle after attending these education sessions?
Probe:
-
What do you think some of the challenges/barriers will be in making changes?
-
Where do you think some of the opportunities are in your lives to make changes?
-
When you were booked onto the course, would you have liked the choice of going to education sessions in English without Gujarati interpretation or preferred to have one with Gujarati interpretation? Probe: Why?
-
How did you find the interpretation, useful or not useful? Why?
Probe: Accuracy and level?
-
Did you find having the stickers/images useful or not useful?
Probe: In what ways, with examples, if they can remember any particular ones?
-
How did you find the educators style of delivery during the education sessions?
Probe: Did you feel that they were open to having questions asked or not?
-
Before you came onto the course, did you know that you were coming to group education sessions?
Probe: How did you feel about that before coming?
How do you feel about it now that you been?
-
DESMOND group education is very different from many other health education courses because patients are asked for what they think and are involved in the way it’s delivered. In some courses, patients are simply told about their condition and what they should do.
Which type group education do you prefer and why?
-
Finally, what would you prefer out the following and why?
-
Two half days such as 9.30 until 1.30.
-
Four half day sessions.
-
Anything else you would like to say?
THANKS SO MUCH FOR YOUR TIME.
Appendix 10 Black and minority ethnic topic guides
Topic guide for patients attending the pre-diabetes black and minority ethnic Diabetes Education and Self-Management for Ongoing and Newly Diagnosed sessions
-
How did you find the timing of the education sessions?
Probe:
-
Were they convenient or inconvenient for you?
-
How did you find the venue for the education sessions?
Probe:
-
Was it easy or difficult for you to get to?
-
How did you find the length of sessions?
-
Before you came onto the course, what did you think caused diabetes?
Probe:
-
What did you think caused pre-diabetes?
-
When you were told that you were at a pre-diabetes stage, how did you feel about that?
-
Did you understand what that meant or not?
-
What were your reasons for attending the education sessions?
Overall views
-
After attending the course, do you feel as if you know more about pre-diabetes than you did before?
Probe:
-
If so, what kind of things?
-
If no, is there a reason why?
-
I would just like to ask what key message/s you have taken away from these education sessions.
-
After coming onto a course like this, some people like to make changes to their diet and lifestyle but can find it difficult.
If you were being very honest with yourself, are you likely to make changes after attending these?
Probe:
-
Did you find it helpful with making changes? In what ways?
-
Did you find it unhelpful with making changes? In what ways?
-
How did you find the content of the course, was it difficult or easy to understand?
Probe:
-
Any particular aspects?
-
How did you find the speed at which the course was delivered?
Probe:
-
Was it too fast or slow for you?
-
Why did you feel it was fast/slow/just right?
-
Did you feel that there was too much patient participation or too little patient participation?
-
How did you feel when the educator asked the group for their ideas, experience and questions?
-
Did you feel like you were being tested or did not feel like that at all? (Ask fluidly)
-
After attending the course, do you think you are able to do something about your risk of developing diabetes or not?
-
Did you feel uncomfortable about anything in the way the course was delivered or did not feel like that all? (Ask fluidly)
Probe:
-
What in particular if anything is mentioned.
-
Did you like being taught in a group or would have preferred one-to-one education sessions?
Probe:
-
Was the group the right size?
-
Why?
Specific aspects of the course
-
Can you remember the section of the course on how pre-diabetes happens? I am not testing you, just seeing whether you remember it?
Explain if not remembered.
-
Do you feel it important or not important to have this explained?
Probe:
-
Why?
-
Was this something that you wanted to know about or not?
-
Can you remember any of the games?
I am not testing you, just seeing whether there are some that you remembered.
If no, remind:
-
100-calorie game.
-
Physical activity games.
-
Types of fat.
-
Food continuum.
-
How did you find the 100-calorie game?
Probe:
-
What did you learn from that game?
-
How did you find the physical activity games?
Probe:
-
What did you learn from that game?
-
How did you find the types of fat game?
Probe:
-
What did you learn from that game?
-
Overall how did you find the games?
-
Did you find it a good way to learn for adults to learn or not?
-
Again I am not testing you but can you remember any of the leaflets to help you with self-management?
Prompt:
-
Health profile (traffic lights)
-
What am I going to do now?
-
Physical exercise
-
Do you think you will look at them again?
Probe:
-
Will you show them to anyone else?
-
Are you likely to use them?
-
Are you likely or unlikely to use the pedometer?
Probe:
-
Do you think you are likely or unlikely to follow the course guidance on recommended amount of steps?
-
Do you think you are likely or unlikely to follow the course guidance on recommended amount of exercise?
-
If you knew someone who was recently diagnosed with pre-diabetes, would you recommend them to come to a pre-diabetes course?
Probe:
-
If so, why?
-
If not, why?
-
Some people have said that going on a course like this, has helped with their confidence in making changes with their diet and lifestyle.
Do you feel the same way?
If so, what helped you to feel this way about your diet?
If so, what helped you to feel this way about your lifestyle?
Appendix 11 Quality development self-reflection and peer-reflection sheets
© Let’s Prevent Diabetes 2009.
Appendix 12 DESMOND observation sheet and DESMOND observer tool sheets
© Let’s Prevent 2009.
Appendix 13 Topic guide for experience of black and minority ethnic educator training
Topic guide for educators for pilot training courses for black and minority ethnic pre-diabetes course
General views about training: the before and after experience
-
When you were asked to take part in the training for the pre-diabetes course, what were your expectations about how the course would differ from the newly diagnosed course?
Prompt: (e.g. learn about pre-diabetes, style, practice).
-
After completing the training do you feel these expectations were met?
Probe:
-
In what ways?
Prompt: curriculum
-
Before you actually took part in the training, how confident were you about being able to deliver a pre-diabetes course with a 1-day training course?
-
After completing the training day, do you feel there is a need for further training or not?
-
Prior to the training, how confident were you about your knowledge of pre-diabetes?
-
Having attended the training has this increased or stayed the same?
Probe:
-
Why and how? (E.g. what part of the training helped, if at all?)
Specific aspects of training
-
Do you feel there is a need for opportunities to practise beyond the days that have decided in November and December 2007 or not?
-
How did you find the timing of the course?
-
How did you find the level of training? Prompt: easy, difficult or appropriate/just right.
Probe: Why?
-
Do you think the pre-diabetes training differed in any way to the standard DESMOND training course you have attended?
Probe: In what ways?
-
Pleased or unhappy with any aspects, discuss these.
-
What do you think the key messages of a pre-diabetes course for patients?
-
Do you think the content of the curriculum addresses these key messages or not?
Probe:
-
Explore reasons for the response (e.g. specific aspects or generally).
-
Do you feel that you will have enough time to familiarise yourself with the resources or not?
-
In a moment I will take you through specific parts of the training day, can you tell me what you found useful or not useful and why?
Probe:
-
Informative.
-
Helpful/unhelpful for delivery.
-
Development of the pre-diabetes module.
-
Philosophy and educator behaviour.
-
Individual and group work exercise to go through the curriculum to make it suitable for patients with pre-diabetes.
-
Guided tour through curriculum and key messages.
-
Preparation for ‘doing it’ for pre-diabetes.
-
‘Doing it’- Physical activity- E.
-
Professional Story C1 V.
-
Probe:
-
Do you feel that you will be able to deliver the physical activity game or not?
-
How well do you feel the game contributes to patient learning objectives?
-
Why? In what ways?
-
-
-
Reflection, confidence and action planning.
-
This course is going to be delivered to South Asian communities using interpreters, are there any particular issues that you feel that you would like some help/support/training on or not?
-
Do you think it will be difficult or easy to remember that you will be delivering to pre-diabetes patients rather than newly diagnosed patients?
-
Appendix 14 Topic guide for trainers’ feedback
Topic guide for trainers for pilot training courses for black and minority ethnic pre-diabetes course
-
Overall, how did you feel the training went?
Probe:
-
Too short or too long?
-
What do you think went worked well, in terms of the individual sessions?
-
Upon reflection which aspects do you think could be improved or revised?
-
Do you think that there is any section/s of the curriculum that educators may need additional training on?
-
How well do you think the section on physical activity went?
-
How confident do you feel after the training in the educators abilities to deliver this part of the course effectively?
-
How effective do you think the training was in helping educators to achieve their learning objectives for each new section of the curriculum?
Appendix 15 Observation notes and topic guides after retraining
Observation notes for training
-
Note down timings of each session within training.
-
Narrative of what is going –brief descriptive accounts of each activity.
-
Followed by observation of educators, trainers, interpreters:
-
problems
-
worked well
-
as per curriculum.
-
Topic guide for educators after retraining
-
Overall views.
-
Specific parts of the training:
-
do they address concerns after the first training?
-
-
Using a summary of the key findings from the last training, for example:
-
more modelling
-
aims and objectives
-
more dietary knowledge and information about South Asian foods
-
more practice with interpreters
-
more working together rather than being split up from interpreters.
-
-
Different going to training after having run some courses (i.e. is retraining useful?).
Observation notes of pilot courses
-
Times of each session.
-
Numbers attending each course including partners/relatives.
-
Times of BME sessions with/out interpreters differences.
-
Note of some of the questions raised by patients.
-
Briefing and reflection interaction between educators and interpreters.
-
New resources and their inclusion.
-
Response of patients to changes in the physical activity sessions.
Was there any difference/improvement since first set of training? This underpins all the courses
Topic guide for educators after pilot sessions
-
Overall key differences between last pilot and this one.
-
Specific bits of the curriculum (after establishing which bits with JT) how did they view the changes?
-
Patient interaction: any issues.
-
Those that did the BME without interpretation – how did they find that?
-
Getting used to working with interpreters.
-
Getting used to working with specific interpreters.
-
Are there still any problems/issues?
-
Overall aim is to find out whether changes have worked or are there any problems?
Suggestions for questions from educators for black and minority ethnic sessions – received during the formal feedback sessions
Particularly about changed sessions?
-
Fewer than four sessions?
-
How did they about being a group with mixed language needs?
-
Any particular activities liked?
-
For those who default why? Or those who attend only some?
-
Any changes – things that could be done better?
-
Did the course meet expectations?
-
Did stickers help or not?
-
How do you feel about parking questions?
-
Link between PDM and diabetes – could you detect change?
-
Was it culturally relevant?
-
Food?
-
Activities?
-
-
How did you feel attending a course with an interpreter?
-
How was action planning for you? Did it work/successful?
Suggestions for questions from educators for standard sessions – received during the formal feedback sessions
Particularly about changed sessions?
-
Have you been in a group learning situation before?
-
Did you want to come to a group education session and what are your thoughts after?
-
How are you using the resources – do they need changing? Practical issues?
-
In information easily found?
-
Feedback on educators:
-
Able to be open?
-
Style?
-
-
What follow-up would you like?
-
Who rang you and gave you information about the course? Did it influence you?
-
Would you recommend the course to friends?
-
Have patients made any changes?
-
Did they make a plan? Did they stick to it or fall by the wayside? Is plan reviewed and altered?
-
Have you talked to anyone about your plan?
-
What did you get out it?
-
What did you learn in relation to PDM?
-
What key messages?
-
Were the diet messages clear?
-
Would they prefer 2 half days or 1 full day?
Topic guide for interpreters
-
Dictionary:
-
useful
-
appropriate translations
-
how used (e.g. prepared before the session or used as you along)?
-
-
Pace of interpreting:
-
working in a group education session.
-
Appendix 16 Topic guides for telephone interviews with educators after retraining
-
How did you find the training, overall?
-
Did the training address any concerns that you may have from the first training session?
Probe each of following individually:
-
More modelling?
-
Aims and objectives of each section clear to you?
-
More dietary knowledge and information about South Asian foods?
-
More practice with interpreters?
-
Working together rather than being split into different group from interpreters?
-
Specific parts of the curriculum such as the activity section?
-
How helpful do you think the training was to help you deliver some of the revisions to the curriculum?
Specific parts of the training
-
How helpful were the following in relation to helping you deliver the revised curriculum?
-
Reviewing the pre-course materials
-
Exploring the changes to the curriculum
-
Preparing to model
-
Modelling the ‘activity’ section
-
Modelling and reflection, professional story and self management plan
-
Food continuum
-
Planning, what’s next?
-
Anything else that you would like to discuss?
Appendix 17 Invitation letter and reply slip
Appendix 18 Patient information sheet
Appendix 19 Patient invitation letter and oral glucose tolerance test instructions
Appendix 20 Consent form
Appendix 21 Patient result letter prediabetes mellitus: intervention group
Appendix 22 Patient results letter prediabetes mellitus: control group
Appendix 23 Results letter to general practitioner: control group
Appendix 24 Results letter to general practitioner: intervention arm
Appendix 25 Patient result letter: rescreen
Appendix 26 Letter to patient: confirmation of diabetes mellitus
Appendix 27 Results letter to general practitioner, patient with type 2 diabetes mellitus
Appendix 28 Baseline case report form
Appendix 29 36-month questionnaire
Appendix 30 Pedometer log
Appendix 31 Results tables
Tables adapted from Preventive Medicine, vol. 84, Melanie J Davis, Laura J Gray, Jacqui Troughton, Alastair Gray, Jaakko Tuomilehto, Azhar Farooqi, Kamlesh Khunti, Thomas Yates, A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let’s Prevent Diabetes cluster randomized controlled trial, pp. 48–56, 2016, table 3, with permission from Elsevier. 210
Biomedical outcomes
| Variable | Number of participants | Mean change from baseline (SD) | Adjusted coefficient (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Standard care | Intervention | Standard care | Intervention | |||
| Fasting glucose (mmol/l) | ||||||
| 12 months | 385 | 371 | –0.02 (0.59) | –0.02 (0.62) | 0.001 (–0.10 to 0.10) | 0.98 |
| 24 months | 348 | 350 | 0.09 (0.65) | 0.02 (0.72) | –0.06 (–0.16 to 0.04) | 0.27 |
| 36 months | 327 | 329 | 0.16 (0.64) | 0.10 (0.76) | –0.05 (–0.18 to 0.07) | 0.38 |
| Overall | 390 | 381 | – | – | 0.0004 (–0.10 to 0.10) | 0.99 |
| 2-hour glucose (mmol/l) | ||||||
| 12 months | 382 | 367 | –1.31 (2.06) | –1.27 (2.20) | 0.08 (–0.23 to 0.39) | 0.61 |
| 24 months | 337 | 333 | –1.02 (2.35) | –1.06 (2.39) | –0.07 (–0.37 to 0.22) | 0.62 |
| 36 months | 317 | 315 | –0.71 (2.45) | –0.82 (2.41) | –0.14 (–0.46 to 0.18) | 0.39 |
| Overall | 390 | 374 | – | – | –0.03 (–0.28 to 0.22) | 0.83 |
| HbA1c (%) | ||||||
| 6 months | 396 | 366 | 0.01 (0.30) | –0.06 (0.25) | –0.07 (–0.12 to –0.01) | 0.02 |
| 12 months | 379 | 361 | 0.01 (0.32) | –0.03 (0.26) | –0.04 (–0.10 to 0.02) | 0.21 |
| 24 months | 342 | 344 | 0.15 (0.37) | 0.04 (0.38) | –0.10 (–0.20 to –0.004) | 0.04 |
| 36 months | 328 | 322 | 0.01 (0.44) | –0.07 (0.39) | –0.07 (–0.18 to 0.04) | 0.19 |
| Overall | 415 | 393 | – | – | –0.06 (–0.11 to –0.01) | 0.03 |
| Total cholesterol (mmol/l) | ||||||
| 6 months | 396 | 367 | –0.31 (0.81) | –0.34 (0.66) | –0.06 (–0.18 to 0.05) | 0.30 |
| 12 months | 381 | 367 | –0.23 (0.74) | –0.28 (0.73) | –0.07 (–0.16 to 0.02) | 0.13 |
| 24 months | 351 | 352 | –0.20 (0.82) | –0.20 (0.70) | –0.02 (–0.12 to 0.08) | 0.69 |
| 36 months | 330 | 331 | –0.18 (0.90) | –0.27 (0.84) | –0.11 (–0.23 to 0.02) | 0.09 |
| Overall | 416 | 398 | – | – | –0.06 (–0.14 to 0.01) | 0.10 |
| HDL cholesterol (mmol/l) | ||||||
| 6 months | 392 | 365 | –0.06 (0.33) | –0.05 (0.40) | 0.003 (–0.05 to 0.06) | 0.92 |
| 12 months | 380 | 364 | –0.01 (0.38) | –0.01 (0.39) | –0.01 (–0.07 to 0.05) | 0.86 |
| 24 months | 345 | 348 | 0.01 (0.46) | 0.03 (0.46) | 0.004 (–0.06 to 0.07) | 0.91 |
| 36 months | 327 | 328 | 0.02 (0.46) | 0.02 (0.39) | –0.02 (–0.08 to 0.05) | 0.60 |
| Overall | 415 | 397 | – | – | 0.01 (–0.04 to 0.05) | 0.76 |
| LDL cholesterol (mmol/l) | ||||||
| 6 months | 384 | 354 | –0.36 (0.61) | –0.40 (0.61) | –0.06 (–0.15 to 0.04) | 0.23 |
| 12 months | 372 | 349 | –0.22 (0.65) | –0.29 (0.67) | –0.10 (–0.018 to –0.02) | 0.02 |
| 24 months | 336 | 336 | –0.24 (0.73) | –0.25 (0.63) | –0.02 (–0.09 to 0.05) | 0.57 |
| 36 months | 320 | 319 | –0.24 (0.78) | –0.33 (0.70) | –0.09 (–0.19 to 0.005) | 0.06 |
| Overall | 414 | 397 | – | – | –0.08 (–0.15 to –0.01) | 0.03 |
| Triglyceride (mmol/l) | ||||||
| 6 months | 394 | 367 | 0.27 (0.82) | 0.24 (0.78) | –0.01 (–0.16 to 0.14) | 0.89 |
| 12 months | 381 | 367 | –0.04 (0.67) | 0.01 (0.81) | 0.05 (–0.05 to 0.15) | 0.32 |
| 24 months | 349 | 352 | 0.03 (0.73) | –0.02 (0.70) | –0.05 (–0.15 to 0.05) | 0.31 |
| 36 months | 328 | 330 | 0.02 (0.80) | –0.05 (0.76) | –0.06 (–0.17 to 0.06) | 0.32 |
| Overall | 416 | 397 | – | – | –0.001 (–0.08 to 0.08) | 0.99 |
| Body weight (kg) | ||||||
| 6 months | 400 | 373 | –0.30 (3.67) | –0.38 (3.71) | –0.10 (–0.72 to 0.51) | 0.74 |
| 12 months | 382 | 368 | 0.02 (4.22) | –0.19 (4.57) | –0.27 (–1.17 to 0.63) | 0.56 |
| 24 months | 335 | 341 | 0.33 (4.45) | –0.14 (4.77) | –0.49 (–1.48 to 0.50) | 0.34 |
| 36 months | 321 | 321 | –0.46 (5.02) | –0.59 (4.59) | –0.26 (–1.17 to 0.65) | 0.58 |
| Overall | 413 | 391 | – | – | –0.10 (–0.85 to 0.66) | 0.80 |
| BMI (kg/m2) | ||||||
| 6 months | 400 | 373 | –0.11 (1.30) | –0.13 (1.32) | –0.03 (–0.24 to 0.19) | 0.81 |
| 12 months | 381 | 368 | 0.01 (1.52) | –0.08 (1.72) | –0.11 (–0.42 to 0.21) | 0.51 |
| 24 months | 335 | 341 | 0.12 (1.57) | –0.04 (1.71) | –0.14 (–0.50 to 0.21) | 0.42 |
| 36 months | 321 | 321 | –0.17 (1.77) | –0.17 (1.66) | –0.05 (–0.38 to 0.27) | 0.75 |
| Overall | 413 | 391 | – | – | –0.02 (–0.28 to 0.25) | 0.91 |
| Waist circumference (cm) | ||||||
| 6 months | 399 | 373 | –2.15 (5.63) | –2.73 (5.93) | –0.91 (–2.03 to 0.20) | 0.11 |
| 12 months | 381 | 369 | –2.73 (5.40) | –2.53 (5.79) | –0.11 (–1.37 to 1.15) | 0.87 |
| 24 months | 332 | 341 | –2.14 (6.03) | –2.67 (5.89) | –0.82 (–2.03 to 0.40) | 0.19 |
| 36 months | 320 | 320 | –3.13 (6.32) | –3.63 (5.80) | –0.79 (–1.73 to 0.14) | 0.10 |
| Overall | 414 | 391 | – | – | –0.45 (–1.32 to 0.42) | 0.31 |
| Systolic BP (mmHg) | ||||||
| 6 months | 401 | 373 | –9.85 (17.10) | –8.64 (17.51) | 1.17 (–1.45 to 3.79) | 0.38 |
| 12 months | 382 | 370 | –8.33 (15.65) | –7.54 (17.00) | 1.22 (–0.85 to 3.30) | 0.25 |
| 24 months | 336 | 343 | –7.11 (15.40) | –9.06 (16.10) | –1.26 (–3.79 to 1.28) | 0.33 |
| 36 months | 322 | 325 | –8.00 (17.36) | –7.57 (16.76) | 0.55 (–2.09 to 3.19) | 0.68 |
| Overall | 414 | 391 | – | – | 0.81 (–0.97 to 2.60) | 0.37 |
| Diastolic BP (mmHg) | ||||||
| 6 months | 401 | 373 | –3.55 (10.36) | –3.65 (10.00) | –0.22 (–1.90 to 1.46) | 0.80 |
| 12 months | 382 | 370 | –4.82 (9.64) | 4.15 (9.99) | 0.80 (–0.66 to 2.26) | 0.28 |
| 24 months | 336 | 343 | –3.69 (9.78) | –4.57 (9.21) | –0.37 (–1.92 to 1.19) | 0.64 |
| 36 months | 322 | 325 | –2.50 (10.92) | –3.52 (9.87) | –0.49 (–2.15 to 1.17) | 0.56 |
| Overall | 414 | 391 | – | – | 0.24 (–0.82 to 1.30) | 0.66 |
| Heart rate (b.p.m.) | ||||||
| 6 months | 396 | 372 | 2.33 (9.84) | 1.59 (10.12) | –1.31 (–2.90 to 0.28) | 0.11 |
| 12 months | 379 | 368 | 0.03 (8.52) | –0.12 (9.31) | –0.61 (–1.84 to 0.61) | 0.33 |
| 24 months | 335 | 338 | 0.53 (9.49) | 0.35 (9.79) | –0.68 (–2.00 to 0.65) | 0.32 |
| 36 months | 319 | 323 | –0.63 (10.12) | –0.70 (9.82) | –0.52 (–1.83 to 0.78) | 0.43 |
| Overall | 413 | 391 | – | – | –0.66 (–1.58 to 0.27) | 0.16 |
Ethrisk/Framingham outcomes
| Variable | Number of participants | Mean change from baseline (SD) | Adjusted coefficient (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Standard care | Intervention | Standard care | Intervention | |||
| CHD 10-year risk | ||||||
| 6 months | 163 | 148 | –0.01 (0.05) | –0.01 (0.04) | –0.0002 (–0.01 to 0.01) | 0.97 |
| 12 months | 168 | 180 | –0.01 (0.05) | –0.01 (0.04) | –0.001 (–0.01 to 0.01) | 0.87 |
| 24 months | 136 | 153 | –0.001 (0.05) | –0.01 (0.04) | –0.01 (–0.02 to 0.002) | 0.12 |
| 36 months | 119 | 144 | –0.004 (0.05) | –0.004 (0.05) | 0.004 (–0.007 to 0.01) | 0.49 |
| Overall | 215 | 218 | – | – | –0.004 (–0.01 to 0.002) | 0.19 |
| CVD-10 year risk | ||||||
| 6 months | 163 | 149 | –0.03 (0.08) | –0.02 (0.08) | 0.01 (–0.01 to 0.02) | 0.38 |
| 12 months | 168 | 180 | –0.02 (0.07) | –0.02 (0.06) | 0.003 (–0.01 to 0.01) | 0.64 |
| 24 months | 136 | 153 | –0.005 (0.08) | –0.02 (0.06) | –0.01 (–0.03 to 0.001) | 0.07 |
| 36 months | 119 | 144 | –0.01 (0.08) | –0.01 (0.07) | 0.01 (–0.004 to 0.02) | 0.16 |
| Overall | 215 | 218 | – | – | 0.002 (–0.02 to 0.03)a | 0.88 |
| Number of participants | Number with CVD > 20% | Adjusted OR (95% CI) | p-value | |||
| Standard care | Intervention | Standard care | Intervention | |||
| CVD 10-year risk > 20% | ||||||
| 6 months | 163 | 148 | 56 | 58 | 1.22 (0.68 to 2.18) | 0.50 |
| 12 months | 168 | 180 | 60 | 68 | 0.94 (0.61 to 1.46) | 0.78 |
| 24 months | 136 | 153 | 49 | 59 | 0.82 (0.45 to 1.52) | 0.54 |
| 36 months | 119 | 144 | 44 | 67 | 1.34 (0.70 to 2.53) | 0.38 |
Metabolic syndrome outcome
| Variable | Number (%) with metabolic syndrome | Adjusted OR (95% CI) | p-value | |
|---|---|---|---|---|
| Standard care | Intervention | |||
| NCEP ATP III Criteria | ||||
| 12 months | 263 (60.7) | 275 (61.5) | 1.05 (0.78 to 1.43) | 0.74 |
| 24 months | 296 (68.4) | 286 (64.0) | 0.81 (0.60 to 1.09) | 0.16 |
| 36 months | 278 (64.2) | 295 (66.0) | 1.10 (0.83 to 1.46) | 0.52 |
Lifestyle outcomes
| Variable | Number of participants | Median (IQR) | Adjusted coefficient (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Standard care | Intervention | Standard care | Intervention | |||
| Illness perception score | ||||||
| 6 months | 334 | 300 | 30 (25–37) | 28 (21–36) | –1.46 (–3.13 to 0.20) | 0.09 |
| 12 months | 314 | 311 | 30 (23–37) | 29 (22–35) | –2.06 (–4.03 to –0.09) | 0.04 |
| 24 months | 305 | 299 | 30 (23–37) | 28 (20–36) | –2.47 (–4.16 to –0.78) | 0.004 |
| 36 months | 285 | 275 | 30 (24–38) | 30 (22–37) | –1.16 (–2.69 to 0.37) | 0.14 |
| Overall | 394 | 373 | – | – | –1.61 (–2.92 to –0.30) | 0.02 |
| Quality of life | ||||||
| 6 months | 316 | 283 | 0.92 (0.85–0.96) | 0.91 (0.83–0.96) | 0.01 (–0.002 to 0.01) | 0.15 |
| 12 months | 313 | 301 | 0.91 (0.85–0.96) | 0.91 (0.84–0.96) | 0.01 (–0.002 to 0.02) | 0.13 |
| 24 months | 266 | 269 | 0.91 (0.83–0.96) | 0.90 (0.83–0.95) | 0.01 (–0.002 to 0.02) | 0.12 |
| 36 months | 250 | 245 | 0.89 (0.82–0.95) | 0.91 (0.84–0.96) | 0.02 (0.01 to 0.03) | 0.01 |
| Overall | 367 | 354 | – | – | 0.01 (0.001 to 0.02) | 0.03 |
| Anxiety score | ||||||
| 6 months | 387 | 360 | 4 (2–7) | 4 (2–7) | –0.21 (–0.57 to 0.15) | 0.25 |
| 12 months | 378 | 368 | 4 (2–7) | 4 (2–7) | –0.40 (–0.77 to –0.03) | 0.03 |
| 24 months | 331 | 340 | 4 (2–6) | 4 (2–6) | –0.09 (–0.40 to 0.21) | 0.55 |
| 36 months | 322 | 322 | 4 (2–7) | 4.5 (2–7) | –0.11 (–0.44 to 0.23) | 0.53 |
| Overall | 407 | 390 | – | – | –0.28 (–0.54 to –0.02) | 0.03 |
| Depression score | ||||||
| 6 months | 387 | 360 | 2 (1–5) | 2 (1–5) | –0.08 (–0.42 to 0.26) | 0.64 |
| 12 months | 378 | 368 | 3 (1–6) | 2 (1–5) | –0.34 (–0.81 to 0.14) | 0.16 |
| 24 months | 331 | 340 | 3 (1–6) | 3 (1–5) | –0.09 (–0.45 to 0.27) | 0.62 |
| 36 months | 322 | 322 | 3 (1–5) | 3 (1–5) | –0.05 (–0.44 to 0.35) | 0.82 |
| Overall | 407 | 390 | – | – | –0.21 (–0.57 to 0.16) | 0.27 |
| Fibre intake | ||||||
| 6 months | 171 | 140 | 33 (25–40) | 33 (24–42.5) | –1.69 (–4.68 to 1.29) | 0.27 |
| 12 months | 194 | 178 | 31 (25–40) | 33 (26–42) | 0.97 (–1.27 to 3.21) | 0.40 |
| 24 months | 153 | 156 | 34 (26–42) | 33 (26–42) | –1.64 (–4.68 to 1.39) | 0.29 |
| 36 months | 153 | 157 | 33 (24–42) | 33 (25–43) | 1.53 (–0.94 to 4.00) | 0.23 |
| Overall | 297 | 286 | – | – | –1.01 (–3.11 to 1.08) | 0.34 |
| Fat intake | ||||||
| 6 months | 122 | 111 | 24 (17–32) | 22 (15–29) | –1.41 (–4.60 to 1.77) | 0.38 |
| 12 months | 155 | 129 | 24 (15–31) | 24 (16–31) | 0.45 (–2.62 to 3.51) | 0.78 |
| 24 months | 117 | 107 | 23 (15–33) | 23 (16–33) | –0.55 (–4.04 to 2.95) | 0.76 |
| 36 months | 139 | 105 | 24 (15–35) | 23 (17–30) | –3.60 (–7.52 to 0.31) | 0.07 |
| Overall | 257 | 228 | – | – | –0.72 (–2.92 to 1.48) | 0.52 |
| Unsaturated fat intake | ||||||
| 6 months | 299 | 276 | 10 (9–11) | 10 (9–11) | 0.18 (–0.11 to 0.48) | 0.23 |
| 12 months | 297 | 300 | 10 (8–11) | 10 (9–11) | 0.32 (0.05 to 0.58) | 0.02 |
| 24 months | 268 | 270 | 10 (8–11) | 10 (9–11) | 0.50 (0.24 to 0.76) | < 0.0001 |
| 36 months | 253 | 271 | 9 (8–11) | 10 (9–11) | 0.38 (0.12 to 0.63)a | 0.004 |
| Overall | 378 | 368 | – | – | 0.33 (0.15 to 0.51) | < 0.0001 |
| Walking METs | ||||||
| 6 months | 311 | 311 | 693 (99–1584) | 693 (132–1980) | 87.68 (–254.26 to 429.63) | 0.62 |
| 12 months | 328 | 326 | 792 (396–1980) | 1188 (396–2772) | 200.54 (–90.68 to 491.76) | 0.18 |
| 24 months | 285 | 307 | 792 (396–1980) | 1188 (495–2772) | 143.18 (–133.40 to 419.75) | 0.31 |
| 36 months | 263 | 283 | 990 (396–2079) | 1188 (462–2310) | –20.03 (–287.00 to 246.93) | 0.88 |
| Overall | 392 | 379 | – | – | 159.60 (–72.89 to 392.10) | 0.18 |
| Moderate METs | ||||||
| 6 months | 302 | 303 | 0 (0–720) | 0 (0–960) | 123.90 (–189.44 to 437.24) | 0.44 |
| 12 months | 316 | 318 | 0 (0–960) | 240 (0–1440) | 102.18 (–64.53 to 268.88) | 0.23 |
| 24 months | 384 | 276 | 0 (0–960) | 240 (0–1160) | 128.16 (–67.06 to 323.37) | 0.20 |
| 36 months | 263 | 261 | 120 (0–1440) | 360 (0–1200) | 28.65 (–180.84 to 238.14) | 0.79 |
| Overall | 393 | 376 | – | – | 144.18 (–25.98 to 314.33) | 0.10 |
| Vigorous METs | ||||||
| 6 months | 313 | 309 | 0 (0–960) | 0 (0–1440) | 154.34 (–174.69 to 483.38) | 0.36 |
| 12 months | 327 | 335 | 0 (0–1080) | 0 (0–1440) | 173.82 (–116.09 to 463.73) | 0.24 |
| 24 months | 287 | 304 | 0 (0–960) | 0 (0–1440) | 214.85 (–95.50 to 525.21) | 0.18 |
| 36 months | 272 | 279 | 0 (0–960) | 0 (0–1440) | –7.14 (–279.77 to 265.49) | 0.96 |
| Overall | 399 | 382 | – | – | 160.05 (–52.07 to 372.17) | 0.14 |
| Total METs | ||||||
| 6 months | 266 | 273 | 1386 (132–3825) | 1782 (330–4518) | 352.71 (–570.24 to 1275.65) | 0.45 |
| 12 months | 265 | 280 | 2079 (693–4425) | 2763 (1157.5–5185.5) | 447.31 (–220.84 to 1115.46) | 0.19 |
| 24 months | 235 | 239 | 1980 (693–4158) | 2439 (1039.5–4878) | 415.06 (–235.47 to 1064.59) | 0.21 |
| 36 months | 206 | 220 | 2102.3 (792–4320) | 2365.5 (1049.3–4638) | –19.82 (–568.05 to 528.41) | 0.94 |
| Overall | 368 | 363 | – | – | 428.37 (–175.19 to 1031.93) | 0.16 |
| Sitting time | ||||||
| 6 months | 284 | 280 | 300 (240–420) | 300 (180–360) | –27.26 (–63.34 to 8.83) | 0.14 |
| 12 months | 293 | 314 | 300 (240–480) | 300 (180–360) | –25.94 (–49.95 to –1.92) | 0.03 |
| 24 months | 266 | 298 | 300 (240–420) | 240 (180–360) | –38.96 (–66.15 to –11.78) | 0.01 |
| 36 months | 258 | 274 | 300 (210–420) | 300 (180–360) | –20.15 (–43.91 to 3.60) | 0.10 |
| Overall | 372 | 367 | – | – | –26.29 (–45.26 to –7.32) | 0.01 |
| Average steps | ||||||
| 6 months | 331 | 313 | 5764 (4043–7624) | 6076 (4321–8251) | 591.38 (63.61 to 1119.16) | 0.03 |
| 12 months | 337 | 302 | 5579 (3992–7713) | 6215 (4364–8414) | 551.76 (117.27 to 986.25) | 0.01 |
| 24 months | 287 | 280 | 5523 (3800–7816) | 5965 (4348–8364) | 466.30 (–65.50 to 998.10) | 0.09 |
| 36 months | 252 | 235 | 4936 (3633–6659) | 5714 (3815–8009) | 535.76 (12.71 to 1058.81) | 0.05 |
| Hours slept last night | ||||||
| 12 months | 363 | 357 | 7 (6–7) | 7 (6–7.5) | 0.04 (–0.15 to 0.22) | 0.68 |
| 24 months | 324 | 325 | 7 (6–7.5) | 7 (6–8) | –0.05 (–0.18 to 0.09) | 0.51 |
| 36 months | 314 | 315 | 7 (6–7.5) | 7 (6–7.5) | –0.10 (–0.26 to 0.06) | 0.23 |
| Overall | 383 | 372 | – | – | –0.05 (–0.18 to 0.08) | 0.47 |
| Average hours asleep in 24 hours | ||||||
| 12 months | 361 | 355 | 7.5 (7–8) | 8 (7–8) | 0.10 (–0.16 to 0.35) | 0.46 |
| 24 months | 324 | 323 | 7.5 (7–8) | 8 (7–8) | –0.03 (–0.23 to 0.17) | 0.77 |
| 36 months | 312 | 311 | 7.5 (7–8) | 8 (7–8) | 0.11 (–0.06 to 0.27) | 0.20 |
| Overall | 383 | 371 | – | – | 0.01 (–0.16 to 0.18) | 0.94 |
Appendix 32 Supplementary tables for health economics calculations
| Category | Cost (£) | Note | Source |
|---|---|---|---|
| Non-inpatient costs | |||
| GP (home) | 114 | Visit time of 23.4 minutes | PSSRU 2012/13221 |
| GP (practice) | 45 | Average patient contact 11.7 minutes | PSSRU 2012/13221 |
| Nurse (practice) | 13.40 | Hourly cost of £40 with average duration of patient contact of 15.5 minutes | PSSRU 2012/13221 |
| Nurse (home) | 70 | £70 per hour of home visiting, with 5.6 patient contacts per day on average and 69% of an 8-hour day spent on home visits | PSSRU 2012/13221 |
| Other HCW (practice) | 13.40 | Nurse cost used | PSSRU 2012/13221 |
| Other HCW (home) | 70 | Nurse cost used | PSSRU 2012/13221 |
| A&E attendance | 114.86 | Weighted mean of type 1 attendances not resulting in admission | NHS reference costs 2012/13222 |
| Outpatient attendance | 108 | Weighted mean of all outpatient visits | NHS reference costs 2012/13222 |
| Day hospital visit | 692 | Day procedure data used | NHS reference costs 2012/13222 |
| Inpatient costs | |||
| Admission cost | 1758 | Elective and non-elective admissions | NHS reference costs 2012/13222 |
| Per bed-day cost | 586 | NHS reference costs 2012/13222 | |
| Medication costs | All costs are weighted means (relative to frequency of national use) of the relevant category, assuming one tablet/day unless otherwise specified | ||
| ACE inhibitor | 18.43 | PCA 2013223 | |
| Alpha blocker | 27.42 | PCA 2013223 | |
| ARB | 49.35 | PCA 2013223 | |
| Beta blocker | 22.33 | PCA 2013223 | |
| CCB | 32.78 | PCA 2013223 | |
| Diuretics | 22.07 | PCA 2013223 | |
| Aspirin | 11.09 | PCA 2013223 | |
| Statins | 50.46 | PCA 2013223 | |
| Fibrates | 105.75 | PCA 2013223 | |
| Thyroid medications | 31.50 | PCA 2013223 | |
| Steroids | 240.57 | Composite cost of oral and inhaled steroids in a 0.3 : 0.7 ratio (ratio based on within-trial steroid route data available). For inhaled 1 unit/month assumed. High cost reflects branded inhaled steroid combination inhalers | PCA 2013223 |
| Other medications | PCA 2013223 | ||
| NSAIDs | 28.04 | PCA 2013223 | |
| H1 blockers | 17.32 | PCA 2013223 | |
| H2 blockers | 17.04 | PCA 2013223 | |
| Bisphosphonates | 14.01 | 1 per week use assumed; only alendronate and risedronate included as no other medications reported | PCA 2013223 |
| Antiplatelet | 55.83 | Aspirin not included | PCA 2013223 |
| Simple analgesics | 13.71 | Not including NSAIDs. Includes weak opiates [not tramadol (Tramal, Grünenthal Ltd)] | PCA 2013223 |
| Opiate analgesia | 53.31 | Includes tramadol | PCA 2013223 |
| Gout medications | 27.79 | PCA 2013223 | |
| Benzodiazepines | 76.29 | PCA 2013223 | |
| Calcium | 34.13 | Calcium supplements prescribed by NHS (e.g. Adcal-D3® chewable tablets, Biokirch, Germany) | PCA 2013223 |
| PPIs | 25.72 | PCA 2013223 | |
| Antibiotics | 2.41 | 7-day course assumed | PCA 2013223 |
| Anticoagulants | 26.16 | PCA 2013223 | |
| Iron supplements | 11.56 | PCA 2013223 | |
| Laxatives | 13.76 | PCA 2013223 | |
| Non-steroid inhalers | 12.33 | 1 unit/month assumed | PCA 2013223 |
| Eyedrops | 6.60 | 1 unit/month assumed | PCA 2013223 |
| Antidepressants | 49.33 | PCA 2013223 | |
| Antiepileptics | 92.38 | PCA 2013223 | |
| Antipsychotics | 150.42 | PCA 2013223 | |
| Antiarrhythmics | 51.49 | PCA 2013223 | |
| Nasal sprays | 22.44 | 1 unit/month assumed | PCA 2013223 |
| Oral contraceptives | 30.80 | PCA 2013223 | |
| Bladder agents | 203.67 | PCA 2013223 | |
| Parkinson’s disease drugs | 129.68 | PCA 2013223 | |
| Triptans | 16.45 | 1 unit/month assumed | PCA 2013223 |
| Long-acting nitrates | 78.75 | PCA 2013223 | |
| Short-acting nitrates | 12.41 | 1 unit/month assumed | PCA 2013223 |
| Oral hypoglycaemics | 18.13 | PCA 2013223 | |
| Sulfa drugs | 107.04 | PCA 2013223 | |
| Rheumatoid drugs | 64.24 | PCA 2013223 | |
| HRT | 75.55 | PCA 2013223 | |
| Antiemetics | 28.26 | PCA 2013223 | |
| Non-categorised | 63.6 | Average cost of all above medications excluding biologics. Medications divided into multiple categories by system and assigned this cost | PCA 2013223 |
| Anti-TNF biologics | 1679 | No other biologics reported by patients | PCA 2013223 |
| Variable name | Missing values (%) | ||
|---|---|---|---|
| Total | Intervention | Control | |
| Age at trial entry | 0 | 0 | 0 |
| Male or female | 0 | 0 | 0 |
| BMI at baseline | 0 | 0 | 0 |
| 15D at baseline | 21 | 20 | 22 |
| Treatment allocation | 0 | 0 | 0 |
| Cluster | 0 | 0 | 0 |
| Smoking status at baseline | 0.1 | 0.2 | 0 |
| Cholesterol at baseline | 0.8 | 1 | 0.5 |
| LDL at baseline | 3 | 3 | 2 |
| HDL at baseline | 1 | 1 | 1 |
| Creatinine at baseline | 0.8 | 1 | 0.5 |
| Ethnicity | 0 | 0 | 0 |
| Atrial fibrillation baseline | 1 | 0 | 2 |
| PVD baseline | 1 | 0 | 2 |
| HbA1c at baseline | 1 | 2 | 0.5 |
| eGFR at baseline | 1 | 1 | 1 |
| Systolic BP at baseline | 0 | 0 | 0 |
| Medication at baseline | 2 | 1 | 3 |
| EQ-5D at 12 months | 98 | 97 | 99 |
| EQ-5D at 24 months | 58 | 60 | 55 |
| EQ-5D at 36 months | 36 | 36 | 33 |
| 15D at 12 months | 30 | 33 | 28 |
| 15D at 24 months | 40 | 41 | 39 |
| 15D at 36 months | 45 | 47 | 43 |
| Non-hospital contacts at 12 months | 98 | 96 | 99 |
| Non-hospital contacts at 24 months | 56 | 57 | 55 |
| Non-hospital contacts at 36 months | 32 | 34 | 30 |
| Hospital admissions at 12 months | 98 | 97 | 99 |
| Hospital admissions at 24 months | 62 | 62 | 62 |
| Hospital admissions at 36 months | 41 | 43 | 39 |
| Medication at 12 months | 19 | 19 | 19 |
| Medication at 24 months | 36 | 40 | 32 |
| Medication at 36 months | 41 | 42 | 40 |
Appendix 33 A report of the outcome of the Walking Away course
List of abbreviations
- 15D
- health-state descriptive system
- A&E
- accident and emergency
- ACTID
- Early Activity in Diabetes
- ADA
- American Diabetes Association
- ADDITION
- Anglo–Danish–Dutch study of Intensive Treatment In people with screen detected diabetes in primary care
- AUC
- area under the curve
- BME
- black and minority ethnic
- BMI
- body mass index
- BP
- blood pressure
- CCG
- clinical commissioning group
- CDRS
- Cambridge Diabetes Risk Score
- CHD
- coronary heart disease
- CI
- confidence interval
- CLAHRC
- Collaboration for Leadership in Applied Health Research and Care
- CVD
- cardiovascular disease
- DEPLAN
- Diabetes in Europe Prevention using Lifestyle, physical Activity and Nutritional intervention
- DESMOND
- Diabetes Education and Self-Management for Ongoing and Newly Diagnosed
- DINE
- Dietary Instrument for Nutritional Education
- DPP
- Diabetes Prevention Program
- DPS
- Finnish Diabetes Prevention Study
- EMIS
- Egton Medical Information Systems
- EQ-5D
- European Quality of Life-5 Dimensions
- FINDRISC
- Finnish Diabetes Risk Score
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HDL
- high-density lipoprotein
- HR
- hazard ratio
- ICER
- incremental cost-effectiveness ratio
- IFG
- impaired fasting glucose
- IGR
- impaired glucose regulation
- IGT
- impaired glucose tolerance
- IL-6
- interleukin 6
- IMAGE
- Development and Implementation of a European Guideline and Training Standards for Diabetes prevention
- IMD
- Index of Multiple Deprivation
- IPAQ
- International Physical Activity Questionnaire
- IQR
- interquartile range
- ITT
- intention to treat
- LDL
- low-density lipoprotein
- LPRS
- Leicester Practice Risk Score
- LR+
- likelihood ratio for a positive test
- LR−
- likelihood ratio for a negative test
- MET
- metabolic equivalent for task
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- OGTT
- oral glucose tolerance test
- PCT
- primary care trust
- PDM
- prediabetes mellitus
- PPV
- positive predictive value
- PREPARE
- Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement
- QALY
- quality-adjusted life-year
- QDScore
- multivariable risk score to predict the 10-year risk of acquiring type 2 diabetes mellitus
- R&D
- research and development
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- SD
- standard deviation
- SE
- standard error
- STAR
- Screening Those At Risk
- T2DM
- type 2 diabetes mellitus
- TNF
- tumour necrosis factor
- WHO
- World Health Organization